User login
A Rare Case of Spontaneous Fusion of the Knee
Take-Home Points
- Post-infectious or post-inflammatory pathological knee arthrodesis is one of the most challenging complications in orthopedics.
- It can result in significant patient distress with some struggling to maintain any range of motion for functionality.
- TKA for the correction of knee ankylosis is an option, but not without significant morbidity and failure rates.
Spontaneous knee fusion is an unusual and rarely reported phenomenon. Progressive stiffness is commonly experienced by patients with arthritis. However, most patients maintain some range of knee motion, which may be enhanced with medical treatment, rehabilitation with physiotherapy, and ambulation devices. To our knowledge, this article is the first report of a case of spontaneous and progressive bony fusion of a knee joint without a prior diagnosis of inflammatory or septic arthritis or surgical arthrodesis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In 2015, a 51-year-old woman presented to the orthopedics department with a 13-year history of complete loss of left knee flexion. She denied a history of trauma to or surgical intervention for the knee and denied a medical history of inflammatory or septic arthritis.
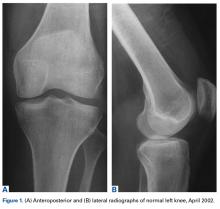
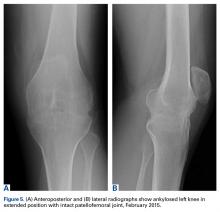
On initial referral to the department, in 2002, the patient, age 38 years at the time, had a 1-year history of progressive left knee stiffness and reduced range of motion (ROM). At the time, she recalled injuring the knee during an aerobics class 2 months prior. A physiotherapy trial (ROM actively and passively assessed 10°-90°) failed. All movement was painful, and 2 crutches were needed for ambulation. The patient was treated nonoperatively with analgesia and was advised to return to physiotherapy. Plain radiographs showed a small effusion but no bony abnormalities or fractures (Figures 1A, 1B).
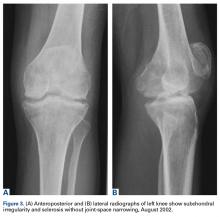
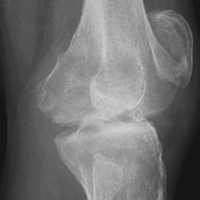
Four months after the initial referral, the patient returned to the outpatient department with persistent knee pain and ROM of 5° to 20°. A repeat radiograph showed extensive left knee joint destruction, cortical irregularity, and narrowing of the joint space (Figures 3A, 3B).
At the latest presentation (2015), the patient had a painless fixed extension deformity of the left knee joint and poor quality of life and wanted surgical intervention.
Discussion
We have reported a rare case of spontaneous knee fusion in a middle-aged patient with no significant predisposing factors and no clear diagnosis. Serologic results were normal and not significant, but imaging was highly suggestive of an inflammatory process and provided a probable diagnosis of an underlying inflammatory condition and/or infection.
In the literature, there are no other reports of similar cases of spontaneous knee joint fusion, though there are some rare cases of the phenomenon in other joints. In 2005, Budoff and Lichtman1 reported a case of spontaneous wrist fusion in an 18-year-old patient with a background of Kienböck disease, which may have predisposed the patient to an underlying synovitis progressing to autofusion of the joint. In 2014, Lui2 described the case of a 64-year-old woman with spontaneous subtalar fusion complicating a subtalar arthroereisis. Although an extensive literature review on the topic is difficult owing to the rarity of the condition, these few cases, unlike our case, appear to describe a predisposing factor or inciting event.
The reversibility of knee arthrodesis remains an issue in our patient’s case and in other cases, and total knee arthroplasty (TKA) may be the most obvious operative intervention. Cameron and Hu3 reported 17 cases of knee fusion take-down with conversion to TKA, and Kim and colleagues4 reported 16 TKAs performed after spontaneous osseous ankylosis and 14 performed after formal knee fusion take-down. Although functional improvements were found in both studies, complication rates were relatively high, at least 53%. Other authors have used TKAs in cases of knee ankylosis after infectious or inflammatory arthritis, but results were suboptimal and unpredictable, and complication rates were 27% and 53.3%.5,6In this difficult scenario, our middle-aged patient’s fixed extension deformity of the knee, likely the result of an idiopathic process, led to severe debilitation and poor quality of life. To perform a TKA in a 51-year-old patient is far from ideal. The reversibility of formally fused and spontaneously fused knees is still in question, and, though there are reports of relatively satisfactory results, most operative options are fraught with complications.
Am J Orthop. 2017;46(2):E83-E85. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Budoff JE, Lichtman DM. Spontaneous wrist fusion: an unusual complication of Kienböck’s disease. J Hand Surg Am. 2005;30(1):59-64.
2. Lui TH. Spontaneous subtalar fusion: an irreversible complication of subtalar arthroereisis. J Foot Ankle Surg. 2014;53(5):652-656.
3. Cameron HU, Hu C. Results of total knee arthroplasty following takedown of formal knee fusion. J Arthroplasty. 1996;11(6):732-737.
4. Kim YH, Kim JS, Cho SH. Total knee arthroplasty after spontaneous osseous ankylosis and takedown of formal knee fusion. J Arthroplasty. 2000;15(4):453-460.
5. Rajgopal A, Ahuja N, Dolai B. Total knee arthroplasty in stiff and ankylosed knees. J Arthroplasty. 2005;20(5):585-590.
6. Kim YH, Cho SH, Kim JS. Total knee arthroplasty in bony ankylosis in gross flexion. J Bone Joint Surg Br. 1999;81(2):296-300.
Take-Home Points
- Post-infectious or post-inflammatory pathological knee arthrodesis is one of the most challenging complications in orthopedics.
- It can result in significant patient distress with some struggling to maintain any range of motion for functionality.
- TKA for the correction of knee ankylosis is an option, but not without significant morbidity and failure rates.
Spontaneous knee fusion is an unusual and rarely reported phenomenon. Progressive stiffness is commonly experienced by patients with arthritis. However, most patients maintain some range of knee motion, which may be enhanced with medical treatment, rehabilitation with physiotherapy, and ambulation devices. To our knowledge, this article is the first report of a case of spontaneous and progressive bony fusion of a knee joint without a prior diagnosis of inflammatory or septic arthritis or surgical arthrodesis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In 2015, a 51-year-old woman presented to the orthopedics department with a 13-year history of complete loss of left knee flexion. She denied a history of trauma to or surgical intervention for the knee and denied a medical history of inflammatory or septic arthritis.
On initial referral to the department, in 2002, the patient, age 38 years at the time, had a 1-year history of progressive left knee stiffness and reduced range of motion (ROM). At the time, she recalled injuring the knee during an aerobics class 2 months prior. A physiotherapy trial (ROM actively and passively assessed 10°-90°) failed. All movement was painful, and 2 crutches were needed for ambulation. The patient was treated nonoperatively with analgesia and was advised to return to physiotherapy. Plain radiographs showed a small effusion but no bony abnormalities or fractures (Figures 1A, 1B).
Four months after the initial referral, the patient returned to the outpatient department with persistent knee pain and ROM of 5° to 20°. A repeat radiograph showed extensive left knee joint destruction, cortical irregularity, and narrowing of the joint space (Figures 3A, 3B).
At the latest presentation (2015), the patient had a painless fixed extension deformity of the left knee joint and poor quality of life and wanted surgical intervention.
Discussion
We have reported a rare case of spontaneous knee fusion in a middle-aged patient with no significant predisposing factors and no clear diagnosis. Serologic results were normal and not significant, but imaging was highly suggestive of an inflammatory process and provided a probable diagnosis of an underlying inflammatory condition and/or infection.
In the literature, there are no other reports of similar cases of spontaneous knee joint fusion, though there are some rare cases of the phenomenon in other joints. In 2005, Budoff and Lichtman1 reported a case of spontaneous wrist fusion in an 18-year-old patient with a background of Kienböck disease, which may have predisposed the patient to an underlying synovitis progressing to autofusion of the joint. In 2014, Lui2 described the case of a 64-year-old woman with spontaneous subtalar fusion complicating a subtalar arthroereisis. Although an extensive literature review on the topic is difficult owing to the rarity of the condition, these few cases, unlike our case, appear to describe a predisposing factor or inciting event.
The reversibility of knee arthrodesis remains an issue in our patient’s case and in other cases, and total knee arthroplasty (TKA) may be the most obvious operative intervention. Cameron and Hu3 reported 17 cases of knee fusion take-down with conversion to TKA, and Kim and colleagues4 reported 16 TKAs performed after spontaneous osseous ankylosis and 14 performed after formal knee fusion take-down. Although functional improvements were found in both studies, complication rates were relatively high, at least 53%. Other authors have used TKAs in cases of knee ankylosis after infectious or inflammatory arthritis, but results were suboptimal and unpredictable, and complication rates were 27% and 53.3%.5,6In this difficult scenario, our middle-aged patient’s fixed extension deformity of the knee, likely the result of an idiopathic process, led to severe debilitation and poor quality of life. To perform a TKA in a 51-year-old patient is far from ideal. The reversibility of formally fused and spontaneously fused knees is still in question, and, though there are reports of relatively satisfactory results, most operative options are fraught with complications.
Am J Orthop. 2017;46(2):E83-E85. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Post-infectious or post-inflammatory pathological knee arthrodesis is one of the most challenging complications in orthopedics.
- It can result in significant patient distress with some struggling to maintain any range of motion for functionality.
- TKA for the correction of knee ankylosis is an option, but not without significant morbidity and failure rates.
Spontaneous knee fusion is an unusual and rarely reported phenomenon. Progressive stiffness is commonly experienced by patients with arthritis. However, most patients maintain some range of knee motion, which may be enhanced with medical treatment, rehabilitation with physiotherapy, and ambulation devices. To our knowledge, this article is the first report of a case of spontaneous and progressive bony fusion of a knee joint without a prior diagnosis of inflammatory or septic arthritis or surgical arthrodesis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In 2015, a 51-year-old woman presented to the orthopedics department with a 13-year history of complete loss of left knee flexion. She denied a history of trauma to or surgical intervention for the knee and denied a medical history of inflammatory or septic arthritis.
On initial referral to the department, in 2002, the patient, age 38 years at the time, had a 1-year history of progressive left knee stiffness and reduced range of motion (ROM). At the time, she recalled injuring the knee during an aerobics class 2 months prior. A physiotherapy trial (ROM actively and passively assessed 10°-90°) failed. All movement was painful, and 2 crutches were needed for ambulation. The patient was treated nonoperatively with analgesia and was advised to return to physiotherapy. Plain radiographs showed a small effusion but no bony abnormalities or fractures (Figures 1A, 1B).
Four months after the initial referral, the patient returned to the outpatient department with persistent knee pain and ROM of 5° to 20°. A repeat radiograph showed extensive left knee joint destruction, cortical irregularity, and narrowing of the joint space (Figures 3A, 3B).
At the latest presentation (2015), the patient had a painless fixed extension deformity of the left knee joint and poor quality of life and wanted surgical intervention.
Discussion
We have reported a rare case of spontaneous knee fusion in a middle-aged patient with no significant predisposing factors and no clear diagnosis. Serologic results were normal and not significant, but imaging was highly suggestive of an inflammatory process and provided a probable diagnosis of an underlying inflammatory condition and/or infection.
In the literature, there are no other reports of similar cases of spontaneous knee joint fusion, though there are some rare cases of the phenomenon in other joints. In 2005, Budoff and Lichtman1 reported a case of spontaneous wrist fusion in an 18-year-old patient with a background of Kienböck disease, which may have predisposed the patient to an underlying synovitis progressing to autofusion of the joint. In 2014, Lui2 described the case of a 64-year-old woman with spontaneous subtalar fusion complicating a subtalar arthroereisis. Although an extensive literature review on the topic is difficult owing to the rarity of the condition, these few cases, unlike our case, appear to describe a predisposing factor or inciting event.
The reversibility of knee arthrodesis remains an issue in our patient’s case and in other cases, and total knee arthroplasty (TKA) may be the most obvious operative intervention. Cameron and Hu3 reported 17 cases of knee fusion take-down with conversion to TKA, and Kim and colleagues4 reported 16 TKAs performed after spontaneous osseous ankylosis and 14 performed after formal knee fusion take-down. Although functional improvements were found in both studies, complication rates were relatively high, at least 53%. Other authors have used TKAs in cases of knee ankylosis after infectious or inflammatory arthritis, but results were suboptimal and unpredictable, and complication rates were 27% and 53.3%.5,6In this difficult scenario, our middle-aged patient’s fixed extension deformity of the knee, likely the result of an idiopathic process, led to severe debilitation and poor quality of life. To perform a TKA in a 51-year-old patient is far from ideal. The reversibility of formally fused and spontaneously fused knees is still in question, and, though there are reports of relatively satisfactory results, most operative options are fraught with complications.
Am J Orthop. 2017;46(2):E83-E85. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Budoff JE, Lichtman DM. Spontaneous wrist fusion: an unusual complication of Kienböck’s disease. J Hand Surg Am. 2005;30(1):59-64.
2. Lui TH. Spontaneous subtalar fusion: an irreversible complication of subtalar arthroereisis. J Foot Ankle Surg. 2014;53(5):652-656.
3. Cameron HU, Hu C. Results of total knee arthroplasty following takedown of formal knee fusion. J Arthroplasty. 1996;11(6):732-737.
4. Kim YH, Kim JS, Cho SH. Total knee arthroplasty after spontaneous osseous ankylosis and takedown of formal knee fusion. J Arthroplasty. 2000;15(4):453-460.
5. Rajgopal A, Ahuja N, Dolai B. Total knee arthroplasty in stiff and ankylosed knees. J Arthroplasty. 2005;20(5):585-590.
6. Kim YH, Cho SH, Kim JS. Total knee arthroplasty in bony ankylosis in gross flexion. J Bone Joint Surg Br. 1999;81(2):296-300.
1. Budoff JE, Lichtman DM. Spontaneous wrist fusion: an unusual complication of Kienböck’s disease. J Hand Surg Am. 2005;30(1):59-64.
2. Lui TH. Spontaneous subtalar fusion: an irreversible complication of subtalar arthroereisis. J Foot Ankle Surg. 2014;53(5):652-656.
3. Cameron HU, Hu C. Results of total knee arthroplasty following takedown of formal knee fusion. J Arthroplasty. 1996;11(6):732-737.
4. Kim YH, Kim JS, Cho SH. Total knee arthroplasty after spontaneous osseous ankylosis and takedown of formal knee fusion. J Arthroplasty. 2000;15(4):453-460.
5. Rajgopal A, Ahuja N, Dolai B. Total knee arthroplasty in stiff and ankylosed knees. J Arthroplasty. 2005;20(5):585-590.
6. Kim YH, Cho SH, Kim JS. Total knee arthroplasty in bony ankylosis in gross flexion. J Bone Joint Surg Br. 1999;81(2):296-300.
Antiphospholipid Syndrome in a Patient With Rheumatoid Arthritis
Case Report
A 39-year-old woman with a 20-year history of rheumatoid arthritis (RA) presented to a university-affiliated tertiary care hospital with painful ulcerations on the bilateral dorsal feet that started as bullae 16 weeks prior to presentation. Initial skin biopsy performed by an outside dermatologist 8 weeks prior to presentation showed vasculitis and culture was positive for methicillin-sensitive Staphylococcus aureus. She was started on a prednisone taper and cephalexin, which did not improve the lower extremity ulcerations and the pain became progressively worse. At the time of presentation to our dermatology department, the patient was taking prednisone, hydroxychloroquine, hydrocodone-acetaminophen, and gabapentin. Prior therapy with sulfasalazine failed; etanercept and methotrexate were discontinued years prior due to side effects. The patient had no history of deep vein thrombosis, pulmonary embolism, or miscarriage.
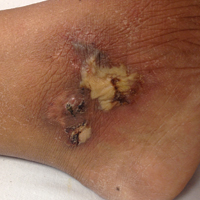
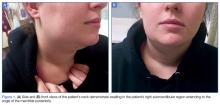
At presentation, the patient was afebrile and her vital signs were stable. Physical examination showed multiple ulcers and erosions on the bilateral dorsal feet with a few scattered retiform red-purple patches (Figure). One bulla was present on the right dorsal foot. All lesions were tender to the touch and edema was present on the bilateral feet. No oral ulcerations were present and no focal neuropathies or palpable cords were appreciated in the lower extremities. There were no other cutaneous abnormalities.
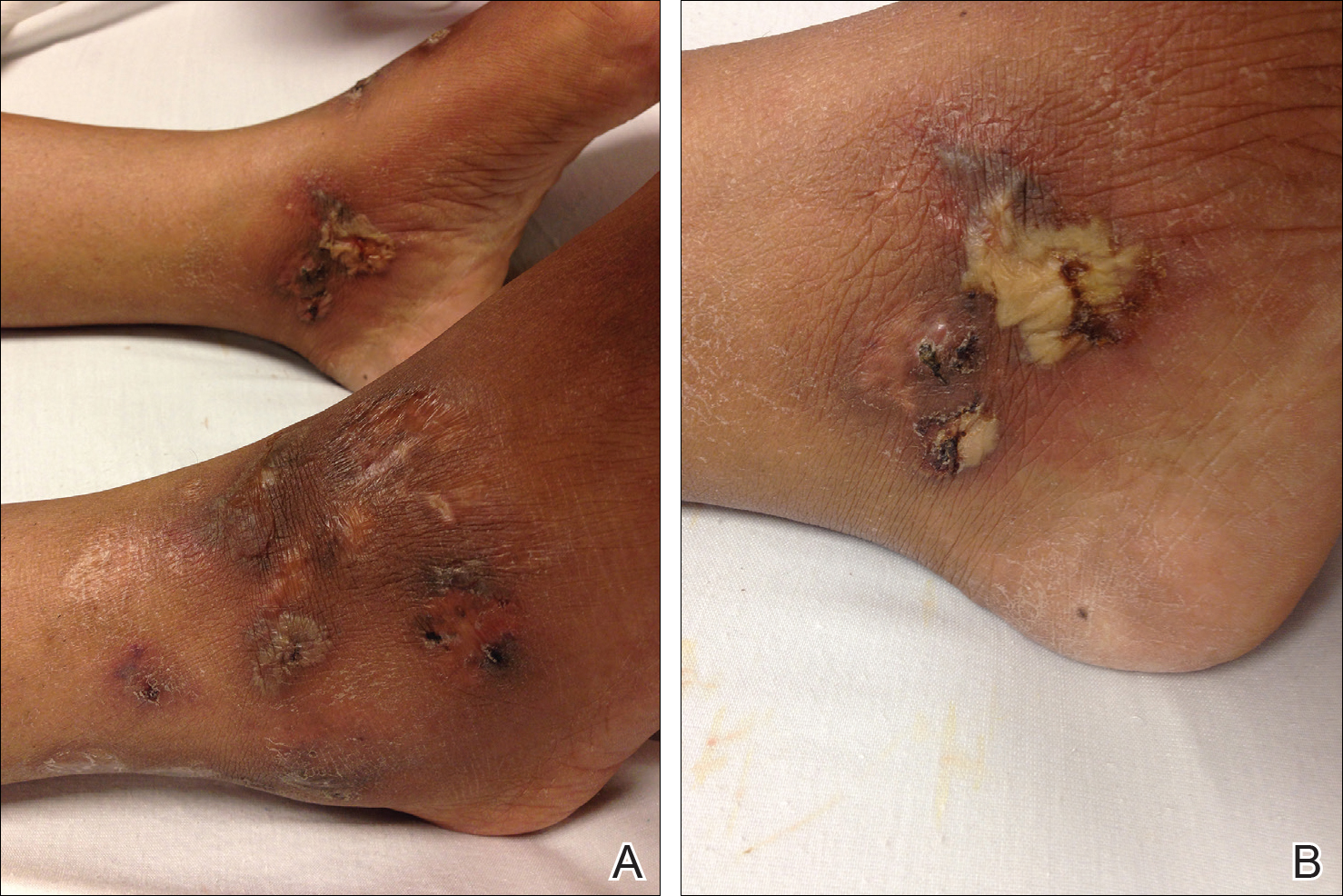
Laboratory studies showed a white blood cell count of 9.54×103/µL (reference range, 4.16-9.95×103/µL), hemoglobin count of 12.4 g/dL (reference range, 11.6-15.2 g/dL), and a platelet count of 175×103/µL (reference range, 143-398×103/µL). A basic metabolic panel was normal except for an elevated glucose level of 185 mg/dL (reference range, 65-100 mg/dL). Urinalysis was normal. Erythrocyte sedimentation rate and C-reactive protein level were not elevated. Antinuclear antibodies and double-stranded DNA antibodies were normal. Prothrombin time was 10.4 seconds (reference range, 9.2-11.5 seconds) and dilute viper's venom time was negative. Rheumatoid factor level was elevated at 76 IU/mL (reference range, <25 IU/mL) and anti-citrullinated peptide antibody was moderately elevated at 42 U/mL (negative, <20 U/mL; weak positive, 20-39 U/mL; moderate positive, 40-59 U/mL; strong positive, >59 U/mL). The cardiolipin antibodies IgG, IgM, and IgA were within reference range. Results of β2-glycoprotein I IgG and IgM antibody tests were normal, but IgA was elevated at 34 µg/mL (reference range, <20 µg/mL). Wound cultures grew moderate Enterobacter cloacae and Staphylococcus lugdunensis.
Slides from 2 prior punch biopsies obtained by an outside hospital approximately 8 weeks prior from the right and left dorsal foot lesions were reviewed. Both biopsies were histologically similar. Postcapillary venules showed extensive vasculitis with numerous fibrin thrombi in the lumens in both biopsy specimens. The biopsy from the right foot showed prominent ulceration of the epidermis, with a few of the affected vessels showing minimal accompanying nuclear dust; however, the predominant pattern was not that of leukocytoclastic vasculitis. Biopsy from the left foot showed prominent epidermal necrosis with focal reepithelialization and scattered eosinophils. The pathologist felt that a vasculitis secondary to coagulopathy was most likely but that a drug reaction and rheumatoid vasculitis would be other entities to consider in the differential. A review of the laboratory findings from the outside hospital from approximately 12 weeks prior to presentation showed IgM was normal but IgG was elevated at 28 U/mL (reference range, 0-15 U/mL) and IgA was elevated at 8 U/mL (reference range, 0-7 U/mL); β2-glycoprotein I IgG antibodies were elevated at 37 mg/dL (reference range, 0-25.0 mg/dL) and β2-glycoprotein I IgA antibodies were elevated at 5 mg/dL (reference range, 0-4.0 mg/dL).
The clinical suspicion of a thrombotic event on the dorsal feet, which was confirmed histologically, and the persistently positive antiphospholipid (aPL) antibody titers helped to establish the diagnosis of antiphospholipid syndrome (APS) in the setting of RA. The dose of prednisone was increased from 10 mg daily on admission to 40 mg daily. The patient was started on enoxaparin 60 mg subcutaneously twice daily at initial presentation and was bridged to oral warfarin 2 mg daily after the diagnosis of APS was established. Oral doxycycline 100 mg twice daily was started for wound infection. The ulcerations gradually improved over the course of her 7-day hospitalization. She was continued on prednisone, hydroxychloroquine, and warfarin as an outpatient and has had no recurrence of lesions after 3 years of follow-up on this regimen.
Comment
Antiphospholipid syndrome is an autoimmune condition defined by a venous and/or arterial thrombotic event and/or pregnancy morbidity in the presence of persistently elevated aPL antibody titers. The most frequently detected subgroups of aPL are anticardiolipin (aCL) antibodies, anti-β2-glycoprotein I antibodies, and lupus anticoagulants.1 Primary APS occurs as an isolated entity, whereas secondary APS occurs in the setting of a preexisting autoimmune disease, infection, malignancy, or medication.2 The diagnostic criteria for APS requires positive aPL titers at least 12 weeks apart and a clinically confirmed thrombotic event or pregnancy morbidity.3
About one-third to half of patients with APS exhibit cutaneous manifestations.4,5 Livedo reticularis is most commonly observed and represents the first clinical sign of APS in 17.5% of cases.6 Cutaneous findings of APS also include anetoderma, cutaneous ulceration and necrosis, necrotizing vasculitis, livedoid vasculitis, thrombophlebitis, purpura, ecchymoses, painful skin nodules, and subungual hemorrhages.7 The various cutaneous manifestations of APS are associated with a range of histopathologic findings, but noninflammatory thrombosis in small arteries and/or veins in the dermis and subcutaneous fat tissue is the most common histologic feature.4 Our patient exhibited cutaneous ulceration and necrosis, and biopsy clearly showed the presence of vasculitis and fibrin thrombi within postcapillary venules. These findings along with the persistently elevated β2-glycoprotein I IgA solidified the diagnosis of APS.
The most common cutaneous manifestations of RA are nodules (32%), Raynaud phenomenon (10%), and vasculitis (3%).8 The mean prevalence of aPL antibodies in patients with RA is 28%, though reports range from 5% to 75%.1 The presence of aPL or aCL does not predict the development of thrombosis and/or thrombocytopenia in RA patients9,10; however, aCL antibodies in RA patients are associated with a higher risk for developing rheumatoid nodules. It is hypothesized that the majority of aCL antibodies identified in RA patients have different specificities than those identified in other diseases that are associated with thrombotic events.1
Anticoagulation has been proven to decrease the risk for recurrent thrombotic events in patients with APS.11 Patients should discontinue the use of estrogen-containing oral contraceptives; avoid smoking cigarettes; and treat hypertension, hyperlipidemia, and diabetes mellitus, if present. The type and duration of anticoagulation therapy, especially for the treatment of the cutaneous manifestations of APS, is less well defined. Antiplatelet therapies such as low-dose aspirin or dipyridamole often are used for less severe cutaneous manifestations such as livedoid vasculopathy. Warfarin with a target international normalized ratio of 2.0 to 3.0 is most commonly used following major thrombotic events, including cutaneous necrosis and digital gangrene. The role of corticosteroids and immunosuppressants is unclear; one study showed that these therapies did not prevent further thrombotic events in patients with systemic lupus erythematosus.4
Conclusion
Although aPL antibodies are most prevalent in patients with systemic lupus erythematosus, an estimated 28% of patients with RA have elevated aPL titers. The aPL antibodies recognized in RA patients are thought to have a different specificity than those recognized in other APS-associated diseases because elevated aPL antibody titers are not associated with an increased incidence of thrombotic events in RA patients; however, larger studies are needed to clarify this phenomenon. It remains to be determined if this case of APS and RA represents a coincidence or a true disease association, but the recognition of the cutaneous and histological features of APS is crucial for establishing a diagnosis and initiating anticoagulation therapy to prevent further morbidity and mortality.
- Olech E, Merrill JT. The prevalence and clinical significance of antiphospholipid antibodies in rheumatoid arthritis. Curr Rheumatol Rep. 2006;8:100-108.
- Thornsberry LA, LoSicco KI, English JC. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306.
- Asherson A, Francès C, Iaccarino FL, et al. Theantiphospholipid antibody syndrome: diagnosis, skin manifestations and current therapy. Clin Exp Rheumatol. 2006;24(1 suppl 40):S46-S51.
- Cervera R, Piette JC, Font J, et al; Euro-Phospholipid Project Group. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019-1027.
- Francès C, Niang S, Laffitte E, et al. Dermatologic manifestations of antiphospholipid syndrome. two hundred consecutive cases. Arthritis Rheum. 2005;52:1785-1793.
- Gibson GE, Su WP, Pittelkow MR. Antiphospholipid syndrome and the skin. J Am Acad Dermatol. 1997;36(6, pt 1):970-982.
- Young A. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:907-927.
- Palomo I, Pinochet C, Alarcón M, et al. Prevalence of antiphospholipid antibodies in Chilean patients with rheumatoid arthritis. J Clin Lab Anal. 2006;20:190-194.
- Wolf P, Gretler J, Aglas F, et al. Anticardiolipin antibodies in rheumatoid arthritis: their relation to rheumatoid nodules and cutaneous vascular manifestations. Br J Dermatol. 1994;131:48-51.
- Lim W, Crowther MA, Eikelboom JW. Management of antiphospholipid antibody syndrome: a systematic review. JAMA. 2006;295:1050-1057.
Case Report
A 39-year-old woman with a 20-year history of rheumatoid arthritis (RA) presented to a university-affiliated tertiary care hospital with painful ulcerations on the bilateral dorsal feet that started as bullae 16 weeks prior to presentation. Initial skin biopsy performed by an outside dermatologist 8 weeks prior to presentation showed vasculitis and culture was positive for methicillin-sensitive Staphylococcus aureus. She was started on a prednisone taper and cephalexin, which did not improve the lower extremity ulcerations and the pain became progressively worse. At the time of presentation to our dermatology department, the patient was taking prednisone, hydroxychloroquine, hydrocodone-acetaminophen, and gabapentin. Prior therapy with sulfasalazine failed; etanercept and methotrexate were discontinued years prior due to side effects. The patient had no history of deep vein thrombosis, pulmonary embolism, or miscarriage.
At presentation, the patient was afebrile and her vital signs were stable. Physical examination showed multiple ulcers and erosions on the bilateral dorsal feet with a few scattered retiform red-purple patches (Figure). One bulla was present on the right dorsal foot. All lesions were tender to the touch and edema was present on the bilateral feet. No oral ulcerations were present and no focal neuropathies or palpable cords were appreciated in the lower extremities. There were no other cutaneous abnormalities.

Laboratory studies showed a white blood cell count of 9.54×103/µL (reference range, 4.16-9.95×103/µL), hemoglobin count of 12.4 g/dL (reference range, 11.6-15.2 g/dL), and a platelet count of 175×103/µL (reference range, 143-398×103/µL). A basic metabolic panel was normal except for an elevated glucose level of 185 mg/dL (reference range, 65-100 mg/dL). Urinalysis was normal. Erythrocyte sedimentation rate and C-reactive protein level were not elevated. Antinuclear antibodies and double-stranded DNA antibodies were normal. Prothrombin time was 10.4 seconds (reference range, 9.2-11.5 seconds) and dilute viper's venom time was negative. Rheumatoid factor level was elevated at 76 IU/mL (reference range, <25 IU/mL) and anti-citrullinated peptide antibody was moderately elevated at 42 U/mL (negative, <20 U/mL; weak positive, 20-39 U/mL; moderate positive, 40-59 U/mL; strong positive, >59 U/mL). The cardiolipin antibodies IgG, IgM, and IgA were within reference range. Results of β2-glycoprotein I IgG and IgM antibody tests were normal, but IgA was elevated at 34 µg/mL (reference range, <20 µg/mL). Wound cultures grew moderate Enterobacter cloacae and Staphylococcus lugdunensis.
Slides from 2 prior punch biopsies obtained by an outside hospital approximately 8 weeks prior from the right and left dorsal foot lesions were reviewed. Both biopsies were histologically similar. Postcapillary venules showed extensive vasculitis with numerous fibrin thrombi in the lumens in both biopsy specimens. The biopsy from the right foot showed prominent ulceration of the epidermis, with a few of the affected vessels showing minimal accompanying nuclear dust; however, the predominant pattern was not that of leukocytoclastic vasculitis. Biopsy from the left foot showed prominent epidermal necrosis with focal reepithelialization and scattered eosinophils. The pathologist felt that a vasculitis secondary to coagulopathy was most likely but that a drug reaction and rheumatoid vasculitis would be other entities to consider in the differential. A review of the laboratory findings from the outside hospital from approximately 12 weeks prior to presentation showed IgM was normal but IgG was elevated at 28 U/mL (reference range, 0-15 U/mL) and IgA was elevated at 8 U/mL (reference range, 0-7 U/mL); β2-glycoprotein I IgG antibodies were elevated at 37 mg/dL (reference range, 0-25.0 mg/dL) and β2-glycoprotein I IgA antibodies were elevated at 5 mg/dL (reference range, 0-4.0 mg/dL).
The clinical suspicion of a thrombotic event on the dorsal feet, which was confirmed histologically, and the persistently positive antiphospholipid (aPL) antibody titers helped to establish the diagnosis of antiphospholipid syndrome (APS) in the setting of RA. The dose of prednisone was increased from 10 mg daily on admission to 40 mg daily. The patient was started on enoxaparin 60 mg subcutaneously twice daily at initial presentation and was bridged to oral warfarin 2 mg daily after the diagnosis of APS was established. Oral doxycycline 100 mg twice daily was started for wound infection. The ulcerations gradually improved over the course of her 7-day hospitalization. She was continued on prednisone, hydroxychloroquine, and warfarin as an outpatient and has had no recurrence of lesions after 3 years of follow-up on this regimen.
Comment
Antiphospholipid syndrome is an autoimmune condition defined by a venous and/or arterial thrombotic event and/or pregnancy morbidity in the presence of persistently elevated aPL antibody titers. The most frequently detected subgroups of aPL are anticardiolipin (aCL) antibodies, anti-β2-glycoprotein I antibodies, and lupus anticoagulants.1 Primary APS occurs as an isolated entity, whereas secondary APS occurs in the setting of a preexisting autoimmune disease, infection, malignancy, or medication.2 The diagnostic criteria for APS requires positive aPL titers at least 12 weeks apart and a clinically confirmed thrombotic event or pregnancy morbidity.3
About one-third to half of patients with APS exhibit cutaneous manifestations.4,5 Livedo reticularis is most commonly observed and represents the first clinical sign of APS in 17.5% of cases.6 Cutaneous findings of APS also include anetoderma, cutaneous ulceration and necrosis, necrotizing vasculitis, livedoid vasculitis, thrombophlebitis, purpura, ecchymoses, painful skin nodules, and subungual hemorrhages.7 The various cutaneous manifestations of APS are associated with a range of histopathologic findings, but noninflammatory thrombosis in small arteries and/or veins in the dermis and subcutaneous fat tissue is the most common histologic feature.4 Our patient exhibited cutaneous ulceration and necrosis, and biopsy clearly showed the presence of vasculitis and fibrin thrombi within postcapillary venules. These findings along with the persistently elevated β2-glycoprotein I IgA solidified the diagnosis of APS.
The most common cutaneous manifestations of RA are nodules (32%), Raynaud phenomenon (10%), and vasculitis (3%).8 The mean prevalence of aPL antibodies in patients with RA is 28%, though reports range from 5% to 75%.1 The presence of aPL or aCL does not predict the development of thrombosis and/or thrombocytopenia in RA patients9,10; however, aCL antibodies in RA patients are associated with a higher risk for developing rheumatoid nodules. It is hypothesized that the majority of aCL antibodies identified in RA patients have different specificities than those identified in other diseases that are associated with thrombotic events.1
Anticoagulation has been proven to decrease the risk for recurrent thrombotic events in patients with APS.11 Patients should discontinue the use of estrogen-containing oral contraceptives; avoid smoking cigarettes; and treat hypertension, hyperlipidemia, and diabetes mellitus, if present. The type and duration of anticoagulation therapy, especially for the treatment of the cutaneous manifestations of APS, is less well defined. Antiplatelet therapies such as low-dose aspirin or dipyridamole often are used for less severe cutaneous manifestations such as livedoid vasculopathy. Warfarin with a target international normalized ratio of 2.0 to 3.0 is most commonly used following major thrombotic events, including cutaneous necrosis and digital gangrene. The role of corticosteroids and immunosuppressants is unclear; one study showed that these therapies did not prevent further thrombotic events in patients with systemic lupus erythematosus.4
Conclusion
Although aPL antibodies are most prevalent in patients with systemic lupus erythematosus, an estimated 28% of patients with RA have elevated aPL titers. The aPL antibodies recognized in RA patients are thought to have a different specificity than those recognized in other APS-associated diseases because elevated aPL antibody titers are not associated with an increased incidence of thrombotic events in RA patients; however, larger studies are needed to clarify this phenomenon. It remains to be determined if this case of APS and RA represents a coincidence or a true disease association, but the recognition of the cutaneous and histological features of APS is crucial for establishing a diagnosis and initiating anticoagulation therapy to prevent further morbidity and mortality.
Case Report
A 39-year-old woman with a 20-year history of rheumatoid arthritis (RA) presented to a university-affiliated tertiary care hospital with painful ulcerations on the bilateral dorsal feet that started as bullae 16 weeks prior to presentation. Initial skin biopsy performed by an outside dermatologist 8 weeks prior to presentation showed vasculitis and culture was positive for methicillin-sensitive Staphylococcus aureus. She was started on a prednisone taper and cephalexin, which did not improve the lower extremity ulcerations and the pain became progressively worse. At the time of presentation to our dermatology department, the patient was taking prednisone, hydroxychloroquine, hydrocodone-acetaminophen, and gabapentin. Prior therapy with sulfasalazine failed; etanercept and methotrexate were discontinued years prior due to side effects. The patient had no history of deep vein thrombosis, pulmonary embolism, or miscarriage.
At presentation, the patient was afebrile and her vital signs were stable. Physical examination showed multiple ulcers and erosions on the bilateral dorsal feet with a few scattered retiform red-purple patches (Figure). One bulla was present on the right dorsal foot. All lesions were tender to the touch and edema was present on the bilateral feet. No oral ulcerations were present and no focal neuropathies or palpable cords were appreciated in the lower extremities. There were no other cutaneous abnormalities.

Laboratory studies showed a white blood cell count of 9.54×103/µL (reference range, 4.16-9.95×103/µL), hemoglobin count of 12.4 g/dL (reference range, 11.6-15.2 g/dL), and a platelet count of 175×103/µL (reference range, 143-398×103/µL). A basic metabolic panel was normal except for an elevated glucose level of 185 mg/dL (reference range, 65-100 mg/dL). Urinalysis was normal. Erythrocyte sedimentation rate and C-reactive protein level were not elevated. Antinuclear antibodies and double-stranded DNA antibodies were normal. Prothrombin time was 10.4 seconds (reference range, 9.2-11.5 seconds) and dilute viper's venom time was negative. Rheumatoid factor level was elevated at 76 IU/mL (reference range, <25 IU/mL) and anti-citrullinated peptide antibody was moderately elevated at 42 U/mL (negative, <20 U/mL; weak positive, 20-39 U/mL; moderate positive, 40-59 U/mL; strong positive, >59 U/mL). The cardiolipin antibodies IgG, IgM, and IgA were within reference range. Results of β2-glycoprotein I IgG and IgM antibody tests were normal, but IgA was elevated at 34 µg/mL (reference range, <20 µg/mL). Wound cultures grew moderate Enterobacter cloacae and Staphylococcus lugdunensis.
Slides from 2 prior punch biopsies obtained by an outside hospital approximately 8 weeks prior from the right and left dorsal foot lesions were reviewed. Both biopsies were histologically similar. Postcapillary venules showed extensive vasculitis with numerous fibrin thrombi in the lumens in both biopsy specimens. The biopsy from the right foot showed prominent ulceration of the epidermis, with a few of the affected vessels showing minimal accompanying nuclear dust; however, the predominant pattern was not that of leukocytoclastic vasculitis. Biopsy from the left foot showed prominent epidermal necrosis with focal reepithelialization and scattered eosinophils. The pathologist felt that a vasculitis secondary to coagulopathy was most likely but that a drug reaction and rheumatoid vasculitis would be other entities to consider in the differential. A review of the laboratory findings from the outside hospital from approximately 12 weeks prior to presentation showed IgM was normal but IgG was elevated at 28 U/mL (reference range, 0-15 U/mL) and IgA was elevated at 8 U/mL (reference range, 0-7 U/mL); β2-glycoprotein I IgG antibodies were elevated at 37 mg/dL (reference range, 0-25.0 mg/dL) and β2-glycoprotein I IgA antibodies were elevated at 5 mg/dL (reference range, 0-4.0 mg/dL).
The clinical suspicion of a thrombotic event on the dorsal feet, which was confirmed histologically, and the persistently positive antiphospholipid (aPL) antibody titers helped to establish the diagnosis of antiphospholipid syndrome (APS) in the setting of RA. The dose of prednisone was increased from 10 mg daily on admission to 40 mg daily. The patient was started on enoxaparin 60 mg subcutaneously twice daily at initial presentation and was bridged to oral warfarin 2 mg daily after the diagnosis of APS was established. Oral doxycycline 100 mg twice daily was started for wound infection. The ulcerations gradually improved over the course of her 7-day hospitalization. She was continued on prednisone, hydroxychloroquine, and warfarin as an outpatient and has had no recurrence of lesions after 3 years of follow-up on this regimen.
Comment
Antiphospholipid syndrome is an autoimmune condition defined by a venous and/or arterial thrombotic event and/or pregnancy morbidity in the presence of persistently elevated aPL antibody titers. The most frequently detected subgroups of aPL are anticardiolipin (aCL) antibodies, anti-β2-glycoprotein I antibodies, and lupus anticoagulants.1 Primary APS occurs as an isolated entity, whereas secondary APS occurs in the setting of a preexisting autoimmune disease, infection, malignancy, or medication.2 The diagnostic criteria for APS requires positive aPL titers at least 12 weeks apart and a clinically confirmed thrombotic event or pregnancy morbidity.3
About one-third to half of patients with APS exhibit cutaneous manifestations.4,5 Livedo reticularis is most commonly observed and represents the first clinical sign of APS in 17.5% of cases.6 Cutaneous findings of APS also include anetoderma, cutaneous ulceration and necrosis, necrotizing vasculitis, livedoid vasculitis, thrombophlebitis, purpura, ecchymoses, painful skin nodules, and subungual hemorrhages.7 The various cutaneous manifestations of APS are associated with a range of histopathologic findings, but noninflammatory thrombosis in small arteries and/or veins in the dermis and subcutaneous fat tissue is the most common histologic feature.4 Our patient exhibited cutaneous ulceration and necrosis, and biopsy clearly showed the presence of vasculitis and fibrin thrombi within postcapillary venules. These findings along with the persistently elevated β2-glycoprotein I IgA solidified the diagnosis of APS.
The most common cutaneous manifestations of RA are nodules (32%), Raynaud phenomenon (10%), and vasculitis (3%).8 The mean prevalence of aPL antibodies in patients with RA is 28%, though reports range from 5% to 75%.1 The presence of aPL or aCL does not predict the development of thrombosis and/or thrombocytopenia in RA patients9,10; however, aCL antibodies in RA patients are associated with a higher risk for developing rheumatoid nodules. It is hypothesized that the majority of aCL antibodies identified in RA patients have different specificities than those identified in other diseases that are associated with thrombotic events.1
Anticoagulation has been proven to decrease the risk for recurrent thrombotic events in patients with APS.11 Patients should discontinue the use of estrogen-containing oral contraceptives; avoid smoking cigarettes; and treat hypertension, hyperlipidemia, and diabetes mellitus, if present. The type and duration of anticoagulation therapy, especially for the treatment of the cutaneous manifestations of APS, is less well defined. Antiplatelet therapies such as low-dose aspirin or dipyridamole often are used for less severe cutaneous manifestations such as livedoid vasculopathy. Warfarin with a target international normalized ratio of 2.0 to 3.0 is most commonly used following major thrombotic events, including cutaneous necrosis and digital gangrene. The role of corticosteroids and immunosuppressants is unclear; one study showed that these therapies did not prevent further thrombotic events in patients with systemic lupus erythematosus.4
Conclusion
Although aPL antibodies are most prevalent in patients with systemic lupus erythematosus, an estimated 28% of patients with RA have elevated aPL titers. The aPL antibodies recognized in RA patients are thought to have a different specificity than those recognized in other APS-associated diseases because elevated aPL antibody titers are not associated with an increased incidence of thrombotic events in RA patients; however, larger studies are needed to clarify this phenomenon. It remains to be determined if this case of APS and RA represents a coincidence or a true disease association, but the recognition of the cutaneous and histological features of APS is crucial for establishing a diagnosis and initiating anticoagulation therapy to prevent further morbidity and mortality.
- Olech E, Merrill JT. The prevalence and clinical significance of antiphospholipid antibodies in rheumatoid arthritis. Curr Rheumatol Rep. 2006;8:100-108.
- Thornsberry LA, LoSicco KI, English JC. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306.
- Asherson A, Francès C, Iaccarino FL, et al. Theantiphospholipid antibody syndrome: diagnosis, skin manifestations and current therapy. Clin Exp Rheumatol. 2006;24(1 suppl 40):S46-S51.
- Cervera R, Piette JC, Font J, et al; Euro-Phospholipid Project Group. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019-1027.
- Francès C, Niang S, Laffitte E, et al. Dermatologic manifestations of antiphospholipid syndrome. two hundred consecutive cases. Arthritis Rheum. 2005;52:1785-1793.
- Gibson GE, Su WP, Pittelkow MR. Antiphospholipid syndrome and the skin. J Am Acad Dermatol. 1997;36(6, pt 1):970-982.
- Young A. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:907-927.
- Palomo I, Pinochet C, Alarcón M, et al. Prevalence of antiphospholipid antibodies in Chilean patients with rheumatoid arthritis. J Clin Lab Anal. 2006;20:190-194.
- Wolf P, Gretler J, Aglas F, et al. Anticardiolipin antibodies in rheumatoid arthritis: their relation to rheumatoid nodules and cutaneous vascular manifestations. Br J Dermatol. 1994;131:48-51.
- Lim W, Crowther MA, Eikelboom JW. Management of antiphospholipid antibody syndrome: a systematic review. JAMA. 2006;295:1050-1057.
- Olech E, Merrill JT. The prevalence and clinical significance of antiphospholipid antibodies in rheumatoid arthritis. Curr Rheumatol Rep. 2006;8:100-108.
- Thornsberry LA, LoSicco KI, English JC. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306.
- Asherson A, Francès C, Iaccarino FL, et al. Theantiphospholipid antibody syndrome: diagnosis, skin manifestations and current therapy. Clin Exp Rheumatol. 2006;24(1 suppl 40):S46-S51.
- Cervera R, Piette JC, Font J, et al; Euro-Phospholipid Project Group. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019-1027.
- Francès C, Niang S, Laffitte E, et al. Dermatologic manifestations of antiphospholipid syndrome. two hundred consecutive cases. Arthritis Rheum. 2005;52:1785-1793.
- Gibson GE, Su WP, Pittelkow MR. Antiphospholipid syndrome and the skin. J Am Acad Dermatol. 1997;36(6, pt 1):970-982.
- Young A. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:907-927.
- Palomo I, Pinochet C, Alarcón M, et al. Prevalence of antiphospholipid antibodies in Chilean patients with rheumatoid arthritis. J Clin Lab Anal. 2006;20:190-194.
- Wolf P, Gretler J, Aglas F, et al. Anticardiolipin antibodies in rheumatoid arthritis: their relation to rheumatoid nodules and cutaneous vascular manifestations. Br J Dermatol. 1994;131:48-51.
- Lim W, Crowther MA, Eikelboom JW. Management of antiphospholipid antibody syndrome: a systematic review. JAMA. 2006;295:1050-1057.
Practice Points
- Antiphospholipid syndrome (APS) is an autoimmune condition defined by a venous and/or arterial thrombotic event and/or pregnancy morbidity in the presence of persistently elevated antiphospholipid antibody titers.
- Cutaneous findings of APS include livedo reticularis most commonly but also anetoderma, cutaneous ulceration and necrosis, necrotizing vasculitis, livedoid vasculitis, thrombophlebitis, purpura, ecchymoses, painful skin nodules, and subungual hemorrhages.
- The various cutaneous manifestations of APS are associated with a range of histopathologic findings, but noninflammatory thrombosis in small arteries and/or veins in the dermis and subcutaneous fat tissue is the most common histologic feature.
Pronator Teres Myotendinous Tear
Take-Home Points
- Pronator teres muscle injuries are rare.
- Injury can be mistaken for MUCL injury in athletes.
- Tenderness and weak/painful forearm pronation are common findings.
- MRI confirms the diagnosis and helps grade the muscle strain injury.
- Conservative treatment is recommended and prognosis is excellent even for high-grade strains.
Pronator teres muscle strain is a rare sporting injury reported only in cricket players, and now in a golfer whose forearm experienced an eccentric force during resisted elbow flexion and pronation.1,2 The injury occurs when the sporting club or racket strikes the ground during a swing, impeding forward progress and subjecting the pronator teres muscle to eccentric forces in excess of what it can withstand. The pronator teres, one of several muscles that comprise the flexor wad of the forearm, consists of 2 heads, originating proximally from the medical epicondyle and attaching distally to the shaft of the radius on its lateral surface and just distal to the supinator. The oblique orientation of the muscle belly allows it to serve in its primary rotatory role as the main pronator of the forearm. Injuries to the soft tissue of the medial forearm are common in both elite and recreational athletes, especially in racket and club sports.3 Often, these injuries are related to overuse and chronic fatigue of the surrounding soft tissue—caused by repetitive flexing, gripping, or swinging. Even when identified early, these injuries can result in a significant loss of training time.4 In this article, we report a case of pronator teres muscle tear at the myotendinous junction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
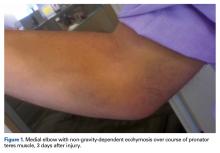
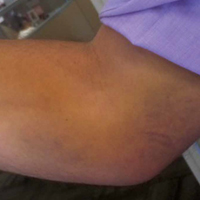
A right-hand–dominant 36-year-old man presented to the clinic with pain on the medial side of his right elbow after sustaining an injury to the elbow while playing golf several days earlier. The patient, an advertising executive, was playing recreational golf several times a month and had no significant medical history or previous symptoms related to the elbow. Initial pain symptoms began during a second round of play, immediately after the patient miss-hit an iron shot, making contact mostly with the ground and causing the club to forcefully stop. The pain was on the medial side of the elbow and forearm. The patient noted progressive swelling and bruising at the pain site and development of forearm weakness. Physical examination during the clinic presentation revealed ecchymosis on the anterior medial forearm, medial elbow, and medial triceps (Figure 1).
Noncontrast magnetic resonance imaging (MRI) showed a high-grade partial tear of the pronator teres myotendinous junction (Figures 2A-2C).
The patient was instructed to rest the elbow from strenuous activity, golf in particular, for 4 weeks. Physical therapy for ROM and forearm strengthening of the surrounding flexor wad was initiated at 2 weeks and continued for 4 weeks. The patient was advised to take over-the-counter nonsteroidal anti-inflammatory drugs as needed for comfort. On repeat examination at 4 weeks, with tenderness or weakness with pronation absent and full ROM regained, the patient was released back to full activity. He was able to return to golf and reported being symptom-free and having no sense of weakness or loss of control.
Discussion
A tear of the pronator teres is an exceedingly rare injury. Our results with conservative treatment and a full return to previous activity level are consistent with the only other case reported in the literature.5 In contrast to our patient, the previous patient sustained a tear of the pronator teres after a prolonged period of batting during a recreational cricket match.
Our patient’s pronator teres injury occurred at the myotendinous junction, a muscle-tendon transition zone often susceptible to injury. What is unusual for this athletic medial elbow injury is that the patient reported no previous symptoms, and it appears that, though the surrounding muscle may have been fatigued by overuse from the round of golf earlier that day, the pathology was caused by an acute eccentric force. During a golf swing, tremendous forces are put on the entire body, from the lower extremities to the forearm and the fingers. Successful completion of the transfer of energy from the golf club to the ball requires both proper technique and proper functioning of key muscles. Specifically, parameters such as ball positioning, club angle, and wrist control play a major role.6 Altered forearm positioning or swing arc can significantly affect club head velocity and energy transfer without putting more stress on the golfer.7 Therefore, it is easy to understand how prolonged or extended play may fatigue the surrounding elbow muscles, leading to altered technique and increased susceptibility to acute injury. Biomechanical analysis of shoulder motion can provide a helpful baseline for assessing injury-related changes in golf swing and developing specific exercise and rehabilitation programs.8,9Although injury to the pronator teres is rare, sport physicians should be aware that, after a valgus stress or force, bruising and swelling along the medial elbow do not always indicate a medial ulnar collateral ligament (MUCL) tear or medial epicondylitis. The key examination findings that differentiate this injury from a MUCL injury are the exact location of pain, the milking maneuver for MUCL incompetence, and the extensive bruising over the muscle course of the pronator teres. MRI plays a pivotal role in proper diagnosis.4 In addition, MRI allows for evaluation of any concomitant injuries that may be obscuring the clinical presentation.
Successful treatment of such injuries is important for both elite and recreational athletes. With rest and physical therapy, our patient recovered from this rare isolated injury to the pronator teres with complete resolution of symptoms and full ROM. In the literature, we found no other reports of isolated full-thickness myotendinous rupture of the pronator teres or avulsion from the medial epicondyle. Therefore, it is unclear whether the same outcome can be expected with conservative therapy. However, because of the good outcomes for partial-thickness injuries treated conservatively and the lack of robust tendinous tissue to repair at the myotendinous junction, we recommend an initial course of conservative treatment. Sports physicians should be aware of this exceedingly rare injury to the elbow and understand the large forces experienced by the soft tissues of the forearm during the golf swing.9,10
Conclusion
Pronator teres muscle strain is a rare sporting injury reported in cricket and golf players. The elbow experiences a large eccentric force during resisted elbow flexion and pronation. The injury appears to occur when the sporting club or racket strikes the ground during a forceful swing impeding forward progress of the arm. The injury can be confused with a MUCL injury, or exacerbation of medial epicondylitis. Physical examination reveals bruising and tenderness over the course of the pronator teres, often distal to the elbow. Advanced imaging confirms the diagnosis and helps grade the severity of muscle strain. Treatment is often conservative, with return to function and sport after 4 to 6 weeks of rest and restricted activities. The patient in this case report had complete return to sporting function, with no residual weakness or pain.
Am J Orthop. 2017;46(2):E105-E107. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Field LD, Savoie FH. Common elbow injuries in sport. Sports Med. 1998;26(3):193-205.
2. Loomer RL. Elbow injuries in athletes. Can J Appl Sport Sci. 1982;7(3):164-166.
3. Dines JS, Bedi A, Williams PN, et al. Tennis injuries: epidemiology, pathophysiology, and treatment. J Am Acad Orthop Surg. 2015;23(3):181-189.
4. Banks KP, Ly JQ, Beall DP, Grayson DE, Bancroft LW, Tall MA. Overuse injuries of the upper extremity in the competitive athlete: magnetic resonance imaging findings associated with repetitive trauma. Curr Probl Diagn Radiol. 2005;34(4):127-142.
5. Niebulski HZ, Richardson ML. High-grade pronator teres tear in a cricket batsman. Radiol Case Rep. 2015;6(3):540.
6. Zhang X, Shan G. Where do golf drive swings go wrong? Factors influencing driver swing consistency. Scand J Med Sci Sports. 2014;24(5):749-757.
7. Nesbit SM, McGinnis RS. Kinetic constrained optimization of the golf swing hub path. J Sports Sci Med. 2014;13(4):859-873.
8. Helton MS. Conservative treatment of a proximal full-thickness biceps brachii muscle tear in a special operations soldier. Phys Ther. 2014;94(4):571-577.
9. Mitchell K, Banks S, Morgan D, Sugaya H. Shoulder motions during the golf swing in male amateur golfers. J Orthop Sports Phys Ther. 2003;33(4):196-203.
10. Grimshaw P, Giles A, Tong R, Grimmer K. Lower back and elbow injuries in golf. Sports Med. 2002;32(10):655-666.
Take-Home Points
- Pronator teres muscle injuries are rare.
- Injury can be mistaken for MUCL injury in athletes.
- Tenderness and weak/painful forearm pronation are common findings.
- MRI confirms the diagnosis and helps grade the muscle strain injury.
- Conservative treatment is recommended and prognosis is excellent even for high-grade strains.
Pronator teres muscle strain is a rare sporting injury reported only in cricket players, and now in a golfer whose forearm experienced an eccentric force during resisted elbow flexion and pronation.1,2 The injury occurs when the sporting club or racket strikes the ground during a swing, impeding forward progress and subjecting the pronator teres muscle to eccentric forces in excess of what it can withstand. The pronator teres, one of several muscles that comprise the flexor wad of the forearm, consists of 2 heads, originating proximally from the medical epicondyle and attaching distally to the shaft of the radius on its lateral surface and just distal to the supinator. The oblique orientation of the muscle belly allows it to serve in its primary rotatory role as the main pronator of the forearm. Injuries to the soft tissue of the medial forearm are common in both elite and recreational athletes, especially in racket and club sports.3 Often, these injuries are related to overuse and chronic fatigue of the surrounding soft tissue—caused by repetitive flexing, gripping, or swinging. Even when identified early, these injuries can result in a significant loss of training time.4 In this article, we report a case of pronator teres muscle tear at the myotendinous junction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand–dominant 36-year-old man presented to the clinic with pain on the medial side of his right elbow after sustaining an injury to the elbow while playing golf several days earlier. The patient, an advertising executive, was playing recreational golf several times a month and had no significant medical history or previous symptoms related to the elbow. Initial pain symptoms began during a second round of play, immediately after the patient miss-hit an iron shot, making contact mostly with the ground and causing the club to forcefully stop. The pain was on the medial side of the elbow and forearm. The patient noted progressive swelling and bruising at the pain site and development of forearm weakness. Physical examination during the clinic presentation revealed ecchymosis on the anterior medial forearm, medial elbow, and medial triceps (Figure 1).
Noncontrast magnetic resonance imaging (MRI) showed a high-grade partial tear of the pronator teres myotendinous junction (Figures 2A-2C).
The patient was instructed to rest the elbow from strenuous activity, golf in particular, for 4 weeks. Physical therapy for ROM and forearm strengthening of the surrounding flexor wad was initiated at 2 weeks and continued for 4 weeks. The patient was advised to take over-the-counter nonsteroidal anti-inflammatory drugs as needed for comfort. On repeat examination at 4 weeks, with tenderness or weakness with pronation absent and full ROM regained, the patient was released back to full activity. He was able to return to golf and reported being symptom-free and having no sense of weakness or loss of control.
Discussion
A tear of the pronator teres is an exceedingly rare injury. Our results with conservative treatment and a full return to previous activity level are consistent with the only other case reported in the literature.5 In contrast to our patient, the previous patient sustained a tear of the pronator teres after a prolonged period of batting during a recreational cricket match.
Our patient’s pronator teres injury occurred at the myotendinous junction, a muscle-tendon transition zone often susceptible to injury. What is unusual for this athletic medial elbow injury is that the patient reported no previous symptoms, and it appears that, though the surrounding muscle may have been fatigued by overuse from the round of golf earlier that day, the pathology was caused by an acute eccentric force. During a golf swing, tremendous forces are put on the entire body, from the lower extremities to the forearm and the fingers. Successful completion of the transfer of energy from the golf club to the ball requires both proper technique and proper functioning of key muscles. Specifically, parameters such as ball positioning, club angle, and wrist control play a major role.6 Altered forearm positioning or swing arc can significantly affect club head velocity and energy transfer without putting more stress on the golfer.7 Therefore, it is easy to understand how prolonged or extended play may fatigue the surrounding elbow muscles, leading to altered technique and increased susceptibility to acute injury. Biomechanical analysis of shoulder motion can provide a helpful baseline for assessing injury-related changes in golf swing and developing specific exercise and rehabilitation programs.8,9Although injury to the pronator teres is rare, sport physicians should be aware that, after a valgus stress or force, bruising and swelling along the medial elbow do not always indicate a medial ulnar collateral ligament (MUCL) tear or medial epicondylitis. The key examination findings that differentiate this injury from a MUCL injury are the exact location of pain, the milking maneuver for MUCL incompetence, and the extensive bruising over the muscle course of the pronator teres. MRI plays a pivotal role in proper diagnosis.4 In addition, MRI allows for evaluation of any concomitant injuries that may be obscuring the clinical presentation.
Successful treatment of such injuries is important for both elite and recreational athletes. With rest and physical therapy, our patient recovered from this rare isolated injury to the pronator teres with complete resolution of symptoms and full ROM. In the literature, we found no other reports of isolated full-thickness myotendinous rupture of the pronator teres or avulsion from the medial epicondyle. Therefore, it is unclear whether the same outcome can be expected with conservative therapy. However, because of the good outcomes for partial-thickness injuries treated conservatively and the lack of robust tendinous tissue to repair at the myotendinous junction, we recommend an initial course of conservative treatment. Sports physicians should be aware of this exceedingly rare injury to the elbow and understand the large forces experienced by the soft tissues of the forearm during the golf swing.9,10
Conclusion
Pronator teres muscle strain is a rare sporting injury reported in cricket and golf players. The elbow experiences a large eccentric force during resisted elbow flexion and pronation. The injury appears to occur when the sporting club or racket strikes the ground during a forceful swing impeding forward progress of the arm. The injury can be confused with a MUCL injury, or exacerbation of medial epicondylitis. Physical examination reveals bruising and tenderness over the course of the pronator teres, often distal to the elbow. Advanced imaging confirms the diagnosis and helps grade the severity of muscle strain. Treatment is often conservative, with return to function and sport after 4 to 6 weeks of rest and restricted activities. The patient in this case report had complete return to sporting function, with no residual weakness or pain.
Am J Orthop. 2017;46(2):E105-E107. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Pronator teres muscle injuries are rare.
- Injury can be mistaken for MUCL injury in athletes.
- Tenderness and weak/painful forearm pronation are common findings.
- MRI confirms the diagnosis and helps grade the muscle strain injury.
- Conservative treatment is recommended and prognosis is excellent even for high-grade strains.
Pronator teres muscle strain is a rare sporting injury reported only in cricket players, and now in a golfer whose forearm experienced an eccentric force during resisted elbow flexion and pronation.1,2 The injury occurs when the sporting club or racket strikes the ground during a swing, impeding forward progress and subjecting the pronator teres muscle to eccentric forces in excess of what it can withstand. The pronator teres, one of several muscles that comprise the flexor wad of the forearm, consists of 2 heads, originating proximally from the medical epicondyle and attaching distally to the shaft of the radius on its lateral surface and just distal to the supinator. The oblique orientation of the muscle belly allows it to serve in its primary rotatory role as the main pronator of the forearm. Injuries to the soft tissue of the medial forearm are common in both elite and recreational athletes, especially in racket and club sports.3 Often, these injuries are related to overuse and chronic fatigue of the surrounding soft tissue—caused by repetitive flexing, gripping, or swinging. Even when identified early, these injuries can result in a significant loss of training time.4 In this article, we report a case of pronator teres muscle tear at the myotendinous junction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand–dominant 36-year-old man presented to the clinic with pain on the medial side of his right elbow after sustaining an injury to the elbow while playing golf several days earlier. The patient, an advertising executive, was playing recreational golf several times a month and had no significant medical history or previous symptoms related to the elbow. Initial pain symptoms began during a second round of play, immediately after the patient miss-hit an iron shot, making contact mostly with the ground and causing the club to forcefully stop. The pain was on the medial side of the elbow and forearm. The patient noted progressive swelling and bruising at the pain site and development of forearm weakness. Physical examination during the clinic presentation revealed ecchymosis on the anterior medial forearm, medial elbow, and medial triceps (Figure 1).
Noncontrast magnetic resonance imaging (MRI) showed a high-grade partial tear of the pronator teres myotendinous junction (Figures 2A-2C).
The patient was instructed to rest the elbow from strenuous activity, golf in particular, for 4 weeks. Physical therapy for ROM and forearm strengthening of the surrounding flexor wad was initiated at 2 weeks and continued for 4 weeks. The patient was advised to take over-the-counter nonsteroidal anti-inflammatory drugs as needed for comfort. On repeat examination at 4 weeks, with tenderness or weakness with pronation absent and full ROM regained, the patient was released back to full activity. He was able to return to golf and reported being symptom-free and having no sense of weakness or loss of control.
Discussion
A tear of the pronator teres is an exceedingly rare injury. Our results with conservative treatment and a full return to previous activity level are consistent with the only other case reported in the literature.5 In contrast to our patient, the previous patient sustained a tear of the pronator teres after a prolonged period of batting during a recreational cricket match.
Our patient’s pronator teres injury occurred at the myotendinous junction, a muscle-tendon transition zone often susceptible to injury. What is unusual for this athletic medial elbow injury is that the patient reported no previous symptoms, and it appears that, though the surrounding muscle may have been fatigued by overuse from the round of golf earlier that day, the pathology was caused by an acute eccentric force. During a golf swing, tremendous forces are put on the entire body, from the lower extremities to the forearm and the fingers. Successful completion of the transfer of energy from the golf club to the ball requires both proper technique and proper functioning of key muscles. Specifically, parameters such as ball positioning, club angle, and wrist control play a major role.6 Altered forearm positioning or swing arc can significantly affect club head velocity and energy transfer without putting more stress on the golfer.7 Therefore, it is easy to understand how prolonged or extended play may fatigue the surrounding elbow muscles, leading to altered technique and increased susceptibility to acute injury. Biomechanical analysis of shoulder motion can provide a helpful baseline for assessing injury-related changes in golf swing and developing specific exercise and rehabilitation programs.8,9Although injury to the pronator teres is rare, sport physicians should be aware that, after a valgus stress or force, bruising and swelling along the medial elbow do not always indicate a medial ulnar collateral ligament (MUCL) tear or medial epicondylitis. The key examination findings that differentiate this injury from a MUCL injury are the exact location of pain, the milking maneuver for MUCL incompetence, and the extensive bruising over the muscle course of the pronator teres. MRI plays a pivotal role in proper diagnosis.4 In addition, MRI allows for evaluation of any concomitant injuries that may be obscuring the clinical presentation.
Successful treatment of such injuries is important for both elite and recreational athletes. With rest and physical therapy, our patient recovered from this rare isolated injury to the pronator teres with complete resolution of symptoms and full ROM. In the literature, we found no other reports of isolated full-thickness myotendinous rupture of the pronator teres or avulsion from the medial epicondyle. Therefore, it is unclear whether the same outcome can be expected with conservative therapy. However, because of the good outcomes for partial-thickness injuries treated conservatively and the lack of robust tendinous tissue to repair at the myotendinous junction, we recommend an initial course of conservative treatment. Sports physicians should be aware of this exceedingly rare injury to the elbow and understand the large forces experienced by the soft tissues of the forearm during the golf swing.9,10
Conclusion
Pronator teres muscle strain is a rare sporting injury reported in cricket and golf players. The elbow experiences a large eccentric force during resisted elbow flexion and pronation. The injury appears to occur when the sporting club or racket strikes the ground during a forceful swing impeding forward progress of the arm. The injury can be confused with a MUCL injury, or exacerbation of medial epicondylitis. Physical examination reveals bruising and tenderness over the course of the pronator teres, often distal to the elbow. Advanced imaging confirms the diagnosis and helps grade the severity of muscle strain. Treatment is often conservative, with return to function and sport after 4 to 6 weeks of rest and restricted activities. The patient in this case report had complete return to sporting function, with no residual weakness or pain.
Am J Orthop. 2017;46(2):E105-E107. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Field LD, Savoie FH. Common elbow injuries in sport. Sports Med. 1998;26(3):193-205.
2. Loomer RL. Elbow injuries in athletes. Can J Appl Sport Sci. 1982;7(3):164-166.
3. Dines JS, Bedi A, Williams PN, et al. Tennis injuries: epidemiology, pathophysiology, and treatment. J Am Acad Orthop Surg. 2015;23(3):181-189.
4. Banks KP, Ly JQ, Beall DP, Grayson DE, Bancroft LW, Tall MA. Overuse injuries of the upper extremity in the competitive athlete: magnetic resonance imaging findings associated with repetitive trauma. Curr Probl Diagn Radiol. 2005;34(4):127-142.
5. Niebulski HZ, Richardson ML. High-grade pronator teres tear in a cricket batsman. Radiol Case Rep. 2015;6(3):540.
6. Zhang X, Shan G. Where do golf drive swings go wrong? Factors influencing driver swing consistency. Scand J Med Sci Sports. 2014;24(5):749-757.
7. Nesbit SM, McGinnis RS. Kinetic constrained optimization of the golf swing hub path. J Sports Sci Med. 2014;13(4):859-873.
8. Helton MS. Conservative treatment of a proximal full-thickness biceps brachii muscle tear in a special operations soldier. Phys Ther. 2014;94(4):571-577.
9. Mitchell K, Banks S, Morgan D, Sugaya H. Shoulder motions during the golf swing in male amateur golfers. J Orthop Sports Phys Ther. 2003;33(4):196-203.
10. Grimshaw P, Giles A, Tong R, Grimmer K. Lower back and elbow injuries in golf. Sports Med. 2002;32(10):655-666.
1. Field LD, Savoie FH. Common elbow injuries in sport. Sports Med. 1998;26(3):193-205.
2. Loomer RL. Elbow injuries in athletes. Can J Appl Sport Sci. 1982;7(3):164-166.
3. Dines JS, Bedi A, Williams PN, et al. Tennis injuries: epidemiology, pathophysiology, and treatment. J Am Acad Orthop Surg. 2015;23(3):181-189.
4. Banks KP, Ly JQ, Beall DP, Grayson DE, Bancroft LW, Tall MA. Overuse injuries of the upper extremity in the competitive athlete: magnetic resonance imaging findings associated with repetitive trauma. Curr Probl Diagn Radiol. 2005;34(4):127-142.
5. Niebulski HZ, Richardson ML. High-grade pronator teres tear in a cricket batsman. Radiol Case Rep. 2015;6(3):540.
6. Zhang X, Shan G. Where do golf drive swings go wrong? Factors influencing driver swing consistency. Scand J Med Sci Sports. 2014;24(5):749-757.
7. Nesbit SM, McGinnis RS. Kinetic constrained optimization of the golf swing hub path. J Sports Sci Med. 2014;13(4):859-873.
8. Helton MS. Conservative treatment of a proximal full-thickness biceps brachii muscle tear in a special operations soldier. Phys Ther. 2014;94(4):571-577.
9. Mitchell K, Banks S, Morgan D, Sugaya H. Shoulder motions during the golf swing in male amateur golfers. J Orthop Sports Phys Ther. 2003;33(4):196-203.
10. Grimshaw P, Giles A, Tong R, Grimmer K. Lower back and elbow injuries in golf. Sports Med. 2002;32(10):655-666.
Colonic Diaphragm Disease: An Important NSAID Complication to Know
Colonic diaphragm disease (CDD) is a rare but serious complication of nonsteroidal anti‐inflammatory drug (NSAID) use in which diaphragm‐like strictures develop in the large and/or small intestines. There have been about 100 reported cases of CDD since this finding was first reported in the medical literature in 1989.1,2 However, given the frequency of NSAID use, providers should consider this diagnosis.
Case Report
An 85-year‐old woman presented to the emergency department (ED) with generalized weakness, occult positive stool, and severe anemia requiring blood transfusions. Eight months prior, she had presented to her primary care physician (PCP) for a routine visit and was found to have anemia. She was referred to gastroenterology by her PCP for an upper endoscopy but refused the referral despite clear warnings of the potential for serious complications. She was started on ferrous sulfate 325 mg po tid at that time. The patient’s hemoglobin (Hbg) levels ranged from 10.8 to 11.2 g/dL prior to her ED presentation, and random fecal occult blood testing had been negative.
In the ED, the patient reported no abdominal pain, nausea emesis or constipation. Her past medical history was significant for compensated diastolic heart failure, paroxysmal atrial fibrillation controlled by medication, and chronic lumbago. The patient had been prescribed NSAIDs for 10 years for the chronic lumbago diagnosis; however, she was inconsistent in taking this medication until more recent years. Surgical history included a hysterectomy and back surgeries in the distant past. She had no symptoms or history of inflammatory bowel disease. The patient’s medications included both the oral and topical forms of diclofenac, conjugated estrogen, nitroglycerin, amlodipine, hydrocodone bitartrate and acetaminophen 5 mg once daily, amiodarone, diazepam, valsartan, furosemide, oxycodone 5 mg nightly prn for severe pain, and ferrous sulfate 325 mg.
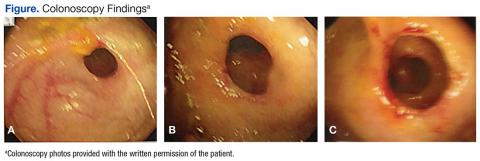
Significant laboratory results in the ED included a Hbg level of 8.3 g/dL; a ferritin level of 11.9 ng/mL, and a serum iron level of 12 mg/dL. A computerized tomography scan of the abdomen and pelvis was normal. The patient was admitted, received a blood transfusion, and a gastroenterology consult was obtained for an upper and lower endoscopy. Although copy was normal, the colonoscopy revealed evidence of CDD of the ascending colon.
Colonic Diaphragm Disease
The gross findings seen on this patient’s colonoscopy showed evidence of the development of an internal colonic stricture from the fibrous overgrowth of the diaphragm (Figures A, B, & C). The narrow lumen is exhibited particularly well in Figure A in the upper left image. The external surface of the intestine appears normal.
Presentation and Evaluation
The presenting symptoms of CDD can vary and include abdominal pain, vomiting, lower gastrointestinal bleeding, anemia, and intestinal obstruction/perforation. Evidence seems to suggest a greater association with use of the oxicams (meloxicam, piroxicam) or diclofenac.1,2 Some researchers have suggested an association with long‐acting NSAID formulations.1 The use of misoprostol or proton pump inhibitors does not seem to lessen the chance of stricture formulation.2 Cox‐2 inhibitors are less likely players in the development of CDD.2 Most diaphragms in the large colon have been noted in the cecum, ascending and transverse colon, though descending colonic lesions have been documented as well.1
The incidence of CDD is higher among women and occurs most often in the seventh decade of life. Correlation with the duration of NSAID use prior to disease onset varies widely, with studies documenting disease onset from 3 months to 5 years after initiating consistent NSAID use.1 The patient in this case study was prescribed NSAIDs for almost 10 years, although she had poor adherence. One study also notes a possible association of CYP2C9*3 genetic polymorphism with the propensity to develop CDD. This finding is particularly interesting since NSAIDs are principally metabolized by CYP2C9 in the liver. CYP2C9 polymorphisms are thought to induce higher plasma concentrations of NSAIDs over time, which may lead to the formation of colonic diaphragms.2
Endoscopy with biopsy is the diagnostic modality of choice. Use of capsule endoscopy is limited because the capsule may not be able to pass through narrow stricture walls.
Pathology and Histology
The mechanism by which NSAIDs induce the formation of colonic diaphragms may be related to these medications’ effects on mucosal integrity, vasoconstriction, and vascular spasm. These effects may lead to “erosions, ulceration, and diaphragm-like strictures.”3
The primary histologic feature of these strictures is submucosal fibrosis with an intact muscularis propria. Prominent features also include areas of ulceration and granulation.1 The widened submucosa results in annular constriction of the intestinal lumen with disorganized bundles of smooth muscle, unmyelinated nerve bundles, scattered ganglion cells, and blood vessels. This morphology can resemble a node or mass.4 The mucosa is typically normal.5 Conditions such as vascular and neuromuscular hamartoma can resemble CDD. Interestingly, the fibrous changes in CDD have been noted apart from NSAID use, leading to speculation that diaphragm formation may be a result of injury and not exclusively a reaction to NSAID use.5
Treatment
Recommendations include NSAID withdrawal, endoscopic/fluoroscopic dilatation, and surgical resection. Outcomes with these treatment approaches have been highly variable. For some patients, NSAID withdrawal alone or following resection has resulted in success. However, even with withdrawal of NSAIDs, symptoms may improve, although the histologic findings of CDD may not be reversed.3,6 In one case report, a colonic stricture persisted endoscopically after NSAID withdrawal but resolved after a 20-week course of prednisone.7
Conclusion
Despite NSAID cessation, the patient in this case study presented a month later with an acute bowel obstruction. The bowel obstruction responded to conservative treatment, and the patient declined surgical intervention. The patient was readmitted again 4 months later with an acute bowel obstruction, and underwent an emergent colonic resection. The pathology report was consistent with CDD. In the 3 years since the resection, the patient has had no recurrences and continues to avoid all NSAIDs. She has refused a follow-up postresection colonoscopy.
1. Munipalle PC, Garud T, Light D. Diaphragmatic disease of the colon: systematic review. Colorectal Dis. 2013;15(9):1063-1069.
2. Ishihara M, Ohmiya N, Nakamura M, et al. Risk factors of symptomatic NSAID-induced small intestinal injury and diaphragm disease. Aliment Pharmacol Ther. 2014;40(5):538-547.
3. Keeling AN, Davis JL, Williams A, Sabharwal T, Adam A. Fluoroscopically guided balloon dilation of NSAID-induced colonic diaphragm. J Vasc Interv Radiol. 2007;18(8):1060-1062.
4. Yousfi MM, De Petris G, Leighton JA, et al. Diaphragm disease after use of nonsteroidal anti-inflammatory agents: first report of diagnosis with capsule endoscopy. J Clin Gastroenterol. 2004;38(8):686-691.
5. Pilgrim S, Velchuru V, Waters G, Tsiamis A, Lal R. Diaphragm disease and small bowel enteropathy due to nonsteroidal anti-inflammatory drugs: a surgical perspective. Colorectal Dis. 2011;13(4):463-466.
6. Munipalle PC, Little M, Garud T, Henderson D. NSAID-induced diaphragmatic disease of the colon. BMJ Case Rep. 2013;2013:pii:bcr2012008448.
7. Penner RM, Williams CN. Resolution of multiple severe nonsteroidal anti-inflammatory drug-induced colonic strictures with prednisone therapy: a case report and review of the literature. Can J Gastroenterol. 2003;17(8):497-500.
Colonic diaphragm disease (CDD) is a rare but serious complication of nonsteroidal anti‐inflammatory drug (NSAID) use in which diaphragm‐like strictures develop in the large and/or small intestines. There have been about 100 reported cases of CDD since this finding was first reported in the medical literature in 1989.1,2 However, given the frequency of NSAID use, providers should consider this diagnosis.
Case Report
An 85-year‐old woman presented to the emergency department (ED) with generalized weakness, occult positive stool, and severe anemia requiring blood transfusions. Eight months prior, she had presented to her primary care physician (PCP) for a routine visit and was found to have anemia. She was referred to gastroenterology by her PCP for an upper endoscopy but refused the referral despite clear warnings of the potential for serious complications. She was started on ferrous sulfate 325 mg po tid at that time. The patient’s hemoglobin (Hbg) levels ranged from 10.8 to 11.2 g/dL prior to her ED presentation, and random fecal occult blood testing had been negative.
In the ED, the patient reported no abdominal pain, nausea emesis or constipation. Her past medical history was significant for compensated diastolic heart failure, paroxysmal atrial fibrillation controlled by medication, and chronic lumbago. The patient had been prescribed NSAIDs for 10 years for the chronic lumbago diagnosis; however, she was inconsistent in taking this medication until more recent years. Surgical history included a hysterectomy and back surgeries in the distant past. She had no symptoms or history of inflammatory bowel disease. The patient’s medications included both the oral and topical forms of diclofenac, conjugated estrogen, nitroglycerin, amlodipine, hydrocodone bitartrate and acetaminophen 5 mg once daily, amiodarone, diazepam, valsartan, furosemide, oxycodone 5 mg nightly prn for severe pain, and ferrous sulfate 325 mg.
Significant laboratory results in the ED included a Hbg level of 8.3 g/dL; a ferritin level of 11.9 ng/mL, and a serum iron level of 12 mg/dL. A computerized tomography scan of the abdomen and pelvis was normal. The patient was admitted, received a blood transfusion, and a gastroenterology consult was obtained for an upper and lower endoscopy. Although copy was normal, the colonoscopy revealed evidence of CDD of the ascending colon.
Colonic Diaphragm Disease
The gross findings seen on this patient’s colonoscopy showed evidence of the development of an internal colonic stricture from the fibrous overgrowth of the diaphragm (Figures A, B, & C). The narrow lumen is exhibited particularly well in Figure A in the upper left image. The external surface of the intestine appears normal.
Presentation and Evaluation
The presenting symptoms of CDD can vary and include abdominal pain, vomiting, lower gastrointestinal bleeding, anemia, and intestinal obstruction/perforation. Evidence seems to suggest a greater association with use of the oxicams (meloxicam, piroxicam) or diclofenac.1,2 Some researchers have suggested an association with long‐acting NSAID formulations.1 The use of misoprostol or proton pump inhibitors does not seem to lessen the chance of stricture formulation.2 Cox‐2 inhibitors are less likely players in the development of CDD.2 Most diaphragms in the large colon have been noted in the cecum, ascending and transverse colon, though descending colonic lesions have been documented as well.1
The incidence of CDD is higher among women and occurs most often in the seventh decade of life. Correlation with the duration of NSAID use prior to disease onset varies widely, with studies documenting disease onset from 3 months to 5 years after initiating consistent NSAID use.1 The patient in this case study was prescribed NSAIDs for almost 10 years, although she had poor adherence. One study also notes a possible association of CYP2C9*3 genetic polymorphism with the propensity to develop CDD. This finding is particularly interesting since NSAIDs are principally metabolized by CYP2C9 in the liver. CYP2C9 polymorphisms are thought to induce higher plasma concentrations of NSAIDs over time, which may lead to the formation of colonic diaphragms.2
Endoscopy with biopsy is the diagnostic modality of choice. Use of capsule endoscopy is limited because the capsule may not be able to pass through narrow stricture walls.
Pathology and Histology
The mechanism by which NSAIDs induce the formation of colonic diaphragms may be related to these medications’ effects on mucosal integrity, vasoconstriction, and vascular spasm. These effects may lead to “erosions, ulceration, and diaphragm-like strictures.”3
The primary histologic feature of these strictures is submucosal fibrosis with an intact muscularis propria. Prominent features also include areas of ulceration and granulation.1 The widened submucosa results in annular constriction of the intestinal lumen with disorganized bundles of smooth muscle, unmyelinated nerve bundles, scattered ganglion cells, and blood vessels. This morphology can resemble a node or mass.4 The mucosa is typically normal.5 Conditions such as vascular and neuromuscular hamartoma can resemble CDD. Interestingly, the fibrous changes in CDD have been noted apart from NSAID use, leading to speculation that diaphragm formation may be a result of injury and not exclusively a reaction to NSAID use.5
Treatment
Recommendations include NSAID withdrawal, endoscopic/fluoroscopic dilatation, and surgical resection. Outcomes with these treatment approaches have been highly variable. For some patients, NSAID withdrawal alone or following resection has resulted in success. However, even with withdrawal of NSAIDs, symptoms may improve, although the histologic findings of CDD may not be reversed.3,6 In one case report, a colonic stricture persisted endoscopically after NSAID withdrawal but resolved after a 20-week course of prednisone.7
Conclusion
Despite NSAID cessation, the patient in this case study presented a month later with an acute bowel obstruction. The bowel obstruction responded to conservative treatment, and the patient declined surgical intervention. The patient was readmitted again 4 months later with an acute bowel obstruction, and underwent an emergent colonic resection. The pathology report was consistent with CDD. In the 3 years since the resection, the patient has had no recurrences and continues to avoid all NSAIDs. She has refused a follow-up postresection colonoscopy.
Colonic diaphragm disease (CDD) is a rare but serious complication of nonsteroidal anti‐inflammatory drug (NSAID) use in which diaphragm‐like strictures develop in the large and/or small intestines. There have been about 100 reported cases of CDD since this finding was first reported in the medical literature in 1989.1,2 However, given the frequency of NSAID use, providers should consider this diagnosis.
Case Report
An 85-year‐old woman presented to the emergency department (ED) with generalized weakness, occult positive stool, and severe anemia requiring blood transfusions. Eight months prior, she had presented to her primary care physician (PCP) for a routine visit and was found to have anemia. She was referred to gastroenterology by her PCP for an upper endoscopy but refused the referral despite clear warnings of the potential for serious complications. She was started on ferrous sulfate 325 mg po tid at that time. The patient’s hemoglobin (Hbg) levels ranged from 10.8 to 11.2 g/dL prior to her ED presentation, and random fecal occult blood testing had been negative.
In the ED, the patient reported no abdominal pain, nausea emesis or constipation. Her past medical history was significant for compensated diastolic heart failure, paroxysmal atrial fibrillation controlled by medication, and chronic lumbago. The patient had been prescribed NSAIDs for 10 years for the chronic lumbago diagnosis; however, she was inconsistent in taking this medication until more recent years. Surgical history included a hysterectomy and back surgeries in the distant past. She had no symptoms or history of inflammatory bowel disease. The patient’s medications included both the oral and topical forms of diclofenac, conjugated estrogen, nitroglycerin, amlodipine, hydrocodone bitartrate and acetaminophen 5 mg once daily, amiodarone, diazepam, valsartan, furosemide, oxycodone 5 mg nightly prn for severe pain, and ferrous sulfate 325 mg.
Significant laboratory results in the ED included a Hbg level of 8.3 g/dL; a ferritin level of 11.9 ng/mL, and a serum iron level of 12 mg/dL. A computerized tomography scan of the abdomen and pelvis was normal. The patient was admitted, received a blood transfusion, and a gastroenterology consult was obtained for an upper and lower endoscopy. Although copy was normal, the colonoscopy revealed evidence of CDD of the ascending colon.
Colonic Diaphragm Disease
The gross findings seen on this patient’s colonoscopy showed evidence of the development of an internal colonic stricture from the fibrous overgrowth of the diaphragm (Figures A, B, & C). The narrow lumen is exhibited particularly well in Figure A in the upper left image. The external surface of the intestine appears normal.
Presentation and Evaluation
The presenting symptoms of CDD can vary and include abdominal pain, vomiting, lower gastrointestinal bleeding, anemia, and intestinal obstruction/perforation. Evidence seems to suggest a greater association with use of the oxicams (meloxicam, piroxicam) or diclofenac.1,2 Some researchers have suggested an association with long‐acting NSAID formulations.1 The use of misoprostol or proton pump inhibitors does not seem to lessen the chance of stricture formulation.2 Cox‐2 inhibitors are less likely players in the development of CDD.2 Most diaphragms in the large colon have been noted in the cecum, ascending and transverse colon, though descending colonic lesions have been documented as well.1
The incidence of CDD is higher among women and occurs most often in the seventh decade of life. Correlation with the duration of NSAID use prior to disease onset varies widely, with studies documenting disease onset from 3 months to 5 years after initiating consistent NSAID use.1 The patient in this case study was prescribed NSAIDs for almost 10 years, although she had poor adherence. One study also notes a possible association of CYP2C9*3 genetic polymorphism with the propensity to develop CDD. This finding is particularly interesting since NSAIDs are principally metabolized by CYP2C9 in the liver. CYP2C9 polymorphisms are thought to induce higher plasma concentrations of NSAIDs over time, which may lead to the formation of colonic diaphragms.2
Endoscopy with biopsy is the diagnostic modality of choice. Use of capsule endoscopy is limited because the capsule may not be able to pass through narrow stricture walls.
Pathology and Histology
The mechanism by which NSAIDs induce the formation of colonic diaphragms may be related to these medications’ effects on mucosal integrity, vasoconstriction, and vascular spasm. These effects may lead to “erosions, ulceration, and diaphragm-like strictures.”3
The primary histologic feature of these strictures is submucosal fibrosis with an intact muscularis propria. Prominent features also include areas of ulceration and granulation.1 The widened submucosa results in annular constriction of the intestinal lumen with disorganized bundles of smooth muscle, unmyelinated nerve bundles, scattered ganglion cells, and blood vessels. This morphology can resemble a node or mass.4 The mucosa is typically normal.5 Conditions such as vascular and neuromuscular hamartoma can resemble CDD. Interestingly, the fibrous changes in CDD have been noted apart from NSAID use, leading to speculation that diaphragm formation may be a result of injury and not exclusively a reaction to NSAID use.5
Treatment
Recommendations include NSAID withdrawal, endoscopic/fluoroscopic dilatation, and surgical resection. Outcomes with these treatment approaches have been highly variable. For some patients, NSAID withdrawal alone or following resection has resulted in success. However, even with withdrawal of NSAIDs, symptoms may improve, although the histologic findings of CDD may not be reversed.3,6 In one case report, a colonic stricture persisted endoscopically after NSAID withdrawal but resolved after a 20-week course of prednisone.7
Conclusion
Despite NSAID cessation, the patient in this case study presented a month later with an acute bowel obstruction. The bowel obstruction responded to conservative treatment, and the patient declined surgical intervention. The patient was readmitted again 4 months later with an acute bowel obstruction, and underwent an emergent colonic resection. The pathology report was consistent with CDD. In the 3 years since the resection, the patient has had no recurrences and continues to avoid all NSAIDs. She has refused a follow-up postresection colonoscopy.
1. Munipalle PC, Garud T, Light D. Diaphragmatic disease of the colon: systematic review. Colorectal Dis. 2013;15(9):1063-1069.
2. Ishihara M, Ohmiya N, Nakamura M, et al. Risk factors of symptomatic NSAID-induced small intestinal injury and diaphragm disease. Aliment Pharmacol Ther. 2014;40(5):538-547.
3. Keeling AN, Davis JL, Williams A, Sabharwal T, Adam A. Fluoroscopically guided balloon dilation of NSAID-induced colonic diaphragm. J Vasc Interv Radiol. 2007;18(8):1060-1062.
4. Yousfi MM, De Petris G, Leighton JA, et al. Diaphragm disease after use of nonsteroidal anti-inflammatory agents: first report of diagnosis with capsule endoscopy. J Clin Gastroenterol. 2004;38(8):686-691.
5. Pilgrim S, Velchuru V, Waters G, Tsiamis A, Lal R. Diaphragm disease and small bowel enteropathy due to nonsteroidal anti-inflammatory drugs: a surgical perspective. Colorectal Dis. 2011;13(4):463-466.
6. Munipalle PC, Little M, Garud T, Henderson D. NSAID-induced diaphragmatic disease of the colon. BMJ Case Rep. 2013;2013:pii:bcr2012008448.
7. Penner RM, Williams CN. Resolution of multiple severe nonsteroidal anti-inflammatory drug-induced colonic strictures with prednisone therapy: a case report and review of the literature. Can J Gastroenterol. 2003;17(8):497-500.
1. Munipalle PC, Garud T, Light D. Diaphragmatic disease of the colon: systematic review. Colorectal Dis. 2013;15(9):1063-1069.
2. Ishihara M, Ohmiya N, Nakamura M, et al. Risk factors of symptomatic NSAID-induced small intestinal injury and diaphragm disease. Aliment Pharmacol Ther. 2014;40(5):538-547.
3. Keeling AN, Davis JL, Williams A, Sabharwal T, Adam A. Fluoroscopically guided balloon dilation of NSAID-induced colonic diaphragm. J Vasc Interv Radiol. 2007;18(8):1060-1062.
4. Yousfi MM, De Petris G, Leighton JA, et al. Diaphragm disease after use of nonsteroidal anti-inflammatory agents: first report of diagnosis with capsule endoscopy. J Clin Gastroenterol. 2004;38(8):686-691.
5. Pilgrim S, Velchuru V, Waters G, Tsiamis A, Lal R. Diaphragm disease and small bowel enteropathy due to nonsteroidal anti-inflammatory drugs: a surgical perspective. Colorectal Dis. 2011;13(4):463-466.
6. Munipalle PC, Little M, Garud T, Henderson D. NSAID-induced diaphragmatic disease of the colon. BMJ Case Rep. 2013;2013:pii:bcr2012008448.
7. Penner RM, Williams CN. Resolution of multiple severe nonsteroidal anti-inflammatory drug-induced colonic strictures with prednisone therapy: a case report and review of the literature. Can J Gastroenterol. 2003;17(8):497-500.
Generalized Vaccinia After Smallpox Vaccination With Concomitant Primary Epstein Barr Virus Infection
Generalized vaccinia (GV) is a rare, self-limiting complication of the smallpox vaccination that is caused by the systemic spread of the virus from the inoculation site. The incidence of GV became rare after routine vaccination was discontinued in the U.S. in 1971 and globally in the 1980s after the disease was eradicated.1,2 However in 2002, heightened concerns for the deliberate release of the smallpox virus as a bioweapon led the U.S. military to restart its smallpox vaccination program for soldiers and public health workers.3,4 Here, the authors describe a patient with concomitant GV and mononucleosis.
Case Report
A 19-year-old active-duty marine presented to his battalion aid station with concern for a spreading vesicular rash 9 days after a primary inoculation with the smallpox vaccine. The rash was limited to the inoculation site on his left shoulder (Figure 1). He had no medical history of eczema, atopic dermatitis, or other rashes and reported no systemic symptoms. His vitals also were within normal limits. A clinical diagnosis of inadvertent inoculation (also termed accidental infection) with satellite lesions was made, and he was discharged with counseling on wound care and close follow-up. Two days later, on postvaccination day 11, he presented with new symptoms of a headache, fever, chills, diffuse myalgia, sore throat, and spreading erythematous macules, papules, and vesicles on his arms, chest, abdomen, back, legs, and face (Figures 2A-2D). His vital signs were remarkable for tachycardia with heart rate of 100 bpm and a fever of 103º F (39.4º C). He was sent to the emergency department with a presumed GV diagnosis.
A complete blood count, liver function tests, and basic metabolic panel were unremarkable. Given his symptom of pharyngitis, a rapid strep test was performed. The test was negative, and a throat culture showed no growth. A mononucleosis screen also was performed and was positive. The patient was diagnosed with mononucleosis and GV. His condition improved, and his vital signs stabilized with conservative treatment without the need for vaccine immune globulin (VIG). He convalesced for 72 hours and was referred to dermatology on the following day. Quarantining him in a single occupancy barracks room until all lesions crusted over addressed the concern for spread of the virus to nonimmunized marines or family members.
On postvaccination day 12, the patient continued to be clinically well, and he remained afebrile. The dermatologist obtained a skin biopsy from a lesion on the patient’s right shin. The biopsy demonstrated marked epidermal necrosis with peripheral keratinocytes showing ballooning degeneration and viral cytopathic changes consistent with GV. Antibody titers showing high levels of Epstein-Barr virus (EBV) capsid IgM and IgG present confirmed mononucleosis infection within the past 6 months. The patient remained clinically well and was released from quarantine on postvaccination day 22 when all lesions crusted over (Figures 3A-3D).
Discussion
The CDC current definition for GV is “the spread of lesions to other parts of the body that are benign in appearance and occur as a result of viremia.”5 Although the exact mechanisms of viral spread are unknown, it may be due to a subtle immunologic defect, specifically in the B-cell line.6,7 Epstein-Barr virus affects the B-cell line, and concurrent infection may depress humoral immunity and allow for systemic spread of the virus.8,9
This case illustrates the potential for a severe reaction after smallpox vaccination in a patient with a concomitant EBV infection. Service members primarily receive the smallpox vaccination early in their career when the risk of mononucleosis is at its highest incidence among young adults, 11 to 48 per 1,000.10-13 Although the potential for disseminated vaccinia following vaccination is rare, clinicians need to remain cognizant of the risk, which may be enhanced by recent or subsequent infection with EBV. However, regular screening for EBV would be of questionable value given the large number of tests needed to prevent a single case of GV.
Generalized vaccinia is a rare complication after smallpox vaccination. Despite its dire appearance, GV typically resolves spontaneously with limited adverse effects (AEs).14 The pre-eradication reported incidence was 17.7 per 1,000,000 recipients in a national survey.15 Posteradication the incidence of GV was 3 times as high with 2 reported cases in 2003 after administration of 38,440 vaccinations.16 Inflammatory reactions can be common; however, these reactions are not due to systemic viral spread.5 When dealing with a vaccinia-specific AE, it is important to distinguish the benign inadvertent inoculations and GV from the more serious reactions of eczema vaccinatum (EV) or progressive vaccinia (PV). 5
Inadvertent inoculations and GV are usually benign and self-limited—requiring only prevention of secondary transmission and nosocomial infection. Eczema vaccinatum occurs among persons with atopic dermatitis or eczema.5 The
Conclusion
The smallpox vaccination is unique among vaccinations. It is the only vaccine that is administered via inoculation with a bifurcated needle, requires regular follow-up care, and can be spread to casual contacts.5
It is important for any practitioner administering the smallpox vaccine to be aware of associated AEs. A greater knowledge of the unique challenges with the smallpox vaccine allows for better patient selection that eliminates those with conditions that impair their immune system and improves patient education.
1. Centers for Disease Control and prevention. Public Health Service recommendation on smallpox vaccination. MMWR Recomm Rep. 1971;20:339
2. The global eradication of smallpox. World Health Organization Web site. http://apps.who.int/iris/bitstream/10665/39253/1/a41438.pdf. Accessed February 8, 2017.
3. Belongia EA, Naleway A. Smallpox vaccine: the good, the bad and the ugly. Clin Med Res. 2003;1(2):87-92.
4. Wharton M, Strikas RA, Harpaz R, et al. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52(RR-7):1-16.
5. Cono J, Casey CG, Bell DM. Smallpox vaccination and adverse reactions. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Updated February 10, 2003. Accessed February 2, 2017.
6. Chahroudi A, Chavan R, Kozyr N, Waller EK, Silvestri G, Feinberg MB. Vaccinia virus tropism for primary hematolymphoid cells is determined by restricted expression of a unique virus receptor. J Virol. 2005;79(16):10397-10407.
7. , , Blasco R. of different leukocyte cell types to Vaccinia virus infection. Virol J. 2004;1(1):10.
8. Küppers R. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. 2003;3(10):801-812.
9. Nemerow G, Cooper N. Infection of B lymphocytes by a human herpesvirus, Epstein-Barr virus, is blocked by calmodulin antagonists. Proc Natl Acad Sci U S A. 1984;81(15):4955-4959.
10. Hallee TJ, Evans AS, Niederman JC, Brooks CM, Voegtly JH. Infectious Mononucleosis at the United States Military Academy. A prospective study of a single class over four years. Yale J Biol Med. 1974;47(3):182-195.
11. Evans AS, Robinton ED. An epidemiological study of infectious mononucleosis. N Engl J Med. 1950;242:492-496.
12. Niederman JC, Evans AS, Subrahmanyan L, McCollum RW. Prevalence, incidence and persistence of EB virus antibody in young adults. N Engl J Med. 1970;282(7):361-365.
13. Sawyer RN, Evans AS, Niederman JC, McCollum RW. Prospective studies of a group of Yale University freshmen. I. Occurrence of infectious mononucleosis. J Infect Dis. 1971;123(3):263-270.
14. Henderson DA, Borio LL, Lane MJ. Smallpox and vaccinia. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 4th ed. Philadelphia, PA: Elsevier; 2004:123-153.
15. Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968—national surveillance in the United States. N Engl J Med. 1969;281(22):1201-1208.
16. Vellozzi C, Lane JM, Averhoff F, et al. Generalized vaccinia, progressive vaccinia and eczema vaccinatum are rare following smallpox (vaccinia) vaccination: United States surveillance, 2003. Clin Infect Dis. 2005;41(5):689-697.
17. Reed J, Scott D. Bray M. Eczema Vaccinatum. Clin Infect Dis. 2012;54(6):832-840.
18. Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36(6):766-774.
19. Fulginiti V, Kempe C, Hathaway W, et al. Progressive vaccinia in immunologically deficient individuals. Birth Defects Orig Artic Ser. 1968;4:129-145.
Generalized vaccinia (GV) is a rare, self-limiting complication of the smallpox vaccination that is caused by the systemic spread of the virus from the inoculation site. The incidence of GV became rare after routine vaccination was discontinued in the U.S. in 1971 and globally in the 1980s after the disease was eradicated.1,2 However in 2002, heightened concerns for the deliberate release of the smallpox virus as a bioweapon led the U.S. military to restart its smallpox vaccination program for soldiers and public health workers.3,4 Here, the authors describe a patient with concomitant GV and mononucleosis.
Case Report
A 19-year-old active-duty marine presented to his battalion aid station with concern for a spreading vesicular rash 9 days after a primary inoculation with the smallpox vaccine. The rash was limited to the inoculation site on his left shoulder (Figure 1). He had no medical history of eczema, atopic dermatitis, or other rashes and reported no systemic symptoms. His vitals also were within normal limits. A clinical diagnosis of inadvertent inoculation (also termed accidental infection) with satellite lesions was made, and he was discharged with counseling on wound care and close follow-up. Two days later, on postvaccination day 11, he presented with new symptoms of a headache, fever, chills, diffuse myalgia, sore throat, and spreading erythematous macules, papules, and vesicles on his arms, chest, abdomen, back, legs, and face (Figures 2A-2D). His vital signs were remarkable for tachycardia with heart rate of 100 bpm and a fever of 103º F (39.4º C). He was sent to the emergency department with a presumed GV diagnosis.
A complete blood count, liver function tests, and basic metabolic panel were unremarkable. Given his symptom of pharyngitis, a rapid strep test was performed. The test was negative, and a throat culture showed no growth. A mononucleosis screen also was performed and was positive. The patient was diagnosed with mononucleosis and GV. His condition improved, and his vital signs stabilized with conservative treatment without the need for vaccine immune globulin (VIG). He convalesced for 72 hours and was referred to dermatology on the following day. Quarantining him in a single occupancy barracks room until all lesions crusted over addressed the concern for spread of the virus to nonimmunized marines or family members.
On postvaccination day 12, the patient continued to be clinically well, and he remained afebrile. The dermatologist obtained a skin biopsy from a lesion on the patient’s right shin. The biopsy demonstrated marked epidermal necrosis with peripheral keratinocytes showing ballooning degeneration and viral cytopathic changes consistent with GV. Antibody titers showing high levels of Epstein-Barr virus (EBV) capsid IgM and IgG present confirmed mononucleosis infection within the past 6 months. The patient remained clinically well and was released from quarantine on postvaccination day 22 when all lesions crusted over (Figures 3A-3D).
Discussion
The CDC current definition for GV is “the spread of lesions to other parts of the body that are benign in appearance and occur as a result of viremia.”5 Although the exact mechanisms of viral spread are unknown, it may be due to a subtle immunologic defect, specifically in the B-cell line.6,7 Epstein-Barr virus affects the B-cell line, and concurrent infection may depress humoral immunity and allow for systemic spread of the virus.8,9
This case illustrates the potential for a severe reaction after smallpox vaccination in a patient with a concomitant EBV infection. Service members primarily receive the smallpox vaccination early in their career when the risk of mononucleosis is at its highest incidence among young adults, 11 to 48 per 1,000.10-13 Although the potential for disseminated vaccinia following vaccination is rare, clinicians need to remain cognizant of the risk, which may be enhanced by recent or subsequent infection with EBV. However, regular screening for EBV would be of questionable value given the large number of tests needed to prevent a single case of GV.
Generalized vaccinia is a rare complication after smallpox vaccination. Despite its dire appearance, GV typically resolves spontaneously with limited adverse effects (AEs).14 The pre-eradication reported incidence was 17.7 per 1,000,000 recipients in a national survey.15 Posteradication the incidence of GV was 3 times as high with 2 reported cases in 2003 after administration of 38,440 vaccinations.16 Inflammatory reactions can be common; however, these reactions are not due to systemic viral spread.5 When dealing with a vaccinia-specific AE, it is important to distinguish the benign inadvertent inoculations and GV from the more serious reactions of eczema vaccinatum (EV) or progressive vaccinia (PV). 5
Inadvertent inoculations and GV are usually benign and self-limited—requiring only prevention of secondary transmission and nosocomial infection. Eczema vaccinatum occurs among persons with atopic dermatitis or eczema.5 The
Conclusion
The smallpox vaccination is unique among vaccinations. It is the only vaccine that is administered via inoculation with a bifurcated needle, requires regular follow-up care, and can be spread to casual contacts.5
It is important for any practitioner administering the smallpox vaccine to be aware of associated AEs. A greater knowledge of the unique challenges with the smallpox vaccine allows for better patient selection that eliminates those with conditions that impair their immune system and improves patient education.
Generalized vaccinia (GV) is a rare, self-limiting complication of the smallpox vaccination that is caused by the systemic spread of the virus from the inoculation site. The incidence of GV became rare after routine vaccination was discontinued in the U.S. in 1971 and globally in the 1980s after the disease was eradicated.1,2 However in 2002, heightened concerns for the deliberate release of the smallpox virus as a bioweapon led the U.S. military to restart its smallpox vaccination program for soldiers and public health workers.3,4 Here, the authors describe a patient with concomitant GV and mononucleosis.
Case Report
A 19-year-old active-duty marine presented to his battalion aid station with concern for a spreading vesicular rash 9 days after a primary inoculation with the smallpox vaccine. The rash was limited to the inoculation site on his left shoulder (Figure 1). He had no medical history of eczema, atopic dermatitis, or other rashes and reported no systemic symptoms. His vitals also were within normal limits. A clinical diagnosis of inadvertent inoculation (also termed accidental infection) with satellite lesions was made, and he was discharged with counseling on wound care and close follow-up. Two days later, on postvaccination day 11, he presented with new symptoms of a headache, fever, chills, diffuse myalgia, sore throat, and spreading erythematous macules, papules, and vesicles on his arms, chest, abdomen, back, legs, and face (Figures 2A-2D). His vital signs were remarkable for tachycardia with heart rate of 100 bpm and a fever of 103º F (39.4º C). He was sent to the emergency department with a presumed GV diagnosis.
A complete blood count, liver function tests, and basic metabolic panel were unremarkable. Given his symptom of pharyngitis, a rapid strep test was performed. The test was negative, and a throat culture showed no growth. A mononucleosis screen also was performed and was positive. The patient was diagnosed with mononucleosis and GV. His condition improved, and his vital signs stabilized with conservative treatment without the need for vaccine immune globulin (VIG). He convalesced for 72 hours and was referred to dermatology on the following day. Quarantining him in a single occupancy barracks room until all lesions crusted over addressed the concern for spread of the virus to nonimmunized marines or family members.
On postvaccination day 12, the patient continued to be clinically well, and he remained afebrile. The dermatologist obtained a skin biopsy from a lesion on the patient’s right shin. The biopsy demonstrated marked epidermal necrosis with peripheral keratinocytes showing ballooning degeneration and viral cytopathic changes consistent with GV. Antibody titers showing high levels of Epstein-Barr virus (EBV) capsid IgM and IgG present confirmed mononucleosis infection within the past 6 months. The patient remained clinically well and was released from quarantine on postvaccination day 22 when all lesions crusted over (Figures 3A-3D).
Discussion
The CDC current definition for GV is “the spread of lesions to other parts of the body that are benign in appearance and occur as a result of viremia.”5 Although the exact mechanisms of viral spread are unknown, it may be due to a subtle immunologic defect, specifically in the B-cell line.6,7 Epstein-Barr virus affects the B-cell line, and concurrent infection may depress humoral immunity and allow for systemic spread of the virus.8,9
This case illustrates the potential for a severe reaction after smallpox vaccination in a patient with a concomitant EBV infection. Service members primarily receive the smallpox vaccination early in their career when the risk of mononucleosis is at its highest incidence among young adults, 11 to 48 per 1,000.10-13 Although the potential for disseminated vaccinia following vaccination is rare, clinicians need to remain cognizant of the risk, which may be enhanced by recent or subsequent infection with EBV. However, regular screening for EBV would be of questionable value given the large number of tests needed to prevent a single case of GV.
Generalized vaccinia is a rare complication after smallpox vaccination. Despite its dire appearance, GV typically resolves spontaneously with limited adverse effects (AEs).14 The pre-eradication reported incidence was 17.7 per 1,000,000 recipients in a national survey.15 Posteradication the incidence of GV was 3 times as high with 2 reported cases in 2003 after administration of 38,440 vaccinations.16 Inflammatory reactions can be common; however, these reactions are not due to systemic viral spread.5 When dealing with a vaccinia-specific AE, it is important to distinguish the benign inadvertent inoculations and GV from the more serious reactions of eczema vaccinatum (EV) or progressive vaccinia (PV). 5
Inadvertent inoculations and GV are usually benign and self-limited—requiring only prevention of secondary transmission and nosocomial infection. Eczema vaccinatum occurs among persons with atopic dermatitis or eczema.5 The
Conclusion
The smallpox vaccination is unique among vaccinations. It is the only vaccine that is administered via inoculation with a bifurcated needle, requires regular follow-up care, and can be spread to casual contacts.5
It is important for any practitioner administering the smallpox vaccine to be aware of associated AEs. A greater knowledge of the unique challenges with the smallpox vaccine allows for better patient selection that eliminates those with conditions that impair their immune system and improves patient education.
1. Centers for Disease Control and prevention. Public Health Service recommendation on smallpox vaccination. MMWR Recomm Rep. 1971;20:339
2. The global eradication of smallpox. World Health Organization Web site. http://apps.who.int/iris/bitstream/10665/39253/1/a41438.pdf. Accessed February 8, 2017.
3. Belongia EA, Naleway A. Smallpox vaccine: the good, the bad and the ugly. Clin Med Res. 2003;1(2):87-92.
4. Wharton M, Strikas RA, Harpaz R, et al. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52(RR-7):1-16.
5. Cono J, Casey CG, Bell DM. Smallpox vaccination and adverse reactions. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Updated February 10, 2003. Accessed February 2, 2017.
6. Chahroudi A, Chavan R, Kozyr N, Waller EK, Silvestri G, Feinberg MB. Vaccinia virus tropism for primary hematolymphoid cells is determined by restricted expression of a unique virus receptor. J Virol. 2005;79(16):10397-10407.
7. , , Blasco R. of different leukocyte cell types to Vaccinia virus infection. Virol J. 2004;1(1):10.
8. Küppers R. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. 2003;3(10):801-812.
9. Nemerow G, Cooper N. Infection of B lymphocytes by a human herpesvirus, Epstein-Barr virus, is blocked by calmodulin antagonists. Proc Natl Acad Sci U S A. 1984;81(15):4955-4959.
10. Hallee TJ, Evans AS, Niederman JC, Brooks CM, Voegtly JH. Infectious Mononucleosis at the United States Military Academy. A prospective study of a single class over four years. Yale J Biol Med. 1974;47(3):182-195.
11. Evans AS, Robinton ED. An epidemiological study of infectious mononucleosis. N Engl J Med. 1950;242:492-496.
12. Niederman JC, Evans AS, Subrahmanyan L, McCollum RW. Prevalence, incidence and persistence of EB virus antibody in young adults. N Engl J Med. 1970;282(7):361-365.
13. Sawyer RN, Evans AS, Niederman JC, McCollum RW. Prospective studies of a group of Yale University freshmen. I. Occurrence of infectious mononucleosis. J Infect Dis. 1971;123(3):263-270.
14. Henderson DA, Borio LL, Lane MJ. Smallpox and vaccinia. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 4th ed. Philadelphia, PA: Elsevier; 2004:123-153.
15. Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968—national surveillance in the United States. N Engl J Med. 1969;281(22):1201-1208.
16. Vellozzi C, Lane JM, Averhoff F, et al. Generalized vaccinia, progressive vaccinia and eczema vaccinatum are rare following smallpox (vaccinia) vaccination: United States surveillance, 2003. Clin Infect Dis. 2005;41(5):689-697.
17. Reed J, Scott D. Bray M. Eczema Vaccinatum. Clin Infect Dis. 2012;54(6):832-840.
18. Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36(6):766-774.
19. Fulginiti V, Kempe C, Hathaway W, et al. Progressive vaccinia in immunologically deficient individuals. Birth Defects Orig Artic Ser. 1968;4:129-145.
1. Centers for Disease Control and prevention. Public Health Service recommendation on smallpox vaccination. MMWR Recomm Rep. 1971;20:339
2. The global eradication of smallpox. World Health Organization Web site. http://apps.who.int/iris/bitstream/10665/39253/1/a41438.pdf. Accessed February 8, 2017.
3. Belongia EA, Naleway A. Smallpox vaccine: the good, the bad and the ugly. Clin Med Res. 2003;1(2):87-92.
4. Wharton M, Strikas RA, Harpaz R, et al. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52(RR-7):1-16.
5. Cono J, Casey CG, Bell DM. Smallpox vaccination and adverse reactions. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Updated February 10, 2003. Accessed February 2, 2017.
6. Chahroudi A, Chavan R, Kozyr N, Waller EK, Silvestri G, Feinberg MB. Vaccinia virus tropism for primary hematolymphoid cells is determined by restricted expression of a unique virus receptor. J Virol. 2005;79(16):10397-10407.
7. , , Blasco R. of different leukocyte cell types to Vaccinia virus infection. Virol J. 2004;1(1):10.
8. Küppers R. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. 2003;3(10):801-812.
9. Nemerow G, Cooper N. Infection of B lymphocytes by a human herpesvirus, Epstein-Barr virus, is blocked by calmodulin antagonists. Proc Natl Acad Sci U S A. 1984;81(15):4955-4959.
10. Hallee TJ, Evans AS, Niederman JC, Brooks CM, Voegtly JH. Infectious Mononucleosis at the United States Military Academy. A prospective study of a single class over four years. Yale J Biol Med. 1974;47(3):182-195.
11. Evans AS, Robinton ED. An epidemiological study of infectious mononucleosis. N Engl J Med. 1950;242:492-496.
12. Niederman JC, Evans AS, Subrahmanyan L, McCollum RW. Prevalence, incidence and persistence of EB virus antibody in young adults. N Engl J Med. 1970;282(7):361-365.
13. Sawyer RN, Evans AS, Niederman JC, McCollum RW. Prospective studies of a group of Yale University freshmen. I. Occurrence of infectious mononucleosis. J Infect Dis. 1971;123(3):263-270.
14. Henderson DA, Borio LL, Lane MJ. Smallpox and vaccinia. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 4th ed. Philadelphia, PA: Elsevier; 2004:123-153.
15. Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968—national surveillance in the United States. N Engl J Med. 1969;281(22):1201-1208.
16. Vellozzi C, Lane JM, Averhoff F, et al. Generalized vaccinia, progressive vaccinia and eczema vaccinatum are rare following smallpox (vaccinia) vaccination: United States surveillance, 2003. Clin Infect Dis. 2005;41(5):689-697.
17. Reed J, Scott D. Bray M. Eczema Vaccinatum. Clin Infect Dis. 2012;54(6):832-840.
18. Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36(6):766-774.
19. Fulginiti V, Kempe C, Hathaway W, et al. Progressive vaccinia in immunologically deficient individuals. Birth Defects Orig Artic Ser. 1968;4:129-145.
One Hundred Case Series of Vocal Cord Dysfunction in a Military Treatment Facility
Vocal cord dysfunction (VCD), also known as paradoxical vocal cord movement, is described as paroxysms of glottis obstruction due to true vocal cord adduction.1 Since VCD presents as a constellation of symptoms associated with dyspnea, it often is misdiagnosed as asthma.2 Vocal cord dysfunction often manifests as episodic dyspnea and wheezing, may occur with exercise, and may be minimally responsive to initial therapies. Flattened inspiratory curves may be noted on pulmonary function tests (PFTs), but direct laryngoscopy is the gold standard for diagnosis.3 A cohort of proven patients with VCD with a plateau in the inspiratory curve of PFTs also had a plateau on expiratory phase in 81% of cases.4
The differential diagnosis of patients presenting with upper airway symptoms is broad. It must include VCD, asthma, angioedema, laryngomalacia, vocal cord polyps, vocal cord tumors, and neurologic conditions such as brain stem compression or movement disorders. Essentially, all movement disorders of vocal cords must be considered, and organic causes of this movement disorder can be evaluated by visualization of the vocal cords. Triggers for VCD include exercise, airborne irritants, gastroesophageal reflux disease (GERD), allergic rhinitis, medications, and psychological conditions.5 Additionally, VCD can coexist with asthma, further complicating accurate diagnoses.6
Therapies are reported in case studies, but no large randomized controlled trials exist to evaluate current therapy options. Primary treatments of asthma therapy were largely ineffective, and ideal therapy includes a multidisciplinary approach, including speech therapy to optimize laryngeal control and treatment of all identified laryngeal irritants.6
The prevalence of VCD is unknown, with no prospective cohort studies completed to date and conflicting diagnostic criteria used in many case studies.7 A prevalence of 2.8% was noted in one particular cohort of 1,028 patients admitted to a rehabilitation center in a calendar year with the primary pulmonary diagnosis on admission.6 Females seemed to be affected at a higher ratio than were males, 2 to 3 females per 1 male diagnosis.7
In the military population, certain risk factors were noted in returning deployed members, including anxiety/high stress, exercise, and acute respiratory illnesses.8 In that particular cohort, 72% positive predictive value was noted for VCD if flattened inspiratory flow loops with negative methacholine challenge were present.
Diagnostic criteria are challenging, as symptoms such as dyspnea may be present acutely, last < 2 minutes, be self-limiting, and completely resolve outside of acute events. Stridor may be noted, primarily above the vocal cords, and less audible on chest auscultation.6 A goal of therapy, in addition to dedicated speech pathologist input, is optimizing comedical conditions, including GERD, allergic rhinitis, concomitant asthma, and any psychological diagnoses.9
Athletes are a particular subset of patients with VCD who are crucial to appropriately diagnose, including a detailed history and physical, PFTs, and proceeding to direct laryngoscopy to confirm diagnoses.10 Behavioral management includes rescue breathing techniques, and speech therapy programs focus on relaxation of the larynx and diaphragmatic breathing techniques, with the goal of establishing sense of control during acute events.10 Military service members are expected to operate at a high-intensity level similar to that of athletes, and treatments considered for athletes are applicable to military service members as well. Military strength and cardiovascular standards are measured by a combination of push-ups, sit-ups, and a run test, in addition to waist measurements. Some of the cohort were identified during physical fitness standard failures, usually in the run test, and ultimately received a pulmonology referral for wheezing or dyspnea with exertion. The objective of this retrospective cohort study was to evaluate 100 consecutively diagnosed cases of VCD in a military treatment facility.
Methods
The authors conducted a retrospective chart review of DoD military medical records of outpatient diagnoses in 100 consecutive diagnoses of VCD from January 2011 to February 2014. Institutional review board approval was obtained under Project RSM20130001E by the Exempt Determination Official at Eglin Air Force Base (AFB), Florida.
All cases were identified at time of VCD visualization and were diagnosed with video stroboscopy by speech therapy or by visual laryngoscopy by the otolaryngology or pulmonology departments via direct visualization.
Cases were collected chronologically, and all diagnosed cases at Eglin AFB hospital were included. Follow-up was scheduled with all patients diagnosed in Speech Therapy, and most patients were concurrently treated by Pulmonology or Allergy/Immunology. Pulmonary function tests were obtained in 98 of the 100 diagnosed cases. Patients eligible for care at Eglin AFB included active-duty and Reserve military members plus dependents and retirees.
The majority of patients diagnosed in this cohort were seen and diagnosed by Speech Therapy. Video stroboscopy is based on the principle that a movement of an object higher than a certain flicker rate appears to stand still to direct visualization, but with a rate of light exposure and imaging above the flicker rate by video, the true movement of the object can be identified.¹¹ Video stroboscopy is considered highly sensitive for organic disorders of vocal cords, but it is not specific for either organic or dysfunctional disorders.¹¹ It is still the gold standard above direct visualization, as it can detect abnormal movement of vocal cords above the critical rate that the human eye would perceive as not moving due to the frequency of movement (Figures 1 & 2).¹¹
In an older study, laryngoscopy was able to diagnose 100% of patients with symptomatic paradoxical vocal cord movement and additional 60% asymptomatic patients with a constellation of symptoms consistent with paradoxical vocal cord movement.¹²
Speech Therapy; Ear, Nose, and Throat (ENT); and Pulmonology may not perform direct visualization in these patients at initial presentation due to other suspected diagnoses. A more common test is the PFT, especially if asthma or other airway tract diseases are suspected (Figure 3).
Patient Descriptions
Study patients were referred for a variety of reasons, often from primary care clinics for concerns for asthma, episodic dyspnea, wheezing, or decreased exercise tolerance thought to be related to pulmonary or allergy causes. Pulmonology worked closely with Speech Therapy and referred VCD cases for speech evaluation, including video stroboscopy. Notably, of the patients in this cohort, although some were suspected to have asthma, those patients were ruled out during part of the pulmonology evaluation, both with PFT testing and methacholine challenges. An asthma diagnosis is important in a military treatment facility, as asthma is often grounds for discharge.
Patients ranged in age from 13 to 68 years, with a median age at 31 years diagnosis. Thirty-nine females and 61 males comprised the total case series. Speech Therapy diagnosed 97 patients, 96 were diagnosed at Eglin AFB hospital via stroboscopy. One patient was diagnosed off-base by Speech Therapy via direct visualization, 1 patient was diagnosed by Pulmonology on-base via direct visualization, and 2 patients were diagnosed by ENT on-base via direct visualization. These patients had direct laryngoscopy completed, often to rule out other organic causes for upper airway disease processes, and were found to have visual paradoxical vocal cord movement. Ninety-eight patients completed PFTs. Several patients were lost to follow-up, as can be common in a military population with frequent moves or members leaving service.
On record review, patient symptoms were present in the range of 2 months to 20 years, with a median duration of symptomatic reports lasting 2 years prior to diagnosis. Common diagnoses prior to visual VCD diagnosis included asthma, exercise-induced asthma, anxiety, and episodic wheezing. Risk factors that were evaluated in this case series included age, sex, body mass index (BMI), GERD, allergic rhinitis, postnasal drip, active smoker, previous smoker, and mental health diagnoses (Figure 4).
Pulmonary function test results were analyzed on 98 patients, including forced expiratory volume in 1 second (FEV1); forced vital capacity (FVC), FEV1/FVC ratio; peak inspiratory flow (PIF) and peak expiratory flow (PEF)—available in 97 studies; forced expiratory flow (FEF) at 25% to 75% of FVC (FEF 25%-75%)—available in 96 studies; and maximum voluntary ventilation (MVV) and MVV/FEV1 ratio—available in 60 of 98 PFTs.
Interventions
All patients diagnosed by Speech Therapy on-base were provided with laryngeal relaxation techniques, diaphragmatic breathing techniques, and controlled inhale/exhale techniques at time of diagnosis, with frequent follow-up scheduled with Speech Therapy and Pulmonology. All diagnoses potentially contributing to laryngeal irritation were treated, including GERD, allergic rhinitis, smoking cessation, weight loss, and exercise recommendations as needed.
Patients reported improvement on follow-up appointments with Speech Therapy in overall control of symptoms, subjectively categorized as poor improvement, partial improvement, and complete improvement. This was a subjective measurement of improvement and fully dependent on follow-up care and patient reporting for improvement. No predefined number of follow-ups was determined; patients were followed monthly until they declined further care, fully improved, moved out of the military treatment system, or were lost to follow-up.
Treatment included structured Speech Therapy sessions. Response to treatment was subjectively qualified by patient report. Fifteen patients reported complete resolution of symptoms, 57 reported partial improvement, 24 reported poor improvement, and 4 patients were lost to follow-up.
Results
Risk factors for the diagnosis of VCD included possible associations with GERD, allergic rhinitis, smoking, prior smoking, BMI, and mental health diagnoses. Body mass index ranged from 17 to 36 in the case series, with median BMI of 27. Mental health diagnoses were present in 35 patients and included diagnoses of anxiety, depression, and adjustment disorders. Gastroesophageal reflux disease diagnosis was present in 59 of the case series patients, 80 had the diagnosis of allergic rhinitis, 63 were diagnosed with postnasal drip. Sixteen case series patients were current smokers. An additional 26 were previous smokers (at least 100 cigarettes in lifetime) for a total of 42 patients that were current or prior smokers.
The chart review was completed to evaluate for the presence of these diagnoses, which included previous treatments; for example, proton pump inhibitors for GERD, antidepressants for depression, or intranasal steroids for allergic rhinitis. The diagnosis was counted as present if the patient was currently being treated for the particular diagnosis in question.
PFT Data
Data from PFTs were available for 98 of 100 cases diagnosed. Review of data across all 98 patients is noted for median FEV1 of 3.6, a median FVC of 4.5, with ratio of 0.80.
Since PFT values vary according to age, sex, and ethnicity, PFTs were analyzed for percent predicted values based on age, gender, and race. Notably, median values for FEV1, FVC, and PEF were all close to 100% of the predicted value. The MVV percent predicted was available in 60 cases and was 93% of predicted values. The most significant difference from expected values was FEF 25% to 75%, at 84% of expected results.
Flow-volume loop evaluations on the 97 PFTs available were completed, and 58 of the 97 were noted for variable extrathoracic airway obstruction consistent with inspiratory inhibition in the patient population. This is 60% of the available PFTs in this cohort study.
Discussion
This retrospective chart review of 100 consecutive VCD diagnoses in a military treatment facility reinforces many of the findings currently available in the literature. As illustrated in a Chest review article, the diagnosis of VCD on history, physical examination, or PFTs remains ellusive.1 The PFT evaluation contains some subjectivity regarding the flattening of inspiratory flow-volume loops and is not routinely reported in PFT results. In patients diagnosed with VCD, a clear consensus of treatment modalities remains lacking. Modification of risk factors (allergic rhinitis, GERD, smoking cessation, weight loss) assisted in self-reported patient improvement, as did focused speech therapy.
The median age of 31 years, likely reflected the younger military population served at Eglin AFB. Seventy-five of these patients were currently on active duty, 6 were retired from active duty (veterans), and 19 were dependents. The median time of symptoms to diagnosis was 2 years. Prior misdiagnosis with other diseases such as asthma was common. Also, referral to Pulmonology and Speech Therapy was usually completed after failed outpatient primary care management for the alternative diagnoses.
Improvement with therapy was mixed, and during the time of documented follow-up, 72 patients reported complete or partial improvement. Most active-duty patients in the partial improvement category based this subjective reporting on their ability to meet military physical fitness standards.
Previous data suggested a female predominance, but this study population was 61% male. Military populations are about 80% to 85% male, so an increase in male diagnosis is expected.
Many patients in the patient cohort arrived as a result of Pulmonology referrals with a presumptive diagnoses of asthma but were determined not to have asthma through PFT results inconsistent with asthma, no improvement with β-agonist therapies, and negative methacholine challenges (if performed). These results prompted evaluations for other conditions and eventually a VCD diagnosis. As noted, exclusion of asthma is of particular importance in a military population, as medical discharges often are pursued in service members with asthma whether controlled or uncontrolled. Lag time to referral also is possible in failures of military physical, which prompted medical evaluation once several failures had occurred over a 1- to 2-year time frame.
The PFT data evaluation was inconclusive for statistically significant changes when compared with age-matched normal PFT values. This also was noted in previous studies of VCD cases. Most notable was percent predicted values of FEF 25% to 75%, with 84% of expected values. The FEV1, FVC, and PEF all fell within predicted values of normal, despite wide ranges in age, sex, and ethnicity among the subjects. Inspiratory flattening consistent with extrathoracic obstruction was present in 58 of the 97 PFTs available for review at Eglin AFB.
Limitations
Limitations to this retrospective case series are illustrated here. Cases were found only when VCD was diagnosed and coded; and it is the authors’ suspicion that many have been misdiagnosed or improperly treated for asthma or other pulmonary/oropharynx conditions. If providers are not familiar with VCD or if PFT readings do not comment on inspiratory findings, diagnosis is less likely. Some of the authors’ colleagues already have determined that postdeployment prevalence of VCD seems to be elevated.8
This cohort was completed on all patients in a military treatment facility, with 75 active-duty personnel, 6 veterans, and 19 dependents of varying ages. This case series is retrospective and tabulates suspected risk factors; stronger and more informative studies could certainly be completed in prospective studies (although likely difficult with low prevalence) or in treatment comparison studies at the time of diagnosis.
Since the cohort had varied and lengthy time to diagnosis from onset of related symptoms, the treatment patients received prior to diagnosis differed extensively. Diagnosis was completed by numerous primary care managers or other subspecialties prior to arrival to Pulmonology and Speech Therapy at Eglin AFB. Once diagnosed in Speech Therapy, consistent treatment options were provided to patients in accordance with standard of care.
It is the authors’ suspicion that VCD may have a higher prevalence than previously reported in the literature. Military service members are tested annually or biannually on physical fitness standards and are evaluated for medical reasons for recurrent fitness standard failures. This selection of patients is more likely to have a VCD evaluation as part of a comprehensive evaluation than is a healthy adult in a civilian population. A prospective study in military service members would be more fruitful and possibly yield a higher prevalence postdeployment.
Conclusion
Vocal cord dysfunction remains a difficult diagnosis to treat, because multiple comorbidities likely contribute to the diagnosis. This retrospective case series attempted to compile common themes and noted that most of the patients had 2 or more risk factors of smoking, allergic rhinitis, GERD, or mental health diagnoses. A prospective trial would be ideal to evaluate VCD further. A focused trial in the particular communities of athletes or of military service members may be of increased benefit to better define VCD. It is notable that 100 cases were found in a relatively short period for a community hospital, and prevalence may be higher than previously reported.
1. Morris MJ, Christopher KL. Diagnostic criteria for the classification of vocal cord dysfunction. Chest. 2010;138(5):1213-1223.
2. National Heart, Lung, and Blood Institute. Expert panel report 3: guidelines for the diagnoses and management of asthma. Full report 2007. https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln .pdf. Published 2007.Accessed February 1, 2017.
3. Newman KB, Mason UG III, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med. 1995;152(4, pt 1):1382-1386.
4. Sanz Santiago V, López Neyra A, Almería Gil E, Villa Asensi JR. Spirometry patterns in vocal cord dysfunction [in Spanish]. An Pediatr (Barc). 2013;78(3):173-177.
5. Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81(2):156-159.
6. Benninger C, Parsons JP, Mastronarde JG. Vocal cord dysfunction and asthma. Curr Opin Pulm Med. 2011;17(1):45-49.
7. Campainha S, Ribeiro C, Guimar M, Lima R. Vocal cord dysfunction: a frequently forgotten entity. Case Rep Pulmonol. 2012;2012:525493.
8. Morris MJ, Oleszewski RT, Sterner JB, Allan PF. Vocal cord dysfunction related to combat deployment. Mil Med. 2013;178(11):1208-1212.
9. Al-Alwan A, Kaminsky D. Vocal cord dysfunction in athletes: clinical presentation and review of the literature. Phys Sportsmed. 2012;40(2):22-27.
10. Kenn K, Schmitz M. Prevalence of vocal cord dysfunction in patients with dyspnea. First prospective clinical study. Am J Respir Crit Care Med. 1997;155:A965.
11. Wendler, J, Nawka, T, Verges, D. Instructional course: videolaryngo-stroboscopy and phonetography—basic tools for diagnostics and documentation in the voice clinic. Poster presented at: 15th European Congress of Oto-Rhino-Laryngology, Head and Neck Surgery; September 11-16, 2004; Rodos-Kos, Greece.
12. Ibrahim WH, Gheriani HA, Almohamed AA, Raza T. Paradoxical vocal cord motion disorder: past, present and future. Postgrad Med J. 2007;83(977):164-172.
Vocal cord dysfunction (VCD), also known as paradoxical vocal cord movement, is described as paroxysms of glottis obstruction due to true vocal cord adduction.1 Since VCD presents as a constellation of symptoms associated with dyspnea, it often is misdiagnosed as asthma.2 Vocal cord dysfunction often manifests as episodic dyspnea and wheezing, may occur with exercise, and may be minimally responsive to initial therapies. Flattened inspiratory curves may be noted on pulmonary function tests (PFTs), but direct laryngoscopy is the gold standard for diagnosis.3 A cohort of proven patients with VCD with a plateau in the inspiratory curve of PFTs also had a plateau on expiratory phase in 81% of cases.4
The differential diagnosis of patients presenting with upper airway symptoms is broad. It must include VCD, asthma, angioedema, laryngomalacia, vocal cord polyps, vocal cord tumors, and neurologic conditions such as brain stem compression or movement disorders. Essentially, all movement disorders of vocal cords must be considered, and organic causes of this movement disorder can be evaluated by visualization of the vocal cords. Triggers for VCD include exercise, airborne irritants, gastroesophageal reflux disease (GERD), allergic rhinitis, medications, and psychological conditions.5 Additionally, VCD can coexist with asthma, further complicating accurate diagnoses.6
Therapies are reported in case studies, but no large randomized controlled trials exist to evaluate current therapy options. Primary treatments of asthma therapy were largely ineffective, and ideal therapy includes a multidisciplinary approach, including speech therapy to optimize laryngeal control and treatment of all identified laryngeal irritants.6
The prevalence of VCD is unknown, with no prospective cohort studies completed to date and conflicting diagnostic criteria used in many case studies.7 A prevalence of 2.8% was noted in one particular cohort of 1,028 patients admitted to a rehabilitation center in a calendar year with the primary pulmonary diagnosis on admission.6 Females seemed to be affected at a higher ratio than were males, 2 to 3 females per 1 male diagnosis.7
In the military population, certain risk factors were noted in returning deployed members, including anxiety/high stress, exercise, and acute respiratory illnesses.8 In that particular cohort, 72% positive predictive value was noted for VCD if flattened inspiratory flow loops with negative methacholine challenge were present.
Diagnostic criteria are challenging, as symptoms such as dyspnea may be present acutely, last < 2 minutes, be self-limiting, and completely resolve outside of acute events. Stridor may be noted, primarily above the vocal cords, and less audible on chest auscultation.6 A goal of therapy, in addition to dedicated speech pathologist input, is optimizing comedical conditions, including GERD, allergic rhinitis, concomitant asthma, and any psychological diagnoses.9
Athletes are a particular subset of patients with VCD who are crucial to appropriately diagnose, including a detailed history and physical, PFTs, and proceeding to direct laryngoscopy to confirm diagnoses.10 Behavioral management includes rescue breathing techniques, and speech therapy programs focus on relaxation of the larynx and diaphragmatic breathing techniques, with the goal of establishing sense of control during acute events.10 Military service members are expected to operate at a high-intensity level similar to that of athletes, and treatments considered for athletes are applicable to military service members as well. Military strength and cardiovascular standards are measured by a combination of push-ups, sit-ups, and a run test, in addition to waist measurements. Some of the cohort were identified during physical fitness standard failures, usually in the run test, and ultimately received a pulmonology referral for wheezing or dyspnea with exertion. The objective of this retrospective cohort study was to evaluate 100 consecutively diagnosed cases of VCD in a military treatment facility.
Methods
The authors conducted a retrospective chart review of DoD military medical records of outpatient diagnoses in 100 consecutive diagnoses of VCD from January 2011 to February 2014. Institutional review board approval was obtained under Project RSM20130001E by the Exempt Determination Official at Eglin Air Force Base (AFB), Florida.
All cases were identified at time of VCD visualization and were diagnosed with video stroboscopy by speech therapy or by visual laryngoscopy by the otolaryngology or pulmonology departments via direct visualization.
Cases were collected chronologically, and all diagnosed cases at Eglin AFB hospital were included. Follow-up was scheduled with all patients diagnosed in Speech Therapy, and most patients were concurrently treated by Pulmonology or Allergy/Immunology. Pulmonary function tests were obtained in 98 of the 100 diagnosed cases. Patients eligible for care at Eglin AFB included active-duty and Reserve military members plus dependents and retirees.
The majority of patients diagnosed in this cohort were seen and diagnosed by Speech Therapy. Video stroboscopy is based on the principle that a movement of an object higher than a certain flicker rate appears to stand still to direct visualization, but with a rate of light exposure and imaging above the flicker rate by video, the true movement of the object can be identified.¹¹ Video stroboscopy is considered highly sensitive for organic disorders of vocal cords, but it is not specific for either organic or dysfunctional disorders.¹¹ It is still the gold standard above direct visualization, as it can detect abnormal movement of vocal cords above the critical rate that the human eye would perceive as not moving due to the frequency of movement (Figures 1 & 2).¹¹
In an older study, laryngoscopy was able to diagnose 100% of patients with symptomatic paradoxical vocal cord movement and additional 60% asymptomatic patients with a constellation of symptoms consistent with paradoxical vocal cord movement.¹²
Speech Therapy; Ear, Nose, and Throat (ENT); and Pulmonology may not perform direct visualization in these patients at initial presentation due to other suspected diagnoses. A more common test is the PFT, especially if asthma or other airway tract diseases are suspected (Figure 3).
Patient Descriptions
Study patients were referred for a variety of reasons, often from primary care clinics for concerns for asthma, episodic dyspnea, wheezing, or decreased exercise tolerance thought to be related to pulmonary or allergy causes. Pulmonology worked closely with Speech Therapy and referred VCD cases for speech evaluation, including video stroboscopy. Notably, of the patients in this cohort, although some were suspected to have asthma, those patients were ruled out during part of the pulmonology evaluation, both with PFT testing and methacholine challenges. An asthma diagnosis is important in a military treatment facility, as asthma is often grounds for discharge.
Patients ranged in age from 13 to 68 years, with a median age at 31 years diagnosis. Thirty-nine females and 61 males comprised the total case series. Speech Therapy diagnosed 97 patients, 96 were diagnosed at Eglin AFB hospital via stroboscopy. One patient was diagnosed off-base by Speech Therapy via direct visualization, 1 patient was diagnosed by Pulmonology on-base via direct visualization, and 2 patients were diagnosed by ENT on-base via direct visualization. These patients had direct laryngoscopy completed, often to rule out other organic causes for upper airway disease processes, and were found to have visual paradoxical vocal cord movement. Ninety-eight patients completed PFTs. Several patients were lost to follow-up, as can be common in a military population with frequent moves or members leaving service.
On record review, patient symptoms were present in the range of 2 months to 20 years, with a median duration of symptomatic reports lasting 2 years prior to diagnosis. Common diagnoses prior to visual VCD diagnosis included asthma, exercise-induced asthma, anxiety, and episodic wheezing. Risk factors that were evaluated in this case series included age, sex, body mass index (BMI), GERD, allergic rhinitis, postnasal drip, active smoker, previous smoker, and mental health diagnoses (Figure 4).
Pulmonary function test results were analyzed on 98 patients, including forced expiratory volume in 1 second (FEV1); forced vital capacity (FVC), FEV1/FVC ratio; peak inspiratory flow (PIF) and peak expiratory flow (PEF)—available in 97 studies; forced expiratory flow (FEF) at 25% to 75% of FVC (FEF 25%-75%)—available in 96 studies; and maximum voluntary ventilation (MVV) and MVV/FEV1 ratio—available in 60 of 98 PFTs.
Interventions
All patients diagnosed by Speech Therapy on-base were provided with laryngeal relaxation techniques, diaphragmatic breathing techniques, and controlled inhale/exhale techniques at time of diagnosis, with frequent follow-up scheduled with Speech Therapy and Pulmonology. All diagnoses potentially contributing to laryngeal irritation were treated, including GERD, allergic rhinitis, smoking cessation, weight loss, and exercise recommendations as needed.
Patients reported improvement on follow-up appointments with Speech Therapy in overall control of symptoms, subjectively categorized as poor improvement, partial improvement, and complete improvement. This was a subjective measurement of improvement and fully dependent on follow-up care and patient reporting for improvement. No predefined number of follow-ups was determined; patients were followed monthly until they declined further care, fully improved, moved out of the military treatment system, or were lost to follow-up.
Treatment included structured Speech Therapy sessions. Response to treatment was subjectively qualified by patient report. Fifteen patients reported complete resolution of symptoms, 57 reported partial improvement, 24 reported poor improvement, and 4 patients were lost to follow-up.
Results
Risk factors for the diagnosis of VCD included possible associations with GERD, allergic rhinitis, smoking, prior smoking, BMI, and mental health diagnoses. Body mass index ranged from 17 to 36 in the case series, with median BMI of 27. Mental health diagnoses were present in 35 patients and included diagnoses of anxiety, depression, and adjustment disorders. Gastroesophageal reflux disease diagnosis was present in 59 of the case series patients, 80 had the diagnosis of allergic rhinitis, 63 were diagnosed with postnasal drip. Sixteen case series patients were current smokers. An additional 26 were previous smokers (at least 100 cigarettes in lifetime) for a total of 42 patients that were current or prior smokers.
The chart review was completed to evaluate for the presence of these diagnoses, which included previous treatments; for example, proton pump inhibitors for GERD, antidepressants for depression, or intranasal steroids for allergic rhinitis. The diagnosis was counted as present if the patient was currently being treated for the particular diagnosis in question.
PFT Data
Data from PFTs were available for 98 of 100 cases diagnosed. Review of data across all 98 patients is noted for median FEV1 of 3.6, a median FVC of 4.5, with ratio of 0.80.
Since PFT values vary according to age, sex, and ethnicity, PFTs were analyzed for percent predicted values based on age, gender, and race. Notably, median values for FEV1, FVC, and PEF were all close to 100% of the predicted value. The MVV percent predicted was available in 60 cases and was 93% of predicted values. The most significant difference from expected values was FEF 25% to 75%, at 84% of expected results.
Flow-volume loop evaluations on the 97 PFTs available were completed, and 58 of the 97 were noted for variable extrathoracic airway obstruction consistent with inspiratory inhibition in the patient population. This is 60% of the available PFTs in this cohort study.
Discussion
This retrospective chart review of 100 consecutive VCD diagnoses in a military treatment facility reinforces many of the findings currently available in the literature. As illustrated in a Chest review article, the diagnosis of VCD on history, physical examination, or PFTs remains ellusive.1 The PFT evaluation contains some subjectivity regarding the flattening of inspiratory flow-volume loops and is not routinely reported in PFT results. In patients diagnosed with VCD, a clear consensus of treatment modalities remains lacking. Modification of risk factors (allergic rhinitis, GERD, smoking cessation, weight loss) assisted in self-reported patient improvement, as did focused speech therapy.
The median age of 31 years, likely reflected the younger military population served at Eglin AFB. Seventy-five of these patients were currently on active duty, 6 were retired from active duty (veterans), and 19 were dependents. The median time of symptoms to diagnosis was 2 years. Prior misdiagnosis with other diseases such as asthma was common. Also, referral to Pulmonology and Speech Therapy was usually completed after failed outpatient primary care management for the alternative diagnoses.
Improvement with therapy was mixed, and during the time of documented follow-up, 72 patients reported complete or partial improvement. Most active-duty patients in the partial improvement category based this subjective reporting on their ability to meet military physical fitness standards.
Previous data suggested a female predominance, but this study population was 61% male. Military populations are about 80% to 85% male, so an increase in male diagnosis is expected.
Many patients in the patient cohort arrived as a result of Pulmonology referrals with a presumptive diagnoses of asthma but were determined not to have asthma through PFT results inconsistent with asthma, no improvement with β-agonist therapies, and negative methacholine challenges (if performed). These results prompted evaluations for other conditions and eventually a VCD diagnosis. As noted, exclusion of asthma is of particular importance in a military population, as medical discharges often are pursued in service members with asthma whether controlled or uncontrolled. Lag time to referral also is possible in failures of military physical, which prompted medical evaluation once several failures had occurred over a 1- to 2-year time frame.
The PFT data evaluation was inconclusive for statistically significant changes when compared with age-matched normal PFT values. This also was noted in previous studies of VCD cases. Most notable was percent predicted values of FEF 25% to 75%, with 84% of expected values. The FEV1, FVC, and PEF all fell within predicted values of normal, despite wide ranges in age, sex, and ethnicity among the subjects. Inspiratory flattening consistent with extrathoracic obstruction was present in 58 of the 97 PFTs available for review at Eglin AFB.
Limitations
Limitations to this retrospective case series are illustrated here. Cases were found only when VCD was diagnosed and coded; and it is the authors’ suspicion that many have been misdiagnosed or improperly treated for asthma or other pulmonary/oropharynx conditions. If providers are not familiar with VCD or if PFT readings do not comment on inspiratory findings, diagnosis is less likely. Some of the authors’ colleagues already have determined that postdeployment prevalence of VCD seems to be elevated.8
This cohort was completed on all patients in a military treatment facility, with 75 active-duty personnel, 6 veterans, and 19 dependents of varying ages. This case series is retrospective and tabulates suspected risk factors; stronger and more informative studies could certainly be completed in prospective studies (although likely difficult with low prevalence) or in treatment comparison studies at the time of diagnosis.
Since the cohort had varied and lengthy time to diagnosis from onset of related symptoms, the treatment patients received prior to diagnosis differed extensively. Diagnosis was completed by numerous primary care managers or other subspecialties prior to arrival to Pulmonology and Speech Therapy at Eglin AFB. Once diagnosed in Speech Therapy, consistent treatment options were provided to patients in accordance with standard of care.
It is the authors’ suspicion that VCD may have a higher prevalence than previously reported in the literature. Military service members are tested annually or biannually on physical fitness standards and are evaluated for medical reasons for recurrent fitness standard failures. This selection of patients is more likely to have a VCD evaluation as part of a comprehensive evaluation than is a healthy adult in a civilian population. A prospective study in military service members would be more fruitful and possibly yield a higher prevalence postdeployment.
Conclusion
Vocal cord dysfunction remains a difficult diagnosis to treat, because multiple comorbidities likely contribute to the diagnosis. This retrospective case series attempted to compile common themes and noted that most of the patients had 2 or more risk factors of smoking, allergic rhinitis, GERD, or mental health diagnoses. A prospective trial would be ideal to evaluate VCD further. A focused trial in the particular communities of athletes or of military service members may be of increased benefit to better define VCD. It is notable that 100 cases were found in a relatively short period for a community hospital, and prevalence may be higher than previously reported.
Vocal cord dysfunction (VCD), also known as paradoxical vocal cord movement, is described as paroxysms of glottis obstruction due to true vocal cord adduction.1 Since VCD presents as a constellation of symptoms associated with dyspnea, it often is misdiagnosed as asthma.2 Vocal cord dysfunction often manifests as episodic dyspnea and wheezing, may occur with exercise, and may be minimally responsive to initial therapies. Flattened inspiratory curves may be noted on pulmonary function tests (PFTs), but direct laryngoscopy is the gold standard for diagnosis.3 A cohort of proven patients with VCD with a plateau in the inspiratory curve of PFTs also had a plateau on expiratory phase in 81% of cases.4
The differential diagnosis of patients presenting with upper airway symptoms is broad. It must include VCD, asthma, angioedema, laryngomalacia, vocal cord polyps, vocal cord tumors, and neurologic conditions such as brain stem compression or movement disorders. Essentially, all movement disorders of vocal cords must be considered, and organic causes of this movement disorder can be evaluated by visualization of the vocal cords. Triggers for VCD include exercise, airborne irritants, gastroesophageal reflux disease (GERD), allergic rhinitis, medications, and psychological conditions.5 Additionally, VCD can coexist with asthma, further complicating accurate diagnoses.6
Therapies are reported in case studies, but no large randomized controlled trials exist to evaluate current therapy options. Primary treatments of asthma therapy were largely ineffective, and ideal therapy includes a multidisciplinary approach, including speech therapy to optimize laryngeal control and treatment of all identified laryngeal irritants.6
The prevalence of VCD is unknown, with no prospective cohort studies completed to date and conflicting diagnostic criteria used in many case studies.7 A prevalence of 2.8% was noted in one particular cohort of 1,028 patients admitted to a rehabilitation center in a calendar year with the primary pulmonary diagnosis on admission.6 Females seemed to be affected at a higher ratio than were males, 2 to 3 females per 1 male diagnosis.7
In the military population, certain risk factors were noted in returning deployed members, including anxiety/high stress, exercise, and acute respiratory illnesses.8 In that particular cohort, 72% positive predictive value was noted for VCD if flattened inspiratory flow loops with negative methacholine challenge were present.
Diagnostic criteria are challenging, as symptoms such as dyspnea may be present acutely, last < 2 minutes, be self-limiting, and completely resolve outside of acute events. Stridor may be noted, primarily above the vocal cords, and less audible on chest auscultation.6 A goal of therapy, in addition to dedicated speech pathologist input, is optimizing comedical conditions, including GERD, allergic rhinitis, concomitant asthma, and any psychological diagnoses.9
Athletes are a particular subset of patients with VCD who are crucial to appropriately diagnose, including a detailed history and physical, PFTs, and proceeding to direct laryngoscopy to confirm diagnoses.10 Behavioral management includes rescue breathing techniques, and speech therapy programs focus on relaxation of the larynx and diaphragmatic breathing techniques, with the goal of establishing sense of control during acute events.10 Military service members are expected to operate at a high-intensity level similar to that of athletes, and treatments considered for athletes are applicable to military service members as well. Military strength and cardiovascular standards are measured by a combination of push-ups, sit-ups, and a run test, in addition to waist measurements. Some of the cohort were identified during physical fitness standard failures, usually in the run test, and ultimately received a pulmonology referral for wheezing or dyspnea with exertion. The objective of this retrospective cohort study was to evaluate 100 consecutively diagnosed cases of VCD in a military treatment facility.
Methods
The authors conducted a retrospective chart review of DoD military medical records of outpatient diagnoses in 100 consecutive diagnoses of VCD from January 2011 to February 2014. Institutional review board approval was obtained under Project RSM20130001E by the Exempt Determination Official at Eglin Air Force Base (AFB), Florida.
All cases were identified at time of VCD visualization and were diagnosed with video stroboscopy by speech therapy or by visual laryngoscopy by the otolaryngology or pulmonology departments via direct visualization.
Cases were collected chronologically, and all diagnosed cases at Eglin AFB hospital were included. Follow-up was scheduled with all patients diagnosed in Speech Therapy, and most patients were concurrently treated by Pulmonology or Allergy/Immunology. Pulmonary function tests were obtained in 98 of the 100 diagnosed cases. Patients eligible for care at Eglin AFB included active-duty and Reserve military members plus dependents and retirees.
The majority of patients diagnosed in this cohort were seen and diagnosed by Speech Therapy. Video stroboscopy is based on the principle that a movement of an object higher than a certain flicker rate appears to stand still to direct visualization, but with a rate of light exposure and imaging above the flicker rate by video, the true movement of the object can be identified.¹¹ Video stroboscopy is considered highly sensitive for organic disorders of vocal cords, but it is not specific for either organic or dysfunctional disorders.¹¹ It is still the gold standard above direct visualization, as it can detect abnormal movement of vocal cords above the critical rate that the human eye would perceive as not moving due to the frequency of movement (Figures 1 & 2).¹¹
In an older study, laryngoscopy was able to diagnose 100% of patients with symptomatic paradoxical vocal cord movement and additional 60% asymptomatic patients with a constellation of symptoms consistent with paradoxical vocal cord movement.¹²
Speech Therapy; Ear, Nose, and Throat (ENT); and Pulmonology may not perform direct visualization in these patients at initial presentation due to other suspected diagnoses. A more common test is the PFT, especially if asthma or other airway tract diseases are suspected (Figure 3).
Patient Descriptions
Study patients were referred for a variety of reasons, often from primary care clinics for concerns for asthma, episodic dyspnea, wheezing, or decreased exercise tolerance thought to be related to pulmonary or allergy causes. Pulmonology worked closely with Speech Therapy and referred VCD cases for speech evaluation, including video stroboscopy. Notably, of the patients in this cohort, although some were suspected to have asthma, those patients were ruled out during part of the pulmonology evaluation, both with PFT testing and methacholine challenges. An asthma diagnosis is important in a military treatment facility, as asthma is often grounds for discharge.
Patients ranged in age from 13 to 68 years, with a median age at 31 years diagnosis. Thirty-nine females and 61 males comprised the total case series. Speech Therapy diagnosed 97 patients, 96 were diagnosed at Eglin AFB hospital via stroboscopy. One patient was diagnosed off-base by Speech Therapy via direct visualization, 1 patient was diagnosed by Pulmonology on-base via direct visualization, and 2 patients were diagnosed by ENT on-base via direct visualization. These patients had direct laryngoscopy completed, often to rule out other organic causes for upper airway disease processes, and were found to have visual paradoxical vocal cord movement. Ninety-eight patients completed PFTs. Several patients were lost to follow-up, as can be common in a military population with frequent moves or members leaving service.
On record review, patient symptoms were present in the range of 2 months to 20 years, with a median duration of symptomatic reports lasting 2 years prior to diagnosis. Common diagnoses prior to visual VCD diagnosis included asthma, exercise-induced asthma, anxiety, and episodic wheezing. Risk factors that were evaluated in this case series included age, sex, body mass index (BMI), GERD, allergic rhinitis, postnasal drip, active smoker, previous smoker, and mental health diagnoses (Figure 4).
Pulmonary function test results were analyzed on 98 patients, including forced expiratory volume in 1 second (FEV1); forced vital capacity (FVC), FEV1/FVC ratio; peak inspiratory flow (PIF) and peak expiratory flow (PEF)—available in 97 studies; forced expiratory flow (FEF) at 25% to 75% of FVC (FEF 25%-75%)—available in 96 studies; and maximum voluntary ventilation (MVV) and MVV/FEV1 ratio—available in 60 of 98 PFTs.
Interventions
All patients diagnosed by Speech Therapy on-base were provided with laryngeal relaxation techniques, diaphragmatic breathing techniques, and controlled inhale/exhale techniques at time of diagnosis, with frequent follow-up scheduled with Speech Therapy and Pulmonology. All diagnoses potentially contributing to laryngeal irritation were treated, including GERD, allergic rhinitis, smoking cessation, weight loss, and exercise recommendations as needed.
Patients reported improvement on follow-up appointments with Speech Therapy in overall control of symptoms, subjectively categorized as poor improvement, partial improvement, and complete improvement. This was a subjective measurement of improvement and fully dependent on follow-up care and patient reporting for improvement. No predefined number of follow-ups was determined; patients were followed monthly until they declined further care, fully improved, moved out of the military treatment system, or were lost to follow-up.
Treatment included structured Speech Therapy sessions. Response to treatment was subjectively qualified by patient report. Fifteen patients reported complete resolution of symptoms, 57 reported partial improvement, 24 reported poor improvement, and 4 patients were lost to follow-up.
Results
Risk factors for the diagnosis of VCD included possible associations with GERD, allergic rhinitis, smoking, prior smoking, BMI, and mental health diagnoses. Body mass index ranged from 17 to 36 in the case series, with median BMI of 27. Mental health diagnoses were present in 35 patients and included diagnoses of anxiety, depression, and adjustment disorders. Gastroesophageal reflux disease diagnosis was present in 59 of the case series patients, 80 had the diagnosis of allergic rhinitis, 63 were diagnosed with postnasal drip. Sixteen case series patients were current smokers. An additional 26 were previous smokers (at least 100 cigarettes in lifetime) for a total of 42 patients that were current or prior smokers.
The chart review was completed to evaluate for the presence of these diagnoses, which included previous treatments; for example, proton pump inhibitors for GERD, antidepressants for depression, or intranasal steroids for allergic rhinitis. The diagnosis was counted as present if the patient was currently being treated for the particular diagnosis in question.
PFT Data
Data from PFTs were available for 98 of 100 cases diagnosed. Review of data across all 98 patients is noted for median FEV1 of 3.6, a median FVC of 4.5, with ratio of 0.80.
Since PFT values vary according to age, sex, and ethnicity, PFTs were analyzed for percent predicted values based on age, gender, and race. Notably, median values for FEV1, FVC, and PEF were all close to 100% of the predicted value. The MVV percent predicted was available in 60 cases and was 93% of predicted values. The most significant difference from expected values was FEF 25% to 75%, at 84% of expected results.
Flow-volume loop evaluations on the 97 PFTs available were completed, and 58 of the 97 were noted for variable extrathoracic airway obstruction consistent with inspiratory inhibition in the patient population. This is 60% of the available PFTs in this cohort study.
Discussion
This retrospective chart review of 100 consecutive VCD diagnoses in a military treatment facility reinforces many of the findings currently available in the literature. As illustrated in a Chest review article, the diagnosis of VCD on history, physical examination, or PFTs remains ellusive.1 The PFT evaluation contains some subjectivity regarding the flattening of inspiratory flow-volume loops and is not routinely reported in PFT results. In patients diagnosed with VCD, a clear consensus of treatment modalities remains lacking. Modification of risk factors (allergic rhinitis, GERD, smoking cessation, weight loss) assisted in self-reported patient improvement, as did focused speech therapy.
The median age of 31 years, likely reflected the younger military population served at Eglin AFB. Seventy-five of these patients were currently on active duty, 6 were retired from active duty (veterans), and 19 were dependents. The median time of symptoms to diagnosis was 2 years. Prior misdiagnosis with other diseases such as asthma was common. Also, referral to Pulmonology and Speech Therapy was usually completed after failed outpatient primary care management for the alternative diagnoses.
Improvement with therapy was mixed, and during the time of documented follow-up, 72 patients reported complete or partial improvement. Most active-duty patients in the partial improvement category based this subjective reporting on their ability to meet military physical fitness standards.
Previous data suggested a female predominance, but this study population was 61% male. Military populations are about 80% to 85% male, so an increase in male diagnosis is expected.
Many patients in the patient cohort arrived as a result of Pulmonology referrals with a presumptive diagnoses of asthma but were determined not to have asthma through PFT results inconsistent with asthma, no improvement with β-agonist therapies, and negative methacholine challenges (if performed). These results prompted evaluations for other conditions and eventually a VCD diagnosis. As noted, exclusion of asthma is of particular importance in a military population, as medical discharges often are pursued in service members with asthma whether controlled or uncontrolled. Lag time to referral also is possible in failures of military physical, which prompted medical evaluation once several failures had occurred over a 1- to 2-year time frame.
The PFT data evaluation was inconclusive for statistically significant changes when compared with age-matched normal PFT values. This also was noted in previous studies of VCD cases. Most notable was percent predicted values of FEF 25% to 75%, with 84% of expected values. The FEV1, FVC, and PEF all fell within predicted values of normal, despite wide ranges in age, sex, and ethnicity among the subjects. Inspiratory flattening consistent with extrathoracic obstruction was present in 58 of the 97 PFTs available for review at Eglin AFB.
Limitations
Limitations to this retrospective case series are illustrated here. Cases were found only when VCD was diagnosed and coded; and it is the authors’ suspicion that many have been misdiagnosed or improperly treated for asthma or other pulmonary/oropharynx conditions. If providers are not familiar with VCD or if PFT readings do not comment on inspiratory findings, diagnosis is less likely. Some of the authors’ colleagues already have determined that postdeployment prevalence of VCD seems to be elevated.8
This cohort was completed on all patients in a military treatment facility, with 75 active-duty personnel, 6 veterans, and 19 dependents of varying ages. This case series is retrospective and tabulates suspected risk factors; stronger and more informative studies could certainly be completed in prospective studies (although likely difficult with low prevalence) or in treatment comparison studies at the time of diagnosis.
Since the cohort had varied and lengthy time to diagnosis from onset of related symptoms, the treatment patients received prior to diagnosis differed extensively. Diagnosis was completed by numerous primary care managers or other subspecialties prior to arrival to Pulmonology and Speech Therapy at Eglin AFB. Once diagnosed in Speech Therapy, consistent treatment options were provided to patients in accordance with standard of care.
It is the authors’ suspicion that VCD may have a higher prevalence than previously reported in the literature. Military service members are tested annually or biannually on physical fitness standards and are evaluated for medical reasons for recurrent fitness standard failures. This selection of patients is more likely to have a VCD evaluation as part of a comprehensive evaluation than is a healthy adult in a civilian population. A prospective study in military service members would be more fruitful and possibly yield a higher prevalence postdeployment.
Conclusion
Vocal cord dysfunction remains a difficult diagnosis to treat, because multiple comorbidities likely contribute to the diagnosis. This retrospective case series attempted to compile common themes and noted that most of the patients had 2 or more risk factors of smoking, allergic rhinitis, GERD, or mental health diagnoses. A prospective trial would be ideal to evaluate VCD further. A focused trial in the particular communities of athletes or of military service members may be of increased benefit to better define VCD. It is notable that 100 cases were found in a relatively short period for a community hospital, and prevalence may be higher than previously reported.
1. Morris MJ, Christopher KL. Diagnostic criteria for the classification of vocal cord dysfunction. Chest. 2010;138(5):1213-1223.
2. National Heart, Lung, and Blood Institute. Expert panel report 3: guidelines for the diagnoses and management of asthma. Full report 2007. https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln .pdf. Published 2007.Accessed February 1, 2017.
3. Newman KB, Mason UG III, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med. 1995;152(4, pt 1):1382-1386.
4. Sanz Santiago V, López Neyra A, Almería Gil E, Villa Asensi JR. Spirometry patterns in vocal cord dysfunction [in Spanish]. An Pediatr (Barc). 2013;78(3):173-177.
5. Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81(2):156-159.
6. Benninger C, Parsons JP, Mastronarde JG. Vocal cord dysfunction and asthma. Curr Opin Pulm Med. 2011;17(1):45-49.
7. Campainha S, Ribeiro C, Guimar M, Lima R. Vocal cord dysfunction: a frequently forgotten entity. Case Rep Pulmonol. 2012;2012:525493.
8. Morris MJ, Oleszewski RT, Sterner JB, Allan PF. Vocal cord dysfunction related to combat deployment. Mil Med. 2013;178(11):1208-1212.
9. Al-Alwan A, Kaminsky D. Vocal cord dysfunction in athletes: clinical presentation and review of the literature. Phys Sportsmed. 2012;40(2):22-27.
10. Kenn K, Schmitz M. Prevalence of vocal cord dysfunction in patients with dyspnea. First prospective clinical study. Am J Respir Crit Care Med. 1997;155:A965.
11. Wendler, J, Nawka, T, Verges, D. Instructional course: videolaryngo-stroboscopy and phonetography—basic tools for diagnostics and documentation in the voice clinic. Poster presented at: 15th European Congress of Oto-Rhino-Laryngology, Head and Neck Surgery; September 11-16, 2004; Rodos-Kos, Greece.
12. Ibrahim WH, Gheriani HA, Almohamed AA, Raza T. Paradoxical vocal cord motion disorder: past, present and future. Postgrad Med J. 2007;83(977):164-172.
1. Morris MJ, Christopher KL. Diagnostic criteria for the classification of vocal cord dysfunction. Chest. 2010;138(5):1213-1223.
2. National Heart, Lung, and Blood Institute. Expert panel report 3: guidelines for the diagnoses and management of asthma. Full report 2007. https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln .pdf. Published 2007.Accessed February 1, 2017.
3. Newman KB, Mason UG III, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med. 1995;152(4, pt 1):1382-1386.
4. Sanz Santiago V, López Neyra A, Almería Gil E, Villa Asensi JR. Spirometry patterns in vocal cord dysfunction [in Spanish]. An Pediatr (Barc). 2013;78(3):173-177.
5. Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81(2):156-159.
6. Benninger C, Parsons JP, Mastronarde JG. Vocal cord dysfunction and asthma. Curr Opin Pulm Med. 2011;17(1):45-49.
7. Campainha S, Ribeiro C, Guimar M, Lima R. Vocal cord dysfunction: a frequently forgotten entity. Case Rep Pulmonol. 2012;2012:525493.
8. Morris MJ, Oleszewski RT, Sterner JB, Allan PF. Vocal cord dysfunction related to combat deployment. Mil Med. 2013;178(11):1208-1212.
9. Al-Alwan A, Kaminsky D. Vocal cord dysfunction in athletes: clinical presentation and review of the literature. Phys Sportsmed. 2012;40(2):22-27.
10. Kenn K, Schmitz M. Prevalence of vocal cord dysfunction in patients with dyspnea. First prospective clinical study. Am J Respir Crit Care Med. 1997;155:A965.
11. Wendler, J, Nawka, T, Verges, D. Instructional course: videolaryngo-stroboscopy and phonetography—basic tools for diagnostics and documentation in the voice clinic. Poster presented at: 15th European Congress of Oto-Rhino-Laryngology, Head and Neck Surgery; September 11-16, 2004; Rodos-Kos, Greece.
12. Ibrahim WH, Gheriani HA, Almohamed AA, Raza T. Paradoxical vocal cord motion disorder: past, present and future. Postgrad Med J. 2007;83(977):164-172.
Odontogenic Sinusitis
A 55-year-old man who had experienced discolored nasal drainage and mucus plugs in the right side of his nose for 5 years was referred to the ear, nose, and throat clinic. A computerized tomography (CT) scan showed opacification of the right ethmoid and maxillary sinuses and periapical radiolucency in the first and second right maxillary molars (Figure 1).
The patient was treated with antibiotics (amoxicillin and clavulanate; moxifloxacin) and nasal rinses but failed to improve.
The maxillary molars were considered the source of the persistent sinus infection, and the patient was referred to oral surgery for extraction. Three months after oral surgery, the extraction sites were completely healed, and the right maxillary sinus appeared free of disease endoscopically (Figure 3).
1 .
2. Patel NA, Ferguson BJ. Odontogenic sinusitis: an ancient but under-appreciated cause of maxillary sinusitis. Curr Opin Otolaryngol Head Neck Surg. 2012;20(1):24-28.
A 55-year-old man who had experienced discolored nasal drainage and mucus plugs in the right side of his nose for 5 years was referred to the ear, nose, and throat clinic. A computerized tomography (CT) scan showed opacification of the right ethmoid and maxillary sinuses and periapical radiolucency in the first and second right maxillary molars (Figure 1).
The patient was treated with antibiotics (amoxicillin and clavulanate; moxifloxacin) and nasal rinses but failed to improve.
The maxillary molars were considered the source of the persistent sinus infection, and the patient was referred to oral surgery for extraction. Three months after oral surgery, the extraction sites were completely healed, and the right maxillary sinus appeared free of disease endoscopically (Figure 3).
A 55-year-old man who had experienced discolored nasal drainage and mucus plugs in the right side of his nose for 5 years was referred to the ear, nose, and throat clinic. A computerized tomography (CT) scan showed opacification of the right ethmoid and maxillary sinuses and periapical radiolucency in the first and second right maxillary molars (Figure 1).
The patient was treated with antibiotics (amoxicillin and clavulanate; moxifloxacin) and nasal rinses but failed to improve.
The maxillary molars were considered the source of the persistent sinus infection, and the patient was referred to oral surgery for extraction. Three months after oral surgery, the extraction sites were completely healed, and the right maxillary sinus appeared free of disease endoscopically (Figure 3).
1 .
2. Patel NA, Ferguson BJ. Odontogenic sinusitis: an ancient but under-appreciated cause of maxillary sinusitis. Curr Opin Otolaryngol Head Neck Surg. 2012;20(1):24-28.
1 .
2. Patel NA, Ferguson BJ. Odontogenic sinusitis: an ancient but under-appreciated cause of maxillary sinusitis. Curr Opin Otolaryngol Head Neck Surg. 2012;20(1):24-28.
Oxybutynin Treatment for Hyperhidrosis in Spinal Cord Injury Patients
Hyperhidrosis (HH) is sweating beyond that which is required for thermoregulation.1 Secondary HH, which is usually caused by an underlying medical condition or drug, may be seen in patients with spinal cord injury (SCI) and can negatively impact psychosocial well-being and quality of life (QOL) if not treated.1
Information on the current prevalence of HH is lacking. In 2004, one study projected the prevalence of HH in the U.S. to be 2.8%.2 A previous study found that about 27% of the 154 patients with SCI reported experiencing HH that was annoying, with 28 (14.6%) of those reporting no contributing somatic causes.3 Somatic causes of HH include autonomic dysreflexia, posttraumatic syringomyelia, or orthostatic hypotension.4
Autonomic dysreflexia is a syndrome that describes a dramatic increase in blood pressure (BP) in patients with spinal cord lesion at or above T6 and is characterized by exaggerated autonomic responses to stimuli that are innocuous to individuals without SCI.5,6 Noxious stimuli that may trigger autonomic dysreflexia include bowel or bladder distension or obstruction, urinary infection, catheter insertion, suprapubic palpation, or skin irritation.6 Syringomyelia, another somatic cause of HH, is a cystic lesion on the spinal cord that may develop secondary to congenital anomalies or SCI.6-8
The following case reports describe 2 patients with SCI with different diagnoses and presentations of HH. Both were treated with oxybutynin for HH.
Case 1 Presentation
Mr. J is a 49-year-old with C6-C7 SCI attributed to a motor vehicle accident 26 years before. He presented to the primary care clinic for a routine visit in a self-propelled wheelchair. His diagnosis included tetraplegia, muscle spasms, osteoporosis, chronic pain syndrome, benign prosthetic hypertrophy, neurogenic bladder, and neurogenic bowel. He was noted to have a bath towel around his neck to wipe sweat from his face and neck. He did not recall when this condition started; however, he reported a prior trial of diazepam 5 mg as needed in 2006 in the mornings and before transfers when sweating was usually worst. He continued to use diazepam because it helped with his muscle spasms but not with sweating. His other medications included oral baclofen, alendronate, ibuprofen, docusate sodium, tamsulosin, calcium/vitamin C supplement, and bisacodyl suppository.
The patient’s surgical history was significant for anterior discectomy with C6-C7 fusion, sphincterotomy, transurethral resection of the prostate, and right urethral stent placement for hydronephrosis in 2004. His cystoscopy and renal sonogram were within normal limits. On physical examination, his vital signs were within normal limits. However, his long-sleeved shirt was wet on the front, and his neck, chest, and arms also were moist from excessive sweating. During his transfer to and from his wheelchair, he was noted to have chattering of his teeth. The remainder of the physical examination was negative for any other acute findings.
Mr. J was prescribed a 30-day trial of oxybutynin 5 mg 1 tablet by mouth per day for HH. During a 3-week follow-up telephone call, Mr. J reported that the oxybutynin was working well; the sweating on his chest had improved and had resolved on his face. Except for mild dryness of mouth, he was tolerating the medication well. There were no changes in his neurogenic bladder, which was managed with an external urinary device.
Six months later, Mr. J reported that oxybutynin continued to work well, and he no longer had to travel with a towel. He was able to go to a football game, social activities were more enjoyable, and he was not embarrassed because of excessive sweating.
Case 2 Presentation
Mr. B is a 48-year-old with T12 paraplegia secondary to a motor vehicle accident in 1994. He called the primary clinic for a visit because he was concerned about cold clammy hands for the past 6 to 7 months when he woke up in the morning and sometimes throughout the day. He was preparing to start his first semester in college. His diagnosis included neurogenic bowel and bladder and muscle spasms. There was no history of posttraumatic syringomyelia, and his medications included baclofen, dantrolene, diazepam, and multivitamins.
Mr. B took tolterodine 4 mg/d for several years, and for unknown reasons, about 6 years previously the prescription was changed to oxybutynin 15-mg extended release for his neurogenic bladder. He continued to have urinary leakage between the every 4-hour intermittent catherization, and oxybutynin was increased to 10-mg tablet twice per day.
About 7 months prior to this appointment, Mr. B independently stopped the oxybutynin as he felt that it was not making a difference in the management of his neurogenic bladder. It was noted that his cold clammy hands started about the same time that he discontinued the oxybutynin. He could not recall whether he had this symptom prior to initiation of any medication. It was mutually decided to restart the oxybutynin at a lower dose, this time not for his bladder but for the HH. He was ordered a 30-day trial of a 5-mg tablet once per day. About 3 weeks later, he sent a secure message to report oxybutynin’s effectiveness and to request a refill.
Discussion
Sweating is a physiological process that involves the active secretion of water by specialized sweat glands in the skin.9-11 There are 2 types of sweat glands in the skin; apocrine and eccrine.9 Collectively the 3 million eccrine sweat glands of the average person approximately equal the mass of a kidney and exceed the secretory rate of exocrine glands.9 They function in evaporative cooling in response to thermal or physiologic stimuli and are widely distributed over the body, especially on the forehead, back, palms, and soles.10
Sympathetic cholinergic nerves are mainly responsible for sweat secretion by the release of acetylcholine to activate muscarinic receptors on the gland.11 Postganglionic fibers from sympathetic nerve cells innervate sweat glands that release cholinergics.6 Postganglionic cholinergic receptors that are activated by muscarinic drugs are termed muscarinic receptors and are readily accessible to antimuscarinic drugs.6,12 Anticholinergic/antimuscarinic agents antagonize muscarinic receptors and suppress premature detrusor contractions to enhance bladder storage.13 They include oxybutynin, tolterodine, trospium, solifenacin, darifenacin, and fesoterodine.13 Oxybutynin was used in both cases because it is on VA formulary. It was effective in treating HH, although the etiology is unclear and the presentations were different.
One retrospective study that analyzed 20 patients who received oxybutynin for primary HH at uncommon sites, such as the back and groin, found that QOL improved in 85% of the subjects after 6 weeks.14 Randomized placebo-controlled trials also have found oxybutynin effective for treatment of palmar and axillary HH and generalized HH.15,16
Syringomyelia was ruled out in both cases based on history and radiologic studies, specifically magnetic resonance imaging. Autonomic dysreflexia was ruled out as the HH was not an acute finding and BP was within normal limits. Orthostatic hypotension is a common finding in SCI, mainly in tetraplegic patients, and could be suspected in both cases. Sweating was usually worse in the mornings in both cases and during transfers, as noted in the first case.17 However, chronic autoregulation allows for chronic adaption to tissue hypoperfusion over time.16
Hyperhidrosis or other disorders of eccrine sweating can occur for various reasons, including changes in the spinal sympathetic preganglionic, ganglionic, or postganglionic neurons; dysfunction of the thermoregulatory centers in the brain’s central autonomic network; or changes in the muscarinic cholinergic synapse on sweat glands.18
Conclusion
Patients with SCI may have an acute or chronic presentation of HH. Removal of the inciting cause in the case of autonomic dysreflexia and/or the administration of a pharmaceutical agent is the usual treatment.
Regardless of the etiology of HH that persists, effective treatment should be a goal, especially in those patients whose QOL is affected by this condition. The outcome of treatment with oxybutynin in these case reports is consistent with the findings of the limited retrospective study and randomized placebo-controlled studies that show oxybutynin is effective for treating bothersome HH.14-16
The results of these case reports are not generalizable to patients with SCI and HH, nor are the results of the limited retrospective study and randomized placebo-controlled studies, as their sample sizes were small.14,16,17 However, information on the use of oxybutynin for the effective treatment of HH in the SCI population is promising. Research studies on the prevalence of HH and randomized placebo-controlled trials with a larger SCI population are considerations for future studies.
1. Strutton DR, Kowalski JW, Glaser DA, Stang PE. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol. 2004;51(2):241-248.
2. Walling HW. Clinical differentiation of primary from secondary hyperhidrosis. J Am Acad Dermatol. 2011;64(4):690-695.
3. Andersen LS, Biering-Sørensen F, Müller PG, Jensen IL, Aggerbeck B. The prevalence of hyperhidrosis in patients with spinal cord injuries and an evaluation of the effect of dextropropoxyphene hydrochloride in therapy. Paraplegia. 1992;(30):184-191.
4. Sato K, Kang WH, Saga K, Sato KT. Biology of sweat glands and their disorders. II. Disorders of sweat gland function. J Am Acad Dermatol. 1989;20(5, pt 1):713-726.
5. Kewalramani LS. Autonomic dysreflexia in traumatic myelopathy. Am J Phys Med. 1980;59(1):1-21.
6. Low PA, Engstrom JW. Disorders of the autonomic nervous system. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015.
7. Milhort TH. Classification of syringomyelia. Neurosurg Focus. 2000;8(3):E1.
8. National Institute of Neurological Disorders and Stroke. Syringomyelia fact sheet. https://www.ninds .nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Syringomyelia-Fact-Sheet. Accessed February 13, 2017.
9. Meschner AL. Junqueira’s Basic Histology. 14th ed. New York, NY: McGraw-Hill; 2016:371-392.
10. Mauro TM. Biology of eccrine and apocrine glands. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K. eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
11. Barrett KE, Barman SM, Boitano S, Brooks HL. Ganong’s Review of Medical Physiology. 25th ed. New York, NY: McGraw-Hill; 2016:261-272.
12. Kellogg DL Jr. Thermoregulation. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
13. Rovner ES, Wyman J, Lam S. Urinary Incontinence. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill; 2014:1377-1396.
14. Teivelis MP, Wolosker N, Krutman M, Kauffman P, Campos JR, Puech-Leão P. Treatment of uncommon sites of focal primary hyperhidrosis: experience with pharmacological therapy using oxybutynin. Clinics (Sao Paulo). 2014;69(9):608-614.
15. Wolosker N, de Campos JR, Kauffman P, Puech-Leão P. A randomized-placebo-controlled trial of oxybutynin for the initial treatment of palmar and axillary hyperhidrosis. J Vasc Surg. 2012;55(6):1696-1700.
16. Schollhammer M, Brenaut E, Menard-Andivot N, et al. Oxybutynin as a treatment for generalized hyperhidrosis. Br J Dermatol. 2015;173(5):1163-1168.
17. Gonzalez F, Chang JY, Banovac K, Messina D, Martinez-Arizala A, Kelley RE. Autoregulation of cerebral blood flow in patients with orthostatic hypotension after spinal cord injury. Paraplegia. 1991;29(1):1-7.
18. Fealey RD, Hebert AA. Disorders of the eccrine sweat glands and sweating. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, eds. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw-Hill; 2012:chap 84.
Hyperhidrosis (HH) is sweating beyond that which is required for thermoregulation.1 Secondary HH, which is usually caused by an underlying medical condition or drug, may be seen in patients with spinal cord injury (SCI) and can negatively impact psychosocial well-being and quality of life (QOL) if not treated.1
Information on the current prevalence of HH is lacking. In 2004, one study projected the prevalence of HH in the U.S. to be 2.8%.2 A previous study found that about 27% of the 154 patients with SCI reported experiencing HH that was annoying, with 28 (14.6%) of those reporting no contributing somatic causes.3 Somatic causes of HH include autonomic dysreflexia, posttraumatic syringomyelia, or orthostatic hypotension.4
Autonomic dysreflexia is a syndrome that describes a dramatic increase in blood pressure (BP) in patients with spinal cord lesion at or above T6 and is characterized by exaggerated autonomic responses to stimuli that are innocuous to individuals without SCI.5,6 Noxious stimuli that may trigger autonomic dysreflexia include bowel or bladder distension or obstruction, urinary infection, catheter insertion, suprapubic palpation, or skin irritation.6 Syringomyelia, another somatic cause of HH, is a cystic lesion on the spinal cord that may develop secondary to congenital anomalies or SCI.6-8
The following case reports describe 2 patients with SCI with different diagnoses and presentations of HH. Both were treated with oxybutynin for HH.
Case 1 Presentation
Mr. J is a 49-year-old with C6-C7 SCI attributed to a motor vehicle accident 26 years before. He presented to the primary care clinic for a routine visit in a self-propelled wheelchair. His diagnosis included tetraplegia, muscle spasms, osteoporosis, chronic pain syndrome, benign prosthetic hypertrophy, neurogenic bladder, and neurogenic bowel. He was noted to have a bath towel around his neck to wipe sweat from his face and neck. He did not recall when this condition started; however, he reported a prior trial of diazepam 5 mg as needed in 2006 in the mornings and before transfers when sweating was usually worst. He continued to use diazepam because it helped with his muscle spasms but not with sweating. His other medications included oral baclofen, alendronate, ibuprofen, docusate sodium, tamsulosin, calcium/vitamin C supplement, and bisacodyl suppository.
The patient’s surgical history was significant for anterior discectomy with C6-C7 fusion, sphincterotomy, transurethral resection of the prostate, and right urethral stent placement for hydronephrosis in 2004. His cystoscopy and renal sonogram were within normal limits. On physical examination, his vital signs were within normal limits. However, his long-sleeved shirt was wet on the front, and his neck, chest, and arms also were moist from excessive sweating. During his transfer to and from his wheelchair, he was noted to have chattering of his teeth. The remainder of the physical examination was negative for any other acute findings.
Mr. J was prescribed a 30-day trial of oxybutynin 5 mg 1 tablet by mouth per day for HH. During a 3-week follow-up telephone call, Mr. J reported that the oxybutynin was working well; the sweating on his chest had improved and had resolved on his face. Except for mild dryness of mouth, he was tolerating the medication well. There were no changes in his neurogenic bladder, which was managed with an external urinary device.
Six months later, Mr. J reported that oxybutynin continued to work well, and he no longer had to travel with a towel. He was able to go to a football game, social activities were more enjoyable, and he was not embarrassed because of excessive sweating.
Case 2 Presentation
Mr. B is a 48-year-old with T12 paraplegia secondary to a motor vehicle accident in 1994. He called the primary clinic for a visit because he was concerned about cold clammy hands for the past 6 to 7 months when he woke up in the morning and sometimes throughout the day. He was preparing to start his first semester in college. His diagnosis included neurogenic bowel and bladder and muscle spasms. There was no history of posttraumatic syringomyelia, and his medications included baclofen, dantrolene, diazepam, and multivitamins.
Mr. B took tolterodine 4 mg/d for several years, and for unknown reasons, about 6 years previously the prescription was changed to oxybutynin 15-mg extended release for his neurogenic bladder. He continued to have urinary leakage between the every 4-hour intermittent catherization, and oxybutynin was increased to 10-mg tablet twice per day.
About 7 months prior to this appointment, Mr. B independently stopped the oxybutynin as he felt that it was not making a difference in the management of his neurogenic bladder. It was noted that his cold clammy hands started about the same time that he discontinued the oxybutynin. He could not recall whether he had this symptom prior to initiation of any medication. It was mutually decided to restart the oxybutynin at a lower dose, this time not for his bladder but for the HH. He was ordered a 30-day trial of a 5-mg tablet once per day. About 3 weeks later, he sent a secure message to report oxybutynin’s effectiveness and to request a refill.
Discussion
Sweating is a physiological process that involves the active secretion of water by specialized sweat glands in the skin.9-11 There are 2 types of sweat glands in the skin; apocrine and eccrine.9 Collectively the 3 million eccrine sweat glands of the average person approximately equal the mass of a kidney and exceed the secretory rate of exocrine glands.9 They function in evaporative cooling in response to thermal or physiologic stimuli and are widely distributed over the body, especially on the forehead, back, palms, and soles.10
Sympathetic cholinergic nerves are mainly responsible for sweat secretion by the release of acetylcholine to activate muscarinic receptors on the gland.11 Postganglionic fibers from sympathetic nerve cells innervate sweat glands that release cholinergics.6 Postganglionic cholinergic receptors that are activated by muscarinic drugs are termed muscarinic receptors and are readily accessible to antimuscarinic drugs.6,12 Anticholinergic/antimuscarinic agents antagonize muscarinic receptors and suppress premature detrusor contractions to enhance bladder storage.13 They include oxybutynin, tolterodine, trospium, solifenacin, darifenacin, and fesoterodine.13 Oxybutynin was used in both cases because it is on VA formulary. It was effective in treating HH, although the etiology is unclear and the presentations were different.
One retrospective study that analyzed 20 patients who received oxybutynin for primary HH at uncommon sites, such as the back and groin, found that QOL improved in 85% of the subjects after 6 weeks.14 Randomized placebo-controlled trials also have found oxybutynin effective for treatment of palmar and axillary HH and generalized HH.15,16
Syringomyelia was ruled out in both cases based on history and radiologic studies, specifically magnetic resonance imaging. Autonomic dysreflexia was ruled out as the HH was not an acute finding and BP was within normal limits. Orthostatic hypotension is a common finding in SCI, mainly in tetraplegic patients, and could be suspected in both cases. Sweating was usually worse in the mornings in both cases and during transfers, as noted in the first case.17 However, chronic autoregulation allows for chronic adaption to tissue hypoperfusion over time.16
Hyperhidrosis or other disorders of eccrine sweating can occur for various reasons, including changes in the spinal sympathetic preganglionic, ganglionic, or postganglionic neurons; dysfunction of the thermoregulatory centers in the brain’s central autonomic network; or changes in the muscarinic cholinergic synapse on sweat glands.18
Conclusion
Patients with SCI may have an acute or chronic presentation of HH. Removal of the inciting cause in the case of autonomic dysreflexia and/or the administration of a pharmaceutical agent is the usual treatment.
Regardless of the etiology of HH that persists, effective treatment should be a goal, especially in those patients whose QOL is affected by this condition. The outcome of treatment with oxybutynin in these case reports is consistent with the findings of the limited retrospective study and randomized placebo-controlled studies that show oxybutynin is effective for treating bothersome HH.14-16
The results of these case reports are not generalizable to patients with SCI and HH, nor are the results of the limited retrospective study and randomized placebo-controlled studies, as their sample sizes were small.14,16,17 However, information on the use of oxybutynin for the effective treatment of HH in the SCI population is promising. Research studies on the prevalence of HH and randomized placebo-controlled trials with a larger SCI population are considerations for future studies.
Hyperhidrosis (HH) is sweating beyond that which is required for thermoregulation.1 Secondary HH, which is usually caused by an underlying medical condition or drug, may be seen in patients with spinal cord injury (SCI) and can negatively impact psychosocial well-being and quality of life (QOL) if not treated.1
Information on the current prevalence of HH is lacking. In 2004, one study projected the prevalence of HH in the U.S. to be 2.8%.2 A previous study found that about 27% of the 154 patients with SCI reported experiencing HH that was annoying, with 28 (14.6%) of those reporting no contributing somatic causes.3 Somatic causes of HH include autonomic dysreflexia, posttraumatic syringomyelia, or orthostatic hypotension.4
Autonomic dysreflexia is a syndrome that describes a dramatic increase in blood pressure (BP) in patients with spinal cord lesion at or above T6 and is characterized by exaggerated autonomic responses to stimuli that are innocuous to individuals without SCI.5,6 Noxious stimuli that may trigger autonomic dysreflexia include bowel or bladder distension or obstruction, urinary infection, catheter insertion, suprapubic palpation, or skin irritation.6 Syringomyelia, another somatic cause of HH, is a cystic lesion on the spinal cord that may develop secondary to congenital anomalies or SCI.6-8
The following case reports describe 2 patients with SCI with different diagnoses and presentations of HH. Both were treated with oxybutynin for HH.
Case 1 Presentation
Mr. J is a 49-year-old with C6-C7 SCI attributed to a motor vehicle accident 26 years before. He presented to the primary care clinic for a routine visit in a self-propelled wheelchair. His diagnosis included tetraplegia, muscle spasms, osteoporosis, chronic pain syndrome, benign prosthetic hypertrophy, neurogenic bladder, and neurogenic bowel. He was noted to have a bath towel around his neck to wipe sweat from his face and neck. He did not recall when this condition started; however, he reported a prior trial of diazepam 5 mg as needed in 2006 in the mornings and before transfers when sweating was usually worst. He continued to use diazepam because it helped with his muscle spasms but not with sweating. His other medications included oral baclofen, alendronate, ibuprofen, docusate sodium, tamsulosin, calcium/vitamin C supplement, and bisacodyl suppository.
The patient’s surgical history was significant for anterior discectomy with C6-C7 fusion, sphincterotomy, transurethral resection of the prostate, and right urethral stent placement for hydronephrosis in 2004. His cystoscopy and renal sonogram were within normal limits. On physical examination, his vital signs were within normal limits. However, his long-sleeved shirt was wet on the front, and his neck, chest, and arms also were moist from excessive sweating. During his transfer to and from his wheelchair, he was noted to have chattering of his teeth. The remainder of the physical examination was negative for any other acute findings.
Mr. J was prescribed a 30-day trial of oxybutynin 5 mg 1 tablet by mouth per day for HH. During a 3-week follow-up telephone call, Mr. J reported that the oxybutynin was working well; the sweating on his chest had improved and had resolved on his face. Except for mild dryness of mouth, he was tolerating the medication well. There were no changes in his neurogenic bladder, which was managed with an external urinary device.
Six months later, Mr. J reported that oxybutynin continued to work well, and he no longer had to travel with a towel. He was able to go to a football game, social activities were more enjoyable, and he was not embarrassed because of excessive sweating.
Case 2 Presentation
Mr. B is a 48-year-old with T12 paraplegia secondary to a motor vehicle accident in 1994. He called the primary clinic for a visit because he was concerned about cold clammy hands for the past 6 to 7 months when he woke up in the morning and sometimes throughout the day. He was preparing to start his first semester in college. His diagnosis included neurogenic bowel and bladder and muscle spasms. There was no history of posttraumatic syringomyelia, and his medications included baclofen, dantrolene, diazepam, and multivitamins.
Mr. B took tolterodine 4 mg/d for several years, and for unknown reasons, about 6 years previously the prescription was changed to oxybutynin 15-mg extended release for his neurogenic bladder. He continued to have urinary leakage between the every 4-hour intermittent catherization, and oxybutynin was increased to 10-mg tablet twice per day.
About 7 months prior to this appointment, Mr. B independently stopped the oxybutynin as he felt that it was not making a difference in the management of his neurogenic bladder. It was noted that his cold clammy hands started about the same time that he discontinued the oxybutynin. He could not recall whether he had this symptom prior to initiation of any medication. It was mutually decided to restart the oxybutynin at a lower dose, this time not for his bladder but for the HH. He was ordered a 30-day trial of a 5-mg tablet once per day. About 3 weeks later, he sent a secure message to report oxybutynin’s effectiveness and to request a refill.
Discussion
Sweating is a physiological process that involves the active secretion of water by specialized sweat glands in the skin.9-11 There are 2 types of sweat glands in the skin; apocrine and eccrine.9 Collectively the 3 million eccrine sweat glands of the average person approximately equal the mass of a kidney and exceed the secretory rate of exocrine glands.9 They function in evaporative cooling in response to thermal or physiologic stimuli and are widely distributed over the body, especially on the forehead, back, palms, and soles.10
Sympathetic cholinergic nerves are mainly responsible for sweat secretion by the release of acetylcholine to activate muscarinic receptors on the gland.11 Postganglionic fibers from sympathetic nerve cells innervate sweat glands that release cholinergics.6 Postganglionic cholinergic receptors that are activated by muscarinic drugs are termed muscarinic receptors and are readily accessible to antimuscarinic drugs.6,12 Anticholinergic/antimuscarinic agents antagonize muscarinic receptors and suppress premature detrusor contractions to enhance bladder storage.13 They include oxybutynin, tolterodine, trospium, solifenacin, darifenacin, and fesoterodine.13 Oxybutynin was used in both cases because it is on VA formulary. It was effective in treating HH, although the etiology is unclear and the presentations were different.
One retrospective study that analyzed 20 patients who received oxybutynin for primary HH at uncommon sites, such as the back and groin, found that QOL improved in 85% of the subjects after 6 weeks.14 Randomized placebo-controlled trials also have found oxybutynin effective for treatment of palmar and axillary HH and generalized HH.15,16
Syringomyelia was ruled out in both cases based on history and radiologic studies, specifically magnetic resonance imaging. Autonomic dysreflexia was ruled out as the HH was not an acute finding and BP was within normal limits. Orthostatic hypotension is a common finding in SCI, mainly in tetraplegic patients, and could be suspected in both cases. Sweating was usually worse in the mornings in both cases and during transfers, as noted in the first case.17 However, chronic autoregulation allows for chronic adaption to tissue hypoperfusion over time.16
Hyperhidrosis or other disorders of eccrine sweating can occur for various reasons, including changes in the spinal sympathetic preganglionic, ganglionic, or postganglionic neurons; dysfunction of the thermoregulatory centers in the brain’s central autonomic network; or changes in the muscarinic cholinergic synapse on sweat glands.18
Conclusion
Patients with SCI may have an acute or chronic presentation of HH. Removal of the inciting cause in the case of autonomic dysreflexia and/or the administration of a pharmaceutical agent is the usual treatment.
Regardless of the etiology of HH that persists, effective treatment should be a goal, especially in those patients whose QOL is affected by this condition. The outcome of treatment with oxybutynin in these case reports is consistent with the findings of the limited retrospective study and randomized placebo-controlled studies that show oxybutynin is effective for treating bothersome HH.14-16
The results of these case reports are not generalizable to patients with SCI and HH, nor are the results of the limited retrospective study and randomized placebo-controlled studies, as their sample sizes were small.14,16,17 However, information on the use of oxybutynin for the effective treatment of HH in the SCI population is promising. Research studies on the prevalence of HH and randomized placebo-controlled trials with a larger SCI population are considerations for future studies.
1. Strutton DR, Kowalski JW, Glaser DA, Stang PE. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol. 2004;51(2):241-248.
2. Walling HW. Clinical differentiation of primary from secondary hyperhidrosis. J Am Acad Dermatol. 2011;64(4):690-695.
3. Andersen LS, Biering-Sørensen F, Müller PG, Jensen IL, Aggerbeck B. The prevalence of hyperhidrosis in patients with spinal cord injuries and an evaluation of the effect of dextropropoxyphene hydrochloride in therapy. Paraplegia. 1992;(30):184-191.
4. Sato K, Kang WH, Saga K, Sato KT. Biology of sweat glands and their disorders. II. Disorders of sweat gland function. J Am Acad Dermatol. 1989;20(5, pt 1):713-726.
5. Kewalramani LS. Autonomic dysreflexia in traumatic myelopathy. Am J Phys Med. 1980;59(1):1-21.
6. Low PA, Engstrom JW. Disorders of the autonomic nervous system. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015.
7. Milhort TH. Classification of syringomyelia. Neurosurg Focus. 2000;8(3):E1.
8. National Institute of Neurological Disorders and Stroke. Syringomyelia fact sheet. https://www.ninds .nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Syringomyelia-Fact-Sheet. Accessed February 13, 2017.
9. Meschner AL. Junqueira’s Basic Histology. 14th ed. New York, NY: McGraw-Hill; 2016:371-392.
10. Mauro TM. Biology of eccrine and apocrine glands. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K. eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
11. Barrett KE, Barman SM, Boitano S, Brooks HL. Ganong’s Review of Medical Physiology. 25th ed. New York, NY: McGraw-Hill; 2016:261-272.
12. Kellogg DL Jr. Thermoregulation. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
13. Rovner ES, Wyman J, Lam S. Urinary Incontinence. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill; 2014:1377-1396.
14. Teivelis MP, Wolosker N, Krutman M, Kauffman P, Campos JR, Puech-Leão P. Treatment of uncommon sites of focal primary hyperhidrosis: experience with pharmacological therapy using oxybutynin. Clinics (Sao Paulo). 2014;69(9):608-614.
15. Wolosker N, de Campos JR, Kauffman P, Puech-Leão P. A randomized-placebo-controlled trial of oxybutynin for the initial treatment of palmar and axillary hyperhidrosis. J Vasc Surg. 2012;55(6):1696-1700.
16. Schollhammer M, Brenaut E, Menard-Andivot N, et al. Oxybutynin as a treatment for generalized hyperhidrosis. Br J Dermatol. 2015;173(5):1163-1168.
17. Gonzalez F, Chang JY, Banovac K, Messina D, Martinez-Arizala A, Kelley RE. Autoregulation of cerebral blood flow in patients with orthostatic hypotension after spinal cord injury. Paraplegia. 1991;29(1):1-7.
18. Fealey RD, Hebert AA. Disorders of the eccrine sweat glands and sweating. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, eds. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw-Hill; 2012:chap 84.
1. Strutton DR, Kowalski JW, Glaser DA, Stang PE. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol. 2004;51(2):241-248.
2. Walling HW. Clinical differentiation of primary from secondary hyperhidrosis. J Am Acad Dermatol. 2011;64(4):690-695.
3. Andersen LS, Biering-Sørensen F, Müller PG, Jensen IL, Aggerbeck B. The prevalence of hyperhidrosis in patients with spinal cord injuries and an evaluation of the effect of dextropropoxyphene hydrochloride in therapy. Paraplegia. 1992;(30):184-191.
4. Sato K, Kang WH, Saga K, Sato KT. Biology of sweat glands and their disorders. II. Disorders of sweat gland function. J Am Acad Dermatol. 1989;20(5, pt 1):713-726.
5. Kewalramani LS. Autonomic dysreflexia in traumatic myelopathy. Am J Phys Med. 1980;59(1):1-21.
6. Low PA, Engstrom JW. Disorders of the autonomic nervous system. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015.
7. Milhort TH. Classification of syringomyelia. Neurosurg Focus. 2000;8(3):E1.
8. National Institute of Neurological Disorders and Stroke. Syringomyelia fact sheet. https://www.ninds .nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Syringomyelia-Fact-Sheet. Accessed February 13, 2017.
9. Meschner AL. Junqueira’s Basic Histology. 14th ed. New York, NY: McGraw-Hill; 2016:371-392.
10. Mauro TM. Biology of eccrine and apocrine glands. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K. eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
11. Barrett KE, Barman SM, Boitano S, Brooks HL. Ganong’s Review of Medical Physiology. 25th ed. New York, NY: McGraw-Hill; 2016:261-272.
12. Kellogg DL Jr. Thermoregulation. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
13. Rovner ES, Wyman J, Lam S. Urinary Incontinence. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill; 2014:1377-1396.
14. Teivelis MP, Wolosker N, Krutman M, Kauffman P, Campos JR, Puech-Leão P. Treatment of uncommon sites of focal primary hyperhidrosis: experience with pharmacological therapy using oxybutynin. Clinics (Sao Paulo). 2014;69(9):608-614.
15. Wolosker N, de Campos JR, Kauffman P, Puech-Leão P. A randomized-placebo-controlled trial of oxybutynin for the initial treatment of palmar and axillary hyperhidrosis. J Vasc Surg. 2012;55(6):1696-1700.
16. Schollhammer M, Brenaut E, Menard-Andivot N, et al. Oxybutynin as a treatment for generalized hyperhidrosis. Br J Dermatol. 2015;173(5):1163-1168.
17. Gonzalez F, Chang JY, Banovac K, Messina D, Martinez-Arizala A, Kelley RE. Autoregulation of cerebral blood flow in patients with orthostatic hypotension after spinal cord injury. Paraplegia. 1991;29(1):1-7.
18. Fealey RD, Hebert AA. Disorders of the eccrine sweat glands and sweating. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, eds. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw-Hill; 2012:chap 84.
Case Studies in Toxicology: Drink the Water, but Don’t Eat the Paint
Case
A 2-year-old boy and his mother were referred to the ED by the child’s pediatrician after a routine venous blood lead level (BLL) taken at the boy’s recent well visit revealed an elevated lead level of 52 mcg/dL (normal range, <5 mcg/dL). The child’s mother reported that although her son had always been a picky eater, he had recently been complaining of abdominal pain.
The patient’s well-child visits had been normal until his recent 2-year checkup, at which time his pediatrician noticed some speech delay. On further history taking, the emergency physician (EP) learned the patient and his mother resided in an older home (built in the 1950s) that was in disrepair. The mother asked the EP if the elevation in the child’s BLL could be due to the drinking water in their town.
What are the most likely sources of environmental lead exposure?
In 2016, the topic of lead poisoning grabbed national attention when a pediatrician in Flint, Michigan detected an abrupt doubling of the number of children with elevated lead levels in her practice.1 Upon further investigation, it was discovered that these kids had one thing in common: the source of their drinking water. The City of Flint had recently switched the source of its potable water from Lake Huron to the Flint River. The lower quality water, which was not properly treated with an anticorrosive agent such as orthophosphate, led to widespread pipe corrosion and lead contamination. This finding resulted in a cascade of water testing by other municipalities and school systems, many of which identified lead concentrations above the currently accepted drinking water standard of 15 parts per billion (ppb).
Thousands of children each year are identified to have elevated BLLs, based on the Centers for Disease Control and Prevention definition of a “level of concern” as more than 5 mcg/dL.2 The majority of these exposures stem from environmental exposure to lead paint dust in the home, but drinking water normally contributes as a low-level, constant, “basal” exposure. While lead-contaminated drinking water is not acceptable, it is unlikely to generate many ED visits. However, there are a variety of other lead sources that may prompt children to present to the ED with acute or subacute lead poisoning.
Lead is a heavy metal whose physical properties indicate its common uses. It provides durability and opacity to pigments, which is why it is found in oil paint, house paint used before 1976, and on paint for large outdoor structures, where it is still used. Lead is also found in the pigments used in cosmetics, stained glass, and painted pottery, and as an adulterant in highly colored foodstuffs such as imported turmeric.3
The physicochemical characteristics of lead make it an ideal component of solder. Many plumbing pipes in use today are not lead, but join one another using lead solder at the joints, sites that are vulnerable to corrosion. The heavy molecular weight of lead makes it a useful component of bullets and munitions.
Tetraethyl lead was used as an “anti-knock” agent to smooth out the combustion of heterogenous compounds in automotive fuel before it was removed in the mid-1970s.4 Prior to its removal, leaded gasoline was the largest source of air, soil, and groundwater contamination leading to environmental exposures.4 At present, the most common source of environmental lead exposure among young children is through peeling paint in deteriorating residential buildings. Hazardous occupational lead exposures arise from work involving munitions, reclamation and salvage, painting, welding, and numerous other settings—particularly sites where industrial hygiene is suboptimal. Lead from these sites can be inadvertently transported home on clothing or shoes, raising the exposure risk for children in the household.4
What are the health effects of lead exposure?
Like most heavy metals, lead is toxic to many organ systems in the body. The signs and symptoms of lead poisoning vary depending on the patient’s BLL and age (Table 1).5 The most common clinical effect of lead in the adult population is hypertension.6 Additional renal effects include a Fanconi-type syndrome with glycosuria and proteinuria. Lead can cause a peripheral neuropathy that is predominantly motor, classically causing foot or wrist drop. Abdominal pain from lead exposure is sometimes termed “lead colic” due to its intermittent and often severe nature. Abnormalities in urate metabolism cause a gouty arthritis referred to as “saturnine gout.” 6
The young pediatric central nervous system (CNS) is much more vulnerable to the effects of lead than the adult CNS. Even low-level lead exposure to the developing brain causes deficits in intelligence quotient, attention, impulse control, and other neurocognitive functions that are largely irreversible.7
Children with an elevated BLL may also develop constipation, anorexia, pallor, and pica.8 The development of geophagia (subtype of pica in which one craves and ingests nonfood clay or soil-like materials), represents a “chicken-or-egg” phenomena as it both causes and results from lead poisoning.
Lead impairs multiple steps of the heme synthesis pathway, causing microcytic anemia with basophilic stippling. Lead-induced anemia exacerbates pica as anemic patients are more likely to eat leaded paint chips and other lead-containing materials such as pottery.8 Of note, leaded white paint is reported to have a pleasant taste due to the sweet-tasting lead acetate used as a pigment.
The most dramatic and consequential manifestation of lead poisoning is lead encephalopathy. This can occur at any age, but manifests in children at much lower BLLs than in adults. Patients can be altered or obtunded, have convulsive activity, and may develop cerebral edema. Encephalopathy is a life-threatening emergency and must be recognized and treated immediately. Lead encephalopathy should be suspected in any young child with hand-to-mouth behavior who has any of the above environmental risk factors.4 The findings of anemia or the other diagnostic signs described below are too unreliable and take too long to be truly helpful in making the diagnosis.
How is the diagnosis of lead poisoning made?
The gold standard for the diagnosis of lead poisoning is the measurement of BLL. However, the turnaround time for this test is usually at least 24 hours, but may take up to several days. As such, adjunctive testing can accelerate obtaining a diagnosis. A complete blood count (CBC) to evaluate for microcytic anemia may demonstrate a characteristic pattern of basophilic stippling.9 A protoporphyrin level—either a free erythrocyte protoporphyrin (FEP) or a zinc protoporphyrin level—will be elevated, a result of heme synthesis disruption.9 Urinalysis may demonstrate glycosuria or proteinuria.6 Hypertension is often present, even in pediatric patients.
An abdominal radiograph is essential in children to determine whether a lead foreign body, such as a paint chip, is present in the intestinal lumen. Long bone films may demonstrate “lead lines” at the metaphysis, which in fact do not reflect lead itself but abnormal calcium deposition in growing bone due to lead’s interference with bone remodeling. A computed tomography (CT) scan of the brain in patients with encephalopathy will often demonstrate cerebral edema.6
Of note, capillary BLLs taken via finger-stick can be falsely elevated due contamination during collection (eg, the presence of lead dust on the skin). However, this screening method is often used by clinicians in the pediatric primary care setting because of its feasibility. Elevated BLLs from capillary testing should always be followed by a BLL obtained by venipuncture.2
Case Continuation
The patient’s mother was counseled on sources of lead contamination. She was informed that although drinking water may contribute some amount to an elevated BLL, the most likely source of contamination is still lead paint found in older homes such as the one in which she and her son resided.
Diagnostic studies to support the diagnosis of lead poisoning were performed. A CBC revealed a hemoglobin of 9.8 g/dL with a mean corpuscular volume of 68 fL. A microscopic smear of blood demonstrated basophilic stippling of red blood cells. An FEP level was 386 mcg/dL. An abdominal radiograph demonstrated small radiopacities throughout the large intestine, without obstruction, which was suggestive of ingested lead paint chips.
What is the best management approach to patients with suspected lead poisoning?
The first-line treatment for patients with lead poisoning is removal from the exposure source, which first and foremost requires identification of the hazard through careful history taking and scene investigation by the local health department. This will avoid recurrent visits following successful chelation for repeat exposure to an unidentified source. Relocation to another dwelling will often be required for patients with presumed exposure until the hazard can be identified and abated.
Patients who have ingested or have embedded leaded foreign bodies will require removal via whole bowel irrigation or surgical means.
Following decontamination, chelation is required for children with a BLL more than 45 mcg/dL, and adults with CNS symptomatology and a BLL more than 70 mcg/dL. Table 2 provides guidelines for chelation therapy based on BLL.5
There are three chelating agents commonly used to reduce the body lead burden (Table 2).5 The most common, owing largely to it being the only agent used orally, is succimer (or dimercaptosuccinic acid, DMSA). The second agent is calcium disodium edetate (CaNa2EDTA), which is given intravenously. In patients with encephalopathy, EDTA should be given after the first dose of the third agent, British anti-Lewisite (BAL; 2,3-dimercaptopropanol), in order to prevent redistribution of lead from the peripheral compartment into the CNS.10 However, BAL is the most difficult of the three agents to administer as it is suspended in peanut oil and is given via intramuscular injection every 4 hours.
Unfortunately, while chelation therapy is highly beneficial for patients with severe lead poisoning, it has not been demonstrated to positively impact children who already have developed neurocognitive sequelae associated with lower level lead exposure.11 This highlights the importance of prevention.
Work-up and Management in the ED
The patient with lead poisoning, while an unusual presentation in the ED, requires specialized management to minimize sequelae of exposure. Careful attention to history is vital. When in doubt, the EP should consult with her or his regional poison control center (800-222-1222) or with a medical toxicologist or other expert.
There are several scenarios in which a patient may present to the ED with lead toxicity. The following scenarios, along with their respective clinical approach strategies, represent three of the most common presentations.
Scenario 1: The Pediatric Patient With Elevated Venous Blood Lead Levels
The EP should employ the following clinical approach when evaluating and managing the pediatric patient with normal mental status whose routine screening reveals a BLL sufficiently elevated to warrant evaluation or admission—perhaps to discontinue exposure or initiate chelation therapy.
- Obtain a history, including possible lead sources; perform a complete physical examination; and obtain a repeat BLL, CBC with microscopic examination, and renal function test.
- Obtain an abdominal film to look for radiopacities, including paint chips or larger ingested foreign bodies.
- If radiopaque foreign bodies are present on abdominal radiograph, whole bowel irrigation with polyethylene glycol solution given via a nasogastric tube at 250 to 500 cc/h for a pediatric patient (1 to 2 L/h for adult patients) should be given until no residual foreign bodies remain.
- Obtain a radiograph of the long bone, which may demonstrate metaphyseal enhancement in the pediatric patient, suggesting long-term exposure.
- Ensure local or state health departments are contacted to arrange for environmental inspection of the home. This is essential prior to discharge to the home environment.
- Begin chelation therapy according to the BLL (Table 2).
Scenario 2: Adult Patients Presenting With Signs and Symptoms of Lead Toxicity
The adult patient who presents to the ED with complaints suggestive of lead poisoning and whose history is indicative of lead exposure should be evaluated and managed as follows:
- Obtain a thorough history, including the occupation and hobbies of the patient and all family members.
- Obtain vital signs to evaluate for hypertension; repeat BLL, CBC with smear, and serum creatinine test. Perform a physical examination to evaluate for lead lines.
- Obtain radiographic images, which may demonstrate a leaded foreign body, such as in the patient with prior history of gunshot wounds.
- If the BLL is sufficiently elevated or clinical findings are sufficiently severe, admit for chelation.
Scenario 3: The Pediatric or Adult Patient Presenting With Altered Mental Status
The patient presenting with altered mental status of unclear etiology—regardless of age—and in whom lead encephalopathy is a possible cause, should be worked-up and managed as follows:
- Obtain BLL, CBC, FEP levels. Consider radiographic imaging to assess for ingested or embedded foreign bodies.
- If abnormalities in the above laboratory studies are consistent with lead poisoning, initiate chelation immediately—prior to receiving repeat BLL result.
- Obtain a CT scan of the head to assess for cerebral edema.
- Provide supportive care for encephalopathy, including airway control and management of increased intracranial pressure.
Case Conclusion
The patient was admitted to the hospital for whole bowel irrigation and chelation therapy with succimer. The local health department conducted an investigation of the home and found multiple areas of peeling lead paint and lead dust, and ordered remediation of the property before it could be re-occupied by the family. A test of the home’s drinking water found no elevation above the 15 ppb standard.
The patient was discharged from the hospital in the care of his mother. They were relocated to a lead-free home, with follow-up by the pediatrician for ongoing monitoring of the BLL and developmental milestones.
1. Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. Elevated blood lead levels in children associated with the flint drinking water crisis: A spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. doi:0.2105/AJPH.2015.303003.
2. Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Low level lead exposure harms children: a renewed call for primary prevention. January 4, 2012. Available at https://www.cdc.gov/nceh/lead/acclpp/final_document_030712.pdf. Accessed February 27, 2017.
3. Food and Drug Administration. Spices USA Inc. issues alert on elevated levels of lead in ground turmeric. http://www.fda.gov/safety/recalls/ucm523561.htm, September 26, 2016. Accessed February 27, 2017.
4. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Toxic substances portal: lead. US Department of Health and Human Services Web site. Available at https://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=96&tid=22. Updated January 21, 2015. Accessed February 27, 2017.
5. Calello DP, Henretig FM. Lead. In: Goldfrank LG, Flomenbaum NE, Lewin NA, Howland MA, Hoffman RS, Nelson LS (eds.). Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2014:1219-1234.
6. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education: lead toxicity. https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=10. Updated August 26, 2016. Accessed February 27, 2017.
7. Canfield RL, Henderson Jr CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. New Engl J Med. 2003;348:1517-1526.
8. Kathuria P, Rowden AK. Lead toxicity. Medscape Web site. Available at http://emedicine.medscape.com/article/1174752-clinical. Updated January 31, 2017. Accessed February 27, 2017.
9. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education. Lead toxicity: what tests can assist with diagnosis of lead toxicity? https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=12. Updated August 25, 2016. Accessed February 27, 2017.
10. Chisholm JJ Jr. The use of chelating agents in the treatment of acute and chronic lead intoxication in childhood. J Pediatr. 1968;73(1):1-38.
11. Rogan WJ, Dietrich KN, Ware JH, et al; Treatment of Lead-Exposed Children Trial Group. The effect of chelation therapy with succimer on neuropsychological development in children exposed to lead. N Engl J Med. 2001;344(19):1421-1426.
Case
A 2-year-old boy and his mother were referred to the ED by the child’s pediatrician after a routine venous blood lead level (BLL) taken at the boy’s recent well visit revealed an elevated lead level of 52 mcg/dL (normal range, <5 mcg/dL). The child’s mother reported that although her son had always been a picky eater, he had recently been complaining of abdominal pain.
The patient’s well-child visits had been normal until his recent 2-year checkup, at which time his pediatrician noticed some speech delay. On further history taking, the emergency physician (EP) learned the patient and his mother resided in an older home (built in the 1950s) that was in disrepair. The mother asked the EP if the elevation in the child’s BLL could be due to the drinking water in their town.
What are the most likely sources of environmental lead exposure?
In 2016, the topic of lead poisoning grabbed national attention when a pediatrician in Flint, Michigan detected an abrupt doubling of the number of children with elevated lead levels in her practice.1 Upon further investigation, it was discovered that these kids had one thing in common: the source of their drinking water. The City of Flint had recently switched the source of its potable water from Lake Huron to the Flint River. The lower quality water, which was not properly treated with an anticorrosive agent such as orthophosphate, led to widespread pipe corrosion and lead contamination. This finding resulted in a cascade of water testing by other municipalities and school systems, many of which identified lead concentrations above the currently accepted drinking water standard of 15 parts per billion (ppb).
Thousands of children each year are identified to have elevated BLLs, based on the Centers for Disease Control and Prevention definition of a “level of concern” as more than 5 mcg/dL.2 The majority of these exposures stem from environmental exposure to lead paint dust in the home, but drinking water normally contributes as a low-level, constant, “basal” exposure. While lead-contaminated drinking water is not acceptable, it is unlikely to generate many ED visits. However, there are a variety of other lead sources that may prompt children to present to the ED with acute or subacute lead poisoning.
Lead is a heavy metal whose physical properties indicate its common uses. It provides durability and opacity to pigments, which is why it is found in oil paint, house paint used before 1976, and on paint for large outdoor structures, where it is still used. Lead is also found in the pigments used in cosmetics, stained glass, and painted pottery, and as an adulterant in highly colored foodstuffs such as imported turmeric.3
The physicochemical characteristics of lead make it an ideal component of solder. Many plumbing pipes in use today are not lead, but join one another using lead solder at the joints, sites that are vulnerable to corrosion. The heavy molecular weight of lead makes it a useful component of bullets and munitions.
Tetraethyl lead was used as an “anti-knock” agent to smooth out the combustion of heterogenous compounds in automotive fuel before it was removed in the mid-1970s.4 Prior to its removal, leaded gasoline was the largest source of air, soil, and groundwater contamination leading to environmental exposures.4 At present, the most common source of environmental lead exposure among young children is through peeling paint in deteriorating residential buildings. Hazardous occupational lead exposures arise from work involving munitions, reclamation and salvage, painting, welding, and numerous other settings—particularly sites where industrial hygiene is suboptimal. Lead from these sites can be inadvertently transported home on clothing or shoes, raising the exposure risk for children in the household.4
What are the health effects of lead exposure?
Like most heavy metals, lead is toxic to many organ systems in the body. The signs and symptoms of lead poisoning vary depending on the patient’s BLL and age (Table 1).5 The most common clinical effect of lead in the adult population is hypertension.6 Additional renal effects include a Fanconi-type syndrome with glycosuria and proteinuria. Lead can cause a peripheral neuropathy that is predominantly motor, classically causing foot or wrist drop. Abdominal pain from lead exposure is sometimes termed “lead colic” due to its intermittent and often severe nature. Abnormalities in urate metabolism cause a gouty arthritis referred to as “saturnine gout.” 6
The young pediatric central nervous system (CNS) is much more vulnerable to the effects of lead than the adult CNS. Even low-level lead exposure to the developing brain causes deficits in intelligence quotient, attention, impulse control, and other neurocognitive functions that are largely irreversible.7
Children with an elevated BLL may also develop constipation, anorexia, pallor, and pica.8 The development of geophagia (subtype of pica in which one craves and ingests nonfood clay or soil-like materials), represents a “chicken-or-egg” phenomena as it both causes and results from lead poisoning.
Lead impairs multiple steps of the heme synthesis pathway, causing microcytic anemia with basophilic stippling. Lead-induced anemia exacerbates pica as anemic patients are more likely to eat leaded paint chips and other lead-containing materials such as pottery.8 Of note, leaded white paint is reported to have a pleasant taste due to the sweet-tasting lead acetate used as a pigment.
The most dramatic and consequential manifestation of lead poisoning is lead encephalopathy. This can occur at any age, but manifests in children at much lower BLLs than in adults. Patients can be altered or obtunded, have convulsive activity, and may develop cerebral edema. Encephalopathy is a life-threatening emergency and must be recognized and treated immediately. Lead encephalopathy should be suspected in any young child with hand-to-mouth behavior who has any of the above environmental risk factors.4 The findings of anemia or the other diagnostic signs described below are too unreliable and take too long to be truly helpful in making the diagnosis.
How is the diagnosis of lead poisoning made?
The gold standard for the diagnosis of lead poisoning is the measurement of BLL. However, the turnaround time for this test is usually at least 24 hours, but may take up to several days. As such, adjunctive testing can accelerate obtaining a diagnosis. A complete blood count (CBC) to evaluate for microcytic anemia may demonstrate a characteristic pattern of basophilic stippling.9 A protoporphyrin level—either a free erythrocyte protoporphyrin (FEP) or a zinc protoporphyrin level—will be elevated, a result of heme synthesis disruption.9 Urinalysis may demonstrate glycosuria or proteinuria.6 Hypertension is often present, even in pediatric patients.
An abdominal radiograph is essential in children to determine whether a lead foreign body, such as a paint chip, is present in the intestinal lumen. Long bone films may demonstrate “lead lines” at the metaphysis, which in fact do not reflect lead itself but abnormal calcium deposition in growing bone due to lead’s interference with bone remodeling. A computed tomography (CT) scan of the brain in patients with encephalopathy will often demonstrate cerebral edema.6
Of note, capillary BLLs taken via finger-stick can be falsely elevated due contamination during collection (eg, the presence of lead dust on the skin). However, this screening method is often used by clinicians in the pediatric primary care setting because of its feasibility. Elevated BLLs from capillary testing should always be followed by a BLL obtained by venipuncture.2
Case Continuation
The patient’s mother was counseled on sources of lead contamination. She was informed that although drinking water may contribute some amount to an elevated BLL, the most likely source of contamination is still lead paint found in older homes such as the one in which she and her son resided.
Diagnostic studies to support the diagnosis of lead poisoning were performed. A CBC revealed a hemoglobin of 9.8 g/dL with a mean corpuscular volume of 68 fL. A microscopic smear of blood demonstrated basophilic stippling of red blood cells. An FEP level was 386 mcg/dL. An abdominal radiograph demonstrated small radiopacities throughout the large intestine, without obstruction, which was suggestive of ingested lead paint chips.
What is the best management approach to patients with suspected lead poisoning?
The first-line treatment for patients with lead poisoning is removal from the exposure source, which first and foremost requires identification of the hazard through careful history taking and scene investigation by the local health department. This will avoid recurrent visits following successful chelation for repeat exposure to an unidentified source. Relocation to another dwelling will often be required for patients with presumed exposure until the hazard can be identified and abated.
Patients who have ingested or have embedded leaded foreign bodies will require removal via whole bowel irrigation or surgical means.
Following decontamination, chelation is required for children with a BLL more than 45 mcg/dL, and adults with CNS symptomatology and a BLL more than 70 mcg/dL. Table 2 provides guidelines for chelation therapy based on BLL.5
There are three chelating agents commonly used to reduce the body lead burden (Table 2).5 The most common, owing largely to it being the only agent used orally, is succimer (or dimercaptosuccinic acid, DMSA). The second agent is calcium disodium edetate (CaNa2EDTA), which is given intravenously. In patients with encephalopathy, EDTA should be given after the first dose of the third agent, British anti-Lewisite (BAL; 2,3-dimercaptopropanol), in order to prevent redistribution of lead from the peripheral compartment into the CNS.10 However, BAL is the most difficult of the three agents to administer as it is suspended in peanut oil and is given via intramuscular injection every 4 hours.
Unfortunately, while chelation therapy is highly beneficial for patients with severe lead poisoning, it has not been demonstrated to positively impact children who already have developed neurocognitive sequelae associated with lower level lead exposure.11 This highlights the importance of prevention.
Work-up and Management in the ED
The patient with lead poisoning, while an unusual presentation in the ED, requires specialized management to minimize sequelae of exposure. Careful attention to history is vital. When in doubt, the EP should consult with her or his regional poison control center (800-222-1222) or with a medical toxicologist or other expert.
There are several scenarios in which a patient may present to the ED with lead toxicity. The following scenarios, along with their respective clinical approach strategies, represent three of the most common presentations.
Scenario 1: The Pediatric Patient With Elevated Venous Blood Lead Levels
The EP should employ the following clinical approach when evaluating and managing the pediatric patient with normal mental status whose routine screening reveals a BLL sufficiently elevated to warrant evaluation or admission—perhaps to discontinue exposure or initiate chelation therapy.
- Obtain a history, including possible lead sources; perform a complete physical examination; and obtain a repeat BLL, CBC with microscopic examination, and renal function test.
- Obtain an abdominal film to look for radiopacities, including paint chips or larger ingested foreign bodies.
- If radiopaque foreign bodies are present on abdominal radiograph, whole bowel irrigation with polyethylene glycol solution given via a nasogastric tube at 250 to 500 cc/h for a pediatric patient (1 to 2 L/h for adult patients) should be given until no residual foreign bodies remain.
- Obtain a radiograph of the long bone, which may demonstrate metaphyseal enhancement in the pediatric patient, suggesting long-term exposure.
- Ensure local or state health departments are contacted to arrange for environmental inspection of the home. This is essential prior to discharge to the home environment.
- Begin chelation therapy according to the BLL (Table 2).
Scenario 2: Adult Patients Presenting With Signs and Symptoms of Lead Toxicity
The adult patient who presents to the ED with complaints suggestive of lead poisoning and whose history is indicative of lead exposure should be evaluated and managed as follows:
- Obtain a thorough history, including the occupation and hobbies of the patient and all family members.
- Obtain vital signs to evaluate for hypertension; repeat BLL, CBC with smear, and serum creatinine test. Perform a physical examination to evaluate for lead lines.
- Obtain radiographic images, which may demonstrate a leaded foreign body, such as in the patient with prior history of gunshot wounds.
- If the BLL is sufficiently elevated or clinical findings are sufficiently severe, admit for chelation.
Scenario 3: The Pediatric or Adult Patient Presenting With Altered Mental Status
The patient presenting with altered mental status of unclear etiology—regardless of age—and in whom lead encephalopathy is a possible cause, should be worked-up and managed as follows:
- Obtain BLL, CBC, FEP levels. Consider radiographic imaging to assess for ingested or embedded foreign bodies.
- If abnormalities in the above laboratory studies are consistent with lead poisoning, initiate chelation immediately—prior to receiving repeat BLL result.
- Obtain a CT scan of the head to assess for cerebral edema.
- Provide supportive care for encephalopathy, including airway control and management of increased intracranial pressure.
Case Conclusion
The patient was admitted to the hospital for whole bowel irrigation and chelation therapy with succimer. The local health department conducted an investigation of the home and found multiple areas of peeling lead paint and lead dust, and ordered remediation of the property before it could be re-occupied by the family. A test of the home’s drinking water found no elevation above the 15 ppb standard.
The patient was discharged from the hospital in the care of his mother. They were relocated to a lead-free home, with follow-up by the pediatrician for ongoing monitoring of the BLL and developmental milestones.
Case
A 2-year-old boy and his mother were referred to the ED by the child’s pediatrician after a routine venous blood lead level (BLL) taken at the boy’s recent well visit revealed an elevated lead level of 52 mcg/dL (normal range, <5 mcg/dL). The child’s mother reported that although her son had always been a picky eater, he had recently been complaining of abdominal pain.
The patient’s well-child visits had been normal until his recent 2-year checkup, at which time his pediatrician noticed some speech delay. On further history taking, the emergency physician (EP) learned the patient and his mother resided in an older home (built in the 1950s) that was in disrepair. The mother asked the EP if the elevation in the child’s BLL could be due to the drinking water in their town.
What are the most likely sources of environmental lead exposure?
In 2016, the topic of lead poisoning grabbed national attention when a pediatrician in Flint, Michigan detected an abrupt doubling of the number of children with elevated lead levels in her practice.1 Upon further investigation, it was discovered that these kids had one thing in common: the source of their drinking water. The City of Flint had recently switched the source of its potable water from Lake Huron to the Flint River. The lower quality water, which was not properly treated with an anticorrosive agent such as orthophosphate, led to widespread pipe corrosion and lead contamination. This finding resulted in a cascade of water testing by other municipalities and school systems, many of which identified lead concentrations above the currently accepted drinking water standard of 15 parts per billion (ppb).
Thousands of children each year are identified to have elevated BLLs, based on the Centers for Disease Control and Prevention definition of a “level of concern” as more than 5 mcg/dL.2 The majority of these exposures stem from environmental exposure to lead paint dust in the home, but drinking water normally contributes as a low-level, constant, “basal” exposure. While lead-contaminated drinking water is not acceptable, it is unlikely to generate many ED visits. However, there are a variety of other lead sources that may prompt children to present to the ED with acute or subacute lead poisoning.
Lead is a heavy metal whose physical properties indicate its common uses. It provides durability and opacity to pigments, which is why it is found in oil paint, house paint used before 1976, and on paint for large outdoor structures, where it is still used. Lead is also found in the pigments used in cosmetics, stained glass, and painted pottery, and as an adulterant in highly colored foodstuffs such as imported turmeric.3
The physicochemical characteristics of lead make it an ideal component of solder. Many plumbing pipes in use today are not lead, but join one another using lead solder at the joints, sites that are vulnerable to corrosion. The heavy molecular weight of lead makes it a useful component of bullets and munitions.
Tetraethyl lead was used as an “anti-knock” agent to smooth out the combustion of heterogenous compounds in automotive fuel before it was removed in the mid-1970s.4 Prior to its removal, leaded gasoline was the largest source of air, soil, and groundwater contamination leading to environmental exposures.4 At present, the most common source of environmental lead exposure among young children is through peeling paint in deteriorating residential buildings. Hazardous occupational lead exposures arise from work involving munitions, reclamation and salvage, painting, welding, and numerous other settings—particularly sites where industrial hygiene is suboptimal. Lead from these sites can be inadvertently transported home on clothing or shoes, raising the exposure risk for children in the household.4
What are the health effects of lead exposure?
Like most heavy metals, lead is toxic to many organ systems in the body. The signs and symptoms of lead poisoning vary depending on the patient’s BLL and age (Table 1).5 The most common clinical effect of lead in the adult population is hypertension.6 Additional renal effects include a Fanconi-type syndrome with glycosuria and proteinuria. Lead can cause a peripheral neuropathy that is predominantly motor, classically causing foot or wrist drop. Abdominal pain from lead exposure is sometimes termed “lead colic” due to its intermittent and often severe nature. Abnormalities in urate metabolism cause a gouty arthritis referred to as “saturnine gout.” 6
The young pediatric central nervous system (CNS) is much more vulnerable to the effects of lead than the adult CNS. Even low-level lead exposure to the developing brain causes deficits in intelligence quotient, attention, impulse control, and other neurocognitive functions that are largely irreversible.7
Children with an elevated BLL may also develop constipation, anorexia, pallor, and pica.8 The development of geophagia (subtype of pica in which one craves and ingests nonfood clay or soil-like materials), represents a “chicken-or-egg” phenomena as it both causes and results from lead poisoning.
Lead impairs multiple steps of the heme synthesis pathway, causing microcytic anemia with basophilic stippling. Lead-induced anemia exacerbates pica as anemic patients are more likely to eat leaded paint chips and other lead-containing materials such as pottery.8 Of note, leaded white paint is reported to have a pleasant taste due to the sweet-tasting lead acetate used as a pigment.
The most dramatic and consequential manifestation of lead poisoning is lead encephalopathy. This can occur at any age, but manifests in children at much lower BLLs than in adults. Patients can be altered or obtunded, have convulsive activity, and may develop cerebral edema. Encephalopathy is a life-threatening emergency and must be recognized and treated immediately. Lead encephalopathy should be suspected in any young child with hand-to-mouth behavior who has any of the above environmental risk factors.4 The findings of anemia or the other diagnostic signs described below are too unreliable and take too long to be truly helpful in making the diagnosis.
How is the diagnosis of lead poisoning made?
The gold standard for the diagnosis of lead poisoning is the measurement of BLL. However, the turnaround time for this test is usually at least 24 hours, but may take up to several days. As such, adjunctive testing can accelerate obtaining a diagnosis. A complete blood count (CBC) to evaluate for microcytic anemia may demonstrate a characteristic pattern of basophilic stippling.9 A protoporphyrin level—either a free erythrocyte protoporphyrin (FEP) or a zinc protoporphyrin level—will be elevated, a result of heme synthesis disruption.9 Urinalysis may demonstrate glycosuria or proteinuria.6 Hypertension is often present, even in pediatric patients.
An abdominal radiograph is essential in children to determine whether a lead foreign body, such as a paint chip, is present in the intestinal lumen. Long bone films may demonstrate “lead lines” at the metaphysis, which in fact do not reflect lead itself but abnormal calcium deposition in growing bone due to lead’s interference with bone remodeling. A computed tomography (CT) scan of the brain in patients with encephalopathy will often demonstrate cerebral edema.6
Of note, capillary BLLs taken via finger-stick can be falsely elevated due contamination during collection (eg, the presence of lead dust on the skin). However, this screening method is often used by clinicians in the pediatric primary care setting because of its feasibility. Elevated BLLs from capillary testing should always be followed by a BLL obtained by venipuncture.2
Case Continuation
The patient’s mother was counseled on sources of lead contamination. She was informed that although drinking water may contribute some amount to an elevated BLL, the most likely source of contamination is still lead paint found in older homes such as the one in which she and her son resided.
Diagnostic studies to support the diagnosis of lead poisoning were performed. A CBC revealed a hemoglobin of 9.8 g/dL with a mean corpuscular volume of 68 fL. A microscopic smear of blood demonstrated basophilic stippling of red blood cells. An FEP level was 386 mcg/dL. An abdominal radiograph demonstrated small radiopacities throughout the large intestine, without obstruction, which was suggestive of ingested lead paint chips.
What is the best management approach to patients with suspected lead poisoning?
The first-line treatment for patients with lead poisoning is removal from the exposure source, which first and foremost requires identification of the hazard through careful history taking and scene investigation by the local health department. This will avoid recurrent visits following successful chelation for repeat exposure to an unidentified source. Relocation to another dwelling will often be required for patients with presumed exposure until the hazard can be identified and abated.
Patients who have ingested or have embedded leaded foreign bodies will require removal via whole bowel irrigation or surgical means.
Following decontamination, chelation is required for children with a BLL more than 45 mcg/dL, and adults with CNS symptomatology and a BLL more than 70 mcg/dL. Table 2 provides guidelines for chelation therapy based on BLL.5
There are three chelating agents commonly used to reduce the body lead burden (Table 2).5 The most common, owing largely to it being the only agent used orally, is succimer (or dimercaptosuccinic acid, DMSA). The second agent is calcium disodium edetate (CaNa2EDTA), which is given intravenously. In patients with encephalopathy, EDTA should be given after the first dose of the third agent, British anti-Lewisite (BAL; 2,3-dimercaptopropanol), in order to prevent redistribution of lead from the peripheral compartment into the CNS.10 However, BAL is the most difficult of the three agents to administer as it is suspended in peanut oil and is given via intramuscular injection every 4 hours.
Unfortunately, while chelation therapy is highly beneficial for patients with severe lead poisoning, it has not been demonstrated to positively impact children who already have developed neurocognitive sequelae associated with lower level lead exposure.11 This highlights the importance of prevention.
Work-up and Management in the ED
The patient with lead poisoning, while an unusual presentation in the ED, requires specialized management to minimize sequelae of exposure. Careful attention to history is vital. When in doubt, the EP should consult with her or his regional poison control center (800-222-1222) or with a medical toxicologist or other expert.
There are several scenarios in which a patient may present to the ED with lead toxicity. The following scenarios, along with their respective clinical approach strategies, represent three of the most common presentations.
Scenario 1: The Pediatric Patient With Elevated Venous Blood Lead Levels
The EP should employ the following clinical approach when evaluating and managing the pediatric patient with normal mental status whose routine screening reveals a BLL sufficiently elevated to warrant evaluation or admission—perhaps to discontinue exposure or initiate chelation therapy.
- Obtain a history, including possible lead sources; perform a complete physical examination; and obtain a repeat BLL, CBC with microscopic examination, and renal function test.
- Obtain an abdominal film to look for radiopacities, including paint chips or larger ingested foreign bodies.
- If radiopaque foreign bodies are present on abdominal radiograph, whole bowel irrigation with polyethylene glycol solution given via a nasogastric tube at 250 to 500 cc/h for a pediatric patient (1 to 2 L/h for adult patients) should be given until no residual foreign bodies remain.
- Obtain a radiograph of the long bone, which may demonstrate metaphyseal enhancement in the pediatric patient, suggesting long-term exposure.
- Ensure local or state health departments are contacted to arrange for environmental inspection of the home. This is essential prior to discharge to the home environment.
- Begin chelation therapy according to the BLL (Table 2).
Scenario 2: Adult Patients Presenting With Signs and Symptoms of Lead Toxicity
The adult patient who presents to the ED with complaints suggestive of lead poisoning and whose history is indicative of lead exposure should be evaluated and managed as follows:
- Obtain a thorough history, including the occupation and hobbies of the patient and all family members.
- Obtain vital signs to evaluate for hypertension; repeat BLL, CBC with smear, and serum creatinine test. Perform a physical examination to evaluate for lead lines.
- Obtain radiographic images, which may demonstrate a leaded foreign body, such as in the patient with prior history of gunshot wounds.
- If the BLL is sufficiently elevated or clinical findings are sufficiently severe, admit for chelation.
Scenario 3: The Pediatric or Adult Patient Presenting With Altered Mental Status
The patient presenting with altered mental status of unclear etiology—regardless of age—and in whom lead encephalopathy is a possible cause, should be worked-up and managed as follows:
- Obtain BLL, CBC, FEP levels. Consider radiographic imaging to assess for ingested or embedded foreign bodies.
- If abnormalities in the above laboratory studies are consistent with lead poisoning, initiate chelation immediately—prior to receiving repeat BLL result.
- Obtain a CT scan of the head to assess for cerebral edema.
- Provide supportive care for encephalopathy, including airway control and management of increased intracranial pressure.
Case Conclusion
The patient was admitted to the hospital for whole bowel irrigation and chelation therapy with succimer. The local health department conducted an investigation of the home and found multiple areas of peeling lead paint and lead dust, and ordered remediation of the property before it could be re-occupied by the family. A test of the home’s drinking water found no elevation above the 15 ppb standard.
The patient was discharged from the hospital in the care of his mother. They were relocated to a lead-free home, with follow-up by the pediatrician for ongoing monitoring of the BLL and developmental milestones.
1. Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. Elevated blood lead levels in children associated with the flint drinking water crisis: A spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. doi:0.2105/AJPH.2015.303003.
2. Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Low level lead exposure harms children: a renewed call for primary prevention. January 4, 2012. Available at https://www.cdc.gov/nceh/lead/acclpp/final_document_030712.pdf. Accessed February 27, 2017.
3. Food and Drug Administration. Spices USA Inc. issues alert on elevated levels of lead in ground turmeric. http://www.fda.gov/safety/recalls/ucm523561.htm, September 26, 2016. Accessed February 27, 2017.
4. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Toxic substances portal: lead. US Department of Health and Human Services Web site. Available at https://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=96&tid=22. Updated January 21, 2015. Accessed February 27, 2017.
5. Calello DP, Henretig FM. Lead. In: Goldfrank LG, Flomenbaum NE, Lewin NA, Howland MA, Hoffman RS, Nelson LS (eds.). Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2014:1219-1234.
6. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education: lead toxicity. https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=10. Updated August 26, 2016. Accessed February 27, 2017.
7. Canfield RL, Henderson Jr CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. New Engl J Med. 2003;348:1517-1526.
8. Kathuria P, Rowden AK. Lead toxicity. Medscape Web site. Available at http://emedicine.medscape.com/article/1174752-clinical. Updated January 31, 2017. Accessed February 27, 2017.
9. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education. Lead toxicity: what tests can assist with diagnosis of lead toxicity? https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=12. Updated August 25, 2016. Accessed February 27, 2017.
10. Chisholm JJ Jr. The use of chelating agents in the treatment of acute and chronic lead intoxication in childhood. J Pediatr. 1968;73(1):1-38.
11. Rogan WJ, Dietrich KN, Ware JH, et al; Treatment of Lead-Exposed Children Trial Group. The effect of chelation therapy with succimer on neuropsychological development in children exposed to lead. N Engl J Med. 2001;344(19):1421-1426.
1. Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. Elevated blood lead levels in children associated with the flint drinking water crisis: A spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. doi:0.2105/AJPH.2015.303003.
2. Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Low level lead exposure harms children: a renewed call for primary prevention. January 4, 2012. Available at https://www.cdc.gov/nceh/lead/acclpp/final_document_030712.pdf. Accessed February 27, 2017.
3. Food and Drug Administration. Spices USA Inc. issues alert on elevated levels of lead in ground turmeric. http://www.fda.gov/safety/recalls/ucm523561.htm, September 26, 2016. Accessed February 27, 2017.
4. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Toxic substances portal: lead. US Department of Health and Human Services Web site. Available at https://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=96&tid=22. Updated January 21, 2015. Accessed February 27, 2017.
5. Calello DP, Henretig FM. Lead. In: Goldfrank LG, Flomenbaum NE, Lewin NA, Howland MA, Hoffman RS, Nelson LS (eds.). Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2014:1219-1234.
6. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education: lead toxicity. https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=10. Updated August 26, 2016. Accessed February 27, 2017.
7. Canfield RL, Henderson Jr CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. New Engl J Med. 2003;348:1517-1526.
8. Kathuria P, Rowden AK. Lead toxicity. Medscape Web site. Available at http://emedicine.medscape.com/article/1174752-clinical. Updated January 31, 2017. Accessed February 27, 2017.
9. US Department of Health and Human Services - Agency for Toxic Substances & Disease Registry. Environmental health and medicine education. Lead toxicity: what tests can assist with diagnosis of lead toxicity? https://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=12. Updated August 25, 2016. Accessed February 27, 2017.
10. Chisholm JJ Jr. The use of chelating agents in the treatment of acute and chronic lead intoxication in childhood. J Pediatr. 1968;73(1):1-38.
11. Rogan WJ, Dietrich KN, Ware JH, et al; Treatment of Lead-Exposed Children Trial Group. The effect of chelation therapy with succimer on neuropsychological development in children exposed to lead. N Engl J Med. 2001;344(19):1421-1426.
Acute Submandibular Sialadenitis
Case
A 21-year-old woman presented to the ED with pain and swelling on the right side of her neck. She stated the pain started earlier that morning and worsened when she ate or swallowed. The patient denied a recent or remote history of drooling, voice changes, or neck swelling. She reported no fevers, chills, or any other complaints, and had no pertinent medical history—specifically, no history of recent dental work. Her surgical history included tonsillectomy and cholecystectomy. There was no family history of diabetes, thyroid disease, autoimmune disease, or any other diseases. The patient stated that she was not on any prescription or over-the-counter medications. Regarding her social history, she denied past or cu
Vital signs at presentation were: blood pressure, 124/63 mm Hg (sitting); heart rate, 73 beats/min; respiratory rate, 15 breaths/min; and temperature, 98°F. Oxygen saturation was 99% on room air. On clinical examination, pain was noted in the patient’s right submandibular area and was tender to palpation. The swelling extended to the angle of the mandible posteriorly (Figure 1a and 1b). There was no erythema or increased surface temperature to suggest overlying cellulitis. The oral examination showed no evidence of dental infection, angioedema, or Ludwig angina. The pharynx was normal in appearance. The otological examination was unremarkable, and there was no evidence of mastoiditis.
Laboratory evaluation included a complete blood count (CBC), basic metabolic profile (BMP), and rapid streptococcal test (RST). The results of the patient’s CBC revealed a white blood cell count (WBC) of 11.1 x 109/L; the BMP was unremarkable; and the RST was negative.
A soft tissue neck computed tomography (CT) scan with contrast was obtained, which revealed mild right submandibular gland enlargement with abnormal enhancement (Figure 2). Stranding was also noted in the right submandibular space along with thickening of the right platysma muscle, and few surrounding lymph nodes were prominent (Figure 3). The findings were consistent with acute submandibular sialadenitis.
The patient received intravenous (IV) normal saline for hydration and IV ketorolac for analgesia, as well as an initial dose of oral amoxicillin/clavulanate 875/125 mg. At discharge, she was given a 10-day course of oral amoxicillin/clavulanate 875/125 mg with instructions to follow-up with her primary care physician and otolaryngologist within 2 days. The patient did well on follow-up, and her symptoms resolved within a few days of discharge.
Discussion
Comparatively little has been published on acute submandibular sialadenitis over the past three decades, and much of that which is cited in the literature comes from a rather small pool of case reports.1 In a literature review, Raad et al1 noted, “Pertinent literature on [this] subject includes case reports but no studies describing the microbial and clinical characteristics of this disease.” Further, many of the published case reports describe neonatal presentations of submandibular sialadenitis, the incidence of which is rare in this patient population.2-4
Submandibular and Parotid Glands
The submandibular gland is the second largest salivary gland, the parotid gland being the largest. The duct of the salivary gland, the Wharton’s duct, opens under the tongue in the area of the lingual frenulum. Ductal obstruction is more frequently seen with the submandibular gland than with the parotid gland.1 The reason for this is unclear, but may be related to several factors. One factor may be that, unlike the Stenson’s duct of the parotid gland, the Wharton’s duct does not pass through a muscle; thus, there is no muscular massage supporting the movement of secretion, as there is with buccinator muscle massage of Stenson’s duct. In addition, submandibular saliva is more viscous than parotid saliva due to its higher protein content and higher concentration of calcium phosphate.1
Etiology
Submandibular gland obstruction can occur in the absence of infection. Noninfectious cases typically present with pain upon eating and swallowing. A bacterial infectious etiology is associated with odynophagia, but also includes persistent pain and tenderness. This presents as pain associated with eating. Bacterial infection of the submandibular gland adds the element of persistent pain, associated with such features as tenderness. In addition, purulent discharge from the Wharton’s duct may be present in infectious cases, and accompanied by fever, chills, and an elevated WBC.1
Several bacteria have been isolated in infectious submandibular sialadenitis, the most common pathogens being Staphylococcus aureus. However, streptococci, Pseudomonas aeruginosa, Moraxella catarrhalis, and Escherichia coli bacteria have also been identified in cases of infectious submandibular sialadenitis.5
Viral etiologies of sialadenitis, such as mumps, are generally bilateral and nonsuppurative. The human immunodeficiency virus can also cause bilateral nonsuppurative salivary gland infections.6
Imaging Studies
As illustrated in our case, CT imaging can assist in confirming the diagnosis of acute submandibular sialadenitis by defining the anatomic involvement and identifying the presence of an abscess. Ultrasound can also be used and has been described as a first-line imaging procedure.7,8
Treatment
Surgical Intervention. Abscesses may require surgical intervention. However, most cases without abscess formation respond to outpatient treatment with antibiotics.5 If ductal obstruction is identified, removal of the calculus may be needed. This may involve ductal dilation, sialolithectomy, or even ductoplasty if a stricture is identified.1
Antibiotic Therapy. With respect to antibiotic selection, Chandak et al5 recommend oral amoxicillin-clavulanic acid. Other antistaphylococcal coverage recommendations have been made in the literature. Gland massage may be helpful after the tenderness has resolved,5 and sialogogues (eg, lemon drops, vitamin C lozenges) can also provide some relief.6 In addition, to avoid disease recurrence and prevent dental complications, Chandak et al5 emphasize the crucial role of hydration and excellent oral hygiene.
Conclusion
We suspected acute submandibular sialadenitis in our patient based on clinical findings, which were confirmed on CT imaging. Patients with acute submandibular sialadenitis may present with submandibular gland obstruction in the absence of bacterial infection. Noninfectious obstruction typically presents as pain associated with eating and swallowing, whereas infectious cases include constant pain and tenderness in the affected area. In addition, patients with infectious etiology may also have purulent discharge from Wharton’s duct, fever, chills, and an elevated WBC. Several bacteria have been isolated, the most common being S aureus. However, streptococci, P aeruginosa, M catarrhalis and E coli have also been identified. Computed tomography studies are helpful in confirming the diagnosis, defining anatomical involvement, and in identifying abscess formation.
Abscesses may require surgical intervention. However, most cases without abscess formation respond to outpatient treatment with antibiotics. Antibiotic selection involves antistaphylococcal coverage, such as amoxicillin-clavulanic acid. Glandular massage may be helpful after the tenderness has resolved. In addition, the literature emphasizes the crucial role of hydration and excellent oral hygiene in disease recurrence and to prevent dental complications.
1. Raad II, Sabbagh MF, Caranasos GJ. Acute bacterial sialadenitis: a study of 29 cases and review. Rev Infect Dis. 1990;12(4):591-601.
2. Banks WW, Handler SD, Glade GB, Turner HD. Neonatal submandibular sialadenitis. Am J Otolaryngol. 1980;1(3):261-263.
3. Wells DH. Suppuration of the submandibular salivary glands in the neonate. Am J Dis Child. 1975;129(5):628-630.
4. Ryan RF, Padmakumar B. Neonatal suppurative sialadenitis: an important clinical diagnosis. BMJ Case Rep. 2015;2015. pii:bcr2014208535. doi:10.1136/bcr-2014-208535.
5. Chandak R, Degwekar S, Chandak M, Rawlani S. Acute submandibular sialadenitis—a case report. Case Rep Dent. 2012;2012:615375. doi:10.1155/2012/615375.
6. Wilson KF, Meier JD, Ward PD. Salivary gland disorders. Am Fam Physician. 2014;89(11):882-888.
7. Alyas F, Lewis K, Williams M, et al. Diseases of the submandibular gland as demonstrated using high resolution ultrasound. Br J Radiol. 2005;78(928):362-369. doi:10.1259/bjr/93120352.
8. Howlett DC, Alyas F, Wong KT, et al. Sonographic assessment of the submandibular space. Clin Radiol. 2004;59(12):1070-1078. doi:10.1016/j.crad.2004.06.025.
Case
A 21-year-old woman presented to the ED with pain and swelling on the right side of her neck. She stated the pain started earlier that morning and worsened when she ate or swallowed. The patient denied a recent or remote history of drooling, voice changes, or neck swelling. She reported no fevers, chills, or any other complaints, and had no pertinent medical history—specifically, no history of recent dental work. Her surgical history included tonsillectomy and cholecystectomy. There was no family history of diabetes, thyroid disease, autoimmune disease, or any other diseases. The patient stated that she was not on any prescription or over-the-counter medications. Regarding her social history, she denied past or cu
Vital signs at presentation were: blood pressure, 124/63 mm Hg (sitting); heart rate, 73 beats/min; respiratory rate, 15 breaths/min; and temperature, 98°F. Oxygen saturation was 99% on room air. On clinical examination, pain was noted in the patient’s right submandibular area and was tender to palpation. The swelling extended to the angle of the mandible posteriorly (Figure 1a and 1b). There was no erythema or increased surface temperature to suggest overlying cellulitis. The oral examination showed no evidence of dental infection, angioedema, or Ludwig angina. The pharynx was normal in appearance. The otological examination was unremarkable, and there was no evidence of mastoiditis.
Laboratory evaluation included a complete blood count (CBC), basic metabolic profile (BMP), and rapid streptococcal test (RST). The results of the patient’s CBC revealed a white blood cell count (WBC) of 11.1 x 109/L; the BMP was unremarkable; and the RST was negative.
A soft tissue neck computed tomography (CT) scan with contrast was obtained, which revealed mild right submandibular gland enlargement with abnormal enhancement (Figure 2). Stranding was also noted in the right submandibular space along with thickening of the right platysma muscle, and few surrounding lymph nodes were prominent (Figure 3). The findings were consistent with acute submandibular sialadenitis.
The patient received intravenous (IV) normal saline for hydration and IV ketorolac for analgesia, as well as an initial dose of oral amoxicillin/clavulanate 875/125 mg. At discharge, she was given a 10-day course of oral amoxicillin/clavulanate 875/125 mg with instructions to follow-up with her primary care physician and otolaryngologist within 2 days. The patient did well on follow-up, and her symptoms resolved within a few days of discharge.
Discussion
Comparatively little has been published on acute submandibular sialadenitis over the past three decades, and much of that which is cited in the literature comes from a rather small pool of case reports.1 In a literature review, Raad et al1 noted, “Pertinent literature on [this] subject includes case reports but no studies describing the microbial and clinical characteristics of this disease.” Further, many of the published case reports describe neonatal presentations of submandibular sialadenitis, the incidence of which is rare in this patient population.2-4
Submandibular and Parotid Glands
The submandibular gland is the second largest salivary gland, the parotid gland being the largest. The duct of the salivary gland, the Wharton’s duct, opens under the tongue in the area of the lingual frenulum. Ductal obstruction is more frequently seen with the submandibular gland than with the parotid gland.1 The reason for this is unclear, but may be related to several factors. One factor may be that, unlike the Stenson’s duct of the parotid gland, the Wharton’s duct does not pass through a muscle; thus, there is no muscular massage supporting the movement of secretion, as there is with buccinator muscle massage of Stenson’s duct. In addition, submandibular saliva is more viscous than parotid saliva due to its higher protein content and higher concentration of calcium phosphate.1
Etiology
Submandibular gland obstruction can occur in the absence of infection. Noninfectious cases typically present with pain upon eating and swallowing. A bacterial infectious etiology is associated with odynophagia, but also includes persistent pain and tenderness. This presents as pain associated with eating. Bacterial infection of the submandibular gland adds the element of persistent pain, associated with such features as tenderness. In addition, purulent discharge from the Wharton’s duct may be present in infectious cases, and accompanied by fever, chills, and an elevated WBC.1
Several bacteria have been isolated in infectious submandibular sialadenitis, the most common pathogens being Staphylococcus aureus. However, streptococci, Pseudomonas aeruginosa, Moraxella catarrhalis, and Escherichia coli bacteria have also been identified in cases of infectious submandibular sialadenitis.5
Viral etiologies of sialadenitis, such as mumps, are generally bilateral and nonsuppurative. The human immunodeficiency virus can also cause bilateral nonsuppurative salivary gland infections.6
Imaging Studies
As illustrated in our case, CT imaging can assist in confirming the diagnosis of acute submandibular sialadenitis by defining the anatomic involvement and identifying the presence of an abscess. Ultrasound can also be used and has been described as a first-line imaging procedure.7,8
Treatment
Surgical Intervention. Abscesses may require surgical intervention. However, most cases without abscess formation respond to outpatient treatment with antibiotics.5 If ductal obstruction is identified, removal of the calculus may be needed. This may involve ductal dilation, sialolithectomy, or even ductoplasty if a stricture is identified.1
Antibiotic Therapy. With respect to antibiotic selection, Chandak et al5 recommend oral amoxicillin-clavulanic acid. Other antistaphylococcal coverage recommendations have been made in the literature. Gland massage may be helpful after the tenderness has resolved,5 and sialogogues (eg, lemon drops, vitamin C lozenges) can also provide some relief.6 In addition, to avoid disease recurrence and prevent dental complications, Chandak et al5 emphasize the crucial role of hydration and excellent oral hygiene.
Conclusion
We suspected acute submandibular sialadenitis in our patient based on clinical findings, which were confirmed on CT imaging. Patients with acute submandibular sialadenitis may present with submandibular gland obstruction in the absence of bacterial infection. Noninfectious obstruction typically presents as pain associated with eating and swallowing, whereas infectious cases include constant pain and tenderness in the affected area. In addition, patients with infectious etiology may also have purulent discharge from Wharton’s duct, fever, chills, and an elevated WBC. Several bacteria have been isolated, the most common being S aureus. However, streptococci, P aeruginosa, M catarrhalis and E coli have also been identified. Computed tomography studies are helpful in confirming the diagnosis, defining anatomical involvement, and in identifying abscess formation.
Abscesses may require surgical intervention. However, most cases without abscess formation respond to outpatient treatment with antibiotics. Antibiotic selection involves antistaphylococcal coverage, such as amoxicillin-clavulanic acid. Glandular massage may be helpful after the tenderness has resolved. In addition, the literature emphasizes the crucial role of hydration and excellent oral hygiene in disease recurrence and to prevent dental complications.
Case
A 21-year-old woman presented to the ED with pain and swelling on the right side of her neck. She stated the pain started earlier that morning and worsened when she ate or swallowed. The patient denied a recent or remote history of drooling, voice changes, or neck swelling. She reported no fevers, chills, or any other complaints, and had no pertinent medical history—specifically, no history of recent dental work. Her surgical history included tonsillectomy and cholecystectomy. There was no family history of diabetes, thyroid disease, autoimmune disease, or any other diseases. The patient stated that she was not on any prescription or over-the-counter medications. Regarding her social history, she denied past or cu
Vital signs at presentation were: blood pressure, 124/63 mm Hg (sitting); heart rate, 73 beats/min; respiratory rate, 15 breaths/min; and temperature, 98°F. Oxygen saturation was 99% on room air. On clinical examination, pain was noted in the patient’s right submandibular area and was tender to palpation. The swelling extended to the angle of the mandible posteriorly (Figure 1a and 1b). There was no erythema or increased surface temperature to suggest overlying cellulitis. The oral examination showed no evidence of dental infection, angioedema, or Ludwig angina. The pharynx was normal in appearance. The otological examination was unremarkable, and there was no evidence of mastoiditis.
Laboratory evaluation included a complete blood count (CBC), basic metabolic profile (BMP), and rapid streptococcal test (RST). The results of the patient’s CBC revealed a white blood cell count (WBC) of 11.1 x 109/L; the BMP was unremarkable; and the RST was negative.
A soft tissue neck computed tomography (CT) scan with contrast was obtained, which revealed mild right submandibular gland enlargement with abnormal enhancement (Figure 2). Stranding was also noted in the right submandibular space along with thickening of the right platysma muscle, and few surrounding lymph nodes were prominent (Figure 3). The findings were consistent with acute submandibular sialadenitis.
The patient received intravenous (IV) normal saline for hydration and IV ketorolac for analgesia, as well as an initial dose of oral amoxicillin/clavulanate 875/125 mg. At discharge, she was given a 10-day course of oral amoxicillin/clavulanate 875/125 mg with instructions to follow-up with her primary care physician and otolaryngologist within 2 days. The patient did well on follow-up, and her symptoms resolved within a few days of discharge.
Discussion
Comparatively little has been published on acute submandibular sialadenitis over the past three decades, and much of that which is cited in the literature comes from a rather small pool of case reports.1 In a literature review, Raad et al1 noted, “Pertinent literature on [this] subject includes case reports but no studies describing the microbial and clinical characteristics of this disease.” Further, many of the published case reports describe neonatal presentations of submandibular sialadenitis, the incidence of which is rare in this patient population.2-4
Submandibular and Parotid Glands
The submandibular gland is the second largest salivary gland, the parotid gland being the largest. The duct of the salivary gland, the Wharton’s duct, opens under the tongue in the area of the lingual frenulum. Ductal obstruction is more frequently seen with the submandibular gland than with the parotid gland.1 The reason for this is unclear, but may be related to several factors. One factor may be that, unlike the Stenson’s duct of the parotid gland, the Wharton’s duct does not pass through a muscle; thus, there is no muscular massage supporting the movement of secretion, as there is with buccinator muscle massage of Stenson’s duct. In addition, submandibular saliva is more viscous than parotid saliva due to its higher protein content and higher concentration of calcium phosphate.1
Etiology
Submandibular gland obstruction can occur in the absence of infection. Noninfectious cases typically present with pain upon eating and swallowing. A bacterial infectious etiology is associated with odynophagia, but also includes persistent pain and tenderness. This presents as pain associated with eating. Bacterial infection of the submandibular gland adds the element of persistent pain, associated with such features as tenderness. In addition, purulent discharge from the Wharton’s duct may be present in infectious cases, and accompanied by fever, chills, and an elevated WBC.1
Several bacteria have been isolated in infectious submandibular sialadenitis, the most common pathogens being Staphylococcus aureus. However, streptococci, Pseudomonas aeruginosa, Moraxella catarrhalis, and Escherichia coli bacteria have also been identified in cases of infectious submandibular sialadenitis.5
Viral etiologies of sialadenitis, such as mumps, are generally bilateral and nonsuppurative. The human immunodeficiency virus can also cause bilateral nonsuppurative salivary gland infections.6
Imaging Studies
As illustrated in our case, CT imaging can assist in confirming the diagnosis of acute submandibular sialadenitis by defining the anatomic involvement and identifying the presence of an abscess. Ultrasound can also be used and has been described as a first-line imaging procedure.7,8
Treatment
Surgical Intervention. Abscesses may require surgical intervention. However, most cases without abscess formation respond to outpatient treatment with antibiotics.5 If ductal obstruction is identified, removal of the calculus may be needed. This may involve ductal dilation, sialolithectomy, or even ductoplasty if a stricture is identified.1
Antibiotic Therapy. With respect to antibiotic selection, Chandak et al5 recommend oral amoxicillin-clavulanic acid. Other antistaphylococcal coverage recommendations have been made in the literature. Gland massage may be helpful after the tenderness has resolved,5 and sialogogues (eg, lemon drops, vitamin C lozenges) can also provide some relief.6 In addition, to avoid disease recurrence and prevent dental complications, Chandak et al5 emphasize the crucial role of hydration and excellent oral hygiene.
Conclusion
We suspected acute submandibular sialadenitis in our patient based on clinical findings, which were confirmed on CT imaging. Patients with acute submandibular sialadenitis may present with submandibular gland obstruction in the absence of bacterial infection. Noninfectious obstruction typically presents as pain associated with eating and swallowing, whereas infectious cases include constant pain and tenderness in the affected area. In addition, patients with infectious etiology may also have purulent discharge from Wharton’s duct, fever, chills, and an elevated WBC. Several bacteria have been isolated, the most common being S aureus. However, streptococci, P aeruginosa, M catarrhalis and E coli have also been identified. Computed tomography studies are helpful in confirming the diagnosis, defining anatomical involvement, and in identifying abscess formation.
Abscesses may require surgical intervention. However, most cases without abscess formation respond to outpatient treatment with antibiotics. Antibiotic selection involves antistaphylococcal coverage, such as amoxicillin-clavulanic acid. Glandular massage may be helpful after the tenderness has resolved. In addition, the literature emphasizes the crucial role of hydration and excellent oral hygiene in disease recurrence and to prevent dental complications.
1. Raad II, Sabbagh MF, Caranasos GJ. Acute bacterial sialadenitis: a study of 29 cases and review. Rev Infect Dis. 1990;12(4):591-601.
2. Banks WW, Handler SD, Glade GB, Turner HD. Neonatal submandibular sialadenitis. Am J Otolaryngol. 1980;1(3):261-263.
3. Wells DH. Suppuration of the submandibular salivary glands in the neonate. Am J Dis Child. 1975;129(5):628-630.
4. Ryan RF, Padmakumar B. Neonatal suppurative sialadenitis: an important clinical diagnosis. BMJ Case Rep. 2015;2015. pii:bcr2014208535. doi:10.1136/bcr-2014-208535.
5. Chandak R, Degwekar S, Chandak M, Rawlani S. Acute submandibular sialadenitis—a case report. Case Rep Dent. 2012;2012:615375. doi:10.1155/2012/615375.
6. Wilson KF, Meier JD, Ward PD. Salivary gland disorders. Am Fam Physician. 2014;89(11):882-888.
7. Alyas F, Lewis K, Williams M, et al. Diseases of the submandibular gland as demonstrated using high resolution ultrasound. Br J Radiol. 2005;78(928):362-369. doi:10.1259/bjr/93120352.
8. Howlett DC, Alyas F, Wong KT, et al. Sonographic assessment of the submandibular space. Clin Radiol. 2004;59(12):1070-1078. doi:10.1016/j.crad.2004.06.025.
1. Raad II, Sabbagh MF, Caranasos GJ. Acute bacterial sialadenitis: a study of 29 cases and review. Rev Infect Dis. 1990;12(4):591-601.
2. Banks WW, Handler SD, Glade GB, Turner HD. Neonatal submandibular sialadenitis. Am J Otolaryngol. 1980;1(3):261-263.
3. Wells DH. Suppuration of the submandibular salivary glands in the neonate. Am J Dis Child. 1975;129(5):628-630.
4. Ryan RF, Padmakumar B. Neonatal suppurative sialadenitis: an important clinical diagnosis. BMJ Case Rep. 2015;2015. pii:bcr2014208535. doi:10.1136/bcr-2014-208535.
5. Chandak R, Degwekar S, Chandak M, Rawlani S. Acute submandibular sialadenitis—a case report. Case Rep Dent. 2012;2012:615375. doi:10.1155/2012/615375.
6. Wilson KF, Meier JD, Ward PD. Salivary gland disorders. Am Fam Physician. 2014;89(11):882-888.
7. Alyas F, Lewis K, Williams M, et al. Diseases of the submandibular gland as demonstrated using high resolution ultrasound. Br J Radiol. 2005;78(928):362-369. doi:10.1259/bjr/93120352.
8. Howlett DC, Alyas F, Wong KT, et al. Sonographic assessment of the submandibular space. Clin Radiol. 2004;59(12):1070-1078. doi:10.1016/j.crad.2004.06.025.