User login
Cardiac Biomarkers: Current Standards, Future Trends
Since the 1950s, researchers and clinicians have sought a simple chemical biomarker that would aid in the diagnosis and treatment of cardiovascular disease (CVD). An initial discovery was that proteins normally found within cardiac myocytes are released into the circulation when the myocardial cell membrane loses its integrity as a result of hypoxic injury.1 Over the past 60 years, major advances have been made to distinguish which proteins are highly cardiac specific, and which are suggestive of other pathologic cardiovascular processes.
In the past 15 to 20 years, significant data have emerged that support the role of cardiac-specific troponins in the cardiac disease process, making their measurement an important consideration in diagnosis and clinical decision-making for patients with various forms of CVD.2-7 With the exception of 1918, CVD has accounted for more deaths in the United States than any other illness in every year since 1900.8 It has been suggested that nearly 2,300 Americans die of CVD each day—an average of one death every 38 seconds. The direct and indirect costs of CVD for 2010 were projected at $503.2 billion—including $155 billion for total hospital costs alone.9 By comparison, a 2010 projection from the National Cancer Institute was $124.57 billion for the direct costs of cancer care.10
It is imperative for practitioners in all areas of medicine to have a working knowledge of the role of cardiac biomarkers and their potential impact on patients’ overall health. Primary care clinicians in particular should be familiar with the release kinetics of certain cardiac biomarkers to aid in the diagnosis and treatment of the cardiac patient. This article is intended as an overview of the cardiac biomarkers that are being used in practice today, and to review the novel biomarkers that may have an impact on the way clinicians practice in the near future.
BIOMARKERS CURRENTLY IN USE
Cardiac Troponins
The cardiac-specific troponins (cTn) are an integral component of the myosin-actin binding complex found in striated muscle tissue. The troponin complex includes three regulatory proteins: troponin C, troponin I, and troponin T. The genes that code for troponin C are identical in skeletal and cardiac tissue; for this reason, troponin C becomes much less cardiospecific.
However, cardiac troponin T (cTnT) and cardiac troponin I (cTnI) have differing amino acid sequences, making it possible to develop highly sensitive immunoassays to detect circulating antibody-troponin complexes after myocardial cell injury, as seen in acute coronary syndrome (ACS).12-14 No cross-reactivity occurs between skeletal and cardiac sources, indicating the unique cardiospecificity of the cTn proteins.11
Of note, there are numerous patient populations in which elevated cTn may be found; the associated conditions are outlined in Table 1.11,15,16 In particular, elevations in cTn (more specifically cTnT) are commonly observed in patients with end-stage renal disease (ESRD).11,17 The pathogenesis of elevated cTnT is not completely understood but has been proposed as a promising prognostic tool for use in patients with ESRD. Elevated levels of cTnT identify patients whose chance of survival is poor, with an increased risk for cardiac death.7 It should be noted that a significant proportion of patients with chronic renal failure succumb to cardiovascular death.17
Recently, deFilippi and colleagues18 reported a correlation between trace amounts of circulating cTnT in older patients not known to have CVD and an increased risk for future heart failure or even cardiovascular-related death. This novel finding may add valuable information to the screening and risk stratification of relatively healthy but sedentary individuals older than 65.
Elevations in cTn can be seen as early as two to four hours after myocardial injury.11 A small portion of cTnT and cTnI is found within the myocardial cells’ cytosol and is not bound to the troponin complex, which begins to degrade after cell injury.1,14 This cytosolic pool allows for earlier recognition of cardiac injury. As increasingly sensitive assays are developed, rises in cTn levels can be detected earlier after symptom onset, thus making the use of less specific early biomarkers, such as myoglobin and creatine kinase–MB, obsolete.
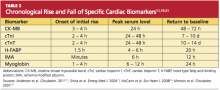
Because of the continuous release of structural proteins from the degrading myocardial tissue, elevations of cTnI may persist for seven to 10 days, while cTnT elevations can continue for 10 to 14 days postinfarction.11 This prolonged period allows for detection of even very slight cardiac damage and provides an advantage for identification and treatment of high-risk patients. When measured 72 hours after infarction, cTnI can also provide prognostic information, such as the potential size of the affected myocardial area.5,6 This, too, facilitates risk stratification. Timing of the release of key biomarkers can be seen in Table 2.11,19-21
Natriuretic Peptides
The natriuretic peptides are cardiac neurohormones that are released in response to myocardial wall stress.11 B-type natriuretic peptide (BNP; previously known as brain natriuretic peptide) is synthesized and released from the ventricular myocardium in times of volume expansion and increased pressure burden. Initially, the prohormone proBNP is released and is enzymatically cleaved to N-terminal proBNP (NT-proBNP) and then to “mature” BNP.11
Today, BNP is used widely as a biomarker for congestive heart failure.22 In the Breathing Not Properly (BNP) study,23 BNP was able to accurately distinguish dyspnea caused by heart failure from that with a pulmonary etiology; it was found to be the strongest predictor for the diagnosis of heart failure. In the evaluation of serum BNP, a level below 100 ng/L makes heart failure unlikely (negative predictive value, 90%). If the value rises above 500 ng/L, heart failure is highly likely (positive predictive value, 90%).23
As for NT-proBNP, levels exceeding 450 ng/L in patients younger than 50 and exceeding 900 ng/L in patients 50 or older have been found highly sensitive and specific for heart failure–related dyspnea.24 Current studies suggest that NT-proBNP may have greater sensitivity and specificity than standard BNP when these age-related cut-off limits are applied.25
Researchers for the International Collaborative of NT-proBNP Study reported that when NT-proBNP was measured in the emergency department (ED) in patients with dyspnea, patients were less likely to require hospitalization for heart failure.26 Instead, patients were presenting with exacerbations of chronic obstructive pulmonary disease, which would best be managed on an outpatient basis. Those who were admitted experienced shorter lengths of stay with subsequent reductions in health care costs, and had no significant difference in readmission or mortality rates.
Use of BNP as a cardiac biomarker does not come without its limitations. In addition to right-sided heart failure, ACS, and MI, elevations in BNP have been associated with septic shock, sepsis, renal dysfunction, and acute pulmonary embolus.27 Correlations have been made between the degree of BNP elevation and the extent of myocardial ischemia; increased levels of both BNP and cTnI were associated with higher mortality rates.28
C-Reactive Protein
Inflammation is known to play a key role in the development of atherosclerotic plaques. Measuring byproducts of the atherosclerotic process, from the initial development of fatty streaks to plaque rupture, can help determine whether a patient is at an increased risk for a cardiovascular event.29 Primary proinflammatory markers from the local site of intravascular inflammation signal messenger cytokines that are altered in the liver via an acute-phase reaction.
One of the major acute-phase reactants, C-reactive protein (CRP) is simply a byproduct of inflammation—yet it has become a major indicator of atherosclerotic plaque stability.30 A high-sensitivity CRP assay (hsCRP) can be a significantly effective predictor for MI, stroke, and peripheral vascular disease, even in patients who appear to be healthy.31 Studies have suggested that hsCRP is a better indicator of unstable plaque, and a better predictor of adverse cardiovascular events, than is low-density lipoprotein cholesterol (LDL-C), when these markers are used independently.32 When detected together, however, hsCRP and LDL-C elevations have been shown to be an even better predictor of adverse events in patients with no overt cardiovascular risk factors.31
As a risk factor, elevated hsCRP has been called as important as smoking or hypertension—highlighting the role of inflammation in formation of atherosclerosis at every level.33
In the evaluation of serum hsCRP, it is important to know that levels tend to be stable over long periods of time, have no circadian rhythms, and are not affected by various prandial states. Levels can be measured conveniently during the standard annual cholesterol screening. Relative cardiovascular risk is deemed low, medium, and high, in patients with an hsCRP measurement of less than 1 mg/L, 1 to 3 mg/L, and greater than 3 mg/L, respectively.2 Values exceeding 8.0 mg/L may be consistent with an acute infectious or inflammatory process, thus exposing the nonspecific nature of this popular biomarker.34 However, when used in conjunction with cTn, BNP, and patient history, hsCRP still proves to be an important clinical tool, offering prognostic information to facilitate clinical decision making.
Chronically elevated hsCRP signifies a very high risk for future cardiovascular events and should prompt the clinician to target the patient’s modifiable risk factors, including consideration of statin therapy as a part of the treatment regimen.35 According to results from the PROVE IT–TIMI 22 trial,35 statin use appears particularly cardioprotective in patients whose hsCRP levels are lower than 2 mg/L and who maintain LDL-C below 70 mg/dL. Researchers for this study group recommend monitoring hsCRP along with serum lipids for a more comprehensive cardiovascular risk profile.
Creatine Kinase
Three isoenzymes of creatine kinase (CK) exist: MM (skeletal muscle type), BB (brain type), and MB (myocardial band). The CK-MB isoenzyme was the diagnostic marker of choice for ACS until the introduction of cardiac-specific troponins in the early 1990s.36,37
CK-MB is an intracellular carrier protein for high-energy phosphates, found in higher concentrations in the myocardium than are the other CK isoenzymes.11 CK-MB accounts for about 15% of total CK, but it also exists in skeletal muscle and to a lesser extent in the small intestine, diaphragm, uterus, and prostate.21 As with most cardiac biomarkers, cardiospecificity of CK-MB is not 100%, and false-positive elevations can occur in a multitude of clinical settings, including significant musculoskeletal injury, heavy exertion, and myopathies.11
CK-MB is detectable three to four hours after myocardial injury, peaks at 24 hours, and returns to normal in 48 to 72 hours. CK-MB may be used to evaluate for ACS if cardiac troponin assays are not accessible,21 but its usefulness is limited during the early hours of ACS onset and after 72 hours.
The relative index of CK-MB to total CK (CK-MB/CK) can help the clinician assess whether the rise in CK-MB is attributable to a skeletal muscle source (CK-MB/CK < 3) or a cardiac source (CK-MB/CK > 5, indicating myocardial release of CK-MB). A relative index that falls between 3 and 5 warrants further investigation with serial analyses.36 It may also be helpful to know that a CK-MB elevation associated with skeletal muscle release tends to persist and plateau over a period of several days—as opposed to CK-MB elevation with a myocardial source, which follows the time course stated above.21
In a 2006 study that included nearly 30,000 patients, it was found that 28% of those with ACS had conflicting results between troponin levels and CK-MB.38 Patients with no elevations in cardiac troponins but elevations in CK-MB had no significant increased risk for in-hospital mortality, compared with patients with negative results for both markers. Thus, an isolated elevation in CK-MB has limited prognostic value in patients with negative troponin levels.
By contrast, however, Lim et al39 recently found that elevations in CK-MB were closely associated with perioperative necrosis and MI after percutaneous coronary intervention (PCI) as a result of currently oversensitive thresholds for cTnI. Thus, CK-MB use may play a role as an independent marker of necrosis in certain situations.
Myoglobin
Because of its small molecular size, myoglobin has timely release kinetics, with elevations appreciable before those of CK-MB or cTn.11,21 Myoglobin typically rises one to four hours after myocardial injury, peaks at six to 12 hours, and returns to normal within 24 hours. For this reason, it has held special interest as an early marker of cardiac injury.
With its early release/degradation kinetics, myoglobin may serve as a marker for reinfarction.21 It can also be used to assess further damage to the myocardium, as seen with distal thrombus embolization or coronary artery manipulation. However, troponin values provide similar information on reinfarction status.4
An established shortcoming of myoglobin as an early marker of necrosis is its lack of cardiac specificity, as it is found extensively in skeletal muscle.21 Patients who present with inflammation, trauma, or significant skeletal muscle injury can have extremely high levels of serum myoglobin without myocardial involvement. Since 2000, when cTn was designated the myocardial biomarker of choice (according to the revised definition of MI, as presented that year by the European Society of Cardiology and American College of Cardiology), the use of myoglobin to identify ACS has been considered obsolete.14
NOVEL BIOMARKERS OF CARDIOVASCULAR DISEASE
Heart-Type Fatty Acid–Binding Protein
A low–molecular-weight protein, heart-type fatty acid–binding protein (H-FABP) is involved in the intracellular uptake and buffering of myocardial free fatty acids. Because its molecular size is similar to that of troponin, it is rapidly released from the cytosol and has been proposed as an early sensitive marker of acute MI.40 In a 2010 study, H-FABP was shown to be of additional prognostic value when used in conjunction with cTnI in patients at low to moderate risk for suspected ACS.41 According to the known release kinetics of H-FABP after myocardial ischemia or infarct, a rise is detectable as early as 1.5 hours after symptom onset. The marker peaks after four to six hours and, because of rapid renal clearance, returns to baseline within 20 hours.20
Interpreting results may be hindered in the patient with impaired renal function—with not only higher levels, but sustained levels of H-FABP.20 Early assays used antibodies to detect circulating H-FABP levels, but cross-reactivity occurring between other fatty acid–binding protein types have limited the clinical applications of H-FABP testing.20 As more highly specific assays are produced, more practical protocols can be implemented to confirm the presence of ACS in patients presenting early to the ED with apparent ACS.
Ischemia-Modified Albumin
A biomarker that could detect ischemia alone would help identify patients at highest risk for infarction; immediate intervention could then prevent the progression of ACS, as evidenced by rising markers of necrosis. Ischemia-modified albumin (IMA), which is produced rapidly when circulating albumin comes into contact with ischemic myocardial tissue, has been touted as such a biomarker.42
Several changes occur in the human albumin molecule in the presence of ischemia, including its ability to bind transition metals—especially cobalt. This discovery led to the creation of an albumin-cobalt–binding assay, approved by the FDA for rapid detection of myocardial ischemia.19 When artificial ischemia is produced by balloon inflation during percutaneous coronary angioplasty, IMA levels can be detected, using the assay, within minutes of coronary artery occlusion. Levels tend to peak within six hours and can be elevated for as long as 12 hours.19
When IMA is used in conjunction with ECG findings and cTnT levels, a sensitivity of 97% for detecting ACS can be achieved. This could reduce the number of patients being discharged from the ED with occult ACS,43 giving IMA a potentially important precautionary and supplemental role. As with most cardiac biomarkers, IMA alone is not 100% specific for ACS since it is also present in other ischemic conditions, thus hindering its usefulness in clinical practice. Elevations had been reported in patients with liver cirrhosis, uncontrolled type 2 diabetes mellitus, obstetric conditions associated with placental ischemia, carbon monoxide poisoning, and cerebrovascular ischemia.44-47
Recent suggestions to use IMA to rule out rather than diagnose ACS show promise, since the absence of this acute-phase reactant should exclude the presence of myocardial ischemia.48 Further studies are needed to determine the exact physiology of IMA production in order to identify its cardiac specificity for clinical use.49
Homocysteine
As a marker of increased cardiovascular risk, homocysteine is thought to have multiple effects on the cardiovascular system. These include endothelial dysfunction, decreased arteriole vasodilation (ie, reduced release of nitric oxide), increased platelet activation, increased production of free radicals, and increased LDL oxidation with arteriole lipid accumulation.50
In patients with severe hyperhomocysteinemia (ie, homocysteine serum levels > 100 mol/L), risk for premature atherothrombosis and venous thromboembolism is increased. In the general public, mildly elevated homocysteine levels (> 15 mol/L) have been attributed to insufficient dietary intake of folic acid. Folate in its natural form has been known to decrease serum homocysteine levels by 25%, if supplemented appropriately with vitamins B6 and B12.50
In recent years, folate deficiency has declined due to enrichment of certain foods with this crucial nutrient, initially mandated to decrease the incidence of neural tube defects in developing embryos. From a cardiovascular standpoint, researchers have been unable to determine whether elevated homocysteine increases CVD risk or is simply a marker of existing disease burden.50 In clinical trials in which subjects took B-vitamins supplemented with folate, homocysteine levels were reduced; yet in one study, stroke risk was not reduced in patients with a history of stroke51; in a second, in-stent restenosis was more common in patients who took the supplement after undergoing angioplasty52; and in a third, patients following the vitamin regimen after a recent acute MI proved to be at higher cardiovascular risk.53
As a result of this conflicting evidence, no recommendations have been made for routine homocysteine screening except in patients with a history of markedly premature atherosclerosis or a family history of early-onset acute MI or stroke.50 Monitoring may be advisable in patients who take a folate antagonist (eg, methotrexate, carbamazepine), considering the risk for folate deficiency and subsequent hyperhomocysteinemia.
ACUTE INFLAMMATORY MARKERS OF PLAQUE RUPTURE OR VULNERABILITY
Myeloperoxidase
Many researchers have taken a particular interest in the acute substances formed as a result of atherothrombotic plaque inflammation or rupture. One such biomarker is myeloperoxidase (MPO), which is thought to be expressed from the degranulation of activated leukocytes found in atherosclerotic plaques. This acute-phase enzyme may convert LDL into a high-uptake form for macrophages, leading to foam cell formation and depletion of nitric oxide, contributing to additional ischemia by way of vasoconstriction.54
Recently, a high systemic MPO level was found to be a more significant marker of plaque at risk for rupture, compared with already-ruptured plaque.55 Although MPO elevations may also occur in a number of inflammatory, infectious, or infiltrative conditions, the association between MPO, inflammation, and oxidative stress supports its use as a marker for plaque that is vulnerable to rupture.56,57
Serum levels of MPO have been shown to predict increased risk for subsequent death or MI in patients who present to the ED with ACS, independent of other cardiac risk factors or cardiac biomarkers. In a 2001 study, Zhang et al54 established an association between elevated MPO levels and angiographically proven coronary atherosclerosis, with a 20-fold higher risk for coronary artery disease; earlier this year, Oemrawsingh et al58 reported an independent association between MPO and long-term adverse outcomes in patients who presented with non–ST-segment elevation ACS. Thus, MPO may be a significant indicator of vascular inflammation.57
Soluble CD40 Ligand
In the 1980s, postmortem studies confirmed that erosions or ruptures in atherosclerotic fibrous caps lead to platelet activation—the main pathophysiologic contributor in ACS.59 This fundamental actuality suggests that biomarkers of platelet activation may provide supplemental information in patients who present with chest pain of cardiac origin. Another acute inflammatory marker, soluble CD40 ligand, is a marker of active platelet stimulation.60 Increased serum levels of soluble CD40 ligand have been correlated to increased risk for cardiovascular events in apparently healthy women.61
Soluble CD40 ligand, expressed within seconds of platelet activation, is also commonly found on various leukocytes, endothelial cells, and smooth-muscle cells.60 This may provide insight into cardiovascular disease progression and plaque deterioration that precedes the events of ACS.
Therapeutic antiplatelet medications are now the mainstay in the treatment and prevention of cardiovascular complications associated with ischemic thrombus formation.11 Platelet biomarkers are likely to play an essential supplemental role in the diagnosis of ACS.
Pregnancy-Associated Plasma Protein A
Additional risk-stratifying biomarkers include those that may determine whether a patient has plaques that are acutely vulnerable to rupture. Pregnancy-associated plasma protein A (PAPP-A), first detected in the 1970s in the circulation of pregnant women, is now widely used in first-trimester screening for fetal trisomy.62
Since then, it has been found that PAPP-A, which is theorized to be produced by vascular smooth-muscle cells, is extensively expressed in unstable coronary artery plaques, while minimally expressed in stable plaques.63 Since a significant proportion of patients who present with symptoms of ACS have normal cTn levels, PAPP-A may help identify patients who are at increased risk for subsequent short-term cardiovascular complications resulting from occult disease.64 This relatively new marker may also prove useful for screening in the office setting, identifying outpatients who are at high cardiovascular risk. Further studies are needed to define the release kinetics of PAPP-A, guiding clinicians in its implementation and clinical use.
CONCLUSION
When used in conjunction with the history and physical exam, cardiac biomarkers can provide a simple, noninvasive means to further the clinician’s exploration into a suspected underlying cardiovascular process. As advances continue in the understanding of the pathogenesis of heart disease, new interpretations of existing markers and discovery of novel markers may allow for specific therapeutic interventions to improve patient outcomes.
It is important to note that the list of biomarkers described here is by no means complete, and there is continued interest in finding more specific and sensitive markers of heart disease. Numerous cardiovascular organizations are now suggesting a shift toward a multi-marker strategy to determine the best etiology in the patient who presents with decompensating cardiovascular disease. A change in cardiac enzyme panels may be inevitable in the near future. Practicing PAs and NPs, particularly those who care for patients at risk for CVD, should remain up-to-date and proficient in interpreting those results to help determine the best course of action for each patient.
REFERENCES
1. Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634-2653.
2. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003; 107(3):499-511.
3. Kavsak PA, MacRae AR, Newman AM, et al. Effects of contemporary troponin assay sensitivity on the utility of the early markers myoglobin and CKMB isoforms in evaluating patients with possible acute myocardial infarction. Clin Chim Acta. 2007; 380(1-2):213-216.
4. Eggers KM, Oldgren J, Nordenskjöld A, Lindahl B. Diagnostic value of serial measurement of cardiac markers in patients with chest pain: limited value of adding myoglobin to troponin I for exclusion of myocardial infarction. Am Heart J. 2004; 148(4):574-581.
5. Licka M, Zimmermann R, Zehelein J, et al. Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart. 2002;87(6):520-524.
6. Panteghini M, Cuccia C, Bonetti G, et al. Single-point cardiac troponin T at coronary care unit discharge after myocardial infarction correlates with infarct size and ejection fraction. Clin Chem. 2002; 48(9):1432-1436.
7. Giannitsis E, Müller-Bardorff M, Lehrke S, et al. Admission troponin T level predicts clinical outcomes, TIMI flow, and myocardial tissue perfusion after primary percutaneous intervention for acute ST-segment elevation myocardial infarction. Circulation. 2001;104(6):630-635.
8. Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25-e146.
9. Lloyd-Jones D, Adams RJ, Brown TM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010; 121(7):e46-e215.
10. Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128.
11. Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non–ST-Elevation Myocardial Infarction: a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123(18):e426-e579.
12. Ohman EM, Armstrong PW, Christenson RH, et al; GUSTO IIA Investigators. Cardiac troponin T levels for risk stratification in acute myocardial ischemia.
N Engl J Med. 1996;335(18):1333-1341.
13. Antman EM, Tanasijevic MJ, Thompson B, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996;335(18):1342-1349.
14. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined: a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. J Am Coll Cardiol. 2000;36(3):959-969.
15. Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol. 2006;48(1):1-11.
16. Galvani M, Ottani F, Ferrini D, et al. Prognostic influence of elevated values of cardiac troponin I in patients with unstable angina. Circulation. 1997;95 (8):2053-2059.
17. Freda BJ, Tang WH, Van Lente F, et al. Cardiac troponins in renal insufficiency: review and clinical implications. J Am Coll Cardiol. 2002;40(12):2065-2071.
18. deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304(22):2494-2502.
19. Sinha MK, Roy D, Gaze DC, et al. Role of “ischemia modified albumin,” a new biochemical marker of myocardial ischaemia, in the early diagnosis of acute coronary syndromes. Emerg Med J. 2004; 21(1):29-34.
20. McCann CJ, Glover BM, Menown IB, et al. Novel biomarkers in early diagnosis of acute myocardial infarction compared with cardiac troponin T. Eur Heart J. 2008;29(23):2843-2850.
21. Morrow DA, Cannon CP, Jesse RL, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13): e356-e375.
22. Maisel AS, Krishnaswamy P, Nowak RM, et al; Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161-167.
23. McCullough PA, Nowak RM, McCord J, et al: B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002;106(4):416-422.
24. Januzzi JL Jr, Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95(8):948-954.
25. Mueller C, Laule-Kilian K, Scholer A, et al. Use of B-type natriuretic peptide for the management of women with dyspnea. Am J Cardiol. 2004; 94(12):1510-1514.
26. Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27(3):330-337.
27. Maisel AS. The diagnosis of acute congestive heart failure: role of BNP measurements. Heart Fail Rev. 2003;8(4):327-334.
28. Morrow DA, de Lemos JA, Sabatine MS, et al. Evaluation of B-type natriuretic peptide for risk assessment in unstable angina/non–ST-elevation myocardial infarction: B-type natriuretic peptide and prognosis in TACTICS-TIMI 18. J Am Coll Cardiol. 2003;41(8):1264-1272.
29. Libby P, Ridker PM. Inflammation and atherothrombosis: from population biology and bench research to clinical practice. J Am Coll Cardiol. 2006; 48(9 suppl A):A33-A46.
30. Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363-369.
31. Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557-1565.
32. Boekholdt SM, Hack CE, Sandhu MS, et al. C-reactive protein levels and coronary artery disease incidence and mortality in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study 1993-2003. Atherosclerosis. 2006; 187(2):415-422.
33. Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350(14):1387-1397.
34. Ridker PM, Cook N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham Risk Scores. Circulation. 2004;109(16):1955-1959.
35. Ridker PM, Cannon CP, Morrow D, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombosis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20-28.
36. Fischer JH, Jeschkeit-Schubbert S, Kuhn-Régnier F, Switkowski R. The origin of CK-MB serum levels and CK-MB/total CK ratios: measurements of CK isoenzyme activities in various tissues. Internet J Thorac Cardiovasc Surg. 2005;7(1).
37. Adams JE 3rd, Abendschein DR, Jaffe AS. Biochemical markers of myocardial injury: is MB creatine kinase the choice for the 1990s? Circulation. 1993;88(2):750-763.
38. Newby LK, Roe MT, Chen AY, et al; CRUSADE Investigators. Frequency and clinical implications of discordant creatine kinase-MB and troponin measurements in acute coronary syndromes. J Am Coll Cardiol. 2006;47(2):312-318.
39. Lim CC, van Gaal WJ, Testa L, et al. With the “universal definition,” measurement of creatine kinase–myocardial band rather than troponin allows more accurate diagnosis of periprocedural necrosis and infarction after coronary intervention. J Am Coll Cardiol. 2011;57(6):653-661.
40. Kilcullen N, Viswanathan K, Das R, et al; EMMACE-2 Investigators. Heart-type fatty acid–binding protein predicts long-term mortality after acute coronary syndrome and identifies high-risk patients across the range of troponin values. J Am Coll Cardiol. 2007;50(21):2061-2067.
41. Viswanathan K, Kilcullen N, Morrell C, et al. Heart-type fatty acid–binding protein predicts long-term mortality and re-infarction in consecutive patients with suspected acute coronary syndrome who are troponin-negative. J Am Coll Cardiol. 2010; 55(23): 2590-2598.
42. Abadie JM, Blassingame CL, Bankson DD. Albumin cobalt binding assay to rule out acute coronary syndrome. Ann Clin Lab Sci. 2005;35(1):66-72.
43. Anwaruddin S, Januzzi JL Jr, Baggish AL, et al. Ischemia-modified albumin improves the usefulness of standard cardiac biomarkers for the diagnosis of myocardial ischemia in the emergency department setting. Am J Clin Pathol. 2005;123(1):140-145.
44. Piwowar A, Knapik-Kordecka M, Warwas M. Ischemia-modified albumin level in type 2 diabetes mellitus: preliminary report. Dis Markers. 2008; 24(6):311-317.
45. Prefumo F, Gaze DC, Papageorghiou AT, et al. First trimester maternal serum ischaemia-modified albumin: a marker of hypoxia-ischaemia-driven early trophoblast development. Hum Reprod. 2007; 22(7):2029-2032.
46. Gunduz A, Turedi S, Mentese A, et al. Ischemia-modified albumin levels in cerebrovascular accidents. Am J Emerg Med. 2008;26(8):874-878.
47. Turedi S, Cinar O, Kaldirim U, et al. Ischemia-modified albumin levels in carbon monoxide poisoning. Am J Emerg Med. 2011;29(6):675-681.
48. Sbarouni E, Georgiadou P, Voudris V. Ischemia modified albumin changes: review and clinical implications. Clin Chem Lab Med. 2011;49(2):177-184.
49. Gaze DC. Ischemia modified albumin: a novel biomarker for the detection of cardiac ischemia. Drug Metab Pharmacokinet. 2009;24(4):333-341.
50. Kaul S, Zadeh AA, Shah PK. Homocysteine hypothesis for atherothrombotic cardiovascular disease: not validated. J Am Coll Cardiol. 2006; 48(5):914-923.
51. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291(5):565-575.
52. Lange H, Suryapranata H, De Luca G, et al. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med. 2004;350(26):2673-2681.
53. Bønaa KH, Njølstad I, Ueland PM, et al; NORVIT Trial Investigators. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578-1588.
54. Zhang R, Brennan ML, Fu X, et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286(17):2136-2142.
55. Ferrante G, Nakano M, Prati F, et al. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes: a clinicopathological study. Circulation. 2010;122(24):2505-2513.
56. Loria V, Dato I, Graziani F, Biasucci LM. Myeloperoxidase: a new biomarker of inflammation in ischemic heart disease and acute coronary syndromes. Mediators Inflamm. 2008;2008:135625.
57. Apple FS, Wu AH, Mair J, et al; Committee on Standardization of Markers of Cardiac Damage of the IFCC. Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin Chem. 2005;51(5):810-824.
58. Oemrawsingh RM, Lenderink T, Akkerhuis KM, et al. Multimarker risk model containing troponin-T, interleukin 10, myeloperoxidase and placental growth factor predicts long-term cardiovascular risk after non–ST-segment elevation acute coronary syndrome. Heart. 2011;97(13):1061-1066.
59. Davies MJ, Thomas AC. Plaque fissuring: the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J. 1985;53(4):363-373.
60. Heeschen C, Dimmeler S, Hamm CW, et al; CAPTURE Study. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med. 2003;348(12): 1104-1111.
61. Schönbeck U, Varo N, Libby P, et al. Soluble CD40L and cardiovascular risk in women. Circulation. 2001;104(19):2266-2268.
62. Lin TM, Galbert SP, Kiefer D, et al. Characterization of four human pregnancy–associated plasma proteins. Am J Obstet Gynecol. 1974; 118(2):223-236.
63. Bayes-Genis A, Conover CA, Overgaard MT, et al. Pregnancy-associated plasma protein A as a marker of acute coronary syndromes. N Engl J Med. 2001;345(14):1022-1029.
64. Lund J, Qin QP, Ilva T, et al. Circulating pregnancy-associated plasma protein A predicts outcome in patients with acute coronary syndrome but no troponin I elevation. Circulation. 2003; 108(16):1924-1926.
Since the 1950s, researchers and clinicians have sought a simple chemical biomarker that would aid in the diagnosis and treatment of cardiovascular disease (CVD). An initial discovery was that proteins normally found within cardiac myocytes are released into the circulation when the myocardial cell membrane loses its integrity as a result of hypoxic injury.1 Over the past 60 years, major advances have been made to distinguish which proteins are highly cardiac specific, and which are suggestive of other pathologic cardiovascular processes.
In the past 15 to 20 years, significant data have emerged that support the role of cardiac-specific troponins in the cardiac disease process, making their measurement an important consideration in diagnosis and clinical decision-making for patients with various forms of CVD.2-7 With the exception of 1918, CVD has accounted for more deaths in the United States than any other illness in every year since 1900.8 It has been suggested that nearly 2,300 Americans die of CVD each day—an average of one death every 38 seconds. The direct and indirect costs of CVD for 2010 were projected at $503.2 billion—including $155 billion for total hospital costs alone.9 By comparison, a 2010 projection from the National Cancer Institute was $124.57 billion for the direct costs of cancer care.10
It is imperative for practitioners in all areas of medicine to have a working knowledge of the role of cardiac biomarkers and their potential impact on patients’ overall health. Primary care clinicians in particular should be familiar with the release kinetics of certain cardiac biomarkers to aid in the diagnosis and treatment of the cardiac patient. This article is intended as an overview of the cardiac biomarkers that are being used in practice today, and to review the novel biomarkers that may have an impact on the way clinicians practice in the near future.
BIOMARKERS CURRENTLY IN USE
Cardiac Troponins
The cardiac-specific troponins (cTn) are an integral component of the myosin-actin binding complex found in striated muscle tissue. The troponin complex includes three regulatory proteins: troponin C, troponin I, and troponin T. The genes that code for troponin C are identical in skeletal and cardiac tissue; for this reason, troponin C becomes much less cardiospecific.
However, cardiac troponin T (cTnT) and cardiac troponin I (cTnI) have differing amino acid sequences, making it possible to develop highly sensitive immunoassays to detect circulating antibody-troponin complexes after myocardial cell injury, as seen in acute coronary syndrome (ACS).12-14 No cross-reactivity occurs between skeletal and cardiac sources, indicating the unique cardiospecificity of the cTn proteins.11
Of note, there are numerous patient populations in which elevated cTn may be found; the associated conditions are outlined in Table 1.11,15,16 In particular, elevations in cTn (more specifically cTnT) are commonly observed in patients with end-stage renal disease (ESRD).11,17 The pathogenesis of elevated cTnT is not completely understood but has been proposed as a promising prognostic tool for use in patients with ESRD. Elevated levels of cTnT identify patients whose chance of survival is poor, with an increased risk for cardiac death.7 It should be noted that a significant proportion of patients with chronic renal failure succumb to cardiovascular death.17
Recently, deFilippi and colleagues18 reported a correlation between trace amounts of circulating cTnT in older patients not known to have CVD and an increased risk for future heart failure or even cardiovascular-related death. This novel finding may add valuable information to the screening and risk stratification of relatively healthy but sedentary individuals older than 65.
Elevations in cTn can be seen as early as two to four hours after myocardial injury.11 A small portion of cTnT and cTnI is found within the myocardial cells’ cytosol and is not bound to the troponin complex, which begins to degrade after cell injury.1,14 This cytosolic pool allows for earlier recognition of cardiac injury. As increasingly sensitive assays are developed, rises in cTn levels can be detected earlier after symptom onset, thus making the use of less specific early biomarkers, such as myoglobin and creatine kinase–MB, obsolete.
Because of the continuous release of structural proteins from the degrading myocardial tissue, elevations of cTnI may persist for seven to 10 days, while cTnT elevations can continue for 10 to 14 days postinfarction.11 This prolonged period allows for detection of even very slight cardiac damage and provides an advantage for identification and treatment of high-risk patients. When measured 72 hours after infarction, cTnI can also provide prognostic information, such as the potential size of the affected myocardial area.5,6 This, too, facilitates risk stratification. Timing of the release of key biomarkers can be seen in Table 2.11,19-21
Natriuretic Peptides
The natriuretic peptides are cardiac neurohormones that are released in response to myocardial wall stress.11 B-type natriuretic peptide (BNP; previously known as brain natriuretic peptide) is synthesized and released from the ventricular myocardium in times of volume expansion and increased pressure burden. Initially, the prohormone proBNP is released and is enzymatically cleaved to N-terminal proBNP (NT-proBNP) and then to “mature” BNP.11
Today, BNP is used widely as a biomarker for congestive heart failure.22 In the Breathing Not Properly (BNP) study,23 BNP was able to accurately distinguish dyspnea caused by heart failure from that with a pulmonary etiology; it was found to be the strongest predictor for the diagnosis of heart failure. In the evaluation of serum BNP, a level below 100 ng/L makes heart failure unlikely (negative predictive value, 90%). If the value rises above 500 ng/L, heart failure is highly likely (positive predictive value, 90%).23
As for NT-proBNP, levels exceeding 450 ng/L in patients younger than 50 and exceeding 900 ng/L in patients 50 or older have been found highly sensitive and specific for heart failure–related dyspnea.24 Current studies suggest that NT-proBNP may have greater sensitivity and specificity than standard BNP when these age-related cut-off limits are applied.25
Researchers for the International Collaborative of NT-proBNP Study reported that when NT-proBNP was measured in the emergency department (ED) in patients with dyspnea, patients were less likely to require hospitalization for heart failure.26 Instead, patients were presenting with exacerbations of chronic obstructive pulmonary disease, which would best be managed on an outpatient basis. Those who were admitted experienced shorter lengths of stay with subsequent reductions in health care costs, and had no significant difference in readmission or mortality rates.
Use of BNP as a cardiac biomarker does not come without its limitations. In addition to right-sided heart failure, ACS, and MI, elevations in BNP have been associated with septic shock, sepsis, renal dysfunction, and acute pulmonary embolus.27 Correlations have been made between the degree of BNP elevation and the extent of myocardial ischemia; increased levels of both BNP and cTnI were associated with higher mortality rates.28
C-Reactive Protein
Inflammation is known to play a key role in the development of atherosclerotic plaques. Measuring byproducts of the atherosclerotic process, from the initial development of fatty streaks to plaque rupture, can help determine whether a patient is at an increased risk for a cardiovascular event.29 Primary proinflammatory markers from the local site of intravascular inflammation signal messenger cytokines that are altered in the liver via an acute-phase reaction.
One of the major acute-phase reactants, C-reactive protein (CRP) is simply a byproduct of inflammation—yet it has become a major indicator of atherosclerotic plaque stability.30 A high-sensitivity CRP assay (hsCRP) can be a significantly effective predictor for MI, stroke, and peripheral vascular disease, even in patients who appear to be healthy.31 Studies have suggested that hsCRP is a better indicator of unstable plaque, and a better predictor of adverse cardiovascular events, than is low-density lipoprotein cholesterol (LDL-C), when these markers are used independently.32 When detected together, however, hsCRP and LDL-C elevations have been shown to be an even better predictor of adverse events in patients with no overt cardiovascular risk factors.31
As a risk factor, elevated hsCRP has been called as important as smoking or hypertension—highlighting the role of inflammation in formation of atherosclerosis at every level.33
In the evaluation of serum hsCRP, it is important to know that levels tend to be stable over long periods of time, have no circadian rhythms, and are not affected by various prandial states. Levels can be measured conveniently during the standard annual cholesterol screening. Relative cardiovascular risk is deemed low, medium, and high, in patients with an hsCRP measurement of less than 1 mg/L, 1 to 3 mg/L, and greater than 3 mg/L, respectively.2 Values exceeding 8.0 mg/L may be consistent with an acute infectious or inflammatory process, thus exposing the nonspecific nature of this popular biomarker.34 However, when used in conjunction with cTn, BNP, and patient history, hsCRP still proves to be an important clinical tool, offering prognostic information to facilitate clinical decision making.
Chronically elevated hsCRP signifies a very high risk for future cardiovascular events and should prompt the clinician to target the patient’s modifiable risk factors, including consideration of statin therapy as a part of the treatment regimen.35 According to results from the PROVE IT–TIMI 22 trial,35 statin use appears particularly cardioprotective in patients whose hsCRP levels are lower than 2 mg/L and who maintain LDL-C below 70 mg/dL. Researchers for this study group recommend monitoring hsCRP along with serum lipids for a more comprehensive cardiovascular risk profile.
Creatine Kinase
Three isoenzymes of creatine kinase (CK) exist: MM (skeletal muscle type), BB (brain type), and MB (myocardial band). The CK-MB isoenzyme was the diagnostic marker of choice for ACS until the introduction of cardiac-specific troponins in the early 1990s.36,37
CK-MB is an intracellular carrier protein for high-energy phosphates, found in higher concentrations in the myocardium than are the other CK isoenzymes.11 CK-MB accounts for about 15% of total CK, but it also exists in skeletal muscle and to a lesser extent in the small intestine, diaphragm, uterus, and prostate.21 As with most cardiac biomarkers, cardiospecificity of CK-MB is not 100%, and false-positive elevations can occur in a multitude of clinical settings, including significant musculoskeletal injury, heavy exertion, and myopathies.11
CK-MB is detectable three to four hours after myocardial injury, peaks at 24 hours, and returns to normal in 48 to 72 hours. CK-MB may be used to evaluate for ACS if cardiac troponin assays are not accessible,21 but its usefulness is limited during the early hours of ACS onset and after 72 hours.
The relative index of CK-MB to total CK (CK-MB/CK) can help the clinician assess whether the rise in CK-MB is attributable to a skeletal muscle source (CK-MB/CK < 3) or a cardiac source (CK-MB/CK > 5, indicating myocardial release of CK-MB). A relative index that falls between 3 and 5 warrants further investigation with serial analyses.36 It may also be helpful to know that a CK-MB elevation associated with skeletal muscle release tends to persist and plateau over a period of several days—as opposed to CK-MB elevation with a myocardial source, which follows the time course stated above.21
In a 2006 study that included nearly 30,000 patients, it was found that 28% of those with ACS had conflicting results between troponin levels and CK-MB.38 Patients with no elevations in cardiac troponins but elevations in CK-MB had no significant increased risk for in-hospital mortality, compared with patients with negative results for both markers. Thus, an isolated elevation in CK-MB has limited prognostic value in patients with negative troponin levels.
By contrast, however, Lim et al39 recently found that elevations in CK-MB were closely associated with perioperative necrosis and MI after percutaneous coronary intervention (PCI) as a result of currently oversensitive thresholds for cTnI. Thus, CK-MB use may play a role as an independent marker of necrosis in certain situations.
Myoglobin
Because of its small molecular size, myoglobin has timely release kinetics, with elevations appreciable before those of CK-MB or cTn.11,21 Myoglobin typically rises one to four hours after myocardial injury, peaks at six to 12 hours, and returns to normal within 24 hours. For this reason, it has held special interest as an early marker of cardiac injury.
With its early release/degradation kinetics, myoglobin may serve as a marker for reinfarction.21 It can also be used to assess further damage to the myocardium, as seen with distal thrombus embolization or coronary artery manipulation. However, troponin values provide similar information on reinfarction status.4
An established shortcoming of myoglobin as an early marker of necrosis is its lack of cardiac specificity, as it is found extensively in skeletal muscle.21 Patients who present with inflammation, trauma, or significant skeletal muscle injury can have extremely high levels of serum myoglobin without myocardial involvement. Since 2000, when cTn was designated the myocardial biomarker of choice (according to the revised definition of MI, as presented that year by the European Society of Cardiology and American College of Cardiology), the use of myoglobin to identify ACS has been considered obsolete.14
NOVEL BIOMARKERS OF CARDIOVASCULAR DISEASE
Heart-Type Fatty Acid–Binding Protein
A low–molecular-weight protein, heart-type fatty acid–binding protein (H-FABP) is involved in the intracellular uptake and buffering of myocardial free fatty acids. Because its molecular size is similar to that of troponin, it is rapidly released from the cytosol and has been proposed as an early sensitive marker of acute MI.40 In a 2010 study, H-FABP was shown to be of additional prognostic value when used in conjunction with cTnI in patients at low to moderate risk for suspected ACS.41 According to the known release kinetics of H-FABP after myocardial ischemia or infarct, a rise is detectable as early as 1.5 hours after symptom onset. The marker peaks after four to six hours and, because of rapid renal clearance, returns to baseline within 20 hours.20
Interpreting results may be hindered in the patient with impaired renal function—with not only higher levels, but sustained levels of H-FABP.20 Early assays used antibodies to detect circulating H-FABP levels, but cross-reactivity occurring between other fatty acid–binding protein types have limited the clinical applications of H-FABP testing.20 As more highly specific assays are produced, more practical protocols can be implemented to confirm the presence of ACS in patients presenting early to the ED with apparent ACS.
Ischemia-Modified Albumin
A biomarker that could detect ischemia alone would help identify patients at highest risk for infarction; immediate intervention could then prevent the progression of ACS, as evidenced by rising markers of necrosis. Ischemia-modified albumin (IMA), which is produced rapidly when circulating albumin comes into contact with ischemic myocardial tissue, has been touted as such a biomarker.42
Several changes occur in the human albumin molecule in the presence of ischemia, including its ability to bind transition metals—especially cobalt. This discovery led to the creation of an albumin-cobalt–binding assay, approved by the FDA for rapid detection of myocardial ischemia.19 When artificial ischemia is produced by balloon inflation during percutaneous coronary angioplasty, IMA levels can be detected, using the assay, within minutes of coronary artery occlusion. Levels tend to peak within six hours and can be elevated for as long as 12 hours.19
When IMA is used in conjunction with ECG findings and cTnT levels, a sensitivity of 97% for detecting ACS can be achieved. This could reduce the number of patients being discharged from the ED with occult ACS,43 giving IMA a potentially important precautionary and supplemental role. As with most cardiac biomarkers, IMA alone is not 100% specific for ACS since it is also present in other ischemic conditions, thus hindering its usefulness in clinical practice. Elevations had been reported in patients with liver cirrhosis, uncontrolled type 2 diabetes mellitus, obstetric conditions associated with placental ischemia, carbon monoxide poisoning, and cerebrovascular ischemia.44-47
Recent suggestions to use IMA to rule out rather than diagnose ACS show promise, since the absence of this acute-phase reactant should exclude the presence of myocardial ischemia.48 Further studies are needed to determine the exact physiology of IMA production in order to identify its cardiac specificity for clinical use.49
Homocysteine
As a marker of increased cardiovascular risk, homocysteine is thought to have multiple effects on the cardiovascular system. These include endothelial dysfunction, decreased arteriole vasodilation (ie, reduced release of nitric oxide), increased platelet activation, increased production of free radicals, and increased LDL oxidation with arteriole lipid accumulation.50
In patients with severe hyperhomocysteinemia (ie, homocysteine serum levels > 100 mol/L), risk for premature atherothrombosis and venous thromboembolism is increased. In the general public, mildly elevated homocysteine levels (> 15 mol/L) have been attributed to insufficient dietary intake of folic acid. Folate in its natural form has been known to decrease serum homocysteine levels by 25%, if supplemented appropriately with vitamins B6 and B12.50
In recent years, folate deficiency has declined due to enrichment of certain foods with this crucial nutrient, initially mandated to decrease the incidence of neural tube defects in developing embryos. From a cardiovascular standpoint, researchers have been unable to determine whether elevated homocysteine increases CVD risk or is simply a marker of existing disease burden.50 In clinical trials in which subjects took B-vitamins supplemented with folate, homocysteine levels were reduced; yet in one study, stroke risk was not reduced in patients with a history of stroke51; in a second, in-stent restenosis was more common in patients who took the supplement after undergoing angioplasty52; and in a third, patients following the vitamin regimen after a recent acute MI proved to be at higher cardiovascular risk.53
As a result of this conflicting evidence, no recommendations have been made for routine homocysteine screening except in patients with a history of markedly premature atherosclerosis or a family history of early-onset acute MI or stroke.50 Monitoring may be advisable in patients who take a folate antagonist (eg, methotrexate, carbamazepine), considering the risk for folate deficiency and subsequent hyperhomocysteinemia.
ACUTE INFLAMMATORY MARKERS OF PLAQUE RUPTURE OR VULNERABILITY
Myeloperoxidase
Many researchers have taken a particular interest in the acute substances formed as a result of atherothrombotic plaque inflammation or rupture. One such biomarker is myeloperoxidase (MPO), which is thought to be expressed from the degranulation of activated leukocytes found in atherosclerotic plaques. This acute-phase enzyme may convert LDL into a high-uptake form for macrophages, leading to foam cell formation and depletion of nitric oxide, contributing to additional ischemia by way of vasoconstriction.54
Recently, a high systemic MPO level was found to be a more significant marker of plaque at risk for rupture, compared with already-ruptured plaque.55 Although MPO elevations may also occur in a number of inflammatory, infectious, or infiltrative conditions, the association between MPO, inflammation, and oxidative stress supports its use as a marker for plaque that is vulnerable to rupture.56,57
Serum levels of MPO have been shown to predict increased risk for subsequent death or MI in patients who present to the ED with ACS, independent of other cardiac risk factors or cardiac biomarkers. In a 2001 study, Zhang et al54 established an association between elevated MPO levels and angiographically proven coronary atherosclerosis, with a 20-fold higher risk for coronary artery disease; earlier this year, Oemrawsingh et al58 reported an independent association between MPO and long-term adverse outcomes in patients who presented with non–ST-segment elevation ACS. Thus, MPO may be a significant indicator of vascular inflammation.57
Soluble CD40 Ligand
In the 1980s, postmortem studies confirmed that erosions or ruptures in atherosclerotic fibrous caps lead to platelet activation—the main pathophysiologic contributor in ACS.59 This fundamental actuality suggests that biomarkers of platelet activation may provide supplemental information in patients who present with chest pain of cardiac origin. Another acute inflammatory marker, soluble CD40 ligand, is a marker of active platelet stimulation.60 Increased serum levels of soluble CD40 ligand have been correlated to increased risk for cardiovascular events in apparently healthy women.61
Soluble CD40 ligand, expressed within seconds of platelet activation, is also commonly found on various leukocytes, endothelial cells, and smooth-muscle cells.60 This may provide insight into cardiovascular disease progression and plaque deterioration that precedes the events of ACS.
Therapeutic antiplatelet medications are now the mainstay in the treatment and prevention of cardiovascular complications associated with ischemic thrombus formation.11 Platelet biomarkers are likely to play an essential supplemental role in the diagnosis of ACS.
Pregnancy-Associated Plasma Protein A
Additional risk-stratifying biomarkers include those that may determine whether a patient has plaques that are acutely vulnerable to rupture. Pregnancy-associated plasma protein A (PAPP-A), first detected in the 1970s in the circulation of pregnant women, is now widely used in first-trimester screening for fetal trisomy.62
Since then, it has been found that PAPP-A, which is theorized to be produced by vascular smooth-muscle cells, is extensively expressed in unstable coronary artery plaques, while minimally expressed in stable plaques.63 Since a significant proportion of patients who present with symptoms of ACS have normal cTn levels, PAPP-A may help identify patients who are at increased risk for subsequent short-term cardiovascular complications resulting from occult disease.64 This relatively new marker may also prove useful for screening in the office setting, identifying outpatients who are at high cardiovascular risk. Further studies are needed to define the release kinetics of PAPP-A, guiding clinicians in its implementation and clinical use.
CONCLUSION
When used in conjunction with the history and physical exam, cardiac biomarkers can provide a simple, noninvasive means to further the clinician’s exploration into a suspected underlying cardiovascular process. As advances continue in the understanding of the pathogenesis of heart disease, new interpretations of existing markers and discovery of novel markers may allow for specific therapeutic interventions to improve patient outcomes.
It is important to note that the list of biomarkers described here is by no means complete, and there is continued interest in finding more specific and sensitive markers of heart disease. Numerous cardiovascular organizations are now suggesting a shift toward a multi-marker strategy to determine the best etiology in the patient who presents with decompensating cardiovascular disease. A change in cardiac enzyme panels may be inevitable in the near future. Practicing PAs and NPs, particularly those who care for patients at risk for CVD, should remain up-to-date and proficient in interpreting those results to help determine the best course of action for each patient.
REFERENCES
1. Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634-2653.
2. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003; 107(3):499-511.
3. Kavsak PA, MacRae AR, Newman AM, et al. Effects of contemporary troponin assay sensitivity on the utility of the early markers myoglobin and CKMB isoforms in evaluating patients with possible acute myocardial infarction. Clin Chim Acta. 2007; 380(1-2):213-216.
4. Eggers KM, Oldgren J, Nordenskjöld A, Lindahl B. Diagnostic value of serial measurement of cardiac markers in patients with chest pain: limited value of adding myoglobin to troponin I for exclusion of myocardial infarction. Am Heart J. 2004; 148(4):574-581.
5. Licka M, Zimmermann R, Zehelein J, et al. Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart. 2002;87(6):520-524.
6. Panteghini M, Cuccia C, Bonetti G, et al. Single-point cardiac troponin T at coronary care unit discharge after myocardial infarction correlates with infarct size and ejection fraction. Clin Chem. 2002; 48(9):1432-1436.
7. Giannitsis E, Müller-Bardorff M, Lehrke S, et al. Admission troponin T level predicts clinical outcomes, TIMI flow, and myocardial tissue perfusion after primary percutaneous intervention for acute ST-segment elevation myocardial infarction. Circulation. 2001;104(6):630-635.
8. Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25-e146.
9. Lloyd-Jones D, Adams RJ, Brown TM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010; 121(7):e46-e215.
10. Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128.
11. Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non–ST-Elevation Myocardial Infarction: a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123(18):e426-e579.
12. Ohman EM, Armstrong PW, Christenson RH, et al; GUSTO IIA Investigators. Cardiac troponin T levels for risk stratification in acute myocardial ischemia.
N Engl J Med. 1996;335(18):1333-1341.
13. Antman EM, Tanasijevic MJ, Thompson B, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996;335(18):1342-1349.
14. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined: a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. J Am Coll Cardiol. 2000;36(3):959-969.
15. Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol. 2006;48(1):1-11.
16. Galvani M, Ottani F, Ferrini D, et al. Prognostic influence of elevated values of cardiac troponin I in patients with unstable angina. Circulation. 1997;95 (8):2053-2059.
17. Freda BJ, Tang WH, Van Lente F, et al. Cardiac troponins in renal insufficiency: review and clinical implications. J Am Coll Cardiol. 2002;40(12):2065-2071.
18. deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304(22):2494-2502.
19. Sinha MK, Roy D, Gaze DC, et al. Role of “ischemia modified albumin,” a new biochemical marker of myocardial ischaemia, in the early diagnosis of acute coronary syndromes. Emerg Med J. 2004; 21(1):29-34.
20. McCann CJ, Glover BM, Menown IB, et al. Novel biomarkers in early diagnosis of acute myocardial infarction compared with cardiac troponin T. Eur Heart J. 2008;29(23):2843-2850.
21. Morrow DA, Cannon CP, Jesse RL, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13): e356-e375.
22. Maisel AS, Krishnaswamy P, Nowak RM, et al; Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161-167.
23. McCullough PA, Nowak RM, McCord J, et al: B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002;106(4):416-422.
24. Januzzi JL Jr, Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95(8):948-954.
25. Mueller C, Laule-Kilian K, Scholer A, et al. Use of B-type natriuretic peptide for the management of women with dyspnea. Am J Cardiol. 2004; 94(12):1510-1514.
26. Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27(3):330-337.
27. Maisel AS. The diagnosis of acute congestive heart failure: role of BNP measurements. Heart Fail Rev. 2003;8(4):327-334.
28. Morrow DA, de Lemos JA, Sabatine MS, et al. Evaluation of B-type natriuretic peptide for risk assessment in unstable angina/non–ST-elevation myocardial infarction: B-type natriuretic peptide and prognosis in TACTICS-TIMI 18. J Am Coll Cardiol. 2003;41(8):1264-1272.
29. Libby P, Ridker PM. Inflammation and atherothrombosis: from population biology and bench research to clinical practice. J Am Coll Cardiol. 2006; 48(9 suppl A):A33-A46.
30. Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363-369.
31. Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557-1565.
32. Boekholdt SM, Hack CE, Sandhu MS, et al. C-reactive protein levels and coronary artery disease incidence and mortality in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study 1993-2003. Atherosclerosis. 2006; 187(2):415-422.
33. Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350(14):1387-1397.
34. Ridker PM, Cook N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham Risk Scores. Circulation. 2004;109(16):1955-1959.
35. Ridker PM, Cannon CP, Morrow D, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombosis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20-28.
36. Fischer JH, Jeschkeit-Schubbert S, Kuhn-Régnier F, Switkowski R. The origin of CK-MB serum levels and CK-MB/total CK ratios: measurements of CK isoenzyme activities in various tissues. Internet J Thorac Cardiovasc Surg. 2005;7(1).
37. Adams JE 3rd, Abendschein DR, Jaffe AS. Biochemical markers of myocardial injury: is MB creatine kinase the choice for the 1990s? Circulation. 1993;88(2):750-763.
38. Newby LK, Roe MT, Chen AY, et al; CRUSADE Investigators. Frequency and clinical implications of discordant creatine kinase-MB and troponin measurements in acute coronary syndromes. J Am Coll Cardiol. 2006;47(2):312-318.
39. Lim CC, van Gaal WJ, Testa L, et al. With the “universal definition,” measurement of creatine kinase–myocardial band rather than troponin allows more accurate diagnosis of periprocedural necrosis and infarction after coronary intervention. J Am Coll Cardiol. 2011;57(6):653-661.
40. Kilcullen N, Viswanathan K, Das R, et al; EMMACE-2 Investigators. Heart-type fatty acid–binding protein predicts long-term mortality after acute coronary syndrome and identifies high-risk patients across the range of troponin values. J Am Coll Cardiol. 2007;50(21):2061-2067.
41. Viswanathan K, Kilcullen N, Morrell C, et al. Heart-type fatty acid–binding protein predicts long-term mortality and re-infarction in consecutive patients with suspected acute coronary syndrome who are troponin-negative. J Am Coll Cardiol. 2010; 55(23): 2590-2598.
42. Abadie JM, Blassingame CL, Bankson DD. Albumin cobalt binding assay to rule out acute coronary syndrome. Ann Clin Lab Sci. 2005;35(1):66-72.
43. Anwaruddin S, Januzzi JL Jr, Baggish AL, et al. Ischemia-modified albumin improves the usefulness of standard cardiac biomarkers for the diagnosis of myocardial ischemia in the emergency department setting. Am J Clin Pathol. 2005;123(1):140-145.
44. Piwowar A, Knapik-Kordecka M, Warwas M. Ischemia-modified albumin level in type 2 diabetes mellitus: preliminary report. Dis Markers. 2008; 24(6):311-317.
45. Prefumo F, Gaze DC, Papageorghiou AT, et al. First trimester maternal serum ischaemia-modified albumin: a marker of hypoxia-ischaemia-driven early trophoblast development. Hum Reprod. 2007; 22(7):2029-2032.
46. Gunduz A, Turedi S, Mentese A, et al. Ischemia-modified albumin levels in cerebrovascular accidents. Am J Emerg Med. 2008;26(8):874-878.
47. Turedi S, Cinar O, Kaldirim U, et al. Ischemia-modified albumin levels in carbon monoxide poisoning. Am J Emerg Med. 2011;29(6):675-681.
48. Sbarouni E, Georgiadou P, Voudris V. Ischemia modified albumin changes: review and clinical implications. Clin Chem Lab Med. 2011;49(2):177-184.
49. Gaze DC. Ischemia modified albumin: a novel biomarker for the detection of cardiac ischemia. Drug Metab Pharmacokinet. 2009;24(4):333-341.
50. Kaul S, Zadeh AA, Shah PK. Homocysteine hypothesis for atherothrombotic cardiovascular disease: not validated. J Am Coll Cardiol. 2006; 48(5):914-923.
51. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291(5):565-575.
52. Lange H, Suryapranata H, De Luca G, et al. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med. 2004;350(26):2673-2681.
53. Bønaa KH, Njølstad I, Ueland PM, et al; NORVIT Trial Investigators. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578-1588.
54. Zhang R, Brennan ML, Fu X, et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286(17):2136-2142.
55. Ferrante G, Nakano M, Prati F, et al. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes: a clinicopathological study. Circulation. 2010;122(24):2505-2513.
56. Loria V, Dato I, Graziani F, Biasucci LM. Myeloperoxidase: a new biomarker of inflammation in ischemic heart disease and acute coronary syndromes. Mediators Inflamm. 2008;2008:135625.
57. Apple FS, Wu AH, Mair J, et al; Committee on Standardization of Markers of Cardiac Damage of the IFCC. Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin Chem. 2005;51(5):810-824.
58. Oemrawsingh RM, Lenderink T, Akkerhuis KM, et al. Multimarker risk model containing troponin-T, interleukin 10, myeloperoxidase and placental growth factor predicts long-term cardiovascular risk after non–ST-segment elevation acute coronary syndrome. Heart. 2011;97(13):1061-1066.
59. Davies MJ, Thomas AC. Plaque fissuring: the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J. 1985;53(4):363-373.
60. Heeschen C, Dimmeler S, Hamm CW, et al; CAPTURE Study. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med. 2003;348(12): 1104-1111.
61. Schönbeck U, Varo N, Libby P, et al. Soluble CD40L and cardiovascular risk in women. Circulation. 2001;104(19):2266-2268.
62. Lin TM, Galbert SP, Kiefer D, et al. Characterization of four human pregnancy–associated plasma proteins. Am J Obstet Gynecol. 1974; 118(2):223-236.
63. Bayes-Genis A, Conover CA, Overgaard MT, et al. Pregnancy-associated plasma protein A as a marker of acute coronary syndromes. N Engl J Med. 2001;345(14):1022-1029.
64. Lund J, Qin QP, Ilva T, et al. Circulating pregnancy-associated plasma protein A predicts outcome in patients with acute coronary syndrome but no troponin I elevation. Circulation. 2003; 108(16):1924-1926.
Since the 1950s, researchers and clinicians have sought a simple chemical biomarker that would aid in the diagnosis and treatment of cardiovascular disease (CVD). An initial discovery was that proteins normally found within cardiac myocytes are released into the circulation when the myocardial cell membrane loses its integrity as a result of hypoxic injury.1 Over the past 60 years, major advances have been made to distinguish which proteins are highly cardiac specific, and which are suggestive of other pathologic cardiovascular processes.
In the past 15 to 20 years, significant data have emerged that support the role of cardiac-specific troponins in the cardiac disease process, making their measurement an important consideration in diagnosis and clinical decision-making for patients with various forms of CVD.2-7 With the exception of 1918, CVD has accounted for more deaths in the United States than any other illness in every year since 1900.8 It has been suggested that nearly 2,300 Americans die of CVD each day—an average of one death every 38 seconds. The direct and indirect costs of CVD for 2010 were projected at $503.2 billion—including $155 billion for total hospital costs alone.9 By comparison, a 2010 projection from the National Cancer Institute was $124.57 billion for the direct costs of cancer care.10
It is imperative for practitioners in all areas of medicine to have a working knowledge of the role of cardiac biomarkers and their potential impact on patients’ overall health. Primary care clinicians in particular should be familiar with the release kinetics of certain cardiac biomarkers to aid in the diagnosis and treatment of the cardiac patient. This article is intended as an overview of the cardiac biomarkers that are being used in practice today, and to review the novel biomarkers that may have an impact on the way clinicians practice in the near future.
BIOMARKERS CURRENTLY IN USE
Cardiac Troponins
The cardiac-specific troponins (cTn) are an integral component of the myosin-actin binding complex found in striated muscle tissue. The troponin complex includes three regulatory proteins: troponin C, troponin I, and troponin T. The genes that code for troponin C are identical in skeletal and cardiac tissue; for this reason, troponin C becomes much less cardiospecific.
However, cardiac troponin T (cTnT) and cardiac troponin I (cTnI) have differing amino acid sequences, making it possible to develop highly sensitive immunoassays to detect circulating antibody-troponin complexes after myocardial cell injury, as seen in acute coronary syndrome (ACS).12-14 No cross-reactivity occurs between skeletal and cardiac sources, indicating the unique cardiospecificity of the cTn proteins.11
Of note, there are numerous patient populations in which elevated cTn may be found; the associated conditions are outlined in Table 1.11,15,16 In particular, elevations in cTn (more specifically cTnT) are commonly observed in patients with end-stage renal disease (ESRD).11,17 The pathogenesis of elevated cTnT is not completely understood but has been proposed as a promising prognostic tool for use in patients with ESRD. Elevated levels of cTnT identify patients whose chance of survival is poor, with an increased risk for cardiac death.7 It should be noted that a significant proportion of patients with chronic renal failure succumb to cardiovascular death.17
Recently, deFilippi and colleagues18 reported a correlation between trace amounts of circulating cTnT in older patients not known to have CVD and an increased risk for future heart failure or even cardiovascular-related death. This novel finding may add valuable information to the screening and risk stratification of relatively healthy but sedentary individuals older than 65.
Elevations in cTn can be seen as early as two to four hours after myocardial injury.11 A small portion of cTnT and cTnI is found within the myocardial cells’ cytosol and is not bound to the troponin complex, which begins to degrade after cell injury.1,14 This cytosolic pool allows for earlier recognition of cardiac injury. As increasingly sensitive assays are developed, rises in cTn levels can be detected earlier after symptom onset, thus making the use of less specific early biomarkers, such as myoglobin and creatine kinase–MB, obsolete.
Because of the continuous release of structural proteins from the degrading myocardial tissue, elevations of cTnI may persist for seven to 10 days, while cTnT elevations can continue for 10 to 14 days postinfarction.11 This prolonged period allows for detection of even very slight cardiac damage and provides an advantage for identification and treatment of high-risk patients. When measured 72 hours after infarction, cTnI can also provide prognostic information, such as the potential size of the affected myocardial area.5,6 This, too, facilitates risk stratification. Timing of the release of key biomarkers can be seen in Table 2.11,19-21
Natriuretic Peptides
The natriuretic peptides are cardiac neurohormones that are released in response to myocardial wall stress.11 B-type natriuretic peptide (BNP; previously known as brain natriuretic peptide) is synthesized and released from the ventricular myocardium in times of volume expansion and increased pressure burden. Initially, the prohormone proBNP is released and is enzymatically cleaved to N-terminal proBNP (NT-proBNP) and then to “mature” BNP.11
Today, BNP is used widely as a biomarker for congestive heart failure.22 In the Breathing Not Properly (BNP) study,23 BNP was able to accurately distinguish dyspnea caused by heart failure from that with a pulmonary etiology; it was found to be the strongest predictor for the diagnosis of heart failure. In the evaluation of serum BNP, a level below 100 ng/L makes heart failure unlikely (negative predictive value, 90%). If the value rises above 500 ng/L, heart failure is highly likely (positive predictive value, 90%).23
As for NT-proBNP, levels exceeding 450 ng/L in patients younger than 50 and exceeding 900 ng/L in patients 50 or older have been found highly sensitive and specific for heart failure–related dyspnea.24 Current studies suggest that NT-proBNP may have greater sensitivity and specificity than standard BNP when these age-related cut-off limits are applied.25
Researchers for the International Collaborative of NT-proBNP Study reported that when NT-proBNP was measured in the emergency department (ED) in patients with dyspnea, patients were less likely to require hospitalization for heart failure.26 Instead, patients were presenting with exacerbations of chronic obstructive pulmonary disease, which would best be managed on an outpatient basis. Those who were admitted experienced shorter lengths of stay with subsequent reductions in health care costs, and had no significant difference in readmission or mortality rates.
Use of BNP as a cardiac biomarker does not come without its limitations. In addition to right-sided heart failure, ACS, and MI, elevations in BNP have been associated with septic shock, sepsis, renal dysfunction, and acute pulmonary embolus.27 Correlations have been made between the degree of BNP elevation and the extent of myocardial ischemia; increased levels of both BNP and cTnI were associated with higher mortality rates.28
C-Reactive Protein
Inflammation is known to play a key role in the development of atherosclerotic plaques. Measuring byproducts of the atherosclerotic process, from the initial development of fatty streaks to plaque rupture, can help determine whether a patient is at an increased risk for a cardiovascular event.29 Primary proinflammatory markers from the local site of intravascular inflammation signal messenger cytokines that are altered in the liver via an acute-phase reaction.
One of the major acute-phase reactants, C-reactive protein (CRP) is simply a byproduct of inflammation—yet it has become a major indicator of atherosclerotic plaque stability.30 A high-sensitivity CRP assay (hsCRP) can be a significantly effective predictor for MI, stroke, and peripheral vascular disease, even in patients who appear to be healthy.31 Studies have suggested that hsCRP is a better indicator of unstable plaque, and a better predictor of adverse cardiovascular events, than is low-density lipoprotein cholesterol (LDL-C), when these markers are used independently.32 When detected together, however, hsCRP and LDL-C elevations have been shown to be an even better predictor of adverse events in patients with no overt cardiovascular risk factors.31
As a risk factor, elevated hsCRP has been called as important as smoking or hypertension—highlighting the role of inflammation in formation of atherosclerosis at every level.33
In the evaluation of serum hsCRP, it is important to know that levels tend to be stable over long periods of time, have no circadian rhythms, and are not affected by various prandial states. Levels can be measured conveniently during the standard annual cholesterol screening. Relative cardiovascular risk is deemed low, medium, and high, in patients with an hsCRP measurement of less than 1 mg/L, 1 to 3 mg/L, and greater than 3 mg/L, respectively.2 Values exceeding 8.0 mg/L may be consistent with an acute infectious or inflammatory process, thus exposing the nonspecific nature of this popular biomarker.34 However, when used in conjunction with cTn, BNP, and patient history, hsCRP still proves to be an important clinical tool, offering prognostic information to facilitate clinical decision making.
Chronically elevated hsCRP signifies a very high risk for future cardiovascular events and should prompt the clinician to target the patient’s modifiable risk factors, including consideration of statin therapy as a part of the treatment regimen.35 According to results from the PROVE IT–TIMI 22 trial,35 statin use appears particularly cardioprotective in patients whose hsCRP levels are lower than 2 mg/L and who maintain LDL-C below 70 mg/dL. Researchers for this study group recommend monitoring hsCRP along with serum lipids for a more comprehensive cardiovascular risk profile.
Creatine Kinase
Three isoenzymes of creatine kinase (CK) exist: MM (skeletal muscle type), BB (brain type), and MB (myocardial band). The CK-MB isoenzyme was the diagnostic marker of choice for ACS until the introduction of cardiac-specific troponins in the early 1990s.36,37
CK-MB is an intracellular carrier protein for high-energy phosphates, found in higher concentrations in the myocardium than are the other CK isoenzymes.11 CK-MB accounts for about 15% of total CK, but it also exists in skeletal muscle and to a lesser extent in the small intestine, diaphragm, uterus, and prostate.21 As with most cardiac biomarkers, cardiospecificity of CK-MB is not 100%, and false-positive elevations can occur in a multitude of clinical settings, including significant musculoskeletal injury, heavy exertion, and myopathies.11
CK-MB is detectable three to four hours after myocardial injury, peaks at 24 hours, and returns to normal in 48 to 72 hours. CK-MB may be used to evaluate for ACS if cardiac troponin assays are not accessible,21 but its usefulness is limited during the early hours of ACS onset and after 72 hours.
The relative index of CK-MB to total CK (CK-MB/CK) can help the clinician assess whether the rise in CK-MB is attributable to a skeletal muscle source (CK-MB/CK < 3) or a cardiac source (CK-MB/CK > 5, indicating myocardial release of CK-MB). A relative index that falls between 3 and 5 warrants further investigation with serial analyses.36 It may also be helpful to know that a CK-MB elevation associated with skeletal muscle release tends to persist and plateau over a period of several days—as opposed to CK-MB elevation with a myocardial source, which follows the time course stated above.21
In a 2006 study that included nearly 30,000 patients, it was found that 28% of those with ACS had conflicting results between troponin levels and CK-MB.38 Patients with no elevations in cardiac troponins but elevations in CK-MB had no significant increased risk for in-hospital mortality, compared with patients with negative results for both markers. Thus, an isolated elevation in CK-MB has limited prognostic value in patients with negative troponin levels.
By contrast, however, Lim et al39 recently found that elevations in CK-MB were closely associated with perioperative necrosis and MI after percutaneous coronary intervention (PCI) as a result of currently oversensitive thresholds for cTnI. Thus, CK-MB use may play a role as an independent marker of necrosis in certain situations.
Myoglobin
Because of its small molecular size, myoglobin has timely release kinetics, with elevations appreciable before those of CK-MB or cTn.11,21 Myoglobin typically rises one to four hours after myocardial injury, peaks at six to 12 hours, and returns to normal within 24 hours. For this reason, it has held special interest as an early marker of cardiac injury.
With its early release/degradation kinetics, myoglobin may serve as a marker for reinfarction.21 It can also be used to assess further damage to the myocardium, as seen with distal thrombus embolization or coronary artery manipulation. However, troponin values provide similar information on reinfarction status.4
An established shortcoming of myoglobin as an early marker of necrosis is its lack of cardiac specificity, as it is found extensively in skeletal muscle.21 Patients who present with inflammation, trauma, or significant skeletal muscle injury can have extremely high levels of serum myoglobin without myocardial involvement. Since 2000, when cTn was designated the myocardial biomarker of choice (according to the revised definition of MI, as presented that year by the European Society of Cardiology and American College of Cardiology), the use of myoglobin to identify ACS has been considered obsolete.14
NOVEL BIOMARKERS OF CARDIOVASCULAR DISEASE
Heart-Type Fatty Acid–Binding Protein
A low–molecular-weight protein, heart-type fatty acid–binding protein (H-FABP) is involved in the intracellular uptake and buffering of myocardial free fatty acids. Because its molecular size is similar to that of troponin, it is rapidly released from the cytosol and has been proposed as an early sensitive marker of acute MI.40 In a 2010 study, H-FABP was shown to be of additional prognostic value when used in conjunction with cTnI in patients at low to moderate risk for suspected ACS.41 According to the known release kinetics of H-FABP after myocardial ischemia or infarct, a rise is detectable as early as 1.5 hours after symptom onset. The marker peaks after four to six hours and, because of rapid renal clearance, returns to baseline within 20 hours.20
Interpreting results may be hindered in the patient with impaired renal function—with not only higher levels, but sustained levels of H-FABP.20 Early assays used antibodies to detect circulating H-FABP levels, but cross-reactivity occurring between other fatty acid–binding protein types have limited the clinical applications of H-FABP testing.20 As more highly specific assays are produced, more practical protocols can be implemented to confirm the presence of ACS in patients presenting early to the ED with apparent ACS.
Ischemia-Modified Albumin
A biomarker that could detect ischemia alone would help identify patients at highest risk for infarction; immediate intervention could then prevent the progression of ACS, as evidenced by rising markers of necrosis. Ischemia-modified albumin (IMA), which is produced rapidly when circulating albumin comes into contact with ischemic myocardial tissue, has been touted as such a biomarker.42
Several changes occur in the human albumin molecule in the presence of ischemia, including its ability to bind transition metals—especially cobalt. This discovery led to the creation of an albumin-cobalt–binding assay, approved by the FDA for rapid detection of myocardial ischemia.19 When artificial ischemia is produced by balloon inflation during percutaneous coronary angioplasty, IMA levels can be detected, using the assay, within minutes of coronary artery occlusion. Levels tend to peak within six hours and can be elevated for as long as 12 hours.19
When IMA is used in conjunction with ECG findings and cTnT levels, a sensitivity of 97% for detecting ACS can be achieved. This could reduce the number of patients being discharged from the ED with occult ACS,43 giving IMA a potentially important precautionary and supplemental role. As with most cardiac biomarkers, IMA alone is not 100% specific for ACS since it is also present in other ischemic conditions, thus hindering its usefulness in clinical practice. Elevations had been reported in patients with liver cirrhosis, uncontrolled type 2 diabetes mellitus, obstetric conditions associated with placental ischemia, carbon monoxide poisoning, and cerebrovascular ischemia.44-47
Recent suggestions to use IMA to rule out rather than diagnose ACS show promise, since the absence of this acute-phase reactant should exclude the presence of myocardial ischemia.48 Further studies are needed to determine the exact physiology of IMA production in order to identify its cardiac specificity for clinical use.49
Homocysteine
As a marker of increased cardiovascular risk, homocysteine is thought to have multiple effects on the cardiovascular system. These include endothelial dysfunction, decreased arteriole vasodilation (ie, reduced release of nitric oxide), increased platelet activation, increased production of free radicals, and increased LDL oxidation with arteriole lipid accumulation.50
In patients with severe hyperhomocysteinemia (ie, homocysteine serum levels > 100 mol/L), risk for premature atherothrombosis and venous thromboembolism is increased. In the general public, mildly elevated homocysteine levels (> 15 mol/L) have been attributed to insufficient dietary intake of folic acid. Folate in its natural form has been known to decrease serum homocysteine levels by 25%, if supplemented appropriately with vitamins B6 and B12.50
In recent years, folate deficiency has declined due to enrichment of certain foods with this crucial nutrient, initially mandated to decrease the incidence of neural tube defects in developing embryos. From a cardiovascular standpoint, researchers have been unable to determine whether elevated homocysteine increases CVD risk or is simply a marker of existing disease burden.50 In clinical trials in which subjects took B-vitamins supplemented with folate, homocysteine levels were reduced; yet in one study, stroke risk was not reduced in patients with a history of stroke51; in a second, in-stent restenosis was more common in patients who took the supplement after undergoing angioplasty52; and in a third, patients following the vitamin regimen after a recent acute MI proved to be at higher cardiovascular risk.53
As a result of this conflicting evidence, no recommendations have been made for routine homocysteine screening except in patients with a history of markedly premature atherosclerosis or a family history of early-onset acute MI or stroke.50 Monitoring may be advisable in patients who take a folate antagonist (eg, methotrexate, carbamazepine), considering the risk for folate deficiency and subsequent hyperhomocysteinemia.
ACUTE INFLAMMATORY MARKERS OF PLAQUE RUPTURE OR VULNERABILITY
Myeloperoxidase
Many researchers have taken a particular interest in the acute substances formed as a result of atherothrombotic plaque inflammation or rupture. One such biomarker is myeloperoxidase (MPO), which is thought to be expressed from the degranulation of activated leukocytes found in atherosclerotic plaques. This acute-phase enzyme may convert LDL into a high-uptake form for macrophages, leading to foam cell formation and depletion of nitric oxide, contributing to additional ischemia by way of vasoconstriction.54
Recently, a high systemic MPO level was found to be a more significant marker of plaque at risk for rupture, compared with already-ruptured plaque.55 Although MPO elevations may also occur in a number of inflammatory, infectious, or infiltrative conditions, the association between MPO, inflammation, and oxidative stress supports its use as a marker for plaque that is vulnerable to rupture.56,57
Serum levels of MPO have been shown to predict increased risk for subsequent death or MI in patients who present to the ED with ACS, independent of other cardiac risk factors or cardiac biomarkers. In a 2001 study, Zhang et al54 established an association between elevated MPO levels and angiographically proven coronary atherosclerosis, with a 20-fold higher risk for coronary artery disease; earlier this year, Oemrawsingh et al58 reported an independent association between MPO and long-term adverse outcomes in patients who presented with non–ST-segment elevation ACS. Thus, MPO may be a significant indicator of vascular inflammation.57
Soluble CD40 Ligand
In the 1980s, postmortem studies confirmed that erosions or ruptures in atherosclerotic fibrous caps lead to platelet activation—the main pathophysiologic contributor in ACS.59 This fundamental actuality suggests that biomarkers of platelet activation may provide supplemental information in patients who present with chest pain of cardiac origin. Another acute inflammatory marker, soluble CD40 ligand, is a marker of active platelet stimulation.60 Increased serum levels of soluble CD40 ligand have been correlated to increased risk for cardiovascular events in apparently healthy women.61
Soluble CD40 ligand, expressed within seconds of platelet activation, is also commonly found on various leukocytes, endothelial cells, and smooth-muscle cells.60 This may provide insight into cardiovascular disease progression and plaque deterioration that precedes the events of ACS.
Therapeutic antiplatelet medications are now the mainstay in the treatment and prevention of cardiovascular complications associated with ischemic thrombus formation.11 Platelet biomarkers are likely to play an essential supplemental role in the diagnosis of ACS.
Pregnancy-Associated Plasma Protein A
Additional risk-stratifying biomarkers include those that may determine whether a patient has plaques that are acutely vulnerable to rupture. Pregnancy-associated plasma protein A (PAPP-A), first detected in the 1970s in the circulation of pregnant women, is now widely used in first-trimester screening for fetal trisomy.62
Since then, it has been found that PAPP-A, which is theorized to be produced by vascular smooth-muscle cells, is extensively expressed in unstable coronary artery plaques, while minimally expressed in stable plaques.63 Since a significant proportion of patients who present with symptoms of ACS have normal cTn levels, PAPP-A may help identify patients who are at increased risk for subsequent short-term cardiovascular complications resulting from occult disease.64 This relatively new marker may also prove useful for screening in the office setting, identifying outpatients who are at high cardiovascular risk. Further studies are needed to define the release kinetics of PAPP-A, guiding clinicians in its implementation and clinical use.
CONCLUSION
When used in conjunction with the history and physical exam, cardiac biomarkers can provide a simple, noninvasive means to further the clinician’s exploration into a suspected underlying cardiovascular process. As advances continue in the understanding of the pathogenesis of heart disease, new interpretations of existing markers and discovery of novel markers may allow for specific therapeutic interventions to improve patient outcomes.
It is important to note that the list of biomarkers described here is by no means complete, and there is continued interest in finding more specific and sensitive markers of heart disease. Numerous cardiovascular organizations are now suggesting a shift toward a multi-marker strategy to determine the best etiology in the patient who presents with decompensating cardiovascular disease. A change in cardiac enzyme panels may be inevitable in the near future. Practicing PAs and NPs, particularly those who care for patients at risk for CVD, should remain up-to-date and proficient in interpreting those results to help determine the best course of action for each patient.
REFERENCES
1. Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634-2653.
2. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003; 107(3):499-511.
3. Kavsak PA, MacRae AR, Newman AM, et al. Effects of contemporary troponin assay sensitivity on the utility of the early markers myoglobin and CKMB isoforms in evaluating patients with possible acute myocardial infarction. Clin Chim Acta. 2007; 380(1-2):213-216.
4. Eggers KM, Oldgren J, Nordenskjöld A, Lindahl B. Diagnostic value of serial measurement of cardiac markers in patients with chest pain: limited value of adding myoglobin to troponin I for exclusion of myocardial infarction. Am Heart J. 2004; 148(4):574-581.
5. Licka M, Zimmermann R, Zehelein J, et al. Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart. 2002;87(6):520-524.
6. Panteghini M, Cuccia C, Bonetti G, et al. Single-point cardiac troponin T at coronary care unit discharge after myocardial infarction correlates with infarct size and ejection fraction. Clin Chem. 2002; 48(9):1432-1436.
7. Giannitsis E, Müller-Bardorff M, Lehrke S, et al. Admission troponin T level predicts clinical outcomes, TIMI flow, and myocardial tissue perfusion after primary percutaneous intervention for acute ST-segment elevation myocardial infarction. Circulation. 2001;104(6):630-635.
8. Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25-e146.
9. Lloyd-Jones D, Adams RJ, Brown TM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010; 121(7):e46-e215.
10. Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128.
11. Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non–ST-Elevation Myocardial Infarction: a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123(18):e426-e579.
12. Ohman EM, Armstrong PW, Christenson RH, et al; GUSTO IIA Investigators. Cardiac troponin T levels for risk stratification in acute myocardial ischemia.
N Engl J Med. 1996;335(18):1333-1341.
13. Antman EM, Tanasijevic MJ, Thompson B, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996;335(18):1342-1349.
14. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined: a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. J Am Coll Cardiol. 2000;36(3):959-969.
15. Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol. 2006;48(1):1-11.
16. Galvani M, Ottani F, Ferrini D, et al. Prognostic influence of elevated values of cardiac troponin I in patients with unstable angina. Circulation. 1997;95 (8):2053-2059.
17. Freda BJ, Tang WH, Van Lente F, et al. Cardiac troponins in renal insufficiency: review and clinical implications. J Am Coll Cardiol. 2002;40(12):2065-2071.
18. deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304(22):2494-2502.
19. Sinha MK, Roy D, Gaze DC, et al. Role of “ischemia modified albumin,” a new biochemical marker of myocardial ischaemia, in the early diagnosis of acute coronary syndromes. Emerg Med J. 2004; 21(1):29-34.
20. McCann CJ, Glover BM, Menown IB, et al. Novel biomarkers in early diagnosis of acute myocardial infarction compared with cardiac troponin T. Eur Heart J. 2008;29(23):2843-2850.
21. Morrow DA, Cannon CP, Jesse RL, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13): e356-e375.
22. Maisel AS, Krishnaswamy P, Nowak RM, et al; Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161-167.
23. McCullough PA, Nowak RM, McCord J, et al: B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002;106(4):416-422.
24. Januzzi JL Jr, Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95(8):948-954.
25. Mueller C, Laule-Kilian K, Scholer A, et al. Use of B-type natriuretic peptide for the management of women with dyspnea. Am J Cardiol. 2004; 94(12):1510-1514.
26. Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27(3):330-337.
27. Maisel AS. The diagnosis of acute congestive heart failure: role of BNP measurements. Heart Fail Rev. 2003;8(4):327-334.
28. Morrow DA, de Lemos JA, Sabatine MS, et al. Evaluation of B-type natriuretic peptide for risk assessment in unstable angina/non–ST-elevation myocardial infarction: B-type natriuretic peptide and prognosis in TACTICS-TIMI 18. J Am Coll Cardiol. 2003;41(8):1264-1272.
29. Libby P, Ridker PM. Inflammation and atherothrombosis: from population biology and bench research to clinical practice. J Am Coll Cardiol. 2006; 48(9 suppl A):A33-A46.
30. Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363-369.
31. Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557-1565.
32. Boekholdt SM, Hack CE, Sandhu MS, et al. C-reactive protein levels and coronary artery disease incidence and mortality in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study 1993-2003. Atherosclerosis. 2006; 187(2):415-422.
33. Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350(14):1387-1397.
34. Ridker PM, Cook N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham Risk Scores. Circulation. 2004;109(16):1955-1959.
35. Ridker PM, Cannon CP, Morrow D, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombosis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20-28.
36. Fischer JH, Jeschkeit-Schubbert S, Kuhn-Régnier F, Switkowski R. The origin of CK-MB serum levels and CK-MB/total CK ratios: measurements of CK isoenzyme activities in various tissues. Internet J Thorac Cardiovasc Surg. 2005;7(1).
37. Adams JE 3rd, Abendschein DR, Jaffe AS. Biochemical markers of myocardial injury: is MB creatine kinase the choice for the 1990s? Circulation. 1993;88(2):750-763.
38. Newby LK, Roe MT, Chen AY, et al; CRUSADE Investigators. Frequency and clinical implications of discordant creatine kinase-MB and troponin measurements in acute coronary syndromes. J Am Coll Cardiol. 2006;47(2):312-318.
39. Lim CC, van Gaal WJ, Testa L, et al. With the “universal definition,” measurement of creatine kinase–myocardial band rather than troponin allows more accurate diagnosis of periprocedural necrosis and infarction after coronary intervention. J Am Coll Cardiol. 2011;57(6):653-661.
40. Kilcullen N, Viswanathan K, Das R, et al; EMMACE-2 Investigators. Heart-type fatty acid–binding protein predicts long-term mortality after acute coronary syndrome and identifies high-risk patients across the range of troponin values. J Am Coll Cardiol. 2007;50(21):2061-2067.
41. Viswanathan K, Kilcullen N, Morrell C, et al. Heart-type fatty acid–binding protein predicts long-term mortality and re-infarction in consecutive patients with suspected acute coronary syndrome who are troponin-negative. J Am Coll Cardiol. 2010; 55(23): 2590-2598.
42. Abadie JM, Blassingame CL, Bankson DD. Albumin cobalt binding assay to rule out acute coronary syndrome. Ann Clin Lab Sci. 2005;35(1):66-72.
43. Anwaruddin S, Januzzi JL Jr, Baggish AL, et al. Ischemia-modified albumin improves the usefulness of standard cardiac biomarkers for the diagnosis of myocardial ischemia in the emergency department setting. Am J Clin Pathol. 2005;123(1):140-145.
44. Piwowar A, Knapik-Kordecka M, Warwas M. Ischemia-modified albumin level in type 2 diabetes mellitus: preliminary report. Dis Markers. 2008; 24(6):311-317.
45. Prefumo F, Gaze DC, Papageorghiou AT, et al. First trimester maternal serum ischaemia-modified albumin: a marker of hypoxia-ischaemia-driven early trophoblast development. Hum Reprod. 2007; 22(7):2029-2032.
46. Gunduz A, Turedi S, Mentese A, et al. Ischemia-modified albumin levels in cerebrovascular accidents. Am J Emerg Med. 2008;26(8):874-878.
47. Turedi S, Cinar O, Kaldirim U, et al. Ischemia-modified albumin levels in carbon monoxide poisoning. Am J Emerg Med. 2011;29(6):675-681.
48. Sbarouni E, Georgiadou P, Voudris V. Ischemia modified albumin changes: review and clinical implications. Clin Chem Lab Med. 2011;49(2):177-184.
49. Gaze DC. Ischemia modified albumin: a novel biomarker for the detection of cardiac ischemia. Drug Metab Pharmacokinet. 2009;24(4):333-341.
50. Kaul S, Zadeh AA, Shah PK. Homocysteine hypothesis for atherothrombotic cardiovascular disease: not validated. J Am Coll Cardiol. 2006; 48(5):914-923.
51. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291(5):565-575.
52. Lange H, Suryapranata H, De Luca G, et al. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med. 2004;350(26):2673-2681.
53. Bønaa KH, Njølstad I, Ueland PM, et al; NORVIT Trial Investigators. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578-1588.
54. Zhang R, Brennan ML, Fu X, et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286(17):2136-2142.
55. Ferrante G, Nakano M, Prati F, et al. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes: a clinicopathological study. Circulation. 2010;122(24):2505-2513.
56. Loria V, Dato I, Graziani F, Biasucci LM. Myeloperoxidase: a new biomarker of inflammation in ischemic heart disease and acute coronary syndromes. Mediators Inflamm. 2008;2008:135625.
57. Apple FS, Wu AH, Mair J, et al; Committee on Standardization of Markers of Cardiac Damage of the IFCC. Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin Chem. 2005;51(5):810-824.
58. Oemrawsingh RM, Lenderink T, Akkerhuis KM, et al. Multimarker risk model containing troponin-T, interleukin 10, myeloperoxidase and placental growth factor predicts long-term cardiovascular risk after non–ST-segment elevation acute coronary syndrome. Heart. 2011;97(13):1061-1066.
59. Davies MJ, Thomas AC. Plaque fissuring: the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J. 1985;53(4):363-373.
60. Heeschen C, Dimmeler S, Hamm CW, et al; CAPTURE Study. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med. 2003;348(12): 1104-1111.
61. Schönbeck U, Varo N, Libby P, et al. Soluble CD40L and cardiovascular risk in women. Circulation. 2001;104(19):2266-2268.
62. Lin TM, Galbert SP, Kiefer D, et al. Characterization of four human pregnancy–associated plasma proteins. Am J Obstet Gynecol. 1974; 118(2):223-236.
63. Bayes-Genis A, Conover CA, Overgaard MT, et al. Pregnancy-associated plasma protein A as a marker of acute coronary syndromes. N Engl J Med. 2001;345(14):1022-1029.
64. Lund J, Qin QP, Ilva T, et al. Circulating pregnancy-associated plasma protein A predicts outcome in patients with acute coronary syndrome but no troponin I elevation. Circulation. 2003; 108(16):1924-1926.
Diabetes Influences ACS Treatment
Diltiazem Exacerbated Myasthenia Gravis
Diabetes Self-Care Education: Cooking Classes as a Basis for Teaching Healthful Eating
October 2011
A Toxic Fish Dinner
Emergent Management of Lightning Injuries
Current Management Options for Osteonecrosis of the Femoral Head: Part II, Operative Management
Occipitocervical Junction: Imaging, Pathology, Instrumentation
UPDATE ON PELVIC FLOOR DYSFUNCTION
Vulvar Pain Syndromes 3-Part Series
- Making the correct diagnosis
(September 2011) - A bounty of treatments-but not all of them are proven
(October 2011) - Provoked vestibulodynia
(Coming in November 2011)
Chronic pelvic pain: 11 critical questions about causes and care
Fred M. Howard, MD (August 2009)
Vague symptoms. Unexpected flares. Inconsistent manifestations. These characteristics can make diagnosis and treatment of chronic pelvic pain frustrating for both patient and physician. Most patients undergo myriad tests and studies to uncover the source of their pain—but a targeted pelvic exam may be all that is necessary to identify a prevalent but commonly overlooked cause of pelvic pain. Levator myalgia, myofascial pelvic pain syndrome, and pelvic floor spasm are all terms that describe a condition that may affect as many as 78% of women who are given a diagnosis of chronic pelvic pain.1 This syndrome may be represented by an array of symptoms, including pelvic pressure, dyspareunia, rectal discomfort, and irritative urinary symptoms such as spasms, frequency, and urgency. It is characterized by the presence of tight, band-like pelvic muscles that reproduce the patient’s pain when palpated.2
Diagnosis of this syndrome often surprises the patient. Although the concept of a muscle spasm is not foreign, the location is unexpected. Patients and physicians alike may forget that there is a large complex of muscles that completely lines the pelvic girdle. To complicate matters, the patient often associates the onset of her symptoms with an acute event such as a “bad” urinary tract infection or pelvic or vaginal surgery, which may divert attention from the musculature. Although a muscle spasm may be the cause of the patient’s pain, it’s important to realize that an underlying process may have triggered the original spasm. To provide effective treatment of pain, therefore, you must identify the fundamental cause, assuming that it is reversible, rather than focus exclusively on symptoms.
Although there are many therapeutic options for levator myalgia, an appraisal of the extensive literature on these medications is beyond the scope of this article. Rather, we will review alternative treatment modalities and summarize the results of five trials that explored physical therapy, trigger-point or chemodenervation injection, and neuromodulation (TABLE).
Weighing the nonpharmaceutical options for treatment
of myofascial pelvic pain
| Treatment | Pros | Cons |
|---|---|---|
| Physical therapy | Minimally invasive Moderate long-term success | Requires highly specialized therapist |
| Trigger-point injection | Minimally invasive Performed in clinic Immediate short-term success | Optimal injectable agent is unknown Botulinum toxin A lacks FDA approval for this indication Limited information on adverse events and long-term efficacy |
| Percutaneous tibial nerve stimulation | Minimally invasive Performed in clinic | Requires numerous office visits for treatment Lacks FDA approval for this indication Limited information on long-term efficacy |
| Sacral neuromodulation | Moderately invasive Permanent implant | Requires implantation in operating room Lacks FDA approval for this indication Limited information on long-term efficacy |
Pelvic myofascial therapy offers relief—but qualified therapists may be scarce
FitzGerald MP, Anderson RU, Potts J, et al; Urological Pelvic Pain Collaborative Research Network. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urology. 2009;182(2):570–580.
Physical therapy of the pelvic floor—otherwise known as pelvic myofascial therapy—requires a therapist who is highly trained and specialized in this technique. It is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers (FIGURE 1).
This pilot study by the Urological Pelvic Pain Collaborative Research Network evaluated the ability of patients to adhere to pelvic myofascial therapy, the response of their pain to therapy, and adverse events associated with manual therapy. It found that patients were willing to undergo the therapy, despite the invasive nature of the maneuvers, because it was significantly effective.
Details of the study
Patients (both men and women) were randomized to myofascial physical therapy or global therapeutic massage. Myofascial therapy consisted of internal or vaginal manipulation of the trigger-point muscle bundles and tissues of the pelvic floor. It also focused on muscles of the hip girdle and abdomen. The comparison group underwent traditional Western full-body massage. In both groups, treatment lasted 1 hour every week, and participants agreed to 10 full treatments.
Patients were eligible for the study if they experienced pelvic pain, urinary frequency, or bladder discomfort in the previous 6 months. In addition, an examiner must have been able to elicit tenderness upon palpation of the pelvic floor during examination. Patients were excluded if they showed signs of urinary tract infection or dysmenorrhea.
A total of 47 patients were randomized—24 to global massage and 23 to myofascial physical therapy. Overall, the myofascial group experienced a significantly higher rate of improvement in the global response at 12 weeks than did patients in the global-massage group (57% vs 21%; P=.03). Patients were willing to engage in myofascial pelvic therapy, and adverse events were minor.
FIGURE 1 Transvaginal myofascial therapy
Physical therapy of the pelvic floor is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers.
Need for specialized training may limit number of therapists
The randomized controlled study design renders these findings fairly reliable. Therapists were unmasked and aware of the treatment arms but were trained to make the different therapy sessions appear as similar as possible.
Although investigators were enthusiastic about their initial findings, additional studies are needed to validate the results. Moreover, these findings may be difficult to generalize because women who volunteer to participate in such a study may differ from the general population.
Nevertheless, patients who suffer from chronic pelvic pain may take heart that there is a nonpharmaceutical alternative to manage their symptoms, although availability is likely limited in many areas. Given the nature of the physical therapy required for this particular location of myofascial pain, specialized training is necessary for therapists. Despite motivated patients and well-informed providers, it may be difficult to find specialized therapists within local vicinities. Referrals to centers where this type of therapy is offered may be necessary.
Pelvic myofascial therapy is an effective and acceptable intervention for the treatment of levator myalgia.
The ideal agent for trigger-point injections remains a mystery
Langford CF, Udvari Nagy S, Ghoniem G M. Levator ani trigger point injections: An underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26(1):59–62.
Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108(4):915–923.
Trigger points are discrete, tender areas within a ridge of contracted muscle. These points may cause focal pain or referred pain upon irritation of the muscle.2 Trigger-point injection therapy aims to anesthetize or relax these points by infiltrating the muscle with medications.
These two studies evaluated the value of trigger-point injections in the treatment of pelvic myofascial pain; they found that the injections provide relief, although the mechanism of action and the ideal agent remain to be determined.
Langford et al: Details of the study
In this prospective study, 18 women who had pelvic pain of at least 6 months’ duration and confirmed trigger points on examination underwent transvaginal injection of a solution of bupivacaine, lidocaine, and triamcinolone. They were assessed by questionnaire at baseline and 3 months after injection. Assessment included a visual analog scale for pain severity. Investigators defined success as a decrease in pain of 50% or more and global-satisfaction and global-cure visual scores of 60% or higher.
Thirteen of the 18 women (72.2%) improved after their first injection, with six women reporting a complete absence of pain. Overall, women reported significant decreases in pain and increases in the rates of satisfaction and cure, meeting the definition of success at 3 months after the injection.
Among the theories proposed to explain the mechanism of action of trigger-point injections are:
- disruption of reflex arcs within skeletal muscle
- release of endorphins
- mechanical changes in abnormally contracted muscle fibers.
This last theory highlights one of the limitations of this study—lack of a placebo arm. Could it be possible that the injection of any fluid produces the same effect?
This study was not designed to investigate the causal relationship between the injection of a particular solution and pain relief, but it does highlight the need for studies to clarify the mechanism of action, including use of a placebo. It also prompts questions about the duration of effect after a single injection.
Goal of chemodenervation is blocking of muscle activity
Botulinum toxin type A (Botox) blocks the release of acetylcholine from presynaptic neurons. The release of acetylcholine stimulates muscle contractions; therefore, blockage of its release reduces muscle activity. This type of chemodenervation has found widespread use, and botulinum toxin A now has approval from the Food and Drug Administration (FDA) for treatment of chronic migraine, limb spasticity, cervical dystonia, strabismus, hyperhidrosis, and facial cosmesis.3 Although it is not approved for pelvic floor levator spasm, its success in treating other myotonic disorders suggests that its application may be relevant.
Abbott et al: Details of the study
Abbott and colleagues performed a double-blind, randomized, controlled trial to compare injection of botulinum toxin A with injection of saline. They measured changes in the pain scale, quality of life, and vaginal pressure.
Women were eligible for the study if they had subjectively reported pelvic pain of more than 2 years’ duration and objective evidence of trigger points (on examination) and elevated vaginal resting pressure (by vaginal manometry). Neither the clinical research staff nor the patient knew the contents of the injections, but all women received a total of four—two at sites in the puborectalis muscle and two in the pubococcygeus muscle.
After periodic assessment by questionnaire and examination through 6 months after injection, no differences were found in the pain score or resting vaginal pressure between the group of women who received botulinum toxin A and the group who received placebo. However, each group experienced a significant reduction in pain and vaginal pressure, compared with baseline. And both groups reported improved quality of life, compared with baseline. Neither group reported voiding dysfunction.
These two studies support the use of trigger-point injection into pelvic floor muscles to reduce pelvic myofascial pain. The findings of Abbott and colleagues, in particular, suggest that the substance that is injected may not be as important as the actual needling of the muscle. Larger studies and comparisons between placebo, botulinum toxin A, and anesthetic solutions are needed to elucidate the therapeutic benefit of these particular medications.
Neuromodulation shows promise as treatment for pelvic myofascial pain
van Balken MR, Vandoninck V, Messelink, BJ, et al. Percutaneous tibial nerve stimulation as neuromodulative treatment of chronic pelvic pain. Eur Urol. 2003;43(2):158–163.
Zabihi N, Mourtzinos A, Maher MG, Raz S, Rodriguez LV. Short-term results of bilateral S2-S4 sacral neuromodulation for the treatment of refractory interstitial cystitis, painful bladder syndrome, and chronic pelvic pain. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(4):553–557.
Neuromodulation is the science of using electrical impulses to alter neuronal activities. The exact mechanisms of action are unclear, but the technology has been utilized to control symptoms of overactive bladder and urinary retention caused by poor relaxation of the urethral and pelvic floor muscles. While studying the effects of sacral nerve root neuromodulation on the bladder, investigators noted improvements in other symptoms, such as pelvic pain.
Neuromodulation of the sacral nerve roots may be achieved by direct conduction of electrical impulses from a lead implanted in the sacrum (sacral neuromodulation) or by the retrograde conduction of these impulses through the posterior tibial nerve (percutaneous tibial nerve stimulation, or PTNS) (FIGURE 2). The tibial nerve arises from sacral nerves L5 to S3 and is one of the larger branches of the sciatic nerve.
FIGURE 2 InterStim therapy
Stimulation of the sacral nerve has been used successfully to manage overactive bladder and urinary retention and may prove useful in the treatment of pelvic myofascial pain.
Van Balken et al: Details of the study
In this prospective observational study, 33 patients (both male and female) who had chronic pelvic pain by history and examination were treated with weekly, 30-minute outpatient sessions of PTNS for 12 weeks. Participants were asked to provide baseline pain scores and keep a diary of their pain. Quality-of-life questionnaires were also administered at baseline and at 12 weeks.
Investigators considered both subjective and objective success in their outcomes. If a patient elected to continue therapy, he or she was classified as a subjective success. Objective success required a decrease of at least 50% in the pain score. At the end of 12 weeks, although 33 patients (42%) wanted to continue therapy, only seven (21%) met the definition for objective success. Of those seven, six elected to continue therapy.
This study sheds light on a treatment modality that has not been studied adequately for the indication of pelvic pain but that may be promising in patients who have levator myalgia. Limitations of this study include the lack of a placebo arm, short-term outcome, and lack of localization of pain. Furthermore, although PTNS has FDA approval for treatment of urinary urgency, frequency, and urge incontinence, it is not approved for the treatment of pelvic pain. These preliminary findings demonstrate potential but, as with any new indication, long-term comparative studies are needed.
Zabihi et al: Details of the study
Patients in this retrospective study had a diagnosis of interstitial cystitis or chronic pelvic pain. Pelvic myofascial pain and trigger points were not required for eligibility. Thirty patients (21 women and nine men) had temporary placement of a lead containing four small electrodes along the S2 to S4 sacral nerve roots on both sides of the sacrum. They were then followed for a trial period of 2 to 4 weeks. To qualify for the final stage of the study, in which the leads were connected internally to a generator implanted in the buttocks, patients had to report improvement of at least 50% in their symptoms. If their improvement did not meet that threshold, the leads were removed.
Twenty-three patients (77%) met the criteria for permanent implantation. Of these patients, 42% reported improvement of more than 50% at 6 postoperative months. Quality-of-life scores also improved significantly.
Sacral neuromodulation is not FDA-approved for the treatment of chronic pelvic pain; further studies are needed before it can be recommended for this indication.
Neither of these studies required objective evidence of myofascial pain for inclusion. Therefore, although the benefits they demonstrated may be theorized to extend to the relief of myofascial pain, this fact cannot be corroborated.
We want to hear from you! Tell us what you think.
1. Bassaly R, Tidwell N, Bertolino S, Hoyte L, Downes K, Hart S. Myofascial pain and pelvic floor dysfunction in patients with interstitial cystitis. Int Urogynecol J. 2011;22(4):413-418.
2. Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician. 2002;65(4):653-660.
3. Allergan, Inc. Medication Guide: BOTOX. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM176360.pdf. Published October 2010. Accessed August 30, 2011.
Vulvar Pain Syndromes 3-Part Series
- Making the correct diagnosis
(September 2011) - A bounty of treatments-but not all of them are proven
(October 2011) - Provoked vestibulodynia
(Coming in November 2011)
Chronic pelvic pain: 11 critical questions about causes and care
Fred M. Howard, MD (August 2009)
Vague symptoms. Unexpected flares. Inconsistent manifestations. These characteristics can make diagnosis and treatment of chronic pelvic pain frustrating for both patient and physician. Most patients undergo myriad tests and studies to uncover the source of their pain—but a targeted pelvic exam may be all that is necessary to identify a prevalent but commonly overlooked cause of pelvic pain. Levator myalgia, myofascial pelvic pain syndrome, and pelvic floor spasm are all terms that describe a condition that may affect as many as 78% of women who are given a diagnosis of chronic pelvic pain.1 This syndrome may be represented by an array of symptoms, including pelvic pressure, dyspareunia, rectal discomfort, and irritative urinary symptoms such as spasms, frequency, and urgency. It is characterized by the presence of tight, band-like pelvic muscles that reproduce the patient’s pain when palpated.2
Diagnosis of this syndrome often surprises the patient. Although the concept of a muscle spasm is not foreign, the location is unexpected. Patients and physicians alike may forget that there is a large complex of muscles that completely lines the pelvic girdle. To complicate matters, the patient often associates the onset of her symptoms with an acute event such as a “bad” urinary tract infection or pelvic or vaginal surgery, which may divert attention from the musculature. Although a muscle spasm may be the cause of the patient’s pain, it’s important to realize that an underlying process may have triggered the original spasm. To provide effective treatment of pain, therefore, you must identify the fundamental cause, assuming that it is reversible, rather than focus exclusively on symptoms.
Although there are many therapeutic options for levator myalgia, an appraisal of the extensive literature on these medications is beyond the scope of this article. Rather, we will review alternative treatment modalities and summarize the results of five trials that explored physical therapy, trigger-point or chemodenervation injection, and neuromodulation (TABLE).
Weighing the nonpharmaceutical options for treatment
of myofascial pelvic pain
| Treatment | Pros | Cons |
|---|---|---|
| Physical therapy | Minimally invasive Moderate long-term success | Requires highly specialized therapist |
| Trigger-point injection | Minimally invasive Performed in clinic Immediate short-term success | Optimal injectable agent is unknown Botulinum toxin A lacks FDA approval for this indication Limited information on adverse events and long-term efficacy |
| Percutaneous tibial nerve stimulation | Minimally invasive Performed in clinic | Requires numerous office visits for treatment Lacks FDA approval for this indication Limited information on long-term efficacy |
| Sacral neuromodulation | Moderately invasive Permanent implant | Requires implantation in operating room Lacks FDA approval for this indication Limited information on long-term efficacy |
Pelvic myofascial therapy offers relief—but qualified therapists may be scarce
FitzGerald MP, Anderson RU, Potts J, et al; Urological Pelvic Pain Collaborative Research Network. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urology. 2009;182(2):570–580.
Physical therapy of the pelvic floor—otherwise known as pelvic myofascial therapy—requires a therapist who is highly trained and specialized in this technique. It is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers (FIGURE 1).
This pilot study by the Urological Pelvic Pain Collaborative Research Network evaluated the ability of patients to adhere to pelvic myofascial therapy, the response of their pain to therapy, and adverse events associated with manual therapy. It found that patients were willing to undergo the therapy, despite the invasive nature of the maneuvers, because it was significantly effective.
Details of the study
Patients (both men and women) were randomized to myofascial physical therapy or global therapeutic massage. Myofascial therapy consisted of internal or vaginal manipulation of the trigger-point muscle bundles and tissues of the pelvic floor. It also focused on muscles of the hip girdle and abdomen. The comparison group underwent traditional Western full-body massage. In both groups, treatment lasted 1 hour every week, and participants agreed to 10 full treatments.
Patients were eligible for the study if they experienced pelvic pain, urinary frequency, or bladder discomfort in the previous 6 months. In addition, an examiner must have been able to elicit tenderness upon palpation of the pelvic floor during examination. Patients were excluded if they showed signs of urinary tract infection or dysmenorrhea.
A total of 47 patients were randomized—24 to global massage and 23 to myofascial physical therapy. Overall, the myofascial group experienced a significantly higher rate of improvement in the global response at 12 weeks than did patients in the global-massage group (57% vs 21%; P=.03). Patients were willing to engage in myofascial pelvic therapy, and adverse events were minor.

FIGURE 1 Transvaginal myofascial therapy
Physical therapy of the pelvic floor is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers.
Need for specialized training may limit number of therapists
The randomized controlled study design renders these findings fairly reliable. Therapists were unmasked and aware of the treatment arms but were trained to make the different therapy sessions appear as similar as possible.
Although investigators were enthusiastic about their initial findings, additional studies are needed to validate the results. Moreover, these findings may be difficult to generalize because women who volunteer to participate in such a study may differ from the general population.
Nevertheless, patients who suffer from chronic pelvic pain may take heart that there is a nonpharmaceutical alternative to manage their symptoms, although availability is likely limited in many areas. Given the nature of the physical therapy required for this particular location of myofascial pain, specialized training is necessary for therapists. Despite motivated patients and well-informed providers, it may be difficult to find specialized therapists within local vicinities. Referrals to centers where this type of therapy is offered may be necessary.
Pelvic myofascial therapy is an effective and acceptable intervention for the treatment of levator myalgia.
The ideal agent for trigger-point injections remains a mystery
Langford CF, Udvari Nagy S, Ghoniem G M. Levator ani trigger point injections: An underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26(1):59–62.
Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108(4):915–923.
Trigger points are discrete, tender areas within a ridge of contracted muscle. These points may cause focal pain or referred pain upon irritation of the muscle.2 Trigger-point injection therapy aims to anesthetize or relax these points by infiltrating the muscle with medications.
These two studies evaluated the value of trigger-point injections in the treatment of pelvic myofascial pain; they found that the injections provide relief, although the mechanism of action and the ideal agent remain to be determined.
Langford et al: Details of the study
In this prospective study, 18 women who had pelvic pain of at least 6 months’ duration and confirmed trigger points on examination underwent transvaginal injection of a solution of bupivacaine, lidocaine, and triamcinolone. They were assessed by questionnaire at baseline and 3 months after injection. Assessment included a visual analog scale for pain severity. Investigators defined success as a decrease in pain of 50% or more and global-satisfaction and global-cure visual scores of 60% or higher.
Thirteen of the 18 women (72.2%) improved after their first injection, with six women reporting a complete absence of pain. Overall, women reported significant decreases in pain and increases in the rates of satisfaction and cure, meeting the definition of success at 3 months after the injection.
Among the theories proposed to explain the mechanism of action of trigger-point injections are:
- disruption of reflex arcs within skeletal muscle
- release of endorphins
- mechanical changes in abnormally contracted muscle fibers.
This last theory highlights one of the limitations of this study—lack of a placebo arm. Could it be possible that the injection of any fluid produces the same effect?
This study was not designed to investigate the causal relationship between the injection of a particular solution and pain relief, but it does highlight the need for studies to clarify the mechanism of action, including use of a placebo. It also prompts questions about the duration of effect after a single injection.
Goal of chemodenervation is blocking of muscle activity
Botulinum toxin type A (Botox) blocks the release of acetylcholine from presynaptic neurons. The release of acetylcholine stimulates muscle contractions; therefore, blockage of its release reduces muscle activity. This type of chemodenervation has found widespread use, and botulinum toxin A now has approval from the Food and Drug Administration (FDA) for treatment of chronic migraine, limb spasticity, cervical dystonia, strabismus, hyperhidrosis, and facial cosmesis.3 Although it is not approved for pelvic floor levator spasm, its success in treating other myotonic disorders suggests that its application may be relevant.
Abbott et al: Details of the study
Abbott and colleagues performed a double-blind, randomized, controlled trial to compare injection of botulinum toxin A with injection of saline. They measured changes in the pain scale, quality of life, and vaginal pressure.
Women were eligible for the study if they had subjectively reported pelvic pain of more than 2 years’ duration and objective evidence of trigger points (on examination) and elevated vaginal resting pressure (by vaginal manometry). Neither the clinical research staff nor the patient knew the contents of the injections, but all women received a total of four—two at sites in the puborectalis muscle and two in the pubococcygeus muscle.
After periodic assessment by questionnaire and examination through 6 months after injection, no differences were found in the pain score or resting vaginal pressure between the group of women who received botulinum toxin A and the group who received placebo. However, each group experienced a significant reduction in pain and vaginal pressure, compared with baseline. And both groups reported improved quality of life, compared with baseline. Neither group reported voiding dysfunction.
These two studies support the use of trigger-point injection into pelvic floor muscles to reduce pelvic myofascial pain. The findings of Abbott and colleagues, in particular, suggest that the substance that is injected may not be as important as the actual needling of the muscle. Larger studies and comparisons between placebo, botulinum toxin A, and anesthetic solutions are needed to elucidate the therapeutic benefit of these particular medications.
Neuromodulation shows promise as treatment for pelvic myofascial pain
van Balken MR, Vandoninck V, Messelink, BJ, et al. Percutaneous tibial nerve stimulation as neuromodulative treatment of chronic pelvic pain. Eur Urol. 2003;43(2):158–163.
Zabihi N, Mourtzinos A, Maher MG, Raz S, Rodriguez LV. Short-term results of bilateral S2-S4 sacral neuromodulation for the treatment of refractory interstitial cystitis, painful bladder syndrome, and chronic pelvic pain. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(4):553–557.
Neuromodulation is the science of using electrical impulses to alter neuronal activities. The exact mechanisms of action are unclear, but the technology has been utilized to control symptoms of overactive bladder and urinary retention caused by poor relaxation of the urethral and pelvic floor muscles. While studying the effects of sacral nerve root neuromodulation on the bladder, investigators noted improvements in other symptoms, such as pelvic pain.
Neuromodulation of the sacral nerve roots may be achieved by direct conduction of electrical impulses from a lead implanted in the sacrum (sacral neuromodulation) or by the retrograde conduction of these impulses through the posterior tibial nerve (percutaneous tibial nerve stimulation, or PTNS) (FIGURE 2). The tibial nerve arises from sacral nerves L5 to S3 and is one of the larger branches of the sciatic nerve.

FIGURE 2 InterStim therapy
Stimulation of the sacral nerve has been used successfully to manage overactive bladder and urinary retention and may prove useful in the treatment of pelvic myofascial pain.
Van Balken et al: Details of the study
In this prospective observational study, 33 patients (both male and female) who had chronic pelvic pain by history and examination were treated with weekly, 30-minute outpatient sessions of PTNS for 12 weeks. Participants were asked to provide baseline pain scores and keep a diary of their pain. Quality-of-life questionnaires were also administered at baseline and at 12 weeks.
Investigators considered both subjective and objective success in their outcomes. If a patient elected to continue therapy, he or she was classified as a subjective success. Objective success required a decrease of at least 50% in the pain score. At the end of 12 weeks, although 33 patients (42%) wanted to continue therapy, only seven (21%) met the definition for objective success. Of those seven, six elected to continue therapy.
This study sheds light on a treatment modality that has not been studied adequately for the indication of pelvic pain but that may be promising in patients who have levator myalgia. Limitations of this study include the lack of a placebo arm, short-term outcome, and lack of localization of pain. Furthermore, although PTNS has FDA approval for treatment of urinary urgency, frequency, and urge incontinence, it is not approved for the treatment of pelvic pain. These preliminary findings demonstrate potential but, as with any new indication, long-term comparative studies are needed.
Zabihi et al: Details of the study
Patients in this retrospective study had a diagnosis of interstitial cystitis or chronic pelvic pain. Pelvic myofascial pain and trigger points were not required for eligibility. Thirty patients (21 women and nine men) had temporary placement of a lead containing four small electrodes along the S2 to S4 sacral nerve roots on both sides of the sacrum. They were then followed for a trial period of 2 to 4 weeks. To qualify for the final stage of the study, in which the leads were connected internally to a generator implanted in the buttocks, patients had to report improvement of at least 50% in their symptoms. If their improvement did not meet that threshold, the leads were removed.
Twenty-three patients (77%) met the criteria for permanent implantation. Of these patients, 42% reported improvement of more than 50% at 6 postoperative months. Quality-of-life scores also improved significantly.
Sacral neuromodulation is not FDA-approved for the treatment of chronic pelvic pain; further studies are needed before it can be recommended for this indication.
Neither of these studies required objective evidence of myofascial pain for inclusion. Therefore, although the benefits they demonstrated may be theorized to extend to the relief of myofascial pain, this fact cannot be corroborated.
We want to hear from you! Tell us what you think.
Vulvar Pain Syndromes 3-Part Series
- Making the correct diagnosis
(September 2011) - A bounty of treatments-but not all of them are proven
(October 2011) - Provoked vestibulodynia
(Coming in November 2011)
Chronic pelvic pain: 11 critical questions about causes and care
Fred M. Howard, MD (August 2009)
Vague symptoms. Unexpected flares. Inconsistent manifestations. These characteristics can make diagnosis and treatment of chronic pelvic pain frustrating for both patient and physician. Most patients undergo myriad tests and studies to uncover the source of their pain—but a targeted pelvic exam may be all that is necessary to identify a prevalent but commonly overlooked cause of pelvic pain. Levator myalgia, myofascial pelvic pain syndrome, and pelvic floor spasm are all terms that describe a condition that may affect as many as 78% of women who are given a diagnosis of chronic pelvic pain.1 This syndrome may be represented by an array of symptoms, including pelvic pressure, dyspareunia, rectal discomfort, and irritative urinary symptoms such as spasms, frequency, and urgency. It is characterized by the presence of tight, band-like pelvic muscles that reproduce the patient’s pain when palpated.2
Diagnosis of this syndrome often surprises the patient. Although the concept of a muscle spasm is not foreign, the location is unexpected. Patients and physicians alike may forget that there is a large complex of muscles that completely lines the pelvic girdle. To complicate matters, the patient often associates the onset of her symptoms with an acute event such as a “bad” urinary tract infection or pelvic or vaginal surgery, which may divert attention from the musculature. Although a muscle spasm may be the cause of the patient’s pain, it’s important to realize that an underlying process may have triggered the original spasm. To provide effective treatment of pain, therefore, you must identify the fundamental cause, assuming that it is reversible, rather than focus exclusively on symptoms.
Although there are many therapeutic options for levator myalgia, an appraisal of the extensive literature on these medications is beyond the scope of this article. Rather, we will review alternative treatment modalities and summarize the results of five trials that explored physical therapy, trigger-point or chemodenervation injection, and neuromodulation (TABLE).
Weighing the nonpharmaceutical options for treatment
of myofascial pelvic pain
| Treatment | Pros | Cons |
|---|---|---|
| Physical therapy | Minimally invasive Moderate long-term success | Requires highly specialized therapist |
| Trigger-point injection | Minimally invasive Performed in clinic Immediate short-term success | Optimal injectable agent is unknown Botulinum toxin A lacks FDA approval for this indication Limited information on adverse events and long-term efficacy |
| Percutaneous tibial nerve stimulation | Minimally invasive Performed in clinic | Requires numerous office visits for treatment Lacks FDA approval for this indication Limited information on long-term efficacy |
| Sacral neuromodulation | Moderately invasive Permanent implant | Requires implantation in operating room Lacks FDA approval for this indication Limited information on long-term efficacy |
Pelvic myofascial therapy offers relief—but qualified therapists may be scarce
FitzGerald MP, Anderson RU, Potts J, et al; Urological Pelvic Pain Collaborative Research Network. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urology. 2009;182(2):570–580.
Physical therapy of the pelvic floor—otherwise known as pelvic myofascial therapy—requires a therapist who is highly trained and specialized in this technique. It is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers (FIGURE 1).
This pilot study by the Urological Pelvic Pain Collaborative Research Network evaluated the ability of patients to adhere to pelvic myofascial therapy, the response of their pain to therapy, and adverse events associated with manual therapy. It found that patients were willing to undergo the therapy, despite the invasive nature of the maneuvers, because it was significantly effective.
Details of the study
Patients (both men and women) were randomized to myofascial physical therapy or global therapeutic massage. Myofascial therapy consisted of internal or vaginal manipulation of the trigger-point muscle bundles and tissues of the pelvic floor. It also focused on muscles of the hip girdle and abdomen. The comparison group underwent traditional Western full-body massage. In both groups, treatment lasted 1 hour every week, and participants agreed to 10 full treatments.
Patients were eligible for the study if they experienced pelvic pain, urinary frequency, or bladder discomfort in the previous 6 months. In addition, an examiner must have been able to elicit tenderness upon palpation of the pelvic floor during examination. Patients were excluded if they showed signs of urinary tract infection or dysmenorrhea.
A total of 47 patients were randomized—24 to global massage and 23 to myofascial physical therapy. Overall, the myofascial group experienced a significantly higher rate of improvement in the global response at 12 weeks than did patients in the global-massage group (57% vs 21%; P=.03). Patients were willing to engage in myofascial pelvic therapy, and adverse events were minor.

FIGURE 1 Transvaginal myofascial therapy
Physical therapy of the pelvic floor is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers.
Need for specialized training may limit number of therapists
The randomized controlled study design renders these findings fairly reliable. Therapists were unmasked and aware of the treatment arms but were trained to make the different therapy sessions appear as similar as possible.
Although investigators were enthusiastic about their initial findings, additional studies are needed to validate the results. Moreover, these findings may be difficult to generalize because women who volunteer to participate in such a study may differ from the general population.
Nevertheless, patients who suffer from chronic pelvic pain may take heart that there is a nonpharmaceutical alternative to manage their symptoms, although availability is likely limited in many areas. Given the nature of the physical therapy required for this particular location of myofascial pain, specialized training is necessary for therapists. Despite motivated patients and well-informed providers, it may be difficult to find specialized therapists within local vicinities. Referrals to centers where this type of therapy is offered may be necessary.
Pelvic myofascial therapy is an effective and acceptable intervention for the treatment of levator myalgia.
The ideal agent for trigger-point injections remains a mystery
Langford CF, Udvari Nagy S, Ghoniem G M. Levator ani trigger point injections: An underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26(1):59–62.
Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108(4):915–923.
Trigger points are discrete, tender areas within a ridge of contracted muscle. These points may cause focal pain or referred pain upon irritation of the muscle.2 Trigger-point injection therapy aims to anesthetize or relax these points by infiltrating the muscle with medications.
These two studies evaluated the value of trigger-point injections in the treatment of pelvic myofascial pain; they found that the injections provide relief, although the mechanism of action and the ideal agent remain to be determined.
Langford et al: Details of the study
In this prospective study, 18 women who had pelvic pain of at least 6 months’ duration and confirmed trigger points on examination underwent transvaginal injection of a solution of bupivacaine, lidocaine, and triamcinolone. They were assessed by questionnaire at baseline and 3 months after injection. Assessment included a visual analog scale for pain severity. Investigators defined success as a decrease in pain of 50% or more and global-satisfaction and global-cure visual scores of 60% or higher.
Thirteen of the 18 women (72.2%) improved after their first injection, with six women reporting a complete absence of pain. Overall, women reported significant decreases in pain and increases in the rates of satisfaction and cure, meeting the definition of success at 3 months after the injection.
Among the theories proposed to explain the mechanism of action of trigger-point injections are:
- disruption of reflex arcs within skeletal muscle
- release of endorphins
- mechanical changes in abnormally contracted muscle fibers.
This last theory highlights one of the limitations of this study—lack of a placebo arm. Could it be possible that the injection of any fluid produces the same effect?
This study was not designed to investigate the causal relationship between the injection of a particular solution and pain relief, but it does highlight the need for studies to clarify the mechanism of action, including use of a placebo. It also prompts questions about the duration of effect after a single injection.
Goal of chemodenervation is blocking of muscle activity
Botulinum toxin type A (Botox) blocks the release of acetylcholine from presynaptic neurons. The release of acetylcholine stimulates muscle contractions; therefore, blockage of its release reduces muscle activity. This type of chemodenervation has found widespread use, and botulinum toxin A now has approval from the Food and Drug Administration (FDA) for treatment of chronic migraine, limb spasticity, cervical dystonia, strabismus, hyperhidrosis, and facial cosmesis.3 Although it is not approved for pelvic floor levator spasm, its success in treating other myotonic disorders suggests that its application may be relevant.
Abbott et al: Details of the study
Abbott and colleagues performed a double-blind, randomized, controlled trial to compare injection of botulinum toxin A with injection of saline. They measured changes in the pain scale, quality of life, and vaginal pressure.
Women were eligible for the study if they had subjectively reported pelvic pain of more than 2 years’ duration and objective evidence of trigger points (on examination) and elevated vaginal resting pressure (by vaginal manometry). Neither the clinical research staff nor the patient knew the contents of the injections, but all women received a total of four—two at sites in the puborectalis muscle and two in the pubococcygeus muscle.
After periodic assessment by questionnaire and examination through 6 months after injection, no differences were found in the pain score or resting vaginal pressure between the group of women who received botulinum toxin A and the group who received placebo. However, each group experienced a significant reduction in pain and vaginal pressure, compared with baseline. And both groups reported improved quality of life, compared with baseline. Neither group reported voiding dysfunction.
These two studies support the use of trigger-point injection into pelvic floor muscles to reduce pelvic myofascial pain. The findings of Abbott and colleagues, in particular, suggest that the substance that is injected may not be as important as the actual needling of the muscle. Larger studies and comparisons between placebo, botulinum toxin A, and anesthetic solutions are needed to elucidate the therapeutic benefit of these particular medications.
Neuromodulation shows promise as treatment for pelvic myofascial pain
van Balken MR, Vandoninck V, Messelink, BJ, et al. Percutaneous tibial nerve stimulation as neuromodulative treatment of chronic pelvic pain. Eur Urol. 2003;43(2):158–163.
Zabihi N, Mourtzinos A, Maher MG, Raz S, Rodriguez LV. Short-term results of bilateral S2-S4 sacral neuromodulation for the treatment of refractory interstitial cystitis, painful bladder syndrome, and chronic pelvic pain. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(4):553–557.
Neuromodulation is the science of using electrical impulses to alter neuronal activities. The exact mechanisms of action are unclear, but the technology has been utilized to control symptoms of overactive bladder and urinary retention caused by poor relaxation of the urethral and pelvic floor muscles. While studying the effects of sacral nerve root neuromodulation on the bladder, investigators noted improvements in other symptoms, such as pelvic pain.
Neuromodulation of the sacral nerve roots may be achieved by direct conduction of electrical impulses from a lead implanted in the sacrum (sacral neuromodulation) or by the retrograde conduction of these impulses through the posterior tibial nerve (percutaneous tibial nerve stimulation, or PTNS) (FIGURE 2). The tibial nerve arises from sacral nerves L5 to S3 and is one of the larger branches of the sciatic nerve.

FIGURE 2 InterStim therapy
Stimulation of the sacral nerve has been used successfully to manage overactive bladder and urinary retention and may prove useful in the treatment of pelvic myofascial pain.
Van Balken et al: Details of the study
In this prospective observational study, 33 patients (both male and female) who had chronic pelvic pain by history and examination were treated with weekly, 30-minute outpatient sessions of PTNS for 12 weeks. Participants were asked to provide baseline pain scores and keep a diary of their pain. Quality-of-life questionnaires were also administered at baseline and at 12 weeks.
Investigators considered both subjective and objective success in their outcomes. If a patient elected to continue therapy, he or she was classified as a subjective success. Objective success required a decrease of at least 50% in the pain score. At the end of 12 weeks, although 33 patients (42%) wanted to continue therapy, only seven (21%) met the definition for objective success. Of those seven, six elected to continue therapy.
This study sheds light on a treatment modality that has not been studied adequately for the indication of pelvic pain but that may be promising in patients who have levator myalgia. Limitations of this study include the lack of a placebo arm, short-term outcome, and lack of localization of pain. Furthermore, although PTNS has FDA approval for treatment of urinary urgency, frequency, and urge incontinence, it is not approved for the treatment of pelvic pain. These preliminary findings demonstrate potential but, as with any new indication, long-term comparative studies are needed.
Zabihi et al: Details of the study
Patients in this retrospective study had a diagnosis of interstitial cystitis or chronic pelvic pain. Pelvic myofascial pain and trigger points were not required for eligibility. Thirty patients (21 women and nine men) had temporary placement of a lead containing four small electrodes along the S2 to S4 sacral nerve roots on both sides of the sacrum. They were then followed for a trial period of 2 to 4 weeks. To qualify for the final stage of the study, in which the leads were connected internally to a generator implanted in the buttocks, patients had to report improvement of at least 50% in their symptoms. If their improvement did not meet that threshold, the leads were removed.
Twenty-three patients (77%) met the criteria for permanent implantation. Of these patients, 42% reported improvement of more than 50% at 6 postoperative months. Quality-of-life scores also improved significantly.
Sacral neuromodulation is not FDA-approved for the treatment of chronic pelvic pain; further studies are needed before it can be recommended for this indication.
Neither of these studies required objective evidence of myofascial pain for inclusion. Therefore, although the benefits they demonstrated may be theorized to extend to the relief of myofascial pain, this fact cannot be corroborated.
We want to hear from you! Tell us what you think.
1. Bassaly R, Tidwell N, Bertolino S, Hoyte L, Downes K, Hart S. Myofascial pain and pelvic floor dysfunction in patients with interstitial cystitis. Int Urogynecol J. 2011;22(4):413-418.
2. Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician. 2002;65(4):653-660.
3. Allergan, Inc. Medication Guide: BOTOX. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM176360.pdf. Published October 2010. Accessed August 30, 2011.
1. Bassaly R, Tidwell N, Bertolino S, Hoyte L, Downes K, Hart S. Myofascial pain and pelvic floor dysfunction in patients with interstitial cystitis. Int Urogynecol J. 2011;22(4):413-418.
2. Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician. 2002;65(4):653-660.
3. Allergan, Inc. Medication Guide: BOTOX. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM176360.pdf. Published October 2010. Accessed August 30, 2011.