User login
Transcervical fibroid radiofrequency ablation: A look inside
Uterine leiomyomas affect 70% to 80% of reproductive-age women. Interventions for symptomatic patients include myomectomy, hysterectomy, uterine artery embolization (UAE), and radiofrequency ablation (RFA). Several RFA devices exist on the market. One such device is the sonography-guided transcervical ablation of uterine fibroids (Sonata), which is unique in its transcervical approach that allows for incisionless treatment.1 It can be used to treat fibroids classified as FIGO 1-6 with a radius up to 5 cm.1 Postablative therapy outcomes at 1 and 2 years have been promising for total volume reduction (mean maximal volume reduction, 63.8%) and improvement in symptoms, including quality-of-life measures and amount of bleeding (95% reported reduction).2,3
In our practice, we find this tool most helpful for medium-sized (3–5 cm) intramural fibroids and large type 2 fibroids.
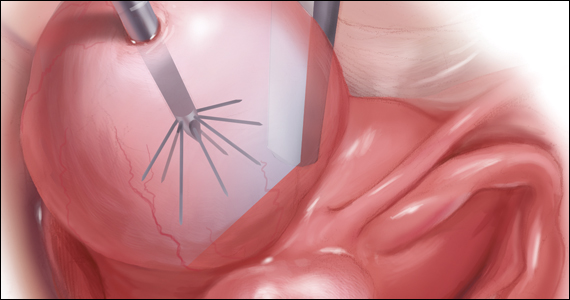
In the accompanying video, we illustrate the steps for use of transcervical ultrasonographic RFA with Sonata treatment and demonstrate its impact on the uterus during simultaneous laparoscopy. We present a patient who underwent Sonata treatment for a 4-cm intramural fibroid and simultaneous laparoscopic myomectomy for a 4-cm pedunculated fibroid. This allowed for the unique ability to view the external effect on the uterus during Sonata use. We review the key surgical steps with this approach, including:
- cervical dilation
- introduction of the Sonata system
- sonographic identification of the target fibroid
- adjust size and shape of Smart Guide overlays
- deploy the introducer
- safety rotation check
- deploy the needle electrodes
- initiate RFA
- withdraw needle electrodes and introducer.
RFA with Sonata treatment is a simple, minimally invasive therapeutic option for fibroids.
We hope that you find this video useful to your clinical practice.
>>DR. ARNOLD P. ADVINCULA AND COLLEAGUES

- Toub DB. A new paradigm for uterine fibroid treatment: transcervical, intrauterine sonography-guided radiofrequency ablation of uterine fibroids with the Sonata system. Curr Obstet Gynecol Rep. 2017;6:67-73.
- Hudgens J, Johns DA, Lukes AS, et al. 12-month outcomes of the US patient cohort in the Sonata pivotal IDE trial of transcervical ablation of uterine fibroids. Int J Womens Health. 2019;11:387-394.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
Uterine leiomyomas affect 70% to 80% of reproductive-age women. Interventions for symptomatic patients include myomectomy, hysterectomy, uterine artery embolization (UAE), and radiofrequency ablation (RFA). Several RFA devices exist on the market. One such device is the sonography-guided transcervical ablation of uterine fibroids (Sonata), which is unique in its transcervical approach that allows for incisionless treatment.1 It can be used to treat fibroids classified as FIGO 1-6 with a radius up to 5 cm.1 Postablative therapy outcomes at 1 and 2 years have been promising for total volume reduction (mean maximal volume reduction, 63.8%) and improvement in symptoms, including quality-of-life measures and amount of bleeding (95% reported reduction).2,3
In our practice, we find this tool most helpful for medium-sized (3–5 cm) intramural fibroids and large type 2 fibroids.
In the accompanying video, we illustrate the steps for use of transcervical ultrasonographic RFA with Sonata treatment and demonstrate its impact on the uterus during simultaneous laparoscopy. We present a patient who underwent Sonata treatment for a 4-cm intramural fibroid and simultaneous laparoscopic myomectomy for a 4-cm pedunculated fibroid. This allowed for the unique ability to view the external effect on the uterus during Sonata use. We review the key surgical steps with this approach, including:
- cervical dilation
- introduction of the Sonata system
- sonographic identification of the target fibroid
- adjust size and shape of Smart Guide overlays
- deploy the introducer
- safety rotation check
- deploy the needle electrodes
- initiate RFA
- withdraw needle electrodes and introducer.
RFA with Sonata treatment is a simple, minimally invasive therapeutic option for fibroids.
We hope that you find this video useful to your clinical practice.
>>DR. ARNOLD P. ADVINCULA AND COLLEAGUES

Uterine leiomyomas affect 70% to 80% of reproductive-age women. Interventions for symptomatic patients include myomectomy, hysterectomy, uterine artery embolization (UAE), and radiofrequency ablation (RFA). Several RFA devices exist on the market. One such device is the sonography-guided transcervical ablation of uterine fibroids (Sonata), which is unique in its transcervical approach that allows for incisionless treatment.1 It can be used to treat fibroids classified as FIGO 1-6 with a radius up to 5 cm.1 Postablative therapy outcomes at 1 and 2 years have been promising for total volume reduction (mean maximal volume reduction, 63.8%) and improvement in symptoms, including quality-of-life measures and amount of bleeding (95% reported reduction).2,3
In our practice, we find this tool most helpful for medium-sized (3–5 cm) intramural fibroids and large type 2 fibroids.
In the accompanying video, we illustrate the steps for use of transcervical ultrasonographic RFA with Sonata treatment and demonstrate its impact on the uterus during simultaneous laparoscopy. We present a patient who underwent Sonata treatment for a 4-cm intramural fibroid and simultaneous laparoscopic myomectomy for a 4-cm pedunculated fibroid. This allowed for the unique ability to view the external effect on the uterus during Sonata use. We review the key surgical steps with this approach, including:
- cervical dilation
- introduction of the Sonata system
- sonographic identification of the target fibroid
- adjust size and shape of Smart Guide overlays
- deploy the introducer
- safety rotation check
- deploy the needle electrodes
- initiate RFA
- withdraw needle electrodes and introducer.
RFA with Sonata treatment is a simple, minimally invasive therapeutic option for fibroids.
We hope that you find this video useful to your clinical practice.
>>DR. ARNOLD P. ADVINCULA AND COLLEAGUES

- Toub DB. A new paradigm for uterine fibroid treatment: transcervical, intrauterine sonography-guided radiofrequency ablation of uterine fibroids with the Sonata system. Curr Obstet Gynecol Rep. 2017;6:67-73.
- Hudgens J, Johns DA, Lukes AS, et al. 12-month outcomes of the US patient cohort in the Sonata pivotal IDE trial of transcervical ablation of uterine fibroids. Int J Womens Health. 2019;11:387-394.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Toub DB. A new paradigm for uterine fibroid treatment: transcervical, intrauterine sonography-guided radiofrequency ablation of uterine fibroids with the Sonata system. Curr Obstet Gynecol Rep. 2017;6:67-73.
- Hudgens J, Johns DA, Lukes AS, et al. 12-month outcomes of the US patient cohort in the Sonata pivotal IDE trial of transcervical ablation of uterine fibroids. Int J Womens Health. 2019;11:387-394.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
Management of Acute and Chronic Pain Associated With Hidradenitis Suppurativa: A Comprehensive Review of Pharmacologic and Therapeutic Considerations in Clinical Practice
Hidradenitis suppurativa (HS) is a chronic inflammatory, androgen gland disorder characterized by recurrent rupture of the hair follicles with a vigorous inflammatory response. This response results in abscess formation and development of draining sinus tracts and hypertrophic fibrous scars.1,2 Pain, discomfort, and odorous discharge from the recalcitrant lesions have a profound impact on patient quality of life.3,4
The morbidity and disease burden associated with HS are particularly underestimated, as patients frequently report debilitating pain that often is overlooked.5,6 Additionally, the quality and intensity of perceived pain are compounded by frequently associated depression and anxiety.7-9 Pain has been reported by patients with HS to be the highest cause of morbidity, despite the disfiguring nature of the disease and its associated psychosocial distress.7,10 Nonetheless, HS lacks an accepted pain management algorithm similar to those that have been developed for the treatment of other acute or chronic pain disorders, such as back pain and sickle cell disease.4,11-13
Given the lack of formal studies regarding pain management in patients with HS, clinicians are limited to general pain guidelines, expert opinion, small trials, and patient preference.3 Furthermore, effective pain management in HS necessitates the treatment of both chronic pain affecting daily function and acute pain present during disease flares, surgical interventions, and dressing changes.3 The result is a wide array of strategies used for HS-associated pain.3,4
Epidemiology and Pathophysiology
Hidradenitis suppurativa historically has been an overlooked and underdiagnosed disease, which limits epidemiology data.5 Current estimates are that HS affects approximately 1% of the general population; however, prevalence rates range from 0.03% to 4.1%.14-16
The exact etiology of HS remains unclear, but it is thought that genetic factors, immune dysregulation, and environmental/behavioral influences all contribute to its pathophysiology.1,17 Up to 40% of patients with HS report a positive family history of the disease.18-20 Hidradenitis suppurativa has been associated with other inflammatory disease states, such as inflammatory bowel disease, spondyloarthropathies, and pyoderma gangrenosum.16,21,22
It is thought that HS is the result of some defect in keratin clearance that leads to follicular hyperkeratinization and occlusion.1 Resultant rupture of pilosebaceous units and spillage of contents (including keratin and bacteria) into the surrounding dermis triggers a vigorous inflammatory response. Sinus tracts and fistulas become the targets of bacterial colonization, biofilm formation, and secondary infection. The result is suppuration and extension of the lesions as well as sustained chronic inflammation.23,24
Although the etiology of HS is complex, several modifiable risk factors for the disease have been identified, most prominently cigarette smoking and obesity. Approximately 70% of patients with HS smoke cigarettes.2,15,25,26 Obesity has a well-known association with HS, and it is possible that weight reduction lowers disease severity.27-30
Clinical Presentation and Diagnosis
Establishing a diagnosis of HS necessitates recognition of disease morphology, topography, and chronicity. Hidradenitis suppurativa most commonly occurs in the axillae, inguinal and anogenital region, perineal region, and inframammary region.5,31 A typical history involves a prolonged disease course with recurrent lesions and intermittent periods of improvement or remission. Primary lesions are deep, inflamed, painful, and sterile. Ultimately, these lesions rupture and track subcutaneously.15,25 Intercommunicating sinus tracts form from multiple recurrent nodules in close proximity and may ultimately lead to fibrotic scarring and local architectural distortion.32 The Hurley staging system helps to guide treatment interventions based on disease severity. Approach to pain management is discussed below.
Pain Management in HS: General Principles
Pain management is complex for clinicians, as there are limited studies from which to draw treatment recommendations. Incomplete understanding of the etiology and pathophysiology of the disease contributes to the lack of established management guidelines.
A PubMed search of articles indexed for MEDLINE using the terms hidradenitis, suppurativa, pain, and management revealed 61 different results dating back to 1980, 52 of which had been published in the last 5 years. When the word acute was added to the search, there were only 6 results identified. These results clearly reflect a better understanding of HS-mediated pain as well as clinical unmet needs and evolving strategies in pain management therapeutics. However, many of these studies reflect therapies focused on the mediation or modulation of HS pathogenesis rather than potential pain management therapies.
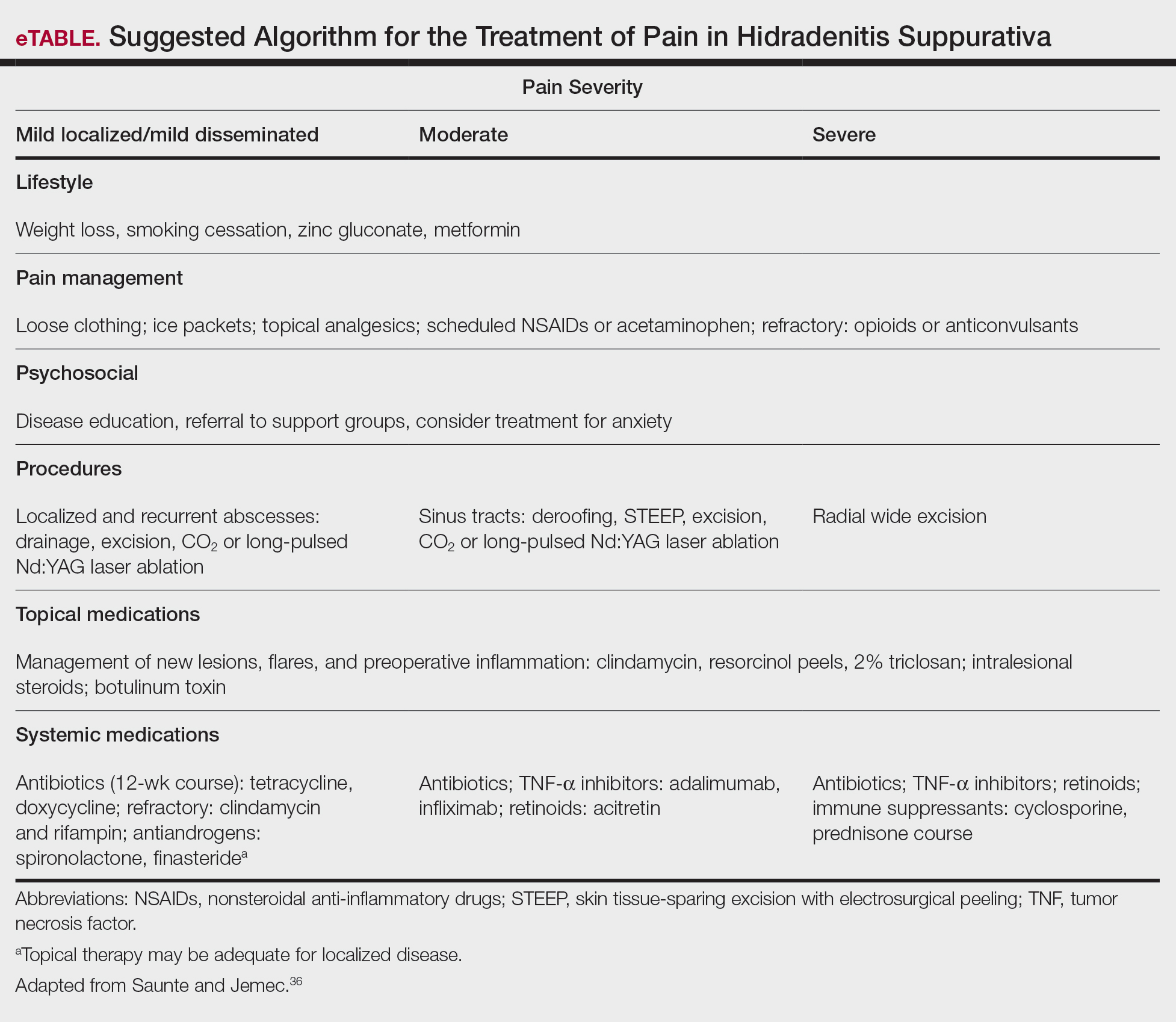
In addition, the heterogenous nature of the pain experience in HS poses a challenge for clinicians. Patients may experience multiple pain types concurrently, including inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic, as well as pain related to arthritis.3,33,34 Pain perception is further complicated by the observation that patients with HS have high rates of psychiatric comorbidities such as depression and anxiety, both of which profoundly alter perception of both the strength and quality of pain.7,8,22,35 A suggested algorithm for treatment of pain in HS is described in the eTable.36
Chronicity is a hallmark of HS. Patients experience a prolonged disease course involving acute painful exacerbations superimposed on chronic pain that affects all aspects of daily life. Changes in self-perception, daily living activities, mood state, physical functioning, and physical comfort frequently are reported to have a major impact on quality of life.1,3,37
In 2018, Thorlacius et al38 created a multistakeholder consensus on a core outcome set of domains detailing what to measure in clinical trials for HS. The authors hoped that the routine adoption of these core domains would promote the collection of consistent and relevant information, bolster the strength of evidence synthesis, and minimize the risk for outcome reporting bias among studies.38 It is important to ascertain the patient’s description of his/her pain to distinguish between stimulus-dependent nociceptive pain vs spontaneous neuropathic pain.3,7,10 The most common pain descriptors used by patients are “shooting,” “itchy,” “blinding,” “cutting,” and “exhausting.”10 In addition to obtaining descriptive factors, it is important for the clinician to obtain information on the timing of the pain, whether or not the pain is relieved with spontaneous or surgical drainage, and if the patient is experiencing chronic background pain secondary to scarring or skin contraction.3 With the routine utilization of a consistent set of core domains, advances in our understanding of the different elements of HS pain, and increased provider awareness of the disease, the future of pain management in patients with HS seems promising.
Acute and Perioperative Pain Management
Acute Pain Management—The pain in HS can range from mild to excruciating.3,7 The difference between acute and chronic pain in this condition may be hard to delineate, as patients may have intense acute flares on top of a baseline level of chronic pain.3,7,14 These factors, in combination with various pain types of differing etiologies, make the treatment of HS-associated pain a therapeutic challenge.
The first-line treatments for acute pain in HS are oral acetaminophen, oral nonsteroidal anti-inflammatory drugs (NSAIDs), and topical analgesics.3 These treatment modalities are especially helpful for nociceptive pain, which often is described as having an aching or tender quality.3 Topical treatment for acute pain episodes includes diclofenac gel and liposomal lidocaine cream.39 Topical lidocaine in particular has the benefit of being rapid acting, and its effect can last 1 to 2 hours. Ketamine has been anecdotally used as a topical treatment. Treatment options for neuropathic pain include topical amitriptyline, gabapentin, and pregabalin.39 Dressings and ice packs may be used in cases of mild acute pain, depending on patient preference.3
First-line therapies may not provide adequate pain control in many patients.3,40,41 Should the first-line treatments fail, oral opiates can be considered as a treatment option, especially if the patient has a history of recurrent pain unresponsive to milder methods of pain control.3,40,41 However, prudence should be exercised, as patients with HS have a higher risk for opioid abuse, and referral to a pain specialist is advisable.40 Generally, use of opioids should be limited to the smallest period of time possible.40,41 Codeine can be used as a first opioid option, with hydromorphone available as an alternative.41
Pain caused by inflamed abscesses and nodules can be treated with either intralesional corticosteroids or incision and drainage. Intralesional triamcinolone has been found to cause substantial pain relief within 1 day of injection in patients with HS.3,42
Prompt discussion about the remitting course of HS will prepare patients for flares. Although the therapies discussed here aim to reduce the clinical severity and inflammation associated with HS, achieving pain-free remission can be challenging. Barriers to developing a long-term treatment regimen include intolerable side effects or simply nonresponsive disease.36,43
Management of Perioperative Pain—Medical treatment of HS often yields only transient or mild results. Hurley stage II or III lesions typically require surgical removal of affected tissues.32,44-46 Surgery may dramatically reduce the primary disease burden and provide substantial pain relief.3,4,44 Complete resection of the affected tissue by wide excision is the most common surgical procedure used.46-48 However, various tissue-sparing techniques, such as skin-tissue-sparing excision with electrosurgical peeling, also have been utilized. Tissue-sparing surgical techniques may lead to shorter healing times and less postoperative pain.48
There currently is little guidance available on the perioperative management of pain as it relates to surgical procedures for HS. The pain experienced from surgery varies based on the area and location of affected tissue; extent of disease; surgical technique used; and whether primary closure, closure by secondary intention, or skin grafting is utilized.47,49 Medical treatment aimed at reducing inflammation prior to surgical intervention may improve postoperative pain and complications.
The use of general vs local anesthesia during surgery depends on the extent of the disease and the amount of tissue being removed; however, the use of local anesthesia has been associated with a higher recurrence of disease, possibly owing to less aggressive tissue removal.50 Intraoperatively, the injection of 0.5% bupivacaine around the wound edges may lead to less postoperative pain.3,48 Postoperative pain usually is managed with acetaminophen and NSAIDs.48 In cases of severe postoperative pain, short- and long-acting opioid oxycodone preparations may be used. The combination of diclofenac and tramadol also has been used postoperatively.3 Patients who do not undergo extensive surgery often can leave the hospital the same day.
Effective strategies for mitigating HS-associated pain must address the chronic pain component of the disease. Long-term management involves lifestyle modifications and pharmacologic agents.
Chronic Pain Management
Although HS is not a curable disease, there are treatments available to minimize symptoms. Long-term management of HS is essential to minimize the effects of chronic pain and physical scarring associated with inflammation.31 In one study from the French Society of Dermatology, pain reported by patients with HS was directly associated with severity and duration of disease, emotional symptoms, and reduced functionality.51 For these reasons, many treatments for HS target reducing clinical severity and achieving remission, often defined as more than 6 months without any recurrence of lesions.52 In addition to lifestyle management, therapies available to manage HS include topical and systemic medications as well as procedures such as surgical excision.36,43,52,53
Lifestyle Modifications
Regardless of the severity of HS, all patients may benefit from basic education on the pathogenesis of the disease.36 The associations with smoking and obesity have been well documented, and treatment of these comorbid conditions is indicated.36,43,52 For example, in relation to obesity, the use of metformin is very well tolerated and seems to positively impact HS symptoms.43 Several studies have suggested that weight reduction lowers disease severity.28-30 Patients should be counseled on the importance of smoking cessation and weight loss.
Finally, the emotional impact of HS is not to be discounted, both the physical and social discomfort as well as the chronicity of the disease and frustration with treatment.51 Chronic pain has been associated with increased rates of depression, and 43% of patients with HS specifically have been diagnosed with major depressive disorder.7 For these reasons, clinician guidance, social support, and websites can improve patient understanding of the disease, adherence to treatment, and comorbid anxiety and depression.52
Topical Therapy
Topical therapy generally is limited to mild disease and is geared at decreasing inflammation or superimposed infection.36,52 Some of the earliest therapies used were topical antibiotics.43 Topical clindamycin has been shown to be as effective as oral tetracyclines in reducing the number of abscesses, but neither treatment substantially reduces pain associated with smaller nodules.54 Intralesional corticosteroids such as triamcinolone acetonide have been shown to decrease both patient-reported pain and physician-assessed severity within 1 to 7 days.42 Routine injection, however, is not a feasible means of long-term treatment both because of inconvenience and the potential adverse effects of corticosteroids.36,52 Both topical clindamycin and intralesional steroids are helpful in reducing inflammation prior to planned surgical intervention.36,52,53
Newer topical therapies include resorcinol peels and combination antimicrobials, such as 2% triclosan and oral zinc gluconate.52,53 Data surrounding the use of resorcinol in mild to moderate HS are promising and have shown decreased severity of both new and long-standing nodules. Fifteen-percent resorcinol peels are helpful tools that allow for self-administration by patients during exacerbations to decrease pain and flare duration.55,56 In a 2016 clinical trial, a combination of oral zinc gluconate with topical triclosan was shown to reduce flare-ups and nodules in mild HS.57 Oral zinc alone may have anti-inflammatory properties and generally is well tolerated.43,53 Topical therapies have a role in reducing HS-associated pain but often are limited to milder disease.
Systemic Agents
Several therapeutic options exist for the treatment of HS; however, a detailed description of their mechanisms and efficacies is beyond the scope of this review, which is focused on pain. Briefly, these systemic agents include antibiotics, retinoids, corticosteroids, antiandrogens, and biologics.43,52,53
Treatment with antibiotics such as tetracyclines or a combination of clindamycin plus rifampin has been shown to produce complete remission in 60% to 80% of users; however, this treatment requires more than 6 months of antibiotic therapy, which can be difficult to tolerate.52,53,58 Relapse is common after antibiotic cessation.2,43,52 Antibiotics have demonstrated efficacy during acute flares and in reducing inflammatory activity prior to surgery.52
Retinoids have been utilized in the treatment of HS because of their action on sebaceous glands and hair follicles.43,53 Acitretin has been shown to be the most effective oral retinoid available in the United States.43 Unfortunately, many of the studies investigating the use of retinoids for treatment of HS are limited by small sample size.36,43,52
Because HS is predominantly an inflammatory condition, immunosuppressants have been adapted to manage patients when antibiotics and topicals have failed. Systemic steroids rarely are used for long-term therapy because of the severe side effects and are preferred only for acute management.36,52 Cyclosporine and dapsone have demonstrated efficacy in treating moderate to severe HS, whereas methotrexate and colchicine have shown little efficacy.52 Both cyclosporine and dapsone are difficult to tolerate, require laboratory monitoring, and lead to only conservative improvement rather than remission in most patients.43
Immune dysregulation in HS involves elevated levels of proinflammatory cytokines such as tumor necrosis factor α (TNF-α), which is a key mediator of inflammation and a stimulator of other inflammatory cytokines.59,60 The first approved biologic treatment of HS was adalimumab, a TNF-α inhibitor, which showed a 50% reduction in total abscess and inflammatory nodule count in 60% of patients with moderate to severe HS.61-63 Of course, TNF-α inhibitor therapy is not without risks, specifically those of infection.43,53,61,62 Maintenance therapy may be required if patients relapse.53,61
Various interleukin inhibitors also have emerged as potential therapies for HS, such as ustekinumab and anakinra.36,64 Both have been subject to numerous small case trials that have reported improvements in clinical severity and pain; however, both drugs were associated with a fair number of nonresponders.36,64,65
Surgical Procedures
Although HS lesions may regress on their own in a matter of weeks, surgical drainage allows an acute alleviation of the severe burning pain associated with HS flares.36,52,53 Because of improved understanding of the disease pathophysiology, recent therapies targeting the hair follicle have been developed and have shown promising results. These therapies include laser- and light-based procedures. Long-pulsed Nd:YAG laser therapy reduces the number of hair follicles and sebaceous glands and has been effective for Hurley stage I or II disease.36,43,52,53,66 Photodynamic therapy offers a less-invasive option compared to surgery and laser therapy.52,53,66 Both Nd:YAG and CO2 laser therapy offer low recurrence rates (<30%) due to destruction of the apocrine unit.43,53 Photodynamic therapy for mild disease offers a less-invasive option compared to surgery and laser therapy.53 There is a need for larger randomized controlled trials involving laser, light, and CO2 therapies.66
Conclusion
Hidradenitis suppurativa is a debilitating condition with an underestimated disease burden. Although the pathophysiology of the disease is not completely understood, it is evident that pain is a major cause of morbidity. Patients experience a multitude of acute and chronic pain types: inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic. Pain perception and quality of life are further impacted by psychiatric conditions such as depression and anxiety, both of which are common comorbidities in patients with HS. Several pharmacologic agents have been used to treat HS-associated pain with mixed results. First-line treatment of acute pain episodes includes oral acetaminophen, NSAIDs, and topical analgesics. Management of chronic pain includes utilization of topical agents, systemic agents, and biologics, as well as addressing lifestyle (eg, obesity, smoking status) and psychiatric comorbidities. Although these therapies have roles in HS pain management, the most effective pain remedies developed thus far are limited to surgery and TNF-α inhibitors. Optimization of pain control in patients with HS requires multidisciplinary collaboration among dermatologists, pain specialists, psychiatrists, and other members of the health care team. Further large-scale studies are needed to create an evidence-based treatment algorithm for the management of pain in HS.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi:10.2147/CCID.S111019
- Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatology Venereol. 2009;23:985-998. doi:10.1111/j.1468-3083.2009.03356.x
- Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S47-S51. doi:10.1016/j.jaad.2015.07.046
- Puza CJ, Wolfe SA, Jaleel T. Pain management in patients with hidradenitis suppurativa requiring surgery. Dermatolog Surg. 2019;45:1327-1330. doi:10.1097/DSS.0000000000001693
- Kurzen H, Kurokawa I, Jemec GBE, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Kelly G, Sweeney CM, Tobin AM, et al. Hidradenitis suppurativa: the role of immune dysregulation. Int J Dermatol. 2014;53:1186-1196. doi:10.1111/ijd.12550
- Patel ZS, Hoffman LK, Buse DC, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep. 2017;21:49. doi:10.1007/s11916-017-0647-3
- Sist TC, Florio GA, Miner MF, et al. The relationship between depression and pain language in cancer and chronic non-cancer pain patients. J Pain Symptom Manage. 1998;15:350-358. doi:10.1016/S0885-3924(98)00006-2
- Jemec GBE. Hidradenitis suppurativa. N Engl J Med. 2012;366:158-164. doi:10.1056/NEJMcp1014163
- Nielsen RM, Lindsø Andersen P, Sigsgaard V, et al. Pain perception in patients with hidradenitis suppurativa. Br J Dermatol. 2019;182:bjd.17935. doi:10.1111/bjd.17935
- Tanabe P, Myers R, Zosel A, et al. Emergency department management of acute pain episodes in sickle cell disease. Acad Emerg Med. 2007;14:419-425. doi:10.1197/j.aem.2006.11.033
- Chou R, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009;34:1066-1077. doi:10.1097/BRS.0b013e3181a1390d
- Enamandram M, Rathmell JP, Kimball AB. Chronic pain management in dermatology: a guide to assessment and nonopioid pharmacotherapy. J Am Acad Dermatol. 2015;73:563-573; quiz 573-574. doi:10.1016/j.jaad.2014.11.039
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7. doi:10.1016/j.jaad.2015.07.052
- Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
- Patil S, Apurwa A, Nadkarni N, et al. Hidradenitis suppurativa: inside and out. Indian J Dermatol. 2018;63:91-98. doi:10.4103/ijd.IJD_412_16
- Woodruff CM, Charlie AM, Leslie KS. Hidradenitis suppurativa: a guide for the practicing physician. Mayo Clin Proc. 2015;90:1679-1693. doi:10.1016/j.mayocp.2015.08.020
- Pink AE, Simpson MA, Desai N, et al. Mutations in the γ-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa). J Invest Dermatol. 2012;132:2459-2461. doi:10.1038/jid.2012.162
- Jemec GBE, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35:191-194. doi:10.1016/s0190-9622(96)90321-7
- Fitzsimmons JS, Guilbert PR. A family study of hidradenitis suppurativa. J Med Genet. 1985;22:367-373. doi:10.1136/jmg.22.5.367
- Kelly G, Prens EP. Inflammatory mechanisms in hidradenitis suppurativa. Dermatol Clin. 2016;34:51-58. doi:10.1016/j.det.2015.08.004
- Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123. doi:10.1097/QCO.0b013e3283428d07
- Kathju S, Lasko LA, Stoodley P. Considering hidradenitis suppurativa as a bacterial biofilm disease. FEMS Immunol Med Microbiol. 2012;65:385-389. doi:10.1111/j.1574-695X.2012.00946.x
- Jahns AC, Killasli H, Nosek D, et al. Microbiology of hidradenitis suppurativa (acne inversa): a histological study of 27 patients. APMIS. 2014;122:804-809. doi:10.1111/apm.12220
- Ralf Paus L, Kurzen H, Kurokawa I, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Kromann CB, Ibler KS, Kristiansen VB, et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol. 2014;94:553-557. doi:10.2340/00015555-1800
- Lindsø Andersen P, Kromann C, Fonvig CE, et al. Hidradenitis suppurativa in a cohort of overweight and obese children and adolescents. Int J Dermatol. 2020;59:47-51. doi:10.1111/ijd.14639
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601. doi:10.1016/j.jaad.2008.06.020
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824. doi:10.1111/bjd.13090
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Hsiao J, Leslie K, McMichael A, et al. Folliculitis and other follicular disorders. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:615-632.
- Scheinfeld N. Treatment of hidradenitis suppurativa associated pain with nonsteroidal anti-inflammatory drugs, acetaminophen, celecoxib, gapapentin, pegabalin, duloxetine, and venlafaxine. Dermatol Online J. 2013;19:20616.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1.
- Rajmohan V, Suresh Kumar S. Psychiatric morbidity, pain perception, and functional status of chronic pain patients in palliative care. Indian J Palliat Care. 2013;19:146-151. doi:10.4103/0973-1075.121527
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032. doi:10.1001/jama.2017.16691
- Wang B, Yang W, Wen W, et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330:1065. doi:10.1126/science.1196284
- Thorlacius L, Ingram JR, Villumsen B, et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol. 2018;179:642-650. doi:10.1111/bjd.16672
- Scheinfeld N. Topical treatments of skin pain: a general review with a focus on hidradenitis suppurativa with topical agents. Dermatol Online J. 2014;20:13030/qt4m57506k.
- Reddy S, Orenstein LAV, Strunk A, et al. Incidence of long-term opioid use among opioid-naive patients with hidradenitis suppurativa in the United States. JAMA Dermatol. 2019;155:1284-1290. doi:10.1001/jamadermatol.2019.2610
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatology Venereol. 2015;29:619-644. doi:10.1111/jdv.12966
- Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155. doi:10.1016/j.jaad.2016.06.049
- Robert E, Bodin F, Paul C, et al. Non-surgical treatments for hidradenitis suppurativa: a systematic review. Ann Chir Plast Esthet. 2017;62:274-294. doi:10.1016/j.anplas.2017.03.012
- Menderes A, Sunay O, Vayvada H, et al. Surgical management of hidradenitis suppurativa. Int J Med Sci. 2010;7:240-247. doi:10.7150/ijms.7.240
- Alharbi Z, Kauczok J, Pallua N. A review of wide surgical excision of hidradenitis suppurativa. BMC Dermatol. 2012;12:9. doi:10.1186/1471-5945-12-9
- Burney RE. 35-year experience with surgical treatment of hidradenitis suppurativa. World J Surg. 2017;41:2723-2730. doi:10.1007/s00268-017-4091-7
- Bocchini SF, Habr-Gama A, Kiss DR, et al. Gluteal and perianal hidradenitis suppurativa: surgical treatment by wide excision. Dis Colon Rectum. 2003;46:944-949. doi:10.1007/s10350-004-6691-1
- Blok JL, Spoo JR, Leeman FWJ, et al. Skin-tissue-sparing excision with electrosurgical peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382. doi:10.1111/jdv.12376
- Bilali S, Todi V, Lila A, et al. Surgical treatment of chronic hidradenitis suppurativa in the gluteal and perianal regions. Acta Chir Iugosl. 2012;59:91-95. doi:10.2298/ACI1202091B
- Walter AC, Meissner M, Kaufmann R, et al. Hidradenitis suppurativa after radical surgery-long-term follow-up for recurrences and associated factors. Dermatol Surg. 2018;44:1323-1331. doi:10.1097/DSS.0000000000001668.
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623. doi:10.1016/j.jaad.2006.08.061
- Alavi A, Lynde C, Alhusayen R, et al. Approach to the management of patients with hidradenitis suppurativa: a consensus document. J Cutan Med Surg. 2017;21:513-524. doi:10.1177/1203475417716117
- Duran C, Baumeister A. Recognition, diagnosis, and treatment of hidradenitis suppurativa. J Am Acad Physician Assist. 2019;32:36-42. doi:10.1097/01.JAA.0000578768.62051.13
- Jemec GBE, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-974. doi:10.1016/S0190-9622(98)70272-5
- Pascual JC, Encabo B, Ruiz de Apodaca RF, et al. Topical 15% resorcinol for hidradenitis suppurativa: an uncontrolled prospective trial with clinical and ultrasonographic follow-up. J Am Acad Dermatol. 2017;77:1175-1178. doi:10.1016/j.jaad.2017.07.008
- Boer J, Jemec GBE. Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa. Clin Exp Dermatol. 2010;35:36-40. doi:10.1111/j.1365-2230.2009.03377.x
- Hessam S, Sand M, Meier NM, et al. Combination of oral zinc gluconate and topical triclosan: an anti-inflammatory treatment modality for initial hidradenitis suppurativa. J Dermatol Sci. 2016;84:197-202. doi:10.1016/j.jdermsci.2016.08.010
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154. doi:10.1159/000228334
- Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
- Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328:222-225. doi:10.1016/j.canlet.2012.10.014
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Morita A, Takahashi H, Ozawa K, et al. Twenty-four-week interim analysis from a phase 3 open-label trial of adalimumab in Japanese patients with moderate to severe hidradenitis suppurativa. J Dermatol. 2019;46:745-751. doi:10.1111/1346-8138.14997
- Ghias MH, Johnston AD, Kutner AJ, et al. High-dose, high-frequency infliximab: a novel treatment paradigm for hidradenitis suppurativa. J Am Acad Dermatol. 2020;82:1094-1101. doi:10.1016/j.jaad.2019.09.071
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa a randomized clinical trial. JAMA Dermatol. 2016;152:52-59. doi:10.1001/jamadermatol.2015.3903
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846. doi:10.1111/bjd.14338
- John H, Manoloudakis N, Stephen Sinclair J. A systematic review of the use of lasers for the treatment of hidradenitis suppurativa. J Plast Reconstr Aesthet Surg. 2016;69:1374-1381. doi:10.1016/j.bjps.2016.05.029
Hidradenitis suppurativa (HS) is a chronic inflammatory, androgen gland disorder characterized by recurrent rupture of the hair follicles with a vigorous inflammatory response. This response results in abscess formation and development of draining sinus tracts and hypertrophic fibrous scars.1,2 Pain, discomfort, and odorous discharge from the recalcitrant lesions have a profound impact on patient quality of life.3,4
The morbidity and disease burden associated with HS are particularly underestimated, as patients frequently report debilitating pain that often is overlooked.5,6 Additionally, the quality and intensity of perceived pain are compounded by frequently associated depression and anxiety.7-9 Pain has been reported by patients with HS to be the highest cause of morbidity, despite the disfiguring nature of the disease and its associated psychosocial distress.7,10 Nonetheless, HS lacks an accepted pain management algorithm similar to those that have been developed for the treatment of other acute or chronic pain disorders, such as back pain and sickle cell disease.4,11-13
Given the lack of formal studies regarding pain management in patients with HS, clinicians are limited to general pain guidelines, expert opinion, small trials, and patient preference.3 Furthermore, effective pain management in HS necessitates the treatment of both chronic pain affecting daily function and acute pain present during disease flares, surgical interventions, and dressing changes.3 The result is a wide array of strategies used for HS-associated pain.3,4
Epidemiology and Pathophysiology
Hidradenitis suppurativa historically has been an overlooked and underdiagnosed disease, which limits epidemiology data.5 Current estimates are that HS affects approximately 1% of the general population; however, prevalence rates range from 0.03% to 4.1%.14-16
The exact etiology of HS remains unclear, but it is thought that genetic factors, immune dysregulation, and environmental/behavioral influences all contribute to its pathophysiology.1,17 Up to 40% of patients with HS report a positive family history of the disease.18-20 Hidradenitis suppurativa has been associated with other inflammatory disease states, such as inflammatory bowel disease, spondyloarthropathies, and pyoderma gangrenosum.16,21,22
It is thought that HS is the result of some defect in keratin clearance that leads to follicular hyperkeratinization and occlusion.1 Resultant rupture of pilosebaceous units and spillage of contents (including keratin and bacteria) into the surrounding dermis triggers a vigorous inflammatory response. Sinus tracts and fistulas become the targets of bacterial colonization, biofilm formation, and secondary infection. The result is suppuration and extension of the lesions as well as sustained chronic inflammation.23,24
Although the etiology of HS is complex, several modifiable risk factors for the disease have been identified, most prominently cigarette smoking and obesity. Approximately 70% of patients with HS smoke cigarettes.2,15,25,26 Obesity has a well-known association with HS, and it is possible that weight reduction lowers disease severity.27-30
Clinical Presentation and Diagnosis
Establishing a diagnosis of HS necessitates recognition of disease morphology, topography, and chronicity. Hidradenitis suppurativa most commonly occurs in the axillae, inguinal and anogenital region, perineal region, and inframammary region.5,31 A typical history involves a prolonged disease course with recurrent lesions and intermittent periods of improvement or remission. Primary lesions are deep, inflamed, painful, and sterile. Ultimately, these lesions rupture and track subcutaneously.15,25 Intercommunicating sinus tracts form from multiple recurrent nodules in close proximity and may ultimately lead to fibrotic scarring and local architectural distortion.32 The Hurley staging system helps to guide treatment interventions based on disease severity. Approach to pain management is discussed below.
Pain Management in HS: General Principles
Pain management is complex for clinicians, as there are limited studies from which to draw treatment recommendations. Incomplete understanding of the etiology and pathophysiology of the disease contributes to the lack of established management guidelines.
A PubMed search of articles indexed for MEDLINE using the terms hidradenitis, suppurativa, pain, and management revealed 61 different results dating back to 1980, 52 of which had been published in the last 5 years. When the word acute was added to the search, there were only 6 results identified. These results clearly reflect a better understanding of HS-mediated pain as well as clinical unmet needs and evolving strategies in pain management therapeutics. However, many of these studies reflect therapies focused on the mediation or modulation of HS pathogenesis rather than potential pain management therapies.
In addition, the heterogenous nature of the pain experience in HS poses a challenge for clinicians. Patients may experience multiple pain types concurrently, including inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic, as well as pain related to arthritis.3,33,34 Pain perception is further complicated by the observation that patients with HS have high rates of psychiatric comorbidities such as depression and anxiety, both of which profoundly alter perception of both the strength and quality of pain.7,8,22,35 A suggested algorithm for treatment of pain in HS is described in the eTable.36
Chronicity is a hallmark of HS. Patients experience a prolonged disease course involving acute painful exacerbations superimposed on chronic pain that affects all aspects of daily life. Changes in self-perception, daily living activities, mood state, physical functioning, and physical comfort frequently are reported to have a major impact on quality of life.1,3,37
In 2018, Thorlacius et al38 created a multistakeholder consensus on a core outcome set of domains detailing what to measure in clinical trials for HS. The authors hoped that the routine adoption of these core domains would promote the collection of consistent and relevant information, bolster the strength of evidence synthesis, and minimize the risk for outcome reporting bias among studies.38 It is important to ascertain the patient’s description of his/her pain to distinguish between stimulus-dependent nociceptive pain vs spontaneous neuropathic pain.3,7,10 The most common pain descriptors used by patients are “shooting,” “itchy,” “blinding,” “cutting,” and “exhausting.”10 In addition to obtaining descriptive factors, it is important for the clinician to obtain information on the timing of the pain, whether or not the pain is relieved with spontaneous or surgical drainage, and if the patient is experiencing chronic background pain secondary to scarring or skin contraction.3 With the routine utilization of a consistent set of core domains, advances in our understanding of the different elements of HS pain, and increased provider awareness of the disease, the future of pain management in patients with HS seems promising.
Acute and Perioperative Pain Management
Acute Pain Management—The pain in HS can range from mild to excruciating.3,7 The difference between acute and chronic pain in this condition may be hard to delineate, as patients may have intense acute flares on top of a baseline level of chronic pain.3,7,14 These factors, in combination with various pain types of differing etiologies, make the treatment of HS-associated pain a therapeutic challenge.
The first-line treatments for acute pain in HS are oral acetaminophen, oral nonsteroidal anti-inflammatory drugs (NSAIDs), and topical analgesics.3 These treatment modalities are especially helpful for nociceptive pain, which often is described as having an aching or tender quality.3 Topical treatment for acute pain episodes includes diclofenac gel and liposomal lidocaine cream.39 Topical lidocaine in particular has the benefit of being rapid acting, and its effect can last 1 to 2 hours. Ketamine has been anecdotally used as a topical treatment. Treatment options for neuropathic pain include topical amitriptyline, gabapentin, and pregabalin.39 Dressings and ice packs may be used in cases of mild acute pain, depending on patient preference.3
First-line therapies may not provide adequate pain control in many patients.3,40,41 Should the first-line treatments fail, oral opiates can be considered as a treatment option, especially if the patient has a history of recurrent pain unresponsive to milder methods of pain control.3,40,41 However, prudence should be exercised, as patients with HS have a higher risk for opioid abuse, and referral to a pain specialist is advisable.40 Generally, use of opioids should be limited to the smallest period of time possible.40,41 Codeine can be used as a first opioid option, with hydromorphone available as an alternative.41
Pain caused by inflamed abscesses and nodules can be treated with either intralesional corticosteroids or incision and drainage. Intralesional triamcinolone has been found to cause substantial pain relief within 1 day of injection in patients with HS.3,42
Prompt discussion about the remitting course of HS will prepare patients for flares. Although the therapies discussed here aim to reduce the clinical severity and inflammation associated with HS, achieving pain-free remission can be challenging. Barriers to developing a long-term treatment regimen include intolerable side effects or simply nonresponsive disease.36,43
Management of Perioperative Pain—Medical treatment of HS often yields only transient or mild results. Hurley stage II or III lesions typically require surgical removal of affected tissues.32,44-46 Surgery may dramatically reduce the primary disease burden and provide substantial pain relief.3,4,44 Complete resection of the affected tissue by wide excision is the most common surgical procedure used.46-48 However, various tissue-sparing techniques, such as skin-tissue-sparing excision with electrosurgical peeling, also have been utilized. Tissue-sparing surgical techniques may lead to shorter healing times and less postoperative pain.48
There currently is little guidance available on the perioperative management of pain as it relates to surgical procedures for HS. The pain experienced from surgery varies based on the area and location of affected tissue; extent of disease; surgical technique used; and whether primary closure, closure by secondary intention, or skin grafting is utilized.47,49 Medical treatment aimed at reducing inflammation prior to surgical intervention may improve postoperative pain and complications.
The use of general vs local anesthesia during surgery depends on the extent of the disease and the amount of tissue being removed; however, the use of local anesthesia has been associated with a higher recurrence of disease, possibly owing to less aggressive tissue removal.50 Intraoperatively, the injection of 0.5% bupivacaine around the wound edges may lead to less postoperative pain.3,48 Postoperative pain usually is managed with acetaminophen and NSAIDs.48 In cases of severe postoperative pain, short- and long-acting opioid oxycodone preparations may be used. The combination of diclofenac and tramadol also has been used postoperatively.3 Patients who do not undergo extensive surgery often can leave the hospital the same day.
Effective strategies for mitigating HS-associated pain must address the chronic pain component of the disease. Long-term management involves lifestyle modifications and pharmacologic agents.
Chronic Pain Management
Although HS is not a curable disease, there are treatments available to minimize symptoms. Long-term management of HS is essential to minimize the effects of chronic pain and physical scarring associated with inflammation.31 In one study from the French Society of Dermatology, pain reported by patients with HS was directly associated with severity and duration of disease, emotional symptoms, and reduced functionality.51 For these reasons, many treatments for HS target reducing clinical severity and achieving remission, often defined as more than 6 months without any recurrence of lesions.52 In addition to lifestyle management, therapies available to manage HS include topical and systemic medications as well as procedures such as surgical excision.36,43,52,53
Lifestyle Modifications
Regardless of the severity of HS, all patients may benefit from basic education on the pathogenesis of the disease.36 The associations with smoking and obesity have been well documented, and treatment of these comorbid conditions is indicated.36,43,52 For example, in relation to obesity, the use of metformin is very well tolerated and seems to positively impact HS symptoms.43 Several studies have suggested that weight reduction lowers disease severity.28-30 Patients should be counseled on the importance of smoking cessation and weight loss.
Finally, the emotional impact of HS is not to be discounted, both the physical and social discomfort as well as the chronicity of the disease and frustration with treatment.51 Chronic pain has been associated with increased rates of depression, and 43% of patients with HS specifically have been diagnosed with major depressive disorder.7 For these reasons, clinician guidance, social support, and websites can improve patient understanding of the disease, adherence to treatment, and comorbid anxiety and depression.52
Topical Therapy
Topical therapy generally is limited to mild disease and is geared at decreasing inflammation or superimposed infection.36,52 Some of the earliest therapies used were topical antibiotics.43 Topical clindamycin has been shown to be as effective as oral tetracyclines in reducing the number of abscesses, but neither treatment substantially reduces pain associated with smaller nodules.54 Intralesional corticosteroids such as triamcinolone acetonide have been shown to decrease both patient-reported pain and physician-assessed severity within 1 to 7 days.42 Routine injection, however, is not a feasible means of long-term treatment both because of inconvenience and the potential adverse effects of corticosteroids.36,52 Both topical clindamycin and intralesional steroids are helpful in reducing inflammation prior to planned surgical intervention.36,52,53
Newer topical therapies include resorcinol peels and combination antimicrobials, such as 2% triclosan and oral zinc gluconate.52,53 Data surrounding the use of resorcinol in mild to moderate HS are promising and have shown decreased severity of both new and long-standing nodules. Fifteen-percent resorcinol peels are helpful tools that allow for self-administration by patients during exacerbations to decrease pain and flare duration.55,56 In a 2016 clinical trial, a combination of oral zinc gluconate with topical triclosan was shown to reduce flare-ups and nodules in mild HS.57 Oral zinc alone may have anti-inflammatory properties and generally is well tolerated.43,53 Topical therapies have a role in reducing HS-associated pain but often are limited to milder disease.
Systemic Agents
Several therapeutic options exist for the treatment of HS; however, a detailed description of their mechanisms and efficacies is beyond the scope of this review, which is focused on pain. Briefly, these systemic agents include antibiotics, retinoids, corticosteroids, antiandrogens, and biologics.43,52,53
Treatment with antibiotics such as tetracyclines or a combination of clindamycin plus rifampin has been shown to produce complete remission in 60% to 80% of users; however, this treatment requires more than 6 months of antibiotic therapy, which can be difficult to tolerate.52,53,58 Relapse is common after antibiotic cessation.2,43,52 Antibiotics have demonstrated efficacy during acute flares and in reducing inflammatory activity prior to surgery.52
Retinoids have been utilized in the treatment of HS because of their action on sebaceous glands and hair follicles.43,53 Acitretin has been shown to be the most effective oral retinoid available in the United States.43 Unfortunately, many of the studies investigating the use of retinoids for treatment of HS are limited by small sample size.36,43,52
Because HS is predominantly an inflammatory condition, immunosuppressants have been adapted to manage patients when antibiotics and topicals have failed. Systemic steroids rarely are used for long-term therapy because of the severe side effects and are preferred only for acute management.36,52 Cyclosporine and dapsone have demonstrated efficacy in treating moderate to severe HS, whereas methotrexate and colchicine have shown little efficacy.52 Both cyclosporine and dapsone are difficult to tolerate, require laboratory monitoring, and lead to only conservative improvement rather than remission in most patients.43
Immune dysregulation in HS involves elevated levels of proinflammatory cytokines such as tumor necrosis factor α (TNF-α), which is a key mediator of inflammation and a stimulator of other inflammatory cytokines.59,60 The first approved biologic treatment of HS was adalimumab, a TNF-α inhibitor, which showed a 50% reduction in total abscess and inflammatory nodule count in 60% of patients with moderate to severe HS.61-63 Of course, TNF-α inhibitor therapy is not without risks, specifically those of infection.43,53,61,62 Maintenance therapy may be required if patients relapse.53,61
Various interleukin inhibitors also have emerged as potential therapies for HS, such as ustekinumab and anakinra.36,64 Both have been subject to numerous small case trials that have reported improvements in clinical severity and pain; however, both drugs were associated with a fair number of nonresponders.36,64,65
Surgical Procedures
Although HS lesions may regress on their own in a matter of weeks, surgical drainage allows an acute alleviation of the severe burning pain associated with HS flares.36,52,53 Because of improved understanding of the disease pathophysiology, recent therapies targeting the hair follicle have been developed and have shown promising results. These therapies include laser- and light-based procedures. Long-pulsed Nd:YAG laser therapy reduces the number of hair follicles and sebaceous glands and has been effective for Hurley stage I or II disease.36,43,52,53,66 Photodynamic therapy offers a less-invasive option compared to surgery and laser therapy.52,53,66 Both Nd:YAG and CO2 laser therapy offer low recurrence rates (<30%) due to destruction of the apocrine unit.43,53 Photodynamic therapy for mild disease offers a less-invasive option compared to surgery and laser therapy.53 There is a need for larger randomized controlled trials involving laser, light, and CO2 therapies.66
Conclusion
Hidradenitis suppurativa is a debilitating condition with an underestimated disease burden. Although the pathophysiology of the disease is not completely understood, it is evident that pain is a major cause of morbidity. Patients experience a multitude of acute and chronic pain types: inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic. Pain perception and quality of life are further impacted by psychiatric conditions such as depression and anxiety, both of which are common comorbidities in patients with HS. Several pharmacologic agents have been used to treat HS-associated pain with mixed results. First-line treatment of acute pain episodes includes oral acetaminophen, NSAIDs, and topical analgesics. Management of chronic pain includes utilization of topical agents, systemic agents, and biologics, as well as addressing lifestyle (eg, obesity, smoking status) and psychiatric comorbidities. Although these therapies have roles in HS pain management, the most effective pain remedies developed thus far are limited to surgery and TNF-α inhibitors. Optimization of pain control in patients with HS requires multidisciplinary collaboration among dermatologists, pain specialists, psychiatrists, and other members of the health care team. Further large-scale studies are needed to create an evidence-based treatment algorithm for the management of pain in HS.
Hidradenitis suppurativa (HS) is a chronic inflammatory, androgen gland disorder characterized by recurrent rupture of the hair follicles with a vigorous inflammatory response. This response results in abscess formation and development of draining sinus tracts and hypertrophic fibrous scars.1,2 Pain, discomfort, and odorous discharge from the recalcitrant lesions have a profound impact on patient quality of life.3,4
The morbidity and disease burden associated with HS are particularly underestimated, as patients frequently report debilitating pain that often is overlooked.5,6 Additionally, the quality and intensity of perceived pain are compounded by frequently associated depression and anxiety.7-9 Pain has been reported by patients with HS to be the highest cause of morbidity, despite the disfiguring nature of the disease and its associated psychosocial distress.7,10 Nonetheless, HS lacks an accepted pain management algorithm similar to those that have been developed for the treatment of other acute or chronic pain disorders, such as back pain and sickle cell disease.4,11-13
Given the lack of formal studies regarding pain management in patients with HS, clinicians are limited to general pain guidelines, expert opinion, small trials, and patient preference.3 Furthermore, effective pain management in HS necessitates the treatment of both chronic pain affecting daily function and acute pain present during disease flares, surgical interventions, and dressing changes.3 The result is a wide array of strategies used for HS-associated pain.3,4
Epidemiology and Pathophysiology
Hidradenitis suppurativa historically has been an overlooked and underdiagnosed disease, which limits epidemiology data.5 Current estimates are that HS affects approximately 1% of the general population; however, prevalence rates range from 0.03% to 4.1%.14-16
The exact etiology of HS remains unclear, but it is thought that genetic factors, immune dysregulation, and environmental/behavioral influences all contribute to its pathophysiology.1,17 Up to 40% of patients with HS report a positive family history of the disease.18-20 Hidradenitis suppurativa has been associated with other inflammatory disease states, such as inflammatory bowel disease, spondyloarthropathies, and pyoderma gangrenosum.16,21,22
It is thought that HS is the result of some defect in keratin clearance that leads to follicular hyperkeratinization and occlusion.1 Resultant rupture of pilosebaceous units and spillage of contents (including keratin and bacteria) into the surrounding dermis triggers a vigorous inflammatory response. Sinus tracts and fistulas become the targets of bacterial colonization, biofilm formation, and secondary infection. The result is suppuration and extension of the lesions as well as sustained chronic inflammation.23,24
Although the etiology of HS is complex, several modifiable risk factors for the disease have been identified, most prominently cigarette smoking and obesity. Approximately 70% of patients with HS smoke cigarettes.2,15,25,26 Obesity has a well-known association with HS, and it is possible that weight reduction lowers disease severity.27-30
Clinical Presentation and Diagnosis
Establishing a diagnosis of HS necessitates recognition of disease morphology, topography, and chronicity. Hidradenitis suppurativa most commonly occurs in the axillae, inguinal and anogenital region, perineal region, and inframammary region.5,31 A typical history involves a prolonged disease course with recurrent lesions and intermittent periods of improvement or remission. Primary lesions are deep, inflamed, painful, and sterile. Ultimately, these lesions rupture and track subcutaneously.15,25 Intercommunicating sinus tracts form from multiple recurrent nodules in close proximity and may ultimately lead to fibrotic scarring and local architectural distortion.32 The Hurley staging system helps to guide treatment interventions based on disease severity. Approach to pain management is discussed below.
Pain Management in HS: General Principles
Pain management is complex for clinicians, as there are limited studies from which to draw treatment recommendations. Incomplete understanding of the etiology and pathophysiology of the disease contributes to the lack of established management guidelines.
A PubMed search of articles indexed for MEDLINE using the terms hidradenitis, suppurativa, pain, and management revealed 61 different results dating back to 1980, 52 of which had been published in the last 5 years. When the word acute was added to the search, there were only 6 results identified. These results clearly reflect a better understanding of HS-mediated pain as well as clinical unmet needs and evolving strategies in pain management therapeutics. However, many of these studies reflect therapies focused on the mediation or modulation of HS pathogenesis rather than potential pain management therapies.
In addition, the heterogenous nature of the pain experience in HS poses a challenge for clinicians. Patients may experience multiple pain types concurrently, including inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic, as well as pain related to arthritis.3,33,34 Pain perception is further complicated by the observation that patients with HS have high rates of psychiatric comorbidities such as depression and anxiety, both of which profoundly alter perception of both the strength and quality of pain.7,8,22,35 A suggested algorithm for treatment of pain in HS is described in the eTable.36
Chronicity is a hallmark of HS. Patients experience a prolonged disease course involving acute painful exacerbations superimposed on chronic pain that affects all aspects of daily life. Changes in self-perception, daily living activities, mood state, physical functioning, and physical comfort frequently are reported to have a major impact on quality of life.1,3,37
In 2018, Thorlacius et al38 created a multistakeholder consensus on a core outcome set of domains detailing what to measure in clinical trials for HS. The authors hoped that the routine adoption of these core domains would promote the collection of consistent and relevant information, bolster the strength of evidence synthesis, and minimize the risk for outcome reporting bias among studies.38 It is important to ascertain the patient’s description of his/her pain to distinguish between stimulus-dependent nociceptive pain vs spontaneous neuropathic pain.3,7,10 The most common pain descriptors used by patients are “shooting,” “itchy,” “blinding,” “cutting,” and “exhausting.”10 In addition to obtaining descriptive factors, it is important for the clinician to obtain information on the timing of the pain, whether or not the pain is relieved with spontaneous or surgical drainage, and if the patient is experiencing chronic background pain secondary to scarring or skin contraction.3 With the routine utilization of a consistent set of core domains, advances in our understanding of the different elements of HS pain, and increased provider awareness of the disease, the future of pain management in patients with HS seems promising.
Acute and Perioperative Pain Management
Acute Pain Management—The pain in HS can range from mild to excruciating.3,7 The difference between acute and chronic pain in this condition may be hard to delineate, as patients may have intense acute flares on top of a baseline level of chronic pain.3,7,14 These factors, in combination with various pain types of differing etiologies, make the treatment of HS-associated pain a therapeutic challenge.
The first-line treatments for acute pain in HS are oral acetaminophen, oral nonsteroidal anti-inflammatory drugs (NSAIDs), and topical analgesics.3 These treatment modalities are especially helpful for nociceptive pain, which often is described as having an aching or tender quality.3 Topical treatment for acute pain episodes includes diclofenac gel and liposomal lidocaine cream.39 Topical lidocaine in particular has the benefit of being rapid acting, and its effect can last 1 to 2 hours. Ketamine has been anecdotally used as a topical treatment. Treatment options for neuropathic pain include topical amitriptyline, gabapentin, and pregabalin.39 Dressings and ice packs may be used in cases of mild acute pain, depending on patient preference.3
First-line therapies may not provide adequate pain control in many patients.3,40,41 Should the first-line treatments fail, oral opiates can be considered as a treatment option, especially if the patient has a history of recurrent pain unresponsive to milder methods of pain control.3,40,41 However, prudence should be exercised, as patients with HS have a higher risk for opioid abuse, and referral to a pain specialist is advisable.40 Generally, use of opioids should be limited to the smallest period of time possible.40,41 Codeine can be used as a first opioid option, with hydromorphone available as an alternative.41
Pain caused by inflamed abscesses and nodules can be treated with either intralesional corticosteroids or incision and drainage. Intralesional triamcinolone has been found to cause substantial pain relief within 1 day of injection in patients with HS.3,42
Prompt discussion about the remitting course of HS will prepare patients for flares. Although the therapies discussed here aim to reduce the clinical severity and inflammation associated with HS, achieving pain-free remission can be challenging. Barriers to developing a long-term treatment regimen include intolerable side effects or simply nonresponsive disease.36,43
Management of Perioperative Pain—Medical treatment of HS often yields only transient or mild results. Hurley stage II or III lesions typically require surgical removal of affected tissues.32,44-46 Surgery may dramatically reduce the primary disease burden and provide substantial pain relief.3,4,44 Complete resection of the affected tissue by wide excision is the most common surgical procedure used.46-48 However, various tissue-sparing techniques, such as skin-tissue-sparing excision with electrosurgical peeling, also have been utilized. Tissue-sparing surgical techniques may lead to shorter healing times and less postoperative pain.48
There currently is little guidance available on the perioperative management of pain as it relates to surgical procedures for HS. The pain experienced from surgery varies based on the area and location of affected tissue; extent of disease; surgical technique used; and whether primary closure, closure by secondary intention, or skin grafting is utilized.47,49 Medical treatment aimed at reducing inflammation prior to surgical intervention may improve postoperative pain and complications.
The use of general vs local anesthesia during surgery depends on the extent of the disease and the amount of tissue being removed; however, the use of local anesthesia has been associated with a higher recurrence of disease, possibly owing to less aggressive tissue removal.50 Intraoperatively, the injection of 0.5% bupivacaine around the wound edges may lead to less postoperative pain.3,48 Postoperative pain usually is managed with acetaminophen and NSAIDs.48 In cases of severe postoperative pain, short- and long-acting opioid oxycodone preparations may be used. The combination of diclofenac and tramadol also has been used postoperatively.3 Patients who do not undergo extensive surgery often can leave the hospital the same day.
Effective strategies for mitigating HS-associated pain must address the chronic pain component of the disease. Long-term management involves lifestyle modifications and pharmacologic agents.
Chronic Pain Management
Although HS is not a curable disease, there are treatments available to minimize symptoms. Long-term management of HS is essential to minimize the effects of chronic pain and physical scarring associated with inflammation.31 In one study from the French Society of Dermatology, pain reported by patients with HS was directly associated with severity and duration of disease, emotional symptoms, and reduced functionality.51 For these reasons, many treatments for HS target reducing clinical severity and achieving remission, often defined as more than 6 months without any recurrence of lesions.52 In addition to lifestyle management, therapies available to manage HS include topical and systemic medications as well as procedures such as surgical excision.36,43,52,53
Lifestyle Modifications
Regardless of the severity of HS, all patients may benefit from basic education on the pathogenesis of the disease.36 The associations with smoking and obesity have been well documented, and treatment of these comorbid conditions is indicated.36,43,52 For example, in relation to obesity, the use of metformin is very well tolerated and seems to positively impact HS symptoms.43 Several studies have suggested that weight reduction lowers disease severity.28-30 Patients should be counseled on the importance of smoking cessation and weight loss.
Finally, the emotional impact of HS is not to be discounted, both the physical and social discomfort as well as the chronicity of the disease and frustration with treatment.51 Chronic pain has been associated with increased rates of depression, and 43% of patients with HS specifically have been diagnosed with major depressive disorder.7 For these reasons, clinician guidance, social support, and websites can improve patient understanding of the disease, adherence to treatment, and comorbid anxiety and depression.52
Topical Therapy
Topical therapy generally is limited to mild disease and is geared at decreasing inflammation or superimposed infection.36,52 Some of the earliest therapies used were topical antibiotics.43 Topical clindamycin has been shown to be as effective as oral tetracyclines in reducing the number of abscesses, but neither treatment substantially reduces pain associated with smaller nodules.54 Intralesional corticosteroids such as triamcinolone acetonide have been shown to decrease both patient-reported pain and physician-assessed severity within 1 to 7 days.42 Routine injection, however, is not a feasible means of long-term treatment both because of inconvenience and the potential adverse effects of corticosteroids.36,52 Both topical clindamycin and intralesional steroids are helpful in reducing inflammation prior to planned surgical intervention.36,52,53
Newer topical therapies include resorcinol peels and combination antimicrobials, such as 2% triclosan and oral zinc gluconate.52,53 Data surrounding the use of resorcinol in mild to moderate HS are promising and have shown decreased severity of both new and long-standing nodules. Fifteen-percent resorcinol peels are helpful tools that allow for self-administration by patients during exacerbations to decrease pain and flare duration.55,56 In a 2016 clinical trial, a combination of oral zinc gluconate with topical triclosan was shown to reduce flare-ups and nodules in mild HS.57 Oral zinc alone may have anti-inflammatory properties and generally is well tolerated.43,53 Topical therapies have a role in reducing HS-associated pain but often are limited to milder disease.
Systemic Agents
Several therapeutic options exist for the treatment of HS; however, a detailed description of their mechanisms and efficacies is beyond the scope of this review, which is focused on pain. Briefly, these systemic agents include antibiotics, retinoids, corticosteroids, antiandrogens, and biologics.43,52,53
Treatment with antibiotics such as tetracyclines or a combination of clindamycin plus rifampin has been shown to produce complete remission in 60% to 80% of users; however, this treatment requires more than 6 months of antibiotic therapy, which can be difficult to tolerate.52,53,58 Relapse is common after antibiotic cessation.2,43,52 Antibiotics have demonstrated efficacy during acute flares and in reducing inflammatory activity prior to surgery.52
Retinoids have been utilized in the treatment of HS because of their action on sebaceous glands and hair follicles.43,53 Acitretin has been shown to be the most effective oral retinoid available in the United States.43 Unfortunately, many of the studies investigating the use of retinoids for treatment of HS are limited by small sample size.36,43,52
Because HS is predominantly an inflammatory condition, immunosuppressants have been adapted to manage patients when antibiotics and topicals have failed. Systemic steroids rarely are used for long-term therapy because of the severe side effects and are preferred only for acute management.36,52 Cyclosporine and dapsone have demonstrated efficacy in treating moderate to severe HS, whereas methotrexate and colchicine have shown little efficacy.52 Both cyclosporine and dapsone are difficult to tolerate, require laboratory monitoring, and lead to only conservative improvement rather than remission in most patients.43
Immune dysregulation in HS involves elevated levels of proinflammatory cytokines such as tumor necrosis factor α (TNF-α), which is a key mediator of inflammation and a stimulator of other inflammatory cytokines.59,60 The first approved biologic treatment of HS was adalimumab, a TNF-α inhibitor, which showed a 50% reduction in total abscess and inflammatory nodule count in 60% of patients with moderate to severe HS.61-63 Of course, TNF-α inhibitor therapy is not without risks, specifically those of infection.43,53,61,62 Maintenance therapy may be required if patients relapse.53,61
Various interleukin inhibitors also have emerged as potential therapies for HS, such as ustekinumab and anakinra.36,64 Both have been subject to numerous small case trials that have reported improvements in clinical severity and pain; however, both drugs were associated with a fair number of nonresponders.36,64,65
Surgical Procedures
Although HS lesions may regress on their own in a matter of weeks, surgical drainage allows an acute alleviation of the severe burning pain associated with HS flares.36,52,53 Because of improved understanding of the disease pathophysiology, recent therapies targeting the hair follicle have been developed and have shown promising results. These therapies include laser- and light-based procedures. Long-pulsed Nd:YAG laser therapy reduces the number of hair follicles and sebaceous glands and has been effective for Hurley stage I or II disease.36,43,52,53,66 Photodynamic therapy offers a less-invasive option compared to surgery and laser therapy.52,53,66 Both Nd:YAG and CO2 laser therapy offer low recurrence rates (<30%) due to destruction of the apocrine unit.43,53 Photodynamic therapy for mild disease offers a less-invasive option compared to surgery and laser therapy.53 There is a need for larger randomized controlled trials involving laser, light, and CO2 therapies.66
Conclusion
Hidradenitis suppurativa is a debilitating condition with an underestimated disease burden. Although the pathophysiology of the disease is not completely understood, it is evident that pain is a major cause of morbidity. Patients experience a multitude of acute and chronic pain types: inflammatory, noninflammatory, nociceptive, neuropathic, and ischemic. Pain perception and quality of life are further impacted by psychiatric conditions such as depression and anxiety, both of which are common comorbidities in patients with HS. Several pharmacologic agents have been used to treat HS-associated pain with mixed results. First-line treatment of acute pain episodes includes oral acetaminophen, NSAIDs, and topical analgesics. Management of chronic pain includes utilization of topical agents, systemic agents, and biologics, as well as addressing lifestyle (eg, obesity, smoking status) and psychiatric comorbidities. Although these therapies have roles in HS pain management, the most effective pain remedies developed thus far are limited to surgery and TNF-α inhibitors. Optimization of pain control in patients with HS requires multidisciplinary collaboration among dermatologists, pain specialists, psychiatrists, and other members of the health care team. Further large-scale studies are needed to create an evidence-based treatment algorithm for the management of pain in HS.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi:10.2147/CCID.S111019
- Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatology Venereol. 2009;23:985-998. doi:10.1111/j.1468-3083.2009.03356.x
- Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S47-S51. doi:10.1016/j.jaad.2015.07.046
- Puza CJ, Wolfe SA, Jaleel T. Pain management in patients with hidradenitis suppurativa requiring surgery. Dermatolog Surg. 2019;45:1327-1330. doi:10.1097/DSS.0000000000001693
- Kurzen H, Kurokawa I, Jemec GBE, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Kelly G, Sweeney CM, Tobin AM, et al. Hidradenitis suppurativa: the role of immune dysregulation. Int J Dermatol. 2014;53:1186-1196. doi:10.1111/ijd.12550
- Patel ZS, Hoffman LK, Buse DC, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep. 2017;21:49. doi:10.1007/s11916-017-0647-3
- Sist TC, Florio GA, Miner MF, et al. The relationship between depression and pain language in cancer and chronic non-cancer pain patients. J Pain Symptom Manage. 1998;15:350-358. doi:10.1016/S0885-3924(98)00006-2
- Jemec GBE. Hidradenitis suppurativa. N Engl J Med. 2012;366:158-164. doi:10.1056/NEJMcp1014163
- Nielsen RM, Lindsø Andersen P, Sigsgaard V, et al. Pain perception in patients with hidradenitis suppurativa. Br J Dermatol. 2019;182:bjd.17935. doi:10.1111/bjd.17935
- Tanabe P, Myers R, Zosel A, et al. Emergency department management of acute pain episodes in sickle cell disease. Acad Emerg Med. 2007;14:419-425. doi:10.1197/j.aem.2006.11.033
- Chou R, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009;34:1066-1077. doi:10.1097/BRS.0b013e3181a1390d
- Enamandram M, Rathmell JP, Kimball AB. Chronic pain management in dermatology: a guide to assessment and nonopioid pharmacotherapy. J Am Acad Dermatol. 2015;73:563-573; quiz 573-574. doi:10.1016/j.jaad.2014.11.039
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7. doi:10.1016/j.jaad.2015.07.052
- Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
- Patil S, Apurwa A, Nadkarni N, et al. Hidradenitis suppurativa: inside and out. Indian J Dermatol. 2018;63:91-98. doi:10.4103/ijd.IJD_412_16
- Woodruff CM, Charlie AM, Leslie KS. Hidradenitis suppurativa: a guide for the practicing physician. Mayo Clin Proc. 2015;90:1679-1693. doi:10.1016/j.mayocp.2015.08.020
- Pink AE, Simpson MA, Desai N, et al. Mutations in the γ-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa). J Invest Dermatol. 2012;132:2459-2461. doi:10.1038/jid.2012.162
- Jemec GBE, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35:191-194. doi:10.1016/s0190-9622(96)90321-7
- Fitzsimmons JS, Guilbert PR. A family study of hidradenitis suppurativa. J Med Genet. 1985;22:367-373. doi:10.1136/jmg.22.5.367
- Kelly G, Prens EP. Inflammatory mechanisms in hidradenitis suppurativa. Dermatol Clin. 2016;34:51-58. doi:10.1016/j.det.2015.08.004
- Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123. doi:10.1097/QCO.0b013e3283428d07
- Kathju S, Lasko LA, Stoodley P. Considering hidradenitis suppurativa as a bacterial biofilm disease. FEMS Immunol Med Microbiol. 2012;65:385-389. doi:10.1111/j.1574-695X.2012.00946.x
- Jahns AC, Killasli H, Nosek D, et al. Microbiology of hidradenitis suppurativa (acne inversa): a histological study of 27 patients. APMIS. 2014;122:804-809. doi:10.1111/apm.12220
- Ralf Paus L, Kurzen H, Kurokawa I, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Kromann CB, Ibler KS, Kristiansen VB, et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol. 2014;94:553-557. doi:10.2340/00015555-1800
- Lindsø Andersen P, Kromann C, Fonvig CE, et al. Hidradenitis suppurativa in a cohort of overweight and obese children and adolescents. Int J Dermatol. 2020;59:47-51. doi:10.1111/ijd.14639
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601. doi:10.1016/j.jaad.2008.06.020
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824. doi:10.1111/bjd.13090
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Hsiao J, Leslie K, McMichael A, et al. Folliculitis and other follicular disorders. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:615-632.
- Scheinfeld N. Treatment of hidradenitis suppurativa associated pain with nonsteroidal anti-inflammatory drugs, acetaminophen, celecoxib, gapapentin, pegabalin, duloxetine, and venlafaxine. Dermatol Online J. 2013;19:20616.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1.
- Rajmohan V, Suresh Kumar S. Psychiatric morbidity, pain perception, and functional status of chronic pain patients in palliative care. Indian J Palliat Care. 2013;19:146-151. doi:10.4103/0973-1075.121527
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032. doi:10.1001/jama.2017.16691
- Wang B, Yang W, Wen W, et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330:1065. doi:10.1126/science.1196284
- Thorlacius L, Ingram JR, Villumsen B, et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol. 2018;179:642-650. doi:10.1111/bjd.16672
- Scheinfeld N. Topical treatments of skin pain: a general review with a focus on hidradenitis suppurativa with topical agents. Dermatol Online J. 2014;20:13030/qt4m57506k.
- Reddy S, Orenstein LAV, Strunk A, et al. Incidence of long-term opioid use among opioid-naive patients with hidradenitis suppurativa in the United States. JAMA Dermatol. 2019;155:1284-1290. doi:10.1001/jamadermatol.2019.2610
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatology Venereol. 2015;29:619-644. doi:10.1111/jdv.12966
- Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155. doi:10.1016/j.jaad.2016.06.049
- Robert E, Bodin F, Paul C, et al. Non-surgical treatments for hidradenitis suppurativa: a systematic review. Ann Chir Plast Esthet. 2017;62:274-294. doi:10.1016/j.anplas.2017.03.012
- Menderes A, Sunay O, Vayvada H, et al. Surgical management of hidradenitis suppurativa. Int J Med Sci. 2010;7:240-247. doi:10.7150/ijms.7.240
- Alharbi Z, Kauczok J, Pallua N. A review of wide surgical excision of hidradenitis suppurativa. BMC Dermatol. 2012;12:9. doi:10.1186/1471-5945-12-9
- Burney RE. 35-year experience with surgical treatment of hidradenitis suppurativa. World J Surg. 2017;41:2723-2730. doi:10.1007/s00268-017-4091-7
- Bocchini SF, Habr-Gama A, Kiss DR, et al. Gluteal and perianal hidradenitis suppurativa: surgical treatment by wide excision. Dis Colon Rectum. 2003;46:944-949. doi:10.1007/s10350-004-6691-1
- Blok JL, Spoo JR, Leeman FWJ, et al. Skin-tissue-sparing excision with electrosurgical peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382. doi:10.1111/jdv.12376
- Bilali S, Todi V, Lila A, et al. Surgical treatment of chronic hidradenitis suppurativa in the gluteal and perianal regions. Acta Chir Iugosl. 2012;59:91-95. doi:10.2298/ACI1202091B
- Walter AC, Meissner M, Kaufmann R, et al. Hidradenitis suppurativa after radical surgery-long-term follow-up for recurrences and associated factors. Dermatol Surg. 2018;44:1323-1331. doi:10.1097/DSS.0000000000001668.
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623. doi:10.1016/j.jaad.2006.08.061
- Alavi A, Lynde C, Alhusayen R, et al. Approach to the management of patients with hidradenitis suppurativa: a consensus document. J Cutan Med Surg. 2017;21:513-524. doi:10.1177/1203475417716117
- Duran C, Baumeister A. Recognition, diagnosis, and treatment of hidradenitis suppurativa. J Am Acad Physician Assist. 2019;32:36-42. doi:10.1097/01.JAA.0000578768.62051.13
- Jemec GBE, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-974. doi:10.1016/S0190-9622(98)70272-5
- Pascual JC, Encabo B, Ruiz de Apodaca RF, et al. Topical 15% resorcinol for hidradenitis suppurativa: an uncontrolled prospective trial with clinical and ultrasonographic follow-up. J Am Acad Dermatol. 2017;77:1175-1178. doi:10.1016/j.jaad.2017.07.008
- Boer J, Jemec GBE. Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa. Clin Exp Dermatol. 2010;35:36-40. doi:10.1111/j.1365-2230.2009.03377.x
- Hessam S, Sand M, Meier NM, et al. Combination of oral zinc gluconate and topical triclosan: an anti-inflammatory treatment modality for initial hidradenitis suppurativa. J Dermatol Sci. 2016;84:197-202. doi:10.1016/j.jdermsci.2016.08.010
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154. doi:10.1159/000228334
- Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
- Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328:222-225. doi:10.1016/j.canlet.2012.10.014
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Morita A, Takahashi H, Ozawa K, et al. Twenty-four-week interim analysis from a phase 3 open-label trial of adalimumab in Japanese patients with moderate to severe hidradenitis suppurativa. J Dermatol. 2019;46:745-751. doi:10.1111/1346-8138.14997
- Ghias MH, Johnston AD, Kutner AJ, et al. High-dose, high-frequency infliximab: a novel treatment paradigm for hidradenitis suppurativa. J Am Acad Dermatol. 2020;82:1094-1101. doi:10.1016/j.jaad.2019.09.071
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa a randomized clinical trial. JAMA Dermatol. 2016;152:52-59. doi:10.1001/jamadermatol.2015.3903
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846. doi:10.1111/bjd.14338
- John H, Manoloudakis N, Stephen Sinclair J. A systematic review of the use of lasers for the treatment of hidradenitis suppurativa. J Plast Reconstr Aesthet Surg. 2016;69:1374-1381. doi:10.1016/j.bjps.2016.05.029
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi:10.2147/CCID.S111019
- Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatology Venereol. 2009;23:985-998. doi:10.1111/j.1468-3083.2009.03356.x
- Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S47-S51. doi:10.1016/j.jaad.2015.07.046
- Puza CJ, Wolfe SA, Jaleel T. Pain management in patients with hidradenitis suppurativa requiring surgery. Dermatolog Surg. 2019;45:1327-1330. doi:10.1097/DSS.0000000000001693
- Kurzen H, Kurokawa I, Jemec GBE, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Kelly G, Sweeney CM, Tobin AM, et al. Hidradenitis suppurativa: the role of immune dysregulation. Int J Dermatol. 2014;53:1186-1196. doi:10.1111/ijd.12550
- Patel ZS, Hoffman LK, Buse DC, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep. 2017;21:49. doi:10.1007/s11916-017-0647-3
- Sist TC, Florio GA, Miner MF, et al. The relationship between depression and pain language in cancer and chronic non-cancer pain patients. J Pain Symptom Manage. 1998;15:350-358. doi:10.1016/S0885-3924(98)00006-2
- Jemec GBE. Hidradenitis suppurativa. N Engl J Med. 2012;366:158-164. doi:10.1056/NEJMcp1014163
- Nielsen RM, Lindsø Andersen P, Sigsgaard V, et al. Pain perception in patients with hidradenitis suppurativa. Br J Dermatol. 2019;182:bjd.17935. doi:10.1111/bjd.17935
- Tanabe P, Myers R, Zosel A, et al. Emergency department management of acute pain episodes in sickle cell disease. Acad Emerg Med. 2007;14:419-425. doi:10.1197/j.aem.2006.11.033
- Chou R, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009;34:1066-1077. doi:10.1097/BRS.0b013e3181a1390d
- Enamandram M, Rathmell JP, Kimball AB. Chronic pain management in dermatology: a guide to assessment and nonopioid pharmacotherapy. J Am Acad Dermatol. 2015;73:563-573; quiz 573-574. doi:10.1016/j.jaad.2014.11.039
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7. doi:10.1016/j.jaad.2015.07.052
- Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
- Patil S, Apurwa A, Nadkarni N, et al. Hidradenitis suppurativa: inside and out. Indian J Dermatol. 2018;63:91-98. doi:10.4103/ijd.IJD_412_16
- Woodruff CM, Charlie AM, Leslie KS. Hidradenitis suppurativa: a guide for the practicing physician. Mayo Clin Proc. 2015;90:1679-1693. doi:10.1016/j.mayocp.2015.08.020
- Pink AE, Simpson MA, Desai N, et al. Mutations in the γ-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa). J Invest Dermatol. 2012;132:2459-2461. doi:10.1038/jid.2012.162
- Jemec GBE, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35:191-194. doi:10.1016/s0190-9622(96)90321-7
- Fitzsimmons JS, Guilbert PR. A family study of hidradenitis suppurativa. J Med Genet. 1985;22:367-373. doi:10.1136/jmg.22.5.367
- Kelly G, Prens EP. Inflammatory mechanisms in hidradenitis suppurativa. Dermatol Clin. 2016;34:51-58. doi:10.1016/j.det.2015.08.004
- Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123. doi:10.1097/QCO.0b013e3283428d07
- Kathju S, Lasko LA, Stoodley P. Considering hidradenitis suppurativa as a bacterial biofilm disease. FEMS Immunol Med Microbiol. 2012;65:385-389. doi:10.1111/j.1574-695X.2012.00946.x
- Jahns AC, Killasli H, Nosek D, et al. Microbiology of hidradenitis suppurativa (acne inversa): a histological study of 27 patients. APMIS. 2014;122:804-809. doi:10.1111/apm.12220
- Ralf Paus L, Kurzen H, Kurokawa I, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456. doi:10.1111/j.1600-0625.2008.00712_1.x
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Kromann CB, Ibler KS, Kristiansen VB, et al. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol. 2014;94:553-557. doi:10.2340/00015555-1800
- Lindsø Andersen P, Kromann C, Fonvig CE, et al. Hidradenitis suppurativa in a cohort of overweight and obese children and adolescents. Int J Dermatol. 2020;59:47-51. doi:10.1111/ijd.14639
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601. doi:10.1016/j.jaad.2008.06.020
- Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824. doi:10.1111/bjd.13090
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Hsiao J, Leslie K, McMichael A, et al. Folliculitis and other follicular disorders. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:615-632.
- Scheinfeld N. Treatment of hidradenitis suppurativa associated pain with nonsteroidal anti-inflammatory drugs, acetaminophen, celecoxib, gapapentin, pegabalin, duloxetine, and venlafaxine. Dermatol Online J. 2013;19:20616.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1.
- Rajmohan V, Suresh Kumar S. Psychiatric morbidity, pain perception, and functional status of chronic pain patients in palliative care. Indian J Palliat Care. 2013;19:146-151. doi:10.4103/0973-1075.121527
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032. doi:10.1001/jama.2017.16691
- Wang B, Yang W, Wen W, et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330:1065. doi:10.1126/science.1196284
- Thorlacius L, Ingram JR, Villumsen B, et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol. 2018;179:642-650. doi:10.1111/bjd.16672
- Scheinfeld N. Topical treatments of skin pain: a general review with a focus on hidradenitis suppurativa with topical agents. Dermatol Online J. 2014;20:13030/qt4m57506k.
- Reddy S, Orenstein LAV, Strunk A, et al. Incidence of long-term opioid use among opioid-naive patients with hidradenitis suppurativa in the United States. JAMA Dermatol. 2019;155:1284-1290. doi:10.1001/jamadermatol.2019.2610
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatology Venereol. 2015;29:619-644. doi:10.1111/jdv.12966
- Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155. doi:10.1016/j.jaad.2016.06.049
- Robert E, Bodin F, Paul C, et al. Non-surgical treatments for hidradenitis suppurativa: a systematic review. Ann Chir Plast Esthet. 2017;62:274-294. doi:10.1016/j.anplas.2017.03.012
- Menderes A, Sunay O, Vayvada H, et al. Surgical management of hidradenitis suppurativa. Int J Med Sci. 2010;7:240-247. doi:10.7150/ijms.7.240
- Alharbi Z, Kauczok J, Pallua N. A review of wide surgical excision of hidradenitis suppurativa. BMC Dermatol. 2012;12:9. doi:10.1186/1471-5945-12-9
- Burney RE. 35-year experience with surgical treatment of hidradenitis suppurativa. World J Surg. 2017;41:2723-2730. doi:10.1007/s00268-017-4091-7
- Bocchini SF, Habr-Gama A, Kiss DR, et al. Gluteal and perianal hidradenitis suppurativa: surgical treatment by wide excision. Dis Colon Rectum. 2003;46:944-949. doi:10.1007/s10350-004-6691-1
- Blok JL, Spoo JR, Leeman FWJ, et al. Skin-tissue-sparing excision with electrosurgical peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382. doi:10.1111/jdv.12376
- Bilali S, Todi V, Lila A, et al. Surgical treatment of chronic hidradenitis suppurativa in the gluteal and perianal regions. Acta Chir Iugosl. 2012;59:91-95. doi:10.2298/ACI1202091B
- Walter AC, Meissner M, Kaufmann R, et al. Hidradenitis suppurativa after radical surgery-long-term follow-up for recurrences and associated factors. Dermatol Surg. 2018;44:1323-1331. doi:10.1097/DSS.0000000000001668.
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623. doi:10.1016/j.jaad.2006.08.061
- Alavi A, Lynde C, Alhusayen R, et al. Approach to the management of patients with hidradenitis suppurativa: a consensus document. J Cutan Med Surg. 2017;21:513-524. doi:10.1177/1203475417716117
- Duran C, Baumeister A. Recognition, diagnosis, and treatment of hidradenitis suppurativa. J Am Acad Physician Assist. 2019;32:36-42. doi:10.1097/01.JAA.0000578768.62051.13
- Jemec GBE, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-974. doi:10.1016/S0190-9622(98)70272-5
- Pascual JC, Encabo B, Ruiz de Apodaca RF, et al. Topical 15% resorcinol for hidradenitis suppurativa: an uncontrolled prospective trial with clinical and ultrasonographic follow-up. J Am Acad Dermatol. 2017;77:1175-1178. doi:10.1016/j.jaad.2017.07.008
- Boer J, Jemec GBE. Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa. Clin Exp Dermatol. 2010;35:36-40. doi:10.1111/j.1365-2230.2009.03377.x
- Hessam S, Sand M, Meier NM, et al. Combination of oral zinc gluconate and topical triclosan: an anti-inflammatory treatment modality for initial hidradenitis suppurativa. J Dermatol Sci. 2016;84:197-202. doi:10.1016/j.jdermsci.2016.08.010
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154. doi:10.1159/000228334
- Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
- Chu WM. Tumor necrosis factor. Cancer Lett. 2013;328:222-225. doi:10.1016/j.canlet.2012.10.014
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Morita A, Takahashi H, Ozawa K, et al. Twenty-four-week interim analysis from a phase 3 open-label trial of adalimumab in Japanese patients with moderate to severe hidradenitis suppurativa. J Dermatol. 2019;46:745-751. doi:10.1111/1346-8138.14997
- Ghias MH, Johnston AD, Kutner AJ, et al. High-dose, high-frequency infliximab: a novel treatment paradigm for hidradenitis suppurativa. J Am Acad Dermatol. 2020;82:1094-1101. doi:10.1016/j.jaad.2019.09.071
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa a randomized clinical trial. JAMA Dermatol. 2016;152:52-59. doi:10.1001/jamadermatol.2015.3903
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846. doi:10.1111/bjd.14338
- John H, Manoloudakis N, Stephen Sinclair J. A systematic review of the use of lasers for the treatment of hidradenitis suppurativa. J Plast Reconstr Aesthet Surg. 2016;69:1374-1381. doi:10.1016/j.bjps.2016.05.029
Practice Points
- First-line therapies may not provide adequate pain control in many patients with hidradenitis suppurativa.
- Pain caused by inflamed abscesses and nodules can be treated with either intralesional corticosteroids or incision and drainage. Tissue-sparing surgical techniques may lead to shorter healing times and less postoperative pain.
- Long-term management involves lifestyle modifications and pharmacologic agents.
- The most effective pain remedies developed thus far are limited to surgery and tumor necrosis factor α inhibitors.
Artificial Intelligence: Review of Current and Future Applications in Medicine
Artificial Intelligence (AI) was first described in 1956 and refers to machines having the ability to learn as they receive and process information, resulting in the ability to “think” like humans.1 AI’s impact in medicine is increasing; currently, at least 29 AI medical devices and algorithms are approved by the US Food and Drug Administration (FDA) in a variety of areas, including radiograph interpretation, managing glucose levels in patients with diabetes mellitus, analyzing electrocardiograms (ECGs), and diagnosing sleep disorders among others.2 Significantly, in 2020, the Centers for Medicare and Medicaid Services (CMS) announced the first reimbursement to hospitals for an AI platform, a model for early detection of strokes.3 AI is rapidly becoming an integral part of health care, and its role will only increase in the future (Table).
As knowledge in medicine is expanding exponentially, AI has great potential to assist with handling complex patient care data. The concept of exponential growth is not a natural one. As Bini described, with exponential growth the volume of knowledge amassed over the past 10 years will now occur in perhaps only 1 year.1 Likewise, equivalent advances over the past year may take just a few months. This phenomenon is partly due to the law of accelerating returns, which states that advances feed on themselves, continually increasing the rate of further advances.4 The volume of medical data doubles every 2 to 5 years.5 Fortunately, the field of AI is growing exponentially as well and can help health care practitioners (HCPs) keep pace, allowing the continued delivery of effective health care.
In this report, we review common terminology, principles, and general applications of AI, followed by current and potential applications of AI for selected medical specialties. Finally, we discuss AI’s future in health care, along with potential risks and pitfalls.
AI Overview
AI refers to machine programs that can “learn” or think based on past experiences. This functionality contrasts with simple rules-based programming available to health care for years. An example of rules-based programming is the warfarindosing.org website developed by Barnes-Jewish Hospital at Washington University Medical Center, which guides initial warfarin dosing.6,7 The prescriber inputs detailed patient information, including age, sex, height, weight, tobacco history, medications, laboratory results, and genotype if available. The application then calculates recommended warfarin dosing regimens to avoid over- or underanticoagulation. While the dosing algorithm may be complex, it depends entirely on preprogrammed rules. The program does not learn to reach its conclusions and recommendations from patient data.
In contrast, one of the most common subsets of AI is machine learning (ML). ML describes a program that “learns from experience and improves its performance as it learns.”1 With ML, the computer is initially provided with a training data set—data with known outcomes or labels. Because the initial data are input from known samples, this type of AI is known as supervised learning.8-10 As an example, we recently reported using ML to diagnose various types of cancer from pathology slides.11 In one experiment, we captured images of colon adenocarcinoma and normal colon (these 2 groups represent the training data set). Unlike traditional programming, we did not define characteristics that would differentiate colon cancer from normal; rather, the machine learned these characteristics independently by assessing the labeled images provided. A second data set (the validation data set) was used to evaluate the program and fine-tune the ML training model’s parameters. Finally, the program was presented with new images of cancer and normal cases for final assessment of accuracy (test data set). Our program learned to recognize differences from the images provided and was able to differentiate normal and cancer images with > 95% accuracy.
Advances in computer processing have allowed for the development of artificial neural networks (ANNs). While there are several types of ANNs, the most common types used for image classification and segmentation are known as convolutional neural networks (CNNs).9,12-14 The programs are designed to work similar to the human brain, specifically the visual cortex.15,16 As data are acquired, they are processed by various layers in the program. Much like neurons in the brain, one layer decides whether to advance information to the next.13,14 CNNs can be many layers deep, leading to the term deep learning: “computational models that are composed of multiple processing layers to learn representations of data with multiple levels of abstraction.”1,13,17
ANNs can process larger volumes of data. This advance has led to the development of unstructured or unsupervised learning. With this type of learning, imputing defined features (ie, predetermined answers) of the training data set described above is no longer required.1,8,10,14 The advantage of unsupervised learning is that the program can be presented raw data and extract meaningful interpretation without human input, often with less bias than may exist with supervised learning.1,18 If shown enough data, the program can extract relevant features to make conclusions independently without predefined definitions, potentially uncovering markers not previously known. For example, several studies have used unsupervised learning to search patient data to assess readmission risks of patients with congestive heart failure.10,19,20 AI compiled features independently and not previously defined, predicting patients at greater risk for readmission superior to traditional methods.
A more detailed description of the various terminologies and techniques of AI is beyond the scope of this review.9,10,17,21 However, in this basic overview, we describe 4 general areas that AI impacts health care (Figure).
Health Care Applications
Image analysis has seen the most AI health care applications.8,15 AI has shown potential in interpreting many types of medical images, including pathology slides, radiographs of various types, retina and other eye scans, and photographs of skin lesions. Many studies have demonstrated that AI can interpret these images as accurately as or even better than experienced clinicians.9,13,22-29 Studies have suggested AI interpretation of radiographs may better distinguish patients infected with COVID-19 from other causes of pneumonia, and AI interpretation of pathology slides may detect specific genetic mutations not previously identified without additional molecular tests.11,14,23,24,30-32
The second area in which AI can impact health care is improving workflow and efficiency. AI has improved surgery scheduling, saving significant revenue, and decreased patient wait times for appointments.1 AI can screen and triage radiographs, allowing attention to be directed to critical patients. This use would be valuable in many busy clinical settings, such as the recent COVID-19 pandemic.8,23 Similarly, AI can screen retina images to prioritize urgent conditions.25 AI has improved pathologists’ efficiency when used to detect breast metastases.33 Finally, AI may reduce medical errors, thereby ensuring patient safety.8,9,34
A third health care benefit of AI is in public health and epidemiology. AI can assist with clinical decision-making and diagnoses in low-income countries and areas with limited health care resources and personnel.25,29 AI can improve identification of infectious outbreaks, such as tuberculosis, malaria, dengue fever, and influenza.29,35-40 AI has been used to predict transmission patterns of the Zika virus and the current COVID-19 pandemic.41,42 Applications can stratify the risk of outbreaks based on multiple factors, including age, income, race, atypical geographic clusters, and seasonal factors like rainfall and temperature.35,36,38,43 AI has been used to assess morbidity and mortality, such as predicting disease severity with malaria and identifying treatment failures in tuberculosis.29
Finally, AI can dramatically impact health care due to processing large data sets or disconnected volumes of patient information—so-called big data.44-46 An example is the widespread use of electronic health records (EHRs) such as the Computerized Patient Record System used in Veteran Affairs medical centers (VAMCs). Much of patient information exists in written text: HCP notes, laboratory and radiology reports, medication records, etc. Natural language processing (NLP) allows platforms to sort through extensive volumes of data on complex patients at rates much faster than human capability, which has great potential to assist with diagnosis and treatment decisions.9
Medical literature is being produced at rates that exceed our ability to digest. More than 200,000 cancer-related articles were published in 2019 alone.14 NLP capabilities of AI have the potential to rapidly sort through this extensive medical literature and relate specific verbiage in patient records guiding therapy.46 IBM Watson, a supercomputer based on ML and NLP, demonstrates this concept with many potential applications, only some of which relate to health care.1,9 Watson has an oncology component to assimilate multiple aspects of patient care, including clinical notes, pathology results, radiograph findings, staging, and a tumor’s genetic profile. It coordinates these inputs from the EHR and mines medical literature and research databases to recommend treatment options.1,46 AI can assess and compile far greater patient data and therapeutic options than would be feasible by individual clinicians, thus providing customized patient care.47 Watson has partnered with numerous medical centers, including MD Anderson Cancer Center and Memorial Sloan Kettering Cancer Center, with variable success.44,47-49 While the full potential of Watson appears not yet realized, these AI-driven approaches will likely play an important role in leveraging the hidden value in the expanding volume of health care information.
Medical Specialty Applications
Radiology
Currently > 70% of FDA-approved AI medical devices are in the field of radiology.2 Most radiology departments have used AI-friendly digital imaging for years, such as the picture archiving and communication systems used by numerous health care systems, including VAMCs.2,15 Gray-scale images common in radiology lend themselves to standardization, although AI is not limited to black-and- white image interpretation.15
An abundance of literature describes plain radiograph interpretation using AI. One FDA-approved platform improved X-ray diagnosis of wrist fractures when used by emergency medicine clinicians.2,50 AI has been applied to chest X-ray (CXR) interpretation of many conditions, including pneumonia, tuberculosis, malignant lung lesions, and COVID-19.23,25,28,44,51-53 For example, Nam and colleagues suggested AI is better at diagnosing malignant pulmonary nodules from CXRs than are trained radiologists.28
In addition to plain radiographs, AI has been applied to many other imaging technologies, including ultrasounds, positron emission tomography, mammograms, computed tomography (CT), and magnetic resonance imaging (MRI).15,26,44,48,54-56 A large study demonstrated that ML platforms significantly reduced the time to diagnose intracranial hemorrhages on CT and identified subtle hemorrhages missed by radiologists.55 Other studies have claimed that AI programs may be better than radiologists in detecting cancer in screening mammograms, and 3 FDA-approved devices focus on mammogram interpretation.2,15,54,57 There is also great interest in MRI applications to detect and predict prognosis for breast cancer based on imaging findings.21,56
Aside from providing accurate diagnoses, other studies focus on AI radiograph interpretation to assist with patient screening, triage, improving time to final diagnosis, providing a rapid “second opinion,” and even monitoring disease progression and offering insights into prognosis.8,21,23,52,55,56,58 These features help in busy urban centers but may play an even greater role in areas with limited access to health care or trained specialists such as radiologists.52
Cardiology
Cardiology has the second highest number of FDA-approved AI applications.2 Many cardiology AI platforms involve image analysis, as described in several recent reviews.45,59,60 AI has been applied to echocardiography to measure ejection fractions, detect valvular disease, and assess heart failure from hypertrophic and restrictive cardiomyopathy and amyloidosis.45,48,59 Applications for cardiac CT scans and CT angiography have successfully quantified both calcified and noncalcified coronary artery plaques and lumen assessments, assessed myocardial perfusion, and performed coronary artery calcium scoring.45,59,60 Likewise, AI applications for cardiac MRI have been used to quantitate ejection fraction, large vessel flow assessment, and cardiac scar burden.45,59
For years ECG devices have provided interpretation with limited accuracy using preprogrammed parameters.48 However, the application of AI allows ECG interpretation on par with trained cardiologists. Numerous such AI applications exist, and 2 FDA-approved devices perform ECG interpretation.2,61-64 One of these devices incorporates an AI-powered stethoscope to detect atrial fibrillation and heart murmurs.65
Pathology
The advancement of whole slide imaging, wherein entire slides can be scanned and digitized at high speed and resolution, creates great potential for AI applications in pathology.12,24,32,33,66 A landmark study demonstrating the potential of AI for assessing whole slide imaging examined sentinel lymph node metastases in patients with breast cancer.22 Multiple algorithms in the study demonstrated that AI was equivalent or better than pathologists in detecting metastases, especially when the pathologists were time-constrained consistent with a normal working environment. Significantly, the most accurate and efficient diagnoses were achieved when the pathologist and AI interpretations were used together.22,33
AI has shown promise in diagnosing many other entities, including cancers of the prostate (including Gleason scoring), lung, colon, breast, and skin.11,12,24,27,32,67 In addition, AI has shown great potential in scoring biomarkers important for prognosis and treatment, such as immunohistochemistry (IHC) labeling of Ki-67 and PD-L1.32 Pathologists can have difficulty classifying certain tumors or determining the site of origin for metastases, often having to rely on IHC with limited success. The unique features of image analysis with AI have the potential to assist in classifying difficult tumors and identifying sites of origin for metastatic disease based on morphology alone.11
Oncology depends heavily on molecular pathology testing to dictate treatment options and determine prognosis. Preliminary studies suggest that AI interpretation alone has the potential to delineate whether certain molecular mutations are present in tumors from various sites.11,14,24,32 One study combined histology and genomic results for AI interpretation that improved prognostic predictions.68 In addition, AI analysis may have potential in predicting tumor recurrence or prognosis based on cellular features, as demonstrated for lung cancer and melanoma.67,69,70
Ophthalmology
AI applications for ophthalmology have focused on diabetic retinopathy, age-related macular degeneration, glaucoma, retinopathy of prematurity, age-related and congenital cataracts, and retinal vein occlusion.71-73 Diabetic retinopathy is a leading cause of blindness and has been studied by numerous platforms with good success, most having used color fundus photography.71,72 One study showed AI could diagnose diabetic retinopathy and diabetic macular edema with specificities similar to ophthalmologists.74 In 2018, the FDA approved the AI platform IDx-DR. This diagnostic system classifies retinal images and recommends referral for patients determined to have “more than mild diabetic retinopathy” and reexamination within a year for other patients.8,75 Significantly, the platform recommendations do not require confirmation by a clinician.8
AI has been applied to other modalities in ophthalmology such as optical coherence tomography (OCT) to diagnose retinal disease and to predict appropriate management of congenital cataracts.25,73,76 For example, an AI application using OCT has been demonstrated to match or exceed the accuracy of retinal experts in diagnosing and triaging patients with a variety of retinal pathologies, including patients needing urgent referrals.77
Dermatology
Multiple studies demonstrate AI performs at least equal to experienced dermatologists in differentiating selected skin lesions.78-81 For example, Esteva and colleagues demonstrated AI could differentiate keratinocyte carcinomas from benign seborrheic keratoses and malignant melanomas from benign nevi with accuracy equal to 21 board-certified dermatologists.78
AI is applicable to various imaging procedures common to dermatology, such as dermoscopy, very high-frequency ultrasound, and reflectance confocal microscopy.82 Several studies have demonstrated that AI interpretation compared favorably to dermatologists evaluating dermoscopy to assess melanocytic lesions.78-81,83
A limitation in these studies is that they differentiate only a few diagnoses.82 Furthermore, dermatologists have sensory input such as touch and visual examination under various conditions, something AI has yet to replicate.15,34,84 Also, most AI devices use no or limited clinical information.81 Dermatologists can recognize rarer conditions for which AI models may have had limited or no training.34 Nevertheless, a recent study assessed AI for the diagnosis of 134 separate skin disorders with promising results, including providing diagnoses with accuracy comparable to that of dermatologists and providing accurate treatment strategies.84 As Topol points out, most skin lesions are diagnosed in the primary care setting where AI can have a greater impact when used in conjunction with the clinical impression, especially where specialists are in limited supply.48,78
Finally, dermatology lends itself to using portable or smartphone applications (apps) wherein the user can photograph a lesion for analysis by AI algorithms to assess the need for further evaluation or make treatment recommendations.34,84,85 Although results from currently available apps are not encouraging, they may play a greater role as the technology advances.34,85
Oncology
Applications of AI in oncology include predicting prognosis for patients with cancer based on histologic and/or genetic information.14,68,86 Programs can predict the risk of complications before and recurrence risks after surgery for malignancies.44,87-89 AI can also assist in treatment planning and predict treatment failure with radiation therapy.90,91
AI has great potential in processing the large volumes of patient data in cancer genomics. Next-generation sequencing has allowed for the identification of millions of DNA sequences in a single tumor to detect genetic anomalies.92 Thousands of mutations can be found in individual tumor samples, and processing this information and determining its significance can be beyond human capability.14 We know little about the effects of various mutation combinations, and most tumors have a heterogeneous molecular profile among different cell populations.14,93 The presence or absence of various mutations can have diagnostic, prognostic, and therapeutic implications.93 AI has great potential to sort through these complex data and identify actionable findings.
More than 200,000 cancer-related articles were published in 2019, and publications in the field of cancer genomics are increasing exponentially.14,92,93 Patel and colleagues assessed the utility of IBM Watson for Genomics against results from a molecular tumor board.93 Watson for Genomics identified potentially significant mutations not identified by the tumor board in 32% of patients. Most mutations were related to new clinical trials not yet added to the tumor board watch list, demonstrating the role AI will have in processing the large volume of genetic data required to deliver personalized medicine moving forward.
Gastroenterology
AI has shown promise in predicting risk or outcomes based on clinical parameters in various common gastroenterology problems, including gastric reflux, acute pancreatitis, gastrointestinal bleeding, celiac disease, and inflammatory bowel disease.94,95 AI endoscopic analysis has demonstrated potential in assessing Barrett’s esophagus, gastric Helicobacter pylori infections, gastric atrophy, and gastric intestinal metaplasia.95 Applications have been used to assess esophageal, gastric, and colonic malignancies, including depth of invasion based on endoscopic images.95 Finally, studies have evaluated AI to assess small colon polyps during colonoscopy, including differentiating benign and premalignant polyps with success comparable to gastroenterologists.94,95 AI has been shown to increase the speed and accuracy of gastroenterologists in detecting small polyps during colonoscopy.48 In a prospective randomized study, colonoscopies performed using an AI device identified significantly more small adenomatous polyps than colonoscopies without AI.96
Neurology
It has been suggested that AI technologies are well suited for application in neurology due to the subtle presentation of many neurologic diseases.16 Viz LVO, the first CMS-approved AI reimbursement for the diagnosis of strokes, analyzes CTs to detect early ischemic strokes and alerts the medical team, thus shortening time to treatment.3,97 Many other AI platforms are in use or development that use CT and MRI for the early detection of strokes as well as for treatment and prognosis.9,97
AI technologies have been applied to neurodegenerative diseases, such as Alzheimer and Parkinson diseases.16,98 For example, several studies have evaluated patient movements in Parkinson disease for both early diagnosis and to assess response to treatment.98 These evaluations included assessment with both external cameras as well as wearable devices and smartphone apps.
AI has also been applied to seizure disorders, attempting to determine seizure type, localize the area of seizure onset, and address the challenges of identifying seizures in neonates.99,100 Other potential applications range from early detection and prognosis predictions for cases of multiple sclerosis to restoring movement in paralysis from a variety of conditions such as spinal cord injury.9,101,102
Mental Health
Due to the interactive nature of mental health care, the field has been slower to develop AI applications.18 With heavy reliance on textual information (eg, clinic notes, mood rating scales, and documentation of conversations), successful AI applications in this field will likely rely heavily on NLP.18 However, studies investigating the application of AI to mental health have also incorporated data such as brain imaging, smartphone monitoring, and social media platforms, such as Facebook and Twitter.18,103,104
The risk of suicide is higher in veteran patients, and ML algorithms have had limited success in predicting suicide risk in both veteran and nonveteran populations.104-106 While early models have low positive predictive values and low sensitivities, they still promise to be a useful tool in conjunction with traditional risk assessments.106 Kessler and colleagues suggest that combining multiple rather than single ML algorithms might lead to greater success.105,106
AI may assist in diagnosing other mental health disorders, including major depressive disorder, attention deficit hyperactivity disorder (ADHD), schizophrenia, posttraumatic stress disorder, and Alzheimer disease.103,104,107 These investigations are in the early stages with limited clinical applicability. However, 2 AI applications awaiting FDA approval relate to ADHD and opioid use.2 Furthermore, potential exists for AI to not only assist with prevention and diagnosis of ADHD, but also to identify optimal treatment options.2,103
General and Personalized Medicine
Additional AI applications include diagnosing patients with suspected sepsis, measuring liver iron concentrations, predicting hospital mortality at the time of admission, and more.2,108,109 AI can guide end-of-life decisions such as resuscitation status or whether to initiate mechanical ventilation.48
AI-driven smartphone apps can be beneficial to both patients and clinicians. Examples include predicting nonadherence to anticoagulation therapy, monitoring heart rhythms for atrial fibrillation or signs of hyperkalemia in patients with renal failure, and improving outcomes for patients with diabetes mellitus by decreasing glycemic variability and reducing hypoglycemia.8,48,110,111 The potential for AI applications to health care and personalized medicine are almost limitless.
Discussion
With ever-increasing expectations for all health care sectors to deliver timely, fiscally-responsible, high-quality health care, AI has the potential to have numerous impacts. AI can improve diagnostic accuracy while limiting errors and impact patient safety such as assisting with prescription delivery.8,9,34 It can screen and triage patients, alerting clinicians to those needing more urgent evaluation.8,23,77,97 AI also may increase a clinician’s efficiency and speed to render a diagnosis.12,13,55,97 AI can provide a rapid second opinion, an ability especially beneficial in underserved areas with shortages of specialists.23,25,26,29,34 Similarly, AI may decrease the inter- and intraobserver variability common in many medical specialties.12,27,45 AI applications can also monitor disease progression, identifying patients at greatest risk, and provide information for prognosis.21,23,56,58 Finally, as described with applications using IBM Watson, AI can allow for an integrated approach to health care that is currently lacking.
We have described many reports suggesting AI can render diagnoses as well as or better than experienced clinicians, and speculation exists that AI will replace many roles currently performed by health care practitioners.9,26 However, most studies demonstrate that AI’s diagnostic benefits are best realized when used to supplement a clinician’s impression.8,22,30,33,52,54,56,69,84 AI is not likely to replace humans in health care in the foreseeable future. The technology can be likened to the impact of CT scans developed in the 1970s in neurology. Prior to such detailed imaging, neurologists spent extensive time performing detailed physicals to render diagnoses and locate lesions before surgery. There was mistrust of this new technology and concern that CT scans would eliminate the need for neurologists.112 On the contrary, neurology is alive and well, frequently being augmented by the technologies once speculated to replace it.
Commercial AI health care platforms represented a $2 billion industry in 2018 and are growing rapidly each year.13,32 Many AI products are offered ready for implementation for various tasks, including diagnostics, patient management, and improved efficiency. Others will likely be provided as templates suitable for modification to meet the specific needs of the facility, practice, or specialty for its patient population.
AI Risks and Limitations
AI has several risks and limitations. Although there is progress in explainable AI, at times we still struggle to understand how the output provided by machine learning algorithms was created.44,48 The many layers associated with deep learning self-determine the criteria to reach its conclusion, and these criteria can continually evolve. The parameters of deep learning are not preprogrammed, and there are too many individual data points to be extrapolated or deconvoluted for evaluation at our current level of knowledge.26,51 These apparent lack of constraints cause concern for patient safety and suggest that greater validation and continued scrutiny of validity is required.8,48 Efforts are underway to create explainable AI programs to make their processes more transparent, but such clarification is limited presently.14,26,48,77
Another challenge of AI is determining the amount of training data required to function optimally. Also, if the output describes multiple variables or diagnoses, are each equally valid?113 Furthermore, many AI applications look for a specific process, such as cancer diagnoses on CXRs. However, how coexisting conditions like cardiomegaly, emphysema, pneumonia, etc, seen on CXRs will affect the diagnosis needs to be considered.51,52 Zech and colleagues provide the example that diagnoses for pneumothorax are frequently rendered on CXRs with chest tubes in place.51 They suggest that CNNs may develop a bias toward diagnosing pneumothorax when chest tubes are present. Many current studies approach an issue in isolation, a situation not realistic in real-world clinical practice.26
Most studies on AI have been retrospective, and frequently data used to train the program are preselected.13,26 The data are typically validated on available databases rather than actual patients in the clinical setting, limiting confidence in the validity of the AI output when applied to real-world situations. Currently, fewer than 12 prospective trials had been published comparing AI with traditional clinical care.13,114 Randomized prospective clinical trials are even fewer, with none currently reported from the United States.13,114 The results from several studies have been shown to diminish when repeated prospectively.114
The FDA has created a new category known as Software as a Medical Device and has a Digital Health Innovation Action Plan to regulate AI platforms. Still, the process of AI regulation is of necessity different from traditional approval processes and is continually evolving.8 The FDA approval process cannot account for the fact that the program’s parameters may continually evolve or adapt.2
Guidelines for investigating and reporting AI research with its unique attributes are being developed. Examples include the TRIPOD-ML statement and others.49,115 In September 2020, 2 publications addressed the paucity of gold-standard randomized clinical trials in clinical AI applications.116,117 The SPIRIT-AI statement expands on the original SPIRIT statement published in 2013 to guide minimal reporting standards for AI clinical trial protocols to promote transparency of design and methodology.116 Similarly, the CONSORT-AI extension, stemming from the original CONSORT statement in 1996, aims to ensure quality reporting of randomized controlled trials in AI.117
Another risk with AI is that while an individual physician making a mistake may adversely affect 1 patient, a single mistake in an AI algorithm could potentially affect thousands of patients.48 Also, AI programs developed for patient populations at a facility may not translate to another. Referred to as overfitting, this phenomenon relates to selection bias in training data sets.15,34,49,51,52 Studies have shown that programs that underrepresent certain group characteristics such as age, sex, or race may be less effective when applied to a population in which these characteristics have differing representations.8,48,49 This problem of underrepresentation has been demonstrated in programs interpreting pathology slides, radiographs, and skin lesions.15,32,51
Admittedly, most of these challenges are not specific to AI and existed in health care previously. Physicians make mistakes, treatments are sometimes used without adequate prospective studies, and medications are given without understanding their mechanism of action, much like AI-facilitated processes reach a conclusion that cannot be fully explained.48
Conclusions
The view that AI will dramatically impact health care in the coming years will likely prove true. However, much work is needed, especially because of the paucity of prospective clinical trials as has been historically required in medical research. Any concern that AI will replace HCPs seems unwarranted. Early studies suggest that even AI programs that appear to exceed human interpretation perform best when working in cooperation with and oversight from clinicians. AI’s greatest potential appears to be its ability to augment care from health professionals, improving efficiency and accuracy, and should be anticipated with enthusiasm as the field moves forward at an exponential rate.
Acknowledgments
The authors thank Makenna G. Thomas for proofreading and review of the manuscript. This material is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital. This research has been approved by the James A. Haley Veteran’s Hospital Office of Communications and Media.
1. Bini SA. Artificial intelligence, machine learning, deep learning, and cognitive computing: what do these terms mean and how will they impact health care? J Arthroplasty. 2018;33(8):2358-2361. doi:10.1016/j.arth.2018.02.067
2. Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med. 2020;3:118. doi:10.1038/s41746-020-00324-0
3. Viz. AI powered synchronized stroke care. Accessed September 15, 2021. https://www.viz.ai/ischemic-stroke
4. Buchanan M. The law of accelerating returns. Nat Phys. 2008;4(7):507. doi:10.1038/nphys1010
5. IBM Watson Health computes a pair of new solutions to improve healthcare data and security. Published September 10, 2015. Accessed October 21, 2020. https://www.techrepublic.com/article/ibm-watson-health-computes-a-pair-of-new-solutions-to-improve-healthcare-data-and-security
6. Borkowski AA, Kardani A, Mastorides SM, Thomas LB. Warfarin pharmacogenomics: recommendations with available patented clinical technologies. Recent Pat Biotechnol. 2014;8(2):110-115. doi:10.2174/1872208309666140904112003
7. Washington University in St. Louis. Warfarin dosing. Accessed September 15, 2021. http://www.warfarindosing.org/Source/Home.aspx
8. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25(1):30-36. doi:10.1038/s41591-018-0307-0
9. Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243. Published 2017 Jun 21. doi:10.1136/svn-2017-000101
10. Johnson KW, Torres Soto J, Glicksberg BS, et al. Artificial intelligence in cardiology. J Am Coll Cardiol. 2018;71(23):2668-2679. doi:10.1016/j.jacc.2018.03.521
11. Borkowski AA, Wilson CP, Borkowski SA, et al. Comparing artificial intelligence platforms for histopathologic cancer diagnosis. Fed Pract. 2019;36(10):456-463.
12. Cruz-Roa A, Gilmore H, Basavanhally A, et al. High-throughput adaptive sampling for whole-slide histopathology image analysis (HASHI) via convolutional neural networks: application to invasive breast cancer detection. PLoS One. 2018;13(5):e0196828. Published 2018 May 24. doi:10.1371/journal.pone.0196828
13. Nagendran M, Chen Y, Lovejoy CA, et al. Artificial intelligence versus clinicians: systematic review of design, reporting standards, and claims of deep learning studies. BMJ. 2020;368:m689. Published 2020 Mar 25. doi:10.1136/bmj.m689
14. Shimizu H, Nakayama KI. Artificial intelligence in oncology. Cancer Sci. 2020;111(5):1452-1460. doi:10.1111/cas.14377
15. Talebi-Liasi F, Markowitz O. Is artificial intelligence going to replace dermatologists?. Cutis. 2020;105(1):28-31.
16. Valliani AA, Ranti D, Oermann EK. Deep learning and neurology: a systematic review. Neurol Ther. 2019;8(2):351-365. doi:10.1007/s40120-019-00153-8
17. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436-444. doi:10.1038/nature14539
18. Graham S, Depp C, Lee EE, et al. Artificial intelligence for mental health and mental illnesses: an overview. Curr Psychiatry Rep. 2019;21(11):116. Published 2019 Nov 7. doi:10.1007/s11920-019-1094-0
19. Golas SB, Shibahara T, Agboola S, et al. A machine learning model to predict the risk of 30-day readmissions in patients with heart failure: a retrospective analysis of electronic medical records data. BMC Med Inform Decis Mak. 2018;18(1):44. Published 2018 Jun 22. doi:10.1186/s12911-018-0620-z
20. Mortazavi BJ, Downing NS, Bucholz EM, et al. Analysis of machine learning techniques for heart failure readmissions. Circ Cardiovasc Qual Outcomes. 2016;9(6):629-640. doi:10.1161/CIRCOUTCOMES.116.003039
21. Meyer-Bäse A, Morra L, Meyer-Bäse U, Pinker K. Current status and future perspectives of artificial intelligence in magnetic resonance breast imaging. Contrast Media Mol Imaging. 2020;2020:6805710. Published 2020 Aug 28. doi:10.1155/2020/6805710
22. Ehteshami Bejnordi B, Veta M, Johannes van Diest P, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318(22):2199-2210. doi:10.1001/jama.2017.14585
23. Borkowski AA, Viswanadhan NA, Thomas LB, Guzman RD, Deland LA, Mastorides SM. Using artificial intelligence for COVID-19 chest X-ray diagnosis. Fed Pract. 2020;37(9):398-404. doi:10.12788/fp.0045
24. Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24(10):1559-1567. doi:10.1038/s41591-018-0177-5
25. Kermany DS, Goldbaum M, Cai W, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172(5):1122-1131.e9. doi:10.1016/j.cell.2018.02.010
26. Liu X, Faes L, Kale AU, et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit Health. 2019;1(6):e271-e297. doi:10.1016/S2589-7500(19)30123-2
27. Nagpal K, Foote D, Liu Y, et al. Development and validation of a deep learning algorithm for improving Gleason scoring of prostate cancer [published correction appears in NPJ Digit Med. 2019 Nov 19;2:113]. NPJ Digit Med. 2019;2:48. Published 2019 Jun 7. doi:10.1038/s41746-019-0112-2
28. Nam JG, Park S, Hwang EJ, et al. Development and validation of deep learning-based automatic detection algorithm for malignant pulmonary nodules on chest radiographs. Radiology. 2019;290(1):218-228. doi:10.1148/radiol.2018180237
29. Schwalbe N, Wahl B. Artificial intelligence and the future of global health. Lancet. 2020;395(10236):1579-1586. doi:10.1016/S0140-6736(20)30226-9
30. Bai HX, Wang R, Xiong Z, et al. Artificial intelligence augmentation of radiologist performance in distinguishing COVID-19 from pneumonia of other origin at chest CT [published correction appears in Radiology. 2021 Apr;299(1):E225]. Radiology. 2020;296(3):E156-E165. doi:10.1148/radiol.2020201491
31. Li L, Qin L, Xu Z, et al. Using artificial intelligence to detect COVID-19 and community-acquired pneumonia based on pulmonary CT: evaluation of the diagnostic accuracy. Radiology. 2020;296(2):E65-E71. doi:10.1148/radiol.2020200905
32. Serag A, Ion-Margineanu A, Qureshi H, et al. Translational AI and deep learning in diagnostic pathology. Front Med (Lausanne). 2019;6:185. Published 2019 Oct 1. doi:10.3389/fmed.2019.00185

33. Wang D, Khosla A, Gargeya R, Irshad H, Beck AH. Deep learning for identifying metastatic breast cancer. ArXiv. 2016 June 18:arXiv:1606.05718v1. Published online June 18, 2016. Accessed September 15, 2021. http://arxiv.org/abs/1606.05718
34. Alabdulkareem A. Artificial intelligence and dermatologists: friends or foes? J Dermatology Dermatol Surg. 2019;23(2):57-60. doi:10.4103/jdds.jdds_19_19
35. Mollalo A, Mao L, Rashidi P, Glass GE. A GIS-based artificial neural network model for spatial distribution of tuberculosis across the continental United States. Int J Environ Res Public Health. 2019;16(1):157. Published 2019 Jan 8. doi:10.3390/ijerph16010157
36. Haddawy P, Hasan AHMI, Kasantikul R, et al. Spatiotemporal Bayesian networks for malaria prediction. Artif Intell Med. 2018;84:127-138. doi:10.1016/j.artmed.2017.12.002
37. Laureano-Rosario AE, Duncan AP, Mendez-Lazaro PA, et al. Application of artificial neural networks for dengue fever outbreak predictions in the northwest coast of Yucatan, Mexico and San Juan, Puerto Rico. Trop Med Infect Dis. 2018;3(1):5. Published 2018 Jan 5. doi:10.3390/tropicalmed3010005
38. Buczak AL, Koshute PT, Babin SM, Feighner BH, Lewis SH. A data-driven epidemiological prediction method for dengue outbreaks using local and remote sensing data. BMC Med Inform Decis Mak. 2012;12:124. Published 2012 Nov 5. doi:10.1186/1472-6947-12-124
39. Scavuzzo JM, Trucco F, Espinosa M, et al. Modeling dengue vector population using remotely sensed data and machine learning. Acta Trop. 2018;185:167-175. doi:10.1016/j.actatropica.2018.05.003
40. Xue H, Bai Y, Hu H, Liang H. Influenza activity surveillance based on multiple regression model and artificial neural network. IEEE Access. 2018;6:563-575. doi:10.1109/ACCESS.2017.2771798
41. Jiang D, Hao M, Ding F, Fu J, Li M. Mapping the transmission risk of Zika virus using machine learning models. Acta Trop. 2018;185:391-399. doi:10.1016/j.actatropica.2018.06.021
42. Bragazzi NL, Dai H, Damiani G, Behzadifar M, Martini M, Wu J. How big data and artificial intelligence can help better manage the COVID-19 pandemic. Int J Environ Res Public Health. 2020;17(9):3176. Published 2020 May 2. doi:10.3390/ijerph17093176
43. Lake IR, Colón-González FJ, Barker GC, Morbey RA, Smith GE, Elliot AJ. Machine learning to refine decision making within a syndromic surveillance service. BMC Public Health. 2019;19(1):559. Published 2019 May 14. doi:10.1186/s12889-019-6916-9
44. Khan OF, Bebb G, Alimohamed NA. Artificial intelligence in medicine: what oncologists need to know about its potential-and its limitations. Oncol Exch. 2017;16(4):8-13. Accessed September 1, 2021. http://www.oncologyex.com/pdf/vol16_no4/feature_khan-ai.pdf
45. Badano LP, Keller DM, Muraru D, Torlasco C, Parati G. Artificial intelligence and cardiovascular imaging: A win-win combination. Anatol J Cardiol. 2020;24(4):214-223. doi:10.14744/AnatolJCardiol.2020.94491
46. Murdoch TB, Detsky AS. The inevitable application of big data to health care. JAMA. 2013;309(13):1351-1352. doi:10.1001/jama.2013.393
47. Greatbatch O, Garrett A, Snape K. The impact of artificial intelligence on the current and future practice of clinical cancer genomics. Genet Res (Camb). 2019;101:e9. Published 2019 Oct 31. doi:10.1017/S0016672319000089
48. Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44-56. doi:10.1038/s41591-018-0300-7
49. Vollmer S, Mateen BA, Bohner G, et al. Machine learning and artificial intelligence research for patient benefit: 20 critical questions on transparency, replicability, ethics, and effectiveness [published correction appears in BMJ. 2020 Apr 1;369:m1312]. BMJ. 2020;368:l6927. Published 2020 Mar 20. doi:10.1136/bmj.l6927
50. Lindsey R, Daluiski A, Chopra S, et al. Deep neural network improves fracture detection by clinicians. Proc Natl Acad Sci U S A. 2018;115(45):11591-11596. doi:10.1073/pnas.1806905115
51. Zech JR, Badgeley MA, Liu M, Costa AB, Titano JJ, Oermann EK. Variable generalization performance of a deep learning model to detect pneumonia in chest radiographs: a cross-sectional study. PLoS Med. 2018;15(11):e1002683. doi:10.1371/journal.pmed.1002683
52. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582. doi:10.1148/radiol.2017162326
53. Rajpurkar P, Joshi A, Pareek A, et al. CheXpedition: investigating generalization challenges for translation of chest x-ray algorithms to the clinical setting. ArXiv. 2020 Feb 26:arXiv:2002.11379v2. Revised March 11, 2020. Accessed September 15, 2021. http://arxiv.org/abs/2002.11379
54. Salim M, Wåhlin E, Dembrower K, et al. External evaluation of 3 commercial artificial intelligence algorithms for independent assessment of screening mammograms. JAMA Oncol. 2020;6(10):1581-1588. doi:10.1001/jamaoncol.2020.3321
55. Arbabshirani MR, Fornwalt BK, Mongelluzzo GJ, et al. Advanced machine learning in action: identification of intracranial hemorrhage on computed tomography scans of the head with clinical workflow integration. NPJ Digit Med. 2018;1:9. doi:10.1038/s41746-017-0015-z
56. Sheth D, Giger ML. Artificial intelligence in the interpretation of breast cancer on MRI. J Magn Reson Imaging. 2020;51(5):1310-1324. doi:10.1002/jmri.26878
57. McKinney SM, Sieniek M, Godbole V, et al. International evaluation of an AI system for breast cancer screening. Nature. 2020;577(7788):89-94. doi:10.1038/s41586-019-1799-6
58. Booth AL, Abels E, McCaffrey P. Development of a prognostic model for mortality in COVID-19 infection using machine learning. Mod Pathol. 2021;34(3):522-531. doi:10.1038/s41379-020-00700-x
59. Xu B, Kocyigit D, Grimm R, Griffin BP, Cheng F. Applications of artificial intelligence in multimodality cardiovascular imaging: a state-of-the-art review. Prog Cardiovasc Dis. 2020;63(3):367-376. doi:10.1016/j.pcad.2020.03.003
60. Dey D, Slomka PJ, Leeson P, et al. Artificial intelligence in cardiovascular imaging: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(11):1317-1335. doi:10.1016/j.jacc.2018.12.054
61. Carewell Health. AI powered ECG diagnosis solutions. Accessed November 2, 2020. https://www.carewellhealth.com/products_aiecg.html
62. Strodthoff N, Strodthoff C. Detecting and interpreting myocardial infarction using fully convolutional neural networks. Physiol Meas. 2019;40(1):015001. doi:10.1088/1361-6579/aaf34d
63. Hannun AY, Rajpurkar P, Haghpanahi M, et al. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019;25(1):65-69. doi:10.1038/s41591-018-0268-3
64. Kwon JM, Jeon KH, Kim HM, et al. Comparing the performance of artificial intelligence and conventional diagnosis criteria for detecting left ventricular hypertrophy using electrocardiography. Europace. 2020;22(3):412-419. doi:10.1093/europace/euz324

65. Eko. FDA clears Eko’s AFib and heart murmur detection algorithms, making it the first AI-powered stethoscope to screen for serious heart conditions [press release]. Published January 28, 2020. Accessed September 15, 2021. https://www.businesswire.com/news/home/20200128005232/en/FDA-Clears-Eko’s-AFib-and-Heart-Murmur-Detection-Algorithms-Making-It-the-First-AI-Powered-Stethoscope-to-Screen-for-Serious-Heart-Conditions
66. Cruz-Roa A, Gilmore H, Basavanhally A, et al. Accurate and reproducible invasive breast cancer detection in whole-slide images: a deep learning approach for quantifying tumor extent. Sci Rep. 2017;7:46450. doi:10.1038/srep46450
67. Acs B, Rantalainen M, Hartman J. Artificial intelligence as the next step towards precision pathology. J Intern Med. 2020;288(1):62-81. doi:10.1111/joim.13030
68. Mobadersany P, Yousefi S, Amgad M, et al. Predicting cancer outcomes from histology and genomics using convolutional networks. Proc Natl Acad Sci U S A. 2018;115(13):E2970-E2979. doi:10.1073/pnas.1717139115
69. Wang X, Janowczyk A, Zhou Y, et al. Prediction of recurrence in early stage non-small cell lung cancer using computer extracted nuclear features from digital H&E images. Sci Rep. 2017;7:13543. doi:10.1038/s41598-017-13773-7
70. Kulkarni PM, Robinson EJ, Pradhan JS, et al. Deep learning based on standard H&E images of primary melanoma tumors identifies patients at risk for visceral recurrence and death. Clin Cancer Res. 2020;26(5):1126-1134. doi:10.1158/1078-0432.CCR-19-1495
71. Du XL, Li WB, Hu BJ. Application of artificial intelligence in ophthalmology. Int J Ophthalmol. 2018;11(9):1555-1561. doi:10.18240/ijo.2018.09.21
72. Gunasekeran DV, Wong TY. Artificial intelligence in ophthalmology in 2020: a technology on the cusp for translation and implementation. Asia Pac J Ophthalmol (Phila). 2020;9(2):61-66. doi:10.1097/01.APO.0000656984.56467.2c
73. Ting DSW, Pasquale LR, Peng L, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019;103(2):167-175. doi:10.1136/bjophthalmol-2018-313173
74. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2410. doi:10.1001/jama.2016.17216
75. US Food and Drug Administration. FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems [press release]. Published April 11, 2018. Accessed September 15, 2021. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye
76. Long E, Chen J, Wu X, et al. Artificial intelligence manages congenital cataract with individualized prediction and telehealth computing. NPJ Digit Med. 2020;3:112. doi:10.1038/s41746-020-00319-x
77. De Fauw J, Ledsam JR, Romera-Paredes B, et al. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat Med. 2018;24(9):1342-1350. doi:10.1038/s41591-018-0107-6
78. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115-118. doi:10.1038/nature21056
79. Brinker TJ, Hekler A, Enk AH, et al. Deep neural networks are superior to dermatologists in melanoma image classification. Eur J Cancer. 2019;119:11-17. doi:10.1016/j.ejca.2019.05.023
80. Brinker TJ, Hekler A, Enk AH, et al. A convolutional neural network trained with dermoscopic images performed on par with 145 dermatologists in a clinical melanoma image classification task. Eur J Cancer. 2019;111:148-154. doi:10.1016/j.ejca.2019.02.005
81. Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists. Ann Oncol. 2018;29(8):1836-1842. doi:10.1093/annonc/mdy166
82. Li CX, Shen CB, Xue K, et al. Artificial intelligence in dermatology: past, present, and future. Chin Med J (Engl). 2019;132(17):2017-2020. doi:10.1097/CM9.0000000000000372
83. Tschandl P, Codella N, Akay BN, et al. Comparison of the accuracy of human readers versus machine-learning algorithms for pigmented skin lesion classification: an open, web-based, international, diagnostic study. Lancet Oncol. 2019;20(7):938-947. doi:10.1016/S1470-2045(19)30333-X
84. Han SS, Park I, Eun Chang SE, et al. Augmented intelligence dermatology: deep neural networks empower medical professionals in diagnosing skin cancer and predicting treatment options for 134 skin disorders. J Invest Dermatol. 2020;140(9):1753-1761. doi:10.1016/j.jid.2020.01.019
85. Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies [published correction appears in BMJ. 2020 Feb 25;368:m645]. BMJ. 2020;368:m127. Published 2020 Feb 10. doi:10.1136/bmj.m127
86. Chen YC, Ke WC, Chiu HW. Risk classification of cancer survival using ANN with gene expression data from multiple laboratories. Comput Biol Med. 2014;48:1-7. doi:10.1016/j.compbiomed.2014.02.006
87. Kim W, Kim KS, Lee JE, et al. Development of novel breast cancer recurrence prediction model using support vector machine. J Breast Cancer. 2012;15(2):230-238. doi:10.4048/jbc.2012.15.2.230
88. Merath K, Hyer JM, Mehta R, et al. Use of machine learning for prediction of patient risk of postoperative complications after liver, pancreatic, and colorectal surgery. J Gastrointest Surg. 2020;24(8):1843-1851. doi:10.1007/s11605-019-04338-2
89. Santos-García G, Varela G, Novoa N, Jiménez MF. Prediction of postoperative morbidity after lung resection using an artificial neural network ensemble. Artif Intell Med. 2004;30(1):61-69. doi:10.1016/S0933-3657(03)00059-9
90. Ibragimov B, Xing L. Segmentation of organs-at-risks in head and neck CT images using convolutional neural networks. Med Phys. 2017;44(2):547-557. doi:10.1002/mp.12045
91. Lou B, Doken S, Zhuang T, et al. An image-based deep learning framework for individualizing radiotherapy dose. Lancet Digit Health. 2019;1(3):e136-e147. doi:10.1016/S2589-7500(19)30058-5
92. Xu J, Yang P, Xue S, et al. Translating cancer genomics into precision medicine with artificial intelligence: applications, challenges and future perspectives. Hum Genet. 2019;138(2):109-124. doi:10.1007/s00439-019-01970-5
93. Patel NM, Michelini VV, Snell JM, et al. Enhancing next‐generation sequencing‐guided cancer care through cognitive computing. Oncologist. 2018;23(2):179-185. doi:10.1634/theoncologist.2017-0170
94. Le Berre C, Sandborn WJ, Aridhi S, et al. Application of artificial intelligence to gastroenterology and hepatology. Gastroenterology. 2020;158(1):76-94.e2. doi:10.1053/j.gastro.2019.08.058
95. Yang YJ, Bang CS. Application of artificial intelligence in gastroenterology. World J Gastroenterol. 2019;25(14):1666-1683. doi:10.3748/wjg.v25.i14.1666
96. Wang P, Berzin TM, Glissen Brown JR, et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut. 2019;68(10):1813-1819. doi:10.1136/gutjnl-2018-317500

97. Gupta R, Krishnam SP, Schaefer PW, Lev MH, Gonzalez RG. An East Coast perspective on artificial intelligence and machine learning: part 2: ischemic stroke imaging and triage. Neuroimaging Clin N Am. 2020;30(4):467-478. doi:10.1016/j.nic.2020.08.002
98. Beli M, Bobi V, Badža M, Šolaja N, Duri-Jovii M, Kosti VS. Artificial intelligence for assisting diagnostics and assessment of Parkinson’s disease—a review. Clin Neurol Neurosurg. 2019;184:105442. doi:10.1016/j.clineuro.2019.105442
99. An S, Kang C, Lee HW. Artificial intelligence and computational approaches for epilepsy. J Epilepsy Res. 2020;10(1):8-17. doi:10.14581/jer.20003
100. Pavel AM, Rennie JM, de Vries LS, et al. A machine-learning algorithm for neonatal seizure recognition: a multicentre, randomised, controlled trial. Lancet Child Adolesc Health. 2020;4(10):740-749. doi:10.1016/S2352-4642(20)30239-X
101. Afzal HMR, Luo S, Ramadan S, Lechner-Scott J. The emerging role of artificial intelligence in multiple sclerosis imaging [published online ahead of print, 2020 Oct 28]. Mult Scler. 2020;1352458520966298. doi:10.1177/1352458520966298
102. Bouton CE. Restoring movement in paralysis with a bioelectronic neural bypass approach: current state and future directions. Cold Spring Harb Perspect Med. 2019;9(11):a034306. doi:10.1101/cshperspect.a034306
103. Durstewitz D, Koppe G, Meyer-Lindenberg A. Deep neural networks in psychiatry. Mol Psychiatry. 2019;24(11):1583-1598. doi:10.1038/s41380-019-0365-9
104. Fonseka TM, Bhat V, Kennedy SH. The utility of artificial intelligence in suicide risk prediction and the management of suicidal behaviors. Aust N Z J Psychiatry. 2019;53(10):954-964. doi:10.1177/0004867419864428
105. Kessler RC, Hwang I, Hoffmire CA, et al. Developing a practical suicide risk prediction model for targeting high-risk patients in the Veterans Health Administration. Int J Methods Psychiatr Res. 2017;26(3):e1575. doi:10.1002/mpr.1575
106. Kessler RC, Bauer MS, Bishop TM, et al. Using administrative data to predict suicide after psychiatric hospitalization in the Veterans Health Administration System. Front Psychiatry. 2020;11:390. doi:10.3389/fpsyt.2020.00390
107. Kessler RC, van Loo HM, Wardenaar KJ, et al. Testing a machine-learning algorithm to predict the persistence and severity of major depressive disorder from baseline self-reports. Mol Psychiatry. 2016;21(10):1366-1371. doi:10.1038/mp.2015.198
108. Horng S, Sontag DA, Halpern Y, Jernite Y, Shapiro NI, Nathanson LA. Creating an automated trigger for sepsis clinical decision support at emergency department triage using machine learning. PLoS One. 2017;12(4):e0174708. doi:10.1371/journal.pone.0174708
109. Soffer S, Klang E, Barash Y, Grossman E, Zimlichman E. Predicting in-hospital mortality at admission to the medical ward: a big-data machine learning model. Am J Med. 2021;134(2):227-234.e4. doi:10.1016/j.amjmed.2020.07.014
110. Labovitz DL, Shafner L, Reyes Gil M, Virmani D, Hanina A. Using artificial intelligence to reduce the risk of nonadherence in patients on anticoagulation therapy. Stroke. 2017;48(5):1416-1419. doi:10.1161/STROKEAHA.116.016281
111. Forlenza GP. Use of artificial intelligence to improve diabetes outcomes in patients using multiple daily injections therapy. Diabetes Technol Ther. 2019;21(S2):S24-S28. doi:10.1089/dia.2019.0077
112. Poser CM. CT scan and the practice of neurology. Arch Neurol. 1977;34(2):132. doi:10.1001/archneur.1977.00500140086023
113. Angus DC. Randomized clinical trials of artificial intelligence. JAMA. 2020;323(11):1043-1045. doi:10.1001/jama.2020.1039
114. Topol EJ. Welcoming new guidelines for AI clinical research. Nat Med. 2020;26(9):1318-1320. doi:10.1038/s41591-020-1042-x
115. Collins GS, Moons KGM. Reporting of artificial intelligence prediction models. Lancet. 2019;393(10181):1577-1579. doi:10.1016/S0140-6736(19)30037-6
116. Cruz Rivera S, Liu X, Chan AW, et al. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Nat Med. 2020;26(9):1351-1363. doi:10.1038/s41591-020-1037-7
117. Liu X, Cruz Rivera S, Moher D, Calvert MJ, Denniston AK; SPIRIT-AI and CONSORT-AI Working Group. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat Med. 2020;26(9):1364-1374. doi:10.1038/s41591-020-1034-x
118. McCulloch WS, Pitts W. A logical calculus of the ideas immanent in nervous activity. Bull Math Biophys. 1943;5(4):115-133. doi:10.1007/BF02478259
119. Samuel AL. Some studies in machine learning using the game of Checkers. IBM J Res Dev. 1959;3(3):535-554. Accessed September 15, 2021. https://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.368.2254
120. Sonoda M, Takano M, Miyahara J, Kato H. Computed radiography utilizing scanning laser stimulated luminescence. Radiology. 1983;148(3):833-838. doi:10.1148/radiology.148.3.6878707
121. Dechter R. Learning while searching in constraint-satisfaction-problems. AAAI’86: proceedings of the fifth AAAI national conference on artificial intelligence. Published 1986. Accessed September 15, 2021. https://www.aaai.org/Papers/AAAI/1986/AAAI86-029.pdf
122. Le Cun Y, Jackel LD, Boser B, et al. Handwritten digit recognition: applications of neural network chips and automatic learning. IEEE Commun Mag. 1989;27(11):41-46. doi:10.1109/35.41400
123. US Food and Drug Administration. FDA allows marketing of first whole slide imaging system for digital pathology [press release]. Published April 12, 2017. Accessed September 15, 2021. https://www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-whole-slide-imaging-system-digital-pathology
Artificial Intelligence (AI) was first described in 1956 and refers to machines having the ability to learn as they receive and process information, resulting in the ability to “think” like humans.1 AI’s impact in medicine is increasing; currently, at least 29 AI medical devices and algorithms are approved by the US Food and Drug Administration (FDA) in a variety of areas, including radiograph interpretation, managing glucose levels in patients with diabetes mellitus, analyzing electrocardiograms (ECGs), and diagnosing sleep disorders among others.2 Significantly, in 2020, the Centers for Medicare and Medicaid Services (CMS) announced the first reimbursement to hospitals for an AI platform, a model for early detection of strokes.3 AI is rapidly becoming an integral part of health care, and its role will only increase in the future (Table).
As knowledge in medicine is expanding exponentially, AI has great potential to assist with handling complex patient care data. The concept of exponential growth is not a natural one. As Bini described, with exponential growth the volume of knowledge amassed over the past 10 years will now occur in perhaps only 1 year.1 Likewise, equivalent advances over the past year may take just a few months. This phenomenon is partly due to the law of accelerating returns, which states that advances feed on themselves, continually increasing the rate of further advances.4 The volume of medical data doubles every 2 to 5 years.5 Fortunately, the field of AI is growing exponentially as well and can help health care practitioners (HCPs) keep pace, allowing the continued delivery of effective health care.
In this report, we review common terminology, principles, and general applications of AI, followed by current and potential applications of AI for selected medical specialties. Finally, we discuss AI’s future in health care, along with potential risks and pitfalls.
AI Overview
AI refers to machine programs that can “learn” or think based on past experiences. This functionality contrasts with simple rules-based programming available to health care for years. An example of rules-based programming is the warfarindosing.org website developed by Barnes-Jewish Hospital at Washington University Medical Center, which guides initial warfarin dosing.6,7 The prescriber inputs detailed patient information, including age, sex, height, weight, tobacco history, medications, laboratory results, and genotype if available. The application then calculates recommended warfarin dosing regimens to avoid over- or underanticoagulation. While the dosing algorithm may be complex, it depends entirely on preprogrammed rules. The program does not learn to reach its conclusions and recommendations from patient data.
In contrast, one of the most common subsets of AI is machine learning (ML). ML describes a program that “learns from experience and improves its performance as it learns.”1 With ML, the computer is initially provided with a training data set—data with known outcomes or labels. Because the initial data are input from known samples, this type of AI is known as supervised learning.8-10 As an example, we recently reported using ML to diagnose various types of cancer from pathology slides.11 In one experiment, we captured images of colon adenocarcinoma and normal colon (these 2 groups represent the training data set). Unlike traditional programming, we did not define characteristics that would differentiate colon cancer from normal; rather, the machine learned these characteristics independently by assessing the labeled images provided. A second data set (the validation data set) was used to evaluate the program and fine-tune the ML training model’s parameters. Finally, the program was presented with new images of cancer and normal cases for final assessment of accuracy (test data set). Our program learned to recognize differences from the images provided and was able to differentiate normal and cancer images with > 95% accuracy.
Advances in computer processing have allowed for the development of artificial neural networks (ANNs). While there are several types of ANNs, the most common types used for image classification and segmentation are known as convolutional neural networks (CNNs).9,12-14 The programs are designed to work similar to the human brain, specifically the visual cortex.15,16 As data are acquired, they are processed by various layers in the program. Much like neurons in the brain, one layer decides whether to advance information to the next.13,14 CNNs can be many layers deep, leading to the term deep learning: “computational models that are composed of multiple processing layers to learn representations of data with multiple levels of abstraction.”1,13,17
ANNs can process larger volumes of data. This advance has led to the development of unstructured or unsupervised learning. With this type of learning, imputing defined features (ie, predetermined answers) of the training data set described above is no longer required.1,8,10,14 The advantage of unsupervised learning is that the program can be presented raw data and extract meaningful interpretation without human input, often with less bias than may exist with supervised learning.1,18 If shown enough data, the program can extract relevant features to make conclusions independently without predefined definitions, potentially uncovering markers not previously known. For example, several studies have used unsupervised learning to search patient data to assess readmission risks of patients with congestive heart failure.10,19,20 AI compiled features independently and not previously defined, predicting patients at greater risk for readmission superior to traditional methods.
A more detailed description of the various terminologies and techniques of AI is beyond the scope of this review.9,10,17,21 However, in this basic overview, we describe 4 general areas that AI impacts health care (Figure).
Health Care Applications
Image analysis has seen the most AI health care applications.8,15 AI has shown potential in interpreting many types of medical images, including pathology slides, radiographs of various types, retina and other eye scans, and photographs of skin lesions. Many studies have demonstrated that AI can interpret these images as accurately as or even better than experienced clinicians.9,13,22-29 Studies have suggested AI interpretation of radiographs may better distinguish patients infected with COVID-19 from other causes of pneumonia, and AI interpretation of pathology slides may detect specific genetic mutations not previously identified without additional molecular tests.11,14,23,24,30-32
The second area in which AI can impact health care is improving workflow and efficiency. AI has improved surgery scheduling, saving significant revenue, and decreased patient wait times for appointments.1 AI can screen and triage radiographs, allowing attention to be directed to critical patients. This use would be valuable in many busy clinical settings, such as the recent COVID-19 pandemic.8,23 Similarly, AI can screen retina images to prioritize urgent conditions.25 AI has improved pathologists’ efficiency when used to detect breast metastases.33 Finally, AI may reduce medical errors, thereby ensuring patient safety.8,9,34
A third health care benefit of AI is in public health and epidemiology. AI can assist with clinical decision-making and diagnoses in low-income countries and areas with limited health care resources and personnel.25,29 AI can improve identification of infectious outbreaks, such as tuberculosis, malaria, dengue fever, and influenza.29,35-40 AI has been used to predict transmission patterns of the Zika virus and the current COVID-19 pandemic.41,42 Applications can stratify the risk of outbreaks based on multiple factors, including age, income, race, atypical geographic clusters, and seasonal factors like rainfall and temperature.35,36,38,43 AI has been used to assess morbidity and mortality, such as predicting disease severity with malaria and identifying treatment failures in tuberculosis.29
Finally, AI can dramatically impact health care due to processing large data sets or disconnected volumes of patient information—so-called big data.44-46 An example is the widespread use of electronic health records (EHRs) such as the Computerized Patient Record System used in Veteran Affairs medical centers (VAMCs). Much of patient information exists in written text: HCP notes, laboratory and radiology reports, medication records, etc. Natural language processing (NLP) allows platforms to sort through extensive volumes of data on complex patients at rates much faster than human capability, which has great potential to assist with diagnosis and treatment decisions.9
Medical literature is being produced at rates that exceed our ability to digest. More than 200,000 cancer-related articles were published in 2019 alone.14 NLP capabilities of AI have the potential to rapidly sort through this extensive medical literature and relate specific verbiage in patient records guiding therapy.46 IBM Watson, a supercomputer based on ML and NLP, demonstrates this concept with many potential applications, only some of which relate to health care.1,9 Watson has an oncology component to assimilate multiple aspects of patient care, including clinical notes, pathology results, radiograph findings, staging, and a tumor’s genetic profile. It coordinates these inputs from the EHR and mines medical literature and research databases to recommend treatment options.1,46 AI can assess and compile far greater patient data and therapeutic options than would be feasible by individual clinicians, thus providing customized patient care.47 Watson has partnered with numerous medical centers, including MD Anderson Cancer Center and Memorial Sloan Kettering Cancer Center, with variable success.44,47-49 While the full potential of Watson appears not yet realized, these AI-driven approaches will likely play an important role in leveraging the hidden value in the expanding volume of health care information.
Medical Specialty Applications
Radiology
Currently > 70% of FDA-approved AI medical devices are in the field of radiology.2 Most radiology departments have used AI-friendly digital imaging for years, such as the picture archiving and communication systems used by numerous health care systems, including VAMCs.2,15 Gray-scale images common in radiology lend themselves to standardization, although AI is not limited to black-and- white image interpretation.15
An abundance of literature describes plain radiograph interpretation using AI. One FDA-approved platform improved X-ray diagnosis of wrist fractures when used by emergency medicine clinicians.2,50 AI has been applied to chest X-ray (CXR) interpretation of many conditions, including pneumonia, tuberculosis, malignant lung lesions, and COVID-19.23,25,28,44,51-53 For example, Nam and colleagues suggested AI is better at diagnosing malignant pulmonary nodules from CXRs than are trained radiologists.28
In addition to plain radiographs, AI has been applied to many other imaging technologies, including ultrasounds, positron emission tomography, mammograms, computed tomography (CT), and magnetic resonance imaging (MRI).15,26,44,48,54-56 A large study demonstrated that ML platforms significantly reduced the time to diagnose intracranial hemorrhages on CT and identified subtle hemorrhages missed by radiologists.55 Other studies have claimed that AI programs may be better than radiologists in detecting cancer in screening mammograms, and 3 FDA-approved devices focus on mammogram interpretation.2,15,54,57 There is also great interest in MRI applications to detect and predict prognosis for breast cancer based on imaging findings.21,56
Aside from providing accurate diagnoses, other studies focus on AI radiograph interpretation to assist with patient screening, triage, improving time to final diagnosis, providing a rapid “second opinion,” and even monitoring disease progression and offering insights into prognosis.8,21,23,52,55,56,58 These features help in busy urban centers but may play an even greater role in areas with limited access to health care or trained specialists such as radiologists.52
Cardiology
Cardiology has the second highest number of FDA-approved AI applications.2 Many cardiology AI platforms involve image analysis, as described in several recent reviews.45,59,60 AI has been applied to echocardiography to measure ejection fractions, detect valvular disease, and assess heart failure from hypertrophic and restrictive cardiomyopathy and amyloidosis.45,48,59 Applications for cardiac CT scans and CT angiography have successfully quantified both calcified and noncalcified coronary artery plaques and lumen assessments, assessed myocardial perfusion, and performed coronary artery calcium scoring.45,59,60 Likewise, AI applications for cardiac MRI have been used to quantitate ejection fraction, large vessel flow assessment, and cardiac scar burden.45,59
For years ECG devices have provided interpretation with limited accuracy using preprogrammed parameters.48 However, the application of AI allows ECG interpretation on par with trained cardiologists. Numerous such AI applications exist, and 2 FDA-approved devices perform ECG interpretation.2,61-64 One of these devices incorporates an AI-powered stethoscope to detect atrial fibrillation and heart murmurs.65
Pathology
The advancement of whole slide imaging, wherein entire slides can be scanned and digitized at high speed and resolution, creates great potential for AI applications in pathology.12,24,32,33,66 A landmark study demonstrating the potential of AI for assessing whole slide imaging examined sentinel lymph node metastases in patients with breast cancer.22 Multiple algorithms in the study demonstrated that AI was equivalent or better than pathologists in detecting metastases, especially when the pathologists were time-constrained consistent with a normal working environment. Significantly, the most accurate and efficient diagnoses were achieved when the pathologist and AI interpretations were used together.22,33
AI has shown promise in diagnosing many other entities, including cancers of the prostate (including Gleason scoring), lung, colon, breast, and skin.11,12,24,27,32,67 In addition, AI has shown great potential in scoring biomarkers important for prognosis and treatment, such as immunohistochemistry (IHC) labeling of Ki-67 and PD-L1.32 Pathologists can have difficulty classifying certain tumors or determining the site of origin for metastases, often having to rely on IHC with limited success. The unique features of image analysis with AI have the potential to assist in classifying difficult tumors and identifying sites of origin for metastatic disease based on morphology alone.11
Oncology depends heavily on molecular pathology testing to dictate treatment options and determine prognosis. Preliminary studies suggest that AI interpretation alone has the potential to delineate whether certain molecular mutations are present in tumors from various sites.11,14,24,32 One study combined histology and genomic results for AI interpretation that improved prognostic predictions.68 In addition, AI analysis may have potential in predicting tumor recurrence or prognosis based on cellular features, as demonstrated for lung cancer and melanoma.67,69,70
Ophthalmology
AI applications for ophthalmology have focused on diabetic retinopathy, age-related macular degeneration, glaucoma, retinopathy of prematurity, age-related and congenital cataracts, and retinal vein occlusion.71-73 Diabetic retinopathy is a leading cause of blindness and has been studied by numerous platforms with good success, most having used color fundus photography.71,72 One study showed AI could diagnose diabetic retinopathy and diabetic macular edema with specificities similar to ophthalmologists.74 In 2018, the FDA approved the AI platform IDx-DR. This diagnostic system classifies retinal images and recommends referral for patients determined to have “more than mild diabetic retinopathy” and reexamination within a year for other patients.8,75 Significantly, the platform recommendations do not require confirmation by a clinician.8
AI has been applied to other modalities in ophthalmology such as optical coherence tomography (OCT) to diagnose retinal disease and to predict appropriate management of congenital cataracts.25,73,76 For example, an AI application using OCT has been demonstrated to match or exceed the accuracy of retinal experts in diagnosing and triaging patients with a variety of retinal pathologies, including patients needing urgent referrals.77
Dermatology
Multiple studies demonstrate AI performs at least equal to experienced dermatologists in differentiating selected skin lesions.78-81 For example, Esteva and colleagues demonstrated AI could differentiate keratinocyte carcinomas from benign seborrheic keratoses and malignant melanomas from benign nevi with accuracy equal to 21 board-certified dermatologists.78
AI is applicable to various imaging procedures common to dermatology, such as dermoscopy, very high-frequency ultrasound, and reflectance confocal microscopy.82 Several studies have demonstrated that AI interpretation compared favorably to dermatologists evaluating dermoscopy to assess melanocytic lesions.78-81,83
A limitation in these studies is that they differentiate only a few diagnoses.82 Furthermore, dermatologists have sensory input such as touch and visual examination under various conditions, something AI has yet to replicate.15,34,84 Also, most AI devices use no or limited clinical information.81 Dermatologists can recognize rarer conditions for which AI models may have had limited or no training.34 Nevertheless, a recent study assessed AI for the diagnosis of 134 separate skin disorders with promising results, including providing diagnoses with accuracy comparable to that of dermatologists and providing accurate treatment strategies.84 As Topol points out, most skin lesions are diagnosed in the primary care setting where AI can have a greater impact when used in conjunction with the clinical impression, especially where specialists are in limited supply.48,78
Finally, dermatology lends itself to using portable or smartphone applications (apps) wherein the user can photograph a lesion for analysis by AI algorithms to assess the need for further evaluation or make treatment recommendations.34,84,85 Although results from currently available apps are not encouraging, they may play a greater role as the technology advances.34,85
Oncology
Applications of AI in oncology include predicting prognosis for patients with cancer based on histologic and/or genetic information.14,68,86 Programs can predict the risk of complications before and recurrence risks after surgery for malignancies.44,87-89 AI can also assist in treatment planning and predict treatment failure with radiation therapy.90,91
AI has great potential in processing the large volumes of patient data in cancer genomics. Next-generation sequencing has allowed for the identification of millions of DNA sequences in a single tumor to detect genetic anomalies.92 Thousands of mutations can be found in individual tumor samples, and processing this information and determining its significance can be beyond human capability.14 We know little about the effects of various mutation combinations, and most tumors have a heterogeneous molecular profile among different cell populations.14,93 The presence or absence of various mutations can have diagnostic, prognostic, and therapeutic implications.93 AI has great potential to sort through these complex data and identify actionable findings.
More than 200,000 cancer-related articles were published in 2019, and publications in the field of cancer genomics are increasing exponentially.14,92,93 Patel and colleagues assessed the utility of IBM Watson for Genomics against results from a molecular tumor board.93 Watson for Genomics identified potentially significant mutations not identified by the tumor board in 32% of patients. Most mutations were related to new clinical trials not yet added to the tumor board watch list, demonstrating the role AI will have in processing the large volume of genetic data required to deliver personalized medicine moving forward.
Gastroenterology
AI has shown promise in predicting risk or outcomes based on clinical parameters in various common gastroenterology problems, including gastric reflux, acute pancreatitis, gastrointestinal bleeding, celiac disease, and inflammatory bowel disease.94,95 AI endoscopic analysis has demonstrated potential in assessing Barrett’s esophagus, gastric Helicobacter pylori infections, gastric atrophy, and gastric intestinal metaplasia.95 Applications have been used to assess esophageal, gastric, and colonic malignancies, including depth of invasion based on endoscopic images.95 Finally, studies have evaluated AI to assess small colon polyps during colonoscopy, including differentiating benign and premalignant polyps with success comparable to gastroenterologists.94,95 AI has been shown to increase the speed and accuracy of gastroenterologists in detecting small polyps during colonoscopy.48 In a prospective randomized study, colonoscopies performed using an AI device identified significantly more small adenomatous polyps than colonoscopies without AI.96
Neurology
It has been suggested that AI technologies are well suited for application in neurology due to the subtle presentation of many neurologic diseases.16 Viz LVO, the first CMS-approved AI reimbursement for the diagnosis of strokes, analyzes CTs to detect early ischemic strokes and alerts the medical team, thus shortening time to treatment.3,97 Many other AI platforms are in use or development that use CT and MRI for the early detection of strokes as well as for treatment and prognosis.9,97
AI technologies have been applied to neurodegenerative diseases, such as Alzheimer and Parkinson diseases.16,98 For example, several studies have evaluated patient movements in Parkinson disease for both early diagnosis and to assess response to treatment.98 These evaluations included assessment with both external cameras as well as wearable devices and smartphone apps.
AI has also been applied to seizure disorders, attempting to determine seizure type, localize the area of seizure onset, and address the challenges of identifying seizures in neonates.99,100 Other potential applications range from early detection and prognosis predictions for cases of multiple sclerosis to restoring movement in paralysis from a variety of conditions such as spinal cord injury.9,101,102
Mental Health
Due to the interactive nature of mental health care, the field has been slower to develop AI applications.18 With heavy reliance on textual information (eg, clinic notes, mood rating scales, and documentation of conversations), successful AI applications in this field will likely rely heavily on NLP.18 However, studies investigating the application of AI to mental health have also incorporated data such as brain imaging, smartphone monitoring, and social media platforms, such as Facebook and Twitter.18,103,104
The risk of suicide is higher in veteran patients, and ML algorithms have had limited success in predicting suicide risk in both veteran and nonveteran populations.104-106 While early models have low positive predictive values and low sensitivities, they still promise to be a useful tool in conjunction with traditional risk assessments.106 Kessler and colleagues suggest that combining multiple rather than single ML algorithms might lead to greater success.105,106
AI may assist in diagnosing other mental health disorders, including major depressive disorder, attention deficit hyperactivity disorder (ADHD), schizophrenia, posttraumatic stress disorder, and Alzheimer disease.103,104,107 These investigations are in the early stages with limited clinical applicability. However, 2 AI applications awaiting FDA approval relate to ADHD and opioid use.2 Furthermore, potential exists for AI to not only assist with prevention and diagnosis of ADHD, but also to identify optimal treatment options.2,103
General and Personalized Medicine
Additional AI applications include diagnosing patients with suspected sepsis, measuring liver iron concentrations, predicting hospital mortality at the time of admission, and more.2,108,109 AI can guide end-of-life decisions such as resuscitation status or whether to initiate mechanical ventilation.48
AI-driven smartphone apps can be beneficial to both patients and clinicians. Examples include predicting nonadherence to anticoagulation therapy, monitoring heart rhythms for atrial fibrillation or signs of hyperkalemia in patients with renal failure, and improving outcomes for patients with diabetes mellitus by decreasing glycemic variability and reducing hypoglycemia.8,48,110,111 The potential for AI applications to health care and personalized medicine are almost limitless.
Discussion
With ever-increasing expectations for all health care sectors to deliver timely, fiscally-responsible, high-quality health care, AI has the potential to have numerous impacts. AI can improve diagnostic accuracy while limiting errors and impact patient safety such as assisting with prescription delivery.8,9,34 It can screen and triage patients, alerting clinicians to those needing more urgent evaluation.8,23,77,97 AI also may increase a clinician’s efficiency and speed to render a diagnosis.12,13,55,97 AI can provide a rapid second opinion, an ability especially beneficial in underserved areas with shortages of specialists.23,25,26,29,34 Similarly, AI may decrease the inter- and intraobserver variability common in many medical specialties.12,27,45 AI applications can also monitor disease progression, identifying patients at greatest risk, and provide information for prognosis.21,23,56,58 Finally, as described with applications using IBM Watson, AI can allow for an integrated approach to health care that is currently lacking.
We have described many reports suggesting AI can render diagnoses as well as or better than experienced clinicians, and speculation exists that AI will replace many roles currently performed by health care practitioners.9,26 However, most studies demonstrate that AI’s diagnostic benefits are best realized when used to supplement a clinician’s impression.8,22,30,33,52,54,56,69,84 AI is not likely to replace humans in health care in the foreseeable future. The technology can be likened to the impact of CT scans developed in the 1970s in neurology. Prior to such detailed imaging, neurologists spent extensive time performing detailed physicals to render diagnoses and locate lesions before surgery. There was mistrust of this new technology and concern that CT scans would eliminate the need for neurologists.112 On the contrary, neurology is alive and well, frequently being augmented by the technologies once speculated to replace it.
Commercial AI health care platforms represented a $2 billion industry in 2018 and are growing rapidly each year.13,32 Many AI products are offered ready for implementation for various tasks, including diagnostics, patient management, and improved efficiency. Others will likely be provided as templates suitable for modification to meet the specific needs of the facility, practice, or specialty for its patient population.
AI Risks and Limitations
AI has several risks and limitations. Although there is progress in explainable AI, at times we still struggle to understand how the output provided by machine learning algorithms was created.44,48 The many layers associated with deep learning self-determine the criteria to reach its conclusion, and these criteria can continually evolve. The parameters of deep learning are not preprogrammed, and there are too many individual data points to be extrapolated or deconvoluted for evaluation at our current level of knowledge.26,51 These apparent lack of constraints cause concern for patient safety and suggest that greater validation and continued scrutiny of validity is required.8,48 Efforts are underway to create explainable AI programs to make their processes more transparent, but such clarification is limited presently.14,26,48,77
Another challenge of AI is determining the amount of training data required to function optimally. Also, if the output describes multiple variables or diagnoses, are each equally valid?113 Furthermore, many AI applications look for a specific process, such as cancer diagnoses on CXRs. However, how coexisting conditions like cardiomegaly, emphysema, pneumonia, etc, seen on CXRs will affect the diagnosis needs to be considered.51,52 Zech and colleagues provide the example that diagnoses for pneumothorax are frequently rendered on CXRs with chest tubes in place.51 They suggest that CNNs may develop a bias toward diagnosing pneumothorax when chest tubes are present. Many current studies approach an issue in isolation, a situation not realistic in real-world clinical practice.26
Most studies on AI have been retrospective, and frequently data used to train the program are preselected.13,26 The data are typically validated on available databases rather than actual patients in the clinical setting, limiting confidence in the validity of the AI output when applied to real-world situations. Currently, fewer than 12 prospective trials had been published comparing AI with traditional clinical care.13,114 Randomized prospective clinical trials are even fewer, with none currently reported from the United States.13,114 The results from several studies have been shown to diminish when repeated prospectively.114
The FDA has created a new category known as Software as a Medical Device and has a Digital Health Innovation Action Plan to regulate AI platforms. Still, the process of AI regulation is of necessity different from traditional approval processes and is continually evolving.8 The FDA approval process cannot account for the fact that the program’s parameters may continually evolve or adapt.2
Guidelines for investigating and reporting AI research with its unique attributes are being developed. Examples include the TRIPOD-ML statement and others.49,115 In September 2020, 2 publications addressed the paucity of gold-standard randomized clinical trials in clinical AI applications.116,117 The SPIRIT-AI statement expands on the original SPIRIT statement published in 2013 to guide minimal reporting standards for AI clinical trial protocols to promote transparency of design and methodology.116 Similarly, the CONSORT-AI extension, stemming from the original CONSORT statement in 1996, aims to ensure quality reporting of randomized controlled trials in AI.117
Another risk with AI is that while an individual physician making a mistake may adversely affect 1 patient, a single mistake in an AI algorithm could potentially affect thousands of patients.48 Also, AI programs developed for patient populations at a facility may not translate to another. Referred to as overfitting, this phenomenon relates to selection bias in training data sets.15,34,49,51,52 Studies have shown that programs that underrepresent certain group characteristics such as age, sex, or race may be less effective when applied to a population in which these characteristics have differing representations.8,48,49 This problem of underrepresentation has been demonstrated in programs interpreting pathology slides, radiographs, and skin lesions.15,32,51
Admittedly, most of these challenges are not specific to AI and existed in health care previously. Physicians make mistakes, treatments are sometimes used without adequate prospective studies, and medications are given without understanding their mechanism of action, much like AI-facilitated processes reach a conclusion that cannot be fully explained.48
Conclusions
The view that AI will dramatically impact health care in the coming years will likely prove true. However, much work is needed, especially because of the paucity of prospective clinical trials as has been historically required in medical research. Any concern that AI will replace HCPs seems unwarranted. Early studies suggest that even AI programs that appear to exceed human interpretation perform best when working in cooperation with and oversight from clinicians. AI’s greatest potential appears to be its ability to augment care from health professionals, improving efficiency and accuracy, and should be anticipated with enthusiasm as the field moves forward at an exponential rate.
Acknowledgments
The authors thank Makenna G. Thomas for proofreading and review of the manuscript. This material is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital. This research has been approved by the James A. Haley Veteran’s Hospital Office of Communications and Media.
Artificial Intelligence (AI) was first described in 1956 and refers to machines having the ability to learn as they receive and process information, resulting in the ability to “think” like humans.1 AI’s impact in medicine is increasing; currently, at least 29 AI medical devices and algorithms are approved by the US Food and Drug Administration (FDA) in a variety of areas, including radiograph interpretation, managing glucose levels in patients with diabetes mellitus, analyzing electrocardiograms (ECGs), and diagnosing sleep disorders among others.2 Significantly, in 2020, the Centers for Medicare and Medicaid Services (CMS) announced the first reimbursement to hospitals for an AI platform, a model for early detection of strokes.3 AI is rapidly becoming an integral part of health care, and its role will only increase in the future (Table).
As knowledge in medicine is expanding exponentially, AI has great potential to assist with handling complex patient care data. The concept of exponential growth is not a natural one. As Bini described, with exponential growth the volume of knowledge amassed over the past 10 years will now occur in perhaps only 1 year.1 Likewise, equivalent advances over the past year may take just a few months. This phenomenon is partly due to the law of accelerating returns, which states that advances feed on themselves, continually increasing the rate of further advances.4 The volume of medical data doubles every 2 to 5 years.5 Fortunately, the field of AI is growing exponentially as well and can help health care practitioners (HCPs) keep pace, allowing the continued delivery of effective health care.
In this report, we review common terminology, principles, and general applications of AI, followed by current and potential applications of AI for selected medical specialties. Finally, we discuss AI’s future in health care, along with potential risks and pitfalls.
AI Overview
AI refers to machine programs that can “learn” or think based on past experiences. This functionality contrasts with simple rules-based programming available to health care for years. An example of rules-based programming is the warfarindosing.org website developed by Barnes-Jewish Hospital at Washington University Medical Center, which guides initial warfarin dosing.6,7 The prescriber inputs detailed patient information, including age, sex, height, weight, tobacco history, medications, laboratory results, and genotype if available. The application then calculates recommended warfarin dosing regimens to avoid over- or underanticoagulation. While the dosing algorithm may be complex, it depends entirely on preprogrammed rules. The program does not learn to reach its conclusions and recommendations from patient data.
In contrast, one of the most common subsets of AI is machine learning (ML). ML describes a program that “learns from experience and improves its performance as it learns.”1 With ML, the computer is initially provided with a training data set—data with known outcomes or labels. Because the initial data are input from known samples, this type of AI is known as supervised learning.8-10 As an example, we recently reported using ML to diagnose various types of cancer from pathology slides.11 In one experiment, we captured images of colon adenocarcinoma and normal colon (these 2 groups represent the training data set). Unlike traditional programming, we did not define characteristics that would differentiate colon cancer from normal; rather, the machine learned these characteristics independently by assessing the labeled images provided. A second data set (the validation data set) was used to evaluate the program and fine-tune the ML training model’s parameters. Finally, the program was presented with new images of cancer and normal cases for final assessment of accuracy (test data set). Our program learned to recognize differences from the images provided and was able to differentiate normal and cancer images with > 95% accuracy.
Advances in computer processing have allowed for the development of artificial neural networks (ANNs). While there are several types of ANNs, the most common types used for image classification and segmentation are known as convolutional neural networks (CNNs).9,12-14 The programs are designed to work similar to the human brain, specifically the visual cortex.15,16 As data are acquired, they are processed by various layers in the program. Much like neurons in the brain, one layer decides whether to advance information to the next.13,14 CNNs can be many layers deep, leading to the term deep learning: “computational models that are composed of multiple processing layers to learn representations of data with multiple levels of abstraction.”1,13,17
ANNs can process larger volumes of data. This advance has led to the development of unstructured or unsupervised learning. With this type of learning, imputing defined features (ie, predetermined answers) of the training data set described above is no longer required.1,8,10,14 The advantage of unsupervised learning is that the program can be presented raw data and extract meaningful interpretation without human input, often with less bias than may exist with supervised learning.1,18 If shown enough data, the program can extract relevant features to make conclusions independently without predefined definitions, potentially uncovering markers not previously known. For example, several studies have used unsupervised learning to search patient data to assess readmission risks of patients with congestive heart failure.10,19,20 AI compiled features independently and not previously defined, predicting patients at greater risk for readmission superior to traditional methods.
A more detailed description of the various terminologies and techniques of AI is beyond the scope of this review.9,10,17,21 However, in this basic overview, we describe 4 general areas that AI impacts health care (Figure).
Health Care Applications
Image analysis has seen the most AI health care applications.8,15 AI has shown potential in interpreting many types of medical images, including pathology slides, radiographs of various types, retina and other eye scans, and photographs of skin lesions. Many studies have demonstrated that AI can interpret these images as accurately as or even better than experienced clinicians.9,13,22-29 Studies have suggested AI interpretation of radiographs may better distinguish patients infected with COVID-19 from other causes of pneumonia, and AI interpretation of pathology slides may detect specific genetic mutations not previously identified without additional molecular tests.11,14,23,24,30-32
The second area in which AI can impact health care is improving workflow and efficiency. AI has improved surgery scheduling, saving significant revenue, and decreased patient wait times for appointments.1 AI can screen and triage radiographs, allowing attention to be directed to critical patients. This use would be valuable in many busy clinical settings, such as the recent COVID-19 pandemic.8,23 Similarly, AI can screen retina images to prioritize urgent conditions.25 AI has improved pathologists’ efficiency when used to detect breast metastases.33 Finally, AI may reduce medical errors, thereby ensuring patient safety.8,9,34
A third health care benefit of AI is in public health and epidemiology. AI can assist with clinical decision-making and diagnoses in low-income countries and areas with limited health care resources and personnel.25,29 AI can improve identification of infectious outbreaks, such as tuberculosis, malaria, dengue fever, and influenza.29,35-40 AI has been used to predict transmission patterns of the Zika virus and the current COVID-19 pandemic.41,42 Applications can stratify the risk of outbreaks based on multiple factors, including age, income, race, atypical geographic clusters, and seasonal factors like rainfall and temperature.35,36,38,43 AI has been used to assess morbidity and mortality, such as predicting disease severity with malaria and identifying treatment failures in tuberculosis.29
Finally, AI can dramatically impact health care due to processing large data sets or disconnected volumes of patient information—so-called big data.44-46 An example is the widespread use of electronic health records (EHRs) such as the Computerized Patient Record System used in Veteran Affairs medical centers (VAMCs). Much of patient information exists in written text: HCP notes, laboratory and radiology reports, medication records, etc. Natural language processing (NLP) allows platforms to sort through extensive volumes of data on complex patients at rates much faster than human capability, which has great potential to assist with diagnosis and treatment decisions.9
Medical literature is being produced at rates that exceed our ability to digest. More than 200,000 cancer-related articles were published in 2019 alone.14 NLP capabilities of AI have the potential to rapidly sort through this extensive medical literature and relate specific verbiage in patient records guiding therapy.46 IBM Watson, a supercomputer based on ML and NLP, demonstrates this concept with many potential applications, only some of which relate to health care.1,9 Watson has an oncology component to assimilate multiple aspects of patient care, including clinical notes, pathology results, radiograph findings, staging, and a tumor’s genetic profile. It coordinates these inputs from the EHR and mines medical literature and research databases to recommend treatment options.1,46 AI can assess and compile far greater patient data and therapeutic options than would be feasible by individual clinicians, thus providing customized patient care.47 Watson has partnered with numerous medical centers, including MD Anderson Cancer Center and Memorial Sloan Kettering Cancer Center, with variable success.44,47-49 While the full potential of Watson appears not yet realized, these AI-driven approaches will likely play an important role in leveraging the hidden value in the expanding volume of health care information.
Medical Specialty Applications
Radiology
Currently > 70% of FDA-approved AI medical devices are in the field of radiology.2 Most radiology departments have used AI-friendly digital imaging for years, such as the picture archiving and communication systems used by numerous health care systems, including VAMCs.2,15 Gray-scale images common in radiology lend themselves to standardization, although AI is not limited to black-and- white image interpretation.15
An abundance of literature describes plain radiograph interpretation using AI. One FDA-approved platform improved X-ray diagnosis of wrist fractures when used by emergency medicine clinicians.2,50 AI has been applied to chest X-ray (CXR) interpretation of many conditions, including pneumonia, tuberculosis, malignant lung lesions, and COVID-19.23,25,28,44,51-53 For example, Nam and colleagues suggested AI is better at diagnosing malignant pulmonary nodules from CXRs than are trained radiologists.28
In addition to plain radiographs, AI has been applied to many other imaging technologies, including ultrasounds, positron emission tomography, mammograms, computed tomography (CT), and magnetic resonance imaging (MRI).15,26,44,48,54-56 A large study demonstrated that ML platforms significantly reduced the time to diagnose intracranial hemorrhages on CT and identified subtle hemorrhages missed by radiologists.55 Other studies have claimed that AI programs may be better than radiologists in detecting cancer in screening mammograms, and 3 FDA-approved devices focus on mammogram interpretation.2,15,54,57 There is also great interest in MRI applications to detect and predict prognosis for breast cancer based on imaging findings.21,56
Aside from providing accurate diagnoses, other studies focus on AI radiograph interpretation to assist with patient screening, triage, improving time to final diagnosis, providing a rapid “second opinion,” and even monitoring disease progression and offering insights into prognosis.8,21,23,52,55,56,58 These features help in busy urban centers but may play an even greater role in areas with limited access to health care or trained specialists such as radiologists.52
Cardiology
Cardiology has the second highest number of FDA-approved AI applications.2 Many cardiology AI platforms involve image analysis, as described in several recent reviews.45,59,60 AI has been applied to echocardiography to measure ejection fractions, detect valvular disease, and assess heart failure from hypertrophic and restrictive cardiomyopathy and amyloidosis.45,48,59 Applications for cardiac CT scans and CT angiography have successfully quantified both calcified and noncalcified coronary artery plaques and lumen assessments, assessed myocardial perfusion, and performed coronary artery calcium scoring.45,59,60 Likewise, AI applications for cardiac MRI have been used to quantitate ejection fraction, large vessel flow assessment, and cardiac scar burden.45,59
For years ECG devices have provided interpretation with limited accuracy using preprogrammed parameters.48 However, the application of AI allows ECG interpretation on par with trained cardiologists. Numerous such AI applications exist, and 2 FDA-approved devices perform ECG interpretation.2,61-64 One of these devices incorporates an AI-powered stethoscope to detect atrial fibrillation and heart murmurs.65
Pathology
The advancement of whole slide imaging, wherein entire slides can be scanned and digitized at high speed and resolution, creates great potential for AI applications in pathology.12,24,32,33,66 A landmark study demonstrating the potential of AI for assessing whole slide imaging examined sentinel lymph node metastases in patients with breast cancer.22 Multiple algorithms in the study demonstrated that AI was equivalent or better than pathologists in detecting metastases, especially when the pathologists were time-constrained consistent with a normal working environment. Significantly, the most accurate and efficient diagnoses were achieved when the pathologist and AI interpretations were used together.22,33
AI has shown promise in diagnosing many other entities, including cancers of the prostate (including Gleason scoring), lung, colon, breast, and skin.11,12,24,27,32,67 In addition, AI has shown great potential in scoring biomarkers important for prognosis and treatment, such as immunohistochemistry (IHC) labeling of Ki-67 and PD-L1.32 Pathologists can have difficulty classifying certain tumors or determining the site of origin for metastases, often having to rely on IHC with limited success. The unique features of image analysis with AI have the potential to assist in classifying difficult tumors and identifying sites of origin for metastatic disease based on morphology alone.11
Oncology depends heavily on molecular pathology testing to dictate treatment options and determine prognosis. Preliminary studies suggest that AI interpretation alone has the potential to delineate whether certain molecular mutations are present in tumors from various sites.11,14,24,32 One study combined histology and genomic results for AI interpretation that improved prognostic predictions.68 In addition, AI analysis may have potential in predicting tumor recurrence or prognosis based on cellular features, as demonstrated for lung cancer and melanoma.67,69,70
Ophthalmology
AI applications for ophthalmology have focused on diabetic retinopathy, age-related macular degeneration, glaucoma, retinopathy of prematurity, age-related and congenital cataracts, and retinal vein occlusion.71-73 Diabetic retinopathy is a leading cause of blindness and has been studied by numerous platforms with good success, most having used color fundus photography.71,72 One study showed AI could diagnose diabetic retinopathy and diabetic macular edema with specificities similar to ophthalmologists.74 In 2018, the FDA approved the AI platform IDx-DR. This diagnostic system classifies retinal images and recommends referral for patients determined to have “more than mild diabetic retinopathy” and reexamination within a year for other patients.8,75 Significantly, the platform recommendations do not require confirmation by a clinician.8
AI has been applied to other modalities in ophthalmology such as optical coherence tomography (OCT) to diagnose retinal disease and to predict appropriate management of congenital cataracts.25,73,76 For example, an AI application using OCT has been demonstrated to match or exceed the accuracy of retinal experts in diagnosing and triaging patients with a variety of retinal pathologies, including patients needing urgent referrals.77
Dermatology
Multiple studies demonstrate AI performs at least equal to experienced dermatologists in differentiating selected skin lesions.78-81 For example, Esteva and colleagues demonstrated AI could differentiate keratinocyte carcinomas from benign seborrheic keratoses and malignant melanomas from benign nevi with accuracy equal to 21 board-certified dermatologists.78
AI is applicable to various imaging procedures common to dermatology, such as dermoscopy, very high-frequency ultrasound, and reflectance confocal microscopy.82 Several studies have demonstrated that AI interpretation compared favorably to dermatologists evaluating dermoscopy to assess melanocytic lesions.78-81,83
A limitation in these studies is that they differentiate only a few diagnoses.82 Furthermore, dermatologists have sensory input such as touch and visual examination under various conditions, something AI has yet to replicate.15,34,84 Also, most AI devices use no or limited clinical information.81 Dermatologists can recognize rarer conditions for which AI models may have had limited or no training.34 Nevertheless, a recent study assessed AI for the diagnosis of 134 separate skin disorders with promising results, including providing diagnoses with accuracy comparable to that of dermatologists and providing accurate treatment strategies.84 As Topol points out, most skin lesions are diagnosed in the primary care setting where AI can have a greater impact when used in conjunction with the clinical impression, especially where specialists are in limited supply.48,78
Finally, dermatology lends itself to using portable or smartphone applications (apps) wherein the user can photograph a lesion for analysis by AI algorithms to assess the need for further evaluation or make treatment recommendations.34,84,85 Although results from currently available apps are not encouraging, they may play a greater role as the technology advances.34,85
Oncology
Applications of AI in oncology include predicting prognosis for patients with cancer based on histologic and/or genetic information.14,68,86 Programs can predict the risk of complications before and recurrence risks after surgery for malignancies.44,87-89 AI can also assist in treatment planning and predict treatment failure with radiation therapy.90,91
AI has great potential in processing the large volumes of patient data in cancer genomics. Next-generation sequencing has allowed for the identification of millions of DNA sequences in a single tumor to detect genetic anomalies.92 Thousands of mutations can be found in individual tumor samples, and processing this information and determining its significance can be beyond human capability.14 We know little about the effects of various mutation combinations, and most tumors have a heterogeneous molecular profile among different cell populations.14,93 The presence or absence of various mutations can have diagnostic, prognostic, and therapeutic implications.93 AI has great potential to sort through these complex data and identify actionable findings.
More than 200,000 cancer-related articles were published in 2019, and publications in the field of cancer genomics are increasing exponentially.14,92,93 Patel and colleagues assessed the utility of IBM Watson for Genomics against results from a molecular tumor board.93 Watson for Genomics identified potentially significant mutations not identified by the tumor board in 32% of patients. Most mutations were related to new clinical trials not yet added to the tumor board watch list, demonstrating the role AI will have in processing the large volume of genetic data required to deliver personalized medicine moving forward.
Gastroenterology
AI has shown promise in predicting risk or outcomes based on clinical parameters in various common gastroenterology problems, including gastric reflux, acute pancreatitis, gastrointestinal bleeding, celiac disease, and inflammatory bowel disease.94,95 AI endoscopic analysis has demonstrated potential in assessing Barrett’s esophagus, gastric Helicobacter pylori infections, gastric atrophy, and gastric intestinal metaplasia.95 Applications have been used to assess esophageal, gastric, and colonic malignancies, including depth of invasion based on endoscopic images.95 Finally, studies have evaluated AI to assess small colon polyps during colonoscopy, including differentiating benign and premalignant polyps with success comparable to gastroenterologists.94,95 AI has been shown to increase the speed and accuracy of gastroenterologists in detecting small polyps during colonoscopy.48 In a prospective randomized study, colonoscopies performed using an AI device identified significantly more small adenomatous polyps than colonoscopies without AI.96
Neurology
It has been suggested that AI technologies are well suited for application in neurology due to the subtle presentation of many neurologic diseases.16 Viz LVO, the first CMS-approved AI reimbursement for the diagnosis of strokes, analyzes CTs to detect early ischemic strokes and alerts the medical team, thus shortening time to treatment.3,97 Many other AI platforms are in use or development that use CT and MRI for the early detection of strokes as well as for treatment and prognosis.9,97
AI technologies have been applied to neurodegenerative diseases, such as Alzheimer and Parkinson diseases.16,98 For example, several studies have evaluated patient movements in Parkinson disease for both early diagnosis and to assess response to treatment.98 These evaluations included assessment with both external cameras as well as wearable devices and smartphone apps.
AI has also been applied to seizure disorders, attempting to determine seizure type, localize the area of seizure onset, and address the challenges of identifying seizures in neonates.99,100 Other potential applications range from early detection and prognosis predictions for cases of multiple sclerosis to restoring movement in paralysis from a variety of conditions such as spinal cord injury.9,101,102
Mental Health
Due to the interactive nature of mental health care, the field has been slower to develop AI applications.18 With heavy reliance on textual information (eg, clinic notes, mood rating scales, and documentation of conversations), successful AI applications in this field will likely rely heavily on NLP.18 However, studies investigating the application of AI to mental health have also incorporated data such as brain imaging, smartphone monitoring, and social media platforms, such as Facebook and Twitter.18,103,104
The risk of suicide is higher in veteran patients, and ML algorithms have had limited success in predicting suicide risk in both veteran and nonveteran populations.104-106 While early models have low positive predictive values and low sensitivities, they still promise to be a useful tool in conjunction with traditional risk assessments.106 Kessler and colleagues suggest that combining multiple rather than single ML algorithms might lead to greater success.105,106
AI may assist in diagnosing other mental health disorders, including major depressive disorder, attention deficit hyperactivity disorder (ADHD), schizophrenia, posttraumatic stress disorder, and Alzheimer disease.103,104,107 These investigations are in the early stages with limited clinical applicability. However, 2 AI applications awaiting FDA approval relate to ADHD and opioid use.2 Furthermore, potential exists for AI to not only assist with prevention and diagnosis of ADHD, but also to identify optimal treatment options.2,103
General and Personalized Medicine
Additional AI applications include diagnosing patients with suspected sepsis, measuring liver iron concentrations, predicting hospital mortality at the time of admission, and more.2,108,109 AI can guide end-of-life decisions such as resuscitation status or whether to initiate mechanical ventilation.48
AI-driven smartphone apps can be beneficial to both patients and clinicians. Examples include predicting nonadherence to anticoagulation therapy, monitoring heart rhythms for atrial fibrillation or signs of hyperkalemia in patients with renal failure, and improving outcomes for patients with diabetes mellitus by decreasing glycemic variability and reducing hypoglycemia.8,48,110,111 The potential for AI applications to health care and personalized medicine are almost limitless.
Discussion
With ever-increasing expectations for all health care sectors to deliver timely, fiscally-responsible, high-quality health care, AI has the potential to have numerous impacts. AI can improve diagnostic accuracy while limiting errors and impact patient safety such as assisting with prescription delivery.8,9,34 It can screen and triage patients, alerting clinicians to those needing more urgent evaluation.8,23,77,97 AI also may increase a clinician’s efficiency and speed to render a diagnosis.12,13,55,97 AI can provide a rapid second opinion, an ability especially beneficial in underserved areas with shortages of specialists.23,25,26,29,34 Similarly, AI may decrease the inter- and intraobserver variability common in many medical specialties.12,27,45 AI applications can also monitor disease progression, identifying patients at greatest risk, and provide information for prognosis.21,23,56,58 Finally, as described with applications using IBM Watson, AI can allow for an integrated approach to health care that is currently lacking.
We have described many reports suggesting AI can render diagnoses as well as or better than experienced clinicians, and speculation exists that AI will replace many roles currently performed by health care practitioners.9,26 However, most studies demonstrate that AI’s diagnostic benefits are best realized when used to supplement a clinician’s impression.8,22,30,33,52,54,56,69,84 AI is not likely to replace humans in health care in the foreseeable future. The technology can be likened to the impact of CT scans developed in the 1970s in neurology. Prior to such detailed imaging, neurologists spent extensive time performing detailed physicals to render diagnoses and locate lesions before surgery. There was mistrust of this new technology and concern that CT scans would eliminate the need for neurologists.112 On the contrary, neurology is alive and well, frequently being augmented by the technologies once speculated to replace it.
Commercial AI health care platforms represented a $2 billion industry in 2018 and are growing rapidly each year.13,32 Many AI products are offered ready for implementation for various tasks, including diagnostics, patient management, and improved efficiency. Others will likely be provided as templates suitable for modification to meet the specific needs of the facility, practice, or specialty for its patient population.
AI Risks and Limitations
AI has several risks and limitations. Although there is progress in explainable AI, at times we still struggle to understand how the output provided by machine learning algorithms was created.44,48 The many layers associated with deep learning self-determine the criteria to reach its conclusion, and these criteria can continually evolve. The parameters of deep learning are not preprogrammed, and there are too many individual data points to be extrapolated or deconvoluted for evaluation at our current level of knowledge.26,51 These apparent lack of constraints cause concern for patient safety and suggest that greater validation and continued scrutiny of validity is required.8,48 Efforts are underway to create explainable AI programs to make their processes more transparent, but such clarification is limited presently.14,26,48,77
Another challenge of AI is determining the amount of training data required to function optimally. Also, if the output describes multiple variables or diagnoses, are each equally valid?113 Furthermore, many AI applications look for a specific process, such as cancer diagnoses on CXRs. However, how coexisting conditions like cardiomegaly, emphysema, pneumonia, etc, seen on CXRs will affect the diagnosis needs to be considered.51,52 Zech and colleagues provide the example that diagnoses for pneumothorax are frequently rendered on CXRs with chest tubes in place.51 They suggest that CNNs may develop a bias toward diagnosing pneumothorax when chest tubes are present. Many current studies approach an issue in isolation, a situation not realistic in real-world clinical practice.26
Most studies on AI have been retrospective, and frequently data used to train the program are preselected.13,26 The data are typically validated on available databases rather than actual patients in the clinical setting, limiting confidence in the validity of the AI output when applied to real-world situations. Currently, fewer than 12 prospective trials had been published comparing AI with traditional clinical care.13,114 Randomized prospective clinical trials are even fewer, with none currently reported from the United States.13,114 The results from several studies have been shown to diminish when repeated prospectively.114
The FDA has created a new category known as Software as a Medical Device and has a Digital Health Innovation Action Plan to regulate AI platforms. Still, the process of AI regulation is of necessity different from traditional approval processes and is continually evolving.8 The FDA approval process cannot account for the fact that the program’s parameters may continually evolve or adapt.2
Guidelines for investigating and reporting AI research with its unique attributes are being developed. Examples include the TRIPOD-ML statement and others.49,115 In September 2020, 2 publications addressed the paucity of gold-standard randomized clinical trials in clinical AI applications.116,117 The SPIRIT-AI statement expands on the original SPIRIT statement published in 2013 to guide minimal reporting standards for AI clinical trial protocols to promote transparency of design and methodology.116 Similarly, the CONSORT-AI extension, stemming from the original CONSORT statement in 1996, aims to ensure quality reporting of randomized controlled trials in AI.117
Another risk with AI is that while an individual physician making a mistake may adversely affect 1 patient, a single mistake in an AI algorithm could potentially affect thousands of patients.48 Also, AI programs developed for patient populations at a facility may not translate to another. Referred to as overfitting, this phenomenon relates to selection bias in training data sets.15,34,49,51,52 Studies have shown that programs that underrepresent certain group characteristics such as age, sex, or race may be less effective when applied to a population in which these characteristics have differing representations.8,48,49 This problem of underrepresentation has been demonstrated in programs interpreting pathology slides, radiographs, and skin lesions.15,32,51
Admittedly, most of these challenges are not specific to AI and existed in health care previously. Physicians make mistakes, treatments are sometimes used without adequate prospective studies, and medications are given without understanding their mechanism of action, much like AI-facilitated processes reach a conclusion that cannot be fully explained.48
Conclusions
The view that AI will dramatically impact health care in the coming years will likely prove true. However, much work is needed, especially because of the paucity of prospective clinical trials as has been historically required in medical research. Any concern that AI will replace HCPs seems unwarranted. Early studies suggest that even AI programs that appear to exceed human interpretation perform best when working in cooperation with and oversight from clinicians. AI’s greatest potential appears to be its ability to augment care from health professionals, improving efficiency and accuracy, and should be anticipated with enthusiasm as the field moves forward at an exponential rate.
Acknowledgments
The authors thank Makenna G. Thomas for proofreading and review of the manuscript. This material is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital. This research has been approved by the James A. Haley Veteran’s Hospital Office of Communications and Media.
1. Bini SA. Artificial intelligence, machine learning, deep learning, and cognitive computing: what do these terms mean and how will they impact health care? J Arthroplasty. 2018;33(8):2358-2361. doi:10.1016/j.arth.2018.02.067
2. Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med. 2020;3:118. doi:10.1038/s41746-020-00324-0
3. Viz. AI powered synchronized stroke care. Accessed September 15, 2021. https://www.viz.ai/ischemic-stroke
4. Buchanan M. The law of accelerating returns. Nat Phys. 2008;4(7):507. doi:10.1038/nphys1010
5. IBM Watson Health computes a pair of new solutions to improve healthcare data and security. Published September 10, 2015. Accessed October 21, 2020. https://www.techrepublic.com/article/ibm-watson-health-computes-a-pair-of-new-solutions-to-improve-healthcare-data-and-security
6. Borkowski AA, Kardani A, Mastorides SM, Thomas LB. Warfarin pharmacogenomics: recommendations with available patented clinical technologies. Recent Pat Biotechnol. 2014;8(2):110-115. doi:10.2174/1872208309666140904112003
7. Washington University in St. Louis. Warfarin dosing. Accessed September 15, 2021. http://www.warfarindosing.org/Source/Home.aspx
8. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25(1):30-36. doi:10.1038/s41591-018-0307-0
9. Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243. Published 2017 Jun 21. doi:10.1136/svn-2017-000101
10. Johnson KW, Torres Soto J, Glicksberg BS, et al. Artificial intelligence in cardiology. J Am Coll Cardiol. 2018;71(23):2668-2679. doi:10.1016/j.jacc.2018.03.521
11. Borkowski AA, Wilson CP, Borkowski SA, et al. Comparing artificial intelligence platforms for histopathologic cancer diagnosis. Fed Pract. 2019;36(10):456-463.
12. Cruz-Roa A, Gilmore H, Basavanhally A, et al. High-throughput adaptive sampling for whole-slide histopathology image analysis (HASHI) via convolutional neural networks: application to invasive breast cancer detection. PLoS One. 2018;13(5):e0196828. Published 2018 May 24. doi:10.1371/journal.pone.0196828
13. Nagendran M, Chen Y, Lovejoy CA, et al. Artificial intelligence versus clinicians: systematic review of design, reporting standards, and claims of deep learning studies. BMJ. 2020;368:m689. Published 2020 Mar 25. doi:10.1136/bmj.m689
14. Shimizu H, Nakayama KI. Artificial intelligence in oncology. Cancer Sci. 2020;111(5):1452-1460. doi:10.1111/cas.14377
15. Talebi-Liasi F, Markowitz O. Is artificial intelligence going to replace dermatologists?. Cutis. 2020;105(1):28-31.
16. Valliani AA, Ranti D, Oermann EK. Deep learning and neurology: a systematic review. Neurol Ther. 2019;8(2):351-365. doi:10.1007/s40120-019-00153-8
17. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436-444. doi:10.1038/nature14539
18. Graham S, Depp C, Lee EE, et al. Artificial intelligence for mental health and mental illnesses: an overview. Curr Psychiatry Rep. 2019;21(11):116. Published 2019 Nov 7. doi:10.1007/s11920-019-1094-0
19. Golas SB, Shibahara T, Agboola S, et al. A machine learning model to predict the risk of 30-day readmissions in patients with heart failure: a retrospective analysis of electronic medical records data. BMC Med Inform Decis Mak. 2018;18(1):44. Published 2018 Jun 22. doi:10.1186/s12911-018-0620-z
20. Mortazavi BJ, Downing NS, Bucholz EM, et al. Analysis of machine learning techniques for heart failure readmissions. Circ Cardiovasc Qual Outcomes. 2016;9(6):629-640. doi:10.1161/CIRCOUTCOMES.116.003039
21. Meyer-Bäse A, Morra L, Meyer-Bäse U, Pinker K. Current status and future perspectives of artificial intelligence in magnetic resonance breast imaging. Contrast Media Mol Imaging. 2020;2020:6805710. Published 2020 Aug 28. doi:10.1155/2020/6805710
22. Ehteshami Bejnordi B, Veta M, Johannes van Diest P, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318(22):2199-2210. doi:10.1001/jama.2017.14585
23. Borkowski AA, Viswanadhan NA, Thomas LB, Guzman RD, Deland LA, Mastorides SM. Using artificial intelligence for COVID-19 chest X-ray diagnosis. Fed Pract. 2020;37(9):398-404. doi:10.12788/fp.0045
24. Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24(10):1559-1567. doi:10.1038/s41591-018-0177-5
25. Kermany DS, Goldbaum M, Cai W, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172(5):1122-1131.e9. doi:10.1016/j.cell.2018.02.010
26. Liu X, Faes L, Kale AU, et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit Health. 2019;1(6):e271-e297. doi:10.1016/S2589-7500(19)30123-2
27. Nagpal K, Foote D, Liu Y, et al. Development and validation of a deep learning algorithm for improving Gleason scoring of prostate cancer [published correction appears in NPJ Digit Med. 2019 Nov 19;2:113]. NPJ Digit Med. 2019;2:48. Published 2019 Jun 7. doi:10.1038/s41746-019-0112-2
28. Nam JG, Park S, Hwang EJ, et al. Development and validation of deep learning-based automatic detection algorithm for malignant pulmonary nodules on chest radiographs. Radiology. 2019;290(1):218-228. doi:10.1148/radiol.2018180237
29. Schwalbe N, Wahl B. Artificial intelligence and the future of global health. Lancet. 2020;395(10236):1579-1586. doi:10.1016/S0140-6736(20)30226-9
30. Bai HX, Wang R, Xiong Z, et al. Artificial intelligence augmentation of radiologist performance in distinguishing COVID-19 from pneumonia of other origin at chest CT [published correction appears in Radiology. 2021 Apr;299(1):E225]. Radiology. 2020;296(3):E156-E165. doi:10.1148/radiol.2020201491
31. Li L, Qin L, Xu Z, et al. Using artificial intelligence to detect COVID-19 and community-acquired pneumonia based on pulmonary CT: evaluation of the diagnostic accuracy. Radiology. 2020;296(2):E65-E71. doi:10.1148/radiol.2020200905
32. Serag A, Ion-Margineanu A, Qureshi H, et al. Translational AI and deep learning in diagnostic pathology. Front Med (Lausanne). 2019;6:185. Published 2019 Oct 1. doi:10.3389/fmed.2019.00185

33. Wang D, Khosla A, Gargeya R, Irshad H, Beck AH. Deep learning for identifying metastatic breast cancer. ArXiv. 2016 June 18:arXiv:1606.05718v1. Published online June 18, 2016. Accessed September 15, 2021. http://arxiv.org/abs/1606.05718
34. Alabdulkareem A. Artificial intelligence and dermatologists: friends or foes? J Dermatology Dermatol Surg. 2019;23(2):57-60. doi:10.4103/jdds.jdds_19_19
35. Mollalo A, Mao L, Rashidi P, Glass GE. A GIS-based artificial neural network model for spatial distribution of tuberculosis across the continental United States. Int J Environ Res Public Health. 2019;16(1):157. Published 2019 Jan 8. doi:10.3390/ijerph16010157
36. Haddawy P, Hasan AHMI, Kasantikul R, et al. Spatiotemporal Bayesian networks for malaria prediction. Artif Intell Med. 2018;84:127-138. doi:10.1016/j.artmed.2017.12.002
37. Laureano-Rosario AE, Duncan AP, Mendez-Lazaro PA, et al. Application of artificial neural networks for dengue fever outbreak predictions in the northwest coast of Yucatan, Mexico and San Juan, Puerto Rico. Trop Med Infect Dis. 2018;3(1):5. Published 2018 Jan 5. doi:10.3390/tropicalmed3010005
38. Buczak AL, Koshute PT, Babin SM, Feighner BH, Lewis SH. A data-driven epidemiological prediction method for dengue outbreaks using local and remote sensing data. BMC Med Inform Decis Mak. 2012;12:124. Published 2012 Nov 5. doi:10.1186/1472-6947-12-124
39. Scavuzzo JM, Trucco F, Espinosa M, et al. Modeling dengue vector population using remotely sensed data and machine learning. Acta Trop. 2018;185:167-175. doi:10.1016/j.actatropica.2018.05.003
40. Xue H, Bai Y, Hu H, Liang H. Influenza activity surveillance based on multiple regression model and artificial neural network. IEEE Access. 2018;6:563-575. doi:10.1109/ACCESS.2017.2771798
41. Jiang D, Hao M, Ding F, Fu J, Li M. Mapping the transmission risk of Zika virus using machine learning models. Acta Trop. 2018;185:391-399. doi:10.1016/j.actatropica.2018.06.021
42. Bragazzi NL, Dai H, Damiani G, Behzadifar M, Martini M, Wu J. How big data and artificial intelligence can help better manage the COVID-19 pandemic. Int J Environ Res Public Health. 2020;17(9):3176. Published 2020 May 2. doi:10.3390/ijerph17093176
43. Lake IR, Colón-González FJ, Barker GC, Morbey RA, Smith GE, Elliot AJ. Machine learning to refine decision making within a syndromic surveillance service. BMC Public Health. 2019;19(1):559. Published 2019 May 14. doi:10.1186/s12889-019-6916-9
44. Khan OF, Bebb G, Alimohamed NA. Artificial intelligence in medicine: what oncologists need to know about its potential-and its limitations. Oncol Exch. 2017;16(4):8-13. Accessed September 1, 2021. http://www.oncologyex.com/pdf/vol16_no4/feature_khan-ai.pdf
45. Badano LP, Keller DM, Muraru D, Torlasco C, Parati G. Artificial intelligence and cardiovascular imaging: A win-win combination. Anatol J Cardiol. 2020;24(4):214-223. doi:10.14744/AnatolJCardiol.2020.94491
46. Murdoch TB, Detsky AS. The inevitable application of big data to health care. JAMA. 2013;309(13):1351-1352. doi:10.1001/jama.2013.393
47. Greatbatch O, Garrett A, Snape K. The impact of artificial intelligence on the current and future practice of clinical cancer genomics. Genet Res (Camb). 2019;101:e9. Published 2019 Oct 31. doi:10.1017/S0016672319000089
48. Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44-56. doi:10.1038/s41591-018-0300-7
49. Vollmer S, Mateen BA, Bohner G, et al. Machine learning and artificial intelligence research for patient benefit: 20 critical questions on transparency, replicability, ethics, and effectiveness [published correction appears in BMJ. 2020 Apr 1;369:m1312]. BMJ. 2020;368:l6927. Published 2020 Mar 20. doi:10.1136/bmj.l6927
50. Lindsey R, Daluiski A, Chopra S, et al. Deep neural network improves fracture detection by clinicians. Proc Natl Acad Sci U S A. 2018;115(45):11591-11596. doi:10.1073/pnas.1806905115
51. Zech JR, Badgeley MA, Liu M, Costa AB, Titano JJ, Oermann EK. Variable generalization performance of a deep learning model to detect pneumonia in chest radiographs: a cross-sectional study. PLoS Med. 2018;15(11):e1002683. doi:10.1371/journal.pmed.1002683
52. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582. doi:10.1148/radiol.2017162326
53. Rajpurkar P, Joshi A, Pareek A, et al. CheXpedition: investigating generalization challenges for translation of chest x-ray algorithms to the clinical setting. ArXiv. 2020 Feb 26:arXiv:2002.11379v2. Revised March 11, 2020. Accessed September 15, 2021. http://arxiv.org/abs/2002.11379
54. Salim M, Wåhlin E, Dembrower K, et al. External evaluation of 3 commercial artificial intelligence algorithms for independent assessment of screening mammograms. JAMA Oncol. 2020;6(10):1581-1588. doi:10.1001/jamaoncol.2020.3321
55. Arbabshirani MR, Fornwalt BK, Mongelluzzo GJ, et al. Advanced machine learning in action: identification of intracranial hemorrhage on computed tomography scans of the head with clinical workflow integration. NPJ Digit Med. 2018;1:9. doi:10.1038/s41746-017-0015-z
56. Sheth D, Giger ML. Artificial intelligence in the interpretation of breast cancer on MRI. J Magn Reson Imaging. 2020;51(5):1310-1324. doi:10.1002/jmri.26878
57. McKinney SM, Sieniek M, Godbole V, et al. International evaluation of an AI system for breast cancer screening. Nature. 2020;577(7788):89-94. doi:10.1038/s41586-019-1799-6
58. Booth AL, Abels E, McCaffrey P. Development of a prognostic model for mortality in COVID-19 infection using machine learning. Mod Pathol. 2021;34(3):522-531. doi:10.1038/s41379-020-00700-x
59. Xu B, Kocyigit D, Grimm R, Griffin BP, Cheng F. Applications of artificial intelligence in multimodality cardiovascular imaging: a state-of-the-art review. Prog Cardiovasc Dis. 2020;63(3):367-376. doi:10.1016/j.pcad.2020.03.003
60. Dey D, Slomka PJ, Leeson P, et al. Artificial intelligence in cardiovascular imaging: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(11):1317-1335. doi:10.1016/j.jacc.2018.12.054
61. Carewell Health. AI powered ECG diagnosis solutions. Accessed November 2, 2020. https://www.carewellhealth.com/products_aiecg.html
62. Strodthoff N, Strodthoff C. Detecting and interpreting myocardial infarction using fully convolutional neural networks. Physiol Meas. 2019;40(1):015001. doi:10.1088/1361-6579/aaf34d
63. Hannun AY, Rajpurkar P, Haghpanahi M, et al. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019;25(1):65-69. doi:10.1038/s41591-018-0268-3
64. Kwon JM, Jeon KH, Kim HM, et al. Comparing the performance of artificial intelligence and conventional diagnosis criteria for detecting left ventricular hypertrophy using electrocardiography. Europace. 2020;22(3):412-419. doi:10.1093/europace/euz324

65. Eko. FDA clears Eko’s AFib and heart murmur detection algorithms, making it the first AI-powered stethoscope to screen for serious heart conditions [press release]. Published January 28, 2020. Accessed September 15, 2021. https://www.businesswire.com/news/home/20200128005232/en/FDA-Clears-Eko’s-AFib-and-Heart-Murmur-Detection-Algorithms-Making-It-the-First-AI-Powered-Stethoscope-to-Screen-for-Serious-Heart-Conditions
66. Cruz-Roa A, Gilmore H, Basavanhally A, et al. Accurate and reproducible invasive breast cancer detection in whole-slide images: a deep learning approach for quantifying tumor extent. Sci Rep. 2017;7:46450. doi:10.1038/srep46450
67. Acs B, Rantalainen M, Hartman J. Artificial intelligence as the next step towards precision pathology. J Intern Med. 2020;288(1):62-81. doi:10.1111/joim.13030
68. Mobadersany P, Yousefi S, Amgad M, et al. Predicting cancer outcomes from histology and genomics using convolutional networks. Proc Natl Acad Sci U S A. 2018;115(13):E2970-E2979. doi:10.1073/pnas.1717139115
69. Wang X, Janowczyk A, Zhou Y, et al. Prediction of recurrence in early stage non-small cell lung cancer using computer extracted nuclear features from digital H&E images. Sci Rep. 2017;7:13543. doi:10.1038/s41598-017-13773-7
70. Kulkarni PM, Robinson EJ, Pradhan JS, et al. Deep learning based on standard H&E images of primary melanoma tumors identifies patients at risk for visceral recurrence and death. Clin Cancer Res. 2020;26(5):1126-1134. doi:10.1158/1078-0432.CCR-19-1495
71. Du XL, Li WB, Hu BJ. Application of artificial intelligence in ophthalmology. Int J Ophthalmol. 2018;11(9):1555-1561. doi:10.18240/ijo.2018.09.21
72. Gunasekeran DV, Wong TY. Artificial intelligence in ophthalmology in 2020: a technology on the cusp for translation and implementation. Asia Pac J Ophthalmol (Phila). 2020;9(2):61-66. doi:10.1097/01.APO.0000656984.56467.2c
73. Ting DSW, Pasquale LR, Peng L, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019;103(2):167-175. doi:10.1136/bjophthalmol-2018-313173
74. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2410. doi:10.1001/jama.2016.17216
75. US Food and Drug Administration. FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems [press release]. Published April 11, 2018. Accessed September 15, 2021. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye
76. Long E, Chen J, Wu X, et al. Artificial intelligence manages congenital cataract with individualized prediction and telehealth computing. NPJ Digit Med. 2020;3:112. doi:10.1038/s41746-020-00319-x
77. De Fauw J, Ledsam JR, Romera-Paredes B, et al. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat Med. 2018;24(9):1342-1350. doi:10.1038/s41591-018-0107-6
78. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115-118. doi:10.1038/nature21056
79. Brinker TJ, Hekler A, Enk AH, et al. Deep neural networks are superior to dermatologists in melanoma image classification. Eur J Cancer. 2019;119:11-17. doi:10.1016/j.ejca.2019.05.023
80. Brinker TJ, Hekler A, Enk AH, et al. A convolutional neural network trained with dermoscopic images performed on par with 145 dermatologists in a clinical melanoma image classification task. Eur J Cancer. 2019;111:148-154. doi:10.1016/j.ejca.2019.02.005
81. Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists. Ann Oncol. 2018;29(8):1836-1842. doi:10.1093/annonc/mdy166
82. Li CX, Shen CB, Xue K, et al. Artificial intelligence in dermatology: past, present, and future. Chin Med J (Engl). 2019;132(17):2017-2020. doi:10.1097/CM9.0000000000000372
83. Tschandl P, Codella N, Akay BN, et al. Comparison of the accuracy of human readers versus machine-learning algorithms for pigmented skin lesion classification: an open, web-based, international, diagnostic study. Lancet Oncol. 2019;20(7):938-947. doi:10.1016/S1470-2045(19)30333-X
84. Han SS, Park I, Eun Chang SE, et al. Augmented intelligence dermatology: deep neural networks empower medical professionals in diagnosing skin cancer and predicting treatment options for 134 skin disorders. J Invest Dermatol. 2020;140(9):1753-1761. doi:10.1016/j.jid.2020.01.019
85. Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies [published correction appears in BMJ. 2020 Feb 25;368:m645]. BMJ. 2020;368:m127. Published 2020 Feb 10. doi:10.1136/bmj.m127
86. Chen YC, Ke WC, Chiu HW. Risk classification of cancer survival using ANN with gene expression data from multiple laboratories. Comput Biol Med. 2014;48:1-7. doi:10.1016/j.compbiomed.2014.02.006
87. Kim W, Kim KS, Lee JE, et al. Development of novel breast cancer recurrence prediction model using support vector machine. J Breast Cancer. 2012;15(2):230-238. doi:10.4048/jbc.2012.15.2.230
88. Merath K, Hyer JM, Mehta R, et al. Use of machine learning for prediction of patient risk of postoperative complications after liver, pancreatic, and colorectal surgery. J Gastrointest Surg. 2020;24(8):1843-1851. doi:10.1007/s11605-019-04338-2
89. Santos-García G, Varela G, Novoa N, Jiménez MF. Prediction of postoperative morbidity after lung resection using an artificial neural network ensemble. Artif Intell Med. 2004;30(1):61-69. doi:10.1016/S0933-3657(03)00059-9
90. Ibragimov B, Xing L. Segmentation of organs-at-risks in head and neck CT images using convolutional neural networks. Med Phys. 2017;44(2):547-557. doi:10.1002/mp.12045
91. Lou B, Doken S, Zhuang T, et al. An image-based deep learning framework for individualizing radiotherapy dose. Lancet Digit Health. 2019;1(3):e136-e147. doi:10.1016/S2589-7500(19)30058-5
92. Xu J, Yang P, Xue S, et al. Translating cancer genomics into precision medicine with artificial intelligence: applications, challenges and future perspectives. Hum Genet. 2019;138(2):109-124. doi:10.1007/s00439-019-01970-5
93. Patel NM, Michelini VV, Snell JM, et al. Enhancing next‐generation sequencing‐guided cancer care through cognitive computing. Oncologist. 2018;23(2):179-185. doi:10.1634/theoncologist.2017-0170
94. Le Berre C, Sandborn WJ, Aridhi S, et al. Application of artificial intelligence to gastroenterology and hepatology. Gastroenterology. 2020;158(1):76-94.e2. doi:10.1053/j.gastro.2019.08.058
95. Yang YJ, Bang CS. Application of artificial intelligence in gastroenterology. World J Gastroenterol. 2019;25(14):1666-1683. doi:10.3748/wjg.v25.i14.1666
96. Wang P, Berzin TM, Glissen Brown JR, et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut. 2019;68(10):1813-1819. doi:10.1136/gutjnl-2018-317500

97. Gupta R, Krishnam SP, Schaefer PW, Lev MH, Gonzalez RG. An East Coast perspective on artificial intelligence and machine learning: part 2: ischemic stroke imaging and triage. Neuroimaging Clin N Am. 2020;30(4):467-478. doi:10.1016/j.nic.2020.08.002
98. Beli M, Bobi V, Badža M, Šolaja N, Duri-Jovii M, Kosti VS. Artificial intelligence for assisting diagnostics and assessment of Parkinson’s disease—a review. Clin Neurol Neurosurg. 2019;184:105442. doi:10.1016/j.clineuro.2019.105442
99. An S, Kang C, Lee HW. Artificial intelligence and computational approaches for epilepsy. J Epilepsy Res. 2020;10(1):8-17. doi:10.14581/jer.20003
100. Pavel AM, Rennie JM, de Vries LS, et al. A machine-learning algorithm for neonatal seizure recognition: a multicentre, randomised, controlled trial. Lancet Child Adolesc Health. 2020;4(10):740-749. doi:10.1016/S2352-4642(20)30239-X
101. Afzal HMR, Luo S, Ramadan S, Lechner-Scott J. The emerging role of artificial intelligence in multiple sclerosis imaging [published online ahead of print, 2020 Oct 28]. Mult Scler. 2020;1352458520966298. doi:10.1177/1352458520966298
102. Bouton CE. Restoring movement in paralysis with a bioelectronic neural bypass approach: current state and future directions. Cold Spring Harb Perspect Med. 2019;9(11):a034306. doi:10.1101/cshperspect.a034306
103. Durstewitz D, Koppe G, Meyer-Lindenberg A. Deep neural networks in psychiatry. Mol Psychiatry. 2019;24(11):1583-1598. doi:10.1038/s41380-019-0365-9
104. Fonseka TM, Bhat V, Kennedy SH. The utility of artificial intelligence in suicide risk prediction and the management of suicidal behaviors. Aust N Z J Psychiatry. 2019;53(10):954-964. doi:10.1177/0004867419864428
105. Kessler RC, Hwang I, Hoffmire CA, et al. Developing a practical suicide risk prediction model for targeting high-risk patients in the Veterans Health Administration. Int J Methods Psychiatr Res. 2017;26(3):e1575. doi:10.1002/mpr.1575
106. Kessler RC, Bauer MS, Bishop TM, et al. Using administrative data to predict suicide after psychiatric hospitalization in the Veterans Health Administration System. Front Psychiatry. 2020;11:390. doi:10.3389/fpsyt.2020.00390
107. Kessler RC, van Loo HM, Wardenaar KJ, et al. Testing a machine-learning algorithm to predict the persistence and severity of major depressive disorder from baseline self-reports. Mol Psychiatry. 2016;21(10):1366-1371. doi:10.1038/mp.2015.198
108. Horng S, Sontag DA, Halpern Y, Jernite Y, Shapiro NI, Nathanson LA. Creating an automated trigger for sepsis clinical decision support at emergency department triage using machine learning. PLoS One. 2017;12(4):e0174708. doi:10.1371/journal.pone.0174708
109. Soffer S, Klang E, Barash Y, Grossman E, Zimlichman E. Predicting in-hospital mortality at admission to the medical ward: a big-data machine learning model. Am J Med. 2021;134(2):227-234.e4. doi:10.1016/j.amjmed.2020.07.014
110. Labovitz DL, Shafner L, Reyes Gil M, Virmani D, Hanina A. Using artificial intelligence to reduce the risk of nonadherence in patients on anticoagulation therapy. Stroke. 2017;48(5):1416-1419. doi:10.1161/STROKEAHA.116.016281
111. Forlenza GP. Use of artificial intelligence to improve diabetes outcomes in patients using multiple daily injections therapy. Diabetes Technol Ther. 2019;21(S2):S24-S28. doi:10.1089/dia.2019.0077
112. Poser CM. CT scan and the practice of neurology. Arch Neurol. 1977;34(2):132. doi:10.1001/archneur.1977.00500140086023
113. Angus DC. Randomized clinical trials of artificial intelligence. JAMA. 2020;323(11):1043-1045. doi:10.1001/jama.2020.1039
114. Topol EJ. Welcoming new guidelines for AI clinical research. Nat Med. 2020;26(9):1318-1320. doi:10.1038/s41591-020-1042-x
115. Collins GS, Moons KGM. Reporting of artificial intelligence prediction models. Lancet. 2019;393(10181):1577-1579. doi:10.1016/S0140-6736(19)30037-6
116. Cruz Rivera S, Liu X, Chan AW, et al. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Nat Med. 2020;26(9):1351-1363. doi:10.1038/s41591-020-1037-7
117. Liu X, Cruz Rivera S, Moher D, Calvert MJ, Denniston AK; SPIRIT-AI and CONSORT-AI Working Group. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat Med. 2020;26(9):1364-1374. doi:10.1038/s41591-020-1034-x
118. McCulloch WS, Pitts W. A logical calculus of the ideas immanent in nervous activity. Bull Math Biophys. 1943;5(4):115-133. doi:10.1007/BF02478259
119. Samuel AL. Some studies in machine learning using the game of Checkers. IBM J Res Dev. 1959;3(3):535-554. Accessed September 15, 2021. https://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.368.2254
120. Sonoda M, Takano M, Miyahara J, Kato H. Computed radiography utilizing scanning laser stimulated luminescence. Radiology. 1983;148(3):833-838. doi:10.1148/radiology.148.3.6878707
121. Dechter R. Learning while searching in constraint-satisfaction-problems. AAAI’86: proceedings of the fifth AAAI national conference on artificial intelligence. Published 1986. Accessed September 15, 2021. https://www.aaai.org/Papers/AAAI/1986/AAAI86-029.pdf
122. Le Cun Y, Jackel LD, Boser B, et al. Handwritten digit recognition: applications of neural network chips and automatic learning. IEEE Commun Mag. 1989;27(11):41-46. doi:10.1109/35.41400
123. US Food and Drug Administration. FDA allows marketing of first whole slide imaging system for digital pathology [press release]. Published April 12, 2017. Accessed September 15, 2021. https://www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-whole-slide-imaging-system-digital-pathology
1. Bini SA. Artificial intelligence, machine learning, deep learning, and cognitive computing: what do these terms mean and how will they impact health care? J Arthroplasty. 2018;33(8):2358-2361. doi:10.1016/j.arth.2018.02.067
2. Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med. 2020;3:118. doi:10.1038/s41746-020-00324-0
3. Viz. AI powered synchronized stroke care. Accessed September 15, 2021. https://www.viz.ai/ischemic-stroke
4. Buchanan M. The law of accelerating returns. Nat Phys. 2008;4(7):507. doi:10.1038/nphys1010
5. IBM Watson Health computes a pair of new solutions to improve healthcare data and security. Published September 10, 2015. Accessed October 21, 2020. https://www.techrepublic.com/article/ibm-watson-health-computes-a-pair-of-new-solutions-to-improve-healthcare-data-and-security
6. Borkowski AA, Kardani A, Mastorides SM, Thomas LB. Warfarin pharmacogenomics: recommendations with available patented clinical technologies. Recent Pat Biotechnol. 2014;8(2):110-115. doi:10.2174/1872208309666140904112003
7. Washington University in St. Louis. Warfarin dosing. Accessed September 15, 2021. http://www.warfarindosing.org/Source/Home.aspx
8. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25(1):30-36. doi:10.1038/s41591-018-0307-0
9. Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243. Published 2017 Jun 21. doi:10.1136/svn-2017-000101
10. Johnson KW, Torres Soto J, Glicksberg BS, et al. Artificial intelligence in cardiology. J Am Coll Cardiol. 2018;71(23):2668-2679. doi:10.1016/j.jacc.2018.03.521
11. Borkowski AA, Wilson CP, Borkowski SA, et al. Comparing artificial intelligence platforms for histopathologic cancer diagnosis. Fed Pract. 2019;36(10):456-463.
12. Cruz-Roa A, Gilmore H, Basavanhally A, et al. High-throughput adaptive sampling for whole-slide histopathology image analysis (HASHI) via convolutional neural networks: application to invasive breast cancer detection. PLoS One. 2018;13(5):e0196828. Published 2018 May 24. doi:10.1371/journal.pone.0196828
13. Nagendran M, Chen Y, Lovejoy CA, et al. Artificial intelligence versus clinicians: systematic review of design, reporting standards, and claims of deep learning studies. BMJ. 2020;368:m689. Published 2020 Mar 25. doi:10.1136/bmj.m689
14. Shimizu H, Nakayama KI. Artificial intelligence in oncology. Cancer Sci. 2020;111(5):1452-1460. doi:10.1111/cas.14377
15. Talebi-Liasi F, Markowitz O. Is artificial intelligence going to replace dermatologists?. Cutis. 2020;105(1):28-31.
16. Valliani AA, Ranti D, Oermann EK. Deep learning and neurology: a systematic review. Neurol Ther. 2019;8(2):351-365. doi:10.1007/s40120-019-00153-8
17. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436-444. doi:10.1038/nature14539
18. Graham S, Depp C, Lee EE, et al. Artificial intelligence for mental health and mental illnesses: an overview. Curr Psychiatry Rep. 2019;21(11):116. Published 2019 Nov 7. doi:10.1007/s11920-019-1094-0
19. Golas SB, Shibahara T, Agboola S, et al. A machine learning model to predict the risk of 30-day readmissions in patients with heart failure: a retrospective analysis of electronic medical records data. BMC Med Inform Decis Mak. 2018;18(1):44. Published 2018 Jun 22. doi:10.1186/s12911-018-0620-z
20. Mortazavi BJ, Downing NS, Bucholz EM, et al. Analysis of machine learning techniques for heart failure readmissions. Circ Cardiovasc Qual Outcomes. 2016;9(6):629-640. doi:10.1161/CIRCOUTCOMES.116.003039
21. Meyer-Bäse A, Morra L, Meyer-Bäse U, Pinker K. Current status and future perspectives of artificial intelligence in magnetic resonance breast imaging. Contrast Media Mol Imaging. 2020;2020:6805710. Published 2020 Aug 28. doi:10.1155/2020/6805710
22. Ehteshami Bejnordi B, Veta M, Johannes van Diest P, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318(22):2199-2210. doi:10.1001/jama.2017.14585
23. Borkowski AA, Viswanadhan NA, Thomas LB, Guzman RD, Deland LA, Mastorides SM. Using artificial intelligence for COVID-19 chest X-ray diagnosis. Fed Pract. 2020;37(9):398-404. doi:10.12788/fp.0045
24. Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24(10):1559-1567. doi:10.1038/s41591-018-0177-5
25. Kermany DS, Goldbaum M, Cai W, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172(5):1122-1131.e9. doi:10.1016/j.cell.2018.02.010
26. Liu X, Faes L, Kale AU, et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit Health. 2019;1(6):e271-e297. doi:10.1016/S2589-7500(19)30123-2
27. Nagpal K, Foote D, Liu Y, et al. Development and validation of a deep learning algorithm for improving Gleason scoring of prostate cancer [published correction appears in NPJ Digit Med. 2019 Nov 19;2:113]. NPJ Digit Med. 2019;2:48. Published 2019 Jun 7. doi:10.1038/s41746-019-0112-2
28. Nam JG, Park S, Hwang EJ, et al. Development and validation of deep learning-based automatic detection algorithm for malignant pulmonary nodules on chest radiographs. Radiology. 2019;290(1):218-228. doi:10.1148/radiol.2018180237
29. Schwalbe N, Wahl B. Artificial intelligence and the future of global health. Lancet. 2020;395(10236):1579-1586. doi:10.1016/S0140-6736(20)30226-9
30. Bai HX, Wang R, Xiong Z, et al. Artificial intelligence augmentation of radiologist performance in distinguishing COVID-19 from pneumonia of other origin at chest CT [published correction appears in Radiology. 2021 Apr;299(1):E225]. Radiology. 2020;296(3):E156-E165. doi:10.1148/radiol.2020201491
31. Li L, Qin L, Xu Z, et al. Using artificial intelligence to detect COVID-19 and community-acquired pneumonia based on pulmonary CT: evaluation of the diagnostic accuracy. Radiology. 2020;296(2):E65-E71. doi:10.1148/radiol.2020200905
32. Serag A, Ion-Margineanu A, Qureshi H, et al. Translational AI and deep learning in diagnostic pathology. Front Med (Lausanne). 2019;6:185. Published 2019 Oct 1. doi:10.3389/fmed.2019.00185

33. Wang D, Khosla A, Gargeya R, Irshad H, Beck AH. Deep learning for identifying metastatic breast cancer. ArXiv. 2016 June 18:arXiv:1606.05718v1. Published online June 18, 2016. Accessed September 15, 2021. http://arxiv.org/abs/1606.05718
34. Alabdulkareem A. Artificial intelligence and dermatologists: friends or foes? J Dermatology Dermatol Surg. 2019;23(2):57-60. doi:10.4103/jdds.jdds_19_19
35. Mollalo A, Mao L, Rashidi P, Glass GE. A GIS-based artificial neural network model for spatial distribution of tuberculosis across the continental United States. Int J Environ Res Public Health. 2019;16(1):157. Published 2019 Jan 8. doi:10.3390/ijerph16010157
36. Haddawy P, Hasan AHMI, Kasantikul R, et al. Spatiotemporal Bayesian networks for malaria prediction. Artif Intell Med. 2018;84:127-138. doi:10.1016/j.artmed.2017.12.002
37. Laureano-Rosario AE, Duncan AP, Mendez-Lazaro PA, et al. Application of artificial neural networks for dengue fever outbreak predictions in the northwest coast of Yucatan, Mexico and San Juan, Puerto Rico. Trop Med Infect Dis. 2018;3(1):5. Published 2018 Jan 5. doi:10.3390/tropicalmed3010005
38. Buczak AL, Koshute PT, Babin SM, Feighner BH, Lewis SH. A data-driven epidemiological prediction method for dengue outbreaks using local and remote sensing data. BMC Med Inform Decis Mak. 2012;12:124. Published 2012 Nov 5. doi:10.1186/1472-6947-12-124
39. Scavuzzo JM, Trucco F, Espinosa M, et al. Modeling dengue vector population using remotely sensed data and machine learning. Acta Trop. 2018;185:167-175. doi:10.1016/j.actatropica.2018.05.003
40. Xue H, Bai Y, Hu H, Liang H. Influenza activity surveillance based on multiple regression model and artificial neural network. IEEE Access. 2018;6:563-575. doi:10.1109/ACCESS.2017.2771798
41. Jiang D, Hao M, Ding F, Fu J, Li M. Mapping the transmission risk of Zika virus using machine learning models. Acta Trop. 2018;185:391-399. doi:10.1016/j.actatropica.2018.06.021
42. Bragazzi NL, Dai H, Damiani G, Behzadifar M, Martini M, Wu J. How big data and artificial intelligence can help better manage the COVID-19 pandemic. Int J Environ Res Public Health. 2020;17(9):3176. Published 2020 May 2. doi:10.3390/ijerph17093176
43. Lake IR, Colón-González FJ, Barker GC, Morbey RA, Smith GE, Elliot AJ. Machine learning to refine decision making within a syndromic surveillance service. BMC Public Health. 2019;19(1):559. Published 2019 May 14. doi:10.1186/s12889-019-6916-9
44. Khan OF, Bebb G, Alimohamed NA. Artificial intelligence in medicine: what oncologists need to know about its potential-and its limitations. Oncol Exch. 2017;16(4):8-13. Accessed September 1, 2021. http://www.oncologyex.com/pdf/vol16_no4/feature_khan-ai.pdf
45. Badano LP, Keller DM, Muraru D, Torlasco C, Parati G. Artificial intelligence and cardiovascular imaging: A win-win combination. Anatol J Cardiol. 2020;24(4):214-223. doi:10.14744/AnatolJCardiol.2020.94491
46. Murdoch TB, Detsky AS. The inevitable application of big data to health care. JAMA. 2013;309(13):1351-1352. doi:10.1001/jama.2013.393
47. Greatbatch O, Garrett A, Snape K. The impact of artificial intelligence on the current and future practice of clinical cancer genomics. Genet Res (Camb). 2019;101:e9. Published 2019 Oct 31. doi:10.1017/S0016672319000089
48. Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44-56. doi:10.1038/s41591-018-0300-7
49. Vollmer S, Mateen BA, Bohner G, et al. Machine learning and artificial intelligence research for patient benefit: 20 critical questions on transparency, replicability, ethics, and effectiveness [published correction appears in BMJ. 2020 Apr 1;369:m1312]. BMJ. 2020;368:l6927. Published 2020 Mar 20. doi:10.1136/bmj.l6927
50. Lindsey R, Daluiski A, Chopra S, et al. Deep neural network improves fracture detection by clinicians. Proc Natl Acad Sci U S A. 2018;115(45):11591-11596. doi:10.1073/pnas.1806905115
51. Zech JR, Badgeley MA, Liu M, Costa AB, Titano JJ, Oermann EK. Variable generalization performance of a deep learning model to detect pneumonia in chest radiographs: a cross-sectional study. PLoS Med. 2018;15(11):e1002683. doi:10.1371/journal.pmed.1002683
52. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582. doi:10.1148/radiol.2017162326
53. Rajpurkar P, Joshi A, Pareek A, et al. CheXpedition: investigating generalization challenges for translation of chest x-ray algorithms to the clinical setting. ArXiv. 2020 Feb 26:arXiv:2002.11379v2. Revised March 11, 2020. Accessed September 15, 2021. http://arxiv.org/abs/2002.11379
54. Salim M, Wåhlin E, Dembrower K, et al. External evaluation of 3 commercial artificial intelligence algorithms for independent assessment of screening mammograms. JAMA Oncol. 2020;6(10):1581-1588. doi:10.1001/jamaoncol.2020.3321
55. Arbabshirani MR, Fornwalt BK, Mongelluzzo GJ, et al. Advanced machine learning in action: identification of intracranial hemorrhage on computed tomography scans of the head with clinical workflow integration. NPJ Digit Med. 2018;1:9. doi:10.1038/s41746-017-0015-z
56. Sheth D, Giger ML. Artificial intelligence in the interpretation of breast cancer on MRI. J Magn Reson Imaging. 2020;51(5):1310-1324. doi:10.1002/jmri.26878
57. McKinney SM, Sieniek M, Godbole V, et al. International evaluation of an AI system for breast cancer screening. Nature. 2020;577(7788):89-94. doi:10.1038/s41586-019-1799-6
58. Booth AL, Abels E, McCaffrey P. Development of a prognostic model for mortality in COVID-19 infection using machine learning. Mod Pathol. 2021;34(3):522-531. doi:10.1038/s41379-020-00700-x
59. Xu B, Kocyigit D, Grimm R, Griffin BP, Cheng F. Applications of artificial intelligence in multimodality cardiovascular imaging: a state-of-the-art review. Prog Cardiovasc Dis. 2020;63(3):367-376. doi:10.1016/j.pcad.2020.03.003
60. Dey D, Slomka PJ, Leeson P, et al. Artificial intelligence in cardiovascular imaging: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(11):1317-1335. doi:10.1016/j.jacc.2018.12.054
61. Carewell Health. AI powered ECG diagnosis solutions. Accessed November 2, 2020. https://www.carewellhealth.com/products_aiecg.html
62. Strodthoff N, Strodthoff C. Detecting and interpreting myocardial infarction using fully convolutional neural networks. Physiol Meas. 2019;40(1):015001. doi:10.1088/1361-6579/aaf34d
63. Hannun AY, Rajpurkar P, Haghpanahi M, et al. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019;25(1):65-69. doi:10.1038/s41591-018-0268-3
64. Kwon JM, Jeon KH, Kim HM, et al. Comparing the performance of artificial intelligence and conventional diagnosis criteria for detecting left ventricular hypertrophy using electrocardiography. Europace. 2020;22(3):412-419. doi:10.1093/europace/euz324

65. Eko. FDA clears Eko’s AFib and heart murmur detection algorithms, making it the first AI-powered stethoscope to screen for serious heart conditions [press release]. Published January 28, 2020. Accessed September 15, 2021. https://www.businesswire.com/news/home/20200128005232/en/FDA-Clears-Eko’s-AFib-and-Heart-Murmur-Detection-Algorithms-Making-It-the-First-AI-Powered-Stethoscope-to-Screen-for-Serious-Heart-Conditions
66. Cruz-Roa A, Gilmore H, Basavanhally A, et al. Accurate and reproducible invasive breast cancer detection in whole-slide images: a deep learning approach for quantifying tumor extent. Sci Rep. 2017;7:46450. doi:10.1038/srep46450
67. Acs B, Rantalainen M, Hartman J. Artificial intelligence as the next step towards precision pathology. J Intern Med. 2020;288(1):62-81. doi:10.1111/joim.13030
68. Mobadersany P, Yousefi S, Amgad M, et al. Predicting cancer outcomes from histology and genomics using convolutional networks. Proc Natl Acad Sci U S A. 2018;115(13):E2970-E2979. doi:10.1073/pnas.1717139115
69. Wang X, Janowczyk A, Zhou Y, et al. Prediction of recurrence in early stage non-small cell lung cancer using computer extracted nuclear features from digital H&E images. Sci Rep. 2017;7:13543. doi:10.1038/s41598-017-13773-7
70. Kulkarni PM, Robinson EJ, Pradhan JS, et al. Deep learning based on standard H&E images of primary melanoma tumors identifies patients at risk for visceral recurrence and death. Clin Cancer Res. 2020;26(5):1126-1134. doi:10.1158/1078-0432.CCR-19-1495
71. Du XL, Li WB, Hu BJ. Application of artificial intelligence in ophthalmology. Int J Ophthalmol. 2018;11(9):1555-1561. doi:10.18240/ijo.2018.09.21
72. Gunasekeran DV, Wong TY. Artificial intelligence in ophthalmology in 2020: a technology on the cusp for translation and implementation. Asia Pac J Ophthalmol (Phila). 2020;9(2):61-66. doi:10.1097/01.APO.0000656984.56467.2c
73. Ting DSW, Pasquale LR, Peng L, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019;103(2):167-175. doi:10.1136/bjophthalmol-2018-313173
74. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2410. doi:10.1001/jama.2016.17216
75. US Food and Drug Administration. FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems [press release]. Published April 11, 2018. Accessed September 15, 2021. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye
76. Long E, Chen J, Wu X, et al. Artificial intelligence manages congenital cataract with individualized prediction and telehealth computing. NPJ Digit Med. 2020;3:112. doi:10.1038/s41746-020-00319-x
77. De Fauw J, Ledsam JR, Romera-Paredes B, et al. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat Med. 2018;24(9):1342-1350. doi:10.1038/s41591-018-0107-6
78. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115-118. doi:10.1038/nature21056
79. Brinker TJ, Hekler A, Enk AH, et al. Deep neural networks are superior to dermatologists in melanoma image classification. Eur J Cancer. 2019;119:11-17. doi:10.1016/j.ejca.2019.05.023
80. Brinker TJ, Hekler A, Enk AH, et al. A convolutional neural network trained with dermoscopic images performed on par with 145 dermatologists in a clinical melanoma image classification task. Eur J Cancer. 2019;111:148-154. doi:10.1016/j.ejca.2019.02.005
81. Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists. Ann Oncol. 2018;29(8):1836-1842. doi:10.1093/annonc/mdy166
82. Li CX, Shen CB, Xue K, et al. Artificial intelligence in dermatology: past, present, and future. Chin Med J (Engl). 2019;132(17):2017-2020. doi:10.1097/CM9.0000000000000372
83. Tschandl P, Codella N, Akay BN, et al. Comparison of the accuracy of human readers versus machine-learning algorithms for pigmented skin lesion classification: an open, web-based, international, diagnostic study. Lancet Oncol. 2019;20(7):938-947. doi:10.1016/S1470-2045(19)30333-X
84. Han SS, Park I, Eun Chang SE, et al. Augmented intelligence dermatology: deep neural networks empower medical professionals in diagnosing skin cancer and predicting treatment options for 134 skin disorders. J Invest Dermatol. 2020;140(9):1753-1761. doi:10.1016/j.jid.2020.01.019
85. Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies [published correction appears in BMJ. 2020 Feb 25;368:m645]. BMJ. 2020;368:m127. Published 2020 Feb 10. doi:10.1136/bmj.m127
86. Chen YC, Ke WC, Chiu HW. Risk classification of cancer survival using ANN with gene expression data from multiple laboratories. Comput Biol Med. 2014;48:1-7. doi:10.1016/j.compbiomed.2014.02.006
87. Kim W, Kim KS, Lee JE, et al. Development of novel breast cancer recurrence prediction model using support vector machine. J Breast Cancer. 2012;15(2):230-238. doi:10.4048/jbc.2012.15.2.230
88. Merath K, Hyer JM, Mehta R, et al. Use of machine learning for prediction of patient risk of postoperative complications after liver, pancreatic, and colorectal surgery. J Gastrointest Surg. 2020;24(8):1843-1851. doi:10.1007/s11605-019-04338-2
89. Santos-García G, Varela G, Novoa N, Jiménez MF. Prediction of postoperative morbidity after lung resection using an artificial neural network ensemble. Artif Intell Med. 2004;30(1):61-69. doi:10.1016/S0933-3657(03)00059-9
90. Ibragimov B, Xing L. Segmentation of organs-at-risks in head and neck CT images using convolutional neural networks. Med Phys. 2017;44(2):547-557. doi:10.1002/mp.12045
91. Lou B, Doken S, Zhuang T, et al. An image-based deep learning framework for individualizing radiotherapy dose. Lancet Digit Health. 2019;1(3):e136-e147. doi:10.1016/S2589-7500(19)30058-5
92. Xu J, Yang P, Xue S, et al. Translating cancer genomics into precision medicine with artificial intelligence: applications, challenges and future perspectives. Hum Genet. 2019;138(2):109-124. doi:10.1007/s00439-019-01970-5
93. Patel NM, Michelini VV, Snell JM, et al. Enhancing next‐generation sequencing‐guided cancer care through cognitive computing. Oncologist. 2018;23(2):179-185. doi:10.1634/theoncologist.2017-0170
94. Le Berre C, Sandborn WJ, Aridhi S, et al. Application of artificial intelligence to gastroenterology and hepatology. Gastroenterology. 2020;158(1):76-94.e2. doi:10.1053/j.gastro.2019.08.058
95. Yang YJ, Bang CS. Application of artificial intelligence in gastroenterology. World J Gastroenterol. 2019;25(14):1666-1683. doi:10.3748/wjg.v25.i14.1666
96. Wang P, Berzin TM, Glissen Brown JR, et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut. 2019;68(10):1813-1819. doi:10.1136/gutjnl-2018-317500

97. Gupta R, Krishnam SP, Schaefer PW, Lev MH, Gonzalez RG. An East Coast perspective on artificial intelligence and machine learning: part 2: ischemic stroke imaging and triage. Neuroimaging Clin N Am. 2020;30(4):467-478. doi:10.1016/j.nic.2020.08.002
98. Beli M, Bobi V, Badža M, Šolaja N, Duri-Jovii M, Kosti VS. Artificial intelligence for assisting diagnostics and assessment of Parkinson’s disease—a review. Clin Neurol Neurosurg. 2019;184:105442. doi:10.1016/j.clineuro.2019.105442
99. An S, Kang C, Lee HW. Artificial intelligence and computational approaches for epilepsy. J Epilepsy Res. 2020;10(1):8-17. doi:10.14581/jer.20003
100. Pavel AM, Rennie JM, de Vries LS, et al. A machine-learning algorithm for neonatal seizure recognition: a multicentre, randomised, controlled trial. Lancet Child Adolesc Health. 2020;4(10):740-749. doi:10.1016/S2352-4642(20)30239-X
101. Afzal HMR, Luo S, Ramadan S, Lechner-Scott J. The emerging role of artificial intelligence in multiple sclerosis imaging [published online ahead of print, 2020 Oct 28]. Mult Scler. 2020;1352458520966298. doi:10.1177/1352458520966298
102. Bouton CE. Restoring movement in paralysis with a bioelectronic neural bypass approach: current state and future directions. Cold Spring Harb Perspect Med. 2019;9(11):a034306. doi:10.1101/cshperspect.a034306
103. Durstewitz D, Koppe G, Meyer-Lindenberg A. Deep neural networks in psychiatry. Mol Psychiatry. 2019;24(11):1583-1598. doi:10.1038/s41380-019-0365-9
104. Fonseka TM, Bhat V, Kennedy SH. The utility of artificial intelligence in suicide risk prediction and the management of suicidal behaviors. Aust N Z J Psychiatry. 2019;53(10):954-964. doi:10.1177/0004867419864428
105. Kessler RC, Hwang I, Hoffmire CA, et al. Developing a practical suicide risk prediction model for targeting high-risk patients in the Veterans Health Administration. Int J Methods Psychiatr Res. 2017;26(3):e1575. doi:10.1002/mpr.1575
106. Kessler RC, Bauer MS, Bishop TM, et al. Using administrative data to predict suicide after psychiatric hospitalization in the Veterans Health Administration System. Front Psychiatry. 2020;11:390. doi:10.3389/fpsyt.2020.00390
107. Kessler RC, van Loo HM, Wardenaar KJ, et al. Testing a machine-learning algorithm to predict the persistence and severity of major depressive disorder from baseline self-reports. Mol Psychiatry. 2016;21(10):1366-1371. doi:10.1038/mp.2015.198
108. Horng S, Sontag DA, Halpern Y, Jernite Y, Shapiro NI, Nathanson LA. Creating an automated trigger for sepsis clinical decision support at emergency department triage using machine learning. PLoS One. 2017;12(4):e0174708. doi:10.1371/journal.pone.0174708
109. Soffer S, Klang E, Barash Y, Grossman E, Zimlichman E. Predicting in-hospital mortality at admission to the medical ward: a big-data machine learning model. Am J Med. 2021;134(2):227-234.e4. doi:10.1016/j.amjmed.2020.07.014
110. Labovitz DL, Shafner L, Reyes Gil M, Virmani D, Hanina A. Using artificial intelligence to reduce the risk of nonadherence in patients on anticoagulation therapy. Stroke. 2017;48(5):1416-1419. doi:10.1161/STROKEAHA.116.016281
111. Forlenza GP. Use of artificial intelligence to improve diabetes outcomes in patients using multiple daily injections therapy. Diabetes Technol Ther. 2019;21(S2):S24-S28. doi:10.1089/dia.2019.0077
112. Poser CM. CT scan and the practice of neurology. Arch Neurol. 1977;34(2):132. doi:10.1001/archneur.1977.00500140086023
113. Angus DC. Randomized clinical trials of artificial intelligence. JAMA. 2020;323(11):1043-1045. doi:10.1001/jama.2020.1039
114. Topol EJ. Welcoming new guidelines for AI clinical research. Nat Med. 2020;26(9):1318-1320. doi:10.1038/s41591-020-1042-x
115. Collins GS, Moons KGM. Reporting of artificial intelligence prediction models. Lancet. 2019;393(10181):1577-1579. doi:10.1016/S0140-6736(19)30037-6
116. Cruz Rivera S, Liu X, Chan AW, et al. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Nat Med. 2020;26(9):1351-1363. doi:10.1038/s41591-020-1037-7
117. Liu X, Cruz Rivera S, Moher D, Calvert MJ, Denniston AK; SPIRIT-AI and CONSORT-AI Working Group. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat Med. 2020;26(9):1364-1374. doi:10.1038/s41591-020-1034-x
118. McCulloch WS, Pitts W. A logical calculus of the ideas immanent in nervous activity. Bull Math Biophys. 1943;5(4):115-133. doi:10.1007/BF02478259
119. Samuel AL. Some studies in machine learning using the game of Checkers. IBM J Res Dev. 1959;3(3):535-554. Accessed September 15, 2021. https://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.368.2254
120. Sonoda M, Takano M, Miyahara J, Kato H. Computed radiography utilizing scanning laser stimulated luminescence. Radiology. 1983;148(3):833-838. doi:10.1148/radiology.148.3.6878707
121. Dechter R. Learning while searching in constraint-satisfaction-problems. AAAI’86: proceedings of the fifth AAAI national conference on artificial intelligence. Published 1986. Accessed September 15, 2021. https://www.aaai.org/Papers/AAAI/1986/AAAI86-029.pdf
122. Le Cun Y, Jackel LD, Boser B, et al. Handwritten digit recognition: applications of neural network chips and automatic learning. IEEE Commun Mag. 1989;27(11):41-46. doi:10.1109/35.41400
123. US Food and Drug Administration. FDA allows marketing of first whole slide imaging system for digital pathology [press release]. Published April 12, 2017. Accessed September 15, 2021. https://www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-whole-slide-imaging-system-digital-pathology
Management of Pediatric Nail Psoriasis
Pediatric nail psoriasis is a condition that has not been extensively studied. The prevalence of nail alterations in pediatric patients with psoriasis varies among different studies, ranging from 17% to 39.2%.1 Nail pitting, onycholysis associated with subungual hyperkeratosis, paronychia, and pachyonychia are the most frequent features of psoriatic nail involvement in children.2,3 The management of nail psoriasis in children and adolescents is critical due to the quality-of-life impact, from potential functional impairment issues to the obvious cosmetic problems, which can aggravate the psychologic distress and social embarrassment of patients with psoriasis. Despite the emergence of modern potent systemic agents to treat chronic plaque psoriasis, nail psoriasis often is refractory to treatment.4 Coupled with the limited on-label options for psoriasis treatment in children, the management of nail psoriasis in this special patient group constitutes an even greater therapeutic challenge. This report aims to summarize the limited existing data on the successful management of nail psoriasis in the pediatric population.
Reviewing the Literature on Nail Psoriasis
We conducted a search of PubMed articles indexed for MEDLINE, Embase, and Scopus using the following Medical Subject Headings key terms: nail psoriasis and children, juvenile, pediatric. Additional articles were identified from the reference lists of the retrieved articles and citations. Our search included reports in the English language published from 2000 to 2019. The selection process included the following 2 steps: screening of the titles and abstracts, followed by evaluation of the selected full-text articles.
Topical Treatments for Nail Psoriasis
Because most systemic antipsoriatic treatments that can be administered in adult patients have not yet been granted an official license for administration in children, topical treatments are considered by many physicians as the preferred first-line therapy for psoriatic nail involvement in pediatric patients.5,6 However, only scarce data are available in the literature concerning the successful use of local agents in pediatric patients with psoriasis.
The main limitation of local treatments relates mostly to their impaired penetration into the affected area (nails). To optimize drug penetration, some authors suggest the use of potent keratolytic topical preparations to reduce the nail volume and facilitate drug absorption.7 A popular suggestion is trimming the onycholytic nail plate followed by 40% urea avulsion to treat subungual hyperkeratosis8 or simply the use of occlusive 40% urea in petroleum jelly.9 Other approaches include clipping the onycholytic nail over the diseased nail bed or processing the nail plate through grinding or even drilling holes with the use of mechanical burrs or ablative lasers to enhance the penetration of the topical agent.7
A frequent approach in pediatric patients is clipping the detached nails combined with daily application of calcipotriene (calcipotriol) and steroids, such as betamethasone dipropionate.5,8 Reports on the use of regimens with clobetasol propionate ointment 0.05% under occlusion, with or without the concomitant use of calcipotriol solution 0.005%, also are present in the literature but not always with satisfactory results.10,11 Another successfully administered topical steroid is mometasone furoate cream 0.1%.12 Although the use of intralesional triamcinolone acetonide also has demonstrated encouraging outcomes in isolated reports,13 associated adverse events, such as pain and hematomas, can result in tolerability issues for pediatric patients.7
Piraccini et al14 described the case of an 8-year-old patient with pustular nail psoriasis who showed improvement within 3 to 6 months of treatment with topical calcipotriol 5 μg/g as monotherapy applied to the nail and periungual tissues twice daily. Another approach, described by Diluvio et al,15 is the use of tazarotene gel 0.05% applied once daily to the affected nail plates, nail folds, and periungual skin without occlusion. In a 6-year-old patient with isolated nail psoriasis, this treatment regimen demonstrated notable improvement within 8 weeks.15
Systemic Treatments for Nail Psoriasis
Data on the successful administration of systemic agents in pediatric patients also are extremely scarce. Due to the lack of clinical trials, everyday practice is mostly based on isolated case series and case reports.
Methotrexate—Lee11 described the case of an 11-year-old girl with severe, symptomatic, 20-nail psoriatic onychodystrophy who showed a complete response to oral methotrexate 5 mg/wk after topical clobetasol propionate and calcipotriol failed. Improvement was seen as early as 4 weeks after therapy initiation, and complete resolution of the lesions was documented after 9 and 13 months of methotrexate therapy for the fingers and toes, respectively.11 The successful use of methotrexate in the improvement of psoriatic nail dystrophy in a pediatric patient also was documented by Teran et al.16 In this case, a 9-year-old girl with erythrodermic psoriasis, psoriatic arthritis, and severe onychodystrophy showed notable amelioration of all psoriatic manifestations, including the nail findings, with systemic methotrexate therapy (dose not specified).16 Notably, the authors reported that the improvement of onychodystrophy occurred with considerable delay compared to the other psoriatic lesions,16 indicating the already-known refractoriness of nail psoriasis to the various therapeutic attempts.9-15
Acitretin—Another agent that has been linked with partial improvement of acrodermatitis continua of Hallopeau (ACH)–associated onychodystrophy is acitretin. In a case series of 15 pediatric patients with pustular psoriasis, a 5-year-old boy with severe nail involvement presented with partial amelioration of nail changes with acitretin within the first 6 weeks of treatment using the following regimen: initial dosage of 0.8 mg/kg/d for 6 weeks, followed by 0.3 mg/kg/d for 4 weeks.17
Biologics—The emerging use of biologics in pediatric psoriasis also has brought important advances in the successful management of nail psoriasis in children and adolescents.18-21 Wells et al18 presented the case of an 8-year-old girl with nail psoriasis, psoriatic arthritis, and plaque psoriasis who showed complete resolution of all psoriatic manifestations, including nail involvement, within 3 months of treatment with secukinumab 150 mg subcutaneously every 4 weeks. Prior failed treatments included various systemic agents (ie, subcutaneous methotrexate 20 mg/m2, etanercept 0.8 mg/kg weekly, adalimumab 40 mg every 2 weeks) as well as topical agents (ie, urea, tazarotene, corticosteroids) and intralesional triamcinolone.18
Infliximab also has been successfully used for pediatric nail psoriasis. Watabe et al19 presented the case of an 8-year-old girl with psoriatic onychodystrophy in addition to psoriatic onycho-pachydermo-periostitis. Prior therapy with adalimumab 20 mg every other week combined with methotrexate 10 mg weekly failed. She experienced notable amelioration of the nail dystrophy within 3 months of using a combination of infliximab and methotrexate (infliximab 5 mg/kg intravenously on weeks 0, 2, and 6, and every 8 weeks thereafter; methotrexate 10 mg/wk).19
Cases in which infliximab has resulted in rapid yet only transient restoration of psoriatic onychodystrophy also are present in the literature. Pereira et al20 reported that a 3-year-old patient with severe 20-digit onychodystrophy in addition to pustular psoriasis had complete resolution of nail lesions within 2 weeks of treatment with infliximab (5 mg/kg at weeks 0, 2, and 6, and then every 7 weeks thereafter), which was sustained over the course of 1 year. The therapy had to be discontinued because of exacerbation of the cutaneous symptoms; thereafter, etanercept was initiated. Although the patient noted major improvement of all skin lesions under etanercept, only moderate amelioration of the psoriatic nail lesions was demonstrated.20
Dini et al21 described a 9-year-old girl with severe ACH-associated psoriatic onychodystrophy who showed complete clearance of all lesions within 8 weeks of treatment with adalimumab (initially 80 mg, followed by 40 mg after 1 week and then 40 mg every other week). Prior treatment with potent topical corticosteroids, cyclosporine (3 mg/kg/d for 6 months), and etanercept (0.4 mg/kg twice weekly for 3 months) was ineffective.21
Phototherapy—Other systemic agents with reported satisfactory outcomes in the treatment of psoriatic onychodystrophy include thalidomide combined with UVB phototherapy. Kiszewski et al22 described a 2-year-old patient with ACH and severe 19-digit onychodystrophy. Prior failed therapies included occluded clobetasol ointment 0.05%, occluded pimecrolimus 0.1%, and systemic methotrexate, while systemic acitretin (0.8 mg⁄kg⁄d) resulted in elevated cholesterol levels and therefore had to be interrupted. Improvement was seen 2 months after the initiation of a combined broadband UVB and thalidomide (50 mg⁄d) treatment, with no documented relapses after discontinuation of therapy.22
Narrowband UVB (311 nm) also has been used as monotherapy for ACH-associated onychodystrophy, as demonstrated by Bordignon et al.23 They reported a 9-year-old patient who showed partial improvement of isolated onychodystrophy of the fourth nail plate of the left hand after 36 sessions of narrowband UVB using a 311-nm filtering handpiece with a square spot size of 19×19 mm.23
Conclusion
Nail psoriasis constitutes a type of psoriasis that is not only refractory to most treatments but is accompanied by substantial psychological and occasionally functional burden for the affected individuals.24 Data concerning therapeutic options in the pediatric population are extremely limited, and therefore the everyday practice often involves administration of off-label medications, which can constitute a dilemma for many physicians, especially for safety.10 We suggest a simple therapeutic algorithm for the management of pediatric nail psoriasis based on the summarized data that are currently available in the literature. This algorithm is shown in the eFigure.
As progressively more agents—especially biologics—receive approval for use in plaque psoriasis in pediatric patients,25 it is expected that gradually more real-life data on their side efficacy for plaque psoriasis of the nails in children also will come to light. Furthermore, their on-label use in pediatric psoriasis patients will facilitate further relevant clinical trials to this target group so that the potential of these medications in the management of nail psoriasis can be fully explored.
- Uber M, Carvalho VO, Abagge KT, et al. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018;35:202-207.
- Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34:58-63.
- Piraccini BM, Triantafyllopoulou I, Prevezas C, et al. Nail psoriasis in children: common or uncommon? results from a 10-year double-center study. Skin Appendage Disord. 2015;1:43-48.
- Baran R. The burden of nail psoriasis: an introduction. Dermatology. 2010;221(suppl 1):1-5.
- Richert B, André J. Nail disorders in children: diagnosis and management. Am J Clin Dermatol. 2011;12:101-112.
- Trüeb RM. Therapies for childhood psoriasis. Curr Probl Dermatol. 2009;38:137-159.
- Haneke E. Nail psoriasis: clinical features, pathogenesis, differential diagnoses, and management. Psoriasis (Auckl). 2017;7:51-63.
- Piraccini BM, Starace M. Nail disorders in infants and children. Curr Opin Pediatr. 2014;26:440-445.
- Duran-McKinster C, Ortiz-Solis D, Granados J, et al. Juvenile psoriatic arthritis with nail psoriasis in the absence of cutaneous lesions. Int J Dermatol. 2000;39:32-35.
- Holzberg M, Ruben BS, Baran R. Psoriasis restricted to the nail in a 7-year-old child. should biologics be an appropriate treatment modality when considering quality of life? J Eur Acad Dermatol Venereol. 2014;28:668-670.
- Lee JY. Severe 20-nail psoriasis successfully treated by low dose methotrexate. Dermatol Online J. 2009;15:8.
- Liao YC, Lee JY. Psoriasis in a 3-month-old infant with Kawasaki disease. Dermatol Online J. 2009;15:10.
- Khoo BP, Giam YC. A pilot study on the role of intralesional triamcinolone acetonide in the treatment of pitted nails in children. Singapore Med J. 2000;41:66-68.
- Piraccini BM, Tosti A, Iorizzo M, et al. Pustular psoriasis of the nails: treatment and long-term follow-up of 46 patients. Br J Dermatol. 2001;144:1000-1005.
- Diluvio L, Campione E, Paternò EJ, et al. Childhood nail psoriasis: a useful treatment with tazarotene 0.05%. Pediatr Dermatol. 2007;24:332-333.
- Teran CG, Teran-Escalera CN, Balderrama C. A severe case of erythrodermic psoriasis associated with advanced nail and joint manifestations: a case report. J Med Case Rep. 2010;4:179.
- Chen P, Li C, Xue R, et al. Efficacy and safety of acitretin monotherapy in children with pustular psoriasis: results from 15 cases and a literature review. J Dermatolog Treat. 2018;29:353-363.
- Wells LE, Evans T, Hilton R, et al. Use of secukinumab in a pediatric patient leads to significant improvement in nail psoriasis and psoriatic arthritis. Pediatr Dermatol. 2019;36:384-385.
- Watabe D, Endoh K, Maeda F, et al. Childhood-onset psoriatic onycho-pachydermo-periostitis treated successfully with infliximab. Eur J Dermatol. 2015;25:506-508.
- Pereira TM, Vieira AP, Fernandes JC, et al. Anti-TNF-alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350-352.
- Dini V, Barbanera S, Romanelli M. Efficacy of adalimumab for the treatment of refractory paediatric acrodermatitis continua of Hallopeau. Acta Derm Venereol. 2013;93:588-589.
- Kiszewski AE, De Villa D, Scheibel I, et al. An infant with acrodermatitis continua of Hallopeau: successful treatment with thalidomide and UVB therapy. Pediatr Dermatol. 2009;26:105-106.
- Bordignon M, Zattra E, Albertin C, et al. Successful treatment of a 9-year-old boy affected by acrodermatitis continua of Hallopeau with targeted ultraviolet B narrow-band phototherapy. Photodermatol Photoimmunol Photomed. 2010;26:41-43.
- Fabroni C, Gori A, Troiano M, et al. Infliximab efficacy in nail psoriasis. a retrospective study in 48 patients. J Eur Acad Dermatol Venereol. 2011;25:549-553.
- Lilly’s Taltz® (ixekizumab) receives U.S. FDA approval for the treatment of pediatric patients with moderate to severe plaque psoriasis. Eli Lilly and Company. March 30, 2020. Accessed September 24, 2021. https://investor.lilly.com/news-releases/news-release-details/lillys-taltzr-ixekizumab-receives-us-fda-approval-treatment-1
Pediatric nail psoriasis is a condition that has not been extensively studied. The prevalence of nail alterations in pediatric patients with psoriasis varies among different studies, ranging from 17% to 39.2%.1 Nail pitting, onycholysis associated with subungual hyperkeratosis, paronychia, and pachyonychia are the most frequent features of psoriatic nail involvement in children.2,3 The management of nail psoriasis in children and adolescents is critical due to the quality-of-life impact, from potential functional impairment issues to the obvious cosmetic problems, which can aggravate the psychologic distress and social embarrassment of patients with psoriasis. Despite the emergence of modern potent systemic agents to treat chronic plaque psoriasis, nail psoriasis often is refractory to treatment.4 Coupled with the limited on-label options for psoriasis treatment in children, the management of nail psoriasis in this special patient group constitutes an even greater therapeutic challenge. This report aims to summarize the limited existing data on the successful management of nail psoriasis in the pediatric population.
Reviewing the Literature on Nail Psoriasis
We conducted a search of PubMed articles indexed for MEDLINE, Embase, and Scopus using the following Medical Subject Headings key terms: nail psoriasis and children, juvenile, pediatric. Additional articles were identified from the reference lists of the retrieved articles and citations. Our search included reports in the English language published from 2000 to 2019. The selection process included the following 2 steps: screening of the titles and abstracts, followed by evaluation of the selected full-text articles.
Topical Treatments for Nail Psoriasis
Because most systemic antipsoriatic treatments that can be administered in adult patients have not yet been granted an official license for administration in children, topical treatments are considered by many physicians as the preferred first-line therapy for psoriatic nail involvement in pediatric patients.5,6 However, only scarce data are available in the literature concerning the successful use of local agents in pediatric patients with psoriasis.
The main limitation of local treatments relates mostly to their impaired penetration into the affected area (nails). To optimize drug penetration, some authors suggest the use of potent keratolytic topical preparations to reduce the nail volume and facilitate drug absorption.7 A popular suggestion is trimming the onycholytic nail plate followed by 40% urea avulsion to treat subungual hyperkeratosis8 or simply the use of occlusive 40% urea in petroleum jelly.9 Other approaches include clipping the onycholytic nail over the diseased nail bed or processing the nail plate through grinding or even drilling holes with the use of mechanical burrs or ablative lasers to enhance the penetration of the topical agent.7
A frequent approach in pediatric patients is clipping the detached nails combined with daily application of calcipotriene (calcipotriol) and steroids, such as betamethasone dipropionate.5,8 Reports on the use of regimens with clobetasol propionate ointment 0.05% under occlusion, with or without the concomitant use of calcipotriol solution 0.005%, also are present in the literature but not always with satisfactory results.10,11 Another successfully administered topical steroid is mometasone furoate cream 0.1%.12 Although the use of intralesional triamcinolone acetonide also has demonstrated encouraging outcomes in isolated reports,13 associated adverse events, such as pain and hematomas, can result in tolerability issues for pediatric patients.7
Piraccini et al14 described the case of an 8-year-old patient with pustular nail psoriasis who showed improvement within 3 to 6 months of treatment with topical calcipotriol 5 μg/g as monotherapy applied to the nail and periungual tissues twice daily. Another approach, described by Diluvio et al,15 is the use of tazarotene gel 0.05% applied once daily to the affected nail plates, nail folds, and periungual skin without occlusion. In a 6-year-old patient with isolated nail psoriasis, this treatment regimen demonstrated notable improvement within 8 weeks.15
Systemic Treatments for Nail Psoriasis
Data on the successful administration of systemic agents in pediatric patients also are extremely scarce. Due to the lack of clinical trials, everyday practice is mostly based on isolated case series and case reports.
Methotrexate—Lee11 described the case of an 11-year-old girl with severe, symptomatic, 20-nail psoriatic onychodystrophy who showed a complete response to oral methotrexate 5 mg/wk after topical clobetasol propionate and calcipotriol failed. Improvement was seen as early as 4 weeks after therapy initiation, and complete resolution of the lesions was documented after 9 and 13 months of methotrexate therapy for the fingers and toes, respectively.11 The successful use of methotrexate in the improvement of psoriatic nail dystrophy in a pediatric patient also was documented by Teran et al.16 In this case, a 9-year-old girl with erythrodermic psoriasis, psoriatic arthritis, and severe onychodystrophy showed notable amelioration of all psoriatic manifestations, including the nail findings, with systemic methotrexate therapy (dose not specified).16 Notably, the authors reported that the improvement of onychodystrophy occurred with considerable delay compared to the other psoriatic lesions,16 indicating the already-known refractoriness of nail psoriasis to the various therapeutic attempts.9-15
Acitretin—Another agent that has been linked with partial improvement of acrodermatitis continua of Hallopeau (ACH)–associated onychodystrophy is acitretin. In a case series of 15 pediatric patients with pustular psoriasis, a 5-year-old boy with severe nail involvement presented with partial amelioration of nail changes with acitretin within the first 6 weeks of treatment using the following regimen: initial dosage of 0.8 mg/kg/d for 6 weeks, followed by 0.3 mg/kg/d for 4 weeks.17
Biologics—The emerging use of biologics in pediatric psoriasis also has brought important advances in the successful management of nail psoriasis in children and adolescents.18-21 Wells et al18 presented the case of an 8-year-old girl with nail psoriasis, psoriatic arthritis, and plaque psoriasis who showed complete resolution of all psoriatic manifestations, including nail involvement, within 3 months of treatment with secukinumab 150 mg subcutaneously every 4 weeks. Prior failed treatments included various systemic agents (ie, subcutaneous methotrexate 20 mg/m2, etanercept 0.8 mg/kg weekly, adalimumab 40 mg every 2 weeks) as well as topical agents (ie, urea, tazarotene, corticosteroids) and intralesional triamcinolone.18
Infliximab also has been successfully used for pediatric nail psoriasis. Watabe et al19 presented the case of an 8-year-old girl with psoriatic onychodystrophy in addition to psoriatic onycho-pachydermo-periostitis. Prior therapy with adalimumab 20 mg every other week combined with methotrexate 10 mg weekly failed. She experienced notable amelioration of the nail dystrophy within 3 months of using a combination of infliximab and methotrexate (infliximab 5 mg/kg intravenously on weeks 0, 2, and 6, and every 8 weeks thereafter; methotrexate 10 mg/wk).19
Cases in which infliximab has resulted in rapid yet only transient restoration of psoriatic onychodystrophy also are present in the literature. Pereira et al20 reported that a 3-year-old patient with severe 20-digit onychodystrophy in addition to pustular psoriasis had complete resolution of nail lesions within 2 weeks of treatment with infliximab (5 mg/kg at weeks 0, 2, and 6, and then every 7 weeks thereafter), which was sustained over the course of 1 year. The therapy had to be discontinued because of exacerbation of the cutaneous symptoms; thereafter, etanercept was initiated. Although the patient noted major improvement of all skin lesions under etanercept, only moderate amelioration of the psoriatic nail lesions was demonstrated.20
Dini et al21 described a 9-year-old girl with severe ACH-associated psoriatic onychodystrophy who showed complete clearance of all lesions within 8 weeks of treatment with adalimumab (initially 80 mg, followed by 40 mg after 1 week and then 40 mg every other week). Prior treatment with potent topical corticosteroids, cyclosporine (3 mg/kg/d for 6 months), and etanercept (0.4 mg/kg twice weekly for 3 months) was ineffective.21
Phototherapy—Other systemic agents with reported satisfactory outcomes in the treatment of psoriatic onychodystrophy include thalidomide combined with UVB phototherapy. Kiszewski et al22 described a 2-year-old patient with ACH and severe 19-digit onychodystrophy. Prior failed therapies included occluded clobetasol ointment 0.05%, occluded pimecrolimus 0.1%, and systemic methotrexate, while systemic acitretin (0.8 mg⁄kg⁄d) resulted in elevated cholesterol levels and therefore had to be interrupted. Improvement was seen 2 months after the initiation of a combined broadband UVB and thalidomide (50 mg⁄d) treatment, with no documented relapses after discontinuation of therapy.22
Narrowband UVB (311 nm) also has been used as monotherapy for ACH-associated onychodystrophy, as demonstrated by Bordignon et al.23 They reported a 9-year-old patient who showed partial improvement of isolated onychodystrophy of the fourth nail plate of the left hand after 36 sessions of narrowband UVB using a 311-nm filtering handpiece with a square spot size of 19×19 mm.23
Conclusion
Nail psoriasis constitutes a type of psoriasis that is not only refractory to most treatments but is accompanied by substantial psychological and occasionally functional burden for the affected individuals.24 Data concerning therapeutic options in the pediatric population are extremely limited, and therefore the everyday practice often involves administration of off-label medications, which can constitute a dilemma for many physicians, especially for safety.10 We suggest a simple therapeutic algorithm for the management of pediatric nail psoriasis based on the summarized data that are currently available in the literature. This algorithm is shown in the eFigure.
As progressively more agents—especially biologics—receive approval for use in plaque psoriasis in pediatric patients,25 it is expected that gradually more real-life data on their side efficacy for plaque psoriasis of the nails in children also will come to light. Furthermore, their on-label use in pediatric psoriasis patients will facilitate further relevant clinical trials to this target group so that the potential of these medications in the management of nail psoriasis can be fully explored.
Pediatric nail psoriasis is a condition that has not been extensively studied. The prevalence of nail alterations in pediatric patients with psoriasis varies among different studies, ranging from 17% to 39.2%.1 Nail pitting, onycholysis associated with subungual hyperkeratosis, paronychia, and pachyonychia are the most frequent features of psoriatic nail involvement in children.2,3 The management of nail psoriasis in children and adolescents is critical due to the quality-of-life impact, from potential functional impairment issues to the obvious cosmetic problems, which can aggravate the psychologic distress and social embarrassment of patients with psoriasis. Despite the emergence of modern potent systemic agents to treat chronic plaque psoriasis, nail psoriasis often is refractory to treatment.4 Coupled with the limited on-label options for psoriasis treatment in children, the management of nail psoriasis in this special patient group constitutes an even greater therapeutic challenge. This report aims to summarize the limited existing data on the successful management of nail psoriasis in the pediatric population.
Reviewing the Literature on Nail Psoriasis
We conducted a search of PubMed articles indexed for MEDLINE, Embase, and Scopus using the following Medical Subject Headings key terms: nail psoriasis and children, juvenile, pediatric. Additional articles were identified from the reference lists of the retrieved articles and citations. Our search included reports in the English language published from 2000 to 2019. The selection process included the following 2 steps: screening of the titles and abstracts, followed by evaluation of the selected full-text articles.
Topical Treatments for Nail Psoriasis
Because most systemic antipsoriatic treatments that can be administered in adult patients have not yet been granted an official license for administration in children, topical treatments are considered by many physicians as the preferred first-line therapy for psoriatic nail involvement in pediatric patients.5,6 However, only scarce data are available in the literature concerning the successful use of local agents in pediatric patients with psoriasis.
The main limitation of local treatments relates mostly to their impaired penetration into the affected area (nails). To optimize drug penetration, some authors suggest the use of potent keratolytic topical preparations to reduce the nail volume and facilitate drug absorption.7 A popular suggestion is trimming the onycholytic nail plate followed by 40% urea avulsion to treat subungual hyperkeratosis8 or simply the use of occlusive 40% urea in petroleum jelly.9 Other approaches include clipping the onycholytic nail over the diseased nail bed or processing the nail plate through grinding or even drilling holes with the use of mechanical burrs or ablative lasers to enhance the penetration of the topical agent.7
A frequent approach in pediatric patients is clipping the detached nails combined with daily application of calcipotriene (calcipotriol) and steroids, such as betamethasone dipropionate.5,8 Reports on the use of regimens with clobetasol propionate ointment 0.05% under occlusion, with or without the concomitant use of calcipotriol solution 0.005%, also are present in the literature but not always with satisfactory results.10,11 Another successfully administered topical steroid is mometasone furoate cream 0.1%.12 Although the use of intralesional triamcinolone acetonide also has demonstrated encouraging outcomes in isolated reports,13 associated adverse events, such as pain and hematomas, can result in tolerability issues for pediatric patients.7
Piraccini et al14 described the case of an 8-year-old patient with pustular nail psoriasis who showed improvement within 3 to 6 months of treatment with topical calcipotriol 5 μg/g as monotherapy applied to the nail and periungual tissues twice daily. Another approach, described by Diluvio et al,15 is the use of tazarotene gel 0.05% applied once daily to the affected nail plates, nail folds, and periungual skin without occlusion. In a 6-year-old patient with isolated nail psoriasis, this treatment regimen demonstrated notable improvement within 8 weeks.15
Systemic Treatments for Nail Psoriasis
Data on the successful administration of systemic agents in pediatric patients also are extremely scarce. Due to the lack of clinical trials, everyday practice is mostly based on isolated case series and case reports.
Methotrexate—Lee11 described the case of an 11-year-old girl with severe, symptomatic, 20-nail psoriatic onychodystrophy who showed a complete response to oral methotrexate 5 mg/wk after topical clobetasol propionate and calcipotriol failed. Improvement was seen as early as 4 weeks after therapy initiation, and complete resolution of the lesions was documented after 9 and 13 months of methotrexate therapy for the fingers and toes, respectively.11 The successful use of methotrexate in the improvement of psoriatic nail dystrophy in a pediatric patient also was documented by Teran et al.16 In this case, a 9-year-old girl with erythrodermic psoriasis, psoriatic arthritis, and severe onychodystrophy showed notable amelioration of all psoriatic manifestations, including the nail findings, with systemic methotrexate therapy (dose not specified).16 Notably, the authors reported that the improvement of onychodystrophy occurred with considerable delay compared to the other psoriatic lesions,16 indicating the already-known refractoriness of nail psoriasis to the various therapeutic attempts.9-15
Acitretin—Another agent that has been linked with partial improvement of acrodermatitis continua of Hallopeau (ACH)–associated onychodystrophy is acitretin. In a case series of 15 pediatric patients with pustular psoriasis, a 5-year-old boy with severe nail involvement presented with partial amelioration of nail changes with acitretin within the first 6 weeks of treatment using the following regimen: initial dosage of 0.8 mg/kg/d for 6 weeks, followed by 0.3 mg/kg/d for 4 weeks.17
Biologics—The emerging use of biologics in pediatric psoriasis also has brought important advances in the successful management of nail psoriasis in children and adolescents.18-21 Wells et al18 presented the case of an 8-year-old girl with nail psoriasis, psoriatic arthritis, and plaque psoriasis who showed complete resolution of all psoriatic manifestations, including nail involvement, within 3 months of treatment with secukinumab 150 mg subcutaneously every 4 weeks. Prior failed treatments included various systemic agents (ie, subcutaneous methotrexate 20 mg/m2, etanercept 0.8 mg/kg weekly, adalimumab 40 mg every 2 weeks) as well as topical agents (ie, urea, tazarotene, corticosteroids) and intralesional triamcinolone.18
Infliximab also has been successfully used for pediatric nail psoriasis. Watabe et al19 presented the case of an 8-year-old girl with psoriatic onychodystrophy in addition to psoriatic onycho-pachydermo-periostitis. Prior therapy with adalimumab 20 mg every other week combined with methotrexate 10 mg weekly failed. She experienced notable amelioration of the nail dystrophy within 3 months of using a combination of infliximab and methotrexate (infliximab 5 mg/kg intravenously on weeks 0, 2, and 6, and every 8 weeks thereafter; methotrexate 10 mg/wk).19
Cases in which infliximab has resulted in rapid yet only transient restoration of psoriatic onychodystrophy also are present in the literature. Pereira et al20 reported that a 3-year-old patient with severe 20-digit onychodystrophy in addition to pustular psoriasis had complete resolution of nail lesions within 2 weeks of treatment with infliximab (5 mg/kg at weeks 0, 2, and 6, and then every 7 weeks thereafter), which was sustained over the course of 1 year. The therapy had to be discontinued because of exacerbation of the cutaneous symptoms; thereafter, etanercept was initiated. Although the patient noted major improvement of all skin lesions under etanercept, only moderate amelioration of the psoriatic nail lesions was demonstrated.20
Dini et al21 described a 9-year-old girl with severe ACH-associated psoriatic onychodystrophy who showed complete clearance of all lesions within 8 weeks of treatment with adalimumab (initially 80 mg, followed by 40 mg after 1 week and then 40 mg every other week). Prior treatment with potent topical corticosteroids, cyclosporine (3 mg/kg/d for 6 months), and etanercept (0.4 mg/kg twice weekly for 3 months) was ineffective.21
Phototherapy—Other systemic agents with reported satisfactory outcomes in the treatment of psoriatic onychodystrophy include thalidomide combined with UVB phototherapy. Kiszewski et al22 described a 2-year-old patient with ACH and severe 19-digit onychodystrophy. Prior failed therapies included occluded clobetasol ointment 0.05%, occluded pimecrolimus 0.1%, and systemic methotrexate, while systemic acitretin (0.8 mg⁄kg⁄d) resulted in elevated cholesterol levels and therefore had to be interrupted. Improvement was seen 2 months after the initiation of a combined broadband UVB and thalidomide (50 mg⁄d) treatment, with no documented relapses after discontinuation of therapy.22
Narrowband UVB (311 nm) also has been used as monotherapy for ACH-associated onychodystrophy, as demonstrated by Bordignon et al.23 They reported a 9-year-old patient who showed partial improvement of isolated onychodystrophy of the fourth nail plate of the left hand after 36 sessions of narrowband UVB using a 311-nm filtering handpiece with a square spot size of 19×19 mm.23
Conclusion
Nail psoriasis constitutes a type of psoriasis that is not only refractory to most treatments but is accompanied by substantial psychological and occasionally functional burden for the affected individuals.24 Data concerning therapeutic options in the pediatric population are extremely limited, and therefore the everyday practice often involves administration of off-label medications, which can constitute a dilemma for many physicians, especially for safety.10 We suggest a simple therapeutic algorithm for the management of pediatric nail psoriasis based on the summarized data that are currently available in the literature. This algorithm is shown in the eFigure.
As progressively more agents—especially biologics—receive approval for use in plaque psoriasis in pediatric patients,25 it is expected that gradually more real-life data on their side efficacy for plaque psoriasis of the nails in children also will come to light. Furthermore, their on-label use in pediatric psoriasis patients will facilitate further relevant clinical trials to this target group so that the potential of these medications in the management of nail psoriasis can be fully explored.
- Uber M, Carvalho VO, Abagge KT, et al. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018;35:202-207.
- Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34:58-63.
- Piraccini BM, Triantafyllopoulou I, Prevezas C, et al. Nail psoriasis in children: common or uncommon? results from a 10-year double-center study. Skin Appendage Disord. 2015;1:43-48.
- Baran R. The burden of nail psoriasis: an introduction. Dermatology. 2010;221(suppl 1):1-5.
- Richert B, André J. Nail disorders in children: diagnosis and management. Am J Clin Dermatol. 2011;12:101-112.
- Trüeb RM. Therapies for childhood psoriasis. Curr Probl Dermatol. 2009;38:137-159.
- Haneke E. Nail psoriasis: clinical features, pathogenesis, differential diagnoses, and management. Psoriasis (Auckl). 2017;7:51-63.
- Piraccini BM, Starace M. Nail disorders in infants and children. Curr Opin Pediatr. 2014;26:440-445.
- Duran-McKinster C, Ortiz-Solis D, Granados J, et al. Juvenile psoriatic arthritis with nail psoriasis in the absence of cutaneous lesions. Int J Dermatol. 2000;39:32-35.
- Holzberg M, Ruben BS, Baran R. Psoriasis restricted to the nail in a 7-year-old child. should biologics be an appropriate treatment modality when considering quality of life? J Eur Acad Dermatol Venereol. 2014;28:668-670.
- Lee JY. Severe 20-nail psoriasis successfully treated by low dose methotrexate. Dermatol Online J. 2009;15:8.
- Liao YC, Lee JY. Psoriasis in a 3-month-old infant with Kawasaki disease. Dermatol Online J. 2009;15:10.
- Khoo BP, Giam YC. A pilot study on the role of intralesional triamcinolone acetonide in the treatment of pitted nails in children. Singapore Med J. 2000;41:66-68.
- Piraccini BM, Tosti A, Iorizzo M, et al. Pustular psoriasis of the nails: treatment and long-term follow-up of 46 patients. Br J Dermatol. 2001;144:1000-1005.
- Diluvio L, Campione E, Paternò EJ, et al. Childhood nail psoriasis: a useful treatment with tazarotene 0.05%. Pediatr Dermatol. 2007;24:332-333.
- Teran CG, Teran-Escalera CN, Balderrama C. A severe case of erythrodermic psoriasis associated with advanced nail and joint manifestations: a case report. J Med Case Rep. 2010;4:179.
- Chen P, Li C, Xue R, et al. Efficacy and safety of acitretin monotherapy in children with pustular psoriasis: results from 15 cases and a literature review. J Dermatolog Treat. 2018;29:353-363.
- Wells LE, Evans T, Hilton R, et al. Use of secukinumab in a pediatric patient leads to significant improvement in nail psoriasis and psoriatic arthritis. Pediatr Dermatol. 2019;36:384-385.
- Watabe D, Endoh K, Maeda F, et al. Childhood-onset psoriatic onycho-pachydermo-periostitis treated successfully with infliximab. Eur J Dermatol. 2015;25:506-508.
- Pereira TM, Vieira AP, Fernandes JC, et al. Anti-TNF-alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350-352.
- Dini V, Barbanera S, Romanelli M. Efficacy of adalimumab for the treatment of refractory paediatric acrodermatitis continua of Hallopeau. Acta Derm Venereol. 2013;93:588-589.
- Kiszewski AE, De Villa D, Scheibel I, et al. An infant with acrodermatitis continua of Hallopeau: successful treatment with thalidomide and UVB therapy. Pediatr Dermatol. 2009;26:105-106.
- Bordignon M, Zattra E, Albertin C, et al. Successful treatment of a 9-year-old boy affected by acrodermatitis continua of Hallopeau with targeted ultraviolet B narrow-band phototherapy. Photodermatol Photoimmunol Photomed. 2010;26:41-43.
- Fabroni C, Gori A, Troiano M, et al. Infliximab efficacy in nail psoriasis. a retrospective study in 48 patients. J Eur Acad Dermatol Venereol. 2011;25:549-553.
- Lilly’s Taltz® (ixekizumab) receives U.S. FDA approval for the treatment of pediatric patients with moderate to severe plaque psoriasis. Eli Lilly and Company. March 30, 2020. Accessed September 24, 2021. https://investor.lilly.com/news-releases/news-release-details/lillys-taltzr-ixekizumab-receives-us-fda-approval-treatment-1
- Uber M, Carvalho VO, Abagge KT, et al. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018;35:202-207.
- Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34:58-63.
- Piraccini BM, Triantafyllopoulou I, Prevezas C, et al. Nail psoriasis in children: common or uncommon? results from a 10-year double-center study. Skin Appendage Disord. 2015;1:43-48.
- Baran R. The burden of nail psoriasis: an introduction. Dermatology. 2010;221(suppl 1):1-5.
- Richert B, André J. Nail disorders in children: diagnosis and management. Am J Clin Dermatol. 2011;12:101-112.
- Trüeb RM. Therapies for childhood psoriasis. Curr Probl Dermatol. 2009;38:137-159.
- Haneke E. Nail psoriasis: clinical features, pathogenesis, differential diagnoses, and management. Psoriasis (Auckl). 2017;7:51-63.
- Piraccini BM, Starace M. Nail disorders in infants and children. Curr Opin Pediatr. 2014;26:440-445.
- Duran-McKinster C, Ortiz-Solis D, Granados J, et al. Juvenile psoriatic arthritis with nail psoriasis in the absence of cutaneous lesions. Int J Dermatol. 2000;39:32-35.
- Holzberg M, Ruben BS, Baran R. Psoriasis restricted to the nail in a 7-year-old child. should biologics be an appropriate treatment modality when considering quality of life? J Eur Acad Dermatol Venereol. 2014;28:668-670.
- Lee JY. Severe 20-nail psoriasis successfully treated by low dose methotrexate. Dermatol Online J. 2009;15:8.
- Liao YC, Lee JY. Psoriasis in a 3-month-old infant with Kawasaki disease. Dermatol Online J. 2009;15:10.
- Khoo BP, Giam YC. A pilot study on the role of intralesional triamcinolone acetonide in the treatment of pitted nails in children. Singapore Med J. 2000;41:66-68.
- Piraccini BM, Tosti A, Iorizzo M, et al. Pustular psoriasis of the nails: treatment and long-term follow-up of 46 patients. Br J Dermatol. 2001;144:1000-1005.
- Diluvio L, Campione E, Paternò EJ, et al. Childhood nail psoriasis: a useful treatment with tazarotene 0.05%. Pediatr Dermatol. 2007;24:332-333.
- Teran CG, Teran-Escalera CN, Balderrama C. A severe case of erythrodermic psoriasis associated with advanced nail and joint manifestations: a case report. J Med Case Rep. 2010;4:179.
- Chen P, Li C, Xue R, et al. Efficacy and safety of acitretin monotherapy in children with pustular psoriasis: results from 15 cases and a literature review. J Dermatolog Treat. 2018;29:353-363.
- Wells LE, Evans T, Hilton R, et al. Use of secukinumab in a pediatric patient leads to significant improvement in nail psoriasis and psoriatic arthritis. Pediatr Dermatol. 2019;36:384-385.
- Watabe D, Endoh K, Maeda F, et al. Childhood-onset psoriatic onycho-pachydermo-periostitis treated successfully with infliximab. Eur J Dermatol. 2015;25:506-508.
- Pereira TM, Vieira AP, Fernandes JC, et al. Anti-TNF-alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350-352.
- Dini V, Barbanera S, Romanelli M. Efficacy of adalimumab for the treatment of refractory paediatric acrodermatitis continua of Hallopeau. Acta Derm Venereol. 2013;93:588-589.
- Kiszewski AE, De Villa D, Scheibel I, et al. An infant with acrodermatitis continua of Hallopeau: successful treatment with thalidomide and UVB therapy. Pediatr Dermatol. 2009;26:105-106.
- Bordignon M, Zattra E, Albertin C, et al. Successful treatment of a 9-year-old boy affected by acrodermatitis continua of Hallopeau with targeted ultraviolet B narrow-band phototherapy. Photodermatol Photoimmunol Photomed. 2010;26:41-43.
- Fabroni C, Gori A, Troiano M, et al. Infliximab efficacy in nail psoriasis. a retrospective study in 48 patients. J Eur Acad Dermatol Venereol. 2011;25:549-553.
- Lilly’s Taltz® (ixekizumab) receives U.S. FDA approval for the treatment of pediatric patients with moderate to severe plaque psoriasis. Eli Lilly and Company. March 30, 2020. Accessed September 24, 2021. https://investor.lilly.com/news-releases/news-release-details/lillys-taltzr-ixekizumab-receives-us-fda-approval-treatment-1
Practice Points
- No clinical trials assessing the management of pediatric nail psoriasis currently are present in the literature. Limited information on the treatment of pediatric nail psoriasis exists, mostly in the form of small case series and case reports.
- As more agents are approved for on-label use in plaque psoriasis in pediatric patients, gradually more real-life data on their efficacy for nail psoriasis in children are expected to come to light.
4 new short-acting hormonal contraceptives offer enhancement over earlier options
Short-term hormonal contraceptives remain the most popular class of reversible contraceptives in the United States, despite the availability of longer-acting methods. Oral contraceptives (OCs), contraceptive patches, and contraceptive vaginal rings are extensively used not only because these methods are easy to initiate but also because their ongoing use remains under the control of the woman herself and also provides her with a wide range of important noncontraceptive benefits.
Despite the more than 60 years of innovation that have made hormonal contraceptives safer, more tolerable, and more convenient, there has been room for improvement. Over the last few years, 4 new hormonal methods have been introduced, and each addresses different limitations and problems associated with the existing, often generic, products.
Compared with the traditional norethindrone pill (Micronor and generics), a new drospirenone progestin-only pill (POP) increases ovulation suppression, offers an improved cyclical bleeding profile, and relaxes the tight missed-pill rules that are usually associated with POPs.
In contrast with the older norelgestromin patch (Evra, Xulane), a new contraceptive transdermal patch significantly decreases total estrogen exposure and pairs its estrogen with levonorgestrel, the progestin associated with the lowest venous thromboembolism (VTE) risk in combined hormonal pills.
While existing combination OCs are formulated with the potent estrogen ethinyl estradiol (EE), a new combination pill, formulated with estetrol (E4) and drospirenone, introduces the first new estrogen (estetrol) used in a contraceptive in more than 50 years. Estetrol, a native estrogen, has selective tissue activity with minimal hepatic and breast impacts. Combined with drospirenone, this formulation offers women good contraceptive efficacy and bleeding patterns.
A new contraceptive vaginal ring introduces a new long-acting, specific progestin (segesterone acetate) and pairs it with low-dose EE. These hormones are packaged in a soft vaginal ring that provides up to 13 cycles of contraceptive protection (3 weeks in/1 week out) with one ring, greatly increasing convenience for women.
Each of these new products represents important incremental improvement over existing options.
Continue to: 1. The drospirenone-only OC...
1. The drospirenone-only OC
The new POP with drospirenone 4 mg (Slynd), which received US Food and Drug Administration (FDA) approval in 2019, is packaged in a 24/4 formulation (24 hormonally active tablets followed by 4 inactive tablets). This formulation results in more predictable bleeding than does the 0.35-mg norethindrone POP, which contains 28 hormonally active tablets in each pack. In the US clinical trials of drospirenone 4 mg, scheduled bleeding decreased from 81% in cycle 1 to 20% in cycle 13. Unscheduled spotting and bleeding decreased from 61% to 40% in the same timeframe. Notably, this bleeding pattern was well tolerated; only 0.4% of trial participants discontinued this drospirenone POP due to problems with irregular bleeding or amenorrhea.
In contrast to the continuous norethindrone POP, which is not sufficiently dosed to consistently suppress ovulation, the 4-mg daily dose of drospirenone in this new POP is higher than the 3 mg used in commonly prescribed combination OCs that contain EE and drospirenone. This results in a POP that has more consistent ovulation suppression. Because this drospirenone POP is appropriately dosed and based on a longer-acting progestin, it is more forgiving of inconsistent pill taking. Accordingly, the missed-pill rules for this pill are the same as with combination estrogen-progestin OCs.1 The package labeling cites a first-year failure rate of 4%, but this includes unconfirmed pregnancies. The Pearl Index from the North American trials, based on confirmed pregnancies in nonbreastfeeding women, was 2.9.2
The package labeling for this drospirenone POP includes few contraindications. Conditions that preclude use include the US Medical Eligibility Criteria for contraception Category 4 condition (breast cancer in the last 5 years), renal impairment, and adrenal insufficiency. Other standard contraindications are listed in the prescribing information. Serum potassium levels should be checked (one time only) in the first cycle only for women who chronically use medications that could cause hyperkalemia, such as nonsteroidal anti-inflammatory drugs.
Given the ovulation suppression associated with this drospirenone POP, the safety of a progestin-only method, and the persistent popularity of OC pills, this pill should greatly increase the use of POPs beyond their traditional niche of postpartum and breastfeeding women. The advent of the drospirenone POP means that clinicians now have better options for women who have contraindications to estrogen and desire to control their own contraceptive use. It would be a logical consideration for over-the-counter accessibility.
2. Transdermal patch with ethinyl estradiol/levonorgestrel
The new EE/levonorgestrel transdermal contraceptive patch (Twirla) is soft and flexible, about the same size as other contraceptive patches, and contains EE 2.3 mg/levonorgestrel 2.6 mg. It provides total estrogen exposure that is similar to that of OCs with EE 30 µg and distinctly lower than estrogen levels seen with the original norelgestromin-containing patch or its 2 subsequent generic versions.3 This EE/levonorgestrel patch uses a new 5-layer drug delivery system that focuses the steroids for absorption beneath the patch; there is no peripheral spread of drug around the patch (FIGURE 1).
Transdermal patches offer the convenience of once-a-week dosing. One patch is used each week for 3 consecutive weeks followed by a patch-free week. Patches can be worn on the abdomen, buttock, or trunk (except breasts). Patches should not be placed consecutively on the same site; after a week’s rest, however, the first site can be reused. All transdermal contraceptive products are indicated for use only by women with a body mass index (BMI) <30 kg/m2.4
While no head-to-head trials have compared this new lower-dose patch with older patches, each patch was compared against a standardized pill, so meaningful comparisons can be made.
In each case, the circulating estrogen levels associated with use of the EE/levonorgestrel patch were considerably lower than those of the comparator pill, while the older norelgestromin patch consistently delivered higher total estrogen levels than its 35-µg comparator pill (TABLE).3 Along these lines, no VTE events occurred in women in the clinical trial of the new patch among women with a BMI <30 kg/m2.4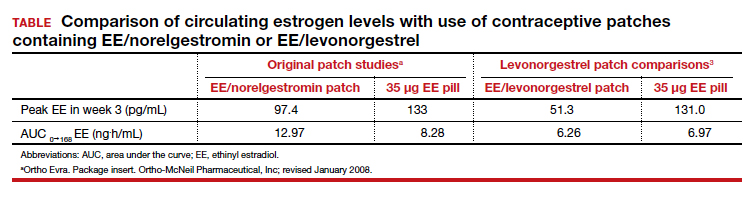
Women with a BMI <25 kg/m2 experienced lower Pearl Index (PI) pregnancy rates (3.5%) compared with women with a BMI between 25 and 30 kg/m2 (5.7%), according to clinical trial data cited in the package labeling. All the modern PI criteria were used to calculate these failure rates. Cycles in which no coitus occurred were excluded. Similarly, cycles in which another contraceptive method (for example, condoms) was added (even once) were excluded. Frequent pregnancy testing was done in the study centers and by the women at home. Bleeding patterns were well accepted; only 2.2% of study participants exited the study early due to menstrual disorders of any kind. Similarly, 3.1% of women discontinued use because of application site disorders. Women should be advised to press down on the patch edges after emerging from water exposure. Replacement patches are rapidly available from the manufacturer should permanent complete patch detachment occur.
Larger-scale phase 4 trials will be conducted to study the impact of this lower-dose patch on VTE rates.
Continue to: 3. A 1-year contraceptive vaginal ring...
3. A 1-year contraceptive vaginal ring
The need to obtain new supplies every month or every 3 months contributes to high rates of contraceptive failure and unintended pregnancy among women using short-acting hormonal contraceptives (pills, patches, and vaginal rings).5 A woman-controlled contraceptive that would provide 1 year of protection against unintended pregnancy represents a step forward. A contraceptive vaginal ring (CVR) that releases the novel progestin segesterone acetate and EE provides woman-controlled contraception for up to 1 year. This CVR (Annovera) received FDA approval in 2018 and has been marketed in the United States since 2020.
The segesterone acetate/EE CVR is a soft, flexible ring that is opaque white in color and fabricated from nonbiodegradable silicone (FIGURE 2). The outside diameter is 5.6 cm, compared with the 5.4-cm outer diameter of the etonogestrel/EE vaginal ring (NuvaRing). The segesterone acetate/EE CVR has 2 channels: one releases segesterone acetate only and the other releases segesterone acetate and EE. In contrast with the etonogestrel/EE CVR, the segesterone acetate/EE CVR does not need to be refrigerated when stored.6
Segesterone is a 19-nor-progesterone derivative that binds in a highly selective fashion to progesterone receptors, and it is potent in suppressing ovulation. During use of the segesterone acetate/EE CVR, mean levels of EE are incrementally higher than those observed with use of the etonogestrel/EE CVR.
Two 13-cycle (1 year) phase 3 clinical trials conducted from 2006 to 2009 enrolled 2,308 women aged 18 to 40 years, including 2,265 women aged 18 to 35 (the age group the FDA considers for efficacy analysis). Trial participants placed the ring vaginally on cycle days 2 to 5 and were asked to keep the ring in place for 21 days, then to remove the CVR for 7 days, during which scheduled bleeding was anticipated. For sexual intercourse, rings could be removed, depending on patient/couple preference, for up to 2 hours.
In the combined trials, the PI was 2.98 per 100 woman-years, a pregnancy rate comparable to those seen in other recent trials of combination estrogen-progestin contraceptives. The incidence of contraceptive failure did not increase over time during the 1-year trials, indicating that contraceptive efficacy of the segesterone acetate/EE was maintained during 1 year of use. While the pregnancy rate was lower in participants who did not report any instances of CVR removal during the 21-day periods of use, the rate was substantially higher among those who reported prolonged episodes of CVR removal.
In the 2 trials, bleeding patterns were similar to those observed with other combination estrogen-progestin contraceptives. Fewer than 2% of trial participants discontinued the trial early due to what they considered unacceptable bleeding.
More than one-half of trial participants reported at least 1 episode of complete or partial CVR expulsion. Most expulsions occurred in the first cycle, suggesting a learning curve with CVR use. Fewer than 2% of participants discontinued trial participation due to expulsions.
Almost 90% of participants reported that they were “highly satisfied” or “satisfied” with the CVR. Although more than two-thirds of participants reported that they never felt the ring during intercourse, if a couple did report feeling the ring during sex, the likelihood of dissatisfaction with the CVR doubled. In addition, feeling the CVR at other times was strongly associated with dissatisfaction. Because a deeply positioned CVR is less likely to be felt by users, these observations underscore the importance of counseling users to place the ring into the upper vagina. Of note, neither prior ring use nor tampon use was associated with CVR satisfaction.
One other important counseling point regarding CVR use relates to the discoloration of the ring that occurs over time. The initially white ring tends to become dark brown during the 1-year usage period. Although this discoloration does not indicate hygiene problems, women who are not advised about this in advance may be put off by the color change.
Four nonfatal VTE events occurred, all in the US trial sites. The overall VTE incidence was higher than expected, particularly among participants with a BMI of 29 kg/m2 or higher. After this association was noted, participants with a BMI >29 kg/m2 were discontinued from the trials. The package labeling for the segesterone acetate/EE CVR states that “Limited data are available in females with a BMI >29.0 kg/m2 because this subpopulation was excluded from the clinical trials after VTEs were reported.”6
A 1-year CVR raises the possibility that users could use their rings in an experimental extended fashion to reduce the frequency of withdrawal bleeding or continuously so as to eliminate withdrawal bleeding. In a randomly chosen sample of CVRs that had been used in the 13-cycle clinical trials, residual steroids in the CVRs were assessed. Sixty percent of segesterone acetate and 80% of EE remained. Using these observations as well as pharmacokinetic data collected from phase 3 trial participants, predicted segesterone acetate levels after 1 year of hypothetical continuous use appear to be sufficient to provide effective contraception.7 These observations suggest that performing clinical trials of extended as well as continuous segesterone acetate/EE CVR use is warranted.
Continue to: 4. An OC with a novel estrogen...
4. An OC with a novel estrogen
Even as use of intrauterine devices and contraceptive implants continues to grow, OCs remain the reversible contraceptive most used by US women. While OCs have been widely studied and represent a safe method of contraception for most reproductive-age women, combination estrogen-progestin OCs are well recognized to increase the risk of VTE. Although the primary role of the progestin component of combination OCs is to suppress ovulation, estrogen is included in combination OCs to stimulate endometrial proliferation, thereby causing predictable bleeding. EE, the potent synthetic estrogen used in the great majority of current OC formulations, induces hepatic production of prothrombotic proteins while inhibiting synthesis of antithrombotic proteins. While the lower EE doses (10–35 µg) in today’s OC formulations are associated with a lower VTE risk than older OCs that contained higher doses of estrogen, VTE continues to represent the principal health risk associated with use of combination OCs. Accordingly, development of a combination OC that has less impact on risk of VTE would be appealing.
In April 2021, the FDA approved an OC formulation that combines 15 mg of the novel estrogen estetrol with 3 mg of drospirenone (Nextstellis). This dose of drospirenone is the same as that used in commonly prescribed EE/drospirenone OC formulations. Also known as E4, estetrol is a natural estrogen synthesized by the fetal liver. Plant-derived E4 is used in this new OC.
Depending on the tissue, E4 acts differently than other estrogens. Similar to other estrogens, E4 acts as an agonist on the nuclear receptor to produce beneficial effects in bone, vaginal mucosa, and heart.8 Unlike other estrogens, E4 inhibits proliferation of mammary gland cells and has a neutral impact on the liver.9
In contrast with EE, E4 is not inhibited by the liver’s P450 enzymes; accordingly, the risk of drug-drug interactions is reduced. Because E4 is primarily excreted through the urine and not through the biliary tract, the risk of gallstone formation may be lower than with an EE OC. Likewise, E4 has substantially less impact on triglycerides, which are increased with EE. Finally, because of E4’s reduced effect on the liver, the impact on clotting parameters is less than that observed with an OC formulated with EE.10 This latter observation raises the possibility that VTE risk is lower with the E4/drospirenone OC than an OC formulated with EE.
A 13-cycle phase 3 trial of the E4/drospirenone OC conducted in the United States and Canada enrolled 1,864 women aged 16 to 50 years, including 1,674 who were aged 16 to 35 years.11 Among women in this latter age group, the PI was 2.65 per 100 woman-years. Bleeding/cycle control patterns were similar to those observed in recent trials of other combination contraceptives. Likewise, the proportion of trial participants who discontinued the study due to adverse effects was similar to or lower than that noted in recent trials of other combination contraceptives. Of particular note, no cases of VTE were noted among trial participants of any BMI, a finding which contrasts with recent phase 3 trials of other combination contraceptives. The result of this pivotal trial suggests that the theoretic advantages of E4 when used in a combination OC formulation may translate into a safer, effective, and well-tolerated contraceptive.
Refinements in hormonal contraceptives continue
The 4 new short-acting hormonal contraceptives we reviewed represent enhancements on existing pills, patches, and rings. We hope that, financially, women will have access to these innovative methods and, in particular, that third-party payers will facilitate women’s access to these enhanced short-acting hormonal contraceptives. ●
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Archer DF, Stanczyk FZ, Rubin A, et al. Ethinyl estradiol and levonorgestrel pharmacokinetics with a low-dose transdermal contraceptive delivery system, AG200-15: a randomized controlled trial. Contraception. 2012;85:595-601.
- Nelson AL, Kaunitz AM, Kroll R, et al; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Westhoff CL, Heartwell S, Edwards S, et al. Oral contraceptive discontinuation: do side effects matter? Am J Obstet Gynecol. 2007;196:412.e1-6; discussion 412.e6-7.
- Nelson AL. Comprehensive overview of the recently FDAapproved contraceptive vaginal ring releasing segesterone acetate and ethinylestradiol: a new year-long, patient controlled, reversible birth control method. Expert Rev Clin Pharmacol. 2019;12:953-963.
- Liu JH, Plagianos M, Archer DF, et al. Segesterone acetate serum levels with a regression model of continuous use of the segesterone acetate/ethinyl estradiol contraceptive vaginal system. Contraception. 2021;104:229-234.
- Mawet M, Maillard C, Klipping C, et al. Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives. Eur J Contracept Reprod Health Care. 2015;20:463-475.
- Gérard C, Blacher S, Communal L, et al. Estetrol is a weak estrogen antagonizing estradiol-dependent mammary gland proliferation. J Endocrinol. 2015;224:85-95.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetroldrospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
Short-term hormonal contraceptives remain the most popular class of reversible contraceptives in the United States, despite the availability of longer-acting methods. Oral contraceptives (OCs), contraceptive patches, and contraceptive vaginal rings are extensively used not only because these methods are easy to initiate but also because their ongoing use remains under the control of the woman herself and also provides her with a wide range of important noncontraceptive benefits.
Despite the more than 60 years of innovation that have made hormonal contraceptives safer, more tolerable, and more convenient, there has been room for improvement. Over the last few years, 4 new hormonal methods have been introduced, and each addresses different limitations and problems associated with the existing, often generic, products.
Compared with the traditional norethindrone pill (Micronor and generics), a new drospirenone progestin-only pill (POP) increases ovulation suppression, offers an improved cyclical bleeding profile, and relaxes the tight missed-pill rules that are usually associated with POPs.
In contrast with the older norelgestromin patch (Evra, Xulane), a new contraceptive transdermal patch significantly decreases total estrogen exposure and pairs its estrogen with levonorgestrel, the progestin associated with the lowest venous thromboembolism (VTE) risk in combined hormonal pills.
While existing combination OCs are formulated with the potent estrogen ethinyl estradiol (EE), a new combination pill, formulated with estetrol (E4) and drospirenone, introduces the first new estrogen (estetrol) used in a contraceptive in more than 50 years. Estetrol, a native estrogen, has selective tissue activity with minimal hepatic and breast impacts. Combined with drospirenone, this formulation offers women good contraceptive efficacy and bleeding patterns.
A new contraceptive vaginal ring introduces a new long-acting, specific progestin (segesterone acetate) and pairs it with low-dose EE. These hormones are packaged in a soft vaginal ring that provides up to 13 cycles of contraceptive protection (3 weeks in/1 week out) with one ring, greatly increasing convenience for women.
Each of these new products represents important incremental improvement over existing options.
Continue to: 1. The drospirenone-only OC...
1. The drospirenone-only OC
The new POP with drospirenone 4 mg (Slynd), which received US Food and Drug Administration (FDA) approval in 2019, is packaged in a 24/4 formulation (24 hormonally active tablets followed by 4 inactive tablets). This formulation results in more predictable bleeding than does the 0.35-mg norethindrone POP, which contains 28 hormonally active tablets in each pack. In the US clinical trials of drospirenone 4 mg, scheduled bleeding decreased from 81% in cycle 1 to 20% in cycle 13. Unscheduled spotting and bleeding decreased from 61% to 40% in the same timeframe. Notably, this bleeding pattern was well tolerated; only 0.4% of trial participants discontinued this drospirenone POP due to problems with irregular bleeding or amenorrhea.
In contrast to the continuous norethindrone POP, which is not sufficiently dosed to consistently suppress ovulation, the 4-mg daily dose of drospirenone in this new POP is higher than the 3 mg used in commonly prescribed combination OCs that contain EE and drospirenone. This results in a POP that has more consistent ovulation suppression. Because this drospirenone POP is appropriately dosed and based on a longer-acting progestin, it is more forgiving of inconsistent pill taking. Accordingly, the missed-pill rules for this pill are the same as with combination estrogen-progestin OCs.1 The package labeling cites a first-year failure rate of 4%, but this includes unconfirmed pregnancies. The Pearl Index from the North American trials, based on confirmed pregnancies in nonbreastfeeding women, was 2.9.2
The package labeling for this drospirenone POP includes few contraindications. Conditions that preclude use include the US Medical Eligibility Criteria for contraception Category 4 condition (breast cancer in the last 5 years), renal impairment, and adrenal insufficiency. Other standard contraindications are listed in the prescribing information. Serum potassium levels should be checked (one time only) in the first cycle only for women who chronically use medications that could cause hyperkalemia, such as nonsteroidal anti-inflammatory drugs.
Given the ovulation suppression associated with this drospirenone POP, the safety of a progestin-only method, and the persistent popularity of OC pills, this pill should greatly increase the use of POPs beyond their traditional niche of postpartum and breastfeeding women. The advent of the drospirenone POP means that clinicians now have better options for women who have contraindications to estrogen and desire to control their own contraceptive use. It would be a logical consideration for over-the-counter accessibility.
2. Transdermal patch with ethinyl estradiol/levonorgestrel
The new EE/levonorgestrel transdermal contraceptive patch (Twirla) is soft and flexible, about the same size as other contraceptive patches, and contains EE 2.3 mg/levonorgestrel 2.6 mg. It provides total estrogen exposure that is similar to that of OCs with EE 30 µg and distinctly lower than estrogen levels seen with the original norelgestromin-containing patch or its 2 subsequent generic versions.3 This EE/levonorgestrel patch uses a new 5-layer drug delivery system that focuses the steroids for absorption beneath the patch; there is no peripheral spread of drug around the patch (FIGURE 1).

Transdermal patches offer the convenience of once-a-week dosing. One patch is used each week for 3 consecutive weeks followed by a patch-free week. Patches can be worn on the abdomen, buttock, or trunk (except breasts). Patches should not be placed consecutively on the same site; after a week’s rest, however, the first site can be reused. All transdermal contraceptive products are indicated for use only by women with a body mass index (BMI) <30 kg/m2.4
While no head-to-head trials have compared this new lower-dose patch with older patches, each patch was compared against a standardized pill, so meaningful comparisons can be made.
In each case, the circulating estrogen levels associated with use of the EE/levonorgestrel patch were considerably lower than those of the comparator pill, while the older norelgestromin patch consistently delivered higher total estrogen levels than its 35-µg comparator pill (TABLE).3 Along these lines, no VTE events occurred in women in the clinical trial of the new patch among women with a BMI <30 kg/m2.4
Women with a BMI <25 kg/m2 experienced lower Pearl Index (PI) pregnancy rates (3.5%) compared with women with a BMI between 25 and 30 kg/m2 (5.7%), according to clinical trial data cited in the package labeling. All the modern PI criteria were used to calculate these failure rates. Cycles in which no coitus occurred were excluded. Similarly, cycles in which another contraceptive method (for example, condoms) was added (even once) were excluded. Frequent pregnancy testing was done in the study centers and by the women at home. Bleeding patterns were well accepted; only 2.2% of study participants exited the study early due to menstrual disorders of any kind. Similarly, 3.1% of women discontinued use because of application site disorders. Women should be advised to press down on the patch edges after emerging from water exposure. Replacement patches are rapidly available from the manufacturer should permanent complete patch detachment occur.
Larger-scale phase 4 trials will be conducted to study the impact of this lower-dose patch on VTE rates.
Continue to: 3. A 1-year contraceptive vaginal ring...
3. A 1-year contraceptive vaginal ring
The need to obtain new supplies every month or every 3 months contributes to high rates of contraceptive failure and unintended pregnancy among women using short-acting hormonal contraceptives (pills, patches, and vaginal rings).5 A woman-controlled contraceptive that would provide 1 year of protection against unintended pregnancy represents a step forward. A contraceptive vaginal ring (CVR) that releases the novel progestin segesterone acetate and EE provides woman-controlled contraception for up to 1 year. This CVR (Annovera) received FDA approval in 2018 and has been marketed in the United States since 2020.
The segesterone acetate/EE CVR is a soft, flexible ring that is opaque white in color and fabricated from nonbiodegradable silicone (FIGURE 2). The outside diameter is 5.6 cm, compared with the 5.4-cm outer diameter of the etonogestrel/EE vaginal ring (NuvaRing). The segesterone acetate/EE CVR has 2 channels: one releases segesterone acetate only and the other releases segesterone acetate and EE. In contrast with the etonogestrel/EE CVR, the segesterone acetate/EE CVR does not need to be refrigerated when stored.6

Segesterone is a 19-nor-progesterone derivative that binds in a highly selective fashion to progesterone receptors, and it is potent in suppressing ovulation. During use of the segesterone acetate/EE CVR, mean levels of EE are incrementally higher than those observed with use of the etonogestrel/EE CVR.
Two 13-cycle (1 year) phase 3 clinical trials conducted from 2006 to 2009 enrolled 2,308 women aged 18 to 40 years, including 2,265 women aged 18 to 35 (the age group the FDA considers for efficacy analysis). Trial participants placed the ring vaginally on cycle days 2 to 5 and were asked to keep the ring in place for 21 days, then to remove the CVR for 7 days, during which scheduled bleeding was anticipated. For sexual intercourse, rings could be removed, depending on patient/couple preference, for up to 2 hours.
In the combined trials, the PI was 2.98 per 100 woman-years, a pregnancy rate comparable to those seen in other recent trials of combination estrogen-progestin contraceptives. The incidence of contraceptive failure did not increase over time during the 1-year trials, indicating that contraceptive efficacy of the segesterone acetate/EE was maintained during 1 year of use. While the pregnancy rate was lower in participants who did not report any instances of CVR removal during the 21-day periods of use, the rate was substantially higher among those who reported prolonged episodes of CVR removal.
In the 2 trials, bleeding patterns were similar to those observed with other combination estrogen-progestin contraceptives. Fewer than 2% of trial participants discontinued the trial early due to what they considered unacceptable bleeding.
More than one-half of trial participants reported at least 1 episode of complete or partial CVR expulsion. Most expulsions occurred in the first cycle, suggesting a learning curve with CVR use. Fewer than 2% of participants discontinued trial participation due to expulsions.
Almost 90% of participants reported that they were “highly satisfied” or “satisfied” with the CVR. Although more than two-thirds of participants reported that they never felt the ring during intercourse, if a couple did report feeling the ring during sex, the likelihood of dissatisfaction with the CVR doubled. In addition, feeling the CVR at other times was strongly associated with dissatisfaction. Because a deeply positioned CVR is less likely to be felt by users, these observations underscore the importance of counseling users to place the ring into the upper vagina. Of note, neither prior ring use nor tampon use was associated with CVR satisfaction.
One other important counseling point regarding CVR use relates to the discoloration of the ring that occurs over time. The initially white ring tends to become dark brown during the 1-year usage period. Although this discoloration does not indicate hygiene problems, women who are not advised about this in advance may be put off by the color change.
Four nonfatal VTE events occurred, all in the US trial sites. The overall VTE incidence was higher than expected, particularly among participants with a BMI of 29 kg/m2 or higher. After this association was noted, participants with a BMI >29 kg/m2 were discontinued from the trials. The package labeling for the segesterone acetate/EE CVR states that “Limited data are available in females with a BMI >29.0 kg/m2 because this subpopulation was excluded from the clinical trials after VTEs were reported.”6
A 1-year CVR raises the possibility that users could use their rings in an experimental extended fashion to reduce the frequency of withdrawal bleeding or continuously so as to eliminate withdrawal bleeding. In a randomly chosen sample of CVRs that had been used in the 13-cycle clinical trials, residual steroids in the CVRs were assessed. Sixty percent of segesterone acetate and 80% of EE remained. Using these observations as well as pharmacokinetic data collected from phase 3 trial participants, predicted segesterone acetate levels after 1 year of hypothetical continuous use appear to be sufficient to provide effective contraception.7 These observations suggest that performing clinical trials of extended as well as continuous segesterone acetate/EE CVR use is warranted.
Continue to: 4. An OC with a novel estrogen...
4. An OC with a novel estrogen
Even as use of intrauterine devices and contraceptive implants continues to grow, OCs remain the reversible contraceptive most used by US women. While OCs have been widely studied and represent a safe method of contraception for most reproductive-age women, combination estrogen-progestin OCs are well recognized to increase the risk of VTE. Although the primary role of the progestin component of combination OCs is to suppress ovulation, estrogen is included in combination OCs to stimulate endometrial proliferation, thereby causing predictable bleeding. EE, the potent synthetic estrogen used in the great majority of current OC formulations, induces hepatic production of prothrombotic proteins while inhibiting synthesis of antithrombotic proteins. While the lower EE doses (10–35 µg) in today’s OC formulations are associated with a lower VTE risk than older OCs that contained higher doses of estrogen, VTE continues to represent the principal health risk associated with use of combination OCs. Accordingly, development of a combination OC that has less impact on risk of VTE would be appealing.
In April 2021, the FDA approved an OC formulation that combines 15 mg of the novel estrogen estetrol with 3 mg of drospirenone (Nextstellis). This dose of drospirenone is the same as that used in commonly prescribed EE/drospirenone OC formulations. Also known as E4, estetrol is a natural estrogen synthesized by the fetal liver. Plant-derived E4 is used in this new OC.
Depending on the tissue, E4 acts differently than other estrogens. Similar to other estrogens, E4 acts as an agonist on the nuclear receptor to produce beneficial effects in bone, vaginal mucosa, and heart.8 Unlike other estrogens, E4 inhibits proliferation of mammary gland cells and has a neutral impact on the liver.9
In contrast with EE, E4 is not inhibited by the liver’s P450 enzymes; accordingly, the risk of drug-drug interactions is reduced. Because E4 is primarily excreted through the urine and not through the biliary tract, the risk of gallstone formation may be lower than with an EE OC. Likewise, E4 has substantially less impact on triglycerides, which are increased with EE. Finally, because of E4’s reduced effect on the liver, the impact on clotting parameters is less than that observed with an OC formulated with EE.10 This latter observation raises the possibility that VTE risk is lower with the E4/drospirenone OC than an OC formulated with EE.
A 13-cycle phase 3 trial of the E4/drospirenone OC conducted in the United States and Canada enrolled 1,864 women aged 16 to 50 years, including 1,674 who were aged 16 to 35 years.11 Among women in this latter age group, the PI was 2.65 per 100 woman-years. Bleeding/cycle control patterns were similar to those observed in recent trials of other combination contraceptives. Likewise, the proportion of trial participants who discontinued the study due to adverse effects was similar to or lower than that noted in recent trials of other combination contraceptives. Of particular note, no cases of VTE were noted among trial participants of any BMI, a finding which contrasts with recent phase 3 trials of other combination contraceptives. The result of this pivotal trial suggests that the theoretic advantages of E4 when used in a combination OC formulation may translate into a safer, effective, and well-tolerated contraceptive.
Refinements in hormonal contraceptives continue
The 4 new short-acting hormonal contraceptives we reviewed represent enhancements on existing pills, patches, and rings. We hope that, financially, women will have access to these innovative methods and, in particular, that third-party payers will facilitate women’s access to these enhanced short-acting hormonal contraceptives. ●
Short-term hormonal contraceptives remain the most popular class of reversible contraceptives in the United States, despite the availability of longer-acting methods. Oral contraceptives (OCs), contraceptive patches, and contraceptive vaginal rings are extensively used not only because these methods are easy to initiate but also because their ongoing use remains under the control of the woman herself and also provides her with a wide range of important noncontraceptive benefits.
Despite the more than 60 years of innovation that have made hormonal contraceptives safer, more tolerable, and more convenient, there has been room for improvement. Over the last few years, 4 new hormonal methods have been introduced, and each addresses different limitations and problems associated with the existing, often generic, products.
Compared with the traditional norethindrone pill (Micronor and generics), a new drospirenone progestin-only pill (POP) increases ovulation suppression, offers an improved cyclical bleeding profile, and relaxes the tight missed-pill rules that are usually associated with POPs.
In contrast with the older norelgestromin patch (Evra, Xulane), a new contraceptive transdermal patch significantly decreases total estrogen exposure and pairs its estrogen with levonorgestrel, the progestin associated with the lowest venous thromboembolism (VTE) risk in combined hormonal pills.
While existing combination OCs are formulated with the potent estrogen ethinyl estradiol (EE), a new combination pill, formulated with estetrol (E4) and drospirenone, introduces the first new estrogen (estetrol) used in a contraceptive in more than 50 years. Estetrol, a native estrogen, has selective tissue activity with minimal hepatic and breast impacts. Combined with drospirenone, this formulation offers women good contraceptive efficacy and bleeding patterns.
A new contraceptive vaginal ring introduces a new long-acting, specific progestin (segesterone acetate) and pairs it with low-dose EE. These hormones are packaged in a soft vaginal ring that provides up to 13 cycles of contraceptive protection (3 weeks in/1 week out) with one ring, greatly increasing convenience for women.
Each of these new products represents important incremental improvement over existing options.
Continue to: 1. The drospirenone-only OC...
1. The drospirenone-only OC
The new POP with drospirenone 4 mg (Slynd), which received US Food and Drug Administration (FDA) approval in 2019, is packaged in a 24/4 formulation (24 hormonally active tablets followed by 4 inactive tablets). This formulation results in more predictable bleeding than does the 0.35-mg norethindrone POP, which contains 28 hormonally active tablets in each pack. In the US clinical trials of drospirenone 4 mg, scheduled bleeding decreased from 81% in cycle 1 to 20% in cycle 13. Unscheduled spotting and bleeding decreased from 61% to 40% in the same timeframe. Notably, this bleeding pattern was well tolerated; only 0.4% of trial participants discontinued this drospirenone POP due to problems with irregular bleeding or amenorrhea.
In contrast to the continuous norethindrone POP, which is not sufficiently dosed to consistently suppress ovulation, the 4-mg daily dose of drospirenone in this new POP is higher than the 3 mg used in commonly prescribed combination OCs that contain EE and drospirenone. This results in a POP that has more consistent ovulation suppression. Because this drospirenone POP is appropriately dosed and based on a longer-acting progestin, it is more forgiving of inconsistent pill taking. Accordingly, the missed-pill rules for this pill are the same as with combination estrogen-progestin OCs.1 The package labeling cites a first-year failure rate of 4%, but this includes unconfirmed pregnancies. The Pearl Index from the North American trials, based on confirmed pregnancies in nonbreastfeeding women, was 2.9.2
The package labeling for this drospirenone POP includes few contraindications. Conditions that preclude use include the US Medical Eligibility Criteria for contraception Category 4 condition (breast cancer in the last 5 years), renal impairment, and adrenal insufficiency. Other standard contraindications are listed in the prescribing information. Serum potassium levels should be checked (one time only) in the first cycle only for women who chronically use medications that could cause hyperkalemia, such as nonsteroidal anti-inflammatory drugs.
Given the ovulation suppression associated with this drospirenone POP, the safety of a progestin-only method, and the persistent popularity of OC pills, this pill should greatly increase the use of POPs beyond their traditional niche of postpartum and breastfeeding women. The advent of the drospirenone POP means that clinicians now have better options for women who have contraindications to estrogen and desire to control their own contraceptive use. It would be a logical consideration for over-the-counter accessibility.
2. Transdermal patch with ethinyl estradiol/levonorgestrel
The new EE/levonorgestrel transdermal contraceptive patch (Twirla) is soft and flexible, about the same size as other contraceptive patches, and contains EE 2.3 mg/levonorgestrel 2.6 mg. It provides total estrogen exposure that is similar to that of OCs with EE 30 µg and distinctly lower than estrogen levels seen with the original norelgestromin-containing patch or its 2 subsequent generic versions.3 This EE/levonorgestrel patch uses a new 5-layer drug delivery system that focuses the steroids for absorption beneath the patch; there is no peripheral spread of drug around the patch (FIGURE 1).

Transdermal patches offer the convenience of once-a-week dosing. One patch is used each week for 3 consecutive weeks followed by a patch-free week. Patches can be worn on the abdomen, buttock, or trunk (except breasts). Patches should not be placed consecutively on the same site; after a week’s rest, however, the first site can be reused. All transdermal contraceptive products are indicated for use only by women with a body mass index (BMI) <30 kg/m2.4
While no head-to-head trials have compared this new lower-dose patch with older patches, each patch was compared against a standardized pill, so meaningful comparisons can be made.
In each case, the circulating estrogen levels associated with use of the EE/levonorgestrel patch were considerably lower than those of the comparator pill, while the older norelgestromin patch consistently delivered higher total estrogen levels than its 35-µg comparator pill (TABLE).3 Along these lines, no VTE events occurred in women in the clinical trial of the new patch among women with a BMI <30 kg/m2.4
Women with a BMI <25 kg/m2 experienced lower Pearl Index (PI) pregnancy rates (3.5%) compared with women with a BMI between 25 and 30 kg/m2 (5.7%), according to clinical trial data cited in the package labeling. All the modern PI criteria were used to calculate these failure rates. Cycles in which no coitus occurred were excluded. Similarly, cycles in which another contraceptive method (for example, condoms) was added (even once) were excluded. Frequent pregnancy testing was done in the study centers and by the women at home. Bleeding patterns were well accepted; only 2.2% of study participants exited the study early due to menstrual disorders of any kind. Similarly, 3.1% of women discontinued use because of application site disorders. Women should be advised to press down on the patch edges after emerging from water exposure. Replacement patches are rapidly available from the manufacturer should permanent complete patch detachment occur.
Larger-scale phase 4 trials will be conducted to study the impact of this lower-dose patch on VTE rates.
Continue to: 3. A 1-year contraceptive vaginal ring...
3. A 1-year contraceptive vaginal ring
The need to obtain new supplies every month or every 3 months contributes to high rates of contraceptive failure and unintended pregnancy among women using short-acting hormonal contraceptives (pills, patches, and vaginal rings).5 A woman-controlled contraceptive that would provide 1 year of protection against unintended pregnancy represents a step forward. A contraceptive vaginal ring (CVR) that releases the novel progestin segesterone acetate and EE provides woman-controlled contraception for up to 1 year. This CVR (Annovera) received FDA approval in 2018 and has been marketed in the United States since 2020.
The segesterone acetate/EE CVR is a soft, flexible ring that is opaque white in color and fabricated from nonbiodegradable silicone (FIGURE 2). The outside diameter is 5.6 cm, compared with the 5.4-cm outer diameter of the etonogestrel/EE vaginal ring (NuvaRing). The segesterone acetate/EE CVR has 2 channels: one releases segesterone acetate only and the other releases segesterone acetate and EE. In contrast with the etonogestrel/EE CVR, the segesterone acetate/EE CVR does not need to be refrigerated when stored.6

Segesterone is a 19-nor-progesterone derivative that binds in a highly selective fashion to progesterone receptors, and it is potent in suppressing ovulation. During use of the segesterone acetate/EE CVR, mean levels of EE are incrementally higher than those observed with use of the etonogestrel/EE CVR.
Two 13-cycle (1 year) phase 3 clinical trials conducted from 2006 to 2009 enrolled 2,308 women aged 18 to 40 years, including 2,265 women aged 18 to 35 (the age group the FDA considers for efficacy analysis). Trial participants placed the ring vaginally on cycle days 2 to 5 and were asked to keep the ring in place for 21 days, then to remove the CVR for 7 days, during which scheduled bleeding was anticipated. For sexual intercourse, rings could be removed, depending on patient/couple preference, for up to 2 hours.
In the combined trials, the PI was 2.98 per 100 woman-years, a pregnancy rate comparable to those seen in other recent trials of combination estrogen-progestin contraceptives. The incidence of contraceptive failure did not increase over time during the 1-year trials, indicating that contraceptive efficacy of the segesterone acetate/EE was maintained during 1 year of use. While the pregnancy rate was lower in participants who did not report any instances of CVR removal during the 21-day periods of use, the rate was substantially higher among those who reported prolonged episodes of CVR removal.
In the 2 trials, bleeding patterns were similar to those observed with other combination estrogen-progestin contraceptives. Fewer than 2% of trial participants discontinued the trial early due to what they considered unacceptable bleeding.
More than one-half of trial participants reported at least 1 episode of complete or partial CVR expulsion. Most expulsions occurred in the first cycle, suggesting a learning curve with CVR use. Fewer than 2% of participants discontinued trial participation due to expulsions.
Almost 90% of participants reported that they were “highly satisfied” or “satisfied” with the CVR. Although more than two-thirds of participants reported that they never felt the ring during intercourse, if a couple did report feeling the ring during sex, the likelihood of dissatisfaction with the CVR doubled. In addition, feeling the CVR at other times was strongly associated with dissatisfaction. Because a deeply positioned CVR is less likely to be felt by users, these observations underscore the importance of counseling users to place the ring into the upper vagina. Of note, neither prior ring use nor tampon use was associated with CVR satisfaction.
One other important counseling point regarding CVR use relates to the discoloration of the ring that occurs over time. The initially white ring tends to become dark brown during the 1-year usage period. Although this discoloration does not indicate hygiene problems, women who are not advised about this in advance may be put off by the color change.
Four nonfatal VTE events occurred, all in the US trial sites. The overall VTE incidence was higher than expected, particularly among participants with a BMI of 29 kg/m2 or higher. After this association was noted, participants with a BMI >29 kg/m2 were discontinued from the trials. The package labeling for the segesterone acetate/EE CVR states that “Limited data are available in females with a BMI >29.0 kg/m2 because this subpopulation was excluded from the clinical trials after VTEs were reported.”6
A 1-year CVR raises the possibility that users could use their rings in an experimental extended fashion to reduce the frequency of withdrawal bleeding or continuously so as to eliminate withdrawal bleeding. In a randomly chosen sample of CVRs that had been used in the 13-cycle clinical trials, residual steroids in the CVRs were assessed. Sixty percent of segesterone acetate and 80% of EE remained. Using these observations as well as pharmacokinetic data collected from phase 3 trial participants, predicted segesterone acetate levels after 1 year of hypothetical continuous use appear to be sufficient to provide effective contraception.7 These observations suggest that performing clinical trials of extended as well as continuous segesterone acetate/EE CVR use is warranted.
Continue to: 4. An OC with a novel estrogen...
4. An OC with a novel estrogen
Even as use of intrauterine devices and contraceptive implants continues to grow, OCs remain the reversible contraceptive most used by US women. While OCs have been widely studied and represent a safe method of contraception for most reproductive-age women, combination estrogen-progestin OCs are well recognized to increase the risk of VTE. Although the primary role of the progestin component of combination OCs is to suppress ovulation, estrogen is included in combination OCs to stimulate endometrial proliferation, thereby causing predictable bleeding. EE, the potent synthetic estrogen used in the great majority of current OC formulations, induces hepatic production of prothrombotic proteins while inhibiting synthesis of antithrombotic proteins. While the lower EE doses (10–35 µg) in today’s OC formulations are associated with a lower VTE risk than older OCs that contained higher doses of estrogen, VTE continues to represent the principal health risk associated with use of combination OCs. Accordingly, development of a combination OC that has less impact on risk of VTE would be appealing.
In April 2021, the FDA approved an OC formulation that combines 15 mg of the novel estrogen estetrol with 3 mg of drospirenone (Nextstellis). This dose of drospirenone is the same as that used in commonly prescribed EE/drospirenone OC formulations. Also known as E4, estetrol is a natural estrogen synthesized by the fetal liver. Plant-derived E4 is used in this new OC.
Depending on the tissue, E4 acts differently than other estrogens. Similar to other estrogens, E4 acts as an agonist on the nuclear receptor to produce beneficial effects in bone, vaginal mucosa, and heart.8 Unlike other estrogens, E4 inhibits proliferation of mammary gland cells and has a neutral impact on the liver.9
In contrast with EE, E4 is not inhibited by the liver’s P450 enzymes; accordingly, the risk of drug-drug interactions is reduced. Because E4 is primarily excreted through the urine and not through the biliary tract, the risk of gallstone formation may be lower than with an EE OC. Likewise, E4 has substantially less impact on triglycerides, which are increased with EE. Finally, because of E4’s reduced effect on the liver, the impact on clotting parameters is less than that observed with an OC formulated with EE.10 This latter observation raises the possibility that VTE risk is lower with the E4/drospirenone OC than an OC formulated with EE.
A 13-cycle phase 3 trial of the E4/drospirenone OC conducted in the United States and Canada enrolled 1,864 women aged 16 to 50 years, including 1,674 who were aged 16 to 35 years.11 Among women in this latter age group, the PI was 2.65 per 100 woman-years. Bleeding/cycle control patterns were similar to those observed in recent trials of other combination contraceptives. Likewise, the proportion of trial participants who discontinued the study due to adverse effects was similar to or lower than that noted in recent trials of other combination contraceptives. Of particular note, no cases of VTE were noted among trial participants of any BMI, a finding which contrasts with recent phase 3 trials of other combination contraceptives. The result of this pivotal trial suggests that the theoretic advantages of E4 when used in a combination OC formulation may translate into a safer, effective, and well-tolerated contraceptive.
Refinements in hormonal contraceptives continue
The 4 new short-acting hormonal contraceptives we reviewed represent enhancements on existing pills, patches, and rings. We hope that, financially, women will have access to these innovative methods and, in particular, that third-party payers will facilitate women’s access to these enhanced short-acting hormonal contraceptives. ●
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Archer DF, Stanczyk FZ, Rubin A, et al. Ethinyl estradiol and levonorgestrel pharmacokinetics with a low-dose transdermal contraceptive delivery system, AG200-15: a randomized controlled trial. Contraception. 2012;85:595-601.
- Nelson AL, Kaunitz AM, Kroll R, et al; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Westhoff CL, Heartwell S, Edwards S, et al. Oral contraceptive discontinuation: do side effects matter? Am J Obstet Gynecol. 2007;196:412.e1-6; discussion 412.e6-7.
- Nelson AL. Comprehensive overview of the recently FDAapproved contraceptive vaginal ring releasing segesterone acetate and ethinylestradiol: a new year-long, patient controlled, reversible birth control method. Expert Rev Clin Pharmacol. 2019;12:953-963.
- Liu JH, Plagianos M, Archer DF, et al. Segesterone acetate serum levels with a regression model of continuous use of the segesterone acetate/ethinyl estradiol contraceptive vaginal system. Contraception. 2021;104:229-234.
- Mawet M, Maillard C, Klipping C, et al. Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives. Eur J Contracept Reprod Health Care. 2015;20:463-475.
- Gérard C, Blacher S, Communal L, et al. Estetrol is a weak estrogen antagonizing estradiol-dependent mammary gland proliferation. J Endocrinol. 2015;224:85-95.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetroldrospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Archer DF, Stanczyk FZ, Rubin A, et al. Ethinyl estradiol and levonorgestrel pharmacokinetics with a low-dose transdermal contraceptive delivery system, AG200-15: a randomized controlled trial. Contraception. 2012;85:595-601.
- Nelson AL, Kaunitz AM, Kroll R, et al; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Westhoff CL, Heartwell S, Edwards S, et al. Oral contraceptive discontinuation: do side effects matter? Am J Obstet Gynecol. 2007;196:412.e1-6; discussion 412.e6-7.
- Nelson AL. Comprehensive overview of the recently FDAapproved contraceptive vaginal ring releasing segesterone acetate and ethinylestradiol: a new year-long, patient controlled, reversible birth control method. Expert Rev Clin Pharmacol. 2019;12:953-963.
- Liu JH, Plagianos M, Archer DF, et al. Segesterone acetate serum levels with a regression model of continuous use of the segesterone acetate/ethinyl estradiol contraceptive vaginal system. Contraception. 2021;104:229-234.
- Mawet M, Maillard C, Klipping C, et al. Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives. Eur J Contracept Reprod Health Care. 2015;20:463-475.
- Gérard C, Blacher S, Communal L, et al. Estetrol is a weak estrogen antagonizing estradiol-dependent mammary gland proliferation. J Endocrinol. 2015;224:85-95.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetroldrospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
2021 Update on minimally invasive gynecologic surgery

Uterine fibroids are a common condition that affects up to 80% of reproductive-age women.1 Many women with fibroids are asymptomatic, but some experience symptoms that profoundly disrupt their lives, such as abnormal uterine bleeding, pelvic pain, and bulk symptoms including bladder and bowel dysfunction.2 Although hysterectomy remains the definitive treatment for symptomatic fibroids, many women seek more conservative management. Hormonal treatment, such as contraceptive pills, levonorgestrel intrauterine devices, and gonadotropin-releasing hormone analogs, can improve heavy menstrual bleeding and anemia.3 Additionally, uterine artery embolization is a nonsurgical uterine-sparing option. However, these treatments are not ideal options for women who want to conceive.4 For reproductive-age women who desire future fertility, myomectomy has been the standard of care. Unfortunately, by the time patients become symptomatic from their fibroids and seek care, they may have numerous and/or sizable fibroids that result in high blood loss, surgical scarring, and the probable need for cesarean delivery (FIGURES 1 and 2).5
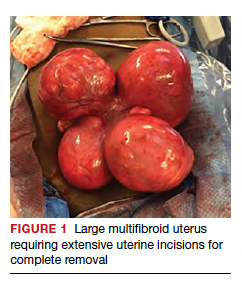
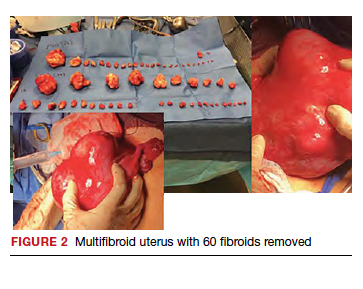
For patients who desire future conception, treatment of uterine fibroids poses a challenge in which optimizing symptomatic improvement must be balanced with protecting fertility and improving reproductive outcomes. In recent years, high-intensity focused ultrasound (FUS) and radiofrequency ablation (RFA) have been presented as less invasive, uterine-sparing alternatives for fibroid treatment that could potentially provide that balance.
In this article, we briefly review the available uterine-sparing fibroid treatments and their outcomes and then focus specifically on RFA as a possible option to address the fibroid treatment gap for reproductive-age women who desire future fertility.
Overview of uterine-sparing treatments
Two approaches can be pursued for conservative fibroid treatment: fibroid removal and fibroid necrosis (TABLE 1). We focus this review on outcomes for the most widely available of these treatments.
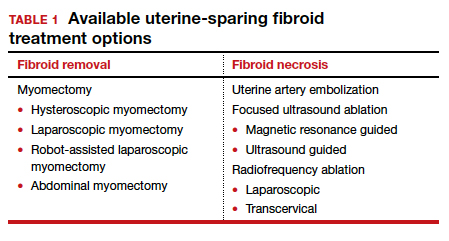
Myomectomy
For reproductive-age women who wish to conceive, surgical removal of fibroids has been the standard of care for symptomatic patients. Myomectomy can be performed via laparotomy, laparoscopy, robot-assisted surgery, and hysteroscopy. The mode of surgery depends on the fibroid characteristics (size, number, and location) and the surgeon’s skill set. Although some variation in the data exists, overall surgical outcomes, including blood loss, postoperative pain, and length of stay, are generally more favorable for minimally invasive approaches compared with laparotomy, with no significant differences in fibroid recurrence or reproductive outcomes (live birth rate, miscarriage rate, and cesarean delivery rate).6 This comes at the expense of longer operating time compared with laparotomy.7
While improvement in abnormal uterine bleeding and pelvic pain is reliable and usually significant after myomectomy,8 reproductive implications also warrant consideration. Myomectomy is associated with subsequent uterine adhesion formation, with some studies finding rates up to 83% to 94% depending on the surgical approach and the number of fibroids removed.9 These adhesions can impair fertility success.10 Myomectomy also is associated with high rates of cesarean delivery,5 invasive placentation (including placenta accreta spectrum),11 and uterine rupture.12 While the latter 2 complications are rare, they potentially can be catastrophic and should be kept in mind.
Continue to: Uterine artery embolization...
Uterine artery embolization
As a nonsurgical alternative to myomectomy, uterine artery embolization (UAE) has gained popularity as a conservative fibroid treatment since it was introduced in 1995. It is less invasive than myomectomy, a benefit for patients who decline surgery or are not ideal candidates for surgery.13 Evidence suggests that UAE produces overall comparable symptomatic improvement compared with myomectomy. One study showed no significant differences between UAE and myomectomy in terms of decreased uterine volume and menstrual bleeding at 6-month follow-up.14 In terms of long-term outcomes, a large multicenter study showed no significant difference in reintervention rates at 7 years posttreatment between UAE and myomectomy (8.9% vs 11.2%, respectively), and a significantly higher rate of improved menstrual bleeding with UAE (79.4% vs 49.5%), with no significant difference in bulk symptoms.15 The evidence is not entirely consistent, as other studies have shown increased rates of reintervention with UAE,8,16 but overall UAE can be considered a reasonable alternative to myomectomy in terms of symptomatic improvement.
Pregnancy outcomes data, however, are mixed, and UAE often is not recommended for patients with future fertility plans. In a large review article that compared minimally invasive fibroid treatments, UAE was associated with a lower live birth rate compared with myomectomy and ablation techniques (60.6% for UAE, 75.6% for myomectomy, and 70.5% for ablation), and it also had the highest rate of miscarriage (27.4% for UAE vs 19.0% for myomectomy and 11.9% for ablation) and abnormal placentation.12 While UAE remains an effective option for conservative treatment of symptomatic fibroids, it appears to have a worse impact on reproductive outcomes compared with myomectomy or ablative treatments.
Magnetic resonance–guided focused ultrasound
Emerging as a noninvasive ablation treatment for fibroids, magnetic resonance–guided focused ultrasound (MRgFUS) uses targeted high-intensity ultrasound pulses to cause thermal and mechanical fibroid tissue disruption.17 Data on this treatment are less robust given that it is newer than myomectomy or UAE. One study showed a decrease in fibroid volume by 12% at 1 month and 15% at 6 months, with 37.1% of patients reporting marked improvement in symptoms and an additional 31.4% reporting partial improvement; these are modest numbers compared with other treatment approaches.18 Another study showed more favorable outcomes, with 74% of patients reporting clinically significant improvement in bleeding and pain, and a 12.7% reintervention rate, comparable to rates reported for UAE and myomectomy.19
Because MRgFUS is newer than UAE or myomectomy, data are limited in terms of pregnancy outcomes, particularly because initial trials excluded women with future fertility plans due to lack of knowledge regarding pregnancy safety. A follow-up case series from one of the initial studies showed a decreased miscarriage rate compared with UAE, a term delivery rate of 93%, and a similar rate of abnormal placentation.20 A more recent systematic review concluded that reproductive outcomes were noninferior to myomectomy; however, the outcomes data for MRgFUS were heterogenous and many studies did not report pregnancy rates.21
Overall, MRgFUS appears to be an effective alternative approach for symptomatic fibroids, but the long-term data are not yet conclusive and information on pregnancy safety and outcomes largely is lacking. Recent reviews have not made definitive statements on whether MRgFUS should be offered to patients desiring future fertility.
Continue to: RFA is a promising option...
RFA is a promising option
RFA is another noninvasive fibroid ablation technique that has become more widely adopted in recent years. Here, we describe the basics of RFA and its impact on fibroid symptoms and reproductive outcomes.
The RFA technique
RFA uses hyperthermic energy from a handpiece and real-time ultrasound for targeted coagulative necrosis via a laparoscopic (L-RFA) or transcervical (TC-RFA) approach.22 A comparison between the 2 devices available on the market in the United States is shown in TABLE 2. Ultrasound guidance allows placement of radiofrequency needles directly into the fibroid to target local treatment to the fibroid tissue only. Once the fibroid undergoes coagulative necrosis, the process of fibroid resorption and volume reduction occurs over weeks to months, depending on the fibroid size.
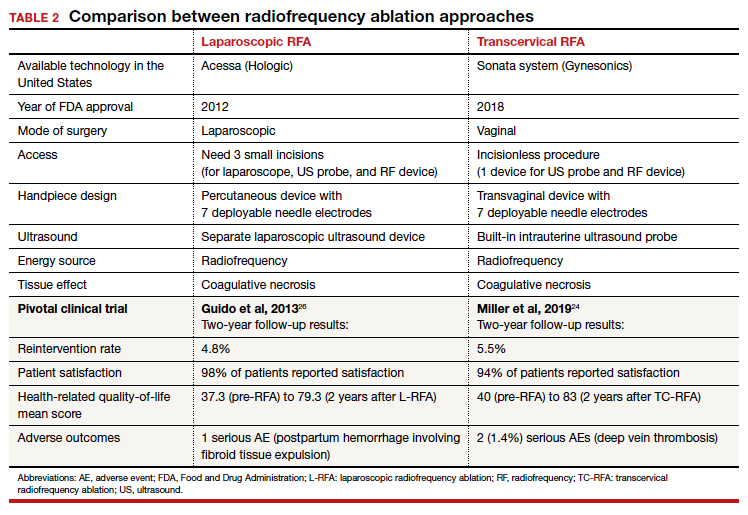
Impact on fibroid symptoms
Both laparoscopic and transcervical RFA approaches have shown significant decreases in pelvic pain and heavy menstrual bleeding associated with fibroids and a low reintervention rate that emphasizes the durability of their impact.
A feasibility and safety study of a TC-RFA device prior to the primary clinical trials found only a 4.3% reintervention rate in the first 18 months postprocedure.23 The pivotal clinical trial of a TC-RFA device that followed also reported a low 5.5% reintervention rate in the first 24 months postprocedure, with significant improvement in health-related quality-of-life and high patient satisfaction24 (results shown in TABLE 2, along with trial results for an L-RFA device). A subsequent study of TC-RFA reported that symptomatic improvement persisted at 3-year follow-up, with a 9.2% reintervention rate comparable to existing fibroid treatments such as myomectomy and UAE.25 The original L-RFA trial also has shown similar positive results at 2-year follow-up, with a low reintervention rate of 4.8% after treatment, and similar patient satisfaction and quality-of-life improvements as TC-RFA.26 While long-term data are limited by only recent approval by the Food and Drug Administration (FDA) of a TC-RFA device in 2018, one study followed clinical trial patients for a mean duration of 64 months. This study found no surgical reinterventions in the first 3.5 years posttreatment and a persistent reduction in fibroid symptoms from baseline 64.9 points to 27.6 points, as assessed by a validated symptom severity scale (out of 100 points).27 Similar improvements in health-related quality-of life-were also found to persist for years posttreatment.4
In a large systematic review that compared L-RFA, MRgFUS, UAE, and myomectomy, L-RFA had similar improvement rates in quality-of-life and symptom severity scores compared with myomectomy, with no significant difference in reintervention rates.28 This review also noted minimal heterogeneity among RFA meta-analyses data in contrast to significant heterogeneity among UAE and myomectomy data.
Reproductive outcomes
Similar to MRgFUS, the initial studies of RFA devices largely excluded women with future fertility plans, as data on safety were lacking. However, many RFA devices are now on the market across the globe, and subsequent pregnancies have been tracked and reported.
A large case series that included clinical trials and commercial settings reported a miscarriage rate (13.3%) similar to that of the general obstetric population and no cases of uterine rupture, invasive placentation, preterm delivery, or placental abruption.29 Other case series have reported live birth rates similar those with myomectomy, and safe and favorable pregnancy outcomes with RFA have been supported by larger systematic reviews of all ablation techniques.12
Continue to: Uterine impact...
Uterine impact
One study of TC-RFA patients showed a greater than 65% reduction in fibroid volume (with a 90% reduction in fibroid volume for fibroids larger than 6 cm prior to RFA), and 54% of patients reported complete resolution of symptoms, with another 36% reporting decreased symptoms.30 Similar decreases in fibroid volume, ranging from 65% to 84%, have been reported in numerous follow-up studies, with significant decreases in bleeding and pain in 78% to 88% of patients.23,31-33 Additionally, a large secondary analysis of a TC-RFA clinical trial showed that patients did not have any significant decrease in uterine wall thickness or integrity on follow-up with magnetic resonance imaging compared with baseline measurements, and they did not have any new myometrial scars (assessed as nonperfused linear areas).22
As with other ablation techniques, most data on RFA pregnancy outcomes come from case series, and further research and evaluation are needed. Existing studies, however, have demonstrated promising aspects of RFA that argue its usefulness in women with fertility plans.
A prospective trial that evaluated intrauterine adhesion formation with use of a TC-RFA device found no new adhesions on 6-week follow-up hysteroscopy compared with baseline pre-RFA hysteroscopy.34 Because intrauterine adhesion formation and uterine rupture are both significant concerns with other uterine-sparing fibroid treatment approaches such as myomectomy, these findings suggest that RFA may be a better alternative for women who are planning future pregnancies, as they may have increased fertility success and decreased catastrophic complications.
The consensus is growing that RFA is a safe and effective option for women who desire minimally invasive fibroid treatment and want to preserve fertility.
Unique benefits of RFA
In this article, we highlight RFA as an emerging treatment option for fibroid management, particularly for women who desire a uterine-sparing approach to preserve their reproductive options. Although myomectomy has been the standard of care for many years, with UAE as the alternative nonsurgical treatment, neither approach provides the best balance between symptomatic improvement and reproductive outcomes, and neither is without pregnancy risks. In addition, many women with symptomatic fibroids do not desire future conception but decline fibroid removal for religious or personal reasons. RFA offers these women an alternative minimally invasive option for uterine-sparing fibroid treatment.
RFA presents a unique “incision-free” fibroid treatment that is truly minimally invasive. This technique minimizes the risks associated with myomectomy, such as intra-abdominal adhesions, intrauterine adhesions (Asherman syndrome), need for cesarean delivery, and pregnancy complications such as uterine rupture or invasive placentation. Furthermore, the evolution of an RFA transcervical approach has enabled treatment with no abdominal or uterine incisions, thus offering all the above reproductive benefits as well as the operative benefits of a faster recovery, less pain, and less risk of intraperitoneal surgical complications.
While many women desire uterine-sparing fibroid treatment even without future fertility plans, the larger question is whether we should treat fibroids more strategically for women who desire future fertility. Myomectomy and UAE are effective and reliable in terms of fibroid symptomatic improvement, but RFA promises more beneficial reproductive outcomes. The ability to avoid uterine myometrial incisions and still attain significant symptomatic improvement should be prioritized in these patients.
Currently, RFA is not approved by the FDA as a fertility-enabling treatment, and these patients have been largely excluded from RFA studies. However, the reproductive-age patient who desires future conception may benefit most from RFA. Furthermore, RFA technology also could address the gap in uterine-sparing treatment for reproductive-age women with adenomyosis. Although a complete review of adenomyosis treatment is beyond the scope of this article, recent studies show that RFA produces similar improvement in both uterine volume and symptom severity in women with adenomyosis.35-37 ●
The RFA data suggest that both laparoscopic and transcervical RFA offer a safe and effective alternative treatment option for patients with symptomatic fibroids who seek uterine-sparing treatment, and transcervical RFA offers the least invasive treatment option. Women with fibroids who wish to conceive currently face a challenging treatment gap in clinical medicine, and future research is needed to address this concern in these patients. RFA is promising and appears to be a better fertility-enabling conservative fibroid treatment than the current options of myomectomy or UAE.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;CD005073.
- Paul GP, Naik SA, Madhu KN, et al. Complications of laparoscopic myomectomy: a single surgeon’s series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385-390.
- Flyckt R, Coyne K, Falcone T. Minimally invasive myomectomy. Clin Obstet Gynecol. 2017;60:252-272.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485-493.
- Broder MS, Goodwin S, Chen G, et al. Comparison of longterm outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100(5 pt 1):864-868.
- Torng PL. Adhesion prevention in laparoscopic myomectomy. Gynecol Minim Invasive Ther. 2014;3:7-11.
- Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190-197.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online. 2020;40:429-444.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
- Goodwin SC, Bradley LD, Lipman JC, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril. 2006;85:14-21
- Jia JB, Nguyen ET, Ravilla A, et al. Comparison of uterine artery embolization and myomectomy: a long-term analysis of 863 patients. Am J Interv Radiol. 2020;5:1.
- Huang JY, Kafy S, Dugas A, et al. Failure of uterine fibroid embolization. Fertil Steril. 2006;85:30-35.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5-13.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771-777.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317-1328.
- Rabinovici J, David M, Fukunishi H, et al; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199-209.
- Anneveldt KJ, Oever HJV, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Bongers M, Gupta J, Garza-Leal JG, et al. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35:299-303.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020;36:228-233.
- Guido RS, Macer JA, Abbott K, et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;11:139.
- Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19-23.
- Cope AG, Young RJ, Stewart EA. Non-extirpative treatments for uterine myomas: measuring success. J Minim Invasive Gynecol. 2021;28:442-452.e4.
- Berman JM, Shashoua A, Olson C, et al. Case series of reproductive outcomes after laparoscopic radiofrequency ablation of symptomatic myomas. J Minim Invasive Gynecol. 2020;27:639-645.
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012:194839.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768-773.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21:2081-2085.
- Szydłowska I, Starczewski A. Laparoscopic coagulation of uterine myomas with the use of a unipolar electrode. Surg Laparosc Endosc Percutan Tech. 2007;17:99-103.
- Bongers M, Quinn SD, Mueller MD et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122-125.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90:201601132.
- Polin M, Krenitsky N, Hur HC. Transcervical radiofrequency ablation for symptomatic adenomyosis: a case report. J Minim Invasive Gyn. 2021;28:S152-S153.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19:e2015.00071.

Uterine fibroids are a common condition that affects up to 80% of reproductive-age women.1 Many women with fibroids are asymptomatic, but some experience symptoms that profoundly disrupt their lives, such as abnormal uterine bleeding, pelvic pain, and bulk symptoms including bladder and bowel dysfunction.2 Although hysterectomy remains the definitive treatment for symptomatic fibroids, many women seek more conservative management. Hormonal treatment, such as contraceptive pills, levonorgestrel intrauterine devices, and gonadotropin-releasing hormone analogs, can improve heavy menstrual bleeding and anemia.3 Additionally, uterine artery embolization is a nonsurgical uterine-sparing option. However, these treatments are not ideal options for women who want to conceive.4 For reproductive-age women who desire future fertility, myomectomy has been the standard of care. Unfortunately, by the time patients become symptomatic from their fibroids and seek care, they may have numerous and/or sizable fibroids that result in high blood loss, surgical scarring, and the probable need for cesarean delivery (FIGURES 1 and 2).5


For patients who desire future conception, treatment of uterine fibroids poses a challenge in which optimizing symptomatic improvement must be balanced with protecting fertility and improving reproductive outcomes. In recent years, high-intensity focused ultrasound (FUS) and radiofrequency ablation (RFA) have been presented as less invasive, uterine-sparing alternatives for fibroid treatment that could potentially provide that balance.
In this article, we briefly review the available uterine-sparing fibroid treatments and their outcomes and then focus specifically on RFA as a possible option to address the fibroid treatment gap for reproductive-age women who desire future fertility.
Overview of uterine-sparing treatments
Two approaches can be pursued for conservative fibroid treatment: fibroid removal and fibroid necrosis (TABLE 1). We focus this review on outcomes for the most widely available of these treatments.

Myomectomy
For reproductive-age women who wish to conceive, surgical removal of fibroids has been the standard of care for symptomatic patients. Myomectomy can be performed via laparotomy, laparoscopy, robot-assisted surgery, and hysteroscopy. The mode of surgery depends on the fibroid characteristics (size, number, and location) and the surgeon’s skill set. Although some variation in the data exists, overall surgical outcomes, including blood loss, postoperative pain, and length of stay, are generally more favorable for minimally invasive approaches compared with laparotomy, with no significant differences in fibroid recurrence or reproductive outcomes (live birth rate, miscarriage rate, and cesarean delivery rate).6 This comes at the expense of longer operating time compared with laparotomy.7
While improvement in abnormal uterine bleeding and pelvic pain is reliable and usually significant after myomectomy,8 reproductive implications also warrant consideration. Myomectomy is associated with subsequent uterine adhesion formation, with some studies finding rates up to 83% to 94% depending on the surgical approach and the number of fibroids removed.9 These adhesions can impair fertility success.10 Myomectomy also is associated with high rates of cesarean delivery,5 invasive placentation (including placenta accreta spectrum),11 and uterine rupture.12 While the latter 2 complications are rare, they potentially can be catastrophic and should be kept in mind.
Continue to: Uterine artery embolization...
Uterine artery embolization
As a nonsurgical alternative to myomectomy, uterine artery embolization (UAE) has gained popularity as a conservative fibroid treatment since it was introduced in 1995. It is less invasive than myomectomy, a benefit for patients who decline surgery or are not ideal candidates for surgery.13 Evidence suggests that UAE produces overall comparable symptomatic improvement compared with myomectomy. One study showed no significant differences between UAE and myomectomy in terms of decreased uterine volume and menstrual bleeding at 6-month follow-up.14 In terms of long-term outcomes, a large multicenter study showed no significant difference in reintervention rates at 7 years posttreatment between UAE and myomectomy (8.9% vs 11.2%, respectively), and a significantly higher rate of improved menstrual bleeding with UAE (79.4% vs 49.5%), with no significant difference in bulk symptoms.15 The evidence is not entirely consistent, as other studies have shown increased rates of reintervention with UAE,8,16 but overall UAE can be considered a reasonable alternative to myomectomy in terms of symptomatic improvement.
Pregnancy outcomes data, however, are mixed, and UAE often is not recommended for patients with future fertility plans. In a large review article that compared minimally invasive fibroid treatments, UAE was associated with a lower live birth rate compared with myomectomy and ablation techniques (60.6% for UAE, 75.6% for myomectomy, and 70.5% for ablation), and it also had the highest rate of miscarriage (27.4% for UAE vs 19.0% for myomectomy and 11.9% for ablation) and abnormal placentation.12 While UAE remains an effective option for conservative treatment of symptomatic fibroids, it appears to have a worse impact on reproductive outcomes compared with myomectomy or ablative treatments.
Magnetic resonance–guided focused ultrasound
Emerging as a noninvasive ablation treatment for fibroids, magnetic resonance–guided focused ultrasound (MRgFUS) uses targeted high-intensity ultrasound pulses to cause thermal and mechanical fibroid tissue disruption.17 Data on this treatment are less robust given that it is newer than myomectomy or UAE. One study showed a decrease in fibroid volume by 12% at 1 month and 15% at 6 months, with 37.1% of patients reporting marked improvement in symptoms and an additional 31.4% reporting partial improvement; these are modest numbers compared with other treatment approaches.18 Another study showed more favorable outcomes, with 74% of patients reporting clinically significant improvement in bleeding and pain, and a 12.7% reintervention rate, comparable to rates reported for UAE and myomectomy.19
Because MRgFUS is newer than UAE or myomectomy, data are limited in terms of pregnancy outcomes, particularly because initial trials excluded women with future fertility plans due to lack of knowledge regarding pregnancy safety. A follow-up case series from one of the initial studies showed a decreased miscarriage rate compared with UAE, a term delivery rate of 93%, and a similar rate of abnormal placentation.20 A more recent systematic review concluded that reproductive outcomes were noninferior to myomectomy; however, the outcomes data for MRgFUS were heterogenous and many studies did not report pregnancy rates.21
Overall, MRgFUS appears to be an effective alternative approach for symptomatic fibroids, but the long-term data are not yet conclusive and information on pregnancy safety and outcomes largely is lacking. Recent reviews have not made definitive statements on whether MRgFUS should be offered to patients desiring future fertility.
Continue to: RFA is a promising option...
RFA is a promising option
RFA is another noninvasive fibroid ablation technique that has become more widely adopted in recent years. Here, we describe the basics of RFA and its impact on fibroid symptoms and reproductive outcomes.
The RFA technique
RFA uses hyperthermic energy from a handpiece and real-time ultrasound for targeted coagulative necrosis via a laparoscopic (L-RFA) or transcervical (TC-RFA) approach.22 A comparison between the 2 devices available on the market in the United States is shown in TABLE 2. Ultrasound guidance allows placement of radiofrequency needles directly into the fibroid to target local treatment to the fibroid tissue only. Once the fibroid undergoes coagulative necrosis, the process of fibroid resorption and volume reduction occurs over weeks to months, depending on the fibroid size.

Impact on fibroid symptoms
Both laparoscopic and transcervical RFA approaches have shown significant decreases in pelvic pain and heavy menstrual bleeding associated with fibroids and a low reintervention rate that emphasizes the durability of their impact.
A feasibility and safety study of a TC-RFA device prior to the primary clinical trials found only a 4.3% reintervention rate in the first 18 months postprocedure.23 The pivotal clinical trial of a TC-RFA device that followed also reported a low 5.5% reintervention rate in the first 24 months postprocedure, with significant improvement in health-related quality-of-life and high patient satisfaction24 (results shown in TABLE 2, along with trial results for an L-RFA device). A subsequent study of TC-RFA reported that symptomatic improvement persisted at 3-year follow-up, with a 9.2% reintervention rate comparable to existing fibroid treatments such as myomectomy and UAE.25 The original L-RFA trial also has shown similar positive results at 2-year follow-up, with a low reintervention rate of 4.8% after treatment, and similar patient satisfaction and quality-of-life improvements as TC-RFA.26 While long-term data are limited by only recent approval by the Food and Drug Administration (FDA) of a TC-RFA device in 2018, one study followed clinical trial patients for a mean duration of 64 months. This study found no surgical reinterventions in the first 3.5 years posttreatment and a persistent reduction in fibroid symptoms from baseline 64.9 points to 27.6 points, as assessed by a validated symptom severity scale (out of 100 points).27 Similar improvements in health-related quality-of life-were also found to persist for years posttreatment.4
In a large systematic review that compared L-RFA, MRgFUS, UAE, and myomectomy, L-RFA had similar improvement rates in quality-of-life and symptom severity scores compared with myomectomy, with no significant difference in reintervention rates.28 This review also noted minimal heterogeneity among RFA meta-analyses data in contrast to significant heterogeneity among UAE and myomectomy data.
Reproductive outcomes
Similar to MRgFUS, the initial studies of RFA devices largely excluded women with future fertility plans, as data on safety were lacking. However, many RFA devices are now on the market across the globe, and subsequent pregnancies have been tracked and reported.
A large case series that included clinical trials and commercial settings reported a miscarriage rate (13.3%) similar to that of the general obstetric population and no cases of uterine rupture, invasive placentation, preterm delivery, or placental abruption.29 Other case series have reported live birth rates similar those with myomectomy, and safe and favorable pregnancy outcomes with RFA have been supported by larger systematic reviews of all ablation techniques.12
Continue to: Uterine impact...
Uterine impact
One study of TC-RFA patients showed a greater than 65% reduction in fibroid volume (with a 90% reduction in fibroid volume for fibroids larger than 6 cm prior to RFA), and 54% of patients reported complete resolution of symptoms, with another 36% reporting decreased symptoms.30 Similar decreases in fibroid volume, ranging from 65% to 84%, have been reported in numerous follow-up studies, with significant decreases in bleeding and pain in 78% to 88% of patients.23,31-33 Additionally, a large secondary analysis of a TC-RFA clinical trial showed that patients did not have any significant decrease in uterine wall thickness or integrity on follow-up with magnetic resonance imaging compared with baseline measurements, and they did not have any new myometrial scars (assessed as nonperfused linear areas).22
As with other ablation techniques, most data on RFA pregnancy outcomes come from case series, and further research and evaluation are needed. Existing studies, however, have demonstrated promising aspects of RFA that argue its usefulness in women with fertility plans.
A prospective trial that evaluated intrauterine adhesion formation with use of a TC-RFA device found no new adhesions on 6-week follow-up hysteroscopy compared with baseline pre-RFA hysteroscopy.34 Because intrauterine adhesion formation and uterine rupture are both significant concerns with other uterine-sparing fibroid treatment approaches such as myomectomy, these findings suggest that RFA may be a better alternative for women who are planning future pregnancies, as they may have increased fertility success and decreased catastrophic complications.
The consensus is growing that RFA is a safe and effective option for women who desire minimally invasive fibroid treatment and want to preserve fertility.
Unique benefits of RFA
In this article, we highlight RFA as an emerging treatment option for fibroid management, particularly for women who desire a uterine-sparing approach to preserve their reproductive options. Although myomectomy has been the standard of care for many years, with UAE as the alternative nonsurgical treatment, neither approach provides the best balance between symptomatic improvement and reproductive outcomes, and neither is without pregnancy risks. In addition, many women with symptomatic fibroids do not desire future conception but decline fibroid removal for religious or personal reasons. RFA offers these women an alternative minimally invasive option for uterine-sparing fibroid treatment.
RFA presents a unique “incision-free” fibroid treatment that is truly minimally invasive. This technique minimizes the risks associated with myomectomy, such as intra-abdominal adhesions, intrauterine adhesions (Asherman syndrome), need for cesarean delivery, and pregnancy complications such as uterine rupture or invasive placentation. Furthermore, the evolution of an RFA transcervical approach has enabled treatment with no abdominal or uterine incisions, thus offering all the above reproductive benefits as well as the operative benefits of a faster recovery, less pain, and less risk of intraperitoneal surgical complications.
While many women desire uterine-sparing fibroid treatment even without future fertility plans, the larger question is whether we should treat fibroids more strategically for women who desire future fertility. Myomectomy and UAE are effective and reliable in terms of fibroid symptomatic improvement, but RFA promises more beneficial reproductive outcomes. The ability to avoid uterine myometrial incisions and still attain significant symptomatic improvement should be prioritized in these patients.
Currently, RFA is not approved by the FDA as a fertility-enabling treatment, and these patients have been largely excluded from RFA studies. However, the reproductive-age patient who desires future conception may benefit most from RFA. Furthermore, RFA technology also could address the gap in uterine-sparing treatment for reproductive-age women with adenomyosis. Although a complete review of adenomyosis treatment is beyond the scope of this article, recent studies show that RFA produces similar improvement in both uterine volume and symptom severity in women with adenomyosis.35-37 ●
The RFA data suggest that both laparoscopic and transcervical RFA offer a safe and effective alternative treatment option for patients with symptomatic fibroids who seek uterine-sparing treatment, and transcervical RFA offers the least invasive treatment option. Women with fibroids who wish to conceive currently face a challenging treatment gap in clinical medicine, and future research is needed to address this concern in these patients. RFA is promising and appears to be a better fertility-enabling conservative fibroid treatment than the current options of myomectomy or UAE.

Uterine fibroids are a common condition that affects up to 80% of reproductive-age women.1 Many women with fibroids are asymptomatic, but some experience symptoms that profoundly disrupt their lives, such as abnormal uterine bleeding, pelvic pain, and bulk symptoms including bladder and bowel dysfunction.2 Although hysterectomy remains the definitive treatment for symptomatic fibroids, many women seek more conservative management. Hormonal treatment, such as contraceptive pills, levonorgestrel intrauterine devices, and gonadotropin-releasing hormone analogs, can improve heavy menstrual bleeding and anemia.3 Additionally, uterine artery embolization is a nonsurgical uterine-sparing option. However, these treatments are not ideal options for women who want to conceive.4 For reproductive-age women who desire future fertility, myomectomy has been the standard of care. Unfortunately, by the time patients become symptomatic from their fibroids and seek care, they may have numerous and/or sizable fibroids that result in high blood loss, surgical scarring, and the probable need for cesarean delivery (FIGURES 1 and 2).5


For patients who desire future conception, treatment of uterine fibroids poses a challenge in which optimizing symptomatic improvement must be balanced with protecting fertility and improving reproductive outcomes. In recent years, high-intensity focused ultrasound (FUS) and radiofrequency ablation (RFA) have been presented as less invasive, uterine-sparing alternatives for fibroid treatment that could potentially provide that balance.
In this article, we briefly review the available uterine-sparing fibroid treatments and their outcomes and then focus specifically on RFA as a possible option to address the fibroid treatment gap for reproductive-age women who desire future fertility.
Overview of uterine-sparing treatments
Two approaches can be pursued for conservative fibroid treatment: fibroid removal and fibroid necrosis (TABLE 1). We focus this review on outcomes for the most widely available of these treatments.

Myomectomy
For reproductive-age women who wish to conceive, surgical removal of fibroids has been the standard of care for symptomatic patients. Myomectomy can be performed via laparotomy, laparoscopy, robot-assisted surgery, and hysteroscopy. The mode of surgery depends on the fibroid characteristics (size, number, and location) and the surgeon’s skill set. Although some variation in the data exists, overall surgical outcomes, including blood loss, postoperative pain, and length of stay, are generally more favorable for minimally invasive approaches compared with laparotomy, with no significant differences in fibroid recurrence or reproductive outcomes (live birth rate, miscarriage rate, and cesarean delivery rate).6 This comes at the expense of longer operating time compared with laparotomy.7
While improvement in abnormal uterine bleeding and pelvic pain is reliable and usually significant after myomectomy,8 reproductive implications also warrant consideration. Myomectomy is associated with subsequent uterine adhesion formation, with some studies finding rates up to 83% to 94% depending on the surgical approach and the number of fibroids removed.9 These adhesions can impair fertility success.10 Myomectomy also is associated with high rates of cesarean delivery,5 invasive placentation (including placenta accreta spectrum),11 and uterine rupture.12 While the latter 2 complications are rare, they potentially can be catastrophic and should be kept in mind.
Continue to: Uterine artery embolization...
Uterine artery embolization
As a nonsurgical alternative to myomectomy, uterine artery embolization (UAE) has gained popularity as a conservative fibroid treatment since it was introduced in 1995. It is less invasive than myomectomy, a benefit for patients who decline surgery or are not ideal candidates for surgery.13 Evidence suggests that UAE produces overall comparable symptomatic improvement compared with myomectomy. One study showed no significant differences between UAE and myomectomy in terms of decreased uterine volume and menstrual bleeding at 6-month follow-up.14 In terms of long-term outcomes, a large multicenter study showed no significant difference in reintervention rates at 7 years posttreatment between UAE and myomectomy (8.9% vs 11.2%, respectively), and a significantly higher rate of improved menstrual bleeding with UAE (79.4% vs 49.5%), with no significant difference in bulk symptoms.15 The evidence is not entirely consistent, as other studies have shown increased rates of reintervention with UAE,8,16 but overall UAE can be considered a reasonable alternative to myomectomy in terms of symptomatic improvement.
Pregnancy outcomes data, however, are mixed, and UAE often is not recommended for patients with future fertility plans. In a large review article that compared minimally invasive fibroid treatments, UAE was associated with a lower live birth rate compared with myomectomy and ablation techniques (60.6% for UAE, 75.6% for myomectomy, and 70.5% for ablation), and it also had the highest rate of miscarriage (27.4% for UAE vs 19.0% for myomectomy and 11.9% for ablation) and abnormal placentation.12 While UAE remains an effective option for conservative treatment of symptomatic fibroids, it appears to have a worse impact on reproductive outcomes compared with myomectomy or ablative treatments.
Magnetic resonance–guided focused ultrasound
Emerging as a noninvasive ablation treatment for fibroids, magnetic resonance–guided focused ultrasound (MRgFUS) uses targeted high-intensity ultrasound pulses to cause thermal and mechanical fibroid tissue disruption.17 Data on this treatment are less robust given that it is newer than myomectomy or UAE. One study showed a decrease in fibroid volume by 12% at 1 month and 15% at 6 months, with 37.1% of patients reporting marked improvement in symptoms and an additional 31.4% reporting partial improvement; these are modest numbers compared with other treatment approaches.18 Another study showed more favorable outcomes, with 74% of patients reporting clinically significant improvement in bleeding and pain, and a 12.7% reintervention rate, comparable to rates reported for UAE and myomectomy.19
Because MRgFUS is newer than UAE or myomectomy, data are limited in terms of pregnancy outcomes, particularly because initial trials excluded women with future fertility plans due to lack of knowledge regarding pregnancy safety. A follow-up case series from one of the initial studies showed a decreased miscarriage rate compared with UAE, a term delivery rate of 93%, and a similar rate of abnormal placentation.20 A more recent systematic review concluded that reproductive outcomes were noninferior to myomectomy; however, the outcomes data for MRgFUS were heterogenous and many studies did not report pregnancy rates.21
Overall, MRgFUS appears to be an effective alternative approach for symptomatic fibroids, but the long-term data are not yet conclusive and information on pregnancy safety and outcomes largely is lacking. Recent reviews have not made definitive statements on whether MRgFUS should be offered to patients desiring future fertility.
Continue to: RFA is a promising option...
RFA is a promising option
RFA is another noninvasive fibroid ablation technique that has become more widely adopted in recent years. Here, we describe the basics of RFA and its impact on fibroid symptoms and reproductive outcomes.
The RFA technique
RFA uses hyperthermic energy from a handpiece and real-time ultrasound for targeted coagulative necrosis via a laparoscopic (L-RFA) or transcervical (TC-RFA) approach.22 A comparison between the 2 devices available on the market in the United States is shown in TABLE 2. Ultrasound guidance allows placement of radiofrequency needles directly into the fibroid to target local treatment to the fibroid tissue only. Once the fibroid undergoes coagulative necrosis, the process of fibroid resorption and volume reduction occurs over weeks to months, depending on the fibroid size.

Impact on fibroid symptoms
Both laparoscopic and transcervical RFA approaches have shown significant decreases in pelvic pain and heavy menstrual bleeding associated with fibroids and a low reintervention rate that emphasizes the durability of their impact.
A feasibility and safety study of a TC-RFA device prior to the primary clinical trials found only a 4.3% reintervention rate in the first 18 months postprocedure.23 The pivotal clinical trial of a TC-RFA device that followed also reported a low 5.5% reintervention rate in the first 24 months postprocedure, with significant improvement in health-related quality-of-life and high patient satisfaction24 (results shown in TABLE 2, along with trial results for an L-RFA device). A subsequent study of TC-RFA reported that symptomatic improvement persisted at 3-year follow-up, with a 9.2% reintervention rate comparable to existing fibroid treatments such as myomectomy and UAE.25 The original L-RFA trial also has shown similar positive results at 2-year follow-up, with a low reintervention rate of 4.8% after treatment, and similar patient satisfaction and quality-of-life improvements as TC-RFA.26 While long-term data are limited by only recent approval by the Food and Drug Administration (FDA) of a TC-RFA device in 2018, one study followed clinical trial patients for a mean duration of 64 months. This study found no surgical reinterventions in the first 3.5 years posttreatment and a persistent reduction in fibroid symptoms from baseline 64.9 points to 27.6 points, as assessed by a validated symptom severity scale (out of 100 points).27 Similar improvements in health-related quality-of life-were also found to persist for years posttreatment.4
In a large systematic review that compared L-RFA, MRgFUS, UAE, and myomectomy, L-RFA had similar improvement rates in quality-of-life and symptom severity scores compared with myomectomy, with no significant difference in reintervention rates.28 This review also noted minimal heterogeneity among RFA meta-analyses data in contrast to significant heterogeneity among UAE and myomectomy data.
Reproductive outcomes
Similar to MRgFUS, the initial studies of RFA devices largely excluded women with future fertility plans, as data on safety were lacking. However, many RFA devices are now on the market across the globe, and subsequent pregnancies have been tracked and reported.
A large case series that included clinical trials and commercial settings reported a miscarriage rate (13.3%) similar to that of the general obstetric population and no cases of uterine rupture, invasive placentation, preterm delivery, or placental abruption.29 Other case series have reported live birth rates similar those with myomectomy, and safe and favorable pregnancy outcomes with RFA have been supported by larger systematic reviews of all ablation techniques.12
Continue to: Uterine impact...
Uterine impact
One study of TC-RFA patients showed a greater than 65% reduction in fibroid volume (with a 90% reduction in fibroid volume for fibroids larger than 6 cm prior to RFA), and 54% of patients reported complete resolution of symptoms, with another 36% reporting decreased symptoms.30 Similar decreases in fibroid volume, ranging from 65% to 84%, have been reported in numerous follow-up studies, with significant decreases in bleeding and pain in 78% to 88% of patients.23,31-33 Additionally, a large secondary analysis of a TC-RFA clinical trial showed that patients did not have any significant decrease in uterine wall thickness or integrity on follow-up with magnetic resonance imaging compared with baseline measurements, and they did not have any new myometrial scars (assessed as nonperfused linear areas).22
As with other ablation techniques, most data on RFA pregnancy outcomes come from case series, and further research and evaluation are needed. Existing studies, however, have demonstrated promising aspects of RFA that argue its usefulness in women with fertility plans.
A prospective trial that evaluated intrauterine adhesion formation with use of a TC-RFA device found no new adhesions on 6-week follow-up hysteroscopy compared with baseline pre-RFA hysteroscopy.34 Because intrauterine adhesion formation and uterine rupture are both significant concerns with other uterine-sparing fibroid treatment approaches such as myomectomy, these findings suggest that RFA may be a better alternative for women who are planning future pregnancies, as they may have increased fertility success and decreased catastrophic complications.
The consensus is growing that RFA is a safe and effective option for women who desire minimally invasive fibroid treatment and want to preserve fertility.
Unique benefits of RFA
In this article, we highlight RFA as an emerging treatment option for fibroid management, particularly for women who desire a uterine-sparing approach to preserve their reproductive options. Although myomectomy has been the standard of care for many years, with UAE as the alternative nonsurgical treatment, neither approach provides the best balance between symptomatic improvement and reproductive outcomes, and neither is without pregnancy risks. In addition, many women with symptomatic fibroids do not desire future conception but decline fibroid removal for religious or personal reasons. RFA offers these women an alternative minimally invasive option for uterine-sparing fibroid treatment.
RFA presents a unique “incision-free” fibroid treatment that is truly minimally invasive. This technique minimizes the risks associated with myomectomy, such as intra-abdominal adhesions, intrauterine adhesions (Asherman syndrome), need for cesarean delivery, and pregnancy complications such as uterine rupture or invasive placentation. Furthermore, the evolution of an RFA transcervical approach has enabled treatment with no abdominal or uterine incisions, thus offering all the above reproductive benefits as well as the operative benefits of a faster recovery, less pain, and less risk of intraperitoneal surgical complications.
While many women desire uterine-sparing fibroid treatment even without future fertility plans, the larger question is whether we should treat fibroids more strategically for women who desire future fertility. Myomectomy and UAE are effective and reliable in terms of fibroid symptomatic improvement, but RFA promises more beneficial reproductive outcomes. The ability to avoid uterine myometrial incisions and still attain significant symptomatic improvement should be prioritized in these patients.
Currently, RFA is not approved by the FDA as a fertility-enabling treatment, and these patients have been largely excluded from RFA studies. However, the reproductive-age patient who desires future conception may benefit most from RFA. Furthermore, RFA technology also could address the gap in uterine-sparing treatment for reproductive-age women with adenomyosis. Although a complete review of adenomyosis treatment is beyond the scope of this article, recent studies show that RFA produces similar improvement in both uterine volume and symptom severity in women with adenomyosis.35-37 ●
The RFA data suggest that both laparoscopic and transcervical RFA offer a safe and effective alternative treatment option for patients with symptomatic fibroids who seek uterine-sparing treatment, and transcervical RFA offers the least invasive treatment option. Women with fibroids who wish to conceive currently face a challenging treatment gap in clinical medicine, and future research is needed to address this concern in these patients. RFA is promising and appears to be a better fertility-enabling conservative fibroid treatment than the current options of myomectomy or UAE.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;CD005073.
- Paul GP, Naik SA, Madhu KN, et al. Complications of laparoscopic myomectomy: a single surgeon’s series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385-390.
- Flyckt R, Coyne K, Falcone T. Minimally invasive myomectomy. Clin Obstet Gynecol. 2017;60:252-272.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485-493.
- Broder MS, Goodwin S, Chen G, et al. Comparison of longterm outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100(5 pt 1):864-868.
- Torng PL. Adhesion prevention in laparoscopic myomectomy. Gynecol Minim Invasive Ther. 2014;3:7-11.
- Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190-197.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online. 2020;40:429-444.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
- Goodwin SC, Bradley LD, Lipman JC, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril. 2006;85:14-21
- Jia JB, Nguyen ET, Ravilla A, et al. Comparison of uterine artery embolization and myomectomy: a long-term analysis of 863 patients. Am J Interv Radiol. 2020;5:1.
- Huang JY, Kafy S, Dugas A, et al. Failure of uterine fibroid embolization. Fertil Steril. 2006;85:30-35.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5-13.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771-777.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317-1328.
- Rabinovici J, David M, Fukunishi H, et al; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199-209.
- Anneveldt KJ, Oever HJV, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Bongers M, Gupta J, Garza-Leal JG, et al. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35:299-303.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020;36:228-233.
- Guido RS, Macer JA, Abbott K, et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;11:139.
- Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19-23.
- Cope AG, Young RJ, Stewart EA. Non-extirpative treatments for uterine myomas: measuring success. J Minim Invasive Gynecol. 2021;28:442-452.e4.
- Berman JM, Shashoua A, Olson C, et al. Case series of reproductive outcomes after laparoscopic radiofrequency ablation of symptomatic myomas. J Minim Invasive Gynecol. 2020;27:639-645.
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012:194839.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768-773.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21:2081-2085.
- Szydłowska I, Starczewski A. Laparoscopic coagulation of uterine myomas with the use of a unipolar electrode. Surg Laparosc Endosc Percutan Tech. 2007;17:99-103.
- Bongers M, Quinn SD, Mueller MD et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122-125.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90:201601132.
- Polin M, Krenitsky N, Hur HC. Transcervical radiofrequency ablation for symptomatic adenomyosis: a case report. J Minim Invasive Gyn. 2021;28:S152-S153.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19:e2015.00071.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;CD005073.
- Paul GP, Naik SA, Madhu KN, et al. Complications of laparoscopic myomectomy: a single surgeon’s series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385-390.
- Flyckt R, Coyne K, Falcone T. Minimally invasive myomectomy. Clin Obstet Gynecol. 2017;60:252-272.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485-493.
- Broder MS, Goodwin S, Chen G, et al. Comparison of longterm outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100(5 pt 1):864-868.
- Torng PL. Adhesion prevention in laparoscopic myomectomy. Gynecol Minim Invasive Ther. 2014;3:7-11.
- Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190-197.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online. 2020;40:429-444.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
- Goodwin SC, Bradley LD, Lipman JC, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril. 2006;85:14-21
- Jia JB, Nguyen ET, Ravilla A, et al. Comparison of uterine artery embolization and myomectomy: a long-term analysis of 863 patients. Am J Interv Radiol. 2020;5:1.
- Huang JY, Kafy S, Dugas A, et al. Failure of uterine fibroid embolization. Fertil Steril. 2006;85:30-35.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5-13.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771-777.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317-1328.
- Rabinovici J, David M, Fukunishi H, et al; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199-209.
- Anneveldt KJ, Oever HJV, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Bongers M, Gupta J, Garza-Leal JG, et al. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35:299-303.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020;36:228-233.
- Guido RS, Macer JA, Abbott K, et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;11:139.
- Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19-23.
- Cope AG, Young RJ, Stewart EA. Non-extirpative treatments for uterine myomas: measuring success. J Minim Invasive Gynecol. 2021;28:442-452.e4.
- Berman JM, Shashoua A, Olson C, et al. Case series of reproductive outcomes after laparoscopic radiofrequency ablation of symptomatic myomas. J Minim Invasive Gynecol. 2020;27:639-645.
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012:194839.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768-773.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21:2081-2085.
- Szydłowska I, Starczewski A. Laparoscopic coagulation of uterine myomas with the use of a unipolar electrode. Surg Laparosc Endosc Percutan Tech. 2007;17:99-103.
- Bongers M, Quinn SD, Mueller MD et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122-125.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90:201601132.
- Polin M, Krenitsky N, Hur HC. Transcervical radiofrequency ablation for symptomatic adenomyosis: a case report. J Minim Invasive Gyn. 2021;28:S152-S153.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19:e2015.00071.
2021 update on contraception
A new contraceptive method should ideally provide improved access or a higher quality and safety option. Although unintended pregnancy rates in the United States are decreasing, significant disparities across race and socioeconomic status remain,1 and these disparities actually doubled from 1994 to 2011 even though the overall unintended pregnancy rate decreased.1-3 Specifically, people of color, those with lower income, and people with lower education levels had higher rates of unintended pregnancies than did White people with higher education and income, suggesting disparate access to contraception services.1 Thus, as new contraceptive methods are introduced, we must assess if they have the potential to address this disparity as well as continue to provide higher quality and safer options.
In this Update, we critically review the phase 3 data on efficacy and safety for 3 new methods that were introduced to the US market over the past year to evaluate their impact on the current contraceptive landscape.
The first method, newly approved by the US Food and Drug Administration (FDA), is a combined oral contraceptive (OC) that contains a novel endogenous estrogen, estetrol, or E4 (Nextstellis). E4 is a natural estrogen produced in the fetal liver that has lower potency and a longer half-life than estradiol. Nextstellis is a monophasic 24/4 OC pill that contains E4 14.2 mg and drospirenone 3 mg in each of the 24 hormone-containing pills. Most combined hormonal contraceptives (CHCs) in the United States today contain synthetically made ethinyl estradiol (EE) due to its high potency and oral bioavailability. Outside of the reproductive system, EE upregulates the production of hepatic proteins and alters procoagulant and anticoagulant factors, which results in an overall increase in venous thromboembolic (VTE) risk among CHC users.2
After widespread use of combined oral contraceptives (COCs) started in the 1960s, data emerged regarding increased VTE risk.3 Subsequent research discovered that the type of estrogen used in CHCs directly correlates with the thrombosis risk due to the hepatic upregulation with both first- and second-pass metabolism. Although this risk was reduced as the EE dose decreased below 50 µg and concurrent VTE risk factors were contraindicated, CHC users still faced a 2-fold increase in VTE risk compared with nonusers.4,5 EE in contraceptive formulations increases VTE risk, likely related to upregulation of procoagulant factors and decreasing anticoagulant proteins.2 By contrast, a phase 2 trial of Nextstellis demonstrated more neutral effects of E4/drospirenone on hemostatic parameters compared with EE/levonorgestrel or EE/drospirenone.6 Furthermore, E4/drospirenone exhibited lower increases in hepatic proteins, such as angiotensinogen, triglycerides, and sex-hormone binding globulin.7 These findings together suggest that this novel CHC pill has a more favorable cardiovascular adverse effect profile compared with currently available CHCs.
The second contraceptive method is a new transdermal patch that contains EE and levonorgestrel (Twirla); this is in contrast to the available EE/norelgestromin contraceptive patch (Xulane). Transdermal contraceptive patches can offer some users easier adherence as compared with a daily OC.8 Until this past year, the only transdermal contraceptive available in the United States was Xulane, which contains a daily dose of EE 35 µg and norelgestromin 150 µg. Norelgestromin is eventually metabolized to levonorgestrel derivatives.9 Twirla is administered in the same manner as Xulane and contains a daily hormone exposure equivalent to a COC containing EE 30 µg and levonorgestrel 120 µg. Similar to EE/norelgestromin, the EE/levonorgestrel patch also is contraindicated in obese patients (body mass index [BMI] ≥30 kg/m2) due to decreased efficacy and increased risk for VTE. Additionally, phase 3 data demonstrated decreasing efficacy of Twirla in overweight users (BMI ≥25–30 kg/m2), perhaps further limiting the population that may benefit from this contraceptive method.10 These issues with efficacy and weight likely are related to the fact that levonorgestrel distribution is weight dependent, with evidence of lower plasma levels in obese individuals.11-13
The third new method is a prescription vaginal contraceptive gel with lactic acid, citric acid, and potassium bitartrate (Phexxi) designed to prevent pregnancy by maintaining an acidic vaginal environment that is inhospitable to sperm. For many decades, vaginal contraceptives, including vaginal spermicidal gels, provided easy access to a nonhormonal and flexible method of moderately effective contraception for many users. Phexxi is a prescription vaginal pH regulator administered as a gel and active for 1 hour after application. All previous vaginal gels sold in the United States are applied similarly, are available over the counter, and include nonoxynol-9, which is a surfactant that damages sperm cell membranes. Recent data from a phase 3 trial demonstrated similar contraceptive effectiveness of Phexxi when compared with available nonoxynol-9 alternatives.14
Continue to: New OC with the novel estrogen E4 demonstrates good safety profile for VTE...
New OC with the novel estrogen E4 demonstrates good safety profile for VTE
Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
The COC E4/drospirenone was evaluated in 2 parallel multinational studies. Here, we review the North American data that are more relevant for the US population; the European-Russian data also are published.15
Study examined 1 year’s use of E4/drospirenone
The US–Canadian trial conducted by Creinin and colleagues enrolled 1,864 participants aged 16 to 50 years to evaluate contraceptive efficacy, bleeding patterns, and adverse events with 1-year use (13 cycles) of E4/drospirenone. The primary efficacy group included 1,524 women aged 16 to 35. This study enrolled healthy, heterosexually active participants with a BMI ≤35 kg/m2 and regular menses from 70 sites in the United States and 7 sites in Canada. The dropout rate was 45%, comparable to that in other contraceptive studies. Participants used E4/drospirenone cyclically, taking 1 hormone-containing pill daily for 24 days followed by 4 days of placebo pills.
Contraceptive efficacious, no VTE observed
The researchers reported efficacy as a Pearl Index (PI) of 2.65 pregnancies per 100 woman-years in participants aged 16 to 35 and an overall 13-cycle life-table pregnancy rate of 2.06%. The PI did not differ among nonobese and obese participants in multivariable analysis. Most users experienced scheduled withdrawal bleeding; only 13% to 18% reported absence of scheduled bleeding. Unscheduled bleeding was typically spotting (55.2%), and this decreased with treatment duration from 30% in cycle 1 to 15% to 20% in cycle 5 and on.
Overall, 28.9% of participants reported treatment-related adverse events (AEs), which most commonly were headache (5.0%), metrorrhagia (4.6%), and nausea (3.8%). Investigators reported a minimal change in mean (SD) BMI of 0.4 (1.7) kg/m2 from baseline after 1 year of E4/drospirenone use, and only 0.5% of participants discontinued use due to weight gain. The most common reasons for AE-related treatment discontinuation included metrorrhagia (0.9%), menorrhagia (0.8%), and vaginal hemorrhage (0.5%). Importantly, no cases of VTE occurred in this study of estetrol despite 23% of participants being obese, a known risk factor for VTE.
Nextstellis provides safe, effective contraception with a PI comparable to that of other available CHCs as well as a favorable bleeding profile in healthy users who are adherent to treatment. Importantly, contraceptive efficacy was maintained in obese users with a BMI up to 35 kg/m2. In contrast to EE or estradiol, E4 demonstrates a lower impact on the hepatic system, and preliminary findings suggest a lower VTE risk compared with other CHCs on the market. The European phase 3 trial of 1,553 participants also demonstrated a low rate of VTE, with only 1 case diagnosed.15 By contrast, similar phase 3 trials of available CHCs demonstrated more frequent VTE events despite low-dose EE formulations (TABLE 1).10,15-18 In general, most US phase 3 trials have 3 to 4 VTE events in the studied population, and the Nextstellis North American trial, of which 92% of participants were from the United States, had 0. However, confirmation of any potential lower VTE risk requires further analysis from large, population-based postmarketing studies.
Continue to: Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women...
Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women
Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
To assess the contraceptive efficacy, tolerability, and safety of the transdermal patch Twirla (EE/levonorgestrel) over 1 year of treatment (13 cycles), Nelson and colleagues conducted an open-label, multicenter, US-based phase 3 trial of participants aged 18 years and older with regular cycles. There were no restrictions based on BMI. On average, the study population was overweight, with a mean BMI of 28.3 kg/m2 , and 35% of the population was considered obese (BMI ≥30 kg/m2).
Study design
A total of 2,032 participants enrolled in the study, with separate populations defined for specific analysis on safety, contraceptive efficacy, and cycle control. The primary efficacy group included 1,736 participants. Fifty-one percent discontinued the study, most commonly due to “women’s decision” (15%) and lost to follow-up (11%). Users received bleeding diaries and returned periodically throughout the study for evaluation for efficacy, adherence, and adverse events.
Efficacy associated with BMI
The study results demonstrated an overall PI of 5.8 pregnancies per 100 woman-years for users aged younger than 35. TABLE 2 demonstrates the overall trend of efficacy in relation to BMI.10,15-19 Participants with a higher BMI were found to have a higher PI, revealing lower contraceptive efficacy in more overweight and obese patients. The overall cumulative pregnancy rate over 13 cycles was 5.3%
Participants reported decreasing frequency of bleeding/spotting days over the treatment duration of 13 cycles, from a mean (SD) of 6.2 (4.5) days in cycle 1 to 4.9 (3.5) days in cycle 13. Unscheduled bleeding episodes remained high throughout the study period. Initially, 60% of users reported 1 or more days of unscheduled bleeding in cycle 1, and 42% still reported unscheduled bleeding in cycle 13. In light of this, only 45 participants (2.2%) discontinued the study due to bleeding issues, suggesting perhaps that the bleeding was light. Overall, users experienced acceptable wearability of the patch, and the rate of detachment decreased over the study period from 9.9% in cycle 1 to 2.4% in cycle 13. There were also low rates (0.5%) of moderate to severe irritation. Itching at the adhesion site decreased slightly from 13.1% in cycle 2 to 9.6% in cycle 13.
In general, 27.2% of patch users experienced a study-related AE, most reported as mild to moderate. Nausea (4.1%) and headaches (3.6%) were the most common hormone-related AE. Importantly, 4 obese users experienced 5 VTEs (deep vein thrombosis, n = 2; pulmonary embolism, n = 3) between cycle 5 and 13. Three of these users had additional VTE risk factors, such as air travel and a family history of clots. No users who were of normal weight or overweight experienced VTE.
Available data demonstrate that the EE/norelgestromin patch exposes users to higher serum levels compared with the pill or the ring.20 The higher estrogen exposure with the patch may explain higher estrogen-related adverse effects and may result in increased VTE risk. Initial pharmacokinetic data of the EE/levonorgestrel patch showed lower EE concentrations, similar to marketed COCs and lower than EE/norelgestromin.21 Despite this lower estrogen exposure, the phase 3 trial by Nelson and colleagues did not demonstrate a safer profile with respect to thromboembolic events.
Further, the high PI of 5.8 pregnancies per 100 woman-years calls into question the efficacy of this patch compared with already available CHC options. Indeed, the efficacy appears reasonable in normal-weight individuals, with a PI of 3.5 pregnancies per 100 woman-years; however, this is still higher than its contemporary counterpart, Nextstellis, which has a PI of 2.65 pregnancies per 100 woman-years and included users with a BMI of up to 35 kg/m2 (Table 2). Given the evidence of decreased efficacy, clinicians may consider reserving this option for only normal-weight women who cannot use or prefer not to use another CHC method. Obese individuals (BMI ≥30 kg/m2 ) should not use this patch due to decreased efficacy and increased VTE risk. Lastly, although use in overweight individuals (BMI ≥25 kg/m2) is not absolutely contraindicated, clinicians should counsel the overweight patient on the possibility of decreased contraceptive efficacy due to weight, and they may choose to reserve use of this patch in overweight individuals only when no other comparable or more effective method is an option.
Continue to: Novel vaginal pH buffering spermicide is a new Rx-only option...
Novel vaginal pH buffering spermicide is a new Rx-only option
Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
In an open-label phase 3 study, Thomas and colleagues enrolled 1,384 participants aged 18 to 35 with regular cycles at 112 sites in the United States to assess the contraceptive efficacy, safety, and acceptability of Phexxi vaginal gel (lactic acid, citric acid, and potassium bitartrate) over 7 cycles (6 months). Participants were required to have at least 3 episodes of heterosexual vaginal intercourse per cycle and return throughout the treatment duration for study visits. Fifty-three percent of participants did not complete the study, most frequently due to loss to follow-up (18.1%) and participant withdrawal (12.3%). Most participants were White (69%) and had an average (SD) age of 27.7 (4.5) years.
Efficacy and AE rates
The investigators reported a cumulative pregnancy rate of 13.7% over 7 cycles (6 months). In this study, 45.2% of women experienced 1 AE, and most were noted to be mild (23.9%) to moderate (18.7%). The most reported AE was vulvovaginal burning (20.0%), followed by vulvovaginal pruritus (11.2%), urinary tract infection (5.7%), and vulvovaginal pain (3.8%). Less than 2% of participants discontinued the study due to an AE. Burning and itching decreased with time and with decreased frequency of use. When used twice per day compared with once per day, burning rates decreased from 4.6% to 2.1%, and itching rates decreased from 1.0% to 0.7%. Serious AEs were uncommon, occurring in 1.3% of users; only 1, cystitis, was noted to be “probably” related to the treatment. ●
Prior to the approval of Phexxi, all currently available vaginal contraceptive gels in the United States contained nonoxynol-9 as the active ingredient, which is a surfactant that is spermicidal by damaging cell membranes. Although Phexxi provides a novel mechanism of action as a spermicide, the contraceptive efficacy is about the same as available spermicides on the market (see TABLE 3).14,22,23 The FDA calculated a 13-cycle PI to include in the label (27.5 pregnancies per 100 woman-years) based on the results of this study; however, no reliable statistical method exists to calculate a true PI from a 7-cycle study. Thus, we recommend that clinicians counsel patients appropriately based on the 6-month rate noted in the study, and that this rate is similar to that with currently available over-the-counter products. This point is important, as Phexxi is available only by prescription, which may impact patient cost and access.
Equally important is Phexxi’s potential for sexually transmitted infection (STI) prevention. In a US-based randomized controlled trial, Phexxi use demonstrated significant risk reduction in gonorrhea and chlamydia infections among participants aged 18 to 45 years.24 That study showed a relative risk reduction of 50% and 78% for chlamydia and gonorrhea, respectively.24 Future research is planned to evaluate this spermicide as a novel STI prevention method. Ultimately, Phexxi may provide an alternative spermicide for users interested in moderately effective contraception and unable to tolerate available nonoxynol-9 formulations. Interested users will have to rely on a prescription, possibly limiting access to this novel spermicide. Further data are required to determine its potential as an STI prevention agent.
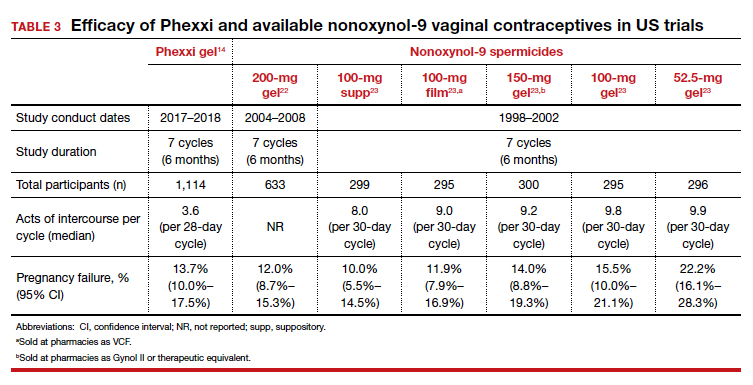
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Meade TW. Oral contraceptives, clotting factors, and thrombosis. Am J Obstet Gynecol. 1982;142(6 pt 2):758-761.
- Royal College of General Practitioners’ Oral Contraception Study. Oral contraceptives, venous thrombosis, and varicose veins. J R Coll Gen Pract. 1978;28:393-399.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception. 2007;75:344-354.
- Heinemann LA, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception. 2007;75:328-336.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Klipping C, Duijkers I, Mawet M, et al. Endocrine and metabolic effects of an oral contraceptive containing estetrol and drospirenone. Contraception. 2021;103:213-221.
- Archer DF, Cullins V, Creasy GW, et al. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra) on contraceptive efficacy. Contraception. 2004;69:189-195.
- Stanczyk FZ, Roy S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception. 1990;42:67-96.
- Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Natavio M, Stanczyk FZ, Molins EAG, et al. Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index. Contraception. 2019;99:306-311.
- Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception. 2017;95:464-469.
- Westhoff CL, Torgal AH, Mayeda ER, et al. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474-480.
- Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
- Gemzell-Danielsson K, Apter D, Zatik J, et al. Estetrol-drospirenone combination oral contraceptive: a clinical study of contraceptive efficacy, bleeding pattern, and safety in Europe and Russia. BJOG. 2021. doi: 10.1111/1471-0528.16840.
- Archer DF, Nakajima ST, Sawyer AT, et al. Norethindrone acetate 1.0 milligram and ethinyl estradiol 10 micrograms as an ultra low-dose oral contraceptive. Obstet Gynecol. 2013;122:601-607.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Safety and efficacy of a contraceptive vaginal ring delivering Nestorone and ethinyl estradiol. Clinicaltrials.gov identifier: NCT00263341. https://clinicaltrials.gov/ct2/show /NCT00263341. Accessed August 23, 2021.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168-174.
- Stanczyk FZ, Rubin A, Flood L, et al. Pharmacokinetics, tolerability and cycle control of three transdermal contraceptive delivery systems containing different doses of ethinyl-estradiol and levonorgestrel. Horm Mol Biol Clin Investig. 2011;6:231-240.
- Burke AE, Barnhart K, Jensen JT, et al. Contraceptive efficacy, acceptability, and safety of C31G and nonoxynol-9 spermicidal gels: a randomized controlled trial. Obstet Gynecol. 2010;116:1265-1273.
- Raymond EG, Chen PL, Luoto J; Spermicidal Trial Group. Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: a randomized trial. Obstet Gynecol. 2004;103:430-439.
- Chappell BT, Mena LA, Maximos B, et al. EVO100 prevents chlamydia and gonorrhea in women at high risk of infection. Am J Obstet Gynecol. 2021;225:162.e1-162.e14.
A new contraceptive method should ideally provide improved access or a higher quality and safety option. Although unintended pregnancy rates in the United States are decreasing, significant disparities across race and socioeconomic status remain,1 and these disparities actually doubled from 1994 to 2011 even though the overall unintended pregnancy rate decreased.1-3 Specifically, people of color, those with lower income, and people with lower education levels had higher rates of unintended pregnancies than did White people with higher education and income, suggesting disparate access to contraception services.1 Thus, as new contraceptive methods are introduced, we must assess if they have the potential to address this disparity as well as continue to provide higher quality and safer options.
In this Update, we critically review the phase 3 data on efficacy and safety for 3 new methods that were introduced to the US market over the past year to evaluate their impact on the current contraceptive landscape.
The first method, newly approved by the US Food and Drug Administration (FDA), is a combined oral contraceptive (OC) that contains a novel endogenous estrogen, estetrol, or E4 (Nextstellis). E4 is a natural estrogen produced in the fetal liver that has lower potency and a longer half-life than estradiol. Nextstellis is a monophasic 24/4 OC pill that contains E4 14.2 mg and drospirenone 3 mg in each of the 24 hormone-containing pills. Most combined hormonal contraceptives (CHCs) in the United States today contain synthetically made ethinyl estradiol (EE) due to its high potency and oral bioavailability. Outside of the reproductive system, EE upregulates the production of hepatic proteins and alters procoagulant and anticoagulant factors, which results in an overall increase in venous thromboembolic (VTE) risk among CHC users.2
After widespread use of combined oral contraceptives (COCs) started in the 1960s, data emerged regarding increased VTE risk.3 Subsequent research discovered that the type of estrogen used in CHCs directly correlates with the thrombosis risk due to the hepatic upregulation with both first- and second-pass metabolism. Although this risk was reduced as the EE dose decreased below 50 µg and concurrent VTE risk factors were contraindicated, CHC users still faced a 2-fold increase in VTE risk compared with nonusers.4,5 EE in contraceptive formulations increases VTE risk, likely related to upregulation of procoagulant factors and decreasing anticoagulant proteins.2 By contrast, a phase 2 trial of Nextstellis demonstrated more neutral effects of E4/drospirenone on hemostatic parameters compared with EE/levonorgestrel or EE/drospirenone.6 Furthermore, E4/drospirenone exhibited lower increases in hepatic proteins, such as angiotensinogen, triglycerides, and sex-hormone binding globulin.7 These findings together suggest that this novel CHC pill has a more favorable cardiovascular adverse effect profile compared with currently available CHCs.
The second contraceptive method is a new transdermal patch that contains EE and levonorgestrel (Twirla); this is in contrast to the available EE/norelgestromin contraceptive patch (Xulane). Transdermal contraceptive patches can offer some users easier adherence as compared with a daily OC.8 Until this past year, the only transdermal contraceptive available in the United States was Xulane, which contains a daily dose of EE 35 µg and norelgestromin 150 µg. Norelgestromin is eventually metabolized to levonorgestrel derivatives.9 Twirla is administered in the same manner as Xulane and contains a daily hormone exposure equivalent to a COC containing EE 30 µg and levonorgestrel 120 µg. Similar to EE/norelgestromin, the EE/levonorgestrel patch also is contraindicated in obese patients (body mass index [BMI] ≥30 kg/m2) due to decreased efficacy and increased risk for VTE. Additionally, phase 3 data demonstrated decreasing efficacy of Twirla in overweight users (BMI ≥25–30 kg/m2), perhaps further limiting the population that may benefit from this contraceptive method.10 These issues with efficacy and weight likely are related to the fact that levonorgestrel distribution is weight dependent, with evidence of lower plasma levels in obese individuals.11-13
The third new method is a prescription vaginal contraceptive gel with lactic acid, citric acid, and potassium bitartrate (Phexxi) designed to prevent pregnancy by maintaining an acidic vaginal environment that is inhospitable to sperm. For many decades, vaginal contraceptives, including vaginal spermicidal gels, provided easy access to a nonhormonal and flexible method of moderately effective contraception for many users. Phexxi is a prescription vaginal pH regulator administered as a gel and active for 1 hour after application. All previous vaginal gels sold in the United States are applied similarly, are available over the counter, and include nonoxynol-9, which is a surfactant that damages sperm cell membranes. Recent data from a phase 3 trial demonstrated similar contraceptive effectiveness of Phexxi when compared with available nonoxynol-9 alternatives.14
Continue to: New OC with the novel estrogen E4 demonstrates good safety profile for VTE...
New OC with the novel estrogen E4 demonstrates good safety profile for VTE
Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
The COC E4/drospirenone was evaluated in 2 parallel multinational studies. Here, we review the North American data that are more relevant for the US population; the European-Russian data also are published.15
Study examined 1 year’s use of E4/drospirenone
The US–Canadian trial conducted by Creinin and colleagues enrolled 1,864 participants aged 16 to 50 years to evaluate contraceptive efficacy, bleeding patterns, and adverse events with 1-year use (13 cycles) of E4/drospirenone. The primary efficacy group included 1,524 women aged 16 to 35. This study enrolled healthy, heterosexually active participants with a BMI ≤35 kg/m2 and regular menses from 70 sites in the United States and 7 sites in Canada. The dropout rate was 45%, comparable to that in other contraceptive studies. Participants used E4/drospirenone cyclically, taking 1 hormone-containing pill daily for 24 days followed by 4 days of placebo pills.
Contraceptive efficacious, no VTE observed
The researchers reported efficacy as a Pearl Index (PI) of 2.65 pregnancies per 100 woman-years in participants aged 16 to 35 and an overall 13-cycle life-table pregnancy rate of 2.06%. The PI did not differ among nonobese and obese participants in multivariable analysis. Most users experienced scheduled withdrawal bleeding; only 13% to 18% reported absence of scheduled bleeding. Unscheduled bleeding was typically spotting (55.2%), and this decreased with treatment duration from 30% in cycle 1 to 15% to 20% in cycle 5 and on.
Overall, 28.9% of participants reported treatment-related adverse events (AEs), which most commonly were headache (5.0%), metrorrhagia (4.6%), and nausea (3.8%). Investigators reported a minimal change in mean (SD) BMI of 0.4 (1.7) kg/m2 from baseline after 1 year of E4/drospirenone use, and only 0.5% of participants discontinued use due to weight gain. The most common reasons for AE-related treatment discontinuation included metrorrhagia (0.9%), menorrhagia (0.8%), and vaginal hemorrhage (0.5%). Importantly, no cases of VTE occurred in this study of estetrol despite 23% of participants being obese, a known risk factor for VTE.
Nextstellis provides safe, effective contraception with a PI comparable to that of other available CHCs as well as a favorable bleeding profile in healthy users who are adherent to treatment. Importantly, contraceptive efficacy was maintained in obese users with a BMI up to 35 kg/m2. In contrast to EE or estradiol, E4 demonstrates a lower impact on the hepatic system, and preliminary findings suggest a lower VTE risk compared with other CHCs on the market. The European phase 3 trial of 1,553 participants also demonstrated a low rate of VTE, with only 1 case diagnosed.15 By contrast, similar phase 3 trials of available CHCs demonstrated more frequent VTE events despite low-dose EE formulations (TABLE 1).10,15-18 In general, most US phase 3 trials have 3 to 4 VTE events in the studied population, and the Nextstellis North American trial, of which 92% of participants were from the United States, had 0. However, confirmation of any potential lower VTE risk requires further analysis from large, population-based postmarketing studies.

Continue to: Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women...
Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women
Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
To assess the contraceptive efficacy, tolerability, and safety of the transdermal patch Twirla (EE/levonorgestrel) over 1 year of treatment (13 cycles), Nelson and colleagues conducted an open-label, multicenter, US-based phase 3 trial of participants aged 18 years and older with regular cycles. There were no restrictions based on BMI. On average, the study population was overweight, with a mean BMI of 28.3 kg/m2 , and 35% of the population was considered obese (BMI ≥30 kg/m2).
Study design
A total of 2,032 participants enrolled in the study, with separate populations defined for specific analysis on safety, contraceptive efficacy, and cycle control. The primary efficacy group included 1,736 participants. Fifty-one percent discontinued the study, most commonly due to “women’s decision” (15%) and lost to follow-up (11%). Users received bleeding diaries and returned periodically throughout the study for evaluation for efficacy, adherence, and adverse events.
Efficacy associated with BMI
The study results demonstrated an overall PI of 5.8 pregnancies per 100 woman-years for users aged younger than 35. TABLE 2 demonstrates the overall trend of efficacy in relation to BMI.10,15-19 Participants with a higher BMI were found to have a higher PI, revealing lower contraceptive efficacy in more overweight and obese patients. The overall cumulative pregnancy rate over 13 cycles was 5.3%

Participants reported decreasing frequency of bleeding/spotting days over the treatment duration of 13 cycles, from a mean (SD) of 6.2 (4.5) days in cycle 1 to 4.9 (3.5) days in cycle 13. Unscheduled bleeding episodes remained high throughout the study period. Initially, 60% of users reported 1 or more days of unscheduled bleeding in cycle 1, and 42% still reported unscheduled bleeding in cycle 13. In light of this, only 45 participants (2.2%) discontinued the study due to bleeding issues, suggesting perhaps that the bleeding was light. Overall, users experienced acceptable wearability of the patch, and the rate of detachment decreased over the study period from 9.9% in cycle 1 to 2.4% in cycle 13. There were also low rates (0.5%) of moderate to severe irritation. Itching at the adhesion site decreased slightly from 13.1% in cycle 2 to 9.6% in cycle 13.
In general, 27.2% of patch users experienced a study-related AE, most reported as mild to moderate. Nausea (4.1%) and headaches (3.6%) were the most common hormone-related AE. Importantly, 4 obese users experienced 5 VTEs (deep vein thrombosis, n = 2; pulmonary embolism, n = 3) between cycle 5 and 13. Three of these users had additional VTE risk factors, such as air travel and a family history of clots. No users who were of normal weight or overweight experienced VTE.
Available data demonstrate that the EE/norelgestromin patch exposes users to higher serum levels compared with the pill or the ring.20 The higher estrogen exposure with the patch may explain higher estrogen-related adverse effects and may result in increased VTE risk. Initial pharmacokinetic data of the EE/levonorgestrel patch showed lower EE concentrations, similar to marketed COCs and lower than EE/norelgestromin.21 Despite this lower estrogen exposure, the phase 3 trial by Nelson and colleagues did not demonstrate a safer profile with respect to thromboembolic events.
Further, the high PI of 5.8 pregnancies per 100 woman-years calls into question the efficacy of this patch compared with already available CHC options. Indeed, the efficacy appears reasonable in normal-weight individuals, with a PI of 3.5 pregnancies per 100 woman-years; however, this is still higher than its contemporary counterpart, Nextstellis, which has a PI of 2.65 pregnancies per 100 woman-years and included users with a BMI of up to 35 kg/m2 (Table 2). Given the evidence of decreased efficacy, clinicians may consider reserving this option for only normal-weight women who cannot use or prefer not to use another CHC method. Obese individuals (BMI ≥30 kg/m2 ) should not use this patch due to decreased efficacy and increased VTE risk. Lastly, although use in overweight individuals (BMI ≥25 kg/m2) is not absolutely contraindicated, clinicians should counsel the overweight patient on the possibility of decreased contraceptive efficacy due to weight, and they may choose to reserve use of this patch in overweight individuals only when no other comparable or more effective method is an option.
Continue to: Novel vaginal pH buffering spermicide is a new Rx-only option...
Novel vaginal pH buffering spermicide is a new Rx-only option
Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
In an open-label phase 3 study, Thomas and colleagues enrolled 1,384 participants aged 18 to 35 with regular cycles at 112 sites in the United States to assess the contraceptive efficacy, safety, and acceptability of Phexxi vaginal gel (lactic acid, citric acid, and potassium bitartrate) over 7 cycles (6 months). Participants were required to have at least 3 episodes of heterosexual vaginal intercourse per cycle and return throughout the treatment duration for study visits. Fifty-three percent of participants did not complete the study, most frequently due to loss to follow-up (18.1%) and participant withdrawal (12.3%). Most participants were White (69%) and had an average (SD) age of 27.7 (4.5) years.
Efficacy and AE rates
The investigators reported a cumulative pregnancy rate of 13.7% over 7 cycles (6 months). In this study, 45.2% of women experienced 1 AE, and most were noted to be mild (23.9%) to moderate (18.7%). The most reported AE was vulvovaginal burning (20.0%), followed by vulvovaginal pruritus (11.2%), urinary tract infection (5.7%), and vulvovaginal pain (3.8%). Less than 2% of participants discontinued the study due to an AE. Burning and itching decreased with time and with decreased frequency of use. When used twice per day compared with once per day, burning rates decreased from 4.6% to 2.1%, and itching rates decreased from 1.0% to 0.7%. Serious AEs were uncommon, occurring in 1.3% of users; only 1, cystitis, was noted to be “probably” related to the treatment. ●
Prior to the approval of Phexxi, all currently available vaginal contraceptive gels in the United States contained nonoxynol-9 as the active ingredient, which is a surfactant that is spermicidal by damaging cell membranes. Although Phexxi provides a novel mechanism of action as a spermicide, the contraceptive efficacy is about the same as available spermicides on the market (see TABLE 3).14,22,23 The FDA calculated a 13-cycle PI to include in the label (27.5 pregnancies per 100 woman-years) based on the results of this study; however, no reliable statistical method exists to calculate a true PI from a 7-cycle study. Thus, we recommend that clinicians counsel patients appropriately based on the 6-month rate noted in the study, and that this rate is similar to that with currently available over-the-counter products. This point is important, as Phexxi is available only by prescription, which may impact patient cost and access.
Equally important is Phexxi’s potential for sexually transmitted infection (STI) prevention. In a US-based randomized controlled trial, Phexxi use demonstrated significant risk reduction in gonorrhea and chlamydia infections among participants aged 18 to 45 years.24 That study showed a relative risk reduction of 50% and 78% for chlamydia and gonorrhea, respectively.24 Future research is planned to evaluate this spermicide as a novel STI prevention method. Ultimately, Phexxi may provide an alternative spermicide for users interested in moderately effective contraception and unable to tolerate available nonoxynol-9 formulations. Interested users will have to rely on a prescription, possibly limiting access to this novel spermicide. Further data are required to determine its potential as an STI prevention agent.

A new contraceptive method should ideally provide improved access or a higher quality and safety option. Although unintended pregnancy rates in the United States are decreasing, significant disparities across race and socioeconomic status remain,1 and these disparities actually doubled from 1994 to 2011 even though the overall unintended pregnancy rate decreased.1-3 Specifically, people of color, those with lower income, and people with lower education levels had higher rates of unintended pregnancies than did White people with higher education and income, suggesting disparate access to contraception services.1 Thus, as new contraceptive methods are introduced, we must assess if they have the potential to address this disparity as well as continue to provide higher quality and safer options.
In this Update, we critically review the phase 3 data on efficacy and safety for 3 new methods that were introduced to the US market over the past year to evaluate their impact on the current contraceptive landscape.
The first method, newly approved by the US Food and Drug Administration (FDA), is a combined oral contraceptive (OC) that contains a novel endogenous estrogen, estetrol, or E4 (Nextstellis). E4 is a natural estrogen produced in the fetal liver that has lower potency and a longer half-life than estradiol. Nextstellis is a monophasic 24/4 OC pill that contains E4 14.2 mg and drospirenone 3 mg in each of the 24 hormone-containing pills. Most combined hormonal contraceptives (CHCs) in the United States today contain synthetically made ethinyl estradiol (EE) due to its high potency and oral bioavailability. Outside of the reproductive system, EE upregulates the production of hepatic proteins and alters procoagulant and anticoagulant factors, which results in an overall increase in venous thromboembolic (VTE) risk among CHC users.2
After widespread use of combined oral contraceptives (COCs) started in the 1960s, data emerged regarding increased VTE risk.3 Subsequent research discovered that the type of estrogen used in CHCs directly correlates with the thrombosis risk due to the hepatic upregulation with both first- and second-pass metabolism. Although this risk was reduced as the EE dose decreased below 50 µg and concurrent VTE risk factors were contraindicated, CHC users still faced a 2-fold increase in VTE risk compared with nonusers.4,5 EE in contraceptive formulations increases VTE risk, likely related to upregulation of procoagulant factors and decreasing anticoagulant proteins.2 By contrast, a phase 2 trial of Nextstellis demonstrated more neutral effects of E4/drospirenone on hemostatic parameters compared with EE/levonorgestrel or EE/drospirenone.6 Furthermore, E4/drospirenone exhibited lower increases in hepatic proteins, such as angiotensinogen, triglycerides, and sex-hormone binding globulin.7 These findings together suggest that this novel CHC pill has a more favorable cardiovascular adverse effect profile compared with currently available CHCs.
The second contraceptive method is a new transdermal patch that contains EE and levonorgestrel (Twirla); this is in contrast to the available EE/norelgestromin contraceptive patch (Xulane). Transdermal contraceptive patches can offer some users easier adherence as compared with a daily OC.8 Until this past year, the only transdermal contraceptive available in the United States was Xulane, which contains a daily dose of EE 35 µg and norelgestromin 150 µg. Norelgestromin is eventually metabolized to levonorgestrel derivatives.9 Twirla is administered in the same manner as Xulane and contains a daily hormone exposure equivalent to a COC containing EE 30 µg and levonorgestrel 120 µg. Similar to EE/norelgestromin, the EE/levonorgestrel patch also is contraindicated in obese patients (body mass index [BMI] ≥30 kg/m2) due to decreased efficacy and increased risk for VTE. Additionally, phase 3 data demonstrated decreasing efficacy of Twirla in overweight users (BMI ≥25–30 kg/m2), perhaps further limiting the population that may benefit from this contraceptive method.10 These issues with efficacy and weight likely are related to the fact that levonorgestrel distribution is weight dependent, with evidence of lower plasma levels in obese individuals.11-13
The third new method is a prescription vaginal contraceptive gel with lactic acid, citric acid, and potassium bitartrate (Phexxi) designed to prevent pregnancy by maintaining an acidic vaginal environment that is inhospitable to sperm. For many decades, vaginal contraceptives, including vaginal spermicidal gels, provided easy access to a nonhormonal and flexible method of moderately effective contraception for many users. Phexxi is a prescription vaginal pH regulator administered as a gel and active for 1 hour after application. All previous vaginal gels sold in the United States are applied similarly, are available over the counter, and include nonoxynol-9, which is a surfactant that damages sperm cell membranes. Recent data from a phase 3 trial demonstrated similar contraceptive effectiveness of Phexxi when compared with available nonoxynol-9 alternatives.14
Continue to: New OC with the novel estrogen E4 demonstrates good safety profile for VTE...
New OC with the novel estrogen E4 demonstrates good safety profile for VTE
Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
The COC E4/drospirenone was evaluated in 2 parallel multinational studies. Here, we review the North American data that are more relevant for the US population; the European-Russian data also are published.15
Study examined 1 year’s use of E4/drospirenone
The US–Canadian trial conducted by Creinin and colleagues enrolled 1,864 participants aged 16 to 50 years to evaluate contraceptive efficacy, bleeding patterns, and adverse events with 1-year use (13 cycles) of E4/drospirenone. The primary efficacy group included 1,524 women aged 16 to 35. This study enrolled healthy, heterosexually active participants with a BMI ≤35 kg/m2 and regular menses from 70 sites in the United States and 7 sites in Canada. The dropout rate was 45%, comparable to that in other contraceptive studies. Participants used E4/drospirenone cyclically, taking 1 hormone-containing pill daily for 24 days followed by 4 days of placebo pills.
Contraceptive efficacious, no VTE observed
The researchers reported efficacy as a Pearl Index (PI) of 2.65 pregnancies per 100 woman-years in participants aged 16 to 35 and an overall 13-cycle life-table pregnancy rate of 2.06%. The PI did not differ among nonobese and obese participants in multivariable analysis. Most users experienced scheduled withdrawal bleeding; only 13% to 18% reported absence of scheduled bleeding. Unscheduled bleeding was typically spotting (55.2%), and this decreased with treatment duration from 30% in cycle 1 to 15% to 20% in cycle 5 and on.
Overall, 28.9% of participants reported treatment-related adverse events (AEs), which most commonly were headache (5.0%), metrorrhagia (4.6%), and nausea (3.8%). Investigators reported a minimal change in mean (SD) BMI of 0.4 (1.7) kg/m2 from baseline after 1 year of E4/drospirenone use, and only 0.5% of participants discontinued use due to weight gain. The most common reasons for AE-related treatment discontinuation included metrorrhagia (0.9%), menorrhagia (0.8%), and vaginal hemorrhage (0.5%). Importantly, no cases of VTE occurred in this study of estetrol despite 23% of participants being obese, a known risk factor for VTE.
Nextstellis provides safe, effective contraception with a PI comparable to that of other available CHCs as well as a favorable bleeding profile in healthy users who are adherent to treatment. Importantly, contraceptive efficacy was maintained in obese users with a BMI up to 35 kg/m2. In contrast to EE or estradiol, E4 demonstrates a lower impact on the hepatic system, and preliminary findings suggest a lower VTE risk compared with other CHCs on the market. The European phase 3 trial of 1,553 participants also demonstrated a low rate of VTE, with only 1 case diagnosed.15 By contrast, similar phase 3 trials of available CHCs demonstrated more frequent VTE events despite low-dose EE formulations (TABLE 1).10,15-18 In general, most US phase 3 trials have 3 to 4 VTE events in the studied population, and the Nextstellis North American trial, of which 92% of participants were from the United States, had 0. However, confirmation of any potential lower VTE risk requires further analysis from large, population-based postmarketing studies.

Continue to: Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women...
Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women
Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
To assess the contraceptive efficacy, tolerability, and safety of the transdermal patch Twirla (EE/levonorgestrel) over 1 year of treatment (13 cycles), Nelson and colleagues conducted an open-label, multicenter, US-based phase 3 trial of participants aged 18 years and older with regular cycles. There were no restrictions based on BMI. On average, the study population was overweight, with a mean BMI of 28.3 kg/m2 , and 35% of the population was considered obese (BMI ≥30 kg/m2).
Study design
A total of 2,032 participants enrolled in the study, with separate populations defined for specific analysis on safety, contraceptive efficacy, and cycle control. The primary efficacy group included 1,736 participants. Fifty-one percent discontinued the study, most commonly due to “women’s decision” (15%) and lost to follow-up (11%). Users received bleeding diaries and returned periodically throughout the study for evaluation for efficacy, adherence, and adverse events.
Efficacy associated with BMI
The study results demonstrated an overall PI of 5.8 pregnancies per 100 woman-years for users aged younger than 35. TABLE 2 demonstrates the overall trend of efficacy in relation to BMI.10,15-19 Participants with a higher BMI were found to have a higher PI, revealing lower contraceptive efficacy in more overweight and obese patients. The overall cumulative pregnancy rate over 13 cycles was 5.3%

Participants reported decreasing frequency of bleeding/spotting days over the treatment duration of 13 cycles, from a mean (SD) of 6.2 (4.5) days in cycle 1 to 4.9 (3.5) days in cycle 13. Unscheduled bleeding episodes remained high throughout the study period. Initially, 60% of users reported 1 or more days of unscheduled bleeding in cycle 1, and 42% still reported unscheduled bleeding in cycle 13. In light of this, only 45 participants (2.2%) discontinued the study due to bleeding issues, suggesting perhaps that the bleeding was light. Overall, users experienced acceptable wearability of the patch, and the rate of detachment decreased over the study period from 9.9% in cycle 1 to 2.4% in cycle 13. There were also low rates (0.5%) of moderate to severe irritation. Itching at the adhesion site decreased slightly from 13.1% in cycle 2 to 9.6% in cycle 13.
In general, 27.2% of patch users experienced a study-related AE, most reported as mild to moderate. Nausea (4.1%) and headaches (3.6%) were the most common hormone-related AE. Importantly, 4 obese users experienced 5 VTEs (deep vein thrombosis, n = 2; pulmonary embolism, n = 3) between cycle 5 and 13. Three of these users had additional VTE risk factors, such as air travel and a family history of clots. No users who were of normal weight or overweight experienced VTE.
Available data demonstrate that the EE/norelgestromin patch exposes users to higher serum levels compared with the pill or the ring.20 The higher estrogen exposure with the patch may explain higher estrogen-related adverse effects and may result in increased VTE risk. Initial pharmacokinetic data of the EE/levonorgestrel patch showed lower EE concentrations, similar to marketed COCs and lower than EE/norelgestromin.21 Despite this lower estrogen exposure, the phase 3 trial by Nelson and colleagues did not demonstrate a safer profile with respect to thromboembolic events.
Further, the high PI of 5.8 pregnancies per 100 woman-years calls into question the efficacy of this patch compared with already available CHC options. Indeed, the efficacy appears reasonable in normal-weight individuals, with a PI of 3.5 pregnancies per 100 woman-years; however, this is still higher than its contemporary counterpart, Nextstellis, which has a PI of 2.65 pregnancies per 100 woman-years and included users with a BMI of up to 35 kg/m2 (Table 2). Given the evidence of decreased efficacy, clinicians may consider reserving this option for only normal-weight women who cannot use or prefer not to use another CHC method. Obese individuals (BMI ≥30 kg/m2 ) should not use this patch due to decreased efficacy and increased VTE risk. Lastly, although use in overweight individuals (BMI ≥25 kg/m2) is not absolutely contraindicated, clinicians should counsel the overweight patient on the possibility of decreased contraceptive efficacy due to weight, and they may choose to reserve use of this patch in overweight individuals only when no other comparable or more effective method is an option.
Continue to: Novel vaginal pH buffering spermicide is a new Rx-only option...
Novel vaginal pH buffering spermicide is a new Rx-only option
Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
In an open-label phase 3 study, Thomas and colleagues enrolled 1,384 participants aged 18 to 35 with regular cycles at 112 sites in the United States to assess the contraceptive efficacy, safety, and acceptability of Phexxi vaginal gel (lactic acid, citric acid, and potassium bitartrate) over 7 cycles (6 months). Participants were required to have at least 3 episodes of heterosexual vaginal intercourse per cycle and return throughout the treatment duration for study visits. Fifty-three percent of participants did not complete the study, most frequently due to loss to follow-up (18.1%) and participant withdrawal (12.3%). Most participants were White (69%) and had an average (SD) age of 27.7 (4.5) years.
Efficacy and AE rates
The investigators reported a cumulative pregnancy rate of 13.7% over 7 cycles (6 months). In this study, 45.2% of women experienced 1 AE, and most were noted to be mild (23.9%) to moderate (18.7%). The most reported AE was vulvovaginal burning (20.0%), followed by vulvovaginal pruritus (11.2%), urinary tract infection (5.7%), and vulvovaginal pain (3.8%). Less than 2% of participants discontinued the study due to an AE. Burning and itching decreased with time and with decreased frequency of use. When used twice per day compared with once per day, burning rates decreased from 4.6% to 2.1%, and itching rates decreased from 1.0% to 0.7%. Serious AEs were uncommon, occurring in 1.3% of users; only 1, cystitis, was noted to be “probably” related to the treatment. ●
Prior to the approval of Phexxi, all currently available vaginal contraceptive gels in the United States contained nonoxynol-9 as the active ingredient, which is a surfactant that is spermicidal by damaging cell membranes. Although Phexxi provides a novel mechanism of action as a spermicide, the contraceptive efficacy is about the same as available spermicides on the market (see TABLE 3).14,22,23 The FDA calculated a 13-cycle PI to include in the label (27.5 pregnancies per 100 woman-years) based on the results of this study; however, no reliable statistical method exists to calculate a true PI from a 7-cycle study. Thus, we recommend that clinicians counsel patients appropriately based on the 6-month rate noted in the study, and that this rate is similar to that with currently available over-the-counter products. This point is important, as Phexxi is available only by prescription, which may impact patient cost and access.
Equally important is Phexxi’s potential for sexually transmitted infection (STI) prevention. In a US-based randomized controlled trial, Phexxi use demonstrated significant risk reduction in gonorrhea and chlamydia infections among participants aged 18 to 45 years.24 That study showed a relative risk reduction of 50% and 78% for chlamydia and gonorrhea, respectively.24 Future research is planned to evaluate this spermicide as a novel STI prevention method. Ultimately, Phexxi may provide an alternative spermicide for users interested in moderately effective contraception and unable to tolerate available nonoxynol-9 formulations. Interested users will have to rely on a prescription, possibly limiting access to this novel spermicide. Further data are required to determine its potential as an STI prevention agent.

- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Meade TW. Oral contraceptives, clotting factors, and thrombosis. Am J Obstet Gynecol. 1982;142(6 pt 2):758-761.
- Royal College of General Practitioners’ Oral Contraception Study. Oral contraceptives, venous thrombosis, and varicose veins. J R Coll Gen Pract. 1978;28:393-399.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception. 2007;75:344-354.
- Heinemann LA, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception. 2007;75:328-336.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Klipping C, Duijkers I, Mawet M, et al. Endocrine and metabolic effects of an oral contraceptive containing estetrol and drospirenone. Contraception. 2021;103:213-221.
- Archer DF, Cullins V, Creasy GW, et al. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra) on contraceptive efficacy. Contraception. 2004;69:189-195.
- Stanczyk FZ, Roy S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception. 1990;42:67-96.
- Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Natavio M, Stanczyk FZ, Molins EAG, et al. Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index. Contraception. 2019;99:306-311.
- Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception. 2017;95:464-469.
- Westhoff CL, Torgal AH, Mayeda ER, et al. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474-480.
- Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
- Gemzell-Danielsson K, Apter D, Zatik J, et al. Estetrol-drospirenone combination oral contraceptive: a clinical study of contraceptive efficacy, bleeding pattern, and safety in Europe and Russia. BJOG. 2021. doi: 10.1111/1471-0528.16840.
- Archer DF, Nakajima ST, Sawyer AT, et al. Norethindrone acetate 1.0 milligram and ethinyl estradiol 10 micrograms as an ultra low-dose oral contraceptive. Obstet Gynecol. 2013;122:601-607.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Safety and efficacy of a contraceptive vaginal ring delivering Nestorone and ethinyl estradiol. Clinicaltrials.gov identifier: NCT00263341. https://clinicaltrials.gov/ct2/show /NCT00263341. Accessed August 23, 2021.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168-174.
- Stanczyk FZ, Rubin A, Flood L, et al. Pharmacokinetics, tolerability and cycle control of three transdermal contraceptive delivery systems containing different doses of ethinyl-estradiol and levonorgestrel. Horm Mol Biol Clin Investig. 2011;6:231-240.
- Burke AE, Barnhart K, Jensen JT, et al. Contraceptive efficacy, acceptability, and safety of C31G and nonoxynol-9 spermicidal gels: a randomized controlled trial. Obstet Gynecol. 2010;116:1265-1273.
- Raymond EG, Chen PL, Luoto J; Spermicidal Trial Group. Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: a randomized trial. Obstet Gynecol. 2004;103:430-439.
- Chappell BT, Mena LA, Maximos B, et al. EVO100 prevents chlamydia and gonorrhea in women at high risk of infection. Am J Obstet Gynecol. 2021;225:162.e1-162.e14.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Meade TW. Oral contraceptives, clotting factors, and thrombosis. Am J Obstet Gynecol. 1982;142(6 pt 2):758-761.
- Royal College of General Practitioners’ Oral Contraception Study. Oral contraceptives, venous thrombosis, and varicose veins. J R Coll Gen Pract. 1978;28:393-399.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception. 2007;75:344-354.
- Heinemann LA, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception. 2007;75:328-336.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Klipping C, Duijkers I, Mawet M, et al. Endocrine and metabolic effects of an oral contraceptive containing estetrol and drospirenone. Contraception. 2021;103:213-221.
- Archer DF, Cullins V, Creasy GW, et al. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra) on contraceptive efficacy. Contraception. 2004;69:189-195.
- Stanczyk FZ, Roy S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception. 1990;42:67-96.
- Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Natavio M, Stanczyk FZ, Molins EAG, et al. Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index. Contraception. 2019;99:306-311.
- Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception. 2017;95:464-469.
- Westhoff CL, Torgal AH, Mayeda ER, et al. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474-480.
- Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
- Gemzell-Danielsson K, Apter D, Zatik J, et al. Estetrol-drospirenone combination oral contraceptive: a clinical study of contraceptive efficacy, bleeding pattern, and safety in Europe and Russia. BJOG. 2021. doi: 10.1111/1471-0528.16840.
- Archer DF, Nakajima ST, Sawyer AT, et al. Norethindrone acetate 1.0 milligram and ethinyl estradiol 10 micrograms as an ultra low-dose oral contraceptive. Obstet Gynecol. 2013;122:601-607.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Safety and efficacy of a contraceptive vaginal ring delivering Nestorone and ethinyl estradiol. Clinicaltrials.gov identifier: NCT00263341. https://clinicaltrials.gov/ct2/show /NCT00263341. Accessed August 23, 2021.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168-174.
- Stanczyk FZ, Rubin A, Flood L, et al. Pharmacokinetics, tolerability and cycle control of three transdermal contraceptive delivery systems containing different doses of ethinyl-estradiol and levonorgestrel. Horm Mol Biol Clin Investig. 2011;6:231-240.
- Burke AE, Barnhart K, Jensen JT, et al. Contraceptive efficacy, acceptability, and safety of C31G and nonoxynol-9 spermicidal gels: a randomized controlled trial. Obstet Gynecol. 2010;116:1265-1273.
- Raymond EG, Chen PL, Luoto J; Spermicidal Trial Group. Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: a randomized trial. Obstet Gynecol. 2004;103:430-439.
- Chappell BT, Mena LA, Maximos B, et al. EVO100 prevents chlamydia and gonorrhea in women at high risk of infection. Am J Obstet Gynecol. 2021;225:162.e1-162.e14.
Vaccinations for the ObGyn’s toolbox
CASE 1st prenatal appointment for young, pregnant migrant
A 21-year-old primigravid woman at 12 weeks’ gestation recently immigrated to the United States from an impoverished rural area of Southeast Asia. On the first prenatal appointment, she is noted to have no evidence of immunity to rubella, measles, or varicella. Her hepatitis B surface antigen and hepatitis C antibody tests are negative. She also has negative test results for gonorrhea, chlamydia, syphilis, and HIV infection. Her pap test is negative.
- What vaccinations should this patient receive during her pregnancy?
- What additional vaccinations are indicated postpartum?
Preventive vaccinations: What to know
As ObGyns, we serve as the primary care physician for many women throughout their early and middle decades of life. Accordingly, we have an obligation to be well informed about preventive health services such as vaccinations. The purpose of this article is to review the principal vaccines with which ObGyns should be familiar. I will discuss the vaccines in alphabetical order and then focus on the indications and timing for each vaccine and the relative cost of each immunization. Key points are summarized in the TABLE.
COVID-19 vaccine
In the latter part of 2020 and early part of 2021, three COVID-19 vaccines received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for individuals 16 years of age and older (Pfizer-BioNTech) and 18 years of age and older (Moderna and Johnson & Johnson).1 The cost of their administration is borne by the federal government. Two of the vaccines are mRNA agents—Moderna and Pfizer-BioNTech. Both are administered in a 2-dose series, separated by 4 and 3 weeks, respectively. The efficacy of these vaccines in preventing serious or critical illness approaches 95%. The Pfizer-BioNTech vaccine has now been fully FDA approved for administration to individuals older than age 16, with EUA for those down to age 12. Full approval of the Moderna vaccine will not be far behind. Because of some evidence suggesting waning immunity over time and because of growing concerns about the increased transmissibility of the delta variant of the virus, the FDA has been strongly considering a recommendation for a third (booster) dose of each of these vaccines, administered 8 months after the second dose for all eligible Americans. On September 17, 2021, the FDA advisory committee recommended a booster for the Pfizer-BioNTech vaccine for people older than age 65 and for those over the age of 16 at high risk for severe COVID-19. Several days later, full FDA approval was granted for this recommendation. Subsequently, the Director of the Centers for Disease Control and Prevention (CDC) included health care workers and pregnant women in the group for whom the booster is recommended.
The third vaccine formulation is the Johnson & Johnson DNA vaccine, which is prepared with a human adenovirus vector. This vaccine is administered in a single intramuscular dose and has a reported efficacy of 66% to 85%, though it may approach 95% in preventing critical illness. The FDA is expected to announce decisions about booster doses for the Johnson & Johnson and Moderna vaccines in the coming weeks.
Although initial trials of the COVID-19vaccines excluded pregnant and lactating women, the vaccines are safe in pregnancy or postpartum. In fact the vaccines do not contain either a killed or attenuated viral particle that is capable of transmitting infection. Therefore, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine now support routine immunization during pregnancy.
A recent report by Shimabukuro and colleagues2 demonstrated that the risk of vaccine-related complications in pregnant women receiving the Pfizer-BioNTech or Moderna vaccines was no different than in nonpregnant patients and that there was no evidence of teratogenic effects. The trial included more than 35,000 pregnant women; 2.3% were vaccinated in the periconception period, 28.6% in the first trimester, 43.3% in the second trimester, and 25.7% in the third trimester. Given this, and in light of isolated reports of unusual thromboembolic complications associated with the Johnson & Johnson vaccine, I strongly recommend use of either the Moderna or Pfizer-BioNTech vaccine in our prenatal and postpartum patients.
Continue to: Hepatitis A vaccine...
Hepatitis A vaccine
The hepatitis A vaccine is an inactivated vaccine and is safe for use in pregnancy. It is available in two monovalent preparations—Havrix (GlaxoSmithKline) and Vaqta (Merck & Co.) and is administered in a 2-dose intramuscular injection at time zero and 6 to 12 months later.3 The vaccine is also available in a bivalent form with recombinant hepatitis B vaccine—Twinrix (GlaxoSmithKline). When administered in this form, the vaccine should be given at time zero, 1 month, and 6 months. The wholesale cost of the monovalent vaccine is $66 to $119, depending upon whether the provider uses a multi-dose or a single-dose vial. The cost of Twinrix is $149.
The hepatitis A vaccine is indicated for select pregnant and nonpregnant patients:
- international travelers
- intravenous drug users
- those with occupational exposure (eg, individuals who work in a primate laboratory)
- residents and staff in chronic care facilities
- individuals with chronic liver disease
- individuals with clotting factor disorders
- residents in endemic areas.
Hepatitis B vaccine
The hepatitis B vaccine is a recombinant vaccine that contains an inactivated portion of the hepatitis B surface antigen. It was originally produced in two monovalent formulations: Engerix B (GlaxoSmithKline) and Recombivax-HB (Merck & Co.). These original formulations are given in a 3-dose series at time zero, 1 month, and 6 months. Recently, a new and more potent formulation was introduced into clinical practice. Heplisav-B (Dynavax Technologies Co.) is also a recombinant vaccine that contains a boosting adjuvant. It is programed to be administered in a 2-dose series at time zero and 1 month.4-6
The wholesale cost of the monovalent vaccines varies from $60 to $173, depending upon use of a multi-dose vial versus a single-use vial. The cost of Heplisav-B varies from $146 to $173, depending upon use of a prefilled syringe versus a single-dose vial.
Although the hepatitis B vaccine should be part of the childhood immunization series, it also should be administered to any pregnant woman who has not been vaccinated previously or who does not already have evidence of immunity as a result of natural infection.
Continue to: Herpes zoster vaccine...
Herpes zoster vaccine
Herpes zoster infection (shingles) can be a particularly disabling condition in older patients and results from reactivation of a latent varicella-zoster infection. Shingles can cause extremely painful skin lesions, threaten the patient’s vision, and result in long-lasting postherpetic neuralgia. Both cellular and hormonal immunity are essential to protect against recurrent infection.
The original herpes zoster vaccine (Zoster Vaccine Live; ZVL, Zostavax) is no longer produced in the United States because it is not as effective as the newer vaccine—Recombinant Zoster Vaccine (Shingrix, GlaxoSmithKline).7,8 The antigen in the new vaccine is a component of the surface glycoprotein E, and it is combined with an adjuvant to enhance immunoreactivity. The vaccine is given intramuscularly in two doses at time zero and again at 2 to 6 months and is indicated for all individuals >50 years, including those who may have had an episode of shingles. This newer vaccine is 97% effective in patients >50 years and 90% effective in patients >70. The wholesale cost of each injection is about $160.
Human papillomavirus vaccine
The HPV vaccine (Gardasil-9, Merck & Co.) is a recombinant 9-valent vaccine directed against the human papillomavirus. It induces immunity to serotypes 6 and 11 (which cause 90% of genital warts), 16 and 18 (which cause 80% of genital cancers), and 31, 33, 45, 52, and 58 (viral strains that are responsible for both genital and oropharyngeal cancers). The vaccine is administered intramuscularly in a 3-dose series at time zero, 1-2 months, and 6 months. The principal target groups for the vaccine are males and females, ages 9 to 45 years. Ideally, children of both sexes should receive this vaccine prior to the onset of sexual activity. The wholesale cost of each vaccine injection is approximately $222.9
Influenza vaccine
The inactivated, intramuscular flu vaccine is recommended for anyone over age 2, including pregnant women. Although pregnant women are not more likely to acquire flu compared with those who are not pregnant, if they do become infected, they are likely to become more seriously ill, with higher mortality. Accordingly, all pregnant women should receive, in any trimester, the inactivated flu vaccine beginning in the late summer and early fall of each year and extending through March of the next year.10,11
Multiple formulations of the inactivated vaccine are marketed, all targeting two strains of influenza A and two strains of influenza B. The components of the vaccine vary each year as scientists try to match the new vaccine with the most highly prevalent strains in the previous flu season. The vaccine should be administered in a single intramuscular dose. The cost varies from approximately $20 to $70.
The intranasal influenza vaccine is a live virus vaccine that is intended primarily for children and should not be administered in pregnancy. In addition, there is a higher dose of the inactivated quadrivalent vaccine that is available for administration to patients over age 65. This higher dose is more likely to cause adverse effects and is not indicated in pregnancy.
Continue to: Measles, mumps, rubella vaccine (MMR)...
Measles, mumps, rubella vaccine (MMR)
The MMR is a standard component of the childhood vaccination series. The trivalent preparation is a live, attenuated vaccine that is typically given subcutaneously in a 2-dose series. The first dose is administered at age 12-15 months, and the second dose at age 4-6 years. The vaccine is highly immunogenic, with vaccine-induced immunity usually life-long. In some patients, however, immunity wanes over time. Accordingly, all pregnant women should be screened for immunity to rubella since, of the 3, this infection poses the greatest risk to the fetus. Women who do not have evidence of immunity should be advised to avoid contact with children who may have a viral exanthem. They should then receive a booster dose of the vaccine immediately postpartum and should practice secure contraception for 1 month. The vaccine cost is approximately $60.
Pneumococcal vaccine
The inactivated pneumococcal vaccine is produced in two forms, both of which are safe for administration in pregnancy.12 The original vaccine, introduced in 1983, was PPSV23 (Pneumovax 23, Merck & Co), a 23-serovalent vaccine that was intended primarily for adults. This vaccine is administered in a single subcutaneous or intramuscular dose. The newest vaccine, introduced in 2010, is PCV13 (Prevnar 13, Pfizer Inc), a 13-serovalent vaccine. It was intended primarily for children, in whom it is administered in a 4-dose series beginning at 6 to 8 weeks of age. The cost of the former is approximately $98 to $120; the cost of the latter is $228.
Vaccination against pneumococcal infection is routinely indicated for those older than the age of 65 and for the following at-risk patients, including those who are pregnant11:
- individuals who have had a splenectomy or who have a medical illness that produces functional asplenia (eg, sickle cell anemia)
- individuals with chronic cardiac, pulmonic, hepatic, or renal disease
- individuals with immunosuppressive conditions such as HIV infection or a disseminated malignancy
- individuals who have a cochlear implant
- individuals who have a chronic leak of cerebrospinal fluid.
The recommendations for timing of these 2 vaccines in adults can initially appear confusing. Put most simply, if a high-risk patient first receives the PCV13 vaccine, she should receive the PPSV23 vaccine in about 8 weeks. The PPSV23 vaccine should be repeated in 5 years. If an at-risk patient initially receives the PPSV23 vaccine, the PCV13 vaccine should be given 1 year later.12
Tdap vaccine
The Tdap vaccine contains tetanus toxoid, reduced diptheria toxoid, and an acellular component of the pertussis bacterium. Although it has long been part of the childhood vaccinations series, immunity to each component, particularly pertussis, tends to wane over time.
Pertussis poses a serious risk to the health of the pregnant woman and the newborn infant. Accordingly, the Advisory Committee on Immunization Practices (ACIP), CDC, and the ACOG now advise administration of a booster dose of this vaccine in the early third trimester of each pregnancy.13-15 The vaccine should be administered as a single intramuscular injection. The approximate cost of the vaccine is $64 to $71, depending upon whether the provider uses a single-dose vial or a single-dose prefilled syringe. In nonpregnant patients, the ACIP currently recommends administration of a booster dose of the vaccine every 10 years, primarily to provide durable protection against tetanus.
Continue to: Varicella vaccine...
Varicella vaccine
The varicella vaccine is also one of the main components of the childhood immunization series. This live virus vaccine can be administered subcutaneously as a monovalent agent or as a quadrivalent agent in association with the MMR vaccine.
Pregnant women who do not have a well-documented history of natural infection should be tested for IgG antibody to the varicella-zoster virus at the time of their first prenatal appointment. Interestingly, approximately 70% of patients with an uncertain history actually have immunity when tested. If the patient lacks immunity, she should be vaccinated immediately postpartum.16,17 The vaccine should be administered in a 2-dose series at time zero and then 4 to 8 weeks later. Patients should adhere to secure contraception from the time of the first dose until 1 month after the second dose. The cost of each dose of the vaccine is approximately $145.
Adverse effects of vaccination
All vaccines have many of the same side effects. The most common is simply a reaction at the site of injection, characterized by pain, increased warmth, erythema, swelling, and tenderness. Other common side effects include systemic manifestations, such as low-grade fever, nausea and vomiting, malaise, fatigue, headache, lymphadenopathy, myalgias, and arthralgias. Some vaccines, notably varicella, herpes zoster, measles, and rubella may cause a disseminated rash. Most of these minor side effects are easily managed by rest, hydration, and administration of an analgesic such as acetaminophen or ibuprofen. More serious side effects include rare complications such as anaphylaxis, Bell palsy, Guillain-Barre syndrome, and venous thromboembolism (Johnson & Johnson COVID-19 vaccine). Any of the vaccines discussed above should not be given, or given only with extreme caution, to an individual who has experienced any of these reactions with a previous vaccine.
Barriers to vaccination
Although the vaccines reviewed above are highly effective in preventing serious illness in recipients, the medical profession’s “report card” in ensuring adherence with vaccine protocols is not optimal. In fact, it probably merits a grade no higher than C+, with vaccination rates in the range of 50% to 70%.
One of the major barriers to vaccination is lack of detailed information about vaccine efficacy and safety on the part of both provider and patient. Another is the problem of misinformation (eg, the persistent belief on the part of some individuals that vaccines may cause a serious problem, such as autism).18,19 Another important barrier to widespread vaccination is the logistical problem associated with proper scheduling of multidose regimens (such as those for hepatitis A and B, varicella, and COVID-19). A final barrier, and in my own university-based practice, the most important obstacle is the expense of vaccination. Most, but not all, private insurance companies provide coverage for vaccines approved by the Centers for Disease Control and Prevention and the US Preventive Services Task Force. However, public insurance agencies often provide disappointingly inconsistent coverage for essential vaccines.
By keeping well informed about the most recent public health recommendations for vaccinations for adults and by leading important initiatives within our own practices, we should be able to overcome the first 3 barriers listed above. For example, Morgan and colleagues20 recently achieved a 97% success rate with Tdap administration in pregnancy by placing a best-practice alert in the patients’ electronic medical records. Surmounting the final barrier will require intense effort on the part of individual practitioners and professional organizations to advocate for coverage for essential vaccinations for our patients.
CASE Resolved
This patient was raised in an area of the world where her family did not have easy access to medical care. Accordingly, she did not receive the usual childhood vaccines, such as measles, mumps, rubella, varicella, hepatitis B, and almost certainly, tetanus, diphtheria, and pertussis (Tdap), and the HPV vaccine. The MMR vaccine and the varicella vaccine are live virus vaccines and should not be given during pregnancy. However, these vaccines should be administered postpartum, and the patient should be instructed to practice secure contraception for a minimum of 1 month following vaccination. She also should be offered the HPV vaccine postpartum. During pregnancy, she definitely should receive the COVID-19 vaccine, the 3-dose hepatitis B vaccine series, the influenza vaccine, and Tdap. If her present living conditions place her at risk for hepatitis A, she also should be vaccinated against this illness. ●
- Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy. What obstetricians need to know. Obstet Gynecol. 2021;137:408-414. doi: 10.1097/AOG.0000000000004290.
- Shimabukuro TT, Kim SY, Myers RT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282. doi: 10.1056/NEJMoa2104983.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471. doi: 10.1016/s0029-7844(97)00669-8.
- Omer SB. Maternal immunization. N Engl J Med. 2017;376:1256-1267. doi: 10.1056/NEJMra1509044.
- Dionne-Odom J, Tita AT, Silverman NS. Society for Maternal-Fetal Medicine Consult Series: #38: hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14. doi: http://dx.doi.org/10.1016/j.ajog.2015.09.100.
- Yawetz S. Immunizations during pregnancy. UpToDate, January 15, 2021.
- Cunningham Al, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016:375:1019-1032. doi: 10.1056/NEJMoa1603800.
- Albrecht MA, Levin MJ. Vaccination for the prevention of shingles (herpes zoster). UpToDate, July 6, 2020.
- ACOG Committee Opinion. Human papillomavirus vaccination. Obstet Gynecol. 2006;108:699-705. doi: 10.1097/00006250-200609000-00047.
- Callaghan WM, Creanga AA, Jamieson DJ. Pregnancy-related mortality resulting from influenza in the United States during the 2009-2010 pandemic. Obstet Gynecol. 2015;126:486-490. doi: 10.1097/AOG.0000000000000996.
- ACOG Committee Opinion. Influenza vaccination during pregnancy. Obstet Gynecol. 2014;124:648-651. doi: 10.1097/01.AOG.0000453599.11566.11.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233. doi: 10.1056/NEJMoa1612296.
- Moumne O, Duff P. Treatment and prevention of pneumococcal infection. Clin Obstet Gynecol. 2019;62:781-789. doi: 10.1097/GRF.0000000000000451.
- ACOG Committee Opinion. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2017;130:668-669. doi: 10.1097/AOG.0000000000002293.
- Sukumaran L, McCarthy NL, Kharbanda EO, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol. 2015;126:1069-1074. doi: 10.1097/AOG.0000000000001066.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165. doi: 10.1155/S1064744994000013.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65. doi: 10.1155/S1064744996000142.
- Desmond A, Offit PA. On the shoulders of giants--from Jenner's cowpox to mRNA COVID vaccines. N Engl. J Med. 2021;384:1081-1083. doi: 10.1056/NEJMp2034334.
- Poland GA, Jacobson RM. The age-old struggle against the antivaccinationists. N Engl J Med. 2011;364:97-99. doi: 10.1056/NEJMp1010594.
- Morgan JL, Baggari SR, Chung W, et al. Association of a best-practice alert and prenatal administration with tetanus toxoid, reduced diptheria toxoid, and acellular pertussis vaccination rates. Obstet Gynecol. 2015;126:333-337. doi: 10.1097/AOG.0000000000000975.

CASE 1st prenatal appointment for young, pregnant migrant
A 21-year-old primigravid woman at 12 weeks’ gestation recently immigrated to the United States from an impoverished rural area of Southeast Asia. On the first prenatal appointment, she is noted to have no evidence of immunity to rubella, measles, or varicella. Her hepatitis B surface antigen and hepatitis C antibody tests are negative. She also has negative test results for gonorrhea, chlamydia, syphilis, and HIV infection. Her pap test is negative.
- What vaccinations should this patient receive during her pregnancy?
- What additional vaccinations are indicated postpartum?
Preventive vaccinations: What to know
As ObGyns, we serve as the primary care physician for many women throughout their early and middle decades of life. Accordingly, we have an obligation to be well informed about preventive health services such as vaccinations. The purpose of this article is to review the principal vaccines with which ObGyns should be familiar. I will discuss the vaccines in alphabetical order and then focus on the indications and timing for each vaccine and the relative cost of each immunization. Key points are summarized in the TABLE.

COVID-19 vaccine
In the latter part of 2020 and early part of 2021, three COVID-19 vaccines received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for individuals 16 years of age and older (Pfizer-BioNTech) and 18 years of age and older (Moderna and Johnson & Johnson).1 The cost of their administration is borne by the federal government. Two of the vaccines are mRNA agents—Moderna and Pfizer-BioNTech. Both are administered in a 2-dose series, separated by 4 and 3 weeks, respectively. The efficacy of these vaccines in preventing serious or critical illness approaches 95%. The Pfizer-BioNTech vaccine has now been fully FDA approved for administration to individuals older than age 16, with EUA for those down to age 12. Full approval of the Moderna vaccine will not be far behind. Because of some evidence suggesting waning immunity over time and because of growing concerns about the increased transmissibility of the delta variant of the virus, the FDA has been strongly considering a recommendation for a third (booster) dose of each of these vaccines, administered 8 months after the second dose for all eligible Americans. On September 17, 2021, the FDA advisory committee recommended a booster for the Pfizer-BioNTech vaccine for people older than age 65 and for those over the age of 16 at high risk for severe COVID-19. Several days later, full FDA approval was granted for this recommendation. Subsequently, the Director of the Centers for Disease Control and Prevention (CDC) included health care workers and pregnant women in the group for whom the booster is recommended.
The third vaccine formulation is the Johnson & Johnson DNA vaccine, which is prepared with a human adenovirus vector. This vaccine is administered in a single intramuscular dose and has a reported efficacy of 66% to 85%, though it may approach 95% in preventing critical illness. The FDA is expected to announce decisions about booster doses for the Johnson & Johnson and Moderna vaccines in the coming weeks.
Although initial trials of the COVID-19vaccines excluded pregnant and lactating women, the vaccines are safe in pregnancy or postpartum. In fact the vaccines do not contain either a killed or attenuated viral particle that is capable of transmitting infection. Therefore, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine now support routine immunization during pregnancy.
A recent report by Shimabukuro and colleagues2 demonstrated that the risk of vaccine-related complications in pregnant women receiving the Pfizer-BioNTech or Moderna vaccines was no different than in nonpregnant patients and that there was no evidence of teratogenic effects. The trial included more than 35,000 pregnant women; 2.3% were vaccinated in the periconception period, 28.6% in the first trimester, 43.3% in the second trimester, and 25.7% in the third trimester. Given this, and in light of isolated reports of unusual thromboembolic complications associated with the Johnson & Johnson vaccine, I strongly recommend use of either the Moderna or Pfizer-BioNTech vaccine in our prenatal and postpartum patients.
Continue to: Hepatitis A vaccine...
Hepatitis A vaccine
The hepatitis A vaccine is an inactivated vaccine and is safe for use in pregnancy. It is available in two monovalent preparations—Havrix (GlaxoSmithKline) and Vaqta (Merck & Co.) and is administered in a 2-dose intramuscular injection at time zero and 6 to 12 months later.3 The vaccine is also available in a bivalent form with recombinant hepatitis B vaccine—Twinrix (GlaxoSmithKline). When administered in this form, the vaccine should be given at time zero, 1 month, and 6 months. The wholesale cost of the monovalent vaccine is $66 to $119, depending upon whether the provider uses a multi-dose or a single-dose vial. The cost of Twinrix is $149.
The hepatitis A vaccine is indicated for select pregnant and nonpregnant patients:
- international travelers
- intravenous drug users
- those with occupational exposure (eg, individuals who work in a primate laboratory)
- residents and staff in chronic care facilities
- individuals with chronic liver disease
- individuals with clotting factor disorders
- residents in endemic areas.
Hepatitis B vaccine
The hepatitis B vaccine is a recombinant vaccine that contains an inactivated portion of the hepatitis B surface antigen. It was originally produced in two monovalent formulations: Engerix B (GlaxoSmithKline) and Recombivax-HB (Merck & Co.). These original formulations are given in a 3-dose series at time zero, 1 month, and 6 months. Recently, a new and more potent formulation was introduced into clinical practice. Heplisav-B (Dynavax Technologies Co.) is also a recombinant vaccine that contains a boosting adjuvant. It is programed to be administered in a 2-dose series at time zero and 1 month.4-6
The wholesale cost of the monovalent vaccines varies from $60 to $173, depending upon use of a multi-dose vial versus a single-use vial. The cost of Heplisav-B varies from $146 to $173, depending upon use of a prefilled syringe versus a single-dose vial.
Although the hepatitis B vaccine should be part of the childhood immunization series, it also should be administered to any pregnant woman who has not been vaccinated previously or who does not already have evidence of immunity as a result of natural infection.
Continue to: Herpes zoster vaccine...
Herpes zoster vaccine
Herpes zoster infection (shingles) can be a particularly disabling condition in older patients and results from reactivation of a latent varicella-zoster infection. Shingles can cause extremely painful skin lesions, threaten the patient’s vision, and result in long-lasting postherpetic neuralgia. Both cellular and hormonal immunity are essential to protect against recurrent infection.
The original herpes zoster vaccine (Zoster Vaccine Live; ZVL, Zostavax) is no longer produced in the United States because it is not as effective as the newer vaccine—Recombinant Zoster Vaccine (Shingrix, GlaxoSmithKline).7,8 The antigen in the new vaccine is a component of the surface glycoprotein E, and it is combined with an adjuvant to enhance immunoreactivity. The vaccine is given intramuscularly in two doses at time zero and again at 2 to 6 months and is indicated for all individuals >50 years, including those who may have had an episode of shingles. This newer vaccine is 97% effective in patients >50 years and 90% effective in patients >70. The wholesale cost of each injection is about $160.
Human papillomavirus vaccine
The HPV vaccine (Gardasil-9, Merck & Co.) is a recombinant 9-valent vaccine directed against the human papillomavirus. It induces immunity to serotypes 6 and 11 (which cause 90% of genital warts), 16 and 18 (which cause 80% of genital cancers), and 31, 33, 45, 52, and 58 (viral strains that are responsible for both genital and oropharyngeal cancers). The vaccine is administered intramuscularly in a 3-dose series at time zero, 1-2 months, and 6 months. The principal target groups for the vaccine are males and females, ages 9 to 45 years. Ideally, children of both sexes should receive this vaccine prior to the onset of sexual activity. The wholesale cost of each vaccine injection is approximately $222.9
Influenza vaccine
The inactivated, intramuscular flu vaccine is recommended for anyone over age 2, including pregnant women. Although pregnant women are not more likely to acquire flu compared with those who are not pregnant, if they do become infected, they are likely to become more seriously ill, with higher mortality. Accordingly, all pregnant women should receive, in any trimester, the inactivated flu vaccine beginning in the late summer and early fall of each year and extending through March of the next year.10,11
Multiple formulations of the inactivated vaccine are marketed, all targeting two strains of influenza A and two strains of influenza B. The components of the vaccine vary each year as scientists try to match the new vaccine with the most highly prevalent strains in the previous flu season. The vaccine should be administered in a single intramuscular dose. The cost varies from approximately $20 to $70.
The intranasal influenza vaccine is a live virus vaccine that is intended primarily for children and should not be administered in pregnancy. In addition, there is a higher dose of the inactivated quadrivalent vaccine that is available for administration to patients over age 65. This higher dose is more likely to cause adverse effects and is not indicated in pregnancy.
Continue to: Measles, mumps, rubella vaccine (MMR)...
Measles, mumps, rubella vaccine (MMR)
The MMR is a standard component of the childhood vaccination series. The trivalent preparation is a live, attenuated vaccine that is typically given subcutaneously in a 2-dose series. The first dose is administered at age 12-15 months, and the second dose at age 4-6 years. The vaccine is highly immunogenic, with vaccine-induced immunity usually life-long. In some patients, however, immunity wanes over time. Accordingly, all pregnant women should be screened for immunity to rubella since, of the 3, this infection poses the greatest risk to the fetus. Women who do not have evidence of immunity should be advised to avoid contact with children who may have a viral exanthem. They should then receive a booster dose of the vaccine immediately postpartum and should practice secure contraception for 1 month. The vaccine cost is approximately $60.
Pneumococcal vaccine
The inactivated pneumococcal vaccine is produced in two forms, both of which are safe for administration in pregnancy.12 The original vaccine, introduced in 1983, was PPSV23 (Pneumovax 23, Merck & Co), a 23-serovalent vaccine that was intended primarily for adults. This vaccine is administered in a single subcutaneous or intramuscular dose. The newest vaccine, introduced in 2010, is PCV13 (Prevnar 13, Pfizer Inc), a 13-serovalent vaccine. It was intended primarily for children, in whom it is administered in a 4-dose series beginning at 6 to 8 weeks of age. The cost of the former is approximately $98 to $120; the cost of the latter is $228.
Vaccination against pneumococcal infection is routinely indicated for those older than the age of 65 and for the following at-risk patients, including those who are pregnant11:
- individuals who have had a splenectomy or who have a medical illness that produces functional asplenia (eg, sickle cell anemia)
- individuals with chronic cardiac, pulmonic, hepatic, or renal disease
- individuals with immunosuppressive conditions such as HIV infection or a disseminated malignancy
- individuals who have a cochlear implant
- individuals who have a chronic leak of cerebrospinal fluid.
The recommendations for timing of these 2 vaccines in adults can initially appear confusing. Put most simply, if a high-risk patient first receives the PCV13 vaccine, she should receive the PPSV23 vaccine in about 8 weeks. The PPSV23 vaccine should be repeated in 5 years. If an at-risk patient initially receives the PPSV23 vaccine, the PCV13 vaccine should be given 1 year later.12
Tdap vaccine
The Tdap vaccine contains tetanus toxoid, reduced diptheria toxoid, and an acellular component of the pertussis bacterium. Although it has long been part of the childhood vaccinations series, immunity to each component, particularly pertussis, tends to wane over time.
Pertussis poses a serious risk to the health of the pregnant woman and the newborn infant. Accordingly, the Advisory Committee on Immunization Practices (ACIP), CDC, and the ACOG now advise administration of a booster dose of this vaccine in the early third trimester of each pregnancy.13-15 The vaccine should be administered as a single intramuscular injection. The approximate cost of the vaccine is $64 to $71, depending upon whether the provider uses a single-dose vial or a single-dose prefilled syringe. In nonpregnant patients, the ACIP currently recommends administration of a booster dose of the vaccine every 10 years, primarily to provide durable protection against tetanus.
Continue to: Varicella vaccine...
Varicella vaccine
The varicella vaccine is also one of the main components of the childhood immunization series. This live virus vaccine can be administered subcutaneously as a monovalent agent or as a quadrivalent agent in association with the MMR vaccine.
Pregnant women who do not have a well-documented history of natural infection should be tested for IgG antibody to the varicella-zoster virus at the time of their first prenatal appointment. Interestingly, approximately 70% of patients with an uncertain history actually have immunity when tested. If the patient lacks immunity, she should be vaccinated immediately postpartum.16,17 The vaccine should be administered in a 2-dose series at time zero and then 4 to 8 weeks later. Patients should adhere to secure contraception from the time of the first dose until 1 month after the second dose. The cost of each dose of the vaccine is approximately $145.
Adverse effects of vaccination
All vaccines have many of the same side effects. The most common is simply a reaction at the site of injection, characterized by pain, increased warmth, erythema, swelling, and tenderness. Other common side effects include systemic manifestations, such as low-grade fever, nausea and vomiting, malaise, fatigue, headache, lymphadenopathy, myalgias, and arthralgias. Some vaccines, notably varicella, herpes zoster, measles, and rubella may cause a disseminated rash. Most of these minor side effects are easily managed by rest, hydration, and administration of an analgesic such as acetaminophen or ibuprofen. More serious side effects include rare complications such as anaphylaxis, Bell palsy, Guillain-Barre syndrome, and venous thromboembolism (Johnson & Johnson COVID-19 vaccine). Any of the vaccines discussed above should not be given, or given only with extreme caution, to an individual who has experienced any of these reactions with a previous vaccine.
Barriers to vaccination
Although the vaccines reviewed above are highly effective in preventing serious illness in recipients, the medical profession’s “report card” in ensuring adherence with vaccine protocols is not optimal. In fact, it probably merits a grade no higher than C+, with vaccination rates in the range of 50% to 70%.
One of the major barriers to vaccination is lack of detailed information about vaccine efficacy and safety on the part of both provider and patient. Another is the problem of misinformation (eg, the persistent belief on the part of some individuals that vaccines may cause a serious problem, such as autism).18,19 Another important barrier to widespread vaccination is the logistical problem associated with proper scheduling of multidose regimens (such as those for hepatitis A and B, varicella, and COVID-19). A final barrier, and in my own university-based practice, the most important obstacle is the expense of vaccination. Most, but not all, private insurance companies provide coverage for vaccines approved by the Centers for Disease Control and Prevention and the US Preventive Services Task Force. However, public insurance agencies often provide disappointingly inconsistent coverage for essential vaccines.
By keeping well informed about the most recent public health recommendations for vaccinations for adults and by leading important initiatives within our own practices, we should be able to overcome the first 3 barriers listed above. For example, Morgan and colleagues20 recently achieved a 97% success rate with Tdap administration in pregnancy by placing a best-practice alert in the patients’ electronic medical records. Surmounting the final barrier will require intense effort on the part of individual practitioners and professional organizations to advocate for coverage for essential vaccinations for our patients.
CASE Resolved
This patient was raised in an area of the world where her family did not have easy access to medical care. Accordingly, she did not receive the usual childhood vaccines, such as measles, mumps, rubella, varicella, hepatitis B, and almost certainly, tetanus, diphtheria, and pertussis (Tdap), and the HPV vaccine. The MMR vaccine and the varicella vaccine are live virus vaccines and should not be given during pregnancy. However, these vaccines should be administered postpartum, and the patient should be instructed to practice secure contraception for a minimum of 1 month following vaccination. She also should be offered the HPV vaccine postpartum. During pregnancy, she definitely should receive the COVID-19 vaccine, the 3-dose hepatitis B vaccine series, the influenza vaccine, and Tdap. If her present living conditions place her at risk for hepatitis A, she also should be vaccinated against this illness. ●

CASE 1st prenatal appointment for young, pregnant migrant
A 21-year-old primigravid woman at 12 weeks’ gestation recently immigrated to the United States from an impoverished rural area of Southeast Asia. On the first prenatal appointment, she is noted to have no evidence of immunity to rubella, measles, or varicella. Her hepatitis B surface antigen and hepatitis C antibody tests are negative. She also has negative test results for gonorrhea, chlamydia, syphilis, and HIV infection. Her pap test is negative.
- What vaccinations should this patient receive during her pregnancy?
- What additional vaccinations are indicated postpartum?
Preventive vaccinations: What to know
As ObGyns, we serve as the primary care physician for many women throughout their early and middle decades of life. Accordingly, we have an obligation to be well informed about preventive health services such as vaccinations. The purpose of this article is to review the principal vaccines with which ObGyns should be familiar. I will discuss the vaccines in alphabetical order and then focus on the indications and timing for each vaccine and the relative cost of each immunization. Key points are summarized in the TABLE.

COVID-19 vaccine
In the latter part of 2020 and early part of 2021, three COVID-19 vaccines received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for individuals 16 years of age and older (Pfizer-BioNTech) and 18 years of age and older (Moderna and Johnson & Johnson).1 The cost of their administration is borne by the federal government. Two of the vaccines are mRNA agents—Moderna and Pfizer-BioNTech. Both are administered in a 2-dose series, separated by 4 and 3 weeks, respectively. The efficacy of these vaccines in preventing serious or critical illness approaches 95%. The Pfizer-BioNTech vaccine has now been fully FDA approved for administration to individuals older than age 16, with EUA for those down to age 12. Full approval of the Moderna vaccine will not be far behind. Because of some evidence suggesting waning immunity over time and because of growing concerns about the increased transmissibility of the delta variant of the virus, the FDA has been strongly considering a recommendation for a third (booster) dose of each of these vaccines, administered 8 months after the second dose for all eligible Americans. On September 17, 2021, the FDA advisory committee recommended a booster for the Pfizer-BioNTech vaccine for people older than age 65 and for those over the age of 16 at high risk for severe COVID-19. Several days later, full FDA approval was granted for this recommendation. Subsequently, the Director of the Centers for Disease Control and Prevention (CDC) included health care workers and pregnant women in the group for whom the booster is recommended.
The third vaccine formulation is the Johnson & Johnson DNA vaccine, which is prepared with a human adenovirus vector. This vaccine is administered in a single intramuscular dose and has a reported efficacy of 66% to 85%, though it may approach 95% in preventing critical illness. The FDA is expected to announce decisions about booster doses for the Johnson & Johnson and Moderna vaccines in the coming weeks.
Although initial trials of the COVID-19vaccines excluded pregnant and lactating women, the vaccines are safe in pregnancy or postpartum. In fact the vaccines do not contain either a killed or attenuated viral particle that is capable of transmitting infection. Therefore, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine now support routine immunization during pregnancy.
A recent report by Shimabukuro and colleagues2 demonstrated that the risk of vaccine-related complications in pregnant women receiving the Pfizer-BioNTech or Moderna vaccines was no different than in nonpregnant patients and that there was no evidence of teratogenic effects. The trial included more than 35,000 pregnant women; 2.3% were vaccinated in the periconception period, 28.6% in the first trimester, 43.3% in the second trimester, and 25.7% in the third trimester. Given this, and in light of isolated reports of unusual thromboembolic complications associated with the Johnson & Johnson vaccine, I strongly recommend use of either the Moderna or Pfizer-BioNTech vaccine in our prenatal and postpartum patients.
Continue to: Hepatitis A vaccine...
Hepatitis A vaccine
The hepatitis A vaccine is an inactivated vaccine and is safe for use in pregnancy. It is available in two monovalent preparations—Havrix (GlaxoSmithKline) and Vaqta (Merck & Co.) and is administered in a 2-dose intramuscular injection at time zero and 6 to 12 months later.3 The vaccine is also available in a bivalent form with recombinant hepatitis B vaccine—Twinrix (GlaxoSmithKline). When administered in this form, the vaccine should be given at time zero, 1 month, and 6 months. The wholesale cost of the monovalent vaccine is $66 to $119, depending upon whether the provider uses a multi-dose or a single-dose vial. The cost of Twinrix is $149.
The hepatitis A vaccine is indicated for select pregnant and nonpregnant patients:
- international travelers
- intravenous drug users
- those with occupational exposure (eg, individuals who work in a primate laboratory)
- residents and staff in chronic care facilities
- individuals with chronic liver disease
- individuals with clotting factor disorders
- residents in endemic areas.
Hepatitis B vaccine
The hepatitis B vaccine is a recombinant vaccine that contains an inactivated portion of the hepatitis B surface antigen. It was originally produced in two monovalent formulations: Engerix B (GlaxoSmithKline) and Recombivax-HB (Merck & Co.). These original formulations are given in a 3-dose series at time zero, 1 month, and 6 months. Recently, a new and more potent formulation was introduced into clinical practice. Heplisav-B (Dynavax Technologies Co.) is also a recombinant vaccine that contains a boosting adjuvant. It is programed to be administered in a 2-dose series at time zero and 1 month.4-6
The wholesale cost of the monovalent vaccines varies from $60 to $173, depending upon use of a multi-dose vial versus a single-use vial. The cost of Heplisav-B varies from $146 to $173, depending upon use of a prefilled syringe versus a single-dose vial.
Although the hepatitis B vaccine should be part of the childhood immunization series, it also should be administered to any pregnant woman who has not been vaccinated previously or who does not already have evidence of immunity as a result of natural infection.
Continue to: Herpes zoster vaccine...
Herpes zoster vaccine
Herpes zoster infection (shingles) can be a particularly disabling condition in older patients and results from reactivation of a latent varicella-zoster infection. Shingles can cause extremely painful skin lesions, threaten the patient’s vision, and result in long-lasting postherpetic neuralgia. Both cellular and hormonal immunity are essential to protect against recurrent infection.
The original herpes zoster vaccine (Zoster Vaccine Live; ZVL, Zostavax) is no longer produced in the United States because it is not as effective as the newer vaccine—Recombinant Zoster Vaccine (Shingrix, GlaxoSmithKline).7,8 The antigen in the new vaccine is a component of the surface glycoprotein E, and it is combined with an adjuvant to enhance immunoreactivity. The vaccine is given intramuscularly in two doses at time zero and again at 2 to 6 months and is indicated for all individuals >50 years, including those who may have had an episode of shingles. This newer vaccine is 97% effective in patients >50 years and 90% effective in patients >70. The wholesale cost of each injection is about $160.
Human papillomavirus vaccine
The HPV vaccine (Gardasil-9, Merck & Co.) is a recombinant 9-valent vaccine directed against the human papillomavirus. It induces immunity to serotypes 6 and 11 (which cause 90% of genital warts), 16 and 18 (which cause 80% of genital cancers), and 31, 33, 45, 52, and 58 (viral strains that are responsible for both genital and oropharyngeal cancers). The vaccine is administered intramuscularly in a 3-dose series at time zero, 1-2 months, and 6 months. The principal target groups for the vaccine are males and females, ages 9 to 45 years. Ideally, children of both sexes should receive this vaccine prior to the onset of sexual activity. The wholesale cost of each vaccine injection is approximately $222.9
Influenza vaccine
The inactivated, intramuscular flu vaccine is recommended for anyone over age 2, including pregnant women. Although pregnant women are not more likely to acquire flu compared with those who are not pregnant, if they do become infected, they are likely to become more seriously ill, with higher mortality. Accordingly, all pregnant women should receive, in any trimester, the inactivated flu vaccine beginning in the late summer and early fall of each year and extending through March of the next year.10,11
Multiple formulations of the inactivated vaccine are marketed, all targeting two strains of influenza A and two strains of influenza B. The components of the vaccine vary each year as scientists try to match the new vaccine with the most highly prevalent strains in the previous flu season. The vaccine should be administered in a single intramuscular dose. The cost varies from approximately $20 to $70.
The intranasal influenza vaccine is a live virus vaccine that is intended primarily for children and should not be administered in pregnancy. In addition, there is a higher dose of the inactivated quadrivalent vaccine that is available for administration to patients over age 65. This higher dose is more likely to cause adverse effects and is not indicated in pregnancy.
Continue to: Measles, mumps, rubella vaccine (MMR)...
Measles, mumps, rubella vaccine (MMR)
The MMR is a standard component of the childhood vaccination series. The trivalent preparation is a live, attenuated vaccine that is typically given subcutaneously in a 2-dose series. The first dose is administered at age 12-15 months, and the second dose at age 4-6 years. The vaccine is highly immunogenic, with vaccine-induced immunity usually life-long. In some patients, however, immunity wanes over time. Accordingly, all pregnant women should be screened for immunity to rubella since, of the 3, this infection poses the greatest risk to the fetus. Women who do not have evidence of immunity should be advised to avoid contact with children who may have a viral exanthem. They should then receive a booster dose of the vaccine immediately postpartum and should practice secure contraception for 1 month. The vaccine cost is approximately $60.
Pneumococcal vaccine
The inactivated pneumococcal vaccine is produced in two forms, both of which are safe for administration in pregnancy.12 The original vaccine, introduced in 1983, was PPSV23 (Pneumovax 23, Merck & Co), a 23-serovalent vaccine that was intended primarily for adults. This vaccine is administered in a single subcutaneous or intramuscular dose. The newest vaccine, introduced in 2010, is PCV13 (Prevnar 13, Pfizer Inc), a 13-serovalent vaccine. It was intended primarily for children, in whom it is administered in a 4-dose series beginning at 6 to 8 weeks of age. The cost of the former is approximately $98 to $120; the cost of the latter is $228.
Vaccination against pneumococcal infection is routinely indicated for those older than the age of 65 and for the following at-risk patients, including those who are pregnant11:
- individuals who have had a splenectomy or who have a medical illness that produces functional asplenia (eg, sickle cell anemia)
- individuals with chronic cardiac, pulmonic, hepatic, or renal disease
- individuals with immunosuppressive conditions such as HIV infection or a disseminated malignancy
- individuals who have a cochlear implant
- individuals who have a chronic leak of cerebrospinal fluid.
The recommendations for timing of these 2 vaccines in adults can initially appear confusing. Put most simply, if a high-risk patient first receives the PCV13 vaccine, she should receive the PPSV23 vaccine in about 8 weeks. The PPSV23 vaccine should be repeated in 5 years. If an at-risk patient initially receives the PPSV23 vaccine, the PCV13 vaccine should be given 1 year later.12
Tdap vaccine
The Tdap vaccine contains tetanus toxoid, reduced diptheria toxoid, and an acellular component of the pertussis bacterium. Although it has long been part of the childhood vaccinations series, immunity to each component, particularly pertussis, tends to wane over time.
Pertussis poses a serious risk to the health of the pregnant woman and the newborn infant. Accordingly, the Advisory Committee on Immunization Practices (ACIP), CDC, and the ACOG now advise administration of a booster dose of this vaccine in the early third trimester of each pregnancy.13-15 The vaccine should be administered as a single intramuscular injection. The approximate cost of the vaccine is $64 to $71, depending upon whether the provider uses a single-dose vial or a single-dose prefilled syringe. In nonpregnant patients, the ACIP currently recommends administration of a booster dose of the vaccine every 10 years, primarily to provide durable protection against tetanus.
Continue to: Varicella vaccine...
Varicella vaccine
The varicella vaccine is also one of the main components of the childhood immunization series. This live virus vaccine can be administered subcutaneously as a monovalent agent or as a quadrivalent agent in association with the MMR vaccine.
Pregnant women who do not have a well-documented history of natural infection should be tested for IgG antibody to the varicella-zoster virus at the time of their first prenatal appointment. Interestingly, approximately 70% of patients with an uncertain history actually have immunity when tested. If the patient lacks immunity, she should be vaccinated immediately postpartum.16,17 The vaccine should be administered in a 2-dose series at time zero and then 4 to 8 weeks later. Patients should adhere to secure contraception from the time of the first dose until 1 month after the second dose. The cost of each dose of the vaccine is approximately $145.
Adverse effects of vaccination
All vaccines have many of the same side effects. The most common is simply a reaction at the site of injection, characterized by pain, increased warmth, erythema, swelling, and tenderness. Other common side effects include systemic manifestations, such as low-grade fever, nausea and vomiting, malaise, fatigue, headache, lymphadenopathy, myalgias, and arthralgias. Some vaccines, notably varicella, herpes zoster, measles, and rubella may cause a disseminated rash. Most of these minor side effects are easily managed by rest, hydration, and administration of an analgesic such as acetaminophen or ibuprofen. More serious side effects include rare complications such as anaphylaxis, Bell palsy, Guillain-Barre syndrome, and venous thromboembolism (Johnson & Johnson COVID-19 vaccine). Any of the vaccines discussed above should not be given, or given only with extreme caution, to an individual who has experienced any of these reactions with a previous vaccine.
Barriers to vaccination
Although the vaccines reviewed above are highly effective in preventing serious illness in recipients, the medical profession’s “report card” in ensuring adherence with vaccine protocols is not optimal. In fact, it probably merits a grade no higher than C+, with vaccination rates in the range of 50% to 70%.
One of the major barriers to vaccination is lack of detailed information about vaccine efficacy and safety on the part of both provider and patient. Another is the problem of misinformation (eg, the persistent belief on the part of some individuals that vaccines may cause a serious problem, such as autism).18,19 Another important barrier to widespread vaccination is the logistical problem associated with proper scheduling of multidose regimens (such as those for hepatitis A and B, varicella, and COVID-19). A final barrier, and in my own university-based practice, the most important obstacle is the expense of vaccination. Most, but not all, private insurance companies provide coverage for vaccines approved by the Centers for Disease Control and Prevention and the US Preventive Services Task Force. However, public insurance agencies often provide disappointingly inconsistent coverage for essential vaccines.
By keeping well informed about the most recent public health recommendations for vaccinations for adults and by leading important initiatives within our own practices, we should be able to overcome the first 3 barriers listed above. For example, Morgan and colleagues20 recently achieved a 97% success rate with Tdap administration in pregnancy by placing a best-practice alert in the patients’ electronic medical records. Surmounting the final barrier will require intense effort on the part of individual practitioners and professional organizations to advocate for coverage for essential vaccinations for our patients.
CASE Resolved
This patient was raised in an area of the world where her family did not have easy access to medical care. Accordingly, she did not receive the usual childhood vaccines, such as measles, mumps, rubella, varicella, hepatitis B, and almost certainly, tetanus, diphtheria, and pertussis (Tdap), and the HPV vaccine. The MMR vaccine and the varicella vaccine are live virus vaccines and should not be given during pregnancy. However, these vaccines should be administered postpartum, and the patient should be instructed to practice secure contraception for a minimum of 1 month following vaccination. She also should be offered the HPV vaccine postpartum. During pregnancy, she definitely should receive the COVID-19 vaccine, the 3-dose hepatitis B vaccine series, the influenza vaccine, and Tdap. If her present living conditions place her at risk for hepatitis A, she also should be vaccinated against this illness. ●
- Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy. What obstetricians need to know. Obstet Gynecol. 2021;137:408-414. doi: 10.1097/AOG.0000000000004290.
- Shimabukuro TT, Kim SY, Myers RT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282. doi: 10.1056/NEJMoa2104983.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471. doi: 10.1016/s0029-7844(97)00669-8.
- Omer SB. Maternal immunization. N Engl J Med. 2017;376:1256-1267. doi: 10.1056/NEJMra1509044.
- Dionne-Odom J, Tita AT, Silverman NS. Society for Maternal-Fetal Medicine Consult Series: #38: hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14. doi: http://dx.doi.org/10.1016/j.ajog.2015.09.100.
- Yawetz S. Immunizations during pregnancy. UpToDate, January 15, 2021.
- Cunningham Al, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016:375:1019-1032. doi: 10.1056/NEJMoa1603800.
- Albrecht MA, Levin MJ. Vaccination for the prevention of shingles (herpes zoster). UpToDate, July 6, 2020.
- ACOG Committee Opinion. Human papillomavirus vaccination. Obstet Gynecol. 2006;108:699-705. doi: 10.1097/00006250-200609000-00047.
- Callaghan WM, Creanga AA, Jamieson DJ. Pregnancy-related mortality resulting from influenza in the United States during the 2009-2010 pandemic. Obstet Gynecol. 2015;126:486-490. doi: 10.1097/AOG.0000000000000996.
- ACOG Committee Opinion. Influenza vaccination during pregnancy. Obstet Gynecol. 2014;124:648-651. doi: 10.1097/01.AOG.0000453599.11566.11.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233. doi: 10.1056/NEJMoa1612296.
- Moumne O, Duff P. Treatment and prevention of pneumococcal infection. Clin Obstet Gynecol. 2019;62:781-789. doi: 10.1097/GRF.0000000000000451.
- ACOG Committee Opinion. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2017;130:668-669. doi: 10.1097/AOG.0000000000002293.
- Sukumaran L, McCarthy NL, Kharbanda EO, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol. 2015;126:1069-1074. doi: 10.1097/AOG.0000000000001066.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165. doi: 10.1155/S1064744994000013.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65. doi: 10.1155/S1064744996000142.
- Desmond A, Offit PA. On the shoulders of giants--from Jenner's cowpox to mRNA COVID vaccines. N Engl. J Med. 2021;384:1081-1083. doi: 10.1056/NEJMp2034334.
- Poland GA, Jacobson RM. The age-old struggle against the antivaccinationists. N Engl J Med. 2011;364:97-99. doi: 10.1056/NEJMp1010594.
- Morgan JL, Baggari SR, Chung W, et al. Association of a best-practice alert and prenatal administration with tetanus toxoid, reduced diptheria toxoid, and acellular pertussis vaccination rates. Obstet Gynecol. 2015;126:333-337. doi: 10.1097/AOG.0000000000000975.
- Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy. What obstetricians need to know. Obstet Gynecol. 2021;137:408-414. doi: 10.1097/AOG.0000000000004290.
- Shimabukuro TT, Kim SY, Myers RT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282. doi: 10.1056/NEJMoa2104983.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471. doi: 10.1016/s0029-7844(97)00669-8.
- Omer SB. Maternal immunization. N Engl J Med. 2017;376:1256-1267. doi: 10.1056/NEJMra1509044.
- Dionne-Odom J, Tita AT, Silverman NS. Society for Maternal-Fetal Medicine Consult Series: #38: hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14. doi: http://dx.doi.org/10.1016/j.ajog.2015.09.100.
- Yawetz S. Immunizations during pregnancy. UpToDate, January 15, 2021.
- Cunningham Al, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016:375:1019-1032. doi: 10.1056/NEJMoa1603800.
- Albrecht MA, Levin MJ. Vaccination for the prevention of shingles (herpes zoster). UpToDate, July 6, 2020.
- ACOG Committee Opinion. Human papillomavirus vaccination. Obstet Gynecol. 2006;108:699-705. doi: 10.1097/00006250-200609000-00047.
- Callaghan WM, Creanga AA, Jamieson DJ. Pregnancy-related mortality resulting from influenza in the United States during the 2009-2010 pandemic. Obstet Gynecol. 2015;126:486-490. doi: 10.1097/AOG.0000000000000996.
- ACOG Committee Opinion. Influenza vaccination during pregnancy. Obstet Gynecol. 2014;124:648-651. doi: 10.1097/01.AOG.0000453599.11566.11.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233. doi: 10.1056/NEJMoa1612296.
- Moumne O, Duff P. Treatment and prevention of pneumococcal infection. Clin Obstet Gynecol. 2019;62:781-789. doi: 10.1097/GRF.0000000000000451.
- ACOG Committee Opinion. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2017;130:668-669. doi: 10.1097/AOG.0000000000002293.
- Sukumaran L, McCarthy NL, Kharbanda EO, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol. 2015;126:1069-1074. doi: 10.1097/AOG.0000000000001066.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165. doi: 10.1155/S1064744994000013.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65. doi: 10.1155/S1064744996000142.
- Desmond A, Offit PA. On the shoulders of giants--from Jenner's cowpox to mRNA COVID vaccines. N Engl. J Med. 2021;384:1081-1083. doi: 10.1056/NEJMp2034334.
- Poland GA, Jacobson RM. The age-old struggle against the antivaccinationists. N Engl J Med. 2011;364:97-99. doi: 10.1056/NEJMp1010594.
- Morgan JL, Baggari SR, Chung W, et al. Association of a best-practice alert and prenatal administration with tetanus toxoid, reduced diptheria toxoid, and acellular pertussis vaccination rates. Obstet Gynecol. 2015;126:333-337. doi: 10.1097/AOG.0000000000000975.
Novel and Alternative Strategies for Management of Panitumumab-Induced Hypomagnesemia
Background
Panitumumab is an epidermal growth factor receptor (EGFR) inhibiting monoclonal antibody approved for the treatment of RAS wild-type metastatic colorectal cancer (mCRC), which has an incidence of hypomagnesemia of approximately 35%. Grade 3 or 4 hypomagnesemia occurs in roughly 7% of patients, which can lead to serious complications such as seizures and arrhythmias. In one study, hypomagnesemia led to discontinuation of targeted therapy in 3% of patients. Currently, there is no standardized prophylactic strategy or treatment protocol for panitumumab-induced hypomagnesemia. In cases of refractory hypomagnesemia, it is recommended to discontinue panitumumab, even if the patient is deriving clinical benefit.
Case Report
This 59-year-old male was diagnosed with RAS wild-type mCRC and had already progressed through multiple lines of treatment. Panitumumab was initiated with good response; however, the drug was discontinued due to grade 4 hypomagnesemia, despite intravenous and oral supplementation. As the patient progressed through further lines of treatment, the decision was made to retry panitumumab. Grade 2-3 hypomagnesemia persisted throughout treatment, requiring frequent magnesium infusions. Innovative and alternative treatment options were investigated in an effort to improve his quality of life. In addition to oral and intravenous magnesium replacement, an ambulatory elastomeric pump, traditionally used for fluorouracil administration, was repurposed to deliver between 6 and 24 grams of magnesium sulfate over 24 to 72 hours. The pump was generally well tolerated with the exception of mild skin irritation around the port site, which prevented a transition to longer infusion times. The ambulatory elastomeric pump decreased the frequency of healthcare visits and improved the hypomagnesemia sufficiently to continue treatment with panitumumab, although levels did not fully normalize. A two-week trial of amiloride was also attempted to decrease renal magnesium wasting. Amiloride normalized magnesium levels but had to be discontinued due to asymptomatic hyperkalemia. This case report suggests that amiloride and magnesium replacement via ambulatory elastomeric pumps may be safe and effective treatment options for panitumumab-induced refractory hypomagnesemia in mCRC, potentially improving quality of life and allowing beneficial anti-cancer treatments to continue. Future studies should further evaluate optimization of amiloride and intravenous magnesium replacement via ambulatory elastomeric pump.
Background
Panitumumab is an epidermal growth factor receptor (EGFR) inhibiting monoclonal antibody approved for the treatment of RAS wild-type metastatic colorectal cancer (mCRC), which has an incidence of hypomagnesemia of approximately 35%. Grade 3 or 4 hypomagnesemia occurs in roughly 7% of patients, which can lead to serious complications such as seizures and arrhythmias. In one study, hypomagnesemia led to discontinuation of targeted therapy in 3% of patients. Currently, there is no standardized prophylactic strategy or treatment protocol for panitumumab-induced hypomagnesemia. In cases of refractory hypomagnesemia, it is recommended to discontinue panitumumab, even if the patient is deriving clinical benefit.
Case Report
This 59-year-old male was diagnosed with RAS wild-type mCRC and had already progressed through multiple lines of treatment. Panitumumab was initiated with good response; however, the drug was discontinued due to grade 4 hypomagnesemia, despite intravenous and oral supplementation. As the patient progressed through further lines of treatment, the decision was made to retry panitumumab. Grade 2-3 hypomagnesemia persisted throughout treatment, requiring frequent magnesium infusions. Innovative and alternative treatment options were investigated in an effort to improve his quality of life. In addition to oral and intravenous magnesium replacement, an ambulatory elastomeric pump, traditionally used for fluorouracil administration, was repurposed to deliver between 6 and 24 grams of magnesium sulfate over 24 to 72 hours. The pump was generally well tolerated with the exception of mild skin irritation around the port site, which prevented a transition to longer infusion times. The ambulatory elastomeric pump decreased the frequency of healthcare visits and improved the hypomagnesemia sufficiently to continue treatment with panitumumab, although levels did not fully normalize. A two-week trial of amiloride was also attempted to decrease renal magnesium wasting. Amiloride normalized magnesium levels but had to be discontinued due to asymptomatic hyperkalemia. This case report suggests that amiloride and magnesium replacement via ambulatory elastomeric pumps may be safe and effective treatment options for panitumumab-induced refractory hypomagnesemia in mCRC, potentially improving quality of life and allowing beneficial anti-cancer treatments to continue. Future studies should further evaluate optimization of amiloride and intravenous magnesium replacement via ambulatory elastomeric pump.
Background
Panitumumab is an epidermal growth factor receptor (EGFR) inhibiting monoclonal antibody approved for the treatment of RAS wild-type metastatic colorectal cancer (mCRC), which has an incidence of hypomagnesemia of approximately 35%. Grade 3 or 4 hypomagnesemia occurs in roughly 7% of patients, which can lead to serious complications such as seizures and arrhythmias. In one study, hypomagnesemia led to discontinuation of targeted therapy in 3% of patients. Currently, there is no standardized prophylactic strategy or treatment protocol for panitumumab-induced hypomagnesemia. In cases of refractory hypomagnesemia, it is recommended to discontinue panitumumab, even if the patient is deriving clinical benefit.
Case Report
This 59-year-old male was diagnosed with RAS wild-type mCRC and had already progressed through multiple lines of treatment. Panitumumab was initiated with good response; however, the drug was discontinued due to grade 4 hypomagnesemia, despite intravenous and oral supplementation. As the patient progressed through further lines of treatment, the decision was made to retry panitumumab. Grade 2-3 hypomagnesemia persisted throughout treatment, requiring frequent magnesium infusions. Innovative and alternative treatment options were investigated in an effort to improve his quality of life. In addition to oral and intravenous magnesium replacement, an ambulatory elastomeric pump, traditionally used for fluorouracil administration, was repurposed to deliver between 6 and 24 grams of magnesium sulfate over 24 to 72 hours. The pump was generally well tolerated with the exception of mild skin irritation around the port site, which prevented a transition to longer infusion times. The ambulatory elastomeric pump decreased the frequency of healthcare visits and improved the hypomagnesemia sufficiently to continue treatment with panitumumab, although levels did not fully normalize. A two-week trial of amiloride was also attempted to decrease renal magnesium wasting. Amiloride normalized magnesium levels but had to be discontinued due to asymptomatic hyperkalemia. This case report suggests that amiloride and magnesium replacement via ambulatory elastomeric pumps may be safe and effective treatment options for panitumumab-induced refractory hypomagnesemia in mCRC, potentially improving quality of life and allowing beneficial anti-cancer treatments to continue. Future studies should further evaluate optimization of amiloride and intravenous magnesium replacement via ambulatory elastomeric pump.