User login
Gastrointestinal Stromal Tumors: Management of Localized Disease
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumor of the gastrointestinal (GI) tract and arise from the interstitial cells of Cajal of the myenteric plexus. These tumors are rare, with about 1 case per 100,000 persons diagnosed in the United States annually, but may be incidentally discovered in up to 1 in 5 autopsy specimens of older adults.1,2 Epidemiologic risk factors include increasing age, with a peak incidence between age 60 and 65 years, male gender, black race, and non-Hispanic white ethnicity. Germline predisposition can also increase the risk of developing GISTs; molecular drivers of GIST include gain-of-function mutations in the KIT proto-oncogene and platelet-derived growth factor receptor α (PDGFRA) gene, which both encode structurally similar tyrosine kinase receptors; germline mutations of succinate dehydrogenase (SDH) subunit genes; and mutations associated with neurofibromatosis type 1.
GISTs most commonly involve the stomach, followed by the small intestine, but can arise anywhere within the GI tract (esophagus, colon, rectum, and anus). They can also develop outside the GI tract, arising from the mesentery, omentum, and retroperitoneum. The majority of cases are localized or locoregional, whereas about 20% are metastatic at presentation.1 GISTs can occur in children, adolescents, and young adults. Pediatric GISTs represent a distinct subset marked by female predominance and gastric origin, are often multifocal, can sometimes have lymph node involvement, and typically lack mutations in the KIT and PDGFRA genes.
This review is the first of 2 articles focusing on the diagnosis and management of GISTs. Here, we review the evaluation and diagnosis of GISTs along with management of localized disease. Management of advanced disease is reviewed in a separate article.
Case Presentation
A 64-year-old African American man with progressive iron deficiency and abdominal discomfort undergoes upper and lower endoscopy and is found to have a bulging mass within his abdominal cavity. He undergoes a computed tomography (CT) evaluation of the chest, abdomen, and pelvis with contrast, which reveals the presence of a 10-cm gastric mass, with no other lesions identified. He undergoes surgical resection of the mass and presents for review of his pathology and to discuss his treatment plan.
What histopathologic features are consistent with GIST?
What factors are used for risk stratification and to predict likelihood of recurrence?
Clinical Presentation and Diagnosis
Most patients present with symptoms of overt or occult GI bleeding or abdominal discomfort, but a significant proportion of GISTs are discovered incidentally. Lymph node involvement is not typical, except for GISTs occurring in children and/or with rare syndromes. Most syndromic GISTs are multifocal and multicentric. After surgical resection, GISTs usually recur or metastasize within the abdominal cavity, including the omentum, peritoneum, or liver. These tumors rarely spread to the lungs, brain, or bones; when tumor spread does occur, it tends to be in heavily pre-treated patients with advanced disease who have been on multiple lines of therapy for a long duration of time.
The diagnosis usually can be made by histopathology. Specimens can be obtained by endoscopic ultrasound (EUS)– or CT-guided methods, the latter of which carries a very small risk of contamination from percutaneous biopsy. In terms of morphology, GISTs can be spindle cell, epithelioid, or mixed neoplasms. Epithelioid tumors are more commonly seen in the stomach and are often PDGFRA-mutated or SDH-deficient. The differential diagnosis includes other soft-tissue GI wall tumors such as leiomyosarcomas/leiomyomas, germ cell tumors, lymphomas, fibromatosis, and neuroendocrine and neurogenic tumors. A unique feature of GISTs that differentiates them from leiomyomas is near universal expression of CD117 by immunohistochemistry (IHC); this characteristic has allowed pathologists and providers to accurately distinguish true GISTs from other GI mesenchymal tumors.3 Recently, DOG1 (discovered on GIST1) immunoreactivity has been found to be helpful in identifying patients with CD117-negative GISTs. Initially identified through gene expression analysis of GISTs, DOG1 IHC can identify the common mutant c-Kit-driven CD117-positive GISTs as well as the rare CD117-negative GISTs, which are often driven by mutated PDGFRA.4 Importantly, IHC for KIT and DOG1 are not surrogates for mutational status, nor are they predictive of tyrosine kinase inhibitor (TKI) sensitivity. If IHC of a tumor specimen is CD117- and DOG1-negative, the specimen can be sent for KIT and PDGFRA mutational analysis to confirm the diagnosis. If analysis reveals that these genes are wild-type, then IHC staining for SDH B (SDHB) should follow to assess for an SDH-deficient GIST (negative staining).
Risk Stratification for Recurrence
The clinical behavior of GISTs can be variable. Some are indolent, while others behave more aggressively, with a greater malignant potential and a higher propensity to recur and metastasize. Clinical and pathologic features can provide important prognostic information that allows providers to risk-stratify patients. Various institutions have assessed prognostic variables for GISTs. In 2001, the National Institutes of Health (NIH) held a GIST workshop that proposed an approach to estimating metastatic risk based on tumor size and mitotic index (NIH or Fletcher criteria).5 Joensuu et al later proposed a modification of the NIH risk classification to include tumor location and tumor rupture (modified NIH criteria or Joensuu criteria).6-8 Similarly, the Armed Forces Institute of Pathology (AFIP) identified tumor site as a prognostic factor, with gastric GISTs having the best prognosis (AFIP-Miettinen criteria).9-11 Tabular schemes were designed which stratified patients into discrete groups with ranges for mitotic rate and tumor size. Nomograms for ease of use were then constructed utilizing a bimodal mitotic rate and included tumor site and size.12 Finally, contour maps were developed, which have the advantage of evaluating mitotic rate and tumor size as continuous nonlinear variables and also include tumor site and rupture (associated with a high risk of peritoneal metastasis) separately, further improving risk assessment. These contour maps have been validated against pooled data from 10 series (2560 patients).13 High-risk features identified from these studies include tumor location, size, mitotic rate and tumor rupture and are now used for deciding on the use of adjuvant imatinib and as requirements to enter clinical trials assessing adjuvant therapy for resected GISTs.
Case Continued
The patient’s operative and pathology reports indicate that the tumor is a spindle cell neoplasm of the stomach that is positive for CD117, DOG1, and CD34 and negative for smooth muscle actin and S-100, consistent with a diagnosis of GIST. Resection margins are negative. There are 10 mitoses per 50 high-power fields (HPF). Per the operative report, there was no intraoperative or intraperitoneal tumor rupture. Thus, while his GIST was gastric, which generally has a more favorable prognosis, the tumor harbors high-risk features based on its size and mitotic index.
What further testing should be requested?
Molecular Alterations
It is recommended that a mutational analysis be performed as part of the diagnostic work-up of all GISTs.14 Mutational analysis can provide prognostic and predictive information for sensitivity to imatinib and should be considered standard of care. It may also be useful for confirming a GIST diagnosis, or, if negative, lead to further evaluation with an IHC stain for SDHB. The c-Kit receptor is a member of the tyrosine kinase family and, through direct interactions with stem cell factor (SCF), can upregulate the PI3K/AKT/mTOR, Ras/Raf/MEK/ERK, and JAK-STAT pathways, resulting in transcription and translation of genes that enhance cell growth and survival.15 The cell of origin of GISTs, the interstitial cells of Cajal, are dependent on the SCF–c-Kit interaction for development.16 Likewise, the large majority of GISTs (about 70%) are driven by upregulation and constitutive activation of c-Kit, which is normally autoinhibited. About 80% of KIT mutations involve exon 11; these GISTs are most often associated with a gastric location and are associated with a favorable recurrence-free survival (RFS) rate with surgery alone.17 KIT exon 9 mutations are much less common, encompassing only about 10% of GIST KIT mutations, and GISTs with these mutations are more likely to arise from the small bowel.17
About 8% of GISTs harbor gain-of-function PDGFRA driver mutations rendering constitutively active PDGFRA.18 PDGFRA mutations are mutually exclusive from KIT mutations, and PDGFRA-mutated tumors most often occur in the stomach. PDGFRA mutations generally are associated with a lower mitotic rate and gastric location. Identification of the PDGFRA D842V mutation on exon 18, which is the most common, is important, as it is associated with imatinib resistance, and these patients should not be offered imatinib.19
Several other mutations associated with GISTs outside of the KIT and PDGFRA spectrum have been identified. About 10% of GISTs are wildtype for KIT and PDGFRA, and not all KIT/PDGFRA-wildtype GISTs are imatinib-sensitive and/or respond to other TKIs.18 These tumors may harbor aberrations in SDH and NF1, or less commonly, BRAF V600E, FGFR, and NTRK.20,21 SDH subunits B, C and D play a role in the Krebs cycle and electron transport chain. Germline mutations in these SDH subunits can result in the Carney-Stratakis syndrome characterized by the dyad of multifocal GISTs and multicentric paragangliomas.22 This syndrome is most likely to manifest in the pediatric or young adult population. In contradistinction is the Carney triad, which is associated with acquired loss of function of the SDHC gene due to promoter hypermethylation. This syndrome classically occurs in young women and is characterized by an indolent-behaving triad of multicentric GISTs, non-adrenal paragangliomas, and pulmonary chondromas.23 Like PDGFRA D842V–mutated GISTs, SDH-deficient and NF1-associated GISTs are considered imatinib resistant, and these patients should not be offered imatinib therapy.14
Case Continued
The patient’s GIST is found to harbor a KIT exon 11 single codon deletion. He appears anxious and asks to have everything done to prevent his GIST from coming back and to improve his lifespan.
What are the next steps in the management of this patient?
Management
A multidisciplinary team approach to the management of all GISTs is essential and includes input from radiology, gastroenterology, pathology, medical and surgical oncology, nuclear medicine, and nursing.
Surgical Resection
Small esophagogastric and duodenal GISTs ≤ 2 cm can be asymptomatic and managed with serial endoscopic surveillance, typically every 6 to 12 months, with biopsies if the tumors increase in size. GISTs larger than 2 cm require surgical resection, with resection of the full pseudocapsule and an R0 resection, if possible, since larger GISTs carry a higher risk of growth and recurrence. If an R0 resection would lead to significant morbidity or functional sequelae, an R1 may suffice. Rectal GISTs are an exception, where microscopic margins have been shown to be associated with an increased risk of local failure.24 It is important to explore the abdomen thoroughly for peritoneal, rectovaginal, and vesicular implants and metastasis to the liver. A formal lymph node dissection is not necessary because lymph nodes are rarely involved and should only be removed when clinically suspicious. Tumor rupture must be avoided. A laparoscopic approach should only be considered for smaller tumors, since there is a risk of tumor rupture with larger tumors.14
When is adjuvant imatinib indicated?
Adjuvant Imatinib
Among patients with local or locally advanced GISTs, the risk of death from recurrence with surgery alone can be high, with a historical 5-year overall survival (OS) of about 35%.25 As a result, multiple studies have assessed the benefit of adjuvant imatinib, which is now considered standard of care for patients with imatinib-sensitive, high-risk GISTs. In addition to inhibiting BCR-ABL, imatinib mesylate inhibits multiple other receptor tyrosine kinases, including PDGFR, SCF and c-Kit. As a result, imatinib has demonstrated in vitro inhibition of cell proliferation and apoptosis and clinical activity against GISTs expressing CD117.26 Importantly, adjuvant imatinib should only be offered to patients with imatinib-sensitive mutations, such as KIT exon 11 and KIT exon 9 mutations. Adjuvant imatinib should not be offered to patients with imatinib-insensitive mutations such as PDGFR 842V, NF1, or BRAF-related or SDH-deficient GISTs.
The ACOSOG Z9000 was the first study of adjuvant imatinib in patients with resected GISTs.25 This was a single-arm, phase 2 study involving 106 patients with surgically resected GISTs deemed high-risk for recurrence, defined as size > 10 cm, tumor rupture, or up to 4 peritoneal implants. Patients were treated with imatinib 400 mg daily for 1 year. The primary and secondary endpoints were OS and RFS, respectively. Long-term follow-up of this study demonstrated 1-, 3-, and 5-year OS of 99%, 97%, and 83%, and 1-, 3-, and 5-year RFS of 96%, 60%, and 40%, which compared favorably with historical controls. In a multivariable analysis, increasing tumor size, small bowel location, KIT exon 9 mutation, high mitotic rate, and older age were independent risk factors for a poor RFS.25 It is important to note that the benefit of adjuvant imatinib waned after discontinuation of therapy, creating a rationale to study adjuvant imatinib for longer periods of time.
As a result of the promising phase 2 data, ACOSOG opened a phase 3 randomized trial (Z9001) comparing 1 year of adjuvant imatinib to placebo among patients with surgically resected GISTs that were > 3 cm in size and that stained positive for CD117 on pathology. The trial accrued 713 patients and was stopped early at a planned interim analysis, which revealed a 1-year RFS of 98% for imatinib versus 83% for placebo (hazard ratio [HR], 0.35; P < 0.001). The 1-year OS did not differ between the 2 arms (92.2% vs 99.7%; HR, 0.66; P = 0.47).27 When comparing the 2 arms, imatinib was associated with a higher RFS among patients with a KIT exon 11 deletion, but not among patients with other KIT mutation types, PDGFRA mutations, or who were KIT/PDGFRA wildtype.28 Imatinib was granted approval by the US Food and Drug Administration (FDA) for the adjuvant treatment of high-risk GISTs based on the results of the ACOSOG Z9001 trial.
The EORTC 62024 study was a randomized placebo-controlled trial assessing the benefit of 2 years of adjuvant imatinib.29 Patients had to be considered intermediate or high risk per the 2002 NIH consensus classification to be eligible. The trial enrolled 918 patients. The 5-year OS rate, the original primary endpoint, did not differ between the 2 groups (100% vs 99%). The 3-year and 5-year RFS rates, secondary endpoints, were significantly longer among patients treated with imatinib (84% vs 66% and 69% vs 63%, respectively). Again, it was noted that the benefit of imatinib waned over time after treatment discontinuation.
The Scandinavian Sarcoma Group (SSG XVIII) trial was a prospective randomized phase 3 trial that compared 3 years versus 1 year of adjuvant imatinib.30 Patients had to be enrolled within 12 weeks of the postoperative period and had to have GISTs that were CD117-positive and with a high estimated risk of recurrence, per the modified NIH consensus criteria (size > 10 cm, > 10 mitoses per 50 HPF, diameter > 5 cm with mitotic count > 5, or tumor rupture before or at surgery). Three years of adjuvant imatinib was associated with a 54% reduction in the hazard for recurrence at 5 years (65.6% vs 47.9%; HR, 0.46; P < 0.001) and a 55% reduction in the hazard for death at 5 years (OS 92% vs 81.7%; HR, 0.45; P = 0.02). Based on the results of this study, the FDA granted approval for the use of 3 years of adjuvant imatinib in patients with high-risk resected GISTs.
The observation that a longer duration of adjuvant imatinib was associated with superior RFS and OS led to studies to further explore longer durations of adjuvant imatinib. The PERSIST-5 (Postresection Evaluation of Recurrence-free Survival for Gastrointestinal Stromal Tumors With 5 Years of Adjuvant Imatinib) was a multicenter, single-arm, phase 2 prospective study of adjuvant imatinib with a primary endpoint of RFS after 5 years.31 Patients had to have an intermediate or high risk of recurrence, which included GISTs at any site > 2 cm with > 5 mitoses per 50 HPF or nongastric GISTs that were ≥ 5 cm. With 91 patients enrolled, the estimated 5-year RFS was 90% and the OS was 95%. Of note, about half of the patients stopped treatment early due to a variety of reasons, including patient choice or adverse events. Importantly, there were no recurrences in patients with imatinib-sensitive mutations while on therapy. We know that in patients at high risk of relapse, adjuvant imatinib delays recurrence and improves survival, but whether any patients are cured, or their survival curves are just shifted to the right, is unknown. Only longer follow-up of existing studies, and the results of newer trials utilizing longer durations of adjuvant treatment, will help to determine the real value of adjuvant therapy for GIST patients.32 Based on this study, it would be reasonable to discuss a longer duration of imatinib with patients deemed to be at very high risk of recurrence and who are tolerating therapy well. We are awaiting the data from the randomized phase 3 Scandinavian Sarcoma Group XII trial comparing 5 years versus 3 years of adjuvant imatinib therapy, and from the French ImadGIST trial of adjuvant imatinib for 3 versus 6 years. A summary of the aforementioned key adjuvant trials is shown in the Table.

When imatinib is commenced, careful monitoring for treatment toxicities and drug interactions should ensue in order to improve compliance. Dose density should be maintained if possible, as retrospective studies suggest suboptimal plasma levels are associated with a worse outcome.33
When should neoadjuvant imatinib be considered?
Neoadjuvant Imatinib
Neoadjuvant imatinib should be considered for patients requiring total gastrectomy, esophagectomy, or abdominoperineal resection of the rectum in order to reduce tumor size, limit subsequent surgical morbidity, mitigate tumor bleeding and rupture, and aid with organ preservation. Patients with rectal GISTs that may otherwise warrant an abdominoperineal resection should be offered a trial of imatinib in the neoadjuvant setting. There is no evidence for the use of any other TKI aside from imatinib in the neoadjuvant or adjuvant setting. With neoadjuvant imatinib, it is difficult to accurately assess the mitotic rate in the resected tumor specimen.
The RTOG 0132/ACRIN 6665 trial was a prospective phase 2 study evaluating the efficacy of imatinib 600 mg daily in the perioperative setting.34 The trial enrolled 50 patients, 30 with primary GISTs (group A) and 22 with recurrent metastatic GISTs (group B). Based on data from the metastatic setting revealing a time to treatment response of about 2.5 months, patients were treated with 8 to 12 weeks of preoperative imatinib followed by 2 years of adjuvant imatinib. Imatinib was stopped 24 hours preoperatively and resumed as soon as possible postoperatively. In group A, 7% of patients achieved a partial response (PR), 83% achieved stable disease, and 2-year progression-free survival (PFS) and OS were 83% and 93%, respectively. In group B, 4.5% of patients achieved a PR, 91% achieved stable disease, and 4.5% experienced progressive disease in the preoperative period; the 2-year PFS and OS were 77% and 91%, respectively. The results of this trial demonstrated the feasibility of using perioperative imatinib with minimal effects on surgical outcomes and set the rationale to use neoadjuvant imatinib in select patients with borderline resectable or rectal GISTs. Another EORTC pooled analysis from 10 sarcoma centers revealed that after a median of 10 months of neoadjuvant imatinib, 83.2% of patients achieved an R0 resection and only 1% progressed during treatment.35 After a median follow-up of 46 months, the 5-year disease-free survival and OS were 65% and 87%, respectively.
Mutational testing should be performed beforehand to ensure the tumor is imatinib-sensitive. If a KIT exon 9 mutation is identified, then 400 mg twice daily should be considered (given the benefit seen with 800 mg imatinib for advanced GIST patients), although there are no studies to confirm this practice. Neoadjuvant imatinib is recommended for a total of 6 to 12 months to ensure maximal tumor debulking, but with very close monitoring and surgical input for disease resistance and growth.14 Imatinib should be stopped 1 to 2 days preoperatively and resumed once the patient has recovered from surgery for a total of 3 years (pre-/postoperatively combined). Neoadjuvant therapy has been shown to be safe and effective, but there have been no randomized trials to assess survival.
What is appropriate surveillance for resected GISTs?
Surveillance
There have been no randomized studies to guide the management of surveillance after surgical resection and adjuvant therapy. There is no known optimal follow-up schedule, but several have been proposed.13,36 Among high-risk patients, it is suggested to image every 3 to 6 months during adjuvant therapy, followed by every 3 months for 2 years after discontinuing therapy, then every 6 months for another 3 years and annually thereafter for an additional 5 years. High-risk patients usually relapse within 1 to 3 years after finishing adjuvant therapy, while low-risk patients can relapse later given that their disease can be slower growing. It has been recommended that low-risk patients undergo imaging every 6 months for 5 years, with follow-up individualized thereafter. Very-low-risk patients may not require more than annual imaging. Because most relapses occur within the peritoneum or liver, imaging should encompass the abdomen and pelvis. Surveillance imaging usually consists of CT scans of the abdomen and pelvis. MRI scans can be utilized for patients at lower risk or who are out several years in order to avoid excess radiation exposure. MRI is also specifically helpful for rectal and esophageal lesions. Chest CT or chest radiograph and bone scan are not routinely required for follow-up.
Case Conclusion
The patient receives adjuvant imatinib and experiences grade 2 myalgias, periorbital edema, and macrocytic anemia, which result in imatinib discontinuation after 3 years of treatment. He is seen every 3 to 6 months and a contrast CT abdomen and pelvis is obtained every 6 months for 5 years. During this 5-year follow-up period, he does not have any clinical or radiographic evidence of disease recurrence.
Further follow-up of this patient is presented in the second article in this 2-part review of management of GISTs.
Key Points
- GISTs are the most common mesenchymal neoplasms of the GI tract and can occasionally occur in extragastrointestinal locations as well.
- GISTs encompass a heterogeneous family of tumor subsets with different natural histories, mutations, and TKI responsiveness.
- Surgery is the mainstay of treatment for localized GISTs, with cure rates greater than 50%.
- For very small (< 2 cm) esophagogastric GISTs, endoscopic ultrasound evaluation and follow-up is recommended.
- For tumors ≥ 2 cm, biopsy and excision is the standard approach.
- For localized GISTs, complete surgical resection (R0) is standard treatment, with no lymphadenectomy for clinically negative lymph nodes.
- Mutational analysis should be considered standard of practice. It can be helpful for confirming the diagnosis and can be predictive and prognostic in determining specific TKI therapy and dose.
- Adjuvant imatinib at a dose of 400 mg for 3 years is standard of care for GISTs that are at high risk of relapse and are imatinib-sensitive, and it is the only TKI approved for adjuvant therapy. Patients with PDGFRA D842V, NF1, BRAF or SDH-deficient GISTs should not receive adjuvant imatinib therapy.
- Neoadjuvant therapy can be utilized for sites where extensive resection would lead to significant morbidity. It should be given for 6 to 12 months, but patients need to be monitored closely for tumor growth.
1. Ma GL, Murphy JD, Martinez ME et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298-302.
2. Agaimy A, Wunsch PH, Hofstaedter F, et al. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol. 2007;31:113-120.
3. Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol. 2000;13:1134-1142.
4. West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutational status. Am J Pathol. 2004;165:107-113.
5. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10:81-89.
6. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419.
7. Hohenberger P, Ronellenfitsch U, Oladeji O, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumor. Br J Surg. 2010;97:1854-1859.
8. Holmenbakk T, Bjerkehagen B, Boye K, et al. Definition and clinical significance of tumor rupture in gastrointestinal stromal tumours of the small intestine. Br J Surg. 2016;103:684-691.
9. Emory TS, Sobin LH, Lukes L, et al. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol. 1999;23:82-87.
10. Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30:477-489.
11. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68.
12. Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localized primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10:1045-1052.
13. Joensuu H, Vehtari A, Rihimaki J et al. Risk of recurrence of gastrointestinal stromal tumor after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274.
14. Casali PG, Abecassis N, Bauer S, et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow up. Ann Oncol. 2018;29(Supplement_4): iv267.
15. Jing L, Yan-Ling W, Bing-Jia C, et al. The c-kit receptor-mediated signal transduction and tumor-related diseases. Int J Biol Sci. 2013;9:435-443.
16. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580.
17. Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol. 2015;33:634-642.
18. Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813-3825.
19. Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342-4349.
20. Huss S, Pasternack H, Ihle MA, et al. Clinicopathological and molecular features of a large cohort of gastrointestinal stromal tumors (GISTs) and review of the literature: BRAF mutations in KIT/PDGFRA wild-type GISTs are rare events. Hum Pathol. 2017;62:206-214.
21. Shi E, Chmielecki J, Tang CM, et al. FGFR1 and NTRK3 actionable alterations in “Wild-Type” gastrointestinal stromal tumors. J Transl Med. 2016;14:339.
22. Carney JA, Stratakis CA. Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet. 2002;108:132-139.
23. Carney JA. Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney Triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc. 1999;74:543-552.
24. Jakob J, Mussi C, Ronellenfitsch U, et al. Gastrointestinal stromal tumor of the rectum: results of surgical and multimodality therapy in the era of imatinib. Ann Surg Oncol. 2013;20:586-592.
25. DeMatteo RP, Ballman KV, Antonescu CR, et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor (GIST): ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg. 2013;258:422-429.
26. Gleevac (imatinib) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals; 2016.
27. DeMatteo RP, Ballman KV, Antonescu CR, et al. Placebo-controlled randomized trial of adjuvant imatinib mesylate following the resection of localized, primary gastrointestinal stromal tumor (GIST). Lancet. 2009;373:1097-1104.
28. Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32:1563-1570.
29. Casali PG, Le Cesne A, Poveda Velasco A, et al. Imatinib failure-free survival (IFS) in patients with localized gastrointestinal stromal tumors (GIST) treated with adjuvant imatinib (IM): the EORTC/AGITG/FSG/GEIS/ISG randomized controlled phase III trial. J Clin Oncol. 2013;31. Abstract 10500.
30. Joensuu H, Eriksson M, Sundby HK, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265-1272.
31. Raut CP, Espat NJ, Maki RG, et al. Efficacy and tolerability of 5-year adjuvant imatinib treatment for patients with resected intermediate- or high-risk primary gastrointestinal stromal tumor: The PERSIST-5 Clinical Trial. JAMA Oncol. 2018: e184060.
32. Benjamin RS, Casali PG. Adjuvant imatinib for GI stromal tumors: when and for how long? J Clin Oncol. 2016;34:215-218.
33. Demetri GD, Wang Y, Wehrle E, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009;27:3141-3147.
34. Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99:42-47.
35. Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol. 2013;20:2937-2943.
36. Joensuu H, Martin-Broto J, Nishida T, et al. Follow-up strategies for patients with gastrointestinal stromal tumour treated with or without adjuvant imatinib after surgery. Eur J Cancer. 2015;51:1611-1617.
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumor of the gastrointestinal (GI) tract and arise from the interstitial cells of Cajal of the myenteric plexus. These tumors are rare, with about 1 case per 100,000 persons diagnosed in the United States annually, but may be incidentally discovered in up to 1 in 5 autopsy specimens of older adults.1,2 Epidemiologic risk factors include increasing age, with a peak incidence between age 60 and 65 years, male gender, black race, and non-Hispanic white ethnicity. Germline predisposition can also increase the risk of developing GISTs; molecular drivers of GIST include gain-of-function mutations in the KIT proto-oncogene and platelet-derived growth factor receptor α (PDGFRA) gene, which both encode structurally similar tyrosine kinase receptors; germline mutations of succinate dehydrogenase (SDH) subunit genes; and mutations associated with neurofibromatosis type 1.
GISTs most commonly involve the stomach, followed by the small intestine, but can arise anywhere within the GI tract (esophagus, colon, rectum, and anus). They can also develop outside the GI tract, arising from the mesentery, omentum, and retroperitoneum. The majority of cases are localized or locoregional, whereas about 20% are metastatic at presentation.1 GISTs can occur in children, adolescents, and young adults. Pediatric GISTs represent a distinct subset marked by female predominance and gastric origin, are often multifocal, can sometimes have lymph node involvement, and typically lack mutations in the KIT and PDGFRA genes.
This review is the first of 2 articles focusing on the diagnosis and management of GISTs. Here, we review the evaluation and diagnosis of GISTs along with management of localized disease. Management of advanced disease is reviewed in a separate article.
Case Presentation
A 64-year-old African American man with progressive iron deficiency and abdominal discomfort undergoes upper and lower endoscopy and is found to have a bulging mass within his abdominal cavity. He undergoes a computed tomography (CT) evaluation of the chest, abdomen, and pelvis with contrast, which reveals the presence of a 10-cm gastric mass, with no other lesions identified. He undergoes surgical resection of the mass and presents for review of his pathology and to discuss his treatment plan.
What histopathologic features are consistent with GIST?
What factors are used for risk stratification and to predict likelihood of recurrence?
Clinical Presentation and Diagnosis
Most patients present with symptoms of overt or occult GI bleeding or abdominal discomfort, but a significant proportion of GISTs are discovered incidentally. Lymph node involvement is not typical, except for GISTs occurring in children and/or with rare syndromes. Most syndromic GISTs are multifocal and multicentric. After surgical resection, GISTs usually recur or metastasize within the abdominal cavity, including the omentum, peritoneum, or liver. These tumors rarely spread to the lungs, brain, or bones; when tumor spread does occur, it tends to be in heavily pre-treated patients with advanced disease who have been on multiple lines of therapy for a long duration of time.
The diagnosis usually can be made by histopathology. Specimens can be obtained by endoscopic ultrasound (EUS)– or CT-guided methods, the latter of which carries a very small risk of contamination from percutaneous biopsy. In terms of morphology, GISTs can be spindle cell, epithelioid, or mixed neoplasms. Epithelioid tumors are more commonly seen in the stomach and are often PDGFRA-mutated or SDH-deficient. The differential diagnosis includes other soft-tissue GI wall tumors such as leiomyosarcomas/leiomyomas, germ cell tumors, lymphomas, fibromatosis, and neuroendocrine and neurogenic tumors. A unique feature of GISTs that differentiates them from leiomyomas is near universal expression of CD117 by immunohistochemistry (IHC); this characteristic has allowed pathologists and providers to accurately distinguish true GISTs from other GI mesenchymal tumors.3 Recently, DOG1 (discovered on GIST1) immunoreactivity has been found to be helpful in identifying patients with CD117-negative GISTs. Initially identified through gene expression analysis of GISTs, DOG1 IHC can identify the common mutant c-Kit-driven CD117-positive GISTs as well as the rare CD117-negative GISTs, which are often driven by mutated PDGFRA.4 Importantly, IHC for KIT and DOG1 are not surrogates for mutational status, nor are they predictive of tyrosine kinase inhibitor (TKI) sensitivity. If IHC of a tumor specimen is CD117- and DOG1-negative, the specimen can be sent for KIT and PDGFRA mutational analysis to confirm the diagnosis. If analysis reveals that these genes are wild-type, then IHC staining for SDH B (SDHB) should follow to assess for an SDH-deficient GIST (negative staining).
Risk Stratification for Recurrence
The clinical behavior of GISTs can be variable. Some are indolent, while others behave more aggressively, with a greater malignant potential and a higher propensity to recur and metastasize. Clinical and pathologic features can provide important prognostic information that allows providers to risk-stratify patients. Various institutions have assessed prognostic variables for GISTs. In 2001, the National Institutes of Health (NIH) held a GIST workshop that proposed an approach to estimating metastatic risk based on tumor size and mitotic index (NIH or Fletcher criteria).5 Joensuu et al later proposed a modification of the NIH risk classification to include tumor location and tumor rupture (modified NIH criteria or Joensuu criteria).6-8 Similarly, the Armed Forces Institute of Pathology (AFIP) identified tumor site as a prognostic factor, with gastric GISTs having the best prognosis (AFIP-Miettinen criteria).9-11 Tabular schemes were designed which stratified patients into discrete groups with ranges for mitotic rate and tumor size. Nomograms for ease of use were then constructed utilizing a bimodal mitotic rate and included tumor site and size.12 Finally, contour maps were developed, which have the advantage of evaluating mitotic rate and tumor size as continuous nonlinear variables and also include tumor site and rupture (associated with a high risk of peritoneal metastasis) separately, further improving risk assessment. These contour maps have been validated against pooled data from 10 series (2560 patients).13 High-risk features identified from these studies include tumor location, size, mitotic rate and tumor rupture and are now used for deciding on the use of adjuvant imatinib and as requirements to enter clinical trials assessing adjuvant therapy for resected GISTs.
Case Continued
The patient’s operative and pathology reports indicate that the tumor is a spindle cell neoplasm of the stomach that is positive for CD117, DOG1, and CD34 and negative for smooth muscle actin and S-100, consistent with a diagnosis of GIST. Resection margins are negative. There are 10 mitoses per 50 high-power fields (HPF). Per the operative report, there was no intraoperative or intraperitoneal tumor rupture. Thus, while his GIST was gastric, which generally has a more favorable prognosis, the tumor harbors high-risk features based on its size and mitotic index.
What further testing should be requested?
Molecular Alterations
It is recommended that a mutational analysis be performed as part of the diagnostic work-up of all GISTs.14 Mutational analysis can provide prognostic and predictive information for sensitivity to imatinib and should be considered standard of care. It may also be useful for confirming a GIST diagnosis, or, if negative, lead to further evaluation with an IHC stain for SDHB. The c-Kit receptor is a member of the tyrosine kinase family and, through direct interactions with stem cell factor (SCF), can upregulate the PI3K/AKT/mTOR, Ras/Raf/MEK/ERK, and JAK-STAT pathways, resulting in transcription and translation of genes that enhance cell growth and survival.15 The cell of origin of GISTs, the interstitial cells of Cajal, are dependent on the SCF–c-Kit interaction for development.16 Likewise, the large majority of GISTs (about 70%) are driven by upregulation and constitutive activation of c-Kit, which is normally autoinhibited. About 80% of KIT mutations involve exon 11; these GISTs are most often associated with a gastric location and are associated with a favorable recurrence-free survival (RFS) rate with surgery alone.17 KIT exon 9 mutations are much less common, encompassing only about 10% of GIST KIT mutations, and GISTs with these mutations are more likely to arise from the small bowel.17
About 8% of GISTs harbor gain-of-function PDGFRA driver mutations rendering constitutively active PDGFRA.18 PDGFRA mutations are mutually exclusive from KIT mutations, and PDGFRA-mutated tumors most often occur in the stomach. PDGFRA mutations generally are associated with a lower mitotic rate and gastric location. Identification of the PDGFRA D842V mutation on exon 18, which is the most common, is important, as it is associated with imatinib resistance, and these patients should not be offered imatinib.19
Several other mutations associated with GISTs outside of the KIT and PDGFRA spectrum have been identified. About 10% of GISTs are wildtype for KIT and PDGFRA, and not all KIT/PDGFRA-wildtype GISTs are imatinib-sensitive and/or respond to other TKIs.18 These tumors may harbor aberrations in SDH and NF1, or less commonly, BRAF V600E, FGFR, and NTRK.20,21 SDH subunits B, C and D play a role in the Krebs cycle and electron transport chain. Germline mutations in these SDH subunits can result in the Carney-Stratakis syndrome characterized by the dyad of multifocal GISTs and multicentric paragangliomas.22 This syndrome is most likely to manifest in the pediatric or young adult population. In contradistinction is the Carney triad, which is associated with acquired loss of function of the SDHC gene due to promoter hypermethylation. This syndrome classically occurs in young women and is characterized by an indolent-behaving triad of multicentric GISTs, non-adrenal paragangliomas, and pulmonary chondromas.23 Like PDGFRA D842V–mutated GISTs, SDH-deficient and NF1-associated GISTs are considered imatinib resistant, and these patients should not be offered imatinib therapy.14
Case Continued
The patient’s GIST is found to harbor a KIT exon 11 single codon deletion. He appears anxious and asks to have everything done to prevent his GIST from coming back and to improve his lifespan.
What are the next steps in the management of this patient?
Management
A multidisciplinary team approach to the management of all GISTs is essential and includes input from radiology, gastroenterology, pathology, medical and surgical oncology, nuclear medicine, and nursing.
Surgical Resection
Small esophagogastric and duodenal GISTs ≤ 2 cm can be asymptomatic and managed with serial endoscopic surveillance, typically every 6 to 12 months, with biopsies if the tumors increase in size. GISTs larger than 2 cm require surgical resection, with resection of the full pseudocapsule and an R0 resection, if possible, since larger GISTs carry a higher risk of growth and recurrence. If an R0 resection would lead to significant morbidity or functional sequelae, an R1 may suffice. Rectal GISTs are an exception, where microscopic margins have been shown to be associated with an increased risk of local failure.24 It is important to explore the abdomen thoroughly for peritoneal, rectovaginal, and vesicular implants and metastasis to the liver. A formal lymph node dissection is not necessary because lymph nodes are rarely involved and should only be removed when clinically suspicious. Tumor rupture must be avoided. A laparoscopic approach should only be considered for smaller tumors, since there is a risk of tumor rupture with larger tumors.14
When is adjuvant imatinib indicated?
Adjuvant Imatinib
Among patients with local or locally advanced GISTs, the risk of death from recurrence with surgery alone can be high, with a historical 5-year overall survival (OS) of about 35%.25 As a result, multiple studies have assessed the benefit of adjuvant imatinib, which is now considered standard of care for patients with imatinib-sensitive, high-risk GISTs. In addition to inhibiting BCR-ABL, imatinib mesylate inhibits multiple other receptor tyrosine kinases, including PDGFR, SCF and c-Kit. As a result, imatinib has demonstrated in vitro inhibition of cell proliferation and apoptosis and clinical activity against GISTs expressing CD117.26 Importantly, adjuvant imatinib should only be offered to patients with imatinib-sensitive mutations, such as KIT exon 11 and KIT exon 9 mutations. Adjuvant imatinib should not be offered to patients with imatinib-insensitive mutations such as PDGFR 842V, NF1, or BRAF-related or SDH-deficient GISTs.
The ACOSOG Z9000 was the first study of adjuvant imatinib in patients with resected GISTs.25 This was a single-arm, phase 2 study involving 106 patients with surgically resected GISTs deemed high-risk for recurrence, defined as size > 10 cm, tumor rupture, or up to 4 peritoneal implants. Patients were treated with imatinib 400 mg daily for 1 year. The primary and secondary endpoints were OS and RFS, respectively. Long-term follow-up of this study demonstrated 1-, 3-, and 5-year OS of 99%, 97%, and 83%, and 1-, 3-, and 5-year RFS of 96%, 60%, and 40%, which compared favorably with historical controls. In a multivariable analysis, increasing tumor size, small bowel location, KIT exon 9 mutation, high mitotic rate, and older age were independent risk factors for a poor RFS.25 It is important to note that the benefit of adjuvant imatinib waned after discontinuation of therapy, creating a rationale to study adjuvant imatinib for longer periods of time.
As a result of the promising phase 2 data, ACOSOG opened a phase 3 randomized trial (Z9001) comparing 1 year of adjuvant imatinib to placebo among patients with surgically resected GISTs that were > 3 cm in size and that stained positive for CD117 on pathology. The trial accrued 713 patients and was stopped early at a planned interim analysis, which revealed a 1-year RFS of 98% for imatinib versus 83% for placebo (hazard ratio [HR], 0.35; P < 0.001). The 1-year OS did not differ between the 2 arms (92.2% vs 99.7%; HR, 0.66; P = 0.47).27 When comparing the 2 arms, imatinib was associated with a higher RFS among patients with a KIT exon 11 deletion, but not among patients with other KIT mutation types, PDGFRA mutations, or who were KIT/PDGFRA wildtype.28 Imatinib was granted approval by the US Food and Drug Administration (FDA) for the adjuvant treatment of high-risk GISTs based on the results of the ACOSOG Z9001 trial.
The EORTC 62024 study was a randomized placebo-controlled trial assessing the benefit of 2 years of adjuvant imatinib.29 Patients had to be considered intermediate or high risk per the 2002 NIH consensus classification to be eligible. The trial enrolled 918 patients. The 5-year OS rate, the original primary endpoint, did not differ between the 2 groups (100% vs 99%). The 3-year and 5-year RFS rates, secondary endpoints, were significantly longer among patients treated with imatinib (84% vs 66% and 69% vs 63%, respectively). Again, it was noted that the benefit of imatinib waned over time after treatment discontinuation.
The Scandinavian Sarcoma Group (SSG XVIII) trial was a prospective randomized phase 3 trial that compared 3 years versus 1 year of adjuvant imatinib.30 Patients had to be enrolled within 12 weeks of the postoperative period and had to have GISTs that were CD117-positive and with a high estimated risk of recurrence, per the modified NIH consensus criteria (size > 10 cm, > 10 mitoses per 50 HPF, diameter > 5 cm with mitotic count > 5, or tumor rupture before or at surgery). Three years of adjuvant imatinib was associated with a 54% reduction in the hazard for recurrence at 5 years (65.6% vs 47.9%; HR, 0.46; P < 0.001) and a 55% reduction in the hazard for death at 5 years (OS 92% vs 81.7%; HR, 0.45; P = 0.02). Based on the results of this study, the FDA granted approval for the use of 3 years of adjuvant imatinib in patients with high-risk resected GISTs.
The observation that a longer duration of adjuvant imatinib was associated with superior RFS and OS led to studies to further explore longer durations of adjuvant imatinib. The PERSIST-5 (Postresection Evaluation of Recurrence-free Survival for Gastrointestinal Stromal Tumors With 5 Years of Adjuvant Imatinib) was a multicenter, single-arm, phase 2 prospective study of adjuvant imatinib with a primary endpoint of RFS after 5 years.31 Patients had to have an intermediate or high risk of recurrence, which included GISTs at any site > 2 cm with > 5 mitoses per 50 HPF or nongastric GISTs that were ≥ 5 cm. With 91 patients enrolled, the estimated 5-year RFS was 90% and the OS was 95%. Of note, about half of the patients stopped treatment early due to a variety of reasons, including patient choice or adverse events. Importantly, there were no recurrences in patients with imatinib-sensitive mutations while on therapy. We know that in patients at high risk of relapse, adjuvant imatinib delays recurrence and improves survival, but whether any patients are cured, or their survival curves are just shifted to the right, is unknown. Only longer follow-up of existing studies, and the results of newer trials utilizing longer durations of adjuvant treatment, will help to determine the real value of adjuvant therapy for GIST patients.32 Based on this study, it would be reasonable to discuss a longer duration of imatinib with patients deemed to be at very high risk of recurrence and who are tolerating therapy well. We are awaiting the data from the randomized phase 3 Scandinavian Sarcoma Group XII trial comparing 5 years versus 3 years of adjuvant imatinib therapy, and from the French ImadGIST trial of adjuvant imatinib for 3 versus 6 years. A summary of the aforementioned key adjuvant trials is shown in the Table.

When imatinib is commenced, careful monitoring for treatment toxicities and drug interactions should ensue in order to improve compliance. Dose density should be maintained if possible, as retrospective studies suggest suboptimal plasma levels are associated with a worse outcome.33
When should neoadjuvant imatinib be considered?
Neoadjuvant Imatinib
Neoadjuvant imatinib should be considered for patients requiring total gastrectomy, esophagectomy, or abdominoperineal resection of the rectum in order to reduce tumor size, limit subsequent surgical morbidity, mitigate tumor bleeding and rupture, and aid with organ preservation. Patients with rectal GISTs that may otherwise warrant an abdominoperineal resection should be offered a trial of imatinib in the neoadjuvant setting. There is no evidence for the use of any other TKI aside from imatinib in the neoadjuvant or adjuvant setting. With neoadjuvant imatinib, it is difficult to accurately assess the mitotic rate in the resected tumor specimen.
The RTOG 0132/ACRIN 6665 trial was a prospective phase 2 study evaluating the efficacy of imatinib 600 mg daily in the perioperative setting.34 The trial enrolled 50 patients, 30 with primary GISTs (group A) and 22 with recurrent metastatic GISTs (group B). Based on data from the metastatic setting revealing a time to treatment response of about 2.5 months, patients were treated with 8 to 12 weeks of preoperative imatinib followed by 2 years of adjuvant imatinib. Imatinib was stopped 24 hours preoperatively and resumed as soon as possible postoperatively. In group A, 7% of patients achieved a partial response (PR), 83% achieved stable disease, and 2-year progression-free survival (PFS) and OS were 83% and 93%, respectively. In group B, 4.5% of patients achieved a PR, 91% achieved stable disease, and 4.5% experienced progressive disease in the preoperative period; the 2-year PFS and OS were 77% and 91%, respectively. The results of this trial demonstrated the feasibility of using perioperative imatinib with minimal effects on surgical outcomes and set the rationale to use neoadjuvant imatinib in select patients with borderline resectable or rectal GISTs. Another EORTC pooled analysis from 10 sarcoma centers revealed that after a median of 10 months of neoadjuvant imatinib, 83.2% of patients achieved an R0 resection and only 1% progressed during treatment.35 After a median follow-up of 46 months, the 5-year disease-free survival and OS were 65% and 87%, respectively.
Mutational testing should be performed beforehand to ensure the tumor is imatinib-sensitive. If a KIT exon 9 mutation is identified, then 400 mg twice daily should be considered (given the benefit seen with 800 mg imatinib for advanced GIST patients), although there are no studies to confirm this practice. Neoadjuvant imatinib is recommended for a total of 6 to 12 months to ensure maximal tumor debulking, but with very close monitoring and surgical input for disease resistance and growth.14 Imatinib should be stopped 1 to 2 days preoperatively and resumed once the patient has recovered from surgery for a total of 3 years (pre-/postoperatively combined). Neoadjuvant therapy has been shown to be safe and effective, but there have been no randomized trials to assess survival.
What is appropriate surveillance for resected GISTs?
Surveillance
There have been no randomized studies to guide the management of surveillance after surgical resection and adjuvant therapy. There is no known optimal follow-up schedule, but several have been proposed.13,36 Among high-risk patients, it is suggested to image every 3 to 6 months during adjuvant therapy, followed by every 3 months for 2 years after discontinuing therapy, then every 6 months for another 3 years and annually thereafter for an additional 5 years. High-risk patients usually relapse within 1 to 3 years after finishing adjuvant therapy, while low-risk patients can relapse later given that their disease can be slower growing. It has been recommended that low-risk patients undergo imaging every 6 months for 5 years, with follow-up individualized thereafter. Very-low-risk patients may not require more than annual imaging. Because most relapses occur within the peritoneum or liver, imaging should encompass the abdomen and pelvis. Surveillance imaging usually consists of CT scans of the abdomen and pelvis. MRI scans can be utilized for patients at lower risk or who are out several years in order to avoid excess radiation exposure. MRI is also specifically helpful for rectal and esophageal lesions. Chest CT or chest radiograph and bone scan are not routinely required for follow-up.
Case Conclusion
The patient receives adjuvant imatinib and experiences grade 2 myalgias, periorbital edema, and macrocytic anemia, which result in imatinib discontinuation after 3 years of treatment. He is seen every 3 to 6 months and a contrast CT abdomen and pelvis is obtained every 6 months for 5 years. During this 5-year follow-up period, he does not have any clinical or radiographic evidence of disease recurrence.
Further follow-up of this patient is presented in the second article in this 2-part review of management of GISTs.
Key Points
- GISTs are the most common mesenchymal neoplasms of the GI tract and can occasionally occur in extragastrointestinal locations as well.
- GISTs encompass a heterogeneous family of tumor subsets with different natural histories, mutations, and TKI responsiveness.
- Surgery is the mainstay of treatment for localized GISTs, with cure rates greater than 50%.
- For very small (< 2 cm) esophagogastric GISTs, endoscopic ultrasound evaluation and follow-up is recommended.
- For tumors ≥ 2 cm, biopsy and excision is the standard approach.
- For localized GISTs, complete surgical resection (R0) is standard treatment, with no lymphadenectomy for clinically negative lymph nodes.
- Mutational analysis should be considered standard of practice. It can be helpful for confirming the diagnosis and can be predictive and prognostic in determining specific TKI therapy and dose.
- Adjuvant imatinib at a dose of 400 mg for 3 years is standard of care for GISTs that are at high risk of relapse and are imatinib-sensitive, and it is the only TKI approved for adjuvant therapy. Patients with PDGFRA D842V, NF1, BRAF or SDH-deficient GISTs should not receive adjuvant imatinib therapy.
- Neoadjuvant therapy can be utilized for sites where extensive resection would lead to significant morbidity. It should be given for 6 to 12 months, but patients need to be monitored closely for tumor growth.
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumor of the gastrointestinal (GI) tract and arise from the interstitial cells of Cajal of the myenteric plexus. These tumors are rare, with about 1 case per 100,000 persons diagnosed in the United States annually, but may be incidentally discovered in up to 1 in 5 autopsy specimens of older adults.1,2 Epidemiologic risk factors include increasing age, with a peak incidence between age 60 and 65 years, male gender, black race, and non-Hispanic white ethnicity. Germline predisposition can also increase the risk of developing GISTs; molecular drivers of GIST include gain-of-function mutations in the KIT proto-oncogene and platelet-derived growth factor receptor α (PDGFRA) gene, which both encode structurally similar tyrosine kinase receptors; germline mutations of succinate dehydrogenase (SDH) subunit genes; and mutations associated with neurofibromatosis type 1.
GISTs most commonly involve the stomach, followed by the small intestine, but can arise anywhere within the GI tract (esophagus, colon, rectum, and anus). They can also develop outside the GI tract, arising from the mesentery, omentum, and retroperitoneum. The majority of cases are localized or locoregional, whereas about 20% are metastatic at presentation.1 GISTs can occur in children, adolescents, and young adults. Pediatric GISTs represent a distinct subset marked by female predominance and gastric origin, are often multifocal, can sometimes have lymph node involvement, and typically lack mutations in the KIT and PDGFRA genes.
This review is the first of 2 articles focusing on the diagnosis and management of GISTs. Here, we review the evaluation and diagnosis of GISTs along with management of localized disease. Management of advanced disease is reviewed in a separate article.
Case Presentation
A 64-year-old African American man with progressive iron deficiency and abdominal discomfort undergoes upper and lower endoscopy and is found to have a bulging mass within his abdominal cavity. He undergoes a computed tomography (CT) evaluation of the chest, abdomen, and pelvis with contrast, which reveals the presence of a 10-cm gastric mass, with no other lesions identified. He undergoes surgical resection of the mass and presents for review of his pathology and to discuss his treatment plan.
What histopathologic features are consistent with GIST?
What factors are used for risk stratification and to predict likelihood of recurrence?
Clinical Presentation and Diagnosis
Most patients present with symptoms of overt or occult GI bleeding or abdominal discomfort, but a significant proportion of GISTs are discovered incidentally. Lymph node involvement is not typical, except for GISTs occurring in children and/or with rare syndromes. Most syndromic GISTs are multifocal and multicentric. After surgical resection, GISTs usually recur or metastasize within the abdominal cavity, including the omentum, peritoneum, or liver. These tumors rarely spread to the lungs, brain, or bones; when tumor spread does occur, it tends to be in heavily pre-treated patients with advanced disease who have been on multiple lines of therapy for a long duration of time.
The diagnosis usually can be made by histopathology. Specimens can be obtained by endoscopic ultrasound (EUS)– or CT-guided methods, the latter of which carries a very small risk of contamination from percutaneous biopsy. In terms of morphology, GISTs can be spindle cell, epithelioid, or mixed neoplasms. Epithelioid tumors are more commonly seen in the stomach and are often PDGFRA-mutated or SDH-deficient. The differential diagnosis includes other soft-tissue GI wall tumors such as leiomyosarcomas/leiomyomas, germ cell tumors, lymphomas, fibromatosis, and neuroendocrine and neurogenic tumors. A unique feature of GISTs that differentiates them from leiomyomas is near universal expression of CD117 by immunohistochemistry (IHC); this characteristic has allowed pathologists and providers to accurately distinguish true GISTs from other GI mesenchymal tumors.3 Recently, DOG1 (discovered on GIST1) immunoreactivity has been found to be helpful in identifying patients with CD117-negative GISTs. Initially identified through gene expression analysis of GISTs, DOG1 IHC can identify the common mutant c-Kit-driven CD117-positive GISTs as well as the rare CD117-negative GISTs, which are often driven by mutated PDGFRA.4 Importantly, IHC for KIT and DOG1 are not surrogates for mutational status, nor are they predictive of tyrosine kinase inhibitor (TKI) sensitivity. If IHC of a tumor specimen is CD117- and DOG1-negative, the specimen can be sent for KIT and PDGFRA mutational analysis to confirm the diagnosis. If analysis reveals that these genes are wild-type, then IHC staining for SDH B (SDHB) should follow to assess for an SDH-deficient GIST (negative staining).
Risk Stratification for Recurrence
The clinical behavior of GISTs can be variable. Some are indolent, while others behave more aggressively, with a greater malignant potential and a higher propensity to recur and metastasize. Clinical and pathologic features can provide important prognostic information that allows providers to risk-stratify patients. Various institutions have assessed prognostic variables for GISTs. In 2001, the National Institutes of Health (NIH) held a GIST workshop that proposed an approach to estimating metastatic risk based on tumor size and mitotic index (NIH or Fletcher criteria).5 Joensuu et al later proposed a modification of the NIH risk classification to include tumor location and tumor rupture (modified NIH criteria or Joensuu criteria).6-8 Similarly, the Armed Forces Institute of Pathology (AFIP) identified tumor site as a prognostic factor, with gastric GISTs having the best prognosis (AFIP-Miettinen criteria).9-11 Tabular schemes were designed which stratified patients into discrete groups with ranges for mitotic rate and tumor size. Nomograms for ease of use were then constructed utilizing a bimodal mitotic rate and included tumor site and size.12 Finally, contour maps were developed, which have the advantage of evaluating mitotic rate and tumor size as continuous nonlinear variables and also include tumor site and rupture (associated with a high risk of peritoneal metastasis) separately, further improving risk assessment. These contour maps have been validated against pooled data from 10 series (2560 patients).13 High-risk features identified from these studies include tumor location, size, mitotic rate and tumor rupture and are now used for deciding on the use of adjuvant imatinib and as requirements to enter clinical trials assessing adjuvant therapy for resected GISTs.
Case Continued
The patient’s operative and pathology reports indicate that the tumor is a spindle cell neoplasm of the stomach that is positive for CD117, DOG1, and CD34 and negative for smooth muscle actin and S-100, consistent with a diagnosis of GIST. Resection margins are negative. There are 10 mitoses per 50 high-power fields (HPF). Per the operative report, there was no intraoperative or intraperitoneal tumor rupture. Thus, while his GIST was gastric, which generally has a more favorable prognosis, the tumor harbors high-risk features based on its size and mitotic index.
What further testing should be requested?
Molecular Alterations
It is recommended that a mutational analysis be performed as part of the diagnostic work-up of all GISTs.14 Mutational analysis can provide prognostic and predictive information for sensitivity to imatinib and should be considered standard of care. It may also be useful for confirming a GIST diagnosis, or, if negative, lead to further evaluation with an IHC stain for SDHB. The c-Kit receptor is a member of the tyrosine kinase family and, through direct interactions with stem cell factor (SCF), can upregulate the PI3K/AKT/mTOR, Ras/Raf/MEK/ERK, and JAK-STAT pathways, resulting in transcription and translation of genes that enhance cell growth and survival.15 The cell of origin of GISTs, the interstitial cells of Cajal, are dependent on the SCF–c-Kit interaction for development.16 Likewise, the large majority of GISTs (about 70%) are driven by upregulation and constitutive activation of c-Kit, which is normally autoinhibited. About 80% of KIT mutations involve exon 11; these GISTs are most often associated with a gastric location and are associated with a favorable recurrence-free survival (RFS) rate with surgery alone.17 KIT exon 9 mutations are much less common, encompassing only about 10% of GIST KIT mutations, and GISTs with these mutations are more likely to arise from the small bowel.17
About 8% of GISTs harbor gain-of-function PDGFRA driver mutations rendering constitutively active PDGFRA.18 PDGFRA mutations are mutually exclusive from KIT mutations, and PDGFRA-mutated tumors most often occur in the stomach. PDGFRA mutations generally are associated with a lower mitotic rate and gastric location. Identification of the PDGFRA D842V mutation on exon 18, which is the most common, is important, as it is associated with imatinib resistance, and these patients should not be offered imatinib.19
Several other mutations associated with GISTs outside of the KIT and PDGFRA spectrum have been identified. About 10% of GISTs are wildtype for KIT and PDGFRA, and not all KIT/PDGFRA-wildtype GISTs are imatinib-sensitive and/or respond to other TKIs.18 These tumors may harbor aberrations in SDH and NF1, or less commonly, BRAF V600E, FGFR, and NTRK.20,21 SDH subunits B, C and D play a role in the Krebs cycle and electron transport chain. Germline mutations in these SDH subunits can result in the Carney-Stratakis syndrome characterized by the dyad of multifocal GISTs and multicentric paragangliomas.22 This syndrome is most likely to manifest in the pediatric or young adult population. In contradistinction is the Carney triad, which is associated with acquired loss of function of the SDHC gene due to promoter hypermethylation. This syndrome classically occurs in young women and is characterized by an indolent-behaving triad of multicentric GISTs, non-adrenal paragangliomas, and pulmonary chondromas.23 Like PDGFRA D842V–mutated GISTs, SDH-deficient and NF1-associated GISTs are considered imatinib resistant, and these patients should not be offered imatinib therapy.14
Case Continued
The patient’s GIST is found to harbor a KIT exon 11 single codon deletion. He appears anxious and asks to have everything done to prevent his GIST from coming back and to improve his lifespan.
What are the next steps in the management of this patient?
Management
A multidisciplinary team approach to the management of all GISTs is essential and includes input from radiology, gastroenterology, pathology, medical and surgical oncology, nuclear medicine, and nursing.
Surgical Resection
Small esophagogastric and duodenal GISTs ≤ 2 cm can be asymptomatic and managed with serial endoscopic surveillance, typically every 6 to 12 months, with biopsies if the tumors increase in size. GISTs larger than 2 cm require surgical resection, with resection of the full pseudocapsule and an R0 resection, if possible, since larger GISTs carry a higher risk of growth and recurrence. If an R0 resection would lead to significant morbidity or functional sequelae, an R1 may suffice. Rectal GISTs are an exception, where microscopic margins have been shown to be associated with an increased risk of local failure.24 It is important to explore the abdomen thoroughly for peritoneal, rectovaginal, and vesicular implants and metastasis to the liver. A formal lymph node dissection is not necessary because lymph nodes are rarely involved and should only be removed when clinically suspicious. Tumor rupture must be avoided. A laparoscopic approach should only be considered for smaller tumors, since there is a risk of tumor rupture with larger tumors.14
When is adjuvant imatinib indicated?
Adjuvant Imatinib
Among patients with local or locally advanced GISTs, the risk of death from recurrence with surgery alone can be high, with a historical 5-year overall survival (OS) of about 35%.25 As a result, multiple studies have assessed the benefit of adjuvant imatinib, which is now considered standard of care for patients with imatinib-sensitive, high-risk GISTs. In addition to inhibiting BCR-ABL, imatinib mesylate inhibits multiple other receptor tyrosine kinases, including PDGFR, SCF and c-Kit. As a result, imatinib has demonstrated in vitro inhibition of cell proliferation and apoptosis and clinical activity against GISTs expressing CD117.26 Importantly, adjuvant imatinib should only be offered to patients with imatinib-sensitive mutations, such as KIT exon 11 and KIT exon 9 mutations. Adjuvant imatinib should not be offered to patients with imatinib-insensitive mutations such as PDGFR 842V, NF1, or BRAF-related or SDH-deficient GISTs.
The ACOSOG Z9000 was the first study of adjuvant imatinib in patients with resected GISTs.25 This was a single-arm, phase 2 study involving 106 patients with surgically resected GISTs deemed high-risk for recurrence, defined as size > 10 cm, tumor rupture, or up to 4 peritoneal implants. Patients were treated with imatinib 400 mg daily for 1 year. The primary and secondary endpoints were OS and RFS, respectively. Long-term follow-up of this study demonstrated 1-, 3-, and 5-year OS of 99%, 97%, and 83%, and 1-, 3-, and 5-year RFS of 96%, 60%, and 40%, which compared favorably with historical controls. In a multivariable analysis, increasing tumor size, small bowel location, KIT exon 9 mutation, high mitotic rate, and older age were independent risk factors for a poor RFS.25 It is important to note that the benefit of adjuvant imatinib waned after discontinuation of therapy, creating a rationale to study adjuvant imatinib for longer periods of time.
As a result of the promising phase 2 data, ACOSOG opened a phase 3 randomized trial (Z9001) comparing 1 year of adjuvant imatinib to placebo among patients with surgically resected GISTs that were > 3 cm in size and that stained positive for CD117 on pathology. The trial accrued 713 patients and was stopped early at a planned interim analysis, which revealed a 1-year RFS of 98% for imatinib versus 83% for placebo (hazard ratio [HR], 0.35; P < 0.001). The 1-year OS did not differ between the 2 arms (92.2% vs 99.7%; HR, 0.66; P = 0.47).27 When comparing the 2 arms, imatinib was associated with a higher RFS among patients with a KIT exon 11 deletion, but not among patients with other KIT mutation types, PDGFRA mutations, or who were KIT/PDGFRA wildtype.28 Imatinib was granted approval by the US Food and Drug Administration (FDA) for the adjuvant treatment of high-risk GISTs based on the results of the ACOSOG Z9001 trial.
The EORTC 62024 study was a randomized placebo-controlled trial assessing the benefit of 2 years of adjuvant imatinib.29 Patients had to be considered intermediate or high risk per the 2002 NIH consensus classification to be eligible. The trial enrolled 918 patients. The 5-year OS rate, the original primary endpoint, did not differ between the 2 groups (100% vs 99%). The 3-year and 5-year RFS rates, secondary endpoints, were significantly longer among patients treated with imatinib (84% vs 66% and 69% vs 63%, respectively). Again, it was noted that the benefit of imatinib waned over time after treatment discontinuation.
The Scandinavian Sarcoma Group (SSG XVIII) trial was a prospective randomized phase 3 trial that compared 3 years versus 1 year of adjuvant imatinib.30 Patients had to be enrolled within 12 weeks of the postoperative period and had to have GISTs that were CD117-positive and with a high estimated risk of recurrence, per the modified NIH consensus criteria (size > 10 cm, > 10 mitoses per 50 HPF, diameter > 5 cm with mitotic count > 5, or tumor rupture before or at surgery). Three years of adjuvant imatinib was associated with a 54% reduction in the hazard for recurrence at 5 years (65.6% vs 47.9%; HR, 0.46; P < 0.001) and a 55% reduction in the hazard for death at 5 years (OS 92% vs 81.7%; HR, 0.45; P = 0.02). Based on the results of this study, the FDA granted approval for the use of 3 years of adjuvant imatinib in patients with high-risk resected GISTs.
The observation that a longer duration of adjuvant imatinib was associated with superior RFS and OS led to studies to further explore longer durations of adjuvant imatinib. The PERSIST-5 (Postresection Evaluation of Recurrence-free Survival for Gastrointestinal Stromal Tumors With 5 Years of Adjuvant Imatinib) was a multicenter, single-arm, phase 2 prospective study of adjuvant imatinib with a primary endpoint of RFS after 5 years.31 Patients had to have an intermediate or high risk of recurrence, which included GISTs at any site > 2 cm with > 5 mitoses per 50 HPF or nongastric GISTs that were ≥ 5 cm. With 91 patients enrolled, the estimated 5-year RFS was 90% and the OS was 95%. Of note, about half of the patients stopped treatment early due to a variety of reasons, including patient choice or adverse events. Importantly, there were no recurrences in patients with imatinib-sensitive mutations while on therapy. We know that in patients at high risk of relapse, adjuvant imatinib delays recurrence and improves survival, but whether any patients are cured, or their survival curves are just shifted to the right, is unknown. Only longer follow-up of existing studies, and the results of newer trials utilizing longer durations of adjuvant treatment, will help to determine the real value of adjuvant therapy for GIST patients.32 Based on this study, it would be reasonable to discuss a longer duration of imatinib with patients deemed to be at very high risk of recurrence and who are tolerating therapy well. We are awaiting the data from the randomized phase 3 Scandinavian Sarcoma Group XII trial comparing 5 years versus 3 years of adjuvant imatinib therapy, and from the French ImadGIST trial of adjuvant imatinib for 3 versus 6 years. A summary of the aforementioned key adjuvant trials is shown in the Table.

When imatinib is commenced, careful monitoring for treatment toxicities and drug interactions should ensue in order to improve compliance. Dose density should be maintained if possible, as retrospective studies suggest suboptimal plasma levels are associated with a worse outcome.33
When should neoadjuvant imatinib be considered?
Neoadjuvant Imatinib
Neoadjuvant imatinib should be considered for patients requiring total gastrectomy, esophagectomy, or abdominoperineal resection of the rectum in order to reduce tumor size, limit subsequent surgical morbidity, mitigate tumor bleeding and rupture, and aid with organ preservation. Patients with rectal GISTs that may otherwise warrant an abdominoperineal resection should be offered a trial of imatinib in the neoadjuvant setting. There is no evidence for the use of any other TKI aside from imatinib in the neoadjuvant or adjuvant setting. With neoadjuvant imatinib, it is difficult to accurately assess the mitotic rate in the resected tumor specimen.
The RTOG 0132/ACRIN 6665 trial was a prospective phase 2 study evaluating the efficacy of imatinib 600 mg daily in the perioperative setting.34 The trial enrolled 50 patients, 30 with primary GISTs (group A) and 22 with recurrent metastatic GISTs (group B). Based on data from the metastatic setting revealing a time to treatment response of about 2.5 months, patients were treated with 8 to 12 weeks of preoperative imatinib followed by 2 years of adjuvant imatinib. Imatinib was stopped 24 hours preoperatively and resumed as soon as possible postoperatively. In group A, 7% of patients achieved a partial response (PR), 83% achieved stable disease, and 2-year progression-free survival (PFS) and OS were 83% and 93%, respectively. In group B, 4.5% of patients achieved a PR, 91% achieved stable disease, and 4.5% experienced progressive disease in the preoperative period; the 2-year PFS and OS were 77% and 91%, respectively. The results of this trial demonstrated the feasibility of using perioperative imatinib with minimal effects on surgical outcomes and set the rationale to use neoadjuvant imatinib in select patients with borderline resectable or rectal GISTs. Another EORTC pooled analysis from 10 sarcoma centers revealed that after a median of 10 months of neoadjuvant imatinib, 83.2% of patients achieved an R0 resection and only 1% progressed during treatment.35 After a median follow-up of 46 months, the 5-year disease-free survival and OS were 65% and 87%, respectively.
Mutational testing should be performed beforehand to ensure the tumor is imatinib-sensitive. If a KIT exon 9 mutation is identified, then 400 mg twice daily should be considered (given the benefit seen with 800 mg imatinib for advanced GIST patients), although there are no studies to confirm this practice. Neoadjuvant imatinib is recommended for a total of 6 to 12 months to ensure maximal tumor debulking, but with very close monitoring and surgical input for disease resistance and growth.14 Imatinib should be stopped 1 to 2 days preoperatively and resumed once the patient has recovered from surgery for a total of 3 years (pre-/postoperatively combined). Neoadjuvant therapy has been shown to be safe and effective, but there have been no randomized trials to assess survival.
What is appropriate surveillance for resected GISTs?
Surveillance
There have been no randomized studies to guide the management of surveillance after surgical resection and adjuvant therapy. There is no known optimal follow-up schedule, but several have been proposed.13,36 Among high-risk patients, it is suggested to image every 3 to 6 months during adjuvant therapy, followed by every 3 months for 2 years after discontinuing therapy, then every 6 months for another 3 years and annually thereafter for an additional 5 years. High-risk patients usually relapse within 1 to 3 years after finishing adjuvant therapy, while low-risk patients can relapse later given that their disease can be slower growing. It has been recommended that low-risk patients undergo imaging every 6 months for 5 years, with follow-up individualized thereafter. Very-low-risk patients may not require more than annual imaging. Because most relapses occur within the peritoneum or liver, imaging should encompass the abdomen and pelvis. Surveillance imaging usually consists of CT scans of the abdomen and pelvis. MRI scans can be utilized for patients at lower risk or who are out several years in order to avoid excess radiation exposure. MRI is also specifically helpful for rectal and esophageal lesions. Chest CT or chest radiograph and bone scan are not routinely required for follow-up.
Case Conclusion
The patient receives adjuvant imatinib and experiences grade 2 myalgias, periorbital edema, and macrocytic anemia, which result in imatinib discontinuation after 3 years of treatment. He is seen every 3 to 6 months and a contrast CT abdomen and pelvis is obtained every 6 months for 5 years. During this 5-year follow-up period, he does not have any clinical or radiographic evidence of disease recurrence.
Further follow-up of this patient is presented in the second article in this 2-part review of management of GISTs.
Key Points
- GISTs are the most common mesenchymal neoplasms of the GI tract and can occasionally occur in extragastrointestinal locations as well.
- GISTs encompass a heterogeneous family of tumor subsets with different natural histories, mutations, and TKI responsiveness.
- Surgery is the mainstay of treatment for localized GISTs, with cure rates greater than 50%.
- For very small (< 2 cm) esophagogastric GISTs, endoscopic ultrasound evaluation and follow-up is recommended.
- For tumors ≥ 2 cm, biopsy and excision is the standard approach.
- For localized GISTs, complete surgical resection (R0) is standard treatment, with no lymphadenectomy for clinically negative lymph nodes.
- Mutational analysis should be considered standard of practice. It can be helpful for confirming the diagnosis and can be predictive and prognostic in determining specific TKI therapy and dose.
- Adjuvant imatinib at a dose of 400 mg for 3 years is standard of care for GISTs that are at high risk of relapse and are imatinib-sensitive, and it is the only TKI approved for adjuvant therapy. Patients with PDGFRA D842V, NF1, BRAF or SDH-deficient GISTs should not receive adjuvant imatinib therapy.
- Neoadjuvant therapy can be utilized for sites where extensive resection would lead to significant morbidity. It should be given for 6 to 12 months, but patients need to be monitored closely for tumor growth.
1. Ma GL, Murphy JD, Martinez ME et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298-302.
2. Agaimy A, Wunsch PH, Hofstaedter F, et al. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol. 2007;31:113-120.
3. Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol. 2000;13:1134-1142.
4. West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutational status. Am J Pathol. 2004;165:107-113.
5. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10:81-89.
6. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419.
7. Hohenberger P, Ronellenfitsch U, Oladeji O, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumor. Br J Surg. 2010;97:1854-1859.
8. Holmenbakk T, Bjerkehagen B, Boye K, et al. Definition and clinical significance of tumor rupture in gastrointestinal stromal tumours of the small intestine. Br J Surg. 2016;103:684-691.
9. Emory TS, Sobin LH, Lukes L, et al. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol. 1999;23:82-87.
10. Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30:477-489.
11. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68.
12. Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localized primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10:1045-1052.
13. Joensuu H, Vehtari A, Rihimaki J et al. Risk of recurrence of gastrointestinal stromal tumor after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274.
14. Casali PG, Abecassis N, Bauer S, et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow up. Ann Oncol. 2018;29(Supplement_4): iv267.
15. Jing L, Yan-Ling W, Bing-Jia C, et al. The c-kit receptor-mediated signal transduction and tumor-related diseases. Int J Biol Sci. 2013;9:435-443.
16. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580.
17. Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol. 2015;33:634-642.
18. Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813-3825.
19. Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342-4349.
20. Huss S, Pasternack H, Ihle MA, et al. Clinicopathological and molecular features of a large cohort of gastrointestinal stromal tumors (GISTs) and review of the literature: BRAF mutations in KIT/PDGFRA wild-type GISTs are rare events. Hum Pathol. 2017;62:206-214.
21. Shi E, Chmielecki J, Tang CM, et al. FGFR1 and NTRK3 actionable alterations in “Wild-Type” gastrointestinal stromal tumors. J Transl Med. 2016;14:339.
22. Carney JA, Stratakis CA. Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet. 2002;108:132-139.
23. Carney JA. Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney Triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc. 1999;74:543-552.
24. Jakob J, Mussi C, Ronellenfitsch U, et al. Gastrointestinal stromal tumor of the rectum: results of surgical and multimodality therapy in the era of imatinib. Ann Surg Oncol. 2013;20:586-592.
25. DeMatteo RP, Ballman KV, Antonescu CR, et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor (GIST): ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg. 2013;258:422-429.
26. Gleevac (imatinib) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals; 2016.
27. DeMatteo RP, Ballman KV, Antonescu CR, et al. Placebo-controlled randomized trial of adjuvant imatinib mesylate following the resection of localized, primary gastrointestinal stromal tumor (GIST). Lancet. 2009;373:1097-1104.
28. Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32:1563-1570.
29. Casali PG, Le Cesne A, Poveda Velasco A, et al. Imatinib failure-free survival (IFS) in patients with localized gastrointestinal stromal tumors (GIST) treated with adjuvant imatinib (IM): the EORTC/AGITG/FSG/GEIS/ISG randomized controlled phase III trial. J Clin Oncol. 2013;31. Abstract 10500.
30. Joensuu H, Eriksson M, Sundby HK, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265-1272.
31. Raut CP, Espat NJ, Maki RG, et al. Efficacy and tolerability of 5-year adjuvant imatinib treatment for patients with resected intermediate- or high-risk primary gastrointestinal stromal tumor: The PERSIST-5 Clinical Trial. JAMA Oncol. 2018: e184060.
32. Benjamin RS, Casali PG. Adjuvant imatinib for GI stromal tumors: when and for how long? J Clin Oncol. 2016;34:215-218.
33. Demetri GD, Wang Y, Wehrle E, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009;27:3141-3147.
34. Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99:42-47.
35. Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol. 2013;20:2937-2943.
36. Joensuu H, Martin-Broto J, Nishida T, et al. Follow-up strategies for patients with gastrointestinal stromal tumour treated with or without adjuvant imatinib after surgery. Eur J Cancer. 2015;51:1611-1617.
1. Ma GL, Murphy JD, Martinez ME et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298-302.
2. Agaimy A, Wunsch PH, Hofstaedter F, et al. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol. 2007;31:113-120.
3. Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol. 2000;13:1134-1142.
4. West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutational status. Am J Pathol. 2004;165:107-113.
5. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10:81-89.
6. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419.
7. Hohenberger P, Ronellenfitsch U, Oladeji O, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumor. Br J Surg. 2010;97:1854-1859.
8. Holmenbakk T, Bjerkehagen B, Boye K, et al. Definition and clinical significance of tumor rupture in gastrointestinal stromal tumours of the small intestine. Br J Surg. 2016;103:684-691.
9. Emory TS, Sobin LH, Lukes L, et al. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol. 1999;23:82-87.
10. Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30:477-489.
11. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68.
12. Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localized primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10:1045-1052.
13. Joensuu H, Vehtari A, Rihimaki J et al. Risk of recurrence of gastrointestinal stromal tumor after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274.
14. Casali PG, Abecassis N, Bauer S, et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow up. Ann Oncol. 2018;29(Supplement_4): iv267.
15. Jing L, Yan-Ling W, Bing-Jia C, et al. The c-kit receptor-mediated signal transduction and tumor-related diseases. Int J Biol Sci. 2013;9:435-443.
16. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580.
17. Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol. 2015;33:634-642.
18. Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813-3825.
19. Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342-4349.
20. Huss S, Pasternack H, Ihle MA, et al. Clinicopathological and molecular features of a large cohort of gastrointestinal stromal tumors (GISTs) and review of the literature: BRAF mutations in KIT/PDGFRA wild-type GISTs are rare events. Hum Pathol. 2017;62:206-214.
21. Shi E, Chmielecki J, Tang CM, et al. FGFR1 and NTRK3 actionable alterations in “Wild-Type” gastrointestinal stromal tumors. J Transl Med. 2016;14:339.
22. Carney JA, Stratakis CA. Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet. 2002;108:132-139.
23. Carney JA. Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney Triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc. 1999;74:543-552.
24. Jakob J, Mussi C, Ronellenfitsch U, et al. Gastrointestinal stromal tumor of the rectum: results of surgical and multimodality therapy in the era of imatinib. Ann Surg Oncol. 2013;20:586-592.
25. DeMatteo RP, Ballman KV, Antonescu CR, et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor (GIST): ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg. 2013;258:422-429.
26. Gleevac (imatinib) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals; 2016.
27. DeMatteo RP, Ballman KV, Antonescu CR, et al. Placebo-controlled randomized trial of adjuvant imatinib mesylate following the resection of localized, primary gastrointestinal stromal tumor (GIST). Lancet. 2009;373:1097-1104.
28. Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32:1563-1570.
29. Casali PG, Le Cesne A, Poveda Velasco A, et al. Imatinib failure-free survival (IFS) in patients with localized gastrointestinal stromal tumors (GIST) treated with adjuvant imatinib (IM): the EORTC/AGITG/FSG/GEIS/ISG randomized controlled phase III trial. J Clin Oncol. 2013;31. Abstract 10500.
30. Joensuu H, Eriksson M, Sundby HK, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265-1272.
31. Raut CP, Espat NJ, Maki RG, et al. Efficacy and tolerability of 5-year adjuvant imatinib treatment for patients with resected intermediate- or high-risk primary gastrointestinal stromal tumor: The PERSIST-5 Clinical Trial. JAMA Oncol. 2018: e184060.
32. Benjamin RS, Casali PG. Adjuvant imatinib for GI stromal tumors: when and for how long? J Clin Oncol. 2016;34:215-218.
33. Demetri GD, Wang Y, Wehrle E, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009;27:3141-3147.
34. Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99:42-47.
35. Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol. 2013;20:2937-2943.
36. Joensuu H, Martin-Broto J, Nishida T, et al. Follow-up strategies for patients with gastrointestinal stromal tumour treated with or without adjuvant imatinib after surgery. Eur J Cancer. 2015;51:1611-1617.
Gastrointestinal Stromal Tumors: Management of Advanced Disease
Most advanced gastrointestinal stromal tumors (GISTs) are due to a recurrence of localized disease, with only a small minority presenting with metastatic disease.1 Compared with chemotherapy, tyrosine kinase inhibitors (TKIs) have significantly improved the natural history of the disease, with median overall survival (OS) increasing from less than 1 year to about 5 years and approximately 1 in 5 patients achieving long-term survival.2 In addition, newer drugs in development and in clinical trials appear promising and have the potential to improve outcomes even further. This article reviews current evidence on options for treating metastatic or recurrent GISTs and GISTs that have progressed following initial therapy. The evaluation and diagnosis of GIST along with management of localized disease are reviewed in a separate article.
Case Presentation
A 64-year-old African American man underwent surgical resection of a 10-cm gastric mass, which pathology reported was positive for CD117, DOG1, and CD34 and negative for smooth muscle actin and S-100, consistent with a diagnosis of GIST. There were 10 mitoses per 50 HPF, and there was no intraoperative or intraperitoneal tumor rupture. The patient was treated with adjuvant imatinib, which was discontinued after 3 years due to grade 2 myalgias, periorbital edema, and macrocytic anemia. Surveillance included office visits every 3 to 6 months and a contrast CT abdomen and pelvis every 6 months. For the past 5 years, he has not had any clinical or radiographic evidence of disease recurrence. New imaging reveals multiple liver metastases and peritoneal implants. He feels fatigued and has lost about 10 lb since his last visit. He is 5 years out from his initial diagnosis and 2 years out from last receiving imatinib. His original tumor harbored a KIT exon 11 deletion.
What treatment should you recommend now?
Imatinib for Advanced GISTs
Before the first report of the efficacy of imatinib for metastatic GISTs in 2002, patients with advanced unresectable or metastatic GISTs were routinely treated with doxorubicin-based chemotherapy regimens, which were largely ineffective, with response rates (RRs) of around 5% and a median overall survival (OS) of less than 1 year.3,4 In 2002 a landmark phase 2 study revealed imatinib’s significant efficacy profile in advanced or metastatic GISTs, resulting in its approval by the US Food and Drug Administration (FDA).5 In this study, 147 patients with CD117-positive GISTs were randomly assigned to receive daily imatinib 400 mg or 600 mg for up to 36 months. The RRs were similar between the 2 groups (68.5% vs 67.6%), with a median time to response of 12 weeks and median duration of response of 118 days. Results of this study were much more favorable when compared to doxorubicin, rendering imatinib the new standard of care for advanced GISTs. A long-term follow-up of this study after a median of 63 months confirmed near identical RRs, progression-free survival (PFS), and median survival of 57 months among the 2 groups.6
Imatinib Daily Dosing
Although 400 mg of daily imatinib proved to be efficacious, it was unclear if a dose-response relationship existed for imatinib. An EORTC phase 2 study demonstrated a benefit of using a higher dose of imatinib at 400 mg twice daily, producing a RR of 71% (4% complete , 67% partial) and 1-year PFS of 73%, which appeared favorable compared with once-daily dosing and set the framework for larger phase 3 studies.7 Two phase 3 studies compared imatinib 400 mg once daily versus twice daily (until disease progression or unacceptable toxicity) among patients with CD117-positive advanced or metastatic GISTs. These studies were eventually combined into a meta-analysis (metaGIST) to compare RR, PFS and OS between the treatment groups. Both studies allowed cross-over to the 800 mg dose for patients who progressed on 400 mg daily.
The first study, conducted jointly by the EORTC, Italian Sarcoma Group, and Australasian Gastro-Intestinal Trials Group (EU-AUS),8 randomly assigned 946 patients to 400 mg once daily or twice daily. There were no differences in response rates between the groups, but the twice-daily group had a predicted 18% reduction in the hazard for progression compared with the once-daily group (estimated HR, 0.82; P = 0.026), which came at the expense of greater toxicities warranting dose reductions (60%) and treatment interruptions (64%). Cross-over to high-dose imatinib was feasible and safe, producing a partial response in 2%, stable disease in 27%, and a median PFS of 81 days. The second study was an intergroup study conducted jointly by SWOG, CALGB, NCI-C, and ECOG (S0033, US-CDN), with a nearly identical study design as the EU-AUS trial.9 The trial enrolled 746 patients. After a median follow up of 4.5 years, the median PFS and OS were not statistically different (18 vs 20 months and 55 vs 51 months, respectively). There were also no differences in response rates. One third of patients initially placed on the once-daily arm who crossed over after progression achieved a treatment response or stable disease.
The combined EU-AUS and US-CDN analysis (metaGIST) included 1640 patients with a median age of 60 years and 58% of whom were men; 818 and 822 patients were assigned to the 400 mg and 800 mg total daily doses, respectively.10 The median follow-up was 37.5 months. There were no differences in OS (49 vs 48.7 months), median PFS (18.9 vs 23.2 months), or overall response rates (51.4% vs 53.9%). Patients who had crossed over (n = 347) to the 800 mg total daily dose arm had a 7.7-month average PFS while on the higher daily dose. An analysis was performed on 377 patients in the EU-AUS trial assessing the impact of mutational status on clinical outcomes among imatinib-treated patients. KIT exon 9 activating mutations were found to be a significant independent prognostic factor for death when compared with KIT exon 11 mutations. However, the adverse prognostic value of KIT exon 9 mutations was partially overcome with higher doses of imatinib, as those who received 800 mg total had a significantly better PFS, with a 61% relative risk reduction, than those who received 400 mg. Altogether, it was concluded that imatinib 400 mg once daily should be the standard-of-care first-line treatment for advanced or metastatic GISTs, unless a KIT exon 9 mutation is present, in which case imatinib 800 mg should be considered, if 400 mg is well tolerated. In addition, patients treated with frontline imatinib at 400 mg once daily, if tolerated well, should be considered for imatinib 800 mg upon progression of disease.
Despite there being problems with secondary resistance, significant progress has occurred in the treatment of metastatic disease over a short period of time. Prior to 2000, median OS for patients with metastatic GISTs was 9 months. With the introduction of imatinib and other TKIs, the median OS has increased to 5 years, with an estimated 10-year OS rate of approximately 20%.2
Imatinib Interruption
Since at this point, imatinib was a well-established standard of care for advanced GISTs, it was questioned whether imatinib therapy could be interrupted. At this time, treatment interruption in a stop-and-go fashion was deemed feasible in other metastatic solid tumors such as colorectal cancer (OPTIMOX1).11 The BFR French trial showed that stopping imatinib therapy in patients who had a response or stable disease after 1, 3, or 5 years was generally followed by relatively rapid tumor progression (approximately 50% of patients within 6 months), even when tumors were previously removed.12 Therefore, it is recommended that treatment in the metastatic setting should be continued indefinitely, unless there is disease progression. Hence, unlike with colorectal cancer or chronic myelogenous leukemia, as of now there is no role for imatinib interruption in metastatic GISTs.
Case Continued
The patient is started on imatinib 400 mg daily, and overall he tolerates therapy well. Interval CT imaging reveals a treatment response. Two years later, imaging reveals an increase in the tumor size and density with a new nodule present within a preexisting mass. There are no clinical trials in the area.
What defines tumor progression?
Disease Progression
When GISTs are responding to treatment, on imaging the tumors can become more cystic and less dense but with an increase in size. In addition, tumor progression may not always be associated with increased size—increased density of the tumor or a nodule within a mass that may indicate progression. If CT imaging is equivocal for progression, positron emission tomography (PET) can play a role in identifying true progression. It is critically important that tumor size and density are carefully assessed when performing interval imaging. Of note, radiofrequency ablation, cryotherapy, or chemoembolization can be used for symptomatic liver metastases or oligometastatic disease. When evaluating for progression, one needs to ask patients about compliance (ie, maintaining dose intensity related to side effects of therapy as well as the financial burden of treatment—copay toxicity).
What are mechanisms of secondary imatinib resistance?
Imatinib resistance can be subtle in patients with GISTs, manifesting with new nodular, enhancing foci enclosed within a preexisting mass (resistant clonal nodule), or can be clinically or radiographically overt.13 Imatinib resistance occurs through multiple mechanisms including acquisition of secondary activating KIT mutations in the intracellular ATP-binding domain (exons 13 and 14) and the activation loop (exons 17 and 18).14
What are the treatment options for this patient?
Second-line Therapy
Sunitinib malate is a multitargeted TKI that not only targets c-Kit and PDGFRA, but also has anti-angiogenic activity through inhibition of vascular endothelial growth factor receptors (VEGFR). Sunitinib gained FDA approval for the second-line treatment of advanced GISTs based on an international double-blind trial that randomized 312 patients with imatinib-resistant metastatic GISTs in a 2:1 fashion to receive sunitinib 50 mg daily for 4 weeks on and 2 weeks off or placebo.15,16 The trial was unblinded early at the planned interim analysis, which revealed a marked benefit, producing a 66% reduction in the hazard risk of progression (27.3 vs 6.4 weeks, HR, 0.33; P < 0.001). The most common treatment-related adverse events were fatigue, diarrhea, skin discoloration, nausea, and hand-foot syndrome. Another open-label phase 2 study assessed a continuous dosing schema of sunitinib 37.5 mg daily, which has been shown to be effective with less toxicity.17 Among the 60 patients enrolled, the primary endpoint of clinical benefit rate at 24 weeks was reached in 53%, which consisted of 13% partial responses and 40% stable disease. Most toxicities were grade 1 or 2 and easily manageable through standard interventions. This has been recommended as an alternative to the initial scheduled regimen.18 Part of sunitinib’s success is its activity against GISTs harboring secondary KIT exon 13 and 14 mutations, and possibly its anti-angiogenic activity.19 Sunitinib is particularly efficacious among GISTs harboring KIT exon 9 mutations.
Third-line Therapy
Patients who have progressed on prior imatinib and sunitinib can receive third-line regorafenib, a multi-TKI that differs chemically from sorafenib by a fluorouracil group (fluoro-sorafenib). FDA approval of regorafenib was based on the phase 3 GRID (GIST Regorafenib In progressive Disease) multicenter international trial.20 This trial randomly assigned 199 patients in a 2:1 fashion to receive regorafenib 160 mg daily for 21 days out of 28-day cycles plus best supportive care (BSC) versus placebo plus BSC. Cross-over was allowed. Regorafenib significantly reduced the hazard risk of progression by 73% compared with placebo (4.8 vs 0.9 months; HR, 0.27; P < 0.001). There was no difference in OS, which may be because of cross-over (median OS, 17.4 months in both arms). As a result, regorafenib is now considered standard third-line treatment for patients with metastatic GISTs. It has a less favorable toxicity profile than imatinib, with hand-foot syndrome, transaminitis, hypertension and fatigue being the most common treatment toxicities. In order to avoid noncompliance, it is recommended to start at 80 mg and carefully titrate upwards to the 160 mg dose.
A list of landmark studies for advanced GISTs is provided in Table 1.
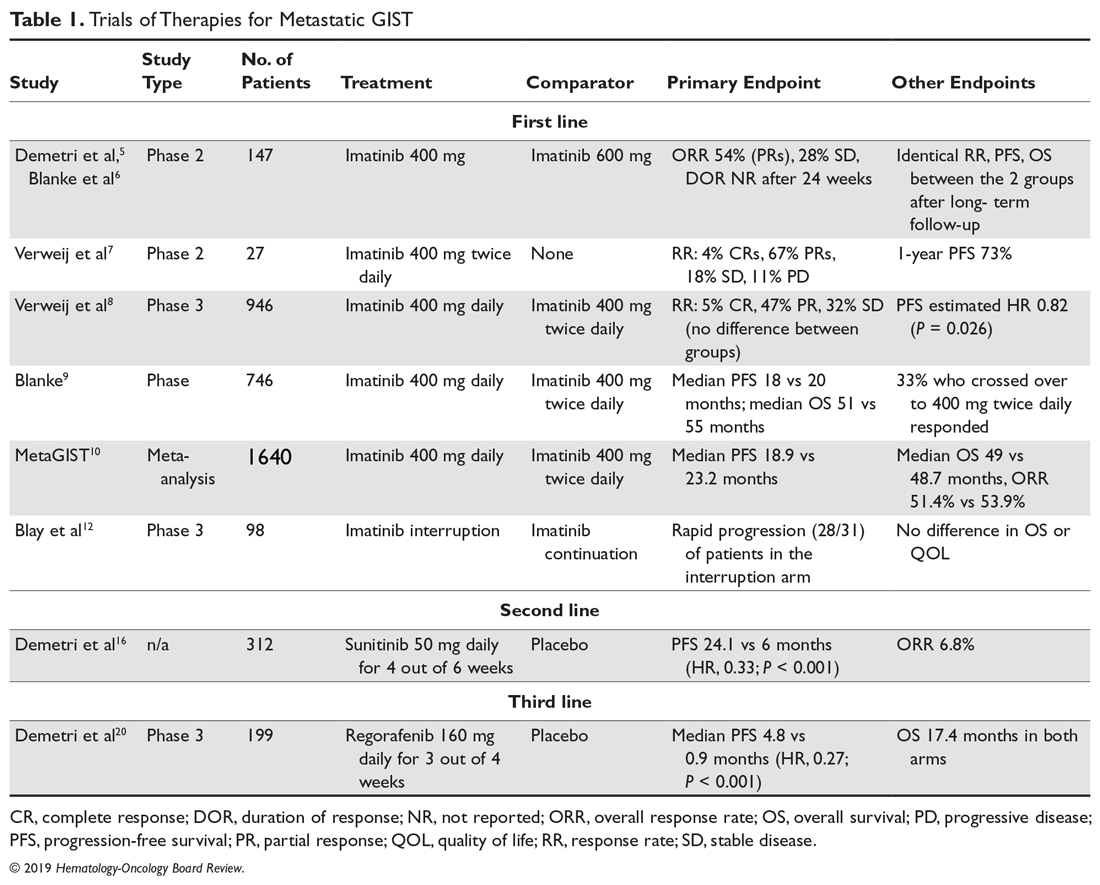
A summary of FDA-approved drugs for treating GISTs is provided in Table 2.
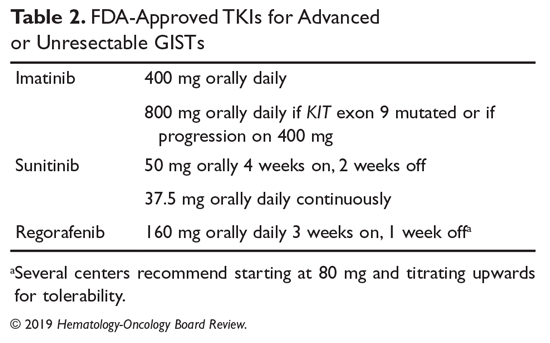
Clinical Trials
Clinical trial enrollment should be considered for all patients with advanced or unresectable GISTs throughout their treatment continuum. Owing to significant advances in genomic profiling through next-generation sequencing, multiple driver mutations have recently been identified, and targeted therapies are being explored in clinical trials.21 For example, the neurotrophic receptor tyrosine kinase (NTRK) gene appears to be mutated in a small number of advanced GISTs, and these can respond to the highly selective TRK inhibitor larotrectinib.22 Additionally, ongoing studies are assessing immunotherapies for sporadic GISTs and treatment for familial GISTs (Table 3). Some notable studies include those assessing the efficacy of agents that target KIT and PDGFR secondary mutations, including avapritinib (BLU-285) and DCC-2618, MEK inhibitors, and the multi-kinase inhibitor crenolanib for GISTs harboring the imatinib-resistant PDGFRA D842V mutation. There are also studies utilizing checkpoint inhibitors alone or in combination with imatinib.
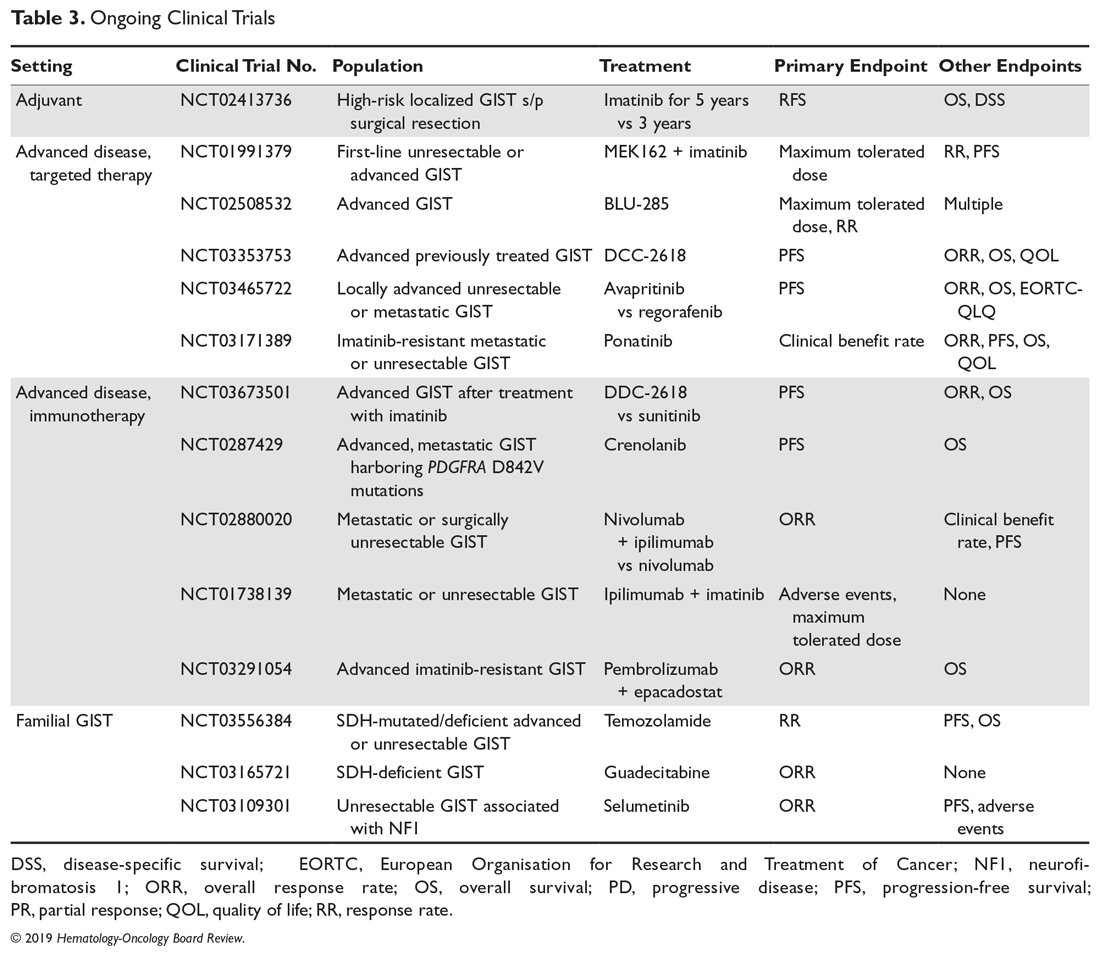
Case Conclusion
Given the patient’s progression on imatinib, he is started on second-line sunitinib malate. He experiences grade 1 fatigue and hand-foot syndrome, which are managed supportively. After he has been on sunitinib for approximately 8 months, his disease progresses. He subsequently undergoes genomic profiling of his tumor and starts BLU-285 on a clinical trial.
Key Points
- For advanced and metastatic disease, TKIs have substantially improved the prognosis of KIT mutated GISTs, with 3 FDA-approved drugs: imatinib, sunitinib, and regorafenib. Imatinib 400 mg is the standard-of-care frontline therapy for locally advanced, unresectable, or metastatic imatinib-sensitive GISTs. If a patient has a KIT exon 9 mutation and 400 mg is well-tolerated, increasing to 800 mg is recommended. Imatinib should be continued indefinitely unless there is intolerance, a specific patient request for interruption, or progression of disease.
- When there is progression of disease in a patient with a sensitive mutation on 400 mg of imatinib, the dose can be increased to 800 mg.
- For patients who are imatinib-intolerant or have progression, standard second line is sunitinib.
- For patients who further progress or are sunitinib-intolerant, regorafenib is the standard third-line treatment.
- There needs to be close attention to side effects, drug and food interactions, and patient copay costs in order to maintain patient compliance while on TKI therapy.
- There are still major limitations in the systemic treatment of GISTs marked by their inherent genetic heterogeneity and secondary resistance. Continued translational and clinical research is needed in order to improve treatment for patients who develop secondary resistance or who have less common primary resistant mutations. Patients are encouraged to participate in clinical trials of new therapies.
Summary
GISTs are the most common mesenchymal tumors of the GI tract. They comprise an expanding landscape of tumors that are heterogenous in terms of natural history, mutations, and response to systemic treatments. The mainstay of treatment for localized GISTs is surgical resection followed by at least 3-years of adjuvant imatinib for patients with high-risk features who are imatinib-sensitive. Patients with GISTs harboring resistance mutations such as PDGFRA D842V or with SDH-deficient or NF1-associated GISTs should not receive adjuvant imatinib. Patients with more advanced GISTs and/or in difficult to resect sites harboring a sensitive mutation can be considered for neoadjuvant imatinib. Those with metastatic GISTs can receive first-, second-, and third-line imatinib, sunitinib, or regorafenib, respectively. Clinical trial enrollment should be encouraged for patients whose GISTs harbor primary imatinib-resistant mutations, and those with advanced or unresectable GISTs with secondary resistance.
1. Ma GL, Murphy JD, Martinez ME et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298-302.
2. Heinrich MC, Rankin C, Blanke CD, et al. Correlation of long-term results of imatinib in advanced gastrointestinal stromal tumors with next-generation sequencing results: analysis of phase 3 SWOG Intergroup Trial S0033. JAMA Oncol. 2017;3:944-952.
3. DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51-58.
4. Goss GA, Merriam P, Manola J, et al. Clinical and pathological characteristics of gastrointestinal stromal tumors (GIST). Prog Proc Am Soc Clin Oncol. 2000;19:599a.
5. Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472-480.
6. Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase ii trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620-625.
7. Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevac) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer. 2003;39:2006-2011.
8. Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomized trial. Lancet. 2004;364:1127-1134.
9. Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626-632.
10. Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010;28:1247-1253.
11. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer –a GERCOR study. J Clin Oncol. 2006;24:394-400.
12. Blay JV, Cesne AL, Ray-Coquard I, et al. Prospective multicentric randomized phase iii study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: The French Sarcoma Group. J Clin Oncol. 2007;25:1107-1113.
13. Desai J, Shankar S, Heinrich MC, et al. Clonal evolution of resistance to imatinib in patients with metastatic gastrointestinal stromal tumors. Clin Cancer Res. 2007;13(18 Pt 1): 5398-5405.
14. Gramza AW, Corless CL, Heinrich MC. Resistance to tyrosine kinase inhibitors in gastrointestinal stromal tumors. Clin Cancer Res. 2009;15:7510-7518.
15. Sutent (sunitinib malate) [package insert]. New York, NY: Pfizer Labs; 2017.
16. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomized controlled trial. Lancet. 2006;368:1329-1338.
17. George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45:1959-1968.
18. Brennan MF, Antonescu CR, Maki RG. Management of Soft Tissue Sarcomas. Switzerland: Springer International Publishing; 2013.
19. Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumors. J Clin Oncol. 2008;26:5352-5359.
20. Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295-302.
21. Wilky BA, Villalobos VM. Emerging role for precision therapy through next-generation sequencing for sarcomas. JCO Precision Oncology. 2018;2:1-4.
22. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in trk fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731-739.
Most advanced gastrointestinal stromal tumors (GISTs) are due to a recurrence of localized disease, with only a small minority presenting with metastatic disease.1 Compared with chemotherapy, tyrosine kinase inhibitors (TKIs) have significantly improved the natural history of the disease, with median overall survival (OS) increasing from less than 1 year to about 5 years and approximately 1 in 5 patients achieving long-term survival.2 In addition, newer drugs in development and in clinical trials appear promising and have the potential to improve outcomes even further. This article reviews current evidence on options for treating metastatic or recurrent GISTs and GISTs that have progressed following initial therapy. The evaluation and diagnosis of GIST along with management of localized disease are reviewed in a separate article.
Case Presentation
A 64-year-old African American man underwent surgical resection of a 10-cm gastric mass, which pathology reported was positive for CD117, DOG1, and CD34 and negative for smooth muscle actin and S-100, consistent with a diagnosis of GIST. There were 10 mitoses per 50 HPF, and there was no intraoperative or intraperitoneal tumor rupture. The patient was treated with adjuvant imatinib, which was discontinued after 3 years due to grade 2 myalgias, periorbital edema, and macrocytic anemia. Surveillance included office visits every 3 to 6 months and a contrast CT abdomen and pelvis every 6 months. For the past 5 years, he has not had any clinical or radiographic evidence of disease recurrence. New imaging reveals multiple liver metastases and peritoneal implants. He feels fatigued and has lost about 10 lb since his last visit. He is 5 years out from his initial diagnosis and 2 years out from last receiving imatinib. His original tumor harbored a KIT exon 11 deletion.
What treatment should you recommend now?
Imatinib for Advanced GISTs
Before the first report of the efficacy of imatinib for metastatic GISTs in 2002, patients with advanced unresectable or metastatic GISTs were routinely treated with doxorubicin-based chemotherapy regimens, which were largely ineffective, with response rates (RRs) of around 5% and a median overall survival (OS) of less than 1 year.3,4 In 2002 a landmark phase 2 study revealed imatinib’s significant efficacy profile in advanced or metastatic GISTs, resulting in its approval by the US Food and Drug Administration (FDA).5 In this study, 147 patients with CD117-positive GISTs were randomly assigned to receive daily imatinib 400 mg or 600 mg for up to 36 months. The RRs were similar between the 2 groups (68.5% vs 67.6%), with a median time to response of 12 weeks and median duration of response of 118 days. Results of this study were much more favorable when compared to doxorubicin, rendering imatinib the new standard of care for advanced GISTs. A long-term follow-up of this study after a median of 63 months confirmed near identical RRs, progression-free survival (PFS), and median survival of 57 months among the 2 groups.6
Imatinib Daily Dosing
Although 400 mg of daily imatinib proved to be efficacious, it was unclear if a dose-response relationship existed for imatinib. An EORTC phase 2 study demonstrated a benefit of using a higher dose of imatinib at 400 mg twice daily, producing a RR of 71% (4% complete , 67% partial) and 1-year PFS of 73%, which appeared favorable compared with once-daily dosing and set the framework for larger phase 3 studies.7 Two phase 3 studies compared imatinib 400 mg once daily versus twice daily (until disease progression or unacceptable toxicity) among patients with CD117-positive advanced or metastatic GISTs. These studies were eventually combined into a meta-analysis (metaGIST) to compare RR, PFS and OS between the treatment groups. Both studies allowed cross-over to the 800 mg dose for patients who progressed on 400 mg daily.
The first study, conducted jointly by the EORTC, Italian Sarcoma Group, and Australasian Gastro-Intestinal Trials Group (EU-AUS),8 randomly assigned 946 patients to 400 mg once daily or twice daily. There were no differences in response rates between the groups, but the twice-daily group had a predicted 18% reduction in the hazard for progression compared with the once-daily group (estimated HR, 0.82; P = 0.026), which came at the expense of greater toxicities warranting dose reductions (60%) and treatment interruptions (64%). Cross-over to high-dose imatinib was feasible and safe, producing a partial response in 2%, stable disease in 27%, and a median PFS of 81 days. The second study was an intergroup study conducted jointly by SWOG, CALGB, NCI-C, and ECOG (S0033, US-CDN), with a nearly identical study design as the EU-AUS trial.9 The trial enrolled 746 patients. After a median follow up of 4.5 years, the median PFS and OS were not statistically different (18 vs 20 months and 55 vs 51 months, respectively). There were also no differences in response rates. One third of patients initially placed on the once-daily arm who crossed over after progression achieved a treatment response or stable disease.
The combined EU-AUS and US-CDN analysis (metaGIST) included 1640 patients with a median age of 60 years and 58% of whom were men; 818 and 822 patients were assigned to the 400 mg and 800 mg total daily doses, respectively.10 The median follow-up was 37.5 months. There were no differences in OS (49 vs 48.7 months), median PFS (18.9 vs 23.2 months), or overall response rates (51.4% vs 53.9%). Patients who had crossed over (n = 347) to the 800 mg total daily dose arm had a 7.7-month average PFS while on the higher daily dose. An analysis was performed on 377 patients in the EU-AUS trial assessing the impact of mutational status on clinical outcomes among imatinib-treated patients. KIT exon 9 activating mutations were found to be a significant independent prognostic factor for death when compared with KIT exon 11 mutations. However, the adverse prognostic value of KIT exon 9 mutations was partially overcome with higher doses of imatinib, as those who received 800 mg total had a significantly better PFS, with a 61% relative risk reduction, than those who received 400 mg. Altogether, it was concluded that imatinib 400 mg once daily should be the standard-of-care first-line treatment for advanced or metastatic GISTs, unless a KIT exon 9 mutation is present, in which case imatinib 800 mg should be considered, if 400 mg is well tolerated. In addition, patients treated with frontline imatinib at 400 mg once daily, if tolerated well, should be considered for imatinib 800 mg upon progression of disease.
Despite there being problems with secondary resistance, significant progress has occurred in the treatment of metastatic disease over a short period of time. Prior to 2000, median OS for patients with metastatic GISTs was 9 months. With the introduction of imatinib and other TKIs, the median OS has increased to 5 years, with an estimated 10-year OS rate of approximately 20%.2
Imatinib Interruption
Since at this point, imatinib was a well-established standard of care for advanced GISTs, it was questioned whether imatinib therapy could be interrupted. At this time, treatment interruption in a stop-and-go fashion was deemed feasible in other metastatic solid tumors such as colorectal cancer (OPTIMOX1).11 The BFR French trial showed that stopping imatinib therapy in patients who had a response or stable disease after 1, 3, or 5 years was generally followed by relatively rapid tumor progression (approximately 50% of patients within 6 months), even when tumors were previously removed.12 Therefore, it is recommended that treatment in the metastatic setting should be continued indefinitely, unless there is disease progression. Hence, unlike with colorectal cancer or chronic myelogenous leukemia, as of now there is no role for imatinib interruption in metastatic GISTs.
Case Continued
The patient is started on imatinib 400 mg daily, and overall he tolerates therapy well. Interval CT imaging reveals a treatment response. Two years later, imaging reveals an increase in the tumor size and density with a new nodule present within a preexisting mass. There are no clinical trials in the area.
What defines tumor progression?
Disease Progression
When GISTs are responding to treatment, on imaging the tumors can become more cystic and less dense but with an increase in size. In addition, tumor progression may not always be associated with increased size—increased density of the tumor or a nodule within a mass that may indicate progression. If CT imaging is equivocal for progression, positron emission tomography (PET) can play a role in identifying true progression. It is critically important that tumor size and density are carefully assessed when performing interval imaging. Of note, radiofrequency ablation, cryotherapy, or chemoembolization can be used for symptomatic liver metastases or oligometastatic disease. When evaluating for progression, one needs to ask patients about compliance (ie, maintaining dose intensity related to side effects of therapy as well as the financial burden of treatment—copay toxicity).
What are mechanisms of secondary imatinib resistance?
Imatinib resistance can be subtle in patients with GISTs, manifesting with new nodular, enhancing foci enclosed within a preexisting mass (resistant clonal nodule), or can be clinically or radiographically overt.13 Imatinib resistance occurs through multiple mechanisms including acquisition of secondary activating KIT mutations in the intracellular ATP-binding domain (exons 13 and 14) and the activation loop (exons 17 and 18).14
What are the treatment options for this patient?
Second-line Therapy
Sunitinib malate is a multitargeted TKI that not only targets c-Kit and PDGFRA, but also has anti-angiogenic activity through inhibition of vascular endothelial growth factor receptors (VEGFR). Sunitinib gained FDA approval for the second-line treatment of advanced GISTs based on an international double-blind trial that randomized 312 patients with imatinib-resistant metastatic GISTs in a 2:1 fashion to receive sunitinib 50 mg daily for 4 weeks on and 2 weeks off or placebo.15,16 The trial was unblinded early at the planned interim analysis, which revealed a marked benefit, producing a 66% reduction in the hazard risk of progression (27.3 vs 6.4 weeks, HR, 0.33; P < 0.001). The most common treatment-related adverse events were fatigue, diarrhea, skin discoloration, nausea, and hand-foot syndrome. Another open-label phase 2 study assessed a continuous dosing schema of sunitinib 37.5 mg daily, which has been shown to be effective with less toxicity.17 Among the 60 patients enrolled, the primary endpoint of clinical benefit rate at 24 weeks was reached in 53%, which consisted of 13% partial responses and 40% stable disease. Most toxicities were grade 1 or 2 and easily manageable through standard interventions. This has been recommended as an alternative to the initial scheduled regimen.18 Part of sunitinib’s success is its activity against GISTs harboring secondary KIT exon 13 and 14 mutations, and possibly its anti-angiogenic activity.19 Sunitinib is particularly efficacious among GISTs harboring KIT exon 9 mutations.
Third-line Therapy
Patients who have progressed on prior imatinib and sunitinib can receive third-line regorafenib, a multi-TKI that differs chemically from sorafenib by a fluorouracil group (fluoro-sorafenib). FDA approval of regorafenib was based on the phase 3 GRID (GIST Regorafenib In progressive Disease) multicenter international trial.20 This trial randomly assigned 199 patients in a 2:1 fashion to receive regorafenib 160 mg daily for 21 days out of 28-day cycles plus best supportive care (BSC) versus placebo plus BSC. Cross-over was allowed. Regorafenib significantly reduced the hazard risk of progression by 73% compared with placebo (4.8 vs 0.9 months; HR, 0.27; P < 0.001). There was no difference in OS, which may be because of cross-over (median OS, 17.4 months in both arms). As a result, regorafenib is now considered standard third-line treatment for patients with metastatic GISTs. It has a less favorable toxicity profile than imatinib, with hand-foot syndrome, transaminitis, hypertension and fatigue being the most common treatment toxicities. In order to avoid noncompliance, it is recommended to start at 80 mg and carefully titrate upwards to the 160 mg dose.
A list of landmark studies for advanced GISTs is provided in Table 1.

A summary of FDA-approved drugs for treating GISTs is provided in Table 2.

Clinical Trials
Clinical trial enrollment should be considered for all patients with advanced or unresectable GISTs throughout their treatment continuum. Owing to significant advances in genomic profiling through next-generation sequencing, multiple driver mutations have recently been identified, and targeted therapies are being explored in clinical trials.21 For example, the neurotrophic receptor tyrosine kinase (NTRK) gene appears to be mutated in a small number of advanced GISTs, and these can respond to the highly selective TRK inhibitor larotrectinib.22 Additionally, ongoing studies are assessing immunotherapies for sporadic GISTs and treatment for familial GISTs (Table 3). Some notable studies include those assessing the efficacy of agents that target KIT and PDGFR secondary mutations, including avapritinib (BLU-285) and DCC-2618, MEK inhibitors, and the multi-kinase inhibitor crenolanib for GISTs harboring the imatinib-resistant PDGFRA D842V mutation. There are also studies utilizing checkpoint inhibitors alone or in combination with imatinib.

Case Conclusion
Given the patient’s progression on imatinib, he is started on second-line sunitinib malate. He experiences grade 1 fatigue and hand-foot syndrome, which are managed supportively. After he has been on sunitinib for approximately 8 months, his disease progresses. He subsequently undergoes genomic profiling of his tumor and starts BLU-285 on a clinical trial.
Key Points
- For advanced and metastatic disease, TKIs have substantially improved the prognosis of KIT mutated GISTs, with 3 FDA-approved drugs: imatinib, sunitinib, and regorafenib. Imatinib 400 mg is the standard-of-care frontline therapy for locally advanced, unresectable, or metastatic imatinib-sensitive GISTs. If a patient has a KIT exon 9 mutation and 400 mg is well-tolerated, increasing to 800 mg is recommended. Imatinib should be continued indefinitely unless there is intolerance, a specific patient request for interruption, or progression of disease.
- When there is progression of disease in a patient with a sensitive mutation on 400 mg of imatinib, the dose can be increased to 800 mg.
- For patients who are imatinib-intolerant or have progression, standard second line is sunitinib.
- For patients who further progress or are sunitinib-intolerant, regorafenib is the standard third-line treatment.
- There needs to be close attention to side effects, drug and food interactions, and patient copay costs in order to maintain patient compliance while on TKI therapy.
- There are still major limitations in the systemic treatment of GISTs marked by their inherent genetic heterogeneity and secondary resistance. Continued translational and clinical research is needed in order to improve treatment for patients who develop secondary resistance or who have less common primary resistant mutations. Patients are encouraged to participate in clinical trials of new therapies.
Summary
GISTs are the most common mesenchymal tumors of the GI tract. They comprise an expanding landscape of tumors that are heterogenous in terms of natural history, mutations, and response to systemic treatments. The mainstay of treatment for localized GISTs is surgical resection followed by at least 3-years of adjuvant imatinib for patients with high-risk features who are imatinib-sensitive. Patients with GISTs harboring resistance mutations such as PDGFRA D842V or with SDH-deficient or NF1-associated GISTs should not receive adjuvant imatinib. Patients with more advanced GISTs and/or in difficult to resect sites harboring a sensitive mutation can be considered for neoadjuvant imatinib. Those with metastatic GISTs can receive first-, second-, and third-line imatinib, sunitinib, or regorafenib, respectively. Clinical trial enrollment should be encouraged for patients whose GISTs harbor primary imatinib-resistant mutations, and those with advanced or unresectable GISTs with secondary resistance.
Most advanced gastrointestinal stromal tumors (GISTs) are due to a recurrence of localized disease, with only a small minority presenting with metastatic disease.1 Compared with chemotherapy, tyrosine kinase inhibitors (TKIs) have significantly improved the natural history of the disease, with median overall survival (OS) increasing from less than 1 year to about 5 years and approximately 1 in 5 patients achieving long-term survival.2 In addition, newer drugs in development and in clinical trials appear promising and have the potential to improve outcomes even further. This article reviews current evidence on options for treating metastatic or recurrent GISTs and GISTs that have progressed following initial therapy. The evaluation and diagnosis of GIST along with management of localized disease are reviewed in a separate article.
Case Presentation
A 64-year-old African American man underwent surgical resection of a 10-cm gastric mass, which pathology reported was positive for CD117, DOG1, and CD34 and negative for smooth muscle actin and S-100, consistent with a diagnosis of GIST. There were 10 mitoses per 50 HPF, and there was no intraoperative or intraperitoneal tumor rupture. The patient was treated with adjuvant imatinib, which was discontinued after 3 years due to grade 2 myalgias, periorbital edema, and macrocytic anemia. Surveillance included office visits every 3 to 6 months and a contrast CT abdomen and pelvis every 6 months. For the past 5 years, he has not had any clinical or radiographic evidence of disease recurrence. New imaging reveals multiple liver metastases and peritoneal implants. He feels fatigued and has lost about 10 lb since his last visit. He is 5 years out from his initial diagnosis and 2 years out from last receiving imatinib. His original tumor harbored a KIT exon 11 deletion.
What treatment should you recommend now?
Imatinib for Advanced GISTs
Before the first report of the efficacy of imatinib for metastatic GISTs in 2002, patients with advanced unresectable or metastatic GISTs were routinely treated with doxorubicin-based chemotherapy regimens, which were largely ineffective, with response rates (RRs) of around 5% and a median overall survival (OS) of less than 1 year.3,4 In 2002 a landmark phase 2 study revealed imatinib’s significant efficacy profile in advanced or metastatic GISTs, resulting in its approval by the US Food and Drug Administration (FDA).5 In this study, 147 patients with CD117-positive GISTs were randomly assigned to receive daily imatinib 400 mg or 600 mg for up to 36 months. The RRs were similar between the 2 groups (68.5% vs 67.6%), with a median time to response of 12 weeks and median duration of response of 118 days. Results of this study were much more favorable when compared to doxorubicin, rendering imatinib the new standard of care for advanced GISTs. A long-term follow-up of this study after a median of 63 months confirmed near identical RRs, progression-free survival (PFS), and median survival of 57 months among the 2 groups.6
Imatinib Daily Dosing
Although 400 mg of daily imatinib proved to be efficacious, it was unclear if a dose-response relationship existed for imatinib. An EORTC phase 2 study demonstrated a benefit of using a higher dose of imatinib at 400 mg twice daily, producing a RR of 71% (4% complete , 67% partial) and 1-year PFS of 73%, which appeared favorable compared with once-daily dosing and set the framework for larger phase 3 studies.7 Two phase 3 studies compared imatinib 400 mg once daily versus twice daily (until disease progression or unacceptable toxicity) among patients with CD117-positive advanced or metastatic GISTs. These studies were eventually combined into a meta-analysis (metaGIST) to compare RR, PFS and OS between the treatment groups. Both studies allowed cross-over to the 800 mg dose for patients who progressed on 400 mg daily.
The first study, conducted jointly by the EORTC, Italian Sarcoma Group, and Australasian Gastro-Intestinal Trials Group (EU-AUS),8 randomly assigned 946 patients to 400 mg once daily or twice daily. There were no differences in response rates between the groups, but the twice-daily group had a predicted 18% reduction in the hazard for progression compared with the once-daily group (estimated HR, 0.82; P = 0.026), which came at the expense of greater toxicities warranting dose reductions (60%) and treatment interruptions (64%). Cross-over to high-dose imatinib was feasible and safe, producing a partial response in 2%, stable disease in 27%, and a median PFS of 81 days. The second study was an intergroup study conducted jointly by SWOG, CALGB, NCI-C, and ECOG (S0033, US-CDN), with a nearly identical study design as the EU-AUS trial.9 The trial enrolled 746 patients. After a median follow up of 4.5 years, the median PFS and OS were not statistically different (18 vs 20 months and 55 vs 51 months, respectively). There were also no differences in response rates. One third of patients initially placed on the once-daily arm who crossed over after progression achieved a treatment response or stable disease.
The combined EU-AUS and US-CDN analysis (metaGIST) included 1640 patients with a median age of 60 years and 58% of whom were men; 818 and 822 patients were assigned to the 400 mg and 800 mg total daily doses, respectively.10 The median follow-up was 37.5 months. There were no differences in OS (49 vs 48.7 months), median PFS (18.9 vs 23.2 months), or overall response rates (51.4% vs 53.9%). Patients who had crossed over (n = 347) to the 800 mg total daily dose arm had a 7.7-month average PFS while on the higher daily dose. An analysis was performed on 377 patients in the EU-AUS trial assessing the impact of mutational status on clinical outcomes among imatinib-treated patients. KIT exon 9 activating mutations were found to be a significant independent prognostic factor for death when compared with KIT exon 11 mutations. However, the adverse prognostic value of KIT exon 9 mutations was partially overcome with higher doses of imatinib, as those who received 800 mg total had a significantly better PFS, with a 61% relative risk reduction, than those who received 400 mg. Altogether, it was concluded that imatinib 400 mg once daily should be the standard-of-care first-line treatment for advanced or metastatic GISTs, unless a KIT exon 9 mutation is present, in which case imatinib 800 mg should be considered, if 400 mg is well tolerated. In addition, patients treated with frontline imatinib at 400 mg once daily, if tolerated well, should be considered for imatinib 800 mg upon progression of disease.
Despite there being problems with secondary resistance, significant progress has occurred in the treatment of metastatic disease over a short period of time. Prior to 2000, median OS for patients with metastatic GISTs was 9 months. With the introduction of imatinib and other TKIs, the median OS has increased to 5 years, with an estimated 10-year OS rate of approximately 20%.2
Imatinib Interruption
Since at this point, imatinib was a well-established standard of care for advanced GISTs, it was questioned whether imatinib therapy could be interrupted. At this time, treatment interruption in a stop-and-go fashion was deemed feasible in other metastatic solid tumors such as colorectal cancer (OPTIMOX1).11 The BFR French trial showed that stopping imatinib therapy in patients who had a response or stable disease after 1, 3, or 5 years was generally followed by relatively rapid tumor progression (approximately 50% of patients within 6 months), even when tumors were previously removed.12 Therefore, it is recommended that treatment in the metastatic setting should be continued indefinitely, unless there is disease progression. Hence, unlike with colorectal cancer or chronic myelogenous leukemia, as of now there is no role for imatinib interruption in metastatic GISTs.
Case Continued
The patient is started on imatinib 400 mg daily, and overall he tolerates therapy well. Interval CT imaging reveals a treatment response. Two years later, imaging reveals an increase in the tumor size and density with a new nodule present within a preexisting mass. There are no clinical trials in the area.
What defines tumor progression?
Disease Progression
When GISTs are responding to treatment, on imaging the tumors can become more cystic and less dense but with an increase in size. In addition, tumor progression may not always be associated with increased size—increased density of the tumor or a nodule within a mass that may indicate progression. If CT imaging is equivocal for progression, positron emission tomography (PET) can play a role in identifying true progression. It is critically important that tumor size and density are carefully assessed when performing interval imaging. Of note, radiofrequency ablation, cryotherapy, or chemoembolization can be used for symptomatic liver metastases or oligometastatic disease. When evaluating for progression, one needs to ask patients about compliance (ie, maintaining dose intensity related to side effects of therapy as well as the financial burden of treatment—copay toxicity).
What are mechanisms of secondary imatinib resistance?
Imatinib resistance can be subtle in patients with GISTs, manifesting with new nodular, enhancing foci enclosed within a preexisting mass (resistant clonal nodule), or can be clinically or radiographically overt.13 Imatinib resistance occurs through multiple mechanisms including acquisition of secondary activating KIT mutations in the intracellular ATP-binding domain (exons 13 and 14) and the activation loop (exons 17 and 18).14
What are the treatment options for this patient?
Second-line Therapy
Sunitinib malate is a multitargeted TKI that not only targets c-Kit and PDGFRA, but also has anti-angiogenic activity through inhibition of vascular endothelial growth factor receptors (VEGFR). Sunitinib gained FDA approval for the second-line treatment of advanced GISTs based on an international double-blind trial that randomized 312 patients with imatinib-resistant metastatic GISTs in a 2:1 fashion to receive sunitinib 50 mg daily for 4 weeks on and 2 weeks off or placebo.15,16 The trial was unblinded early at the planned interim analysis, which revealed a marked benefit, producing a 66% reduction in the hazard risk of progression (27.3 vs 6.4 weeks, HR, 0.33; P < 0.001). The most common treatment-related adverse events were fatigue, diarrhea, skin discoloration, nausea, and hand-foot syndrome. Another open-label phase 2 study assessed a continuous dosing schema of sunitinib 37.5 mg daily, which has been shown to be effective with less toxicity.17 Among the 60 patients enrolled, the primary endpoint of clinical benefit rate at 24 weeks was reached in 53%, which consisted of 13% partial responses and 40% stable disease. Most toxicities were grade 1 or 2 and easily manageable through standard interventions. This has been recommended as an alternative to the initial scheduled regimen.18 Part of sunitinib’s success is its activity against GISTs harboring secondary KIT exon 13 and 14 mutations, and possibly its anti-angiogenic activity.19 Sunitinib is particularly efficacious among GISTs harboring KIT exon 9 mutations.
Third-line Therapy
Patients who have progressed on prior imatinib and sunitinib can receive third-line regorafenib, a multi-TKI that differs chemically from sorafenib by a fluorouracil group (fluoro-sorafenib). FDA approval of regorafenib was based on the phase 3 GRID (GIST Regorafenib In progressive Disease) multicenter international trial.20 This trial randomly assigned 199 patients in a 2:1 fashion to receive regorafenib 160 mg daily for 21 days out of 28-day cycles plus best supportive care (BSC) versus placebo plus BSC. Cross-over was allowed. Regorafenib significantly reduced the hazard risk of progression by 73% compared with placebo (4.8 vs 0.9 months; HR, 0.27; P < 0.001). There was no difference in OS, which may be because of cross-over (median OS, 17.4 months in both arms). As a result, regorafenib is now considered standard third-line treatment for patients with metastatic GISTs. It has a less favorable toxicity profile than imatinib, with hand-foot syndrome, transaminitis, hypertension and fatigue being the most common treatment toxicities. In order to avoid noncompliance, it is recommended to start at 80 mg and carefully titrate upwards to the 160 mg dose.
A list of landmark studies for advanced GISTs is provided in Table 1.

A summary of FDA-approved drugs for treating GISTs is provided in Table 2.

Clinical Trials
Clinical trial enrollment should be considered for all patients with advanced or unresectable GISTs throughout their treatment continuum. Owing to significant advances in genomic profiling through next-generation sequencing, multiple driver mutations have recently been identified, and targeted therapies are being explored in clinical trials.21 For example, the neurotrophic receptor tyrosine kinase (NTRK) gene appears to be mutated in a small number of advanced GISTs, and these can respond to the highly selective TRK inhibitor larotrectinib.22 Additionally, ongoing studies are assessing immunotherapies for sporadic GISTs and treatment for familial GISTs (Table 3). Some notable studies include those assessing the efficacy of agents that target KIT and PDGFR secondary mutations, including avapritinib (BLU-285) and DCC-2618, MEK inhibitors, and the multi-kinase inhibitor crenolanib for GISTs harboring the imatinib-resistant PDGFRA D842V mutation. There are also studies utilizing checkpoint inhibitors alone or in combination with imatinib.

Case Conclusion
Given the patient’s progression on imatinib, he is started on second-line sunitinib malate. He experiences grade 1 fatigue and hand-foot syndrome, which are managed supportively. After he has been on sunitinib for approximately 8 months, his disease progresses. He subsequently undergoes genomic profiling of his tumor and starts BLU-285 on a clinical trial.
Key Points
- For advanced and metastatic disease, TKIs have substantially improved the prognosis of KIT mutated GISTs, with 3 FDA-approved drugs: imatinib, sunitinib, and regorafenib. Imatinib 400 mg is the standard-of-care frontline therapy for locally advanced, unresectable, or metastatic imatinib-sensitive GISTs. If a patient has a KIT exon 9 mutation and 400 mg is well-tolerated, increasing to 800 mg is recommended. Imatinib should be continued indefinitely unless there is intolerance, a specific patient request for interruption, or progression of disease.
- When there is progression of disease in a patient with a sensitive mutation on 400 mg of imatinib, the dose can be increased to 800 mg.
- For patients who are imatinib-intolerant or have progression, standard second line is sunitinib.
- For patients who further progress or are sunitinib-intolerant, regorafenib is the standard third-line treatment.
- There needs to be close attention to side effects, drug and food interactions, and patient copay costs in order to maintain patient compliance while on TKI therapy.
- There are still major limitations in the systemic treatment of GISTs marked by their inherent genetic heterogeneity and secondary resistance. Continued translational and clinical research is needed in order to improve treatment for patients who develop secondary resistance or who have less common primary resistant mutations. Patients are encouraged to participate in clinical trials of new therapies.
Summary
GISTs are the most common mesenchymal tumors of the GI tract. They comprise an expanding landscape of tumors that are heterogenous in terms of natural history, mutations, and response to systemic treatments. The mainstay of treatment for localized GISTs is surgical resection followed by at least 3-years of adjuvant imatinib for patients with high-risk features who are imatinib-sensitive. Patients with GISTs harboring resistance mutations such as PDGFRA D842V or with SDH-deficient or NF1-associated GISTs should not receive adjuvant imatinib. Patients with more advanced GISTs and/or in difficult to resect sites harboring a sensitive mutation can be considered for neoadjuvant imatinib. Those with metastatic GISTs can receive first-, second-, and third-line imatinib, sunitinib, or regorafenib, respectively. Clinical trial enrollment should be encouraged for patients whose GISTs harbor primary imatinib-resistant mutations, and those with advanced or unresectable GISTs with secondary resistance.
1. Ma GL, Murphy JD, Martinez ME et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298-302.
2. Heinrich MC, Rankin C, Blanke CD, et al. Correlation of long-term results of imatinib in advanced gastrointestinal stromal tumors with next-generation sequencing results: analysis of phase 3 SWOG Intergroup Trial S0033. JAMA Oncol. 2017;3:944-952.
3. DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51-58.
4. Goss GA, Merriam P, Manola J, et al. Clinical and pathological characteristics of gastrointestinal stromal tumors (GIST). Prog Proc Am Soc Clin Oncol. 2000;19:599a.
5. Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472-480.
6. Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase ii trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620-625.
7. Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevac) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer. 2003;39:2006-2011.
8. Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomized trial. Lancet. 2004;364:1127-1134.
9. Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626-632.
10. Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010;28:1247-1253.
11. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer –a GERCOR study. J Clin Oncol. 2006;24:394-400.
12. Blay JV, Cesne AL, Ray-Coquard I, et al. Prospective multicentric randomized phase iii study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: The French Sarcoma Group. J Clin Oncol. 2007;25:1107-1113.
13. Desai J, Shankar S, Heinrich MC, et al. Clonal evolution of resistance to imatinib in patients with metastatic gastrointestinal stromal tumors. Clin Cancer Res. 2007;13(18 Pt 1): 5398-5405.
14. Gramza AW, Corless CL, Heinrich MC. Resistance to tyrosine kinase inhibitors in gastrointestinal stromal tumors. Clin Cancer Res. 2009;15:7510-7518.
15. Sutent (sunitinib malate) [package insert]. New York, NY: Pfizer Labs; 2017.
16. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomized controlled trial. Lancet. 2006;368:1329-1338.
17. George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45:1959-1968.
18. Brennan MF, Antonescu CR, Maki RG. Management of Soft Tissue Sarcomas. Switzerland: Springer International Publishing; 2013.
19. Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumors. J Clin Oncol. 2008;26:5352-5359.
20. Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295-302.
21. Wilky BA, Villalobos VM. Emerging role for precision therapy through next-generation sequencing for sarcomas. JCO Precision Oncology. 2018;2:1-4.
22. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in trk fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731-739.
1. Ma GL, Murphy JD, Martinez ME et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298-302.
2. Heinrich MC, Rankin C, Blanke CD, et al. Correlation of long-term results of imatinib in advanced gastrointestinal stromal tumors with next-generation sequencing results: analysis of phase 3 SWOG Intergroup Trial S0033. JAMA Oncol. 2017;3:944-952.
3. DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51-58.
4. Goss GA, Merriam P, Manola J, et al. Clinical and pathological characteristics of gastrointestinal stromal tumors (GIST). Prog Proc Am Soc Clin Oncol. 2000;19:599a.
5. Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472-480.
6. Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase ii trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620-625.
7. Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevac) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer. 2003;39:2006-2011.
8. Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomized trial. Lancet. 2004;364:1127-1134.
9. Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626-632.
10. Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010;28:1247-1253.
11. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer –a GERCOR study. J Clin Oncol. 2006;24:394-400.
12. Blay JV, Cesne AL, Ray-Coquard I, et al. Prospective multicentric randomized phase iii study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: The French Sarcoma Group. J Clin Oncol. 2007;25:1107-1113.
13. Desai J, Shankar S, Heinrich MC, et al. Clonal evolution of resistance to imatinib in patients with metastatic gastrointestinal stromal tumors. Clin Cancer Res. 2007;13(18 Pt 1): 5398-5405.
14. Gramza AW, Corless CL, Heinrich MC. Resistance to tyrosine kinase inhibitors in gastrointestinal stromal tumors. Clin Cancer Res. 2009;15:7510-7518.
15. Sutent (sunitinib malate) [package insert]. New York, NY: Pfizer Labs; 2017.
16. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomized controlled trial. Lancet. 2006;368:1329-1338.
17. George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45:1959-1968.
18. Brennan MF, Antonescu CR, Maki RG. Management of Soft Tissue Sarcomas. Switzerland: Springer International Publishing; 2013.
19. Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumors. J Clin Oncol. 2008;26:5352-5359.
20. Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295-302.
21. Wilky BA, Villalobos VM. Emerging role for precision therapy through next-generation sequencing for sarcomas. JCO Precision Oncology. 2018;2:1-4.
22. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in trk fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731-739.
Artificial intelligence in psychiatry
For many people, artificial intelligence (AI) brings to mind some form of humanoid robot that speaks and acts like a human. However, AI is much more than merely robotics and machines. Professor John McCarthy of Stanford University, who first coined the term “artificial intelligence” in the early 1950s, defined it as “the science and engineering of making intelligent machines, especially intelligent computer programs”; he defined intelligence as “the computational part of the ability to achieve goals.”1 Artificial intelligence also is commonly defined as the development of computer systems able to perform tasks that normally require human intelligence.2 English Mathematician Alan Turing is considered one of the forefathers of AI research, and devised the first test to determine if a computer program was intelligent (Box 13). Today, AI has established itself as an integral part of medicine and psychiatry.
Box 1
During World War II, the English Mathematician Alan Turing helped the British government crack the Enigma machine, a coding device used by the Nazi army. He went on to pioneer many research projects in the field of artificial intelligence, including developing the Turing Test, which can determine if a computer program is intelligent.3 In this test, a human questioner uses a computer interface to pose questions to 2 respondents in different rooms; one of the respondents is a human and the other a computer program. If the questioner cannot tell the difference between the 2 respondents’ answers, then the computer program is deemed to be “artificially intelligent” because it can pass
The semantics of AI
Two subsets of AI are machine learning and deep learning.4,5 Machine learning is defined as a set of methods that can automatically detect patterns in data and then use the uncovered pattern to predict future data.4 Deep learning is a form of machine learning that enables computers to learn from experience and understand the world in terms of a hierarchy of concepts.5
Machine learning can be supervised, semi-supervised, or unsupervised. The majority of practical machine learning uses supervised learning, where all data are labeled and an algorithm is used to learn the mapping function from the input to the output. In unsupervised learning, all data are unlabeled and the algorithm models the underlying structure of the data by itself. Semi-supervised learning is a mixture of both.6
Many researchers also categorize AI into 2 types: general or “strong” AI, and narrow or “weak” AI. Strong AI is defined as computers that can think on a level at least equal to humans and are able to experience emotions and even consciousness.7 Weak AI includes adding “thinking-like” features to computers to make them more useful tools. Almost all AI technologies available today are considered to be weak AI.
AI in medicine
AI is being developed for a broad range of applications in medicine. This includes informatics approaches, including learning in health management systems such as electronic health records, and actively guiding physicians in their treatment decisions.8
AI has been applied to assist administrative workflows that reach beyond automated non-patient care activities such as chart documentation and placing orders. One example is the Judy Reitz Capacity Command Center, which was designed and built with GE Healthcare Partners.9 It combines AI technology in the form of systems engineering and predictive analytics to better manage multiple workflows in different administrative settings, including patient safety, volume, flow, and access to care.9
In April 2018, Intel Corporation surveyed 200 health-care decision makers in the United States regarding their use of AI in practice and their attitudes toward it.10 Overall, 37% of respondents reported using AI and 54% expected to increase their use of AI in the next 5 years. Clinical use of AI (77%) was more common than administrative use (41%) or financial use (26 %).10
Continue to: Box 2
Box 211-19 describes studies that evaluated the clinical use of AI in specialties other than psychiatry.
Box 2
Ophthalmology. Multiple studies have evaluated using artificial intelligence (AI) to screen for diabetic retinopathy, which is one of the fastest growing causes of blindness worldwide.11 In a recent study, researchers used a deep learning algorithm to automatically detect diabetic retinopathy and diabetic macular edema by analyzing retinal images. It was trained over a dataset of 128,000 images that were evaluated by 3 to 7 ophthalmologists. The algorithm showed high sensitivity and specificity for detecting referable diabetic retinopathy.11
Cardiology. One study looked at training a deep learning algorithm to predict cardiovascular risk based on analysis of retinal fundus images from 284,335 patients. In this study, the algorithm was able to predict a cardiovascular event in the next 5 years with 70% accuracy.12 The results were based on risk factors not previously thought to be quantifiable in retinal images, such as age, gender, smoking status, systolic blood pressure, and major adverse cardiac events.12 Similarly, researchers in the United Kingdom wanted to assess AI’s ability to predict a first cardiovascular event over 10 years by comparing a machine-learning algorithm to current guidelines from the American College of Cardiology, which include age, smoking history, cholesterol levels, and diabetes history.13 The algorithm was applied to data from approximately 82,000 patients known to have a future cardiac event. It was able to significantly improve the accuracy of cardiovascular risk prediction.13
Radiology. Researchers in the Department of Radiology at Thomas Jefferson University Hospital trained 2 convolutional neural networks (CNNs), AlexNet and GoogleNet, on 150 chest X-ray images to diagnose the presence or absence of tuberculosis (TB).14 They found that the CNNs could accurately classify TB on chest X-ray, with an area under the curve of 0.99.14 The best-performing AI model was a combination of the 2 networks, which had an accuracy of 96%.14
Stroke. The ALADIN trial compared an AI algorithm vs 2 trained neuroradiologists for detecting large artery occlusions on 300 CT scans.15 The algorithm had a sensitivity of 97%, a specificity of 52%, and an accuracy of 78%.15
Surgery. AI in the form of surgical robots has been around for many decades. Probably the best-known surgical robot is the da Vinci Surgical System, which was FDA-approved in 2000 for laparoscopic procedures.16 The da Vinci Surgical System functions as an extension of the human surgeon, who controls the device from a nearby console. Researchers at McGill University developed an anesthesia robot called “McSleepy” that can analyze biological information and recognize malfunctions while constantly adapting its own behavior.17
Dermatology. One study compared the use of deep CNNs vs 21 board-certified dermatologists to identify skin cancer on 2,000 biopsy-proven clinical images.18 The CNNs were capable of classifying skin cancer with a level of competence comparable to that of the dermatologists.18
Pathology. One study compared the efficacy of a CNN to that of human pathologists in detecting breast cancer metastasis to lymph nodes on microscopy images.19 The CNN detected 92.4% of the tumors, whereas the pathologists had a sensitivity of 73.2%.19
How can AI be used in psychiatry?
Artificially intelligent technologies have been used in psychiatry for several decades. One of the earliest examples is ELIZA, a computer program published by Professor Joseph Weizenbaum of the Massachusetts Institute of Technology in 1966.20 ELIZA consisted of a language analyzer and a script or a set of rules to improvise around a certain theme; the script DOCTOR was used to simulate a Rogerian psychotherapist.20
The application of AI in psychiatry has come a long way since the pioneering work of Weizenbaum. A recent study examined AI’s ability to distinguish between an individual who had suicidal ideation vs a control group. Machine-learning algorithms were used to evaluate functional MRI scans of 34 participants (17 who had suicidal ideation and 17 controls) to identify certain neural signatures of concepts related to life and death.21 The machine-learning algorithms were able to distinguish between these 2 groups with 91% accuracy. They also were able to distinguish between individuals who attempted suicide and those who did not with 94% accuracy.21
A study from the University of Cincinnati looked at using machine learning and natural language processing to distinguish genuine suicide notes from “fake” suicide notes that had been written by a healthy control group.22 Sixty-six notes were evaluated and categorized by 11 mental health professionals (psychiatrists, social workers, and emergency medicine physicians) and 31 PGY-3 residents. The accuracy of their results was compared with that of 9 machine-learning algorithms.22 The best machine-learning algorithm accurately classified the notes 78% of the time, compared with 63% of the time for the mental health professionals and 49% of the time for the residents.22
Researchers at Vanderbilt University examined using machine learning to predict suicide risk.23 They developed algorithms to scan electronic health records of 5,167 adults, 3,250 of whom had attempted suicide. In a review of the patients’ data from 1 week to 2 years before the attempt, the algorithms looked for certain predictors of suicide attempts, including recurrent depression, psychotic disorder, and substance use. The algorithm was 80% accurate at predicting whether a patient would attempt suicide within the next 2 years, and 84% accurate at predicting an attempt within the next week.23
Continue to: In a prospective study...
In a prospective study, researchers at Cincinnati Children’s Hospital used a machine-learning algorithm to evaluate 379 patients who were categorized into 3 groups: suicidal, mentally ill but not suicidal, or controls.24 All participants completed a standardized behavioral rating scale and participated in a semi-structured interview. Based on the participants’ linguistic and acoustic characteristics, the algorithm was able to classify them into the 3 groups with 85% accuracy.24
Many studies have looked at using language analysis to predicting the risk of psychosis in at-risk individuals. In one study, researchers evaluated individuals known to be at high risk for developing psychosis, some of whom eventually did develop psychosis.25 Participants were asked to retell a story and to answer questions about that story. Researchers fed the transcripts of these interviews into a language analysis program that looked at semantic coherence, syntactic complexity, and other factors. The algorithm was able to predict the future occurrence of psychosis with 82% accuracy. Participants who converted to psychosis had decreased semantic coherence and reduced syntactic complexity.25
A similar study looked at 34 at-risk youth in an attempt to predict who would develop psychosis based on speech pattern analysis.26 The participants underwent baseline interviews and were assessed quarterly for 2.5 years. The algorithm was able to predict who would develop psychosis with 100% accuracy.26
Challenges and limitations
The amount of research about applying machine learning to various fields of psychiatry continues to grow. With this increased interest, there have been reports of bias and human influence in the various stages of machine learning. Therefore, being aware of these challenges and engaging in practices to minimize their effects are necessary. Such practices include providing more details on data collection and processing, and constantly evaluating machine learning models for their relevance and utility to the research question proposed.27
As is the case with most innovative, fast-growing technologies, AI has its fair share of criticisms and concerns. Critics have focused on the potential threat of privacy issues, medical errors, and ethical concerns. Researchers at the Stanford Center for Biomedical Ethics emphasize the importance of being aware of the different types of bias that humans and algorithm designs can introduce into health data.28
Continue to: The Nuffield Council on Bioethics...
The Nuffield Council on Bioethics also emphasizes the importance of identifying the ethical issues raised by using AI in health care. Concerns include erroneous decisions made by AI and determining who is responsible for such errors, difficulty in validating the outputs of AI systems, and the potential for AI to be used for malicious purposes.29
For clinicians who are considering implementing AI into their practice, it is vital to recognize where this technology belongs in a workflow and in the decision-making process. Jeffery Axt, a researcher on the clinical applications of AI, encourages clinicians to view using AI as a consulting tool to eliminate the element of fear associated with not having control over diagnostics and management.30
What’s on the horizon
Research into using AI in psychiatry has drawn the attention of large companies. IBM is building an automated speech analysis application that uses machine learning to provide a real-time overview of a patient’s mental health.31 Social media platforms are also starting to incorporate AI technologies to scan posts for language and image patterns suggestive of suicidal thoughts or behavior.32
“Chat bots”—AI that can conduct a conversation in natural language—are becoming popular as well. Woebot is a cognitive-behavioral therapy–based chat bot designed by a Stanford psychologist that can be accessed through Facebook Messenger. In a 2-week study, 70 young adults (age 18 to 28) with depression were randomly assigned to use Woebot or to read mental health e-books.33 Participants who used Woebot experienced a significant reduction in depressive symptoms as measured by change in score on the Patient Health Questionnaire-9, while those assigned to the reading group did not.33
Other researchers have focused on identifying patterns of inattention, hyperactivity, and impulsivity in children using AI technologies such as computer vision, machine learning, and data mining. For example, researchers at the University of Texas at Arlington and Yale University are analyzing data from watching children perform certain tasks involving attention, decision making, and emotion management.34 There have been several advances in using AI to note abnormalities in a child’s gaze pattern that might suggest autism.35
Continue to: A project at...
A project at the University of Southern California called SimSensei/Multisense uses software to track real-time behavior descriptors such as facial expressions, body postures, and acoustic features that can help identify psychological distress.36 This software is combined with a virtual human platform that communicates with the patient as a therapist would.36
The future of AI in health care appears to have great possibilities. Putting aside irrational fears of being replaced by computers one day, AI may someday be highly transformative, leading to vast improvements in patient care.
Bottom Line
Artificial intelligence (AI) —the development of computer systems able to perform tasks that normally require human intelligence—is being developed for use in a wide range of medical specialties. Potential uses in psychiatry include predicting a patient’s risk for suicide or psychosis. Privacy concerns, ethical issues, and the potential for medical errors are among the challenges of AI use in psychiatry.
Related Resources
- Durstewitz D, Koppe G, Meyer-Lindenberg A. Deep neural networks in psychiatry. Mol Psychiatry. 2019. doi:10.1038/s41380-019-0365-9.
- Kretzschmar K, Tyroll H, Pavarini G, et al; NeurOx Young People’s Advisory Group. Can your phone be your therapist? Young people’s ethical perspectives on the use of fully automated conversational agents (chatbots) in mental health support. Biomed Inform Insights. 2019;11:1178222619829083. doi: 10.1177/1178222619829083.
1. McCarthy J. What is AI? Basic questions. http://jmc.stanford.edu/artificial-intelligence/what-is-ai/index.html. Accessed July 19, 2019.
2. Oxford Reference. Artificial intelligence. http://www.oxfordreference.com/view/10.1093/oi/authority.20110803095426960. Accessed July 19, 2019.
3. Turing AM. Computing machinery and intelligence. Mind. 1950;49:433-460.
4. Robert C. Book review: machine learning, a probabilistic perspective. CHANCE. 2014;27:2:62-63.
5. Goodfellow I, Bengio Y, Courville A. Deep learning. Cambridge, MA: The MIT Press; 2016.
6. Brownlee J. Supervised and unsupervised machine learning algorithms. https://machinelearningmastery.com/supervised-and-unsupervised-machine-learning-algorithms/. Published March 16, 2016. Accessed July 19, 2019.
7. Russell S, Norvig P. Artificial intelligence: a modern approach. Upper Saddle River, NJ: Pearson; 1995.
8. Hamet P, Tremblay J. Artificial intelligence in medicine. Metabolism. 2017;69S:S36-S40.
9. The Johns Hopkins hospital launches capacity command center to enhance hospital operations. Johns Hopkins Medicine. https://www.hopkinsmedicine.org/news/media/releases/the_johns_hopkins_hospital_launches_capacity_command_center_to_enhance_hospital_operations. Published October 26, 2016. Accessed July, 19 2019.
10. U.S. healthcare leaders expect widespread adoption of artificial intelligence by 2023. Intel. https://newsroom.intel.com/news-releases/u-s-healthcare-leaders-expect-widespread-adoption-artificial-intelligence-2023/#gs.7j7yjk. Published July 2, 2018. Accessed July, 19 2019.
11. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2410.
12. Poplin R, Varadarajan AV, Blumer K, et al. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nature Biomedical Engineering. 2018;2:158-164.
13. Weng SF, Reps J, Kai J, et al. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS One. 2017;12(4):e0174944. doi: 10.1371/journal.pone. 0174944.
14. Lakhani P, Sundaram B. Deep learning at chest radiography: Automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582.
15. Bluemke DA. Radiology in 2018: Are you working with ai or being replaced by AI? Radiology. 2018;287(2):365-366.
16. Kakar PN, Das J, Roy PM, et al. Robotic invasion of operation theatre and associated anaesthetic issues: A review. Indian J Anaesth. 2011;55(1):18-25.
17. World first: researchers develop completely automated anesthesia system. McGill University. https://www.mcgill.ca/newsroom/channels/news/world-first-researchers-develop-completely-automated-anesthesia-system-100263. Published May 1, 2008. Accessed July 19, 2019.
18. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115-118.
19. Liu Y, Gadepalli K, Norouzi M, et al. Detecting cancer metastases on gigapixel pathology images. https://arxiv.org/abs/1703.02442. Published March 8, 2017. Accessed July 19, 2019.
20. Bassett C. The computational therapeutic: exploring Weizenbaum’s ELIZA as a history of the present. AI & Soc. 2018. https://doi.org/10.1007/s00146-018-0825-9.
21. Just MA, Pan L, Cherkassky VL, et al. Machine learning of neural representations of suicide and emotion concepts identifies suicidal youth. Nat Hum Behav. 2017;1:911-919.
22. Pestian J, Nasrallah H, Matykiewicz P, et al. Suicide note classification using natural language processing: a content analysis. Biomed Inform Insights. 2010;2010(3):19-28.
23. Walsh CG, Ribeiro JD, Franklin JC. Predicting risk of suicide attempts over time through machine learning. Clinical Psychological Science. 2017;5(3):457-469.
24. Pestian JP, Sorter M, Connolly B, et al; STM Research Group. A machine learning approach to identifying the thought markers of suicidal subjects: a prospective multicenter trial. Suicide Life Threat Behav. 2017;47(1):112-121.
25. Corcoran CM, Carrillo F, Fernández-Slezak D, et al. Prediction of psychosis across protocols and risk cohorts using automated language analysis. World Psychiatry. 2018;17(1):67-75.
26. Bedi G, Carrillo F, Cecchi GA, et al. Automated analysis of free speech predicts psychosis onset in high-risk youths. NPJ Schizophr. 2015;1:15030. doi:10.1038/npjschz.2015.30.
27. Tandon N, Tandon R. Will machine learning enable us to finally cut the Gordian Knot of schizophrenia. Schizophr Bull. 2018;44(5):939-941.
28. Char DS, Shah NH, Magnus D. Implementing machine learning in health care - addressing ethical challenges. N Engl J Med. 2018;378(11):981-983.
29. Nuffield Council on Bioethics. The big ethical questions for artificial intelligence (AI) in healthcare. http://nuffieldbioethics.org/news/2018/big-ethical-questions-artificial-intelligence-ai-healthcare. Published May 15, 2018. Accessed July 19, 2019.
30. Axt J. Artificial neural networks: a systematic review of their efficacy as an innovative resource for health care practice managers. https://www.researchgate.net/publication/322101587_Running_head_ANN_EFFICACY_IN_HEALTHCARE-A_SYSTEMATIC_REVIEW_1_Artificial_Neural_Networks_A_systematic_review_of_their_efficacy_as_an_innovative_resource_for_healthcare_practice_managers. Published October 2017. Accessed July 19, 2019.
31. Cecchi G. IBM 5 in 5: with AI, our words will be a window into our mental health. IBM Research Blog. https://www.ibm.com/blogs/research/2017/1/ibm-5-in-5-our-words-will-be-the-windows-to-our-mental-health/. Published January 5, 2017. Accessed July 19, 2019.
32. Constine J. Facebook rolls out AI to detect suicidal posts before they’re reported. TechCrunch. http://tcrn.ch/2hUBi3B. Published November 27, 2017. Accessed July 19, 2019.
33. Fitzpatrick KK, Darcy A, Vierhile M. Delivering cognitive behavior therapy to young adults with symptoms of depression and anxiety using a fully automated conversational agent (Woebot): a randomized controlled trial. JMIR Ment Health. 2017;4(2):e19. doi:10.2196/mental.7785.
34. UTA researchers use artificial intelligence to assess, enhance cognitive abilities in school-aged children. University of Texas at Arlington. https://www.uta.edu/news/releases/2016/10/makedon-children-learning-difficulties.php. Published October 13, 2016. Accessed July 19, 2019.
35. Nealon C. App for early autism detection launched on World Autism Awareness Day, April 2. University at Buffalo. http://www.buffalo.edu/news/releases/2018/04/001.html. Published April 2, 2018. Accessed July 19, 2019.
36. SimSensei. University of Southern California Institute for Creative Technologies. http://ict.usc.edu/prototypes/simsensei/. Accessed July 19, 2019.
For many people, artificial intelligence (AI) brings to mind some form of humanoid robot that speaks and acts like a human. However, AI is much more than merely robotics and machines. Professor John McCarthy of Stanford University, who first coined the term “artificial intelligence” in the early 1950s, defined it as “the science and engineering of making intelligent machines, especially intelligent computer programs”; he defined intelligence as “the computational part of the ability to achieve goals.”1 Artificial intelligence also is commonly defined as the development of computer systems able to perform tasks that normally require human intelligence.2 English Mathematician Alan Turing is considered one of the forefathers of AI research, and devised the first test to determine if a computer program was intelligent (Box 13). Today, AI has established itself as an integral part of medicine and psychiatry.
Box 1
During World War II, the English Mathematician Alan Turing helped the British government crack the Enigma machine, a coding device used by the Nazi army. He went on to pioneer many research projects in the field of artificial intelligence, including developing the Turing Test, which can determine if a computer program is intelligent.3 In this test, a human questioner uses a computer interface to pose questions to 2 respondents in different rooms; one of the respondents is a human and the other a computer program. If the questioner cannot tell the difference between the 2 respondents’ answers, then the computer program is deemed to be “artificially intelligent” because it can pass
The semantics of AI
Two subsets of AI are machine learning and deep learning.4,5 Machine learning is defined as a set of methods that can automatically detect patterns in data and then use the uncovered pattern to predict future data.4 Deep learning is a form of machine learning that enables computers to learn from experience and understand the world in terms of a hierarchy of concepts.5
Machine learning can be supervised, semi-supervised, or unsupervised. The majority of practical machine learning uses supervised learning, where all data are labeled and an algorithm is used to learn the mapping function from the input to the output. In unsupervised learning, all data are unlabeled and the algorithm models the underlying structure of the data by itself. Semi-supervised learning is a mixture of both.6
Many researchers also categorize AI into 2 types: general or “strong” AI, and narrow or “weak” AI. Strong AI is defined as computers that can think on a level at least equal to humans and are able to experience emotions and even consciousness.7 Weak AI includes adding “thinking-like” features to computers to make them more useful tools. Almost all AI technologies available today are considered to be weak AI.
AI in medicine
AI is being developed for a broad range of applications in medicine. This includes informatics approaches, including learning in health management systems such as electronic health records, and actively guiding physicians in their treatment decisions.8
AI has been applied to assist administrative workflows that reach beyond automated non-patient care activities such as chart documentation and placing orders. One example is the Judy Reitz Capacity Command Center, which was designed and built with GE Healthcare Partners.9 It combines AI technology in the form of systems engineering and predictive analytics to better manage multiple workflows in different administrative settings, including patient safety, volume, flow, and access to care.9
In April 2018, Intel Corporation surveyed 200 health-care decision makers in the United States regarding their use of AI in practice and their attitudes toward it.10 Overall, 37% of respondents reported using AI and 54% expected to increase their use of AI in the next 5 years. Clinical use of AI (77%) was more common than administrative use (41%) or financial use (26 %).10
Continue to: Box 2
Box 211-19 describes studies that evaluated the clinical use of AI in specialties other than psychiatry.
Box 2
Ophthalmology. Multiple studies have evaluated using artificial intelligence (AI) to screen for diabetic retinopathy, which is one of the fastest growing causes of blindness worldwide.11 In a recent study, researchers used a deep learning algorithm to automatically detect diabetic retinopathy and diabetic macular edema by analyzing retinal images. It was trained over a dataset of 128,000 images that were evaluated by 3 to 7 ophthalmologists. The algorithm showed high sensitivity and specificity for detecting referable diabetic retinopathy.11
Cardiology. One study looked at training a deep learning algorithm to predict cardiovascular risk based on analysis of retinal fundus images from 284,335 patients. In this study, the algorithm was able to predict a cardiovascular event in the next 5 years with 70% accuracy.12 The results were based on risk factors not previously thought to be quantifiable in retinal images, such as age, gender, smoking status, systolic blood pressure, and major adverse cardiac events.12 Similarly, researchers in the United Kingdom wanted to assess AI’s ability to predict a first cardiovascular event over 10 years by comparing a machine-learning algorithm to current guidelines from the American College of Cardiology, which include age, smoking history, cholesterol levels, and diabetes history.13 The algorithm was applied to data from approximately 82,000 patients known to have a future cardiac event. It was able to significantly improve the accuracy of cardiovascular risk prediction.13
Radiology. Researchers in the Department of Radiology at Thomas Jefferson University Hospital trained 2 convolutional neural networks (CNNs), AlexNet and GoogleNet, on 150 chest X-ray images to diagnose the presence or absence of tuberculosis (TB).14 They found that the CNNs could accurately classify TB on chest X-ray, with an area under the curve of 0.99.14 The best-performing AI model was a combination of the 2 networks, which had an accuracy of 96%.14
Stroke. The ALADIN trial compared an AI algorithm vs 2 trained neuroradiologists for detecting large artery occlusions on 300 CT scans.15 The algorithm had a sensitivity of 97%, a specificity of 52%, and an accuracy of 78%.15
Surgery. AI in the form of surgical robots has been around for many decades. Probably the best-known surgical robot is the da Vinci Surgical System, which was FDA-approved in 2000 for laparoscopic procedures.16 The da Vinci Surgical System functions as an extension of the human surgeon, who controls the device from a nearby console. Researchers at McGill University developed an anesthesia robot called “McSleepy” that can analyze biological information and recognize malfunctions while constantly adapting its own behavior.17
Dermatology. One study compared the use of deep CNNs vs 21 board-certified dermatologists to identify skin cancer on 2,000 biopsy-proven clinical images.18 The CNNs were capable of classifying skin cancer with a level of competence comparable to that of the dermatologists.18
Pathology. One study compared the efficacy of a CNN to that of human pathologists in detecting breast cancer metastasis to lymph nodes on microscopy images.19 The CNN detected 92.4% of the tumors, whereas the pathologists had a sensitivity of 73.2%.19
How can AI be used in psychiatry?
Artificially intelligent technologies have been used in psychiatry for several decades. One of the earliest examples is ELIZA, a computer program published by Professor Joseph Weizenbaum of the Massachusetts Institute of Technology in 1966.20 ELIZA consisted of a language analyzer and a script or a set of rules to improvise around a certain theme; the script DOCTOR was used to simulate a Rogerian psychotherapist.20
The application of AI in psychiatry has come a long way since the pioneering work of Weizenbaum. A recent study examined AI’s ability to distinguish between an individual who had suicidal ideation vs a control group. Machine-learning algorithms were used to evaluate functional MRI scans of 34 participants (17 who had suicidal ideation and 17 controls) to identify certain neural signatures of concepts related to life and death.21 The machine-learning algorithms were able to distinguish between these 2 groups with 91% accuracy. They also were able to distinguish between individuals who attempted suicide and those who did not with 94% accuracy.21
A study from the University of Cincinnati looked at using machine learning and natural language processing to distinguish genuine suicide notes from “fake” suicide notes that had been written by a healthy control group.22 Sixty-six notes were evaluated and categorized by 11 mental health professionals (psychiatrists, social workers, and emergency medicine physicians) and 31 PGY-3 residents. The accuracy of their results was compared with that of 9 machine-learning algorithms.22 The best machine-learning algorithm accurately classified the notes 78% of the time, compared with 63% of the time for the mental health professionals and 49% of the time for the residents.22
Researchers at Vanderbilt University examined using machine learning to predict suicide risk.23 They developed algorithms to scan electronic health records of 5,167 adults, 3,250 of whom had attempted suicide. In a review of the patients’ data from 1 week to 2 years before the attempt, the algorithms looked for certain predictors of suicide attempts, including recurrent depression, psychotic disorder, and substance use. The algorithm was 80% accurate at predicting whether a patient would attempt suicide within the next 2 years, and 84% accurate at predicting an attempt within the next week.23
Continue to: In a prospective study...
In a prospective study, researchers at Cincinnati Children’s Hospital used a machine-learning algorithm to evaluate 379 patients who were categorized into 3 groups: suicidal, mentally ill but not suicidal, or controls.24 All participants completed a standardized behavioral rating scale and participated in a semi-structured interview. Based on the participants’ linguistic and acoustic characteristics, the algorithm was able to classify them into the 3 groups with 85% accuracy.24
Many studies have looked at using language analysis to predicting the risk of psychosis in at-risk individuals. In one study, researchers evaluated individuals known to be at high risk for developing psychosis, some of whom eventually did develop psychosis.25 Participants were asked to retell a story and to answer questions about that story. Researchers fed the transcripts of these interviews into a language analysis program that looked at semantic coherence, syntactic complexity, and other factors. The algorithm was able to predict the future occurrence of psychosis with 82% accuracy. Participants who converted to psychosis had decreased semantic coherence and reduced syntactic complexity.25
A similar study looked at 34 at-risk youth in an attempt to predict who would develop psychosis based on speech pattern analysis.26 The participants underwent baseline interviews and were assessed quarterly for 2.5 years. The algorithm was able to predict who would develop psychosis with 100% accuracy.26
Challenges and limitations
The amount of research about applying machine learning to various fields of psychiatry continues to grow. With this increased interest, there have been reports of bias and human influence in the various stages of machine learning. Therefore, being aware of these challenges and engaging in practices to minimize their effects are necessary. Such practices include providing more details on data collection and processing, and constantly evaluating machine learning models for their relevance and utility to the research question proposed.27
As is the case with most innovative, fast-growing technologies, AI has its fair share of criticisms and concerns. Critics have focused on the potential threat of privacy issues, medical errors, and ethical concerns. Researchers at the Stanford Center for Biomedical Ethics emphasize the importance of being aware of the different types of bias that humans and algorithm designs can introduce into health data.28
Continue to: The Nuffield Council on Bioethics...
The Nuffield Council on Bioethics also emphasizes the importance of identifying the ethical issues raised by using AI in health care. Concerns include erroneous decisions made by AI and determining who is responsible for such errors, difficulty in validating the outputs of AI systems, and the potential for AI to be used for malicious purposes.29
For clinicians who are considering implementing AI into their practice, it is vital to recognize where this technology belongs in a workflow and in the decision-making process. Jeffery Axt, a researcher on the clinical applications of AI, encourages clinicians to view using AI as a consulting tool to eliminate the element of fear associated with not having control over diagnostics and management.30
What’s on the horizon
Research into using AI in psychiatry has drawn the attention of large companies. IBM is building an automated speech analysis application that uses machine learning to provide a real-time overview of a patient’s mental health.31 Social media platforms are also starting to incorporate AI technologies to scan posts for language and image patterns suggestive of suicidal thoughts or behavior.32
“Chat bots”—AI that can conduct a conversation in natural language—are becoming popular as well. Woebot is a cognitive-behavioral therapy–based chat bot designed by a Stanford psychologist that can be accessed through Facebook Messenger. In a 2-week study, 70 young adults (age 18 to 28) with depression were randomly assigned to use Woebot or to read mental health e-books.33 Participants who used Woebot experienced a significant reduction in depressive symptoms as measured by change in score on the Patient Health Questionnaire-9, while those assigned to the reading group did not.33
Other researchers have focused on identifying patterns of inattention, hyperactivity, and impulsivity in children using AI technologies such as computer vision, machine learning, and data mining. For example, researchers at the University of Texas at Arlington and Yale University are analyzing data from watching children perform certain tasks involving attention, decision making, and emotion management.34 There have been several advances in using AI to note abnormalities in a child’s gaze pattern that might suggest autism.35
Continue to: A project at...
A project at the University of Southern California called SimSensei/Multisense uses software to track real-time behavior descriptors such as facial expressions, body postures, and acoustic features that can help identify psychological distress.36 This software is combined with a virtual human platform that communicates with the patient as a therapist would.36
The future of AI in health care appears to have great possibilities. Putting aside irrational fears of being replaced by computers one day, AI may someday be highly transformative, leading to vast improvements in patient care.
Bottom Line
Artificial intelligence (AI) —the development of computer systems able to perform tasks that normally require human intelligence—is being developed for use in a wide range of medical specialties. Potential uses in psychiatry include predicting a patient’s risk for suicide or psychosis. Privacy concerns, ethical issues, and the potential for medical errors are among the challenges of AI use in psychiatry.
Related Resources
- Durstewitz D, Koppe G, Meyer-Lindenberg A. Deep neural networks in psychiatry. Mol Psychiatry. 2019. doi:10.1038/s41380-019-0365-9.
- Kretzschmar K, Tyroll H, Pavarini G, et al; NeurOx Young People’s Advisory Group. Can your phone be your therapist? Young people’s ethical perspectives on the use of fully automated conversational agents (chatbots) in mental health support. Biomed Inform Insights. 2019;11:1178222619829083. doi: 10.1177/1178222619829083.
For many people, artificial intelligence (AI) brings to mind some form of humanoid robot that speaks and acts like a human. However, AI is much more than merely robotics and machines. Professor John McCarthy of Stanford University, who first coined the term “artificial intelligence” in the early 1950s, defined it as “the science and engineering of making intelligent machines, especially intelligent computer programs”; he defined intelligence as “the computational part of the ability to achieve goals.”1 Artificial intelligence also is commonly defined as the development of computer systems able to perform tasks that normally require human intelligence.2 English Mathematician Alan Turing is considered one of the forefathers of AI research, and devised the first test to determine if a computer program was intelligent (Box 13). Today, AI has established itself as an integral part of medicine and psychiatry.
Box 1
During World War II, the English Mathematician Alan Turing helped the British government crack the Enigma machine, a coding device used by the Nazi army. He went on to pioneer many research projects in the field of artificial intelligence, including developing the Turing Test, which can determine if a computer program is intelligent.3 In this test, a human questioner uses a computer interface to pose questions to 2 respondents in different rooms; one of the respondents is a human and the other a computer program. If the questioner cannot tell the difference between the 2 respondents’ answers, then the computer program is deemed to be “artificially intelligent” because it can pass
The semantics of AI
Two subsets of AI are machine learning and deep learning.4,5 Machine learning is defined as a set of methods that can automatically detect patterns in data and then use the uncovered pattern to predict future data.4 Deep learning is a form of machine learning that enables computers to learn from experience and understand the world in terms of a hierarchy of concepts.5
Machine learning can be supervised, semi-supervised, or unsupervised. The majority of practical machine learning uses supervised learning, where all data are labeled and an algorithm is used to learn the mapping function from the input to the output. In unsupervised learning, all data are unlabeled and the algorithm models the underlying structure of the data by itself. Semi-supervised learning is a mixture of both.6
Many researchers also categorize AI into 2 types: general or “strong” AI, and narrow or “weak” AI. Strong AI is defined as computers that can think on a level at least equal to humans and are able to experience emotions and even consciousness.7 Weak AI includes adding “thinking-like” features to computers to make them more useful tools. Almost all AI technologies available today are considered to be weak AI.
AI in medicine
AI is being developed for a broad range of applications in medicine. This includes informatics approaches, including learning in health management systems such as electronic health records, and actively guiding physicians in their treatment decisions.8
AI has been applied to assist administrative workflows that reach beyond automated non-patient care activities such as chart documentation and placing orders. One example is the Judy Reitz Capacity Command Center, which was designed and built with GE Healthcare Partners.9 It combines AI technology in the form of systems engineering and predictive analytics to better manage multiple workflows in different administrative settings, including patient safety, volume, flow, and access to care.9
In April 2018, Intel Corporation surveyed 200 health-care decision makers in the United States regarding their use of AI in practice and their attitudes toward it.10 Overall, 37% of respondents reported using AI and 54% expected to increase their use of AI in the next 5 years. Clinical use of AI (77%) was more common than administrative use (41%) or financial use (26 %).10
Continue to: Box 2
Box 211-19 describes studies that evaluated the clinical use of AI in specialties other than psychiatry.
Box 2
Ophthalmology. Multiple studies have evaluated using artificial intelligence (AI) to screen for diabetic retinopathy, which is one of the fastest growing causes of blindness worldwide.11 In a recent study, researchers used a deep learning algorithm to automatically detect diabetic retinopathy and diabetic macular edema by analyzing retinal images. It was trained over a dataset of 128,000 images that were evaluated by 3 to 7 ophthalmologists. The algorithm showed high sensitivity and specificity for detecting referable diabetic retinopathy.11
Cardiology. One study looked at training a deep learning algorithm to predict cardiovascular risk based on analysis of retinal fundus images from 284,335 patients. In this study, the algorithm was able to predict a cardiovascular event in the next 5 years with 70% accuracy.12 The results were based on risk factors not previously thought to be quantifiable in retinal images, such as age, gender, smoking status, systolic blood pressure, and major adverse cardiac events.12 Similarly, researchers in the United Kingdom wanted to assess AI’s ability to predict a first cardiovascular event over 10 years by comparing a machine-learning algorithm to current guidelines from the American College of Cardiology, which include age, smoking history, cholesterol levels, and diabetes history.13 The algorithm was applied to data from approximately 82,000 patients known to have a future cardiac event. It was able to significantly improve the accuracy of cardiovascular risk prediction.13
Radiology. Researchers in the Department of Radiology at Thomas Jefferson University Hospital trained 2 convolutional neural networks (CNNs), AlexNet and GoogleNet, on 150 chest X-ray images to diagnose the presence or absence of tuberculosis (TB).14 They found that the CNNs could accurately classify TB on chest X-ray, with an area under the curve of 0.99.14 The best-performing AI model was a combination of the 2 networks, which had an accuracy of 96%.14
Stroke. The ALADIN trial compared an AI algorithm vs 2 trained neuroradiologists for detecting large artery occlusions on 300 CT scans.15 The algorithm had a sensitivity of 97%, a specificity of 52%, and an accuracy of 78%.15
Surgery. AI in the form of surgical robots has been around for many decades. Probably the best-known surgical robot is the da Vinci Surgical System, which was FDA-approved in 2000 for laparoscopic procedures.16 The da Vinci Surgical System functions as an extension of the human surgeon, who controls the device from a nearby console. Researchers at McGill University developed an anesthesia robot called “McSleepy” that can analyze biological information and recognize malfunctions while constantly adapting its own behavior.17
Dermatology. One study compared the use of deep CNNs vs 21 board-certified dermatologists to identify skin cancer on 2,000 biopsy-proven clinical images.18 The CNNs were capable of classifying skin cancer with a level of competence comparable to that of the dermatologists.18
Pathology. One study compared the efficacy of a CNN to that of human pathologists in detecting breast cancer metastasis to lymph nodes on microscopy images.19 The CNN detected 92.4% of the tumors, whereas the pathologists had a sensitivity of 73.2%.19
How can AI be used in psychiatry?
Artificially intelligent technologies have been used in psychiatry for several decades. One of the earliest examples is ELIZA, a computer program published by Professor Joseph Weizenbaum of the Massachusetts Institute of Technology in 1966.20 ELIZA consisted of a language analyzer and a script or a set of rules to improvise around a certain theme; the script DOCTOR was used to simulate a Rogerian psychotherapist.20
The application of AI in psychiatry has come a long way since the pioneering work of Weizenbaum. A recent study examined AI’s ability to distinguish between an individual who had suicidal ideation vs a control group. Machine-learning algorithms were used to evaluate functional MRI scans of 34 participants (17 who had suicidal ideation and 17 controls) to identify certain neural signatures of concepts related to life and death.21 The machine-learning algorithms were able to distinguish between these 2 groups with 91% accuracy. They also were able to distinguish between individuals who attempted suicide and those who did not with 94% accuracy.21
A study from the University of Cincinnati looked at using machine learning and natural language processing to distinguish genuine suicide notes from “fake” suicide notes that had been written by a healthy control group.22 Sixty-six notes were evaluated and categorized by 11 mental health professionals (psychiatrists, social workers, and emergency medicine physicians) and 31 PGY-3 residents. The accuracy of their results was compared with that of 9 machine-learning algorithms.22 The best machine-learning algorithm accurately classified the notes 78% of the time, compared with 63% of the time for the mental health professionals and 49% of the time for the residents.22
Researchers at Vanderbilt University examined using machine learning to predict suicide risk.23 They developed algorithms to scan electronic health records of 5,167 adults, 3,250 of whom had attempted suicide. In a review of the patients’ data from 1 week to 2 years before the attempt, the algorithms looked for certain predictors of suicide attempts, including recurrent depression, psychotic disorder, and substance use. The algorithm was 80% accurate at predicting whether a patient would attempt suicide within the next 2 years, and 84% accurate at predicting an attempt within the next week.23
Continue to: In a prospective study...
In a prospective study, researchers at Cincinnati Children’s Hospital used a machine-learning algorithm to evaluate 379 patients who were categorized into 3 groups: suicidal, mentally ill but not suicidal, or controls.24 All participants completed a standardized behavioral rating scale and participated in a semi-structured interview. Based on the participants’ linguistic and acoustic characteristics, the algorithm was able to classify them into the 3 groups with 85% accuracy.24
Many studies have looked at using language analysis to predicting the risk of psychosis in at-risk individuals. In one study, researchers evaluated individuals known to be at high risk for developing psychosis, some of whom eventually did develop psychosis.25 Participants were asked to retell a story and to answer questions about that story. Researchers fed the transcripts of these interviews into a language analysis program that looked at semantic coherence, syntactic complexity, and other factors. The algorithm was able to predict the future occurrence of psychosis with 82% accuracy. Participants who converted to psychosis had decreased semantic coherence and reduced syntactic complexity.25
A similar study looked at 34 at-risk youth in an attempt to predict who would develop psychosis based on speech pattern analysis.26 The participants underwent baseline interviews and were assessed quarterly for 2.5 years. The algorithm was able to predict who would develop psychosis with 100% accuracy.26
Challenges and limitations
The amount of research about applying machine learning to various fields of psychiatry continues to grow. With this increased interest, there have been reports of bias and human influence in the various stages of machine learning. Therefore, being aware of these challenges and engaging in practices to minimize their effects are necessary. Such practices include providing more details on data collection and processing, and constantly evaluating machine learning models for their relevance and utility to the research question proposed.27
As is the case with most innovative, fast-growing technologies, AI has its fair share of criticisms and concerns. Critics have focused on the potential threat of privacy issues, medical errors, and ethical concerns. Researchers at the Stanford Center for Biomedical Ethics emphasize the importance of being aware of the different types of bias that humans and algorithm designs can introduce into health data.28
Continue to: The Nuffield Council on Bioethics...
The Nuffield Council on Bioethics also emphasizes the importance of identifying the ethical issues raised by using AI in health care. Concerns include erroneous decisions made by AI and determining who is responsible for such errors, difficulty in validating the outputs of AI systems, and the potential for AI to be used for malicious purposes.29
For clinicians who are considering implementing AI into their practice, it is vital to recognize where this technology belongs in a workflow and in the decision-making process. Jeffery Axt, a researcher on the clinical applications of AI, encourages clinicians to view using AI as a consulting tool to eliminate the element of fear associated with not having control over diagnostics and management.30
What’s on the horizon
Research into using AI in psychiatry has drawn the attention of large companies. IBM is building an automated speech analysis application that uses machine learning to provide a real-time overview of a patient’s mental health.31 Social media platforms are also starting to incorporate AI technologies to scan posts for language and image patterns suggestive of suicidal thoughts or behavior.32
“Chat bots”—AI that can conduct a conversation in natural language—are becoming popular as well. Woebot is a cognitive-behavioral therapy–based chat bot designed by a Stanford psychologist that can be accessed through Facebook Messenger. In a 2-week study, 70 young adults (age 18 to 28) with depression were randomly assigned to use Woebot or to read mental health e-books.33 Participants who used Woebot experienced a significant reduction in depressive symptoms as measured by change in score on the Patient Health Questionnaire-9, while those assigned to the reading group did not.33
Other researchers have focused on identifying patterns of inattention, hyperactivity, and impulsivity in children using AI technologies such as computer vision, machine learning, and data mining. For example, researchers at the University of Texas at Arlington and Yale University are analyzing data from watching children perform certain tasks involving attention, decision making, and emotion management.34 There have been several advances in using AI to note abnormalities in a child’s gaze pattern that might suggest autism.35
Continue to: A project at...
A project at the University of Southern California called SimSensei/Multisense uses software to track real-time behavior descriptors such as facial expressions, body postures, and acoustic features that can help identify psychological distress.36 This software is combined with a virtual human platform that communicates with the patient as a therapist would.36
The future of AI in health care appears to have great possibilities. Putting aside irrational fears of being replaced by computers one day, AI may someday be highly transformative, leading to vast improvements in patient care.
Bottom Line
Artificial intelligence (AI) —the development of computer systems able to perform tasks that normally require human intelligence—is being developed for use in a wide range of medical specialties. Potential uses in psychiatry include predicting a patient’s risk for suicide or psychosis. Privacy concerns, ethical issues, and the potential for medical errors are among the challenges of AI use in psychiatry.
Related Resources
- Durstewitz D, Koppe G, Meyer-Lindenberg A. Deep neural networks in psychiatry. Mol Psychiatry. 2019. doi:10.1038/s41380-019-0365-9.
- Kretzschmar K, Tyroll H, Pavarini G, et al; NeurOx Young People’s Advisory Group. Can your phone be your therapist? Young people’s ethical perspectives on the use of fully automated conversational agents (chatbots) in mental health support. Biomed Inform Insights. 2019;11:1178222619829083. doi: 10.1177/1178222619829083.
1. McCarthy J. What is AI? Basic questions. http://jmc.stanford.edu/artificial-intelligence/what-is-ai/index.html. Accessed July 19, 2019.
2. Oxford Reference. Artificial intelligence. http://www.oxfordreference.com/view/10.1093/oi/authority.20110803095426960. Accessed July 19, 2019.
3. Turing AM. Computing machinery and intelligence. Mind. 1950;49:433-460.
4. Robert C. Book review: machine learning, a probabilistic perspective. CHANCE. 2014;27:2:62-63.
5. Goodfellow I, Bengio Y, Courville A. Deep learning. Cambridge, MA: The MIT Press; 2016.
6. Brownlee J. Supervised and unsupervised machine learning algorithms. https://machinelearningmastery.com/supervised-and-unsupervised-machine-learning-algorithms/. Published March 16, 2016. Accessed July 19, 2019.
7. Russell S, Norvig P. Artificial intelligence: a modern approach. Upper Saddle River, NJ: Pearson; 1995.
8. Hamet P, Tremblay J. Artificial intelligence in medicine. Metabolism. 2017;69S:S36-S40.
9. The Johns Hopkins hospital launches capacity command center to enhance hospital operations. Johns Hopkins Medicine. https://www.hopkinsmedicine.org/news/media/releases/the_johns_hopkins_hospital_launches_capacity_command_center_to_enhance_hospital_operations. Published October 26, 2016. Accessed July, 19 2019.
10. U.S. healthcare leaders expect widespread adoption of artificial intelligence by 2023. Intel. https://newsroom.intel.com/news-releases/u-s-healthcare-leaders-expect-widespread-adoption-artificial-intelligence-2023/#gs.7j7yjk. Published July 2, 2018. Accessed July, 19 2019.
11. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2410.
12. Poplin R, Varadarajan AV, Blumer K, et al. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nature Biomedical Engineering. 2018;2:158-164.
13. Weng SF, Reps J, Kai J, et al. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS One. 2017;12(4):e0174944. doi: 10.1371/journal.pone. 0174944.
14. Lakhani P, Sundaram B. Deep learning at chest radiography: Automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582.
15. Bluemke DA. Radiology in 2018: Are you working with ai or being replaced by AI? Radiology. 2018;287(2):365-366.
16. Kakar PN, Das J, Roy PM, et al. Robotic invasion of operation theatre and associated anaesthetic issues: A review. Indian J Anaesth. 2011;55(1):18-25.
17. World first: researchers develop completely automated anesthesia system. McGill University. https://www.mcgill.ca/newsroom/channels/news/world-first-researchers-develop-completely-automated-anesthesia-system-100263. Published May 1, 2008. Accessed July 19, 2019.
18. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115-118.
19. Liu Y, Gadepalli K, Norouzi M, et al. Detecting cancer metastases on gigapixel pathology images. https://arxiv.org/abs/1703.02442. Published March 8, 2017. Accessed July 19, 2019.
20. Bassett C. The computational therapeutic: exploring Weizenbaum’s ELIZA as a history of the present. AI & Soc. 2018. https://doi.org/10.1007/s00146-018-0825-9.
21. Just MA, Pan L, Cherkassky VL, et al. Machine learning of neural representations of suicide and emotion concepts identifies suicidal youth. Nat Hum Behav. 2017;1:911-919.
22. Pestian J, Nasrallah H, Matykiewicz P, et al. Suicide note classification using natural language processing: a content analysis. Biomed Inform Insights. 2010;2010(3):19-28.
23. Walsh CG, Ribeiro JD, Franklin JC. Predicting risk of suicide attempts over time through machine learning. Clinical Psychological Science. 2017;5(3):457-469.
24. Pestian JP, Sorter M, Connolly B, et al; STM Research Group. A machine learning approach to identifying the thought markers of suicidal subjects: a prospective multicenter trial. Suicide Life Threat Behav. 2017;47(1):112-121.
25. Corcoran CM, Carrillo F, Fernández-Slezak D, et al. Prediction of psychosis across protocols and risk cohorts using automated language analysis. World Psychiatry. 2018;17(1):67-75.
26. Bedi G, Carrillo F, Cecchi GA, et al. Automated analysis of free speech predicts psychosis onset in high-risk youths. NPJ Schizophr. 2015;1:15030. doi:10.1038/npjschz.2015.30.
27. Tandon N, Tandon R. Will machine learning enable us to finally cut the Gordian Knot of schizophrenia. Schizophr Bull. 2018;44(5):939-941.
28. Char DS, Shah NH, Magnus D. Implementing machine learning in health care - addressing ethical challenges. N Engl J Med. 2018;378(11):981-983.
29. Nuffield Council on Bioethics. The big ethical questions for artificial intelligence (AI) in healthcare. http://nuffieldbioethics.org/news/2018/big-ethical-questions-artificial-intelligence-ai-healthcare. Published May 15, 2018. Accessed July 19, 2019.
30. Axt J. Artificial neural networks: a systematic review of their efficacy as an innovative resource for health care practice managers. https://www.researchgate.net/publication/322101587_Running_head_ANN_EFFICACY_IN_HEALTHCARE-A_SYSTEMATIC_REVIEW_1_Artificial_Neural_Networks_A_systematic_review_of_their_efficacy_as_an_innovative_resource_for_healthcare_practice_managers. Published October 2017. Accessed July 19, 2019.
31. Cecchi G. IBM 5 in 5: with AI, our words will be a window into our mental health. IBM Research Blog. https://www.ibm.com/blogs/research/2017/1/ibm-5-in-5-our-words-will-be-the-windows-to-our-mental-health/. Published January 5, 2017. Accessed July 19, 2019.
32. Constine J. Facebook rolls out AI to detect suicidal posts before they’re reported. TechCrunch. http://tcrn.ch/2hUBi3B. Published November 27, 2017. Accessed July 19, 2019.
33. Fitzpatrick KK, Darcy A, Vierhile M. Delivering cognitive behavior therapy to young adults with symptoms of depression and anxiety using a fully automated conversational agent (Woebot): a randomized controlled trial. JMIR Ment Health. 2017;4(2):e19. doi:10.2196/mental.7785.
34. UTA researchers use artificial intelligence to assess, enhance cognitive abilities in school-aged children. University of Texas at Arlington. https://www.uta.edu/news/releases/2016/10/makedon-children-learning-difficulties.php. Published October 13, 2016. Accessed July 19, 2019.
35. Nealon C. App for early autism detection launched on World Autism Awareness Day, April 2. University at Buffalo. http://www.buffalo.edu/news/releases/2018/04/001.html. Published April 2, 2018. Accessed July 19, 2019.
36. SimSensei. University of Southern California Institute for Creative Technologies. http://ict.usc.edu/prototypes/simsensei/. Accessed July 19, 2019.
1. McCarthy J. What is AI? Basic questions. http://jmc.stanford.edu/artificial-intelligence/what-is-ai/index.html. Accessed July 19, 2019.
2. Oxford Reference. Artificial intelligence. http://www.oxfordreference.com/view/10.1093/oi/authority.20110803095426960. Accessed July 19, 2019.
3. Turing AM. Computing machinery and intelligence. Mind. 1950;49:433-460.
4. Robert C. Book review: machine learning, a probabilistic perspective. CHANCE. 2014;27:2:62-63.
5. Goodfellow I, Bengio Y, Courville A. Deep learning. Cambridge, MA: The MIT Press; 2016.
6. Brownlee J. Supervised and unsupervised machine learning algorithms. https://machinelearningmastery.com/supervised-and-unsupervised-machine-learning-algorithms/. Published March 16, 2016. Accessed July 19, 2019.
7. Russell S, Norvig P. Artificial intelligence: a modern approach. Upper Saddle River, NJ: Pearson; 1995.
8. Hamet P, Tremblay J. Artificial intelligence in medicine. Metabolism. 2017;69S:S36-S40.
9. The Johns Hopkins hospital launches capacity command center to enhance hospital operations. Johns Hopkins Medicine. https://www.hopkinsmedicine.org/news/media/releases/the_johns_hopkins_hospital_launches_capacity_command_center_to_enhance_hospital_operations. Published October 26, 2016. Accessed July, 19 2019.
10. U.S. healthcare leaders expect widespread adoption of artificial intelligence by 2023. Intel. https://newsroom.intel.com/news-releases/u-s-healthcare-leaders-expect-widespread-adoption-artificial-intelligence-2023/#gs.7j7yjk. Published July 2, 2018. Accessed July, 19 2019.
11. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2410.
12. Poplin R, Varadarajan AV, Blumer K, et al. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nature Biomedical Engineering. 2018;2:158-164.
13. Weng SF, Reps J, Kai J, et al. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS One. 2017;12(4):e0174944. doi: 10.1371/journal.pone. 0174944.
14. Lakhani P, Sundaram B. Deep learning at chest radiography: Automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582.
15. Bluemke DA. Radiology in 2018: Are you working with ai or being replaced by AI? Radiology. 2018;287(2):365-366.
16. Kakar PN, Das J, Roy PM, et al. Robotic invasion of operation theatre and associated anaesthetic issues: A review. Indian J Anaesth. 2011;55(1):18-25.
17. World first: researchers develop completely automated anesthesia system. McGill University. https://www.mcgill.ca/newsroom/channels/news/world-first-researchers-develop-completely-automated-anesthesia-system-100263. Published May 1, 2008. Accessed July 19, 2019.
18. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115-118.
19. Liu Y, Gadepalli K, Norouzi M, et al. Detecting cancer metastases on gigapixel pathology images. https://arxiv.org/abs/1703.02442. Published March 8, 2017. Accessed July 19, 2019.
20. Bassett C. The computational therapeutic: exploring Weizenbaum’s ELIZA as a history of the present. AI & Soc. 2018. https://doi.org/10.1007/s00146-018-0825-9.
21. Just MA, Pan L, Cherkassky VL, et al. Machine learning of neural representations of suicide and emotion concepts identifies suicidal youth. Nat Hum Behav. 2017;1:911-919.
22. Pestian J, Nasrallah H, Matykiewicz P, et al. Suicide note classification using natural language processing: a content analysis. Biomed Inform Insights. 2010;2010(3):19-28.
23. Walsh CG, Ribeiro JD, Franklin JC. Predicting risk of suicide attempts over time through machine learning. Clinical Psychological Science. 2017;5(3):457-469.
24. Pestian JP, Sorter M, Connolly B, et al; STM Research Group. A machine learning approach to identifying the thought markers of suicidal subjects: a prospective multicenter trial. Suicide Life Threat Behav. 2017;47(1):112-121.
25. Corcoran CM, Carrillo F, Fernández-Slezak D, et al. Prediction of psychosis across protocols and risk cohorts using automated language analysis. World Psychiatry. 2018;17(1):67-75.
26. Bedi G, Carrillo F, Cecchi GA, et al. Automated analysis of free speech predicts psychosis onset in high-risk youths. NPJ Schizophr. 2015;1:15030. doi:10.1038/npjschz.2015.30.
27. Tandon N, Tandon R. Will machine learning enable us to finally cut the Gordian Knot of schizophrenia. Schizophr Bull. 2018;44(5):939-941.
28. Char DS, Shah NH, Magnus D. Implementing machine learning in health care - addressing ethical challenges. N Engl J Med. 2018;378(11):981-983.
29. Nuffield Council on Bioethics. The big ethical questions for artificial intelligence (AI) in healthcare. http://nuffieldbioethics.org/news/2018/big-ethical-questions-artificial-intelligence-ai-healthcare. Published May 15, 2018. Accessed July 19, 2019.
30. Axt J. Artificial neural networks: a systematic review of their efficacy as an innovative resource for health care practice managers. https://www.researchgate.net/publication/322101587_Running_head_ANN_EFFICACY_IN_HEALTHCARE-A_SYSTEMATIC_REVIEW_1_Artificial_Neural_Networks_A_systematic_review_of_their_efficacy_as_an_innovative_resource_for_healthcare_practice_managers. Published October 2017. Accessed July 19, 2019.
31. Cecchi G. IBM 5 in 5: with AI, our words will be a window into our mental health. IBM Research Blog. https://www.ibm.com/blogs/research/2017/1/ibm-5-in-5-our-words-will-be-the-windows-to-our-mental-health/. Published January 5, 2017. Accessed July 19, 2019.
32. Constine J. Facebook rolls out AI to detect suicidal posts before they’re reported. TechCrunch. http://tcrn.ch/2hUBi3B. Published November 27, 2017. Accessed July 19, 2019.
33. Fitzpatrick KK, Darcy A, Vierhile M. Delivering cognitive behavior therapy to young adults with symptoms of depression and anxiety using a fully automated conversational agent (Woebot): a randomized controlled trial. JMIR Ment Health. 2017;4(2):e19. doi:10.2196/mental.7785.
34. UTA researchers use artificial intelligence to assess, enhance cognitive abilities in school-aged children. University of Texas at Arlington. https://www.uta.edu/news/releases/2016/10/makedon-children-learning-difficulties.php. Published October 13, 2016. Accessed July 19, 2019.
35. Nealon C. App for early autism detection launched on World Autism Awareness Day, April 2. University at Buffalo. http://www.buffalo.edu/news/releases/2018/04/001.html. Published April 2, 2018. Accessed July 19, 2019.
36. SimSensei. University of Southern California Institute for Creative Technologies. http://ict.usc.edu/prototypes/simsensei/. Accessed July 19, 2019.
Is it time to taper that opioid? (And how best to do it)
The opioid crisis has brought added scrutiny to opioid prescribing, particularly to health care providers, whom many blame for the genesis of the opioid overdose epidemic. Family physicians are acutely aware of these complexities: By sheer volume, family physicians prescribe more opioid analgesics than any other subspecialist.1
Overwhelmed by opioid prescriptions
Because of a complexity of factors (notably, the influence of the US pharmaceutical industry), the quantity of opioid prescriptions has risen substantially—enough so that, in 2010, opioids were prescribed in great enough quantity to medicate every American around the clock for a month.2 Among people who began abusing opioids in the 2000s, 75% reported that their first opioid was a prescription drug; this is a shift from prior decades, when heroin was the gateway to opioid addiction.3 As the reality of the size of the opioid problem sunk in, many were hopeful that the epidemic would reverse itself as quickly as it began if the medical community would simply prescribe fewer opioids.

Since 2010, the opioid overdose fatality rate has risen dramatically, even though prescription opioid overdose mortality has leveled off, or even declined. 2 One explanation for this paradox? As availability of prescription opioids declined, people suffering from an underlying opioid use disorder (OUD) turned instead first to heroin, then later to potent fentanyl analogues to fuel their addiction. In most communities, the prevalence of fentanyl analogues—alone or more commonly mixed with other opioids—has driven the staggering rise in opioid-related fatalities in recent years.
No question: Prescription opioids played a critical role in the origins of this epidemic, but just withdrawing prescriptions will not result in marked reduction in the epidemic. This quandary is no more apparent than in primary care, where the considerable risk of continuing opioids—especially at high dosages—must be weighed against the potential risks of discontinuation. Adding to this dilemma are lack of access to treatment for patients with an OUD and the continued stigma and misunderstanding of substance use disorders.
In this article, we describe the challenges of long-term opioid use and review necessary protocols and precautions for maintaining or tapering an opioid regimen in patients who suffer chronic pain.
Managing chronic pain is fraught with complexity
Chronic pain is both real and a disease in its own right. Although definitions of chronic pain vary, pain that lasts > 3 months or past the duration of normal tissue healing is typically considered chronic.4 Approximations of prevalence vary, but in 1 study that examined a representative sample, it was estimated that 14.6% of US adults experience chronic pain.5
Patients who report symptoms or a history of chronic pain can elicit negative reactions from physicians—stemming from our biases, which can inadvertently provoke emotions on our part.6 Unflattering portrayals of patients in the media can further fuel unwarranted biases and prejudices.7
Continue to: Preventing, assessing, and treating...
Preventing, assessing, and treating chronic pain can be difficult, at the level of both the individual physician and the larger system of care, even without adding in complications of the opioid epidemic. For racial and ethnic minority groups, women, older people, and people with cognitive impairment or cancer, pain can be underrecognized and go inadequately treated.
Chronic pain itself has clinical, psychological, and social consequences and is associated with limitations in activity, work productivity, quality of life, and stigma.8 Treatment of chronic pain—with opioids or other modalities—remains an important component of patient-centered primary care. Interestingly, however, many patients struggling through chronic pain report that efforts to curb the opioid epidemic have inadvertently led to lower-quality pain management and, therefore, understandable concern among patients whose chronic pain is well managed with opioid pain medications.9,10
When is it appropriate to continue opioids for chronic pain?
Apart from the treatment of active cancer, palliative care, and end-of-life care, the appropriate use of opioids for chronic and acute pain has become clouded in recent years. To assist with this problem, the Centers for Disease Control and Prevention issued guidelines in 2016 for primary care physicians who are faced with this clinical dilemma.11 The guidelines (1) address circumstances in which it is safe to consider opioid prescribing and (2) provide ongoing reassessment of indications for chronic opioid prescribing within the context of potential risk to the patient and society. Because appropriate use of opioids has grown murky, nonpharmacotherapeutic management and nonopioid pharmacotherapy are preferred for chronic pain.
Plan ahead. Establish goals of treatment that focus on both pain and function when starting opioid therapy. This will facilitate decision-making when it comes time to continue—or discontinue—opioids down the road. Opioids should be prescribed at the lowest effective dosage; ongoing reassessment of benefit should be made, and particular caution should be exercised, if the daily opioid dosage reaches ≥ 50 morphine milligram equivalents (MME) and especially as the dosage approaches ≥ 90 MME/d. Prescribers should ensure that patients are educated about known risks and the limited evidence of benefit of opioid therapy.
An age-related concern. Special consideration is warranted in older patients, who might have reduced renal function even in the absence of renal disease; this can lead to a reduction in clearance of pain medication. Because of that increased risk of drug accumulation, the therapeutic window—between safe dosages and those that could lead to respiratory depression or overdose—is narrow for these patients.11
Continue to: Use in pregnancy
Use in pregnancy. Treatment with opioid medication in pregnancy warrants special consideration. In general, it’s wise to avoid opioid use in pregnant women because data on long- and short-term safety are limited.12 In 2015, the US Food and Drug Administration issued a safety announcement that further investigation is needed to determine whether the fetus is at increased risk of a neural tube defect related to opioid exposure during the first trimester.13 In women with an OUD, both methadone and buprenorphine are safe to use. Buprenorphine is associated with slightly better outcomes for neonatal abstinence syndrome and length of hospital stay.14
Ongoing monitoring of risk. Periodically assessing risk factors for opioid-related harm during continuation of opioid treatment is important. Tools such as the Opioid Risk Tool (ORT) or the Screener and Opioid Assessment for Patients with Pain-Revised, or SOAPP-R, can be used to evaluate the risk of misuse in adults who are prescribed opioids for chronic pain,15 although the evidence for utilizing these tools is inconclusive.11
Offering naloxone should be considered when factors that increase the risk of opioid overdose are present, such as a history of substance use disorder, a daily opioid dosage > 50 MME, concurrent use of benzodiazepines, and medical comorbidities that increase the risk of overdose (eg, sleep apnea, pulmonary disease, heart failure).16 Prescribers should review prescription drug monitoring program data, when available, to assess treatment adherence and to obtain a collateral history that might suggest abuse or diversion. Urine drug testing can be a useful adjunct to ongoing therapy—again, to assess treatment adherence and look for evidence of other substance use disorders.
Watchfulness for misuse and OUD. Opioid misuse—the nontherapeutic use of opioids—includes taking opioids in amounts other than prescribed, for indications other than prescribed, and administering by alternative routes other than prescribed (eg, crushing and snorting, rather than ingesting). The presence of opioid misuse does not always signify OUD. However, The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,17 defines OUD as out-of-control use; devoting increasing mental and physical resources to obtaining, using, and recovering from substances; and continued use despite adverse consequences.
Behaviors that increase the risk of, and might signal, opioid misuse and OUD include18
- seeking early refills
- obtaining opioids from the emergency room
- using medications prescribed to others
- using opioids to treat symptoms other than pain, such as anxiety or insomnia
- “doctor-shopping.”
Continue to: Furthermore...
Furthermore, psychiatric comorbidities,19 a personal or family history of substance use disorder,20 and a preadolescent history of sexual abuse21 are associated with a higher risk of a substance use disorder.
If OUD is identified, remain nonjudgmental and acknowledge that addiction is a chronic disease. Assumptions about a patient’s character or morality have no place in the appropriate management of OUD; remain mindful of your own implicit biases.
When is it appropriateto start an opioid taper?
The decision to taper opioids is difficult and can provoke anxiety for both prescriber and patient. Complicating matters is that there is insufficient evidence to evaluate opioid dosage-reduction interventions for patients with chronic noncancer pain.22
Safety concerns. Even in patients who are taking opioids as prescribed and for whom no red flags have been raised, the long-term safety of high-dosage opioids remains unclear. There is no “safe” dosage of opioids; however, evidence is clear that the risk of death from overdose increases with dosage. Compared with patients taking a dosage anywhere from 1 to 20 MME/d, those taking 50 to 99 MME/d have a 3.7-fold increased risk of overdose; patients taking ≥ 100 MME/d had an 8.9-fold increased risk.23 Patients for whom concomitant benzodiazepines are prescribed are also at higher risk of overdose and death. In studies of opioid overdose deaths, there was evidence of concurrent benzodiazepine use in 31% to 61% of cases.11
Inadequate analgesia. Given the well-established risk of drug tolerance, the inability to achieve or maintain pain relief or functional improvement can still occur—even when the opioid dosage is escalated reasonably. It might be prudent in that situation to taper opioids while also considering alternative modalities, including ones that were deferred previously.
Continue to: Intolerable adverse effects
Intolerable adverse effects. Adverse effects are common. Constipation has a reported prevalence of 15% to 90% among patients on long-term opioid treatment.24 Short-term, mild constipation is often manageable; long-term opioid use, however, can produce constipation refractory to bowel regimens and, in rare cases, lead to bowel obstruction, perforation, and even death. Other adverse effects include25
- sedation and drowsiness
- impaired memory or concentration
- mood changes
- dry mouth
- abdominal pain and nausea
- sexual dysfunction.
When these effects limit the tolerability of treatment, tapering might be indicated.
How are opioids tapered?
There is no definitive evidence of an optimal rate of taper or frequency of follow-up. Most guidelines suggest tapering opioids at 10% of the dosage each week; patients who have been taking opioids for many years, however, might require a slower taper (eg, a dosage decrease of 5%-20% every 2-4 weeks).11
Psychosocial support and maximizing nonopioid pain management techniques are critical to successful opioid tapering. When tapering is part of a comprehensive pain and rehabilitative plan, patients might find their symptoms alleviated.26 Given the potential risks in patients taking both short- and long-acting opioids, tapering the long-acting opioid should be the initial priority.
A more rapid taper—eg, a 20% reduction each week or even abrupt discontinuation of opioids—might be necessary if diversion is suspected or if there is concern that continued use of the medication presents high risk. In such cases, consultation with an addiction medicine specialist can be helpful—to assess whether medication-assisted therapy for OUD would be appropriate and how to support patients who are having withdrawal symptoms.
Continue to: For all patients...
For all patients, frequent follow-up visits with their primary care clinician, as well as referrals to mental health, physical therapy, and pain or rehabilitation services, can promote a successful taper. It is advised that, before beginning a taper, a treatment plan should be written out with the patient so that expectations are shared by physician and patient for the goals of the taper, the speed of dosage decreases, and the frequency of follow-up after each dosage change. At each follow-up visit, education regarding self-management and individualized recommendations for psychosocial support, mental health services, and substance use disorder services should be updated.
Assessing risk when tapering chronic opioid therapy
The goals of tapering should be to (1) reduce adverse effects of treatment and (2) mitigate short- and long-term risks.
Three short-term risks
Unmasking OUD. Tapering prescribed opioids, or even just discussing tapering, can unmask OUD in some patients. Follow-up visits during the tapering schedule should include frequent screening for OUD. If OUD is diagnosed, we recommend beginning medication-assisted treatment or referring the patient to a substance use treatment center. There is strong evidence of the safety and efficacy of medication-assisted treatment, even with a coexisting chronic pain disorder.27

Withdrawal syndrome. Opioid withdrawal syndrome is characterized by signs and symptoms of sympathetic stimulation, resulting from decreased sympathetic blockade by opioids (TABLE).28 (See “Changes in the locus ceruleus lead to withdrawal.”29) Symptoms start 2 to 3 half-lives after the last dose of opioid. Oxycodone, for example, has a half-life of 3 to 4 hours; withdrawal symptoms should therefore be anticipated in 6 to 12 hours. Because mixing opioids is commonplace, it can be difficult to predict exactly when withdrawal symptoms will begin. Patients are often most helpful in predicting the onset and severity of withdrawal symptoms.
SIDEBAR
Changes in the locus ceruleus lead to withdrawal
Normally, the locus ceruleus (LC), a pontine nucleus within the brainstem, produces noradrenaline (NA), which stimulates alertness, breathing, and blood pressure, among other physiologic functions. When opioids bind to the mu-opioid receptors in the LC and decrease the release of NA, the result is diminished alertness, lower blood pressure, and slower respiration.
With chronic exposure to opioids, the LC acts to increase levels of NA to counteract suppression. When a patient stops taking opioids, the increased NA levels become excessive and produce symptoms of opioid withdrawal. 29
Withdrawal can be measured using any of a number of validated tools, including
- the Subjective Opiate Withdrawal Scale, or SOWS30 (FIGURE 1), which utilizes a patient self-report
- the Clinical Opiate Withdrawal Scale, or COWS31 (FIGURE 2), which relies on assessment made by the physician.
Continue to: Although withdrawal...
Although withdrawal is generally not considered life-threatening in patients without significant comorbidities, do not underestimate the severity of withdrawal symptoms. Often, the desire to avoid these intense symptoms drives patients with OUD to continue to overuse.
Increased pain. Patients might fear that pain will become worse if opioids are tapered. Although it is important to acknowledge this fear, studies of patients undergoing a long-term opioid taper report improvements in function without loss of adequate pain control; some even report that pain control improves.32
Three long-term risks
Relapse. The most dangerous risk of tapering opioids is use of illicit opioids, a danger made worse by the increasing presence of highly lethal synthetic fentanyl analogues in the community. Risk factors for relapse following a full taper include the presence of depressive symptoms at initiation of tapering and higher pain scores at initiation and conclusion of the taper.33 Having low pain at the end of an opioid taper, on the other hand, is predictive of long-term abstinence from opioids.32
Declining function. As is the case while prescribing opioids for pain, maintenance of function remains a priority when tapering opioids. Function can be difficult to assess, given the many variables that can influence an individual’s function. Psychosocial factors, such as coping strategies and mood, strongly influence function; so do psychiatric morbidities, which are more prevalent in patients with chronic pain and disability, compared with the general population.34
Medicolegal matters. Although difficult to characterize, medicolegal risk is an inevitable consideration when tapering opioids:
- In a study of closed malpractice claims involving all medical specialties, narcotic pain medications were the most common drug class involved, representing 1% of claims.35
- In a study of closed malpractice claims involving pain medicine specialists, 3% were related to medication management. Most claims arose following death from opioid overdose.36
Continue to: What else is needed in this area of practice?
What else is needed in this area of practice?
Increasingly, family physicians face the inherent tension of wanting to provide patient-centered, compassionate care for patients in pain while being mindful of opioid prescription stewardship. To support their work and help allay this tension, clinical research on this topic in the future should focus on
- new options for nonopioid pharmacotherapy for pain
- best practices for using opioids in noncancer chronic pain.
In addition, health care systems can help—by providing insurance coverage of nonpharmacotherapeutic options for treating pain.
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 111 Westfall Road, Room 952, Rochester, NY 14620; MichaelMendoza@ monroecounty.gov
1. Chen J, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
2. Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:697-704.
3. Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
4. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-S226.
5. Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803-812.
6. Wilson HD, Dansie EJ, Kim MS, et al. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14:613-627.
7. Peppin JF. The marginalization of chronic pain patients on chronic opioid therapy. Pain Physician. 2009;12:493-498.
8. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
9. Bonnie RJ. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017.
10. Sherman KJ, Walker RL, Saunders K, et al. Doctor-patient trust among chronic pain patients on chronic opioid therapy after opioid risk reduction initiatives: a survey. J Am Board Fam Med. 2018;31:578-587.
11. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
12. Broussard CS, Rasmussen SA, Reefhuis J, et al; National Birth Defects Prevention Study. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1-e11.
13. FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. US Food and Drug Administration website. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy. Published January 9, 2015. Accessed May 27, 2019.
14. Tran TH, Griffin BL, Stone RH, et al. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824-839.
15. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131-146.
16. Kuryshev YA, Bruening-Wright A, Brown AM, et al. Increased cardiac risk in concomitant methadone and diazepam treatment: pharmacodynamic interactions in cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:420-430.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
19. Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71-80.
20. Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973-979.
21. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953-959.
22. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2017;11:CD010323.
23. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686-691.
24. Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23:616-622.
25. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;1:CD006605.
26. Murphy JL, Clark ME, Banou E. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain. 2013;29:109-117.
27. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59-80.
28. Farrell M. Opiate withdrawal. Addiction. 1994;89:1471-1475.
29. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20.
30. Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293-308.
31. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259.
32. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277-282.
33. Heiwe S, Lönnquist I, Källmén H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain. 2011;15:966-970.
34. Dersh J, Gatchel RJ, Polatin P, et al. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44:459-468.
35. Troxel DB. REMS: Opioid-Related Patient Safety and Liability. Richardson, TX: The Doctors Company; 2012.
36. Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948-956.
The opioid crisis has brought added scrutiny to opioid prescribing, particularly to health care providers, whom many blame for the genesis of the opioid overdose epidemic. Family physicians are acutely aware of these complexities: By sheer volume, family physicians prescribe more opioid analgesics than any other subspecialist.1
Overwhelmed by opioid prescriptions
Because of a complexity of factors (notably, the influence of the US pharmaceutical industry), the quantity of opioid prescriptions has risen substantially—enough so that, in 2010, opioids were prescribed in great enough quantity to medicate every American around the clock for a month.2 Among people who began abusing opioids in the 2000s, 75% reported that their first opioid was a prescription drug; this is a shift from prior decades, when heroin was the gateway to opioid addiction.3 As the reality of the size of the opioid problem sunk in, many were hopeful that the epidemic would reverse itself as quickly as it began if the medical community would simply prescribe fewer opioids.

Since 2010, the opioid overdose fatality rate has risen dramatically, even though prescription opioid overdose mortality has leveled off, or even declined. 2 One explanation for this paradox? As availability of prescription opioids declined, people suffering from an underlying opioid use disorder (OUD) turned instead first to heroin, then later to potent fentanyl analogues to fuel their addiction. In most communities, the prevalence of fentanyl analogues—alone or more commonly mixed with other opioids—has driven the staggering rise in opioid-related fatalities in recent years.
No question: Prescription opioids played a critical role in the origins of this epidemic, but just withdrawing prescriptions will not result in marked reduction in the epidemic. This quandary is no more apparent than in primary care, where the considerable risk of continuing opioids—especially at high dosages—must be weighed against the potential risks of discontinuation. Adding to this dilemma are lack of access to treatment for patients with an OUD and the continued stigma and misunderstanding of substance use disorders.
In this article, we describe the challenges of long-term opioid use and review necessary protocols and precautions for maintaining or tapering an opioid regimen in patients who suffer chronic pain.
Managing chronic pain is fraught with complexity
Chronic pain is both real and a disease in its own right. Although definitions of chronic pain vary, pain that lasts > 3 months or past the duration of normal tissue healing is typically considered chronic.4 Approximations of prevalence vary, but in 1 study that examined a representative sample, it was estimated that 14.6% of US adults experience chronic pain.5
Patients who report symptoms or a history of chronic pain can elicit negative reactions from physicians—stemming from our biases, which can inadvertently provoke emotions on our part.6 Unflattering portrayals of patients in the media can further fuel unwarranted biases and prejudices.7
Continue to: Preventing, assessing, and treating...
Preventing, assessing, and treating chronic pain can be difficult, at the level of both the individual physician and the larger system of care, even without adding in complications of the opioid epidemic. For racial and ethnic minority groups, women, older people, and people with cognitive impairment or cancer, pain can be underrecognized and go inadequately treated.
Chronic pain itself has clinical, psychological, and social consequences and is associated with limitations in activity, work productivity, quality of life, and stigma.8 Treatment of chronic pain—with opioids or other modalities—remains an important component of patient-centered primary care. Interestingly, however, many patients struggling through chronic pain report that efforts to curb the opioid epidemic have inadvertently led to lower-quality pain management and, therefore, understandable concern among patients whose chronic pain is well managed with opioid pain medications.9,10
When is it appropriate to continue opioids for chronic pain?
Apart from the treatment of active cancer, palliative care, and end-of-life care, the appropriate use of opioids for chronic and acute pain has become clouded in recent years. To assist with this problem, the Centers for Disease Control and Prevention issued guidelines in 2016 for primary care physicians who are faced with this clinical dilemma.11 The guidelines (1) address circumstances in which it is safe to consider opioid prescribing and (2) provide ongoing reassessment of indications for chronic opioid prescribing within the context of potential risk to the patient and society. Because appropriate use of opioids has grown murky, nonpharmacotherapeutic management and nonopioid pharmacotherapy are preferred for chronic pain.
Plan ahead. Establish goals of treatment that focus on both pain and function when starting opioid therapy. This will facilitate decision-making when it comes time to continue—or discontinue—opioids down the road. Opioids should be prescribed at the lowest effective dosage; ongoing reassessment of benefit should be made, and particular caution should be exercised, if the daily opioid dosage reaches ≥ 50 morphine milligram equivalents (MME) and especially as the dosage approaches ≥ 90 MME/d. Prescribers should ensure that patients are educated about known risks and the limited evidence of benefit of opioid therapy.
An age-related concern. Special consideration is warranted in older patients, who might have reduced renal function even in the absence of renal disease; this can lead to a reduction in clearance of pain medication. Because of that increased risk of drug accumulation, the therapeutic window—between safe dosages and those that could lead to respiratory depression or overdose—is narrow for these patients.11
Continue to: Use in pregnancy
Use in pregnancy. Treatment with opioid medication in pregnancy warrants special consideration. In general, it’s wise to avoid opioid use in pregnant women because data on long- and short-term safety are limited.12 In 2015, the US Food and Drug Administration issued a safety announcement that further investigation is needed to determine whether the fetus is at increased risk of a neural tube defect related to opioid exposure during the first trimester.13 In women with an OUD, both methadone and buprenorphine are safe to use. Buprenorphine is associated with slightly better outcomes for neonatal abstinence syndrome and length of hospital stay.14
Ongoing monitoring of risk. Periodically assessing risk factors for opioid-related harm during continuation of opioid treatment is important. Tools such as the Opioid Risk Tool (ORT) or the Screener and Opioid Assessment for Patients with Pain-Revised, or SOAPP-R, can be used to evaluate the risk of misuse in adults who are prescribed opioids for chronic pain,15 although the evidence for utilizing these tools is inconclusive.11
Offering naloxone should be considered when factors that increase the risk of opioid overdose are present, such as a history of substance use disorder, a daily opioid dosage > 50 MME, concurrent use of benzodiazepines, and medical comorbidities that increase the risk of overdose (eg, sleep apnea, pulmonary disease, heart failure).16 Prescribers should review prescription drug monitoring program data, when available, to assess treatment adherence and to obtain a collateral history that might suggest abuse or diversion. Urine drug testing can be a useful adjunct to ongoing therapy—again, to assess treatment adherence and look for evidence of other substance use disorders.
Watchfulness for misuse and OUD. Opioid misuse—the nontherapeutic use of opioids—includes taking opioids in amounts other than prescribed, for indications other than prescribed, and administering by alternative routes other than prescribed (eg, crushing and snorting, rather than ingesting). The presence of opioid misuse does not always signify OUD. However, The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,17 defines OUD as out-of-control use; devoting increasing mental and physical resources to obtaining, using, and recovering from substances; and continued use despite adverse consequences.
Behaviors that increase the risk of, and might signal, opioid misuse and OUD include18
- seeking early refills
- obtaining opioids from the emergency room
- using medications prescribed to others
- using opioids to treat symptoms other than pain, such as anxiety or insomnia
- “doctor-shopping.”
Continue to: Furthermore...
Furthermore, psychiatric comorbidities,19 a personal or family history of substance use disorder,20 and a preadolescent history of sexual abuse21 are associated with a higher risk of a substance use disorder.
If OUD is identified, remain nonjudgmental and acknowledge that addiction is a chronic disease. Assumptions about a patient’s character or morality have no place in the appropriate management of OUD; remain mindful of your own implicit biases.
When is it appropriateto start an opioid taper?
The decision to taper opioids is difficult and can provoke anxiety for both prescriber and patient. Complicating matters is that there is insufficient evidence to evaluate opioid dosage-reduction interventions for patients with chronic noncancer pain.22
Safety concerns. Even in patients who are taking opioids as prescribed and for whom no red flags have been raised, the long-term safety of high-dosage opioids remains unclear. There is no “safe” dosage of opioids; however, evidence is clear that the risk of death from overdose increases with dosage. Compared with patients taking a dosage anywhere from 1 to 20 MME/d, those taking 50 to 99 MME/d have a 3.7-fold increased risk of overdose; patients taking ≥ 100 MME/d had an 8.9-fold increased risk.23 Patients for whom concomitant benzodiazepines are prescribed are also at higher risk of overdose and death. In studies of opioid overdose deaths, there was evidence of concurrent benzodiazepine use in 31% to 61% of cases.11
Inadequate analgesia. Given the well-established risk of drug tolerance, the inability to achieve or maintain pain relief or functional improvement can still occur—even when the opioid dosage is escalated reasonably. It might be prudent in that situation to taper opioids while also considering alternative modalities, including ones that were deferred previously.
Continue to: Intolerable adverse effects
Intolerable adverse effects. Adverse effects are common. Constipation has a reported prevalence of 15% to 90% among patients on long-term opioid treatment.24 Short-term, mild constipation is often manageable; long-term opioid use, however, can produce constipation refractory to bowel regimens and, in rare cases, lead to bowel obstruction, perforation, and even death. Other adverse effects include25
- sedation and drowsiness
- impaired memory or concentration
- mood changes
- dry mouth
- abdominal pain and nausea
- sexual dysfunction.
When these effects limit the tolerability of treatment, tapering might be indicated.
How are opioids tapered?
There is no definitive evidence of an optimal rate of taper or frequency of follow-up. Most guidelines suggest tapering opioids at 10% of the dosage each week; patients who have been taking opioids for many years, however, might require a slower taper (eg, a dosage decrease of 5%-20% every 2-4 weeks).11
Psychosocial support and maximizing nonopioid pain management techniques are critical to successful opioid tapering. When tapering is part of a comprehensive pain and rehabilitative plan, patients might find their symptoms alleviated.26 Given the potential risks in patients taking both short- and long-acting opioids, tapering the long-acting opioid should be the initial priority.
A more rapid taper—eg, a 20% reduction each week or even abrupt discontinuation of opioids—might be necessary if diversion is suspected or if there is concern that continued use of the medication presents high risk. In such cases, consultation with an addiction medicine specialist can be helpful—to assess whether medication-assisted therapy for OUD would be appropriate and how to support patients who are having withdrawal symptoms.
Continue to: For all patients...
For all patients, frequent follow-up visits with their primary care clinician, as well as referrals to mental health, physical therapy, and pain or rehabilitation services, can promote a successful taper. It is advised that, before beginning a taper, a treatment plan should be written out with the patient so that expectations are shared by physician and patient for the goals of the taper, the speed of dosage decreases, and the frequency of follow-up after each dosage change. At each follow-up visit, education regarding self-management and individualized recommendations for psychosocial support, mental health services, and substance use disorder services should be updated.
Assessing risk when tapering chronic opioid therapy
The goals of tapering should be to (1) reduce adverse effects of treatment and (2) mitigate short- and long-term risks.
Three short-term risks
Unmasking OUD. Tapering prescribed opioids, or even just discussing tapering, can unmask OUD in some patients. Follow-up visits during the tapering schedule should include frequent screening for OUD. If OUD is diagnosed, we recommend beginning medication-assisted treatment or referring the patient to a substance use treatment center. There is strong evidence of the safety and efficacy of medication-assisted treatment, even with a coexisting chronic pain disorder.27

Withdrawal syndrome. Opioid withdrawal syndrome is characterized by signs and symptoms of sympathetic stimulation, resulting from decreased sympathetic blockade by opioids (TABLE).28 (See “Changes in the locus ceruleus lead to withdrawal.”29) Symptoms start 2 to 3 half-lives after the last dose of opioid. Oxycodone, for example, has a half-life of 3 to 4 hours; withdrawal symptoms should therefore be anticipated in 6 to 12 hours. Because mixing opioids is commonplace, it can be difficult to predict exactly when withdrawal symptoms will begin. Patients are often most helpful in predicting the onset and severity of withdrawal symptoms.
SIDEBAR
Changes in the locus ceruleus lead to withdrawal
Normally, the locus ceruleus (LC), a pontine nucleus within the brainstem, produces noradrenaline (NA), which stimulates alertness, breathing, and blood pressure, among other physiologic functions. When opioids bind to the mu-opioid receptors in the LC and decrease the release of NA, the result is diminished alertness, lower blood pressure, and slower respiration.
With chronic exposure to opioids, the LC acts to increase levels of NA to counteract suppression. When a patient stops taking opioids, the increased NA levels become excessive and produce symptoms of opioid withdrawal. 29

Withdrawal can be measured using any of a number of validated tools, including
- the Subjective Opiate Withdrawal Scale, or SOWS30 (FIGURE 1), which utilizes a patient self-report
- the Clinical Opiate Withdrawal Scale, or COWS31 (FIGURE 2), which relies on assessment made by the physician.

Continue to: Although withdrawal...
Although withdrawal is generally not considered life-threatening in patients without significant comorbidities, do not underestimate the severity of withdrawal symptoms. Often, the desire to avoid these intense symptoms drives patients with OUD to continue to overuse.
Increased pain. Patients might fear that pain will become worse if opioids are tapered. Although it is important to acknowledge this fear, studies of patients undergoing a long-term opioid taper report improvements in function without loss of adequate pain control; some even report that pain control improves.32
Three long-term risks
Relapse. The most dangerous risk of tapering opioids is use of illicit opioids, a danger made worse by the increasing presence of highly lethal synthetic fentanyl analogues in the community. Risk factors for relapse following a full taper include the presence of depressive symptoms at initiation of tapering and higher pain scores at initiation and conclusion of the taper.33 Having low pain at the end of an opioid taper, on the other hand, is predictive of long-term abstinence from opioids.32
Declining function. As is the case while prescribing opioids for pain, maintenance of function remains a priority when tapering opioids. Function can be difficult to assess, given the many variables that can influence an individual’s function. Psychosocial factors, such as coping strategies and mood, strongly influence function; so do psychiatric morbidities, which are more prevalent in patients with chronic pain and disability, compared with the general population.34
Medicolegal matters. Although difficult to characterize, medicolegal risk is an inevitable consideration when tapering opioids:
- In a study of closed malpractice claims involving all medical specialties, narcotic pain medications were the most common drug class involved, representing 1% of claims.35
- In a study of closed malpractice claims involving pain medicine specialists, 3% were related to medication management. Most claims arose following death from opioid overdose.36
Continue to: What else is needed in this area of practice?
What else is needed in this area of practice?
Increasingly, family physicians face the inherent tension of wanting to provide patient-centered, compassionate care for patients in pain while being mindful of opioid prescription stewardship. To support their work and help allay this tension, clinical research on this topic in the future should focus on
- new options for nonopioid pharmacotherapy for pain
- best practices for using opioids in noncancer chronic pain.
In addition, health care systems can help—by providing insurance coverage of nonpharmacotherapeutic options for treating pain.
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 111 Westfall Road, Room 952, Rochester, NY 14620; MichaelMendoza@ monroecounty.gov
The opioid crisis has brought added scrutiny to opioid prescribing, particularly to health care providers, whom many blame for the genesis of the opioid overdose epidemic. Family physicians are acutely aware of these complexities: By sheer volume, family physicians prescribe more opioid analgesics than any other subspecialist.1
Overwhelmed by opioid prescriptions
Because of a complexity of factors (notably, the influence of the US pharmaceutical industry), the quantity of opioid prescriptions has risen substantially—enough so that, in 2010, opioids were prescribed in great enough quantity to medicate every American around the clock for a month.2 Among people who began abusing opioids in the 2000s, 75% reported that their first opioid was a prescription drug; this is a shift from prior decades, when heroin was the gateway to opioid addiction.3 As the reality of the size of the opioid problem sunk in, many were hopeful that the epidemic would reverse itself as quickly as it began if the medical community would simply prescribe fewer opioids.

Since 2010, the opioid overdose fatality rate has risen dramatically, even though prescription opioid overdose mortality has leveled off, or even declined. 2 One explanation for this paradox? As availability of prescription opioids declined, people suffering from an underlying opioid use disorder (OUD) turned instead first to heroin, then later to potent fentanyl analogues to fuel their addiction. In most communities, the prevalence of fentanyl analogues—alone or more commonly mixed with other opioids—has driven the staggering rise in opioid-related fatalities in recent years.
No question: Prescription opioids played a critical role in the origins of this epidemic, but just withdrawing prescriptions will not result in marked reduction in the epidemic. This quandary is no more apparent than in primary care, where the considerable risk of continuing opioids—especially at high dosages—must be weighed against the potential risks of discontinuation. Adding to this dilemma are lack of access to treatment for patients with an OUD and the continued stigma and misunderstanding of substance use disorders.
In this article, we describe the challenges of long-term opioid use and review necessary protocols and precautions for maintaining or tapering an opioid regimen in patients who suffer chronic pain.
Managing chronic pain is fraught with complexity
Chronic pain is both real and a disease in its own right. Although definitions of chronic pain vary, pain that lasts > 3 months or past the duration of normal tissue healing is typically considered chronic.4 Approximations of prevalence vary, but in 1 study that examined a representative sample, it was estimated that 14.6% of US adults experience chronic pain.5
Patients who report symptoms or a history of chronic pain can elicit negative reactions from physicians—stemming from our biases, which can inadvertently provoke emotions on our part.6 Unflattering portrayals of patients in the media can further fuel unwarranted biases and prejudices.7
Continue to: Preventing, assessing, and treating...
Preventing, assessing, and treating chronic pain can be difficult, at the level of both the individual physician and the larger system of care, even without adding in complications of the opioid epidemic. For racial and ethnic minority groups, women, older people, and people with cognitive impairment or cancer, pain can be underrecognized and go inadequately treated.
Chronic pain itself has clinical, psychological, and social consequences and is associated with limitations in activity, work productivity, quality of life, and stigma.8 Treatment of chronic pain—with opioids or other modalities—remains an important component of patient-centered primary care. Interestingly, however, many patients struggling through chronic pain report that efforts to curb the opioid epidemic have inadvertently led to lower-quality pain management and, therefore, understandable concern among patients whose chronic pain is well managed with opioid pain medications.9,10
When is it appropriate to continue opioids for chronic pain?
Apart from the treatment of active cancer, palliative care, and end-of-life care, the appropriate use of opioids for chronic and acute pain has become clouded in recent years. To assist with this problem, the Centers for Disease Control and Prevention issued guidelines in 2016 for primary care physicians who are faced with this clinical dilemma.11 The guidelines (1) address circumstances in which it is safe to consider opioid prescribing and (2) provide ongoing reassessment of indications for chronic opioid prescribing within the context of potential risk to the patient and society. Because appropriate use of opioids has grown murky, nonpharmacotherapeutic management and nonopioid pharmacotherapy are preferred for chronic pain.
Plan ahead. Establish goals of treatment that focus on both pain and function when starting opioid therapy. This will facilitate decision-making when it comes time to continue—or discontinue—opioids down the road. Opioids should be prescribed at the lowest effective dosage; ongoing reassessment of benefit should be made, and particular caution should be exercised, if the daily opioid dosage reaches ≥ 50 morphine milligram equivalents (MME) and especially as the dosage approaches ≥ 90 MME/d. Prescribers should ensure that patients are educated about known risks and the limited evidence of benefit of opioid therapy.
An age-related concern. Special consideration is warranted in older patients, who might have reduced renal function even in the absence of renal disease; this can lead to a reduction in clearance of pain medication. Because of that increased risk of drug accumulation, the therapeutic window—between safe dosages and those that could lead to respiratory depression or overdose—is narrow for these patients.11
Continue to: Use in pregnancy
Use in pregnancy. Treatment with opioid medication in pregnancy warrants special consideration. In general, it’s wise to avoid opioid use in pregnant women because data on long- and short-term safety are limited.12 In 2015, the US Food and Drug Administration issued a safety announcement that further investigation is needed to determine whether the fetus is at increased risk of a neural tube defect related to opioid exposure during the first trimester.13 In women with an OUD, both methadone and buprenorphine are safe to use. Buprenorphine is associated with slightly better outcomes for neonatal abstinence syndrome and length of hospital stay.14
Ongoing monitoring of risk. Periodically assessing risk factors for opioid-related harm during continuation of opioid treatment is important. Tools such as the Opioid Risk Tool (ORT) or the Screener and Opioid Assessment for Patients with Pain-Revised, or SOAPP-R, can be used to evaluate the risk of misuse in adults who are prescribed opioids for chronic pain,15 although the evidence for utilizing these tools is inconclusive.11
Offering naloxone should be considered when factors that increase the risk of opioid overdose are present, such as a history of substance use disorder, a daily opioid dosage > 50 MME, concurrent use of benzodiazepines, and medical comorbidities that increase the risk of overdose (eg, sleep apnea, pulmonary disease, heart failure).16 Prescribers should review prescription drug monitoring program data, when available, to assess treatment adherence and to obtain a collateral history that might suggest abuse or diversion. Urine drug testing can be a useful adjunct to ongoing therapy—again, to assess treatment adherence and look for evidence of other substance use disorders.
Watchfulness for misuse and OUD. Opioid misuse—the nontherapeutic use of opioids—includes taking opioids in amounts other than prescribed, for indications other than prescribed, and administering by alternative routes other than prescribed (eg, crushing and snorting, rather than ingesting). The presence of opioid misuse does not always signify OUD. However, The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,17 defines OUD as out-of-control use; devoting increasing mental and physical resources to obtaining, using, and recovering from substances; and continued use despite adverse consequences.
Behaviors that increase the risk of, and might signal, opioid misuse and OUD include18
- seeking early refills
- obtaining opioids from the emergency room
- using medications prescribed to others
- using opioids to treat symptoms other than pain, such as anxiety or insomnia
- “doctor-shopping.”
Continue to: Furthermore...
Furthermore, psychiatric comorbidities,19 a personal or family history of substance use disorder,20 and a preadolescent history of sexual abuse21 are associated with a higher risk of a substance use disorder.
If OUD is identified, remain nonjudgmental and acknowledge that addiction is a chronic disease. Assumptions about a patient’s character or morality have no place in the appropriate management of OUD; remain mindful of your own implicit biases.
When is it appropriateto start an opioid taper?
The decision to taper opioids is difficult and can provoke anxiety for both prescriber and patient. Complicating matters is that there is insufficient evidence to evaluate opioid dosage-reduction interventions for patients with chronic noncancer pain.22
Safety concerns. Even in patients who are taking opioids as prescribed and for whom no red flags have been raised, the long-term safety of high-dosage opioids remains unclear. There is no “safe” dosage of opioids; however, evidence is clear that the risk of death from overdose increases with dosage. Compared with patients taking a dosage anywhere from 1 to 20 MME/d, those taking 50 to 99 MME/d have a 3.7-fold increased risk of overdose; patients taking ≥ 100 MME/d had an 8.9-fold increased risk.23 Patients for whom concomitant benzodiazepines are prescribed are also at higher risk of overdose and death. In studies of opioid overdose deaths, there was evidence of concurrent benzodiazepine use in 31% to 61% of cases.11
Inadequate analgesia. Given the well-established risk of drug tolerance, the inability to achieve or maintain pain relief or functional improvement can still occur—even when the opioid dosage is escalated reasonably. It might be prudent in that situation to taper opioids while also considering alternative modalities, including ones that were deferred previously.
Continue to: Intolerable adverse effects
Intolerable adverse effects. Adverse effects are common. Constipation has a reported prevalence of 15% to 90% among patients on long-term opioid treatment.24 Short-term, mild constipation is often manageable; long-term opioid use, however, can produce constipation refractory to bowel regimens and, in rare cases, lead to bowel obstruction, perforation, and even death. Other adverse effects include25
- sedation and drowsiness
- impaired memory or concentration
- mood changes
- dry mouth
- abdominal pain and nausea
- sexual dysfunction.
When these effects limit the tolerability of treatment, tapering might be indicated.
How are opioids tapered?
There is no definitive evidence of an optimal rate of taper or frequency of follow-up. Most guidelines suggest tapering opioids at 10% of the dosage each week; patients who have been taking opioids for many years, however, might require a slower taper (eg, a dosage decrease of 5%-20% every 2-4 weeks).11
Psychosocial support and maximizing nonopioid pain management techniques are critical to successful opioid tapering. When tapering is part of a comprehensive pain and rehabilitative plan, patients might find their symptoms alleviated.26 Given the potential risks in patients taking both short- and long-acting opioids, tapering the long-acting opioid should be the initial priority.
A more rapid taper—eg, a 20% reduction each week or even abrupt discontinuation of opioids—might be necessary if diversion is suspected or if there is concern that continued use of the medication presents high risk. In such cases, consultation with an addiction medicine specialist can be helpful—to assess whether medication-assisted therapy for OUD would be appropriate and how to support patients who are having withdrawal symptoms.
Continue to: For all patients...
For all patients, frequent follow-up visits with their primary care clinician, as well as referrals to mental health, physical therapy, and pain or rehabilitation services, can promote a successful taper. It is advised that, before beginning a taper, a treatment plan should be written out with the patient so that expectations are shared by physician and patient for the goals of the taper, the speed of dosage decreases, and the frequency of follow-up after each dosage change. At each follow-up visit, education regarding self-management and individualized recommendations for psychosocial support, mental health services, and substance use disorder services should be updated.
Assessing risk when tapering chronic opioid therapy
The goals of tapering should be to (1) reduce adverse effects of treatment and (2) mitigate short- and long-term risks.
Three short-term risks
Unmasking OUD. Tapering prescribed opioids, or even just discussing tapering, can unmask OUD in some patients. Follow-up visits during the tapering schedule should include frequent screening for OUD. If OUD is diagnosed, we recommend beginning medication-assisted treatment or referring the patient to a substance use treatment center. There is strong evidence of the safety and efficacy of medication-assisted treatment, even with a coexisting chronic pain disorder.27

Withdrawal syndrome. Opioid withdrawal syndrome is characterized by signs and symptoms of sympathetic stimulation, resulting from decreased sympathetic blockade by opioids (TABLE).28 (See “Changes in the locus ceruleus lead to withdrawal.”29) Symptoms start 2 to 3 half-lives after the last dose of opioid. Oxycodone, for example, has a half-life of 3 to 4 hours; withdrawal symptoms should therefore be anticipated in 6 to 12 hours. Because mixing opioids is commonplace, it can be difficult to predict exactly when withdrawal symptoms will begin. Patients are often most helpful in predicting the onset and severity of withdrawal symptoms.
SIDEBAR
Changes in the locus ceruleus lead to withdrawal
Normally, the locus ceruleus (LC), a pontine nucleus within the brainstem, produces noradrenaline (NA), which stimulates alertness, breathing, and blood pressure, among other physiologic functions. When opioids bind to the mu-opioid receptors in the LC and decrease the release of NA, the result is diminished alertness, lower blood pressure, and slower respiration.
With chronic exposure to opioids, the LC acts to increase levels of NA to counteract suppression. When a patient stops taking opioids, the increased NA levels become excessive and produce symptoms of opioid withdrawal. 29

Withdrawal can be measured using any of a number of validated tools, including
- the Subjective Opiate Withdrawal Scale, or SOWS30 (FIGURE 1), which utilizes a patient self-report
- the Clinical Opiate Withdrawal Scale, or COWS31 (FIGURE 2), which relies on assessment made by the physician.

Continue to: Although withdrawal...
Although withdrawal is generally not considered life-threatening in patients without significant comorbidities, do not underestimate the severity of withdrawal symptoms. Often, the desire to avoid these intense symptoms drives patients with OUD to continue to overuse.
Increased pain. Patients might fear that pain will become worse if opioids are tapered. Although it is important to acknowledge this fear, studies of patients undergoing a long-term opioid taper report improvements in function without loss of adequate pain control; some even report that pain control improves.32
Three long-term risks
Relapse. The most dangerous risk of tapering opioids is use of illicit opioids, a danger made worse by the increasing presence of highly lethal synthetic fentanyl analogues in the community. Risk factors for relapse following a full taper include the presence of depressive symptoms at initiation of tapering and higher pain scores at initiation and conclusion of the taper.33 Having low pain at the end of an opioid taper, on the other hand, is predictive of long-term abstinence from opioids.32
Declining function. As is the case while prescribing opioids for pain, maintenance of function remains a priority when tapering opioids. Function can be difficult to assess, given the many variables that can influence an individual’s function. Psychosocial factors, such as coping strategies and mood, strongly influence function; so do psychiatric morbidities, which are more prevalent in patients with chronic pain and disability, compared with the general population.34
Medicolegal matters. Although difficult to characterize, medicolegal risk is an inevitable consideration when tapering opioids:
- In a study of closed malpractice claims involving all medical specialties, narcotic pain medications were the most common drug class involved, representing 1% of claims.35
- In a study of closed malpractice claims involving pain medicine specialists, 3% were related to medication management. Most claims arose following death from opioid overdose.36
Continue to: What else is needed in this area of practice?
What else is needed in this area of practice?
Increasingly, family physicians face the inherent tension of wanting to provide patient-centered, compassionate care for patients in pain while being mindful of opioid prescription stewardship. To support their work and help allay this tension, clinical research on this topic in the future should focus on
- new options for nonopioid pharmacotherapy for pain
- best practices for using opioids in noncancer chronic pain.
In addition, health care systems can help—by providing insurance coverage of nonpharmacotherapeutic options for treating pain.
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 111 Westfall Road, Room 952, Rochester, NY 14620; MichaelMendoza@ monroecounty.gov
1. Chen J, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
2. Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:697-704.
3. Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
4. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-S226.
5. Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803-812.
6. Wilson HD, Dansie EJ, Kim MS, et al. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14:613-627.
7. Peppin JF. The marginalization of chronic pain patients on chronic opioid therapy. Pain Physician. 2009;12:493-498.
8. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
9. Bonnie RJ. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017.
10. Sherman KJ, Walker RL, Saunders K, et al. Doctor-patient trust among chronic pain patients on chronic opioid therapy after opioid risk reduction initiatives: a survey. J Am Board Fam Med. 2018;31:578-587.
11. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
12. Broussard CS, Rasmussen SA, Reefhuis J, et al; National Birth Defects Prevention Study. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1-e11.
13. FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. US Food and Drug Administration website. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy. Published January 9, 2015. Accessed May 27, 2019.
14. Tran TH, Griffin BL, Stone RH, et al. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824-839.
15. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131-146.
16. Kuryshev YA, Bruening-Wright A, Brown AM, et al. Increased cardiac risk in concomitant methadone and diazepam treatment: pharmacodynamic interactions in cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:420-430.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
19. Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71-80.
20. Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973-979.
21. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953-959.
22. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2017;11:CD010323.
23. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686-691.
24. Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23:616-622.
25. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;1:CD006605.
26. Murphy JL, Clark ME, Banou E. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain. 2013;29:109-117.
27. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59-80.
28. Farrell M. Opiate withdrawal. Addiction. 1994;89:1471-1475.
29. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20.
30. Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293-308.
31. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259.
32. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277-282.
33. Heiwe S, Lönnquist I, Källmén H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain. 2011;15:966-970.
34. Dersh J, Gatchel RJ, Polatin P, et al. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44:459-468.
35. Troxel DB. REMS: Opioid-Related Patient Safety and Liability. Richardson, TX: The Doctors Company; 2012.
36. Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948-956.
1. Chen J, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
2. Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:697-704.
3. Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
4. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-S226.
5. Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803-812.
6. Wilson HD, Dansie EJ, Kim MS, et al. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14:613-627.
7. Peppin JF. The marginalization of chronic pain patients on chronic opioid therapy. Pain Physician. 2009;12:493-498.
8. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
9. Bonnie RJ. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017.
10. Sherman KJ, Walker RL, Saunders K, et al. Doctor-patient trust among chronic pain patients on chronic opioid therapy after opioid risk reduction initiatives: a survey. J Am Board Fam Med. 2018;31:578-587.
11. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
12. Broussard CS, Rasmussen SA, Reefhuis J, et al; National Birth Defects Prevention Study. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1-e11.
13. FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. US Food and Drug Administration website. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy. Published January 9, 2015. Accessed May 27, 2019.
14. Tran TH, Griffin BL, Stone RH, et al. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824-839.
15. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131-146.
16. Kuryshev YA, Bruening-Wright A, Brown AM, et al. Increased cardiac risk in concomitant methadone and diazepam treatment: pharmacodynamic interactions in cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:420-430.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
19. Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71-80.
20. Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973-979.
21. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953-959.
22. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2017;11:CD010323.
23. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686-691.
24. Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23:616-622.
25. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;1:CD006605.
26. Murphy JL, Clark ME, Banou E. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain. 2013;29:109-117.
27. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59-80.
28. Farrell M. Opiate withdrawal. Addiction. 1994;89:1471-1475.
29. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20.
30. Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293-308.
31. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259.
32. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277-282.
33. Heiwe S, Lönnquist I, Källmén H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain. 2011;15:966-970.
34. Dersh J, Gatchel RJ, Polatin P, et al. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44:459-468.
35. Troxel DB. REMS: Opioid-Related Patient Safety and Liability. Richardson, TX: The Doctors Company; 2012.
36. Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948-956.
PRACTICE RECOMMENDATIONS
› Continue opioid therapy only when it has brought clinically meaningful improvement in pain and function and when the benefits outweigh adverse events or risks. C
› Review the selected opioid tapering plan in detail with the patient and provide close follow-up monitoring of ongoing or emerging risks. C
› Be vigilant: Enacting an opioid-tapering plan can unmask opioid use disorder, which can cause the patient to seek alternative forms of opioids, including illicit, potentially lethal fentanyl analogues. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Update on Rosacea Classification and Its Controversies
Rosacea is an inflammatory skin condition that affects approximately 5% of the adult population, with the highest prevalence in Europe and North America.1 Despite its prevalence, rosacea remains poorly understood from a pathophysiologic perspective, with no diagnostic laboratory markers.2 Because diagnosis relies on clinical judgment, the nomenclature for describing and characterizing rosacea becomes paramount in ensuring that patients are given an accurate diagnosis and subsequent treatment. We review the shortfalls in the recent history of rosacea classification and discuss their implications.
Subtype to Phenotype Classification
In 2002, the National Rosacea Society (NRS) Expert Committee published a standardized classification schema for rosacea (Table).3 The authors described primary and secondary diagnostic criteria. The presence of 1 or more primary features in a central facial distribution was indicative of rosacea. Primary characteristics included flushing (transient erythema), nontransient erythema, papules and pustules, and telangiectasia. Secondary features, which could occur with or independently of primary features, included burning or stinging of the face, dry appearance, facial edema, ocular manifestations, peripheral (nonfacial) occurrence, phymatous changes, and red facial plaques. Whereas these features often present simultaneously in a characteristic pattern, they were grouped into 4 main subtypes—erythematotelangiectatic (ETR), papulopustular, phymatous, and ocular—and 1 variant, granulomatous rosacea.3
To enhance clinical and research applications of this categorization system as well as offer further standardization, the NRS released a supplementary clinical grading scorecard in 2004 in which each of the primary and secondary characteristics could be assigned a subjective severity score of absent, mild, moderate, or severe. The goal was that the subtype classification and clinical grading system, when used in conjunction with each other, would establish a common language for patients, clinicians, and researchers to describe and further investigate rosacea.4
The 2002 categorization system was certainly an impactful first step in the organization of rosacea. It was not without its critics, however, namely rosacea-oriented dermatologists who were concerned about its lack of specificity.5-7 For instance, the NRS Expert Committee did not address the time frame for flushing, which typically has a long duration in rosacea patients, or for the nontransient erythema; telangiectasia secondary to heliodermatitis; or the often-observed periocular sparing. Additionally, the schema did not account for conditions such as gram-negative folliculitis (pustules characteristically located on the central face) or discuss the need to rule out carcinoid, mastocytosis, or connective-tissue disease, which can lead to nontransient facial erythema. Without strict definitions and exclusions, nonrosacea disorders could be incorrectly labeled as rosacea.
Beyond the lack of specificity, there was additional concern if a subtype system was the ideal way to capture disease presentation and severity. By subtyping, there was unnecessary division of interrelated disease into individual disorders; an individual’s clinical presentation might fall along a spectrum rather than within a discrete box.8
Furthermore, from a research standpoint, subtyping rosacea could hinder or confuse epidemiologic studies. For instance, if patients present with phenotypes from different subtypes, into which subtype would they fall?8-10
The global ROSacea COnsensus (ROSCO) panel, comprising 17 international dermatologists and ophthalmologists, convened in 2016 to address this matter. The panel proposed a new system (published in 2017) based on individual phenotypes.9 In this new system, diagnostic features include persistent centrofacial erythema with periods of increased intensity and phymatous changes. Major features, which are diagnostic when there are at least 2, include flushing (transient erythema), inflammatory papules and pustules, centrofacial telangiectasia, and ocular manifestations. Each feature could then be graded on a severity spectrum independent of concurrent phenotypes (Table).8
The panel concluded that this system would provide a stronger foundation for standardization as new knowledge of rosacea continues to be elucidated.8 In support of their argument, ROSCO also released a treatment algorithm that depended on a phenotype scheme.11 The panel emphasized that by focusing on individual lesions rather than a subtype encompassing many characteristics, treatment could be tailored to the patient. Using this à-la-carte therapy option, physicians could choose those rosacea aspects that are particularly concerning to the patient and manage only those aspects or overlap treatments to improve multiple aspects.11
In 2017, 15 years after the original classification system was proposed, the NRS updated their classification system (published in 2018), taking into consideration some of the criticisms as well as new scientific data on rosacea. Similar to the schema proposed by ROSCO, this system was based on phenotype. Inclusion and exclusion criteria were more robust in this update compared to the original classification in 2002. The criteria provide a timeline for transient flushing—it must occur within seconds or minutes in response to a neurovascular stimulant—and state that it is characteristically prolonged (Table).12
However, the Expert Committee still did not define either the length of time of flushing or nontransient erythema. It also did not specify convex surfaces of the face with periocular sparing as the characteristic pattern or provide additional information on how photoaging fits into the definition. The updated classification stated that centrofacial erythema must not be from cutaneous lupus or seborrheic eczema, and steroid-induced rosacea was still excluded.12 However, there is still the need to exclude other systemic conditions, such as mastocytosis, carcinoid, polycythemia vera, and dermatomyositis. Therefore, the potential for subjective error and inclusion of nonrosacea diseases persists.
A critical change was elimination of the granulomatous rosacea variant. In 2002, this variant was defined by monomorphic, yellow-brown to red papules and nodules that led to scarring. This variant, however, did not share the commonalities of the other subtypes, including persistent facial erythema, limitation to convex surfaces, periocular sparing, and transient flushing.3,13 At the time, Crawford et al6 proposed that the variant be recategorized as granulomatous facial dermatitis. In the updated NRS classification, this variant and phenotypic description was eliminated from the schema.12 It is unclear if it was removed because of these discrepancies or if the NRS panel felt it had a distinct pathogenesis from the proposed rosacea pathophysiology; however, we applaud this change.
Subtype Progression
Both the ROSCO and NRS classification schemes mention progression between the various phenotypes,10,12 suggesting that rosacea phenotypes exist along a continuum, progressing and regressing with disease severity. The main study addressing this point was based on the self-reported retrospective patient memory of disease features in rosacea patients. The authors used a modified criterion of centrofacial erythema alone to define ETR; therefore, a person who began their disease with this finding but then acquired inflammatory lesions or phymas was defined as progressing along a spectrum.14 Given that persistent erythema of convex surfaces of the face is common in all subtypes, we do not find it surprising that the authors found (using their modified criteria) that ETR appeared to progress to papulopustular and phymatous subtypes in a small number of patients. We strongly disagree with their interpretation and conclusion.
In our experience, ETR patients have fine textured skin without sebaceous quality or a history of extensive acne (Figure 1). Flushing is common and usually lasts 10 minutes to 1 hour. There might be concurrent burning or stinging; however, there is no associated sweating, lightheadedness, palpitations, or diagnostic laboratory findings, which distinguishes ETR from other common causes of flushing. The persistent centrofacial erythema involves convex surfaces, spares periocular skin, and can be best defined as present for longer than 3 months.
In contrast, phymas occur commonly in patients with thick and sebaceous (glandular) skin (Figure 2).6,15-17 Men are most often affected and usually have a history of moderate to severe acne. It is not uncommon to observe nodules, cysts, and scarring in addition to papules and pustules. These eruptions primarily cluster on the central face and present in areas of nontransient erythema. Flushing, although less prominent than in other phenotypes, also can be seen.
Taken together, we find no convincing evidence from published studies or extensive experience caring for rosacea patients that classic ETR progresses to phymatous rosacea, or the other way around, as displayed in the ROSCO panel report.8 The type of skin seen in Figure 1 will not “progress” to the type seen in Figure 2. Furthermore, treatment will not “reverse” the phymatous skin into thin, ETR-type skin. The implications are important: If a female patient is given a diagnosis of ETR, she will not develop an enlarged phymatous nose. Patients with thick sebaceous skin, as in Figure 2, usually tolerate treatments such as benzoyl peroxide that other rosacea patients do not and frequently respond well to such intervention.
Implications and Future Directions
We present an overview of 2 rosacea classification systems, hoping to stimulate further refinement. Looking forward, there are many directions for further investigation into the pathophysiology of rosacea. From a genetic standpoint, there needs to be continued molecular and epidemiologic data to determine the underlying genetic contributions to disease.
There has been some progress in the realm of understanding the mechanisms of inflammation; we urge further investigation to elucidate how “subclinical neuroinflammation” might lead to glandular hyperplasia.12 We also see value in examining the genetic and hormonal contributions to phymas, as they may be different than those seen in the ETR-type patients. Last, more studies focusing on comorbidities that contribute to or arise from rosacea are welcomed.
The ultimate goal is to develop a classification system that integrates clinical descriptions, pathophysiologic mechanisms, and benchmark indicators of disease. Only then can we have a true gold standard for the diagnosis of rosacea, one that allows for improved personalized treatment and better outcomes.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta‐analysis. Br J Dermatol. 2018;179:282-289.
- van Zuuren EJ. Rosacea. N Engl J Med. 2017;377:1754-1764.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Saleem MD. Revisiting rosacea criteria: where have we been, where are we going, and how will we get there? Dermatol Clin. 2018;36:161-165.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Tan J, Almeida LMC, Bewley A, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:431-438.
- Wilkin J. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes. Br J Dermatol. 2017;177:597-598.
- Tan J; ROSCO coauthors. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes: reply from the author. Br J Dermatol. 2017;177:598-599.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management and treatment. Dermatol Clin. 2015;33:447-455.
- Tan J, Blume‐Peytavi U, Ortonne JP, et al. An observational cross‐sectional survey of rosacea: clinical associations and progression between subtypes. Br J Dermatol. 2013;169:555-562.
- James WD, Elston D, Treat JR, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. New York, NY: Elsevier; 2019.
- Reinholz M, Tietze JK, Kilian K, et al. Rosacea - S1 guideline. J Dtsch Dermatol Ges. 2013;11:768-780.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom‐oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
Rosacea is an inflammatory skin condition that affects approximately 5% of the adult population, with the highest prevalence in Europe and North America.1 Despite its prevalence, rosacea remains poorly understood from a pathophysiologic perspective, with no diagnostic laboratory markers.2 Because diagnosis relies on clinical judgment, the nomenclature for describing and characterizing rosacea becomes paramount in ensuring that patients are given an accurate diagnosis and subsequent treatment. We review the shortfalls in the recent history of rosacea classification and discuss their implications.
Subtype to Phenotype Classification
In 2002, the National Rosacea Society (NRS) Expert Committee published a standardized classification schema for rosacea (Table).3 The authors described primary and secondary diagnostic criteria. The presence of 1 or more primary features in a central facial distribution was indicative of rosacea. Primary characteristics included flushing (transient erythema), nontransient erythema, papules and pustules, and telangiectasia. Secondary features, which could occur with or independently of primary features, included burning or stinging of the face, dry appearance, facial edema, ocular manifestations, peripheral (nonfacial) occurrence, phymatous changes, and red facial plaques. Whereas these features often present simultaneously in a characteristic pattern, they were grouped into 4 main subtypes—erythematotelangiectatic (ETR), papulopustular, phymatous, and ocular—and 1 variant, granulomatous rosacea.3
To enhance clinical and research applications of this categorization system as well as offer further standardization, the NRS released a supplementary clinical grading scorecard in 2004 in which each of the primary and secondary characteristics could be assigned a subjective severity score of absent, mild, moderate, or severe. The goal was that the subtype classification and clinical grading system, when used in conjunction with each other, would establish a common language for patients, clinicians, and researchers to describe and further investigate rosacea.4
The 2002 categorization system was certainly an impactful first step in the organization of rosacea. It was not without its critics, however, namely rosacea-oriented dermatologists who were concerned about its lack of specificity.5-7 For instance, the NRS Expert Committee did not address the time frame for flushing, which typically has a long duration in rosacea patients, or for the nontransient erythema; telangiectasia secondary to heliodermatitis; or the often-observed periocular sparing. Additionally, the schema did not account for conditions such as gram-negative folliculitis (pustules characteristically located on the central face) or discuss the need to rule out carcinoid, mastocytosis, or connective-tissue disease, which can lead to nontransient facial erythema. Without strict definitions and exclusions, nonrosacea disorders could be incorrectly labeled as rosacea.
Beyond the lack of specificity, there was additional concern if a subtype system was the ideal way to capture disease presentation and severity. By subtyping, there was unnecessary division of interrelated disease into individual disorders; an individual’s clinical presentation might fall along a spectrum rather than within a discrete box.8
Furthermore, from a research standpoint, subtyping rosacea could hinder or confuse epidemiologic studies. For instance, if patients present with phenotypes from different subtypes, into which subtype would they fall?8-10
The global ROSacea COnsensus (ROSCO) panel, comprising 17 international dermatologists and ophthalmologists, convened in 2016 to address this matter. The panel proposed a new system (published in 2017) based on individual phenotypes.9 In this new system, diagnostic features include persistent centrofacial erythema with periods of increased intensity and phymatous changes. Major features, which are diagnostic when there are at least 2, include flushing (transient erythema), inflammatory papules and pustules, centrofacial telangiectasia, and ocular manifestations. Each feature could then be graded on a severity spectrum independent of concurrent phenotypes (Table).8
The panel concluded that this system would provide a stronger foundation for standardization as new knowledge of rosacea continues to be elucidated.8 In support of their argument, ROSCO also released a treatment algorithm that depended on a phenotype scheme.11 The panel emphasized that by focusing on individual lesions rather than a subtype encompassing many characteristics, treatment could be tailored to the patient. Using this à-la-carte therapy option, physicians could choose those rosacea aspects that are particularly concerning to the patient and manage only those aspects or overlap treatments to improve multiple aspects.11
In 2017, 15 years after the original classification system was proposed, the NRS updated their classification system (published in 2018), taking into consideration some of the criticisms as well as new scientific data on rosacea. Similar to the schema proposed by ROSCO, this system was based on phenotype. Inclusion and exclusion criteria were more robust in this update compared to the original classification in 2002. The criteria provide a timeline for transient flushing—it must occur within seconds or minutes in response to a neurovascular stimulant—and state that it is characteristically prolonged (Table).12
However, the Expert Committee still did not define either the length of time of flushing or nontransient erythema. It also did not specify convex surfaces of the face with periocular sparing as the characteristic pattern or provide additional information on how photoaging fits into the definition. The updated classification stated that centrofacial erythema must not be from cutaneous lupus or seborrheic eczema, and steroid-induced rosacea was still excluded.12 However, there is still the need to exclude other systemic conditions, such as mastocytosis, carcinoid, polycythemia vera, and dermatomyositis. Therefore, the potential for subjective error and inclusion of nonrosacea diseases persists.
A critical change was elimination of the granulomatous rosacea variant. In 2002, this variant was defined by monomorphic, yellow-brown to red papules and nodules that led to scarring. This variant, however, did not share the commonalities of the other subtypes, including persistent facial erythema, limitation to convex surfaces, periocular sparing, and transient flushing.3,13 At the time, Crawford et al6 proposed that the variant be recategorized as granulomatous facial dermatitis. In the updated NRS classification, this variant and phenotypic description was eliminated from the schema.12 It is unclear if it was removed because of these discrepancies or if the NRS panel felt it had a distinct pathogenesis from the proposed rosacea pathophysiology; however, we applaud this change.
Subtype Progression
Both the ROSCO and NRS classification schemes mention progression between the various phenotypes,10,12 suggesting that rosacea phenotypes exist along a continuum, progressing and regressing with disease severity. The main study addressing this point was based on the self-reported retrospective patient memory of disease features in rosacea patients. The authors used a modified criterion of centrofacial erythema alone to define ETR; therefore, a person who began their disease with this finding but then acquired inflammatory lesions or phymas was defined as progressing along a spectrum.14 Given that persistent erythema of convex surfaces of the face is common in all subtypes, we do not find it surprising that the authors found (using their modified criteria) that ETR appeared to progress to papulopustular and phymatous subtypes in a small number of patients. We strongly disagree with their interpretation and conclusion.
In our experience, ETR patients have fine textured skin without sebaceous quality or a history of extensive acne (Figure 1). Flushing is common and usually lasts 10 minutes to 1 hour. There might be concurrent burning or stinging; however, there is no associated sweating, lightheadedness, palpitations, or diagnostic laboratory findings, which distinguishes ETR from other common causes of flushing. The persistent centrofacial erythema involves convex surfaces, spares periocular skin, and can be best defined as present for longer than 3 months.

In contrast, phymas occur commonly in patients with thick and sebaceous (glandular) skin (Figure 2).6,15-17 Men are most often affected and usually have a history of moderate to severe acne. It is not uncommon to observe nodules, cysts, and scarring in addition to papules and pustules. These eruptions primarily cluster on the central face and present in areas of nontransient erythema. Flushing, although less prominent than in other phenotypes, also can be seen.

Taken together, we find no convincing evidence from published studies or extensive experience caring for rosacea patients that classic ETR progresses to phymatous rosacea, or the other way around, as displayed in the ROSCO panel report.8 The type of skin seen in Figure 1 will not “progress” to the type seen in Figure 2. Furthermore, treatment will not “reverse” the phymatous skin into thin, ETR-type skin. The implications are important: If a female patient is given a diagnosis of ETR, she will not develop an enlarged phymatous nose. Patients with thick sebaceous skin, as in Figure 2, usually tolerate treatments such as benzoyl peroxide that other rosacea patients do not and frequently respond well to such intervention.
Implications and Future Directions
We present an overview of 2 rosacea classification systems, hoping to stimulate further refinement. Looking forward, there are many directions for further investigation into the pathophysiology of rosacea. From a genetic standpoint, there needs to be continued molecular and epidemiologic data to determine the underlying genetic contributions to disease.
There has been some progress in the realm of understanding the mechanisms of inflammation; we urge further investigation to elucidate how “subclinical neuroinflammation” might lead to glandular hyperplasia.12 We also see value in examining the genetic and hormonal contributions to phymas, as they may be different than those seen in the ETR-type patients. Last, more studies focusing on comorbidities that contribute to or arise from rosacea are welcomed.
The ultimate goal is to develop a classification system that integrates clinical descriptions, pathophysiologic mechanisms, and benchmark indicators of disease. Only then can we have a true gold standard for the diagnosis of rosacea, one that allows for improved personalized treatment and better outcomes.
Rosacea is an inflammatory skin condition that affects approximately 5% of the adult population, with the highest prevalence in Europe and North America.1 Despite its prevalence, rosacea remains poorly understood from a pathophysiologic perspective, with no diagnostic laboratory markers.2 Because diagnosis relies on clinical judgment, the nomenclature for describing and characterizing rosacea becomes paramount in ensuring that patients are given an accurate diagnosis and subsequent treatment. We review the shortfalls in the recent history of rosacea classification and discuss their implications.
Subtype to Phenotype Classification
In 2002, the National Rosacea Society (NRS) Expert Committee published a standardized classification schema for rosacea (Table).3 The authors described primary and secondary diagnostic criteria. The presence of 1 or more primary features in a central facial distribution was indicative of rosacea. Primary characteristics included flushing (transient erythema), nontransient erythema, papules and pustules, and telangiectasia. Secondary features, which could occur with or independently of primary features, included burning or stinging of the face, dry appearance, facial edema, ocular manifestations, peripheral (nonfacial) occurrence, phymatous changes, and red facial plaques. Whereas these features often present simultaneously in a characteristic pattern, they were grouped into 4 main subtypes—erythematotelangiectatic (ETR), papulopustular, phymatous, and ocular—and 1 variant, granulomatous rosacea.3
To enhance clinical and research applications of this categorization system as well as offer further standardization, the NRS released a supplementary clinical grading scorecard in 2004 in which each of the primary and secondary characteristics could be assigned a subjective severity score of absent, mild, moderate, or severe. The goal was that the subtype classification and clinical grading system, when used in conjunction with each other, would establish a common language for patients, clinicians, and researchers to describe and further investigate rosacea.4
The 2002 categorization system was certainly an impactful first step in the organization of rosacea. It was not without its critics, however, namely rosacea-oriented dermatologists who were concerned about its lack of specificity.5-7 For instance, the NRS Expert Committee did not address the time frame for flushing, which typically has a long duration in rosacea patients, or for the nontransient erythema; telangiectasia secondary to heliodermatitis; or the often-observed periocular sparing. Additionally, the schema did not account for conditions such as gram-negative folliculitis (pustules characteristically located on the central face) or discuss the need to rule out carcinoid, mastocytosis, or connective-tissue disease, which can lead to nontransient facial erythema. Without strict definitions and exclusions, nonrosacea disorders could be incorrectly labeled as rosacea.
Beyond the lack of specificity, there was additional concern if a subtype system was the ideal way to capture disease presentation and severity. By subtyping, there was unnecessary division of interrelated disease into individual disorders; an individual’s clinical presentation might fall along a spectrum rather than within a discrete box.8
Furthermore, from a research standpoint, subtyping rosacea could hinder or confuse epidemiologic studies. For instance, if patients present with phenotypes from different subtypes, into which subtype would they fall?8-10
The global ROSacea COnsensus (ROSCO) panel, comprising 17 international dermatologists and ophthalmologists, convened in 2016 to address this matter. The panel proposed a new system (published in 2017) based on individual phenotypes.9 In this new system, diagnostic features include persistent centrofacial erythema with periods of increased intensity and phymatous changes. Major features, which are diagnostic when there are at least 2, include flushing (transient erythema), inflammatory papules and pustules, centrofacial telangiectasia, and ocular manifestations. Each feature could then be graded on a severity spectrum independent of concurrent phenotypes (Table).8
The panel concluded that this system would provide a stronger foundation for standardization as new knowledge of rosacea continues to be elucidated.8 In support of their argument, ROSCO also released a treatment algorithm that depended on a phenotype scheme.11 The panel emphasized that by focusing on individual lesions rather than a subtype encompassing many characteristics, treatment could be tailored to the patient. Using this à-la-carte therapy option, physicians could choose those rosacea aspects that are particularly concerning to the patient and manage only those aspects or overlap treatments to improve multiple aspects.11
In 2017, 15 years after the original classification system was proposed, the NRS updated their classification system (published in 2018), taking into consideration some of the criticisms as well as new scientific data on rosacea. Similar to the schema proposed by ROSCO, this system was based on phenotype. Inclusion and exclusion criteria were more robust in this update compared to the original classification in 2002. The criteria provide a timeline for transient flushing—it must occur within seconds or minutes in response to a neurovascular stimulant—and state that it is characteristically prolonged (Table).12
However, the Expert Committee still did not define either the length of time of flushing or nontransient erythema. It also did not specify convex surfaces of the face with periocular sparing as the characteristic pattern or provide additional information on how photoaging fits into the definition. The updated classification stated that centrofacial erythema must not be from cutaneous lupus or seborrheic eczema, and steroid-induced rosacea was still excluded.12 However, there is still the need to exclude other systemic conditions, such as mastocytosis, carcinoid, polycythemia vera, and dermatomyositis. Therefore, the potential for subjective error and inclusion of nonrosacea diseases persists.
A critical change was elimination of the granulomatous rosacea variant. In 2002, this variant was defined by monomorphic, yellow-brown to red papules and nodules that led to scarring. This variant, however, did not share the commonalities of the other subtypes, including persistent facial erythema, limitation to convex surfaces, periocular sparing, and transient flushing.3,13 At the time, Crawford et al6 proposed that the variant be recategorized as granulomatous facial dermatitis. In the updated NRS classification, this variant and phenotypic description was eliminated from the schema.12 It is unclear if it was removed because of these discrepancies or if the NRS panel felt it had a distinct pathogenesis from the proposed rosacea pathophysiology; however, we applaud this change.
Subtype Progression
Both the ROSCO and NRS classification schemes mention progression between the various phenotypes,10,12 suggesting that rosacea phenotypes exist along a continuum, progressing and regressing with disease severity. The main study addressing this point was based on the self-reported retrospective patient memory of disease features in rosacea patients. The authors used a modified criterion of centrofacial erythema alone to define ETR; therefore, a person who began their disease with this finding but then acquired inflammatory lesions or phymas was defined as progressing along a spectrum.14 Given that persistent erythema of convex surfaces of the face is common in all subtypes, we do not find it surprising that the authors found (using their modified criteria) that ETR appeared to progress to papulopustular and phymatous subtypes in a small number of patients. We strongly disagree with their interpretation and conclusion.
In our experience, ETR patients have fine textured skin without sebaceous quality or a history of extensive acne (Figure 1). Flushing is common and usually lasts 10 minutes to 1 hour. There might be concurrent burning or stinging; however, there is no associated sweating, lightheadedness, palpitations, or diagnostic laboratory findings, which distinguishes ETR from other common causes of flushing. The persistent centrofacial erythema involves convex surfaces, spares periocular skin, and can be best defined as present for longer than 3 months.

In contrast, phymas occur commonly in patients with thick and sebaceous (glandular) skin (Figure 2).6,15-17 Men are most often affected and usually have a history of moderate to severe acne. It is not uncommon to observe nodules, cysts, and scarring in addition to papules and pustules. These eruptions primarily cluster on the central face and present in areas of nontransient erythema. Flushing, although less prominent than in other phenotypes, also can be seen.

Taken together, we find no convincing evidence from published studies or extensive experience caring for rosacea patients that classic ETR progresses to phymatous rosacea, or the other way around, as displayed in the ROSCO panel report.8 The type of skin seen in Figure 1 will not “progress” to the type seen in Figure 2. Furthermore, treatment will not “reverse” the phymatous skin into thin, ETR-type skin. The implications are important: If a female patient is given a diagnosis of ETR, she will not develop an enlarged phymatous nose. Patients with thick sebaceous skin, as in Figure 2, usually tolerate treatments such as benzoyl peroxide that other rosacea patients do not and frequently respond well to such intervention.
Implications and Future Directions
We present an overview of 2 rosacea classification systems, hoping to stimulate further refinement. Looking forward, there are many directions for further investigation into the pathophysiology of rosacea. From a genetic standpoint, there needs to be continued molecular and epidemiologic data to determine the underlying genetic contributions to disease.
There has been some progress in the realm of understanding the mechanisms of inflammation; we urge further investigation to elucidate how “subclinical neuroinflammation” might lead to glandular hyperplasia.12 We also see value in examining the genetic and hormonal contributions to phymas, as they may be different than those seen in the ETR-type patients. Last, more studies focusing on comorbidities that contribute to or arise from rosacea are welcomed.
The ultimate goal is to develop a classification system that integrates clinical descriptions, pathophysiologic mechanisms, and benchmark indicators of disease. Only then can we have a true gold standard for the diagnosis of rosacea, one that allows for improved personalized treatment and better outcomes.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta‐analysis. Br J Dermatol. 2018;179:282-289.
- van Zuuren EJ. Rosacea. N Engl J Med. 2017;377:1754-1764.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Saleem MD. Revisiting rosacea criteria: where have we been, where are we going, and how will we get there? Dermatol Clin. 2018;36:161-165.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Tan J, Almeida LMC, Bewley A, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:431-438.
- Wilkin J. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes. Br J Dermatol. 2017;177:597-598.
- Tan J; ROSCO coauthors. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes: reply from the author. Br J Dermatol. 2017;177:598-599.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management and treatment. Dermatol Clin. 2015;33:447-455.
- Tan J, Blume‐Peytavi U, Ortonne JP, et al. An observational cross‐sectional survey of rosacea: clinical associations and progression between subtypes. Br J Dermatol. 2013;169:555-562.
- James WD, Elston D, Treat JR, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. New York, NY: Elsevier; 2019.
- Reinholz M, Tietze JK, Kilian K, et al. Rosacea - S1 guideline. J Dtsch Dermatol Ges. 2013;11:768-780.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom‐oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta‐analysis. Br J Dermatol. 2018;179:282-289.
- van Zuuren EJ. Rosacea. N Engl J Med. 2017;377:1754-1764.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Saleem MD. Revisiting rosacea criteria: where have we been, where are we going, and how will we get there? Dermatol Clin. 2018;36:161-165.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Tan J, Almeida LMC, Bewley A, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:431-438.
- Wilkin J. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes. Br J Dermatol. 2017;177:597-598.
- Tan J; ROSCO coauthors. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes: reply from the author. Br J Dermatol. 2017;177:598-599.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management and treatment. Dermatol Clin. 2015;33:447-455.
- Tan J, Blume‐Peytavi U, Ortonne JP, et al. An observational cross‐sectional survey of rosacea: clinical associations and progression between subtypes. Br J Dermatol. 2013;169:555-562.
- James WD, Elston D, Treat JR, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. New York, NY: Elsevier; 2019.
- Reinholz M, Tietze JK, Kilian K, et al. Rosacea - S1 guideline. J Dtsch Dermatol Ges. 2013;11:768-780.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom‐oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
Practice Points
- Rosacea therapy is based on a phenotype classification system, in which patients can have major and minor features across all previously denoted subtypes. This system allows for greater flexibility in treatment regimens.
- Despite mention of progression between subtypes, there has not been convincing evidence that patients can progress or regress from one end of the rosacea spectrum (erythematotelangiectatic) to the other (phymatous).
What’s New in the Management of Acne Vulgaris
Inflammation is a backdrop to the commonly cited elements of the pathophysiology of acne: Propionibacterium acnes proliferation, increased sebum production with an increase in circulating androgens, and faulty keratinization.1,2 In fact, research shows that the initiating lesion of acne vulgaris—the microcomedone—is, in essence, an inflammatory lesion.3 This realization has clearly influenced the approach to acne treatment but has not yielded a bevy of new treatments.
A better understanding of acne pathophysiology and the role of inflammation has, however, yielded a better understanding of how existing therapies treat the disease and have led to more comprehensive treatment strategies that are multitargeted. Nonetheless, topical and oral antibiotics remain mainstays of acne therapy, along with topical retinoids and benzoyl peroxide. Current guidelines of care for acne emphasize strategies that reduce dependence on antibiotics and minimize the risk for resistance.4 The therapeutic landscape might at last be shifting, with new chemical entities for acne and several novel formulations in development.
Sarecycline: A Novel Tetracycline
Tetracycline antibiotics have been used to manage acne since the 1950s, but their method of action in the disease has not been fully elucidated.5 In addition to antibiotic effects, tetracyclines have been shown to confer anti-inflammatory properties and other biologic effects.6,7
First-generation tetracycline is broad spectrum. As such, it is associated with increased potential for antibiotic resistance and greater impact on gastrointestinal health. The novel compound sarecycline is a tetracycline with a narrower spectrum of activity compared to other tetracyclines and with reduced activity against enteric gram-negative bacteria8 (Figure 1). Sarecycline recently was approved by the US Food and Drug Administration (FDA) in a once-daily oral formulation for the treatment of inflammatory lesions of nonnodular moderate to severe acne vulgaris in patients 9 years and older. Sarecycline is dosed at 1.5 mg/kg daily. The FDA approval marks the first new antibiotic approved for acne in 4 decades.

In 2 phase 3 clinical trials, sarecycline demonstrated efficacy in reducing both inflammatory and noninflammatory lesions.9 At week 12, investigator global assessment (IGA) success (≥2 point reduction in IGA and score 0 [clear] or 1 [almost clear]) rates were 21.9% and 22.6% for active treatment (n=483 and n=519), respectively, in the 2 trials compared to 10.5% and 15.3% (n=485 and n=515), respectively, for controls. Sarecycline demonstrated rapid anti-inflammatory effect. Onset of action against inflammatory lesions was notable by week 3. At week 12, inflammatory lesions were reduced in the active treatment arms by 51.8% and 49.9%, respectively, compared to 35.1% and 35.4%, respectively, for controls.9
The most common reported treatment-emergent adverse events (TEAEs) were nausea, nasopharyngitis, headache, and vomiting.9 Vestibular (dizziness, tinnitus, vertigo) and phototoxic (sunburn, photosensitivity) TEAEs both occurred in 1% or fewer of sarecycline patients. Gastrointestinal TEAE rates for sarecycline were low.9
Sarecycline also was assessed in the 2 trials for efficacy in the treatment of back and chest acne; in the active treatment group, IGA success was achieved by 29.6% and 36.6%, respectively, compared to 19.6% and 21.6%, respectively, of controls.9
Tazarotene Foam in Focus
Topical tazarotene is commercially available in cream, gel, and foam formulations. Tazarotene foam 0.1% was FDA approved in 2012 for the treatment of acne vulgaris in patients 12 years and older. However, the product was recently relaunched to the market and therefore warrants discussion.
Similar to other retinoids, topical tazarotene has been associated with the potential for application-site irritation. This aqueous foam formulation of tazarotene was designed for ease of application and to attempt to impart moisturizing effects to offset potential irritation. It contains noncomedogenic light mineral oil, which is an emollient. The foam spreads easily, including on hair-bearing skin, with demonstrated penetration of the active drug into the epidermis and dermis. Nonetheless, compared to the gel formulation of tazarotene, the foam formulation was associated with reduced systemic exposure.10
The tazarotene foam formulation does not contain alcohol, fragrance, propylene glycol, or parabens. Clinical trial participants, blinded to whether they were on active treatment or vehicle foam, consistently rated the foam formulation favorably for ease of application and spreadability, lack of stickiness or residue, and moisturizing effect. The foam vehicle is suggested to increase compliance and satisfaction in some patients.11The efficacy and tolerability of tazarotene foam 0.1% was investigated in 2 randomized, double-blind, vehicle-controlled, parallel-group studies in the United States and Canada.12 The studies involved participants aged 2 to 45 years who were randomized to receive treatment with either tazarotene foam 0.1% or vehicle foam once daily for 12 weeks (N=1486). Lesion counts, investigator static global assessment, and subject global assessment were evaluated at baseline and at weeks 2, 4, 8, and 12. At week 12, mean reduction from baseline in noninflammatory lesions was 55.9% for active treatment, mean reduction in inflammatory lesions was 56.1%, and mean total lesion reduction was 56% compared to mean reductions of 37.7%, 45.3%, and 40.8%, respectively, for vehicle. In all, 28.2% of participants achieved treatment success with active treatment compared to 14.7% of controls. There was a greater proportion of active-treatment participants with investigator static global assessment scores of 0 or 1 compared to vehicle. The only adverse events reported by more than 5% of participants in the active-treatment groups in both studies were application-site skin irritation and dryness.12
Topical Minocycline
Systemic minocycline is the most commonly prescribed oral antibiotic for acne management.13 Despite its widespread use, it is not without potential safety concerns. Minocycline is distinct among tetracyclines for posing a small risk for systemic lupus erythematosus and autoimmune TEAE.Gastrointestinal side effects and bluish discoloration also are reported.14 Topical application of minocycline for acne would optimize the therapeutic effect while reducing systemic effects. FMX101 4%, an investigational minocycline foam, is being studied for the treatment of moderate to severe acne.
In a pharmacokinetic study, minocycline exposure was 730- to 765-times lower with foam application vs oral minocycline.15 No evidence of minocycline accumulation was identified over the 21 days of application of minocycline foam 4%. Minocycline foam 4% appeared to be safe and well tolerated, without serious TEAEs, treatment-related TEAEs, or TEAEs that led to treatment discontinuation.15
In 2 identical phase 3 studies in which 961 participants were randomized (2:1) to once-daily minocycline foam 4% or foam vehicle for 12 weeks, participants in the active-treatment group demonstrated a significantly greater reduction in both inflammatory and noninflammatory lesions in both studies (both P<.05) and a greater rate of treatment success (≥2 point reduction in IGA and score of 0 [clear] or 1 [almost clear]) in 1 study. Treatment was generally safe and well tolerated, with skin-related adverse events reported in fewer than 1% of participants receiving active treatment.16
In an open-label safety extension study that enrolled 657 patients, treatment with FMX101 continued for as long as 40 weeks.17 In total, 291 participants completed 52 weeks of therapy. Rates and types of reported TEAEs in the open-label extension phase were similar to those seen in the phase 3 trials. Application-site TEAEs occurred in fewer than 2% of participants. Participants reported a high level of treatment satisfaction at week 52.17
In a more recent phase 3 study, 1507 participants were randomized (1:1) to once-daily minocycline foam 4% or foam vehicle for 12 weeks to further evaluate the efficacy and safety of FMX101 4% for moderate to severe acne vulgaris.18 The study met both primary end points: absolute change from baseline in the inflammatory lesion count (−16.93 vs −13.40; P<.0001) and the noninflammatory lesion count (−18.80 vs −15.89; P<.05), as well as percentage of participants with IGA treatment success at week 12 (30.80% vs 19.63%; P<.0001). The percentage reduction in the inflammatory lesion count was statistically significantly greater for minocycline foam 4% compared to vehicle as early as week 3 (P<.0001). The safety profile was found to be consistent with the 2 earlier phase 3 studies.18
Topical Minocycline in Rosacea
A similar foam formulation of minocycline (1.5% concentration) has shown benefit in 2 identical phase 3 studies.19 A total of 1522 participants were enrolled in 2 phase 3, randomized, multicenter, double-blind, vehicle-controlled, 2-arm studies in participants 18 years and older with moderate to severe papulopustular rosacea. Participants were randomized (2:1) to either minocycline foam 1.5% or vehicle once daily to the face for 12 weeks.19
Treatment was associated with a statistically significant reduction in counts of inflammatory lesions of rosacea (Study FX2016-11: −17.57 vs −15.65 [P=.003]; Study FX2016-12: −18.54 vs −14.88 [P<.0001]) and a significantly higher rate of IGA treatment success compared to vehicle (Study FX2016-11: 52.1% vs 43.0% [P=.027]; Study FX2016-12: 49.1% vs 39.0% [P=.008]), highlighting the anti-inflammatory action of the topically applied agent.19
The most common TEAE for both studies was upper respiratory tract infection; there were no serious TEAEs. Overall, 9 participants across both studies discontinued because of a TEAE (foam, 7 participants; vehicle, 2 participants).19
Clascoterone: First-in-Class Topical
Clascoterone cream 1% is a new chemical entity under investigation for the treatment of moderate to severe acne in patients 9 years and older. Clascoterone targets androgen receptors in the skin to block the effects of circulating endogenous androgens; chemically, it shares a 4-ring backbone identical to dihydrotestosterone and spironolactone (Figure 2). Clascoterone competes with dihydrotestosterone for binding to the androgen receptor to limit or block transcription of androgen-responsive genes and modify specific gene expression.20

Androgens are known to promote both sebum production and inflammatory responses within the follicle, contributing to the cycle of acne.21 Antiandrogen therapy would, therefore, inhibit excess sebum production and directly reduce the presence of certain inflammatory mediators in skin. This effect is expected to lead to reduced follicular plugging and a reduction in growth of P acnes and its inflammatory by-products.
Direct and indirect hormonal modulation have been successfully employed to manage acne in women; however, such therapies have not been considered first-line interventions for the disease.22 Although systemic antiandrogens and hormonal modulation are effective for certain women with acne, there may be concerns about systemic exposure23; no hormone-modulating agent has been adopted for use in men with acne.
As an androgen inhibitor, clascoterone is thought to displace androgen hormones from androgen receptors located at the sebaceous gland and hair follicle, thus inhibiting the cycle of physiologic events that leads to acne formation. Clascoterone is applied topically and acts locally on androgen receptors in the skin, with no systemic exposure seen. In phase 2 trials, clascoterone was found to be safe and effective with no systemic exposure and was suggested to have better tolerability than topical tretinoin.
Preliminary individual study analysis of data from 2 phase 3 trials showed that topical clascoterone met its primary end points, achieving statistically significantly greater rates of IGA treatment success (≥2 point reduction in IGA and score of 0 [clear] or 1 [almost clear]) at week 12 (P<.0001).24 Rates of treatment success for actively treated participants were 16.1% and 18.7%, respectively, compared to 7% and 4.7%, respectively, for vehicle. The study population included both males and nonpregnant females 9 years and older who had a baseline IGA score of 3 (moderate) or 4 (severe). At baseline, participants had a mix of inflammatory lesions (≥30, to a maximum of 75) and noninflammatory lesions (≥30, to a maximum of 100).24
Intention-to-treat analysis at week 12 showed a mean total lesion reduction from baseline for active treatment of 37.1% and 37.7%, respectively, compared to 28.5% and 22.2%, respectively, for controls.24 Mean reductions from baseline in noninflammatory lesions for active treatment were 30.7% and 29.3%, respectively, compared to 21.9% and 15.8%, respectively, for controls. Mean reductions from baseline in inflammatory lesions for active treatment were 44.8% and 47%, respectively, compared to 36.6% and 29.8%, respectively, for controls. Similarly low rates of TEAEs were reported in active and placebo groups in both studies. No TEAE suggested systemic antiandrogen exposure.24
Advancements in Cannabinoids
Advancements in pharmaceutical development of cannabinoid compounds have largely coincided with the controversial national movement to legalize medical marijuana and decriminalize recreational marijuana use. Despite the temporal connection, the 2 topics are entirely distinct. Importantly, pharmaceutical development is largely focused on the effects of cannabidiol (CBD), which is 1 of approximately 113 cannabinoids identified from Cannabis sativa. Cannabidiol is not tetrahydrocannabinol, or THC, the compound responsible for marijuana’s psychoactive effects and addictive properties; CBD does not have any psychoactive effects and is not addictive (Figure 3).25

A CBD oral solution agent recently gained FDA approval for seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients 2 years and older; it is estimated that more than 180 trials of CBD are ongoing in the United States for various indications.26 A notable question in the development of CBD-based therapies is: What is the role of natural plant-derived CBD compared to a pure synthetic form of CBD? The latter is akin to a pharmaceutical process in which a single molecule is developed as the active drug.27 Although the potency and composition of plant-derived CBD can vary with crop conditions, plant strains, and the extraction process, a synthetic molecule would allow for consistency in safety, potency, and pharmacokinetic properties, as well as efficacy, as a consequence.26
There are intriguing data to suggest a potential use for topical CBD in the management of skin diseases, including acne vulgaris. Researchers have, for at least a decade, been investigating the role of the endocannabinoid system, which has physiologic regulatory functions in proliferation, differentiation, apoptosis and cytokine, mediator, and hormone production of various cell types in skin, hair follicles, and sebaceous glands.28 Cannabidiol has been shown to suppress proliferation of sebocytes through activation of transient receptor potential vanilloid 4 ion channels and to have anti-inflammatory effects on sebocytes.29 It has been shown to inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism30 and to possess potent antimicrobial activity against gram-positive bacteria such as P acnes.31
Given these effects on sebocytes, modulation of keratinocyte proliferation, and anti-inflammatory and antibacterial effects, CBD could prove beneficial in the management of acne vulgaris. A new synthetic CBD topical formulation, BTX 1503, is under investigation for the treatment of acne vulgaris.
Early clinical data confirm both the anti-inflammatory effects of topical BTX 1503 as well as its effects on noninflammatory lesions, with 4-week reductions in inflammatory lesion counts similar to what are reported in clinical trials for leading FDA-approved topical therapies in the same time frame.
The phase 1b trial was a 4-week, open-label study in participants with moderate to severe acne vulgaris.32 The primary end point was safety, as demonstrated by the incidence of TEAE, laboratory monitoring, and assessment of cutaneous tolerability. Exploratory end points included changes in inflammatory and noninflammatory lesion counts and IGA score. A total of 21 participants aged 18 to 65 years with moderate to severe acne vulgaris were enrolled. BTX 1503 was applied topically twice daily. At baseline, eligible participants had 20 to 50 inflammatory lesions and 20 to 100 noninflammatory acne lesions on the face, an IGA of 3 (moderate) or 4 (severe), and 3 or fewer nodular or cystic lesions (>5 mm in diameter). No serious or severe TEAEs were reported; no participants withdrew due to a TEAE. Slight erythema, slight scaling, slight dryness, and slight burning and stinging were reported; there were no reports of irritant or allergic contact dermatitis. Only 1 TEAE was thought to be possibly related to treatment: mild pain at the application site.32
In addition to presenting a potential new chemical entity for the topical treatment of acne, the novel topical vehicle formulation of BTX 1503 represents an innovative approach to drug delivery. The formulation utilizes proprietary technology to deliver high doses of drug into the skin without controversial penetration enhancers, preservatives, or other potential irritating additives. Instead, volatile excipients are used that evaporate upon application to the skin, leaving a so-called superconcentrated secondary formulation on the skin. The concentration gradient effect then drives the concentrated drug into skin. Although the formulation efficiently delivers active drug into the skin and its appendages, systemic exposure has been reported to be very low. A phase 2 randomized, double-blind, vehicle-controlled trial ongoing in the United States and Australia in 360 patients with moderate to severe acne vulgaris will provide key data to confirm the efficacy and safety of BTX 1503 (ClinicalTrials.gov Identifier NCT03573518).
Conclusion
Drug development continues to focus on the challenge of treating acne effectively and safely. Vehicle innovations are optimizing existing active drugs and creating opportunities to deliver new compounds to the skin. The approval of sarecycline as the first new chemical entity approved for acne in several years may be followed in coming years by other new actives, including clascoterone and CBD.
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Burkhart CN, Gottwald L. Assessment of etiologic agents in acne pathogenesis. Skinmed. 2003;2:222-228.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169:337-352.
- Griffin MO, Ceballos G, Villarreal FJ. Tetracycline compounds with non-antimicrobial organ protective properties: possible mechanisms of action. Pharmacol Res. 2011;63:102-107.
- Weinberg JM. The anti-inflammatory effects of tetracyclines. Cutis. 2005;75(4 suppl):6-11.
- Leyden JJ, Sniukiene V, Berk DR, et al. Efficacy and safety of sarecycline, a novel, once-daily, narrow spectrum antibiotic for the treatment of moderate to severe facial acne vulgaris: results of a phase 2, dose-ranging study. J Drugs Dermatol. 2018;17:333-338.
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Jarratt M, Werner CP, Alió Saenz AB. Tazarotene foam versus tazarotene gel: a randomized relative bioavailability study in acne vulgaris. Clin Drug Investig. 2013;33:283-289.
- Smith JA, Narahari S, Hill D, et al. Tazarotene foam, 0.1%, for the treatment of acne. Expert Opin Drug Saf. 2016;15:99-103.
- Feldman SR, Werner CP, Alió Saenz AB. The efficacy and tolerability of tazarotene foam, 0.1%, in the treatment of acne vulgaris in 2 multicenter, randomized, vehicle-controlled, double-blind studies. J Drugs Dermatol. 2013;12:438-446.
- Lee YH, Liu G, Thiboutot DM, et al. A retrospective analysis of the duration of oral antibiotic therapy for the treatment of acne among adolescents: investigating practice gaps and potential cost-savings. J Am Acad Dermatol. 2014;71:70-76.
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012(8):CD002086.
- Jones TM, Ellman H, deVries T. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16:1022-1028.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177.17.
- Gold LS, Dhawan S, Weiss J, et al. FMX101 4% minocycline foam for the treatment of acne vulgaris: safety and patient satisfaction from the open-label extension of 2 phase 3 studies. Poster presented at: 2018 Winter Clinical Dermatology Conference; January 12-17, 2018; Maui, HI.
- Raoof J, Hooper D, Moore A, et al. FMX101 4% topical minocycline foam for the treatment of moderate to severe acne vulgaris: efficacy and safety from a phase 3 randomized, double-blind, vehicle-controlled study. Poster presented at: 2018 Fall Clinical Dermatology Conference; October 18-21, 2018; Las Vegas, NV.
- Gold LS, Del Rosso JQ, Bhatia ND, et al. Efficacy and safety of FMX103 (1.5% minocycline foam) in the treatment of moderate-to-severe papulopustular rosacea: results from two phase 3 randomized, multicenter, double-blind, vehicle-controlled studies. Poster presented at: 2019 Winter Clinical Dermatology Conference; January 18-23; 2019; Koloa, HI.
- Data on file. CB-03-01 2017. Milan, Italy: Cassiopea SpA; 2017.
- Ju Q, Tao T, Hu T, et al. Sex hormones and acne. Clin Dermatol. 2017;35:130-137.
- Park JH, Bienenfeld A, Orlow SJ, et al. The use of hormonal antiandrogen therapy in female patients with acne: a 10-year retrospective study. Am J Clin Dermatol. 2018;19:449-455.
- Barros B, Thiboutot D. Hormonal therapies for acne. Clin Dermatol. 2017;35:168-172.
- Hebert A. Clascoterone topical cream, 1%: a novel, topical, local, selective androgen receptor antagonist: results from two phase 3 studies treating children and adult patients with facial acne vulgaris. Presented at: 2019 American Academy of Dermatology Annual Meeting; March 2, 2019; Washington, DC.
- Noreen N, Muhammad F, Akhtar B, et al. Is cannabidiol a promising substance for new drug development? a review of its potential therapeutic applications. Crit Rev Eukaryot Gene Expr. 2018;28:73-86.
- White CM. A review of human studies assessing cannabidiol’s (CBD) therapeutic actions and potential [published online February 7, 2019]. J Clin Pharmacol. 2019;59:923-934.
- Bonn-Miller MO, ElSohly MA, Loflin MJE, et al. Cannabis and cannabinoid drug development: evaluating botanical versus single molecule approaches. Int Rev Psychiatry. 2018;30:277-284.
- Bíró T, Tóth BI, Haskó G, et al. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30:411-420.
- Oláh A, Tóth BI, Borbíró I, et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J Clin Invest. 2014;124:3713-3724.
- Wilkinson JD, Williamson EM. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J Dermatol Sci. 2007;45:87-92.
- Appendino G, Gibbons S, Giana A, et al. Antibacterial cannabinoids from Cannabis sativa: a structure-activity study. J Nat Prod. 2008;71:1427-1430.
- Spleman L, Sinclair R, Freeman M, et al. The safety of topical cannabidiol (CBD) for the treatment of acne. J Invest Dermatol. 2018;138:S180.
Inflammation is a backdrop to the commonly cited elements of the pathophysiology of acne: Propionibacterium acnes proliferation, increased sebum production with an increase in circulating androgens, and faulty keratinization.1,2 In fact, research shows that the initiating lesion of acne vulgaris—the microcomedone—is, in essence, an inflammatory lesion.3 This realization has clearly influenced the approach to acne treatment but has not yielded a bevy of new treatments.
A better understanding of acne pathophysiology and the role of inflammation has, however, yielded a better understanding of how existing therapies treat the disease and have led to more comprehensive treatment strategies that are multitargeted. Nonetheless, topical and oral antibiotics remain mainstays of acne therapy, along with topical retinoids and benzoyl peroxide. Current guidelines of care for acne emphasize strategies that reduce dependence on antibiotics and minimize the risk for resistance.4 The therapeutic landscape might at last be shifting, with new chemical entities for acne and several novel formulations in development.
Sarecycline: A Novel Tetracycline
Tetracycline antibiotics have been used to manage acne since the 1950s, but their method of action in the disease has not been fully elucidated.5 In addition to antibiotic effects, tetracyclines have been shown to confer anti-inflammatory properties and other biologic effects.6,7
First-generation tetracycline is broad spectrum. As such, it is associated with increased potential for antibiotic resistance and greater impact on gastrointestinal health. The novel compound sarecycline is a tetracycline with a narrower spectrum of activity compared to other tetracyclines and with reduced activity against enteric gram-negative bacteria8 (Figure 1). Sarecycline recently was approved by the US Food and Drug Administration (FDA) in a once-daily oral formulation for the treatment of inflammatory lesions of nonnodular moderate to severe acne vulgaris in patients 9 years and older. Sarecycline is dosed at 1.5 mg/kg daily. The FDA approval marks the first new antibiotic approved for acne in 4 decades.

In 2 phase 3 clinical trials, sarecycline demonstrated efficacy in reducing both inflammatory and noninflammatory lesions.9 At week 12, investigator global assessment (IGA) success (≥2 point reduction in IGA and score 0 [clear] or 1 [almost clear]) rates were 21.9% and 22.6% for active treatment (n=483 and n=519), respectively, in the 2 trials compared to 10.5% and 15.3% (n=485 and n=515), respectively, for controls. Sarecycline demonstrated rapid anti-inflammatory effect. Onset of action against inflammatory lesions was notable by week 3. At week 12, inflammatory lesions were reduced in the active treatment arms by 51.8% and 49.9%, respectively, compared to 35.1% and 35.4%, respectively, for controls.9
The most common reported treatment-emergent adverse events (TEAEs) were nausea, nasopharyngitis, headache, and vomiting.9 Vestibular (dizziness, tinnitus, vertigo) and phototoxic (sunburn, photosensitivity) TEAEs both occurred in 1% or fewer of sarecycline patients. Gastrointestinal TEAE rates for sarecycline were low.9
Sarecycline also was assessed in the 2 trials for efficacy in the treatment of back and chest acne; in the active treatment group, IGA success was achieved by 29.6% and 36.6%, respectively, compared to 19.6% and 21.6%, respectively, of controls.9
Tazarotene Foam in Focus
Topical tazarotene is commercially available in cream, gel, and foam formulations. Tazarotene foam 0.1% was FDA approved in 2012 for the treatment of acne vulgaris in patients 12 years and older. However, the product was recently relaunched to the market and therefore warrants discussion.
Similar to other retinoids, topical tazarotene has been associated with the potential for application-site irritation. This aqueous foam formulation of tazarotene was designed for ease of application and to attempt to impart moisturizing effects to offset potential irritation. It contains noncomedogenic light mineral oil, which is an emollient. The foam spreads easily, including on hair-bearing skin, with demonstrated penetration of the active drug into the epidermis and dermis. Nonetheless, compared to the gel formulation of tazarotene, the foam formulation was associated with reduced systemic exposure.10
The tazarotene foam formulation does not contain alcohol, fragrance, propylene glycol, or parabens. Clinical trial participants, blinded to whether they were on active treatment or vehicle foam, consistently rated the foam formulation favorably for ease of application and spreadability, lack of stickiness or residue, and moisturizing effect. The foam vehicle is suggested to increase compliance and satisfaction in some patients.11The efficacy and tolerability of tazarotene foam 0.1% was investigated in 2 randomized, double-blind, vehicle-controlled, parallel-group studies in the United States and Canada.12 The studies involved participants aged 2 to 45 years who were randomized to receive treatment with either tazarotene foam 0.1% or vehicle foam once daily for 12 weeks (N=1486). Lesion counts, investigator static global assessment, and subject global assessment were evaluated at baseline and at weeks 2, 4, 8, and 12. At week 12, mean reduction from baseline in noninflammatory lesions was 55.9% for active treatment, mean reduction in inflammatory lesions was 56.1%, and mean total lesion reduction was 56% compared to mean reductions of 37.7%, 45.3%, and 40.8%, respectively, for vehicle. In all, 28.2% of participants achieved treatment success with active treatment compared to 14.7% of controls. There was a greater proportion of active-treatment participants with investigator static global assessment scores of 0 or 1 compared to vehicle. The only adverse events reported by more than 5% of participants in the active-treatment groups in both studies were application-site skin irritation and dryness.12
Topical Minocycline
Systemic minocycline is the most commonly prescribed oral antibiotic for acne management.13 Despite its widespread use, it is not without potential safety concerns. Minocycline is distinct among tetracyclines for posing a small risk for systemic lupus erythematosus and autoimmune TEAE.Gastrointestinal side effects and bluish discoloration also are reported.14 Topical application of minocycline for acne would optimize the therapeutic effect while reducing systemic effects. FMX101 4%, an investigational minocycline foam, is being studied for the treatment of moderate to severe acne.
In a pharmacokinetic study, minocycline exposure was 730- to 765-times lower with foam application vs oral minocycline.15 No evidence of minocycline accumulation was identified over the 21 days of application of minocycline foam 4%. Minocycline foam 4% appeared to be safe and well tolerated, without serious TEAEs, treatment-related TEAEs, or TEAEs that led to treatment discontinuation.15
In 2 identical phase 3 studies in which 961 participants were randomized (2:1) to once-daily minocycline foam 4% or foam vehicle for 12 weeks, participants in the active-treatment group demonstrated a significantly greater reduction in both inflammatory and noninflammatory lesions in both studies (both P<.05) and a greater rate of treatment success (≥2 point reduction in IGA and score of 0 [clear] or 1 [almost clear]) in 1 study. Treatment was generally safe and well tolerated, with skin-related adverse events reported in fewer than 1% of participants receiving active treatment.16
In an open-label safety extension study that enrolled 657 patients, treatment with FMX101 continued for as long as 40 weeks.17 In total, 291 participants completed 52 weeks of therapy. Rates and types of reported TEAEs in the open-label extension phase were similar to those seen in the phase 3 trials. Application-site TEAEs occurred in fewer than 2% of participants. Participants reported a high level of treatment satisfaction at week 52.17
In a more recent phase 3 study, 1507 participants were randomized (1:1) to once-daily minocycline foam 4% or foam vehicle for 12 weeks to further evaluate the efficacy and safety of FMX101 4% for moderate to severe acne vulgaris.18 The study met both primary end points: absolute change from baseline in the inflammatory lesion count (−16.93 vs −13.40; P<.0001) and the noninflammatory lesion count (−18.80 vs −15.89; P<.05), as well as percentage of participants with IGA treatment success at week 12 (30.80% vs 19.63%; P<.0001). The percentage reduction in the inflammatory lesion count was statistically significantly greater for minocycline foam 4% compared to vehicle as early as week 3 (P<.0001). The safety profile was found to be consistent with the 2 earlier phase 3 studies.18
Topical Minocycline in Rosacea
A similar foam formulation of minocycline (1.5% concentration) has shown benefit in 2 identical phase 3 studies.19 A total of 1522 participants were enrolled in 2 phase 3, randomized, multicenter, double-blind, vehicle-controlled, 2-arm studies in participants 18 years and older with moderate to severe papulopustular rosacea. Participants were randomized (2:1) to either minocycline foam 1.5% or vehicle once daily to the face for 12 weeks.19
Treatment was associated with a statistically significant reduction in counts of inflammatory lesions of rosacea (Study FX2016-11: −17.57 vs −15.65 [P=.003]; Study FX2016-12: −18.54 vs −14.88 [P<.0001]) and a significantly higher rate of IGA treatment success compared to vehicle (Study FX2016-11: 52.1% vs 43.0% [P=.027]; Study FX2016-12: 49.1% vs 39.0% [P=.008]), highlighting the anti-inflammatory action of the topically applied agent.19
The most common TEAE for both studies was upper respiratory tract infection; there were no serious TEAEs. Overall, 9 participants across both studies discontinued because of a TEAE (foam, 7 participants; vehicle, 2 participants).19
Clascoterone: First-in-Class Topical
Clascoterone cream 1% is a new chemical entity under investigation for the treatment of moderate to severe acne in patients 9 years and older. Clascoterone targets androgen receptors in the skin to block the effects of circulating endogenous androgens; chemically, it shares a 4-ring backbone identical to dihydrotestosterone and spironolactone (Figure 2). Clascoterone competes with dihydrotestosterone for binding to the androgen receptor to limit or block transcription of androgen-responsive genes and modify specific gene expression.20

Androgens are known to promote both sebum production and inflammatory responses within the follicle, contributing to the cycle of acne.21 Antiandrogen therapy would, therefore, inhibit excess sebum production and directly reduce the presence of certain inflammatory mediators in skin. This effect is expected to lead to reduced follicular plugging and a reduction in growth of P acnes and its inflammatory by-products.
Direct and indirect hormonal modulation have been successfully employed to manage acne in women; however, such therapies have not been considered first-line interventions for the disease.22 Although systemic antiandrogens and hormonal modulation are effective for certain women with acne, there may be concerns about systemic exposure23; no hormone-modulating agent has been adopted for use in men with acne.
As an androgen inhibitor, clascoterone is thought to displace androgen hormones from androgen receptors located at the sebaceous gland and hair follicle, thus inhibiting the cycle of physiologic events that leads to acne formation. Clascoterone is applied topically and acts locally on androgen receptors in the skin, with no systemic exposure seen. In phase 2 trials, clascoterone was found to be safe and effective with no systemic exposure and was suggested to have better tolerability than topical tretinoin.
Preliminary individual study analysis of data from 2 phase 3 trials showed that topical clascoterone met its primary end points, achieving statistically significantly greater rates of IGA treatment success (≥2 point reduction in IGA and score of 0 [clear] or 1 [almost clear]) at week 12 (P<.0001).24 Rates of treatment success for actively treated participants were 16.1% and 18.7%, respectively, compared to 7% and 4.7%, respectively, for vehicle. The study population included both males and nonpregnant females 9 years and older who had a baseline IGA score of 3 (moderate) or 4 (severe). At baseline, participants had a mix of inflammatory lesions (≥30, to a maximum of 75) and noninflammatory lesions (≥30, to a maximum of 100).24
Intention-to-treat analysis at week 12 showed a mean total lesion reduction from baseline for active treatment of 37.1% and 37.7%, respectively, compared to 28.5% and 22.2%, respectively, for controls.24 Mean reductions from baseline in noninflammatory lesions for active treatment were 30.7% and 29.3%, respectively, compared to 21.9% and 15.8%, respectively, for controls. Mean reductions from baseline in inflammatory lesions for active treatment were 44.8% and 47%, respectively, compared to 36.6% and 29.8%, respectively, for controls. Similarly low rates of TEAEs were reported in active and placebo groups in both studies. No TEAE suggested systemic antiandrogen exposure.24
Advancements in Cannabinoids
Advancements in pharmaceutical development of cannabinoid compounds have largely coincided with the controversial national movement to legalize medical marijuana and decriminalize recreational marijuana use. Despite the temporal connection, the 2 topics are entirely distinct. Importantly, pharmaceutical development is largely focused on the effects of cannabidiol (CBD), which is 1 of approximately 113 cannabinoids identified from Cannabis sativa. Cannabidiol is not tetrahydrocannabinol, or THC, the compound responsible for marijuana’s psychoactive effects and addictive properties; CBD does not have any psychoactive effects and is not addictive (Figure 3).25

A CBD oral solution agent recently gained FDA approval for seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients 2 years and older; it is estimated that more than 180 trials of CBD are ongoing in the United States for various indications.26 A notable question in the development of CBD-based therapies is: What is the role of natural plant-derived CBD compared to a pure synthetic form of CBD? The latter is akin to a pharmaceutical process in which a single molecule is developed as the active drug.27 Although the potency and composition of plant-derived CBD can vary with crop conditions, plant strains, and the extraction process, a synthetic molecule would allow for consistency in safety, potency, and pharmacokinetic properties, as well as efficacy, as a consequence.26
There are intriguing data to suggest a potential use for topical CBD in the management of skin diseases, including acne vulgaris. Researchers have, for at least a decade, been investigating the role of the endocannabinoid system, which has physiologic regulatory functions in proliferation, differentiation, apoptosis and cytokine, mediator, and hormone production of various cell types in skin, hair follicles, and sebaceous glands.28 Cannabidiol has been shown to suppress proliferation of sebocytes through activation of transient receptor potential vanilloid 4 ion channels and to have anti-inflammatory effects on sebocytes.29 It has been shown to inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism30 and to possess potent antimicrobial activity against gram-positive bacteria such as P acnes.31
Given these effects on sebocytes, modulation of keratinocyte proliferation, and anti-inflammatory and antibacterial effects, CBD could prove beneficial in the management of acne vulgaris. A new synthetic CBD topical formulation, BTX 1503, is under investigation for the treatment of acne vulgaris.
Early clinical data confirm both the anti-inflammatory effects of topical BTX 1503 as well as its effects on noninflammatory lesions, with 4-week reductions in inflammatory lesion counts similar to what are reported in clinical trials for leading FDA-approved topical therapies in the same time frame.
The phase 1b trial was a 4-week, open-label study in participants with moderate to severe acne vulgaris.32 The primary end point was safety, as demonstrated by the incidence of TEAE, laboratory monitoring, and assessment of cutaneous tolerability. Exploratory end points included changes in inflammatory and noninflammatory lesion counts and IGA score. A total of 21 participants aged 18 to 65 years with moderate to severe acne vulgaris were enrolled. BTX 1503 was applied topically twice daily. At baseline, eligible participants had 20 to 50 inflammatory lesions and 20 to 100 noninflammatory acne lesions on the face, an IGA of 3 (moderate) or 4 (severe), and 3 or fewer nodular or cystic lesions (>5 mm in diameter). No serious or severe TEAEs were reported; no participants withdrew due to a TEAE. Slight erythema, slight scaling, slight dryness, and slight burning and stinging were reported; there were no reports of irritant or allergic contact dermatitis. Only 1 TEAE was thought to be possibly related to treatment: mild pain at the application site.32
In addition to presenting a potential new chemical entity for the topical treatment of acne, the novel topical vehicle formulation of BTX 1503 represents an innovative approach to drug delivery. The formulation utilizes proprietary technology to deliver high doses of drug into the skin without controversial penetration enhancers, preservatives, or other potential irritating additives. Instead, volatile excipients are used that evaporate upon application to the skin, leaving a so-called superconcentrated secondary formulation on the skin. The concentration gradient effect then drives the concentrated drug into skin. Although the formulation efficiently delivers active drug into the skin and its appendages, systemic exposure has been reported to be very low. A phase 2 randomized, double-blind, vehicle-controlled trial ongoing in the United States and Australia in 360 patients with moderate to severe acne vulgaris will provide key data to confirm the efficacy and safety of BTX 1503 (ClinicalTrials.gov Identifier NCT03573518).
Conclusion
Drug development continues to focus on the challenge of treating acne effectively and safely. Vehicle innovations are optimizing existing active drugs and creating opportunities to deliver new compounds to the skin. The approval of sarecycline as the first new chemical entity approved for acne in several years may be followed in coming years by other new actives, including clascoterone and CBD.
Inflammation is a backdrop to the commonly cited elements of the pathophysiology of acne: Propionibacterium acnes proliferation, increased sebum production with an increase in circulating androgens, and faulty keratinization.1,2 In fact, research shows that the initiating lesion of acne vulgaris—the microcomedone—is, in essence, an inflammatory lesion.3 This realization has clearly influenced the approach to acne treatment but has not yielded a bevy of new treatments.
A better understanding of acne pathophysiology and the role of inflammation has, however, yielded a better understanding of how existing therapies treat the disease and have led to more comprehensive treatment strategies that are multitargeted. Nonetheless, topical and oral antibiotics remain mainstays of acne therapy, along with topical retinoids and benzoyl peroxide. Current guidelines of care for acne emphasize strategies that reduce dependence on antibiotics and minimize the risk for resistance.4 The therapeutic landscape might at last be shifting, with new chemical entities for acne and several novel formulations in development.
Sarecycline: A Novel Tetracycline
Tetracycline antibiotics have been used to manage acne since the 1950s, but their method of action in the disease has not been fully elucidated.5 In addition to antibiotic effects, tetracyclines have been shown to confer anti-inflammatory properties and other biologic effects.6,7
First-generation tetracycline is broad spectrum. As such, it is associated with increased potential for antibiotic resistance and greater impact on gastrointestinal health. The novel compound sarecycline is a tetracycline with a narrower spectrum of activity compared to other tetracyclines and with reduced activity against enteric gram-negative bacteria8 (Figure 1). Sarecycline recently was approved by the US Food and Drug Administration (FDA) in a once-daily oral formulation for the treatment of inflammatory lesions of nonnodular moderate to severe acne vulgaris in patients 9 years and older. Sarecycline is dosed at 1.5 mg/kg daily. The FDA approval marks the first new antibiotic approved for acne in 4 decades.

In 2 phase 3 clinical trials, sarecycline demonstrated efficacy in reducing both inflammatory and noninflammatory lesions.9 At week 12, investigator global assessment (IGA) success (≥2 point reduction in IGA and score 0 [clear] or 1 [almost clear]) rates were 21.9% and 22.6% for active treatment (n=483 and n=519), respectively, in the 2 trials compared to 10.5% and 15.3% (n=485 and n=515), respectively, for controls. Sarecycline demonstrated rapid anti-inflammatory effect. Onset of action against inflammatory lesions was notable by week 3. At week 12, inflammatory lesions were reduced in the active treatment arms by 51.8% and 49.9%, respectively, compared to 35.1% and 35.4%, respectively, for controls.9
The most common reported treatment-emergent adverse events (TEAEs) were nausea, nasopharyngitis, headache, and vomiting.9 Vestibular (dizziness, tinnitus, vertigo) and phototoxic (sunburn, photosensitivity) TEAEs both occurred in 1% or fewer of sarecycline patients. Gastrointestinal TEAE rates for sarecycline were low.9
Sarecycline also was assessed in the 2 trials for efficacy in the treatment of back and chest acne; in the active treatment group, IGA success was achieved by 29.6% and 36.6%, respectively, compared to 19.6% and 21.6%, respectively, of controls.9
Tazarotene Foam in Focus
Topical tazarotene is commercially available in cream, gel, and foam formulations. Tazarotene foam 0.1% was FDA approved in 2012 for the treatment of acne vulgaris in patients 12 years and older. However, the product was recently relaunched to the market and therefore warrants discussion.
Similar to other retinoids, topical tazarotene has been associated with the potential for application-site irritation. This aqueous foam formulation of tazarotene was designed for ease of application and to attempt to impart moisturizing effects to offset potential irritation. It contains noncomedogenic light mineral oil, which is an emollient. The foam spreads easily, including on hair-bearing skin, with demonstrated penetration of the active drug into the epidermis and dermis. Nonetheless, compared to the gel formulation of tazarotene, the foam formulation was associated with reduced systemic exposure.10
The tazarotene foam formulation does not contain alcohol, fragrance, propylene glycol, or parabens. Clinical trial participants, blinded to whether they were on active treatment or vehicle foam, consistently rated the foam formulation favorably for ease of application and spreadability, lack of stickiness or residue, and moisturizing effect. The foam vehicle is suggested to increase compliance and satisfaction in some patients.11The efficacy and tolerability of tazarotene foam 0.1% was investigated in 2 randomized, double-blind, vehicle-controlled, parallel-group studies in the United States and Canada.12 The studies involved participants aged 2 to 45 years who were randomized to receive treatment with either tazarotene foam 0.1% or vehicle foam once daily for 12 weeks (N=1486). Lesion counts, investigator static global assessment, and subject global assessment were evaluated at baseline and at weeks 2, 4, 8, and 12. At week 12, mean reduction from baseline in noninflammatory lesions was 55.9% for active treatment, mean reduction in inflammatory lesions was 56.1%, and mean total lesion reduction was 56% compared to mean reductions of 37.7%, 45.3%, and 40.8%, respectively, for vehicle. In all, 28.2% of participants achieved treatment success with active treatment compared to 14.7% of controls. There was a greater proportion of active-treatment participants with investigator static global assessment scores of 0 or 1 compared to vehicle. The only adverse events reported by more than 5% of participants in the active-treatment groups in both studies were application-site skin irritation and dryness.12
Topical Minocycline
Systemic minocycline is the most commonly prescribed oral antibiotic for acne management.13 Despite its widespread use, it is not without potential safety concerns. Minocycline is distinct among tetracyclines for posing a small risk for systemic lupus erythematosus and autoimmune TEAE.Gastrointestinal side effects and bluish discoloration also are reported.14 Topical application of minocycline for acne would optimize the therapeutic effect while reducing systemic effects. FMX101 4%, an investigational minocycline foam, is being studied for the treatment of moderate to severe acne.
In a pharmacokinetic study, minocycline exposure was 730- to 765-times lower with foam application vs oral minocycline.15 No evidence of minocycline accumulation was identified over the 21 days of application of minocycline foam 4%. Minocycline foam 4% appeared to be safe and well tolerated, without serious TEAEs, treatment-related TEAEs, or TEAEs that led to treatment discontinuation.15
In 2 identical phase 3 studies in which 961 participants were randomized (2:1) to once-daily minocycline foam 4% or foam vehicle for 12 weeks, participants in the active-treatment group demonstrated a significantly greater reduction in both inflammatory and noninflammatory lesions in both studies (both P<.05) and a greater rate of treatment success (≥2 point reduction in IGA and score of 0 [clear] or 1 [almost clear]) in 1 study. Treatment was generally safe and well tolerated, with skin-related adverse events reported in fewer than 1% of participants receiving active treatment.16
In an open-label safety extension study that enrolled 657 patients, treatment with FMX101 continued for as long as 40 weeks.17 In total, 291 participants completed 52 weeks of therapy. Rates and types of reported TEAEs in the open-label extension phase were similar to those seen in the phase 3 trials. Application-site TEAEs occurred in fewer than 2% of participants. Participants reported a high level of treatment satisfaction at week 52.17
In a more recent phase 3 study, 1507 participants were randomized (1:1) to once-daily minocycline foam 4% or foam vehicle for 12 weeks to further evaluate the efficacy and safety of FMX101 4% for moderate to severe acne vulgaris.18 The study met both primary end points: absolute change from baseline in the inflammatory lesion count (−16.93 vs −13.40; P<.0001) and the noninflammatory lesion count (−18.80 vs −15.89; P<.05), as well as percentage of participants with IGA treatment success at week 12 (30.80% vs 19.63%; P<.0001). The percentage reduction in the inflammatory lesion count was statistically significantly greater for minocycline foam 4% compared to vehicle as early as week 3 (P<.0001). The safety profile was found to be consistent with the 2 earlier phase 3 studies.18
Topical Minocycline in Rosacea
A similar foam formulation of minocycline (1.5% concentration) has shown benefit in 2 identical phase 3 studies.19 A total of 1522 participants were enrolled in 2 phase 3, randomized, multicenter, double-blind, vehicle-controlled, 2-arm studies in participants 18 years and older with moderate to severe papulopustular rosacea. Participants were randomized (2:1) to either minocycline foam 1.5% or vehicle once daily to the face for 12 weeks.19
Treatment was associated with a statistically significant reduction in counts of inflammatory lesions of rosacea (Study FX2016-11: −17.57 vs −15.65 [P=.003]; Study FX2016-12: −18.54 vs −14.88 [P<.0001]) and a significantly higher rate of IGA treatment success compared to vehicle (Study FX2016-11: 52.1% vs 43.0% [P=.027]; Study FX2016-12: 49.1% vs 39.0% [P=.008]), highlighting the anti-inflammatory action of the topically applied agent.19
The most common TEAE for both studies was upper respiratory tract infection; there were no serious TEAEs. Overall, 9 participants across both studies discontinued because of a TEAE (foam, 7 participants; vehicle, 2 participants).19
Clascoterone: First-in-Class Topical
Clascoterone cream 1% is a new chemical entity under investigation for the treatment of moderate to severe acne in patients 9 years and older. Clascoterone targets androgen receptors in the skin to block the effects of circulating endogenous androgens; chemically, it shares a 4-ring backbone identical to dihydrotestosterone and spironolactone (Figure 2). Clascoterone competes with dihydrotestosterone for binding to the androgen receptor to limit or block transcription of androgen-responsive genes and modify specific gene expression.20

Androgens are known to promote both sebum production and inflammatory responses within the follicle, contributing to the cycle of acne.21 Antiandrogen therapy would, therefore, inhibit excess sebum production and directly reduce the presence of certain inflammatory mediators in skin. This effect is expected to lead to reduced follicular plugging and a reduction in growth of P acnes and its inflammatory by-products.
Direct and indirect hormonal modulation have been successfully employed to manage acne in women; however, such therapies have not been considered first-line interventions for the disease.22 Although systemic antiandrogens and hormonal modulation are effective for certain women with acne, there may be concerns about systemic exposure23; no hormone-modulating agent has been adopted for use in men with acne.
As an androgen inhibitor, clascoterone is thought to displace androgen hormones from androgen receptors located at the sebaceous gland and hair follicle, thus inhibiting the cycle of physiologic events that leads to acne formation. Clascoterone is applied topically and acts locally on androgen receptors in the skin, with no systemic exposure seen. In phase 2 trials, clascoterone was found to be safe and effective with no systemic exposure and was suggested to have better tolerability than topical tretinoin.
Preliminary individual study analysis of data from 2 phase 3 trials showed that topical clascoterone met its primary end points, achieving statistically significantly greater rates of IGA treatment success (≥2 point reduction in IGA and score of 0 [clear] or 1 [almost clear]) at week 12 (P<.0001).24 Rates of treatment success for actively treated participants were 16.1% and 18.7%, respectively, compared to 7% and 4.7%, respectively, for vehicle. The study population included both males and nonpregnant females 9 years and older who had a baseline IGA score of 3 (moderate) or 4 (severe). At baseline, participants had a mix of inflammatory lesions (≥30, to a maximum of 75) and noninflammatory lesions (≥30, to a maximum of 100).24
Intention-to-treat analysis at week 12 showed a mean total lesion reduction from baseline for active treatment of 37.1% and 37.7%, respectively, compared to 28.5% and 22.2%, respectively, for controls.24 Mean reductions from baseline in noninflammatory lesions for active treatment were 30.7% and 29.3%, respectively, compared to 21.9% and 15.8%, respectively, for controls. Mean reductions from baseline in inflammatory lesions for active treatment were 44.8% and 47%, respectively, compared to 36.6% and 29.8%, respectively, for controls. Similarly low rates of TEAEs were reported in active and placebo groups in both studies. No TEAE suggested systemic antiandrogen exposure.24
Advancements in Cannabinoids
Advancements in pharmaceutical development of cannabinoid compounds have largely coincided with the controversial national movement to legalize medical marijuana and decriminalize recreational marijuana use. Despite the temporal connection, the 2 topics are entirely distinct. Importantly, pharmaceutical development is largely focused on the effects of cannabidiol (CBD), which is 1 of approximately 113 cannabinoids identified from Cannabis sativa. Cannabidiol is not tetrahydrocannabinol, or THC, the compound responsible for marijuana’s psychoactive effects and addictive properties; CBD does not have any psychoactive effects and is not addictive (Figure 3).25

A CBD oral solution agent recently gained FDA approval for seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients 2 years and older; it is estimated that more than 180 trials of CBD are ongoing in the United States for various indications.26 A notable question in the development of CBD-based therapies is: What is the role of natural plant-derived CBD compared to a pure synthetic form of CBD? The latter is akin to a pharmaceutical process in which a single molecule is developed as the active drug.27 Although the potency and composition of plant-derived CBD can vary with crop conditions, plant strains, and the extraction process, a synthetic molecule would allow for consistency in safety, potency, and pharmacokinetic properties, as well as efficacy, as a consequence.26
There are intriguing data to suggest a potential use for topical CBD in the management of skin diseases, including acne vulgaris. Researchers have, for at least a decade, been investigating the role of the endocannabinoid system, which has physiologic regulatory functions in proliferation, differentiation, apoptosis and cytokine, mediator, and hormone production of various cell types in skin, hair follicles, and sebaceous glands.28 Cannabidiol has been shown to suppress proliferation of sebocytes through activation of transient receptor potential vanilloid 4 ion channels and to have anti-inflammatory effects on sebocytes.29 It has been shown to inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism30 and to possess potent antimicrobial activity against gram-positive bacteria such as P acnes.31
Given these effects on sebocytes, modulation of keratinocyte proliferation, and anti-inflammatory and antibacterial effects, CBD could prove beneficial in the management of acne vulgaris. A new synthetic CBD topical formulation, BTX 1503, is under investigation for the treatment of acne vulgaris.
Early clinical data confirm both the anti-inflammatory effects of topical BTX 1503 as well as its effects on noninflammatory lesions, with 4-week reductions in inflammatory lesion counts similar to what are reported in clinical trials for leading FDA-approved topical therapies in the same time frame.
The phase 1b trial was a 4-week, open-label study in participants with moderate to severe acne vulgaris.32 The primary end point was safety, as demonstrated by the incidence of TEAE, laboratory monitoring, and assessment of cutaneous tolerability. Exploratory end points included changes in inflammatory and noninflammatory lesion counts and IGA score. A total of 21 participants aged 18 to 65 years with moderate to severe acne vulgaris were enrolled. BTX 1503 was applied topically twice daily. At baseline, eligible participants had 20 to 50 inflammatory lesions and 20 to 100 noninflammatory acne lesions on the face, an IGA of 3 (moderate) or 4 (severe), and 3 or fewer nodular or cystic lesions (>5 mm in diameter). No serious or severe TEAEs were reported; no participants withdrew due to a TEAE. Slight erythema, slight scaling, slight dryness, and slight burning and stinging were reported; there were no reports of irritant or allergic contact dermatitis. Only 1 TEAE was thought to be possibly related to treatment: mild pain at the application site.32
In addition to presenting a potential new chemical entity for the topical treatment of acne, the novel topical vehicle formulation of BTX 1503 represents an innovative approach to drug delivery. The formulation utilizes proprietary technology to deliver high doses of drug into the skin without controversial penetration enhancers, preservatives, or other potential irritating additives. Instead, volatile excipients are used that evaporate upon application to the skin, leaving a so-called superconcentrated secondary formulation on the skin. The concentration gradient effect then drives the concentrated drug into skin. Although the formulation efficiently delivers active drug into the skin and its appendages, systemic exposure has been reported to be very low. A phase 2 randomized, double-blind, vehicle-controlled trial ongoing in the United States and Australia in 360 patients with moderate to severe acne vulgaris will provide key data to confirm the efficacy and safety of BTX 1503 (ClinicalTrials.gov Identifier NCT03573518).
Conclusion
Drug development continues to focus on the challenge of treating acne effectively and safely. Vehicle innovations are optimizing existing active drugs and creating opportunities to deliver new compounds to the skin. The approval of sarecycline as the first new chemical entity approved for acne in several years may be followed in coming years by other new actives, including clascoterone and CBD.
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Burkhart CN, Gottwald L. Assessment of etiologic agents in acne pathogenesis. Skinmed. 2003;2:222-228.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169:337-352.
- Griffin MO, Ceballos G, Villarreal FJ. Tetracycline compounds with non-antimicrobial organ protective properties: possible mechanisms of action. Pharmacol Res. 2011;63:102-107.
- Weinberg JM. The anti-inflammatory effects of tetracyclines. Cutis. 2005;75(4 suppl):6-11.
- Leyden JJ, Sniukiene V, Berk DR, et al. Efficacy and safety of sarecycline, a novel, once-daily, narrow spectrum antibiotic for the treatment of moderate to severe facial acne vulgaris: results of a phase 2, dose-ranging study. J Drugs Dermatol. 2018;17:333-338.
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Jarratt M, Werner CP, Alió Saenz AB. Tazarotene foam versus tazarotene gel: a randomized relative bioavailability study in acne vulgaris. Clin Drug Investig. 2013;33:283-289.
- Smith JA, Narahari S, Hill D, et al. Tazarotene foam, 0.1%, for the treatment of acne. Expert Opin Drug Saf. 2016;15:99-103.
- Feldman SR, Werner CP, Alió Saenz AB. The efficacy and tolerability of tazarotene foam, 0.1%, in the treatment of acne vulgaris in 2 multicenter, randomized, vehicle-controlled, double-blind studies. J Drugs Dermatol. 2013;12:438-446.
- Lee YH, Liu G, Thiboutot DM, et al. A retrospective analysis of the duration of oral antibiotic therapy for the treatment of acne among adolescents: investigating practice gaps and potential cost-savings. J Am Acad Dermatol. 2014;71:70-76.
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012(8):CD002086.
- Jones TM, Ellman H, deVries T. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16:1022-1028.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177.17.
- Gold LS, Dhawan S, Weiss J, et al. FMX101 4% minocycline foam for the treatment of acne vulgaris: safety and patient satisfaction from the open-label extension of 2 phase 3 studies. Poster presented at: 2018 Winter Clinical Dermatology Conference; January 12-17, 2018; Maui, HI.
- Raoof J, Hooper D, Moore A, et al. FMX101 4% topical minocycline foam for the treatment of moderate to severe acne vulgaris: efficacy and safety from a phase 3 randomized, double-blind, vehicle-controlled study. Poster presented at: 2018 Fall Clinical Dermatology Conference; October 18-21, 2018; Las Vegas, NV.
- Gold LS, Del Rosso JQ, Bhatia ND, et al. Efficacy and safety of FMX103 (1.5% minocycline foam) in the treatment of moderate-to-severe papulopustular rosacea: results from two phase 3 randomized, multicenter, double-blind, vehicle-controlled studies. Poster presented at: 2019 Winter Clinical Dermatology Conference; January 18-23; 2019; Koloa, HI.
- Data on file. CB-03-01 2017. Milan, Italy: Cassiopea SpA; 2017.
- Ju Q, Tao T, Hu T, et al. Sex hormones and acne. Clin Dermatol. 2017;35:130-137.
- Park JH, Bienenfeld A, Orlow SJ, et al. The use of hormonal antiandrogen therapy in female patients with acne: a 10-year retrospective study. Am J Clin Dermatol. 2018;19:449-455.
- Barros B, Thiboutot D. Hormonal therapies for acne. Clin Dermatol. 2017;35:168-172.
- Hebert A. Clascoterone topical cream, 1%: a novel, topical, local, selective androgen receptor antagonist: results from two phase 3 studies treating children and adult patients with facial acne vulgaris. Presented at: 2019 American Academy of Dermatology Annual Meeting; March 2, 2019; Washington, DC.
- Noreen N, Muhammad F, Akhtar B, et al. Is cannabidiol a promising substance for new drug development? a review of its potential therapeutic applications. Crit Rev Eukaryot Gene Expr. 2018;28:73-86.
- White CM. A review of human studies assessing cannabidiol’s (CBD) therapeutic actions and potential [published online February 7, 2019]. J Clin Pharmacol. 2019;59:923-934.
- Bonn-Miller MO, ElSohly MA, Loflin MJE, et al. Cannabis and cannabinoid drug development: evaluating botanical versus single molecule approaches. Int Rev Psychiatry. 2018;30:277-284.
- Bíró T, Tóth BI, Haskó G, et al. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30:411-420.
- Oláh A, Tóth BI, Borbíró I, et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J Clin Invest. 2014;124:3713-3724.
- Wilkinson JD, Williamson EM. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J Dermatol Sci. 2007;45:87-92.
- Appendino G, Gibbons S, Giana A, et al. Antibacterial cannabinoids from Cannabis sativa: a structure-activity study. J Nat Prod. 2008;71:1427-1430.
- Spleman L, Sinclair R, Freeman M, et al. The safety of topical cannabidiol (CBD) for the treatment of acne. J Invest Dermatol. 2018;138:S180.
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Burkhart CN, Gottwald L. Assessment of etiologic agents in acne pathogenesis. Skinmed. 2003;2:222-228.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169:337-352.
- Griffin MO, Ceballos G, Villarreal FJ. Tetracycline compounds with non-antimicrobial organ protective properties: possible mechanisms of action. Pharmacol Res. 2011;63:102-107.
- Weinberg JM. The anti-inflammatory effects of tetracyclines. Cutis. 2005;75(4 suppl):6-11.
- Leyden JJ, Sniukiene V, Berk DR, et al. Efficacy and safety of sarecycline, a novel, once-daily, narrow spectrum antibiotic for the treatment of moderate to severe facial acne vulgaris: results of a phase 2, dose-ranging study. J Drugs Dermatol. 2018;17:333-338.
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Jarratt M, Werner CP, Alió Saenz AB. Tazarotene foam versus tazarotene gel: a randomized relative bioavailability study in acne vulgaris. Clin Drug Investig. 2013;33:283-289.
- Smith JA, Narahari S, Hill D, et al. Tazarotene foam, 0.1%, for the treatment of acne. Expert Opin Drug Saf. 2016;15:99-103.
- Feldman SR, Werner CP, Alió Saenz AB. The efficacy and tolerability of tazarotene foam, 0.1%, in the treatment of acne vulgaris in 2 multicenter, randomized, vehicle-controlled, double-blind studies. J Drugs Dermatol. 2013;12:438-446.
- Lee YH, Liu G, Thiboutot DM, et al. A retrospective analysis of the duration of oral antibiotic therapy for the treatment of acne among adolescents: investigating practice gaps and potential cost-savings. J Am Acad Dermatol. 2014;71:70-76.
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012(8):CD002086.
- Jones TM, Ellman H, deVries T. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16:1022-1028.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177.17.
- Gold LS, Dhawan S, Weiss J, et al. FMX101 4% minocycline foam for the treatment of acne vulgaris: safety and patient satisfaction from the open-label extension of 2 phase 3 studies. Poster presented at: 2018 Winter Clinical Dermatology Conference; January 12-17, 2018; Maui, HI.
- Raoof J, Hooper D, Moore A, et al. FMX101 4% topical minocycline foam for the treatment of moderate to severe acne vulgaris: efficacy and safety from a phase 3 randomized, double-blind, vehicle-controlled study. Poster presented at: 2018 Fall Clinical Dermatology Conference; October 18-21, 2018; Las Vegas, NV.
- Gold LS, Del Rosso JQ, Bhatia ND, et al. Efficacy and safety of FMX103 (1.5% minocycline foam) in the treatment of moderate-to-severe papulopustular rosacea: results from two phase 3 randomized, multicenter, double-blind, vehicle-controlled studies. Poster presented at: 2019 Winter Clinical Dermatology Conference; January 18-23; 2019; Koloa, HI.
- Data on file. CB-03-01 2017. Milan, Italy: Cassiopea SpA; 2017.
- Ju Q, Tao T, Hu T, et al. Sex hormones and acne. Clin Dermatol. 2017;35:130-137.
- Park JH, Bienenfeld A, Orlow SJ, et al. The use of hormonal antiandrogen therapy in female patients with acne: a 10-year retrospective study. Am J Clin Dermatol. 2018;19:449-455.
- Barros B, Thiboutot D. Hormonal therapies for acne. Clin Dermatol. 2017;35:168-172.
- Hebert A. Clascoterone topical cream, 1%: a novel, topical, local, selective androgen receptor antagonist: results from two phase 3 studies treating children and adult patients with facial acne vulgaris. Presented at: 2019 American Academy of Dermatology Annual Meeting; March 2, 2019; Washington, DC.
- Noreen N, Muhammad F, Akhtar B, et al. Is cannabidiol a promising substance for new drug development? a review of its potential therapeutic applications. Crit Rev Eukaryot Gene Expr. 2018;28:73-86.
- White CM. A review of human studies assessing cannabidiol’s (CBD) therapeutic actions and potential [published online February 7, 2019]. J Clin Pharmacol. 2019;59:923-934.
- Bonn-Miller MO, ElSohly MA, Loflin MJE, et al. Cannabis and cannabinoid drug development: evaluating botanical versus single molecule approaches. Int Rev Psychiatry. 2018;30:277-284.
- Bíró T, Tóth BI, Haskó G, et al. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30:411-420.
- Oláh A, Tóth BI, Borbíró I, et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J Clin Invest. 2014;124:3713-3724.
- Wilkinson JD, Williamson EM. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J Dermatol Sci. 2007;45:87-92.
- Appendino G, Gibbons S, Giana A, et al. Antibacterial cannabinoids from Cannabis sativa: a structure-activity study. J Nat Prod. 2008;71:1427-1430.
- Spleman L, Sinclair R, Freeman M, et al. The safety of topical cannabidiol (CBD) for the treatment of acne. J Invest Dermatol. 2018;138:S180.
Practice Points
- Sarecycline is the first new antibiotic approved for acne in several years.
- Tazarotene foam 0.1% was relaunched to the market. The foam formulation attempts to impart moisturizing effects to offset potential irritation.
- Topical minocycline for acne optimizes the therapeutic effects while reducing systemic effects.
- Clascoterone and cannabidiol currently are under investigation for acne treatment.
The Affordable Care Act, closing in on a decade
The Affordable Care Act (ACA) was enacted on March 23, 2010. Controversies, complaints, and detractors have and continue to abound. But the ACA’s landmark women’s health gains are unmistakable. Contraceptive coverage, maternity coverage, Medicaid coverage of low-income women, coverage for individuals with preexisting conditions, and gender-neutral premiums are now a part of the fabric of our society. For most.
Many physicians and patients—many lawmakers, too—do not remember the serious problems people had with their insurance companies before the ACA. Maternity coverage was usually a free-standing rider to an insurance policy, making it very expensive. Insurance plans did not have to, and often did not, cover contraceptives, and none did without copays or deductibles. Women were routinely denied coverage if they had ever had a cesarean delivery, had once been the victim of domestic violence, or had any one of many common conditions, like diabetes. The many exclusionary conditions are so common, in fact, that one study estimated that around 52 million adults in the United States (27% of those younger than age 65 years) have preexisting conditions that would potentially make them uninsurable without the ACA’s protections.1
Before the ACA, it also was common for women with insurance policies to find their coverage rescinded, often with no explanation, even though they paid their premiums every month. And women with serious medical conditions often saw their coverage ended midway through their course of treatment. That placed their ObGyns in a terrible situation, too.
The insurance industry as a whole was running rough-shod over its customers, and making a lot of money by creatively and routinely denying coverage and payment for care. People were often insured, but not covered. The ACA halted many of these practices, and required insurers to meet high medical loss ratios, guaranteeing that 80% of the premiums’ for individual and small market insurers (and 85% for large insurers) are returned to patients in care payments or even in checks. In fact, nearly $4 billion in premiums have been rebated to insured individuals over the last 7 years under the ACA.2
The commitment of the American College of Obstetricians and Gynecologists (ACOG) to women’s health and to our members’ ability to provide the best care has centered on preserving the critical gains of the ACA for women, improving them when we can, and making sure politicians don’t turn back the clock on women’s health. We have been busy.
In this article, we will look at what has happened to these landmark gains and promises of improved women’s health, specifically preexisting condition protections and contraceptive coverage, under a new Administration. What happens when good health care policy and political enmity collide?

Preexisting coverage protections
The 1996 Health Insurance Portability and Accountability Act (HIPAA) defines a preexisting condition exclusionas a “limitation or exclusion of benefits relating to a condition based on the fact that the condition was present before the date of enrollment for the coverage, whether or not any medical advice, diagnosis, care, or treatment was recommended or received before that date.” HIPPA prohibited employer-sponsored health plans from discriminating against individuals through denying them coverage or charging them more based on their or their family members’ health problems. The ACA expanded protections to prohibit the insurance practice of denying coverage altogether to an individual with a preexisting condition.3
Continue to: Under Congress...
Under Congress
Republicans held the majority in both chambers of the 115th Congress (2017–2018), and hoped to use their majority status to get an ACA repeal bill to the Republican President’s desk for speedy enactment. It was not easy, and they were not successful. Four major bills—the American Health Care Act, the Better Care Reconciliation Act, the Health Care Freedom Act, and the Graham-Cassidy Amendment—never made it over the finish line, with some not even making it to a vote. The Health Care Freedom Act was voted down in the Senate 51-49 when Senator John McCain came back from brain surgery to cast his famous thumbs-down vote.4 These bills all would have repealed or hobbled guaranteed issue, community rating, and essential health benefits of the ACA. Of all the legislative attempts to undermine the ACA, only the 2017 Tax Cuts and Jobs Act (TCJA) was signed into law, repealing the ACA individual mandate.
Handling by the courts
The TCJA gave ACA opponents their opening in court. Twenty Republican state attorneys general and governors brought suit in February 2018 (Texas v Azar), arguing that because the ACA relies on the mandate, and the mandate has been repealed, the rest of the ACA also should be struck down. A federal district judge agreed, on December 15, 2018, declaring the entire ACA unconstitutional.5
That decision has been limited in its practical effect so far, and maybe it was not altogether unexpected. What was unexpected was that the US Department of Justice (DOJ) refused to defend a federal law, in this case, the ACA. In June 2018, the DOJ declined to defend the individual mandate, as well as guaranteed issue, community rating, the ban on preexisting condition exclusions, and discrimination based on health status in the ACA. The DOJ at that time, however, did not agree with the plaintiffs that without the mandate the entire ACA should be struck down. It said, “There is no reason why the ACA’s particular expansion of Medicaid hinges on the individual mandate.” Later, after the December 15 ruling, the DOJ changed its position and agreed with the judge, in a two-sentence letter to the court, that the ACA should be stricken altogether—shortly after which 3 career DOJ attorneys resigned.6
A legal expert observed: “The DOJ’s decision not to defend the ACA breaks with the Department’s long-standing bipartisan commitment to defend federal laws if reasonable arguments can be made in their defense. Decisions not to defend federal law are exceedingly rare. It seems even rarer to change the government’s position mid-appeal in such a high-profile lawsuit that risks disrupting the entire health care system and health insurance coverage for millions of Americans.”7
Regulatory tactics
What a policy maker cannot do by law, he or she can try to accomplish by regulation. The Administration is using 3 regulatory routes to undercut the ACA preexisting coverage protections and market stability.
Route 1: Short-Term Limited Duration (STLD) plans. These plans were created in the ACA to provide bridge coverage for up to 3 months for individuals in between health insurance plans. These plans do not have to comply with ACA patient protections, can deny coverage for preexisting conditions, and do not cover maternity care. In 2018, the Administration moved to allow these plans to be marketed broadly and renewed for up to 3 years. Because these plans provide less coverage and often come with high deductibles, they can be marketed with lower premiums, skimming off healthier younger people who do not expect to need much care, as well as lower-income families. This destabilizes the market and leaves people insured but not covered, exactly the situation before the ACA. Seven public health and medical groups sued to challenge the Administration’s STLD regulation; the lawsuit is presently pending.
Continue to: Route 2: Association Health Plans (AHPs)...
Route 2: Association Health Plans (AHPs). The Administration also has allowed the sale of AHPs, marketed to small employers and self-employed individuals. These plans also do not have to comply with ACA consumer protections. They often do not cover maternity care or other essential benefits, and can charge women higher premiums for the same insurance. This regulation, too, resulted in litigation and a federal judge enjoined the rule, but the case is now on appeal.
Route 3: ACA Section 1332 waivers. These waivers were created in the ACA to encourage state innovation to increase access to health coverage, under certain guardrails: states must ensure coverage is at least as comprehensive as the Essential Health Benefits; cost sharing protections must be at least as affordable as under the ACA; the plan must cover at least a comparable number of its residents; and the plan must not increase the federal deficit.
The Adminstration has come under fire for approving 1332 waiver plans that do not meet these guardrails, and allow insurers to exclude coverage for individuals with preexisting conditions, as well as skirt other important ACA patient protections. In response, Seema Verma, Administrator of the Centers for Medicare & Medicaid Services, promised as recently as April 23, that the Administration will not allow any weakening of the ACA preexisting coverage guarantee.8 So far, however, we do not know what action this means, and not surprisingly, House Democrats, now in the majority, are waiting to see those assurances come true. Consistent polling shows that a large majority of Americans, across political parties, think preexisting coverage protections are very important.9
Already, the House passed HR986, to repeal the Administration’s changes to the 1332 waiver rules. The bill won only 4 Republican votes in the House and now waits a Senate vote.
The House is ready to vote on HR1010, which returns the STLD rules to the original ACA version. The Congressional Budget Office has determined that this bill will reduce the federal deficit by $8.9 billion over 10 years, in part by reestablishing a large risk pool. Lower ACA premiums would mean lower federal subsidies and small federal outlays.
Contraceptive coverage
Since 2012, the ACA has required non-grandfathered individual and group health plans to cover, with no copays or deductibles, women’s preventive services, as determined by the Health Resources and Services Administration (HRSA). HRSA asked the National Academy of Medicine (the Institute of Medicine [IOM] at the time) to develop these coverage guidelines based on clinical and scientific relevance. The IOM relied heavily on ACOG’s testimony and women’s health guidelines. The guidelines are updated every 5 years, based on extensive review by the Women’s Preventive Services Initiative, led by ACOG. By law and regulation, covered services include:
- well-woman visits
- contraceptive methods and counseling, including all methods approved for women by the FDA
- breast and cervical cancer screening
- counseling for sexually transmitted infections
- counseling and screening for HIV
- screening for gestational diabetes
- breastfeeding support, supplies, and counseling
- screening and counseling for interpersonal and domestic violence.
Continue to: The previous administration offered a narrow exemption...
The previous administration offered a narrow exemption—an accommodation—for churches, religious orders, and integrated auxiliaries (organizations with financial support primarily from churches). That accommodation was expanded in the Supreme Court’s decision in Hobby Lobby, for closely held for-profit organizations that had religious objections to covering some or all contraceptives. Under the accommodation, the entity’s insurer or third-party administrator was responsible for providing contraceptive services to the entity’s plan participants and beneficiaries.
In October 2017, the Trump administration acted to greatly expand the ability of any employer, college or university, individual, or insurer to opt out of the ACA’s contraceptive coverage requirement. You will read more about this later.
ACOG’s business case for contraception
Early in the Trump Administration, the White House released a statement saying, “Ensuring affordable, accessible, and quality healthcare is critical to improving women’s health and ensuring that it fits their priorities at any stage of life.”10 ACOG could not agree more, and we encouraged the President to accomplish this important goal by protecting the landmark women’s health gains of the ACA. Our call to the President and the US Congress was: “Don’t turn back the clock on women’s health.”
We made a business case for continued contraceptive coverage:
Contraception reduces unintended pregnancies and saves federal dollars.
- Approximately 45% of US pregnancies are unintended.11
- No-copay coverage of contraception has contributed to a dramatic decline in the unintended pregnancy rate in the United States, now at a 30-year low.12
- When cost is not a barrier, women choose more effective forms of contraception, such as intrauterine devices and implants.13
- Unintended pregnancies cost approximately $12.5 billion in government expenditures in 2008.14
- Private health plans spend as much as $4.6 billion annually in costs related to unintended pregnancies.15
Contraception means healthier women and healthier families.
- Under the ACA, the uninsured rate among women ages 18 to 64 almost halved, decreasing from 19.3% to 10.8%.16
- More than 55 million women gained access to preventive services, including contraception, without a copay or a deductible.16
- Women with unintended pregnancies are more likely to delay prenatal care. Infants are at greater risk of birth defects, low birth weight, and poor mental and physical functioning in early childhood.17
Increased access to contraception helps families and improves economic security.
- Women saved $1.4 billion in out-of-pocket costs for contraception in 1 year.18
- Before the ACA, women were spending between 30% and 44% of their total out-of-pocket health costs just on birth control.19
- The ability to plan a pregnancy increases engagement of women in the workforce and improves economic stability for women and their families.20
Administration expands religious exemptions to contraception coverage
Still, on October 6, 2017, the Trump Administration moved to curtail women’s access to and coverage of contraception with the Religious Exemptions and Accommodations for Coverage of Certain Preventive Services under the Affordable Care Act and Moral Exemptions and Accommodations for Coverage of Certain Preventive Services Under the Affordable Care Act. In November 2018, the Administration published a revised rule, to take effect in January 2019.21 The rule immediately was taken to court by more than a dozen states and, 1 month later, was subject to an injunction by the US Court of Appeals for the Ninth Circuit, blocking the rules from going into effect in those states.
Continue to: The rule vastly expands the Obama Administration’s religious accommodation...
The rule vastly expands the Obama Administration’s religious accommodation to include “nonprofit organizations, small businesses, and individuals that have nonreligious moral convictions opposing services covered by the contraceptive mandate.” The covered entities include21:
- churches, integrated auxiliaries, and religious orders with religious objections
- nonprofit organizations with religious or moral objections
- for-profit entities that are not publicly traded, with religious or moral objections
- for-profit entities that are publicly traded, with religious objections
- other nongovernmental employers with religious objections
- nongovernmental institutions of higher education with religious or moral objections
- individuals with religious or moral objections, with employer sponsored or individual market coverage, where the plan sponsor and/or issuer (as applicable) are willing to offer them a plan omitting contraceptive coverage to which they object
- issuers with religious or moral objections, to the extent they provide coverage to a plan sponsor or individual that is also exempt.
The Administration says women losing coverage can get contraceptives through Title X clinics or other government programs. Of course, many women losing coverage are employed, and earn above the low income (100% of the federal poverty level) eligibility requirement for Title X assistance. To address that, the Administration, through its proposed Title X regulations, broadens the definition of “low income” in that program to include women who lose their contraceptive coverage through the employer-base health insurance plan. This move further limits the ability of the Title X program to adequately care for already-qualified individuals.
The Administration’s rule also relied on major inaccuracies, which ACOG corrected.22 First, ACOG pointed out that, in fact, FDA-approved contraceptive methods are not abortifacients, countering the Administration’s contention that contraception is an abortifacient, and that contraceptives cause abortions or miscarriages. Every FDA-approved contraceptive acts before implantation, does not interfere with a pregnancy, and is not effective after a fertilized egg has implanted successfully in the uterus.23 No credible research supports the false statement that birth control causes miscarriages.24
Second, ACOG offered data proving that increased access to contraception is not associated with increased unsafe sexual behavior or increased sexual activity.25,26 The facts are that:
- The percentage of teens who are having sex has declined significantly, by 14% for female and 22% for male teenagers, over the past 25 years.27
- More women are using contraception the first time they have sex. Young women who do not use birth control at first sexual intercourse are twice as likely to become teen mothers.28
- Increased access to and use of contraception has contributed to a dramatic decline in rates of adolescent pregnancy.29
- School-based health centers that provide access to contraceptives are proven to increase use of contraceptives by already sexually active students, not to increase onset of sexual activity.30,31
Third, ACOG made clear the benefits to women’s health from contraception. ACOG asserted: As with any medication, certain types of contraception may be contraindicated for patients with certain medical conditions, including high blood pressure, lupus, or a history of breast cancer.32,33 For these and many other reasons, access to the full range of FDA-approved contraception, with no cost sharing or other barriers, is critical to women’s health. Regarding VTE, the risk among oral contraceptive users is very low. In fact, it is much lower than the risk of VTE during pregnancy or in the immediate postpartum period.34
Continue to: Regarding breast cancer: there is no proven increased risk...
Regarding breast cancer: there is no proven increased risk of breast cancer among contraceptive users, particularly among those younger than age 40. For women older than 40, health care providers must consider both the risks of becoming pregnant at advanced reproductive age and the risks of continuing contraception use until menopause.35
ACOG has 2 clear messages for politicians
ACOG has remained steadfast in its opposition to the Administration’s proposals to block access to contraception. ACOG expressed its strong opposition to political interference in medical care, saying “Every woman, regardless of her insurer, employer, state of residence, or income, should have affordable, seamless access to the right form of contraception for her, free from interference from her employer or politicians.”22
ACOG’s voice has been joined by 5 other major medical associations—American Academy of Family Physicians, American Academy of Pediatrics, American Psychiatric Association, American Academy of Pediatrics, and American Osteopathic Association—together representing more than 560,000 physicians and medical students, in urging the Administration to immediately withdraw its proposals. This broad coalition unequivocally stated36:
Contraception is an integral part of preventive care and a medical necessity for women during approximately 30 years of their lives. Access to no-copay contraception leads to healthier women and families. Changes to our healthcare system come with very high stakes – impacting tens of millions of our patients. Access to contraception allows women to achieve, lead and reach their full potentials, becoming key drivers of our Nation’s economic success. These rules would create a new standard whereby employers can deny their employees coverage, based on their own moral objections. This interferes in the personal health care decisions of our patients, and inappropriately inserts a patient’s employer into the physician-patient relationship. In addition, these rules open the door to moral exemptions for other essential health care, including vaccinations.
These are challenging days for women’s health policy and legislation federally, and in many states. ACOG has two clear messages for politicians: Don’t turn back the clock on women’s health, and stay out of our exam rooms.
- Claxton G, Cox C, Damico A, et al. Pre-existing conditions and medical underwriting in the individual insurance market prior to the ACA. Kaiser Family Foundation website. Published December 12, 2016. Accessed June 25, 2019.
- Norris L. Billions in ACA rebates show 80/20 rule’s impact. HealthInsurance.org website. Published May 10, 2019. Accessed June 25, 2019.
- Patient Protection and Affordable Care Act: Preexisting condition exclusions, lifetime and annual limits, rescissions, and patient protections. Regulations.gov website. Accessed June 25, 2019.
- Jost T. The Senate’s Health Care Freedom Act. Health Affairs website. Updated July 28, 2017. Accessed June 25, 2019.
- Texas v Azar decision. American Medical Association website. Accessed June 25, 2019.
- Keith K. DOJ, plaintiffs file in Texas v United States. Health Affairs website. Published May 2 2019. Accessed June 25, 2019.
- John & Rusty Report. Trump Administration asks court to strike down entire ACA. March 26, 2019. https://jrreport.wordandbrown.com/2019/03/26/trump-administration-asks-court-to-strike-down-entire-aca/. Accessed June 29, 2019.
- Speech: Remarks by Administrator Seema Verma at the CMS National Forum on State Relief and Empowerment Waivers. Centers for Medicare & Medicaid website. Published April 23, 2019. Accessed June 25, 2019.
- Poll: The ACA’s pre-existing condition protections remain popular with the public, including republicans, as legal challenge looms this week. Kaiser Family Foundation website. Published September 5, 2018. Accessed June 25, 2019.
- Statement from President Donald J. Trump on Women’s Health Week. White House website. Issued May 14, 2017. Accessed June 26, 2019.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374:843-852.
- Insurance coverage of contraception. Guttmacher Institute website. Published August 2018. Accessed June 26, 2019.
- Carlin CS, Fertig AR, Dowd BE. Affordable Care Act’s mandate eliminating contraceptive cost sharing influenced choices of women with employer coverage. Health Affairs. 2016;35:1608-1615.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250–255.
- Canestaro W, et al. Implications of employer coverage of contraception: cost-effectiveness analysis of contraception coverage under an employer mandate. Contraception. 2017;95:77-89.
- Simmons A, et al. The Affordable Care Act: Promoting better health for women. Office of the Assistant Secretary for Planning and Evaluation Issue Brief, Department of Health and Human Services. June 14, 2016. Accessed June 25, 2019.
- Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809–1823.
- Becker NV, Polsky D. Women saw large decrease in out-of-pocket spending for contraceptives after ACA mandate removed cost sharing. Health Affairs. 2015;34:1204-1211. Accessed June 25, 2019.
- Becker NV, Polsky D. Women saw large decrease in out-of-pocket spending for contraceptives after ACA mandate removed cost sharing. Health Affairs. 2015;34(7).
- Sonfield A, Hasstedt K, Kavanaugh ML, Anderson R. The social and economic benefits of women’s ability to determine whether and when to have children. New York, NY: Guttmacher Institute; 2013.
- Department of Health and Human Services. Fact sheet: Final rules on religious and moral exemptions and accommodation for coverage of certain preventive services under the Affordable Care Act. November 7, 2018. Accessed June 26, 2019.
- American College of Obstetricians and Gynecologists. Facts are important: Correcting the record on the Administration’s contraceptive coverage roll back rule. October 2017. Accessed June 26, 2019.
- Brief for Physicians for Reproductive Health, American College of Obstetricians and Gynecologists et al. as Amici Curiae Supporting Respondents, Sebelius v. Hobby Lobby, 573 U.S. XXX. 2014. (No. 13-354).
- Early pregnancy loss. FAQ No. 90. American College of Obstetricians and Gynecologists. August 2015.
- Kirby D. Emerging answers 2007: Research findings on programs to reduce teen pregnancy and sexually transmitted diseases. Washington, DC: The National Campaign to Prevent Teen and Unplanned Pregnancy; 2009.
- Meyer JL, Gold MA, Haggerty CL. Advance provision of emergency contraception among adolescent and young adult women: a systematic review of literature. J Pediatr Adolesc Gynecol. 2011;24:2-9.
- Martinez GM and Abma JC. Sexual activity, contraceptive use, and childbearing of teenagers aged 15–19 in the United States. NCHS Data Brief, 2015, No. 209. Hyattsville, MD: National Center for Health Statistics; 2015.
- Martinez GM, Abma JC. Sexual activity, contraceptive use, and childbearing of teenagers aged 15-19 in the United States. NCHS Data Brief. July 2015. Accessed June 26, 2019.
- Lindberg L, Santelli J, Desai S. Understanding the decline in adolescent fertility in the United States, 2007–2012. J Adolesc Health. 2016;59:577-583.
- Minguez M, Santelli JS, Gibson E, et al. Reproductive health impact of a school health center. J Adolesc Health. 2015;56:338-344.
- Knopf JA, Finnie RK, Peng Y, et al. Community Preventive Services Task Force. School-based health centers to advance health equity: a Community Guide systematic review. Am J Preventive Med. 2016;51:114-126.
- Progestin-only hormonal birth control: pill and injection. FAQ No. 86. American College of Obstetricians and Gynecologists. July 2014.
- Combined hormonal birth control: pill, patch, and ring. FAQ No. 185. American College of Obstetricians and Gynecologists. July 2014.
- Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Committee Opinion No. 540. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2012;120:1239-1242.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(No. RR-4):1–66.
- Letter to President Donald J. Trump. October 6, 2017. https://www.aafp.org/dam/AAFP/documents/advocacy/coverage/aca/LT-Group6-President-ContraceptionIFRs-100617.pdf. Accessed June 26, 2019.
The Affordable Care Act (ACA) was enacted on March 23, 2010. Controversies, complaints, and detractors have and continue to abound. But the ACA’s landmark women’s health gains are unmistakable. Contraceptive coverage, maternity coverage, Medicaid coverage of low-income women, coverage for individuals with preexisting conditions, and gender-neutral premiums are now a part of the fabric of our society. For most.
Many physicians and patients—many lawmakers, too—do not remember the serious problems people had with their insurance companies before the ACA. Maternity coverage was usually a free-standing rider to an insurance policy, making it very expensive. Insurance plans did not have to, and often did not, cover contraceptives, and none did without copays or deductibles. Women were routinely denied coverage if they had ever had a cesarean delivery, had once been the victim of domestic violence, or had any one of many common conditions, like diabetes. The many exclusionary conditions are so common, in fact, that one study estimated that around 52 million adults in the United States (27% of those younger than age 65 years) have preexisting conditions that would potentially make them uninsurable without the ACA’s protections.1
Before the ACA, it also was common for women with insurance policies to find their coverage rescinded, often with no explanation, even though they paid their premiums every month. And women with serious medical conditions often saw their coverage ended midway through their course of treatment. That placed their ObGyns in a terrible situation, too.
The insurance industry as a whole was running rough-shod over its customers, and making a lot of money by creatively and routinely denying coverage and payment for care. People were often insured, but not covered. The ACA halted many of these practices, and required insurers to meet high medical loss ratios, guaranteeing that 80% of the premiums’ for individual and small market insurers (and 85% for large insurers) are returned to patients in care payments or even in checks. In fact, nearly $4 billion in premiums have been rebated to insured individuals over the last 7 years under the ACA.2
The commitment of the American College of Obstetricians and Gynecologists (ACOG) to women’s health and to our members’ ability to provide the best care has centered on preserving the critical gains of the ACA for women, improving them when we can, and making sure politicians don’t turn back the clock on women’s health. We have been busy.
In this article, we will look at what has happened to these landmark gains and promises of improved women’s health, specifically preexisting condition protections and contraceptive coverage, under a new Administration. What happens when good health care policy and political enmity collide?

Preexisting coverage protections
The 1996 Health Insurance Portability and Accountability Act (HIPAA) defines a preexisting condition exclusionas a “limitation or exclusion of benefits relating to a condition based on the fact that the condition was present before the date of enrollment for the coverage, whether or not any medical advice, diagnosis, care, or treatment was recommended or received before that date.” HIPPA prohibited employer-sponsored health plans from discriminating against individuals through denying them coverage or charging them more based on their or their family members’ health problems. The ACA expanded protections to prohibit the insurance practice of denying coverage altogether to an individual with a preexisting condition.3
Continue to: Under Congress...
Under Congress
Republicans held the majority in both chambers of the 115th Congress (2017–2018), and hoped to use their majority status to get an ACA repeal bill to the Republican President’s desk for speedy enactment. It was not easy, and they were not successful. Four major bills—the American Health Care Act, the Better Care Reconciliation Act, the Health Care Freedom Act, and the Graham-Cassidy Amendment—never made it over the finish line, with some not even making it to a vote. The Health Care Freedom Act was voted down in the Senate 51-49 when Senator John McCain came back from brain surgery to cast his famous thumbs-down vote.4 These bills all would have repealed or hobbled guaranteed issue, community rating, and essential health benefits of the ACA. Of all the legislative attempts to undermine the ACA, only the 2017 Tax Cuts and Jobs Act (TCJA) was signed into law, repealing the ACA individual mandate.
Handling by the courts
The TCJA gave ACA opponents their opening in court. Twenty Republican state attorneys general and governors brought suit in February 2018 (Texas v Azar), arguing that because the ACA relies on the mandate, and the mandate has been repealed, the rest of the ACA also should be struck down. A federal district judge agreed, on December 15, 2018, declaring the entire ACA unconstitutional.5
That decision has been limited in its practical effect so far, and maybe it was not altogether unexpected. What was unexpected was that the US Department of Justice (DOJ) refused to defend a federal law, in this case, the ACA. In June 2018, the DOJ declined to defend the individual mandate, as well as guaranteed issue, community rating, the ban on preexisting condition exclusions, and discrimination based on health status in the ACA. The DOJ at that time, however, did not agree with the plaintiffs that without the mandate the entire ACA should be struck down. It said, “There is no reason why the ACA’s particular expansion of Medicaid hinges on the individual mandate.” Later, after the December 15 ruling, the DOJ changed its position and agreed with the judge, in a two-sentence letter to the court, that the ACA should be stricken altogether—shortly after which 3 career DOJ attorneys resigned.6
A legal expert observed: “The DOJ’s decision not to defend the ACA breaks with the Department’s long-standing bipartisan commitment to defend federal laws if reasonable arguments can be made in their defense. Decisions not to defend federal law are exceedingly rare. It seems even rarer to change the government’s position mid-appeal in such a high-profile lawsuit that risks disrupting the entire health care system and health insurance coverage for millions of Americans.”7
Regulatory tactics
What a policy maker cannot do by law, he or she can try to accomplish by regulation. The Administration is using 3 regulatory routes to undercut the ACA preexisting coverage protections and market stability.
Route 1: Short-Term Limited Duration (STLD) plans. These plans were created in the ACA to provide bridge coverage for up to 3 months for individuals in between health insurance plans. These plans do not have to comply with ACA patient protections, can deny coverage for preexisting conditions, and do not cover maternity care. In 2018, the Administration moved to allow these plans to be marketed broadly and renewed for up to 3 years. Because these plans provide less coverage and often come with high deductibles, they can be marketed with lower premiums, skimming off healthier younger people who do not expect to need much care, as well as lower-income families. This destabilizes the market and leaves people insured but not covered, exactly the situation before the ACA. Seven public health and medical groups sued to challenge the Administration’s STLD regulation; the lawsuit is presently pending.
Continue to: Route 2: Association Health Plans (AHPs)...
Route 2: Association Health Plans (AHPs). The Administration also has allowed the sale of AHPs, marketed to small employers and self-employed individuals. These plans also do not have to comply with ACA consumer protections. They often do not cover maternity care or other essential benefits, and can charge women higher premiums for the same insurance. This regulation, too, resulted in litigation and a federal judge enjoined the rule, but the case is now on appeal.
Route 3: ACA Section 1332 waivers. These waivers were created in the ACA to encourage state innovation to increase access to health coverage, under certain guardrails: states must ensure coverage is at least as comprehensive as the Essential Health Benefits; cost sharing protections must be at least as affordable as under the ACA; the plan must cover at least a comparable number of its residents; and the plan must not increase the federal deficit.
The Adminstration has come under fire for approving 1332 waiver plans that do not meet these guardrails, and allow insurers to exclude coverage for individuals with preexisting conditions, as well as skirt other important ACA patient protections. In response, Seema Verma, Administrator of the Centers for Medicare & Medicaid Services, promised as recently as April 23, that the Administration will not allow any weakening of the ACA preexisting coverage guarantee.8 So far, however, we do not know what action this means, and not surprisingly, House Democrats, now in the majority, are waiting to see those assurances come true. Consistent polling shows that a large majority of Americans, across political parties, think preexisting coverage protections are very important.9
Already, the House passed HR986, to repeal the Administration’s changes to the 1332 waiver rules. The bill won only 4 Republican votes in the House and now waits a Senate vote.
The House is ready to vote on HR1010, which returns the STLD rules to the original ACA version. The Congressional Budget Office has determined that this bill will reduce the federal deficit by $8.9 billion over 10 years, in part by reestablishing a large risk pool. Lower ACA premiums would mean lower federal subsidies and small federal outlays.
Contraceptive coverage
Since 2012, the ACA has required non-grandfathered individual and group health plans to cover, with no copays or deductibles, women’s preventive services, as determined by the Health Resources and Services Administration (HRSA). HRSA asked the National Academy of Medicine (the Institute of Medicine [IOM] at the time) to develop these coverage guidelines based on clinical and scientific relevance. The IOM relied heavily on ACOG’s testimony and women’s health guidelines. The guidelines are updated every 5 years, based on extensive review by the Women’s Preventive Services Initiative, led by ACOG. By law and regulation, covered services include:
- well-woman visits
- contraceptive methods and counseling, including all methods approved for women by the FDA
- breast and cervical cancer screening
- counseling for sexually transmitted infections
- counseling and screening for HIV
- screening for gestational diabetes
- breastfeeding support, supplies, and counseling
- screening and counseling for interpersonal and domestic violence.
Continue to: The previous administration offered a narrow exemption...
The previous administration offered a narrow exemption—an accommodation—for churches, religious orders, and integrated auxiliaries (organizations with financial support primarily from churches). That accommodation was expanded in the Supreme Court’s decision in Hobby Lobby, for closely held for-profit organizations that had religious objections to covering some or all contraceptives. Under the accommodation, the entity’s insurer or third-party administrator was responsible for providing contraceptive services to the entity’s plan participants and beneficiaries.
In October 2017, the Trump administration acted to greatly expand the ability of any employer, college or university, individual, or insurer to opt out of the ACA’s contraceptive coverage requirement. You will read more about this later.
ACOG’s business case for contraception
Early in the Trump Administration, the White House released a statement saying, “Ensuring affordable, accessible, and quality healthcare is critical to improving women’s health and ensuring that it fits their priorities at any stage of life.”10 ACOG could not agree more, and we encouraged the President to accomplish this important goal by protecting the landmark women’s health gains of the ACA. Our call to the President and the US Congress was: “Don’t turn back the clock on women’s health.”
We made a business case for continued contraceptive coverage:
Contraception reduces unintended pregnancies and saves federal dollars.
- Approximately 45% of US pregnancies are unintended.11
- No-copay coverage of contraception has contributed to a dramatic decline in the unintended pregnancy rate in the United States, now at a 30-year low.12
- When cost is not a barrier, women choose more effective forms of contraception, such as intrauterine devices and implants.13
- Unintended pregnancies cost approximately $12.5 billion in government expenditures in 2008.14
- Private health plans spend as much as $4.6 billion annually in costs related to unintended pregnancies.15
Contraception means healthier women and healthier families.
- Under the ACA, the uninsured rate among women ages 18 to 64 almost halved, decreasing from 19.3% to 10.8%.16
- More than 55 million women gained access to preventive services, including contraception, without a copay or a deductible.16
- Women with unintended pregnancies are more likely to delay prenatal care. Infants are at greater risk of birth defects, low birth weight, and poor mental and physical functioning in early childhood.17
Increased access to contraception helps families and improves economic security.
- Women saved $1.4 billion in out-of-pocket costs for contraception in 1 year.18
- Before the ACA, women were spending between 30% and 44% of their total out-of-pocket health costs just on birth control.19
- The ability to plan a pregnancy increases engagement of women in the workforce and improves economic stability for women and their families.20
Administration expands religious exemptions to contraception coverage
Still, on October 6, 2017, the Trump Administration moved to curtail women’s access to and coverage of contraception with the Religious Exemptions and Accommodations for Coverage of Certain Preventive Services under the Affordable Care Act and Moral Exemptions and Accommodations for Coverage of Certain Preventive Services Under the Affordable Care Act. In November 2018, the Administration published a revised rule, to take effect in January 2019.21 The rule immediately was taken to court by more than a dozen states and, 1 month later, was subject to an injunction by the US Court of Appeals for the Ninth Circuit, blocking the rules from going into effect in those states.
Continue to: The rule vastly expands the Obama Administration’s religious accommodation...
The rule vastly expands the Obama Administration’s religious accommodation to include “nonprofit organizations, small businesses, and individuals that have nonreligious moral convictions opposing services covered by the contraceptive mandate.” The covered entities include21:
- churches, integrated auxiliaries, and religious orders with religious objections
- nonprofit organizations with religious or moral objections
- for-profit entities that are not publicly traded, with religious or moral objections
- for-profit entities that are publicly traded, with religious objections
- other nongovernmental employers with religious objections
- nongovernmental institutions of higher education with religious or moral objections
- individuals with religious or moral objections, with employer sponsored or individual market coverage, where the plan sponsor and/or issuer (as applicable) are willing to offer them a plan omitting contraceptive coverage to which they object
- issuers with religious or moral objections, to the extent they provide coverage to a plan sponsor or individual that is also exempt.
The Administration says women losing coverage can get contraceptives through Title X clinics or other government programs. Of course, many women losing coverage are employed, and earn above the low income (100% of the federal poverty level) eligibility requirement for Title X assistance. To address that, the Administration, through its proposed Title X regulations, broadens the definition of “low income” in that program to include women who lose their contraceptive coverage through the employer-base health insurance plan. This move further limits the ability of the Title X program to adequately care for already-qualified individuals.
The Administration’s rule also relied on major inaccuracies, which ACOG corrected.22 First, ACOG pointed out that, in fact, FDA-approved contraceptive methods are not abortifacients, countering the Administration’s contention that contraception is an abortifacient, and that contraceptives cause abortions or miscarriages. Every FDA-approved contraceptive acts before implantation, does not interfere with a pregnancy, and is not effective after a fertilized egg has implanted successfully in the uterus.23 No credible research supports the false statement that birth control causes miscarriages.24
Second, ACOG offered data proving that increased access to contraception is not associated with increased unsafe sexual behavior or increased sexual activity.25,26 The facts are that:
- The percentage of teens who are having sex has declined significantly, by 14% for female and 22% for male teenagers, over the past 25 years.27
- More women are using contraception the first time they have sex. Young women who do not use birth control at first sexual intercourse are twice as likely to become teen mothers.28
- Increased access to and use of contraception has contributed to a dramatic decline in rates of adolescent pregnancy.29
- School-based health centers that provide access to contraceptives are proven to increase use of contraceptives by already sexually active students, not to increase onset of sexual activity.30,31
Third, ACOG made clear the benefits to women’s health from contraception. ACOG asserted: As with any medication, certain types of contraception may be contraindicated for patients with certain medical conditions, including high blood pressure, lupus, or a history of breast cancer.32,33 For these and many other reasons, access to the full range of FDA-approved contraception, with no cost sharing or other barriers, is critical to women’s health. Regarding VTE, the risk among oral contraceptive users is very low. In fact, it is much lower than the risk of VTE during pregnancy or in the immediate postpartum period.34
Continue to: Regarding breast cancer: there is no proven increased risk...
Regarding breast cancer: there is no proven increased risk of breast cancer among contraceptive users, particularly among those younger than age 40. For women older than 40, health care providers must consider both the risks of becoming pregnant at advanced reproductive age and the risks of continuing contraception use until menopause.35
ACOG has 2 clear messages for politicians
ACOG has remained steadfast in its opposition to the Administration’s proposals to block access to contraception. ACOG expressed its strong opposition to political interference in medical care, saying “Every woman, regardless of her insurer, employer, state of residence, or income, should have affordable, seamless access to the right form of contraception for her, free from interference from her employer or politicians.”22
ACOG’s voice has been joined by 5 other major medical associations—American Academy of Family Physicians, American Academy of Pediatrics, American Psychiatric Association, American Academy of Pediatrics, and American Osteopathic Association—together representing more than 560,000 physicians and medical students, in urging the Administration to immediately withdraw its proposals. This broad coalition unequivocally stated36:
Contraception is an integral part of preventive care and a medical necessity for women during approximately 30 years of their lives. Access to no-copay contraception leads to healthier women and families. Changes to our healthcare system come with very high stakes – impacting tens of millions of our patients. Access to contraception allows women to achieve, lead and reach their full potentials, becoming key drivers of our Nation’s economic success. These rules would create a new standard whereby employers can deny their employees coverage, based on their own moral objections. This interferes in the personal health care decisions of our patients, and inappropriately inserts a patient’s employer into the physician-patient relationship. In addition, these rules open the door to moral exemptions for other essential health care, including vaccinations.
These are challenging days for women’s health policy and legislation federally, and in many states. ACOG has two clear messages for politicians: Don’t turn back the clock on women’s health, and stay out of our exam rooms.
The Affordable Care Act (ACA) was enacted on March 23, 2010. Controversies, complaints, and detractors have and continue to abound. But the ACA’s landmark women’s health gains are unmistakable. Contraceptive coverage, maternity coverage, Medicaid coverage of low-income women, coverage for individuals with preexisting conditions, and gender-neutral premiums are now a part of the fabric of our society. For most.
Many physicians and patients—many lawmakers, too—do not remember the serious problems people had with their insurance companies before the ACA. Maternity coverage was usually a free-standing rider to an insurance policy, making it very expensive. Insurance plans did not have to, and often did not, cover contraceptives, and none did without copays or deductibles. Women were routinely denied coverage if they had ever had a cesarean delivery, had once been the victim of domestic violence, or had any one of many common conditions, like diabetes. The many exclusionary conditions are so common, in fact, that one study estimated that around 52 million adults in the United States (27% of those younger than age 65 years) have preexisting conditions that would potentially make them uninsurable without the ACA’s protections.1
Before the ACA, it also was common for women with insurance policies to find their coverage rescinded, often with no explanation, even though they paid their premiums every month. And women with serious medical conditions often saw their coverage ended midway through their course of treatment. That placed their ObGyns in a terrible situation, too.
The insurance industry as a whole was running rough-shod over its customers, and making a lot of money by creatively and routinely denying coverage and payment for care. People were often insured, but not covered. The ACA halted many of these practices, and required insurers to meet high medical loss ratios, guaranteeing that 80% of the premiums’ for individual and small market insurers (and 85% for large insurers) are returned to patients in care payments or even in checks. In fact, nearly $4 billion in premiums have been rebated to insured individuals over the last 7 years under the ACA.2
The commitment of the American College of Obstetricians and Gynecologists (ACOG) to women’s health and to our members’ ability to provide the best care has centered on preserving the critical gains of the ACA for women, improving them when we can, and making sure politicians don’t turn back the clock on women’s health. We have been busy.
In this article, we will look at what has happened to these landmark gains and promises of improved women’s health, specifically preexisting condition protections and contraceptive coverage, under a new Administration. What happens when good health care policy and political enmity collide?

Preexisting coverage protections
The 1996 Health Insurance Portability and Accountability Act (HIPAA) defines a preexisting condition exclusionas a “limitation or exclusion of benefits relating to a condition based on the fact that the condition was present before the date of enrollment for the coverage, whether or not any medical advice, diagnosis, care, or treatment was recommended or received before that date.” HIPPA prohibited employer-sponsored health plans from discriminating against individuals through denying them coverage or charging them more based on their or their family members’ health problems. The ACA expanded protections to prohibit the insurance practice of denying coverage altogether to an individual with a preexisting condition.3
Continue to: Under Congress...
Under Congress
Republicans held the majority in both chambers of the 115th Congress (2017–2018), and hoped to use their majority status to get an ACA repeal bill to the Republican President’s desk for speedy enactment. It was not easy, and they were not successful. Four major bills—the American Health Care Act, the Better Care Reconciliation Act, the Health Care Freedom Act, and the Graham-Cassidy Amendment—never made it over the finish line, with some not even making it to a vote. The Health Care Freedom Act was voted down in the Senate 51-49 when Senator John McCain came back from brain surgery to cast his famous thumbs-down vote.4 These bills all would have repealed or hobbled guaranteed issue, community rating, and essential health benefits of the ACA. Of all the legislative attempts to undermine the ACA, only the 2017 Tax Cuts and Jobs Act (TCJA) was signed into law, repealing the ACA individual mandate.
Handling by the courts
The TCJA gave ACA opponents their opening in court. Twenty Republican state attorneys general and governors brought suit in February 2018 (Texas v Azar), arguing that because the ACA relies on the mandate, and the mandate has been repealed, the rest of the ACA also should be struck down. A federal district judge agreed, on December 15, 2018, declaring the entire ACA unconstitutional.5
That decision has been limited in its practical effect so far, and maybe it was not altogether unexpected. What was unexpected was that the US Department of Justice (DOJ) refused to defend a federal law, in this case, the ACA. In June 2018, the DOJ declined to defend the individual mandate, as well as guaranteed issue, community rating, the ban on preexisting condition exclusions, and discrimination based on health status in the ACA. The DOJ at that time, however, did not agree with the plaintiffs that without the mandate the entire ACA should be struck down. It said, “There is no reason why the ACA’s particular expansion of Medicaid hinges on the individual mandate.” Later, after the December 15 ruling, the DOJ changed its position and agreed with the judge, in a two-sentence letter to the court, that the ACA should be stricken altogether—shortly after which 3 career DOJ attorneys resigned.6
A legal expert observed: “The DOJ’s decision not to defend the ACA breaks with the Department’s long-standing bipartisan commitment to defend federal laws if reasonable arguments can be made in their defense. Decisions not to defend federal law are exceedingly rare. It seems even rarer to change the government’s position mid-appeal in such a high-profile lawsuit that risks disrupting the entire health care system and health insurance coverage for millions of Americans.”7
Regulatory tactics
What a policy maker cannot do by law, he or she can try to accomplish by regulation. The Administration is using 3 regulatory routes to undercut the ACA preexisting coverage protections and market stability.
Route 1: Short-Term Limited Duration (STLD) plans. These plans were created in the ACA to provide bridge coverage for up to 3 months for individuals in between health insurance plans. These plans do not have to comply with ACA patient protections, can deny coverage for preexisting conditions, and do not cover maternity care. In 2018, the Administration moved to allow these plans to be marketed broadly and renewed for up to 3 years. Because these plans provide less coverage and often come with high deductibles, they can be marketed with lower premiums, skimming off healthier younger people who do not expect to need much care, as well as lower-income families. This destabilizes the market and leaves people insured but not covered, exactly the situation before the ACA. Seven public health and medical groups sued to challenge the Administration’s STLD regulation; the lawsuit is presently pending.
Continue to: Route 2: Association Health Plans (AHPs)...
Route 2: Association Health Plans (AHPs). The Administration also has allowed the sale of AHPs, marketed to small employers and self-employed individuals. These plans also do not have to comply with ACA consumer protections. They often do not cover maternity care or other essential benefits, and can charge women higher premiums for the same insurance. This regulation, too, resulted in litigation and a federal judge enjoined the rule, but the case is now on appeal.
Route 3: ACA Section 1332 waivers. These waivers were created in the ACA to encourage state innovation to increase access to health coverage, under certain guardrails: states must ensure coverage is at least as comprehensive as the Essential Health Benefits; cost sharing protections must be at least as affordable as under the ACA; the plan must cover at least a comparable number of its residents; and the plan must not increase the federal deficit.
The Adminstration has come under fire for approving 1332 waiver plans that do not meet these guardrails, and allow insurers to exclude coverage for individuals with preexisting conditions, as well as skirt other important ACA patient protections. In response, Seema Verma, Administrator of the Centers for Medicare & Medicaid Services, promised as recently as April 23, that the Administration will not allow any weakening of the ACA preexisting coverage guarantee.8 So far, however, we do not know what action this means, and not surprisingly, House Democrats, now in the majority, are waiting to see those assurances come true. Consistent polling shows that a large majority of Americans, across political parties, think preexisting coverage protections are very important.9
Already, the House passed HR986, to repeal the Administration’s changes to the 1332 waiver rules. The bill won only 4 Republican votes in the House and now waits a Senate vote.
The House is ready to vote on HR1010, which returns the STLD rules to the original ACA version. The Congressional Budget Office has determined that this bill will reduce the federal deficit by $8.9 billion over 10 years, in part by reestablishing a large risk pool. Lower ACA premiums would mean lower federal subsidies and small federal outlays.
Contraceptive coverage
Since 2012, the ACA has required non-grandfathered individual and group health plans to cover, with no copays or deductibles, women’s preventive services, as determined by the Health Resources and Services Administration (HRSA). HRSA asked the National Academy of Medicine (the Institute of Medicine [IOM] at the time) to develop these coverage guidelines based on clinical and scientific relevance. The IOM relied heavily on ACOG’s testimony and women’s health guidelines. The guidelines are updated every 5 years, based on extensive review by the Women’s Preventive Services Initiative, led by ACOG. By law and regulation, covered services include:
- well-woman visits
- contraceptive methods and counseling, including all methods approved for women by the FDA
- breast and cervical cancer screening
- counseling for sexually transmitted infections
- counseling and screening for HIV
- screening for gestational diabetes
- breastfeeding support, supplies, and counseling
- screening and counseling for interpersonal and domestic violence.
Continue to: The previous administration offered a narrow exemption...
The previous administration offered a narrow exemption—an accommodation—for churches, religious orders, and integrated auxiliaries (organizations with financial support primarily from churches). That accommodation was expanded in the Supreme Court’s decision in Hobby Lobby, for closely held for-profit organizations that had religious objections to covering some or all contraceptives. Under the accommodation, the entity’s insurer or third-party administrator was responsible for providing contraceptive services to the entity’s plan participants and beneficiaries.
In October 2017, the Trump administration acted to greatly expand the ability of any employer, college or university, individual, or insurer to opt out of the ACA’s contraceptive coverage requirement. You will read more about this later.
ACOG’s business case for contraception
Early in the Trump Administration, the White House released a statement saying, “Ensuring affordable, accessible, and quality healthcare is critical to improving women’s health and ensuring that it fits their priorities at any stage of life.”10 ACOG could not agree more, and we encouraged the President to accomplish this important goal by protecting the landmark women’s health gains of the ACA. Our call to the President and the US Congress was: “Don’t turn back the clock on women’s health.”
We made a business case for continued contraceptive coverage:
Contraception reduces unintended pregnancies and saves federal dollars.
- Approximately 45% of US pregnancies are unintended.11
- No-copay coverage of contraception has contributed to a dramatic decline in the unintended pregnancy rate in the United States, now at a 30-year low.12
- When cost is not a barrier, women choose more effective forms of contraception, such as intrauterine devices and implants.13
- Unintended pregnancies cost approximately $12.5 billion in government expenditures in 2008.14
- Private health plans spend as much as $4.6 billion annually in costs related to unintended pregnancies.15
Contraception means healthier women and healthier families.
- Under the ACA, the uninsured rate among women ages 18 to 64 almost halved, decreasing from 19.3% to 10.8%.16
- More than 55 million women gained access to preventive services, including contraception, without a copay or a deductible.16
- Women with unintended pregnancies are more likely to delay prenatal care. Infants are at greater risk of birth defects, low birth weight, and poor mental and physical functioning in early childhood.17
Increased access to contraception helps families and improves economic security.
- Women saved $1.4 billion in out-of-pocket costs for contraception in 1 year.18
- Before the ACA, women were spending between 30% and 44% of their total out-of-pocket health costs just on birth control.19
- The ability to plan a pregnancy increases engagement of women in the workforce and improves economic stability for women and their families.20
Administration expands religious exemptions to contraception coverage
Still, on October 6, 2017, the Trump Administration moved to curtail women’s access to and coverage of contraception with the Religious Exemptions and Accommodations for Coverage of Certain Preventive Services under the Affordable Care Act and Moral Exemptions and Accommodations for Coverage of Certain Preventive Services Under the Affordable Care Act. In November 2018, the Administration published a revised rule, to take effect in January 2019.21 The rule immediately was taken to court by more than a dozen states and, 1 month later, was subject to an injunction by the US Court of Appeals for the Ninth Circuit, blocking the rules from going into effect in those states.
Continue to: The rule vastly expands the Obama Administration’s religious accommodation...
The rule vastly expands the Obama Administration’s religious accommodation to include “nonprofit organizations, small businesses, and individuals that have nonreligious moral convictions opposing services covered by the contraceptive mandate.” The covered entities include21:
- churches, integrated auxiliaries, and religious orders with religious objections
- nonprofit organizations with religious or moral objections
- for-profit entities that are not publicly traded, with religious or moral objections
- for-profit entities that are publicly traded, with religious objections
- other nongovernmental employers with religious objections
- nongovernmental institutions of higher education with religious or moral objections
- individuals with religious or moral objections, with employer sponsored or individual market coverage, where the plan sponsor and/or issuer (as applicable) are willing to offer them a plan omitting contraceptive coverage to which they object
- issuers with religious or moral objections, to the extent they provide coverage to a plan sponsor or individual that is also exempt.
The Administration says women losing coverage can get contraceptives through Title X clinics or other government programs. Of course, many women losing coverage are employed, and earn above the low income (100% of the federal poverty level) eligibility requirement for Title X assistance. To address that, the Administration, through its proposed Title X regulations, broadens the definition of “low income” in that program to include women who lose their contraceptive coverage through the employer-base health insurance plan. This move further limits the ability of the Title X program to adequately care for already-qualified individuals.
The Administration’s rule also relied on major inaccuracies, which ACOG corrected.22 First, ACOG pointed out that, in fact, FDA-approved contraceptive methods are not abortifacients, countering the Administration’s contention that contraception is an abortifacient, and that contraceptives cause abortions or miscarriages. Every FDA-approved contraceptive acts before implantation, does not interfere with a pregnancy, and is not effective after a fertilized egg has implanted successfully in the uterus.23 No credible research supports the false statement that birth control causes miscarriages.24
Second, ACOG offered data proving that increased access to contraception is not associated with increased unsafe sexual behavior or increased sexual activity.25,26 The facts are that:
- The percentage of teens who are having sex has declined significantly, by 14% for female and 22% for male teenagers, over the past 25 years.27
- More women are using contraception the first time they have sex. Young women who do not use birth control at first sexual intercourse are twice as likely to become teen mothers.28
- Increased access to and use of contraception has contributed to a dramatic decline in rates of adolescent pregnancy.29
- School-based health centers that provide access to contraceptives are proven to increase use of contraceptives by already sexually active students, not to increase onset of sexual activity.30,31
Third, ACOG made clear the benefits to women’s health from contraception. ACOG asserted: As with any medication, certain types of contraception may be contraindicated for patients with certain medical conditions, including high blood pressure, lupus, or a history of breast cancer.32,33 For these and many other reasons, access to the full range of FDA-approved contraception, with no cost sharing or other barriers, is critical to women’s health. Regarding VTE, the risk among oral contraceptive users is very low. In fact, it is much lower than the risk of VTE during pregnancy or in the immediate postpartum period.34
Continue to: Regarding breast cancer: there is no proven increased risk...
Regarding breast cancer: there is no proven increased risk of breast cancer among contraceptive users, particularly among those younger than age 40. For women older than 40, health care providers must consider both the risks of becoming pregnant at advanced reproductive age and the risks of continuing contraception use until menopause.35
ACOG has 2 clear messages for politicians
ACOG has remained steadfast in its opposition to the Administration’s proposals to block access to contraception. ACOG expressed its strong opposition to political interference in medical care, saying “Every woman, regardless of her insurer, employer, state of residence, or income, should have affordable, seamless access to the right form of contraception for her, free from interference from her employer or politicians.”22
ACOG’s voice has been joined by 5 other major medical associations—American Academy of Family Physicians, American Academy of Pediatrics, American Psychiatric Association, American Academy of Pediatrics, and American Osteopathic Association—together representing more than 560,000 physicians and medical students, in urging the Administration to immediately withdraw its proposals. This broad coalition unequivocally stated36:
Contraception is an integral part of preventive care and a medical necessity for women during approximately 30 years of their lives. Access to no-copay contraception leads to healthier women and families. Changes to our healthcare system come with very high stakes – impacting tens of millions of our patients. Access to contraception allows women to achieve, lead and reach their full potentials, becoming key drivers of our Nation’s economic success. These rules would create a new standard whereby employers can deny their employees coverage, based on their own moral objections. This interferes in the personal health care decisions of our patients, and inappropriately inserts a patient’s employer into the physician-patient relationship. In addition, these rules open the door to moral exemptions for other essential health care, including vaccinations.
These are challenging days for women’s health policy and legislation federally, and in many states. ACOG has two clear messages for politicians: Don’t turn back the clock on women’s health, and stay out of our exam rooms.
- Claxton G, Cox C, Damico A, et al. Pre-existing conditions and medical underwriting in the individual insurance market prior to the ACA. Kaiser Family Foundation website. Published December 12, 2016. Accessed June 25, 2019.
- Norris L. Billions in ACA rebates show 80/20 rule’s impact. HealthInsurance.org website. Published May 10, 2019. Accessed June 25, 2019.
- Patient Protection and Affordable Care Act: Preexisting condition exclusions, lifetime and annual limits, rescissions, and patient protections. Regulations.gov website. Accessed June 25, 2019.
- Jost T. The Senate’s Health Care Freedom Act. Health Affairs website. Updated July 28, 2017. Accessed June 25, 2019.
- Texas v Azar decision. American Medical Association website. Accessed June 25, 2019.
- Keith K. DOJ, plaintiffs file in Texas v United States. Health Affairs website. Published May 2 2019. Accessed June 25, 2019.
- John & Rusty Report. Trump Administration asks court to strike down entire ACA. March 26, 2019. https://jrreport.wordandbrown.com/2019/03/26/trump-administration-asks-court-to-strike-down-entire-aca/. Accessed June 29, 2019.
- Speech: Remarks by Administrator Seema Verma at the CMS National Forum on State Relief and Empowerment Waivers. Centers for Medicare & Medicaid website. Published April 23, 2019. Accessed June 25, 2019.
- Poll: The ACA’s pre-existing condition protections remain popular with the public, including republicans, as legal challenge looms this week. Kaiser Family Foundation website. Published September 5, 2018. Accessed June 25, 2019.
- Statement from President Donald J. Trump on Women’s Health Week. White House website. Issued May 14, 2017. Accessed June 26, 2019.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374:843-852.
- Insurance coverage of contraception. Guttmacher Institute website. Published August 2018. Accessed June 26, 2019.
- Carlin CS, Fertig AR, Dowd BE. Affordable Care Act’s mandate eliminating contraceptive cost sharing influenced choices of women with employer coverage. Health Affairs. 2016;35:1608-1615.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250–255.
- Canestaro W, et al. Implications of employer coverage of contraception: cost-effectiveness analysis of contraception coverage under an employer mandate. Contraception. 2017;95:77-89.
- Simmons A, et al. The Affordable Care Act: Promoting better health for women. Office of the Assistant Secretary for Planning and Evaluation Issue Brief, Department of Health and Human Services. June 14, 2016. Accessed June 25, 2019.
- Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809–1823.
- Becker NV, Polsky D. Women saw large decrease in out-of-pocket spending for contraceptives after ACA mandate removed cost sharing. Health Affairs. 2015;34:1204-1211. Accessed June 25, 2019.
- Becker NV, Polsky D. Women saw large decrease in out-of-pocket spending for contraceptives after ACA mandate removed cost sharing. Health Affairs. 2015;34(7).
- Sonfield A, Hasstedt K, Kavanaugh ML, Anderson R. The social and economic benefits of women’s ability to determine whether and when to have children. New York, NY: Guttmacher Institute; 2013.
- Department of Health and Human Services. Fact sheet: Final rules on religious and moral exemptions and accommodation for coverage of certain preventive services under the Affordable Care Act. November 7, 2018. Accessed June 26, 2019.
- American College of Obstetricians and Gynecologists. Facts are important: Correcting the record on the Administration’s contraceptive coverage roll back rule. October 2017. Accessed June 26, 2019.
- Brief for Physicians for Reproductive Health, American College of Obstetricians and Gynecologists et al. as Amici Curiae Supporting Respondents, Sebelius v. Hobby Lobby, 573 U.S. XXX. 2014. (No. 13-354).
- Early pregnancy loss. FAQ No. 90. American College of Obstetricians and Gynecologists. August 2015.
- Kirby D. Emerging answers 2007: Research findings on programs to reduce teen pregnancy and sexually transmitted diseases. Washington, DC: The National Campaign to Prevent Teen and Unplanned Pregnancy; 2009.
- Meyer JL, Gold MA, Haggerty CL. Advance provision of emergency contraception among adolescent and young adult women: a systematic review of literature. J Pediatr Adolesc Gynecol. 2011;24:2-9.
- Martinez GM and Abma JC. Sexual activity, contraceptive use, and childbearing of teenagers aged 15–19 in the United States. NCHS Data Brief, 2015, No. 209. Hyattsville, MD: National Center for Health Statistics; 2015.
- Martinez GM, Abma JC. Sexual activity, contraceptive use, and childbearing of teenagers aged 15-19 in the United States. NCHS Data Brief. July 2015. Accessed June 26, 2019.
- Lindberg L, Santelli J, Desai S. Understanding the decline in adolescent fertility in the United States, 2007–2012. J Adolesc Health. 2016;59:577-583.
- Minguez M, Santelli JS, Gibson E, et al. Reproductive health impact of a school health center. J Adolesc Health. 2015;56:338-344.
- Knopf JA, Finnie RK, Peng Y, et al. Community Preventive Services Task Force. School-based health centers to advance health equity: a Community Guide systematic review. Am J Preventive Med. 2016;51:114-126.
- Progestin-only hormonal birth control: pill and injection. FAQ No. 86. American College of Obstetricians and Gynecologists. July 2014.
- Combined hormonal birth control: pill, patch, and ring. FAQ No. 185. American College of Obstetricians and Gynecologists. July 2014.
- Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Committee Opinion No. 540. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2012;120:1239-1242.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(No. RR-4):1–66.
- Letter to President Donald J. Trump. October 6, 2017. https://www.aafp.org/dam/AAFP/documents/advocacy/coverage/aca/LT-Group6-President-ContraceptionIFRs-100617.pdf. Accessed June 26, 2019.
- Claxton G, Cox C, Damico A, et al. Pre-existing conditions and medical underwriting in the individual insurance market prior to the ACA. Kaiser Family Foundation website. Published December 12, 2016. Accessed June 25, 2019.
- Norris L. Billions in ACA rebates show 80/20 rule’s impact. HealthInsurance.org website. Published May 10, 2019. Accessed June 25, 2019.
- Patient Protection and Affordable Care Act: Preexisting condition exclusions, lifetime and annual limits, rescissions, and patient protections. Regulations.gov website. Accessed June 25, 2019.
- Jost T. The Senate’s Health Care Freedom Act. Health Affairs website. Updated July 28, 2017. Accessed June 25, 2019.
- Texas v Azar decision. American Medical Association website. Accessed June 25, 2019.
- Keith K. DOJ, plaintiffs file in Texas v United States. Health Affairs website. Published May 2 2019. Accessed June 25, 2019.
- John & Rusty Report. Trump Administration asks court to strike down entire ACA. March 26, 2019. https://jrreport.wordandbrown.com/2019/03/26/trump-administration-asks-court-to-strike-down-entire-aca/. Accessed June 29, 2019.
- Speech: Remarks by Administrator Seema Verma at the CMS National Forum on State Relief and Empowerment Waivers. Centers for Medicare & Medicaid website. Published April 23, 2019. Accessed June 25, 2019.
- Poll: The ACA’s pre-existing condition protections remain popular with the public, including republicans, as legal challenge looms this week. Kaiser Family Foundation website. Published September 5, 2018. Accessed June 25, 2019.
- Statement from President Donald J. Trump on Women’s Health Week. White House website. Issued May 14, 2017. Accessed June 26, 2019.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374:843-852.
- Insurance coverage of contraception. Guttmacher Institute website. Published August 2018. Accessed June 26, 2019.
- Carlin CS, Fertig AR, Dowd BE. Affordable Care Act’s mandate eliminating contraceptive cost sharing influenced choices of women with employer coverage. Health Affairs. 2016;35:1608-1615.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250–255.
- Canestaro W, et al. Implications of employer coverage of contraception: cost-effectiveness analysis of contraception coverage under an employer mandate. Contraception. 2017;95:77-89.
- Simmons A, et al. The Affordable Care Act: Promoting better health for women. Office of the Assistant Secretary for Planning and Evaluation Issue Brief, Department of Health and Human Services. June 14, 2016. Accessed June 25, 2019.
- Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809–1823.
- Becker NV, Polsky D. Women saw large decrease in out-of-pocket spending for contraceptives after ACA mandate removed cost sharing. Health Affairs. 2015;34:1204-1211. Accessed June 25, 2019.
- Becker NV, Polsky D. Women saw large decrease in out-of-pocket spending for contraceptives after ACA mandate removed cost sharing. Health Affairs. 2015;34(7).
- Sonfield A, Hasstedt K, Kavanaugh ML, Anderson R. The social and economic benefits of women’s ability to determine whether and when to have children. New York, NY: Guttmacher Institute; 2013.
- Department of Health and Human Services. Fact sheet: Final rules on religious and moral exemptions and accommodation for coverage of certain preventive services under the Affordable Care Act. November 7, 2018. Accessed June 26, 2019.
- American College of Obstetricians and Gynecologists. Facts are important: Correcting the record on the Administration’s contraceptive coverage roll back rule. October 2017. Accessed June 26, 2019.
- Brief for Physicians for Reproductive Health, American College of Obstetricians and Gynecologists et al. as Amici Curiae Supporting Respondents, Sebelius v. Hobby Lobby, 573 U.S. XXX. 2014. (No. 13-354).
- Early pregnancy loss. FAQ No. 90. American College of Obstetricians and Gynecologists. August 2015.
- Kirby D. Emerging answers 2007: Research findings on programs to reduce teen pregnancy and sexually transmitted diseases. Washington, DC: The National Campaign to Prevent Teen and Unplanned Pregnancy; 2009.
- Meyer JL, Gold MA, Haggerty CL. Advance provision of emergency contraception among adolescent and young adult women: a systematic review of literature. J Pediatr Adolesc Gynecol. 2011;24:2-9.
- Martinez GM and Abma JC. Sexual activity, contraceptive use, and childbearing of teenagers aged 15–19 in the United States. NCHS Data Brief, 2015, No. 209. Hyattsville, MD: National Center for Health Statistics; 2015.
- Martinez GM, Abma JC. Sexual activity, contraceptive use, and childbearing of teenagers aged 15-19 in the United States. NCHS Data Brief. July 2015. Accessed June 26, 2019.
- Lindberg L, Santelli J, Desai S. Understanding the decline in adolescent fertility in the United States, 2007–2012. J Adolesc Health. 2016;59:577-583.
- Minguez M, Santelli JS, Gibson E, et al. Reproductive health impact of a school health center. J Adolesc Health. 2015;56:338-344.
- Knopf JA, Finnie RK, Peng Y, et al. Community Preventive Services Task Force. School-based health centers to advance health equity: a Community Guide systematic review. Am J Preventive Med. 2016;51:114-126.
- Progestin-only hormonal birth control: pill and injection. FAQ No. 86. American College of Obstetricians and Gynecologists. July 2014.
- Combined hormonal birth control: pill, patch, and ring. FAQ No. 185. American College of Obstetricians and Gynecologists. July 2014.
- Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Committee Opinion No. 540. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2012;120:1239-1242.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(No. RR-4):1–66.
- Letter to President Donald J. Trump. October 6, 2017. https://www.aafp.org/dam/AAFP/documents/advocacy/coverage/aca/LT-Group6-President-ContraceptionIFRs-100617.pdf. Accessed June 26, 2019.
Testicular Cancer: Diagnosis and Treatment
Malignant testicular neoplasms can arise from either the germ cells or sex-cord stromal cells, with the former comprising approximately 95% of all testicular cancers (Table 1). Germ cell tumors may contain a single histology or a mix of multiple histologies. For clinical decision making, testicular tumors are categorized as either pure seminoma (no nonseminomatous elements present) or nonseminomatous germ cell tumors (NSGCT). The prevalence of seminoma and NSGCT is roughly equal. If a testicular tumor contains both seminomatous and nonseminomatous components, it is called a mixed germ cell tumor. Because of similarities in biological behavior, the approach to treatment of mixed germ cell tumors is similar to that for NSGCT.

The key points to remember for testicular cancer are:
- With early diagnosis and aggressive multidisciplinary therapy, the overwhelming majority of patients can be cured;
- Specialized care is often critical and affects outcomes; and
- Survivorship, or post-treatment care, is very important for these patients, as they often have lifespan of several decades and a unique set of short- and long-term treatment-related complications.
Developmental Biology and Genetics
The developmental biology of germ cells and germ cell neoplasms is beyond the scope of this review, and interested readers are recommended to refer to pertinent articles on the topic.1,2 A characteristic genetic marker of all germ cell tumors is an isochromosome of the short arm of chromosome 12, i(12p). This is present in testicular tumors regardless of histologic subtype as well as in carcinoma-in-situ. In germ cell tumors without i(12p) karyotype, excess 12p genetic material consisting of repetitive segments has been found, suggesting that this is an early and potentially critical change in oncogenesis.3 Several recent studies have revealed a diverse genomic landscape in testicular cancers, including KIT, KRAS and NRAS mutations in addition to a hyperdiploid karyotype.4,5
Evaluation and Diagnosis
Case Presentation
A 23-year-old Caucasian man presents to a primary care clinic for a pre-employment history and physical exam. He reports testicular pain on the sexually transmitted infections screening questionnaire. On examination, the physician finds a firm, mobile, minimally-tender, 1.5-cm mass in the inferior aspect of left testicle. No contralateral testicular mass or inguinal lymphadenopathy is noted, and a detailed physical exam is otherwise unremarkable. The physician immediately orders an ultrasound of the testicles, which shows a 1.5-cm hypoechoic mass in the inferior aspect of the left testicle, with an unremarkable contralateral testicle. After discussion of the results, the patient is referred a urologic oncologist with expertise in testicular cancer for further care.
The urologic oncologist orders a computed tomography (CT) abdomen and pelvis with and without contrast, which shows a 1.8-cm pathologic-appearing retroperitoneal lymph node at the level of the left renal vein. Chest radiograph with anteroposterior and lateral views is unremarkable. Tumor markers are as follows: beta human chorionic gonadotropin (beta-HCG) 8 mIU/mL (normal range, 0–4 mIU/mL), alpha-fetoprotein (AFP) 2 ng/mL (normal range, 0–8.5 ng/mL), and lactate dehydrogenase (LDH) 195 U/L (normal range, 119–213 U/L).
What is the approach to the initial workup and diagnosis of testicular cancer?
Clinical Presentation and Physical Exam
The majority of testicular cancers are diagnosed on work-up of a nodule or painless swelling of one testicle, usually noted incidentally by the patient. Approximately 30% to 40% of patients complain of a dull ache or heavy sensation in the lower abdomen, perianal area, or scrotum, while acute pain is the presenting symptom in 10%.3
In approximately 10% of patients, the presenting symptom is a result of distant metastatic involvement, such as cough and dyspnea on exertion (pulmonary or mediastinal metastasis), intractable bone pain (skeletal metastasis), intractable back/flank pain, presence of psoas sign or unexplained lower extremity deep vein thrombosis (bulky retroperitoneal metastasis), or central nervous system symptoms (vertebral, spinal or brain metastasis). Constitutional symptoms (unexplained weight loss, anorexia, fatigue) often accompany these symptoms.3
Rarely (5% or less), testicular cancer may present with systemic endocrine symptoms or paraneoplastic symptoms. Gynecomastia is the most common in this category, occurring in approximately 2% of germ cell tumors and more commonly (20%–30%) in Leydig cell tumors of testis.6 Classically, these patients are either 6- to 10-year-old boys with precocious puberty or young men (mid 20s-mid 30s) with a combination of testicular mass, gynecomastia, loss of libido, and impotence. Workup typically reveals increased beta-HCG levels in blood.
Anti-Ma2-antibody-associated limbic encephalitis is the most common (and still quite rare) paraneoplastic complication associated with testicular germ cell tumors. The Ma2 antigen is selectively expressed in the neuronal nucleoli of normal brain tissue and the testicular tumor of the patient. Importantly, in a subset of these patients, the treatment of testicular cancer may result in improvement of symptoms of encephalitis.7
The first step in the diagnosis of testicular neoplasm is a physical exam. This should include a bimanual examination of the scrotal contents, starting with the normal contralateral testis. Normal testicle has a homogeneous texture and consistency, is freely movable, and is separable from the epididymis. Any firm, hard, or fixed mass within the substance of the tunica albuginea should be considered suspicious until proven otherwise. Spread to the epididymis or spermatic cord occurs in 10% to 15% of patients and examination should include these structures as well.3 A comprehensive system-wise examination for features of metastatic spread as discussed above should then be performed. If the patient has cryptorchidism, ultrasound is a mandatory part of the diagnostic workup.
If clinical evaluation suggests a possibility of testicular cancer, the patient must be counseled to undergo an expedited diagnostic workup and specialist evaluation, as a prompt diagnosis and treatment is key to not only improving the likelihood of cure, but also minimizing the treatments needed to achieve it.
Role of Imaging
Scrotal Ultrasound
Scrotal ultrasound is the first imaging modality used in the diagnostic workup of patient with suspected testicular cancer. Bilateral scrotal ultrasound can detect lesions as small as 1 to 2 mm in diameter and help differentiate intratesticular lesions from extrinsic masses. A cystic mass on ultrasound is unlikely to be malignant. Seminomas appear as well-defined hypoechoic lesions without cystic areas, while NSGCTs are typically inhomogeneous with calcifications, cystic areas, and indistinct margins. However, this distinction is not always apparent or reliable. Ultrasound alone is also insufficient for tumor staging.8 For these reasons, a radical inguinal orchiectomy must be pursued for accurate determination of histology and local stage.
If testicular ultrasound shows a suspicious intratesticular mass, the following workup is typically done:
- Measurement of serum tumor markers (beta-HCG, AFP and LDH);
- CT abdomen and pelvis with and without contrast;
- Chest radiograph anteroposterior and lateral views, or CT chest with and without contrast if clinically indicated;
- Any additional focal imaging based on symptoms (eg, magnetic resonance imaging [MRI] scan with and without contrast to evaluate the brain if the patient has CNS symptoms).
CT Scan
CT scan is the preferred imaging modality for staging of testicular cancers, specifically for evaluation of the retroperitoneum, as it is the predominant site for metastases.9 CT scan should encompass the abdomen and pelvis, and contrast-enhanced sequences should be obtained unless medically contraindicated. CT scan of the chest (if not initially done) is compulsory should a CT of abdomen and pelvis and/or a chest radiograph show abnormal findings.
The sensitivity and specificity of CT scans for detection of nodal metastases can vary significantly based on the cutoff. For example, in a series of 70 patients using a cutoff of 10 mm, the sensitivity and specificity of CT scans for patients undergoing retroperitoneal lymph node dissection were 37% and 100%, respectively.10 In the same study, a cutoff of 4 mm increased the sensitivity to 93% and decreased the specificity to 58%. The current general consensus for this cutoff value is 8 to 10 mm measured in the short axis in the transverse (axial) plane.
Approximately 20% of men with clinical stage I testicular cancer (ie, those with non-enlarged retroperitoneal lymph nodes) who do not undergo any adjuvant therapy will have disease relapse in the retroperitoneum, suggesting that they had occult micrometastases that were missed on the initial CT scans.11,12
MRI/Radionuclide Bone Scan/PET Scan
Abdominal or pelvic MRI, whole-body radionuclide bone scan, and positron emission tomography (PET) scans are almost never needed as part of the initial staging workup for testicular cancers due to several limitations, including a high false-negative rate, specifically for the PET scans, and lack of any additional value compared with CT and testicular ultrasound alone.9,13,14 If necessary, these should only be ordered after a multidisciplinary oncology consultation to prevent unnecessary delays in treatment, inappropriate changes to treatment, and unnecessary increases in cost of care.
Tumor Markers, Biopsy, and Staging
What is the role of tumor markers in the management of testicular cancers?
Serum AFP, beta-hCG, and LDH have a well-established role as tumor markers in testicular cancer. The alpha subunit of hCG is shared between multiple pituitary hormones and hence does not serve as a specific marker for testicular cancer. Serum levels of AFP and/or beta-hCG are elevated in approximately 80% percent of men with NSGCTs, even in absence of metastatic spread. On the other hand, serum beta-hCG is elevated in less than 20% and AFP is not elevated in pure seminomas.3
Tumor markers by themselves are not sufficiently sensitive or specific for the diagnosis of testicular cancer, in general, or to differentiate among its subtypes. Despite this limitation, marked elevations in these markers are rarely due to causes other than germ cell tumor. For example, serum beta-hCG concentrations greater than 10,000 mIU/mL occur only in germ cell tumors, trophoblastic differentiation of a primary lung or gastric cancer, gestational trophoblastic disease, or pregnancy. Serum AFP concentrations greater than 10,000 ng/mL occur almost exclusively in germ cell tumors and hepatocellular carcinoma.15
The pattern of marker elevation may play an important role in management of testicular cancer patients. For example, in our practice, several patients have had discordant serum tumor markers and pathology results (eg, elevated AFP with pure seminoma on orchiectomy). One of these patients was treated with adjuvant retroperitoneal lymph node dissection, which confirmed that he had a NSGCT with a seminoma, choriocarcinoma, and teratoma on pathology evaluation of retroperitoneal lymph nodes.
Serum tumor markers have 2 additional critical roles—(1) in the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging16 and International Germ Cell Cancer Collaboration Group (IGCCCG) risk stratification of testicular cancer,17 and (2) in post-treatment disease monitoring.
Is a testicular biopsy necessary for diagnosis?
A testicular biopsy is almost never pursued to confirm the diagnosis of testicular cancer. There is a concern that percutaneous testicular biopsy, which is associated with scrotal skin violation, can adversely affect outcomes due to tumor seeding of scrotal sac or metastatic spread into the inguinal nodes via scrotal skin lymphatics.
Tissue diagnosis is made by radical orchiectomy in a majority of cases. Rarely in our practice, we obtain a biopsy of metastatic lesion for a tissue diagnosis. This is only done in cases where chemotherapy must be started urgently to prevent worsening of complications from metastatic spread. This decision should be made only after a multidisciplinary consultation with urologic and medical oncology teams.
How is testicular cancer staged?
Both seminomatous and nonseminomatous germ cell tumors of the testis are staged using the AJCC/UICC staging system, which incorporates assessments of the primary tumor (T), lymph nodes (N), and distant metastases (M) and serum tumor marker values (S). Details of this staging system are beyond the scope of this review and further information can be obtained through the AJCC website (www.cancerstaging.org). This TNMS staging enables a prognostic assessment and helps with the therapeutic approach.
For patients with advanced germ cell tumors, a risk group classification developed by the IGCCCG is used to classify patients into good-risk, intermediate-risk, and poor-risk category (Table 2). This classification has been extensively validated for the past 2 decades, provides important prognostic information, and helps inform therapy decisions.
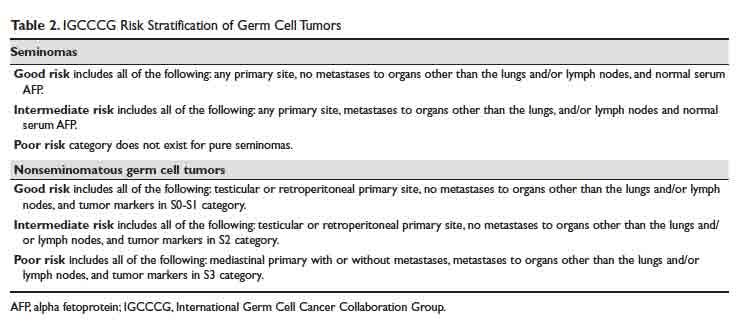
Treatment
Case 1 Continued
Based on the patient’s imaging and biomarker results, the patient undergoes a left radical inguinal orchiectomy. The physician’s operative note mentions that the left testicle was delivered without violation of scrotal integrity. A pathology report shows pure spermatocytic seminoma (unifocal, 1.4 cm size) with negative margins and no evidence of lymphovascular invasion. No lymph nodes are identified in the resection specimen. Post-orchiectomy markers are “negative,” meaning within normal range. After discussions with medical and radiation oncology physicians, the patient opts to pursue active surveillance.
Surgery alone followed by active surveillance is an appropriate option for this patient, as the likelihood of recurrence is low and most recurrences can be subsequently salvaged using treatment options detailed below.
What are the therapeutic options for testicular cancer?
An overview of management for most testicular cancers is presented in Table 3. Note that the actual treatments are significantly more complex and need a comprehensive multidisciplinary consultation (urologic, medical and radiation oncology) at centers with specialized testicular cancer teams, if possible.
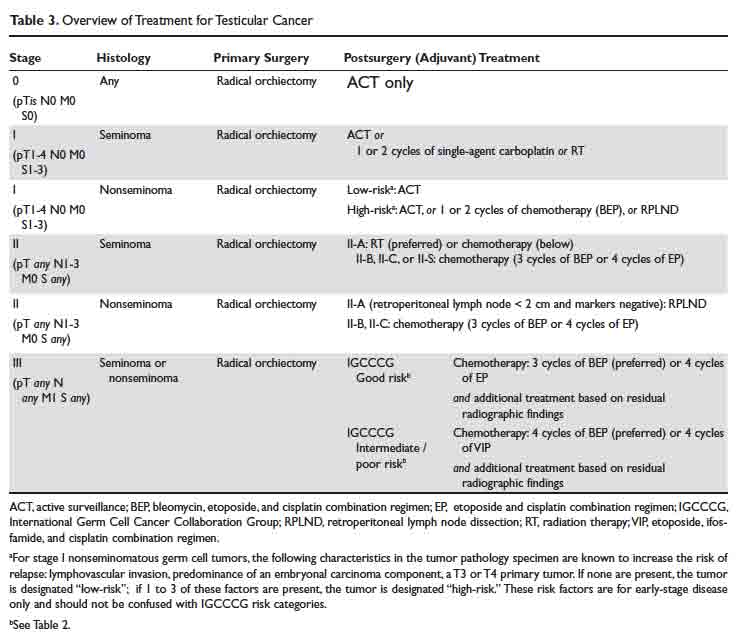
Fertility Preservation
All patients initiating treatment for testicular cancer must be offered options for fertility preservation and consultation with a reproductive health team, if available. At the time of diagnosis, approximately 50% patients have some degree of impairment in spermatogenesis, but with effective fertility preservation, successful pregnancy can occur for as many as 30% to 60% of patients.18,19
Orchiectomy
Radical inguinal orchiectomy with high ligation of the spermatic cord at the level of the internal ring is the procedure of choice for suspected testicular cancer. The goal is to provide a definitive tissue diagnosis and local tumor control with minimal morbidity. It can be performed under general, regional, or local anesthesia. Depending on the complexity and surgical expertise, it can be done in an inpatient or outpatient setting. During the procedure, the testicle is delivered from the scrotum through an incision in the inguinal region and then resected. A testicular prosthesis is usually inserted, with resultant excellent cosmetic and patient satisfaction outcomes.20
Testicular sparing surgery (TSS) has been explored as an alternative to radical orchiectomy but is not considered a standard-of-care option at this time. Small studies have shown evidence for comparable short-term oncologic outcomes in a very select group of patients, generally with solitary tumors < 2 cm in size and solitary testicle. If this is being considered as an option, we recommended obtaining a consultation from a urologist at a high-volume center. For a majority of patients, the value of a TSS is diminished due to excellent anatomic/cosmetic outcomes with a testicular prosthesis implanted during the radical orchiectomy, and resumption of sexual functions by the unaffected contralateral testicle.
Retroperitoneal Lymph Node Dissection
As discussed, conventional cross-sectional imaging has a high false-negative rate for detection of retroperitoneal involvement. General indications for RPLND in various stages and histologies of testicular cancer germ cell tumors are outlined in Table 3. Seminoma tends to most commonly metastasize to retroperitoneum, but RPLND for seminoma is generally reserved for a very small subset of these patients. Patterns of metastases of NSGCT (except choriocarcinoma) are considered to be well-defined. In a series of patients with stage II NSGCTs, left-sided tumors metastasized to the pre- and para-aortic nodes in 88% and 86% of cases, respectively (drainage basin of left testicular vein); and right-sided tumors involved the interaortocaval nodes in 93% of patients.3 Inguinal and pelvic nodal metastases may rarely be seen and should not be used to rule out the diagnosis of testicular cancer.
Choriocarcinoma is an exception to this pattern of retroperitoneal spread, as it tends to have a higher likelihood of hematogenous metastases to distant organs. Compared with NSGCTs, pure seminomas are either localized to the testis (80% of all cases) or limited to the retroperitoneum (an additional 15% of all cases) at presentation.3
Depending on the case and expertise of the surgical team, robotic or open RPLND can be performed.21 Regardless of the approach used, RPLND remains a technically challenging surgery. The retroperitoneal “landing zone” lymph nodes lie in close proximity to, and are often densely adherent to, the abdominal great vessels. Complication rates vary widely in the reported literature, but can be as high as 50%.21-23 As detailed in Table 2, the number and size of involved retroperitoneal lymph nodes have prognostic importance.
In summary, RPLND is considered to be a viable option for a subset of early-stage NSGCT (T1-3, N0-2, M0) and for those with advanced seminoma, NSGCT, or mixed germ cell tumors with post-chemotherapy residual disease.
Systemic Chemotherapy
Except for the single-agent carboplatin, most chemotherapy regimens used to treat testicular cancer are combinations of 2 or more chemotherapy agents. For this review, we will focus on the 3 most commonly used regimens: bleomycin, etoposide, and cisplatin (BEP), etoposide and cisplatin (EP), and etoposide, ifosfamide, and cisplatin (VIP).
The core principles of testicular cancer chemotherapy are:
- Minimize dose interruptions, delays, or reductions, as these adversely affect outcomes without clearly improving side effect profile;
- Do not substitute carboplatin for cisplatin in combination regimens because carboplatin-containing combination regimens have been shown to result in significantly poorer outcomes in multiple trials of adults with germ cell tumors;24-27 and
- Give myeloid growth factor support, if necessary.
BEP
The standard BEP regimen comprises a 21-day cycle with bleomycin 30 units on days 1, 8, and 15; etoposide 100 mg/m2 on days 1 to 5; and cisplatin 20 mg/m2 on days 1 to 5. Number of cycles varies based on histology and stage (Table 3). A strong justification to maintain treatment intensity comes from the Australian and New Zealand Germ Cell Trial Group trial. In this study, 166 men were randomly assigned to treatment using 3 cycles of standard BEP or 4 cycles of a modified BEP regimen (bleomycin 30 units day 1; etoposide 120 mg/m2 days 1 to 3; cisplatin 100 mg/m2 day 1) every 21 days. This trial was stopped at interim analyses because the modified BEP arm was inferior to the standard BEP arm. With a median follow-up of 8.5 years, 8-year overall survival was 92% with standard BEP and 83% with modified BEP (P = 0.037).28
Bleomycin used in the BEP regimen has been associated with uncommon but potentially fatal pulmonary toxicity that tends to present as interstitial pneumonitis, which may ultimately progress to fibrosis or bronchiolitis obliterans with organizing pneumonia.29 This has led to evaluation of EP as an alternative to BEP.
EP
The standard EP regimen consists of a 21-day cycle with etoposide 100 mg/m2 on days 1 to 5, and cisplatin 20 mg/m2 on days 1 to 5. Due to conflicting data from multiple randomized trials, there is considerable debate in the field regarding whether 4 cycles of EP are equivalent to 3 cycles of BEP.30,31 The benefit of the EP regimen is that it avoids the higher rates of pulmonary, cutaneous, and neurologic toxicities associated bleomycin, but it does result in the patient receiving an up to 33% higher cumulative dose of cisplatin and etoposide due to the extra cycle of treatment. This has important implications in terms of tolerability and side effects, including delayed toxicities such as second malignancies, which increase with a higher cumulative dose of these agents (etoposide in particular).
VIP
The standard VIP regimen consists of a 21-day cycle with etoposide 75 mg/m2 on days 1 to 5; cisplatin 20 mg/m2 on days 1 to 5; ifosfamide 1200 mg/m2 on days 1 to 5; and mesna 120 mg/m2 IV push on day 1 followed by 1200 mg/m2 on days 1 to 5. For patients with intermediate- or poor-risk disease, 4 cycles of VIP has demonstrated comparable efficacy but higher rates of hematologic toxicities compared with 4 cycles of BEP.32-34 It remains an option for upfront treatment of patients who are not good candidates for a bleomycin-based regimen, and for patients who need salvage chemotherapy.
Adverse Effects of Chemotherapy
Acute and late chemotherapy toxicities vary significantly between regimens depending on the chemotherapy drugs used. Bleomycin-induced pneumonitis may masquerade as a “pneumonia,” which can lead to a delay in diagnosis or institution of treatment, as well as institution of an incorrect treatment (for example, there is a concern that bleomycin toxicity can be precipitated or worsened by a high fraction of inspired oxygen). Chemotherapy-associated neutropenia tends to occur a few days (7–10 days) after initiation of chemotherapy, and neutrophil counts recover without intervention in most patients after an additional 7 to 10 days. Myeloid growth factor support (eg, filgrastim, pegfilgrastim) can be given to patients either prophylactically (if they had an episode of febrile or prolonged neutropenia with the preceding cycle) or secondarily if they present with neutropenia (an absolute neutrophil count ≤ 500 cells/µL) with fever or active infection. Such interventions tend to shorten the duration of neutropenia but does not affect overall survival. Patients with asymptomatic neutropenia do not benefit from growth factor use.35
Stem Cell Transplant
Autologous stem cell transplant (SCT) is the preferred type of SCT for patients with testicular cancer and involves delivery of high doses of chemotherapy followed by infusion of patient-derived myeloid stem cells. While the details of this treatment are outside the scope of this review, decades of experience has shown that this is an effective curative option for a subset of patients with poor prognosis, such as those with platinum-refractory or relapsed disease.36
Clinical Trials
Due to excellent clinical outcomes with front-line therapy, as described, and the relatively low incidence of testicular and other germ cell tumors, clinical trial options for patients with testicular cancer are limited. The TIGER trial is an ongoing international, randomized, phase 3 trial comparing conventional TIP (paclitaxel, ifosfamide, and cisplatin) chemotherapy with high-dose chemotherapy with SCT as the first salvage treatment for relapsed/refractory germ cell tumors (NCT02375204). It is enrolling at multiple centers in the United States and results are expected in 2022. At least 2 ongoing trials are evaluating the role of immunotherapy in patients with relapsed/refractory germ cell tumors (NCT03081923 and NCT03726281). Cluster of differentiation antigen-30 (CD30) has emerged as a potential target of interest in germ cell tumors, and brentuximab vedotin, an anti-CD30 monoclonal antibody, is undergoing evaluation in a phase 2 trial of CD-30–expressing germ cell tumors (NCT01851200). This trial has completed enrollment and results are expected to be available in late 2019 or early 2020.
When possible, patients with relapsed/refractory germ cell tumors should be referred to centers of excellence with access to either testicular/germ-cell tumor specific clinical trials or phase 1 clinical trials.
Radiation Therapy
Adjuvant radiation to the retroperitoneum has a role in the management of stage I and IIA seminomas (Table 3). In a randomized noninferiority trial of radiation therapy versus single-dose carboplatin in stage I seminoma patients, 5-year recurrence-free survival was comparable at approximately 95% in either arm.37,38 In a retrospective database review of 2437 patients receiving either radiation therapy or multi-agent chemotherapy for stage II seminoma, the 5-year survival exceeded 90% in both treatment groups.39 Typically, a total of 30 to 36 Gy of radiation is delivered to para-aortic and ipsilateral external iliac lymph nodes (“dog-leg” field), followed by an optional boost to the involved nodal areas.40 Radiation is associated with acute side effects such as fatigue, gastrointestinal effects, myelosuppression as well as late side effects such as second cancers in the irradiated field (eg, sarcoma, bladder cancer).
Evaluation of Treatment Response
Monitoring of treatment response is fairly straightforward for patients with testicular cancer. Our practice is the following:
- Measure tumor markers on day 1 of each chemotherapy cycle and 3 to 4 weeks after completion of treatment.
- CT of the chest, abdomen, and pelvis with intravenous contrast prior to chemotherapy and upon completion of chemotherapy. Interim imaging is only needed for a small subset of patients with additional clinical indications (eg, new symptoms, lack of improvement in existing symptoms).
- For patients with stage II/III seminoma who have a residual mass ≥ 3 cm on post-treatment CT scan, a PET-CT scan is indicated 6 to 8 weeks after the completion of chemotherapy to determine the need for further treatment.
Active Surveillance
Because testicular cancer has high cure rates even when patients have disease relapse after primary therapy, and additional therapies have significant short- and long-term side effects in these generally young patients, active surveillance is a critical option used in the management of testicular cancer.41
Patients must be counseled that active surveillance is a form of treatment itself in that it involves close clinical and radiographic monitoring. Because there is a risk of disease relapse, patients opting to undergo active surveillance must fully understand the risks of disease recurrence and be willing to abide by the recommended follow-up schedule.
Surveillance is necessary for a minimum of 5 years and possibly 10 years following orchiectomy, and most relapses tend to occur within the first 2 years. Late relapses such as skeletal metastatic disease from seminoma have been reported to occur more than 15 years after orchiectomy, but are generally rare and unpredictable.
The general guidelines for active surveillance are as follows:
For patients with seminoma, history and physical exam and tumor marker assessment should be performed every 3 to 6 months for the first year, then every 6 to 12 months in years 2 and 3, and then annually. CT of the abdomen and pelvis should be done at 3, 6, and 12 months, every 6 to 12 months in years 2 and 3, and then every 12 to 24 months in years 4 and 5. A chest radiograph is performed only if clinically indicated, as the likelihood of distant metastatic recurrence is low.
For patients with nonseminoma, history and physical exam and tumor markers assessment should be performed every 2 to 3 months for first 2 years, every 4 to 6 months in years 3 and 4, and then annually. CT of the abdomen and pelvis should be obtained every 4 to 6 months in year 1, gradually decreasing to annually in year 3 or 4. Chest radiograph is indicated at 4 and 12 months and annually thereafter for stage IA disease. For those with stage IB disease, chest radiograph is indicated every 2 months during the first year and then gradually decreasing to annually beginning year 5.
These recommendations are expected to change over time, and treating physicians are recommended to exercise discretion and consider the patient and tumor characteristics to develop the optimal surveillance plan.
Conclusion
Testicular cancer is the most common cancer afflicting young men. Prompt diagnostic workup initiated in a primary care or hospital setting followed by a referral to a multidisciplinary team of urologists, medical oncologists, and radiation oncologists enables cure in a majority of patients. For patients with stage I seminoma, a radical inguinal orchiectomy followed by active surveillance may offer the best long-term outcome with minimal side effects. For patients with relapsed/refractory testicular cancers, clinical trial participation is strongly encouraged. Patients with a history of testicular cancer benefit from robust survivorship care tailored to their prior therapies. This can be safely delivered through their primary care providers in collaboration with the multidisciplinary oncology team.
1. van der Zwan YG, Biermann K, Wolffenbuttel KP, et al. Gonadal maldevelopment as risk factor for germ cell cancer: towards a clinical decision model. Eur Urol. 2015; 67:692–701.
2. Pierce JL, Frazier AL, Amatruda JF. Pediatric germ cell tumors: a developmental perspective. Adv Urol. 2018 Feb 4;2018.
3. Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242-253.
4. Pyle LC, Nathanson KL. Genetic changes associated with testicular cancer susceptibility. Semin Oncol. 2016;43:575-581.
5. Shen H, Shih J, Hollern DP, et al. Integrated molecular characterization of testicular germ cell tumors. Cell Rep. 2018;23:3392-3406.
6. Barry M, Rao A, Lauer R. Sex cord-stromal tumors of the testis. In: Pagliaro L, ed. Rare Genitourinary Tumors. Cham: Springer International Publishing; 2016: 231-251.
7. Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain J Neurol. 2004;127:1831-1844.
8. Coursey Moreno C, Small WC, Camacho JC, et al. Testicular tumors: what radiologists need to know—differential diagnosis, staging, and management. RadioGraphics. 2015;35:400-415.
9. Kreydin EI, Barrisford GW, Feldman AS, Preston MA. Testicular cancer: what the radiologist needs to know. Am J Roentgenol. 2013;200:1215-1225.
10. Hilton S, Herr HW, Teitcher JB, et al. CT detection of retroperitoneal lymph node metastases in patients with clinical stage I testicular nonseminomatous germ cell cancer: assessment of size and distribution criteria. Am J Roentgenol. 1997;169:521-525.
11. Thompson PI, Nixon J, Harvey VJ. Disease relapse in patients with stage I nonseminomatous germ cell tumor of the testis on active surveillance. J Clin Oncol. 1988;6:1597-1603.
12. Nicolai N, Pizzocaro G. A surveillance study of clinical stage I nonseminomatous germ cell tumors of the testis: 10-year followup. J Urol. 1995;154:1045-1049.
13. Kok HK, Leong S, Torreggiani WC. Is magnetic resonance imaging comparable with computed tomography in the diagnosis of retroperitoneal metastasis in patients with testicular cancer? Can Assoc Radiol J. 2014;65:196-198.
14. Hale GR, Teplitsky S, Truong H, et al. Lymph node imaging in testicular cancer. Transl Androl Urol. 2018;7:864-874.
15. Honecker F, Aparicio J, Berney D, et al. ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol. 2018;29:1658-1686.
16. Paner GP, Stadler WM, Hansel DE, et al. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur Urol. 2018;73:560-569.
17. International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15:594-603.
18. Lopategui DM, Ibrahim E, Aballa TC, et al. Effect of a formal oncofertility program on fertility preservation rates-first year experience. Transl Androl Urol. 2018;7:S271-S275.
19. Moody JA, Ahmed K, Horsfield C, et al. Fertility preservation in testicular cancer - predictors of spermatogenesis. BJU Int. 2018;122:236-242.
20. Dieckmann KP, Anheuser P, Schmidt S, et al. Testicular prostheses in patients with testicular cancer - acceptance rate and patient satisfaction. BMC Urol. 2015;15:16.
21. Schwen ZR, Gupta M, Pierorazio PM. A review of outcomes and technique for the robotic-assisted laparoscopic retroperitoneal lymph node dissection for testicular cancer. Adv Urol. 2018;2146080.
22. Singh P, Yadav S, Mahapatra S, Seth A. Outcomes following retroperitoneal lymph node dissection in postchemotherapy residual masses in advanced testicular germ cell tumors. Indian J Urol. 2016;32:40-44.
23. Heidenreich A, Thüer D, Polyakov S. Postchemotherapy retroperitoneal lymph node dissection in advanced germ cell tumours of the testis. Eur Urol. 2008;53:260-272.
24. Bajorin DF, Sarosdy MF, Pfister DG, et al. Randomized trial of etoposide and cisplatin versus etoposide and carboplatin in patients with good-risk germ cell tumors: a multiinstitutional study. J Clin Oncol. 1993;11:598-606.
25. Bokemeyer C, Köhrmann O, Tischler J, et al. A randomized trial of cisplatin, etoposide and bleomycin (PEB) versus carboplatin, etoposide and bleomycin (CEB) for patients with “good-risk” metastatic non-seminomatous germ cell tumors. Ann Oncol. 1996;7:1015-1021.
26. Horwich A, Sleijfer DT, Fosså SD, et al. Randomized trial of bleomycin, etoposide, and cisplatin compared with bleomycin, etoposide, and carboplatin in good-prognosis metastatic nonseminomatous germ cell cancer: a Multiinstitutional Medical Research Council/European Organization for Research and Treatment of Cancer Trial. J Clin Oncol. 1997;15:1844-1852.
27. Shaikh F, Nathan PC, Hale J, et al. Is there a role for carboplatin in the treatment of malignant germ cell tumors? A systematic review of adult and pediatric trials. Pediatr Blood Cancer. 2013;60:587-592.
28. Grimison PS, Stockler MR, Thomson DB, et al. Comparison of two standard chemotherapy regimens for good-prognosis germ cell tumors: updated analysis of a randomized trial. J Natl Cancer Inst. 2010;102:1253-1262.
29. Reinert T, da Rocha Baldotto CS, Nunes FAP, de Souza Scheliga AA. Bleomycin-induced lung injury. J Cancer Res. 2013;480608.
30. Jones RH, Vasey PA. Part II: Testicular cancer—management of advanced disease. Lancet Oncol. 2003;4:738-747.
31. Jankilevich G. BEP versus EP for treatment of metastatic germ-cell tumours. Lancet Oncol. 2004;5, 146.
32. Nichols CR, Catalano PJ, Crawford ED, et al. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: an Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B Study. J Clin Oncol. 1998;16:12871293.
33. Hinton S, Catalano PJ, Einhorn LH, et al. Cisplatin, etoposide and either bleomycin or ifosfamide in the treatment of disseminated germ cell tumors: final analysis of an intergroup trial. Cancer. 2003;97: 1869-1875.
34. de Wit R, Stoter G, Sleijfer DT, et al. Four cycles of BEP vs four cycles of VIP in patients with intermediate-prognosis metastatic testicular non-seminoma: a randomized study of the EORTC Genitourinary Tract Cancer Cooperative Group. European Organization for Research and Treatment of Cancer. Br J Cancer. 1998;78:828-832.
35. Mhaskar R, Clark OA, Lyman G, et al. Colony-stimulating factors for chemotherapy-induced febrile neutropenia. Cochrane Database Syst. Rev. 2014;CD003039.
36. Adra N, Abonour R, Althouse SK, et al. High-dose chemotherapy and autologous peripheral-blood stem-cell transplantation for relapsed metastatic germ cell tumors: The Indiana University experience. J Clin Oncol. 2017;35:1096-1102.
37. Oliver RT, Mason MD, Mead GM, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005;366:293-300.
38. Oliver RT, Mead GM, Rustin GJ, et al. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214). J Clin Oncol. 2011;29:957-962.
39. Glaser SM, Vargo JA, Balasubramani GK, Beriwal S. Stage II testicular seminoma: patterns of care and survival by treatment strategy. Clin Oncol. 2016;28:513-521.
40. Boujelbene N, Cosinschi A, Boujelbene N, et al. Pure seminoma: A review and update. Radiat Oncol. 2011;6:90.
41. Nichols CR, Roth B, Albers P, et al. Active surveillance is the preferred approach to clinical stage I testicular cancer. J Clin Oncol. 2013;31;3490-3493.
Malignant testicular neoplasms can arise from either the germ cells or sex-cord stromal cells, with the former comprising approximately 95% of all testicular cancers (Table 1). Germ cell tumors may contain a single histology or a mix of multiple histologies. For clinical decision making, testicular tumors are categorized as either pure seminoma (no nonseminomatous elements present) or nonseminomatous germ cell tumors (NSGCT). The prevalence of seminoma and NSGCT is roughly equal. If a testicular tumor contains both seminomatous and nonseminomatous components, it is called a mixed germ cell tumor. Because of similarities in biological behavior, the approach to treatment of mixed germ cell tumors is similar to that for NSGCT.

The key points to remember for testicular cancer are:
- With early diagnosis and aggressive multidisciplinary therapy, the overwhelming majority of patients can be cured;
- Specialized care is often critical and affects outcomes; and
- Survivorship, or post-treatment care, is very important for these patients, as they often have lifespan of several decades and a unique set of short- and long-term treatment-related complications.
Developmental Biology and Genetics
The developmental biology of germ cells and germ cell neoplasms is beyond the scope of this review, and interested readers are recommended to refer to pertinent articles on the topic.1,2 A characteristic genetic marker of all germ cell tumors is an isochromosome of the short arm of chromosome 12, i(12p). This is present in testicular tumors regardless of histologic subtype as well as in carcinoma-in-situ. In germ cell tumors without i(12p) karyotype, excess 12p genetic material consisting of repetitive segments has been found, suggesting that this is an early and potentially critical change in oncogenesis.3 Several recent studies have revealed a diverse genomic landscape in testicular cancers, including KIT, KRAS and NRAS mutations in addition to a hyperdiploid karyotype.4,5
Evaluation and Diagnosis
Case Presentation
A 23-year-old Caucasian man presents to a primary care clinic for a pre-employment history and physical exam. He reports testicular pain on the sexually transmitted infections screening questionnaire. On examination, the physician finds a firm, mobile, minimally-tender, 1.5-cm mass in the inferior aspect of left testicle. No contralateral testicular mass or inguinal lymphadenopathy is noted, and a detailed physical exam is otherwise unremarkable. The physician immediately orders an ultrasound of the testicles, which shows a 1.5-cm hypoechoic mass in the inferior aspect of the left testicle, with an unremarkable contralateral testicle. After discussion of the results, the patient is referred a urologic oncologist with expertise in testicular cancer for further care.
The urologic oncologist orders a computed tomography (CT) abdomen and pelvis with and without contrast, which shows a 1.8-cm pathologic-appearing retroperitoneal lymph node at the level of the left renal vein. Chest radiograph with anteroposterior and lateral views is unremarkable. Tumor markers are as follows: beta human chorionic gonadotropin (beta-HCG) 8 mIU/mL (normal range, 0–4 mIU/mL), alpha-fetoprotein (AFP) 2 ng/mL (normal range, 0–8.5 ng/mL), and lactate dehydrogenase (LDH) 195 U/L (normal range, 119–213 U/L).
What is the approach to the initial workup and diagnosis of testicular cancer?
Clinical Presentation and Physical Exam
The majority of testicular cancers are diagnosed on work-up of a nodule or painless swelling of one testicle, usually noted incidentally by the patient. Approximately 30% to 40% of patients complain of a dull ache or heavy sensation in the lower abdomen, perianal area, or scrotum, while acute pain is the presenting symptom in 10%.3
In approximately 10% of patients, the presenting symptom is a result of distant metastatic involvement, such as cough and dyspnea on exertion (pulmonary or mediastinal metastasis), intractable bone pain (skeletal metastasis), intractable back/flank pain, presence of psoas sign or unexplained lower extremity deep vein thrombosis (bulky retroperitoneal metastasis), or central nervous system symptoms (vertebral, spinal or brain metastasis). Constitutional symptoms (unexplained weight loss, anorexia, fatigue) often accompany these symptoms.3
Rarely (5% or less), testicular cancer may present with systemic endocrine symptoms or paraneoplastic symptoms. Gynecomastia is the most common in this category, occurring in approximately 2% of germ cell tumors and more commonly (20%–30%) in Leydig cell tumors of testis.6 Classically, these patients are either 6- to 10-year-old boys with precocious puberty or young men (mid 20s-mid 30s) with a combination of testicular mass, gynecomastia, loss of libido, and impotence. Workup typically reveals increased beta-HCG levels in blood.
Anti-Ma2-antibody-associated limbic encephalitis is the most common (and still quite rare) paraneoplastic complication associated with testicular germ cell tumors. The Ma2 antigen is selectively expressed in the neuronal nucleoli of normal brain tissue and the testicular tumor of the patient. Importantly, in a subset of these patients, the treatment of testicular cancer may result in improvement of symptoms of encephalitis.7
The first step in the diagnosis of testicular neoplasm is a physical exam. This should include a bimanual examination of the scrotal contents, starting with the normal contralateral testis. Normal testicle has a homogeneous texture and consistency, is freely movable, and is separable from the epididymis. Any firm, hard, or fixed mass within the substance of the tunica albuginea should be considered suspicious until proven otherwise. Spread to the epididymis or spermatic cord occurs in 10% to 15% of patients and examination should include these structures as well.3 A comprehensive system-wise examination for features of metastatic spread as discussed above should then be performed. If the patient has cryptorchidism, ultrasound is a mandatory part of the diagnostic workup.
If clinical evaluation suggests a possibility of testicular cancer, the patient must be counseled to undergo an expedited diagnostic workup and specialist evaluation, as a prompt diagnosis and treatment is key to not only improving the likelihood of cure, but also minimizing the treatments needed to achieve it.
Role of Imaging
Scrotal Ultrasound
Scrotal ultrasound is the first imaging modality used in the diagnostic workup of patient with suspected testicular cancer. Bilateral scrotal ultrasound can detect lesions as small as 1 to 2 mm in diameter and help differentiate intratesticular lesions from extrinsic masses. A cystic mass on ultrasound is unlikely to be malignant. Seminomas appear as well-defined hypoechoic lesions without cystic areas, while NSGCTs are typically inhomogeneous with calcifications, cystic areas, and indistinct margins. However, this distinction is not always apparent or reliable. Ultrasound alone is also insufficient for tumor staging.8 For these reasons, a radical inguinal orchiectomy must be pursued for accurate determination of histology and local stage.
If testicular ultrasound shows a suspicious intratesticular mass, the following workup is typically done:
- Measurement of serum tumor markers (beta-HCG, AFP and LDH);
- CT abdomen and pelvis with and without contrast;
- Chest radiograph anteroposterior and lateral views, or CT chest with and without contrast if clinically indicated;
- Any additional focal imaging based on symptoms (eg, magnetic resonance imaging [MRI] scan with and without contrast to evaluate the brain if the patient has CNS symptoms).
CT Scan
CT scan is the preferred imaging modality for staging of testicular cancers, specifically for evaluation of the retroperitoneum, as it is the predominant site for metastases.9 CT scan should encompass the abdomen and pelvis, and contrast-enhanced sequences should be obtained unless medically contraindicated. CT scan of the chest (if not initially done) is compulsory should a CT of abdomen and pelvis and/or a chest radiograph show abnormal findings.
The sensitivity and specificity of CT scans for detection of nodal metastases can vary significantly based on the cutoff. For example, in a series of 70 patients using a cutoff of 10 mm, the sensitivity and specificity of CT scans for patients undergoing retroperitoneal lymph node dissection were 37% and 100%, respectively.10 In the same study, a cutoff of 4 mm increased the sensitivity to 93% and decreased the specificity to 58%. The current general consensus for this cutoff value is 8 to 10 mm measured in the short axis in the transverse (axial) plane.
Approximately 20% of men with clinical stage I testicular cancer (ie, those with non-enlarged retroperitoneal lymph nodes) who do not undergo any adjuvant therapy will have disease relapse in the retroperitoneum, suggesting that they had occult micrometastases that were missed on the initial CT scans.11,12
MRI/Radionuclide Bone Scan/PET Scan
Abdominal or pelvic MRI, whole-body radionuclide bone scan, and positron emission tomography (PET) scans are almost never needed as part of the initial staging workup for testicular cancers due to several limitations, including a high false-negative rate, specifically for the PET scans, and lack of any additional value compared with CT and testicular ultrasound alone.9,13,14 If necessary, these should only be ordered after a multidisciplinary oncology consultation to prevent unnecessary delays in treatment, inappropriate changes to treatment, and unnecessary increases in cost of care.
Tumor Markers, Biopsy, and Staging
What is the role of tumor markers in the management of testicular cancers?
Serum AFP, beta-hCG, and LDH have a well-established role as tumor markers in testicular cancer. The alpha subunit of hCG is shared between multiple pituitary hormones and hence does not serve as a specific marker for testicular cancer. Serum levels of AFP and/or beta-hCG are elevated in approximately 80% percent of men with NSGCTs, even in absence of metastatic spread. On the other hand, serum beta-hCG is elevated in less than 20% and AFP is not elevated in pure seminomas.3
Tumor markers by themselves are not sufficiently sensitive or specific for the diagnosis of testicular cancer, in general, or to differentiate among its subtypes. Despite this limitation, marked elevations in these markers are rarely due to causes other than germ cell tumor. For example, serum beta-hCG concentrations greater than 10,000 mIU/mL occur only in germ cell tumors, trophoblastic differentiation of a primary lung or gastric cancer, gestational trophoblastic disease, or pregnancy. Serum AFP concentrations greater than 10,000 ng/mL occur almost exclusively in germ cell tumors and hepatocellular carcinoma.15
The pattern of marker elevation may play an important role in management of testicular cancer patients. For example, in our practice, several patients have had discordant serum tumor markers and pathology results (eg, elevated AFP with pure seminoma on orchiectomy). One of these patients was treated with adjuvant retroperitoneal lymph node dissection, which confirmed that he had a NSGCT with a seminoma, choriocarcinoma, and teratoma on pathology evaluation of retroperitoneal lymph nodes.
Serum tumor markers have 2 additional critical roles—(1) in the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging16 and International Germ Cell Cancer Collaboration Group (IGCCCG) risk stratification of testicular cancer,17 and (2) in post-treatment disease monitoring.
Is a testicular biopsy necessary for diagnosis?
A testicular biopsy is almost never pursued to confirm the diagnosis of testicular cancer. There is a concern that percutaneous testicular biopsy, which is associated with scrotal skin violation, can adversely affect outcomes due to tumor seeding of scrotal sac or metastatic spread into the inguinal nodes via scrotal skin lymphatics.
Tissue diagnosis is made by radical orchiectomy in a majority of cases. Rarely in our practice, we obtain a biopsy of metastatic lesion for a tissue diagnosis. This is only done in cases where chemotherapy must be started urgently to prevent worsening of complications from metastatic spread. This decision should be made only after a multidisciplinary consultation with urologic and medical oncology teams.
How is testicular cancer staged?
Both seminomatous and nonseminomatous germ cell tumors of the testis are staged using the AJCC/UICC staging system, which incorporates assessments of the primary tumor (T), lymph nodes (N), and distant metastases (M) and serum tumor marker values (S). Details of this staging system are beyond the scope of this review and further information can be obtained through the AJCC website (www.cancerstaging.org). This TNMS staging enables a prognostic assessment and helps with the therapeutic approach.
For patients with advanced germ cell tumors, a risk group classification developed by the IGCCCG is used to classify patients into good-risk, intermediate-risk, and poor-risk category (Table 2). This classification has been extensively validated for the past 2 decades, provides important prognostic information, and helps inform therapy decisions.

Treatment
Case 1 Continued
Based on the patient’s imaging and biomarker results, the patient undergoes a left radical inguinal orchiectomy. The physician’s operative note mentions that the left testicle was delivered without violation of scrotal integrity. A pathology report shows pure spermatocytic seminoma (unifocal, 1.4 cm size) with negative margins and no evidence of lymphovascular invasion. No lymph nodes are identified in the resection specimen. Post-orchiectomy markers are “negative,” meaning within normal range. After discussions with medical and radiation oncology physicians, the patient opts to pursue active surveillance.
Surgery alone followed by active surveillance is an appropriate option for this patient, as the likelihood of recurrence is low and most recurrences can be subsequently salvaged using treatment options detailed below.
What are the therapeutic options for testicular cancer?
An overview of management for most testicular cancers is presented in Table 3. Note that the actual treatments are significantly more complex and need a comprehensive multidisciplinary consultation (urologic, medical and radiation oncology) at centers with specialized testicular cancer teams, if possible.

Fertility Preservation
All patients initiating treatment for testicular cancer must be offered options for fertility preservation and consultation with a reproductive health team, if available. At the time of diagnosis, approximately 50% patients have some degree of impairment in spermatogenesis, but with effective fertility preservation, successful pregnancy can occur for as many as 30% to 60% of patients.18,19
Orchiectomy
Radical inguinal orchiectomy with high ligation of the spermatic cord at the level of the internal ring is the procedure of choice for suspected testicular cancer. The goal is to provide a definitive tissue diagnosis and local tumor control with minimal morbidity. It can be performed under general, regional, or local anesthesia. Depending on the complexity and surgical expertise, it can be done in an inpatient or outpatient setting. During the procedure, the testicle is delivered from the scrotum through an incision in the inguinal region and then resected. A testicular prosthesis is usually inserted, with resultant excellent cosmetic and patient satisfaction outcomes.20
Testicular sparing surgery (TSS) has been explored as an alternative to radical orchiectomy but is not considered a standard-of-care option at this time. Small studies have shown evidence for comparable short-term oncologic outcomes in a very select group of patients, generally with solitary tumors < 2 cm in size and solitary testicle. If this is being considered as an option, we recommended obtaining a consultation from a urologist at a high-volume center. For a majority of patients, the value of a TSS is diminished due to excellent anatomic/cosmetic outcomes with a testicular prosthesis implanted during the radical orchiectomy, and resumption of sexual functions by the unaffected contralateral testicle.
Retroperitoneal Lymph Node Dissection
As discussed, conventional cross-sectional imaging has a high false-negative rate for detection of retroperitoneal involvement. General indications for RPLND in various stages and histologies of testicular cancer germ cell tumors are outlined in Table 3. Seminoma tends to most commonly metastasize to retroperitoneum, but RPLND for seminoma is generally reserved for a very small subset of these patients. Patterns of metastases of NSGCT (except choriocarcinoma) are considered to be well-defined. In a series of patients with stage II NSGCTs, left-sided tumors metastasized to the pre- and para-aortic nodes in 88% and 86% of cases, respectively (drainage basin of left testicular vein); and right-sided tumors involved the interaortocaval nodes in 93% of patients.3 Inguinal and pelvic nodal metastases may rarely be seen and should not be used to rule out the diagnosis of testicular cancer.
Choriocarcinoma is an exception to this pattern of retroperitoneal spread, as it tends to have a higher likelihood of hematogenous metastases to distant organs. Compared with NSGCTs, pure seminomas are either localized to the testis (80% of all cases) or limited to the retroperitoneum (an additional 15% of all cases) at presentation.3
Depending on the case and expertise of the surgical team, robotic or open RPLND can be performed.21 Regardless of the approach used, RPLND remains a technically challenging surgery. The retroperitoneal “landing zone” lymph nodes lie in close proximity to, and are often densely adherent to, the abdominal great vessels. Complication rates vary widely in the reported literature, but can be as high as 50%.21-23 As detailed in Table 2, the number and size of involved retroperitoneal lymph nodes have prognostic importance.
In summary, RPLND is considered to be a viable option for a subset of early-stage NSGCT (T1-3, N0-2, M0) and for those with advanced seminoma, NSGCT, or mixed germ cell tumors with post-chemotherapy residual disease.
Systemic Chemotherapy
Except for the single-agent carboplatin, most chemotherapy regimens used to treat testicular cancer are combinations of 2 or more chemotherapy agents. For this review, we will focus on the 3 most commonly used regimens: bleomycin, etoposide, and cisplatin (BEP), etoposide and cisplatin (EP), and etoposide, ifosfamide, and cisplatin (VIP).
The core principles of testicular cancer chemotherapy are:
- Minimize dose interruptions, delays, or reductions, as these adversely affect outcomes without clearly improving side effect profile;
- Do not substitute carboplatin for cisplatin in combination regimens because carboplatin-containing combination regimens have been shown to result in significantly poorer outcomes in multiple trials of adults with germ cell tumors;24-27 and
- Give myeloid growth factor support, if necessary.
BEP
The standard BEP regimen comprises a 21-day cycle with bleomycin 30 units on days 1, 8, and 15; etoposide 100 mg/m2 on days 1 to 5; and cisplatin 20 mg/m2 on days 1 to 5. Number of cycles varies based on histology and stage (Table 3). A strong justification to maintain treatment intensity comes from the Australian and New Zealand Germ Cell Trial Group trial. In this study, 166 men were randomly assigned to treatment using 3 cycles of standard BEP or 4 cycles of a modified BEP regimen (bleomycin 30 units day 1; etoposide 120 mg/m2 days 1 to 3; cisplatin 100 mg/m2 day 1) every 21 days. This trial was stopped at interim analyses because the modified BEP arm was inferior to the standard BEP arm. With a median follow-up of 8.5 years, 8-year overall survival was 92% with standard BEP and 83% with modified BEP (P = 0.037).28
Bleomycin used in the BEP regimen has been associated with uncommon but potentially fatal pulmonary toxicity that tends to present as interstitial pneumonitis, which may ultimately progress to fibrosis or bronchiolitis obliterans with organizing pneumonia.29 This has led to evaluation of EP as an alternative to BEP.
EP
The standard EP regimen consists of a 21-day cycle with etoposide 100 mg/m2 on days 1 to 5, and cisplatin 20 mg/m2 on days 1 to 5. Due to conflicting data from multiple randomized trials, there is considerable debate in the field regarding whether 4 cycles of EP are equivalent to 3 cycles of BEP.30,31 The benefit of the EP regimen is that it avoids the higher rates of pulmonary, cutaneous, and neurologic toxicities associated bleomycin, but it does result in the patient receiving an up to 33% higher cumulative dose of cisplatin and etoposide due to the extra cycle of treatment. This has important implications in terms of tolerability and side effects, including delayed toxicities such as second malignancies, which increase with a higher cumulative dose of these agents (etoposide in particular).
VIP
The standard VIP regimen consists of a 21-day cycle with etoposide 75 mg/m2 on days 1 to 5; cisplatin 20 mg/m2 on days 1 to 5; ifosfamide 1200 mg/m2 on days 1 to 5; and mesna 120 mg/m2 IV push on day 1 followed by 1200 mg/m2 on days 1 to 5. For patients with intermediate- or poor-risk disease, 4 cycles of VIP has demonstrated comparable efficacy but higher rates of hematologic toxicities compared with 4 cycles of BEP.32-34 It remains an option for upfront treatment of patients who are not good candidates for a bleomycin-based regimen, and for patients who need salvage chemotherapy.
Adverse Effects of Chemotherapy
Acute and late chemotherapy toxicities vary significantly between regimens depending on the chemotherapy drugs used. Bleomycin-induced pneumonitis may masquerade as a “pneumonia,” which can lead to a delay in diagnosis or institution of treatment, as well as institution of an incorrect treatment (for example, there is a concern that bleomycin toxicity can be precipitated or worsened by a high fraction of inspired oxygen). Chemotherapy-associated neutropenia tends to occur a few days (7–10 days) after initiation of chemotherapy, and neutrophil counts recover without intervention in most patients after an additional 7 to 10 days. Myeloid growth factor support (eg, filgrastim, pegfilgrastim) can be given to patients either prophylactically (if they had an episode of febrile or prolonged neutropenia with the preceding cycle) or secondarily if they present with neutropenia (an absolute neutrophil count ≤ 500 cells/µL) with fever or active infection. Such interventions tend to shorten the duration of neutropenia but does not affect overall survival. Patients with asymptomatic neutropenia do not benefit from growth factor use.35
Stem Cell Transplant
Autologous stem cell transplant (SCT) is the preferred type of SCT for patients with testicular cancer and involves delivery of high doses of chemotherapy followed by infusion of patient-derived myeloid stem cells. While the details of this treatment are outside the scope of this review, decades of experience has shown that this is an effective curative option for a subset of patients with poor prognosis, such as those with platinum-refractory or relapsed disease.36
Clinical Trials
Due to excellent clinical outcomes with front-line therapy, as described, and the relatively low incidence of testicular and other germ cell tumors, clinical trial options for patients with testicular cancer are limited. The TIGER trial is an ongoing international, randomized, phase 3 trial comparing conventional TIP (paclitaxel, ifosfamide, and cisplatin) chemotherapy with high-dose chemotherapy with SCT as the first salvage treatment for relapsed/refractory germ cell tumors (NCT02375204). It is enrolling at multiple centers in the United States and results are expected in 2022. At least 2 ongoing trials are evaluating the role of immunotherapy in patients with relapsed/refractory germ cell tumors (NCT03081923 and NCT03726281). Cluster of differentiation antigen-30 (CD30) has emerged as a potential target of interest in germ cell tumors, and brentuximab vedotin, an anti-CD30 monoclonal antibody, is undergoing evaluation in a phase 2 trial of CD-30–expressing germ cell tumors (NCT01851200). This trial has completed enrollment and results are expected to be available in late 2019 or early 2020.
When possible, patients with relapsed/refractory germ cell tumors should be referred to centers of excellence with access to either testicular/germ-cell tumor specific clinical trials or phase 1 clinical trials.
Radiation Therapy
Adjuvant radiation to the retroperitoneum has a role in the management of stage I and IIA seminomas (Table 3). In a randomized noninferiority trial of radiation therapy versus single-dose carboplatin in stage I seminoma patients, 5-year recurrence-free survival was comparable at approximately 95% in either arm.37,38 In a retrospective database review of 2437 patients receiving either radiation therapy or multi-agent chemotherapy for stage II seminoma, the 5-year survival exceeded 90% in both treatment groups.39 Typically, a total of 30 to 36 Gy of radiation is delivered to para-aortic and ipsilateral external iliac lymph nodes (“dog-leg” field), followed by an optional boost to the involved nodal areas.40 Radiation is associated with acute side effects such as fatigue, gastrointestinal effects, myelosuppression as well as late side effects such as second cancers in the irradiated field (eg, sarcoma, bladder cancer).
Evaluation of Treatment Response
Monitoring of treatment response is fairly straightforward for patients with testicular cancer. Our practice is the following:
- Measure tumor markers on day 1 of each chemotherapy cycle and 3 to 4 weeks after completion of treatment.
- CT of the chest, abdomen, and pelvis with intravenous contrast prior to chemotherapy and upon completion of chemotherapy. Interim imaging is only needed for a small subset of patients with additional clinical indications (eg, new symptoms, lack of improvement in existing symptoms).
- For patients with stage II/III seminoma who have a residual mass ≥ 3 cm on post-treatment CT scan, a PET-CT scan is indicated 6 to 8 weeks after the completion of chemotherapy to determine the need for further treatment.
Active Surveillance
Because testicular cancer has high cure rates even when patients have disease relapse after primary therapy, and additional therapies have significant short- and long-term side effects in these generally young patients, active surveillance is a critical option used in the management of testicular cancer.41
Patients must be counseled that active surveillance is a form of treatment itself in that it involves close clinical and radiographic monitoring. Because there is a risk of disease relapse, patients opting to undergo active surveillance must fully understand the risks of disease recurrence and be willing to abide by the recommended follow-up schedule.
Surveillance is necessary for a minimum of 5 years and possibly 10 years following orchiectomy, and most relapses tend to occur within the first 2 years. Late relapses such as skeletal metastatic disease from seminoma have been reported to occur more than 15 years after orchiectomy, but are generally rare and unpredictable.
The general guidelines for active surveillance are as follows:
For patients with seminoma, history and physical exam and tumor marker assessment should be performed every 3 to 6 months for the first year, then every 6 to 12 months in years 2 and 3, and then annually. CT of the abdomen and pelvis should be done at 3, 6, and 12 months, every 6 to 12 months in years 2 and 3, and then every 12 to 24 months in years 4 and 5. A chest radiograph is performed only if clinically indicated, as the likelihood of distant metastatic recurrence is low.
For patients with nonseminoma, history and physical exam and tumor markers assessment should be performed every 2 to 3 months for first 2 years, every 4 to 6 months in years 3 and 4, and then annually. CT of the abdomen and pelvis should be obtained every 4 to 6 months in year 1, gradually decreasing to annually in year 3 or 4. Chest radiograph is indicated at 4 and 12 months and annually thereafter for stage IA disease. For those with stage IB disease, chest radiograph is indicated every 2 months during the first year and then gradually decreasing to annually beginning year 5.
These recommendations are expected to change over time, and treating physicians are recommended to exercise discretion and consider the patient and tumor characteristics to develop the optimal surveillance plan.
Conclusion
Testicular cancer is the most common cancer afflicting young men. Prompt diagnostic workup initiated in a primary care or hospital setting followed by a referral to a multidisciplinary team of urologists, medical oncologists, and radiation oncologists enables cure in a majority of patients. For patients with stage I seminoma, a radical inguinal orchiectomy followed by active surveillance may offer the best long-term outcome with minimal side effects. For patients with relapsed/refractory testicular cancers, clinical trial participation is strongly encouraged. Patients with a history of testicular cancer benefit from robust survivorship care tailored to their prior therapies. This can be safely delivered through their primary care providers in collaboration with the multidisciplinary oncology team.
Malignant testicular neoplasms can arise from either the germ cells or sex-cord stromal cells, with the former comprising approximately 95% of all testicular cancers (Table 1). Germ cell tumors may contain a single histology or a mix of multiple histologies. For clinical decision making, testicular tumors are categorized as either pure seminoma (no nonseminomatous elements present) or nonseminomatous germ cell tumors (NSGCT). The prevalence of seminoma and NSGCT is roughly equal. If a testicular tumor contains both seminomatous and nonseminomatous components, it is called a mixed germ cell tumor. Because of similarities in biological behavior, the approach to treatment of mixed germ cell tumors is similar to that for NSGCT.

The key points to remember for testicular cancer are:
- With early diagnosis and aggressive multidisciplinary therapy, the overwhelming majority of patients can be cured;
- Specialized care is often critical and affects outcomes; and
- Survivorship, or post-treatment care, is very important for these patients, as they often have lifespan of several decades and a unique set of short- and long-term treatment-related complications.
Developmental Biology and Genetics
The developmental biology of germ cells and germ cell neoplasms is beyond the scope of this review, and interested readers are recommended to refer to pertinent articles on the topic.1,2 A characteristic genetic marker of all germ cell tumors is an isochromosome of the short arm of chromosome 12, i(12p). This is present in testicular tumors regardless of histologic subtype as well as in carcinoma-in-situ. In germ cell tumors without i(12p) karyotype, excess 12p genetic material consisting of repetitive segments has been found, suggesting that this is an early and potentially critical change in oncogenesis.3 Several recent studies have revealed a diverse genomic landscape in testicular cancers, including KIT, KRAS and NRAS mutations in addition to a hyperdiploid karyotype.4,5
Evaluation and Diagnosis
Case Presentation
A 23-year-old Caucasian man presents to a primary care clinic for a pre-employment history and physical exam. He reports testicular pain on the sexually transmitted infections screening questionnaire. On examination, the physician finds a firm, mobile, minimally-tender, 1.5-cm mass in the inferior aspect of left testicle. No contralateral testicular mass or inguinal lymphadenopathy is noted, and a detailed physical exam is otherwise unremarkable. The physician immediately orders an ultrasound of the testicles, which shows a 1.5-cm hypoechoic mass in the inferior aspect of the left testicle, with an unremarkable contralateral testicle. After discussion of the results, the patient is referred a urologic oncologist with expertise in testicular cancer for further care.
The urologic oncologist orders a computed tomography (CT) abdomen and pelvis with and without contrast, which shows a 1.8-cm pathologic-appearing retroperitoneal lymph node at the level of the left renal vein. Chest radiograph with anteroposterior and lateral views is unremarkable. Tumor markers are as follows: beta human chorionic gonadotropin (beta-HCG) 8 mIU/mL (normal range, 0–4 mIU/mL), alpha-fetoprotein (AFP) 2 ng/mL (normal range, 0–8.5 ng/mL), and lactate dehydrogenase (LDH) 195 U/L (normal range, 119–213 U/L).
What is the approach to the initial workup and diagnosis of testicular cancer?
Clinical Presentation and Physical Exam
The majority of testicular cancers are diagnosed on work-up of a nodule or painless swelling of one testicle, usually noted incidentally by the patient. Approximately 30% to 40% of patients complain of a dull ache or heavy sensation in the lower abdomen, perianal area, or scrotum, while acute pain is the presenting symptom in 10%.3
In approximately 10% of patients, the presenting symptom is a result of distant metastatic involvement, such as cough and dyspnea on exertion (pulmonary or mediastinal metastasis), intractable bone pain (skeletal metastasis), intractable back/flank pain, presence of psoas sign or unexplained lower extremity deep vein thrombosis (bulky retroperitoneal metastasis), or central nervous system symptoms (vertebral, spinal or brain metastasis). Constitutional symptoms (unexplained weight loss, anorexia, fatigue) often accompany these symptoms.3
Rarely (5% or less), testicular cancer may present with systemic endocrine symptoms or paraneoplastic symptoms. Gynecomastia is the most common in this category, occurring in approximately 2% of germ cell tumors and more commonly (20%–30%) in Leydig cell tumors of testis.6 Classically, these patients are either 6- to 10-year-old boys with precocious puberty or young men (mid 20s-mid 30s) with a combination of testicular mass, gynecomastia, loss of libido, and impotence. Workup typically reveals increased beta-HCG levels in blood.
Anti-Ma2-antibody-associated limbic encephalitis is the most common (and still quite rare) paraneoplastic complication associated with testicular germ cell tumors. The Ma2 antigen is selectively expressed in the neuronal nucleoli of normal brain tissue and the testicular tumor of the patient. Importantly, in a subset of these patients, the treatment of testicular cancer may result in improvement of symptoms of encephalitis.7
The first step in the diagnosis of testicular neoplasm is a physical exam. This should include a bimanual examination of the scrotal contents, starting with the normal contralateral testis. Normal testicle has a homogeneous texture and consistency, is freely movable, and is separable from the epididymis. Any firm, hard, or fixed mass within the substance of the tunica albuginea should be considered suspicious until proven otherwise. Spread to the epididymis or spermatic cord occurs in 10% to 15% of patients and examination should include these structures as well.3 A comprehensive system-wise examination for features of metastatic spread as discussed above should then be performed. If the patient has cryptorchidism, ultrasound is a mandatory part of the diagnostic workup.
If clinical evaluation suggests a possibility of testicular cancer, the patient must be counseled to undergo an expedited diagnostic workup and specialist evaluation, as a prompt diagnosis and treatment is key to not only improving the likelihood of cure, but also minimizing the treatments needed to achieve it.
Role of Imaging
Scrotal Ultrasound
Scrotal ultrasound is the first imaging modality used in the diagnostic workup of patient with suspected testicular cancer. Bilateral scrotal ultrasound can detect lesions as small as 1 to 2 mm in diameter and help differentiate intratesticular lesions from extrinsic masses. A cystic mass on ultrasound is unlikely to be malignant. Seminomas appear as well-defined hypoechoic lesions without cystic areas, while NSGCTs are typically inhomogeneous with calcifications, cystic areas, and indistinct margins. However, this distinction is not always apparent or reliable. Ultrasound alone is also insufficient for tumor staging.8 For these reasons, a radical inguinal orchiectomy must be pursued for accurate determination of histology and local stage.
If testicular ultrasound shows a suspicious intratesticular mass, the following workup is typically done:
- Measurement of serum tumor markers (beta-HCG, AFP and LDH);
- CT abdomen and pelvis with and without contrast;
- Chest radiograph anteroposterior and lateral views, or CT chest with and without contrast if clinically indicated;
- Any additional focal imaging based on symptoms (eg, magnetic resonance imaging [MRI] scan with and without contrast to evaluate the brain if the patient has CNS symptoms).
CT Scan
CT scan is the preferred imaging modality for staging of testicular cancers, specifically for evaluation of the retroperitoneum, as it is the predominant site for metastases.9 CT scan should encompass the abdomen and pelvis, and contrast-enhanced sequences should be obtained unless medically contraindicated. CT scan of the chest (if not initially done) is compulsory should a CT of abdomen and pelvis and/or a chest radiograph show abnormal findings.
The sensitivity and specificity of CT scans for detection of nodal metastases can vary significantly based on the cutoff. For example, in a series of 70 patients using a cutoff of 10 mm, the sensitivity and specificity of CT scans for patients undergoing retroperitoneal lymph node dissection were 37% and 100%, respectively.10 In the same study, a cutoff of 4 mm increased the sensitivity to 93% and decreased the specificity to 58%. The current general consensus for this cutoff value is 8 to 10 mm measured in the short axis in the transverse (axial) plane.
Approximately 20% of men with clinical stage I testicular cancer (ie, those with non-enlarged retroperitoneal lymph nodes) who do not undergo any adjuvant therapy will have disease relapse in the retroperitoneum, suggesting that they had occult micrometastases that were missed on the initial CT scans.11,12
MRI/Radionuclide Bone Scan/PET Scan
Abdominal or pelvic MRI, whole-body radionuclide bone scan, and positron emission tomography (PET) scans are almost never needed as part of the initial staging workup for testicular cancers due to several limitations, including a high false-negative rate, specifically for the PET scans, and lack of any additional value compared with CT and testicular ultrasound alone.9,13,14 If necessary, these should only be ordered after a multidisciplinary oncology consultation to prevent unnecessary delays in treatment, inappropriate changes to treatment, and unnecessary increases in cost of care.
Tumor Markers, Biopsy, and Staging
What is the role of tumor markers in the management of testicular cancers?
Serum AFP, beta-hCG, and LDH have a well-established role as tumor markers in testicular cancer. The alpha subunit of hCG is shared between multiple pituitary hormones and hence does not serve as a specific marker for testicular cancer. Serum levels of AFP and/or beta-hCG are elevated in approximately 80% percent of men with NSGCTs, even in absence of metastatic spread. On the other hand, serum beta-hCG is elevated in less than 20% and AFP is not elevated in pure seminomas.3
Tumor markers by themselves are not sufficiently sensitive or specific for the diagnosis of testicular cancer, in general, or to differentiate among its subtypes. Despite this limitation, marked elevations in these markers are rarely due to causes other than germ cell tumor. For example, serum beta-hCG concentrations greater than 10,000 mIU/mL occur only in germ cell tumors, trophoblastic differentiation of a primary lung or gastric cancer, gestational trophoblastic disease, or pregnancy. Serum AFP concentrations greater than 10,000 ng/mL occur almost exclusively in germ cell tumors and hepatocellular carcinoma.15
The pattern of marker elevation may play an important role in management of testicular cancer patients. For example, in our practice, several patients have had discordant serum tumor markers and pathology results (eg, elevated AFP with pure seminoma on orchiectomy). One of these patients was treated with adjuvant retroperitoneal lymph node dissection, which confirmed that he had a NSGCT with a seminoma, choriocarcinoma, and teratoma on pathology evaluation of retroperitoneal lymph nodes.
Serum tumor markers have 2 additional critical roles—(1) in the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging16 and International Germ Cell Cancer Collaboration Group (IGCCCG) risk stratification of testicular cancer,17 and (2) in post-treatment disease monitoring.
Is a testicular biopsy necessary for diagnosis?
A testicular biopsy is almost never pursued to confirm the diagnosis of testicular cancer. There is a concern that percutaneous testicular biopsy, which is associated with scrotal skin violation, can adversely affect outcomes due to tumor seeding of scrotal sac or metastatic spread into the inguinal nodes via scrotal skin lymphatics.
Tissue diagnosis is made by radical orchiectomy in a majority of cases. Rarely in our practice, we obtain a biopsy of metastatic lesion for a tissue diagnosis. This is only done in cases where chemotherapy must be started urgently to prevent worsening of complications from metastatic spread. This decision should be made only after a multidisciplinary consultation with urologic and medical oncology teams.
How is testicular cancer staged?
Both seminomatous and nonseminomatous germ cell tumors of the testis are staged using the AJCC/UICC staging system, which incorporates assessments of the primary tumor (T), lymph nodes (N), and distant metastases (M) and serum tumor marker values (S). Details of this staging system are beyond the scope of this review and further information can be obtained through the AJCC website (www.cancerstaging.org). This TNMS staging enables a prognostic assessment and helps with the therapeutic approach.
For patients with advanced germ cell tumors, a risk group classification developed by the IGCCCG is used to classify patients into good-risk, intermediate-risk, and poor-risk category (Table 2). This classification has been extensively validated for the past 2 decades, provides important prognostic information, and helps inform therapy decisions.

Treatment
Case 1 Continued
Based on the patient’s imaging and biomarker results, the patient undergoes a left radical inguinal orchiectomy. The physician’s operative note mentions that the left testicle was delivered without violation of scrotal integrity. A pathology report shows pure spermatocytic seminoma (unifocal, 1.4 cm size) with negative margins and no evidence of lymphovascular invasion. No lymph nodes are identified in the resection specimen. Post-orchiectomy markers are “negative,” meaning within normal range. After discussions with medical and radiation oncology physicians, the patient opts to pursue active surveillance.
Surgery alone followed by active surveillance is an appropriate option for this patient, as the likelihood of recurrence is low and most recurrences can be subsequently salvaged using treatment options detailed below.
What are the therapeutic options for testicular cancer?
An overview of management for most testicular cancers is presented in Table 3. Note that the actual treatments are significantly more complex and need a comprehensive multidisciplinary consultation (urologic, medical and radiation oncology) at centers with specialized testicular cancer teams, if possible.

Fertility Preservation
All patients initiating treatment for testicular cancer must be offered options for fertility preservation and consultation with a reproductive health team, if available. At the time of diagnosis, approximately 50% patients have some degree of impairment in spermatogenesis, but with effective fertility preservation, successful pregnancy can occur for as many as 30% to 60% of patients.18,19
Orchiectomy
Radical inguinal orchiectomy with high ligation of the spermatic cord at the level of the internal ring is the procedure of choice for suspected testicular cancer. The goal is to provide a definitive tissue diagnosis and local tumor control with minimal morbidity. It can be performed under general, regional, or local anesthesia. Depending on the complexity and surgical expertise, it can be done in an inpatient or outpatient setting. During the procedure, the testicle is delivered from the scrotum through an incision in the inguinal region and then resected. A testicular prosthesis is usually inserted, with resultant excellent cosmetic and patient satisfaction outcomes.20
Testicular sparing surgery (TSS) has been explored as an alternative to radical orchiectomy but is not considered a standard-of-care option at this time. Small studies have shown evidence for comparable short-term oncologic outcomes in a very select group of patients, generally with solitary tumors < 2 cm in size and solitary testicle. If this is being considered as an option, we recommended obtaining a consultation from a urologist at a high-volume center. For a majority of patients, the value of a TSS is diminished due to excellent anatomic/cosmetic outcomes with a testicular prosthesis implanted during the radical orchiectomy, and resumption of sexual functions by the unaffected contralateral testicle.
Retroperitoneal Lymph Node Dissection
As discussed, conventional cross-sectional imaging has a high false-negative rate for detection of retroperitoneal involvement. General indications for RPLND in various stages and histologies of testicular cancer germ cell tumors are outlined in Table 3. Seminoma tends to most commonly metastasize to retroperitoneum, but RPLND for seminoma is generally reserved for a very small subset of these patients. Patterns of metastases of NSGCT (except choriocarcinoma) are considered to be well-defined. In a series of patients with stage II NSGCTs, left-sided tumors metastasized to the pre- and para-aortic nodes in 88% and 86% of cases, respectively (drainage basin of left testicular vein); and right-sided tumors involved the interaortocaval nodes in 93% of patients.3 Inguinal and pelvic nodal metastases may rarely be seen and should not be used to rule out the diagnosis of testicular cancer.
Choriocarcinoma is an exception to this pattern of retroperitoneal spread, as it tends to have a higher likelihood of hematogenous metastases to distant organs. Compared with NSGCTs, pure seminomas are either localized to the testis (80% of all cases) or limited to the retroperitoneum (an additional 15% of all cases) at presentation.3
Depending on the case and expertise of the surgical team, robotic or open RPLND can be performed.21 Regardless of the approach used, RPLND remains a technically challenging surgery. The retroperitoneal “landing zone” lymph nodes lie in close proximity to, and are often densely adherent to, the abdominal great vessels. Complication rates vary widely in the reported literature, but can be as high as 50%.21-23 As detailed in Table 2, the number and size of involved retroperitoneal lymph nodes have prognostic importance.
In summary, RPLND is considered to be a viable option for a subset of early-stage NSGCT (T1-3, N0-2, M0) and for those with advanced seminoma, NSGCT, or mixed germ cell tumors with post-chemotherapy residual disease.
Systemic Chemotherapy
Except for the single-agent carboplatin, most chemotherapy regimens used to treat testicular cancer are combinations of 2 or more chemotherapy agents. For this review, we will focus on the 3 most commonly used regimens: bleomycin, etoposide, and cisplatin (BEP), etoposide and cisplatin (EP), and etoposide, ifosfamide, and cisplatin (VIP).
The core principles of testicular cancer chemotherapy are:
- Minimize dose interruptions, delays, or reductions, as these adversely affect outcomes without clearly improving side effect profile;
- Do not substitute carboplatin for cisplatin in combination regimens because carboplatin-containing combination regimens have been shown to result in significantly poorer outcomes in multiple trials of adults with germ cell tumors;24-27 and
- Give myeloid growth factor support, if necessary.
BEP
The standard BEP regimen comprises a 21-day cycle with bleomycin 30 units on days 1, 8, and 15; etoposide 100 mg/m2 on days 1 to 5; and cisplatin 20 mg/m2 on days 1 to 5. Number of cycles varies based on histology and stage (Table 3). A strong justification to maintain treatment intensity comes from the Australian and New Zealand Germ Cell Trial Group trial. In this study, 166 men were randomly assigned to treatment using 3 cycles of standard BEP or 4 cycles of a modified BEP regimen (bleomycin 30 units day 1; etoposide 120 mg/m2 days 1 to 3; cisplatin 100 mg/m2 day 1) every 21 days. This trial was stopped at interim analyses because the modified BEP arm was inferior to the standard BEP arm. With a median follow-up of 8.5 years, 8-year overall survival was 92% with standard BEP and 83% with modified BEP (P = 0.037).28
Bleomycin used in the BEP regimen has been associated with uncommon but potentially fatal pulmonary toxicity that tends to present as interstitial pneumonitis, which may ultimately progress to fibrosis or bronchiolitis obliterans with organizing pneumonia.29 This has led to evaluation of EP as an alternative to BEP.
EP
The standard EP regimen consists of a 21-day cycle with etoposide 100 mg/m2 on days 1 to 5, and cisplatin 20 mg/m2 on days 1 to 5. Due to conflicting data from multiple randomized trials, there is considerable debate in the field regarding whether 4 cycles of EP are equivalent to 3 cycles of BEP.30,31 The benefit of the EP regimen is that it avoids the higher rates of pulmonary, cutaneous, and neurologic toxicities associated bleomycin, but it does result in the patient receiving an up to 33% higher cumulative dose of cisplatin and etoposide due to the extra cycle of treatment. This has important implications in terms of tolerability and side effects, including delayed toxicities such as second malignancies, which increase with a higher cumulative dose of these agents (etoposide in particular).
VIP
The standard VIP regimen consists of a 21-day cycle with etoposide 75 mg/m2 on days 1 to 5; cisplatin 20 mg/m2 on days 1 to 5; ifosfamide 1200 mg/m2 on days 1 to 5; and mesna 120 mg/m2 IV push on day 1 followed by 1200 mg/m2 on days 1 to 5. For patients with intermediate- or poor-risk disease, 4 cycles of VIP has demonstrated comparable efficacy but higher rates of hematologic toxicities compared with 4 cycles of BEP.32-34 It remains an option for upfront treatment of patients who are not good candidates for a bleomycin-based regimen, and for patients who need salvage chemotherapy.
Adverse Effects of Chemotherapy
Acute and late chemotherapy toxicities vary significantly between regimens depending on the chemotherapy drugs used. Bleomycin-induced pneumonitis may masquerade as a “pneumonia,” which can lead to a delay in diagnosis or institution of treatment, as well as institution of an incorrect treatment (for example, there is a concern that bleomycin toxicity can be precipitated or worsened by a high fraction of inspired oxygen). Chemotherapy-associated neutropenia tends to occur a few days (7–10 days) after initiation of chemotherapy, and neutrophil counts recover without intervention in most patients after an additional 7 to 10 days. Myeloid growth factor support (eg, filgrastim, pegfilgrastim) can be given to patients either prophylactically (if they had an episode of febrile or prolonged neutropenia with the preceding cycle) or secondarily if they present with neutropenia (an absolute neutrophil count ≤ 500 cells/µL) with fever or active infection. Such interventions tend to shorten the duration of neutropenia but does not affect overall survival. Patients with asymptomatic neutropenia do not benefit from growth factor use.35
Stem Cell Transplant
Autologous stem cell transplant (SCT) is the preferred type of SCT for patients with testicular cancer and involves delivery of high doses of chemotherapy followed by infusion of patient-derived myeloid stem cells. While the details of this treatment are outside the scope of this review, decades of experience has shown that this is an effective curative option for a subset of patients with poor prognosis, such as those with platinum-refractory or relapsed disease.36
Clinical Trials
Due to excellent clinical outcomes with front-line therapy, as described, and the relatively low incidence of testicular and other germ cell tumors, clinical trial options for patients with testicular cancer are limited. The TIGER trial is an ongoing international, randomized, phase 3 trial comparing conventional TIP (paclitaxel, ifosfamide, and cisplatin) chemotherapy with high-dose chemotherapy with SCT as the first salvage treatment for relapsed/refractory germ cell tumors (NCT02375204). It is enrolling at multiple centers in the United States and results are expected in 2022. At least 2 ongoing trials are evaluating the role of immunotherapy in patients with relapsed/refractory germ cell tumors (NCT03081923 and NCT03726281). Cluster of differentiation antigen-30 (CD30) has emerged as a potential target of interest in germ cell tumors, and brentuximab vedotin, an anti-CD30 monoclonal antibody, is undergoing evaluation in a phase 2 trial of CD-30–expressing germ cell tumors (NCT01851200). This trial has completed enrollment and results are expected to be available in late 2019 or early 2020.
When possible, patients with relapsed/refractory germ cell tumors should be referred to centers of excellence with access to either testicular/germ-cell tumor specific clinical trials or phase 1 clinical trials.
Radiation Therapy
Adjuvant radiation to the retroperitoneum has a role in the management of stage I and IIA seminomas (Table 3). In a randomized noninferiority trial of radiation therapy versus single-dose carboplatin in stage I seminoma patients, 5-year recurrence-free survival was comparable at approximately 95% in either arm.37,38 In a retrospective database review of 2437 patients receiving either radiation therapy or multi-agent chemotherapy for stage II seminoma, the 5-year survival exceeded 90% in both treatment groups.39 Typically, a total of 30 to 36 Gy of radiation is delivered to para-aortic and ipsilateral external iliac lymph nodes (“dog-leg” field), followed by an optional boost to the involved nodal areas.40 Radiation is associated with acute side effects such as fatigue, gastrointestinal effects, myelosuppression as well as late side effects such as second cancers in the irradiated field (eg, sarcoma, bladder cancer).
Evaluation of Treatment Response
Monitoring of treatment response is fairly straightforward for patients with testicular cancer. Our practice is the following:
- Measure tumor markers on day 1 of each chemotherapy cycle and 3 to 4 weeks after completion of treatment.
- CT of the chest, abdomen, and pelvis with intravenous contrast prior to chemotherapy and upon completion of chemotherapy. Interim imaging is only needed for a small subset of patients with additional clinical indications (eg, new symptoms, lack of improvement in existing symptoms).
- For patients with stage II/III seminoma who have a residual mass ≥ 3 cm on post-treatment CT scan, a PET-CT scan is indicated 6 to 8 weeks after the completion of chemotherapy to determine the need for further treatment.
Active Surveillance
Because testicular cancer has high cure rates even when patients have disease relapse after primary therapy, and additional therapies have significant short- and long-term side effects in these generally young patients, active surveillance is a critical option used in the management of testicular cancer.41
Patients must be counseled that active surveillance is a form of treatment itself in that it involves close clinical and radiographic monitoring. Because there is a risk of disease relapse, patients opting to undergo active surveillance must fully understand the risks of disease recurrence and be willing to abide by the recommended follow-up schedule.
Surveillance is necessary for a minimum of 5 years and possibly 10 years following orchiectomy, and most relapses tend to occur within the first 2 years. Late relapses such as skeletal metastatic disease from seminoma have been reported to occur more than 15 years after orchiectomy, but are generally rare and unpredictable.
The general guidelines for active surveillance are as follows:
For patients with seminoma, history and physical exam and tumor marker assessment should be performed every 3 to 6 months for the first year, then every 6 to 12 months in years 2 and 3, and then annually. CT of the abdomen and pelvis should be done at 3, 6, and 12 months, every 6 to 12 months in years 2 and 3, and then every 12 to 24 months in years 4 and 5. A chest radiograph is performed only if clinically indicated, as the likelihood of distant metastatic recurrence is low.
For patients with nonseminoma, history and physical exam and tumor markers assessment should be performed every 2 to 3 months for first 2 years, every 4 to 6 months in years 3 and 4, and then annually. CT of the abdomen and pelvis should be obtained every 4 to 6 months in year 1, gradually decreasing to annually in year 3 or 4. Chest radiograph is indicated at 4 and 12 months and annually thereafter for stage IA disease. For those with stage IB disease, chest radiograph is indicated every 2 months during the first year and then gradually decreasing to annually beginning year 5.
These recommendations are expected to change over time, and treating physicians are recommended to exercise discretion and consider the patient and tumor characteristics to develop the optimal surveillance plan.
Conclusion
Testicular cancer is the most common cancer afflicting young men. Prompt diagnostic workup initiated in a primary care or hospital setting followed by a referral to a multidisciplinary team of urologists, medical oncologists, and radiation oncologists enables cure in a majority of patients. For patients with stage I seminoma, a radical inguinal orchiectomy followed by active surveillance may offer the best long-term outcome with minimal side effects. For patients with relapsed/refractory testicular cancers, clinical trial participation is strongly encouraged. Patients with a history of testicular cancer benefit from robust survivorship care tailored to their prior therapies. This can be safely delivered through their primary care providers in collaboration with the multidisciplinary oncology team.
1. van der Zwan YG, Biermann K, Wolffenbuttel KP, et al. Gonadal maldevelopment as risk factor for germ cell cancer: towards a clinical decision model. Eur Urol. 2015; 67:692–701.
2. Pierce JL, Frazier AL, Amatruda JF. Pediatric germ cell tumors: a developmental perspective. Adv Urol. 2018 Feb 4;2018.
3. Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242-253.
4. Pyle LC, Nathanson KL. Genetic changes associated with testicular cancer susceptibility. Semin Oncol. 2016;43:575-581.
5. Shen H, Shih J, Hollern DP, et al. Integrated molecular characterization of testicular germ cell tumors. Cell Rep. 2018;23:3392-3406.
6. Barry M, Rao A, Lauer R. Sex cord-stromal tumors of the testis. In: Pagliaro L, ed. Rare Genitourinary Tumors. Cham: Springer International Publishing; 2016: 231-251.
7. Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain J Neurol. 2004;127:1831-1844.
8. Coursey Moreno C, Small WC, Camacho JC, et al. Testicular tumors: what radiologists need to know—differential diagnosis, staging, and management. RadioGraphics. 2015;35:400-415.
9. Kreydin EI, Barrisford GW, Feldman AS, Preston MA. Testicular cancer: what the radiologist needs to know. Am J Roentgenol. 2013;200:1215-1225.
10. Hilton S, Herr HW, Teitcher JB, et al. CT detection of retroperitoneal lymph node metastases in patients with clinical stage I testicular nonseminomatous germ cell cancer: assessment of size and distribution criteria. Am J Roentgenol. 1997;169:521-525.
11. Thompson PI, Nixon J, Harvey VJ. Disease relapse in patients with stage I nonseminomatous germ cell tumor of the testis on active surveillance. J Clin Oncol. 1988;6:1597-1603.
12. Nicolai N, Pizzocaro G. A surveillance study of clinical stage I nonseminomatous germ cell tumors of the testis: 10-year followup. J Urol. 1995;154:1045-1049.
13. Kok HK, Leong S, Torreggiani WC. Is magnetic resonance imaging comparable with computed tomography in the diagnosis of retroperitoneal metastasis in patients with testicular cancer? Can Assoc Radiol J. 2014;65:196-198.
14. Hale GR, Teplitsky S, Truong H, et al. Lymph node imaging in testicular cancer. Transl Androl Urol. 2018;7:864-874.
15. Honecker F, Aparicio J, Berney D, et al. ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol. 2018;29:1658-1686.
16. Paner GP, Stadler WM, Hansel DE, et al. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur Urol. 2018;73:560-569.
17. International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15:594-603.
18. Lopategui DM, Ibrahim E, Aballa TC, et al. Effect of a formal oncofertility program on fertility preservation rates-first year experience. Transl Androl Urol. 2018;7:S271-S275.
19. Moody JA, Ahmed K, Horsfield C, et al. Fertility preservation in testicular cancer - predictors of spermatogenesis. BJU Int. 2018;122:236-242.
20. Dieckmann KP, Anheuser P, Schmidt S, et al. Testicular prostheses in patients with testicular cancer - acceptance rate and patient satisfaction. BMC Urol. 2015;15:16.
21. Schwen ZR, Gupta M, Pierorazio PM. A review of outcomes and technique for the robotic-assisted laparoscopic retroperitoneal lymph node dissection for testicular cancer. Adv Urol. 2018;2146080.
22. Singh P, Yadav S, Mahapatra S, Seth A. Outcomes following retroperitoneal lymph node dissection in postchemotherapy residual masses in advanced testicular germ cell tumors. Indian J Urol. 2016;32:40-44.
23. Heidenreich A, Thüer D, Polyakov S. Postchemotherapy retroperitoneal lymph node dissection in advanced germ cell tumours of the testis. Eur Urol. 2008;53:260-272.
24. Bajorin DF, Sarosdy MF, Pfister DG, et al. Randomized trial of etoposide and cisplatin versus etoposide and carboplatin in patients with good-risk germ cell tumors: a multiinstitutional study. J Clin Oncol. 1993;11:598-606.
25. Bokemeyer C, Köhrmann O, Tischler J, et al. A randomized trial of cisplatin, etoposide and bleomycin (PEB) versus carboplatin, etoposide and bleomycin (CEB) for patients with “good-risk” metastatic non-seminomatous germ cell tumors. Ann Oncol. 1996;7:1015-1021.
26. Horwich A, Sleijfer DT, Fosså SD, et al. Randomized trial of bleomycin, etoposide, and cisplatin compared with bleomycin, etoposide, and carboplatin in good-prognosis metastatic nonseminomatous germ cell cancer: a Multiinstitutional Medical Research Council/European Organization for Research and Treatment of Cancer Trial. J Clin Oncol. 1997;15:1844-1852.
27. Shaikh F, Nathan PC, Hale J, et al. Is there a role for carboplatin in the treatment of malignant germ cell tumors? A systematic review of adult and pediatric trials. Pediatr Blood Cancer. 2013;60:587-592.
28. Grimison PS, Stockler MR, Thomson DB, et al. Comparison of two standard chemotherapy regimens for good-prognosis germ cell tumors: updated analysis of a randomized trial. J Natl Cancer Inst. 2010;102:1253-1262.
29. Reinert T, da Rocha Baldotto CS, Nunes FAP, de Souza Scheliga AA. Bleomycin-induced lung injury. J Cancer Res. 2013;480608.
30. Jones RH, Vasey PA. Part II: Testicular cancer—management of advanced disease. Lancet Oncol. 2003;4:738-747.
31. Jankilevich G. BEP versus EP for treatment of metastatic germ-cell tumours. Lancet Oncol. 2004;5, 146.
32. Nichols CR, Catalano PJ, Crawford ED, et al. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: an Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B Study. J Clin Oncol. 1998;16:12871293.
33. Hinton S, Catalano PJ, Einhorn LH, et al. Cisplatin, etoposide and either bleomycin or ifosfamide in the treatment of disseminated germ cell tumors: final analysis of an intergroup trial. Cancer. 2003;97: 1869-1875.
34. de Wit R, Stoter G, Sleijfer DT, et al. Four cycles of BEP vs four cycles of VIP in patients with intermediate-prognosis metastatic testicular non-seminoma: a randomized study of the EORTC Genitourinary Tract Cancer Cooperative Group. European Organization for Research and Treatment of Cancer. Br J Cancer. 1998;78:828-832.
35. Mhaskar R, Clark OA, Lyman G, et al. Colony-stimulating factors for chemotherapy-induced febrile neutropenia. Cochrane Database Syst. Rev. 2014;CD003039.
36. Adra N, Abonour R, Althouse SK, et al. High-dose chemotherapy and autologous peripheral-blood stem-cell transplantation for relapsed metastatic germ cell tumors: The Indiana University experience. J Clin Oncol. 2017;35:1096-1102.
37. Oliver RT, Mason MD, Mead GM, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005;366:293-300.
38. Oliver RT, Mead GM, Rustin GJ, et al. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214). J Clin Oncol. 2011;29:957-962.
39. Glaser SM, Vargo JA, Balasubramani GK, Beriwal S. Stage II testicular seminoma: patterns of care and survival by treatment strategy. Clin Oncol. 2016;28:513-521.
40. Boujelbene N, Cosinschi A, Boujelbene N, et al. Pure seminoma: A review and update. Radiat Oncol. 2011;6:90.
41. Nichols CR, Roth B, Albers P, et al. Active surveillance is the preferred approach to clinical stage I testicular cancer. J Clin Oncol. 2013;31;3490-3493.
1. van der Zwan YG, Biermann K, Wolffenbuttel KP, et al. Gonadal maldevelopment as risk factor for germ cell cancer: towards a clinical decision model. Eur Urol. 2015; 67:692–701.
2. Pierce JL, Frazier AL, Amatruda JF. Pediatric germ cell tumors: a developmental perspective. Adv Urol. 2018 Feb 4;2018.
3. Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242-253.
4. Pyle LC, Nathanson KL. Genetic changes associated with testicular cancer susceptibility. Semin Oncol. 2016;43:575-581.
5. Shen H, Shih J, Hollern DP, et al. Integrated molecular characterization of testicular germ cell tumors. Cell Rep. 2018;23:3392-3406.
6. Barry M, Rao A, Lauer R. Sex cord-stromal tumors of the testis. In: Pagliaro L, ed. Rare Genitourinary Tumors. Cham: Springer International Publishing; 2016: 231-251.
7. Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain J Neurol. 2004;127:1831-1844.
8. Coursey Moreno C, Small WC, Camacho JC, et al. Testicular tumors: what radiologists need to know—differential diagnosis, staging, and management. RadioGraphics. 2015;35:400-415.
9. Kreydin EI, Barrisford GW, Feldman AS, Preston MA. Testicular cancer: what the radiologist needs to know. Am J Roentgenol. 2013;200:1215-1225.
10. Hilton S, Herr HW, Teitcher JB, et al. CT detection of retroperitoneal lymph node metastases in patients with clinical stage I testicular nonseminomatous germ cell cancer: assessment of size and distribution criteria. Am J Roentgenol. 1997;169:521-525.
11. Thompson PI, Nixon J, Harvey VJ. Disease relapse in patients with stage I nonseminomatous germ cell tumor of the testis on active surveillance. J Clin Oncol. 1988;6:1597-1603.
12. Nicolai N, Pizzocaro G. A surveillance study of clinical stage I nonseminomatous germ cell tumors of the testis: 10-year followup. J Urol. 1995;154:1045-1049.
13. Kok HK, Leong S, Torreggiani WC. Is magnetic resonance imaging comparable with computed tomography in the diagnosis of retroperitoneal metastasis in patients with testicular cancer? Can Assoc Radiol J. 2014;65:196-198.
14. Hale GR, Teplitsky S, Truong H, et al. Lymph node imaging in testicular cancer. Transl Androl Urol. 2018;7:864-874.
15. Honecker F, Aparicio J, Berney D, et al. ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol. 2018;29:1658-1686.
16. Paner GP, Stadler WM, Hansel DE, et al. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur Urol. 2018;73:560-569.
17. International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15:594-603.
18. Lopategui DM, Ibrahim E, Aballa TC, et al. Effect of a formal oncofertility program on fertility preservation rates-first year experience. Transl Androl Urol. 2018;7:S271-S275.
19. Moody JA, Ahmed K, Horsfield C, et al. Fertility preservation in testicular cancer - predictors of spermatogenesis. BJU Int. 2018;122:236-242.
20. Dieckmann KP, Anheuser P, Schmidt S, et al. Testicular prostheses in patients with testicular cancer - acceptance rate and patient satisfaction. BMC Urol. 2015;15:16.
21. Schwen ZR, Gupta M, Pierorazio PM. A review of outcomes and technique for the robotic-assisted laparoscopic retroperitoneal lymph node dissection for testicular cancer. Adv Urol. 2018;2146080.
22. Singh P, Yadav S, Mahapatra S, Seth A. Outcomes following retroperitoneal lymph node dissection in postchemotherapy residual masses in advanced testicular germ cell tumors. Indian J Urol. 2016;32:40-44.
23. Heidenreich A, Thüer D, Polyakov S. Postchemotherapy retroperitoneal lymph node dissection in advanced germ cell tumours of the testis. Eur Urol. 2008;53:260-272.
24. Bajorin DF, Sarosdy MF, Pfister DG, et al. Randomized trial of etoposide and cisplatin versus etoposide and carboplatin in patients with good-risk germ cell tumors: a multiinstitutional study. J Clin Oncol. 1993;11:598-606.
25. Bokemeyer C, Köhrmann O, Tischler J, et al. A randomized trial of cisplatin, etoposide and bleomycin (PEB) versus carboplatin, etoposide and bleomycin (CEB) for patients with “good-risk” metastatic non-seminomatous germ cell tumors. Ann Oncol. 1996;7:1015-1021.
26. Horwich A, Sleijfer DT, Fosså SD, et al. Randomized trial of bleomycin, etoposide, and cisplatin compared with bleomycin, etoposide, and carboplatin in good-prognosis metastatic nonseminomatous germ cell cancer: a Multiinstitutional Medical Research Council/European Organization for Research and Treatment of Cancer Trial. J Clin Oncol. 1997;15:1844-1852.
27. Shaikh F, Nathan PC, Hale J, et al. Is there a role for carboplatin in the treatment of malignant germ cell tumors? A systematic review of adult and pediatric trials. Pediatr Blood Cancer. 2013;60:587-592.
28. Grimison PS, Stockler MR, Thomson DB, et al. Comparison of two standard chemotherapy regimens for good-prognosis germ cell tumors: updated analysis of a randomized trial. J Natl Cancer Inst. 2010;102:1253-1262.
29. Reinert T, da Rocha Baldotto CS, Nunes FAP, de Souza Scheliga AA. Bleomycin-induced lung injury. J Cancer Res. 2013;480608.
30. Jones RH, Vasey PA. Part II: Testicular cancer—management of advanced disease. Lancet Oncol. 2003;4:738-747.
31. Jankilevich G. BEP versus EP for treatment of metastatic germ-cell tumours. Lancet Oncol. 2004;5, 146.
32. Nichols CR, Catalano PJ, Crawford ED, et al. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: an Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B Study. J Clin Oncol. 1998;16:12871293.
33. Hinton S, Catalano PJ, Einhorn LH, et al. Cisplatin, etoposide and either bleomycin or ifosfamide in the treatment of disseminated germ cell tumors: final analysis of an intergroup trial. Cancer. 2003;97: 1869-1875.
34. de Wit R, Stoter G, Sleijfer DT, et al. Four cycles of BEP vs four cycles of VIP in patients with intermediate-prognosis metastatic testicular non-seminoma: a randomized study of the EORTC Genitourinary Tract Cancer Cooperative Group. European Organization for Research and Treatment of Cancer. Br J Cancer. 1998;78:828-832.
35. Mhaskar R, Clark OA, Lyman G, et al. Colony-stimulating factors for chemotherapy-induced febrile neutropenia. Cochrane Database Syst. Rev. 2014;CD003039.
36. Adra N, Abonour R, Althouse SK, et al. High-dose chemotherapy and autologous peripheral-blood stem-cell transplantation for relapsed metastatic germ cell tumors: The Indiana University experience. J Clin Oncol. 2017;35:1096-1102.
37. Oliver RT, Mason MD, Mead GM, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005;366:293-300.
38. Oliver RT, Mead GM, Rustin GJ, et al. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214). J Clin Oncol. 2011;29:957-962.
39. Glaser SM, Vargo JA, Balasubramani GK, Beriwal S. Stage II testicular seminoma: patterns of care and survival by treatment strategy. Clin Oncol. 2016;28:513-521.
40. Boujelbene N, Cosinschi A, Boujelbene N, et al. Pure seminoma: A review and update. Radiat Oncol. 2011;6:90.
41. Nichols CR, Roth B, Albers P, et al. Active surveillance is the preferred approach to clinical stage I testicular cancer. J Clin Oncol. 2013;31;3490-3493.
Feasibility—and safety—of reducing the traditional 14 prenatal visits to 8 or 10
CASE Low-risk maternity patient wants fewer prenatal visits
A recently pregnant patient asks her obstetrician if she can schedule fewer prenatal visits given that she is at low risk, wants to minimize missing work, and lives an hour away from the clinic office. Her physician tells her that she needs the standard 13 to 15 visits to have a healthy pregnancy.
Obstetric care in the United States largely remains a “one-size fits all” approach despite compelling data that fewer visits for low-risk women are medically acceptable and may be more cost-effective.
Prenatal care: One size does not fit all
With nearly 4 million births annually in the United States, prenatal care is one of the most widely used preventive health care strategies.1,2 The ideal method for providing prenatal care, however, remains controversial. At the inception of early 20th century prenatal care in the United States, preventive strategies focused in part on eclampsia-related maternal morbidity and mortality, which in turn informed the content and frequency of prenatal visits.2 Despite the dramatic changes in medical practice over the last 100 years, the basic timing and quantity of prenatal care has not changed substantively.
The lack of change is not because we have not explored other models of prenatal care and sought to introduce evidence-based change. Several studies have assessed the impact of reduced prenatal care visits for low-risk women.3-7 Systematic reviews evaluated 7 randomized trials, with more than 60,000 women enrolled, of prenatal care models with a reduced number of planned antenatal visits (4 to 9 visits vs the traditional 13 to 15 visits).3,8 There were no demonstrable differences in maternal or perinatal morbidity or mortality, particularly in higher resource settings.
Despite strong safety data and the potential cost-effectiveness of a reduced schedule of prenatal visits, US prenatal care practices generally continue to have a one-size-fits-all approach. Several organizations, however, have called for a change in practice.
Endorsing a reduced number of prenatal visits for low-risk women, the US Department of Health and Human Services Expert Panel on Prenatal Care issued a report in 1989 that stated “the specific content and timing of prenatal visits, contacts, and education should vary depending on the risk status of the pregnant woman and her fetus.”9 Consistent with that recommendation, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (ACOG) jointly published guidelines that recommend a system of goal-oriented antenatal visits at specific gestational ages and that support a reduced schedule of prenatal visits, compared with traditional models, for low-risk, parous women.10 The World Health Organization also published recommendations for an 8 “contact” prenatal care system to reduce perinatal mortality and improve women’s prenatal experience.11
Is obstetric dogma the reason for lack of change?
Concerns about patient satisfaction may play a role in limiting the use of a reduced prenatal care visit model. In trials that evaluated a model of reduced prenatal care visits, women were less satisfied with a reduced visit schedule and the gap between provider contacts.3,8 Anecdotally, providers have expressed concerns about perceived liability. Most compelling, perhaps, is the idea that the traditional prenatal schedule has become obstetric dogma.
Continue to: Consciously or unconsciously, clinicians may feel...
Consciously or unconsciously, clinicians may feel uncomfortable diverging from a schedule of visits that is firmly entrenched in obstetric practice. Continuing the status quo is easier than restructuring prenatal care practice. Ultimately, a paradigm shift may be required to broadly adopt a model of fewer prenatal visits for low-risk pregnancies.12 With these issues propelling the historic patterns of prenatal care, it is easy to see why we have not yet changed despite convincing reasons to do so.
In this article, we detail the reduced-visit prenatal care models developed at 3 institutions and how they incorporate use of today’s technology.
Approach #1: University of Utah Virtual Prenatal Care Program
The University of Utah Virtual Prenatal Care Program was conceived as a “baby step” toward developing a model of fewer total prenatal visits. Virtual visits were intended to reduce the number of prenatal face-to-face visits while maintaining the same total number of visits. Since large clinical trials had established the safety of reduced visits, the primary objectives were to retain patient satisfaction and to facilitate provider adoption.
Would women be satisfied with remote prenatal care? A prospective randomized controlled trial was designed in which 200 women were assigned to receive either a combination of telemedicine and 5 scheduled in-clinic prenatal visits (remote care group) or traditional in-clinic prenatal care (usual care group). Low-risk multigravida pregnant women who were between 6 0/7 and 16 0/7 weeks’ gestation were enrolled. The primary outcome was patient satisfaction.
The face-to-face visits were goal oriented, with scheduled physical examination, laboratory tests, or ultrasonography, and were conducted by the patient’s established obstetric provider (physician or nurse midwife) to maintain continuity of care. The remote care group self-collected measurements for weight, blood pressure, and fetal heart rate by handheld Doppler device prior to each telemedicine visit and entered the information into the electronic medical record. The purpose of the self-collected data was patient engagement and satisfaction, as well as increased provider comfort with the change in prenatal care schedule, rather than medical necessity.
The primary outcome of overall patient satisfaction with prenatal care was ascertained by questionnaire after delivery. The sample size calculation of 200 patients was based on noninferiority testing, and analysis was by intent-to-treat. The details of the trial are pending publication.
As expected, the remote care group had significantly fewer in-clinic prenatal care visits compared with the usual care group (7.2 vs 11.3 visits); the total number of prenatal visits was not different between groups. Overall satisfaction with prenatal care was very high in both the remote care and the usual care group (100% vs 97%).
The virtual prenatal care model for low-risk pregnancies, consisting of a novel remote monitoring strategy and a reduced number of in-clinic visits, was not associated with lower patient satisfaction compared with traditional care.
New care strategy gives patients a choice. The success of this clinical trial has led to its programmatic adoption at the University of Utah, and low-risk women currently are offered a choice between participating in the Virtual Prenatal Care Program or receiving traditional prenatal care. The University of Utah is moving on from the one-size-fits-all approach to adopt new strategies that provide personalized evidence-based prenatal care at the lowest cost, while retaining high patient satisfaction. Formal cost-effectiveness analyses are underway.
Continue to: Approach #2: Mayo Clinic OB Nest...
Approach #2: Mayo Clinic OB Nest
In 2011, the Mayo Clinic Obstetric Division partnered with 2 other Mayo Clinic divisions, the Center for Innovation and the Center for the Science of Health Care Delivery, to redesign prenatal care for low-risk expectant mothers.Pregnant women and their obstetric health care teams (including obstetricians, certified nurse midwives, registered nurses, and clinical support staff) were convened to develop a novel model of prenatal care.4 The goal of this collaboration centered on:
- creating an evidence-driven prenatal care model for low-risk expectant women designed by relevant stakeholders
- focusing on meeting the on-demand needs of expectant mothers
- integrating innovative 21st century technology, and
- reducing the burden of prescheduled, low-value office visits.
Exploratory efforts to develop a novel care program. Based on feedback from the collaboration and guided by these goals, 141 expectant mothers participated in 19 different experiments, enabling the health care team to understand the impact of changing various components of prenatal care.
The experiments included integration of home monitoring (home fetal Doppler devices, drop-in fetal Doppler stations, home blood pressure monitoring devices), technology-enhanced communication with obstetric team members (video chats, tummy photos, virtual prenatal clinic appointments, proactive calls), and social media engagement (secure online prenatal care community).
Recommendations for the final components of OB Nest were based on feasibility and the potential impact on care. The recommendations included decreasing scheduled clinic appointments from 14 to 8, providing home monitoring devices to measure maternal blood pressure and fetal heart rate, establishing OB Nest virtual connected care visits with a registered nurse, and offering a secure online community of expectant mothers.
Trial assessed program’s efficacy, safety, satisfaction. A mixed-methods randomized controlled trial subsequently was conducted to evaluate the components of OB Nest.6 The trial included 300 pregnant women who were randomly assigned to standard prenatal care as recommended by ACOG or to OB Nest care.
OB Nest care consisted of 8 scheduled clinic appointments, 6 planned virtual (phone or online) connected care visits with a registered nurse dedicated to OB Nest, home monitoring of blood pressure (with a home digital sphygmomanometer) and fetal heart rate, and access to an online prenatal care community designated for OB Nest participants.
While publication of the trial results currently is pending, the OB Nest program appears to safely and effectively decrease the number of scheduled prenatal care visits for low-risk expectant mothers while improving the overall patient experience. OB Nest care now is offered as one of several options for low-risk expectant mothers at Mayo Clinic.
Additional avenues of study. Studies evaluating the impact of OB Nest in various nonacademic settings are now underway. Also under review is the potential cost savings of OB Nest as related to the productive lives of expectant mothers, while prenatal care safety is maintained.
The focus shift from a sick to a wellness perspective, stakeholder inclusion in the program design, and the integration of home monitoring tools are all major contributing factors to the success of OB Nest.
Continue to: Approach #3: Prisma Health utilizes mobile app technology...
Approach #3: Prisma Health utilizes mobile app technology
A third approach to reducing unnecessary visits for routine maternity care is to employ mobile app technology. Technology companies have developed app platforms for providers to use to educate and connect with patients; such apps reduce the number of routine obstetric office visits while maintaining patient satisfaction.
One group’s app experience. In a pilot study at a Prisma Health practice (South Carolina), 100 patients were placed on a reduced appointment schedule of 9 prenatal visits; the women self-monitored their weight gain and blood pressure using a remote monitoring system via an app called Babyscripts.7 Patient feedback was collected, with 45 of 100 patients responding.
Ninety-five percent of patients were satisfied with the mobile app, 94% reported positivity around pregnancy readiness, 90% were satisfied with their health care team, and 89% were happy with remote monitoring. Patients visited the app 3 times per week on average, and the top categories of interest were travel, exercise, genetics, and eating right.
One patient using the Babyscripts mobile health app and schedule optimization platform commented, “I am on my second pregnancy and wish this had been available for the first! The app is easy to use and I love seeing my weight on a graph. And I very much like the quality of the cuff” (personal data generated from Babyscripts).
In with the new
As clinicians strive to provide more patient-centered care, offering expectant families more than one way to receive their prenatal care is appropriate. Beyond the traditional 14-visit care model, we should offer use of novel options like mobile health apps, which improve the patient experience while decreasing the cost of care by reducing unnecessary visits.12 Note also that reducing visits for low-risk mothers opens space in the provider schedule for patients who need services more quickly.
Benefits for postpartum care. Traditionally, clinicians see the low-risk patient for a single follow-up appointment at 6 weeks postpartum. However, the World Health Organization recommends evaluating women at 3 days, 1 to 2 weeks, and 6 weeks postpartum.13 Further, the National Institute for Health and Care Excellence guidance recommends screening all women for resolution of postpartum blues at 10 to 14 days.14
ACOG also has made recommendations on optimizing postpartum care. In a committee opinion, ACOG recommends that all women have contact with their provider within the first 3 weeks postpartum.15 Recognizing that such an in-person visit may be difficult, ACOG has endorsed communication via text messaging, app-based support, and remote monitoring.15 An app such as Babyscripts would fill this need conveniently for both patient and provider.
In 2019, patients want choice. As maternity care providers, we should be open to considering novel, evidence-based options that may provide more cost-effective obstetric care.
- Martin JA, Hamilton BE, Osterman MJK, et al. Births: final data for 2017. Natl Vital Stat Rep. 2018;67:1-50.
- Alexander GR, Kotelchuck M. Assessing the role and effectiveness of prenatal care: history, challenges, and directions for future research. Public Health Rep. 2001;116:306-316.
- Dowswell T, Carroli G, Duley L, et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst Rev. 2015; (7):CD000934.
- de Mooij MJM, Hodny RL, O'Neil DA, et al. OB Nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Pflugeisen BM, McCarren C, Poore S, et al. Virtual visits: managing prenatal care with modern technology. MCN Am J Matern Child Nurs. 2016;41:24-30.
- Ridgeway JL, LeBlanc A, Branda M, et al. Implementation of a new prenatal care model to reduce office visits and increase connectivity and continuity of care: protocol for a mixed-methods study. BMC Pregnancy Childbirth. 2015;15:323.
- Marko KI, Krapf JM, Meltzer AC, et al. Testing the feasibility of remote patient monitoring in prenatal care using a mobile app and connected devices: a prospective observational trial. JMIR Res Protoc. 2016;5:e200.
- Carroli G, Villar J, Piaggio G, et al. WHO systematic review of randomised controlled trials of routine antenatal care. Lancet. 2001;357:1565-1570.
- Rosen MG, Merkatz IR, Hill JG. Caring for our future: a report by the expert panel on the content of prenatal care. Obstet Gynecol. 1991;77:782-787.
- American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 8th edition. Elk Grove Village, IL: American Academy of Pediatrics, American College of Obstetricians and Gynecologists; 2017.
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva, Switzerland: World Health Organization; 2016. https://apps.who.int/iris/bitstream/handle/10665/250796 /9789241549912-eng.pdf;jsessionid=C740C52F8AA1D7694CD9463152C193BA?sequence=1. Accessed June 19, 2019.
- Woo VG, Lundeen T, Matula S, et al. Achieving higher-value obstetrical care. Am J Obstet Gynecol. 2017;216:240e1-250e14.
- World Health Organization. WHO Recommendations on Postnatal Care of the Mother and Newborn. Geneva, Switzerland: WHO; 2014. https://apps.who.int/iris/bitstream/handle/10665/97603/9789241506649_eng.pdf?sequence=1. Accessed June 19, 2019.
- National Institute for Health and Care Excellence. Postnatal care up to 8 weeks after birth. Updated February 2015. https://www.nice.org.uk/guidance/cg37/chapter/1-Recommendations#maternal-health. Accessed June 19, 2019.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 736. Optimizing postpartum care. Washington, DC: ACOG; 2018.
CASE Low-risk maternity patient wants fewer prenatal visits
A recently pregnant patient asks her obstetrician if she can schedule fewer prenatal visits given that she is at low risk, wants to minimize missing work, and lives an hour away from the clinic office. Her physician tells her that she needs the standard 13 to 15 visits to have a healthy pregnancy.
Obstetric care in the United States largely remains a “one-size fits all” approach despite compelling data that fewer visits for low-risk women are medically acceptable and may be more cost-effective.
Prenatal care: One size does not fit all
With nearly 4 million births annually in the United States, prenatal care is one of the most widely used preventive health care strategies.1,2 The ideal method for providing prenatal care, however, remains controversial. At the inception of early 20th century prenatal care in the United States, preventive strategies focused in part on eclampsia-related maternal morbidity and mortality, which in turn informed the content and frequency of prenatal visits.2 Despite the dramatic changes in medical practice over the last 100 years, the basic timing and quantity of prenatal care has not changed substantively.
The lack of change is not because we have not explored other models of prenatal care and sought to introduce evidence-based change. Several studies have assessed the impact of reduced prenatal care visits for low-risk women.3-7 Systematic reviews evaluated 7 randomized trials, with more than 60,000 women enrolled, of prenatal care models with a reduced number of planned antenatal visits (4 to 9 visits vs the traditional 13 to 15 visits).3,8 There were no demonstrable differences in maternal or perinatal morbidity or mortality, particularly in higher resource settings.
Despite strong safety data and the potential cost-effectiveness of a reduced schedule of prenatal visits, US prenatal care practices generally continue to have a one-size-fits-all approach. Several organizations, however, have called for a change in practice.
Endorsing a reduced number of prenatal visits for low-risk women, the US Department of Health and Human Services Expert Panel on Prenatal Care issued a report in 1989 that stated “the specific content and timing of prenatal visits, contacts, and education should vary depending on the risk status of the pregnant woman and her fetus.”9 Consistent with that recommendation, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (ACOG) jointly published guidelines that recommend a system of goal-oriented antenatal visits at specific gestational ages and that support a reduced schedule of prenatal visits, compared with traditional models, for low-risk, parous women.10 The World Health Organization also published recommendations for an 8 “contact” prenatal care system to reduce perinatal mortality and improve women’s prenatal experience.11
Is obstetric dogma the reason for lack of change?
Concerns about patient satisfaction may play a role in limiting the use of a reduced prenatal care visit model. In trials that evaluated a model of reduced prenatal care visits, women were less satisfied with a reduced visit schedule and the gap between provider contacts.3,8 Anecdotally, providers have expressed concerns about perceived liability. Most compelling, perhaps, is the idea that the traditional prenatal schedule has become obstetric dogma.
Continue to: Consciously or unconsciously, clinicians may feel...
Consciously or unconsciously, clinicians may feel uncomfortable diverging from a schedule of visits that is firmly entrenched in obstetric practice. Continuing the status quo is easier than restructuring prenatal care practice. Ultimately, a paradigm shift may be required to broadly adopt a model of fewer prenatal visits for low-risk pregnancies.12 With these issues propelling the historic patterns of prenatal care, it is easy to see why we have not yet changed despite convincing reasons to do so.
In this article, we detail the reduced-visit prenatal care models developed at 3 institutions and how they incorporate use of today’s technology.
Approach #1: University of Utah Virtual Prenatal Care Program
The University of Utah Virtual Prenatal Care Program was conceived as a “baby step” toward developing a model of fewer total prenatal visits. Virtual visits were intended to reduce the number of prenatal face-to-face visits while maintaining the same total number of visits. Since large clinical trials had established the safety of reduced visits, the primary objectives were to retain patient satisfaction and to facilitate provider adoption.
Would women be satisfied with remote prenatal care? A prospective randomized controlled trial was designed in which 200 women were assigned to receive either a combination of telemedicine and 5 scheduled in-clinic prenatal visits (remote care group) or traditional in-clinic prenatal care (usual care group). Low-risk multigravida pregnant women who were between 6 0/7 and 16 0/7 weeks’ gestation were enrolled. The primary outcome was patient satisfaction.
The face-to-face visits were goal oriented, with scheduled physical examination, laboratory tests, or ultrasonography, and were conducted by the patient’s established obstetric provider (physician or nurse midwife) to maintain continuity of care. The remote care group self-collected measurements for weight, blood pressure, and fetal heart rate by handheld Doppler device prior to each telemedicine visit and entered the information into the electronic medical record. The purpose of the self-collected data was patient engagement and satisfaction, as well as increased provider comfort with the change in prenatal care schedule, rather than medical necessity.
The primary outcome of overall patient satisfaction with prenatal care was ascertained by questionnaire after delivery. The sample size calculation of 200 patients was based on noninferiority testing, and analysis was by intent-to-treat. The details of the trial are pending publication.
As expected, the remote care group had significantly fewer in-clinic prenatal care visits compared with the usual care group (7.2 vs 11.3 visits); the total number of prenatal visits was not different between groups. Overall satisfaction with prenatal care was very high in both the remote care and the usual care group (100% vs 97%).
The virtual prenatal care model for low-risk pregnancies, consisting of a novel remote monitoring strategy and a reduced number of in-clinic visits, was not associated with lower patient satisfaction compared with traditional care.
New care strategy gives patients a choice. The success of this clinical trial has led to its programmatic adoption at the University of Utah, and low-risk women currently are offered a choice between participating in the Virtual Prenatal Care Program or receiving traditional prenatal care. The University of Utah is moving on from the one-size-fits-all approach to adopt new strategies that provide personalized evidence-based prenatal care at the lowest cost, while retaining high patient satisfaction. Formal cost-effectiveness analyses are underway.
Continue to: Approach #2: Mayo Clinic OB Nest...
Approach #2: Mayo Clinic OB Nest
In 2011, the Mayo Clinic Obstetric Division partnered with 2 other Mayo Clinic divisions, the Center for Innovation and the Center for the Science of Health Care Delivery, to redesign prenatal care for low-risk expectant mothers.Pregnant women and their obstetric health care teams (including obstetricians, certified nurse midwives, registered nurses, and clinical support staff) were convened to develop a novel model of prenatal care.4 The goal of this collaboration centered on:
- creating an evidence-driven prenatal care model for low-risk expectant women designed by relevant stakeholders
- focusing on meeting the on-demand needs of expectant mothers
- integrating innovative 21st century technology, and
- reducing the burden of prescheduled, low-value office visits.
Exploratory efforts to develop a novel care program. Based on feedback from the collaboration and guided by these goals, 141 expectant mothers participated in 19 different experiments, enabling the health care team to understand the impact of changing various components of prenatal care.
The experiments included integration of home monitoring (home fetal Doppler devices, drop-in fetal Doppler stations, home blood pressure monitoring devices), technology-enhanced communication with obstetric team members (video chats, tummy photos, virtual prenatal clinic appointments, proactive calls), and social media engagement (secure online prenatal care community).
Recommendations for the final components of OB Nest were based on feasibility and the potential impact on care. The recommendations included decreasing scheduled clinic appointments from 14 to 8, providing home monitoring devices to measure maternal blood pressure and fetal heart rate, establishing OB Nest virtual connected care visits with a registered nurse, and offering a secure online community of expectant mothers.
Trial assessed program’s efficacy, safety, satisfaction. A mixed-methods randomized controlled trial subsequently was conducted to evaluate the components of OB Nest.6 The trial included 300 pregnant women who were randomly assigned to standard prenatal care as recommended by ACOG or to OB Nest care.
OB Nest care consisted of 8 scheduled clinic appointments, 6 planned virtual (phone or online) connected care visits with a registered nurse dedicated to OB Nest, home monitoring of blood pressure (with a home digital sphygmomanometer) and fetal heart rate, and access to an online prenatal care community designated for OB Nest participants.
While publication of the trial results currently is pending, the OB Nest program appears to safely and effectively decrease the number of scheduled prenatal care visits for low-risk expectant mothers while improving the overall patient experience. OB Nest care now is offered as one of several options for low-risk expectant mothers at Mayo Clinic.
Additional avenues of study. Studies evaluating the impact of OB Nest in various nonacademic settings are now underway. Also under review is the potential cost savings of OB Nest as related to the productive lives of expectant mothers, while prenatal care safety is maintained.
The focus shift from a sick to a wellness perspective, stakeholder inclusion in the program design, and the integration of home monitoring tools are all major contributing factors to the success of OB Nest.
Continue to: Approach #3: Prisma Health utilizes mobile app technology...
Approach #3: Prisma Health utilizes mobile app technology
A third approach to reducing unnecessary visits for routine maternity care is to employ mobile app technology. Technology companies have developed app platforms for providers to use to educate and connect with patients; such apps reduce the number of routine obstetric office visits while maintaining patient satisfaction.
One group’s app experience. In a pilot study at a Prisma Health practice (South Carolina), 100 patients were placed on a reduced appointment schedule of 9 prenatal visits; the women self-monitored their weight gain and blood pressure using a remote monitoring system via an app called Babyscripts.7 Patient feedback was collected, with 45 of 100 patients responding.
Ninety-five percent of patients were satisfied with the mobile app, 94% reported positivity around pregnancy readiness, 90% were satisfied with their health care team, and 89% were happy with remote monitoring. Patients visited the app 3 times per week on average, and the top categories of interest were travel, exercise, genetics, and eating right.
One patient using the Babyscripts mobile health app and schedule optimization platform commented, “I am on my second pregnancy and wish this had been available for the first! The app is easy to use and I love seeing my weight on a graph. And I very much like the quality of the cuff” (personal data generated from Babyscripts).
In with the new
As clinicians strive to provide more patient-centered care, offering expectant families more than one way to receive their prenatal care is appropriate. Beyond the traditional 14-visit care model, we should offer use of novel options like mobile health apps, which improve the patient experience while decreasing the cost of care by reducing unnecessary visits.12 Note also that reducing visits for low-risk mothers opens space in the provider schedule for patients who need services more quickly.
Benefits for postpartum care. Traditionally, clinicians see the low-risk patient for a single follow-up appointment at 6 weeks postpartum. However, the World Health Organization recommends evaluating women at 3 days, 1 to 2 weeks, and 6 weeks postpartum.13 Further, the National Institute for Health and Care Excellence guidance recommends screening all women for resolution of postpartum blues at 10 to 14 days.14
ACOG also has made recommendations on optimizing postpartum care. In a committee opinion, ACOG recommends that all women have contact with their provider within the first 3 weeks postpartum.15 Recognizing that such an in-person visit may be difficult, ACOG has endorsed communication via text messaging, app-based support, and remote monitoring.15 An app such as Babyscripts would fill this need conveniently for both patient and provider.
In 2019, patients want choice. As maternity care providers, we should be open to considering novel, evidence-based options that may provide more cost-effective obstetric care.
CASE Low-risk maternity patient wants fewer prenatal visits
A recently pregnant patient asks her obstetrician if she can schedule fewer prenatal visits given that she is at low risk, wants to minimize missing work, and lives an hour away from the clinic office. Her physician tells her that she needs the standard 13 to 15 visits to have a healthy pregnancy.
Obstetric care in the United States largely remains a “one-size fits all” approach despite compelling data that fewer visits for low-risk women are medically acceptable and may be more cost-effective.
Prenatal care: One size does not fit all
With nearly 4 million births annually in the United States, prenatal care is one of the most widely used preventive health care strategies.1,2 The ideal method for providing prenatal care, however, remains controversial. At the inception of early 20th century prenatal care in the United States, preventive strategies focused in part on eclampsia-related maternal morbidity and mortality, which in turn informed the content and frequency of prenatal visits.2 Despite the dramatic changes in medical practice over the last 100 years, the basic timing and quantity of prenatal care has not changed substantively.
The lack of change is not because we have not explored other models of prenatal care and sought to introduce evidence-based change. Several studies have assessed the impact of reduced prenatal care visits for low-risk women.3-7 Systematic reviews evaluated 7 randomized trials, with more than 60,000 women enrolled, of prenatal care models with a reduced number of planned antenatal visits (4 to 9 visits vs the traditional 13 to 15 visits).3,8 There were no demonstrable differences in maternal or perinatal morbidity or mortality, particularly in higher resource settings.
Despite strong safety data and the potential cost-effectiveness of a reduced schedule of prenatal visits, US prenatal care practices generally continue to have a one-size-fits-all approach. Several organizations, however, have called for a change in practice.
Endorsing a reduced number of prenatal visits for low-risk women, the US Department of Health and Human Services Expert Panel on Prenatal Care issued a report in 1989 that stated “the specific content and timing of prenatal visits, contacts, and education should vary depending on the risk status of the pregnant woman and her fetus.”9 Consistent with that recommendation, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (ACOG) jointly published guidelines that recommend a system of goal-oriented antenatal visits at specific gestational ages and that support a reduced schedule of prenatal visits, compared with traditional models, for low-risk, parous women.10 The World Health Organization also published recommendations for an 8 “contact” prenatal care system to reduce perinatal mortality and improve women’s prenatal experience.11
Is obstetric dogma the reason for lack of change?
Concerns about patient satisfaction may play a role in limiting the use of a reduced prenatal care visit model. In trials that evaluated a model of reduced prenatal care visits, women were less satisfied with a reduced visit schedule and the gap between provider contacts.3,8 Anecdotally, providers have expressed concerns about perceived liability. Most compelling, perhaps, is the idea that the traditional prenatal schedule has become obstetric dogma.
Continue to: Consciously or unconsciously, clinicians may feel...
Consciously or unconsciously, clinicians may feel uncomfortable diverging from a schedule of visits that is firmly entrenched in obstetric practice. Continuing the status quo is easier than restructuring prenatal care practice. Ultimately, a paradigm shift may be required to broadly adopt a model of fewer prenatal visits for low-risk pregnancies.12 With these issues propelling the historic patterns of prenatal care, it is easy to see why we have not yet changed despite convincing reasons to do so.
In this article, we detail the reduced-visit prenatal care models developed at 3 institutions and how they incorporate use of today’s technology.
Approach #1: University of Utah Virtual Prenatal Care Program
The University of Utah Virtual Prenatal Care Program was conceived as a “baby step” toward developing a model of fewer total prenatal visits. Virtual visits were intended to reduce the number of prenatal face-to-face visits while maintaining the same total number of visits. Since large clinical trials had established the safety of reduced visits, the primary objectives were to retain patient satisfaction and to facilitate provider adoption.
Would women be satisfied with remote prenatal care? A prospective randomized controlled trial was designed in which 200 women were assigned to receive either a combination of telemedicine and 5 scheduled in-clinic prenatal visits (remote care group) or traditional in-clinic prenatal care (usual care group). Low-risk multigravida pregnant women who were between 6 0/7 and 16 0/7 weeks’ gestation were enrolled. The primary outcome was patient satisfaction.
The face-to-face visits were goal oriented, with scheduled physical examination, laboratory tests, or ultrasonography, and were conducted by the patient’s established obstetric provider (physician or nurse midwife) to maintain continuity of care. The remote care group self-collected measurements for weight, blood pressure, and fetal heart rate by handheld Doppler device prior to each telemedicine visit and entered the information into the electronic medical record. The purpose of the self-collected data was patient engagement and satisfaction, as well as increased provider comfort with the change in prenatal care schedule, rather than medical necessity.
The primary outcome of overall patient satisfaction with prenatal care was ascertained by questionnaire after delivery. The sample size calculation of 200 patients was based on noninferiority testing, and analysis was by intent-to-treat. The details of the trial are pending publication.
As expected, the remote care group had significantly fewer in-clinic prenatal care visits compared with the usual care group (7.2 vs 11.3 visits); the total number of prenatal visits was not different between groups. Overall satisfaction with prenatal care was very high in both the remote care and the usual care group (100% vs 97%).
The virtual prenatal care model for low-risk pregnancies, consisting of a novel remote monitoring strategy and a reduced number of in-clinic visits, was not associated with lower patient satisfaction compared with traditional care.
New care strategy gives patients a choice. The success of this clinical trial has led to its programmatic adoption at the University of Utah, and low-risk women currently are offered a choice between participating in the Virtual Prenatal Care Program or receiving traditional prenatal care. The University of Utah is moving on from the one-size-fits-all approach to adopt new strategies that provide personalized evidence-based prenatal care at the lowest cost, while retaining high patient satisfaction. Formal cost-effectiveness analyses are underway.
Continue to: Approach #2: Mayo Clinic OB Nest...
Approach #2: Mayo Clinic OB Nest
In 2011, the Mayo Clinic Obstetric Division partnered with 2 other Mayo Clinic divisions, the Center for Innovation and the Center for the Science of Health Care Delivery, to redesign prenatal care for low-risk expectant mothers.Pregnant women and their obstetric health care teams (including obstetricians, certified nurse midwives, registered nurses, and clinical support staff) were convened to develop a novel model of prenatal care.4 The goal of this collaboration centered on:
- creating an evidence-driven prenatal care model for low-risk expectant women designed by relevant stakeholders
- focusing on meeting the on-demand needs of expectant mothers
- integrating innovative 21st century technology, and
- reducing the burden of prescheduled, low-value office visits.
Exploratory efforts to develop a novel care program. Based on feedback from the collaboration and guided by these goals, 141 expectant mothers participated in 19 different experiments, enabling the health care team to understand the impact of changing various components of prenatal care.
The experiments included integration of home monitoring (home fetal Doppler devices, drop-in fetal Doppler stations, home blood pressure monitoring devices), technology-enhanced communication with obstetric team members (video chats, tummy photos, virtual prenatal clinic appointments, proactive calls), and social media engagement (secure online prenatal care community).
Recommendations for the final components of OB Nest were based on feasibility and the potential impact on care. The recommendations included decreasing scheduled clinic appointments from 14 to 8, providing home monitoring devices to measure maternal blood pressure and fetal heart rate, establishing OB Nest virtual connected care visits with a registered nurse, and offering a secure online community of expectant mothers.
Trial assessed program’s efficacy, safety, satisfaction. A mixed-methods randomized controlled trial subsequently was conducted to evaluate the components of OB Nest.6 The trial included 300 pregnant women who were randomly assigned to standard prenatal care as recommended by ACOG or to OB Nest care.
OB Nest care consisted of 8 scheduled clinic appointments, 6 planned virtual (phone or online) connected care visits with a registered nurse dedicated to OB Nest, home monitoring of blood pressure (with a home digital sphygmomanometer) and fetal heart rate, and access to an online prenatal care community designated for OB Nest participants.
While publication of the trial results currently is pending, the OB Nest program appears to safely and effectively decrease the number of scheduled prenatal care visits for low-risk expectant mothers while improving the overall patient experience. OB Nest care now is offered as one of several options for low-risk expectant mothers at Mayo Clinic.
Additional avenues of study. Studies evaluating the impact of OB Nest in various nonacademic settings are now underway. Also under review is the potential cost savings of OB Nest as related to the productive lives of expectant mothers, while prenatal care safety is maintained.
The focus shift from a sick to a wellness perspective, stakeholder inclusion in the program design, and the integration of home monitoring tools are all major contributing factors to the success of OB Nest.
Continue to: Approach #3: Prisma Health utilizes mobile app technology...
Approach #3: Prisma Health utilizes mobile app technology
A third approach to reducing unnecessary visits for routine maternity care is to employ mobile app technology. Technology companies have developed app platforms for providers to use to educate and connect with patients; such apps reduce the number of routine obstetric office visits while maintaining patient satisfaction.
One group’s app experience. In a pilot study at a Prisma Health practice (South Carolina), 100 patients were placed on a reduced appointment schedule of 9 prenatal visits; the women self-monitored their weight gain and blood pressure using a remote monitoring system via an app called Babyscripts.7 Patient feedback was collected, with 45 of 100 patients responding.
Ninety-five percent of patients were satisfied with the mobile app, 94% reported positivity around pregnancy readiness, 90% were satisfied with their health care team, and 89% were happy with remote monitoring. Patients visited the app 3 times per week on average, and the top categories of interest were travel, exercise, genetics, and eating right.
One patient using the Babyscripts mobile health app and schedule optimization platform commented, “I am on my second pregnancy and wish this had been available for the first! The app is easy to use and I love seeing my weight on a graph. And I very much like the quality of the cuff” (personal data generated from Babyscripts).
In with the new
As clinicians strive to provide more patient-centered care, offering expectant families more than one way to receive their prenatal care is appropriate. Beyond the traditional 14-visit care model, we should offer use of novel options like mobile health apps, which improve the patient experience while decreasing the cost of care by reducing unnecessary visits.12 Note also that reducing visits for low-risk mothers opens space in the provider schedule for patients who need services more quickly.
Benefits for postpartum care. Traditionally, clinicians see the low-risk patient for a single follow-up appointment at 6 weeks postpartum. However, the World Health Organization recommends evaluating women at 3 days, 1 to 2 weeks, and 6 weeks postpartum.13 Further, the National Institute for Health and Care Excellence guidance recommends screening all women for resolution of postpartum blues at 10 to 14 days.14
ACOG also has made recommendations on optimizing postpartum care. In a committee opinion, ACOG recommends that all women have contact with their provider within the first 3 weeks postpartum.15 Recognizing that such an in-person visit may be difficult, ACOG has endorsed communication via text messaging, app-based support, and remote monitoring.15 An app such as Babyscripts would fill this need conveniently for both patient and provider.
In 2019, patients want choice. As maternity care providers, we should be open to considering novel, evidence-based options that may provide more cost-effective obstetric care.
- Martin JA, Hamilton BE, Osterman MJK, et al. Births: final data for 2017. Natl Vital Stat Rep. 2018;67:1-50.
- Alexander GR, Kotelchuck M. Assessing the role and effectiveness of prenatal care: history, challenges, and directions for future research. Public Health Rep. 2001;116:306-316.
- Dowswell T, Carroli G, Duley L, et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst Rev. 2015; (7):CD000934.
- de Mooij MJM, Hodny RL, O'Neil DA, et al. OB Nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Pflugeisen BM, McCarren C, Poore S, et al. Virtual visits: managing prenatal care with modern technology. MCN Am J Matern Child Nurs. 2016;41:24-30.
- Ridgeway JL, LeBlanc A, Branda M, et al. Implementation of a new prenatal care model to reduce office visits and increase connectivity and continuity of care: protocol for a mixed-methods study. BMC Pregnancy Childbirth. 2015;15:323.
- Marko KI, Krapf JM, Meltzer AC, et al. Testing the feasibility of remote patient monitoring in prenatal care using a mobile app and connected devices: a prospective observational trial. JMIR Res Protoc. 2016;5:e200.
- Carroli G, Villar J, Piaggio G, et al. WHO systematic review of randomised controlled trials of routine antenatal care. Lancet. 2001;357:1565-1570.
- Rosen MG, Merkatz IR, Hill JG. Caring for our future: a report by the expert panel on the content of prenatal care. Obstet Gynecol. 1991;77:782-787.
- American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 8th edition. Elk Grove Village, IL: American Academy of Pediatrics, American College of Obstetricians and Gynecologists; 2017.
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva, Switzerland: World Health Organization; 2016. https://apps.who.int/iris/bitstream/handle/10665/250796 /9789241549912-eng.pdf;jsessionid=C740C52F8AA1D7694CD9463152C193BA?sequence=1. Accessed June 19, 2019.
- Woo VG, Lundeen T, Matula S, et al. Achieving higher-value obstetrical care. Am J Obstet Gynecol. 2017;216:240e1-250e14.
- World Health Organization. WHO Recommendations on Postnatal Care of the Mother and Newborn. Geneva, Switzerland: WHO; 2014. https://apps.who.int/iris/bitstream/handle/10665/97603/9789241506649_eng.pdf?sequence=1. Accessed June 19, 2019.
- National Institute for Health and Care Excellence. Postnatal care up to 8 weeks after birth. Updated February 2015. https://www.nice.org.uk/guidance/cg37/chapter/1-Recommendations#maternal-health. Accessed June 19, 2019.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 736. Optimizing postpartum care. Washington, DC: ACOG; 2018.
- Martin JA, Hamilton BE, Osterman MJK, et al. Births: final data for 2017. Natl Vital Stat Rep. 2018;67:1-50.
- Alexander GR, Kotelchuck M. Assessing the role and effectiveness of prenatal care: history, challenges, and directions for future research. Public Health Rep. 2001;116:306-316.
- Dowswell T, Carroli G, Duley L, et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst Rev. 2015; (7):CD000934.
- de Mooij MJM, Hodny RL, O'Neil DA, et al. OB Nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Pflugeisen BM, McCarren C, Poore S, et al. Virtual visits: managing prenatal care with modern technology. MCN Am J Matern Child Nurs. 2016;41:24-30.
- Ridgeway JL, LeBlanc A, Branda M, et al. Implementation of a new prenatal care model to reduce office visits and increase connectivity and continuity of care: protocol for a mixed-methods study. BMC Pregnancy Childbirth. 2015;15:323.
- Marko KI, Krapf JM, Meltzer AC, et al. Testing the feasibility of remote patient monitoring in prenatal care using a mobile app and connected devices: a prospective observational trial. JMIR Res Protoc. 2016;5:e200.
- Carroli G, Villar J, Piaggio G, et al. WHO systematic review of randomised controlled trials of routine antenatal care. Lancet. 2001;357:1565-1570.
- Rosen MG, Merkatz IR, Hill JG. Caring for our future: a report by the expert panel on the content of prenatal care. Obstet Gynecol. 1991;77:782-787.
- American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 8th edition. Elk Grove Village, IL: American Academy of Pediatrics, American College of Obstetricians and Gynecologists; 2017.
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva, Switzerland: World Health Organization; 2016. https://apps.who.int/iris/bitstream/handle/10665/250796 /9789241549912-eng.pdf;jsessionid=C740C52F8AA1D7694CD9463152C193BA?sequence=1. Accessed June 19, 2019.
- Woo VG, Lundeen T, Matula S, et al. Achieving higher-value obstetrical care. Am J Obstet Gynecol. 2017;216:240e1-250e14.
- World Health Organization. WHO Recommendations on Postnatal Care of the Mother and Newborn. Geneva, Switzerland: WHO; 2014. https://apps.who.int/iris/bitstream/handle/10665/97603/9789241506649_eng.pdf?sequence=1. Accessed June 19, 2019.
- National Institute for Health and Care Excellence. Postnatal care up to 8 weeks after birth. Updated February 2015. https://www.nice.org.uk/guidance/cg37/chapter/1-Recommendations#maternal-health. Accessed June 19, 2019.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 736. Optimizing postpartum care. Washington, DC: ACOG; 2018.