User login
Hematocrit, White Blood Cells, and Thrombotic Events in the Veteran Population With Polycythemia Vera
Polycythemia vera (PV) is a rare myeloproliferative neoplasm affecting 44 to 57 individuals per 100,000 in the United States.1,2 It is characterized by somatic mutations in the hematopoietic stem cell, resulting in hyperproliferation of mature myeloid lineage cells.2 Sustained erythrocytosis is a hallmark of PV, although many patients also have leukocytosis and thrombocytosis.2,3 These patients have increased inherent thrombotic risk with arterial events reported to occur at rates of 7 to 21/1000 person-years and venous thrombotic events at 5 to 20/1000 person-years.4-7 Thrombotic and cardiovascular events are leading causes of morbidity and mortality, resulting in a reduced overall survival of patients with PV compared with the general population.3,8-10
Blood Cell Counts and Thrombotic Events in PV
Treatment strategies for patients with PV mainly aim to prevent or manage thrombotic and bleeding complications through normalization of blood counts.11 Hematocrit (Hct) control has been reported to be associated with reduced thrombotic risk in patients with PV. This was shown and popularized by the prospective, randomized Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) trial in which participants were randomized 1:1 to maintaining either a low (< 45%) or high (45%-50%) Hct for 5 years to examine the long-term effects of more- or less-intensive cytoreductive therapy.12 Patients in the low-Hct group were found to have a lower rate of death from cardiovascular events or major thrombosis (1.1/100 person-years in the low-Hct group vs 4.4 in the high-Hct group; hazard ratio [HR], 3.91; 95% confidence interval [CI], 1.45-10.53; P = .007). Likewise, cardiovascular events occurred at a lower rate in patients in the low-Hct group compared with the high-Hct group (4.4% vs 10.9% of patients, respectively; HR, 2.69; 95% CI, 1.19-6.12; P = .02).12
Leukocytosis has also been linked to elevated risk for vascular events as shown in several studies, including the real-world European Collaboration on Low-Dose Aspirin in PV (ECLAP) observational study and a post hoc subanalysis of the CYTO-PV study.13,14 In a multivariate, time-dependent analysis in ECLAP, patients with white blood cell (WBC) counts > 15 × 109/L had a significant increase in the risk of thrombosis compared with those who had lower WBC counts, with higher WBC count more strongly associated with arterial than venous thromboembolism.13 In CYTO-PV, a significant correlation between elevated WBC count (≥ 11 × 109/L vs reference level of < 7 × 109/L) and time-dependent risk of major thrombosis was shown (HR, 3.9; 95% CI, 1.24-12.3; P = .02).14 Likewise, WBC count ≥ 11 × 109/L was found to be a predictor of subsequent venous events in a separate single-center multivariate analysis of patients with PV.8
Although CYTO-PV remains one of the largest prospective landmark studies in PV demonstrating the impact of Hct control on thrombosis, it is worthwhile to note that the patients in the high-Hct group who received less frequent myelosuppressive therapy with hydroxyurea than the low-Hct group also had higher WBC counts.12,15 Work is needed to determine the relative effects of high Hct and high WBC counts on PV independent of each other.
The Veteran Population with PV
Two recently published retrospective analyses from Parasuraman and colleagues used data from the Veterans Health Administration (VHA), the largest integrated health care system in the US, with an aim to replicate findings from CYTO-PV in a real-world population.16,17 The 2 analyses focused independently on the effects of Hct control and WBC count on the risk of a thrombotic event in patients with PV.
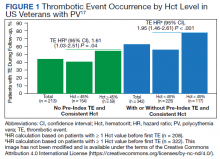
In the first retrospective analysis, 213 patients with PV and no prior thrombosis were placed into groups based on whether Hct levels were consistently either < 45% or ≥ 45% throughout the study period.17 The mean follow-up time was 2.3 years, during which 44.1% of patients experienced a thrombotic event (Figure 1). Patients with Hct levels < 45% had a lower rate of thrombotic events compared to those with levels ≥ 45% (40.3% vs 54.2%, respectively; HR, 1.61; 95% CI, 1.03-2.51; P = .04). In a sensitivity analysis that included patients with pre-index thrombotic events (N = 342), similar results were noted (55.6% vs 76.9% between the < 45% and ≥ 45% groups, respectively; HR, 1.95; 95% CI, 1.46-2.61; P < .001).
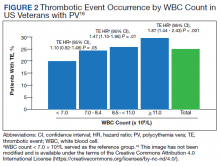
In the second analysis, the authors investigated the relationship between WBC counts and thrombotic events.16 Evaluable patients (N = 1565) were grouped into 1 of 4 cohorts based on the last WBC measurement taken during the study period before a thrombotic event or through the end of follow-up: (1) WBC < 7.0 × 109/L, (2) 7.0 to 8.4 × 109/L, (3) 8.5 to < 11.0 × 109/L, or (4) ≥ 11.0 × 109/L. Mean follow-up time ranged from 3.6 to 4.5 years among WBC count cohorts, during which 24.9% of patients experienced a thrombotic event. Compared with the reference cohort (WBC < 7.0 × 109/L), a significant positive association between WBC counts and thrombotic event occurrence was observed among patients with WBC counts of 8.5 to < 11.0 × 109/L (HR, 1.47; 95% CI, 1.10-1.96; P < .01) and ≥ 11 × 109/L (HR, 1.87; 95% CI, 1.44-2.43; P < .001) (Figure 2).16 When including all patients in a sensitivity analysis regardless of whether they experienced thrombotic events before the index date (N = 1876), similar results were obtained (7.0-8.4 × 109/L group: HR, 1.22; 95% CI, 0.97-1.55; P = .0959; 8.5 - 11.0 × 109/L group: HR, 1.41; 95% CI, 1.10-1.81; P = .0062; ≥ 11.0 × 109/L group: HR, 1.53; 95% CI, 1.23-1.91; P < .001; compared with < 7.0 × 109/L reference group). Rates of phlebotomy and cytoreductive treatments were similar across groups.16
Some limitations to these studies are attributable to their retrospective design, reliance on health records, and the VHA population characteristics, which differ from the general population. For example, in this analysis, patients with PV in the VHA population had significantly increased risk of thrombotic events, even at a lower WBC count threshold (≥ 8.5 × 109/L) compared with those reported in CYTO-PV (≥ 11 × 109/L). Furthermore, approximately one-third of patients had elevated WBC levels, compared with 25.5% in the CYTO-PV study.14,16 This is most likely due to the unique nature of the VHA patient population, who are predominantly older adult men and generally have a higher comorbidity burden. A notable pre-index comorbidity burden was reported in the VHA population in the Hct analysis, even when compared to patients with PV in the general US population (Charlson Comorbidity Index score, 1.3 vs 0.8).6,17 Comorbid conditions such as hypertension, diabetes, and tobacco use, which are most common among the VHA population, are independently associated with higher risk of cardiovascular and thrombotic events.18,19 However, whether these higher levels of comorbidities affected the type of treatments they received was not elucidated, and the effectiveness of treatments to maintain target Hct levels was not addressed in the study.
Current PV Management and Future Implications
The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology in myeloproliferative neoplasms recommend maintaining Hct levels < 45% in patients with PV.11 Patients with high-risk disease (age ≥ 60 years and/or history of thrombosis) are monitored for new thrombosis or bleeding and are managed for their cardiovascular risk factors. In addition, they receive low-dose aspirin (81-100 mg/day), undergo phlebotomy to maintain an Hct < 45%, and are managed with pharmacologic cytoreductive therapy. Cytoreductive therapy primarily consists of hydroxyurea or peginterferon alfa-2a for younger patients. Ruxolitinib, a Janus kinase (JAK1)/JAK2 inhibitor, is now approved by the US Food and Drug Administration as second-line treatment for those with PV that is intolerant or unresponsive to hydroxyurea or peginterferon alfa-2a treatments.11,20 However, the role of cytoreductive therapy is not clear for patients with low-risk disease (age < 60 years and no history of thrombosis). These patients are managed for their cardiovascular risk factors, undergo phlebotomy to maintain an Hct < 45%, are maintained on low-dose aspirin (81-100 mg/day), and are monitored for indications for cytoreductive therapy, which include any new thrombosis or disease-related major bleeding, frequent or persistent need for phlebotomy with poor tolerance for the procedure, splenomegaly, thrombocytosis, leukocytosis, and disease-related symptoms (eg, aquagenic pruritus, night sweats, fatigue).
Even though the current guidelines recommend maintaining a target Hct of < 45% in patients with high-risk PV, the role of Hct as the main determinant of thrombotic risk in patients with PV is still debated.21 In JAK2V617F-positive essential thrombocythemia, Hct levels are usually normal but risk of thrombosis is nevertheless still significant.22 The risk of thrombosis is significantly lower in primary familial and congenital polycythemia and much lower in secondary erythrocytosis such as cyanotic heart disease, long-term native dwellers of high altitude, and those with high-oxygen–affinity hemoglobins.21,23 In secondary erythrocytosis from hypoxia or upregulated hypoxic pathway such as hypoxia inducible factor-2α (HIF-2α) mutation and Chuvash erythrocytosis, the risk of thrombosis is more associated with the upregulated HIF pathway and its downstream consequences, rather than the elevated Hct level.24
However, most current literature supports the association of increased risk of thrombosis with higher Hct and high WBC count in patients with PV. In addition, the underlying mechanism of thrombogenesis still remains elusive; it is likely a complex process that involves interactions among multiple components, including elevated blood counts arising from clonal hematopoiesis, JAK2V617F allele burden, and platelet and WBC activation and their interaction with endothelial cells and inflammatory cytokines.25
Nevertheless, Hct control and aspirin use are current standard of care for patients with PV to mitigate thrombotic risk, and the results from the 2 analyses by Parasuraman and colleagues, using real-world data from the VHA, support the current practice guidelines to maintain Hct < 45% in these patients. They also provide additional support for considering WBC counts when determining patient risk and treatment plans. Although treatment response criteria from the European LeukemiaNet include achieving normal WBC levels to decrease the risk of thrombosis, current NCCN guidelines do not include WBC counts as a component for establishing patient risk or provide a target WBC count to guide patient management.11,26,27 Updates to these practice guidelines may be warranted. In addition, further study is needed to understand the mechanism of thrombogenesis in PV and other myeloproliferative disorders in order to develop novel therapeutic targets and improve patient outcomes.
Acknowledgments
Writing assistance was provided by Tania Iqbal, PhD, an employee of ICON (North Wales, PA), and was funded by Incyte Corporation (Wilmington, DE).
1. Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55(3):595-600. doi:10.3109/10428194.2013.813500
2. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. doi:10.1182/blood-2016-03-643544
3. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874-1881. doi:10.1038/leu.2013.163
4. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224-2232. doi:10.1200/JCO.2005.07.062
5. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110(3):840-846. doi:10.1182/blood-2006-12-064287
6. Goyal RK, Davis KL, Cote I, Mounedji N, Kaye JA. Increased incidence of thromboembolic event rates in patients diagnosed with polycythemia vera: results from an observational cohort study. Blood (ASH Annual Meeting Abstracts). 2014;124:4840. doi:10.1182/blood.V124.21.4840.4840
7. Barbui T, Carobbio A, Rumi E, et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014;124(19):3021-3023. doi:10.1182/blood-2014-07-591610 8. Cerquozzi S, Barraco D, Lasho T, et al. Risk factors for arterial versus venous thrombosis in polycythemia vera: a single center experience in 587 patients. Blood Cancer J. 2017;7(12):662. doi:10.1038/s41408-017-0035-6
9. Stein BL, Moliterno AR, Tiu RV. Polycythemia vera disease burden: contributing factors, impact on quality of life, and emerging treatment options. Ann Hematol. 2014;93(12):1965-1976. doi:10.1007/s00277-014-2205-y
10. Hultcrantz M, Kristinsson SY, Andersson TM-L, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30(24):2995-3001. doi:10.1200/JCO.2012.42.1925
11. National Comprehensive Cancer Network. NCCN clinical practice guidelines in myeloproliferative neoplasms (Version 1.2020). Accessed March 3, 2022. https://www.nccn.org/professionals/physician_gls/pdf/mpn.pdf
12. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33. doi:10.1056/NEJMoa1208500
13. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109(6):2446-2452. doi:10.1182/blood-2006-08-042515
14. Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015;126(4):560-561. doi:10.1182/blood-2015-04-638593
15. Prchal JT, Gordeuk VR. Treatment target in polycythemia vera. N Engl J Med. 2013;368(16):1555-1556. doi:10.1056/NEJMc1301262
16. Parasuraman S, Yu J, Paranagama D, et al. Elevated white blood cell levels and thrombotic events in patients with polycythemia vera: a real-world analysis of Veterans Health Administration data. Clin Lymphoma Myeloma Leuk. 2020;20(2):63-69. doi:10.1016/j.clml.2019.11.010
17. Parasuraman S, Yu J, Paranagama D, et al. Hematocrit levels and thrombotic events in patients with polycythemia vera: an analysis of Veterans Health Administration data. Ann Hematol. 2019;98(11):2533-2539. doi:10.1007/s00277-019-03793-w
18. WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7(10):e1332-e1345. doi:10.1016/S2214-109X(19)30318-3.
19. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. doi:10.1161/CIRCULATIONAHA.107.699579
20. Jakafi. Package insert. Incyte Corporation; 2020.
21. Gordeuk VR, Key NS, Prchal JT. Re-evaluation of hematocrit as a determinant of thrombotic risk in erythrocytosis. Haematologica. 2019;104(4):653-658. doi:10.3324/haematol.2018.210732
22. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117(22):5857-5859. doi:10.1182/blood-2011-02-339002
23. Perloff JK, Marelli AJ, Miner PD. Risk of stroke in adults with cyanotic congenital heart disease. Circulation. 1993;87(6):1954-1959. doi:10.1161/01.cir.87.6.1954
24. Gordeuk VR, Miasnikova GY, Sergueeva AI, et al. Thrombotic risk in congenital erythrocytosis due to up-regulated hypoxia sensing is not associated with elevated hematocrit. Haematologica. 2020;105(3):e87-e90. doi:10.3324/haematol.2019.216267
25. Kroll MH, Michaelis LC, Verstovsek S. Mechanisms of thrombogenesis in polycythemia vera. Blood Rev. 2015;29(4):215-221. doi:10.1016/j.blre.2014.12.002
26. Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057-1069. doi:10.1038/s41375-018-0077-1
27. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121(23):4778-4781. doi:10.1182/blood-2013-01-478891
Polycythemia vera (PV) is a rare myeloproliferative neoplasm affecting 44 to 57 individuals per 100,000 in the United States.1,2 It is characterized by somatic mutations in the hematopoietic stem cell, resulting in hyperproliferation of mature myeloid lineage cells.2 Sustained erythrocytosis is a hallmark of PV, although many patients also have leukocytosis and thrombocytosis.2,3 These patients have increased inherent thrombotic risk with arterial events reported to occur at rates of 7 to 21/1000 person-years and venous thrombotic events at 5 to 20/1000 person-years.4-7 Thrombotic and cardiovascular events are leading causes of morbidity and mortality, resulting in a reduced overall survival of patients with PV compared with the general population.3,8-10
Blood Cell Counts and Thrombotic Events in PV
Treatment strategies for patients with PV mainly aim to prevent or manage thrombotic and bleeding complications through normalization of blood counts.11 Hematocrit (Hct) control has been reported to be associated with reduced thrombotic risk in patients with PV. This was shown and popularized by the prospective, randomized Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) trial in which participants were randomized 1:1 to maintaining either a low (< 45%) or high (45%-50%) Hct for 5 years to examine the long-term effects of more- or less-intensive cytoreductive therapy.12 Patients in the low-Hct group were found to have a lower rate of death from cardiovascular events or major thrombosis (1.1/100 person-years in the low-Hct group vs 4.4 in the high-Hct group; hazard ratio [HR], 3.91; 95% confidence interval [CI], 1.45-10.53; P = .007). Likewise, cardiovascular events occurred at a lower rate in patients in the low-Hct group compared with the high-Hct group (4.4% vs 10.9% of patients, respectively; HR, 2.69; 95% CI, 1.19-6.12; P = .02).12
Leukocytosis has also been linked to elevated risk for vascular events as shown in several studies, including the real-world European Collaboration on Low-Dose Aspirin in PV (ECLAP) observational study and a post hoc subanalysis of the CYTO-PV study.13,14 In a multivariate, time-dependent analysis in ECLAP, patients with white blood cell (WBC) counts > 15 × 109/L had a significant increase in the risk of thrombosis compared with those who had lower WBC counts, with higher WBC count more strongly associated with arterial than venous thromboembolism.13 In CYTO-PV, a significant correlation between elevated WBC count (≥ 11 × 109/L vs reference level of < 7 × 109/L) and time-dependent risk of major thrombosis was shown (HR, 3.9; 95% CI, 1.24-12.3; P = .02).14 Likewise, WBC count ≥ 11 × 109/L was found to be a predictor of subsequent venous events in a separate single-center multivariate analysis of patients with PV.8
Although CYTO-PV remains one of the largest prospective landmark studies in PV demonstrating the impact of Hct control on thrombosis, it is worthwhile to note that the patients in the high-Hct group who received less frequent myelosuppressive therapy with hydroxyurea than the low-Hct group also had higher WBC counts.12,15 Work is needed to determine the relative effects of high Hct and high WBC counts on PV independent of each other.
The Veteran Population with PV
Two recently published retrospective analyses from Parasuraman and colleagues used data from the Veterans Health Administration (VHA), the largest integrated health care system in the US, with an aim to replicate findings from CYTO-PV in a real-world population.16,17 The 2 analyses focused independently on the effects of Hct control and WBC count on the risk of a thrombotic event in patients with PV.
In the first retrospective analysis, 213 patients with PV and no prior thrombosis were placed into groups based on whether Hct levels were consistently either < 45% or ≥ 45% throughout the study period.17 The mean follow-up time was 2.3 years, during which 44.1% of patients experienced a thrombotic event (Figure 1). Patients with Hct levels < 45% had a lower rate of thrombotic events compared to those with levels ≥ 45% (40.3% vs 54.2%, respectively; HR, 1.61; 95% CI, 1.03-2.51; P = .04). In a sensitivity analysis that included patients with pre-index thrombotic events (N = 342), similar results were noted (55.6% vs 76.9% between the < 45% and ≥ 45% groups, respectively; HR, 1.95; 95% CI, 1.46-2.61; P < .001).
In the second analysis, the authors investigated the relationship between WBC counts and thrombotic events.16 Evaluable patients (N = 1565) were grouped into 1 of 4 cohorts based on the last WBC measurement taken during the study period before a thrombotic event or through the end of follow-up: (1) WBC < 7.0 × 109/L, (2) 7.0 to 8.4 × 109/L, (3) 8.5 to < 11.0 × 109/L, or (4) ≥ 11.0 × 109/L. Mean follow-up time ranged from 3.6 to 4.5 years among WBC count cohorts, during which 24.9% of patients experienced a thrombotic event. Compared with the reference cohort (WBC < 7.0 × 109/L), a significant positive association between WBC counts and thrombotic event occurrence was observed among patients with WBC counts of 8.5 to < 11.0 × 109/L (HR, 1.47; 95% CI, 1.10-1.96; P < .01) and ≥ 11 × 109/L (HR, 1.87; 95% CI, 1.44-2.43; P < .001) (Figure 2).16 When including all patients in a sensitivity analysis regardless of whether they experienced thrombotic events before the index date (N = 1876), similar results were obtained (7.0-8.4 × 109/L group: HR, 1.22; 95% CI, 0.97-1.55; P = .0959; 8.5 - 11.0 × 109/L group: HR, 1.41; 95% CI, 1.10-1.81; P = .0062; ≥ 11.0 × 109/L group: HR, 1.53; 95% CI, 1.23-1.91; P < .001; compared with < 7.0 × 109/L reference group). Rates of phlebotomy and cytoreductive treatments were similar across groups.16
Some limitations to these studies are attributable to their retrospective design, reliance on health records, and the VHA population characteristics, which differ from the general population. For example, in this analysis, patients with PV in the VHA population had significantly increased risk of thrombotic events, even at a lower WBC count threshold (≥ 8.5 × 109/L) compared with those reported in CYTO-PV (≥ 11 × 109/L). Furthermore, approximately one-third of patients had elevated WBC levels, compared with 25.5% in the CYTO-PV study.14,16 This is most likely due to the unique nature of the VHA patient population, who are predominantly older adult men and generally have a higher comorbidity burden. A notable pre-index comorbidity burden was reported in the VHA population in the Hct analysis, even when compared to patients with PV in the general US population (Charlson Comorbidity Index score, 1.3 vs 0.8).6,17 Comorbid conditions such as hypertension, diabetes, and tobacco use, which are most common among the VHA population, are independently associated with higher risk of cardiovascular and thrombotic events.18,19 However, whether these higher levels of comorbidities affected the type of treatments they received was not elucidated, and the effectiveness of treatments to maintain target Hct levels was not addressed in the study.
Current PV Management and Future Implications
The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology in myeloproliferative neoplasms recommend maintaining Hct levels < 45% in patients with PV.11 Patients with high-risk disease (age ≥ 60 years and/or history of thrombosis) are monitored for new thrombosis or bleeding and are managed for their cardiovascular risk factors. In addition, they receive low-dose aspirin (81-100 mg/day), undergo phlebotomy to maintain an Hct < 45%, and are managed with pharmacologic cytoreductive therapy. Cytoreductive therapy primarily consists of hydroxyurea or peginterferon alfa-2a for younger patients. Ruxolitinib, a Janus kinase (JAK1)/JAK2 inhibitor, is now approved by the US Food and Drug Administration as second-line treatment for those with PV that is intolerant or unresponsive to hydroxyurea or peginterferon alfa-2a treatments.11,20 However, the role of cytoreductive therapy is not clear for patients with low-risk disease (age < 60 years and no history of thrombosis). These patients are managed for their cardiovascular risk factors, undergo phlebotomy to maintain an Hct < 45%, are maintained on low-dose aspirin (81-100 mg/day), and are monitored for indications for cytoreductive therapy, which include any new thrombosis or disease-related major bleeding, frequent or persistent need for phlebotomy with poor tolerance for the procedure, splenomegaly, thrombocytosis, leukocytosis, and disease-related symptoms (eg, aquagenic pruritus, night sweats, fatigue).
Even though the current guidelines recommend maintaining a target Hct of < 45% in patients with high-risk PV, the role of Hct as the main determinant of thrombotic risk in patients with PV is still debated.21 In JAK2V617F-positive essential thrombocythemia, Hct levels are usually normal but risk of thrombosis is nevertheless still significant.22 The risk of thrombosis is significantly lower in primary familial and congenital polycythemia and much lower in secondary erythrocytosis such as cyanotic heart disease, long-term native dwellers of high altitude, and those with high-oxygen–affinity hemoglobins.21,23 In secondary erythrocytosis from hypoxia or upregulated hypoxic pathway such as hypoxia inducible factor-2α (HIF-2α) mutation and Chuvash erythrocytosis, the risk of thrombosis is more associated with the upregulated HIF pathway and its downstream consequences, rather than the elevated Hct level.24
However, most current literature supports the association of increased risk of thrombosis with higher Hct and high WBC count in patients with PV. In addition, the underlying mechanism of thrombogenesis still remains elusive; it is likely a complex process that involves interactions among multiple components, including elevated blood counts arising from clonal hematopoiesis, JAK2V617F allele burden, and platelet and WBC activation and their interaction with endothelial cells and inflammatory cytokines.25
Nevertheless, Hct control and aspirin use are current standard of care for patients with PV to mitigate thrombotic risk, and the results from the 2 analyses by Parasuraman and colleagues, using real-world data from the VHA, support the current practice guidelines to maintain Hct < 45% in these patients. They also provide additional support for considering WBC counts when determining patient risk and treatment plans. Although treatment response criteria from the European LeukemiaNet include achieving normal WBC levels to decrease the risk of thrombosis, current NCCN guidelines do not include WBC counts as a component for establishing patient risk or provide a target WBC count to guide patient management.11,26,27 Updates to these practice guidelines may be warranted. In addition, further study is needed to understand the mechanism of thrombogenesis in PV and other myeloproliferative disorders in order to develop novel therapeutic targets and improve patient outcomes.
Acknowledgments
Writing assistance was provided by Tania Iqbal, PhD, an employee of ICON (North Wales, PA), and was funded by Incyte Corporation (Wilmington, DE).
Polycythemia vera (PV) is a rare myeloproliferative neoplasm affecting 44 to 57 individuals per 100,000 in the United States.1,2 It is characterized by somatic mutations in the hematopoietic stem cell, resulting in hyperproliferation of mature myeloid lineage cells.2 Sustained erythrocytosis is a hallmark of PV, although many patients also have leukocytosis and thrombocytosis.2,3 These patients have increased inherent thrombotic risk with arterial events reported to occur at rates of 7 to 21/1000 person-years and venous thrombotic events at 5 to 20/1000 person-years.4-7 Thrombotic and cardiovascular events are leading causes of morbidity and mortality, resulting in a reduced overall survival of patients with PV compared with the general population.3,8-10
Blood Cell Counts and Thrombotic Events in PV
Treatment strategies for patients with PV mainly aim to prevent or manage thrombotic and bleeding complications through normalization of blood counts.11 Hematocrit (Hct) control has been reported to be associated with reduced thrombotic risk in patients with PV. This was shown and popularized by the prospective, randomized Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) trial in which participants were randomized 1:1 to maintaining either a low (< 45%) or high (45%-50%) Hct for 5 years to examine the long-term effects of more- or less-intensive cytoreductive therapy.12 Patients in the low-Hct group were found to have a lower rate of death from cardiovascular events or major thrombosis (1.1/100 person-years in the low-Hct group vs 4.4 in the high-Hct group; hazard ratio [HR], 3.91; 95% confidence interval [CI], 1.45-10.53; P = .007). Likewise, cardiovascular events occurred at a lower rate in patients in the low-Hct group compared with the high-Hct group (4.4% vs 10.9% of patients, respectively; HR, 2.69; 95% CI, 1.19-6.12; P = .02).12
Leukocytosis has also been linked to elevated risk for vascular events as shown in several studies, including the real-world European Collaboration on Low-Dose Aspirin in PV (ECLAP) observational study and a post hoc subanalysis of the CYTO-PV study.13,14 In a multivariate, time-dependent analysis in ECLAP, patients with white blood cell (WBC) counts > 15 × 109/L had a significant increase in the risk of thrombosis compared with those who had lower WBC counts, with higher WBC count more strongly associated with arterial than venous thromboembolism.13 In CYTO-PV, a significant correlation between elevated WBC count (≥ 11 × 109/L vs reference level of < 7 × 109/L) and time-dependent risk of major thrombosis was shown (HR, 3.9; 95% CI, 1.24-12.3; P = .02).14 Likewise, WBC count ≥ 11 × 109/L was found to be a predictor of subsequent venous events in a separate single-center multivariate analysis of patients with PV.8
Although CYTO-PV remains one of the largest prospective landmark studies in PV demonstrating the impact of Hct control on thrombosis, it is worthwhile to note that the patients in the high-Hct group who received less frequent myelosuppressive therapy with hydroxyurea than the low-Hct group also had higher WBC counts.12,15 Work is needed to determine the relative effects of high Hct and high WBC counts on PV independent of each other.
The Veteran Population with PV
Two recently published retrospective analyses from Parasuraman and colleagues used data from the Veterans Health Administration (VHA), the largest integrated health care system in the US, with an aim to replicate findings from CYTO-PV in a real-world population.16,17 The 2 analyses focused independently on the effects of Hct control and WBC count on the risk of a thrombotic event in patients with PV.
In the first retrospective analysis, 213 patients with PV and no prior thrombosis were placed into groups based on whether Hct levels were consistently either < 45% or ≥ 45% throughout the study period.17 The mean follow-up time was 2.3 years, during which 44.1% of patients experienced a thrombotic event (Figure 1). Patients with Hct levels < 45% had a lower rate of thrombotic events compared to those with levels ≥ 45% (40.3% vs 54.2%, respectively; HR, 1.61; 95% CI, 1.03-2.51; P = .04). In a sensitivity analysis that included patients with pre-index thrombotic events (N = 342), similar results were noted (55.6% vs 76.9% between the < 45% and ≥ 45% groups, respectively; HR, 1.95; 95% CI, 1.46-2.61; P < .001).
In the second analysis, the authors investigated the relationship between WBC counts and thrombotic events.16 Evaluable patients (N = 1565) were grouped into 1 of 4 cohorts based on the last WBC measurement taken during the study period before a thrombotic event or through the end of follow-up: (1) WBC < 7.0 × 109/L, (2) 7.0 to 8.4 × 109/L, (3) 8.5 to < 11.0 × 109/L, or (4) ≥ 11.0 × 109/L. Mean follow-up time ranged from 3.6 to 4.5 years among WBC count cohorts, during which 24.9% of patients experienced a thrombotic event. Compared with the reference cohort (WBC < 7.0 × 109/L), a significant positive association between WBC counts and thrombotic event occurrence was observed among patients with WBC counts of 8.5 to < 11.0 × 109/L (HR, 1.47; 95% CI, 1.10-1.96; P < .01) and ≥ 11 × 109/L (HR, 1.87; 95% CI, 1.44-2.43; P < .001) (Figure 2).16 When including all patients in a sensitivity analysis regardless of whether they experienced thrombotic events before the index date (N = 1876), similar results were obtained (7.0-8.4 × 109/L group: HR, 1.22; 95% CI, 0.97-1.55; P = .0959; 8.5 - 11.0 × 109/L group: HR, 1.41; 95% CI, 1.10-1.81; P = .0062; ≥ 11.0 × 109/L group: HR, 1.53; 95% CI, 1.23-1.91; P < .001; compared with < 7.0 × 109/L reference group). Rates of phlebotomy and cytoreductive treatments were similar across groups.16
Some limitations to these studies are attributable to their retrospective design, reliance on health records, and the VHA population characteristics, which differ from the general population. For example, in this analysis, patients with PV in the VHA population had significantly increased risk of thrombotic events, even at a lower WBC count threshold (≥ 8.5 × 109/L) compared with those reported in CYTO-PV (≥ 11 × 109/L). Furthermore, approximately one-third of patients had elevated WBC levels, compared with 25.5% in the CYTO-PV study.14,16 This is most likely due to the unique nature of the VHA patient population, who are predominantly older adult men and generally have a higher comorbidity burden. A notable pre-index comorbidity burden was reported in the VHA population in the Hct analysis, even when compared to patients with PV in the general US population (Charlson Comorbidity Index score, 1.3 vs 0.8).6,17 Comorbid conditions such as hypertension, diabetes, and tobacco use, which are most common among the VHA population, are independently associated with higher risk of cardiovascular and thrombotic events.18,19 However, whether these higher levels of comorbidities affected the type of treatments they received was not elucidated, and the effectiveness of treatments to maintain target Hct levels was not addressed in the study.
Current PV Management and Future Implications
The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology in myeloproliferative neoplasms recommend maintaining Hct levels < 45% in patients with PV.11 Patients with high-risk disease (age ≥ 60 years and/or history of thrombosis) are monitored for new thrombosis or bleeding and are managed for their cardiovascular risk factors. In addition, they receive low-dose aspirin (81-100 mg/day), undergo phlebotomy to maintain an Hct < 45%, and are managed with pharmacologic cytoreductive therapy. Cytoreductive therapy primarily consists of hydroxyurea or peginterferon alfa-2a for younger patients. Ruxolitinib, a Janus kinase (JAK1)/JAK2 inhibitor, is now approved by the US Food and Drug Administration as second-line treatment for those with PV that is intolerant or unresponsive to hydroxyurea or peginterferon alfa-2a treatments.11,20 However, the role of cytoreductive therapy is not clear for patients with low-risk disease (age < 60 years and no history of thrombosis). These patients are managed for their cardiovascular risk factors, undergo phlebotomy to maintain an Hct < 45%, are maintained on low-dose aspirin (81-100 mg/day), and are monitored for indications for cytoreductive therapy, which include any new thrombosis or disease-related major bleeding, frequent or persistent need for phlebotomy with poor tolerance for the procedure, splenomegaly, thrombocytosis, leukocytosis, and disease-related symptoms (eg, aquagenic pruritus, night sweats, fatigue).
Even though the current guidelines recommend maintaining a target Hct of < 45% in patients with high-risk PV, the role of Hct as the main determinant of thrombotic risk in patients with PV is still debated.21 In JAK2V617F-positive essential thrombocythemia, Hct levels are usually normal but risk of thrombosis is nevertheless still significant.22 The risk of thrombosis is significantly lower in primary familial and congenital polycythemia and much lower in secondary erythrocytosis such as cyanotic heart disease, long-term native dwellers of high altitude, and those with high-oxygen–affinity hemoglobins.21,23 In secondary erythrocytosis from hypoxia or upregulated hypoxic pathway such as hypoxia inducible factor-2α (HIF-2α) mutation and Chuvash erythrocytosis, the risk of thrombosis is more associated with the upregulated HIF pathway and its downstream consequences, rather than the elevated Hct level.24
However, most current literature supports the association of increased risk of thrombosis with higher Hct and high WBC count in patients with PV. In addition, the underlying mechanism of thrombogenesis still remains elusive; it is likely a complex process that involves interactions among multiple components, including elevated blood counts arising from clonal hematopoiesis, JAK2V617F allele burden, and platelet and WBC activation and their interaction with endothelial cells and inflammatory cytokines.25
Nevertheless, Hct control and aspirin use are current standard of care for patients with PV to mitigate thrombotic risk, and the results from the 2 analyses by Parasuraman and colleagues, using real-world data from the VHA, support the current practice guidelines to maintain Hct < 45% in these patients. They also provide additional support for considering WBC counts when determining patient risk and treatment plans. Although treatment response criteria from the European LeukemiaNet include achieving normal WBC levels to decrease the risk of thrombosis, current NCCN guidelines do not include WBC counts as a component for establishing patient risk or provide a target WBC count to guide patient management.11,26,27 Updates to these practice guidelines may be warranted. In addition, further study is needed to understand the mechanism of thrombogenesis in PV and other myeloproliferative disorders in order to develop novel therapeutic targets and improve patient outcomes.
Acknowledgments
Writing assistance was provided by Tania Iqbal, PhD, an employee of ICON (North Wales, PA), and was funded by Incyte Corporation (Wilmington, DE).
1. Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55(3):595-600. doi:10.3109/10428194.2013.813500
2. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. doi:10.1182/blood-2016-03-643544
3. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874-1881. doi:10.1038/leu.2013.163
4. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224-2232. doi:10.1200/JCO.2005.07.062
5. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110(3):840-846. doi:10.1182/blood-2006-12-064287
6. Goyal RK, Davis KL, Cote I, Mounedji N, Kaye JA. Increased incidence of thromboembolic event rates in patients diagnosed with polycythemia vera: results from an observational cohort study. Blood (ASH Annual Meeting Abstracts). 2014;124:4840. doi:10.1182/blood.V124.21.4840.4840
7. Barbui T, Carobbio A, Rumi E, et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014;124(19):3021-3023. doi:10.1182/blood-2014-07-591610 8. Cerquozzi S, Barraco D, Lasho T, et al. Risk factors for arterial versus venous thrombosis in polycythemia vera: a single center experience in 587 patients. Blood Cancer J. 2017;7(12):662. doi:10.1038/s41408-017-0035-6
9. Stein BL, Moliterno AR, Tiu RV. Polycythemia vera disease burden: contributing factors, impact on quality of life, and emerging treatment options. Ann Hematol. 2014;93(12):1965-1976. doi:10.1007/s00277-014-2205-y
10. Hultcrantz M, Kristinsson SY, Andersson TM-L, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30(24):2995-3001. doi:10.1200/JCO.2012.42.1925
11. National Comprehensive Cancer Network. NCCN clinical practice guidelines in myeloproliferative neoplasms (Version 1.2020). Accessed March 3, 2022. https://www.nccn.org/professionals/physician_gls/pdf/mpn.pdf
12. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33. doi:10.1056/NEJMoa1208500
13. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109(6):2446-2452. doi:10.1182/blood-2006-08-042515
14. Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015;126(4):560-561. doi:10.1182/blood-2015-04-638593
15. Prchal JT, Gordeuk VR. Treatment target in polycythemia vera. N Engl J Med. 2013;368(16):1555-1556. doi:10.1056/NEJMc1301262
16. Parasuraman S, Yu J, Paranagama D, et al. Elevated white blood cell levels and thrombotic events in patients with polycythemia vera: a real-world analysis of Veterans Health Administration data. Clin Lymphoma Myeloma Leuk. 2020;20(2):63-69. doi:10.1016/j.clml.2019.11.010
17. Parasuraman S, Yu J, Paranagama D, et al. Hematocrit levels and thrombotic events in patients with polycythemia vera: an analysis of Veterans Health Administration data. Ann Hematol. 2019;98(11):2533-2539. doi:10.1007/s00277-019-03793-w
18. WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7(10):e1332-e1345. doi:10.1016/S2214-109X(19)30318-3.
19. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. doi:10.1161/CIRCULATIONAHA.107.699579
20. Jakafi. Package insert. Incyte Corporation; 2020.
21. Gordeuk VR, Key NS, Prchal JT. Re-evaluation of hematocrit as a determinant of thrombotic risk in erythrocytosis. Haematologica. 2019;104(4):653-658. doi:10.3324/haematol.2018.210732
22. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117(22):5857-5859. doi:10.1182/blood-2011-02-339002
23. Perloff JK, Marelli AJ, Miner PD. Risk of stroke in adults with cyanotic congenital heart disease. Circulation. 1993;87(6):1954-1959. doi:10.1161/01.cir.87.6.1954
24. Gordeuk VR, Miasnikova GY, Sergueeva AI, et al. Thrombotic risk in congenital erythrocytosis due to up-regulated hypoxia sensing is not associated with elevated hematocrit. Haematologica. 2020;105(3):e87-e90. doi:10.3324/haematol.2019.216267
25. Kroll MH, Michaelis LC, Verstovsek S. Mechanisms of thrombogenesis in polycythemia vera. Blood Rev. 2015;29(4):215-221. doi:10.1016/j.blre.2014.12.002
26. Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057-1069. doi:10.1038/s41375-018-0077-1
27. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121(23):4778-4781. doi:10.1182/blood-2013-01-478891
1. Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55(3):595-600. doi:10.3109/10428194.2013.813500
2. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. doi:10.1182/blood-2016-03-643544
3. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874-1881. doi:10.1038/leu.2013.163
4. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224-2232. doi:10.1200/JCO.2005.07.062
5. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110(3):840-846. doi:10.1182/blood-2006-12-064287
6. Goyal RK, Davis KL, Cote I, Mounedji N, Kaye JA. Increased incidence of thromboembolic event rates in patients diagnosed with polycythemia vera: results from an observational cohort study. Blood (ASH Annual Meeting Abstracts). 2014;124:4840. doi:10.1182/blood.V124.21.4840.4840
7. Barbui T, Carobbio A, Rumi E, et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014;124(19):3021-3023. doi:10.1182/blood-2014-07-591610 8. Cerquozzi S, Barraco D, Lasho T, et al. Risk factors for arterial versus venous thrombosis in polycythemia vera: a single center experience in 587 patients. Blood Cancer J. 2017;7(12):662. doi:10.1038/s41408-017-0035-6
9. Stein BL, Moliterno AR, Tiu RV. Polycythemia vera disease burden: contributing factors, impact on quality of life, and emerging treatment options. Ann Hematol. 2014;93(12):1965-1976. doi:10.1007/s00277-014-2205-y
10. Hultcrantz M, Kristinsson SY, Andersson TM-L, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30(24):2995-3001. doi:10.1200/JCO.2012.42.1925
11. National Comprehensive Cancer Network. NCCN clinical practice guidelines in myeloproliferative neoplasms (Version 1.2020). Accessed March 3, 2022. https://www.nccn.org/professionals/physician_gls/pdf/mpn.pdf
12. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33. doi:10.1056/NEJMoa1208500
13. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109(6):2446-2452. doi:10.1182/blood-2006-08-042515
14. Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015;126(4):560-561. doi:10.1182/blood-2015-04-638593
15. Prchal JT, Gordeuk VR. Treatment target in polycythemia vera. N Engl J Med. 2013;368(16):1555-1556. doi:10.1056/NEJMc1301262
16. Parasuraman S, Yu J, Paranagama D, et al. Elevated white blood cell levels and thrombotic events in patients with polycythemia vera: a real-world analysis of Veterans Health Administration data. Clin Lymphoma Myeloma Leuk. 2020;20(2):63-69. doi:10.1016/j.clml.2019.11.010
17. Parasuraman S, Yu J, Paranagama D, et al. Hematocrit levels and thrombotic events in patients with polycythemia vera: an analysis of Veterans Health Administration data. Ann Hematol. 2019;98(11):2533-2539. doi:10.1007/s00277-019-03793-w
18. WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7(10):e1332-e1345. doi:10.1016/S2214-109X(19)30318-3.
19. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. doi:10.1161/CIRCULATIONAHA.107.699579
20. Jakafi. Package insert. Incyte Corporation; 2020.
21. Gordeuk VR, Key NS, Prchal JT. Re-evaluation of hematocrit as a determinant of thrombotic risk in erythrocytosis. Haematologica. 2019;104(4):653-658. doi:10.3324/haematol.2018.210732
22. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117(22):5857-5859. doi:10.1182/blood-2011-02-339002
23. Perloff JK, Marelli AJ, Miner PD. Risk of stroke in adults with cyanotic congenital heart disease. Circulation. 1993;87(6):1954-1959. doi:10.1161/01.cir.87.6.1954
24. Gordeuk VR, Miasnikova GY, Sergueeva AI, et al. Thrombotic risk in congenital erythrocytosis due to up-regulated hypoxia sensing is not associated with elevated hematocrit. Haematologica. 2020;105(3):e87-e90. doi:10.3324/haematol.2019.216267
25. Kroll MH, Michaelis LC, Verstovsek S. Mechanisms of thrombogenesis in polycythemia vera. Blood Rev. 2015;29(4):215-221. doi:10.1016/j.blre.2014.12.002
26. Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057-1069. doi:10.1038/s41375-018-0077-1
27. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121(23):4778-4781. doi:10.1182/blood-2013-01-478891
2022 Update on gynecologic cancer
Despite the challenges of an ongoing COVID-19 pandemic, researchers in 2021 delivered practice-changing studies in gynecologic oncology. In this cancer Update, we highlight 4 studies that shed light on the surgical and systemic therapies that may improve outcomes for patients with cancers of the ovary, endometrium, and cervix. We review DESKTOP III, a trial that investigated the role of cytoreductive surgery in patients with recurrent ovarian cancer, and SENTOR, a study that evaluated the performance of sentinel lymph node biopsy in patients with high-grade endometrial cancers. Additionally, we examine 2 studies of systemic therapy that reveal the growing role of targeted therapies and immuno-oncology in the treatment of gynecologic malignancies.
A new era for patients with BRCA mutation–associated ovarian cancer
Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
Ovarian cancer remains the most lethal gynecologic malignancy due to the frequency of advanced-stage diagnosis and frequent relapse after primary therapy. But for ovarian cancer patients with inherited mutations of the BRCA1 or BRCA2 genes, poly(ADP-ribose) polymerase (PARP) inhibitors, a class of oral anticancer medicines that target DNA repair, have ushered in a new era in which the possibility of long-term remission, and even cure, is more likely than at any other time.
Olaparib trial details
The SOLO1 study was a double-blind, placebo-controlled, phase 3 trial that investigated the role of PARP inhibitor maintenance therapy with olaparib in patients with pathologic BRCA1 or BRCA2 mutations who responded to platinum-based chemotherapy administered for a newly diagnosed, advanced-stage ovarian cancer.1 The study enrolled 391 patients, with 260 randomly assigned to receive olaparib for 24 months and 131 patients randomly assigned to receive placebo tablets. Most patients in the study had a mutation in the BRCA1 gene (72%), 27% had a BRCA2 mutation, and 1% had mutations in both genes.
The primary analysis of SOLO1 was published in 2018 and was based on a median follow-up of 3.4 years.1 That study showed that olaparib maintenance therapy resulted in a large progression-free survival benefit and led to its approval by the US Food and Drug Administration (FDA) as a maintenance therapy for patients with BRCA-mutated advanced ovarian cancer who responded to first-line platinum-based chemotherapy.
In 2021, Banerjee and colleagues updated the progression-free survival results for the SOLO1 trial after 5 years of follow-up.2 In this study, the patients randomly assigned to olaparib maintenance therapy had a persistent and statistically significant progression-free survival benefit, with the median progression-free survival reaching 56 months among the olaparib group compared with 13.8 months in the placebo group (hazard ratio [HR], 0.33; 95% confidence interval [CI], 0.25–0.43).2 Olaparib maintenance therapy resulted in more clinically significant adverse events, including anemia and neutropenia. Serious adverse events occurred in 55 (21%) of the olaparib-treated patients and 17 (13%) of the placebo-treated patients, but no treatment-related adverse events were fatal.
The updated progression-free survival data from the SOLO1 study provides important and promising evidence that frontline PARP inhibitor maintenance therapy may affect long-term remission in an unprecedented proportion of patients with BRCA-related ovarian cancer. Significant, sustained benefit was seen well beyond the end of treatment, and median progression-free survival was an astonishing 3.5 years longer in the olaparib treatment group than among patients who received placebo therapy.
Continue to: Cytoreductive surgery for recurrent ovarian cancer improves survival in well-selected patients...
Cytoreductive surgery for recurrent ovarian cancer improves survival in well-selected patients
Harter P, Sehouli J, Vergote I, et al; DESKTOP III Investigators. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123- 2131.
In the DESKTOP III trial, Harter and colleagues contribute results to the ongoing discourse surrounding treatment options for patients with recurrent, platinum-sensitive ovarian cancer.3 Systemic therapies continue to be the mainstay of treatment in this setting; however, several groups have attempted to evaluate the role of secondary cytoreductive surgery in this setting.4,5
Specific inclusion criteria employed
The DESKTOP III investigators randomly assigned 407 patients with platinum-sensitive recurrent ovarian cancer to secondary cytoreductive surgery followed by platinum-based chemotherapy (n = 206) or platinum-based chemotherapy alone (n = 201).3 An essential aspect of the study’s design was the use of specific inclusion criteria known to identify patients with a high likelihood of complete resection at the time of secondary cytoreduction.6,7 Patients were eligible only if they had at least a 6-month remission following platinum-based chemotherapy, had a complete resection at their previous surgery, had no restriction on physical activity, and had ascites of no more than 500 mL.
Surgery group had superior overall and progression-free survival
After a median follow-up of approximately 70 months, patients randomly assigned to surgery had superior overall survival (53.7 months) compared with those assigned to chemotherapy alone (46.0 months; HR, 0.75; 95% CI, 0.59–0.96).3 Progression-free survival also was improved among patients who underwent surgery (median 18.4 vs 12.7 months; HR, 0.66; 95% CI, 0.54–0.82). Subgroup analyses did not identify any subset of patients who did not benefit from surgery. Whether a complete resection was achieved at secondary cytoreduction was highly prognostic: Patients who had a complete resection had a median overall survival of 61.9 months compared with 27.7 months in patients with residual disease. There were no deaths within 90 days of surgery.
The DESKTOP III trial provides compelling evidence that secondary cytoreductive surgery improves overall and progression-free survival among well-selected patients with recurrent, platinum-sensitive ovarian cancer. These results differ from those of a recently reported Gynecologic Oncology Group (GOG) trial that failed to detect a survival benefit for secondary cytoreductive surgery among patients with platinum-sensitive recurrent ovarian cancer.5 Key differences, which might explain the studies’ seemingly contradictory results, were that the GOG study had fewer specific eligibility criteria than the DESKTOP III trial, and that bevacizumab was administered much more frequently in the GOG study. It is therefore reasonable to discuss the possible benefits of secondary cytoreductive surgery with patients who meet DESKTOP III eligibility criteria, with a focus toward shared decision making and a candid discussion of the potential risks and benefits of secondary cytoreduction.
Continue to: Immunotherapy enters first-line treatment regimen for advanced cervical cancer...
Immunotherapy enters first-line treatment regimen for advanced cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Persistent, recurrent, and metastatic cervical cancer carries a very poor prognosis: Most patients progress less than a year after starting treatment, and fewer than half survive for 2 years. First-line treatment in this setting has been platinum-based chemotherapy, often given with bevacizumab, a humanized monoclonal antibody that inhibits tumor growth by blocking angiogenesis.8 Pembrolizumab, an immune checkpoint inhibitor, targets cancer cells by blocking their ability to evade the immune system, and it is FDA approved and widely administered to patients with advanced cervical cancer who progress after first-line treatment.9
Addition of pembrolizumab extended survival
In the KEYNOTE-826 trial, Colombo and colleagues investigated the efficacy of incorporating an immune checkpoint inhibitor into the first-line treatment regimen for patients with persistent, recurrent, and metastatic cervical cancer.10 Researchers in this double-blinded, phase 3, randomized controlled trial assigned 617 patients to receive pembrolizumab or placebo concurrently with the investigator’s choice platinum-based chemotherapy. Bevacizumab was administered at the discretion of the treating oncologist.
The proportion of patients who survived at least 2 years following randomization was significantly higher among those assigned to pembrolizumab compared with placebo (53% vs 42%; HR, 0.67, 95% CI, 0.54–0.84).10 Similarly, median progression-free survival was superior among patients who received pembrolizumab compared with those who received placebo (10.4 months vs 8.2 months; HR, 0.65; 95% CI, 0.53–0.79). The role of bevacizumab in conjunction with pembrolizumab and platinum-based chemotherapy was not elucidated in this study because bevacizumab administration was not randomly assigned.
Anemia and neutropenia were the most common adverse events and were more frequent in the pembrolizumab group, but there were no new safety concerns related to concurrent use of pembrolizumab with cytotoxic chemotherapy and bevacizumab. Importantly, subgroup analysis results suggested that pembrolizumab was effective only in patients whose tumors expressed PD-L1 (programmed death ligand 1), a biomarker of pembrolizumab sensitivity in cervical cancer.
In light of the significant improvements in overall and progression-free survival demonstrated in the KEYNOTE-826 trial, in October 2021, the FDA approved the use of frontline pembrolizumab alongside platinum-based chemotherapy, with or without bevacizumab, for treatment of patients with persistent, recurrent, or metastatic cervical cancers that express PD-L1.
Continue to: Endometrial cancer surgical staging...
Endometrial cancer surgical staging: Is sentinel lymph node biopsy a viable option for high-risk histologies?
Cusimano MC, Vicus D, Pulman K, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157-164.
The use of intraoperative sentinel lymph node mapping and biopsy to identify lymph node metastases among patients undergoing surgical staging for endometrial cancer has become increasingly common. Lymph node status is an important prognostic factor, and it guides adjuvant treatment decisions in endometrial cancer. However, traditional pelvic and para-aortic lymphadenectomy is associated with increased risk of lower-extremity lymphedema, postoperative complications, and intraoperative injury.
Sentinel lymph node biopsy seeks to identify lymph node metastases while minimizing surgical morbidity by identifying and excising only lymph nodes that directly receive lymphatic drainage from the uterus. The combination of a fluorescent dye (indocyanine green) and near infrared cameras have led to the broad adoption of sentinel lymph node biopsy in endometrial cancer staging surgery. This practice is supported by prospective studies that demonstrate the high diagnostic accuracy of this approach.11,12 However, because most patients included in prior studies had low-grade endometrial cancer, the utility of sentinel lymph node biopsy in cases of high-grade histology has been less clear.
Sentinel lymph node biopsy vs lymphadenectomy for staging
In the SENTOR trial, Cusimano and colleagues examined the diagnostic accuracy of sentinel lymph node mapping and biopsy, using indocyanine green, in patients with intermediate- or high-grade early-stage endometrial cancer.13
All eligible patients (N = 156) underwent traditional or robot-assisted laparoscopic hysterectomy with sentinel lymph node biopsy. Subsequently, patients with grade 2 endometrioid carcinoma underwent bilateral pelvic lymphadenectomy, and those with high-grade histology (grade 3 endometrioid, serous, carcinosarcoma, clear cell, undifferentiated or dedifferentiated, and mixed high grade) underwent bilateral pelvic and para-aortic lymphadenectomy. The investigators evaluated the diagnostic characteristics of sentinel lymph node biopsy, treating complete lymphadenectomy as the gold standard.
Of the 156 patients enrolled, the median age was 65.5 and median body mass index was 27.5; 126 patients (81%) had high-grade histology. The sentinel lymph node biopsy had a sensitivity of 96% (95% CI, 81%–100%), identifying 26 of the 27 patients with nodal metastases. The false-negative rate was 4% (95% CI, 0%–9%) and the negative predictive value was 99% (95% CI, 96%–100%). Intraoperative adverse events occurred in 5 patients (3%), but none occurred during the sentinel lymph node biopsy. ●
The high sensitivity and negative predictive value of sentinel lymph node biopsy in the intermediate- and high-grade cohort included in the SENTOR trial are concordant with prior studies that predominantly included patients with low-grade endometrial cancer. These findings suggest that sentinel lymph node mapping and biopsy is a reasonable option for surgical staging, not only for patients with low-grade endometrial cancer but also for those with intermediate- and high-grade disease.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
- Harter P, Sehouli J, Vergote I, et al; DESKTOP III Investigators. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123-2131.
- Shi T, Zhu J, Feng Y, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:439-449.
- Coleman RL, Spiritos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Harter P, du Bois A, Hahmann M, et al; Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Committee; AGO Ovarian Cancer Study Group. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13:1702-1710.
- Harter P, Sehouli J, Reuss A, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. 2011;21: 289-295.
- Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663.
- Frenel JS, Le Tourneau C, O’Neil B, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol. 2017;35:4035-4041.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
- Rossi EC, Kowalski L, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18:384-392.
- Ballester M, Dubernard G, Lecuru F, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTIENDO). Lancet Oncol. 2011;12: 469-476.
- Cusimano MC, Vicus D, Pulman K, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157-164.
Despite the challenges of an ongoing COVID-19 pandemic, researchers in 2021 delivered practice-changing studies in gynecologic oncology. In this cancer Update, we highlight 4 studies that shed light on the surgical and systemic therapies that may improve outcomes for patients with cancers of the ovary, endometrium, and cervix. We review DESKTOP III, a trial that investigated the role of cytoreductive surgery in patients with recurrent ovarian cancer, and SENTOR, a study that evaluated the performance of sentinel lymph node biopsy in patients with high-grade endometrial cancers. Additionally, we examine 2 studies of systemic therapy that reveal the growing role of targeted therapies and immuno-oncology in the treatment of gynecologic malignancies.
A new era for patients with BRCA mutation–associated ovarian cancer
Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
Ovarian cancer remains the most lethal gynecologic malignancy due to the frequency of advanced-stage diagnosis and frequent relapse after primary therapy. But for ovarian cancer patients with inherited mutations of the BRCA1 or BRCA2 genes, poly(ADP-ribose) polymerase (PARP) inhibitors, a class of oral anticancer medicines that target DNA repair, have ushered in a new era in which the possibility of long-term remission, and even cure, is more likely than at any other time.
Olaparib trial details
The SOLO1 study was a double-blind, placebo-controlled, phase 3 trial that investigated the role of PARP inhibitor maintenance therapy with olaparib in patients with pathologic BRCA1 or BRCA2 mutations who responded to platinum-based chemotherapy administered for a newly diagnosed, advanced-stage ovarian cancer.1 The study enrolled 391 patients, with 260 randomly assigned to receive olaparib for 24 months and 131 patients randomly assigned to receive placebo tablets. Most patients in the study had a mutation in the BRCA1 gene (72%), 27% had a BRCA2 mutation, and 1% had mutations in both genes.
The primary analysis of SOLO1 was published in 2018 and was based on a median follow-up of 3.4 years.1 That study showed that olaparib maintenance therapy resulted in a large progression-free survival benefit and led to its approval by the US Food and Drug Administration (FDA) as a maintenance therapy for patients with BRCA-mutated advanced ovarian cancer who responded to first-line platinum-based chemotherapy.
In 2021, Banerjee and colleagues updated the progression-free survival results for the SOLO1 trial after 5 years of follow-up.2 In this study, the patients randomly assigned to olaparib maintenance therapy had a persistent and statistically significant progression-free survival benefit, with the median progression-free survival reaching 56 months among the olaparib group compared with 13.8 months in the placebo group (hazard ratio [HR], 0.33; 95% confidence interval [CI], 0.25–0.43).2 Olaparib maintenance therapy resulted in more clinically significant adverse events, including anemia and neutropenia. Serious adverse events occurred in 55 (21%) of the olaparib-treated patients and 17 (13%) of the placebo-treated patients, but no treatment-related adverse events were fatal.
The updated progression-free survival data from the SOLO1 study provides important and promising evidence that frontline PARP inhibitor maintenance therapy may affect long-term remission in an unprecedented proportion of patients with BRCA-related ovarian cancer. Significant, sustained benefit was seen well beyond the end of treatment, and median progression-free survival was an astonishing 3.5 years longer in the olaparib treatment group than among patients who received placebo therapy.
Continue to: Cytoreductive surgery for recurrent ovarian cancer improves survival in well-selected patients...
Cytoreductive surgery for recurrent ovarian cancer improves survival in well-selected patients
Harter P, Sehouli J, Vergote I, et al; DESKTOP III Investigators. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123- 2131.
In the DESKTOP III trial, Harter and colleagues contribute results to the ongoing discourse surrounding treatment options for patients with recurrent, platinum-sensitive ovarian cancer.3 Systemic therapies continue to be the mainstay of treatment in this setting; however, several groups have attempted to evaluate the role of secondary cytoreductive surgery in this setting.4,5
Specific inclusion criteria employed
The DESKTOP III investigators randomly assigned 407 patients with platinum-sensitive recurrent ovarian cancer to secondary cytoreductive surgery followed by platinum-based chemotherapy (n = 206) or platinum-based chemotherapy alone (n = 201).3 An essential aspect of the study’s design was the use of specific inclusion criteria known to identify patients with a high likelihood of complete resection at the time of secondary cytoreduction.6,7 Patients were eligible only if they had at least a 6-month remission following platinum-based chemotherapy, had a complete resection at their previous surgery, had no restriction on physical activity, and had ascites of no more than 500 mL.
Surgery group had superior overall and progression-free survival
After a median follow-up of approximately 70 months, patients randomly assigned to surgery had superior overall survival (53.7 months) compared with those assigned to chemotherapy alone (46.0 months; HR, 0.75; 95% CI, 0.59–0.96).3 Progression-free survival also was improved among patients who underwent surgery (median 18.4 vs 12.7 months; HR, 0.66; 95% CI, 0.54–0.82). Subgroup analyses did not identify any subset of patients who did not benefit from surgery. Whether a complete resection was achieved at secondary cytoreduction was highly prognostic: Patients who had a complete resection had a median overall survival of 61.9 months compared with 27.7 months in patients with residual disease. There were no deaths within 90 days of surgery.
The DESKTOP III trial provides compelling evidence that secondary cytoreductive surgery improves overall and progression-free survival among well-selected patients with recurrent, platinum-sensitive ovarian cancer. These results differ from those of a recently reported Gynecologic Oncology Group (GOG) trial that failed to detect a survival benefit for secondary cytoreductive surgery among patients with platinum-sensitive recurrent ovarian cancer.5 Key differences, which might explain the studies’ seemingly contradictory results, were that the GOG study had fewer specific eligibility criteria than the DESKTOP III trial, and that bevacizumab was administered much more frequently in the GOG study. It is therefore reasonable to discuss the possible benefits of secondary cytoreductive surgery with patients who meet DESKTOP III eligibility criteria, with a focus toward shared decision making and a candid discussion of the potential risks and benefits of secondary cytoreduction.
Continue to: Immunotherapy enters first-line treatment regimen for advanced cervical cancer...
Immunotherapy enters first-line treatment regimen for advanced cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Persistent, recurrent, and metastatic cervical cancer carries a very poor prognosis: Most patients progress less than a year after starting treatment, and fewer than half survive for 2 years. First-line treatment in this setting has been platinum-based chemotherapy, often given with bevacizumab, a humanized monoclonal antibody that inhibits tumor growth by blocking angiogenesis.8 Pembrolizumab, an immune checkpoint inhibitor, targets cancer cells by blocking their ability to evade the immune system, and it is FDA approved and widely administered to patients with advanced cervical cancer who progress after first-line treatment.9
Addition of pembrolizumab extended survival
In the KEYNOTE-826 trial, Colombo and colleagues investigated the efficacy of incorporating an immune checkpoint inhibitor into the first-line treatment regimen for patients with persistent, recurrent, and metastatic cervical cancer.10 Researchers in this double-blinded, phase 3, randomized controlled trial assigned 617 patients to receive pembrolizumab or placebo concurrently with the investigator’s choice platinum-based chemotherapy. Bevacizumab was administered at the discretion of the treating oncologist.
The proportion of patients who survived at least 2 years following randomization was significantly higher among those assigned to pembrolizumab compared with placebo (53% vs 42%; HR, 0.67, 95% CI, 0.54–0.84).10 Similarly, median progression-free survival was superior among patients who received pembrolizumab compared with those who received placebo (10.4 months vs 8.2 months; HR, 0.65; 95% CI, 0.53–0.79). The role of bevacizumab in conjunction with pembrolizumab and platinum-based chemotherapy was not elucidated in this study because bevacizumab administration was not randomly assigned.
Anemia and neutropenia were the most common adverse events and were more frequent in the pembrolizumab group, but there were no new safety concerns related to concurrent use of pembrolizumab with cytotoxic chemotherapy and bevacizumab. Importantly, subgroup analysis results suggested that pembrolizumab was effective only in patients whose tumors expressed PD-L1 (programmed death ligand 1), a biomarker of pembrolizumab sensitivity in cervical cancer.
In light of the significant improvements in overall and progression-free survival demonstrated in the KEYNOTE-826 trial, in October 2021, the FDA approved the use of frontline pembrolizumab alongside platinum-based chemotherapy, with or without bevacizumab, for treatment of patients with persistent, recurrent, or metastatic cervical cancers that express PD-L1.
Continue to: Endometrial cancer surgical staging...
Endometrial cancer surgical staging: Is sentinel lymph node biopsy a viable option for high-risk histologies?
Cusimano MC, Vicus D, Pulman K, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157-164.
The use of intraoperative sentinel lymph node mapping and biopsy to identify lymph node metastases among patients undergoing surgical staging for endometrial cancer has become increasingly common. Lymph node status is an important prognostic factor, and it guides adjuvant treatment decisions in endometrial cancer. However, traditional pelvic and para-aortic lymphadenectomy is associated with increased risk of lower-extremity lymphedema, postoperative complications, and intraoperative injury.
Sentinel lymph node biopsy seeks to identify lymph node metastases while minimizing surgical morbidity by identifying and excising only lymph nodes that directly receive lymphatic drainage from the uterus. The combination of a fluorescent dye (indocyanine green) and near infrared cameras have led to the broad adoption of sentinel lymph node biopsy in endometrial cancer staging surgery. This practice is supported by prospective studies that demonstrate the high diagnostic accuracy of this approach.11,12 However, because most patients included in prior studies had low-grade endometrial cancer, the utility of sentinel lymph node biopsy in cases of high-grade histology has been less clear.
Sentinel lymph node biopsy vs lymphadenectomy for staging
In the SENTOR trial, Cusimano and colleagues examined the diagnostic accuracy of sentinel lymph node mapping and biopsy, using indocyanine green, in patients with intermediate- or high-grade early-stage endometrial cancer.13
All eligible patients (N = 156) underwent traditional or robot-assisted laparoscopic hysterectomy with sentinel lymph node biopsy. Subsequently, patients with grade 2 endometrioid carcinoma underwent bilateral pelvic lymphadenectomy, and those with high-grade histology (grade 3 endometrioid, serous, carcinosarcoma, clear cell, undifferentiated or dedifferentiated, and mixed high grade) underwent bilateral pelvic and para-aortic lymphadenectomy. The investigators evaluated the diagnostic characteristics of sentinel lymph node biopsy, treating complete lymphadenectomy as the gold standard.
Of the 156 patients enrolled, the median age was 65.5 and median body mass index was 27.5; 126 patients (81%) had high-grade histology. The sentinel lymph node biopsy had a sensitivity of 96% (95% CI, 81%–100%), identifying 26 of the 27 patients with nodal metastases. The false-negative rate was 4% (95% CI, 0%–9%) and the negative predictive value was 99% (95% CI, 96%–100%). Intraoperative adverse events occurred in 5 patients (3%), but none occurred during the sentinel lymph node biopsy. ●
The high sensitivity and negative predictive value of sentinel lymph node biopsy in the intermediate- and high-grade cohort included in the SENTOR trial are concordant with prior studies that predominantly included patients with low-grade endometrial cancer. These findings suggest that sentinel lymph node mapping and biopsy is a reasonable option for surgical staging, not only for patients with low-grade endometrial cancer but also for those with intermediate- and high-grade disease.
Despite the challenges of an ongoing COVID-19 pandemic, researchers in 2021 delivered practice-changing studies in gynecologic oncology. In this cancer Update, we highlight 4 studies that shed light on the surgical and systemic therapies that may improve outcomes for patients with cancers of the ovary, endometrium, and cervix. We review DESKTOP III, a trial that investigated the role of cytoreductive surgery in patients with recurrent ovarian cancer, and SENTOR, a study that evaluated the performance of sentinel lymph node biopsy in patients with high-grade endometrial cancers. Additionally, we examine 2 studies of systemic therapy that reveal the growing role of targeted therapies and immuno-oncology in the treatment of gynecologic malignancies.
A new era for patients with BRCA mutation–associated ovarian cancer
Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
Ovarian cancer remains the most lethal gynecologic malignancy due to the frequency of advanced-stage diagnosis and frequent relapse after primary therapy. But for ovarian cancer patients with inherited mutations of the BRCA1 or BRCA2 genes, poly(ADP-ribose) polymerase (PARP) inhibitors, a class of oral anticancer medicines that target DNA repair, have ushered in a new era in which the possibility of long-term remission, and even cure, is more likely than at any other time.
Olaparib trial details
The SOLO1 study was a double-blind, placebo-controlled, phase 3 trial that investigated the role of PARP inhibitor maintenance therapy with olaparib in patients with pathologic BRCA1 or BRCA2 mutations who responded to platinum-based chemotherapy administered for a newly diagnosed, advanced-stage ovarian cancer.1 The study enrolled 391 patients, with 260 randomly assigned to receive olaparib for 24 months and 131 patients randomly assigned to receive placebo tablets. Most patients in the study had a mutation in the BRCA1 gene (72%), 27% had a BRCA2 mutation, and 1% had mutations in both genes.
The primary analysis of SOLO1 was published in 2018 and was based on a median follow-up of 3.4 years.1 That study showed that olaparib maintenance therapy resulted in a large progression-free survival benefit and led to its approval by the US Food and Drug Administration (FDA) as a maintenance therapy for patients with BRCA-mutated advanced ovarian cancer who responded to first-line platinum-based chemotherapy.
In 2021, Banerjee and colleagues updated the progression-free survival results for the SOLO1 trial after 5 years of follow-up.2 In this study, the patients randomly assigned to olaparib maintenance therapy had a persistent and statistically significant progression-free survival benefit, with the median progression-free survival reaching 56 months among the olaparib group compared with 13.8 months in the placebo group (hazard ratio [HR], 0.33; 95% confidence interval [CI], 0.25–0.43).2 Olaparib maintenance therapy resulted in more clinically significant adverse events, including anemia and neutropenia. Serious adverse events occurred in 55 (21%) of the olaparib-treated patients and 17 (13%) of the placebo-treated patients, but no treatment-related adverse events were fatal.
The updated progression-free survival data from the SOLO1 study provides important and promising evidence that frontline PARP inhibitor maintenance therapy may affect long-term remission in an unprecedented proportion of patients with BRCA-related ovarian cancer. Significant, sustained benefit was seen well beyond the end of treatment, and median progression-free survival was an astonishing 3.5 years longer in the olaparib treatment group than among patients who received placebo therapy.
Continue to: Cytoreductive surgery for recurrent ovarian cancer improves survival in well-selected patients...
Cytoreductive surgery for recurrent ovarian cancer improves survival in well-selected patients
Harter P, Sehouli J, Vergote I, et al; DESKTOP III Investigators. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123- 2131.
In the DESKTOP III trial, Harter and colleagues contribute results to the ongoing discourse surrounding treatment options for patients with recurrent, platinum-sensitive ovarian cancer.3 Systemic therapies continue to be the mainstay of treatment in this setting; however, several groups have attempted to evaluate the role of secondary cytoreductive surgery in this setting.4,5
Specific inclusion criteria employed
The DESKTOP III investigators randomly assigned 407 patients with platinum-sensitive recurrent ovarian cancer to secondary cytoreductive surgery followed by platinum-based chemotherapy (n = 206) or platinum-based chemotherapy alone (n = 201).3 An essential aspect of the study’s design was the use of specific inclusion criteria known to identify patients with a high likelihood of complete resection at the time of secondary cytoreduction.6,7 Patients were eligible only if they had at least a 6-month remission following platinum-based chemotherapy, had a complete resection at their previous surgery, had no restriction on physical activity, and had ascites of no more than 500 mL.
Surgery group had superior overall and progression-free survival
After a median follow-up of approximately 70 months, patients randomly assigned to surgery had superior overall survival (53.7 months) compared with those assigned to chemotherapy alone (46.0 months; HR, 0.75; 95% CI, 0.59–0.96).3 Progression-free survival also was improved among patients who underwent surgery (median 18.4 vs 12.7 months; HR, 0.66; 95% CI, 0.54–0.82). Subgroup analyses did not identify any subset of patients who did not benefit from surgery. Whether a complete resection was achieved at secondary cytoreduction was highly prognostic: Patients who had a complete resection had a median overall survival of 61.9 months compared with 27.7 months in patients with residual disease. There were no deaths within 90 days of surgery.
The DESKTOP III trial provides compelling evidence that secondary cytoreductive surgery improves overall and progression-free survival among well-selected patients with recurrent, platinum-sensitive ovarian cancer. These results differ from those of a recently reported Gynecologic Oncology Group (GOG) trial that failed to detect a survival benefit for secondary cytoreductive surgery among patients with platinum-sensitive recurrent ovarian cancer.5 Key differences, which might explain the studies’ seemingly contradictory results, were that the GOG study had fewer specific eligibility criteria than the DESKTOP III trial, and that bevacizumab was administered much more frequently in the GOG study. It is therefore reasonable to discuss the possible benefits of secondary cytoreductive surgery with patients who meet DESKTOP III eligibility criteria, with a focus toward shared decision making and a candid discussion of the potential risks and benefits of secondary cytoreduction.
Continue to: Immunotherapy enters first-line treatment regimen for advanced cervical cancer...
Immunotherapy enters first-line treatment regimen for advanced cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Persistent, recurrent, and metastatic cervical cancer carries a very poor prognosis: Most patients progress less than a year after starting treatment, and fewer than half survive for 2 years. First-line treatment in this setting has been platinum-based chemotherapy, often given with bevacizumab, a humanized monoclonal antibody that inhibits tumor growth by blocking angiogenesis.8 Pembrolizumab, an immune checkpoint inhibitor, targets cancer cells by blocking their ability to evade the immune system, and it is FDA approved and widely administered to patients with advanced cervical cancer who progress after first-line treatment.9
Addition of pembrolizumab extended survival
In the KEYNOTE-826 trial, Colombo and colleagues investigated the efficacy of incorporating an immune checkpoint inhibitor into the first-line treatment regimen for patients with persistent, recurrent, and metastatic cervical cancer.10 Researchers in this double-blinded, phase 3, randomized controlled trial assigned 617 patients to receive pembrolizumab or placebo concurrently with the investigator’s choice platinum-based chemotherapy. Bevacizumab was administered at the discretion of the treating oncologist.
The proportion of patients who survived at least 2 years following randomization was significantly higher among those assigned to pembrolizumab compared with placebo (53% vs 42%; HR, 0.67, 95% CI, 0.54–0.84).10 Similarly, median progression-free survival was superior among patients who received pembrolizumab compared with those who received placebo (10.4 months vs 8.2 months; HR, 0.65; 95% CI, 0.53–0.79). The role of bevacizumab in conjunction with pembrolizumab and platinum-based chemotherapy was not elucidated in this study because bevacizumab administration was not randomly assigned.
Anemia and neutropenia were the most common adverse events and were more frequent in the pembrolizumab group, but there were no new safety concerns related to concurrent use of pembrolizumab with cytotoxic chemotherapy and bevacizumab. Importantly, subgroup analysis results suggested that pembrolizumab was effective only in patients whose tumors expressed PD-L1 (programmed death ligand 1), a biomarker of pembrolizumab sensitivity in cervical cancer.
In light of the significant improvements in overall and progression-free survival demonstrated in the KEYNOTE-826 trial, in October 2021, the FDA approved the use of frontline pembrolizumab alongside platinum-based chemotherapy, with or without bevacizumab, for treatment of patients with persistent, recurrent, or metastatic cervical cancers that express PD-L1.
Continue to: Endometrial cancer surgical staging...
Endometrial cancer surgical staging: Is sentinel lymph node biopsy a viable option for high-risk histologies?
Cusimano MC, Vicus D, Pulman K, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157-164.
The use of intraoperative sentinel lymph node mapping and biopsy to identify lymph node metastases among patients undergoing surgical staging for endometrial cancer has become increasingly common. Lymph node status is an important prognostic factor, and it guides adjuvant treatment decisions in endometrial cancer. However, traditional pelvic and para-aortic lymphadenectomy is associated with increased risk of lower-extremity lymphedema, postoperative complications, and intraoperative injury.
Sentinel lymph node biopsy seeks to identify lymph node metastases while minimizing surgical morbidity by identifying and excising only lymph nodes that directly receive lymphatic drainage from the uterus. The combination of a fluorescent dye (indocyanine green) and near infrared cameras have led to the broad adoption of sentinel lymph node biopsy in endometrial cancer staging surgery. This practice is supported by prospective studies that demonstrate the high diagnostic accuracy of this approach.11,12 However, because most patients included in prior studies had low-grade endometrial cancer, the utility of sentinel lymph node biopsy in cases of high-grade histology has been less clear.
Sentinel lymph node biopsy vs lymphadenectomy for staging
In the SENTOR trial, Cusimano and colleagues examined the diagnostic accuracy of sentinel lymph node mapping and biopsy, using indocyanine green, in patients with intermediate- or high-grade early-stage endometrial cancer.13
All eligible patients (N = 156) underwent traditional or robot-assisted laparoscopic hysterectomy with sentinel lymph node biopsy. Subsequently, patients with grade 2 endometrioid carcinoma underwent bilateral pelvic lymphadenectomy, and those with high-grade histology (grade 3 endometrioid, serous, carcinosarcoma, clear cell, undifferentiated or dedifferentiated, and mixed high grade) underwent bilateral pelvic and para-aortic lymphadenectomy. The investigators evaluated the diagnostic characteristics of sentinel lymph node biopsy, treating complete lymphadenectomy as the gold standard.
Of the 156 patients enrolled, the median age was 65.5 and median body mass index was 27.5; 126 patients (81%) had high-grade histology. The sentinel lymph node biopsy had a sensitivity of 96% (95% CI, 81%–100%), identifying 26 of the 27 patients with nodal metastases. The false-negative rate was 4% (95% CI, 0%–9%) and the negative predictive value was 99% (95% CI, 96%–100%). Intraoperative adverse events occurred in 5 patients (3%), but none occurred during the sentinel lymph node biopsy. ●
The high sensitivity and negative predictive value of sentinel lymph node biopsy in the intermediate- and high-grade cohort included in the SENTOR trial are concordant with prior studies that predominantly included patients with low-grade endometrial cancer. These findings suggest that sentinel lymph node mapping and biopsy is a reasonable option for surgical staging, not only for patients with low-grade endometrial cancer but also for those with intermediate- and high-grade disease.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
- Harter P, Sehouli J, Vergote I, et al; DESKTOP III Investigators. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123-2131.
- Shi T, Zhu J, Feng Y, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:439-449.
- Coleman RL, Spiritos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Harter P, du Bois A, Hahmann M, et al; Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Committee; AGO Ovarian Cancer Study Group. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13:1702-1710.
- Harter P, Sehouli J, Reuss A, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. 2011;21: 289-295.
- Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663.
- Frenel JS, Le Tourneau C, O’Neil B, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol. 2017;35:4035-4041.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
- Rossi EC, Kowalski L, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18:384-392.
- Ballester M, Dubernard G, Lecuru F, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTIENDO). Lancet Oncol. 2011;12: 469-476.
- Cusimano MC, Vicus D, Pulman K, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157-164.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
- Harter P, Sehouli J, Vergote I, et al; DESKTOP III Investigators. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123-2131.
- Shi T, Zhu J, Feng Y, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:439-449.
- Coleman RL, Spiritos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Harter P, du Bois A, Hahmann M, et al; Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Committee; AGO Ovarian Cancer Study Group. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13:1702-1710.
- Harter P, Sehouli J, Reuss A, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. 2011;21: 289-295.
- Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663.
- Frenel JS, Le Tourneau C, O’Neil B, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol. 2017;35:4035-4041.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
- Rossi EC, Kowalski L, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18:384-392.
- Ballester M, Dubernard G, Lecuru F, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTIENDO). Lancet Oncol. 2011;12: 469-476.
- Cusimano MC, Vicus D, Pulman K, et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157-164.
Nonstress test and maximal vertical pocket vs the biophysical profile: Equivocal or equivalent?
CASE 1 Pregnant patient endures extensive wait and travel times to have antenatal testing
Pregnant at age 35 without comorbidities, Ms. H was instructed to schedule weekly biophysical profiles (BPP) after 36 weeks’ gestation for advanced maternal age. She receives care at a community office 25 miles from the hospital where she will deliver. Ms. H must complete her antenatal testing at the hospital where the sonographer performs BPPs. She sees her physician at the nearby clinic and then takes public transit to the hospital. She waits 2 hours to be seen then makes her way back home. Her prenatal care visit, which usually takes 30 minutes, turns into a 5-hour ordeal. Ms. H delivered a healthy baby at 39 weeks. Unfortunately, she was fired from her job for missing too many workdays.
Antenatal testing has become routine, and it is costly
For the prescriber, antenatal testing is simple: Order a weekly ultrasound exam to reduce the risk of stillbirth, decrease litigation, generate income, and maximize patient satisfaction (with the assumption that everyone likes to peek at their baby). Recommending antenatal testing has—with the best intentions—become a habit and therefore is difficult to break. However, the American College of Obstetricians and Gynecologists (ACOG) recognizes that “there is a paucity of evidenced-based recommendations on the timing and frequency of antenatal fetal surveillance because of the challenges of conducting prospective trials in pregnancies complicated by stillbirths and the varying conditions that place pregnancies at high risk for stillbirth. As a result, evidence for the efficacy of antenatal fetal surveillance, when available, is largely circumstantial.”1
Antenatal testing without an evidence-based indication can be costly for the health care system, insurance companies, and patients. Many clinics, especially those in rural communities, do not have the equipment or personnel to complete antenatal testing on site. Asking a pregnant patient to travel repeatedly to another location for antenatal testing can increase her time off from work, complicate childcare, pose a financial burden, and lead to nonadherence. As clinicians, it is imperative that we work with our patients to create an individualized care plan to minimize these burdens and increase adherence.
Antenatal fetal surveillance can be considered for conditions in which stillbirth is reported more frequently than 0.8 per 1,000.
Advanced maternal age and stillbirth risk
One of the most common reasons for antenatal testing is advanced maternal age, that is, age older than 35. According to the Centers for Disease Control and Prevention and the National Vital Statistics System, from 2000 to 2012, 46 states and the District of Columbia (DC) reported an increase in first birth rates for women aged 35 to 39. Thirty-one states and DC saw a rise among women aged 40 to 44 in the same period (FIGURE).2
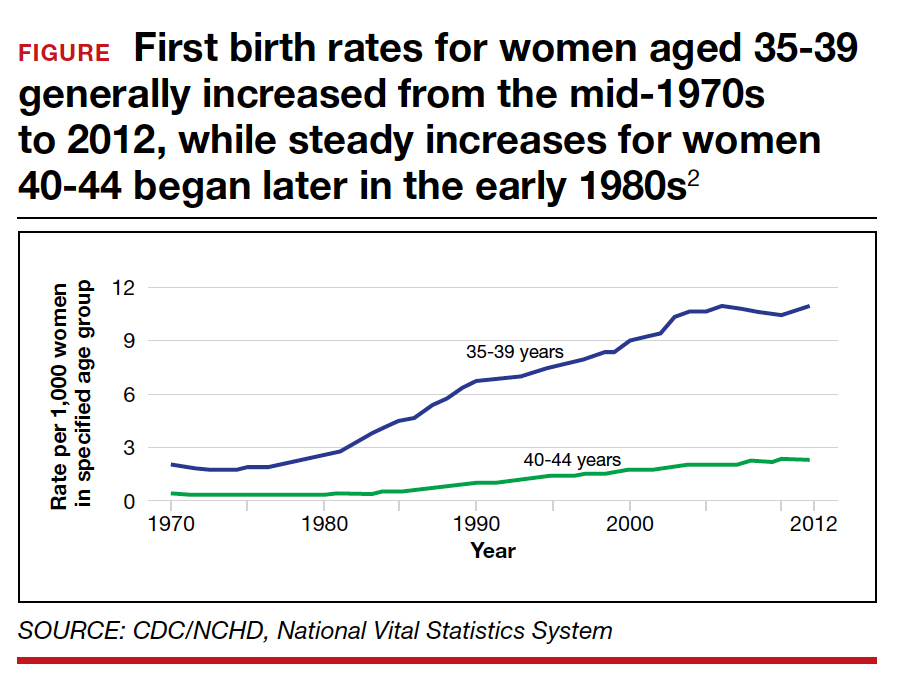
Advanced maternal age is an independent risk factor for stillbirth, with women aged 35 to 39 at 1.9-fold increased risk and women older than age 40 with a 2.4-fold higher risk compared with women younger than age 30.3 In a review of 44 studies including nearly 45,000,000 births, case-control studies, versus cohort studies, demonstrated a higher odds for stillbirth among women aged 35 and older (odds ratio [OR], 2.39; 95% confidence interval [CI], 1.57-3.66 vs OR, 1.73; 95% CI, 1.6-1.87).4 Now, many women older than age 35 may have a concomitant risk factor, such as diabetes or hypertension, that requires antenatal testing. However, for those without other risk factors, nearly 863 antenatal tests and 71 inductions would need to be completed to reduce the number of stillbirths by 1. Antenatal testing for women older than age 35 without other risk factors should be individualized through shared decision making.5 See the ACOG committee opinion for a table that outlines factors associated with an increased risk of stillbirth and suggested strategies for antenatal surveillance after viability.1
Continue to: CASE 2 Patient with high BPP score and altered...
CASE 2 Patient with high BPP score and altered fetal movements delivered for nonreassuring fetal heart rate
Ms. Q was undergoing weekly BPPs for diet-controlled gestational diabetes and a prepregnancy body mass index (BMI) of 52. At 37 weeks’ gestation, she had a BPP score of 8/8. However, it took almost 30 minutes to see 2 discrete body or limb movements. Ms. Q mentioned to the nurse taking her vitals after the BPP that the baby’s movements had changed over the previous few days, especially after contractions. Ms. Q then completed a nonstress test (NST); she had 2 contractions and 2 fetal heart rate decelerations, each lasting approximately 60 seconds. Ms. Q was sent to labor and delivery for prolonged monitoring, and she was delivered that day for a nonreassuring fetal heart rate tracing. Meconium-stained amniotic fluid and a tight triple nuchal cord were noted at delivery.
BPP considerations
While considered an in-depth look at the fetal status, BPPs may not predict overall fetal well-being during acute changes, such as umbilical cord compression or placental abruption. BPPs take longer to complete, require a trained sonographer, and include components like fetal breathing that may be influenced by such factors as nicotine,6-8 labor,9 rupture of membranes,10 magnesium sulfate,11 and infection.12
If medically indicated, which antenatal surveillance technique is right for your patient?
Frequently used antepartum fetal surveillance techniques include maternal perception of fetal movement or “kick counting,” NST, BPP, modified BPP, contraction stress test (CST), and umbilical artery Doppler velocimetry.
Worldwide, the most common form of antenatal surveillance is fetal kick counting. It is noninvasive, can be completed frequently, may decrease maternal anxiety, may improve maternal-fetal bonding, and is free.13 According to the results of a 2020 meta-analysis of 468,601 fetuses, however, there was no difference in perinatal death among patients who assessed fetal movements (0.54%) and those who did not (0.59%).14 There was a statistically significant increase in induction of labor, cesarean delivery, and preterm delivery among patients who counted fetal movements. Women who perceive a decrease in fetal movement should seek medical attention from a health care provider.
An evaluation for decreased fetal movement typically includes taking a history that focuses on risk factors that may increase stillbirth, including hypertension, growth restriction, fetal anomalies, diabetes, and substance use, and auscultation with a fetal Doppler. In the absence of risk factors and the presence of a normal fetal heartbeat, pregnant women should be reassured of fetal well-being. In a pregnancy at greater than 28 weeks, a 20-minute NST can be completed as well; this has become part of the standard workup of decreased fetal movement in developed countries. A reactive NST indicates normal fetal autonomic function in real time and a low incidence of stillbirth (1.9/1,000) within 1 week.15
Additionally, by measuring the amniotic fluid volume using the largest maximal vertical pocket (MVP), clinicians can gain insight into overall uteroplacental function. The combination of the NST and the MVP—otherwise known as a modified BPP—provides both short-term acid-base status and long-term uteroplacental function. The incidence of stillbirth in the 1 week after a modified BPP has been reported to be 0.8/1,000, which is equivalent to stillbirth incidence using a full BPP (0.8/1,000).16 The negative predictive value for both the modified BPP and the BPP is 99.9%—equivalent.
The case for modified BPP use
The modified BPP requires less time, is less costly (cost savings of approximately 50%), does not require a specialized sonographer, and can be performed in local community clinics.
Perhaps the initial antepartum surveillance test of choice should be the modified BPP, with the BPP used in cases in which the results of a modified BPP are abnormal. ●
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for MaternalFetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197.
- Mathews TJ, Hamilton BE. First births to older women continue to rise. NCHS Data Brief, No. 152. Hyattsville, MD: National Center for Health Statistics; 2014.
- Fretts RC, Schmittdiel J, McLean FH, et al. Increased maternal age and the risk of fetal death. N Engl J Med. 1995;333: 953-957.
- Lean SC, Derricott H, Jones RL, et al. Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS One. 2017;12:e0186287.
- Fretts RC, Elkins EB, Myers ER, et al. Should older women have antepartum testing to prevent unexplained stillbirth? Obstet Gynecol. 2004;104:56-64.
- Manning F, Wyn Pugh E, Boddy K. Effect of cigarette smoking on fetal breathing movements in normal pregnancies. Br Med J. 1975;1:552-553.
- Manning FA, Feyerabend C. Cigarette smoking and fetal breathing movements. Br J Obstet Gynecol. 1976;83:262-270.
- Gennser G, Marsal K, Brantmark B. Maternal smoking and fetal breathing movements. Am J Obstet Gynecol. 1975;123:861-867.
- Boylan P, O’Donovan P, Owens OJ. Fetal breathing movements and the diagnosis of labor: a prospective analysis of 100 cases. Obstet Gynecol. 1985;66:517-520.
- Kivikoski AI, Amon E, Vaalamo PO, et al. Effect of thirdtrimester premature rupture of membranes on fetal breathing movements: a prospective case-control study. Am J Obstet Gynecol. 1988;159:1474-1477.
- Peaceman AM, Meyer BA, Thorp JA, et al. The effect of magnesium sulfate tocolysis on the fetal biophysical profile. Am J Obstet Gynecol. 1989;161:771-774.
- Vintzileos AM, Campbell WA, Nochimson DJ, et al. The fetal biophysical profile in patients with premature rupture of the membranes—an early predictor of fetal infection. Am J Obstet Gynecol. 1985;152:501-516.
- Liston RM, Bloom K, Zimmer P. The psychological effects of counting fetal movements. Birth. 1994;21:135-140.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality: a systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462.
- Freeman RK, Anderson G, Dorchester W. A prospective multiinstitutional study of antepartum fetal heart rate monitoring. I. Risk of perinatal mortality and morbidity according to antepartum fetal heart rate test results. Am J Obstet Gynecol. 1982;143:771-777.
- Miller DA , Rabello YA, Paul RH. The modified biophysical profile: antepartum testing in the 1990s. Am J Obstet Gynecol. 1996;174:812-817.
CASE 1 Pregnant patient endures extensive wait and travel times to have antenatal testing
Pregnant at age 35 without comorbidities, Ms. H was instructed to schedule weekly biophysical profiles (BPP) after 36 weeks’ gestation for advanced maternal age. She receives care at a community office 25 miles from the hospital where she will deliver. Ms. H must complete her antenatal testing at the hospital where the sonographer performs BPPs. She sees her physician at the nearby clinic and then takes public transit to the hospital. She waits 2 hours to be seen then makes her way back home. Her prenatal care visit, which usually takes 30 minutes, turns into a 5-hour ordeal. Ms. H delivered a healthy baby at 39 weeks. Unfortunately, she was fired from her job for missing too many workdays.
Antenatal testing has become routine, and it is costly
For the prescriber, antenatal testing is simple: Order a weekly ultrasound exam to reduce the risk of stillbirth, decrease litigation, generate income, and maximize patient satisfaction (with the assumption that everyone likes to peek at their baby). Recommending antenatal testing has—with the best intentions—become a habit and therefore is difficult to break. However, the American College of Obstetricians and Gynecologists (ACOG) recognizes that “there is a paucity of evidenced-based recommendations on the timing and frequency of antenatal fetal surveillance because of the challenges of conducting prospective trials in pregnancies complicated by stillbirths and the varying conditions that place pregnancies at high risk for stillbirth. As a result, evidence for the efficacy of antenatal fetal surveillance, when available, is largely circumstantial.”1
Antenatal testing without an evidence-based indication can be costly for the health care system, insurance companies, and patients. Many clinics, especially those in rural communities, do not have the equipment or personnel to complete antenatal testing on site. Asking a pregnant patient to travel repeatedly to another location for antenatal testing can increase her time off from work, complicate childcare, pose a financial burden, and lead to nonadherence. As clinicians, it is imperative that we work with our patients to create an individualized care plan to minimize these burdens and increase adherence.
Antenatal fetal surveillance can be considered for conditions in which stillbirth is reported more frequently than 0.8 per 1,000.
Advanced maternal age and stillbirth risk
One of the most common reasons for antenatal testing is advanced maternal age, that is, age older than 35. According to the Centers for Disease Control and Prevention and the National Vital Statistics System, from 2000 to 2012, 46 states and the District of Columbia (DC) reported an increase in first birth rates for women aged 35 to 39. Thirty-one states and DC saw a rise among women aged 40 to 44 in the same period (FIGURE).2

Advanced maternal age is an independent risk factor for stillbirth, with women aged 35 to 39 at 1.9-fold increased risk and women older than age 40 with a 2.4-fold higher risk compared with women younger than age 30.3 In a review of 44 studies including nearly 45,000,000 births, case-control studies, versus cohort studies, demonstrated a higher odds for stillbirth among women aged 35 and older (odds ratio [OR], 2.39; 95% confidence interval [CI], 1.57-3.66 vs OR, 1.73; 95% CI, 1.6-1.87).4 Now, many women older than age 35 may have a concomitant risk factor, such as diabetes or hypertension, that requires antenatal testing. However, for those without other risk factors, nearly 863 antenatal tests and 71 inductions would need to be completed to reduce the number of stillbirths by 1. Antenatal testing for women older than age 35 without other risk factors should be individualized through shared decision making.5 See the ACOG committee opinion for a table that outlines factors associated with an increased risk of stillbirth and suggested strategies for antenatal surveillance after viability.1
Continue to: CASE 2 Patient with high BPP score and altered...
CASE 2 Patient with high BPP score and altered fetal movements delivered for nonreassuring fetal heart rate
Ms. Q was undergoing weekly BPPs for diet-controlled gestational diabetes and a prepregnancy body mass index (BMI) of 52. At 37 weeks’ gestation, she had a BPP score of 8/8. However, it took almost 30 minutes to see 2 discrete body or limb movements. Ms. Q mentioned to the nurse taking her vitals after the BPP that the baby’s movements had changed over the previous few days, especially after contractions. Ms. Q then completed a nonstress test (NST); she had 2 contractions and 2 fetal heart rate decelerations, each lasting approximately 60 seconds. Ms. Q was sent to labor and delivery for prolonged monitoring, and she was delivered that day for a nonreassuring fetal heart rate tracing. Meconium-stained amniotic fluid and a tight triple nuchal cord were noted at delivery.
BPP considerations
While considered an in-depth look at the fetal status, BPPs may not predict overall fetal well-being during acute changes, such as umbilical cord compression or placental abruption. BPPs take longer to complete, require a trained sonographer, and include components like fetal breathing that may be influenced by such factors as nicotine,6-8 labor,9 rupture of membranes,10 magnesium sulfate,11 and infection.12
If medically indicated, which antenatal surveillance technique is right for your patient?
Frequently used antepartum fetal surveillance techniques include maternal perception of fetal movement or “kick counting,” NST, BPP, modified BPP, contraction stress test (CST), and umbilical artery Doppler velocimetry.
Worldwide, the most common form of antenatal surveillance is fetal kick counting. It is noninvasive, can be completed frequently, may decrease maternal anxiety, may improve maternal-fetal bonding, and is free.13 According to the results of a 2020 meta-analysis of 468,601 fetuses, however, there was no difference in perinatal death among patients who assessed fetal movements (0.54%) and those who did not (0.59%).14 There was a statistically significant increase in induction of labor, cesarean delivery, and preterm delivery among patients who counted fetal movements. Women who perceive a decrease in fetal movement should seek medical attention from a health care provider.
An evaluation for decreased fetal movement typically includes taking a history that focuses on risk factors that may increase stillbirth, including hypertension, growth restriction, fetal anomalies, diabetes, and substance use, and auscultation with a fetal Doppler. In the absence of risk factors and the presence of a normal fetal heartbeat, pregnant women should be reassured of fetal well-being. In a pregnancy at greater than 28 weeks, a 20-minute NST can be completed as well; this has become part of the standard workup of decreased fetal movement in developed countries. A reactive NST indicates normal fetal autonomic function in real time and a low incidence of stillbirth (1.9/1,000) within 1 week.15
Additionally, by measuring the amniotic fluid volume using the largest maximal vertical pocket (MVP), clinicians can gain insight into overall uteroplacental function. The combination of the NST and the MVP—otherwise known as a modified BPP—provides both short-term acid-base status and long-term uteroplacental function. The incidence of stillbirth in the 1 week after a modified BPP has been reported to be 0.8/1,000, which is equivalent to stillbirth incidence using a full BPP (0.8/1,000).16 The negative predictive value for both the modified BPP and the BPP is 99.9%—equivalent.
The case for modified BPP use
The modified BPP requires less time, is less costly (cost savings of approximately 50%), does not require a specialized sonographer, and can be performed in local community clinics.
Perhaps the initial antepartum surveillance test of choice should be the modified BPP, with the BPP used in cases in which the results of a modified BPP are abnormal. ●
CASE 1 Pregnant patient endures extensive wait and travel times to have antenatal testing
Pregnant at age 35 without comorbidities, Ms. H was instructed to schedule weekly biophysical profiles (BPP) after 36 weeks’ gestation for advanced maternal age. She receives care at a community office 25 miles from the hospital where she will deliver. Ms. H must complete her antenatal testing at the hospital where the sonographer performs BPPs. She sees her physician at the nearby clinic and then takes public transit to the hospital. She waits 2 hours to be seen then makes her way back home. Her prenatal care visit, which usually takes 30 minutes, turns into a 5-hour ordeal. Ms. H delivered a healthy baby at 39 weeks. Unfortunately, she was fired from her job for missing too many workdays.
Antenatal testing has become routine, and it is costly
For the prescriber, antenatal testing is simple: Order a weekly ultrasound exam to reduce the risk of stillbirth, decrease litigation, generate income, and maximize patient satisfaction (with the assumption that everyone likes to peek at their baby). Recommending antenatal testing has—with the best intentions—become a habit and therefore is difficult to break. However, the American College of Obstetricians and Gynecologists (ACOG) recognizes that “there is a paucity of evidenced-based recommendations on the timing and frequency of antenatal fetal surveillance because of the challenges of conducting prospective trials in pregnancies complicated by stillbirths and the varying conditions that place pregnancies at high risk for stillbirth. As a result, evidence for the efficacy of antenatal fetal surveillance, when available, is largely circumstantial.”1
Antenatal testing without an evidence-based indication can be costly for the health care system, insurance companies, and patients. Many clinics, especially those in rural communities, do not have the equipment or personnel to complete antenatal testing on site. Asking a pregnant patient to travel repeatedly to another location for antenatal testing can increase her time off from work, complicate childcare, pose a financial burden, and lead to nonadherence. As clinicians, it is imperative that we work with our patients to create an individualized care plan to minimize these burdens and increase adherence.
Antenatal fetal surveillance can be considered for conditions in which stillbirth is reported more frequently than 0.8 per 1,000.
Advanced maternal age and stillbirth risk
One of the most common reasons for antenatal testing is advanced maternal age, that is, age older than 35. According to the Centers for Disease Control and Prevention and the National Vital Statistics System, from 2000 to 2012, 46 states and the District of Columbia (DC) reported an increase in first birth rates for women aged 35 to 39. Thirty-one states and DC saw a rise among women aged 40 to 44 in the same period (FIGURE).2

Advanced maternal age is an independent risk factor for stillbirth, with women aged 35 to 39 at 1.9-fold increased risk and women older than age 40 with a 2.4-fold higher risk compared with women younger than age 30.3 In a review of 44 studies including nearly 45,000,000 births, case-control studies, versus cohort studies, demonstrated a higher odds for stillbirth among women aged 35 and older (odds ratio [OR], 2.39; 95% confidence interval [CI], 1.57-3.66 vs OR, 1.73; 95% CI, 1.6-1.87).4 Now, many women older than age 35 may have a concomitant risk factor, such as diabetes or hypertension, that requires antenatal testing. However, for those without other risk factors, nearly 863 antenatal tests and 71 inductions would need to be completed to reduce the number of stillbirths by 1. Antenatal testing for women older than age 35 without other risk factors should be individualized through shared decision making.5 See the ACOG committee opinion for a table that outlines factors associated with an increased risk of stillbirth and suggested strategies for antenatal surveillance after viability.1
Continue to: CASE 2 Patient with high BPP score and altered...
CASE 2 Patient with high BPP score and altered fetal movements delivered for nonreassuring fetal heart rate
Ms. Q was undergoing weekly BPPs for diet-controlled gestational diabetes and a prepregnancy body mass index (BMI) of 52. At 37 weeks’ gestation, she had a BPP score of 8/8. However, it took almost 30 minutes to see 2 discrete body or limb movements. Ms. Q mentioned to the nurse taking her vitals after the BPP that the baby’s movements had changed over the previous few days, especially after contractions. Ms. Q then completed a nonstress test (NST); she had 2 contractions and 2 fetal heart rate decelerations, each lasting approximately 60 seconds. Ms. Q was sent to labor and delivery for prolonged monitoring, and she was delivered that day for a nonreassuring fetal heart rate tracing. Meconium-stained amniotic fluid and a tight triple nuchal cord were noted at delivery.
BPP considerations
While considered an in-depth look at the fetal status, BPPs may not predict overall fetal well-being during acute changes, such as umbilical cord compression or placental abruption. BPPs take longer to complete, require a trained sonographer, and include components like fetal breathing that may be influenced by such factors as nicotine,6-8 labor,9 rupture of membranes,10 magnesium sulfate,11 and infection.12
If medically indicated, which antenatal surveillance technique is right for your patient?
Frequently used antepartum fetal surveillance techniques include maternal perception of fetal movement or “kick counting,” NST, BPP, modified BPP, contraction stress test (CST), and umbilical artery Doppler velocimetry.
Worldwide, the most common form of antenatal surveillance is fetal kick counting. It is noninvasive, can be completed frequently, may decrease maternal anxiety, may improve maternal-fetal bonding, and is free.13 According to the results of a 2020 meta-analysis of 468,601 fetuses, however, there was no difference in perinatal death among patients who assessed fetal movements (0.54%) and those who did not (0.59%).14 There was a statistically significant increase in induction of labor, cesarean delivery, and preterm delivery among patients who counted fetal movements. Women who perceive a decrease in fetal movement should seek medical attention from a health care provider.
An evaluation for decreased fetal movement typically includes taking a history that focuses on risk factors that may increase stillbirth, including hypertension, growth restriction, fetal anomalies, diabetes, and substance use, and auscultation with a fetal Doppler. In the absence of risk factors and the presence of a normal fetal heartbeat, pregnant women should be reassured of fetal well-being. In a pregnancy at greater than 28 weeks, a 20-minute NST can be completed as well; this has become part of the standard workup of decreased fetal movement in developed countries. A reactive NST indicates normal fetal autonomic function in real time and a low incidence of stillbirth (1.9/1,000) within 1 week.15
Additionally, by measuring the amniotic fluid volume using the largest maximal vertical pocket (MVP), clinicians can gain insight into overall uteroplacental function. The combination of the NST and the MVP—otherwise known as a modified BPP—provides both short-term acid-base status and long-term uteroplacental function. The incidence of stillbirth in the 1 week after a modified BPP has been reported to be 0.8/1,000, which is equivalent to stillbirth incidence using a full BPP (0.8/1,000).16 The negative predictive value for both the modified BPP and the BPP is 99.9%—equivalent.
The case for modified BPP use
The modified BPP requires less time, is less costly (cost savings of approximately 50%), does not require a specialized sonographer, and can be performed in local community clinics.
Perhaps the initial antepartum surveillance test of choice should be the modified BPP, with the BPP used in cases in which the results of a modified BPP are abnormal. ●
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for MaternalFetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197.
- Mathews TJ, Hamilton BE. First births to older women continue to rise. NCHS Data Brief, No. 152. Hyattsville, MD: National Center for Health Statistics; 2014.
- Fretts RC, Schmittdiel J, McLean FH, et al. Increased maternal age and the risk of fetal death. N Engl J Med. 1995;333: 953-957.
- Lean SC, Derricott H, Jones RL, et al. Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS One. 2017;12:e0186287.
- Fretts RC, Elkins EB, Myers ER, et al. Should older women have antepartum testing to prevent unexplained stillbirth? Obstet Gynecol. 2004;104:56-64.
- Manning F, Wyn Pugh E, Boddy K. Effect of cigarette smoking on fetal breathing movements in normal pregnancies. Br Med J. 1975;1:552-553.
- Manning FA, Feyerabend C. Cigarette smoking and fetal breathing movements. Br J Obstet Gynecol. 1976;83:262-270.
- Gennser G, Marsal K, Brantmark B. Maternal smoking and fetal breathing movements. Am J Obstet Gynecol. 1975;123:861-867.
- Boylan P, O’Donovan P, Owens OJ. Fetal breathing movements and the diagnosis of labor: a prospective analysis of 100 cases. Obstet Gynecol. 1985;66:517-520.
- Kivikoski AI, Amon E, Vaalamo PO, et al. Effect of thirdtrimester premature rupture of membranes on fetal breathing movements: a prospective case-control study. Am J Obstet Gynecol. 1988;159:1474-1477.
- Peaceman AM, Meyer BA, Thorp JA, et al. The effect of magnesium sulfate tocolysis on the fetal biophysical profile. Am J Obstet Gynecol. 1989;161:771-774.
- Vintzileos AM, Campbell WA, Nochimson DJ, et al. The fetal biophysical profile in patients with premature rupture of the membranes—an early predictor of fetal infection. Am J Obstet Gynecol. 1985;152:501-516.
- Liston RM, Bloom K, Zimmer P. The psychological effects of counting fetal movements. Birth. 1994;21:135-140.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality: a systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462.
- Freeman RK, Anderson G, Dorchester W. A prospective multiinstitutional study of antepartum fetal heart rate monitoring. I. Risk of perinatal mortality and morbidity according to antepartum fetal heart rate test results. Am J Obstet Gynecol. 1982;143:771-777.
- Miller DA , Rabello YA, Paul RH. The modified biophysical profile: antepartum testing in the 1990s. Am J Obstet Gynecol. 1996;174:812-817.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for MaternalFetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197.
- Mathews TJ, Hamilton BE. First births to older women continue to rise. NCHS Data Brief, No. 152. Hyattsville, MD: National Center for Health Statistics; 2014.
- Fretts RC, Schmittdiel J, McLean FH, et al. Increased maternal age and the risk of fetal death. N Engl J Med. 1995;333: 953-957.
- Lean SC, Derricott H, Jones RL, et al. Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS One. 2017;12:e0186287.
- Fretts RC, Elkins EB, Myers ER, et al. Should older women have antepartum testing to prevent unexplained stillbirth? Obstet Gynecol. 2004;104:56-64.
- Manning F, Wyn Pugh E, Boddy K. Effect of cigarette smoking on fetal breathing movements in normal pregnancies. Br Med J. 1975;1:552-553.
- Manning FA, Feyerabend C. Cigarette smoking and fetal breathing movements. Br J Obstet Gynecol. 1976;83:262-270.
- Gennser G, Marsal K, Brantmark B. Maternal smoking and fetal breathing movements. Am J Obstet Gynecol. 1975;123:861-867.
- Boylan P, O’Donovan P, Owens OJ. Fetal breathing movements and the diagnosis of labor: a prospective analysis of 100 cases. Obstet Gynecol. 1985;66:517-520.
- Kivikoski AI, Amon E, Vaalamo PO, et al. Effect of thirdtrimester premature rupture of membranes on fetal breathing movements: a prospective case-control study. Am J Obstet Gynecol. 1988;159:1474-1477.
- Peaceman AM, Meyer BA, Thorp JA, et al. The effect of magnesium sulfate tocolysis on the fetal biophysical profile. Am J Obstet Gynecol. 1989;161:771-774.
- Vintzileos AM, Campbell WA, Nochimson DJ, et al. The fetal biophysical profile in patients with premature rupture of the membranes—an early predictor of fetal infection. Am J Obstet Gynecol. 1985;152:501-516.
- Liston RM, Bloom K, Zimmer P. The psychological effects of counting fetal movements. Birth. 1994;21:135-140.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality: a systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462.
- Freeman RK, Anderson G, Dorchester W. A prospective multiinstitutional study of antepartum fetal heart rate monitoring. I. Risk of perinatal mortality and morbidity according to antepartum fetal heart rate test results. Am J Obstet Gynecol. 1982;143:771-777.
- Miller DA , Rabello YA, Paul RH. The modified biophysical profile: antepartum testing in the 1990s. Am J Obstet Gynecol. 1996;174:812-817.
COVID-19 vaccination and pregnancy: What’s the latest?

COVID-19 vaccination is recommended for all reproductive-aged women, regardless of pregnancy status.1 Yet, national vaccination rates in pregnancy remain woefully low—lower than vaccine coverage rates for other recommended vaccines during pregnancy.2,3 COVID-19 infection has clearly documented risks for maternal and fetal health, and data continue to accumulate on the maternal and neonatal benefits of COVID-19 vaccination in pregnancy, as well as the safety of vaccination during pregnancy.
Maternal and neonatal benefits of COVID-19 vaccination
Does vaccination in pregnancy result in decreased rates of severe COVID-19 infection? Results from a study from a Louisiana health system comparing maternal outcomes between fully vaccinated (defined as 2 weeks after the final vaccine dose) and unvaccinated or partially vaccinated pregnant women during the delta variant—predominant COVID-19 surge clearly answer this question. Vaccination in pregnancy resulted in a 90% risk reduction in severe or critical COVID-19 infection and a 70% risk reduction in COVID-19 infection of any severity among fully vaccinated women. The study also provides some useful absolute numbers for patient counseling: Although none of the 1,332 vaccinated pregnant women in the study required supplemental oxygen or intensive care unit (ICU) admission, there was 1 maternal death, 5 ICU admissions, and 6 stillbirths among the 8,760 unvaccinated pregnant women.4
A larger population-based data set from Scotland and Israel demonstrated similar findings.5 Most importantly, the Scotland data, with most patients having had an mRNA-based vaccine, showed that, while 77% of all COVID-19 infections occurred in unvaccinated pregnant women, 91% of all hospital admissions occurred in unvaccinated women, and 98% of all critical care admissions occurred in unvaccinated women. Furthermore, although 13% of all COVID-19 hospitalizations in pregnancy occurred among vaccinated women, only 2% of critical care admissions occurred among vaccinated women. The Israeli experience (which identified nearly 30,000 eligible pregnancies from 1 of 4 state-mandated health funds in the country), demonstrated that the efficacy of the Pfizer/BioNTech vaccine to prevent a SARS-CoV-2 infection of any severity once fully vaccinated is more than 80%.6
Breakthrough infections, which were more prevalent during the omicron surge, have caused some patients to question the utility of COVID-19 vaccination. Recent data from South Africa, where the omicron variant was first identified, noted that efficacy of the Pfizer/ BioNTech vaccine to prevent hospitalization with COVID-19 infection during an omicron-predominant period was 70%—versus 93% efficacy in a delta-predominant period.7 These data, however, were in the absence of a booster dose, and in vitro studies suggest increased vaccine efficacy with a booster dose.8
Continue to: Counseling women on vaccination benefits and risks...
Counseling women on vaccination benefits and risks. No matter the specific numeric rate of efficacy against a COVID-19 infection, it is important to counsel women that the goal of vaccination is to prevent severe or critical COVID-19 infections, and these data all demonstrate that COVID-19 vaccination meets this goal. However, women may have additional questions regarding both fetal/neonatal benefits and safety with immunization in pregnancy.
Let us address the question of benefit first. In a large cohort of more than 1,300 women vaccinated during pregnancy and delivering at >34 weeks’ gestation, a few observations are worth noting.9 The first is that women who were fully vaccinated by the time of delivery had detectable antibodies at birth, even with first trimester vaccination, and these antibodies did cross the placenta to the neonate. Although higher maternal and neonatal antibody levels are achieved with early third trimester vaccination, it is key that women interpret this finding in light of 2 important points:
- women cannot know what gestational age they will deliver, thus waiting until the early third trimester for vaccination to optimize neonatal antibody levels could result in delivery prior to planned vaccination, with benefit for neither the woman nor the baby
- partial vaccination in the early third trimester resulted in lower maternal and neonatal antibody levels than full vaccination in the first trimester.
In addition, while the data were limited, a booster dose in the third trimester results in the highest antibody levels at delivery. Given the recommendation to initiate a booster dose 5 months after the completion of the primary vaccine series,10 many women will be eligible for a booster prior to delivery and thus can achieve the goals of high maternal and neonatal antibody levels simultaneously. One caveat to these data is that, while higher antibody levels seem comforting and may be better, we do not yet know the level of neonatal antibody necessary to decrease risks of COVID-19 infection in early newborn life.9 Recent data from the Centers for Disease Control and Prevention provide real-world evidence that maternal vaccination decreases the risk of hospitalization from COVID-19 for infants aged <6 months, with vaccine efficacy estimated to be 61% during a period of both Delta and Omicron predominance.11
The evidence is clear—the time for COVID-19 vaccination is now. There is no “optimal” time of vaccination in pregnancy for neonatal benefit that would be worth risking any amount of time a woman is susceptible to COVID-19, especially given the promising data regarding maternal and neonatal antibody levels achieved after a booster dose.
Although the COVID-19 vaccine is currently approved by the US Food and Drug Administration for ages 5 and above, Pfizer-BioNTech has plans to submit for approval for their vaccine’s use among kids as young as 6 months.1 Assuming that this approval occurs, this will leave newborns as the only group without possible vaccination against COVID-19. But can vaccination during pregnancy protect these infants against infection, as vaccination with the flu vaccine during pregnancy confers protective benefit to newborns?2
In a recent research letter published in Journal of the American Medical Association, Shook and colleagues present their data on antibody levels against COVID-19 present in newborns of women who were either naturally infected with COVID-19 at 20 to 32 weeks’ gestation (12 women) or who received mRNA vaccination during pregnancy at 20 to 32 weeks’ gestation (77 women).3 (They chose the 20- to 32-week timeframe during pregnancy because it had “demonstrated superior transplacental transfer of antibodies during this window.”)
They found that COVID-19 antibody levels were higher in both maternal and cord blood at birth in the women who were vaccinated versus the women who had infection. At 6 months, 16 of the 28 infants from the vaccinated-mother group had detectable antibodies compared with 1 of 12 infants from the infected-mother group. The researchers pointed out that the “antibody titer known to be protective against COVID-19 in infants is unknown;” however, they say that their findings provide further supportive evidence for COVID-19 vaccination in pregnant women.3
References
- Pfizer-BioNTech coronavirus vaccine for children under 5 could be available by the end of February, people with knowledge say. The Washington Post. https://www.washingtonpost.com /health/2022/01/31/coronavirus-vaccine-children-under-5/. Accessed February 11, 2022.
- Sakala IG, Honda-Okubo Y, Fung J, et al. Influenza immunization during pregnancy: benefits for mother and infant. Hum Vaccin Immunother. 2016;12:3065-3071. doi:10.1080/21645515.2016 .1215392.
- Shook LL, Atyeo CG, Yonker LM, et al. Durability of anti-spike antibodies in infants after maternal COVID-19 vaccination or natural infection. JAMA. doi:10.1001/jama.2022.1206.
Safety of COVID-19 vaccination: Current data
Risks for pregnancy loss, birth defects, and preterm delivery often are concerns of pregnant women considering a COVID-19 vaccination. Data from more than 2,400 women who submitted their information to the v-SAFE registry demonstrated a 14% risk for pregnancy loss between 6 and 20 weeks’ gestation—well within the expected rate of pregnancy loss in this gestational age range.12
Data from more than 46,000 pregnancies included in the Vaccine Safety Datalink, which includes data from health care organizations in 6 states, demonstrated a preterm birth rate of 6.6% and a small-for-gestational-age rate of 8.2% among fully vaccinated women, rates that were no different among unvaccinated women. There were no differences in the outcomes by trimester of vaccination, and these rates are comparable to the expected rates of these outcomes.13
Women also worry about the risks of vaccine side effects, such as fever or rare adverse events. Although all adverse events (ie, Guillain-Barre syndrome, pericarditis/myocarditis, thrombosis with thrombocytopenia syndrome [TTS]) are very rare, the American College of Obstetricians and Gynecologists does recommend that women get an mRNA COVID-19 vaccine, as the Johnson & Johnson/Janssen vaccine is associated with TTS, which occurred more commonly (although still rare) in women of reproductive age.14
Two large studies of typical side effects experienced after COVID-19 vaccination in pregnancy are incredibly reassuring. In the first, authors of a large study of more than 12,000 pregnant women enrolled in the v-SAFE registry reported that the most common side effect after each mRNA dose was injection site pain (88% after dose 1, 92% after dose 2).15 Self-reported fever occurred in 4% of women after dose 1 and 35% after dose 2. Although this frequency may seem high, a fever of 38.0°C (100.4°F) or higher only occurred among 8% of all participants.
In another study of almost 8,000 women self-reporting side effects (some of whom also may have contributed data to the v-SAFE study), fever occurred in approximately 5% after dose 1 and in about 20% after dose 2.16 In this study, the highest mean temperature was 38.1°C (100.6°F) after dose 1 and 38.2°C (100.7°F) after dose 2. Although it is a reasonable expectation for fever to follow COVID-19 vaccination, particularly after the second dose, the typical fever is a low-grade temperature that will not harm a developing fetus and will be responsive to acetaminophen administration. Moreover, if the fever were the harbinger of harm, then it might stand to reason that an increased signal of preterm delivery may be observed, but data from nearly 10,000 pregnant women vaccinated during the second or third trimesters showed no association with preterm birth (adjusted hazard ratio, 0.91; 95% confidence interval, 0.82–1.01).13
The bottom line
The data are clear. COVID-19 vaccination decreases the risks of severe infection in pregnancy, confers antibodies to neonates with at least some level of protection, and has no demonstrated harmful side effects in pregnancy. ●
- Interim clinical considerations for use of COVID-19 vaccines. CDC website. Published January 24, 2022. Accessed February 22, 2022. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
- Cumulative data: percent of pregnant people aged 18-49 years receiving at least one dose of a COVID-19 vaccine during pregnancy overall, by race/ethnicity, and date reported to CDC—Vaccine Safety Datalink, United States. CDC website. Accessed February 22, 2022. https://data.cdc.gov/Vaccinations/Cumulative-Data-Percent-of-Pregnant-People-aged-18/4ht3-nbmd/data
- Razzaghi H, Kahn KE, Black CL, et al. Influenza and Tdap vaccination coverage among pregnant women—United States, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1391-1397.
- Morgan JA, Biggio JRJ, Martin JK, et al. Maternal outcomes after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in vaccinated compared with unvaccinated pregnant patients. Obstet Gynecol. 2022;139:107-109.
- Stock SJ, Carruthers J, Calvert C, et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland [published online January 13, 2022]. Nat Med. doi:10.1038/s41591-021-01666-2
- Goldshtein I, Nevo D, Steinberg DM, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326:728-735.
- Collie S, Champion J, Moultrie H, et al. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa [published online December 29, 2021]. N Engl J Med. doi:10.1056/NEJMc2119270
- Nemet I, Kliker L, Lustig Y, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 omicron infection [published online December 29, 2021]. N Engl J Med. doi:10.1056/NEJMc2119358
- Yang YJ, Murphy EA, Singh S, et al. Association of gestational age at coronavirus disease 2019 (COVID-19) vaccination, history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and a vaccine booster dose with maternal and umbilical cord antibody levels at delivery [published online December 28, 2021]. Obstet Gynecol. doi:10.1097/AOG.0000000000004693
- COVID-19 vaccine booster shots. Centers for Disease Control and Prevention web site. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html. Accessed March 2, 2022.
- Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19–associated hospitalization in infants aged <6 months—17 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264–270. doi: http://dx.doi.org/10.15585/mmwr.mm7107e3external icon.
- Zauche LH, Wallace B, Smoots AN, et al. Receipt of mRNA COVID-19 vaccines and risk of spontaneous abortion. N Engl J Med. 2021;385:1533-1535.
- Lipkind HS. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth—eight integrated health care organizations, United States, December 15, 2020–July 22, 2021. MMWR Morb Mortal Wkly Rep. doi:10.15585/mmwr.mm7101e1
- COVID-19 vaccination considerations for obstetric-gynecologic care. ACOG website. Updated February 8, 2022. Accessed February 22, 2022. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282.
- Kachikis A, Englund JA, Singleton M, et al. Short-term reactions among pregnant and lactating individuals in the first wave of the COVID-19 vaccine rollout. JAMA Netw Open. 2021;4:E2121310.

COVID-19 vaccination is recommended for all reproductive-aged women, regardless of pregnancy status.1 Yet, national vaccination rates in pregnancy remain woefully low—lower than vaccine coverage rates for other recommended vaccines during pregnancy.2,3 COVID-19 infection has clearly documented risks for maternal and fetal health, and data continue to accumulate on the maternal and neonatal benefits of COVID-19 vaccination in pregnancy, as well as the safety of vaccination during pregnancy.
Maternal and neonatal benefits of COVID-19 vaccination
Does vaccination in pregnancy result in decreased rates of severe COVID-19 infection? Results from a study from a Louisiana health system comparing maternal outcomes between fully vaccinated (defined as 2 weeks after the final vaccine dose) and unvaccinated or partially vaccinated pregnant women during the delta variant—predominant COVID-19 surge clearly answer this question. Vaccination in pregnancy resulted in a 90% risk reduction in severe or critical COVID-19 infection and a 70% risk reduction in COVID-19 infection of any severity among fully vaccinated women. The study also provides some useful absolute numbers for patient counseling: Although none of the 1,332 vaccinated pregnant women in the study required supplemental oxygen or intensive care unit (ICU) admission, there was 1 maternal death, 5 ICU admissions, and 6 stillbirths among the 8,760 unvaccinated pregnant women.4
A larger population-based data set from Scotland and Israel demonstrated similar findings.5 Most importantly, the Scotland data, with most patients having had an mRNA-based vaccine, showed that, while 77% of all COVID-19 infections occurred in unvaccinated pregnant women, 91% of all hospital admissions occurred in unvaccinated women, and 98% of all critical care admissions occurred in unvaccinated women. Furthermore, although 13% of all COVID-19 hospitalizations in pregnancy occurred among vaccinated women, only 2% of critical care admissions occurred among vaccinated women. The Israeli experience (which identified nearly 30,000 eligible pregnancies from 1 of 4 state-mandated health funds in the country), demonstrated that the efficacy of the Pfizer/BioNTech vaccine to prevent a SARS-CoV-2 infection of any severity once fully vaccinated is more than 80%.6
Breakthrough infections, which were more prevalent during the omicron surge, have caused some patients to question the utility of COVID-19 vaccination. Recent data from South Africa, where the omicron variant was first identified, noted that efficacy of the Pfizer/ BioNTech vaccine to prevent hospitalization with COVID-19 infection during an omicron-predominant period was 70%—versus 93% efficacy in a delta-predominant period.7 These data, however, were in the absence of a booster dose, and in vitro studies suggest increased vaccine efficacy with a booster dose.8
Continue to: Counseling women on vaccination benefits and risks...
Counseling women on vaccination benefits and risks. No matter the specific numeric rate of efficacy against a COVID-19 infection, it is important to counsel women that the goal of vaccination is to prevent severe or critical COVID-19 infections, and these data all demonstrate that COVID-19 vaccination meets this goal. However, women may have additional questions regarding both fetal/neonatal benefits and safety with immunization in pregnancy.
Let us address the question of benefit first. In a large cohort of more than 1,300 women vaccinated during pregnancy and delivering at >34 weeks’ gestation, a few observations are worth noting.9 The first is that women who were fully vaccinated by the time of delivery had detectable antibodies at birth, even with first trimester vaccination, and these antibodies did cross the placenta to the neonate. Although higher maternal and neonatal antibody levels are achieved with early third trimester vaccination, it is key that women interpret this finding in light of 2 important points:
- women cannot know what gestational age they will deliver, thus waiting until the early third trimester for vaccination to optimize neonatal antibody levels could result in delivery prior to planned vaccination, with benefit for neither the woman nor the baby
- partial vaccination in the early third trimester resulted in lower maternal and neonatal antibody levels than full vaccination in the first trimester.
In addition, while the data were limited, a booster dose in the third trimester results in the highest antibody levels at delivery. Given the recommendation to initiate a booster dose 5 months after the completion of the primary vaccine series,10 many women will be eligible for a booster prior to delivery and thus can achieve the goals of high maternal and neonatal antibody levels simultaneously. One caveat to these data is that, while higher antibody levels seem comforting and may be better, we do not yet know the level of neonatal antibody necessary to decrease risks of COVID-19 infection in early newborn life.9 Recent data from the Centers for Disease Control and Prevention provide real-world evidence that maternal vaccination decreases the risk of hospitalization from COVID-19 for infants aged <6 months, with vaccine efficacy estimated to be 61% during a period of both Delta and Omicron predominance.11
The evidence is clear—the time for COVID-19 vaccination is now. There is no “optimal” time of vaccination in pregnancy for neonatal benefit that would be worth risking any amount of time a woman is susceptible to COVID-19, especially given the promising data regarding maternal and neonatal antibody levels achieved after a booster dose.
Although the COVID-19 vaccine is currently approved by the US Food and Drug Administration for ages 5 and above, Pfizer-BioNTech has plans to submit for approval for their vaccine’s use among kids as young as 6 months.1 Assuming that this approval occurs, this will leave newborns as the only group without possible vaccination against COVID-19. But can vaccination during pregnancy protect these infants against infection, as vaccination with the flu vaccine during pregnancy confers protective benefit to newborns?2
In a recent research letter published in Journal of the American Medical Association, Shook and colleagues present their data on antibody levels against COVID-19 present in newborns of women who were either naturally infected with COVID-19 at 20 to 32 weeks’ gestation (12 women) or who received mRNA vaccination during pregnancy at 20 to 32 weeks’ gestation (77 women).3 (They chose the 20- to 32-week timeframe during pregnancy because it had “demonstrated superior transplacental transfer of antibodies during this window.”)
They found that COVID-19 antibody levels were higher in both maternal and cord blood at birth in the women who were vaccinated versus the women who had infection. At 6 months, 16 of the 28 infants from the vaccinated-mother group had detectable antibodies compared with 1 of 12 infants from the infected-mother group. The researchers pointed out that the “antibody titer known to be protective against COVID-19 in infants is unknown;” however, they say that their findings provide further supportive evidence for COVID-19 vaccination in pregnant women.3
References
- Pfizer-BioNTech coronavirus vaccine for children under 5 could be available by the end of February, people with knowledge say. The Washington Post. https://www.washingtonpost.com /health/2022/01/31/coronavirus-vaccine-children-under-5/. Accessed February 11, 2022.
- Sakala IG, Honda-Okubo Y, Fung J, et al. Influenza immunization during pregnancy: benefits for mother and infant. Hum Vaccin Immunother. 2016;12:3065-3071. doi:10.1080/21645515.2016 .1215392.
- Shook LL, Atyeo CG, Yonker LM, et al. Durability of anti-spike antibodies in infants after maternal COVID-19 vaccination or natural infection. JAMA. doi:10.1001/jama.2022.1206.
Safety of COVID-19 vaccination: Current data
Risks for pregnancy loss, birth defects, and preterm delivery often are concerns of pregnant women considering a COVID-19 vaccination. Data from more than 2,400 women who submitted their information to the v-SAFE registry demonstrated a 14% risk for pregnancy loss between 6 and 20 weeks’ gestation—well within the expected rate of pregnancy loss in this gestational age range.12
Data from more than 46,000 pregnancies included in the Vaccine Safety Datalink, which includes data from health care organizations in 6 states, demonstrated a preterm birth rate of 6.6% and a small-for-gestational-age rate of 8.2% among fully vaccinated women, rates that were no different among unvaccinated women. There were no differences in the outcomes by trimester of vaccination, and these rates are comparable to the expected rates of these outcomes.13
Women also worry about the risks of vaccine side effects, such as fever or rare adverse events. Although all adverse events (ie, Guillain-Barre syndrome, pericarditis/myocarditis, thrombosis with thrombocytopenia syndrome [TTS]) are very rare, the American College of Obstetricians and Gynecologists does recommend that women get an mRNA COVID-19 vaccine, as the Johnson & Johnson/Janssen vaccine is associated with TTS, which occurred more commonly (although still rare) in women of reproductive age.14
Two large studies of typical side effects experienced after COVID-19 vaccination in pregnancy are incredibly reassuring. In the first, authors of a large study of more than 12,000 pregnant women enrolled in the v-SAFE registry reported that the most common side effect after each mRNA dose was injection site pain (88% after dose 1, 92% after dose 2).15 Self-reported fever occurred in 4% of women after dose 1 and 35% after dose 2. Although this frequency may seem high, a fever of 38.0°C (100.4°F) or higher only occurred among 8% of all participants.
In another study of almost 8,000 women self-reporting side effects (some of whom also may have contributed data to the v-SAFE study), fever occurred in approximately 5% after dose 1 and in about 20% after dose 2.16 In this study, the highest mean temperature was 38.1°C (100.6°F) after dose 1 and 38.2°C (100.7°F) after dose 2. Although it is a reasonable expectation for fever to follow COVID-19 vaccination, particularly after the second dose, the typical fever is a low-grade temperature that will not harm a developing fetus and will be responsive to acetaminophen administration. Moreover, if the fever were the harbinger of harm, then it might stand to reason that an increased signal of preterm delivery may be observed, but data from nearly 10,000 pregnant women vaccinated during the second or third trimesters showed no association with preterm birth (adjusted hazard ratio, 0.91; 95% confidence interval, 0.82–1.01).13
The bottom line
The data are clear. COVID-19 vaccination decreases the risks of severe infection in pregnancy, confers antibodies to neonates with at least some level of protection, and has no demonstrated harmful side effects in pregnancy. ●

COVID-19 vaccination is recommended for all reproductive-aged women, regardless of pregnancy status.1 Yet, national vaccination rates in pregnancy remain woefully low—lower than vaccine coverage rates for other recommended vaccines during pregnancy.2,3 COVID-19 infection has clearly documented risks for maternal and fetal health, and data continue to accumulate on the maternal and neonatal benefits of COVID-19 vaccination in pregnancy, as well as the safety of vaccination during pregnancy.
Maternal and neonatal benefits of COVID-19 vaccination
Does vaccination in pregnancy result in decreased rates of severe COVID-19 infection? Results from a study from a Louisiana health system comparing maternal outcomes between fully vaccinated (defined as 2 weeks after the final vaccine dose) and unvaccinated or partially vaccinated pregnant women during the delta variant—predominant COVID-19 surge clearly answer this question. Vaccination in pregnancy resulted in a 90% risk reduction in severe or critical COVID-19 infection and a 70% risk reduction in COVID-19 infection of any severity among fully vaccinated women. The study also provides some useful absolute numbers for patient counseling: Although none of the 1,332 vaccinated pregnant women in the study required supplemental oxygen or intensive care unit (ICU) admission, there was 1 maternal death, 5 ICU admissions, and 6 stillbirths among the 8,760 unvaccinated pregnant women.4
A larger population-based data set from Scotland and Israel demonstrated similar findings.5 Most importantly, the Scotland data, with most patients having had an mRNA-based vaccine, showed that, while 77% of all COVID-19 infections occurred in unvaccinated pregnant women, 91% of all hospital admissions occurred in unvaccinated women, and 98% of all critical care admissions occurred in unvaccinated women. Furthermore, although 13% of all COVID-19 hospitalizations in pregnancy occurred among vaccinated women, only 2% of critical care admissions occurred among vaccinated women. The Israeli experience (which identified nearly 30,000 eligible pregnancies from 1 of 4 state-mandated health funds in the country), demonstrated that the efficacy of the Pfizer/BioNTech vaccine to prevent a SARS-CoV-2 infection of any severity once fully vaccinated is more than 80%.6
Breakthrough infections, which were more prevalent during the omicron surge, have caused some patients to question the utility of COVID-19 vaccination. Recent data from South Africa, where the omicron variant was first identified, noted that efficacy of the Pfizer/ BioNTech vaccine to prevent hospitalization with COVID-19 infection during an omicron-predominant period was 70%—versus 93% efficacy in a delta-predominant period.7 These data, however, were in the absence of a booster dose, and in vitro studies suggest increased vaccine efficacy with a booster dose.8
Continue to: Counseling women on vaccination benefits and risks...
Counseling women on vaccination benefits and risks. No matter the specific numeric rate of efficacy against a COVID-19 infection, it is important to counsel women that the goal of vaccination is to prevent severe or critical COVID-19 infections, and these data all demonstrate that COVID-19 vaccination meets this goal. However, women may have additional questions regarding both fetal/neonatal benefits and safety with immunization in pregnancy.
Let us address the question of benefit first. In a large cohort of more than 1,300 women vaccinated during pregnancy and delivering at >34 weeks’ gestation, a few observations are worth noting.9 The first is that women who were fully vaccinated by the time of delivery had detectable antibodies at birth, even with first trimester vaccination, and these antibodies did cross the placenta to the neonate. Although higher maternal and neonatal antibody levels are achieved with early third trimester vaccination, it is key that women interpret this finding in light of 2 important points:
- women cannot know what gestational age they will deliver, thus waiting until the early third trimester for vaccination to optimize neonatal antibody levels could result in delivery prior to planned vaccination, with benefit for neither the woman nor the baby
- partial vaccination in the early third trimester resulted in lower maternal and neonatal antibody levels than full vaccination in the first trimester.
In addition, while the data were limited, a booster dose in the third trimester results in the highest antibody levels at delivery. Given the recommendation to initiate a booster dose 5 months after the completion of the primary vaccine series,10 many women will be eligible for a booster prior to delivery and thus can achieve the goals of high maternal and neonatal antibody levels simultaneously. One caveat to these data is that, while higher antibody levels seem comforting and may be better, we do not yet know the level of neonatal antibody necessary to decrease risks of COVID-19 infection in early newborn life.9 Recent data from the Centers for Disease Control and Prevention provide real-world evidence that maternal vaccination decreases the risk of hospitalization from COVID-19 for infants aged <6 months, with vaccine efficacy estimated to be 61% during a period of both Delta and Omicron predominance.11
The evidence is clear—the time for COVID-19 vaccination is now. There is no “optimal” time of vaccination in pregnancy for neonatal benefit that would be worth risking any amount of time a woman is susceptible to COVID-19, especially given the promising data regarding maternal and neonatal antibody levels achieved after a booster dose.
Although the COVID-19 vaccine is currently approved by the US Food and Drug Administration for ages 5 and above, Pfizer-BioNTech has plans to submit for approval for their vaccine’s use among kids as young as 6 months.1 Assuming that this approval occurs, this will leave newborns as the only group without possible vaccination against COVID-19. But can vaccination during pregnancy protect these infants against infection, as vaccination with the flu vaccine during pregnancy confers protective benefit to newborns?2
In a recent research letter published in Journal of the American Medical Association, Shook and colleagues present their data on antibody levels against COVID-19 present in newborns of women who were either naturally infected with COVID-19 at 20 to 32 weeks’ gestation (12 women) or who received mRNA vaccination during pregnancy at 20 to 32 weeks’ gestation (77 women).3 (They chose the 20- to 32-week timeframe during pregnancy because it had “demonstrated superior transplacental transfer of antibodies during this window.”)
They found that COVID-19 antibody levels were higher in both maternal and cord blood at birth in the women who were vaccinated versus the women who had infection. At 6 months, 16 of the 28 infants from the vaccinated-mother group had detectable antibodies compared with 1 of 12 infants from the infected-mother group. The researchers pointed out that the “antibody titer known to be protective against COVID-19 in infants is unknown;” however, they say that their findings provide further supportive evidence for COVID-19 vaccination in pregnant women.3
References
- Pfizer-BioNTech coronavirus vaccine for children under 5 could be available by the end of February, people with knowledge say. The Washington Post. https://www.washingtonpost.com /health/2022/01/31/coronavirus-vaccine-children-under-5/. Accessed February 11, 2022.
- Sakala IG, Honda-Okubo Y, Fung J, et al. Influenza immunization during pregnancy: benefits for mother and infant. Hum Vaccin Immunother. 2016;12:3065-3071. doi:10.1080/21645515.2016 .1215392.
- Shook LL, Atyeo CG, Yonker LM, et al. Durability of anti-spike antibodies in infants after maternal COVID-19 vaccination or natural infection. JAMA. doi:10.1001/jama.2022.1206.
Safety of COVID-19 vaccination: Current data
Risks for pregnancy loss, birth defects, and preterm delivery often are concerns of pregnant women considering a COVID-19 vaccination. Data from more than 2,400 women who submitted their information to the v-SAFE registry demonstrated a 14% risk for pregnancy loss between 6 and 20 weeks’ gestation—well within the expected rate of pregnancy loss in this gestational age range.12
Data from more than 46,000 pregnancies included in the Vaccine Safety Datalink, which includes data from health care organizations in 6 states, demonstrated a preterm birth rate of 6.6% and a small-for-gestational-age rate of 8.2% among fully vaccinated women, rates that were no different among unvaccinated women. There were no differences in the outcomes by trimester of vaccination, and these rates are comparable to the expected rates of these outcomes.13
Women also worry about the risks of vaccine side effects, such as fever or rare adverse events. Although all adverse events (ie, Guillain-Barre syndrome, pericarditis/myocarditis, thrombosis with thrombocytopenia syndrome [TTS]) are very rare, the American College of Obstetricians and Gynecologists does recommend that women get an mRNA COVID-19 vaccine, as the Johnson & Johnson/Janssen vaccine is associated with TTS, which occurred more commonly (although still rare) in women of reproductive age.14
Two large studies of typical side effects experienced after COVID-19 vaccination in pregnancy are incredibly reassuring. In the first, authors of a large study of more than 12,000 pregnant women enrolled in the v-SAFE registry reported that the most common side effect after each mRNA dose was injection site pain (88% after dose 1, 92% after dose 2).15 Self-reported fever occurred in 4% of women after dose 1 and 35% after dose 2. Although this frequency may seem high, a fever of 38.0°C (100.4°F) or higher only occurred among 8% of all participants.
In another study of almost 8,000 women self-reporting side effects (some of whom also may have contributed data to the v-SAFE study), fever occurred in approximately 5% after dose 1 and in about 20% after dose 2.16 In this study, the highest mean temperature was 38.1°C (100.6°F) after dose 1 and 38.2°C (100.7°F) after dose 2. Although it is a reasonable expectation for fever to follow COVID-19 vaccination, particularly after the second dose, the typical fever is a low-grade temperature that will not harm a developing fetus and will be responsive to acetaminophen administration. Moreover, if the fever were the harbinger of harm, then it might stand to reason that an increased signal of preterm delivery may be observed, but data from nearly 10,000 pregnant women vaccinated during the second or third trimesters showed no association with preterm birth (adjusted hazard ratio, 0.91; 95% confidence interval, 0.82–1.01).13
The bottom line
The data are clear. COVID-19 vaccination decreases the risks of severe infection in pregnancy, confers antibodies to neonates with at least some level of protection, and has no demonstrated harmful side effects in pregnancy. ●
- Interim clinical considerations for use of COVID-19 vaccines. CDC website. Published January 24, 2022. Accessed February 22, 2022. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
- Cumulative data: percent of pregnant people aged 18-49 years receiving at least one dose of a COVID-19 vaccine during pregnancy overall, by race/ethnicity, and date reported to CDC—Vaccine Safety Datalink, United States. CDC website. Accessed February 22, 2022. https://data.cdc.gov/Vaccinations/Cumulative-Data-Percent-of-Pregnant-People-aged-18/4ht3-nbmd/data
- Razzaghi H, Kahn KE, Black CL, et al. Influenza and Tdap vaccination coverage among pregnant women—United States, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1391-1397.
- Morgan JA, Biggio JRJ, Martin JK, et al. Maternal outcomes after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in vaccinated compared with unvaccinated pregnant patients. Obstet Gynecol. 2022;139:107-109.
- Stock SJ, Carruthers J, Calvert C, et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland [published online January 13, 2022]. Nat Med. doi:10.1038/s41591-021-01666-2
- Goldshtein I, Nevo D, Steinberg DM, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326:728-735.
- Collie S, Champion J, Moultrie H, et al. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa [published online December 29, 2021]. N Engl J Med. doi:10.1056/NEJMc2119270
- Nemet I, Kliker L, Lustig Y, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 omicron infection [published online December 29, 2021]. N Engl J Med. doi:10.1056/NEJMc2119358
- Yang YJ, Murphy EA, Singh S, et al. Association of gestational age at coronavirus disease 2019 (COVID-19) vaccination, history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and a vaccine booster dose with maternal and umbilical cord antibody levels at delivery [published online December 28, 2021]. Obstet Gynecol. doi:10.1097/AOG.0000000000004693
- COVID-19 vaccine booster shots. Centers for Disease Control and Prevention web site. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html. Accessed March 2, 2022.
- Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19–associated hospitalization in infants aged <6 months—17 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264–270. doi: http://dx.doi.org/10.15585/mmwr.mm7107e3external icon.
- Zauche LH, Wallace B, Smoots AN, et al. Receipt of mRNA COVID-19 vaccines and risk of spontaneous abortion. N Engl J Med. 2021;385:1533-1535.
- Lipkind HS. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth—eight integrated health care organizations, United States, December 15, 2020–July 22, 2021. MMWR Morb Mortal Wkly Rep. doi:10.15585/mmwr.mm7101e1
- COVID-19 vaccination considerations for obstetric-gynecologic care. ACOG website. Updated February 8, 2022. Accessed February 22, 2022. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282.
- Kachikis A, Englund JA, Singleton M, et al. Short-term reactions among pregnant and lactating individuals in the first wave of the COVID-19 vaccine rollout. JAMA Netw Open. 2021;4:E2121310.
- Interim clinical considerations for use of COVID-19 vaccines. CDC website. Published January 24, 2022. Accessed February 22, 2022. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
- Cumulative data: percent of pregnant people aged 18-49 years receiving at least one dose of a COVID-19 vaccine during pregnancy overall, by race/ethnicity, and date reported to CDC—Vaccine Safety Datalink, United States. CDC website. Accessed February 22, 2022. https://data.cdc.gov/Vaccinations/Cumulative-Data-Percent-of-Pregnant-People-aged-18/4ht3-nbmd/data
- Razzaghi H, Kahn KE, Black CL, et al. Influenza and Tdap vaccination coverage among pregnant women—United States, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1391-1397.
- Morgan JA, Biggio JRJ, Martin JK, et al. Maternal outcomes after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in vaccinated compared with unvaccinated pregnant patients. Obstet Gynecol. 2022;139:107-109.
- Stock SJ, Carruthers J, Calvert C, et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland [published online January 13, 2022]. Nat Med. doi:10.1038/s41591-021-01666-2
- Goldshtein I, Nevo D, Steinberg DM, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326:728-735.
- Collie S, Champion J, Moultrie H, et al. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa [published online December 29, 2021]. N Engl J Med. doi:10.1056/NEJMc2119270
- Nemet I, Kliker L, Lustig Y, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 omicron infection [published online December 29, 2021]. N Engl J Med. doi:10.1056/NEJMc2119358
- Yang YJ, Murphy EA, Singh S, et al. Association of gestational age at coronavirus disease 2019 (COVID-19) vaccination, history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and a vaccine booster dose with maternal and umbilical cord antibody levels at delivery [published online December 28, 2021]. Obstet Gynecol. doi:10.1097/AOG.0000000000004693
- COVID-19 vaccine booster shots. Centers for Disease Control and Prevention web site. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html. Accessed March 2, 2022.
- Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19–associated hospitalization in infants aged <6 months—17 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264–270. doi: http://dx.doi.org/10.15585/mmwr.mm7107e3external icon.
- Zauche LH, Wallace B, Smoots AN, et al. Receipt of mRNA COVID-19 vaccines and risk of spontaneous abortion. N Engl J Med. 2021;385:1533-1535.
- Lipkind HS. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth—eight integrated health care organizations, United States, December 15, 2020–July 22, 2021. MMWR Morb Mortal Wkly Rep. doi:10.15585/mmwr.mm7101e1
- COVID-19 vaccination considerations for obstetric-gynecologic care. ACOG website. Updated February 8, 2022. Accessed February 22, 2022. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282.
- Kachikis A, Englund JA, Singleton M, et al. Short-term reactions among pregnant and lactating individuals in the first wave of the COVID-19 vaccine rollout. JAMA Netw Open. 2021;4:E2121310.
Dermatologic Management of Hidradenitis Suppurativa and Impact on Pregnancy and Breastfeeding
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease associated with hyperandrogenism and is caused by occlusion or rupture of follicular units and inflammation of the apocrine glands.1-3 The disease most commonly affects women (female to male ratio of 3:1) of childbearing age.1,2,4,5 Body areas affected include the axillae and groin, and less commonly the perineum; perianal region; and skin folds, such as gluteal, inframammary, and infraumbilical folds.1,2 Symptoms manifest as painful subcutaneous nodules with possible accompanying purulent drainage, sinus tracts, and/or dermal contractures. Although the pathophysiology is unclear, androgens affect the course of HS during pregnancy by stimulating the affected glands and altering cytokines.1,2,6
During pregnancy, maternal immune function switches from cell-mediated T helper cell (TH1) to humoral TH2 cytokine production. The activity of sebaceous and eccrine glands increases while the activity of apocrine glands decreases, thus changing the inflammatory course of HS during pregnancy.3 Approximately 20% of women with HS experience improvement of symptoms during pregnancy, while the remainder either experience no relief or deterioration of symptoms.1 Improvement in symptoms during pregnancy was found to occur more frequently in those who had worsening symptoms during menses owing to the possible hormonal effect estrogen has on inhibiting TH1 and TH17 proinflammatory cytokines, which promotes an immunosuppressive environment.4
Lactation and breastfeeding abilities may be hindered if a woman has HS affecting the apocrine glands of breast tissue and a symptom flare in the postpartum period. If HS causes notable inflammation in the nipple-areolar complex during pregnancy, the patient may experience difficulties with lactation and milk fistula formation, leading to inability to breastfeed.2 Another reason why mothers with HS may not be able to breastfeed is that the medications required to treat the disease are unsafe if passed to the infant via breast milk. In addition, the teratogenic effects of HS medications may necessitate therapy adjustments in pregnancy.1 Here, we provide a brief overview of the medical management considerations of HS in the setting of pregnancy and the impact on breastfeeding.
MEDICAL MANAGEMENT AND DRUG SAFETY
Dermatologists prescribe a myriad of topical and systemic medications to ameliorate symptoms of HS. Therapy regimens often are multimodal and include antibiotics, biologics, and immunosuppressants.1,3
Antibiotics
First-line antibiotics include clindamycin, metronidazole, tetracyclines, erythromycin, rifampin, dapsone, and fluoroquinolones. Topical clindamycin 1%, metronidazole 0.75%, and erythromycin 2% are used for open or active HS lesions and are all safe to use in pregnancy since there is minimal systemic absorption and minimal excretion into breast milk.1 Topical antimicrobial washes such as benzoyl peroxide and chlorhexidine often are used in combination with systemic medications to treat HS. These washes are safe during pregnancy and lactation, as they have minimal systemic absorption.7
Of these first-line antibiotics, only tetracyclines are contraindicated during pregnancy and lactation, as they are deemed to be in category D by the US Food and Drug Administration (FDA).1 Aside from tetracyclines, these antibiotics do not cause birth defects and are safe for nursing infants.1,8 Systemic clindamycin is safe during pregnancy and breastfeeding. Systemic metronidazole also is safe for use in pregnant patients but needs to be discontinued 12 to 24 hours prior to breastfeeding, which often prohibits appropriate dosing.1
Systemic Erythromycin—There are several forms of systemic erythromycin, including erythromycin base, erythromycin estolate, erythromycin ethylsuccinate (EES), and erythromycin stearate. Erythromycin estolate is contraindicated in pregnancy because it is associated with reversible maternal hepatoxicity and jaundice.9-11 Erythromycin ethylsuccinate is the preferred form for pregnant patients. Providers should exercise caution when prescribing EES to lactating mothers, as small amounts are still secreted through breast milk.11 Some studies have shown an increased risk for development of infantile hypertrophic pyloric stenosis with systemic erythromycin use, especially if a neonate is exposed in the first 14 days of life. Thus, we recommend withholding EES for 2 weeks after delivery if the patient is breastfeeding. A follow-up study did not find any association between erythromycin and infantile hypertrophic pyloric stenosis; however, the American Academy of Pediatrics still recommends short-term use only of erythromycin if it is to be used in the systemic form.8
Rifampin—Rifampin is excreted into breast milk but without adverse effects to the infant. Rifampin also is safe in pregnancy but should be used on a case-by-case basis in pregnant or nursing women because it is a cytochrome P450 inducer.
Dapsone—Dapsone has no increased risk for congenital anomalies. However, it is associated with hemolytic anemia and neonatal hyperbilirubinemia, especially in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.12 Newborns exposed to dapsone are at an increased risk for methemoglobinemia owing to increased sensitivity of fetal erythrocytes to oxidizing agents.13 If dapsone use is necessary, stopping dapsone treatment in the last month of gestation is recommended to minimize risk for kernicterus.9 Dapsone can be found in high concentrations in breast milk at 14.3% of the maternal dose. It is still safe to use during breastfeeding, but there is a risk of the infant developing hyperbilirubinemia/G6PD deficiency.1,8 Thus, physicians may consider performing a G6PD screen on infants to determine if breastfeeding is safe.12
Fluoroquinolones—Quinolones are not contraindicated during pregnancy, but they can damage fetal cartilage and thus should be reserved for use in complicated infections when the benefits outweigh the risks.12 Quinolones are believed to increase risk for arthropathy but are safe for use in lactation. When quinolones are digested with milk, exposure decreases below pediatric doses because of the ionized property of calcium in milk.8
Tumor Necrosis Factor α Inhibitors—The safety of anti–tumor necrosis factor (TNF) α biologics in pregnancy is less certain when compared with antibiotics.1 Anti–TNF-α inhibitors such as etanercept, adalimumab, and infliximab are all labeled as FDA category B, meaning there are no well-controlled human studies of the drugs.9 There are limited data that support safe use of TNF-α inhibitors prior to the third trimester before maternal IgG antibodies are transferred to the fetus via the placenta.1,13 Anti–TNF-α inhibitors may be safe when breastfeeding because the drugs have large molecular weights that prevent them from entering breast milk in large amounts. Absorption also is limited due to the infant’s digestive acids and enzymes breaking down the protein structure of the medication.8 Overall, TNF-α inhibitor use is still controversial and only used if the benefits outweigh the risks during pregnancy or if there is no alternative treatment.1,3,9
Ustekinumab and Anakinra—Ustekinumab (an IL-12/IL-23 inhibitor) and anakinra (an IL-1α and IL-1β inhibitor) also are FDA category B drugs and have limited data supporting their use as HS treatment in pregnancy. Anakinra may have evidence of compatibility with breastfeeding, as endogenous IL-1α inhibitor is found in colostrum and mature breast milk.1
Immunosuppressants
Immunosuppressants that are used to treat HS include corticosteroids and cyclosporine.
Corticosteroids—Topical corticosteroids can be used safely in lactation if they are not applied directly to the nipple or any area that makes direct contact with the infant’s mouth. Intralesional corticosteroid injections are safe for use during both pregnancy and breastfeeding to decrease inflammation of acutely flaring lesions and can be considered first-line treatment.1 Oral glucocorticoids also can be safely used for acute flares during pregnancy; however, prolonged use is associated with pregnancy complications such as preeclampsia, eclampsia, premature delivery, and gestational diabetes.12 There also is a small risk of oral cleft deformity in the infant; thus, potent corticosteroids are recommended in short durations during pregnancy, and there are no adverse effects if the maternal dose is less than 10 mg daily.8,12 Systemic steroids are safe to use with breastfeeding, but patients should be advised to wait 4 hours after ingesting medication before breastfeeding.1,8
Cyclosporine—Topical and oral calcineurin inhibitors such as cyclosporine have low risk for transmission into breast milk; however, potential effects of exposure through breast milk are unknown. For that reason, manufacturers state that cyclosporine use is contraindicated during lactation.8 If cyclosporine is to be used by a breastfeeding woman, monitoring cyclosporine concentrations in the infant is suggested to ensure that the exposure is less than 5% to 10% of the therapeutic dose.13 The use of cyclosporine has been extensively studied in pregnant transplant patients and is considered relatively safe for use in pregnancy.14 Cyclosporine is lipid soluble and thus is quickly metabolized and spread throughout the body; it can easily cross the placenta.9,13 Blood concentration in the fetus is 30% to 64% that of the maternal circulation. However, cyclosporine is only toxic to the fetus at maternally toxic doses, which can result in low birth weight and increased prenatal and postnatal mortality.13
Isotretinoin, Oral Contraceptive Pills, and Spironolactone
Isotretinoin and hormonal treatments such as oral contraceptive pills and spironolactone (an androgen receptor blocker) commonly are used to treat HS, but all are contraindicated in pregnancy and lactation. Isotretinoin is a well-established teratogen, but adverse effects on nursing babies have not been described. However, the manufacturer of isotretinoin advises against its use in lactation. Oral contraceptive pill use in early pregnancy is associated with increased risk for Down syndrome. Oral contraceptive pill use also is contraindicated in lactation for 2 reasons: decreased milk production and risk for fetal feminization. Antiandrogenic agents such as spironolactone have been shown to be associated with hypospadias and feminization of the male fetus.7
COMMENT
Women with HS usually require ongoing medical treatment during pregnancy and immediately postpartum; thus, it is important that treatments are proven to be safe for use in this specific population. Current management guidelines are not entirely suitable for pregnant and breastfeeding women given that many HS drugs have teratogenic effects and/or can be excreted into breast milk.1 Several treatments have uncertain safety profiles in pregnancy and breastfeeding, which calls for dermatologists to change or create new regimens for their patients. Close management also is necessary to prevent excess inflammation of breast tissue and milk fistula formation, which would hinder normal breastfeeding.
The eTable lists medications used to treat HS. The FDA category is listed next to each drug. However, it should be noted that these FDA letter categories were replaced with the Pregnancy and Lactation Labeling Rule in 2015. The letter ratings were deemed overly simplistic and replaced with narrative-based labeling that provides more detailed adverse effects and clinical considerations.9
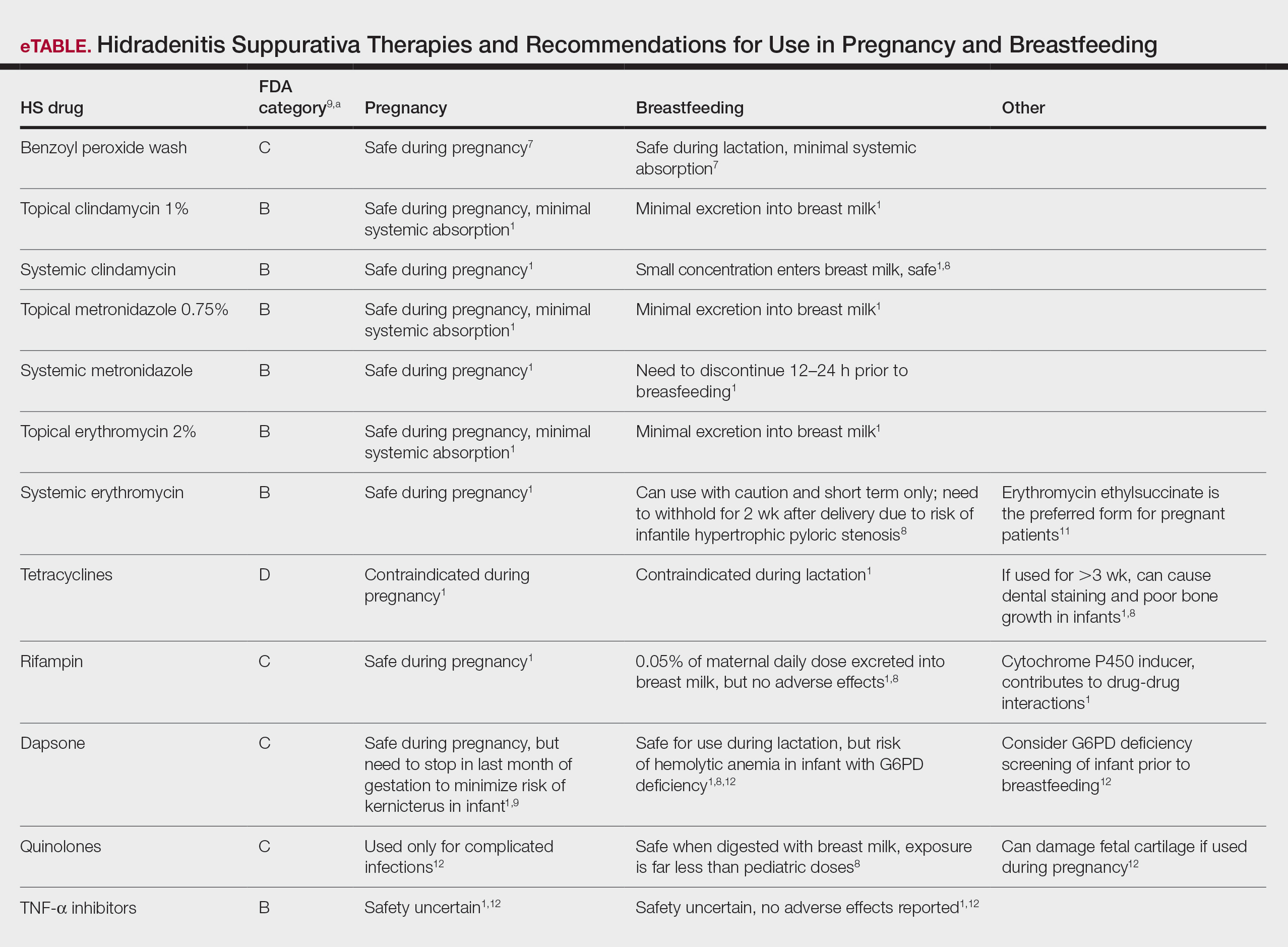
Risk Factors of HS—Predisposing risk factors for HS flares that are controllable include obesity and smoking.2 Pregnancy weight gain may cause increased skin maceration at intertriginous sites, which can contribute to worsening HS symptoms.1,5 Adipocytes play a role in HS exacerbation by promoting secretion of TNF-α, leading to increased inflammation.5 Dermatologists can help prevent postpartum HS flares by monitoring weight gain during pregnancy, encouraging smoking cessation, and promoting weight and nutrition goals as set by an obstetrician.1 In addition to medications, management of HS should include emotional support and education on wearing loose-fitting clothing to avoid irritation of the affected areas.3 An emphasis on dermatologist counseling for all patients with HS, even for those with milder disease, can reduce exacerbations during pregnancy.5
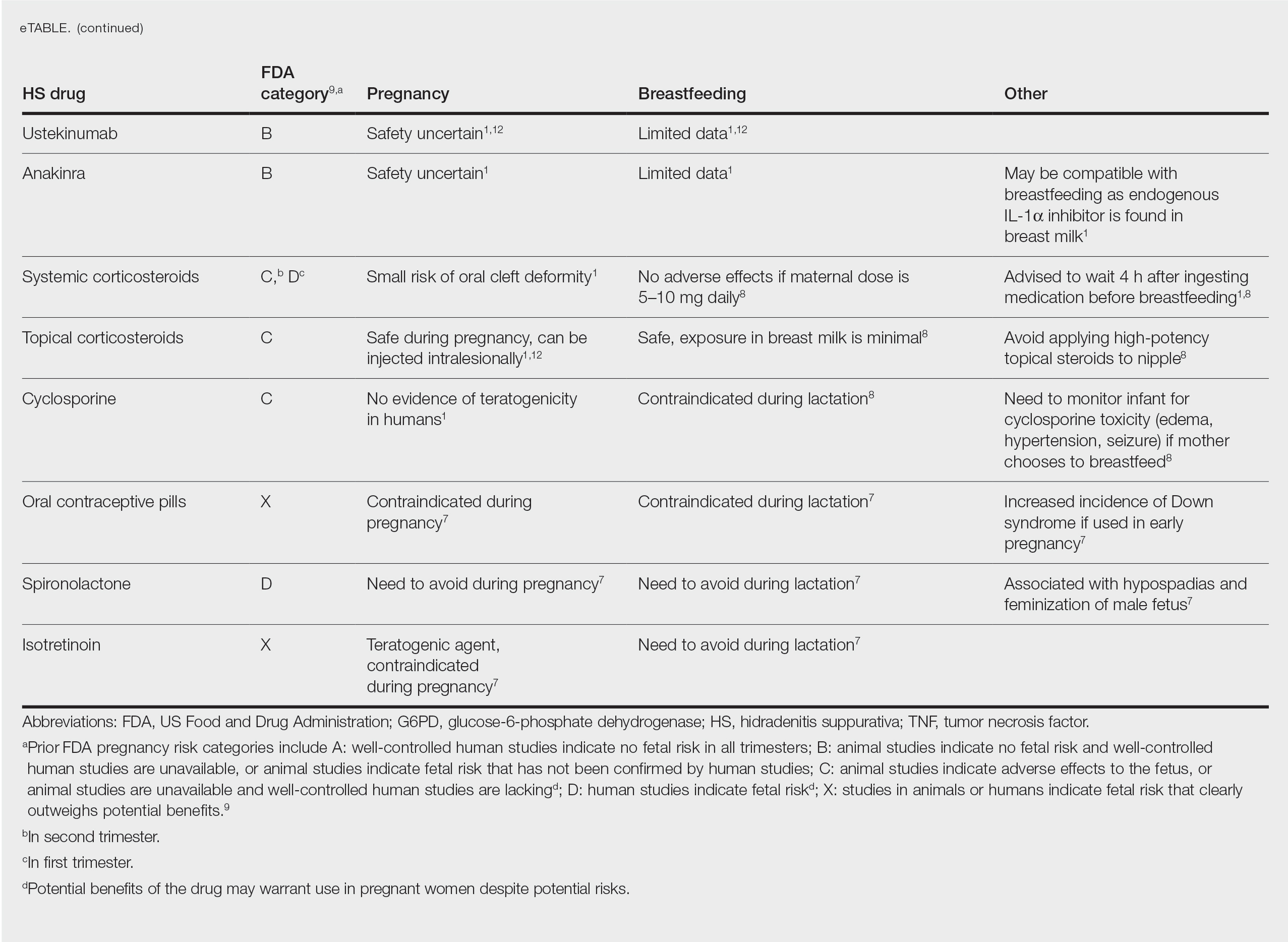
CONCLUSION
The selection of dermatologic drugs for the treatment of HS in the setting of pregnancy involves complex decision-making. Dermatologists need more guidelines and proven safety data in human trials, especially regarding use of biologics and immunosuppressants to better treat HS in pregnancy. With more data, they can create more evidence-based treatment regimens to help prevent postpartum exacerbations of HS. Thus, patients can breastfeed their infants comfortably and without any risks of impaired child development. In the meantime, dermatologists can continue to work together with obstetricians and psychiatrists to decrease disease flares through counseling patients on nutrition and weight gain and providing emotional support.
- Perng P, Zampella JG, Okoye GA. Management of hidradenitis suppurativa in pregnancy. J Am Acad Dermatol. 2017;76:979-989. doi:10.1016/j.jaad.2016.10.032
- Samuel S, Tremelling A, Murray M. Presentation and surgical management of hidradenitis suppurativa of the breast during pregnancy: a case report. Int J Surg Case Rep. 2018;51:21-24. doi:10.1016/j.ijscr.2018.08.013
- Yang CS, Teeple M, Muglia J, et al. Inflammatory and glandular skin disease in pregnancy. Clin Dermatol. 2016;34:335-343. doi:10.1016/j.clindermatol.2016.02.005
- Vossen AR, van Straalen KR, Prens EP, et al. Menses and pregnancy affect symptoms in hidradenitis suppurativa: a cross-sectional study. J Am Acad Dermatol. 2017;76:155-156. doi:10.1016/j.jaad.2016.07.024
- Lyons AB, Peacock A, McKenzie SA, et al. Evaluation of hidradenitis suppurativa disease course during pregnancy and postpartum. JAMA Dermatol. 2020;156:681-685. doi:10.1001/jamadermatol.2020.0777
- Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa—a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
- Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787. doi:10.1007/s40265-013-0060-0
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. lactation. J Am Acad Dermatol. 2014;70:417:E1-E10. doi:10.1016/j.jaad.2013.09.009
- Wilmer E, Chai S, Kroumpouzos G. Drug safety: pregnancy rating classifications and controversies. Clin Dermatol. 2016;34:401-409. doi:10.1016/j.clindermatol.2016.02.013
- Inman WH, Rawson NS. Erythromycin estolate and jaundice. Br Med J (Clin Res Ed). 1983;286:1954-1955. doi:10.1136/bmj.286.6382.1954
- Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. pregnancy. J Am Acad Dermatol. 2014;70:401.e1-14; quiz 415. doi:10.1016/j.jaad.2013.09.010
- Brown SM, Aljefri K, Waas R, et al. Systemic medications used in treatment of common dermatological conditions: safety profile with respect to pregnancy, breast feeding and content in seminal fluid. J Dermatolog Treat. 2019;30:2-18. doi:10.1080/09546634.2016.1202402
- Kamarajah SK, Arntdz K, Bundred J, et al. Outcomes of pregnancy in recipients of liver transplants. Clin Gastroenterol Hepatol. 2019;17:1398-1404.e1. doi:10.1016/j.cgh.2018.11.055
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease associated with hyperandrogenism and is caused by occlusion or rupture of follicular units and inflammation of the apocrine glands.1-3 The disease most commonly affects women (female to male ratio of 3:1) of childbearing age.1,2,4,5 Body areas affected include the axillae and groin, and less commonly the perineum; perianal region; and skin folds, such as gluteal, inframammary, and infraumbilical folds.1,2 Symptoms manifest as painful subcutaneous nodules with possible accompanying purulent drainage, sinus tracts, and/or dermal contractures. Although the pathophysiology is unclear, androgens affect the course of HS during pregnancy by stimulating the affected glands and altering cytokines.1,2,6
During pregnancy, maternal immune function switches from cell-mediated T helper cell (TH1) to humoral TH2 cytokine production. The activity of sebaceous and eccrine glands increases while the activity of apocrine glands decreases, thus changing the inflammatory course of HS during pregnancy.3 Approximately 20% of women with HS experience improvement of symptoms during pregnancy, while the remainder either experience no relief or deterioration of symptoms.1 Improvement in symptoms during pregnancy was found to occur more frequently in those who had worsening symptoms during menses owing to the possible hormonal effect estrogen has on inhibiting TH1 and TH17 proinflammatory cytokines, which promotes an immunosuppressive environment.4
Lactation and breastfeeding abilities may be hindered if a woman has HS affecting the apocrine glands of breast tissue and a symptom flare in the postpartum period. If HS causes notable inflammation in the nipple-areolar complex during pregnancy, the patient may experience difficulties with lactation and milk fistula formation, leading to inability to breastfeed.2 Another reason why mothers with HS may not be able to breastfeed is that the medications required to treat the disease are unsafe if passed to the infant via breast milk. In addition, the teratogenic effects of HS medications may necessitate therapy adjustments in pregnancy.1 Here, we provide a brief overview of the medical management considerations of HS in the setting of pregnancy and the impact on breastfeeding.
MEDICAL MANAGEMENT AND DRUG SAFETY
Dermatologists prescribe a myriad of topical and systemic medications to ameliorate symptoms of HS. Therapy regimens often are multimodal and include antibiotics, biologics, and immunosuppressants.1,3
Antibiotics
First-line antibiotics include clindamycin, metronidazole, tetracyclines, erythromycin, rifampin, dapsone, and fluoroquinolones. Topical clindamycin 1%, metronidazole 0.75%, and erythromycin 2% are used for open or active HS lesions and are all safe to use in pregnancy since there is minimal systemic absorption and minimal excretion into breast milk.1 Topical antimicrobial washes such as benzoyl peroxide and chlorhexidine often are used in combination with systemic medications to treat HS. These washes are safe during pregnancy and lactation, as they have minimal systemic absorption.7
Of these first-line antibiotics, only tetracyclines are contraindicated during pregnancy and lactation, as they are deemed to be in category D by the US Food and Drug Administration (FDA).1 Aside from tetracyclines, these antibiotics do not cause birth defects and are safe for nursing infants.1,8 Systemic clindamycin is safe during pregnancy and breastfeeding. Systemic metronidazole also is safe for use in pregnant patients but needs to be discontinued 12 to 24 hours prior to breastfeeding, which often prohibits appropriate dosing.1
Systemic Erythromycin—There are several forms of systemic erythromycin, including erythromycin base, erythromycin estolate, erythromycin ethylsuccinate (EES), and erythromycin stearate. Erythromycin estolate is contraindicated in pregnancy because it is associated with reversible maternal hepatoxicity and jaundice.9-11 Erythromycin ethylsuccinate is the preferred form for pregnant patients. Providers should exercise caution when prescribing EES to lactating mothers, as small amounts are still secreted through breast milk.11 Some studies have shown an increased risk for development of infantile hypertrophic pyloric stenosis with systemic erythromycin use, especially if a neonate is exposed in the first 14 days of life. Thus, we recommend withholding EES for 2 weeks after delivery if the patient is breastfeeding. A follow-up study did not find any association between erythromycin and infantile hypertrophic pyloric stenosis; however, the American Academy of Pediatrics still recommends short-term use only of erythromycin if it is to be used in the systemic form.8
Rifampin—Rifampin is excreted into breast milk but without adverse effects to the infant. Rifampin also is safe in pregnancy but should be used on a case-by-case basis in pregnant or nursing women because it is a cytochrome P450 inducer.
Dapsone—Dapsone has no increased risk for congenital anomalies. However, it is associated with hemolytic anemia and neonatal hyperbilirubinemia, especially in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.12 Newborns exposed to dapsone are at an increased risk for methemoglobinemia owing to increased sensitivity of fetal erythrocytes to oxidizing agents.13 If dapsone use is necessary, stopping dapsone treatment in the last month of gestation is recommended to minimize risk for kernicterus.9 Dapsone can be found in high concentrations in breast milk at 14.3% of the maternal dose. It is still safe to use during breastfeeding, but there is a risk of the infant developing hyperbilirubinemia/G6PD deficiency.1,8 Thus, physicians may consider performing a G6PD screen on infants to determine if breastfeeding is safe.12
Fluoroquinolones—Quinolones are not contraindicated during pregnancy, but they can damage fetal cartilage and thus should be reserved for use in complicated infections when the benefits outweigh the risks.12 Quinolones are believed to increase risk for arthropathy but are safe for use in lactation. When quinolones are digested with milk, exposure decreases below pediatric doses because of the ionized property of calcium in milk.8
Tumor Necrosis Factor α Inhibitors—The safety of anti–tumor necrosis factor (TNF) α biologics in pregnancy is less certain when compared with antibiotics.1 Anti–TNF-α inhibitors such as etanercept, adalimumab, and infliximab are all labeled as FDA category B, meaning there are no well-controlled human studies of the drugs.9 There are limited data that support safe use of TNF-α inhibitors prior to the third trimester before maternal IgG antibodies are transferred to the fetus via the placenta.1,13 Anti–TNF-α inhibitors may be safe when breastfeeding because the drugs have large molecular weights that prevent them from entering breast milk in large amounts. Absorption also is limited due to the infant’s digestive acids and enzymes breaking down the protein structure of the medication.8 Overall, TNF-α inhibitor use is still controversial and only used if the benefits outweigh the risks during pregnancy or if there is no alternative treatment.1,3,9
Ustekinumab and Anakinra—Ustekinumab (an IL-12/IL-23 inhibitor) and anakinra (an IL-1α and IL-1β inhibitor) also are FDA category B drugs and have limited data supporting their use as HS treatment in pregnancy. Anakinra may have evidence of compatibility with breastfeeding, as endogenous IL-1α inhibitor is found in colostrum and mature breast milk.1
Immunosuppressants
Immunosuppressants that are used to treat HS include corticosteroids and cyclosporine.
Corticosteroids—Topical corticosteroids can be used safely in lactation if they are not applied directly to the nipple or any area that makes direct contact with the infant’s mouth. Intralesional corticosteroid injections are safe for use during both pregnancy and breastfeeding to decrease inflammation of acutely flaring lesions and can be considered first-line treatment.1 Oral glucocorticoids also can be safely used for acute flares during pregnancy; however, prolonged use is associated with pregnancy complications such as preeclampsia, eclampsia, premature delivery, and gestational diabetes.12 There also is a small risk of oral cleft deformity in the infant; thus, potent corticosteroids are recommended in short durations during pregnancy, and there are no adverse effects if the maternal dose is less than 10 mg daily.8,12 Systemic steroids are safe to use with breastfeeding, but patients should be advised to wait 4 hours after ingesting medication before breastfeeding.1,8
Cyclosporine—Topical and oral calcineurin inhibitors such as cyclosporine have low risk for transmission into breast milk; however, potential effects of exposure through breast milk are unknown. For that reason, manufacturers state that cyclosporine use is contraindicated during lactation.8 If cyclosporine is to be used by a breastfeeding woman, monitoring cyclosporine concentrations in the infant is suggested to ensure that the exposure is less than 5% to 10% of the therapeutic dose.13 The use of cyclosporine has been extensively studied in pregnant transplant patients and is considered relatively safe for use in pregnancy.14 Cyclosporine is lipid soluble and thus is quickly metabolized and spread throughout the body; it can easily cross the placenta.9,13 Blood concentration in the fetus is 30% to 64% that of the maternal circulation. However, cyclosporine is only toxic to the fetus at maternally toxic doses, which can result in low birth weight and increased prenatal and postnatal mortality.13
Isotretinoin, Oral Contraceptive Pills, and Spironolactone
Isotretinoin and hormonal treatments such as oral contraceptive pills and spironolactone (an androgen receptor blocker) commonly are used to treat HS, but all are contraindicated in pregnancy and lactation. Isotretinoin is a well-established teratogen, but adverse effects on nursing babies have not been described. However, the manufacturer of isotretinoin advises against its use in lactation. Oral contraceptive pill use in early pregnancy is associated with increased risk for Down syndrome. Oral contraceptive pill use also is contraindicated in lactation for 2 reasons: decreased milk production and risk for fetal feminization. Antiandrogenic agents such as spironolactone have been shown to be associated with hypospadias and feminization of the male fetus.7
COMMENT
Women with HS usually require ongoing medical treatment during pregnancy and immediately postpartum; thus, it is important that treatments are proven to be safe for use in this specific population. Current management guidelines are not entirely suitable for pregnant and breastfeeding women given that many HS drugs have teratogenic effects and/or can be excreted into breast milk.1 Several treatments have uncertain safety profiles in pregnancy and breastfeeding, which calls for dermatologists to change or create new regimens for their patients. Close management also is necessary to prevent excess inflammation of breast tissue and milk fistula formation, which would hinder normal breastfeeding.
The eTable lists medications used to treat HS. The FDA category is listed next to each drug. However, it should be noted that these FDA letter categories were replaced with the Pregnancy and Lactation Labeling Rule in 2015. The letter ratings were deemed overly simplistic and replaced with narrative-based labeling that provides more detailed adverse effects and clinical considerations.9

Risk Factors of HS—Predisposing risk factors for HS flares that are controllable include obesity and smoking.2 Pregnancy weight gain may cause increased skin maceration at intertriginous sites, which can contribute to worsening HS symptoms.1,5 Adipocytes play a role in HS exacerbation by promoting secretion of TNF-α, leading to increased inflammation.5 Dermatologists can help prevent postpartum HS flares by monitoring weight gain during pregnancy, encouraging smoking cessation, and promoting weight and nutrition goals as set by an obstetrician.1 In addition to medications, management of HS should include emotional support and education on wearing loose-fitting clothing to avoid irritation of the affected areas.3 An emphasis on dermatologist counseling for all patients with HS, even for those with milder disease, can reduce exacerbations during pregnancy.5

CONCLUSION
The selection of dermatologic drugs for the treatment of HS in the setting of pregnancy involves complex decision-making. Dermatologists need more guidelines and proven safety data in human trials, especially regarding use of biologics and immunosuppressants to better treat HS in pregnancy. With more data, they can create more evidence-based treatment regimens to help prevent postpartum exacerbations of HS. Thus, patients can breastfeed their infants comfortably and without any risks of impaired child development. In the meantime, dermatologists can continue to work together with obstetricians and psychiatrists to decrease disease flares through counseling patients on nutrition and weight gain and providing emotional support.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease associated with hyperandrogenism and is caused by occlusion or rupture of follicular units and inflammation of the apocrine glands.1-3 The disease most commonly affects women (female to male ratio of 3:1) of childbearing age.1,2,4,5 Body areas affected include the axillae and groin, and less commonly the perineum; perianal region; and skin folds, such as gluteal, inframammary, and infraumbilical folds.1,2 Symptoms manifest as painful subcutaneous nodules with possible accompanying purulent drainage, sinus tracts, and/or dermal contractures. Although the pathophysiology is unclear, androgens affect the course of HS during pregnancy by stimulating the affected glands and altering cytokines.1,2,6
During pregnancy, maternal immune function switches from cell-mediated T helper cell (TH1) to humoral TH2 cytokine production. The activity of sebaceous and eccrine glands increases while the activity of apocrine glands decreases, thus changing the inflammatory course of HS during pregnancy.3 Approximately 20% of women with HS experience improvement of symptoms during pregnancy, while the remainder either experience no relief or deterioration of symptoms.1 Improvement in symptoms during pregnancy was found to occur more frequently in those who had worsening symptoms during menses owing to the possible hormonal effect estrogen has on inhibiting TH1 and TH17 proinflammatory cytokines, which promotes an immunosuppressive environment.4
Lactation and breastfeeding abilities may be hindered if a woman has HS affecting the apocrine glands of breast tissue and a symptom flare in the postpartum period. If HS causes notable inflammation in the nipple-areolar complex during pregnancy, the patient may experience difficulties with lactation and milk fistula formation, leading to inability to breastfeed.2 Another reason why mothers with HS may not be able to breastfeed is that the medications required to treat the disease are unsafe if passed to the infant via breast milk. In addition, the teratogenic effects of HS medications may necessitate therapy adjustments in pregnancy.1 Here, we provide a brief overview of the medical management considerations of HS in the setting of pregnancy and the impact on breastfeeding.
MEDICAL MANAGEMENT AND DRUG SAFETY
Dermatologists prescribe a myriad of topical and systemic medications to ameliorate symptoms of HS. Therapy regimens often are multimodal and include antibiotics, biologics, and immunosuppressants.1,3
Antibiotics
First-line antibiotics include clindamycin, metronidazole, tetracyclines, erythromycin, rifampin, dapsone, and fluoroquinolones. Topical clindamycin 1%, metronidazole 0.75%, and erythromycin 2% are used for open or active HS lesions and are all safe to use in pregnancy since there is minimal systemic absorption and minimal excretion into breast milk.1 Topical antimicrobial washes such as benzoyl peroxide and chlorhexidine often are used in combination with systemic medications to treat HS. These washes are safe during pregnancy and lactation, as they have minimal systemic absorption.7
Of these first-line antibiotics, only tetracyclines are contraindicated during pregnancy and lactation, as they are deemed to be in category D by the US Food and Drug Administration (FDA).1 Aside from tetracyclines, these antibiotics do not cause birth defects and are safe for nursing infants.1,8 Systemic clindamycin is safe during pregnancy and breastfeeding. Systemic metronidazole also is safe for use in pregnant patients but needs to be discontinued 12 to 24 hours prior to breastfeeding, which often prohibits appropriate dosing.1
Systemic Erythromycin—There are several forms of systemic erythromycin, including erythromycin base, erythromycin estolate, erythromycin ethylsuccinate (EES), and erythromycin stearate. Erythromycin estolate is contraindicated in pregnancy because it is associated with reversible maternal hepatoxicity and jaundice.9-11 Erythromycin ethylsuccinate is the preferred form for pregnant patients. Providers should exercise caution when prescribing EES to lactating mothers, as small amounts are still secreted through breast milk.11 Some studies have shown an increased risk for development of infantile hypertrophic pyloric stenosis with systemic erythromycin use, especially if a neonate is exposed in the first 14 days of life. Thus, we recommend withholding EES for 2 weeks after delivery if the patient is breastfeeding. A follow-up study did not find any association between erythromycin and infantile hypertrophic pyloric stenosis; however, the American Academy of Pediatrics still recommends short-term use only of erythromycin if it is to be used in the systemic form.8
Rifampin—Rifampin is excreted into breast milk but without adverse effects to the infant. Rifampin also is safe in pregnancy but should be used on a case-by-case basis in pregnant or nursing women because it is a cytochrome P450 inducer.
Dapsone—Dapsone has no increased risk for congenital anomalies. However, it is associated with hemolytic anemia and neonatal hyperbilirubinemia, especially in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.12 Newborns exposed to dapsone are at an increased risk for methemoglobinemia owing to increased sensitivity of fetal erythrocytes to oxidizing agents.13 If dapsone use is necessary, stopping dapsone treatment in the last month of gestation is recommended to minimize risk for kernicterus.9 Dapsone can be found in high concentrations in breast milk at 14.3% of the maternal dose. It is still safe to use during breastfeeding, but there is a risk of the infant developing hyperbilirubinemia/G6PD deficiency.1,8 Thus, physicians may consider performing a G6PD screen on infants to determine if breastfeeding is safe.12
Fluoroquinolones—Quinolones are not contraindicated during pregnancy, but they can damage fetal cartilage and thus should be reserved for use in complicated infections when the benefits outweigh the risks.12 Quinolones are believed to increase risk for arthropathy but are safe for use in lactation. When quinolones are digested with milk, exposure decreases below pediatric doses because of the ionized property of calcium in milk.8
Tumor Necrosis Factor α Inhibitors—The safety of anti–tumor necrosis factor (TNF) α biologics in pregnancy is less certain when compared with antibiotics.1 Anti–TNF-α inhibitors such as etanercept, adalimumab, and infliximab are all labeled as FDA category B, meaning there are no well-controlled human studies of the drugs.9 There are limited data that support safe use of TNF-α inhibitors prior to the third trimester before maternal IgG antibodies are transferred to the fetus via the placenta.1,13 Anti–TNF-α inhibitors may be safe when breastfeeding because the drugs have large molecular weights that prevent them from entering breast milk in large amounts. Absorption also is limited due to the infant’s digestive acids and enzymes breaking down the protein structure of the medication.8 Overall, TNF-α inhibitor use is still controversial and only used if the benefits outweigh the risks during pregnancy or if there is no alternative treatment.1,3,9
Ustekinumab and Anakinra—Ustekinumab (an IL-12/IL-23 inhibitor) and anakinra (an IL-1α and IL-1β inhibitor) also are FDA category B drugs and have limited data supporting their use as HS treatment in pregnancy. Anakinra may have evidence of compatibility with breastfeeding, as endogenous IL-1α inhibitor is found in colostrum and mature breast milk.1
Immunosuppressants
Immunosuppressants that are used to treat HS include corticosteroids and cyclosporine.
Corticosteroids—Topical corticosteroids can be used safely in lactation if they are not applied directly to the nipple or any area that makes direct contact with the infant’s mouth. Intralesional corticosteroid injections are safe for use during both pregnancy and breastfeeding to decrease inflammation of acutely flaring lesions and can be considered first-line treatment.1 Oral glucocorticoids also can be safely used for acute flares during pregnancy; however, prolonged use is associated with pregnancy complications such as preeclampsia, eclampsia, premature delivery, and gestational diabetes.12 There also is a small risk of oral cleft deformity in the infant; thus, potent corticosteroids are recommended in short durations during pregnancy, and there are no adverse effects if the maternal dose is less than 10 mg daily.8,12 Systemic steroids are safe to use with breastfeeding, but patients should be advised to wait 4 hours after ingesting medication before breastfeeding.1,8
Cyclosporine—Topical and oral calcineurin inhibitors such as cyclosporine have low risk for transmission into breast milk; however, potential effects of exposure through breast milk are unknown. For that reason, manufacturers state that cyclosporine use is contraindicated during lactation.8 If cyclosporine is to be used by a breastfeeding woman, monitoring cyclosporine concentrations in the infant is suggested to ensure that the exposure is less than 5% to 10% of the therapeutic dose.13 The use of cyclosporine has been extensively studied in pregnant transplant patients and is considered relatively safe for use in pregnancy.14 Cyclosporine is lipid soluble and thus is quickly metabolized and spread throughout the body; it can easily cross the placenta.9,13 Blood concentration in the fetus is 30% to 64% that of the maternal circulation. However, cyclosporine is only toxic to the fetus at maternally toxic doses, which can result in low birth weight and increased prenatal and postnatal mortality.13
Isotretinoin, Oral Contraceptive Pills, and Spironolactone
Isotretinoin and hormonal treatments such as oral contraceptive pills and spironolactone (an androgen receptor blocker) commonly are used to treat HS, but all are contraindicated in pregnancy and lactation. Isotretinoin is a well-established teratogen, but adverse effects on nursing babies have not been described. However, the manufacturer of isotretinoin advises against its use in lactation. Oral contraceptive pill use in early pregnancy is associated with increased risk for Down syndrome. Oral contraceptive pill use also is contraindicated in lactation for 2 reasons: decreased milk production and risk for fetal feminization. Antiandrogenic agents such as spironolactone have been shown to be associated with hypospadias and feminization of the male fetus.7
COMMENT
Women with HS usually require ongoing medical treatment during pregnancy and immediately postpartum; thus, it is important that treatments are proven to be safe for use in this specific population. Current management guidelines are not entirely suitable for pregnant and breastfeeding women given that many HS drugs have teratogenic effects and/or can be excreted into breast milk.1 Several treatments have uncertain safety profiles in pregnancy and breastfeeding, which calls for dermatologists to change or create new regimens for their patients. Close management also is necessary to prevent excess inflammation of breast tissue and milk fistula formation, which would hinder normal breastfeeding.
The eTable lists medications used to treat HS. The FDA category is listed next to each drug. However, it should be noted that these FDA letter categories were replaced with the Pregnancy and Lactation Labeling Rule in 2015. The letter ratings were deemed overly simplistic and replaced with narrative-based labeling that provides more detailed adverse effects and clinical considerations.9

Risk Factors of HS—Predisposing risk factors for HS flares that are controllable include obesity and smoking.2 Pregnancy weight gain may cause increased skin maceration at intertriginous sites, which can contribute to worsening HS symptoms.1,5 Adipocytes play a role in HS exacerbation by promoting secretion of TNF-α, leading to increased inflammation.5 Dermatologists can help prevent postpartum HS flares by monitoring weight gain during pregnancy, encouraging smoking cessation, and promoting weight and nutrition goals as set by an obstetrician.1 In addition to medications, management of HS should include emotional support and education on wearing loose-fitting clothing to avoid irritation of the affected areas.3 An emphasis on dermatologist counseling for all patients with HS, even for those with milder disease, can reduce exacerbations during pregnancy.5

CONCLUSION
The selection of dermatologic drugs for the treatment of HS in the setting of pregnancy involves complex decision-making. Dermatologists need more guidelines and proven safety data in human trials, especially regarding use of biologics and immunosuppressants to better treat HS in pregnancy. With more data, they can create more evidence-based treatment regimens to help prevent postpartum exacerbations of HS. Thus, patients can breastfeed their infants comfortably and without any risks of impaired child development. In the meantime, dermatologists can continue to work together with obstetricians and psychiatrists to decrease disease flares through counseling patients on nutrition and weight gain and providing emotional support.
- Perng P, Zampella JG, Okoye GA. Management of hidradenitis suppurativa in pregnancy. J Am Acad Dermatol. 2017;76:979-989. doi:10.1016/j.jaad.2016.10.032
- Samuel S, Tremelling A, Murray M. Presentation and surgical management of hidradenitis suppurativa of the breast during pregnancy: a case report. Int J Surg Case Rep. 2018;51:21-24. doi:10.1016/j.ijscr.2018.08.013
- Yang CS, Teeple M, Muglia J, et al. Inflammatory and glandular skin disease in pregnancy. Clin Dermatol. 2016;34:335-343. doi:10.1016/j.clindermatol.2016.02.005
- Vossen AR, van Straalen KR, Prens EP, et al. Menses and pregnancy affect symptoms in hidradenitis suppurativa: a cross-sectional study. J Am Acad Dermatol. 2017;76:155-156. doi:10.1016/j.jaad.2016.07.024
- Lyons AB, Peacock A, McKenzie SA, et al. Evaluation of hidradenitis suppurativa disease course during pregnancy and postpartum. JAMA Dermatol. 2020;156:681-685. doi:10.1001/jamadermatol.2020.0777
- Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa—a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
- Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787. doi:10.1007/s40265-013-0060-0
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. lactation. J Am Acad Dermatol. 2014;70:417:E1-E10. doi:10.1016/j.jaad.2013.09.009
- Wilmer E, Chai S, Kroumpouzos G. Drug safety: pregnancy rating classifications and controversies. Clin Dermatol. 2016;34:401-409. doi:10.1016/j.clindermatol.2016.02.013
- Inman WH, Rawson NS. Erythromycin estolate and jaundice. Br Med J (Clin Res Ed). 1983;286:1954-1955. doi:10.1136/bmj.286.6382.1954
- Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. pregnancy. J Am Acad Dermatol. 2014;70:401.e1-14; quiz 415. doi:10.1016/j.jaad.2013.09.010
- Brown SM, Aljefri K, Waas R, et al. Systemic medications used in treatment of common dermatological conditions: safety profile with respect to pregnancy, breast feeding and content in seminal fluid. J Dermatolog Treat. 2019;30:2-18. doi:10.1080/09546634.2016.1202402
- Kamarajah SK, Arntdz K, Bundred J, et al. Outcomes of pregnancy in recipients of liver transplants. Clin Gastroenterol Hepatol. 2019;17:1398-1404.e1. doi:10.1016/j.cgh.2018.11.055
- Perng P, Zampella JG, Okoye GA. Management of hidradenitis suppurativa in pregnancy. J Am Acad Dermatol. 2017;76:979-989. doi:10.1016/j.jaad.2016.10.032
- Samuel S, Tremelling A, Murray M. Presentation and surgical management of hidradenitis suppurativa of the breast during pregnancy: a case report. Int J Surg Case Rep. 2018;51:21-24. doi:10.1016/j.ijscr.2018.08.013
- Yang CS, Teeple M, Muglia J, et al. Inflammatory and glandular skin disease in pregnancy. Clin Dermatol. 2016;34:335-343. doi:10.1016/j.clindermatol.2016.02.005
- Vossen AR, van Straalen KR, Prens EP, et al. Menses and pregnancy affect symptoms in hidradenitis suppurativa: a cross-sectional study. J Am Acad Dermatol. 2017;76:155-156. doi:10.1016/j.jaad.2016.07.024
- Lyons AB, Peacock A, McKenzie SA, et al. Evaluation of hidradenitis suppurativa disease course during pregnancy and postpartum. JAMA Dermatol. 2020;156:681-685. doi:10.1001/jamadermatol.2020.0777
- Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa—a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
- Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787. doi:10.1007/s40265-013-0060-0
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. lactation. J Am Acad Dermatol. 2014;70:417:E1-E10. doi:10.1016/j.jaad.2013.09.009
- Wilmer E, Chai S, Kroumpouzos G. Drug safety: pregnancy rating classifications and controversies. Clin Dermatol. 2016;34:401-409. doi:10.1016/j.clindermatol.2016.02.013
- Inman WH, Rawson NS. Erythromycin estolate and jaundice. Br Med J (Clin Res Ed). 1983;286:1954-1955. doi:10.1136/bmj.286.6382.1954
- Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. pregnancy. J Am Acad Dermatol. 2014;70:401.e1-14; quiz 415. doi:10.1016/j.jaad.2013.09.010
- Brown SM, Aljefri K, Waas R, et al. Systemic medications used in treatment of common dermatological conditions: safety profile with respect to pregnancy, breast feeding and content in seminal fluid. J Dermatolog Treat. 2019;30:2-18. doi:10.1080/09546634.2016.1202402
- Kamarajah SK, Arntdz K, Bundred J, et al. Outcomes of pregnancy in recipients of liver transplants. Clin Gastroenterol Hepatol. 2019;17:1398-1404.e1. doi:10.1016/j.cgh.2018.11.055
Practice Points
- Some medications used to treat hidradenitis suppurativa (HS) may have teratogenic effects and be contraindicated during breastfeeding.
- We summarize what treatments are proven to be safe in pregnancy and breastfeeding and highlight the need for more guidelines and safety data for dermatologists to manage their pregnant patients with HS.
Bullous Dermatoses and Quality of Life: A Summary of Tools to Assess Psychosocial Health
Autoimmune bullous dermatoses (ABDs) develop due to antibodies directed against antigens within the epidermis or at the dermoepidermal junction. They are categorized histologically by the location of acantholysis (separation of keratinocytes), clinical presentation, and presence of autoantibodies. The most common ABDs include pemphigus vulgaris, pemphigus foliaceus, and bullous pemphigoid (BP). These conditions present on a spectrum of symptoms and severity.1
Although multiple studies have evaluated the impact of bullous dermatoses on mental health, most were designed with a small sample size, thus limiting the generalizability of each study. Sebaratnam et al2 summarized several studies in 2012. In this review, we will analyze additional relevant literature and systematically combine the data to determine the psychological burden of disease of ABDs. We also will discuss the existing questionnaires frequently used in the dermatology setting to assess adverse psychosocial symptoms.
Methods
We searched PubMed, MEDLINE, and Google Scholar for articles published within the last 15 years using the terms bullous pemphigoid, pemphigus, quality of life, anxiety, and depression. We reviewed the citations in each article to further our search.
Criteria for Inclusion and Exclusion—Studies that utilized validated questionnaires to evaluate the effects of pemphigus vulgaris, pemphigus foliaceus, and/or BP on mental health were included. All research participants were 18 years and older. For the questionnaires administered, each study must have included numerical scores in the results. The studies all reported statistically significant results (P<.05), but no studies were excluded on the basis of statistical significance.
Studies were excluded if they did not use a validated questionnaire to examine quality of life (QOL) or psychological status. We also excluded database, retrospective, qualitative, and observational studies. We did not include studies with a sample size less than 20. Studies that administered questionnaires that were uncommon in this realm of research such as the Attitude to Appearance Scale or The Anxiety Questionnaire also were excluded. We did not exclude articles based on their primary language.
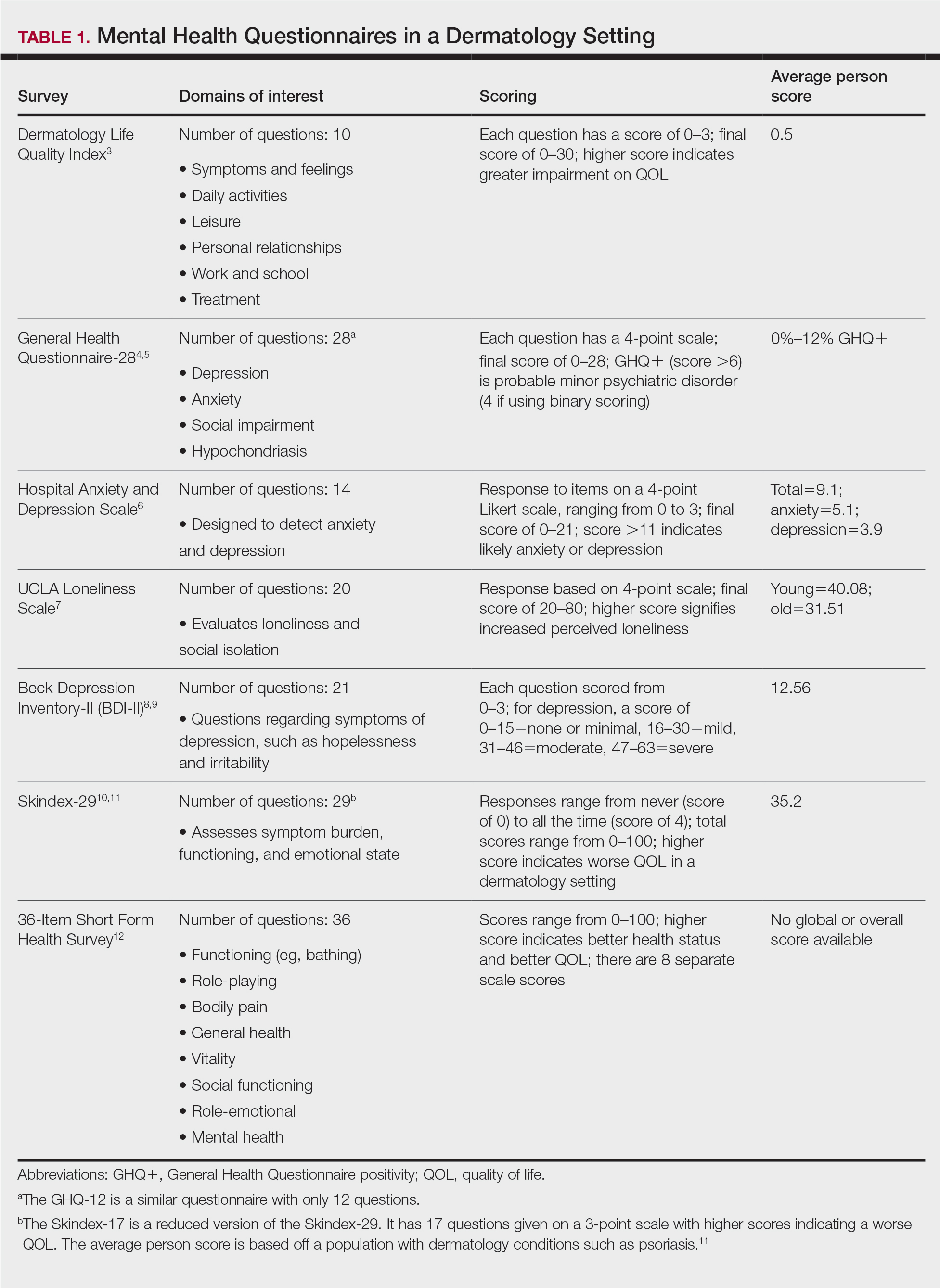
Results
A total of 13 studies met the inclusion criteria with a total of 1716 participants enrolled in the trials. The questionnaires most commonly used are summarized in Table 1. Tables 2 and 3 demonstrate the studies that evaluate QOL and psychological state in patients with bullous dermatoses, respectively.
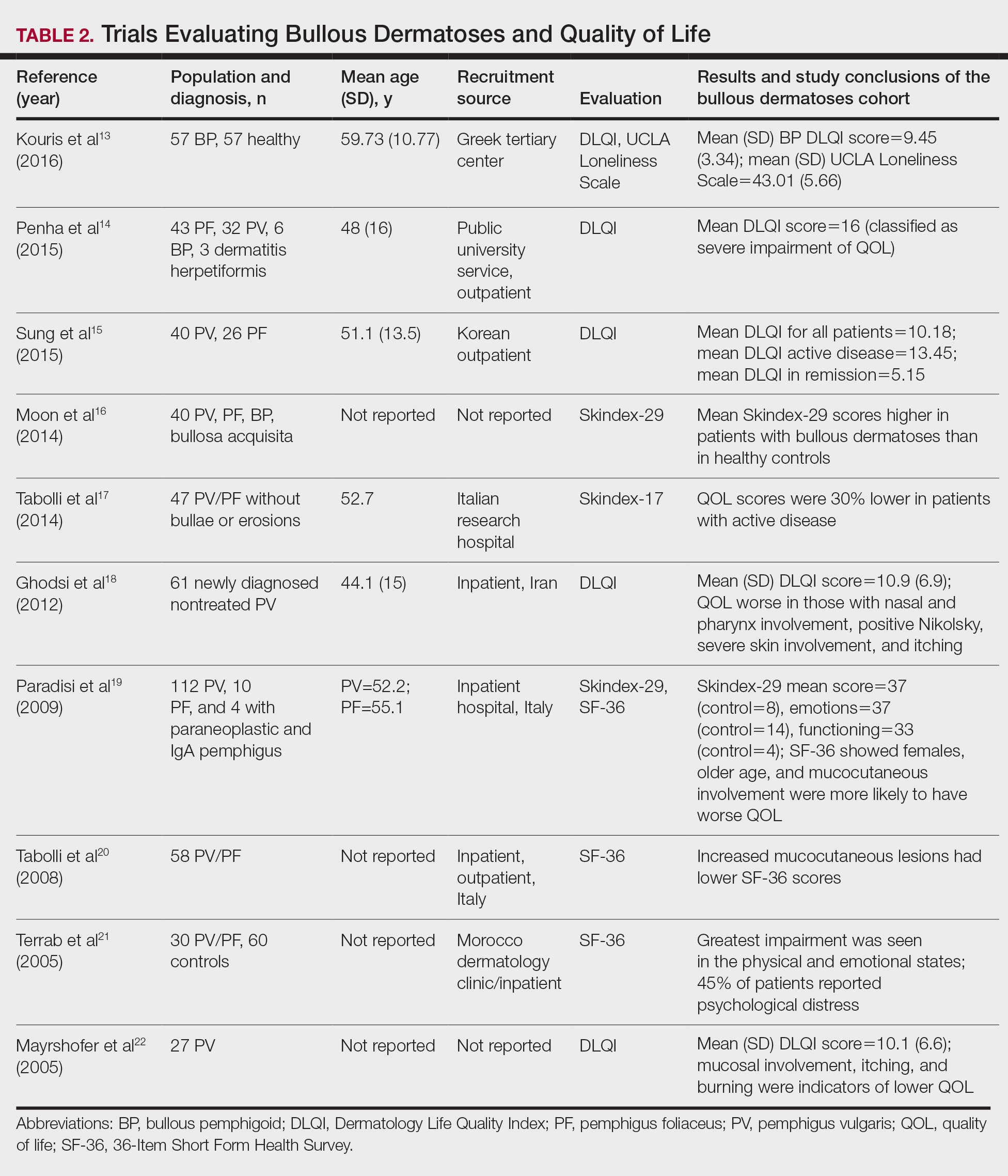
The Dermatology Life Quality Index (DLQI) was the most utilized method for analyzing QOL followed by the Skindex-17, Skindex-29, and 36-Item Short Form Health Survey. The DLQI is a skin-specific measurement tool with higher scores translating to greater impairment in QOL. Healthy patients have an average score of 0.5.3 The mean DLQI scores for ABD patients as seen in Table 2 were 9.45, 10.18, 16, 10.9, and 10.1.13-15,18,22 The most commonly reported concerns among patients included feelings about appearance and disturbances in daily activities.18 Symptoms of mucosal involvement, itching, and burning also were indicators of lower QOL.15,18,20,22 Furthermore, women consistently had lower scores than men.15,17,19,25 Multiple studies concluded that severity of the disease correlated with a lower QOL, though the subtype of pemphigus did not have an effect on QOL scores.15,19,20,21 Lastly, recent onset of symptoms was associated with a worse QOL score.15,18-20 Age, education level, and marital status did not have an effect on QOL.
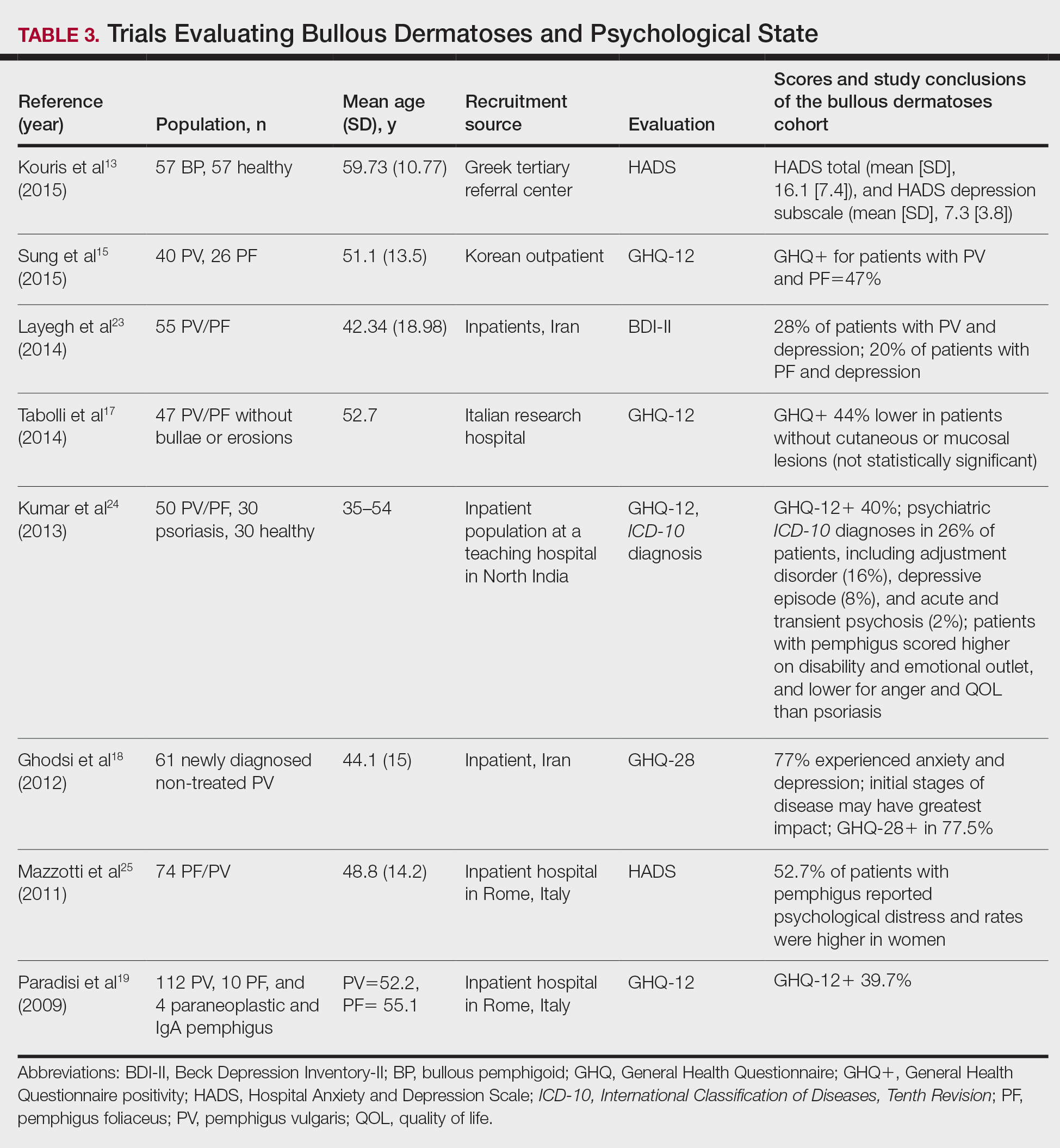
To evaluate psychological state, the General Health Questionnaire (GHQ)-28 and -12 primarily were used, in addition to the Hospital Anxiety and Depression Scale; the International Classification of Diseases, Tenth Revision; the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; and the Beck Depression Inventory-II. As seen in Table 3, GHQ-12 positivity, reflecting probable minor nonpsychotic psychiatric disorders such as depression and anxiety, was identified in 47%, 39.7%, and 40% of patients with pemphigus15,19,24; GHQ-28 positivity was seen in 77.5% of pemphigus patients.18 In the average population, GHQ positivity was found in up to 12% of patients.26,27 Similar to the QOL scores, no significant differences were seen based on subtype of pemphigus for symptoms of depression or anxiety.20,23
Comment
Mental Health of Patients With ABDs—Immunobullous diseases are painful, potentially lifelong conditions that have no definitive cure. These conditions are characterized by bullae and erosions of the skin and mucosae that physically are disabling and often create a stigma for patients. Across multiple different validated psychosocial assessments, the 13 studies included in this review consistently reported that ABDs have a negative effect on mental well-being of patients that is more pronounced in women and worse at the onset of symptoms.13-25
QOL Scores in Patients With ABDs—Quality of life is a broad term that encompasses a general sense of psychological and overall well-being. A score of approximately 10 on the DLQI most often was reported in patients with ABDs, which translates to a moderate impact on QOL. Incomparison, a large cohort study reported the mean (SD) DLQI scores for patients with atopic dermatitis and psoriasis as 7.31 (5.98) and 5.93 (5.66), respectively.28 In another study, Penha et al14 found that patients with psoriasis have a mean DLQI score of 10. Reasons for the similarly low QOL scores in patients with ABDs include long hospitalization periods, disease chronicity, social anxiety, inability to control symptoms, difficulty with activities of daily living, and the belief that the disease is incurable.17,19,23 Although there is a need for increased family and social support with performing necessary daily tasks, personal relationships often are negatively affected, resulting in social isolation, loneliness, and worsening of cutaneous symptoms.
Severity of cutaneous disease and recent onset of symptoms correlated with worse QOL scores. Tabolli et al20 proposed the reason for this relates to not having had enough time to find the best treatment regimen. We believe there also may be an element of habituation involved, whereby patients become accustomed to the appearance of the lesions over time and therefore they become less distressing. Interestingly, Tabolli et al17 determined that patients in the quiescent phase of the disease—without any mucosal or cutaneous lesions—still maintained lower QOL scores than the average population, particularly on the psychosocial section of the 36-Item Short Form Health Survey, which may be due to a concern of disease relapse or from adverse effects of treatment. Providers should monitor patients for mental health complications not only in the disease infancy but throughout the disease course.
Future Directions—Cause and effect of the relationship between the psychosocial variables and ABD disease state has yet to be determined. Most studies included in this review were cross-sectional in design. Although many studies concluded that bullous dermatoses were the cause of impaired QOL, Ren and colleagues29 proposed that medications used to treat neuropsychiatric disorders may trigger the autoimmune antigens of BP. Possible triggers for BP have been reported including hydrochlorothiazide, ciprofloxacin, and dipeptidyl peptidase-4 inhibitors.27,30-32 A longitudinal study design would better evaluate the causal relationship.
The effects of the medications were included in 2 cases, one in which the steroid dose was not found to have a significant impact on rates of depression23 and another in which patients treated with a higher dose of corticosteroids (>10 mg) had worse QOL scores.17 Sung et al15 suggested this may be because patients who took higher doses of steroids had worse symptoms and therefore also had a worse QOL. It also is possible that those patients taking higher doses had increased side effects.17 Further studies that evaluate treatment modalities and timing in relation to the disease onset would be helpful.
Study Limitations—There are potential barriers to combining these data. Multiple different questionnaires were used, and it was difficult to ascertain if all the participants were experiencing active disease. Additionally, questionnaires are not always the best proxy for what is happening in everyday life. Lastly, the sample size of each individual study was small, and the studies only included adults.
Conclusion
As demonstrated by the 13 studies in this review, patients with ABDs have lower QOL scores and higher numbers of psychological symptoms. Clinicians should be mindful of this at-risk population and create opportunities in clinic to discuss personal hardship associated with the disease process and recommend psychiatric intervention if indicated. Additionally, family members often are overburdened with the chronicity of ABDs, and they should not be forgotten. Using one of the aforementioned questionnaires is a practical way to screen patients for lower QOL scores. We agree with Paradisi and colleagues19 that although these questionnaires may be helpful, clinicians still need to determine if the use of a dermatologic QOL evaluation tool in clinical practice improves patient satisfaction.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489. https://doi.org/10.1016/j.autrev.2014.01.047
- Sebaratnam DF, McMillan JR, Werth VP, et al. Quality of life in patients with bullous dermatoses. Clin Dermatol. 2012;30:103-107. doi:10.1016/j.clindermatol.2011.03.016
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Goldberg DP. The Detection of Psychiatric Illness by Questionnaire. Oxford University Press; 1972.
- Cano A, Sprafkin RP, Scaturo DJ, et al. Mental health screening in primary care: a comparison of 3 brief measures of psychological distress. Prim Care Companion J Clin Psychiatry. 2001;3:206-210.
- Zigmond A, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361-370.
- Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66:20-40. doi:10.1207/s15327752jpa6601_2
- Beck A, Alford B. Depression: Causes and Treatment. 2nd ed. Philadelphia University of Pennsylvania Press; 2009.
- Ghassemzadeh H, Mojtabai R, Karamghadiri N, et al. Psychometric properties of a Persian-language version of the Beck Depression Inventory—Second Edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21:185-192. doi:10.1002/da.20070
- Chren MM, Lasek RJ, Sahay AP, et al. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5:105-110.
- Nijsten TEC, Sampogna F, Chren M, et al. Testing and reducing Skindex-29 using Rasch analysis: Skindex-17. J Invest Dermatol. 2006;126:1244-1250. https://doi.org/10.1038/sj.jid.5700212
- Ware JE Jr, Sherbourne C. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care. 1992;30:473-483.
- Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life, depression, anxiety and loneliness in patients with bullous pemphigoid: a case control study. An Bras Dermatol. 2016;91:601-603. doi:10.1590/abd1806-4841.2016493
- Penha MA, Farat JG, Miot HA, et al. Quality of life index in autoimmune bullous dermatosis patients. An Bras Dermatol. 2015;90:190-194. https://dx.doi.org/10.1590/abd1806-4841.20153372
- Sung JY, Roh MR, Kim SC. Quality of life assessment in Korean patients with pemphigus. Ann Dermatol. 2015;27:492-498.
- Moon SH, Kwon HI, Park HC, et al. Assessment of the quality of life in autoimmune blistering skin disease patients. Korean J Dermatol. 2014;52:402-409.
- Tabolli S, Pagliarello C, Paradisi A, et al. Burden of disease during quiescent periods in patients with pemphigus. Br J Dermatol. 2014;170:1087-1091. doi:10.1111/bjd.12836
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and general health questionnaires. J Dermatol. 2012;39:141-144. doi:10.1111/j.1346-8138.2011.01382
- Paradisi A, Sampogna F, Di Pietro C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269. doi:10.1016/j.jaad.2008.09.014
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034. doi:10.1111/j.1365-2133.2008.08481.x
- Terrab Z, Benchikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris: results from the German Bullous Skin Disease (BSD) Study Group [in German]. J Dtsch Dermatol Ges. 2005;3:431-435. doi:10.1111/j.1610-0387.2005.05722.x
- Layegh P, Mokhber N, Javidi Z, et al. Depression in patients with pemphigus: is it a major concern? J Dermatol. 2014;40:434-437. doi:10.1111/1346-8138.12067
- Kumar V, Mattoo SK, Handa S. Psychiatric morbidity in pemphigus and psoriasis: a comparative study from India. Asian J Psychiatr. 2013;6:151-156. doi:10.1016/j.ajp.2012.10.005
- Mazzotti E, Mozzetta A, Antinone V, et al. Psychological distress and investment in one’s appearance in patients with pemphigus. J Eur Acad Dermatol Venereol. 2011;25:285-289. doi:10.1111/j.1468-3083.2010.03780.x
- Regier DA, Boyd JH, Burke JD, et al. One-month prevalence of mental disorders in the United States: based on five epidemiologic catchment area sites. Arch Gen Psychiatr. 1988;45:977-986. doi:10.1001/archpsyc.1988.01800350011002
- Cozzani E, Chinazzo C, Burlando M, et al. Ciprofloxacin as a trigger for bullous pemphigoid: the second case in the literature. Am J Ther. 2016;23:E1202-E1204. doi:10.1097/MJT.0000000000000283
- Lundberg L, Johannesson M, Silverdahl M, et al. Health-related quality of life in patients with psoriasis and atopic dermatitis measured with SF-36, DLQI and a subjective measure of disease activity. Acta Derm Venereol. 2000;80:430-434.
- Ren Z, Hsu DY, Brieva J, et al. Hospitalization, inpatient burden and comorbidities associated with bullous pemphigoid in the U.S.A. Br J Dermatol. 2017;176:87-99. doi:10.1111/bjd.14821
- Warner C, Kwak Y, Glover MH, et al. Bullous pemphigoid induced by hydrochlorothiazide therapy. J Drugs Dermatol. 2014;13:360-362.
- Mendonca FM, Martin-Gutierrez FJ, Rios-Martin JJ, et al. Three cases of bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors—one due to linagliptin. Dermatology. 2016;232:249-253. doi:10.1159/000443330
- Attaway A, Mersfelder TL, Vaishnav S, et al. Bullous pemphigoid associated with dipeptidyl peptidase IV inhibitors: a case report and review of literature. J Dermatol Case Rep. 2014;8:24-28.
Autoimmune bullous dermatoses (ABDs) develop due to antibodies directed against antigens within the epidermis or at the dermoepidermal junction. They are categorized histologically by the location of acantholysis (separation of keratinocytes), clinical presentation, and presence of autoantibodies. The most common ABDs include pemphigus vulgaris, pemphigus foliaceus, and bullous pemphigoid (BP). These conditions present on a spectrum of symptoms and severity.1
Although multiple studies have evaluated the impact of bullous dermatoses on mental health, most were designed with a small sample size, thus limiting the generalizability of each study. Sebaratnam et al2 summarized several studies in 2012. In this review, we will analyze additional relevant literature and systematically combine the data to determine the psychological burden of disease of ABDs. We also will discuss the existing questionnaires frequently used in the dermatology setting to assess adverse psychosocial symptoms.
Methods
We searched PubMed, MEDLINE, and Google Scholar for articles published within the last 15 years using the terms bullous pemphigoid, pemphigus, quality of life, anxiety, and depression. We reviewed the citations in each article to further our search.
Criteria for Inclusion and Exclusion—Studies that utilized validated questionnaires to evaluate the effects of pemphigus vulgaris, pemphigus foliaceus, and/or BP on mental health were included. All research participants were 18 years and older. For the questionnaires administered, each study must have included numerical scores in the results. The studies all reported statistically significant results (P<.05), but no studies were excluded on the basis of statistical significance.
Studies were excluded if they did not use a validated questionnaire to examine quality of life (QOL) or psychological status. We also excluded database, retrospective, qualitative, and observational studies. We did not include studies with a sample size less than 20. Studies that administered questionnaires that were uncommon in this realm of research such as the Attitude to Appearance Scale or The Anxiety Questionnaire also were excluded. We did not exclude articles based on their primary language.

Results
A total of 13 studies met the inclusion criteria with a total of 1716 participants enrolled in the trials. The questionnaires most commonly used are summarized in Table 1. Tables 2 and 3 demonstrate the studies that evaluate QOL and psychological state in patients with bullous dermatoses, respectively.

The Dermatology Life Quality Index (DLQI) was the most utilized method for analyzing QOL followed by the Skindex-17, Skindex-29, and 36-Item Short Form Health Survey. The DLQI is a skin-specific measurement tool with higher scores translating to greater impairment in QOL. Healthy patients have an average score of 0.5.3 The mean DLQI scores for ABD patients as seen in Table 2 were 9.45, 10.18, 16, 10.9, and 10.1.13-15,18,22 The most commonly reported concerns among patients included feelings about appearance and disturbances in daily activities.18 Symptoms of mucosal involvement, itching, and burning also were indicators of lower QOL.15,18,20,22 Furthermore, women consistently had lower scores than men.15,17,19,25 Multiple studies concluded that severity of the disease correlated with a lower QOL, though the subtype of pemphigus did not have an effect on QOL scores.15,19,20,21 Lastly, recent onset of symptoms was associated with a worse QOL score.15,18-20 Age, education level, and marital status did not have an effect on QOL.

To evaluate psychological state, the General Health Questionnaire (GHQ)-28 and -12 primarily were used, in addition to the Hospital Anxiety and Depression Scale; the International Classification of Diseases, Tenth Revision; the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; and the Beck Depression Inventory-II. As seen in Table 3, GHQ-12 positivity, reflecting probable minor nonpsychotic psychiatric disorders such as depression and anxiety, was identified in 47%, 39.7%, and 40% of patients with pemphigus15,19,24; GHQ-28 positivity was seen in 77.5% of pemphigus patients.18 In the average population, GHQ positivity was found in up to 12% of patients.26,27 Similar to the QOL scores, no significant differences were seen based on subtype of pemphigus for symptoms of depression or anxiety.20,23
Comment
Mental Health of Patients With ABDs—Immunobullous diseases are painful, potentially lifelong conditions that have no definitive cure. These conditions are characterized by bullae and erosions of the skin and mucosae that physically are disabling and often create a stigma for patients. Across multiple different validated psychosocial assessments, the 13 studies included in this review consistently reported that ABDs have a negative effect on mental well-being of patients that is more pronounced in women and worse at the onset of symptoms.13-25
QOL Scores in Patients With ABDs—Quality of life is a broad term that encompasses a general sense of psychological and overall well-being. A score of approximately 10 on the DLQI most often was reported in patients with ABDs, which translates to a moderate impact on QOL. Incomparison, a large cohort study reported the mean (SD) DLQI scores for patients with atopic dermatitis and psoriasis as 7.31 (5.98) and 5.93 (5.66), respectively.28 In another study, Penha et al14 found that patients with psoriasis have a mean DLQI score of 10. Reasons for the similarly low QOL scores in patients with ABDs include long hospitalization periods, disease chronicity, social anxiety, inability to control symptoms, difficulty with activities of daily living, and the belief that the disease is incurable.17,19,23 Although there is a need for increased family and social support with performing necessary daily tasks, personal relationships often are negatively affected, resulting in social isolation, loneliness, and worsening of cutaneous symptoms.
Severity of cutaneous disease and recent onset of symptoms correlated with worse QOL scores. Tabolli et al20 proposed the reason for this relates to not having had enough time to find the best treatment regimen. We believe there also may be an element of habituation involved, whereby patients become accustomed to the appearance of the lesions over time and therefore they become less distressing. Interestingly, Tabolli et al17 determined that patients in the quiescent phase of the disease—without any mucosal or cutaneous lesions—still maintained lower QOL scores than the average population, particularly on the psychosocial section of the 36-Item Short Form Health Survey, which may be due to a concern of disease relapse or from adverse effects of treatment. Providers should monitor patients for mental health complications not only in the disease infancy but throughout the disease course.
Future Directions—Cause and effect of the relationship between the psychosocial variables and ABD disease state has yet to be determined. Most studies included in this review were cross-sectional in design. Although many studies concluded that bullous dermatoses were the cause of impaired QOL, Ren and colleagues29 proposed that medications used to treat neuropsychiatric disorders may trigger the autoimmune antigens of BP. Possible triggers for BP have been reported including hydrochlorothiazide, ciprofloxacin, and dipeptidyl peptidase-4 inhibitors.27,30-32 A longitudinal study design would better evaluate the causal relationship.
The effects of the medications were included in 2 cases, one in which the steroid dose was not found to have a significant impact on rates of depression23 and another in which patients treated with a higher dose of corticosteroids (>10 mg) had worse QOL scores.17 Sung et al15 suggested this may be because patients who took higher doses of steroids had worse symptoms and therefore also had a worse QOL. It also is possible that those patients taking higher doses had increased side effects.17 Further studies that evaluate treatment modalities and timing in relation to the disease onset would be helpful.
Study Limitations—There are potential barriers to combining these data. Multiple different questionnaires were used, and it was difficult to ascertain if all the participants were experiencing active disease. Additionally, questionnaires are not always the best proxy for what is happening in everyday life. Lastly, the sample size of each individual study was small, and the studies only included adults.
Conclusion
As demonstrated by the 13 studies in this review, patients with ABDs have lower QOL scores and higher numbers of psychological symptoms. Clinicians should be mindful of this at-risk population and create opportunities in clinic to discuss personal hardship associated with the disease process and recommend psychiatric intervention if indicated. Additionally, family members often are overburdened with the chronicity of ABDs, and they should not be forgotten. Using one of the aforementioned questionnaires is a practical way to screen patients for lower QOL scores. We agree with Paradisi and colleagues19 that although these questionnaires may be helpful, clinicians still need to determine if the use of a dermatologic QOL evaluation tool in clinical practice improves patient satisfaction.
Autoimmune bullous dermatoses (ABDs) develop due to antibodies directed against antigens within the epidermis or at the dermoepidermal junction. They are categorized histologically by the location of acantholysis (separation of keratinocytes), clinical presentation, and presence of autoantibodies. The most common ABDs include pemphigus vulgaris, pemphigus foliaceus, and bullous pemphigoid (BP). These conditions present on a spectrum of symptoms and severity.1
Although multiple studies have evaluated the impact of bullous dermatoses on mental health, most were designed with a small sample size, thus limiting the generalizability of each study. Sebaratnam et al2 summarized several studies in 2012. In this review, we will analyze additional relevant literature and systematically combine the data to determine the psychological burden of disease of ABDs. We also will discuss the existing questionnaires frequently used in the dermatology setting to assess adverse psychosocial symptoms.
Methods
We searched PubMed, MEDLINE, and Google Scholar for articles published within the last 15 years using the terms bullous pemphigoid, pemphigus, quality of life, anxiety, and depression. We reviewed the citations in each article to further our search.
Criteria for Inclusion and Exclusion—Studies that utilized validated questionnaires to evaluate the effects of pemphigus vulgaris, pemphigus foliaceus, and/or BP on mental health were included. All research participants were 18 years and older. For the questionnaires administered, each study must have included numerical scores in the results. The studies all reported statistically significant results (P<.05), but no studies were excluded on the basis of statistical significance.
Studies were excluded if they did not use a validated questionnaire to examine quality of life (QOL) or psychological status. We also excluded database, retrospective, qualitative, and observational studies. We did not include studies with a sample size less than 20. Studies that administered questionnaires that were uncommon in this realm of research such as the Attitude to Appearance Scale or The Anxiety Questionnaire also were excluded. We did not exclude articles based on their primary language.

Results
A total of 13 studies met the inclusion criteria with a total of 1716 participants enrolled in the trials. The questionnaires most commonly used are summarized in Table 1. Tables 2 and 3 demonstrate the studies that evaluate QOL and psychological state in patients with bullous dermatoses, respectively.

The Dermatology Life Quality Index (DLQI) was the most utilized method for analyzing QOL followed by the Skindex-17, Skindex-29, and 36-Item Short Form Health Survey. The DLQI is a skin-specific measurement tool with higher scores translating to greater impairment in QOL. Healthy patients have an average score of 0.5.3 The mean DLQI scores for ABD patients as seen in Table 2 were 9.45, 10.18, 16, 10.9, and 10.1.13-15,18,22 The most commonly reported concerns among patients included feelings about appearance and disturbances in daily activities.18 Symptoms of mucosal involvement, itching, and burning also were indicators of lower QOL.15,18,20,22 Furthermore, women consistently had lower scores than men.15,17,19,25 Multiple studies concluded that severity of the disease correlated with a lower QOL, though the subtype of pemphigus did not have an effect on QOL scores.15,19,20,21 Lastly, recent onset of symptoms was associated with a worse QOL score.15,18-20 Age, education level, and marital status did not have an effect on QOL.

To evaluate psychological state, the General Health Questionnaire (GHQ)-28 and -12 primarily were used, in addition to the Hospital Anxiety and Depression Scale; the International Classification of Diseases, Tenth Revision; the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; and the Beck Depression Inventory-II. As seen in Table 3, GHQ-12 positivity, reflecting probable minor nonpsychotic psychiatric disorders such as depression and anxiety, was identified in 47%, 39.7%, and 40% of patients with pemphigus15,19,24; GHQ-28 positivity was seen in 77.5% of pemphigus patients.18 In the average population, GHQ positivity was found in up to 12% of patients.26,27 Similar to the QOL scores, no significant differences were seen based on subtype of pemphigus for symptoms of depression or anxiety.20,23
Comment
Mental Health of Patients With ABDs—Immunobullous diseases are painful, potentially lifelong conditions that have no definitive cure. These conditions are characterized by bullae and erosions of the skin and mucosae that physically are disabling and often create a stigma for patients. Across multiple different validated psychosocial assessments, the 13 studies included in this review consistently reported that ABDs have a negative effect on mental well-being of patients that is more pronounced in women and worse at the onset of symptoms.13-25
QOL Scores in Patients With ABDs—Quality of life is a broad term that encompasses a general sense of psychological and overall well-being. A score of approximately 10 on the DLQI most often was reported in patients with ABDs, which translates to a moderate impact on QOL. Incomparison, a large cohort study reported the mean (SD) DLQI scores for patients with atopic dermatitis and psoriasis as 7.31 (5.98) and 5.93 (5.66), respectively.28 In another study, Penha et al14 found that patients with psoriasis have a mean DLQI score of 10. Reasons for the similarly low QOL scores in patients with ABDs include long hospitalization periods, disease chronicity, social anxiety, inability to control symptoms, difficulty with activities of daily living, and the belief that the disease is incurable.17,19,23 Although there is a need for increased family and social support with performing necessary daily tasks, personal relationships often are negatively affected, resulting in social isolation, loneliness, and worsening of cutaneous symptoms.
Severity of cutaneous disease and recent onset of symptoms correlated with worse QOL scores. Tabolli et al20 proposed the reason for this relates to not having had enough time to find the best treatment regimen. We believe there also may be an element of habituation involved, whereby patients become accustomed to the appearance of the lesions over time and therefore they become less distressing. Interestingly, Tabolli et al17 determined that patients in the quiescent phase of the disease—without any mucosal or cutaneous lesions—still maintained lower QOL scores than the average population, particularly on the psychosocial section of the 36-Item Short Form Health Survey, which may be due to a concern of disease relapse or from adverse effects of treatment. Providers should monitor patients for mental health complications not only in the disease infancy but throughout the disease course.
Future Directions—Cause and effect of the relationship between the psychosocial variables and ABD disease state has yet to be determined. Most studies included in this review were cross-sectional in design. Although many studies concluded that bullous dermatoses were the cause of impaired QOL, Ren and colleagues29 proposed that medications used to treat neuropsychiatric disorders may trigger the autoimmune antigens of BP. Possible triggers for BP have been reported including hydrochlorothiazide, ciprofloxacin, and dipeptidyl peptidase-4 inhibitors.27,30-32 A longitudinal study design would better evaluate the causal relationship.
The effects of the medications were included in 2 cases, one in which the steroid dose was not found to have a significant impact on rates of depression23 and another in which patients treated with a higher dose of corticosteroids (>10 mg) had worse QOL scores.17 Sung et al15 suggested this may be because patients who took higher doses of steroids had worse symptoms and therefore also had a worse QOL. It also is possible that those patients taking higher doses had increased side effects.17 Further studies that evaluate treatment modalities and timing in relation to the disease onset would be helpful.
Study Limitations—There are potential barriers to combining these data. Multiple different questionnaires were used, and it was difficult to ascertain if all the participants were experiencing active disease. Additionally, questionnaires are not always the best proxy for what is happening in everyday life. Lastly, the sample size of each individual study was small, and the studies only included adults.
Conclusion
As demonstrated by the 13 studies in this review, patients with ABDs have lower QOL scores and higher numbers of psychological symptoms. Clinicians should be mindful of this at-risk population and create opportunities in clinic to discuss personal hardship associated with the disease process and recommend psychiatric intervention if indicated. Additionally, family members often are overburdened with the chronicity of ABDs, and they should not be forgotten. Using one of the aforementioned questionnaires is a practical way to screen patients for lower QOL scores. We agree with Paradisi and colleagues19 that although these questionnaires may be helpful, clinicians still need to determine if the use of a dermatologic QOL evaluation tool in clinical practice improves patient satisfaction.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489. https://doi.org/10.1016/j.autrev.2014.01.047
- Sebaratnam DF, McMillan JR, Werth VP, et al. Quality of life in patients with bullous dermatoses. Clin Dermatol. 2012;30:103-107. doi:10.1016/j.clindermatol.2011.03.016
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Goldberg DP. The Detection of Psychiatric Illness by Questionnaire. Oxford University Press; 1972.
- Cano A, Sprafkin RP, Scaturo DJ, et al. Mental health screening in primary care: a comparison of 3 brief measures of psychological distress. Prim Care Companion J Clin Psychiatry. 2001;3:206-210.
- Zigmond A, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361-370.
- Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66:20-40. doi:10.1207/s15327752jpa6601_2
- Beck A, Alford B. Depression: Causes and Treatment. 2nd ed. Philadelphia University of Pennsylvania Press; 2009.
- Ghassemzadeh H, Mojtabai R, Karamghadiri N, et al. Psychometric properties of a Persian-language version of the Beck Depression Inventory—Second Edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21:185-192. doi:10.1002/da.20070
- Chren MM, Lasek RJ, Sahay AP, et al. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5:105-110.
- Nijsten TEC, Sampogna F, Chren M, et al. Testing and reducing Skindex-29 using Rasch analysis: Skindex-17. J Invest Dermatol. 2006;126:1244-1250. https://doi.org/10.1038/sj.jid.5700212
- Ware JE Jr, Sherbourne C. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care. 1992;30:473-483.
- Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life, depression, anxiety and loneliness in patients with bullous pemphigoid: a case control study. An Bras Dermatol. 2016;91:601-603. doi:10.1590/abd1806-4841.2016493
- Penha MA, Farat JG, Miot HA, et al. Quality of life index in autoimmune bullous dermatosis patients. An Bras Dermatol. 2015;90:190-194. https://dx.doi.org/10.1590/abd1806-4841.20153372
- Sung JY, Roh MR, Kim SC. Quality of life assessment in Korean patients with pemphigus. Ann Dermatol. 2015;27:492-498.
- Moon SH, Kwon HI, Park HC, et al. Assessment of the quality of life in autoimmune blistering skin disease patients. Korean J Dermatol. 2014;52:402-409.
- Tabolli S, Pagliarello C, Paradisi A, et al. Burden of disease during quiescent periods in patients with pemphigus. Br J Dermatol. 2014;170:1087-1091. doi:10.1111/bjd.12836
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and general health questionnaires. J Dermatol. 2012;39:141-144. doi:10.1111/j.1346-8138.2011.01382
- Paradisi A, Sampogna F, Di Pietro C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269. doi:10.1016/j.jaad.2008.09.014
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034. doi:10.1111/j.1365-2133.2008.08481.x
- Terrab Z, Benchikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris: results from the German Bullous Skin Disease (BSD) Study Group [in German]. J Dtsch Dermatol Ges. 2005;3:431-435. doi:10.1111/j.1610-0387.2005.05722.x
- Layegh P, Mokhber N, Javidi Z, et al. Depression in patients with pemphigus: is it a major concern? J Dermatol. 2014;40:434-437. doi:10.1111/1346-8138.12067
- Kumar V, Mattoo SK, Handa S. Psychiatric morbidity in pemphigus and psoriasis: a comparative study from India. Asian J Psychiatr. 2013;6:151-156. doi:10.1016/j.ajp.2012.10.005
- Mazzotti E, Mozzetta A, Antinone V, et al. Psychological distress and investment in one’s appearance in patients with pemphigus. J Eur Acad Dermatol Venereol. 2011;25:285-289. doi:10.1111/j.1468-3083.2010.03780.x
- Regier DA, Boyd JH, Burke JD, et al. One-month prevalence of mental disorders in the United States: based on five epidemiologic catchment area sites. Arch Gen Psychiatr. 1988;45:977-986. doi:10.1001/archpsyc.1988.01800350011002
- Cozzani E, Chinazzo C, Burlando M, et al. Ciprofloxacin as a trigger for bullous pemphigoid: the second case in the literature. Am J Ther. 2016;23:E1202-E1204. doi:10.1097/MJT.0000000000000283
- Lundberg L, Johannesson M, Silverdahl M, et al. Health-related quality of life in patients with psoriasis and atopic dermatitis measured with SF-36, DLQI and a subjective measure of disease activity. Acta Derm Venereol. 2000;80:430-434.
- Ren Z, Hsu DY, Brieva J, et al. Hospitalization, inpatient burden and comorbidities associated with bullous pemphigoid in the U.S.A. Br J Dermatol. 2017;176:87-99. doi:10.1111/bjd.14821
- Warner C, Kwak Y, Glover MH, et al. Bullous pemphigoid induced by hydrochlorothiazide therapy. J Drugs Dermatol. 2014;13:360-362.
- Mendonca FM, Martin-Gutierrez FJ, Rios-Martin JJ, et al. Three cases of bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors—one due to linagliptin. Dermatology. 2016;232:249-253. doi:10.1159/000443330
- Attaway A, Mersfelder TL, Vaishnav S, et al. Bullous pemphigoid associated with dipeptidyl peptidase IV inhibitors: a case report and review of literature. J Dermatol Case Rep. 2014;8:24-28.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489. https://doi.org/10.1016/j.autrev.2014.01.047
- Sebaratnam DF, McMillan JR, Werth VP, et al. Quality of life in patients with bullous dermatoses. Clin Dermatol. 2012;30:103-107. doi:10.1016/j.clindermatol.2011.03.016
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Goldberg DP. The Detection of Psychiatric Illness by Questionnaire. Oxford University Press; 1972.
- Cano A, Sprafkin RP, Scaturo DJ, et al. Mental health screening in primary care: a comparison of 3 brief measures of psychological distress. Prim Care Companion J Clin Psychiatry. 2001;3:206-210.
- Zigmond A, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361-370.
- Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66:20-40. doi:10.1207/s15327752jpa6601_2
- Beck A, Alford B. Depression: Causes and Treatment. 2nd ed. Philadelphia University of Pennsylvania Press; 2009.
- Ghassemzadeh H, Mojtabai R, Karamghadiri N, et al. Psychometric properties of a Persian-language version of the Beck Depression Inventory—Second Edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21:185-192. doi:10.1002/da.20070
- Chren MM, Lasek RJ, Sahay AP, et al. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5:105-110.
- Nijsten TEC, Sampogna F, Chren M, et al. Testing and reducing Skindex-29 using Rasch analysis: Skindex-17. J Invest Dermatol. 2006;126:1244-1250. https://doi.org/10.1038/sj.jid.5700212
- Ware JE Jr, Sherbourne C. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care. 1992;30:473-483.
- Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life, depression, anxiety and loneliness in patients with bullous pemphigoid: a case control study. An Bras Dermatol. 2016;91:601-603. doi:10.1590/abd1806-4841.2016493
- Penha MA, Farat JG, Miot HA, et al. Quality of life index in autoimmune bullous dermatosis patients. An Bras Dermatol. 2015;90:190-194. https://dx.doi.org/10.1590/abd1806-4841.20153372
- Sung JY, Roh MR, Kim SC. Quality of life assessment in Korean patients with pemphigus. Ann Dermatol. 2015;27:492-498.
- Moon SH, Kwon HI, Park HC, et al. Assessment of the quality of life in autoimmune blistering skin disease patients. Korean J Dermatol. 2014;52:402-409.
- Tabolli S, Pagliarello C, Paradisi A, et al. Burden of disease during quiescent periods in patients with pemphigus. Br J Dermatol. 2014;170:1087-1091. doi:10.1111/bjd.12836
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and general health questionnaires. J Dermatol. 2012;39:141-144. doi:10.1111/j.1346-8138.2011.01382
- Paradisi A, Sampogna F, Di Pietro C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269. doi:10.1016/j.jaad.2008.09.014
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034. doi:10.1111/j.1365-2133.2008.08481.x
- Terrab Z, Benchikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris: results from the German Bullous Skin Disease (BSD) Study Group [in German]. J Dtsch Dermatol Ges. 2005;3:431-435. doi:10.1111/j.1610-0387.2005.05722.x
- Layegh P, Mokhber N, Javidi Z, et al. Depression in patients with pemphigus: is it a major concern? J Dermatol. 2014;40:434-437. doi:10.1111/1346-8138.12067
- Kumar V, Mattoo SK, Handa S. Psychiatric morbidity in pemphigus and psoriasis: a comparative study from India. Asian J Psychiatr. 2013;6:151-156. doi:10.1016/j.ajp.2012.10.005
- Mazzotti E, Mozzetta A, Antinone V, et al. Psychological distress and investment in one’s appearance in patients with pemphigus. J Eur Acad Dermatol Venereol. 2011;25:285-289. doi:10.1111/j.1468-3083.2010.03780.x
- Regier DA, Boyd JH, Burke JD, et al. One-month prevalence of mental disorders in the United States: based on five epidemiologic catchment area sites. Arch Gen Psychiatr. 1988;45:977-986. doi:10.1001/archpsyc.1988.01800350011002
- Cozzani E, Chinazzo C, Burlando M, et al. Ciprofloxacin as a trigger for bullous pemphigoid: the second case in the literature. Am J Ther. 2016;23:E1202-E1204. doi:10.1097/MJT.0000000000000283
- Lundberg L, Johannesson M, Silverdahl M, et al. Health-related quality of life in patients with psoriasis and atopic dermatitis measured with SF-36, DLQI and a subjective measure of disease activity. Acta Derm Venereol. 2000;80:430-434.
- Ren Z, Hsu DY, Brieva J, et al. Hospitalization, inpatient burden and comorbidities associated with bullous pemphigoid in the U.S.A. Br J Dermatol. 2017;176:87-99. doi:10.1111/bjd.14821
- Warner C, Kwak Y, Glover MH, et al. Bullous pemphigoid induced by hydrochlorothiazide therapy. J Drugs Dermatol. 2014;13:360-362.
- Mendonca FM, Martin-Gutierrez FJ, Rios-Martin JJ, et al. Three cases of bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors—one due to linagliptin. Dermatology. 2016;232:249-253. doi:10.1159/000443330
- Attaway A, Mersfelder TL, Vaishnav S, et al. Bullous pemphigoid associated with dipeptidyl peptidase IV inhibitors: a case report and review of literature. J Dermatol Case Rep. 2014;8:24-28.
Practice Points
- Autoimmune bullous dermatoses cause cutaneous lesions that are painful and disfiguring. These conditions affect a patient’s ability to perform everyday tasks, and individual lesions can take years to heal.
- Providers should take necessary steps to address patient well-being, especially at disease onset in patients with bullous dermatoses.
2022 Update on fertility
In this Update, the authors discuss 2 important areas that impact fertility. First, with in vitro fertilization (IVF), successful implantation that leads to live birth requires a normal embryo and a receptive endometrium. While research using advanced molecular array technology has resulted in a clinical test to identify the optimal window of implantation, recent evidence has questioned its clinical effectiveness. Second, recognizing the importance of endometriosis—a common disease with high burden that causes pain, infertility, and other symptoms—the World Health Organization (WHO) last year published an informative fact sheet that highlights the diagnosis, treatment options, and challenges of this significant disease.
Endometrial receptivity array and the quest for optimal endometrial preparation prior to embryo transfer in IVF
Bergin K, Eliner Y, Duvall DW Jr, et al. The use of propensity score matching to assess the benefit of the endometrial receptivity analysis in frozen embryo transfers. Fertil Steril. 2021;116:396-403.
Riestenberg C, Kroener L, Quinn M, et al. Routine endometrial receptivity array in first embryo transfer cycles does not improve live birth rate. Fertil Steril. 2021;115:1001-1006.
Doyle N, Jahandideh S, Hill MJ, et al. A randomized controlled trial comparing live birth from single euploid frozen blastocyst transfer using standardized timing versus timing by endometrial receptivity analysis. Fertil Steril. 2021;116(suppl):e101.
A successful pregnancy requires optimal crosstalk between the embryo and the endometrium. Over the past several decades, research efforts to improve IVF outcomes have been focused mainly on the embryo factor and methods to improve embryo selection, such as extended culture to blastocyst, time-lapse imaging (morphokinetic assessment), and more notably, preimplantation genetic testing for aneuploidy (PGT-A). However, the other half of the equation, the endometrium, has not garnered the attention that it deserves. Effort has therefore been renewed to optimize the endometrial factor by better diagnosing and treating various forms of endometrial dysfunction that could lead to infertility in general and lack of success with IVF and euploid embryo transfers in particular.
Historical background on endometrial function
Progesterone has long been recognized as the main effector that transforms the estrogen-primed endometrium into a receptive state that results in successful embryo implantation. Progesterone exposure is required at appropriate levels and duration before the endometrium becomes receptive to the embryo. If implantation does not occur soon after the endometrium has attained receptive status (7–10 days after ovulation), further progesterone exposure results in progression of endometrial changes that no longer permit successful implantation.
As early as the 1950s, “luteal phase deficiency” was defined as due to inadequate progesterone secretion and resulted in a short luteal phase. In the 1970s, histologic “dating” of the endometrium became the gold standard for diagnosing luteal phase defects; this relied on a classic histologic appearance of secretory phase endometrium and its changes throughout the luteal phase. Subsequently, however, results of prospective randomized controlled trials published in 2004 cast significant doubt on the accuracy and reproducibility of these endometrial biopsies and did not show any clinical diagnostic benefit or correlation with pregnancy outcomes.
21st century advances: Endometrial dating 2.0
A decade later, with the advancement of molecular biology tools such as microarray technology, researchers were able to study endometrial gene expression patterns at different stages of the menstrual cycle. They identified different phases of endometrial development with molecular profiles, or “signatures,” for the luteal phase, endometriosis, polycystic ovary syndrome, and uterine fibroids.
In 2013, researchers in Spain introduced a diagnostic test called endometrial receptivity array (ERA) with the stated goal of being able to temporally define the receptive endometrium and identify prereceptive as well as postreceptive states.1 In other words, instead of the histologic dating of the endometrium used in the 1970s, it represented “molecular dating” of the endometrium. Although the initial studies were conducted among women who experienced prior unsuccessful embryo transfers (the so-called recurrent implantation failure, or RIF), the test’s scope was subsequently expanded to include any individual planning on a frozen embryo transfer (FET), regardless of any prior attempts. The term personalized embryo transfer (pET) was coined to suggest the ability to define the best time (up to hours) for embryo transfers on an individual basis. Despite lack of independent validation studies, ERA was then widely adopted by many clinicians (and requested by some patients) with the hope of improving IVF outcomes.
However, not unlike many other novel innovations in assisted reproductive technology, ERA regrettably did not withstand the test of time. Three independent studies in 2021, 1 randomized clinical trial and 2 observational cohort studies, did not show any benefit with regard to implantation rates, pregnancy rates, or live birth rates when ERA was performed in the general infertility population.2-4
Continue to: Study results...
Study results
The cohort study that matched 133 ERA patients with 353 non-ERA patients showed live birth rates of 49.62% for the ERA group and 54.96% for the non-ERA group (odds ratio [OR], 0.8074; 95% confidence interval [CI], 0.5424–1.2018).2 Of note, no difference occurred between subgroups based on the prior number of FETs or the receptivity status (TABLE 1).
Another cohort study from the University of California, Los Angeles, published in 2021 analyzed 228 single euploid FET cycles.3 This study did not show any benefit for routine ERA testing, with a live birth rate of 56.6% in the non-ERA group and 56.5% in the ERA group.
Still, the most convincing evidence for the lack of benefit from routine ERA was noted from the results of the randomized clinical trial.4 A total of 767 patients were randomly allocated, 381 to the ERA group and 386 to the control group. There was no difference in ongoing pregnancy rates between the 2 groups. Perhaps more important, even after limiting the analysis to individuals with a nonreceptive ERA result, there was no difference in ongoing pregnancy rates between the 2 groups: 62.5% in the control group (default timing of transfer) and 55.5% in the study group (transfer timing adjusted based on ERA) (rate ratio [RR], 0.9; 95% CI, 0.70–1.14).
ERA usefulness is unsupported in general infertility population
The studies discussed collectively suggest with a high degree of certainty that there is no indication for routine ERA testing in the general infertility population prior to frozen embryo transfers.
Although these studies all were conducted in the general infertility population and did not specifically evaluate the performance of ERA in women with recurrent pregnancy loss or recurrent implantation failure, it is important to acknowledge that if ERA were truly able to define the window of receptivity, one would expect a lower implantation rate if the embryos were transferred outside of the window suggested by the ERA. This was not the case in these studies, as they all showed equivalent pregnancy rates in the control (nonadjusted) groups even when ERA suggested a nonreceptive status.
This observation seriously questions the validity of ERA regarding its ability to temporally define the window of receptivity. On the other hand, as stated earlier, there is still a possibility for ERA to be beneficial for a small subgroup of patients whose window of receptivity may not be as wide as expected in the general population. The challenging question would be how best to identify the particular group with a narrow, or displaced, window of receptivity.
The optimal timing for implantation of a normal embryo requires a receptive endometrium. The endometrial biopsy was used widely for many years before research showed it was not clinically useful. More recently, the endometrial receptivity array has been suggested to help time the frozen embryo transfer. Unfortunately, recent studies have shown that this test is not clinically useful for the general infertility population.
Continue to: WHO raises awareness of endometriosis burden and...
WHO raises awareness of endometriosis burden and highlights need to address diagnosis and treatment for women’s reproductive health
World Health Organization. Endometriosis fact sheet. March 31, 2021. https://www.who.int/news-room /fact-sheets/detail/endometriosis. Accessed January 3, 2022.
The WHO published its first fact sheet on endometriosis in March 2021, recognizing endometriosis as a severe disease that affects almost 190 million women with life-impacting pain, infertility, other symptoms, and especially with chronic, significant emotional sequelae (TABLE 2).5 The disease’s variable and broad symptoms result in a lack of awareness and diagnosis by both women and health care providers, especially in low- and middle-income countries and in disadvantaged populations in developed countries. Increased awareness to promote earlier diagnosis, improved training for better management, expanded research for greater understanding, and policies that increase access to quality care are needed to ensure the reproductive health and rights of tens of millions of women with endometriosis.
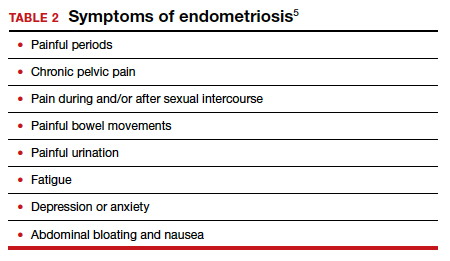
Endometriosis characteristics and symptoms
Endometriosis is characterized by the presence of tissue resembling endometrium outside the uterus, where it causes a chronic inflammatory reaction that may result in the formation of scar tissue. Endometriotic lesions may be superficial, cystic ovarian endometriomas, or deep lesions, causing a myriad of pain and related symptoms.6.7
Chronic pain may occur because pain centers in the brain become hyperresponsive over time (central sensitization); this can occur at any point throughout the life course of endometriosis, even when endometriosis lesions are no longer visible. Sometimes, endometriosis is asymptomatic. In addition, endometriosis can cause infertility through anatomic distortion and inflammatory, endocrinologic, and other pathways.
The origins of endometriosis are thought to be multifactorial and include retrograde menstruation, cellular metaplasia, and/or stem cells that spread through blood and lymphatic vessels. Endometriosis is estrogen dependent, but lesion growth also is affected by altered or impaired immunity, localized complex hormonal influences, genetics, and possibly environmental contaminants.
Impact on public health and reproductive rights
Endometriosis has significant social, public health, and economic implications. It can decrease quality of life and prevent girls and women from attending work or school.8 Painful sex can affect sexual health. The WHO states that, “Addressing endometriosis will empower those affected by it, by supporting their human right to the highest standard of sexual and reproductive health, quality of life, and overall well-being.”5
At present, no known way is available to prevent or cure endometriosis. Early diagnosis and treatment, however, may slow or halt its natural progression and associated symptoms.
Diagnostic steps and treatment options
Early suspicion of endometriosis is the most important factor, followed by a careful history of menstrual symptoms and chronic pelvic pain, early referral to specialists for ultrasonography or other imaging, and sometimes surgical or laparoscopic visualization. Empirical treatment can be begun without histologic or laparoscopic confirmation.
Endometriosis can be treated with medications and/or surgery depending on symptoms, lesions, desired outcome, and patient choice.5,6 Common therapies include contraceptive steroids, nonsteroidal anti-inflammatory medications, and analgesics. Medical treatments focus on either lowering estrogen or increasing progesterone levels.
Surgery can remove endometriosis lesions, adhesions, and scar tissue. However, success in reducing pain symptoms and increasing pregnancy rates often depends on the extent of disease.
For infertility due to endometriosis, treatment options include laparoscopic surgical removal of endometriosis, ovarian stimulation with intrauterine insemination (IUI), and IVF. Multidisciplinary treatment addressing different symptoms and overall health often requires referral to pain experts and other specialists.9
The WHO perspective on endometriosis
Recognizing the importance of endometriosis and its impact on people’s sexual and reproductive health, quality of life, and overall well-being, the WHO is taking action to improve awareness, diagnosis, and treatment of endometriosis (TABLE 3).5 ●
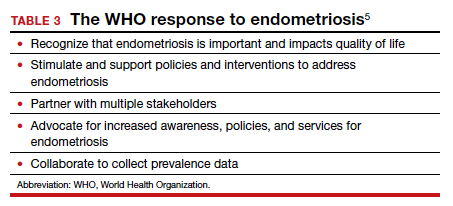
Endometriosis is now recognized as a disease with significant burden for women everywhere. Widespread lack of awareness of presenting symptoms and management options means that all women’s health care clinicians need to become better informed about endometriosis so they can improve the quality of care they provide.
- Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100:818-824.
- Bergin K, Eliner Y, Duvall DW Jr, et al. The use of propensity score matching to assess the benefit of the endometrial receptivity analysis in frozen embryo transfers. Fertil Steril. 2021;116:396-403.
- Riestenberg C, Kroener L, Quinn M, et al. Routine endometrial receptivity array in first embryo transfer cycles does not improve live birth rate. Fertil Steril. 2021;115:1001-1006.
- Doyle N, Jahandideh S, Hill MJ, et al. A randomized controlled trial comparing live birth from single euploid frozen blastocyst transfer using standardized timing versus timing by endometrial receptivity analysis. Fertil Steril. 2021;116(suppl):e101.
- World Health Organization. Endometriosis fact sheet. March 31, 2021. https://www.who.int/news-room/fact-sheets/detail /endometriosis. Accessed January 3, 2022.
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382:1244-1256.
- Johnson NP, Hummelshoj L, Adamson GD, et al. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32:315-324.
- Nnoaham K, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96:366-373.e8.
- Carey ET, Till SR, As-Sanie S. Pharmacological management of chronic pelvic pain in women. Drugs. 2017;77:285-301.
In this Update, the authors discuss 2 important areas that impact fertility. First, with in vitro fertilization (IVF), successful implantation that leads to live birth requires a normal embryo and a receptive endometrium. While research using advanced molecular array technology has resulted in a clinical test to identify the optimal window of implantation, recent evidence has questioned its clinical effectiveness. Second, recognizing the importance of endometriosis—a common disease with high burden that causes pain, infertility, and other symptoms—the World Health Organization (WHO) last year published an informative fact sheet that highlights the diagnosis, treatment options, and challenges of this significant disease.
Endometrial receptivity array and the quest for optimal endometrial preparation prior to embryo transfer in IVF
Bergin K, Eliner Y, Duvall DW Jr, et al. The use of propensity score matching to assess the benefit of the endometrial receptivity analysis in frozen embryo transfers. Fertil Steril. 2021;116:396-403.
Riestenberg C, Kroener L, Quinn M, et al. Routine endometrial receptivity array in first embryo transfer cycles does not improve live birth rate. Fertil Steril. 2021;115:1001-1006.
Doyle N, Jahandideh S, Hill MJ, et al. A randomized controlled trial comparing live birth from single euploid frozen blastocyst transfer using standardized timing versus timing by endometrial receptivity analysis. Fertil Steril. 2021;116(suppl):e101.
A successful pregnancy requires optimal crosstalk between the embryo and the endometrium. Over the past several decades, research efforts to improve IVF outcomes have been focused mainly on the embryo factor and methods to improve embryo selection, such as extended culture to blastocyst, time-lapse imaging (morphokinetic assessment), and more notably, preimplantation genetic testing for aneuploidy (PGT-A). However, the other half of the equation, the endometrium, has not garnered the attention that it deserves. Effort has therefore been renewed to optimize the endometrial factor by better diagnosing and treating various forms of endometrial dysfunction that could lead to infertility in general and lack of success with IVF and euploid embryo transfers in particular.
Historical background on endometrial function
Progesterone has long been recognized as the main effector that transforms the estrogen-primed endometrium into a receptive state that results in successful embryo implantation. Progesterone exposure is required at appropriate levels and duration before the endometrium becomes receptive to the embryo. If implantation does not occur soon after the endometrium has attained receptive status (7–10 days after ovulation), further progesterone exposure results in progression of endometrial changes that no longer permit successful implantation.
As early as the 1950s, “luteal phase deficiency” was defined as due to inadequate progesterone secretion and resulted in a short luteal phase. In the 1970s, histologic “dating” of the endometrium became the gold standard for diagnosing luteal phase defects; this relied on a classic histologic appearance of secretory phase endometrium and its changes throughout the luteal phase. Subsequently, however, results of prospective randomized controlled trials published in 2004 cast significant doubt on the accuracy and reproducibility of these endometrial biopsies and did not show any clinical diagnostic benefit or correlation with pregnancy outcomes.
21st century advances: Endometrial dating 2.0
A decade later, with the advancement of molecular biology tools such as microarray technology, researchers were able to study endometrial gene expression patterns at different stages of the menstrual cycle. They identified different phases of endometrial development with molecular profiles, or “signatures,” for the luteal phase, endometriosis, polycystic ovary syndrome, and uterine fibroids.
In 2013, researchers in Spain introduced a diagnostic test called endometrial receptivity array (ERA) with the stated goal of being able to temporally define the receptive endometrium and identify prereceptive as well as postreceptive states.1 In other words, instead of the histologic dating of the endometrium used in the 1970s, it represented “molecular dating” of the endometrium. Although the initial studies were conducted among women who experienced prior unsuccessful embryo transfers (the so-called recurrent implantation failure, or RIF), the test’s scope was subsequently expanded to include any individual planning on a frozen embryo transfer (FET), regardless of any prior attempts. The term personalized embryo transfer (pET) was coined to suggest the ability to define the best time (up to hours) for embryo transfers on an individual basis. Despite lack of independent validation studies, ERA was then widely adopted by many clinicians (and requested by some patients) with the hope of improving IVF outcomes.
However, not unlike many other novel innovations in assisted reproductive technology, ERA regrettably did not withstand the test of time. Three independent studies in 2021, 1 randomized clinical trial and 2 observational cohort studies, did not show any benefit with regard to implantation rates, pregnancy rates, or live birth rates when ERA was performed in the general infertility population.2-4
Continue to: Study results...
Study results
The cohort study that matched 133 ERA patients with 353 non-ERA patients showed live birth rates of 49.62% for the ERA group and 54.96% for the non-ERA group (odds ratio [OR], 0.8074; 95% confidence interval [CI], 0.5424–1.2018).2 Of note, no difference occurred between subgroups based on the prior number of FETs or the receptivity status (TABLE 1).

Another cohort study from the University of California, Los Angeles, published in 2021 analyzed 228 single euploid FET cycles.3 This study did not show any benefit for routine ERA testing, with a live birth rate of 56.6% in the non-ERA group and 56.5% in the ERA group.
Still, the most convincing evidence for the lack of benefit from routine ERA was noted from the results of the randomized clinical trial.4 A total of 767 patients were randomly allocated, 381 to the ERA group and 386 to the control group. There was no difference in ongoing pregnancy rates between the 2 groups. Perhaps more important, even after limiting the analysis to individuals with a nonreceptive ERA result, there was no difference in ongoing pregnancy rates between the 2 groups: 62.5% in the control group (default timing of transfer) and 55.5% in the study group (transfer timing adjusted based on ERA) (rate ratio [RR], 0.9; 95% CI, 0.70–1.14).
ERA usefulness is unsupported in general infertility population
The studies discussed collectively suggest with a high degree of certainty that there is no indication for routine ERA testing in the general infertility population prior to frozen embryo transfers.
Although these studies all were conducted in the general infertility population and did not specifically evaluate the performance of ERA in women with recurrent pregnancy loss or recurrent implantation failure, it is important to acknowledge that if ERA were truly able to define the window of receptivity, one would expect a lower implantation rate if the embryos were transferred outside of the window suggested by the ERA. This was not the case in these studies, as they all showed equivalent pregnancy rates in the control (nonadjusted) groups even when ERA suggested a nonreceptive status.
This observation seriously questions the validity of ERA regarding its ability to temporally define the window of receptivity. On the other hand, as stated earlier, there is still a possibility for ERA to be beneficial for a small subgroup of patients whose window of receptivity may not be as wide as expected in the general population. The challenging question would be how best to identify the particular group with a narrow, or displaced, window of receptivity.
The optimal timing for implantation of a normal embryo requires a receptive endometrium. The endometrial biopsy was used widely for many years before research showed it was not clinically useful. More recently, the endometrial receptivity array has been suggested to help time the frozen embryo transfer. Unfortunately, recent studies have shown that this test is not clinically useful for the general infertility population.
Continue to: WHO raises awareness of endometriosis burden and...
WHO raises awareness of endometriosis burden and highlights need to address diagnosis and treatment for women’s reproductive health
World Health Organization. Endometriosis fact sheet. March 31, 2021. https://www.who.int/news-room /fact-sheets/detail/endometriosis. Accessed January 3, 2022.
The WHO published its first fact sheet on endometriosis in March 2021, recognizing endometriosis as a severe disease that affects almost 190 million women with life-impacting pain, infertility, other symptoms, and especially with chronic, significant emotional sequelae (TABLE 2).5 The disease’s variable and broad symptoms result in a lack of awareness and diagnosis by both women and health care providers, especially in low- and middle-income countries and in disadvantaged populations in developed countries. Increased awareness to promote earlier diagnosis, improved training for better management, expanded research for greater understanding, and policies that increase access to quality care are needed to ensure the reproductive health and rights of tens of millions of women with endometriosis.

Endometriosis characteristics and symptoms
Endometriosis is characterized by the presence of tissue resembling endometrium outside the uterus, where it causes a chronic inflammatory reaction that may result in the formation of scar tissue. Endometriotic lesions may be superficial, cystic ovarian endometriomas, or deep lesions, causing a myriad of pain and related symptoms.6.7
Chronic pain may occur because pain centers in the brain become hyperresponsive over time (central sensitization); this can occur at any point throughout the life course of endometriosis, even when endometriosis lesions are no longer visible. Sometimes, endometriosis is asymptomatic. In addition, endometriosis can cause infertility through anatomic distortion and inflammatory, endocrinologic, and other pathways.
The origins of endometriosis are thought to be multifactorial and include retrograde menstruation, cellular metaplasia, and/or stem cells that spread through blood and lymphatic vessels. Endometriosis is estrogen dependent, but lesion growth also is affected by altered or impaired immunity, localized complex hormonal influences, genetics, and possibly environmental contaminants.
Impact on public health and reproductive rights
Endometriosis has significant social, public health, and economic implications. It can decrease quality of life and prevent girls and women from attending work or school.8 Painful sex can affect sexual health. The WHO states that, “Addressing endometriosis will empower those affected by it, by supporting their human right to the highest standard of sexual and reproductive health, quality of life, and overall well-being.”5
At present, no known way is available to prevent or cure endometriosis. Early diagnosis and treatment, however, may slow or halt its natural progression and associated symptoms.
Diagnostic steps and treatment options
Early suspicion of endometriosis is the most important factor, followed by a careful history of menstrual symptoms and chronic pelvic pain, early referral to specialists for ultrasonography or other imaging, and sometimes surgical or laparoscopic visualization. Empirical treatment can be begun without histologic or laparoscopic confirmation.
Endometriosis can be treated with medications and/or surgery depending on symptoms, lesions, desired outcome, and patient choice.5,6 Common therapies include contraceptive steroids, nonsteroidal anti-inflammatory medications, and analgesics. Medical treatments focus on either lowering estrogen or increasing progesterone levels.
Surgery can remove endometriosis lesions, adhesions, and scar tissue. However, success in reducing pain symptoms and increasing pregnancy rates often depends on the extent of disease.
For infertility due to endometriosis, treatment options include laparoscopic surgical removal of endometriosis, ovarian stimulation with intrauterine insemination (IUI), and IVF. Multidisciplinary treatment addressing different symptoms and overall health often requires referral to pain experts and other specialists.9
The WHO perspective on endometriosis
Recognizing the importance of endometriosis and its impact on people’s sexual and reproductive health, quality of life, and overall well-being, the WHO is taking action to improve awareness, diagnosis, and treatment of endometriosis (TABLE 3).5 ●

Endometriosis is now recognized as a disease with significant burden for women everywhere. Widespread lack of awareness of presenting symptoms and management options means that all women’s health care clinicians need to become better informed about endometriosis so they can improve the quality of care they provide.
In this Update, the authors discuss 2 important areas that impact fertility. First, with in vitro fertilization (IVF), successful implantation that leads to live birth requires a normal embryo and a receptive endometrium. While research using advanced molecular array technology has resulted in a clinical test to identify the optimal window of implantation, recent evidence has questioned its clinical effectiveness. Second, recognizing the importance of endometriosis—a common disease with high burden that causes pain, infertility, and other symptoms—the World Health Organization (WHO) last year published an informative fact sheet that highlights the diagnosis, treatment options, and challenges of this significant disease.
Endometrial receptivity array and the quest for optimal endometrial preparation prior to embryo transfer in IVF
Bergin K, Eliner Y, Duvall DW Jr, et al. The use of propensity score matching to assess the benefit of the endometrial receptivity analysis in frozen embryo transfers. Fertil Steril. 2021;116:396-403.
Riestenberg C, Kroener L, Quinn M, et al. Routine endometrial receptivity array in first embryo transfer cycles does not improve live birth rate. Fertil Steril. 2021;115:1001-1006.
Doyle N, Jahandideh S, Hill MJ, et al. A randomized controlled trial comparing live birth from single euploid frozen blastocyst transfer using standardized timing versus timing by endometrial receptivity analysis. Fertil Steril. 2021;116(suppl):e101.
A successful pregnancy requires optimal crosstalk between the embryo and the endometrium. Over the past several decades, research efforts to improve IVF outcomes have been focused mainly on the embryo factor and methods to improve embryo selection, such as extended culture to blastocyst, time-lapse imaging (morphokinetic assessment), and more notably, preimplantation genetic testing for aneuploidy (PGT-A). However, the other half of the equation, the endometrium, has not garnered the attention that it deserves. Effort has therefore been renewed to optimize the endometrial factor by better diagnosing and treating various forms of endometrial dysfunction that could lead to infertility in general and lack of success with IVF and euploid embryo transfers in particular.
Historical background on endometrial function
Progesterone has long been recognized as the main effector that transforms the estrogen-primed endometrium into a receptive state that results in successful embryo implantation. Progesterone exposure is required at appropriate levels and duration before the endometrium becomes receptive to the embryo. If implantation does not occur soon after the endometrium has attained receptive status (7–10 days after ovulation), further progesterone exposure results in progression of endometrial changes that no longer permit successful implantation.
As early as the 1950s, “luteal phase deficiency” was defined as due to inadequate progesterone secretion and resulted in a short luteal phase. In the 1970s, histologic “dating” of the endometrium became the gold standard for diagnosing luteal phase defects; this relied on a classic histologic appearance of secretory phase endometrium and its changes throughout the luteal phase. Subsequently, however, results of prospective randomized controlled trials published in 2004 cast significant doubt on the accuracy and reproducibility of these endometrial biopsies and did not show any clinical diagnostic benefit or correlation with pregnancy outcomes.
21st century advances: Endometrial dating 2.0
A decade later, with the advancement of molecular biology tools such as microarray technology, researchers were able to study endometrial gene expression patterns at different stages of the menstrual cycle. They identified different phases of endometrial development with molecular profiles, or “signatures,” for the luteal phase, endometriosis, polycystic ovary syndrome, and uterine fibroids.
In 2013, researchers in Spain introduced a diagnostic test called endometrial receptivity array (ERA) with the stated goal of being able to temporally define the receptive endometrium and identify prereceptive as well as postreceptive states.1 In other words, instead of the histologic dating of the endometrium used in the 1970s, it represented “molecular dating” of the endometrium. Although the initial studies were conducted among women who experienced prior unsuccessful embryo transfers (the so-called recurrent implantation failure, or RIF), the test’s scope was subsequently expanded to include any individual planning on a frozen embryo transfer (FET), regardless of any prior attempts. The term personalized embryo transfer (pET) was coined to suggest the ability to define the best time (up to hours) for embryo transfers on an individual basis. Despite lack of independent validation studies, ERA was then widely adopted by many clinicians (and requested by some patients) with the hope of improving IVF outcomes.
However, not unlike many other novel innovations in assisted reproductive technology, ERA regrettably did not withstand the test of time. Three independent studies in 2021, 1 randomized clinical trial and 2 observational cohort studies, did not show any benefit with regard to implantation rates, pregnancy rates, or live birth rates when ERA was performed in the general infertility population.2-4
Continue to: Study results...
Study results
The cohort study that matched 133 ERA patients with 353 non-ERA patients showed live birth rates of 49.62% for the ERA group and 54.96% for the non-ERA group (odds ratio [OR], 0.8074; 95% confidence interval [CI], 0.5424–1.2018).2 Of note, no difference occurred between subgroups based on the prior number of FETs or the receptivity status (TABLE 1).

Another cohort study from the University of California, Los Angeles, published in 2021 analyzed 228 single euploid FET cycles.3 This study did not show any benefit for routine ERA testing, with a live birth rate of 56.6% in the non-ERA group and 56.5% in the ERA group.
Still, the most convincing evidence for the lack of benefit from routine ERA was noted from the results of the randomized clinical trial.4 A total of 767 patients were randomly allocated, 381 to the ERA group and 386 to the control group. There was no difference in ongoing pregnancy rates between the 2 groups. Perhaps more important, even after limiting the analysis to individuals with a nonreceptive ERA result, there was no difference in ongoing pregnancy rates between the 2 groups: 62.5% in the control group (default timing of transfer) and 55.5% in the study group (transfer timing adjusted based on ERA) (rate ratio [RR], 0.9; 95% CI, 0.70–1.14).
ERA usefulness is unsupported in general infertility population
The studies discussed collectively suggest with a high degree of certainty that there is no indication for routine ERA testing in the general infertility population prior to frozen embryo transfers.
Although these studies all were conducted in the general infertility population and did not specifically evaluate the performance of ERA in women with recurrent pregnancy loss or recurrent implantation failure, it is important to acknowledge that if ERA were truly able to define the window of receptivity, one would expect a lower implantation rate if the embryos were transferred outside of the window suggested by the ERA. This was not the case in these studies, as they all showed equivalent pregnancy rates in the control (nonadjusted) groups even when ERA suggested a nonreceptive status.
This observation seriously questions the validity of ERA regarding its ability to temporally define the window of receptivity. On the other hand, as stated earlier, there is still a possibility for ERA to be beneficial for a small subgroup of patients whose window of receptivity may not be as wide as expected in the general population. The challenging question would be how best to identify the particular group with a narrow, or displaced, window of receptivity.
The optimal timing for implantation of a normal embryo requires a receptive endometrium. The endometrial biopsy was used widely for many years before research showed it was not clinically useful. More recently, the endometrial receptivity array has been suggested to help time the frozen embryo transfer. Unfortunately, recent studies have shown that this test is not clinically useful for the general infertility population.
Continue to: WHO raises awareness of endometriosis burden and...
WHO raises awareness of endometriosis burden and highlights need to address diagnosis and treatment for women’s reproductive health
World Health Organization. Endometriosis fact sheet. March 31, 2021. https://www.who.int/news-room /fact-sheets/detail/endometriosis. Accessed January 3, 2022.
The WHO published its first fact sheet on endometriosis in March 2021, recognizing endometriosis as a severe disease that affects almost 190 million women with life-impacting pain, infertility, other symptoms, and especially with chronic, significant emotional sequelae (TABLE 2).5 The disease’s variable and broad symptoms result in a lack of awareness and diagnosis by both women and health care providers, especially in low- and middle-income countries and in disadvantaged populations in developed countries. Increased awareness to promote earlier diagnosis, improved training for better management, expanded research for greater understanding, and policies that increase access to quality care are needed to ensure the reproductive health and rights of tens of millions of women with endometriosis.

Endometriosis characteristics and symptoms
Endometriosis is characterized by the presence of tissue resembling endometrium outside the uterus, where it causes a chronic inflammatory reaction that may result in the formation of scar tissue. Endometriotic lesions may be superficial, cystic ovarian endometriomas, or deep lesions, causing a myriad of pain and related symptoms.6.7
Chronic pain may occur because pain centers in the brain become hyperresponsive over time (central sensitization); this can occur at any point throughout the life course of endometriosis, even when endometriosis lesions are no longer visible. Sometimes, endometriosis is asymptomatic. In addition, endometriosis can cause infertility through anatomic distortion and inflammatory, endocrinologic, and other pathways.
The origins of endometriosis are thought to be multifactorial and include retrograde menstruation, cellular metaplasia, and/or stem cells that spread through blood and lymphatic vessels. Endometriosis is estrogen dependent, but lesion growth also is affected by altered or impaired immunity, localized complex hormonal influences, genetics, and possibly environmental contaminants.
Impact on public health and reproductive rights
Endometriosis has significant social, public health, and economic implications. It can decrease quality of life and prevent girls and women from attending work or school.8 Painful sex can affect sexual health. The WHO states that, “Addressing endometriosis will empower those affected by it, by supporting their human right to the highest standard of sexual and reproductive health, quality of life, and overall well-being.”5
At present, no known way is available to prevent or cure endometriosis. Early diagnosis and treatment, however, may slow or halt its natural progression and associated symptoms.
Diagnostic steps and treatment options
Early suspicion of endometriosis is the most important factor, followed by a careful history of menstrual symptoms and chronic pelvic pain, early referral to specialists for ultrasonography or other imaging, and sometimes surgical or laparoscopic visualization. Empirical treatment can be begun without histologic or laparoscopic confirmation.
Endometriosis can be treated with medications and/or surgery depending on symptoms, lesions, desired outcome, and patient choice.5,6 Common therapies include contraceptive steroids, nonsteroidal anti-inflammatory medications, and analgesics. Medical treatments focus on either lowering estrogen or increasing progesterone levels.
Surgery can remove endometriosis lesions, adhesions, and scar tissue. However, success in reducing pain symptoms and increasing pregnancy rates often depends on the extent of disease.
For infertility due to endometriosis, treatment options include laparoscopic surgical removal of endometriosis, ovarian stimulation with intrauterine insemination (IUI), and IVF. Multidisciplinary treatment addressing different symptoms and overall health often requires referral to pain experts and other specialists.9
The WHO perspective on endometriosis
Recognizing the importance of endometriosis and its impact on people’s sexual and reproductive health, quality of life, and overall well-being, the WHO is taking action to improve awareness, diagnosis, and treatment of endometriosis (TABLE 3).5 ●

Endometriosis is now recognized as a disease with significant burden for women everywhere. Widespread lack of awareness of presenting symptoms and management options means that all women’s health care clinicians need to become better informed about endometriosis so they can improve the quality of care they provide.
- Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100:818-824.
- Bergin K, Eliner Y, Duvall DW Jr, et al. The use of propensity score matching to assess the benefit of the endometrial receptivity analysis in frozen embryo transfers. Fertil Steril. 2021;116:396-403.
- Riestenberg C, Kroener L, Quinn M, et al. Routine endometrial receptivity array in first embryo transfer cycles does not improve live birth rate. Fertil Steril. 2021;115:1001-1006.
- Doyle N, Jahandideh S, Hill MJ, et al. A randomized controlled trial comparing live birth from single euploid frozen blastocyst transfer using standardized timing versus timing by endometrial receptivity analysis. Fertil Steril. 2021;116(suppl):e101.
- World Health Organization. Endometriosis fact sheet. March 31, 2021. https://www.who.int/news-room/fact-sheets/detail /endometriosis. Accessed January 3, 2022.
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382:1244-1256.
- Johnson NP, Hummelshoj L, Adamson GD, et al. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32:315-324.
- Nnoaham K, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96:366-373.e8.
- Carey ET, Till SR, As-Sanie S. Pharmacological management of chronic pelvic pain in women. Drugs. 2017;77:285-301.
- Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100:818-824.
- Bergin K, Eliner Y, Duvall DW Jr, et al. The use of propensity score matching to assess the benefit of the endometrial receptivity analysis in frozen embryo transfers. Fertil Steril. 2021;116:396-403.
- Riestenberg C, Kroener L, Quinn M, et al. Routine endometrial receptivity array in first embryo transfer cycles does not improve live birth rate. Fertil Steril. 2021;115:1001-1006.
- Doyle N, Jahandideh S, Hill MJ, et al. A randomized controlled trial comparing live birth from single euploid frozen blastocyst transfer using standardized timing versus timing by endometrial receptivity analysis. Fertil Steril. 2021;116(suppl):e101.
- World Health Organization. Endometriosis fact sheet. March 31, 2021. https://www.who.int/news-room/fact-sheets/detail /endometriosis. Accessed January 3, 2022.
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382:1244-1256.
- Johnson NP, Hummelshoj L, Adamson GD, et al. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32:315-324.
- Nnoaham K, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96:366-373.e8.
- Carey ET, Till SR, As-Sanie S. Pharmacological management of chronic pelvic pain in women. Drugs. 2017;77:285-301.
HT for women who have had BSO before the age of natural menopause: Discerning the nuances
Women who undergo bilateral salpingo-oophorectomy (BSO) for various indications prior to menopause experience a rapid decline in ovarian hormone levels and consequent vasomotor and other menopausal symptoms. In addition, the resulting estrogen deprivation is associated with such long-term adverse outcomes as osteoporosis and cardiovascular morbidity.
OBG M
Surgical vs natural menopause
Stephanie Faubion, MD, MBA, NCMP: Since the Women’s Health Initiative study was published in 2002,2 many clinicians have been fearful of using systemic HT in menopausal women, and HT use has declined dramatically such that only about 4% to 6% of menopausal women are now receiving systemic HT. Importantly, however, a group of younger menopausal women also are not receiving HT, and that is women who undergo BSO before they reach the average age of menopause, which in the United States is about age 52; this is sometimes referred to as surgical menopause or early surgical menopause. Early surgical menopause has different connotations for long-term health risks than natural menopause at the average age, and we are here to discuss these health effects and their management.
My name is Stephanie Faubion, and I am a women’s health internist and the Chair of the Department of Medicine at Mayo Clinic in Jacksonville, Florida, and Director of Mayo Clinic Women’s Health. I am here with 2 of my esteemed colleagues, Dr. Andrew Kaunitz and Dr. Ekta Kapoor.
Andrew M. Kaunitz, MD, NCMP: Hello, I am an ObGyn with the University of Florida College of Medicine in Jacksonville, with particular interests in contraception, menopause, and gynecologic ultrasonography.
Ekta Kapoor, MBBS, NCMP: And I am an endocrinologist at Mayo Clinic in Rochester with a specific interest in menopause and hormone therapy. I am also the Assistant Director for Mayo Clinic Women’s Health.
Higher-than-standard estrogen doses needed in younger menopausal women
Dr. Faubion: Let’s consider a couple of cases so that we can illustrate some important points regarding hormone management in women who have undergone BSO before the age of natural menopause.
Our first case patient is a woman who is 41 years of age and, because of adenomyosis, she will undergo a hysterectomy. She tells her clinician that she is very concerned about ovarian cancer risk because one of her good friends recently was diagnosed with ovarian cancer, and together they decide to remove her ovaries at the time of hysterectomy. Notably, her ovaries were healthy.
The patient is now menopausal postsurgery, and she is having significant hot flashes and night sweats. She visits her local internist, who is concerned about initiating HT. She is otherwise a healthy woman and does not have any contraindications to HT. Dr. Kaunitz, what would you tell her internist?
Dr. Kaunitz: We are dealing with 2 different issues in terms of decision making about systemic HT for this 41-year-old who has undergone BSO. First, as you mentioned, Dr. Faubion, she has bothersome hot flashes, or vasomotor symptoms. Unless there are contraindications, systemic HT would be appropriate. Although I might start treatment at standard doses, and the accompanying TABLE depicts standard doses for the 2 most common oral estrogen formulations as well as transdermal estradiol, it’s important to recognize that younger menopausal women often will need to use higher-than-standard doses.
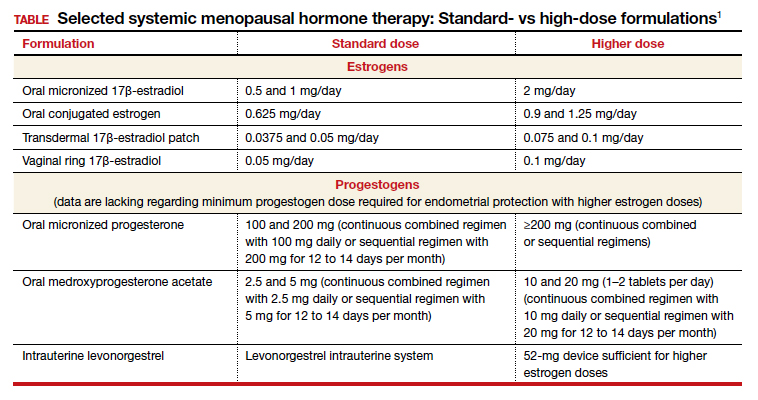
For example, for a 53-year-old woman who has been menopausal for a year or 2 and now has bothersome symptoms, I might start her on estradiol 1 mg tablets with progestin if a uterus is present. However, in this 41-year-old case patient, while I might start treatment at a standard dose, I would anticipate increasing to higher doses, such as 1.5 or 2 mg of daily estradiol until she feels her menopausal symptoms are adequately addressed.
Dr. Faubion: It is important to note that sometimes women with early BSO tend to have more severe vasomotor symptoms. Do you find that sometimes a higher dose is required just to manage symptoms, Dr. Kaunitz?
Dr. Kaunitz: Absolutely, yes. The decision whether or not to use systemic HT might be considered discretionary or elective in the classic 53-year-old woman recently menopausal with hot flashes, a so-called spontaneously or naturally menopausal woman. But my perspective is that unless there are clear contraindications, the decision to start systemic HT in the 41-year-old BSO case patient is actually not discretionary. Unless contraindications are present, it is important not only to treat symptoms but also to prevent an array of chronic major health concerns that are more likely if we don’t prescribe systemic HT.
Continue to: Health effects of not using HT...
Health effects of not using HT
Dr. Faubion: Dr. Kapoor, can you describe the potential long-term adverse health consequences of not using estrogen therapy? Say the same 41-year-old woman does not have many bothersome symptoms. What would you do?
Dr. Kapoor: Thank you for that important question. Building on what Dr. Kaunitz said, in these patients there are really 2 issues that can seem to be independent but are not: The first relates to the immediate consequences of lack of estrogen, ie, the menopause-related symptoms, but the second and perhaps the bigger issue is the long-term risk associated with estrogen deprivation.
The symptoms in these women are often obvious as they can be quite severe and abrupt; one day these women have normal hormone levels and the next day, after BSO, suddenly their hormones are very low. So if symptoms occur, they are usually hard to miss, simply because they are very drastic and very severe.
Historically, patients and their clinicians have targeted these symptoms. Patients experience menopausal symptoms, they seek treatment, and then the clinicians basically titrate the treatment to manage these symptoms. That misses the bigger issue, however, which is that premature estrogen deprivation leads to a host of chronic health conditions, as Dr. Kaunitz mentioned. These mainly include increased risk for cardiovascular disease, diabetes, hypertension, dyslipidemia, increased risk of mortality, dementia, and osteoporosis.
Fairly strong observational evidence suggests that use of estrogen therapy given in replacement doses—doses higher than those typically used in women after natural menopause, therefore considered replacement doses—helps mitigate the risk of some of these adverse health conditions.
In these women, the bigger goal really is to reinstate the hormonal milieu that exists prior to menopause. To your point, Dr. Faubion, if I have a patient who is younger than 46 years, who has her ovaries taken out, and even if she has zero symptoms (and sometimes that does happen), I would still make a case for this patient to utilize hormone therapy unless there is a contraindication such as breast cancer or other estrogen-sensitive cancers.
Dr. Faubion: Again, would you aim for those higher doses rather than treat with the “lowest dose”?
Dr. Kapoor: Absolutely. My punchline to the patients and clinicians in these discussions is that the rules of the game are different for these women. We cannot extrapolate the risks and benefits of HT use in women after natural menopause to younger women who have surgical menopause. Those rules just do not apply with respect to both benefits and risks.
Dr. Faubion: I think it’s important to say that these same “rules” would apply if the women were to go through premature menopause for any other reason, too, such as chemotherapy, radiation therapy, or premature ovarian insufficiency for any number of reasons, including toxic, metabolic, or genetic causes and so on. Would that be true?
Dr. Kapoor: Yes, absolutely so.
Dr. Faubion: Dr. Kaunitz, do you want to add anything?
Dr. Kaunitz: In terms of practical or clinical issues regarding systemic HT management, for the woman in her early 50s who has experienced normal or natural spontaneous menopause, a starting dose of transdermal estradiol would be, for instance, a 0.05-mg patch, which is a patch that over 24 hours releases 0.05 mg of estradiol daily; or standard oral estrogen, including conjugated equine estrogen, a 0.625-mg tablet daily, or estradiol, a 1-mg tablet daily.
But in younger patients, we want to use higher doses. For a patch, for instance, I would aim for a 0.075- or 0.1-mg estradiol patch, which releases a higher daily dose of estradiol than the standard dose. For oral estrogen, the dose would be 0.9- or even 1.25-mg tablets of conjugated equine estrogen or 1.5 mg, which is a 1-mg plus a 0.5-mg estradiol tablet, or a 2-mg estradiol tablet. Estradiol does come in a 2-mg strength.
For oral estrogen, I prefer estradiol because it’s available as a generic medication and often available at a very low cost, sometimes as low as $4 a month from chain pharmacies.
Continue to: Usefulness of monitoring estradiol levels for dosage adjustment...
Usefulness of monitoring estradiol levels for dosage adjustment
Dr. Faubion: That’s a great point, and again it is important to emphasize that we are aiming to recreate the premenopausal hormonal milieu. If you were to check estradiol levels, that would be aiming for a premenopausal range of approximately 80 to 120 pg per mL. Dr. Kapoor, is there utility in monitoring estrogen levels?
Dr. Kapoor: Great question, Dr. Faubion, and as you know it’s a loaded one. We base this on empiric evidence. We know that if the hormonal milieu in a young patient is changed to a postmenopausal one, her risk for many chronic conditions is increased. So if we were to reinstate a premenopausal hormonal milieu, that risk would probably be reduced. It makes good sense to target an empiric goal of 80 to 120 pg per mL of estradiol, which is the average estradiol level in a premenopausal woman. If you were to ask me, however, are there randomized, controlled trial data to support this practice—that is, if you target that level, can you make sure that the risk of diabetes is lower or that the risk of heart disease is lower—that study has yet to be done, and it may not ever be done on a large scale. However, it intuitively makes good sense to target premenopausal estradiol levels.
Dr. Faubion: When might you check an estradiol level in this population? For example, if you are treating a patient with a 0.1-mg estradiol patch and she still has significant hot flashes, would it be useful to check the level?
Dr. Kapoor: It would. In my practice, I check estradiol levels on these patients on an annual basis, regardless of symptoms, but definitely in the patient who has symptoms. It makes good sense, because sometimes these patients don’t absorb the estrogen well, particularly if administered by the transdermal route.
A general rule of thumb is that in the average population, if a patient is on the 0.1-mg patch, for example, you would expect her level to be around 100. If it is much lower than that, which sometimes happens, that speaks for poor absorption. Options at that point would be to treat her with a higher dose patch, depending on what the level is, or switch to a different formulation, such as oral.
In instances in which I have treated patients with a 0.1-mg patch for example, and their estradiol levels are undetectable, that speaks for very poor absorption. For such patients I make a case for switching them to oral therapy. Most definitely that makes sense in a patient who is symptomatic despite treatment. But even for patients who don’t have symptoms, I like to target that level, acknowledging that there is no evidence as such to support this practice.
Dr. Faubion: Dr. Kaunitz, do you want to add anything?
Dr. Kaunitz: Yes, a few practical points. Although patches are available in a wider array of doses than oral estrogen formulations, the highest dose available is 0.1 mg. It’s important for clinicians to recognize that while checking serum levels when indicated can be performed in women using transdermal estradiol or patches, in women who are using oral estrogen, checking blood levels is not going to work well because serum estrogen levels have a daily peak and valley in women who use oral versus transdermal estradiol.
I also wanted to talk about progestins. Although many patients who have had a BSO prior to spontaneous menopause also have had a hysterectomy, others have an intact uterus associated with their BSO, so progestins must be used along with estrogen. And if we are using higher-than-standard doses of estrogen, we also need to use higher-than-standard doses of progestin.
In that classic 53-year-old woman I referred to who had spontaneous normal menopause, if she is taking 1 mg of estradiol daily, or a 0.05-mg patch, or 0.625 mg of conjugated equine estrogen, 2.5 mg of medroxyprogesterone is fine. In fact, that showed excellent progestational protection of the endometrium in the Women’s Health Initiative and in other studies.
However, if we are going to use double the estrogen dose, we should increase the progestin dose too. In some of my patients on higher estrogen doses who have an intact uterus, I’ll use 5 or even 10 mg of daily medroxyprogesterone acetate to ensure adequate progestational suppression.
Dr. Faubion: Another practical tip is that if one is using conjugated equine estrogens, measuring the serum estradiol levels is not useful either.
Dr. Kaunitz: I agree.
Continue to: Oral contraceptives as replacement HT...
Oral contraceptives as replacement HT
Dr. Faubion: Would you comment on use of a birth control pill in this circumstance? Would it be optimal to use a postmenopausal HT regimen as opposed to a birth control pill or combined hormonal contraception?
Dr. Kapoor: In this younger population, sometimes it seems like a more socially acceptable decision to be on a birth control option than on menopausal HT. But there are some issues with being on a contraceptive regimen. One is that we end up using estrogen doses much higher than what is really needed for replacement purposes. It is also a nonphysiologic way of replacement in another sense—as opposed to estradiol, which is the main hormone made by the ovaries, the hormonal contraceptive regimens contain the synthetic estrogen ethinyl estradiol for the most part.
The other issue that is based on some weak evidence is that it appears that the bone health outcomes are probably inferior with combined hormonal contraception. For these reasons, regimens that are based on replacement doses of estradiol are preferred.
Dr. Faubion: Right, although the data are somewhat weak, I agree that thus far it seems optimal to utilize a postmenopausal regimen for various reasons. Dr. Kaunitz, anything to add?
Dr. Kaunitz: Yes, to underscore Dr. Kapoor’s point, a common oral contraceptive that contains 20 µg of ethinyl estradiol is substantially more estrogenic than 1.0 or 2.0 mg of micronized oral estradiol.
Also consider that a 20-µg ethinyl estradiol oral contraceptive may increase the risk of venous thromboembolism more than menopausal doses of oral estradiol, whether it be a micronized estradiol or conjugated equine estrogen.
Dr. Faubion: So the risk may be greater with oral combined hormonal contraception as well?
Dr. Kaunitz: One thing we can do is explain to our patients that their ovaries, prior to surgery or prior to induced menopause, were making substantial quantities of estradiol. Whether we prescribe a patch or oral micronized estradiol, this estrogen is identical to the hormone that their ovaries were making prior to surgery or induced menopause.
Breast cancer concerns
Dr. Faubion: Let’s consider a more complicated case. A 35-year-old woman has an identified BRCA1 mutation; she has not had any cancers but has undergone risk-reducing BSO and her uterus remains. Is this woman a candidate for HT? At what dose, and for how long? Dr. Kaunitz, why don’t you start.
Dr. Kaunitz: That is a challenging case but one that I think our readers will find interesting and maybe even provocative.
We know that women with BRCA1 mutations, the more common of the 2 BRCA mutations, have a very high risk of developing epithelial ovarian cancer at a young age. For this reason, our colleagues in medical oncology who specialize in hereditary ovarian/breast cancer syndromes recommend prophylactic risk-reducing—and I would also say lifesaving—BSO with or without hysterectomy for women with BRCA1 mutations.
However, over the years there has been tremendous reluctance among physicians caring for BRCA patients and the women themselves—I use the term “previvors” to describe BRCA carriers who have not been diagnosed with breast or ovarian cancer—to use HT after BSO because of concerns that HT might increase breast cancer risk in women who are already at high risk for breast cancer.
I assume, Dr. Faubion, that in this case the woman had gynecologic surgery but continues to have intact breasts. Is that correct?
Dr. Faubion: That is correct.
Dr. Kaunitz: Although the assumption has been that it is not safe to prescribe HT in this setting, in fact, the reported cohort studies that have looked at this issue have not found an elevated risk of breast cancer when replacement estrogen, with or without progestin, is prescribed to BRCA1 previvors with intact breasts.
Given what Dr. Kapoor said regarding the morbidity that is associated with BSO without replacement of physiologic estrogen, and also given the severe symptoms that so many of these young menopausal women experience, in my practice I do prescribe estrogen or estrogen-progestin therapy and focus on the higher target doses that we discussed for the earlier case patient who had a hysterectomy for abnormal uterine bleeding with adenomyosis.
Dr. Faubion: Dr. Kapoor, do you agree with this approach? How long would you continue therapy?
Dr. Kapoor: First, in this BRCA1 case we need to appreciate that the indication for the BSO is a legitimate one, in contrast to the first case in which the ovaries were removed in a patient whose average risk of ovarian cancer was low. It is important to recognize that surgery performed in this context is the right thing to do because it does significantly reduce the risk of ovarian cancer.
The second thing to appreciate is that while we reduce the risk of ovarian cancer significantly and make sure that these patients survive longer, it’s striking a fine balance in that you want to make sure that their morbidity is not increased as a result of premature estrogen deprivation.
As Dr. Kaunitz told us, the evidence that we have so far, which granted is not very robust but is fairly strong observational evidence, suggests that the risk of breast cancer is not elevated when these patients are treated with replacement doses of HT.
Having said that, I do have very strong discussions with my patients in this category about having the risk-reducing bilateral mastectomy also, because if they were to get breast cancer because of their increased genetic predisposition, the cancer is likely to grow faster if the patient is on HT. So one of my counseling points to patients is that they strongly consider bilateral mastectomy, which reduces their breast cancer risk by more than 90%. At the same time, I also strongly endorse using HT in replacement doses for the reasons that we have already stated.
Dr. Faubion: Continue HT until age 50 or 52?
Dr. Kapoor: Definitely until that age, and possibly longer, depending on their symptoms. The indications for treating beyond the age of natural menopause are much the same as for women who experience natural menopause.
Dr. Faubion: That is assuming they had a bilateral mastectomy?
Dr. Kapoor: Yes.
Continue to: Continuing HT until the age of natural menopause...
Continuing HT until the age of natural menopause
Dr. Kaunitz: Dr. Kapoor brings up the important point of duration of systemic HT. I agree that similar considerations apply both to the healthy 41-year-old who had a hysterectomy for abnormal uterine bleeding and to the 35-year-old who had risk-reducing surgery because of her BRCA1 mutation.
In the 2 cases, both to treat symptoms and to prevent chronic diseases, it makes sense to continue HT at least until the age of natural menopause. That is consistent with 2017 guidance from The North American Menopause Society (NAMS) position statement on the use of systemic HT, that is, continuing systemic HT at least until the age of natural menopause.3 Then at that point, continuing or discontinuing systemic HT becomes discretionary, and that would be true for both cases. If the patient is slender or has a strong family history of osteoporosis, that tends to push the patient more in terms of continuing systemic HT. Those are just some examples, and Dr. Kapoor may want to detail other relevant considerations.
Dr. Kapoor: I completely agree. The decision is driven by symptoms that are not otherwise well managed, for example, with nonhormone strategies. If we have any concerns utilizing HT beyond the age of natural menopause, then nonhormonal options can be considered; but sometimes those are not as effective. And bone health is very important. You want to avoid using bisphosphonates in younger women and reserve them for older patients in their late 60s and 70s. Hormone therapy use is a very reasonable strategy to prevent bone loss.
Dr. Kaunitz: It is also worth mentioning that sometimes the woman involved in shared decision making with her clinician decides to stop systemic HT. In that setting, should the patient start developing new-onset dyspareunia, vaginal dryness, or other genital or sexuality-related concerns, it takes very little for me to advise that she start low-dose local vaginal estrogen therapy.
Dr. Faubion: In either scenario, if a woman were to develop symptoms consistent with genitourinary syndrome of menopause (GSM), would you use vaginal estrogen in addition to the systemic estrogen or alone after the woman elected to discontinue systemic therapy?
Dr. Kapoor: Yes to both, I would say.
Dr. Kaunitz: As my patients using systemic HT age, often I will lower the dose. For instance, the dose I use in a 53-year-old will be higher than when she is 59 or 62. At the same time, as we lower the dose of systemic estrogen therapy, symptoms of vaginal atrophy or GSM often will appear, and these can be effectively treated by adding low-dose vaginal estrogen therapy. A number of my patients, particularly those who are on lower-than-standard doses of systemic HT, are also using low-dose vaginal estrogen therapy.
There is a “hybrid” product available: the 90-day estradiol vaginal ring. Estring is a low-dose, 2-mg, 90-day estradiol ring that is very useful, but it is effective only for treating GSM or vaginal atrophy. A second menopausal vaginal estradiol ring, Femring, is available in 2 doses: 0.05 mg/day and 0.1 mg/day. These are very effective in treating both systemic issues, such as vasomotor symptoms or prevention of osteoporosis, and very effective in treating GSM or vaginal atrophy. One problem is that Femring, depending on insurance coverage, can be very expensive. It’s not available as a generic, so for insurance or financial reasons I don’t often prescribe it. If I could remove those financial barriers, I would prescribe Femring more often because it is very useful.
Dr. Faubion: You raise an important point, and that is, for women who have been on HT for some time, clinicians often feel the need to slowly reduce the dose. Would you do that same thing, Dr. Kapoor, for a 40-year-old woman? Would you reduce the dose as she approaches age 50? Is there pressure that “she shouldn’t be on that much estrogen”?
Dr. Kapoor: No, I would not feel pressured until the patient turns at least 46. I bring up age 46 because the average age range for menopause is 46 to 55. After that, if there is any concern, we can decrease the dose to half and keep the patient on that until she turns 50 or 51. But most of my patients are on replacement doses until the average age of menopause, which is around 51 years, and that’s when you reduce the dose to that of the typical HT regimens used after natural menopause.
Sometimes patients are told something by a friend or they have read something and they worry about the risk of 2 things. One is breast cancer and the other is venous thromboembolism (VTE), and that may be why they want to be on a lower dose. I counsel patients that while the risk of VTE is real with HT, it is the women after natural menopause who are at risk—because age itself is a risk for VTE—and it also has to do with the kind of HT regimen that a patient is on. High doses of oral estrogens and certain progestogens increase the risk. But again, for estradiol used in replacement doses and the more common progestogens that we now use in practice, such as micronized progesterone, the risk is not the same. The same goes for breast cancer. My biggest message to patients and clinicians who take care of these patients is that the rules that apply to women after natural menopause just do not apply to this very different patient population.
Dr. Faubion: Thank you, Dr. Kaunitz and Dr. Kapoor, for sharing your knowledge and experience. ●
Systemic HT past the age of 65
Dr. Kaunitz: Another practical issue relates to long-term or extended use of systemic HT. It’s not infrequent in my practice to receive mail and faxes from insurance carriers of systemic HT users who are age 65 and older in which the company refers to the American Geriatrics Society’s Beers criteria for potentially inappropriate medication use in older adults,1 suggesting that systemic HT is inappropriate for all women over age 65. In this age group, I use lower doses if I am continuing systemic HT. But the good news is that both NAMS and the American College Obstetricians and Gynecologists indicate that arbitrarily stopping systemic HT at age 65 or for any other arbitrary reason is inappropriate, and that decisions about continuing or discontinuing therapy should be made on an individualized basis using shared decision making. That’s an important message for our readers.
Counseling regarding elective BSO
Dr. Faubion: One final note about elective BSO in the absence of a genetic mutation that predisposes to increased ovarian or breast cancer risk. Fortunately, we have seen rates of oophorectomy before the age of natural menopause decline, but what would your advice be to women or clinicians of these women who say they are “just afraid of ovarian cancer and would like to have their ovaries removed before the age of natural menopause”?
Dr. Kaunitz: If patients have increased anxiety about ovarian cancer and yet they themselves are not known to be at elevated risk, I emphasize that, fortunately, ovarian cancer is uncommon. It is much less common than other cancers the patient might be familiar with, such as breast or colon or lung cancer. I also emphasize that women who have given birth, particularly multiple times; women who nursed their infants; and women who have used combination hormonal contraceptives, particularly if long term, are at markedly lower risk for ovarian cancer as they get older. We are talking about an uncommon cancer that is even less common if women have given birth, nursed their infants, or used combination contraceptives long term.
Dr. Faubion: Dr. Kapoor, what would you say regarding the increased risk they might incur if they do have their ovaries out?
Dr. Kapoor: As Dr. Kaunitz said, this is an uncommon cancer, and pursuing something to reduce the risk of an uncommon cancer does not benefit the community. That is also my counseling point to patients.
I also talk to them extensively about the risk associated with the ovaries being removed, and I tell them that although we have the option of giving them HT, it is hard to replicate the magic of nature. No matter what concoction or regimen we use, we cannot ensure reinstating health to what it was in the premenopausal state, because estrogen has such myriad effects on the body in so many different organ systems.
Reference
1. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
- North American Menopause Society. The 2017 hormone therapy position statement of The North American Menopause Society. J North Am Menopause Soc. 2017;24: 728-753.

Women who undergo bilateral salpingo-oophorectomy (BSO) for various indications prior to menopause experience a rapid decline in ovarian hormone levels and consequent vasomotor and other menopausal symptoms. In addition, the resulting estrogen deprivation is associated with such long-term adverse outcomes as osteoporosis and cardiovascular morbidity.
OBG M
Surgical vs natural menopause
Stephanie Faubion, MD, MBA, NCMP: Since the Women’s Health Initiative study was published in 2002,2 many clinicians have been fearful of using systemic HT in menopausal women, and HT use has declined dramatically such that only about 4% to 6% of menopausal women are now receiving systemic HT. Importantly, however, a group of younger menopausal women also are not receiving HT, and that is women who undergo BSO before they reach the average age of menopause, which in the United States is about age 52; this is sometimes referred to as surgical menopause or early surgical menopause. Early surgical menopause has different connotations for long-term health risks than natural menopause at the average age, and we are here to discuss these health effects and their management.
My name is Stephanie Faubion, and I am a women’s health internist and the Chair of the Department of Medicine at Mayo Clinic in Jacksonville, Florida, and Director of Mayo Clinic Women’s Health. I am here with 2 of my esteemed colleagues, Dr. Andrew Kaunitz and Dr. Ekta Kapoor.
Andrew M. Kaunitz, MD, NCMP: Hello, I am an ObGyn with the University of Florida College of Medicine in Jacksonville, with particular interests in contraception, menopause, and gynecologic ultrasonography.
Ekta Kapoor, MBBS, NCMP: And I am an endocrinologist at Mayo Clinic in Rochester with a specific interest in menopause and hormone therapy. I am also the Assistant Director for Mayo Clinic Women’s Health.
Higher-than-standard estrogen doses needed in younger menopausal women
Dr. Faubion: Let’s consider a couple of cases so that we can illustrate some important points regarding hormone management in women who have undergone BSO before the age of natural menopause.
Our first case patient is a woman who is 41 years of age and, because of adenomyosis, she will undergo a hysterectomy. She tells her clinician that she is very concerned about ovarian cancer risk because one of her good friends recently was diagnosed with ovarian cancer, and together they decide to remove her ovaries at the time of hysterectomy. Notably, her ovaries were healthy.
The patient is now menopausal postsurgery, and she is having significant hot flashes and night sweats. She visits her local internist, who is concerned about initiating HT. She is otherwise a healthy woman and does not have any contraindications to HT. Dr. Kaunitz, what would you tell her internist?
Dr. Kaunitz: We are dealing with 2 different issues in terms of decision making about systemic HT for this 41-year-old who has undergone BSO. First, as you mentioned, Dr. Faubion, she has bothersome hot flashes, or vasomotor symptoms. Unless there are contraindications, systemic HT would be appropriate. Although I might start treatment at standard doses, and the accompanying TABLE depicts standard doses for the 2 most common oral estrogen formulations as well as transdermal estradiol, it’s important to recognize that younger menopausal women often will need to use higher-than-standard doses.

For example, for a 53-year-old woman who has been menopausal for a year or 2 and now has bothersome symptoms, I might start her on estradiol 1 mg tablets with progestin if a uterus is present. However, in this 41-year-old case patient, while I might start treatment at a standard dose, I would anticipate increasing to higher doses, such as 1.5 or 2 mg of daily estradiol until she feels her menopausal symptoms are adequately addressed.
Dr. Faubion: It is important to note that sometimes women with early BSO tend to have more severe vasomotor symptoms. Do you find that sometimes a higher dose is required just to manage symptoms, Dr. Kaunitz?
Dr. Kaunitz: Absolutely, yes. The decision whether or not to use systemic HT might be considered discretionary or elective in the classic 53-year-old woman recently menopausal with hot flashes, a so-called spontaneously or naturally menopausal woman. But my perspective is that unless there are clear contraindications, the decision to start systemic HT in the 41-year-old BSO case patient is actually not discretionary. Unless contraindications are present, it is important not only to treat symptoms but also to prevent an array of chronic major health concerns that are more likely if we don’t prescribe systemic HT.
Continue to: Health effects of not using HT...
Health effects of not using HT
Dr. Faubion: Dr. Kapoor, can you describe the potential long-term adverse health consequences of not using estrogen therapy? Say the same 41-year-old woman does not have many bothersome symptoms. What would you do?
Dr. Kapoor: Thank you for that important question. Building on what Dr. Kaunitz said, in these patients there are really 2 issues that can seem to be independent but are not: The first relates to the immediate consequences of lack of estrogen, ie, the menopause-related symptoms, but the second and perhaps the bigger issue is the long-term risk associated with estrogen deprivation.
The symptoms in these women are often obvious as they can be quite severe and abrupt; one day these women have normal hormone levels and the next day, after BSO, suddenly their hormones are very low. So if symptoms occur, they are usually hard to miss, simply because they are very drastic and very severe.
Historically, patients and their clinicians have targeted these symptoms. Patients experience menopausal symptoms, they seek treatment, and then the clinicians basically titrate the treatment to manage these symptoms. That misses the bigger issue, however, which is that premature estrogen deprivation leads to a host of chronic health conditions, as Dr. Kaunitz mentioned. These mainly include increased risk for cardiovascular disease, diabetes, hypertension, dyslipidemia, increased risk of mortality, dementia, and osteoporosis.
Fairly strong observational evidence suggests that use of estrogen therapy given in replacement doses—doses higher than those typically used in women after natural menopause, therefore considered replacement doses—helps mitigate the risk of some of these adverse health conditions.
In these women, the bigger goal really is to reinstate the hormonal milieu that exists prior to menopause. To your point, Dr. Faubion, if I have a patient who is younger than 46 years, who has her ovaries taken out, and even if she has zero symptoms (and sometimes that does happen), I would still make a case for this patient to utilize hormone therapy unless there is a contraindication such as breast cancer or other estrogen-sensitive cancers.
Dr. Faubion: Again, would you aim for those higher doses rather than treat with the “lowest dose”?
Dr. Kapoor: Absolutely. My punchline to the patients and clinicians in these discussions is that the rules of the game are different for these women. We cannot extrapolate the risks and benefits of HT use in women after natural menopause to younger women who have surgical menopause. Those rules just do not apply with respect to both benefits and risks.
Dr. Faubion: I think it’s important to say that these same “rules” would apply if the women were to go through premature menopause for any other reason, too, such as chemotherapy, radiation therapy, or premature ovarian insufficiency for any number of reasons, including toxic, metabolic, or genetic causes and so on. Would that be true?
Dr. Kapoor: Yes, absolutely so.
Dr. Faubion: Dr. Kaunitz, do you want to add anything?
Dr. Kaunitz: In terms of practical or clinical issues regarding systemic HT management, for the woman in her early 50s who has experienced normal or natural spontaneous menopause, a starting dose of transdermal estradiol would be, for instance, a 0.05-mg patch, which is a patch that over 24 hours releases 0.05 mg of estradiol daily; or standard oral estrogen, including conjugated equine estrogen, a 0.625-mg tablet daily, or estradiol, a 1-mg tablet daily.
But in younger patients, we want to use higher doses. For a patch, for instance, I would aim for a 0.075- or 0.1-mg estradiol patch, which releases a higher daily dose of estradiol than the standard dose. For oral estrogen, the dose would be 0.9- or even 1.25-mg tablets of conjugated equine estrogen or 1.5 mg, which is a 1-mg plus a 0.5-mg estradiol tablet, or a 2-mg estradiol tablet. Estradiol does come in a 2-mg strength.
For oral estrogen, I prefer estradiol because it’s available as a generic medication and often available at a very low cost, sometimes as low as $4 a month from chain pharmacies.
Continue to: Usefulness of monitoring estradiol levels for dosage adjustment...
Usefulness of monitoring estradiol levels for dosage adjustment
Dr. Faubion: That’s a great point, and again it is important to emphasize that we are aiming to recreate the premenopausal hormonal milieu. If you were to check estradiol levels, that would be aiming for a premenopausal range of approximately 80 to 120 pg per mL. Dr. Kapoor, is there utility in monitoring estrogen levels?
Dr. Kapoor: Great question, Dr. Faubion, and as you know it’s a loaded one. We base this on empiric evidence. We know that if the hormonal milieu in a young patient is changed to a postmenopausal one, her risk for many chronic conditions is increased. So if we were to reinstate a premenopausal hormonal milieu, that risk would probably be reduced. It makes good sense to target an empiric goal of 80 to 120 pg per mL of estradiol, which is the average estradiol level in a premenopausal woman. If you were to ask me, however, are there randomized, controlled trial data to support this practice—that is, if you target that level, can you make sure that the risk of diabetes is lower or that the risk of heart disease is lower—that study has yet to be done, and it may not ever be done on a large scale. However, it intuitively makes good sense to target premenopausal estradiol levels.
Dr. Faubion: When might you check an estradiol level in this population? For example, if you are treating a patient with a 0.1-mg estradiol patch and she still has significant hot flashes, would it be useful to check the level?
Dr. Kapoor: It would. In my practice, I check estradiol levels on these patients on an annual basis, regardless of symptoms, but definitely in the patient who has symptoms. It makes good sense, because sometimes these patients don’t absorb the estrogen well, particularly if administered by the transdermal route.
A general rule of thumb is that in the average population, if a patient is on the 0.1-mg patch, for example, you would expect her level to be around 100. If it is much lower than that, which sometimes happens, that speaks for poor absorption. Options at that point would be to treat her with a higher dose patch, depending on what the level is, or switch to a different formulation, such as oral.
In instances in which I have treated patients with a 0.1-mg patch for example, and their estradiol levels are undetectable, that speaks for very poor absorption. For such patients I make a case for switching them to oral therapy. Most definitely that makes sense in a patient who is symptomatic despite treatment. But even for patients who don’t have symptoms, I like to target that level, acknowledging that there is no evidence as such to support this practice.
Dr. Faubion: Dr. Kaunitz, do you want to add anything?
Dr. Kaunitz: Yes, a few practical points. Although patches are available in a wider array of doses than oral estrogen formulations, the highest dose available is 0.1 mg. It’s important for clinicians to recognize that while checking serum levels when indicated can be performed in women using transdermal estradiol or patches, in women who are using oral estrogen, checking blood levels is not going to work well because serum estrogen levels have a daily peak and valley in women who use oral versus transdermal estradiol.
I also wanted to talk about progestins. Although many patients who have had a BSO prior to spontaneous menopause also have had a hysterectomy, others have an intact uterus associated with their BSO, so progestins must be used along with estrogen. And if we are using higher-than-standard doses of estrogen, we also need to use higher-than-standard doses of progestin.
In that classic 53-year-old woman I referred to who had spontaneous normal menopause, if she is taking 1 mg of estradiol daily, or a 0.05-mg patch, or 0.625 mg of conjugated equine estrogen, 2.5 mg of medroxyprogesterone is fine. In fact, that showed excellent progestational protection of the endometrium in the Women’s Health Initiative and in other studies.
However, if we are going to use double the estrogen dose, we should increase the progestin dose too. In some of my patients on higher estrogen doses who have an intact uterus, I’ll use 5 or even 10 mg of daily medroxyprogesterone acetate to ensure adequate progestational suppression.
Dr. Faubion: Another practical tip is that if one is using conjugated equine estrogens, measuring the serum estradiol levels is not useful either.
Dr. Kaunitz: I agree.
Continue to: Oral contraceptives as replacement HT...
Oral contraceptives as replacement HT
Dr. Faubion: Would you comment on use of a birth control pill in this circumstance? Would it be optimal to use a postmenopausal HT regimen as opposed to a birth control pill or combined hormonal contraception?
Dr. Kapoor: In this younger population, sometimes it seems like a more socially acceptable decision to be on a birth control option than on menopausal HT. But there are some issues with being on a contraceptive regimen. One is that we end up using estrogen doses much higher than what is really needed for replacement purposes. It is also a nonphysiologic way of replacement in another sense—as opposed to estradiol, which is the main hormone made by the ovaries, the hormonal contraceptive regimens contain the synthetic estrogen ethinyl estradiol for the most part.
The other issue that is based on some weak evidence is that it appears that the bone health outcomes are probably inferior with combined hormonal contraception. For these reasons, regimens that are based on replacement doses of estradiol are preferred.
Dr. Faubion: Right, although the data are somewhat weak, I agree that thus far it seems optimal to utilize a postmenopausal regimen for various reasons. Dr. Kaunitz, anything to add?
Dr. Kaunitz: Yes, to underscore Dr. Kapoor’s point, a common oral contraceptive that contains 20 µg of ethinyl estradiol is substantially more estrogenic than 1.0 or 2.0 mg of micronized oral estradiol.
Also consider that a 20-µg ethinyl estradiol oral contraceptive may increase the risk of venous thromboembolism more than menopausal doses of oral estradiol, whether it be a micronized estradiol or conjugated equine estrogen.
Dr. Faubion: So the risk may be greater with oral combined hormonal contraception as well?
Dr. Kaunitz: One thing we can do is explain to our patients that their ovaries, prior to surgery or prior to induced menopause, were making substantial quantities of estradiol. Whether we prescribe a patch or oral micronized estradiol, this estrogen is identical to the hormone that their ovaries were making prior to surgery or induced menopause.
Breast cancer concerns
Dr. Faubion: Let’s consider a more complicated case. A 35-year-old woman has an identified BRCA1 mutation; she has not had any cancers but has undergone risk-reducing BSO and her uterus remains. Is this woman a candidate for HT? At what dose, and for how long? Dr. Kaunitz, why don’t you start.
Dr. Kaunitz: That is a challenging case but one that I think our readers will find interesting and maybe even provocative.
We know that women with BRCA1 mutations, the more common of the 2 BRCA mutations, have a very high risk of developing epithelial ovarian cancer at a young age. For this reason, our colleagues in medical oncology who specialize in hereditary ovarian/breast cancer syndromes recommend prophylactic risk-reducing—and I would also say lifesaving—BSO with or without hysterectomy for women with BRCA1 mutations.
However, over the years there has been tremendous reluctance among physicians caring for BRCA patients and the women themselves—I use the term “previvors” to describe BRCA carriers who have not been diagnosed with breast or ovarian cancer—to use HT after BSO because of concerns that HT might increase breast cancer risk in women who are already at high risk for breast cancer.
I assume, Dr. Faubion, that in this case the woman had gynecologic surgery but continues to have intact breasts. Is that correct?
Dr. Faubion: That is correct.
Dr. Kaunitz: Although the assumption has been that it is not safe to prescribe HT in this setting, in fact, the reported cohort studies that have looked at this issue have not found an elevated risk of breast cancer when replacement estrogen, with or without progestin, is prescribed to BRCA1 previvors with intact breasts.
Given what Dr. Kapoor said regarding the morbidity that is associated with BSO without replacement of physiologic estrogen, and also given the severe symptoms that so many of these young menopausal women experience, in my practice I do prescribe estrogen or estrogen-progestin therapy and focus on the higher target doses that we discussed for the earlier case patient who had a hysterectomy for abnormal uterine bleeding with adenomyosis.
Dr. Faubion: Dr. Kapoor, do you agree with this approach? How long would you continue therapy?
Dr. Kapoor: First, in this BRCA1 case we need to appreciate that the indication for the BSO is a legitimate one, in contrast to the first case in which the ovaries were removed in a patient whose average risk of ovarian cancer was low. It is important to recognize that surgery performed in this context is the right thing to do because it does significantly reduce the risk of ovarian cancer.
The second thing to appreciate is that while we reduce the risk of ovarian cancer significantly and make sure that these patients survive longer, it’s striking a fine balance in that you want to make sure that their morbidity is not increased as a result of premature estrogen deprivation.
As Dr. Kaunitz told us, the evidence that we have so far, which granted is not very robust but is fairly strong observational evidence, suggests that the risk of breast cancer is not elevated when these patients are treated with replacement doses of HT.
Having said that, I do have very strong discussions with my patients in this category about having the risk-reducing bilateral mastectomy also, because if they were to get breast cancer because of their increased genetic predisposition, the cancer is likely to grow faster if the patient is on HT. So one of my counseling points to patients is that they strongly consider bilateral mastectomy, which reduces their breast cancer risk by more than 90%. At the same time, I also strongly endorse using HT in replacement doses for the reasons that we have already stated.
Dr. Faubion: Continue HT until age 50 or 52?
Dr. Kapoor: Definitely until that age, and possibly longer, depending on their symptoms. The indications for treating beyond the age of natural menopause are much the same as for women who experience natural menopause.
Dr. Faubion: That is assuming they had a bilateral mastectomy?
Dr. Kapoor: Yes.
Continue to: Continuing HT until the age of natural menopause...
Continuing HT until the age of natural menopause
Dr. Kaunitz: Dr. Kapoor brings up the important point of duration of systemic HT. I agree that similar considerations apply both to the healthy 41-year-old who had a hysterectomy for abnormal uterine bleeding and to the 35-year-old who had risk-reducing surgery because of her BRCA1 mutation.
In the 2 cases, both to treat symptoms and to prevent chronic diseases, it makes sense to continue HT at least until the age of natural menopause. That is consistent with 2017 guidance from The North American Menopause Society (NAMS) position statement on the use of systemic HT, that is, continuing systemic HT at least until the age of natural menopause.3 Then at that point, continuing or discontinuing systemic HT becomes discretionary, and that would be true for both cases. If the patient is slender or has a strong family history of osteoporosis, that tends to push the patient more in terms of continuing systemic HT. Those are just some examples, and Dr. Kapoor may want to detail other relevant considerations.
Dr. Kapoor: I completely agree. The decision is driven by symptoms that are not otherwise well managed, for example, with nonhormone strategies. If we have any concerns utilizing HT beyond the age of natural menopause, then nonhormonal options can be considered; but sometimes those are not as effective. And bone health is very important. You want to avoid using bisphosphonates in younger women and reserve them for older patients in their late 60s and 70s. Hormone therapy use is a very reasonable strategy to prevent bone loss.
Dr. Kaunitz: It is also worth mentioning that sometimes the woman involved in shared decision making with her clinician decides to stop systemic HT. In that setting, should the patient start developing new-onset dyspareunia, vaginal dryness, or other genital or sexuality-related concerns, it takes very little for me to advise that she start low-dose local vaginal estrogen therapy.
Dr. Faubion: In either scenario, if a woman were to develop symptoms consistent with genitourinary syndrome of menopause (GSM), would you use vaginal estrogen in addition to the systemic estrogen or alone after the woman elected to discontinue systemic therapy?
Dr. Kapoor: Yes to both, I would say.
Dr. Kaunitz: As my patients using systemic HT age, often I will lower the dose. For instance, the dose I use in a 53-year-old will be higher than when she is 59 or 62. At the same time, as we lower the dose of systemic estrogen therapy, symptoms of vaginal atrophy or GSM often will appear, and these can be effectively treated by adding low-dose vaginal estrogen therapy. A number of my patients, particularly those who are on lower-than-standard doses of systemic HT, are also using low-dose vaginal estrogen therapy.
There is a “hybrid” product available: the 90-day estradiol vaginal ring. Estring is a low-dose, 2-mg, 90-day estradiol ring that is very useful, but it is effective only for treating GSM or vaginal atrophy. A second menopausal vaginal estradiol ring, Femring, is available in 2 doses: 0.05 mg/day and 0.1 mg/day. These are very effective in treating both systemic issues, such as vasomotor symptoms or prevention of osteoporosis, and very effective in treating GSM or vaginal atrophy. One problem is that Femring, depending on insurance coverage, can be very expensive. It’s not available as a generic, so for insurance or financial reasons I don’t often prescribe it. If I could remove those financial barriers, I would prescribe Femring more often because it is very useful.
Dr. Faubion: You raise an important point, and that is, for women who have been on HT for some time, clinicians often feel the need to slowly reduce the dose. Would you do that same thing, Dr. Kapoor, for a 40-year-old woman? Would you reduce the dose as she approaches age 50? Is there pressure that “she shouldn’t be on that much estrogen”?
Dr. Kapoor: No, I would not feel pressured until the patient turns at least 46. I bring up age 46 because the average age range for menopause is 46 to 55. After that, if there is any concern, we can decrease the dose to half and keep the patient on that until she turns 50 or 51. But most of my patients are on replacement doses until the average age of menopause, which is around 51 years, and that’s when you reduce the dose to that of the typical HT regimens used after natural menopause.
Sometimes patients are told something by a friend or they have read something and they worry about the risk of 2 things. One is breast cancer and the other is venous thromboembolism (VTE), and that may be why they want to be on a lower dose. I counsel patients that while the risk of VTE is real with HT, it is the women after natural menopause who are at risk—because age itself is a risk for VTE—and it also has to do with the kind of HT regimen that a patient is on. High doses of oral estrogens and certain progestogens increase the risk. But again, for estradiol used in replacement doses and the more common progestogens that we now use in practice, such as micronized progesterone, the risk is not the same. The same goes for breast cancer. My biggest message to patients and clinicians who take care of these patients is that the rules that apply to women after natural menopause just do not apply to this very different patient population.
Dr. Faubion: Thank you, Dr. Kaunitz and Dr. Kapoor, for sharing your knowledge and experience. ●
Systemic HT past the age of 65
Dr. Kaunitz: Another practical issue relates to long-term or extended use of systemic HT. It’s not infrequent in my practice to receive mail and faxes from insurance carriers of systemic HT users who are age 65 and older in which the company refers to the American Geriatrics Society’s Beers criteria for potentially inappropriate medication use in older adults,1 suggesting that systemic HT is inappropriate for all women over age 65. In this age group, I use lower doses if I am continuing systemic HT. But the good news is that both NAMS and the American College Obstetricians and Gynecologists indicate that arbitrarily stopping systemic HT at age 65 or for any other arbitrary reason is inappropriate, and that decisions about continuing or discontinuing therapy should be made on an individualized basis using shared decision making. That’s an important message for our readers.
Counseling regarding elective BSO
Dr. Faubion: One final note about elective BSO in the absence of a genetic mutation that predisposes to increased ovarian or breast cancer risk. Fortunately, we have seen rates of oophorectomy before the age of natural menopause decline, but what would your advice be to women or clinicians of these women who say they are “just afraid of ovarian cancer and would like to have their ovaries removed before the age of natural menopause”?
Dr. Kaunitz: If patients have increased anxiety about ovarian cancer and yet they themselves are not known to be at elevated risk, I emphasize that, fortunately, ovarian cancer is uncommon. It is much less common than other cancers the patient might be familiar with, such as breast or colon or lung cancer. I also emphasize that women who have given birth, particularly multiple times; women who nursed their infants; and women who have used combination hormonal contraceptives, particularly if long term, are at markedly lower risk for ovarian cancer as they get older. We are talking about an uncommon cancer that is even less common if women have given birth, nursed their infants, or used combination contraceptives long term.
Dr. Faubion: Dr. Kapoor, what would you say regarding the increased risk they might incur if they do have their ovaries out?
Dr. Kapoor: As Dr. Kaunitz said, this is an uncommon cancer, and pursuing something to reduce the risk of an uncommon cancer does not benefit the community. That is also my counseling point to patients.
I also talk to them extensively about the risk associated with the ovaries being removed, and I tell them that although we have the option of giving them HT, it is hard to replicate the magic of nature. No matter what concoction or regimen we use, we cannot ensure reinstating health to what it was in the premenopausal state, because estrogen has such myriad effects on the body in so many different organ systems.
Reference
1. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.

Women who undergo bilateral salpingo-oophorectomy (BSO) for various indications prior to menopause experience a rapid decline in ovarian hormone levels and consequent vasomotor and other menopausal symptoms. In addition, the resulting estrogen deprivation is associated with such long-term adverse outcomes as osteoporosis and cardiovascular morbidity.
OBG M
Surgical vs natural menopause
Stephanie Faubion, MD, MBA, NCMP: Since the Women’s Health Initiative study was published in 2002,2 many clinicians have been fearful of using systemic HT in menopausal women, and HT use has declined dramatically such that only about 4% to 6% of menopausal women are now receiving systemic HT. Importantly, however, a group of younger menopausal women also are not receiving HT, and that is women who undergo BSO before they reach the average age of menopause, which in the United States is about age 52; this is sometimes referred to as surgical menopause or early surgical menopause. Early surgical menopause has different connotations for long-term health risks than natural menopause at the average age, and we are here to discuss these health effects and their management.
My name is Stephanie Faubion, and I am a women’s health internist and the Chair of the Department of Medicine at Mayo Clinic in Jacksonville, Florida, and Director of Mayo Clinic Women’s Health. I am here with 2 of my esteemed colleagues, Dr. Andrew Kaunitz and Dr. Ekta Kapoor.
Andrew M. Kaunitz, MD, NCMP: Hello, I am an ObGyn with the University of Florida College of Medicine in Jacksonville, with particular interests in contraception, menopause, and gynecologic ultrasonography.
Ekta Kapoor, MBBS, NCMP: And I am an endocrinologist at Mayo Clinic in Rochester with a specific interest in menopause and hormone therapy. I am also the Assistant Director for Mayo Clinic Women’s Health.
Higher-than-standard estrogen doses needed in younger menopausal women
Dr. Faubion: Let’s consider a couple of cases so that we can illustrate some important points regarding hormone management in women who have undergone BSO before the age of natural menopause.
Our first case patient is a woman who is 41 years of age and, because of adenomyosis, she will undergo a hysterectomy. She tells her clinician that she is very concerned about ovarian cancer risk because one of her good friends recently was diagnosed with ovarian cancer, and together they decide to remove her ovaries at the time of hysterectomy. Notably, her ovaries were healthy.
The patient is now menopausal postsurgery, and she is having significant hot flashes and night sweats. She visits her local internist, who is concerned about initiating HT. She is otherwise a healthy woman and does not have any contraindications to HT. Dr. Kaunitz, what would you tell her internist?
Dr. Kaunitz: We are dealing with 2 different issues in terms of decision making about systemic HT for this 41-year-old who has undergone BSO. First, as you mentioned, Dr. Faubion, she has bothersome hot flashes, or vasomotor symptoms. Unless there are contraindications, systemic HT would be appropriate. Although I might start treatment at standard doses, and the accompanying TABLE depicts standard doses for the 2 most common oral estrogen formulations as well as transdermal estradiol, it’s important to recognize that younger menopausal women often will need to use higher-than-standard doses.

For example, for a 53-year-old woman who has been menopausal for a year or 2 and now has bothersome symptoms, I might start her on estradiol 1 mg tablets with progestin if a uterus is present. However, in this 41-year-old case patient, while I might start treatment at a standard dose, I would anticipate increasing to higher doses, such as 1.5 or 2 mg of daily estradiol until she feels her menopausal symptoms are adequately addressed.
Dr. Faubion: It is important to note that sometimes women with early BSO tend to have more severe vasomotor symptoms. Do you find that sometimes a higher dose is required just to manage symptoms, Dr. Kaunitz?
Dr. Kaunitz: Absolutely, yes. The decision whether or not to use systemic HT might be considered discretionary or elective in the classic 53-year-old woman recently menopausal with hot flashes, a so-called spontaneously or naturally menopausal woman. But my perspective is that unless there are clear contraindications, the decision to start systemic HT in the 41-year-old BSO case patient is actually not discretionary. Unless contraindications are present, it is important not only to treat symptoms but also to prevent an array of chronic major health concerns that are more likely if we don’t prescribe systemic HT.
Continue to: Health effects of not using HT...
Health effects of not using HT
Dr. Faubion: Dr. Kapoor, can you describe the potential long-term adverse health consequences of not using estrogen therapy? Say the same 41-year-old woman does not have many bothersome symptoms. What would you do?
Dr. Kapoor: Thank you for that important question. Building on what Dr. Kaunitz said, in these patients there are really 2 issues that can seem to be independent but are not: The first relates to the immediate consequences of lack of estrogen, ie, the menopause-related symptoms, but the second and perhaps the bigger issue is the long-term risk associated with estrogen deprivation.
The symptoms in these women are often obvious as they can be quite severe and abrupt; one day these women have normal hormone levels and the next day, after BSO, suddenly their hormones are very low. So if symptoms occur, they are usually hard to miss, simply because they are very drastic and very severe.
Historically, patients and their clinicians have targeted these symptoms. Patients experience menopausal symptoms, they seek treatment, and then the clinicians basically titrate the treatment to manage these symptoms. That misses the bigger issue, however, which is that premature estrogen deprivation leads to a host of chronic health conditions, as Dr. Kaunitz mentioned. These mainly include increased risk for cardiovascular disease, diabetes, hypertension, dyslipidemia, increased risk of mortality, dementia, and osteoporosis.
Fairly strong observational evidence suggests that use of estrogen therapy given in replacement doses—doses higher than those typically used in women after natural menopause, therefore considered replacement doses—helps mitigate the risk of some of these adverse health conditions.
In these women, the bigger goal really is to reinstate the hormonal milieu that exists prior to menopause. To your point, Dr. Faubion, if I have a patient who is younger than 46 years, who has her ovaries taken out, and even if she has zero symptoms (and sometimes that does happen), I would still make a case for this patient to utilize hormone therapy unless there is a contraindication such as breast cancer or other estrogen-sensitive cancers.
Dr. Faubion: Again, would you aim for those higher doses rather than treat with the “lowest dose”?
Dr. Kapoor: Absolutely. My punchline to the patients and clinicians in these discussions is that the rules of the game are different for these women. We cannot extrapolate the risks and benefits of HT use in women after natural menopause to younger women who have surgical menopause. Those rules just do not apply with respect to both benefits and risks.
Dr. Faubion: I think it’s important to say that these same “rules” would apply if the women were to go through premature menopause for any other reason, too, such as chemotherapy, radiation therapy, or premature ovarian insufficiency for any number of reasons, including toxic, metabolic, or genetic causes and so on. Would that be true?
Dr. Kapoor: Yes, absolutely so.
Dr. Faubion: Dr. Kaunitz, do you want to add anything?
Dr. Kaunitz: In terms of practical or clinical issues regarding systemic HT management, for the woman in her early 50s who has experienced normal or natural spontaneous menopause, a starting dose of transdermal estradiol would be, for instance, a 0.05-mg patch, which is a patch that over 24 hours releases 0.05 mg of estradiol daily; or standard oral estrogen, including conjugated equine estrogen, a 0.625-mg tablet daily, or estradiol, a 1-mg tablet daily.
But in younger patients, we want to use higher doses. For a patch, for instance, I would aim for a 0.075- or 0.1-mg estradiol patch, which releases a higher daily dose of estradiol than the standard dose. For oral estrogen, the dose would be 0.9- or even 1.25-mg tablets of conjugated equine estrogen or 1.5 mg, which is a 1-mg plus a 0.5-mg estradiol tablet, or a 2-mg estradiol tablet. Estradiol does come in a 2-mg strength.
For oral estrogen, I prefer estradiol because it’s available as a generic medication and often available at a very low cost, sometimes as low as $4 a month from chain pharmacies.
Continue to: Usefulness of monitoring estradiol levels for dosage adjustment...
Usefulness of monitoring estradiol levels for dosage adjustment
Dr. Faubion: That’s a great point, and again it is important to emphasize that we are aiming to recreate the premenopausal hormonal milieu. If you were to check estradiol levels, that would be aiming for a premenopausal range of approximately 80 to 120 pg per mL. Dr. Kapoor, is there utility in monitoring estrogen levels?
Dr. Kapoor: Great question, Dr. Faubion, and as you know it’s a loaded one. We base this on empiric evidence. We know that if the hormonal milieu in a young patient is changed to a postmenopausal one, her risk for many chronic conditions is increased. So if we were to reinstate a premenopausal hormonal milieu, that risk would probably be reduced. It makes good sense to target an empiric goal of 80 to 120 pg per mL of estradiol, which is the average estradiol level in a premenopausal woman. If you were to ask me, however, are there randomized, controlled trial data to support this practice—that is, if you target that level, can you make sure that the risk of diabetes is lower or that the risk of heart disease is lower—that study has yet to be done, and it may not ever be done on a large scale. However, it intuitively makes good sense to target premenopausal estradiol levels.
Dr. Faubion: When might you check an estradiol level in this population? For example, if you are treating a patient with a 0.1-mg estradiol patch and she still has significant hot flashes, would it be useful to check the level?
Dr. Kapoor: It would. In my practice, I check estradiol levels on these patients on an annual basis, regardless of symptoms, but definitely in the patient who has symptoms. It makes good sense, because sometimes these patients don’t absorb the estrogen well, particularly if administered by the transdermal route.
A general rule of thumb is that in the average population, if a patient is on the 0.1-mg patch, for example, you would expect her level to be around 100. If it is much lower than that, which sometimes happens, that speaks for poor absorption. Options at that point would be to treat her with a higher dose patch, depending on what the level is, or switch to a different formulation, such as oral.
In instances in which I have treated patients with a 0.1-mg patch for example, and their estradiol levels are undetectable, that speaks for very poor absorption. For such patients I make a case for switching them to oral therapy. Most definitely that makes sense in a patient who is symptomatic despite treatment. But even for patients who don’t have symptoms, I like to target that level, acknowledging that there is no evidence as such to support this practice.
Dr. Faubion: Dr. Kaunitz, do you want to add anything?
Dr. Kaunitz: Yes, a few practical points. Although patches are available in a wider array of doses than oral estrogen formulations, the highest dose available is 0.1 mg. It’s important for clinicians to recognize that while checking serum levels when indicated can be performed in women using transdermal estradiol or patches, in women who are using oral estrogen, checking blood levels is not going to work well because serum estrogen levels have a daily peak and valley in women who use oral versus transdermal estradiol.
I also wanted to talk about progestins. Although many patients who have had a BSO prior to spontaneous menopause also have had a hysterectomy, others have an intact uterus associated with their BSO, so progestins must be used along with estrogen. And if we are using higher-than-standard doses of estrogen, we also need to use higher-than-standard doses of progestin.
In that classic 53-year-old woman I referred to who had spontaneous normal menopause, if she is taking 1 mg of estradiol daily, or a 0.05-mg patch, or 0.625 mg of conjugated equine estrogen, 2.5 mg of medroxyprogesterone is fine. In fact, that showed excellent progestational protection of the endometrium in the Women’s Health Initiative and in other studies.
However, if we are going to use double the estrogen dose, we should increase the progestin dose too. In some of my patients on higher estrogen doses who have an intact uterus, I’ll use 5 or even 10 mg of daily medroxyprogesterone acetate to ensure adequate progestational suppression.
Dr. Faubion: Another practical tip is that if one is using conjugated equine estrogens, measuring the serum estradiol levels is not useful either.
Dr. Kaunitz: I agree.
Continue to: Oral contraceptives as replacement HT...
Oral contraceptives as replacement HT
Dr. Faubion: Would you comment on use of a birth control pill in this circumstance? Would it be optimal to use a postmenopausal HT regimen as opposed to a birth control pill or combined hormonal contraception?
Dr. Kapoor: In this younger population, sometimes it seems like a more socially acceptable decision to be on a birth control option than on menopausal HT. But there are some issues with being on a contraceptive regimen. One is that we end up using estrogen doses much higher than what is really needed for replacement purposes. It is also a nonphysiologic way of replacement in another sense—as opposed to estradiol, which is the main hormone made by the ovaries, the hormonal contraceptive regimens contain the synthetic estrogen ethinyl estradiol for the most part.
The other issue that is based on some weak evidence is that it appears that the bone health outcomes are probably inferior with combined hormonal contraception. For these reasons, regimens that are based on replacement doses of estradiol are preferred.
Dr. Faubion: Right, although the data are somewhat weak, I agree that thus far it seems optimal to utilize a postmenopausal regimen for various reasons. Dr. Kaunitz, anything to add?
Dr. Kaunitz: Yes, to underscore Dr. Kapoor’s point, a common oral contraceptive that contains 20 µg of ethinyl estradiol is substantially more estrogenic than 1.0 or 2.0 mg of micronized oral estradiol.
Also consider that a 20-µg ethinyl estradiol oral contraceptive may increase the risk of venous thromboembolism more than menopausal doses of oral estradiol, whether it be a micronized estradiol or conjugated equine estrogen.
Dr. Faubion: So the risk may be greater with oral combined hormonal contraception as well?
Dr. Kaunitz: One thing we can do is explain to our patients that their ovaries, prior to surgery or prior to induced menopause, were making substantial quantities of estradiol. Whether we prescribe a patch or oral micronized estradiol, this estrogen is identical to the hormone that their ovaries were making prior to surgery or induced menopause.
Breast cancer concerns
Dr. Faubion: Let’s consider a more complicated case. A 35-year-old woman has an identified BRCA1 mutation; she has not had any cancers but has undergone risk-reducing BSO and her uterus remains. Is this woman a candidate for HT? At what dose, and for how long? Dr. Kaunitz, why don’t you start.
Dr. Kaunitz: That is a challenging case but one that I think our readers will find interesting and maybe even provocative.
We know that women with BRCA1 mutations, the more common of the 2 BRCA mutations, have a very high risk of developing epithelial ovarian cancer at a young age. For this reason, our colleagues in medical oncology who specialize in hereditary ovarian/breast cancer syndromes recommend prophylactic risk-reducing—and I would also say lifesaving—BSO with or without hysterectomy for women with BRCA1 mutations.
However, over the years there has been tremendous reluctance among physicians caring for BRCA patients and the women themselves—I use the term “previvors” to describe BRCA carriers who have not been diagnosed with breast or ovarian cancer—to use HT after BSO because of concerns that HT might increase breast cancer risk in women who are already at high risk for breast cancer.
I assume, Dr. Faubion, that in this case the woman had gynecologic surgery but continues to have intact breasts. Is that correct?
Dr. Faubion: That is correct.
Dr. Kaunitz: Although the assumption has been that it is not safe to prescribe HT in this setting, in fact, the reported cohort studies that have looked at this issue have not found an elevated risk of breast cancer when replacement estrogen, with or without progestin, is prescribed to BRCA1 previvors with intact breasts.
Given what Dr. Kapoor said regarding the morbidity that is associated with BSO without replacement of physiologic estrogen, and also given the severe symptoms that so many of these young menopausal women experience, in my practice I do prescribe estrogen or estrogen-progestin therapy and focus on the higher target doses that we discussed for the earlier case patient who had a hysterectomy for abnormal uterine bleeding with adenomyosis.
Dr. Faubion: Dr. Kapoor, do you agree with this approach? How long would you continue therapy?
Dr. Kapoor: First, in this BRCA1 case we need to appreciate that the indication for the BSO is a legitimate one, in contrast to the first case in which the ovaries were removed in a patient whose average risk of ovarian cancer was low. It is important to recognize that surgery performed in this context is the right thing to do because it does significantly reduce the risk of ovarian cancer.
The second thing to appreciate is that while we reduce the risk of ovarian cancer significantly and make sure that these patients survive longer, it’s striking a fine balance in that you want to make sure that their morbidity is not increased as a result of premature estrogen deprivation.
As Dr. Kaunitz told us, the evidence that we have so far, which granted is not very robust but is fairly strong observational evidence, suggests that the risk of breast cancer is not elevated when these patients are treated with replacement doses of HT.
Having said that, I do have very strong discussions with my patients in this category about having the risk-reducing bilateral mastectomy also, because if they were to get breast cancer because of their increased genetic predisposition, the cancer is likely to grow faster if the patient is on HT. So one of my counseling points to patients is that they strongly consider bilateral mastectomy, which reduces their breast cancer risk by more than 90%. At the same time, I also strongly endorse using HT in replacement doses for the reasons that we have already stated.
Dr. Faubion: Continue HT until age 50 or 52?
Dr. Kapoor: Definitely until that age, and possibly longer, depending on their symptoms. The indications for treating beyond the age of natural menopause are much the same as for women who experience natural menopause.
Dr. Faubion: That is assuming they had a bilateral mastectomy?
Dr. Kapoor: Yes.
Continue to: Continuing HT until the age of natural menopause...
Continuing HT until the age of natural menopause
Dr. Kaunitz: Dr. Kapoor brings up the important point of duration of systemic HT. I agree that similar considerations apply both to the healthy 41-year-old who had a hysterectomy for abnormal uterine bleeding and to the 35-year-old who had risk-reducing surgery because of her BRCA1 mutation.
In the 2 cases, both to treat symptoms and to prevent chronic diseases, it makes sense to continue HT at least until the age of natural menopause. That is consistent with 2017 guidance from The North American Menopause Society (NAMS) position statement on the use of systemic HT, that is, continuing systemic HT at least until the age of natural menopause.3 Then at that point, continuing or discontinuing systemic HT becomes discretionary, and that would be true for both cases. If the patient is slender or has a strong family history of osteoporosis, that tends to push the patient more in terms of continuing systemic HT. Those are just some examples, and Dr. Kapoor may want to detail other relevant considerations.
Dr. Kapoor: I completely agree. The decision is driven by symptoms that are not otherwise well managed, for example, with nonhormone strategies. If we have any concerns utilizing HT beyond the age of natural menopause, then nonhormonal options can be considered; but sometimes those are not as effective. And bone health is very important. You want to avoid using bisphosphonates in younger women and reserve them for older patients in their late 60s and 70s. Hormone therapy use is a very reasonable strategy to prevent bone loss.
Dr. Kaunitz: It is also worth mentioning that sometimes the woman involved in shared decision making with her clinician decides to stop systemic HT. In that setting, should the patient start developing new-onset dyspareunia, vaginal dryness, or other genital or sexuality-related concerns, it takes very little for me to advise that she start low-dose local vaginal estrogen therapy.
Dr. Faubion: In either scenario, if a woman were to develop symptoms consistent with genitourinary syndrome of menopause (GSM), would you use vaginal estrogen in addition to the systemic estrogen or alone after the woman elected to discontinue systemic therapy?
Dr. Kapoor: Yes to both, I would say.
Dr. Kaunitz: As my patients using systemic HT age, often I will lower the dose. For instance, the dose I use in a 53-year-old will be higher than when she is 59 or 62. At the same time, as we lower the dose of systemic estrogen therapy, symptoms of vaginal atrophy or GSM often will appear, and these can be effectively treated by adding low-dose vaginal estrogen therapy. A number of my patients, particularly those who are on lower-than-standard doses of systemic HT, are also using low-dose vaginal estrogen therapy.
There is a “hybrid” product available: the 90-day estradiol vaginal ring. Estring is a low-dose, 2-mg, 90-day estradiol ring that is very useful, but it is effective only for treating GSM or vaginal atrophy. A second menopausal vaginal estradiol ring, Femring, is available in 2 doses: 0.05 mg/day and 0.1 mg/day. These are very effective in treating both systemic issues, such as vasomotor symptoms or prevention of osteoporosis, and very effective in treating GSM or vaginal atrophy. One problem is that Femring, depending on insurance coverage, can be very expensive. It’s not available as a generic, so for insurance or financial reasons I don’t often prescribe it. If I could remove those financial barriers, I would prescribe Femring more often because it is very useful.
Dr. Faubion: You raise an important point, and that is, for women who have been on HT for some time, clinicians often feel the need to slowly reduce the dose. Would you do that same thing, Dr. Kapoor, for a 40-year-old woman? Would you reduce the dose as she approaches age 50? Is there pressure that “she shouldn’t be on that much estrogen”?
Dr. Kapoor: No, I would not feel pressured until the patient turns at least 46. I bring up age 46 because the average age range for menopause is 46 to 55. After that, if there is any concern, we can decrease the dose to half and keep the patient on that until she turns 50 or 51. But most of my patients are on replacement doses until the average age of menopause, which is around 51 years, and that’s when you reduce the dose to that of the typical HT regimens used after natural menopause.
Sometimes patients are told something by a friend or they have read something and they worry about the risk of 2 things. One is breast cancer and the other is venous thromboembolism (VTE), and that may be why they want to be on a lower dose. I counsel patients that while the risk of VTE is real with HT, it is the women after natural menopause who are at risk—because age itself is a risk for VTE—and it also has to do with the kind of HT regimen that a patient is on. High doses of oral estrogens and certain progestogens increase the risk. But again, for estradiol used in replacement doses and the more common progestogens that we now use in practice, such as micronized progesterone, the risk is not the same. The same goes for breast cancer. My biggest message to patients and clinicians who take care of these patients is that the rules that apply to women after natural menopause just do not apply to this very different patient population.
Dr. Faubion: Thank you, Dr. Kaunitz and Dr. Kapoor, for sharing your knowledge and experience. ●
Systemic HT past the age of 65
Dr. Kaunitz: Another practical issue relates to long-term or extended use of systemic HT. It’s not infrequent in my practice to receive mail and faxes from insurance carriers of systemic HT users who are age 65 and older in which the company refers to the American Geriatrics Society’s Beers criteria for potentially inappropriate medication use in older adults,1 suggesting that systemic HT is inappropriate for all women over age 65. In this age group, I use lower doses if I am continuing systemic HT. But the good news is that both NAMS and the American College Obstetricians and Gynecologists indicate that arbitrarily stopping systemic HT at age 65 or for any other arbitrary reason is inappropriate, and that decisions about continuing or discontinuing therapy should be made on an individualized basis using shared decision making. That’s an important message for our readers.
Counseling regarding elective BSO
Dr. Faubion: One final note about elective BSO in the absence of a genetic mutation that predisposes to increased ovarian or breast cancer risk. Fortunately, we have seen rates of oophorectomy before the age of natural menopause decline, but what would your advice be to women or clinicians of these women who say they are “just afraid of ovarian cancer and would like to have their ovaries removed before the age of natural menopause”?
Dr. Kaunitz: If patients have increased anxiety about ovarian cancer and yet they themselves are not known to be at elevated risk, I emphasize that, fortunately, ovarian cancer is uncommon. It is much less common than other cancers the patient might be familiar with, such as breast or colon or lung cancer. I also emphasize that women who have given birth, particularly multiple times; women who nursed their infants; and women who have used combination hormonal contraceptives, particularly if long term, are at markedly lower risk for ovarian cancer as they get older. We are talking about an uncommon cancer that is even less common if women have given birth, nursed their infants, or used combination contraceptives long term.
Dr. Faubion: Dr. Kapoor, what would you say regarding the increased risk they might incur if they do have their ovaries out?
Dr. Kapoor: As Dr. Kaunitz said, this is an uncommon cancer, and pursuing something to reduce the risk of an uncommon cancer does not benefit the community. That is also my counseling point to patients.
I also talk to them extensively about the risk associated with the ovaries being removed, and I tell them that although we have the option of giving them HT, it is hard to replicate the magic of nature. No matter what concoction or regimen we use, we cannot ensure reinstating health to what it was in the premenopausal state, because estrogen has such myriad effects on the body in so many different organ systems.
Reference
1. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
- North American Menopause Society. The 2017 hormone therapy position statement of The North American Menopause Society. J North Am Menopause Soc. 2017;24: 728-753.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430.
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
- North American Menopause Society. The 2017 hormone therapy position statement of The North American Menopause Society. J North Am Menopause Soc. 2017;24: 728-753.