User login
Pediatric Orthopedic Basics
Case 1
A mother presented to the ED with her 8-year-old daughter after she witnessed the child fall off her bicycle onto the sidewalk. When she fell, the girl landed onto her outstretched arms and sustained minor abrasions to her palms and knees, but did not hit her head or lose consciousness. Upon falling, the child immediately cried that her left arm hurt and kept holding it guarded near her body.
She was seated on her mother’s lap in the examination room, appearing anxious but in no acute distress. The treating EP observed the superficial abrasions from across the room and obtained a detailed history.
The patient was afebrile, and her vital signs were stable with the exception of mild tachycardia. After a couple of minutes, the EP slowly approached the child and was able to perform a basic examination. There was no obvious deformity to her left upper extremity, and only mild swelling over the wrist. She was able to move her fingers well and had excellent capillary refill distally. The child remained calm during manual palpation of the anatomic snuff box. However, she immediately pulled away and began to cry upon palpation more proximally over the distal forearm. The EP discussed his concerns with the child’s mother and explained that further evaluation was necessary.
Case 2
A mother and father presented to the ED carrying their 6-year-old boy, stating that the child had been limping since they picked him up from a neighbor’s house an hour earlier and was now refusing to walk. The father noted that a group of children had been jumping on a trampoline unsupervised, but he did not witness any injury to his son. Both parents said that the boy had been well up until that day.
At presentation, the child was afebrile and his vital signs were stable. The EP asked the parents to coax the child to walk across the room. During the walk, the boy was reluctant to bear weight on his right foot. Careful inspection of his lower limb revealed no external signs of trauma, and it appeared neurovascularly intact. Careful palpation elicited tenderness directly over the physis at the distal fibula and near the lateral malleolus. While considering the broad differential for a limping child, the physician was primarily concerned with point tenderness on examination and informed the parents that radiographic imaging was warranted.
Overview
Pediatric musculoskeletal (MSK) injury and orthopedic trauma now comprise more than 10% of visits to the ED.1 Fractures in particular are becoming more commonplace with the increasing number of children actively involved in athletic sports and high-risk activities.
The general approach to acute-care management of these children has evolved, trending more toward splinting the fractured extremity and away from traditional casting. There are many benefits to splinting. The most important is arguably the reduced risk of developing compartment syndrome due to a splint’s ability to expand with accompanied swelling.2 This article reviews the unique characteristics of pediatric bone development and initial management of pediatric fractures, as well as basic splinting techniques and unique indications that require further orthopedic consultation.
Physiological Differences in Children
The MSK system of a child differs greatly from that of an adult. The bones themselves are much more porous and malleable during childhood, making them more susceptible to traumatic injury. The growing periosteum and the developing physes are particularly vulnerable, accounting for up to 20% of pediatric fractures (see the Figure illustrating the Salter-Harris classification in the next section).3 This is particularly true at a young age, when ligamentous adherence out-performs the bony integrity itself, making fractures more common than sprains and tears. The opposite is true in adults, who are much more likely to experience sprains before succumbing to fracture. Furthermore, since the periosteum is still very active in children, the fractured bone is much more likely to remodel, lending to less deformity and overall better outcomes in most cases.3 Nonunion is extremely rare in children.
Initial Management
Approaching the Pediatric Patient
Special consideration should be given when initially approaching an injured child, so as not to cause additional undue fear or anxiety to the patient. It is helpful to take an extra moment upon entering the room to simply observe the child’s positioning, posture, or reluctance to move a particular limb. Obtaining a careful, detailed history from a distance is recommended before too quickly approaching the patient. In addition, asking the caregiver to serve as proxy during the initial physical examination may also prove helpful in localizing the pain. In the obscure case, such as the child refusing to bear weight, it is good to keep a broad differential and inspect for non-MSK injury (eg, painful hernia, testicular torsion, foreign body lodged in the bottom of the foot). Utilizing a “log-rolling” technique with the palms of one’s hands on the patient’s thigh may reveal hip pathology. Simply observing the preoccupied child walk around the unit while watching from behind may also aid in the evaluation.
However, when the injury such as an open fracture or severe displacement is obvious, immediate stabilization is critical so as not to permit any additional harm. An arm board is typically used to accomplish this. In addition, pain control should never be overlooked, either with intravenous opioids or more appropriate oral or intranasal analgesia.4,5 In cases of significant trauma, always remember ABC assessment (airway, breathing/oxygenation, and circulation), despite the eagerness to give attention to what may be an obvious fracture.
Workup
Although the use of ultrasound and other modalities is becoming more popular in some settings, it is still commonplace to begin the evaluation of a potential fracture or dislocation with plain film X-rays. Before sending a patient to radiology, always stabilize any unstable fracture to avoid further injury or potentiate neurovascular compromise. In most cases, three views, including anteroposterior, true lateral, and oblique, are obtained. If a fracture is unclear, it may be helpful to image the opposite extremity for comparison. The location of a fracture and its characteristics greatly influence acute-care management, as well as patient disposition, the need for consultation with orthopedics, and follow-up expectations and instructions.
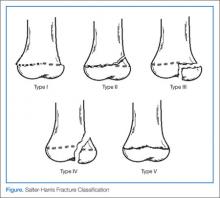
Salter-Harris Fracture Classification
The physes of bones in growing children are particularly vulnerable sites of fracture since they have not yet fused. The five generally accepted types of fracture according to risk of growth disturbance are illustrated in the above Figure and are differentiated as follows:
Type I. This type of fracture is exclusive involvement of the physis itself, separating the metaphysis from the epiphysis. Since plain films may not reveal any visible fracture, the clinician should have a high index of suspicion if the physical examination elicits point tenderness over the growth plate. When in doubt if a fracture is present, always splint. Type I fractures of the physis tend to heal well, without significant consequence.
Type II. As with type I fractures, type II involve the physis, but also have a fragment of displaced metaphysis—the most common of all physeal fractures. Without significant displacement, these fractures also tend to have good outcomes.
Type III. Rather than involving the physis and metaphysis, type III fractures involve the epiphysis and therefore the joint itself. It is because of the epiphyseal displacement that these fractures tend to have a worse prognosis with joint disability and growth arrest. Thus, establishing alignment is imperative. The distal tibial Tillaux fracture is an example and requires internal fixation for optimal healing.
Type IV. Similar to a type III fracture, with the fracture extending proximally through a segment of the metaphysis, type IV fractures are treated similar to type III fractures. Due to joint involvement, an orthopedic consultation is warranted.
Type V. This type represents compression fractures of the physis. As the visibility of these fractures is poor on plain films, diagnosis can be challenging. However, history of axial compression injury may help lead the clinician to an accurate diagnosis. Since there is a high incidence of growth disturbance in type V fractures, compression affecting other areas such as the spine should also be considered.
Certainly not all pediatric fractures will involve a physis. A detailed description and management of other unique types of pediatric fractures is discussed in other articles in this feature.
Splinting Basics
Once the decision is made to apply a splint to a fracture, certain basic precautions should first be taken. Initially, any significant lacerations or abrasions should be thoroughly irrigated, cleansed, and dressed appropriately. Next, the physician should reevaluate and document both neurological status and perfusion of the area, particularly distal to the fracture site.
One commonly overlooked step in management of any fracture is pain control. It is advisable to consider administering medication prior to splinting on a case-by-case basis and for all fractures requiring reduction.
Materials and Methods
Prepackaged fiberglass splints have become a popular, efficient, and less-messy material of choice in pediatric splinting. Alternatively, plaster of Paris—although a bit more cumbersome—has some advantages, including low cost and a tendency to mold more easily to the extremity being splinted.7 When using plaster, strips should be cut a little longer than the anticipated length needed since they may shrink during curing. The unaffected limb should be used to gauge the measurement needed.
Regardless of the material chosen, all splinting should begin with the application of a stockinette tube dressing over the skin, leaving a distal opening over fingers or toes. This should be followed by a padding material (eg, Webril), beginning distally and rolling proximally, being sure to have approximately 50% overlap of each roll. Extra padding should be rolled over any bony prominence (eg, ulnar stylus) to avoid discomfort or pressure sores once the splint is applied.2
Between 8 and 10 layers of plaster (additional layers for lower extremity splints) should be wetted with room-temperature water. Hot water should never be used as this will intensify the exothermic reaction that occurs when curing and could cause burns.2 The limb should be kept in the anatomic position while the plaster is being molded to the shape of the extremity, allowing 15 to 20 minutes to dry.1 Once dry, an elastic bandage such as an Ace wrap may be placed over the entire cast to hold it secure in place. If fiberglass is used, it is helpful to squeeze out extra water before molding to the extremity. Again, an additional padding roll should be employed to avoid any discomfort or pressure beneath the splint.
In both fiberglass and plaster splinting, the edges of either type of material should not be abrasive to the skin; this can be avoided by rolling over excess padding and stockinette to create a round soft edge on either end.7 Finally, the patient should be fitted with a shoulder sling or crutches (if age appropriate) to further immobilize the injured extremity and avoid any movement or weight bearing.
Types of Splints
The type of splint depends of the location and characteristics of the fracture being immobilized. The following are a few examples of the more popular splinting techniques indicated for common pediatric fractures.
Long-Arm Posterior Splint. This splint is useful for most forearm and elbow fractures. The splint length should extend from midlength of the humerus to the palmar crease, and the width should be semicircular. In addition, an anatomic position of 90˚ flexion of the elbow should be maintained, with the hand in a neutral position and slight dorsiflexion. It is generally accepted to slightly pronate the forearm when splinting a supracondylar fracture. Orthopedics should always be consulted if the fracture involves the elbow.
Ulnar “Gutter” Splint. Useful for nondisplaced, minimally-angulated metacarpal “boxer’s fracture” or fourth and fifth phalangeal fractures, the length of the ulnar splint should extend from the distal phalanx to proximal forearm. Splint width should enclose both the volar and dorsum surfaces of the fourth and fifth metacarpals. In addition, padding should be placed between the digits for comfort. The metacarpophalangeal joints should be positioned at 70˚, and the proximal phalangeal angle at approximately 20˚ flexion2; this will help minimize the risk of contractures.
Forearm “Sugar-Tong” Splint. These splints are indicated for immobilization of a distal radius fracture or wrist injury. Distal radial fractures are by far the most common fractures encountered in the pediatric population,8 and splinting for angulation less than 15˚ is preferred.9,10 For proper stabilization, a long U-shaped splint should originate at the palmar crease, wrap around the elbow, and end at the metacarpophalangeal joint dorsally. Again, the hand should be dorsiflexed, and a soft rolled edge should be kept on the palmar crease to allow full finger flexion to near 90˚.
Thumb Spica Splint. A thumb spica splint is useful to immobilize uncomplicated fracture of the first metacarpal or proximal phalanx or when scaphoid (navicular) bone fracture is suspected. A semicircumferential molding of the radial forearm should be formed, extending to the thumbnail bed, and wrapping around the thumb. The proper hand positioning is slightly dorsiflexed, with thumb abducted slightly, as if holding a glass of water.2 If there is any doubt of a navicular fracture (rare in prepubescent children), the clinician should never hesitate to splint!
Long-Leg Posterior Splint. This type of splint is appropriate for immobilization of midshaft tibia/fibula fractures or most knee injuries. Full length of the splint should start beneath the inferior gluteal fold and extend to the ball of the foot, leaving the toes free. The ankle should be at 90˚ flexion and the knee should remain just slightly flexed, never locked straight. Orthopedics should always be consulted in cases of proximal tibia/fibula fractures or knee joint involvement.
Posterior Ankle Splint. Essentially a shorter version of a long-leg splint extending proximally to just below the knee, the posterior ankle splint is useful to immobilize ankle fractures, foot fractures, and severe ankle sprains. The distal fibula and occasional tibia physes are another common site of pediatric fractures, particularly in obese or more active children.11,12 When using either a long- or short-leg posterior ankle splint, it is helpful to hold the foot at 90˚ flexion until the material hardens or the proper angle may be lost. A recall that displaced or Salter-Harris type III or IV physeal fractures justify orthopedics consult. Nonweight-bearing, use of crutches, ice, and elevation are all important points for recovery in 3 to 6 weeks.
Lower Extremity Stirrup “Sugar-Tong” Splint. This splint is indicated for additional ankle stabilization. It runs in a U-shape (not unlike a forearm sugar-tong splint) from just below the knee around the calcaneus, and it must be wide enough to encase the ankle but not so wide that the two sides overlap when molded. It is very important to add extra padding around both malleoli and beneath the calcaneus to reduce the likelihood of pressure sores. Crutches are essential to avoid weight-bearing in patients old enough to use them. Some pediatric orthopedists advise avoiding this type of splint in the smaller, noncompliant, active child.
Complications
Although splinting has many advantages over casting in the acute-care setting, several potential complications may develop. Although rare, thermal burns to the underlying skin may occur if excessively warm or hot water is used on plaster or fiberglass due to the exothermic reaction during the hardening process. Therefore, the use of room-temperature water is always recommended. Despite the noncircumferential nature of a splint, it is still possible to develop significant swelling following splint application, which can lead to neurovascular compromise, compartment syndrome, infection, or pressure ulcers.7 The patient and caregiver should be advised to return to the ED immediately for evaluation if serious signs and symptoms such as pain, numbness, tingling, dusky color of skin, or poor capillary refill develop.
Case 1 Conclusion
The EP in this case elected to obtain plain X-rays of the patient’s left forearm, including the wrist and elbow. The results demonstrated a disruption of the cortical integrity of the distal radius, consistent with a buckle fracture. The angulation was estimated at merely 10˚. The bones of the wrist and elbow appeared normal. The EP concluded that a consult with orthopedics was not required urgently, and immobilized the patient’s arm using a fiberglass sugar-tong splint, keeping her elbow at 90˚, the forearm in a neutral position, and hand slightly dorsiflexed. A nurse assisted in keeping the child still to ensure the splint was shaped around the arm and hardened in this position. The child was provided with a sling, and supportive-care measures, including analgesia with nonsteroidal anti-inflammatory drugs as needed, ice, rest, and the importance of keeping the splint dry, were reviewed with her parents. The EP also stressed the importance of surveying for any loss of sensation or perfusion to the patient’s hand and fingers, and recommended follow up with orthopedics 1 week from discharge.
Case 2 Conclusion
Multiple views of the patient’s ankle were obtained on X-ray and showed no apparent fracture or dislocation. Additional films of the opposite ankle were obtained for comparison, but both appeared quite similar except for mild soft-tissue swelling of the affected side. Since point tenderness was reproducible over the distal fibular physis, the EP elected to place a short-leg posterior splint, maintaining good anatomic position with extra padding around the malleoli. The parents were instructed on proper elevation, ice to reduce inflammation, and the use of pain medication if needed.
One week after discharge, the treating EP received a letter from the child’s orthopedist, informing him that at the follow-up appointment, a repeat ankle film revealed periosteal changes and a type I Salter-Harris distal fibula fracture. Immobilization for an additional 3 weeks and supportive care was indicated.
Dr Del Re is an instructor of pediatrics and an intermediate care pediatrician, Rady Children’s Hospital, San Diego, California. Dr Clingenpeel is a fellowship director, pediatric emergency medicine, and associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
- Bachman D, Santora S. Musculoskeletal trauma. In: Fleisher GR, Ludwig S, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2010:1335-1375.
- Klig JE. Splinting procedures. In: King C, Henretig FM, eds. Texbook of Pediatric Emergency Procedures. 2nd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008:919-931.
- Wilkins KE. The incidence of fractures in children. In: Rockwood CA, Wilkins KE, Beaty JH, eds. Fractures in Children. 4th ed. Philadelphia, PA: Lippincott-Raven; 1996:3-17.
- Mahar PJ, Rana JA, Kennedy CS, Christopher NC. A randomized clinical trial of oral transmucosal fentanyl citrate versus intravenous morphine sulfate for initial control of pain in children with extremity injuries. Pediatr Emerg Care. 2007;23(8):544-548.
- Saunders M, Adelgais K, Nelson D. Use of intranasal fentanyl for the relief of pediatric orthopedic trauma pain. Acad Emerg Med. 2010;17(11):1155-1161.
- Salter RB, Harris WR. Injuries involving the epiphyseal plate. J Bone Joint Surg Am. 1963;45:587-622.
- Boyd AS, Benjamin HJ, Asplund C. Principles of casting and splinting. Am Fam Physician. 2009;79(1):16-22.
- Solan MC, Rees R, Daly K. Current management of torus fractures of the distal radius. Injury. 2002;33(6):503-505.
- Boutis K, Willan A, Babyn P, Goeree R, Howard A. Cast versus splint in children with minimally angulated fractures of the distal radius: a randomized controlled trial. CMAJ. 2010;182(14):1507-1512.
- Firmin F, Crouch R. Splinting versus casting of “torus” fractures to the distal radius in the paediatric patient presenting at the emergency department (ED): a literature review. Int Emerg Nurs. 2009;17(3):173-178.
- Peterson HA, Madhok R, Benson JT, Ilstrup DM, Melton LJ 3rd. Physeal fractures: Part 1. Epidemiology in Olmsted County, Minnesota, 1979-1988. J Pediatr Orthop. 1994;14(4):423-430.
- Blackburn EW, Aronsson DD, Rubright JH, Lisle JW. Ankle fractures in children. J Bone Joint Surg Am. 2012; 94(13):1234-1244.
Case 1
A mother presented to the ED with her 8-year-old daughter after she witnessed the child fall off her bicycle onto the sidewalk. When she fell, the girl landed onto her outstretched arms and sustained minor abrasions to her palms and knees, but did not hit her head or lose consciousness. Upon falling, the child immediately cried that her left arm hurt and kept holding it guarded near her body.
She was seated on her mother’s lap in the examination room, appearing anxious but in no acute distress. The treating EP observed the superficial abrasions from across the room and obtained a detailed history.
The patient was afebrile, and her vital signs were stable with the exception of mild tachycardia. After a couple of minutes, the EP slowly approached the child and was able to perform a basic examination. There was no obvious deformity to her left upper extremity, and only mild swelling over the wrist. She was able to move her fingers well and had excellent capillary refill distally. The child remained calm during manual palpation of the anatomic snuff box. However, she immediately pulled away and began to cry upon palpation more proximally over the distal forearm. The EP discussed his concerns with the child’s mother and explained that further evaluation was necessary.
Case 2
A mother and father presented to the ED carrying their 6-year-old boy, stating that the child had been limping since they picked him up from a neighbor’s house an hour earlier and was now refusing to walk. The father noted that a group of children had been jumping on a trampoline unsupervised, but he did not witness any injury to his son. Both parents said that the boy had been well up until that day.
At presentation, the child was afebrile and his vital signs were stable. The EP asked the parents to coax the child to walk across the room. During the walk, the boy was reluctant to bear weight on his right foot. Careful inspection of his lower limb revealed no external signs of trauma, and it appeared neurovascularly intact. Careful palpation elicited tenderness directly over the physis at the distal fibula and near the lateral malleolus. While considering the broad differential for a limping child, the physician was primarily concerned with point tenderness on examination and informed the parents that radiographic imaging was warranted.
Overview
Pediatric musculoskeletal (MSK) injury and orthopedic trauma now comprise more than 10% of visits to the ED.1 Fractures in particular are becoming more commonplace with the increasing number of children actively involved in athletic sports and high-risk activities.
The general approach to acute-care management of these children has evolved, trending more toward splinting the fractured extremity and away from traditional casting. There are many benefits to splinting. The most important is arguably the reduced risk of developing compartment syndrome due to a splint’s ability to expand with accompanied swelling.2 This article reviews the unique characteristics of pediatric bone development and initial management of pediatric fractures, as well as basic splinting techniques and unique indications that require further orthopedic consultation.
Physiological Differences in Children
The MSK system of a child differs greatly from that of an adult. The bones themselves are much more porous and malleable during childhood, making them more susceptible to traumatic injury. The growing periosteum and the developing physes are particularly vulnerable, accounting for up to 20% of pediatric fractures (see the Figure illustrating the Salter-Harris classification in the next section).3 This is particularly true at a young age, when ligamentous adherence out-performs the bony integrity itself, making fractures more common than sprains and tears. The opposite is true in adults, who are much more likely to experience sprains before succumbing to fracture. Furthermore, since the periosteum is still very active in children, the fractured bone is much more likely to remodel, lending to less deformity and overall better outcomes in most cases.3 Nonunion is extremely rare in children.
Initial Management
Approaching the Pediatric Patient
Special consideration should be given when initially approaching an injured child, so as not to cause additional undue fear or anxiety to the patient. It is helpful to take an extra moment upon entering the room to simply observe the child’s positioning, posture, or reluctance to move a particular limb. Obtaining a careful, detailed history from a distance is recommended before too quickly approaching the patient. In addition, asking the caregiver to serve as proxy during the initial physical examination may also prove helpful in localizing the pain. In the obscure case, such as the child refusing to bear weight, it is good to keep a broad differential and inspect for non-MSK injury (eg, painful hernia, testicular torsion, foreign body lodged in the bottom of the foot). Utilizing a “log-rolling” technique with the palms of one’s hands on the patient’s thigh may reveal hip pathology. Simply observing the preoccupied child walk around the unit while watching from behind may also aid in the evaluation.
However, when the injury such as an open fracture or severe displacement is obvious, immediate stabilization is critical so as not to permit any additional harm. An arm board is typically used to accomplish this. In addition, pain control should never be overlooked, either with intravenous opioids or more appropriate oral or intranasal analgesia.4,5 In cases of significant trauma, always remember ABC assessment (airway, breathing/oxygenation, and circulation), despite the eagerness to give attention to what may be an obvious fracture.
Workup
Although the use of ultrasound and other modalities is becoming more popular in some settings, it is still commonplace to begin the evaluation of a potential fracture or dislocation with plain film X-rays. Before sending a patient to radiology, always stabilize any unstable fracture to avoid further injury or potentiate neurovascular compromise. In most cases, three views, including anteroposterior, true lateral, and oblique, are obtained. If a fracture is unclear, it may be helpful to image the opposite extremity for comparison. The location of a fracture and its characteristics greatly influence acute-care management, as well as patient disposition, the need for consultation with orthopedics, and follow-up expectations and instructions.
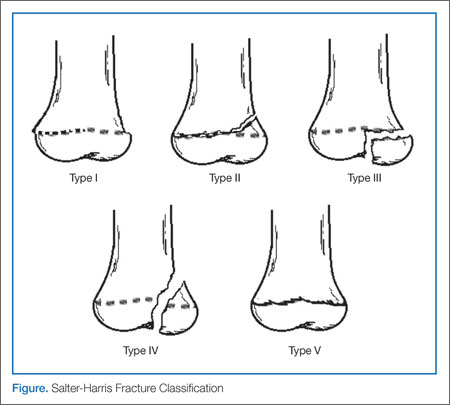
Salter-Harris Fracture Classification
The physes of bones in growing children are particularly vulnerable sites of fracture since they have not yet fused. The five generally accepted types of fracture according to risk of growth disturbance are illustrated in the above Figure and are differentiated as follows:
Type I. This type of fracture is exclusive involvement of the physis itself, separating the metaphysis from the epiphysis. Since plain films may not reveal any visible fracture, the clinician should have a high index of suspicion if the physical examination elicits point tenderness over the growth plate. When in doubt if a fracture is present, always splint. Type I fractures of the physis tend to heal well, without significant consequence.
Type II. As with type I fractures, type II involve the physis, but also have a fragment of displaced metaphysis—the most common of all physeal fractures. Without significant displacement, these fractures also tend to have good outcomes.
Type III. Rather than involving the physis and metaphysis, type III fractures involve the epiphysis and therefore the joint itself. It is because of the epiphyseal displacement that these fractures tend to have a worse prognosis with joint disability and growth arrest. Thus, establishing alignment is imperative. The distal tibial Tillaux fracture is an example and requires internal fixation for optimal healing.
Type IV. Similar to a type III fracture, with the fracture extending proximally through a segment of the metaphysis, type IV fractures are treated similar to type III fractures. Due to joint involvement, an orthopedic consultation is warranted.
Type V. This type represents compression fractures of the physis. As the visibility of these fractures is poor on plain films, diagnosis can be challenging. However, history of axial compression injury may help lead the clinician to an accurate diagnosis. Since there is a high incidence of growth disturbance in type V fractures, compression affecting other areas such as the spine should also be considered.
Certainly not all pediatric fractures will involve a physis. A detailed description and management of other unique types of pediatric fractures is discussed in other articles in this feature.
Splinting Basics
Once the decision is made to apply a splint to a fracture, certain basic precautions should first be taken. Initially, any significant lacerations or abrasions should be thoroughly irrigated, cleansed, and dressed appropriately. Next, the physician should reevaluate and document both neurological status and perfusion of the area, particularly distal to the fracture site.
One commonly overlooked step in management of any fracture is pain control. It is advisable to consider administering medication prior to splinting on a case-by-case basis and for all fractures requiring reduction.
Materials and Methods
Prepackaged fiberglass splints have become a popular, efficient, and less-messy material of choice in pediatric splinting. Alternatively, plaster of Paris—although a bit more cumbersome—has some advantages, including low cost and a tendency to mold more easily to the extremity being splinted.7 When using plaster, strips should be cut a little longer than the anticipated length needed since they may shrink during curing. The unaffected limb should be used to gauge the measurement needed.
Regardless of the material chosen, all splinting should begin with the application of a stockinette tube dressing over the skin, leaving a distal opening over fingers or toes. This should be followed by a padding material (eg, Webril), beginning distally and rolling proximally, being sure to have approximately 50% overlap of each roll. Extra padding should be rolled over any bony prominence (eg, ulnar stylus) to avoid discomfort or pressure sores once the splint is applied.2
Between 8 and 10 layers of plaster (additional layers for lower extremity splints) should be wetted with room-temperature water. Hot water should never be used as this will intensify the exothermic reaction that occurs when curing and could cause burns.2 The limb should be kept in the anatomic position while the plaster is being molded to the shape of the extremity, allowing 15 to 20 minutes to dry.1 Once dry, an elastic bandage such as an Ace wrap may be placed over the entire cast to hold it secure in place. If fiberglass is used, it is helpful to squeeze out extra water before molding to the extremity. Again, an additional padding roll should be employed to avoid any discomfort or pressure beneath the splint.
In both fiberglass and plaster splinting, the edges of either type of material should not be abrasive to the skin; this can be avoided by rolling over excess padding and stockinette to create a round soft edge on either end.7 Finally, the patient should be fitted with a shoulder sling or crutches (if age appropriate) to further immobilize the injured extremity and avoid any movement or weight bearing.
Types of Splints
The type of splint depends of the location and characteristics of the fracture being immobilized. The following are a few examples of the more popular splinting techniques indicated for common pediatric fractures.
Long-Arm Posterior Splint. This splint is useful for most forearm and elbow fractures. The splint length should extend from midlength of the humerus to the palmar crease, and the width should be semicircular. In addition, an anatomic position of 90˚ flexion of the elbow should be maintained, with the hand in a neutral position and slight dorsiflexion. It is generally accepted to slightly pronate the forearm when splinting a supracondylar fracture. Orthopedics should always be consulted if the fracture involves the elbow.
Ulnar “Gutter” Splint. Useful for nondisplaced, minimally-angulated metacarpal “boxer’s fracture” or fourth and fifth phalangeal fractures, the length of the ulnar splint should extend from the distal phalanx to proximal forearm. Splint width should enclose both the volar and dorsum surfaces of the fourth and fifth metacarpals. In addition, padding should be placed between the digits for comfort. The metacarpophalangeal joints should be positioned at 70˚, and the proximal phalangeal angle at approximately 20˚ flexion2; this will help minimize the risk of contractures.
Forearm “Sugar-Tong” Splint. These splints are indicated for immobilization of a distal radius fracture or wrist injury. Distal radial fractures are by far the most common fractures encountered in the pediatric population,8 and splinting for angulation less than 15˚ is preferred.9,10 For proper stabilization, a long U-shaped splint should originate at the palmar crease, wrap around the elbow, and end at the metacarpophalangeal joint dorsally. Again, the hand should be dorsiflexed, and a soft rolled edge should be kept on the palmar crease to allow full finger flexion to near 90˚.
Thumb Spica Splint. A thumb spica splint is useful to immobilize uncomplicated fracture of the first metacarpal or proximal phalanx or when scaphoid (navicular) bone fracture is suspected. A semicircumferential molding of the radial forearm should be formed, extending to the thumbnail bed, and wrapping around the thumb. The proper hand positioning is slightly dorsiflexed, with thumb abducted slightly, as if holding a glass of water.2 If there is any doubt of a navicular fracture (rare in prepubescent children), the clinician should never hesitate to splint!
Long-Leg Posterior Splint. This type of splint is appropriate for immobilization of midshaft tibia/fibula fractures or most knee injuries. Full length of the splint should start beneath the inferior gluteal fold and extend to the ball of the foot, leaving the toes free. The ankle should be at 90˚ flexion and the knee should remain just slightly flexed, never locked straight. Orthopedics should always be consulted in cases of proximal tibia/fibula fractures or knee joint involvement.
Posterior Ankle Splint. Essentially a shorter version of a long-leg splint extending proximally to just below the knee, the posterior ankle splint is useful to immobilize ankle fractures, foot fractures, and severe ankle sprains. The distal fibula and occasional tibia physes are another common site of pediatric fractures, particularly in obese or more active children.11,12 When using either a long- or short-leg posterior ankle splint, it is helpful to hold the foot at 90˚ flexion until the material hardens or the proper angle may be lost. A recall that displaced or Salter-Harris type III or IV physeal fractures justify orthopedics consult. Nonweight-bearing, use of crutches, ice, and elevation are all important points for recovery in 3 to 6 weeks.
Lower Extremity Stirrup “Sugar-Tong” Splint. This splint is indicated for additional ankle stabilization. It runs in a U-shape (not unlike a forearm sugar-tong splint) from just below the knee around the calcaneus, and it must be wide enough to encase the ankle but not so wide that the two sides overlap when molded. It is very important to add extra padding around both malleoli and beneath the calcaneus to reduce the likelihood of pressure sores. Crutches are essential to avoid weight-bearing in patients old enough to use them. Some pediatric orthopedists advise avoiding this type of splint in the smaller, noncompliant, active child.
Complications
Although splinting has many advantages over casting in the acute-care setting, several potential complications may develop. Although rare, thermal burns to the underlying skin may occur if excessively warm or hot water is used on plaster or fiberglass due to the exothermic reaction during the hardening process. Therefore, the use of room-temperature water is always recommended. Despite the noncircumferential nature of a splint, it is still possible to develop significant swelling following splint application, which can lead to neurovascular compromise, compartment syndrome, infection, or pressure ulcers.7 The patient and caregiver should be advised to return to the ED immediately for evaluation if serious signs and symptoms such as pain, numbness, tingling, dusky color of skin, or poor capillary refill develop.
Case 1 Conclusion
The EP in this case elected to obtain plain X-rays of the patient’s left forearm, including the wrist and elbow. The results demonstrated a disruption of the cortical integrity of the distal radius, consistent with a buckle fracture. The angulation was estimated at merely 10˚. The bones of the wrist and elbow appeared normal. The EP concluded that a consult with orthopedics was not required urgently, and immobilized the patient’s arm using a fiberglass sugar-tong splint, keeping her elbow at 90˚, the forearm in a neutral position, and hand slightly dorsiflexed. A nurse assisted in keeping the child still to ensure the splint was shaped around the arm and hardened in this position. The child was provided with a sling, and supportive-care measures, including analgesia with nonsteroidal anti-inflammatory drugs as needed, ice, rest, and the importance of keeping the splint dry, were reviewed with her parents. The EP also stressed the importance of surveying for any loss of sensation or perfusion to the patient’s hand and fingers, and recommended follow up with orthopedics 1 week from discharge.
Case 2 Conclusion
Multiple views of the patient’s ankle were obtained on X-ray and showed no apparent fracture or dislocation. Additional films of the opposite ankle were obtained for comparison, but both appeared quite similar except for mild soft-tissue swelling of the affected side. Since point tenderness was reproducible over the distal fibular physis, the EP elected to place a short-leg posterior splint, maintaining good anatomic position with extra padding around the malleoli. The parents were instructed on proper elevation, ice to reduce inflammation, and the use of pain medication if needed.
One week after discharge, the treating EP received a letter from the child’s orthopedist, informing him that at the follow-up appointment, a repeat ankle film revealed periosteal changes and a type I Salter-Harris distal fibula fracture. Immobilization for an additional 3 weeks and supportive care was indicated.
Dr Del Re is an instructor of pediatrics and an intermediate care pediatrician, Rady Children’s Hospital, San Diego, California. Dr Clingenpeel is a fellowship director, pediatric emergency medicine, and associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
Case 1
A mother presented to the ED with her 8-year-old daughter after she witnessed the child fall off her bicycle onto the sidewalk. When she fell, the girl landed onto her outstretched arms and sustained minor abrasions to her palms and knees, but did not hit her head or lose consciousness. Upon falling, the child immediately cried that her left arm hurt and kept holding it guarded near her body.
She was seated on her mother’s lap in the examination room, appearing anxious but in no acute distress. The treating EP observed the superficial abrasions from across the room and obtained a detailed history.
The patient was afebrile, and her vital signs were stable with the exception of mild tachycardia. After a couple of minutes, the EP slowly approached the child and was able to perform a basic examination. There was no obvious deformity to her left upper extremity, and only mild swelling over the wrist. She was able to move her fingers well and had excellent capillary refill distally. The child remained calm during manual palpation of the anatomic snuff box. However, she immediately pulled away and began to cry upon palpation more proximally over the distal forearm. The EP discussed his concerns with the child’s mother and explained that further evaluation was necessary.
Case 2
A mother and father presented to the ED carrying their 6-year-old boy, stating that the child had been limping since they picked him up from a neighbor’s house an hour earlier and was now refusing to walk. The father noted that a group of children had been jumping on a trampoline unsupervised, but he did not witness any injury to his son. Both parents said that the boy had been well up until that day.
At presentation, the child was afebrile and his vital signs were stable. The EP asked the parents to coax the child to walk across the room. During the walk, the boy was reluctant to bear weight on his right foot. Careful inspection of his lower limb revealed no external signs of trauma, and it appeared neurovascularly intact. Careful palpation elicited tenderness directly over the physis at the distal fibula and near the lateral malleolus. While considering the broad differential for a limping child, the physician was primarily concerned with point tenderness on examination and informed the parents that radiographic imaging was warranted.
Overview
Pediatric musculoskeletal (MSK) injury and orthopedic trauma now comprise more than 10% of visits to the ED.1 Fractures in particular are becoming more commonplace with the increasing number of children actively involved in athletic sports and high-risk activities.
The general approach to acute-care management of these children has evolved, trending more toward splinting the fractured extremity and away from traditional casting. There are many benefits to splinting. The most important is arguably the reduced risk of developing compartment syndrome due to a splint’s ability to expand with accompanied swelling.2 This article reviews the unique characteristics of pediatric bone development and initial management of pediatric fractures, as well as basic splinting techniques and unique indications that require further orthopedic consultation.
Physiological Differences in Children
The MSK system of a child differs greatly from that of an adult. The bones themselves are much more porous and malleable during childhood, making them more susceptible to traumatic injury. The growing periosteum and the developing physes are particularly vulnerable, accounting for up to 20% of pediatric fractures (see the Figure illustrating the Salter-Harris classification in the next section).3 This is particularly true at a young age, when ligamentous adherence out-performs the bony integrity itself, making fractures more common than sprains and tears. The opposite is true in adults, who are much more likely to experience sprains before succumbing to fracture. Furthermore, since the periosteum is still very active in children, the fractured bone is much more likely to remodel, lending to less deformity and overall better outcomes in most cases.3 Nonunion is extremely rare in children.
Initial Management
Approaching the Pediatric Patient
Special consideration should be given when initially approaching an injured child, so as not to cause additional undue fear or anxiety to the patient. It is helpful to take an extra moment upon entering the room to simply observe the child’s positioning, posture, or reluctance to move a particular limb. Obtaining a careful, detailed history from a distance is recommended before too quickly approaching the patient. In addition, asking the caregiver to serve as proxy during the initial physical examination may also prove helpful in localizing the pain. In the obscure case, such as the child refusing to bear weight, it is good to keep a broad differential and inspect for non-MSK injury (eg, painful hernia, testicular torsion, foreign body lodged in the bottom of the foot). Utilizing a “log-rolling” technique with the palms of one’s hands on the patient’s thigh may reveal hip pathology. Simply observing the preoccupied child walk around the unit while watching from behind may also aid in the evaluation.
However, when the injury such as an open fracture or severe displacement is obvious, immediate stabilization is critical so as not to permit any additional harm. An arm board is typically used to accomplish this. In addition, pain control should never be overlooked, either with intravenous opioids or more appropriate oral or intranasal analgesia.4,5 In cases of significant trauma, always remember ABC assessment (airway, breathing/oxygenation, and circulation), despite the eagerness to give attention to what may be an obvious fracture.
Workup
Although the use of ultrasound and other modalities is becoming more popular in some settings, it is still commonplace to begin the evaluation of a potential fracture or dislocation with plain film X-rays. Before sending a patient to radiology, always stabilize any unstable fracture to avoid further injury or potentiate neurovascular compromise. In most cases, three views, including anteroposterior, true lateral, and oblique, are obtained. If a fracture is unclear, it may be helpful to image the opposite extremity for comparison. The location of a fracture and its characteristics greatly influence acute-care management, as well as patient disposition, the need for consultation with orthopedics, and follow-up expectations and instructions.
Salter-Harris Fracture Classification
The physes of bones in growing children are particularly vulnerable sites of fracture since they have not yet fused. The five generally accepted types of fracture according to risk of growth disturbance are illustrated in the above Figure and are differentiated as follows:
Type I. This type of fracture is exclusive involvement of the physis itself, separating the metaphysis from the epiphysis. Since plain films may not reveal any visible fracture, the clinician should have a high index of suspicion if the physical examination elicits point tenderness over the growth plate. When in doubt if a fracture is present, always splint. Type I fractures of the physis tend to heal well, without significant consequence.
Type II. As with type I fractures, type II involve the physis, but also have a fragment of displaced metaphysis—the most common of all physeal fractures. Without significant displacement, these fractures also tend to have good outcomes.
Type III. Rather than involving the physis and metaphysis, type III fractures involve the epiphysis and therefore the joint itself. It is because of the epiphyseal displacement that these fractures tend to have a worse prognosis with joint disability and growth arrest. Thus, establishing alignment is imperative. The distal tibial Tillaux fracture is an example and requires internal fixation for optimal healing.
Type IV. Similar to a type III fracture, with the fracture extending proximally through a segment of the metaphysis, type IV fractures are treated similar to type III fractures. Due to joint involvement, an orthopedic consultation is warranted.
Type V. This type represents compression fractures of the physis. As the visibility of these fractures is poor on plain films, diagnosis can be challenging. However, history of axial compression injury may help lead the clinician to an accurate diagnosis. Since there is a high incidence of growth disturbance in type V fractures, compression affecting other areas such as the spine should also be considered.
Certainly not all pediatric fractures will involve a physis. A detailed description and management of other unique types of pediatric fractures is discussed in other articles in this feature.
Splinting Basics
Once the decision is made to apply a splint to a fracture, certain basic precautions should first be taken. Initially, any significant lacerations or abrasions should be thoroughly irrigated, cleansed, and dressed appropriately. Next, the physician should reevaluate and document both neurological status and perfusion of the area, particularly distal to the fracture site.
One commonly overlooked step in management of any fracture is pain control. It is advisable to consider administering medication prior to splinting on a case-by-case basis and for all fractures requiring reduction.
Materials and Methods
Prepackaged fiberglass splints have become a popular, efficient, and less-messy material of choice in pediatric splinting. Alternatively, plaster of Paris—although a bit more cumbersome—has some advantages, including low cost and a tendency to mold more easily to the extremity being splinted.7 When using plaster, strips should be cut a little longer than the anticipated length needed since they may shrink during curing. The unaffected limb should be used to gauge the measurement needed.
Regardless of the material chosen, all splinting should begin with the application of a stockinette tube dressing over the skin, leaving a distal opening over fingers or toes. This should be followed by a padding material (eg, Webril), beginning distally and rolling proximally, being sure to have approximately 50% overlap of each roll. Extra padding should be rolled over any bony prominence (eg, ulnar stylus) to avoid discomfort or pressure sores once the splint is applied.2
Between 8 and 10 layers of plaster (additional layers for lower extremity splints) should be wetted with room-temperature water. Hot water should never be used as this will intensify the exothermic reaction that occurs when curing and could cause burns.2 The limb should be kept in the anatomic position while the plaster is being molded to the shape of the extremity, allowing 15 to 20 minutes to dry.1 Once dry, an elastic bandage such as an Ace wrap may be placed over the entire cast to hold it secure in place. If fiberglass is used, it is helpful to squeeze out extra water before molding to the extremity. Again, an additional padding roll should be employed to avoid any discomfort or pressure beneath the splint.
In both fiberglass and plaster splinting, the edges of either type of material should not be abrasive to the skin; this can be avoided by rolling over excess padding and stockinette to create a round soft edge on either end.7 Finally, the patient should be fitted with a shoulder sling or crutches (if age appropriate) to further immobilize the injured extremity and avoid any movement or weight bearing.
Types of Splints
The type of splint depends of the location and characteristics of the fracture being immobilized. The following are a few examples of the more popular splinting techniques indicated for common pediatric fractures.
Long-Arm Posterior Splint. This splint is useful for most forearm and elbow fractures. The splint length should extend from midlength of the humerus to the palmar crease, and the width should be semicircular. In addition, an anatomic position of 90˚ flexion of the elbow should be maintained, with the hand in a neutral position and slight dorsiflexion. It is generally accepted to slightly pronate the forearm when splinting a supracondylar fracture. Orthopedics should always be consulted if the fracture involves the elbow.
Ulnar “Gutter” Splint. Useful for nondisplaced, minimally-angulated metacarpal “boxer’s fracture” or fourth and fifth phalangeal fractures, the length of the ulnar splint should extend from the distal phalanx to proximal forearm. Splint width should enclose both the volar and dorsum surfaces of the fourth and fifth metacarpals. In addition, padding should be placed between the digits for comfort. The metacarpophalangeal joints should be positioned at 70˚, and the proximal phalangeal angle at approximately 20˚ flexion2; this will help minimize the risk of contractures.
Forearm “Sugar-Tong” Splint. These splints are indicated for immobilization of a distal radius fracture or wrist injury. Distal radial fractures are by far the most common fractures encountered in the pediatric population,8 and splinting for angulation less than 15˚ is preferred.9,10 For proper stabilization, a long U-shaped splint should originate at the palmar crease, wrap around the elbow, and end at the metacarpophalangeal joint dorsally. Again, the hand should be dorsiflexed, and a soft rolled edge should be kept on the palmar crease to allow full finger flexion to near 90˚.
Thumb Spica Splint. A thumb spica splint is useful to immobilize uncomplicated fracture of the first metacarpal or proximal phalanx or when scaphoid (navicular) bone fracture is suspected. A semicircumferential molding of the radial forearm should be formed, extending to the thumbnail bed, and wrapping around the thumb. The proper hand positioning is slightly dorsiflexed, with thumb abducted slightly, as if holding a glass of water.2 If there is any doubt of a navicular fracture (rare in prepubescent children), the clinician should never hesitate to splint!
Long-Leg Posterior Splint. This type of splint is appropriate for immobilization of midshaft tibia/fibula fractures or most knee injuries. Full length of the splint should start beneath the inferior gluteal fold and extend to the ball of the foot, leaving the toes free. The ankle should be at 90˚ flexion and the knee should remain just slightly flexed, never locked straight. Orthopedics should always be consulted in cases of proximal tibia/fibula fractures or knee joint involvement.
Posterior Ankle Splint. Essentially a shorter version of a long-leg splint extending proximally to just below the knee, the posterior ankle splint is useful to immobilize ankle fractures, foot fractures, and severe ankle sprains. The distal fibula and occasional tibia physes are another common site of pediatric fractures, particularly in obese or more active children.11,12 When using either a long- or short-leg posterior ankle splint, it is helpful to hold the foot at 90˚ flexion until the material hardens or the proper angle may be lost. A recall that displaced or Salter-Harris type III or IV physeal fractures justify orthopedics consult. Nonweight-bearing, use of crutches, ice, and elevation are all important points for recovery in 3 to 6 weeks.
Lower Extremity Stirrup “Sugar-Tong” Splint. This splint is indicated for additional ankle stabilization. It runs in a U-shape (not unlike a forearm sugar-tong splint) from just below the knee around the calcaneus, and it must be wide enough to encase the ankle but not so wide that the two sides overlap when molded. It is very important to add extra padding around both malleoli and beneath the calcaneus to reduce the likelihood of pressure sores. Crutches are essential to avoid weight-bearing in patients old enough to use them. Some pediatric orthopedists advise avoiding this type of splint in the smaller, noncompliant, active child.
Complications
Although splinting has many advantages over casting in the acute-care setting, several potential complications may develop. Although rare, thermal burns to the underlying skin may occur if excessively warm or hot water is used on plaster or fiberglass due to the exothermic reaction during the hardening process. Therefore, the use of room-temperature water is always recommended. Despite the noncircumferential nature of a splint, it is still possible to develop significant swelling following splint application, which can lead to neurovascular compromise, compartment syndrome, infection, or pressure ulcers.7 The patient and caregiver should be advised to return to the ED immediately for evaluation if serious signs and symptoms such as pain, numbness, tingling, dusky color of skin, or poor capillary refill develop.
Case 1 Conclusion
The EP in this case elected to obtain plain X-rays of the patient’s left forearm, including the wrist and elbow. The results demonstrated a disruption of the cortical integrity of the distal radius, consistent with a buckle fracture. The angulation was estimated at merely 10˚. The bones of the wrist and elbow appeared normal. The EP concluded that a consult with orthopedics was not required urgently, and immobilized the patient’s arm using a fiberglass sugar-tong splint, keeping her elbow at 90˚, the forearm in a neutral position, and hand slightly dorsiflexed. A nurse assisted in keeping the child still to ensure the splint was shaped around the arm and hardened in this position. The child was provided with a sling, and supportive-care measures, including analgesia with nonsteroidal anti-inflammatory drugs as needed, ice, rest, and the importance of keeping the splint dry, were reviewed with her parents. The EP also stressed the importance of surveying for any loss of sensation or perfusion to the patient’s hand and fingers, and recommended follow up with orthopedics 1 week from discharge.
Case 2 Conclusion
Multiple views of the patient’s ankle were obtained on X-ray and showed no apparent fracture or dislocation. Additional films of the opposite ankle were obtained for comparison, but both appeared quite similar except for mild soft-tissue swelling of the affected side. Since point tenderness was reproducible over the distal fibular physis, the EP elected to place a short-leg posterior splint, maintaining good anatomic position with extra padding around the malleoli. The parents were instructed on proper elevation, ice to reduce inflammation, and the use of pain medication if needed.
One week after discharge, the treating EP received a letter from the child’s orthopedist, informing him that at the follow-up appointment, a repeat ankle film revealed periosteal changes and a type I Salter-Harris distal fibula fracture. Immobilization for an additional 3 weeks and supportive care was indicated.
Dr Del Re is an instructor of pediatrics and an intermediate care pediatrician, Rady Children’s Hospital, San Diego, California. Dr Clingenpeel is a fellowship director, pediatric emergency medicine, and associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
- Bachman D, Santora S. Musculoskeletal trauma. In: Fleisher GR, Ludwig S, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2010:1335-1375.
- Klig JE. Splinting procedures. In: King C, Henretig FM, eds. Texbook of Pediatric Emergency Procedures. 2nd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008:919-931.
- Wilkins KE. The incidence of fractures in children. In: Rockwood CA, Wilkins KE, Beaty JH, eds. Fractures in Children. 4th ed. Philadelphia, PA: Lippincott-Raven; 1996:3-17.
- Mahar PJ, Rana JA, Kennedy CS, Christopher NC. A randomized clinical trial of oral transmucosal fentanyl citrate versus intravenous morphine sulfate for initial control of pain in children with extremity injuries. Pediatr Emerg Care. 2007;23(8):544-548.
- Saunders M, Adelgais K, Nelson D. Use of intranasal fentanyl for the relief of pediatric orthopedic trauma pain. Acad Emerg Med. 2010;17(11):1155-1161.
- Salter RB, Harris WR. Injuries involving the epiphyseal plate. J Bone Joint Surg Am. 1963;45:587-622.
- Boyd AS, Benjamin HJ, Asplund C. Principles of casting and splinting. Am Fam Physician. 2009;79(1):16-22.
- Solan MC, Rees R, Daly K. Current management of torus fractures of the distal radius. Injury. 2002;33(6):503-505.
- Boutis K, Willan A, Babyn P, Goeree R, Howard A. Cast versus splint in children with minimally angulated fractures of the distal radius: a randomized controlled trial. CMAJ. 2010;182(14):1507-1512.
- Firmin F, Crouch R. Splinting versus casting of “torus” fractures to the distal radius in the paediatric patient presenting at the emergency department (ED): a literature review. Int Emerg Nurs. 2009;17(3):173-178.
- Peterson HA, Madhok R, Benson JT, Ilstrup DM, Melton LJ 3rd. Physeal fractures: Part 1. Epidemiology in Olmsted County, Minnesota, 1979-1988. J Pediatr Orthop. 1994;14(4):423-430.
- Blackburn EW, Aronsson DD, Rubright JH, Lisle JW. Ankle fractures in children. J Bone Joint Surg Am. 2012; 94(13):1234-1244.
- Bachman D, Santora S. Musculoskeletal trauma. In: Fleisher GR, Ludwig S, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2010:1335-1375.
- Klig JE. Splinting procedures. In: King C, Henretig FM, eds. Texbook of Pediatric Emergency Procedures. 2nd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008:919-931.
- Wilkins KE. The incidence of fractures in children. In: Rockwood CA, Wilkins KE, Beaty JH, eds. Fractures in Children. 4th ed. Philadelphia, PA: Lippincott-Raven; 1996:3-17.
- Mahar PJ, Rana JA, Kennedy CS, Christopher NC. A randomized clinical trial of oral transmucosal fentanyl citrate versus intravenous morphine sulfate for initial control of pain in children with extremity injuries. Pediatr Emerg Care. 2007;23(8):544-548.
- Saunders M, Adelgais K, Nelson D. Use of intranasal fentanyl for the relief of pediatric orthopedic trauma pain. Acad Emerg Med. 2010;17(11):1155-1161.
- Salter RB, Harris WR. Injuries involving the epiphyseal plate. J Bone Joint Surg Am. 1963;45:587-622.
- Boyd AS, Benjamin HJ, Asplund C. Principles of casting and splinting. Am Fam Physician. 2009;79(1):16-22.
- Solan MC, Rees R, Daly K. Current management of torus fractures of the distal radius. Injury. 2002;33(6):503-505.
- Boutis K, Willan A, Babyn P, Goeree R, Howard A. Cast versus splint in children with minimally angulated fractures of the distal radius: a randomized controlled trial. CMAJ. 2010;182(14):1507-1512.
- Firmin F, Crouch R. Splinting versus casting of “torus” fractures to the distal radius in the paediatric patient presenting at the emergency department (ED): a literature review. Int Emerg Nurs. 2009;17(3):173-178.
- Peterson HA, Madhok R, Benson JT, Ilstrup DM, Melton LJ 3rd. Physeal fractures: Part 1. Epidemiology in Olmsted County, Minnesota, 1979-1988. J Pediatr Orthop. 1994;14(4):423-430.
- Blackburn EW, Aronsson DD, Rubright JH, Lisle JW. Ankle fractures in children. J Bone Joint Surg Am. 2012; 94(13):1234-1244.
Pediatric Orthopedic Injuries
| Pediatric orthopedic injuries are a common presentation to the ED, representing 12% of pediatric visits.1 In this special feature, our authors focus on the challenge of evaluating and managing the pediatric orthopedic patient and spotlight conditions where the emergency physician (EP) might not have significant clinical experience. |
While many pediatric orthopedic injuries are the simple “bruises and bumps” of active childhood and need little more than pain control and parental education, there are some age-specific injuries that require truly emergent care in order to salvage an extremity or prevent loss of function. Differentiation between the urgent and emergent patient may not be obvious in the preverbal child or in a child whose radiographs show open growth plates and ossification centers obscuring interpretation.
This educational series begins by covering the physiological differences in pediatric musculoskeletal injuries and reviews both the general approach to examination and some pediatric splinting basics. With an enhanced awareness of the structural differences between growing and mature bones (along with their tendons and ligaments), subtle age-related injuries are less likely to be missed.
With the basics firmly in hand, we turn your attention to several common orthopedic injuries unique to children and review acute-care management. The third article deals with the frustrating diagnostic dilemma of “my child won’t walk” and explores some nontraumatic pediatric orthopedic presentations. Our series concludes with a review of some “high risk/can’t miss” pediatric injury patterns and presenting symptoms and also reminds us of injuries that might be suggestive of nonaccidental trauma as the underlying etiology.
While pediatric bones are indeed not simply little adult bones, EPs need not be intimidated in caring for these patients. A basic understanding of pediatric musculoskeletal physiology and an enhanced clinical awareness as to common injury patterns will equip most EPs with the knowledge necessary to ensure the best possible outcome for these children.
Reference
1. Chamberlain JM, Patel KM, Pollack MM, et al. Recalibration of the pediatric risk of admission (PRISA) score using a multi-institutional sample. Ann Emerg Med. 2004;43(4):461-486.
| Pediatric orthopedic injuries are a common presentation to the ED, representing 12% of pediatric visits.1 In this special feature, our authors focus on the challenge of evaluating and managing the pediatric orthopedic patient and spotlight conditions where the emergency physician (EP) might not have significant clinical experience. |
While many pediatric orthopedic injuries are the simple “bruises and bumps” of active childhood and need little more than pain control and parental education, there are some age-specific injuries that require truly emergent care in order to salvage an extremity or prevent loss of function. Differentiation between the urgent and emergent patient may not be obvious in the preverbal child or in a child whose radiographs show open growth plates and ossification centers obscuring interpretation.
This educational series begins by covering the physiological differences in pediatric musculoskeletal injuries and reviews both the general approach to examination and some pediatric splinting basics. With an enhanced awareness of the structural differences between growing and mature bones (along with their tendons and ligaments), subtle age-related injuries are less likely to be missed.
With the basics firmly in hand, we turn your attention to several common orthopedic injuries unique to children and review acute-care management. The third article deals with the frustrating diagnostic dilemma of “my child won’t walk” and explores some nontraumatic pediatric orthopedic presentations. Our series concludes with a review of some “high risk/can’t miss” pediatric injury patterns and presenting symptoms and also reminds us of injuries that might be suggestive of nonaccidental trauma as the underlying etiology.
While pediatric bones are indeed not simply little adult bones, EPs need not be intimidated in caring for these patients. A basic understanding of pediatric musculoskeletal physiology and an enhanced clinical awareness as to common injury patterns will equip most EPs with the knowledge necessary to ensure the best possible outcome for these children.
| Pediatric orthopedic injuries are a common presentation to the ED, representing 12% of pediatric visits.1 In this special feature, our authors focus on the challenge of evaluating and managing the pediatric orthopedic patient and spotlight conditions where the emergency physician (EP) might not have significant clinical experience. |
While many pediatric orthopedic injuries are the simple “bruises and bumps” of active childhood and need little more than pain control and parental education, there are some age-specific injuries that require truly emergent care in order to salvage an extremity or prevent loss of function. Differentiation between the urgent and emergent patient may not be obvious in the preverbal child or in a child whose radiographs show open growth plates and ossification centers obscuring interpretation.
This educational series begins by covering the physiological differences in pediatric musculoskeletal injuries and reviews both the general approach to examination and some pediatric splinting basics. With an enhanced awareness of the structural differences between growing and mature bones (along with their tendons and ligaments), subtle age-related injuries are less likely to be missed.
With the basics firmly in hand, we turn your attention to several common orthopedic injuries unique to children and review acute-care management. The third article deals with the frustrating diagnostic dilemma of “my child won’t walk” and explores some nontraumatic pediatric orthopedic presentations. Our series concludes with a review of some “high risk/can’t miss” pediatric injury patterns and presenting symptoms and also reminds us of injuries that might be suggestive of nonaccidental trauma as the underlying etiology.
While pediatric bones are indeed not simply little adult bones, EPs need not be intimidated in caring for these patients. A basic understanding of pediatric musculoskeletal physiology and an enhanced clinical awareness as to common injury patterns will equip most EPs with the knowledge necessary to ensure the best possible outcome for these children.
Reference
1. Chamberlain JM, Patel KM, Pollack MM, et al. Recalibration of the pediatric risk of admission (PRISA) score using a multi-institutional sample. Ann Emerg Med. 2004;43(4):461-486.
Reference
1. Chamberlain JM, Patel KM, Pollack MM, et al. Recalibration of the pediatric risk of admission (PRISA) score using a multi-institutional sample. Ann Emerg Med. 2004;43(4):461-486.
Management of Gastroenteropancreatic Neuroendocrine Tumors
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Neuroendocrine tumors (NETs) are a rare, heterogeneous group of neoplasms that arise from neuroendocrine cells located throughout the body. These tumors are characterized by variable but most often indolent biologic behavior. They are also classically characterized by their ability to secrete peptides, resulting in distinctive hormonal syndromes. Although NETs have been considered rare, recent studies suggest that they are more common than previously suspected. An analysis of the Surveillance, Epidemiology, and End Results (SEER) database demonstrated a significant increase in the incidence of NETs over time with an age-adjusted annual incidence in the United States of 5.25 cases per 100,000 population. The increase in incidence is likely attributable to increasing awareness, improved diagnostic strategies, and possibly other undetermined environmental and genetic factors.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Neuroendocrine tumors (NETs) are a rare, heterogeneous group of neoplasms that arise from neuroendocrine cells located throughout the body. These tumors are characterized by variable but most often indolent biologic behavior. They are also classically characterized by their ability to secrete peptides, resulting in distinctive hormonal syndromes. Although NETs have been considered rare, recent studies suggest that they are more common than previously suspected. An analysis of the Surveillance, Epidemiology, and End Results (SEER) database demonstrated a significant increase in the incidence of NETs over time with an age-adjusted annual incidence in the United States of 5.25 cases per 100,000 population. The increase in incidence is likely attributable to increasing awareness, improved diagnostic strategies, and possibly other undetermined environmental and genetic factors.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Neuroendocrine tumors (NETs) are a rare, heterogeneous group of neoplasms that arise from neuroendocrine cells located throughout the body. These tumors are characterized by variable but most often indolent biologic behavior. They are also classically characterized by their ability to secrete peptides, resulting in distinctive hormonal syndromes. Although NETs have been considered rare, recent studies suggest that they are more common than previously suspected. An analysis of the Surveillance, Epidemiology, and End Results (SEER) database demonstrated a significant increase in the incidence of NETs over time with an age-adjusted annual incidence in the United States of 5.25 cases per 100,000 population. The increase in incidence is likely attributable to increasing awareness, improved diagnostic strategies, and possibly other undetermined environmental and genetic factors.
To read the full article in PDF:
A summary of the new ACOG report on neonatal brachial plexus palsy. Part 1: Can it be predicted?
Neonatal brachial plexus palsy (NBPP) after a delivery involving shoulder dystocia is not only a clinical disaster—it constitutes the second largest category of litigation in obstetrics.1
Lawsuits that center on NBPP often feature plaintiff expert witnesses who claim that the only way a permanent brachial plexus injury can occur is by a clinician applying “excessive” traction on the fetal head during delivery. The same experts often claim that the mother had multiple risk factors for shoulder dystocia and should never have been allowed a trial of labor in the first place.
The jury is left suspecting that the NBPP was a disaster waiting to happen, with warning signs that were ignored by the clinician. Jurors also may be convinced that, when the dystocia occurred, the defendant handled it badly, causing a severe, lifelong injury to the beautiful child whose images they are shown in the courtroom.
But this scenario is far from accurate.
ACOG publishes new guidance on NBPPThe American College of Obstetricians and Gynecologists (ACOG) periodically issues practice bulletins on the subject of shoulder dystocia, the most recent one written in 2002 and reaffirmed in 2013.2 These bulletins are, of necessity, relatively brief summaries of current thinking about the causes, pathophysiology, treatment, and preventability of shoulder dystocia and associated brachial plexus injuries.
In 2011, James Breeden, MD, then president-elect of ACOG, called for formation of a task force on NBPP. The task force’s report, Neonatal Brachial Plexus Palsy,3 was published earlier this year and represents ACOG’s official position on the important—but still controversial—subjects of shoulder dystocia and NBPP. This report should serve not only to help clinicians better understand and manage these entities but also as a foundational document in the prolific and complex medicolegal suits involving them.
Given the length of this report, however, a concise summary of the key takeaways is in order.
NBPP and shoulder dystocia are not always linked
Early in the report, ACOG presents three very important statements, all of which challenge claims that are frequently made by plaintiffs in brachial plexus injury cases:
- NBPP can occur without concomitant, clinically recognizable shoulder dystocia, although it often is associated with shoulder dystocia.
- In the presence of shoulder dystocia, all ancillary maneuvers necessarily increase strain on the brachial plexus, no matter how expertly the maneuvers are performed.
- Recent multidisciplinary research now indicates that the existence of NBPP after birth does not prove that exogenous forces are the sole cause of this injury.
These findings raise a number of questions, including:
- Can NBPP be predicted and prevented?
- What is the pathophysiologic mechanism for NBPP with and without shoulder dystocia?
- Are there specific interventions that may reduce the frequency of NBPP?
In Part 1 of this article, I summarize ACOG data on whether and how NBPP might be predicted. Part 2, to follow in October 2014, will discuss the pathophysiologic mechanism for NBPP and discuss potential interventions.
The data on NBPP without shoulder dystocia
The results of 12 reports published between 1990 and 2011 describe NBPP (temporary and persistent) that occurred without concomitant shoulder dystocia. These reports indicate that 46% of NBPP cases occurred without documented shoulder dystocia (0.9 cases/1,000 births).
Persistent NBPP. Two of these reports provide data on persistent NBPP without shoulder dystocia. Even when injury to the brachial plexus was documented as lasting more than 1 year, 26% of cases occurred in the absence of documented shoulder dystocia.
NBPP sometimes can occur during cesarean delivery. Four studies evaluated more than 240,000 births and found a rate of NBPP with cesarean delivery ranging from 0.3 to 1.5 cases per 1,000 live births.
All of these studies are described in the ACOG report.
When NBPP is related to shoulder dystocia
Shoulder dystocia may occur when there is a lack of fit of the transverse diameter of the fetal shoulders through the different pelvic diameters the shoulders encounter as they descend through the pelvis during the course of labor and delivery. This lack of fit can be related to excessive size of the fetal shoulders, inadequacy of pelvic dimensions to allow passage of a given fetus, or both. Abnormalities of fetal anatomy, fetal presentation, and soft tissue obstruction are rarely the cause of shoulder dystocia.
The difference between anterior shoulder obstruction behind the symphysis pubis and posterior shoulder obstruction from arrest at the level of the sacral promontory also is discussed in the ACOG report. In both cases, it is this obstruction of the affected shoulder while the long axis of the body continues to be pushed downward that widens the angle between the neck and impacted shoulder and stretches the brachial plexus.
The ACOG report acknowledges that may cases of NBPP do occur in conjunction with shoulder dystocia and that the same biomechanical factors that predispose a fetus to develop NBPP are associated with shoulder dystocia as well. However, the report takes pains to point out that the frequent conjunction of these two entities—NBPP and shoulder dystocia—may lead to an “erroneous retrospective inference of causation.”
Risk and predictive factors
The ACOG report states: “Various risk factors have been described in association with NBPP. Overall, however, these risk factors have not been shown to be statistically reliable or clinically useful predictors for...NBPP.”
For example, fetal macrosomia, defined as a birth weight of 4,000 g or more, has been reported as a risk factor for NBPP either alone or in conjunction with maternal diabetes. Although NBPP does occur more frequently as birth weight increases, seven studies over the past 20 years have shown that most cases of NBPP occur in infants of mothers without diabetes and in infants who weigh less than 4,000 g.
Other studies have shown that, if cesarean delivery were performed in cases of suspected macrosomia, it would have only a limited effect on reducing the incidence of NBPP. Specifically, in women with diabetes who have an estimated fetal weight of more than 4,500 g, the positive predictive value for NBPP is only 5%. Without maternal diabetes, that figure is less than 2%.
Estimating fetal weight by ultrasound does not significantly enhance our ability to predict NBPP. Ultrasound estimates of birth weight usually fall within 15% to 20% of actual birth weight, and the sensitivity of ultrasound in detecting birth weights more than 4,500 g is only 40%.
Therefore, ultrasound estimates of birth weight are of limited utility for contemporaneous clinical management. Furthermore, no data exist to support the claim that estimated fetal weight can be used prophylactically to reduce the incidence of NBPP.
Recurrent shoulder dystocia may be predictive of future NBPP
Whether studied alone or with NBPP, risk factors for shoulder dystocia are not reliable predictors of its occurrence. This is not the case, however, for recurrent shoulder dystocia, where the risk of neonatal brachial plexus palsy can be as high as 4.5%, compared with 1% to 2% for a first episode of shoulder dystocia.
NBPP is a rare phenomenon
The frequency of NBPP is “rare,” according to the ACOG report, which cites a rate of 1.5 cases for every 1,000 births. Favorable outcomes with complete recovery are estimated to range from 50% to 80%.3
Brachial plexus injuries are classically defined as Erb’s palsy—involving C5 and C6 nerve roots—or Klumpke’s palsy, in which there is damage to the C8 and T1 nerve roots.
Erb’s palsy is recognizable by the characteristic “waiter’s tip” position of the hand, which is caused by muscle imbalance in the shoulder and upper arm. Most NBPP injuries are Erb’s palsy, which affect 1.2 infants in every 1,000 births.
Klumpke’s palsy results in weakness of the hand and medial forearm muscles. It affects 0.05 infants in every 1,000 births. The remaining cases involve a combination of the two types of palsy.
These injuries can be temporary, resolving by 12 months after birth, or permanent. The rate of persistence of NBPP at 12 months ranges from 3% to 33%.
Can clinician maneuvers increase the likelihood of NBPP?
The ACOG report addresses the direction and angle of clinician traction at delivery. The report confirms what clinicians generally have been taught: The application of fundal pressure during a delivery in which shoulder dystocia is recognized can exacerbate shoulder impaction and can lead to an increased risk of NBPP.
Traction applied by the clinician and lateral bending of the fetal neck often are implicated as causative factors of NBPP. However, ACOG presents evidence that NBPP can occur entirely unrelated to clinician traction. The report cites studies involving both transient and persistent NBPP in fetuses delivered vaginally without evident shoulder dystocia. The same types of injury are sometimes seen in fetuses delivered by cesarean, as has been mentioned.
The report goes on to state:
Recommendations for practice
At the close of its second chapter (“Risk and predictive factors”), the ACOG report offers the same official recommendations that appear in its current practice bulletin on shoulder dystocia. It notes that there are three clinical situations in which it may be prudent to alter usual obstetric management, with an aim of reducing the risk of shoulder dystocia and NBPP:
- when fetal macrosomia is suspected, with fetal weight estimated to exceed 5,000 g in a woman without diabetes or 4,500 g in a woman with diabetes
- when the mother has a history of recognized shoulder dystocia, especially when neonatal injury was severe
- when midpelvic operative vaginal delivery is contemplated with a fetus estimated to weigh more than 4,000 g.
It is interesting to note that these recommendations are made, according to the report, “notwithstanding the unreliability of specific risk factors to predict NBPP or clinically apparent shoulder dystocia in a specific case.” The report further adds:
More to come
For ACOG’s conclusions on the pathophysiology and causation of NBPP, with a view toward formulating specific protective interventions, see Part 2 of this article, which will appear in the October 2014 issue of OBG Management.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Physician Insurers Association of America. http://www.piaa.us. Accessed August 21, 2014.
2. American College of Obstetricians and Gynecologists. Practice Bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
3. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
Neonatal brachial plexus palsy (NBPP) after a delivery involving shoulder dystocia is not only a clinical disaster—it constitutes the second largest category of litigation in obstetrics.1
Lawsuits that center on NBPP often feature plaintiff expert witnesses who claim that the only way a permanent brachial plexus injury can occur is by a clinician applying “excessive” traction on the fetal head during delivery. The same experts often claim that the mother had multiple risk factors for shoulder dystocia and should never have been allowed a trial of labor in the first place.
The jury is left suspecting that the NBPP was a disaster waiting to happen, with warning signs that were ignored by the clinician. Jurors also may be convinced that, when the dystocia occurred, the defendant handled it badly, causing a severe, lifelong injury to the beautiful child whose images they are shown in the courtroom.
But this scenario is far from accurate.
ACOG publishes new guidance on NBPPThe American College of Obstetricians and Gynecologists (ACOG) periodically issues practice bulletins on the subject of shoulder dystocia, the most recent one written in 2002 and reaffirmed in 2013.2 These bulletins are, of necessity, relatively brief summaries of current thinking about the causes, pathophysiology, treatment, and preventability of shoulder dystocia and associated brachial plexus injuries.
In 2011, James Breeden, MD, then president-elect of ACOG, called for formation of a task force on NBPP. The task force’s report, Neonatal Brachial Plexus Palsy,3 was published earlier this year and represents ACOG’s official position on the important—but still controversial—subjects of shoulder dystocia and NBPP. This report should serve not only to help clinicians better understand and manage these entities but also as a foundational document in the prolific and complex medicolegal suits involving them.
Given the length of this report, however, a concise summary of the key takeaways is in order.
NBPP and shoulder dystocia are not always linked
Early in the report, ACOG presents three very important statements, all of which challenge claims that are frequently made by plaintiffs in brachial plexus injury cases:
- NBPP can occur without concomitant, clinically recognizable shoulder dystocia, although it often is associated with shoulder dystocia.
- In the presence of shoulder dystocia, all ancillary maneuvers necessarily increase strain on the brachial plexus, no matter how expertly the maneuvers are performed.
- Recent multidisciplinary research now indicates that the existence of NBPP after birth does not prove that exogenous forces are the sole cause of this injury.
These findings raise a number of questions, including:
- Can NBPP be predicted and prevented?
- What is the pathophysiologic mechanism for NBPP with and without shoulder dystocia?
- Are there specific interventions that may reduce the frequency of NBPP?
In Part 1 of this article, I summarize ACOG data on whether and how NBPP might be predicted. Part 2, to follow in October 2014, will discuss the pathophysiologic mechanism for NBPP and discuss potential interventions.
The data on NBPP without shoulder dystocia
The results of 12 reports published between 1990 and 2011 describe NBPP (temporary and persistent) that occurred without concomitant shoulder dystocia. These reports indicate that 46% of NBPP cases occurred without documented shoulder dystocia (0.9 cases/1,000 births).
Persistent NBPP. Two of these reports provide data on persistent NBPP without shoulder dystocia. Even when injury to the brachial plexus was documented as lasting more than 1 year, 26% of cases occurred in the absence of documented shoulder dystocia.
NBPP sometimes can occur during cesarean delivery. Four studies evaluated more than 240,000 births and found a rate of NBPP with cesarean delivery ranging from 0.3 to 1.5 cases per 1,000 live births.
All of these studies are described in the ACOG report.
When NBPP is related to shoulder dystocia
Shoulder dystocia may occur when there is a lack of fit of the transverse diameter of the fetal shoulders through the different pelvic diameters the shoulders encounter as they descend through the pelvis during the course of labor and delivery. This lack of fit can be related to excessive size of the fetal shoulders, inadequacy of pelvic dimensions to allow passage of a given fetus, or both. Abnormalities of fetal anatomy, fetal presentation, and soft tissue obstruction are rarely the cause of shoulder dystocia.
The difference between anterior shoulder obstruction behind the symphysis pubis and posterior shoulder obstruction from arrest at the level of the sacral promontory also is discussed in the ACOG report. In both cases, it is this obstruction of the affected shoulder while the long axis of the body continues to be pushed downward that widens the angle between the neck and impacted shoulder and stretches the brachial plexus.
The ACOG report acknowledges that may cases of NBPP do occur in conjunction with shoulder dystocia and that the same biomechanical factors that predispose a fetus to develop NBPP are associated with shoulder dystocia as well. However, the report takes pains to point out that the frequent conjunction of these two entities—NBPP and shoulder dystocia—may lead to an “erroneous retrospective inference of causation.”
Risk and predictive factors
The ACOG report states: “Various risk factors have been described in association with NBPP. Overall, however, these risk factors have not been shown to be statistically reliable or clinically useful predictors for...NBPP.”
For example, fetal macrosomia, defined as a birth weight of 4,000 g or more, has been reported as a risk factor for NBPP either alone or in conjunction with maternal diabetes. Although NBPP does occur more frequently as birth weight increases, seven studies over the past 20 years have shown that most cases of NBPP occur in infants of mothers without diabetes and in infants who weigh less than 4,000 g.
Other studies have shown that, if cesarean delivery were performed in cases of suspected macrosomia, it would have only a limited effect on reducing the incidence of NBPP. Specifically, in women with diabetes who have an estimated fetal weight of more than 4,500 g, the positive predictive value for NBPP is only 5%. Without maternal diabetes, that figure is less than 2%.
Estimating fetal weight by ultrasound does not significantly enhance our ability to predict NBPP. Ultrasound estimates of birth weight usually fall within 15% to 20% of actual birth weight, and the sensitivity of ultrasound in detecting birth weights more than 4,500 g is only 40%.
Therefore, ultrasound estimates of birth weight are of limited utility for contemporaneous clinical management. Furthermore, no data exist to support the claim that estimated fetal weight can be used prophylactically to reduce the incidence of NBPP.
Recurrent shoulder dystocia may be predictive of future NBPP
Whether studied alone or with NBPP, risk factors for shoulder dystocia are not reliable predictors of its occurrence. This is not the case, however, for recurrent shoulder dystocia, where the risk of neonatal brachial plexus palsy can be as high as 4.5%, compared with 1% to 2% for a first episode of shoulder dystocia.
NBPP is a rare phenomenon
The frequency of NBPP is “rare,” according to the ACOG report, which cites a rate of 1.5 cases for every 1,000 births. Favorable outcomes with complete recovery are estimated to range from 50% to 80%.3
Brachial plexus injuries are classically defined as Erb’s palsy—involving C5 and C6 nerve roots—or Klumpke’s palsy, in which there is damage to the C8 and T1 nerve roots.
Erb’s palsy is recognizable by the characteristic “waiter’s tip” position of the hand, which is caused by muscle imbalance in the shoulder and upper arm. Most NBPP injuries are Erb’s palsy, which affect 1.2 infants in every 1,000 births.
Klumpke’s palsy results in weakness of the hand and medial forearm muscles. It affects 0.05 infants in every 1,000 births. The remaining cases involve a combination of the two types of palsy.
These injuries can be temporary, resolving by 12 months after birth, or permanent. The rate of persistence of NBPP at 12 months ranges from 3% to 33%.
Can clinician maneuvers increase the likelihood of NBPP?
The ACOG report addresses the direction and angle of clinician traction at delivery. The report confirms what clinicians generally have been taught: The application of fundal pressure during a delivery in which shoulder dystocia is recognized can exacerbate shoulder impaction and can lead to an increased risk of NBPP.
Traction applied by the clinician and lateral bending of the fetal neck often are implicated as causative factors of NBPP. However, ACOG presents evidence that NBPP can occur entirely unrelated to clinician traction. The report cites studies involving both transient and persistent NBPP in fetuses delivered vaginally without evident shoulder dystocia. The same types of injury are sometimes seen in fetuses delivered by cesarean, as has been mentioned.
The report goes on to state:
Recommendations for practice
At the close of its second chapter (“Risk and predictive factors”), the ACOG report offers the same official recommendations that appear in its current practice bulletin on shoulder dystocia. It notes that there are three clinical situations in which it may be prudent to alter usual obstetric management, with an aim of reducing the risk of shoulder dystocia and NBPP:
- when fetal macrosomia is suspected, with fetal weight estimated to exceed 5,000 g in a woman without diabetes or 4,500 g in a woman with diabetes
- when the mother has a history of recognized shoulder dystocia, especially when neonatal injury was severe
- when midpelvic operative vaginal delivery is contemplated with a fetus estimated to weigh more than 4,000 g.
It is interesting to note that these recommendations are made, according to the report, “notwithstanding the unreliability of specific risk factors to predict NBPP or clinically apparent shoulder dystocia in a specific case.” The report further adds:
More to come
For ACOG’s conclusions on the pathophysiology and causation of NBPP, with a view toward formulating specific protective interventions, see Part 2 of this article, which will appear in the October 2014 issue of OBG Management.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Neonatal brachial plexus palsy (NBPP) after a delivery involving shoulder dystocia is not only a clinical disaster—it constitutes the second largest category of litigation in obstetrics.1
Lawsuits that center on NBPP often feature plaintiff expert witnesses who claim that the only way a permanent brachial plexus injury can occur is by a clinician applying “excessive” traction on the fetal head during delivery. The same experts often claim that the mother had multiple risk factors for shoulder dystocia and should never have been allowed a trial of labor in the first place.
The jury is left suspecting that the NBPP was a disaster waiting to happen, with warning signs that were ignored by the clinician. Jurors also may be convinced that, when the dystocia occurred, the defendant handled it badly, causing a severe, lifelong injury to the beautiful child whose images they are shown in the courtroom.
But this scenario is far from accurate.
ACOG publishes new guidance on NBPPThe American College of Obstetricians and Gynecologists (ACOG) periodically issues practice bulletins on the subject of shoulder dystocia, the most recent one written in 2002 and reaffirmed in 2013.2 These bulletins are, of necessity, relatively brief summaries of current thinking about the causes, pathophysiology, treatment, and preventability of shoulder dystocia and associated brachial plexus injuries.
In 2011, James Breeden, MD, then president-elect of ACOG, called for formation of a task force on NBPP. The task force’s report, Neonatal Brachial Plexus Palsy,3 was published earlier this year and represents ACOG’s official position on the important—but still controversial—subjects of shoulder dystocia and NBPP. This report should serve not only to help clinicians better understand and manage these entities but also as a foundational document in the prolific and complex medicolegal suits involving them.
Given the length of this report, however, a concise summary of the key takeaways is in order.
NBPP and shoulder dystocia are not always linked
Early in the report, ACOG presents three very important statements, all of which challenge claims that are frequently made by plaintiffs in brachial plexus injury cases:
- NBPP can occur without concomitant, clinically recognizable shoulder dystocia, although it often is associated with shoulder dystocia.
- In the presence of shoulder dystocia, all ancillary maneuvers necessarily increase strain on the brachial plexus, no matter how expertly the maneuvers are performed.
- Recent multidisciplinary research now indicates that the existence of NBPP after birth does not prove that exogenous forces are the sole cause of this injury.
These findings raise a number of questions, including:
- Can NBPP be predicted and prevented?
- What is the pathophysiologic mechanism for NBPP with and without shoulder dystocia?
- Are there specific interventions that may reduce the frequency of NBPP?
In Part 1 of this article, I summarize ACOG data on whether and how NBPP might be predicted. Part 2, to follow in October 2014, will discuss the pathophysiologic mechanism for NBPP and discuss potential interventions.
The data on NBPP without shoulder dystocia
The results of 12 reports published between 1990 and 2011 describe NBPP (temporary and persistent) that occurred without concomitant shoulder dystocia. These reports indicate that 46% of NBPP cases occurred without documented shoulder dystocia (0.9 cases/1,000 births).
Persistent NBPP. Two of these reports provide data on persistent NBPP without shoulder dystocia. Even when injury to the brachial plexus was documented as lasting more than 1 year, 26% of cases occurred in the absence of documented shoulder dystocia.
NBPP sometimes can occur during cesarean delivery. Four studies evaluated more than 240,000 births and found a rate of NBPP with cesarean delivery ranging from 0.3 to 1.5 cases per 1,000 live births.
All of these studies are described in the ACOG report.
When NBPP is related to shoulder dystocia
Shoulder dystocia may occur when there is a lack of fit of the transverse diameter of the fetal shoulders through the different pelvic diameters the shoulders encounter as they descend through the pelvis during the course of labor and delivery. This lack of fit can be related to excessive size of the fetal shoulders, inadequacy of pelvic dimensions to allow passage of a given fetus, or both. Abnormalities of fetal anatomy, fetal presentation, and soft tissue obstruction are rarely the cause of shoulder dystocia.
The difference between anterior shoulder obstruction behind the symphysis pubis and posterior shoulder obstruction from arrest at the level of the sacral promontory also is discussed in the ACOG report. In both cases, it is this obstruction of the affected shoulder while the long axis of the body continues to be pushed downward that widens the angle between the neck and impacted shoulder and stretches the brachial plexus.
The ACOG report acknowledges that may cases of NBPP do occur in conjunction with shoulder dystocia and that the same biomechanical factors that predispose a fetus to develop NBPP are associated with shoulder dystocia as well. However, the report takes pains to point out that the frequent conjunction of these two entities—NBPP and shoulder dystocia—may lead to an “erroneous retrospective inference of causation.”
Risk and predictive factors
The ACOG report states: “Various risk factors have been described in association with NBPP. Overall, however, these risk factors have not been shown to be statistically reliable or clinically useful predictors for...NBPP.”
For example, fetal macrosomia, defined as a birth weight of 4,000 g or more, has been reported as a risk factor for NBPP either alone or in conjunction with maternal diabetes. Although NBPP does occur more frequently as birth weight increases, seven studies over the past 20 years have shown that most cases of NBPP occur in infants of mothers without diabetes and in infants who weigh less than 4,000 g.
Other studies have shown that, if cesarean delivery were performed in cases of suspected macrosomia, it would have only a limited effect on reducing the incidence of NBPP. Specifically, in women with diabetes who have an estimated fetal weight of more than 4,500 g, the positive predictive value for NBPP is only 5%. Without maternal diabetes, that figure is less than 2%.
Estimating fetal weight by ultrasound does not significantly enhance our ability to predict NBPP. Ultrasound estimates of birth weight usually fall within 15% to 20% of actual birth weight, and the sensitivity of ultrasound in detecting birth weights more than 4,500 g is only 40%.
Therefore, ultrasound estimates of birth weight are of limited utility for contemporaneous clinical management. Furthermore, no data exist to support the claim that estimated fetal weight can be used prophylactically to reduce the incidence of NBPP.
Recurrent shoulder dystocia may be predictive of future NBPP
Whether studied alone or with NBPP, risk factors for shoulder dystocia are not reliable predictors of its occurrence. This is not the case, however, for recurrent shoulder dystocia, where the risk of neonatal brachial plexus palsy can be as high as 4.5%, compared with 1% to 2% for a first episode of shoulder dystocia.
NBPP is a rare phenomenon
The frequency of NBPP is “rare,” according to the ACOG report, which cites a rate of 1.5 cases for every 1,000 births. Favorable outcomes with complete recovery are estimated to range from 50% to 80%.3
Brachial plexus injuries are classically defined as Erb’s palsy—involving C5 and C6 nerve roots—or Klumpke’s palsy, in which there is damage to the C8 and T1 nerve roots.
Erb’s palsy is recognizable by the characteristic “waiter’s tip” position of the hand, which is caused by muscle imbalance in the shoulder and upper arm. Most NBPP injuries are Erb’s palsy, which affect 1.2 infants in every 1,000 births.
Klumpke’s palsy results in weakness of the hand and medial forearm muscles. It affects 0.05 infants in every 1,000 births. The remaining cases involve a combination of the two types of palsy.
These injuries can be temporary, resolving by 12 months after birth, or permanent. The rate of persistence of NBPP at 12 months ranges from 3% to 33%.
Can clinician maneuvers increase the likelihood of NBPP?
The ACOG report addresses the direction and angle of clinician traction at delivery. The report confirms what clinicians generally have been taught: The application of fundal pressure during a delivery in which shoulder dystocia is recognized can exacerbate shoulder impaction and can lead to an increased risk of NBPP.
Traction applied by the clinician and lateral bending of the fetal neck often are implicated as causative factors of NBPP. However, ACOG presents evidence that NBPP can occur entirely unrelated to clinician traction. The report cites studies involving both transient and persistent NBPP in fetuses delivered vaginally without evident shoulder dystocia. The same types of injury are sometimes seen in fetuses delivered by cesarean, as has been mentioned.
The report goes on to state:
Recommendations for practice
At the close of its second chapter (“Risk and predictive factors”), the ACOG report offers the same official recommendations that appear in its current practice bulletin on shoulder dystocia. It notes that there are three clinical situations in which it may be prudent to alter usual obstetric management, with an aim of reducing the risk of shoulder dystocia and NBPP:
- when fetal macrosomia is suspected, with fetal weight estimated to exceed 5,000 g in a woman without diabetes or 4,500 g in a woman with diabetes
- when the mother has a history of recognized shoulder dystocia, especially when neonatal injury was severe
- when midpelvic operative vaginal delivery is contemplated with a fetus estimated to weigh more than 4,000 g.
It is interesting to note that these recommendations are made, according to the report, “notwithstanding the unreliability of specific risk factors to predict NBPP or clinically apparent shoulder dystocia in a specific case.” The report further adds:
More to come
For ACOG’s conclusions on the pathophysiology and causation of NBPP, with a view toward formulating specific protective interventions, see Part 2 of this article, which will appear in the October 2014 issue of OBG Management.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Physician Insurers Association of America. http://www.piaa.us. Accessed August 21, 2014.
2. American College of Obstetricians and Gynecologists. Practice Bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
3. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
1. Physician Insurers Association of America. http://www.piaa.us. Accessed August 21, 2014.
2. American College of Obstetricians and Gynecologists. Practice Bulletin #40: shoulder dystocia. Obstet Gynecol. 2002;100(5 pt 1):1045–1050.
3. American College of Obstetricians and Gynecologists. Executive summary: neonatal brachial plexus palsy. Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal brachial plexus palsy. Obstet Gynecol. 2014;123(4):902–904.
Chest Pain: Tools to Improve Your In-office Evaluation
Your patient, Amy Z., age 58, was given a diagnosis of hypertension 10 years ago and since then has been maintained on hydrochlorothiazide 50 mg/d and lisinopril 10 mg/d. In the office today, she reports intermittent chest tightness and heaviness. She has no history of coronary artery disease (CAD), cerebrovascular disease, or peripheral vascular disease. She attributes her chest discomfort to emotional stress. She recently started a job after having been unemployed but still has no health insurance and is concerned about losing her house.
She denies orthopnea and resting or exertional dyspnea and says she never gets chest pain while climbing stairs. Her blood pressure is elevated at 180/110 mm Hg, but her other vital signs are normal (pulse, 70 beats/min; respiratory rate, 18 breaths/min). On physical examination, she has no venous distension in her neck and her lungs are clear. A cardiac exam reveals a regular rate and rhythm, with a normally split S1 and S2 and no murmurs, rubs, or gallops. Palpation of the chest does not reproduce her chest pain.
You are concerned that your patient’s chest pain could be from heart disease, but she wants to defer additional testing because of the cost, stating, “It’s all due to my stress.”
HOW WOULD YOU PROCEED?
Whether they go to the emergency department (ED) or to their primary care provider’s office, most patients who seek treatment for chest pain don’t have life-threatening cardiac illness. Of the 8 million patients who visit an ED for chest pain each year, only 13% are diagnosed with acute coronary syndrome (ACS).1,2 Among those seen for chest pain in a primary care office, only a minority (approximately 1.5%) have unstable heart disease.3-5 Cross-sectional studies indicate that musculoskeletal chest wall pain (or “chest wall syndrome [CWS]”) is the most common cause of chest pain in patients who seek treatment in the office, followed by gastrointestinal (GI) disease, stable heart disease, psychosocial or psychiatric conditions, pulmonary disease, and other cardiovascular conditions (see Table 1).3,6,7
When evaluating patients with chest pain in the office, the challenge is to appropriately evaluate and manage those who are at low risk for ACS, while at the same time identifying and arranging prompt transfer or referral for the minority of patients who are at high cardiac risk. This article describes how to determine which patients require emergency treatment, which tools to use to screen for ACS and other potential causes of chest pain, and how to proceed when initial evaluation and testing do not point to a diagnosis.
One the next page: Start with the "ABCs" >>
START WITH THE ABCS
When a patient presents in primary care with a chief complaint of chest pain, it’s of course critical that you quickly determine if he or she is stable by evaluating the “ABCs” (airway, breathing, and circulation). Any potentially unstable patient should be immediately transferred for emergency care.8 A patient who shows no signs of respiratory distress and whose vital signs are within a normal range is unlikely to be acutely unstable and can be further evaluated in the office.
If the patient is stable, obtain a history of the onset and evolution of the chest pain, especially its location, quality, duration, and aggravating or alleviating factors. Also ask about a personal or family history of heart disease, hypertension, diabetes, or hypercholesterolemia and about tobacco use. While any of these cardiac risk factors may increase suspicion for a cardiac cause for chest pain, the absence of such factors does not eliminate the need for a careful diagnostic evaluation.
Patients with “typical” chest pain have a higher risk for ACS. In a 2005 review of observational prospective and retrospective studies and systematic reviews, Swap et al9 corroborated the description of “typical” anginal chest pain, indicating that patients whose chest pain is exertional, radiating to one or both arms, similar to or worse than prior cardiac chest pain, or associated with nausea, vomiting, or diaphoresis are at high risk for ACS (see Table 2).9 These researchers also found that chest pain that is stabbing, pleuritic, positional, or reproducible with palpation suggests that a patient is at low risk for ACS. Pain that is not exertional or that is in a small inframammary area of the chest also suggests a low risk for ACS.9
Marburg Heart Score and other tests can help rule out ACS
As part of your initial examination, assess the patient’s overall condition and stability. Be aware, however, that an older literature review found that a physical exam is only minimally helpful in assessing ACS risk in a patient with chest pain. Findings that may increase the risk for ACS are a third heart sound (positive likelihood ratio [LR+] = 3.2), systolic blood pressure < 80 mm/Hg (LR+
= 3.1), and pulmonary crackles on auscultation (LR+ = 2.1); however, the absence of these findings does not exclude ACS.10 The most helpful sign or symptom in evaluating a patient with chest pain is chest wall tenderness on palpation, which largely rules out ACS in low-prevalence settings (eg, a primary care office).11
Bösner et al12 developed the Marburg Heart Score (MHS) to help primary care clinicians evaluate the risk for CAD in patients with chest pain (see Table 3).12,13 A subsequent validation study found that an MHS ≥ 3 had a sensitivity of 89.1% and a specificity of 63.5% for CAD.13 The test’s negative predictive value (NPV) of 97.9% means that patients with an MHS ≤ 2 are very unlikely to have CAD; however, the low positive predictive value (PPV) of only 23.3% means an MHS ≥ 3 is not particularly helpful in diagnosing CAD.12,13
Unless it is clear that your patient’s chest pain is unlikely to have a cardiac cause (eg, pain is reproducible on palpation, or an MHS ≤ 2), order an ECG. If the ECG shows ST-segment elevation in two or more contiguous leads, presumed new left bundle branch block, ischemic ST-segment depression > .5 mm (.05 mV), or dynamic T-wave inversion with pain or discomfort, the patient needs urgent referral for emergency care.8 If the ECG is nondiagnostic but the chest pain is suspicious for CAD, then further testing with cardiac biomarkers (eg, troponin I or T) is recommended to evaluate for non-ST elevation myocardial infarction. Consider chest radiography if there is evidence of respiratory disease (cough, dyspnea, or a history of pulmonary disease).
Don’t overlook chest wall syndrome, GERD, or panic disorder
There are several conditions to consider in the differential diagnosis of patients whose chest pain does not appear to have a cardiac cause
CWS is the most common cause of chest pain in primary care patients.14,15 While there are several specific types of chest wall pain—including musculoskeletal pain, parietal or intercostal pain, Tietze’s syndrome, and costochondral pain—all are manifestations of a musculoskeletal disorder and associated with tenderness of the chest wall. CWS is not life threatening, but one study found high rates of anxiety (54%-93%) among patients with moderate to severe CWS.14,15
Few trials have evaluated treatments for chest wall pain or costochondritis, though typical recommendations include NSAIDs, use of heat or cold, physical therapy, or injection of local anesthetic.16 One study found that stretching exercises might benefit patients with costochondritis.17
GI disorders. Patients with esophagitis or gastroesophageal reflux disease (GERD) often report heartburn, chronic cough, chronic laryngitis, and asthma.18 However, the sensitivity and specificity of these symptoms are too low to allow diagnosis or exclusion of GERD based on history alone.18
Acid suppression therapy can be used to test for GERD. A 2005 meta-analysis of six studies found the sensitivity and specificity of a proton-pump inhibitor (PPI) acid suppression test for the diagnosis of GERD in patients with noncardiac chest pain were 80% and 74%, respectively.19 One study demonstrated that relief of chest pain after a 14-day course of omeprazole 40 mg/d was more sensitive than endoscopy, manometry, or 24-hour esophageal pH monitoring in diagnosing GERD.20 Another study found that in patients with noncardiac chest pain and normal upper endoscopy, symptomatic relief with lansoprazole 30 mg/d for four weeks can be used to diagnose endoscopy-negative GERD.21
It is appropriate to experiment with a high-dose course of a PPI (ie, omeprazole 40 mg bid, lansoprazole30 mg/d, or esomeprazole 40 mg bid) to evaluate for GERD as the cause of chest pain in patients who20-22
• Do not initially describe typical reflux symptoms (eg, heartburn, chronic regurgitation, chronic cough, or a sore or burning throat)
• Have no history of surgery in the upper GI tract, esophagus, or thorax, and
• Have no signs or symptoms that indicate they have a serious or malignant disease (eg, weight loss, anemia, or dysphagia).
Panic disorder. Several tools have been proposed for screening for panic disorder (PD),23,24 but none have been tested in patients with chest pain. Dammen et al25 developed a three-item questionnaire to assess for PD among patients with chest pain who were referred for cardiac evaluation (see Table 4).25 A score ≥ 5 on the Dammen questionnaire had 55% sensitivity and 86% specificity for PD, with a PPV of 71% and an NPV of 76%.25 Although this instrument has not been subjected to validation studies, using it may help clarify whether further investigation for PD is warranted.
Psychotherapeutic interventions may be effective for patients whose chest pain is caused by PD. A Cochrane review of 15 randomized controlled trials of psychologic interventions for chest pain in patients with normal coronary anatomy found that cognitive-behavioral therapy, and possibly hypnotherapy, reduced patient reports of chest pain, reduced chest pain frequency, and increased the number of chest pain-free days, at least for three months.26
On the next page: When the diagnosis is unclear and case conclusion >>
WHAT TO DO WHEN THE DIAGNOSIS REMAINS UNCLEAR
When your initial evaluation and diagnostic testing yield no clear diagnosis, appropriate follow-up is vital because in the year after primary care patients first develop chest pain, they are 1.5 to 3 times more likely than the general population to be diagnosed with musculoskeletal, GI, psychological, or respiratory problems, nearly five times as likely to be diagnosed with heart failure, and nearly 15 times as likely to be diagnosed with coronary heart disease.27,28
Consider ordering exercise or chemical stress testing within three to seven days for a patient with chest pain that suggests ACS but who has normal results on ECG and biomarker testing.8 Interestingly, though, in a study of 4,181 patients in an ED chest pain unit who had two sets of normal serum troponins during a six-hour period followed by exercise or chemical stress testing, only 470 patients (11%) had abnormal stress test results and only 37 (.9%) had obstructive CAD that would have potentially benefited from revascularization.29 Thus, testing troponin levels twice in six hours is a reasonable alternative to stress testing for a primary care patient with chest pain; stress testing would be unnecessary if both troponin values were normal.
CASE OUTCOME
Based on her current chest pain symptoms, Ms. Z.’s MHS is a reassuringly low 1, so CAD is unlikely. However, she scores 5 on the Dammen panic disorder screen. Due to her financial concerns, you decide to forgo stress testing and instead draw a serum troponin now, with plans to repeat later in the afternoon at your clinic lab if the initial result is normal. You encourage her to try a high-dose PPI for two weeks to determine whether GERD may be contributing to her symptoms, and offer to help her explore counseling options to address her emotional stressors.
REFERENCES
1. Amsterdam EA, Kirk JD, Bluemke DA, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122:1756-7176.
2. Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999-2008. NCHS Data Brief. 2010;43:1-8.
3. Klinkman MS, Stevens D, Gorenflo DW. Episodes of care for chest pain: a preliminary report from MIRNET. Michigan Research Network. J Fam Pract. 1994;38:345-352.
4. McConaghy JR, Oza RS. Outpatient diagnosis of acute chest pain in adults. Am Fam Physician. 2013;87:177-82.
5. Nilsson S, Scheike M, Engblom D, et al. Chest pain and ischaemic heart disease in primary care. Br J Gen Pract. 2003;53:378-382.
6. Buntinx F, Knockaert D, Bruyninckx R, et al. Chest pain in general practice or in the hospital emergency department: is it the same? Fam Pract. 2001;18:586-589.
7. Jonsbu E, Dammen T, Morken G, et al. Cardiac and psychiatric diagnoses among patients referred for chest pain and palpitations. Scand Cardiovasc J. 2009;43:256-259.
8. O’Connor RE, Brady W, Brooks SC, et al. Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S787-S817.
9. Swap CJ, Nagurney JT. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA. 2005;294:2623-2629.
10. Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263.
11. Bruyninckx R, Aertgeerts B, Bruyninckx P, et al. Signs and symptoms in diagnosing acute myocardial infarction and acute coronary syndrome: a diagnostic meta-analysis. Br J Gen Pract. 2008;58:105-111.
12. Bösner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182:1295-1300.
13. Haasenritter J, Bösner S, Vaucher P, et al. Ruling out coronary heart disease in primary care: external validation of a clinical prediction rule. Br J Gen Pract. 2012;62:e415-e421.
14. Bösner S, Becker A, Hani MA, et al. Chest wall syndrome in primary care patients with chest pain: presentation, associated features and diagnosis. Fam Pract. 2010;27:363-369.
15. Verdon F, Burnand B, Herzig L, et al. Chest wall syndrome among primary care patients: a cohort study. BMC Fam Pract. 2007;8:51.
16. Proulx AM, Zryd TW. Costochondritis: diagnosis and treatment. Am Fam Physician. 2009;80:617-620.
17. Rovetta G, Sessarego P, Monteforte P. Stretching exercises for costochondritis pain. G Ital Med Lav Ergon. 2009;31:169-171.
18. Lacy BE, Weiser K, Chertoff J, et al. The diagnosis of gastroesophageal reflux disease. Am J Med. 2010;123:583-592.
19. Wang WH, Huang JQ, Zheng GF, et al. Is proton pump inhibitor testing an effective approach to diagnose gastroesophageal reflux disease in patients with noncardiac chest pain?: a meta-analysis. Arch Intern Med. 2005;165:1222-1228.
20. Pandak WM, Arezo S, Everett S, et al. Short course of omeprazole: a better first diagnostic approach to noncardiac chest pain than endoscopy, manometry, or 24-hour esophageal pH monitoring. J Clin Gastroenterol. 2002;35:307-314.
21. Xia HH, Lai KC, Lam SK, et al. Symptomatic response to lansoprazole predicts abnormal acid reflux in endoscopy-negative patients with non-cardiac chest pain. Aliment Pharmacol Ther. 2003;17:369-377.
22. Flook NW, Moayyedi P, Dent J, et al. Acid-suppressive therapy with esomeprazole for relief of unexplained chest pain in primary care: a randomized, double-blind, placebo-controlled trial. Am J Gastroenterol. 2013;108:56-64.
23. Stein MB, Roy-Byrne PP, McQuaid JR, et al. Development of a brief diagnostic screen for panic disorder in primary care. Psychosom Med. 1999;61:359-364.
24. Ballenger JC. Treatment of panic disorder in the general medical setting. J Psychosom Res. 1998;44:5-15.
25. Dammen T, Ekeberg O, Arnesen H, et al. The detection of panic disorder in chest pain patients. Gen Hosp Psychiatry. 1999;21:323-332.
26. Kisely SR, Campbell LA, Yelland MJ, et al. Psychological interventions for symptomatic management of non-specific chest pain in patients with normal coronary anatomy. Cochrane Database Syst Rev. 2012;
6:CD004101.
27. Ruigómez A, Rodríguez LA, Wallander MA, et al. Chest pain in general practice: incidence, comorbidity and mortality. Fam Pract. 2006;23:
167-174.
28. Ruigómez A, Massó-González EL, Johansson S, et al. Chest pain without established ischaemic heart disease in primary care patients: associated comorbidities and mortality. Br J Gen Pract. 2009;59:e78-e86.
29. Hermann LK, Newman DH, Pleasant WA, et al. Yield of routine provocative cardiac testing among patients in an emergency department-based chest pain unit. JAMA Intern Med. 2013;173:1128-1133.
Your patient, Amy Z., age 58, was given a diagnosis of hypertension 10 years ago and since then has been maintained on hydrochlorothiazide 50 mg/d and lisinopril 10 mg/d. In the office today, she reports intermittent chest tightness and heaviness. She has no history of coronary artery disease (CAD), cerebrovascular disease, or peripheral vascular disease. She attributes her chest discomfort to emotional stress. She recently started a job after having been unemployed but still has no health insurance and is concerned about losing her house.
She denies orthopnea and resting or exertional dyspnea and says she never gets chest pain while climbing stairs. Her blood pressure is elevated at 180/110 mm Hg, but her other vital signs are normal (pulse, 70 beats/min; respiratory rate, 18 breaths/min). On physical examination, she has no venous distension in her neck and her lungs are clear. A cardiac exam reveals a regular rate and rhythm, with a normally split S1 and S2 and no murmurs, rubs, or gallops. Palpation of the chest does not reproduce her chest pain.
You are concerned that your patient’s chest pain could be from heart disease, but she wants to defer additional testing because of the cost, stating, “It’s all due to my stress.”
HOW WOULD YOU PROCEED?
Whether they go to the emergency department (ED) or to their primary care provider’s office, most patients who seek treatment for chest pain don’t have life-threatening cardiac illness. Of the 8 million patients who visit an ED for chest pain each year, only 13% are diagnosed with acute coronary syndrome (ACS).1,2 Among those seen for chest pain in a primary care office, only a minority (approximately 1.5%) have unstable heart disease.3-5 Cross-sectional studies indicate that musculoskeletal chest wall pain (or “chest wall syndrome [CWS]”) is the most common cause of chest pain in patients who seek treatment in the office, followed by gastrointestinal (GI) disease, stable heart disease, psychosocial or psychiatric conditions, pulmonary disease, and other cardiovascular conditions (see Table 1).3,6,7
When evaluating patients with chest pain in the office, the challenge is to appropriately evaluate and manage those who are at low risk for ACS, while at the same time identifying and arranging prompt transfer or referral for the minority of patients who are at high cardiac risk. This article describes how to determine which patients require emergency treatment, which tools to use to screen for ACS and other potential causes of chest pain, and how to proceed when initial evaluation and testing do not point to a diagnosis.
One the next page: Start with the "ABCs" >>
START WITH THE ABCS
When a patient presents in primary care with a chief complaint of chest pain, it’s of course critical that you quickly determine if he or she is stable by evaluating the “ABCs” (airway, breathing, and circulation). Any potentially unstable patient should be immediately transferred for emergency care.8 A patient who shows no signs of respiratory distress and whose vital signs are within a normal range is unlikely to be acutely unstable and can be further evaluated in the office.
If the patient is stable, obtain a history of the onset and evolution of the chest pain, especially its location, quality, duration, and aggravating or alleviating factors. Also ask about a personal or family history of heart disease, hypertension, diabetes, or hypercholesterolemia and about tobacco use. While any of these cardiac risk factors may increase suspicion for a cardiac cause for chest pain, the absence of such factors does not eliminate the need for a careful diagnostic evaluation.
Patients with “typical” chest pain have a higher risk for ACS. In a 2005 review of observational prospective and retrospective studies and systematic reviews, Swap et al9 corroborated the description of “typical” anginal chest pain, indicating that patients whose chest pain is exertional, radiating to one or both arms, similar to or worse than prior cardiac chest pain, or associated with nausea, vomiting, or diaphoresis are at high risk for ACS (see Table 2).9 These researchers also found that chest pain that is stabbing, pleuritic, positional, or reproducible with palpation suggests that a patient is at low risk for ACS. Pain that is not exertional or that is in a small inframammary area of the chest also suggests a low risk for ACS.9
Marburg Heart Score and other tests can help rule out ACS
As part of your initial examination, assess the patient’s overall condition and stability. Be aware, however, that an older literature review found that a physical exam is only minimally helpful in assessing ACS risk in a patient with chest pain. Findings that may increase the risk for ACS are a third heart sound (positive likelihood ratio [LR+] = 3.2), systolic blood pressure < 80 mm/Hg (LR+
= 3.1), and pulmonary crackles on auscultation (LR+ = 2.1); however, the absence of these findings does not exclude ACS.10 The most helpful sign or symptom in evaluating a patient with chest pain is chest wall tenderness on palpation, which largely rules out ACS in low-prevalence settings (eg, a primary care office).11
Bösner et al12 developed the Marburg Heart Score (MHS) to help primary care clinicians evaluate the risk for CAD in patients with chest pain (see Table 3).12,13 A subsequent validation study found that an MHS ≥ 3 had a sensitivity of 89.1% and a specificity of 63.5% for CAD.13 The test’s negative predictive value (NPV) of 97.9% means that patients with an MHS ≤ 2 are very unlikely to have CAD; however, the low positive predictive value (PPV) of only 23.3% means an MHS ≥ 3 is not particularly helpful in diagnosing CAD.12,13
Unless it is clear that your patient’s chest pain is unlikely to have a cardiac cause (eg, pain is reproducible on palpation, or an MHS ≤ 2), order an ECG. If the ECG shows ST-segment elevation in two or more contiguous leads, presumed new left bundle branch block, ischemic ST-segment depression > .5 mm (.05 mV), or dynamic T-wave inversion with pain or discomfort, the patient needs urgent referral for emergency care.8 If the ECG is nondiagnostic but the chest pain is suspicious for CAD, then further testing with cardiac biomarkers (eg, troponin I or T) is recommended to evaluate for non-ST elevation myocardial infarction. Consider chest radiography if there is evidence of respiratory disease (cough, dyspnea, or a history of pulmonary disease).
Don’t overlook chest wall syndrome, GERD, or panic disorder
There are several conditions to consider in the differential diagnosis of patients whose chest pain does not appear to have a cardiac cause
CWS is the most common cause of chest pain in primary care patients.14,15 While there are several specific types of chest wall pain—including musculoskeletal pain, parietal or intercostal pain, Tietze’s syndrome, and costochondral pain—all are manifestations of a musculoskeletal disorder and associated with tenderness of the chest wall. CWS is not life threatening, but one study found high rates of anxiety (54%-93%) among patients with moderate to severe CWS.14,15
Few trials have evaluated treatments for chest wall pain or costochondritis, though typical recommendations include NSAIDs, use of heat or cold, physical therapy, or injection of local anesthetic.16 One study found that stretching exercises might benefit patients with costochondritis.17
GI disorders. Patients with esophagitis or gastroesophageal reflux disease (GERD) often report heartburn, chronic cough, chronic laryngitis, and asthma.18 However, the sensitivity and specificity of these symptoms are too low to allow diagnosis or exclusion of GERD based on history alone.18
Acid suppression therapy can be used to test for GERD. A 2005 meta-analysis of six studies found the sensitivity and specificity of a proton-pump inhibitor (PPI) acid suppression test for the diagnosis of GERD in patients with noncardiac chest pain were 80% and 74%, respectively.19 One study demonstrated that relief of chest pain after a 14-day course of omeprazole 40 mg/d was more sensitive than endoscopy, manometry, or 24-hour esophageal pH monitoring in diagnosing GERD.20 Another study found that in patients with noncardiac chest pain and normal upper endoscopy, symptomatic relief with lansoprazole 30 mg/d for four weeks can be used to diagnose endoscopy-negative GERD.21
It is appropriate to experiment with a high-dose course of a PPI (ie, omeprazole 40 mg bid, lansoprazole30 mg/d, or esomeprazole 40 mg bid) to evaluate for GERD as the cause of chest pain in patients who20-22
• Do not initially describe typical reflux symptoms (eg, heartburn, chronic regurgitation, chronic cough, or a sore or burning throat)
• Have no history of surgery in the upper GI tract, esophagus, or thorax, and
• Have no signs or symptoms that indicate they have a serious or malignant disease (eg, weight loss, anemia, or dysphagia).
Panic disorder. Several tools have been proposed for screening for panic disorder (PD),23,24 but none have been tested in patients with chest pain. Dammen et al25 developed a three-item questionnaire to assess for PD among patients with chest pain who were referred for cardiac evaluation (see Table 4).25 A score ≥ 5 on the Dammen questionnaire had 55% sensitivity and 86% specificity for PD, with a PPV of 71% and an NPV of 76%.25 Although this instrument has not been subjected to validation studies, using it may help clarify whether further investigation for PD is warranted.
Psychotherapeutic interventions may be effective for patients whose chest pain is caused by PD. A Cochrane review of 15 randomized controlled trials of psychologic interventions for chest pain in patients with normal coronary anatomy found that cognitive-behavioral therapy, and possibly hypnotherapy, reduced patient reports of chest pain, reduced chest pain frequency, and increased the number of chest pain-free days, at least for three months.26
On the next page: When the diagnosis is unclear and case conclusion >>
WHAT TO DO WHEN THE DIAGNOSIS REMAINS UNCLEAR
When your initial evaluation and diagnostic testing yield no clear diagnosis, appropriate follow-up is vital because in the year after primary care patients first develop chest pain, they are 1.5 to 3 times more likely than the general population to be diagnosed with musculoskeletal, GI, psychological, or respiratory problems, nearly five times as likely to be diagnosed with heart failure, and nearly 15 times as likely to be diagnosed with coronary heart disease.27,28
Consider ordering exercise or chemical stress testing within three to seven days for a patient with chest pain that suggests ACS but who has normal results on ECG and biomarker testing.8 Interestingly, though, in a study of 4,181 patients in an ED chest pain unit who had two sets of normal serum troponins during a six-hour period followed by exercise or chemical stress testing, only 470 patients (11%) had abnormal stress test results and only 37 (.9%) had obstructive CAD that would have potentially benefited from revascularization.29 Thus, testing troponin levels twice in six hours is a reasonable alternative to stress testing for a primary care patient with chest pain; stress testing would be unnecessary if both troponin values were normal.
CASE OUTCOME
Based on her current chest pain symptoms, Ms. Z.’s MHS is a reassuringly low 1, so CAD is unlikely. However, she scores 5 on the Dammen panic disorder screen. Due to her financial concerns, you decide to forgo stress testing and instead draw a serum troponin now, with plans to repeat later in the afternoon at your clinic lab if the initial result is normal. You encourage her to try a high-dose PPI for two weeks to determine whether GERD may be contributing to her symptoms, and offer to help her explore counseling options to address her emotional stressors.
REFERENCES
1. Amsterdam EA, Kirk JD, Bluemke DA, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122:1756-7176.
2. Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999-2008. NCHS Data Brief. 2010;43:1-8.
3. Klinkman MS, Stevens D, Gorenflo DW. Episodes of care for chest pain: a preliminary report from MIRNET. Michigan Research Network. J Fam Pract. 1994;38:345-352.
4. McConaghy JR, Oza RS. Outpatient diagnosis of acute chest pain in adults. Am Fam Physician. 2013;87:177-82.
5. Nilsson S, Scheike M, Engblom D, et al. Chest pain and ischaemic heart disease in primary care. Br J Gen Pract. 2003;53:378-382.
6. Buntinx F, Knockaert D, Bruyninckx R, et al. Chest pain in general practice or in the hospital emergency department: is it the same? Fam Pract. 2001;18:586-589.
7. Jonsbu E, Dammen T, Morken G, et al. Cardiac and psychiatric diagnoses among patients referred for chest pain and palpitations. Scand Cardiovasc J. 2009;43:256-259.
8. O’Connor RE, Brady W, Brooks SC, et al. Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S787-S817.
9. Swap CJ, Nagurney JT. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA. 2005;294:2623-2629.
10. Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263.
11. Bruyninckx R, Aertgeerts B, Bruyninckx P, et al. Signs and symptoms in diagnosing acute myocardial infarction and acute coronary syndrome: a diagnostic meta-analysis. Br J Gen Pract. 2008;58:105-111.
12. Bösner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182:1295-1300.
13. Haasenritter J, Bösner S, Vaucher P, et al. Ruling out coronary heart disease in primary care: external validation of a clinical prediction rule. Br J Gen Pract. 2012;62:e415-e421.
14. Bösner S, Becker A, Hani MA, et al. Chest wall syndrome in primary care patients with chest pain: presentation, associated features and diagnosis. Fam Pract. 2010;27:363-369.
15. Verdon F, Burnand B, Herzig L, et al. Chest wall syndrome among primary care patients: a cohort study. BMC Fam Pract. 2007;8:51.
16. Proulx AM, Zryd TW. Costochondritis: diagnosis and treatment. Am Fam Physician. 2009;80:617-620.
17. Rovetta G, Sessarego P, Monteforte P. Stretching exercises for costochondritis pain. G Ital Med Lav Ergon. 2009;31:169-171.
18. Lacy BE, Weiser K, Chertoff J, et al. The diagnosis of gastroesophageal reflux disease. Am J Med. 2010;123:583-592.
19. Wang WH, Huang JQ, Zheng GF, et al. Is proton pump inhibitor testing an effective approach to diagnose gastroesophageal reflux disease in patients with noncardiac chest pain?: a meta-analysis. Arch Intern Med. 2005;165:1222-1228.
20. Pandak WM, Arezo S, Everett S, et al. Short course of omeprazole: a better first diagnostic approach to noncardiac chest pain than endoscopy, manometry, or 24-hour esophageal pH monitoring. J Clin Gastroenterol. 2002;35:307-314.
21. Xia HH, Lai KC, Lam SK, et al. Symptomatic response to lansoprazole predicts abnormal acid reflux in endoscopy-negative patients with non-cardiac chest pain. Aliment Pharmacol Ther. 2003;17:369-377.
22. Flook NW, Moayyedi P, Dent J, et al. Acid-suppressive therapy with esomeprazole for relief of unexplained chest pain in primary care: a randomized, double-blind, placebo-controlled trial. Am J Gastroenterol. 2013;108:56-64.
23. Stein MB, Roy-Byrne PP, McQuaid JR, et al. Development of a brief diagnostic screen for panic disorder in primary care. Psychosom Med. 1999;61:359-364.
24. Ballenger JC. Treatment of panic disorder in the general medical setting. J Psychosom Res. 1998;44:5-15.
25. Dammen T, Ekeberg O, Arnesen H, et al. The detection of panic disorder in chest pain patients. Gen Hosp Psychiatry. 1999;21:323-332.
26. Kisely SR, Campbell LA, Yelland MJ, et al. Psychological interventions for symptomatic management of non-specific chest pain in patients with normal coronary anatomy. Cochrane Database Syst Rev. 2012;
6:CD004101.
27. Ruigómez A, Rodríguez LA, Wallander MA, et al. Chest pain in general practice: incidence, comorbidity and mortality. Fam Pract. 2006;23:
167-174.
28. Ruigómez A, Massó-González EL, Johansson S, et al. Chest pain without established ischaemic heart disease in primary care patients: associated comorbidities and mortality. Br J Gen Pract. 2009;59:e78-e86.
29. Hermann LK, Newman DH, Pleasant WA, et al. Yield of routine provocative cardiac testing among patients in an emergency department-based chest pain unit. JAMA Intern Med. 2013;173:1128-1133.
Your patient, Amy Z., age 58, was given a diagnosis of hypertension 10 years ago and since then has been maintained on hydrochlorothiazide 50 mg/d and lisinopril 10 mg/d. In the office today, she reports intermittent chest tightness and heaviness. She has no history of coronary artery disease (CAD), cerebrovascular disease, or peripheral vascular disease. She attributes her chest discomfort to emotional stress. She recently started a job after having been unemployed but still has no health insurance and is concerned about losing her house.
She denies orthopnea and resting or exertional dyspnea and says she never gets chest pain while climbing stairs. Her blood pressure is elevated at 180/110 mm Hg, but her other vital signs are normal (pulse, 70 beats/min; respiratory rate, 18 breaths/min). On physical examination, she has no venous distension in her neck and her lungs are clear. A cardiac exam reveals a regular rate and rhythm, with a normally split S1 and S2 and no murmurs, rubs, or gallops. Palpation of the chest does not reproduce her chest pain.
You are concerned that your patient’s chest pain could be from heart disease, but she wants to defer additional testing because of the cost, stating, “It’s all due to my stress.”
HOW WOULD YOU PROCEED?
Whether they go to the emergency department (ED) or to their primary care provider’s office, most patients who seek treatment for chest pain don’t have life-threatening cardiac illness. Of the 8 million patients who visit an ED for chest pain each year, only 13% are diagnosed with acute coronary syndrome (ACS).1,2 Among those seen for chest pain in a primary care office, only a minority (approximately 1.5%) have unstable heart disease.3-5 Cross-sectional studies indicate that musculoskeletal chest wall pain (or “chest wall syndrome [CWS]”) is the most common cause of chest pain in patients who seek treatment in the office, followed by gastrointestinal (GI) disease, stable heart disease, psychosocial or psychiatric conditions, pulmonary disease, and other cardiovascular conditions (see Table 1).3,6,7
When evaluating patients with chest pain in the office, the challenge is to appropriately evaluate and manage those who are at low risk for ACS, while at the same time identifying and arranging prompt transfer or referral for the minority of patients who are at high cardiac risk. This article describes how to determine which patients require emergency treatment, which tools to use to screen for ACS and other potential causes of chest pain, and how to proceed when initial evaluation and testing do not point to a diagnosis.
One the next page: Start with the "ABCs" >>
START WITH THE ABCS
When a patient presents in primary care with a chief complaint of chest pain, it’s of course critical that you quickly determine if he or she is stable by evaluating the “ABCs” (airway, breathing, and circulation). Any potentially unstable patient should be immediately transferred for emergency care.8 A patient who shows no signs of respiratory distress and whose vital signs are within a normal range is unlikely to be acutely unstable and can be further evaluated in the office.
If the patient is stable, obtain a history of the onset and evolution of the chest pain, especially its location, quality, duration, and aggravating or alleviating factors. Also ask about a personal or family history of heart disease, hypertension, diabetes, or hypercholesterolemia and about tobacco use. While any of these cardiac risk factors may increase suspicion for a cardiac cause for chest pain, the absence of such factors does not eliminate the need for a careful diagnostic evaluation.
Patients with “typical” chest pain have a higher risk for ACS. In a 2005 review of observational prospective and retrospective studies and systematic reviews, Swap et al9 corroborated the description of “typical” anginal chest pain, indicating that patients whose chest pain is exertional, radiating to one or both arms, similar to or worse than prior cardiac chest pain, or associated with nausea, vomiting, or diaphoresis are at high risk for ACS (see Table 2).9 These researchers also found that chest pain that is stabbing, pleuritic, positional, or reproducible with palpation suggests that a patient is at low risk for ACS. Pain that is not exertional or that is in a small inframammary area of the chest also suggests a low risk for ACS.9
Marburg Heart Score and other tests can help rule out ACS
As part of your initial examination, assess the patient’s overall condition and stability. Be aware, however, that an older literature review found that a physical exam is only minimally helpful in assessing ACS risk in a patient with chest pain. Findings that may increase the risk for ACS are a third heart sound (positive likelihood ratio [LR+] = 3.2), systolic blood pressure < 80 mm/Hg (LR+
= 3.1), and pulmonary crackles on auscultation (LR+ = 2.1); however, the absence of these findings does not exclude ACS.10 The most helpful sign or symptom in evaluating a patient with chest pain is chest wall tenderness on palpation, which largely rules out ACS in low-prevalence settings (eg, a primary care office).11
Bösner et al12 developed the Marburg Heart Score (MHS) to help primary care clinicians evaluate the risk for CAD in patients with chest pain (see Table 3).12,13 A subsequent validation study found that an MHS ≥ 3 had a sensitivity of 89.1% and a specificity of 63.5% for CAD.13 The test’s negative predictive value (NPV) of 97.9% means that patients with an MHS ≤ 2 are very unlikely to have CAD; however, the low positive predictive value (PPV) of only 23.3% means an MHS ≥ 3 is not particularly helpful in diagnosing CAD.12,13
Unless it is clear that your patient’s chest pain is unlikely to have a cardiac cause (eg, pain is reproducible on palpation, or an MHS ≤ 2), order an ECG. If the ECG shows ST-segment elevation in two or more contiguous leads, presumed new left bundle branch block, ischemic ST-segment depression > .5 mm (.05 mV), or dynamic T-wave inversion with pain or discomfort, the patient needs urgent referral for emergency care.8 If the ECG is nondiagnostic but the chest pain is suspicious for CAD, then further testing with cardiac biomarkers (eg, troponin I or T) is recommended to evaluate for non-ST elevation myocardial infarction. Consider chest radiography if there is evidence of respiratory disease (cough, dyspnea, or a history of pulmonary disease).
Don’t overlook chest wall syndrome, GERD, or panic disorder
There are several conditions to consider in the differential diagnosis of patients whose chest pain does not appear to have a cardiac cause
CWS is the most common cause of chest pain in primary care patients.14,15 While there are several specific types of chest wall pain—including musculoskeletal pain, parietal or intercostal pain, Tietze’s syndrome, and costochondral pain—all are manifestations of a musculoskeletal disorder and associated with tenderness of the chest wall. CWS is not life threatening, but one study found high rates of anxiety (54%-93%) among patients with moderate to severe CWS.14,15
Few trials have evaluated treatments for chest wall pain or costochondritis, though typical recommendations include NSAIDs, use of heat or cold, physical therapy, or injection of local anesthetic.16 One study found that stretching exercises might benefit patients with costochondritis.17
GI disorders. Patients with esophagitis or gastroesophageal reflux disease (GERD) often report heartburn, chronic cough, chronic laryngitis, and asthma.18 However, the sensitivity and specificity of these symptoms are too low to allow diagnosis or exclusion of GERD based on history alone.18
Acid suppression therapy can be used to test for GERD. A 2005 meta-analysis of six studies found the sensitivity and specificity of a proton-pump inhibitor (PPI) acid suppression test for the diagnosis of GERD in patients with noncardiac chest pain were 80% and 74%, respectively.19 One study demonstrated that relief of chest pain after a 14-day course of omeprazole 40 mg/d was more sensitive than endoscopy, manometry, or 24-hour esophageal pH monitoring in diagnosing GERD.20 Another study found that in patients with noncardiac chest pain and normal upper endoscopy, symptomatic relief with lansoprazole 30 mg/d for four weeks can be used to diagnose endoscopy-negative GERD.21
It is appropriate to experiment with a high-dose course of a PPI (ie, omeprazole 40 mg bid, lansoprazole30 mg/d, or esomeprazole 40 mg bid) to evaluate for GERD as the cause of chest pain in patients who20-22
• Do not initially describe typical reflux symptoms (eg, heartburn, chronic regurgitation, chronic cough, or a sore or burning throat)
• Have no history of surgery in the upper GI tract, esophagus, or thorax, and
• Have no signs or symptoms that indicate they have a serious or malignant disease (eg, weight loss, anemia, or dysphagia).
Panic disorder. Several tools have been proposed for screening for panic disorder (PD),23,24 but none have been tested in patients with chest pain. Dammen et al25 developed a three-item questionnaire to assess for PD among patients with chest pain who were referred for cardiac evaluation (see Table 4).25 A score ≥ 5 on the Dammen questionnaire had 55% sensitivity and 86% specificity for PD, with a PPV of 71% and an NPV of 76%.25 Although this instrument has not been subjected to validation studies, using it may help clarify whether further investigation for PD is warranted.
Psychotherapeutic interventions may be effective for patients whose chest pain is caused by PD. A Cochrane review of 15 randomized controlled trials of psychologic interventions for chest pain in patients with normal coronary anatomy found that cognitive-behavioral therapy, and possibly hypnotherapy, reduced patient reports of chest pain, reduced chest pain frequency, and increased the number of chest pain-free days, at least for three months.26
On the next page: When the diagnosis is unclear and case conclusion >>
WHAT TO DO WHEN THE DIAGNOSIS REMAINS UNCLEAR
When your initial evaluation and diagnostic testing yield no clear diagnosis, appropriate follow-up is vital because in the year after primary care patients first develop chest pain, they are 1.5 to 3 times more likely than the general population to be diagnosed with musculoskeletal, GI, psychological, or respiratory problems, nearly five times as likely to be diagnosed with heart failure, and nearly 15 times as likely to be diagnosed with coronary heart disease.27,28
Consider ordering exercise or chemical stress testing within three to seven days for a patient with chest pain that suggests ACS but who has normal results on ECG and biomarker testing.8 Interestingly, though, in a study of 4,181 patients in an ED chest pain unit who had two sets of normal serum troponins during a six-hour period followed by exercise or chemical stress testing, only 470 patients (11%) had abnormal stress test results and only 37 (.9%) had obstructive CAD that would have potentially benefited from revascularization.29 Thus, testing troponin levels twice in six hours is a reasonable alternative to stress testing for a primary care patient with chest pain; stress testing would be unnecessary if both troponin values were normal.
CASE OUTCOME
Based on her current chest pain symptoms, Ms. Z.’s MHS is a reassuringly low 1, so CAD is unlikely. However, she scores 5 on the Dammen panic disorder screen. Due to her financial concerns, you decide to forgo stress testing and instead draw a serum troponin now, with plans to repeat later in the afternoon at your clinic lab if the initial result is normal. You encourage her to try a high-dose PPI for two weeks to determine whether GERD may be contributing to her symptoms, and offer to help her explore counseling options to address her emotional stressors.
REFERENCES
1. Amsterdam EA, Kirk JD, Bluemke DA, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122:1756-7176.
2. Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999-2008. NCHS Data Brief. 2010;43:1-8.
3. Klinkman MS, Stevens D, Gorenflo DW. Episodes of care for chest pain: a preliminary report from MIRNET. Michigan Research Network. J Fam Pract. 1994;38:345-352.
4. McConaghy JR, Oza RS. Outpatient diagnosis of acute chest pain in adults. Am Fam Physician. 2013;87:177-82.
5. Nilsson S, Scheike M, Engblom D, et al. Chest pain and ischaemic heart disease in primary care. Br J Gen Pract. 2003;53:378-382.
6. Buntinx F, Knockaert D, Bruyninckx R, et al. Chest pain in general practice or in the hospital emergency department: is it the same? Fam Pract. 2001;18:586-589.
7. Jonsbu E, Dammen T, Morken G, et al. Cardiac and psychiatric diagnoses among patients referred for chest pain and palpitations. Scand Cardiovasc J. 2009;43:256-259.
8. O’Connor RE, Brady W, Brooks SC, et al. Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S787-S817.
9. Swap CJ, Nagurney JT. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA. 2005;294:2623-2629.
10. Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263.
11. Bruyninckx R, Aertgeerts B, Bruyninckx P, et al. Signs and symptoms in diagnosing acute myocardial infarction and acute coronary syndrome: a diagnostic meta-analysis. Br J Gen Pract. 2008;58:105-111.
12. Bösner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182:1295-1300.
13. Haasenritter J, Bösner S, Vaucher P, et al. Ruling out coronary heart disease in primary care: external validation of a clinical prediction rule. Br J Gen Pract. 2012;62:e415-e421.
14. Bösner S, Becker A, Hani MA, et al. Chest wall syndrome in primary care patients with chest pain: presentation, associated features and diagnosis. Fam Pract. 2010;27:363-369.
15. Verdon F, Burnand B, Herzig L, et al. Chest wall syndrome among primary care patients: a cohort study. BMC Fam Pract. 2007;8:51.
16. Proulx AM, Zryd TW. Costochondritis: diagnosis and treatment. Am Fam Physician. 2009;80:617-620.
17. Rovetta G, Sessarego P, Monteforte P. Stretching exercises for costochondritis pain. G Ital Med Lav Ergon. 2009;31:169-171.
18. Lacy BE, Weiser K, Chertoff J, et al. The diagnosis of gastroesophageal reflux disease. Am J Med. 2010;123:583-592.
19. Wang WH, Huang JQ, Zheng GF, et al. Is proton pump inhibitor testing an effective approach to diagnose gastroesophageal reflux disease in patients with noncardiac chest pain?: a meta-analysis. Arch Intern Med. 2005;165:1222-1228.
20. Pandak WM, Arezo S, Everett S, et al. Short course of omeprazole: a better first diagnostic approach to noncardiac chest pain than endoscopy, manometry, or 24-hour esophageal pH monitoring. J Clin Gastroenterol. 2002;35:307-314.
21. Xia HH, Lai KC, Lam SK, et al. Symptomatic response to lansoprazole predicts abnormal acid reflux in endoscopy-negative patients with non-cardiac chest pain. Aliment Pharmacol Ther. 2003;17:369-377.
22. Flook NW, Moayyedi P, Dent J, et al. Acid-suppressive therapy with esomeprazole for relief of unexplained chest pain in primary care: a randomized, double-blind, placebo-controlled trial. Am J Gastroenterol. 2013;108:56-64.
23. Stein MB, Roy-Byrne PP, McQuaid JR, et al. Development of a brief diagnostic screen for panic disorder in primary care. Psychosom Med. 1999;61:359-364.
24. Ballenger JC. Treatment of panic disorder in the general medical setting. J Psychosom Res. 1998;44:5-15.
25. Dammen T, Ekeberg O, Arnesen H, et al. The detection of panic disorder in chest pain patients. Gen Hosp Psychiatry. 1999;21:323-332.
26. Kisely SR, Campbell LA, Yelland MJ, et al. Psychological interventions for symptomatic management of non-specific chest pain in patients with normal coronary anatomy. Cochrane Database Syst Rev. 2012;
6:CD004101.
27. Ruigómez A, Rodríguez LA, Wallander MA, et al. Chest pain in general practice: incidence, comorbidity and mortality. Fam Pract. 2006;23:
167-174.
28. Ruigómez A, Massó-González EL, Johansson S, et al. Chest pain without established ischaemic heart disease in primary care patients: associated comorbidities and mortality. Br J Gen Pract. 2009;59:e78-e86.
29. Hermann LK, Newman DH, Pleasant WA, et al. Yield of routine provocative cardiac testing among patients in an emergency department-based chest pain unit. JAMA Intern Med. 2013;173:1128-1133.
When Patients Ask for Antibiotics, Arm Them With Handouts
Drug store and supermarket shelves display aisle after aisle of OTC medications that alleviate the common symptoms of upper respiratory infections. Despite the easy availability of symptom relief, a significant number of people consult clinicians in primary care offices, emergency departments (EDs), and walk-in or convenient care clinics for help when they feel that they’re “coming down with something.” During these visits, many of these patients expect, and sometimes demand, antibiotics.
Antibiotics may be viewed by the patient as a quick fix, with the demand undoubtedly fueled by busy lifestyles, long work hours, and little time for patients to stay home while ill. Patients so inclined may “doctor shop” if their demands are not met; clinicians know better but may feel pressure to “satisfy the customer” and may rationalize that an antibiotic might prove helpful in a particular case.
Lee et al studied outpatient antibiotic prescribing in the United States for acute respiratory tract infections (ARTI), including acute nasopharyngitis, upper respiratory tract infection, bronchitis, influenza, pharyngitis, and sinusitis. In 2000, antibiotics were prescribed during outpatient visits for ARTI to 64% of patients; by 2010, that percentage had increased to 73%.1 Although antibiotics are neither effective nor appropriate for the treatment of ARTI, most of which are viral infections, they are commonly prescribed. Further, while 17% of ARTI prescriptions in 2000 were for broad-spectrum antibiotics, that percentage jumped to 46% in 2010.1,2
Patient insistence on antibiotics may stem either from little knowledge of or little regard for the health problems caused by unnecessary antibiotic use. For example, one study found that 19.3% of drug-related ED visits were related to systemic antibiotics; nearly 80% of those were for allergic reactions.3,4 With an estimated 50% of antibiotic prescriptions considered inappropriate, the overuse of antibiotics creates unnecessary personal health risks and health care expenditures. Further, a more serious consequence of this overuse is the growing public health problem of antibiotic resistance (see Figure 1).1,2
Clinicians are ideally positioned to address these issues by incorporating effective, proactive strategies into selected patient encounters to specifically explain appropriate versus inappropriate antibiotic use.
Continue for patient handouts >>
One approach is to merge accurate, powerful messages about antibiotics with helpful information about effective OTC products to both enlighten patients and offer them the symptomatic relief they seek. The CDC has taken the lead in this area with its “Get Smart: Know When Antibiotics Work” initiative, which includes a variety of materials for both health care providers and patients.5
Inspired by the unmet need for written communications that explain viral and bacterial illnesses in simple terms and the reasons why taking antibiotics for the former is a bad idea, we developed two handouts for adult patients who present to the clinician’s office with viral respiratory illnesses.
The first is entitled “Prescription for Recovery From Your Viral Respiratory Illness” [download PDF]. Intended to be duplicated and designed with primary care use in mind, it can be customized for specialty use as well.
This “prescription” handout addresses common complaints of fever, pain (eg, sore throat, body aches, headache), cough, congestion (chest, nose, sinuses), and sneezing/runny nose. Clinicians can check off the appropriate treatments for an individual patient, who can use it as a handy reference to purchase the recommended OTC products and/or for selecting products or preparing helpful remedies at home. Blank lines at the bottom provide space for you to write in your OTC preferences and allow you to customize patient instructions.
The second patient handout is entitled “Antibiotics: When You Need Them, When You Don’t, and What to Take When You Don’t” [download PDF]. This focused patient teaching tool offers an overview of
• Viral and bacterial respiratory illnesses and what the patient should do if he or she has one or the other (including when to see a clinician)
• Some helpful OTC and home treatments for symptoms of viral respiratory illnesses
• When antibiotics are indicated and when they’re not—and why
• The serious problems caused by unnecessary use of antibiotics.
This brief guide can be used as a general informational handout that can, for example, be given, mailed, or e-mailed to all adult patients at the start of cold and flu season and retained by them for reference. It can also be provided along with the “prescription” handout to patients with ARTIs who visit your office. In the latter situation, the patient leaves your office with concrete information and guidance for appropriate care of his or her viral respiratory illness—but without a prescription for antibiotics. As with the “prescription” handout, this brief guide may be duplicated or customized as needed.
To download the handouts: Click here.
REFERENCES
1. Lee GC, Reveles KR, Attridge RT, et al. Outpatient antibiotic prescribing in the United States: 2000-2010. BMC Med. 2014;12:96.
2. CDC. Antibiotics: will they work when you really need them? www.cdc.gov/getsmart/healthcare/factsheets/antibiotics.html#MustAct. Accessed August 14, 2014.
3. Shehab N, Patel P, Srinivasan A, Budnitz D. Emergency department visits for antibiotic associated adverse events. Clin Infect Dis. 2008;47:735-743.
4. CDC. Adverse drug events from select medication classes. www.cdc.gov/MedicationSafety/program_focus_activities.html. Accessed August 4, 2014.
5. CDC. Get smart: know when antibiotics work. www.cdc.gov/getsmart/. Accessed August 14, 2014.
Drug store and supermarket shelves display aisle after aisle of OTC medications that alleviate the common symptoms of upper respiratory infections. Despite the easy availability of symptom relief, a significant number of people consult clinicians in primary care offices, emergency departments (EDs), and walk-in or convenient care clinics for help when they feel that they’re “coming down with something.” During these visits, many of these patients expect, and sometimes demand, antibiotics.
Antibiotics may be viewed by the patient as a quick fix, with the demand undoubtedly fueled by busy lifestyles, long work hours, and little time for patients to stay home while ill. Patients so inclined may “doctor shop” if their demands are not met; clinicians know better but may feel pressure to “satisfy the customer” and may rationalize that an antibiotic might prove helpful in a particular case.
Lee et al studied outpatient antibiotic prescribing in the United States for acute respiratory tract infections (ARTI), including acute nasopharyngitis, upper respiratory tract infection, bronchitis, influenza, pharyngitis, and sinusitis. In 2000, antibiotics were prescribed during outpatient visits for ARTI to 64% of patients; by 2010, that percentage had increased to 73%.1 Although antibiotics are neither effective nor appropriate for the treatment of ARTI, most of which are viral infections, they are commonly prescribed. Further, while 17% of ARTI prescriptions in 2000 were for broad-spectrum antibiotics, that percentage jumped to 46% in 2010.1,2
Patient insistence on antibiotics may stem either from little knowledge of or little regard for the health problems caused by unnecessary antibiotic use. For example, one study found that 19.3% of drug-related ED visits were related to systemic antibiotics; nearly 80% of those were for allergic reactions.3,4 With an estimated 50% of antibiotic prescriptions considered inappropriate, the overuse of antibiotics creates unnecessary personal health risks and health care expenditures. Further, a more serious consequence of this overuse is the growing public health problem of antibiotic resistance (see Figure 1).1,2
Clinicians are ideally positioned to address these issues by incorporating effective, proactive strategies into selected patient encounters to specifically explain appropriate versus inappropriate antibiotic use.
Continue for patient handouts >>
One approach is to merge accurate, powerful messages about antibiotics with helpful information about effective OTC products to both enlighten patients and offer them the symptomatic relief they seek. The CDC has taken the lead in this area with its “Get Smart: Know When Antibiotics Work” initiative, which includes a variety of materials for both health care providers and patients.5
Inspired by the unmet need for written communications that explain viral and bacterial illnesses in simple terms and the reasons why taking antibiotics for the former is a bad idea, we developed two handouts for adult patients who present to the clinician’s office with viral respiratory illnesses.
The first is entitled “Prescription for Recovery From Your Viral Respiratory Illness” [download PDF]. Intended to be duplicated and designed with primary care use in mind, it can be customized for specialty use as well.
This “prescription” handout addresses common complaints of fever, pain (eg, sore throat, body aches, headache), cough, congestion (chest, nose, sinuses), and sneezing/runny nose. Clinicians can check off the appropriate treatments for an individual patient, who can use it as a handy reference to purchase the recommended OTC products and/or for selecting products or preparing helpful remedies at home. Blank lines at the bottom provide space for you to write in your OTC preferences and allow you to customize patient instructions.
The second patient handout is entitled “Antibiotics: When You Need Them, When You Don’t, and What to Take When You Don’t” [download PDF]. This focused patient teaching tool offers an overview of
• Viral and bacterial respiratory illnesses and what the patient should do if he or she has one or the other (including when to see a clinician)
• Some helpful OTC and home treatments for symptoms of viral respiratory illnesses
• When antibiotics are indicated and when they’re not—and why
• The serious problems caused by unnecessary use of antibiotics.
This brief guide can be used as a general informational handout that can, for example, be given, mailed, or e-mailed to all adult patients at the start of cold and flu season and retained by them for reference. It can also be provided along with the “prescription” handout to patients with ARTIs who visit your office. In the latter situation, the patient leaves your office with concrete information and guidance for appropriate care of his or her viral respiratory illness—but without a prescription for antibiotics. As with the “prescription” handout, this brief guide may be duplicated or customized as needed.
To download the handouts: Click here.
REFERENCES
1. Lee GC, Reveles KR, Attridge RT, et al. Outpatient antibiotic prescribing in the United States: 2000-2010. BMC Med. 2014;12:96.
2. CDC. Antibiotics: will they work when you really need them? www.cdc.gov/getsmart/healthcare/factsheets/antibiotics.html#MustAct. Accessed August 14, 2014.
3. Shehab N, Patel P, Srinivasan A, Budnitz D. Emergency department visits for antibiotic associated adverse events. Clin Infect Dis. 2008;47:735-743.
4. CDC. Adverse drug events from select medication classes. www.cdc.gov/MedicationSafety/program_focus_activities.html. Accessed August 4, 2014.
5. CDC. Get smart: know when antibiotics work. www.cdc.gov/getsmart/. Accessed August 14, 2014.
Drug store and supermarket shelves display aisle after aisle of OTC medications that alleviate the common symptoms of upper respiratory infections. Despite the easy availability of symptom relief, a significant number of people consult clinicians in primary care offices, emergency departments (EDs), and walk-in or convenient care clinics for help when they feel that they’re “coming down with something.” During these visits, many of these patients expect, and sometimes demand, antibiotics.
Antibiotics may be viewed by the patient as a quick fix, with the demand undoubtedly fueled by busy lifestyles, long work hours, and little time for patients to stay home while ill. Patients so inclined may “doctor shop” if their demands are not met; clinicians know better but may feel pressure to “satisfy the customer” and may rationalize that an antibiotic might prove helpful in a particular case.
Lee et al studied outpatient antibiotic prescribing in the United States for acute respiratory tract infections (ARTI), including acute nasopharyngitis, upper respiratory tract infection, bronchitis, influenza, pharyngitis, and sinusitis. In 2000, antibiotics were prescribed during outpatient visits for ARTI to 64% of patients; by 2010, that percentage had increased to 73%.1 Although antibiotics are neither effective nor appropriate for the treatment of ARTI, most of which are viral infections, they are commonly prescribed. Further, while 17% of ARTI prescriptions in 2000 were for broad-spectrum antibiotics, that percentage jumped to 46% in 2010.1,2
Patient insistence on antibiotics may stem either from little knowledge of or little regard for the health problems caused by unnecessary antibiotic use. For example, one study found that 19.3% of drug-related ED visits were related to systemic antibiotics; nearly 80% of those were for allergic reactions.3,4 With an estimated 50% of antibiotic prescriptions considered inappropriate, the overuse of antibiotics creates unnecessary personal health risks and health care expenditures. Further, a more serious consequence of this overuse is the growing public health problem of antibiotic resistance (see Figure 1).1,2
Clinicians are ideally positioned to address these issues by incorporating effective, proactive strategies into selected patient encounters to specifically explain appropriate versus inappropriate antibiotic use.
Continue for patient handouts >>
One approach is to merge accurate, powerful messages about antibiotics with helpful information about effective OTC products to both enlighten patients and offer them the symptomatic relief they seek. The CDC has taken the lead in this area with its “Get Smart: Know When Antibiotics Work” initiative, which includes a variety of materials for both health care providers and patients.5
Inspired by the unmet need for written communications that explain viral and bacterial illnesses in simple terms and the reasons why taking antibiotics for the former is a bad idea, we developed two handouts for adult patients who present to the clinician’s office with viral respiratory illnesses.
The first is entitled “Prescription for Recovery From Your Viral Respiratory Illness” [download PDF]. Intended to be duplicated and designed with primary care use in mind, it can be customized for specialty use as well.
This “prescription” handout addresses common complaints of fever, pain (eg, sore throat, body aches, headache), cough, congestion (chest, nose, sinuses), and sneezing/runny nose. Clinicians can check off the appropriate treatments for an individual patient, who can use it as a handy reference to purchase the recommended OTC products and/or for selecting products or preparing helpful remedies at home. Blank lines at the bottom provide space for you to write in your OTC preferences and allow you to customize patient instructions.
The second patient handout is entitled “Antibiotics: When You Need Them, When You Don’t, and What to Take When You Don’t” [download PDF]. This focused patient teaching tool offers an overview of
• Viral and bacterial respiratory illnesses and what the patient should do if he or she has one or the other (including when to see a clinician)
• Some helpful OTC and home treatments for symptoms of viral respiratory illnesses
• When antibiotics are indicated and when they’re not—and why
• The serious problems caused by unnecessary use of antibiotics.
This brief guide can be used as a general informational handout that can, for example, be given, mailed, or e-mailed to all adult patients at the start of cold and flu season and retained by them for reference. It can also be provided along with the “prescription” handout to patients with ARTIs who visit your office. In the latter situation, the patient leaves your office with concrete information and guidance for appropriate care of his or her viral respiratory illness—but without a prescription for antibiotics. As with the “prescription” handout, this brief guide may be duplicated or customized as needed.
To download the handouts: Click here.
REFERENCES
1. Lee GC, Reveles KR, Attridge RT, et al. Outpatient antibiotic prescribing in the United States: 2000-2010. BMC Med. 2014;12:96.
2. CDC. Antibiotics: will they work when you really need them? www.cdc.gov/getsmart/healthcare/factsheets/antibiotics.html#MustAct. Accessed August 14, 2014.
3. Shehab N, Patel P, Srinivasan A, Budnitz D. Emergency department visits for antibiotic associated adverse events. Clin Infect Dis. 2008;47:735-743.
4. CDC. Adverse drug events from select medication classes. www.cdc.gov/MedicationSafety/program_focus_activities.html. Accessed August 4, 2014.
5. CDC. Get smart: know when antibiotics work. www.cdc.gov/getsmart/. Accessed August 14, 2014.
Pica: An Age-old Eating Disorder That’s Often Missed
› Ask about pica behavior or unusual cravings in certain high-risk groups: pregnant women, immigrants or refugees, and children and adults with autism or other developmental disabilities. C
› Obtain serum hemoglobin and hematocrit levels along with iron levels, if necessary, in patients who report cravings for unusual substances. B
› Check serum lead levels and consider testing for ova and parasites in patients who eat dirt. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 6-year-old African girl, developing and growing appropriately for age, was brought to our clinic by her father with the chief complaint of “eating the textbooks at school.” The child had eaten paper for years, the father said; he never thought it unusual until her teacher brought it to his attention. The father reported that his daughter had met all developmental milestones and was up to date with her immunizations. When asked why she ate paper, the child responded, “I don’t know.”
The child was diagnosed with pica and, because we were concerned that she was eating other nonnutritive foods, we ordered hematologic studies. Her lead level (2 mcg/dL) was within the normal range; her hemoglobin/hematocrit was 10.4 g/dL/32.3%. Iron therapy was started. At follow-up 4 weeks later, the child’s paper-eating behavior had resolved.
The word pica comes from the Latin word for magpie, a bird with a reputation for eating practically anything. The Diagnostic and Statistical Manual of Mental Disorders, 5th edition, defines pica as persistent eating of nonnutritive substances for at least 1 month that is inappropriate to developmental level and not part of a culturally supported or socially normative practice.1
Case reports on paper pica are few, but numerous reports describe other forms of the behavior, including eating ice; dirt, soil, and clay; starch; burnt matches; cardboard; hair; laundry detergent; chalk; soap; firecrackers; and metal artifacts such as coins.2-16
Pica has been described in the literature as “underreported” and “unrecognized.” Its true prevalence is difficult to assess because most people don’t report it and the methodology of data collection varies among populations, as does the definition of pica. According to some estimates, more than 50% of children ages 18 to 36 months seek and ingest nonfood items. The practice reportedly decreases as a child ages, but an estimated 10% of children older than 12 years may engage in it.17
Pica has been reported since antiquity. Many medical and anthropological studies refer to the practice of geophagia, or dirt eating, which is prevalent in Africa and among small children and women, particularly women who are native to the southern United States, African-American, or pregnant.5-10,18,19
Pica often occurs in people with developmental disabilities such as autism and is considered a psychiatric condition in that context.3,11,15,20-31 However, because many forms of pica, especially geophagia, aren’t associated with mental health issues, researchers disagree about whether to consider it an abnormal behavior. A 2000 workshop on pica organized by the Agency for Toxic Substances and Disease Registry concluded that geophagia is not an abnormal behavior.17 One of the most compelling arguments for this view is that dirt eating is far too common around the world to be considered abnormal, and dirt is held in some cultures to have therapeutic powers.7,13,24
Adverse outcomes linked to pica
Pica is associated with adverse outcomes, however. A study by the Agency for Healthcare Research and Quality found that despite an overall decline in hospitalizations for eating disorders, hospitalizations for pica have risen.25 From 1999 to 2009, pica-related hospitalizations jumped 93%, although the overall number of patients hospitalized for the condition remains small (964 in 1999 to 2000, 1862 in 2008-2009).
Documented adverse effects of pica include potassium abnormalities and gastrointestinal conditions ranging from irritation and abdominal pain to perforation, blockage, and colon ischemia.3,11,26-29 Reported bidirectional effects (which both result from and contribute to pica) include iron deficiency, parasitic infections, and heavy metal exposure—particularly lead, mercury, and arsenic.4,6,9,20,30-38
Diagnosis: Focus on history and selective testing
Pica is a clinical diagnosis, confirmed by the patient’s history, not any single laboratory test. Providers should ask about pica behavior or unusual cravings in certain high-risk groups: pregnant women, particularly women from the southern United States, immigrants or refugees, and children and adults with autism or other developmental disabilities.18,22
Testing should be based on the type of pica behavior. Because various forms of pica are commonly associated with iron-deficiency anemia, obtain serum hemoglobin and hematocrit levels along with iron levels if necessary in patients who report cravings for unusual substances. Pica in pregnancy is a sign of iron deficiency, but it also may signal iron deficiency in patients who aren’t pregnant. In one study of 262 nonpregnant adults with iron-deficiency anemia, 45% reported pica behaviors; of these, 87.3% reported eating ice.34
Check serum lead levels in children who engage in geophagia since dirt may contain lead. Because ingestion of soil or clay is associated with soil-borne parasitic infections, also consider testing for ova and parasites if clinically indicated. Patients who eat paper may be exposed to mercury poisoning, so a serum mercury level is advisable.
Management: Prevention and behavior modification are key
Treatment for pica varies by patient and the specific behavior. Management approaches are primarily preventive, educational, and directed toward behavior modification.
Prevention. Residential facilities and primary care offices that care for people with developmental disabilities may screen for pica by means of prevalence surveys, direct observation, stool checks, review of medical history records, and interviews with caregivers.
Residential facilities can create a pica-safe environment by training staff in pica prevention, instituting regular on-site monitoring to ensure that no dangerous objects are available, and developing procedures to guide staff behavior, such as safe disposal of rubber gloves.22 Parents and caregivers of young children or children with developmental disabilities who don’t live in residential facilities should be aware of pica and monitor what their children are ingesting.
Behavior modification. Behavior-based approaches have proved effective for treating pica in developmentally disabled patients. Applied behavioral analysis “was found to have the most robust empirical support to treat this behavior.”39 Patients found to have pica may be referred for further assessment to a behavior specialist or a psychologist with experience in treating the condition.22,39
A review of 26 studies found that, in 25 studies, behavioral therapy reduced pica behavior by 80% or more.23 Behavioral treatments included reinforcement procedures alone, response reduction procedures alone, and combined reinforcement and response reduction procedures. Reinforcement shapes behavior by controlling the consequences of the behavior using a combination of rewards and punishments.23 Response reduction, or blocking, involves obstructing every attempt to eat inedible items.22
Treatments that combined reinforcement and response reduction showed good efficacy.23 An example of the combined approach would be to stop the patient from eating nonnutritive items while redirecting him to eat food instead.22
Supplementation. Iron supplementation has decreased or even reversed pica in patients whose clinical symptoms and behavior were associated with iron deficiency.35,40
Medications. Successful treatment with selective serotonin reuptake inhibitors (escitalopram), atypical neuroleptics (olanzapine), and attention-deficit/hyperactivity disorder medications (methylphenidate) has been reported in some patients, but case reports are few, and the evidence for the drugs’ efficacy is limited.41-43
Be alert for pica. Primary care physicians need to be aware of pica and proactively seek information about cravings or behaviors suggesting the condition from patients in high-risk populations—pregnant women, children, immigrants and refugees, people with developmental disabilities—or their caregivers. Once pica is identified, clinicians should undertake appropriate laboratory investigation and behavior modification attempts.
CORRESPONDENCE
Ranit Mishori, MD, MHS, Department of Family Medicine, Georgetown University School of Medicine, Pre-Clinical Building, GB-01D, 3900 Reservoir Road, NW, Washington, DC 20007; [email protected]
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
2. Yalug I, Kirmizi-Alsan E, Tufan AE. Adult-onset paper pica in the context of anorexia nervosa with major depressive disorder and a history of childhood geophagia: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1341-1342.
3. Spaniolas K, Ou S, Findeis-Hosey J, et al. Paper pica: an unusual cause of colonic ischemia. J Gastrointest Surg. 2010;14:1065-1066.
4. Olynyk F, Sharpe DH. Mercury poisoning in paper pica. N Engl J Med. 1982;306:1056-1057.
5. Guney M, Zagury GJ, Dogan N, et al. Exposure assessment and risk characterization from trace elements following soil ingestion by children exposed to playgrounds, parks and picnic areas. J Hazard Mater. 2010;182:656-664.
6. Kawai K, Saathoff E, Antelman G, et al. Geophagy (soil-eating) in relation to anemia and helminth infection among HIV-infected pregnant women in Tanzania. Am J Trop Med Hyg. 2009;80:36-43.
7. Woywodt A, Kiss A. Geophagia: the history of earth-eating. J R Soc Med. 2002;95:143-146.
8. Stokes T. The earth-eaters. Nature. 2006;444:543-544.
9. Kutalek R, Wewalka G, Gundacker C, et al. Geophagy and potential health implications: geohelminths, microbes and heavy metals. Trans R Soc Trop Med Hyg. 2010;104:787-795.
10. Keith D, Keith L, Berger GS, et al. Amylophagia during pregnancy: some maternal and perinatal correlations. Mt Sinai J Med. 1975;42:410-414.
11. Abu-Hamdan DK, Sondheimer JH, Mahajan SK. Cautopyreiophagia. Cause of life-threatening hyperkalemia in a patient undergoing hemodialysis. Am J Med. 1985;79:517-519.
12. Ewert P, Keim L, Schulte-Markwort M. Trichobezoar. A rare cause of recurrent upper abdominal pain [in German]. Monatsschr Kinderheilkd. 1992;140:811-813.
13. Grigsby RK, Thyer BA, Waller RJ, et al. Chalk eating in middle Georgia: a culture-bound syndrome of pica? South Med J. 1999;92:190-192.
14. Ahishali E, Boynueğrı B, Dabak R, et al. A case of severe acute hepatitis due to oral intake of firecrackers. Turk J Gastroenterol. 2010;21:325-326.
15. Rashid F, Davies L, Iftikhar SY. Magnetised intragastric foreign body collection and autism: An advice for careers and literature review. Autism. 2010;14:139-145.
16. Martindale JL, Bunker CJ, Noble VE. Ingested foreign bodies in a patient with pica. Gastroenterol Hepatol (N Y). 2010;6:582-584.
17. Agency for Toxic Substances and Disease Registry. Summary Report for the ATSDR Soil-Pica Workshop June 2000, Atlanta, Georgia. Agency for Toxic Substances and Disease Registry Web site. Available at: www.atsdr.cdc.gov/child/soilpica.html. Accessed June 2, 2012.
18. Njiru H, Elchalal U, Paltiel O. Geophagy during pregnancy in Africa: a literature review. Obstet Gynecol Surv. 2011;66:452-459.
19. Young SL. Pica in pregnancy: new ideas about an old condition. Annu Rev Nutr. 2010;30:403-422.
20. Clark B, Vandermeer B, Simonetti A, et al. Is lead a concern in Canadian autistic children? Paediatr Child Health. 2010;15:17-22.
21. Matson JL, Sipes M, Fodstad JC, et al. Issues in the management of challenging behaviours of adults with autism spectrum disorder. CNS Drugs. 2011;25:597-606.
22. Williams DE, McAdam D. Assessment, behavioral treatment, and prevention of pica: clinical guidelines and recommendations for practitioners. Res Dev Disabil. 2012;33:2050-2057.
23. Hagopian LP, Rooker GW, Rolider NU. Identifying empirically supported treatments for pica in individuals with intellectual disabilities. Res Dev Disabil. 2011;32:2114-2120.
24. Engberg DE. Geophagy: adaptive or aberrant behavior. Nebraska Anthropologist. 1995;12:57-68.
25. Agency for Healthcare Research and Quality. Hospitalizations for eating disorder decline, but big increase seen in pica disorder. Agency for Healthcare Research and Quality Web site. Available at: www.ahrq.gov/news/nn/nn090811.htm. Accessed June 2, 2014.
26. Stroman D, Young C, Rubano AR, et al. Adult-onset pica leading to acute intestinal obstruction. Psychosomatics. 2011;52:393-394.
27. Young SL, Khalfan SS, Farag TH, et al. Association of pica with anemia and gastrointestinal distress among pregnant women in Zanzibar, Tanzania. Am J Trop Med Hyg. 2010;83:144-151.
28. Altepeter T, Annes J, Meller J. Foam bezoar: resection of perforated terminal ileum in a 17-year-old with sickle b+thalassemia and pica. J Pediatr Surg. 2011;46:E31-E32.
29. Chatzimavroudis G, Christopoulos P, Atmatzidis S, et al. Pica: an uncommon cause of acute abdominal pain in children. Indian J Pediatr. 2011;78:886-887.
30. Rector WG Jr. Pica: its frequency and significance in patients with iron-deficiency anemia due to chronic gastrointestinal blood loss. J Gen Intern Med. 1989;4:512-513.
31. Sontag C, Kettaneh A, Fain O, et al. Rapid regression of prolonged pagophagia after treatment of iron deficiency [in French]. Presse Med. 2001;30:321-323.
32. Sharma TR, Kavuru B, Aly M. Coprophagia and pica in individuals with mild to moderate dementia and mixed (iron deficiency and macrocytic) anemia. J Am Geriatr Soc. 2011;59:2375-2377.
33. Kushner RF, Shanta Retelny V. Emergence of pica (ingestion of non-food substances) accompanying iron deficiency anemia after gastric bypass surgery. Obes Surg. 2005;15:1491-1495.
34. Barton JC, Barton JC, Bertoli LF. Pica associated with iron deficiency or depletion: clinical and laboratory correlates in 262 nonpregnant adult outpatients. BMC Blood Disord. 2010;10:9.
35. Khan Y, Tisman G. Pica in iron deficiency: a case series. J Med Case Rep. 2010;4:86.
36. Bakhireva LN, Rowland AS, Young BN, et al. Sources of potential lead exposure among pregnant women in New Mexico. Matern Child Health J. 2013;17:172-179.
37. Thihalolipavan S, Candalla BM, Ehrlich J. Examining pica in NYC pregnant women with elevated blood lead levels. Matern Child Health J. 2013;17:49-55.
38. Al-Rmalli SW, Jenkins RO, Watts MJ, et al. Risk of human exposure to arsenic and other toxic elements from geophagy: trace element analysis of baked clay using inductively coupled plasma mass spectrometry. Environ Health. 2010;9:79.
39. Matson JL, Hattier MA, Belva B, et al. Pica in persons with developmental disabilities: approaches to treatment. Res Dev Disabil. 2013;34:2564-2571.
40. Bryant BJ1, Yau YY, Arceo SM, et al. Ascertainment of iron deficiency and depletion in blood donors through screening questions for pica and restless legs syndrome. Transfusion. 2013;53:1637-1644.
41. Lerner AJ. Treatment of pica behavior with olanzapine. CNS Spectr. 2008;13:19.
42. Hergüner S, Hergüner AS. Pica in a child with attention deficit hyperactivity disorder and successful treatment with methylphenidate. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1155-1156.
43. Bhatia MS, Gupta R. Pica responding to SSRI: an OCD Spectrum Disorder? World J Biol Psychiatry. 2009;10(4 pt 3):936-938.
› Ask about pica behavior or unusual cravings in certain high-risk groups: pregnant women, immigrants or refugees, and children and adults with autism or other developmental disabilities. C
› Obtain serum hemoglobin and hematocrit levels along with iron levels, if necessary, in patients who report cravings for unusual substances. B
› Check serum lead levels and consider testing for ova and parasites in patients who eat dirt. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 6-year-old African girl, developing and growing appropriately for age, was brought to our clinic by her father with the chief complaint of “eating the textbooks at school.” The child had eaten paper for years, the father said; he never thought it unusual until her teacher brought it to his attention. The father reported that his daughter had met all developmental milestones and was up to date with her immunizations. When asked why she ate paper, the child responded, “I don’t know.”
The child was diagnosed with pica and, because we were concerned that she was eating other nonnutritive foods, we ordered hematologic studies. Her lead level (2 mcg/dL) was within the normal range; her hemoglobin/hematocrit was 10.4 g/dL/32.3%. Iron therapy was started. At follow-up 4 weeks later, the child’s paper-eating behavior had resolved.
The word pica comes from the Latin word for magpie, a bird with a reputation for eating practically anything. The Diagnostic and Statistical Manual of Mental Disorders, 5th edition, defines pica as persistent eating of nonnutritive substances for at least 1 month that is inappropriate to developmental level and not part of a culturally supported or socially normative practice.1
Case reports on paper pica are few, but numerous reports describe other forms of the behavior, including eating ice; dirt, soil, and clay; starch; burnt matches; cardboard; hair; laundry detergent; chalk; soap; firecrackers; and metal artifacts such as coins.2-16
Pica has been described in the literature as “underreported” and “unrecognized.” Its true prevalence is difficult to assess because most people don’t report it and the methodology of data collection varies among populations, as does the definition of pica. According to some estimates, more than 50% of children ages 18 to 36 months seek and ingest nonfood items. The practice reportedly decreases as a child ages, but an estimated 10% of children older than 12 years may engage in it.17
Pica has been reported since antiquity. Many medical and anthropological studies refer to the practice of geophagia, or dirt eating, which is prevalent in Africa and among small children and women, particularly women who are native to the southern United States, African-American, or pregnant.5-10,18,19
Pica often occurs in people with developmental disabilities such as autism and is considered a psychiatric condition in that context.3,11,15,20-31 However, because many forms of pica, especially geophagia, aren’t associated with mental health issues, researchers disagree about whether to consider it an abnormal behavior. A 2000 workshop on pica organized by the Agency for Toxic Substances and Disease Registry concluded that geophagia is not an abnormal behavior.17 One of the most compelling arguments for this view is that dirt eating is far too common around the world to be considered abnormal, and dirt is held in some cultures to have therapeutic powers.7,13,24
Adverse outcomes linked to pica
Pica is associated with adverse outcomes, however. A study by the Agency for Healthcare Research and Quality found that despite an overall decline in hospitalizations for eating disorders, hospitalizations for pica have risen.25 From 1999 to 2009, pica-related hospitalizations jumped 93%, although the overall number of patients hospitalized for the condition remains small (964 in 1999 to 2000, 1862 in 2008-2009).
Documented adverse effects of pica include potassium abnormalities and gastrointestinal conditions ranging from irritation and abdominal pain to perforation, blockage, and colon ischemia.3,11,26-29 Reported bidirectional effects (which both result from and contribute to pica) include iron deficiency, parasitic infections, and heavy metal exposure—particularly lead, mercury, and arsenic.4,6,9,20,30-38
Diagnosis: Focus on history and selective testing
Pica is a clinical diagnosis, confirmed by the patient’s history, not any single laboratory test. Providers should ask about pica behavior or unusual cravings in certain high-risk groups: pregnant women, particularly women from the southern United States, immigrants or refugees, and children and adults with autism or other developmental disabilities.18,22
Testing should be based on the type of pica behavior. Because various forms of pica are commonly associated with iron-deficiency anemia, obtain serum hemoglobin and hematocrit levels along with iron levels if necessary in patients who report cravings for unusual substances. Pica in pregnancy is a sign of iron deficiency, but it also may signal iron deficiency in patients who aren’t pregnant. In one study of 262 nonpregnant adults with iron-deficiency anemia, 45% reported pica behaviors; of these, 87.3% reported eating ice.34
Check serum lead levels in children who engage in geophagia since dirt may contain lead. Because ingestion of soil or clay is associated with soil-borne parasitic infections, also consider testing for ova and parasites if clinically indicated. Patients who eat paper may be exposed to mercury poisoning, so a serum mercury level is advisable.
Management: Prevention and behavior modification are key
Treatment for pica varies by patient and the specific behavior. Management approaches are primarily preventive, educational, and directed toward behavior modification.
Prevention. Residential facilities and primary care offices that care for people with developmental disabilities may screen for pica by means of prevalence surveys, direct observation, stool checks, review of medical history records, and interviews with caregivers.
Residential facilities can create a pica-safe environment by training staff in pica prevention, instituting regular on-site monitoring to ensure that no dangerous objects are available, and developing procedures to guide staff behavior, such as safe disposal of rubber gloves.22 Parents and caregivers of young children or children with developmental disabilities who don’t live in residential facilities should be aware of pica and monitor what their children are ingesting.
Behavior modification. Behavior-based approaches have proved effective for treating pica in developmentally disabled patients. Applied behavioral analysis “was found to have the most robust empirical support to treat this behavior.”39 Patients found to have pica may be referred for further assessment to a behavior specialist or a psychologist with experience in treating the condition.22,39
A review of 26 studies found that, in 25 studies, behavioral therapy reduced pica behavior by 80% or more.23 Behavioral treatments included reinforcement procedures alone, response reduction procedures alone, and combined reinforcement and response reduction procedures. Reinforcement shapes behavior by controlling the consequences of the behavior using a combination of rewards and punishments.23 Response reduction, or blocking, involves obstructing every attempt to eat inedible items.22
Treatments that combined reinforcement and response reduction showed good efficacy.23 An example of the combined approach would be to stop the patient from eating nonnutritive items while redirecting him to eat food instead.22
Supplementation. Iron supplementation has decreased or even reversed pica in patients whose clinical symptoms and behavior were associated with iron deficiency.35,40
Medications. Successful treatment with selective serotonin reuptake inhibitors (escitalopram), atypical neuroleptics (olanzapine), and attention-deficit/hyperactivity disorder medications (methylphenidate) has been reported in some patients, but case reports are few, and the evidence for the drugs’ efficacy is limited.41-43
Be alert for pica. Primary care physicians need to be aware of pica and proactively seek information about cravings or behaviors suggesting the condition from patients in high-risk populations—pregnant women, children, immigrants and refugees, people with developmental disabilities—or their caregivers. Once pica is identified, clinicians should undertake appropriate laboratory investigation and behavior modification attempts.
CORRESPONDENCE
Ranit Mishori, MD, MHS, Department of Family Medicine, Georgetown University School of Medicine, Pre-Clinical Building, GB-01D, 3900 Reservoir Road, NW, Washington, DC 20007; [email protected]
› Ask about pica behavior or unusual cravings in certain high-risk groups: pregnant women, immigrants or refugees, and children and adults with autism or other developmental disabilities. C
› Obtain serum hemoglobin and hematocrit levels along with iron levels, if necessary, in patients who report cravings for unusual substances. B
› Check serum lead levels and consider testing for ova and parasites in patients who eat dirt. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 6-year-old African girl, developing and growing appropriately for age, was brought to our clinic by her father with the chief complaint of “eating the textbooks at school.” The child had eaten paper for years, the father said; he never thought it unusual until her teacher brought it to his attention. The father reported that his daughter had met all developmental milestones and was up to date with her immunizations. When asked why she ate paper, the child responded, “I don’t know.”
The child was diagnosed with pica and, because we were concerned that she was eating other nonnutritive foods, we ordered hematologic studies. Her lead level (2 mcg/dL) was within the normal range; her hemoglobin/hematocrit was 10.4 g/dL/32.3%. Iron therapy was started. At follow-up 4 weeks later, the child’s paper-eating behavior had resolved.
The word pica comes from the Latin word for magpie, a bird with a reputation for eating practically anything. The Diagnostic and Statistical Manual of Mental Disorders, 5th edition, defines pica as persistent eating of nonnutritive substances for at least 1 month that is inappropriate to developmental level and not part of a culturally supported or socially normative practice.1
Case reports on paper pica are few, but numerous reports describe other forms of the behavior, including eating ice; dirt, soil, and clay; starch; burnt matches; cardboard; hair; laundry detergent; chalk; soap; firecrackers; and metal artifacts such as coins.2-16
Pica has been described in the literature as “underreported” and “unrecognized.” Its true prevalence is difficult to assess because most people don’t report it and the methodology of data collection varies among populations, as does the definition of pica. According to some estimates, more than 50% of children ages 18 to 36 months seek and ingest nonfood items. The practice reportedly decreases as a child ages, but an estimated 10% of children older than 12 years may engage in it.17
Pica has been reported since antiquity. Many medical and anthropological studies refer to the practice of geophagia, or dirt eating, which is prevalent in Africa and among small children and women, particularly women who are native to the southern United States, African-American, or pregnant.5-10,18,19
Pica often occurs in people with developmental disabilities such as autism and is considered a psychiatric condition in that context.3,11,15,20-31 However, because many forms of pica, especially geophagia, aren’t associated with mental health issues, researchers disagree about whether to consider it an abnormal behavior. A 2000 workshop on pica organized by the Agency for Toxic Substances and Disease Registry concluded that geophagia is not an abnormal behavior.17 One of the most compelling arguments for this view is that dirt eating is far too common around the world to be considered abnormal, and dirt is held in some cultures to have therapeutic powers.7,13,24
Adverse outcomes linked to pica
Pica is associated with adverse outcomes, however. A study by the Agency for Healthcare Research and Quality found that despite an overall decline in hospitalizations for eating disorders, hospitalizations for pica have risen.25 From 1999 to 2009, pica-related hospitalizations jumped 93%, although the overall number of patients hospitalized for the condition remains small (964 in 1999 to 2000, 1862 in 2008-2009).
Documented adverse effects of pica include potassium abnormalities and gastrointestinal conditions ranging from irritation and abdominal pain to perforation, blockage, and colon ischemia.3,11,26-29 Reported bidirectional effects (which both result from and contribute to pica) include iron deficiency, parasitic infections, and heavy metal exposure—particularly lead, mercury, and arsenic.4,6,9,20,30-38
Diagnosis: Focus on history and selective testing
Pica is a clinical diagnosis, confirmed by the patient’s history, not any single laboratory test. Providers should ask about pica behavior or unusual cravings in certain high-risk groups: pregnant women, particularly women from the southern United States, immigrants or refugees, and children and adults with autism or other developmental disabilities.18,22
Testing should be based on the type of pica behavior. Because various forms of pica are commonly associated with iron-deficiency anemia, obtain serum hemoglobin and hematocrit levels along with iron levels if necessary in patients who report cravings for unusual substances. Pica in pregnancy is a sign of iron deficiency, but it also may signal iron deficiency in patients who aren’t pregnant. In one study of 262 nonpregnant adults with iron-deficiency anemia, 45% reported pica behaviors; of these, 87.3% reported eating ice.34
Check serum lead levels in children who engage in geophagia since dirt may contain lead. Because ingestion of soil or clay is associated with soil-borne parasitic infections, also consider testing for ova and parasites if clinically indicated. Patients who eat paper may be exposed to mercury poisoning, so a serum mercury level is advisable.
Management: Prevention and behavior modification are key
Treatment for pica varies by patient and the specific behavior. Management approaches are primarily preventive, educational, and directed toward behavior modification.
Prevention. Residential facilities and primary care offices that care for people with developmental disabilities may screen for pica by means of prevalence surveys, direct observation, stool checks, review of medical history records, and interviews with caregivers.
Residential facilities can create a pica-safe environment by training staff in pica prevention, instituting regular on-site monitoring to ensure that no dangerous objects are available, and developing procedures to guide staff behavior, such as safe disposal of rubber gloves.22 Parents and caregivers of young children or children with developmental disabilities who don’t live in residential facilities should be aware of pica and monitor what their children are ingesting.
Behavior modification. Behavior-based approaches have proved effective for treating pica in developmentally disabled patients. Applied behavioral analysis “was found to have the most robust empirical support to treat this behavior.”39 Patients found to have pica may be referred for further assessment to a behavior specialist or a psychologist with experience in treating the condition.22,39
A review of 26 studies found that, in 25 studies, behavioral therapy reduced pica behavior by 80% or more.23 Behavioral treatments included reinforcement procedures alone, response reduction procedures alone, and combined reinforcement and response reduction procedures. Reinforcement shapes behavior by controlling the consequences of the behavior using a combination of rewards and punishments.23 Response reduction, or blocking, involves obstructing every attempt to eat inedible items.22
Treatments that combined reinforcement and response reduction showed good efficacy.23 An example of the combined approach would be to stop the patient from eating nonnutritive items while redirecting him to eat food instead.22
Supplementation. Iron supplementation has decreased or even reversed pica in patients whose clinical symptoms and behavior were associated with iron deficiency.35,40
Medications. Successful treatment with selective serotonin reuptake inhibitors (escitalopram), atypical neuroleptics (olanzapine), and attention-deficit/hyperactivity disorder medications (methylphenidate) has been reported in some patients, but case reports are few, and the evidence for the drugs’ efficacy is limited.41-43
Be alert for pica. Primary care physicians need to be aware of pica and proactively seek information about cravings or behaviors suggesting the condition from patients in high-risk populations—pregnant women, children, immigrants and refugees, people with developmental disabilities—or their caregivers. Once pica is identified, clinicians should undertake appropriate laboratory investigation and behavior modification attempts.
CORRESPONDENCE
Ranit Mishori, MD, MHS, Department of Family Medicine, Georgetown University School of Medicine, Pre-Clinical Building, GB-01D, 3900 Reservoir Road, NW, Washington, DC 20007; [email protected]
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
2. Yalug I, Kirmizi-Alsan E, Tufan AE. Adult-onset paper pica in the context of anorexia nervosa with major depressive disorder and a history of childhood geophagia: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1341-1342.
3. Spaniolas K, Ou S, Findeis-Hosey J, et al. Paper pica: an unusual cause of colonic ischemia. J Gastrointest Surg. 2010;14:1065-1066.
4. Olynyk F, Sharpe DH. Mercury poisoning in paper pica. N Engl J Med. 1982;306:1056-1057.
5. Guney M, Zagury GJ, Dogan N, et al. Exposure assessment and risk characterization from trace elements following soil ingestion by children exposed to playgrounds, parks and picnic areas. J Hazard Mater. 2010;182:656-664.
6. Kawai K, Saathoff E, Antelman G, et al. Geophagy (soil-eating) in relation to anemia and helminth infection among HIV-infected pregnant women in Tanzania. Am J Trop Med Hyg. 2009;80:36-43.
7. Woywodt A, Kiss A. Geophagia: the history of earth-eating. J R Soc Med. 2002;95:143-146.
8. Stokes T. The earth-eaters. Nature. 2006;444:543-544.
9. Kutalek R, Wewalka G, Gundacker C, et al. Geophagy and potential health implications: geohelminths, microbes and heavy metals. Trans R Soc Trop Med Hyg. 2010;104:787-795.
10. Keith D, Keith L, Berger GS, et al. Amylophagia during pregnancy: some maternal and perinatal correlations. Mt Sinai J Med. 1975;42:410-414.
11. Abu-Hamdan DK, Sondheimer JH, Mahajan SK. Cautopyreiophagia. Cause of life-threatening hyperkalemia in a patient undergoing hemodialysis. Am J Med. 1985;79:517-519.
12. Ewert P, Keim L, Schulte-Markwort M. Trichobezoar. A rare cause of recurrent upper abdominal pain [in German]. Monatsschr Kinderheilkd. 1992;140:811-813.
13. Grigsby RK, Thyer BA, Waller RJ, et al. Chalk eating in middle Georgia: a culture-bound syndrome of pica? South Med J. 1999;92:190-192.
14. Ahishali E, Boynueğrı B, Dabak R, et al. A case of severe acute hepatitis due to oral intake of firecrackers. Turk J Gastroenterol. 2010;21:325-326.
15. Rashid F, Davies L, Iftikhar SY. Magnetised intragastric foreign body collection and autism: An advice for careers and literature review. Autism. 2010;14:139-145.
16. Martindale JL, Bunker CJ, Noble VE. Ingested foreign bodies in a patient with pica. Gastroenterol Hepatol (N Y). 2010;6:582-584.
17. Agency for Toxic Substances and Disease Registry. Summary Report for the ATSDR Soil-Pica Workshop June 2000, Atlanta, Georgia. Agency for Toxic Substances and Disease Registry Web site. Available at: www.atsdr.cdc.gov/child/soilpica.html. Accessed June 2, 2012.
18. Njiru H, Elchalal U, Paltiel O. Geophagy during pregnancy in Africa: a literature review. Obstet Gynecol Surv. 2011;66:452-459.
19. Young SL. Pica in pregnancy: new ideas about an old condition. Annu Rev Nutr. 2010;30:403-422.
20. Clark B, Vandermeer B, Simonetti A, et al. Is lead a concern in Canadian autistic children? Paediatr Child Health. 2010;15:17-22.
21. Matson JL, Sipes M, Fodstad JC, et al. Issues in the management of challenging behaviours of adults with autism spectrum disorder. CNS Drugs. 2011;25:597-606.
22. Williams DE, McAdam D. Assessment, behavioral treatment, and prevention of pica: clinical guidelines and recommendations for practitioners. Res Dev Disabil. 2012;33:2050-2057.
23. Hagopian LP, Rooker GW, Rolider NU. Identifying empirically supported treatments for pica in individuals with intellectual disabilities. Res Dev Disabil. 2011;32:2114-2120.
24. Engberg DE. Geophagy: adaptive or aberrant behavior. Nebraska Anthropologist. 1995;12:57-68.
25. Agency for Healthcare Research and Quality. Hospitalizations for eating disorder decline, but big increase seen in pica disorder. Agency for Healthcare Research and Quality Web site. Available at: www.ahrq.gov/news/nn/nn090811.htm. Accessed June 2, 2014.
26. Stroman D, Young C, Rubano AR, et al. Adult-onset pica leading to acute intestinal obstruction. Psychosomatics. 2011;52:393-394.
27. Young SL, Khalfan SS, Farag TH, et al. Association of pica with anemia and gastrointestinal distress among pregnant women in Zanzibar, Tanzania. Am J Trop Med Hyg. 2010;83:144-151.
28. Altepeter T, Annes J, Meller J. Foam bezoar: resection of perforated terminal ileum in a 17-year-old with sickle b+thalassemia and pica. J Pediatr Surg. 2011;46:E31-E32.
29. Chatzimavroudis G, Christopoulos P, Atmatzidis S, et al. Pica: an uncommon cause of acute abdominal pain in children. Indian J Pediatr. 2011;78:886-887.
30. Rector WG Jr. Pica: its frequency and significance in patients with iron-deficiency anemia due to chronic gastrointestinal blood loss. J Gen Intern Med. 1989;4:512-513.
31. Sontag C, Kettaneh A, Fain O, et al. Rapid regression of prolonged pagophagia after treatment of iron deficiency [in French]. Presse Med. 2001;30:321-323.
32. Sharma TR, Kavuru B, Aly M. Coprophagia and pica in individuals with mild to moderate dementia and mixed (iron deficiency and macrocytic) anemia. J Am Geriatr Soc. 2011;59:2375-2377.
33. Kushner RF, Shanta Retelny V. Emergence of pica (ingestion of non-food substances) accompanying iron deficiency anemia after gastric bypass surgery. Obes Surg. 2005;15:1491-1495.
34. Barton JC, Barton JC, Bertoli LF. Pica associated with iron deficiency or depletion: clinical and laboratory correlates in 262 nonpregnant adult outpatients. BMC Blood Disord. 2010;10:9.
35. Khan Y, Tisman G. Pica in iron deficiency: a case series. J Med Case Rep. 2010;4:86.
36. Bakhireva LN, Rowland AS, Young BN, et al. Sources of potential lead exposure among pregnant women in New Mexico. Matern Child Health J. 2013;17:172-179.
37. Thihalolipavan S, Candalla BM, Ehrlich J. Examining pica in NYC pregnant women with elevated blood lead levels. Matern Child Health J. 2013;17:49-55.
38. Al-Rmalli SW, Jenkins RO, Watts MJ, et al. Risk of human exposure to arsenic and other toxic elements from geophagy: trace element analysis of baked clay using inductively coupled plasma mass spectrometry. Environ Health. 2010;9:79.
39. Matson JL, Hattier MA, Belva B, et al. Pica in persons with developmental disabilities: approaches to treatment. Res Dev Disabil. 2013;34:2564-2571.
40. Bryant BJ1, Yau YY, Arceo SM, et al. Ascertainment of iron deficiency and depletion in blood donors through screening questions for pica and restless legs syndrome. Transfusion. 2013;53:1637-1644.
41. Lerner AJ. Treatment of pica behavior with olanzapine. CNS Spectr. 2008;13:19.
42. Hergüner S, Hergüner AS. Pica in a child with attention deficit hyperactivity disorder and successful treatment with methylphenidate. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1155-1156.
43. Bhatia MS, Gupta R. Pica responding to SSRI: an OCD Spectrum Disorder? World J Biol Psychiatry. 2009;10(4 pt 3):936-938.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
2. Yalug I, Kirmizi-Alsan E, Tufan AE. Adult-onset paper pica in the context of anorexia nervosa with major depressive disorder and a history of childhood geophagia: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1341-1342.
3. Spaniolas K, Ou S, Findeis-Hosey J, et al. Paper pica: an unusual cause of colonic ischemia. J Gastrointest Surg. 2010;14:1065-1066.
4. Olynyk F, Sharpe DH. Mercury poisoning in paper pica. N Engl J Med. 1982;306:1056-1057.
5. Guney M, Zagury GJ, Dogan N, et al. Exposure assessment and risk characterization from trace elements following soil ingestion by children exposed to playgrounds, parks and picnic areas. J Hazard Mater. 2010;182:656-664.
6. Kawai K, Saathoff E, Antelman G, et al. Geophagy (soil-eating) in relation to anemia and helminth infection among HIV-infected pregnant women in Tanzania. Am J Trop Med Hyg. 2009;80:36-43.
7. Woywodt A, Kiss A. Geophagia: the history of earth-eating. J R Soc Med. 2002;95:143-146.
8. Stokes T. The earth-eaters. Nature. 2006;444:543-544.
9. Kutalek R, Wewalka G, Gundacker C, et al. Geophagy and potential health implications: geohelminths, microbes and heavy metals. Trans R Soc Trop Med Hyg. 2010;104:787-795.
10. Keith D, Keith L, Berger GS, et al. Amylophagia during pregnancy: some maternal and perinatal correlations. Mt Sinai J Med. 1975;42:410-414.
11. Abu-Hamdan DK, Sondheimer JH, Mahajan SK. Cautopyreiophagia. Cause of life-threatening hyperkalemia in a patient undergoing hemodialysis. Am J Med. 1985;79:517-519.
12. Ewert P, Keim L, Schulte-Markwort M. Trichobezoar. A rare cause of recurrent upper abdominal pain [in German]. Monatsschr Kinderheilkd. 1992;140:811-813.
13. Grigsby RK, Thyer BA, Waller RJ, et al. Chalk eating in middle Georgia: a culture-bound syndrome of pica? South Med J. 1999;92:190-192.
14. Ahishali E, Boynueğrı B, Dabak R, et al. A case of severe acute hepatitis due to oral intake of firecrackers. Turk J Gastroenterol. 2010;21:325-326.
15. Rashid F, Davies L, Iftikhar SY. Magnetised intragastric foreign body collection and autism: An advice for careers and literature review. Autism. 2010;14:139-145.
16. Martindale JL, Bunker CJ, Noble VE. Ingested foreign bodies in a patient with pica. Gastroenterol Hepatol (N Y). 2010;6:582-584.
17. Agency for Toxic Substances and Disease Registry. Summary Report for the ATSDR Soil-Pica Workshop June 2000, Atlanta, Georgia. Agency for Toxic Substances and Disease Registry Web site. Available at: www.atsdr.cdc.gov/child/soilpica.html. Accessed June 2, 2012.
18. Njiru H, Elchalal U, Paltiel O. Geophagy during pregnancy in Africa: a literature review. Obstet Gynecol Surv. 2011;66:452-459.
19. Young SL. Pica in pregnancy: new ideas about an old condition. Annu Rev Nutr. 2010;30:403-422.
20. Clark B, Vandermeer B, Simonetti A, et al. Is lead a concern in Canadian autistic children? Paediatr Child Health. 2010;15:17-22.
21. Matson JL, Sipes M, Fodstad JC, et al. Issues in the management of challenging behaviours of adults with autism spectrum disorder. CNS Drugs. 2011;25:597-606.
22. Williams DE, McAdam D. Assessment, behavioral treatment, and prevention of pica: clinical guidelines and recommendations for practitioners. Res Dev Disabil. 2012;33:2050-2057.
23. Hagopian LP, Rooker GW, Rolider NU. Identifying empirically supported treatments for pica in individuals with intellectual disabilities. Res Dev Disabil. 2011;32:2114-2120.
24. Engberg DE. Geophagy: adaptive or aberrant behavior. Nebraska Anthropologist. 1995;12:57-68.
25. Agency for Healthcare Research and Quality. Hospitalizations for eating disorder decline, but big increase seen in pica disorder. Agency for Healthcare Research and Quality Web site. Available at: www.ahrq.gov/news/nn/nn090811.htm. Accessed June 2, 2014.
26. Stroman D, Young C, Rubano AR, et al. Adult-onset pica leading to acute intestinal obstruction. Psychosomatics. 2011;52:393-394.
27. Young SL, Khalfan SS, Farag TH, et al. Association of pica with anemia and gastrointestinal distress among pregnant women in Zanzibar, Tanzania. Am J Trop Med Hyg. 2010;83:144-151.
28. Altepeter T, Annes J, Meller J. Foam bezoar: resection of perforated terminal ileum in a 17-year-old with sickle b+thalassemia and pica. J Pediatr Surg. 2011;46:E31-E32.
29. Chatzimavroudis G, Christopoulos P, Atmatzidis S, et al. Pica: an uncommon cause of acute abdominal pain in children. Indian J Pediatr. 2011;78:886-887.
30. Rector WG Jr. Pica: its frequency and significance in patients with iron-deficiency anemia due to chronic gastrointestinal blood loss. J Gen Intern Med. 1989;4:512-513.
31. Sontag C, Kettaneh A, Fain O, et al. Rapid regression of prolonged pagophagia after treatment of iron deficiency [in French]. Presse Med. 2001;30:321-323.
32. Sharma TR, Kavuru B, Aly M. Coprophagia and pica in individuals with mild to moderate dementia and mixed (iron deficiency and macrocytic) anemia. J Am Geriatr Soc. 2011;59:2375-2377.
33. Kushner RF, Shanta Retelny V. Emergence of pica (ingestion of non-food substances) accompanying iron deficiency anemia after gastric bypass surgery. Obes Surg. 2005;15:1491-1495.
34. Barton JC, Barton JC, Bertoli LF. Pica associated with iron deficiency or depletion: clinical and laboratory correlates in 262 nonpregnant adult outpatients. BMC Blood Disord. 2010;10:9.
35. Khan Y, Tisman G. Pica in iron deficiency: a case series. J Med Case Rep. 2010;4:86.
36. Bakhireva LN, Rowland AS, Young BN, et al. Sources of potential lead exposure among pregnant women in New Mexico. Matern Child Health J. 2013;17:172-179.
37. Thihalolipavan S, Candalla BM, Ehrlich J. Examining pica in NYC pregnant women with elevated blood lead levels. Matern Child Health J. 2013;17:49-55.
38. Al-Rmalli SW, Jenkins RO, Watts MJ, et al. Risk of human exposure to arsenic and other toxic elements from geophagy: trace element analysis of baked clay using inductively coupled plasma mass spectrometry. Environ Health. 2010;9:79.
39. Matson JL, Hattier MA, Belva B, et al. Pica in persons with developmental disabilities: approaches to treatment. Res Dev Disabil. 2013;34:2564-2571.
40. Bryant BJ1, Yau YY, Arceo SM, et al. Ascertainment of iron deficiency and depletion in blood donors through screening questions for pica and restless legs syndrome. Transfusion. 2013;53:1637-1644.
41. Lerner AJ. Treatment of pica behavior with olanzapine. CNS Spectr. 2008;13:19.
42. Hergüner S, Hergüner AS. Pica in a child with attention deficit hyperactivity disorder and successful treatment with methylphenidate. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1155-1156.
43. Bhatia MS, Gupta R. Pica responding to SSRI: an OCD Spectrum Disorder? World J Biol Psychiatry. 2009;10(4 pt 3):936-938.
Prostate Cancer Survivorship Care
Prostate cancer is the most common cancer diagnosis among U.S. veterans.1 More than 12,000 veterans will be diagnosed with prostate cancer in 2014, to join more than 200,000 veteran survivors.1 Because its incidence increases with age and nearly half of veterans are aged ≥ 65 years, the clinical and economic burdens of prostate cancer are expected to increase.2 Fortunately, > 80% of these men will have local disease with 5-year cancer-specific survivals of 98%.3 Even among the small population of veterans whose disease returns after treatment, < 1 in 5 will die of prostate cancer within 10 years.4
Thus, most men live with prostate cancer and its sequelae rather than die of it, similar to other chronic diseases. In 2003, the VHA outlined a National Cancer Strategy, indicating priorities for quality cancer care and access to care for all veterans with cancer.5 Importantly, this directive recognized prostate cancer as a service-connected condition for men exposed to the herbicide Agent Orange.6 For all these reasons, understanding the delivery of prostate cancer survivorship care has tremendous cost and quality implications for the VHA.
SURVIVORSHIP CARE
Due to the extensive focus on screening and initial treatment, very little prostate cancer survivorship research exists either within or outside VHA. In fact, a 2011 literature review found that < 10 prostate cancer survivorship studies were published annually.7 Because long-term survival is increasingly common after any cancer diagnosis, better understanding cancer survivorship (ie, the chronic care following diagnosis and treatment) and the distinct needs of cancer survivors are central to cancer care quality.8,9
A 2005 breakthrough report from the Institute of Medicine, From Cancer Patient to Cancer Survivor: Lost in Transition, emphasized the distinct issues facing cancer survivors and called for an increased emphasis on cancer survivors and their care from both clinical and research perspectives (Table).10
Due to the expanding population of veteran prostate cancer survivors, this report has increasing relevance to VHA.11 For prostate cancer survivors in particular, up to 70% have persistent symptoms (eg, incontinence, impotence) with some symptoms persisting 15 years after treatment, indicating the need for ongoing care and similarity to other chronic diseases.12,13
Despite this growing need and the universal provider access to electronic medical records, VHA, like most other integrated delivery systems, does not have a systematic organizational approach to deal with its prostate cancer survivors, indicating a tremendous opportunity.
One recent proposal for supporting survivorship care in the VHA is a Patient-Aligned Specialty Team for oncology to provide comprehensive cancer care through tumor boards, multispecialty clinics, care coordinators/navigators, and patient education.14
Symptom Burden
The 3 usual approaches to treatment of prostate cancer are (1) surgery (radical prostatectomy); (2) radiation therapy (brachytherapy or external “beam” radiation); and (3) observation (watchful waiting and active surveillance).15-18 While some men do choose observation initially, ultimately many undergo some form of surgical or radiation treatment.19 Unfortunately, long-term adverse effects (AEs) of these treatments are common and vary by treatment type. Men may experience ongoing problems with urinary control (eg, urinary incontinence), sexual function (eg, impotence), hormonal (eg, fatigue, depression), and bowel function (eg, diarrhea and fecal incontinence) far beyond that of age-matched controls.13,15,20-27
Up to 75% of men report problems with erectile dysfunction after prostatectomy, compared with 25% who receive brachytherapy, and 40% who receive brachytherapy plus external beam radiation.20,22,26,28 Urinary problems include both incontinence and pain with urination, which may improve over time with medical and nonmedical management approaches.26,27 Among patients treated with radiation therapy, between 40% and 55% report urinary problems as long as 8 years posttreatment (incontinence and/or pain).26,27,29,30 Unlike surgery, radiation therapy is also associated with bowel problems posttreatment, including rectal urgency and diarrhea.25,31
Although the greatest symptom burden and associated reduction in quality of life (QOL) occurs initially following treatment, many prostate cancer survivors experience considerable symptom burden for years following treatment.21,22,26,32-35 This persistence of symptoms is documented among thousands of patients after prostate cancer treatment, most of which are nonveterans. For example, among men with prostate cancer and no sexual, urinary, or hormonal problems at baseline, 9% to 83% reported severe problems in at least 1 domain 3 years after treatment with surgery or radiation.36
Gore and colleagues demonstrated persistent symptoms among 475 prostate cancer patients for up to 48 months following initial treatment.27 The Michigan Prostate Cancer Survivor Study, a registry-based survey of 2,500 prostate cancer survivors responding about 9 years postdiagnosis, found that up to 70% reported ongoing problems with AEs, some of whom were more than 15 years removed from primary treatment.12 Addressing these symptoms through medical and self-management approaches is one way to reduce their impact and improve QOL among prostate cancer survivors.
Despite the size of the veteran prostate cancer survivor population, most research documenting symptom burden and reduced QOL is from nonveterans. Because veterans often experience greater disease burden than that of the general population, their symptom burden would be expected to be similar or greater than that reported among nonveterans. Although there has been no comprehensive assessment of symptom burden across the VHA as a whole, research to understand optimal approaches to support veteran prostate cancer survivors with self- and medical management of their treatment related symptoms seems warranted.
Self-Management
Though there have been no comprehensive self-management interventions directed to help survivors limit the impact of prostate cancer treatment sequelae in everyday life, evidence suggests that such an intervention is likely to have a positive impact.37 For example, urinary symptoms can be self-managed through a variety of approaches, including emptying the bladder at regular intervals before it gets too full and pelvic floor (ie, Kegel) exercises to help decrease urinary leakage episodes. In fact, a randomized trial demonstrated a 50% decrease in incontinence episodes among prostate cancer survivors who used pelvic floor muscle training and bladder control strategies.38 A recent systematic review suggests that exercise, another self-management strategy, improves incontinence, energy level, body constitution, and QOL after treatment for prostate cancer.37 Exercise among prostate cancer survivors is also associated with decreased prostate cancer-specific and overall mortality.39
For sexual function after prostate cancer treatment, minimizing tobacco and excessive alcohol use and communicating with partners about feelings and sex are self-management strategies for improving sexual relationships.40 Avoiding spicy and greasy foods, coffee and alcohol, and staying well-hydrated may help limit the adverse bowel effects of radiation (ie, radiation proctitis) among prostate cancer survivors.41 However, there are no systematic mechanisms to share these strategies with veterans or nonveterans.
Medical Management
Recommendations for the medical management of prostate cancer-related AEs have recently been updated by the Michigan Cancer Consortium’s Prostate Cancer Action Committee and are available at www.prostatecancerdecision.org.42 Originally developed in 2009, these recommendations were directed toward the management of common posttreatment problems to minimize their impact on men who have been treated for prostate cancer, their families, caregivers, partners, and primary care providers (PCPs).
The recommendations combine expert opinion and evidence-based strategies for identifying recurrence and managing specific symptoms, including erectile dysfunction, urinary incontinence, bowel problems, hot flashes, bone health, gynecomastia, relationship issues, and metabolic syndrome. The increasing recognition that comprehensive, point-of-care resources are needed to direct survivorship care is fueling tremendous efforts targeting primary and specialty care providers from many major cancer stakeholder organizations (ie, American Cancer Society, National Comprehensive Cancer Network, etc).43-45
Primary care providers often consult prostate cancer specialists (urologists and radiation and medical oncologists) for assistance in managing prostate cancer survivors.46 However, it is not clear whether the supply of cancer specialists is capable of meeting the increasing needs of cancer survivors and their PCPs.47 VHA urologists vary tremendously in their regional availability from < 1 per 100,000 patients in Little Rock, Arkansas, to > 10 urologists per 100,000 patients in New York City.48 Similar variation exists for medical oncologists in the VHA. For prostate cancer, the urologist workforce impacts screening rates and cancer-related mortality.49,50 Yet how this workforce variation influences quality of survivorship care, particularly among PCPs dependent on specialist expertise, is unknown.
A better understanding of these relationships will help inform whether interventions to improve survivorship quality of care need to target PCPs with less access to prostate cancer specialists (eg, rural providers through telemedicine initiatives); survivorship care coordination at sites with more cancer specialists; or other potential barriers, such as knowledge gaps pertaining to AE evaluation and management. Each of these barriers to optimal care would be addressed through different interventions.
The long natural history of prostate cancer coupled with the number of survivors basically ensures that PCPs are faced with managing these men and their symptom burdens.51 However, it is often undecided who has primary responsibility for survivorship care.52,53 When queried regarding responsibility for prostate cancer survivorship care, about half of PCPs from one state-based survey felt that it was appropriate for either the cancer specialist or themselves to provide such care.12 Another study revealed high discordance among cancer specialists and PCPs regarding who should provide follow-up care, cancer screening, and general preventive care.54 Without clear role identification, poor communication between primary and specialty care fosters fragmented, expensive, and even poor quality survivorship care.55
Optimizing the delivery of survivorship care among cancer specialists and PCPs is also difficult, because comprehensive prostate cancer survivorship guidelines that might delineate responsibilities and recommend referral practices are just becoming available. In fact, the American Cancer Society just released its Prostate Cancer Survivorship Guidelines in June 2014.10,56,57 Primary care providers may be willing to take on increased responsibility for survivorship care with appropriate specialist support, including timely access to specialist evaluation.54,58 Moreover, PCPs are usually better at supporting cancer survivors’ general health as well.51,58 Therefore, defining the interface between PCPs, their medical home (ie, Patient-Aligned Care Team), and the limited supply of cancer specialists is necessary to streamline information exchange and care transitions.59
Understanding symptom management (eg, incontinence, impotence) across this interface is also critical to the design and implementation of survivorship quality improvement interventions. Promoting clear responsibilities for prostate-specific antigen surveillance, symptom management, and bone density testing for men treated with androgen deprivation therapy across the primary-specialty care interface is a potential starting point.
Transformative Tools
Whether targeting cancer care or not, quality improvement interventions often lack insight into the causal mechanisms by which they effect change.60-62 This is particularly true for interventions targeting clinician behavior change, such as improving uptake of evidence-based practice.63,64 For example, the effectiveness of audit with feedback interventions to improve guideline adherence ranges from 1% to 16%.65-69 The same intervention can vary in its effectiveness, depending on context.70-72 Barriers and enablers that vary by provider, facility, and other contextual factors (eg, workforce, location) contribute to this variable effectiveness.73-79 For this reason, a guiding theoretical framework is useful to understand an intervention’s transferability among different settings, as well as to ensure comprehensive assessment of the factors that can prevent uptake of evidence-based practice.80-83 For example, a theoretical framework might provide insight into how causal mechanisms of an intervention to improve cancer survivorship care might vary in a community-based outpatient clinic vs a tertiary center.84-86
A guiding theoretical framework is even more useful when used to design quality improvement interventions.82,83,87,88 Mapping barriers to theoretical constructs, and theoretical constructs to interventions to facilitate clinician behavior change can assist in planning strategies for effective implementation across a range of settings.88 While psychological theories like the Theoretical Domains Framework and Theory of Planned Behavior are pertinent for individual behavior change, understanding how best to implement interventions targeted at the facility level requires a broader perspective focused on context.83,88-92
The Consolidated Framework for Implementation Research (CFIR) provides a comprehensive, practical taxonomy for understanding important organizational, individual, and intervention characteristics to consider during an implementation process.75,76 The CFIR framework provides the broader contextual milieu contributing to the quality of survivorship care at the facility level across 5 domains: (1) intervention characteristics—evidence, complexity, relative advantage; (2) outer setting—peer pressure, external policies; (3) inner setting—structural characteristics, readiness for implementation, culture; (4) individual characteristics—knowledge about intervention, self-efficacy; and (5) process—planning, engaging stakeholders, champions, execution.
Using both individual and organizational constructs to effectively characterize the relationships, needs, intentions, and organizational characteristics of primary and cancer care providers throughout VHA will be key to designing successful interventions to broadly ensure quality survivorship care. The best interventions to improve survivorship care will likely vary across facilities based on contextual factors such as cancer specialist availability, facility characteristics, and the current delivery system for survivorship care.
Intervention modalities currently being used by the VHA Office of Specialty Care Transformation to improve access to specialty care are indeed transformative tools to optimize the quality of survivorship care. The latter builds on a successful approach developed and widely used in New Mexico, which makes the expertise of academic specialists at the University of New Mexico available throughout the state, using video teleconferencing.93,94 The opportunities for video-enabled interaction between specialists and PCPs in VHA, both in consultation about specific patients and in educational sessions to enhance PCP knowledge and self-efficacy in managing patients requiring specialty knowledge, are revolutionary for cancer care.93,95
Conclusions
Due to the expanding population of veteran prostate cancer survivors, improving their QOL by ensuring proper cancer surveillance, effectively managing their treatment complications and transitions of cancer care will reduce risk and provide timely management of symptoms and disease recurrence.
Understanding how variation in the VHA cancer specialist workforce impacts the quality of cancer survivorship care is a critical step towards optimizing veteran cancer care. Through this understanding, communication between PCPs, PACT, and cancer specialists can be improved via theory-based quality improvement tools to address gaps in the quality of prostate and other VHA cancer survivorship care. Interventions designed to enhance PCP self-efficacy in delivering high-quality prostate cancer survivor care may improve job satisfaction among PCPs and specialists.
Clarifying issues in the delivery of optimal prostate cancer survivorship care may inform models for other cancer survivorship care in the VHA. The contextual factors contributing to a VHA facility’s performance for prostate cancer survivorship care may be very relevant to the facility’s performance for other types of cancer survivorship care. A facility’s primary care organizational structure, cancer specialist workforce, and oncology-specific facility characteristics vary little across cancer types, suggesting that a better understanding of how to improve PSA surveillance for prostate cancer, the most common cancer treated in the VHA, should apply to carcinoembryonic antigen surveillance for colon cancer, hematology studies for lymphoma, and the surveillance of other malignancies in the VHA.96,97
The VHA National Cancer Strategy stressed the importance of meeting or exceeding accepted national standards of quality cancer care. Therefore, understanding the relationship between quality of cancer survivorship care and the cancer specialist workforce and its interface with primary care is critical to this goal, as is elucidation of the other barriers preventing optimal care. Last, embracing VHA’s latest telemedicine initiatives, including video teleconferencing to improve prostate cancer care, has the potential to transform this system into a national leader in prostate cancer survivorship care.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article. Dr. Skolarus is supported by a VA HSR&D Career Development Award - 2 (CDA 12-171). Drs. Hawley (PI) and Skolarus (Co-I) are supported by VA HSR&D IIR (12-116).
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect an endorsement by or opinion of Federal Practitioner, Frontline Medical Communications, the U.S. Air Force, the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drug combinations–including indications, contraindications, warnings, and adverse effects–before administering pharmacologic therapy to patients.
1. Veterans Affairs Central Cancer Registry (VACCR) [intranet database]. Washington, DC: US Department of Veterans Affairs; 1995.
2. U.S. Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. Profile of Veterans: 2009. Data from the American Community Survey. U.S. Department of Veterans Affairs Website. http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2009_FINAL.pdf. Accessed July 23, 2014.
3. National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. SEER Website. http://www.seer.cancer.gov. Accessed July 23, 2014.
4. Uchio EM, Aslan M, Wells CK, Calderone J, Concato J. Impact of biochemical recurrence in prostate cancer among US veterans. Arch Intern Med. 2010;170(15):1390-1395.
5. U.S. Department of Veterans Affairs. VHA DIRECTIVE 2003-034. Department of Veterans Affairs, Veterans Health Administration Website. http://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=261. Accessed July 23, 2014.
6. Chamie K, DeVere White RW, Lee D, Ok JH, Ellison LM. Agent Orange exposure, Vietnam War veterans, and the risk of prostate cancer. Cancer. 2008;113(9):2464-2470.
7. Harrop JP, Dean JA, Paskett ED. Cancer survivorship research: A review of the literature and summary of current NCI-designated cancer center projects. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2042-2047.
8. Centers for Disease Control and Prevention. Cancer survivors—United States, 2007. MMWR Morb Mortal Wkly Rep. 2011;60(9):269-272.
9. Ganz PA. Survivorship: Adult cancer survivors. Prim Care. 2009;36(4):721-741.
10. Hewitt M, Greenfield S, Stovall E, eds. From Cancer Patient to Cancer Survivor: Lost in Translation. Institute of Medicine and National Research Council of the National Academies. Washington, DC: The National Academies Press; 2005.
11. Moye J, Schuster JL, Latini DM, Naik AD. The future of cancer survivorship care for veterans. Fed Pract. 2010;27(3):36-43.
12. Darwish-Yassine M, Berenji M, Wing D, et al. Evaluating long-term patient-centered outcomes following prostate cancer treatment: findings from the Michigan Prostate Cancer Survivor study. J Cancer Surviv. 2014;8(1):121-130.
13. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250-1261.
14. Kelley M. VA Proposes Team-Based Model for Prostate Cancer Care. U.S. Medicine Website. http://www.usmedicine.com/agencies/department-of-veterans-affairs/va-proposes-team-based-model-for-prostate-cancer-care. Published May 2013. Accessed April 28, 2014.
15. Bill-Axelson A, Holmberg L, Ruutu M, et al; Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352(19):1977-1984.
16. Skolarus TA, Miller DC, Zhang Y, Hollingsworth JM, Hollenbeck BK. The delivery of prostate cancer care in the United States: Implications for delivery system reform. J Urol. 2010;184(6):2279-2284.
17. NCCN Clinical Practice Guidelines in Oncology Prostate Cancer, Version 2.2014. NCCN Website. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed April 1, 2014.
18. Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360(9327):103-106.
19. Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28(1):126-131.
20. Alemozaffar M, Regan MM, Cooperberg MR, et al. Prediction of erectile function following treatment for prostate cancer. JAMA. 2011;306(11):1205-1214.
21. Dandapani SV, Sanda MG. Measuring health-related quality of life consequences from primary treatment for early-state prostate cancer. Semin Radiat Oncol. 2008;18(1):67-72.
22. Johansson E, Steineck G, Holmberg L, et al; SPCG-4 Investigators. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Proste Cancer Group-4 randomised trial. Lancet Oncol. 2011;12(9):891-899.
23. Johansson E, Bill-Axelson A, Holmberg L, Onelöv E, Johansson JE, Steineck G; Scandinavian Prostate Cancer Group Study No 4. Time, symptom burden, androgen deprivation, and self-assessed quality of life after radical prostatectomy or watchful waiting: the Randomized Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4) clinical trial. Eur Urol. 2009;55(2):422-430.
24. Steineck G, Helgesen F, Adofsson J, et al; Scandanavian Prostatic Cancer Group Study Number 4. Quality of life after radial prostatectomy or watchful waiting. N Engl J Med. 2002;347(11):790-796.
25. Penson DF, Litwin MS. Quality of life after treatment for prostate cancer. Curr Urol Rep. 2003;4(3):185-195.
26. Ferrer M, Suárez JF, Guedea F, et al; Multicentric Spanish Group of Clinically Localized Prostate Cancer. Health-related quality of life 2 years after treatment with radical prostatectomy, prostate brachytherapy, or external beam radiotherapy in patients with clinically localized prostate cancer. Int J Radiation Oncology Bio Phys. 2008;72(2):421-432.
27. Gore JL, Kwan L, Lee SP, Reiter RE, Litwin MS. Survivorship beyond convalescence: 48-month quality-of-life outcomes after treatment for localized prostate cancer. J Natl Cancer Inst. 2009;101(12):888-892.
28. Miller DC, Wei JT, Dunn RL, et al. Use of medications or devices for erectile dysfunction among long-term prostate cancer treatment survivors: potential influence of sexual motivation and/or indifference. Urology. 2006;68(1):166-171.
29. Wilt TJ, Shamliyan TA, Taylor BC, MacDonald R, Kane RL. Association between hospital and surgeon radical prostatectomy volume and patient outcomes: A systematic review. J Urol. 2008;180(3):820-828; discussion 828-829.
30. Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: Health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772-2780.
31. Michaelson MD, Cotter SE, Gargollo PC, Zietman AL, Dahl DM, Smith MR. Management of complications of prostate cancer treatment. CA Cancer J Clin. 2008;58(4):196-213.
32. Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: Results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2000;92(19):1582-1592.
33. Potosky AL, Davis WW, Hoffman RM, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: The prostate cancer outcomes study. J Natl Cancer Inst. 2004;96(18):1358-1367.
34. Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20(2):557-566.
35. Harrington CB, Hansen JA, Moskowitz M, Todd BL, Fuerestein M. It’s not over when it’s over: Long-term symptoms in cancer survivors—a systematic review. Int J Psychiatry Med. 2010;40(2):163-181.
36. Pardo Y, Guedea F, Aguiló F, et al. Quality-of-life impact of primary treatments for localized prostate cancer in patients without hormonal treatment [published correction appears in J Clin Oncol. 2011;29(6):779]. J Clin Oncol. 2010;28(31):4687-4696.
37. Baumann FT, Zoph EM, Bloch W. Clinical exercise interventions in prostate cancer patients—a systematic review of randomized controlled trials. Support Care Cancer. 2011;20(2):221-233.
38. Goode PS, Burgio KL, Johnson TM II, et al. Behavioral therapy with or without biofeedback and pelvic floor electrical stimulation for persistent postprostatectomy incontinence: A randomized controlled trial. JAMA. 2011;305(2):151-159.
39. Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29(6):726-732.
40. Meldrum DR, Gambone JC, Morris MA, Ignarro LJ. A multifaceted approach to maximize erectile function and vascular health. Fertil Steril. 2010;94(7):2514-2520.
41. National Cancer Institute. Gastrointestinal Complications. NCCN Website. http://www.cancer.gov/cancertopics/pdq/supportivecare/gastrointestinalcomplications/HealthProfessional. Accessed June 27, 2014.
42. Michigan Cancer Consortium. Michigan Cancer Consortium Recommendations for Prostate Cancer Survivorship Care. Prostate Cancer Decision Website. http://www.prostatecancerdecision.org/PDFs/Algorithms2013/RecommProstateCancerCare-09182013.pdf. Accessed April 10, 2014.
43. Cowens-Alvarado R, Sharpe K, Pratt-Chapman M, et al. Advancing survivorship care through the National Cancer Survivorship Resource Center: Developing American Cancer Society guidelines for primary care providers. CA Cancer J Clin. 2013;63(3):147-150.
44. American Society of Clinical Oncology. Cancer Survivorship. American Society of Clinical Cancer Website. http://www.asco.org/practice-research
/cancer-survivorship. Accessed May 1, 2014.
45. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Survivorship. V1. 2014. NCCN Website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#survivorship. Accessed April 24, 2014.
46. Skolarus TA, Holmes-Rovner M, Northouse LL, et al. Primary care perspectives on prostate cancer survivorship: Implications for improving quality of care. Urol Oncol. 2013;31(6):727-732.
47. Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future supply and demand for oncologists: Challenges to assuring access to oncology services. J Oncol Pract. 2007;3(2):79-86.
48. VHA Office of Productivity, Efficiency, & Staffing (OPES). Fiscal Year 2011 VHA Physician Workforce & Support Staff Data by VISN Facility. Washington, DC: VHA Office of Productivity, Efficiency, & Staffing; 2011.
49. Odisho AY, Cooperberg MR, Fradet V, Ahmad AE, Carroll PR. Urologist density and county-level urologic cancer mortality. J Clin Oncol. 2010;28(15):2499-2504.
50. Odisho AY, Fradet V, Cooperberg MR, Ahmad AE, Carroll PR. Geographic distribution of urologists throughout the United States using a county level approach. J Urol. 2009;181(2):760-765; discussion 765-766.
51. Pollack LA, Adamache W, Ryerson AB, Eheman CR, Richardson LC. Care of long-term cancer survivors: Physicians seen by Medicare enrollees surviving longer than 5 years. Cancer. 2009;115(22):5284-5295.
52. Ganz PA, Casillas J, Hahn EE. Ensuring quality care for cancer survivors: Implementing the survivorship care plan. Semin Oncology Nurs. 2008;24(3):208-217.
53. Bober SL, Recklitis CJ, Campbell EG, et al. Caring for cancer survivors: A survey of primary care physicians. Cancer. 2009;115(suppl 18):4409-4418.
54. Cheung WY, Neville BA, Cameron DB, Cook EF, Earle CC. Comparisons of patient and physician expectations for cancer survivorship care. J Clin Oncol. 2009;27(15):2489-2495.
55. Skolarus TA, Zhang Y, Hollenbeck BK. Understanding fragmentation of prostate cancer survivorship care: Implications for cost and quality. Cancer. 2012;118(11):2837-2845.
56. Grimshaw JM, Winkens RA, Shirran L, et al. Interventions to improve outpatient referrals from primary care to secondary care. Cochrane Database Syst Rev. 2005;(3):CD005471.
57. Skolarus TA, Wolf AM, Erb NL, et al. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin. 2014;64(4):225-249.
58. Jacobs LA, Palmer SC, Schwartz LA, et al. Adult cancer survivorship: Evolution, research, and planning care. CA Cancer J Clin. 2009;59(6):391-410.
59. O’Malley AS, Reschovsky JD. Referral and consultation communication between primary care and specialist physicians: Finding common ground. Arch Intern Med. 2011;171(1):56-65.
60. Krein SL, Damschroder LJ, Kowalski CP, Forman J, Hofer TP, Saint S. The influence of organizational context on quality improvement and patient safety efforts in infection prevention: A multi-center qualitative study. Soc Sci Med. 2010;71(9):1692-1701.
61. McDermott KA, Helfrich CD, Sales AE, Rumsfeld JS, Ho PM, Fihn SD. A review of interventions and system changes to improve time to reperfusion for ST-segment elevation myocardial infarction. J Gen Intern Med. 2008;23(8):1246-1256.
62. Grimshaw JM, Zwarenstein M, Tetroe JM, et al. Looking inside the black box: A theory-based process evaluation alongside a randomised controlled trial of printed educational materials (the Ontario printed educational message, OPEM) to improve referral and prescribing practices in primary care in Ontario, Canada. Implement Sci. 2007;2:38.
63. Gagliardi AR, Brouwers MC, Palda VA, Lemieux-Charles L, Grimshaw JM. How can we improve guideline use? A conceptual framework of implementability. Implement Sci. 2011;6:26.
64. Godin G, Bélanger-Gravel A, Eccles M, Grimshaw J. Healthcare professionals’ intentions and behaviours: a systematic review of studies based on social cognitive theories. Implement Sci. 2008;2008(3):36.
65. Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8(6):iii-iv, 1-72.
66. Hysong SJ, Teal CR, Khan MJ, Haidet P. Improving quality of care through improved audit and feedback. Implement Sci. 2012;7(1):45.
67. Hysong SJ. Meta-analysis: Audit and feedback features impact effectiveness on care quality. Med Care. 2009;47(3):356-363.
68. Hysong SJ, Best RG, Pugh JA. Audit and feedback and clinical practice guideline adherence: Making feedback actionable. Implement Sci. 2006;1:9.
69. Sales AE, Schalm C. Data for improvement and clinical excellence: Protocol for an audit with feedback intervention in long-term care. Implement Sci. 2010;5(1):74.
70. Helfrich CD, Blevins D, Smith JL, et al. Predicting implementation from organizational readiness for change: A study protocol. Implement Sci. 2011;6(1):76.
71. Pineros SL, Sales AE, Li YF, Sharp ND. Improving care to patients with ischemic heart disease: Experiences in a single network of the Veterans Health Administration. Worldviews Evid Based Nurs. 2004;1(suppl 1):S33-S40.
72. Sales AE, Pineros SL, Magid DJ, Every NR, Sharp ND, Rumsfeld JS. The association between clinical integration of care and transfer of veterans with acute coronary syndromes from primary care VHA hospitals. BMC Health Serv Res. 2005;5(1):2.
73. Grimshaw JM, Eccles MP, Lavis JN, Hill SJ, Squires JE. Knowledge translation of research findings. Implement Sci. 2012;7(1):50.
74. Sales AE, Lapham GG, Squires J, et al. Organizational factors associated with decreased mortality among Veterans Affairs patients with an ICU stay. Comput Inform Nurs. 2011;29(9):496-501.
75. Damschroder LJ, Hagedorn HJ. A guiding framework and approach for implementation research in substance use disorders treatment. Psychol Addict Behav. 2011;25(2):194-205.
76. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50.
77. Salkeld E, Leaver CA, Guttmann A, et al. Barriers and facilitators to the implementation of Ontario’s emergency department clinical decision unit pilot program: A qualitative study. CJEM. 2011;13(6):363-371.
78. Sharp ND, Pineros SL, Hsu C, Starks H, Sales AE. A qualitative study to identify barriers and facilitators to implementation of pilot interventions in the Veterans Health Administration (VHA) Northwest Network. Worldviews Evid Based Nurs. 2004;1(2):129-139.
79. Render ML, Hasselbeck R, Freyberg RW, Hofer TP, Sales AE, Almenoff PL; VA ICU Clinical Advisory Group. Reduction of central line infections in Veterans Administration intensive care units: An observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725-732.
80. Grimshaw JM, Eccles MP, Steen N, et al. Applying psychological theories to evidence-based clinical practice: Identifying factors predictive of lumbar spine x-ray for low back pain in UK primary care practice. Implement Sci. 2011;6(1):55.
81. Eccles M, Grimshaw J, Walker A, Johnston M, Pitts N. Changing the behavior of healthcare professionals: The use of theory in promoting the uptake of research findings. J Clin Epidemiol. 2005;58(2):107-112.
82. Davies P, Walker AE, Grimshaw JM. A systematic review of the use of theory in the design of guideline dissemination and implementation strategies and interpretation of the results of rigorous evaluations. Implement Sci. 2010;5(1):14.
83. Sales A, Smith J, Curran G, Kochevar L. Models, strategies, and tools. Theory in implementing evidence-based findings into health care practice. J Gen Intern Med. 2006;21(suppl 2):S43-S49.
84. Dixon-Woods M, Bosk CL, Aveling EL, Goeschel CA, Pronovost PJ. Explaining Michigan: Developing an ex post theory of a quality improvement program. Milbank Q. 2011;89(2):167-205.
85. Damschroder LJ, Goodrich DE, Robinson CH, Fletcher CE, Lowery JC. A systematic exploration of differences in contextual factors related to implementing the MOVE! weight management program in VA: A mixed methods study. BMC Health Serv Res. 2011;11(1):248.
86. Ramsay CR, Thomas RE, Croal BL, Grimshaw JM, Eccles MP. Using the theory of planned behaviour as a process evaluation tool in randomised trials of knowledge translation strategies: A case study from UK primary care. Implement Sci. 2010;5(1):71.
87. Légaré F, Borduas F, Jacques A, et al. Developing a theory-based instrument to assess the impact of continuing professional development activities on clinical practice: A study protocol. Implement Sci. 2011;6(1):17.
88. French SD, Green SE, O’Connor DA, et al. Developing theory-informed behaviour change interventions to implement evidence into practice: A systematic approach using the Theoretical Domains Framework. Implement Sci. 2012;7(1):38.
89. Ajzen I. The theory of planned behaviour: Reactions and reflections. Psychol Health. 2011;26(9):1113-1127.
90. Fishbein M, Ajzen I. Theory-based behavior change interventions: Comments on Hobbis and Sutton. J Health Psychol. 2005;10(1):27-31; discussion 37-43.
91. Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50(2):179-211.
92. Francis JJ, O’Connor D, Curran J. Theories of behaviour change synthesised into a set of theoretical groupings: Introducing a thematic series on the theoretical domains framework. Implement Sci. 2012;7(1):35.
93. Arora S, Kalishman S, Thornton K, et al. Expanding access to hepatitis C virus treatment-Extension for Community Healthcare Outcomes (ECHO) project: Disruptive innovation in specialty care. Hepatology. 2010;52(3):1124-1133.
94. Arora S, Kalishman S, Dion D, et al. Partnering urban academic medical centers and rural primary care clinicians to provide complex chronic disease care. Health Aff (Milwood). 2011;30(6):1176-1184.
95. Horner K, Wagner E, Tufano J. Electronic consultations between primary and specialty care clinicians: Early insights. Issue Brief (Commonw Fund). 2011;23:1-14.
96. Kunitake H, Zheng P, Yothers G, et al. Routine preventive care and cancer surveillance in long-term survivors of colorectal cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol LTS-01. J Clin Oncol. 2010;28(36):5274-5279.
97. Ng A, Constine LS, Advani R; Expert Panel on Radiation Oncology-Hodgkin’s Lymphoma. ACR Appropriateness Criteria follow-up of Hodgkin’s lymphoma. [online publication]. Reston (VA): American College of Radiology (ACR); 2010.
radiation therapy, brachytherapy or external “beam” radiation, observation, sequelae, SYMPTOM BURDEN, SELF-MANAGEMENT, MEDICAL MANAGEMENT, Primary care providers, PCPs, TRANSFORMATIVE TOOLS, Consolidated Framework for Implementation Research, CFIR
Prostate cancer is the most common cancer diagnosis among U.S. veterans.1 More than 12,000 veterans will be diagnosed with prostate cancer in 2014, to join more than 200,000 veteran survivors.1 Because its incidence increases with age and nearly half of veterans are aged ≥ 65 years, the clinical and economic burdens of prostate cancer are expected to increase.2 Fortunately, > 80% of these men will have local disease with 5-year cancer-specific survivals of 98%.3 Even among the small population of veterans whose disease returns after treatment, < 1 in 5 will die of prostate cancer within 10 years.4
Thus, most men live with prostate cancer and its sequelae rather than die of it, similar to other chronic diseases. In 2003, the VHA outlined a National Cancer Strategy, indicating priorities for quality cancer care and access to care for all veterans with cancer.5 Importantly, this directive recognized prostate cancer as a service-connected condition for men exposed to the herbicide Agent Orange.6 For all these reasons, understanding the delivery of prostate cancer survivorship care has tremendous cost and quality implications for the VHA.
SURVIVORSHIP CARE
Due to the extensive focus on screening and initial treatment, very little prostate cancer survivorship research exists either within or outside VHA. In fact, a 2011 literature review found that < 10 prostate cancer survivorship studies were published annually.7 Because long-term survival is increasingly common after any cancer diagnosis, better understanding cancer survivorship (ie, the chronic care following diagnosis and treatment) and the distinct needs of cancer survivors are central to cancer care quality.8,9
A 2005 breakthrough report from the Institute of Medicine, From Cancer Patient to Cancer Survivor: Lost in Transition, emphasized the distinct issues facing cancer survivors and called for an increased emphasis on cancer survivors and their care from both clinical and research perspectives (Table).10
Due to the expanding population of veteran prostate cancer survivors, this report has increasing relevance to VHA.11 For prostate cancer survivors in particular, up to 70% have persistent symptoms (eg, incontinence, impotence) with some symptoms persisting 15 years after treatment, indicating the need for ongoing care and similarity to other chronic diseases.12,13
Despite this growing need and the universal provider access to electronic medical records, VHA, like most other integrated delivery systems, does not have a systematic organizational approach to deal with its prostate cancer survivors, indicating a tremendous opportunity.
One recent proposal for supporting survivorship care in the VHA is a Patient-Aligned Specialty Team for oncology to provide comprehensive cancer care through tumor boards, multispecialty clinics, care coordinators/navigators, and patient education.14
Symptom Burden
The 3 usual approaches to treatment of prostate cancer are (1) surgery (radical prostatectomy); (2) radiation therapy (brachytherapy or external “beam” radiation); and (3) observation (watchful waiting and active surveillance).15-18 While some men do choose observation initially, ultimately many undergo some form of surgical or radiation treatment.19 Unfortunately, long-term adverse effects (AEs) of these treatments are common and vary by treatment type. Men may experience ongoing problems with urinary control (eg, urinary incontinence), sexual function (eg, impotence), hormonal (eg, fatigue, depression), and bowel function (eg, diarrhea and fecal incontinence) far beyond that of age-matched controls.13,15,20-27
Up to 75% of men report problems with erectile dysfunction after prostatectomy, compared with 25% who receive brachytherapy, and 40% who receive brachytherapy plus external beam radiation.20,22,26,28 Urinary problems include both incontinence and pain with urination, which may improve over time with medical and nonmedical management approaches.26,27 Among patients treated with radiation therapy, between 40% and 55% report urinary problems as long as 8 years posttreatment (incontinence and/or pain).26,27,29,30 Unlike surgery, radiation therapy is also associated with bowel problems posttreatment, including rectal urgency and diarrhea.25,31
Although the greatest symptom burden and associated reduction in quality of life (QOL) occurs initially following treatment, many prostate cancer survivors experience considerable symptom burden for years following treatment.21,22,26,32-35 This persistence of symptoms is documented among thousands of patients after prostate cancer treatment, most of which are nonveterans. For example, among men with prostate cancer and no sexual, urinary, or hormonal problems at baseline, 9% to 83% reported severe problems in at least 1 domain 3 years after treatment with surgery or radiation.36
Gore and colleagues demonstrated persistent symptoms among 475 prostate cancer patients for up to 48 months following initial treatment.27 The Michigan Prostate Cancer Survivor Study, a registry-based survey of 2,500 prostate cancer survivors responding about 9 years postdiagnosis, found that up to 70% reported ongoing problems with AEs, some of whom were more than 15 years removed from primary treatment.12 Addressing these symptoms through medical and self-management approaches is one way to reduce their impact and improve QOL among prostate cancer survivors.
Despite the size of the veteran prostate cancer survivor population, most research documenting symptom burden and reduced QOL is from nonveterans. Because veterans often experience greater disease burden than that of the general population, their symptom burden would be expected to be similar or greater than that reported among nonveterans. Although there has been no comprehensive assessment of symptom burden across the VHA as a whole, research to understand optimal approaches to support veteran prostate cancer survivors with self- and medical management of their treatment related symptoms seems warranted.
Self-Management
Though there have been no comprehensive self-management interventions directed to help survivors limit the impact of prostate cancer treatment sequelae in everyday life, evidence suggests that such an intervention is likely to have a positive impact.37 For example, urinary symptoms can be self-managed through a variety of approaches, including emptying the bladder at regular intervals before it gets too full and pelvic floor (ie, Kegel) exercises to help decrease urinary leakage episodes. In fact, a randomized trial demonstrated a 50% decrease in incontinence episodes among prostate cancer survivors who used pelvic floor muscle training and bladder control strategies.38 A recent systematic review suggests that exercise, another self-management strategy, improves incontinence, energy level, body constitution, and QOL after treatment for prostate cancer.37 Exercise among prostate cancer survivors is also associated with decreased prostate cancer-specific and overall mortality.39
For sexual function after prostate cancer treatment, minimizing tobacco and excessive alcohol use and communicating with partners about feelings and sex are self-management strategies for improving sexual relationships.40 Avoiding spicy and greasy foods, coffee and alcohol, and staying well-hydrated may help limit the adverse bowel effects of radiation (ie, radiation proctitis) among prostate cancer survivors.41 However, there are no systematic mechanisms to share these strategies with veterans or nonveterans.
Medical Management
Recommendations for the medical management of prostate cancer-related AEs have recently been updated by the Michigan Cancer Consortium’s Prostate Cancer Action Committee and are available at www.prostatecancerdecision.org.42 Originally developed in 2009, these recommendations were directed toward the management of common posttreatment problems to minimize their impact on men who have been treated for prostate cancer, their families, caregivers, partners, and primary care providers (PCPs).
The recommendations combine expert opinion and evidence-based strategies for identifying recurrence and managing specific symptoms, including erectile dysfunction, urinary incontinence, bowel problems, hot flashes, bone health, gynecomastia, relationship issues, and metabolic syndrome. The increasing recognition that comprehensive, point-of-care resources are needed to direct survivorship care is fueling tremendous efforts targeting primary and specialty care providers from many major cancer stakeholder organizations (ie, American Cancer Society, National Comprehensive Cancer Network, etc).43-45
Primary care providers often consult prostate cancer specialists (urologists and radiation and medical oncologists) for assistance in managing prostate cancer survivors.46 However, it is not clear whether the supply of cancer specialists is capable of meeting the increasing needs of cancer survivors and their PCPs.47 VHA urologists vary tremendously in their regional availability from < 1 per 100,000 patients in Little Rock, Arkansas, to > 10 urologists per 100,000 patients in New York City.48 Similar variation exists for medical oncologists in the VHA. For prostate cancer, the urologist workforce impacts screening rates and cancer-related mortality.49,50 Yet how this workforce variation influences quality of survivorship care, particularly among PCPs dependent on specialist expertise, is unknown.
A better understanding of these relationships will help inform whether interventions to improve survivorship quality of care need to target PCPs with less access to prostate cancer specialists (eg, rural providers through telemedicine initiatives); survivorship care coordination at sites with more cancer specialists; or other potential barriers, such as knowledge gaps pertaining to AE evaluation and management. Each of these barriers to optimal care would be addressed through different interventions.
The long natural history of prostate cancer coupled with the number of survivors basically ensures that PCPs are faced with managing these men and their symptom burdens.51 However, it is often undecided who has primary responsibility for survivorship care.52,53 When queried regarding responsibility for prostate cancer survivorship care, about half of PCPs from one state-based survey felt that it was appropriate for either the cancer specialist or themselves to provide such care.12 Another study revealed high discordance among cancer specialists and PCPs regarding who should provide follow-up care, cancer screening, and general preventive care.54 Without clear role identification, poor communication between primary and specialty care fosters fragmented, expensive, and even poor quality survivorship care.55
Optimizing the delivery of survivorship care among cancer specialists and PCPs is also difficult, because comprehensive prostate cancer survivorship guidelines that might delineate responsibilities and recommend referral practices are just becoming available. In fact, the American Cancer Society just released its Prostate Cancer Survivorship Guidelines in June 2014.10,56,57 Primary care providers may be willing to take on increased responsibility for survivorship care with appropriate specialist support, including timely access to specialist evaluation.54,58 Moreover, PCPs are usually better at supporting cancer survivors’ general health as well.51,58 Therefore, defining the interface between PCPs, their medical home (ie, Patient-Aligned Care Team), and the limited supply of cancer specialists is necessary to streamline information exchange and care transitions.59
Understanding symptom management (eg, incontinence, impotence) across this interface is also critical to the design and implementation of survivorship quality improvement interventions. Promoting clear responsibilities for prostate-specific antigen surveillance, symptom management, and bone density testing for men treated with androgen deprivation therapy across the primary-specialty care interface is a potential starting point.
Transformative Tools
Whether targeting cancer care or not, quality improvement interventions often lack insight into the causal mechanisms by which they effect change.60-62 This is particularly true for interventions targeting clinician behavior change, such as improving uptake of evidence-based practice.63,64 For example, the effectiveness of audit with feedback interventions to improve guideline adherence ranges from 1% to 16%.65-69 The same intervention can vary in its effectiveness, depending on context.70-72 Barriers and enablers that vary by provider, facility, and other contextual factors (eg, workforce, location) contribute to this variable effectiveness.73-79 For this reason, a guiding theoretical framework is useful to understand an intervention’s transferability among different settings, as well as to ensure comprehensive assessment of the factors that can prevent uptake of evidence-based practice.80-83 For example, a theoretical framework might provide insight into how causal mechanisms of an intervention to improve cancer survivorship care might vary in a community-based outpatient clinic vs a tertiary center.84-86
A guiding theoretical framework is even more useful when used to design quality improvement interventions.82,83,87,88 Mapping barriers to theoretical constructs, and theoretical constructs to interventions to facilitate clinician behavior change can assist in planning strategies for effective implementation across a range of settings.88 While psychological theories like the Theoretical Domains Framework and Theory of Planned Behavior are pertinent for individual behavior change, understanding how best to implement interventions targeted at the facility level requires a broader perspective focused on context.83,88-92
The Consolidated Framework for Implementation Research (CFIR) provides a comprehensive, practical taxonomy for understanding important organizational, individual, and intervention characteristics to consider during an implementation process.75,76 The CFIR framework provides the broader contextual milieu contributing to the quality of survivorship care at the facility level across 5 domains: (1) intervention characteristics—evidence, complexity, relative advantage; (2) outer setting—peer pressure, external policies; (3) inner setting—structural characteristics, readiness for implementation, culture; (4) individual characteristics—knowledge about intervention, self-efficacy; and (5) process—planning, engaging stakeholders, champions, execution.
Using both individual and organizational constructs to effectively characterize the relationships, needs, intentions, and organizational characteristics of primary and cancer care providers throughout VHA will be key to designing successful interventions to broadly ensure quality survivorship care. The best interventions to improve survivorship care will likely vary across facilities based on contextual factors such as cancer specialist availability, facility characteristics, and the current delivery system for survivorship care.
Intervention modalities currently being used by the VHA Office of Specialty Care Transformation to improve access to specialty care are indeed transformative tools to optimize the quality of survivorship care. The latter builds on a successful approach developed and widely used in New Mexico, which makes the expertise of academic specialists at the University of New Mexico available throughout the state, using video teleconferencing.93,94 The opportunities for video-enabled interaction between specialists and PCPs in VHA, both in consultation about specific patients and in educational sessions to enhance PCP knowledge and self-efficacy in managing patients requiring specialty knowledge, are revolutionary for cancer care.93,95
Conclusions
Due to the expanding population of veteran prostate cancer survivors, improving their QOL by ensuring proper cancer surveillance, effectively managing their treatment complications and transitions of cancer care will reduce risk and provide timely management of symptoms and disease recurrence.
Understanding how variation in the VHA cancer specialist workforce impacts the quality of cancer survivorship care is a critical step towards optimizing veteran cancer care. Through this understanding, communication between PCPs, PACT, and cancer specialists can be improved via theory-based quality improvement tools to address gaps in the quality of prostate and other VHA cancer survivorship care. Interventions designed to enhance PCP self-efficacy in delivering high-quality prostate cancer survivor care may improve job satisfaction among PCPs and specialists.
Clarifying issues in the delivery of optimal prostate cancer survivorship care may inform models for other cancer survivorship care in the VHA. The contextual factors contributing to a VHA facility’s performance for prostate cancer survivorship care may be very relevant to the facility’s performance for other types of cancer survivorship care. A facility’s primary care organizational structure, cancer specialist workforce, and oncology-specific facility characteristics vary little across cancer types, suggesting that a better understanding of how to improve PSA surveillance for prostate cancer, the most common cancer treated in the VHA, should apply to carcinoembryonic antigen surveillance for colon cancer, hematology studies for lymphoma, and the surveillance of other malignancies in the VHA.96,97
The VHA National Cancer Strategy stressed the importance of meeting or exceeding accepted national standards of quality cancer care. Therefore, understanding the relationship between quality of cancer survivorship care and the cancer specialist workforce and its interface with primary care is critical to this goal, as is elucidation of the other barriers preventing optimal care. Last, embracing VHA’s latest telemedicine initiatives, including video teleconferencing to improve prostate cancer care, has the potential to transform this system into a national leader in prostate cancer survivorship care.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article. Dr. Skolarus is supported by a VA HSR&D Career Development Award - 2 (CDA 12-171). Drs. Hawley (PI) and Skolarus (Co-I) are supported by VA HSR&D IIR (12-116).
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect an endorsement by or opinion of Federal Practitioner, Frontline Medical Communications, the U.S. Air Force, the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drug combinations–including indications, contraindications, warnings, and adverse effects–before administering pharmacologic therapy to patients.
Prostate cancer is the most common cancer diagnosis among U.S. veterans.1 More than 12,000 veterans will be diagnosed with prostate cancer in 2014, to join more than 200,000 veteran survivors.1 Because its incidence increases with age and nearly half of veterans are aged ≥ 65 years, the clinical and economic burdens of prostate cancer are expected to increase.2 Fortunately, > 80% of these men will have local disease with 5-year cancer-specific survivals of 98%.3 Even among the small population of veterans whose disease returns after treatment, < 1 in 5 will die of prostate cancer within 10 years.4
Thus, most men live with prostate cancer and its sequelae rather than die of it, similar to other chronic diseases. In 2003, the VHA outlined a National Cancer Strategy, indicating priorities for quality cancer care and access to care for all veterans with cancer.5 Importantly, this directive recognized prostate cancer as a service-connected condition for men exposed to the herbicide Agent Orange.6 For all these reasons, understanding the delivery of prostate cancer survivorship care has tremendous cost and quality implications for the VHA.
SURVIVORSHIP CARE
Due to the extensive focus on screening and initial treatment, very little prostate cancer survivorship research exists either within or outside VHA. In fact, a 2011 literature review found that < 10 prostate cancer survivorship studies were published annually.7 Because long-term survival is increasingly common after any cancer diagnosis, better understanding cancer survivorship (ie, the chronic care following diagnosis and treatment) and the distinct needs of cancer survivors are central to cancer care quality.8,9
A 2005 breakthrough report from the Institute of Medicine, From Cancer Patient to Cancer Survivor: Lost in Transition, emphasized the distinct issues facing cancer survivors and called for an increased emphasis on cancer survivors and their care from both clinical and research perspectives (Table).10
Due to the expanding population of veteran prostate cancer survivors, this report has increasing relevance to VHA.11 For prostate cancer survivors in particular, up to 70% have persistent symptoms (eg, incontinence, impotence) with some symptoms persisting 15 years after treatment, indicating the need for ongoing care and similarity to other chronic diseases.12,13
Despite this growing need and the universal provider access to electronic medical records, VHA, like most other integrated delivery systems, does not have a systematic organizational approach to deal with its prostate cancer survivors, indicating a tremendous opportunity.
One recent proposal for supporting survivorship care in the VHA is a Patient-Aligned Specialty Team for oncology to provide comprehensive cancer care through tumor boards, multispecialty clinics, care coordinators/navigators, and patient education.14
Symptom Burden
The 3 usual approaches to treatment of prostate cancer are (1) surgery (radical prostatectomy); (2) radiation therapy (brachytherapy or external “beam” radiation); and (3) observation (watchful waiting and active surveillance).15-18 While some men do choose observation initially, ultimately many undergo some form of surgical or radiation treatment.19 Unfortunately, long-term adverse effects (AEs) of these treatments are common and vary by treatment type. Men may experience ongoing problems with urinary control (eg, urinary incontinence), sexual function (eg, impotence), hormonal (eg, fatigue, depression), and bowel function (eg, diarrhea and fecal incontinence) far beyond that of age-matched controls.13,15,20-27
Up to 75% of men report problems with erectile dysfunction after prostatectomy, compared with 25% who receive brachytherapy, and 40% who receive brachytherapy plus external beam radiation.20,22,26,28 Urinary problems include both incontinence and pain with urination, which may improve over time with medical and nonmedical management approaches.26,27 Among patients treated with radiation therapy, between 40% and 55% report urinary problems as long as 8 years posttreatment (incontinence and/or pain).26,27,29,30 Unlike surgery, radiation therapy is also associated with bowel problems posttreatment, including rectal urgency and diarrhea.25,31
Although the greatest symptom burden and associated reduction in quality of life (QOL) occurs initially following treatment, many prostate cancer survivors experience considerable symptom burden for years following treatment.21,22,26,32-35 This persistence of symptoms is documented among thousands of patients after prostate cancer treatment, most of which are nonveterans. For example, among men with prostate cancer and no sexual, urinary, or hormonal problems at baseline, 9% to 83% reported severe problems in at least 1 domain 3 years after treatment with surgery or radiation.36
Gore and colleagues demonstrated persistent symptoms among 475 prostate cancer patients for up to 48 months following initial treatment.27 The Michigan Prostate Cancer Survivor Study, a registry-based survey of 2,500 prostate cancer survivors responding about 9 years postdiagnosis, found that up to 70% reported ongoing problems with AEs, some of whom were more than 15 years removed from primary treatment.12 Addressing these symptoms through medical and self-management approaches is one way to reduce their impact and improve QOL among prostate cancer survivors.
Despite the size of the veteran prostate cancer survivor population, most research documenting symptom burden and reduced QOL is from nonveterans. Because veterans often experience greater disease burden than that of the general population, their symptom burden would be expected to be similar or greater than that reported among nonveterans. Although there has been no comprehensive assessment of symptom burden across the VHA as a whole, research to understand optimal approaches to support veteran prostate cancer survivors with self- and medical management of their treatment related symptoms seems warranted.
Self-Management
Though there have been no comprehensive self-management interventions directed to help survivors limit the impact of prostate cancer treatment sequelae in everyday life, evidence suggests that such an intervention is likely to have a positive impact.37 For example, urinary symptoms can be self-managed through a variety of approaches, including emptying the bladder at regular intervals before it gets too full and pelvic floor (ie, Kegel) exercises to help decrease urinary leakage episodes. In fact, a randomized trial demonstrated a 50% decrease in incontinence episodes among prostate cancer survivors who used pelvic floor muscle training and bladder control strategies.38 A recent systematic review suggests that exercise, another self-management strategy, improves incontinence, energy level, body constitution, and QOL after treatment for prostate cancer.37 Exercise among prostate cancer survivors is also associated with decreased prostate cancer-specific and overall mortality.39
For sexual function after prostate cancer treatment, minimizing tobacco and excessive alcohol use and communicating with partners about feelings and sex are self-management strategies for improving sexual relationships.40 Avoiding spicy and greasy foods, coffee and alcohol, and staying well-hydrated may help limit the adverse bowel effects of radiation (ie, radiation proctitis) among prostate cancer survivors.41 However, there are no systematic mechanisms to share these strategies with veterans or nonveterans.
Medical Management
Recommendations for the medical management of prostate cancer-related AEs have recently been updated by the Michigan Cancer Consortium’s Prostate Cancer Action Committee and are available at www.prostatecancerdecision.org.42 Originally developed in 2009, these recommendations were directed toward the management of common posttreatment problems to minimize their impact on men who have been treated for prostate cancer, their families, caregivers, partners, and primary care providers (PCPs).
The recommendations combine expert opinion and evidence-based strategies for identifying recurrence and managing specific symptoms, including erectile dysfunction, urinary incontinence, bowel problems, hot flashes, bone health, gynecomastia, relationship issues, and metabolic syndrome. The increasing recognition that comprehensive, point-of-care resources are needed to direct survivorship care is fueling tremendous efforts targeting primary and specialty care providers from many major cancer stakeholder organizations (ie, American Cancer Society, National Comprehensive Cancer Network, etc).43-45
Primary care providers often consult prostate cancer specialists (urologists and radiation and medical oncologists) for assistance in managing prostate cancer survivors.46 However, it is not clear whether the supply of cancer specialists is capable of meeting the increasing needs of cancer survivors and their PCPs.47 VHA urologists vary tremendously in their regional availability from < 1 per 100,000 patients in Little Rock, Arkansas, to > 10 urologists per 100,000 patients in New York City.48 Similar variation exists for medical oncologists in the VHA. For prostate cancer, the urologist workforce impacts screening rates and cancer-related mortality.49,50 Yet how this workforce variation influences quality of survivorship care, particularly among PCPs dependent on specialist expertise, is unknown.
A better understanding of these relationships will help inform whether interventions to improve survivorship quality of care need to target PCPs with less access to prostate cancer specialists (eg, rural providers through telemedicine initiatives); survivorship care coordination at sites with more cancer specialists; or other potential barriers, such as knowledge gaps pertaining to AE evaluation and management. Each of these barriers to optimal care would be addressed through different interventions.
The long natural history of prostate cancer coupled with the number of survivors basically ensures that PCPs are faced with managing these men and their symptom burdens.51 However, it is often undecided who has primary responsibility for survivorship care.52,53 When queried regarding responsibility for prostate cancer survivorship care, about half of PCPs from one state-based survey felt that it was appropriate for either the cancer specialist or themselves to provide such care.12 Another study revealed high discordance among cancer specialists and PCPs regarding who should provide follow-up care, cancer screening, and general preventive care.54 Without clear role identification, poor communication between primary and specialty care fosters fragmented, expensive, and even poor quality survivorship care.55
Optimizing the delivery of survivorship care among cancer specialists and PCPs is also difficult, because comprehensive prostate cancer survivorship guidelines that might delineate responsibilities and recommend referral practices are just becoming available. In fact, the American Cancer Society just released its Prostate Cancer Survivorship Guidelines in June 2014.10,56,57 Primary care providers may be willing to take on increased responsibility for survivorship care with appropriate specialist support, including timely access to specialist evaluation.54,58 Moreover, PCPs are usually better at supporting cancer survivors’ general health as well.51,58 Therefore, defining the interface between PCPs, their medical home (ie, Patient-Aligned Care Team), and the limited supply of cancer specialists is necessary to streamline information exchange and care transitions.59
Understanding symptom management (eg, incontinence, impotence) across this interface is also critical to the design and implementation of survivorship quality improvement interventions. Promoting clear responsibilities for prostate-specific antigen surveillance, symptom management, and bone density testing for men treated with androgen deprivation therapy across the primary-specialty care interface is a potential starting point.
Transformative Tools
Whether targeting cancer care or not, quality improvement interventions often lack insight into the causal mechanisms by which they effect change.60-62 This is particularly true for interventions targeting clinician behavior change, such as improving uptake of evidence-based practice.63,64 For example, the effectiveness of audit with feedback interventions to improve guideline adherence ranges from 1% to 16%.65-69 The same intervention can vary in its effectiveness, depending on context.70-72 Barriers and enablers that vary by provider, facility, and other contextual factors (eg, workforce, location) contribute to this variable effectiveness.73-79 For this reason, a guiding theoretical framework is useful to understand an intervention’s transferability among different settings, as well as to ensure comprehensive assessment of the factors that can prevent uptake of evidence-based practice.80-83 For example, a theoretical framework might provide insight into how causal mechanisms of an intervention to improve cancer survivorship care might vary in a community-based outpatient clinic vs a tertiary center.84-86
A guiding theoretical framework is even more useful when used to design quality improvement interventions.82,83,87,88 Mapping barriers to theoretical constructs, and theoretical constructs to interventions to facilitate clinician behavior change can assist in planning strategies for effective implementation across a range of settings.88 While psychological theories like the Theoretical Domains Framework and Theory of Planned Behavior are pertinent for individual behavior change, understanding how best to implement interventions targeted at the facility level requires a broader perspective focused on context.83,88-92
The Consolidated Framework for Implementation Research (CFIR) provides a comprehensive, practical taxonomy for understanding important organizational, individual, and intervention characteristics to consider during an implementation process.75,76 The CFIR framework provides the broader contextual milieu contributing to the quality of survivorship care at the facility level across 5 domains: (1) intervention characteristics—evidence, complexity, relative advantage; (2) outer setting—peer pressure, external policies; (3) inner setting—structural characteristics, readiness for implementation, culture; (4) individual characteristics—knowledge about intervention, self-efficacy; and (5) process—planning, engaging stakeholders, champions, execution.
Using both individual and organizational constructs to effectively characterize the relationships, needs, intentions, and organizational characteristics of primary and cancer care providers throughout VHA will be key to designing successful interventions to broadly ensure quality survivorship care. The best interventions to improve survivorship care will likely vary across facilities based on contextual factors such as cancer specialist availability, facility characteristics, and the current delivery system for survivorship care.
Intervention modalities currently being used by the VHA Office of Specialty Care Transformation to improve access to specialty care are indeed transformative tools to optimize the quality of survivorship care. The latter builds on a successful approach developed and widely used in New Mexico, which makes the expertise of academic specialists at the University of New Mexico available throughout the state, using video teleconferencing.93,94 The opportunities for video-enabled interaction between specialists and PCPs in VHA, both in consultation about specific patients and in educational sessions to enhance PCP knowledge and self-efficacy in managing patients requiring specialty knowledge, are revolutionary for cancer care.93,95
Conclusions
Due to the expanding population of veteran prostate cancer survivors, improving their QOL by ensuring proper cancer surveillance, effectively managing their treatment complications and transitions of cancer care will reduce risk and provide timely management of symptoms and disease recurrence.
Understanding how variation in the VHA cancer specialist workforce impacts the quality of cancer survivorship care is a critical step towards optimizing veteran cancer care. Through this understanding, communication between PCPs, PACT, and cancer specialists can be improved via theory-based quality improvement tools to address gaps in the quality of prostate and other VHA cancer survivorship care. Interventions designed to enhance PCP self-efficacy in delivering high-quality prostate cancer survivor care may improve job satisfaction among PCPs and specialists.
Clarifying issues in the delivery of optimal prostate cancer survivorship care may inform models for other cancer survivorship care in the VHA. The contextual factors contributing to a VHA facility’s performance for prostate cancer survivorship care may be very relevant to the facility’s performance for other types of cancer survivorship care. A facility’s primary care organizational structure, cancer specialist workforce, and oncology-specific facility characteristics vary little across cancer types, suggesting that a better understanding of how to improve PSA surveillance for prostate cancer, the most common cancer treated in the VHA, should apply to carcinoembryonic antigen surveillance for colon cancer, hematology studies for lymphoma, and the surveillance of other malignancies in the VHA.96,97
The VHA National Cancer Strategy stressed the importance of meeting or exceeding accepted national standards of quality cancer care. Therefore, understanding the relationship between quality of cancer survivorship care and the cancer specialist workforce and its interface with primary care is critical to this goal, as is elucidation of the other barriers preventing optimal care. Last, embracing VHA’s latest telemedicine initiatives, including video teleconferencing to improve prostate cancer care, has the potential to transform this system into a national leader in prostate cancer survivorship care.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article. Dr. Skolarus is supported by a VA HSR&D Career Development Award - 2 (CDA 12-171). Drs. Hawley (PI) and Skolarus (Co-I) are supported by VA HSR&D IIR (12-116).
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect an endorsement by or opinion of Federal Practitioner, Frontline Medical Communications, the U.S. Air Force, the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drug combinations–including indications, contraindications, warnings, and adverse effects–before administering pharmacologic therapy to patients.
1. Veterans Affairs Central Cancer Registry (VACCR) [intranet database]. Washington, DC: US Department of Veterans Affairs; 1995.
2. U.S. Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. Profile of Veterans: 2009. Data from the American Community Survey. U.S. Department of Veterans Affairs Website. http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2009_FINAL.pdf. Accessed July 23, 2014.
3. National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. SEER Website. http://www.seer.cancer.gov. Accessed July 23, 2014.
4. Uchio EM, Aslan M, Wells CK, Calderone J, Concato J. Impact of biochemical recurrence in prostate cancer among US veterans. Arch Intern Med. 2010;170(15):1390-1395.
5. U.S. Department of Veterans Affairs. VHA DIRECTIVE 2003-034. Department of Veterans Affairs, Veterans Health Administration Website. http://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=261. Accessed July 23, 2014.
6. Chamie K, DeVere White RW, Lee D, Ok JH, Ellison LM. Agent Orange exposure, Vietnam War veterans, and the risk of prostate cancer. Cancer. 2008;113(9):2464-2470.
7. Harrop JP, Dean JA, Paskett ED. Cancer survivorship research: A review of the literature and summary of current NCI-designated cancer center projects. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2042-2047.
8. Centers for Disease Control and Prevention. Cancer survivors—United States, 2007. MMWR Morb Mortal Wkly Rep. 2011;60(9):269-272.
9. Ganz PA. Survivorship: Adult cancer survivors. Prim Care. 2009;36(4):721-741.
10. Hewitt M, Greenfield S, Stovall E, eds. From Cancer Patient to Cancer Survivor: Lost in Translation. Institute of Medicine and National Research Council of the National Academies. Washington, DC: The National Academies Press; 2005.
11. Moye J, Schuster JL, Latini DM, Naik AD. The future of cancer survivorship care for veterans. Fed Pract. 2010;27(3):36-43.
12. Darwish-Yassine M, Berenji M, Wing D, et al. Evaluating long-term patient-centered outcomes following prostate cancer treatment: findings from the Michigan Prostate Cancer Survivor study. J Cancer Surviv. 2014;8(1):121-130.
13. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250-1261.
14. Kelley M. VA Proposes Team-Based Model for Prostate Cancer Care. U.S. Medicine Website. http://www.usmedicine.com/agencies/department-of-veterans-affairs/va-proposes-team-based-model-for-prostate-cancer-care. Published May 2013. Accessed April 28, 2014.
15. Bill-Axelson A, Holmberg L, Ruutu M, et al; Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352(19):1977-1984.
16. Skolarus TA, Miller DC, Zhang Y, Hollingsworth JM, Hollenbeck BK. The delivery of prostate cancer care in the United States: Implications for delivery system reform. J Urol. 2010;184(6):2279-2284.
17. NCCN Clinical Practice Guidelines in Oncology Prostate Cancer, Version 2.2014. NCCN Website. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed April 1, 2014.
18. Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360(9327):103-106.
19. Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28(1):126-131.
20. Alemozaffar M, Regan MM, Cooperberg MR, et al. Prediction of erectile function following treatment for prostate cancer. JAMA. 2011;306(11):1205-1214.
21. Dandapani SV, Sanda MG. Measuring health-related quality of life consequences from primary treatment for early-state prostate cancer. Semin Radiat Oncol. 2008;18(1):67-72.
22. Johansson E, Steineck G, Holmberg L, et al; SPCG-4 Investigators. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Proste Cancer Group-4 randomised trial. Lancet Oncol. 2011;12(9):891-899.
23. Johansson E, Bill-Axelson A, Holmberg L, Onelöv E, Johansson JE, Steineck G; Scandinavian Prostate Cancer Group Study No 4. Time, symptom burden, androgen deprivation, and self-assessed quality of life after radical prostatectomy or watchful waiting: the Randomized Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4) clinical trial. Eur Urol. 2009;55(2):422-430.
24. Steineck G, Helgesen F, Adofsson J, et al; Scandanavian Prostatic Cancer Group Study Number 4. Quality of life after radial prostatectomy or watchful waiting. N Engl J Med. 2002;347(11):790-796.
25. Penson DF, Litwin MS. Quality of life after treatment for prostate cancer. Curr Urol Rep. 2003;4(3):185-195.
26. Ferrer M, Suárez JF, Guedea F, et al; Multicentric Spanish Group of Clinically Localized Prostate Cancer. Health-related quality of life 2 years after treatment with radical prostatectomy, prostate brachytherapy, or external beam radiotherapy in patients with clinically localized prostate cancer. Int J Radiation Oncology Bio Phys. 2008;72(2):421-432.
27. Gore JL, Kwan L, Lee SP, Reiter RE, Litwin MS. Survivorship beyond convalescence: 48-month quality-of-life outcomes after treatment for localized prostate cancer. J Natl Cancer Inst. 2009;101(12):888-892.
28. Miller DC, Wei JT, Dunn RL, et al. Use of medications or devices for erectile dysfunction among long-term prostate cancer treatment survivors: potential influence of sexual motivation and/or indifference. Urology. 2006;68(1):166-171.
29. Wilt TJ, Shamliyan TA, Taylor BC, MacDonald R, Kane RL. Association between hospital and surgeon radical prostatectomy volume and patient outcomes: A systematic review. J Urol. 2008;180(3):820-828; discussion 828-829.
30. Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: Health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772-2780.
31. Michaelson MD, Cotter SE, Gargollo PC, Zietman AL, Dahl DM, Smith MR. Management of complications of prostate cancer treatment. CA Cancer J Clin. 2008;58(4):196-213.
32. Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: Results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2000;92(19):1582-1592.
33. Potosky AL, Davis WW, Hoffman RM, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: The prostate cancer outcomes study. J Natl Cancer Inst. 2004;96(18):1358-1367.
34. Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20(2):557-566.
35. Harrington CB, Hansen JA, Moskowitz M, Todd BL, Fuerestein M. It’s not over when it’s over: Long-term symptoms in cancer survivors—a systematic review. Int J Psychiatry Med. 2010;40(2):163-181.
36. Pardo Y, Guedea F, Aguiló F, et al. Quality-of-life impact of primary treatments for localized prostate cancer in patients without hormonal treatment [published correction appears in J Clin Oncol. 2011;29(6):779]. J Clin Oncol. 2010;28(31):4687-4696.
37. Baumann FT, Zoph EM, Bloch W. Clinical exercise interventions in prostate cancer patients—a systematic review of randomized controlled trials. Support Care Cancer. 2011;20(2):221-233.
38. Goode PS, Burgio KL, Johnson TM II, et al. Behavioral therapy with or without biofeedback and pelvic floor electrical stimulation for persistent postprostatectomy incontinence: A randomized controlled trial. JAMA. 2011;305(2):151-159.
39. Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29(6):726-732.
40. Meldrum DR, Gambone JC, Morris MA, Ignarro LJ. A multifaceted approach to maximize erectile function and vascular health. Fertil Steril. 2010;94(7):2514-2520.
41. National Cancer Institute. Gastrointestinal Complications. NCCN Website. http://www.cancer.gov/cancertopics/pdq/supportivecare/gastrointestinalcomplications/HealthProfessional. Accessed June 27, 2014.
42. Michigan Cancer Consortium. Michigan Cancer Consortium Recommendations for Prostate Cancer Survivorship Care. Prostate Cancer Decision Website. http://www.prostatecancerdecision.org/PDFs/Algorithms2013/RecommProstateCancerCare-09182013.pdf. Accessed April 10, 2014.
43. Cowens-Alvarado R, Sharpe K, Pratt-Chapman M, et al. Advancing survivorship care through the National Cancer Survivorship Resource Center: Developing American Cancer Society guidelines for primary care providers. CA Cancer J Clin. 2013;63(3):147-150.
44. American Society of Clinical Oncology. Cancer Survivorship. American Society of Clinical Cancer Website. http://www.asco.org/practice-research
/cancer-survivorship. Accessed May 1, 2014.
45. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Survivorship. V1. 2014. NCCN Website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#survivorship. Accessed April 24, 2014.
46. Skolarus TA, Holmes-Rovner M, Northouse LL, et al. Primary care perspectives on prostate cancer survivorship: Implications for improving quality of care. Urol Oncol. 2013;31(6):727-732.
47. Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future supply and demand for oncologists: Challenges to assuring access to oncology services. J Oncol Pract. 2007;3(2):79-86.
48. VHA Office of Productivity, Efficiency, & Staffing (OPES). Fiscal Year 2011 VHA Physician Workforce & Support Staff Data by VISN Facility. Washington, DC: VHA Office of Productivity, Efficiency, & Staffing; 2011.
49. Odisho AY, Cooperberg MR, Fradet V, Ahmad AE, Carroll PR. Urologist density and county-level urologic cancer mortality. J Clin Oncol. 2010;28(15):2499-2504.
50. Odisho AY, Fradet V, Cooperberg MR, Ahmad AE, Carroll PR. Geographic distribution of urologists throughout the United States using a county level approach. J Urol. 2009;181(2):760-765; discussion 765-766.
51. Pollack LA, Adamache W, Ryerson AB, Eheman CR, Richardson LC. Care of long-term cancer survivors: Physicians seen by Medicare enrollees surviving longer than 5 years. Cancer. 2009;115(22):5284-5295.
52. Ganz PA, Casillas J, Hahn EE. Ensuring quality care for cancer survivors: Implementing the survivorship care plan. Semin Oncology Nurs. 2008;24(3):208-217.
53. Bober SL, Recklitis CJ, Campbell EG, et al. Caring for cancer survivors: A survey of primary care physicians. Cancer. 2009;115(suppl 18):4409-4418.
54. Cheung WY, Neville BA, Cameron DB, Cook EF, Earle CC. Comparisons of patient and physician expectations for cancer survivorship care. J Clin Oncol. 2009;27(15):2489-2495.
55. Skolarus TA, Zhang Y, Hollenbeck BK. Understanding fragmentation of prostate cancer survivorship care: Implications for cost and quality. Cancer. 2012;118(11):2837-2845.
56. Grimshaw JM, Winkens RA, Shirran L, et al. Interventions to improve outpatient referrals from primary care to secondary care. Cochrane Database Syst Rev. 2005;(3):CD005471.
57. Skolarus TA, Wolf AM, Erb NL, et al. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin. 2014;64(4):225-249.
58. Jacobs LA, Palmer SC, Schwartz LA, et al. Adult cancer survivorship: Evolution, research, and planning care. CA Cancer J Clin. 2009;59(6):391-410.
59. O’Malley AS, Reschovsky JD. Referral and consultation communication between primary care and specialist physicians: Finding common ground. Arch Intern Med. 2011;171(1):56-65.
60. Krein SL, Damschroder LJ, Kowalski CP, Forman J, Hofer TP, Saint S. The influence of organizational context on quality improvement and patient safety efforts in infection prevention: A multi-center qualitative study. Soc Sci Med. 2010;71(9):1692-1701.
61. McDermott KA, Helfrich CD, Sales AE, Rumsfeld JS, Ho PM, Fihn SD. A review of interventions and system changes to improve time to reperfusion for ST-segment elevation myocardial infarction. J Gen Intern Med. 2008;23(8):1246-1256.
62. Grimshaw JM, Zwarenstein M, Tetroe JM, et al. Looking inside the black box: A theory-based process evaluation alongside a randomised controlled trial of printed educational materials (the Ontario printed educational message, OPEM) to improve referral and prescribing practices in primary care in Ontario, Canada. Implement Sci. 2007;2:38.
63. Gagliardi AR, Brouwers MC, Palda VA, Lemieux-Charles L, Grimshaw JM. How can we improve guideline use? A conceptual framework of implementability. Implement Sci. 2011;6:26.
64. Godin G, Bélanger-Gravel A, Eccles M, Grimshaw J. Healthcare professionals’ intentions and behaviours: a systematic review of studies based on social cognitive theories. Implement Sci. 2008;2008(3):36.
65. Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8(6):iii-iv, 1-72.
66. Hysong SJ, Teal CR, Khan MJ, Haidet P. Improving quality of care through improved audit and feedback. Implement Sci. 2012;7(1):45.
67. Hysong SJ. Meta-analysis: Audit and feedback features impact effectiveness on care quality. Med Care. 2009;47(3):356-363.
68. Hysong SJ, Best RG, Pugh JA. Audit and feedback and clinical practice guideline adherence: Making feedback actionable. Implement Sci. 2006;1:9.
69. Sales AE, Schalm C. Data for improvement and clinical excellence: Protocol for an audit with feedback intervention in long-term care. Implement Sci. 2010;5(1):74.
70. Helfrich CD, Blevins D, Smith JL, et al. Predicting implementation from organizational readiness for change: A study protocol. Implement Sci. 2011;6(1):76.
71. Pineros SL, Sales AE, Li YF, Sharp ND. Improving care to patients with ischemic heart disease: Experiences in a single network of the Veterans Health Administration. Worldviews Evid Based Nurs. 2004;1(suppl 1):S33-S40.
72. Sales AE, Pineros SL, Magid DJ, Every NR, Sharp ND, Rumsfeld JS. The association between clinical integration of care and transfer of veterans with acute coronary syndromes from primary care VHA hospitals. BMC Health Serv Res. 2005;5(1):2.
73. Grimshaw JM, Eccles MP, Lavis JN, Hill SJ, Squires JE. Knowledge translation of research findings. Implement Sci. 2012;7(1):50.
74. Sales AE, Lapham GG, Squires J, et al. Organizational factors associated with decreased mortality among Veterans Affairs patients with an ICU stay. Comput Inform Nurs. 2011;29(9):496-501.
75. Damschroder LJ, Hagedorn HJ. A guiding framework and approach for implementation research in substance use disorders treatment. Psychol Addict Behav. 2011;25(2):194-205.
76. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50.
77. Salkeld E, Leaver CA, Guttmann A, et al. Barriers and facilitators to the implementation of Ontario’s emergency department clinical decision unit pilot program: A qualitative study. CJEM. 2011;13(6):363-371.
78. Sharp ND, Pineros SL, Hsu C, Starks H, Sales AE. A qualitative study to identify barriers and facilitators to implementation of pilot interventions in the Veterans Health Administration (VHA) Northwest Network. Worldviews Evid Based Nurs. 2004;1(2):129-139.
79. Render ML, Hasselbeck R, Freyberg RW, Hofer TP, Sales AE, Almenoff PL; VA ICU Clinical Advisory Group. Reduction of central line infections in Veterans Administration intensive care units: An observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725-732.
80. Grimshaw JM, Eccles MP, Steen N, et al. Applying psychological theories to evidence-based clinical practice: Identifying factors predictive of lumbar spine x-ray for low back pain in UK primary care practice. Implement Sci. 2011;6(1):55.
81. Eccles M, Grimshaw J, Walker A, Johnston M, Pitts N. Changing the behavior of healthcare professionals: The use of theory in promoting the uptake of research findings. J Clin Epidemiol. 2005;58(2):107-112.
82. Davies P, Walker AE, Grimshaw JM. A systematic review of the use of theory in the design of guideline dissemination and implementation strategies and interpretation of the results of rigorous evaluations. Implement Sci. 2010;5(1):14.
83. Sales A, Smith J, Curran G, Kochevar L. Models, strategies, and tools. Theory in implementing evidence-based findings into health care practice. J Gen Intern Med. 2006;21(suppl 2):S43-S49.
84. Dixon-Woods M, Bosk CL, Aveling EL, Goeschel CA, Pronovost PJ. Explaining Michigan: Developing an ex post theory of a quality improvement program. Milbank Q. 2011;89(2):167-205.
85. Damschroder LJ, Goodrich DE, Robinson CH, Fletcher CE, Lowery JC. A systematic exploration of differences in contextual factors related to implementing the MOVE! weight management program in VA: A mixed methods study. BMC Health Serv Res. 2011;11(1):248.
86. Ramsay CR, Thomas RE, Croal BL, Grimshaw JM, Eccles MP. Using the theory of planned behaviour as a process evaluation tool in randomised trials of knowledge translation strategies: A case study from UK primary care. Implement Sci. 2010;5(1):71.
87. Légaré F, Borduas F, Jacques A, et al. Developing a theory-based instrument to assess the impact of continuing professional development activities on clinical practice: A study protocol. Implement Sci. 2011;6(1):17.
88. French SD, Green SE, O’Connor DA, et al. Developing theory-informed behaviour change interventions to implement evidence into practice: A systematic approach using the Theoretical Domains Framework. Implement Sci. 2012;7(1):38.
89. Ajzen I. The theory of planned behaviour: Reactions and reflections. Psychol Health. 2011;26(9):1113-1127.
90. Fishbein M, Ajzen I. Theory-based behavior change interventions: Comments on Hobbis and Sutton. J Health Psychol. 2005;10(1):27-31; discussion 37-43.
91. Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50(2):179-211.
92. Francis JJ, O’Connor D, Curran J. Theories of behaviour change synthesised into a set of theoretical groupings: Introducing a thematic series on the theoretical domains framework. Implement Sci. 2012;7(1):35.
93. Arora S, Kalishman S, Thornton K, et al. Expanding access to hepatitis C virus treatment-Extension for Community Healthcare Outcomes (ECHO) project: Disruptive innovation in specialty care. Hepatology. 2010;52(3):1124-1133.
94. Arora S, Kalishman S, Dion D, et al. Partnering urban academic medical centers and rural primary care clinicians to provide complex chronic disease care. Health Aff (Milwood). 2011;30(6):1176-1184.
95. Horner K, Wagner E, Tufano J. Electronic consultations between primary and specialty care clinicians: Early insights. Issue Brief (Commonw Fund). 2011;23:1-14.
96. Kunitake H, Zheng P, Yothers G, et al. Routine preventive care and cancer surveillance in long-term survivors of colorectal cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol LTS-01. J Clin Oncol. 2010;28(36):5274-5279.
97. Ng A, Constine LS, Advani R; Expert Panel on Radiation Oncology-Hodgkin’s Lymphoma. ACR Appropriateness Criteria follow-up of Hodgkin’s lymphoma. [online publication]. Reston (VA): American College of Radiology (ACR); 2010.
1. Veterans Affairs Central Cancer Registry (VACCR) [intranet database]. Washington, DC: US Department of Veterans Affairs; 1995.
2. U.S. Department of Veterans Affairs. National Center for Veterans Analysis and Statistics. Profile of Veterans: 2009. Data from the American Community Survey. U.S. Department of Veterans Affairs Website. http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2009_FINAL.pdf. Accessed July 23, 2014.
3. National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. SEER Website. http://www.seer.cancer.gov. Accessed July 23, 2014.
4. Uchio EM, Aslan M, Wells CK, Calderone J, Concato J. Impact of biochemical recurrence in prostate cancer among US veterans. Arch Intern Med. 2010;170(15):1390-1395.
5. U.S. Department of Veterans Affairs. VHA DIRECTIVE 2003-034. Department of Veterans Affairs, Veterans Health Administration Website. http://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=261. Accessed July 23, 2014.
6. Chamie K, DeVere White RW, Lee D, Ok JH, Ellison LM. Agent Orange exposure, Vietnam War veterans, and the risk of prostate cancer. Cancer. 2008;113(9):2464-2470.
7. Harrop JP, Dean JA, Paskett ED. Cancer survivorship research: A review of the literature and summary of current NCI-designated cancer center projects. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2042-2047.
8. Centers for Disease Control and Prevention. Cancer survivors—United States, 2007. MMWR Morb Mortal Wkly Rep. 2011;60(9):269-272.
9. Ganz PA. Survivorship: Adult cancer survivors. Prim Care. 2009;36(4):721-741.
10. Hewitt M, Greenfield S, Stovall E, eds. From Cancer Patient to Cancer Survivor: Lost in Translation. Institute of Medicine and National Research Council of the National Academies. Washington, DC: The National Academies Press; 2005.
11. Moye J, Schuster JL, Latini DM, Naik AD. The future of cancer survivorship care for veterans. Fed Pract. 2010;27(3):36-43.
12. Darwish-Yassine M, Berenji M, Wing D, et al. Evaluating long-term patient-centered outcomes following prostate cancer treatment: findings from the Michigan Prostate Cancer Survivor study. J Cancer Surviv. 2014;8(1):121-130.
13. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250-1261.
14. Kelley M. VA Proposes Team-Based Model for Prostate Cancer Care. U.S. Medicine Website. http://www.usmedicine.com/agencies/department-of-veterans-affairs/va-proposes-team-based-model-for-prostate-cancer-care. Published May 2013. Accessed April 28, 2014.
15. Bill-Axelson A, Holmberg L, Ruutu M, et al; Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352(19):1977-1984.
16. Skolarus TA, Miller DC, Zhang Y, Hollingsworth JM, Hollenbeck BK. The delivery of prostate cancer care in the United States: Implications for delivery system reform. J Urol. 2010;184(6):2279-2284.
17. NCCN Clinical Practice Guidelines in Oncology Prostate Cancer, Version 2.2014. NCCN Website. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed April 1, 2014.
18. Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360(9327):103-106.
19. Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28(1):126-131.
20. Alemozaffar M, Regan MM, Cooperberg MR, et al. Prediction of erectile function following treatment for prostate cancer. JAMA. 2011;306(11):1205-1214.
21. Dandapani SV, Sanda MG. Measuring health-related quality of life consequences from primary treatment for early-state prostate cancer. Semin Radiat Oncol. 2008;18(1):67-72.
22. Johansson E, Steineck G, Holmberg L, et al; SPCG-4 Investigators. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Proste Cancer Group-4 randomised trial. Lancet Oncol. 2011;12(9):891-899.
23. Johansson E, Bill-Axelson A, Holmberg L, Onelöv E, Johansson JE, Steineck G; Scandinavian Prostate Cancer Group Study No 4. Time, symptom burden, androgen deprivation, and self-assessed quality of life after radical prostatectomy or watchful waiting: the Randomized Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4) clinical trial. Eur Urol. 2009;55(2):422-430.
24. Steineck G, Helgesen F, Adofsson J, et al; Scandanavian Prostatic Cancer Group Study Number 4. Quality of life after radial prostatectomy or watchful waiting. N Engl J Med. 2002;347(11):790-796.
25. Penson DF, Litwin MS. Quality of life after treatment for prostate cancer. Curr Urol Rep. 2003;4(3):185-195.
26. Ferrer M, Suárez JF, Guedea F, et al; Multicentric Spanish Group of Clinically Localized Prostate Cancer. Health-related quality of life 2 years after treatment with radical prostatectomy, prostate brachytherapy, or external beam radiotherapy in patients with clinically localized prostate cancer. Int J Radiation Oncology Bio Phys. 2008;72(2):421-432.
27. Gore JL, Kwan L, Lee SP, Reiter RE, Litwin MS. Survivorship beyond convalescence: 48-month quality-of-life outcomes after treatment for localized prostate cancer. J Natl Cancer Inst. 2009;101(12):888-892.
28. Miller DC, Wei JT, Dunn RL, et al. Use of medications or devices for erectile dysfunction among long-term prostate cancer treatment survivors: potential influence of sexual motivation and/or indifference. Urology. 2006;68(1):166-171.
29. Wilt TJ, Shamliyan TA, Taylor BC, MacDonald R, Kane RL. Association between hospital and surgeon radical prostatectomy volume and patient outcomes: A systematic review. J Urol. 2008;180(3):820-828; discussion 828-829.
30. Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: Health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772-2780.
31. Michaelson MD, Cotter SE, Gargollo PC, Zietman AL, Dahl DM, Smith MR. Management of complications of prostate cancer treatment. CA Cancer J Clin. 2008;58(4):196-213.
32. Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: Results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2000;92(19):1582-1592.
33. Potosky AL, Davis WW, Hoffman RM, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: The prostate cancer outcomes study. J Natl Cancer Inst. 2004;96(18):1358-1367.
34. Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20(2):557-566.
35. Harrington CB, Hansen JA, Moskowitz M, Todd BL, Fuerestein M. It’s not over when it’s over: Long-term symptoms in cancer survivors—a systematic review. Int J Psychiatry Med. 2010;40(2):163-181.
36. Pardo Y, Guedea F, Aguiló F, et al. Quality-of-life impact of primary treatments for localized prostate cancer in patients without hormonal treatment [published correction appears in J Clin Oncol. 2011;29(6):779]. J Clin Oncol. 2010;28(31):4687-4696.
37. Baumann FT, Zoph EM, Bloch W. Clinical exercise interventions in prostate cancer patients—a systematic review of randomized controlled trials. Support Care Cancer. 2011;20(2):221-233.
38. Goode PS, Burgio KL, Johnson TM II, et al. Behavioral therapy with or without biofeedback and pelvic floor electrical stimulation for persistent postprostatectomy incontinence: A randomized controlled trial. JAMA. 2011;305(2):151-159.
39. Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29(6):726-732.
40. Meldrum DR, Gambone JC, Morris MA, Ignarro LJ. A multifaceted approach to maximize erectile function and vascular health. Fertil Steril. 2010;94(7):2514-2520.
41. National Cancer Institute. Gastrointestinal Complications. NCCN Website. http://www.cancer.gov/cancertopics/pdq/supportivecare/gastrointestinalcomplications/HealthProfessional. Accessed June 27, 2014.
42. Michigan Cancer Consortium. Michigan Cancer Consortium Recommendations for Prostate Cancer Survivorship Care. Prostate Cancer Decision Website. http://www.prostatecancerdecision.org/PDFs/Algorithms2013/RecommProstateCancerCare-09182013.pdf. Accessed April 10, 2014.
43. Cowens-Alvarado R, Sharpe K, Pratt-Chapman M, et al. Advancing survivorship care through the National Cancer Survivorship Resource Center: Developing American Cancer Society guidelines for primary care providers. CA Cancer J Clin. 2013;63(3):147-150.
44. American Society of Clinical Oncology. Cancer Survivorship. American Society of Clinical Cancer Website. http://www.asco.org/practice-research
/cancer-survivorship. Accessed May 1, 2014.
45. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Survivorship. V1. 2014. NCCN Website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#survivorship. Accessed April 24, 2014.
46. Skolarus TA, Holmes-Rovner M, Northouse LL, et al. Primary care perspectives on prostate cancer survivorship: Implications for improving quality of care. Urol Oncol. 2013;31(6):727-732.
47. Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future supply and demand for oncologists: Challenges to assuring access to oncology services. J Oncol Pract. 2007;3(2):79-86.
48. VHA Office of Productivity, Efficiency, & Staffing (OPES). Fiscal Year 2011 VHA Physician Workforce & Support Staff Data by VISN Facility. Washington, DC: VHA Office of Productivity, Efficiency, & Staffing; 2011.
49. Odisho AY, Cooperberg MR, Fradet V, Ahmad AE, Carroll PR. Urologist density and county-level urologic cancer mortality. J Clin Oncol. 2010;28(15):2499-2504.
50. Odisho AY, Fradet V, Cooperberg MR, Ahmad AE, Carroll PR. Geographic distribution of urologists throughout the United States using a county level approach. J Urol. 2009;181(2):760-765; discussion 765-766.
51. Pollack LA, Adamache W, Ryerson AB, Eheman CR, Richardson LC. Care of long-term cancer survivors: Physicians seen by Medicare enrollees surviving longer than 5 years. Cancer. 2009;115(22):5284-5295.
52. Ganz PA, Casillas J, Hahn EE. Ensuring quality care for cancer survivors: Implementing the survivorship care plan. Semin Oncology Nurs. 2008;24(3):208-217.
53. Bober SL, Recklitis CJ, Campbell EG, et al. Caring for cancer survivors: A survey of primary care physicians. Cancer. 2009;115(suppl 18):4409-4418.
54. Cheung WY, Neville BA, Cameron DB, Cook EF, Earle CC. Comparisons of patient and physician expectations for cancer survivorship care. J Clin Oncol. 2009;27(15):2489-2495.
55. Skolarus TA, Zhang Y, Hollenbeck BK. Understanding fragmentation of prostate cancer survivorship care: Implications for cost and quality. Cancer. 2012;118(11):2837-2845.
56. Grimshaw JM, Winkens RA, Shirran L, et al. Interventions to improve outpatient referrals from primary care to secondary care. Cochrane Database Syst Rev. 2005;(3):CD005471.
57. Skolarus TA, Wolf AM, Erb NL, et al. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin. 2014;64(4):225-249.
58. Jacobs LA, Palmer SC, Schwartz LA, et al. Adult cancer survivorship: Evolution, research, and planning care. CA Cancer J Clin. 2009;59(6):391-410.
59. O’Malley AS, Reschovsky JD. Referral and consultation communication between primary care and specialist physicians: Finding common ground. Arch Intern Med. 2011;171(1):56-65.
60. Krein SL, Damschroder LJ, Kowalski CP, Forman J, Hofer TP, Saint S. The influence of organizational context on quality improvement and patient safety efforts in infection prevention: A multi-center qualitative study. Soc Sci Med. 2010;71(9):1692-1701.
61. McDermott KA, Helfrich CD, Sales AE, Rumsfeld JS, Ho PM, Fihn SD. A review of interventions and system changes to improve time to reperfusion for ST-segment elevation myocardial infarction. J Gen Intern Med. 2008;23(8):1246-1256.
62. Grimshaw JM, Zwarenstein M, Tetroe JM, et al. Looking inside the black box: A theory-based process evaluation alongside a randomised controlled trial of printed educational materials (the Ontario printed educational message, OPEM) to improve referral and prescribing practices in primary care in Ontario, Canada. Implement Sci. 2007;2:38.
63. Gagliardi AR, Brouwers MC, Palda VA, Lemieux-Charles L, Grimshaw JM. How can we improve guideline use? A conceptual framework of implementability. Implement Sci. 2011;6:26.
64. Godin G, Bélanger-Gravel A, Eccles M, Grimshaw J. Healthcare professionals’ intentions and behaviours: a systematic review of studies based on social cognitive theories. Implement Sci. 2008;2008(3):36.
65. Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8(6):iii-iv, 1-72.
66. Hysong SJ, Teal CR, Khan MJ, Haidet P. Improving quality of care through improved audit and feedback. Implement Sci. 2012;7(1):45.
67. Hysong SJ. Meta-analysis: Audit and feedback features impact effectiveness on care quality. Med Care. 2009;47(3):356-363.
68. Hysong SJ, Best RG, Pugh JA. Audit and feedback and clinical practice guideline adherence: Making feedback actionable. Implement Sci. 2006;1:9.
69. Sales AE, Schalm C. Data for improvement and clinical excellence: Protocol for an audit with feedback intervention in long-term care. Implement Sci. 2010;5(1):74.
70. Helfrich CD, Blevins D, Smith JL, et al. Predicting implementation from organizational readiness for change: A study protocol. Implement Sci. 2011;6(1):76.
71. Pineros SL, Sales AE, Li YF, Sharp ND. Improving care to patients with ischemic heart disease: Experiences in a single network of the Veterans Health Administration. Worldviews Evid Based Nurs. 2004;1(suppl 1):S33-S40.
72. Sales AE, Pineros SL, Magid DJ, Every NR, Sharp ND, Rumsfeld JS. The association between clinical integration of care and transfer of veterans with acute coronary syndromes from primary care VHA hospitals. BMC Health Serv Res. 2005;5(1):2.
73. Grimshaw JM, Eccles MP, Lavis JN, Hill SJ, Squires JE. Knowledge translation of research findings. Implement Sci. 2012;7(1):50.
74. Sales AE, Lapham GG, Squires J, et al. Organizational factors associated with decreased mortality among Veterans Affairs patients with an ICU stay. Comput Inform Nurs. 2011;29(9):496-501.
75. Damschroder LJ, Hagedorn HJ. A guiding framework and approach for implementation research in substance use disorders treatment. Psychol Addict Behav. 2011;25(2):194-205.
76. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50.
77. Salkeld E, Leaver CA, Guttmann A, et al. Barriers and facilitators to the implementation of Ontario’s emergency department clinical decision unit pilot program: A qualitative study. CJEM. 2011;13(6):363-371.
78. Sharp ND, Pineros SL, Hsu C, Starks H, Sales AE. A qualitative study to identify barriers and facilitators to implementation of pilot interventions in the Veterans Health Administration (VHA) Northwest Network. Worldviews Evid Based Nurs. 2004;1(2):129-139.
79. Render ML, Hasselbeck R, Freyberg RW, Hofer TP, Sales AE, Almenoff PL; VA ICU Clinical Advisory Group. Reduction of central line infections in Veterans Administration intensive care units: An observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725-732.
80. Grimshaw JM, Eccles MP, Steen N, et al. Applying psychological theories to evidence-based clinical practice: Identifying factors predictive of lumbar spine x-ray for low back pain in UK primary care practice. Implement Sci. 2011;6(1):55.
81. Eccles M, Grimshaw J, Walker A, Johnston M, Pitts N. Changing the behavior of healthcare professionals: The use of theory in promoting the uptake of research findings. J Clin Epidemiol. 2005;58(2):107-112.
82. Davies P, Walker AE, Grimshaw JM. A systematic review of the use of theory in the design of guideline dissemination and implementation strategies and interpretation of the results of rigorous evaluations. Implement Sci. 2010;5(1):14.
83. Sales A, Smith J, Curran G, Kochevar L. Models, strategies, and tools. Theory in implementing evidence-based findings into health care practice. J Gen Intern Med. 2006;21(suppl 2):S43-S49.
84. Dixon-Woods M, Bosk CL, Aveling EL, Goeschel CA, Pronovost PJ. Explaining Michigan: Developing an ex post theory of a quality improvement program. Milbank Q. 2011;89(2):167-205.
85. Damschroder LJ, Goodrich DE, Robinson CH, Fletcher CE, Lowery JC. A systematic exploration of differences in contextual factors related to implementing the MOVE! weight management program in VA: A mixed methods study. BMC Health Serv Res. 2011;11(1):248.
86. Ramsay CR, Thomas RE, Croal BL, Grimshaw JM, Eccles MP. Using the theory of planned behaviour as a process evaluation tool in randomised trials of knowledge translation strategies: A case study from UK primary care. Implement Sci. 2010;5(1):71.
87. Légaré F, Borduas F, Jacques A, et al. Developing a theory-based instrument to assess the impact of continuing professional development activities on clinical practice: A study protocol. Implement Sci. 2011;6(1):17.
88. French SD, Green SE, O’Connor DA, et al. Developing theory-informed behaviour change interventions to implement evidence into practice: A systematic approach using the Theoretical Domains Framework. Implement Sci. 2012;7(1):38.
89. Ajzen I. The theory of planned behaviour: Reactions and reflections. Psychol Health. 2011;26(9):1113-1127.
90. Fishbein M, Ajzen I. Theory-based behavior change interventions: Comments on Hobbis and Sutton. J Health Psychol. 2005;10(1):27-31; discussion 37-43.
91. Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50(2):179-211.
92. Francis JJ, O’Connor D, Curran J. Theories of behaviour change synthesised into a set of theoretical groupings: Introducing a thematic series on the theoretical domains framework. Implement Sci. 2012;7(1):35.
93. Arora S, Kalishman S, Thornton K, et al. Expanding access to hepatitis C virus treatment-Extension for Community Healthcare Outcomes (ECHO) project: Disruptive innovation in specialty care. Hepatology. 2010;52(3):1124-1133.
94. Arora S, Kalishman S, Dion D, et al. Partnering urban academic medical centers and rural primary care clinicians to provide complex chronic disease care. Health Aff (Milwood). 2011;30(6):1176-1184.
95. Horner K, Wagner E, Tufano J. Electronic consultations between primary and specialty care clinicians: Early insights. Issue Brief (Commonw Fund). 2011;23:1-14.
96. Kunitake H, Zheng P, Yothers G, et al. Routine preventive care and cancer surveillance in long-term survivors of colorectal cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol LTS-01. J Clin Oncol. 2010;28(36):5274-5279.
97. Ng A, Constine LS, Advani R; Expert Panel on Radiation Oncology-Hodgkin’s Lymphoma. ACR Appropriateness Criteria follow-up of Hodgkin’s lymphoma. [online publication]. Reston (VA): American College of Radiology (ACR); 2010.
radiation therapy, brachytherapy or external “beam” radiation, observation, sequelae, SYMPTOM BURDEN, SELF-MANAGEMENT, MEDICAL MANAGEMENT, Primary care providers, PCPs, TRANSFORMATIVE TOOLS, Consolidated Framework for Implementation Research, CFIR
radiation therapy, brachytherapy or external “beam” radiation, observation, sequelae, SYMPTOM BURDEN, SELF-MANAGEMENT, MEDICAL MANAGEMENT, Primary care providers, PCPs, TRANSFORMATIVE TOOLS, Consolidated Framework for Implementation Research, CFIR
Systemic Treatment and Outcomes of Metastatic Pancreatic Cancer
Pancreatic cancer incidence rates have been increasing among men and women since 2000. From 2006 to 2010, incidence rates increased by 1.3% per year, and the death rate for pancreatic cancer increased by 0.4% per year. An estimated 39,590 deaths are expected to occur in 2014, with similar numbers expected in women (19,420) and in men (20,170).1
Pancreatic cancer incidence and mortality rates vary across different racial/ethnic groups, with the highest rates in African Americans and the lowest rates in Asian Americans/Pacific Islanders. During 2005-2009, the incidence rate (per 100,000 persons) was 15.3 for African Americans, 11.6 for whites, and 8.8 for Asian Americans/Pacific Islanders. According to Surveillance, Epidemiology, and End Results (SEER) 18, for the period 2007-2011, the incidence rate was 17.2 for black men and 14 for white men; the incidence rate was 14.2 for black women and 10.7 for white women.2
Well-known risk factors for pancreatic cancer include tobacco use, chronic pancreatitis, obesity, diabetes, and alcohol use. Individuals with Lynch syndrome and certain other hereditary syndromes are at increased risk.3 Newer risk factors of interest include chronic infection with hepatitis B, hepatitis C, or Helicobacter pylori. These risk factors have in common a backdrop of inflammation, which may predispose the patient to developing pancreatic cancer.
Whether veterans as a group are at increased risk for developing pancreatic cancer is unclear. One study suggests there may be an increased risk in obese veterans.4 Certain high-risk groups among veterans have been identified, such as veterans with hepatitis C, diabetes, Gaucher disease, and women nurses from the Vietnam War era.5-8
Comorbidity, an area of increasing interest, was not a predictor of survival in a pilot study from one VA medical center.9
MANAGEMENT OF METASTATIC PANCREATIC CANCER
Management of metastatic pancreatic cancer has 2 components: systemic disease treatment and supportive care.
Systemic Disease Treatment
The standard of care at the VA for patients with metastatic pancreatic cancer follows the national guidelines, including the National Comprehensive Cancer Network (NCCN) guidelines.10
• For first-line therapy, currently recognized regimens include 5-fluorouracil (5-FU), with irinotecan, leucovorin, and oxaliplatin (FOLFIRINOX), gemcitabine with albumin-bound paclitaxel, and gemcitabine with erlotinib. Single- agent gemcitabine or supportive care is for patients with poor performance status.
• For second-line therapy, switching from a gemcitabine to a fluoropyrimidine-based regimen, and vice versa is a recognized option.
• When possible, participation in clinical trials is recommended.
Treatment for metastatic pancreatic cancer has not been satisfactory. In 1997, single-agent gemcitabine was approved by the FDA and became the standard of care when a randomized controlled trial of gemcitabine in symptomatic patients showed an increased survival compared with that of 5-FU.11 The median survival durations were 5.65 and 4.41 months for gemcitabine-treated and 5-FU-treated patients, respectively (P = .0025).
Many attempts to combine gemcitabine with other medications were unsuccessful until 2005 when gemcitabine in combination with erlotinib was approved by the FDA as a first-line therapy. A randomized trial showed a 2-week prolongation in survival with the addition of erlotinib. The median survivalwas 6.24 months in the combination group compared with 5.91 months in the gemcitabine group.12 Erlotinib is associatedwith increased skin toxicity anddiarrhea. Interestingly, trials with many other targeted agents, such as bevacizumab, have not demonstrated any survival improvement.13
In 2011, a French study showed that the multidrug combination of FOLFIRINOX in patients with good performance status (Eastern Cooperative Oncology Group Performance Status [ECOG PS] 0 or 1) increased overall mean survival to 11.1 months compared with 6.7 months in the gemcitabine group, but with more neutropenia, diarrhea, and sensory neuropathy.11
In 2013, a comparison of nab-paclitaxel plus gemcitabine and gemcitabine alone for patients with a Karnofsky performance-status score of 70 or more showed a median survival of 8.5 months vs 6.7 months, with more neutropenia, fatigue, and neurotoxicity in the combination group.14,15 This regimen was approved in September 2013 by the FDA as first-line treatment for patients with metastatic pancreatic cancer.
For second-line therapy, a recent phase 2 study of ruxolitinib and capecitabine demonstrated prolongation of overall survival in patients who had an elevated C-reactive protein (CRP). In the high CRP subgroup, 3- and 6-month survival was 48% and 42% with ruxolitinib, but was 29% and 11% with placebo.16 Further studies are planned.
Although these developments, summarized in the Table, are exciting, determining how they will apply to clinical practice will require careful patient selection and shared decision making about anticipated toxicities.
List of New Agents
Erlotinib is an oral agent and a small molecule that inhibits the tyrosine kinase activity of the human epidermal growth factor receptor (EGFR or HER1) pathway.
Albumin-bound paclitaxel (nab-paclitaxel) is effective in tumors that overexpress the albumin-binding protein secreted protein, acidic and rich in cysteine (SPARC), such as breast cancer, and used in pancreatic cancer (as this cancer also overexpresses SPARC). It is hypothesized, but not yet proven, that blockage of SPARC proteins in pancreatic cancer patients can affect tumor growth. The strategy here is to attack inflammatory stromal tissue. Pegylated recombinant human hyaluronidase (PEGPH20) represents another way to target the tumor stroma.17
Ruxolitinib, a janus kinase (JAK) inhibitor, decreases the production of inflammatory cytokines.
Immunotherapy research is ongoing, using anti-cytotoxic-leukocyte associated antigens 4 (anti-CTLA-4) and cancer vaccines.18,19
Research is increasing along many different avenues, and many other research approaches beyond the scope of this article are also underway, funded by the NCCN, private foundations such as the Lustgarten Foundation, and other groups.
SUPPORTIVE CARE
Supportive care aspects relevant to pancreatic cancer are biliary drainage and pain control.
Biliary Drainage
It is often necessary to place stents to improve bilirubin levels to relieve jaundice as well as obtain tissue or cells for diagnosis.20
Pain Control
Recent studies have shown that an inflammatory perineural invasion by pancreatic cancer cells leads to increased arborization and hypertrophy of the sensory nerves, helping to explain in part the severe pain experienced by patients.21 In addition to opioids, other options include radiation therapy and an image-guided celiac plexus block.22 The timing of when to start radiation treatments or use a block is not settled, but there is a consensus to consider these interventions earlier rather than later.
IMPLEMENTATION IN THE VA
There are no major barriers for obtaining the newer agents or combinations at the VA when they are indicated. Most of the agents in the Table are on the VA National Formulary, with the exception of nab-paclitaxel and erlotinib. When the requested agents are not in the formulary, the case is reviewed, and the agents are usually approved for use if they are appropriate for treatment according to national guidelines. Patient education about the risks and benefits of chemotherapy is important because some patients may decide against chemotherapy based on past undesirable experiences of relatives or friends with cancer. The major barrier to using the newer regimens is the patient’s poor general condition at diagnosis. If the diagnosis can be made earlier, before their general condition deteriorates significantly, the patients will have more treatment options and an improved outcome. More research is needed to find better treatments for this deadly disease.
CONCLUSIONS
Systemic chemotherapy provides benefit to patients with advanced pancreatic cancer. For the patientswith good performance status (ECOG PS 0 or 1), FOLFIRINOX or gemcitabine plus nab-paclitaxel are reasonable choices for first-line treatment. Gemcitabine plus nab-paclitaxel is an option for the patients with modest performance status who cannot tolerate the FOLFIRINOX regimen. For patients with poor performance status, gemcitabine as a single-agent or as supportive care may be offered. Although the new combination regimens are more effective than single-agent gemcitabine, the median survival is still < 1 year. More effective therapy is needed. Participation in clinical trials is encouraged.
Acknowledgements
I want to thank my colleagues Dr. Victor Chang and Dr. Basil Kasimis for their comments.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9-29.
2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
3. Solomon S, Das S, Brand R, Whitcomb DC. Inherited pancreatic cancer syndromes. Cancer J. 2012;18(6):485-491.
4. Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni JF Jr. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15(1):35-43.
5. El-Serag HB, Engels EA, Landgren O, et al. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology. 2009;49(1):116-123.
6. Atchison EA, Gridley G, Carreon JD, Leitzmann MF, McGlynn KA. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer. 2011;128(3):635-643.
7. Landgren O, Turesson I, Gridley G, Caporaso NE. Risk of malignant disease among 1525 adult male US Veterans with Gaucher disease. Arch Intern Med. 2007;167(11):1189-1194.
8. Kang HK, Cypel Y, Kilbourne AM, et al. HealthViEWS: Mortality study of female US Vietnam era veterans, 1965-2010. Am J Epidemiol. 2014;179(6):721-730.
9. Kim K, Zhong F, Chang VT, et al. Clinical characteristics and comorbidity of veterans with pancreatic cancer. J Clin Oncol. 2011;29(15)(suppl):Abstract e14549.
10. National Comprehensive Cancer Network. NCCN Guidelines in the treatment of pancreatic adenocarcinoma. http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed June 15, 2014.
11. Burris HA III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15(6):2403-2413.
12. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960-1966.
13. Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28(22):3617-3622.
14. Conroy T, Desseigne F, Ychou M. Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825.
15. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691-1703.
16. Hurwitz H, Uppal N, Wagner SA, et al. A randomized double-blind phase 2 study of ruxolitinib (RUX) or placebo (PBO) with capecitabine (CAPE) as second-line therapy in patients (pts) with metastatic pancreatic cancer (mPC). Abstract 4000. J Clin Oncol. 2014;32(suppl):5s.
17. Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418-429.
18. Le DT, Lutz E, Uram JN, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36(7):382-389.
19. Plate JM. Advances in therapeutic vaccines for pancreatic cancer. Discov Med. 2012;14(75):89-94.
20. Sharaiha RZ, Widmer J, Kahaleh M. Palliation of pancreatic ductal obstruction in pancreatic cancer. Gastrointest Endosc Clin N Am. 2013;23(4):917-923.
21. Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11(10):695-707.
22. Erdek MA, King LM, Ellsworth SG. Pain management and palliative care in pancreatic cancer. Curr Probl Cancer. 2013;37(5):266-272.
Pancreatic cancer incidence rates have been increasing among men and women since 2000. From 2006 to 2010, incidence rates increased by 1.3% per year, and the death rate for pancreatic cancer increased by 0.4% per year. An estimated 39,590 deaths are expected to occur in 2014, with similar numbers expected in women (19,420) and in men (20,170).1
Pancreatic cancer incidence and mortality rates vary across different racial/ethnic groups, with the highest rates in African Americans and the lowest rates in Asian Americans/Pacific Islanders. During 2005-2009, the incidence rate (per 100,000 persons) was 15.3 for African Americans, 11.6 for whites, and 8.8 for Asian Americans/Pacific Islanders. According to Surveillance, Epidemiology, and End Results (SEER) 18, for the period 2007-2011, the incidence rate was 17.2 for black men and 14 for white men; the incidence rate was 14.2 for black women and 10.7 for white women.2
Well-known risk factors for pancreatic cancer include tobacco use, chronic pancreatitis, obesity, diabetes, and alcohol use. Individuals with Lynch syndrome and certain other hereditary syndromes are at increased risk.3 Newer risk factors of interest include chronic infection with hepatitis B, hepatitis C, or Helicobacter pylori. These risk factors have in common a backdrop of inflammation, which may predispose the patient to developing pancreatic cancer.
Whether veterans as a group are at increased risk for developing pancreatic cancer is unclear. One study suggests there may be an increased risk in obese veterans.4 Certain high-risk groups among veterans have been identified, such as veterans with hepatitis C, diabetes, Gaucher disease, and women nurses from the Vietnam War era.5-8
Comorbidity, an area of increasing interest, was not a predictor of survival in a pilot study from one VA medical center.9
MANAGEMENT OF METASTATIC PANCREATIC CANCER
Management of metastatic pancreatic cancer has 2 components: systemic disease treatment and supportive care.
Systemic Disease Treatment
The standard of care at the VA for patients with metastatic pancreatic cancer follows the national guidelines, including the National Comprehensive Cancer Network (NCCN) guidelines.10
• For first-line therapy, currently recognized regimens include 5-fluorouracil (5-FU), with irinotecan, leucovorin, and oxaliplatin (FOLFIRINOX), gemcitabine with albumin-bound paclitaxel, and gemcitabine with erlotinib. Single- agent gemcitabine or supportive care is for patients with poor performance status.
• For second-line therapy, switching from a gemcitabine to a fluoropyrimidine-based regimen, and vice versa is a recognized option.
• When possible, participation in clinical trials is recommended.
Treatment for metastatic pancreatic cancer has not been satisfactory. In 1997, single-agent gemcitabine was approved by the FDA and became the standard of care when a randomized controlled trial of gemcitabine in symptomatic patients showed an increased survival compared with that of 5-FU.11 The median survival durations were 5.65 and 4.41 months for gemcitabine-treated and 5-FU-treated patients, respectively (P = .0025).
Many attempts to combine gemcitabine with other medications were unsuccessful until 2005 when gemcitabine in combination with erlotinib was approved by the FDA as a first-line therapy. A randomized trial showed a 2-week prolongation in survival with the addition of erlotinib. The median survivalwas 6.24 months in the combination group compared with 5.91 months in the gemcitabine group.12 Erlotinib is associatedwith increased skin toxicity anddiarrhea. Interestingly, trials with many other targeted agents, such as bevacizumab, have not demonstrated any survival improvement.13
In 2011, a French study showed that the multidrug combination of FOLFIRINOX in patients with good performance status (Eastern Cooperative Oncology Group Performance Status [ECOG PS] 0 or 1) increased overall mean survival to 11.1 months compared with 6.7 months in the gemcitabine group, but with more neutropenia, diarrhea, and sensory neuropathy.11
In 2013, a comparison of nab-paclitaxel plus gemcitabine and gemcitabine alone for patients with a Karnofsky performance-status score of 70 or more showed a median survival of 8.5 months vs 6.7 months, with more neutropenia, fatigue, and neurotoxicity in the combination group.14,15 This regimen was approved in September 2013 by the FDA as first-line treatment for patients with metastatic pancreatic cancer.
For second-line therapy, a recent phase 2 study of ruxolitinib and capecitabine demonstrated prolongation of overall survival in patients who had an elevated C-reactive protein (CRP). In the high CRP subgroup, 3- and 6-month survival was 48% and 42% with ruxolitinib, but was 29% and 11% with placebo.16 Further studies are planned.
Although these developments, summarized in the Table, are exciting, determining how they will apply to clinical practice will require careful patient selection and shared decision making about anticipated toxicities.
List of New Agents
Erlotinib is an oral agent and a small molecule that inhibits the tyrosine kinase activity of the human epidermal growth factor receptor (EGFR or HER1) pathway.
Albumin-bound paclitaxel (nab-paclitaxel) is effective in tumors that overexpress the albumin-binding protein secreted protein, acidic and rich in cysteine (SPARC), such as breast cancer, and used in pancreatic cancer (as this cancer also overexpresses SPARC). It is hypothesized, but not yet proven, that blockage of SPARC proteins in pancreatic cancer patients can affect tumor growth. The strategy here is to attack inflammatory stromal tissue. Pegylated recombinant human hyaluronidase (PEGPH20) represents another way to target the tumor stroma.17
Ruxolitinib, a janus kinase (JAK) inhibitor, decreases the production of inflammatory cytokines.
Immunotherapy research is ongoing, using anti-cytotoxic-leukocyte associated antigens 4 (anti-CTLA-4) and cancer vaccines.18,19
Research is increasing along many different avenues, and many other research approaches beyond the scope of this article are also underway, funded by the NCCN, private foundations such as the Lustgarten Foundation, and other groups.
SUPPORTIVE CARE
Supportive care aspects relevant to pancreatic cancer are biliary drainage and pain control.
Biliary Drainage
It is often necessary to place stents to improve bilirubin levels to relieve jaundice as well as obtain tissue or cells for diagnosis.20
Pain Control
Recent studies have shown that an inflammatory perineural invasion by pancreatic cancer cells leads to increased arborization and hypertrophy of the sensory nerves, helping to explain in part the severe pain experienced by patients.21 In addition to opioids, other options include radiation therapy and an image-guided celiac plexus block.22 The timing of when to start radiation treatments or use a block is not settled, but there is a consensus to consider these interventions earlier rather than later.
IMPLEMENTATION IN THE VA
There are no major barriers for obtaining the newer agents or combinations at the VA when they are indicated. Most of the agents in the Table are on the VA National Formulary, with the exception of nab-paclitaxel and erlotinib. When the requested agents are not in the formulary, the case is reviewed, and the agents are usually approved for use if they are appropriate for treatment according to national guidelines. Patient education about the risks and benefits of chemotherapy is important because some patients may decide against chemotherapy based on past undesirable experiences of relatives or friends with cancer. The major barrier to using the newer regimens is the patient’s poor general condition at diagnosis. If the diagnosis can be made earlier, before their general condition deteriorates significantly, the patients will have more treatment options and an improved outcome. More research is needed to find better treatments for this deadly disease.
CONCLUSIONS
Systemic chemotherapy provides benefit to patients with advanced pancreatic cancer. For the patientswith good performance status (ECOG PS 0 or 1), FOLFIRINOX or gemcitabine plus nab-paclitaxel are reasonable choices for first-line treatment. Gemcitabine plus nab-paclitaxel is an option for the patients with modest performance status who cannot tolerate the FOLFIRINOX regimen. For patients with poor performance status, gemcitabine as a single-agent or as supportive care may be offered. Although the new combination regimens are more effective than single-agent gemcitabine, the median survival is still < 1 year. More effective therapy is needed. Participation in clinical trials is encouraged.
Acknowledgements
I want to thank my colleagues Dr. Victor Chang and Dr. Basil Kasimis for their comments.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Pancreatic cancer incidence rates have been increasing among men and women since 2000. From 2006 to 2010, incidence rates increased by 1.3% per year, and the death rate for pancreatic cancer increased by 0.4% per year. An estimated 39,590 deaths are expected to occur in 2014, with similar numbers expected in women (19,420) and in men (20,170).1
Pancreatic cancer incidence and mortality rates vary across different racial/ethnic groups, with the highest rates in African Americans and the lowest rates in Asian Americans/Pacific Islanders. During 2005-2009, the incidence rate (per 100,000 persons) was 15.3 for African Americans, 11.6 for whites, and 8.8 for Asian Americans/Pacific Islanders. According to Surveillance, Epidemiology, and End Results (SEER) 18, for the period 2007-2011, the incidence rate was 17.2 for black men and 14 for white men; the incidence rate was 14.2 for black women and 10.7 for white women.2
Well-known risk factors for pancreatic cancer include tobacco use, chronic pancreatitis, obesity, diabetes, and alcohol use. Individuals with Lynch syndrome and certain other hereditary syndromes are at increased risk.3 Newer risk factors of interest include chronic infection with hepatitis B, hepatitis C, or Helicobacter pylori. These risk factors have in common a backdrop of inflammation, which may predispose the patient to developing pancreatic cancer.
Whether veterans as a group are at increased risk for developing pancreatic cancer is unclear. One study suggests there may be an increased risk in obese veterans.4 Certain high-risk groups among veterans have been identified, such as veterans with hepatitis C, diabetes, Gaucher disease, and women nurses from the Vietnam War era.5-8
Comorbidity, an area of increasing interest, was not a predictor of survival in a pilot study from one VA medical center.9
MANAGEMENT OF METASTATIC PANCREATIC CANCER
Management of metastatic pancreatic cancer has 2 components: systemic disease treatment and supportive care.
Systemic Disease Treatment
The standard of care at the VA for patients with metastatic pancreatic cancer follows the national guidelines, including the National Comprehensive Cancer Network (NCCN) guidelines.10
• For first-line therapy, currently recognized regimens include 5-fluorouracil (5-FU), with irinotecan, leucovorin, and oxaliplatin (FOLFIRINOX), gemcitabine with albumin-bound paclitaxel, and gemcitabine with erlotinib. Single- agent gemcitabine or supportive care is for patients with poor performance status.
• For second-line therapy, switching from a gemcitabine to a fluoropyrimidine-based regimen, and vice versa is a recognized option.
• When possible, participation in clinical trials is recommended.
Treatment for metastatic pancreatic cancer has not been satisfactory. In 1997, single-agent gemcitabine was approved by the FDA and became the standard of care when a randomized controlled trial of gemcitabine in symptomatic patients showed an increased survival compared with that of 5-FU.11 The median survival durations were 5.65 and 4.41 months for gemcitabine-treated and 5-FU-treated patients, respectively (P = .0025).
Many attempts to combine gemcitabine with other medications were unsuccessful until 2005 when gemcitabine in combination with erlotinib was approved by the FDA as a first-line therapy. A randomized trial showed a 2-week prolongation in survival with the addition of erlotinib. The median survivalwas 6.24 months in the combination group compared with 5.91 months in the gemcitabine group.12 Erlotinib is associatedwith increased skin toxicity anddiarrhea. Interestingly, trials with many other targeted agents, such as bevacizumab, have not demonstrated any survival improvement.13
In 2011, a French study showed that the multidrug combination of FOLFIRINOX in patients with good performance status (Eastern Cooperative Oncology Group Performance Status [ECOG PS] 0 or 1) increased overall mean survival to 11.1 months compared with 6.7 months in the gemcitabine group, but with more neutropenia, diarrhea, and sensory neuropathy.11
In 2013, a comparison of nab-paclitaxel plus gemcitabine and gemcitabine alone for patients with a Karnofsky performance-status score of 70 or more showed a median survival of 8.5 months vs 6.7 months, with more neutropenia, fatigue, and neurotoxicity in the combination group.14,15 This regimen was approved in September 2013 by the FDA as first-line treatment for patients with metastatic pancreatic cancer.
For second-line therapy, a recent phase 2 study of ruxolitinib and capecitabine demonstrated prolongation of overall survival in patients who had an elevated C-reactive protein (CRP). In the high CRP subgroup, 3- and 6-month survival was 48% and 42% with ruxolitinib, but was 29% and 11% with placebo.16 Further studies are planned.
Although these developments, summarized in the Table, are exciting, determining how they will apply to clinical practice will require careful patient selection and shared decision making about anticipated toxicities.
List of New Agents
Erlotinib is an oral agent and a small molecule that inhibits the tyrosine kinase activity of the human epidermal growth factor receptor (EGFR or HER1) pathway.
Albumin-bound paclitaxel (nab-paclitaxel) is effective in tumors that overexpress the albumin-binding protein secreted protein, acidic and rich in cysteine (SPARC), such as breast cancer, and used in pancreatic cancer (as this cancer also overexpresses SPARC). It is hypothesized, but not yet proven, that blockage of SPARC proteins in pancreatic cancer patients can affect tumor growth. The strategy here is to attack inflammatory stromal tissue. Pegylated recombinant human hyaluronidase (PEGPH20) represents another way to target the tumor stroma.17
Ruxolitinib, a janus kinase (JAK) inhibitor, decreases the production of inflammatory cytokines.
Immunotherapy research is ongoing, using anti-cytotoxic-leukocyte associated antigens 4 (anti-CTLA-4) and cancer vaccines.18,19
Research is increasing along many different avenues, and many other research approaches beyond the scope of this article are also underway, funded by the NCCN, private foundations such as the Lustgarten Foundation, and other groups.
SUPPORTIVE CARE
Supportive care aspects relevant to pancreatic cancer are biliary drainage and pain control.
Biliary Drainage
It is often necessary to place stents to improve bilirubin levels to relieve jaundice as well as obtain tissue or cells for diagnosis.20
Pain Control
Recent studies have shown that an inflammatory perineural invasion by pancreatic cancer cells leads to increased arborization and hypertrophy of the sensory nerves, helping to explain in part the severe pain experienced by patients.21 In addition to opioids, other options include radiation therapy and an image-guided celiac plexus block.22 The timing of when to start radiation treatments or use a block is not settled, but there is a consensus to consider these interventions earlier rather than later.
IMPLEMENTATION IN THE VA
There are no major barriers for obtaining the newer agents or combinations at the VA when they are indicated. Most of the agents in the Table are on the VA National Formulary, with the exception of nab-paclitaxel and erlotinib. When the requested agents are not in the formulary, the case is reviewed, and the agents are usually approved for use if they are appropriate for treatment according to national guidelines. Patient education about the risks and benefits of chemotherapy is important because some patients may decide against chemotherapy based on past undesirable experiences of relatives or friends with cancer. The major barrier to using the newer regimens is the patient’s poor general condition at diagnosis. If the diagnosis can be made earlier, before their general condition deteriorates significantly, the patients will have more treatment options and an improved outcome. More research is needed to find better treatments for this deadly disease.
CONCLUSIONS
Systemic chemotherapy provides benefit to patients with advanced pancreatic cancer. For the patientswith good performance status (ECOG PS 0 or 1), FOLFIRINOX or gemcitabine plus nab-paclitaxel are reasonable choices for first-line treatment. Gemcitabine plus nab-paclitaxel is an option for the patients with modest performance status who cannot tolerate the FOLFIRINOX regimen. For patients with poor performance status, gemcitabine as a single-agent or as supportive care may be offered. Although the new combination regimens are more effective than single-agent gemcitabine, the median survival is still < 1 year. More effective therapy is needed. Participation in clinical trials is encouraged.
Acknowledgements
I want to thank my colleagues Dr. Victor Chang and Dr. Basil Kasimis for their comments.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9-29.
2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
3. Solomon S, Das S, Brand R, Whitcomb DC. Inherited pancreatic cancer syndromes. Cancer J. 2012;18(6):485-491.
4. Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni JF Jr. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15(1):35-43.
5. El-Serag HB, Engels EA, Landgren O, et al. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology. 2009;49(1):116-123.
6. Atchison EA, Gridley G, Carreon JD, Leitzmann MF, McGlynn KA. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer. 2011;128(3):635-643.
7. Landgren O, Turesson I, Gridley G, Caporaso NE. Risk of malignant disease among 1525 adult male US Veterans with Gaucher disease. Arch Intern Med. 2007;167(11):1189-1194.
8. Kang HK, Cypel Y, Kilbourne AM, et al. HealthViEWS: Mortality study of female US Vietnam era veterans, 1965-2010. Am J Epidemiol. 2014;179(6):721-730.
9. Kim K, Zhong F, Chang VT, et al. Clinical characteristics and comorbidity of veterans with pancreatic cancer. J Clin Oncol. 2011;29(15)(suppl):Abstract e14549.
10. National Comprehensive Cancer Network. NCCN Guidelines in the treatment of pancreatic adenocarcinoma. http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed June 15, 2014.
11. Burris HA III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15(6):2403-2413.
12. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960-1966.
13. Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28(22):3617-3622.
14. Conroy T, Desseigne F, Ychou M. Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825.
15. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691-1703.
16. Hurwitz H, Uppal N, Wagner SA, et al. A randomized double-blind phase 2 study of ruxolitinib (RUX) or placebo (PBO) with capecitabine (CAPE) as second-line therapy in patients (pts) with metastatic pancreatic cancer (mPC). Abstract 4000. J Clin Oncol. 2014;32(suppl):5s.
17. Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418-429.
18. Le DT, Lutz E, Uram JN, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36(7):382-389.
19. Plate JM. Advances in therapeutic vaccines for pancreatic cancer. Discov Med. 2012;14(75):89-94.
20. Sharaiha RZ, Widmer J, Kahaleh M. Palliation of pancreatic ductal obstruction in pancreatic cancer. Gastrointest Endosc Clin N Am. 2013;23(4):917-923.
21. Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11(10):695-707.
22. Erdek MA, King LM, Ellsworth SG. Pain management and palliative care in pancreatic cancer. Curr Probl Cancer. 2013;37(5):266-272.
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9-29.
2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
3. Solomon S, Das S, Brand R, Whitcomb DC. Inherited pancreatic cancer syndromes. Cancer J. 2012;18(6):485-491.
4. Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni JF Jr. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15(1):35-43.
5. El-Serag HB, Engels EA, Landgren O, et al. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology. 2009;49(1):116-123.
6. Atchison EA, Gridley G, Carreon JD, Leitzmann MF, McGlynn KA. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer. 2011;128(3):635-643.
7. Landgren O, Turesson I, Gridley G, Caporaso NE. Risk of malignant disease among 1525 adult male US Veterans with Gaucher disease. Arch Intern Med. 2007;167(11):1189-1194.
8. Kang HK, Cypel Y, Kilbourne AM, et al. HealthViEWS: Mortality study of female US Vietnam era veterans, 1965-2010. Am J Epidemiol. 2014;179(6):721-730.
9. Kim K, Zhong F, Chang VT, et al. Clinical characteristics and comorbidity of veterans with pancreatic cancer. J Clin Oncol. 2011;29(15)(suppl):Abstract e14549.
10. National Comprehensive Cancer Network. NCCN Guidelines in the treatment of pancreatic adenocarcinoma. http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed June 15, 2014.
11. Burris HA III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15(6):2403-2413.
12. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960-1966.
13. Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28(22):3617-3622.
14. Conroy T, Desseigne F, Ychou M. Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825.
15. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691-1703.
16. Hurwitz H, Uppal N, Wagner SA, et al. A randomized double-blind phase 2 study of ruxolitinib (RUX) or placebo (PBO) with capecitabine (CAPE) as second-line therapy in patients (pts) with metastatic pancreatic cancer (mPC). Abstract 4000. J Clin Oncol. 2014;32(suppl):5s.
17. Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418-429.
18. Le DT, Lutz E, Uram JN, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36(7):382-389.
19. Plate JM. Advances in therapeutic vaccines for pancreatic cancer. Discov Med. 2012;14(75):89-94.
20. Sharaiha RZ, Widmer J, Kahaleh M. Palliation of pancreatic ductal obstruction in pancreatic cancer. Gastrointest Endosc Clin N Am. 2013;23(4):917-923.
21. Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11(10):695-707.
22. Erdek MA, King LM, Ellsworth SG. Pain management and palliative care in pancreatic cancer. Curr Probl Cancer. 2013;37(5):266-272.
Maternal Morbidity: Higher Risks for Minorities
Minority women have a disproportionate risk of serious complications during delivery, according to researchers from the Centers for Disease Control and Prevention in Atlanta, Georgia; Brigham and Women’s Hospital; and Harvard Medical School, both in Boston, Massachusetts. Whereas maternal death has been the “traditional sentinel event” for monitoring maternal health, they say, maternal morbidity is more common.
The researchers used data from > 90% of all births between 2008 and 2010 in 7 states: Arizona, California, Florida, Michigan, New Jersey, New York, and North Carolina. Of nearly 3.5 million delivery hospitalizations, 43% were among non-Hispanic whites, 33% Hispanics, 13% non-Hispanic blacks, 7% Asian/Pacific Islanders, 0.6% American Indian/Alaska Native women, and 4.3% other or multiple race/ethnicity groups. The researchers analyzed the rates of morbidity indicators, including blood transfusion, heart failure during procedure or surgery, ventilation, eclampsia, shock, and sepsis.
Blood transfusion was the most common indicator of severe morbidity with rates ranging from about 79 to 187 per 10,000 delivery hospitalizations among non-Hispanic white and non-Hispanic black women, respectively. Overall, for every 10,000 delivery hospitalizations, 151 involved at least 1 severe complication (114 non-Hispanic whites, 284 non-Hispanic blacks). Non-Hispanic black women had 2.1 times higher rates of severe morbidity (measured with blood transfusion), compared with non-Hispanic white women. American Indian/Alaskan Native women had 1.7 times higher rates, Hispanic women had 1.3 times, and Asian/Pacific Islander women had 1.2 times higher rates.
Some indicators in particular stood out. In earlier analyses, the researchers say, they had found that “traditional” causes of pregnancy-related deaths (such as hemorrhage and sepsis) had given way to cardiovascular disease, cardiomyopathy, and other medical conditions. Those data were borne out by this study’s findings. For instance, 22 of the 25 specific severe morbidity indicators were significantly higher among non-Hispanic black women than that among non-Hispanic white women (all P < .05); notably, the rate of cardio monitoring was > 20 times higher than that among non-Hispanic white women. That finding, the researchers believe, points to significant differences in mortality rates contributed by cardiovascular conditions, especially cardiomyopathy, between U.S.-born black and white women. Cardiovascular-specific mortality ratios were 5.1 and 1.9 deaths per 100,000 live births, respectively. And as in other studies, this study found a rate 3 to 4 times higher for in-hospital maternal mortality among non-Hispanic black women.
Clinically, the researchers say their findings suggest a need for better screening. They advise combining maternal race and ethnicity with other maternal characteristics and obstetric history to get a clearer picture of the clinical risk for adverse outcomes.
Source
Creanga AA, Bateman BT, Kuklina EV, Callaghan WM. Am J Obstet Gynecol. 2014;210:435.e1-435.e8.
doi: j.ajog.2013.11.039.
Minority women have a disproportionate risk of serious complications during delivery, according to researchers from the Centers for Disease Control and Prevention in Atlanta, Georgia; Brigham and Women’s Hospital; and Harvard Medical School, both in Boston, Massachusetts. Whereas maternal death has been the “traditional sentinel event” for monitoring maternal health, they say, maternal morbidity is more common.
The researchers used data from > 90% of all births between 2008 and 2010 in 7 states: Arizona, California, Florida, Michigan, New Jersey, New York, and North Carolina. Of nearly 3.5 million delivery hospitalizations, 43% were among non-Hispanic whites, 33% Hispanics, 13% non-Hispanic blacks, 7% Asian/Pacific Islanders, 0.6% American Indian/Alaska Native women, and 4.3% other or multiple race/ethnicity groups. The researchers analyzed the rates of morbidity indicators, including blood transfusion, heart failure during procedure or surgery, ventilation, eclampsia, shock, and sepsis.
Blood transfusion was the most common indicator of severe morbidity with rates ranging from about 79 to 187 per 10,000 delivery hospitalizations among non-Hispanic white and non-Hispanic black women, respectively. Overall, for every 10,000 delivery hospitalizations, 151 involved at least 1 severe complication (114 non-Hispanic whites, 284 non-Hispanic blacks). Non-Hispanic black women had 2.1 times higher rates of severe morbidity (measured with blood transfusion), compared with non-Hispanic white women. American Indian/Alaskan Native women had 1.7 times higher rates, Hispanic women had 1.3 times, and Asian/Pacific Islander women had 1.2 times higher rates.
Some indicators in particular stood out. In earlier analyses, the researchers say, they had found that “traditional” causes of pregnancy-related deaths (such as hemorrhage and sepsis) had given way to cardiovascular disease, cardiomyopathy, and other medical conditions. Those data were borne out by this study’s findings. For instance, 22 of the 25 specific severe morbidity indicators were significantly higher among non-Hispanic black women than that among non-Hispanic white women (all P < .05); notably, the rate of cardio monitoring was > 20 times higher than that among non-Hispanic white women. That finding, the researchers believe, points to significant differences in mortality rates contributed by cardiovascular conditions, especially cardiomyopathy, between U.S.-born black and white women. Cardiovascular-specific mortality ratios were 5.1 and 1.9 deaths per 100,000 live births, respectively. And as in other studies, this study found a rate 3 to 4 times higher for in-hospital maternal mortality among non-Hispanic black women.
Clinically, the researchers say their findings suggest a need for better screening. They advise combining maternal race and ethnicity with other maternal characteristics and obstetric history to get a clearer picture of the clinical risk for adverse outcomes.
Source
Creanga AA, Bateman BT, Kuklina EV, Callaghan WM. Am J Obstet Gynecol. 2014;210:435.e1-435.e8.
doi: j.ajog.2013.11.039.
Minority women have a disproportionate risk of serious complications during delivery, according to researchers from the Centers for Disease Control and Prevention in Atlanta, Georgia; Brigham and Women’s Hospital; and Harvard Medical School, both in Boston, Massachusetts. Whereas maternal death has been the “traditional sentinel event” for monitoring maternal health, they say, maternal morbidity is more common.
The researchers used data from > 90% of all births between 2008 and 2010 in 7 states: Arizona, California, Florida, Michigan, New Jersey, New York, and North Carolina. Of nearly 3.5 million delivery hospitalizations, 43% were among non-Hispanic whites, 33% Hispanics, 13% non-Hispanic blacks, 7% Asian/Pacific Islanders, 0.6% American Indian/Alaska Native women, and 4.3% other or multiple race/ethnicity groups. The researchers analyzed the rates of morbidity indicators, including blood transfusion, heart failure during procedure or surgery, ventilation, eclampsia, shock, and sepsis.
Blood transfusion was the most common indicator of severe morbidity with rates ranging from about 79 to 187 per 10,000 delivery hospitalizations among non-Hispanic white and non-Hispanic black women, respectively. Overall, for every 10,000 delivery hospitalizations, 151 involved at least 1 severe complication (114 non-Hispanic whites, 284 non-Hispanic blacks). Non-Hispanic black women had 2.1 times higher rates of severe morbidity (measured with blood transfusion), compared with non-Hispanic white women. American Indian/Alaskan Native women had 1.7 times higher rates, Hispanic women had 1.3 times, and Asian/Pacific Islander women had 1.2 times higher rates.
Some indicators in particular stood out. In earlier analyses, the researchers say, they had found that “traditional” causes of pregnancy-related deaths (such as hemorrhage and sepsis) had given way to cardiovascular disease, cardiomyopathy, and other medical conditions. Those data were borne out by this study’s findings. For instance, 22 of the 25 specific severe morbidity indicators were significantly higher among non-Hispanic black women than that among non-Hispanic white women (all P < .05); notably, the rate of cardio monitoring was > 20 times higher than that among non-Hispanic white women. That finding, the researchers believe, points to significant differences in mortality rates contributed by cardiovascular conditions, especially cardiomyopathy, between U.S.-born black and white women. Cardiovascular-specific mortality ratios were 5.1 and 1.9 deaths per 100,000 live births, respectively. And as in other studies, this study found a rate 3 to 4 times higher for in-hospital maternal mortality among non-Hispanic black women.
Clinically, the researchers say their findings suggest a need for better screening. They advise combining maternal race and ethnicity with other maternal characteristics and obstetric history to get a clearer picture of the clinical risk for adverse outcomes.
Source
Creanga AA, Bateman BT, Kuklina EV, Callaghan WM. Am J Obstet Gynecol. 2014;210:435.e1-435.e8.
doi: j.ajog.2013.11.039.