User login
Fibroids: Growing management options for a prevalent problem
OBG Manag. 33(12). | doi 10.12788/obgm.0169
2022 Update on obstetrics
Obstetrical practice saw updates in 2021 to 3 major areas of pregnancy management: preterm birth prevention, antepartum fetal surveillance, and the use of antenatal corticosteroids.
Updated guidance on predicting and preventing spontaneous PTB
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
Preterm birth (PTB) continues to pose a challenge in clinical obstetrics, with the most recently reported rate of 10.2% in the United States.1 This accounts for almost 75% of perinatal mortality and more than half of neonatal morbidity, in which effects last well past the neonatal period. PTB is classified as spontaneous (following preterm labor, preterm prelabor rupture of membranes, or cervical insufficiency) or iatrogenic (indicated due to maternal and/or fetal complications).
Assessing risk for PTB
The single strongest predictor of subsequent PTB is a history of spontaneous PTB. Recurrence risk is further increased by the number of prior PTBs and the gestational age at prior PTB. Identification of and intervention for a short cervix has been shown to prolong gestation. Transvaginal ultrasonography of the cervix is the most accurate method for evaluating cervical length (CL). Specific examination criteria exist to ensure that CL measurements are reproducible and reliable.2 A short CL is generally defined as a measurement of less than 25 mm between 16 and 24 weeks’ gestation.
Screening strategies
The American College of Obstetricians and Gynecologists (ACOG), with an endorsement from the Society for Maternal-Fetal Medicine (SMFM), recommends cervical evaluation during the anatomy ultrasound exam between 18 0/7 and 22 6/7 weeks’ gestation in all pregnant patients regardless of prior PTB.3 If transabdominal imaging is concerning for a shortened cervix, transvaginal ultrasonography should be performed to assess the CL.
Serial transvaginal CL measurements are recommended between 16 0/7 and 24 0/7 weeks’ gestation for patients with a current singleton pregnancy and history of a spontaneous PTB, but not for patients with a history of iatrogenic or indicated PTB.
Interventions: Mind your p’s and c’s
Interventions to reduce the risk of spontaneous PTB depend on whether the current pregnancy is a singleton, twins, or higher-order multiples; CL measurement; and history of spontaneous PTB. Preconception optimization of underlying medical conditions also is important to reduce the risk of recurrent indicated PTB.
Continue to: Progesterone...
Progesterone
Vaginal administration. Several trials have shown that vaginal progesterone can be used to reduce the risk of spontaneous PTB in asymptomatic patients with a singleton pregnancy, incidental finding of a short cervix (<25 mm), and no history of spontaneous PTB. This is a change from the prior recommendation of CL of less than 20 mm. In the setting of a twin pregnancy, regardless of CL, data do not definitively support the use of vaginal progesterone.
Intramuscular administration.4,5 The popularity of intramuscular progesterone has waxed and waned. At present, ACOG recommends that all patients with a singleton pregnancy and history of spontaneous PTB be offered progesterone beginning at 16 0/7 weeks’ gestation following a shared decision-making process that includes the limited data of efficacy noted in existing studies.
In a twin pregnancy with no history of spontaneous PTB, the use of intramuscular progesterone has been shown to potentially increase the risk of PTB and admission to the neonatal intensive care unit. As such, intramuscular progesterone in the setting of a twin gestation without a history of spontaneous PTB is not recommended. When a prior spontaneous PTB has occurred, there may be some benefit to intramuscular progesterone in twin gestations.
Cerclage
Ultrasound indicated. In a singleton pregnancy with an incidental finding of short cervix (<25 mm) and no history of PTB, the use of cerclage is of uncertain benefit. Effectiveness may be seen if the cervix is less than 10 mm. Ultrasound-indicated cerclage should be considered in a singleton pregnancy with a CL less than 25 mm and a history of spontaneous PTB.
Possibly one of the most controversial topics is ultrasound-indicated cerclage placement in twin gestation. As with many situations in obstetrics, data regarding ultrasound-indicated cerclage in twin gestation is based on small retrospective studies fraught with bias. Results from these studies range from no benefit, to potential benefit, to even possible increased risk of PTB. Since data are limited, as we await more evidence, it is recommended that the clinician and patient use shared decision making to decide on cerclage placement in a twin gestation.
Exam indicated. In a singleton pregnancy with a dilated cervix on digital or speculum exam between 16 0/7 to 23 6/7 weeks’ gestation, a physical exam–indicated cerclage should be offered. Exam-indicated cerclage also may reduce the incidence of PTB in twin gestations with cervical dilation between 16 0/7 and 23 6/7 weeks’ gestation. Indomethacin tocolysis and perioperative antibiotics should be considered when an exam-indicated cerclage is placed.
As the limits of viability are continually pushed earlier, more in-depth conversation is needed with patients who are considering an exam-indicated cerclage. The nuances of periviability and the likelihood that an exam-indicated cerclage will commit a pregnancy to a periviable or extremely preterm birth should be discussed in detail using a shared decision making model.
Regardless of whether the cerclage is ultrasound or exam indicated, once it is placed there is no utility in additional CL ultrasound monitoring.
Pessary
Vaginal pessaries for prevention of PTB have not gained popularity in the United States as they have in other countries. Trials are being conducted to determine the utility of vaginal pessary, but current data have not proven its effectiveness in preventing PTB in the setting of singleton pregnancy, short cervix, and no history of spontaneous PTB. So for now, pessary is not recommended. The same can be said for use in the twin gestation.
- All patients should have cervical evaluation during pregnancy. Serial imaging is reserved for those with a history of spontaneous PTB.
- Progesterone supplementation should be offered to patients with a singleton pregnancy and a history of spontaneous PTB or to patients with a singleton pregnancy and no history of spontaneous PTB who have cervical shortening at less than 24 weeks.
- Cerclage may be offered between 16 and 24 weeks for a cervical length less than 25 mm in a patient with a singleton gestation who has a history of spontaneous PTB (<10 mm if no history of spontaneous PTB) or for a dilated cervix on exam regardless of history.
- Women who have a twin gestation with cervical dilation may be offered physical exam–indicated cerclage.
Which patients may benefit from antepartum fetal surveillance and when to initiate it
American College of Obstetricians and Gynecologists’ Committee on Obstetrics Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Antepartum fetal surveillance: ACOG practice bulletin, number 229. Obstet Gynecol. 2021;137:e116-e127.
The ultimate purpose of antenatal fetal surveillance is to prevent stillbirth. However, stillbirth has multiple etiologies, not all of which are preventable with testing. In June 2021, ACOG released a new Committee Opinion containing guidelines for fetal surveillance, including suggested gestational age at initiation and frequency of testing, for the most common high-risk conditions. ACOG also released an update to the Practice Bulletin on antepartum fetal surveillance; additions include randomized controlled trial level data on the utility of fetal kick counts (FKCs) and recommendations that align with the new Committee Opinion.
Data for the efficacy of antepartum fetal surveillance are lacking, mainly due to the difficulty of performing prospective studies in stillbirth. The existing evidence is subject to intervention bias, as deliveries increase in tested patients, and recommendations rely heavily on expert consensus and nonrandomized studies. Antenatal testing is also time, cost, and labor intensive, with the risk of intervention for a false-positive result. Despite these limitations, obstetrical practices routinely perform antenatal fetal surveillance.
The new guidelines: The why, when, and how often
Why. Antepartum fetal surveillance is suggested for conditions that have a risk of stillbirth greater than 0.8 per 1,000 (that is, the false-negative rate of a biophysical profile or a modified biophysical profile) and the relative risk or odds ratio is greater than 2.0 for stillbirth compared with unaffected pregnancies.
When. For most conditions, ACOG recommends initiation of testing at 32 weeks or later, with notable earlier exceptions for some of the highest-risk patients. For certain conditions, such as fetal growth restriction and hypertensive disorders of pregnancy, the recommendation is to start “at diagnosis,” with the corollary “or at a gestational age when delivery would be considered because of abnormal results.” Shared decision making with the patient about pregnancy goals therefore is required, particularly in cases of fetal anomalies, genetic conditions, or at very early gestational ages.
How often. The recommended frequency of testing is at least weekly. Testing frequency should be increased to twice-weekly outpatient or daily inpatient for the most complicated pregnancies (for example, fetal growth restriction with abnormal umbilical artery Doppler studies, preeclampsia with severe features).
Once or twice weekly is an option for many conditions, which gives the clinician the opportunity to assess clinical stability as well as the patient’s input in terms of logistics and anxiety.
Patients with multiple conditions may fall into the “individualized” category, as do patients with suboptimal control of conditions (for example, diabetes, hypertension) that may affect the fetus as the pregnancy progresses.
New diagnoses included for surveillance
Several diagnoses not previously included now qualify for antepartum fetal surveillance under the new guidelines, most notably:
- history of obstetrical complications in the immediate preceding pregnancy
—history of prior fetal growth restriction requiring preterm delivery
—history of prior preeclampsia requiring delivery
- alcohol use of 5 or more drinks per day
- in vitro fertilization
- abnormal serum markers
—pregnancy-associated plasma protein A (PAPP-A) in the fifth or lower percentile or 0.4 multiples of the median (MoM)
—second trimester inhibin A of 2 or greater MoM
- prepregnancy body mass index (BMI)
—this is divided into 2 categories for timing of initiation of testing:
- 37 weeks for BMI of 35 to 39.9 kg/m2
- 34 weeks for BMI of or greater than 40 kg/m2.
Fetal kick counts
The major change to the updated Practice Bulletin on antenatal surveillance is the inclusion of data on FKCs, a simple modality of fetal surveillance that does not require a clinical visit. For FKCs, a meta-analysis of more than 450,000 patients did not demonstrate a difference in perinatal death between the FKC intervention group (0.54%) and the control group (0.59%). Of note, there were small but statistically significant increases in the rates of induction of labor, cesarean delivery, and preterm delivery in the FKC intervention group. Therefore, this update does not recommend a formal program of FKCs for all patients.
- The antenatal fetal surveillance guidelines are just that—guidelines, not mandates. Their use will need to be adapted for specific patient populations and practice management patterns.
- Many conditions qualify for “individualized” surveillance, which offers the opportunity for detailed discussions on the patient’s care. This includes shared decision making with patients to meet their goals for the pregnancy.
- Although patient-perceived decreased fetal movement always warrants clinical evaluation, a regular program of fetal kick count monitoring is not recommended for all patients due to lack of data supporting its benefit in reducing perinatal death.
- As with any change, new guidelines potentially are a source of frustration, so a concerted effort by obstetrical clinicians to agree on adoption of the guidelines is needed. Additional clinical resources and both clinician and patient education may be required depending on current practice style, as the new strategy may increase the number of appointments and ultrasound exams required.
Continue to: Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation...
Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation
American College of Obstetricians and Gynecologists. Use of antenatal corticosteroids at 22 weeks of gestation: ACOG practice advisory. September 2021. https://www .acog.org/clinical/clinical-guidance/practice-advisory /articles/2021/09/use-of-antenatal-corticosteroids-at -22-weeks-of-gestation. Accessed December 11, 2021.
In September 2021, ACOG and SMFM released a Practice Advisory updating the current recommendations for the administration of antenatal corticosteroids in the periviable period (22 to 25 6/7 weeks’ gestation). Whereas the prior lower limit of gestational age for consideration of steroids was 23 weeks, the new recommendation now extends this consideration down to 22 weeks.
The cited data include a meta-analysis of more than 2,200 patients in which the survival rate of infants born between 22 and 22 6/7 weeks who were exposed to antenatal steroids was 39% compared with 19.5% in the unexposed group. Another study of more than 1,000 patients demonstrated a statistically significant increase in overall survival in patients treated with antenatal steroids plus life support compared with life support only (38.5% vs 17.7%). Survival without major morbidity in this study, although increased from 1% to 4.4%, was still low.
Recommendation carries caveats
Given this information, the Practice Advisory offers a 2C level recommendation (weak recommendation, low quality of evidence) for antenatal steroids at 22 to 22 6/7 weeks’ gestation if neonatal resuscitation is planned, acknowledging the limitations and potential bias of the available data.
The Practice Advisory emphasizes the importance of counseling and patient involvement in the decision making. This requires a multidisciplinary collaboration among the neonatology and obstetrical teams, flexibility in the plan after birth depending on the infant’s condition, and redirection of care if appropriate. Estimated fetal weight, the presence of multiple gestations, fetal biologic sex, and any anomalies are also important in helping families make an informed decision for their particular pregnancy. As described in the Obstetric Care Consensus on periviable birth,6 it is important to remember that considerations and recommendations are not the same as requirements, and redirection of care to comfort and family memory making is not the same as withholding care.
The rest of the recommendations for the administration of antenatal steroids remain the same: Antenatal steroids are not recommended at less than 22 weeks due to lack of evidence of benefit, and they continue to be recommended at 24 weeks and beyond. ●
- Antenatal corticosteroids may be considered at 22 to 22 6/7 weeks’ gestation if, after thorough patient counseling, neonatal resuscitation is desired and planned by the family.
- The overall likelihood of survival and survival without major morbidities continues to be very low in the periviable period, especially at 22 weeks. Gestational age is only one of the many factors that must be considered in the shared decision making for this very difficult decision.
- Palliative care is a valid and appropriate option for patients facing a periviable delivery after appropriate counseling or after evaluation of the infant has occurred after birth.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2019. NCHS Data Brief, no 387. Hyattsville, MD: National Center for Health Statistics. October 2020. www.cdc.gov/nchs /data/databriefs/db387-H.pdf. Accessed December 20, 2021.
- To MS, Skentou C, Chan C, et al. Cervical assessment at the routine 23-week scan: standardizing techniques. Ultrasound Obstet Gynecol. 2001;17:217-219.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. Am J Obstet Gynecol. 2020;223:B16-B18.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 6: Periviable birth. Obstet Gynecol. 2017;130:e187-e199.
Obstetrical practice saw updates in 2021 to 3 major areas of pregnancy management: preterm birth prevention, antepartum fetal surveillance, and the use of antenatal corticosteroids.
Updated guidance on predicting and preventing spontaneous PTB
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
Preterm birth (PTB) continues to pose a challenge in clinical obstetrics, with the most recently reported rate of 10.2% in the United States.1 This accounts for almost 75% of perinatal mortality and more than half of neonatal morbidity, in which effects last well past the neonatal period. PTB is classified as spontaneous (following preterm labor, preterm prelabor rupture of membranes, or cervical insufficiency) or iatrogenic (indicated due to maternal and/or fetal complications).
Assessing risk for PTB
The single strongest predictor of subsequent PTB is a history of spontaneous PTB. Recurrence risk is further increased by the number of prior PTBs and the gestational age at prior PTB. Identification of and intervention for a short cervix has been shown to prolong gestation. Transvaginal ultrasonography of the cervix is the most accurate method for evaluating cervical length (CL). Specific examination criteria exist to ensure that CL measurements are reproducible and reliable.2 A short CL is generally defined as a measurement of less than 25 mm between 16 and 24 weeks’ gestation.
Screening strategies
The American College of Obstetricians and Gynecologists (ACOG), with an endorsement from the Society for Maternal-Fetal Medicine (SMFM), recommends cervical evaluation during the anatomy ultrasound exam between 18 0/7 and 22 6/7 weeks’ gestation in all pregnant patients regardless of prior PTB.3 If transabdominal imaging is concerning for a shortened cervix, transvaginal ultrasonography should be performed to assess the CL.
Serial transvaginal CL measurements are recommended between 16 0/7 and 24 0/7 weeks’ gestation for patients with a current singleton pregnancy and history of a spontaneous PTB, but not for patients with a history of iatrogenic or indicated PTB.
Interventions: Mind your p’s and c’s
Interventions to reduce the risk of spontaneous PTB depend on whether the current pregnancy is a singleton, twins, or higher-order multiples; CL measurement; and history of spontaneous PTB. Preconception optimization of underlying medical conditions also is important to reduce the risk of recurrent indicated PTB.
Continue to: Progesterone...
Progesterone
Vaginal administration. Several trials have shown that vaginal progesterone can be used to reduce the risk of spontaneous PTB in asymptomatic patients with a singleton pregnancy, incidental finding of a short cervix (<25 mm), and no history of spontaneous PTB. This is a change from the prior recommendation of CL of less than 20 mm. In the setting of a twin pregnancy, regardless of CL, data do not definitively support the use of vaginal progesterone.
Intramuscular administration.4,5 The popularity of intramuscular progesterone has waxed and waned. At present, ACOG recommends that all patients with a singleton pregnancy and history of spontaneous PTB be offered progesterone beginning at 16 0/7 weeks’ gestation following a shared decision-making process that includes the limited data of efficacy noted in existing studies.
In a twin pregnancy with no history of spontaneous PTB, the use of intramuscular progesterone has been shown to potentially increase the risk of PTB and admission to the neonatal intensive care unit. As such, intramuscular progesterone in the setting of a twin gestation without a history of spontaneous PTB is not recommended. When a prior spontaneous PTB has occurred, there may be some benefit to intramuscular progesterone in twin gestations.
Cerclage
Ultrasound indicated. In a singleton pregnancy with an incidental finding of short cervix (<25 mm) and no history of PTB, the use of cerclage is of uncertain benefit. Effectiveness may be seen if the cervix is less than 10 mm. Ultrasound-indicated cerclage should be considered in a singleton pregnancy with a CL less than 25 mm and a history of spontaneous PTB.
Possibly one of the most controversial topics is ultrasound-indicated cerclage placement in twin gestation. As with many situations in obstetrics, data regarding ultrasound-indicated cerclage in twin gestation is based on small retrospective studies fraught with bias. Results from these studies range from no benefit, to potential benefit, to even possible increased risk of PTB. Since data are limited, as we await more evidence, it is recommended that the clinician and patient use shared decision making to decide on cerclage placement in a twin gestation.
Exam indicated. In a singleton pregnancy with a dilated cervix on digital or speculum exam between 16 0/7 to 23 6/7 weeks’ gestation, a physical exam–indicated cerclage should be offered. Exam-indicated cerclage also may reduce the incidence of PTB in twin gestations with cervical dilation between 16 0/7 and 23 6/7 weeks’ gestation. Indomethacin tocolysis and perioperative antibiotics should be considered when an exam-indicated cerclage is placed.
As the limits of viability are continually pushed earlier, more in-depth conversation is needed with patients who are considering an exam-indicated cerclage. The nuances of periviability and the likelihood that an exam-indicated cerclage will commit a pregnancy to a periviable or extremely preterm birth should be discussed in detail using a shared decision making model.
Regardless of whether the cerclage is ultrasound or exam indicated, once it is placed there is no utility in additional CL ultrasound monitoring.
Pessary
Vaginal pessaries for prevention of PTB have not gained popularity in the United States as they have in other countries. Trials are being conducted to determine the utility of vaginal pessary, but current data have not proven its effectiveness in preventing PTB in the setting of singleton pregnancy, short cervix, and no history of spontaneous PTB. So for now, pessary is not recommended. The same can be said for use in the twin gestation.
- All patients should have cervical evaluation during pregnancy. Serial imaging is reserved for those with a history of spontaneous PTB.
- Progesterone supplementation should be offered to patients with a singleton pregnancy and a history of spontaneous PTB or to patients with a singleton pregnancy and no history of spontaneous PTB who have cervical shortening at less than 24 weeks.
- Cerclage may be offered between 16 and 24 weeks for a cervical length less than 25 mm in a patient with a singleton gestation who has a history of spontaneous PTB (<10 mm if no history of spontaneous PTB) or for a dilated cervix on exam regardless of history.
- Women who have a twin gestation with cervical dilation may be offered physical exam–indicated cerclage.
Which patients may benefit from antepartum fetal surveillance and when to initiate it
American College of Obstetricians and Gynecologists’ Committee on Obstetrics Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Antepartum fetal surveillance: ACOG practice bulletin, number 229. Obstet Gynecol. 2021;137:e116-e127.
The ultimate purpose of antenatal fetal surveillance is to prevent stillbirth. However, stillbirth has multiple etiologies, not all of which are preventable with testing. In June 2021, ACOG released a new Committee Opinion containing guidelines for fetal surveillance, including suggested gestational age at initiation and frequency of testing, for the most common high-risk conditions. ACOG also released an update to the Practice Bulletin on antepartum fetal surveillance; additions include randomized controlled trial level data on the utility of fetal kick counts (FKCs) and recommendations that align with the new Committee Opinion.
Data for the efficacy of antepartum fetal surveillance are lacking, mainly due to the difficulty of performing prospective studies in stillbirth. The existing evidence is subject to intervention bias, as deliveries increase in tested patients, and recommendations rely heavily on expert consensus and nonrandomized studies. Antenatal testing is also time, cost, and labor intensive, with the risk of intervention for a false-positive result. Despite these limitations, obstetrical practices routinely perform antenatal fetal surveillance.
The new guidelines: The why, when, and how often
Why. Antepartum fetal surveillance is suggested for conditions that have a risk of stillbirth greater than 0.8 per 1,000 (that is, the false-negative rate of a biophysical profile or a modified biophysical profile) and the relative risk or odds ratio is greater than 2.0 for stillbirth compared with unaffected pregnancies.
When. For most conditions, ACOG recommends initiation of testing at 32 weeks or later, with notable earlier exceptions for some of the highest-risk patients. For certain conditions, such as fetal growth restriction and hypertensive disorders of pregnancy, the recommendation is to start “at diagnosis,” with the corollary “or at a gestational age when delivery would be considered because of abnormal results.” Shared decision making with the patient about pregnancy goals therefore is required, particularly in cases of fetal anomalies, genetic conditions, or at very early gestational ages.
How often. The recommended frequency of testing is at least weekly. Testing frequency should be increased to twice-weekly outpatient or daily inpatient for the most complicated pregnancies (for example, fetal growth restriction with abnormal umbilical artery Doppler studies, preeclampsia with severe features).
Once or twice weekly is an option for many conditions, which gives the clinician the opportunity to assess clinical stability as well as the patient’s input in terms of logistics and anxiety.
Patients with multiple conditions may fall into the “individualized” category, as do patients with suboptimal control of conditions (for example, diabetes, hypertension) that may affect the fetus as the pregnancy progresses.
New diagnoses included for surveillance
Several diagnoses not previously included now qualify for antepartum fetal surveillance under the new guidelines, most notably:
- history of obstetrical complications in the immediate preceding pregnancy
—history of prior fetal growth restriction requiring preterm delivery
—history of prior preeclampsia requiring delivery
- alcohol use of 5 or more drinks per day
- in vitro fertilization
- abnormal serum markers
—pregnancy-associated plasma protein A (PAPP-A) in the fifth or lower percentile or 0.4 multiples of the median (MoM)
—second trimester inhibin A of 2 or greater MoM
- prepregnancy body mass index (BMI)
—this is divided into 2 categories for timing of initiation of testing:
- 37 weeks for BMI of 35 to 39.9 kg/m2
- 34 weeks for BMI of or greater than 40 kg/m2.
Fetal kick counts
The major change to the updated Practice Bulletin on antenatal surveillance is the inclusion of data on FKCs, a simple modality of fetal surveillance that does not require a clinical visit. For FKCs, a meta-analysis of more than 450,000 patients did not demonstrate a difference in perinatal death between the FKC intervention group (0.54%) and the control group (0.59%). Of note, there were small but statistically significant increases in the rates of induction of labor, cesarean delivery, and preterm delivery in the FKC intervention group. Therefore, this update does not recommend a formal program of FKCs for all patients.
- The antenatal fetal surveillance guidelines are just that—guidelines, not mandates. Their use will need to be adapted for specific patient populations and practice management patterns.
- Many conditions qualify for “individualized” surveillance, which offers the opportunity for detailed discussions on the patient’s care. This includes shared decision making with patients to meet their goals for the pregnancy.
- Although patient-perceived decreased fetal movement always warrants clinical evaluation, a regular program of fetal kick count monitoring is not recommended for all patients due to lack of data supporting its benefit in reducing perinatal death.
- As with any change, new guidelines potentially are a source of frustration, so a concerted effort by obstetrical clinicians to agree on adoption of the guidelines is needed. Additional clinical resources and both clinician and patient education may be required depending on current practice style, as the new strategy may increase the number of appointments and ultrasound exams required.
Continue to: Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation...
Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation
American College of Obstetricians and Gynecologists. Use of antenatal corticosteroids at 22 weeks of gestation: ACOG practice advisory. September 2021. https://www .acog.org/clinical/clinical-guidance/practice-advisory /articles/2021/09/use-of-antenatal-corticosteroids-at -22-weeks-of-gestation. Accessed December 11, 2021.
In September 2021, ACOG and SMFM released a Practice Advisory updating the current recommendations for the administration of antenatal corticosteroids in the periviable period (22 to 25 6/7 weeks’ gestation). Whereas the prior lower limit of gestational age for consideration of steroids was 23 weeks, the new recommendation now extends this consideration down to 22 weeks.
The cited data include a meta-analysis of more than 2,200 patients in which the survival rate of infants born between 22 and 22 6/7 weeks who were exposed to antenatal steroids was 39% compared with 19.5% in the unexposed group. Another study of more than 1,000 patients demonstrated a statistically significant increase in overall survival in patients treated with antenatal steroids plus life support compared with life support only (38.5% vs 17.7%). Survival without major morbidity in this study, although increased from 1% to 4.4%, was still low.
Recommendation carries caveats
Given this information, the Practice Advisory offers a 2C level recommendation (weak recommendation, low quality of evidence) for antenatal steroids at 22 to 22 6/7 weeks’ gestation if neonatal resuscitation is planned, acknowledging the limitations and potential bias of the available data.
The Practice Advisory emphasizes the importance of counseling and patient involvement in the decision making. This requires a multidisciplinary collaboration among the neonatology and obstetrical teams, flexibility in the plan after birth depending on the infant’s condition, and redirection of care if appropriate. Estimated fetal weight, the presence of multiple gestations, fetal biologic sex, and any anomalies are also important in helping families make an informed decision for their particular pregnancy. As described in the Obstetric Care Consensus on periviable birth,6 it is important to remember that considerations and recommendations are not the same as requirements, and redirection of care to comfort and family memory making is not the same as withholding care.
The rest of the recommendations for the administration of antenatal steroids remain the same: Antenatal steroids are not recommended at less than 22 weeks due to lack of evidence of benefit, and they continue to be recommended at 24 weeks and beyond. ●
- Antenatal corticosteroids may be considered at 22 to 22 6/7 weeks’ gestation if, after thorough patient counseling, neonatal resuscitation is desired and planned by the family.
- The overall likelihood of survival and survival without major morbidities continues to be very low in the periviable period, especially at 22 weeks. Gestational age is only one of the many factors that must be considered in the shared decision making for this very difficult decision.
- Palliative care is a valid and appropriate option for patients facing a periviable delivery after appropriate counseling or after evaluation of the infant has occurred after birth.
Obstetrical practice saw updates in 2021 to 3 major areas of pregnancy management: preterm birth prevention, antepartum fetal surveillance, and the use of antenatal corticosteroids.
Updated guidance on predicting and preventing spontaneous PTB
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
Preterm birth (PTB) continues to pose a challenge in clinical obstetrics, with the most recently reported rate of 10.2% in the United States.1 This accounts for almost 75% of perinatal mortality and more than half of neonatal morbidity, in which effects last well past the neonatal period. PTB is classified as spontaneous (following preterm labor, preterm prelabor rupture of membranes, or cervical insufficiency) or iatrogenic (indicated due to maternal and/or fetal complications).
Assessing risk for PTB
The single strongest predictor of subsequent PTB is a history of spontaneous PTB. Recurrence risk is further increased by the number of prior PTBs and the gestational age at prior PTB. Identification of and intervention for a short cervix has been shown to prolong gestation. Transvaginal ultrasonography of the cervix is the most accurate method for evaluating cervical length (CL). Specific examination criteria exist to ensure that CL measurements are reproducible and reliable.2 A short CL is generally defined as a measurement of less than 25 mm between 16 and 24 weeks’ gestation.
Screening strategies
The American College of Obstetricians and Gynecologists (ACOG), with an endorsement from the Society for Maternal-Fetal Medicine (SMFM), recommends cervical evaluation during the anatomy ultrasound exam between 18 0/7 and 22 6/7 weeks’ gestation in all pregnant patients regardless of prior PTB.3 If transabdominal imaging is concerning for a shortened cervix, transvaginal ultrasonography should be performed to assess the CL.
Serial transvaginal CL measurements are recommended between 16 0/7 and 24 0/7 weeks’ gestation for patients with a current singleton pregnancy and history of a spontaneous PTB, but not for patients with a history of iatrogenic or indicated PTB.
Interventions: Mind your p’s and c’s
Interventions to reduce the risk of spontaneous PTB depend on whether the current pregnancy is a singleton, twins, or higher-order multiples; CL measurement; and history of spontaneous PTB. Preconception optimization of underlying medical conditions also is important to reduce the risk of recurrent indicated PTB.
Continue to: Progesterone...
Progesterone
Vaginal administration. Several trials have shown that vaginal progesterone can be used to reduce the risk of spontaneous PTB in asymptomatic patients with a singleton pregnancy, incidental finding of a short cervix (<25 mm), and no history of spontaneous PTB. This is a change from the prior recommendation of CL of less than 20 mm. In the setting of a twin pregnancy, regardless of CL, data do not definitively support the use of vaginal progesterone.
Intramuscular administration.4,5 The popularity of intramuscular progesterone has waxed and waned. At present, ACOG recommends that all patients with a singleton pregnancy and history of spontaneous PTB be offered progesterone beginning at 16 0/7 weeks’ gestation following a shared decision-making process that includes the limited data of efficacy noted in existing studies.
In a twin pregnancy with no history of spontaneous PTB, the use of intramuscular progesterone has been shown to potentially increase the risk of PTB and admission to the neonatal intensive care unit. As such, intramuscular progesterone in the setting of a twin gestation without a history of spontaneous PTB is not recommended. When a prior spontaneous PTB has occurred, there may be some benefit to intramuscular progesterone in twin gestations.
Cerclage
Ultrasound indicated. In a singleton pregnancy with an incidental finding of short cervix (<25 mm) and no history of PTB, the use of cerclage is of uncertain benefit. Effectiveness may be seen if the cervix is less than 10 mm. Ultrasound-indicated cerclage should be considered in a singleton pregnancy with a CL less than 25 mm and a history of spontaneous PTB.
Possibly one of the most controversial topics is ultrasound-indicated cerclage placement in twin gestation. As with many situations in obstetrics, data regarding ultrasound-indicated cerclage in twin gestation is based on small retrospective studies fraught with bias. Results from these studies range from no benefit, to potential benefit, to even possible increased risk of PTB. Since data are limited, as we await more evidence, it is recommended that the clinician and patient use shared decision making to decide on cerclage placement in a twin gestation.
Exam indicated. In a singleton pregnancy with a dilated cervix on digital or speculum exam between 16 0/7 to 23 6/7 weeks’ gestation, a physical exam–indicated cerclage should be offered. Exam-indicated cerclage also may reduce the incidence of PTB in twin gestations with cervical dilation between 16 0/7 and 23 6/7 weeks’ gestation. Indomethacin tocolysis and perioperative antibiotics should be considered when an exam-indicated cerclage is placed.
As the limits of viability are continually pushed earlier, more in-depth conversation is needed with patients who are considering an exam-indicated cerclage. The nuances of periviability and the likelihood that an exam-indicated cerclage will commit a pregnancy to a periviable or extremely preterm birth should be discussed in detail using a shared decision making model.
Regardless of whether the cerclage is ultrasound or exam indicated, once it is placed there is no utility in additional CL ultrasound monitoring.
Pessary
Vaginal pessaries for prevention of PTB have not gained popularity in the United States as they have in other countries. Trials are being conducted to determine the utility of vaginal pessary, but current data have not proven its effectiveness in preventing PTB in the setting of singleton pregnancy, short cervix, and no history of spontaneous PTB. So for now, pessary is not recommended. The same can be said for use in the twin gestation.
- All patients should have cervical evaluation during pregnancy. Serial imaging is reserved for those with a history of spontaneous PTB.
- Progesterone supplementation should be offered to patients with a singleton pregnancy and a history of spontaneous PTB or to patients with a singleton pregnancy and no history of spontaneous PTB who have cervical shortening at less than 24 weeks.
- Cerclage may be offered between 16 and 24 weeks for a cervical length less than 25 mm in a patient with a singleton gestation who has a history of spontaneous PTB (<10 mm if no history of spontaneous PTB) or for a dilated cervix on exam regardless of history.
- Women who have a twin gestation with cervical dilation may be offered physical exam–indicated cerclage.
Which patients may benefit from antepartum fetal surveillance and when to initiate it
American College of Obstetricians and Gynecologists’ Committee on Obstetrics Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Antepartum fetal surveillance: ACOG practice bulletin, number 229. Obstet Gynecol. 2021;137:e116-e127.
The ultimate purpose of antenatal fetal surveillance is to prevent stillbirth. However, stillbirth has multiple etiologies, not all of which are preventable with testing. In June 2021, ACOG released a new Committee Opinion containing guidelines for fetal surveillance, including suggested gestational age at initiation and frequency of testing, for the most common high-risk conditions. ACOG also released an update to the Practice Bulletin on antepartum fetal surveillance; additions include randomized controlled trial level data on the utility of fetal kick counts (FKCs) and recommendations that align with the new Committee Opinion.
Data for the efficacy of antepartum fetal surveillance are lacking, mainly due to the difficulty of performing prospective studies in stillbirth. The existing evidence is subject to intervention bias, as deliveries increase in tested patients, and recommendations rely heavily on expert consensus and nonrandomized studies. Antenatal testing is also time, cost, and labor intensive, with the risk of intervention for a false-positive result. Despite these limitations, obstetrical practices routinely perform antenatal fetal surveillance.
The new guidelines: The why, when, and how often
Why. Antepartum fetal surveillance is suggested for conditions that have a risk of stillbirth greater than 0.8 per 1,000 (that is, the false-negative rate of a biophysical profile or a modified biophysical profile) and the relative risk or odds ratio is greater than 2.0 for stillbirth compared with unaffected pregnancies.
When. For most conditions, ACOG recommends initiation of testing at 32 weeks or later, with notable earlier exceptions for some of the highest-risk patients. For certain conditions, such as fetal growth restriction and hypertensive disorders of pregnancy, the recommendation is to start “at diagnosis,” with the corollary “or at a gestational age when delivery would be considered because of abnormal results.” Shared decision making with the patient about pregnancy goals therefore is required, particularly in cases of fetal anomalies, genetic conditions, or at very early gestational ages.
How often. The recommended frequency of testing is at least weekly. Testing frequency should be increased to twice-weekly outpatient or daily inpatient for the most complicated pregnancies (for example, fetal growth restriction with abnormal umbilical artery Doppler studies, preeclampsia with severe features).
Once or twice weekly is an option for many conditions, which gives the clinician the opportunity to assess clinical stability as well as the patient’s input in terms of logistics and anxiety.
Patients with multiple conditions may fall into the “individualized” category, as do patients with suboptimal control of conditions (for example, diabetes, hypertension) that may affect the fetus as the pregnancy progresses.
New diagnoses included for surveillance
Several diagnoses not previously included now qualify for antepartum fetal surveillance under the new guidelines, most notably:
- history of obstetrical complications in the immediate preceding pregnancy
—history of prior fetal growth restriction requiring preterm delivery
—history of prior preeclampsia requiring delivery
- alcohol use of 5 or more drinks per day
- in vitro fertilization
- abnormal serum markers
—pregnancy-associated plasma protein A (PAPP-A) in the fifth or lower percentile or 0.4 multiples of the median (MoM)
—second trimester inhibin A of 2 or greater MoM
- prepregnancy body mass index (BMI)
—this is divided into 2 categories for timing of initiation of testing:
- 37 weeks for BMI of 35 to 39.9 kg/m2
- 34 weeks for BMI of or greater than 40 kg/m2.
Fetal kick counts
The major change to the updated Practice Bulletin on antenatal surveillance is the inclusion of data on FKCs, a simple modality of fetal surveillance that does not require a clinical visit. For FKCs, a meta-analysis of more than 450,000 patients did not demonstrate a difference in perinatal death between the FKC intervention group (0.54%) and the control group (0.59%). Of note, there were small but statistically significant increases in the rates of induction of labor, cesarean delivery, and preterm delivery in the FKC intervention group. Therefore, this update does not recommend a formal program of FKCs for all patients.
- The antenatal fetal surveillance guidelines are just that—guidelines, not mandates. Their use will need to be adapted for specific patient populations and practice management patterns.
- Many conditions qualify for “individualized” surveillance, which offers the opportunity for detailed discussions on the patient’s care. This includes shared decision making with patients to meet their goals for the pregnancy.
- Although patient-perceived decreased fetal movement always warrants clinical evaluation, a regular program of fetal kick count monitoring is not recommended for all patients due to lack of data supporting its benefit in reducing perinatal death.
- As with any change, new guidelines potentially are a source of frustration, so a concerted effort by obstetrical clinicians to agree on adoption of the guidelines is needed. Additional clinical resources and both clinician and patient education may be required depending on current practice style, as the new strategy may increase the number of appointments and ultrasound exams required.
Continue to: Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation...
Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation
American College of Obstetricians and Gynecologists. Use of antenatal corticosteroids at 22 weeks of gestation: ACOG practice advisory. September 2021. https://www .acog.org/clinical/clinical-guidance/practice-advisory /articles/2021/09/use-of-antenatal-corticosteroids-at -22-weeks-of-gestation. Accessed December 11, 2021.
In September 2021, ACOG and SMFM released a Practice Advisory updating the current recommendations for the administration of antenatal corticosteroids in the periviable period (22 to 25 6/7 weeks’ gestation). Whereas the prior lower limit of gestational age for consideration of steroids was 23 weeks, the new recommendation now extends this consideration down to 22 weeks.
The cited data include a meta-analysis of more than 2,200 patients in which the survival rate of infants born between 22 and 22 6/7 weeks who were exposed to antenatal steroids was 39% compared with 19.5% in the unexposed group. Another study of more than 1,000 patients demonstrated a statistically significant increase in overall survival in patients treated with antenatal steroids plus life support compared with life support only (38.5% vs 17.7%). Survival without major morbidity in this study, although increased from 1% to 4.4%, was still low.
Recommendation carries caveats
Given this information, the Practice Advisory offers a 2C level recommendation (weak recommendation, low quality of evidence) for antenatal steroids at 22 to 22 6/7 weeks’ gestation if neonatal resuscitation is planned, acknowledging the limitations and potential bias of the available data.
The Practice Advisory emphasizes the importance of counseling and patient involvement in the decision making. This requires a multidisciplinary collaboration among the neonatology and obstetrical teams, flexibility in the plan after birth depending on the infant’s condition, and redirection of care if appropriate. Estimated fetal weight, the presence of multiple gestations, fetal biologic sex, and any anomalies are also important in helping families make an informed decision for their particular pregnancy. As described in the Obstetric Care Consensus on periviable birth,6 it is important to remember that considerations and recommendations are not the same as requirements, and redirection of care to comfort and family memory making is not the same as withholding care.
The rest of the recommendations for the administration of antenatal steroids remain the same: Antenatal steroids are not recommended at less than 22 weeks due to lack of evidence of benefit, and they continue to be recommended at 24 weeks and beyond. ●
- Antenatal corticosteroids may be considered at 22 to 22 6/7 weeks’ gestation if, after thorough patient counseling, neonatal resuscitation is desired and planned by the family.
- The overall likelihood of survival and survival without major morbidities continues to be very low in the periviable period, especially at 22 weeks. Gestational age is only one of the many factors that must be considered in the shared decision making for this very difficult decision.
- Palliative care is a valid and appropriate option for patients facing a periviable delivery after appropriate counseling or after evaluation of the infant has occurred after birth.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2019. NCHS Data Brief, no 387. Hyattsville, MD: National Center for Health Statistics. October 2020. www.cdc.gov/nchs /data/databriefs/db387-H.pdf. Accessed December 20, 2021.
- To MS, Skentou C, Chan C, et al. Cervical assessment at the routine 23-week scan: standardizing techniques. Ultrasound Obstet Gynecol. 2001;17:217-219.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. Am J Obstet Gynecol. 2020;223:B16-B18.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 6: Periviable birth. Obstet Gynecol. 2017;130:e187-e199.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2019. NCHS Data Brief, no 387. Hyattsville, MD: National Center for Health Statistics. October 2020. www.cdc.gov/nchs /data/databriefs/db387-H.pdf. Accessed December 20, 2021.
- To MS, Skentou C, Chan C, et al. Cervical assessment at the routine 23-week scan: standardizing techniques. Ultrasound Obstet Gynecol. 2001;17:217-219.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. Am J Obstet Gynecol. 2020;223:B16-B18.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 6: Periviable birth. Obstet Gynecol. 2017;130:e187-e199.
The troubling trend of repackaging feminine hygiene products for the next generation
Feminine hygiene products have been commercially available for decades. They are commonly marketed to reduce odor or clean vaginal discharge and menses. Multiple formulas are available as topical washes, wipes, creams, sprays, powders, deodorants, and douches.1 Products on the market range from those used externally on the vulva, such as wipes and sprays, to liquid solutions used intravaginally, such as washes and douches.
Who uses feminine hygiene products?
According to a 2006 study, the majority of women who use douches started using them between age 15 and 19 years, but some women initiate this practice habit as early as age 10 to 14.1 Predictably, women who douche are more likely to perceive douche products as safe.1
Demographic data on douche utilization are mixed: Some studies show that there are no significant racial differences in douching practices,2 while others have found that Black and African American women are more likely to practice douching than White and Hispanic women.1,3 Studies have shown a significant difference in attitudes toward douching and knowledge of normal vaginal symptoms among US racial demographics, although this must be examined through the historical context of racism and the lens of medical anthropology.4
Women cite that common reasons they use feminine hygiene products are to feel clean, to control odor, and to use after menses and intercourse.1,2
Modern marketing approaches
From wipes to soaps to douches, feminine hygiene products often are advertised to promote “funk-free periods”5 and “freshness,” fostering an environment in which women and men develop unrealistic standards for what is considered normal genital odor and resulting in poor body image.6
Recently, Vagisil (Combe Incorporated) marketing efforts faced backlash from the ObGyn community for targeting younger populations with a specific product line for adolescents called OMV! In addition, attention has been drawn to VCF vaginal odor eliminating film (Apothecus Pharmaceutical Corp), small stamp-sized dissolving films that are placed in the vaginal canal in contact with the epithelium. This product has entered the market of feminine hygiene products accompanied by slogans of eliminating “feminine odor” and providing “confidence for women to be intimate.”
Continue to: Effects of feminine hygiene products on the vaginal microbiome...
Effects of feminine hygiene products on the vaginal microbiome
Frequent use of feminine hygiene products has been associated with recurrent vaginitis, bacterial vaginosis, and general irritation/itch,7,8 which can cause more discharge and odor. Ironically, this may result in women using the product more frequently since they often seek out these products to eliminate odor and discharge.1,2
The pH of the vagina changes during a woman’s lifetime, but in the reproductive years, the normal pH range is typically 3.8 to 4.4.9 This range allows for a normal vaginal flora to form with bacteria such as Lactobacillus species and Gardnerella vaginalis, while feminine hygiene products have a wide range of pH.9,10
Regardless of the formulation, most feminine hygiene products contain ingredients and compositions that potentially are detrimental to the health of the vulva and vagina. Many products contain acidic ingredients, such as citric acid, lactic acid, and dehydroacetic acid, that can alter the vaginal pH and weaken the vaginal barrier by wiping out normal vaginal flora10 despite being advertised for use on “sensitive areas” (TABLE). Lactic acid also has been found to increase diverse anaerobic bacteria in the vaginal microbiome.11 Some feminine hygiene products have been shown to suppress Lactobacillus growth at 2 hours after use and to kill all lactobacilli at 24 hours.10 Shifts in microbiota numbers often occur when the vaginal pH has been altered, as is frequently the case with feminine hygiene products. In the absence of microbiome bacteria, the presence of vaginal hygiene products has been shown to increase interleukin-8 (IL-8), suggesting a proinflammatory reaction.10
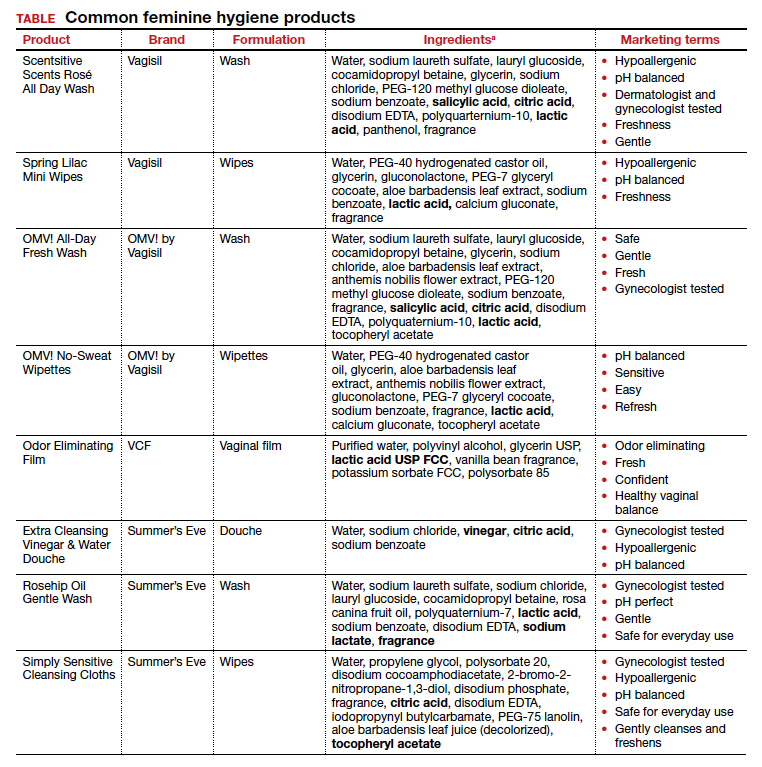
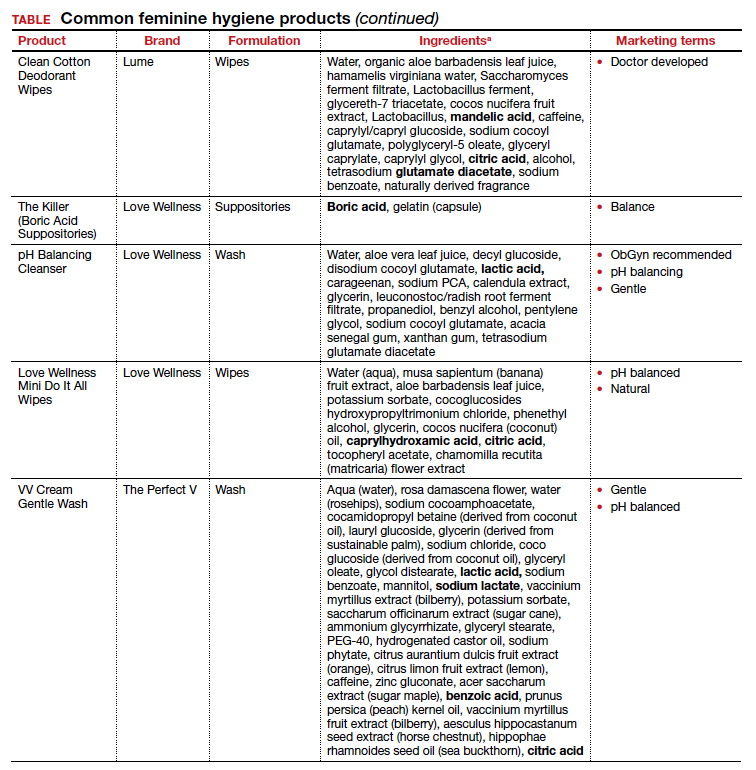
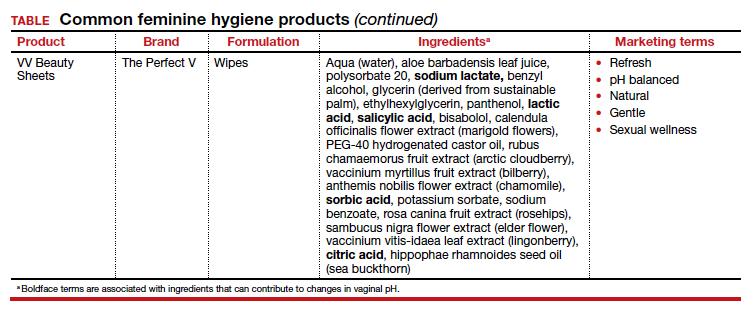
A study in the United Kingdom found that women who used bubble bath, antiseptics, or douche products had a higher incidence of bacterial vaginosis compared with women who did not use such products.7 Women in Canada who used feminine hygiene products were more likely to report adverse conditions, including yeast infections, bacterial vaginosis, urinary tract infections, and sexually transmitted diseases.8 Furthermore, a significant association exists between vaginal douching and endometrial infection by bacterial vaginosis–associated organisms.12
Additionally, a study that analyzed volatile organic compound levels in the blood with the use of feminine hygiene products revealed a significant positive dose-exposure relationship between the frequency of vaginal douching in the last 6 months and concentrations of 1,4-dichloromethane, one of the volatile organic compounds.3 This points to the issue of not only disruption of pH and vaginal flora but also to the introduction of harmful substances that can further disrupt the vaginal barrier.
Understand the products to help educate patients
Use of feminine hygiene products is common among women. While women depend on the market to filter out products that are considered unsafe or may have harmful side effects,1 unfortunately that is not necessarily the case. With increasingly more feminine products on the market and the target demographic becoming younger, women of all ages are susceptible to misinformation that could affect their vaginal health long term.
It is vital that clinicians understand the topical effects of these products in order to properly educate and counsel patients. Ultimately, research on feminine hygiene products is limited and, as more products come to market, we must continue to reassess the effects of topical products on the vaginal epithelium and vulvar tissues. ●
- Grimley DM, Annang L, Foushee HR, et al. Vaginal douches and other feminine hygiene products: women’s practices and perceptions of product safety. Matern Child Health J. 2006;10:303-310. doi: 10.1007/s10995-005-0054-y.
- Foch BJ, McDaniel ND, Chacko MR. Racial differences in vaginal douching knowledge, attitude, and practices among sexually active adolescents. J Pediatr Adolesc Gynecol. 2001;14:29-33. doi: 10.1016/S1083-3188(00)00080-2.
- Lin N, Ding N, Meza-Wilson E, et al. Volatile organic compounds in feminine hygiene products sold in the US market: a survey of products and health risks. Environ Int. 2020;144:105740. doi: 10.1016/j.envint.2020.105740.
- Wayne State University Digital Commons. Guy-Lee AK. Rituals reproducing race: African American women’s feminine hygiene practices, shared experiences, and power. 2017. http://digitalcommons.wayne.edu/oa_dissertations/1806. Accessed December 13, 2021.
- YouTube. OMV! by Vagisil—Intimate care products designed by teens. July 10, 2020. www.youtube.com/ watch?v=VkVsCagrAw0. Accessed December 13, 2021.
- Jenkins A, O’Doherty KC. The clean vagina, the healthy vagina, and the dirty vagina: exploring women’s portrayals of the vagina in relation to vaginal cleansing product use. Fem Psychol. 2021;31:192-211. doi: 10.1177/0959353520944144.
- Rajamanoharan S, Low N, Jones SB, et al. Bacterial vaginosis, ethnicity, and the use of genital cleansing agents: a case control study. Sex Transm Dis. 1999;26:404-409.
- Crann SE, Cunningham S, Albert A, et al. Vaginal health and hygiene practices and product use in Canada: a national cross-sectional survey. BMC Womens Health. 2018;18:52. doi: 10.1186/s12905-018-0543-y.
- Chen Y, Bruning E, Rubino J, et al. Role of female intimate hygiene in vulvovaginal health: global hygiene practices and product usage. Womens Health (London). 2017;13:58-67. doi: 10.1177/1745505717731011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd. v24i0.19703.
- Van der Veer C, Bruisten SM, Van Houdt R, et al. Effects of an over-the-counter lactic-acid containing intra-vaginal douching product on the vaginal microbiota. BMC Microbiol. 2019;19:168. doi: 10.1186/s12866-019-1545-0.
- Gondwe T, Ness R, Totten PA, et al. Novel bacterial vaginosis-associated organisms mediate the relationship between vaginal douching and pelvic inflammatory disease. Sex Transm Infect. 2020;96:439-444. doi: 10.1136/ sextrans-2019-054191.
Feminine hygiene products have been commercially available for decades. They are commonly marketed to reduce odor or clean vaginal discharge and menses. Multiple formulas are available as topical washes, wipes, creams, sprays, powders, deodorants, and douches.1 Products on the market range from those used externally on the vulva, such as wipes and sprays, to liquid solutions used intravaginally, such as washes and douches.
Who uses feminine hygiene products?
According to a 2006 study, the majority of women who use douches started using them between age 15 and 19 years, but some women initiate this practice habit as early as age 10 to 14.1 Predictably, women who douche are more likely to perceive douche products as safe.1
Demographic data on douche utilization are mixed: Some studies show that there are no significant racial differences in douching practices,2 while others have found that Black and African American women are more likely to practice douching than White and Hispanic women.1,3 Studies have shown a significant difference in attitudes toward douching and knowledge of normal vaginal symptoms among US racial demographics, although this must be examined through the historical context of racism and the lens of medical anthropology.4
Women cite that common reasons they use feminine hygiene products are to feel clean, to control odor, and to use after menses and intercourse.1,2
Modern marketing approaches
From wipes to soaps to douches, feminine hygiene products often are advertised to promote “funk-free periods”5 and “freshness,” fostering an environment in which women and men develop unrealistic standards for what is considered normal genital odor and resulting in poor body image.6
Recently, Vagisil (Combe Incorporated) marketing efforts faced backlash from the ObGyn community for targeting younger populations with a specific product line for adolescents called OMV! In addition, attention has been drawn to VCF vaginal odor eliminating film (Apothecus Pharmaceutical Corp), small stamp-sized dissolving films that are placed in the vaginal canal in contact with the epithelium. This product has entered the market of feminine hygiene products accompanied by slogans of eliminating “feminine odor” and providing “confidence for women to be intimate.”
Continue to: Effects of feminine hygiene products on the vaginal microbiome...
Effects of feminine hygiene products on the vaginal microbiome
Frequent use of feminine hygiene products has been associated with recurrent vaginitis, bacterial vaginosis, and general irritation/itch,7,8 which can cause more discharge and odor. Ironically, this may result in women using the product more frequently since they often seek out these products to eliminate odor and discharge.1,2
The pH of the vagina changes during a woman’s lifetime, but in the reproductive years, the normal pH range is typically 3.8 to 4.4.9 This range allows for a normal vaginal flora to form with bacteria such as Lactobacillus species and Gardnerella vaginalis, while feminine hygiene products have a wide range of pH.9,10
Regardless of the formulation, most feminine hygiene products contain ingredients and compositions that potentially are detrimental to the health of the vulva and vagina. Many products contain acidic ingredients, such as citric acid, lactic acid, and dehydroacetic acid, that can alter the vaginal pH and weaken the vaginal barrier by wiping out normal vaginal flora10 despite being advertised for use on “sensitive areas” (TABLE). Lactic acid also has been found to increase diverse anaerobic bacteria in the vaginal microbiome.11 Some feminine hygiene products have been shown to suppress Lactobacillus growth at 2 hours after use and to kill all lactobacilli at 24 hours.10 Shifts in microbiota numbers often occur when the vaginal pH has been altered, as is frequently the case with feminine hygiene products. In the absence of microbiome bacteria, the presence of vaginal hygiene products has been shown to increase interleukin-8 (IL-8), suggesting a proinflammatory reaction.10



A study in the United Kingdom found that women who used bubble bath, antiseptics, or douche products had a higher incidence of bacterial vaginosis compared with women who did not use such products.7 Women in Canada who used feminine hygiene products were more likely to report adverse conditions, including yeast infections, bacterial vaginosis, urinary tract infections, and sexually transmitted diseases.8 Furthermore, a significant association exists between vaginal douching and endometrial infection by bacterial vaginosis–associated organisms.12
Additionally, a study that analyzed volatile organic compound levels in the blood with the use of feminine hygiene products revealed a significant positive dose-exposure relationship between the frequency of vaginal douching in the last 6 months and concentrations of 1,4-dichloromethane, one of the volatile organic compounds.3 This points to the issue of not only disruption of pH and vaginal flora but also to the introduction of harmful substances that can further disrupt the vaginal barrier.
Understand the products to help educate patients
Use of feminine hygiene products is common among women. While women depend on the market to filter out products that are considered unsafe or may have harmful side effects,1 unfortunately that is not necessarily the case. With increasingly more feminine products on the market and the target demographic becoming younger, women of all ages are susceptible to misinformation that could affect their vaginal health long term.
It is vital that clinicians understand the topical effects of these products in order to properly educate and counsel patients. Ultimately, research on feminine hygiene products is limited and, as more products come to market, we must continue to reassess the effects of topical products on the vaginal epithelium and vulvar tissues. ●
Feminine hygiene products have been commercially available for decades. They are commonly marketed to reduce odor or clean vaginal discharge and menses. Multiple formulas are available as topical washes, wipes, creams, sprays, powders, deodorants, and douches.1 Products on the market range from those used externally on the vulva, such as wipes and sprays, to liquid solutions used intravaginally, such as washes and douches.
Who uses feminine hygiene products?
According to a 2006 study, the majority of women who use douches started using them between age 15 and 19 years, but some women initiate this practice habit as early as age 10 to 14.1 Predictably, women who douche are more likely to perceive douche products as safe.1
Demographic data on douche utilization are mixed: Some studies show that there are no significant racial differences in douching practices,2 while others have found that Black and African American women are more likely to practice douching than White and Hispanic women.1,3 Studies have shown a significant difference in attitudes toward douching and knowledge of normal vaginal symptoms among US racial demographics, although this must be examined through the historical context of racism and the lens of medical anthropology.4
Women cite that common reasons they use feminine hygiene products are to feel clean, to control odor, and to use after menses and intercourse.1,2
Modern marketing approaches
From wipes to soaps to douches, feminine hygiene products often are advertised to promote “funk-free periods”5 and “freshness,” fostering an environment in which women and men develop unrealistic standards for what is considered normal genital odor and resulting in poor body image.6
Recently, Vagisil (Combe Incorporated) marketing efforts faced backlash from the ObGyn community for targeting younger populations with a specific product line for adolescents called OMV! In addition, attention has been drawn to VCF vaginal odor eliminating film (Apothecus Pharmaceutical Corp), small stamp-sized dissolving films that are placed in the vaginal canal in contact with the epithelium. This product has entered the market of feminine hygiene products accompanied by slogans of eliminating “feminine odor” and providing “confidence for women to be intimate.”
Continue to: Effects of feminine hygiene products on the vaginal microbiome...
Effects of feminine hygiene products on the vaginal microbiome
Frequent use of feminine hygiene products has been associated with recurrent vaginitis, bacterial vaginosis, and general irritation/itch,7,8 which can cause more discharge and odor. Ironically, this may result in women using the product more frequently since they often seek out these products to eliminate odor and discharge.1,2
The pH of the vagina changes during a woman’s lifetime, but in the reproductive years, the normal pH range is typically 3.8 to 4.4.9 This range allows for a normal vaginal flora to form with bacteria such as Lactobacillus species and Gardnerella vaginalis, while feminine hygiene products have a wide range of pH.9,10
Regardless of the formulation, most feminine hygiene products contain ingredients and compositions that potentially are detrimental to the health of the vulva and vagina. Many products contain acidic ingredients, such as citric acid, lactic acid, and dehydroacetic acid, that can alter the vaginal pH and weaken the vaginal barrier by wiping out normal vaginal flora10 despite being advertised for use on “sensitive areas” (TABLE). Lactic acid also has been found to increase diverse anaerobic bacteria in the vaginal microbiome.11 Some feminine hygiene products have been shown to suppress Lactobacillus growth at 2 hours after use and to kill all lactobacilli at 24 hours.10 Shifts in microbiota numbers often occur when the vaginal pH has been altered, as is frequently the case with feminine hygiene products. In the absence of microbiome bacteria, the presence of vaginal hygiene products has been shown to increase interleukin-8 (IL-8), suggesting a proinflammatory reaction.10



A study in the United Kingdom found that women who used bubble bath, antiseptics, or douche products had a higher incidence of bacterial vaginosis compared with women who did not use such products.7 Women in Canada who used feminine hygiene products were more likely to report adverse conditions, including yeast infections, bacterial vaginosis, urinary tract infections, and sexually transmitted diseases.8 Furthermore, a significant association exists between vaginal douching and endometrial infection by bacterial vaginosis–associated organisms.12
Additionally, a study that analyzed volatile organic compound levels in the blood with the use of feminine hygiene products revealed a significant positive dose-exposure relationship between the frequency of vaginal douching in the last 6 months and concentrations of 1,4-dichloromethane, one of the volatile organic compounds.3 This points to the issue of not only disruption of pH and vaginal flora but also to the introduction of harmful substances that can further disrupt the vaginal barrier.
Understand the products to help educate patients
Use of feminine hygiene products is common among women. While women depend on the market to filter out products that are considered unsafe or may have harmful side effects,1 unfortunately that is not necessarily the case. With increasingly more feminine products on the market and the target demographic becoming younger, women of all ages are susceptible to misinformation that could affect their vaginal health long term.
It is vital that clinicians understand the topical effects of these products in order to properly educate and counsel patients. Ultimately, research on feminine hygiene products is limited and, as more products come to market, we must continue to reassess the effects of topical products on the vaginal epithelium and vulvar tissues. ●
- Grimley DM, Annang L, Foushee HR, et al. Vaginal douches and other feminine hygiene products: women’s practices and perceptions of product safety. Matern Child Health J. 2006;10:303-310. doi: 10.1007/s10995-005-0054-y.
- Foch BJ, McDaniel ND, Chacko MR. Racial differences in vaginal douching knowledge, attitude, and practices among sexually active adolescents. J Pediatr Adolesc Gynecol. 2001;14:29-33. doi: 10.1016/S1083-3188(00)00080-2.
- Lin N, Ding N, Meza-Wilson E, et al. Volatile organic compounds in feminine hygiene products sold in the US market: a survey of products and health risks. Environ Int. 2020;144:105740. doi: 10.1016/j.envint.2020.105740.
- Wayne State University Digital Commons. Guy-Lee AK. Rituals reproducing race: African American women’s feminine hygiene practices, shared experiences, and power. 2017. http://digitalcommons.wayne.edu/oa_dissertations/1806. Accessed December 13, 2021.
- YouTube. OMV! by Vagisil—Intimate care products designed by teens. July 10, 2020. www.youtube.com/ watch?v=VkVsCagrAw0. Accessed December 13, 2021.
- Jenkins A, O’Doherty KC. The clean vagina, the healthy vagina, and the dirty vagina: exploring women’s portrayals of the vagina in relation to vaginal cleansing product use. Fem Psychol. 2021;31:192-211. doi: 10.1177/0959353520944144.
- Rajamanoharan S, Low N, Jones SB, et al. Bacterial vaginosis, ethnicity, and the use of genital cleansing agents: a case control study. Sex Transm Dis. 1999;26:404-409.
- Crann SE, Cunningham S, Albert A, et al. Vaginal health and hygiene practices and product use in Canada: a national cross-sectional survey. BMC Womens Health. 2018;18:52. doi: 10.1186/s12905-018-0543-y.
- Chen Y, Bruning E, Rubino J, et al. Role of female intimate hygiene in vulvovaginal health: global hygiene practices and product usage. Womens Health (London). 2017;13:58-67. doi: 10.1177/1745505717731011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd. v24i0.19703.
- Van der Veer C, Bruisten SM, Van Houdt R, et al. Effects of an over-the-counter lactic-acid containing intra-vaginal douching product on the vaginal microbiota. BMC Microbiol. 2019;19:168. doi: 10.1186/s12866-019-1545-0.
- Gondwe T, Ness R, Totten PA, et al. Novel bacterial vaginosis-associated organisms mediate the relationship between vaginal douching and pelvic inflammatory disease. Sex Transm Infect. 2020;96:439-444. doi: 10.1136/ sextrans-2019-054191.
- Grimley DM, Annang L, Foushee HR, et al. Vaginal douches and other feminine hygiene products: women’s practices and perceptions of product safety. Matern Child Health J. 2006;10:303-310. doi: 10.1007/s10995-005-0054-y.
- Foch BJ, McDaniel ND, Chacko MR. Racial differences in vaginal douching knowledge, attitude, and practices among sexually active adolescents. J Pediatr Adolesc Gynecol. 2001;14:29-33. doi: 10.1016/S1083-3188(00)00080-2.
- Lin N, Ding N, Meza-Wilson E, et al. Volatile organic compounds in feminine hygiene products sold in the US market: a survey of products and health risks. Environ Int. 2020;144:105740. doi: 10.1016/j.envint.2020.105740.
- Wayne State University Digital Commons. Guy-Lee AK. Rituals reproducing race: African American women’s feminine hygiene practices, shared experiences, and power. 2017. http://digitalcommons.wayne.edu/oa_dissertations/1806. Accessed December 13, 2021.
- YouTube. OMV! by Vagisil—Intimate care products designed by teens. July 10, 2020. www.youtube.com/ watch?v=VkVsCagrAw0. Accessed December 13, 2021.
- Jenkins A, O’Doherty KC. The clean vagina, the healthy vagina, and the dirty vagina: exploring women’s portrayals of the vagina in relation to vaginal cleansing product use. Fem Psychol. 2021;31:192-211. doi: 10.1177/0959353520944144.
- Rajamanoharan S, Low N, Jones SB, et al. Bacterial vaginosis, ethnicity, and the use of genital cleansing agents: a case control study. Sex Transm Dis. 1999;26:404-409.
- Crann SE, Cunningham S, Albert A, et al. Vaginal health and hygiene practices and product use in Canada: a national cross-sectional survey. BMC Womens Health. 2018;18:52. doi: 10.1186/s12905-018-0543-y.
- Chen Y, Bruning E, Rubino J, et al. Role of female intimate hygiene in vulvovaginal health: global hygiene practices and product usage. Womens Health (London). 2017;13:58-67. doi: 10.1177/1745505717731011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd. v24i0.19703.
- Van der Veer C, Bruisten SM, Van Houdt R, et al. Effects of an over-the-counter lactic-acid containing intra-vaginal douching product on the vaginal microbiota. BMC Microbiol. 2019;19:168. doi: 10.1186/s12866-019-1545-0.
- Gondwe T, Ness R, Totten PA, et al. Novel bacterial vaginosis-associated organisms mediate the relationship between vaginal douching and pelvic inflammatory disease. Sex Transm Infect. 2020;96:439-444. doi: 10.1136/ sextrans-2019-054191.
Buccal Fat Pad Reduction With Intraoperative Fat Transfer to the Temple
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.
Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.

Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
The buccal fat pad (Bichat fat pad) is a tubular-shaped collection of adipose tissue that occupies a prominent position in the midface. The buccal fat pad has been described as having 3 lobes: an anterior lobe, which is anterior to the masseter muscle; an intermediate lobe between the masseter and buccinator muscles; and a posterior lobe between the temporal masticatory space.1 There are 4 extensions from the body of the buccal fat pad: the buccal, the sublevator, the melolabial, and the pterygoid. It is the buccal extension and main body that are removed intraorally to achieve midfacial and lower facial contouring, as these support the contours of the cheeks. The deep fat pad within the temporal fossa is a true extension of the buccal fat pad (Figure).2 It has a complex relationship to the facial structures, with known variability in the positions of the buccal branch of the facial nerve and the parotid duct.3 The parotid duct travels over, superior to, or through the buccal extension 42%, 32%, and 26% of the time, respectively. The duct travels along the surface of the masseter, then pierces the buccinator to drain into the vestibule of the mouth at the second superior molar tooth. The buccal branch of the facial nerve travels on the surface of the buccal fat pad 73% of the time, whereas 27% of the time it travels deeper through the buccal extension.4 A study that used ultrasonography to map the surface anatomy path of the parotid duct in 50 healthy patients showed that the duct was within 1.5 cm of the middle half of a line between the lower border of the tragus and the oral commissure in 93% of individuals.5 We describe a technique in which part of the buccal fat pad is removed and the fat is transferred to the temple to achieve aesthetically pleasing facial contouring. We used a vertical line from the lateral canthus as a surface anatomy landmark to determine when the duct emerges from the gland and is most susceptible to injury.

Operative Technique
Correct instrumentation is important to obtain appropriate anatomic exposure for this procedure. The surgical tray should include 4-0 poliglecaprone 25 suture, bite guards, a needle driver, a hemostat, surgical scissors, toothed forceps, a Beaver surgical handle with #15 blade, a protected diathermy needle, cotton tip applicators, and gauze.
Fat Harvest—With the patient supine, bite blocks are placed, and the buccal fat pad incision line is marked with a surgical marker. A 1-cm line is drawn approximately 4 cm posterior to the oral commissure by the buccal bite marks. The location is verified by balloting externally on the buccal fat pad on the cheek. The incision line is then anesthetized transorally with lidocaine and epinephrine-containing solution. The cheek is retracted laterally with Caldwell-Luc retractors, and a 1-cm incision is made and carried through the mucosa and superficial muscle using the Colorado needle. Scissors are then used to spread the deeper muscle fibers to expose the deeper fascia and fat pads. Metzenbaum scissors are used to gently spread the fat while the surgeon places pressure on the external cheek, manipulating the fat into the wound. Without excess traction, the walnut-sized portion of the fat pad that protrudes is grasped with Debakey forceps, gently teased into the field, clamped at its base with a curved hemostat, and excised. The stump is electrocoagulated with an extendable protected Colorado needle, with care to prevent inadvertent cauterization of the lips. The wound is closed with a single 4-0 poliglecaprone-25 suture.
A 5-cc Luer lock syringe is preloaded with 2 cc of normal saline and attached to another 5-cc Luer lock syringe via a female-female attachment. The excised fat is then placed in a 5-cc Luer lock syringe by removing the plunger. The plunger is then reinstalled, and the fat is injected back and forth approximately 30 times. The fat is centrifuged at 3500 rpm for 3 minutes. The purified fat is then transferred to a 1-cc Luer lock syringe attached to an 18-gauge needle.
Fat Injection—The authors use an 18-gauge needle to perform depot injections into the temporal fossae above the periosteum. This is a relatively safe area of the face to inject, but care must be taken to avoid injury to the superficial temporal artery. Between 1.5 and 3 cc of high-quality fat usually are administered to each temple.
Aftercare Instructions—The patient is instructed to have a soft diet for 24 to 48 hours and can return to work the next day. The patient also is given prophylactic antibiotics with Gram-negative coverage for 7 days (amoxicillin-clavulanate 875 mg/125 mg orally twice daily for 7 days).
Candidates for Buccal Fat Pad Reduction
Buccal fat pad reduction has become an increasingly popular technique for midface and lower face shaping to decrease the appearance of a round face. To achieve an aesthetically pleasing midface, surgeons should consider enhancing zygomatic eminences while emphasizing the border between the zygomatic prominence and cheek hollow.6 Selection criteria for buccal fat pad reduction are not well established. One study recommended avoiding the procedure in pregnant or lactating patients, patients with chronic illnesses, patients on blood-thinning agents, and patients younger than 18 years. In addition, this study suggested ensuring the malar fullness is in the anteromedial portion of the face, as posterolateral fullness may be due to masseter hypertrophy.6
Complications From Buccal Fat Pad Reduction
Complications associated with buccal fat pad reduction include inadvertent damage to surrounding structures, including the buccal branch of the facial nerve and parotid duct. Because the location of the facial nerve in relation to the parotid duct is highly variable, surgeons must be aware of its anatomy to avoid unintentional damage. Hwang et al7 reported that the parotid duct and buccal branches of the facial nerves passed through the buccal extension in 26.3% of cadavers. The transbuccal approach is preferred over the sub–superficial muscular aponeurotic system approach largely because it avoids these structures. In addition, blunt dissection may further decrease chances of injury. Although the long-term effects are unknown, there is a potential risk for facial hollowing.3 The use of preprocedure ultrasonography to quantify the buccal fat pad may avoid overresection and enhanced potential for facial hollowing.6
Avoidance of Temporal Hollowing
Because the buccal fat pad extends into the temporal space, buccal fat pad reduction may lead to further temporal hollowing, contributing to an aged appearance. The authors’ technique addresses both midface and upper face contouring in one minimally invasive procedure. Temporal hollowing commonly has been corrected with autologous fat grafting from the thigh or abdomen, which leads to an additional scar at the donor site. Our technique relies on autologous adjacent fat transfer from previously removed buccal fat. In addition, compared with the use of hyaluronic acid fillers for temple reflation, fat transfer largely is safe and biocompatible. Major complications of autologous fat transfer to the temples include nodularity or fat clumping, fat necrosis, sensory or motor nerve damage, and edema or ecchymosis.4 Also, with time there will be ongoing hollowing of the temples as part of the aging process with soft tissue and bone resorption. Therefore, further volume restoration procedures may be required in the future to address these dynamic changes.
Conclusion
The buccal fat pad has been extensively used to reconstruct oral defects, including oroantral and cranial base defects, owing to its high vascularity.6 However, there also is great potential to utilize buccal fat for autologous fat transfer to improve temporal wasting. Further studies are needed to determine optimal technique as well as longer-term safety and efficacy of this procedure.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
- Zhang HM, Yan YP, Qi KM, et al. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast Reconstr Surg. 2002;109:2509-2518.
- Yousuf S, Tubbs RS, Wartmann CT, et al. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427-436.
- Benjamin M, Reish RG. Buccal fat pad excision: proceed with caution. Plast Reconstr Surg Glob Open. 2018;6:E1970.
- Tzikas TL. Fat grafting volume restoration to the brow and temporal regions. Facial Plast Surg. 2018;34:164-172.
- Stringer MD, Mirjalili SA, Meredith SJ, et al. Redefining the surface anatomy of the parotid duct: an in vivo ultrasound study. Plast Reconstr Surg. 2012;130:1032-1037.
- Sezgin B, Tatar S, Boge M, et al. The excision of the buccal fat pad for cheek refinement: volumetric considerations. Aesthet Surg J. 2019;39:585-592.
- Hwang K, Cho HJ, Battuvshin D, et al. Interrelated buccal fat pad with facial buccal branches and parotid duct. J Craniofac Surg. 2005;16:658-660.
Practice Points
- Buccal fat pad reduction is an increasingly popular procedure for facial shaping.
- Buccal fat pad reduction in addition to natural aging can result in volume depletion of the temporal fossae.
- Removed buccal fat can be transferred to the temples for increased volume.
Nicotine and Nicotine Replacement Therapy Use During Myocardial Perfusion Imaging
Chest pain is one of the most common concerns in patients presenting to the emergency department in the United States, accounting for approximately 7.6 million visits annually.1 Given the high mortality rate associated with acute coronary syndromes, prompt evaluation of chest pain is essential.2 Even in mild cases, recognition of newly onset or worsening coronary artery disease (CAD) is crucial to ensure that patients receive optimal medication therapy.
In symptomatic patients with risk factors for CAD, such as advanced age, hypertension, hyperlipidemia, obesity, and diabetes mellitus, myocardial perfusion imaging (MPI) is frequently used as a modality to assess the presence, location, and severity of ischemic or infarcted myocardium.2 MPI requires administration of a radiopharmaceutical before and after the patient undergoes a form of stress.2 This radiopharmaceutical is then detected in the myocardium with a nuclear camera, and images are obtained of the heart to assess myocardial blood flow.2
MPI can be performed using exercise-induced stress via a treadmill, or medication-induced stress (Table 1). In both strategies, healthy coronary arteries dilate to provide the myocardium with more blood flow to meet the increasing myocardial oxygen demand during this period of stress. While healthy vessels are able to dilate appropriately, coronary arteries with flow-limiting stenoses are unable to dilate to the same extent in response to stress.2 Because radioactive isotope uptake by the myocardium is directly related to arterial blood flow, MPI is able to demonstrate a mismatch in coronary blood flow between healthy and diseased coronary arteries indicated by differences in radioisotope uptake.2 The presence of such a mismatch, in conjunction with clinical history, potentially suggests the presence of CAD.
Prior to conducting MPI with a medication, certain substances should be avoided. For instance, methylxanthines, such as caffeine, aminophylline, and theophylline, antagonize adenosine receptors and can have major drug interactions with regadenoson, adenosine, and dipyridamole. Therefore, it is advised that these substances be stopped for at least 12 hours before testing.3 In some cases, other medications that can affect coronary blood flow, such as long-acting nitrates, β-blockers, and calcium channel blockers, are recommended to be avoided for 12 to 48 hours in order to obtain the most accurate depiction of underlying coronary disease.4
Because nicotine and nicotine replacement therapy (NRT) may have substantial effects on coronary circulation, a current area of controversy is whether these should be stopped prior to the use of a stress-inducing medication during MPI. To date, no formal drug interaction studies have been conducted between nicotine and regadenoson.5 Similarly, the ADVANCE MPI 2 Trial, which led to the US Food and Drug Administration approval of regadenoson, did not specify restrictions on the use of nicotine prior to stress testing in the protocol.6 However, as this trial was multicenter, investigators admit that individual study sites could have had their own restrictions on the use of nicotine prior to stress testing with regadenoson, but this information was not collected.6 The current review focuses on how the simultaneous use of nicotine or NRT during MPI with pharmacologic agents, such as regadenoson, may affect the accuracy of imaging results and the clinical impact of this interaction.
Nicotine Coronary Artery Effect
It is well documented that long-term cigarette smoking is a major risk factor for CAD.7 Compared with nonsmokers, cigarette smokers experience 2 times greater risk of morbidity and mortality from ischemic heart disease.7 There are several mechanisms by which nicotine induces damage to the myocardium (Figure). Nicotine has direct effects on both the sympathetic nervous system (SNS) and myocardial endothelium.8 Together, these factors result in reduced coronary blood flow, leading to less oxygen supply to meet an increased oxygen demand, resulting in myocardial ischemia.
Nicotine’s effect on coronary vasomotor tone occurs primarily through noradrenergic stimulation of α and β receptors associated with coronary vasoconstriction or vasodilation, respectively.9,10 These competing influences on coronary blood flow appear to manifest differently based on whether patients are at rest or in a stressed state. A study by Czerin and colleagues demonstrated that in healthy patients with relatively short smoking histories and in a healthy nonsmoker control group, coronary blood flow increased by 25% and 40%, respectively, with nicotine use at rest.9 However, when these patients were stressed with dipyramidole and while smoking during the examination, myocardial blood flow was reduced by 11% in the study group and 14% in the control group.9 This is likely because the patients studied had relatively healthy coronary arteries that were able to maximally dilate when stressed. In this scenario, nicotine’s dilatory effects are offset by nicotine’s α-receptor–mediated vasoconstriction effects.9 Of note, patients in the study group experienced a somewhat diminished increase in coronary blood flow at rest with nicotine use, suggesting that even a short smoking history may damage the myocardial endothelium, rendering it less responsive to nicotine’s vasodilatory effects.9
These principles similarly apply to patients with underlying moderate-to-severe cardiovascular disease (CVD). With nicotine use at rest, patients with significant CAD do not experience as dramatic of an increase in coronary blood flow, which typically decreases or remains the same despite increased myocardial work.10 This may be because patients with moderate-to-severe CAD often have flow-limiting stenoses and damaged endothelium that do not allow vessels to respond as efficiently to increased myocardial demand or to nicotine’s β-receptor–mediated vasodilatory effects.10,11 Moreover, when stressed, diseased coronary arteries are not able to further dilate and nicotine’s α-receptor–mediated vasoconstriction effects dominate.10,11
In a study by Quillen and colleagues of patients with moderate-to-severe CAD, the mean diameter of proximal coronary artery segments decreased by 5%, the distal coronary diameter decreased 8%, and the coronary vascular resistance increased by 21% while smoking at rest.12 The investigators did not analyze how parameters changed when these diseased coronary arteries were stressed using a medication during MPI. However, it can be predicted that coronary arteries would have constricted to a similar or greater degree than observed in Czerin and colleagues’ study, given that the underlying myocardium was diseased and more susceptible to nicotine’s vasoconstriction effects.9 Importantly, these studies have several limitations, most notably that they are older and have small sample sizes. Additionally, while statistically significant differences were found in the degree of changes in coronary circulation with nicotine use at rest and during stress, it is unclear whether this translates to a clinically significant and impactful finding.9-12
Nicotine Replacement Therapy and Stress Testing
Given the association between cigarette smoking and CAD, medical practitioners strongly encourage patients to quit smoking to reduce their risk of adverse cardiovascular outcomes. Various smoking cessation treatments are available for patients. Common, readily accessible forms of therapy include nicotine replacement products (Table 2).
Early studies of NRT in patients with underlying CVD found an increased risk of cardiovascular events, such as myocardial infarction, presumably due to the nicotine content of these products.13,14 However, the concentration of nicotine in NRT is substantially lower than that found in cigarettes and in some formulations, such as transdermal patches, nicotine is delivered over a prolonged period of time.15 For this reason, NRT is thought to be safe in patients with underlying CVD and stable ischemic heart disease. A recent systematic review and meta-analysis found that while NRT may be associated with tachycardia, it did not increase the risk of more serious cardiovascular adverse effects (AEs).16,17
Given the lower nicotine concentration in NRT products, the associated hemodynamic effect of nicotine also is thought to be less pronounced. In a study conducted by Tzivoni and colleagues in patients with CAD using transdermal nicotine patches, no differences in blood pressure, heart rate, ischemia, or arrhythmias were found from baseline to 2 weeks.18 These findings were further confirmed in a small study by Lucini and colleagues, which found that nicotine patches produced slight hemodynamic effects, but to a lesser extent than cigarette smoking.19 For the NRT gum formulation, while a small study found that 4 mg produced coronary vasoconstriction in patients with underlying CAD, a study by Nitenberg and Antony demonstrated that healthy and diseased coronary arteries did not significantly constrict while patients were using nicotine gum both before and after a cold pressor test, suggesting a lesser degree of coronary vasoconstriction than nicotine from cigarette smoking.20,21 Similar findings have been described with the nicotine intranasal spray in a study by Keeley and colleagues, which showed no additional AEs on myocardial demand or vasoconstriction when an intranasal nicotine spray was added to cigarette smoking.22 Importantly, a review of the transdermal and gum formulations found that these less pronounced hemodynamic effects were observed across different doses of NRT; however, further studies are needed to clarify the relationship between NRT dose and cardiovascular effects.23
Overall, NRT does not seem to activate the SNS to the same degree as nicotine obtained via cigarette smoking and likely does not increase the myocardial oxygen demand as much. Additionally, by containing a lower concentration of nicotine, NRT may not impair the myocardium’s ability to supply oxygen to coronary arteries to the same extent as nicotine from cigarette smoking. Therefore, the effects of NRT on MPI using a stress-inducing medication may not be as pronounced. However, due to study limitations, results should be interpreted cautiously.18-23
Conclusions
Because of the close relationship between cigarette smoking and CAD, many patients with underlying CVD are either current smokers or may be using NRT for smoking cessation. Therefore, the question of whether to refrain from nicotine use prior to MPI is clinically relevant. Currently, there is a lack of high-quality studies demonstrating the effects of nicotine and NRT on coronary perfusion. Because of this, the impact of nicotine and NRT use on the accuracy of MPI using stress-inducing medications remains uncertain. Nevertheless, given that nicotine and NRT may largely affect the accuracy of imaging results, several institutions have adopted protocols that prohibit patients from using these drugs on the day of nuclear stress testing.
There are currently no data specifying the number of hours to hold nicotine products prior to cardiac stress testing. It is generally recommended that other medications that affect coronary blood flow be held for 5 half-lives before conducting MPI.4 Following the same guidance for nicotine and NRT may present a reasonable approach to ensure accurate imaging results. Based on the discussed literature, patients should be instructed to refrain from cigarette smoking for at least 5 to 10 hours prior to MPI, given nicotine’s half-life of about 1 to 2 hours.24
The data for NRT are less clear. While use of NRT may not be an absolute contraindication to conducting MPI, it is important to consider that this may affect the accuracy of results. Given this uncertainty, it is likely ideal to hold NRT prior to MPI, based on the specific formulation of NRT and that product's half-life. Further robust studies are needed to analyze the impact of nicotine and NRT on the accuracy of nuclear stress testing using a medication.
1. Rui P, Kang K, Ashman JJ. National Hospital Ambulatory Medical Care Survey: 2016 emergency department summary tables. Published 2016. Accessed March 30, 2020. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2016_ed_web_tables.pdf
2. Lange RA. Cardiovascular testing. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 10th ed. McGraw Hill; 2017.
3. Mace S. Observation Medicine: Principles and Protocols. Cambridge University Press; 2017.
4. Currie GM. Pharmacology, part 4: nuclear cardiology. J Nucl Med Technol. 2019;47(2):97-110. doi:10.2967/jnmt.118.219675
5. Regadenoson; Package insert. Astellas Pharma US Inc; 2008.
6. Iskandrian AE, Bateman TM, Belardinelli L, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14(5):645-658. doi:10.1016/j.nuclcard.2007.06.114
7. Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18(3):109-114. doi:10.4103/HEARTVIEWS.HEARTVIEWS_106_17
8. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med. 2016;26(6):515-523. doi:10.1016/j.tcm.2016.03.001
9. Czernin J, Sun K, Brunken R, Böttcher M, Phelps M, Schelbert H. Effect of acute and long-term smoking on myocardial blood flow and flow reserve. Circulation. 1995;91:2891-2897. doi:10.1161/01.CIR.91.12.2891
10. Winniford MD, Wheelan KR, Kremers MS, et al. Smoking-induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: evidence for adrenergically mediated alterations in coronary artery tone. Circulation. 1986;73(4):662-667. doi:10.1161/01.cir.73.4.662
11. Klein LW, Ambrose J, Pichard A, Holt J, Gorlin R, Teichholz LE. Acute coronary hemodynamic response to cigarette smoking in patients with coronary artery disease. J Am Coll Cardiol. 1984;3(4):879-886. doi:10.1016/s0735-1097(84)80344-7
12. Quillen JE, Rossen JD, Oskarsson HJ, Minor RL Jr, Lopez AG, Winniford MD. Acute effect of cigarette smoking on the coronary circulation: constriction of epicardial and resistance vessels. J Am Coll Cardiol. 1993;22(3):642-647. doi:10.1016/0735-1097(93)90170-6
13. Dacosta A, Guy JM, Tardy B, et al. Myocardial infarction and nicotine patch: a contributing or causative factor?. Eur Heart J. 1993;14(12):1709-1711. doi:10.1093/eurheartj/14.12.1709
14. Ottervanger JP, Festen JM, de Vries AG, Stricker BH. Acute myocardial infarction while using the nicotine patch. Chest. 1995;107(6):1765-1766. doi:10.1378/chest.107.6.1765
15. Dollerup J, Vestbo J, Murray-Thomas T, et al. Cardiovascular risks in smokers treated with nicotine replacement therapy: a historical cohort study. Clin Epidemiol. 2017;9:231-243. Published 2017 Apr 26. doi:10.2147/CLEP.S127775
16. Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals. Tob Induc Dis. 2010;8(1):8. Published 2010 Jul 13. doi:10.1186/1617-9625-8-8
17. Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129(1):28-41. doi:10.1161/CIRCULATIONAHA.113.003961
18. Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc Drugs Ther. 1998;12(3):239-244. doi:10.1023/a:1007757530765
19. Lucini D, Bertocchi F, Malliani A, Pagani M. Autonomic effects of nicotine patch administration in habitual cigarette smokers: a double-blind, placebo-controlled study using spectral analysis of RR interval and systolic arterial pressure variabilities. J Cardiovasc Pharmacol. 1998;31(5):714-720. doi:10.1097/00005344-199805000-00010
20. Kaijser L, Berglund B. Effect of nicotine on coronary blood-flow in man. Clin Physiol. 1985;5(6):541-552. doi:10.1111/j.1475-097x.1985.tb00767.x
21. Nitenberg A, Antony I. Effects of nicotine gum on coronary vasomotor responses during sympathetic stimulation in patients with coronary artery stenosis. J Cardiovasc Pharmacol. 1999;34(5):694-699. doi:10.1097/00005344-199911000-00011
22. Keeley EC, Pirwitz MJ, Landau C, et al. Intranasal nicotine spray does not augment the adverse effects of cigarette smoking on myocardial oxygen demand or coronary arterial dimensions. Am J Med. 1996;101(4):357-363. doi:10.1016/s0002-9343(96)00237-9
23. Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29(7):1422-1431. doi:10.1016/s0735-1097(97)00079-x
24. Flowers L. Nicotine replacement therapy. Amer J Psych. 2017;11(6):4-7.
25. Adenosine; Package insert. Astellas Pharma US Inc; 1989.
26. Dipyridamole; Package insert. Boehringer Ingelheim Pharmaceuticals Inc; 2019.
27. Dobutamine; Package insert. Baxter Healthcare Corporation; 2012.
Chest pain is one of the most common concerns in patients presenting to the emergency department in the United States, accounting for approximately 7.6 million visits annually.1 Given the high mortality rate associated with acute coronary syndromes, prompt evaluation of chest pain is essential.2 Even in mild cases, recognition of newly onset or worsening coronary artery disease (CAD) is crucial to ensure that patients receive optimal medication therapy.
In symptomatic patients with risk factors for CAD, such as advanced age, hypertension, hyperlipidemia, obesity, and diabetes mellitus, myocardial perfusion imaging (MPI) is frequently used as a modality to assess the presence, location, and severity of ischemic or infarcted myocardium.2 MPI requires administration of a radiopharmaceutical before and after the patient undergoes a form of stress.2 This radiopharmaceutical is then detected in the myocardium with a nuclear camera, and images are obtained of the heart to assess myocardial blood flow.2
MPI can be performed using exercise-induced stress via a treadmill, or medication-induced stress (Table 1). In both strategies, healthy coronary arteries dilate to provide the myocardium with more blood flow to meet the increasing myocardial oxygen demand during this period of stress. While healthy vessels are able to dilate appropriately, coronary arteries with flow-limiting stenoses are unable to dilate to the same extent in response to stress.2 Because radioactive isotope uptake by the myocardium is directly related to arterial blood flow, MPI is able to demonstrate a mismatch in coronary blood flow between healthy and diseased coronary arteries indicated by differences in radioisotope uptake.2 The presence of such a mismatch, in conjunction with clinical history, potentially suggests the presence of CAD.
Prior to conducting MPI with a medication, certain substances should be avoided. For instance, methylxanthines, such as caffeine, aminophylline, and theophylline, antagonize adenosine receptors and can have major drug interactions with regadenoson, adenosine, and dipyridamole. Therefore, it is advised that these substances be stopped for at least 12 hours before testing.3 In some cases, other medications that can affect coronary blood flow, such as long-acting nitrates, β-blockers, and calcium channel blockers, are recommended to be avoided for 12 to 48 hours in order to obtain the most accurate depiction of underlying coronary disease.4
Because nicotine and nicotine replacement therapy (NRT) may have substantial effects on coronary circulation, a current area of controversy is whether these should be stopped prior to the use of a stress-inducing medication during MPI. To date, no formal drug interaction studies have been conducted between nicotine and regadenoson.5 Similarly, the ADVANCE MPI 2 Trial, which led to the US Food and Drug Administration approval of regadenoson, did not specify restrictions on the use of nicotine prior to stress testing in the protocol.6 However, as this trial was multicenter, investigators admit that individual study sites could have had their own restrictions on the use of nicotine prior to stress testing with regadenoson, but this information was not collected.6 The current review focuses on how the simultaneous use of nicotine or NRT during MPI with pharmacologic agents, such as regadenoson, may affect the accuracy of imaging results and the clinical impact of this interaction.
Nicotine Coronary Artery Effect
It is well documented that long-term cigarette smoking is a major risk factor for CAD.7 Compared with nonsmokers, cigarette smokers experience 2 times greater risk of morbidity and mortality from ischemic heart disease.7 There are several mechanisms by which nicotine induces damage to the myocardium (Figure). Nicotine has direct effects on both the sympathetic nervous system (SNS) and myocardial endothelium.8 Together, these factors result in reduced coronary blood flow, leading to less oxygen supply to meet an increased oxygen demand, resulting in myocardial ischemia.
Nicotine’s effect on coronary vasomotor tone occurs primarily through noradrenergic stimulation of α and β receptors associated with coronary vasoconstriction or vasodilation, respectively.9,10 These competing influences on coronary blood flow appear to manifest differently based on whether patients are at rest or in a stressed state. A study by Czerin and colleagues demonstrated that in healthy patients with relatively short smoking histories and in a healthy nonsmoker control group, coronary blood flow increased by 25% and 40%, respectively, with nicotine use at rest.9 However, when these patients were stressed with dipyramidole and while smoking during the examination, myocardial blood flow was reduced by 11% in the study group and 14% in the control group.9 This is likely because the patients studied had relatively healthy coronary arteries that were able to maximally dilate when stressed. In this scenario, nicotine’s dilatory effects are offset by nicotine’s α-receptor–mediated vasoconstriction effects.9 Of note, patients in the study group experienced a somewhat diminished increase in coronary blood flow at rest with nicotine use, suggesting that even a short smoking history may damage the myocardial endothelium, rendering it less responsive to nicotine’s vasodilatory effects.9
These principles similarly apply to patients with underlying moderate-to-severe cardiovascular disease (CVD). With nicotine use at rest, patients with significant CAD do not experience as dramatic of an increase in coronary blood flow, which typically decreases or remains the same despite increased myocardial work.10 This may be because patients with moderate-to-severe CAD often have flow-limiting stenoses and damaged endothelium that do not allow vessels to respond as efficiently to increased myocardial demand or to nicotine’s β-receptor–mediated vasodilatory effects.10,11 Moreover, when stressed, diseased coronary arteries are not able to further dilate and nicotine’s α-receptor–mediated vasoconstriction effects dominate.10,11
In a study by Quillen and colleagues of patients with moderate-to-severe CAD, the mean diameter of proximal coronary artery segments decreased by 5%, the distal coronary diameter decreased 8%, and the coronary vascular resistance increased by 21% while smoking at rest.12 The investigators did not analyze how parameters changed when these diseased coronary arteries were stressed using a medication during MPI. However, it can be predicted that coronary arteries would have constricted to a similar or greater degree than observed in Czerin and colleagues’ study, given that the underlying myocardium was diseased and more susceptible to nicotine’s vasoconstriction effects.9 Importantly, these studies have several limitations, most notably that they are older and have small sample sizes. Additionally, while statistically significant differences were found in the degree of changes in coronary circulation with nicotine use at rest and during stress, it is unclear whether this translates to a clinically significant and impactful finding.9-12
Nicotine Replacement Therapy and Stress Testing
Given the association between cigarette smoking and CAD, medical practitioners strongly encourage patients to quit smoking to reduce their risk of adverse cardiovascular outcomes. Various smoking cessation treatments are available for patients. Common, readily accessible forms of therapy include nicotine replacement products (Table 2).
Early studies of NRT in patients with underlying CVD found an increased risk of cardiovascular events, such as myocardial infarction, presumably due to the nicotine content of these products.13,14 However, the concentration of nicotine in NRT is substantially lower than that found in cigarettes and in some formulations, such as transdermal patches, nicotine is delivered over a prolonged period of time.15 For this reason, NRT is thought to be safe in patients with underlying CVD and stable ischemic heart disease. A recent systematic review and meta-analysis found that while NRT may be associated with tachycardia, it did not increase the risk of more serious cardiovascular adverse effects (AEs).16,17
Given the lower nicotine concentration in NRT products, the associated hemodynamic effect of nicotine also is thought to be less pronounced. In a study conducted by Tzivoni and colleagues in patients with CAD using transdermal nicotine patches, no differences in blood pressure, heart rate, ischemia, or arrhythmias were found from baseline to 2 weeks.18 These findings were further confirmed in a small study by Lucini and colleagues, which found that nicotine patches produced slight hemodynamic effects, but to a lesser extent than cigarette smoking.19 For the NRT gum formulation, while a small study found that 4 mg produced coronary vasoconstriction in patients with underlying CAD, a study by Nitenberg and Antony demonstrated that healthy and diseased coronary arteries did not significantly constrict while patients were using nicotine gum both before and after a cold pressor test, suggesting a lesser degree of coronary vasoconstriction than nicotine from cigarette smoking.20,21 Similar findings have been described with the nicotine intranasal spray in a study by Keeley and colleagues, which showed no additional AEs on myocardial demand or vasoconstriction when an intranasal nicotine spray was added to cigarette smoking.22 Importantly, a review of the transdermal and gum formulations found that these less pronounced hemodynamic effects were observed across different doses of NRT; however, further studies are needed to clarify the relationship between NRT dose and cardiovascular effects.23
Overall, NRT does not seem to activate the SNS to the same degree as nicotine obtained via cigarette smoking and likely does not increase the myocardial oxygen demand as much. Additionally, by containing a lower concentration of nicotine, NRT may not impair the myocardium’s ability to supply oxygen to coronary arteries to the same extent as nicotine from cigarette smoking. Therefore, the effects of NRT on MPI using a stress-inducing medication may not be as pronounced. However, due to study limitations, results should be interpreted cautiously.18-23
Conclusions
Because of the close relationship between cigarette smoking and CAD, many patients with underlying CVD are either current smokers or may be using NRT for smoking cessation. Therefore, the question of whether to refrain from nicotine use prior to MPI is clinically relevant. Currently, there is a lack of high-quality studies demonstrating the effects of nicotine and NRT on coronary perfusion. Because of this, the impact of nicotine and NRT use on the accuracy of MPI using stress-inducing medications remains uncertain. Nevertheless, given that nicotine and NRT may largely affect the accuracy of imaging results, several institutions have adopted protocols that prohibit patients from using these drugs on the day of nuclear stress testing.
There are currently no data specifying the number of hours to hold nicotine products prior to cardiac stress testing. It is generally recommended that other medications that affect coronary blood flow be held for 5 half-lives before conducting MPI.4 Following the same guidance for nicotine and NRT may present a reasonable approach to ensure accurate imaging results. Based on the discussed literature, patients should be instructed to refrain from cigarette smoking for at least 5 to 10 hours prior to MPI, given nicotine’s half-life of about 1 to 2 hours.24
The data for NRT are less clear. While use of NRT may not be an absolute contraindication to conducting MPI, it is important to consider that this may affect the accuracy of results. Given this uncertainty, it is likely ideal to hold NRT prior to MPI, based on the specific formulation of NRT and that product's half-life. Further robust studies are needed to analyze the impact of nicotine and NRT on the accuracy of nuclear stress testing using a medication.
Chest pain is one of the most common concerns in patients presenting to the emergency department in the United States, accounting for approximately 7.6 million visits annually.1 Given the high mortality rate associated with acute coronary syndromes, prompt evaluation of chest pain is essential.2 Even in mild cases, recognition of newly onset or worsening coronary artery disease (CAD) is crucial to ensure that patients receive optimal medication therapy.
In symptomatic patients with risk factors for CAD, such as advanced age, hypertension, hyperlipidemia, obesity, and diabetes mellitus, myocardial perfusion imaging (MPI) is frequently used as a modality to assess the presence, location, and severity of ischemic or infarcted myocardium.2 MPI requires administration of a radiopharmaceutical before and after the patient undergoes a form of stress.2 This radiopharmaceutical is then detected in the myocardium with a nuclear camera, and images are obtained of the heart to assess myocardial blood flow.2
MPI can be performed using exercise-induced stress via a treadmill, or medication-induced stress (Table 1). In both strategies, healthy coronary arteries dilate to provide the myocardium with more blood flow to meet the increasing myocardial oxygen demand during this period of stress. While healthy vessels are able to dilate appropriately, coronary arteries with flow-limiting stenoses are unable to dilate to the same extent in response to stress.2 Because radioactive isotope uptake by the myocardium is directly related to arterial blood flow, MPI is able to demonstrate a mismatch in coronary blood flow between healthy and diseased coronary arteries indicated by differences in radioisotope uptake.2 The presence of such a mismatch, in conjunction with clinical history, potentially suggests the presence of CAD.
Prior to conducting MPI with a medication, certain substances should be avoided. For instance, methylxanthines, such as caffeine, aminophylline, and theophylline, antagonize adenosine receptors and can have major drug interactions with regadenoson, adenosine, and dipyridamole. Therefore, it is advised that these substances be stopped for at least 12 hours before testing.3 In some cases, other medications that can affect coronary blood flow, such as long-acting nitrates, β-blockers, and calcium channel blockers, are recommended to be avoided for 12 to 48 hours in order to obtain the most accurate depiction of underlying coronary disease.4
Because nicotine and nicotine replacement therapy (NRT) may have substantial effects on coronary circulation, a current area of controversy is whether these should be stopped prior to the use of a stress-inducing medication during MPI. To date, no formal drug interaction studies have been conducted between nicotine and regadenoson.5 Similarly, the ADVANCE MPI 2 Trial, which led to the US Food and Drug Administration approval of regadenoson, did not specify restrictions on the use of nicotine prior to stress testing in the protocol.6 However, as this trial was multicenter, investigators admit that individual study sites could have had their own restrictions on the use of nicotine prior to stress testing with regadenoson, but this information was not collected.6 The current review focuses on how the simultaneous use of nicotine or NRT during MPI with pharmacologic agents, such as regadenoson, may affect the accuracy of imaging results and the clinical impact of this interaction.
Nicotine Coronary Artery Effect
It is well documented that long-term cigarette smoking is a major risk factor for CAD.7 Compared with nonsmokers, cigarette smokers experience 2 times greater risk of morbidity and mortality from ischemic heart disease.7 There are several mechanisms by which nicotine induces damage to the myocardium (Figure). Nicotine has direct effects on both the sympathetic nervous system (SNS) and myocardial endothelium.8 Together, these factors result in reduced coronary blood flow, leading to less oxygen supply to meet an increased oxygen demand, resulting in myocardial ischemia.
Nicotine’s effect on coronary vasomotor tone occurs primarily through noradrenergic stimulation of α and β receptors associated with coronary vasoconstriction or vasodilation, respectively.9,10 These competing influences on coronary blood flow appear to manifest differently based on whether patients are at rest or in a stressed state. A study by Czerin and colleagues demonstrated that in healthy patients with relatively short smoking histories and in a healthy nonsmoker control group, coronary blood flow increased by 25% and 40%, respectively, with nicotine use at rest.9 However, when these patients were stressed with dipyramidole and while smoking during the examination, myocardial blood flow was reduced by 11% in the study group and 14% in the control group.9 This is likely because the patients studied had relatively healthy coronary arteries that were able to maximally dilate when stressed. In this scenario, nicotine’s dilatory effects are offset by nicotine’s α-receptor–mediated vasoconstriction effects.9 Of note, patients in the study group experienced a somewhat diminished increase in coronary blood flow at rest with nicotine use, suggesting that even a short smoking history may damage the myocardial endothelium, rendering it less responsive to nicotine’s vasodilatory effects.9
These principles similarly apply to patients with underlying moderate-to-severe cardiovascular disease (CVD). With nicotine use at rest, patients with significant CAD do not experience as dramatic of an increase in coronary blood flow, which typically decreases or remains the same despite increased myocardial work.10 This may be because patients with moderate-to-severe CAD often have flow-limiting stenoses and damaged endothelium that do not allow vessels to respond as efficiently to increased myocardial demand or to nicotine’s β-receptor–mediated vasodilatory effects.10,11 Moreover, when stressed, diseased coronary arteries are not able to further dilate and nicotine’s α-receptor–mediated vasoconstriction effects dominate.10,11
In a study by Quillen and colleagues of patients with moderate-to-severe CAD, the mean diameter of proximal coronary artery segments decreased by 5%, the distal coronary diameter decreased 8%, and the coronary vascular resistance increased by 21% while smoking at rest.12 The investigators did not analyze how parameters changed when these diseased coronary arteries were stressed using a medication during MPI. However, it can be predicted that coronary arteries would have constricted to a similar or greater degree than observed in Czerin and colleagues’ study, given that the underlying myocardium was diseased and more susceptible to nicotine’s vasoconstriction effects.9 Importantly, these studies have several limitations, most notably that they are older and have small sample sizes. Additionally, while statistically significant differences were found in the degree of changes in coronary circulation with nicotine use at rest and during stress, it is unclear whether this translates to a clinically significant and impactful finding.9-12
Nicotine Replacement Therapy and Stress Testing
Given the association between cigarette smoking and CAD, medical practitioners strongly encourage patients to quit smoking to reduce their risk of adverse cardiovascular outcomes. Various smoking cessation treatments are available for patients. Common, readily accessible forms of therapy include nicotine replacement products (Table 2).
Early studies of NRT in patients with underlying CVD found an increased risk of cardiovascular events, such as myocardial infarction, presumably due to the nicotine content of these products.13,14 However, the concentration of nicotine in NRT is substantially lower than that found in cigarettes and in some formulations, such as transdermal patches, nicotine is delivered over a prolonged period of time.15 For this reason, NRT is thought to be safe in patients with underlying CVD and stable ischemic heart disease. A recent systematic review and meta-analysis found that while NRT may be associated with tachycardia, it did not increase the risk of more serious cardiovascular adverse effects (AEs).16,17
Given the lower nicotine concentration in NRT products, the associated hemodynamic effect of nicotine also is thought to be less pronounced. In a study conducted by Tzivoni and colleagues in patients with CAD using transdermal nicotine patches, no differences in blood pressure, heart rate, ischemia, or arrhythmias were found from baseline to 2 weeks.18 These findings were further confirmed in a small study by Lucini and colleagues, which found that nicotine patches produced slight hemodynamic effects, but to a lesser extent than cigarette smoking.19 For the NRT gum formulation, while a small study found that 4 mg produced coronary vasoconstriction in patients with underlying CAD, a study by Nitenberg and Antony demonstrated that healthy and diseased coronary arteries did not significantly constrict while patients were using nicotine gum both before and after a cold pressor test, suggesting a lesser degree of coronary vasoconstriction than nicotine from cigarette smoking.20,21 Similar findings have been described with the nicotine intranasal spray in a study by Keeley and colleagues, which showed no additional AEs on myocardial demand or vasoconstriction when an intranasal nicotine spray was added to cigarette smoking.22 Importantly, a review of the transdermal and gum formulations found that these less pronounced hemodynamic effects were observed across different doses of NRT; however, further studies are needed to clarify the relationship between NRT dose and cardiovascular effects.23
Overall, NRT does not seem to activate the SNS to the same degree as nicotine obtained via cigarette smoking and likely does not increase the myocardial oxygen demand as much. Additionally, by containing a lower concentration of nicotine, NRT may not impair the myocardium’s ability to supply oxygen to coronary arteries to the same extent as nicotine from cigarette smoking. Therefore, the effects of NRT on MPI using a stress-inducing medication may not be as pronounced. However, due to study limitations, results should be interpreted cautiously.18-23
Conclusions
Because of the close relationship between cigarette smoking and CAD, many patients with underlying CVD are either current smokers or may be using NRT for smoking cessation. Therefore, the question of whether to refrain from nicotine use prior to MPI is clinically relevant. Currently, there is a lack of high-quality studies demonstrating the effects of nicotine and NRT on coronary perfusion. Because of this, the impact of nicotine and NRT use on the accuracy of MPI using stress-inducing medications remains uncertain. Nevertheless, given that nicotine and NRT may largely affect the accuracy of imaging results, several institutions have adopted protocols that prohibit patients from using these drugs on the day of nuclear stress testing.
There are currently no data specifying the number of hours to hold nicotine products prior to cardiac stress testing. It is generally recommended that other medications that affect coronary blood flow be held for 5 half-lives before conducting MPI.4 Following the same guidance for nicotine and NRT may present a reasonable approach to ensure accurate imaging results. Based on the discussed literature, patients should be instructed to refrain from cigarette smoking for at least 5 to 10 hours prior to MPI, given nicotine’s half-life of about 1 to 2 hours.24
The data for NRT are less clear. While use of NRT may not be an absolute contraindication to conducting MPI, it is important to consider that this may affect the accuracy of results. Given this uncertainty, it is likely ideal to hold NRT prior to MPI, based on the specific formulation of NRT and that product's half-life. Further robust studies are needed to analyze the impact of nicotine and NRT on the accuracy of nuclear stress testing using a medication.
1. Rui P, Kang K, Ashman JJ. National Hospital Ambulatory Medical Care Survey: 2016 emergency department summary tables. Published 2016. Accessed March 30, 2020. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2016_ed_web_tables.pdf
2. Lange RA. Cardiovascular testing. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 10th ed. McGraw Hill; 2017.
3. Mace S. Observation Medicine: Principles and Protocols. Cambridge University Press; 2017.
4. Currie GM. Pharmacology, part 4: nuclear cardiology. J Nucl Med Technol. 2019;47(2):97-110. doi:10.2967/jnmt.118.219675
5. Regadenoson; Package insert. Astellas Pharma US Inc; 2008.
6. Iskandrian AE, Bateman TM, Belardinelli L, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14(5):645-658. doi:10.1016/j.nuclcard.2007.06.114
7. Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18(3):109-114. doi:10.4103/HEARTVIEWS.HEARTVIEWS_106_17
8. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med. 2016;26(6):515-523. doi:10.1016/j.tcm.2016.03.001
9. Czernin J, Sun K, Brunken R, Böttcher M, Phelps M, Schelbert H. Effect of acute and long-term smoking on myocardial blood flow and flow reserve. Circulation. 1995;91:2891-2897. doi:10.1161/01.CIR.91.12.2891
10. Winniford MD, Wheelan KR, Kremers MS, et al. Smoking-induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: evidence for adrenergically mediated alterations in coronary artery tone. Circulation. 1986;73(4):662-667. doi:10.1161/01.cir.73.4.662
11. Klein LW, Ambrose J, Pichard A, Holt J, Gorlin R, Teichholz LE. Acute coronary hemodynamic response to cigarette smoking in patients with coronary artery disease. J Am Coll Cardiol. 1984;3(4):879-886. doi:10.1016/s0735-1097(84)80344-7
12. Quillen JE, Rossen JD, Oskarsson HJ, Minor RL Jr, Lopez AG, Winniford MD. Acute effect of cigarette smoking on the coronary circulation: constriction of epicardial and resistance vessels. J Am Coll Cardiol. 1993;22(3):642-647. doi:10.1016/0735-1097(93)90170-6
13. Dacosta A, Guy JM, Tardy B, et al. Myocardial infarction and nicotine patch: a contributing or causative factor?. Eur Heart J. 1993;14(12):1709-1711. doi:10.1093/eurheartj/14.12.1709
14. Ottervanger JP, Festen JM, de Vries AG, Stricker BH. Acute myocardial infarction while using the nicotine patch. Chest. 1995;107(6):1765-1766. doi:10.1378/chest.107.6.1765
15. Dollerup J, Vestbo J, Murray-Thomas T, et al. Cardiovascular risks in smokers treated with nicotine replacement therapy: a historical cohort study. Clin Epidemiol. 2017;9:231-243. Published 2017 Apr 26. doi:10.2147/CLEP.S127775
16. Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals. Tob Induc Dis. 2010;8(1):8. Published 2010 Jul 13. doi:10.1186/1617-9625-8-8
17. Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129(1):28-41. doi:10.1161/CIRCULATIONAHA.113.003961
18. Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc Drugs Ther. 1998;12(3):239-244. doi:10.1023/a:1007757530765
19. Lucini D, Bertocchi F, Malliani A, Pagani M. Autonomic effects of nicotine patch administration in habitual cigarette smokers: a double-blind, placebo-controlled study using spectral analysis of RR interval and systolic arterial pressure variabilities. J Cardiovasc Pharmacol. 1998;31(5):714-720. doi:10.1097/00005344-199805000-00010
20. Kaijser L, Berglund B. Effect of nicotine on coronary blood-flow in man. Clin Physiol. 1985;5(6):541-552. doi:10.1111/j.1475-097x.1985.tb00767.x
21. Nitenberg A, Antony I. Effects of nicotine gum on coronary vasomotor responses during sympathetic stimulation in patients with coronary artery stenosis. J Cardiovasc Pharmacol. 1999;34(5):694-699. doi:10.1097/00005344-199911000-00011
22. Keeley EC, Pirwitz MJ, Landau C, et al. Intranasal nicotine spray does not augment the adverse effects of cigarette smoking on myocardial oxygen demand or coronary arterial dimensions. Am J Med. 1996;101(4):357-363. doi:10.1016/s0002-9343(96)00237-9
23. Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29(7):1422-1431. doi:10.1016/s0735-1097(97)00079-x
24. Flowers L. Nicotine replacement therapy. Amer J Psych. 2017;11(6):4-7.
25. Adenosine; Package insert. Astellas Pharma US Inc; 1989.
26. Dipyridamole; Package insert. Boehringer Ingelheim Pharmaceuticals Inc; 2019.
27. Dobutamine; Package insert. Baxter Healthcare Corporation; 2012.
1. Rui P, Kang K, Ashman JJ. National Hospital Ambulatory Medical Care Survey: 2016 emergency department summary tables. Published 2016. Accessed March 30, 2020. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2016_ed_web_tables.pdf
2. Lange RA. Cardiovascular testing. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 10th ed. McGraw Hill; 2017.
3. Mace S. Observation Medicine: Principles and Protocols. Cambridge University Press; 2017.
4. Currie GM. Pharmacology, part 4: nuclear cardiology. J Nucl Med Technol. 2019;47(2):97-110. doi:10.2967/jnmt.118.219675
5. Regadenoson; Package insert. Astellas Pharma US Inc; 2008.
6. Iskandrian AE, Bateman TM, Belardinelli L, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14(5):645-658. doi:10.1016/j.nuclcard.2007.06.114
7. Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18(3):109-114. doi:10.4103/HEARTVIEWS.HEARTVIEWS_106_17
8. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med. 2016;26(6):515-523. doi:10.1016/j.tcm.2016.03.001
9. Czernin J, Sun K, Brunken R, Böttcher M, Phelps M, Schelbert H. Effect of acute and long-term smoking on myocardial blood flow and flow reserve. Circulation. 1995;91:2891-2897. doi:10.1161/01.CIR.91.12.2891
10. Winniford MD, Wheelan KR, Kremers MS, et al. Smoking-induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: evidence for adrenergically mediated alterations in coronary artery tone. Circulation. 1986;73(4):662-667. doi:10.1161/01.cir.73.4.662
11. Klein LW, Ambrose J, Pichard A, Holt J, Gorlin R, Teichholz LE. Acute coronary hemodynamic response to cigarette smoking in patients with coronary artery disease. J Am Coll Cardiol. 1984;3(4):879-886. doi:10.1016/s0735-1097(84)80344-7
12. Quillen JE, Rossen JD, Oskarsson HJ, Minor RL Jr, Lopez AG, Winniford MD. Acute effect of cigarette smoking on the coronary circulation: constriction of epicardial and resistance vessels. J Am Coll Cardiol. 1993;22(3):642-647. doi:10.1016/0735-1097(93)90170-6
13. Dacosta A, Guy JM, Tardy B, et al. Myocardial infarction and nicotine patch: a contributing or causative factor?. Eur Heart J. 1993;14(12):1709-1711. doi:10.1093/eurheartj/14.12.1709
14. Ottervanger JP, Festen JM, de Vries AG, Stricker BH. Acute myocardial infarction while using the nicotine patch. Chest. 1995;107(6):1765-1766. doi:10.1378/chest.107.6.1765
15. Dollerup J, Vestbo J, Murray-Thomas T, et al. Cardiovascular risks in smokers treated with nicotine replacement therapy: a historical cohort study. Clin Epidemiol. 2017;9:231-243. Published 2017 Apr 26. doi:10.2147/CLEP.S127775
16. Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals. Tob Induc Dis. 2010;8(1):8. Published 2010 Jul 13. doi:10.1186/1617-9625-8-8
17. Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129(1):28-41. doi:10.1161/CIRCULATIONAHA.113.003961
18. Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc Drugs Ther. 1998;12(3):239-244. doi:10.1023/a:1007757530765
19. Lucini D, Bertocchi F, Malliani A, Pagani M. Autonomic effects of nicotine patch administration in habitual cigarette smokers: a double-blind, placebo-controlled study using spectral analysis of RR interval and systolic arterial pressure variabilities. J Cardiovasc Pharmacol. 1998;31(5):714-720. doi:10.1097/00005344-199805000-00010
20. Kaijser L, Berglund B. Effect of nicotine on coronary blood-flow in man. Clin Physiol. 1985;5(6):541-552. doi:10.1111/j.1475-097x.1985.tb00767.x
21. Nitenberg A, Antony I. Effects of nicotine gum on coronary vasomotor responses during sympathetic stimulation in patients with coronary artery stenosis. J Cardiovasc Pharmacol. 1999;34(5):694-699. doi:10.1097/00005344-199911000-00011
22. Keeley EC, Pirwitz MJ, Landau C, et al. Intranasal nicotine spray does not augment the adverse effects of cigarette smoking on myocardial oxygen demand or coronary arterial dimensions. Am J Med. 1996;101(4):357-363. doi:10.1016/s0002-9343(96)00237-9
23. Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29(7):1422-1431. doi:10.1016/s0735-1097(97)00079-x
24. Flowers L. Nicotine replacement therapy. Amer J Psych. 2017;11(6):4-7.
25. Adenosine; Package insert. Astellas Pharma US Inc; 1989.
26. Dipyridamole; Package insert. Boehringer Ingelheim Pharmaceuticals Inc; 2019.
27. Dobutamine; Package insert. Baxter Healthcare Corporation; 2012.
A Practical Approach for Primary Care Practitioners to Evaluate and Manage Lower Urinary Tract Symptoms and Benign Prostatic Hyperplasia
Lower urinary tract symptoms (LUTS)are common and tend to increase in frequency with age. Managing LUTS can be complicated, requires an informed discussion between the primary care practitioner (PCP) and patient, and is best achieved by a thorough understanding of the many medical and surgical options available. Over the past 3 decades, medications have become the most common therapy; but recently, newer minimally invasive surgeries have challenged this paradigm. This article provides a comprehensive review for PCPs regarding the evaluation and management of LUTS in men and when to consider a urology referral.
Benign prostatic hyperplasia (BPH) and LUTS are common clinical encounters for most PCPs. About 50% of men will develop LUTS associated with BPH, and symptoms associated with these conditions increase as men age.1,2 Studies have estimated that 90% of men aged 45 to 80 years demonstrate some symptoms of LUTS.3 Strong genetic influence seems to suggest heritability, but BPH also occurs in sporadic forms and is heavily influenced by androgens.4
BPH is a histologic diagnosis, whereas LUTS consists of complex symptomatology related to both static or dynamic components.1 The enlarged prostate gland obstructs the urethra, simultaneously causing an increase in muscle tone and resistance at the bladder neck and prostatic urethra, leading to increased resistance to urine flow. As a result, there is a thickening of the detrusor muscles in the bladder wall and an overall decreased compliance. Urine becomes stored under increased pressure. These changes result in a weak or intermittent urine stream, incomplete emptying of the bladder, postvoid dribble, hesitancy, and irritative symptoms, such as urgency, frequency, and nocturia.
For many patients, BPH associated with LUTS is a quality of life (QOL) issue. The stigma associated with these symptoms often leads to delays in patients seeking care. Many patients do not seek treatment until symptoms have become so severe that changes in bladder health are often irreversible. Early intervention can dramatically improve a patient’s QOL. Also, early intervention has the potential to reduce overall health care expenditures. BPH-related spending exceeds $1 billion each year in the Medicare program alone.5
PCPs are in a unique position to help many patients who present with early-stage LUTS. Given the substantial impact this disease has on QOL, early recognition of symptoms and prompt treatment play a major role. Paramount to this effort is awareness and understanding of various treatments, their advantages, and adverse effects (AEs). This article highlights evidence-based evaluation and treatment of BPH/LUTS for PCPs who treat veterans and recommendations as to when to refer a patient to a urologist.
Evaluation of LUTS and BPH
Evaluation begins with a thorough medical history and physical examination. Particular attention should focus on ruling out other causes of LUTS, such as a urinary tract infection (UTI), acute prostatitis, malignancy, bladder dysfunction, neurogenic bladder, and other obstructive pathology, such as urethral stricture disease. The differential diagnosis of LUTS includes BPH, UTI, bladder neck obstruction, urethral stricture, bladder stones, polydipsia, overactive bladder (OAB), nocturnal polyuria, neurologic disease, genitourinary malignancy, renal failure, and acute/chronic urinary retention.6
Relevant medical history influencing urinary symptoms includes diabetes mellitus, underlying neurologic diseases, previous trauma, sexually transmitted infections, and certain medications. Symptom severity may be obtained using a validated questionnaire, such as the International Prostate Symptom Score (IPSS), which also aids clinicians in assessing the impact of LUTS on QOL. Additionally, urinary frequency or volume records (voiding diary) may help establish the severity of the patient’s symptoms and provide insight into other potential causes for LUTS. Patients with BPH often have concurrent erectile dysfunction (ED) or other sexual dysfunction symptoms. Patients should be evaluated for baseline sexual dysfunction before the initiation of treatment as many therapies worsen symptoms of ED or ejaculatory dysfunction.
A comprehensive physical examination with a focus on the genitourinary system should, at minimum, assess for abnormalities of the urethral meatus, prepuce, penis, groin nodes, and prior surgical scars. A digital rectal examination also should be performed. Although controversial, a digital rectal examination for prostate cancer screening may provide a rough estimate of prostate size, help rule out prostatitis, and detect incident prostate nodules. Prostate size does not necessarily correlate well with the degree of urinary obstruction or LUTS but is an important consideration when deciding among different therapies.1
Laboratory and Adjunctive Tests
A urinalysis with microscopy helps identify other potential causes for urinary symptoms, including infection, proteinuria, or glucosuria. In patients who present with gross or microscopic hematuria, additional consideration should be given to bladder calculi and genitourinary cancer.2 When a reversible source for the hematuria is not identified, these patients require referral to a urologist for a hematuria evaluation.
There is some controversy regarding prostate specific antigen (PSA) testing. Most professional organizations advocate for a shared decision-making approach before testing. The American Cancer Society recommends this informed discussion occur between the patient and the PCP for men aged > 50 years at average risk, men aged > 45 years at high risk of developing prostate cancer (African Americans or first-degree relative with early prostate cancer diagnosis), and aged 40 years for men with more than one first-degree relative with an early prostate cancer diagnosis.7
Adjunctive tests include postvoid residual (PVR), cystoscopy, uroflowmetry, urodynamics, and transrectal ultrasound. However, these are mostly performed by urologists. In some patients with bladder decompensation after prolonged partial bladder outlet obstruction, urodynamics may be used by urologists to determine whether a patient may benefit from an outlet obstruction procedure. Ordering additional imaging or serum studies for the assessment of LUTS is rarely helpful.
Treatment
Treatment includes management with or without lifestyle modification, medication administration, and surgical therapy. New to this paradigm are in-office minimally invasive surgical options. The goal of treatment is not only to reduce patient symptoms and improve QOL, but also to prevent the secondary sequala of urinary retention, bladder failure, and eventual renal impairment.7A basic understanding of these treatments can aid PCPs with appropriate patient counseling and urologic referral.8
Lifestyle and Behavior Modification
Behavior modification is the starting point for all patients with LUTS. Lifestyle modifications for LUTS include avoiding substances that exacerbate symptoms, such as α-agonists (decongestants), caffeine, alcohol, spicy/acidic foods, chocolate, and soda. These substances are known to be bladder irritants. Common medications contributing to LUTS include antidepressants, decongestants, antihistamines, bronchodilators, anticholinergics, and sympathomimetics. To decrease nocturia, behavioral modifications include limiting evening fluid intake, timed diuretic administration for patients already on a diuretic, and elevating legs 1 hour before bedtime. Counseling obese patients to lose weight and increasing physical activity have been linked to reduced LUTS.9 Other behavioral techniques include double voiding: a technique where patients void normally then change positions and return to void to empty the bladder. Another technique is timed voiding: Many patients have impaired sensation when the bladder is full. These patients are encouraged to void at regular intervals.
Complementary and Alternative Medicine
Multiple nutraceutical compounds claim improved urinary health and symptom reduction. These compounds are marketed to patients with little regulation and oversight since supplements are not regulated or held to the same standard as prescription medications. The most popular nutraceutical for prostate health and LUTS is saw palmetto. Despite its common usage for the treatment of LUTS, little data support saw palmetto health claims. In 2012, a systematic review of 32 randomized trials including 5666 patients compared saw palmetto with a placebo. The study found no difference in urinary symptom scores, urinary flow, or prostate size.10,11 Other phytotherapy compounds often considered for urinary symptoms include stinging nettle extract and β-sitosterol compounds. The mechanism of action of these agents is unknown and efficacy data are lacking.
Historically, acupuncture and pelvic floor physical therapy have been used successfully for OAB symptoms. A meta-analysis found positive beneficial effects of acupuncture compared with a sham control for short- and medium-term follow-up in both IPSS and urine flow rates in some studies; however, when combining the studies for more statistical power, the benefits were less clear.12 Physical therapists with specialized training and certification in pelvic health can incorporate certain bladder training techniques. These include voiding positional changes (double voiding and postvoid urethral milking) and timed voiding.13,14 These interventions often address etiologies of LUTS for which medical therapies are not effective as the sole treatment option.
Medication Management
Medical management includes α-blockers, 5-α-reductase inhibitors (5-α-RIs), antimuscarinic or anticholinergic medicines, β-3 agonists, and phosphodiesterase inhibitors (Table). These medications work independently as well as synergistically. The use of medications to improve symptoms must be balanced against potential AEs and the consequences of a lifetime of drug usage, which can be additive.15,16
First-line pharmacological therapy for BPH is α-blockers, which work by blocking α1A receptors in the prostate and bladder neck, leading to smooth muscle relaxation, increased diameter of the channel, and improved urinary flow. α-receptors in the bladder neck and prostate are expressed with increased frequency with age and are a potential cause for worsening symptoms as men age. Studies demonstrate that these medications reduce symptoms by 30 to 40% and increase flow rates by 16 to 25%.17 Commonly prescribed α-blockers include tamsulosin, alfuzosin, silodosin, doxazosin, and terazosin. Doxazosin and terazosin require dose titrations because they may cause significant hypotension. Orthostatic hypotension typically improves with time and is avoided if the patient takes the medication at bedtime. Both doxazosin and terazosin are on the American Geriatric Society’s Beers Criteria list and should be avoided in older patients.18 Tamsulosin, alfuzosin, and silodosin have a standardized dosing regimen and lower rates of hypotension. Significant AEs include ejaculation dysfunction, nasal congestion, and orthostatic hypotension. Duan and colleagues have linked tamsulosin with dementia. However, this association is not causal and further studies are necessary.19,20 Patients who have taken these agents also are at risk for intraoperative floppy iris syndrome (IFIS). Permanent visual problems can arise if the intraoperative management is not managed to account for IFIS. These medications have a rapid onset of action and work immediately. However, to reach maximum benefit, patients must take the medication for several weeks. Unfortunately, up to one-third of patients will have no improvement with α-blocker therapy, and many patients will discontinue these medications because of significant AEs.6,21
5-α-RIs (finasteride and dutasteride) inhibit the conversion of testosterone to more potent dihydrotestosterone. They effectively reduce prostate volume by 25 to 30%.22 The results occur slowly and can take 6 to 12 months to reach the desired outcome. These medications are effective in men with larger prostates and not as effective in men with smaller prostates.23 These medications can improve urinary flow rates by about 10%, reduce IPSS scores by 20 to 30%, reduce the risk of urinary retention by 50%, and reduce the progression of BPH to the point where surgery is required by 50%.24 Furthermore, 5-α-RIs lower PSA by > 50% after 12 months of treatment.25
A baseline PSA should be established before administration and after 6 months of treatment. Any increase in the PSA even if the level is within normal limits should be evaluated for prostate cancer. Sarkar and colleagues recently published a study evaluating prostate cancer diagnosis in patients treated with 5-α-RI and found there was a delay in diagnosing prostate cancer in this population. Controversy also exists as to the potential of these medications increasing the risk for high-grade prostate cancer, which has led to a US Food and Drug Administration (FDA) warning. AEs include decreased libido (1.5%), ejaculatory dysfunction (3.4%), gynecomastia (1.3%), and/or ED (1.6%).26-28 A recent study evaluating 5-α-RIs demonstrated about a 2-fold increased risk of depression.29
There are well-established studies that note increased effectiveness when using combined α-blocker therapy with 5-α-RI medications. The Medical Therapy of Prostate Symptoms (MTOPS) and Combination Avodart and Tamsulosin (CombAT) trials showed that the combination of both medications was more effective in improving voiding symptoms and flow rates than either agent alone.15,16 Combination therapy resulted in a 66% reduction in disease progression, 81% reduction in urinary retention, and a 67% reduction in the need for surgery compared with placebo.
Anticholinergic medication use in BPH with LUTS is well established, and their use is often combined with other therapies. Anticholinergics work by inhibiting muscarinic M3 receptors to reduce detrusor muscle contraction. This effectively decreases bladder contractions and delays the desire to void. Kaplan and colleagues showed that tolterodine significantly improved a patient’s QOL when added to α-blocker therapy.30 Patients reported a positive outcome at 12 weeks, which resulted in a reduction in urgency incontinence, urgency, nocturia, and the overall number of voiding episodes within 24 hours.
β-3 agonists are a class of medications for OAB; mirabegron and vibegron have proven effective in reducing similar symptoms. In phase 3 clinical trials, mirabegron improved urinary incontinence episodes by 50% and reduced the number of voids in 24 hours.31 Mirabegron is well tolerated and avoids many common anticholinergic effects.32 Vibegron is the newest medication in the class and could soon become the preferred agent given it does not have cytochrome P450 interactions and does not cause hypertension like mirabegron.33
Anticholinergics should be used with caution in patients with a history of urinary retention, elevated after-void residual, or other medications with known anticholinergic effects. AEs include sedation, confusion, dry mouth, constipation, and potential falls in older patients.18 Recent studies have noted an association with dementia in the prolonged use of these medications in older patients and should be used cautiously.20
Phosphodiesterase-5 enzyme inhibitors (PDE-5) are adjunctive medications shown to improve LUTS. This class of medication is prescribed mostly for ED. However, tadalafil 5 mg taken daily also is FDA approved for the treatment of LUTS secondary to BPH given its prolonged half-life. The exact mechanism for improved BPH symptoms is unknown. Possibly the effects are due to an increase mediated by PDE-5 in cyclic guanosine monophosphate (cGMP), which increases smooth muscle relaxation and tissue perfusion of the prostate and bladder.34 There have been limited studies on objective improvement in uroflowmetry parameters compared with other treatments. The daily dosing of tadalafil should not be prescribed in men with a creatinine clearance < 30 mL/min.29 Tadalafil is not considered a first-line agent and is usually reserved for patients who experience ED in addition to BPH. When initiating BPH pharmacologic therapy, the PCP should be aware of adherence and high discontinuation rates.35
Surgical Treatments
Surgical treatments are often delayed out of fear of potential AEs or considered a last resort when symptoms are too severe.36 Early intervention is required to prevent irreversible deleterious changes to detrusor muscle structure and function (Figure). Patients fear urinary incontinence, ED or ejaculatory dysfunction, and anesthesia complications associated with surgical interventions.6,37 Multiple studies show that patients fare better with early surgical intervention, experiencing improved IPSS scores, urinary flow, and QOL. The following is an overview of the most popular procedures.
Prostatic urethral lift (PUL) using the UroLift System is an FDA-approved, minimally-invasive treatment of LUTS secondary to BPH. This procedure treats prostates < 80 g with an absent median lobe.6,21,38 Permanent implants are placed per the prostatic urethra to displace obstructing prostate tissue laterally. This opens the urethra directly without cutting, heating, or removing any prostate tissue. This procedure is minimally invasive, often done in the office as an outpatient procedure, and offers better symptom relief than medication with a lower risk profile than transurethral resection of the prostate (TURP).39,40 The LIFT study was a multicenter, randomized, blinded trial; patients were randomized 2:1 to undergo UroLift or a sham operation. At 3 years, average improvements were statistically significant for total IPSS reduction (41%), QOL improvement (49%), and improved maximum flow rates by (51%).41 Risk for urinary incontinence is low, and the procedure has been shown to preserve erectile and ejaculatory function. Furthermore, patients report significant improvement in their QOL without the need for medications. Surgical retreatment rates at 5 years are 13.6%, with an additional 10.7% of subjects back on medication therapy with α-blockers or 5-α-RIs.42
Water vapor thermal therapy or Rez¯um uses steam as thermal energy to destroy obstructing prostate tissue and relieve the obstruction.43 The procedure differs from older conductive heat thermotherapies because the steam penetrates prostate zonal anatomy without affecting areas outside the targeted treatment zone. The procedure is done in the office with local anesthesia and provides long-lasting relief of LUTS with minimal risks. Following the procedure, patients require an indwelling urethral catheter for 3 to 7 days, and most patients begin to experience symptom improvement 2 to 4 weeks following the procedure.44 The procedure received FDA approval in 2015. Four-year data show significant improvement in maximal flow rate (50%), IPSS (47%), and QOL (43%).45 Surgical retreatment rates were 4.4%. Criticisms of this treatment include patient discomfort with the office procedure, the requirement for an indwelling catheter for a short period, and lack of long-term outcomes data. Guidelines support use in prostate volumes > 80 g with or without median lobe anatomy.
TURP is the gold standard to which other treatments are compared.46 The surgery is performed in the operating room where urologists use a rigid cystoscope and resection element to effectively carve out and cauterize obstructing prostate tissue. Patients typically recover for a short period with an indwelling urethral catheter that is often removed 12 to 24 hours after surgery. New research points out that despite increasing mean age (55% of patients are aged > 70 years with associated comorbidities), the morbidity of TURP was < 1% and mortality rate of 0 to 0.3%.47 Postoperative complications include bleeding that requires a transfusion (3%), retrograde ejaculation (65%), and rare urinary incontinence (2%).47 Surgical retreatment rates for patients following a TURP are approximately 13 to 15% at 8 years.34
Laser surgery for BPH includes multiple techniques: photovaporization of the prostate using a Greenlight XPS laser, holmium laser ablation, and holmium laser enucleation (HoLEP). Proponents of these treatments cite lower bleeding risks compared with TURP, but the operation is largely surgeon dependent on the technology chosen. Most studies comparing these technologies with TURP show similar outcomes of IPSS reports, quality of life improvements, and complications.
Patients with extremely large prostates, > 100 g or 4 times the normal size, pose a unique challenge to surgical treatment. Historically, patients were treated with an open simple prostatectomy operation or staged TURP procedures. Today, urologists use newer, safer ways to treat these patients. Both HoLEP and robot-assisted simple prostatectomy work well in relieving urinary symptoms with lower complications compared with older open surgery. Other minimally invasive procedures, such as prostatic artery embolism, have been described for the treatment of BPH specifically in men who may be unfit for surgery.48Future treatments are constantly evolving. Many unanswered questions remain about BPH and the role of inflammation, metabolic dysfunction, obesity, and other genetic factors driving BPH and prostate growth. Pharmaceutical opportunities exist in mechanisms aimed to reduce prostate growth, induce cellular apoptosis, as well as other drugs to reduce bladder symptoms. Newer, minimally invasive therapies also will become more readily available, such as Aquablation, which is the first FDA-granted surgical robot for the autonomous removal of prostatic tissue due to BPH.49 However, the goal of all future therapies should include the balance of alleviating disruptive symptoms while demonstrating a favorable risk profile. Many men discontinue taking medications, yet few present for surgery. Most concerning is the significant population of men who will develop irreversible bladder dysfunction while waiting for the perfect treatment. There are many opportunities for an effective treatment that is less invasive than surgery, provides durable relief, has minimal AEs, and is affordable.
Conclusions
There is no perfect treatment for patients with LUTS. All interventions have potential AEs and associated complications. Medications are often started as first-line therapy but are often discontinued at the onset of significant AEs. This process is often repeated. Many patients will try different medications without any significant improvement in their symptoms or short-term relief, which results in the gradual progression of the disease.
The PCP plays a significant role in the initial evaluation and management of BPH. These frontline clinicians can recognize patients who may already be experiencing sequela of prolonged bladder outlet obstruction and refer these men to urologists promptly. Counseling patients about their treatment options is an important duty for all PCPs.
A clear understanding of the available treatment options will help PCPs counsel patients appropriately about lifestyle modification, medications, and surgical treatment options for their symptoms. The treatment of this disorder is a rapidly evolving topic with the constant introduction of new technologies and medications, which are certain to continue to play an important role for PCPs and urologists.
1. Roehrborn CG. Benign prostatic hyperplasia: an overview. Rev Urol. 2005;7 Suppl 9(Suppl 9):S3-S14
2. McVary KT. Clinical manifestations and diagnostic evaluation of benign prostatic hyperplasia. UpToDate. Updated November 18, 2021. Accessed November 23, 2021. https:// www.uptodate.com/contents/clinical-manifestations-and -diagnostic-evaluation-of-benign-prostatic-hyperplasia
3. McVary KT. BPH: epidemiology and comorbidities. Am J Manag Care. 2006;12(5 Suppl):S122-S128.
4. Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011;8(1):29-41. doi:10.1038/nrurol.2010.207
5. Rensing AJ, Kuxhausen A, Vetter J, Strope SA. Differences in the treatment of benign prostatic hyperplasia: comparing the primary care physician and the urologist. Urol Pract. 2017;4(3):193-199. doi:10.1016/j.urpr.2016.07.002
6. Foster HE, Barry MJ, Dahm P, et al. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline. J Urol. 2018;200(3):612- 619. doi:10.1016/j.juro.2018.05.048
7. Landau A, Welliver C. Analyzing and characterizing why men seek care for lower urinary tract symptoms. Curr Urol Rep. 2020;21(12):58. Published 2020 Oct 30. doi:10.1007/s11934-020-01006-w
8. Das AK, Leong JY, Roehrborn CG. Office-based therapies for benign prostatic hyperplasia: a review and update. Can J Urol. 2019;26(4 Suppl 1):2-7.
9. Parsons JK, Sarma AV, McVary K, Wei JT. Obesity and benign prostatic hyperplasia: clinical connections, emerging etiological paradigms and future directions. J Urol. 2013;189(1 Suppl):S102-S106. doi:10.1016/j.juro.2012.11.029
10. Pattanaik S, Mavuduru RS, Panda A, et al. Phosphodiesterase inhibitors for lower urinary tract symptoms consistent with benign prostatic hyperplasia. Cochrane Database Syst Rev. 2018;11(11):CD010060. Published 2018 Nov 16. doi:10.1002/14651858.CD010060.pub2
11. McVary KT. Medical treatment of benign prostatic hyperplasia. UpToDate. Updated October 4, 2021. Accessed November 23, 2021. https://www.uptodate.com/contents /medical-treatment-of-benign-prostatic-hyperplasia
12. Zhang W, Ma L, Bauer BA, Liu Z, Lu Y. Acupuncture for benign prostatic hyperplasia: A systematic review and metaanalysis. PLoS One. 2017;12(4):e0174586. Published 2017 Apr 4. doi:10.1371/journal.pone.0174586
13. Newman DK, Guzzo T, Lee D, Jayadevappa R. An evidence- based strategy for the conservative management of the male patient with incontinence. Curr Opin Urol. 2014;24(6):553-559. doi:10.1097/MOU.0000000000000115
14. Newman DK, Wein AJ. Office-based behavioral therapy for management of incontinence and other pelvic disorders. Urol Clin North Am. 2013;40(4):613-635. doi:10.1016/j.ucl.2013.07.010
15. McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349(25):2387-2398. doi:10.1056/NEJMoa030656
16. Roehrborn CG, Barkin J, Siami P, et al. Clinical outcomes after combined therapy with dutasteride plus tamsulosin or either monotherapy in men with benign prostatic hyperplasia (BPH) by baseline characteristics: 4-year results from the randomized, double-blind Combination of Avodart and Tamsulosin (CombAT) trial. BJU Int. 2011;107(6):946-954. doi:10.1111/j.1464-410X.2011.10124.x
17. Djavan B, Marberger M. A meta-analysis on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol. 1999;36(1):1-13. doi:10.1159/000019919
18. By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi:10.1111/jgs.13702
19. Duan Y, Grady JJ, Albertsen PC, Helen Wu Z. Tamsulosin and the risk of dementia in older men with benign prostatic hyperplasia. Pharmacoepidemiol Drug Saf. 2018;27(3):340- 348. doi:10.1002/pds.4361
20. Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med. 2019;179(8):1084-1093. doi:10.1001/jamainternmed.2019.0677
21. Parsons JK, Dahm P, Köhler TS, Lerner LB, Wilt TJ. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline amendment 2020. J Urol. 2020;204(4):799-804. doi:10.1097/JU.0000000000001298
22. Smith AB, Carson CC. Finasteride in the treatment of patients with benign prostatic hyperplasia: a review. Ther Clin Risk Manag. 2009;5(3):535-545. doi:10.2147/tcrm.s6195
23. Andriole GL, Guess HA, Epstein JI, et al. Treatment with finasteride preserves usefulness of prostate-specific antigen in the detection of prostate cancer: results of a randomized, double-blind, placebo-controlled clinical trial. PLESS Study Group. Proscar Long-term Efficacy and Safety Study. Urology. 1998;52(2):195-202. doi:10.1016/s0090-4295(98)00184-8
24. McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med. 1998;338(9):557-563. doi:10.1056/NEJM199802263380901
25. Rittmaster RS. 5alpha-reductase inhibitors in benign prostatic hyperplasia and prostate cancer risk reduction. Best Pract Res Clin Endocrinol Metab. 2008;22(2):389-402. doi:10.1016/j.beem.2008.01.016
26. La Torre A, Giupponi G, Duffy D, Conca A, Cai T, Scardigli A. Sexual dysfunction related to drugs: a critical review. Part V: α-blocker and 5-ARI drugs. Pharmacopsychiatry. 2016;49(1):3-13. doi:10.1055/s-0035-1565100
27. Corona G, Tirabassi G, Santi D, et al. Sexual dysfunction in subjects treated with inhibitors of 5α-reductase for benign prostatic hyperplasia: a comprehensive review and meta-analysis. Andrology. 2017;5(4):671-678. doi:10.1111/andr.12353
28. Trost L, Saitz TR, Hellstrom WJ. Side effects of 5-alpha reductase inhibitors: a comprehensive review. Sex Med Rev. 2013;1(1):24-41. doi:10.1002/smrj.3
29. Welk B, McArthur E, Ordon M, Anderson KK, Hayward J, Dixon S. Association of suicidality and depression with 5α-reductase inhibitors. JAMA Intern Med. 2017;177(5):683-691. doi:10.1001/jamainternmed.2017.0089
30. Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial [published correction appears in JAMA. 2007 Mar 21:297(11):1195] [published correction appears in JAMA. 2007 Oct 24;298(16):1864]. JAMA. 2006;296(19):2319-2328. doi:10.1001/jama.296.19.2319
31. Nitti VW, Auerbach S, Martin N, Calhoun A, Lee M, Herschorn S. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol. 2013;189(4):1388-1395. doi:10.1016/j.juro.2012.10.017
32. Chapple CR, Cardozo L, Nitti VW, Siddiqui E, Michel MC. Mirabegron in overactive bladder: a review of efficacy, safety, and tolerability. Neurourol Urodyn. 2014;33(1):17-30. doi:10.1002/nau.22505
33. Rutman MP, King JR, Bennett N, Ankrom W, Mudd PN. PD14-01 once-daily vibegron, a novel oral β3 agonist does not inhibit CYP2D6, a common pathway for drug metabolism in patients on OAB medications. J Urol. 2019;201(Suppl 4):e231. doi:10.1097/01.JU.0000555478.73162.19
34. Bo K, Frawley HC, Haylen BT, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Neurourol Urodyn. 2017;36(2):221- 244. doi:10.1002/nau.23107
35. Cindolo L, Pirozzi L, Fanizza C, et al. Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: population-based cohort study. Eur Urol. 2015;68(3):418-425. doi:10.1016/j.eururo.2014.11.006
36. Ruhaiyem ME, Alshehri AA, Saade M, Shoabi TA, Zahoor H, Tawfeeq NA. Fear of going under general anesthesia: a cross-sectional study. Saudi J Anaesth. 2016;10(3):317- 321. doi:10.4103/1658-354X.179094
37. Hashim MJ. Patient-centered communication: basic skills. Am Fam Physician. 2017;95(1):29-34.
38. Roehrborn CG, Barkin J, Gange SN, et al. Five year results of the prospective randomized controlled prostatic urethral L.I.F.T. study. Can J Urol. 2017;24(3):8802-8813.
39. Gratzke C, Barber N, Speakman MJ, et al. Prostatic urethral lift vs transurethral resection of the prostate: 2-year results of the BPH6 prospective, multicentre, randomized study. BJU Int. 2017;119(5):767-775.doi:10.1111/bju.13714
40. Sønksen J, Barber NJ, Speakman MJ, et al. Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study. Eur Urol. 2015;68(4):643-652. doi:10.1016/j.eururo.2015.04.024
41. Roehrborn CG, Gange SN, Shore ND, et al. The prostatic urethral lift for the treatment of lower urinary tract symptoms associated with prostate enlargement due to benign prostatic hyperplasia: the L.I.F.T. Study. J Urol. 2013;190(6):2161-2167. doi:10.1016/j.juro.2013.05.116
42. McNicholas TA. Benign prostatic hyperplasia and new treatment options - a critical appraisal of the UroLift system. Med Devices (Auckl). 2016;9:115-123. Published 2016 May 19. doi:10.2147/MDER.S60780
43. McVary KT, Rogers T, Roehrborn CG. Rezuˉm Water Vapor thermal therapy for lower urinary tract symptoms associated with benign prostatic hyperplasia: 4-year results from randomized controlled study. Urology. 2019;126:171-179. doi:10.1016/j.urology.2018.12.041
44. Bole R, Gopalakrishna A, Kuang R, et al. Comparative postoperative outcomes of Rezˉum prostate ablation in patients with large versus small glands. J Endourol. 2020;34(7):778-781. doi:10.1089/end.2020.0177
45. Darson MF, Alexander EE, Schiffman ZJ, et al. Procedural techniques and multicenter postmarket experience using minimally invasive convective radiofrequency thermal therapy with Rezˉum system for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Res Rep Urol. 2017;9:159-168. Published 2017 Aug 21. doi:10.2147/RRU.S143679
46. Baazeem A, Elhilali MM. Surgical management of benign prostatic hyperplasia: current evidence. Nat Clin Pract Urol. 2008;5(10):540-549. doi:10.1038/ncpuro1214
47. Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP)- -incidence, management, and prevention. Eur Urol. 2006;50(5):969-980. doi:10.1016/j.eururo.2005.12.042
48. Abt D, Schmid HP, Speakman MJ. Reasons to consider prostatic artery embolization. World J Urol. 2021;39(7):2301-2306. doi:10.1007/s00345-021-03601-z
49. Nguyen DD, Barber N, Bidair M, et al. Waterjet Ablation Therapy for Endoscopic Resection of prostate tissue trial (WATER) vs WATER II: comparing Aquablation therapy for benign prostatic hyperplasia in30-80and80-150mLprostates. BJUInt. 2020;125(1):112-122. doi:10.1111/bju.14917.
Lower urinary tract symptoms (LUTS)are common and tend to increase in frequency with age. Managing LUTS can be complicated, requires an informed discussion between the primary care practitioner (PCP) and patient, and is best achieved by a thorough understanding of the many medical and surgical options available. Over the past 3 decades, medications have become the most common therapy; but recently, newer minimally invasive surgeries have challenged this paradigm. This article provides a comprehensive review for PCPs regarding the evaluation and management of LUTS in men and when to consider a urology referral.
Benign prostatic hyperplasia (BPH) and LUTS are common clinical encounters for most PCPs. About 50% of men will develop LUTS associated with BPH, and symptoms associated with these conditions increase as men age.1,2 Studies have estimated that 90% of men aged 45 to 80 years demonstrate some symptoms of LUTS.3 Strong genetic influence seems to suggest heritability, but BPH also occurs in sporadic forms and is heavily influenced by androgens.4
BPH is a histologic diagnosis, whereas LUTS consists of complex symptomatology related to both static or dynamic components.1 The enlarged prostate gland obstructs the urethra, simultaneously causing an increase in muscle tone and resistance at the bladder neck and prostatic urethra, leading to increased resistance to urine flow. As a result, there is a thickening of the detrusor muscles in the bladder wall and an overall decreased compliance. Urine becomes stored under increased pressure. These changes result in a weak or intermittent urine stream, incomplete emptying of the bladder, postvoid dribble, hesitancy, and irritative symptoms, such as urgency, frequency, and nocturia.
For many patients, BPH associated with LUTS is a quality of life (QOL) issue. The stigma associated with these symptoms often leads to delays in patients seeking care. Many patients do not seek treatment until symptoms have become so severe that changes in bladder health are often irreversible. Early intervention can dramatically improve a patient’s QOL. Also, early intervention has the potential to reduce overall health care expenditures. BPH-related spending exceeds $1 billion each year in the Medicare program alone.5
PCPs are in a unique position to help many patients who present with early-stage LUTS. Given the substantial impact this disease has on QOL, early recognition of symptoms and prompt treatment play a major role. Paramount to this effort is awareness and understanding of various treatments, their advantages, and adverse effects (AEs). This article highlights evidence-based evaluation and treatment of BPH/LUTS for PCPs who treat veterans and recommendations as to when to refer a patient to a urologist.
Evaluation of LUTS and BPH
Evaluation begins with a thorough medical history and physical examination. Particular attention should focus on ruling out other causes of LUTS, such as a urinary tract infection (UTI), acute prostatitis, malignancy, bladder dysfunction, neurogenic bladder, and other obstructive pathology, such as urethral stricture disease. The differential diagnosis of LUTS includes BPH, UTI, bladder neck obstruction, urethral stricture, bladder stones, polydipsia, overactive bladder (OAB), nocturnal polyuria, neurologic disease, genitourinary malignancy, renal failure, and acute/chronic urinary retention.6
Relevant medical history influencing urinary symptoms includes diabetes mellitus, underlying neurologic diseases, previous trauma, sexually transmitted infections, and certain medications. Symptom severity may be obtained using a validated questionnaire, such as the International Prostate Symptom Score (IPSS), which also aids clinicians in assessing the impact of LUTS on QOL. Additionally, urinary frequency or volume records (voiding diary) may help establish the severity of the patient’s symptoms and provide insight into other potential causes for LUTS. Patients with BPH often have concurrent erectile dysfunction (ED) or other sexual dysfunction symptoms. Patients should be evaluated for baseline sexual dysfunction before the initiation of treatment as many therapies worsen symptoms of ED or ejaculatory dysfunction.
A comprehensive physical examination with a focus on the genitourinary system should, at minimum, assess for abnormalities of the urethral meatus, prepuce, penis, groin nodes, and prior surgical scars. A digital rectal examination also should be performed. Although controversial, a digital rectal examination for prostate cancer screening may provide a rough estimate of prostate size, help rule out prostatitis, and detect incident prostate nodules. Prostate size does not necessarily correlate well with the degree of urinary obstruction or LUTS but is an important consideration when deciding among different therapies.1
Laboratory and Adjunctive Tests
A urinalysis with microscopy helps identify other potential causes for urinary symptoms, including infection, proteinuria, or glucosuria. In patients who present with gross or microscopic hematuria, additional consideration should be given to bladder calculi and genitourinary cancer.2 When a reversible source for the hematuria is not identified, these patients require referral to a urologist for a hematuria evaluation.
There is some controversy regarding prostate specific antigen (PSA) testing. Most professional organizations advocate for a shared decision-making approach before testing. The American Cancer Society recommends this informed discussion occur between the patient and the PCP for men aged > 50 years at average risk, men aged > 45 years at high risk of developing prostate cancer (African Americans or first-degree relative with early prostate cancer diagnosis), and aged 40 years for men with more than one first-degree relative with an early prostate cancer diagnosis.7
Adjunctive tests include postvoid residual (PVR), cystoscopy, uroflowmetry, urodynamics, and transrectal ultrasound. However, these are mostly performed by urologists. In some patients with bladder decompensation after prolonged partial bladder outlet obstruction, urodynamics may be used by urologists to determine whether a patient may benefit from an outlet obstruction procedure. Ordering additional imaging or serum studies for the assessment of LUTS is rarely helpful.
Treatment
Treatment includes management with or without lifestyle modification, medication administration, and surgical therapy. New to this paradigm are in-office minimally invasive surgical options. The goal of treatment is not only to reduce patient symptoms and improve QOL, but also to prevent the secondary sequala of urinary retention, bladder failure, and eventual renal impairment.7A basic understanding of these treatments can aid PCPs with appropriate patient counseling and urologic referral.8
Lifestyle and Behavior Modification
Behavior modification is the starting point for all patients with LUTS. Lifestyle modifications for LUTS include avoiding substances that exacerbate symptoms, such as α-agonists (decongestants), caffeine, alcohol, spicy/acidic foods, chocolate, and soda. These substances are known to be bladder irritants. Common medications contributing to LUTS include antidepressants, decongestants, antihistamines, bronchodilators, anticholinergics, and sympathomimetics. To decrease nocturia, behavioral modifications include limiting evening fluid intake, timed diuretic administration for patients already on a diuretic, and elevating legs 1 hour before bedtime. Counseling obese patients to lose weight and increasing physical activity have been linked to reduced LUTS.9 Other behavioral techniques include double voiding: a technique where patients void normally then change positions and return to void to empty the bladder. Another technique is timed voiding: Many patients have impaired sensation when the bladder is full. These patients are encouraged to void at regular intervals.
Complementary and Alternative Medicine
Multiple nutraceutical compounds claim improved urinary health and symptom reduction. These compounds are marketed to patients with little regulation and oversight since supplements are not regulated or held to the same standard as prescription medications. The most popular nutraceutical for prostate health and LUTS is saw palmetto. Despite its common usage for the treatment of LUTS, little data support saw palmetto health claims. In 2012, a systematic review of 32 randomized trials including 5666 patients compared saw palmetto with a placebo. The study found no difference in urinary symptom scores, urinary flow, or prostate size.10,11 Other phytotherapy compounds often considered for urinary symptoms include stinging nettle extract and β-sitosterol compounds. The mechanism of action of these agents is unknown and efficacy data are lacking.
Historically, acupuncture and pelvic floor physical therapy have been used successfully for OAB symptoms. A meta-analysis found positive beneficial effects of acupuncture compared with a sham control for short- and medium-term follow-up in both IPSS and urine flow rates in some studies; however, when combining the studies for more statistical power, the benefits were less clear.12 Physical therapists with specialized training and certification in pelvic health can incorporate certain bladder training techniques. These include voiding positional changes (double voiding and postvoid urethral milking) and timed voiding.13,14 These interventions often address etiologies of LUTS for which medical therapies are not effective as the sole treatment option.
Medication Management
Medical management includes α-blockers, 5-α-reductase inhibitors (5-α-RIs), antimuscarinic or anticholinergic medicines, β-3 agonists, and phosphodiesterase inhibitors (Table). These medications work independently as well as synergistically. The use of medications to improve symptoms must be balanced against potential AEs and the consequences of a lifetime of drug usage, which can be additive.15,16
First-line pharmacological therapy for BPH is α-blockers, which work by blocking α1A receptors in the prostate and bladder neck, leading to smooth muscle relaxation, increased diameter of the channel, and improved urinary flow. α-receptors in the bladder neck and prostate are expressed with increased frequency with age and are a potential cause for worsening symptoms as men age. Studies demonstrate that these medications reduce symptoms by 30 to 40% and increase flow rates by 16 to 25%.17 Commonly prescribed α-blockers include tamsulosin, alfuzosin, silodosin, doxazosin, and terazosin. Doxazosin and terazosin require dose titrations because they may cause significant hypotension. Orthostatic hypotension typically improves with time and is avoided if the patient takes the medication at bedtime. Both doxazosin and terazosin are on the American Geriatric Society’s Beers Criteria list and should be avoided in older patients.18 Tamsulosin, alfuzosin, and silodosin have a standardized dosing regimen and lower rates of hypotension. Significant AEs include ejaculation dysfunction, nasal congestion, and orthostatic hypotension. Duan and colleagues have linked tamsulosin with dementia. However, this association is not causal and further studies are necessary.19,20 Patients who have taken these agents also are at risk for intraoperative floppy iris syndrome (IFIS). Permanent visual problems can arise if the intraoperative management is not managed to account for IFIS. These medications have a rapid onset of action and work immediately. However, to reach maximum benefit, patients must take the medication for several weeks. Unfortunately, up to one-third of patients will have no improvement with α-blocker therapy, and many patients will discontinue these medications because of significant AEs.6,21
5-α-RIs (finasteride and dutasteride) inhibit the conversion of testosterone to more potent dihydrotestosterone. They effectively reduce prostate volume by 25 to 30%.22 The results occur slowly and can take 6 to 12 months to reach the desired outcome. These medications are effective in men with larger prostates and not as effective in men with smaller prostates.23 These medications can improve urinary flow rates by about 10%, reduce IPSS scores by 20 to 30%, reduce the risk of urinary retention by 50%, and reduce the progression of BPH to the point where surgery is required by 50%.24 Furthermore, 5-α-RIs lower PSA by > 50% after 12 months of treatment.25
A baseline PSA should be established before administration and after 6 months of treatment. Any increase in the PSA even if the level is within normal limits should be evaluated for prostate cancer. Sarkar and colleagues recently published a study evaluating prostate cancer diagnosis in patients treated with 5-α-RI and found there was a delay in diagnosing prostate cancer in this population. Controversy also exists as to the potential of these medications increasing the risk for high-grade prostate cancer, which has led to a US Food and Drug Administration (FDA) warning. AEs include decreased libido (1.5%), ejaculatory dysfunction (3.4%), gynecomastia (1.3%), and/or ED (1.6%).26-28 A recent study evaluating 5-α-RIs demonstrated about a 2-fold increased risk of depression.29
There are well-established studies that note increased effectiveness when using combined α-blocker therapy with 5-α-RI medications. The Medical Therapy of Prostate Symptoms (MTOPS) and Combination Avodart and Tamsulosin (CombAT) trials showed that the combination of both medications was more effective in improving voiding symptoms and flow rates than either agent alone.15,16 Combination therapy resulted in a 66% reduction in disease progression, 81% reduction in urinary retention, and a 67% reduction in the need for surgery compared with placebo.
Anticholinergic medication use in BPH with LUTS is well established, and their use is often combined with other therapies. Anticholinergics work by inhibiting muscarinic M3 receptors to reduce detrusor muscle contraction. This effectively decreases bladder contractions and delays the desire to void. Kaplan and colleagues showed that tolterodine significantly improved a patient’s QOL when added to α-blocker therapy.30 Patients reported a positive outcome at 12 weeks, which resulted in a reduction in urgency incontinence, urgency, nocturia, and the overall number of voiding episodes within 24 hours.
β-3 agonists are a class of medications for OAB; mirabegron and vibegron have proven effective in reducing similar symptoms. In phase 3 clinical trials, mirabegron improved urinary incontinence episodes by 50% and reduced the number of voids in 24 hours.31 Mirabegron is well tolerated and avoids many common anticholinergic effects.32 Vibegron is the newest medication in the class and could soon become the preferred agent given it does not have cytochrome P450 interactions and does not cause hypertension like mirabegron.33
Anticholinergics should be used with caution in patients with a history of urinary retention, elevated after-void residual, or other medications with known anticholinergic effects. AEs include sedation, confusion, dry mouth, constipation, and potential falls in older patients.18 Recent studies have noted an association with dementia in the prolonged use of these medications in older patients and should be used cautiously.20
Phosphodiesterase-5 enzyme inhibitors (PDE-5) are adjunctive medications shown to improve LUTS. This class of medication is prescribed mostly for ED. However, tadalafil 5 mg taken daily also is FDA approved for the treatment of LUTS secondary to BPH given its prolonged half-life. The exact mechanism for improved BPH symptoms is unknown. Possibly the effects are due to an increase mediated by PDE-5 in cyclic guanosine monophosphate (cGMP), which increases smooth muscle relaxation and tissue perfusion of the prostate and bladder.34 There have been limited studies on objective improvement in uroflowmetry parameters compared with other treatments. The daily dosing of tadalafil should not be prescribed in men with a creatinine clearance < 30 mL/min.29 Tadalafil is not considered a first-line agent and is usually reserved for patients who experience ED in addition to BPH. When initiating BPH pharmacologic therapy, the PCP should be aware of adherence and high discontinuation rates.35
Surgical Treatments
Surgical treatments are often delayed out of fear of potential AEs or considered a last resort when symptoms are too severe.36 Early intervention is required to prevent irreversible deleterious changes to detrusor muscle structure and function (Figure). Patients fear urinary incontinence, ED or ejaculatory dysfunction, and anesthesia complications associated with surgical interventions.6,37 Multiple studies show that patients fare better with early surgical intervention, experiencing improved IPSS scores, urinary flow, and QOL. The following is an overview of the most popular procedures.
Prostatic urethral lift (PUL) using the UroLift System is an FDA-approved, minimally-invasive treatment of LUTS secondary to BPH. This procedure treats prostates < 80 g with an absent median lobe.6,21,38 Permanent implants are placed per the prostatic urethra to displace obstructing prostate tissue laterally. This opens the urethra directly without cutting, heating, or removing any prostate tissue. This procedure is minimally invasive, often done in the office as an outpatient procedure, and offers better symptom relief than medication with a lower risk profile than transurethral resection of the prostate (TURP).39,40 The LIFT study was a multicenter, randomized, blinded trial; patients were randomized 2:1 to undergo UroLift or a sham operation. At 3 years, average improvements were statistically significant for total IPSS reduction (41%), QOL improvement (49%), and improved maximum flow rates by (51%).41 Risk for urinary incontinence is low, and the procedure has been shown to preserve erectile and ejaculatory function. Furthermore, patients report significant improvement in their QOL without the need for medications. Surgical retreatment rates at 5 years are 13.6%, with an additional 10.7% of subjects back on medication therapy with α-blockers or 5-α-RIs.42
Water vapor thermal therapy or Rez¯um uses steam as thermal energy to destroy obstructing prostate tissue and relieve the obstruction.43 The procedure differs from older conductive heat thermotherapies because the steam penetrates prostate zonal anatomy without affecting areas outside the targeted treatment zone. The procedure is done in the office with local anesthesia and provides long-lasting relief of LUTS with minimal risks. Following the procedure, patients require an indwelling urethral catheter for 3 to 7 days, and most patients begin to experience symptom improvement 2 to 4 weeks following the procedure.44 The procedure received FDA approval in 2015. Four-year data show significant improvement in maximal flow rate (50%), IPSS (47%), and QOL (43%).45 Surgical retreatment rates were 4.4%. Criticisms of this treatment include patient discomfort with the office procedure, the requirement for an indwelling catheter for a short period, and lack of long-term outcomes data. Guidelines support use in prostate volumes > 80 g with or without median lobe anatomy.
TURP is the gold standard to which other treatments are compared.46 The surgery is performed in the operating room where urologists use a rigid cystoscope and resection element to effectively carve out and cauterize obstructing prostate tissue. Patients typically recover for a short period with an indwelling urethral catheter that is often removed 12 to 24 hours after surgery. New research points out that despite increasing mean age (55% of patients are aged > 70 years with associated comorbidities), the morbidity of TURP was < 1% and mortality rate of 0 to 0.3%.47 Postoperative complications include bleeding that requires a transfusion (3%), retrograde ejaculation (65%), and rare urinary incontinence (2%).47 Surgical retreatment rates for patients following a TURP are approximately 13 to 15% at 8 years.34
Laser surgery for BPH includes multiple techniques: photovaporization of the prostate using a Greenlight XPS laser, holmium laser ablation, and holmium laser enucleation (HoLEP). Proponents of these treatments cite lower bleeding risks compared with TURP, but the operation is largely surgeon dependent on the technology chosen. Most studies comparing these technologies with TURP show similar outcomes of IPSS reports, quality of life improvements, and complications.
Patients with extremely large prostates, > 100 g or 4 times the normal size, pose a unique challenge to surgical treatment. Historically, patients were treated with an open simple prostatectomy operation or staged TURP procedures. Today, urologists use newer, safer ways to treat these patients. Both HoLEP and robot-assisted simple prostatectomy work well in relieving urinary symptoms with lower complications compared with older open surgery. Other minimally invasive procedures, such as prostatic artery embolism, have been described for the treatment of BPH specifically in men who may be unfit for surgery.48Future treatments are constantly evolving. Many unanswered questions remain about BPH and the role of inflammation, metabolic dysfunction, obesity, and other genetic factors driving BPH and prostate growth. Pharmaceutical opportunities exist in mechanisms aimed to reduce prostate growth, induce cellular apoptosis, as well as other drugs to reduce bladder symptoms. Newer, minimally invasive therapies also will become more readily available, such as Aquablation, which is the first FDA-granted surgical robot for the autonomous removal of prostatic tissue due to BPH.49 However, the goal of all future therapies should include the balance of alleviating disruptive symptoms while demonstrating a favorable risk profile. Many men discontinue taking medications, yet few present for surgery. Most concerning is the significant population of men who will develop irreversible bladder dysfunction while waiting for the perfect treatment. There are many opportunities for an effective treatment that is less invasive than surgery, provides durable relief, has minimal AEs, and is affordable.
Conclusions
There is no perfect treatment for patients with LUTS. All interventions have potential AEs and associated complications. Medications are often started as first-line therapy but are often discontinued at the onset of significant AEs. This process is often repeated. Many patients will try different medications without any significant improvement in their symptoms or short-term relief, which results in the gradual progression of the disease.
The PCP plays a significant role in the initial evaluation and management of BPH. These frontline clinicians can recognize patients who may already be experiencing sequela of prolonged bladder outlet obstruction and refer these men to urologists promptly. Counseling patients about their treatment options is an important duty for all PCPs.
A clear understanding of the available treatment options will help PCPs counsel patients appropriately about lifestyle modification, medications, and surgical treatment options for their symptoms. The treatment of this disorder is a rapidly evolving topic with the constant introduction of new technologies and medications, which are certain to continue to play an important role for PCPs and urologists.
Lower urinary tract symptoms (LUTS)are common and tend to increase in frequency with age. Managing LUTS can be complicated, requires an informed discussion between the primary care practitioner (PCP) and patient, and is best achieved by a thorough understanding of the many medical and surgical options available. Over the past 3 decades, medications have become the most common therapy; but recently, newer minimally invasive surgeries have challenged this paradigm. This article provides a comprehensive review for PCPs regarding the evaluation and management of LUTS in men and when to consider a urology referral.
Benign prostatic hyperplasia (BPH) and LUTS are common clinical encounters for most PCPs. About 50% of men will develop LUTS associated with BPH, and symptoms associated with these conditions increase as men age.1,2 Studies have estimated that 90% of men aged 45 to 80 years demonstrate some symptoms of LUTS.3 Strong genetic influence seems to suggest heritability, but BPH also occurs in sporadic forms and is heavily influenced by androgens.4
BPH is a histologic diagnosis, whereas LUTS consists of complex symptomatology related to both static or dynamic components.1 The enlarged prostate gland obstructs the urethra, simultaneously causing an increase in muscle tone and resistance at the bladder neck and prostatic urethra, leading to increased resistance to urine flow. As a result, there is a thickening of the detrusor muscles in the bladder wall and an overall decreased compliance. Urine becomes stored under increased pressure. These changes result in a weak or intermittent urine stream, incomplete emptying of the bladder, postvoid dribble, hesitancy, and irritative symptoms, such as urgency, frequency, and nocturia.
For many patients, BPH associated with LUTS is a quality of life (QOL) issue. The stigma associated with these symptoms often leads to delays in patients seeking care. Many patients do not seek treatment until symptoms have become so severe that changes in bladder health are often irreversible. Early intervention can dramatically improve a patient’s QOL. Also, early intervention has the potential to reduce overall health care expenditures. BPH-related spending exceeds $1 billion each year in the Medicare program alone.5
PCPs are in a unique position to help many patients who present with early-stage LUTS. Given the substantial impact this disease has on QOL, early recognition of symptoms and prompt treatment play a major role. Paramount to this effort is awareness and understanding of various treatments, their advantages, and adverse effects (AEs). This article highlights evidence-based evaluation and treatment of BPH/LUTS for PCPs who treat veterans and recommendations as to when to refer a patient to a urologist.
Evaluation of LUTS and BPH
Evaluation begins with a thorough medical history and physical examination. Particular attention should focus on ruling out other causes of LUTS, such as a urinary tract infection (UTI), acute prostatitis, malignancy, bladder dysfunction, neurogenic bladder, and other obstructive pathology, such as urethral stricture disease. The differential diagnosis of LUTS includes BPH, UTI, bladder neck obstruction, urethral stricture, bladder stones, polydipsia, overactive bladder (OAB), nocturnal polyuria, neurologic disease, genitourinary malignancy, renal failure, and acute/chronic urinary retention.6
Relevant medical history influencing urinary symptoms includes diabetes mellitus, underlying neurologic diseases, previous trauma, sexually transmitted infections, and certain medications. Symptom severity may be obtained using a validated questionnaire, such as the International Prostate Symptom Score (IPSS), which also aids clinicians in assessing the impact of LUTS on QOL. Additionally, urinary frequency or volume records (voiding diary) may help establish the severity of the patient’s symptoms and provide insight into other potential causes for LUTS. Patients with BPH often have concurrent erectile dysfunction (ED) or other sexual dysfunction symptoms. Patients should be evaluated for baseline sexual dysfunction before the initiation of treatment as many therapies worsen symptoms of ED or ejaculatory dysfunction.
A comprehensive physical examination with a focus on the genitourinary system should, at minimum, assess for abnormalities of the urethral meatus, prepuce, penis, groin nodes, and prior surgical scars. A digital rectal examination also should be performed. Although controversial, a digital rectal examination for prostate cancer screening may provide a rough estimate of prostate size, help rule out prostatitis, and detect incident prostate nodules. Prostate size does not necessarily correlate well with the degree of urinary obstruction or LUTS but is an important consideration when deciding among different therapies.1
Laboratory and Adjunctive Tests
A urinalysis with microscopy helps identify other potential causes for urinary symptoms, including infection, proteinuria, or glucosuria. In patients who present with gross or microscopic hematuria, additional consideration should be given to bladder calculi and genitourinary cancer.2 When a reversible source for the hematuria is not identified, these patients require referral to a urologist for a hematuria evaluation.
There is some controversy regarding prostate specific antigen (PSA) testing. Most professional organizations advocate for a shared decision-making approach before testing. The American Cancer Society recommends this informed discussion occur between the patient and the PCP for men aged > 50 years at average risk, men aged > 45 years at high risk of developing prostate cancer (African Americans or first-degree relative with early prostate cancer diagnosis), and aged 40 years for men with more than one first-degree relative with an early prostate cancer diagnosis.7
Adjunctive tests include postvoid residual (PVR), cystoscopy, uroflowmetry, urodynamics, and transrectal ultrasound. However, these are mostly performed by urologists. In some patients with bladder decompensation after prolonged partial bladder outlet obstruction, urodynamics may be used by urologists to determine whether a patient may benefit from an outlet obstruction procedure. Ordering additional imaging or serum studies for the assessment of LUTS is rarely helpful.
Treatment
Treatment includes management with or without lifestyle modification, medication administration, and surgical therapy. New to this paradigm are in-office minimally invasive surgical options. The goal of treatment is not only to reduce patient symptoms and improve QOL, but also to prevent the secondary sequala of urinary retention, bladder failure, and eventual renal impairment.7A basic understanding of these treatments can aid PCPs with appropriate patient counseling and urologic referral.8
Lifestyle and Behavior Modification
Behavior modification is the starting point for all patients with LUTS. Lifestyle modifications for LUTS include avoiding substances that exacerbate symptoms, such as α-agonists (decongestants), caffeine, alcohol, spicy/acidic foods, chocolate, and soda. These substances are known to be bladder irritants. Common medications contributing to LUTS include antidepressants, decongestants, antihistamines, bronchodilators, anticholinergics, and sympathomimetics. To decrease nocturia, behavioral modifications include limiting evening fluid intake, timed diuretic administration for patients already on a diuretic, and elevating legs 1 hour before bedtime. Counseling obese patients to lose weight and increasing physical activity have been linked to reduced LUTS.9 Other behavioral techniques include double voiding: a technique where patients void normally then change positions and return to void to empty the bladder. Another technique is timed voiding: Many patients have impaired sensation when the bladder is full. These patients are encouraged to void at regular intervals.
Complementary and Alternative Medicine
Multiple nutraceutical compounds claim improved urinary health and symptom reduction. These compounds are marketed to patients with little regulation and oversight since supplements are not regulated or held to the same standard as prescription medications. The most popular nutraceutical for prostate health and LUTS is saw palmetto. Despite its common usage for the treatment of LUTS, little data support saw palmetto health claims. In 2012, a systematic review of 32 randomized trials including 5666 patients compared saw palmetto with a placebo. The study found no difference in urinary symptom scores, urinary flow, or prostate size.10,11 Other phytotherapy compounds often considered for urinary symptoms include stinging nettle extract and β-sitosterol compounds. The mechanism of action of these agents is unknown and efficacy data are lacking.
Historically, acupuncture and pelvic floor physical therapy have been used successfully for OAB symptoms. A meta-analysis found positive beneficial effects of acupuncture compared with a sham control for short- and medium-term follow-up in both IPSS and urine flow rates in some studies; however, when combining the studies for more statistical power, the benefits were less clear.12 Physical therapists with specialized training and certification in pelvic health can incorporate certain bladder training techniques. These include voiding positional changes (double voiding and postvoid urethral milking) and timed voiding.13,14 These interventions often address etiologies of LUTS for which medical therapies are not effective as the sole treatment option.
Medication Management
Medical management includes α-blockers, 5-α-reductase inhibitors (5-α-RIs), antimuscarinic or anticholinergic medicines, β-3 agonists, and phosphodiesterase inhibitors (Table). These medications work independently as well as synergistically. The use of medications to improve symptoms must be balanced against potential AEs and the consequences of a lifetime of drug usage, which can be additive.15,16
First-line pharmacological therapy for BPH is α-blockers, which work by blocking α1A receptors in the prostate and bladder neck, leading to smooth muscle relaxation, increased diameter of the channel, and improved urinary flow. α-receptors in the bladder neck and prostate are expressed with increased frequency with age and are a potential cause for worsening symptoms as men age. Studies demonstrate that these medications reduce symptoms by 30 to 40% and increase flow rates by 16 to 25%.17 Commonly prescribed α-blockers include tamsulosin, alfuzosin, silodosin, doxazosin, and terazosin. Doxazosin and terazosin require dose titrations because they may cause significant hypotension. Orthostatic hypotension typically improves with time and is avoided if the patient takes the medication at bedtime. Both doxazosin and terazosin are on the American Geriatric Society’s Beers Criteria list and should be avoided in older patients.18 Tamsulosin, alfuzosin, and silodosin have a standardized dosing regimen and lower rates of hypotension. Significant AEs include ejaculation dysfunction, nasal congestion, and orthostatic hypotension. Duan and colleagues have linked tamsulosin with dementia. However, this association is not causal and further studies are necessary.19,20 Patients who have taken these agents also are at risk for intraoperative floppy iris syndrome (IFIS). Permanent visual problems can arise if the intraoperative management is not managed to account for IFIS. These medications have a rapid onset of action and work immediately. However, to reach maximum benefit, patients must take the medication for several weeks. Unfortunately, up to one-third of patients will have no improvement with α-blocker therapy, and many patients will discontinue these medications because of significant AEs.6,21
5-α-RIs (finasteride and dutasteride) inhibit the conversion of testosterone to more potent dihydrotestosterone. They effectively reduce prostate volume by 25 to 30%.22 The results occur slowly and can take 6 to 12 months to reach the desired outcome. These medications are effective in men with larger prostates and not as effective in men with smaller prostates.23 These medications can improve urinary flow rates by about 10%, reduce IPSS scores by 20 to 30%, reduce the risk of urinary retention by 50%, and reduce the progression of BPH to the point where surgery is required by 50%.24 Furthermore, 5-α-RIs lower PSA by > 50% after 12 months of treatment.25
A baseline PSA should be established before administration and after 6 months of treatment. Any increase in the PSA even if the level is within normal limits should be evaluated for prostate cancer. Sarkar and colleagues recently published a study evaluating prostate cancer diagnosis in patients treated with 5-α-RI and found there was a delay in diagnosing prostate cancer in this population. Controversy also exists as to the potential of these medications increasing the risk for high-grade prostate cancer, which has led to a US Food and Drug Administration (FDA) warning. AEs include decreased libido (1.5%), ejaculatory dysfunction (3.4%), gynecomastia (1.3%), and/or ED (1.6%).26-28 A recent study evaluating 5-α-RIs demonstrated about a 2-fold increased risk of depression.29
There are well-established studies that note increased effectiveness when using combined α-blocker therapy with 5-α-RI medications. The Medical Therapy of Prostate Symptoms (MTOPS) and Combination Avodart and Tamsulosin (CombAT) trials showed that the combination of both medications was more effective in improving voiding symptoms and flow rates than either agent alone.15,16 Combination therapy resulted in a 66% reduction in disease progression, 81% reduction in urinary retention, and a 67% reduction in the need for surgery compared with placebo.
Anticholinergic medication use in BPH with LUTS is well established, and their use is often combined with other therapies. Anticholinergics work by inhibiting muscarinic M3 receptors to reduce detrusor muscle contraction. This effectively decreases bladder contractions and delays the desire to void. Kaplan and colleagues showed that tolterodine significantly improved a patient’s QOL when added to α-blocker therapy.30 Patients reported a positive outcome at 12 weeks, which resulted in a reduction in urgency incontinence, urgency, nocturia, and the overall number of voiding episodes within 24 hours.
β-3 agonists are a class of medications for OAB; mirabegron and vibegron have proven effective in reducing similar symptoms. In phase 3 clinical trials, mirabegron improved urinary incontinence episodes by 50% and reduced the number of voids in 24 hours.31 Mirabegron is well tolerated and avoids many common anticholinergic effects.32 Vibegron is the newest medication in the class and could soon become the preferred agent given it does not have cytochrome P450 interactions and does not cause hypertension like mirabegron.33
Anticholinergics should be used with caution in patients with a history of urinary retention, elevated after-void residual, or other medications with known anticholinergic effects. AEs include sedation, confusion, dry mouth, constipation, and potential falls in older patients.18 Recent studies have noted an association with dementia in the prolonged use of these medications in older patients and should be used cautiously.20
Phosphodiesterase-5 enzyme inhibitors (PDE-5) are adjunctive medications shown to improve LUTS. This class of medication is prescribed mostly for ED. However, tadalafil 5 mg taken daily also is FDA approved for the treatment of LUTS secondary to BPH given its prolonged half-life. The exact mechanism for improved BPH symptoms is unknown. Possibly the effects are due to an increase mediated by PDE-5 in cyclic guanosine monophosphate (cGMP), which increases smooth muscle relaxation and tissue perfusion of the prostate and bladder.34 There have been limited studies on objective improvement in uroflowmetry parameters compared with other treatments. The daily dosing of tadalafil should not be prescribed in men with a creatinine clearance < 30 mL/min.29 Tadalafil is not considered a first-line agent and is usually reserved for patients who experience ED in addition to BPH. When initiating BPH pharmacologic therapy, the PCP should be aware of adherence and high discontinuation rates.35
Surgical Treatments
Surgical treatments are often delayed out of fear of potential AEs or considered a last resort when symptoms are too severe.36 Early intervention is required to prevent irreversible deleterious changes to detrusor muscle structure and function (Figure). Patients fear urinary incontinence, ED or ejaculatory dysfunction, and anesthesia complications associated with surgical interventions.6,37 Multiple studies show that patients fare better with early surgical intervention, experiencing improved IPSS scores, urinary flow, and QOL. The following is an overview of the most popular procedures.
Prostatic urethral lift (PUL) using the UroLift System is an FDA-approved, minimally-invasive treatment of LUTS secondary to BPH. This procedure treats prostates < 80 g with an absent median lobe.6,21,38 Permanent implants are placed per the prostatic urethra to displace obstructing prostate tissue laterally. This opens the urethra directly without cutting, heating, or removing any prostate tissue. This procedure is minimally invasive, often done in the office as an outpatient procedure, and offers better symptom relief than medication with a lower risk profile than transurethral resection of the prostate (TURP).39,40 The LIFT study was a multicenter, randomized, blinded trial; patients were randomized 2:1 to undergo UroLift or a sham operation. At 3 years, average improvements were statistically significant for total IPSS reduction (41%), QOL improvement (49%), and improved maximum flow rates by (51%).41 Risk for urinary incontinence is low, and the procedure has been shown to preserve erectile and ejaculatory function. Furthermore, patients report significant improvement in their QOL without the need for medications. Surgical retreatment rates at 5 years are 13.6%, with an additional 10.7% of subjects back on medication therapy with α-blockers or 5-α-RIs.42
Water vapor thermal therapy or Rez¯um uses steam as thermal energy to destroy obstructing prostate tissue and relieve the obstruction.43 The procedure differs from older conductive heat thermotherapies because the steam penetrates prostate zonal anatomy without affecting areas outside the targeted treatment zone. The procedure is done in the office with local anesthesia and provides long-lasting relief of LUTS with minimal risks. Following the procedure, patients require an indwelling urethral catheter for 3 to 7 days, and most patients begin to experience symptom improvement 2 to 4 weeks following the procedure.44 The procedure received FDA approval in 2015. Four-year data show significant improvement in maximal flow rate (50%), IPSS (47%), and QOL (43%).45 Surgical retreatment rates were 4.4%. Criticisms of this treatment include patient discomfort with the office procedure, the requirement for an indwelling catheter for a short period, and lack of long-term outcomes data. Guidelines support use in prostate volumes > 80 g with or without median lobe anatomy.
TURP is the gold standard to which other treatments are compared.46 The surgery is performed in the operating room where urologists use a rigid cystoscope and resection element to effectively carve out and cauterize obstructing prostate tissue. Patients typically recover for a short period with an indwelling urethral catheter that is often removed 12 to 24 hours after surgery. New research points out that despite increasing mean age (55% of patients are aged > 70 years with associated comorbidities), the morbidity of TURP was < 1% and mortality rate of 0 to 0.3%.47 Postoperative complications include bleeding that requires a transfusion (3%), retrograde ejaculation (65%), and rare urinary incontinence (2%).47 Surgical retreatment rates for patients following a TURP are approximately 13 to 15% at 8 years.34
Laser surgery for BPH includes multiple techniques: photovaporization of the prostate using a Greenlight XPS laser, holmium laser ablation, and holmium laser enucleation (HoLEP). Proponents of these treatments cite lower bleeding risks compared with TURP, but the operation is largely surgeon dependent on the technology chosen. Most studies comparing these technologies with TURP show similar outcomes of IPSS reports, quality of life improvements, and complications.
Patients with extremely large prostates, > 100 g or 4 times the normal size, pose a unique challenge to surgical treatment. Historically, patients were treated with an open simple prostatectomy operation or staged TURP procedures. Today, urologists use newer, safer ways to treat these patients. Both HoLEP and robot-assisted simple prostatectomy work well in relieving urinary symptoms with lower complications compared with older open surgery. Other minimally invasive procedures, such as prostatic artery embolism, have been described for the treatment of BPH specifically in men who may be unfit for surgery.48Future treatments are constantly evolving. Many unanswered questions remain about BPH and the role of inflammation, metabolic dysfunction, obesity, and other genetic factors driving BPH and prostate growth. Pharmaceutical opportunities exist in mechanisms aimed to reduce prostate growth, induce cellular apoptosis, as well as other drugs to reduce bladder symptoms. Newer, minimally invasive therapies also will become more readily available, such as Aquablation, which is the first FDA-granted surgical robot for the autonomous removal of prostatic tissue due to BPH.49 However, the goal of all future therapies should include the balance of alleviating disruptive symptoms while demonstrating a favorable risk profile. Many men discontinue taking medications, yet few present for surgery. Most concerning is the significant population of men who will develop irreversible bladder dysfunction while waiting for the perfect treatment. There are many opportunities for an effective treatment that is less invasive than surgery, provides durable relief, has minimal AEs, and is affordable.
Conclusions
There is no perfect treatment for patients with LUTS. All interventions have potential AEs and associated complications. Medications are often started as first-line therapy but are often discontinued at the onset of significant AEs. This process is often repeated. Many patients will try different medications without any significant improvement in their symptoms or short-term relief, which results in the gradual progression of the disease.
The PCP plays a significant role in the initial evaluation and management of BPH. These frontline clinicians can recognize patients who may already be experiencing sequela of prolonged bladder outlet obstruction and refer these men to urologists promptly. Counseling patients about their treatment options is an important duty for all PCPs.
A clear understanding of the available treatment options will help PCPs counsel patients appropriately about lifestyle modification, medications, and surgical treatment options for their symptoms. The treatment of this disorder is a rapidly evolving topic with the constant introduction of new technologies and medications, which are certain to continue to play an important role for PCPs and urologists.
1. Roehrborn CG. Benign prostatic hyperplasia: an overview. Rev Urol. 2005;7 Suppl 9(Suppl 9):S3-S14
2. McVary KT. Clinical manifestations and diagnostic evaluation of benign prostatic hyperplasia. UpToDate. Updated November 18, 2021. Accessed November 23, 2021. https:// www.uptodate.com/contents/clinical-manifestations-and -diagnostic-evaluation-of-benign-prostatic-hyperplasia
3. McVary KT. BPH: epidemiology and comorbidities. Am J Manag Care. 2006;12(5 Suppl):S122-S128.
4. Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011;8(1):29-41. doi:10.1038/nrurol.2010.207
5. Rensing AJ, Kuxhausen A, Vetter J, Strope SA. Differences in the treatment of benign prostatic hyperplasia: comparing the primary care physician and the urologist. Urol Pract. 2017;4(3):193-199. doi:10.1016/j.urpr.2016.07.002
6. Foster HE, Barry MJ, Dahm P, et al. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline. J Urol. 2018;200(3):612- 619. doi:10.1016/j.juro.2018.05.048
7. Landau A, Welliver C. Analyzing and characterizing why men seek care for lower urinary tract symptoms. Curr Urol Rep. 2020;21(12):58. Published 2020 Oct 30. doi:10.1007/s11934-020-01006-w
8. Das AK, Leong JY, Roehrborn CG. Office-based therapies for benign prostatic hyperplasia: a review and update. Can J Urol. 2019;26(4 Suppl 1):2-7.
9. Parsons JK, Sarma AV, McVary K, Wei JT. Obesity and benign prostatic hyperplasia: clinical connections, emerging etiological paradigms and future directions. J Urol. 2013;189(1 Suppl):S102-S106. doi:10.1016/j.juro.2012.11.029
10. Pattanaik S, Mavuduru RS, Panda A, et al. Phosphodiesterase inhibitors for lower urinary tract symptoms consistent with benign prostatic hyperplasia. Cochrane Database Syst Rev. 2018;11(11):CD010060. Published 2018 Nov 16. doi:10.1002/14651858.CD010060.pub2
11. McVary KT. Medical treatment of benign prostatic hyperplasia. UpToDate. Updated October 4, 2021. Accessed November 23, 2021. https://www.uptodate.com/contents /medical-treatment-of-benign-prostatic-hyperplasia
12. Zhang W, Ma L, Bauer BA, Liu Z, Lu Y. Acupuncture for benign prostatic hyperplasia: A systematic review and metaanalysis. PLoS One. 2017;12(4):e0174586. Published 2017 Apr 4. doi:10.1371/journal.pone.0174586
13. Newman DK, Guzzo T, Lee D, Jayadevappa R. An evidence- based strategy for the conservative management of the male patient with incontinence. Curr Opin Urol. 2014;24(6):553-559. doi:10.1097/MOU.0000000000000115
14. Newman DK, Wein AJ. Office-based behavioral therapy for management of incontinence and other pelvic disorders. Urol Clin North Am. 2013;40(4):613-635. doi:10.1016/j.ucl.2013.07.010
15. McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349(25):2387-2398. doi:10.1056/NEJMoa030656
16. Roehrborn CG, Barkin J, Siami P, et al. Clinical outcomes after combined therapy with dutasteride plus tamsulosin or either monotherapy in men with benign prostatic hyperplasia (BPH) by baseline characteristics: 4-year results from the randomized, double-blind Combination of Avodart and Tamsulosin (CombAT) trial. BJU Int. 2011;107(6):946-954. doi:10.1111/j.1464-410X.2011.10124.x
17. Djavan B, Marberger M. A meta-analysis on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol. 1999;36(1):1-13. doi:10.1159/000019919
18. By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi:10.1111/jgs.13702
19. Duan Y, Grady JJ, Albertsen PC, Helen Wu Z. Tamsulosin and the risk of dementia in older men with benign prostatic hyperplasia. Pharmacoepidemiol Drug Saf. 2018;27(3):340- 348. doi:10.1002/pds.4361
20. Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med. 2019;179(8):1084-1093. doi:10.1001/jamainternmed.2019.0677
21. Parsons JK, Dahm P, Köhler TS, Lerner LB, Wilt TJ. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline amendment 2020. J Urol. 2020;204(4):799-804. doi:10.1097/JU.0000000000001298
22. Smith AB, Carson CC. Finasteride in the treatment of patients with benign prostatic hyperplasia: a review. Ther Clin Risk Manag. 2009;5(3):535-545. doi:10.2147/tcrm.s6195
23. Andriole GL, Guess HA, Epstein JI, et al. Treatment with finasteride preserves usefulness of prostate-specific antigen in the detection of prostate cancer: results of a randomized, double-blind, placebo-controlled clinical trial. PLESS Study Group. Proscar Long-term Efficacy and Safety Study. Urology. 1998;52(2):195-202. doi:10.1016/s0090-4295(98)00184-8
24. McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med. 1998;338(9):557-563. doi:10.1056/NEJM199802263380901
25. Rittmaster RS. 5alpha-reductase inhibitors in benign prostatic hyperplasia and prostate cancer risk reduction. Best Pract Res Clin Endocrinol Metab. 2008;22(2):389-402. doi:10.1016/j.beem.2008.01.016
26. La Torre A, Giupponi G, Duffy D, Conca A, Cai T, Scardigli A. Sexual dysfunction related to drugs: a critical review. Part V: α-blocker and 5-ARI drugs. Pharmacopsychiatry. 2016;49(1):3-13. doi:10.1055/s-0035-1565100
27. Corona G, Tirabassi G, Santi D, et al. Sexual dysfunction in subjects treated with inhibitors of 5α-reductase for benign prostatic hyperplasia: a comprehensive review and meta-analysis. Andrology. 2017;5(4):671-678. doi:10.1111/andr.12353
28. Trost L, Saitz TR, Hellstrom WJ. Side effects of 5-alpha reductase inhibitors: a comprehensive review. Sex Med Rev. 2013;1(1):24-41. doi:10.1002/smrj.3
29. Welk B, McArthur E, Ordon M, Anderson KK, Hayward J, Dixon S. Association of suicidality and depression with 5α-reductase inhibitors. JAMA Intern Med. 2017;177(5):683-691. doi:10.1001/jamainternmed.2017.0089
30. Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial [published correction appears in JAMA. 2007 Mar 21:297(11):1195] [published correction appears in JAMA. 2007 Oct 24;298(16):1864]. JAMA. 2006;296(19):2319-2328. doi:10.1001/jama.296.19.2319
31. Nitti VW, Auerbach S, Martin N, Calhoun A, Lee M, Herschorn S. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol. 2013;189(4):1388-1395. doi:10.1016/j.juro.2012.10.017
32. Chapple CR, Cardozo L, Nitti VW, Siddiqui E, Michel MC. Mirabegron in overactive bladder: a review of efficacy, safety, and tolerability. Neurourol Urodyn. 2014;33(1):17-30. doi:10.1002/nau.22505
33. Rutman MP, King JR, Bennett N, Ankrom W, Mudd PN. PD14-01 once-daily vibegron, a novel oral β3 agonist does not inhibit CYP2D6, a common pathway for drug metabolism in patients on OAB medications. J Urol. 2019;201(Suppl 4):e231. doi:10.1097/01.JU.0000555478.73162.19
34. Bo K, Frawley HC, Haylen BT, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Neurourol Urodyn. 2017;36(2):221- 244. doi:10.1002/nau.23107
35. Cindolo L, Pirozzi L, Fanizza C, et al. Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: population-based cohort study. Eur Urol. 2015;68(3):418-425. doi:10.1016/j.eururo.2014.11.006
36. Ruhaiyem ME, Alshehri AA, Saade M, Shoabi TA, Zahoor H, Tawfeeq NA. Fear of going under general anesthesia: a cross-sectional study. Saudi J Anaesth. 2016;10(3):317- 321. doi:10.4103/1658-354X.179094
37. Hashim MJ. Patient-centered communication: basic skills. Am Fam Physician. 2017;95(1):29-34.
38. Roehrborn CG, Barkin J, Gange SN, et al. Five year results of the prospective randomized controlled prostatic urethral L.I.F.T. study. Can J Urol. 2017;24(3):8802-8813.
39. Gratzke C, Barber N, Speakman MJ, et al. Prostatic urethral lift vs transurethral resection of the prostate: 2-year results of the BPH6 prospective, multicentre, randomized study. BJU Int. 2017;119(5):767-775.doi:10.1111/bju.13714
40. Sønksen J, Barber NJ, Speakman MJ, et al. Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study. Eur Urol. 2015;68(4):643-652. doi:10.1016/j.eururo.2015.04.024
41. Roehrborn CG, Gange SN, Shore ND, et al. The prostatic urethral lift for the treatment of lower urinary tract symptoms associated with prostate enlargement due to benign prostatic hyperplasia: the L.I.F.T. Study. J Urol. 2013;190(6):2161-2167. doi:10.1016/j.juro.2013.05.116
42. McNicholas TA. Benign prostatic hyperplasia and new treatment options - a critical appraisal of the UroLift system. Med Devices (Auckl). 2016;9:115-123. Published 2016 May 19. doi:10.2147/MDER.S60780
43. McVary KT, Rogers T, Roehrborn CG. Rezuˉm Water Vapor thermal therapy for lower urinary tract symptoms associated with benign prostatic hyperplasia: 4-year results from randomized controlled study. Urology. 2019;126:171-179. doi:10.1016/j.urology.2018.12.041
44. Bole R, Gopalakrishna A, Kuang R, et al. Comparative postoperative outcomes of Rezˉum prostate ablation in patients with large versus small glands. J Endourol. 2020;34(7):778-781. doi:10.1089/end.2020.0177
45. Darson MF, Alexander EE, Schiffman ZJ, et al. Procedural techniques and multicenter postmarket experience using minimally invasive convective radiofrequency thermal therapy with Rezˉum system for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Res Rep Urol. 2017;9:159-168. Published 2017 Aug 21. doi:10.2147/RRU.S143679
46. Baazeem A, Elhilali MM. Surgical management of benign prostatic hyperplasia: current evidence. Nat Clin Pract Urol. 2008;5(10):540-549. doi:10.1038/ncpuro1214
47. Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP)- -incidence, management, and prevention. Eur Urol. 2006;50(5):969-980. doi:10.1016/j.eururo.2005.12.042
48. Abt D, Schmid HP, Speakman MJ. Reasons to consider prostatic artery embolization. World J Urol. 2021;39(7):2301-2306. doi:10.1007/s00345-021-03601-z
49. Nguyen DD, Barber N, Bidair M, et al. Waterjet Ablation Therapy for Endoscopic Resection of prostate tissue trial (WATER) vs WATER II: comparing Aquablation therapy for benign prostatic hyperplasia in30-80and80-150mLprostates. BJUInt. 2020;125(1):112-122. doi:10.1111/bju.14917.
1. Roehrborn CG. Benign prostatic hyperplasia: an overview. Rev Urol. 2005;7 Suppl 9(Suppl 9):S3-S14
2. McVary KT. Clinical manifestations and diagnostic evaluation of benign prostatic hyperplasia. UpToDate. Updated November 18, 2021. Accessed November 23, 2021. https:// www.uptodate.com/contents/clinical-manifestations-and -diagnostic-evaluation-of-benign-prostatic-hyperplasia
3. McVary KT. BPH: epidemiology and comorbidities. Am J Manag Care. 2006;12(5 Suppl):S122-S128.
4. Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011;8(1):29-41. doi:10.1038/nrurol.2010.207
5. Rensing AJ, Kuxhausen A, Vetter J, Strope SA. Differences in the treatment of benign prostatic hyperplasia: comparing the primary care physician and the urologist. Urol Pract. 2017;4(3):193-199. doi:10.1016/j.urpr.2016.07.002
6. Foster HE, Barry MJ, Dahm P, et al. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline. J Urol. 2018;200(3):612- 619. doi:10.1016/j.juro.2018.05.048
7. Landau A, Welliver C. Analyzing and characterizing why men seek care for lower urinary tract symptoms. Curr Urol Rep. 2020;21(12):58. Published 2020 Oct 30. doi:10.1007/s11934-020-01006-w
8. Das AK, Leong JY, Roehrborn CG. Office-based therapies for benign prostatic hyperplasia: a review and update. Can J Urol. 2019;26(4 Suppl 1):2-7.
9. Parsons JK, Sarma AV, McVary K, Wei JT. Obesity and benign prostatic hyperplasia: clinical connections, emerging etiological paradigms and future directions. J Urol. 2013;189(1 Suppl):S102-S106. doi:10.1016/j.juro.2012.11.029
10. Pattanaik S, Mavuduru RS, Panda A, et al. Phosphodiesterase inhibitors for lower urinary tract symptoms consistent with benign prostatic hyperplasia. Cochrane Database Syst Rev. 2018;11(11):CD010060. Published 2018 Nov 16. doi:10.1002/14651858.CD010060.pub2
11. McVary KT. Medical treatment of benign prostatic hyperplasia. UpToDate. Updated October 4, 2021. Accessed November 23, 2021. https://www.uptodate.com/contents /medical-treatment-of-benign-prostatic-hyperplasia
12. Zhang W, Ma L, Bauer BA, Liu Z, Lu Y. Acupuncture for benign prostatic hyperplasia: A systematic review and metaanalysis. PLoS One. 2017;12(4):e0174586. Published 2017 Apr 4. doi:10.1371/journal.pone.0174586
13. Newman DK, Guzzo T, Lee D, Jayadevappa R. An evidence- based strategy for the conservative management of the male patient with incontinence. Curr Opin Urol. 2014;24(6):553-559. doi:10.1097/MOU.0000000000000115
14. Newman DK, Wein AJ. Office-based behavioral therapy for management of incontinence and other pelvic disorders. Urol Clin North Am. 2013;40(4):613-635. doi:10.1016/j.ucl.2013.07.010
15. McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349(25):2387-2398. doi:10.1056/NEJMoa030656
16. Roehrborn CG, Barkin J, Siami P, et al. Clinical outcomes after combined therapy with dutasteride plus tamsulosin or either monotherapy in men with benign prostatic hyperplasia (BPH) by baseline characteristics: 4-year results from the randomized, double-blind Combination of Avodart and Tamsulosin (CombAT) trial. BJU Int. 2011;107(6):946-954. doi:10.1111/j.1464-410X.2011.10124.x
17. Djavan B, Marberger M. A meta-analysis on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol. 1999;36(1):1-13. doi:10.1159/000019919
18. By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi:10.1111/jgs.13702
19. Duan Y, Grady JJ, Albertsen PC, Helen Wu Z. Tamsulosin and the risk of dementia in older men with benign prostatic hyperplasia. Pharmacoepidemiol Drug Saf. 2018;27(3):340- 348. doi:10.1002/pds.4361
20. Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med. 2019;179(8):1084-1093. doi:10.1001/jamainternmed.2019.0677
21. Parsons JK, Dahm P, Köhler TS, Lerner LB, Wilt TJ. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline amendment 2020. J Urol. 2020;204(4):799-804. doi:10.1097/JU.0000000000001298
22. Smith AB, Carson CC. Finasteride in the treatment of patients with benign prostatic hyperplasia: a review. Ther Clin Risk Manag. 2009;5(3):535-545. doi:10.2147/tcrm.s6195
23. Andriole GL, Guess HA, Epstein JI, et al. Treatment with finasteride preserves usefulness of prostate-specific antigen in the detection of prostate cancer: results of a randomized, double-blind, placebo-controlled clinical trial. PLESS Study Group. Proscar Long-term Efficacy and Safety Study. Urology. 1998;52(2):195-202. doi:10.1016/s0090-4295(98)00184-8
24. McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med. 1998;338(9):557-563. doi:10.1056/NEJM199802263380901
25. Rittmaster RS. 5alpha-reductase inhibitors in benign prostatic hyperplasia and prostate cancer risk reduction. Best Pract Res Clin Endocrinol Metab. 2008;22(2):389-402. doi:10.1016/j.beem.2008.01.016
26. La Torre A, Giupponi G, Duffy D, Conca A, Cai T, Scardigli A. Sexual dysfunction related to drugs: a critical review. Part V: α-blocker and 5-ARI drugs. Pharmacopsychiatry. 2016;49(1):3-13. doi:10.1055/s-0035-1565100
27. Corona G, Tirabassi G, Santi D, et al. Sexual dysfunction in subjects treated with inhibitors of 5α-reductase for benign prostatic hyperplasia: a comprehensive review and meta-analysis. Andrology. 2017;5(4):671-678. doi:10.1111/andr.12353
28. Trost L, Saitz TR, Hellstrom WJ. Side effects of 5-alpha reductase inhibitors: a comprehensive review. Sex Med Rev. 2013;1(1):24-41. doi:10.1002/smrj.3
29. Welk B, McArthur E, Ordon M, Anderson KK, Hayward J, Dixon S. Association of suicidality and depression with 5α-reductase inhibitors. JAMA Intern Med. 2017;177(5):683-691. doi:10.1001/jamainternmed.2017.0089
30. Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial [published correction appears in JAMA. 2007 Mar 21:297(11):1195] [published correction appears in JAMA. 2007 Oct 24;298(16):1864]. JAMA. 2006;296(19):2319-2328. doi:10.1001/jama.296.19.2319
31. Nitti VW, Auerbach S, Martin N, Calhoun A, Lee M, Herschorn S. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol. 2013;189(4):1388-1395. doi:10.1016/j.juro.2012.10.017
32. Chapple CR, Cardozo L, Nitti VW, Siddiqui E, Michel MC. Mirabegron in overactive bladder: a review of efficacy, safety, and tolerability. Neurourol Urodyn. 2014;33(1):17-30. doi:10.1002/nau.22505
33. Rutman MP, King JR, Bennett N, Ankrom W, Mudd PN. PD14-01 once-daily vibegron, a novel oral β3 agonist does not inhibit CYP2D6, a common pathway for drug metabolism in patients on OAB medications. J Urol. 2019;201(Suppl 4):e231. doi:10.1097/01.JU.0000555478.73162.19
34. Bo K, Frawley HC, Haylen BT, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Neurourol Urodyn. 2017;36(2):221- 244. doi:10.1002/nau.23107
35. Cindolo L, Pirozzi L, Fanizza C, et al. Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: population-based cohort study. Eur Urol. 2015;68(3):418-425. doi:10.1016/j.eururo.2014.11.006
36. Ruhaiyem ME, Alshehri AA, Saade M, Shoabi TA, Zahoor H, Tawfeeq NA. Fear of going under general anesthesia: a cross-sectional study. Saudi J Anaesth. 2016;10(3):317- 321. doi:10.4103/1658-354X.179094
37. Hashim MJ. Patient-centered communication: basic skills. Am Fam Physician. 2017;95(1):29-34.
38. Roehrborn CG, Barkin J, Gange SN, et al. Five year results of the prospective randomized controlled prostatic urethral L.I.F.T. study. Can J Urol. 2017;24(3):8802-8813.
39. Gratzke C, Barber N, Speakman MJ, et al. Prostatic urethral lift vs transurethral resection of the prostate: 2-year results of the BPH6 prospective, multicentre, randomized study. BJU Int. 2017;119(5):767-775.doi:10.1111/bju.13714
40. Sønksen J, Barber NJ, Speakman MJ, et al. Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study. Eur Urol. 2015;68(4):643-652. doi:10.1016/j.eururo.2015.04.024
41. Roehrborn CG, Gange SN, Shore ND, et al. The prostatic urethral lift for the treatment of lower urinary tract symptoms associated with prostate enlargement due to benign prostatic hyperplasia: the L.I.F.T. Study. J Urol. 2013;190(6):2161-2167. doi:10.1016/j.juro.2013.05.116
42. McNicholas TA. Benign prostatic hyperplasia and new treatment options - a critical appraisal of the UroLift system. Med Devices (Auckl). 2016;9:115-123. Published 2016 May 19. doi:10.2147/MDER.S60780
43. McVary KT, Rogers T, Roehrborn CG. Rezuˉm Water Vapor thermal therapy for lower urinary tract symptoms associated with benign prostatic hyperplasia: 4-year results from randomized controlled study. Urology. 2019;126:171-179. doi:10.1016/j.urology.2018.12.041
44. Bole R, Gopalakrishna A, Kuang R, et al. Comparative postoperative outcomes of Rezˉum prostate ablation in patients with large versus small glands. J Endourol. 2020;34(7):778-781. doi:10.1089/end.2020.0177
45. Darson MF, Alexander EE, Schiffman ZJ, et al. Procedural techniques and multicenter postmarket experience using minimally invasive convective radiofrequency thermal therapy with Rezˉum system for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Res Rep Urol. 2017;9:159-168. Published 2017 Aug 21. doi:10.2147/RRU.S143679
46. Baazeem A, Elhilali MM. Surgical management of benign prostatic hyperplasia: current evidence. Nat Clin Pract Urol. 2008;5(10):540-549. doi:10.1038/ncpuro1214
47. Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP)- -incidence, management, and prevention. Eur Urol. 2006;50(5):969-980. doi:10.1016/j.eururo.2005.12.042
48. Abt D, Schmid HP, Speakman MJ. Reasons to consider prostatic artery embolization. World J Urol. 2021;39(7):2301-2306. doi:10.1007/s00345-021-03601-z
49. Nguyen DD, Barber N, Bidair M, et al. Waterjet Ablation Therapy for Endoscopic Resection of prostate tissue trial (WATER) vs WATER II: comparing Aquablation therapy for benign prostatic hyperplasia in30-80and80-150mLprostates. BJUInt. 2020;125(1):112-122. doi:10.1111/bju.14917.
2021 Update on bone health
Recently, the National Osteoporosis Foundation (NOF) changed its name to the Bone Health and Osteoporosis Foundation (BHOF). Several years ago, in 2016 at my urging, this column was renamed from “Update on osteoporosis” to “Update on bone health.” I believe we were on the leading edge of this movement. As expressed in last year’s Update, our patients’ bone health must be emphasized more than it has been in the past.1
Consider that localized breast cancer carries a 5-year survival rate of 99%.2 Most of my patients are keenly aware that periodic competent breast imaging is the key to the earliest possible diagnosis. By contrast, in this country a hip fracture carries a mortality in the first year of 21%!3 Furthermore, approximately one-third of women who fracture their hip do not have osteoporosis.4 While the risk of hip fracture is greatest in women with osteoporosis, it is not absent in those without the condition. Finally, the role of muscle mass, strength, and performance in bone health is a rapidly emerging topic and one that constitutes the core of this year’s Update.
Muscle mass and strength play key role in bone health
de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society white paper 2021. Climacteric. 2021;24:498-504. doi:10.1080/13697137.2021.1950967.
Recently, de Villiers and Goldstein offered an overview of osteoporosis.5 What is worthy of reporting here is the role of muscle in bone health.
The bone-muscle relationship
Most clinicians know that osteoporosis and osteopenia are well-defined conditions with known risks associated with fracture. According to a review of PubMed, the first article with the keyword “osteoporosis” was published in 1894; through May 2020, 93,335 articles used that keyword. “Osteoporosis” is derived from the Greek osteon (bone) and poros (little hole). Thus, osteoporosis means “porous bone.”
Sarcopenia is characterized by progressive and generalized loss of skeletal muscle mass, strength, and function, and the condition is associated with a risk of adverse outcomes that include physical disabilities, poor quality of life, and death.6,7 “Sarcopenia” has its roots in the Greek words sarx (flesh) and penia (loss), and the term was coined in 1989.8 A PubMed review that included “sarcopenia” as the keyword revealed that the first article was published in 1993, with 12,068 articles published through May 2020.
Notably, muscle accounts for about 60% of the body’s protein. Muscle mass decreases with age, but younger patients with malnutrition, cachexia, or inflammatory diseases are also prone to decreased muscle mass. While osteoporosis has a well-accepted definition based on dual-energy x-ray absorptiometry (DXA) measurements, sarcopenia has no universally accepted definition, consensus diagnostic criteria, or treatment guidelines. In 2016, however, the International Classification of Diseases, Tenth Revision, Clinical Modification (CD-10-CM) finally recognized sarcopenia as a disease entity.
Currently, the most widely accepted definition comes from the European Working Group on Sarcopenia in Older People, which labeled presarcopenia as low muscle mass without impact on muscle strength or performance; sarcopenia as low muscle mass with either low muscle strength or low physical performance; and severe sarcopenia has all 3 criteria being present.9
When osteosarcopenia (osteoporosis or osteopenia combined with sarcopenia) exists, it can result in a threefold increase in risk of falls and a fourfold increase in fracture risk compared with women who have osteopenia or osteoporosis alone.10
The morbidity and mortality from fragility fractures are well known. Initially, diagnosis of risk seemed to be mainly T-scores on bone mineral density (BMD) testing (normal, osteopenic, osteoporosis). The FRAX fracture risk assessment tool, which includes a number of variables, further refined risk assessment. Increasingly, there is evidence of crosstalk between muscle and bone. Sarcopenia, the loss of skeletal muscle mass, strength, and performance, appears to play an important role as well for fracture risk. Simple tools to evaluate a patient’s muscle status exist. At the very least, resistance and balance exercises should be part of all clinicians’ patient counseling for bone health.
Continue to: Denosumab decreased falls risk, improved sarcopenia measures vs comparator antiresorptives...
Denosumab decreased falls risk, improved sarcopenia measures vs comparator antiresorptives
El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232. doi: 10.1007/s10067-021 -05757-w.
Osteosarcopenia, the combination of osteoporosis or osteopenia with sarcopenia, has been shown to increase the overall rate of falls and fracture when compared with fall and fracture rates in women with osteopenia or osteoporosis alone.10 A study by El Miedany and colleagues examined whether denosumab treatment had a possible dual therapeutic effect on osteoporosis and sarcopenia.11
Study details
The investigators looked at 135 patients diagnosed with postmenopausal osteoporosis and who were prescribed denosumab and compared them with a control group of 272 patients stratified into 2 subgroups: 136 were prescribed alendronate and 136 were prescribed zoledronate.
Assessments were performed for all participants for BMD (DXA), fall risk (falls risk assessment score [FRAS]), fracture risk (FRAX assessment tool), and sarcopenia measures. Reassessments were conducted after 5 years of denosumab or alendronate therapy, 3 years of zoledronate therapy, and 1 year after stopping the osteoporosis therapy.
The FRAS uses the clinical variables of history of falls in the last 12 months, impaired sight, weak hand grip, history of loss of balance in the last 12 months, and slowing of the walking speed/change in gait to yield a percent chance of sustaining a fall.12 Sarcopenic measures include grip strength, timed up and go (TUG) mobility test, and gait speed. There were no significant demographic differences between the 3 groups.
Denosumab reduced risk of falls and positively affected muscle strength
On completion of the 5-year denosumab therapy, falls risk was significantly decreased (P = .001) and significant improvements were seen in all sarcopenia measures (P = .01). One year after denosumab was discontinued, a significant worsening of both falls risk and sarcopenia measures (P = .01) occurred. This was in contrast to results in both control groups (alendronate and zoledronate), in which there was an improvement, although less robust in gait speed and the TUG test (P = .05) but no improvement in risk of falls. Thus, the results of this study showed that denosumab not only improved bone mass but also reduced falls risk.
Compared with bisphosphonates, denosumab showed the highest significant positive effect on both physical performance and skeletal muscle strength. This is evidenced by improvement of the gait speed, TUG test, and 4-m walk test (P<.001) in the denosumab group versus in the alendronate and zoledronate group (P<.05).
These results agree with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis 6 months) trial, which revealed that not only did denosumab treatment reduce the risk of vertebral, nonvertebral, and hip fracture over 36 months, but also that the denosumab-treated group had fewer falls (4.5%) compared with the other groups (5.7%) (P = .02).13
These data highlight that osteoporosis and sarcopenia may share similar underlying risk factors and that muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. While all 3 antiresorptives (denosumab, alendronate, zoledronate) improved measures of BMD and sarcopenia, only denosumab resulted in a reduction in the FRAS risk of falls score.
Continue to: Estrogen’s role in bone health and its therapeutic potential in osteosarcopenia...
Estrogen’s role in bone health and its therapeutic potential in osteosarcopenia
Mandelli A, Tacconi E, Levinger I, et al. The role of estrogens in osteosarcopenia: from biology to potential dual therapeutic effects. Climacteric. 2021;1-7. doi: 10.1080/13697137.2021.1965118.
Osteosarcopenia is a particular term used to describe the coexistence of 2 pathologies, osteopenia/ osteoporosis and sarcopenia.14 Sarcopenia is characterized by a loss of muscle mass, strength, and performance. Numerous studies indicate that higher lean body mass is related to increased BMD and reduced fracture risk, especially in postmenopausal women.15
Menopause, muscle, and estrogen’s physiologic effects
Estrogens play a critical role in maintaining bone and muscle mass in women. Women experience a decline in musculoskeletal quantity and quality at the onset of menopause.16 Muscle mass and strength decrease rapidly after menopause, which suggests that degradation of muscle protein begins to exert a more significant effect due to a decrease in protein synthesis. Indeed, a reduced response to anabolic stimuli has been shown in postmenopausal women.17 Normalization of the protein synthesis response after restoring estrogen levels with estrogen therapy supports this hypothesis.18
In a meta-analysis to identify the role of estrogen therapy on muscle strength, the authors concluded that estrogens benefit muscle strength not by increasing the skeletal mass but by improving muscle quality and its ability to generate force.19 In addition, however, it has been demonstrated that exercise prevents and delays the onset of osteosarcopenia.20
Estrogens play a crucial role in maintaining bone and skeletal muscle health in women. Estrogen therapy is an accepted treatment for osteoporosis, whereas its effects on sarcopenia, although promising, indicate that additional studies are required before it can be recommended solely for that purpose. Given the well-described benefits of exercise on muscle and bone health, postmenopausal women should be encouraged to engage in regular physical exercise as a preventive or disease-modifying treatment for osteosarcopenia.
When should bone mass be measured in premenopausal women?
Conradie M, de Villiers T. Premenopausal osteoporosis. Climacteric. 2021:1-14. doi: 10.1080/13697137 .2021.1926974.
Most women’s clinicians are somewhat well acquainted with the increasing importance of preventing, diagnosing, and treating postmenopausal osteoporosis, which predisposes to fragility fracture and the morbidity and even mortality that brings. Increasingly, some younger women are asking for and receiving both bone mass measurements that may be inappropriately ordered and/or wrongly interpreted. Conradie and de Villiers provided an overview of premenopausal osteoporosis, containing important facts that all clinicians who care for women should be aware of.21
Indications for testing
BMD testing is only indicated in younger women in settings in which the result may influence management decisions, such as:
- a history of fragility fracture
- diseases associated with low bone mass, such as anorexia nervosa, hypogonadism, hyperparathyroidism, hyperthyroidism, celiac disease, irritable bowel disease, rheumatoid arthritis, lupus, renal disease, Marfan syndrome
- medications, such as glucocorticoids, aromatase inhibitors, premenopausal tamoxifen, excess thyroid hormone replacement, progesterone contraception
- excessive alcohol consumption, heavy smoking, vitamin D deficiency, calcium deficiency, occasionally veganism or vegetarianism.
BMD interpretation in premenopausal women does not use the T-scores developed for postmenopausal women in which standard deviations (SD) from the mean for a young reference population are employed. In that population, the normal range is up to -1.0 SD; osteopenia > -1.0 < -2.5 SD; and osteoporosis > -2.5 SD. Instead, in premenopausal patients, Z-scores, which compare the measured bone mass to an age- and gender-matched cohort, are employed. Z-scores > 2 SD below the matched population should be used rather than the T-scores that are already familiar to most clinicians.
Up to 90% of these premenopausal women with such skeletal fragility will display the secondary causes described above. ●
Very specific indications are required to consider bone mass measurements in premenopausal women. When measurements are indicated, the values are evaluated by Z-scores that compare them to those of matched-aged women and not by T-scores meant for postmenopausal women. When fragility or low-trauma fractures or Z-scores more than 2 SD below their peers are present, secondary causes of premenopausal osteoporosis include a variety of disease states, medications, and lifestyle situations. When such factors are present, many general women’s health clinicians may want to refer patients for consultation to a metabolic bone specialist for workup and management.
- Goldstein SR. Update on bone health. OBG Manag. 2020;32:16-20, 22-23.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, GA: American Cancer Society; 2020. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/annual-cancer-facts-and-figures/2020/cancer -facts-and-figures-2020.pdf. Accessed November 11, 2021.
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195-202.
- de Villiers, TJ, Goldstein SR. Update on bone health: the International Menopause Society white paper 2021. Climacteric. 2021;24:498-504.
- Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059-1064.
- Santilli V, Bernetti A, Mangone M, et al. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177-180.
- Rosenberg I. Epidemiological and methodological problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1989. Am J Clin Nutr. 1989;50:1231-1233.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis—report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423.
- Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21:220-225.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- El Miedany Y, El Gaafary M, Toth M, et al. Falls risk assessment score (FRAS): time to rethink. J Clin Gerontol Geriatr. 2011;21-26.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361: 756-765.
- Inoue T, Maeda K, Nagano A, et al. Related factors and clinical outcomes of osteosarcopenia: a narrative review. Nutrients. 2021;13:291.
- Kaji H. Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care. 2013;16:272-277.
- Sipilä S, Törmäkangas T, Sillanpää E, et al. Muscle and bone mass in middle‐aged women: role of menopausal status and physical activity. J Cachexia Sarcopenia Muscle. 2020;11: 698-709.
- Bamman MM, Hill VJ, Adams GR, et al. Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J Gerontol A Biol Sci Med Sci. 2003;58:108-116.
- Hansen M, Skovgaard D, Reitelseder S, et al. Effects of estrogen replacement and lower androgen status on skeletal muscle collagen and myofibrillar protein synthesis in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2012;67:1005-1013.
- Greising SM, Baltgalvis KA, Lowe DA, et al. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64:1071-1081.
- Cariati I, Bonanni R, Onorato F, et al. Role of physical activity in bone-muscle crosstalk: biological aspects and clinical implications. J Funct Morphol Kinesiol. 2021;6:55.
- Conradie M, de Villiers T. Premenopausal osteoporosis. Climacteric. 2021:1-14.
Recently, the National Osteoporosis Foundation (NOF) changed its name to the Bone Health and Osteoporosis Foundation (BHOF). Several years ago, in 2016 at my urging, this column was renamed from “Update on osteoporosis” to “Update on bone health.” I believe we were on the leading edge of this movement. As expressed in last year’s Update, our patients’ bone health must be emphasized more than it has been in the past.1
Consider that localized breast cancer carries a 5-year survival rate of 99%.2 Most of my patients are keenly aware that periodic competent breast imaging is the key to the earliest possible diagnosis. By contrast, in this country a hip fracture carries a mortality in the first year of 21%!3 Furthermore, approximately one-third of women who fracture their hip do not have osteoporosis.4 While the risk of hip fracture is greatest in women with osteoporosis, it is not absent in those without the condition. Finally, the role of muscle mass, strength, and performance in bone health is a rapidly emerging topic and one that constitutes the core of this year’s Update.
Muscle mass and strength play key role in bone health
de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society white paper 2021. Climacteric. 2021;24:498-504. doi:10.1080/13697137.2021.1950967.
Recently, de Villiers and Goldstein offered an overview of osteoporosis.5 What is worthy of reporting here is the role of muscle in bone health.
The bone-muscle relationship
Most clinicians know that osteoporosis and osteopenia are well-defined conditions with known risks associated with fracture. According to a review of PubMed, the first article with the keyword “osteoporosis” was published in 1894; through May 2020, 93,335 articles used that keyword. “Osteoporosis” is derived from the Greek osteon (bone) and poros (little hole). Thus, osteoporosis means “porous bone.”
Sarcopenia is characterized by progressive and generalized loss of skeletal muscle mass, strength, and function, and the condition is associated with a risk of adverse outcomes that include physical disabilities, poor quality of life, and death.6,7 “Sarcopenia” has its roots in the Greek words sarx (flesh) and penia (loss), and the term was coined in 1989.8 A PubMed review that included “sarcopenia” as the keyword revealed that the first article was published in 1993, with 12,068 articles published through May 2020.
Notably, muscle accounts for about 60% of the body’s protein. Muscle mass decreases with age, but younger patients with malnutrition, cachexia, or inflammatory diseases are also prone to decreased muscle mass. While osteoporosis has a well-accepted definition based on dual-energy x-ray absorptiometry (DXA) measurements, sarcopenia has no universally accepted definition, consensus diagnostic criteria, or treatment guidelines. In 2016, however, the International Classification of Diseases, Tenth Revision, Clinical Modification (CD-10-CM) finally recognized sarcopenia as a disease entity.
Currently, the most widely accepted definition comes from the European Working Group on Sarcopenia in Older People, which labeled presarcopenia as low muscle mass without impact on muscle strength or performance; sarcopenia as low muscle mass with either low muscle strength or low physical performance; and severe sarcopenia has all 3 criteria being present.9
When osteosarcopenia (osteoporosis or osteopenia combined with sarcopenia) exists, it can result in a threefold increase in risk of falls and a fourfold increase in fracture risk compared with women who have osteopenia or osteoporosis alone.10
The morbidity and mortality from fragility fractures are well known. Initially, diagnosis of risk seemed to be mainly T-scores on bone mineral density (BMD) testing (normal, osteopenic, osteoporosis). The FRAX fracture risk assessment tool, which includes a number of variables, further refined risk assessment. Increasingly, there is evidence of crosstalk between muscle and bone. Sarcopenia, the loss of skeletal muscle mass, strength, and performance, appears to play an important role as well for fracture risk. Simple tools to evaluate a patient’s muscle status exist. At the very least, resistance and balance exercises should be part of all clinicians’ patient counseling for bone health.
Continue to: Denosumab decreased falls risk, improved sarcopenia measures vs comparator antiresorptives...
Denosumab decreased falls risk, improved sarcopenia measures vs comparator antiresorptives
El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232. doi: 10.1007/s10067-021 -05757-w.
Osteosarcopenia, the combination of osteoporosis or osteopenia with sarcopenia, has been shown to increase the overall rate of falls and fracture when compared with fall and fracture rates in women with osteopenia or osteoporosis alone.10 A study by El Miedany and colleagues examined whether denosumab treatment had a possible dual therapeutic effect on osteoporosis and sarcopenia.11
Study details
The investigators looked at 135 patients diagnosed with postmenopausal osteoporosis and who were prescribed denosumab and compared them with a control group of 272 patients stratified into 2 subgroups: 136 were prescribed alendronate and 136 were prescribed zoledronate.
Assessments were performed for all participants for BMD (DXA), fall risk (falls risk assessment score [FRAS]), fracture risk (FRAX assessment tool), and sarcopenia measures. Reassessments were conducted after 5 years of denosumab or alendronate therapy, 3 years of zoledronate therapy, and 1 year after stopping the osteoporosis therapy.
The FRAS uses the clinical variables of history of falls in the last 12 months, impaired sight, weak hand grip, history of loss of balance in the last 12 months, and slowing of the walking speed/change in gait to yield a percent chance of sustaining a fall.12 Sarcopenic measures include grip strength, timed up and go (TUG) mobility test, and gait speed. There were no significant demographic differences between the 3 groups.
Denosumab reduced risk of falls and positively affected muscle strength
On completion of the 5-year denosumab therapy, falls risk was significantly decreased (P = .001) and significant improvements were seen in all sarcopenia measures (P = .01). One year after denosumab was discontinued, a significant worsening of both falls risk and sarcopenia measures (P = .01) occurred. This was in contrast to results in both control groups (alendronate and zoledronate), in which there was an improvement, although less robust in gait speed and the TUG test (P = .05) but no improvement in risk of falls. Thus, the results of this study showed that denosumab not only improved bone mass but also reduced falls risk.
Compared with bisphosphonates, denosumab showed the highest significant positive effect on both physical performance and skeletal muscle strength. This is evidenced by improvement of the gait speed, TUG test, and 4-m walk test (P<.001) in the denosumab group versus in the alendronate and zoledronate group (P<.05).
These results agree with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis 6 months) trial, which revealed that not only did denosumab treatment reduce the risk of vertebral, nonvertebral, and hip fracture over 36 months, but also that the denosumab-treated group had fewer falls (4.5%) compared with the other groups (5.7%) (P = .02).13
These data highlight that osteoporosis and sarcopenia may share similar underlying risk factors and that muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. While all 3 antiresorptives (denosumab, alendronate, zoledronate) improved measures of BMD and sarcopenia, only denosumab resulted in a reduction in the FRAS risk of falls score.
Continue to: Estrogen’s role in bone health and its therapeutic potential in osteosarcopenia...
Estrogen’s role in bone health and its therapeutic potential in osteosarcopenia
Mandelli A, Tacconi E, Levinger I, et al. The role of estrogens in osteosarcopenia: from biology to potential dual therapeutic effects. Climacteric. 2021;1-7. doi: 10.1080/13697137.2021.1965118.
Osteosarcopenia is a particular term used to describe the coexistence of 2 pathologies, osteopenia/ osteoporosis and sarcopenia.14 Sarcopenia is characterized by a loss of muscle mass, strength, and performance. Numerous studies indicate that higher lean body mass is related to increased BMD and reduced fracture risk, especially in postmenopausal women.15
Menopause, muscle, and estrogen’s physiologic effects
Estrogens play a critical role in maintaining bone and muscle mass in women. Women experience a decline in musculoskeletal quantity and quality at the onset of menopause.16 Muscle mass and strength decrease rapidly after menopause, which suggests that degradation of muscle protein begins to exert a more significant effect due to a decrease in protein synthesis. Indeed, a reduced response to anabolic stimuli has been shown in postmenopausal women.17 Normalization of the protein synthesis response after restoring estrogen levels with estrogen therapy supports this hypothesis.18
In a meta-analysis to identify the role of estrogen therapy on muscle strength, the authors concluded that estrogens benefit muscle strength not by increasing the skeletal mass but by improving muscle quality and its ability to generate force.19 In addition, however, it has been demonstrated that exercise prevents and delays the onset of osteosarcopenia.20
Estrogens play a crucial role in maintaining bone and skeletal muscle health in women. Estrogen therapy is an accepted treatment for osteoporosis, whereas its effects on sarcopenia, although promising, indicate that additional studies are required before it can be recommended solely for that purpose. Given the well-described benefits of exercise on muscle and bone health, postmenopausal women should be encouraged to engage in regular physical exercise as a preventive or disease-modifying treatment for osteosarcopenia.
When should bone mass be measured in premenopausal women?
Conradie M, de Villiers T. Premenopausal osteoporosis. Climacteric. 2021:1-14. doi: 10.1080/13697137 .2021.1926974.
Most women’s clinicians are somewhat well acquainted with the increasing importance of preventing, diagnosing, and treating postmenopausal osteoporosis, which predisposes to fragility fracture and the morbidity and even mortality that brings. Increasingly, some younger women are asking for and receiving both bone mass measurements that may be inappropriately ordered and/or wrongly interpreted. Conradie and de Villiers provided an overview of premenopausal osteoporosis, containing important facts that all clinicians who care for women should be aware of.21
Indications for testing
BMD testing is only indicated in younger women in settings in which the result may influence management decisions, such as:
- a history of fragility fracture
- diseases associated with low bone mass, such as anorexia nervosa, hypogonadism, hyperparathyroidism, hyperthyroidism, celiac disease, irritable bowel disease, rheumatoid arthritis, lupus, renal disease, Marfan syndrome
- medications, such as glucocorticoids, aromatase inhibitors, premenopausal tamoxifen, excess thyroid hormone replacement, progesterone contraception
- excessive alcohol consumption, heavy smoking, vitamin D deficiency, calcium deficiency, occasionally veganism or vegetarianism.
BMD interpretation in premenopausal women does not use the T-scores developed for postmenopausal women in which standard deviations (SD) from the mean for a young reference population are employed. In that population, the normal range is up to -1.0 SD; osteopenia > -1.0 < -2.5 SD; and osteoporosis > -2.5 SD. Instead, in premenopausal patients, Z-scores, which compare the measured bone mass to an age- and gender-matched cohort, are employed. Z-scores > 2 SD below the matched population should be used rather than the T-scores that are already familiar to most clinicians.
Up to 90% of these premenopausal women with such skeletal fragility will display the secondary causes described above. ●
Very specific indications are required to consider bone mass measurements in premenopausal women. When measurements are indicated, the values are evaluated by Z-scores that compare them to those of matched-aged women and not by T-scores meant for postmenopausal women. When fragility or low-trauma fractures or Z-scores more than 2 SD below their peers are present, secondary causes of premenopausal osteoporosis include a variety of disease states, medications, and lifestyle situations. When such factors are present, many general women’s health clinicians may want to refer patients for consultation to a metabolic bone specialist for workup and management.
Recently, the National Osteoporosis Foundation (NOF) changed its name to the Bone Health and Osteoporosis Foundation (BHOF). Several years ago, in 2016 at my urging, this column was renamed from “Update on osteoporosis” to “Update on bone health.” I believe we were on the leading edge of this movement. As expressed in last year’s Update, our patients’ bone health must be emphasized more than it has been in the past.1
Consider that localized breast cancer carries a 5-year survival rate of 99%.2 Most of my patients are keenly aware that periodic competent breast imaging is the key to the earliest possible diagnosis. By contrast, in this country a hip fracture carries a mortality in the first year of 21%!3 Furthermore, approximately one-third of women who fracture their hip do not have osteoporosis.4 While the risk of hip fracture is greatest in women with osteoporosis, it is not absent in those without the condition. Finally, the role of muscle mass, strength, and performance in bone health is a rapidly emerging topic and one that constitutes the core of this year’s Update.
Muscle mass and strength play key role in bone health
de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society white paper 2021. Climacteric. 2021;24:498-504. doi:10.1080/13697137.2021.1950967.
Recently, de Villiers and Goldstein offered an overview of osteoporosis.5 What is worthy of reporting here is the role of muscle in bone health.
The bone-muscle relationship
Most clinicians know that osteoporosis and osteopenia are well-defined conditions with known risks associated with fracture. According to a review of PubMed, the first article with the keyword “osteoporosis” was published in 1894; through May 2020, 93,335 articles used that keyword. “Osteoporosis” is derived from the Greek osteon (bone) and poros (little hole). Thus, osteoporosis means “porous bone.”
Sarcopenia is characterized by progressive and generalized loss of skeletal muscle mass, strength, and function, and the condition is associated with a risk of adverse outcomes that include physical disabilities, poor quality of life, and death.6,7 “Sarcopenia” has its roots in the Greek words sarx (flesh) and penia (loss), and the term was coined in 1989.8 A PubMed review that included “sarcopenia” as the keyword revealed that the first article was published in 1993, with 12,068 articles published through May 2020.
Notably, muscle accounts for about 60% of the body’s protein. Muscle mass decreases with age, but younger patients with malnutrition, cachexia, or inflammatory diseases are also prone to decreased muscle mass. While osteoporosis has a well-accepted definition based on dual-energy x-ray absorptiometry (DXA) measurements, sarcopenia has no universally accepted definition, consensus diagnostic criteria, or treatment guidelines. In 2016, however, the International Classification of Diseases, Tenth Revision, Clinical Modification (CD-10-CM) finally recognized sarcopenia as a disease entity.
Currently, the most widely accepted definition comes from the European Working Group on Sarcopenia in Older People, which labeled presarcopenia as low muscle mass without impact on muscle strength or performance; sarcopenia as low muscle mass with either low muscle strength or low physical performance; and severe sarcopenia has all 3 criteria being present.9
When osteosarcopenia (osteoporosis or osteopenia combined with sarcopenia) exists, it can result in a threefold increase in risk of falls and a fourfold increase in fracture risk compared with women who have osteopenia or osteoporosis alone.10
The morbidity and mortality from fragility fractures are well known. Initially, diagnosis of risk seemed to be mainly T-scores on bone mineral density (BMD) testing (normal, osteopenic, osteoporosis). The FRAX fracture risk assessment tool, which includes a number of variables, further refined risk assessment. Increasingly, there is evidence of crosstalk between muscle and bone. Sarcopenia, the loss of skeletal muscle mass, strength, and performance, appears to play an important role as well for fracture risk. Simple tools to evaluate a patient’s muscle status exist. At the very least, resistance and balance exercises should be part of all clinicians’ patient counseling for bone health.
Continue to: Denosumab decreased falls risk, improved sarcopenia measures vs comparator antiresorptives...
Denosumab decreased falls risk, improved sarcopenia measures vs comparator antiresorptives
El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232. doi: 10.1007/s10067-021 -05757-w.
Osteosarcopenia, the combination of osteoporosis or osteopenia with sarcopenia, has been shown to increase the overall rate of falls and fracture when compared with fall and fracture rates in women with osteopenia or osteoporosis alone.10 A study by El Miedany and colleagues examined whether denosumab treatment had a possible dual therapeutic effect on osteoporosis and sarcopenia.11
Study details
The investigators looked at 135 patients diagnosed with postmenopausal osteoporosis and who were prescribed denosumab and compared them with a control group of 272 patients stratified into 2 subgroups: 136 were prescribed alendronate and 136 were prescribed zoledronate.
Assessments were performed for all participants for BMD (DXA), fall risk (falls risk assessment score [FRAS]), fracture risk (FRAX assessment tool), and sarcopenia measures. Reassessments were conducted after 5 years of denosumab or alendronate therapy, 3 years of zoledronate therapy, and 1 year after stopping the osteoporosis therapy.
The FRAS uses the clinical variables of history of falls in the last 12 months, impaired sight, weak hand grip, history of loss of balance in the last 12 months, and slowing of the walking speed/change in gait to yield a percent chance of sustaining a fall.12 Sarcopenic measures include grip strength, timed up and go (TUG) mobility test, and gait speed. There were no significant demographic differences between the 3 groups.
Denosumab reduced risk of falls and positively affected muscle strength
On completion of the 5-year denosumab therapy, falls risk was significantly decreased (P = .001) and significant improvements were seen in all sarcopenia measures (P = .01). One year after denosumab was discontinued, a significant worsening of both falls risk and sarcopenia measures (P = .01) occurred. This was in contrast to results in both control groups (alendronate and zoledronate), in which there was an improvement, although less robust in gait speed and the TUG test (P = .05) but no improvement in risk of falls. Thus, the results of this study showed that denosumab not only improved bone mass but also reduced falls risk.
Compared with bisphosphonates, denosumab showed the highest significant positive effect on both physical performance and skeletal muscle strength. This is evidenced by improvement of the gait speed, TUG test, and 4-m walk test (P<.001) in the denosumab group versus in the alendronate and zoledronate group (P<.05).
These results agree with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis 6 months) trial, which revealed that not only did denosumab treatment reduce the risk of vertebral, nonvertebral, and hip fracture over 36 months, but also that the denosumab-treated group had fewer falls (4.5%) compared with the other groups (5.7%) (P = .02).13
These data highlight that osteoporosis and sarcopenia may share similar underlying risk factors and that muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. While all 3 antiresorptives (denosumab, alendronate, zoledronate) improved measures of BMD and sarcopenia, only denosumab resulted in a reduction in the FRAS risk of falls score.
Continue to: Estrogen’s role in bone health and its therapeutic potential in osteosarcopenia...
Estrogen’s role in bone health and its therapeutic potential in osteosarcopenia
Mandelli A, Tacconi E, Levinger I, et al. The role of estrogens in osteosarcopenia: from biology to potential dual therapeutic effects. Climacteric. 2021;1-7. doi: 10.1080/13697137.2021.1965118.
Osteosarcopenia is a particular term used to describe the coexistence of 2 pathologies, osteopenia/ osteoporosis and sarcopenia.14 Sarcopenia is characterized by a loss of muscle mass, strength, and performance. Numerous studies indicate that higher lean body mass is related to increased BMD and reduced fracture risk, especially in postmenopausal women.15
Menopause, muscle, and estrogen’s physiologic effects
Estrogens play a critical role in maintaining bone and muscle mass in women. Women experience a decline in musculoskeletal quantity and quality at the onset of menopause.16 Muscle mass and strength decrease rapidly after menopause, which suggests that degradation of muscle protein begins to exert a more significant effect due to a decrease in protein synthesis. Indeed, a reduced response to anabolic stimuli has been shown in postmenopausal women.17 Normalization of the protein synthesis response after restoring estrogen levels with estrogen therapy supports this hypothesis.18
In a meta-analysis to identify the role of estrogen therapy on muscle strength, the authors concluded that estrogens benefit muscle strength not by increasing the skeletal mass but by improving muscle quality and its ability to generate force.19 In addition, however, it has been demonstrated that exercise prevents and delays the onset of osteosarcopenia.20
Estrogens play a crucial role in maintaining bone and skeletal muscle health in women. Estrogen therapy is an accepted treatment for osteoporosis, whereas its effects on sarcopenia, although promising, indicate that additional studies are required before it can be recommended solely for that purpose. Given the well-described benefits of exercise on muscle and bone health, postmenopausal women should be encouraged to engage in regular physical exercise as a preventive or disease-modifying treatment for osteosarcopenia.
When should bone mass be measured in premenopausal women?
Conradie M, de Villiers T. Premenopausal osteoporosis. Climacteric. 2021:1-14. doi: 10.1080/13697137 .2021.1926974.
Most women’s clinicians are somewhat well acquainted with the increasing importance of preventing, diagnosing, and treating postmenopausal osteoporosis, which predisposes to fragility fracture and the morbidity and even mortality that brings. Increasingly, some younger women are asking for and receiving both bone mass measurements that may be inappropriately ordered and/or wrongly interpreted. Conradie and de Villiers provided an overview of premenopausal osteoporosis, containing important facts that all clinicians who care for women should be aware of.21
Indications for testing
BMD testing is only indicated in younger women in settings in which the result may influence management decisions, such as:
- a history of fragility fracture
- diseases associated with low bone mass, such as anorexia nervosa, hypogonadism, hyperparathyroidism, hyperthyroidism, celiac disease, irritable bowel disease, rheumatoid arthritis, lupus, renal disease, Marfan syndrome
- medications, such as glucocorticoids, aromatase inhibitors, premenopausal tamoxifen, excess thyroid hormone replacement, progesterone contraception
- excessive alcohol consumption, heavy smoking, vitamin D deficiency, calcium deficiency, occasionally veganism or vegetarianism.
BMD interpretation in premenopausal women does not use the T-scores developed for postmenopausal women in which standard deviations (SD) from the mean for a young reference population are employed. In that population, the normal range is up to -1.0 SD; osteopenia > -1.0 < -2.5 SD; and osteoporosis > -2.5 SD. Instead, in premenopausal patients, Z-scores, which compare the measured bone mass to an age- and gender-matched cohort, are employed. Z-scores > 2 SD below the matched population should be used rather than the T-scores that are already familiar to most clinicians.
Up to 90% of these premenopausal women with such skeletal fragility will display the secondary causes described above. ●
Very specific indications are required to consider bone mass measurements in premenopausal women. When measurements are indicated, the values are evaluated by Z-scores that compare them to those of matched-aged women and not by T-scores meant for postmenopausal women. When fragility or low-trauma fractures or Z-scores more than 2 SD below their peers are present, secondary causes of premenopausal osteoporosis include a variety of disease states, medications, and lifestyle situations. When such factors are present, many general women’s health clinicians may want to refer patients for consultation to a metabolic bone specialist for workup and management.
- Goldstein SR. Update on bone health. OBG Manag. 2020;32:16-20, 22-23.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, GA: American Cancer Society; 2020. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/annual-cancer-facts-and-figures/2020/cancer -facts-and-figures-2020.pdf. Accessed November 11, 2021.
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195-202.
- de Villiers, TJ, Goldstein SR. Update on bone health: the International Menopause Society white paper 2021. Climacteric. 2021;24:498-504.
- Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059-1064.
- Santilli V, Bernetti A, Mangone M, et al. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177-180.
- Rosenberg I. Epidemiological and methodological problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1989. Am J Clin Nutr. 1989;50:1231-1233.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis—report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423.
- Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21:220-225.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- El Miedany Y, El Gaafary M, Toth M, et al. Falls risk assessment score (FRAS): time to rethink. J Clin Gerontol Geriatr. 2011;21-26.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361: 756-765.
- Inoue T, Maeda K, Nagano A, et al. Related factors and clinical outcomes of osteosarcopenia: a narrative review. Nutrients. 2021;13:291.
- Kaji H. Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care. 2013;16:272-277.
- Sipilä S, Törmäkangas T, Sillanpää E, et al. Muscle and bone mass in middle‐aged women: role of menopausal status and physical activity. J Cachexia Sarcopenia Muscle. 2020;11: 698-709.
- Bamman MM, Hill VJ, Adams GR, et al. Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J Gerontol A Biol Sci Med Sci. 2003;58:108-116.
- Hansen M, Skovgaard D, Reitelseder S, et al. Effects of estrogen replacement and lower androgen status on skeletal muscle collagen and myofibrillar protein synthesis in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2012;67:1005-1013.
- Greising SM, Baltgalvis KA, Lowe DA, et al. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64:1071-1081.
- Cariati I, Bonanni R, Onorato F, et al. Role of physical activity in bone-muscle crosstalk: biological aspects and clinical implications. J Funct Morphol Kinesiol. 2021;6:55.
- Conradie M, de Villiers T. Premenopausal osteoporosis. Climacteric. 2021:1-14.
- Goldstein SR. Update on bone health. OBG Manag. 2020;32:16-20, 22-23.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, GA: American Cancer Society; 2020. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/annual-cancer-facts-and-figures/2020/cancer -facts-and-figures-2020.pdf. Accessed November 11, 2021.
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195-202.
- de Villiers, TJ, Goldstein SR. Update on bone health: the International Menopause Society white paper 2021. Climacteric. 2021;24:498-504.
- Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059-1064.
- Santilli V, Bernetti A, Mangone M, et al. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177-180.
- Rosenberg I. Epidemiological and methodological problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1989. Am J Clin Nutr. 1989;50:1231-1233.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis—report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423.
- Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21:220-225.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- El Miedany Y, El Gaafary M, Toth M, et al. Falls risk assessment score (FRAS): time to rethink. J Clin Gerontol Geriatr. 2011;21-26.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361: 756-765.
- Inoue T, Maeda K, Nagano A, et al. Related factors and clinical outcomes of osteosarcopenia: a narrative review. Nutrients. 2021;13:291.
- Kaji H. Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care. 2013;16:272-277.
- Sipilä S, Törmäkangas T, Sillanpää E, et al. Muscle and bone mass in middle‐aged women: role of menopausal status and physical activity. J Cachexia Sarcopenia Muscle. 2020;11: 698-709.
- Bamman MM, Hill VJ, Adams GR, et al. Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J Gerontol A Biol Sci Med Sci. 2003;58:108-116.
- Hansen M, Skovgaard D, Reitelseder S, et al. Effects of estrogen replacement and lower androgen status on skeletal muscle collagen and myofibrillar protein synthesis in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2012;67:1005-1013.
- Greising SM, Baltgalvis KA, Lowe DA, et al. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64:1071-1081.
- Cariati I, Bonanni R, Onorato F, et al. Role of physical activity in bone-muscle crosstalk: biological aspects and clinical implications. J Funct Morphol Kinesiol. 2021;6:55.
- Conradie M, de Villiers T. Premenopausal osteoporosis. Climacteric. 2021:1-14.
Cancer risk-reducing strategies: Focus on chemoprevention
In her presentation at The North American Menopause Society (NAMS) 2021 annual meeting (September 22–25, 2021, in Washington, DC), Dr. Holly J. Pederson offered her expert perspectives on breast cancer prevention in at-risk women in “Chemoprevention for risk reduction: Women’s health clinicians have a role.”
Which patients would benefit from chemoprevention?
Holly J. Pederson, MD: Obviously, women with significant family history are at risk. And approximately 10% of biopsies that are done for other reasons incidentally show atypical hyperplasia (AH) or lobular carcinoma in situ (LCIS)—which are not precancers or cancers but are markers for the development of the disease—and they markedly increase risk. Atypical hyperplasia confers a 30% risk for developing breast cancer over the next 25 years, and LCIS is associated with up to a 2% per year risk. In this setting, preventive medication has been shown to cut risk by 56% to 86%; this is a targeted population that is often overlooked.
Mathematical risk models can be used to assess risk by assessing women’s risk factors. The United States Preventive Services Task Force (USPSTF) has set forth a threshold at which they believe the benefits outweigh the risks of preventive medications. That threshold is 3% or greater over the next 5 years using the Gail breast cancer risk assessment tool.1 The American Society of Clinical Oncology (ASCO) uses the Tyrer-Cuzick breast cancer risk evaluation model with a threshold of 5% over the next 10 years.2 In general, those are the situations in which chemoprevention is a no-brainer.
Certain genetic mutations also predispose to estrogen-sensitive breast cancer. While preventive medications specifically have not been studied in large groups of gene carriers, chemoprevention makes sense because these medications prevent estrogen-sensitive breast cancers that those patients are prone to. Examples would be patients with ATM and CHEK2 gene mutations, which are very common, and patients with BRCA2 and even BRCA1 variants in the postmenopausal years. Those are the big targets.
Risk assessment models
Dr. Pederson: Yes, I almost exclusively use the Tyrer-Cuzick risk model, version 8, which incorporates breast density. This model is intimidating to some practitioners initially, but once you get used to it, you can complete it very quickly.
The Gail model is very limited. It assesses only first-degree relatives, so you don’t get the paternal information at all, and you don’t use age at diagnosis, family structure, genetic testing, results of breast density, or body mass index (BMI). There are many limitations of the Gail model, but most people use it because it is so easy and they are familiar with it.
Possibly the best model is the CanRisk tool, which incorporates the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA), but it takes too much time to use in clinic; it’s too complicated. The Tyrer-Cuzick model is easy to use once you get used to it.
Dr. Pederson: Risk doesn’t always need to be formally calculated, which can be time-consuming. It’s one of those situations where most practitioners know it when they see it. Benign atypical biopsies, a strong family history, or, obviously, the presence of a genetic mutation are huge red flags.
If a practitioner has a nearby high-risk center where they can refer patients, that can be so useful, even for a one-time consultation to guide management. For example, with the virtual world now, I do a lot of consultations for patients and outline a plan, and then the referring practitioner can carry out the plan with confidence and then send the patient back periodically. There are so many more options now that previously did not exist for the busy ObGyn or primary care provider to rely on.
Continue to: Chemoprevention uptake in at-risk women...
Chemoprevention uptake in at-risk women
Dr. Pederson: We really never practice medicine using numbers. We use clinical judgment, and we use relationships with patients in terms of developing confidence and trust. I think that the uptake that we exhibit in our center probably is more based on the patients’ perception that we are confident in our recommendations. I think that many practitioners simply are not comfortable with explaining medications, explaining and managing adverse effects, and using alternative medications. While the modeling helps, I think the personal expertise really makes the difference.
Going forward, the addition of the polygenic risk score to the mathematical risk models is going to make a big difference. Right now, the mathematical risk model is simply that: it takes the traditional risk factors that a patient has and spits out a number. But adding the patient’s genomic data—that is, a weighted summation of SNPs, or single nucleotide polymorphisms, now numbering over 300 for breast cancer—can explain more about their personalized risk, which is going to be more powerful in influencing a woman to take medication or not to take medication, in my opinion. Knowing their actual genomic risk will be a big step forward in individualized risk stratification and increased medication uptake as well as vigilance with high risk screening and attention to diet, exercise, and drinking alcohol in moderation.
Dr. Pederson: The only drug that can be used in the premenopausal setting is tamoxifen (TABLE 1). Women can’t take it if they are pregnant, planning to become pregnant, or if they don’t use a reliable form of birth control because it is teratogenic. Women also cannot take tamoxifen if they have had a history of blood clots, stroke, or transient ischemic attack; if they are on warfarin or estrogen preparations; or if they have had atypical endometrial biopsies or endometrial cancer. Those are the absolute contraindications for tamoxifen use.
Tamoxifen is generally very well tolerated in most women; some women experience hot flashes and night sweats that often will subside (or become tolerable) over the first 90 days. In addition, some women experience vaginal discharge rather than dryness, but it is not as bothersome to patients as dryness can be.
Tamoxifen can be used in the pre- or postmenopausal setting. In healthy premenopausal women, there’s no increased risk of the serious adverse effects that are seen with tamoxifen use in postmenopausal women, such as the 1% risk of blood clots and the 1% risk of endometrial cancer.
In postmenopausal women who still have their uterus, I’ll preferentially use raloxifene over tamoxifen. If they don’t have their uterus, tamoxifen is slightly more effective than the raloxifene, and I’ll use that.
Tamoxifen and raloxifene are both selective estrogen receptor modulators, or SERMs, which means that they stimulate receptors in some tissues, like bone, keeping bones strong, and block the receptors in other tissues, like the breast, reducing risk. And so you get kind of a two-for-one in terms of breast cancer risk reduction and osteoporosis prevention.
Another class of preventive drugs is the aromatase inhibitors (AIs). They block the enzyme aromatase, which converts androgens to estrogens peripherally; that is, the androgens that are produced primarily in the adrenal gland, but in part in postmenopausal ovaries.
In general, AIs are less well tolerated. There are generally more hot flashes and night sweats, and more vaginal dryness than with the SERMs. Anastrozole use is associated with arthralgias; and with exemestane use, there can be some hair loss (TABLE 2). Relative contraindications to SERMs become more important in the postmenopausal setting because of the increased frequency of both blood clots and uterine cancer in the postmenopausal years. I won’t give it to smokers. I won’t give tamoxifen to smokers in the premenopausal period either. With obese women, care must be taken because of the risk of blood clots with the SERMS, so then I’ll resort to the AIs. In the postmenopausal setting, you have to think a lot harder about the choices you use for preventive medication. Preferentially, I’ll use the SERMS if possible as they have fewer adverse effects.
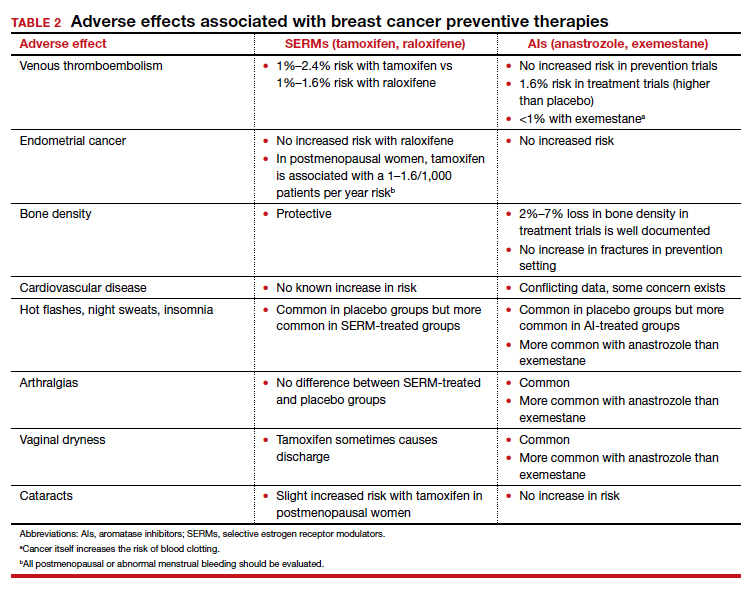
Dr. Pederson: All of them are recommended to be given for 5 years, but the MAP.3 trial, which studied exemestane compared with placebo, showed a 65% risk reduction with 3 years of therapy.3 So occasionally, we’ll use 3 years of therapy. Why the treatment recommendation is universally 5 years is unclear, given that the trial with that particular drug was done in 3 years. And with low-dose tamoxifen, the recommended duration is 3 years. That study was done in Italy with 5 mg daily for 3 years.4 In the United States we use 10 mg every other day for 3 years because the 5-mg tablet is not available here.
Continue to: Counseling points...
Counseling points
Dr. Pederson: Patients’ fears about adverse effects are often worse than the adverse effects themselves. Women will fester over, Should I take it? Should I take it possibly for years? And then they take the medication and they tell me, “I don’t even notice that I’m taking it, and I know I’m being proactive.” The majority of patients who take these medications don’t have a lot of significant adverse effects.
Severe hot flashes can be managed in a number of ways, primarily and most effectively with certain antidepressants. Oxybutynin use is another good way to manage vasomotor symptoms. Sometimes we use local vaginal estrogen if a patient has vaginal dryness. In general, however, I would say at least 80% of my patients who take preventive medications do not require management of adverse side effects, that they are tolerable.
I counsel women this way, “Don’t think of this as a 5-year course of medication. Think of it as a 90-day trial, and let’s see how you do. If you hate it, then we don’t do it.” They often are pleasantly surprised that the medication is much easier to tolerate than they thought it would be.
Dr. Pederson: It would be neat if a trial would directly compare lifestyle interventions with medications, because probably lifestyle change is as effective as medication is—but we don’t know that and probably will never have that data. We do know that alcohol consumption, every drink per day, increases risk by 10%. We know that obesity is responsible for 30% of breast cancers in this country, and that hormone replacement probably is overrated as a significant risk factor. Updated data from the Women’s Health Initiative study suggest that hormone replacement may actually reduce both breast cancer and cardiovascular risk in women in their 50s, but that’s in average-risk women and not in high-risk women, so we can’t generalize. We do recommend lifestyle measures including weight loss, exercise, and limiting alcohol consumption for all of our patients and certainly for our high-risk patients.
The only 2 things a woman can do to reduce the risk of triple negative breast cancer are to achieve and maintain ideal body weight and to breastfeed. The medications that I have mentioned don’t reduce the risk of triple negative breast cancer. Staying thin and breastfeeding do. It’s a problem in this country because at least 35% of all women and 58% of Black women are obese in America, and Black women tend to be prone to triple-negative breast cancer. That’s a real public health issue that we need to address. If we were going to focus on one thing, it would be focusing on obesity in terms of risk reduction.
Final thoughts
Dr. Pederson: I would like to direct attention to the American Heart Association scientific statement published at the end of 2020 that reported that hormone replacement in average-risk women reduced both cardiovascular events and overall mortality in women in their 50s by 30%.5 While that’s not directly related to what we are talking about, we need to weigh the pros and cons of estrogen versus estrogen blockade in women in terms of breast cancer risk management discussions. Part of shared decision making now needs to include cardiovascular risk factors and how estrogen is going to play into that.
In women with atypical hyperplasia or LCIS, they may benefit from the preventive medications we discussed. But in women with family history or in women with genetic mutations who have not had benign atypical biopsies, they may choose to consider estrogen during their 50s and perhaps take tamoxifen either beforehand or raloxifene afterward.
We need to look at patients holistically and consider all their risk factors together. We can’t look at one dimension alone.
- US Preventive Services Task Force. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867.
- Visvanathan K, Fabian CJ, Bantug E, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:3152-3165.
- Goss PE, Ingle JN, Alex-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391.
- DeCensi A, Puntoni M, Guerrieri-Gonzaga A, et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J Clin Oncol. 2019;37:1629-1637.
- El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention, and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532.
In her presentation at The North American Menopause Society (NAMS) 2021 annual meeting (September 22–25, 2021, in Washington, DC), Dr. Holly J. Pederson offered her expert perspectives on breast cancer prevention in at-risk women in “Chemoprevention for risk reduction: Women’s health clinicians have a role.”
Which patients would benefit from chemoprevention?
Holly J. Pederson, MD: Obviously, women with significant family history are at risk. And approximately 10% of biopsies that are done for other reasons incidentally show atypical hyperplasia (AH) or lobular carcinoma in situ (LCIS)—which are not precancers or cancers but are markers for the development of the disease—and they markedly increase risk. Atypical hyperplasia confers a 30% risk for developing breast cancer over the next 25 years, and LCIS is associated with up to a 2% per year risk. In this setting, preventive medication has been shown to cut risk by 56% to 86%; this is a targeted population that is often overlooked.
Mathematical risk models can be used to assess risk by assessing women’s risk factors. The United States Preventive Services Task Force (USPSTF) has set forth a threshold at which they believe the benefits outweigh the risks of preventive medications. That threshold is 3% or greater over the next 5 years using the Gail breast cancer risk assessment tool.1 The American Society of Clinical Oncology (ASCO) uses the Tyrer-Cuzick breast cancer risk evaluation model with a threshold of 5% over the next 10 years.2 In general, those are the situations in which chemoprevention is a no-brainer.
Certain genetic mutations also predispose to estrogen-sensitive breast cancer. While preventive medications specifically have not been studied in large groups of gene carriers, chemoprevention makes sense because these medications prevent estrogen-sensitive breast cancers that those patients are prone to. Examples would be patients with ATM and CHEK2 gene mutations, which are very common, and patients with BRCA2 and even BRCA1 variants in the postmenopausal years. Those are the big targets.
Risk assessment models
Dr. Pederson: Yes, I almost exclusively use the Tyrer-Cuzick risk model, version 8, which incorporates breast density. This model is intimidating to some practitioners initially, but once you get used to it, you can complete it very quickly.
The Gail model is very limited. It assesses only first-degree relatives, so you don’t get the paternal information at all, and you don’t use age at diagnosis, family structure, genetic testing, results of breast density, or body mass index (BMI). There are many limitations of the Gail model, but most people use it because it is so easy and they are familiar with it.
Possibly the best model is the CanRisk tool, which incorporates the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA), but it takes too much time to use in clinic; it’s too complicated. The Tyrer-Cuzick model is easy to use once you get used to it.
Dr. Pederson: Risk doesn’t always need to be formally calculated, which can be time-consuming. It’s one of those situations where most practitioners know it when they see it. Benign atypical biopsies, a strong family history, or, obviously, the presence of a genetic mutation are huge red flags.
If a practitioner has a nearby high-risk center where they can refer patients, that can be so useful, even for a one-time consultation to guide management. For example, with the virtual world now, I do a lot of consultations for patients and outline a plan, and then the referring practitioner can carry out the plan with confidence and then send the patient back periodically. There are so many more options now that previously did not exist for the busy ObGyn or primary care provider to rely on.
Continue to: Chemoprevention uptake in at-risk women...
Chemoprevention uptake in at-risk women
Dr. Pederson: We really never practice medicine using numbers. We use clinical judgment, and we use relationships with patients in terms of developing confidence and trust. I think that the uptake that we exhibit in our center probably is more based on the patients’ perception that we are confident in our recommendations. I think that many practitioners simply are not comfortable with explaining medications, explaining and managing adverse effects, and using alternative medications. While the modeling helps, I think the personal expertise really makes the difference.
Going forward, the addition of the polygenic risk score to the mathematical risk models is going to make a big difference. Right now, the mathematical risk model is simply that: it takes the traditional risk factors that a patient has and spits out a number. But adding the patient’s genomic data—that is, a weighted summation of SNPs, or single nucleotide polymorphisms, now numbering over 300 for breast cancer—can explain more about their personalized risk, which is going to be more powerful in influencing a woman to take medication or not to take medication, in my opinion. Knowing their actual genomic risk will be a big step forward in individualized risk stratification and increased medication uptake as well as vigilance with high risk screening and attention to diet, exercise, and drinking alcohol in moderation.
Dr. Pederson: The only drug that can be used in the premenopausal setting is tamoxifen (TABLE 1). Women can’t take it if they are pregnant, planning to become pregnant, or if they don’t use a reliable form of birth control because it is teratogenic. Women also cannot take tamoxifen if they have had a history of blood clots, stroke, or transient ischemic attack; if they are on warfarin or estrogen preparations; or if they have had atypical endometrial biopsies or endometrial cancer. Those are the absolute contraindications for tamoxifen use.
Tamoxifen is generally very well tolerated in most women; some women experience hot flashes and night sweats that often will subside (or become tolerable) over the first 90 days. In addition, some women experience vaginal discharge rather than dryness, but it is not as bothersome to patients as dryness can be.
Tamoxifen can be used in the pre- or postmenopausal setting. In healthy premenopausal women, there’s no increased risk of the serious adverse effects that are seen with tamoxifen use in postmenopausal women, such as the 1% risk of blood clots and the 1% risk of endometrial cancer.
In postmenopausal women who still have their uterus, I’ll preferentially use raloxifene over tamoxifen. If they don’t have their uterus, tamoxifen is slightly more effective than the raloxifene, and I’ll use that.
Tamoxifen and raloxifene are both selective estrogen receptor modulators, or SERMs, which means that they stimulate receptors in some tissues, like bone, keeping bones strong, and block the receptors in other tissues, like the breast, reducing risk. And so you get kind of a two-for-one in terms of breast cancer risk reduction and osteoporosis prevention.
Another class of preventive drugs is the aromatase inhibitors (AIs). They block the enzyme aromatase, which converts androgens to estrogens peripherally; that is, the androgens that are produced primarily in the adrenal gland, but in part in postmenopausal ovaries.
In general, AIs are less well tolerated. There are generally more hot flashes and night sweats, and more vaginal dryness than with the SERMs. Anastrozole use is associated with arthralgias; and with exemestane use, there can be some hair loss (TABLE 2). Relative contraindications to SERMs become more important in the postmenopausal setting because of the increased frequency of both blood clots and uterine cancer in the postmenopausal years. I won’t give it to smokers. I won’t give tamoxifen to smokers in the premenopausal period either. With obese women, care must be taken because of the risk of blood clots with the SERMS, so then I’ll resort to the AIs. In the postmenopausal setting, you have to think a lot harder about the choices you use for preventive medication. Preferentially, I’ll use the SERMS if possible as they have fewer adverse effects.

Dr. Pederson: All of them are recommended to be given for 5 years, but the MAP.3 trial, which studied exemestane compared with placebo, showed a 65% risk reduction with 3 years of therapy.3 So occasionally, we’ll use 3 years of therapy. Why the treatment recommendation is universally 5 years is unclear, given that the trial with that particular drug was done in 3 years. And with low-dose tamoxifen, the recommended duration is 3 years. That study was done in Italy with 5 mg daily for 3 years.4 In the United States we use 10 mg every other day for 3 years because the 5-mg tablet is not available here.
Continue to: Counseling points...
Counseling points
Dr. Pederson: Patients’ fears about adverse effects are often worse than the adverse effects themselves. Women will fester over, Should I take it? Should I take it possibly for years? And then they take the medication and they tell me, “I don’t even notice that I’m taking it, and I know I’m being proactive.” The majority of patients who take these medications don’t have a lot of significant adverse effects.
Severe hot flashes can be managed in a number of ways, primarily and most effectively with certain antidepressants. Oxybutynin use is another good way to manage vasomotor symptoms. Sometimes we use local vaginal estrogen if a patient has vaginal dryness. In general, however, I would say at least 80% of my patients who take preventive medications do not require management of adverse side effects, that they are tolerable.
I counsel women this way, “Don’t think of this as a 5-year course of medication. Think of it as a 90-day trial, and let’s see how you do. If you hate it, then we don’t do it.” They often are pleasantly surprised that the medication is much easier to tolerate than they thought it would be.
Dr. Pederson: It would be neat if a trial would directly compare lifestyle interventions with medications, because probably lifestyle change is as effective as medication is—but we don’t know that and probably will never have that data. We do know that alcohol consumption, every drink per day, increases risk by 10%. We know that obesity is responsible for 30% of breast cancers in this country, and that hormone replacement probably is overrated as a significant risk factor. Updated data from the Women’s Health Initiative study suggest that hormone replacement may actually reduce both breast cancer and cardiovascular risk in women in their 50s, but that’s in average-risk women and not in high-risk women, so we can’t generalize. We do recommend lifestyle measures including weight loss, exercise, and limiting alcohol consumption for all of our patients and certainly for our high-risk patients.
The only 2 things a woman can do to reduce the risk of triple negative breast cancer are to achieve and maintain ideal body weight and to breastfeed. The medications that I have mentioned don’t reduce the risk of triple negative breast cancer. Staying thin and breastfeeding do. It’s a problem in this country because at least 35% of all women and 58% of Black women are obese in America, and Black women tend to be prone to triple-negative breast cancer. That’s a real public health issue that we need to address. If we were going to focus on one thing, it would be focusing on obesity in terms of risk reduction.
Final thoughts
Dr. Pederson: I would like to direct attention to the American Heart Association scientific statement published at the end of 2020 that reported that hormone replacement in average-risk women reduced both cardiovascular events and overall mortality in women in their 50s by 30%.5 While that’s not directly related to what we are talking about, we need to weigh the pros and cons of estrogen versus estrogen blockade in women in terms of breast cancer risk management discussions. Part of shared decision making now needs to include cardiovascular risk factors and how estrogen is going to play into that.
In women with atypical hyperplasia or LCIS, they may benefit from the preventive medications we discussed. But in women with family history or in women with genetic mutations who have not had benign atypical biopsies, they may choose to consider estrogen during their 50s and perhaps take tamoxifen either beforehand or raloxifene afterward.
We need to look at patients holistically and consider all their risk factors together. We can’t look at one dimension alone.
In her presentation at The North American Menopause Society (NAMS) 2021 annual meeting (September 22–25, 2021, in Washington, DC), Dr. Holly J. Pederson offered her expert perspectives on breast cancer prevention in at-risk women in “Chemoprevention for risk reduction: Women’s health clinicians have a role.”
Which patients would benefit from chemoprevention?
Holly J. Pederson, MD: Obviously, women with significant family history are at risk. And approximately 10% of biopsies that are done for other reasons incidentally show atypical hyperplasia (AH) or lobular carcinoma in situ (LCIS)—which are not precancers or cancers but are markers for the development of the disease—and they markedly increase risk. Atypical hyperplasia confers a 30% risk for developing breast cancer over the next 25 years, and LCIS is associated with up to a 2% per year risk. In this setting, preventive medication has been shown to cut risk by 56% to 86%; this is a targeted population that is often overlooked.
Mathematical risk models can be used to assess risk by assessing women’s risk factors. The United States Preventive Services Task Force (USPSTF) has set forth a threshold at which they believe the benefits outweigh the risks of preventive medications. That threshold is 3% or greater over the next 5 years using the Gail breast cancer risk assessment tool.1 The American Society of Clinical Oncology (ASCO) uses the Tyrer-Cuzick breast cancer risk evaluation model with a threshold of 5% over the next 10 years.2 In general, those are the situations in which chemoprevention is a no-brainer.
Certain genetic mutations also predispose to estrogen-sensitive breast cancer. While preventive medications specifically have not been studied in large groups of gene carriers, chemoprevention makes sense because these medications prevent estrogen-sensitive breast cancers that those patients are prone to. Examples would be patients with ATM and CHEK2 gene mutations, which are very common, and patients with BRCA2 and even BRCA1 variants in the postmenopausal years. Those are the big targets.
Risk assessment models
Dr. Pederson: Yes, I almost exclusively use the Tyrer-Cuzick risk model, version 8, which incorporates breast density. This model is intimidating to some practitioners initially, but once you get used to it, you can complete it very quickly.
The Gail model is very limited. It assesses only first-degree relatives, so you don’t get the paternal information at all, and you don’t use age at diagnosis, family structure, genetic testing, results of breast density, or body mass index (BMI). There are many limitations of the Gail model, but most people use it because it is so easy and they are familiar with it.
Possibly the best model is the CanRisk tool, which incorporates the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA), but it takes too much time to use in clinic; it’s too complicated. The Tyrer-Cuzick model is easy to use once you get used to it.
Dr. Pederson: Risk doesn’t always need to be formally calculated, which can be time-consuming. It’s one of those situations where most practitioners know it when they see it. Benign atypical biopsies, a strong family history, or, obviously, the presence of a genetic mutation are huge red flags.
If a practitioner has a nearby high-risk center where they can refer patients, that can be so useful, even for a one-time consultation to guide management. For example, with the virtual world now, I do a lot of consultations for patients and outline a plan, and then the referring practitioner can carry out the plan with confidence and then send the patient back periodically. There are so many more options now that previously did not exist for the busy ObGyn or primary care provider to rely on.
Continue to: Chemoprevention uptake in at-risk women...
Chemoprevention uptake in at-risk women
Dr. Pederson: We really never practice medicine using numbers. We use clinical judgment, and we use relationships with patients in terms of developing confidence and trust. I think that the uptake that we exhibit in our center probably is more based on the patients’ perception that we are confident in our recommendations. I think that many practitioners simply are not comfortable with explaining medications, explaining and managing adverse effects, and using alternative medications. While the modeling helps, I think the personal expertise really makes the difference.
Going forward, the addition of the polygenic risk score to the mathematical risk models is going to make a big difference. Right now, the mathematical risk model is simply that: it takes the traditional risk factors that a patient has and spits out a number. But adding the patient’s genomic data—that is, a weighted summation of SNPs, or single nucleotide polymorphisms, now numbering over 300 for breast cancer—can explain more about their personalized risk, which is going to be more powerful in influencing a woman to take medication or not to take medication, in my opinion. Knowing their actual genomic risk will be a big step forward in individualized risk stratification and increased medication uptake as well as vigilance with high risk screening and attention to diet, exercise, and drinking alcohol in moderation.
Dr. Pederson: The only drug that can be used in the premenopausal setting is tamoxifen (TABLE 1). Women can’t take it if they are pregnant, planning to become pregnant, or if they don’t use a reliable form of birth control because it is teratogenic. Women also cannot take tamoxifen if they have had a history of blood clots, stroke, or transient ischemic attack; if they are on warfarin or estrogen preparations; or if they have had atypical endometrial biopsies or endometrial cancer. Those are the absolute contraindications for tamoxifen use.
Tamoxifen is generally very well tolerated in most women; some women experience hot flashes and night sweats that often will subside (or become tolerable) over the first 90 days. In addition, some women experience vaginal discharge rather than dryness, but it is not as bothersome to patients as dryness can be.
Tamoxifen can be used in the pre- or postmenopausal setting. In healthy premenopausal women, there’s no increased risk of the serious adverse effects that are seen with tamoxifen use in postmenopausal women, such as the 1% risk of blood clots and the 1% risk of endometrial cancer.
In postmenopausal women who still have their uterus, I’ll preferentially use raloxifene over tamoxifen. If they don’t have their uterus, tamoxifen is slightly more effective than the raloxifene, and I’ll use that.
Tamoxifen and raloxifene are both selective estrogen receptor modulators, or SERMs, which means that they stimulate receptors in some tissues, like bone, keeping bones strong, and block the receptors in other tissues, like the breast, reducing risk. And so you get kind of a two-for-one in terms of breast cancer risk reduction and osteoporosis prevention.
Another class of preventive drugs is the aromatase inhibitors (AIs). They block the enzyme aromatase, which converts androgens to estrogens peripherally; that is, the androgens that are produced primarily in the adrenal gland, but in part in postmenopausal ovaries.
In general, AIs are less well tolerated. There are generally more hot flashes and night sweats, and more vaginal dryness than with the SERMs. Anastrozole use is associated with arthralgias; and with exemestane use, there can be some hair loss (TABLE 2). Relative contraindications to SERMs become more important in the postmenopausal setting because of the increased frequency of both blood clots and uterine cancer in the postmenopausal years. I won’t give it to smokers. I won’t give tamoxifen to smokers in the premenopausal period either. With obese women, care must be taken because of the risk of blood clots with the SERMS, so then I’ll resort to the AIs. In the postmenopausal setting, you have to think a lot harder about the choices you use for preventive medication. Preferentially, I’ll use the SERMS if possible as they have fewer adverse effects.

Dr. Pederson: All of them are recommended to be given for 5 years, but the MAP.3 trial, which studied exemestane compared with placebo, showed a 65% risk reduction with 3 years of therapy.3 So occasionally, we’ll use 3 years of therapy. Why the treatment recommendation is universally 5 years is unclear, given that the trial with that particular drug was done in 3 years. And with low-dose tamoxifen, the recommended duration is 3 years. That study was done in Italy with 5 mg daily for 3 years.4 In the United States we use 10 mg every other day for 3 years because the 5-mg tablet is not available here.
Continue to: Counseling points...
Counseling points
Dr. Pederson: Patients’ fears about adverse effects are often worse than the adverse effects themselves. Women will fester over, Should I take it? Should I take it possibly for years? And then they take the medication and they tell me, “I don’t even notice that I’m taking it, and I know I’m being proactive.” The majority of patients who take these medications don’t have a lot of significant adverse effects.
Severe hot flashes can be managed in a number of ways, primarily and most effectively with certain antidepressants. Oxybutynin use is another good way to manage vasomotor symptoms. Sometimes we use local vaginal estrogen if a patient has vaginal dryness. In general, however, I would say at least 80% of my patients who take preventive medications do not require management of adverse side effects, that they are tolerable.
I counsel women this way, “Don’t think of this as a 5-year course of medication. Think of it as a 90-day trial, and let’s see how you do. If you hate it, then we don’t do it.” They often are pleasantly surprised that the medication is much easier to tolerate than they thought it would be.
Dr. Pederson: It would be neat if a trial would directly compare lifestyle interventions with medications, because probably lifestyle change is as effective as medication is—but we don’t know that and probably will never have that data. We do know that alcohol consumption, every drink per day, increases risk by 10%. We know that obesity is responsible for 30% of breast cancers in this country, and that hormone replacement probably is overrated as a significant risk factor. Updated data from the Women’s Health Initiative study suggest that hormone replacement may actually reduce both breast cancer and cardiovascular risk in women in their 50s, but that’s in average-risk women and not in high-risk women, so we can’t generalize. We do recommend lifestyle measures including weight loss, exercise, and limiting alcohol consumption for all of our patients and certainly for our high-risk patients.
The only 2 things a woman can do to reduce the risk of triple negative breast cancer are to achieve and maintain ideal body weight and to breastfeed. The medications that I have mentioned don’t reduce the risk of triple negative breast cancer. Staying thin and breastfeeding do. It’s a problem in this country because at least 35% of all women and 58% of Black women are obese in America, and Black women tend to be prone to triple-negative breast cancer. That’s a real public health issue that we need to address. If we were going to focus on one thing, it would be focusing on obesity in terms of risk reduction.
Final thoughts
Dr. Pederson: I would like to direct attention to the American Heart Association scientific statement published at the end of 2020 that reported that hormone replacement in average-risk women reduced both cardiovascular events and overall mortality in women in their 50s by 30%.5 While that’s not directly related to what we are talking about, we need to weigh the pros and cons of estrogen versus estrogen blockade in women in terms of breast cancer risk management discussions. Part of shared decision making now needs to include cardiovascular risk factors and how estrogen is going to play into that.
In women with atypical hyperplasia or LCIS, they may benefit from the preventive medications we discussed. But in women with family history or in women with genetic mutations who have not had benign atypical biopsies, they may choose to consider estrogen during their 50s and perhaps take tamoxifen either beforehand or raloxifene afterward.
We need to look at patients holistically and consider all their risk factors together. We can’t look at one dimension alone.
- US Preventive Services Task Force. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867.
- Visvanathan K, Fabian CJ, Bantug E, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:3152-3165.
- Goss PE, Ingle JN, Alex-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391.
- DeCensi A, Puntoni M, Guerrieri-Gonzaga A, et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J Clin Oncol. 2019;37:1629-1637.
- El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention, and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532.
- US Preventive Services Task Force. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867.
- Visvanathan K, Fabian CJ, Bantug E, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:3152-3165.
- Goss PE, Ingle JN, Alex-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391.
- DeCensi A, Puntoni M, Guerrieri-Gonzaga A, et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J Clin Oncol. 2019;37:1629-1637.
- El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention, and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532.
Cancer prevention through cascade genetic testing: A review of the current practice guidelines, barriers to testing and proposed solutions

CASE Woman with BRCA2 mutation
An 80-year-old woman presents for evaluation of newly diagnosed metastatic pancreatic adenocarcinoma. Her medical history is notable for breast cancer. Genetic testing of pancreatic tumor tissue detected a pathogenic variant in BRCA2. Family history revealed a history of melanoma as well as bladder, prostate, breast, and colon cancer. The patient subsequently underwent germline genetic testing with an 86-gene panel and a pathogenic mutation in BRCA2 was identified.
Watch a video of this patient and her clinician, Dr. Andrea Hagemann: https://www.youtube.com/watch?
Methods of genetic testing
It is estimated that 1 in 300 to 1 in 500 women in the United States carry a deleterious mutation in BRCA1 or BRCA2. This equates to between 250,000 and 415,000 women who are at high risk for breast and ovarian cancer.1 Looking at all women with cancer, 20% with ovarian,2 10% with breast,3 2% to 3% with endometrial,4 and 5% with colon cancer5 will have a germline mutation predisposing them to cancer. Identification of germline or somatic (tumor) mutations now inform treatment for patients with cancer. An equally important goal of germline genetic testing is cancer prevention. Cancer prevention strategies include risk-based screening for breast, colon, melanoma, and pancreatic cancer and prophylactic surgeries to reduce the risk of breast and ovarian cancer based on mutation type. Evidence-based screening guidelines by mutation type and absolute risk of associated cancers can be found on the National Comprehensive Cancer Network (NCCN).6,7
Multiple strategies have been proposed to identify patients for germline genetic testing. Patients can be identified based on a detailed multigenerational family history. This strategy requires clinicians or genetic counselors to take and update family histories, to recognize when a patient requires referral for testing, and for such testing to be completed. Even then the generation of a detailed pedigree is not very sensitive or specific. Population-based screening for high-penetrance breast and ovarian cancer susceptibility genes, regardless of family history, also has been proposed.8 Such a strategy has become increasingly realistic with decreasing cost and increasing availability of genetic testing. However, it would require increased genetic counseling resources to feasibly and equitably reach the target population and to explain the results to those patients and their relatives.
An alternative is to test the enriched population of family members of a patient with cancer who has been found to carry a pathogenic variant in a clinically relevant cancer susceptibility gene. This type of testing is termed cascade genetic testing. Cascade testing in first-degree family members carries a 50% probability of detecting the same pathogenic mutation. A related testing model is traceback testing where genetic testing is performed on pathology or tumor registry specimens from deceased patients with cancer.9 This genetic testing information is then provided to the family. Traceback models of genetic testing are an active area of research but can introduce ethical dilemmas. The more widely accepted cascade testing starts with the testing of a living patient affected with cancer. A recent article demonstrated the feasibility of a cascade testing model. Using a multiple linear regression model, the authors determined that all carriers of pathogenic mutations in 18 clinically relevant cancer susceptibility genes in the United States could be identified in 9.9 years if there was a 70% cascade testing rate of first-, second- and third-degree relatives, compared to 59.5 years with no cascade testing.10
Gaps in practice
Identification of mutation carriers, either through screening triggered by family history or through testing of patients affected with cancer, represents a gap between guidelines and clinical practice. Current NCCN guidelines outline genetic testing criteria for hereditary breast and ovarian cancer syndrome and for hereditary colorectal cancer. Despite well-established criteria, a survey in the United States revealed that only 19% of primary care providers were able to accurately assess family history for BRCA1 and 2 testing.11 Looking at patients who meet criteria for testing for Lynch syndrome, only 1 in 4 individuals have undergone genetic testing.12 Among patients diagnosed with breast and ovarian cancer, current NCCN guidelines recommend germline genetic testing for all patients with epithelial ovarian cancer; emerging evidence suggests all patients with breast cancer should be offered germline genetic testing.7,13 Large population-based studies have repeatedly demonstrated that testing rates fall short of this goal, with only 10% to 30% of patients undergoing genetic testing.9,14
Among families with a known hereditary mutation, rates of cascade genetic testing are also low, ranging from 17% to 50%.15-18 Evidence-based management guidelines, for both hereditary breast and ovarian cancer as well as Lynch syndrome, have been shown to reduce mortality.19,20 Failure to identify patients who carry these genetic mutations equates to increased mortality for our patients.
Barriers to cascade genetic testing
Cascade genetic testing ideally would be performed on entire families. Actual practice is far from ideal, and barriers to cascade testing exist. Barriers encompass resistance on the part of the family and provider as well as environmental or system factors.
Family factors
Because of privacy laws, the responsibility of disclosure of genetic testing results to family members falls primarily to the patient. Proband education is critical to ensure disclosure amongst family members. Family dynamics and geographic distribution of family members can further complicate disclosure. Following disclosure, family member gender, education, and demographics as well as personal views, attitudes, and emotions affect whether a family member decides to undergo testing.21 Furthermore, insurance status and awareness of and access to specialty-specific care for the proband’s family members may influence cascade genetic testing rates.
Provider factors
Provider factors that affect cascade genetic testing include awareness of testing guidelines, interpretation of genetic testing results, and education and knowledge of specific mutations. For instance, providers must recognize that cascade testing is not appropriate for variants of uncertain significance. This can lead to unnecessary surveillance testing and prophylactic surgeries. Providers, however, must continue to follow patients and periodically update testing results as variants may be reclassified over time. Additionally, providers must be knowledgeable about the complex and nuanced nature of the screening guidelines for each mutation. The NCCN provides detailed recommendations by mutation.7 Patients may benefit from care with cancer specialists who are aware of the guidelines, particularly for moderate-penetrance genes like BRIP1 and PALB2, as discussions about the timing of risk-reducing surgery are more nuanced in this population. Finally, which providers are responsible for facilitating cascade testing may be unclear; oncologists and genetic counselors not primarily treating probands’ relatives may assume the proper information has been passed along to family members without a practical means to follow up, and primary care providers may assume it is being taken care of by the oncology provider.
Continue to: Environmental or system factors...
Environmental or system factors
Accessibility of genetic counseling and testing is a common barrier to cascade testing. Family members may be geographically remote and connecting them to counseling and testing can be challenging. Working with local genetic counselors can facilitate this process. Insurance coverage of testing is a common perceived barrier; however, many testing companies now provide cascade testing free of charge if within a certain window from the initial test. Despite this, patients often site cost as a barrier to undergoing testing. Concerns about insurance coverage are common after a positive result. The Genetic Information Nondiscrimination Act of 2008 prohibits discrimination against employees or insurance applicants because of genetic information. Life insurance or long-term care policies, however, can incorporate genetic testing information into policy rates, so patients should be recommended to consider purchasing life insurance prior to undergoing genetic testing. This is especially important if the person considering testing has not yet been diagnosed with cancer.
Implications of a positive result
Family members who receive a positive test result should be referred for genetic counseling and to the appropriate specialists for evidence-based screening and discussion for risk-reducing surgery (FIGURE).7 For mutations associated with hereditary breast and ovarian cancer, referral to breast and gynecologic surgeons with expertise in risk reducing surgery is critical as the risk of diagnosing an occult malignancy is approximately 1%.22 Surgical technique with a 2-cm margin on the
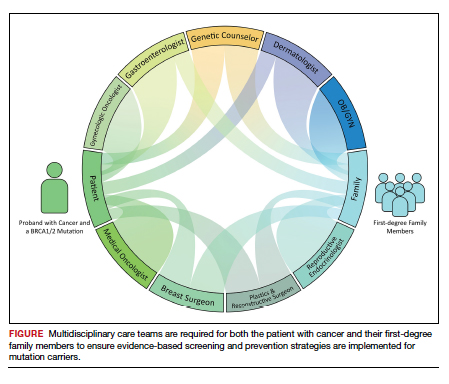
Patient resources: decision aids, websites
As genetic testing becomes more accessible and people are tested at younger ages, studies examining the balance of risk reduction and quality of life (QOL) are increasingly important. Fertility concerns, effects of early menopause, and the interrelatedness between decisions for breast and gynecologic risk reduction should all be considered in the counseling for surgical risk reduction. Patient decision aids can help mutation carriers navigate the complex information and decisions.25 Websites specifically designed by advocacy groups can be useful adjuncts to in-office counseling (Facing Our Risk Empowered, FORCE; Facingourrisk.org).
Family letters
The American College of Obstetricians and Gynecologists recommends an ObGyn have a letter or documentation stating that the patient’s relative has a specific mutation before initiating cascade testing for an at-risk family member. The indicated test (such as BRCA1) should be ordered only after the patient has been counseled about potential outcomes and has expressly decided to be tested.26 Letters, such as the example given in the American College of Obstetricians and Gynecologists practice bulletin,26 are a key component of communication between oncology providers, probands, family members, and their primary care providers. ObGyn providers should work together with genetic counselors and gynecologic oncologists to determine the most efficient strategies in their communities.
Technology
Access to genetic testing and genetic counseling has been improved with the rise in telemedicine. Geographically remote patients can now access genetic counseling through medical center–based counselors as well as company-provided genetic counseling over the phone. Patients also can submit samples remotely without needing to be tested in a doctor’s office.
Databases from cancer centers that detail cascade genetic testing rates. As the preventive impact of cascade genetic testing becomes clearer, strategies to have recurrent discussions with cancer patients regarding their family members’ risk should be implemented. It is still unclear which providers—genetic counselors, gynecologic oncologists, medical oncologists, breast surgeons, ObGyns, to name a few—are primarily responsible for remembering to have these follow-up discussions, and despite advances, the burden still rests on the cancer patient themselves. Databases with automated follow-up surveys done every 6 to 12 months could provide some aid to busy providers in this regard.
Emerging research
If gynecologic risk-reducing surgery is chosen, clinical trial involvement should be encouraged. The Women Choosing Surgical Prevention (NCT02760849) in the United States and the TUBA study (NCT02321228) in the Netherlands were designed to compare menopause-related QOL between standard risk-reducing salpingo-oophorectomy (RRSO) and the innovative risk-reducing salpingectomy with delayed oophorectomy for mutation carriers. Results from the nonrandomized controlled TUBA trial suggest that patients have better menopause-related QOL after risk-reducing salpingectomy than after RRSO, regardless of hormone replacement therapy.27 International collaboration is continuing to better understand oncologic safety. In the United States, the SOROCk trial (NCT04251052) is a noninferiority surgical choice study underway for BRCA1 mutation carriers aged 35 to 50, powered to determine oncologic outcome differences in addition to QOL outcomes between RRSO and delayed oophorectomy arms.
Returning to the case
The patient and her family underwent genetic counseling. The patient’s 2 daughters, each in their 50s, underwent cascade genetic testing and were found to carry the same pathogenic mutation in BRCA2. After counseling from both breast and gynecologic surgeons, they both elected to undergo risk reducing bilateral salpingo-oophorectomy with hysterectomy. Both now complete regular screening for breast cancer and melanoma with plans to start screening for pancreatic cancer. Both are currently cancer free.
Summary
Cascade genetic testing is an efficient strategy to identify mutation carriers for hereditary breast and ovarian cancer syndrome. Implementation of the best patient-centric care will require continued collaboration and communication across and within disciplines. ●
Cascade, or targeted, genetic testing within families known to carry a hereditary mutation in a cancer susceptibility gene should be performed on all living first-degree family members over the age of 18. All mutation carriers should be connected to a multidisciplinary care team (FIGURE) to ensure implementation of evidence-based screening and risk-reducing surgery for cancer prevention. If gynecologic risk-reducing surgery is chosen, clinical trial involvement should be encouraged.
- Gabai-Kapara E, Lahad A, Kaufman B, et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A. 2014;111:14205-14210.
- Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482-490.
- Yamauchi H, Takei J. Management of hereditary breast and ovarian cancer. Int J Clin Oncol. 2018;23:45-51.
- Kahn RM, Gordhandas S, Maddy BP, et al. Universal endometrial cancer tumor typing: how much has immunohistochemistry, microsatellite instability, and MLH1 methylation improved the diagnosis of Lynch syndrome across the population? Cancer. 2019;125:3172-3183.
- Jasperson KW, Tuohy TM, Neklason DW, et al. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
- Gupta S, Provenzale D, Llor X, et al. NCCN guidelines insights: genetic/familial high-risk assessment: colorectal, version 2.2019. J Natl Compr Canc Netw. 2019;17:1032-1041.
- Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:77-102.
- King MC, Levy-Lahad E, Lahad A. Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA. 2014;312:1091-1092.
- Samimi G, et al. Traceback: a proposed framework to increase identification and genetic counseling of BRCA1 and BRCA2 mutation carriers through family-based outreach. J Clin Oncol. 2017;35:2329-2337.
- Offit K, Tkachuk KA, Stadler ZK, et al. Cascading after peridiagnostic cancer genetic testing: an alternative to population-based screening. J Clin Oncol. 2020;38:1398-1408.
- Bellcross CA, Kolor K, Goddard KAB, et al. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 2011;40:61-66.
- Cross DS, Rahm AK, Kauffman TL, et al. Underutilization of Lynch syndrome screening in a multisite study of patients with colorectal cancer. Genet Med. 2013;15:933-940.
- Beitsch PD, Whitworth PW, Hughes K, et al. Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? J Clin Oncol. 2019;37:453-460.
- Childers CP, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800-3806.
- Samadder NJ, Riegert-Johnson D, Boardman L, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021;7:230-237.
- Sharaf RN, Myer P, Stave CD, et al. Uptake of genetic testing by relatives of Lynch syndrome probands: a systematic review. Clin Gastroenterol Hepatol. 2013;11:1093-1100.
- Menko FH, Ter Stege JA, van der Kolk LE, et al. The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and Lynch syndrome: a systematic review of the literature and implications for clinical practice. Fam Cancer. 2019;18:127-135.
- Griffin NE, Buchanan TR, Smith SH, et al. Low rates of cascade genetic testing among families with hereditary gynecologic cancer: an opportunity to improve cancer prevention. Gynecol Oncol. 2020;156:140-146.
- Roberts MC, Dotson WD, DeVore CS, et al. Delivery of cascade screening for hereditary conditions: a scoping review of the literature. Health Aff (Millwood). 2018;37:801-808.
- Finch AP, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Srinivasan S, Won NY, Dotson WD, et al. Barriers and facilitators for cascade testing in genetic conditions: a systematic review. Eur J Hum Genet. 2020;28:1631-1644.
- Piedimonte S, Frank C, Laprise C, et al. Occult tubal carcinoma after risk-reducing salpingo-oophorectomy: a systematic review. Obstet Gynecol. 2020;135:498-508.
- Shu CA, Pike MC, Jotwani AR, et al. Uterine cancer after risk-reducing salpingo-oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncol. 2016;2:1434-1440.
- Gordhandas S, Norquist BM, Pennington KP, et al. Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol Oncol. 2019;153:192-200.
- Steenbeek MP, van Bommel MHD, Harmsen MG, et al. Evaluation of a patient decision aid for BRCA1/2 pathogenic variant carriers choosing an ovarian cancer prevention strategy. Gynecol Oncol. 2021;163:371-377.
- Committee on Gynecologic Practice. ACOG committee opinion No. 727: Cascade testing: testing women for known hereditary genetic mutations associated with cancer. Obstet Gynecol. 2018;131:E31-E34.
- Steenbeek MP, Harmsen MG, Hoogerbrugge N, et al. Association of salpingectomy with delayed oophorectomy versus salpingo-oophorectomy with quality of life in BRCA1/2 pathogenic variant carriers: a nonrandomized controlled trial. JAMA Oncol. 2021;7:1203-1212.

CASE Woman with BRCA2 mutation
An 80-year-old woman presents for evaluation of newly diagnosed metastatic pancreatic adenocarcinoma. Her medical history is notable for breast cancer. Genetic testing of pancreatic tumor tissue detected a pathogenic variant in BRCA2. Family history revealed a history of melanoma as well as bladder, prostate, breast, and colon cancer. The patient subsequently underwent germline genetic testing with an 86-gene panel and a pathogenic mutation in BRCA2 was identified.
Watch a video of this patient and her clinician, Dr. Andrea Hagemann: https://www.youtube.com/watch?
Methods of genetic testing
It is estimated that 1 in 300 to 1 in 500 women in the United States carry a deleterious mutation in BRCA1 or BRCA2. This equates to between 250,000 and 415,000 women who are at high risk for breast and ovarian cancer.1 Looking at all women with cancer, 20% with ovarian,2 10% with breast,3 2% to 3% with endometrial,4 and 5% with colon cancer5 will have a germline mutation predisposing them to cancer. Identification of germline or somatic (tumor) mutations now inform treatment for patients with cancer. An equally important goal of germline genetic testing is cancer prevention. Cancer prevention strategies include risk-based screening for breast, colon, melanoma, and pancreatic cancer and prophylactic surgeries to reduce the risk of breast and ovarian cancer based on mutation type. Evidence-based screening guidelines by mutation type and absolute risk of associated cancers can be found on the National Comprehensive Cancer Network (NCCN).6,7
Multiple strategies have been proposed to identify patients for germline genetic testing. Patients can be identified based on a detailed multigenerational family history. This strategy requires clinicians or genetic counselors to take and update family histories, to recognize when a patient requires referral for testing, and for such testing to be completed. Even then the generation of a detailed pedigree is not very sensitive or specific. Population-based screening for high-penetrance breast and ovarian cancer susceptibility genes, regardless of family history, also has been proposed.8 Such a strategy has become increasingly realistic with decreasing cost and increasing availability of genetic testing. However, it would require increased genetic counseling resources to feasibly and equitably reach the target population and to explain the results to those patients and their relatives.
An alternative is to test the enriched population of family members of a patient with cancer who has been found to carry a pathogenic variant in a clinically relevant cancer susceptibility gene. This type of testing is termed cascade genetic testing. Cascade testing in first-degree family members carries a 50% probability of detecting the same pathogenic mutation. A related testing model is traceback testing where genetic testing is performed on pathology or tumor registry specimens from deceased patients with cancer.9 This genetic testing information is then provided to the family. Traceback models of genetic testing are an active area of research but can introduce ethical dilemmas. The more widely accepted cascade testing starts with the testing of a living patient affected with cancer. A recent article demonstrated the feasibility of a cascade testing model. Using a multiple linear regression model, the authors determined that all carriers of pathogenic mutations in 18 clinically relevant cancer susceptibility genes in the United States could be identified in 9.9 years if there was a 70% cascade testing rate of first-, second- and third-degree relatives, compared to 59.5 years with no cascade testing.10
Gaps in practice
Identification of mutation carriers, either through screening triggered by family history or through testing of patients affected with cancer, represents a gap between guidelines and clinical practice. Current NCCN guidelines outline genetic testing criteria for hereditary breast and ovarian cancer syndrome and for hereditary colorectal cancer. Despite well-established criteria, a survey in the United States revealed that only 19% of primary care providers were able to accurately assess family history for BRCA1 and 2 testing.11 Looking at patients who meet criteria for testing for Lynch syndrome, only 1 in 4 individuals have undergone genetic testing.12 Among patients diagnosed with breast and ovarian cancer, current NCCN guidelines recommend germline genetic testing for all patients with epithelial ovarian cancer; emerging evidence suggests all patients with breast cancer should be offered germline genetic testing.7,13 Large population-based studies have repeatedly demonstrated that testing rates fall short of this goal, with only 10% to 30% of patients undergoing genetic testing.9,14
Among families with a known hereditary mutation, rates of cascade genetic testing are also low, ranging from 17% to 50%.15-18 Evidence-based management guidelines, for both hereditary breast and ovarian cancer as well as Lynch syndrome, have been shown to reduce mortality.19,20 Failure to identify patients who carry these genetic mutations equates to increased mortality for our patients.
Barriers to cascade genetic testing
Cascade genetic testing ideally would be performed on entire families. Actual practice is far from ideal, and barriers to cascade testing exist. Barriers encompass resistance on the part of the family and provider as well as environmental or system factors.
Family factors
Because of privacy laws, the responsibility of disclosure of genetic testing results to family members falls primarily to the patient. Proband education is critical to ensure disclosure amongst family members. Family dynamics and geographic distribution of family members can further complicate disclosure. Following disclosure, family member gender, education, and demographics as well as personal views, attitudes, and emotions affect whether a family member decides to undergo testing.21 Furthermore, insurance status and awareness of and access to specialty-specific care for the proband’s family members may influence cascade genetic testing rates.
Provider factors
Provider factors that affect cascade genetic testing include awareness of testing guidelines, interpretation of genetic testing results, and education and knowledge of specific mutations. For instance, providers must recognize that cascade testing is not appropriate for variants of uncertain significance. This can lead to unnecessary surveillance testing and prophylactic surgeries. Providers, however, must continue to follow patients and periodically update testing results as variants may be reclassified over time. Additionally, providers must be knowledgeable about the complex and nuanced nature of the screening guidelines for each mutation. The NCCN provides detailed recommendations by mutation.7 Patients may benefit from care with cancer specialists who are aware of the guidelines, particularly for moderate-penetrance genes like BRIP1 and PALB2, as discussions about the timing of risk-reducing surgery are more nuanced in this population. Finally, which providers are responsible for facilitating cascade testing may be unclear; oncologists and genetic counselors not primarily treating probands’ relatives may assume the proper information has been passed along to family members without a practical means to follow up, and primary care providers may assume it is being taken care of by the oncology provider.
Continue to: Environmental or system factors...
Environmental or system factors
Accessibility of genetic counseling and testing is a common barrier to cascade testing. Family members may be geographically remote and connecting them to counseling and testing can be challenging. Working with local genetic counselors can facilitate this process. Insurance coverage of testing is a common perceived barrier; however, many testing companies now provide cascade testing free of charge if within a certain window from the initial test. Despite this, patients often site cost as a barrier to undergoing testing. Concerns about insurance coverage are common after a positive result. The Genetic Information Nondiscrimination Act of 2008 prohibits discrimination against employees or insurance applicants because of genetic information. Life insurance or long-term care policies, however, can incorporate genetic testing information into policy rates, so patients should be recommended to consider purchasing life insurance prior to undergoing genetic testing. This is especially important if the person considering testing has not yet been diagnosed with cancer.
Implications of a positive result
Family members who receive a positive test result should be referred for genetic counseling and to the appropriate specialists for evidence-based screening and discussion for risk-reducing surgery (FIGURE).7 For mutations associated with hereditary breast and ovarian cancer, referral to breast and gynecologic surgeons with expertise in risk reducing surgery is critical as the risk of diagnosing an occult malignancy is approximately 1%.22 Surgical technique with a 2-cm margin on the

Patient resources: decision aids, websites
As genetic testing becomes more accessible and people are tested at younger ages, studies examining the balance of risk reduction and quality of life (QOL) are increasingly important. Fertility concerns, effects of early menopause, and the interrelatedness between decisions for breast and gynecologic risk reduction should all be considered in the counseling for surgical risk reduction. Patient decision aids can help mutation carriers navigate the complex information and decisions.25 Websites specifically designed by advocacy groups can be useful adjuncts to in-office counseling (Facing Our Risk Empowered, FORCE; Facingourrisk.org).
Family letters
The American College of Obstetricians and Gynecologists recommends an ObGyn have a letter or documentation stating that the patient’s relative has a specific mutation before initiating cascade testing for an at-risk family member. The indicated test (such as BRCA1) should be ordered only after the patient has been counseled about potential outcomes and has expressly decided to be tested.26 Letters, such as the example given in the American College of Obstetricians and Gynecologists practice bulletin,26 are a key component of communication between oncology providers, probands, family members, and their primary care providers. ObGyn providers should work together with genetic counselors and gynecologic oncologists to determine the most efficient strategies in their communities.
Technology
Access to genetic testing and genetic counseling has been improved with the rise in telemedicine. Geographically remote patients can now access genetic counseling through medical center–based counselors as well as company-provided genetic counseling over the phone. Patients also can submit samples remotely without needing to be tested in a doctor’s office.
Databases from cancer centers that detail cascade genetic testing rates. As the preventive impact of cascade genetic testing becomes clearer, strategies to have recurrent discussions with cancer patients regarding their family members’ risk should be implemented. It is still unclear which providers—genetic counselors, gynecologic oncologists, medical oncologists, breast surgeons, ObGyns, to name a few—are primarily responsible for remembering to have these follow-up discussions, and despite advances, the burden still rests on the cancer patient themselves. Databases with automated follow-up surveys done every 6 to 12 months could provide some aid to busy providers in this regard.
Emerging research
If gynecologic risk-reducing surgery is chosen, clinical trial involvement should be encouraged. The Women Choosing Surgical Prevention (NCT02760849) in the United States and the TUBA study (NCT02321228) in the Netherlands were designed to compare menopause-related QOL between standard risk-reducing salpingo-oophorectomy (RRSO) and the innovative risk-reducing salpingectomy with delayed oophorectomy for mutation carriers. Results from the nonrandomized controlled TUBA trial suggest that patients have better menopause-related QOL after risk-reducing salpingectomy than after RRSO, regardless of hormone replacement therapy.27 International collaboration is continuing to better understand oncologic safety. In the United States, the SOROCk trial (NCT04251052) is a noninferiority surgical choice study underway for BRCA1 mutation carriers aged 35 to 50, powered to determine oncologic outcome differences in addition to QOL outcomes between RRSO and delayed oophorectomy arms.
Returning to the case
The patient and her family underwent genetic counseling. The patient’s 2 daughters, each in their 50s, underwent cascade genetic testing and were found to carry the same pathogenic mutation in BRCA2. After counseling from both breast and gynecologic surgeons, they both elected to undergo risk reducing bilateral salpingo-oophorectomy with hysterectomy. Both now complete regular screening for breast cancer and melanoma with plans to start screening for pancreatic cancer. Both are currently cancer free.
Summary
Cascade genetic testing is an efficient strategy to identify mutation carriers for hereditary breast and ovarian cancer syndrome. Implementation of the best patient-centric care will require continued collaboration and communication across and within disciplines. ●
Cascade, or targeted, genetic testing within families known to carry a hereditary mutation in a cancer susceptibility gene should be performed on all living first-degree family members over the age of 18. All mutation carriers should be connected to a multidisciplinary care team (FIGURE) to ensure implementation of evidence-based screening and risk-reducing surgery for cancer prevention. If gynecologic risk-reducing surgery is chosen, clinical trial involvement should be encouraged.

CASE Woman with BRCA2 mutation
An 80-year-old woman presents for evaluation of newly diagnosed metastatic pancreatic adenocarcinoma. Her medical history is notable for breast cancer. Genetic testing of pancreatic tumor tissue detected a pathogenic variant in BRCA2. Family history revealed a history of melanoma as well as bladder, prostate, breast, and colon cancer. The patient subsequently underwent germline genetic testing with an 86-gene panel and a pathogenic mutation in BRCA2 was identified.
Watch a video of this patient and her clinician, Dr. Andrea Hagemann: https://www.youtube.com/watch?
Methods of genetic testing
It is estimated that 1 in 300 to 1 in 500 women in the United States carry a deleterious mutation in BRCA1 or BRCA2. This equates to between 250,000 and 415,000 women who are at high risk for breast and ovarian cancer.1 Looking at all women with cancer, 20% with ovarian,2 10% with breast,3 2% to 3% with endometrial,4 and 5% with colon cancer5 will have a germline mutation predisposing them to cancer. Identification of germline or somatic (tumor) mutations now inform treatment for patients with cancer. An equally important goal of germline genetic testing is cancer prevention. Cancer prevention strategies include risk-based screening for breast, colon, melanoma, and pancreatic cancer and prophylactic surgeries to reduce the risk of breast and ovarian cancer based on mutation type. Evidence-based screening guidelines by mutation type and absolute risk of associated cancers can be found on the National Comprehensive Cancer Network (NCCN).6,7
Multiple strategies have been proposed to identify patients for germline genetic testing. Patients can be identified based on a detailed multigenerational family history. This strategy requires clinicians or genetic counselors to take and update family histories, to recognize when a patient requires referral for testing, and for such testing to be completed. Even then the generation of a detailed pedigree is not very sensitive or specific. Population-based screening for high-penetrance breast and ovarian cancer susceptibility genes, regardless of family history, also has been proposed.8 Such a strategy has become increasingly realistic with decreasing cost and increasing availability of genetic testing. However, it would require increased genetic counseling resources to feasibly and equitably reach the target population and to explain the results to those patients and their relatives.
An alternative is to test the enriched population of family members of a patient with cancer who has been found to carry a pathogenic variant in a clinically relevant cancer susceptibility gene. This type of testing is termed cascade genetic testing. Cascade testing in first-degree family members carries a 50% probability of detecting the same pathogenic mutation. A related testing model is traceback testing where genetic testing is performed on pathology or tumor registry specimens from deceased patients with cancer.9 This genetic testing information is then provided to the family. Traceback models of genetic testing are an active area of research but can introduce ethical dilemmas. The more widely accepted cascade testing starts with the testing of a living patient affected with cancer. A recent article demonstrated the feasibility of a cascade testing model. Using a multiple linear regression model, the authors determined that all carriers of pathogenic mutations in 18 clinically relevant cancer susceptibility genes in the United States could be identified in 9.9 years if there was a 70% cascade testing rate of first-, second- and third-degree relatives, compared to 59.5 years with no cascade testing.10
Gaps in practice
Identification of mutation carriers, either through screening triggered by family history or through testing of patients affected with cancer, represents a gap between guidelines and clinical practice. Current NCCN guidelines outline genetic testing criteria for hereditary breast and ovarian cancer syndrome and for hereditary colorectal cancer. Despite well-established criteria, a survey in the United States revealed that only 19% of primary care providers were able to accurately assess family history for BRCA1 and 2 testing.11 Looking at patients who meet criteria for testing for Lynch syndrome, only 1 in 4 individuals have undergone genetic testing.12 Among patients diagnosed with breast and ovarian cancer, current NCCN guidelines recommend germline genetic testing for all patients with epithelial ovarian cancer; emerging evidence suggests all patients with breast cancer should be offered germline genetic testing.7,13 Large population-based studies have repeatedly demonstrated that testing rates fall short of this goal, with only 10% to 30% of patients undergoing genetic testing.9,14
Among families with a known hereditary mutation, rates of cascade genetic testing are also low, ranging from 17% to 50%.15-18 Evidence-based management guidelines, for both hereditary breast and ovarian cancer as well as Lynch syndrome, have been shown to reduce mortality.19,20 Failure to identify patients who carry these genetic mutations equates to increased mortality for our patients.
Barriers to cascade genetic testing
Cascade genetic testing ideally would be performed on entire families. Actual practice is far from ideal, and barriers to cascade testing exist. Barriers encompass resistance on the part of the family and provider as well as environmental or system factors.
Family factors
Because of privacy laws, the responsibility of disclosure of genetic testing results to family members falls primarily to the patient. Proband education is critical to ensure disclosure amongst family members. Family dynamics and geographic distribution of family members can further complicate disclosure. Following disclosure, family member gender, education, and demographics as well as personal views, attitudes, and emotions affect whether a family member decides to undergo testing.21 Furthermore, insurance status and awareness of and access to specialty-specific care for the proband’s family members may influence cascade genetic testing rates.
Provider factors
Provider factors that affect cascade genetic testing include awareness of testing guidelines, interpretation of genetic testing results, and education and knowledge of specific mutations. For instance, providers must recognize that cascade testing is not appropriate for variants of uncertain significance. This can lead to unnecessary surveillance testing and prophylactic surgeries. Providers, however, must continue to follow patients and periodically update testing results as variants may be reclassified over time. Additionally, providers must be knowledgeable about the complex and nuanced nature of the screening guidelines for each mutation. The NCCN provides detailed recommendations by mutation.7 Patients may benefit from care with cancer specialists who are aware of the guidelines, particularly for moderate-penetrance genes like BRIP1 and PALB2, as discussions about the timing of risk-reducing surgery are more nuanced in this population. Finally, which providers are responsible for facilitating cascade testing may be unclear; oncologists and genetic counselors not primarily treating probands’ relatives may assume the proper information has been passed along to family members without a practical means to follow up, and primary care providers may assume it is being taken care of by the oncology provider.
Continue to: Environmental or system factors...
Environmental or system factors
Accessibility of genetic counseling and testing is a common barrier to cascade testing. Family members may be geographically remote and connecting them to counseling and testing can be challenging. Working with local genetic counselors can facilitate this process. Insurance coverage of testing is a common perceived barrier; however, many testing companies now provide cascade testing free of charge if within a certain window from the initial test. Despite this, patients often site cost as a barrier to undergoing testing. Concerns about insurance coverage are common after a positive result. The Genetic Information Nondiscrimination Act of 2008 prohibits discrimination against employees or insurance applicants because of genetic information. Life insurance or long-term care policies, however, can incorporate genetic testing information into policy rates, so patients should be recommended to consider purchasing life insurance prior to undergoing genetic testing. This is especially important if the person considering testing has not yet been diagnosed with cancer.
Implications of a positive result
Family members who receive a positive test result should be referred for genetic counseling and to the appropriate specialists for evidence-based screening and discussion for risk-reducing surgery (FIGURE).7 For mutations associated with hereditary breast and ovarian cancer, referral to breast and gynecologic surgeons with expertise in risk reducing surgery is critical as the risk of diagnosing an occult malignancy is approximately 1%.22 Surgical technique with a 2-cm margin on the

Patient resources: decision aids, websites
As genetic testing becomes more accessible and people are tested at younger ages, studies examining the balance of risk reduction and quality of life (QOL) are increasingly important. Fertility concerns, effects of early menopause, and the interrelatedness between decisions for breast and gynecologic risk reduction should all be considered in the counseling for surgical risk reduction. Patient decision aids can help mutation carriers navigate the complex information and decisions.25 Websites specifically designed by advocacy groups can be useful adjuncts to in-office counseling (Facing Our Risk Empowered, FORCE; Facingourrisk.org).
Family letters
The American College of Obstetricians and Gynecologists recommends an ObGyn have a letter or documentation stating that the patient’s relative has a specific mutation before initiating cascade testing for an at-risk family member. The indicated test (such as BRCA1) should be ordered only after the patient has been counseled about potential outcomes and has expressly decided to be tested.26 Letters, such as the example given in the American College of Obstetricians and Gynecologists practice bulletin,26 are a key component of communication between oncology providers, probands, family members, and their primary care providers. ObGyn providers should work together with genetic counselors and gynecologic oncologists to determine the most efficient strategies in their communities.
Technology
Access to genetic testing and genetic counseling has been improved with the rise in telemedicine. Geographically remote patients can now access genetic counseling through medical center–based counselors as well as company-provided genetic counseling over the phone. Patients also can submit samples remotely without needing to be tested in a doctor’s office.
Databases from cancer centers that detail cascade genetic testing rates. As the preventive impact of cascade genetic testing becomes clearer, strategies to have recurrent discussions with cancer patients regarding their family members’ risk should be implemented. It is still unclear which providers—genetic counselors, gynecologic oncologists, medical oncologists, breast surgeons, ObGyns, to name a few—are primarily responsible for remembering to have these follow-up discussions, and despite advances, the burden still rests on the cancer patient themselves. Databases with automated follow-up surveys done every 6 to 12 months could provide some aid to busy providers in this regard.
Emerging research
If gynecologic risk-reducing surgery is chosen, clinical trial involvement should be encouraged. The Women Choosing Surgical Prevention (NCT02760849) in the United States and the TUBA study (NCT02321228) in the Netherlands were designed to compare menopause-related QOL between standard risk-reducing salpingo-oophorectomy (RRSO) and the innovative risk-reducing salpingectomy with delayed oophorectomy for mutation carriers. Results from the nonrandomized controlled TUBA trial suggest that patients have better menopause-related QOL after risk-reducing salpingectomy than after RRSO, regardless of hormone replacement therapy.27 International collaboration is continuing to better understand oncologic safety. In the United States, the SOROCk trial (NCT04251052) is a noninferiority surgical choice study underway for BRCA1 mutation carriers aged 35 to 50, powered to determine oncologic outcome differences in addition to QOL outcomes between RRSO and delayed oophorectomy arms.
Returning to the case
The patient and her family underwent genetic counseling. The patient’s 2 daughters, each in their 50s, underwent cascade genetic testing and were found to carry the same pathogenic mutation in BRCA2. After counseling from both breast and gynecologic surgeons, they both elected to undergo risk reducing bilateral salpingo-oophorectomy with hysterectomy. Both now complete regular screening for breast cancer and melanoma with plans to start screening for pancreatic cancer. Both are currently cancer free.
Summary
Cascade genetic testing is an efficient strategy to identify mutation carriers for hereditary breast and ovarian cancer syndrome. Implementation of the best patient-centric care will require continued collaboration and communication across and within disciplines. ●
Cascade, or targeted, genetic testing within families known to carry a hereditary mutation in a cancer susceptibility gene should be performed on all living first-degree family members over the age of 18. All mutation carriers should be connected to a multidisciplinary care team (FIGURE) to ensure implementation of evidence-based screening and risk-reducing surgery for cancer prevention. If gynecologic risk-reducing surgery is chosen, clinical trial involvement should be encouraged.
- Gabai-Kapara E, Lahad A, Kaufman B, et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A. 2014;111:14205-14210.
- Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482-490.
- Yamauchi H, Takei J. Management of hereditary breast and ovarian cancer. Int J Clin Oncol. 2018;23:45-51.
- Kahn RM, Gordhandas S, Maddy BP, et al. Universal endometrial cancer tumor typing: how much has immunohistochemistry, microsatellite instability, and MLH1 methylation improved the diagnosis of Lynch syndrome across the population? Cancer. 2019;125:3172-3183.
- Jasperson KW, Tuohy TM, Neklason DW, et al. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
- Gupta S, Provenzale D, Llor X, et al. NCCN guidelines insights: genetic/familial high-risk assessment: colorectal, version 2.2019. J Natl Compr Canc Netw. 2019;17:1032-1041.
- Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:77-102.
- King MC, Levy-Lahad E, Lahad A. Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA. 2014;312:1091-1092.
- Samimi G, et al. Traceback: a proposed framework to increase identification and genetic counseling of BRCA1 and BRCA2 mutation carriers through family-based outreach. J Clin Oncol. 2017;35:2329-2337.
- Offit K, Tkachuk KA, Stadler ZK, et al. Cascading after peridiagnostic cancer genetic testing: an alternative to population-based screening. J Clin Oncol. 2020;38:1398-1408.
- Bellcross CA, Kolor K, Goddard KAB, et al. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 2011;40:61-66.
- Cross DS, Rahm AK, Kauffman TL, et al. Underutilization of Lynch syndrome screening in a multisite study of patients with colorectal cancer. Genet Med. 2013;15:933-940.
- Beitsch PD, Whitworth PW, Hughes K, et al. Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? J Clin Oncol. 2019;37:453-460.
- Childers CP, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800-3806.
- Samadder NJ, Riegert-Johnson D, Boardman L, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021;7:230-237.
- Sharaf RN, Myer P, Stave CD, et al. Uptake of genetic testing by relatives of Lynch syndrome probands: a systematic review. Clin Gastroenterol Hepatol. 2013;11:1093-1100.
- Menko FH, Ter Stege JA, van der Kolk LE, et al. The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and Lynch syndrome: a systematic review of the literature and implications for clinical practice. Fam Cancer. 2019;18:127-135.
- Griffin NE, Buchanan TR, Smith SH, et al. Low rates of cascade genetic testing among families with hereditary gynecologic cancer: an opportunity to improve cancer prevention. Gynecol Oncol. 2020;156:140-146.
- Roberts MC, Dotson WD, DeVore CS, et al. Delivery of cascade screening for hereditary conditions: a scoping review of the literature. Health Aff (Millwood). 2018;37:801-808.
- Finch AP, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Srinivasan S, Won NY, Dotson WD, et al. Barriers and facilitators for cascade testing in genetic conditions: a systematic review. Eur J Hum Genet. 2020;28:1631-1644.
- Piedimonte S, Frank C, Laprise C, et al. Occult tubal carcinoma after risk-reducing salpingo-oophorectomy: a systematic review. Obstet Gynecol. 2020;135:498-508.
- Shu CA, Pike MC, Jotwani AR, et al. Uterine cancer after risk-reducing salpingo-oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncol. 2016;2:1434-1440.
- Gordhandas S, Norquist BM, Pennington KP, et al. Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol Oncol. 2019;153:192-200.
- Steenbeek MP, van Bommel MHD, Harmsen MG, et al. Evaluation of a patient decision aid for BRCA1/2 pathogenic variant carriers choosing an ovarian cancer prevention strategy. Gynecol Oncol. 2021;163:371-377.
- Committee on Gynecologic Practice. ACOG committee opinion No. 727: Cascade testing: testing women for known hereditary genetic mutations associated with cancer. Obstet Gynecol. 2018;131:E31-E34.
- Steenbeek MP, Harmsen MG, Hoogerbrugge N, et al. Association of salpingectomy with delayed oophorectomy versus salpingo-oophorectomy with quality of life in BRCA1/2 pathogenic variant carriers: a nonrandomized controlled trial. JAMA Oncol. 2021;7:1203-1212.
- Gabai-Kapara E, Lahad A, Kaufman B, et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A. 2014;111:14205-14210.
- Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482-490.
- Yamauchi H, Takei J. Management of hereditary breast and ovarian cancer. Int J Clin Oncol. 2018;23:45-51.
- Kahn RM, Gordhandas S, Maddy BP, et al. Universal endometrial cancer tumor typing: how much has immunohistochemistry, microsatellite instability, and MLH1 methylation improved the diagnosis of Lynch syndrome across the population? Cancer. 2019;125:3172-3183.
- Jasperson KW, Tuohy TM, Neklason DW, et al. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
- Gupta S, Provenzale D, Llor X, et al. NCCN guidelines insights: genetic/familial high-risk assessment: colorectal, version 2.2019. J Natl Compr Canc Netw. 2019;17:1032-1041.
- Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:77-102.
- King MC, Levy-Lahad E, Lahad A. Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA. 2014;312:1091-1092.
- Samimi G, et al. Traceback: a proposed framework to increase identification and genetic counseling of BRCA1 and BRCA2 mutation carriers through family-based outreach. J Clin Oncol. 2017;35:2329-2337.
- Offit K, Tkachuk KA, Stadler ZK, et al. Cascading after peridiagnostic cancer genetic testing: an alternative to population-based screening. J Clin Oncol. 2020;38:1398-1408.
- Bellcross CA, Kolor K, Goddard KAB, et al. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 2011;40:61-66.
- Cross DS, Rahm AK, Kauffman TL, et al. Underutilization of Lynch syndrome screening in a multisite study of patients with colorectal cancer. Genet Med. 2013;15:933-940.
- Beitsch PD, Whitworth PW, Hughes K, et al. Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? J Clin Oncol. 2019;37:453-460.
- Childers CP, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800-3806.
- Samadder NJ, Riegert-Johnson D, Boardman L, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021;7:230-237.
- Sharaf RN, Myer P, Stave CD, et al. Uptake of genetic testing by relatives of Lynch syndrome probands: a systematic review. Clin Gastroenterol Hepatol. 2013;11:1093-1100.
- Menko FH, Ter Stege JA, van der Kolk LE, et al. The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and Lynch syndrome: a systematic review of the literature and implications for clinical practice. Fam Cancer. 2019;18:127-135.
- Griffin NE, Buchanan TR, Smith SH, et al. Low rates of cascade genetic testing among families with hereditary gynecologic cancer: an opportunity to improve cancer prevention. Gynecol Oncol. 2020;156:140-146.
- Roberts MC, Dotson WD, DeVore CS, et al. Delivery of cascade screening for hereditary conditions: a scoping review of the literature. Health Aff (Millwood). 2018;37:801-808.
- Finch AP, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Srinivasan S, Won NY, Dotson WD, et al. Barriers and facilitators for cascade testing in genetic conditions: a systematic review. Eur J Hum Genet. 2020;28:1631-1644.
- Piedimonte S, Frank C, Laprise C, et al. Occult tubal carcinoma after risk-reducing salpingo-oophorectomy: a systematic review. Obstet Gynecol. 2020;135:498-508.
- Shu CA, Pike MC, Jotwani AR, et al. Uterine cancer after risk-reducing salpingo-oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncol. 2016;2:1434-1440.
- Gordhandas S, Norquist BM, Pennington KP, et al. Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol Oncol. 2019;153:192-200.
- Steenbeek MP, van Bommel MHD, Harmsen MG, et al. Evaluation of a patient decision aid for BRCA1/2 pathogenic variant carriers choosing an ovarian cancer prevention strategy. Gynecol Oncol. 2021;163:371-377.
- Committee on Gynecologic Practice. ACOG committee opinion No. 727: Cascade testing: testing women for known hereditary genetic mutations associated with cancer. Obstet Gynecol. 2018;131:E31-E34.
- Steenbeek MP, Harmsen MG, Hoogerbrugge N, et al. Association of salpingectomy with delayed oophorectomy versus salpingo-oophorectomy with quality of life in BRCA1/2 pathogenic variant carriers: a nonrandomized controlled trial. JAMA Oncol. 2021;7:1203-1212.