User login
Myelodysplastic Syndromes
Myelodysplastic syndromes (MDS) are a spectrum of clonal myeloid disorders characterized by ineffective hematopoiesis, cytopenias, qualitative disorders of blood cells, clonal chromosomal abnormalities, and the potential for clonal evolution to acute myeloid leukemia (AML). In this review, we discuss the various pathogenic conditions included in the spectrum of MDS and the associated risk stratification for these conditions. We further discuss the treatment recommendations based on the risk status and the expected prognosis.
To read the full article in PDF:
Myelodysplastic syndromes (MDS) are a spectrum of clonal myeloid disorders characterized by ineffective hematopoiesis, cytopenias, qualitative disorders of blood cells, clonal chromosomal abnormalities, and the potential for clonal evolution to acute myeloid leukemia (AML). In this review, we discuss the various pathogenic conditions included in the spectrum of MDS and the associated risk stratification for these conditions. We further discuss the treatment recommendations based on the risk status and the expected prognosis.
To read the full article in PDF:
Myelodysplastic syndromes (MDS) are a spectrum of clonal myeloid disorders characterized by ineffective hematopoiesis, cytopenias, qualitative disorders of blood cells, clonal chromosomal abnormalities, and the potential for clonal evolution to acute myeloid leukemia (AML). In this review, we discuss the various pathogenic conditions included in the spectrum of MDS and the associated risk stratification for these conditions. We further discuss the treatment recommendations based on the risk status and the expected prognosis.
To read the full article in PDF:
Primary Brain Tumors
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Primary central nervous system tumors are relatively rare, but they can cause significant morbidity. They are also among the most lethal of all neoplasms. Brain tumors are the second most common cause of death due to intracranial disease, second only to stroke. The estimated annual incidence of primary brain tumors is approximately 21 per 100,000 individuals in the United States. The incidence of brain tumors varies by gender, age, race, ethnicity, and geography and has increased over time. Gliomas and germ cell tumors are more common in men, whereas meningiomas are twice as common in women. The only validated environmental risk factor for primary brain tumors is exposure to ionizing radiation.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Primary central nervous system tumors are relatively rare, but they can cause significant morbidity. They are also among the most lethal of all neoplasms. Brain tumors are the second most common cause of death due to intracranial disease, second only to stroke. The estimated annual incidence of primary brain tumors is approximately 21 per 100,000 individuals in the United States. The incidence of brain tumors varies by gender, age, race, ethnicity, and geography and has increased over time. Gliomas and germ cell tumors are more common in men, whereas meningiomas are twice as common in women. The only validated environmental risk factor for primary brain tumors is exposure to ionizing radiation.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Primary central nervous system tumors are relatively rare, but they can cause significant morbidity. They are also among the most lethal of all neoplasms. Brain tumors are the second most common cause of death due to intracranial disease, second only to stroke. The estimated annual incidence of primary brain tumors is approximately 21 per 100,000 individuals in the United States. The incidence of brain tumors varies by gender, age, race, ethnicity, and geography and has increased over time. Gliomas and germ cell tumors are more common in men, whereas meningiomas are twice as common in women. The only validated environmental risk factor for primary brain tumors is exposure to ionizing radiation.
To read the full article in PDF:
Woman With Blue-Gray Palate and Nail Beds
A 62-year-old African-American woman presented for evaluation of a bluish discoloration of the hard palate and nail beds, noticeable for several months. In addition, she had complaints of fatigue and arthralgia. She reported that she had been taking hydroxychloroquine 400 mg/d and quinacrine 100 mg/d for several years for the treatment of systemic lupus erythematosus (SLE). Her medical history was also significant for dry mouth syndrome treated with pilocarpine.
The patient’s vital signs included a temperature of 97°F;
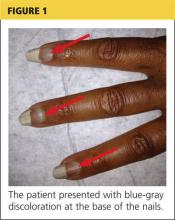
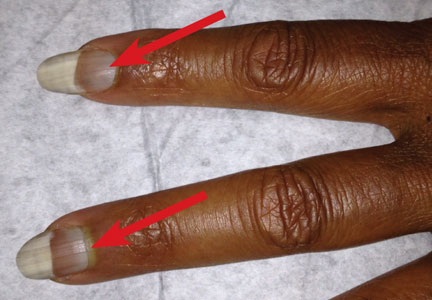
respiratory rate, 15 breaths/min; pulse, 72 beats/min; and blood pressure, 130/80 mm Hg. Height was 62 in, weight was 189 lb, and BMI was 34.56. A bluish gray color was noted in the subungual areas of her nails (see Figure 1). There were several circumferential areas of skin hyperpigmentation resulting from healed lupus skin lesions on her arms. Nailfold capillaroscopy revealed several dilated blood vessels. The sclerae appeared dry, but no erythema or inflammation was noted.
Examination of the mouth revealed a bluish discoloration of the hard palate (see Figure 2) and decreased salivary pool. Respiratory, cardiovascular, and abdominal examination findings were normal. Musculoskeletal examination was unremarkable for acute joint tenderness or synovitis. Crepitation and bony changes were noted in the left knee, without effusion or decreased range of motion.
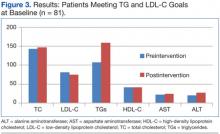
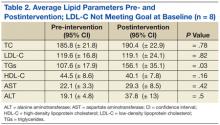
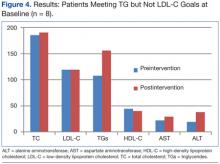
Laboratory studies were ordered, and the results are listed in the table.
DISCUSSION
Hyperpigmentation of the oral mucosa can be associated with a number of conditions, including adrenal insufficiency, Peutz-Jeghers syndrome, hemochromatosis, polyostotic fibrous dysplasia, hyperparathyroidism, neurofibromatosis, and bronchogenic malignancy.1,2 Other causes of oral hyperpigmentation include physiologic pigmentary or postinflammatory changes, oral melanoacanthosis, blue nevus, and melanoma.2,3 While these diagnoses should be considered when encountering a mucosal lesion, they were unlikely in this patient because of the color changes in her nail beds.
Systemic skin and mucous membrane discoloration can also occur with the use of certain drugs and other substances, including chemotherapeutic agents, benzodiazepines, hormones, carotenoids, phenolphthalein, heavy metal salts, and several antimicrobial agents.1 In dark-skinned individuals, hyperpigmentation of the oral mucosa can be caused by a physiologic deposition of melanin.4
Pigmentary Changes
The use of antimalarial drugs, such as quinacrine, chloroquine, and hydroxychloroquine, has long been associated with pigmentary changes to the palatal mucosa and subungual areas.1,3 These drugs can stimulate melanin production and cause hemosiderin deposition, resulting in pigmentary changes.5 Skin discoloration is believed to be the result of the formation of a melanin-drug complex in areas with an elevated affinity for melanin.1 Besides malaria, these drugs are commonly used to treat SLE and discoid lupus erythematosus, rheumatoid arthritis, and other rheumatologic conditions.5
The diagnosis of drug-induced hyperpigmentation is generally clinical, supported by the patient’s history—which often includes the use of antimalarial drugs—and presentation.1 If a clear cause cannot be determined by clinical evaluation, then a biopsy to confirm a drug-induced cause may be necessary.2 A classic study by Tuffanelli et al reported that the onset of hyperpigmentation related to antimalarial drug therapy may not occur until 4 to 70 months after initiation of treatment.6 Once the offending drug is discontinued, pigmentation changes slowly fade but often do not completely resolve,7 and patients should be advised of this.
Ocular Retinopathy
While pigmentary changes associated with antimalarial drugs are benign,3 a rare but serious adverse effect of antimalarials is retinal toxicity. Ocular retinopathy related to chloroquine and hydroxychloroquine therapy has been well documented and may result in irreversible vision loss.8,9 The most recent recommendations from the American Academy of Ophthalmology suggest a baseline eye examination at initiation of antimalarial treatment and annual examinations starting after five years of therapy because the risk for toxicity relates to the cumulative dose.8 More frequent ophthalmologic evaluations are recommended for individuals at higher risk, such as those with preexisting retinal or macular disease.9
Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
A biopsy of the roof of the patient’s mouth confirmed that the palatal hyperpigmentation was caused by her antimalarial medications. Since the patient displayed no evidence of active lupus skin lesions and laboratory results indicated that her SLE was inactive, one of the drugs, quinacrine, was discontinued.
The patient was referred for an ophthalmologic evaluation. No evidence of retinal toxicity was found.
Follow-up evaluations at two months and six months revealed no significant improvement in the discoloration of the patient’s oral mucosa or nail beds. At the six-month visit, her dosage of hydroxychloroquine was reevaluated.
The patient’s hydroxychloroquine dosage was determined based on 7.3 mg/kg/d. In the case of an overweight patient, especially one of shorter-than-average stature, hydroxychloroquine dosing should be based on ideal body weight to minimize the risk for overdosage; in general, a maximum dosage of 6.5 mg/kg/d is recommended.8,9 As a result, the patient’s dosage was decreased to 300 mg/d.
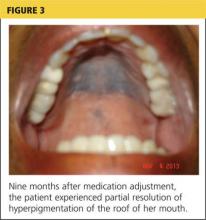
At her nine-month follow-up evaluation, the discoloration to the patient’s oral mucosa had faded but had not resolved completely (see Figure 3). No significant change was noted in the subungual discoloration. The patient had experienced no exacerbations of lupus-related symptoms since her medication adjustments.
CONCLUSION
Although this patient’s hyperpigmentation was benign, staying alert to this potential adverse effect of antimalarial drugs is important in making a diagnosis. As with many skin lesions, if the clinical evaluation does not provide a clear cause, a biopsy may be needed. For anyone taking antimalarial drugs, regular ophthalmologic evaluations are recommended to facilitate early detection of the rare adverse effect of retinal toxicity. Nevertheless, with careful monitoring, antimalarial drugs are safe and effective for the treatment of inflammatory conditions such as SLE and rheumatoid arthritis.
REFERENCES
1. Kleinegger CL, Hammond HL, Finkelstein MW. Oral mucosal hyperpigmentation secondary to antimalarial drug therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(2):189-194.
2. Gondak R-O, da Silva-Jorge R, Jorge J, et al. Oral pigmented lesions: clinicopathologic features and review of the literature. Med Oral Pathol Oral Cir Bucal. 2012;17(6):e919-e924.
3. Lerman MA, Karimbux N, Guze KA, Woo SB. Pigmentation of the hard palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;
107:8-12.
4. Kalampalikis A, Goetze S, Elsner P. Isolated hyperpigmentation of the oral mucosa due to hydroxychloroquine. J Dtsch Dermatol Ges. 2012; 10(12):921-922.
5. de Andrade BA, Fonseca FP, Pires FR, et al. Hard palate hyperpigmentation secondary to chronic chloroquine therapy: report of five cases.
J Cutan Pathol. 2013;40(9):833-838.
6. Tuffanelli D, Abraham RK, Dubois EI. Pigmentation from antimalarial therapy: its possible relationship to the ocular lesions. Arch Derm. 1963; 88:419-426.
7. Melikoglu MA, Melikoglu M, Gurbuz U, et al. Hydroxychloroquine-induced hyperpigmentation: a case report. J Clin Pharm Ther. 2008; 33(6):699-701.
8. Marmor MF, Kellner U, Lai YY, et al; American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118(2):
415-422.
9. Screening for hydroxychloroquine retinopathy. Position statement, American College of Rheumatology. www.rheumatology.org/Practice/Clinical/Position/Position_Statements/. Accessed July 17, 2014.
A 62-year-old African-American woman presented for evaluation of a bluish discoloration of the hard palate and nail beds, noticeable for several months. In addition, she had complaints of fatigue and arthralgia. She reported that she had been taking hydroxychloroquine 400 mg/d and quinacrine 100 mg/d for several years for the treatment of systemic lupus erythematosus (SLE). Her medical history was also significant for dry mouth syndrome treated with pilocarpine.
The patient’s vital signs included a temperature of 97°F;
respiratory rate, 15 breaths/min; pulse, 72 beats/min; and blood pressure, 130/80 mm Hg. Height was 62 in, weight was 189 lb, and BMI was 34.56. A bluish gray color was noted in the subungual areas of her nails (see Figure 1). There were several circumferential areas of skin hyperpigmentation resulting from healed lupus skin lesions on her arms. Nailfold capillaroscopy revealed several dilated blood vessels. The sclerae appeared dry, but no erythema or inflammation was noted.
Examination of the mouth revealed a bluish discoloration of the hard palate (see Figure 2) and decreased salivary pool. Respiratory, cardiovascular, and abdominal examination findings were normal. Musculoskeletal examination was unremarkable for acute joint tenderness or synovitis. Crepitation and bony changes were noted in the left knee, without effusion or decreased range of motion.
Laboratory studies were ordered, and the results are listed in the table.
DISCUSSION
Hyperpigmentation of the oral mucosa can be associated with a number of conditions, including adrenal insufficiency, Peutz-Jeghers syndrome, hemochromatosis, polyostotic fibrous dysplasia, hyperparathyroidism, neurofibromatosis, and bronchogenic malignancy.1,2 Other causes of oral hyperpigmentation include physiologic pigmentary or postinflammatory changes, oral melanoacanthosis, blue nevus, and melanoma.2,3 While these diagnoses should be considered when encountering a mucosal lesion, they were unlikely in this patient because of the color changes in her nail beds.
Systemic skin and mucous membrane discoloration can also occur with the use of certain drugs and other substances, including chemotherapeutic agents, benzodiazepines, hormones, carotenoids, phenolphthalein, heavy metal salts, and several antimicrobial agents.1 In dark-skinned individuals, hyperpigmentation of the oral mucosa can be caused by a physiologic deposition of melanin.4
Pigmentary Changes
The use of antimalarial drugs, such as quinacrine, chloroquine, and hydroxychloroquine, has long been associated with pigmentary changes to the palatal mucosa and subungual areas.1,3 These drugs can stimulate melanin production and cause hemosiderin deposition, resulting in pigmentary changes.5 Skin discoloration is believed to be the result of the formation of a melanin-drug complex in areas with an elevated affinity for melanin.1 Besides malaria, these drugs are commonly used to treat SLE and discoid lupus erythematosus, rheumatoid arthritis, and other rheumatologic conditions.5
The diagnosis of drug-induced hyperpigmentation is generally clinical, supported by the patient’s history—which often includes the use of antimalarial drugs—and presentation.1 If a clear cause cannot be determined by clinical evaluation, then a biopsy to confirm a drug-induced cause may be necessary.2 A classic study by Tuffanelli et al reported that the onset of hyperpigmentation related to antimalarial drug therapy may not occur until 4 to 70 months after initiation of treatment.6 Once the offending drug is discontinued, pigmentation changes slowly fade but often do not completely resolve,7 and patients should be advised of this.
Ocular Retinopathy
While pigmentary changes associated with antimalarial drugs are benign,3 a rare but serious adverse effect of antimalarials is retinal toxicity. Ocular retinopathy related to chloroquine and hydroxychloroquine therapy has been well documented and may result in irreversible vision loss.8,9 The most recent recommendations from the American Academy of Ophthalmology suggest a baseline eye examination at initiation of antimalarial treatment and annual examinations starting after five years of therapy because the risk for toxicity relates to the cumulative dose.8 More frequent ophthalmologic evaluations are recommended for individuals at higher risk, such as those with preexisting retinal or macular disease.9
Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
A biopsy of the roof of the patient’s mouth confirmed that the palatal hyperpigmentation was caused by her antimalarial medications. Since the patient displayed no evidence of active lupus skin lesions and laboratory results indicated that her SLE was inactive, one of the drugs, quinacrine, was discontinued.
The patient was referred for an ophthalmologic evaluation. No evidence of retinal toxicity was found.
Follow-up evaluations at two months and six months revealed no significant improvement in the discoloration of the patient’s oral mucosa or nail beds. At the six-month visit, her dosage of hydroxychloroquine was reevaluated.
The patient’s hydroxychloroquine dosage was determined based on 7.3 mg/kg/d. In the case of an overweight patient, especially one of shorter-than-average stature, hydroxychloroquine dosing should be based on ideal body weight to minimize the risk for overdosage; in general, a maximum dosage of 6.5 mg/kg/d is recommended.8,9 As a result, the patient’s dosage was decreased to 300 mg/d.
At her nine-month follow-up evaluation, the discoloration to the patient’s oral mucosa had faded but had not resolved completely (see Figure 3). No significant change was noted in the subungual discoloration. The patient had experienced no exacerbations of lupus-related symptoms since her medication adjustments.
CONCLUSION
Although this patient’s hyperpigmentation was benign, staying alert to this potential adverse effect of antimalarial drugs is important in making a diagnosis. As with many skin lesions, if the clinical evaluation does not provide a clear cause, a biopsy may be needed. For anyone taking antimalarial drugs, regular ophthalmologic evaluations are recommended to facilitate early detection of the rare adverse effect of retinal toxicity. Nevertheless, with careful monitoring, antimalarial drugs are safe and effective for the treatment of inflammatory conditions such as SLE and rheumatoid arthritis.
REFERENCES
1. Kleinegger CL, Hammond HL, Finkelstein MW. Oral mucosal hyperpigmentation secondary to antimalarial drug therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(2):189-194.
2. Gondak R-O, da Silva-Jorge R, Jorge J, et al. Oral pigmented lesions: clinicopathologic features and review of the literature. Med Oral Pathol Oral Cir Bucal. 2012;17(6):e919-e924.
3. Lerman MA, Karimbux N, Guze KA, Woo SB. Pigmentation of the hard palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;
107:8-12.
4. Kalampalikis A, Goetze S, Elsner P. Isolated hyperpigmentation of the oral mucosa due to hydroxychloroquine. J Dtsch Dermatol Ges. 2012; 10(12):921-922.
5. de Andrade BA, Fonseca FP, Pires FR, et al. Hard palate hyperpigmentation secondary to chronic chloroquine therapy: report of five cases.
J Cutan Pathol. 2013;40(9):833-838.
6. Tuffanelli D, Abraham RK, Dubois EI. Pigmentation from antimalarial therapy: its possible relationship to the ocular lesions. Arch Derm. 1963; 88:419-426.
7. Melikoglu MA, Melikoglu M, Gurbuz U, et al. Hydroxychloroquine-induced hyperpigmentation: a case report. J Clin Pharm Ther. 2008; 33(6):699-701.
8. Marmor MF, Kellner U, Lai YY, et al; American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118(2):
415-422.
9. Screening for hydroxychloroquine retinopathy. Position statement, American College of Rheumatology. www.rheumatology.org/Practice/Clinical/Position/Position_Statements/. Accessed July 17, 2014.
A 62-year-old African-American woman presented for evaluation of a bluish discoloration of the hard palate and nail beds, noticeable for several months. In addition, she had complaints of fatigue and arthralgia. She reported that she had been taking hydroxychloroquine 400 mg/d and quinacrine 100 mg/d for several years for the treatment of systemic lupus erythematosus (SLE). Her medical history was also significant for dry mouth syndrome treated with pilocarpine.
The patient’s vital signs included a temperature of 97°F;
respiratory rate, 15 breaths/min; pulse, 72 beats/min; and blood pressure, 130/80 mm Hg. Height was 62 in, weight was 189 lb, and BMI was 34.56. A bluish gray color was noted in the subungual areas of her nails (see Figure 1). There were several circumferential areas of skin hyperpigmentation resulting from healed lupus skin lesions on her arms. Nailfold capillaroscopy revealed several dilated blood vessels. The sclerae appeared dry, but no erythema or inflammation was noted.
Examination of the mouth revealed a bluish discoloration of the hard palate (see Figure 2) and decreased salivary pool. Respiratory, cardiovascular, and abdominal examination findings were normal. Musculoskeletal examination was unremarkable for acute joint tenderness or synovitis. Crepitation and bony changes were noted in the left knee, without effusion or decreased range of motion.
Laboratory studies were ordered, and the results are listed in the table.
DISCUSSION
Hyperpigmentation of the oral mucosa can be associated with a number of conditions, including adrenal insufficiency, Peutz-Jeghers syndrome, hemochromatosis, polyostotic fibrous dysplasia, hyperparathyroidism, neurofibromatosis, and bronchogenic malignancy.1,2 Other causes of oral hyperpigmentation include physiologic pigmentary or postinflammatory changes, oral melanoacanthosis, blue nevus, and melanoma.2,3 While these diagnoses should be considered when encountering a mucosal lesion, they were unlikely in this patient because of the color changes in her nail beds.
Systemic skin and mucous membrane discoloration can also occur with the use of certain drugs and other substances, including chemotherapeutic agents, benzodiazepines, hormones, carotenoids, phenolphthalein, heavy metal salts, and several antimicrobial agents.1 In dark-skinned individuals, hyperpigmentation of the oral mucosa can be caused by a physiologic deposition of melanin.4
Pigmentary Changes
The use of antimalarial drugs, such as quinacrine, chloroquine, and hydroxychloroquine, has long been associated with pigmentary changes to the palatal mucosa and subungual areas.1,3 These drugs can stimulate melanin production and cause hemosiderin deposition, resulting in pigmentary changes.5 Skin discoloration is believed to be the result of the formation of a melanin-drug complex in areas with an elevated affinity for melanin.1 Besides malaria, these drugs are commonly used to treat SLE and discoid lupus erythematosus, rheumatoid arthritis, and other rheumatologic conditions.5
The diagnosis of drug-induced hyperpigmentation is generally clinical, supported by the patient’s history—which often includes the use of antimalarial drugs—and presentation.1 If a clear cause cannot be determined by clinical evaluation, then a biopsy to confirm a drug-induced cause may be necessary.2 A classic study by Tuffanelli et al reported that the onset of hyperpigmentation related to antimalarial drug therapy may not occur until 4 to 70 months after initiation of treatment.6 Once the offending drug is discontinued, pigmentation changes slowly fade but often do not completely resolve,7 and patients should be advised of this.
Ocular Retinopathy
While pigmentary changes associated with antimalarial drugs are benign,3 a rare but serious adverse effect of antimalarials is retinal toxicity. Ocular retinopathy related to chloroquine and hydroxychloroquine therapy has been well documented and may result in irreversible vision loss.8,9 The most recent recommendations from the American Academy of Ophthalmology suggest a baseline eye examination at initiation of antimalarial treatment and annual examinations starting after five years of therapy because the risk for toxicity relates to the cumulative dose.8 More frequent ophthalmologic evaluations are recommended for individuals at higher risk, such as those with preexisting retinal or macular disease.9
Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
A biopsy of the roof of the patient’s mouth confirmed that the palatal hyperpigmentation was caused by her antimalarial medications. Since the patient displayed no evidence of active lupus skin lesions and laboratory results indicated that her SLE was inactive, one of the drugs, quinacrine, was discontinued.
The patient was referred for an ophthalmologic evaluation. No evidence of retinal toxicity was found.
Follow-up evaluations at two months and six months revealed no significant improvement in the discoloration of the patient’s oral mucosa or nail beds. At the six-month visit, her dosage of hydroxychloroquine was reevaluated.
The patient’s hydroxychloroquine dosage was determined based on 7.3 mg/kg/d. In the case of an overweight patient, especially one of shorter-than-average stature, hydroxychloroquine dosing should be based on ideal body weight to minimize the risk for overdosage; in general, a maximum dosage of 6.5 mg/kg/d is recommended.8,9 As a result, the patient’s dosage was decreased to 300 mg/d.
At her nine-month follow-up evaluation, the discoloration to the patient’s oral mucosa had faded but had not resolved completely (see Figure 3). No significant change was noted in the subungual discoloration. The patient had experienced no exacerbations of lupus-related symptoms since her medication adjustments.
CONCLUSION
Although this patient’s hyperpigmentation was benign, staying alert to this potential adverse effect of antimalarial drugs is important in making a diagnosis. As with many skin lesions, if the clinical evaluation does not provide a clear cause, a biopsy may be needed. For anyone taking antimalarial drugs, regular ophthalmologic evaluations are recommended to facilitate early detection of the rare adverse effect of retinal toxicity. Nevertheless, with careful monitoring, antimalarial drugs are safe and effective for the treatment of inflammatory conditions such as SLE and rheumatoid arthritis.
REFERENCES
1. Kleinegger CL, Hammond HL, Finkelstein MW. Oral mucosal hyperpigmentation secondary to antimalarial drug therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(2):189-194.
2. Gondak R-O, da Silva-Jorge R, Jorge J, et al. Oral pigmented lesions: clinicopathologic features and review of the literature. Med Oral Pathol Oral Cir Bucal. 2012;17(6):e919-e924.
3. Lerman MA, Karimbux N, Guze KA, Woo SB. Pigmentation of the hard palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;
107:8-12.
4. Kalampalikis A, Goetze S, Elsner P. Isolated hyperpigmentation of the oral mucosa due to hydroxychloroquine. J Dtsch Dermatol Ges. 2012; 10(12):921-922.
5. de Andrade BA, Fonseca FP, Pires FR, et al. Hard palate hyperpigmentation secondary to chronic chloroquine therapy: report of five cases.
J Cutan Pathol. 2013;40(9):833-838.
6. Tuffanelli D, Abraham RK, Dubois EI. Pigmentation from antimalarial therapy: its possible relationship to the ocular lesions. Arch Derm. 1963; 88:419-426.
7. Melikoglu MA, Melikoglu M, Gurbuz U, et al. Hydroxychloroquine-induced hyperpigmentation: a case report. J Clin Pharm Ther. 2008; 33(6):699-701.
8. Marmor MF, Kellner U, Lai YY, et al; American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118(2):
415-422.
9. Screening for hydroxychloroquine retinopathy. Position statement, American College of Rheumatology. www.rheumatology.org/Practice/Clinical/Position/Position_Statements/. Accessed July 17, 2014.
Skip the Compression Stockings Following DVT
PRACTICE CHANGER
Do not recommend elastic compression stockings to decrease the incidence of postthrombotic syndrome after deep vein thrombosis.1
STRENGTH OF RECOMMENDATION
B: Based on a large randomized controlled trial1
ILLUSTRATIVE CASE
A 56-year-old man presents to your clinic three days after receiving a diagnosis of lower extremity deep vein thrombosis (DVT). He was prescribed warfarin (5 mg/d) with enoxaparin bridging (120 mg/d). He has read about postthrombotic syndrome (PTS) online and is very concerned about this possible adverse effect. He asks about using elastic compression stockings (ECS). What should you tell him?
PTS can be a frustrating, debilitating condition. Its clinical features range from minor limb swelling to severe edema and pain, irreversible skin changes, and leg ulcerations.2 It occurs in 25% to 50% of patients after DVT.3 Because current PTS treatments are not very effective, prevention is essential.4,5
Patients are frequently encouraged to wear ECS after DVT to reduce the incidence of PTS by decreasing venous hypertension and reflux. These stockings are expensive and uncomfortable. Prior research suggested that use of ECS can reduce PTS incidence by half, but the studies were small, single-center, and not placebo-controlled.6,7
On the next page: Study summary >>
STUDY SUMMARY
RCT sets aside a common practice
Kahn et al1 conducted a randomized, placebo-controlled trial of active versus placebo ECS in patients from 24 centers in the United States and Canada who’d had an ultrasound-confirmed proximal DVT (in the popliteal or more proximal deep leg vein) within the previous 14 days. Most patients received standard anticoagulation therapy to treat their DVT (five to 10 days of heparin and three to six months of warfarin). Patients were excluded if they had received thrombolytics, had arterial claudication, had a life expectancy of less than six months, were unable to put on ECS due to physical disabilities or allergy, or were unable to participate in follow-up visits.
Patients were randomly assigned to wear active (30 to 40 mm Hg graduated) ECS or identical-looking placebo ECS (< 5 mm Hg compression at the ankle) for two years. Providers, study personnel and statisticians, and patients were all blinded to treatment allocation. Patients were asked to wear the stocking on the affected leg each day from waking until bedtime.
Follow-up occurred at one, six, 12, 18, and 24 months. The primary outcome was cumulative incidence of PTS diagnosed at six months or later using the Ginsberg criteria of ipsilateral pain and swelling of at least one month’s duration.8 Secondary outcomes included severity of PTS, leg ulcers, recurrence of venous thromboembolism (VTE), death, adverse events, venous valvular reflux, and quality of life (QOL). Outcomes were measured objectively through use of a validated scale (the Villalta scale) for PTS severity and two questionnaires to assess QOL.9-11
There were 409 patients in the ECS group and 394 in the placebo group. Baseline characteristics, including BMI, VTE risk factors, and anticoagulation treatment regimens, were similar between groups. The average age of participants in the study group was 55.4 years and in the placebo group, 54.8 years. Men comprised 62.4% of the active group and 57.9% of the placebo group. Approximately 90% of the participants in both groups were white.
At one month, approximately 95% of participants in both groups used the stockings; at 24 months, that was reduced to a little less than 70%. The percentage of people who used the stockings for at least three days per week was similar in both groups.
The cumulative incidence of PTS during follow-up was 14.2% in the active group and 12.7% in the placebo group (hazard ratio, 1.13). There were no differences in any of the secondary outcomes. Prespecified subgroup analyses found that age, BMI, and severity of DVT had no effect on outcomes. There was a marginal benefit for ECS for women versus men, but this does not likely reflect a true difference because the confidence intervals surrounding the hazard ratios for men and women overlapped and crossed the null value.
On the next page: What's new & challenges to implementation >>
WHAT’S NEW
New evidence contradicts previous studies
Two prior studies showed that using 30 to 40 mm Hg ECS decreased the incidence of PTS after proximal DVT.6,7 However, these were smaller, open-label, single-center studies. This study by Kahn et al1 was the first placebo-controlled, randomized, multicenter study that used validated instruments to measure PTS and QOL. It found no benefit in using ECS, thus contradicting the results of the prior studies.
There are currently no guidelines or consensus statements that recommend for or against the use of ECS after DVT.
CAVEATS
High nonadherence rates might have affected results
In both groups, adherence to the assigned intervention diminished throughout the study (from 95% at one month to slightly less than 70% at two years). Theoretically, this could have affected efficacy outcomes. However, the decrease was similar in both groups and represents what is observed in clinical practice. A prespecified per protocol analysis of patients who wore their ECS more regularly found no benefit.
It is possible that a “placebo effect” could explain the lack of difference between groups. However, the placebo stockings provided virtually no compression, and the two-year cumulative incidence of PTS in both the treatment and placebo groups was similar to that seen in control groups in prior studies.6,7
Finally, the incidence of PTS in this study was much lower than the 25% to 50% incidence reported previously. Kahn et al1 suggested that this was because they used more stringent and standardized criteria for PTS than was used in previous research.
CHALLENGES TO IMPLEMENTATION
There are no barriers to ending this practice
We can identify no challenges to implementation of this recommendation.
On the next page: References >>
REFERENCES
1. Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
2. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698-707.
3. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996; 125:1-7.
4. Cohen JM, Akl EA, Kahn SR. Pharmacologic and compression therapies for postthrombotic syndrome: a systematic review of randomized controlled trials. Chest. 2012;141: 308-320.
5. Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. 2011;53:
500-509.
6. Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:
759-762.
7. Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249-256.
8. Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med. 2001;161:2105-2109.
9. Villalta S, Bagatella P, Piccioli A, et al. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24:158a.
10. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247-263.
11. Kahn SR, Lamping DL, Ducruet T, et al; VETO Study Investigators. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006; 59:1049-1056.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(7):388-390.
PRACTICE CHANGER
Do not recommend elastic compression stockings to decrease the incidence of postthrombotic syndrome after deep vein thrombosis.1
STRENGTH OF RECOMMENDATION
B: Based on a large randomized controlled trial1
ILLUSTRATIVE CASE
A 56-year-old man presents to your clinic three days after receiving a diagnosis of lower extremity deep vein thrombosis (DVT). He was prescribed warfarin (5 mg/d) with enoxaparin bridging (120 mg/d). He has read about postthrombotic syndrome (PTS) online and is very concerned about this possible adverse effect. He asks about using elastic compression stockings (ECS). What should you tell him?
PTS can be a frustrating, debilitating condition. Its clinical features range from minor limb swelling to severe edema and pain, irreversible skin changes, and leg ulcerations.2 It occurs in 25% to 50% of patients after DVT.3 Because current PTS treatments are not very effective, prevention is essential.4,5
Patients are frequently encouraged to wear ECS after DVT to reduce the incidence of PTS by decreasing venous hypertension and reflux. These stockings are expensive and uncomfortable. Prior research suggested that use of ECS can reduce PTS incidence by half, but the studies were small, single-center, and not placebo-controlled.6,7
On the next page: Study summary >>
STUDY SUMMARY
RCT sets aside a common practice
Kahn et al1 conducted a randomized, placebo-controlled trial of active versus placebo ECS in patients from 24 centers in the United States and Canada who’d had an ultrasound-confirmed proximal DVT (in the popliteal or more proximal deep leg vein) within the previous 14 days. Most patients received standard anticoagulation therapy to treat their DVT (five to 10 days of heparin and three to six months of warfarin). Patients were excluded if they had received thrombolytics, had arterial claudication, had a life expectancy of less than six months, were unable to put on ECS due to physical disabilities or allergy, or were unable to participate in follow-up visits.
Patients were randomly assigned to wear active (30 to 40 mm Hg graduated) ECS or identical-looking placebo ECS (< 5 mm Hg compression at the ankle) for two years. Providers, study personnel and statisticians, and patients were all blinded to treatment allocation. Patients were asked to wear the stocking on the affected leg each day from waking until bedtime.
Follow-up occurred at one, six, 12, 18, and 24 months. The primary outcome was cumulative incidence of PTS diagnosed at six months or later using the Ginsberg criteria of ipsilateral pain and swelling of at least one month’s duration.8 Secondary outcomes included severity of PTS, leg ulcers, recurrence of venous thromboembolism (VTE), death, adverse events, venous valvular reflux, and quality of life (QOL). Outcomes were measured objectively through use of a validated scale (the Villalta scale) for PTS severity and two questionnaires to assess QOL.9-11
There were 409 patients in the ECS group and 394 in the placebo group. Baseline characteristics, including BMI, VTE risk factors, and anticoagulation treatment regimens, were similar between groups. The average age of participants in the study group was 55.4 years and in the placebo group, 54.8 years. Men comprised 62.4% of the active group and 57.9% of the placebo group. Approximately 90% of the participants in both groups were white.
At one month, approximately 95% of participants in both groups used the stockings; at 24 months, that was reduced to a little less than 70%. The percentage of people who used the stockings for at least three days per week was similar in both groups.
The cumulative incidence of PTS during follow-up was 14.2% in the active group and 12.7% in the placebo group (hazard ratio, 1.13). There were no differences in any of the secondary outcomes. Prespecified subgroup analyses found that age, BMI, and severity of DVT had no effect on outcomes. There was a marginal benefit for ECS for women versus men, but this does not likely reflect a true difference because the confidence intervals surrounding the hazard ratios for men and women overlapped and crossed the null value.
On the next page: What's new & challenges to implementation >>
WHAT’S NEW
New evidence contradicts previous studies
Two prior studies showed that using 30 to 40 mm Hg ECS decreased the incidence of PTS after proximal DVT.6,7 However, these were smaller, open-label, single-center studies. This study by Kahn et al1 was the first placebo-controlled, randomized, multicenter study that used validated instruments to measure PTS and QOL. It found no benefit in using ECS, thus contradicting the results of the prior studies.
There are currently no guidelines or consensus statements that recommend for or against the use of ECS after DVT.
CAVEATS
High nonadherence rates might have affected results
In both groups, adherence to the assigned intervention diminished throughout the study (from 95% at one month to slightly less than 70% at two years). Theoretically, this could have affected efficacy outcomes. However, the decrease was similar in both groups and represents what is observed in clinical practice. A prespecified per protocol analysis of patients who wore their ECS more regularly found no benefit.
It is possible that a “placebo effect” could explain the lack of difference between groups. However, the placebo stockings provided virtually no compression, and the two-year cumulative incidence of PTS in both the treatment and placebo groups was similar to that seen in control groups in prior studies.6,7
Finally, the incidence of PTS in this study was much lower than the 25% to 50% incidence reported previously. Kahn et al1 suggested that this was because they used more stringent and standardized criteria for PTS than was used in previous research.
CHALLENGES TO IMPLEMENTATION
There are no barriers to ending this practice
We can identify no challenges to implementation of this recommendation.
On the next page: References >>
REFERENCES
1. Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
2. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698-707.
3. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996; 125:1-7.
4. Cohen JM, Akl EA, Kahn SR. Pharmacologic and compression therapies for postthrombotic syndrome: a systematic review of randomized controlled trials. Chest. 2012;141: 308-320.
5. Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. 2011;53:
500-509.
6. Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:
759-762.
7. Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249-256.
8. Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med. 2001;161:2105-2109.
9. Villalta S, Bagatella P, Piccioli A, et al. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24:158a.
10. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247-263.
11. Kahn SR, Lamping DL, Ducruet T, et al; VETO Study Investigators. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006; 59:1049-1056.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(7):388-390.
PRACTICE CHANGER
Do not recommend elastic compression stockings to decrease the incidence of postthrombotic syndrome after deep vein thrombosis.1
STRENGTH OF RECOMMENDATION
B: Based on a large randomized controlled trial1
ILLUSTRATIVE CASE
A 56-year-old man presents to your clinic three days after receiving a diagnosis of lower extremity deep vein thrombosis (DVT). He was prescribed warfarin (5 mg/d) with enoxaparin bridging (120 mg/d). He has read about postthrombotic syndrome (PTS) online and is very concerned about this possible adverse effect. He asks about using elastic compression stockings (ECS). What should you tell him?
PTS can be a frustrating, debilitating condition. Its clinical features range from minor limb swelling to severe edema and pain, irreversible skin changes, and leg ulcerations.2 It occurs in 25% to 50% of patients after DVT.3 Because current PTS treatments are not very effective, prevention is essential.4,5
Patients are frequently encouraged to wear ECS after DVT to reduce the incidence of PTS by decreasing venous hypertension and reflux. These stockings are expensive and uncomfortable. Prior research suggested that use of ECS can reduce PTS incidence by half, but the studies were small, single-center, and not placebo-controlled.6,7
On the next page: Study summary >>
STUDY SUMMARY
RCT sets aside a common practice
Kahn et al1 conducted a randomized, placebo-controlled trial of active versus placebo ECS in patients from 24 centers in the United States and Canada who’d had an ultrasound-confirmed proximal DVT (in the popliteal or more proximal deep leg vein) within the previous 14 days. Most patients received standard anticoagulation therapy to treat their DVT (five to 10 days of heparin and three to six months of warfarin). Patients were excluded if they had received thrombolytics, had arterial claudication, had a life expectancy of less than six months, were unable to put on ECS due to physical disabilities or allergy, or were unable to participate in follow-up visits.
Patients were randomly assigned to wear active (30 to 40 mm Hg graduated) ECS or identical-looking placebo ECS (< 5 mm Hg compression at the ankle) for two years. Providers, study personnel and statisticians, and patients were all blinded to treatment allocation. Patients were asked to wear the stocking on the affected leg each day from waking until bedtime.
Follow-up occurred at one, six, 12, 18, and 24 months. The primary outcome was cumulative incidence of PTS diagnosed at six months or later using the Ginsberg criteria of ipsilateral pain and swelling of at least one month’s duration.8 Secondary outcomes included severity of PTS, leg ulcers, recurrence of venous thromboembolism (VTE), death, adverse events, venous valvular reflux, and quality of life (QOL). Outcomes were measured objectively through use of a validated scale (the Villalta scale) for PTS severity and two questionnaires to assess QOL.9-11
There were 409 patients in the ECS group and 394 in the placebo group. Baseline characteristics, including BMI, VTE risk factors, and anticoagulation treatment regimens, were similar between groups. The average age of participants in the study group was 55.4 years and in the placebo group, 54.8 years. Men comprised 62.4% of the active group and 57.9% of the placebo group. Approximately 90% of the participants in both groups were white.
At one month, approximately 95% of participants in both groups used the stockings; at 24 months, that was reduced to a little less than 70%. The percentage of people who used the stockings for at least three days per week was similar in both groups.
The cumulative incidence of PTS during follow-up was 14.2% in the active group and 12.7% in the placebo group (hazard ratio, 1.13). There were no differences in any of the secondary outcomes. Prespecified subgroup analyses found that age, BMI, and severity of DVT had no effect on outcomes. There was a marginal benefit for ECS for women versus men, but this does not likely reflect a true difference because the confidence intervals surrounding the hazard ratios for men and women overlapped and crossed the null value.
On the next page: What's new & challenges to implementation >>
WHAT’S NEW
New evidence contradicts previous studies
Two prior studies showed that using 30 to 40 mm Hg ECS decreased the incidence of PTS after proximal DVT.6,7 However, these were smaller, open-label, single-center studies. This study by Kahn et al1 was the first placebo-controlled, randomized, multicenter study that used validated instruments to measure PTS and QOL. It found no benefit in using ECS, thus contradicting the results of the prior studies.
There are currently no guidelines or consensus statements that recommend for or against the use of ECS after DVT.
CAVEATS
High nonadherence rates might have affected results
In both groups, adherence to the assigned intervention diminished throughout the study (from 95% at one month to slightly less than 70% at two years). Theoretically, this could have affected efficacy outcomes. However, the decrease was similar in both groups and represents what is observed in clinical practice. A prespecified per protocol analysis of patients who wore their ECS more regularly found no benefit.
It is possible that a “placebo effect” could explain the lack of difference between groups. However, the placebo stockings provided virtually no compression, and the two-year cumulative incidence of PTS in both the treatment and placebo groups was similar to that seen in control groups in prior studies.6,7
Finally, the incidence of PTS in this study was much lower than the 25% to 50% incidence reported previously. Kahn et al1 suggested that this was because they used more stringent and standardized criteria for PTS than was used in previous research.
CHALLENGES TO IMPLEMENTATION
There are no barriers to ending this practice
We can identify no challenges to implementation of this recommendation.
On the next page: References >>
REFERENCES
1. Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
2. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698-707.
3. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996; 125:1-7.
4. Cohen JM, Akl EA, Kahn SR. Pharmacologic and compression therapies for postthrombotic syndrome: a systematic review of randomized controlled trials. Chest. 2012;141: 308-320.
5. Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. 2011;53:
500-509.
6. Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:
759-762.
7. Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249-256.
8. Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med. 2001;161:2105-2109.
9. Villalta S, Bagatella P, Piccioli A, et al. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24:158a.
10. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247-263.
11. Kahn SR, Lamping DL, Ducruet T, et al; VETO Study Investigators. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006; 59:1049-1056.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(7):388-390.
Esophageal Cancer: Current Diagnosis and Management
From the University of Utah School of Medicine, Salt Lake City, UT.
Abstract
- Objective: To review the evaluation, diagnosis, and management of patients with esophageal cancer.
- Methods: Review of the literature.
- Results: Esophageal adenocarcinoma and esophageal squamous cell carcinoma (SCC) are aggressive cancers with a poor prognosis. GERD is the most common cause of esophageal adenocarcinoma, whereas increased alcohol consumption and tobacco commonly lead to esophageal SCC. Diagnosis is made via esophagogastroduodenoscopy and biopsies, and endoscopic ultrasound is typically used for locoregional staging. The endoscopic treatment of dysphagia is complex and several treatment options are available. Patients with locally advanced esophageal cancers are usually treated with neoadjuvant chemoradiation in combination with surgery. Improvement of quality of life is a major goal in patients with unresectable disease.
- Conclusion: Esophageal cancer remains a commonly encountered clinical entity requiring multidisciplinary evaluation and treatment.
Esophageal cancer is an aggressive disease with an overall poor outcome. It is the eighth most common cancer and sixth most common cause of cancer-related death worldwide [1]. In 2012, there were an estimated 456,000 new diagnoses of esophageal cancer and 400,000 deaths worldwide [1]. In the United States alone, an estimated 18,170 cases of esophageal cancer will be diagnosed in 2014, with 15,450 expected deaths [2].
Esophageal cancer includes 2 distinct histologic diseases: esophageal adenocarcinoma and esophageal squamous cell carcinoma (SCC). Overall, esophageal adenocarcinoma has increased in incidence, while the incidence of SCC has decreased in the Western world due to long-term reductions in smoking and alcohol consumption and increased incidence of gastroesophageal reflux disease (GERD) and obesity [3,4]. Esophageal adenocarcinoma accounted for less than 15% of esophageal cancers in the early 1980s, but now represents more than 60% of all esophageal cancers in the United States [5]. Esophageal SCC is still more common in China, central Asia, sub-Saharan Africa, and India and among the African-American and Caucasian female population in the United States [3,5].
Etiology
Esophageal Adenocarcinoma
While GERD is the most common cause of esophageal adenocarcinoma, other important causes/risk factors have been identified such as male sex, Caucasian race, older age, and obesity [8,12].In a prospective study by Abnet et al, patients who had a body mass index (BMI) greater than 35 kg/m2 had a significantly increased risk of esophageal adenocarcinoma when compared to patients with a BMI of 18.5 to 25 kg/m2 (hazard ratio [HR], 2.27; 95% CI, 1.44 to 3.59) [13]. Similarly, a recent meta-analysis found that patients with a BMI of 30 kg/m2 or greater had a relative risk for esophageal adenocarcinoma of 2.71 (95% CI, 2.16 to 3.46) [14]. Despite the strong correlation, the etiology of esophageal adenocarcinoma is complex and cannot be fully explained by obesity trends [15].
Smoking is another important risk factor associated with the development of esophageal adenocarcinoma. A study from the Barrett’s and Esophageal Adenocarcinoma Consortium revealed strong associations with esophageal adenocarcinoma and cigarette smoking (OR, 1.96; 95% CI, 1.64 to 2.34) [16]. Furthermore, the study found a statistically significant dose-response association between cigarette smoking and esophageal adenocarcinoma (P < 0.001).
Finally, dietary intake of vegetables and fruits has been shown to reduce the risk of Barrett’s esophagus. In a case-control study, patients with a median intake of 8.3 servings per day of vegetables and fruits had a 73% lower risk of developing Barrett’s esophagus versus those with 2.0 servings per day (OR, 0.27; 95% CI, 0.15 to 0.50) [17]. Each additional serving of vegetables and fruit was associated with a 14% reduction of risk (OR, 0.86; 95% CI, 0.80 to 0.93).
Esophageal Squamous Cell Carcinoma
In the study by Freedman et al, when compared with nonsmokers, current cigarette smokers were at significantly increased risk for esophageal SCC (HR, 9.27; 95% CI, 4.04 to 21.29) [18].Smoking has a stronger correlation with esophageal SCC than with esophageal adenocarcinoma [20]. In current smokers, the risk for developing esophageal SCC increases approximately three- to sevenfold [20]. The duration and intensity of smoking has been shown to increase the risk of esophageal SCC as well [21]. Smoking cessation has been shown to reduce the risk of esophageal SCC, but data shows that former cigarette smokers still are at a significant risk [18,21]. In a population-based case-control study, the risk of esophageal SCC in ex-smokers remained elevated for up to 30 years (OR, 1.44; 95% CI, 0.82 to 2.52) [21].
There are only limited studies that have examined the relationship between esophageal SCC and smokeless tobacco and other smoking products. Despite the limited number of studies, smokeless tobacco has been associated with esophageal SCC [22]. In a 2012 study of patients from India, chewing nass (a mix of tobacco, ash, oil, lime, and coloring and flavoring agents) and smoking hookah were associated with an increased risk of developing esophageal SCC [23].
Other risk factors associated with esophageal SCC include poor oral hygiene, atrophic gastritis, caustic esophageal injuries, and achalasia (likely due to stasis of esophageal contents in the case of achalasia) [24–27].Dietary causes of esophageal SCC have also been implicated in many international studies. Foods containing N-nitroso compounds and diets with selenium and zinc mineral deficiencies have been found to be risk factors for esophageal SCC [20,28–30].Thermal injury to the esophageal mucosa caused by food and beverages served at high temperatures has been shown to increase the risk of esophageal cancer [31]. Also, as seen in esophageal adenocarcinoma, diets rich with vegetables and fruits have been associated with a reduced risk of esophageal SCC [32].
In a meta-analysis of 1813 esophageal cancer cases by Corley et al, the use of aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) was found to be protective against both esophageal SCC and esophageal adenocarcinoma [33]. The study found a dose-dependent effect in the protective association between aspirin/NSAID use and esophageal cancer. Frequent aspirin/NSAID use was associated with a 46% reduction of the odds for developing any esophageal cancer, whereas intermittent use provided an 18% reduction in the odds. However, any use of aspirin or NSAIDs offered some degree of protection against both esophageal SCC (OR, 0.58; 95% CI, 0.43 to 0.78) and esophageal adenocarcinoma (OR, 0.67; 95% CI, 0.51 to 0.87). The mechanism of the risk reduction with aspirin and NSAIDs is still unclear but may be associated with inhibition of the cyclooxygenase-2 enzyme and the reduction of inflammation [33–35].
Clinical Manifestations
Esophageal cancer commonly presents with dysphagia, weight loss, gastrointestinal reflux, and/or odynophagia. In a study by Daly et al, 74% of esophageal cancer patients reported dysphagia and 16.6% reported having odynophagia at the time of initial diagnosis [36]. Patients can have the sensation of food getting “stuck,” which initially can be overcome by careful chewing and/or dietary modification [37]. A history of trouble swallowing solid foods followed by difficulty with drinking liquids is frequently seen. Some patients complain of regurgitation of undigested foods, and approximately 20% of patients have reported having GERD symptoms [36,37]. Due to the complete or partial esophageal obstruction combined with tumor effects, patients with esophageal cancer often develop significant weight loss. In the study by Daly et al, 57.3% of patients reported weight loss at the time of their cancer diagnosis [36]. Weight loss of more than 10% body mass has been identified as an independent indicator for poor prognosis [36,38]. Pain, dyspnea, hoarseness, and cough occur less frequently but may reflect extensive cancer burden [39]. Some patients with advanced tumors have hematemesis from tumor erosion or have recurrent pneumonias due to tracheobronchial fistulas.
Hepatomegaly, pleural effusion, and lymphadenopathy, especially in Virchow’s node (left supraclavicular fossa), are physical examination findings suggestive of metastatic disease [39]. However, most patients with esophageal cancer will have unremarkable physical examination findings.
It should be noted that patients with early stage lesions (ie, stage T1 lesions) may have minimal or no symptoms, with lesions detected either incidentally or as part of endoscopic screening/surveillance programs.
Diagnostic Studies
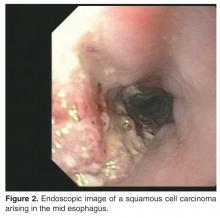
For patients with suspected esophageal cancer, a barium swallow is an inexpensive and readily available diagnostic study [39]. A barium swallow may show a mass lesion and/or a stricture. If the barium swallow is suggestive of cancer, the diagnosis is usually confirmed via an esophagogastroduodenoscopy (EGD) and biopsies, although in practice many patients with dysphagia and/or a history suspicious for esophageal cancer will proceed directly to EGD [40]. Findings suspicious for cancer are routinely biopsied [39].Traditionally, the more biopsies obtained (up to 7), the higher the diagnostic yield of cancer [41]. The addition of brush cytology to biopsies has also been found to increase the diagnostic accuracy, although this is not widely performed [41].
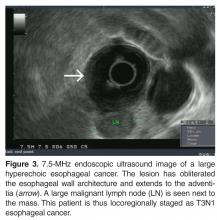
Once the diagnosis of cancer is confirmed, a computed tomography (CT) scan of the chest, abdomen, and pelvis with intravenous (IV) contrast is usually the next step in the patient’s evaluation, primarily to detect distant metastasis and to look for peritumoral adenopathy [39]. However, in terms of locoregional tumor staging, CT scans are less sensitive and specific than endoscopic ultrasonography (EUS) [42]. Patients who do not have evidence of metastasis on CT scan typically undergo EUS for definitive locoregional staging.
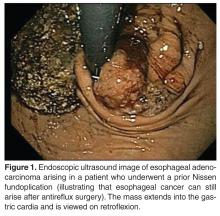
EUS does have its limitations. Between 25% and 36% of patients with esophageal carcinoma present with high-grade malignant strictures that do not allow passage of the scope, although if the exam can show malignant adenopathy and/or tumor extension through the muscularis propria, further evaluation is often of little additional benefit [46]. Dilation of malignant esophageal strictures to facilitate EUS is uncommon as there is a high risk of perforation (up to 24%) [47]. High-frequency (12.5 MHz) EUS mini-probes have been used to interrogate tumors with a very narrow lumen; however, the mini-probes are limited by the penetration depth of the transducer, which can lead to an incomplete locoregional tumor assessment [48]. EUS is not usually used for restaging after neoadjuvant therapy [49].
Endoscopic mucosal resection (EMR) is another technique for staging and treatment of superficial neoplasms (see Treatment section for more details). EMR is critical for distinguishing between T1a lesions (often candidates for definitive endoscopic therapy given the low likelihood of nodal involvement) versus T1b lesions (invasive to submucosa and more likely to prompt surgical esophagectomy with lymph node sampling). The distinction between T1a and T1b disease cannot be established as reliably by EUS when compared with EMR. The American Society for Gastrointestinal Endoscopy 2013 guidelines recommend EMR for the treatment and staging of nodular Barrett’s esophagus and suspected intramucosal adenocarcinoma [50].
Looking for distant metastasis, or M staging, is carried out with EUS, diagnostic laparoscopy/thoracoscopy, and CT and/or positron emission tomography (PET) scans. Despite the high accuracy of esophageal cancer staging with laparoscopy and thoracoscopy, these are invasive procedures and have generally been replaced by PET scan [39,51,52]. PET with 18F-fludeoxyglucose has been shown to significantly improve the detection rate of metastatic disease compared with the conventional staging methods (CT scan and EUS) [53]. In a prospective study, PET scans detected metastasis in 15% of patients who were thought to have localized cancer by conventional staging modalities [39,54].
Unlike several other cancers, tumor markers such as carbohydrate antigen (CA) 19-9, CA 125, and carcinoembryonic antigen (CEA) have low specificity and sensitivity in esophageal cancer and are not routinely obtained and/or followed [39,55].
Staging
Treatment
Early Stage
Historically, patients with early stage esophageal cancer (those without evidence of deep invasion into the esophageal wall and no evidence of peritumoral malignant adenopathy or metastases, typically T1N0M0) were referred for esophagectomy [59]. Recent treatment trends suggest proportionately more patients with T1 disease are being treated endoscopically (up to 29% of patients) and proportionately fewer with esophagectomy [60]. EMR has emerged as a viable alternative treatment to esophagectomy when the lesion is staged T1aN0 (tumor invading the lamina propria or muscularis mucosae but not the submucosa) [3]. EMR is performed via several techniques, but most commonly as follows. First, saline is injected under the lesion to create a submucosal cushion, separating the lesion from the underlying muscularis propria. The actual endoscopic resection of the lesion is usually accomplished via snare electrocautery and the resected lesion is sent for pathologic analysis. Endoscopic caps and band ligation devices are available to facilitate removal of the lesion in one or more pieces [61].
In a retrospective cohort study by Prasad et al of 178 patients from 1998 to 2007, the cumulative mortality in the EMR group was comparable to that of the surgery group (17% vs. 20%, respectively, P = 0.75) [62]. Recurrent cancer was detected in 12% of EMR patients; however, all patients were successfully re-treated without affecting overall survival.
In another study of 742 patients, long-term survival in those with early esophageal cancer managed with endoscopic therapy was comparable to that in patients treated with surgical resection [63]. The median cancer-free survival in the endoscopic group was not significantly different from that in the surgical group (56 and 59 months, respectively, P = 0.41) The study found that the relative hazard for 1esophageal cancer–specific mortality in the endoscopic group did not differ from that of the surgical group (relative hazard, 0.89; 95% CI, 0.51 to 1.56; P = 0.68).
Locally Advanced Disease
Neoadjuvant Therapy
For patients with locally advanced cancer (ie, patients without distant metastases who have extension of the primary tumor into the deeper layers of the esophageal wall, including the muscularis propria and the adventitia with or without peritumoral malignant adenopathy, or T2 or T3 lesions with N0 or N1, N2, or N3 status, neoadjuvant therapy is the norm, although the optimal management remains controversial and treatment protocols vary around the world [3,62]. Most neoadjuvant therapy regimens in the United States combine chemotherapy and external beam radiation therapy.
Neoadjuvant treatment with chemoradiation has been found to be beneficial in all esophageal cancers [3,64]. A meta-analysis of 1209 patients found a significant survival benefit for preoperative chemoradiotherapy and, to a lesser extent, for chemotherapy when compared to surgery alone [65]. When comparing neoadjuvant chemoradiotherapy to surgery alone, there was a 19% decrease in the risk of death corresponding to a 13% absolute difference in 2-year survival in the neoadjuvant chemotherapy group. HR for all-cause mortality with neoadjuvant chemoradiotherapy versus surgery alone was 0.81 (95% CI, 0.70 to 0.93; P = 0.002). The benefits of neoadjuvant chemoradiotherapy were similar for both esophageal SCC and adenocarcinoma. The benefits of chemotherapy, however, were less than chemoradiotherapy. When comparing neoadjuvant chemotherapy to surgery alone, there was an absolute survival benefit of 7%.
Following neoadjuvant therapy, patients typically undergo restaging via cross-sectional imaging, most commonly PET/CT scans. If the patient is felt to have active residual disease and has not developed metastases or contraindications to surgery, esophagectomy is appropriate. Some data suggests that patients with esophageal SCC who have complete clinical response after chemoradiation can be observed closely rather than proceed to surgery [3,62,66]. However, the data concerning the usefulness of definitive chemoradiotherapy in esophageal adenocarcinoma is lacking at this time. In a retrospective study of nonmetastatic esophageal adenocarcinoma patients by Tougeron et al comparing surgical patients (± preoperative treatment) to definitive chemoradiotherapy patients, a complete resection was achieved in 92.5% of patients in the surgical group and a clinical complete response was observed in 49.4% of patients with definitive chemoradiotherapy [67]. The overall survival was 36.2 ± 2 months for the surgery group versus 16.5 ± 0.8 months for the definitive chemoradiotherapy group (P = 0.02).
Stenting Prior to Neoadjuvant Therapy
In a meta-analysis of 9 studies comprising 180 patients, placement of esophageal stents in patients with locally advanced esophageal cancer significantly improved dysphagia and allowed for oral nutrition during neoadjuvant therapy [69]. There was a substantial decrease in the dysphagia scores standard difference in means (SDM) of –0.81 (standard error, 0.15; 95% CI, –1.1 to –0.51), an increase in weight SDM of 0.591 (standard error, 0.434; 95% CI, –0.261 to 1.442), and an increase in serum albumin SDM of 0.35 (standard error, 0.271; 95% CI, –0.181 to 0.881). The overall procedural success rate was 95% (95% CI, 0.895 to 0.977). Major adverse events included stent migration in 32% of patients (95% CI, 0.258 to 0.395) and chest discomfort in 51.4% (95% CI, 0.206 to 0.812). However, it was believed that the stent migration may have been a sign of tumor response to neoadjuvant therapy.
In a prospective nonrandomized study of 13 patients with polyflex stents (polyester mesh stents covered in a silicone membrane) placed prior to neoadjuvant therapy, similar improvements with dysphagia scores were observed after stent placement [70]. In the study, the mean baseline dysphagia score at the time of stent placement was 3. Dysphagia scores were subsequently obtained at 1, 2, 3, and 4 weeks after stent placement and were 1.1, 0.8, 0.9, and 1.0, respectively (P = 0.005, P = 0.01, P = 0.02, and P = 0.008, respectively). There were no episodes of bleeding or esophageal perforation. Immediate complications from stenting included chest discomfort, seen in 12 of the 13 patients. Stent migration occurred at some point in 6 of 13 patients, although not all patients with a migrated stent required stent replacement. Again, it was thought that the stent migration could be a sign of tumor response to neoadjuvant therapy.
Surgery
Surgery is an essential part of treatment of esophageal cancer [3,71]. Transthoracic, transhiatal, and radical (en bloc) are the 3 different basic approaches for esophagectomy [3]. Because it does not require a thoracotomy, the transhiatal approach has a theoretical advantage of decreased morbidity and mortality, although several studies have shown no differences in outcome between the transthoracic and transhiatal approach [3,72,73]. In a study by Chang et al comparing the transhiatal to the transthoracic approach, the 5-year survival was higher for patients undergoing transhiatal versus transthoracic esophagectomy (30.5% vs. 22.7%, P = 0.02) [73]. However, after adjusting for differences in tumor stage and patient and provider factors the survival advantage was no longer statistically significant (adjusted HR for mortality, 0.95; 95% CI, 0.75 to 1.20).
Adjuvant Therapy
Despite the benefits of chemoradiation as a neoadjuvant treatment, the data for chemoradiation as adjuvant therapy after resection is lacking in most clinical situations [74].
Metastatic Disease
Between 25% and 40% of esophageal cancer patients will present with metastases to liver, bone, and lung or widespread nodal metastases [61].Improvement of quality of life is a major goal in patients with unresectable disease. Patients with nonsurgical esophageal cancer who have an estimated life expectancy of greater than a few weeks are recommended to have concurrent chemoradiotherapy as most patients have symptomatic obstructive disease and dysphagia [62]. A study by Harvey et al examined the palliative benefit of chemoradiotherapy on dysphagia versus toxicity in patients with invasive esophageal carcinoma [75]. The study found that treatment was well tolerated, with only 5% of patients failing to complete treatment. The study used the DeMeester (4-point) symptom scores for the assessment of dysphagia. The median baseline score at presentation was 2 (moderate: difficulty with soft food, predominately liquid diet). After chemoradiotherapy, 49% of patients were assessed as having a dysphagia score of 0 (no dysphagia). Of those patients who received chemoradiotherapy, 78% had an improvement of at least 1 grade in their DeMeester dysphagia, while only 14% of patients did not improve with therapy. The median survival for the study population was 7 months, with a 6% treatment-related mortality. Chemoradiation therapy as a primary treatment for dysphagia can take days to weeks to take effect, and can be associated with significant pain, usually from radiation esophagitis.
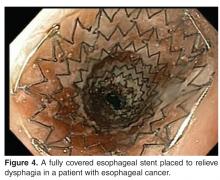
Other alternatives for palliation of nonresectable esophageal cancer include esophageal stenting with SEMS and brachytherapy. SEMS are effective and safe for palliation of dysphagia caused by primary esophageal tumors, postoperative cancer recurrence, esophagorespiratory fistulae, and tumors near the upper esophageal sphincter [76]. A study looking at the use of esophageal SEMS in cancer found that after SEMS placement, the dysphagia score improved from a mean of 3.6 to 1.6 (P < 0.001) [75]. The procedure was technically successful in 96% of the patients. In all cases, esophagorespiratory fistulas were occluded. Pain, reflux, and stent migration are the most common complications of esophageal SEMS.
In a study comparing single-dose brachytherapy versus SEMS, the SEMS group had quicker improvement of dysphagia symptoms than the brachytherapy group, but the long-term relief of dysphagia was better after brachytherapy [77]. In addition, SEMS placement had more complications than brachytherapy (33% vs. 21%, respectively; P = 0.02), which was mainly due to an increased incidence of late hemorrhage. However, brachytherapy and SEMS did not differ in terms of median survival (P = 0.23) or recurrent or persistent dysphagia (P = 0.81).
Tracheoesophageal fistulas may develop in the setting of a locally advanced tumor, or as a complication of RT or chemoradiotherapy. SEMS can also be used successfully in the palliation therapy for tracheoesophageal fistulas or post-esophagectomy anastomotic strictures [78].
Prognosis
The overall survival for patients with resectable esophageal cancer has improved significantly over the past 30 years; however, more than 50% of patients presenting with esophageal cancer will have unresectable or metastatic disease at the time of presentation [3,39,79].Prognosis is primarily TMN stage–dependent, as patients with early stage cancer limited to the mucosa are expected to have curable disease [3]. Poor prognostic predictors include advanced stage cancer, dysphagia, advanced age, large tumors, more than 10% loss in body mass, and malignant adenopathy [39,80–84].
In 2010, the American Joint Committee on Cancer/International Union against Cancer Staging system looked at the prognosis of 4627 patients who underwent esophagectomy alone without radiation or chemotherapy [3,56]. For stage Tis (tumor in situ or high-grade dysplasia) and 1A cancers, there was an approximate 80% 5-year risk-adjusted survival rate [3,56]. The survival rate was marginally better for esophageal adenocarcinoma than for esophageal SCC. With surgery alone, stage 1B disease had a 5-year survival of 62% with SCC and 64% with adenocarcinoma [3,56]. For patients with stage 2A cancer, the 5-year survival was 55% for SCC and 50% for adenocarcinoma as long as there was not nodal involvement [3,56]. If there was nodal involvement, the survival rate dropped to 40% for stage 2B cancer, 25% for stage 3A cancer, and 15% to 17% for stage 3B to 3C cancer [3,56]. As stated earlier, neoadjuvant chemoradiation helps improve outcomes when compared to surgery alone (see Neoadjuvant Therapy in the Treatment section). Thus, one would expect a slightly better prognosis with neoadjuvant therapy and surgery than the previously stated data for surgery alone. Unfortunately, patients with unresectable or metastatic disease at time of diagnosis have a poor prognosis, with a 1-year survival rate less than 20% [3].
Esophageal cancer patients who are treated successfully need to be followed closely because a majority of esophageal cancers will recur within 3 years of treatment [61]. For the first 3 years post treatment, patients should be followed every 3 to 6 months [61]. For 3 to 5 years after treatment, patients should be followed every 6 months and annually thereafter [61]. During each visit, patients should have a thorough history and physical exam and assessment of quality of life [61]. Laboratory studies and EGD are performed as clinically indicated [61]. The importance of intensive post-treatment endoscopic surveillance should be emphasized given a defined rate of disease recurrence. Additionally, radiographic imaging such as CT of the chest and abdomen with contrast or PET/CT may be needed for restaging purposes [61].
Conclusion
Esophageal adenocarcinoma and esophageal SCC are aggressive cancers with poor prognosis. Overall, esophageal adenocarcinoma has increased in incidence, while the incidence of SCC has decreased in the Western world. GERD is the most common cause of esophageal adenocarcinoma, whereas increased alcohol consumption and tobacco commonly lead to esophageal SCC.
For patients with suspected esophageal cancer, a barium swallow is an inexpensive initial diagnostic study that is usually followed up with EGD with biopsies if suggestive of cancer. Once cancer is confirmed, a CT scan with intravenous contrast is obtained to look for adenopathy and metastasis. Those who do not have evidence of metastasis on CT scan typically undergo EUS for definitive locoregional staging.
In the past, patients with early stage esophageal cancer were referred for esophagectomy, but recently EMR has emerged as a viable alternative. Patients with locally advanced esophageal cancers are usually treated with neoadjuvant chemoradiation in combination with surgery. In addition, several studies have showed that esophageal stenting prior to neoadjuvant treatment significantly improves patients’ dysphagia. Unfortunately, many patients still initially present with metastatic or nonresectable disease. Improvement of quality of life is a major goal in patients with unresectable disease. Chemoradiotherapy, esophageal stenting, and brachytherapy are options for improvement of quality of life. Further studies are still needed to evaluate current and new therapeutic guidelines for resectable and nonresectable disease.
Corresponding author: Douglas G. Adler, MD, 30N 1900E 4R118, Salt Lake City, UT 84132, [email protected].
Financial disclosures: None.
1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed on May 5, 2014.
2. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29.
3. Nieman DR, Peters JH. Treatment strategies for esophageal cancer. Gastroenterol Clin North Am 2013;42:187–97.
4. Shridhar R, Almhanna K, Meredith KL, et al. Radiation therapy and esophageal cancer. Cancer Control 2013;20:97–110.
5. Baquet CR, Commiskey P, Mack K, et al. Esophageal cancer epidemiology in blacks and whites: racial and gender disparities in incidence, mortality, survival rates and histology. J Natl Med Assoc 2005;97:1471–8.
6. Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophagealadenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222–7.
7. Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31.
8. Pohl H, Wrobel K, Bojarski C, et al. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol 2013;108:200–7.
9. Coleman HG, Bhat SK, Murray LJ, et al. Symptoms and endoscopic features at Barrett’s esophagus diagnosis: implications for neoplastic progression risk. Am J Gastroenterol 2014;109:527–34.
10. Nguyen DM, El-Serag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2009;7:1299–304.
11. Lagergren J, Ye W, Lagergren P, Lu Y. The risk of esophageal adenocarcinomaafter antireflux surgery. Gastroenterology 2010;138:1297–301.
12. el-Serag HB. The epidemic of esophageal adenocarcinoma. Gastroenterol Clin North Am 2002;31:421–40, viii.
13. Abnet CC, Freedman ND, Hollenbeck AR, et al. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer 2008;44:465–71.
14. Turati F, Tramacere I, La Vecchia C, Negri E. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol 2013;24:609–17.
15. Kroep S, Lansdorp-Vogelaar I, Rubenstein JH, et al. Comparing trends in esophageal adenocarcinoma incidence and lifestyle factors between the United States, Spain, and the Netherlands. Am J Gastroenterol 2014;109:336–43.
16. Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst 2010;102:1344–53.
17. Kubo A, Levin TR, Block G, et al. Dietary antioxidants, fruits, and vegetables and the risk of Barrett’s esophagus. Am J Gastroenterol 2008;103:1614–23.
18. Freedman ND, Abnet CC, Leitzmann MF, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol 2007;165:1424–33.
19. Pandeya N, Williams G, Green AC, et al; Australian Cancer Study. Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology 2009;136:1215–24, e1-2.
20. Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am 2009;38:27–57, vii.
21. Pandeya N, Williams GM, Sadhegi S, et al. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am J Epidemiol 2008;168:105–14.
22. Boffetta P, Hecht S, Gray N, et al. Smokeless tobacco and cancer. Lancet Oncol 2008;9:667–75.
23. Dar NA, Bhat GA, Shah IA, eta l. Hookah smoking, nass chewing, and oesophageal squamous cell carcinoma in Kashmir, India. Br J Cancer 2012;107:1618–23.
24. Abnet CC, Kamangar F, Islami F, et al. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2008;17:3062–8.
25. Islami F, Sheikhattari P, Ren JS, Kamangar F. Gastric atrophy and risk of oesophageal cancer and gastric cardia adenocarcinoma--a systematic review and meta-analysis. Ann Oncol 2011;22:754–60.
26. Appelqvist P, Salmo M. Lye corrosion carcinoma of the esophagus: a review of 63 cases. Cancer 1980;45:2655–8.
27. Sandler RS, Nyrén O, Ekbom A, et al. The risk of esophageal cancer in patients with achalasia. A population-based study. JAMA 1995;274:1359–62.
28. Lu SH, Montesano R, Zhang MS, et al. Relevance of N-nitrosamines to esophageal cancer in China. J Cell Physiol Suppl 1986;4:51–8.
29. Steevens J, van den Brandt PA, Goldbohm RA, Schouten LJ. Selenium status and the risk of esophageal and gastric cancer subtypes: the Netherlands cohort study. Gastroenterology 2010;138:1704–13.
30. Abnet CC, Lai B, Qiao YL, et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst 2005;97:301–6.
31. Islami F, Boffetta P, Ren JS, et al. High-temperature beverages and foods and esophageal cancer risk--a systematic review. Int J Cancer 2009;125:491–524.
32. Liu J, Wang J, Leng Y, Lv C. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer 2013;133:473–85.
33. Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 2003;124:47–56.
34. Ratnasinghe D, Tangrea J, Roth MJ, et al. Expression of cyclooxygenase-2 in human squamous cell carcinoma of the esophagus; an immunohistochemical survey. Anticancer Res 1999;19:171–4.
35. Morris CD, Armstrong GR, Bigley G, et al. Cyclooxygenase-2 expression in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol 2001;96:990–6.
36. Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000;190:562–72.
37. Gastrointestinal cancer. In: Fishman MC, Hoffman R, Klausner RD, Thaler MS, editors. Medicine. 5th ed. Baltimore: Lippincott Williams & Wilkins; 2002:423–6.
38. Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512–8.
39. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241–52.
40. Lightdale CJ. Esophageal cancer. Am J Gastroenterol 1999;94:20–9.
41. Graham DY, Schwartz JT, Cain GD, Gyorkey F. Prospective evaluation of biopsy number in the diagnosis of esophageal and gastric carcinoma. Gastroenterology 1982;82:228–31.
42. Lowe VJ, Booya F, Fletcher JG, et al. Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol Imaging Biol 2005;7:422–30.
43. Röesch T. Endoscopic ultrasonography: equipment and technique. Gastrointest Endosc Clin N Am 2005;15:13–31, vii.
44. Vazquez-Sequeiros E, Norton ID, Clain JE, et al. Impact of EUS-guided fine-needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest Endosc 2001;53:751–7.
45. Van Dam J. Endosonographic evaluation of the patient with esophageal cancer. Chest 1997;112(4 Suppl):184S–190S.
46. Catalano MF, Van Dam J, Sivak MV Jr. Malignant esophageal strictures: staging accuracy of endoscopic ultrasonography. Gastrointest Endosc 1995;41:535–9.
47. Van Dam J, Rice TW, Catalano MF, et al. High-grade malignant stricture is predictive of esophageal tumor stage. Risks of endosonographic evaluation. Cancer 1993;71:2910–7.
48. Hünerbein M, Ghadimi BM, Haensch W, Schlag PM. Transendoscopic ultrasound of esophageal and gastric cancer using miniaturized ultrasound catheter probes. Gastrointest Endosc 1998;48:371–5.
49. Zuccaro G Jr, Rice TW, Goldblum J, et al. Endoscopic ultrasound cannot determine suitability for esophagectomy after aggressive chemoradiotherapy for esophageal cancer. Am J Gastroenterol 1999;94:906–12.
50. ASGE Standards of Practice Committee, Evans JA, Early DS, Chandraskhara V, et al; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013;77:328–34.
51. Luketich JD, Schauer P, Landreneau R, et al. Minimally invasive surgical staging is superior to endoscopic ultrasound in detecting lymph node metastases in esophageal cancer. J Thorac Cardiovasc Surg 1997;114:817–21.
52. Krasna MJ, Flowers JL, Attar S, McLaughlin J. Combined thoracoscopic/laparoscopic staging of esophageal cancer. J Thorac Cardiovasc Surg 1996;111:800–6.
53. Flamen P, Lerut A, Van Cutsem E, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 2000;18:3202–10.
54. Downey RJ, Akhurst T, Ilson D, et al. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol 2003;21:428–32.
55. Mealy K, Feely J, Reid I, et al. Tumour marker detection in oesophageal carcinoma. Eur J Surg Oncol 1996;22:505–7.
56. Rice TW, Rusch VW, Ishwaran H, Blackstone EH; Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763–73.
57. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag; 2009:103–5.
58. Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1–8.
59. Villaflor VM, Allaix ME, Minsky B, et al. Multidisciplinary approach for patients with esophageal cancer. World J Gastroenterol 2012;18:6737–46.
60. Ngamruengphong S, Wolfsen HC, Wallace MB. Survival of patients with superficial esophageal adenocarcinoma after endoscopic treatment vs surgery. Clin Gastroenterol Hepatol 2013;11:1424–9.e2.
61. Lin SH, Chang JY. Esophageal cancer: diagnosis and management. Chin J Cancer 2010;29:843–54.
62. Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815–23.
63. Das A, Singh V, Fleischer DE, Sharma VK. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol 2008;103:1340–5.
64. Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593–8.
65. Gebski V, Burmeister B, Smithers BM, et al; Australasian Gastro-Intestinal Trials Group. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226–34.
66. Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160–8.
67. Tougeron D, Scotté M, Hamidou H, et al. Definitive chemoradiotherapy in patients with esophageal adenocarcinoma: an alternative to surgery? J Surg Oncol 2012;105: 761–6.
68. Siddiqui AA, Sarkar A, Beltz S, et al. Placement of fully covered self-expandable metal stents in patients with locally advanced esophageal cancer before neoadjuvant therapy. Gastrointest Endosc 2012;76:44–51.
69. Nagaraja V, Cox MR, Eslick GD. Safety and efficacy of esophageal stents preceding or during neoadjuvant chemotherapy for esophageal cancer: a systematic review and meta-analysis. J Gastrointest Oncol 2014;5:119–26.
70. Adler DG, Fang J, Wong R, et al. Placement of Polyflex stents in patients with locally advanced esophageal cancer is safe and improves dysphagia during neoadjuvant therapy. Gastrointest Endosc 2009;70:614–9.
71. Dubecz A, Sepesi B, Salvador R, et al. Surgical resection for locoregional esophageal cancer is underutilized in the United States. J Am Coll Surg 2010;211:754–61.
72. Hulscher JB, Tijssen JG, Obertop H, van Lanschot JJ. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 2001;72:306–13.
73. Chang AC, Ji H, Birkmeyer NJ, et al. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg 2008;85:424–9.
74. Almhanna K, Shridhar R, Meredith KL. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: is there a standard of care? Cancer Control 2013;20:89–96.
75. Harvey JA, Bessell JR, Beller E, et al. Chemoradiation therapy is effective for the palliative treatment of malignant dysphagia. Dis Esophagus 2004;17:260–5.
76. Siersema PD, Schrauwen SL, van Blankenstein M, et al; Rotterdam Esophageal Tumor Study Group. Self-expanding metal stents for complicated and recurrent esophagogastric cancer. Gastrointest Endosc 2001;54:579–86.
77. Homs MY, Steyerberg EW, Eijkenboom WM, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet 2004;364(9444):1497–504.
78. van den Bongard HJ, Boot H, Baas P, Taal BG. The role of parallel stent insertion in patients with esophagorespiratory fistulas. Gastrointest Endosc 2002;55:110–5.
79. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220–41.
80. Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512–8.
81. Mariette C, Maurel A, Fabre S, et al. [Preoperative prognostic factors for squamous cell carcinomas of the thoracic esophagus]. Gastroenterol Clin Biol 2001;25:468–72.
82. Swanson SJ, Batirel HF, Bueno R, et al. Transthoracic esophagectomy with radical mediastinal and abdominal lymph node dissection and cervical esophagogastrostomy for esophageal carcinoma. Ann Thorac Surg 2001;72:1918–24.
83. Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305–13.
84. Hosch SB, Stoecklein NH, Pichlmeier U, et al. Esophageal cancer: the mode of lymphatic tumor cell spread and its prognostic significance. J Clin Oncol 2001;19:1970–5.
From the University of Utah School of Medicine, Salt Lake City, UT.
Abstract
- Objective: To review the evaluation, diagnosis, and management of patients with esophageal cancer.
- Methods: Review of the literature.
- Results: Esophageal adenocarcinoma and esophageal squamous cell carcinoma (SCC) are aggressive cancers with a poor prognosis. GERD is the most common cause of esophageal adenocarcinoma, whereas increased alcohol consumption and tobacco commonly lead to esophageal SCC. Diagnosis is made via esophagogastroduodenoscopy and biopsies, and endoscopic ultrasound is typically used for locoregional staging. The endoscopic treatment of dysphagia is complex and several treatment options are available. Patients with locally advanced esophageal cancers are usually treated with neoadjuvant chemoradiation in combination with surgery. Improvement of quality of life is a major goal in patients with unresectable disease.
- Conclusion: Esophageal cancer remains a commonly encountered clinical entity requiring multidisciplinary evaluation and treatment.
Esophageal cancer is an aggressive disease with an overall poor outcome. It is the eighth most common cancer and sixth most common cause of cancer-related death worldwide [1]. In 2012, there were an estimated 456,000 new diagnoses of esophageal cancer and 400,000 deaths worldwide [1]. In the United States alone, an estimated 18,170 cases of esophageal cancer will be diagnosed in 2014, with 15,450 expected deaths [2].
Esophageal cancer includes 2 distinct histologic diseases: esophageal adenocarcinoma and esophageal squamous cell carcinoma (SCC). Overall, esophageal adenocarcinoma has increased in incidence, while the incidence of SCC has decreased in the Western world due to long-term reductions in smoking and alcohol consumption and increased incidence of gastroesophageal reflux disease (GERD) and obesity [3,4]. Esophageal adenocarcinoma accounted for less than 15% of esophageal cancers in the early 1980s, but now represents more than 60% of all esophageal cancers in the United States [5]. Esophageal SCC is still more common in China, central Asia, sub-Saharan Africa, and India and among the African-American and Caucasian female population in the United States [3,5].
Etiology
Esophageal Adenocarcinoma
While GERD is the most common cause of esophageal adenocarcinoma, other important causes/risk factors have been identified such as male sex, Caucasian race, older age, and obesity [8,12].In a prospective study by Abnet et al, patients who had a body mass index (BMI) greater than 35 kg/m2 had a significantly increased risk of esophageal adenocarcinoma when compared to patients with a BMI of 18.5 to 25 kg/m2 (hazard ratio [HR], 2.27; 95% CI, 1.44 to 3.59) [13]. Similarly, a recent meta-analysis found that patients with a BMI of 30 kg/m2 or greater had a relative risk for esophageal adenocarcinoma of 2.71 (95% CI, 2.16 to 3.46) [14]. Despite the strong correlation, the etiology of esophageal adenocarcinoma is complex and cannot be fully explained by obesity trends [15].
Smoking is another important risk factor associated with the development of esophageal adenocarcinoma. A study from the Barrett’s and Esophageal Adenocarcinoma Consortium revealed strong associations with esophageal adenocarcinoma and cigarette smoking (OR, 1.96; 95% CI, 1.64 to 2.34) [16]. Furthermore, the study found a statistically significant dose-response association between cigarette smoking and esophageal adenocarcinoma (P < 0.001).
Finally, dietary intake of vegetables and fruits has been shown to reduce the risk of Barrett’s esophagus. In a case-control study, patients with a median intake of 8.3 servings per day of vegetables and fruits had a 73% lower risk of developing Barrett’s esophagus versus those with 2.0 servings per day (OR, 0.27; 95% CI, 0.15 to 0.50) [17]. Each additional serving of vegetables and fruit was associated with a 14% reduction of risk (OR, 0.86; 95% CI, 0.80 to 0.93).
Esophageal Squamous Cell Carcinoma
In the study by Freedman et al, when compared with nonsmokers, current cigarette smokers were at significantly increased risk for esophageal SCC (HR, 9.27; 95% CI, 4.04 to 21.29) [18].Smoking has a stronger correlation with esophageal SCC than with esophageal adenocarcinoma [20]. In current smokers, the risk for developing esophageal SCC increases approximately three- to sevenfold [20]. The duration and intensity of smoking has been shown to increase the risk of esophageal SCC as well [21]. Smoking cessation has been shown to reduce the risk of esophageal SCC, but data shows that former cigarette smokers still are at a significant risk [18,21]. In a population-based case-control study, the risk of esophageal SCC in ex-smokers remained elevated for up to 30 years (OR, 1.44; 95% CI, 0.82 to 2.52) [21].
There are only limited studies that have examined the relationship between esophageal SCC and smokeless tobacco and other smoking products. Despite the limited number of studies, smokeless tobacco has been associated with esophageal SCC [22]. In a 2012 study of patients from India, chewing nass (a mix of tobacco, ash, oil, lime, and coloring and flavoring agents) and smoking hookah were associated with an increased risk of developing esophageal SCC [23].
Other risk factors associated with esophageal SCC include poor oral hygiene, atrophic gastritis, caustic esophageal injuries, and achalasia (likely due to stasis of esophageal contents in the case of achalasia) [24–27].Dietary causes of esophageal SCC have also been implicated in many international studies. Foods containing N-nitroso compounds and diets with selenium and zinc mineral deficiencies have been found to be risk factors for esophageal SCC [20,28–30].Thermal injury to the esophageal mucosa caused by food and beverages served at high temperatures has been shown to increase the risk of esophageal cancer [31]. Also, as seen in esophageal adenocarcinoma, diets rich with vegetables and fruits have been associated with a reduced risk of esophageal SCC [32].
In a meta-analysis of 1813 esophageal cancer cases by Corley et al, the use of aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) was found to be protective against both esophageal SCC and esophageal adenocarcinoma [33]. The study found a dose-dependent effect in the protective association between aspirin/NSAID use and esophageal cancer. Frequent aspirin/NSAID use was associated with a 46% reduction of the odds for developing any esophageal cancer, whereas intermittent use provided an 18% reduction in the odds. However, any use of aspirin or NSAIDs offered some degree of protection against both esophageal SCC (OR, 0.58; 95% CI, 0.43 to 0.78) and esophageal adenocarcinoma (OR, 0.67; 95% CI, 0.51 to 0.87). The mechanism of the risk reduction with aspirin and NSAIDs is still unclear but may be associated with inhibition of the cyclooxygenase-2 enzyme and the reduction of inflammation [33–35].
Clinical Manifestations
Esophageal cancer commonly presents with dysphagia, weight loss, gastrointestinal reflux, and/or odynophagia. In a study by Daly et al, 74% of esophageal cancer patients reported dysphagia and 16.6% reported having odynophagia at the time of initial diagnosis [36]. Patients can have the sensation of food getting “stuck,” which initially can be overcome by careful chewing and/or dietary modification [37]. A history of trouble swallowing solid foods followed by difficulty with drinking liquids is frequently seen. Some patients complain of regurgitation of undigested foods, and approximately 20% of patients have reported having GERD symptoms [36,37]. Due to the complete or partial esophageal obstruction combined with tumor effects, patients with esophageal cancer often develop significant weight loss. In the study by Daly et al, 57.3% of patients reported weight loss at the time of their cancer diagnosis [36]. Weight loss of more than 10% body mass has been identified as an independent indicator for poor prognosis [36,38]. Pain, dyspnea, hoarseness, and cough occur less frequently but may reflect extensive cancer burden [39]. Some patients with advanced tumors have hematemesis from tumor erosion or have recurrent pneumonias due to tracheobronchial fistulas.
Hepatomegaly, pleural effusion, and lymphadenopathy, especially in Virchow’s node (left supraclavicular fossa), are physical examination findings suggestive of metastatic disease [39]. However, most patients with esophageal cancer will have unremarkable physical examination findings.
It should be noted that patients with early stage lesions (ie, stage T1 lesions) may have minimal or no symptoms, with lesions detected either incidentally or as part of endoscopic screening/surveillance programs.
Diagnostic Studies
For patients with suspected esophageal cancer, a barium swallow is an inexpensive and readily available diagnostic study [39]. A barium swallow may show a mass lesion and/or a stricture. If the barium swallow is suggestive of cancer, the diagnosis is usually confirmed via an esophagogastroduodenoscopy (EGD) and biopsies, although in practice many patients with dysphagia and/or a history suspicious for esophageal cancer will proceed directly to EGD [40]. Findings suspicious for cancer are routinely biopsied [39].Traditionally, the more biopsies obtained (up to 7), the higher the diagnostic yield of cancer [41]. The addition of brush cytology to biopsies has also been found to increase the diagnostic accuracy, although this is not widely performed [41].
Once the diagnosis of cancer is confirmed, a computed tomography (CT) scan of the chest, abdomen, and pelvis with intravenous (IV) contrast is usually the next step in the patient’s evaluation, primarily to detect distant metastasis and to look for peritumoral adenopathy [39]. However, in terms of locoregional tumor staging, CT scans are less sensitive and specific than endoscopic ultrasonography (EUS) [42]. Patients who do not have evidence of metastasis on CT scan typically undergo EUS for definitive locoregional staging.
EUS does have its limitations. Between 25% and 36% of patients with esophageal carcinoma present with high-grade malignant strictures that do not allow passage of the scope, although if the exam can show malignant adenopathy and/or tumor extension through the muscularis propria, further evaluation is often of little additional benefit [46]. Dilation of malignant esophageal strictures to facilitate EUS is uncommon as there is a high risk of perforation (up to 24%) [47]. High-frequency (12.5 MHz) EUS mini-probes have been used to interrogate tumors with a very narrow lumen; however, the mini-probes are limited by the penetration depth of the transducer, which can lead to an incomplete locoregional tumor assessment [48]. EUS is not usually used for restaging after neoadjuvant therapy [49].
Endoscopic mucosal resection (EMR) is another technique for staging and treatment of superficial neoplasms (see Treatment section for more details). EMR is critical for distinguishing between T1a lesions (often candidates for definitive endoscopic therapy given the low likelihood of nodal involvement) versus T1b lesions (invasive to submucosa and more likely to prompt surgical esophagectomy with lymph node sampling). The distinction between T1a and T1b disease cannot be established as reliably by EUS when compared with EMR. The American Society for Gastrointestinal Endoscopy 2013 guidelines recommend EMR for the treatment and staging of nodular Barrett’s esophagus and suspected intramucosal adenocarcinoma [50].
Looking for distant metastasis, or M staging, is carried out with EUS, diagnostic laparoscopy/thoracoscopy, and CT and/or positron emission tomography (PET) scans. Despite the high accuracy of esophageal cancer staging with laparoscopy and thoracoscopy, these are invasive procedures and have generally been replaced by PET scan [39,51,52]. PET with 18F-fludeoxyglucose has been shown to significantly improve the detection rate of metastatic disease compared with the conventional staging methods (CT scan and EUS) [53]. In a prospective study, PET scans detected metastasis in 15% of patients who were thought to have localized cancer by conventional staging modalities [39,54].
Unlike several other cancers, tumor markers such as carbohydrate antigen (CA) 19-9, CA 125, and carcinoembryonic antigen (CEA) have low specificity and sensitivity in esophageal cancer and are not routinely obtained and/or followed [39,55].
Staging
Treatment
Early Stage
Historically, patients with early stage esophageal cancer (those without evidence of deep invasion into the esophageal wall and no evidence of peritumoral malignant adenopathy or metastases, typically T1N0M0) were referred for esophagectomy [59]. Recent treatment trends suggest proportionately more patients with T1 disease are being treated endoscopically (up to 29% of patients) and proportionately fewer with esophagectomy [60]. EMR has emerged as a viable alternative treatment to esophagectomy when the lesion is staged T1aN0 (tumor invading the lamina propria or muscularis mucosae but not the submucosa) [3]. EMR is performed via several techniques, but most commonly as follows. First, saline is injected under the lesion to create a submucosal cushion, separating the lesion from the underlying muscularis propria. The actual endoscopic resection of the lesion is usually accomplished via snare electrocautery and the resected lesion is sent for pathologic analysis. Endoscopic caps and band ligation devices are available to facilitate removal of the lesion in one or more pieces [61].
In a retrospective cohort study by Prasad et al of 178 patients from 1998 to 2007, the cumulative mortality in the EMR group was comparable to that of the surgery group (17% vs. 20%, respectively, P = 0.75) [62]. Recurrent cancer was detected in 12% of EMR patients; however, all patients were successfully re-treated without affecting overall survival.
In another study of 742 patients, long-term survival in those with early esophageal cancer managed with endoscopic therapy was comparable to that in patients treated with surgical resection [63]. The median cancer-free survival in the endoscopic group was not significantly different from that in the surgical group (56 and 59 months, respectively, P = 0.41) The study found that the relative hazard for 1esophageal cancer–specific mortality in the endoscopic group did not differ from that of the surgical group (relative hazard, 0.89; 95% CI, 0.51 to 1.56; P = 0.68).
Locally Advanced Disease
Neoadjuvant Therapy
For patients with locally advanced cancer (ie, patients without distant metastases who have extension of the primary tumor into the deeper layers of the esophageal wall, including the muscularis propria and the adventitia with or without peritumoral malignant adenopathy, or T2 or T3 lesions with N0 or N1, N2, or N3 status, neoadjuvant therapy is the norm, although the optimal management remains controversial and treatment protocols vary around the world [3,62]. Most neoadjuvant therapy regimens in the United States combine chemotherapy and external beam radiation therapy.
Neoadjuvant treatment with chemoradiation has been found to be beneficial in all esophageal cancers [3,64]. A meta-analysis of 1209 patients found a significant survival benefit for preoperative chemoradiotherapy and, to a lesser extent, for chemotherapy when compared to surgery alone [65]. When comparing neoadjuvant chemoradiotherapy to surgery alone, there was a 19% decrease in the risk of death corresponding to a 13% absolute difference in 2-year survival in the neoadjuvant chemotherapy group. HR for all-cause mortality with neoadjuvant chemoradiotherapy versus surgery alone was 0.81 (95% CI, 0.70 to 0.93; P = 0.002). The benefits of neoadjuvant chemoradiotherapy were similar for both esophageal SCC and adenocarcinoma. The benefits of chemotherapy, however, were less than chemoradiotherapy. When comparing neoadjuvant chemotherapy to surgery alone, there was an absolute survival benefit of 7%.
Following neoadjuvant therapy, patients typically undergo restaging via cross-sectional imaging, most commonly PET/CT scans. If the patient is felt to have active residual disease and has not developed metastases or contraindications to surgery, esophagectomy is appropriate. Some data suggests that patients with esophageal SCC who have complete clinical response after chemoradiation can be observed closely rather than proceed to surgery [3,62,66]. However, the data concerning the usefulness of definitive chemoradiotherapy in esophageal adenocarcinoma is lacking at this time. In a retrospective study of nonmetastatic esophageal adenocarcinoma patients by Tougeron et al comparing surgical patients (± preoperative treatment) to definitive chemoradiotherapy patients, a complete resection was achieved in 92.5% of patients in the surgical group and a clinical complete response was observed in 49.4% of patients with definitive chemoradiotherapy [67]. The overall survival was 36.2 ± 2 months for the surgery group versus 16.5 ± 0.8 months for the definitive chemoradiotherapy group (P = 0.02).
Stenting Prior to Neoadjuvant Therapy
In a meta-analysis of 9 studies comprising 180 patients, placement of esophageal stents in patients with locally advanced esophageal cancer significantly improved dysphagia and allowed for oral nutrition during neoadjuvant therapy [69]. There was a substantial decrease in the dysphagia scores standard difference in means (SDM) of –0.81 (standard error, 0.15; 95% CI, –1.1 to –0.51), an increase in weight SDM of 0.591 (standard error, 0.434; 95% CI, –0.261 to 1.442), and an increase in serum albumin SDM of 0.35 (standard error, 0.271; 95% CI, –0.181 to 0.881). The overall procedural success rate was 95% (95% CI, 0.895 to 0.977). Major adverse events included stent migration in 32% of patients (95% CI, 0.258 to 0.395) and chest discomfort in 51.4% (95% CI, 0.206 to 0.812). However, it was believed that the stent migration may have been a sign of tumor response to neoadjuvant therapy.
In a prospective nonrandomized study of 13 patients with polyflex stents (polyester mesh stents covered in a silicone membrane) placed prior to neoadjuvant therapy, similar improvements with dysphagia scores were observed after stent placement [70]. In the study, the mean baseline dysphagia score at the time of stent placement was 3. Dysphagia scores were subsequently obtained at 1, 2, 3, and 4 weeks after stent placement and were 1.1, 0.8, 0.9, and 1.0, respectively (P = 0.005, P = 0.01, P = 0.02, and P = 0.008, respectively). There were no episodes of bleeding or esophageal perforation. Immediate complications from stenting included chest discomfort, seen in 12 of the 13 patients. Stent migration occurred at some point in 6 of 13 patients, although not all patients with a migrated stent required stent replacement. Again, it was thought that the stent migration could be a sign of tumor response to neoadjuvant therapy.
Surgery
Surgery is an essential part of treatment of esophageal cancer [3,71]. Transthoracic, transhiatal, and radical (en bloc) are the 3 different basic approaches for esophagectomy [3]. Because it does not require a thoracotomy, the transhiatal approach has a theoretical advantage of decreased morbidity and mortality, although several studies have shown no differences in outcome between the transthoracic and transhiatal approach [3,72,73]. In a study by Chang et al comparing the transhiatal to the transthoracic approach, the 5-year survival was higher for patients undergoing transhiatal versus transthoracic esophagectomy (30.5% vs. 22.7%, P = 0.02) [73]. However, after adjusting for differences in tumor stage and patient and provider factors the survival advantage was no longer statistically significant (adjusted HR for mortality, 0.95; 95% CI, 0.75 to 1.20).
Adjuvant Therapy
Despite the benefits of chemoradiation as a neoadjuvant treatment, the data for chemoradiation as adjuvant therapy after resection is lacking in most clinical situations [74].
Metastatic Disease
Between 25% and 40% of esophageal cancer patients will present with metastases to liver, bone, and lung or widespread nodal metastases [61].Improvement of quality of life is a major goal in patients with unresectable disease. Patients with nonsurgical esophageal cancer who have an estimated life expectancy of greater than a few weeks are recommended to have concurrent chemoradiotherapy as most patients have symptomatic obstructive disease and dysphagia [62]. A study by Harvey et al examined the palliative benefit of chemoradiotherapy on dysphagia versus toxicity in patients with invasive esophageal carcinoma [75]. The study found that treatment was well tolerated, with only 5% of patients failing to complete treatment. The study used the DeMeester (4-point) symptom scores for the assessment of dysphagia. The median baseline score at presentation was 2 (moderate: difficulty with soft food, predominately liquid diet). After chemoradiotherapy, 49% of patients were assessed as having a dysphagia score of 0 (no dysphagia). Of those patients who received chemoradiotherapy, 78% had an improvement of at least 1 grade in their DeMeester dysphagia, while only 14% of patients did not improve with therapy. The median survival for the study population was 7 months, with a 6% treatment-related mortality. Chemoradiation therapy as a primary treatment for dysphagia can take days to weeks to take effect, and can be associated with significant pain, usually from radiation esophagitis.
Other alternatives for palliation of nonresectable esophageal cancer include esophageal stenting with SEMS and brachytherapy. SEMS are effective and safe for palliation of dysphagia caused by primary esophageal tumors, postoperative cancer recurrence, esophagorespiratory fistulae, and tumors near the upper esophageal sphincter [76]. A study looking at the use of esophageal SEMS in cancer found that after SEMS placement, the dysphagia score improved from a mean of 3.6 to 1.6 (P < 0.001) [75]. The procedure was technically successful in 96% of the patients. In all cases, esophagorespiratory fistulas were occluded. Pain, reflux, and stent migration are the most common complications of esophageal SEMS.
In a study comparing single-dose brachytherapy versus SEMS, the SEMS group had quicker improvement of dysphagia symptoms than the brachytherapy group, but the long-term relief of dysphagia was better after brachytherapy [77]. In addition, SEMS placement had more complications than brachytherapy (33% vs. 21%, respectively; P = 0.02), which was mainly due to an increased incidence of late hemorrhage. However, brachytherapy and SEMS did not differ in terms of median survival (P = 0.23) or recurrent or persistent dysphagia (P = 0.81).
Tracheoesophageal fistulas may develop in the setting of a locally advanced tumor, or as a complication of RT or chemoradiotherapy. SEMS can also be used successfully in the palliation therapy for tracheoesophageal fistulas or post-esophagectomy anastomotic strictures [78].
Prognosis
The overall survival for patients with resectable esophageal cancer has improved significantly over the past 30 years; however, more than 50% of patients presenting with esophageal cancer will have unresectable or metastatic disease at the time of presentation [3,39,79].Prognosis is primarily TMN stage–dependent, as patients with early stage cancer limited to the mucosa are expected to have curable disease [3]. Poor prognostic predictors include advanced stage cancer, dysphagia, advanced age, large tumors, more than 10% loss in body mass, and malignant adenopathy [39,80–84].
In 2010, the American Joint Committee on Cancer/International Union against Cancer Staging system looked at the prognosis of 4627 patients who underwent esophagectomy alone without radiation or chemotherapy [3,56]. For stage Tis (tumor in situ or high-grade dysplasia) and 1A cancers, there was an approximate 80% 5-year risk-adjusted survival rate [3,56]. The survival rate was marginally better for esophageal adenocarcinoma than for esophageal SCC. With surgery alone, stage 1B disease had a 5-year survival of 62% with SCC and 64% with adenocarcinoma [3,56]. For patients with stage 2A cancer, the 5-year survival was 55% for SCC and 50% for adenocarcinoma as long as there was not nodal involvement [3,56]. If there was nodal involvement, the survival rate dropped to 40% for stage 2B cancer, 25% for stage 3A cancer, and 15% to 17% for stage 3B to 3C cancer [3,56]. As stated earlier, neoadjuvant chemoradiation helps improve outcomes when compared to surgery alone (see Neoadjuvant Therapy in the Treatment section). Thus, one would expect a slightly better prognosis with neoadjuvant therapy and surgery than the previously stated data for surgery alone. Unfortunately, patients with unresectable or metastatic disease at time of diagnosis have a poor prognosis, with a 1-year survival rate less than 20% [3].
Esophageal cancer patients who are treated successfully need to be followed closely because a majority of esophageal cancers will recur within 3 years of treatment [61]. For the first 3 years post treatment, patients should be followed every 3 to 6 months [61]. For 3 to 5 years after treatment, patients should be followed every 6 months and annually thereafter [61]. During each visit, patients should have a thorough history and physical exam and assessment of quality of life [61]. Laboratory studies and EGD are performed as clinically indicated [61]. The importance of intensive post-treatment endoscopic surveillance should be emphasized given a defined rate of disease recurrence. Additionally, radiographic imaging such as CT of the chest and abdomen with contrast or PET/CT may be needed for restaging purposes [61].
Conclusion
Esophageal adenocarcinoma and esophageal SCC are aggressive cancers with poor prognosis. Overall, esophageal adenocarcinoma has increased in incidence, while the incidence of SCC has decreased in the Western world. GERD is the most common cause of esophageal adenocarcinoma, whereas increased alcohol consumption and tobacco commonly lead to esophageal SCC.
For patients with suspected esophageal cancer, a barium swallow is an inexpensive initial diagnostic study that is usually followed up with EGD with biopsies if suggestive of cancer. Once cancer is confirmed, a CT scan with intravenous contrast is obtained to look for adenopathy and metastasis. Those who do not have evidence of metastasis on CT scan typically undergo EUS for definitive locoregional staging.
In the past, patients with early stage esophageal cancer were referred for esophagectomy, but recently EMR has emerged as a viable alternative. Patients with locally advanced esophageal cancers are usually treated with neoadjuvant chemoradiation in combination with surgery. In addition, several studies have showed that esophageal stenting prior to neoadjuvant treatment significantly improves patients’ dysphagia. Unfortunately, many patients still initially present with metastatic or nonresectable disease. Improvement of quality of life is a major goal in patients with unresectable disease. Chemoradiotherapy, esophageal stenting, and brachytherapy are options for improvement of quality of life. Further studies are still needed to evaluate current and new therapeutic guidelines for resectable and nonresectable disease.
Corresponding author: Douglas G. Adler, MD, 30N 1900E 4R118, Salt Lake City, UT 84132, [email protected].
Financial disclosures: None.
From the University of Utah School of Medicine, Salt Lake City, UT.
Abstract
- Objective: To review the evaluation, diagnosis, and management of patients with esophageal cancer.
- Methods: Review of the literature.
- Results: Esophageal adenocarcinoma and esophageal squamous cell carcinoma (SCC) are aggressive cancers with a poor prognosis. GERD is the most common cause of esophageal adenocarcinoma, whereas increased alcohol consumption and tobacco commonly lead to esophageal SCC. Diagnosis is made via esophagogastroduodenoscopy and biopsies, and endoscopic ultrasound is typically used for locoregional staging. The endoscopic treatment of dysphagia is complex and several treatment options are available. Patients with locally advanced esophageal cancers are usually treated with neoadjuvant chemoradiation in combination with surgery. Improvement of quality of life is a major goal in patients with unresectable disease.
- Conclusion: Esophageal cancer remains a commonly encountered clinical entity requiring multidisciplinary evaluation and treatment.
Esophageal cancer is an aggressive disease with an overall poor outcome. It is the eighth most common cancer and sixth most common cause of cancer-related death worldwide [1]. In 2012, there were an estimated 456,000 new diagnoses of esophageal cancer and 400,000 deaths worldwide [1]. In the United States alone, an estimated 18,170 cases of esophageal cancer will be diagnosed in 2014, with 15,450 expected deaths [2].
Esophageal cancer includes 2 distinct histologic diseases: esophageal adenocarcinoma and esophageal squamous cell carcinoma (SCC). Overall, esophageal adenocarcinoma has increased in incidence, while the incidence of SCC has decreased in the Western world due to long-term reductions in smoking and alcohol consumption and increased incidence of gastroesophageal reflux disease (GERD) and obesity [3,4]. Esophageal adenocarcinoma accounted for less than 15% of esophageal cancers in the early 1980s, but now represents more than 60% of all esophageal cancers in the United States [5]. Esophageal SCC is still more common in China, central Asia, sub-Saharan Africa, and India and among the African-American and Caucasian female population in the United States [3,5].
Etiology
Esophageal Adenocarcinoma
While GERD is the most common cause of esophageal adenocarcinoma, other important causes/risk factors have been identified such as male sex, Caucasian race, older age, and obesity [8,12].In a prospective study by Abnet et al, patients who had a body mass index (BMI) greater than 35 kg/m2 had a significantly increased risk of esophageal adenocarcinoma when compared to patients with a BMI of 18.5 to 25 kg/m2 (hazard ratio [HR], 2.27; 95% CI, 1.44 to 3.59) [13]. Similarly, a recent meta-analysis found that patients with a BMI of 30 kg/m2 or greater had a relative risk for esophageal adenocarcinoma of 2.71 (95% CI, 2.16 to 3.46) [14]. Despite the strong correlation, the etiology of esophageal adenocarcinoma is complex and cannot be fully explained by obesity trends [15].
Smoking is another important risk factor associated with the development of esophageal adenocarcinoma. A study from the Barrett’s and Esophageal Adenocarcinoma Consortium revealed strong associations with esophageal adenocarcinoma and cigarette smoking (OR, 1.96; 95% CI, 1.64 to 2.34) [16]. Furthermore, the study found a statistically significant dose-response association between cigarette smoking and esophageal adenocarcinoma (P < 0.001).
Finally, dietary intake of vegetables and fruits has been shown to reduce the risk of Barrett’s esophagus. In a case-control study, patients with a median intake of 8.3 servings per day of vegetables and fruits had a 73% lower risk of developing Barrett’s esophagus versus those with 2.0 servings per day (OR, 0.27; 95% CI, 0.15 to 0.50) [17]. Each additional serving of vegetables and fruit was associated with a 14% reduction of risk (OR, 0.86; 95% CI, 0.80 to 0.93).
Esophageal Squamous Cell Carcinoma
In the study by Freedman et al, when compared with nonsmokers, current cigarette smokers were at significantly increased risk for esophageal SCC (HR, 9.27; 95% CI, 4.04 to 21.29) [18].Smoking has a stronger correlation with esophageal SCC than with esophageal adenocarcinoma [20]. In current smokers, the risk for developing esophageal SCC increases approximately three- to sevenfold [20]. The duration and intensity of smoking has been shown to increase the risk of esophageal SCC as well [21]. Smoking cessation has been shown to reduce the risk of esophageal SCC, but data shows that former cigarette smokers still are at a significant risk [18,21]. In a population-based case-control study, the risk of esophageal SCC in ex-smokers remained elevated for up to 30 years (OR, 1.44; 95% CI, 0.82 to 2.52) [21].
There are only limited studies that have examined the relationship between esophageal SCC and smokeless tobacco and other smoking products. Despite the limited number of studies, smokeless tobacco has been associated with esophageal SCC [22]. In a 2012 study of patients from India, chewing nass (a mix of tobacco, ash, oil, lime, and coloring and flavoring agents) and smoking hookah were associated with an increased risk of developing esophageal SCC [23].
Other risk factors associated with esophageal SCC include poor oral hygiene, atrophic gastritis, caustic esophageal injuries, and achalasia (likely due to stasis of esophageal contents in the case of achalasia) [24–27].Dietary causes of esophageal SCC have also been implicated in many international studies. Foods containing N-nitroso compounds and diets with selenium and zinc mineral deficiencies have been found to be risk factors for esophageal SCC [20,28–30].Thermal injury to the esophageal mucosa caused by food and beverages served at high temperatures has been shown to increase the risk of esophageal cancer [31]. Also, as seen in esophageal adenocarcinoma, diets rich with vegetables and fruits have been associated with a reduced risk of esophageal SCC [32].
In a meta-analysis of 1813 esophageal cancer cases by Corley et al, the use of aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) was found to be protective against both esophageal SCC and esophageal adenocarcinoma [33]. The study found a dose-dependent effect in the protective association between aspirin/NSAID use and esophageal cancer. Frequent aspirin/NSAID use was associated with a 46% reduction of the odds for developing any esophageal cancer, whereas intermittent use provided an 18% reduction in the odds. However, any use of aspirin or NSAIDs offered some degree of protection against both esophageal SCC (OR, 0.58; 95% CI, 0.43 to 0.78) and esophageal adenocarcinoma (OR, 0.67; 95% CI, 0.51 to 0.87). The mechanism of the risk reduction with aspirin and NSAIDs is still unclear but may be associated with inhibition of the cyclooxygenase-2 enzyme and the reduction of inflammation [33–35].
Clinical Manifestations
Esophageal cancer commonly presents with dysphagia, weight loss, gastrointestinal reflux, and/or odynophagia. In a study by Daly et al, 74% of esophageal cancer patients reported dysphagia and 16.6% reported having odynophagia at the time of initial diagnosis [36]. Patients can have the sensation of food getting “stuck,” which initially can be overcome by careful chewing and/or dietary modification [37]. A history of trouble swallowing solid foods followed by difficulty with drinking liquids is frequently seen. Some patients complain of regurgitation of undigested foods, and approximately 20% of patients have reported having GERD symptoms [36,37]. Due to the complete or partial esophageal obstruction combined with tumor effects, patients with esophageal cancer often develop significant weight loss. In the study by Daly et al, 57.3% of patients reported weight loss at the time of their cancer diagnosis [36]. Weight loss of more than 10% body mass has been identified as an independent indicator for poor prognosis [36,38]. Pain, dyspnea, hoarseness, and cough occur less frequently but may reflect extensive cancer burden [39]. Some patients with advanced tumors have hematemesis from tumor erosion or have recurrent pneumonias due to tracheobronchial fistulas.
Hepatomegaly, pleural effusion, and lymphadenopathy, especially in Virchow’s node (left supraclavicular fossa), are physical examination findings suggestive of metastatic disease [39]. However, most patients with esophageal cancer will have unremarkable physical examination findings.
It should be noted that patients with early stage lesions (ie, stage T1 lesions) may have minimal or no symptoms, with lesions detected either incidentally or as part of endoscopic screening/surveillance programs.
Diagnostic Studies
For patients with suspected esophageal cancer, a barium swallow is an inexpensive and readily available diagnostic study [39]. A barium swallow may show a mass lesion and/or a stricture. If the barium swallow is suggestive of cancer, the diagnosis is usually confirmed via an esophagogastroduodenoscopy (EGD) and biopsies, although in practice many patients with dysphagia and/or a history suspicious for esophageal cancer will proceed directly to EGD [40]. Findings suspicious for cancer are routinely biopsied [39].Traditionally, the more biopsies obtained (up to 7), the higher the diagnostic yield of cancer [41]. The addition of brush cytology to biopsies has also been found to increase the diagnostic accuracy, although this is not widely performed [41].
Once the diagnosis of cancer is confirmed, a computed tomography (CT) scan of the chest, abdomen, and pelvis with intravenous (IV) contrast is usually the next step in the patient’s evaluation, primarily to detect distant metastasis and to look for peritumoral adenopathy [39]. However, in terms of locoregional tumor staging, CT scans are less sensitive and specific than endoscopic ultrasonography (EUS) [42]. Patients who do not have evidence of metastasis on CT scan typically undergo EUS for definitive locoregional staging.
EUS does have its limitations. Between 25% and 36% of patients with esophageal carcinoma present with high-grade malignant strictures that do not allow passage of the scope, although if the exam can show malignant adenopathy and/or tumor extension through the muscularis propria, further evaluation is often of little additional benefit [46]. Dilation of malignant esophageal strictures to facilitate EUS is uncommon as there is a high risk of perforation (up to 24%) [47]. High-frequency (12.5 MHz) EUS mini-probes have been used to interrogate tumors with a very narrow lumen; however, the mini-probes are limited by the penetration depth of the transducer, which can lead to an incomplete locoregional tumor assessment [48]. EUS is not usually used for restaging after neoadjuvant therapy [49].
Endoscopic mucosal resection (EMR) is another technique for staging and treatment of superficial neoplasms (see Treatment section for more details). EMR is critical for distinguishing between T1a lesions (often candidates for definitive endoscopic therapy given the low likelihood of nodal involvement) versus T1b lesions (invasive to submucosa and more likely to prompt surgical esophagectomy with lymph node sampling). The distinction between T1a and T1b disease cannot be established as reliably by EUS when compared with EMR. The American Society for Gastrointestinal Endoscopy 2013 guidelines recommend EMR for the treatment and staging of nodular Barrett’s esophagus and suspected intramucosal adenocarcinoma [50].
Looking for distant metastasis, or M staging, is carried out with EUS, diagnostic laparoscopy/thoracoscopy, and CT and/or positron emission tomography (PET) scans. Despite the high accuracy of esophageal cancer staging with laparoscopy and thoracoscopy, these are invasive procedures and have generally been replaced by PET scan [39,51,52]. PET with 18F-fludeoxyglucose has been shown to significantly improve the detection rate of metastatic disease compared with the conventional staging methods (CT scan and EUS) [53]. In a prospective study, PET scans detected metastasis in 15% of patients who were thought to have localized cancer by conventional staging modalities [39,54].
Unlike several other cancers, tumor markers such as carbohydrate antigen (CA) 19-9, CA 125, and carcinoembryonic antigen (CEA) have low specificity and sensitivity in esophageal cancer and are not routinely obtained and/or followed [39,55].
Staging
Treatment
Early Stage
Historically, patients with early stage esophageal cancer (those without evidence of deep invasion into the esophageal wall and no evidence of peritumoral malignant adenopathy or metastases, typically T1N0M0) were referred for esophagectomy [59]. Recent treatment trends suggest proportionately more patients with T1 disease are being treated endoscopically (up to 29% of patients) and proportionately fewer with esophagectomy [60]. EMR has emerged as a viable alternative treatment to esophagectomy when the lesion is staged T1aN0 (tumor invading the lamina propria or muscularis mucosae but not the submucosa) [3]. EMR is performed via several techniques, but most commonly as follows. First, saline is injected under the lesion to create a submucosal cushion, separating the lesion from the underlying muscularis propria. The actual endoscopic resection of the lesion is usually accomplished via snare electrocautery and the resected lesion is sent for pathologic analysis. Endoscopic caps and band ligation devices are available to facilitate removal of the lesion in one or more pieces [61].
In a retrospective cohort study by Prasad et al of 178 patients from 1998 to 2007, the cumulative mortality in the EMR group was comparable to that of the surgery group (17% vs. 20%, respectively, P = 0.75) [62]. Recurrent cancer was detected in 12% of EMR patients; however, all patients were successfully re-treated without affecting overall survival.
In another study of 742 patients, long-term survival in those with early esophageal cancer managed with endoscopic therapy was comparable to that in patients treated with surgical resection [63]. The median cancer-free survival in the endoscopic group was not significantly different from that in the surgical group (56 and 59 months, respectively, P = 0.41) The study found that the relative hazard for 1esophageal cancer–specific mortality in the endoscopic group did not differ from that of the surgical group (relative hazard, 0.89; 95% CI, 0.51 to 1.56; P = 0.68).
Locally Advanced Disease
Neoadjuvant Therapy
For patients with locally advanced cancer (ie, patients without distant metastases who have extension of the primary tumor into the deeper layers of the esophageal wall, including the muscularis propria and the adventitia with or without peritumoral malignant adenopathy, or T2 or T3 lesions with N0 or N1, N2, or N3 status, neoadjuvant therapy is the norm, although the optimal management remains controversial and treatment protocols vary around the world [3,62]. Most neoadjuvant therapy regimens in the United States combine chemotherapy and external beam radiation therapy.
Neoadjuvant treatment with chemoradiation has been found to be beneficial in all esophageal cancers [3,64]. A meta-analysis of 1209 patients found a significant survival benefit for preoperative chemoradiotherapy and, to a lesser extent, for chemotherapy when compared to surgery alone [65]. When comparing neoadjuvant chemoradiotherapy to surgery alone, there was a 19% decrease in the risk of death corresponding to a 13% absolute difference in 2-year survival in the neoadjuvant chemotherapy group. HR for all-cause mortality with neoadjuvant chemoradiotherapy versus surgery alone was 0.81 (95% CI, 0.70 to 0.93; P = 0.002). The benefits of neoadjuvant chemoradiotherapy were similar for both esophageal SCC and adenocarcinoma. The benefits of chemotherapy, however, were less than chemoradiotherapy. When comparing neoadjuvant chemotherapy to surgery alone, there was an absolute survival benefit of 7%.
Following neoadjuvant therapy, patients typically undergo restaging via cross-sectional imaging, most commonly PET/CT scans. If the patient is felt to have active residual disease and has not developed metastases or contraindications to surgery, esophagectomy is appropriate. Some data suggests that patients with esophageal SCC who have complete clinical response after chemoradiation can be observed closely rather than proceed to surgery [3,62,66]. However, the data concerning the usefulness of definitive chemoradiotherapy in esophageal adenocarcinoma is lacking at this time. In a retrospective study of nonmetastatic esophageal adenocarcinoma patients by Tougeron et al comparing surgical patients (± preoperative treatment) to definitive chemoradiotherapy patients, a complete resection was achieved in 92.5% of patients in the surgical group and a clinical complete response was observed in 49.4% of patients with definitive chemoradiotherapy [67]. The overall survival was 36.2 ± 2 months for the surgery group versus 16.5 ± 0.8 months for the definitive chemoradiotherapy group (P = 0.02).
Stenting Prior to Neoadjuvant Therapy
In a meta-analysis of 9 studies comprising 180 patients, placement of esophageal stents in patients with locally advanced esophageal cancer significantly improved dysphagia and allowed for oral nutrition during neoadjuvant therapy [69]. There was a substantial decrease in the dysphagia scores standard difference in means (SDM) of –0.81 (standard error, 0.15; 95% CI, –1.1 to –0.51), an increase in weight SDM of 0.591 (standard error, 0.434; 95% CI, –0.261 to 1.442), and an increase in serum albumin SDM of 0.35 (standard error, 0.271; 95% CI, –0.181 to 0.881). The overall procedural success rate was 95% (95% CI, 0.895 to 0.977). Major adverse events included stent migration in 32% of patients (95% CI, 0.258 to 0.395) and chest discomfort in 51.4% (95% CI, 0.206 to 0.812). However, it was believed that the stent migration may have been a sign of tumor response to neoadjuvant therapy.
In a prospective nonrandomized study of 13 patients with polyflex stents (polyester mesh stents covered in a silicone membrane) placed prior to neoadjuvant therapy, similar improvements with dysphagia scores were observed after stent placement [70]. In the study, the mean baseline dysphagia score at the time of stent placement was 3. Dysphagia scores were subsequently obtained at 1, 2, 3, and 4 weeks after stent placement and were 1.1, 0.8, 0.9, and 1.0, respectively (P = 0.005, P = 0.01, P = 0.02, and P = 0.008, respectively). There were no episodes of bleeding or esophageal perforation. Immediate complications from stenting included chest discomfort, seen in 12 of the 13 patients. Stent migration occurred at some point in 6 of 13 patients, although not all patients with a migrated stent required stent replacement. Again, it was thought that the stent migration could be a sign of tumor response to neoadjuvant therapy.
Surgery
Surgery is an essential part of treatment of esophageal cancer [3,71]. Transthoracic, transhiatal, and radical (en bloc) are the 3 different basic approaches for esophagectomy [3]. Because it does not require a thoracotomy, the transhiatal approach has a theoretical advantage of decreased morbidity and mortality, although several studies have shown no differences in outcome between the transthoracic and transhiatal approach [3,72,73]. In a study by Chang et al comparing the transhiatal to the transthoracic approach, the 5-year survival was higher for patients undergoing transhiatal versus transthoracic esophagectomy (30.5% vs. 22.7%, P = 0.02) [73]. However, after adjusting for differences in tumor stage and patient and provider factors the survival advantage was no longer statistically significant (adjusted HR for mortality, 0.95; 95% CI, 0.75 to 1.20).
Adjuvant Therapy
Despite the benefits of chemoradiation as a neoadjuvant treatment, the data for chemoradiation as adjuvant therapy after resection is lacking in most clinical situations [74].
Metastatic Disease
Between 25% and 40% of esophageal cancer patients will present with metastases to liver, bone, and lung or widespread nodal metastases [61].Improvement of quality of life is a major goal in patients with unresectable disease. Patients with nonsurgical esophageal cancer who have an estimated life expectancy of greater than a few weeks are recommended to have concurrent chemoradiotherapy as most patients have symptomatic obstructive disease and dysphagia [62]. A study by Harvey et al examined the palliative benefit of chemoradiotherapy on dysphagia versus toxicity in patients with invasive esophageal carcinoma [75]. The study found that treatment was well tolerated, with only 5% of patients failing to complete treatment. The study used the DeMeester (4-point) symptom scores for the assessment of dysphagia. The median baseline score at presentation was 2 (moderate: difficulty with soft food, predominately liquid diet). After chemoradiotherapy, 49% of patients were assessed as having a dysphagia score of 0 (no dysphagia). Of those patients who received chemoradiotherapy, 78% had an improvement of at least 1 grade in their DeMeester dysphagia, while only 14% of patients did not improve with therapy. The median survival for the study population was 7 months, with a 6% treatment-related mortality. Chemoradiation therapy as a primary treatment for dysphagia can take days to weeks to take effect, and can be associated with significant pain, usually from radiation esophagitis.
Other alternatives for palliation of nonresectable esophageal cancer include esophageal stenting with SEMS and brachytherapy. SEMS are effective and safe for palliation of dysphagia caused by primary esophageal tumors, postoperative cancer recurrence, esophagorespiratory fistulae, and tumors near the upper esophageal sphincter [76]. A study looking at the use of esophageal SEMS in cancer found that after SEMS placement, the dysphagia score improved from a mean of 3.6 to 1.6 (P < 0.001) [75]. The procedure was technically successful in 96% of the patients. In all cases, esophagorespiratory fistulas were occluded. Pain, reflux, and stent migration are the most common complications of esophageal SEMS.
In a study comparing single-dose brachytherapy versus SEMS, the SEMS group had quicker improvement of dysphagia symptoms than the brachytherapy group, but the long-term relief of dysphagia was better after brachytherapy [77]. In addition, SEMS placement had more complications than brachytherapy (33% vs. 21%, respectively; P = 0.02), which was mainly due to an increased incidence of late hemorrhage. However, brachytherapy and SEMS did not differ in terms of median survival (P = 0.23) or recurrent or persistent dysphagia (P = 0.81).
Tracheoesophageal fistulas may develop in the setting of a locally advanced tumor, or as a complication of RT or chemoradiotherapy. SEMS can also be used successfully in the palliation therapy for tracheoesophageal fistulas or post-esophagectomy anastomotic strictures [78].
Prognosis
The overall survival for patients with resectable esophageal cancer has improved significantly over the past 30 years; however, more than 50% of patients presenting with esophageal cancer will have unresectable or metastatic disease at the time of presentation [3,39,79].Prognosis is primarily TMN stage–dependent, as patients with early stage cancer limited to the mucosa are expected to have curable disease [3]. Poor prognostic predictors include advanced stage cancer, dysphagia, advanced age, large tumors, more than 10% loss in body mass, and malignant adenopathy [39,80–84].
In 2010, the American Joint Committee on Cancer/International Union against Cancer Staging system looked at the prognosis of 4627 patients who underwent esophagectomy alone without radiation or chemotherapy [3,56]. For stage Tis (tumor in situ or high-grade dysplasia) and 1A cancers, there was an approximate 80% 5-year risk-adjusted survival rate [3,56]. The survival rate was marginally better for esophageal adenocarcinoma than for esophageal SCC. With surgery alone, stage 1B disease had a 5-year survival of 62% with SCC and 64% with adenocarcinoma [3,56]. For patients with stage 2A cancer, the 5-year survival was 55% for SCC and 50% for adenocarcinoma as long as there was not nodal involvement [3,56]. If there was nodal involvement, the survival rate dropped to 40% for stage 2B cancer, 25% for stage 3A cancer, and 15% to 17% for stage 3B to 3C cancer [3,56]. As stated earlier, neoadjuvant chemoradiation helps improve outcomes when compared to surgery alone (see Neoadjuvant Therapy in the Treatment section). Thus, one would expect a slightly better prognosis with neoadjuvant therapy and surgery than the previously stated data for surgery alone. Unfortunately, patients with unresectable or metastatic disease at time of diagnosis have a poor prognosis, with a 1-year survival rate less than 20% [3].
Esophageal cancer patients who are treated successfully need to be followed closely because a majority of esophageal cancers will recur within 3 years of treatment [61]. For the first 3 years post treatment, patients should be followed every 3 to 6 months [61]. For 3 to 5 years after treatment, patients should be followed every 6 months and annually thereafter [61]. During each visit, patients should have a thorough history and physical exam and assessment of quality of life [61]. Laboratory studies and EGD are performed as clinically indicated [61]. The importance of intensive post-treatment endoscopic surveillance should be emphasized given a defined rate of disease recurrence. Additionally, radiographic imaging such as CT of the chest and abdomen with contrast or PET/CT may be needed for restaging purposes [61].
Conclusion
Esophageal adenocarcinoma and esophageal SCC are aggressive cancers with poor prognosis. Overall, esophageal adenocarcinoma has increased in incidence, while the incidence of SCC has decreased in the Western world. GERD is the most common cause of esophageal adenocarcinoma, whereas increased alcohol consumption and tobacco commonly lead to esophageal SCC.
For patients with suspected esophageal cancer, a barium swallow is an inexpensive initial diagnostic study that is usually followed up with EGD with biopsies if suggestive of cancer. Once cancer is confirmed, a CT scan with intravenous contrast is obtained to look for adenopathy and metastasis. Those who do not have evidence of metastasis on CT scan typically undergo EUS for definitive locoregional staging.
In the past, patients with early stage esophageal cancer were referred for esophagectomy, but recently EMR has emerged as a viable alternative. Patients with locally advanced esophageal cancers are usually treated with neoadjuvant chemoradiation in combination with surgery. In addition, several studies have showed that esophageal stenting prior to neoadjuvant treatment significantly improves patients’ dysphagia. Unfortunately, many patients still initially present with metastatic or nonresectable disease. Improvement of quality of life is a major goal in patients with unresectable disease. Chemoradiotherapy, esophageal stenting, and brachytherapy are options for improvement of quality of life. Further studies are still needed to evaluate current and new therapeutic guidelines for resectable and nonresectable disease.
Corresponding author: Douglas G. Adler, MD, 30N 1900E 4R118, Salt Lake City, UT 84132, [email protected].
Financial disclosures: None.
1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed on May 5, 2014.
2. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29.
3. Nieman DR, Peters JH. Treatment strategies for esophageal cancer. Gastroenterol Clin North Am 2013;42:187–97.
4. Shridhar R, Almhanna K, Meredith KL, et al. Radiation therapy and esophageal cancer. Cancer Control 2013;20:97–110.
5. Baquet CR, Commiskey P, Mack K, et al. Esophageal cancer epidemiology in blacks and whites: racial and gender disparities in incidence, mortality, survival rates and histology. J Natl Med Assoc 2005;97:1471–8.
6. Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophagealadenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222–7.
7. Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31.
8. Pohl H, Wrobel K, Bojarski C, et al. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol 2013;108:200–7.
9. Coleman HG, Bhat SK, Murray LJ, et al. Symptoms and endoscopic features at Barrett’s esophagus diagnosis: implications for neoplastic progression risk. Am J Gastroenterol 2014;109:527–34.
10. Nguyen DM, El-Serag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2009;7:1299–304.
11. Lagergren J, Ye W, Lagergren P, Lu Y. The risk of esophageal adenocarcinomaafter antireflux surgery. Gastroenterology 2010;138:1297–301.
12. el-Serag HB. The epidemic of esophageal adenocarcinoma. Gastroenterol Clin North Am 2002;31:421–40, viii.
13. Abnet CC, Freedman ND, Hollenbeck AR, et al. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer 2008;44:465–71.
14. Turati F, Tramacere I, La Vecchia C, Negri E. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol 2013;24:609–17.
15. Kroep S, Lansdorp-Vogelaar I, Rubenstein JH, et al. Comparing trends in esophageal adenocarcinoma incidence and lifestyle factors between the United States, Spain, and the Netherlands. Am J Gastroenterol 2014;109:336–43.
16. Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst 2010;102:1344–53.
17. Kubo A, Levin TR, Block G, et al. Dietary antioxidants, fruits, and vegetables and the risk of Barrett’s esophagus. Am J Gastroenterol 2008;103:1614–23.
18. Freedman ND, Abnet CC, Leitzmann MF, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol 2007;165:1424–33.
19. Pandeya N, Williams G, Green AC, et al; Australian Cancer Study. Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology 2009;136:1215–24, e1-2.
20. Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am 2009;38:27–57, vii.
21. Pandeya N, Williams GM, Sadhegi S, et al. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am J Epidemiol 2008;168:105–14.
22. Boffetta P, Hecht S, Gray N, et al. Smokeless tobacco and cancer. Lancet Oncol 2008;9:667–75.
23. Dar NA, Bhat GA, Shah IA, eta l. Hookah smoking, nass chewing, and oesophageal squamous cell carcinoma in Kashmir, India. Br J Cancer 2012;107:1618–23.
24. Abnet CC, Kamangar F, Islami F, et al. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2008;17:3062–8.
25. Islami F, Sheikhattari P, Ren JS, Kamangar F. Gastric atrophy and risk of oesophageal cancer and gastric cardia adenocarcinoma--a systematic review and meta-analysis. Ann Oncol 2011;22:754–60.
26. Appelqvist P, Salmo M. Lye corrosion carcinoma of the esophagus: a review of 63 cases. Cancer 1980;45:2655–8.
27. Sandler RS, Nyrén O, Ekbom A, et al. The risk of esophageal cancer in patients with achalasia. A population-based study. JAMA 1995;274:1359–62.
28. Lu SH, Montesano R, Zhang MS, et al. Relevance of N-nitrosamines to esophageal cancer in China. J Cell Physiol Suppl 1986;4:51–8.
29. Steevens J, van den Brandt PA, Goldbohm RA, Schouten LJ. Selenium status and the risk of esophageal and gastric cancer subtypes: the Netherlands cohort study. Gastroenterology 2010;138:1704–13.
30. Abnet CC, Lai B, Qiao YL, et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst 2005;97:301–6.
31. Islami F, Boffetta P, Ren JS, et al. High-temperature beverages and foods and esophageal cancer risk--a systematic review. Int J Cancer 2009;125:491–524.
32. Liu J, Wang J, Leng Y, Lv C. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer 2013;133:473–85.
33. Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 2003;124:47–56.
34. Ratnasinghe D, Tangrea J, Roth MJ, et al. Expression of cyclooxygenase-2 in human squamous cell carcinoma of the esophagus; an immunohistochemical survey. Anticancer Res 1999;19:171–4.
35. Morris CD, Armstrong GR, Bigley G, et al. Cyclooxygenase-2 expression in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol 2001;96:990–6.
36. Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000;190:562–72.
37. Gastrointestinal cancer. In: Fishman MC, Hoffman R, Klausner RD, Thaler MS, editors. Medicine. 5th ed. Baltimore: Lippincott Williams & Wilkins; 2002:423–6.
38. Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512–8.
39. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241–52.
40. Lightdale CJ. Esophageal cancer. Am J Gastroenterol 1999;94:20–9.
41. Graham DY, Schwartz JT, Cain GD, Gyorkey F. Prospective evaluation of biopsy number in the diagnosis of esophageal and gastric carcinoma. Gastroenterology 1982;82:228–31.
42. Lowe VJ, Booya F, Fletcher JG, et al. Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol Imaging Biol 2005;7:422–30.
43. Röesch T. Endoscopic ultrasonography: equipment and technique. Gastrointest Endosc Clin N Am 2005;15:13–31, vii.
44. Vazquez-Sequeiros E, Norton ID, Clain JE, et al. Impact of EUS-guided fine-needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest Endosc 2001;53:751–7.
45. Van Dam J. Endosonographic evaluation of the patient with esophageal cancer. Chest 1997;112(4 Suppl):184S–190S.
46. Catalano MF, Van Dam J, Sivak MV Jr. Malignant esophageal strictures: staging accuracy of endoscopic ultrasonography. Gastrointest Endosc 1995;41:535–9.
47. Van Dam J, Rice TW, Catalano MF, et al. High-grade malignant stricture is predictive of esophageal tumor stage. Risks of endosonographic evaluation. Cancer 1993;71:2910–7.
48. Hünerbein M, Ghadimi BM, Haensch W, Schlag PM. Transendoscopic ultrasound of esophageal and gastric cancer using miniaturized ultrasound catheter probes. Gastrointest Endosc 1998;48:371–5.
49. Zuccaro G Jr, Rice TW, Goldblum J, et al. Endoscopic ultrasound cannot determine suitability for esophagectomy after aggressive chemoradiotherapy for esophageal cancer. Am J Gastroenterol 1999;94:906–12.
50. ASGE Standards of Practice Committee, Evans JA, Early DS, Chandraskhara V, et al; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013;77:328–34.
51. Luketich JD, Schauer P, Landreneau R, et al. Minimally invasive surgical staging is superior to endoscopic ultrasound in detecting lymph node metastases in esophageal cancer. J Thorac Cardiovasc Surg 1997;114:817–21.
52. Krasna MJ, Flowers JL, Attar S, McLaughlin J. Combined thoracoscopic/laparoscopic staging of esophageal cancer. J Thorac Cardiovasc Surg 1996;111:800–6.
53. Flamen P, Lerut A, Van Cutsem E, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 2000;18:3202–10.
54. Downey RJ, Akhurst T, Ilson D, et al. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol 2003;21:428–32.
55. Mealy K, Feely J, Reid I, et al. Tumour marker detection in oesophageal carcinoma. Eur J Surg Oncol 1996;22:505–7.
56. Rice TW, Rusch VW, Ishwaran H, Blackstone EH; Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763–73.
57. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag; 2009:103–5.
58. Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1–8.
59. Villaflor VM, Allaix ME, Minsky B, et al. Multidisciplinary approach for patients with esophageal cancer. World J Gastroenterol 2012;18:6737–46.
60. Ngamruengphong S, Wolfsen HC, Wallace MB. Survival of patients with superficial esophageal adenocarcinoma after endoscopic treatment vs surgery. Clin Gastroenterol Hepatol 2013;11:1424–9.e2.
61. Lin SH, Chang JY. Esophageal cancer: diagnosis and management. Chin J Cancer 2010;29:843–54.
62. Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815–23.
63. Das A, Singh V, Fleischer DE, Sharma VK. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol 2008;103:1340–5.
64. Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593–8.
65. Gebski V, Burmeister B, Smithers BM, et al; Australasian Gastro-Intestinal Trials Group. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226–34.
66. Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160–8.
67. Tougeron D, Scotté M, Hamidou H, et al. Definitive chemoradiotherapy in patients with esophageal adenocarcinoma: an alternative to surgery? J Surg Oncol 2012;105: 761–6.
68. Siddiqui AA, Sarkar A, Beltz S, et al. Placement of fully covered self-expandable metal stents in patients with locally advanced esophageal cancer before neoadjuvant therapy. Gastrointest Endosc 2012;76:44–51.
69. Nagaraja V, Cox MR, Eslick GD. Safety and efficacy of esophageal stents preceding or during neoadjuvant chemotherapy for esophageal cancer: a systematic review and meta-analysis. J Gastrointest Oncol 2014;5:119–26.
70. Adler DG, Fang J, Wong R, et al. Placement of Polyflex stents in patients with locally advanced esophageal cancer is safe and improves dysphagia during neoadjuvant therapy. Gastrointest Endosc 2009;70:614–9.
71. Dubecz A, Sepesi B, Salvador R, et al. Surgical resection for locoregional esophageal cancer is underutilized in the United States. J Am Coll Surg 2010;211:754–61.
72. Hulscher JB, Tijssen JG, Obertop H, van Lanschot JJ. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 2001;72:306–13.
73. Chang AC, Ji H, Birkmeyer NJ, et al. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg 2008;85:424–9.
74. Almhanna K, Shridhar R, Meredith KL. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: is there a standard of care? Cancer Control 2013;20:89–96.
75. Harvey JA, Bessell JR, Beller E, et al. Chemoradiation therapy is effective for the palliative treatment of malignant dysphagia. Dis Esophagus 2004;17:260–5.
76. Siersema PD, Schrauwen SL, van Blankenstein M, et al; Rotterdam Esophageal Tumor Study Group. Self-expanding metal stents for complicated and recurrent esophagogastric cancer. Gastrointest Endosc 2001;54:579–86.
77. Homs MY, Steyerberg EW, Eijkenboom WM, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet 2004;364(9444):1497–504.
78. van den Bongard HJ, Boot H, Baas P, Taal BG. The role of parallel stent insertion in patients with esophagorespiratory fistulas. Gastrointest Endosc 2002;55:110–5.
79. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220–41.
80. Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512–8.
81. Mariette C, Maurel A, Fabre S, et al. [Preoperative prognostic factors for squamous cell carcinomas of the thoracic esophagus]. Gastroenterol Clin Biol 2001;25:468–72.
82. Swanson SJ, Batirel HF, Bueno R, et al. Transthoracic esophagectomy with radical mediastinal and abdominal lymph node dissection and cervical esophagogastrostomy for esophageal carcinoma. Ann Thorac Surg 2001;72:1918–24.
83. Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305–13.
84. Hosch SB, Stoecklein NH, Pichlmeier U, et al. Esophageal cancer: the mode of lymphatic tumor cell spread and its prognostic significance. J Clin Oncol 2001;19:1970–5.
1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed on May 5, 2014.
2. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29.
3. Nieman DR, Peters JH. Treatment strategies for esophageal cancer. Gastroenterol Clin North Am 2013;42:187–97.
4. Shridhar R, Almhanna K, Meredith KL, et al. Radiation therapy and esophageal cancer. Cancer Control 2013;20:97–110.
5. Baquet CR, Commiskey P, Mack K, et al. Esophageal cancer epidemiology in blacks and whites: racial and gender disparities in incidence, mortality, survival rates and histology. J Natl Med Assoc 2005;97:1471–8.
6. Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophagealadenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222–7.
7. Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31.
8. Pohl H, Wrobel K, Bojarski C, et al. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol 2013;108:200–7.
9. Coleman HG, Bhat SK, Murray LJ, et al. Symptoms and endoscopic features at Barrett’s esophagus diagnosis: implications for neoplastic progression risk. Am J Gastroenterol 2014;109:527–34.
10. Nguyen DM, El-Serag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2009;7:1299–304.
11. Lagergren J, Ye W, Lagergren P, Lu Y. The risk of esophageal adenocarcinomaafter antireflux surgery. Gastroenterology 2010;138:1297–301.
12. el-Serag HB. The epidemic of esophageal adenocarcinoma. Gastroenterol Clin North Am 2002;31:421–40, viii.
13. Abnet CC, Freedman ND, Hollenbeck AR, et al. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer 2008;44:465–71.
14. Turati F, Tramacere I, La Vecchia C, Negri E. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol 2013;24:609–17.
15. Kroep S, Lansdorp-Vogelaar I, Rubenstein JH, et al. Comparing trends in esophageal adenocarcinoma incidence and lifestyle factors between the United States, Spain, and the Netherlands. Am J Gastroenterol 2014;109:336–43.
16. Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst 2010;102:1344–53.
17. Kubo A, Levin TR, Block G, et al. Dietary antioxidants, fruits, and vegetables and the risk of Barrett’s esophagus. Am J Gastroenterol 2008;103:1614–23.
18. Freedman ND, Abnet CC, Leitzmann MF, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol 2007;165:1424–33.
19. Pandeya N, Williams G, Green AC, et al; Australian Cancer Study. Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology 2009;136:1215–24, e1-2.
20. Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am 2009;38:27–57, vii.
21. Pandeya N, Williams GM, Sadhegi S, et al. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am J Epidemiol 2008;168:105–14.
22. Boffetta P, Hecht S, Gray N, et al. Smokeless tobacco and cancer. Lancet Oncol 2008;9:667–75.
23. Dar NA, Bhat GA, Shah IA, eta l. Hookah smoking, nass chewing, and oesophageal squamous cell carcinoma in Kashmir, India. Br J Cancer 2012;107:1618–23.
24. Abnet CC, Kamangar F, Islami F, et al. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2008;17:3062–8.
25. Islami F, Sheikhattari P, Ren JS, Kamangar F. Gastric atrophy and risk of oesophageal cancer and gastric cardia adenocarcinoma--a systematic review and meta-analysis. Ann Oncol 2011;22:754–60.
26. Appelqvist P, Salmo M. Lye corrosion carcinoma of the esophagus: a review of 63 cases. Cancer 1980;45:2655–8.
27. Sandler RS, Nyrén O, Ekbom A, et al. The risk of esophageal cancer in patients with achalasia. A population-based study. JAMA 1995;274:1359–62.
28. Lu SH, Montesano R, Zhang MS, et al. Relevance of N-nitrosamines to esophageal cancer in China. J Cell Physiol Suppl 1986;4:51–8.
29. Steevens J, van den Brandt PA, Goldbohm RA, Schouten LJ. Selenium status and the risk of esophageal and gastric cancer subtypes: the Netherlands cohort study. Gastroenterology 2010;138:1704–13.
30. Abnet CC, Lai B, Qiao YL, et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst 2005;97:301–6.
31. Islami F, Boffetta P, Ren JS, et al. High-temperature beverages and foods and esophageal cancer risk--a systematic review. Int J Cancer 2009;125:491–524.
32. Liu J, Wang J, Leng Y, Lv C. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer 2013;133:473–85.
33. Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 2003;124:47–56.
34. Ratnasinghe D, Tangrea J, Roth MJ, et al. Expression of cyclooxygenase-2 in human squamous cell carcinoma of the esophagus; an immunohistochemical survey. Anticancer Res 1999;19:171–4.
35. Morris CD, Armstrong GR, Bigley G, et al. Cyclooxygenase-2 expression in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol 2001;96:990–6.
36. Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000;190:562–72.
37. Gastrointestinal cancer. In: Fishman MC, Hoffman R, Klausner RD, Thaler MS, editors. Medicine. 5th ed. Baltimore: Lippincott Williams & Wilkins; 2002:423–6.
38. Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512–8.
39. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241–52.
40. Lightdale CJ. Esophageal cancer. Am J Gastroenterol 1999;94:20–9.
41. Graham DY, Schwartz JT, Cain GD, Gyorkey F. Prospective evaluation of biopsy number in the diagnosis of esophageal and gastric carcinoma. Gastroenterology 1982;82:228–31.
42. Lowe VJ, Booya F, Fletcher JG, et al. Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol Imaging Biol 2005;7:422–30.
43. Röesch T. Endoscopic ultrasonography: equipment and technique. Gastrointest Endosc Clin N Am 2005;15:13–31, vii.
44. Vazquez-Sequeiros E, Norton ID, Clain JE, et al. Impact of EUS-guided fine-needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest Endosc 2001;53:751–7.
45. Van Dam J. Endosonographic evaluation of the patient with esophageal cancer. Chest 1997;112(4 Suppl):184S–190S.
46. Catalano MF, Van Dam J, Sivak MV Jr. Malignant esophageal strictures: staging accuracy of endoscopic ultrasonography. Gastrointest Endosc 1995;41:535–9.
47. Van Dam J, Rice TW, Catalano MF, et al. High-grade malignant stricture is predictive of esophageal tumor stage. Risks of endosonographic evaluation. Cancer 1993;71:2910–7.
48. Hünerbein M, Ghadimi BM, Haensch W, Schlag PM. Transendoscopic ultrasound of esophageal and gastric cancer using miniaturized ultrasound catheter probes. Gastrointest Endosc 1998;48:371–5.
49. Zuccaro G Jr, Rice TW, Goldblum J, et al. Endoscopic ultrasound cannot determine suitability for esophagectomy after aggressive chemoradiotherapy for esophageal cancer. Am J Gastroenterol 1999;94:906–12.
50. ASGE Standards of Practice Committee, Evans JA, Early DS, Chandraskhara V, et al; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013;77:328–34.
51. Luketich JD, Schauer P, Landreneau R, et al. Minimally invasive surgical staging is superior to endoscopic ultrasound in detecting lymph node metastases in esophageal cancer. J Thorac Cardiovasc Surg 1997;114:817–21.
52. Krasna MJ, Flowers JL, Attar S, McLaughlin J. Combined thoracoscopic/laparoscopic staging of esophageal cancer. J Thorac Cardiovasc Surg 1996;111:800–6.
53. Flamen P, Lerut A, Van Cutsem E, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 2000;18:3202–10.
54. Downey RJ, Akhurst T, Ilson D, et al. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol 2003;21:428–32.
55. Mealy K, Feely J, Reid I, et al. Tumour marker detection in oesophageal carcinoma. Eur J Surg Oncol 1996;22:505–7.
56. Rice TW, Rusch VW, Ishwaran H, Blackstone EH; Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763–73.
57. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag; 2009:103–5.
58. Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1–8.
59. Villaflor VM, Allaix ME, Minsky B, et al. Multidisciplinary approach for patients with esophageal cancer. World J Gastroenterol 2012;18:6737–46.
60. Ngamruengphong S, Wolfsen HC, Wallace MB. Survival of patients with superficial esophageal adenocarcinoma after endoscopic treatment vs surgery. Clin Gastroenterol Hepatol 2013;11:1424–9.e2.
61. Lin SH, Chang JY. Esophageal cancer: diagnosis and management. Chin J Cancer 2010;29:843–54.
62. Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815–23.
63. Das A, Singh V, Fleischer DE, Sharma VK. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol 2008;103:1340–5.
64. Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593–8.
65. Gebski V, Burmeister B, Smithers BM, et al; Australasian Gastro-Intestinal Trials Group. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226–34.
66. Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160–8.
67. Tougeron D, Scotté M, Hamidou H, et al. Definitive chemoradiotherapy in patients with esophageal adenocarcinoma: an alternative to surgery? J Surg Oncol 2012;105: 761–6.
68. Siddiqui AA, Sarkar A, Beltz S, et al. Placement of fully covered self-expandable metal stents in patients with locally advanced esophageal cancer before neoadjuvant therapy. Gastrointest Endosc 2012;76:44–51.
69. Nagaraja V, Cox MR, Eslick GD. Safety and efficacy of esophageal stents preceding or during neoadjuvant chemotherapy for esophageal cancer: a systematic review and meta-analysis. J Gastrointest Oncol 2014;5:119–26.
70. Adler DG, Fang J, Wong R, et al. Placement of Polyflex stents in patients with locally advanced esophageal cancer is safe and improves dysphagia during neoadjuvant therapy. Gastrointest Endosc 2009;70:614–9.
71. Dubecz A, Sepesi B, Salvador R, et al. Surgical resection for locoregional esophageal cancer is underutilized in the United States. J Am Coll Surg 2010;211:754–61.
72. Hulscher JB, Tijssen JG, Obertop H, van Lanschot JJ. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 2001;72:306–13.
73. Chang AC, Ji H, Birkmeyer NJ, et al. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg 2008;85:424–9.
74. Almhanna K, Shridhar R, Meredith KL. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: is there a standard of care? Cancer Control 2013;20:89–96.
75. Harvey JA, Bessell JR, Beller E, et al. Chemoradiation therapy is effective for the palliative treatment of malignant dysphagia. Dis Esophagus 2004;17:260–5.
76. Siersema PD, Schrauwen SL, van Blankenstein M, et al; Rotterdam Esophageal Tumor Study Group. Self-expanding metal stents for complicated and recurrent esophagogastric cancer. Gastrointest Endosc 2001;54:579–86.
77. Homs MY, Steyerberg EW, Eijkenboom WM, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet 2004;364(9444):1497–504.
78. van den Bongard HJ, Boot H, Baas P, Taal BG. The role of parallel stent insertion in patients with esophagorespiratory fistulas. Gastrointest Endosc 2002;55:110–5.
79. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220–41.
80. Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512–8.
81. Mariette C, Maurel A, Fabre S, et al. [Preoperative prognostic factors for squamous cell carcinomas of the thoracic esophagus]. Gastroenterol Clin Biol 2001;25:468–72.
82. Swanson SJ, Batirel HF, Bueno R, et al. Transthoracic esophagectomy with radical mediastinal and abdominal lymph node dissection and cervical esophagogastrostomy for esophageal carcinoma. Ann Thorac Surg 2001;72:1918–24.
83. Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305–13.
84. Hosch SB, Stoecklein NH, Pichlmeier U, et al. Esophageal cancer: the mode of lymphatic tumor cell spread and its prognostic significance. J Clin Oncol 2001;19:1970–5.
2014 Update on Contraception
Unintended pregnancy remains an important public health priority in the United States. Correct and consistent use of effective contraception can help women achieve appropriate interpregnancy intervals and desired family size, whereas inconsistent or non-use of contraceptive methods contributes to the majority of unintended pregnancies.
Long-acting reversible contraceptive (LARC) methods, such as implants and intrauterine devices, have effectiveness rates similar to those of permanent sterilization, and these methods are becoming more popular among American women. The proportion of women using LARC methods increased from 2.4% in 2002 to 8.5% in 2009.1
Sterilization continues to be a common method of contraception, with 32% of women relying on female or male sterilization in 2009.1 For women who are not using contraception regularly or who experience a failure in their method, emergency contraception is a viable back-up plan.
In this article, we will review the latest data on contraceptive efficacy in three different contexts:
- implant placement in the immediate postpartum period
- emergency contraception (EC) with the copper intrauterine device (IUD)
- sterilization via hysteroscopic versus laparoscopic approaches.
Immediate postpartum placement of the contraceptive implant saves money
Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: Do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206(6):481.e1–e7.
Han L, Teal SB, Sheeder J, Tocce K. Preventing repeat pregnancy in adolescents: Is immediate postpartum insertion of the contraceptive implant cost effective? [published online ahead of print March 11, 2014]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2014.03.015.
Although teen birth rates have been declining in the United States in recent years, repeat teen births still pose significant health and socioeconomic challenges for young mothers, their children, and society. Adolescent mothers face barriers in completing their education and obtaining work experience. Repeat teen mothers are also more likely to experience adverse pregnancy outcomes, including preterm birth or delivery of a low-birth-weight infant. Families of adolescent mothers are not the only ones who are affected by teen childbearing. In fact, US taxpayers spend about $11 billion each year on costs related to teen pregnancy.2
The immediate postpartum period is a time when effective LARC methods can be initiated to decrease the risk of rapid repeat pregnancy.
Details of the study by Tocce and colleagues
Tocce and colleagues report the results of a prospective observational study that compared adolescents who chose postpartum etonogestrel implant insertion with those who elected to use no contraception or initiate contraception at the usual interval (condoms, depot medroxyprogesterone acetate, and progestin-only pills at any time after delivery, combined hormonal contraception after 4 weeks postpartum, implant insertion after 4 weeks postpartum, and intrauterine device placement at 6 weeks after delivery).
Of adolescents who chose immediate postpartum implant placement, 88.6% were still using this method at 12 months postpartum. In comparison, only 53.6% of adolescents in the control group were using a highly effective contraceptive method at 12 months postpartum (TABLE).
| Contraceptive method | Immediate postpartum implant (n = 149) | Control* (n = 166) |
| Implant | 132 (88.6%) | 35 (21.1%) |
| Intrauterine device | 6 (4.0%) | 51 (30.7%) |
| Female sterilization | 0 | 3 (01.8%) |
| Total using highly effective method† | 138 (92.6%) | 89 (53.6%) |
* The control group consisted of women who elected to use no contraception or initiate contraception at the usual postpartum interval (condoms, depot medroxyprogesterone acetate, and progestin-only pills at any time after delivery, combined hormonal contraception after 4 weeks postpartum, implant insertion after 4 weeks postpartum, and intrauterine device placement at 6 weeks after delivery). † P<.0001, Fisher exact test.Adapted from: Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206(6):481.e1–e7. | ||
The difference in repeat pregnancy rates was even more compelling. At 12 months postpartum, the pregnancy rate was 2.6% for women who had chosen an immediate postpartum implant, compared with 18.6% in the control group (P<.001).
One significant barrier to immediate postpartum LARC placement is reimbursement policies; hospitals are reimbursed a single global fee for all of the hospital care, so insertion of an expensive contraceptive implant during the hospital stay is not reimbursed. However, if a woman returns to the office for insertion, the provider receives full reimbursement if she has coverage for the product.
A look at cost effectiveness
With this in mind, Han and colleagues determined the cost effectiveness of immediate postpartum implant placement using the results from the observational study from Tocce et al. The costs of implant insertion and removal were calculated, as well as the costs associated with various obstetric or gynecologic outcomes, including prenatal care, vaginal or cesarean delivery, infant medical care for the first year of life, and management of ectopic pregnancy or spontaneous miscarriage. The contraceptive costs for the comparison group were not included in the analysis because these costs would represent baseline contraceptive costs incurred by Medicaid.
Significant cost savings were found with immediate postpartum implant placement over time; specifically, $0.78, $3.54, and $6.50 were saved for every dollar spent at 12, 24, and 36 months, respectively. To be clear, this analysis was limited to contraceptive implant placement, and cannot be directly applied to immediate postpartum intrauterine device insertion.
What this evidence means for practice
Among adolescents who received immediate postpartum implant placement, contraceptive continuation rates were higher and repeat teen birth rates were lower, translating into overall cost savings for state Medicaid programs. Furthermore, young mothers and their families also experience health, social, and economic benefits from a delay in childbearing.
In accordance with the findings of Han and colleagues, the South Carolina Medicaid program is the first to implement reimbursement for inpatient postpartum LARC insertion. Other states should evaluate their own policies for inpatient LARC reimbursement and take into consideration the potential for cost savings
More evidence suggests the copper IUD is the preferred emergency contraceptive
Turok DK, Jacobson JC, Dermish Amna I, et al. Emergency contraception with a copper IUD or oral levonorgestrel: An observational study of 1-year pregnancy rates. Contraception. 2014;89(3):222–228.
Several options for EC exist, but only the copper IUD also can be continued as an effective method of contraception. Despite its dual roles in pregnancy prevention, the copper IUD remains underutilized, compared with oral EC methods. Women who seek EC are motivated to reduce their risk of pregnancy. However, they may not be receiving the most effective method to avoid pregnancy. A survey of 816 emergency contraception providers revealed that 85% of respondents had never offered the copper IUD as a method of EC to their patients.3 This represents a lost opportunity, as the copper IUD would be ideal for women who desire an effective form of EC that also can be continued as contraception.
Details of the trial
Turok and colleagues conducted a prospective observational trial comparing oral levonorgestrel (LNG) with copper IUD insertion in women seeking EC. Women who were interested in participating received scripted counseling on both methods and were given their desired method free of charge.
In this study, almost 40% (215/542) of women chose the copper IUD for EC. However, the providers in this study were unable to place the IUD in 20% of these women. The women who chose not to receive an IUD or who did not have an IUD placed received LNG EC.
There were four pregnancies from EC failures in the first month in the LNG group, compared with none in the IUD group. After 1 year, the risk of pregnancy in women who chose the copper IUD (including the women who were unable to have the device placed) was lower than in women who chose LNG (odds ratio [OR], 0.50; 95% confidence interval [CI], 0.26–0.96).
In an analysis based on the actual method received, the risk of pregnancy in the IUD group was even lower (OR, 0.38; 95% CI, 0.18–0.80).
At 1 year, 60% of women in the copper IUD group were using a highly effective method of contraception, specifically an IUD, implant, or sterilization, compared with 10% in the LNG group.
What this evidence means for practice
When given the option, almost 50% of women chose the copper IUD to reduce their risk of pregnancy. Women who received a copper IUD were more likely to be using a highly effective method of contraception and less likely to experience an unintended pregnancy at 1 year than women who chose LNG EC.
We need to counsel our patients on the differences in efficacy between the methods and offer copper IUDs to eligible women.
Hysteroscopic sterilization may not be as effective as we thought
Gariepy AM, Creinin MD, Smith KJ, Xu X. Probability of pregnancy after sterilization: A comparison of hysteroscopic versus laparoscopic sterilization [published online ahead of print April 24, 2014]. Contraception. doi:10.1016/j.contraception.2014.03.010.
Since its introduction in 2002, hysteroscopic sterilization has become a popular method of sterilization. It has many potential advantages over laparoscopic sterilization, including the ability to perform the procedure in an office setting without general anesthesia or abdominal incisions. However, there are also disadvantages to hysteroscopic sterilization, as we pointed out in this Update last year, such as a risk of unsuccessful procedure completion on the first attempt and the need for contraception until tubal occlusion is confirmed.
There are limited data on the effectiveness of hysteroscopic sterilization, and there are no prospective studies comparing hysteroscopic and laparoscopic sterilization. Given the rare outcome of unintended pregnancy with both procedures, based on published literature, a prospective study is unfeasible. An inherent weakness of large clinical trials or retrospective reports of hysteroscopic sterilization success is that only women who had successful completion of the procedure can be included. Two recent reports that demonstrate that completed hysteroscopic sterilization procedures are highly effective highlighted this “weakness.”4,5 However, these data do not reflect “real-life” practice; there are no intent-to-treat data on pregnancy rates among women who choose this option but are unable to fully complete the procedure.
Details of the study
To evaluate real-life outcomes, Gariepy and colleagues performed a decision analysis to estimate the probability of pregnancy after hysteroscopic sterilization and laparoscopic approaches with silicone rubber band application and bipolar coagulation. Using a Markov state-transition model, the authors could determine the probability of pregnancy over a 10-year period for all types of sterilization. For hysteroscopic sterilization, each of the multiple steps, from coil placement to use of alternative contraception in the interim period to follow-up confirmation of tubal occlusion, could be included.
At 10 years, the expected cumulative pregnancy rates per 1,000 women were 96, 24, and 30 for hysteroscopic sterilization, laparoscopic silicone rubber band application, and laparoscopic bipolar coagulation, respectively. For hysteroscopic and laparoscopic sterilization to be equal in effectiveness, the success of laparoscopic sterilization would need to decrease to less than 90% from 99% and hysteroscopic coil placement or follow-up would need to improve.
The authors concluded that the effectiveness of sterilization does vary significantly bythe method used, and rankings of effectiveness should differentiate between hysteroscopic and laparoscopic sterilization.
What this evidence means for practice
When counseling women about sterilization, we should discuss the advantages and disadvantages of hysteroscopic versus laparoscopic approaches and disclose the efficacy rates of each method.
The issue is not that the hysteroscopic sterilization procedure is less effective than laparoscopic sterilization. The real take-home point is that women choosing to attempt hysteroscopic sterilization are more likely to experience an unintended pregnancy within the next 10 years than women presenting for laparoscopic sterilization.
Each year, 345,000 US women undergo interval sterilization.6 If hysteroscopic sterilization were attempted as the preferred method for all of these women (as compared with laparoscopic sterilization) in just 1 year, then an additional 22,770 pregnancies would occur for this group of women over the ensuing 10 years. With the current technology, hysteroscopic sterilization should be reserved for appropriate candidates, such as women who may face higher risks from laparoscopy.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. Finer LB, Jerman J, Kavanaugh ML. Changes in use of long-acting contraceptive methods in the United States, 2007-2009. Fertil Steril. 2012;98(4):893–897.
2. Centers for Disease Control. Vital signs: Repeat births among teens—United States, 2007–2010. MMWR. 2013;62(13):249–255.
3. Harper CC, Speidel JJ, Drey EA, Trussell J, Blum M, Darney PD. Copper intrauterine device for emergency contraception: Clinical practice among contraceptive providers. Obstet Gynecol. 2012;119(2 Pt 1):220–226.
4. Munro MG, Nichols JE, Levy B, Vleugels MP, Veersema S. Hysteroscopic sterilization: 10-year retrospective analysis of worldwide pregnancy reports. J Minim Invasive Gynecol. 2014;21(2):245–251.
5. Fernandez H, Legendre G, Blein C, Lamarsalle L, Panel P. Tubal sterilization: Pregnancy rates after hysteroscopic versus laparoscopic sterilization in France, 2006–2010 [published online ahead of print May 14, 2014]. Eur J Obstet Gynecol Reprod Biol. doi:10.1016/j.ejogrb.2014.04.043.
6. Bartz D, Greenberg JA. Sterilization in the United States. Rev Obstet Gynecol. 2008;1(1):23–32.
Unintended pregnancy remains an important public health priority in the United States. Correct and consistent use of effective contraception can help women achieve appropriate interpregnancy intervals and desired family size, whereas inconsistent or non-use of contraceptive methods contributes to the majority of unintended pregnancies.
Long-acting reversible contraceptive (LARC) methods, such as implants and intrauterine devices, have effectiveness rates similar to those of permanent sterilization, and these methods are becoming more popular among American women. The proportion of women using LARC methods increased from 2.4% in 2002 to 8.5% in 2009.1
Sterilization continues to be a common method of contraception, with 32% of women relying on female or male sterilization in 2009.1 For women who are not using contraception regularly or who experience a failure in their method, emergency contraception is a viable back-up plan.
In this article, we will review the latest data on contraceptive efficacy in three different contexts:
- implant placement in the immediate postpartum period
- emergency contraception (EC) with the copper intrauterine device (IUD)
- sterilization via hysteroscopic versus laparoscopic approaches.
Immediate postpartum placement of the contraceptive implant saves money
Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: Do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206(6):481.e1–e7.
Han L, Teal SB, Sheeder J, Tocce K. Preventing repeat pregnancy in adolescents: Is immediate postpartum insertion of the contraceptive implant cost effective? [published online ahead of print March 11, 2014]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2014.03.015.
Although teen birth rates have been declining in the United States in recent years, repeat teen births still pose significant health and socioeconomic challenges for young mothers, their children, and society. Adolescent mothers face barriers in completing their education and obtaining work experience. Repeat teen mothers are also more likely to experience adverse pregnancy outcomes, including preterm birth or delivery of a low-birth-weight infant. Families of adolescent mothers are not the only ones who are affected by teen childbearing. In fact, US taxpayers spend about $11 billion each year on costs related to teen pregnancy.2
The immediate postpartum period is a time when effective LARC methods can be initiated to decrease the risk of rapid repeat pregnancy.
Details of the study by Tocce and colleagues
Tocce and colleagues report the results of a prospective observational study that compared adolescents who chose postpartum etonogestrel implant insertion with those who elected to use no contraception or initiate contraception at the usual interval (condoms, depot medroxyprogesterone acetate, and progestin-only pills at any time after delivery, combined hormonal contraception after 4 weeks postpartum, implant insertion after 4 weeks postpartum, and intrauterine device placement at 6 weeks after delivery).
Of adolescents who chose immediate postpartum implant placement, 88.6% were still using this method at 12 months postpartum. In comparison, only 53.6% of adolescents in the control group were using a highly effective contraceptive method at 12 months postpartum (TABLE).
| Contraceptive method | Immediate postpartum implant (n = 149) | Control* (n = 166) |
| Implant | 132 (88.6%) | 35 (21.1%) |
| Intrauterine device | 6 (4.0%) | 51 (30.7%) |
| Female sterilization | 0 | 3 (01.8%) |
| Total using highly effective method† | 138 (92.6%) | 89 (53.6%) |
* The control group consisted of women who elected to use no contraception or initiate contraception at the usual postpartum interval (condoms, depot medroxyprogesterone acetate, and progestin-only pills at any time after delivery, combined hormonal contraception after 4 weeks postpartum, implant insertion after 4 weeks postpartum, and intrauterine device placement at 6 weeks after delivery). † P<.0001, Fisher exact test.Adapted from: Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206(6):481.e1–e7. | ||
The difference in repeat pregnancy rates was even more compelling. At 12 months postpartum, the pregnancy rate was 2.6% for women who had chosen an immediate postpartum implant, compared with 18.6% in the control group (P<.001).
One significant barrier to immediate postpartum LARC placement is reimbursement policies; hospitals are reimbursed a single global fee for all of the hospital care, so insertion of an expensive contraceptive implant during the hospital stay is not reimbursed. However, if a woman returns to the office for insertion, the provider receives full reimbursement if she has coverage for the product.
A look at cost effectiveness
With this in mind, Han and colleagues determined the cost effectiveness of immediate postpartum implant placement using the results from the observational study from Tocce et al. The costs of implant insertion and removal were calculated, as well as the costs associated with various obstetric or gynecologic outcomes, including prenatal care, vaginal or cesarean delivery, infant medical care for the first year of life, and management of ectopic pregnancy or spontaneous miscarriage. The contraceptive costs for the comparison group were not included in the analysis because these costs would represent baseline contraceptive costs incurred by Medicaid.
Significant cost savings were found with immediate postpartum implant placement over time; specifically, $0.78, $3.54, and $6.50 were saved for every dollar spent at 12, 24, and 36 months, respectively. To be clear, this analysis was limited to contraceptive implant placement, and cannot be directly applied to immediate postpartum intrauterine device insertion.
What this evidence means for practice
Among adolescents who received immediate postpartum implant placement, contraceptive continuation rates were higher and repeat teen birth rates were lower, translating into overall cost savings for state Medicaid programs. Furthermore, young mothers and their families also experience health, social, and economic benefits from a delay in childbearing.
In accordance with the findings of Han and colleagues, the South Carolina Medicaid program is the first to implement reimbursement for inpatient postpartum LARC insertion. Other states should evaluate their own policies for inpatient LARC reimbursement and take into consideration the potential for cost savings
More evidence suggests the copper IUD is the preferred emergency contraceptive
Turok DK, Jacobson JC, Dermish Amna I, et al. Emergency contraception with a copper IUD or oral levonorgestrel: An observational study of 1-year pregnancy rates. Contraception. 2014;89(3):222–228.
Several options for EC exist, but only the copper IUD also can be continued as an effective method of contraception. Despite its dual roles in pregnancy prevention, the copper IUD remains underutilized, compared with oral EC methods. Women who seek EC are motivated to reduce their risk of pregnancy. However, they may not be receiving the most effective method to avoid pregnancy. A survey of 816 emergency contraception providers revealed that 85% of respondents had never offered the copper IUD as a method of EC to their patients.3 This represents a lost opportunity, as the copper IUD would be ideal for women who desire an effective form of EC that also can be continued as contraception.
Details of the trial
Turok and colleagues conducted a prospective observational trial comparing oral levonorgestrel (LNG) with copper IUD insertion in women seeking EC. Women who were interested in participating received scripted counseling on both methods and were given their desired method free of charge.
In this study, almost 40% (215/542) of women chose the copper IUD for EC. However, the providers in this study were unable to place the IUD in 20% of these women. The women who chose not to receive an IUD or who did not have an IUD placed received LNG EC.
There were four pregnancies from EC failures in the first month in the LNG group, compared with none in the IUD group. After 1 year, the risk of pregnancy in women who chose the copper IUD (including the women who were unable to have the device placed) was lower than in women who chose LNG (odds ratio [OR], 0.50; 95% confidence interval [CI], 0.26–0.96).
In an analysis based on the actual method received, the risk of pregnancy in the IUD group was even lower (OR, 0.38; 95% CI, 0.18–0.80).
At 1 year, 60% of women in the copper IUD group were using a highly effective method of contraception, specifically an IUD, implant, or sterilization, compared with 10% in the LNG group.
What this evidence means for practice
When given the option, almost 50% of women chose the copper IUD to reduce their risk of pregnancy. Women who received a copper IUD were more likely to be using a highly effective method of contraception and less likely to experience an unintended pregnancy at 1 year than women who chose LNG EC.
We need to counsel our patients on the differences in efficacy between the methods and offer copper IUDs to eligible women.
Hysteroscopic sterilization may not be as effective as we thought
Gariepy AM, Creinin MD, Smith KJ, Xu X. Probability of pregnancy after sterilization: A comparison of hysteroscopic versus laparoscopic sterilization [published online ahead of print April 24, 2014]. Contraception. doi:10.1016/j.contraception.2014.03.010.
Since its introduction in 2002, hysteroscopic sterilization has become a popular method of sterilization. It has many potential advantages over laparoscopic sterilization, including the ability to perform the procedure in an office setting without general anesthesia or abdominal incisions. However, there are also disadvantages to hysteroscopic sterilization, as we pointed out in this Update last year, such as a risk of unsuccessful procedure completion on the first attempt and the need for contraception until tubal occlusion is confirmed.
There are limited data on the effectiveness of hysteroscopic sterilization, and there are no prospective studies comparing hysteroscopic and laparoscopic sterilization. Given the rare outcome of unintended pregnancy with both procedures, based on published literature, a prospective study is unfeasible. An inherent weakness of large clinical trials or retrospective reports of hysteroscopic sterilization success is that only women who had successful completion of the procedure can be included. Two recent reports that demonstrate that completed hysteroscopic sterilization procedures are highly effective highlighted this “weakness.”4,5 However, these data do not reflect “real-life” practice; there are no intent-to-treat data on pregnancy rates among women who choose this option but are unable to fully complete the procedure.
Details of the study
To evaluate real-life outcomes, Gariepy and colleagues performed a decision analysis to estimate the probability of pregnancy after hysteroscopic sterilization and laparoscopic approaches with silicone rubber band application and bipolar coagulation. Using a Markov state-transition model, the authors could determine the probability of pregnancy over a 10-year period for all types of sterilization. For hysteroscopic sterilization, each of the multiple steps, from coil placement to use of alternative contraception in the interim period to follow-up confirmation of tubal occlusion, could be included.
At 10 years, the expected cumulative pregnancy rates per 1,000 women were 96, 24, and 30 for hysteroscopic sterilization, laparoscopic silicone rubber band application, and laparoscopic bipolar coagulation, respectively. For hysteroscopic and laparoscopic sterilization to be equal in effectiveness, the success of laparoscopic sterilization would need to decrease to less than 90% from 99% and hysteroscopic coil placement or follow-up would need to improve.
The authors concluded that the effectiveness of sterilization does vary significantly bythe method used, and rankings of effectiveness should differentiate between hysteroscopic and laparoscopic sterilization.
What this evidence means for practice
When counseling women about sterilization, we should discuss the advantages and disadvantages of hysteroscopic versus laparoscopic approaches and disclose the efficacy rates of each method.
The issue is not that the hysteroscopic sterilization procedure is less effective than laparoscopic sterilization. The real take-home point is that women choosing to attempt hysteroscopic sterilization are more likely to experience an unintended pregnancy within the next 10 years than women presenting for laparoscopic sterilization.
Each year, 345,000 US women undergo interval sterilization.6 If hysteroscopic sterilization were attempted as the preferred method for all of these women (as compared with laparoscopic sterilization) in just 1 year, then an additional 22,770 pregnancies would occur for this group of women over the ensuing 10 years. With the current technology, hysteroscopic sterilization should be reserved for appropriate candidates, such as women who may face higher risks from laparoscopy.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
Unintended pregnancy remains an important public health priority in the United States. Correct and consistent use of effective contraception can help women achieve appropriate interpregnancy intervals and desired family size, whereas inconsistent or non-use of contraceptive methods contributes to the majority of unintended pregnancies.
Long-acting reversible contraceptive (LARC) methods, such as implants and intrauterine devices, have effectiveness rates similar to those of permanent sterilization, and these methods are becoming more popular among American women. The proportion of women using LARC methods increased from 2.4% in 2002 to 8.5% in 2009.1
Sterilization continues to be a common method of contraception, with 32% of women relying on female or male sterilization in 2009.1 For women who are not using contraception regularly or who experience a failure in their method, emergency contraception is a viable back-up plan.
In this article, we will review the latest data on contraceptive efficacy in three different contexts:
- implant placement in the immediate postpartum period
- emergency contraception (EC) with the copper intrauterine device (IUD)
- sterilization via hysteroscopic versus laparoscopic approaches.
Immediate postpartum placement of the contraceptive implant saves money
Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: Do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206(6):481.e1–e7.
Han L, Teal SB, Sheeder J, Tocce K. Preventing repeat pregnancy in adolescents: Is immediate postpartum insertion of the contraceptive implant cost effective? [published online ahead of print March 11, 2014]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2014.03.015.
Although teen birth rates have been declining in the United States in recent years, repeat teen births still pose significant health and socioeconomic challenges for young mothers, their children, and society. Adolescent mothers face barriers in completing their education and obtaining work experience. Repeat teen mothers are also more likely to experience adverse pregnancy outcomes, including preterm birth or delivery of a low-birth-weight infant. Families of adolescent mothers are not the only ones who are affected by teen childbearing. In fact, US taxpayers spend about $11 billion each year on costs related to teen pregnancy.2
The immediate postpartum period is a time when effective LARC methods can be initiated to decrease the risk of rapid repeat pregnancy.
Details of the study by Tocce and colleagues
Tocce and colleagues report the results of a prospective observational study that compared adolescents who chose postpartum etonogestrel implant insertion with those who elected to use no contraception or initiate contraception at the usual interval (condoms, depot medroxyprogesterone acetate, and progestin-only pills at any time after delivery, combined hormonal contraception after 4 weeks postpartum, implant insertion after 4 weeks postpartum, and intrauterine device placement at 6 weeks after delivery).
Of adolescents who chose immediate postpartum implant placement, 88.6% were still using this method at 12 months postpartum. In comparison, only 53.6% of adolescents in the control group were using a highly effective contraceptive method at 12 months postpartum (TABLE).
| Contraceptive method | Immediate postpartum implant (n = 149) | Control* (n = 166) |
| Implant | 132 (88.6%) | 35 (21.1%) |
| Intrauterine device | 6 (4.0%) | 51 (30.7%) |
| Female sterilization | 0 | 3 (01.8%) |
| Total using highly effective method† | 138 (92.6%) | 89 (53.6%) |
* The control group consisted of women who elected to use no contraception or initiate contraception at the usual postpartum interval (condoms, depot medroxyprogesterone acetate, and progestin-only pills at any time after delivery, combined hormonal contraception after 4 weeks postpartum, implant insertion after 4 weeks postpartum, and intrauterine device placement at 6 weeks after delivery). † P<.0001, Fisher exact test.Adapted from: Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206(6):481.e1–e7. | ||
The difference in repeat pregnancy rates was even more compelling. At 12 months postpartum, the pregnancy rate was 2.6% for women who had chosen an immediate postpartum implant, compared with 18.6% in the control group (P<.001).
One significant barrier to immediate postpartum LARC placement is reimbursement policies; hospitals are reimbursed a single global fee for all of the hospital care, so insertion of an expensive contraceptive implant during the hospital stay is not reimbursed. However, if a woman returns to the office for insertion, the provider receives full reimbursement if she has coverage for the product.
A look at cost effectiveness
With this in mind, Han and colleagues determined the cost effectiveness of immediate postpartum implant placement using the results from the observational study from Tocce et al. The costs of implant insertion and removal were calculated, as well as the costs associated with various obstetric or gynecologic outcomes, including prenatal care, vaginal or cesarean delivery, infant medical care for the first year of life, and management of ectopic pregnancy or spontaneous miscarriage. The contraceptive costs for the comparison group were not included in the analysis because these costs would represent baseline contraceptive costs incurred by Medicaid.
Significant cost savings were found with immediate postpartum implant placement over time; specifically, $0.78, $3.54, and $6.50 were saved for every dollar spent at 12, 24, and 36 months, respectively. To be clear, this analysis was limited to contraceptive implant placement, and cannot be directly applied to immediate postpartum intrauterine device insertion.
What this evidence means for practice
Among adolescents who received immediate postpartum implant placement, contraceptive continuation rates were higher and repeat teen birth rates were lower, translating into overall cost savings for state Medicaid programs. Furthermore, young mothers and their families also experience health, social, and economic benefits from a delay in childbearing.
In accordance with the findings of Han and colleagues, the South Carolina Medicaid program is the first to implement reimbursement for inpatient postpartum LARC insertion. Other states should evaluate their own policies for inpatient LARC reimbursement and take into consideration the potential for cost savings
More evidence suggests the copper IUD is the preferred emergency contraceptive
Turok DK, Jacobson JC, Dermish Amna I, et al. Emergency contraception with a copper IUD or oral levonorgestrel: An observational study of 1-year pregnancy rates. Contraception. 2014;89(3):222–228.
Several options for EC exist, but only the copper IUD also can be continued as an effective method of contraception. Despite its dual roles in pregnancy prevention, the copper IUD remains underutilized, compared with oral EC methods. Women who seek EC are motivated to reduce their risk of pregnancy. However, they may not be receiving the most effective method to avoid pregnancy. A survey of 816 emergency contraception providers revealed that 85% of respondents had never offered the copper IUD as a method of EC to their patients.3 This represents a lost opportunity, as the copper IUD would be ideal for women who desire an effective form of EC that also can be continued as contraception.
Details of the trial
Turok and colleagues conducted a prospective observational trial comparing oral levonorgestrel (LNG) with copper IUD insertion in women seeking EC. Women who were interested in participating received scripted counseling on both methods and were given their desired method free of charge.
In this study, almost 40% (215/542) of women chose the copper IUD for EC. However, the providers in this study were unable to place the IUD in 20% of these women. The women who chose not to receive an IUD or who did not have an IUD placed received LNG EC.
There were four pregnancies from EC failures in the first month in the LNG group, compared with none in the IUD group. After 1 year, the risk of pregnancy in women who chose the copper IUD (including the women who were unable to have the device placed) was lower than in women who chose LNG (odds ratio [OR], 0.50; 95% confidence interval [CI], 0.26–0.96).
In an analysis based on the actual method received, the risk of pregnancy in the IUD group was even lower (OR, 0.38; 95% CI, 0.18–0.80).
At 1 year, 60% of women in the copper IUD group were using a highly effective method of contraception, specifically an IUD, implant, or sterilization, compared with 10% in the LNG group.
What this evidence means for practice
When given the option, almost 50% of women chose the copper IUD to reduce their risk of pregnancy. Women who received a copper IUD were more likely to be using a highly effective method of contraception and less likely to experience an unintended pregnancy at 1 year than women who chose LNG EC.
We need to counsel our patients on the differences in efficacy between the methods and offer copper IUDs to eligible women.
Hysteroscopic sterilization may not be as effective as we thought
Gariepy AM, Creinin MD, Smith KJ, Xu X. Probability of pregnancy after sterilization: A comparison of hysteroscopic versus laparoscopic sterilization [published online ahead of print April 24, 2014]. Contraception. doi:10.1016/j.contraception.2014.03.010.
Since its introduction in 2002, hysteroscopic sterilization has become a popular method of sterilization. It has many potential advantages over laparoscopic sterilization, including the ability to perform the procedure in an office setting without general anesthesia or abdominal incisions. However, there are also disadvantages to hysteroscopic sterilization, as we pointed out in this Update last year, such as a risk of unsuccessful procedure completion on the first attempt and the need for contraception until tubal occlusion is confirmed.
There are limited data on the effectiveness of hysteroscopic sterilization, and there are no prospective studies comparing hysteroscopic and laparoscopic sterilization. Given the rare outcome of unintended pregnancy with both procedures, based on published literature, a prospective study is unfeasible. An inherent weakness of large clinical trials or retrospective reports of hysteroscopic sterilization success is that only women who had successful completion of the procedure can be included. Two recent reports that demonstrate that completed hysteroscopic sterilization procedures are highly effective highlighted this “weakness.”4,5 However, these data do not reflect “real-life” practice; there are no intent-to-treat data on pregnancy rates among women who choose this option but are unable to fully complete the procedure.
Details of the study
To evaluate real-life outcomes, Gariepy and colleagues performed a decision analysis to estimate the probability of pregnancy after hysteroscopic sterilization and laparoscopic approaches with silicone rubber band application and bipolar coagulation. Using a Markov state-transition model, the authors could determine the probability of pregnancy over a 10-year period for all types of sterilization. For hysteroscopic sterilization, each of the multiple steps, from coil placement to use of alternative contraception in the interim period to follow-up confirmation of tubal occlusion, could be included.
At 10 years, the expected cumulative pregnancy rates per 1,000 women were 96, 24, and 30 for hysteroscopic sterilization, laparoscopic silicone rubber band application, and laparoscopic bipolar coagulation, respectively. For hysteroscopic and laparoscopic sterilization to be equal in effectiveness, the success of laparoscopic sterilization would need to decrease to less than 90% from 99% and hysteroscopic coil placement or follow-up would need to improve.
The authors concluded that the effectiveness of sterilization does vary significantly bythe method used, and rankings of effectiveness should differentiate between hysteroscopic and laparoscopic sterilization.
What this evidence means for practice
When counseling women about sterilization, we should discuss the advantages and disadvantages of hysteroscopic versus laparoscopic approaches and disclose the efficacy rates of each method.
The issue is not that the hysteroscopic sterilization procedure is less effective than laparoscopic sterilization. The real take-home point is that women choosing to attempt hysteroscopic sterilization are more likely to experience an unintended pregnancy within the next 10 years than women presenting for laparoscopic sterilization.
Each year, 345,000 US women undergo interval sterilization.6 If hysteroscopic sterilization were attempted as the preferred method for all of these women (as compared with laparoscopic sterilization) in just 1 year, then an additional 22,770 pregnancies would occur for this group of women over the ensuing 10 years. With the current technology, hysteroscopic sterilization should be reserved for appropriate candidates, such as women who may face higher risks from laparoscopy.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. Finer LB, Jerman J, Kavanaugh ML. Changes in use of long-acting contraceptive methods in the United States, 2007-2009. Fertil Steril. 2012;98(4):893–897.
2. Centers for Disease Control. Vital signs: Repeat births among teens—United States, 2007–2010. MMWR. 2013;62(13):249–255.
3. Harper CC, Speidel JJ, Drey EA, Trussell J, Blum M, Darney PD. Copper intrauterine device for emergency contraception: Clinical practice among contraceptive providers. Obstet Gynecol. 2012;119(2 Pt 1):220–226.
4. Munro MG, Nichols JE, Levy B, Vleugels MP, Veersema S. Hysteroscopic sterilization: 10-year retrospective analysis of worldwide pregnancy reports. J Minim Invasive Gynecol. 2014;21(2):245–251.
5. Fernandez H, Legendre G, Blein C, Lamarsalle L, Panel P. Tubal sterilization: Pregnancy rates after hysteroscopic versus laparoscopic sterilization in France, 2006–2010 [published online ahead of print May 14, 2014]. Eur J Obstet Gynecol Reprod Biol. doi:10.1016/j.ejogrb.2014.04.043.
6. Bartz D, Greenberg JA. Sterilization in the United States. Rev Obstet Gynecol. 2008;1(1):23–32.
1. Finer LB, Jerman J, Kavanaugh ML. Changes in use of long-acting contraceptive methods in the United States, 2007-2009. Fertil Steril. 2012;98(4):893–897.
2. Centers for Disease Control. Vital signs: Repeat births among teens—United States, 2007–2010. MMWR. 2013;62(13):249–255.
3. Harper CC, Speidel JJ, Drey EA, Trussell J, Blum M, Darney PD. Copper intrauterine device for emergency contraception: Clinical practice among contraceptive providers. Obstet Gynecol. 2012;119(2 Pt 1):220–226.
4. Munro MG, Nichols JE, Levy B, Vleugels MP, Veersema S. Hysteroscopic sterilization: 10-year retrospective analysis of worldwide pregnancy reports. J Minim Invasive Gynecol. 2014;21(2):245–251.
5. Fernandez H, Legendre G, Blein C, Lamarsalle L, Panel P. Tubal sterilization: Pregnancy rates after hysteroscopic versus laparoscopic sterilization in France, 2006–2010 [published online ahead of print May 14, 2014]. Eur J Obstet Gynecol Reprod Biol. doi:10.1016/j.ejogrb.2014.04.043.
6. Bartz D, Greenberg JA. Sterilization in the United States. Rev Obstet Gynecol. 2008;1(1):23–32.
Niacin-laropiprant combo plus statin didn’t cut vascular events
Adding extended-release niacin plus laropiprant to effective statin-based LDL-lowering therapy failed to prevent major vascular events in patients with atherosclerotic vascular disease, but it did raise the rate of serious adverse events, according to a report published online July 16 in the New England Journal of Medicine.
"We believe that [our] findings are likely to be generalizable to all high-dose niacin formulations," and any potential benefits of the therapy must be weighed against those important hazards, reported Martin J. Landray, Ph.D., of the University of Oxford (England), and his associates in the HPS2-THRIVE (Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events) clinical trial.
The study involved 25,673 high-risk men and women at 245 sites in the United Kingdom, Scandinavia, and China whose LDL cholesterol was already controlled by statins and who were randomly assigned to receive either oral niacin plus laropiprant (a prostaglandin-receptor antagonist that improves adherence to niacin by reducing flushing) or a matching placebo (N. Engl. J. Med. 2014;371:203-12).
The goal was to determine whether raising the HDL level with niacin would prevent major coronary events, stroke of any type, or revascularization procedures. Participants were followed for a median of 4 years.
Even though the study drug reduced LDL cholesterol by an average of 10 mg/dL, raised HDL by an average of 6 mg/dL, and reduced triglyceride levels by an average of 33 mg/dL, it failed to significantly decrease the incidence of major vascular events, compared with placebo (13.2% vs. 13.7%, respectively), either overall or in any of more than 30 subgroups of patients.
In fact, using the niacin-laropiprant combination raised the risk of death from any cause by 9%, compared with placebo, a difference that was not significant (P = .08), the investigators reported.
Niacin plus laropiprant was associated with numerous adverse events already linked to niacin therapy, such as serious gastrointestinal, musculoskeletal, and skin-related disorders. Of particular concern was the 55% increase in severe disturbances of metabolic control among patients who had concomitant diabetes (most of whom required hospitalization), and a 32% increase in new-onset diabetes. That contradicts previous assurances that niacin is safe for people who have diabetes, Dr. Landray and his associates noted.
Two serious adverse events that haven’t previously been associated with niacin or laropiprant also were identified: a wide range of infections affecting many body sites, and a similarly wide range of bleeding events, including intracranial hemorrhage and severe gastrointestinal bleeding.
HPS2-THRIVE was supported by Merck, maker of the study drugs; the U.K. Medical Research Council; the British Heart Foundation; and Cancer Research UK. Dr. Landray and several associates reported receiving grant support from Merck; no other relevant conflicts of interest were reported.
The findings of Dr. Landray’s study show that "niacin must be considered to have an unacceptable toxicity profile for the majority of patients, and it should not be used routinely," said Dr. Donald M. Lloyd-Jones. The findings also seriously undermine the hypothesis that HDL cholesterol plays a causal role in major atherosclerotic vascular events, he added.
Taken together with the failure of drugs such as torcetrapib and dalcetrapib in reducing cardiovascular risk despite markedly increasing HDL cholesterol levels, the HPS2-THRIVE results demonstrate that HDL level is solely a risk marker, not a risk factor that merits intervention.
"It is time to face the fact that increasing HDL cholesterol level in isolation" is unlikely to benefit most patients, Dr. Lloyd-Jones cautioned. However, niacin may still have a role in patients at very high risk for cardiovascular events who truly have contraindications to statins and other less toxic drugs, and as a possible fourth-line agent for patients with severe hypertriglyceridemia who are at risk for developing pancreatitis, he added.
Dr. Lloyd-Jones is in the department of preventive medicine and the division of cardiology at Northwestern University, Chicago. He reported no relevant financial conflicts of interest. These remarks were taken from his editorial accompanying Dr. Landray’s report (N. Engl. J. Med. 2014;371:271-3).
The findings of Dr. Landray’s study show that "niacin must be considered to have an unacceptable toxicity profile for the majority of patients, and it should not be used routinely," said Dr. Donald M. Lloyd-Jones. The findings also seriously undermine the hypothesis that HDL cholesterol plays a causal role in major atherosclerotic vascular events, he added.
Taken together with the failure of drugs such as torcetrapib and dalcetrapib in reducing cardiovascular risk despite markedly increasing HDL cholesterol levels, the HPS2-THRIVE results demonstrate that HDL level is solely a risk marker, not a risk factor that merits intervention.
"It is time to face the fact that increasing HDL cholesterol level in isolation" is unlikely to benefit most patients, Dr. Lloyd-Jones cautioned. However, niacin may still have a role in patients at very high risk for cardiovascular events who truly have contraindications to statins and other less toxic drugs, and as a possible fourth-line agent for patients with severe hypertriglyceridemia who are at risk for developing pancreatitis, he added.
Dr. Lloyd-Jones is in the department of preventive medicine and the division of cardiology at Northwestern University, Chicago. He reported no relevant financial conflicts of interest. These remarks were taken from his editorial accompanying Dr. Landray’s report (N. Engl. J. Med. 2014;371:271-3).
The findings of Dr. Landray’s study show that "niacin must be considered to have an unacceptable toxicity profile for the majority of patients, and it should not be used routinely," said Dr. Donald M. Lloyd-Jones. The findings also seriously undermine the hypothesis that HDL cholesterol plays a causal role in major atherosclerotic vascular events, he added.
Taken together with the failure of drugs such as torcetrapib and dalcetrapib in reducing cardiovascular risk despite markedly increasing HDL cholesterol levels, the HPS2-THRIVE results demonstrate that HDL level is solely a risk marker, not a risk factor that merits intervention.
"It is time to face the fact that increasing HDL cholesterol level in isolation" is unlikely to benefit most patients, Dr. Lloyd-Jones cautioned. However, niacin may still have a role in patients at very high risk for cardiovascular events who truly have contraindications to statins and other less toxic drugs, and as a possible fourth-line agent for patients with severe hypertriglyceridemia who are at risk for developing pancreatitis, he added.
Dr. Lloyd-Jones is in the department of preventive medicine and the division of cardiology at Northwestern University, Chicago. He reported no relevant financial conflicts of interest. These remarks were taken from his editorial accompanying Dr. Landray’s report (N. Engl. J. Med. 2014;371:271-3).
Adding extended-release niacin plus laropiprant to effective statin-based LDL-lowering therapy failed to prevent major vascular events in patients with atherosclerotic vascular disease, but it did raise the rate of serious adverse events, according to a report published online July 16 in the New England Journal of Medicine.
"We believe that [our] findings are likely to be generalizable to all high-dose niacin formulations," and any potential benefits of the therapy must be weighed against those important hazards, reported Martin J. Landray, Ph.D., of the University of Oxford (England), and his associates in the HPS2-THRIVE (Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events) clinical trial.
The study involved 25,673 high-risk men and women at 245 sites in the United Kingdom, Scandinavia, and China whose LDL cholesterol was already controlled by statins and who were randomly assigned to receive either oral niacin plus laropiprant (a prostaglandin-receptor antagonist that improves adherence to niacin by reducing flushing) or a matching placebo (N. Engl. J. Med. 2014;371:203-12).
The goal was to determine whether raising the HDL level with niacin would prevent major coronary events, stroke of any type, or revascularization procedures. Participants were followed for a median of 4 years.
Even though the study drug reduced LDL cholesterol by an average of 10 mg/dL, raised HDL by an average of 6 mg/dL, and reduced triglyceride levels by an average of 33 mg/dL, it failed to significantly decrease the incidence of major vascular events, compared with placebo (13.2% vs. 13.7%, respectively), either overall or in any of more than 30 subgroups of patients.
In fact, using the niacin-laropiprant combination raised the risk of death from any cause by 9%, compared with placebo, a difference that was not significant (P = .08), the investigators reported.
Niacin plus laropiprant was associated with numerous adverse events already linked to niacin therapy, such as serious gastrointestinal, musculoskeletal, and skin-related disorders. Of particular concern was the 55% increase in severe disturbances of metabolic control among patients who had concomitant diabetes (most of whom required hospitalization), and a 32% increase in new-onset diabetes. That contradicts previous assurances that niacin is safe for people who have diabetes, Dr. Landray and his associates noted.
Two serious adverse events that haven’t previously been associated with niacin or laropiprant also were identified: a wide range of infections affecting many body sites, and a similarly wide range of bleeding events, including intracranial hemorrhage and severe gastrointestinal bleeding.
HPS2-THRIVE was supported by Merck, maker of the study drugs; the U.K. Medical Research Council; the British Heart Foundation; and Cancer Research UK. Dr. Landray and several associates reported receiving grant support from Merck; no other relevant conflicts of interest were reported.
Adding extended-release niacin plus laropiprant to effective statin-based LDL-lowering therapy failed to prevent major vascular events in patients with atherosclerotic vascular disease, but it did raise the rate of serious adverse events, according to a report published online July 16 in the New England Journal of Medicine.
"We believe that [our] findings are likely to be generalizable to all high-dose niacin formulations," and any potential benefits of the therapy must be weighed against those important hazards, reported Martin J. Landray, Ph.D., of the University of Oxford (England), and his associates in the HPS2-THRIVE (Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events) clinical trial.
The study involved 25,673 high-risk men and women at 245 sites in the United Kingdom, Scandinavia, and China whose LDL cholesterol was already controlled by statins and who were randomly assigned to receive either oral niacin plus laropiprant (a prostaglandin-receptor antagonist that improves adherence to niacin by reducing flushing) or a matching placebo (N. Engl. J. Med. 2014;371:203-12).
The goal was to determine whether raising the HDL level with niacin would prevent major coronary events, stroke of any type, or revascularization procedures. Participants were followed for a median of 4 years.
Even though the study drug reduced LDL cholesterol by an average of 10 mg/dL, raised HDL by an average of 6 mg/dL, and reduced triglyceride levels by an average of 33 mg/dL, it failed to significantly decrease the incidence of major vascular events, compared with placebo (13.2% vs. 13.7%, respectively), either overall or in any of more than 30 subgroups of patients.
In fact, using the niacin-laropiprant combination raised the risk of death from any cause by 9%, compared with placebo, a difference that was not significant (P = .08), the investigators reported.
Niacin plus laropiprant was associated with numerous adverse events already linked to niacin therapy, such as serious gastrointestinal, musculoskeletal, and skin-related disorders. Of particular concern was the 55% increase in severe disturbances of metabolic control among patients who had concomitant diabetes (most of whom required hospitalization), and a 32% increase in new-onset diabetes. That contradicts previous assurances that niacin is safe for people who have diabetes, Dr. Landray and his associates noted.
Two serious adverse events that haven’t previously been associated with niacin or laropiprant also were identified: a wide range of infections affecting many body sites, and a similarly wide range of bleeding events, including intracranial hemorrhage and severe gastrointestinal bleeding.
HPS2-THRIVE was supported by Merck, maker of the study drugs; the U.K. Medical Research Council; the British Heart Foundation; and Cancer Research UK. Dr. Landray and several associates reported receiving grant support from Merck; no other relevant conflicts of interest were reported.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Giving patients on statins high-dose niacin and laropiprant won’t reduce their risk of cardiovascular events.
Major finding: Niacin plus laropiprant reduced LDL cholesterol an additional 10 mg/dL, raised HDL an average of 6 mg/dL, and reduced triglyceride level an average of 33 mg/dL; but it failed to decrease the incidence of major vascular events, compared with placebo (13.2% vs. 13.7%, respectively).
Data source: An international randomized, double-blind clinical trial involving 25,673 high-risk adults with atherosclerotic vascular disease who received either niacin plus laropiprant or a matching placebo and were followed for a median of 4 years.
Disclosures: HPS2-THRIVE was supported by Merck, maker of the study drugs; the U.K. Medical Research Council; the British Heart Foundation; and Cancer Research UK. Dr. Landray and several associates reported receiving grant support from Merck; no other relevant conflicts of interest were reported.
Microprocessor Knee and Power Foot Combination in a Transfemoral Amputee
Rapid advances in technology have brought improvements in prosthetic components. In particular, prosthetic knees and ankle/foot complexes have made substantial advancements with the incorporation of computer technology. For example, microprocessor knees are relatively new; the X2 knee from Ottobock (Minneapolis, Minnesota) represents one of the latest and most advanced units and has just been upgraded.
Until recently, there have been no similarly functioning ankle/foot components except for the Proprio Foot from Össur (Foothill Ranch, California), which also provides powered dorsiflexion.
Also, recently BiOM introduced the BiOM T2 foot and ankle system with the added technology of powered plantarflexion to further normalize amputee prosthetic gait. Active patients who have successfully used a microprocessor knee, such as the X2, have generally paired that technology with a variety of foot/ankle components, ranging from passive-elastic units to advanced-energy storing units.
To normalize gait and improve biomechanics even further in select above-knee amputees, experts in the field have suggested combining a microprocessor knee with a powered foot/ankle complex. One potential obstacle to this combination, however, concerns the possible conflict between the active components of the individual units, such as over- or underengagement of component sensors. This situation, theoretically, could compromise patient safety. BiOM, however, provides training to prosthetic providers to address possible component integration issues, including microprocessor conflict and methods to safely use the components together. Once the prosthetist received this training, the patient in this study was fitted with the T2 foot and the X2 knee with excellent results and no perceived disadvantages.
Case Presentation
The patient was a 32-year-old man with a right transfemoral amputation due to trauma from a blast injury, which occurred during Marine service in Iraq. He also had a gunshot wound to his left leg, which resulted in severe injury, but this limb was salvaged and now has good residual function. Before the trauma, the patient was very athletic and involved in long-distance running and bicycling. Once he recovered from his acute injuries, the patient expressed a desire to return to his previous high level of activity and sport participation.
The experiences of these limitations pushed him to look for other prosthetic options that would offer better performance in these situations. Ultimately, he received the T2 ankle/foot with the X2 microprocessor knee after using a different combination for 2 years. He felt substantial improvements in all the aforementioned limitations and has been using the X2 and T2 combination ever since. The prosthetist provided training in both instances. For distance running, the patient uses the Flex-Run (Össur) Foot.
The Trinity Amputation and Prosthesis Experience Scale (TAPES) and the Locomotor Capabilities Index in Amputees (LCI) were used to assess his adjustment to the prosthetic and performance, respectively, before and after use of the aforementioned combination.
The LCI is a validated measure of lower-extremity amputees’ ability to perform activities with a prosthesis.1 The patient scored the maximum of 7 for all parameters of the LCI (a total of 28 parameters) while using his baseline prosthetic configuration of the X2 knee and the Triton foot (Ottobock). This score did not change when he used the X2/T2 combination (Figure 1; Table).
The TAPES Index is a validated measure of psychological adjustment to prosthetic integration.2 The measure consists of 12 items, rated 1 to 3 (1 = limited a lot; 2 = limited a little; and 3 = not limited at all). His total score was 25 using the X2 alone without the T2 but with the Triton foot. The patient reported that he was “limited a lot” on 2 activity measures (climbing several flights of stairs and running to catch a bus). This measure was reapplied after the patient used the T2 ankle/foot and X2 knee for several weeks. His new sum score was 36, the highest possible for this measure, indicating no functional, social, or athletic restrictions.
Furthermore, the patient reported other improvements, including an almost complete elimination of long-standing back pain, present since amputation. He reported he was able to climb hills with increased speed and less fatigue. The patient also reported he could stand more comfortably and don his shoes more easily, because the T2 would “bend.” Other subjective activity improvements included the ability to easily pick an object off the floor, step up curbs, walk on uneven ground, perform a mountain-climber exercise, and go through small spaces. He reported he was able to do all these activities previously, but the X2/T2 combination made these tasks easier than before to accomplish (Figures 2A and 2B).
Discussion
The subject of this case report is a physically active traumatic transfemoral amputee who had previous experience with several prosthetic components with the ultimate preference and use of the X2 microprocessor knee. Because of the patient’s desire for the most natural and energy-sparing gait he could achieve, a T2 foot and ankle system was added. Though objective measures of locomotion (LCI) did not change, he reported significant improvement in subjective measures of function and prosthetic acceptance (TAPES).
Reported objective advantages favoring the use of microprocessor prosthetic components most often refer to the decrease in energy consumption during locomotion. Several small studies have compared powered with nonpowered, energy-storing, or passive-elastic components and demonstrated at least modest energy savings. In a study of transtibial amputees, researchers compared oxygen consumption during locomotion in patients fitted with a passive-elastic ankle/foot with patients fitted with the powered T2.3 The researchers reported an average decrease in overall energy consumption of 8.4%. Plantarflexion and p
eak ankle-power production at push-off were both increased. The authors of this study conclude that the T2 ankle/foot allows achievement of greater biological realism.
A 2010 review by Highsmith and colleagues concluded that the microprocessor knee C-Leg demonstrated increased efficacy in safety and energy efficiency compared with other prosthetic knees for transfemoral amputees.4
Subjectively, the study patient reported less fatigue when using the X2/T2 combination than when using the X2 knee without the T2 ankle/foot. It is currently unknown whether the combination provided additive energy savings, and this area would be a good course for future investigation.
The study patient reported several subjective improvements, including reduced back pain, a more natural gait, and improved mobility. Hammarlund and colleagues found a significant prevalence of postamputation lower-extremity back pain compared with preamputation symptoms.5 This pain resulted in at least moderate disability in all subjects during prosthetic use. Morgenroth and colleagues went on to speculate that abnormal lumbar spinal kinematics could be a contributing factor for back pain in transfemoral amputees.6
Though not specifically causative, the study found that those transfemoral amputees with increased lumbar spine transverse plane motion experienced significantly more back pain than did similar amputees without lumbar spine transverse plane motion. An abnormal gait would promote more transverse plane motion than that seen in a normal gait. Normalizing prosthetic gait to best simulate the patient’s preamputation biomechanical baseline could reduce transverse lumbar spine motion, reduce back and other mechanical pain, and ultimately, reduce overall disability.
Similarly, the patient in this study also reported increased ease with hills and stairs. Many studies exist that attest to the advantages of microprocessor knees in providing improvements such as decreased stumbles, increased ability to multitask, increased satisfaction with the prosthesis, and improved stair and stance functions, such as with the Genium (Ottobock).7,8 Whether the combination of a microprocessor knee with a powered ankle/foot would further improve these aspects is yet to be objectively investigated. The report of this study patient who used the combination suggests these types of advantages but certainly as a single case report does not provide definitive answers.
The patient achieved the highest possible score on the LCI before using the X2/T2 combination and thus demonstrated a ceiling effect that has been discussed in several studies.9 Furthermore, Larsson and colleagues noted that because of the ceiling effect, the LCI was more useful for amputees of low to moderate activity levels.10 The TAPES, however, showed an improvement in before and after measurements, and assessment with it was not hindered by a ceiling effect.
Conclusion
The patient in this case report noted substantial subjective functional improvements when using the X2 compared with prior mechanical prosthetic knees paired with the T2 foot/ankle. The functional gains were further verified by significant improvement in the TAPES Index score, a validated measure of prosthetic integration. Specific subjective advantages included energy savings, almost complete resolution of back pain, and improved facility with hills, stairs, and crawl spaces. No perceived disadvantages were reported.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Franchignoni F, Orlandini D, Ferriero G, Moscato TA. Reliability, validity, and responsiveness of the locomotor capabilities index in adults with lower-limb amputation undergoing prosthetic training. Arch Phys Med Rehabil. 2004;85(5):743-748.
2. Gallagher P, MacLachlan M. Positive meaning in amputation and thoughts about the amputated limb. Prosthet Orthot Int. 2000;24(3):196-204.
3. Mancinelli C, Patritti BL, Tropea P, et al. Comparing a passive-elastic and a powered prosthesis in transtibial amputees. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:8255-8258.
4. Highsmith MJ, Kahle JT, Bongiorni DR, Sutton BS, Groer S, Kaufman KR. Safety, energy efficiency, and cost efficacy of the C-Leg for transfemoral amputees: A review of the literature. Prosthet Orthot Int. 2010;34(4):362-377.
5. Hammarlund CS, Carlström M, Melchior R, Persson BM. Prevalence of back pain, its effect on functional ability and health-related quality of life in lower limb amputees secondary to trauma or tumour: A comparison across three levels of amputation. Prosthet Orthot Int. 2011;35(1):97-105.
6. Morgenroth DC, Orendurff MS, Shakir A, Segal A, Shofer J, Czerniecki JM. The relationship between lumbar spine kinematics during gait and low-back pain in transfemoral amputees. Am J Phys Med Rehabil. 2010;89(8):635-643.
7. Hafner BJ, Willingham LL, Buell NC, Allyn KJ, Smith DG. Evaluation of function, performance, and preference as transfemoral amputees transition from mechanical to microprocessor control of the prosthetic knee. Arch Phys Med Rehabil. 2007;88(2):207-217.
8. Bellmann M, Schmalz T, Ludwigs E, Blumentritt S. Immediate effects of a new microprocessor-controlled prosthetic knee joint: A comparative biomechanical evaluation. Arch Phys Med Rehabil. 2012;93(3):541-549.
9. Gailey RS, Scoville C, Raya M, et al. The comprehensive high level mobility predictor (CHAMP): A performance-based measure of functional ability of people with lower limb loss. Paper presented at: American Academy of Orthotists & Prosthetists 37th Academy Annual Meeting and Scientific Symposium; March 16-19, 2011; Orlando, FL.
10. Larsson B, Johannesson A, Andersson IH, Atroshi I. The Locomotor Capabilities Index; validity and reliability of the Swedish version in adults with lower limb amputation. Health Qual Life Outcomes. 2009;7:44.
Rapid advances in technology have brought improvements in prosthetic components. In particular, prosthetic knees and ankle/foot complexes have made substantial advancements with the incorporation of computer technology. For example, microprocessor knees are relatively new; the X2 knee from Ottobock (Minneapolis, Minnesota) represents one of the latest and most advanced units and has just been upgraded.
Until recently, there have been no similarly functioning ankle/foot components except for the Proprio Foot from Össur (Foothill Ranch, California), which also provides powered dorsiflexion.
Also, recently BiOM introduced the BiOM T2 foot and ankle system with the added technology of powered plantarflexion to further normalize amputee prosthetic gait. Active patients who have successfully used a microprocessor knee, such as the X2, have generally paired that technology with a variety of foot/ankle components, ranging from passive-elastic units to advanced-energy storing units.
To normalize gait and improve biomechanics even further in select above-knee amputees, experts in the field have suggested combining a microprocessor knee with a powered foot/ankle complex. One potential obstacle to this combination, however, concerns the possible conflict between the active components of the individual units, such as over- or underengagement of component sensors. This situation, theoretically, could compromise patient safety. BiOM, however, provides training to prosthetic providers to address possible component integration issues, including microprocessor conflict and methods to safely use the components together. Once the prosthetist received this training, the patient in this study was fitted with the T2 foot and the X2 knee with excellent results and no perceived disadvantages.
Case Presentation
The patient was a 32-year-old man with a right transfemoral amputation due to trauma from a blast injury, which occurred during Marine service in Iraq. He also had a gunshot wound to his left leg, which resulted in severe injury, but this limb was salvaged and now has good residual function. Before the trauma, the patient was very athletic and involved in long-distance running and bicycling. Once he recovered from his acute injuries, the patient expressed a desire to return to his previous high level of activity and sport participation.
The experiences of these limitations pushed him to look for other prosthetic options that would offer better performance in these situations. Ultimately, he received the T2 ankle/foot with the X2 microprocessor knee after using a different combination for 2 years. He felt substantial improvements in all the aforementioned limitations and has been using the X2 and T2 combination ever since. The prosthetist provided training in both instances. For distance running, the patient uses the Flex-Run (Össur) Foot.
The Trinity Amputation and Prosthesis Experience Scale (TAPES) and the Locomotor Capabilities Index in Amputees (LCI) were used to assess his adjustment to the prosthetic and performance, respectively, before and after use of the aforementioned combination.
The LCI is a validated measure of lower-extremity amputees’ ability to perform activities with a prosthesis.1 The patient scored the maximum of 7 for all parameters of the LCI (a total of 28 parameters) while using his baseline prosthetic configuration of the X2 knee and the Triton foot (Ottobock). This score did not change when he used the X2/T2 combination (Figure 1; Table).
The TAPES Index is a validated measure of psychological adjustment to prosthetic integration.2 The measure consists of 12 items, rated 1 to 3 (1 = limited a lot; 2 = limited a little; and 3 = not limited at all). His total score was 25 using the X2 alone without the T2 but with the Triton foot. The patient reported that he was “limited a lot” on 2 activity measures (climbing several flights of stairs and running to catch a bus). This measure was reapplied after the patient used the T2 ankle/foot and X2 knee for several weeks. His new sum score was 36, the highest possible for this measure, indicating no functional, social, or athletic restrictions.
Furthermore, the patient reported other improvements, including an almost complete elimination of long-standing back pain, present since amputation. He reported he was able to climb hills with increased speed and less fatigue. The patient also reported he could stand more comfortably and don his shoes more easily, because the T2 would “bend.” Other subjective activity improvements included the ability to easily pick an object off the floor, step up curbs, walk on uneven ground, perform a mountain-climber exercise, and go through small spaces. He reported he was able to do all these activities previously, but the X2/T2 combination made these tasks easier than before to accomplish (Figures 2A and 2B).
Discussion
The subject of this case report is a physically active traumatic transfemoral amputee who had previous experience with several prosthetic components with the ultimate preference and use of the X2 microprocessor knee. Because of the patient’s desire for the most natural and energy-sparing gait he could achieve, a T2 foot and ankle system was added. Though objective measures of locomotion (LCI) did not change, he reported significant improvement in subjective measures of function and prosthetic acceptance (TAPES).
Reported objective advantages favoring the use of microprocessor prosthetic components most often refer to the decrease in energy consumption during locomotion. Several small studies have compared powered with nonpowered, energy-storing, or passive-elastic components and demonstrated at least modest energy savings. In a study of transtibial amputees, researchers compared oxygen consumption during locomotion in patients fitted with a passive-elastic ankle/foot with patients fitted with the powered T2.3 The researchers reported an average decrease in overall energy consumption of 8.4%. Plantarflexion and p
eak ankle-power production at push-off were both increased. The authors of this study conclude that the T2 ankle/foot allows achievement of greater biological realism.
A 2010 review by Highsmith and colleagues concluded that the microprocessor knee C-Leg demonstrated increased efficacy in safety and energy efficiency compared with other prosthetic knees for transfemoral amputees.4
Subjectively, the study patient reported less fatigue when using the X2/T2 combination than when using the X2 knee without the T2 ankle/foot. It is currently unknown whether the combination provided additive energy savings, and this area would be a good course for future investigation.
The study patient reported several subjective improvements, including reduced back pain, a more natural gait, and improved mobility. Hammarlund and colleagues found a significant prevalence of postamputation lower-extremity back pain compared with preamputation symptoms.5 This pain resulted in at least moderate disability in all subjects during prosthetic use. Morgenroth and colleagues went on to speculate that abnormal lumbar spinal kinematics could be a contributing factor for back pain in transfemoral amputees.6
Though not specifically causative, the study found that those transfemoral amputees with increased lumbar spine transverse plane motion experienced significantly more back pain than did similar amputees without lumbar spine transverse plane motion. An abnormal gait would promote more transverse plane motion than that seen in a normal gait. Normalizing prosthetic gait to best simulate the patient’s preamputation biomechanical baseline could reduce transverse lumbar spine motion, reduce back and other mechanical pain, and ultimately, reduce overall disability.
Similarly, the patient in this study also reported increased ease with hills and stairs. Many studies exist that attest to the advantages of microprocessor knees in providing improvements such as decreased stumbles, increased ability to multitask, increased satisfaction with the prosthesis, and improved stair and stance functions, such as with the Genium (Ottobock).7,8 Whether the combination of a microprocessor knee with a powered ankle/foot would further improve these aspects is yet to be objectively investigated. The report of this study patient who used the combination suggests these types of advantages but certainly as a single case report does not provide definitive answers.
The patient achieved the highest possible score on the LCI before using the X2/T2 combination and thus demonstrated a ceiling effect that has been discussed in several studies.9 Furthermore, Larsson and colleagues noted that because of the ceiling effect, the LCI was more useful for amputees of low to moderate activity levels.10 The TAPES, however, showed an improvement in before and after measurements, and assessment with it was not hindered by a ceiling effect.
Conclusion
The patient in this case report noted substantial subjective functional improvements when using the X2 compared with prior mechanical prosthetic knees paired with the T2 foot/ankle. The functional gains were further verified by significant improvement in the TAPES Index score, a validated measure of prosthetic integration. Specific subjective advantages included energy savings, almost complete resolution of back pain, and improved facility with hills, stairs, and crawl spaces. No perceived disadvantages were reported.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Rapid advances in technology have brought improvements in prosthetic components. In particular, prosthetic knees and ankle/foot complexes have made substantial advancements with the incorporation of computer technology. For example, microprocessor knees are relatively new; the X2 knee from Ottobock (Minneapolis, Minnesota) represents one of the latest and most advanced units and has just been upgraded.
Until recently, there have been no similarly functioning ankle/foot components except for the Proprio Foot from Össur (Foothill Ranch, California), which also provides powered dorsiflexion.
Also, recently BiOM introduced the BiOM T2 foot and ankle system with the added technology of powered plantarflexion to further normalize amputee prosthetic gait. Active patients who have successfully used a microprocessor knee, such as the X2, have generally paired that technology with a variety of foot/ankle components, ranging from passive-elastic units to advanced-energy storing units.
To normalize gait and improve biomechanics even further in select above-knee amputees, experts in the field have suggested combining a microprocessor knee with a powered foot/ankle complex. One potential obstacle to this combination, however, concerns the possible conflict between the active components of the individual units, such as over- or underengagement of component sensors. This situation, theoretically, could compromise patient safety. BiOM, however, provides training to prosthetic providers to address possible component integration issues, including microprocessor conflict and methods to safely use the components together. Once the prosthetist received this training, the patient in this study was fitted with the T2 foot and the X2 knee with excellent results and no perceived disadvantages.
Case Presentation
The patient was a 32-year-old man with a right transfemoral amputation due to trauma from a blast injury, which occurred during Marine service in Iraq. He also had a gunshot wound to his left leg, which resulted in severe injury, but this limb was salvaged and now has good residual function. Before the trauma, the patient was very athletic and involved in long-distance running and bicycling. Once he recovered from his acute injuries, the patient expressed a desire to return to his previous high level of activity and sport participation.
The experiences of these limitations pushed him to look for other prosthetic options that would offer better performance in these situations. Ultimately, he received the T2 ankle/foot with the X2 microprocessor knee after using a different combination for 2 years. He felt substantial improvements in all the aforementioned limitations and has been using the X2 and T2 combination ever since. The prosthetist provided training in both instances. For distance running, the patient uses the Flex-Run (Össur) Foot.
The Trinity Amputation and Prosthesis Experience Scale (TAPES) and the Locomotor Capabilities Index in Amputees (LCI) were used to assess his adjustment to the prosthetic and performance, respectively, before and after use of the aforementioned combination.
The LCI is a validated measure of lower-extremity amputees’ ability to perform activities with a prosthesis.1 The patient scored the maximum of 7 for all parameters of the LCI (a total of 28 parameters) while using his baseline prosthetic configuration of the X2 knee and the Triton foot (Ottobock). This score did not change when he used the X2/T2 combination (Figure 1; Table).
The TAPES Index is a validated measure of psychological adjustment to prosthetic integration.2 The measure consists of 12 items, rated 1 to 3 (1 = limited a lot; 2 = limited a little; and 3 = not limited at all). His total score was 25 using the X2 alone without the T2 but with the Triton foot. The patient reported that he was “limited a lot” on 2 activity measures (climbing several flights of stairs and running to catch a bus). This measure was reapplied after the patient used the T2 ankle/foot and X2 knee for several weeks. His new sum score was 36, the highest possible for this measure, indicating no functional, social, or athletic restrictions.
Furthermore, the patient reported other improvements, including an almost complete elimination of long-standing back pain, present since amputation. He reported he was able to climb hills with increased speed and less fatigue. The patient also reported he could stand more comfortably and don his shoes more easily, because the T2 would “bend.” Other subjective activity improvements included the ability to easily pick an object off the floor, step up curbs, walk on uneven ground, perform a mountain-climber exercise, and go through small spaces. He reported he was able to do all these activities previously, but the X2/T2 combination made these tasks easier than before to accomplish (Figures 2A and 2B).
Discussion
The subject of this case report is a physically active traumatic transfemoral amputee who had previous experience with several prosthetic components with the ultimate preference and use of the X2 microprocessor knee. Because of the patient’s desire for the most natural and energy-sparing gait he could achieve, a T2 foot and ankle system was added. Though objective measures of locomotion (LCI) did not change, he reported significant improvement in subjective measures of function and prosthetic acceptance (TAPES).
Reported objective advantages favoring the use of microprocessor prosthetic components most often refer to the decrease in energy consumption during locomotion. Several small studies have compared powered with nonpowered, energy-storing, or passive-elastic components and demonstrated at least modest energy savings. In a study of transtibial amputees, researchers compared oxygen consumption during locomotion in patients fitted with a passive-elastic ankle/foot with patients fitted with the powered T2.3 The researchers reported an average decrease in overall energy consumption of 8.4%. Plantarflexion and p
eak ankle-power production at push-off were both increased. The authors of this study conclude that the T2 ankle/foot allows achievement of greater biological realism.
A 2010 review by Highsmith and colleagues concluded that the microprocessor knee C-Leg demonstrated increased efficacy in safety and energy efficiency compared with other prosthetic knees for transfemoral amputees.4
Subjectively, the study patient reported less fatigue when using the X2/T2 combination than when using the X2 knee without the T2 ankle/foot. It is currently unknown whether the combination provided additive energy savings, and this area would be a good course for future investigation.
The study patient reported several subjective improvements, including reduced back pain, a more natural gait, and improved mobility. Hammarlund and colleagues found a significant prevalence of postamputation lower-extremity back pain compared with preamputation symptoms.5 This pain resulted in at least moderate disability in all subjects during prosthetic use. Morgenroth and colleagues went on to speculate that abnormal lumbar spinal kinematics could be a contributing factor for back pain in transfemoral amputees.6
Though not specifically causative, the study found that those transfemoral amputees with increased lumbar spine transverse plane motion experienced significantly more back pain than did similar amputees without lumbar spine transverse plane motion. An abnormal gait would promote more transverse plane motion than that seen in a normal gait. Normalizing prosthetic gait to best simulate the patient’s preamputation biomechanical baseline could reduce transverse lumbar spine motion, reduce back and other mechanical pain, and ultimately, reduce overall disability.
Similarly, the patient in this study also reported increased ease with hills and stairs. Many studies exist that attest to the advantages of microprocessor knees in providing improvements such as decreased stumbles, increased ability to multitask, increased satisfaction with the prosthesis, and improved stair and stance functions, such as with the Genium (Ottobock).7,8 Whether the combination of a microprocessor knee with a powered ankle/foot would further improve these aspects is yet to be objectively investigated. The report of this study patient who used the combination suggests these types of advantages but certainly as a single case report does not provide definitive answers.
The patient achieved the highest possible score on the LCI before using the X2/T2 combination and thus demonstrated a ceiling effect that has been discussed in several studies.9 Furthermore, Larsson and colleagues noted that because of the ceiling effect, the LCI was more useful for amputees of low to moderate activity levels.10 The TAPES, however, showed an improvement in before and after measurements, and assessment with it was not hindered by a ceiling effect.
Conclusion
The patient in this case report noted substantial subjective functional improvements when using the X2 compared with prior mechanical prosthetic knees paired with the T2 foot/ankle. The functional gains were further verified by significant improvement in the TAPES Index score, a validated measure of prosthetic integration. Specific subjective advantages included energy savings, almost complete resolution of back pain, and improved facility with hills, stairs, and crawl spaces. No perceived disadvantages were reported.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Franchignoni F, Orlandini D, Ferriero G, Moscato TA. Reliability, validity, and responsiveness of the locomotor capabilities index in adults with lower-limb amputation undergoing prosthetic training. Arch Phys Med Rehabil. 2004;85(5):743-748.
2. Gallagher P, MacLachlan M. Positive meaning in amputation and thoughts about the amputated limb. Prosthet Orthot Int. 2000;24(3):196-204.
3. Mancinelli C, Patritti BL, Tropea P, et al. Comparing a passive-elastic and a powered prosthesis in transtibial amputees. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:8255-8258.
4. Highsmith MJ, Kahle JT, Bongiorni DR, Sutton BS, Groer S, Kaufman KR. Safety, energy efficiency, and cost efficacy of the C-Leg for transfemoral amputees: A review of the literature. Prosthet Orthot Int. 2010;34(4):362-377.
5. Hammarlund CS, Carlström M, Melchior R, Persson BM. Prevalence of back pain, its effect on functional ability and health-related quality of life in lower limb amputees secondary to trauma or tumour: A comparison across three levels of amputation. Prosthet Orthot Int. 2011;35(1):97-105.
6. Morgenroth DC, Orendurff MS, Shakir A, Segal A, Shofer J, Czerniecki JM. The relationship between lumbar spine kinematics during gait and low-back pain in transfemoral amputees. Am J Phys Med Rehabil. 2010;89(8):635-643.
7. Hafner BJ, Willingham LL, Buell NC, Allyn KJ, Smith DG. Evaluation of function, performance, and preference as transfemoral amputees transition from mechanical to microprocessor control of the prosthetic knee. Arch Phys Med Rehabil. 2007;88(2):207-217.
8. Bellmann M, Schmalz T, Ludwigs E, Blumentritt S. Immediate effects of a new microprocessor-controlled prosthetic knee joint: A comparative biomechanical evaluation. Arch Phys Med Rehabil. 2012;93(3):541-549.
9. Gailey RS, Scoville C, Raya M, et al. The comprehensive high level mobility predictor (CHAMP): A performance-based measure of functional ability of people with lower limb loss. Paper presented at: American Academy of Orthotists & Prosthetists 37th Academy Annual Meeting and Scientific Symposium; March 16-19, 2011; Orlando, FL.
10. Larsson B, Johannesson A, Andersson IH, Atroshi I. The Locomotor Capabilities Index; validity and reliability of the Swedish version in adults with lower limb amputation. Health Qual Life Outcomes. 2009;7:44.
1. Franchignoni F, Orlandini D, Ferriero G, Moscato TA. Reliability, validity, and responsiveness of the locomotor capabilities index in adults with lower-limb amputation undergoing prosthetic training. Arch Phys Med Rehabil. 2004;85(5):743-748.
2. Gallagher P, MacLachlan M. Positive meaning in amputation and thoughts about the amputated limb. Prosthet Orthot Int. 2000;24(3):196-204.
3. Mancinelli C, Patritti BL, Tropea P, et al. Comparing a passive-elastic and a powered prosthesis in transtibial amputees. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:8255-8258.
4. Highsmith MJ, Kahle JT, Bongiorni DR, Sutton BS, Groer S, Kaufman KR. Safety, energy efficiency, and cost efficacy of the C-Leg for transfemoral amputees: A review of the literature. Prosthet Orthot Int. 2010;34(4):362-377.
5. Hammarlund CS, Carlström M, Melchior R, Persson BM. Prevalence of back pain, its effect on functional ability and health-related quality of life in lower limb amputees secondary to trauma or tumour: A comparison across three levels of amputation. Prosthet Orthot Int. 2011;35(1):97-105.
6. Morgenroth DC, Orendurff MS, Shakir A, Segal A, Shofer J, Czerniecki JM. The relationship between lumbar spine kinematics during gait and low-back pain in transfemoral amputees. Am J Phys Med Rehabil. 2010;89(8):635-643.
7. Hafner BJ, Willingham LL, Buell NC, Allyn KJ, Smith DG. Evaluation of function, performance, and preference as transfemoral amputees transition from mechanical to microprocessor control of the prosthetic knee. Arch Phys Med Rehabil. 2007;88(2):207-217.
8. Bellmann M, Schmalz T, Ludwigs E, Blumentritt S. Immediate effects of a new microprocessor-controlled prosthetic knee joint: A comparative biomechanical evaluation. Arch Phys Med Rehabil. 2012;93(3):541-549.
9. Gailey RS, Scoville C, Raya M, et al. The comprehensive high level mobility predictor (CHAMP): A performance-based measure of functional ability of people with lower limb loss. Paper presented at: American Academy of Orthotists & Prosthetists 37th Academy Annual Meeting and Scientific Symposium; March 16-19, 2011; Orlando, FL.
10. Larsson B, Johannesson A, Andersson IH, Atroshi I. The Locomotor Capabilities Index; validity and reliability of the Swedish version in adults with lower limb amputation. Health Qual Life Outcomes. 2009;7:44.
A Conversion Protocol for Simvastatin and Gemfibrozil
Elevated low-density lipoprotein cholesterol (LDL-C) is a major risk factor for the development of coronary artery disease (CAD). For the past decade, lowering LDL-C has been the main focus in the treatment of dyslipidemia.1-3 Significant evidence also exists that hypertriglyceridemia is related to complications, including pancreatitis, and may also be independently linked to cardiovascular risk.4,5
Current treatment guidelines, published by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III), establish LDL-C reduction as the primary goal and triglyceride (TG) reduction as the secondary goal. If TG levels are significantly elevated (> 500 mg/dL), then TG becomes the primary goal due to increased risk of pancreatitis.3
Simvastatin, a 3-hydroxy-3-methylglutaryl-coenzyme (HMG-CoA) reductase inhibitor, has long been the drug of choice in the VA system for the treatment of dyslipidemia, despite its risks, which include myalgias, myopathy, and rhabdomyolysis.6 Incidence of true statin-induced myopathy or rhabdomyolysis is very low, estimated to occur in < 1% of high-dose simvastatin users; the benefits of statin use are often thought to outweigh the risks of therapy.6,7
In the Helsinki Heart Study and the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT), gemfibrozil reduced TG concentrations by 20% to 50%. Gemfibrozil was shown to be useful in both the primary and secondary prevention of CAD and demonstrated mortality reduction in patients with CAD.8,9 The combination of gemfibrozil with simvastatin has been discouraged due to the increased risk of muscle-related complications; however, in practice, the medications are often prescribed concomitantly. A pharmacokinetic study reported that gemfibrozil increased the measured area under the curve concentration of simvastatin 2-fold, likely the reason that rates of myopathy and rhabdomyolysis are 6 times greater when simvastatin is used in combination with gemfibrozil.10
In June 2011, the FDA released new recommendations on the use of simvastatin, which included a dose limit of 40 mg (previously the maximum simvastatin dose was 80 mg) and new drug combination contraindications.11 These recommendations were made in light of the SEARCH (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine) trial, a large, randomized, placebo-controlled trial that revealed a significantly higher rate of myopathy and rhabdomyolysis with simvastatin than had other previous studies.12 Medications cited to increase risk of simvastatin-induced muscle injury included gemfibrozil, and combination therapy is now considered a contraindicated treatment option.10-13
In August 2011, the Pharmacy and Therapeutics (P&T) Committee at the Ralph H. Johnson VA Medical Center in Charleston, South Carolina, reviewed the FDA warning and approved an automatic conversion protocol to be applied to patients prescribed both gemfibrozil and simvastatin. First, the primary goal of therapy (either TG or LDL-C reduction) was determined. TG reduction was considered the primary goal if patients had a history of TG level of > 500 mg/dL or TG-induced pancreatitis; LDL-C was the primary goal in all other patients.
Once the goal of therapy was determined, options for intervention included discontinuation of gemfibrozil, statin dose escalation, and/or addition of niacin or fish oil. In patients whose TG concentration met NCEP goal < 150 mg/dL, gemfibrozil was discontinued. Statin dose was escalated if needed for further LDL-C reduction. Per P&T recommendations, a clinical pharmacist evaluated each patient, made an intervention based on the P&T approved protocol, and sent a letter, which described the intervention and the reason for the action, to each patient.
This study evaluated the outcome of the P&T committee-approved automatic conversion protocol in subgroups of patients prescribed simvastatin and gemfibrozil with a TG serum concentration ≤ 150 mg/dL at baseline (Figure 1).
Methods
A retrospective chart review was conducted on all patients who had prescriptions for gemfibrozil and simvastatin, prescribed in the 52 weeks before August 15, 2011; patient records were reviewed between September 1, 2011, and April 30, 2012. Patients were included for study analysis if they underwent a P&T committee-approved conversion algorithm between September 1, 2011, and December 31, 2011, were aged 18 to 88 years, and their most recent TG measurement was ≤ 150 mg/dL.
Only those patients who had a fasting lipid panel documented in the 56 weeks preceding the study intervention and who returned for a follow-up lipid panel within 6 to 24 weeks following the intervention were included in the study analysis. Patients were excluded from the analysis if they received prescriptions for simvastatin or gemfibrozil from pharmacies outside the VA system, if their cholesterol medications were adjusted during the observation period outside of the initial intervention, if they had any lifetime history of TG > 500 mg/dL or pancreatitis, or if the subjects were incarcerated or pregnant at any time during the study period.
Relevant data were collected, using the VistA (Veterans Health Information Systems and Technology Architecture) and CPRS medical record documentation systems. Patient demographic data, including age, gender, treatment indication, past medical history, adverse drug reaction history, and prescription history were collected for analysis of the study population baseline characteristics. In addition, pertinent laboratory data were examined, including lipid parameters (total cholesterol [TC]; LDL-C; high-density lipoprotein cholesterol [HDL-C]; and TG) and liver function (LF) markers (aspartate aminotransferase [AST]/alanine aminotransferase [ALT]), both before and after the intervention. Patient charts were manually reviewed at follow-up for any adverse events (AEs) attributed to the cholesterol medications.
The primary endpoints included the average change from baseline in lipid parameters, including TC, LDL-C, HDL-C, and TG in each group. Secondary endpoints included descriptions of any safety concerns or AEs. The study was approved on December 15, 2011, by the Medical University of South Carolina internal review board and the VA research and development committee. Statistical analysis was completed, using the paired Student t test for continuous data, and a descriptive analysis was performed for AEs.
Results
Initial patient retrieval included 151 patients who had received prescriptions for gemfibrozil and simvastatin during 2010-2011 and who also had a TG level of ≤ 150 mg/dL obtained in the previous year. Of these patients, 32 patients were missing laboratory data, 20 patients had a lifetime history of a TG level of > 500 mg/dL or pancreatitis, 9 patients had off protocol medication changes, and 1 patient had a nonfasting lipid panel. A total of 62 patients were excluded from the final analysis (Figure 2).
Eighty-nine patients were included in the primary analysis; 8 of those patients were meeting their TG goal but had an LDL-C elevated from goal at baseline, and 81 patients were meeting both their TG and LDL-C goals at baseline. The baseline demographics were reflective of a typical VA population: 99% male, average age 67.3 years, 38% white and 10% African American (52% did not disclose a racial identification).
The primary efficacy goal of change in average lipid parameters after intervention was assessed at 6 to 24 weeks postintervention. Eighty-one patients who met both TG and LDL-C goals at baseline were evaluated (Table 1, Figure 3). Average TC, LDL-C, HDL-C, and AST were not significantly different before and after intervention. There was a statistically significant difference in the average TG levels preintervention and postintervention (107.5 mg/dL vs 159.5 mg/dL; P < .001). Average ALT was significantly higher in the postintervention group (19.6 vs 27; P < .001).
In patients with TGs ≤ 150 mg/dL who did not meet the LDL-C goal at baseline, there was also a statistically significant difference in average TG levels pre- and postintervention (107.6 mg/dL vs 156.1 mg/dL; P = .01). Average LDL-C did not significantly change pre- and postintervention (119.6 mg/dL vs 119.1 mg/dL; P = .82), nor did TC, HDL-C, or AST/ALT (Table 2, Figure 4).
There were no AEs reported in the 6 to 24 weeks following the intervention that could have been attributed to the cholesterol medications. In addition, there were no new diagnoses of pancreatitis entered in the patient’s medical records in the follow-up period. Of note, the range of TG levels after discontinuation of gemfibrozil was 38 mg/dL to 341 mg/dL. After the intervention, 17 of 89 patients (19%) had TG levels of > 200 mg/dL; however, only 1 of 89 patients had a TG level of > 300 mg/dL. No patients had a measured TG level of > 500 mg/dL after the intervention.
Discussion
There was a statistically significant increase in TG concentrations in both groups after discontinuation of gemfibrozil, regardless of change in statin dose. However, the clinical significance of this increase is debatable. The NCEP ATP III guidelines define a TG goal of < 150 mg/dL, and in this study, the average TG level after discontinuation of gemfibrozil was 159.5 mg/dL in the group meeting LDL-C goal and 156.1 mg/dL in the group not meeting LDL-C goal. Following this strict definition, patients did not maintain TG control after the study intervention. Nevertheless, 80% of patients moved from the “normal” category (TG < 150 mg/dL) preintervention to the “borderline high” category (TG 150-200 mg/dL) postintervention per ATP III definitions.3
The importance of TGs as an independent marker for cardiovascular risk has been debated for decades. Data from the Copenhagen City Heart Study indicated that plasma TG levels were significantly associated with increased risk of nonhemorrhagic ischemic events. The relative risk (RR) of ischemic stroke was increased 1.12 (95% confidence interval [CI], 1.07 – 1.16) for every 88.6 mg/dL increase in TGs in that population.14
A meta-analysis of 17 prospective studies of Western subjects found TGs to be an independent risk factor for cardiovascular disease (CVD) endpoints; RR was 1.14 in men (95% CI, 1.05 – 1.28) and 1.37 in women (95% CI, 1.13 – 1.66) per 88.6 mg/dL increase in TGs.15 The association of increased TG concentrations with increased risk of stroke has been validated in 2 other meta-analyses.16,17 Based on these data, there is a correlation between elevated TGs and CVD; however, as Dr. Jerzy-Roch Nofer discusses in an editorial published in 2011 in Current Opinion in Lipidology, the importance of a risk factor is contingent on finding benefit with treatment.18
Two large interventional studies, ACCORD (Action to Control Cardiovascular Risk in Diabetes trial) and FIELDS (Fenofibrate Intervention and Event Lowering in Diabetes trial), did not show a beneficial effect on cardiovascular morbidity and mortality after a clear TG reduction.19,20 The ACCORD and FIELDS studies suggest that although elevated TGs may be associated with elevated cardiovascular risk, no clear protective relationship exists when TGs are reduced to the goal level. Patients in this study who had gemfibrozil discontinued exhibited an increase in TG concentrations; however, the clinical significance of the change is minimal.
Additionally, none of the patients in this study had a TG concentration ≥ 500 mg/dL after the intervention; only 1 of 89 had a TG ≥ 300 mg/dL. This information indicates that most of the patients who were receiving both gemfibrozil and simvastatin likely did not need medical management of their TG levels to begin with. As mentioned earlier, numerous studies did not find a direct causal relationship between the reduction of cardiovascular risk after treating TG concentrations and the NCEP ATP III goal < 150 mg/dL.
There was a small yet significant increase in average ALT in the group of patients who met both LDL-C and TG goals at baseline at follow-up. The increase in ALT met criteria for statistical significance, but both values were maintained within the range of normal values (defined as 7 to 55 units/L by the Mayo Clinic).21 Additionally, the FDA no longer recommends routine monitoring of LF tests during statin therapy in patients with no history of abnormal results.22 The authors concluded that the change in ALT was an incidental finding and did not require further investigation.
The retrospective study design and small patient population limit the external validity of the study. Additionally, the authors did not assess patient compliance with therapy before or after the intervention, which may have skewed the results. Future studies may be designed in a randomized, controlled fashion and would ideally include a broader patient population.
This study provides evidence that can be used in future clinical decisions. Patients in this study may have had slightly elevated TG levels when gemfibrozil was initiated; however, all patients with a history of TG ≥ 500 mg/dL were excluded in this study. None of the patients reached the critical threshold at study follow-up despite a history of TG ≥150 mg/dL (but ≤ 500 mg/dL). This study provides further compelling information that practitioners should aggressively focus on reaching LDL-C and non−HDL-C goals before addressing TG concentrations.
Conclusions
Implementation of an automatic conversion protocol in patients prescribed both simvastatin and gemfibrozil with a baseline TG ≤ 150 mg/dL did not adversely affect lipid control. Patients whose LDL-C met goal preintervention maintained their LDL-C goal at follow-up. Additionally, patients who were not meeting LDL-C goals did not have an increase in LDL-C after the intervention, although there was not a significant improvement in LDL-C either. Both groups demonstrated a statistically significant increase in TG levels after discontinuation of gemfibrozil; however, the clinical significance of the TG change was limited. The results of this study support eliminating gemfibrozil from a statin-containing regimen in patients with low TG who are prescribed the combination.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22.
2. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian simvastatin survival study (4S). Lancet. 1994;344(8934):1383-1389.
3. Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
4. Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high density lipoprotein cholesterol level: A meta-analysis of population based prospective studies. J Cardiovasc Risk. 1996;3(2):213-219.
5 Gotto AM Jr. High-density lipoprotein cholesterol and triglycerides as therapeutic targets for preventing and treating coronary artery disease. Am Heart J. 2002;144(suppl 6):S33-S42.
6 Pedersen TR, Tobert JA. Benefits and risks of HMG-CoA reductase inhibitors in the prevention of coronary heart disease: A reappraisal. Drug Saf. 1996;14(1):11-24.
7. Zocor [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2014.
8. Rubins HB, Robins SJ, Collins D, et al; for the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341(6):410-418.
9. Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: Primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317(20):1237-1245.
10. Backman JT, Kyrklund C, Kivistö KT, Wang JS, Neuvonen PJ. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther. 2000;68(2):122-129.
11. U.S. Food and Drug Administration. FDA Drug Safety Communication: High Dose Simvastatin. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/ucm256581.htm. Updated January 3, 2013. Accessed June 13, 2014.
12. SEARCH Collaborative Group, Armitage J, Bowman L, Wallendszus K, et al. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: A double-blind, randomised trial. Lancet. 2010;376(9753):1658-1669.
13. Gaist D, Rodríguez LA, Huerta C, Hallas J, Sindrup SH. Lipid-lowering drugs and risk of myopathy: A population based follow-up study. Epidemiology. 2001;12(1):565-569.
14. Lindenstrøm E, Boysen G, Nyboe J. Influence of total cholesterol, high density lipoprotein cholesterol, and triglycerides on risk of cerebrovascular disease: The Copenhagen City Heart Study. BMJ. 1994;309(6946):11-15.
15. Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies.J Cardiovasc Risk. 1996;3(2):213-219.
16. Labreuche J, Deplanque D, Touboul PJ, Bruckert E, Amarenco P. Association between change in plasma triglyceride levels and risk of stroke and carotid atherosclerosis: Systematic review and meta-regression analysis. Atherosclerosis. 2010;212(1):9-15.
17. De Caterina R, Scarano M, Marfisi R, et al. Cholesterol-lowering interventions and stroke: Insights from a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2010;55(3):198-211.
18. Nofer JR. Hyperlipidemia and cardiovascular disease: Triglycerides—a revival of cardiovascular risk factor? Curr Opin Lipidol. 2011;22(4):319-321.
19. ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Eng J Med. 2010;362(17):1563-1574.
20. Keech A, Simes RJ, Barter P, et al; FIELD Study Investigators. Effects of long term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet. 2005;366(9500):1849-1861.
21. Mayo Clinic. Liver function tests. Mayo Clinic Website. http://www.mayoclinic.org/tests-procedures/liver-function-tests/basics/results/prc-20012602. Accessed May 30, 2014.
22. U.S. Food and Drug Administration. FDA drug safety communication: Important safety label changes to cholesterol-lowering statin drugs. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm#sa. Update July 7, 2012. Accessed May 30, 2014.
Elevated low-density lipoprotein cholesterol (LDL-C) is a major risk factor for the development of coronary artery disease (CAD). For the past decade, lowering LDL-C has been the main focus in the treatment of dyslipidemia.1-3 Significant evidence also exists that hypertriglyceridemia is related to complications, including pancreatitis, and may also be independently linked to cardiovascular risk.4,5
Current treatment guidelines, published by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III), establish LDL-C reduction as the primary goal and triglyceride (TG) reduction as the secondary goal. If TG levels are significantly elevated (> 500 mg/dL), then TG becomes the primary goal due to increased risk of pancreatitis.3
Simvastatin, a 3-hydroxy-3-methylglutaryl-coenzyme (HMG-CoA) reductase inhibitor, has long been the drug of choice in the VA system for the treatment of dyslipidemia, despite its risks, which include myalgias, myopathy, and rhabdomyolysis.6 Incidence of true statin-induced myopathy or rhabdomyolysis is very low, estimated to occur in < 1% of high-dose simvastatin users; the benefits of statin use are often thought to outweigh the risks of therapy.6,7
In the Helsinki Heart Study and the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT), gemfibrozil reduced TG concentrations by 20% to 50%. Gemfibrozil was shown to be useful in both the primary and secondary prevention of CAD and demonstrated mortality reduction in patients with CAD.8,9 The combination of gemfibrozil with simvastatin has been discouraged due to the increased risk of muscle-related complications; however, in practice, the medications are often prescribed concomitantly. A pharmacokinetic study reported that gemfibrozil increased the measured area under the curve concentration of simvastatin 2-fold, likely the reason that rates of myopathy and rhabdomyolysis are 6 times greater when simvastatin is used in combination with gemfibrozil.10
In June 2011, the FDA released new recommendations on the use of simvastatin, which included a dose limit of 40 mg (previously the maximum simvastatin dose was 80 mg) and new drug combination contraindications.11 These recommendations were made in light of the SEARCH (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine) trial, a large, randomized, placebo-controlled trial that revealed a significantly higher rate of myopathy and rhabdomyolysis with simvastatin than had other previous studies.12 Medications cited to increase risk of simvastatin-induced muscle injury included gemfibrozil, and combination therapy is now considered a contraindicated treatment option.10-13
In August 2011, the Pharmacy and Therapeutics (P&T) Committee at the Ralph H. Johnson VA Medical Center in Charleston, South Carolina, reviewed the FDA warning and approved an automatic conversion protocol to be applied to patients prescribed both gemfibrozil and simvastatin. First, the primary goal of therapy (either TG or LDL-C reduction) was determined. TG reduction was considered the primary goal if patients had a history of TG level of > 500 mg/dL or TG-induced pancreatitis; LDL-C was the primary goal in all other patients.
Once the goal of therapy was determined, options for intervention included discontinuation of gemfibrozil, statin dose escalation, and/or addition of niacin or fish oil. In patients whose TG concentration met NCEP goal < 150 mg/dL, gemfibrozil was discontinued. Statin dose was escalated if needed for further LDL-C reduction. Per P&T recommendations, a clinical pharmacist evaluated each patient, made an intervention based on the P&T approved protocol, and sent a letter, which described the intervention and the reason for the action, to each patient.
This study evaluated the outcome of the P&T committee-approved automatic conversion protocol in subgroups of patients prescribed simvastatin and gemfibrozil with a TG serum concentration ≤ 150 mg/dL at baseline (Figure 1).
Methods
A retrospective chart review was conducted on all patients who had prescriptions for gemfibrozil and simvastatin, prescribed in the 52 weeks before August 15, 2011; patient records were reviewed between September 1, 2011, and April 30, 2012. Patients were included for study analysis if they underwent a P&T committee-approved conversion algorithm between September 1, 2011, and December 31, 2011, were aged 18 to 88 years, and their most recent TG measurement was ≤ 150 mg/dL.
Only those patients who had a fasting lipid panel documented in the 56 weeks preceding the study intervention and who returned for a follow-up lipid panel within 6 to 24 weeks following the intervention were included in the study analysis. Patients were excluded from the analysis if they received prescriptions for simvastatin or gemfibrozil from pharmacies outside the VA system, if their cholesterol medications were adjusted during the observation period outside of the initial intervention, if they had any lifetime history of TG > 500 mg/dL or pancreatitis, or if the subjects were incarcerated or pregnant at any time during the study period.
Relevant data were collected, using the VistA (Veterans Health Information Systems and Technology Architecture) and CPRS medical record documentation systems. Patient demographic data, including age, gender, treatment indication, past medical history, adverse drug reaction history, and prescription history were collected for analysis of the study population baseline characteristics. In addition, pertinent laboratory data were examined, including lipid parameters (total cholesterol [TC]; LDL-C; high-density lipoprotein cholesterol [HDL-C]; and TG) and liver function (LF) markers (aspartate aminotransferase [AST]/alanine aminotransferase [ALT]), both before and after the intervention. Patient charts were manually reviewed at follow-up for any adverse events (AEs) attributed to the cholesterol medications.
The primary endpoints included the average change from baseline in lipid parameters, including TC, LDL-C, HDL-C, and TG in each group. Secondary endpoints included descriptions of any safety concerns or AEs. The study was approved on December 15, 2011, by the Medical University of South Carolina internal review board and the VA research and development committee. Statistical analysis was completed, using the paired Student t test for continuous data, and a descriptive analysis was performed for AEs.
Results
Initial patient retrieval included 151 patients who had received prescriptions for gemfibrozil and simvastatin during 2010-2011 and who also had a TG level of ≤ 150 mg/dL obtained in the previous year. Of these patients, 32 patients were missing laboratory data, 20 patients had a lifetime history of a TG level of > 500 mg/dL or pancreatitis, 9 patients had off protocol medication changes, and 1 patient had a nonfasting lipid panel. A total of 62 patients were excluded from the final analysis (Figure 2).
Eighty-nine patients were included in the primary analysis; 8 of those patients were meeting their TG goal but had an LDL-C elevated from goal at baseline, and 81 patients were meeting both their TG and LDL-C goals at baseline. The baseline demographics were reflective of a typical VA population: 99% male, average age 67.3 years, 38% white and 10% African American (52% did not disclose a racial identification).
The primary efficacy goal of change in average lipid parameters after intervention was assessed at 6 to 24 weeks postintervention. Eighty-one patients who met both TG and LDL-C goals at baseline were evaluated (Table 1, Figure 3). Average TC, LDL-C, HDL-C, and AST were not significantly different before and after intervention. There was a statistically significant difference in the average TG levels preintervention and postintervention (107.5 mg/dL vs 159.5 mg/dL; P < .001). Average ALT was significantly higher in the postintervention group (19.6 vs 27; P < .001).
In patients with TGs ≤ 150 mg/dL who did not meet the LDL-C goal at baseline, there was also a statistically significant difference in average TG levels pre- and postintervention (107.6 mg/dL vs 156.1 mg/dL; P = .01). Average LDL-C did not significantly change pre- and postintervention (119.6 mg/dL vs 119.1 mg/dL; P = .82), nor did TC, HDL-C, or AST/ALT (Table 2, Figure 4).
There were no AEs reported in the 6 to 24 weeks following the intervention that could have been attributed to the cholesterol medications. In addition, there were no new diagnoses of pancreatitis entered in the patient’s medical records in the follow-up period. Of note, the range of TG levels after discontinuation of gemfibrozil was 38 mg/dL to 341 mg/dL. After the intervention, 17 of 89 patients (19%) had TG levels of > 200 mg/dL; however, only 1 of 89 patients had a TG level of > 300 mg/dL. No patients had a measured TG level of > 500 mg/dL after the intervention.
Discussion
There was a statistically significant increase in TG concentrations in both groups after discontinuation of gemfibrozil, regardless of change in statin dose. However, the clinical significance of this increase is debatable. The NCEP ATP III guidelines define a TG goal of < 150 mg/dL, and in this study, the average TG level after discontinuation of gemfibrozil was 159.5 mg/dL in the group meeting LDL-C goal and 156.1 mg/dL in the group not meeting LDL-C goal. Following this strict definition, patients did not maintain TG control after the study intervention. Nevertheless, 80% of patients moved from the “normal” category (TG < 150 mg/dL) preintervention to the “borderline high” category (TG 150-200 mg/dL) postintervention per ATP III definitions.3
The importance of TGs as an independent marker for cardiovascular risk has been debated for decades. Data from the Copenhagen City Heart Study indicated that plasma TG levels were significantly associated with increased risk of nonhemorrhagic ischemic events. The relative risk (RR) of ischemic stroke was increased 1.12 (95% confidence interval [CI], 1.07 – 1.16) for every 88.6 mg/dL increase in TGs in that population.14
A meta-analysis of 17 prospective studies of Western subjects found TGs to be an independent risk factor for cardiovascular disease (CVD) endpoints; RR was 1.14 in men (95% CI, 1.05 – 1.28) and 1.37 in women (95% CI, 1.13 – 1.66) per 88.6 mg/dL increase in TGs.15 The association of increased TG concentrations with increased risk of stroke has been validated in 2 other meta-analyses.16,17 Based on these data, there is a correlation between elevated TGs and CVD; however, as Dr. Jerzy-Roch Nofer discusses in an editorial published in 2011 in Current Opinion in Lipidology, the importance of a risk factor is contingent on finding benefit with treatment.18
Two large interventional studies, ACCORD (Action to Control Cardiovascular Risk in Diabetes trial) and FIELDS (Fenofibrate Intervention and Event Lowering in Diabetes trial), did not show a beneficial effect on cardiovascular morbidity and mortality after a clear TG reduction.19,20 The ACCORD and FIELDS studies suggest that although elevated TGs may be associated with elevated cardiovascular risk, no clear protective relationship exists when TGs are reduced to the goal level. Patients in this study who had gemfibrozil discontinued exhibited an increase in TG concentrations; however, the clinical significance of the change is minimal.
Additionally, none of the patients in this study had a TG concentration ≥ 500 mg/dL after the intervention; only 1 of 89 had a TG ≥ 300 mg/dL. This information indicates that most of the patients who were receiving both gemfibrozil and simvastatin likely did not need medical management of their TG levels to begin with. As mentioned earlier, numerous studies did not find a direct causal relationship between the reduction of cardiovascular risk after treating TG concentrations and the NCEP ATP III goal < 150 mg/dL.
There was a small yet significant increase in average ALT in the group of patients who met both LDL-C and TG goals at baseline at follow-up. The increase in ALT met criteria for statistical significance, but both values were maintained within the range of normal values (defined as 7 to 55 units/L by the Mayo Clinic).21 Additionally, the FDA no longer recommends routine monitoring of LF tests during statin therapy in patients with no history of abnormal results.22 The authors concluded that the change in ALT was an incidental finding and did not require further investigation.
The retrospective study design and small patient population limit the external validity of the study. Additionally, the authors did not assess patient compliance with therapy before or after the intervention, which may have skewed the results. Future studies may be designed in a randomized, controlled fashion and would ideally include a broader patient population.
This study provides evidence that can be used in future clinical decisions. Patients in this study may have had slightly elevated TG levels when gemfibrozil was initiated; however, all patients with a history of TG ≥ 500 mg/dL were excluded in this study. None of the patients reached the critical threshold at study follow-up despite a history of TG ≥150 mg/dL (but ≤ 500 mg/dL). This study provides further compelling information that practitioners should aggressively focus on reaching LDL-C and non−HDL-C goals before addressing TG concentrations.
Conclusions
Implementation of an automatic conversion protocol in patients prescribed both simvastatin and gemfibrozil with a baseline TG ≤ 150 mg/dL did not adversely affect lipid control. Patients whose LDL-C met goal preintervention maintained their LDL-C goal at follow-up. Additionally, patients who were not meeting LDL-C goals did not have an increase in LDL-C after the intervention, although there was not a significant improvement in LDL-C either. Both groups demonstrated a statistically significant increase in TG levels after discontinuation of gemfibrozil; however, the clinical significance of the TG change was limited. The results of this study support eliminating gemfibrozil from a statin-containing regimen in patients with low TG who are prescribed the combination.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Elevated low-density lipoprotein cholesterol (LDL-C) is a major risk factor for the development of coronary artery disease (CAD). For the past decade, lowering LDL-C has been the main focus in the treatment of dyslipidemia.1-3 Significant evidence also exists that hypertriglyceridemia is related to complications, including pancreatitis, and may also be independently linked to cardiovascular risk.4,5
Current treatment guidelines, published by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III), establish LDL-C reduction as the primary goal and triglyceride (TG) reduction as the secondary goal. If TG levels are significantly elevated (> 500 mg/dL), then TG becomes the primary goal due to increased risk of pancreatitis.3
Simvastatin, a 3-hydroxy-3-methylglutaryl-coenzyme (HMG-CoA) reductase inhibitor, has long been the drug of choice in the VA system for the treatment of dyslipidemia, despite its risks, which include myalgias, myopathy, and rhabdomyolysis.6 Incidence of true statin-induced myopathy or rhabdomyolysis is very low, estimated to occur in < 1% of high-dose simvastatin users; the benefits of statin use are often thought to outweigh the risks of therapy.6,7
In the Helsinki Heart Study and the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT), gemfibrozil reduced TG concentrations by 20% to 50%. Gemfibrozil was shown to be useful in both the primary and secondary prevention of CAD and demonstrated mortality reduction in patients with CAD.8,9 The combination of gemfibrozil with simvastatin has been discouraged due to the increased risk of muscle-related complications; however, in practice, the medications are often prescribed concomitantly. A pharmacokinetic study reported that gemfibrozil increased the measured area under the curve concentration of simvastatin 2-fold, likely the reason that rates of myopathy and rhabdomyolysis are 6 times greater when simvastatin is used in combination with gemfibrozil.10
In June 2011, the FDA released new recommendations on the use of simvastatin, which included a dose limit of 40 mg (previously the maximum simvastatin dose was 80 mg) and new drug combination contraindications.11 These recommendations were made in light of the SEARCH (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine) trial, a large, randomized, placebo-controlled trial that revealed a significantly higher rate of myopathy and rhabdomyolysis with simvastatin than had other previous studies.12 Medications cited to increase risk of simvastatin-induced muscle injury included gemfibrozil, and combination therapy is now considered a contraindicated treatment option.10-13
In August 2011, the Pharmacy and Therapeutics (P&T) Committee at the Ralph H. Johnson VA Medical Center in Charleston, South Carolina, reviewed the FDA warning and approved an automatic conversion protocol to be applied to patients prescribed both gemfibrozil and simvastatin. First, the primary goal of therapy (either TG or LDL-C reduction) was determined. TG reduction was considered the primary goal if patients had a history of TG level of > 500 mg/dL or TG-induced pancreatitis; LDL-C was the primary goal in all other patients.
Once the goal of therapy was determined, options for intervention included discontinuation of gemfibrozil, statin dose escalation, and/or addition of niacin or fish oil. In patients whose TG concentration met NCEP goal < 150 mg/dL, gemfibrozil was discontinued. Statin dose was escalated if needed for further LDL-C reduction. Per P&T recommendations, a clinical pharmacist evaluated each patient, made an intervention based on the P&T approved protocol, and sent a letter, which described the intervention and the reason for the action, to each patient.
This study evaluated the outcome of the P&T committee-approved automatic conversion protocol in subgroups of patients prescribed simvastatin and gemfibrozil with a TG serum concentration ≤ 150 mg/dL at baseline (Figure 1).
Methods
A retrospective chart review was conducted on all patients who had prescriptions for gemfibrozil and simvastatin, prescribed in the 52 weeks before August 15, 2011; patient records were reviewed between September 1, 2011, and April 30, 2012. Patients were included for study analysis if they underwent a P&T committee-approved conversion algorithm between September 1, 2011, and December 31, 2011, were aged 18 to 88 years, and their most recent TG measurement was ≤ 150 mg/dL.
Only those patients who had a fasting lipid panel documented in the 56 weeks preceding the study intervention and who returned for a follow-up lipid panel within 6 to 24 weeks following the intervention were included in the study analysis. Patients were excluded from the analysis if they received prescriptions for simvastatin or gemfibrozil from pharmacies outside the VA system, if their cholesterol medications were adjusted during the observation period outside of the initial intervention, if they had any lifetime history of TG > 500 mg/dL or pancreatitis, or if the subjects were incarcerated or pregnant at any time during the study period.
Relevant data were collected, using the VistA (Veterans Health Information Systems and Technology Architecture) and CPRS medical record documentation systems. Patient demographic data, including age, gender, treatment indication, past medical history, adverse drug reaction history, and prescription history were collected for analysis of the study population baseline characteristics. In addition, pertinent laboratory data were examined, including lipid parameters (total cholesterol [TC]; LDL-C; high-density lipoprotein cholesterol [HDL-C]; and TG) and liver function (LF) markers (aspartate aminotransferase [AST]/alanine aminotransferase [ALT]), both before and after the intervention. Patient charts were manually reviewed at follow-up for any adverse events (AEs) attributed to the cholesterol medications.
The primary endpoints included the average change from baseline in lipid parameters, including TC, LDL-C, HDL-C, and TG in each group. Secondary endpoints included descriptions of any safety concerns or AEs. The study was approved on December 15, 2011, by the Medical University of South Carolina internal review board and the VA research and development committee. Statistical analysis was completed, using the paired Student t test for continuous data, and a descriptive analysis was performed for AEs.
Results
Initial patient retrieval included 151 patients who had received prescriptions for gemfibrozil and simvastatin during 2010-2011 and who also had a TG level of ≤ 150 mg/dL obtained in the previous year. Of these patients, 32 patients were missing laboratory data, 20 patients had a lifetime history of a TG level of > 500 mg/dL or pancreatitis, 9 patients had off protocol medication changes, and 1 patient had a nonfasting lipid panel. A total of 62 patients were excluded from the final analysis (Figure 2).
Eighty-nine patients were included in the primary analysis; 8 of those patients were meeting their TG goal but had an LDL-C elevated from goal at baseline, and 81 patients were meeting both their TG and LDL-C goals at baseline. The baseline demographics were reflective of a typical VA population: 99% male, average age 67.3 years, 38% white and 10% African American (52% did not disclose a racial identification).
The primary efficacy goal of change in average lipid parameters after intervention was assessed at 6 to 24 weeks postintervention. Eighty-one patients who met both TG and LDL-C goals at baseline were evaluated (Table 1, Figure 3). Average TC, LDL-C, HDL-C, and AST were not significantly different before and after intervention. There was a statistically significant difference in the average TG levels preintervention and postintervention (107.5 mg/dL vs 159.5 mg/dL; P < .001). Average ALT was significantly higher in the postintervention group (19.6 vs 27; P < .001).
In patients with TGs ≤ 150 mg/dL who did not meet the LDL-C goal at baseline, there was also a statistically significant difference in average TG levels pre- and postintervention (107.6 mg/dL vs 156.1 mg/dL; P = .01). Average LDL-C did not significantly change pre- and postintervention (119.6 mg/dL vs 119.1 mg/dL; P = .82), nor did TC, HDL-C, or AST/ALT (Table 2, Figure 4).
There were no AEs reported in the 6 to 24 weeks following the intervention that could have been attributed to the cholesterol medications. In addition, there were no new diagnoses of pancreatitis entered in the patient’s medical records in the follow-up period. Of note, the range of TG levels after discontinuation of gemfibrozil was 38 mg/dL to 341 mg/dL. After the intervention, 17 of 89 patients (19%) had TG levels of > 200 mg/dL; however, only 1 of 89 patients had a TG level of > 300 mg/dL. No patients had a measured TG level of > 500 mg/dL after the intervention.
Discussion
There was a statistically significant increase in TG concentrations in both groups after discontinuation of gemfibrozil, regardless of change in statin dose. However, the clinical significance of this increase is debatable. The NCEP ATP III guidelines define a TG goal of < 150 mg/dL, and in this study, the average TG level after discontinuation of gemfibrozil was 159.5 mg/dL in the group meeting LDL-C goal and 156.1 mg/dL in the group not meeting LDL-C goal. Following this strict definition, patients did not maintain TG control after the study intervention. Nevertheless, 80% of patients moved from the “normal” category (TG < 150 mg/dL) preintervention to the “borderline high” category (TG 150-200 mg/dL) postintervention per ATP III definitions.3
The importance of TGs as an independent marker for cardiovascular risk has been debated for decades. Data from the Copenhagen City Heart Study indicated that plasma TG levels were significantly associated with increased risk of nonhemorrhagic ischemic events. The relative risk (RR) of ischemic stroke was increased 1.12 (95% confidence interval [CI], 1.07 – 1.16) for every 88.6 mg/dL increase in TGs in that population.14
A meta-analysis of 17 prospective studies of Western subjects found TGs to be an independent risk factor for cardiovascular disease (CVD) endpoints; RR was 1.14 in men (95% CI, 1.05 – 1.28) and 1.37 in women (95% CI, 1.13 – 1.66) per 88.6 mg/dL increase in TGs.15 The association of increased TG concentrations with increased risk of stroke has been validated in 2 other meta-analyses.16,17 Based on these data, there is a correlation between elevated TGs and CVD; however, as Dr. Jerzy-Roch Nofer discusses in an editorial published in 2011 in Current Opinion in Lipidology, the importance of a risk factor is contingent on finding benefit with treatment.18
Two large interventional studies, ACCORD (Action to Control Cardiovascular Risk in Diabetes trial) and FIELDS (Fenofibrate Intervention and Event Lowering in Diabetes trial), did not show a beneficial effect on cardiovascular morbidity and mortality after a clear TG reduction.19,20 The ACCORD and FIELDS studies suggest that although elevated TGs may be associated with elevated cardiovascular risk, no clear protective relationship exists when TGs are reduced to the goal level. Patients in this study who had gemfibrozil discontinued exhibited an increase in TG concentrations; however, the clinical significance of the change is minimal.
Additionally, none of the patients in this study had a TG concentration ≥ 500 mg/dL after the intervention; only 1 of 89 had a TG ≥ 300 mg/dL. This information indicates that most of the patients who were receiving both gemfibrozil and simvastatin likely did not need medical management of their TG levels to begin with. As mentioned earlier, numerous studies did not find a direct causal relationship between the reduction of cardiovascular risk after treating TG concentrations and the NCEP ATP III goal < 150 mg/dL.
There was a small yet significant increase in average ALT in the group of patients who met both LDL-C and TG goals at baseline at follow-up. The increase in ALT met criteria for statistical significance, but both values were maintained within the range of normal values (defined as 7 to 55 units/L by the Mayo Clinic).21 Additionally, the FDA no longer recommends routine monitoring of LF tests during statin therapy in patients with no history of abnormal results.22 The authors concluded that the change in ALT was an incidental finding and did not require further investigation.
The retrospective study design and small patient population limit the external validity of the study. Additionally, the authors did not assess patient compliance with therapy before or after the intervention, which may have skewed the results. Future studies may be designed in a randomized, controlled fashion and would ideally include a broader patient population.
This study provides evidence that can be used in future clinical decisions. Patients in this study may have had slightly elevated TG levels when gemfibrozil was initiated; however, all patients with a history of TG ≥ 500 mg/dL were excluded in this study. None of the patients reached the critical threshold at study follow-up despite a history of TG ≥150 mg/dL (but ≤ 500 mg/dL). This study provides further compelling information that practitioners should aggressively focus on reaching LDL-C and non−HDL-C goals before addressing TG concentrations.
Conclusions
Implementation of an automatic conversion protocol in patients prescribed both simvastatin and gemfibrozil with a baseline TG ≤ 150 mg/dL did not adversely affect lipid control. Patients whose LDL-C met goal preintervention maintained their LDL-C goal at follow-up. Additionally, patients who were not meeting LDL-C goals did not have an increase in LDL-C after the intervention, although there was not a significant improvement in LDL-C either. Both groups demonstrated a statistically significant increase in TG levels after discontinuation of gemfibrozil; however, the clinical significance of the TG change was limited. The results of this study support eliminating gemfibrozil from a statin-containing regimen in patients with low TG who are prescribed the combination.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22.
2. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian simvastatin survival study (4S). Lancet. 1994;344(8934):1383-1389.
3. Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
4. Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high density lipoprotein cholesterol level: A meta-analysis of population based prospective studies. J Cardiovasc Risk. 1996;3(2):213-219.
5 Gotto AM Jr. High-density lipoprotein cholesterol and triglycerides as therapeutic targets for preventing and treating coronary artery disease. Am Heart J. 2002;144(suppl 6):S33-S42.
6 Pedersen TR, Tobert JA. Benefits and risks of HMG-CoA reductase inhibitors in the prevention of coronary heart disease: A reappraisal. Drug Saf. 1996;14(1):11-24.
7. Zocor [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2014.
8. Rubins HB, Robins SJ, Collins D, et al; for the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341(6):410-418.
9. Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: Primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317(20):1237-1245.
10. Backman JT, Kyrklund C, Kivistö KT, Wang JS, Neuvonen PJ. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther. 2000;68(2):122-129.
11. U.S. Food and Drug Administration. FDA Drug Safety Communication: High Dose Simvastatin. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/ucm256581.htm. Updated January 3, 2013. Accessed June 13, 2014.
12. SEARCH Collaborative Group, Armitage J, Bowman L, Wallendszus K, et al. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: A double-blind, randomised trial. Lancet. 2010;376(9753):1658-1669.
13. Gaist D, Rodríguez LA, Huerta C, Hallas J, Sindrup SH. Lipid-lowering drugs and risk of myopathy: A population based follow-up study. Epidemiology. 2001;12(1):565-569.
14. Lindenstrøm E, Boysen G, Nyboe J. Influence of total cholesterol, high density lipoprotein cholesterol, and triglycerides on risk of cerebrovascular disease: The Copenhagen City Heart Study. BMJ. 1994;309(6946):11-15.
15. Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies.J Cardiovasc Risk. 1996;3(2):213-219.
16. Labreuche J, Deplanque D, Touboul PJ, Bruckert E, Amarenco P. Association between change in plasma triglyceride levels and risk of stroke and carotid atherosclerosis: Systematic review and meta-regression analysis. Atherosclerosis. 2010;212(1):9-15.
17. De Caterina R, Scarano M, Marfisi R, et al. Cholesterol-lowering interventions and stroke: Insights from a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2010;55(3):198-211.
18. Nofer JR. Hyperlipidemia and cardiovascular disease: Triglycerides—a revival of cardiovascular risk factor? Curr Opin Lipidol. 2011;22(4):319-321.
19. ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Eng J Med. 2010;362(17):1563-1574.
20. Keech A, Simes RJ, Barter P, et al; FIELD Study Investigators. Effects of long term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet. 2005;366(9500):1849-1861.
21. Mayo Clinic. Liver function tests. Mayo Clinic Website. http://www.mayoclinic.org/tests-procedures/liver-function-tests/basics/results/prc-20012602. Accessed May 30, 2014.
22. U.S. Food and Drug Administration. FDA drug safety communication: Important safety label changes to cholesterol-lowering statin drugs. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm#sa. Update July 7, 2012. Accessed May 30, 2014.
1. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22.
2. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian simvastatin survival study (4S). Lancet. 1994;344(8934):1383-1389.
3. Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
4. Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high density lipoprotein cholesterol level: A meta-analysis of population based prospective studies. J Cardiovasc Risk. 1996;3(2):213-219.
5 Gotto AM Jr. High-density lipoprotein cholesterol and triglycerides as therapeutic targets for preventing and treating coronary artery disease. Am Heart J. 2002;144(suppl 6):S33-S42.
6 Pedersen TR, Tobert JA. Benefits and risks of HMG-CoA reductase inhibitors in the prevention of coronary heart disease: A reappraisal. Drug Saf. 1996;14(1):11-24.
7. Zocor [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2014.
8. Rubins HB, Robins SJ, Collins D, et al; for the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341(6):410-418.
9. Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: Primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317(20):1237-1245.
10. Backman JT, Kyrklund C, Kivistö KT, Wang JS, Neuvonen PJ. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther. 2000;68(2):122-129.
11. U.S. Food and Drug Administration. FDA Drug Safety Communication: High Dose Simvastatin. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/ucm256581.htm. Updated January 3, 2013. Accessed June 13, 2014.
12. SEARCH Collaborative Group, Armitage J, Bowman L, Wallendszus K, et al. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: A double-blind, randomised trial. Lancet. 2010;376(9753):1658-1669.
13. Gaist D, Rodríguez LA, Huerta C, Hallas J, Sindrup SH. Lipid-lowering drugs and risk of myopathy: A population based follow-up study. Epidemiology. 2001;12(1):565-569.
14. Lindenstrøm E, Boysen G, Nyboe J. Influence of total cholesterol, high density lipoprotein cholesterol, and triglycerides on risk of cerebrovascular disease: The Copenhagen City Heart Study. BMJ. 1994;309(6946):11-15.
15. Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies.J Cardiovasc Risk. 1996;3(2):213-219.
16. Labreuche J, Deplanque D, Touboul PJ, Bruckert E, Amarenco P. Association between change in plasma triglyceride levels and risk of stroke and carotid atherosclerosis: Systematic review and meta-regression analysis. Atherosclerosis. 2010;212(1):9-15.
17. De Caterina R, Scarano M, Marfisi R, et al. Cholesterol-lowering interventions and stroke: Insights from a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2010;55(3):198-211.
18. Nofer JR. Hyperlipidemia and cardiovascular disease: Triglycerides—a revival of cardiovascular risk factor? Curr Opin Lipidol. 2011;22(4):319-321.
19. ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Eng J Med. 2010;362(17):1563-1574.
20. Keech A, Simes RJ, Barter P, et al; FIELD Study Investigators. Effects of long term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet. 2005;366(9500):1849-1861.
21. Mayo Clinic. Liver function tests. Mayo Clinic Website. http://www.mayoclinic.org/tests-procedures/liver-function-tests/basics/results/prc-20012602. Accessed May 30, 2014.
22. U.S. Food and Drug Administration. FDA drug safety communication: Important safety label changes to cholesterol-lowering statin drugs. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm#sa. Update July 7, 2012. Accessed May 30, 2014.
Bacteremia From an Unlikely Source
The most common microbes causing postoperative wound infection are Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus epidermidis, and Enterococcus faecalis.1 Pasteurella multocida (P multocida) is an uncommon organism causing surgical-site infections.2 This article reports a case of a patient who developed a postoperative wound infection due to P multocida complicated by a bloodstream infection.
CASE REPORT
A 54-year-old man presented with pain and discharge surrounding his recent surgical site and a 1-day history of fever and chills. Four weeks prior to the presentation, he underwent nodal dissection and reconstructive surgery with a muscle flap on his right leg for a superficial spreading melanoma. The patient noticed wound dehiscence the previous 2 to 3 days and a white-yellowish discharge from the wound.
Six years previously, the patient was diagnosed with a superficial spreading melanoma on his right thigh. At that time, he underwent wide excision for the localized disease. The patient was doing well until 3 months prior to the admission when he noticed a nodule in his right groin area, which had enlarged to a lump. Fine needle aspiration of the lump and immunohistochemistry showed the presence of malignant cells, positive for S100 and MART-1.These markers are useful for a diagnosis of metastatic melanoma.3,4
A positron emission tomography (PET) scan revealed localized disease with increased uptake only in the groin region. Superficial nodal dissection and sartorius muscle flap placement were performed, and the patient was discharged to his home with drains in place.
Two weeks after his node dissection, the patient was seen in the surgery clinic, and his drains were removed. Three days after his clinic visit, he noticed erythema around the suture line and discharge from the wound, followed by increasing pain, high fever, and chills. The patient reported no history of nausea, vomiting, diarrhea, painful urination, cough, shortness of breath, chest pain, or abdominal pain. His medical history was also significant for hypertension and multiple lipomas on his back and extremities, which were removed surgically 15 years previously. He had been a chronic smoker and occasionally used alcohol but did not use illicit drugs. The patient was unmarried and lived alone with his domestic cats.
The patient was febrile on admission (38.9°C); heart rate 110 bpm. His blood pressure and respiratory rate were normal. An examination of the right groin revealed an incision mark of about 20 cm in length from his right anterior superior iliac spine travelling inferomedially into his groin with breakdown at the infero-medial aspect, extending one-half to one-third the wound distance. The wound had clean borders with minimal amount of purulent drainage. Induration was noted, extending down the thigh to above the knee level. Sutures remained in place along the superior aspect of the incision site. No other abnormalities were found.
Laboratory studies revealed leucocytosis (white blood cell count, 12,200/mm3). Blood glucose, blood urea nitrogen, serum creatinine, and electrolytes were within normal range. Computerized tomography (CT) scans of the pelvis and thighs with IV contrast revealed normal right common femoral artery and vein with postsurgical changes and thickening of the skin and small air lucencies in the right groin. The patient was started on empiric treatment with IV vancomycin and piperacillin/tazobactam.
Due to initial worsening of erythema, piperacillin/tazobactam were stopped and ertapenem was initiated. Blood cultures sets and the culture of the surgical wound were positive for P multocida. Vancomycin was discontinued, and the patient rapidly improved. He was discharged on oral moxifloxacin for 2 weeks. After 10 months of follow-up, the patient was doing well and the surgical site wound had healed.
DISCUSSION
Pasteurella multocida is a small, Gram-negative, nonmotile, non–spore-forming coccobacillus with bipolar staining features.5 It often exists as a commensal in the upper respiratory tracts of domestic pet species, especially cats and dogs, and may cause hemorrhagic septicemia in cattle, fowl cholera in chickens, and atrophic rhinitis in pigs.6,7Pasteurella multocida infection in humans is often associated with an animal bite, scratch, or lick, but infection without epidemiologic evidence of animal contact may occur.8
The majority of animal bites involve dogs (85%-90%), followed by cats (5%-10%). Infectious complications occur in about 15% to 20% of dog-related bites and > 50% of cat-related bites. The sharp and long teeth of cats can easily penetrate human skin, create a deep puncture wound, and even inoculate the periosteal component of bones. Cat-related wounds more commonly progress to serious and deeper tissue infections, including osteomyelitis and meningitis.9 Human-to-human transmission is rare, but there are reported cases of respiratory transmission and maternal-to-fetal transmission.10,11
The most common presentation of P multocida infection in humans is soft-tissue infection, appearing as purulent wounds (48%), cellulitis (36%), or abscesses (16%).12 The infection is characterized by the development of soft-tissue inflammation at the site of contact, which may progress to diffuse, localized cellulitis. High leukocyte and neutrophil counts are typically observed.13 Complications of localized infection include rapidly progressive cellulitis, abscesses, tenosynovitis, osteomyelitis, and septic arthritis. The localized infection can also lead to septicemia, which carries a high mortality rate (31%).12,14
Pasteurella multocida septicemia commonly occurs in patients with an immune-compromised status, but septicemia in healthy individuals has also been reported.15,16 Apart from local skin and soft-tissue infections, P multocida can cause upper respiratory tract infections, lower respiratory tract infections leading to pneumonia, trachea-bronchitis, lung abscess, and empyema, usually in individuals with underlying pulmonary disease. Pasteurella multocida meningitis has been associated with cat licks and bites occurring on the face in both the young and the elderly.17-19
Gram stains of purulent material or other fluid specimens, including blood, sputum, and cerebrospinal fluid, may show small, Gram-negative, nonmotile, non–spore-forming pleomorphic coccobacilli. Wright, Giemsa, and Wayson stains enhance bipolar staining. The quickest and most accurate method for confirming an active P multocida infection is molecular detection using polymerase chain reaction.20 Evaluations of tenosynovitis, septic arthritis, osteomyelitis, and meningeal enhancement, when appropriate, should be done with CT scans or MRIs.
In < 10% of cases, localized infection by P multocida may lead to bacteremia. The most common predisposing factors associated with bacteremia identified in a review of cases over 20 years at an urban medical center included old age and chronic medical conditions.21 Chronic medical conditions involved most commonly were diabetes mellitus, hypertension, and congestive heart failure. Liver dysfunction has also been reported as a significant risk factor in cases of P multocida bacteremia. The most common antibiotics used to treat the patients with bacteremia were ampicillin/sulbactam, cephalosporins, and fluoroquinolones.
A brief review of the literature of 21 cases of bacteremia showed that the most common antibiotics used for treatment were penicillins (ampicillin, amoxicillin, and piperacillin) in 11 of 21 cases. After penicillin, ciprofloxacin was the most commonly used antibiotic to treat bacteremia secondary to P multocida. The review also identified chronic medical conditions, such as diabetes mellitus, hypertension, congestive heart failure, and chronic obstructive pulmonary disease, as the most common risk factors associated in these cases with bacteremia.
In 17 of 20 cases, patients reported being present in an environment with pets, mostly cats and dogs. Twelve out of 17 patients reported having contact with pet cats and dogs, mostly in the form of bites or pets licking their wounds, and 3 of these were postoperative patients with external wounds.22-24
This patient reported that after discharge from the hospital following surgery for his spreading melanoma, domestic cats at his home repeatedly licked the postoperative wound. This almost certainly was the source of the infection and bacteremia in this patient. These findings stress the importance of educating patients about proper postoperative wound care and precautions needed if there is potential exposure to domestic animals, such as cats and dogs.
CONCLUSIONS
Pasteurella multocida is an unusual cause of postoperative wound infection. The most common method of acquiring a P multocida infection is through contact with pet animals, mostly cats and dogs. Infection can occur not only with animal bites and scratches, but also with licking of open wounds. The rate of infections can be decreased significantly by educating patients about the mode of transmission of infection, its complications, and safety measures needed if they have pets at home.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Giacometti A, Cirioni O, Schimizzi AM, et al. Epidemiology and microbiology of surgical wound infection. J Clin Microbiol. 2000;38(2):918-919.
2. Cook PP. Persistent postoperative wound infection with Pasteurella multocida: Case report and literature review. Infection. 1995;23(4):252.
3. Hodi FS. Well-defined melanoma antigens as progression markers for melanoma: Insights into differential expression and host response based on stage. Clin Cancer Res. 2006;12(3, pt 1):673-678.
4. Zubovits J, Buzney E, Yu L, Duncan LM. HMB-45, S-100, NK1/C3, and MART-1 in metastatic melanoma. Hum Pathol. 2004;35(2):217-223.
5. Zurlo JJ. Pasteurella species. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 6th ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2005:2687-2691.
6. Waghorn DJ, Robson M. Occupational risk of Pasteurella multocida septicaemia and premature labor in a pregnant vet. BJOG. 2003;110(8):780-781.
7. Francis DP, Holmes MA, Brandon G. Pasteurella multocida infections after domestic animal bites and scratches. JAMA. 1975;233(1):42-45.
8. Cordoba A, Bueno I, Monterrubio J, Corcho G. Surgical wound infection due to Pasteurella multocida [article in Spanish]. Enferm Infecc Microbiol Clin. 2002;20(10):536-537.
9. Kopita JM, Handshoe D, Kussin PS, Kelemen M. Cat germs! Pleuropulmonary pasteurella infection in an old man. N C Med J. 1993;54(7):308-311.
10. Hubert WT, Rosen MN. Pasteurella multocida infections. II. Pasteurella multocida infection in man unrelated to animal bite. Am J Pub Health Nations Health. 1970;60(6):1109-1117.
11. Escande F, Borde M, Pateyron F. Infection maternelle et néonatale à Pasteurella multocida. Arch Pediatr. 1997;4(11):1116-1118.
12. Luchansky M, Bergman M, Djaldetti R, Salman H. Cat bite in an old patient: Is it a simple injury? Eur J Emerg Med. 2003;10(2):130-132.
13. Ryan KJ, Ray CG, eds. Sherris Medical Microbiology. 4th ed. New York, NY: McGraw Hill; 2000.
14. Weber DJ, Wolfson JS, Swartz MN, Hooper DC. Pasteurella multocida infections. Report of 34 cases and review of the literature. Medicine (Baltimore). 1984;63(3):133-154.
15. Kimura R, Hayashi Y, Takeuchi T, Shimizu M, et al. Pasteurella multocida septicemia caused by close contact with a domestic cat: Case report and literature review. J Infect Chemother. 2004;10(4):250-252.
16. Albert TJ, Stevens DL. The first case of Pasteurella canis bacteremia: A cirrhotic patient with an open leg wound. Infection. 2010;38(6):483-485.
17. Lion C, Lozniewski A, Rosner V, Weber M. Lung abscess due to beta-lactamase-producing Pasteurella multocida. Clin Infect Dis. 1999;29(5):1345-1346.
18. Fernández-Esparrach G, Mascaró J, Rota R, Valerio L. Septicemia, peritonitis, and empyema due to Pasteurella multocida in a cirrhotic patient. Clin Infect Dis. 1994;18(3):486.
19. Wade T, Booy R, Teare EL, Kroll S. Pasteurella multocida meningitis in infancy—(a lick may be as bad as a bite). Eur J Pediatr. 1999;158(11):875-878.
20. Miflin JK, Balckall PJ. Development of a 23S rRNA-based PCR assay for the identification of Pasteurella multocida. Lett Appl Microbiol. 2001;33(3):216-221.
21. Ebright J, Frey AB, Fairfax MR. Pasteurella multocida infections and bacteremia: A twenty-year experience at an urban medical center. Infect Dis Clin Pract. 2009;17(2):102-104.
22. Baillot R, Voisine P, Côté LM, Longtin Y. Deep sternal wound infection due to Pasteurella multocida: The first case report and review of literature. Infection. 2011;39(6):575-578.
23. Baskar B, Desai SP, Parsons MA. Postoperative endophthalmitis due to Pasteurella multocida. Br J Ophthalmol. 1997;81(2):172-173.
24. Chun ML, Buekers TE, Sood AK, Sorosky JI. Postoperative wound infection with Pasteurella multocida from a pet cat. Am J Obstet Gynecol. 2003;188(4):1115-1116.
The most common microbes causing postoperative wound infection are Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus epidermidis, and Enterococcus faecalis.1 Pasteurella multocida (P multocida) is an uncommon organism causing surgical-site infections.2 This article reports a case of a patient who developed a postoperative wound infection due to P multocida complicated by a bloodstream infection.
CASE REPORT
A 54-year-old man presented with pain and discharge surrounding his recent surgical site and a 1-day history of fever and chills. Four weeks prior to the presentation, he underwent nodal dissection and reconstructive surgery with a muscle flap on his right leg for a superficial spreading melanoma. The patient noticed wound dehiscence the previous 2 to 3 days and a white-yellowish discharge from the wound.
Six years previously, the patient was diagnosed with a superficial spreading melanoma on his right thigh. At that time, he underwent wide excision for the localized disease. The patient was doing well until 3 months prior to the admission when he noticed a nodule in his right groin area, which had enlarged to a lump. Fine needle aspiration of the lump and immunohistochemistry showed the presence of malignant cells, positive for S100 and MART-1.These markers are useful for a diagnosis of metastatic melanoma.3,4
A positron emission tomography (PET) scan revealed localized disease with increased uptake only in the groin region. Superficial nodal dissection and sartorius muscle flap placement were performed, and the patient was discharged to his home with drains in place.
Two weeks after his node dissection, the patient was seen in the surgery clinic, and his drains were removed. Three days after his clinic visit, he noticed erythema around the suture line and discharge from the wound, followed by increasing pain, high fever, and chills. The patient reported no history of nausea, vomiting, diarrhea, painful urination, cough, shortness of breath, chest pain, or abdominal pain. His medical history was also significant for hypertension and multiple lipomas on his back and extremities, which were removed surgically 15 years previously. He had been a chronic smoker and occasionally used alcohol but did not use illicit drugs. The patient was unmarried and lived alone with his domestic cats.
The patient was febrile on admission (38.9°C); heart rate 110 bpm. His blood pressure and respiratory rate were normal. An examination of the right groin revealed an incision mark of about 20 cm in length from his right anterior superior iliac spine travelling inferomedially into his groin with breakdown at the infero-medial aspect, extending one-half to one-third the wound distance. The wound had clean borders with minimal amount of purulent drainage. Induration was noted, extending down the thigh to above the knee level. Sutures remained in place along the superior aspect of the incision site. No other abnormalities were found.
Laboratory studies revealed leucocytosis (white blood cell count, 12,200/mm3). Blood glucose, blood urea nitrogen, serum creatinine, and electrolytes were within normal range. Computerized tomography (CT) scans of the pelvis and thighs with IV contrast revealed normal right common femoral artery and vein with postsurgical changes and thickening of the skin and small air lucencies in the right groin. The patient was started on empiric treatment with IV vancomycin and piperacillin/tazobactam.
Due to initial worsening of erythema, piperacillin/tazobactam were stopped and ertapenem was initiated. Blood cultures sets and the culture of the surgical wound were positive for P multocida. Vancomycin was discontinued, and the patient rapidly improved. He was discharged on oral moxifloxacin for 2 weeks. After 10 months of follow-up, the patient was doing well and the surgical site wound had healed.
DISCUSSION
Pasteurella multocida is a small, Gram-negative, nonmotile, non–spore-forming coccobacillus with bipolar staining features.5 It often exists as a commensal in the upper respiratory tracts of domestic pet species, especially cats and dogs, and may cause hemorrhagic septicemia in cattle, fowl cholera in chickens, and atrophic rhinitis in pigs.6,7Pasteurella multocida infection in humans is often associated with an animal bite, scratch, or lick, but infection without epidemiologic evidence of animal contact may occur.8
The majority of animal bites involve dogs (85%-90%), followed by cats (5%-10%). Infectious complications occur in about 15% to 20% of dog-related bites and > 50% of cat-related bites. The sharp and long teeth of cats can easily penetrate human skin, create a deep puncture wound, and even inoculate the periosteal component of bones. Cat-related wounds more commonly progress to serious and deeper tissue infections, including osteomyelitis and meningitis.9 Human-to-human transmission is rare, but there are reported cases of respiratory transmission and maternal-to-fetal transmission.10,11
The most common presentation of P multocida infection in humans is soft-tissue infection, appearing as purulent wounds (48%), cellulitis (36%), or abscesses (16%).12 The infection is characterized by the development of soft-tissue inflammation at the site of contact, which may progress to diffuse, localized cellulitis. High leukocyte and neutrophil counts are typically observed.13 Complications of localized infection include rapidly progressive cellulitis, abscesses, tenosynovitis, osteomyelitis, and septic arthritis. The localized infection can also lead to septicemia, which carries a high mortality rate (31%).12,14
Pasteurella multocida septicemia commonly occurs in patients with an immune-compromised status, but septicemia in healthy individuals has also been reported.15,16 Apart from local skin and soft-tissue infections, P multocida can cause upper respiratory tract infections, lower respiratory tract infections leading to pneumonia, trachea-bronchitis, lung abscess, and empyema, usually in individuals with underlying pulmonary disease. Pasteurella multocida meningitis has been associated with cat licks and bites occurring on the face in both the young and the elderly.17-19
Gram stains of purulent material or other fluid specimens, including blood, sputum, and cerebrospinal fluid, may show small, Gram-negative, nonmotile, non–spore-forming pleomorphic coccobacilli. Wright, Giemsa, and Wayson stains enhance bipolar staining. The quickest and most accurate method for confirming an active P multocida infection is molecular detection using polymerase chain reaction.20 Evaluations of tenosynovitis, septic arthritis, osteomyelitis, and meningeal enhancement, when appropriate, should be done with CT scans or MRIs.
In < 10% of cases, localized infection by P multocida may lead to bacteremia. The most common predisposing factors associated with bacteremia identified in a review of cases over 20 years at an urban medical center included old age and chronic medical conditions.21 Chronic medical conditions involved most commonly were diabetes mellitus, hypertension, and congestive heart failure. Liver dysfunction has also been reported as a significant risk factor in cases of P multocida bacteremia. The most common antibiotics used to treat the patients with bacteremia were ampicillin/sulbactam, cephalosporins, and fluoroquinolones.
A brief review of the literature of 21 cases of bacteremia showed that the most common antibiotics used for treatment were penicillins (ampicillin, amoxicillin, and piperacillin) in 11 of 21 cases. After penicillin, ciprofloxacin was the most commonly used antibiotic to treat bacteremia secondary to P multocida. The review also identified chronic medical conditions, such as diabetes mellitus, hypertension, congestive heart failure, and chronic obstructive pulmonary disease, as the most common risk factors associated in these cases with bacteremia.
In 17 of 20 cases, patients reported being present in an environment with pets, mostly cats and dogs. Twelve out of 17 patients reported having contact with pet cats and dogs, mostly in the form of bites or pets licking their wounds, and 3 of these were postoperative patients with external wounds.22-24
This patient reported that after discharge from the hospital following surgery for his spreading melanoma, domestic cats at his home repeatedly licked the postoperative wound. This almost certainly was the source of the infection and bacteremia in this patient. These findings stress the importance of educating patients about proper postoperative wound care and precautions needed if there is potential exposure to domestic animals, such as cats and dogs.
CONCLUSIONS
Pasteurella multocida is an unusual cause of postoperative wound infection. The most common method of acquiring a P multocida infection is through contact with pet animals, mostly cats and dogs. Infection can occur not only with animal bites and scratches, but also with licking of open wounds. The rate of infections can be decreased significantly by educating patients about the mode of transmission of infection, its complications, and safety measures needed if they have pets at home.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The most common microbes causing postoperative wound infection are Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus epidermidis, and Enterococcus faecalis.1 Pasteurella multocida (P multocida) is an uncommon organism causing surgical-site infections.2 This article reports a case of a patient who developed a postoperative wound infection due to P multocida complicated by a bloodstream infection.
CASE REPORT
A 54-year-old man presented with pain and discharge surrounding his recent surgical site and a 1-day history of fever and chills. Four weeks prior to the presentation, he underwent nodal dissection and reconstructive surgery with a muscle flap on his right leg for a superficial spreading melanoma. The patient noticed wound dehiscence the previous 2 to 3 days and a white-yellowish discharge from the wound.
Six years previously, the patient was diagnosed with a superficial spreading melanoma on his right thigh. At that time, he underwent wide excision for the localized disease. The patient was doing well until 3 months prior to the admission when he noticed a nodule in his right groin area, which had enlarged to a lump. Fine needle aspiration of the lump and immunohistochemistry showed the presence of malignant cells, positive for S100 and MART-1.These markers are useful for a diagnosis of metastatic melanoma.3,4
A positron emission tomography (PET) scan revealed localized disease with increased uptake only in the groin region. Superficial nodal dissection and sartorius muscle flap placement were performed, and the patient was discharged to his home with drains in place.
Two weeks after his node dissection, the patient was seen in the surgery clinic, and his drains were removed. Three days after his clinic visit, he noticed erythema around the suture line and discharge from the wound, followed by increasing pain, high fever, and chills. The patient reported no history of nausea, vomiting, diarrhea, painful urination, cough, shortness of breath, chest pain, or abdominal pain. His medical history was also significant for hypertension and multiple lipomas on his back and extremities, which were removed surgically 15 years previously. He had been a chronic smoker and occasionally used alcohol but did not use illicit drugs. The patient was unmarried and lived alone with his domestic cats.
The patient was febrile on admission (38.9°C); heart rate 110 bpm. His blood pressure and respiratory rate were normal. An examination of the right groin revealed an incision mark of about 20 cm in length from his right anterior superior iliac spine travelling inferomedially into his groin with breakdown at the infero-medial aspect, extending one-half to one-third the wound distance. The wound had clean borders with minimal amount of purulent drainage. Induration was noted, extending down the thigh to above the knee level. Sutures remained in place along the superior aspect of the incision site. No other abnormalities were found.
Laboratory studies revealed leucocytosis (white blood cell count, 12,200/mm3). Blood glucose, blood urea nitrogen, serum creatinine, and electrolytes were within normal range. Computerized tomography (CT) scans of the pelvis and thighs with IV contrast revealed normal right common femoral artery and vein with postsurgical changes and thickening of the skin and small air lucencies in the right groin. The patient was started on empiric treatment with IV vancomycin and piperacillin/tazobactam.
Due to initial worsening of erythema, piperacillin/tazobactam were stopped and ertapenem was initiated. Blood cultures sets and the culture of the surgical wound were positive for P multocida. Vancomycin was discontinued, and the patient rapidly improved. He was discharged on oral moxifloxacin for 2 weeks. After 10 months of follow-up, the patient was doing well and the surgical site wound had healed.
DISCUSSION
Pasteurella multocida is a small, Gram-negative, nonmotile, non–spore-forming coccobacillus with bipolar staining features.5 It often exists as a commensal in the upper respiratory tracts of domestic pet species, especially cats and dogs, and may cause hemorrhagic septicemia in cattle, fowl cholera in chickens, and atrophic rhinitis in pigs.6,7Pasteurella multocida infection in humans is often associated with an animal bite, scratch, or lick, but infection without epidemiologic evidence of animal contact may occur.8
The majority of animal bites involve dogs (85%-90%), followed by cats (5%-10%). Infectious complications occur in about 15% to 20% of dog-related bites and > 50% of cat-related bites. The sharp and long teeth of cats can easily penetrate human skin, create a deep puncture wound, and even inoculate the periosteal component of bones. Cat-related wounds more commonly progress to serious and deeper tissue infections, including osteomyelitis and meningitis.9 Human-to-human transmission is rare, but there are reported cases of respiratory transmission and maternal-to-fetal transmission.10,11
The most common presentation of P multocida infection in humans is soft-tissue infection, appearing as purulent wounds (48%), cellulitis (36%), or abscesses (16%).12 The infection is characterized by the development of soft-tissue inflammation at the site of contact, which may progress to diffuse, localized cellulitis. High leukocyte and neutrophil counts are typically observed.13 Complications of localized infection include rapidly progressive cellulitis, abscesses, tenosynovitis, osteomyelitis, and septic arthritis. The localized infection can also lead to septicemia, which carries a high mortality rate (31%).12,14
Pasteurella multocida septicemia commonly occurs in patients with an immune-compromised status, but septicemia in healthy individuals has also been reported.15,16 Apart from local skin and soft-tissue infections, P multocida can cause upper respiratory tract infections, lower respiratory tract infections leading to pneumonia, trachea-bronchitis, lung abscess, and empyema, usually in individuals with underlying pulmonary disease. Pasteurella multocida meningitis has been associated with cat licks and bites occurring on the face in both the young and the elderly.17-19
Gram stains of purulent material or other fluid specimens, including blood, sputum, and cerebrospinal fluid, may show small, Gram-negative, nonmotile, non–spore-forming pleomorphic coccobacilli. Wright, Giemsa, and Wayson stains enhance bipolar staining. The quickest and most accurate method for confirming an active P multocida infection is molecular detection using polymerase chain reaction.20 Evaluations of tenosynovitis, septic arthritis, osteomyelitis, and meningeal enhancement, when appropriate, should be done with CT scans or MRIs.
In < 10% of cases, localized infection by P multocida may lead to bacteremia. The most common predisposing factors associated with bacteremia identified in a review of cases over 20 years at an urban medical center included old age and chronic medical conditions.21 Chronic medical conditions involved most commonly were diabetes mellitus, hypertension, and congestive heart failure. Liver dysfunction has also been reported as a significant risk factor in cases of P multocida bacteremia. The most common antibiotics used to treat the patients with bacteremia were ampicillin/sulbactam, cephalosporins, and fluoroquinolones.
A brief review of the literature of 21 cases of bacteremia showed that the most common antibiotics used for treatment were penicillins (ampicillin, amoxicillin, and piperacillin) in 11 of 21 cases. After penicillin, ciprofloxacin was the most commonly used antibiotic to treat bacteremia secondary to P multocida. The review also identified chronic medical conditions, such as diabetes mellitus, hypertension, congestive heart failure, and chronic obstructive pulmonary disease, as the most common risk factors associated in these cases with bacteremia.
In 17 of 20 cases, patients reported being present in an environment with pets, mostly cats and dogs. Twelve out of 17 patients reported having contact with pet cats and dogs, mostly in the form of bites or pets licking their wounds, and 3 of these were postoperative patients with external wounds.22-24
This patient reported that after discharge from the hospital following surgery for his spreading melanoma, domestic cats at his home repeatedly licked the postoperative wound. This almost certainly was the source of the infection and bacteremia in this patient. These findings stress the importance of educating patients about proper postoperative wound care and precautions needed if there is potential exposure to domestic animals, such as cats and dogs.
CONCLUSIONS
Pasteurella multocida is an unusual cause of postoperative wound infection. The most common method of acquiring a P multocida infection is through contact with pet animals, mostly cats and dogs. Infection can occur not only with animal bites and scratches, but also with licking of open wounds. The rate of infections can be decreased significantly by educating patients about the mode of transmission of infection, its complications, and safety measures needed if they have pets at home.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Giacometti A, Cirioni O, Schimizzi AM, et al. Epidemiology and microbiology of surgical wound infection. J Clin Microbiol. 2000;38(2):918-919.
2. Cook PP. Persistent postoperative wound infection with Pasteurella multocida: Case report and literature review. Infection. 1995;23(4):252.
3. Hodi FS. Well-defined melanoma antigens as progression markers for melanoma: Insights into differential expression and host response based on stage. Clin Cancer Res. 2006;12(3, pt 1):673-678.
4. Zubovits J, Buzney E, Yu L, Duncan LM. HMB-45, S-100, NK1/C3, and MART-1 in metastatic melanoma. Hum Pathol. 2004;35(2):217-223.
5. Zurlo JJ. Pasteurella species. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 6th ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2005:2687-2691.
6. Waghorn DJ, Robson M. Occupational risk of Pasteurella multocida septicaemia and premature labor in a pregnant vet. BJOG. 2003;110(8):780-781.
7. Francis DP, Holmes MA, Brandon G. Pasteurella multocida infections after domestic animal bites and scratches. JAMA. 1975;233(1):42-45.
8. Cordoba A, Bueno I, Monterrubio J, Corcho G. Surgical wound infection due to Pasteurella multocida [article in Spanish]. Enferm Infecc Microbiol Clin. 2002;20(10):536-537.
9. Kopita JM, Handshoe D, Kussin PS, Kelemen M. Cat germs! Pleuropulmonary pasteurella infection in an old man. N C Med J. 1993;54(7):308-311.
10. Hubert WT, Rosen MN. Pasteurella multocida infections. II. Pasteurella multocida infection in man unrelated to animal bite. Am J Pub Health Nations Health. 1970;60(6):1109-1117.
11. Escande F, Borde M, Pateyron F. Infection maternelle et néonatale à Pasteurella multocida. Arch Pediatr. 1997;4(11):1116-1118.
12. Luchansky M, Bergman M, Djaldetti R, Salman H. Cat bite in an old patient: Is it a simple injury? Eur J Emerg Med. 2003;10(2):130-132.
13. Ryan KJ, Ray CG, eds. Sherris Medical Microbiology. 4th ed. New York, NY: McGraw Hill; 2000.
14. Weber DJ, Wolfson JS, Swartz MN, Hooper DC. Pasteurella multocida infections. Report of 34 cases and review of the literature. Medicine (Baltimore). 1984;63(3):133-154.
15. Kimura R, Hayashi Y, Takeuchi T, Shimizu M, et al. Pasteurella multocida septicemia caused by close contact with a domestic cat: Case report and literature review. J Infect Chemother. 2004;10(4):250-252.
16. Albert TJ, Stevens DL. The first case of Pasteurella canis bacteremia: A cirrhotic patient with an open leg wound. Infection. 2010;38(6):483-485.
17. Lion C, Lozniewski A, Rosner V, Weber M. Lung abscess due to beta-lactamase-producing Pasteurella multocida. Clin Infect Dis. 1999;29(5):1345-1346.
18. Fernández-Esparrach G, Mascaró J, Rota R, Valerio L. Septicemia, peritonitis, and empyema due to Pasteurella multocida in a cirrhotic patient. Clin Infect Dis. 1994;18(3):486.
19. Wade T, Booy R, Teare EL, Kroll S. Pasteurella multocida meningitis in infancy—(a lick may be as bad as a bite). Eur J Pediatr. 1999;158(11):875-878.
20. Miflin JK, Balckall PJ. Development of a 23S rRNA-based PCR assay for the identification of Pasteurella multocida. Lett Appl Microbiol. 2001;33(3):216-221.
21. Ebright J, Frey AB, Fairfax MR. Pasteurella multocida infections and bacteremia: A twenty-year experience at an urban medical center. Infect Dis Clin Pract. 2009;17(2):102-104.
22. Baillot R, Voisine P, Côté LM, Longtin Y. Deep sternal wound infection due to Pasteurella multocida: The first case report and review of literature. Infection. 2011;39(6):575-578.
23. Baskar B, Desai SP, Parsons MA. Postoperative endophthalmitis due to Pasteurella multocida. Br J Ophthalmol. 1997;81(2):172-173.
24. Chun ML, Buekers TE, Sood AK, Sorosky JI. Postoperative wound infection with Pasteurella multocida from a pet cat. Am J Obstet Gynecol. 2003;188(4):1115-1116.
1. Giacometti A, Cirioni O, Schimizzi AM, et al. Epidemiology and microbiology of surgical wound infection. J Clin Microbiol. 2000;38(2):918-919.
2. Cook PP. Persistent postoperative wound infection with Pasteurella multocida: Case report and literature review. Infection. 1995;23(4):252.
3. Hodi FS. Well-defined melanoma antigens as progression markers for melanoma: Insights into differential expression and host response based on stage. Clin Cancer Res. 2006;12(3, pt 1):673-678.
4. Zubovits J, Buzney E, Yu L, Duncan LM. HMB-45, S-100, NK1/C3, and MART-1 in metastatic melanoma. Hum Pathol. 2004;35(2):217-223.
5. Zurlo JJ. Pasteurella species. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 6th ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2005:2687-2691.
6. Waghorn DJ, Robson M. Occupational risk of Pasteurella multocida septicaemia and premature labor in a pregnant vet. BJOG. 2003;110(8):780-781.
7. Francis DP, Holmes MA, Brandon G. Pasteurella multocida infections after domestic animal bites and scratches. JAMA. 1975;233(1):42-45.
8. Cordoba A, Bueno I, Monterrubio J, Corcho G. Surgical wound infection due to Pasteurella multocida [article in Spanish]. Enferm Infecc Microbiol Clin. 2002;20(10):536-537.
9. Kopita JM, Handshoe D, Kussin PS, Kelemen M. Cat germs! Pleuropulmonary pasteurella infection in an old man. N C Med J. 1993;54(7):308-311.
10. Hubert WT, Rosen MN. Pasteurella multocida infections. II. Pasteurella multocida infection in man unrelated to animal bite. Am J Pub Health Nations Health. 1970;60(6):1109-1117.
11. Escande F, Borde M, Pateyron F. Infection maternelle et néonatale à Pasteurella multocida. Arch Pediatr. 1997;4(11):1116-1118.
12. Luchansky M, Bergman M, Djaldetti R, Salman H. Cat bite in an old patient: Is it a simple injury? Eur J Emerg Med. 2003;10(2):130-132.
13. Ryan KJ, Ray CG, eds. Sherris Medical Microbiology. 4th ed. New York, NY: McGraw Hill; 2000.
14. Weber DJ, Wolfson JS, Swartz MN, Hooper DC. Pasteurella multocida infections. Report of 34 cases and review of the literature. Medicine (Baltimore). 1984;63(3):133-154.
15. Kimura R, Hayashi Y, Takeuchi T, Shimizu M, et al. Pasteurella multocida septicemia caused by close contact with a domestic cat: Case report and literature review. J Infect Chemother. 2004;10(4):250-252.
16. Albert TJ, Stevens DL. The first case of Pasteurella canis bacteremia: A cirrhotic patient with an open leg wound. Infection. 2010;38(6):483-485.
17. Lion C, Lozniewski A, Rosner V, Weber M. Lung abscess due to beta-lactamase-producing Pasteurella multocida. Clin Infect Dis. 1999;29(5):1345-1346.
18. Fernández-Esparrach G, Mascaró J, Rota R, Valerio L. Septicemia, peritonitis, and empyema due to Pasteurella multocida in a cirrhotic patient. Clin Infect Dis. 1994;18(3):486.
19. Wade T, Booy R, Teare EL, Kroll S. Pasteurella multocida meningitis in infancy—(a lick may be as bad as a bite). Eur J Pediatr. 1999;158(11):875-878.
20. Miflin JK, Balckall PJ. Development of a 23S rRNA-based PCR assay for the identification of Pasteurella multocida. Lett Appl Microbiol. 2001;33(3):216-221.
21. Ebright J, Frey AB, Fairfax MR. Pasteurella multocida infections and bacteremia: A twenty-year experience at an urban medical center. Infect Dis Clin Pract. 2009;17(2):102-104.
22. Baillot R, Voisine P, Côté LM, Longtin Y. Deep sternal wound infection due to Pasteurella multocida: The first case report and review of literature. Infection. 2011;39(6):575-578.
23. Baskar B, Desai SP, Parsons MA. Postoperative endophthalmitis due to Pasteurella multocida. Br J Ophthalmol. 1997;81(2):172-173.
24. Chun ML, Buekers TE, Sood AK, Sorosky JI. Postoperative wound infection with Pasteurella multocida from a pet cat. Am J Obstet Gynecol. 2003;188(4):1115-1116.