User login
2022 Update on cervical disease
Cervical cancer is an important global health problem with an estimated 604,127 new cases and 341,831 deaths in 2020.1 Nearly 85% of the disease burden affects individuals from low and middle-income countries. The World Health Organization (WHO) set forth the goal for all countries to reach and maintain an incidence rate of below 4 per 100,000 women by 2030 as part of the Global Strategy to Accelerate the Elimination of Cervical Cancer.
Although traditional Pap cytology has been the cornerstone of screening programs, its poor sensitivity of approximately 50% and limitations in accessibility require new strategies to achieve the elimination of cervical cancer.2 The discovery that persistent infection with oncogenic human papillomavirus (HPV) is an essential step in the development of cervical cancer led to the development of diagnostic HPV tests, which have higher sensitivity than cytology (96.1% vs 53.0%) but somewhat lower specificity (90.7% vs 96.3%) for the detection of cervical intraepithelial neoplasia (CIN) 2 or worse lesions.2 Initially, HPV testing was incorporated as a method to triage atypical squamous cells of undetermined significance (ASCUS) cytology results.3 Later, the concept of cotesting with cytology emerged,4,5 and since then, several clinical trials have demonstrated the effectiveness of primary HPV screening.6-9
In 2020, the WHO recommended HPV DNA testing as the primary screening method starting at the age of 30 years, with regular testing every 5 to 10 years, for the general population.10 Currently, primary HPV has been adopted in multiple countries, including Australia, the Netherlands, Turkey, England, and Argentina.
In the United States, there are 3 currently acceptable screening strategies: cytology, cytology plus HPV (cotesting), and primary HPV testing (TABLE). The American Cancer Society (ACS) specifically states that HPV testing alone every 5 years is preferred starting at age 25 years; cotesting every 5 years or cytology alone every 3 years are also acceptable.11 The US Preventive Services Task Force (USPSTF) states that cytology alone every 3 years starting at 21 years and then HPV testing alone or cotesting every 5 years or cytology every 3 years starting at age 30 are all acceptable strategies.12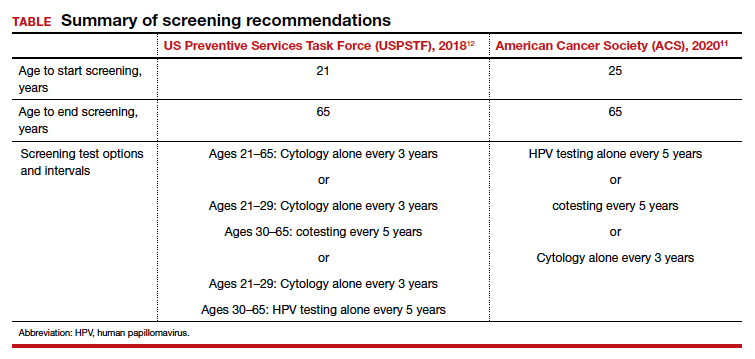
When applying these guidelines, it is important to note that they are intended for the screening of patients with all prior normal results with no symptoms. These routine screening guidelines do not apply to special populations, such as those with a history of abnormal results or treatment, a history of immunosuppression,13 a history of HPV-related vulvar or vaginal dysplasia,14-16 or a history of hysterectomy with removal of the cervix and no prior history of cervical dysplasia.17,18 By contrast, surveillance is interval testing for those who have either an abnormal prior test result or treatment; these may be managed per risk-based estimates provided by the American Society for Colposcopy and Cervical Pathology (ASCCP).18,19 Finally, diagnosis is evaluation (which may include diagnostic cytology) of a patient with abnormal signs and/or symptoms (such as bleeding, pain, discharge, or cervical mass).
In this Update, we present the evidence for primary HPV testing, the management options for a positive result in the United States, and research that will improve uptake of primary HPV testing as well as accessibility.
Change in screening paradigm: Evidence for primary HPV testing
HPV DNA tests are multiplex assays that detect the DNA of targeted high-risk HPV types, using multiple probes, either by direct genomic detection or by amplification of a viral DNA fragment using polymerase chain reaction (PCR).20,21 Alternatively, HPV mRNA-based tests detect the expression of E6 and E7 oncoproteins, a marker of viral integration.20 In examining the data from well-conducted clinical trials, 2 important observations are that different HPV assays were used and that direct comparison may not be valid. In addition, not all tests used in the studies are approved by the US Food and Drug Administration (FDA) for primary HPV testing.
Continue to: FDA-approved HPV tests...
FDA-approved HPV tests
Currently, 2 tests are FDA approved for primary HPV screening. The Cobas HPV test (Roche Molecular Diagnostics) was the first FDA-approved test for primary HPV screening in women aged 25 years and older.6 This test reports pooled results from 12 high-risk (hr) HPV types (31/33/35/39/45/51/52/56/58/59/66/68) with reflex genotyping for HPV 16/18, and thus it provides an immediate triage option for HPV-positive women. Of note, it is also approved for cotesting. The second FDA-approved test is the BD Onclarity HPV assay (Becton, Dickinson and Company) for primary HPV screening.22 It detects 14 hrHPV types, types 16/18/45 specifically as well as types 31/33/35/39/51/52/56/58/59/66/68.
Other HPV tests are FDA approved for cotesting and reflex testing but not for primary HPV testing. The Hybrid Capture test, or HC2 (Qiagen Inc), was the first HPV test to be approved by the FDA in 1997 for reflex testing of women with ASCUS cytology. In 2003, it was approved for cotesting along with cytology in women aged 30 years and older.20,21 In 2009, the Cervista HPV HR test (Hologic Inc) was approved for cotesting. The Aptima HPV assay (Hologic Inc), which is also approved for cotesting, is an RNA-based assay that allows detection of E6/E7 mRNA transcripts of 14 HPV types.23
Comparing HPV testing with cytology
Ronco and colleagues pooled data from 4 European randomized controlled trials (RCTs)—Swedescreen, POBASCAM, NTCC, ARTISTIC—with a total of 176,464 participants randomly assigned to HPV or cytology screening.24 Swedescreen and POBASCAM used GP5/GP6 PCR, while ARTISTIC and NTCC used HC2 for primary HPV screening. The screening interval was 3 years in all except 5 years in POBASCAM. The pooled detection rate of invasive disease was similar in the 2 arms, with pooled rate ratio for cancer detection being 0.79 (95% confidence interval [CI], 0.46–1.36) in the first 2.5 years, but was 0.45 (95% CI, 0.25–0.81), favoring the HPV arm, after 2.5 years. HPV testing was more effective in preventing cases of adenocarcinoma than squamous cell carcinoma (0.31 [95% CI, 0.14–0.69] vs 0.78 [95% CI, 0.49–1.25]). The authors concluded that HPV-based screening from age 30 years provided 60% to 70% better protection than cytology.
The result of the above meta-analysis was confirmed by the HPV FOCAL RCT that investigated the efficacy of HPV testing (HC2) in comparison with cytology.25 The detection rates for CIN 3 lesions supported primary HPV screening, with an absolute difference in incidence rate of 2.67/1,000 (95% CI, 0.53–4.88) at study randomization and 3.22/1,000 (95% CI, 5.12–1.48) at study exit 4 years later.
Cotesting using HPV and cytology: Marginal benefit
Dillner and colleagues were one of the first groups to report on the risk of CIN 3 based on both HPV and cytology status.26 Using pooled analysis of data from multiple countries, these investigators reported that the cumulative incidence rates (CIR) of CIN 3 after 6 years of follow-up increased consistently in HPV-positive subjects, and an HPV-positive result more accurately predicted CIN 3+ at 5 years than cytology alone. Furthermore, HPV negativity provided greater reassurance than cytology alone. At 5 years of follow-up, the rates of CIN 3+ were 0.25% (0.12%–0.41%) for women negative for HPV compared with 0.83% (0.50%–1.13%) for women with negative cytology results. There was little difference in rates for CIN 3+ between women with negative results on both tests and women who were negative for HPV.
The important question is then the marginal benefit of cotesting, which is the most costly screening option. A study of 331,818 women enrolled for cotesting at Kaiser Permanente found that the risk of CIN 3+ predicted by HPV testing alone when compared with cytology was significantly higher at both 3 years (5.0% vs 3.8%; P = .046) and 5 years (7.6% vs 4.7%; P = .001).27 A negative cytology result did not decrease the risk of CIN 3 further for HPV-negative patients (3 years: 0.047% vs 0.063%, P = .6; 5 years: 0.16% vs 0.17%, P = .8). They concluded that a negative HPV test was enough reassurance for low risk of CIN 3+ and that an additional negative cytology result does not provide extra reassurance.
Furthermore, a systematic meta-analysis of 48 studies, including 8 RCTs, found that the addition of cytology to HPV testing raised the sensitivity by 2% for CIN 3 compared with HPV testing alone. This improvement in sensitivity was at the expense of considerable loss of specificity, with a ratio of 0.93 (95% CI, 0.92–0.95) for CIN 3.28 Schiffman and colleagues also assessed the relative contribution of HPV testing and cytology in detection of CIN 3 and cancer.29 The HPV component alone identified a significantly higher proportion of preinvasive and invasive disease than cytology. Only 3.5% of precancers and 5.9% of cancers were preceded by HPV-negative, cytology-positive results. Thus, cytology contributed only 5 cases per million women per year to the sensitivity of the combined test, at the cost of significantly more colposcopies. Hence, the evidence suggests that there is limited benefit of adding cytology to HPV testing.30
Continue to: Triage of a positive HPV result...
Triage of a positive HPV result
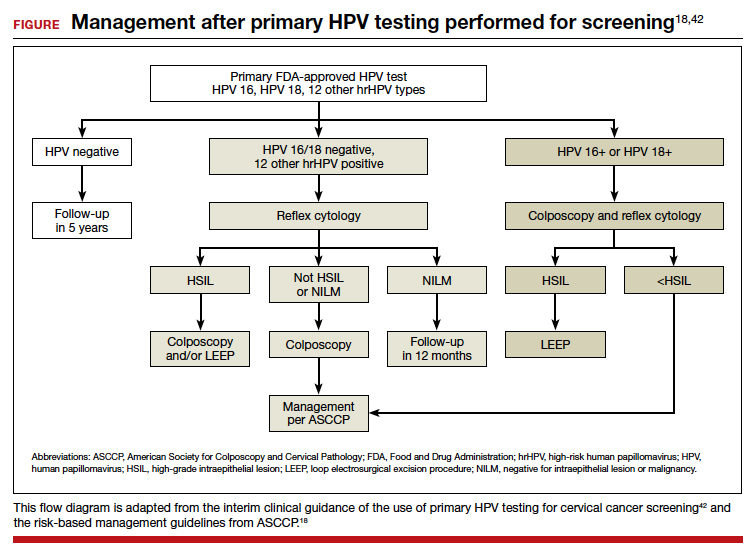
An important limitation of HPV testing is its inability to discriminate between transient and persistent infections. Referral of all HPV-positive cases to colposcopy would overburden the system with associated unnecessary procedures. Hence, a triage strategy is essential to identify clinically important infections that truly require colposcopic evaluation. The FIGURE illustrates the management of a primary HPV test result performed for screening.
HPV genotyping
One strategy for triaging a positive HPV test result is genotyping. HPV 16 and 18 have the highest risk of persistence and progression and merit immediate referral to colposcopy. In the ATHENA trial, CIN 3 was identified in 17.8% (95% CI, 14.8–20.7%) of HPV 16 positive women at baseline, and the CIR increased to 25.2% (95% CI, 21.7–28.7%) after 3 years. The 3-year CIR of CIN 3 was only 5.4% (95% CI, 4.5–6.3%) in women with HPV genotypes other than 16/18. HPV 18–positive women had a 3-year CIR that was intermediate between women with HPV 16 and women with the 12 other genotypes.6 Hence, HPV 16/18–positive cases should be referred for immediate colposcopy, and negative cases should be followed up with cytology and referred for colposcopy if the cytology is ASCUS or worse.31
In July 2020, extended genotyping was approved by the FDA with individual detection of HPV 31, 51, 52 (in addition to 16, 18, and 45) and pooled detection of 33/58, 35/39/68, and 56/59/66. One study found that individual genotypes HPV 16 and 31 carry baseline risk values for CIN 3+ (8.1% and 7.5%, respectively) that are above the 5-year risk threshold for referral to colposcopy following the ASCCP risk-based management guideline.32
Cytology
The higher specificity of cytology makes it an option for triaging HPV-positive cases, and current management guidelines recommend triage to both genotyping and cytology for all patients who are HPV positive, and especially if they are HPV positive but HPV 16/18 negative. Of note, cytology results remain more subjective than those of primary HPV testing, but the combination of initial HPV testing with reflex to cytology is a reasonable and cost effective next step.18 The VASCAR trial found higher colposcopy referrals in the HPV screening and cytology triage group compared with the cytology alone group (19.36 vs 14.54 per 1,000 women).33 The ATHENA trial investigated various triage strategies for HPV-positive cases and its impact on colposcopy referrals.6 Using HPV genotyping and reflex cytology, if HPV 16/18 was positive, colposcopy was advised, but if any of the other 12 HPV types were positive, reflex cytology was done. If reported as ASCUS or worse, colposcopy was performed; conversely, if it was normal, women were rescreened with cotesting after 1 year. Although this strategy led to a reduction in the number of colposcopies, referrals were still higher in the primary HPV arm (3,769 colposcopies per 294 cases) compared with cytology (1,934 colposcopies per 179 cases) or cotesting (3,097 colposcopies per 240 cases) in women aged 25 years.14
p16/Ki-67 Dual-Stain
Diffused p16 immunohistochemical staining, as opposed to focal staining, is associated with active HPV infection but can be present in low-grade as well as high-grade lesions.34 Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, these tests are supportive of active HPV infection and of a high-grade lesion. Incorporation of these stains to cytology alone provides additional objective reassurance to cytology, where there is much inter- and intra-observer variability. These stains can be done by laboratories using the stains alone or they can use the FDA-approved p16/Ki-67 Dual-Stain immunohistochemistry (DS), CINtec PLUS Cytology (Roche Diagnostics). However, DS is not yet formally incorporated into triage algorithms by national guidelines.
The IMPACT trial assessed the performance of DS compared with cytology in the triage of HPV-positive results, with or without HPV 16/18 genotyping.35 This was a prospective observational screening study of 35,263 women aged 25 to 65 years across 32 sites in the United States. Of the 4,927 HPV-positive patients with DS results, the sensitivity of DS for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) and 86.0% (95% CI, 77.5%–91.6%) in HPV 16/18–positive and in the 12 other genotypes, respectively. Using DS alone to triage HPV-positive results showed significantly higher sensitivity and specificity than HPV 16/18 genotyping with cytology triage of 12 “other” genotypes, and substantially higher sensitivity but lower specificity than using cytology alone. Of note, triage with DS alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Similarly, a retrospective analysis of the ATHENA trial cohort of HPV-positive results of 7,727 patients aged 25 years or older also demonstrated increased sensitivity of DS compared with cytology (74.9% vs 51.9%; P<.0001) and similar specificities (74.1% vs 75%; P = .3198).36 The European PALMS study, which included 27,349 women aged 18 years or older across 5 countries who underwent routine screening with HPV testing, cytology, and DS, confirmed these findings.37 The sensitivity of DS was higher than that of cytology (86.7% vs 68.5%; P<.001) for CIN 3+ with comparable specificities (95.2% vs 95.4%; P = .15).
Challenges and opportunities to improve access to primary HPV screening
The historical success of the Pap test in reducing the incidence of cervical cancer relied on individuals having access to the test. This remains true as screening transitions to primary HPV testing. Limitations of HPV-based screening include provider and patient knowledge; access to tests; cost; need for new laboratory infrastructure; need to leverage the electronic health record to record results, calculate a patient’s risk and determine next steps; and the need to re-educate patients and providers about this new model of care. The American Cancer Society and the Centers for Disease Control and Prevention are currently leading initiatives to help adopt primary HPV screening in the United States and to facilitate new care approaches.
Self-collection and independence from subjective cytology would further improve access. Multiple effectiveness studies and patient acceptability studies have shown that primary HPV screening via self-collection is effective, cost effective, and acceptable to women, especially among underscreened populations.38 Sensitivity is comparable to clinician-obtained samples with polymerase chain reaction–based HPV tests. Furthermore, newer molecular tests that detect methylated target host genes or methylated viral genome can be used to triage HPV-positive cases. Several host methylation markers that identify the specific host genes (for example, CADM1, MAL, and miR-124-2) have been shown to be more specific, reproducible, and can be used in self-collected samples as they are based on molecular methylation analysis.39 The ASCCP monitors these new developments and will incorporate promising tests and approaches once validated and FDA approved into the risk-based management guidelines. An erratum was recently published, and the risk-calculator is also available on the ASCCP website free of charge (https://app.asccp.org).40
In conclusion, transition to primary HPV testing from Pap cytology in cervical cancer screening has many challenges but also opportunities. Learning from the experience of countries that have already adopted primary HPV testing is crucial to successful implementation of this new screening paradigm.41 The evidence supporting primary HPV screening with its improved sensitivity is clear, and the existing triage options and innovations will continue to improve triage of patients with clinically important lesions as well as accessibility. With strong advocacy and sound implementation, the WHO goal of cervical cancer elimination and 70% of women being screened with a high-performance test by age 35 and again by age 45 is achievable. ●
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71: 209-249.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101.
- Wright TC Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346-355.
- Tota JE, Bentley J, Blake J, et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: acting on evidence to change the current paradigm. Prev Med. 2017;98:5-14.
- Ronco G, Giorgi Rossi P. Role of HPV DNA testing in modern gynaecological practice. Best Prac Res Clin Obstet Gynaecol. 2018;47:107-118.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579-1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- Bulkmans NW, Rozendaal L, Snijders PJ, et al. POBASCAM, a population-based randomized controlled trial for implementation of high-risk HPV testing in cervical screening: design, methods and baseline data of 44,102 women. Int J Cancer. 2004;110:94-101.
- World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. 2nd edition. Geneva: 2021. https://www .who.int/publications/i/item/9789240030824. Accessed April 28, 2022.
- American Cancer Society. The American Cancer Society guidelines for the prevention and early detection of cervical cancer. American Cancer Society; 2020. https://www.cancer .org/cancer/cervical-cancer/detection-diagnosis-staging /cervical-cancer-screening-guidelines.html. Accessed April 28, 2022.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens KD, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Moscicki AB, Flowers L, Huchko MJ, et al. Guidelines for cervical cancer screening in immunosuppressed women without HIV infection. J Low Gen Tract Dis. 2019;23:87-101.
- Committee opinion no. 675. Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2016;128:e178-e182.
- Satmary W, Holschneider CH, Brunette LL, et al. Vulvar intraepithelial neoplasia: risk factors for recurrence. Gynecol Oncol. 2018;148:126-131.
- Preti M, Scurry J, Marchitelli CE, et al. Vulvar intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol. 2014;28:10511062.
- Khan MJ, Massad LS, Kinney W, et al. A common clinical dilemma: management of abnormal vaginal cytology and human papillomavirus test results. Gynecol Oncol. 2016;141:364-370.
- Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2020;24:102-131.
- Egemen D, Cheung LC, Chen X, et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Low Gen Tract Dis. 2020;24:132-143.
- Bhatla N, Singla S, Awasthi D. Human papillomavirus deoxyribonucleic acid testing in developed countries. Best Pract Res Clin Obstet Gynaecol. 2012;26:209-220.
- Meijer CJ, Berkhof J, Castle PE, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124:516-520.
- Ejegod D, Bottari F, Pedersen H, et al. The BD Onclarity HPV assay on samples collected in SurePath medium meets the international guidelines for human papillomavirus test requirements for cervical screening. J Clin Microbiol. 2016;54:2267-2272.
- Richardson LA, Tota J, Franco EL. Optimizing technology for cervical cancer screening in high-resource settings. Expert Rev Obstet Gynecol. 2011;6:343-353.
- Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: followup of four European randomised controlled trials. Lancet. 2014;383:524-532.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663-672.
- Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(suppl 5):F88-99.
- Schiffman M, Kinney WK, et al. Relative performance of HPV and cytology components of cotesting in cervical screening. J Nat Cancer Inst. 2018;110:501-508.
- Jin XW, Lipold L, Foucher J, et al. Cost-effectiveness of primary HPV testing, cytology and co-testing as cervical cancer screening for women above age 30 years. J Gen Intern Med. 2016;31:1338-1344.
- Tota JE, Bentley J, Blake J, et al. Approaches for triaging women who test positive for human papillomavirus in cervical cancer screening. Prev Med. 2017;98:15-20.
- Stoler MH, Wright TC Jr, Parvu V, et al. Stratified risk of high-grade cervical disease using onclarity HPV extended genotyping in women, ≥25 years of age, with NILM cytology. Gynecol Oncol. 2019;153:26-33.
- Louvanto K, Chevarie-Davis M, Ramanakumar AV, et al. HPV testing with cytology triage for cervical cancer screening in routine practice. Am J Obstet Gynecol. 2014;210:474.e1-7.
- Keating JT, Cviko A, Riethdorf S, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884-891.
- Wright TC Jr, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471.
- Wright TC Jr, Behrens CM, Ranger-Moore J, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144:51-56.
- Ikenberg H, Bergeron C, Schmidt D, et al. Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study. J Nat Cancer Inst. 2013;105:15501557.
- Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
- Verhoef VMJ, Bosgraaf RP, van Kemenade FJ, et al. Triage by methylation-marker testing versus cytology in women who test HPV-positive on self-collected cervicovaginal specimens (PROHTECT-3): a randomised controlled non-inferiority trial. Lancet Oncol. 2014;15:315-322.
- Perkins RB, Guido RS, Castle PE, et al. Erratum: 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2021;25:330-331.
- Hall MT, Simms KT, Lew JB, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4:e19-e27.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178-182.
Cervical cancer is an important global health problem with an estimated 604,127 new cases and 341,831 deaths in 2020.1 Nearly 85% of the disease burden affects individuals from low and middle-income countries. The World Health Organization (WHO) set forth the goal for all countries to reach and maintain an incidence rate of below 4 per 100,000 women by 2030 as part of the Global Strategy to Accelerate the Elimination of Cervical Cancer.
Although traditional Pap cytology has been the cornerstone of screening programs, its poor sensitivity of approximately 50% and limitations in accessibility require new strategies to achieve the elimination of cervical cancer.2 The discovery that persistent infection with oncogenic human papillomavirus (HPV) is an essential step in the development of cervical cancer led to the development of diagnostic HPV tests, which have higher sensitivity than cytology (96.1% vs 53.0%) but somewhat lower specificity (90.7% vs 96.3%) for the detection of cervical intraepithelial neoplasia (CIN) 2 or worse lesions.2 Initially, HPV testing was incorporated as a method to triage atypical squamous cells of undetermined significance (ASCUS) cytology results.3 Later, the concept of cotesting with cytology emerged,4,5 and since then, several clinical trials have demonstrated the effectiveness of primary HPV screening.6-9
In 2020, the WHO recommended HPV DNA testing as the primary screening method starting at the age of 30 years, with regular testing every 5 to 10 years, for the general population.10 Currently, primary HPV has been adopted in multiple countries, including Australia, the Netherlands, Turkey, England, and Argentina.
In the United States, there are 3 currently acceptable screening strategies: cytology, cytology plus HPV (cotesting), and primary HPV testing (TABLE). The American Cancer Society (ACS) specifically states that HPV testing alone every 5 years is preferred starting at age 25 years; cotesting every 5 years or cytology alone every 3 years are also acceptable.11 The US Preventive Services Task Force (USPSTF) states that cytology alone every 3 years starting at 21 years and then HPV testing alone or cotesting every 5 years or cytology every 3 years starting at age 30 are all acceptable strategies.12
When applying these guidelines, it is important to note that they are intended for the screening of patients with all prior normal results with no symptoms. These routine screening guidelines do not apply to special populations, such as those with a history of abnormal results or treatment, a history of immunosuppression,13 a history of HPV-related vulvar or vaginal dysplasia,14-16 or a history of hysterectomy with removal of the cervix and no prior history of cervical dysplasia.17,18 By contrast, surveillance is interval testing for those who have either an abnormal prior test result or treatment; these may be managed per risk-based estimates provided by the American Society for Colposcopy and Cervical Pathology (ASCCP).18,19 Finally, diagnosis is evaluation (which may include diagnostic cytology) of a patient with abnormal signs and/or symptoms (such as bleeding, pain, discharge, or cervical mass).
In this Update, we present the evidence for primary HPV testing, the management options for a positive result in the United States, and research that will improve uptake of primary HPV testing as well as accessibility.
Change in screening paradigm: Evidence for primary HPV testing
HPV DNA tests are multiplex assays that detect the DNA of targeted high-risk HPV types, using multiple probes, either by direct genomic detection or by amplification of a viral DNA fragment using polymerase chain reaction (PCR).20,21 Alternatively, HPV mRNA-based tests detect the expression of E6 and E7 oncoproteins, a marker of viral integration.20 In examining the data from well-conducted clinical trials, 2 important observations are that different HPV assays were used and that direct comparison may not be valid. In addition, not all tests used in the studies are approved by the US Food and Drug Administration (FDA) for primary HPV testing.
Continue to: FDA-approved HPV tests...
FDA-approved HPV tests
Currently, 2 tests are FDA approved for primary HPV screening. The Cobas HPV test (Roche Molecular Diagnostics) was the first FDA-approved test for primary HPV screening in women aged 25 years and older.6 This test reports pooled results from 12 high-risk (hr) HPV types (31/33/35/39/45/51/52/56/58/59/66/68) with reflex genotyping for HPV 16/18, and thus it provides an immediate triage option for HPV-positive women. Of note, it is also approved for cotesting. The second FDA-approved test is the BD Onclarity HPV assay (Becton, Dickinson and Company) for primary HPV screening.22 It detects 14 hrHPV types, types 16/18/45 specifically as well as types 31/33/35/39/51/52/56/58/59/66/68.
Other HPV tests are FDA approved for cotesting and reflex testing but not for primary HPV testing. The Hybrid Capture test, or HC2 (Qiagen Inc), was the first HPV test to be approved by the FDA in 1997 for reflex testing of women with ASCUS cytology. In 2003, it was approved for cotesting along with cytology in women aged 30 years and older.20,21 In 2009, the Cervista HPV HR test (Hologic Inc) was approved for cotesting. The Aptima HPV assay (Hologic Inc), which is also approved for cotesting, is an RNA-based assay that allows detection of E6/E7 mRNA transcripts of 14 HPV types.23
Comparing HPV testing with cytology
Ronco and colleagues pooled data from 4 European randomized controlled trials (RCTs)—Swedescreen, POBASCAM, NTCC, ARTISTIC—with a total of 176,464 participants randomly assigned to HPV or cytology screening.24 Swedescreen and POBASCAM used GP5/GP6 PCR, while ARTISTIC and NTCC used HC2 for primary HPV screening. The screening interval was 3 years in all except 5 years in POBASCAM. The pooled detection rate of invasive disease was similar in the 2 arms, with pooled rate ratio for cancer detection being 0.79 (95% confidence interval [CI], 0.46–1.36) in the first 2.5 years, but was 0.45 (95% CI, 0.25–0.81), favoring the HPV arm, after 2.5 years. HPV testing was more effective in preventing cases of adenocarcinoma than squamous cell carcinoma (0.31 [95% CI, 0.14–0.69] vs 0.78 [95% CI, 0.49–1.25]). The authors concluded that HPV-based screening from age 30 years provided 60% to 70% better protection than cytology.
The result of the above meta-analysis was confirmed by the HPV FOCAL RCT that investigated the efficacy of HPV testing (HC2) in comparison with cytology.25 The detection rates for CIN 3 lesions supported primary HPV screening, with an absolute difference in incidence rate of 2.67/1,000 (95% CI, 0.53–4.88) at study randomization and 3.22/1,000 (95% CI, 5.12–1.48) at study exit 4 years later.
Cotesting using HPV and cytology: Marginal benefit
Dillner and colleagues were one of the first groups to report on the risk of CIN 3 based on both HPV and cytology status.26 Using pooled analysis of data from multiple countries, these investigators reported that the cumulative incidence rates (CIR) of CIN 3 after 6 years of follow-up increased consistently in HPV-positive subjects, and an HPV-positive result more accurately predicted CIN 3+ at 5 years than cytology alone. Furthermore, HPV negativity provided greater reassurance than cytology alone. At 5 years of follow-up, the rates of CIN 3+ were 0.25% (0.12%–0.41%) for women negative for HPV compared with 0.83% (0.50%–1.13%) for women with negative cytology results. There was little difference in rates for CIN 3+ between women with negative results on both tests and women who were negative for HPV.
The important question is then the marginal benefit of cotesting, which is the most costly screening option. A study of 331,818 women enrolled for cotesting at Kaiser Permanente found that the risk of CIN 3+ predicted by HPV testing alone when compared with cytology was significantly higher at both 3 years (5.0% vs 3.8%; P = .046) and 5 years (7.6% vs 4.7%; P = .001).27 A negative cytology result did not decrease the risk of CIN 3 further for HPV-negative patients (3 years: 0.047% vs 0.063%, P = .6; 5 years: 0.16% vs 0.17%, P = .8). They concluded that a negative HPV test was enough reassurance for low risk of CIN 3+ and that an additional negative cytology result does not provide extra reassurance.
Furthermore, a systematic meta-analysis of 48 studies, including 8 RCTs, found that the addition of cytology to HPV testing raised the sensitivity by 2% for CIN 3 compared with HPV testing alone. This improvement in sensitivity was at the expense of considerable loss of specificity, with a ratio of 0.93 (95% CI, 0.92–0.95) for CIN 3.28 Schiffman and colleagues also assessed the relative contribution of HPV testing and cytology in detection of CIN 3 and cancer.29 The HPV component alone identified a significantly higher proportion of preinvasive and invasive disease than cytology. Only 3.5% of precancers and 5.9% of cancers were preceded by HPV-negative, cytology-positive results. Thus, cytology contributed only 5 cases per million women per year to the sensitivity of the combined test, at the cost of significantly more colposcopies. Hence, the evidence suggests that there is limited benefit of adding cytology to HPV testing.30
Continue to: Triage of a positive HPV result...
Triage of a positive HPV result
An important limitation of HPV testing is its inability to discriminate between transient and persistent infections. Referral of all HPV-positive cases to colposcopy would overburden the system with associated unnecessary procedures. Hence, a triage strategy is essential to identify clinically important infections that truly require colposcopic evaluation. The FIGURE illustrates the management of a primary HPV test result performed for screening.

HPV genotyping
One strategy for triaging a positive HPV test result is genotyping. HPV 16 and 18 have the highest risk of persistence and progression and merit immediate referral to colposcopy. In the ATHENA trial, CIN 3 was identified in 17.8% (95% CI, 14.8–20.7%) of HPV 16 positive women at baseline, and the CIR increased to 25.2% (95% CI, 21.7–28.7%) after 3 years. The 3-year CIR of CIN 3 was only 5.4% (95% CI, 4.5–6.3%) in women with HPV genotypes other than 16/18. HPV 18–positive women had a 3-year CIR that was intermediate between women with HPV 16 and women with the 12 other genotypes.6 Hence, HPV 16/18–positive cases should be referred for immediate colposcopy, and negative cases should be followed up with cytology and referred for colposcopy if the cytology is ASCUS or worse.31
In July 2020, extended genotyping was approved by the FDA with individual detection of HPV 31, 51, 52 (in addition to 16, 18, and 45) and pooled detection of 33/58, 35/39/68, and 56/59/66. One study found that individual genotypes HPV 16 and 31 carry baseline risk values for CIN 3+ (8.1% and 7.5%, respectively) that are above the 5-year risk threshold for referral to colposcopy following the ASCCP risk-based management guideline.32
Cytology
The higher specificity of cytology makes it an option for triaging HPV-positive cases, and current management guidelines recommend triage to both genotyping and cytology for all patients who are HPV positive, and especially if they are HPV positive but HPV 16/18 negative. Of note, cytology results remain more subjective than those of primary HPV testing, but the combination of initial HPV testing with reflex to cytology is a reasonable and cost effective next step.18 The VASCAR trial found higher colposcopy referrals in the HPV screening and cytology triage group compared with the cytology alone group (19.36 vs 14.54 per 1,000 women).33 The ATHENA trial investigated various triage strategies for HPV-positive cases and its impact on colposcopy referrals.6 Using HPV genotyping and reflex cytology, if HPV 16/18 was positive, colposcopy was advised, but if any of the other 12 HPV types were positive, reflex cytology was done. If reported as ASCUS or worse, colposcopy was performed; conversely, if it was normal, women were rescreened with cotesting after 1 year. Although this strategy led to a reduction in the number of colposcopies, referrals were still higher in the primary HPV arm (3,769 colposcopies per 294 cases) compared with cytology (1,934 colposcopies per 179 cases) or cotesting (3,097 colposcopies per 240 cases) in women aged 25 years.14
p16/Ki-67 Dual-Stain
Diffused p16 immunohistochemical staining, as opposed to focal staining, is associated with active HPV infection but can be present in low-grade as well as high-grade lesions.34 Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, these tests are supportive of active HPV infection and of a high-grade lesion. Incorporation of these stains to cytology alone provides additional objective reassurance to cytology, where there is much inter- and intra-observer variability. These stains can be done by laboratories using the stains alone or they can use the FDA-approved p16/Ki-67 Dual-Stain immunohistochemistry (DS), CINtec PLUS Cytology (Roche Diagnostics). However, DS is not yet formally incorporated into triage algorithms by national guidelines.
The IMPACT trial assessed the performance of DS compared with cytology in the triage of HPV-positive results, with or without HPV 16/18 genotyping.35 This was a prospective observational screening study of 35,263 women aged 25 to 65 years across 32 sites in the United States. Of the 4,927 HPV-positive patients with DS results, the sensitivity of DS for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) and 86.0% (95% CI, 77.5%–91.6%) in HPV 16/18–positive and in the 12 other genotypes, respectively. Using DS alone to triage HPV-positive results showed significantly higher sensitivity and specificity than HPV 16/18 genotyping with cytology triage of 12 “other” genotypes, and substantially higher sensitivity but lower specificity than using cytology alone. Of note, triage with DS alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Similarly, a retrospective analysis of the ATHENA trial cohort of HPV-positive results of 7,727 patients aged 25 years or older also demonstrated increased sensitivity of DS compared with cytology (74.9% vs 51.9%; P<.0001) and similar specificities (74.1% vs 75%; P = .3198).36 The European PALMS study, which included 27,349 women aged 18 years or older across 5 countries who underwent routine screening with HPV testing, cytology, and DS, confirmed these findings.37 The sensitivity of DS was higher than that of cytology (86.7% vs 68.5%; P<.001) for CIN 3+ with comparable specificities (95.2% vs 95.4%; P = .15).
Challenges and opportunities to improve access to primary HPV screening
The historical success of the Pap test in reducing the incidence of cervical cancer relied on individuals having access to the test. This remains true as screening transitions to primary HPV testing. Limitations of HPV-based screening include provider and patient knowledge; access to tests; cost; need for new laboratory infrastructure; need to leverage the electronic health record to record results, calculate a patient’s risk and determine next steps; and the need to re-educate patients and providers about this new model of care. The American Cancer Society and the Centers for Disease Control and Prevention are currently leading initiatives to help adopt primary HPV screening in the United States and to facilitate new care approaches.
Self-collection and independence from subjective cytology would further improve access. Multiple effectiveness studies and patient acceptability studies have shown that primary HPV screening via self-collection is effective, cost effective, and acceptable to women, especially among underscreened populations.38 Sensitivity is comparable to clinician-obtained samples with polymerase chain reaction–based HPV tests. Furthermore, newer molecular tests that detect methylated target host genes or methylated viral genome can be used to triage HPV-positive cases. Several host methylation markers that identify the specific host genes (for example, CADM1, MAL, and miR-124-2) have been shown to be more specific, reproducible, and can be used in self-collected samples as they are based on molecular methylation analysis.39 The ASCCP monitors these new developments and will incorporate promising tests and approaches once validated and FDA approved into the risk-based management guidelines. An erratum was recently published, and the risk-calculator is also available on the ASCCP website free of charge (https://app.asccp.org).40
In conclusion, transition to primary HPV testing from Pap cytology in cervical cancer screening has many challenges but also opportunities. Learning from the experience of countries that have already adopted primary HPV testing is crucial to successful implementation of this new screening paradigm.41 The evidence supporting primary HPV screening with its improved sensitivity is clear, and the existing triage options and innovations will continue to improve triage of patients with clinically important lesions as well as accessibility. With strong advocacy and sound implementation, the WHO goal of cervical cancer elimination and 70% of women being screened with a high-performance test by age 35 and again by age 45 is achievable. ●
Cervical cancer is an important global health problem with an estimated 604,127 new cases and 341,831 deaths in 2020.1 Nearly 85% of the disease burden affects individuals from low and middle-income countries. The World Health Organization (WHO) set forth the goal for all countries to reach and maintain an incidence rate of below 4 per 100,000 women by 2030 as part of the Global Strategy to Accelerate the Elimination of Cervical Cancer.
Although traditional Pap cytology has been the cornerstone of screening programs, its poor sensitivity of approximately 50% and limitations in accessibility require new strategies to achieve the elimination of cervical cancer.2 The discovery that persistent infection with oncogenic human papillomavirus (HPV) is an essential step in the development of cervical cancer led to the development of diagnostic HPV tests, which have higher sensitivity than cytology (96.1% vs 53.0%) but somewhat lower specificity (90.7% vs 96.3%) for the detection of cervical intraepithelial neoplasia (CIN) 2 or worse lesions.2 Initially, HPV testing was incorporated as a method to triage atypical squamous cells of undetermined significance (ASCUS) cytology results.3 Later, the concept of cotesting with cytology emerged,4,5 and since then, several clinical trials have demonstrated the effectiveness of primary HPV screening.6-9
In 2020, the WHO recommended HPV DNA testing as the primary screening method starting at the age of 30 years, with regular testing every 5 to 10 years, for the general population.10 Currently, primary HPV has been adopted in multiple countries, including Australia, the Netherlands, Turkey, England, and Argentina.
In the United States, there are 3 currently acceptable screening strategies: cytology, cytology plus HPV (cotesting), and primary HPV testing (TABLE). The American Cancer Society (ACS) specifically states that HPV testing alone every 5 years is preferred starting at age 25 years; cotesting every 5 years or cytology alone every 3 years are also acceptable.11 The US Preventive Services Task Force (USPSTF) states that cytology alone every 3 years starting at 21 years and then HPV testing alone or cotesting every 5 years or cytology every 3 years starting at age 30 are all acceptable strategies.12
When applying these guidelines, it is important to note that they are intended for the screening of patients with all prior normal results with no symptoms. These routine screening guidelines do not apply to special populations, such as those with a history of abnormal results or treatment, a history of immunosuppression,13 a history of HPV-related vulvar or vaginal dysplasia,14-16 or a history of hysterectomy with removal of the cervix and no prior history of cervical dysplasia.17,18 By contrast, surveillance is interval testing for those who have either an abnormal prior test result or treatment; these may be managed per risk-based estimates provided by the American Society for Colposcopy and Cervical Pathology (ASCCP).18,19 Finally, diagnosis is evaluation (which may include diagnostic cytology) of a patient with abnormal signs and/or symptoms (such as bleeding, pain, discharge, or cervical mass).
In this Update, we present the evidence for primary HPV testing, the management options for a positive result in the United States, and research that will improve uptake of primary HPV testing as well as accessibility.
Change in screening paradigm: Evidence for primary HPV testing
HPV DNA tests are multiplex assays that detect the DNA of targeted high-risk HPV types, using multiple probes, either by direct genomic detection or by amplification of a viral DNA fragment using polymerase chain reaction (PCR).20,21 Alternatively, HPV mRNA-based tests detect the expression of E6 and E7 oncoproteins, a marker of viral integration.20 In examining the data from well-conducted clinical trials, 2 important observations are that different HPV assays were used and that direct comparison may not be valid. In addition, not all tests used in the studies are approved by the US Food and Drug Administration (FDA) for primary HPV testing.
Continue to: FDA-approved HPV tests...
FDA-approved HPV tests
Currently, 2 tests are FDA approved for primary HPV screening. The Cobas HPV test (Roche Molecular Diagnostics) was the first FDA-approved test for primary HPV screening in women aged 25 years and older.6 This test reports pooled results from 12 high-risk (hr) HPV types (31/33/35/39/45/51/52/56/58/59/66/68) with reflex genotyping for HPV 16/18, and thus it provides an immediate triage option for HPV-positive women. Of note, it is also approved for cotesting. The second FDA-approved test is the BD Onclarity HPV assay (Becton, Dickinson and Company) for primary HPV screening.22 It detects 14 hrHPV types, types 16/18/45 specifically as well as types 31/33/35/39/51/52/56/58/59/66/68.
Other HPV tests are FDA approved for cotesting and reflex testing but not for primary HPV testing. The Hybrid Capture test, or HC2 (Qiagen Inc), was the first HPV test to be approved by the FDA in 1997 for reflex testing of women with ASCUS cytology. In 2003, it was approved for cotesting along with cytology in women aged 30 years and older.20,21 In 2009, the Cervista HPV HR test (Hologic Inc) was approved for cotesting. The Aptima HPV assay (Hologic Inc), which is also approved for cotesting, is an RNA-based assay that allows detection of E6/E7 mRNA transcripts of 14 HPV types.23
Comparing HPV testing with cytology
Ronco and colleagues pooled data from 4 European randomized controlled trials (RCTs)—Swedescreen, POBASCAM, NTCC, ARTISTIC—with a total of 176,464 participants randomly assigned to HPV or cytology screening.24 Swedescreen and POBASCAM used GP5/GP6 PCR, while ARTISTIC and NTCC used HC2 for primary HPV screening. The screening interval was 3 years in all except 5 years in POBASCAM. The pooled detection rate of invasive disease was similar in the 2 arms, with pooled rate ratio for cancer detection being 0.79 (95% confidence interval [CI], 0.46–1.36) in the first 2.5 years, but was 0.45 (95% CI, 0.25–0.81), favoring the HPV arm, after 2.5 years. HPV testing was more effective in preventing cases of adenocarcinoma than squamous cell carcinoma (0.31 [95% CI, 0.14–0.69] vs 0.78 [95% CI, 0.49–1.25]). The authors concluded that HPV-based screening from age 30 years provided 60% to 70% better protection than cytology.
The result of the above meta-analysis was confirmed by the HPV FOCAL RCT that investigated the efficacy of HPV testing (HC2) in comparison with cytology.25 The detection rates for CIN 3 lesions supported primary HPV screening, with an absolute difference in incidence rate of 2.67/1,000 (95% CI, 0.53–4.88) at study randomization and 3.22/1,000 (95% CI, 5.12–1.48) at study exit 4 years later.
Cotesting using HPV and cytology: Marginal benefit
Dillner and colleagues were one of the first groups to report on the risk of CIN 3 based on both HPV and cytology status.26 Using pooled analysis of data from multiple countries, these investigators reported that the cumulative incidence rates (CIR) of CIN 3 after 6 years of follow-up increased consistently in HPV-positive subjects, and an HPV-positive result more accurately predicted CIN 3+ at 5 years than cytology alone. Furthermore, HPV negativity provided greater reassurance than cytology alone. At 5 years of follow-up, the rates of CIN 3+ were 0.25% (0.12%–0.41%) for women negative for HPV compared with 0.83% (0.50%–1.13%) for women with negative cytology results. There was little difference in rates for CIN 3+ between women with negative results on both tests and women who were negative for HPV.
The important question is then the marginal benefit of cotesting, which is the most costly screening option. A study of 331,818 women enrolled for cotesting at Kaiser Permanente found that the risk of CIN 3+ predicted by HPV testing alone when compared with cytology was significantly higher at both 3 years (5.0% vs 3.8%; P = .046) and 5 years (7.6% vs 4.7%; P = .001).27 A negative cytology result did not decrease the risk of CIN 3 further for HPV-negative patients (3 years: 0.047% vs 0.063%, P = .6; 5 years: 0.16% vs 0.17%, P = .8). They concluded that a negative HPV test was enough reassurance for low risk of CIN 3+ and that an additional negative cytology result does not provide extra reassurance.
Furthermore, a systematic meta-analysis of 48 studies, including 8 RCTs, found that the addition of cytology to HPV testing raised the sensitivity by 2% for CIN 3 compared with HPV testing alone. This improvement in sensitivity was at the expense of considerable loss of specificity, with a ratio of 0.93 (95% CI, 0.92–0.95) for CIN 3.28 Schiffman and colleagues also assessed the relative contribution of HPV testing and cytology in detection of CIN 3 and cancer.29 The HPV component alone identified a significantly higher proportion of preinvasive and invasive disease than cytology. Only 3.5% of precancers and 5.9% of cancers were preceded by HPV-negative, cytology-positive results. Thus, cytology contributed only 5 cases per million women per year to the sensitivity of the combined test, at the cost of significantly more colposcopies. Hence, the evidence suggests that there is limited benefit of adding cytology to HPV testing.30
Continue to: Triage of a positive HPV result...
Triage of a positive HPV result
An important limitation of HPV testing is its inability to discriminate between transient and persistent infections. Referral of all HPV-positive cases to colposcopy would overburden the system with associated unnecessary procedures. Hence, a triage strategy is essential to identify clinically important infections that truly require colposcopic evaluation. The FIGURE illustrates the management of a primary HPV test result performed for screening.

HPV genotyping
One strategy for triaging a positive HPV test result is genotyping. HPV 16 and 18 have the highest risk of persistence and progression and merit immediate referral to colposcopy. In the ATHENA trial, CIN 3 was identified in 17.8% (95% CI, 14.8–20.7%) of HPV 16 positive women at baseline, and the CIR increased to 25.2% (95% CI, 21.7–28.7%) after 3 years. The 3-year CIR of CIN 3 was only 5.4% (95% CI, 4.5–6.3%) in women with HPV genotypes other than 16/18. HPV 18–positive women had a 3-year CIR that was intermediate between women with HPV 16 and women with the 12 other genotypes.6 Hence, HPV 16/18–positive cases should be referred for immediate colposcopy, and negative cases should be followed up with cytology and referred for colposcopy if the cytology is ASCUS or worse.31
In July 2020, extended genotyping was approved by the FDA with individual detection of HPV 31, 51, 52 (in addition to 16, 18, and 45) and pooled detection of 33/58, 35/39/68, and 56/59/66. One study found that individual genotypes HPV 16 and 31 carry baseline risk values for CIN 3+ (8.1% and 7.5%, respectively) that are above the 5-year risk threshold for referral to colposcopy following the ASCCP risk-based management guideline.32
Cytology
The higher specificity of cytology makes it an option for triaging HPV-positive cases, and current management guidelines recommend triage to both genotyping and cytology for all patients who are HPV positive, and especially if they are HPV positive but HPV 16/18 negative. Of note, cytology results remain more subjective than those of primary HPV testing, but the combination of initial HPV testing with reflex to cytology is a reasonable and cost effective next step.18 The VASCAR trial found higher colposcopy referrals in the HPV screening and cytology triage group compared with the cytology alone group (19.36 vs 14.54 per 1,000 women).33 The ATHENA trial investigated various triage strategies for HPV-positive cases and its impact on colposcopy referrals.6 Using HPV genotyping and reflex cytology, if HPV 16/18 was positive, colposcopy was advised, but if any of the other 12 HPV types were positive, reflex cytology was done. If reported as ASCUS or worse, colposcopy was performed; conversely, if it was normal, women were rescreened with cotesting after 1 year. Although this strategy led to a reduction in the number of colposcopies, referrals were still higher in the primary HPV arm (3,769 colposcopies per 294 cases) compared with cytology (1,934 colposcopies per 179 cases) or cotesting (3,097 colposcopies per 240 cases) in women aged 25 years.14
p16/Ki-67 Dual-Stain
Diffused p16 immunohistochemical staining, as opposed to focal staining, is associated with active HPV infection but can be present in low-grade as well as high-grade lesions.34 Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, these tests are supportive of active HPV infection and of a high-grade lesion. Incorporation of these stains to cytology alone provides additional objective reassurance to cytology, where there is much inter- and intra-observer variability. These stains can be done by laboratories using the stains alone or they can use the FDA-approved p16/Ki-67 Dual-Stain immunohistochemistry (DS), CINtec PLUS Cytology (Roche Diagnostics). However, DS is not yet formally incorporated into triage algorithms by national guidelines.
The IMPACT trial assessed the performance of DS compared with cytology in the triage of HPV-positive results, with or without HPV 16/18 genotyping.35 This was a prospective observational screening study of 35,263 women aged 25 to 65 years across 32 sites in the United States. Of the 4,927 HPV-positive patients with DS results, the sensitivity of DS for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) and 86.0% (95% CI, 77.5%–91.6%) in HPV 16/18–positive and in the 12 other genotypes, respectively. Using DS alone to triage HPV-positive results showed significantly higher sensitivity and specificity than HPV 16/18 genotyping with cytology triage of 12 “other” genotypes, and substantially higher sensitivity but lower specificity than using cytology alone. Of note, triage with DS alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Similarly, a retrospective analysis of the ATHENA trial cohort of HPV-positive results of 7,727 patients aged 25 years or older also demonstrated increased sensitivity of DS compared with cytology (74.9% vs 51.9%; P<.0001) and similar specificities (74.1% vs 75%; P = .3198).36 The European PALMS study, which included 27,349 women aged 18 years or older across 5 countries who underwent routine screening with HPV testing, cytology, and DS, confirmed these findings.37 The sensitivity of DS was higher than that of cytology (86.7% vs 68.5%; P<.001) for CIN 3+ with comparable specificities (95.2% vs 95.4%; P = .15).
Challenges and opportunities to improve access to primary HPV screening
The historical success of the Pap test in reducing the incidence of cervical cancer relied on individuals having access to the test. This remains true as screening transitions to primary HPV testing. Limitations of HPV-based screening include provider and patient knowledge; access to tests; cost; need for new laboratory infrastructure; need to leverage the electronic health record to record results, calculate a patient’s risk and determine next steps; and the need to re-educate patients and providers about this new model of care. The American Cancer Society and the Centers for Disease Control and Prevention are currently leading initiatives to help adopt primary HPV screening in the United States and to facilitate new care approaches.
Self-collection and independence from subjective cytology would further improve access. Multiple effectiveness studies and patient acceptability studies have shown that primary HPV screening via self-collection is effective, cost effective, and acceptable to women, especially among underscreened populations.38 Sensitivity is comparable to clinician-obtained samples with polymerase chain reaction–based HPV tests. Furthermore, newer molecular tests that detect methylated target host genes or methylated viral genome can be used to triage HPV-positive cases. Several host methylation markers that identify the specific host genes (for example, CADM1, MAL, and miR-124-2) have been shown to be more specific, reproducible, and can be used in self-collected samples as they are based on molecular methylation analysis.39 The ASCCP monitors these new developments and will incorporate promising tests and approaches once validated and FDA approved into the risk-based management guidelines. An erratum was recently published, and the risk-calculator is also available on the ASCCP website free of charge (https://app.asccp.org).40
In conclusion, transition to primary HPV testing from Pap cytology in cervical cancer screening has many challenges but also opportunities. Learning from the experience of countries that have already adopted primary HPV testing is crucial to successful implementation of this new screening paradigm.41 The evidence supporting primary HPV screening with its improved sensitivity is clear, and the existing triage options and innovations will continue to improve triage of patients with clinically important lesions as well as accessibility. With strong advocacy and sound implementation, the WHO goal of cervical cancer elimination and 70% of women being screened with a high-performance test by age 35 and again by age 45 is achievable. ●
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71: 209-249.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101.
- Wright TC Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346-355.
- Tota JE, Bentley J, Blake J, et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: acting on evidence to change the current paradigm. Prev Med. 2017;98:5-14.
- Ronco G, Giorgi Rossi P. Role of HPV DNA testing in modern gynaecological practice. Best Prac Res Clin Obstet Gynaecol. 2018;47:107-118.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579-1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- Bulkmans NW, Rozendaal L, Snijders PJ, et al. POBASCAM, a population-based randomized controlled trial for implementation of high-risk HPV testing in cervical screening: design, methods and baseline data of 44,102 women. Int J Cancer. 2004;110:94-101.
- World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. 2nd edition. Geneva: 2021. https://www .who.int/publications/i/item/9789240030824. Accessed April 28, 2022.
- American Cancer Society. The American Cancer Society guidelines for the prevention and early detection of cervical cancer. American Cancer Society; 2020. https://www.cancer .org/cancer/cervical-cancer/detection-diagnosis-staging /cervical-cancer-screening-guidelines.html. Accessed April 28, 2022.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens KD, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Moscicki AB, Flowers L, Huchko MJ, et al. Guidelines for cervical cancer screening in immunosuppressed women without HIV infection. J Low Gen Tract Dis. 2019;23:87-101.
- Committee opinion no. 675. Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2016;128:e178-e182.
- Satmary W, Holschneider CH, Brunette LL, et al. Vulvar intraepithelial neoplasia: risk factors for recurrence. Gynecol Oncol. 2018;148:126-131.
- Preti M, Scurry J, Marchitelli CE, et al. Vulvar intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol. 2014;28:10511062.
- Khan MJ, Massad LS, Kinney W, et al. A common clinical dilemma: management of abnormal vaginal cytology and human papillomavirus test results. Gynecol Oncol. 2016;141:364-370.
- Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2020;24:102-131.
- Egemen D, Cheung LC, Chen X, et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Low Gen Tract Dis. 2020;24:132-143.
- Bhatla N, Singla S, Awasthi D. Human papillomavirus deoxyribonucleic acid testing in developed countries. Best Pract Res Clin Obstet Gynaecol. 2012;26:209-220.
- Meijer CJ, Berkhof J, Castle PE, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124:516-520.
- Ejegod D, Bottari F, Pedersen H, et al. The BD Onclarity HPV assay on samples collected in SurePath medium meets the international guidelines for human papillomavirus test requirements for cervical screening. J Clin Microbiol. 2016;54:2267-2272.
- Richardson LA, Tota J, Franco EL. Optimizing technology for cervical cancer screening in high-resource settings. Expert Rev Obstet Gynecol. 2011;6:343-353.
- Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: followup of four European randomised controlled trials. Lancet. 2014;383:524-532.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663-672.
- Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(suppl 5):F88-99.
- Schiffman M, Kinney WK, et al. Relative performance of HPV and cytology components of cotesting in cervical screening. J Nat Cancer Inst. 2018;110:501-508.
- Jin XW, Lipold L, Foucher J, et al. Cost-effectiveness of primary HPV testing, cytology and co-testing as cervical cancer screening for women above age 30 years. J Gen Intern Med. 2016;31:1338-1344.
- Tota JE, Bentley J, Blake J, et al. Approaches for triaging women who test positive for human papillomavirus in cervical cancer screening. Prev Med. 2017;98:15-20.
- Stoler MH, Wright TC Jr, Parvu V, et al. Stratified risk of high-grade cervical disease using onclarity HPV extended genotyping in women, ≥25 years of age, with NILM cytology. Gynecol Oncol. 2019;153:26-33.
- Louvanto K, Chevarie-Davis M, Ramanakumar AV, et al. HPV testing with cytology triage for cervical cancer screening in routine practice. Am J Obstet Gynecol. 2014;210:474.e1-7.
- Keating JT, Cviko A, Riethdorf S, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884-891.
- Wright TC Jr, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471.
- Wright TC Jr, Behrens CM, Ranger-Moore J, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144:51-56.
- Ikenberg H, Bergeron C, Schmidt D, et al. Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study. J Nat Cancer Inst. 2013;105:15501557.
- Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
- Verhoef VMJ, Bosgraaf RP, van Kemenade FJ, et al. Triage by methylation-marker testing versus cytology in women who test HPV-positive on self-collected cervicovaginal specimens (PROHTECT-3): a randomised controlled non-inferiority trial. Lancet Oncol. 2014;15:315-322.
- Perkins RB, Guido RS, Castle PE, et al. Erratum: 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2021;25:330-331.
- Hall MT, Simms KT, Lew JB, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4:e19-e27.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178-182.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71: 209-249.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101.
- Wright TC Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346-355.
- Tota JE, Bentley J, Blake J, et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: acting on evidence to change the current paradigm. Prev Med. 2017;98:5-14.
- Ronco G, Giorgi Rossi P. Role of HPV DNA testing in modern gynaecological practice. Best Prac Res Clin Obstet Gynaecol. 2018;47:107-118.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579-1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- Bulkmans NW, Rozendaal L, Snijders PJ, et al. POBASCAM, a population-based randomized controlled trial for implementation of high-risk HPV testing in cervical screening: design, methods and baseline data of 44,102 women. Int J Cancer. 2004;110:94-101.
- World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. 2nd edition. Geneva: 2021. https://www .who.int/publications/i/item/9789240030824. Accessed April 28, 2022.
- American Cancer Society. The American Cancer Society guidelines for the prevention and early detection of cervical cancer. American Cancer Society; 2020. https://www.cancer .org/cancer/cervical-cancer/detection-diagnosis-staging /cervical-cancer-screening-guidelines.html. Accessed April 28, 2022.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens KD, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Moscicki AB, Flowers L, Huchko MJ, et al. Guidelines for cervical cancer screening in immunosuppressed women without HIV infection. J Low Gen Tract Dis. 2019;23:87-101.
- Committee opinion no. 675. Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2016;128:e178-e182.
- Satmary W, Holschneider CH, Brunette LL, et al. Vulvar intraepithelial neoplasia: risk factors for recurrence. Gynecol Oncol. 2018;148:126-131.
- Preti M, Scurry J, Marchitelli CE, et al. Vulvar intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol. 2014;28:10511062.
- Khan MJ, Massad LS, Kinney W, et al. A common clinical dilemma: management of abnormal vaginal cytology and human papillomavirus test results. Gynecol Oncol. 2016;141:364-370.
- Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2020;24:102-131.
- Egemen D, Cheung LC, Chen X, et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Low Gen Tract Dis. 2020;24:132-143.
- Bhatla N, Singla S, Awasthi D. Human papillomavirus deoxyribonucleic acid testing in developed countries. Best Pract Res Clin Obstet Gynaecol. 2012;26:209-220.
- Meijer CJ, Berkhof J, Castle PE, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124:516-520.
- Ejegod D, Bottari F, Pedersen H, et al. The BD Onclarity HPV assay on samples collected in SurePath medium meets the international guidelines for human papillomavirus test requirements for cervical screening. J Clin Microbiol. 2016;54:2267-2272.
- Richardson LA, Tota J, Franco EL. Optimizing technology for cervical cancer screening in high-resource settings. Expert Rev Obstet Gynecol. 2011;6:343-353.
- Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: followup of four European randomised controlled trials. Lancet. 2014;383:524-532.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663-672.
- Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(suppl 5):F88-99.
- Schiffman M, Kinney WK, et al. Relative performance of HPV and cytology components of cotesting in cervical screening. J Nat Cancer Inst. 2018;110:501-508.
- Jin XW, Lipold L, Foucher J, et al. Cost-effectiveness of primary HPV testing, cytology and co-testing as cervical cancer screening for women above age 30 years. J Gen Intern Med. 2016;31:1338-1344.
- Tota JE, Bentley J, Blake J, et al. Approaches for triaging women who test positive for human papillomavirus in cervical cancer screening. Prev Med. 2017;98:15-20.
- Stoler MH, Wright TC Jr, Parvu V, et al. Stratified risk of high-grade cervical disease using onclarity HPV extended genotyping in women, ≥25 years of age, with NILM cytology. Gynecol Oncol. 2019;153:26-33.
- Louvanto K, Chevarie-Davis M, Ramanakumar AV, et al. HPV testing with cytology triage for cervical cancer screening in routine practice. Am J Obstet Gynecol. 2014;210:474.e1-7.
- Keating JT, Cviko A, Riethdorf S, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884-891.
- Wright TC Jr, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471.
- Wright TC Jr, Behrens CM, Ranger-Moore J, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144:51-56.
- Ikenberg H, Bergeron C, Schmidt D, et al. Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study. J Nat Cancer Inst. 2013;105:15501557.
- Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
- Verhoef VMJ, Bosgraaf RP, van Kemenade FJ, et al. Triage by methylation-marker testing versus cytology in women who test HPV-positive on self-collected cervicovaginal specimens (PROHTECT-3): a randomised controlled non-inferiority trial. Lancet Oncol. 2014;15:315-322.
- Perkins RB, Guido RS, Castle PE, et al. Erratum: 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2021;25:330-331.
- Hall MT, Simms KT, Lew JB, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4:e19-e27.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178-182.
Cervical cancer: A path to eradication
David G. Mutch, MD: The cervical cancer screening guidelines, using Pap testing, have changed significantly since the times of yearly Paps and exams. Coupled with vaccination and new management guidelines (recommending HPV testing, etc), we actually hope that we are on the way to eradicating cervical cancer from our environment.
Screening: Current recommendations
Dr. Mutch: Warner, the American Society of Colposcopy and Cervical Pathology (ASCCP)1 endorses the cervical cancer screening guidelines for several professional organizations, including the American Cancer Society (ACS),2 the US Preventive Services Task Force (USPSTF),3 and the American College of Obstetricians and Gynecologists (ACOG).4 What are the current screening recommendations, as these organizations have disparate views?
Warner Huh, MD: There was a time, around 2012-2013, when for the first time ever, we had significant harmonization of the guidelines between ACOG and the USPSTF and ACS. But in the last 10 years there has been an explosion of data in terms of how to best screen patients.
The move to primary HPV testing. The USPSTF3 initially had recommended looking into primary HPV screening, which is just using HPV testing by itself as the screening modality. But there was a lot of pushback at that time. I think a lot of people thought that we were not prepared to make that leap. Therefore, they endorsed screening with cytology by itself as well as HPV testing by itself, as well as co-testing; but in their recommendations, they made it very clear that they were leaning toward primary HPV screening.
A new patient age to begin screening. In 2020, the ACS put out their new guidelines,2 which are a significant departure from what we are used to—they are recommending that we start screening at 25 years of age. Like you said, Dr. Mutch, it doesn’t seem that long ago when we were screening people at age 18, or within 3 years of sexual intercourse. But the reason for it is that the rate of cervical cancer is extremely low under age 25, and other countries like the United Kingdom already do this.
The other major departure in the ACS guidelines is that they really are asking clinicians and screeners to focus on primary HPV screening. Overall, they have sort of doubled down on why they think primary HPV screening is so important.
ACOG sits sort of in the middle of the other recommendations. ACOG understands the value of primary HPV screening, but I don’t think that they are quite ready to recommend screening at age 25. If you look at their updated guidelines from April 2021,4 they state that we should continue a screening-starting age of 21 years. So there are some disparate views, but I am confident, Dr. Mutch, that in the next 2 to 3 years, there will be greater harmonization of these guidelines and less confusion for our providers. The greatest barrier is understanding the science and the comfort level of clinicians to go with just an HPV test, since for the last 40 years the Pap test has anchored gynecologic care in this country. And it took at least 10 years to get to what I consider to be widespread adoption to co-testing. The other thing that readers should recognize is that the Task Force is actually revisiting their cervical cancer screening guidance now, so expect another major revision.
Reimbursement and access are barriers. Reimbursement is a further real issue. We are now using one less test, but insurance companies may not reimburse when just the HPV test is used. The other issue is access to labs that can do the HPV testing.
Dr. Mutch: We used to see patients yearly and picked up a lot of adjunctive or additional illnesses. Now they are not being seen yearly it could impact negatively their overall health care. We need to understand that cervical cancer screening is simply a test, which should not eliminate other health care.
Dr. Huh: Yes, I think the extended interval between recommended HPV screenings scares people. I have been involved in these screening guidelines (and I can only speak for myself, not for my colleagues), but even I do think we made a leap to a longer interval way too quickly in this country. Screening changes are slow, and sometimes a glacial process. I think it can worry providers when we make rapid changes.
But this is a test that should not anchor the yearly visit. There are plenty of other reasons—and ACOG actually states this4—why patients should come for a wellness exam on a yearly basis. So I think our ObGyns in the United States need to recognize that, but I understand there are underlying concerns that if you extend intervals too long, (a) will patients come back, and (b), as a consequence, is the interval going to miss something in between? Those are real legitimate concerns.
Continue to: Management guidelines...
Management guidelines: The latest
Dr. Mutch: The ASCCP issued new management guidelines in 2019.5 Can you address what you feel are the most important updates?
Dr. Huh: Going back to 2002, we have revised these guidelines every 5 years. For this one, the revision came out a little bit later for various reasons, but the reason we revised it is because we collect new data that we think markedly changes our understanding of the disease process and natural history and the interventions for women that have preinvasive disease of the cervix.
Briefly, I think the biggest changes based on what we were hearing from our providers and users of our apps and algorithms was that our algorithms were becoming way too complicated, and they were. If you look over the last 10 years, the number of branch points on our algorithms basically quadrupled. If we incorporated the new data this time, the algorithms would be unworkable, and you could not use them on your phone because they would be too complicated.
So, we created a system where, in essence, providers have 5 choices for patients:
- treatment
- colposcopy
- follow-up in 1 year
- follow-up in 3 years
- follow-up in 5 years.
Those recommendations are based on what we call “clinically actionable thresholds”—basically, the percent chance of developing immediate CIN3 or worse. That threshold will probably change over time, but what we did is create a system that (a) makes it easier for the provider, (although they have to trust the system—and they can look under the hood and understand how we did this) and (b) allows us to create a foundation where we can add future technologies that use the same rubric or paradigm so that they still wind up getting the same result without having to go to another algorithm.
This new system is probably the most marked change in the history of the ASCCP management guidelines, but we did it to make it ultimately easier for providers going forward for the next 10 to 20 years. There are real opportunities, Dr. Mutch, in terms of how do we integrate this into the electronic medical record (EMR), and how do we pull data so clinicians don’t have to manually enter it.
The other difference is now there is a web-based application. Back in 2012, there were a lot of people that were not using EMRs. Now the majority of the country is, and so they actually are on a browser more than they are on their phone. We actually have an equally robust web platform that allows them to get the information that they need.
Dr. Mutch: I think that is really important—the utility of utilizing a mobile app, if you will, for triaging your patient with a specific test result so that patients are followed up at the proper interval, and that ultimately becomes cost-effective.
Dr. Huh: Yes, the app now is very different than the app that I think people are used to using for the last almost 10 years. You don’t put inputs, pull up the algorithm, and look at the outcome. This is different. You enter the patient’s age. You add their cytology, their HPV results, the clinical scenario that you are in, and then it puts out a recommendation of what to do next. Over time, we want to get away from an algorithm and for our providers to understand what the risk is and how that risk calculation then translates into a clinical recommendation.
Dr. Mutch: I think to utilize an app is almost necessary given the complexity of the triaging process so that it does become, in fact, the most cost-effective way to screen patients.
Dr. Huh: I would agree with that. There is a learning curve for whenever you see new technology. There was a learning curve for even ASCCP leadership as they tried to educate providers. I think people will ultimately see that this is a much better way of managing patients with cervical abnormalities, and I am hoping actually that we will use a similar platform for many other diseases that we manage in women’s health.
Continue to: Chipping away of the yearly exam...
Chipping away of the yearly exam
Dr. Mutch: With this moving away from the yearly exam and Pap test, women may not get yearly examinations. Do you feel that this could affect a stage migration to a higher stage at diagnosis, for instance, of a cervical cancer? Or that it might adversely impact other health issues?
Dr. Huh: I think that’s a good question. I am worried about the interval—I think 5 years is a bit long. I am more worried that patients will miss out on visits because they may think that they need to only come back for their Pap, even though they should be re-educated on that.
COVID-19 has made this a little hard for us to analyze because, clearly, we have had access to care issues. But I am a little concerned that we could see an uptick in invasive cancer rates in this country, including an uptick in the stage and more locally advanced cervical cancer because of the changes in the screening paradigms. But we don’t know that to be the case.
As with all screenings, the bottom line is you have to worry about what the false-negative and false-positive rates of screening are, and that affects everything. I want the readers to know that primary HPV should be used for screenings. It is not perfect, but it is much better than cytology alone. We need to think about how to better adapt screening in the age that we live in.
HPV self-sampling
Dr. Mutch: Could self-sampling for HPV testing, which obviously would be easier for the patient, and certainly useful in terms of screening, address some health care disparities with regard to cervical cancer?
Dr. Huh: The short answer is, yes. Self-sampling is not US Food and Drug Administration-approved in this country. It’s not being widely used without that approval. But there are multiple countries, including the United States, that have done lots of studies on this topic. There are many public health experts and champions for HPV self-sampling. I think we have learned, based on some studies, that the sensitivity is reasonable.6
I live in a part of the country that is woefully underserved; where you are there are pockets in Missouri that are woefully underserved as well. So the issue is, can we reduce these disparities and access to care with something like self-sampling? My personal feeling is I think that we can make a dent in that, and it is never going to fully replace screening, but it at least will allow us to reallocate our resources and attention to those women that are at highest risk for developing cervical cancer or precancer based on the self-sampling result.
I don’t think it will ever replace screening per se, but if we have an abnormal self-sampling test, we might say to that patient, “You really do need to come in to get re-tested or to get re-evaluated.” So it could be a better resource and use of our health care dollars and investments in terms of trying to reduce the incidence of cervical cancer. Of course the verdict is out, but I think there are a lot of people who would love to see this scenario.
If we screen and treat perfectly in this country, we would not even need the HPV vaccine when it comes to cervical cancer. That is how effective screening is. But, up to 50% to 60% of women in this country now still are underscreened or unscreened. We were talking about that number almost 25-30 years ago, Dr. Mutch. So access to screening is a big problem, but the other problem is how do you get patients in to be seen if they have an abnormal screening test? It’s not just about screening. It’s about screening, evaluation, and treatment; all 3 components are really important.
Continue to: Where do we stand with HPV vaccination?...
Where do we stand with HPV vaccination?
Dr. Mutch: Those are great points.
You brought up vaccination. We have a long way to go with regard to that, certainly in the United States, because of the various factions opposed to vaccination and so on. But do you think that vaccination has allowed us to decrease the incidence of cervical cancer?
Dr. Huh: Yes. There is clear evidence from the Nordic countries.7 There is emerging evidence from Australia.8 There is emerging evidence from other industrialized nations that clearly demonstrate vaccination’s positive effect in reducing the incidence of cervical cancer. None of this should be a surprise. Every population-based study that has been published with the HPV vaccine in populations that have a low frequency of vaccination have demonstrated substantial reductions in things like genital warts, abnormal Pap tests, precancer, and now evidence that there is a downward trend in terms of the incidence of cervical cancer.9
I don’t think that there is any debate anymore that vaccination is the way to go. Our challenge is about implementation and getting the vaccine to people. We still have a long way to go with that. There are parts of the world that are so affected by invasive cervical cancers; we need to get the vaccine to those parts of the world.
Dr. Mutch: What are the barriers to vaccination? How can we overcome those barriers?
Dr. Huh: There is a lot of criticism that we are not vaccinating more in the United States. However, the rates of vaccination are going up every single year. The pandemic may have blunted that rise a bit, but if you look at the vaccination curves, they are going up, not down. We need to continue to educate patients, parents, and pediatricians on the importance of vaccination.
Boys still get vaccinated less frequently than girls, so we have some work to do there. I think globally it is the issue of getting the vaccine to people, making sure that vaccine is available. The thing that I think will be the game-changer going forward is whether or not we will have evidence to indicate that 1 dose is as effective as 2 doses or 3 doses. If we can vaccinate boys and girls with just 1 dose, then in the next generation or two, we seriously might eradicate not just cervical cancer but a lot of HPV-related malignancies worldwide.
Educating patients, clinicians is key
Dr. Mutch: So it seems education, education, education, with regard to screening guidelines, with regard to the need for continued examinations, and that HPV testing is only a test, it does not supplant overall care. Finally, education regarding eradication of cervical cancer through vaccination.
Dr. Huh: That summarizes it well. We are still going to screen for cervical cancer. We are still going to vaccinate, and providers are still going to manage abnormal Pap tests. It is confusing because we are changing it up it seems every year or 2, so this conversation you and I are having is particularly important for clinicians to understand the basis of that. There has been an explosion of data that has come out in this area in the last decade.
Dr. Mutch: Thank you, Dr. Huh. I really appreciate your thoughts on this. As you all know, Dr. Huh has been President of the ASCCP and is instrumental in writing and disseminating these guidelines, so we are very grateful that he has consented to agree to come and talk with us today.
Dr. Huh: My pleasure. Thank you for inviting me. This was fun, and I have really enjoyed talking to you and participating. ●
- ASCCP Screening Guidelines. https://www.asccp.org /screening-guidelines. Accessed April 25, 2021.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guidelines update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force. Screening for cervical cancer. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686. doi:10.1001/jama .2018.10897.
- American College of Obstetricians and Gynecologists. Practice Advisory: Updated cervical cancer screening guidelines. April 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/04/updated-cervical -cancer-screening-guidelines. Accessed April 25, 2022.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP RiskBased Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10 34 .1097/LGT.0000000000000525.
- Yeh PT, Kennedy CE, de Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/ bmjgh-2018-001351.
- Kjaer SK, Nygard M, Dillner J, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis. 2018;66:339-345. doi: 10.1093/cid/cix797.
- Patel C, Brotherton JM, Pillsbury A, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonvalent vaccine prevent? Euro Surveill. 2018;23:1700737. doi: 10 .2807/1560-7917.
- Falcaro M, Castanon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. 2021;398:2084-2092. doi.org/10.1016/S0140-6736(21) 02178-4.

David G. Mutch, MD: The cervical cancer screening guidelines, using Pap testing, have changed significantly since the times of yearly Paps and exams. Coupled with vaccination and new management guidelines (recommending HPV testing, etc), we actually hope that we are on the way to eradicating cervical cancer from our environment.
Screening: Current recommendations
Dr. Mutch: Warner, the American Society of Colposcopy and Cervical Pathology (ASCCP)1 endorses the cervical cancer screening guidelines for several professional organizations, including the American Cancer Society (ACS),2 the US Preventive Services Task Force (USPSTF),3 and the American College of Obstetricians and Gynecologists (ACOG).4 What are the current screening recommendations, as these organizations have disparate views?
Warner Huh, MD: There was a time, around 2012-2013, when for the first time ever, we had significant harmonization of the guidelines between ACOG and the USPSTF and ACS. But in the last 10 years there has been an explosion of data in terms of how to best screen patients.
The move to primary HPV testing. The USPSTF3 initially had recommended looking into primary HPV screening, which is just using HPV testing by itself as the screening modality. But there was a lot of pushback at that time. I think a lot of people thought that we were not prepared to make that leap. Therefore, they endorsed screening with cytology by itself as well as HPV testing by itself, as well as co-testing; but in their recommendations, they made it very clear that they were leaning toward primary HPV screening.
A new patient age to begin screening. In 2020, the ACS put out their new guidelines,2 which are a significant departure from what we are used to—they are recommending that we start screening at 25 years of age. Like you said, Dr. Mutch, it doesn’t seem that long ago when we were screening people at age 18, or within 3 years of sexual intercourse. But the reason for it is that the rate of cervical cancer is extremely low under age 25, and other countries like the United Kingdom already do this.
The other major departure in the ACS guidelines is that they really are asking clinicians and screeners to focus on primary HPV screening. Overall, they have sort of doubled down on why they think primary HPV screening is so important.
ACOG sits sort of in the middle of the other recommendations. ACOG understands the value of primary HPV screening, but I don’t think that they are quite ready to recommend screening at age 25. If you look at their updated guidelines from April 2021,4 they state that we should continue a screening-starting age of 21 years. So there are some disparate views, but I am confident, Dr. Mutch, that in the next 2 to 3 years, there will be greater harmonization of these guidelines and less confusion for our providers. The greatest barrier is understanding the science and the comfort level of clinicians to go with just an HPV test, since for the last 40 years the Pap test has anchored gynecologic care in this country. And it took at least 10 years to get to what I consider to be widespread adoption to co-testing. The other thing that readers should recognize is that the Task Force is actually revisiting their cervical cancer screening guidance now, so expect another major revision.
Reimbursement and access are barriers. Reimbursement is a further real issue. We are now using one less test, but insurance companies may not reimburse when just the HPV test is used. The other issue is access to labs that can do the HPV testing.
Dr. Mutch: We used to see patients yearly and picked up a lot of adjunctive or additional illnesses. Now they are not being seen yearly it could impact negatively their overall health care. We need to understand that cervical cancer screening is simply a test, which should not eliminate other health care.
Dr. Huh: Yes, I think the extended interval between recommended HPV screenings scares people. I have been involved in these screening guidelines (and I can only speak for myself, not for my colleagues), but even I do think we made a leap to a longer interval way too quickly in this country. Screening changes are slow, and sometimes a glacial process. I think it can worry providers when we make rapid changes.
But this is a test that should not anchor the yearly visit. There are plenty of other reasons—and ACOG actually states this4—why patients should come for a wellness exam on a yearly basis. So I think our ObGyns in the United States need to recognize that, but I understand there are underlying concerns that if you extend intervals too long, (a) will patients come back, and (b), as a consequence, is the interval going to miss something in between? Those are real legitimate concerns.
Continue to: Management guidelines...
Management guidelines: The latest
Dr. Mutch: The ASCCP issued new management guidelines in 2019.5 Can you address what you feel are the most important updates?
Dr. Huh: Going back to 2002, we have revised these guidelines every 5 years. For this one, the revision came out a little bit later for various reasons, but the reason we revised it is because we collect new data that we think markedly changes our understanding of the disease process and natural history and the interventions for women that have preinvasive disease of the cervix.
Briefly, I think the biggest changes based on what we were hearing from our providers and users of our apps and algorithms was that our algorithms were becoming way too complicated, and they were. If you look over the last 10 years, the number of branch points on our algorithms basically quadrupled. If we incorporated the new data this time, the algorithms would be unworkable, and you could not use them on your phone because they would be too complicated.
So, we created a system where, in essence, providers have 5 choices for patients:
- treatment
- colposcopy
- follow-up in 1 year
- follow-up in 3 years
- follow-up in 5 years.
Those recommendations are based on what we call “clinically actionable thresholds”—basically, the percent chance of developing immediate CIN3 or worse. That threshold will probably change over time, but what we did is create a system that (a) makes it easier for the provider, (although they have to trust the system—and they can look under the hood and understand how we did this) and (b) allows us to create a foundation where we can add future technologies that use the same rubric or paradigm so that they still wind up getting the same result without having to go to another algorithm.
This new system is probably the most marked change in the history of the ASCCP management guidelines, but we did it to make it ultimately easier for providers going forward for the next 10 to 20 years. There are real opportunities, Dr. Mutch, in terms of how do we integrate this into the electronic medical record (EMR), and how do we pull data so clinicians don’t have to manually enter it.
The other difference is now there is a web-based application. Back in 2012, there were a lot of people that were not using EMRs. Now the majority of the country is, and so they actually are on a browser more than they are on their phone. We actually have an equally robust web platform that allows them to get the information that they need.
Dr. Mutch: I think that is really important—the utility of utilizing a mobile app, if you will, for triaging your patient with a specific test result so that patients are followed up at the proper interval, and that ultimately becomes cost-effective.
Dr. Huh: Yes, the app now is very different than the app that I think people are used to using for the last almost 10 years. You don’t put inputs, pull up the algorithm, and look at the outcome. This is different. You enter the patient’s age. You add their cytology, their HPV results, the clinical scenario that you are in, and then it puts out a recommendation of what to do next. Over time, we want to get away from an algorithm and for our providers to understand what the risk is and how that risk calculation then translates into a clinical recommendation.
Dr. Mutch: I think to utilize an app is almost necessary given the complexity of the triaging process so that it does become, in fact, the most cost-effective way to screen patients.
Dr. Huh: I would agree with that. There is a learning curve for whenever you see new technology. There was a learning curve for even ASCCP leadership as they tried to educate providers. I think people will ultimately see that this is a much better way of managing patients with cervical abnormalities, and I am hoping actually that we will use a similar platform for many other diseases that we manage in women’s health.
Continue to: Chipping away of the yearly exam...
Chipping away of the yearly exam
Dr. Mutch: With this moving away from the yearly exam and Pap test, women may not get yearly examinations. Do you feel that this could affect a stage migration to a higher stage at diagnosis, for instance, of a cervical cancer? Or that it might adversely impact other health issues?
Dr. Huh: I think that’s a good question. I am worried about the interval—I think 5 years is a bit long. I am more worried that patients will miss out on visits because they may think that they need to only come back for their Pap, even though they should be re-educated on that.
COVID-19 has made this a little hard for us to analyze because, clearly, we have had access to care issues. But I am a little concerned that we could see an uptick in invasive cancer rates in this country, including an uptick in the stage and more locally advanced cervical cancer because of the changes in the screening paradigms. But we don’t know that to be the case.
As with all screenings, the bottom line is you have to worry about what the false-negative and false-positive rates of screening are, and that affects everything. I want the readers to know that primary HPV should be used for screenings. It is not perfect, but it is much better than cytology alone. We need to think about how to better adapt screening in the age that we live in.
HPV self-sampling
Dr. Mutch: Could self-sampling for HPV testing, which obviously would be easier for the patient, and certainly useful in terms of screening, address some health care disparities with regard to cervical cancer?
Dr. Huh: The short answer is, yes. Self-sampling is not US Food and Drug Administration-approved in this country. It’s not being widely used without that approval. But there are multiple countries, including the United States, that have done lots of studies on this topic. There are many public health experts and champions for HPV self-sampling. I think we have learned, based on some studies, that the sensitivity is reasonable.6
I live in a part of the country that is woefully underserved; where you are there are pockets in Missouri that are woefully underserved as well. So the issue is, can we reduce these disparities and access to care with something like self-sampling? My personal feeling is I think that we can make a dent in that, and it is never going to fully replace screening, but it at least will allow us to reallocate our resources and attention to those women that are at highest risk for developing cervical cancer or precancer based on the self-sampling result.
I don’t think it will ever replace screening per se, but if we have an abnormal self-sampling test, we might say to that patient, “You really do need to come in to get re-tested or to get re-evaluated.” So it could be a better resource and use of our health care dollars and investments in terms of trying to reduce the incidence of cervical cancer. Of course the verdict is out, but I think there are a lot of people who would love to see this scenario.
If we screen and treat perfectly in this country, we would not even need the HPV vaccine when it comes to cervical cancer. That is how effective screening is. But, up to 50% to 60% of women in this country now still are underscreened or unscreened. We were talking about that number almost 25-30 years ago, Dr. Mutch. So access to screening is a big problem, but the other problem is how do you get patients in to be seen if they have an abnormal screening test? It’s not just about screening. It’s about screening, evaluation, and treatment; all 3 components are really important.
Continue to: Where do we stand with HPV vaccination?...
Where do we stand with HPV vaccination?
Dr. Mutch: Those are great points.
You brought up vaccination. We have a long way to go with regard to that, certainly in the United States, because of the various factions opposed to vaccination and so on. But do you think that vaccination has allowed us to decrease the incidence of cervical cancer?
Dr. Huh: Yes. There is clear evidence from the Nordic countries.7 There is emerging evidence from Australia.8 There is emerging evidence from other industrialized nations that clearly demonstrate vaccination’s positive effect in reducing the incidence of cervical cancer. None of this should be a surprise. Every population-based study that has been published with the HPV vaccine in populations that have a low frequency of vaccination have demonstrated substantial reductions in things like genital warts, abnormal Pap tests, precancer, and now evidence that there is a downward trend in terms of the incidence of cervical cancer.9
I don’t think that there is any debate anymore that vaccination is the way to go. Our challenge is about implementation and getting the vaccine to people. We still have a long way to go with that. There are parts of the world that are so affected by invasive cervical cancers; we need to get the vaccine to those parts of the world.
Dr. Mutch: What are the barriers to vaccination? How can we overcome those barriers?
Dr. Huh: There is a lot of criticism that we are not vaccinating more in the United States. However, the rates of vaccination are going up every single year. The pandemic may have blunted that rise a bit, but if you look at the vaccination curves, they are going up, not down. We need to continue to educate patients, parents, and pediatricians on the importance of vaccination.
Boys still get vaccinated less frequently than girls, so we have some work to do there. I think globally it is the issue of getting the vaccine to people, making sure that vaccine is available. The thing that I think will be the game-changer going forward is whether or not we will have evidence to indicate that 1 dose is as effective as 2 doses or 3 doses. If we can vaccinate boys and girls with just 1 dose, then in the next generation or two, we seriously might eradicate not just cervical cancer but a lot of HPV-related malignancies worldwide.
Educating patients, clinicians is key
Dr. Mutch: So it seems education, education, education, with regard to screening guidelines, with regard to the need for continued examinations, and that HPV testing is only a test, it does not supplant overall care. Finally, education regarding eradication of cervical cancer through vaccination.
Dr. Huh: That summarizes it well. We are still going to screen for cervical cancer. We are still going to vaccinate, and providers are still going to manage abnormal Pap tests. It is confusing because we are changing it up it seems every year or 2, so this conversation you and I are having is particularly important for clinicians to understand the basis of that. There has been an explosion of data that has come out in this area in the last decade.
Dr. Mutch: Thank you, Dr. Huh. I really appreciate your thoughts on this. As you all know, Dr. Huh has been President of the ASCCP and is instrumental in writing and disseminating these guidelines, so we are very grateful that he has consented to agree to come and talk with us today.
Dr. Huh: My pleasure. Thank you for inviting me. This was fun, and I have really enjoyed talking to you and participating. ●

David G. Mutch, MD: The cervical cancer screening guidelines, using Pap testing, have changed significantly since the times of yearly Paps and exams. Coupled with vaccination and new management guidelines (recommending HPV testing, etc), we actually hope that we are on the way to eradicating cervical cancer from our environment.
Screening: Current recommendations
Dr. Mutch: Warner, the American Society of Colposcopy and Cervical Pathology (ASCCP)1 endorses the cervical cancer screening guidelines for several professional organizations, including the American Cancer Society (ACS),2 the US Preventive Services Task Force (USPSTF),3 and the American College of Obstetricians and Gynecologists (ACOG).4 What are the current screening recommendations, as these organizations have disparate views?
Warner Huh, MD: There was a time, around 2012-2013, when for the first time ever, we had significant harmonization of the guidelines between ACOG and the USPSTF and ACS. But in the last 10 years there has been an explosion of data in terms of how to best screen patients.
The move to primary HPV testing. The USPSTF3 initially had recommended looking into primary HPV screening, which is just using HPV testing by itself as the screening modality. But there was a lot of pushback at that time. I think a lot of people thought that we were not prepared to make that leap. Therefore, they endorsed screening with cytology by itself as well as HPV testing by itself, as well as co-testing; but in their recommendations, they made it very clear that they were leaning toward primary HPV screening.
A new patient age to begin screening. In 2020, the ACS put out their new guidelines,2 which are a significant departure from what we are used to—they are recommending that we start screening at 25 years of age. Like you said, Dr. Mutch, it doesn’t seem that long ago when we were screening people at age 18, or within 3 years of sexual intercourse. But the reason for it is that the rate of cervical cancer is extremely low under age 25, and other countries like the United Kingdom already do this.
The other major departure in the ACS guidelines is that they really are asking clinicians and screeners to focus on primary HPV screening. Overall, they have sort of doubled down on why they think primary HPV screening is so important.
ACOG sits sort of in the middle of the other recommendations. ACOG understands the value of primary HPV screening, but I don’t think that they are quite ready to recommend screening at age 25. If you look at their updated guidelines from April 2021,4 they state that we should continue a screening-starting age of 21 years. So there are some disparate views, but I am confident, Dr. Mutch, that in the next 2 to 3 years, there will be greater harmonization of these guidelines and less confusion for our providers. The greatest barrier is understanding the science and the comfort level of clinicians to go with just an HPV test, since for the last 40 years the Pap test has anchored gynecologic care in this country. And it took at least 10 years to get to what I consider to be widespread adoption to co-testing. The other thing that readers should recognize is that the Task Force is actually revisiting their cervical cancer screening guidance now, so expect another major revision.
Reimbursement and access are barriers. Reimbursement is a further real issue. We are now using one less test, but insurance companies may not reimburse when just the HPV test is used. The other issue is access to labs that can do the HPV testing.
Dr. Mutch: We used to see patients yearly and picked up a lot of adjunctive or additional illnesses. Now they are not being seen yearly it could impact negatively their overall health care. We need to understand that cervical cancer screening is simply a test, which should not eliminate other health care.
Dr. Huh: Yes, I think the extended interval between recommended HPV screenings scares people. I have been involved in these screening guidelines (and I can only speak for myself, not for my colleagues), but even I do think we made a leap to a longer interval way too quickly in this country. Screening changes are slow, and sometimes a glacial process. I think it can worry providers when we make rapid changes.
But this is a test that should not anchor the yearly visit. There are plenty of other reasons—and ACOG actually states this4—why patients should come for a wellness exam on a yearly basis. So I think our ObGyns in the United States need to recognize that, but I understand there are underlying concerns that if you extend intervals too long, (a) will patients come back, and (b), as a consequence, is the interval going to miss something in between? Those are real legitimate concerns.
Continue to: Management guidelines...
Management guidelines: The latest
Dr. Mutch: The ASCCP issued new management guidelines in 2019.5 Can you address what you feel are the most important updates?
Dr. Huh: Going back to 2002, we have revised these guidelines every 5 years. For this one, the revision came out a little bit later for various reasons, but the reason we revised it is because we collect new data that we think markedly changes our understanding of the disease process and natural history and the interventions for women that have preinvasive disease of the cervix.
Briefly, I think the biggest changes based on what we were hearing from our providers and users of our apps and algorithms was that our algorithms were becoming way too complicated, and they were. If you look over the last 10 years, the number of branch points on our algorithms basically quadrupled. If we incorporated the new data this time, the algorithms would be unworkable, and you could not use them on your phone because they would be too complicated.
So, we created a system where, in essence, providers have 5 choices for patients:
- treatment
- colposcopy
- follow-up in 1 year
- follow-up in 3 years
- follow-up in 5 years.
Those recommendations are based on what we call “clinically actionable thresholds”—basically, the percent chance of developing immediate CIN3 or worse. That threshold will probably change over time, but what we did is create a system that (a) makes it easier for the provider, (although they have to trust the system—and they can look under the hood and understand how we did this) and (b) allows us to create a foundation where we can add future technologies that use the same rubric or paradigm so that they still wind up getting the same result without having to go to another algorithm.
This new system is probably the most marked change in the history of the ASCCP management guidelines, but we did it to make it ultimately easier for providers going forward for the next 10 to 20 years. There are real opportunities, Dr. Mutch, in terms of how do we integrate this into the electronic medical record (EMR), and how do we pull data so clinicians don’t have to manually enter it.
The other difference is now there is a web-based application. Back in 2012, there were a lot of people that were not using EMRs. Now the majority of the country is, and so they actually are on a browser more than they are on their phone. We actually have an equally robust web platform that allows them to get the information that they need.
Dr. Mutch: I think that is really important—the utility of utilizing a mobile app, if you will, for triaging your patient with a specific test result so that patients are followed up at the proper interval, and that ultimately becomes cost-effective.
Dr. Huh: Yes, the app now is very different than the app that I think people are used to using for the last almost 10 years. You don’t put inputs, pull up the algorithm, and look at the outcome. This is different. You enter the patient’s age. You add their cytology, their HPV results, the clinical scenario that you are in, and then it puts out a recommendation of what to do next. Over time, we want to get away from an algorithm and for our providers to understand what the risk is and how that risk calculation then translates into a clinical recommendation.
Dr. Mutch: I think to utilize an app is almost necessary given the complexity of the triaging process so that it does become, in fact, the most cost-effective way to screen patients.
Dr. Huh: I would agree with that. There is a learning curve for whenever you see new technology. There was a learning curve for even ASCCP leadership as they tried to educate providers. I think people will ultimately see that this is a much better way of managing patients with cervical abnormalities, and I am hoping actually that we will use a similar platform for many other diseases that we manage in women’s health.
Continue to: Chipping away of the yearly exam...
Chipping away of the yearly exam
Dr. Mutch: With this moving away from the yearly exam and Pap test, women may not get yearly examinations. Do you feel that this could affect a stage migration to a higher stage at diagnosis, for instance, of a cervical cancer? Or that it might adversely impact other health issues?
Dr. Huh: I think that’s a good question. I am worried about the interval—I think 5 years is a bit long. I am more worried that patients will miss out on visits because they may think that they need to only come back for their Pap, even though they should be re-educated on that.
COVID-19 has made this a little hard for us to analyze because, clearly, we have had access to care issues. But I am a little concerned that we could see an uptick in invasive cancer rates in this country, including an uptick in the stage and more locally advanced cervical cancer because of the changes in the screening paradigms. But we don’t know that to be the case.
As with all screenings, the bottom line is you have to worry about what the false-negative and false-positive rates of screening are, and that affects everything. I want the readers to know that primary HPV should be used for screenings. It is not perfect, but it is much better than cytology alone. We need to think about how to better adapt screening in the age that we live in.
HPV self-sampling
Dr. Mutch: Could self-sampling for HPV testing, which obviously would be easier for the patient, and certainly useful in terms of screening, address some health care disparities with regard to cervical cancer?
Dr. Huh: The short answer is, yes. Self-sampling is not US Food and Drug Administration-approved in this country. It’s not being widely used without that approval. But there are multiple countries, including the United States, that have done lots of studies on this topic. There are many public health experts and champions for HPV self-sampling. I think we have learned, based on some studies, that the sensitivity is reasonable.6
I live in a part of the country that is woefully underserved; where you are there are pockets in Missouri that are woefully underserved as well. So the issue is, can we reduce these disparities and access to care with something like self-sampling? My personal feeling is I think that we can make a dent in that, and it is never going to fully replace screening, but it at least will allow us to reallocate our resources and attention to those women that are at highest risk for developing cervical cancer or precancer based on the self-sampling result.
I don’t think it will ever replace screening per se, but if we have an abnormal self-sampling test, we might say to that patient, “You really do need to come in to get re-tested or to get re-evaluated.” So it could be a better resource and use of our health care dollars and investments in terms of trying to reduce the incidence of cervical cancer. Of course the verdict is out, but I think there are a lot of people who would love to see this scenario.
If we screen and treat perfectly in this country, we would not even need the HPV vaccine when it comes to cervical cancer. That is how effective screening is. But, up to 50% to 60% of women in this country now still are underscreened or unscreened. We were talking about that number almost 25-30 years ago, Dr. Mutch. So access to screening is a big problem, but the other problem is how do you get patients in to be seen if they have an abnormal screening test? It’s not just about screening. It’s about screening, evaluation, and treatment; all 3 components are really important.
Continue to: Where do we stand with HPV vaccination?...
Where do we stand with HPV vaccination?
Dr. Mutch: Those are great points.
You brought up vaccination. We have a long way to go with regard to that, certainly in the United States, because of the various factions opposed to vaccination and so on. But do you think that vaccination has allowed us to decrease the incidence of cervical cancer?
Dr. Huh: Yes. There is clear evidence from the Nordic countries.7 There is emerging evidence from Australia.8 There is emerging evidence from other industrialized nations that clearly demonstrate vaccination’s positive effect in reducing the incidence of cervical cancer. None of this should be a surprise. Every population-based study that has been published with the HPV vaccine in populations that have a low frequency of vaccination have demonstrated substantial reductions in things like genital warts, abnormal Pap tests, precancer, and now evidence that there is a downward trend in terms of the incidence of cervical cancer.9
I don’t think that there is any debate anymore that vaccination is the way to go. Our challenge is about implementation and getting the vaccine to people. We still have a long way to go with that. There are parts of the world that are so affected by invasive cervical cancers; we need to get the vaccine to those parts of the world.
Dr. Mutch: What are the barriers to vaccination? How can we overcome those barriers?
Dr. Huh: There is a lot of criticism that we are not vaccinating more in the United States. However, the rates of vaccination are going up every single year. The pandemic may have blunted that rise a bit, but if you look at the vaccination curves, they are going up, not down. We need to continue to educate patients, parents, and pediatricians on the importance of vaccination.
Boys still get vaccinated less frequently than girls, so we have some work to do there. I think globally it is the issue of getting the vaccine to people, making sure that vaccine is available. The thing that I think will be the game-changer going forward is whether or not we will have evidence to indicate that 1 dose is as effective as 2 doses or 3 doses. If we can vaccinate boys and girls with just 1 dose, then in the next generation or two, we seriously might eradicate not just cervical cancer but a lot of HPV-related malignancies worldwide.
Educating patients, clinicians is key
Dr. Mutch: So it seems education, education, education, with regard to screening guidelines, with regard to the need for continued examinations, and that HPV testing is only a test, it does not supplant overall care. Finally, education regarding eradication of cervical cancer through vaccination.
Dr. Huh: That summarizes it well. We are still going to screen for cervical cancer. We are still going to vaccinate, and providers are still going to manage abnormal Pap tests. It is confusing because we are changing it up it seems every year or 2, so this conversation you and I are having is particularly important for clinicians to understand the basis of that. There has been an explosion of data that has come out in this area in the last decade.
Dr. Mutch: Thank you, Dr. Huh. I really appreciate your thoughts on this. As you all know, Dr. Huh has been President of the ASCCP and is instrumental in writing and disseminating these guidelines, so we are very grateful that he has consented to agree to come and talk with us today.
Dr. Huh: My pleasure. Thank you for inviting me. This was fun, and I have really enjoyed talking to you and participating. ●
- ASCCP Screening Guidelines. https://www.asccp.org /screening-guidelines. Accessed April 25, 2021.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guidelines update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force. Screening for cervical cancer. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686. doi:10.1001/jama .2018.10897.
- American College of Obstetricians and Gynecologists. Practice Advisory: Updated cervical cancer screening guidelines. April 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/04/updated-cervical -cancer-screening-guidelines. Accessed April 25, 2022.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP RiskBased Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10 34 .1097/LGT.0000000000000525.
- Yeh PT, Kennedy CE, de Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/ bmjgh-2018-001351.
- Kjaer SK, Nygard M, Dillner J, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis. 2018;66:339-345. doi: 10.1093/cid/cix797.
- Patel C, Brotherton JM, Pillsbury A, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonvalent vaccine prevent? Euro Surveill. 2018;23:1700737. doi: 10 .2807/1560-7917.
- Falcaro M, Castanon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. 2021;398:2084-2092. doi.org/10.1016/S0140-6736(21) 02178-4.
- ASCCP Screening Guidelines. https://www.asccp.org /screening-guidelines. Accessed April 25, 2021.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guidelines update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force. Screening for cervical cancer. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686. doi:10.1001/jama .2018.10897.
- American College of Obstetricians and Gynecologists. Practice Advisory: Updated cervical cancer screening guidelines. April 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/04/updated-cervical -cancer-screening-guidelines. Accessed April 25, 2022.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP RiskBased Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10 34 .1097/LGT.0000000000000525.
- Yeh PT, Kennedy CE, de Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/ bmjgh-2018-001351.
- Kjaer SK, Nygard M, Dillner J, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis. 2018;66:339-345. doi: 10.1093/cid/cix797.
- Patel C, Brotherton JM, Pillsbury A, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonvalent vaccine prevent? Euro Surveill. 2018;23:1700737. doi: 10 .2807/1560-7917.
- Falcaro M, Castanon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. 2021;398:2084-2092. doi.org/10.1016/S0140-6736(21) 02178-4.
Antibiotic treatment alone less effective in children with more appendicitis pain
Children who have greater acute appendicitis pain may be less likely to improve if they’re treated with antibiotics alone, according to a secondary analysis of a nonrandomized clinical trial.
“While approximately 35% of families chose nonoperative management, a high pain score between 7-10 on a 10-point scale nearly doubled in-hospital treatment failure,” Rebecca M. Rentea, MD, a pediatric surgeon and the director of the Comprehensive Colorectal Center at Children’s Mercy Kansas City, Mo., told this news organization in an email.
“Even if nonoperative management of pediatric appendicitis did not work – resulting in the need to remove the appendix in 34% of cases – families were happy with their decisions 1 year later,” added Dr. Rentea, who coauthored an invited commentary about the study.
Lead study author Peter C. Minneci, MD, MHSc, a pediatric surgeon at Nationwide Children’s Hospital, Columbus, Ohio, and colleagues analyzed a subgroup of patients from a larger study in 10 tertiary children’s hospitals in the Midwest Pediatric Surgery Consortium.
As they reported in JAMA Network Open, the larger prospective, nonrandomized clinical trial enrolled 1,068 children between 2015 and 2018. The children ranged in age from 7 to 17 years, and they had imaging-confirmed appendicitis with an appendix diameter of 1.1 cm or less, no abscess, no appendicolith, and no phlegmon. White blood cell count was between 5,000 and 18,000 cells/μL, and abdominal pain began less than 48 hours before they received antibiotic therapy.
Caregivers chose either surgery or nonoperative antibiotic management. Patients who were treated first with antibiotics alone and who did not undergo appendectomy within 1 year were considered to have successfully completed nonoperative treatment.
The secondary analysis included the 370 children enrolled in the nonoperative group. Of these, 229 were boys, and the median age was 12.3 years. In this subgroup, the researchers compared outcomes after nonoperative, antibiotic management vs. surgery.
At 1 year, treatment failure had occurred in 125 patients, with 53 having undergone appendectomy during their first hospitalization, and 72 having experienced delayed treatment failure after being discharged.
- Higher patient-reported pain at presentation was linked to higher risk for in-hospital treatment failure (relative risk, 2.1; 95% confidence interval, 1.0-4.4) but not for delayed treatment failure (RR, 1.3; 95% CI, 0.7-2.3) or overall treatment failure at 1 year (RR, 1.5; 95% CI, 1.0-2.2).
- Pain lasting longer than 24 hours was linked to lower risk for delayed treatment failure (RR, 0.3; 95% CI, 0.1-1.0) but not for in-hospital treatment failure (RR, 1.2; 95% CI, 0.5-2.7) or treatment failure at 1 year (RR, 0.7; 95% CI, 0.4-1.2).
- Satisfaction with the decision was higher with successful nonoperative management at 30 days (28.0 vs. 27.0; difference, 1.0; 95% CI, 0.01-2.0) and at 1 year (28.1 vs 27.0; difference, 1.1; 95% CI, 0.2-2.0).
The researchers found no increased risk for treatment failure based on age, sex, race, ethnicity, white blood cell count, primary language, insurance status, transfer status, presentation symptoms, or imaging results.
Antibiotics-only is a safe option for children
“This study suggests that pediatric patients with uncomplicated acute appendicitis should be offered treatment options, including nonoperative management,” the authors write. “Treatment with antibiotics alone is a safe and equitable option for children, with no increased risk of treatment failure based on sociodemographic or objective clinical characteristics at presentation.”
But, the authors advise: “Families need to be made aware that treatment failure is not uncommon, and they should be provided with anticipatory guidance on how to proceed should symptoms recur.”
The investigators acknowledged limitations to the study, including the nonrandomized design that may have introduced bias, the loss to follow-up, and the study population being U.S. Midwest children, who may differ from children elsewhere in the country.
Shawn D. St Peter, MD, a pediatric surgeon, medical chair, and a senior vice president at Children’s Mercy Kansas City told this news organization in an email that having a nonoperative alternative to surgical appendectomy is important.
“Antibiotics are the initial treatment for appendicitis and can be the definitive treatment,” he said.
“Surprisingly, no sociodemographic or clinical characteristics were associated with an increased risk of nonoperative appendicitis treatment failure,” added Dr. St Peter, who coauthored the commentary with Dr. Rentea.
Howard C. Jen, MD, a pediatric surgeon at University of California, Los Angeles, Mattel Children’s Hospital, was not surprised by the findings.
“Nonoperative management for acute noncomplicated appendicitis in children continues to be safe and effective in highly selected patients,” he said in an email. “This alternative to surgery should be offered routinely to patients with early acute appendicitis.”
Dr. Jen, who was not involved with the current study, noted that it did not address the impact and costs to families of nonoperative management vs. surgery.
“For the most vulnerable children who had difficulties accessing medical care, what is the best treatment option? What factors are important to the families when making this decision?” he asked.
All study and editorial authors report no relevant financial relationships. The study was funded by the Patient-Centered Outcomes Research Institute and the National Center for Advancing Translational Sciences.
A version of this article first appeared on Medscape.com.
Children who have greater acute appendicitis pain may be less likely to improve if they’re treated with antibiotics alone, according to a secondary analysis of a nonrandomized clinical trial.
“While approximately 35% of families chose nonoperative management, a high pain score between 7-10 on a 10-point scale nearly doubled in-hospital treatment failure,” Rebecca M. Rentea, MD, a pediatric surgeon and the director of the Comprehensive Colorectal Center at Children’s Mercy Kansas City, Mo., told this news organization in an email.
“Even if nonoperative management of pediatric appendicitis did not work – resulting in the need to remove the appendix in 34% of cases – families were happy with their decisions 1 year later,” added Dr. Rentea, who coauthored an invited commentary about the study.
Lead study author Peter C. Minneci, MD, MHSc, a pediatric surgeon at Nationwide Children’s Hospital, Columbus, Ohio, and colleagues analyzed a subgroup of patients from a larger study in 10 tertiary children’s hospitals in the Midwest Pediatric Surgery Consortium.
As they reported in JAMA Network Open, the larger prospective, nonrandomized clinical trial enrolled 1,068 children between 2015 and 2018. The children ranged in age from 7 to 17 years, and they had imaging-confirmed appendicitis with an appendix diameter of 1.1 cm or less, no abscess, no appendicolith, and no phlegmon. White blood cell count was between 5,000 and 18,000 cells/μL, and abdominal pain began less than 48 hours before they received antibiotic therapy.
Caregivers chose either surgery or nonoperative antibiotic management. Patients who were treated first with antibiotics alone and who did not undergo appendectomy within 1 year were considered to have successfully completed nonoperative treatment.
The secondary analysis included the 370 children enrolled in the nonoperative group. Of these, 229 were boys, and the median age was 12.3 years. In this subgroup, the researchers compared outcomes after nonoperative, antibiotic management vs. surgery.
At 1 year, treatment failure had occurred in 125 patients, with 53 having undergone appendectomy during their first hospitalization, and 72 having experienced delayed treatment failure after being discharged.
- Higher patient-reported pain at presentation was linked to higher risk for in-hospital treatment failure (relative risk, 2.1; 95% confidence interval, 1.0-4.4) but not for delayed treatment failure (RR, 1.3; 95% CI, 0.7-2.3) or overall treatment failure at 1 year (RR, 1.5; 95% CI, 1.0-2.2).
- Pain lasting longer than 24 hours was linked to lower risk for delayed treatment failure (RR, 0.3; 95% CI, 0.1-1.0) but not for in-hospital treatment failure (RR, 1.2; 95% CI, 0.5-2.7) or treatment failure at 1 year (RR, 0.7; 95% CI, 0.4-1.2).
- Satisfaction with the decision was higher with successful nonoperative management at 30 days (28.0 vs. 27.0; difference, 1.0; 95% CI, 0.01-2.0) and at 1 year (28.1 vs 27.0; difference, 1.1; 95% CI, 0.2-2.0).
The researchers found no increased risk for treatment failure based on age, sex, race, ethnicity, white blood cell count, primary language, insurance status, transfer status, presentation symptoms, or imaging results.
Antibiotics-only is a safe option for children
“This study suggests that pediatric patients with uncomplicated acute appendicitis should be offered treatment options, including nonoperative management,” the authors write. “Treatment with antibiotics alone is a safe and equitable option for children, with no increased risk of treatment failure based on sociodemographic or objective clinical characteristics at presentation.”
But, the authors advise: “Families need to be made aware that treatment failure is not uncommon, and they should be provided with anticipatory guidance on how to proceed should symptoms recur.”
The investigators acknowledged limitations to the study, including the nonrandomized design that may have introduced bias, the loss to follow-up, and the study population being U.S. Midwest children, who may differ from children elsewhere in the country.
Shawn D. St Peter, MD, a pediatric surgeon, medical chair, and a senior vice president at Children’s Mercy Kansas City told this news organization in an email that having a nonoperative alternative to surgical appendectomy is important.
“Antibiotics are the initial treatment for appendicitis and can be the definitive treatment,” he said.
“Surprisingly, no sociodemographic or clinical characteristics were associated with an increased risk of nonoperative appendicitis treatment failure,” added Dr. St Peter, who coauthored the commentary with Dr. Rentea.
Howard C. Jen, MD, a pediatric surgeon at University of California, Los Angeles, Mattel Children’s Hospital, was not surprised by the findings.
“Nonoperative management for acute noncomplicated appendicitis in children continues to be safe and effective in highly selected patients,” he said in an email. “This alternative to surgery should be offered routinely to patients with early acute appendicitis.”
Dr. Jen, who was not involved with the current study, noted that it did not address the impact and costs to families of nonoperative management vs. surgery.
“For the most vulnerable children who had difficulties accessing medical care, what is the best treatment option? What factors are important to the families when making this decision?” he asked.
All study and editorial authors report no relevant financial relationships. The study was funded by the Patient-Centered Outcomes Research Institute and the National Center for Advancing Translational Sciences.
A version of this article first appeared on Medscape.com.
Children who have greater acute appendicitis pain may be less likely to improve if they’re treated with antibiotics alone, according to a secondary analysis of a nonrandomized clinical trial.
“While approximately 35% of families chose nonoperative management, a high pain score between 7-10 on a 10-point scale nearly doubled in-hospital treatment failure,” Rebecca M. Rentea, MD, a pediatric surgeon and the director of the Comprehensive Colorectal Center at Children’s Mercy Kansas City, Mo., told this news organization in an email.
“Even if nonoperative management of pediatric appendicitis did not work – resulting in the need to remove the appendix in 34% of cases – families were happy with their decisions 1 year later,” added Dr. Rentea, who coauthored an invited commentary about the study.
Lead study author Peter C. Minneci, MD, MHSc, a pediatric surgeon at Nationwide Children’s Hospital, Columbus, Ohio, and colleagues analyzed a subgroup of patients from a larger study in 10 tertiary children’s hospitals in the Midwest Pediatric Surgery Consortium.
As they reported in JAMA Network Open, the larger prospective, nonrandomized clinical trial enrolled 1,068 children between 2015 and 2018. The children ranged in age from 7 to 17 years, and they had imaging-confirmed appendicitis with an appendix diameter of 1.1 cm or less, no abscess, no appendicolith, and no phlegmon. White blood cell count was between 5,000 and 18,000 cells/μL, and abdominal pain began less than 48 hours before they received antibiotic therapy.
Caregivers chose either surgery or nonoperative antibiotic management. Patients who were treated first with antibiotics alone and who did not undergo appendectomy within 1 year were considered to have successfully completed nonoperative treatment.
The secondary analysis included the 370 children enrolled in the nonoperative group. Of these, 229 were boys, and the median age was 12.3 years. In this subgroup, the researchers compared outcomes after nonoperative, antibiotic management vs. surgery.
At 1 year, treatment failure had occurred in 125 patients, with 53 having undergone appendectomy during their first hospitalization, and 72 having experienced delayed treatment failure after being discharged.
- Higher patient-reported pain at presentation was linked to higher risk for in-hospital treatment failure (relative risk, 2.1; 95% confidence interval, 1.0-4.4) but not for delayed treatment failure (RR, 1.3; 95% CI, 0.7-2.3) or overall treatment failure at 1 year (RR, 1.5; 95% CI, 1.0-2.2).
- Pain lasting longer than 24 hours was linked to lower risk for delayed treatment failure (RR, 0.3; 95% CI, 0.1-1.0) but not for in-hospital treatment failure (RR, 1.2; 95% CI, 0.5-2.7) or treatment failure at 1 year (RR, 0.7; 95% CI, 0.4-1.2).
- Satisfaction with the decision was higher with successful nonoperative management at 30 days (28.0 vs. 27.0; difference, 1.0; 95% CI, 0.01-2.0) and at 1 year (28.1 vs 27.0; difference, 1.1; 95% CI, 0.2-2.0).
The researchers found no increased risk for treatment failure based on age, sex, race, ethnicity, white blood cell count, primary language, insurance status, transfer status, presentation symptoms, or imaging results.
Antibiotics-only is a safe option for children
“This study suggests that pediatric patients with uncomplicated acute appendicitis should be offered treatment options, including nonoperative management,” the authors write. “Treatment with antibiotics alone is a safe and equitable option for children, with no increased risk of treatment failure based on sociodemographic or objective clinical characteristics at presentation.”
But, the authors advise: “Families need to be made aware that treatment failure is not uncommon, and they should be provided with anticipatory guidance on how to proceed should symptoms recur.”
The investigators acknowledged limitations to the study, including the nonrandomized design that may have introduced bias, the loss to follow-up, and the study population being U.S. Midwest children, who may differ from children elsewhere in the country.
Shawn D. St Peter, MD, a pediatric surgeon, medical chair, and a senior vice president at Children’s Mercy Kansas City told this news organization in an email that having a nonoperative alternative to surgical appendectomy is important.
“Antibiotics are the initial treatment for appendicitis and can be the definitive treatment,” he said.
“Surprisingly, no sociodemographic or clinical characteristics were associated with an increased risk of nonoperative appendicitis treatment failure,” added Dr. St Peter, who coauthored the commentary with Dr. Rentea.
Howard C. Jen, MD, a pediatric surgeon at University of California, Los Angeles, Mattel Children’s Hospital, was not surprised by the findings.
“Nonoperative management for acute noncomplicated appendicitis in children continues to be safe and effective in highly selected patients,” he said in an email. “This alternative to surgery should be offered routinely to patients with early acute appendicitis.”
Dr. Jen, who was not involved with the current study, noted that it did not address the impact and costs to families of nonoperative management vs. surgery.
“For the most vulnerable children who had difficulties accessing medical care, what is the best treatment option? What factors are important to the families when making this decision?” he asked.
All study and editorial authors report no relevant financial relationships. The study was funded by the Patient-Centered Outcomes Research Institute and the National Center for Advancing Translational Sciences.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
How are maternal and neonatal outcomes impacted by the contemporary practice of operative vaginal delivery?
Muraca GM, Boutin A, Razaz N, et al. Maternal and neonatal trauma following operative vaginal delivery. CMAJ. 2022;194:E1-E12. doi: 10.1503/cmaj.210841.
EXPERT COMMENTARY
Operative vaginal delivery is used to achieve and expedite safe vaginal birth while avoiding CD and its associated morbidities.1,2 Despite support from the American College of Obstetricians and Gynecologists (ACOG) for the use of OVD as an alternative to CD, OVD was used in only 3% of all US births in 2013, a shift from approximately 30% in 1987.1,3 Reported complications of OVD are biased by the level of experience of the operator, changes in practice, and by misinterpretation of the counterfactual.1
Outcomes of OVD should be compared with appropriate reference groups, namely, with second-stage CD births rather than with spontaneous vaginal births.4 With decreasing rates of OVD, evidence of contemporary data is needed on appropriately compared perinatal outcomes.4
Details of the study
Muraca and colleagues conducted an observational cohort study of births in Canada between 2013 and 2019 to assess the incidence of maternal and neonatal trauma following OVD. They used composites defined a priori— stratified by instrument, region, level of obstetric care, and institutional OVD volume.
Results. Among 1,326,191 live or stillbirths, 2.9% were attempted forceps deliveries and 8.4% were attempted vacuum deliveries. Following forceps delivery, the maternal trauma rate was 25.3% (95% confidence interval [CI], 24.8%–25.7%), and the neonatal trauma rate was 9.6 per 1,000 live births (95% CI, 8.6–10.6). Following vacuum delivery, maternal and neonatal trauma rates were 13.2% (95% CI, 13.0%–13.4%) and 9.6 per 1,000 live births (95% CI, 9.0–10.2), respectively. Maternal trauma was driven by higher order perineal lacerations. Some association was seen between increased forceps volume and decreased maternal trauma rates.
The authors concluded that in Canada, rates of maternal and neonatal trauma following OVD are higher than previously reported in consensus statements.
Study strengths and limitations
This large contemporary study uniquely stratified perinatal outcomes following OVD. The outcomes are well defined and meaningful, but some limitations affect the generalizability of the findings.
First, stillbirths were included for the maternal composite outcome, yet the incidence of this within the study population is not reported. Operative vaginal deliveries that involve stillbirths can be complex; a subgroup analysis excluding these would aid in interpretation.
Second, complicated OVDs, including sequential use of forceps and vacuum and OVDs from midpelvic station, were included; ACOG recommends against both these practices in routine circumstances due to known increases in maternal and neonatal morbidity.1 As such, the inclusion of these OVDs may bias results away from the null.
Finally, despite discussing the role of episiotomy, the episiotomy rate in this cohort is not reported.
Despite these limitations, the study by Muraca and colleagues is a positive step forward toward understanding the role of OVD in contemporary obstetric practice, and it uniquely ascertains the impact of OVD volume outcomes that previously had been an elusive exposure ●
While it is important to understand perinatal outcomes following OVD in a contemporary cohort, utilizing the correct cohort and reference group is critical.4 Risks for maternal and neonatal trauma follow OVD; however, outcomes vary based on appropriate selection of OVD candidates and adherence to recommended national guidelines.1,4 The infrequency of OVD raises concerns regarding adequate training for obstetricians, which should be prioritized so that they can offer OVD as a safe alternative to CD birth.3
HAYLEY E. MILLER, MD, AND DANIELLE M. PANELLI, MD
- American College of Obstetricians and Gynecologists. Operative vaginal birth: ACOG practice bulletin, number 219. Obstet Gynecol. 2020;135:e149-e159.
- Spong CY, Berghella V, Wenstrom KD, et al. Preventing the first cesarean delivery: summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists workshop. Obstet Gynecol. 2012;120:1181-1193.
- Zahniser SC, Kendrick JS, Franks AL, et al. Trends in obstetric operative procedures, 1980 to 1987. Am J Public Health. 1992;82:1340-1344.
- Panelli DM, Leonard SA, Joudi N, et al. Severe maternal and neonatal morbidity after attempted operative vaginal delivery. Am J Obstet Gynecol MFM. 2021;3: 100339.
Muraca GM, Boutin A, Razaz N, et al. Maternal and neonatal trauma following operative vaginal delivery. CMAJ. 2022;194:E1-E12. doi: 10.1503/cmaj.210841.
EXPERT COMMENTARY
Operative vaginal delivery is used to achieve and expedite safe vaginal birth while avoiding CD and its associated morbidities.1,2 Despite support from the American College of Obstetricians and Gynecologists (ACOG) for the use of OVD as an alternative to CD, OVD was used in only 3% of all US births in 2013, a shift from approximately 30% in 1987.1,3 Reported complications of OVD are biased by the level of experience of the operator, changes in practice, and by misinterpretation of the counterfactual.1
Outcomes of OVD should be compared with appropriate reference groups, namely, with second-stage CD births rather than with spontaneous vaginal births.4 With decreasing rates of OVD, evidence of contemporary data is needed on appropriately compared perinatal outcomes.4
Details of the study
Muraca and colleagues conducted an observational cohort study of births in Canada between 2013 and 2019 to assess the incidence of maternal and neonatal trauma following OVD. They used composites defined a priori— stratified by instrument, region, level of obstetric care, and institutional OVD volume.
Results. Among 1,326,191 live or stillbirths, 2.9% were attempted forceps deliveries and 8.4% were attempted vacuum deliveries. Following forceps delivery, the maternal trauma rate was 25.3% (95% confidence interval [CI], 24.8%–25.7%), and the neonatal trauma rate was 9.6 per 1,000 live births (95% CI, 8.6–10.6). Following vacuum delivery, maternal and neonatal trauma rates were 13.2% (95% CI, 13.0%–13.4%) and 9.6 per 1,000 live births (95% CI, 9.0–10.2), respectively. Maternal trauma was driven by higher order perineal lacerations. Some association was seen between increased forceps volume and decreased maternal trauma rates.
The authors concluded that in Canada, rates of maternal and neonatal trauma following OVD are higher than previously reported in consensus statements.
Study strengths and limitations
This large contemporary study uniquely stratified perinatal outcomes following OVD. The outcomes are well defined and meaningful, but some limitations affect the generalizability of the findings.
First, stillbirths were included for the maternal composite outcome, yet the incidence of this within the study population is not reported. Operative vaginal deliveries that involve stillbirths can be complex; a subgroup analysis excluding these would aid in interpretation.
Second, complicated OVDs, including sequential use of forceps and vacuum and OVDs from midpelvic station, were included; ACOG recommends against both these practices in routine circumstances due to known increases in maternal and neonatal morbidity.1 As such, the inclusion of these OVDs may bias results away from the null.
Finally, despite discussing the role of episiotomy, the episiotomy rate in this cohort is not reported.
Despite these limitations, the study by Muraca and colleagues is a positive step forward toward understanding the role of OVD in contemporary obstetric practice, and it uniquely ascertains the impact of OVD volume outcomes that previously had been an elusive exposure ●
While it is important to understand perinatal outcomes following OVD in a contemporary cohort, utilizing the correct cohort and reference group is critical.4 Risks for maternal and neonatal trauma follow OVD; however, outcomes vary based on appropriate selection of OVD candidates and adherence to recommended national guidelines.1,4 The infrequency of OVD raises concerns regarding adequate training for obstetricians, which should be prioritized so that they can offer OVD as a safe alternative to CD birth.3
HAYLEY E. MILLER, MD, AND DANIELLE M. PANELLI, MD
Muraca GM, Boutin A, Razaz N, et al. Maternal and neonatal trauma following operative vaginal delivery. CMAJ. 2022;194:E1-E12. doi: 10.1503/cmaj.210841.
EXPERT COMMENTARY
Operative vaginal delivery is used to achieve and expedite safe vaginal birth while avoiding CD and its associated morbidities.1,2 Despite support from the American College of Obstetricians and Gynecologists (ACOG) for the use of OVD as an alternative to CD, OVD was used in only 3% of all US births in 2013, a shift from approximately 30% in 1987.1,3 Reported complications of OVD are biased by the level of experience of the operator, changes in practice, and by misinterpretation of the counterfactual.1
Outcomes of OVD should be compared with appropriate reference groups, namely, with second-stage CD births rather than with spontaneous vaginal births.4 With decreasing rates of OVD, evidence of contemporary data is needed on appropriately compared perinatal outcomes.4
Details of the study
Muraca and colleagues conducted an observational cohort study of births in Canada between 2013 and 2019 to assess the incidence of maternal and neonatal trauma following OVD. They used composites defined a priori— stratified by instrument, region, level of obstetric care, and institutional OVD volume.
Results. Among 1,326,191 live or stillbirths, 2.9% were attempted forceps deliveries and 8.4% were attempted vacuum deliveries. Following forceps delivery, the maternal trauma rate was 25.3% (95% confidence interval [CI], 24.8%–25.7%), and the neonatal trauma rate was 9.6 per 1,000 live births (95% CI, 8.6–10.6). Following vacuum delivery, maternal and neonatal trauma rates were 13.2% (95% CI, 13.0%–13.4%) and 9.6 per 1,000 live births (95% CI, 9.0–10.2), respectively. Maternal trauma was driven by higher order perineal lacerations. Some association was seen between increased forceps volume and decreased maternal trauma rates.
The authors concluded that in Canada, rates of maternal and neonatal trauma following OVD are higher than previously reported in consensus statements.
Study strengths and limitations
This large contemporary study uniquely stratified perinatal outcomes following OVD. The outcomes are well defined and meaningful, but some limitations affect the generalizability of the findings.
First, stillbirths were included for the maternal composite outcome, yet the incidence of this within the study population is not reported. Operative vaginal deliveries that involve stillbirths can be complex; a subgroup analysis excluding these would aid in interpretation.
Second, complicated OVDs, including sequential use of forceps and vacuum and OVDs from midpelvic station, were included; ACOG recommends against both these practices in routine circumstances due to known increases in maternal and neonatal morbidity.1 As such, the inclusion of these OVDs may bias results away from the null.
Finally, despite discussing the role of episiotomy, the episiotomy rate in this cohort is not reported.
Despite these limitations, the study by Muraca and colleagues is a positive step forward toward understanding the role of OVD in contemporary obstetric practice, and it uniquely ascertains the impact of OVD volume outcomes that previously had been an elusive exposure ●
While it is important to understand perinatal outcomes following OVD in a contemporary cohort, utilizing the correct cohort and reference group is critical.4 Risks for maternal and neonatal trauma follow OVD; however, outcomes vary based on appropriate selection of OVD candidates and adherence to recommended national guidelines.1,4 The infrequency of OVD raises concerns regarding adequate training for obstetricians, which should be prioritized so that they can offer OVD as a safe alternative to CD birth.3
HAYLEY E. MILLER, MD, AND DANIELLE M. PANELLI, MD
- American College of Obstetricians and Gynecologists. Operative vaginal birth: ACOG practice bulletin, number 219. Obstet Gynecol. 2020;135:e149-e159.
- Spong CY, Berghella V, Wenstrom KD, et al. Preventing the first cesarean delivery: summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists workshop. Obstet Gynecol. 2012;120:1181-1193.
- Zahniser SC, Kendrick JS, Franks AL, et al. Trends in obstetric operative procedures, 1980 to 1987. Am J Public Health. 1992;82:1340-1344.
- Panelli DM, Leonard SA, Joudi N, et al. Severe maternal and neonatal morbidity after attempted operative vaginal delivery. Am J Obstet Gynecol MFM. 2021;3: 100339.
- American College of Obstetricians and Gynecologists. Operative vaginal birth: ACOG practice bulletin, number 219. Obstet Gynecol. 2020;135:e149-e159.
- Spong CY, Berghella V, Wenstrom KD, et al. Preventing the first cesarean delivery: summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists workshop. Obstet Gynecol. 2012;120:1181-1193.
- Zahniser SC, Kendrick JS, Franks AL, et al. Trends in obstetric operative procedures, 1980 to 1987. Am J Public Health. 1992;82:1340-1344.
- Panelli DM, Leonard SA, Joudi N, et al. Severe maternal and neonatal morbidity after attempted operative vaginal delivery. Am J Obstet Gynecol MFM. 2021;3: 100339.
Neuroimaging in the Era of Artificial Intelligence: Current Applications
Artificial intelligence (AI) in medicine has shown significant promise, particularly in neuroimaging. AI refers to computer systems designed to perform tasks that normally require human intelligence.1 Machine learning (ML), a field in which computers learn from data without being specifically programmed, is the AI subset responsible for its success in matching or even surpassing humans in certain tasks.2
Supervised learning, a subset of ML, uses an algorithm with annotated data from which to learn.3 The program will use the characteristics of a training data set to predict a specific outcome or target when exposed to a sample data set of the same type. Unsupervised learning finds naturally occurring patterns or groupings within the data.4 With deep learning (DL) algorithms, computers learn the features that optimally represent the data for the problem at hand.5 Both ML and DL are meant to emulate neural networks in the brain, giving rise to artificial neural networks composed of nodes structured within input, hidden, and output layers.
The DL neural network differs from a conventional one by having many hidden layers instead of just 1 layer that extracts patterns within the data.6 Convolutional neural networks (CNNs) are the most prevalent DL architecture used in medical imaging. CNN’s hidden layers apply convolution and pooling operations to break down an image into features containing the most valuable information. The connecting layer applies high-level reasoning before the output layer provides predictions for the image. This framework has applications within radiology, such as predicting a lesion category or condition from an image, determining whether a specific pixel belongs to background or a target class, and predicting the location of lesions.1
AI promises to increase efficiency and reduces errors. With increased data processing and image interpretation, AI technology may help radiologists improve the quality of patient care.6 This article discusses the current applications and future integration of AI in neuroradiology.
Neuroimaging Applications
AI can improve the quality of neuroimaging and reduce the clinical and systemic loads of other imaging modalities. AI can predict patient wait times for computed tomography (CT), magnetic resonance imaging (MRI), ultrasound, and X-ray imaging.7 A ML-based AI has detected the variables that most affected patient wait times, including proximity to federal holidays and severity of the patient’s condition, and calculated how long patients would be delayed after their scheduled appointment time. This AI modality could allow more efficient patient scheduling and reveal areas of patient processing that could be changed, potentially improving patient satisfaction and outcomes for time-sensitive neurologic conditions.
AI can save patient and health care practitioner time for repeat MRIs. An estimated 20% of MRI scans require a repeat series—a massive loss of time and funds for both patients and the health care system.8 A DL approach can determine whether an MRI is usable clinically or unclear enough to require repetition.9 This initial screening measure can prevent patients from making return visits and neuroradiologists from reading inconclusive images. AI offers the opportunity to reduce time and costs incurred by optimizing the health care process before imaging is obtained.
Speeding Up Neuroimaging
AI can reduce the time spent performing imaging. Because MRIs consume time and resources, compressed sensing (CS) is commonly used. CS preferentially maintains in-plane resolution at the expense of through-plane resolution to produce a scan with a single, usable viewpoint that preserves signal-to-noise ratio (SNR). CS, however, limits interpretation to single directions and can create aliasing artifacts. An AI algorithm known as synthetic multi-orientation resolution enhancement works in real time to reduce aliasing and improve resolution in these compressed scans.10 This AI improved resolution of white matter lesions in patients with multiple sclerosis (MS) on FLAIR (fluid-attenuated inversion recovery) images, and permitted multiview reconstruction from these limited scans.
Tasks of reconstructing and anti-aliasing come with high computational costs that vary inversely with the extent of scanning compression, potentially negating the time and resource savings of CS. DL AI modalities have been developed to reduce operational loads and further improve image resolution in several directions from CS. One such deep residual learning AI was trained with compressed MRIs and used the framelet method to create a CNN that could rapidly remove global and deeply coherent aliasing artifacts.11 This system, compared with synthetic multi-orientation resolution enhancement, uses a pretrained, pretested AI that does not require additional time during scanning for computational analysis, thereby multiplying the time benefit of CS while retaining the benefits of multidirectional reconstruction and increased resolution. This methodology suffers from inherent degradation of perceptual image quality in its reconstructions because of the L2 loss function the CNN uses to reduce mean squared error, which causes blurring by averaging all possible outcomes of signal distribution during reconstruction. To combat this, researchers have developed another AI to reduce reconstruction times that uses a different loss function in a generative adversarial network to retain image quality, while offering reconstruction times several hundred times faster than current CS-MRI structures.12 So-called sparse-coding methods promise further reduction in reconstruction times, with the possibility of processing completed online with a lightweight architecture rather than on a local system.13
Neuroimaging of acute cases benefits most directly from these technologies because MRIs and their high resolution and SNR begin to approach CT imaging time scales. This could have important implications in clinical care, particularly for stroke imaging and evaluating spinal cord compression. CS-MRI optimization represents one of the greatest areas of neuroimaging cost savings and neurologic care improvement in the modern radiology era.
Reducing Contrast and Radiation Doses
AI has the ability to read CT, MRI, and positron emission tomography (PET) with reduced or without contrast without significant loss in sensitivity for detecting lesions. With MRI, gadolinium-based contrast can cause injection site reactions, allergic reactions, metal deposition throughout the body, and nephrogenic systemic fibrosis in the most severe instances.14 DL has been applied to brain MRIs performed with 10% of a full dose of contrast without significant degradation of image quality. Neuroradiologists did not rate the AI-synthesized images for several MRI indications lower than their full-dose counterparts.15 Low-dose contrast imaging, regardless of modality, generates greater noise with a significantly reduced signal. However, with AI applied, researchers found that the software suppressed motion and aliasing artifacts and improved image quality, perhaps evidence that this low-dose modality is less vulnerable to the most common pitfalls of MRI.
Recently, low-dose MRI moved into the spotlight when Subtle Medical SubtleGAD software received a National Institutes of Health grant and an expedited pathway to phase 2 clinical trials.16 SubtleGAD, a DL AI that enables low-dose MRI interpretation, might allow contrast MRI for patients with advanced kidney disease or contrast allergies. At some point, contrast with MRI might not be necessary because DL AI applied to noncontrast MRs for detecting MS lesions was found to be preliminarily effective with 78% lesion detection sensitivity.17
PET-MRI combines simultaneous PET and MRI and has been used to evaluate neurologic disorders. PET-MRI can detect amyloid plaques in Alzheimer disease 10 to 20 years before clinical signs of dementia emerge.18 PET-MRI has sparked DL AI development to decrease the dose of the IV radioactive tracer 18F-florbetaben used in imaging to reduce radiation exposure and imaging costs.This reduction is critical if PET-MRI is to become used widely.19-21
An initial CNN could reconstruct low-dose amyloid scans to full-dose resolution, albeit with a greater susceptibility to some artifacts and motion blurring.22 Similar to the synthetic multi-orientation resolution enhancement CNN, this program showed signal blurring from the L2 loss function, which was corrected in a later AI that used a generative adversarial network to minimize perceptual loss.23 This new AI demonstrated greater image resolution, feature preservation, and radiologist rating over the previous AI and was capable of reconstructing low-dose PET scans to full-dose resolution without an accompanying MRI. Applications of this algorithm are far-reaching, potentially allowing neuroimaging of brain tumors at more frequent intervals with higher resolution and lower total radiation exposure.
AI also has been applied to neurologic CT to reduce radiation exposure.24 Because it is critical to abide by the principles of ALARA (as low as reasonably achievable), the ability of AI to reduce radiation exposure holds significant promise. A CNN has been used to transform low-dose CTs of anthropomorphic models with calcium inserts and cardiac patients to normal-dose CTs, with the goal of improving the SNR.25 By training a noise-discriminating CNN and a noise-generating CNN together in a generative adversarial network, the AI improved image feature preservation during transformation. This algorithm has a direct application in imaging cerebral vasculature, including calcification that can explain lacunar infarcts and tracking systemic atherosclerosis.26
Another CNN has been applied to remove more complex noise patterns from the phenomena of beam hardening and photon starvation common in low-dose CT. This algorithm extracts the directional components of artifacts and compares them to known artifact patterns, allowing for highly specific suppression of unwanted signals.27 In June 2019, the US Food and Drug Administration (FDA) approved ClariPi, a deep CNN program for advanced denoising and resolution improvement of low- and ultra low-dose CTs.28 Aside from only low-dose settings, this AI could reduce artifacts in all CT imaging modalities and improve therapeutic value of procedures, including cerebral angiograms and emergency cranial scans. As the average CT radiation dose decreased from 12 mSv in 2009 to 1.5 mSv in 2014 and continues to fall, these algorithms will become increasingly necessary to retain the high resolution and diagnostic power expected of neurologic CTs.29,30
Downstream Applications
Downstream applications refer to AI use after a radiologic study is acquired, mostly image interpretation. More than 70% of FDA-approved AI medical devices are in radiology, and many of these relate to image analysis.6,31 Although AI is not limited to black-and-white image interpretation, it is hypothesized that one of the reasons radiology is inviting to AI is because gray-scale images lend themselves to standardization.3 Moreover, most radiology departments already use AI-friendly picture archiving and communication systems.31,32
AI has been applied to a range of radiologic modalities, including MRI, CT, ultrasonography, PET, and mammography.32-38 AI also has been specifically applied to radiography, including the interpretation of tuberculosis, pneumonia, lung lesions, and COVID-19.33,39-45 AI also can assist triage, patient screening, providing a “second opinion” rapidly, shortening the time needed for attaining a diagnosis, monitoring disease progression, and predicting prognosis.37-39,43,45-47 Downstream applications of AI in neuroradiology and neurology include using CT to aid in detecting hemorrhage or ischemic stroke; using MRI to automatically segment lesions, such as tumors or MS lesions; assisting in early diagnosis and predicting prognosis in MS; assisting in treating paralysis, including from spinal cord injury; determining seizure type and localizing area of seizure onset; and using cameras, wearable devices, and smartphone applications to diagnose and assess treatment response in neurodegenerative disorders, such as Parkinson or Alzheimer diseases (Figure).37,48-56
Several AI tools have been deployed in the clinical setting, particularly triaging intracranial hemorrhage and moving these studies to the top of the radiologist’s worklist. In 2020 the Centers for Medicare and Medicaid Services (CMS) began reimbursing Viz.ai software’s AI-based Viz ContaCT (Viz LVO) with a new International Statistical Classification of Diseases, Tenth Revision procedure code.57
Viz LVO automatically detects large vessel occlusions, flags the occlusion on CT angiogram, alerts the stroke team (interventional radiologist, neuroradiologist, and neurologist), and transmits images through a secure application to the stroke team members’ mobile devices—all in less than 6 minutes from study acquisition to alarm notification.48 Additional software can quantify and measure perfusion in affected brain areas.48 This could have implications for quantifying and targeting areas of ischemic penumbra that could be salvaged after a stroke and then using that information to plan targeted treatment and/or intervention. Because many trials (DAWN/DEFUSE3) have shown benefits in stroke outcome by extending the therapeutic window for the endovascular thrombectomy, the ability to identify appropriate candidates is essential.58,59 Development of AI tools in assessing ischemic penumbra with quantitative parameters (mean transit time, cerebral blood volume, cerebral blood flow, mismatch ratio) using AI has benefited image interpretation. Medtronic RAPID software can provide quantitative assessment of CT perfusion. AI tools could be used to provide an automatic ASPECT score, which provides a quantitative measure for assessing potential ischemic zones and aids in assessing appropriate candidates for thrombectomy.
Several FDA-approved AI tools help quantify brain structures in neuroradiology, including quantitative analysis through MRI for analysis of anatomy and PET for analysis of functional uptake, assisting in more accurate and more objective detection and monitoring of conditions such as atrophy, dementia, trauma, seizure disorders, and MS.48 The growing number of FDA-approved AI technologies and the recent CMS-approved reimbursement for an AI tool indicate a changing landscape that is more accepting of downstream applications of AI in neuroradiology. As AI continues to integrate into medical regulation and finance, we predict AI will continue to play a prominent role in neuroradiology.
Practical and Ethical Considerations
In any discussion of the benefits of AI, it is prudent to address its shortcomings. Chief among these is overfitting, which occurs when an AI is too closely aligned with its training dataset and prone to error when applied to novel cases. Often this is a byproduct of a small training set.60 Neuroradiology, particularly with uncommon, advanced imaging methods, has a smaller number of available studies.61 Even with more prevalent imaging modalities, such as head CT, the work of collecting training scans from patients with the prerequisite disease processes, particularly if these processes are rare, can limit the number of datapoints collected. Neuroradiologists should understand how an AI tool was generated, including the size and variety of the training dataset used, to best gauge the clinical applicability and fitness of the system.
Another point of concern for AI clinical decision support tools’ implementation is automation bias—the tendency for clinicians to favor machine-generated decisions and ignore contrary data or conflicting human decisions.62 This situation often arises when radiologists experience overwhelming patient loads or are in underresourced settings, where there is little ability to review every AI-based diagnosis. Although AI might be of benefit in such conditions by reducing physician workload and streamlining the diagnostic process, there is the propensity to improperly rely on a tool meant to augment, not replace, a radiologist’s judgment. Such cases have led to adverse outcomes for patients, and legal precedence shows that this constitutes negligence.63 Maintaining awareness of each tool’s limitations and proper application is the only remedy for such situations.
Ethically, we must consider the opaqueness of ML-developed neuroimaging AIs. For many systems, the specific process by which an AI arrives at its conclusions is unknown. This AI “black box” can conceal potential errors and biases that are masked by overall positive performance metrics. The lack of understanding about how a tool functions in the zero-failure clinical setting understandably gives radiologists pause. The question must be asked: Is it ethical to use a system that is a relatively unknown quantity? Entities, including state governments, Canada, and the European Union, have produced an answer. Each of these governments have implemented policies requiring that health care AIs use some method to display to end users the process by which they arrive at conclusions.64-68
The 21st Century Cures Act declares that to attain approval, clinical AIs must demonstrate this explainability to clinicians and patients.69 The response has been an explosion in the development of explainable AI. Systems that visualize the areas where AI attention most often rests with heatmaps, generate labels for the most heavily weighted features of radiographic images, and create full diagnostic reports to justify AI conclusions aim to meet the goal of transparency and inspiring confidence in clinical end users.70 The ability to understand the “thought process” of a system proves useful for error correction and retooling. A trend toward under- or overdetecting conditions, flagging seemingly irrelevant image regions, or low reproducibility can be better addressed when it is clear how the AI is drawing its false conclusions. With an iterative process of testing and redesigning, false positive and negative rates can be reduced, the need for human intervention can be lowered to an appropriate minimum, and patient outcomes can be improved.71
Data collection raises another ethical concern. To train functional clinical decision support tools, massive amounts of patient demographic, laboratory, and imaging data are required. With incentives to develop the most powerful AI systems, record collection can venture down a path where patient autonomy and privacy are threatened. Radiologists have a duty to ensure data mining serves patients and improves the practice of radiology while protecting patients’ personal information.62 Policies have placed similar limits on the access to and use of patient records.64-69 Patients have the right to request explanation of the AI systems their data have been used to train. Approval for data acquisition requires the use of explainable AI, standardized data security protocol implementation, and adequate proof of communal benefit from the clinical decision support tool. Establishment of state-mandated protections bodes well for a future when developers can access enormous caches of data while patients and health care professionals are assured that no identifying information has escaped a well-regulated space. On the level of the individual radiologist, the knowledge that each datum represents a human life. These are people who has made themselves vulnerable by seeking relief for what ails them, which should serve as a lasting reminder to operate with utmost care when handling sensitive information.
Conclusions
The demonstrated applications of AI in neuroimaging are numerous and varied, and it is reasonable to assume that its implementation will increase as the technology matures. AI use for detecting important neurologic conditions holds promise in combatting ever greater imaging volumes and providing timely diagnoses. As medicine witnesses the continuing adoption of AI, it is important that practitioners possess an understanding of its current and emerging uses.
1. Chartrand G, Cheng PM, Vorontsov E, et al. Deep learning: a primer for radiologists. Radiographics. 2017;37(7):2113-2131. doi:10.1148/rg.2017170077
2. King BF Jr. Guest editorial: discovery and artificial intelligence. AJR Am J Roentgenol. 2017;209(6):1189-1190. doi:10.2214/AJR.17.19178
3. Syed AB, Zoga AC. Artificial intelligence in radiology: current technology and future directions. Semin Musculoskelet Radiol. 2018;22(5):540-545. doi:10.1055/s-0038-1673383
4. Deo RC. Machine learning in medicine. Circulation. 2015;132(20):1920-1930. doi:10.1161/CIRCULATIONAHA.115.001593 5. Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60-88. doi:10.1016/j.media.2017.07.005
6. Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2(1):35. doi:10.1186/s41747-018-0061-6
7. Curtis C, Liu C, Bollerman TJ, Pianykh OS. Machine learning for predicting patient wait times and appointment delays. J Am Coll Radiol. 2018;15(9):1310-1316. doi:10.1016/j.jacr.2017.08.021
8. Andre JB, Bresnahan BW, Mossa-Basha M, et al. Toward quantifying the prevalence, severity, and cost associated with patient motion during clinical MR examinations. J Am Coll Radiol. 2015;12(7):689-695. doi:10.1016/j.jacr.2015.03.007
9. Sreekumari A, Shanbhag D, Yeo D, et al. A deep learning-based approach to reduce rescan and recall rates in clinical MRI examinations. AJNR Am J Neuroradiol. 2019;40(2):217-223. doi:10.3174/ajnr.A5926
10. Zhao C, Shao M, Carass A, et al. Applications of a deep learning method for anti-aliasing and super-resolution in MRI. Magn Reson Imaging. 2019;64:132-141. doi:10.1016/j.mri.2019.05.038
11. Lee D, Yoo J, Tak S, Ye JC. Deep residual learning for accelerated MRI using magnitude and phase networks. IEEE Trans Biomed Eng. 2018;65(9):1985-1995. doi:10.1109/TBME.2018.2821699
12. Mardani M, Gong E, Cheng JY, et al. Deep generative adversarial neural networks for compressive sensing MRI. IEEE Trans Med Imaging. 2019;38(1):167-179. doi:10.1109/TMI.2018.2858752
13. Dong C, Loy CC, He K, Tang X. Image super-resolution using deep convolutional networks. IEEE Trans Pattern Anal Mach Intell. 2016;38(2):295-307. doi:10.1109/TPAMI.2015.2439281
14. Sammet S. Magnetic resonance safety. Abdom Radiol (NY). 2016;41(3):444-451. doi:10.1007/s00261-016-0680-4
15. Gong E, Pauly JM, Wintermark M, Zaharchuk G. Deep learning enables reduced gadolinium dose for contrast-enhanced brain MRI. J Magn Reson Imaging. 2018;48(2):330-340. doi:10.1002/jmri.25970
16. Subtle Medical NIH awards Subtle Medical, Inc. $1.6 million grant to improve safety of MRI exams by reducing gadolinium dose using AI. Press release. September 18, 2019. Accessed March 14, 2022. https://www.biospace.com/article/releases/nih-awards-subtle-medical-inc-1-6-million-grant-to-improve-safety-of-mri-exams-by-reducing-gadolinium-dose-using-ai
17. Narayana PA, Coronado I, Sujit SJ, Wolinsky JS, Lublin FD, Gabr RE. Deep learning for predicting enhancing lesions in multiple sclerosis from noncontrast MRI. Radiology. 2020;294(2):398-404. doi:10.1148/radiol.2019191061
18. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128. doi:10.1016/S1474-4422(09)70299-6
19. Gatidis S, Würslin C, Seith F, et al. Towards tracer dose reduction in PET studies: simulation of dose reduction by retrospective randomized undersampling of list-mode data. Hell J Nucl Med. 2016;19(1):15-18. doi:10.1967/s002449910333
20. Kaplan S, Zhu YM. Full-dose PET image estimation from low-dose PET image using deep learning: a pilot study. J Digit Imaging. 2019;32(5):773-778. doi:10.1007/s10278-018-0150-3
21. Xu J, Gong E, Pauly J, Zaharchuk G. 200x low-dose PET reconstruction using deep learning. arXiv: 1712.04119. Accessed 2/16/2022. https://arxiv.org/pdf/1712.04119.pdf
22. Chen KT, Gong E, de Carvalho Macruz FB, et al. Ultra-low-dose 18F-florbetaben amyloid PET imaging using deep learning with multi-contrast MRI inputs. Radiology. 2019;290(3):649-656. doi:10.1148/radiol.2018180940
23. Ouyang J, Chen KT, Gong E, Pauly J, Zaharchuk G. Ultra-low-dose PET reconstruction using generative adversarial network with feature matching and task-specific perceptual loss. Med Phys. 2019;46(8):3555-3564. doi:10.1002/mp.13626
24. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi:10.1056/NEJMra072149
25. Wolterink JM, Leiner T, Viergever MA, Isgum I. Generative adversarial networks for noise reduction in low-dose CT. IEEE Trans Med Imaging. 2017;36(12):2536-2545. doi:10.1109/TMI.2017.2708987
26. Sohn YH, Cheon HY, Jeon P, Kang SY. Clinical implication of cerebral artery calcification on brain CT. Cerebrovasc Dis. 2004;18(4):332-337. doi:10.1159/000080772
27. Kang E, Min J, Ye JC. A deep convolutional neural network using directional wavelets for low-dose X-ray CT reconstruction. Med Phys. 2017;44(10):e360-e375. doi:10.1002/mp.12344
28. ClariPi gets FDA clearance for AI-powered CT image denoising solution. Published June 24, 2019. Accessed February 16, 2022. https://www.itnonline.com/content/claripi-gets-fda-clearance-ai-powered-ct-image-denoising-solution
29. Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301(5):500-507. doi:10.1001/jama.2009.54
30. Al-Mallah M, Aljizeeri A, Alharthi M, Alsaileek A. Routine low-radiation-dose coronary computed tomography angiography. Eur Heart J Suppl. 2014;16(suppl B):B12-B16. doi:10.1093/eurheartj/suu024
31. Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med. 2020;3:118. doi:10.1038/s41746-020-00324-0
32. Talebi-Liasi F, Markowitz O. Is artificial intelligence going to replace dermatologists? Cutis. 2020;105(1):28-31.
33. Khan O, Bebb G, Alimohamed NA. Artificial intelligence in medicine: what oncologists need to know about its potential—and its limitations. Oncology Exchange. 2017;16(4):8-13. http://www.oncologyex.com/pdf/vol16_no4/feature_khan-ai.pdf
34. Liu X, Faes L, Kale AU, et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit Health. 2019;1(6):e271-e297. doi:10.1016/S2589-7500(19)30123-2
35. Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44-56. doi:10.1038/s41591-018-0300-7
36. Salim M, Wåhlin E, Dembrower K, et al. External evaluation of 3 commercial artificial intelligence algorithms for independent assessment of screening mammograms. JAMA Oncol. 2020;6(10):1581-1588. doi:10.1001/jamaoncol.2020.3321
37. Arbabshirani MR, Fornwalt BK, Mongelluzzo GJ, et al. Advanced machine learning in action: identification of intracranial hemorrhage on computed tomography scans of the head with clinical workflow integration. NPJ Digit Med. 2018;1(1):1-7. doi:10.1038/s41746-017-0015-z
38. Sheth D, Giger ML. Artificial intelligence in the interpretation of breast cancer on MRI. J Magn Reson Imaging. 2020;51(5):1310-1324. doi:10.1002/jmri.26878
39. Borkowski AA, Viswanadhan NA, Thomas LB, Guzman RD, Deland LA, Mastorides SM. Using artificial intelligence for COVID-19 chest X-ray diagnosis. Fed Pract. 2020;37(9):398-404. doi:10.12788/fp.0045
40. Kermany DS, Goldbaum M, Cai W, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172(5):1122-1131.e9. doi:10.1016/j.cell.2018.02.010
41. Nam JG, Park S, Hwang EJ, et al. Development and validation of deep learning-based automatic detection algorithm for malignant pulmonary nodules on chest radiographs. Radiology. 2019;290(1):218-228. doi:10.1148/radiol.2018180237
42. Zech JR, Badgeley MA, Liu M, Costa AB, Titano JJ, Oermann EK. Variable generalization performance of a deep learning model to detect pneumonia in chest radiographs: a cross-sectional study. PLoS Med. 2018;15(11):e1002683. doi:10.1371/journal.pmed.1002683
43. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582. doi:10.1148/radiol.2017162326
44. Rajpurkar P, Joshi A, Pareek A, et al. CheXpedition: investigating generalization challenges for translation of chest X-Ray algorithms to the clinical setting. arXiv preprint arXiv:200211379. Accessed February 16, 2022. https://arxiv.org/pdf/2002.11379.pdf
45. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25(1):30-36. doi:10.1038/s41591-018-0307-0
46. Meyer-Bäse A, Morra L, Meyer-Bäse U, Pinker K. Current status and future perspectives of artificial intelligence in magnetic resonance breast imaging. Contrast Media Mol Imaging. 2020;2020:6805710. doi:10.1155/2020/6805710
47. Booth AL, Abels E, McCaffrey P. Development of a prognostic model for mortality in COVID-19 infection using machine learning. Mod Pathol. 2020;4(3):522-531. doi:10.1038/s41379-020-00700-x
48. Bash S. Enhancing neuroimaging with artificial intelligence. Applied Radiology. 2020;49(1):20-21.
49. Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243. doi:10.1136/svn-2017-000101
50. Valliani AA, Ranti D, Oermann EK. Deep learning and neurology: a systematic review. Neurol Ther. 2019;8(2):351-365. doi:10.1007/s40120-019-00153-8
51. Gupta R, Krishnam SP, Schaefer PW, Lev MH, Gonzalez RG. An east coast perspective on artificial intelligence and machine learning: part 2: ischemic stroke imaging and triage. Neuroimaging Clin N Am. 2020;30(4):467-478. doi:10.1016/j.nic.2020.08.002
52. Belić M, Bobić V, Badža M, Šolaja N, Đurić-Jovičić M, Kostić VS. Artificial intelligence for assisting diagnostics and assessment of Parkinson’s disease-A review. Clin Neurol Neurosurg. 2019;184:105442. doi:10.1016/j.clineuro.2019.105442
53. An S, Kang C, Lee HW. Artificial intelligence and computational approaches for epilepsy. J Epilepsy Res. 2020;10(1):8-17. doi:10.14581/jer.20003
54. Pavel AM, Rennie JM, de Vries LS, et al. A machine-learning algorithm for neonatal seizure recognition: a multicentre, randomised, controlled trial. Lancet Child Adolesc Health. 2020;4(10):740-749. doi:10.1016/S2352-4642(20)30239-X
55. Afzal HMR, Luo S, Ramadan S, Lechner-Scott J. The emerging role of artificial intelligence in multiple sclerosis imaging. Mult Scler. 2020;1352458520966298. doi:10.1177/1352458520966298
56. Bouton CE. Restoring movement in paralysis with a bioelectronic neural bypass approach: current state and future directions. Cold Spring Harb Perspect Med. 2019;9(11):a034306. doi:10.1101/cshperspect.a034306
57. Hassan AE. New technology add-on payment (NTAP) for Viz LVO: a win for stroke care. J Neurointerv Surg. 2020;neurintsurg-2020-016897. doi:10.1136/neurintsurg-2020-016897
58. Nogueira RG , Jadhav AP , Haussen DC , et al; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi:10.1056/NEJMoa1706442
59. Albers GW , Marks MP , Kemp S , et al; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–18. doi:10.1056/NEJMoa1713973
60. Bi WL, Hosny A, Schabath MB, et al. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin. 2019;69(2):127-157. doi:10.3322/caac.21552
61. Wagner MW, Namdar K, Biswas A, Monah S, Khalvati F, Ertl-Wagner BB. Radiomics, machine learning, and artificial intelligence-what the neuroradiologist needs to know. Neuroradiology. 2021;63(12):1957-1967. doi:10.1007/s00234-021-02813-9
62. Geis JR, Brady AP, Wu CC, et al. Ethics of artificial intelligence in radiology: summary of the Joint European and North American Multisociety Statement. J Am Coll Radiol. 2019;16(11):1516-1521. doi:10.1016/j.jacr.2019.07.028
63. Kingston J. Artificial intelligence and legal liability. arXiv:1802.07782. https://arxiv.org/ftp/arxiv/papers/1802/1802.07782.pdf
64. Council of the European Union, General Data Protection Regulation. Official Journal of the European Union. Accessed February 16, 2022. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0679
65. Consumer Privacy Protection Act of 2017, HR 4081, 115th Cong (2017). Accessed February 10, 2022. https://www.congress.gov/bill/115th-congress/house-bill/4081
66. Cal. Civ. Code § 1798.198(a) (2018). California Consumer Privacy Act of 2018.
67. Va. Code Ann. § 59.1 (2021). Consumer Data Protection Act. Accessed February 10, 2022. https://lis.virginia.gov/cgi-bin/legp604.exe?212+ful+SB1392ER+pdf
68. Colo. Rev. Stat. § 6-1-1301 (2021). Colorado Privacy Act. Accessed February 10, 2022. https://leg.colorado.gov/sites/default/files/2021a_190_signed.pdf
69. 21st Century Cures Act, Pub L No. 114-255 (2016). Accessed February 10, 2022. https://www.govinfo.gov/content/pkg/PLAW-114publ255/html/PLAW-114publ255.htm
70. Huff DT, Weisman AJ, Jeraj R. Interpretation and visualization techniques for deep learning models in medical imaging. Phys Med Biol. 2021;66(4):04TR01. doi:10.1088/1361-6560/abcd17
71. Thrall JH, Li X, Li Q, et al. Artificial intelligence and machine learning in radiology: opportunities, challenges, pitfalls, and criteria for success. J Am Coll Radiol. 2018;15(3, pt B):504-508. doi:10.1016/j.jacr.2017.12.026
Artificial intelligence (AI) in medicine has shown significant promise, particularly in neuroimaging. AI refers to computer systems designed to perform tasks that normally require human intelligence.1 Machine learning (ML), a field in which computers learn from data without being specifically programmed, is the AI subset responsible for its success in matching or even surpassing humans in certain tasks.2
Supervised learning, a subset of ML, uses an algorithm with annotated data from which to learn.3 The program will use the characteristics of a training data set to predict a specific outcome or target when exposed to a sample data set of the same type. Unsupervised learning finds naturally occurring patterns or groupings within the data.4 With deep learning (DL) algorithms, computers learn the features that optimally represent the data for the problem at hand.5 Both ML and DL are meant to emulate neural networks in the brain, giving rise to artificial neural networks composed of nodes structured within input, hidden, and output layers.
The DL neural network differs from a conventional one by having many hidden layers instead of just 1 layer that extracts patterns within the data.6 Convolutional neural networks (CNNs) are the most prevalent DL architecture used in medical imaging. CNN’s hidden layers apply convolution and pooling operations to break down an image into features containing the most valuable information. The connecting layer applies high-level reasoning before the output layer provides predictions for the image. This framework has applications within radiology, such as predicting a lesion category or condition from an image, determining whether a specific pixel belongs to background or a target class, and predicting the location of lesions.1
AI promises to increase efficiency and reduces errors. With increased data processing and image interpretation, AI technology may help radiologists improve the quality of patient care.6 This article discusses the current applications and future integration of AI in neuroradiology.
Neuroimaging Applications
AI can improve the quality of neuroimaging and reduce the clinical and systemic loads of other imaging modalities. AI can predict patient wait times for computed tomography (CT), magnetic resonance imaging (MRI), ultrasound, and X-ray imaging.7 A ML-based AI has detected the variables that most affected patient wait times, including proximity to federal holidays and severity of the patient’s condition, and calculated how long patients would be delayed after their scheduled appointment time. This AI modality could allow more efficient patient scheduling and reveal areas of patient processing that could be changed, potentially improving patient satisfaction and outcomes for time-sensitive neurologic conditions.
AI can save patient and health care practitioner time for repeat MRIs. An estimated 20% of MRI scans require a repeat series—a massive loss of time and funds for both patients and the health care system.8 A DL approach can determine whether an MRI is usable clinically or unclear enough to require repetition.9 This initial screening measure can prevent patients from making return visits and neuroradiologists from reading inconclusive images. AI offers the opportunity to reduce time and costs incurred by optimizing the health care process before imaging is obtained.
Speeding Up Neuroimaging
AI can reduce the time spent performing imaging. Because MRIs consume time and resources, compressed sensing (CS) is commonly used. CS preferentially maintains in-plane resolution at the expense of through-plane resolution to produce a scan with a single, usable viewpoint that preserves signal-to-noise ratio (SNR). CS, however, limits interpretation to single directions and can create aliasing artifacts. An AI algorithm known as synthetic multi-orientation resolution enhancement works in real time to reduce aliasing and improve resolution in these compressed scans.10 This AI improved resolution of white matter lesions in patients with multiple sclerosis (MS) on FLAIR (fluid-attenuated inversion recovery) images, and permitted multiview reconstruction from these limited scans.
Tasks of reconstructing and anti-aliasing come with high computational costs that vary inversely with the extent of scanning compression, potentially negating the time and resource savings of CS. DL AI modalities have been developed to reduce operational loads and further improve image resolution in several directions from CS. One such deep residual learning AI was trained with compressed MRIs and used the framelet method to create a CNN that could rapidly remove global and deeply coherent aliasing artifacts.11 This system, compared with synthetic multi-orientation resolution enhancement, uses a pretrained, pretested AI that does not require additional time during scanning for computational analysis, thereby multiplying the time benefit of CS while retaining the benefits of multidirectional reconstruction and increased resolution. This methodology suffers from inherent degradation of perceptual image quality in its reconstructions because of the L2 loss function the CNN uses to reduce mean squared error, which causes blurring by averaging all possible outcomes of signal distribution during reconstruction. To combat this, researchers have developed another AI to reduce reconstruction times that uses a different loss function in a generative adversarial network to retain image quality, while offering reconstruction times several hundred times faster than current CS-MRI structures.12 So-called sparse-coding methods promise further reduction in reconstruction times, with the possibility of processing completed online with a lightweight architecture rather than on a local system.13
Neuroimaging of acute cases benefits most directly from these technologies because MRIs and their high resolution and SNR begin to approach CT imaging time scales. This could have important implications in clinical care, particularly for stroke imaging and evaluating spinal cord compression. CS-MRI optimization represents one of the greatest areas of neuroimaging cost savings and neurologic care improvement in the modern radiology era.
Reducing Contrast and Radiation Doses
AI has the ability to read CT, MRI, and positron emission tomography (PET) with reduced or without contrast without significant loss in sensitivity for detecting lesions. With MRI, gadolinium-based contrast can cause injection site reactions, allergic reactions, metal deposition throughout the body, and nephrogenic systemic fibrosis in the most severe instances.14 DL has been applied to brain MRIs performed with 10% of a full dose of contrast without significant degradation of image quality. Neuroradiologists did not rate the AI-synthesized images for several MRI indications lower than their full-dose counterparts.15 Low-dose contrast imaging, regardless of modality, generates greater noise with a significantly reduced signal. However, with AI applied, researchers found that the software suppressed motion and aliasing artifacts and improved image quality, perhaps evidence that this low-dose modality is less vulnerable to the most common pitfalls of MRI.
Recently, low-dose MRI moved into the spotlight when Subtle Medical SubtleGAD software received a National Institutes of Health grant and an expedited pathway to phase 2 clinical trials.16 SubtleGAD, a DL AI that enables low-dose MRI interpretation, might allow contrast MRI for patients with advanced kidney disease or contrast allergies. At some point, contrast with MRI might not be necessary because DL AI applied to noncontrast MRs for detecting MS lesions was found to be preliminarily effective with 78% lesion detection sensitivity.17
PET-MRI combines simultaneous PET and MRI and has been used to evaluate neurologic disorders. PET-MRI can detect amyloid plaques in Alzheimer disease 10 to 20 years before clinical signs of dementia emerge.18 PET-MRI has sparked DL AI development to decrease the dose of the IV radioactive tracer 18F-florbetaben used in imaging to reduce radiation exposure and imaging costs.This reduction is critical if PET-MRI is to become used widely.19-21
An initial CNN could reconstruct low-dose amyloid scans to full-dose resolution, albeit with a greater susceptibility to some artifacts and motion blurring.22 Similar to the synthetic multi-orientation resolution enhancement CNN, this program showed signal blurring from the L2 loss function, which was corrected in a later AI that used a generative adversarial network to minimize perceptual loss.23 This new AI demonstrated greater image resolution, feature preservation, and radiologist rating over the previous AI and was capable of reconstructing low-dose PET scans to full-dose resolution without an accompanying MRI. Applications of this algorithm are far-reaching, potentially allowing neuroimaging of brain tumors at more frequent intervals with higher resolution and lower total radiation exposure.
AI also has been applied to neurologic CT to reduce radiation exposure.24 Because it is critical to abide by the principles of ALARA (as low as reasonably achievable), the ability of AI to reduce radiation exposure holds significant promise. A CNN has been used to transform low-dose CTs of anthropomorphic models with calcium inserts and cardiac patients to normal-dose CTs, with the goal of improving the SNR.25 By training a noise-discriminating CNN and a noise-generating CNN together in a generative adversarial network, the AI improved image feature preservation during transformation. This algorithm has a direct application in imaging cerebral vasculature, including calcification that can explain lacunar infarcts and tracking systemic atherosclerosis.26
Another CNN has been applied to remove more complex noise patterns from the phenomena of beam hardening and photon starvation common in low-dose CT. This algorithm extracts the directional components of artifacts and compares them to known artifact patterns, allowing for highly specific suppression of unwanted signals.27 In June 2019, the US Food and Drug Administration (FDA) approved ClariPi, a deep CNN program for advanced denoising and resolution improvement of low- and ultra low-dose CTs.28 Aside from only low-dose settings, this AI could reduce artifacts in all CT imaging modalities and improve therapeutic value of procedures, including cerebral angiograms and emergency cranial scans. As the average CT radiation dose decreased from 12 mSv in 2009 to 1.5 mSv in 2014 and continues to fall, these algorithms will become increasingly necessary to retain the high resolution and diagnostic power expected of neurologic CTs.29,30
Downstream Applications
Downstream applications refer to AI use after a radiologic study is acquired, mostly image interpretation. More than 70% of FDA-approved AI medical devices are in radiology, and many of these relate to image analysis.6,31 Although AI is not limited to black-and-white image interpretation, it is hypothesized that one of the reasons radiology is inviting to AI is because gray-scale images lend themselves to standardization.3 Moreover, most radiology departments already use AI-friendly picture archiving and communication systems.31,32
AI has been applied to a range of radiologic modalities, including MRI, CT, ultrasonography, PET, and mammography.32-38 AI also has been specifically applied to radiography, including the interpretation of tuberculosis, pneumonia, lung lesions, and COVID-19.33,39-45 AI also can assist triage, patient screening, providing a “second opinion” rapidly, shortening the time needed for attaining a diagnosis, monitoring disease progression, and predicting prognosis.37-39,43,45-47 Downstream applications of AI in neuroradiology and neurology include using CT to aid in detecting hemorrhage or ischemic stroke; using MRI to automatically segment lesions, such as tumors or MS lesions; assisting in early diagnosis and predicting prognosis in MS; assisting in treating paralysis, including from spinal cord injury; determining seizure type and localizing area of seizure onset; and using cameras, wearable devices, and smartphone applications to diagnose and assess treatment response in neurodegenerative disorders, such as Parkinson or Alzheimer diseases (Figure).37,48-56
Several AI tools have been deployed in the clinical setting, particularly triaging intracranial hemorrhage and moving these studies to the top of the radiologist’s worklist. In 2020 the Centers for Medicare and Medicaid Services (CMS) began reimbursing Viz.ai software’s AI-based Viz ContaCT (Viz LVO) with a new International Statistical Classification of Diseases, Tenth Revision procedure code.57
Viz LVO automatically detects large vessel occlusions, flags the occlusion on CT angiogram, alerts the stroke team (interventional radiologist, neuroradiologist, and neurologist), and transmits images through a secure application to the stroke team members’ mobile devices—all in less than 6 minutes from study acquisition to alarm notification.48 Additional software can quantify and measure perfusion in affected brain areas.48 This could have implications for quantifying and targeting areas of ischemic penumbra that could be salvaged after a stroke and then using that information to plan targeted treatment and/or intervention. Because many trials (DAWN/DEFUSE3) have shown benefits in stroke outcome by extending the therapeutic window for the endovascular thrombectomy, the ability to identify appropriate candidates is essential.58,59 Development of AI tools in assessing ischemic penumbra with quantitative parameters (mean transit time, cerebral blood volume, cerebral blood flow, mismatch ratio) using AI has benefited image interpretation. Medtronic RAPID software can provide quantitative assessment of CT perfusion. AI tools could be used to provide an automatic ASPECT score, which provides a quantitative measure for assessing potential ischemic zones and aids in assessing appropriate candidates for thrombectomy.
Several FDA-approved AI tools help quantify brain structures in neuroradiology, including quantitative analysis through MRI for analysis of anatomy and PET for analysis of functional uptake, assisting in more accurate and more objective detection and monitoring of conditions such as atrophy, dementia, trauma, seizure disorders, and MS.48 The growing number of FDA-approved AI technologies and the recent CMS-approved reimbursement for an AI tool indicate a changing landscape that is more accepting of downstream applications of AI in neuroradiology. As AI continues to integrate into medical regulation and finance, we predict AI will continue to play a prominent role in neuroradiology.
Practical and Ethical Considerations
In any discussion of the benefits of AI, it is prudent to address its shortcomings. Chief among these is overfitting, which occurs when an AI is too closely aligned with its training dataset and prone to error when applied to novel cases. Often this is a byproduct of a small training set.60 Neuroradiology, particularly with uncommon, advanced imaging methods, has a smaller number of available studies.61 Even with more prevalent imaging modalities, such as head CT, the work of collecting training scans from patients with the prerequisite disease processes, particularly if these processes are rare, can limit the number of datapoints collected. Neuroradiologists should understand how an AI tool was generated, including the size and variety of the training dataset used, to best gauge the clinical applicability and fitness of the system.
Another point of concern for AI clinical decision support tools’ implementation is automation bias—the tendency for clinicians to favor machine-generated decisions and ignore contrary data or conflicting human decisions.62 This situation often arises when radiologists experience overwhelming patient loads or are in underresourced settings, where there is little ability to review every AI-based diagnosis. Although AI might be of benefit in such conditions by reducing physician workload and streamlining the diagnostic process, there is the propensity to improperly rely on a tool meant to augment, not replace, a radiologist’s judgment. Such cases have led to adverse outcomes for patients, and legal precedence shows that this constitutes negligence.63 Maintaining awareness of each tool’s limitations and proper application is the only remedy for such situations.
Ethically, we must consider the opaqueness of ML-developed neuroimaging AIs. For many systems, the specific process by which an AI arrives at its conclusions is unknown. This AI “black box” can conceal potential errors and biases that are masked by overall positive performance metrics. The lack of understanding about how a tool functions in the zero-failure clinical setting understandably gives radiologists pause. The question must be asked: Is it ethical to use a system that is a relatively unknown quantity? Entities, including state governments, Canada, and the European Union, have produced an answer. Each of these governments have implemented policies requiring that health care AIs use some method to display to end users the process by which they arrive at conclusions.64-68
The 21st Century Cures Act declares that to attain approval, clinical AIs must demonstrate this explainability to clinicians and patients.69 The response has been an explosion in the development of explainable AI. Systems that visualize the areas where AI attention most often rests with heatmaps, generate labels for the most heavily weighted features of radiographic images, and create full diagnostic reports to justify AI conclusions aim to meet the goal of transparency and inspiring confidence in clinical end users.70 The ability to understand the “thought process” of a system proves useful for error correction and retooling. A trend toward under- or overdetecting conditions, flagging seemingly irrelevant image regions, or low reproducibility can be better addressed when it is clear how the AI is drawing its false conclusions. With an iterative process of testing and redesigning, false positive and negative rates can be reduced, the need for human intervention can be lowered to an appropriate minimum, and patient outcomes can be improved.71
Data collection raises another ethical concern. To train functional clinical decision support tools, massive amounts of patient demographic, laboratory, and imaging data are required. With incentives to develop the most powerful AI systems, record collection can venture down a path where patient autonomy and privacy are threatened. Radiologists have a duty to ensure data mining serves patients and improves the practice of radiology while protecting patients’ personal information.62 Policies have placed similar limits on the access to and use of patient records.64-69 Patients have the right to request explanation of the AI systems their data have been used to train. Approval for data acquisition requires the use of explainable AI, standardized data security protocol implementation, and adequate proof of communal benefit from the clinical decision support tool. Establishment of state-mandated protections bodes well for a future when developers can access enormous caches of data while patients and health care professionals are assured that no identifying information has escaped a well-regulated space. On the level of the individual radiologist, the knowledge that each datum represents a human life. These are people who has made themselves vulnerable by seeking relief for what ails them, which should serve as a lasting reminder to operate with utmost care when handling sensitive information.
Conclusions
The demonstrated applications of AI in neuroimaging are numerous and varied, and it is reasonable to assume that its implementation will increase as the technology matures. AI use for detecting important neurologic conditions holds promise in combatting ever greater imaging volumes and providing timely diagnoses. As medicine witnesses the continuing adoption of AI, it is important that practitioners possess an understanding of its current and emerging uses.
Artificial intelligence (AI) in medicine has shown significant promise, particularly in neuroimaging. AI refers to computer systems designed to perform tasks that normally require human intelligence.1 Machine learning (ML), a field in which computers learn from data without being specifically programmed, is the AI subset responsible for its success in matching or even surpassing humans in certain tasks.2
Supervised learning, a subset of ML, uses an algorithm with annotated data from which to learn.3 The program will use the characteristics of a training data set to predict a specific outcome or target when exposed to a sample data set of the same type. Unsupervised learning finds naturally occurring patterns or groupings within the data.4 With deep learning (DL) algorithms, computers learn the features that optimally represent the data for the problem at hand.5 Both ML and DL are meant to emulate neural networks in the brain, giving rise to artificial neural networks composed of nodes structured within input, hidden, and output layers.
The DL neural network differs from a conventional one by having many hidden layers instead of just 1 layer that extracts patterns within the data.6 Convolutional neural networks (CNNs) are the most prevalent DL architecture used in medical imaging. CNN’s hidden layers apply convolution and pooling operations to break down an image into features containing the most valuable information. The connecting layer applies high-level reasoning before the output layer provides predictions for the image. This framework has applications within radiology, such as predicting a lesion category or condition from an image, determining whether a specific pixel belongs to background or a target class, and predicting the location of lesions.1
AI promises to increase efficiency and reduces errors. With increased data processing and image interpretation, AI technology may help radiologists improve the quality of patient care.6 This article discusses the current applications and future integration of AI in neuroradiology.
Neuroimaging Applications
AI can improve the quality of neuroimaging and reduce the clinical and systemic loads of other imaging modalities. AI can predict patient wait times for computed tomography (CT), magnetic resonance imaging (MRI), ultrasound, and X-ray imaging.7 A ML-based AI has detected the variables that most affected patient wait times, including proximity to federal holidays and severity of the patient’s condition, and calculated how long patients would be delayed after their scheduled appointment time. This AI modality could allow more efficient patient scheduling and reveal areas of patient processing that could be changed, potentially improving patient satisfaction and outcomes for time-sensitive neurologic conditions.
AI can save patient and health care practitioner time for repeat MRIs. An estimated 20% of MRI scans require a repeat series—a massive loss of time and funds for both patients and the health care system.8 A DL approach can determine whether an MRI is usable clinically or unclear enough to require repetition.9 This initial screening measure can prevent patients from making return visits and neuroradiologists from reading inconclusive images. AI offers the opportunity to reduce time and costs incurred by optimizing the health care process before imaging is obtained.
Speeding Up Neuroimaging
AI can reduce the time spent performing imaging. Because MRIs consume time and resources, compressed sensing (CS) is commonly used. CS preferentially maintains in-plane resolution at the expense of through-plane resolution to produce a scan with a single, usable viewpoint that preserves signal-to-noise ratio (SNR). CS, however, limits interpretation to single directions and can create aliasing artifacts. An AI algorithm known as synthetic multi-orientation resolution enhancement works in real time to reduce aliasing and improve resolution in these compressed scans.10 This AI improved resolution of white matter lesions in patients with multiple sclerosis (MS) on FLAIR (fluid-attenuated inversion recovery) images, and permitted multiview reconstruction from these limited scans.
Tasks of reconstructing and anti-aliasing come with high computational costs that vary inversely with the extent of scanning compression, potentially negating the time and resource savings of CS. DL AI modalities have been developed to reduce operational loads and further improve image resolution in several directions from CS. One such deep residual learning AI was trained with compressed MRIs and used the framelet method to create a CNN that could rapidly remove global and deeply coherent aliasing artifacts.11 This system, compared with synthetic multi-orientation resolution enhancement, uses a pretrained, pretested AI that does not require additional time during scanning for computational analysis, thereby multiplying the time benefit of CS while retaining the benefits of multidirectional reconstruction and increased resolution. This methodology suffers from inherent degradation of perceptual image quality in its reconstructions because of the L2 loss function the CNN uses to reduce mean squared error, which causes blurring by averaging all possible outcomes of signal distribution during reconstruction. To combat this, researchers have developed another AI to reduce reconstruction times that uses a different loss function in a generative adversarial network to retain image quality, while offering reconstruction times several hundred times faster than current CS-MRI structures.12 So-called sparse-coding methods promise further reduction in reconstruction times, with the possibility of processing completed online with a lightweight architecture rather than on a local system.13
Neuroimaging of acute cases benefits most directly from these technologies because MRIs and their high resolution and SNR begin to approach CT imaging time scales. This could have important implications in clinical care, particularly for stroke imaging and evaluating spinal cord compression. CS-MRI optimization represents one of the greatest areas of neuroimaging cost savings and neurologic care improvement in the modern radiology era.
Reducing Contrast and Radiation Doses
AI has the ability to read CT, MRI, and positron emission tomography (PET) with reduced or without contrast without significant loss in sensitivity for detecting lesions. With MRI, gadolinium-based contrast can cause injection site reactions, allergic reactions, metal deposition throughout the body, and nephrogenic systemic fibrosis in the most severe instances.14 DL has been applied to brain MRIs performed with 10% of a full dose of contrast without significant degradation of image quality. Neuroradiologists did not rate the AI-synthesized images for several MRI indications lower than their full-dose counterparts.15 Low-dose contrast imaging, regardless of modality, generates greater noise with a significantly reduced signal. However, with AI applied, researchers found that the software suppressed motion and aliasing artifacts and improved image quality, perhaps evidence that this low-dose modality is less vulnerable to the most common pitfalls of MRI.
Recently, low-dose MRI moved into the spotlight when Subtle Medical SubtleGAD software received a National Institutes of Health grant and an expedited pathway to phase 2 clinical trials.16 SubtleGAD, a DL AI that enables low-dose MRI interpretation, might allow contrast MRI for patients with advanced kidney disease or contrast allergies. At some point, contrast with MRI might not be necessary because DL AI applied to noncontrast MRs for detecting MS lesions was found to be preliminarily effective with 78% lesion detection sensitivity.17
PET-MRI combines simultaneous PET and MRI and has been used to evaluate neurologic disorders. PET-MRI can detect amyloid plaques in Alzheimer disease 10 to 20 years before clinical signs of dementia emerge.18 PET-MRI has sparked DL AI development to decrease the dose of the IV radioactive tracer 18F-florbetaben used in imaging to reduce radiation exposure and imaging costs.This reduction is critical if PET-MRI is to become used widely.19-21
An initial CNN could reconstruct low-dose amyloid scans to full-dose resolution, albeit with a greater susceptibility to some artifacts and motion blurring.22 Similar to the synthetic multi-orientation resolution enhancement CNN, this program showed signal blurring from the L2 loss function, which was corrected in a later AI that used a generative adversarial network to minimize perceptual loss.23 This new AI demonstrated greater image resolution, feature preservation, and radiologist rating over the previous AI and was capable of reconstructing low-dose PET scans to full-dose resolution without an accompanying MRI. Applications of this algorithm are far-reaching, potentially allowing neuroimaging of brain tumors at more frequent intervals with higher resolution and lower total radiation exposure.
AI also has been applied to neurologic CT to reduce radiation exposure.24 Because it is critical to abide by the principles of ALARA (as low as reasonably achievable), the ability of AI to reduce radiation exposure holds significant promise. A CNN has been used to transform low-dose CTs of anthropomorphic models with calcium inserts and cardiac patients to normal-dose CTs, with the goal of improving the SNR.25 By training a noise-discriminating CNN and a noise-generating CNN together in a generative adversarial network, the AI improved image feature preservation during transformation. This algorithm has a direct application in imaging cerebral vasculature, including calcification that can explain lacunar infarcts and tracking systemic atherosclerosis.26
Another CNN has been applied to remove more complex noise patterns from the phenomena of beam hardening and photon starvation common in low-dose CT. This algorithm extracts the directional components of artifacts and compares them to known artifact patterns, allowing for highly specific suppression of unwanted signals.27 In June 2019, the US Food and Drug Administration (FDA) approved ClariPi, a deep CNN program for advanced denoising and resolution improvement of low- and ultra low-dose CTs.28 Aside from only low-dose settings, this AI could reduce artifacts in all CT imaging modalities and improve therapeutic value of procedures, including cerebral angiograms and emergency cranial scans. As the average CT radiation dose decreased from 12 mSv in 2009 to 1.5 mSv in 2014 and continues to fall, these algorithms will become increasingly necessary to retain the high resolution and diagnostic power expected of neurologic CTs.29,30
Downstream Applications
Downstream applications refer to AI use after a radiologic study is acquired, mostly image interpretation. More than 70% of FDA-approved AI medical devices are in radiology, and many of these relate to image analysis.6,31 Although AI is not limited to black-and-white image interpretation, it is hypothesized that one of the reasons radiology is inviting to AI is because gray-scale images lend themselves to standardization.3 Moreover, most radiology departments already use AI-friendly picture archiving and communication systems.31,32
AI has been applied to a range of radiologic modalities, including MRI, CT, ultrasonography, PET, and mammography.32-38 AI also has been specifically applied to radiography, including the interpretation of tuberculosis, pneumonia, lung lesions, and COVID-19.33,39-45 AI also can assist triage, patient screening, providing a “second opinion” rapidly, shortening the time needed for attaining a diagnosis, monitoring disease progression, and predicting prognosis.37-39,43,45-47 Downstream applications of AI in neuroradiology and neurology include using CT to aid in detecting hemorrhage or ischemic stroke; using MRI to automatically segment lesions, such as tumors or MS lesions; assisting in early diagnosis and predicting prognosis in MS; assisting in treating paralysis, including from spinal cord injury; determining seizure type and localizing area of seizure onset; and using cameras, wearable devices, and smartphone applications to diagnose and assess treatment response in neurodegenerative disorders, such as Parkinson or Alzheimer diseases (Figure).37,48-56
Several AI tools have been deployed in the clinical setting, particularly triaging intracranial hemorrhage and moving these studies to the top of the radiologist’s worklist. In 2020 the Centers for Medicare and Medicaid Services (CMS) began reimbursing Viz.ai software’s AI-based Viz ContaCT (Viz LVO) with a new International Statistical Classification of Diseases, Tenth Revision procedure code.57
Viz LVO automatically detects large vessel occlusions, flags the occlusion on CT angiogram, alerts the stroke team (interventional radiologist, neuroradiologist, and neurologist), and transmits images through a secure application to the stroke team members’ mobile devices—all in less than 6 minutes from study acquisition to alarm notification.48 Additional software can quantify and measure perfusion in affected brain areas.48 This could have implications for quantifying and targeting areas of ischemic penumbra that could be salvaged after a stroke and then using that information to plan targeted treatment and/or intervention. Because many trials (DAWN/DEFUSE3) have shown benefits in stroke outcome by extending the therapeutic window for the endovascular thrombectomy, the ability to identify appropriate candidates is essential.58,59 Development of AI tools in assessing ischemic penumbra with quantitative parameters (mean transit time, cerebral blood volume, cerebral blood flow, mismatch ratio) using AI has benefited image interpretation. Medtronic RAPID software can provide quantitative assessment of CT perfusion. AI tools could be used to provide an automatic ASPECT score, which provides a quantitative measure for assessing potential ischemic zones and aids in assessing appropriate candidates for thrombectomy.
Several FDA-approved AI tools help quantify brain structures in neuroradiology, including quantitative analysis through MRI for analysis of anatomy and PET for analysis of functional uptake, assisting in more accurate and more objective detection and monitoring of conditions such as atrophy, dementia, trauma, seizure disorders, and MS.48 The growing number of FDA-approved AI technologies and the recent CMS-approved reimbursement for an AI tool indicate a changing landscape that is more accepting of downstream applications of AI in neuroradiology. As AI continues to integrate into medical regulation and finance, we predict AI will continue to play a prominent role in neuroradiology.
Practical and Ethical Considerations
In any discussion of the benefits of AI, it is prudent to address its shortcomings. Chief among these is overfitting, which occurs when an AI is too closely aligned with its training dataset and prone to error when applied to novel cases. Often this is a byproduct of a small training set.60 Neuroradiology, particularly with uncommon, advanced imaging methods, has a smaller number of available studies.61 Even with more prevalent imaging modalities, such as head CT, the work of collecting training scans from patients with the prerequisite disease processes, particularly if these processes are rare, can limit the number of datapoints collected. Neuroradiologists should understand how an AI tool was generated, including the size and variety of the training dataset used, to best gauge the clinical applicability and fitness of the system.
Another point of concern for AI clinical decision support tools’ implementation is automation bias—the tendency for clinicians to favor machine-generated decisions and ignore contrary data or conflicting human decisions.62 This situation often arises when radiologists experience overwhelming patient loads or are in underresourced settings, where there is little ability to review every AI-based diagnosis. Although AI might be of benefit in such conditions by reducing physician workload and streamlining the diagnostic process, there is the propensity to improperly rely on a tool meant to augment, not replace, a radiologist’s judgment. Such cases have led to adverse outcomes for patients, and legal precedence shows that this constitutes negligence.63 Maintaining awareness of each tool’s limitations and proper application is the only remedy for such situations.
Ethically, we must consider the opaqueness of ML-developed neuroimaging AIs. For many systems, the specific process by which an AI arrives at its conclusions is unknown. This AI “black box” can conceal potential errors and biases that are masked by overall positive performance metrics. The lack of understanding about how a tool functions in the zero-failure clinical setting understandably gives radiologists pause. The question must be asked: Is it ethical to use a system that is a relatively unknown quantity? Entities, including state governments, Canada, and the European Union, have produced an answer. Each of these governments have implemented policies requiring that health care AIs use some method to display to end users the process by which they arrive at conclusions.64-68
The 21st Century Cures Act declares that to attain approval, clinical AIs must demonstrate this explainability to clinicians and patients.69 The response has been an explosion in the development of explainable AI. Systems that visualize the areas where AI attention most often rests with heatmaps, generate labels for the most heavily weighted features of radiographic images, and create full diagnostic reports to justify AI conclusions aim to meet the goal of transparency and inspiring confidence in clinical end users.70 The ability to understand the “thought process” of a system proves useful for error correction and retooling. A trend toward under- or overdetecting conditions, flagging seemingly irrelevant image regions, or low reproducibility can be better addressed when it is clear how the AI is drawing its false conclusions. With an iterative process of testing and redesigning, false positive and negative rates can be reduced, the need for human intervention can be lowered to an appropriate minimum, and patient outcomes can be improved.71
Data collection raises another ethical concern. To train functional clinical decision support tools, massive amounts of patient demographic, laboratory, and imaging data are required. With incentives to develop the most powerful AI systems, record collection can venture down a path where patient autonomy and privacy are threatened. Radiologists have a duty to ensure data mining serves patients and improves the practice of radiology while protecting patients’ personal information.62 Policies have placed similar limits on the access to and use of patient records.64-69 Patients have the right to request explanation of the AI systems their data have been used to train. Approval for data acquisition requires the use of explainable AI, standardized data security protocol implementation, and adequate proof of communal benefit from the clinical decision support tool. Establishment of state-mandated protections bodes well for a future when developers can access enormous caches of data while patients and health care professionals are assured that no identifying information has escaped a well-regulated space. On the level of the individual radiologist, the knowledge that each datum represents a human life. These are people who has made themselves vulnerable by seeking relief for what ails them, which should serve as a lasting reminder to operate with utmost care when handling sensitive information.
Conclusions
The demonstrated applications of AI in neuroimaging are numerous and varied, and it is reasonable to assume that its implementation will increase as the technology matures. AI use for detecting important neurologic conditions holds promise in combatting ever greater imaging volumes and providing timely diagnoses. As medicine witnesses the continuing adoption of AI, it is important that practitioners possess an understanding of its current and emerging uses.
1. Chartrand G, Cheng PM, Vorontsov E, et al. Deep learning: a primer for radiologists. Radiographics. 2017;37(7):2113-2131. doi:10.1148/rg.2017170077
2. King BF Jr. Guest editorial: discovery and artificial intelligence. AJR Am J Roentgenol. 2017;209(6):1189-1190. doi:10.2214/AJR.17.19178
3. Syed AB, Zoga AC. Artificial intelligence in radiology: current technology and future directions. Semin Musculoskelet Radiol. 2018;22(5):540-545. doi:10.1055/s-0038-1673383
4. Deo RC. Machine learning in medicine. Circulation. 2015;132(20):1920-1930. doi:10.1161/CIRCULATIONAHA.115.001593 5. Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60-88. doi:10.1016/j.media.2017.07.005
6. Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2(1):35. doi:10.1186/s41747-018-0061-6
7. Curtis C, Liu C, Bollerman TJ, Pianykh OS. Machine learning for predicting patient wait times and appointment delays. J Am Coll Radiol. 2018;15(9):1310-1316. doi:10.1016/j.jacr.2017.08.021
8. Andre JB, Bresnahan BW, Mossa-Basha M, et al. Toward quantifying the prevalence, severity, and cost associated with patient motion during clinical MR examinations. J Am Coll Radiol. 2015;12(7):689-695. doi:10.1016/j.jacr.2015.03.007
9. Sreekumari A, Shanbhag D, Yeo D, et al. A deep learning-based approach to reduce rescan and recall rates in clinical MRI examinations. AJNR Am J Neuroradiol. 2019;40(2):217-223. doi:10.3174/ajnr.A5926
10. Zhao C, Shao M, Carass A, et al. Applications of a deep learning method for anti-aliasing and super-resolution in MRI. Magn Reson Imaging. 2019;64:132-141. doi:10.1016/j.mri.2019.05.038
11. Lee D, Yoo J, Tak S, Ye JC. Deep residual learning for accelerated MRI using magnitude and phase networks. IEEE Trans Biomed Eng. 2018;65(9):1985-1995. doi:10.1109/TBME.2018.2821699
12. Mardani M, Gong E, Cheng JY, et al. Deep generative adversarial neural networks for compressive sensing MRI. IEEE Trans Med Imaging. 2019;38(1):167-179. doi:10.1109/TMI.2018.2858752
13. Dong C, Loy CC, He K, Tang X. Image super-resolution using deep convolutional networks. IEEE Trans Pattern Anal Mach Intell. 2016;38(2):295-307. doi:10.1109/TPAMI.2015.2439281
14. Sammet S. Magnetic resonance safety. Abdom Radiol (NY). 2016;41(3):444-451. doi:10.1007/s00261-016-0680-4
15. Gong E, Pauly JM, Wintermark M, Zaharchuk G. Deep learning enables reduced gadolinium dose for contrast-enhanced brain MRI. J Magn Reson Imaging. 2018;48(2):330-340. doi:10.1002/jmri.25970
16. Subtle Medical NIH awards Subtle Medical, Inc. $1.6 million grant to improve safety of MRI exams by reducing gadolinium dose using AI. Press release. September 18, 2019. Accessed March 14, 2022. https://www.biospace.com/article/releases/nih-awards-subtle-medical-inc-1-6-million-grant-to-improve-safety-of-mri-exams-by-reducing-gadolinium-dose-using-ai
17. Narayana PA, Coronado I, Sujit SJ, Wolinsky JS, Lublin FD, Gabr RE. Deep learning for predicting enhancing lesions in multiple sclerosis from noncontrast MRI. Radiology. 2020;294(2):398-404. doi:10.1148/radiol.2019191061
18. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128. doi:10.1016/S1474-4422(09)70299-6
19. Gatidis S, Würslin C, Seith F, et al. Towards tracer dose reduction in PET studies: simulation of dose reduction by retrospective randomized undersampling of list-mode data. Hell J Nucl Med. 2016;19(1):15-18. doi:10.1967/s002449910333
20. Kaplan S, Zhu YM. Full-dose PET image estimation from low-dose PET image using deep learning: a pilot study. J Digit Imaging. 2019;32(5):773-778. doi:10.1007/s10278-018-0150-3
21. Xu J, Gong E, Pauly J, Zaharchuk G. 200x low-dose PET reconstruction using deep learning. arXiv: 1712.04119. Accessed 2/16/2022. https://arxiv.org/pdf/1712.04119.pdf
22. Chen KT, Gong E, de Carvalho Macruz FB, et al. Ultra-low-dose 18F-florbetaben amyloid PET imaging using deep learning with multi-contrast MRI inputs. Radiology. 2019;290(3):649-656. doi:10.1148/radiol.2018180940
23. Ouyang J, Chen KT, Gong E, Pauly J, Zaharchuk G. Ultra-low-dose PET reconstruction using generative adversarial network with feature matching and task-specific perceptual loss. Med Phys. 2019;46(8):3555-3564. doi:10.1002/mp.13626
24. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi:10.1056/NEJMra072149
25. Wolterink JM, Leiner T, Viergever MA, Isgum I. Generative adversarial networks for noise reduction in low-dose CT. IEEE Trans Med Imaging. 2017;36(12):2536-2545. doi:10.1109/TMI.2017.2708987
26. Sohn YH, Cheon HY, Jeon P, Kang SY. Clinical implication of cerebral artery calcification on brain CT. Cerebrovasc Dis. 2004;18(4):332-337. doi:10.1159/000080772
27. Kang E, Min J, Ye JC. A deep convolutional neural network using directional wavelets for low-dose X-ray CT reconstruction. Med Phys. 2017;44(10):e360-e375. doi:10.1002/mp.12344
28. ClariPi gets FDA clearance for AI-powered CT image denoising solution. Published June 24, 2019. Accessed February 16, 2022. https://www.itnonline.com/content/claripi-gets-fda-clearance-ai-powered-ct-image-denoising-solution
29. Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301(5):500-507. doi:10.1001/jama.2009.54
30. Al-Mallah M, Aljizeeri A, Alharthi M, Alsaileek A. Routine low-radiation-dose coronary computed tomography angiography. Eur Heart J Suppl. 2014;16(suppl B):B12-B16. doi:10.1093/eurheartj/suu024
31. Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med. 2020;3:118. doi:10.1038/s41746-020-00324-0
32. Talebi-Liasi F, Markowitz O. Is artificial intelligence going to replace dermatologists? Cutis. 2020;105(1):28-31.
33. Khan O, Bebb G, Alimohamed NA. Artificial intelligence in medicine: what oncologists need to know about its potential—and its limitations. Oncology Exchange. 2017;16(4):8-13. http://www.oncologyex.com/pdf/vol16_no4/feature_khan-ai.pdf
34. Liu X, Faes L, Kale AU, et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit Health. 2019;1(6):e271-e297. doi:10.1016/S2589-7500(19)30123-2
35. Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44-56. doi:10.1038/s41591-018-0300-7
36. Salim M, Wåhlin E, Dembrower K, et al. External evaluation of 3 commercial artificial intelligence algorithms for independent assessment of screening mammograms. JAMA Oncol. 2020;6(10):1581-1588. doi:10.1001/jamaoncol.2020.3321
37. Arbabshirani MR, Fornwalt BK, Mongelluzzo GJ, et al. Advanced machine learning in action: identification of intracranial hemorrhage on computed tomography scans of the head with clinical workflow integration. NPJ Digit Med. 2018;1(1):1-7. doi:10.1038/s41746-017-0015-z
38. Sheth D, Giger ML. Artificial intelligence in the interpretation of breast cancer on MRI. J Magn Reson Imaging. 2020;51(5):1310-1324. doi:10.1002/jmri.26878
39. Borkowski AA, Viswanadhan NA, Thomas LB, Guzman RD, Deland LA, Mastorides SM. Using artificial intelligence for COVID-19 chest X-ray diagnosis. Fed Pract. 2020;37(9):398-404. doi:10.12788/fp.0045
40. Kermany DS, Goldbaum M, Cai W, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172(5):1122-1131.e9. doi:10.1016/j.cell.2018.02.010
41. Nam JG, Park S, Hwang EJ, et al. Development and validation of deep learning-based automatic detection algorithm for malignant pulmonary nodules on chest radiographs. Radiology. 2019;290(1):218-228. doi:10.1148/radiol.2018180237
42. Zech JR, Badgeley MA, Liu M, Costa AB, Titano JJ, Oermann EK. Variable generalization performance of a deep learning model to detect pneumonia in chest radiographs: a cross-sectional study. PLoS Med. 2018;15(11):e1002683. doi:10.1371/journal.pmed.1002683
43. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582. doi:10.1148/radiol.2017162326
44. Rajpurkar P, Joshi A, Pareek A, et al. CheXpedition: investigating generalization challenges for translation of chest X-Ray algorithms to the clinical setting. arXiv preprint arXiv:200211379. Accessed February 16, 2022. https://arxiv.org/pdf/2002.11379.pdf
45. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25(1):30-36. doi:10.1038/s41591-018-0307-0
46. Meyer-Bäse A, Morra L, Meyer-Bäse U, Pinker K. Current status and future perspectives of artificial intelligence in magnetic resonance breast imaging. Contrast Media Mol Imaging. 2020;2020:6805710. doi:10.1155/2020/6805710
47. Booth AL, Abels E, McCaffrey P. Development of a prognostic model for mortality in COVID-19 infection using machine learning. Mod Pathol. 2020;4(3):522-531. doi:10.1038/s41379-020-00700-x
48. Bash S. Enhancing neuroimaging with artificial intelligence. Applied Radiology. 2020;49(1):20-21.
49. Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243. doi:10.1136/svn-2017-000101
50. Valliani AA, Ranti D, Oermann EK. Deep learning and neurology: a systematic review. Neurol Ther. 2019;8(2):351-365. doi:10.1007/s40120-019-00153-8
51. Gupta R, Krishnam SP, Schaefer PW, Lev MH, Gonzalez RG. An east coast perspective on artificial intelligence and machine learning: part 2: ischemic stroke imaging and triage. Neuroimaging Clin N Am. 2020;30(4):467-478. doi:10.1016/j.nic.2020.08.002
52. Belić M, Bobić V, Badža M, Šolaja N, Đurić-Jovičić M, Kostić VS. Artificial intelligence for assisting diagnostics and assessment of Parkinson’s disease-A review. Clin Neurol Neurosurg. 2019;184:105442. doi:10.1016/j.clineuro.2019.105442
53. An S, Kang C, Lee HW. Artificial intelligence and computational approaches for epilepsy. J Epilepsy Res. 2020;10(1):8-17. doi:10.14581/jer.20003
54. Pavel AM, Rennie JM, de Vries LS, et al. A machine-learning algorithm for neonatal seizure recognition: a multicentre, randomised, controlled trial. Lancet Child Adolesc Health. 2020;4(10):740-749. doi:10.1016/S2352-4642(20)30239-X
55. Afzal HMR, Luo S, Ramadan S, Lechner-Scott J. The emerging role of artificial intelligence in multiple sclerosis imaging. Mult Scler. 2020;1352458520966298. doi:10.1177/1352458520966298
56. Bouton CE. Restoring movement in paralysis with a bioelectronic neural bypass approach: current state and future directions. Cold Spring Harb Perspect Med. 2019;9(11):a034306. doi:10.1101/cshperspect.a034306
57. Hassan AE. New technology add-on payment (NTAP) for Viz LVO: a win for stroke care. J Neurointerv Surg. 2020;neurintsurg-2020-016897. doi:10.1136/neurintsurg-2020-016897
58. Nogueira RG , Jadhav AP , Haussen DC , et al; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi:10.1056/NEJMoa1706442
59. Albers GW , Marks MP , Kemp S , et al; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–18. doi:10.1056/NEJMoa1713973
60. Bi WL, Hosny A, Schabath MB, et al. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin. 2019;69(2):127-157. doi:10.3322/caac.21552
61. Wagner MW, Namdar K, Biswas A, Monah S, Khalvati F, Ertl-Wagner BB. Radiomics, machine learning, and artificial intelligence-what the neuroradiologist needs to know. Neuroradiology. 2021;63(12):1957-1967. doi:10.1007/s00234-021-02813-9
62. Geis JR, Brady AP, Wu CC, et al. Ethics of artificial intelligence in radiology: summary of the Joint European and North American Multisociety Statement. J Am Coll Radiol. 2019;16(11):1516-1521. doi:10.1016/j.jacr.2019.07.028
63. Kingston J. Artificial intelligence and legal liability. arXiv:1802.07782. https://arxiv.org/ftp/arxiv/papers/1802/1802.07782.pdf
64. Council of the European Union, General Data Protection Regulation. Official Journal of the European Union. Accessed February 16, 2022. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0679
65. Consumer Privacy Protection Act of 2017, HR 4081, 115th Cong (2017). Accessed February 10, 2022. https://www.congress.gov/bill/115th-congress/house-bill/4081
66. Cal. Civ. Code § 1798.198(a) (2018). California Consumer Privacy Act of 2018.
67. Va. Code Ann. § 59.1 (2021). Consumer Data Protection Act. Accessed February 10, 2022. https://lis.virginia.gov/cgi-bin/legp604.exe?212+ful+SB1392ER+pdf
68. Colo. Rev. Stat. § 6-1-1301 (2021). Colorado Privacy Act. Accessed February 10, 2022. https://leg.colorado.gov/sites/default/files/2021a_190_signed.pdf
69. 21st Century Cures Act, Pub L No. 114-255 (2016). Accessed February 10, 2022. https://www.govinfo.gov/content/pkg/PLAW-114publ255/html/PLAW-114publ255.htm
70. Huff DT, Weisman AJ, Jeraj R. Interpretation and visualization techniques for deep learning models in medical imaging. Phys Med Biol. 2021;66(4):04TR01. doi:10.1088/1361-6560/abcd17
71. Thrall JH, Li X, Li Q, et al. Artificial intelligence and machine learning in radiology: opportunities, challenges, pitfalls, and criteria for success. J Am Coll Radiol. 2018;15(3, pt B):504-508. doi:10.1016/j.jacr.2017.12.026
1. Chartrand G, Cheng PM, Vorontsov E, et al. Deep learning: a primer for radiologists. Radiographics. 2017;37(7):2113-2131. doi:10.1148/rg.2017170077
2. King BF Jr. Guest editorial: discovery and artificial intelligence. AJR Am J Roentgenol. 2017;209(6):1189-1190. doi:10.2214/AJR.17.19178
3. Syed AB, Zoga AC. Artificial intelligence in radiology: current technology and future directions. Semin Musculoskelet Radiol. 2018;22(5):540-545. doi:10.1055/s-0038-1673383
4. Deo RC. Machine learning in medicine. Circulation. 2015;132(20):1920-1930. doi:10.1161/CIRCULATIONAHA.115.001593 5. Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60-88. doi:10.1016/j.media.2017.07.005
6. Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2(1):35. doi:10.1186/s41747-018-0061-6
7. Curtis C, Liu C, Bollerman TJ, Pianykh OS. Machine learning for predicting patient wait times and appointment delays. J Am Coll Radiol. 2018;15(9):1310-1316. doi:10.1016/j.jacr.2017.08.021
8. Andre JB, Bresnahan BW, Mossa-Basha M, et al. Toward quantifying the prevalence, severity, and cost associated with patient motion during clinical MR examinations. J Am Coll Radiol. 2015;12(7):689-695. doi:10.1016/j.jacr.2015.03.007
9. Sreekumari A, Shanbhag D, Yeo D, et al. A deep learning-based approach to reduce rescan and recall rates in clinical MRI examinations. AJNR Am J Neuroradiol. 2019;40(2):217-223. doi:10.3174/ajnr.A5926
10. Zhao C, Shao M, Carass A, et al. Applications of a deep learning method for anti-aliasing and super-resolution in MRI. Magn Reson Imaging. 2019;64:132-141. doi:10.1016/j.mri.2019.05.038
11. Lee D, Yoo J, Tak S, Ye JC. Deep residual learning for accelerated MRI using magnitude and phase networks. IEEE Trans Biomed Eng. 2018;65(9):1985-1995. doi:10.1109/TBME.2018.2821699
12. Mardani M, Gong E, Cheng JY, et al. Deep generative adversarial neural networks for compressive sensing MRI. IEEE Trans Med Imaging. 2019;38(1):167-179. doi:10.1109/TMI.2018.2858752
13. Dong C, Loy CC, He K, Tang X. Image super-resolution using deep convolutional networks. IEEE Trans Pattern Anal Mach Intell. 2016;38(2):295-307. doi:10.1109/TPAMI.2015.2439281
14. Sammet S. Magnetic resonance safety. Abdom Radiol (NY). 2016;41(3):444-451. doi:10.1007/s00261-016-0680-4
15. Gong E, Pauly JM, Wintermark M, Zaharchuk G. Deep learning enables reduced gadolinium dose for contrast-enhanced brain MRI. J Magn Reson Imaging. 2018;48(2):330-340. doi:10.1002/jmri.25970
16. Subtle Medical NIH awards Subtle Medical, Inc. $1.6 million grant to improve safety of MRI exams by reducing gadolinium dose using AI. Press release. September 18, 2019. Accessed March 14, 2022. https://www.biospace.com/article/releases/nih-awards-subtle-medical-inc-1-6-million-grant-to-improve-safety-of-mri-exams-by-reducing-gadolinium-dose-using-ai
17. Narayana PA, Coronado I, Sujit SJ, Wolinsky JS, Lublin FD, Gabr RE. Deep learning for predicting enhancing lesions in multiple sclerosis from noncontrast MRI. Radiology. 2020;294(2):398-404. doi:10.1148/radiol.2019191061
18. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128. doi:10.1016/S1474-4422(09)70299-6
19. Gatidis S, Würslin C, Seith F, et al. Towards tracer dose reduction in PET studies: simulation of dose reduction by retrospective randomized undersampling of list-mode data. Hell J Nucl Med. 2016;19(1):15-18. doi:10.1967/s002449910333
20. Kaplan S, Zhu YM. Full-dose PET image estimation from low-dose PET image using deep learning: a pilot study. J Digit Imaging. 2019;32(5):773-778. doi:10.1007/s10278-018-0150-3
21. Xu J, Gong E, Pauly J, Zaharchuk G. 200x low-dose PET reconstruction using deep learning. arXiv: 1712.04119. Accessed 2/16/2022. https://arxiv.org/pdf/1712.04119.pdf
22. Chen KT, Gong E, de Carvalho Macruz FB, et al. Ultra-low-dose 18F-florbetaben amyloid PET imaging using deep learning with multi-contrast MRI inputs. Radiology. 2019;290(3):649-656. doi:10.1148/radiol.2018180940
23. Ouyang J, Chen KT, Gong E, Pauly J, Zaharchuk G. Ultra-low-dose PET reconstruction using generative adversarial network with feature matching and task-specific perceptual loss. Med Phys. 2019;46(8):3555-3564. doi:10.1002/mp.13626
24. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi:10.1056/NEJMra072149
25. Wolterink JM, Leiner T, Viergever MA, Isgum I. Generative adversarial networks for noise reduction in low-dose CT. IEEE Trans Med Imaging. 2017;36(12):2536-2545. doi:10.1109/TMI.2017.2708987
26. Sohn YH, Cheon HY, Jeon P, Kang SY. Clinical implication of cerebral artery calcification on brain CT. Cerebrovasc Dis. 2004;18(4):332-337. doi:10.1159/000080772
27. Kang E, Min J, Ye JC. A deep convolutional neural network using directional wavelets for low-dose X-ray CT reconstruction. Med Phys. 2017;44(10):e360-e375. doi:10.1002/mp.12344
28. ClariPi gets FDA clearance for AI-powered CT image denoising solution. Published June 24, 2019. Accessed February 16, 2022. https://www.itnonline.com/content/claripi-gets-fda-clearance-ai-powered-ct-image-denoising-solution
29. Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301(5):500-507. doi:10.1001/jama.2009.54
30. Al-Mallah M, Aljizeeri A, Alharthi M, Alsaileek A. Routine low-radiation-dose coronary computed tomography angiography. Eur Heart J Suppl. 2014;16(suppl B):B12-B16. doi:10.1093/eurheartj/suu024
31. Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med. 2020;3:118. doi:10.1038/s41746-020-00324-0
32. Talebi-Liasi F, Markowitz O. Is artificial intelligence going to replace dermatologists? Cutis. 2020;105(1):28-31.
33. Khan O, Bebb G, Alimohamed NA. Artificial intelligence in medicine: what oncologists need to know about its potential—and its limitations. Oncology Exchange. 2017;16(4):8-13. http://www.oncologyex.com/pdf/vol16_no4/feature_khan-ai.pdf
34. Liu X, Faes L, Kale AU, et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit Health. 2019;1(6):e271-e297. doi:10.1016/S2589-7500(19)30123-2
35. Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44-56. doi:10.1038/s41591-018-0300-7
36. Salim M, Wåhlin E, Dembrower K, et al. External evaluation of 3 commercial artificial intelligence algorithms for independent assessment of screening mammograms. JAMA Oncol. 2020;6(10):1581-1588. doi:10.1001/jamaoncol.2020.3321
37. Arbabshirani MR, Fornwalt BK, Mongelluzzo GJ, et al. Advanced machine learning in action: identification of intracranial hemorrhage on computed tomography scans of the head with clinical workflow integration. NPJ Digit Med. 2018;1(1):1-7. doi:10.1038/s41746-017-0015-z
38. Sheth D, Giger ML. Artificial intelligence in the interpretation of breast cancer on MRI. J Magn Reson Imaging. 2020;51(5):1310-1324. doi:10.1002/jmri.26878
39. Borkowski AA, Viswanadhan NA, Thomas LB, Guzman RD, Deland LA, Mastorides SM. Using artificial intelligence for COVID-19 chest X-ray diagnosis. Fed Pract. 2020;37(9):398-404. doi:10.12788/fp.0045
40. Kermany DS, Goldbaum M, Cai W, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172(5):1122-1131.e9. doi:10.1016/j.cell.2018.02.010
41. Nam JG, Park S, Hwang EJ, et al. Development and validation of deep learning-based automatic detection algorithm for malignant pulmonary nodules on chest radiographs. Radiology. 2019;290(1):218-228. doi:10.1148/radiol.2018180237
42. Zech JR, Badgeley MA, Liu M, Costa AB, Titano JJ, Oermann EK. Variable generalization performance of a deep learning model to detect pneumonia in chest radiographs: a cross-sectional study. PLoS Med. 2018;15(11):e1002683. doi:10.1371/journal.pmed.1002683
43. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582. doi:10.1148/radiol.2017162326
44. Rajpurkar P, Joshi A, Pareek A, et al. CheXpedition: investigating generalization challenges for translation of chest X-Ray algorithms to the clinical setting. arXiv preprint arXiv:200211379. Accessed February 16, 2022. https://arxiv.org/pdf/2002.11379.pdf
45. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25(1):30-36. doi:10.1038/s41591-018-0307-0
46. Meyer-Bäse A, Morra L, Meyer-Bäse U, Pinker K. Current status and future perspectives of artificial intelligence in magnetic resonance breast imaging. Contrast Media Mol Imaging. 2020;2020:6805710. doi:10.1155/2020/6805710
47. Booth AL, Abels E, McCaffrey P. Development of a prognostic model for mortality in COVID-19 infection using machine learning. Mod Pathol. 2020;4(3):522-531. doi:10.1038/s41379-020-00700-x
48. Bash S. Enhancing neuroimaging with artificial intelligence. Applied Radiology. 2020;49(1):20-21.
49. Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243. doi:10.1136/svn-2017-000101
50. Valliani AA, Ranti D, Oermann EK. Deep learning and neurology: a systematic review. Neurol Ther. 2019;8(2):351-365. doi:10.1007/s40120-019-00153-8
51. Gupta R, Krishnam SP, Schaefer PW, Lev MH, Gonzalez RG. An east coast perspective on artificial intelligence and machine learning: part 2: ischemic stroke imaging and triage. Neuroimaging Clin N Am. 2020;30(4):467-478. doi:10.1016/j.nic.2020.08.002
52. Belić M, Bobić V, Badža M, Šolaja N, Đurić-Jovičić M, Kostić VS. Artificial intelligence for assisting diagnostics and assessment of Parkinson’s disease-A review. Clin Neurol Neurosurg. 2019;184:105442. doi:10.1016/j.clineuro.2019.105442
53. An S, Kang C, Lee HW. Artificial intelligence and computational approaches for epilepsy. J Epilepsy Res. 2020;10(1):8-17. doi:10.14581/jer.20003
54. Pavel AM, Rennie JM, de Vries LS, et al. A machine-learning algorithm for neonatal seizure recognition: a multicentre, randomised, controlled trial. Lancet Child Adolesc Health. 2020;4(10):740-749. doi:10.1016/S2352-4642(20)30239-X
55. Afzal HMR, Luo S, Ramadan S, Lechner-Scott J. The emerging role of artificial intelligence in multiple sclerosis imaging. Mult Scler. 2020;1352458520966298. doi:10.1177/1352458520966298
56. Bouton CE. Restoring movement in paralysis with a bioelectronic neural bypass approach: current state and future directions. Cold Spring Harb Perspect Med. 2019;9(11):a034306. doi:10.1101/cshperspect.a034306
57. Hassan AE. New technology add-on payment (NTAP) for Viz LVO: a win for stroke care. J Neurointerv Surg. 2020;neurintsurg-2020-016897. doi:10.1136/neurintsurg-2020-016897
58. Nogueira RG , Jadhav AP , Haussen DC , et al; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi:10.1056/NEJMoa1706442
59. Albers GW , Marks MP , Kemp S , et al; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–18. doi:10.1056/NEJMoa1713973
60. Bi WL, Hosny A, Schabath MB, et al. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin. 2019;69(2):127-157. doi:10.3322/caac.21552
61. Wagner MW, Namdar K, Biswas A, Monah S, Khalvati F, Ertl-Wagner BB. Radiomics, machine learning, and artificial intelligence-what the neuroradiologist needs to know. Neuroradiology. 2021;63(12):1957-1967. doi:10.1007/s00234-021-02813-9
62. Geis JR, Brady AP, Wu CC, et al. Ethics of artificial intelligence in radiology: summary of the Joint European and North American Multisociety Statement. J Am Coll Radiol. 2019;16(11):1516-1521. doi:10.1016/j.jacr.2019.07.028
63. Kingston J. Artificial intelligence and legal liability. arXiv:1802.07782. https://arxiv.org/ftp/arxiv/papers/1802/1802.07782.pdf
64. Council of the European Union, General Data Protection Regulation. Official Journal of the European Union. Accessed February 16, 2022. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0679
65. Consumer Privacy Protection Act of 2017, HR 4081, 115th Cong (2017). Accessed February 10, 2022. https://www.congress.gov/bill/115th-congress/house-bill/4081
66. Cal. Civ. Code § 1798.198(a) (2018). California Consumer Privacy Act of 2018.
67. Va. Code Ann. § 59.1 (2021). Consumer Data Protection Act. Accessed February 10, 2022. https://lis.virginia.gov/cgi-bin/legp604.exe?212+ful+SB1392ER+pdf
68. Colo. Rev. Stat. § 6-1-1301 (2021). Colorado Privacy Act. Accessed February 10, 2022. https://leg.colorado.gov/sites/default/files/2021a_190_signed.pdf
69. 21st Century Cures Act, Pub L No. 114-255 (2016). Accessed February 10, 2022. https://www.govinfo.gov/content/pkg/PLAW-114publ255/html/PLAW-114publ255.htm
70. Huff DT, Weisman AJ, Jeraj R. Interpretation and visualization techniques for deep learning models in medical imaging. Phys Med Biol. 2021;66(4):04TR01. doi:10.1088/1361-6560/abcd17
71. Thrall JH, Li X, Li Q, et al. Artificial intelligence and machine learning in radiology: opportunities, challenges, pitfalls, and criteria for success. J Am Coll Radiol. 2018;15(3, pt B):504-508. doi:10.1016/j.jacr.2017.12.026
SERMs revisited: Can they improve menopausal care?
Selective estrogen receptor modulators (SERMs) are unique synthetic compounds that bind to the estrogen receptor and initiate either estrogenic agonistic or antagonistic activity, depending on the confirmational change they produce on binding to the receptor. Many SERMs have come to market, others have not. Unlike estrogens, which regardless of dose or route of administration all carry risks as a boxed warning on the label, referred to as class labeling,1 various SERMs exert various effects in some tissues (uterus, vagina) while they have apparent class properties in others (bone, breast).2
The first SERM, for all practical purposes, was tamoxifen (although clomiphene citrate is often considered a SERM). Tamoxifen was approved by the US Food and Drug Administration (FDA) in 1978 for the treatment of breast cancer and, subsequently, for breast cancer risk reduction. It became the most widely prescribed anticancer drug worldwide.
Subsequently, when data showed that tamoxifen could produce a small number of endometrial cancers and a larger number of endometrial polyps,3,4 there was renewed interest in raloxifene. In preclinical animal studies, raloxifene behaved differently than tamoxifen in the uterus. After clinical trials with raloxifene showed uterine safety,5 the drug was FDA approved for prevention of osteoporosis in 1997, for treatment of osteoporosis in 1999, and for breast cancer risk reduction in 2009. Most clinicians are familiar with these 2 SERMs, which have been in clinical use for more than 4 and 2 decades, respectively.
Ospemifene: A third-generation SERM and its indications
Hormone deficiency from menopause causes vulvovaginal and urogenital changes as well as a multitude of symptoms and signs, including vulvar and vaginal thinning, loss of rugal folds, diminished elasticity, increased pH, and most notably dyspareunia. The nomenclature that previously described vulvovaginal atrophy (VVA) has been expanded to include genitourinary syndrome of menopause (GSM).6 Unfortunately, many health care providers do not ask patients about GSM symptoms, and few women report their symptoms to their clinician.7 Furthermore, although low-dose local estrogens applied vaginally have been the mainstay of therapy for VVA/GSM, only 7% of symptomatic women use any pharmacologic agent,8 mainly because of fear of estrogens due to the class labeling mentioned above.
Ospemifene, a newer SERM, improved superficial cells and reduced parabasal cells as seen on a maturation index compared with placebo, according to results of multiple phase 3 clinical trials9,10; it also lowered vaginal pH and improved most bothersome symptoms (original studies were for dyspareunia). As a result, the FDA approved ospemifene for treatment of moderate to severe dyspareunia from VVA of menopause.
Subsequent studies allowed for a broadened indication to include treatment of moderate to severe dryness due to menopause.11 The ospemifene label contains a boxed warning that states, “In the endometrium, [ospemifene] has estrogen agonistic effects.”12 Although ospemifene is not an estrogen (it’s a SERM), the label goes on to state, “There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens.” This statement caused The Medical Letter to initially suggest that patients who receive ospemifene also should receive a progestational agent—a suggestion they later retracted.13,14
To understand why the ospemifene labeling might be worded in such a way, one must review the data regarding the poorly named entity “weakly proliferative endometrium.” The package labeling combines any proliferative endometrium (“weakly” plus “actively” plus “disordered”) that occurred in the clinical trial. Thus, 86.1 per 1,000 of the ospemifene-treated patients (vs 13.3 per 1,000 of those taking placebo) had any one of the proliferative types. The problem is that “actively proliferative” endometrial glands will have mitotic activity in virtually every nucleus of the gland as well as abundant glandular progression (FIGURE 1), whereas “weakly proliferative” is actually closer to inactive or atrophic endometrium with an occasional mitotic figure in only a few nuclei of each gland (FIGURE 2).
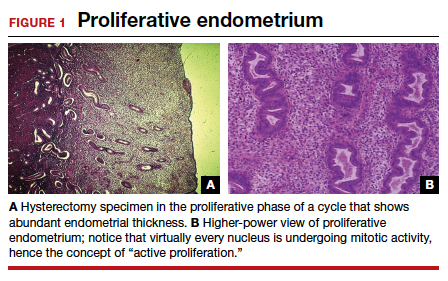
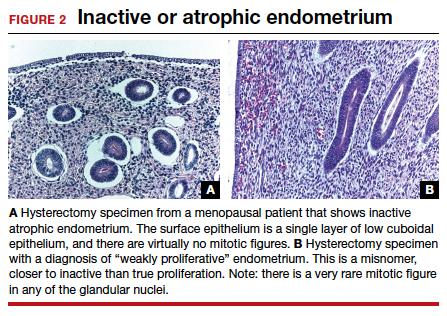
In addition, at 1 year, the incidence of active proliferation with ospemifene was 1%.15 In examining the uterine safety study for raloxifene, both doses of that agent had an active proliferation incidence of 3% at 1 year.5 Furthermore, that study had an estrogen-only arm in which, at end point, the incidence of endometrial proliferation was 39%, and hyperplasia, 23%!5 It therefore is evident that, in the endometrium, ospemifene is much more like the SERM raloxifene than it is like estrogen. The American College of Obstetricians and Gynecologists (ACOG) endorsed ospemifene (level A evidence) as a first-line therapy for dyspareunia, noting absent endometrial stimulation.16
Continue to: Ospemifene effects on breast and bone...
Ospemifene effects on breast and bone
Although ospemifene is approved for treatment of moderate to severe VVA/GSM, it has other SERM effects typical of its class. The label currently states that ospemifene “has not been adequately studied in women with breast cancer; therefore, it should not be used in women with known or suspected breast cancer.”12 We know that tamoxifen reduced breast cancer 49% in high-risk women in the Breast Cancer Prevention Trial (BCPT).17 We also know that in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, raloxifene reduced breast cancer 77% in osteoporotic women,18 and in the Study of Tamoxifen and Raloxifene (STAR) trial, it performed virtually identically to tamoxifen in breast cancer prevention.19 Previous studies demonstrated that ospemifene inhibits breast cancer cell growth in in vitro cultures as well as in animal studies20 and inhibits proliferation of human breast tissue epithelial cells,21 with breast effects similar to those seen with tamoxifen and raloxifene.
Thus, although one would not choose ospemifene as a primary treatment or risk-reducing agent for a patient with breast cancer, the direction of its activity in breast tissue is indisputable and is likely the reason that in the European Union (unlike in the United States) it is approved to treat dyspareunia from VVA/GSM in women with a prior history of breast cancer.
Virtually all SERMs have estrogen agonistic activity in bone. Bone is a dynamic organ, constantly being laid down and taken away (resorption). Estrogen and SERMs are potent antiresorptives in bone metabolism. Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to that of estradiol and raloxifene.22 Clinical data from 3 phase 1 or 2 clinical trials found that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.23 Actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, but there is a good correlation between biochemical markers for bone turnover and the occurrence of fracture.24 Once again, women who need treatment for osteoporosis should not be treated primarily with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
Clinical application
Ospemifene is an oral SERM approved for the treatment of moderate to severe dyspareunia as well as dryness from VVA due to menopause. In addition, it appears one can safely surmise that the direction of ospemifene’s activity in bone and breast is virtually indisputable. The magnitude of that activity, however, is unstudied. Therefore, in selecting an agent to treat women with dyspareunia or vaginal dryness from VVA of menopause, determining any potential add-on benefit for that particular patient in either bone and/or breast is clinically appropriate.
The SERM bazedoxifene
A meta-analysis of 4 randomized, placebo-controlled trials showed that another SERM, bazedoxifene, can significantly decrease the incidence of vertebral fracture in postmenopausal women at follow-up of 3 and 7 years.25 That meta-analysis also confirmed the long-term favorable safety and tolerability of bazedoxifene, with no increase in adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic events, or breast carcinoma in patients using bazedoxifene. However, bazedoxifene use did result in an increased incidence of hot flushes and leg cramps across 7 years.25 Bazedoxifene is available in a 20-mg dose for treatment of postmenopausal osteoporosis in Israel and a number of European Union countries.
Continue to: Enter the concept of tissue-selective estrogen complex (TSEC)...
Enter the concept of tissue-selective estrogen complex (TSEC)
Some postmenopausal women are extremely intolerant of any progestogen added to estrogen therapy to confer endometrial protection in those with a uterus. According to the results of a clinical trial of postmenopausal women, bazedoxifene is the only SERM shown to decrease endometrial thickness compared with placebo.26 This is the basis for thinking that perhaps a SERM like bazedoxifene, instead of a progestogen, could be used to confer endometrial protection.
A further consideration comes out of the evaluation of data derived from the 2 arms of the Women’s Health Initiative (WHI).27 In the arm that combined conjugated estrogen with medroxyprogesterone acetate through 11.3 years, there was a 25% increase in the incidence of invasive breast cancer, which was statistically significant. Contrast that with the arm in hysterectomized women who received only conjugated estrogen (often inaccurately referred to as the “estrogen only” arm of the WHI). In that study arm, the relative risk of invasive breast cancer was reduced 23%, also statistically significant. Thus, the culprit in the breast cancer incidence difference in these 2 arms appears to be the addition of the progestogen medroxyprogesterone acetate.27
Since the progestogen was used only for endometrial protection, could such endometrial protection be provided by a SERM like bazedoxifene? Preclinical trials showed that a combination of bazedoxifene and conjugated estrogen (in various estrogen doses) resulted in uterine wet weight in an ovariectomized rat model that was no different than that with placebo.28
In terms of effects on breast, preclinical models showed that conjugated estrogen use resulted in less mammary duct elongation and end bud proliferation than estradiol by itself, and that the combination of conjugated estrogen and bazedoxifene resulted in mammary duct elongation and end bud proliferation that was similar to that in the ovariectomized animals and considerably less than a combination of estradiol with bazedoxifene.29
Five phase 3 studies known as the SMART (Selective estrogens, Menopause, And Response to Therapy) trials were then conducted. Collectively, these studies examined the frequency and severity of vasomotor symptoms (VMS), BMD, bone turnover markers, lipid profiles, sleep, quality of life, breast density, and endometrial safety with conjugated estrogen/bazedoxifene treatment.30 Based on these trials with more than 7,500 women, in 2013 the FDA approved a compound of conjugated estrogen 0.45 mg and bazedoxifene 20 mg (Duavee in the United States and Duavive outside the United States).
The incidence of endometrial hyperplasia at 12 months was consistently less than 1%, which is the FDA guidance for approval of hormone therapies. The incidence of bleeding or spotting with conjugated estrogen/bazedoxifene (FIGURE 3) in each 4-week interval over 12 months mirror-imaged that of placebo and ranged from 3.9% in the first 4-week interval to 1.7% in the last 4 weeks, compared with conjugated estrogen 0.45 mg/medroxyprogesterone acetate 1.5 mg, which had a 20.8% incidence of bleeding or spotting in the first 4-week interval and was still at an 8.8% incidence in the last 4 weeks.31 This is extremely relevant in clinical practice. There was no difference from placebo in breast cancer incidence, breast pain or tenderness, abnormal mammograms, or breast density at month 12.32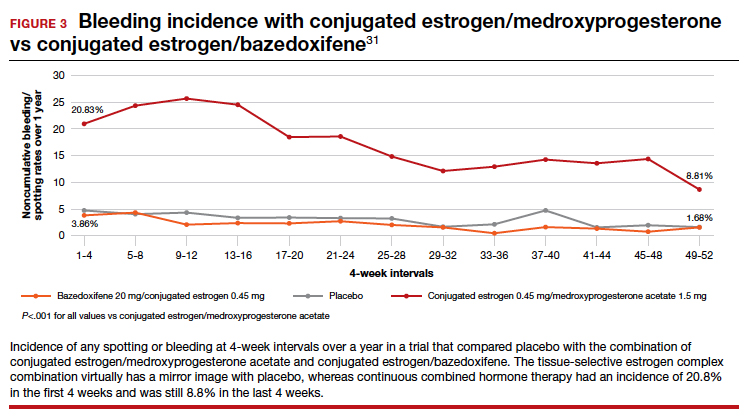
In terms of frequency of VMS, there was a 74% reduction from baseline at 12 weeks compared with placebo (P<.001), as well as a 37% reduction in the VMS severity score (P<.001).32 Statistically significant improvements occurred in lumbar spine and hip BMD (P<.01) for women who were 1 to 5 years since menopause as well as for those who were more than 5 years since menopause.33
Packaging issue puts TSEC on back order
In May 2020, Pfizer voluntarily recalled its conjugated estrogen/bazedoxifene product after identifying a “flaw in the drug’s foil laminate pouch that introduced oxygen and lowered the dissolution rate of active pharmaceutical ingredient bazedoxifene acetate.”34 The manufacturer then wrote a letter to health care professionals in September 2021 stating, “Duavee continues to be out of stock due to an unexpected and complex packaging issue, resulting in manufacturing delays. This has nothing to do with the safety or quality of the product itself but could affect product stability throughout its shelf life… Given regulatory approval timelines for any new packaging, it is unlikely that Duavee will return to stock in 2022.”35
Other TSECs?
The conjugated estrogen/bazedoxifene combination is the first FDA-approved TSEC. Other attempts have been made to achieve similar results with combined raloxifene and 17β-estradiol.36 That study was meant to be a 52-week treatment trial with either raloxifene 60 mg alone or in combination with 17β-estradiol 1 mg per day to assess effects on VMS and endometrial safety. The study was stopped early because signs of endometrial stimulation were observed in the raloxifene plus estradiol group. Thus, one cannot combine any estrogen with any SERM and assume similar results.
Clinical application
The combination of conjugated estrogen/bazedoxifene is approved for treatment of VMS of menopause as well as prevention of osteoporosis. Although it is not approved for treatment of moderate to severe VVA, in younger women who initiate treatment it should prevent the development of moderate to severe symptoms of VVA.
Finally, this drug should be protective of the breast. Conjugated estrogen has clearly shown a reduction in breast cancer incidence and mortality, and bazedoxifene is a SERM. All SERMs have, as a class effect, been shown to be antiestrogens in breast tissue, and abundant preclinical data point in that direction.
This combination of conjugated estrogen/bazedoxifene, when it is once again clinically available, may well provide a new paradigm of hormone therapy that is progestogen free and has a benefit/risk ratio that tilts toward its benefits.
Potential for wider therapeutic benefits
Newer SERMs like ospemifene, approved for treatment of VVA/GSM, and bazedoxifene/conjugated estrogen combination, approved for treatment of VMS and prevention of bone loss, have other beneficial properties that can and should result in their more widespread use. ●
- Stuenkel CA. More evidence why the product labeling for low-dose vaginal estrogen should be changed? Menopause. 2018;25:4-6.
- Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
- Neven P, De Muylder X, Van Belle Y, et al. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
- Schwartz LB, Snyder J, Horan C, et al. The use of transvaginal ultrasound and saline infusion sonohysterography for the evaluation of asymptomatic postmenopausal breast cancer patients on tamoxifen. Ultrasound Obstet Gynecol. 1998;11:48-53.
- Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
- Portman DJ, Gass MLS. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437-447.
- Kingsberg SA, Krychman M, Graham S, et al. The Women’s EMPOWER Survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480-486.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20:623-630.
- Archer DF, Goldstein SR, Simon JA, et al. Efficacy and safety of ospemifene in postmenopausal women with moderateto-severe vaginal dryness: a phase 3, randomized, doubleblind, placebo-controlled, multicenter trial. Menopause. 2019;26:611-621.
- Osphena. Package insert. Shionogi Inc; 2018.
- Ospemifene (Osphena) for dyspareunia. Med Lett Drugs Ther. 2013;55:55-56.
- Addendum: Ospemifene (Osphena) for dyspareunia (Med Lett Drugs Ther 2013;55:55). Med Lett Drugs Ther. 2013;55:84.
- Goldstein SR, Bachmann G, Lin V, et al. Endometrial safety profile of ospemifene 60 mg when used for long-term treatment of vulvar and vaginal atrophy for up to 1 year. Abstract. Climacteric. 2011;14(suppl 1):S57.
- ACOG practice bulletin no. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189-2197.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141:809-820.
- Eigeliene N, Kangas L, Hellmer C, et al. Effects of ospemifene, a novel selective estrogen-receptor modulator, on human breast tissue ex vivo. Menopause. 2016;23:719-730.
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78:1273-1280.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Peng L, Luo Q, Lu H. Efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: a systematic review and meta-analysis. Medicine. 2017;96(49):e8659.
- Ronkin S, Northington R, Baracat E, et al. Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol. 2005;105:1397-1404.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;13:476-486.
- Kharode Y, Bodine PV, Miller CP, et al. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149:6084-6091.
- Song Y, Santen RJ, Wang JP, et al. Effects of the conjugated equine estrogen/bazedoxifene tissue-selective estrogen complex (TSEC) on mammary gland and breast cancer in mice. Endocrinology. 2012;153:5706-5715.
- Umland EM, Karel L, Santoro N. Bazedoxifene and conjugated equine estrogen: a combination product for the management of vasomotor symptoms and osteoporosis prevention associated with menopause. Pharmacotherapy. 2016;36:548-561.
- Kagan R, Goldstein SR, Pickar JH, et al. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag. 2016;12:549–562.
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol. 2013;121:959-968.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/ conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Fierce Pharma. Pfizer continues recalls of menopause drug Duavee on faulty packaging concerns. https:// www.fiercepharma.com/manufacturing/pfizer-recallsmenopause-drug-duavive-uk-due-to-faulty-packagingworries. June 9, 2020. Accessed February 8, 2022.
- Pfizer. Letter to health care provider. Subject: Duavee (conjugated estrogens/bazedoxifene) extended drug shortage. September 10, 2021.
- Stovall DW, Utian WH, Gass MLS, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause. 2007; 14(3 pt 1):510-517.
Selective estrogen receptor modulators (SERMs) are unique synthetic compounds that bind to the estrogen receptor and initiate either estrogenic agonistic or antagonistic activity, depending on the confirmational change they produce on binding to the receptor. Many SERMs have come to market, others have not. Unlike estrogens, which regardless of dose or route of administration all carry risks as a boxed warning on the label, referred to as class labeling,1 various SERMs exert various effects in some tissues (uterus, vagina) while they have apparent class properties in others (bone, breast).2
The first SERM, for all practical purposes, was tamoxifen (although clomiphene citrate is often considered a SERM). Tamoxifen was approved by the US Food and Drug Administration (FDA) in 1978 for the treatment of breast cancer and, subsequently, for breast cancer risk reduction. It became the most widely prescribed anticancer drug worldwide.
Subsequently, when data showed that tamoxifen could produce a small number of endometrial cancers and a larger number of endometrial polyps,3,4 there was renewed interest in raloxifene. In preclinical animal studies, raloxifene behaved differently than tamoxifen in the uterus. After clinical trials with raloxifene showed uterine safety,5 the drug was FDA approved for prevention of osteoporosis in 1997, for treatment of osteoporosis in 1999, and for breast cancer risk reduction in 2009. Most clinicians are familiar with these 2 SERMs, which have been in clinical use for more than 4 and 2 decades, respectively.
Ospemifene: A third-generation SERM and its indications
Hormone deficiency from menopause causes vulvovaginal and urogenital changes as well as a multitude of symptoms and signs, including vulvar and vaginal thinning, loss of rugal folds, diminished elasticity, increased pH, and most notably dyspareunia. The nomenclature that previously described vulvovaginal atrophy (VVA) has been expanded to include genitourinary syndrome of menopause (GSM).6 Unfortunately, many health care providers do not ask patients about GSM symptoms, and few women report their symptoms to their clinician.7 Furthermore, although low-dose local estrogens applied vaginally have been the mainstay of therapy for VVA/GSM, only 7% of symptomatic women use any pharmacologic agent,8 mainly because of fear of estrogens due to the class labeling mentioned above.
Ospemifene, a newer SERM, improved superficial cells and reduced parabasal cells as seen on a maturation index compared with placebo, according to results of multiple phase 3 clinical trials9,10; it also lowered vaginal pH and improved most bothersome symptoms (original studies were for dyspareunia). As a result, the FDA approved ospemifene for treatment of moderate to severe dyspareunia from VVA of menopause.
Subsequent studies allowed for a broadened indication to include treatment of moderate to severe dryness due to menopause.11 The ospemifene label contains a boxed warning that states, “In the endometrium, [ospemifene] has estrogen agonistic effects.”12 Although ospemifene is not an estrogen (it’s a SERM), the label goes on to state, “There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens.” This statement caused The Medical Letter to initially suggest that patients who receive ospemifene also should receive a progestational agent—a suggestion they later retracted.13,14
To understand why the ospemifene labeling might be worded in such a way, one must review the data regarding the poorly named entity “weakly proliferative endometrium.” The package labeling combines any proliferative endometrium (“weakly” plus “actively” plus “disordered”) that occurred in the clinical trial. Thus, 86.1 per 1,000 of the ospemifene-treated patients (vs 13.3 per 1,000 of those taking placebo) had any one of the proliferative types. The problem is that “actively proliferative” endometrial glands will have mitotic activity in virtually every nucleus of the gland as well as abundant glandular progression (FIGURE 1), whereas “weakly proliferative” is actually closer to inactive or atrophic endometrium with an occasional mitotic figure in only a few nuclei of each gland (FIGURE 2).


In addition, at 1 year, the incidence of active proliferation with ospemifene was 1%.15 In examining the uterine safety study for raloxifene, both doses of that agent had an active proliferation incidence of 3% at 1 year.5 Furthermore, that study had an estrogen-only arm in which, at end point, the incidence of endometrial proliferation was 39%, and hyperplasia, 23%!5 It therefore is evident that, in the endometrium, ospemifene is much more like the SERM raloxifene than it is like estrogen. The American College of Obstetricians and Gynecologists (ACOG) endorsed ospemifene (level A evidence) as a first-line therapy for dyspareunia, noting absent endometrial stimulation.16
Continue to: Ospemifene effects on breast and bone...
Ospemifene effects on breast and bone
Although ospemifene is approved for treatment of moderate to severe VVA/GSM, it has other SERM effects typical of its class. The label currently states that ospemifene “has not been adequately studied in women with breast cancer; therefore, it should not be used in women with known or suspected breast cancer.”12 We know that tamoxifen reduced breast cancer 49% in high-risk women in the Breast Cancer Prevention Trial (BCPT).17 We also know that in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, raloxifene reduced breast cancer 77% in osteoporotic women,18 and in the Study of Tamoxifen and Raloxifene (STAR) trial, it performed virtually identically to tamoxifen in breast cancer prevention.19 Previous studies demonstrated that ospemifene inhibits breast cancer cell growth in in vitro cultures as well as in animal studies20 and inhibits proliferation of human breast tissue epithelial cells,21 with breast effects similar to those seen with tamoxifen and raloxifene.
Thus, although one would not choose ospemifene as a primary treatment or risk-reducing agent for a patient with breast cancer, the direction of its activity in breast tissue is indisputable and is likely the reason that in the European Union (unlike in the United States) it is approved to treat dyspareunia from VVA/GSM in women with a prior history of breast cancer.
Virtually all SERMs have estrogen agonistic activity in bone. Bone is a dynamic organ, constantly being laid down and taken away (resorption). Estrogen and SERMs are potent antiresorptives in bone metabolism. Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to that of estradiol and raloxifene.22 Clinical data from 3 phase 1 or 2 clinical trials found that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.23 Actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, but there is a good correlation between biochemical markers for bone turnover and the occurrence of fracture.24 Once again, women who need treatment for osteoporosis should not be treated primarily with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
Clinical application
Ospemifene is an oral SERM approved for the treatment of moderate to severe dyspareunia as well as dryness from VVA due to menopause. In addition, it appears one can safely surmise that the direction of ospemifene’s activity in bone and breast is virtually indisputable. The magnitude of that activity, however, is unstudied. Therefore, in selecting an agent to treat women with dyspareunia or vaginal dryness from VVA of menopause, determining any potential add-on benefit for that particular patient in either bone and/or breast is clinically appropriate.
The SERM bazedoxifene
A meta-analysis of 4 randomized, placebo-controlled trials showed that another SERM, bazedoxifene, can significantly decrease the incidence of vertebral fracture in postmenopausal women at follow-up of 3 and 7 years.25 That meta-analysis also confirmed the long-term favorable safety and tolerability of bazedoxifene, with no increase in adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic events, or breast carcinoma in patients using bazedoxifene. However, bazedoxifene use did result in an increased incidence of hot flushes and leg cramps across 7 years.25 Bazedoxifene is available in a 20-mg dose for treatment of postmenopausal osteoporosis in Israel and a number of European Union countries.
Continue to: Enter the concept of tissue-selective estrogen complex (TSEC)...
Enter the concept of tissue-selective estrogen complex (TSEC)
Some postmenopausal women are extremely intolerant of any progestogen added to estrogen therapy to confer endometrial protection in those with a uterus. According to the results of a clinical trial of postmenopausal women, bazedoxifene is the only SERM shown to decrease endometrial thickness compared with placebo.26 This is the basis for thinking that perhaps a SERM like bazedoxifene, instead of a progestogen, could be used to confer endometrial protection.
A further consideration comes out of the evaluation of data derived from the 2 arms of the Women’s Health Initiative (WHI).27 In the arm that combined conjugated estrogen with medroxyprogesterone acetate through 11.3 years, there was a 25% increase in the incidence of invasive breast cancer, which was statistically significant. Contrast that with the arm in hysterectomized women who received only conjugated estrogen (often inaccurately referred to as the “estrogen only” arm of the WHI). In that study arm, the relative risk of invasive breast cancer was reduced 23%, also statistically significant. Thus, the culprit in the breast cancer incidence difference in these 2 arms appears to be the addition of the progestogen medroxyprogesterone acetate.27
Since the progestogen was used only for endometrial protection, could such endometrial protection be provided by a SERM like bazedoxifene? Preclinical trials showed that a combination of bazedoxifene and conjugated estrogen (in various estrogen doses) resulted in uterine wet weight in an ovariectomized rat model that was no different than that with placebo.28
In terms of effects on breast, preclinical models showed that conjugated estrogen use resulted in less mammary duct elongation and end bud proliferation than estradiol by itself, and that the combination of conjugated estrogen and bazedoxifene resulted in mammary duct elongation and end bud proliferation that was similar to that in the ovariectomized animals and considerably less than a combination of estradiol with bazedoxifene.29
Five phase 3 studies known as the SMART (Selective estrogens, Menopause, And Response to Therapy) trials were then conducted. Collectively, these studies examined the frequency and severity of vasomotor symptoms (VMS), BMD, bone turnover markers, lipid profiles, sleep, quality of life, breast density, and endometrial safety with conjugated estrogen/bazedoxifene treatment.30 Based on these trials with more than 7,500 women, in 2013 the FDA approved a compound of conjugated estrogen 0.45 mg and bazedoxifene 20 mg (Duavee in the United States and Duavive outside the United States).
The incidence of endometrial hyperplasia at 12 months was consistently less than 1%, which is the FDA guidance for approval of hormone therapies. The incidence of bleeding or spotting with conjugated estrogen/bazedoxifene (FIGURE 3) in each 4-week interval over 12 months mirror-imaged that of placebo and ranged from 3.9% in the first 4-week interval to 1.7% in the last 4 weeks, compared with conjugated estrogen 0.45 mg/medroxyprogesterone acetate 1.5 mg, which had a 20.8% incidence of bleeding or spotting in the first 4-week interval and was still at an 8.8% incidence in the last 4 weeks.31 This is extremely relevant in clinical practice. There was no difference from placebo in breast cancer incidence, breast pain or tenderness, abnormal mammograms, or breast density at month 12.32
In terms of frequency of VMS, there was a 74% reduction from baseline at 12 weeks compared with placebo (P<.001), as well as a 37% reduction in the VMS severity score (P<.001).32 Statistically significant improvements occurred in lumbar spine and hip BMD (P<.01) for women who were 1 to 5 years since menopause as well as for those who were more than 5 years since menopause.33
Packaging issue puts TSEC on back order
In May 2020, Pfizer voluntarily recalled its conjugated estrogen/bazedoxifene product after identifying a “flaw in the drug’s foil laminate pouch that introduced oxygen and lowered the dissolution rate of active pharmaceutical ingredient bazedoxifene acetate.”34 The manufacturer then wrote a letter to health care professionals in September 2021 stating, “Duavee continues to be out of stock due to an unexpected and complex packaging issue, resulting in manufacturing delays. This has nothing to do with the safety or quality of the product itself but could affect product stability throughout its shelf life… Given regulatory approval timelines for any new packaging, it is unlikely that Duavee will return to stock in 2022.”35
Other TSECs?
The conjugated estrogen/bazedoxifene combination is the first FDA-approved TSEC. Other attempts have been made to achieve similar results with combined raloxifene and 17β-estradiol.36 That study was meant to be a 52-week treatment trial with either raloxifene 60 mg alone or in combination with 17β-estradiol 1 mg per day to assess effects on VMS and endometrial safety. The study was stopped early because signs of endometrial stimulation were observed in the raloxifene plus estradiol group. Thus, one cannot combine any estrogen with any SERM and assume similar results.
Clinical application
The combination of conjugated estrogen/bazedoxifene is approved for treatment of VMS of menopause as well as prevention of osteoporosis. Although it is not approved for treatment of moderate to severe VVA, in younger women who initiate treatment it should prevent the development of moderate to severe symptoms of VVA.
Finally, this drug should be protective of the breast. Conjugated estrogen has clearly shown a reduction in breast cancer incidence and mortality, and bazedoxifene is a SERM. All SERMs have, as a class effect, been shown to be antiestrogens in breast tissue, and abundant preclinical data point in that direction.
This combination of conjugated estrogen/bazedoxifene, when it is once again clinically available, may well provide a new paradigm of hormone therapy that is progestogen free and has a benefit/risk ratio that tilts toward its benefits.
Potential for wider therapeutic benefits
Newer SERMs like ospemifene, approved for treatment of VVA/GSM, and bazedoxifene/conjugated estrogen combination, approved for treatment of VMS and prevention of bone loss, have other beneficial properties that can and should result in their more widespread use. ●
Selective estrogen receptor modulators (SERMs) are unique synthetic compounds that bind to the estrogen receptor and initiate either estrogenic agonistic or antagonistic activity, depending on the confirmational change they produce on binding to the receptor. Many SERMs have come to market, others have not. Unlike estrogens, which regardless of dose or route of administration all carry risks as a boxed warning on the label, referred to as class labeling,1 various SERMs exert various effects in some tissues (uterus, vagina) while they have apparent class properties in others (bone, breast).2
The first SERM, for all practical purposes, was tamoxifen (although clomiphene citrate is often considered a SERM). Tamoxifen was approved by the US Food and Drug Administration (FDA) in 1978 for the treatment of breast cancer and, subsequently, for breast cancer risk reduction. It became the most widely prescribed anticancer drug worldwide.
Subsequently, when data showed that tamoxifen could produce a small number of endometrial cancers and a larger number of endometrial polyps,3,4 there was renewed interest in raloxifene. In preclinical animal studies, raloxifene behaved differently than tamoxifen in the uterus. After clinical trials with raloxifene showed uterine safety,5 the drug was FDA approved for prevention of osteoporosis in 1997, for treatment of osteoporosis in 1999, and for breast cancer risk reduction in 2009. Most clinicians are familiar with these 2 SERMs, which have been in clinical use for more than 4 and 2 decades, respectively.
Ospemifene: A third-generation SERM and its indications
Hormone deficiency from menopause causes vulvovaginal and urogenital changes as well as a multitude of symptoms and signs, including vulvar and vaginal thinning, loss of rugal folds, diminished elasticity, increased pH, and most notably dyspareunia. The nomenclature that previously described vulvovaginal atrophy (VVA) has been expanded to include genitourinary syndrome of menopause (GSM).6 Unfortunately, many health care providers do not ask patients about GSM symptoms, and few women report their symptoms to their clinician.7 Furthermore, although low-dose local estrogens applied vaginally have been the mainstay of therapy for VVA/GSM, only 7% of symptomatic women use any pharmacologic agent,8 mainly because of fear of estrogens due to the class labeling mentioned above.
Ospemifene, a newer SERM, improved superficial cells and reduced parabasal cells as seen on a maturation index compared with placebo, according to results of multiple phase 3 clinical trials9,10; it also lowered vaginal pH and improved most bothersome symptoms (original studies were for dyspareunia). As a result, the FDA approved ospemifene for treatment of moderate to severe dyspareunia from VVA of menopause.
Subsequent studies allowed for a broadened indication to include treatment of moderate to severe dryness due to menopause.11 The ospemifene label contains a boxed warning that states, “In the endometrium, [ospemifene] has estrogen agonistic effects.”12 Although ospemifene is not an estrogen (it’s a SERM), the label goes on to state, “There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens.” This statement caused The Medical Letter to initially suggest that patients who receive ospemifene also should receive a progestational agent—a suggestion they later retracted.13,14
To understand why the ospemifene labeling might be worded in such a way, one must review the data regarding the poorly named entity “weakly proliferative endometrium.” The package labeling combines any proliferative endometrium (“weakly” plus “actively” plus “disordered”) that occurred in the clinical trial. Thus, 86.1 per 1,000 of the ospemifene-treated patients (vs 13.3 per 1,000 of those taking placebo) had any one of the proliferative types. The problem is that “actively proliferative” endometrial glands will have mitotic activity in virtually every nucleus of the gland as well as abundant glandular progression (FIGURE 1), whereas “weakly proliferative” is actually closer to inactive or atrophic endometrium with an occasional mitotic figure in only a few nuclei of each gland (FIGURE 2).


In addition, at 1 year, the incidence of active proliferation with ospemifene was 1%.15 In examining the uterine safety study for raloxifene, both doses of that agent had an active proliferation incidence of 3% at 1 year.5 Furthermore, that study had an estrogen-only arm in which, at end point, the incidence of endometrial proliferation was 39%, and hyperplasia, 23%!5 It therefore is evident that, in the endometrium, ospemifene is much more like the SERM raloxifene than it is like estrogen. The American College of Obstetricians and Gynecologists (ACOG) endorsed ospemifene (level A evidence) as a first-line therapy for dyspareunia, noting absent endometrial stimulation.16
Continue to: Ospemifene effects on breast and bone...
Ospemifene effects on breast and bone
Although ospemifene is approved for treatment of moderate to severe VVA/GSM, it has other SERM effects typical of its class. The label currently states that ospemifene “has not been adequately studied in women with breast cancer; therefore, it should not be used in women with known or suspected breast cancer.”12 We know that tamoxifen reduced breast cancer 49% in high-risk women in the Breast Cancer Prevention Trial (BCPT).17 We also know that in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, raloxifene reduced breast cancer 77% in osteoporotic women,18 and in the Study of Tamoxifen and Raloxifene (STAR) trial, it performed virtually identically to tamoxifen in breast cancer prevention.19 Previous studies demonstrated that ospemifene inhibits breast cancer cell growth in in vitro cultures as well as in animal studies20 and inhibits proliferation of human breast tissue epithelial cells,21 with breast effects similar to those seen with tamoxifen and raloxifene.
Thus, although one would not choose ospemifene as a primary treatment or risk-reducing agent for a patient with breast cancer, the direction of its activity in breast tissue is indisputable and is likely the reason that in the European Union (unlike in the United States) it is approved to treat dyspareunia from VVA/GSM in women with a prior history of breast cancer.
Virtually all SERMs have estrogen agonistic activity in bone. Bone is a dynamic organ, constantly being laid down and taken away (resorption). Estrogen and SERMs are potent antiresorptives in bone metabolism. Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to that of estradiol and raloxifene.22 Clinical data from 3 phase 1 or 2 clinical trials found that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.23 Actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, but there is a good correlation between biochemical markers for bone turnover and the occurrence of fracture.24 Once again, women who need treatment for osteoporosis should not be treated primarily with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
Clinical application
Ospemifene is an oral SERM approved for the treatment of moderate to severe dyspareunia as well as dryness from VVA due to menopause. In addition, it appears one can safely surmise that the direction of ospemifene’s activity in bone and breast is virtually indisputable. The magnitude of that activity, however, is unstudied. Therefore, in selecting an agent to treat women with dyspareunia or vaginal dryness from VVA of menopause, determining any potential add-on benefit for that particular patient in either bone and/or breast is clinically appropriate.
The SERM bazedoxifene
A meta-analysis of 4 randomized, placebo-controlled trials showed that another SERM, bazedoxifene, can significantly decrease the incidence of vertebral fracture in postmenopausal women at follow-up of 3 and 7 years.25 That meta-analysis also confirmed the long-term favorable safety and tolerability of bazedoxifene, with no increase in adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic events, or breast carcinoma in patients using bazedoxifene. However, bazedoxifene use did result in an increased incidence of hot flushes and leg cramps across 7 years.25 Bazedoxifene is available in a 20-mg dose for treatment of postmenopausal osteoporosis in Israel and a number of European Union countries.
Continue to: Enter the concept of tissue-selective estrogen complex (TSEC)...
Enter the concept of tissue-selective estrogen complex (TSEC)
Some postmenopausal women are extremely intolerant of any progestogen added to estrogen therapy to confer endometrial protection in those with a uterus. According to the results of a clinical trial of postmenopausal women, bazedoxifene is the only SERM shown to decrease endometrial thickness compared with placebo.26 This is the basis for thinking that perhaps a SERM like bazedoxifene, instead of a progestogen, could be used to confer endometrial protection.
A further consideration comes out of the evaluation of data derived from the 2 arms of the Women’s Health Initiative (WHI).27 In the arm that combined conjugated estrogen with medroxyprogesterone acetate through 11.3 years, there was a 25% increase in the incidence of invasive breast cancer, which was statistically significant. Contrast that with the arm in hysterectomized women who received only conjugated estrogen (often inaccurately referred to as the “estrogen only” arm of the WHI). In that study arm, the relative risk of invasive breast cancer was reduced 23%, also statistically significant. Thus, the culprit in the breast cancer incidence difference in these 2 arms appears to be the addition of the progestogen medroxyprogesterone acetate.27
Since the progestogen was used only for endometrial protection, could such endometrial protection be provided by a SERM like bazedoxifene? Preclinical trials showed that a combination of bazedoxifene and conjugated estrogen (in various estrogen doses) resulted in uterine wet weight in an ovariectomized rat model that was no different than that with placebo.28
In terms of effects on breast, preclinical models showed that conjugated estrogen use resulted in less mammary duct elongation and end bud proliferation than estradiol by itself, and that the combination of conjugated estrogen and bazedoxifene resulted in mammary duct elongation and end bud proliferation that was similar to that in the ovariectomized animals and considerably less than a combination of estradiol with bazedoxifene.29
Five phase 3 studies known as the SMART (Selective estrogens, Menopause, And Response to Therapy) trials were then conducted. Collectively, these studies examined the frequency and severity of vasomotor symptoms (VMS), BMD, bone turnover markers, lipid profiles, sleep, quality of life, breast density, and endometrial safety with conjugated estrogen/bazedoxifene treatment.30 Based on these trials with more than 7,500 women, in 2013 the FDA approved a compound of conjugated estrogen 0.45 mg and bazedoxifene 20 mg (Duavee in the United States and Duavive outside the United States).
The incidence of endometrial hyperplasia at 12 months was consistently less than 1%, which is the FDA guidance for approval of hormone therapies. The incidence of bleeding or spotting with conjugated estrogen/bazedoxifene (FIGURE 3) in each 4-week interval over 12 months mirror-imaged that of placebo and ranged from 3.9% in the first 4-week interval to 1.7% in the last 4 weeks, compared with conjugated estrogen 0.45 mg/medroxyprogesterone acetate 1.5 mg, which had a 20.8% incidence of bleeding or spotting in the first 4-week interval and was still at an 8.8% incidence in the last 4 weeks.31 This is extremely relevant in clinical practice. There was no difference from placebo in breast cancer incidence, breast pain or tenderness, abnormal mammograms, or breast density at month 12.32
In terms of frequency of VMS, there was a 74% reduction from baseline at 12 weeks compared with placebo (P<.001), as well as a 37% reduction in the VMS severity score (P<.001).32 Statistically significant improvements occurred in lumbar spine and hip BMD (P<.01) for women who were 1 to 5 years since menopause as well as for those who were more than 5 years since menopause.33
Packaging issue puts TSEC on back order
In May 2020, Pfizer voluntarily recalled its conjugated estrogen/bazedoxifene product after identifying a “flaw in the drug’s foil laminate pouch that introduced oxygen and lowered the dissolution rate of active pharmaceutical ingredient bazedoxifene acetate.”34 The manufacturer then wrote a letter to health care professionals in September 2021 stating, “Duavee continues to be out of stock due to an unexpected and complex packaging issue, resulting in manufacturing delays. This has nothing to do with the safety or quality of the product itself but could affect product stability throughout its shelf life… Given regulatory approval timelines for any new packaging, it is unlikely that Duavee will return to stock in 2022.”35
Other TSECs?
The conjugated estrogen/bazedoxifene combination is the first FDA-approved TSEC. Other attempts have been made to achieve similar results with combined raloxifene and 17β-estradiol.36 That study was meant to be a 52-week treatment trial with either raloxifene 60 mg alone or in combination with 17β-estradiol 1 mg per day to assess effects on VMS and endometrial safety. The study was stopped early because signs of endometrial stimulation were observed in the raloxifene plus estradiol group. Thus, one cannot combine any estrogen with any SERM and assume similar results.
Clinical application
The combination of conjugated estrogen/bazedoxifene is approved for treatment of VMS of menopause as well as prevention of osteoporosis. Although it is not approved for treatment of moderate to severe VVA, in younger women who initiate treatment it should prevent the development of moderate to severe symptoms of VVA.
Finally, this drug should be protective of the breast. Conjugated estrogen has clearly shown a reduction in breast cancer incidence and mortality, and bazedoxifene is a SERM. All SERMs have, as a class effect, been shown to be antiestrogens in breast tissue, and abundant preclinical data point in that direction.
This combination of conjugated estrogen/bazedoxifene, when it is once again clinically available, may well provide a new paradigm of hormone therapy that is progestogen free and has a benefit/risk ratio that tilts toward its benefits.
Potential for wider therapeutic benefits
Newer SERMs like ospemifene, approved for treatment of VVA/GSM, and bazedoxifene/conjugated estrogen combination, approved for treatment of VMS and prevention of bone loss, have other beneficial properties that can and should result in their more widespread use. ●
- Stuenkel CA. More evidence why the product labeling for low-dose vaginal estrogen should be changed? Menopause. 2018;25:4-6.
- Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
- Neven P, De Muylder X, Van Belle Y, et al. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
- Schwartz LB, Snyder J, Horan C, et al. The use of transvaginal ultrasound and saline infusion sonohysterography for the evaluation of asymptomatic postmenopausal breast cancer patients on tamoxifen. Ultrasound Obstet Gynecol. 1998;11:48-53.
- Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
- Portman DJ, Gass MLS. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437-447.
- Kingsberg SA, Krychman M, Graham S, et al. The Women’s EMPOWER Survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480-486.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20:623-630.
- Archer DF, Goldstein SR, Simon JA, et al. Efficacy and safety of ospemifene in postmenopausal women with moderateto-severe vaginal dryness: a phase 3, randomized, doubleblind, placebo-controlled, multicenter trial. Menopause. 2019;26:611-621.
- Osphena. Package insert. Shionogi Inc; 2018.
- Ospemifene (Osphena) for dyspareunia. Med Lett Drugs Ther. 2013;55:55-56.
- Addendum: Ospemifene (Osphena) for dyspareunia (Med Lett Drugs Ther 2013;55:55). Med Lett Drugs Ther. 2013;55:84.
- Goldstein SR, Bachmann G, Lin V, et al. Endometrial safety profile of ospemifene 60 mg when used for long-term treatment of vulvar and vaginal atrophy for up to 1 year. Abstract. Climacteric. 2011;14(suppl 1):S57.
- ACOG practice bulletin no. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189-2197.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141:809-820.
- Eigeliene N, Kangas L, Hellmer C, et al. Effects of ospemifene, a novel selective estrogen-receptor modulator, on human breast tissue ex vivo. Menopause. 2016;23:719-730.
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78:1273-1280.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Peng L, Luo Q, Lu H. Efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: a systematic review and meta-analysis. Medicine. 2017;96(49):e8659.
- Ronkin S, Northington R, Baracat E, et al. Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol. 2005;105:1397-1404.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;13:476-486.
- Kharode Y, Bodine PV, Miller CP, et al. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149:6084-6091.
- Song Y, Santen RJ, Wang JP, et al. Effects of the conjugated equine estrogen/bazedoxifene tissue-selective estrogen complex (TSEC) on mammary gland and breast cancer in mice. Endocrinology. 2012;153:5706-5715.
- Umland EM, Karel L, Santoro N. Bazedoxifene and conjugated equine estrogen: a combination product for the management of vasomotor symptoms and osteoporosis prevention associated with menopause. Pharmacotherapy. 2016;36:548-561.
- Kagan R, Goldstein SR, Pickar JH, et al. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag. 2016;12:549–562.
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol. 2013;121:959-968.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/ conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Fierce Pharma. Pfizer continues recalls of menopause drug Duavee on faulty packaging concerns. https:// www.fiercepharma.com/manufacturing/pfizer-recallsmenopause-drug-duavive-uk-due-to-faulty-packagingworries. June 9, 2020. Accessed February 8, 2022.
- Pfizer. Letter to health care provider. Subject: Duavee (conjugated estrogens/bazedoxifene) extended drug shortage. September 10, 2021.
- Stovall DW, Utian WH, Gass MLS, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause. 2007; 14(3 pt 1):510-517.
- Stuenkel CA. More evidence why the product labeling for low-dose vaginal estrogen should be changed? Menopause. 2018;25:4-6.
- Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
- Neven P, De Muylder X, Van Belle Y, et al. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
- Schwartz LB, Snyder J, Horan C, et al. The use of transvaginal ultrasound and saline infusion sonohysterography for the evaluation of asymptomatic postmenopausal breast cancer patients on tamoxifen. Ultrasound Obstet Gynecol. 1998;11:48-53.
- Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
- Portman DJ, Gass MLS. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437-447.
- Kingsberg SA, Krychman M, Graham S, et al. The Women’s EMPOWER Survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480-486.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20:623-630.
- Archer DF, Goldstein SR, Simon JA, et al. Efficacy and safety of ospemifene in postmenopausal women with moderateto-severe vaginal dryness: a phase 3, randomized, doubleblind, placebo-controlled, multicenter trial. Menopause. 2019;26:611-621.
- Osphena. Package insert. Shionogi Inc; 2018.
- Ospemifene (Osphena) for dyspareunia. Med Lett Drugs Ther. 2013;55:55-56.
- Addendum: Ospemifene (Osphena) for dyspareunia (Med Lett Drugs Ther 2013;55:55). Med Lett Drugs Ther. 2013;55:84.
- Goldstein SR, Bachmann G, Lin V, et al. Endometrial safety profile of ospemifene 60 mg when used for long-term treatment of vulvar and vaginal atrophy for up to 1 year. Abstract. Climacteric. 2011;14(suppl 1):S57.
- ACOG practice bulletin no. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189-2197.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141:809-820.
- Eigeliene N, Kangas L, Hellmer C, et al. Effects of ospemifene, a novel selective estrogen-receptor modulator, on human breast tissue ex vivo. Menopause. 2016;23:719-730.
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78:1273-1280.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Peng L, Luo Q, Lu H. Efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: a systematic review and meta-analysis. Medicine. 2017;96(49):e8659.
- Ronkin S, Northington R, Baracat E, et al. Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol. 2005;105:1397-1404.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;13:476-486.
- Kharode Y, Bodine PV, Miller CP, et al. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149:6084-6091.
- Song Y, Santen RJ, Wang JP, et al. Effects of the conjugated equine estrogen/bazedoxifene tissue-selective estrogen complex (TSEC) on mammary gland and breast cancer in mice. Endocrinology. 2012;153:5706-5715.
- Umland EM, Karel L, Santoro N. Bazedoxifene and conjugated equine estrogen: a combination product for the management of vasomotor symptoms and osteoporosis prevention associated with menopause. Pharmacotherapy. 2016;36:548-561.
- Kagan R, Goldstein SR, Pickar JH, et al. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag. 2016;12:549–562.
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol. 2013;121:959-968.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/ conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Fierce Pharma. Pfizer continues recalls of menopause drug Duavee on faulty packaging concerns. https:// www.fiercepharma.com/manufacturing/pfizer-recallsmenopause-drug-duavive-uk-due-to-faulty-packagingworries. June 9, 2020. Accessed February 8, 2022.
- Pfizer. Letter to health care provider. Subject: Duavee (conjugated estrogens/bazedoxifene) extended drug shortage. September 10, 2021.
- Stovall DW, Utian WH, Gass MLS, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause. 2007; 14(3 pt 1):510-517.
Uterine incision closure: Is it the culprit in the cesarean scar niche and related complications?
While its etiology remains uncertain, cesarean scar niche (CSN) is well publicized, as are its pathological clinical manifestations. In a future pregnancy, they include cesarean scar pregnancy (CSP), which in turn can lead to placenta accreta spectrum, and possible uterine rupture/dehiscence of a residual thin myometrial layer. CSP refers to the implantation of an early pregnancy on the scar or in the niche at the site of a prior cesarean delivery (CD); it has an incidence of 1 per 1,000 pregnancies. An estimated 52% of CSPs occur after even just one CD.1 CSP has been linked to placenta accreta spectrum and has been shown to be its precursor.2 Both CSP and placenta accreta spectrum can be consequences of CD and share a common histology of villous or placental attachment/invasion into the cesarean scar.3 The incidence of placenta accreta spectrum has risen from about 1 in 4,000 live births in the 1970s to 1 in 2,500 in the 1980s; in 2016, the incidence of placenta accreta spectrum was reported as 1 per 272 live births.4
Placenta accreta spectrum denotes the attachment of the placenta into and through the myometrium,5 and it can result in severe complications, including hemorrhage, hysterectomy, and intensive care treatment. The increasing rate of placenta accreta spectrum parallels the increasing CD rate, which rose from 5.8% in 1970 to 31.9% in 2016.6 Multiple repeat CDs are increasing in frequency as well. At the beginning of the century, placenta accreta spectrum mainly occurred after manual removal of the placenta, uterine curettage, or endometritis. Recently, experts are in agreement that the main determinant of placenta accreta spectrum is the uterine scar and niche formation after a previous CD.5 Larger niches are associated with an increased incidence of uterine rupture or dehiscence in a subsequent pregnancy.7
In the nonpregnant state, such niches are associated with intermenstrual bleeding, pelvic pain, painful intercourse, painful menses, and subfertility, becoming increasingly more severe in women with greater numbers of CDs.8-10 Conception rate with assisted reproductive treatment is notably reduced.11
Understanding its etiology
Monteagudo and colleagues first described a “niche” in 100% of 44 women evaluated for postmenopausal bleeding who had a prior CD.12 CSN has been the subject of well over 3,000 publications over the past 30 years. While the topic generates much interest among researchers, it is garnering little traction among practicing obstetricians. Such “niches,” also referred to as isthmocele, cesarean scar defect, or a diverticulum, was first described in 196113 and later defined on ultrasonography as a hypoechoic triangular-shaped uterine defect outlined by saline instillation sonohysterogram (SIS), reflecting a discontinuation of the myometrium at the site of a previous CD.12 In 2019, a European task force further defined a CSN as an “indentation at the site in the cesarean section scar with a depth of at least 2 mm” and extended the classification to include branches as extensions toward the anterior uterine serosa.14 Using this criterion, sonographic postoperative evaluation after one CD revealed a CSN in 68.9% of women with one single-layer uterine closure and in 73.6% of women after a double-layer closure.15 Larger niche sizes with thinner residual myometrial thickness appeared more frequently when a single-layer closure technique was used, without closure of the peritoneum. Its prevalence varies from 56% to 84%.16,17
Etiology of CSN formation: Our hypotheses
The precise pathophysiology of CSN remains elusive. Speculations attributed niche formation to numerous factors: timing of surgery, cervical incision, incomplete closure of the uterine incision, adhesion formation between the CD scar and the abdominal wall, and inherent maternal conditions which may impair healing, such as smoking, obesity, diabetes, maternal age, and labor status.18-20 Retroflexion of the uterus is reportedly associated with increased incidence and size of the niche, with CSN 50% more likely to develop in women with a retroflexed versus an anteverted uterus.21 We demonstrated the origin of niche formation in real-time from the start to the completion of uterine closure by a video capture of a single-layer closure followed by an immediate SIS of the ex vivo hysterectomized uterus, and histopathologic proof of the presence of endometrial cells defining the “niche.”22 This case exposes the misalignment of the uterine wall, while including the endometrium in the closure (FIGURE 1). Similarly, pathologic studies of hysteroscopy-resected isthmocele ridges of symptomatic women with niche-related subfertility revealed the tissue edges lined by endocervical, endometrial, or isthmic mucosa either combined or isolated in the scar.23 The presence of endometrial/cervical tissue in the myometrial closure has been debated for over a century.24,25
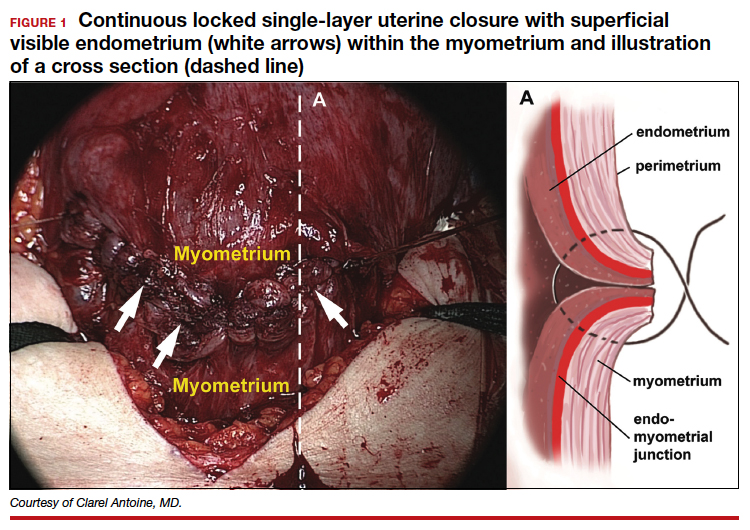
Continue to: Uterine closure techniques...
Uterine closure techniques: Historical perspective
In 1882, Max Sanger introduced a vertical uterine closure of a classical cesarean operation in response to hysterectomy as the contemporaneous alternative to prevent infection, bleeding, and death.24 Dr. Sanger emphasized layer approximation, suturing, and the avoidance of decidua in the first layer (FIGURE 2). This became the teaching of the classical CD until the 1970s. In 1926, Munro Kerr addressed uterine rupture with labor after a classical CD by introducing the lower uterine segment transverse incision. He cautioned to maintain the decidua inside the uterine 2-layer closure of the cavity.25 These pioneers were joined by others to rally for endometrium exclusion while promoting layer approximation. These techniques became universally standard and were taught across teaching medical centers in the United States and abroad until about 50 years ago.
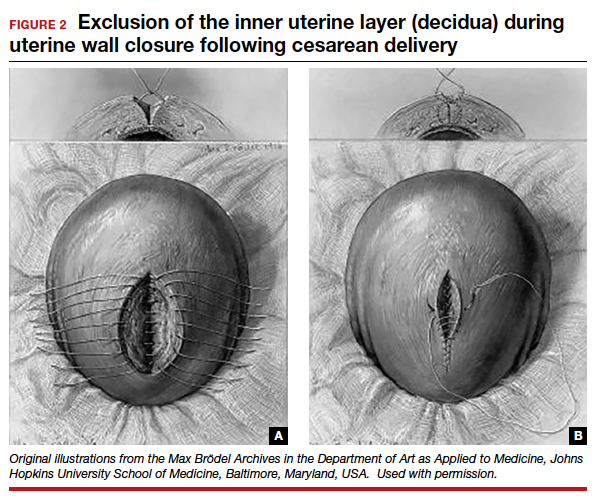
In the 1970s, newer developments brought significant changes to uterine closure techniques. Initiated by Joel-Cohen,26 blunt dissection of the abdominal incision was adapted by Michael Stark, creating what came to be known as the Misgav-Ladach cesarean technique.27 Stark emphasized blunt dissection and introduced single-layer closure. Thereby the exclusion of the endometrium, used for more than 70 years, was abandoned by the present-day single- or double-layer uterine closure in favor of cost and time savings. Systematic reviews and meta-analyses comparing the two contrasting techniques were inconclusive, noting that the niche prevalence and size were similar in both groups. These studies did not take into account the variety of individual techniques or the position of the endometrium in the final closures.28
Endometrium and uterine closure
Our recent study examining uterine scar defect in women after one primary CD by SIS concluded that a specific endometrium-free closure technique (EFCT) (FIGURE 3) is associated with fewer and less severe defects and a thicker residual myometrial thickness when compared with closures with unknown or endometrium inclusion.29 The study found non-specific closure techniques to be 6 times more likely to form a niche of 2-mm deep or greater than the EFCT.
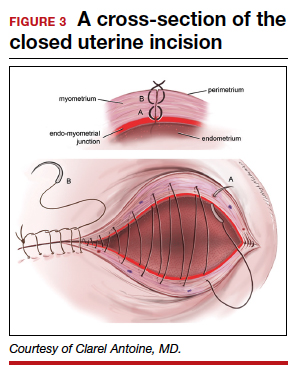
Furthermore, we surveyed the diversity of uterine closures and the location of the endometrium among obstetricians in one institution.30 Presence of endometrium on the surface of the final uterine closure was reported by 20% of respondents (see Figure 1). When asked for their opinion on the impact of CD techniques on placenta accreta spectrum, without available evidence 80% of the survey respondents reported no relationship to techniques, and only 20% suggested an association. This particular study demonstrates that the surgical techniques just described are random, unfettered, and applied without consideration of clinical outcomes.
Our recent retrospective study that spanned 30 years and examined the EFCT—performed anywhere between 3 to 9 consecutive CDs—revealed no abnormal placentation in any subsequent pregnancies.31 This was one of the few clinical studies of the long-term consequences of a uterine closure technique. In this study, the endometrium was excluded during the uterine closure, allowing its free edges to abut and heal. This step avoids scarring the endometrial-myometrial (EM) interface and unintentional inclusion of endometrium in the closed uterine wall. In this context, Jauniaux and colleagues cited the destruction of the EM interface as the main factor for placenta-adherent disorders.32 Sholapurkar and others highlight the need to further examine intrinsic details of uterine closure beyond single- and double-layer techniques to better understand the etiology of cesarean scar formation.19 The search for the pathophysiology of CSN continues to present significant challenges imposed by the variety of currently practiced uterine closures.
Continue to: Focus on prevention...
Research: Focus on prevention
Our research aims to address the endometrium, a specific layer that was the topic of concern in nascent CD techniques, as a renewed and contemporary one. The presence of the endometrium in ectopic locations or its destruction from intrauterine surgeries or infections has been implicated in abnormal placentation.13,24 Our approach, in theory, is to limit the position of the endometrium to its innermost location and avoid its iatrogenic suturing and inclusion into the uterine wall closure. The rationale of sparing the endometrium in a layer-by-layer approximation is to allow for a closer restoration to normal anatomy and physiology than a random “en masse” uterine wall closure would permit. For this reason, the EM junction, the perimetrium, and the serosa must be identified and realigned for a more effective closure that incorporates the entire myometrial thickness. As evidence supports technical impact on the development of uterine scar defect in women after one CD, future studies are needed to evaluate uterine integrity by saline infusion sonohysterography in multiparous women with a prior random closure technique or a prior EFCT.
The potential long-term risks of blunt dissection for opening the uterus have not been studied. There are no physiologic lines in the uterine wall to facilitate a regular-bordered uterine stretch. The tissue stretch, which depends on the individual surgeon’s strength applied during the procedure and patient’s labor status, may result in an irregular tear and a difficult repair. The EFCT technique shows a more optimized risk-benefit ratio for an anatomical repair and is replicable. The safety of uterine layer re-approximation has been demonstrated and can be studied in large populations using strict uniform criteria.
Current and future challenges
Residency training
Most recently, teachers of resident trainees are mostly familiar with blunt dissection, techniques of which are passed on unchallenged from resident to resident. The endometrium and peritoneum are neither identified nor treated as separate layers, thus becoming obsolete as surgical and anatomical landmarks.
Standardization of CD techniques
Front-line obstetricians are persuaded to practice a standardized approach that relies on the benefits of cost related to operating room turnover as well as surgeons’ time savings without consideration of outcomes in subsequent pregnancies. Sholapurkar has warned that “wrong standardization” is far worse than no standardization, worse for the training of junior obstetricians, as it can inhibit critical reasoning about safe surgical techniques that can optimize outcomes of the condition of the lower uterine segment.33
Emergence of cost and time savings in clinical practice
A time-cost savings argument is relatively negligeable in an estimated 40-minute CD. By contrast, deliberate surgical technique and carrying out the appropriate steps for the particular condition at hand to achieve the best outcomes assume more weight.32 Furthermore, this short-term cost benefit is challenged by the comparatively larger costs associated with the diagnosis, the treatment of post-CD adverse consequences (outlined above), as well as the emotional impact on women and their families. Additionally, the emphasis on time savings creates a generation of surgeons fixated with total operative time without consideration of long-term risks and adverse maternal outcomes.
Physician autonomy has led to the unmonitored freedom of obstetricians to choose their own technique for a CD, with some employing the commonly practiced culture of fastest turnaround even in nonurgent circumstances.
Documentation and terminology
Current documenting systems are not detail-oriented enough to assist in a thorough correlation between surgical techniques and outcomes. The use of single- or double-layer closure terminology is insufficient and has proven to be flawed, without describing the handling of the endometrium in terms of its inclusion or exclusion in the closure.
Quality improvement feedback
Long-term post-CD complications are often not reported to the physician or institution involved in the prior CD. In our opinion, some sort of registry would be of value. Perhaps then subsequent CD outcomes could be traced back and reported to the prior institution and surgeon. Feedback is critical to understanding the correlation between techniques and outcomes and more specifically to gathering learning points and using data for quality improvement of future cases.
Patient education
While women continue to have complications following the presently used surgical techniques, they often have expectations not discussed with their obstetricians. Women should be educated and empowered to realize the different approaches to all aspects and consequences of CDs.
Conclusion
The technique of excluding the endometrium in closing the uterine incision appears to reduce subsequent abnormal placentation and diminish the frequency and size of post-CD scar defect. The revival of the endometrium-free closure technique may allow significant change in the postoperative results. Currently, standardization of CD technique is being promoted on the basis of time- and cost-savings rather than clinical outcomes. Simultaneously, inroads are being made to better understand the risks and consequences of CD.
Emerging evidence suggests that a post-CD niche is the result of poor layer approximation as well as inclusion of the endometrium, which prevent healing of the uterine wall and often enables faulty implantation of the fertilized oocyte in the next pregnancy, potentially giving rise to placenta accreta spectrum. The prevalence and size of the defect can be minimized by techniques aimed at restoring the anatomy of the uterine wall and the physiology of the endometrium. Specialized training and education are necessary to stress the importance of anatomical assessment and decision making at the time of uterine closure. ●
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107:1373-1381.
- Timor-Tritsch IE, Monteagudo A, Calì G, et al. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol. 2014;44:346-353. doi:10.1002/ uog.13426.
- Timor-Tritsch IE, Monteagudo A, Cali G, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014;43:383-395. doi: 10.1002/uog.13282.
- Mogos MF, Salemi JL, Ashley M, et al. Recent trends in placenta accreta in the United States and its impact on maternal-fetal morbidity and healthcare-associated costs, 1998-2011. J Matern Fetal Neonatal Med. 2016;29:1077-1082.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75-87.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2016. NCHS Data Brief. 2017(287):1-8.
- Vikhareva Osser O, Valentin L. Clinical importance of appearance of cesarean hysterotomy scar at transvaginal ultrasonography in nonpregnant women. Obstet Gynecol. 2011;117:525-532.
- Chen YY, Tsai CC, Kung FT, et al. Association between hysteroscopic findings of previous cesarean delivery scar defects and abnormal uterine bleeding. Taiwanese J Obstet Gynecol. 2019;58:541-544.
- Stegwee SI, Beij A, de Leeuw RA, et al. Niche-related outcomes after caesarean section and quality of life: a focus group study and review of literature. Qual Life Res. 2020;29:1013-1025.
- Vissers J, Hehenkamp W, Lambalk CB, et al. Post-caesarean section niche-related impaired fertility: hypothetical mechanisms. Hum Reprod. 2020;35:1484-1494.
- Vissers J, Sluckin TC, van Driel-Delprat CCR, et al. Reduced pregnancy and live birth rates after in vitro fertilization in women with previous caesarean section: a retrospective cohort study. Hum Reprod. 2020;35:595-604.
- Monteagudo A, Carreno C, Timor-Tritsch IE. Saline infusion sonohysterography in nonpregnant women with previous cesarean delivery: the “niche” in the scar. J Ultrasound Med. 2001;20:1105-1115.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67-71.
- Jordans IPM, de Leeuw RA, Stegwee SI, et al. Sonographic examination of uterine niche in non-pregnant women: a modified Delphi procedure. Ultrasound Obstet Gynecol. 2019;53:107-115.
- Stegwee SI, van der Voet LF, Ben AJ, et al. Effect of single- versus double-layer uterine closure during caesarean section on postmenstrual spotting (2Close): multicentre, double-blind, randomised controlled superiority trial. BJOG. 2021;128:866-878.
- Bij de Vaate AJ, van der Voet LF, Naji O, et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following cesarean section: systematic review. Ultrasound Obstet Gynecol. 2014;43:372-382.
- van der Voet LF, Bij de Vaate AM, Veersema S, et al. Long-term complications of caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121:236-244.
- Vervoort AJ, Uittenbogaard LB, Hehenkamp WJ, et al. Why do niches develop in caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum Reprod. 2015;30:2695-2702.
- Sholapurkar SL. Etiology of cesarean uterine scar defect (niche): detailed critical analysis of hypotheses and prevention strategies and peritoneal closure debate. J Clin Med Res. 2018;10:166-173.
- Kamel R, Eissa T, Sharaf M, et al. Position and integrity of uterine scar are determined by degree of cervical dilatation at time of cesarean section. Ultrasound Obstet Gynecol. 2021;57:466-470.
- Sanders RC, Parsons AK. Anteverted retroflexed uterus: a common consequence of cesarean delivery. AJR Am J Roentgenol. 2014;203:W117-124.
- Antoine C, Pimentel RN, Timor-Tritsch IE, et al. Origin of a post-cesarean delivery niche: diagnosis, pathophysiologic characteristics, and video documentation. J Ultrasound Med. 2021;40:205-208.
- AbdullGaffar B, Almulla A. A histopathologic approach to uterine niche: what to expect and to report in hysteroscopy-resected isthmocele specimens. Int J Surg Pathol. 2021:10668969211039415. doi: 10.1177/10668969211039415.
- Nagy S, Papp Z. Global approach of the cesarean section rates. J Perinatal Med. 2020;49:1-4.
- Kerr JM. The technic of cesarean section, with special reference to the lower uterine segment incision. Am J Obstet Gynecol. 1926;12:729-734.
- Joel-Cohen S. Abdominal and vaginal hysterectomy: new techniques based on time and motion studies. Lippincott Williams & Wilkins; 1977.
- Holmgren G, Sjoholm L, Stark M. The Misgav Ladach method for cesarean section: method description. Acta Obstet Gynecol Scand. 1999;78:615-621.
- Abalos E, Addo V, Brocklehurst P, et al. Caesarean section surgical techniques: 3-year follow-up of the CORONIS fractional, factorial, unmasked, randomised controlled trial. Lancet. 2016;388:62-72.
- Antoine C, Meyer JA, Silverstein JS, et al. The impact of uterine incision closure techniques on post-cesarean delivery niche formation and size: sonohysterographic examination of nonpregnant women. J Ultrasound Med. 2021. doi: 10.1002/ jum.15859.
- Antoine C AJ, Yaghoubian Y, Harary J. Variations in uterine closure technique: an institutional survey of obstetricians and implications for patient counseling and prevention of adverse sequelae [Abstract]. 2021.
- Antoine C, Pimentel RN, Reece EA, et al. Endometrium-free uterine closure technique and abnormal placental implantation in subsequent pregnancies. J Matern-Fetal Neonatal Med. 2019:1-9.
- Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33:244-251.
- Sholapurkar S. Review of unsafe changes in the practice of cesarean section with analysis of flaws in the interpretation of statistics and the evidence. Surgical Case Reports. 2021;4:2-6.

While its etiology remains uncertain, cesarean scar niche (CSN) is well publicized, as are its pathological clinical manifestations. In a future pregnancy, they include cesarean scar pregnancy (CSP), which in turn can lead to placenta accreta spectrum, and possible uterine rupture/dehiscence of a residual thin myometrial layer. CSP refers to the implantation of an early pregnancy on the scar or in the niche at the site of a prior cesarean delivery (CD); it has an incidence of 1 per 1,000 pregnancies. An estimated 52% of CSPs occur after even just one CD.1 CSP has been linked to placenta accreta spectrum and has been shown to be its precursor.2 Both CSP and placenta accreta spectrum can be consequences of CD and share a common histology of villous or placental attachment/invasion into the cesarean scar.3 The incidence of placenta accreta spectrum has risen from about 1 in 4,000 live births in the 1970s to 1 in 2,500 in the 1980s; in 2016, the incidence of placenta accreta spectrum was reported as 1 per 272 live births.4
Placenta accreta spectrum denotes the attachment of the placenta into and through the myometrium,5 and it can result in severe complications, including hemorrhage, hysterectomy, and intensive care treatment. The increasing rate of placenta accreta spectrum parallels the increasing CD rate, which rose from 5.8% in 1970 to 31.9% in 2016.6 Multiple repeat CDs are increasing in frequency as well. At the beginning of the century, placenta accreta spectrum mainly occurred after manual removal of the placenta, uterine curettage, or endometritis. Recently, experts are in agreement that the main determinant of placenta accreta spectrum is the uterine scar and niche formation after a previous CD.5 Larger niches are associated with an increased incidence of uterine rupture or dehiscence in a subsequent pregnancy.7
In the nonpregnant state, such niches are associated with intermenstrual bleeding, pelvic pain, painful intercourse, painful menses, and subfertility, becoming increasingly more severe in women with greater numbers of CDs.8-10 Conception rate with assisted reproductive treatment is notably reduced.11
Understanding its etiology
Monteagudo and colleagues first described a “niche” in 100% of 44 women evaluated for postmenopausal bleeding who had a prior CD.12 CSN has been the subject of well over 3,000 publications over the past 30 years. While the topic generates much interest among researchers, it is garnering little traction among practicing obstetricians. Such “niches,” also referred to as isthmocele, cesarean scar defect, or a diverticulum, was first described in 196113 and later defined on ultrasonography as a hypoechoic triangular-shaped uterine defect outlined by saline instillation sonohysterogram (SIS), reflecting a discontinuation of the myometrium at the site of a previous CD.12 In 2019, a European task force further defined a CSN as an “indentation at the site in the cesarean section scar with a depth of at least 2 mm” and extended the classification to include branches as extensions toward the anterior uterine serosa.14 Using this criterion, sonographic postoperative evaluation after one CD revealed a CSN in 68.9% of women with one single-layer uterine closure and in 73.6% of women after a double-layer closure.15 Larger niche sizes with thinner residual myometrial thickness appeared more frequently when a single-layer closure technique was used, without closure of the peritoneum. Its prevalence varies from 56% to 84%.16,17
Etiology of CSN formation: Our hypotheses
The precise pathophysiology of CSN remains elusive. Speculations attributed niche formation to numerous factors: timing of surgery, cervical incision, incomplete closure of the uterine incision, adhesion formation between the CD scar and the abdominal wall, and inherent maternal conditions which may impair healing, such as smoking, obesity, diabetes, maternal age, and labor status.18-20 Retroflexion of the uterus is reportedly associated with increased incidence and size of the niche, with CSN 50% more likely to develop in women with a retroflexed versus an anteverted uterus.21 We demonstrated the origin of niche formation in real-time from the start to the completion of uterine closure by a video capture of a single-layer closure followed by an immediate SIS of the ex vivo hysterectomized uterus, and histopathologic proof of the presence of endometrial cells defining the “niche.”22 This case exposes the misalignment of the uterine wall, while including the endometrium in the closure (FIGURE 1). Similarly, pathologic studies of hysteroscopy-resected isthmocele ridges of symptomatic women with niche-related subfertility revealed the tissue edges lined by endocervical, endometrial, or isthmic mucosa either combined or isolated in the scar.23 The presence of endometrial/cervical tissue in the myometrial closure has been debated for over a century.24,25

Continue to: Uterine closure techniques...
Uterine closure techniques: Historical perspective
In 1882, Max Sanger introduced a vertical uterine closure of a classical cesarean operation in response to hysterectomy as the contemporaneous alternative to prevent infection, bleeding, and death.24 Dr. Sanger emphasized layer approximation, suturing, and the avoidance of decidua in the first layer (FIGURE 2). This became the teaching of the classical CD until the 1970s. In 1926, Munro Kerr addressed uterine rupture with labor after a classical CD by introducing the lower uterine segment transverse incision. He cautioned to maintain the decidua inside the uterine 2-layer closure of the cavity.25 These pioneers were joined by others to rally for endometrium exclusion while promoting layer approximation. These techniques became universally standard and were taught across teaching medical centers in the United States and abroad until about 50 years ago.

In the 1970s, newer developments brought significant changes to uterine closure techniques. Initiated by Joel-Cohen,26 blunt dissection of the abdominal incision was adapted by Michael Stark, creating what came to be known as the Misgav-Ladach cesarean technique.27 Stark emphasized blunt dissection and introduced single-layer closure. Thereby the exclusion of the endometrium, used for more than 70 years, was abandoned by the present-day single- or double-layer uterine closure in favor of cost and time savings. Systematic reviews and meta-analyses comparing the two contrasting techniques were inconclusive, noting that the niche prevalence and size were similar in both groups. These studies did not take into account the variety of individual techniques or the position of the endometrium in the final closures.28
Endometrium and uterine closure
Our recent study examining uterine scar defect in women after one primary CD by SIS concluded that a specific endometrium-free closure technique (EFCT) (FIGURE 3) is associated with fewer and less severe defects and a thicker residual myometrial thickness when compared with closures with unknown or endometrium inclusion.29 The study found non-specific closure techniques to be 6 times more likely to form a niche of 2-mm deep or greater than the EFCT.

Furthermore, we surveyed the diversity of uterine closures and the location of the endometrium among obstetricians in one institution.30 Presence of endometrium on the surface of the final uterine closure was reported by 20% of respondents (see Figure 1). When asked for their opinion on the impact of CD techniques on placenta accreta spectrum, without available evidence 80% of the survey respondents reported no relationship to techniques, and only 20% suggested an association. This particular study demonstrates that the surgical techniques just described are random, unfettered, and applied without consideration of clinical outcomes.
Our recent retrospective study that spanned 30 years and examined the EFCT—performed anywhere between 3 to 9 consecutive CDs—revealed no abnormal placentation in any subsequent pregnancies.31 This was one of the few clinical studies of the long-term consequences of a uterine closure technique. In this study, the endometrium was excluded during the uterine closure, allowing its free edges to abut and heal. This step avoids scarring the endometrial-myometrial (EM) interface and unintentional inclusion of endometrium in the closed uterine wall. In this context, Jauniaux and colleagues cited the destruction of the EM interface as the main factor for placenta-adherent disorders.32 Sholapurkar and others highlight the need to further examine intrinsic details of uterine closure beyond single- and double-layer techniques to better understand the etiology of cesarean scar formation.19 The search for the pathophysiology of CSN continues to present significant challenges imposed by the variety of currently practiced uterine closures.
Continue to: Focus on prevention...
Research: Focus on prevention
Our research aims to address the endometrium, a specific layer that was the topic of concern in nascent CD techniques, as a renewed and contemporary one. The presence of the endometrium in ectopic locations or its destruction from intrauterine surgeries or infections has been implicated in abnormal placentation.13,24 Our approach, in theory, is to limit the position of the endometrium to its innermost location and avoid its iatrogenic suturing and inclusion into the uterine wall closure. The rationale of sparing the endometrium in a layer-by-layer approximation is to allow for a closer restoration to normal anatomy and physiology than a random “en masse” uterine wall closure would permit. For this reason, the EM junction, the perimetrium, and the serosa must be identified and realigned for a more effective closure that incorporates the entire myometrial thickness. As evidence supports technical impact on the development of uterine scar defect in women after one CD, future studies are needed to evaluate uterine integrity by saline infusion sonohysterography in multiparous women with a prior random closure technique or a prior EFCT.
The potential long-term risks of blunt dissection for opening the uterus have not been studied. There are no physiologic lines in the uterine wall to facilitate a regular-bordered uterine stretch. The tissue stretch, which depends on the individual surgeon’s strength applied during the procedure and patient’s labor status, may result in an irregular tear and a difficult repair. The EFCT technique shows a more optimized risk-benefit ratio for an anatomical repair and is replicable. The safety of uterine layer re-approximation has been demonstrated and can be studied in large populations using strict uniform criteria.
Current and future challenges
Residency training
Most recently, teachers of resident trainees are mostly familiar with blunt dissection, techniques of which are passed on unchallenged from resident to resident. The endometrium and peritoneum are neither identified nor treated as separate layers, thus becoming obsolete as surgical and anatomical landmarks.
Standardization of CD techniques
Front-line obstetricians are persuaded to practice a standardized approach that relies on the benefits of cost related to operating room turnover as well as surgeons’ time savings without consideration of outcomes in subsequent pregnancies. Sholapurkar has warned that “wrong standardization” is far worse than no standardization, worse for the training of junior obstetricians, as it can inhibit critical reasoning about safe surgical techniques that can optimize outcomes of the condition of the lower uterine segment.33
Emergence of cost and time savings in clinical practice
A time-cost savings argument is relatively negligeable in an estimated 40-minute CD. By contrast, deliberate surgical technique and carrying out the appropriate steps for the particular condition at hand to achieve the best outcomes assume more weight.32 Furthermore, this short-term cost benefit is challenged by the comparatively larger costs associated with the diagnosis, the treatment of post-CD adverse consequences (outlined above), as well as the emotional impact on women and their families. Additionally, the emphasis on time savings creates a generation of surgeons fixated with total operative time without consideration of long-term risks and adverse maternal outcomes.
Physician autonomy has led to the unmonitored freedom of obstetricians to choose their own technique for a CD, with some employing the commonly practiced culture of fastest turnaround even in nonurgent circumstances.
Documentation and terminology
Current documenting systems are not detail-oriented enough to assist in a thorough correlation between surgical techniques and outcomes. The use of single- or double-layer closure terminology is insufficient and has proven to be flawed, without describing the handling of the endometrium in terms of its inclusion or exclusion in the closure.
Quality improvement feedback
Long-term post-CD complications are often not reported to the physician or institution involved in the prior CD. In our opinion, some sort of registry would be of value. Perhaps then subsequent CD outcomes could be traced back and reported to the prior institution and surgeon. Feedback is critical to understanding the correlation between techniques and outcomes and more specifically to gathering learning points and using data for quality improvement of future cases.
Patient education
While women continue to have complications following the presently used surgical techniques, they often have expectations not discussed with their obstetricians. Women should be educated and empowered to realize the different approaches to all aspects and consequences of CDs.
Conclusion
The technique of excluding the endometrium in closing the uterine incision appears to reduce subsequent abnormal placentation and diminish the frequency and size of post-CD scar defect. The revival of the endometrium-free closure technique may allow significant change in the postoperative results. Currently, standardization of CD technique is being promoted on the basis of time- and cost-savings rather than clinical outcomes. Simultaneously, inroads are being made to better understand the risks and consequences of CD.
Emerging evidence suggests that a post-CD niche is the result of poor layer approximation as well as inclusion of the endometrium, which prevent healing of the uterine wall and often enables faulty implantation of the fertilized oocyte in the next pregnancy, potentially giving rise to placenta accreta spectrum. The prevalence and size of the defect can be minimized by techniques aimed at restoring the anatomy of the uterine wall and the physiology of the endometrium. Specialized training and education are necessary to stress the importance of anatomical assessment and decision making at the time of uterine closure. ●

While its etiology remains uncertain, cesarean scar niche (CSN) is well publicized, as are its pathological clinical manifestations. In a future pregnancy, they include cesarean scar pregnancy (CSP), which in turn can lead to placenta accreta spectrum, and possible uterine rupture/dehiscence of a residual thin myometrial layer. CSP refers to the implantation of an early pregnancy on the scar or in the niche at the site of a prior cesarean delivery (CD); it has an incidence of 1 per 1,000 pregnancies. An estimated 52% of CSPs occur after even just one CD.1 CSP has been linked to placenta accreta spectrum and has been shown to be its precursor.2 Both CSP and placenta accreta spectrum can be consequences of CD and share a common histology of villous or placental attachment/invasion into the cesarean scar.3 The incidence of placenta accreta spectrum has risen from about 1 in 4,000 live births in the 1970s to 1 in 2,500 in the 1980s; in 2016, the incidence of placenta accreta spectrum was reported as 1 per 272 live births.4
Placenta accreta spectrum denotes the attachment of the placenta into and through the myometrium,5 and it can result in severe complications, including hemorrhage, hysterectomy, and intensive care treatment. The increasing rate of placenta accreta spectrum parallels the increasing CD rate, which rose from 5.8% in 1970 to 31.9% in 2016.6 Multiple repeat CDs are increasing in frequency as well. At the beginning of the century, placenta accreta spectrum mainly occurred after manual removal of the placenta, uterine curettage, or endometritis. Recently, experts are in agreement that the main determinant of placenta accreta spectrum is the uterine scar and niche formation after a previous CD.5 Larger niches are associated with an increased incidence of uterine rupture or dehiscence in a subsequent pregnancy.7
In the nonpregnant state, such niches are associated with intermenstrual bleeding, pelvic pain, painful intercourse, painful menses, and subfertility, becoming increasingly more severe in women with greater numbers of CDs.8-10 Conception rate with assisted reproductive treatment is notably reduced.11
Understanding its etiology
Monteagudo and colleagues first described a “niche” in 100% of 44 women evaluated for postmenopausal bleeding who had a prior CD.12 CSN has been the subject of well over 3,000 publications over the past 30 years. While the topic generates much interest among researchers, it is garnering little traction among practicing obstetricians. Such “niches,” also referred to as isthmocele, cesarean scar defect, or a diverticulum, was first described in 196113 and later defined on ultrasonography as a hypoechoic triangular-shaped uterine defect outlined by saline instillation sonohysterogram (SIS), reflecting a discontinuation of the myometrium at the site of a previous CD.12 In 2019, a European task force further defined a CSN as an “indentation at the site in the cesarean section scar with a depth of at least 2 mm” and extended the classification to include branches as extensions toward the anterior uterine serosa.14 Using this criterion, sonographic postoperative evaluation after one CD revealed a CSN in 68.9% of women with one single-layer uterine closure and in 73.6% of women after a double-layer closure.15 Larger niche sizes with thinner residual myometrial thickness appeared more frequently when a single-layer closure technique was used, without closure of the peritoneum. Its prevalence varies from 56% to 84%.16,17
Etiology of CSN formation: Our hypotheses
The precise pathophysiology of CSN remains elusive. Speculations attributed niche formation to numerous factors: timing of surgery, cervical incision, incomplete closure of the uterine incision, adhesion formation between the CD scar and the abdominal wall, and inherent maternal conditions which may impair healing, such as smoking, obesity, diabetes, maternal age, and labor status.18-20 Retroflexion of the uterus is reportedly associated with increased incidence and size of the niche, with CSN 50% more likely to develop in women with a retroflexed versus an anteverted uterus.21 We demonstrated the origin of niche formation in real-time from the start to the completion of uterine closure by a video capture of a single-layer closure followed by an immediate SIS of the ex vivo hysterectomized uterus, and histopathologic proof of the presence of endometrial cells defining the “niche.”22 This case exposes the misalignment of the uterine wall, while including the endometrium in the closure (FIGURE 1). Similarly, pathologic studies of hysteroscopy-resected isthmocele ridges of symptomatic women with niche-related subfertility revealed the tissue edges lined by endocervical, endometrial, or isthmic mucosa either combined or isolated in the scar.23 The presence of endometrial/cervical tissue in the myometrial closure has been debated for over a century.24,25

Continue to: Uterine closure techniques...
Uterine closure techniques: Historical perspective
In 1882, Max Sanger introduced a vertical uterine closure of a classical cesarean operation in response to hysterectomy as the contemporaneous alternative to prevent infection, bleeding, and death.24 Dr. Sanger emphasized layer approximation, suturing, and the avoidance of decidua in the first layer (FIGURE 2). This became the teaching of the classical CD until the 1970s. In 1926, Munro Kerr addressed uterine rupture with labor after a classical CD by introducing the lower uterine segment transverse incision. He cautioned to maintain the decidua inside the uterine 2-layer closure of the cavity.25 These pioneers were joined by others to rally for endometrium exclusion while promoting layer approximation. These techniques became universally standard and were taught across teaching medical centers in the United States and abroad until about 50 years ago.

In the 1970s, newer developments brought significant changes to uterine closure techniques. Initiated by Joel-Cohen,26 blunt dissection of the abdominal incision was adapted by Michael Stark, creating what came to be known as the Misgav-Ladach cesarean technique.27 Stark emphasized blunt dissection and introduced single-layer closure. Thereby the exclusion of the endometrium, used for more than 70 years, was abandoned by the present-day single- or double-layer uterine closure in favor of cost and time savings. Systematic reviews and meta-analyses comparing the two contrasting techniques were inconclusive, noting that the niche prevalence and size were similar in both groups. These studies did not take into account the variety of individual techniques or the position of the endometrium in the final closures.28
Endometrium and uterine closure
Our recent study examining uterine scar defect in women after one primary CD by SIS concluded that a specific endometrium-free closure technique (EFCT) (FIGURE 3) is associated with fewer and less severe defects and a thicker residual myometrial thickness when compared with closures with unknown or endometrium inclusion.29 The study found non-specific closure techniques to be 6 times more likely to form a niche of 2-mm deep or greater than the EFCT.

Furthermore, we surveyed the diversity of uterine closures and the location of the endometrium among obstetricians in one institution.30 Presence of endometrium on the surface of the final uterine closure was reported by 20% of respondents (see Figure 1). When asked for their opinion on the impact of CD techniques on placenta accreta spectrum, without available evidence 80% of the survey respondents reported no relationship to techniques, and only 20% suggested an association. This particular study demonstrates that the surgical techniques just described are random, unfettered, and applied without consideration of clinical outcomes.
Our recent retrospective study that spanned 30 years and examined the EFCT—performed anywhere between 3 to 9 consecutive CDs—revealed no abnormal placentation in any subsequent pregnancies.31 This was one of the few clinical studies of the long-term consequences of a uterine closure technique. In this study, the endometrium was excluded during the uterine closure, allowing its free edges to abut and heal. This step avoids scarring the endometrial-myometrial (EM) interface and unintentional inclusion of endometrium in the closed uterine wall. In this context, Jauniaux and colleagues cited the destruction of the EM interface as the main factor for placenta-adherent disorders.32 Sholapurkar and others highlight the need to further examine intrinsic details of uterine closure beyond single- and double-layer techniques to better understand the etiology of cesarean scar formation.19 The search for the pathophysiology of CSN continues to present significant challenges imposed by the variety of currently practiced uterine closures.
Continue to: Focus on prevention...
Research: Focus on prevention
Our research aims to address the endometrium, a specific layer that was the topic of concern in nascent CD techniques, as a renewed and contemporary one. The presence of the endometrium in ectopic locations or its destruction from intrauterine surgeries or infections has been implicated in abnormal placentation.13,24 Our approach, in theory, is to limit the position of the endometrium to its innermost location and avoid its iatrogenic suturing and inclusion into the uterine wall closure. The rationale of sparing the endometrium in a layer-by-layer approximation is to allow for a closer restoration to normal anatomy and physiology than a random “en masse” uterine wall closure would permit. For this reason, the EM junction, the perimetrium, and the serosa must be identified and realigned for a more effective closure that incorporates the entire myometrial thickness. As evidence supports technical impact on the development of uterine scar defect in women after one CD, future studies are needed to evaluate uterine integrity by saline infusion sonohysterography in multiparous women with a prior random closure technique or a prior EFCT.
The potential long-term risks of blunt dissection for opening the uterus have not been studied. There are no physiologic lines in the uterine wall to facilitate a regular-bordered uterine stretch. The tissue stretch, which depends on the individual surgeon’s strength applied during the procedure and patient’s labor status, may result in an irregular tear and a difficult repair. The EFCT technique shows a more optimized risk-benefit ratio for an anatomical repair and is replicable. The safety of uterine layer re-approximation has been demonstrated and can be studied in large populations using strict uniform criteria.
Current and future challenges
Residency training
Most recently, teachers of resident trainees are mostly familiar with blunt dissection, techniques of which are passed on unchallenged from resident to resident. The endometrium and peritoneum are neither identified nor treated as separate layers, thus becoming obsolete as surgical and anatomical landmarks.
Standardization of CD techniques
Front-line obstetricians are persuaded to practice a standardized approach that relies on the benefits of cost related to operating room turnover as well as surgeons’ time savings without consideration of outcomes in subsequent pregnancies. Sholapurkar has warned that “wrong standardization” is far worse than no standardization, worse for the training of junior obstetricians, as it can inhibit critical reasoning about safe surgical techniques that can optimize outcomes of the condition of the lower uterine segment.33
Emergence of cost and time savings in clinical practice
A time-cost savings argument is relatively negligeable in an estimated 40-minute CD. By contrast, deliberate surgical technique and carrying out the appropriate steps for the particular condition at hand to achieve the best outcomes assume more weight.32 Furthermore, this short-term cost benefit is challenged by the comparatively larger costs associated with the diagnosis, the treatment of post-CD adverse consequences (outlined above), as well as the emotional impact on women and their families. Additionally, the emphasis on time savings creates a generation of surgeons fixated with total operative time without consideration of long-term risks and adverse maternal outcomes.
Physician autonomy has led to the unmonitored freedom of obstetricians to choose their own technique for a CD, with some employing the commonly practiced culture of fastest turnaround even in nonurgent circumstances.
Documentation and terminology
Current documenting systems are not detail-oriented enough to assist in a thorough correlation between surgical techniques and outcomes. The use of single- or double-layer closure terminology is insufficient and has proven to be flawed, without describing the handling of the endometrium in terms of its inclusion or exclusion in the closure.
Quality improvement feedback
Long-term post-CD complications are often not reported to the physician or institution involved in the prior CD. In our opinion, some sort of registry would be of value. Perhaps then subsequent CD outcomes could be traced back and reported to the prior institution and surgeon. Feedback is critical to understanding the correlation between techniques and outcomes and more specifically to gathering learning points and using data for quality improvement of future cases.
Patient education
While women continue to have complications following the presently used surgical techniques, they often have expectations not discussed with their obstetricians. Women should be educated and empowered to realize the different approaches to all aspects and consequences of CDs.
Conclusion
The technique of excluding the endometrium in closing the uterine incision appears to reduce subsequent abnormal placentation and diminish the frequency and size of post-CD scar defect. The revival of the endometrium-free closure technique may allow significant change in the postoperative results. Currently, standardization of CD technique is being promoted on the basis of time- and cost-savings rather than clinical outcomes. Simultaneously, inroads are being made to better understand the risks and consequences of CD.
Emerging evidence suggests that a post-CD niche is the result of poor layer approximation as well as inclusion of the endometrium, which prevent healing of the uterine wall and often enables faulty implantation of the fertilized oocyte in the next pregnancy, potentially giving rise to placenta accreta spectrum. The prevalence and size of the defect can be minimized by techniques aimed at restoring the anatomy of the uterine wall and the physiology of the endometrium. Specialized training and education are necessary to stress the importance of anatomical assessment and decision making at the time of uterine closure. ●
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107:1373-1381.
- Timor-Tritsch IE, Monteagudo A, Calì G, et al. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol. 2014;44:346-353. doi:10.1002/ uog.13426.
- Timor-Tritsch IE, Monteagudo A, Cali G, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014;43:383-395. doi: 10.1002/uog.13282.
- Mogos MF, Salemi JL, Ashley M, et al. Recent trends in placenta accreta in the United States and its impact on maternal-fetal morbidity and healthcare-associated costs, 1998-2011. J Matern Fetal Neonatal Med. 2016;29:1077-1082.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75-87.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2016. NCHS Data Brief. 2017(287):1-8.
- Vikhareva Osser O, Valentin L. Clinical importance of appearance of cesarean hysterotomy scar at transvaginal ultrasonography in nonpregnant women. Obstet Gynecol. 2011;117:525-532.
- Chen YY, Tsai CC, Kung FT, et al. Association between hysteroscopic findings of previous cesarean delivery scar defects and abnormal uterine bleeding. Taiwanese J Obstet Gynecol. 2019;58:541-544.
- Stegwee SI, Beij A, de Leeuw RA, et al. Niche-related outcomes after caesarean section and quality of life: a focus group study and review of literature. Qual Life Res. 2020;29:1013-1025.
- Vissers J, Hehenkamp W, Lambalk CB, et al. Post-caesarean section niche-related impaired fertility: hypothetical mechanisms. Hum Reprod. 2020;35:1484-1494.
- Vissers J, Sluckin TC, van Driel-Delprat CCR, et al. Reduced pregnancy and live birth rates after in vitro fertilization in women with previous caesarean section: a retrospective cohort study. Hum Reprod. 2020;35:595-604.
- Monteagudo A, Carreno C, Timor-Tritsch IE. Saline infusion sonohysterography in nonpregnant women with previous cesarean delivery: the “niche” in the scar. J Ultrasound Med. 2001;20:1105-1115.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67-71.
- Jordans IPM, de Leeuw RA, Stegwee SI, et al. Sonographic examination of uterine niche in non-pregnant women: a modified Delphi procedure. Ultrasound Obstet Gynecol. 2019;53:107-115.
- Stegwee SI, van der Voet LF, Ben AJ, et al. Effect of single- versus double-layer uterine closure during caesarean section on postmenstrual spotting (2Close): multicentre, double-blind, randomised controlled superiority trial. BJOG. 2021;128:866-878.
- Bij de Vaate AJ, van der Voet LF, Naji O, et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following cesarean section: systematic review. Ultrasound Obstet Gynecol. 2014;43:372-382.
- van der Voet LF, Bij de Vaate AM, Veersema S, et al. Long-term complications of caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121:236-244.
- Vervoort AJ, Uittenbogaard LB, Hehenkamp WJ, et al. Why do niches develop in caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum Reprod. 2015;30:2695-2702.
- Sholapurkar SL. Etiology of cesarean uterine scar defect (niche): detailed critical analysis of hypotheses and prevention strategies and peritoneal closure debate. J Clin Med Res. 2018;10:166-173.
- Kamel R, Eissa T, Sharaf M, et al. Position and integrity of uterine scar are determined by degree of cervical dilatation at time of cesarean section. Ultrasound Obstet Gynecol. 2021;57:466-470.
- Sanders RC, Parsons AK. Anteverted retroflexed uterus: a common consequence of cesarean delivery. AJR Am J Roentgenol. 2014;203:W117-124.
- Antoine C, Pimentel RN, Timor-Tritsch IE, et al. Origin of a post-cesarean delivery niche: diagnosis, pathophysiologic characteristics, and video documentation. J Ultrasound Med. 2021;40:205-208.
- AbdullGaffar B, Almulla A. A histopathologic approach to uterine niche: what to expect and to report in hysteroscopy-resected isthmocele specimens. Int J Surg Pathol. 2021:10668969211039415. doi: 10.1177/10668969211039415.
- Nagy S, Papp Z. Global approach of the cesarean section rates. J Perinatal Med. 2020;49:1-4.
- Kerr JM. The technic of cesarean section, with special reference to the lower uterine segment incision. Am J Obstet Gynecol. 1926;12:729-734.
- Joel-Cohen S. Abdominal and vaginal hysterectomy: new techniques based on time and motion studies. Lippincott Williams & Wilkins; 1977.
- Holmgren G, Sjoholm L, Stark M. The Misgav Ladach method for cesarean section: method description. Acta Obstet Gynecol Scand. 1999;78:615-621.
- Abalos E, Addo V, Brocklehurst P, et al. Caesarean section surgical techniques: 3-year follow-up of the CORONIS fractional, factorial, unmasked, randomised controlled trial. Lancet. 2016;388:62-72.
- Antoine C, Meyer JA, Silverstein JS, et al. The impact of uterine incision closure techniques on post-cesarean delivery niche formation and size: sonohysterographic examination of nonpregnant women. J Ultrasound Med. 2021. doi: 10.1002/ jum.15859.
- Antoine C AJ, Yaghoubian Y, Harary J. Variations in uterine closure technique: an institutional survey of obstetricians and implications for patient counseling and prevention of adverse sequelae [Abstract]. 2021.
- Antoine C, Pimentel RN, Reece EA, et al. Endometrium-free uterine closure technique and abnormal placental implantation in subsequent pregnancies. J Matern-Fetal Neonatal Med. 2019:1-9.
- Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33:244-251.
- Sholapurkar S. Review of unsafe changes in the practice of cesarean section with analysis of flaws in the interpretation of statistics and the evidence. Surgical Case Reports. 2021;4:2-6.
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107:1373-1381.
- Timor-Tritsch IE, Monteagudo A, Calì G, et al. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol. 2014;44:346-353. doi:10.1002/ uog.13426.
- Timor-Tritsch IE, Monteagudo A, Cali G, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014;43:383-395. doi: 10.1002/uog.13282.
- Mogos MF, Salemi JL, Ashley M, et al. Recent trends in placenta accreta in the United States and its impact on maternal-fetal morbidity and healthcare-associated costs, 1998-2011. J Matern Fetal Neonatal Med. 2016;29:1077-1082.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75-87.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2016. NCHS Data Brief. 2017(287):1-8.
- Vikhareva Osser O, Valentin L. Clinical importance of appearance of cesarean hysterotomy scar at transvaginal ultrasonography in nonpregnant women. Obstet Gynecol. 2011;117:525-532.
- Chen YY, Tsai CC, Kung FT, et al. Association between hysteroscopic findings of previous cesarean delivery scar defects and abnormal uterine bleeding. Taiwanese J Obstet Gynecol. 2019;58:541-544.
- Stegwee SI, Beij A, de Leeuw RA, et al. Niche-related outcomes after caesarean section and quality of life: a focus group study and review of literature. Qual Life Res. 2020;29:1013-1025.
- Vissers J, Hehenkamp W, Lambalk CB, et al. Post-caesarean section niche-related impaired fertility: hypothetical mechanisms. Hum Reprod. 2020;35:1484-1494.
- Vissers J, Sluckin TC, van Driel-Delprat CCR, et al. Reduced pregnancy and live birth rates after in vitro fertilization in women with previous caesarean section: a retrospective cohort study. Hum Reprod. 2020;35:595-604.
- Monteagudo A, Carreno C, Timor-Tritsch IE. Saline infusion sonohysterography in nonpregnant women with previous cesarean delivery: the “niche” in the scar. J Ultrasound Med. 2001;20:1105-1115.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67-71.
- Jordans IPM, de Leeuw RA, Stegwee SI, et al. Sonographic examination of uterine niche in non-pregnant women: a modified Delphi procedure. Ultrasound Obstet Gynecol. 2019;53:107-115.
- Stegwee SI, van der Voet LF, Ben AJ, et al. Effect of single- versus double-layer uterine closure during caesarean section on postmenstrual spotting (2Close): multicentre, double-blind, randomised controlled superiority trial. BJOG. 2021;128:866-878.
- Bij de Vaate AJ, van der Voet LF, Naji O, et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following cesarean section: systematic review. Ultrasound Obstet Gynecol. 2014;43:372-382.
- van der Voet LF, Bij de Vaate AM, Veersema S, et al. Long-term complications of caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121:236-244.
- Vervoort AJ, Uittenbogaard LB, Hehenkamp WJ, et al. Why do niches develop in caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum Reprod. 2015;30:2695-2702.
- Sholapurkar SL. Etiology of cesarean uterine scar defect (niche): detailed critical analysis of hypotheses and prevention strategies and peritoneal closure debate. J Clin Med Res. 2018;10:166-173.
- Kamel R, Eissa T, Sharaf M, et al. Position and integrity of uterine scar are determined by degree of cervical dilatation at time of cesarean section. Ultrasound Obstet Gynecol. 2021;57:466-470.
- Sanders RC, Parsons AK. Anteverted retroflexed uterus: a common consequence of cesarean delivery. AJR Am J Roentgenol. 2014;203:W117-124.
- Antoine C, Pimentel RN, Timor-Tritsch IE, et al. Origin of a post-cesarean delivery niche: diagnosis, pathophysiologic characteristics, and video documentation. J Ultrasound Med. 2021;40:205-208.
- AbdullGaffar B, Almulla A. A histopathologic approach to uterine niche: what to expect and to report in hysteroscopy-resected isthmocele specimens. Int J Surg Pathol. 2021:10668969211039415. doi: 10.1177/10668969211039415.
- Nagy S, Papp Z. Global approach of the cesarean section rates. J Perinatal Med. 2020;49:1-4.
- Kerr JM. The technic of cesarean section, with special reference to the lower uterine segment incision. Am J Obstet Gynecol. 1926;12:729-734.
- Joel-Cohen S. Abdominal and vaginal hysterectomy: new techniques based on time and motion studies. Lippincott Williams & Wilkins; 1977.
- Holmgren G, Sjoholm L, Stark M. The Misgav Ladach method for cesarean section: method description. Acta Obstet Gynecol Scand. 1999;78:615-621.
- Abalos E, Addo V, Brocklehurst P, et al. Caesarean section surgical techniques: 3-year follow-up of the CORONIS fractional, factorial, unmasked, randomised controlled trial. Lancet. 2016;388:62-72.
- Antoine C, Meyer JA, Silverstein JS, et al. The impact of uterine incision closure techniques on post-cesarean delivery niche formation and size: sonohysterographic examination of nonpregnant women. J Ultrasound Med. 2021. doi: 10.1002/ jum.15859.
- Antoine C AJ, Yaghoubian Y, Harary J. Variations in uterine closure technique: an institutional survey of obstetricians and implications for patient counseling and prevention of adverse sequelae [Abstract]. 2021.
- Antoine C, Pimentel RN, Reece EA, et al. Endometrium-free uterine closure technique and abnormal placental implantation in subsequent pregnancies. J Matern-Fetal Neonatal Med. 2019:1-9.
- Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33:244-251.
- Sholapurkar S. Review of unsafe changes in the practice of cesarean section with analysis of flaws in the interpretation of statistics and the evidence. Surgical Case Reports. 2021;4:2-6.
Direct-to-Consumer Teledermatology Growth: A Review and Outlook for the Future
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.
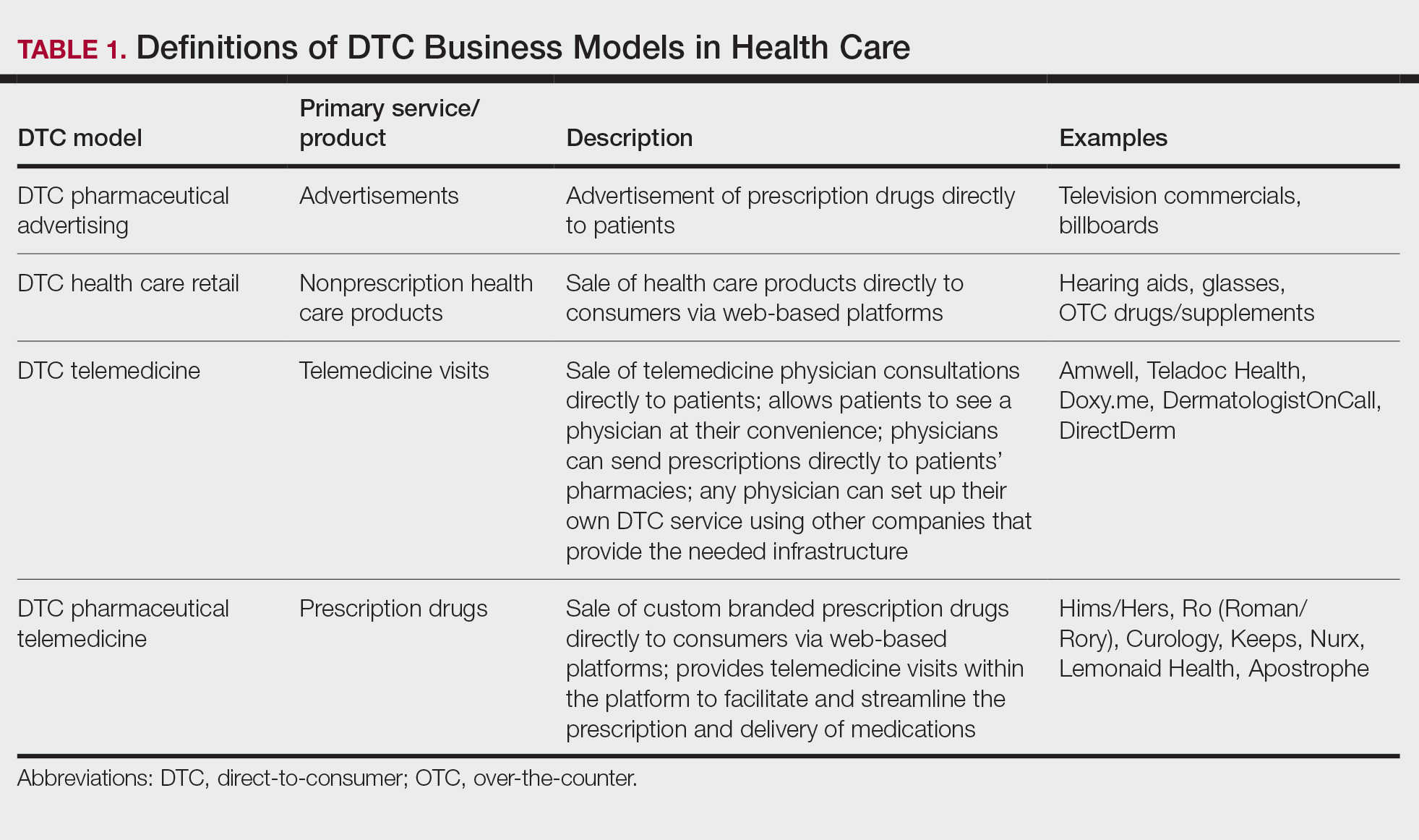
The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.
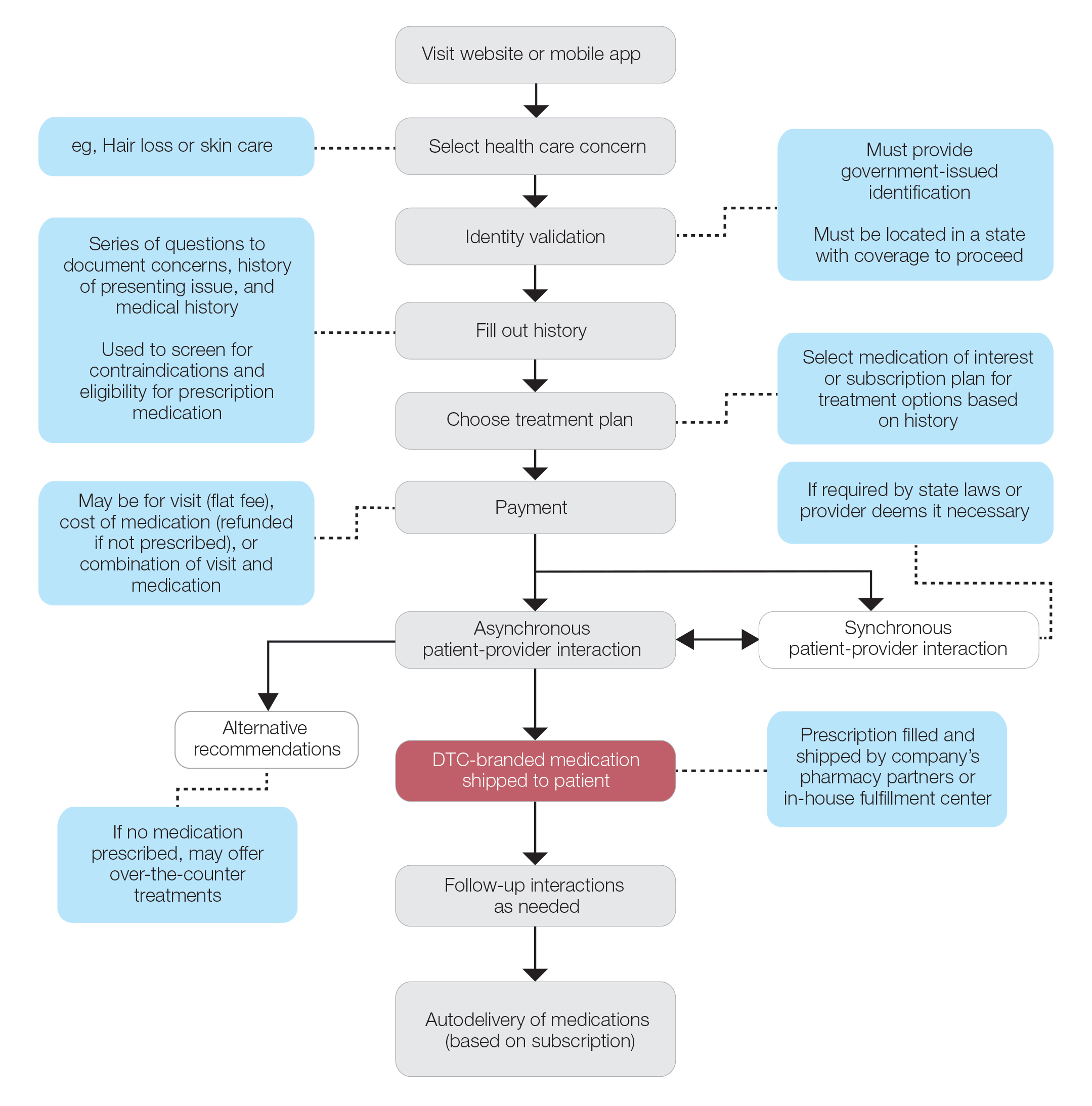
Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.
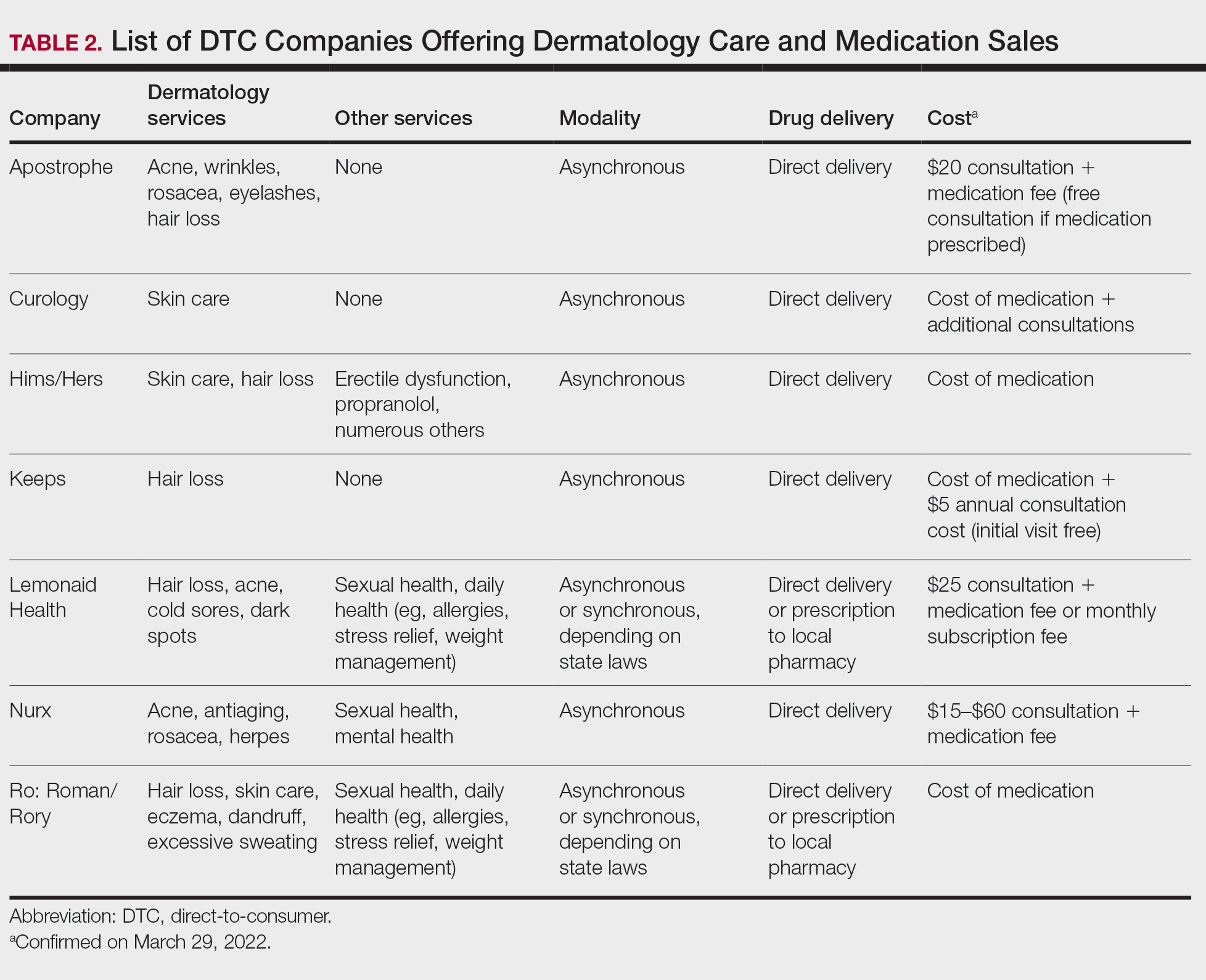
Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.

The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.

Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.

Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.

The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.

Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.

Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
Practice Points
- Direct-to-consumer (DTC) teledermatology platforms are for-profit companies that provide telemedicine visits and sell prescription drugs directly to patients.
- Although they are growing in popularity, DTC teledermatology platforms may lead to overdiagnosis, overtreatment, and fragmentation of health care. Knowledge of teledermatology will be vital to counsel patients on the risks and benefits of these platforms.