User login
Supporting the Needs of Stroke Caregivers Across the Care Continuum
From the School of Nursing, University of North Carolina-Wilmington, Wilmington, NC (Dr. Lutz), and the Kaiser Foundation Rehabilitation Center, Kaiser Permanente, Vallejo, CA (Ms. Camicia).
Abstract
- Objectives: To describe issues faced by stroke family caregivers, discuss evidence-based interventions to improve caregiver outcomes, and provide recommendations for clinicians caring for stroke survivors and their family caregivers.
- Methods: Literature review.
- Results: Caregiver health is linked to the stroke survivor’s degree of functional recovery; the more severe the level of disability, the more likely the caregiver will experience higher levels of strain, increased depression, and poor health. Inadequate caregiver preparation contributes to poorer outcomes. Caregivers describe many unmet needs including skills training; communicating with providers; resource identification and activation; finances; respite; and emotional support. Caregivers need to be assessed for gaps in preparation to provide care. Interventions are recommended that combine skill-building and psycho-educational strategies; are tailored to individual caregiver needs; are face-to-face when feasible; and include 5 to 9 sessions. Family counseling may also be indicated. Intermittent assessment of caregiving outcomes should be conducted so that changing needs can be addressed.
- Conclusions: Stroke caregiving affects the caregiver’s physical, mental, and emotional health, and these effects are sustained over time. Poorly prepared caregivers are more likely to experience negative outcomes and their needs are high during the transition from inpatient care to home. Ongoing support is also important, especially for caregivers who are caring for a stroke survivor with moderate to severe functional limitations. In order to better address unmet needs of stroke caregivers, intermittent assessments should be conducted so that interventions can be tailored to their changing needs over time.
Key words: stroke; family caregivers; care transitions; patient-centered care.
Stroke is a leading cause of major disability in the United States [1] and around the world [2]. Of the estimated 6.6 million stroke survivors living in the US, more than 4.5 million have some level of disability following stroke [1]. In 2009, more than 970,000 persons were hospitalized with stroke in the US with an average length of stay of 5.3 days [3]. Approximately 44% of stroke survivors are discharged home directly from acute care without post-acute care [4]. Only about 25% of stroke survivors receive care in inpatient rehabilitation facilities [4] even though the American Heart Association (AHA) stroke rehabilitation guidelines recommend this level of care for qualified patients [5]. Regardless of the care trajectory, when stroke survivors return home they frequently require assistance with basic and instrumental activities of daily living (BADL/IADL), usually provided by family members who often feel unprepared and overwhelmed by the demands and responsibilities of this caregiving role.
The deleterious effects of caregiving have been identified as a major public health concern [6]. A robust body of literature has established that caregivers are often adversely affected by the demands of their caregiving role. However, much of this literature focuses on caregivers for persons with dementia. Needs of stroke caregivers are categorically different from caregivers of persons with dementia in that stroke is an unpredictable, life-disrupting, crisis event that occurs suddenly leaving family members with insufficient time to prepare for the new roles and caregiving responsibilities. The patient typically transitions from being cared for by multiple providers in an acute care, inpatient rehabilitation facility, or skilled nursing facility (SNF)—24 hours a day, 7 days a week—to relying fully on one person (most often a spouse or adult child) who may not be ready to handle the overwhelming demands and constant vigilance required for adequate care at home. Studies have repeatedly demonstrated the damaging health effects of caregiving. Caregivers describe feeling isolated, abandoned, and alone [7–9], and what frequently follows is a predictable trajectory of depression and deteriorating health and well-being [7,10–13]. The purpose of this article is to describe difficulties and issues faced by family members who are caring for a loved one following stroke, discuss evidence-based interventions designed to improve stroke caregiver outcomes, and provide recommendations for clinicians who care for stroke survivors and their family caregivers post-stroke.
Difficulties and Issues Faced by Caregivers
With an aging population and increasing incidence of stroke, it is imperative that we identify and address the ongoing needs of stroke survivors and their family caregivers in the post-stroke recovery period. Multiple studies acknowledge that stroke is a life-changing event for patients and their family members [9,14] that often results in overwhelming feelings of uncertainty, fear [15], grief, and loss [9]. Stroke also can have long-term effects on the health of stroke survivors and their family caregivers. Studies have identified the effects of caregiving on the health of caregivers and subsequent links between stroke survivor and caregiver outcomes over time [12,16,17]; the ongoing needs of stroke caregivers post-discharge [18,19]; and the importance of assessing caregiver preparedness and subsequent caregiving outcomes [5,20].
Effects of Caregiving on the Health of Caregivers and Stroke Survivors
Research on stroke caregiving consistently indicates that caregiver health is inextricably linked to the stroke survivor’s degree of physical, cognitive, psychological, and emotional recovery. The more severe the patient’s level of disability, the more likely the caregiver will experience higher levels of strain, increased depression, and poor health outcomes [21]. Studies also indicate that certain caregiver characteristics, such as being female or having lower educational level, pre-existing health conditions [7,22,23], poor family functioning, lack of social support [22,24], or lack of preparation [25], are all risk factors for poorer caregiver outcomes.
Stroke family caregivers often experience overwhelming physical and emotional strain, depressive symptoms, sleep deprivation, decline in physical and mental health, reduced quality of life, and increased isolation [7,10,11,14,26,27]. Perceived burden has been positively associated with caregiver depressive symptoms [12,14,28,29]. Depressive symptoms in caregivers, with a reported incidence of 14% [30] to 33% [31], may persist for several years post-stroke. In a study of the long-term effects of caregiving with 235 stroke caregivers when compared with non-caregivers, researchers found that caregivers had more depressive symptoms and poorer life satisfaction and mental health quality of life at 9 months post-stroke, and many of these differences continued for 3 years post-discharge [23].
Lower stroke survivor functioning and higher depressive symptoms are correlated with higher caregiver depressive symptoms and burden, and poorer coping skills and mental health [12,21]. A review of stroke caregiving literature by van Heugten et al [32] indicated that long-term caregiver functioning was influenced by stroke survivor physical and cognitive functioning and behavioral issues; caregiver psychological and emotional health; quality of family relationships; social support; and caregiver demographics. Caregivers of stroke survivors with aphasia may have more difficulties providing care, increased burden and strain, higher depressive symptoms, and other negative stroke-related outcomes [33].
Gaugler [34] conducted a systematic review of 117 studies and reported that caring for stroke survivors who were older, in poorer health, and had greater stroke severity increased the likelihood of poorer emotional and psychological family caregiver outcomes. Caregivers who had “negative problem orientation and less social support” were more likely to have depressive symptoms and poorer self-rated health at 1-year post-stroke. One of the best predictors of caregiver stress and poor health in the first year post-stroke was lack of caregiver preparation [25,34].
Research also suggests that stroke survivor outcomes are influenced by the ability of the family caregiver to provide emotional and instrumental support as well as assistance with BADL/IADL [6,35]. As the caregiver’s health decreases, the stroke survivor’s health and recovery will also likely suffer and ultimately may result in re-hospitalization or nursing home placement. For example, Perrin et al found a consistent reciprocal relationship between caregiver health and stroke survivor functioning, such that the quality of caregiving may be affected by caregiver burden and depressive symptoms, which in turn can impair the functional, psychological, and emotional recovery of the stroke survivor [21]. Studies have also linked poorer caregiver well-being to increased depressive symptoms in stroke survivors [36,37].
Positive effects of caregiving have also been reported, including a feeling of confidence, satisfaction in providing good quality care [30,39,40], an improved relationship with the care recipient [30,40,41], having greater life appreciation, and feeling needed and appreciated [40]. In a systematic review of 9 studies, improvements in the stroke survivor’s condition was a source of positive caregiving experiences [40]. In 2 studies, two-thirds of caregivers surveyed affirmed all survey items related to positive aspects of caregiving [30,42]. Additionally, studies have demonstrated that caregivers who engaged in emotion- and problem-focused coping strategies had positive caregiving experiences [40]. Haley et al found that by 3 years post-stroke many of the ill effects of caregiving had resolved, suggesting that some caregivers may be successful in adapting to their “new” post-stroke lives [23].
Understanding the difficulties and issues faced by caregivers throughout the trajectory, from immediately following the stroke through the transition home and, ideally, the adaptation of the caregiver to this new life, provides an opportunity for health care professionals to intervene with strategies to support this major life change.
Caregiving Trajectory and Ongoing Needs of Stroke Caregivers
Stroke survivors and their family caregivers rapidly move from intensive therapy and nursing case management while in a facility to little or no assistance following discharge. Despite case management and discharge planning services received while in an institutional setting, the transition from inpatient care to home can be a crisis point for caregivers [9]. They describe having to figure things out for themselves with little or no formal support after discharge [9,43,44], leaving them feeling overwhelmed, exhausted, and abandoned once they return home [9].
These family members rarely make an active choice to become caregivers; rather, they take on the role because they are unable to perceive or access any other suitable alternatives [8,45]. Whatever their circumstances, these devoted family members are particularly vulnerable as they transition into the caregiving role without an adequate support system for assessing and addressing their needs [7–9,46]. Without this assistance, caregivers develop their own solutions and strategies to meet the needs of the care recipient after discharge [47,48]. Unfortunately, these strategies are often ineffective and may result in safety risks for patients (eg, falls, skin breakdown, choking), and care-related injuries (eg, falls, muscle strains, bruises) and increased stress and anxiety for caregivers [48–50].
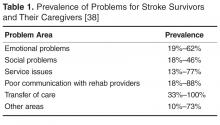
Caregivers have described unmet needs in many domains including skills training, communicating with providers, resource identification and activation, finances, respite, and emotional support [35,44,48,51,52]. Bakas et al found that in the first 6 months post-discharge, stroke caregivers had needs and concerns related to information, emotions and behaviors, physical care, instrumental care, and personal responses to caregiving [48], and that their information needs change during the course of the patient’s recovery [53]. In a study by Lutz et al [44], caregivers identified multiple areas where they felt they were unprepared to assume the caregiving role post-discharge. These included identifying and activating resources; making home and transportation modifications to improve accessibility; developing skills in providing physical care and therapies; managing medications and behavioral issues; preventing falls; coordinating care across settings; attending to other family responsibilities; and caring for themselves.
In a study of interactions between rehabilitation providers and stroke caregivers, Creasy et al [52] noted that caregivers have needs, which were often not recognized, in the following areas: information; providing emotional support for the stroke survivor and having their own emotional support needs met; being involved in treatment decisions; and being adequately prepared for discharge home. Caregivers’ interaction styles with providers, which ranged from passive to active/directing, affected their abilities to have their needs recognized and addressed. These findings highlight the importance of recognizing the caregiver’s interaction style and tailoring communication strategies accordingly.
Cameron et al [54] noted that caregiver support needs change over time, with needs being highest during the inpatient phase as they prepare for discharge home. Moreover, caregivers who are providing care for stroke survivors with more severe functional limitations need more support over a longer period of time. Recognizing the needs of stroke caregivers, the 2016 Canadian Stroke Best Practice Recommendations on Managing Transitions of Care Following Stroke includes recommendations related to assessing, educating, and supporting stroke family caregivers [55].
Assessing Caregiver Readiness and Related Outcomes
Young et al [58] recommend specific domains for a comprehensive readiness assessment of stroke family caregivers. Caregiver domains include strength of the caregiver/care recipient relationship; caregiver willingness to provide care; pre-existing health conditions, previous responsibilities, caregiving experience, home and transportation accessibility, available resources, emotional response to the stroke, and ability to sustain the caregiving role. This type of readiness assessment should be completed early in the care trajectory, while the stroke survivor is receiving inpatient care, so that care plans can be tailored to address gaps in caregiver preparation prior to discharge. It is especially important for new caregivers and those caring for stroke survivors with significant functional limitations [44]. Currently there are no tools designed to assess a family member’s readiness to assume the caregiver role.
Validated instruments have been developed to assess caregiving outcomes, including preparedness, with caregivers who have been providing care for a period of time. For example, the Mutuality and Preparedness Scales of the Family Caregiver Inventory was developed with caregivers 6 months post-discharge [59] and has been validated with stroke caregivers at 3 months post-discharge [60].
Several validated tools are available to assess the caregiver’s changing needs and the effects of care provision on well-being [8,45,61]. For example, the Caregiver Strain Index [62] has been validated in studies with stroke family caregivers [11,28]. Bakas developed 2 scales to specifically assess stroke caregivers post-discharge. The Bakas Caregiving Outcomes Scale assesses caregiver life changes [63] and the Needs and Concerns Checklist assesses post-discharge caregiver needs [48]. There are many other instruments designed to assess general caregiving outcomes, including depressive symptoms, burden, anxiety, and well-being. For a list relevant tools see Deeken et al [61] and The Selected Caregiver Assessment Measures from the Family Caregiver Alliance [64].
While these scales are helpful for assessing caregivers who are already providing care, they do not capture the gaps in caregiver readiness prior to patient discharge from the institutional setting. Taken together, these studies suggest that assessing readiness and implementing interventions to improve caregiver preparation prior to discharge and assessing and addressing their changing needs over time, from inpatient care to community reintegration, may be important strategies for improving both caregiver and stroke survivor outcomes. These strategies may also facilitate sustainability of the caregiver role over time.
Interventions to Improve Caregiver Outcomes
In a review of 39 articles representing 32 caregiver and dyad intervention studies, researchers from the AHA made 13 evidence-based recommendations. Recommendations with the highest level of evidence indicated that (1) interventions that combined skill-building with psycho-educational programs were better than psycho-educational interventions alone; (2) interventions that are tailored to the individual are preferred over “one-size-fits-all” interventions; (3) face-to-face interventions are preferred, but telephone interventions can be useful when face-to-face is not feasible; and (4) interventions with 5 to 9 sessions are recommended [65]. In a review of 18 studies, Cheng et al confirmed the recommendation that psychoeducational interventions that focused on skill building improved caregiver well-being and reduced stroke survivor heath care utilization [66].
Studies also recommend that families may need family counseling to help them develop positive coping strategies and adjust to their lives after stroke [66]. Stroke survivors and their families experience grief and loss as they begin to realize how the stroke has changed their relationships, roles, responsibilities, and future plans for their lives (eg, work, retirement). While many inpatient rehabilitation facilities may provide services from a neuro-psychologist to discuss post-stroke changes in the brain and possible behavioral and emotional manifestations, referrals for family counseling to address the impact of stroke on the family and community reintegration are seldom provided [9].
Recent interventions have shown promise in improving stroke caregiver outcomes. For example, Bakas et al. completed a randomized controlled trial of an 8-week, nurse-delivered, Telephone Assessment and Skill-Building Kit (TASK) intervention [67]. Caregivers in the intervention group with moderate to severe depressive symptoms at baseline demonstrated significant improvements in depressive symptoms and life changes at 8, 24, and 52 weeks. The TASK shows promise because it can reach caregivers in rural and urban areas at a relatively low cost [67].
Recognizing the need to improve post-acute care for stroke survivors and their family caregivers, several large funded clinical trials are being tested in the US and globally. For example, the ATTEND Trial in India is testing a home-based, caregiver-led rehabilitation intervention [68]. The Comprehensive Post-Acute Stroke Services (COMPASS) study in North Carolina, is a state-wide pragmatic, randomized controlled trial testing a comprehensive community-based patient-centered post-acute care intervention with stroke survivors and their caregivers (www.nccompass-study.org). Results of these and other studies will continue to identify evidence-based strategies to improve care coordination, quality of care, and post-stroke outcomes for stroke survivors and their caregivers
Recommendations for Clinicians
Based on this review we have identified strategies that clinicians can implement across the care continuum that may help reduce caregiver strain and burden, and improve outcomes for family caregivers and the stroke survivors for whom they provide care. The evidence suggests that caregivers need assistance in building skills, not only in providing the care needed by the stroke survivor but also in solving problems as they arise; navigating the multiple systems of care, including understanding options for post-acute care; accessing community resources; communicating effectively with health care and social support providers; and dealing with the emotional effects of stroke [44,52].
Caregivers need help in navigating the multiple providers and systems of care to get the services the stroke survivor needs as well as to secure support services. They need information from trusted sources about stroke prevention and available community resources. Providinga list of resources is often insufficient, especially in the first few weeks or months post-stroke; these caregivers are already overwhelmed with the enormity of the tasks and responsibilities that they have taken on as a caregiver. Instead they need someone who can advocate for them and connect them with the appropriate resources at the right time.
They also need assistance developing and maintaining self-care strategies so they can sustain the caregiving role long-term. Identifying opportunities for respite and helping them activate informal and formal resources, such as other family members, friends, church groups, neighbors, and services from local senior centers, independent living centers, or area agencies on aging can help them identify assistance with the breadth of duties including care of the stroke survivor, meal preparation, transportation, or a supportive listening ear. It is important for the caregiver, in addition to any other close support person as available, to have a facilitated discussion withthe healthcare team to brainstorm activities where assistance may be provided and who might be approached to help.
The timing of providing support and resources is also critical. Becoming a caregiver is a process and often family members who are new to the role need more intense direct assistance and support when the stroke survivor first comes home, but many may need ongoing support over time. Research suggests it can take caregivers up to 3 years to figure out how to manage the new responsibilities, learn to navigate the multiple systems for careand services, establish confidence in their abilities, deal with the emotional upheaval, and to adapt to their new lives [23].
Research indicates the 44% of stroke patients receive no post-acute care. Clinicians also need to advocate for patients to get the most appropriate level of organized, coordinated, and inter-professional post-acute care [5]. This requires that they understand the different levels of post-acute care, including the criteria for admission, the scope and intensity of nursing, therapy, physician and other services provided in each setting, and the associated clinical outcomes. This knowledge is also necessary to enable clinicians to educate stroke survivors and their caregivers on post-acute care so that they understand the process and can effectively self-advocate for the provision of appropriate services as needed.
Approximately 45% of stroke survivors in the US are discharged either to an inpatient rehabilitation facility or SNF for rehabilitation [4]. Patients discharged to an inpatient rehabilitation facility receive a minimum of 3 hours of therapy per day and are cared for 24 hours/day by a staff led by registered nurses (RNs) with rehabilitation expertise. SNFs do not have minimum requirements for hours of therapy, 24-hour RN staffing, nor a requirement for nurses with specialty training in rehabilitation. Pressure to reduce the length of stay in acute care often results in providers transitioning stroke survivors to the post-acute care setting that accepts the patient first. Because SNFs have fewer criteria for admission, they are more likely to rapidly accept a patient for care when compared to an inpatient rehabilitation facility. Providers must determine and make recommendations for the most appropriate level of post-acute care to ensure the stroke patients’ rehabilitation needs can be met in the recommended setting [5,69]. It is also essential that family caregivers have the knowledge and skills to advocate for the appropriate level of post-acute care based on the stroke survivor’s expected recovery trajectory. Research has demonstrated that that stroke survivors admitted to an inpatient rehabilitation facility, when compared to similar patients in a SNF, have better outcomes, including improved function [70] and lower re-hospitalization and death rates [71,72]. The Association of Rehabilitation Nurses provides resources for health care professionals and patients regarding rehabilitation. For more information for professionals about levels of post-acute care, see www.rehabnurse.org/uploads/files/healthpolicy/ARN_Care_Transitions_White_Paper_Journal_Copy_FINAL.pdf [73]. For information for patients and caregivers, see www.restartrecovery.org.
Providers must also be knowledgeable about community resources in order to provide connections to services and agencies that are relevant to the changing needs of the caregiver over time. Initially, caregivers may need assistance in meeting the stroke survivor’s BADL/IADL, and later needs may expand to include support groups, respite, and opportunities for a greater community engagement.
Training in time management provides room in the busy caregiving schedule for self-care for the caregiver. Providers must assist with determining routines that meet the needs of both the caregiver and stroke survivor, as the health of each is dependent on the other. Assistance in developing a wellness program that is feasible for the caregiver to maintain will improve adoption of health promoting practices.
As discussed above, the needs of both the stroke survivor and caregiver vary along the post-stroke trajectory. Therefore, both caregivers and stroke survivors should be assessed intermittently over time: caregivers for evidence of effective coping strategies and confidence in the sustaining the caregiving role, and stroke survivors for improvement in their functional abilities and compensatory strategies in BADL/IADL. The opportunity for the stroke survivor to assume household tasks that decrease the caregiver burden, in addition to providing a greater sense of purpose for the stroke survivor, must be explored. For example, the stroke survivor may be able to assist with activities such as meal planning and components of meal preparation or light housekeeping utilizing adaptive devices as needed.
Additional research is necessary to understand how the needs of caregivers change over time, the appropriate timing of reassessment, and the evaluation of interventions to facilitate the transition into this role, while preventing the adverse effects of caregiving on the health of the caregiver and stroke survivor during this transition period.
Conclusion
There is clear evidence that stroke caregiving can have detrimental effects on the physical, mental, and emotional health of caregivers, and that these effects are sustained over time. Evidence also indicates that caregivers who are not well-prepared to assume the caregiving role are more likely to experience negative outcomes. Studies suggest that the time of transition from inpatient care to home is a time of crisis for caregivers and that their support needs are high during this time. However, research also indicates that while needs may change over time, caregivers need ongoing support, especially if they are providing care for a stroke survivor who has moderate to severe physical, cognitive, and/or communication limitations.
In order to better understand the needs of stroke caregivers, a pre-discharge assessment of their readiness to provide care should be conducted so that interventions can be tailored to address their needs to minimize negative effects of a poorly planned transition [69]. Currently, there are assessment tools that can be used with caregivers post-discharge to assess their self-reported needs (after they have an understanding of the role) and caregiving outcomes. Research is needed to develop a valid and reliable tool thatpre-emptively assesses the gaps in caregiver readiness that can be utilized prior to the transition from the institutional setting to home. This will enable the identification and evaluation of primary prevention strategies to improve caregiver preparation so that the adaption to the new caregiving role can be expedited, minimizing the adverse health effects on both the caregiver and stroke survivor.
Providers must be aware of the changing needs of stroke survivors and tailor plans of care accordingly, using evidenced-based interventions. Policy makers must consider research on the long term effects of caregiving and consider legislation to support the health and respite needs of the growing population of caregivers. This will contribute to attaining the 3 aims of the National Quality Strategy: improving quality of care, improving health, and reducing health care system costs [74].
Corresponding author: Barbara J. Lutz, PhD, 601 S. College Rd., Wilmington, NC 28403, [email protected].
Financial disclosures: None.
1. Mozaffarian D, Benjamin EJ, Go AS, et al. on behalf of the American Heart Association Statistics Committee & Stroke Statistics Subcommittee. Heart disease and stroke statistics - 2016 update: A report from the American Heart Association. Circulation 2016;132:e38–e360.
2. Feigin VL, Krishnamurthi RV, Parmar P, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: The GBD 2013 Study. Neuroepidemiology 2015;45:161–76.
3. Hall MJ, Levant S, DeFrances CJ. Hospitalization for stroke in U.S. hospitals, 1989-2009. Hyattsville, MD: National Center for Heatlh Statistics; 2012.
4. Prvu Bettger J, McCoy L, Smith EE, et al. Contemporary trends and predictors of postacute service use and routine discharge home after stroke. J Am Heart Assoc 2015;4(2).
5. Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016;47:e98–e169.
6. Talley RC, Crews JE. Framing the public health of caregiving. Am J Public Health 2007;97:224–8.
7. van Exel NJ, Koopmanschap MA, van den Berg B, et al. Burden of informal caregiving for stroke patients: odentification of caregivers at risk of adverse health effects. Cerebrovasc Dis 2005;19:11–7.
8. van Exel NJ, Scholte op Reimer WJ, Brouwer WB, et al. Instruments for assessing the burden of informal caregiving for stroke patients in clinical practice: a comparison of CSI, CRA, SCQ and self-rated burden. Clin Rehabil 2004;18:203–14.
9. Lutz BJ, Young ME, Cox KJ, et al. The crisis of stroke: experiences of patients and their family caregivers. Top Stroke Rehabil 2011;18:786–97.
10. McCullagh E, Brigstocke G, Donaldson N, Kalra L. Determinants of caregiving burden and quality of life in caregivers of stroke patients. Stroke 2005;36:2181–6.
11. van Exel NJ, Brouwer WB, van den Berg B, et al. What really matters: an inquiry into the relative importance of dimensions of informal caregiver burden. Clin Rehabil 2004;18:683–93.
12. Perrin PB, Heesacker M, Hinojosa MS et al. Identifying at-risk, ethnically diverse stroke caregivers for counseling: a longitudinal study of mental health. Rehabil Psychol 2009;54:138–49.
13. Greenwood N, Mackenzie A, Cloud GC, Wilson N. Informal carers of stroke survivors--factors influencing carers: a systematic review of quantitative studies. Disabil Rehabil 2008;30:1329–49.
14. Camak DJ. Addressing the burden of stroke caregivers: a literature review. J Clin Nurs 2015;24:2376–82.
15. White CL, Barrientos R, Dunn K. Dimensions of uncertainty after stroke: perspectives of the stroke survivor and family caregiver. J Neurosci Nurs 2014;46:233–40.
16. Saban KL, Sherwood PR, DeVon HA, Hynes DM. Measures of psychological stress and physical health in family caregivers of stroke survivors: a literature review. J Neurosci Nurs 2010;42:128–38.
17. Kruithof WJ, Post, MWM, van Mierlo M, et al. Caregiver burden and emotional problems in partners of stroke patients at two months and one year post-stroke: determinants and prediction. Patient Educ Couns 2016. Forthcoming.
18. Sarre S, Redlich C, Tinker A, et al. A systematic review of qualitative studies on adjusting after stroke: lessons for the study of resilience. Disabil Rehabil 2014;36:716–26.
19. Cameron JI, Naglie G, Dick T, et al. Are we meeting the changing needs of family caregivers to stroke survivors across the care continuum? A systematic review of the caregiver intervention literature. Stroke 2012;43:E143-E.
20. Miller EL, Murray L, Richards L, et al. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke ptient: a scientific statement from the American Heart Association. Stroke 2010;41:2402–48.
21. Perrin PB, Heesacker M, Stidham BS, et al. Structural equation modeling of the relationship between caregiver psychosocial variables and functioning of individuals with stroke. Rehabil Psychol 2008;53:54–62.
22. Roth DL, Perkins M, Wadley VG, et al. Family caregiving and emotional strain: associations with quality of life in a large national sample of middle-aged and older adults. Qual Life Res 2009;18:679–88.
23. Haley WE, Roth DL, Hovater M, Clay OJ. Long-term impact of stroke on family caregiver well-being: a population-based case-control study. Neurology 2015;84:1323–9.
24. Greenwood N, Mackenzie A. Informal caring for stroke survivors: meta-ethnographic review of qualitative literature. Maturitas 2010;66:268–76.
25. Ostwald SK, Bernal MP, Cron SG, Godwin KM. Stress experienced by stroke survivors and spousal caregivers during the first year after discharge from inpatient rehabilitation. Top Stroke Rehabil 2009;16:93–104.
26. Brereton L, Carroll C, Barnston S. Interventions for adult family carers of people who have had a stroke: a systematic review. Clin Rehabil 2007;21:867–84.
27. Greenwood N, Mackenzie A, Cloud G, Wilson N. Loss of autonomy, control and independence when caring: a qualitative study of informal carers of stroke survivors in the first three months after discharge. Disabil Rehabil 2009:1–9.
28. Visser-Meily A, Post M, van de Port I, et al. Psychosocial functioning of spouses of patients with stroke from initial inpatient rehabilitation to 3 years poststroke: course and relations with coping strategies. Stroke 2009;40:1399–404.
29. Visser-Meily A, Post M, van de Port I, et al. Psychosocial functioning of spouses in the chronic phase after stroke: improvement or deterioration between 1 and 3 years after stroke? Patient Educ Couns 2008;73:153–8.
30. Haley WE, Allen JY, Grant JS, et al. Problems and benefits reported by stroke family caregivers: results from a prospective epidemiological study. Stroke 2009;40:2129–33.
31. Berg A, Palomaki H, Lonnqvist J, et al. Depression among caregivers of stroke survivors. Stroke 2005;36:639–43.
32. van Heugten C, Visser-Meily A, Post M, Lindeman E. Care for carers of stroke patients: evidence-based clinical practice guidelines. J Rehabil Med 2006;38:153–8.
33. Bakas T, Kroenke K, Plue LD, et al. Outcomes among family caregivers of aphasic versus nonaphasic stroke survivors. Rehabil Nurs 2006;31:33–42.
34. Gaugler JE. The longitudinal ramifications of stroke caregiving: a systematic review. Rehabil Psychol 2010;55:108–25.
35. Andrew NE, Kilkenny MF, Naylor R, et al. The relationship between caregiver impacts and the unmet needs of survivors of stroke. Patient Prefer Adher 2015;9:1065–73.
36. Chung ML, Bakas T, Plue LD, Williams LS. Effects of self-esteem, optimism, and perceived control on depressive symptoms in stroke survivor-spouse dyads. J Cardiovasc Nurs 2016;31:E8–E16.
37. Grant JS, Clay OJ, Keltner NL, et al. Does caregiver well-being predict stroke survivor depressive symptoms? a mediation analysis. Top Stroke Rehabil 2013;20:44–51.
38. Murray J, Young J, Forster A, Ashworth R. Developing a primary care-based stroke model: the prevalence of longer-term problems experienced by patients and carers. Br J Gen Pract 2003;53:803–7.
39. Pierce LL, Steiner V, Govoni A, et al. Two sides to the caregiving story. Top Stroke Rehabil 2007;14:13–20.
40. Mackenzie A, Greenwood N. Positive experiences of caregiving in stroke: a systematic review. Disabil Rehabil 2012;34:1413–22.
41. Parag V, Hackett ML, Yapa CM, et al. The impact of stroke on unpaid caregivers: results from The Auckland Regional Community Stroke study, 2002-2003. Cerebrovasc Dis (Basel, Switzerland) 2008;25:548–54.
42. Kruithof WJ, Post MWM, Visser-Meily JMA. Measuring negative and positive caregiving experiences: a psychometric analysis of the Caregiver Strain Index Expanded. Clin Rehabil 2015;29:1224–33.
43. Lutz BJ. Determinants of discharge destination for stroke patients. Rehabil Nurs 2004;29:154–63.
44. Lutz BJ, Young ME, Creasy KR, et al. Improving stroke caregiver readiness for transition from inpatinet rehabilitation to home. Gerontologist 2016. Forthcoming.
45. Visser-Meily JM, Post MW, Riphagen II, Lindeman E. Measures used to assess burden among caregivers of stroke patients: a review. Clin Rehabil 2004;18:601–23.
46. Moon M. The unprepared caregiver. Gerontologist 2016 Apr 21.
47. Pierce LL, Steiner V, Govoni AL, et al. Internet-based support for rural caregivers of persons with stroke shows promise. Rehabil Nurs 2004;29:95–9,103.
48. Bakas T, Austin JK, Okonkwo KF, et al. Needs, concerns, strategies, and advice of stroke caregivers the first 6 months after discharge. J Neurosci Nurs 2002;34:242–51.
49. Lutz BJ, Chumbler NR, Lyles T, et al. Testing a home-telehealth programme for US veterans recovering from stroke and their family caregivers. Disabil Rehabil 2009;31:402–9.
50. Hayes J, Chapman P, Young LJ, Rittman M. The prevalence of injury for stroke caregivers and associated risk factors. Top Stroke Rehabil 2009;16:300–7.
51. Cameron JI, Gignac MA. “Timing It Right”: a conceptual framework for addressing the support needs of family caregivers to stroke survivors from the hospital to the home. Patient Educ Couns 2008;70:305–14.
52. Creasy KR, Lutz BJ, Young ME, et al. The impact of interactions with providers on stroke caregivers’ needs. Rehabil Nurs 2013;38:88–98.
53. Bakas T, Farran CJ, Austin JK, et al. Stroke caregiver outcomes from the Telephone Assessment and Skill-Building Kit (TASK). Top Stroke Rehabil 2009;16:105–21.
54. Cameron JI, Naglie G, Silver FL, Gignac MA. Stroke family caregivers’ support needs change across the care continuum: a qualitative study using the timing it right framework. Disabil Rehabil 2013;35:315–24.
55. Cameron JI, O’Connell C, Foley N, et al. Canadian Stroke Best Practice Recommendations: Managing transitions of care following Stroke. Guidelines Update 2016. Int J Stroke. 2016.
56. Family Caregiver Alliance. Caregivers Count Too! A toolkit to help practitioners assess the needs of family caregivers. San Francisco: 2006. Accessed 16 Aug 2016 at www.caregiver.org/caregivers-count-too-toolkit.
57. Messecar DC. Nursing standard of practice protocol: family caregiving [Internet]. Accessed at www.consultgeri.org/geriatric-topics/family-caregiving.
58. Young ME, Lutz BJ, Creasy KR et al. A comprehensive assessment of family caregivers of stroke survivors during inpatient rehabilitation. Disabil Rehabil 2014;36:1892–902.
59. Archbold PG, Stewart BJ, Greenlick MR, Harvath T. Mutuality and preparedness as predictors of caregiver role strain. Res Nurs Health 1990;13:375–84.
60. Pucciarelli G, Savini S, Byun E, et al. Psychometric properties of the Caregiver Preparedness Scale in caregivers of stroke survivors. Heart & Lung 2014;43:555–60.
61. Deeken JF, Taylor KL, Mangan P et al. Care for the caregivers: a review of self-report instruments developed to measure the burden, needs, and quality of life of informal caregivers. J Pain Symptom Manage 2003;26:922–53.
62. Haley WE, Roth DL, Howard G, Safford MM. Caregiving strain and estimated risk for stroke and coronary heart disease among spouse caregivers: differential effects by race and sex. Stroke 2010;41:331–6.
63. Bakas T, Champion V, Perkins SM, et al. Psychometric testing of the revised 15-item Bakas Caregiving Outcomes Scale. Nurs Res 2006;55:346–55.
64. Family Caregiver Alliance. Selected caregiver assessment measures: A resource inventory for practitioners. 2d ed. Accessed at www.caregiver.org/selected-caregiver-assessment-measures-resource-inventory-practitioners-2012.
65. Bakas T, Clark PC, Kelly-Hayes M, et al. Evidence for stroke family caregiver and dyad interventions: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2836–52.
66. Cheng HY, Chair SY, Chau JPC. The effectiveness of psychosocial interventions for stroke family caregivers and stroke survivors: a systematic review and meta-analysis. Patient Educ Couns 2014;95:30–44.
67. Bakas T, Austin JK, Habermann B, et al. Telephone assessment and skill-building kit for stroke caregivers a randomized controlled clinical trial. Stroke 2015;46:3478–87.
68. Alim M, Lindley R, Felix C, et al. Family-led rehabilitation after stroke in India: the ATTEND trial, study protocol for a randomized controlled trial. Trials 2016;17:13.
69. Camicia M, Lutz B. Nursing’s role in successful transitions across settings. Stroke 2016. Forthcoming.
70. Chan L, Sandel ME, Jette AM, et al. Does postacute care site matter? A longitudinal study assessing functional recovery after a stroke. Arch Phys Med Rehabil 2013;94:622–9.
71. Kind AJ, Smith MA, Pandhi N, et al. Bouncing-back: rehospitalization in patients with complicated transitions in the first thirty days after hospital discharge for acute stroke. Home Health Care Serv Q 2007;26:37–55.
72. Bettger JP, Liang L, Xian Y, et al. Inpatient rehabilitation facility care reduces the likelihood of death and rehospitalization after stroke compared with skilled nursing facility care [abstract]. Stroke 2015; A146.
73. Camicia M, Black T, Farrell J, et al. The essential role of the rehabilitation nurse in facilitating care transitions: a white paper by the Association of Rehabilitation Nurses. Rehabil Nurs 2014;39:3–15.
74. Centers for Medicare and Medicaid. CMS quality strategy 2016. Accessed at www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/qualityinitiativesgeninfo/downloads/cms-quality-strategy.pdf.
From the School of Nursing, University of North Carolina-Wilmington, Wilmington, NC (Dr. Lutz), and the Kaiser Foundation Rehabilitation Center, Kaiser Permanente, Vallejo, CA (Ms. Camicia).
Abstract
- Objectives: To describe issues faced by stroke family caregivers, discuss evidence-based interventions to improve caregiver outcomes, and provide recommendations for clinicians caring for stroke survivors and their family caregivers.
- Methods: Literature review.
- Results: Caregiver health is linked to the stroke survivor’s degree of functional recovery; the more severe the level of disability, the more likely the caregiver will experience higher levels of strain, increased depression, and poor health. Inadequate caregiver preparation contributes to poorer outcomes. Caregivers describe many unmet needs including skills training; communicating with providers; resource identification and activation; finances; respite; and emotional support. Caregivers need to be assessed for gaps in preparation to provide care. Interventions are recommended that combine skill-building and psycho-educational strategies; are tailored to individual caregiver needs; are face-to-face when feasible; and include 5 to 9 sessions. Family counseling may also be indicated. Intermittent assessment of caregiving outcomes should be conducted so that changing needs can be addressed.
- Conclusions: Stroke caregiving affects the caregiver’s physical, mental, and emotional health, and these effects are sustained over time. Poorly prepared caregivers are more likely to experience negative outcomes and their needs are high during the transition from inpatient care to home. Ongoing support is also important, especially for caregivers who are caring for a stroke survivor with moderate to severe functional limitations. In order to better address unmet needs of stroke caregivers, intermittent assessments should be conducted so that interventions can be tailored to their changing needs over time.
Key words: stroke; family caregivers; care transitions; patient-centered care.
Stroke is a leading cause of major disability in the United States [1] and around the world [2]. Of the estimated 6.6 million stroke survivors living in the US, more than 4.5 million have some level of disability following stroke [1]. In 2009, more than 970,000 persons were hospitalized with stroke in the US with an average length of stay of 5.3 days [3]. Approximately 44% of stroke survivors are discharged home directly from acute care without post-acute care [4]. Only about 25% of stroke survivors receive care in inpatient rehabilitation facilities [4] even though the American Heart Association (AHA) stroke rehabilitation guidelines recommend this level of care for qualified patients [5]. Regardless of the care trajectory, when stroke survivors return home they frequently require assistance with basic and instrumental activities of daily living (BADL/IADL), usually provided by family members who often feel unprepared and overwhelmed by the demands and responsibilities of this caregiving role.
The deleterious effects of caregiving have been identified as a major public health concern [6]. A robust body of literature has established that caregivers are often adversely affected by the demands of their caregiving role. However, much of this literature focuses on caregivers for persons with dementia. Needs of stroke caregivers are categorically different from caregivers of persons with dementia in that stroke is an unpredictable, life-disrupting, crisis event that occurs suddenly leaving family members with insufficient time to prepare for the new roles and caregiving responsibilities. The patient typically transitions from being cared for by multiple providers in an acute care, inpatient rehabilitation facility, or skilled nursing facility (SNF)—24 hours a day, 7 days a week—to relying fully on one person (most often a spouse or adult child) who may not be ready to handle the overwhelming demands and constant vigilance required for adequate care at home. Studies have repeatedly demonstrated the damaging health effects of caregiving. Caregivers describe feeling isolated, abandoned, and alone [7–9], and what frequently follows is a predictable trajectory of depression and deteriorating health and well-being [7,10–13]. The purpose of this article is to describe difficulties and issues faced by family members who are caring for a loved one following stroke, discuss evidence-based interventions designed to improve stroke caregiver outcomes, and provide recommendations for clinicians who care for stroke survivors and their family caregivers post-stroke.
Difficulties and Issues Faced by Caregivers
With an aging population and increasing incidence of stroke, it is imperative that we identify and address the ongoing needs of stroke survivors and their family caregivers in the post-stroke recovery period. Multiple studies acknowledge that stroke is a life-changing event for patients and their family members [9,14] that often results in overwhelming feelings of uncertainty, fear [15], grief, and loss [9]. Stroke also can have long-term effects on the health of stroke survivors and their family caregivers. Studies have identified the effects of caregiving on the health of caregivers and subsequent links between stroke survivor and caregiver outcomes over time [12,16,17]; the ongoing needs of stroke caregivers post-discharge [18,19]; and the importance of assessing caregiver preparedness and subsequent caregiving outcomes [5,20].
Effects of Caregiving on the Health of Caregivers and Stroke Survivors
Research on stroke caregiving consistently indicates that caregiver health is inextricably linked to the stroke survivor’s degree of physical, cognitive, psychological, and emotional recovery. The more severe the patient’s level of disability, the more likely the caregiver will experience higher levels of strain, increased depression, and poor health outcomes [21]. Studies also indicate that certain caregiver characteristics, such as being female or having lower educational level, pre-existing health conditions [7,22,23], poor family functioning, lack of social support [22,24], or lack of preparation [25], are all risk factors for poorer caregiver outcomes.
Stroke family caregivers often experience overwhelming physical and emotional strain, depressive symptoms, sleep deprivation, decline in physical and mental health, reduced quality of life, and increased isolation [7,10,11,14,26,27]. Perceived burden has been positively associated with caregiver depressive symptoms [12,14,28,29]. Depressive symptoms in caregivers, with a reported incidence of 14% [30] to 33% [31], may persist for several years post-stroke. In a study of the long-term effects of caregiving with 235 stroke caregivers when compared with non-caregivers, researchers found that caregivers had more depressive symptoms and poorer life satisfaction and mental health quality of life at 9 months post-stroke, and many of these differences continued for 3 years post-discharge [23].
Lower stroke survivor functioning and higher depressive symptoms are correlated with higher caregiver depressive symptoms and burden, and poorer coping skills and mental health [12,21]. A review of stroke caregiving literature by van Heugten et al [32] indicated that long-term caregiver functioning was influenced by stroke survivor physical and cognitive functioning and behavioral issues; caregiver psychological and emotional health; quality of family relationships; social support; and caregiver demographics. Caregivers of stroke survivors with aphasia may have more difficulties providing care, increased burden and strain, higher depressive symptoms, and other negative stroke-related outcomes [33].
Gaugler [34] conducted a systematic review of 117 studies and reported that caring for stroke survivors who were older, in poorer health, and had greater stroke severity increased the likelihood of poorer emotional and psychological family caregiver outcomes. Caregivers who had “negative problem orientation and less social support” were more likely to have depressive symptoms and poorer self-rated health at 1-year post-stroke. One of the best predictors of caregiver stress and poor health in the first year post-stroke was lack of caregiver preparation [25,34].
Research also suggests that stroke survivor outcomes are influenced by the ability of the family caregiver to provide emotional and instrumental support as well as assistance with BADL/IADL [6,35]. As the caregiver’s health decreases, the stroke survivor’s health and recovery will also likely suffer and ultimately may result in re-hospitalization or nursing home placement. For example, Perrin et al found a consistent reciprocal relationship between caregiver health and stroke survivor functioning, such that the quality of caregiving may be affected by caregiver burden and depressive symptoms, which in turn can impair the functional, psychological, and emotional recovery of the stroke survivor [21]. Studies have also linked poorer caregiver well-being to increased depressive symptoms in stroke survivors [36,37].
Positive effects of caregiving have also been reported, including a feeling of confidence, satisfaction in providing good quality care [30,39,40], an improved relationship with the care recipient [30,40,41], having greater life appreciation, and feeling needed and appreciated [40]. In a systematic review of 9 studies, improvements in the stroke survivor’s condition was a source of positive caregiving experiences [40]. In 2 studies, two-thirds of caregivers surveyed affirmed all survey items related to positive aspects of caregiving [30,42]. Additionally, studies have demonstrated that caregivers who engaged in emotion- and problem-focused coping strategies had positive caregiving experiences [40]. Haley et al found that by 3 years post-stroke many of the ill effects of caregiving had resolved, suggesting that some caregivers may be successful in adapting to their “new” post-stroke lives [23].
Understanding the difficulties and issues faced by caregivers throughout the trajectory, from immediately following the stroke through the transition home and, ideally, the adaptation of the caregiver to this new life, provides an opportunity for health care professionals to intervene with strategies to support this major life change.
Caregiving Trajectory and Ongoing Needs of Stroke Caregivers
Stroke survivors and their family caregivers rapidly move from intensive therapy and nursing case management while in a facility to little or no assistance following discharge. Despite case management and discharge planning services received while in an institutional setting, the transition from inpatient care to home can be a crisis point for caregivers [9]. They describe having to figure things out for themselves with little or no formal support after discharge [9,43,44], leaving them feeling overwhelmed, exhausted, and abandoned once they return home [9].
These family members rarely make an active choice to become caregivers; rather, they take on the role because they are unable to perceive or access any other suitable alternatives [8,45]. Whatever their circumstances, these devoted family members are particularly vulnerable as they transition into the caregiving role without an adequate support system for assessing and addressing their needs [7–9,46]. Without this assistance, caregivers develop their own solutions and strategies to meet the needs of the care recipient after discharge [47,48]. Unfortunately, these strategies are often ineffective and may result in safety risks for patients (eg, falls, skin breakdown, choking), and care-related injuries (eg, falls, muscle strains, bruises) and increased stress and anxiety for caregivers [48–50].
Caregivers have described unmet needs in many domains including skills training, communicating with providers, resource identification and activation, finances, respite, and emotional support [35,44,48,51,52]. Bakas et al found that in the first 6 months post-discharge, stroke caregivers had needs and concerns related to information, emotions and behaviors, physical care, instrumental care, and personal responses to caregiving [48], and that their information needs change during the course of the patient’s recovery [53]. In a study by Lutz et al [44], caregivers identified multiple areas where they felt they were unprepared to assume the caregiving role post-discharge. These included identifying and activating resources; making home and transportation modifications to improve accessibility; developing skills in providing physical care and therapies; managing medications and behavioral issues; preventing falls; coordinating care across settings; attending to other family responsibilities; and caring for themselves.
In a study of interactions between rehabilitation providers and stroke caregivers, Creasy et al [52] noted that caregivers have needs, which were often not recognized, in the following areas: information; providing emotional support for the stroke survivor and having their own emotional support needs met; being involved in treatment decisions; and being adequately prepared for discharge home. Caregivers’ interaction styles with providers, which ranged from passive to active/directing, affected their abilities to have their needs recognized and addressed. These findings highlight the importance of recognizing the caregiver’s interaction style and tailoring communication strategies accordingly.
Cameron et al [54] noted that caregiver support needs change over time, with needs being highest during the inpatient phase as they prepare for discharge home. Moreover, caregivers who are providing care for stroke survivors with more severe functional limitations need more support over a longer period of time. Recognizing the needs of stroke caregivers, the 2016 Canadian Stroke Best Practice Recommendations on Managing Transitions of Care Following Stroke includes recommendations related to assessing, educating, and supporting stroke family caregivers [55].
Assessing Caregiver Readiness and Related Outcomes
Young et al [58] recommend specific domains for a comprehensive readiness assessment of stroke family caregivers. Caregiver domains include strength of the caregiver/care recipient relationship; caregiver willingness to provide care; pre-existing health conditions, previous responsibilities, caregiving experience, home and transportation accessibility, available resources, emotional response to the stroke, and ability to sustain the caregiving role. This type of readiness assessment should be completed early in the care trajectory, while the stroke survivor is receiving inpatient care, so that care plans can be tailored to address gaps in caregiver preparation prior to discharge. It is especially important for new caregivers and those caring for stroke survivors with significant functional limitations [44]. Currently there are no tools designed to assess a family member’s readiness to assume the caregiver role.
Validated instruments have been developed to assess caregiving outcomes, including preparedness, with caregivers who have been providing care for a period of time. For example, the Mutuality and Preparedness Scales of the Family Caregiver Inventory was developed with caregivers 6 months post-discharge [59] and has been validated with stroke caregivers at 3 months post-discharge [60].
Several validated tools are available to assess the caregiver’s changing needs and the effects of care provision on well-being [8,45,61]. For example, the Caregiver Strain Index [62] has been validated in studies with stroke family caregivers [11,28]. Bakas developed 2 scales to specifically assess stroke caregivers post-discharge. The Bakas Caregiving Outcomes Scale assesses caregiver life changes [63] and the Needs and Concerns Checklist assesses post-discharge caregiver needs [48]. There are many other instruments designed to assess general caregiving outcomes, including depressive symptoms, burden, anxiety, and well-being. For a list relevant tools see Deeken et al [61] and The Selected Caregiver Assessment Measures from the Family Caregiver Alliance [64].
While these scales are helpful for assessing caregivers who are already providing care, they do not capture the gaps in caregiver readiness prior to patient discharge from the institutional setting. Taken together, these studies suggest that assessing readiness and implementing interventions to improve caregiver preparation prior to discharge and assessing and addressing their changing needs over time, from inpatient care to community reintegration, may be important strategies for improving both caregiver and stroke survivor outcomes. These strategies may also facilitate sustainability of the caregiver role over time.
Interventions to Improve Caregiver Outcomes
In a review of 39 articles representing 32 caregiver and dyad intervention studies, researchers from the AHA made 13 evidence-based recommendations. Recommendations with the highest level of evidence indicated that (1) interventions that combined skill-building with psycho-educational programs were better than psycho-educational interventions alone; (2) interventions that are tailored to the individual are preferred over “one-size-fits-all” interventions; (3) face-to-face interventions are preferred, but telephone interventions can be useful when face-to-face is not feasible; and (4) interventions with 5 to 9 sessions are recommended [65]. In a review of 18 studies, Cheng et al confirmed the recommendation that psychoeducational interventions that focused on skill building improved caregiver well-being and reduced stroke survivor heath care utilization [66].
Studies also recommend that families may need family counseling to help them develop positive coping strategies and adjust to their lives after stroke [66]. Stroke survivors and their families experience grief and loss as they begin to realize how the stroke has changed their relationships, roles, responsibilities, and future plans for their lives (eg, work, retirement). While many inpatient rehabilitation facilities may provide services from a neuro-psychologist to discuss post-stroke changes in the brain and possible behavioral and emotional manifestations, referrals for family counseling to address the impact of stroke on the family and community reintegration are seldom provided [9].
Recent interventions have shown promise in improving stroke caregiver outcomes. For example, Bakas et al. completed a randomized controlled trial of an 8-week, nurse-delivered, Telephone Assessment and Skill-Building Kit (TASK) intervention [67]. Caregivers in the intervention group with moderate to severe depressive symptoms at baseline demonstrated significant improvements in depressive symptoms and life changes at 8, 24, and 52 weeks. The TASK shows promise because it can reach caregivers in rural and urban areas at a relatively low cost [67].
Recognizing the need to improve post-acute care for stroke survivors and their family caregivers, several large funded clinical trials are being tested in the US and globally. For example, the ATTEND Trial in India is testing a home-based, caregiver-led rehabilitation intervention [68]. The Comprehensive Post-Acute Stroke Services (COMPASS) study in North Carolina, is a state-wide pragmatic, randomized controlled trial testing a comprehensive community-based patient-centered post-acute care intervention with stroke survivors and their caregivers (www.nccompass-study.org). Results of these and other studies will continue to identify evidence-based strategies to improve care coordination, quality of care, and post-stroke outcomes for stroke survivors and their caregivers
Recommendations for Clinicians
Based on this review we have identified strategies that clinicians can implement across the care continuum that may help reduce caregiver strain and burden, and improve outcomes for family caregivers and the stroke survivors for whom they provide care. The evidence suggests that caregivers need assistance in building skills, not only in providing the care needed by the stroke survivor but also in solving problems as they arise; navigating the multiple systems of care, including understanding options for post-acute care; accessing community resources; communicating effectively with health care and social support providers; and dealing with the emotional effects of stroke [44,52].
Caregivers need help in navigating the multiple providers and systems of care to get the services the stroke survivor needs as well as to secure support services. They need information from trusted sources about stroke prevention and available community resources. Providinga list of resources is often insufficient, especially in the first few weeks or months post-stroke; these caregivers are already overwhelmed with the enormity of the tasks and responsibilities that they have taken on as a caregiver. Instead they need someone who can advocate for them and connect them with the appropriate resources at the right time.
They also need assistance developing and maintaining self-care strategies so they can sustain the caregiving role long-term. Identifying opportunities for respite and helping them activate informal and formal resources, such as other family members, friends, church groups, neighbors, and services from local senior centers, independent living centers, or area agencies on aging can help them identify assistance with the breadth of duties including care of the stroke survivor, meal preparation, transportation, or a supportive listening ear. It is important for the caregiver, in addition to any other close support person as available, to have a facilitated discussion withthe healthcare team to brainstorm activities where assistance may be provided and who might be approached to help.
The timing of providing support and resources is also critical. Becoming a caregiver is a process and often family members who are new to the role need more intense direct assistance and support when the stroke survivor first comes home, but many may need ongoing support over time. Research suggests it can take caregivers up to 3 years to figure out how to manage the new responsibilities, learn to navigate the multiple systems for careand services, establish confidence in their abilities, deal with the emotional upheaval, and to adapt to their new lives [23].
Research indicates the 44% of stroke patients receive no post-acute care. Clinicians also need to advocate for patients to get the most appropriate level of organized, coordinated, and inter-professional post-acute care [5]. This requires that they understand the different levels of post-acute care, including the criteria for admission, the scope and intensity of nursing, therapy, physician and other services provided in each setting, and the associated clinical outcomes. This knowledge is also necessary to enable clinicians to educate stroke survivors and their caregivers on post-acute care so that they understand the process and can effectively self-advocate for the provision of appropriate services as needed.
Approximately 45% of stroke survivors in the US are discharged either to an inpatient rehabilitation facility or SNF for rehabilitation [4]. Patients discharged to an inpatient rehabilitation facility receive a minimum of 3 hours of therapy per day and are cared for 24 hours/day by a staff led by registered nurses (RNs) with rehabilitation expertise. SNFs do not have minimum requirements for hours of therapy, 24-hour RN staffing, nor a requirement for nurses with specialty training in rehabilitation. Pressure to reduce the length of stay in acute care often results in providers transitioning stroke survivors to the post-acute care setting that accepts the patient first. Because SNFs have fewer criteria for admission, they are more likely to rapidly accept a patient for care when compared to an inpatient rehabilitation facility. Providers must determine and make recommendations for the most appropriate level of post-acute care to ensure the stroke patients’ rehabilitation needs can be met in the recommended setting [5,69]. It is also essential that family caregivers have the knowledge and skills to advocate for the appropriate level of post-acute care based on the stroke survivor’s expected recovery trajectory. Research has demonstrated that that stroke survivors admitted to an inpatient rehabilitation facility, when compared to similar patients in a SNF, have better outcomes, including improved function [70] and lower re-hospitalization and death rates [71,72]. The Association of Rehabilitation Nurses provides resources for health care professionals and patients regarding rehabilitation. For more information for professionals about levels of post-acute care, see www.rehabnurse.org/uploads/files/healthpolicy/ARN_Care_Transitions_White_Paper_Journal_Copy_FINAL.pdf [73]. For information for patients and caregivers, see www.restartrecovery.org.
Providers must also be knowledgeable about community resources in order to provide connections to services and agencies that are relevant to the changing needs of the caregiver over time. Initially, caregivers may need assistance in meeting the stroke survivor’s BADL/IADL, and later needs may expand to include support groups, respite, and opportunities for a greater community engagement.
Training in time management provides room in the busy caregiving schedule for self-care for the caregiver. Providers must assist with determining routines that meet the needs of both the caregiver and stroke survivor, as the health of each is dependent on the other. Assistance in developing a wellness program that is feasible for the caregiver to maintain will improve adoption of health promoting practices.
As discussed above, the needs of both the stroke survivor and caregiver vary along the post-stroke trajectory. Therefore, both caregivers and stroke survivors should be assessed intermittently over time: caregivers for evidence of effective coping strategies and confidence in the sustaining the caregiving role, and stroke survivors for improvement in their functional abilities and compensatory strategies in BADL/IADL. The opportunity for the stroke survivor to assume household tasks that decrease the caregiver burden, in addition to providing a greater sense of purpose for the stroke survivor, must be explored. For example, the stroke survivor may be able to assist with activities such as meal planning and components of meal preparation or light housekeeping utilizing adaptive devices as needed.
Additional research is necessary to understand how the needs of caregivers change over time, the appropriate timing of reassessment, and the evaluation of interventions to facilitate the transition into this role, while preventing the adverse effects of caregiving on the health of the caregiver and stroke survivor during this transition period.
Conclusion
There is clear evidence that stroke caregiving can have detrimental effects on the physical, mental, and emotional health of caregivers, and that these effects are sustained over time. Evidence also indicates that caregivers who are not well-prepared to assume the caregiving role are more likely to experience negative outcomes. Studies suggest that the time of transition from inpatient care to home is a time of crisis for caregivers and that their support needs are high during this time. However, research also indicates that while needs may change over time, caregivers need ongoing support, especially if they are providing care for a stroke survivor who has moderate to severe physical, cognitive, and/or communication limitations.
In order to better understand the needs of stroke caregivers, a pre-discharge assessment of their readiness to provide care should be conducted so that interventions can be tailored to address their needs to minimize negative effects of a poorly planned transition [69]. Currently, there are assessment tools that can be used with caregivers post-discharge to assess their self-reported needs (after they have an understanding of the role) and caregiving outcomes. Research is needed to develop a valid and reliable tool thatpre-emptively assesses the gaps in caregiver readiness that can be utilized prior to the transition from the institutional setting to home. This will enable the identification and evaluation of primary prevention strategies to improve caregiver preparation so that the adaption to the new caregiving role can be expedited, minimizing the adverse health effects on both the caregiver and stroke survivor.
Providers must be aware of the changing needs of stroke survivors and tailor plans of care accordingly, using evidenced-based interventions. Policy makers must consider research on the long term effects of caregiving and consider legislation to support the health and respite needs of the growing population of caregivers. This will contribute to attaining the 3 aims of the National Quality Strategy: improving quality of care, improving health, and reducing health care system costs [74].
Corresponding author: Barbara J. Lutz, PhD, 601 S. College Rd., Wilmington, NC 28403, [email protected].
Financial disclosures: None.
From the School of Nursing, University of North Carolina-Wilmington, Wilmington, NC (Dr. Lutz), and the Kaiser Foundation Rehabilitation Center, Kaiser Permanente, Vallejo, CA (Ms. Camicia).
Abstract
- Objectives: To describe issues faced by stroke family caregivers, discuss evidence-based interventions to improve caregiver outcomes, and provide recommendations for clinicians caring for stroke survivors and their family caregivers.
- Methods: Literature review.
- Results: Caregiver health is linked to the stroke survivor’s degree of functional recovery; the more severe the level of disability, the more likely the caregiver will experience higher levels of strain, increased depression, and poor health. Inadequate caregiver preparation contributes to poorer outcomes. Caregivers describe many unmet needs including skills training; communicating with providers; resource identification and activation; finances; respite; and emotional support. Caregivers need to be assessed for gaps in preparation to provide care. Interventions are recommended that combine skill-building and psycho-educational strategies; are tailored to individual caregiver needs; are face-to-face when feasible; and include 5 to 9 sessions. Family counseling may also be indicated. Intermittent assessment of caregiving outcomes should be conducted so that changing needs can be addressed.
- Conclusions: Stroke caregiving affects the caregiver’s physical, mental, and emotional health, and these effects are sustained over time. Poorly prepared caregivers are more likely to experience negative outcomes and their needs are high during the transition from inpatient care to home. Ongoing support is also important, especially for caregivers who are caring for a stroke survivor with moderate to severe functional limitations. In order to better address unmet needs of stroke caregivers, intermittent assessments should be conducted so that interventions can be tailored to their changing needs over time.
Key words: stroke; family caregivers; care transitions; patient-centered care.
Stroke is a leading cause of major disability in the United States [1] and around the world [2]. Of the estimated 6.6 million stroke survivors living in the US, more than 4.5 million have some level of disability following stroke [1]. In 2009, more than 970,000 persons were hospitalized with stroke in the US with an average length of stay of 5.3 days [3]. Approximately 44% of stroke survivors are discharged home directly from acute care without post-acute care [4]. Only about 25% of stroke survivors receive care in inpatient rehabilitation facilities [4] even though the American Heart Association (AHA) stroke rehabilitation guidelines recommend this level of care for qualified patients [5]. Regardless of the care trajectory, when stroke survivors return home they frequently require assistance with basic and instrumental activities of daily living (BADL/IADL), usually provided by family members who often feel unprepared and overwhelmed by the demands and responsibilities of this caregiving role.
The deleterious effects of caregiving have been identified as a major public health concern [6]. A robust body of literature has established that caregivers are often adversely affected by the demands of their caregiving role. However, much of this literature focuses on caregivers for persons with dementia. Needs of stroke caregivers are categorically different from caregivers of persons with dementia in that stroke is an unpredictable, life-disrupting, crisis event that occurs suddenly leaving family members with insufficient time to prepare for the new roles and caregiving responsibilities. The patient typically transitions from being cared for by multiple providers in an acute care, inpatient rehabilitation facility, or skilled nursing facility (SNF)—24 hours a day, 7 days a week—to relying fully on one person (most often a spouse or adult child) who may not be ready to handle the overwhelming demands and constant vigilance required for adequate care at home. Studies have repeatedly demonstrated the damaging health effects of caregiving. Caregivers describe feeling isolated, abandoned, and alone [7–9], and what frequently follows is a predictable trajectory of depression and deteriorating health and well-being [7,10–13]. The purpose of this article is to describe difficulties and issues faced by family members who are caring for a loved one following stroke, discuss evidence-based interventions designed to improve stroke caregiver outcomes, and provide recommendations for clinicians who care for stroke survivors and their family caregivers post-stroke.
Difficulties and Issues Faced by Caregivers
With an aging population and increasing incidence of stroke, it is imperative that we identify and address the ongoing needs of stroke survivors and their family caregivers in the post-stroke recovery period. Multiple studies acknowledge that stroke is a life-changing event for patients and their family members [9,14] that often results in overwhelming feelings of uncertainty, fear [15], grief, and loss [9]. Stroke also can have long-term effects on the health of stroke survivors and their family caregivers. Studies have identified the effects of caregiving on the health of caregivers and subsequent links between stroke survivor and caregiver outcomes over time [12,16,17]; the ongoing needs of stroke caregivers post-discharge [18,19]; and the importance of assessing caregiver preparedness and subsequent caregiving outcomes [5,20].
Effects of Caregiving on the Health of Caregivers and Stroke Survivors
Research on stroke caregiving consistently indicates that caregiver health is inextricably linked to the stroke survivor’s degree of physical, cognitive, psychological, and emotional recovery. The more severe the patient’s level of disability, the more likely the caregiver will experience higher levels of strain, increased depression, and poor health outcomes [21]. Studies also indicate that certain caregiver characteristics, such as being female or having lower educational level, pre-existing health conditions [7,22,23], poor family functioning, lack of social support [22,24], or lack of preparation [25], are all risk factors for poorer caregiver outcomes.
Stroke family caregivers often experience overwhelming physical and emotional strain, depressive symptoms, sleep deprivation, decline in physical and mental health, reduced quality of life, and increased isolation [7,10,11,14,26,27]. Perceived burden has been positively associated with caregiver depressive symptoms [12,14,28,29]. Depressive symptoms in caregivers, with a reported incidence of 14% [30] to 33% [31], may persist for several years post-stroke. In a study of the long-term effects of caregiving with 235 stroke caregivers when compared with non-caregivers, researchers found that caregivers had more depressive symptoms and poorer life satisfaction and mental health quality of life at 9 months post-stroke, and many of these differences continued for 3 years post-discharge [23].
Lower stroke survivor functioning and higher depressive symptoms are correlated with higher caregiver depressive symptoms and burden, and poorer coping skills and mental health [12,21]. A review of stroke caregiving literature by van Heugten et al [32] indicated that long-term caregiver functioning was influenced by stroke survivor physical and cognitive functioning and behavioral issues; caregiver psychological and emotional health; quality of family relationships; social support; and caregiver demographics. Caregivers of stroke survivors with aphasia may have more difficulties providing care, increased burden and strain, higher depressive symptoms, and other negative stroke-related outcomes [33].
Gaugler [34] conducted a systematic review of 117 studies and reported that caring for stroke survivors who were older, in poorer health, and had greater stroke severity increased the likelihood of poorer emotional and psychological family caregiver outcomes. Caregivers who had “negative problem orientation and less social support” were more likely to have depressive symptoms and poorer self-rated health at 1-year post-stroke. One of the best predictors of caregiver stress and poor health in the first year post-stroke was lack of caregiver preparation [25,34].
Research also suggests that stroke survivor outcomes are influenced by the ability of the family caregiver to provide emotional and instrumental support as well as assistance with BADL/IADL [6,35]. As the caregiver’s health decreases, the stroke survivor’s health and recovery will also likely suffer and ultimately may result in re-hospitalization or nursing home placement. For example, Perrin et al found a consistent reciprocal relationship between caregiver health and stroke survivor functioning, such that the quality of caregiving may be affected by caregiver burden and depressive symptoms, which in turn can impair the functional, psychological, and emotional recovery of the stroke survivor [21]. Studies have also linked poorer caregiver well-being to increased depressive symptoms in stroke survivors [36,37].
Positive effects of caregiving have also been reported, including a feeling of confidence, satisfaction in providing good quality care [30,39,40], an improved relationship with the care recipient [30,40,41], having greater life appreciation, and feeling needed and appreciated [40]. In a systematic review of 9 studies, improvements in the stroke survivor’s condition was a source of positive caregiving experiences [40]. In 2 studies, two-thirds of caregivers surveyed affirmed all survey items related to positive aspects of caregiving [30,42]. Additionally, studies have demonstrated that caregivers who engaged in emotion- and problem-focused coping strategies had positive caregiving experiences [40]. Haley et al found that by 3 years post-stroke many of the ill effects of caregiving had resolved, suggesting that some caregivers may be successful in adapting to their “new” post-stroke lives [23].
Understanding the difficulties and issues faced by caregivers throughout the trajectory, from immediately following the stroke through the transition home and, ideally, the adaptation of the caregiver to this new life, provides an opportunity for health care professionals to intervene with strategies to support this major life change.
Caregiving Trajectory and Ongoing Needs of Stroke Caregivers
Stroke survivors and their family caregivers rapidly move from intensive therapy and nursing case management while in a facility to little or no assistance following discharge. Despite case management and discharge planning services received while in an institutional setting, the transition from inpatient care to home can be a crisis point for caregivers [9]. They describe having to figure things out for themselves with little or no formal support after discharge [9,43,44], leaving them feeling overwhelmed, exhausted, and abandoned once they return home [9].
These family members rarely make an active choice to become caregivers; rather, they take on the role because they are unable to perceive or access any other suitable alternatives [8,45]. Whatever their circumstances, these devoted family members are particularly vulnerable as they transition into the caregiving role without an adequate support system for assessing and addressing their needs [7–9,46]. Without this assistance, caregivers develop their own solutions and strategies to meet the needs of the care recipient after discharge [47,48]. Unfortunately, these strategies are often ineffective and may result in safety risks for patients (eg, falls, skin breakdown, choking), and care-related injuries (eg, falls, muscle strains, bruises) and increased stress and anxiety for caregivers [48–50].
Caregivers have described unmet needs in many domains including skills training, communicating with providers, resource identification and activation, finances, respite, and emotional support [35,44,48,51,52]. Bakas et al found that in the first 6 months post-discharge, stroke caregivers had needs and concerns related to information, emotions and behaviors, physical care, instrumental care, and personal responses to caregiving [48], and that their information needs change during the course of the patient’s recovery [53]. In a study by Lutz et al [44], caregivers identified multiple areas where they felt they were unprepared to assume the caregiving role post-discharge. These included identifying and activating resources; making home and transportation modifications to improve accessibility; developing skills in providing physical care and therapies; managing medications and behavioral issues; preventing falls; coordinating care across settings; attending to other family responsibilities; and caring for themselves.
In a study of interactions between rehabilitation providers and stroke caregivers, Creasy et al [52] noted that caregivers have needs, which were often not recognized, in the following areas: information; providing emotional support for the stroke survivor and having their own emotional support needs met; being involved in treatment decisions; and being adequately prepared for discharge home. Caregivers’ interaction styles with providers, which ranged from passive to active/directing, affected their abilities to have their needs recognized and addressed. These findings highlight the importance of recognizing the caregiver’s interaction style and tailoring communication strategies accordingly.
Cameron et al [54] noted that caregiver support needs change over time, with needs being highest during the inpatient phase as they prepare for discharge home. Moreover, caregivers who are providing care for stroke survivors with more severe functional limitations need more support over a longer period of time. Recognizing the needs of stroke caregivers, the 2016 Canadian Stroke Best Practice Recommendations on Managing Transitions of Care Following Stroke includes recommendations related to assessing, educating, and supporting stroke family caregivers [55].
Assessing Caregiver Readiness and Related Outcomes
Young et al [58] recommend specific domains for a comprehensive readiness assessment of stroke family caregivers. Caregiver domains include strength of the caregiver/care recipient relationship; caregiver willingness to provide care; pre-existing health conditions, previous responsibilities, caregiving experience, home and transportation accessibility, available resources, emotional response to the stroke, and ability to sustain the caregiving role. This type of readiness assessment should be completed early in the care trajectory, while the stroke survivor is receiving inpatient care, so that care plans can be tailored to address gaps in caregiver preparation prior to discharge. It is especially important for new caregivers and those caring for stroke survivors with significant functional limitations [44]. Currently there are no tools designed to assess a family member’s readiness to assume the caregiver role.
Validated instruments have been developed to assess caregiving outcomes, including preparedness, with caregivers who have been providing care for a period of time. For example, the Mutuality and Preparedness Scales of the Family Caregiver Inventory was developed with caregivers 6 months post-discharge [59] and has been validated with stroke caregivers at 3 months post-discharge [60].
Several validated tools are available to assess the caregiver’s changing needs and the effects of care provision on well-being [8,45,61]. For example, the Caregiver Strain Index [62] has been validated in studies with stroke family caregivers [11,28]. Bakas developed 2 scales to specifically assess stroke caregivers post-discharge. The Bakas Caregiving Outcomes Scale assesses caregiver life changes [63] and the Needs and Concerns Checklist assesses post-discharge caregiver needs [48]. There are many other instruments designed to assess general caregiving outcomes, including depressive symptoms, burden, anxiety, and well-being. For a list relevant tools see Deeken et al [61] and The Selected Caregiver Assessment Measures from the Family Caregiver Alliance [64].
While these scales are helpful for assessing caregivers who are already providing care, they do not capture the gaps in caregiver readiness prior to patient discharge from the institutional setting. Taken together, these studies suggest that assessing readiness and implementing interventions to improve caregiver preparation prior to discharge and assessing and addressing their changing needs over time, from inpatient care to community reintegration, may be important strategies for improving both caregiver and stroke survivor outcomes. These strategies may also facilitate sustainability of the caregiver role over time.
Interventions to Improve Caregiver Outcomes
In a review of 39 articles representing 32 caregiver and dyad intervention studies, researchers from the AHA made 13 evidence-based recommendations. Recommendations with the highest level of evidence indicated that (1) interventions that combined skill-building with psycho-educational programs were better than psycho-educational interventions alone; (2) interventions that are tailored to the individual are preferred over “one-size-fits-all” interventions; (3) face-to-face interventions are preferred, but telephone interventions can be useful when face-to-face is not feasible; and (4) interventions with 5 to 9 sessions are recommended [65]. In a review of 18 studies, Cheng et al confirmed the recommendation that psychoeducational interventions that focused on skill building improved caregiver well-being and reduced stroke survivor heath care utilization [66].
Studies also recommend that families may need family counseling to help them develop positive coping strategies and adjust to their lives after stroke [66]. Stroke survivors and their families experience grief and loss as they begin to realize how the stroke has changed their relationships, roles, responsibilities, and future plans for their lives (eg, work, retirement). While many inpatient rehabilitation facilities may provide services from a neuro-psychologist to discuss post-stroke changes in the brain and possible behavioral and emotional manifestations, referrals for family counseling to address the impact of stroke on the family and community reintegration are seldom provided [9].
Recent interventions have shown promise in improving stroke caregiver outcomes. For example, Bakas et al. completed a randomized controlled trial of an 8-week, nurse-delivered, Telephone Assessment and Skill-Building Kit (TASK) intervention [67]. Caregivers in the intervention group with moderate to severe depressive symptoms at baseline demonstrated significant improvements in depressive symptoms and life changes at 8, 24, and 52 weeks. The TASK shows promise because it can reach caregivers in rural and urban areas at a relatively low cost [67].
Recognizing the need to improve post-acute care for stroke survivors and their family caregivers, several large funded clinical trials are being tested in the US and globally. For example, the ATTEND Trial in India is testing a home-based, caregiver-led rehabilitation intervention [68]. The Comprehensive Post-Acute Stroke Services (COMPASS) study in North Carolina, is a state-wide pragmatic, randomized controlled trial testing a comprehensive community-based patient-centered post-acute care intervention with stroke survivors and their caregivers (www.nccompass-study.org). Results of these and other studies will continue to identify evidence-based strategies to improve care coordination, quality of care, and post-stroke outcomes for stroke survivors and their caregivers
Recommendations for Clinicians
Based on this review we have identified strategies that clinicians can implement across the care continuum that may help reduce caregiver strain and burden, and improve outcomes for family caregivers and the stroke survivors for whom they provide care. The evidence suggests that caregivers need assistance in building skills, not only in providing the care needed by the stroke survivor but also in solving problems as they arise; navigating the multiple systems of care, including understanding options for post-acute care; accessing community resources; communicating effectively with health care and social support providers; and dealing with the emotional effects of stroke [44,52].
Caregivers need help in navigating the multiple providers and systems of care to get the services the stroke survivor needs as well as to secure support services. They need information from trusted sources about stroke prevention and available community resources. Providinga list of resources is often insufficient, especially in the first few weeks or months post-stroke; these caregivers are already overwhelmed with the enormity of the tasks and responsibilities that they have taken on as a caregiver. Instead they need someone who can advocate for them and connect them with the appropriate resources at the right time.
They also need assistance developing and maintaining self-care strategies so they can sustain the caregiving role long-term. Identifying opportunities for respite and helping them activate informal and formal resources, such as other family members, friends, church groups, neighbors, and services from local senior centers, independent living centers, or area agencies on aging can help them identify assistance with the breadth of duties including care of the stroke survivor, meal preparation, transportation, or a supportive listening ear. It is important for the caregiver, in addition to any other close support person as available, to have a facilitated discussion withthe healthcare team to brainstorm activities where assistance may be provided and who might be approached to help.
The timing of providing support and resources is also critical. Becoming a caregiver is a process and often family members who are new to the role need more intense direct assistance and support when the stroke survivor first comes home, but many may need ongoing support over time. Research suggests it can take caregivers up to 3 years to figure out how to manage the new responsibilities, learn to navigate the multiple systems for careand services, establish confidence in their abilities, deal with the emotional upheaval, and to adapt to their new lives [23].
Research indicates the 44% of stroke patients receive no post-acute care. Clinicians also need to advocate for patients to get the most appropriate level of organized, coordinated, and inter-professional post-acute care [5]. This requires that they understand the different levels of post-acute care, including the criteria for admission, the scope and intensity of nursing, therapy, physician and other services provided in each setting, and the associated clinical outcomes. This knowledge is also necessary to enable clinicians to educate stroke survivors and their caregivers on post-acute care so that they understand the process and can effectively self-advocate for the provision of appropriate services as needed.
Approximately 45% of stroke survivors in the US are discharged either to an inpatient rehabilitation facility or SNF for rehabilitation [4]. Patients discharged to an inpatient rehabilitation facility receive a minimum of 3 hours of therapy per day and are cared for 24 hours/day by a staff led by registered nurses (RNs) with rehabilitation expertise. SNFs do not have minimum requirements for hours of therapy, 24-hour RN staffing, nor a requirement for nurses with specialty training in rehabilitation. Pressure to reduce the length of stay in acute care often results in providers transitioning stroke survivors to the post-acute care setting that accepts the patient first. Because SNFs have fewer criteria for admission, they are more likely to rapidly accept a patient for care when compared to an inpatient rehabilitation facility. Providers must determine and make recommendations for the most appropriate level of post-acute care to ensure the stroke patients’ rehabilitation needs can be met in the recommended setting [5,69]. It is also essential that family caregivers have the knowledge and skills to advocate for the appropriate level of post-acute care based on the stroke survivor’s expected recovery trajectory. Research has demonstrated that that stroke survivors admitted to an inpatient rehabilitation facility, when compared to similar patients in a SNF, have better outcomes, including improved function [70] and lower re-hospitalization and death rates [71,72]. The Association of Rehabilitation Nurses provides resources for health care professionals and patients regarding rehabilitation. For more information for professionals about levels of post-acute care, see www.rehabnurse.org/uploads/files/healthpolicy/ARN_Care_Transitions_White_Paper_Journal_Copy_FINAL.pdf [73]. For information for patients and caregivers, see www.restartrecovery.org.
Providers must also be knowledgeable about community resources in order to provide connections to services and agencies that are relevant to the changing needs of the caregiver over time. Initially, caregivers may need assistance in meeting the stroke survivor’s BADL/IADL, and later needs may expand to include support groups, respite, and opportunities for a greater community engagement.
Training in time management provides room in the busy caregiving schedule for self-care for the caregiver. Providers must assist with determining routines that meet the needs of both the caregiver and stroke survivor, as the health of each is dependent on the other. Assistance in developing a wellness program that is feasible for the caregiver to maintain will improve adoption of health promoting practices.
As discussed above, the needs of both the stroke survivor and caregiver vary along the post-stroke trajectory. Therefore, both caregivers and stroke survivors should be assessed intermittently over time: caregivers for evidence of effective coping strategies and confidence in the sustaining the caregiving role, and stroke survivors for improvement in their functional abilities and compensatory strategies in BADL/IADL. The opportunity for the stroke survivor to assume household tasks that decrease the caregiver burden, in addition to providing a greater sense of purpose for the stroke survivor, must be explored. For example, the stroke survivor may be able to assist with activities such as meal planning and components of meal preparation or light housekeeping utilizing adaptive devices as needed.
Additional research is necessary to understand how the needs of caregivers change over time, the appropriate timing of reassessment, and the evaluation of interventions to facilitate the transition into this role, while preventing the adverse effects of caregiving on the health of the caregiver and stroke survivor during this transition period.
Conclusion
There is clear evidence that stroke caregiving can have detrimental effects on the physical, mental, and emotional health of caregivers, and that these effects are sustained over time. Evidence also indicates that caregivers who are not well-prepared to assume the caregiving role are more likely to experience negative outcomes. Studies suggest that the time of transition from inpatient care to home is a time of crisis for caregivers and that their support needs are high during this time. However, research also indicates that while needs may change over time, caregivers need ongoing support, especially if they are providing care for a stroke survivor who has moderate to severe physical, cognitive, and/or communication limitations.
In order to better understand the needs of stroke caregivers, a pre-discharge assessment of their readiness to provide care should be conducted so that interventions can be tailored to address their needs to minimize negative effects of a poorly planned transition [69]. Currently, there are assessment tools that can be used with caregivers post-discharge to assess their self-reported needs (after they have an understanding of the role) and caregiving outcomes. Research is needed to develop a valid and reliable tool thatpre-emptively assesses the gaps in caregiver readiness that can be utilized prior to the transition from the institutional setting to home. This will enable the identification and evaluation of primary prevention strategies to improve caregiver preparation so that the adaption to the new caregiving role can be expedited, minimizing the adverse health effects on both the caregiver and stroke survivor.
Providers must be aware of the changing needs of stroke survivors and tailor plans of care accordingly, using evidenced-based interventions. Policy makers must consider research on the long term effects of caregiving and consider legislation to support the health and respite needs of the growing population of caregivers. This will contribute to attaining the 3 aims of the National Quality Strategy: improving quality of care, improving health, and reducing health care system costs [74].
Corresponding author: Barbara J. Lutz, PhD, 601 S. College Rd., Wilmington, NC 28403, [email protected].
Financial disclosures: None.
1. Mozaffarian D, Benjamin EJ, Go AS, et al. on behalf of the American Heart Association Statistics Committee & Stroke Statistics Subcommittee. Heart disease and stroke statistics - 2016 update: A report from the American Heart Association. Circulation 2016;132:e38–e360.
2. Feigin VL, Krishnamurthi RV, Parmar P, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: The GBD 2013 Study. Neuroepidemiology 2015;45:161–76.
3. Hall MJ, Levant S, DeFrances CJ. Hospitalization for stroke in U.S. hospitals, 1989-2009. Hyattsville, MD: National Center for Heatlh Statistics; 2012.
4. Prvu Bettger J, McCoy L, Smith EE, et al. Contemporary trends and predictors of postacute service use and routine discharge home after stroke. J Am Heart Assoc 2015;4(2).
5. Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016;47:e98–e169.
6. Talley RC, Crews JE. Framing the public health of caregiving. Am J Public Health 2007;97:224–8.
7. van Exel NJ, Koopmanschap MA, van den Berg B, et al. Burden of informal caregiving for stroke patients: odentification of caregivers at risk of adverse health effects. Cerebrovasc Dis 2005;19:11–7.
8. van Exel NJ, Scholte op Reimer WJ, Brouwer WB, et al. Instruments for assessing the burden of informal caregiving for stroke patients in clinical practice: a comparison of CSI, CRA, SCQ and self-rated burden. Clin Rehabil 2004;18:203–14.
9. Lutz BJ, Young ME, Cox KJ, et al. The crisis of stroke: experiences of patients and their family caregivers. Top Stroke Rehabil 2011;18:786–97.
10. McCullagh E, Brigstocke G, Donaldson N, Kalra L. Determinants of caregiving burden and quality of life in caregivers of stroke patients. Stroke 2005;36:2181–6.
11. van Exel NJ, Brouwer WB, van den Berg B, et al. What really matters: an inquiry into the relative importance of dimensions of informal caregiver burden. Clin Rehabil 2004;18:683–93.
12. Perrin PB, Heesacker M, Hinojosa MS et al. Identifying at-risk, ethnically diverse stroke caregivers for counseling: a longitudinal study of mental health. Rehabil Psychol 2009;54:138–49.
13. Greenwood N, Mackenzie A, Cloud GC, Wilson N. Informal carers of stroke survivors--factors influencing carers: a systematic review of quantitative studies. Disabil Rehabil 2008;30:1329–49.
14. Camak DJ. Addressing the burden of stroke caregivers: a literature review. J Clin Nurs 2015;24:2376–82.
15. White CL, Barrientos R, Dunn K. Dimensions of uncertainty after stroke: perspectives of the stroke survivor and family caregiver. J Neurosci Nurs 2014;46:233–40.
16. Saban KL, Sherwood PR, DeVon HA, Hynes DM. Measures of psychological stress and physical health in family caregivers of stroke survivors: a literature review. J Neurosci Nurs 2010;42:128–38.
17. Kruithof WJ, Post, MWM, van Mierlo M, et al. Caregiver burden and emotional problems in partners of stroke patients at two months and one year post-stroke: determinants and prediction. Patient Educ Couns 2016. Forthcoming.
18. Sarre S, Redlich C, Tinker A, et al. A systematic review of qualitative studies on adjusting after stroke: lessons for the study of resilience. Disabil Rehabil 2014;36:716–26.
19. Cameron JI, Naglie G, Dick T, et al. Are we meeting the changing needs of family caregivers to stroke survivors across the care continuum? A systematic review of the caregiver intervention literature. Stroke 2012;43:E143-E.
20. Miller EL, Murray L, Richards L, et al. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke ptient: a scientific statement from the American Heart Association. Stroke 2010;41:2402–48.
21. Perrin PB, Heesacker M, Stidham BS, et al. Structural equation modeling of the relationship between caregiver psychosocial variables and functioning of individuals with stroke. Rehabil Psychol 2008;53:54–62.
22. Roth DL, Perkins M, Wadley VG, et al. Family caregiving and emotional strain: associations with quality of life in a large national sample of middle-aged and older adults. Qual Life Res 2009;18:679–88.
23. Haley WE, Roth DL, Hovater M, Clay OJ. Long-term impact of stroke on family caregiver well-being: a population-based case-control study. Neurology 2015;84:1323–9.
24. Greenwood N, Mackenzie A. Informal caring for stroke survivors: meta-ethnographic review of qualitative literature. Maturitas 2010;66:268–76.
25. Ostwald SK, Bernal MP, Cron SG, Godwin KM. Stress experienced by stroke survivors and spousal caregivers during the first year after discharge from inpatient rehabilitation. Top Stroke Rehabil 2009;16:93–104.
26. Brereton L, Carroll C, Barnston S. Interventions for adult family carers of people who have had a stroke: a systematic review. Clin Rehabil 2007;21:867–84.
27. Greenwood N, Mackenzie A, Cloud G, Wilson N. Loss of autonomy, control and independence when caring: a qualitative study of informal carers of stroke survivors in the first three months after discharge. Disabil Rehabil 2009:1–9.
28. Visser-Meily A, Post M, van de Port I, et al. Psychosocial functioning of spouses of patients with stroke from initial inpatient rehabilitation to 3 years poststroke: course and relations with coping strategies. Stroke 2009;40:1399–404.
29. Visser-Meily A, Post M, van de Port I, et al. Psychosocial functioning of spouses in the chronic phase after stroke: improvement or deterioration between 1 and 3 years after stroke? Patient Educ Couns 2008;73:153–8.
30. Haley WE, Allen JY, Grant JS, et al. Problems and benefits reported by stroke family caregivers: results from a prospective epidemiological study. Stroke 2009;40:2129–33.
31. Berg A, Palomaki H, Lonnqvist J, et al. Depression among caregivers of stroke survivors. Stroke 2005;36:639–43.
32. van Heugten C, Visser-Meily A, Post M, Lindeman E. Care for carers of stroke patients: evidence-based clinical practice guidelines. J Rehabil Med 2006;38:153–8.
33. Bakas T, Kroenke K, Plue LD, et al. Outcomes among family caregivers of aphasic versus nonaphasic stroke survivors. Rehabil Nurs 2006;31:33–42.
34. Gaugler JE. The longitudinal ramifications of stroke caregiving: a systematic review. Rehabil Psychol 2010;55:108–25.
35. Andrew NE, Kilkenny MF, Naylor R, et al. The relationship between caregiver impacts and the unmet needs of survivors of stroke. Patient Prefer Adher 2015;9:1065–73.
36. Chung ML, Bakas T, Plue LD, Williams LS. Effects of self-esteem, optimism, and perceived control on depressive symptoms in stroke survivor-spouse dyads. J Cardiovasc Nurs 2016;31:E8–E16.
37. Grant JS, Clay OJ, Keltner NL, et al. Does caregiver well-being predict stroke survivor depressive symptoms? a mediation analysis. Top Stroke Rehabil 2013;20:44–51.
38. Murray J, Young J, Forster A, Ashworth R. Developing a primary care-based stroke model: the prevalence of longer-term problems experienced by patients and carers. Br J Gen Pract 2003;53:803–7.
39. Pierce LL, Steiner V, Govoni A, et al. Two sides to the caregiving story. Top Stroke Rehabil 2007;14:13–20.
40. Mackenzie A, Greenwood N. Positive experiences of caregiving in stroke: a systematic review. Disabil Rehabil 2012;34:1413–22.
41. Parag V, Hackett ML, Yapa CM, et al. The impact of stroke on unpaid caregivers: results from The Auckland Regional Community Stroke study, 2002-2003. Cerebrovasc Dis (Basel, Switzerland) 2008;25:548–54.
42. Kruithof WJ, Post MWM, Visser-Meily JMA. Measuring negative and positive caregiving experiences: a psychometric analysis of the Caregiver Strain Index Expanded. Clin Rehabil 2015;29:1224–33.
43. Lutz BJ. Determinants of discharge destination for stroke patients. Rehabil Nurs 2004;29:154–63.
44. Lutz BJ, Young ME, Creasy KR, et al. Improving stroke caregiver readiness for transition from inpatinet rehabilitation to home. Gerontologist 2016. Forthcoming.
45. Visser-Meily JM, Post MW, Riphagen II, Lindeman E. Measures used to assess burden among caregivers of stroke patients: a review. Clin Rehabil 2004;18:601–23.
46. Moon M. The unprepared caregiver. Gerontologist 2016 Apr 21.
47. Pierce LL, Steiner V, Govoni AL, et al. Internet-based support for rural caregivers of persons with stroke shows promise. Rehabil Nurs 2004;29:95–9,103.
48. Bakas T, Austin JK, Okonkwo KF, et al. Needs, concerns, strategies, and advice of stroke caregivers the first 6 months after discharge. J Neurosci Nurs 2002;34:242–51.
49. Lutz BJ, Chumbler NR, Lyles T, et al. Testing a home-telehealth programme for US veterans recovering from stroke and their family caregivers. Disabil Rehabil 2009;31:402–9.
50. Hayes J, Chapman P, Young LJ, Rittman M. The prevalence of injury for stroke caregivers and associated risk factors. Top Stroke Rehabil 2009;16:300–7.
51. Cameron JI, Gignac MA. “Timing It Right”: a conceptual framework for addressing the support needs of family caregivers to stroke survivors from the hospital to the home. Patient Educ Couns 2008;70:305–14.
52. Creasy KR, Lutz BJ, Young ME, et al. The impact of interactions with providers on stroke caregivers’ needs. Rehabil Nurs 2013;38:88–98.
53. Bakas T, Farran CJ, Austin JK, et al. Stroke caregiver outcomes from the Telephone Assessment and Skill-Building Kit (TASK). Top Stroke Rehabil 2009;16:105–21.
54. Cameron JI, Naglie G, Silver FL, Gignac MA. Stroke family caregivers’ support needs change across the care continuum: a qualitative study using the timing it right framework. Disabil Rehabil 2013;35:315–24.
55. Cameron JI, O’Connell C, Foley N, et al. Canadian Stroke Best Practice Recommendations: Managing transitions of care following Stroke. Guidelines Update 2016. Int J Stroke. 2016.
56. Family Caregiver Alliance. Caregivers Count Too! A toolkit to help practitioners assess the needs of family caregivers. San Francisco: 2006. Accessed 16 Aug 2016 at www.caregiver.org/caregivers-count-too-toolkit.
57. Messecar DC. Nursing standard of practice protocol: family caregiving [Internet]. Accessed at www.consultgeri.org/geriatric-topics/family-caregiving.
58. Young ME, Lutz BJ, Creasy KR et al. A comprehensive assessment of family caregivers of stroke survivors during inpatient rehabilitation. Disabil Rehabil 2014;36:1892–902.
59. Archbold PG, Stewart BJ, Greenlick MR, Harvath T. Mutuality and preparedness as predictors of caregiver role strain. Res Nurs Health 1990;13:375–84.
60. Pucciarelli G, Savini S, Byun E, et al. Psychometric properties of the Caregiver Preparedness Scale in caregivers of stroke survivors. Heart & Lung 2014;43:555–60.
61. Deeken JF, Taylor KL, Mangan P et al. Care for the caregivers: a review of self-report instruments developed to measure the burden, needs, and quality of life of informal caregivers. J Pain Symptom Manage 2003;26:922–53.
62. Haley WE, Roth DL, Howard G, Safford MM. Caregiving strain and estimated risk for stroke and coronary heart disease among spouse caregivers: differential effects by race and sex. Stroke 2010;41:331–6.
63. Bakas T, Champion V, Perkins SM, et al. Psychometric testing of the revised 15-item Bakas Caregiving Outcomes Scale. Nurs Res 2006;55:346–55.
64. Family Caregiver Alliance. Selected caregiver assessment measures: A resource inventory for practitioners. 2d ed. Accessed at www.caregiver.org/selected-caregiver-assessment-measures-resource-inventory-practitioners-2012.
65. Bakas T, Clark PC, Kelly-Hayes M, et al. Evidence for stroke family caregiver and dyad interventions: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2836–52.
66. Cheng HY, Chair SY, Chau JPC. The effectiveness of psychosocial interventions for stroke family caregivers and stroke survivors: a systematic review and meta-analysis. Patient Educ Couns 2014;95:30–44.
67. Bakas T, Austin JK, Habermann B, et al. Telephone assessment and skill-building kit for stroke caregivers a randomized controlled clinical trial. Stroke 2015;46:3478–87.
68. Alim M, Lindley R, Felix C, et al. Family-led rehabilitation after stroke in India: the ATTEND trial, study protocol for a randomized controlled trial. Trials 2016;17:13.
69. Camicia M, Lutz B. Nursing’s role in successful transitions across settings. Stroke 2016. Forthcoming.
70. Chan L, Sandel ME, Jette AM, et al. Does postacute care site matter? A longitudinal study assessing functional recovery after a stroke. Arch Phys Med Rehabil 2013;94:622–9.
71. Kind AJ, Smith MA, Pandhi N, et al. Bouncing-back: rehospitalization in patients with complicated transitions in the first thirty days after hospital discharge for acute stroke. Home Health Care Serv Q 2007;26:37–55.
72. Bettger JP, Liang L, Xian Y, et al. Inpatient rehabilitation facility care reduces the likelihood of death and rehospitalization after stroke compared with skilled nursing facility care [abstract]. Stroke 2015; A146.
73. Camicia M, Black T, Farrell J, et al. The essential role of the rehabilitation nurse in facilitating care transitions: a white paper by the Association of Rehabilitation Nurses. Rehabil Nurs 2014;39:3–15.
74. Centers for Medicare and Medicaid. CMS quality strategy 2016. Accessed at www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/qualityinitiativesgeninfo/downloads/cms-quality-strategy.pdf.
1. Mozaffarian D, Benjamin EJ, Go AS, et al. on behalf of the American Heart Association Statistics Committee & Stroke Statistics Subcommittee. Heart disease and stroke statistics - 2016 update: A report from the American Heart Association. Circulation 2016;132:e38–e360.
2. Feigin VL, Krishnamurthi RV, Parmar P, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: The GBD 2013 Study. Neuroepidemiology 2015;45:161–76.
3. Hall MJ, Levant S, DeFrances CJ. Hospitalization for stroke in U.S. hospitals, 1989-2009. Hyattsville, MD: National Center for Heatlh Statistics; 2012.
4. Prvu Bettger J, McCoy L, Smith EE, et al. Contemporary trends and predictors of postacute service use and routine discharge home after stroke. J Am Heart Assoc 2015;4(2).
5. Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016;47:e98–e169.
6. Talley RC, Crews JE. Framing the public health of caregiving. Am J Public Health 2007;97:224–8.
7. van Exel NJ, Koopmanschap MA, van den Berg B, et al. Burden of informal caregiving for stroke patients: odentification of caregivers at risk of adverse health effects. Cerebrovasc Dis 2005;19:11–7.
8. van Exel NJ, Scholte op Reimer WJ, Brouwer WB, et al. Instruments for assessing the burden of informal caregiving for stroke patients in clinical practice: a comparison of CSI, CRA, SCQ and self-rated burden. Clin Rehabil 2004;18:203–14.
9. Lutz BJ, Young ME, Cox KJ, et al. The crisis of stroke: experiences of patients and their family caregivers. Top Stroke Rehabil 2011;18:786–97.
10. McCullagh E, Brigstocke G, Donaldson N, Kalra L. Determinants of caregiving burden and quality of life in caregivers of stroke patients. Stroke 2005;36:2181–6.
11. van Exel NJ, Brouwer WB, van den Berg B, et al. What really matters: an inquiry into the relative importance of dimensions of informal caregiver burden. Clin Rehabil 2004;18:683–93.
12. Perrin PB, Heesacker M, Hinojosa MS et al. Identifying at-risk, ethnically diverse stroke caregivers for counseling: a longitudinal study of mental health. Rehabil Psychol 2009;54:138–49.
13. Greenwood N, Mackenzie A, Cloud GC, Wilson N. Informal carers of stroke survivors--factors influencing carers: a systematic review of quantitative studies. Disabil Rehabil 2008;30:1329–49.
14. Camak DJ. Addressing the burden of stroke caregivers: a literature review. J Clin Nurs 2015;24:2376–82.
15. White CL, Barrientos R, Dunn K. Dimensions of uncertainty after stroke: perspectives of the stroke survivor and family caregiver. J Neurosci Nurs 2014;46:233–40.
16. Saban KL, Sherwood PR, DeVon HA, Hynes DM. Measures of psychological stress and physical health in family caregivers of stroke survivors: a literature review. J Neurosci Nurs 2010;42:128–38.
17. Kruithof WJ, Post, MWM, van Mierlo M, et al. Caregiver burden and emotional problems in partners of stroke patients at two months and one year post-stroke: determinants and prediction. Patient Educ Couns 2016. Forthcoming.
18. Sarre S, Redlich C, Tinker A, et al. A systematic review of qualitative studies on adjusting after stroke: lessons for the study of resilience. Disabil Rehabil 2014;36:716–26.
19. Cameron JI, Naglie G, Dick T, et al. Are we meeting the changing needs of family caregivers to stroke survivors across the care continuum? A systematic review of the caregiver intervention literature. Stroke 2012;43:E143-E.
20. Miller EL, Murray L, Richards L, et al. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke ptient: a scientific statement from the American Heart Association. Stroke 2010;41:2402–48.
21. Perrin PB, Heesacker M, Stidham BS, et al. Structural equation modeling of the relationship between caregiver psychosocial variables and functioning of individuals with stroke. Rehabil Psychol 2008;53:54–62.
22. Roth DL, Perkins M, Wadley VG, et al. Family caregiving and emotional strain: associations with quality of life in a large national sample of middle-aged and older adults. Qual Life Res 2009;18:679–88.
23. Haley WE, Roth DL, Hovater M, Clay OJ. Long-term impact of stroke on family caregiver well-being: a population-based case-control study. Neurology 2015;84:1323–9.
24. Greenwood N, Mackenzie A. Informal caring for stroke survivors: meta-ethnographic review of qualitative literature. Maturitas 2010;66:268–76.
25. Ostwald SK, Bernal MP, Cron SG, Godwin KM. Stress experienced by stroke survivors and spousal caregivers during the first year after discharge from inpatient rehabilitation. Top Stroke Rehabil 2009;16:93–104.
26. Brereton L, Carroll C, Barnston S. Interventions for adult family carers of people who have had a stroke: a systematic review. Clin Rehabil 2007;21:867–84.
27. Greenwood N, Mackenzie A, Cloud G, Wilson N. Loss of autonomy, control and independence when caring: a qualitative study of informal carers of stroke survivors in the first three months after discharge. Disabil Rehabil 2009:1–9.
28. Visser-Meily A, Post M, van de Port I, et al. Psychosocial functioning of spouses of patients with stroke from initial inpatient rehabilitation to 3 years poststroke: course and relations with coping strategies. Stroke 2009;40:1399–404.
29. Visser-Meily A, Post M, van de Port I, et al. Psychosocial functioning of spouses in the chronic phase after stroke: improvement or deterioration between 1 and 3 years after stroke? Patient Educ Couns 2008;73:153–8.
30. Haley WE, Allen JY, Grant JS, et al. Problems and benefits reported by stroke family caregivers: results from a prospective epidemiological study. Stroke 2009;40:2129–33.
31. Berg A, Palomaki H, Lonnqvist J, et al. Depression among caregivers of stroke survivors. Stroke 2005;36:639–43.
32. van Heugten C, Visser-Meily A, Post M, Lindeman E. Care for carers of stroke patients: evidence-based clinical practice guidelines. J Rehabil Med 2006;38:153–8.
33. Bakas T, Kroenke K, Plue LD, et al. Outcomes among family caregivers of aphasic versus nonaphasic stroke survivors. Rehabil Nurs 2006;31:33–42.
34. Gaugler JE. The longitudinal ramifications of stroke caregiving: a systematic review. Rehabil Psychol 2010;55:108–25.
35. Andrew NE, Kilkenny MF, Naylor R, et al. The relationship between caregiver impacts and the unmet needs of survivors of stroke. Patient Prefer Adher 2015;9:1065–73.
36. Chung ML, Bakas T, Plue LD, Williams LS. Effects of self-esteem, optimism, and perceived control on depressive symptoms in stroke survivor-spouse dyads. J Cardiovasc Nurs 2016;31:E8–E16.
37. Grant JS, Clay OJ, Keltner NL, et al. Does caregiver well-being predict stroke survivor depressive symptoms? a mediation analysis. Top Stroke Rehabil 2013;20:44–51.
38. Murray J, Young J, Forster A, Ashworth R. Developing a primary care-based stroke model: the prevalence of longer-term problems experienced by patients and carers. Br J Gen Pract 2003;53:803–7.
39. Pierce LL, Steiner V, Govoni A, et al. Two sides to the caregiving story. Top Stroke Rehabil 2007;14:13–20.
40. Mackenzie A, Greenwood N. Positive experiences of caregiving in stroke: a systematic review. Disabil Rehabil 2012;34:1413–22.
41. Parag V, Hackett ML, Yapa CM, et al. The impact of stroke on unpaid caregivers: results from The Auckland Regional Community Stroke study, 2002-2003. Cerebrovasc Dis (Basel, Switzerland) 2008;25:548–54.
42. Kruithof WJ, Post MWM, Visser-Meily JMA. Measuring negative and positive caregiving experiences: a psychometric analysis of the Caregiver Strain Index Expanded. Clin Rehabil 2015;29:1224–33.
43. Lutz BJ. Determinants of discharge destination for stroke patients. Rehabil Nurs 2004;29:154–63.
44. Lutz BJ, Young ME, Creasy KR, et al. Improving stroke caregiver readiness for transition from inpatinet rehabilitation to home. Gerontologist 2016. Forthcoming.
45. Visser-Meily JM, Post MW, Riphagen II, Lindeman E. Measures used to assess burden among caregivers of stroke patients: a review. Clin Rehabil 2004;18:601–23.
46. Moon M. The unprepared caregiver. Gerontologist 2016 Apr 21.
47. Pierce LL, Steiner V, Govoni AL, et al. Internet-based support for rural caregivers of persons with stroke shows promise. Rehabil Nurs 2004;29:95–9,103.
48. Bakas T, Austin JK, Okonkwo KF, et al. Needs, concerns, strategies, and advice of stroke caregivers the first 6 months after discharge. J Neurosci Nurs 2002;34:242–51.
49. Lutz BJ, Chumbler NR, Lyles T, et al. Testing a home-telehealth programme for US veterans recovering from stroke and their family caregivers. Disabil Rehabil 2009;31:402–9.
50. Hayes J, Chapman P, Young LJ, Rittman M. The prevalence of injury for stroke caregivers and associated risk factors. Top Stroke Rehabil 2009;16:300–7.
51. Cameron JI, Gignac MA. “Timing It Right”: a conceptual framework for addressing the support needs of family caregivers to stroke survivors from the hospital to the home. Patient Educ Couns 2008;70:305–14.
52. Creasy KR, Lutz BJ, Young ME, et al. The impact of interactions with providers on stroke caregivers’ needs. Rehabil Nurs 2013;38:88–98.
53. Bakas T, Farran CJ, Austin JK, et al. Stroke caregiver outcomes from the Telephone Assessment and Skill-Building Kit (TASK). Top Stroke Rehabil 2009;16:105–21.
54. Cameron JI, Naglie G, Silver FL, Gignac MA. Stroke family caregivers’ support needs change across the care continuum: a qualitative study using the timing it right framework. Disabil Rehabil 2013;35:315–24.
55. Cameron JI, O’Connell C, Foley N, et al. Canadian Stroke Best Practice Recommendations: Managing transitions of care following Stroke. Guidelines Update 2016. Int J Stroke. 2016.
56. Family Caregiver Alliance. Caregivers Count Too! A toolkit to help practitioners assess the needs of family caregivers. San Francisco: 2006. Accessed 16 Aug 2016 at www.caregiver.org/caregivers-count-too-toolkit.
57. Messecar DC. Nursing standard of practice protocol: family caregiving [Internet]. Accessed at www.consultgeri.org/geriatric-topics/family-caregiving.
58. Young ME, Lutz BJ, Creasy KR et al. A comprehensive assessment of family caregivers of stroke survivors during inpatient rehabilitation. Disabil Rehabil 2014;36:1892–902.
59. Archbold PG, Stewart BJ, Greenlick MR, Harvath T. Mutuality and preparedness as predictors of caregiver role strain. Res Nurs Health 1990;13:375–84.
60. Pucciarelli G, Savini S, Byun E, et al. Psychometric properties of the Caregiver Preparedness Scale in caregivers of stroke survivors. Heart & Lung 2014;43:555–60.
61. Deeken JF, Taylor KL, Mangan P et al. Care for the caregivers: a review of self-report instruments developed to measure the burden, needs, and quality of life of informal caregivers. J Pain Symptom Manage 2003;26:922–53.
62. Haley WE, Roth DL, Howard G, Safford MM. Caregiving strain and estimated risk for stroke and coronary heart disease among spouse caregivers: differential effects by race and sex. Stroke 2010;41:331–6.
63. Bakas T, Champion V, Perkins SM, et al. Psychometric testing of the revised 15-item Bakas Caregiving Outcomes Scale. Nurs Res 2006;55:346–55.
64. Family Caregiver Alliance. Selected caregiver assessment measures: A resource inventory for practitioners. 2d ed. Accessed at www.caregiver.org/selected-caregiver-assessment-measures-resource-inventory-practitioners-2012.
65. Bakas T, Clark PC, Kelly-Hayes M, et al. Evidence for stroke family caregiver and dyad interventions: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2836–52.
66. Cheng HY, Chair SY, Chau JPC. The effectiveness of psychosocial interventions for stroke family caregivers and stroke survivors: a systematic review and meta-analysis. Patient Educ Couns 2014;95:30–44.
67. Bakas T, Austin JK, Habermann B, et al. Telephone assessment and skill-building kit for stroke caregivers a randomized controlled clinical trial. Stroke 2015;46:3478–87.
68. Alim M, Lindley R, Felix C, et al. Family-led rehabilitation after stroke in India: the ATTEND trial, study protocol for a randomized controlled trial. Trials 2016;17:13.
69. Camicia M, Lutz B. Nursing’s role in successful transitions across settings. Stroke 2016. Forthcoming.
70. Chan L, Sandel ME, Jette AM, et al. Does postacute care site matter? A longitudinal study assessing functional recovery after a stroke. Arch Phys Med Rehabil 2013;94:622–9.
71. Kind AJ, Smith MA, Pandhi N, et al. Bouncing-back: rehospitalization in patients with complicated transitions in the first thirty days after hospital discharge for acute stroke. Home Health Care Serv Q 2007;26:37–55.
72. Bettger JP, Liang L, Xian Y, et al. Inpatient rehabilitation facility care reduces the likelihood of death and rehospitalization after stroke compared with skilled nursing facility care [abstract]. Stroke 2015; A146.
73. Camicia M, Black T, Farrell J, et al. The essential role of the rehabilitation nurse in facilitating care transitions: a white paper by the Association of Rehabilitation Nurses. Rehabil Nurs 2014;39:3–15.
74. Centers for Medicare and Medicaid. CMS quality strategy 2016. Accessed at www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/qualityinitiativesgeninfo/downloads/cms-quality-strategy.pdf.
First EDition: Retail Clinics and Rate of ED Visits, more
Retail Clinics Have Not Decreased the Rate of Low-Acuity ED Visits
BY JEFF BAUER
The number of retail clinics—those located in pharmacies, supermarkets, and other retail settings—in the United States increased from 130 in 2006 to nearly 1,400 in 2012. However, this proliferation of retail clinics has not lead to a meaningful reduction in low-acuity ED visits, according to a recent observational study published in Annals of Emergency Medicine.1
Using information from the Healthcare Cost and Utilization Project State Emergency Department Databases, which include data on more than 2,000 EDs in 23 states from 2006 through 2013, researchers looked at the association between retail clinic penetration and the rate of treat-and-release ED visits for 11 low-acuity conditions (allergic rhinitis, bronchitis, conjunctivitis, other eye conditions, influenza, otitis externa, otitis media, pharyngitis, upper respiratory infections/sinusitis, urinary tract infections, and viral infections).
Retail clinic penetration was defined as the percentage of an ED’s catchment area (areas that accounted for up to 75% of patients who visited for low-acuity conditions) that overlapped with the 10-minute-drive radius of a retail clinic. The results were calculated as a rate ratio, which reflected the change in the rate of low-acuity ED visits associated with an ED having no retail clinic penetration to having approximately the average penetration rate within 2012. Results were controlled for the number of urgent care centers that were present in each ED catchment area, but only for hospital-associated urgent care centers, as there are no reliable data to identify all urgent care centers.
Retail clinic penetration more than doubled during the study period. Overall, increased retail clinic penetration was not associated with a change in the rate of low-acuity ED visits. Among patients with private insurance, there was a small reduction (0.3% per calendar quarter) in ED visits for low-acuity conditions, but this translated into an estimated 17 fewer ED visits by privately insured patients over 1 year for the average ED, assuming the retail clinic penetration rate increased by 40% in that year.
In an accompanying editorial,2 Jesse M. Pines, MD, suggests that visits to retail clinics may be mostly “new-use” visits, meaning many individuals who would not have otherwise received treatment seek care in a retail clinic because such clinics are available. Dr Pines proposed three reasons retail clinics may create new-use visits: they meet unmet demands for care; motivations for seeking care differ in EDs and retail clinics; and people who are more likely to use EDs for low-acuity conditions do so because they have limited access to other types of care, including retail clinics.
1. Martsolf G, Fingar KR, Coffey R, et al. Association between the opening of retail clinics and low-acuity emergency department visits. Ann Emerg Med. 2016. In press. http://dx.doi.org/10.1016/j.annemergmed.2016.08.462.
2. Pines JM. Why retail clinics do not substitute for emergency department visits and what this means for value-based care. Ann Emerg Med. 2016. In press. http://dx.doi.org/10.1016/j.annemergmed.2016.09.047.
Hypotension During Transport to ED Drives Mortality in Traumatic Brain Injury
MITCHEL L. ZOLER
FRONTLINE MEDICAL NEWS
The severity and duration of hypotension in traumatic brain injury (TBI) patients during emergency medical service (EMS) transport to an ED has a tight and essentially linear relationship to mortality rate during subsequent weeks of recovery, according to an analysis of more than 7,500 brain-injured patients.
For each doubling of the combined severity and duration of hypotension during the prehospital period, when systolic blood pressure (BP) was <90 mm Hg, patient mortality rose by 19%, Daniel W. Spaite, MD, reported at the American Heart Association scientific sessions.
However, the results do not address whether aggressive treatment of hypotension by EMS technicians in a patient with TBI leads to reduced mortality. That question is being assessed as part of the primary endpoint of the Excellence in Prehospital Injury Care-Traumatic Brain Injury (EPIC-TBI) study, which should be completed by the end of 2017, said Dr Spaite, professor of emergency medicine at the University of Arizona in Tucson.Results from prior studies have clearly linked prehospital hypotension with worse survival in TBI patients. Until now, however, there was no appreciation of the fact that not all hypotensive episodes are equal, and that both the severity of hypotension and its duration incrementally contribute to mortality as the “dose” of hypotension a patient experiences increases. In large part, this is because prehospital hypotension has been recorded simply as a dichotomous, yes/no condition.
The innovation introduced by Dr Spaite and his associates in their analysis of the EPIC-TBI data was to drill down into each patient’s hypotensive event, made possible by the 16,711 patients enrolled in EPIC-TBI. Their calculations were limited to patients with EMS records of at least two BP measurements during prehospital transport. These data allowed Spaite et al to utilize both the extent to which systolic BP dropped below 90 mm Hg and the amount of time systolic BP was below this threshold to better define the total hypotension exposure each patient received.
This meant that a patient with a TBI and a systolic BP of 80 mm Hg for 10 minutes had twice the hypotension exposure of both a patient with a systolic BP of 85 mm Hg for 10 minutes and a patient with a systolic BP of 80 mm Hg for 5 minutes.
The analysis by Spaite et al also adjusted the relationship of total hypotensive severity and duration and subsequent mortality based on several baseline demographic and clinical variables, including age, sex, injury severity, trauma type, and head-region severity score. After adjustment, the researchers found a “strikingly linear relationship” between hypotension severity and duration and mortality, Dr Spaite said.
The EPIC-TBI enrolled TBI patients aged 10 years or older during 2007 to 2014 through participation of dozens of EMS providers throughout Arizona. For the current analysis, the researchers identified 7,521 patients from the total group who had at least two BP measurements taken during their prehospital EMS care and also met other inclusion criteria.
The best way to manage hypotension in TBI patients during the prehospital period remains unclear. Simply raising BP via intravenous (IV) fluid infusion may not necessarily help, because it could exacerbate a patient’s bleeding, Dr Spaite noted during an interview.
The primary goal of EPIC-TBI is to assess the implementation of the third edition of the TBI guidelines released in 2007 by the Brain Trauma Foundation. (The fourth edition of these guidelines came out in August 2016.) The new finding by Dr Spaite and his associates will allow the full EPIC-TBI analysis to correlate patient outcomes with the impact that acute, prehospital treatment had on the hypotension severity and duration each patient experienced, he noted.
“What’s remarkable is that the single prehospital parameter of hypotension for just a few minutes during transport can have such a strong impact on survival, given all the other factors that can influence outcomes” in TBI patients once they reach a hospital and during the period they remain hospitalized, Dr Spaite said.
1. Spaite DW. Presentation at: American Heart Association Scientific Sessions 2016. November 12-16, 2016; New Orleans, LA.
Fluid Administration in Sepsis Did Not Increase Need for Dialysis
M. Alexander Otto
FRONTLINE MEDICAL NEWS
Fluid administration of at least 1 L did not increase the incidence of acute respiratory or heart failure in severe sepsis, and actually seemed to decrease the need for dialysis in a review of 164 patients at Scott and White Memorial Hospital in Temple, Texas.
For every 1 mL of fluid administered per kilogram of body weight, the likelihood of dialysis decreased by 8.5% (odds ratio [OR], 0.915; 95% confidence interval [CI], 0.854-0.980; P = .0111), with no increase in heart or respiratory failure on univariate analysis. The 126 patients (77%) who received at least 1 L of fluid had a 68% reduction in the need for dialysis (OR, 0.32; CI, 0.117-0.890; P = .0288).
These findings come from a quality improvement project the hospital launched after researchers there realized that the benchmark Surviving Sepsis Campaign guidelines were not being met. The patients in the study had a systolic BP below 90 mm Hg or lactate level of at least 4 mmol/L. The guidelines would have called for these patients to receive 30 mL/kg of crystalloid fluids within 3 hours of presentation, but only 28 patients (17%) met that mark.
“The No. 1 reason we weren’t meeting benchmarks was fluid administration,” explained lead investigator Aruna Jahoor, MD, a pulmonary critical care and sleep medicine fellow at Texas Tech University Health Sciences Center.
Seventeen percent of patients received ≥30 mL/kg of fluid resuscitation, while 28% received ≥20 mL/kg of IV fluid resuscitation. It turned out that staff in the ED—where most of the patients were treated in the critical first 6 hours—were concerned about fluid overload and putting patients into respiratory, heart, or renal failure, Dr Jahoor said. The team found no difference in mortality rates when patients received 30 mL/kg—just over 2 L in a patient weighing 70 kg—vs 20 mL/kg or 1 L. The patients’ in-hospital mortality rate and 28-day mortality rate were 27% and 32%, respectively.
There also were no increased rates of heart failure, acute respiratory failure, or mechanical ventilation when patients received at least 1 L of fluid. “There were [also] lower rates of dialysis, which indicated that we weren’t overloading patients. Even when we looked at fluid as a continuous variable, we still didn’t see” complications, Dr Jahoor said.
The findings should be reassuring to treating physicians. “When you have pushback against 30-mL/kg administration, you can say ‘well, at least let’s give a liter.’ You don’t have to worry as much about some of the complications you are citing,’ ” she said.
For very obese patients, “it can get a little uncomfortable to be given” enough fluid to meet the 30-mL/kg goal, “but you can give at least a liter” without having to worry too much, she said. The patients in the study were treated from 2010 to 2013; normal saline was the most common resuscitation fluid. The hospital has since added the 30-mL/kg fluid resuscitation to its sepsis admission orders, and compliance has increased significantly.
A multivariate analysis is in the works to control for confounders. “We will probably [still] see you are not having increased rates of congestive heart or respiratory failure, or needing dialysis,” Dr Jahoor said. The protective effect against dialysis might drop out, “but I am hoping it doesn’t,” she said.
1. Jahoor A, Delmas T, Giri B, et al. Fluid resuscitation of at least 1 liter in septic patients decreases the need for renal replacement therapy without increasing the risk of acute congestive heart failure or acute respiratory failure. Chest. 2016;150(4_S):349A. doi:10.1016/j.chest.2016.08.362.
Survey: Antibiotic Shortages Are the New Norm
SHARON WORCESTER
FRONTLINE MEDICAL NEWS
Antibiotic shortages reported by the Emerging Infections Network (EIN) in 2011 persist in 2016, according to a Web-based follow-up survey of infectious disease physicians.
Of 701 network members who responded to the EIN survey in early 2016, 70% reported needing to modify their antimicrobial choice because of a shortage in the past 2 years. They did so by using broader-spectrum agents (75% of respondents), more costly agents (58%), less effective second-line agents (45%), and more toxic agents (37%), Adi Gundlapalli, MD, PhD, reported at an annual scientific meeting on infectious diseases.
In addition, 73% of respondents reported that the shortages affected patient care or outcomes, reported Dr Gundlapalli of the University of Utah, Salt Lake City.
The percentage of respondents reporting adverse patient outcomes related to shortages increased from 2011 to 2016 (51% vs 73%), he noted at the combined annual meetings of the Infectious Diseases Society of America, the Society of Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In the 2016 survey, the top 10 antimicrobials reported as being in short supply over the past 2 years were piperacillin-tazobactam, ampicillin-sulbactam, meropenem, cefotaxime, cefepime, trimethoprim-sulfamethoxazole (TMP-SMX), doxycycline, imipenem, acyclovir, and amikacin. Trimethoprim-sulfamethoxazole and acyclovir were in short supply in 2011 and 2016.
According to respondents, the most common ways they learned about drug shortages were from hospital notification (76%), from a colleague (56%), from a pharmacy that contacted them regarding a prescription for the agent (53%), or from the US Food and Drug Administration (FDA) Web site or another Web site (23%). The most common ways of learning about a shortage changed—from notification after trying to prescribe a drug in 2011, to proactive hospital/system (local) notification in 2016; 71% of respondents said that communications in 2016 were sufficient.
Most respondents (83%) reported that guidelines for dealing with shortages had been developed by an antimicrobial stewardship program (ASP) at their institution.
“This, I think, is one of the highlight results,” said Dr Gundlapalli, who is also a staff physician at the VA Salt Lake City Health System. “In 2011, we had no specific question or comments received about [ASPs], and here in 2016, 83% of respondents’ institutions had developed guidelines related to drug shortages.”
Respondents also had the opportunity to submit free-text responses, and among the themes that emerged was concern regarding toxicity and adverse outcomes associated with increased use of aminoglycosides because of the shortage of piperacillin-tazobactam. Another was the shortage of meropenem, which led one ASP to “institute restrictions on its use, which have continued,” he said.
“Another theme was ‘simpler agents seem more likely to be in shortage,’ ” Dr Gundlapalli said, noting ampicillin-sulbactam in 2016 and penicillin G procaine as examples.
“And then, of course, the other theme across the board...was our new asset,” he said, explaining that some respondents commented on the value of ASP pharmacists and programs to help with drug shortage issues.
The overall theme of this follow-up survey, in the context of prior surveys in 2001 and 2011, is that antibiotic shortages are the “new normal—a way of life,” Dr Gundlapalli said.
“The concerns do persist, and we feel there is further work to be done here,” he said. He specifically noted that there is a need to inform and educate fellows and colleagues in hospitals, increase awareness generally, improve communication strategies, and conduct detailed studies on adverse effects and outcomes.
“And now, since ASPs are very pervasive...maybe it’s time to formalize and delineate the role of ASPs in antimicrobial shortages,” he said.
Donald Graham, MD, one of the study’s coauthors, said he believes the problem is in part the result of economics, and in part because of “the higher standards that the FDA imposes upon these manufacturing concerns.” These drugs often are low-profit items, and it is not always in the financial best interest of a pharmaceutical company to upgrade their facilities.
1. Gundlapalli A. Presentation at: IDWeek 2016. October 26-30, 2016. New Orleans, LA.
Hospitalizations for Opioid Poisoning Tripled in Preschool Children
Richard Franki
FRONTLINE MEDICAL NEWS
From 1997 to 2012, the annual number of hospitalizations for opioid poisoning rose 178% among children aged 1 to 19 years, according to data from 13,052 discharges in the Agency for Healthcare Research and Quality’s Kids’ Inpatient Database.
In 2012, there were 2,918 hospitalizations for opioid poisoning among children aged 1 to 19 years, compared with 1,049 in 1997, reported Julie R. Gaither, PhD, MPH, RN, and her associates at Yale University in New Haven, Connecticut.
The greatest change occurred among the youngest children, as the number of those aged 1 to 4 years rose from 133 in 1997 to 421 in 2012—an increase of 217%. For those aged 15 to 19 years, the annual number of hospitalizations went from 715 to 2,171 (204%) over that time period, which included a slight drop from 2009 to 2012, according to the investigators,
The increase in hospitalizations for prescription opioid poisoning in children aged 10 to 14 years was 58% from 1997 to 2012 (rising from 171 to 272), while estimates for 5- to 9-year-old children did not meet the criteria for statistical reliability and were not included in the analysis, Dr Gaither and her associates said.
1. Gaither JR, Leventhal JM, Ryan SA, Camenga DR. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997 to 2012. JAMA Pediatr. 2016 Oct 31. Epub ahead of print. doi:10.1001/jamapediatrics.2016.2154.
Pelvic Fracture Pattern Predicts the Need for Hemorrhage Control
Doug Brunk
FRONTLINE MEDICAL NEWS
Blunt trauma patients admitted in shock with anterior posterior compression III or vertical shear fracture patterns, or patients with open pelvic fracture are at greatest risk of severe bleeding requiring pelvic hemorrhage-control intervention, results from a multicenter trial demonstrated.
Thirty years ago, researchers defined a classification of pelvic fracture based on a pattern of force applied to the pelvis, Todd W. Costantini, MD, said at the annual meeting of the American Association for the Surgery of Trauma (AAST). They identified three main force patterns: lateral compression, anterior posterior compression, and vertical shear.
“They were able to show that certain pelvic fractures were associated with soft-tissue injury and pelvic hemorrhage,” said Dr Costantini, of the division of trauma, surgical critical care, burns and acute care surgery at the University of California, San Diego. “Since then, several single-center studies have been conducted in an attempt to correlate fracture pattern with the risk of pelvic hemorrhage. A majority of these studies evaluated angiogram [and embolization] as the endpoint for hemorrhage control. Modern trauma care has evolved to include multiple modalities to control hemorrhage, which include pelvic external fixator placement, pelvic angiography and embolization, preperitoneal pelvic packing, and the use of the REBOA [Resuscitative Endovascular Balloon Occlusion of the Aorta] catheter as an adjunct to hemorrhage control.”
In a recently published study, Dr Costantini and his associates found wide variability in the use of pelvic hemorrhage-control methods.1 “While angioembolization alone and external fixator placement alone were the most common methods used, there were various combinations of these methods used at different times by different institutions,” he said.
These results prompted the researchers to prospectively evaluate the correlation between pelvic fracture pattern and modern care of pelvic hemorrhage control at 11 Level 1 trauma centers over a 2-year period.2 Inclusion criteria for the study, which was sponsored by the AAST Multi-institutional Trials Committee, were patients over age 18 years, blunt mechanism of injury, and shock on admission defined as “...systolic blood pressure <90 mm Hg or heart rate >120 beats per minute or base deficit <-5.”1 Exclusion criteria included isolated hip fracture, pregnancy, and lack of pelvic imaging.
The researchers evaluated the pelvic fracture pattern for each patient in the study. “Each pelvic image was evaluated by a trauma surgeon, orthopedic surgeon, or radiologist and classified using the Young-Burgess Classification system,” Dr Costantini said. Next, they used univariate and multivariate logistic regression analyses to examine predictors for hemorrhage control intervention and mortality. The objective was to determine whether pelvic fracture pattern would predict the need for a hemorrhage control intervention.
Of the 46,716 trauma patients admitted over the 2-year period, 1,339 sustained a pelvic fracture. Of these, 178 met criteria for shock. The researchers excluded 15 patients due to lack of pelvic imaging, which left 163 patients in the final analysis. Their mean age was 44 years and 58% were male. On admission, their mean systolic BP was 93 mm Hg, their mean HR was 117 beats/min, and their median Injury Severity Score was 28. The mean hospital length of stay was 12 days and the mortality rate was 30%. The three most common mechanisms of injury were motor vehicle crash (42%), followed by pedestrian vs auto (23%), and falls (18%).
Compared with patients who did not require hemorrhage-control intervention, those who did received more transfusion of packed red blood cells (13 vs 7 units, respectively; P < .01) and fresh frozen plasma (10 U vs 5 U; P = .01). In addition, 67% of patients with open pelvic fracture required a hemorrhage control intervention. The rate of mortality was similar between the patients who required a pelvic hemorrhage control intervention and those who did not (34% vs 28%; P = .47).
The three most common types of pelvic fracture patterns were lateral compression I (36%) and II (23%), followed by vertical shear (13%). Patients with lateral compression I and II fractures were least likely to require hemorrhage-control intervention (22% and 19%, respectively). However, on univariate analysis, patients with anterior posterior compression III fractures and those with vertical shear fractures were more likely to require a pelvic hemorrhage control intervention, compared with those who sustained other types of pelvic fractures (83% and 55%, respectively).
On multivariate analysis, the three main independent predictors of need for a hemorrhagic control intervention were anterior posterior compression III fracture (OR, 109.43; P < .001), open pelvic fracture (OR, 7.36; P = .014), and vertical shear fracture (OR, 6.99; P = .002). Pelvic fracture pattern did not predict mortality on multivariate analysis.
The invited discussant, Joseph M. Galante, MD, trauma medical director for the University of California, Davis Health System, characterized the study as important “because it examines all forms of hemorrhage control, not just arterioembolism in the treatment of pelvic fractures,” he said. “The ability to predict who will need hemorrhage control allows for earlier mobilization to resources, both in the operating room or interventional suite and in the resuscitation bay.”
1. Costantini TW, Coimbra R, Holcomb JB, et al. Current management of hemorrhage from severe pelvic fractures: Results of an American Association for the Surgery of Trauma multi-institutional trial. J Trauma Acute Care Surg. 2016;80(5):717-723; discussion 723-725. doi:10.1097/TA.0000000000001034.2. Costantini TW. Presentation at: 75th Annual Meeting of American Association for the Surgery of Trauma (AAST) and Clinical Congress of Acute Care Surgery. September 14-17, 2016. Waikoloa, Hawaii.
Retail Clinics Have Not Decreased the Rate of Low-Acuity ED Visits
BY JEFF BAUER
The number of retail clinics—those located in pharmacies, supermarkets, and other retail settings—in the United States increased from 130 in 2006 to nearly 1,400 in 2012. However, this proliferation of retail clinics has not lead to a meaningful reduction in low-acuity ED visits, according to a recent observational study published in Annals of Emergency Medicine.1
Using information from the Healthcare Cost and Utilization Project State Emergency Department Databases, which include data on more than 2,000 EDs in 23 states from 2006 through 2013, researchers looked at the association between retail clinic penetration and the rate of treat-and-release ED visits for 11 low-acuity conditions (allergic rhinitis, bronchitis, conjunctivitis, other eye conditions, influenza, otitis externa, otitis media, pharyngitis, upper respiratory infections/sinusitis, urinary tract infections, and viral infections).
Retail clinic penetration was defined as the percentage of an ED’s catchment area (areas that accounted for up to 75% of patients who visited for low-acuity conditions) that overlapped with the 10-minute-drive radius of a retail clinic. The results were calculated as a rate ratio, which reflected the change in the rate of low-acuity ED visits associated with an ED having no retail clinic penetration to having approximately the average penetration rate within 2012. Results were controlled for the number of urgent care centers that were present in each ED catchment area, but only for hospital-associated urgent care centers, as there are no reliable data to identify all urgent care centers.
Retail clinic penetration more than doubled during the study period. Overall, increased retail clinic penetration was not associated with a change in the rate of low-acuity ED visits. Among patients with private insurance, there was a small reduction (0.3% per calendar quarter) in ED visits for low-acuity conditions, but this translated into an estimated 17 fewer ED visits by privately insured patients over 1 year for the average ED, assuming the retail clinic penetration rate increased by 40% in that year.
In an accompanying editorial,2 Jesse M. Pines, MD, suggests that visits to retail clinics may be mostly “new-use” visits, meaning many individuals who would not have otherwise received treatment seek care in a retail clinic because such clinics are available. Dr Pines proposed three reasons retail clinics may create new-use visits: they meet unmet demands for care; motivations for seeking care differ in EDs and retail clinics; and people who are more likely to use EDs for low-acuity conditions do so because they have limited access to other types of care, including retail clinics.
1. Martsolf G, Fingar KR, Coffey R, et al. Association between the opening of retail clinics and low-acuity emergency department visits. Ann Emerg Med. 2016. In press. http://dx.doi.org/10.1016/j.annemergmed.2016.08.462.
2. Pines JM. Why retail clinics do not substitute for emergency department visits and what this means for value-based care. Ann Emerg Med. 2016. In press. http://dx.doi.org/10.1016/j.annemergmed.2016.09.047.
Hypotension During Transport to ED Drives Mortality in Traumatic Brain Injury
MITCHEL L. ZOLER
FRONTLINE MEDICAL NEWS
The severity and duration of hypotension in traumatic brain injury (TBI) patients during emergency medical service (EMS) transport to an ED has a tight and essentially linear relationship to mortality rate during subsequent weeks of recovery, according to an analysis of more than 7,500 brain-injured patients.
For each doubling of the combined severity and duration of hypotension during the prehospital period, when systolic blood pressure (BP) was <90 mm Hg, patient mortality rose by 19%, Daniel W. Spaite, MD, reported at the American Heart Association scientific sessions.
However, the results do not address whether aggressive treatment of hypotension by EMS technicians in a patient with TBI leads to reduced mortality. That question is being assessed as part of the primary endpoint of the Excellence in Prehospital Injury Care-Traumatic Brain Injury (EPIC-TBI) study, which should be completed by the end of 2017, said Dr Spaite, professor of emergency medicine at the University of Arizona in Tucson.Results from prior studies have clearly linked prehospital hypotension with worse survival in TBI patients. Until now, however, there was no appreciation of the fact that not all hypotensive episodes are equal, and that both the severity of hypotension and its duration incrementally contribute to mortality as the “dose” of hypotension a patient experiences increases. In large part, this is because prehospital hypotension has been recorded simply as a dichotomous, yes/no condition.
The innovation introduced by Dr Spaite and his associates in their analysis of the EPIC-TBI data was to drill down into each patient’s hypotensive event, made possible by the 16,711 patients enrolled in EPIC-TBI. Their calculations were limited to patients with EMS records of at least two BP measurements during prehospital transport. These data allowed Spaite et al to utilize both the extent to which systolic BP dropped below 90 mm Hg and the amount of time systolic BP was below this threshold to better define the total hypotension exposure each patient received.
This meant that a patient with a TBI and a systolic BP of 80 mm Hg for 10 minutes had twice the hypotension exposure of both a patient with a systolic BP of 85 mm Hg for 10 minutes and a patient with a systolic BP of 80 mm Hg for 5 minutes.
The analysis by Spaite et al also adjusted the relationship of total hypotensive severity and duration and subsequent mortality based on several baseline demographic and clinical variables, including age, sex, injury severity, trauma type, and head-region severity score. After adjustment, the researchers found a “strikingly linear relationship” between hypotension severity and duration and mortality, Dr Spaite said.
The EPIC-TBI enrolled TBI patients aged 10 years or older during 2007 to 2014 through participation of dozens of EMS providers throughout Arizona. For the current analysis, the researchers identified 7,521 patients from the total group who had at least two BP measurements taken during their prehospital EMS care and also met other inclusion criteria.
The best way to manage hypotension in TBI patients during the prehospital period remains unclear. Simply raising BP via intravenous (IV) fluid infusion may not necessarily help, because it could exacerbate a patient’s bleeding, Dr Spaite noted during an interview.
The primary goal of EPIC-TBI is to assess the implementation of the third edition of the TBI guidelines released in 2007 by the Brain Trauma Foundation. (The fourth edition of these guidelines came out in August 2016.) The new finding by Dr Spaite and his associates will allow the full EPIC-TBI analysis to correlate patient outcomes with the impact that acute, prehospital treatment had on the hypotension severity and duration each patient experienced, he noted.
“What’s remarkable is that the single prehospital parameter of hypotension for just a few minutes during transport can have such a strong impact on survival, given all the other factors that can influence outcomes” in TBI patients once they reach a hospital and during the period they remain hospitalized, Dr Spaite said.
1. Spaite DW. Presentation at: American Heart Association Scientific Sessions 2016. November 12-16, 2016; New Orleans, LA.
Fluid Administration in Sepsis Did Not Increase Need for Dialysis
M. Alexander Otto
FRONTLINE MEDICAL NEWS
Fluid administration of at least 1 L did not increase the incidence of acute respiratory or heart failure in severe sepsis, and actually seemed to decrease the need for dialysis in a review of 164 patients at Scott and White Memorial Hospital in Temple, Texas.
For every 1 mL of fluid administered per kilogram of body weight, the likelihood of dialysis decreased by 8.5% (odds ratio [OR], 0.915; 95% confidence interval [CI], 0.854-0.980; P = .0111), with no increase in heart or respiratory failure on univariate analysis. The 126 patients (77%) who received at least 1 L of fluid had a 68% reduction in the need for dialysis (OR, 0.32; CI, 0.117-0.890; P = .0288).
These findings come from a quality improvement project the hospital launched after researchers there realized that the benchmark Surviving Sepsis Campaign guidelines were not being met. The patients in the study had a systolic BP below 90 mm Hg or lactate level of at least 4 mmol/L. The guidelines would have called for these patients to receive 30 mL/kg of crystalloid fluids within 3 hours of presentation, but only 28 patients (17%) met that mark.
“The No. 1 reason we weren’t meeting benchmarks was fluid administration,” explained lead investigator Aruna Jahoor, MD, a pulmonary critical care and sleep medicine fellow at Texas Tech University Health Sciences Center.
Seventeen percent of patients received ≥30 mL/kg of fluid resuscitation, while 28% received ≥20 mL/kg of IV fluid resuscitation. It turned out that staff in the ED—where most of the patients were treated in the critical first 6 hours—were concerned about fluid overload and putting patients into respiratory, heart, or renal failure, Dr Jahoor said. The team found no difference in mortality rates when patients received 30 mL/kg—just over 2 L in a patient weighing 70 kg—vs 20 mL/kg or 1 L. The patients’ in-hospital mortality rate and 28-day mortality rate were 27% and 32%, respectively.
There also were no increased rates of heart failure, acute respiratory failure, or mechanical ventilation when patients received at least 1 L of fluid. “There were [also] lower rates of dialysis, which indicated that we weren’t overloading patients. Even when we looked at fluid as a continuous variable, we still didn’t see” complications, Dr Jahoor said.
The findings should be reassuring to treating physicians. “When you have pushback against 30-mL/kg administration, you can say ‘well, at least let’s give a liter.’ You don’t have to worry as much about some of the complications you are citing,’ ” she said.
For very obese patients, “it can get a little uncomfortable to be given” enough fluid to meet the 30-mL/kg goal, “but you can give at least a liter” without having to worry too much, she said. The patients in the study were treated from 2010 to 2013; normal saline was the most common resuscitation fluid. The hospital has since added the 30-mL/kg fluid resuscitation to its sepsis admission orders, and compliance has increased significantly.
A multivariate analysis is in the works to control for confounders. “We will probably [still] see you are not having increased rates of congestive heart or respiratory failure, or needing dialysis,” Dr Jahoor said. The protective effect against dialysis might drop out, “but I am hoping it doesn’t,” she said.
1. Jahoor A, Delmas T, Giri B, et al. Fluid resuscitation of at least 1 liter in septic patients decreases the need for renal replacement therapy without increasing the risk of acute congestive heart failure or acute respiratory failure. Chest. 2016;150(4_S):349A. doi:10.1016/j.chest.2016.08.362.
Survey: Antibiotic Shortages Are the New Norm
SHARON WORCESTER
FRONTLINE MEDICAL NEWS
Antibiotic shortages reported by the Emerging Infections Network (EIN) in 2011 persist in 2016, according to a Web-based follow-up survey of infectious disease physicians.
Of 701 network members who responded to the EIN survey in early 2016, 70% reported needing to modify their antimicrobial choice because of a shortage in the past 2 years. They did so by using broader-spectrum agents (75% of respondents), more costly agents (58%), less effective second-line agents (45%), and more toxic agents (37%), Adi Gundlapalli, MD, PhD, reported at an annual scientific meeting on infectious diseases.
In addition, 73% of respondents reported that the shortages affected patient care or outcomes, reported Dr Gundlapalli of the University of Utah, Salt Lake City.
The percentage of respondents reporting adverse patient outcomes related to shortages increased from 2011 to 2016 (51% vs 73%), he noted at the combined annual meetings of the Infectious Diseases Society of America, the Society of Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In the 2016 survey, the top 10 antimicrobials reported as being in short supply over the past 2 years were piperacillin-tazobactam, ampicillin-sulbactam, meropenem, cefotaxime, cefepime, trimethoprim-sulfamethoxazole (TMP-SMX), doxycycline, imipenem, acyclovir, and amikacin. Trimethoprim-sulfamethoxazole and acyclovir were in short supply in 2011 and 2016.
According to respondents, the most common ways they learned about drug shortages were from hospital notification (76%), from a colleague (56%), from a pharmacy that contacted them regarding a prescription for the agent (53%), or from the US Food and Drug Administration (FDA) Web site or another Web site (23%). The most common ways of learning about a shortage changed—from notification after trying to prescribe a drug in 2011, to proactive hospital/system (local) notification in 2016; 71% of respondents said that communications in 2016 were sufficient.
Most respondents (83%) reported that guidelines for dealing with shortages had been developed by an antimicrobial stewardship program (ASP) at their institution.
“This, I think, is one of the highlight results,” said Dr Gundlapalli, who is also a staff physician at the VA Salt Lake City Health System. “In 2011, we had no specific question or comments received about [ASPs], and here in 2016, 83% of respondents’ institutions had developed guidelines related to drug shortages.”
Respondents also had the opportunity to submit free-text responses, and among the themes that emerged was concern regarding toxicity and adverse outcomes associated with increased use of aminoglycosides because of the shortage of piperacillin-tazobactam. Another was the shortage of meropenem, which led one ASP to “institute restrictions on its use, which have continued,” he said.
“Another theme was ‘simpler agents seem more likely to be in shortage,’ ” Dr Gundlapalli said, noting ampicillin-sulbactam in 2016 and penicillin G procaine as examples.
“And then, of course, the other theme across the board...was our new asset,” he said, explaining that some respondents commented on the value of ASP pharmacists and programs to help with drug shortage issues.
The overall theme of this follow-up survey, in the context of prior surveys in 2001 and 2011, is that antibiotic shortages are the “new normal—a way of life,” Dr Gundlapalli said.
“The concerns do persist, and we feel there is further work to be done here,” he said. He specifically noted that there is a need to inform and educate fellows and colleagues in hospitals, increase awareness generally, improve communication strategies, and conduct detailed studies on adverse effects and outcomes.
“And now, since ASPs are very pervasive...maybe it’s time to formalize and delineate the role of ASPs in antimicrobial shortages,” he said.
Donald Graham, MD, one of the study’s coauthors, said he believes the problem is in part the result of economics, and in part because of “the higher standards that the FDA imposes upon these manufacturing concerns.” These drugs often are low-profit items, and it is not always in the financial best interest of a pharmaceutical company to upgrade their facilities.
1. Gundlapalli A. Presentation at: IDWeek 2016. October 26-30, 2016. New Orleans, LA.
Hospitalizations for Opioid Poisoning Tripled in Preschool Children
Richard Franki
FRONTLINE MEDICAL NEWS
From 1997 to 2012, the annual number of hospitalizations for opioid poisoning rose 178% among children aged 1 to 19 years, according to data from 13,052 discharges in the Agency for Healthcare Research and Quality’s Kids’ Inpatient Database.
In 2012, there were 2,918 hospitalizations for opioid poisoning among children aged 1 to 19 years, compared with 1,049 in 1997, reported Julie R. Gaither, PhD, MPH, RN, and her associates at Yale University in New Haven, Connecticut.
The greatest change occurred among the youngest children, as the number of those aged 1 to 4 years rose from 133 in 1997 to 421 in 2012—an increase of 217%. For those aged 15 to 19 years, the annual number of hospitalizations went from 715 to 2,171 (204%) over that time period, which included a slight drop from 2009 to 2012, according to the investigators,
The increase in hospitalizations for prescription opioid poisoning in children aged 10 to 14 years was 58% from 1997 to 2012 (rising from 171 to 272), while estimates for 5- to 9-year-old children did not meet the criteria for statistical reliability and were not included in the analysis, Dr Gaither and her associates said.
1. Gaither JR, Leventhal JM, Ryan SA, Camenga DR. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997 to 2012. JAMA Pediatr. 2016 Oct 31. Epub ahead of print. doi:10.1001/jamapediatrics.2016.2154.
Pelvic Fracture Pattern Predicts the Need for Hemorrhage Control
Doug Brunk
FRONTLINE MEDICAL NEWS
Blunt trauma patients admitted in shock with anterior posterior compression III or vertical shear fracture patterns, or patients with open pelvic fracture are at greatest risk of severe bleeding requiring pelvic hemorrhage-control intervention, results from a multicenter trial demonstrated.
Thirty years ago, researchers defined a classification of pelvic fracture based on a pattern of force applied to the pelvis, Todd W. Costantini, MD, said at the annual meeting of the American Association for the Surgery of Trauma (AAST). They identified three main force patterns: lateral compression, anterior posterior compression, and vertical shear.
“They were able to show that certain pelvic fractures were associated with soft-tissue injury and pelvic hemorrhage,” said Dr Costantini, of the division of trauma, surgical critical care, burns and acute care surgery at the University of California, San Diego. “Since then, several single-center studies have been conducted in an attempt to correlate fracture pattern with the risk of pelvic hemorrhage. A majority of these studies evaluated angiogram [and embolization] as the endpoint for hemorrhage control. Modern trauma care has evolved to include multiple modalities to control hemorrhage, which include pelvic external fixator placement, pelvic angiography and embolization, preperitoneal pelvic packing, and the use of the REBOA [Resuscitative Endovascular Balloon Occlusion of the Aorta] catheter as an adjunct to hemorrhage control.”
In a recently published study, Dr Costantini and his associates found wide variability in the use of pelvic hemorrhage-control methods.1 “While angioembolization alone and external fixator placement alone were the most common methods used, there were various combinations of these methods used at different times by different institutions,” he said.
These results prompted the researchers to prospectively evaluate the correlation between pelvic fracture pattern and modern care of pelvic hemorrhage control at 11 Level 1 trauma centers over a 2-year period.2 Inclusion criteria for the study, which was sponsored by the AAST Multi-institutional Trials Committee, were patients over age 18 years, blunt mechanism of injury, and shock on admission defined as “...systolic blood pressure <90 mm Hg or heart rate >120 beats per minute or base deficit <-5.”1 Exclusion criteria included isolated hip fracture, pregnancy, and lack of pelvic imaging.
The researchers evaluated the pelvic fracture pattern for each patient in the study. “Each pelvic image was evaluated by a trauma surgeon, orthopedic surgeon, or radiologist and classified using the Young-Burgess Classification system,” Dr Costantini said. Next, they used univariate and multivariate logistic regression analyses to examine predictors for hemorrhage control intervention and mortality. The objective was to determine whether pelvic fracture pattern would predict the need for a hemorrhage control intervention.
Of the 46,716 trauma patients admitted over the 2-year period, 1,339 sustained a pelvic fracture. Of these, 178 met criteria for shock. The researchers excluded 15 patients due to lack of pelvic imaging, which left 163 patients in the final analysis. Their mean age was 44 years and 58% were male. On admission, their mean systolic BP was 93 mm Hg, their mean HR was 117 beats/min, and their median Injury Severity Score was 28. The mean hospital length of stay was 12 days and the mortality rate was 30%. The three most common mechanisms of injury were motor vehicle crash (42%), followed by pedestrian vs auto (23%), and falls (18%).
Compared with patients who did not require hemorrhage-control intervention, those who did received more transfusion of packed red blood cells (13 vs 7 units, respectively; P < .01) and fresh frozen plasma (10 U vs 5 U; P = .01). In addition, 67% of patients with open pelvic fracture required a hemorrhage control intervention. The rate of mortality was similar between the patients who required a pelvic hemorrhage control intervention and those who did not (34% vs 28%; P = .47).
The three most common types of pelvic fracture patterns were lateral compression I (36%) and II (23%), followed by vertical shear (13%). Patients with lateral compression I and II fractures were least likely to require hemorrhage-control intervention (22% and 19%, respectively). However, on univariate analysis, patients with anterior posterior compression III fractures and those with vertical shear fractures were more likely to require a pelvic hemorrhage control intervention, compared with those who sustained other types of pelvic fractures (83% and 55%, respectively).
On multivariate analysis, the three main independent predictors of need for a hemorrhagic control intervention were anterior posterior compression III fracture (OR, 109.43; P < .001), open pelvic fracture (OR, 7.36; P = .014), and vertical shear fracture (OR, 6.99; P = .002). Pelvic fracture pattern did not predict mortality on multivariate analysis.
The invited discussant, Joseph M. Galante, MD, trauma medical director for the University of California, Davis Health System, characterized the study as important “because it examines all forms of hemorrhage control, not just arterioembolism in the treatment of pelvic fractures,” he said. “The ability to predict who will need hemorrhage control allows for earlier mobilization to resources, both in the operating room or interventional suite and in the resuscitation bay.”
1. Costantini TW, Coimbra R, Holcomb JB, et al. Current management of hemorrhage from severe pelvic fractures: Results of an American Association for the Surgery of Trauma multi-institutional trial. J Trauma Acute Care Surg. 2016;80(5):717-723; discussion 723-725. doi:10.1097/TA.0000000000001034.2. Costantini TW. Presentation at: 75th Annual Meeting of American Association for the Surgery of Trauma (AAST) and Clinical Congress of Acute Care Surgery. September 14-17, 2016. Waikoloa, Hawaii.
Retail Clinics Have Not Decreased the Rate of Low-Acuity ED Visits
BY JEFF BAUER
The number of retail clinics—those located in pharmacies, supermarkets, and other retail settings—in the United States increased from 130 in 2006 to nearly 1,400 in 2012. However, this proliferation of retail clinics has not lead to a meaningful reduction in low-acuity ED visits, according to a recent observational study published in Annals of Emergency Medicine.1
Using information from the Healthcare Cost and Utilization Project State Emergency Department Databases, which include data on more than 2,000 EDs in 23 states from 2006 through 2013, researchers looked at the association between retail clinic penetration and the rate of treat-and-release ED visits for 11 low-acuity conditions (allergic rhinitis, bronchitis, conjunctivitis, other eye conditions, influenza, otitis externa, otitis media, pharyngitis, upper respiratory infections/sinusitis, urinary tract infections, and viral infections).
Retail clinic penetration was defined as the percentage of an ED’s catchment area (areas that accounted for up to 75% of patients who visited for low-acuity conditions) that overlapped with the 10-minute-drive radius of a retail clinic. The results were calculated as a rate ratio, which reflected the change in the rate of low-acuity ED visits associated with an ED having no retail clinic penetration to having approximately the average penetration rate within 2012. Results were controlled for the number of urgent care centers that were present in each ED catchment area, but only for hospital-associated urgent care centers, as there are no reliable data to identify all urgent care centers.
Retail clinic penetration more than doubled during the study period. Overall, increased retail clinic penetration was not associated with a change in the rate of low-acuity ED visits. Among patients with private insurance, there was a small reduction (0.3% per calendar quarter) in ED visits for low-acuity conditions, but this translated into an estimated 17 fewer ED visits by privately insured patients over 1 year for the average ED, assuming the retail clinic penetration rate increased by 40% in that year.
In an accompanying editorial,2 Jesse M. Pines, MD, suggests that visits to retail clinics may be mostly “new-use” visits, meaning many individuals who would not have otherwise received treatment seek care in a retail clinic because such clinics are available. Dr Pines proposed three reasons retail clinics may create new-use visits: they meet unmet demands for care; motivations for seeking care differ in EDs and retail clinics; and people who are more likely to use EDs for low-acuity conditions do so because they have limited access to other types of care, including retail clinics.
1. Martsolf G, Fingar KR, Coffey R, et al. Association between the opening of retail clinics and low-acuity emergency department visits. Ann Emerg Med. 2016. In press. http://dx.doi.org/10.1016/j.annemergmed.2016.08.462.
2. Pines JM. Why retail clinics do not substitute for emergency department visits and what this means for value-based care. Ann Emerg Med. 2016. In press. http://dx.doi.org/10.1016/j.annemergmed.2016.09.047.
Hypotension During Transport to ED Drives Mortality in Traumatic Brain Injury
MITCHEL L. ZOLER
FRONTLINE MEDICAL NEWS
The severity and duration of hypotension in traumatic brain injury (TBI) patients during emergency medical service (EMS) transport to an ED has a tight and essentially linear relationship to mortality rate during subsequent weeks of recovery, according to an analysis of more than 7,500 brain-injured patients.
For each doubling of the combined severity and duration of hypotension during the prehospital period, when systolic blood pressure (BP) was <90 mm Hg, patient mortality rose by 19%, Daniel W. Spaite, MD, reported at the American Heart Association scientific sessions.
However, the results do not address whether aggressive treatment of hypotension by EMS technicians in a patient with TBI leads to reduced mortality. That question is being assessed as part of the primary endpoint of the Excellence in Prehospital Injury Care-Traumatic Brain Injury (EPIC-TBI) study, which should be completed by the end of 2017, said Dr Spaite, professor of emergency medicine at the University of Arizona in Tucson.Results from prior studies have clearly linked prehospital hypotension with worse survival in TBI patients. Until now, however, there was no appreciation of the fact that not all hypotensive episodes are equal, and that both the severity of hypotension and its duration incrementally contribute to mortality as the “dose” of hypotension a patient experiences increases. In large part, this is because prehospital hypotension has been recorded simply as a dichotomous, yes/no condition.
The innovation introduced by Dr Spaite and his associates in their analysis of the EPIC-TBI data was to drill down into each patient’s hypotensive event, made possible by the 16,711 patients enrolled in EPIC-TBI. Their calculations were limited to patients with EMS records of at least two BP measurements during prehospital transport. These data allowed Spaite et al to utilize both the extent to which systolic BP dropped below 90 mm Hg and the amount of time systolic BP was below this threshold to better define the total hypotension exposure each patient received.
This meant that a patient with a TBI and a systolic BP of 80 mm Hg for 10 minutes had twice the hypotension exposure of both a patient with a systolic BP of 85 mm Hg for 10 minutes and a patient with a systolic BP of 80 mm Hg for 5 minutes.
The analysis by Spaite et al also adjusted the relationship of total hypotensive severity and duration and subsequent mortality based on several baseline demographic and clinical variables, including age, sex, injury severity, trauma type, and head-region severity score. After adjustment, the researchers found a “strikingly linear relationship” between hypotension severity and duration and mortality, Dr Spaite said.
The EPIC-TBI enrolled TBI patients aged 10 years or older during 2007 to 2014 through participation of dozens of EMS providers throughout Arizona. For the current analysis, the researchers identified 7,521 patients from the total group who had at least two BP measurements taken during their prehospital EMS care and also met other inclusion criteria.
The best way to manage hypotension in TBI patients during the prehospital period remains unclear. Simply raising BP via intravenous (IV) fluid infusion may not necessarily help, because it could exacerbate a patient’s bleeding, Dr Spaite noted during an interview.
The primary goal of EPIC-TBI is to assess the implementation of the third edition of the TBI guidelines released in 2007 by the Brain Trauma Foundation. (The fourth edition of these guidelines came out in August 2016.) The new finding by Dr Spaite and his associates will allow the full EPIC-TBI analysis to correlate patient outcomes with the impact that acute, prehospital treatment had on the hypotension severity and duration each patient experienced, he noted.
“What’s remarkable is that the single prehospital parameter of hypotension for just a few minutes during transport can have such a strong impact on survival, given all the other factors that can influence outcomes” in TBI patients once they reach a hospital and during the period they remain hospitalized, Dr Spaite said.
1. Spaite DW. Presentation at: American Heart Association Scientific Sessions 2016. November 12-16, 2016; New Orleans, LA.
Fluid Administration in Sepsis Did Not Increase Need for Dialysis
M. Alexander Otto
FRONTLINE MEDICAL NEWS
Fluid administration of at least 1 L did not increase the incidence of acute respiratory or heart failure in severe sepsis, and actually seemed to decrease the need for dialysis in a review of 164 patients at Scott and White Memorial Hospital in Temple, Texas.
For every 1 mL of fluid administered per kilogram of body weight, the likelihood of dialysis decreased by 8.5% (odds ratio [OR], 0.915; 95% confidence interval [CI], 0.854-0.980; P = .0111), with no increase in heart or respiratory failure on univariate analysis. The 126 patients (77%) who received at least 1 L of fluid had a 68% reduction in the need for dialysis (OR, 0.32; CI, 0.117-0.890; P = .0288).
These findings come from a quality improvement project the hospital launched after researchers there realized that the benchmark Surviving Sepsis Campaign guidelines were not being met. The patients in the study had a systolic BP below 90 mm Hg or lactate level of at least 4 mmol/L. The guidelines would have called for these patients to receive 30 mL/kg of crystalloid fluids within 3 hours of presentation, but only 28 patients (17%) met that mark.
“The No. 1 reason we weren’t meeting benchmarks was fluid administration,” explained lead investigator Aruna Jahoor, MD, a pulmonary critical care and sleep medicine fellow at Texas Tech University Health Sciences Center.
Seventeen percent of patients received ≥30 mL/kg of fluid resuscitation, while 28% received ≥20 mL/kg of IV fluid resuscitation. It turned out that staff in the ED—where most of the patients were treated in the critical first 6 hours—were concerned about fluid overload and putting patients into respiratory, heart, or renal failure, Dr Jahoor said. The team found no difference in mortality rates when patients received 30 mL/kg—just over 2 L in a patient weighing 70 kg—vs 20 mL/kg or 1 L. The patients’ in-hospital mortality rate and 28-day mortality rate were 27% and 32%, respectively.
There also were no increased rates of heart failure, acute respiratory failure, or mechanical ventilation when patients received at least 1 L of fluid. “There were [also] lower rates of dialysis, which indicated that we weren’t overloading patients. Even when we looked at fluid as a continuous variable, we still didn’t see” complications, Dr Jahoor said.
The findings should be reassuring to treating physicians. “When you have pushback against 30-mL/kg administration, you can say ‘well, at least let’s give a liter.’ You don’t have to worry as much about some of the complications you are citing,’ ” she said.
For very obese patients, “it can get a little uncomfortable to be given” enough fluid to meet the 30-mL/kg goal, “but you can give at least a liter” without having to worry too much, she said. The patients in the study were treated from 2010 to 2013; normal saline was the most common resuscitation fluid. The hospital has since added the 30-mL/kg fluid resuscitation to its sepsis admission orders, and compliance has increased significantly.
A multivariate analysis is in the works to control for confounders. “We will probably [still] see you are not having increased rates of congestive heart or respiratory failure, or needing dialysis,” Dr Jahoor said. The protective effect against dialysis might drop out, “but I am hoping it doesn’t,” she said.
1. Jahoor A, Delmas T, Giri B, et al. Fluid resuscitation of at least 1 liter in septic patients decreases the need for renal replacement therapy without increasing the risk of acute congestive heart failure or acute respiratory failure. Chest. 2016;150(4_S):349A. doi:10.1016/j.chest.2016.08.362.
Survey: Antibiotic Shortages Are the New Norm
SHARON WORCESTER
FRONTLINE MEDICAL NEWS
Antibiotic shortages reported by the Emerging Infections Network (EIN) in 2011 persist in 2016, according to a Web-based follow-up survey of infectious disease physicians.
Of 701 network members who responded to the EIN survey in early 2016, 70% reported needing to modify their antimicrobial choice because of a shortage in the past 2 years. They did so by using broader-spectrum agents (75% of respondents), more costly agents (58%), less effective second-line agents (45%), and more toxic agents (37%), Adi Gundlapalli, MD, PhD, reported at an annual scientific meeting on infectious diseases.
In addition, 73% of respondents reported that the shortages affected patient care or outcomes, reported Dr Gundlapalli of the University of Utah, Salt Lake City.
The percentage of respondents reporting adverse patient outcomes related to shortages increased from 2011 to 2016 (51% vs 73%), he noted at the combined annual meetings of the Infectious Diseases Society of America, the Society of Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
In the 2016 survey, the top 10 antimicrobials reported as being in short supply over the past 2 years were piperacillin-tazobactam, ampicillin-sulbactam, meropenem, cefotaxime, cefepime, trimethoprim-sulfamethoxazole (TMP-SMX), doxycycline, imipenem, acyclovir, and amikacin. Trimethoprim-sulfamethoxazole and acyclovir were in short supply in 2011 and 2016.
According to respondents, the most common ways they learned about drug shortages were from hospital notification (76%), from a colleague (56%), from a pharmacy that contacted them regarding a prescription for the agent (53%), or from the US Food and Drug Administration (FDA) Web site or another Web site (23%). The most common ways of learning about a shortage changed—from notification after trying to prescribe a drug in 2011, to proactive hospital/system (local) notification in 2016; 71% of respondents said that communications in 2016 were sufficient.
Most respondents (83%) reported that guidelines for dealing with shortages had been developed by an antimicrobial stewardship program (ASP) at their institution.
“This, I think, is one of the highlight results,” said Dr Gundlapalli, who is also a staff physician at the VA Salt Lake City Health System. “In 2011, we had no specific question or comments received about [ASPs], and here in 2016, 83% of respondents’ institutions had developed guidelines related to drug shortages.”
Respondents also had the opportunity to submit free-text responses, and among the themes that emerged was concern regarding toxicity and adverse outcomes associated with increased use of aminoglycosides because of the shortage of piperacillin-tazobactam. Another was the shortage of meropenem, which led one ASP to “institute restrictions on its use, which have continued,” he said.
“Another theme was ‘simpler agents seem more likely to be in shortage,’ ” Dr Gundlapalli said, noting ampicillin-sulbactam in 2016 and penicillin G procaine as examples.
“And then, of course, the other theme across the board...was our new asset,” he said, explaining that some respondents commented on the value of ASP pharmacists and programs to help with drug shortage issues.
The overall theme of this follow-up survey, in the context of prior surveys in 2001 and 2011, is that antibiotic shortages are the “new normal—a way of life,” Dr Gundlapalli said.
“The concerns do persist, and we feel there is further work to be done here,” he said. He specifically noted that there is a need to inform and educate fellows and colleagues in hospitals, increase awareness generally, improve communication strategies, and conduct detailed studies on adverse effects and outcomes.
“And now, since ASPs are very pervasive...maybe it’s time to formalize and delineate the role of ASPs in antimicrobial shortages,” he said.
Donald Graham, MD, one of the study’s coauthors, said he believes the problem is in part the result of economics, and in part because of “the higher standards that the FDA imposes upon these manufacturing concerns.” These drugs often are low-profit items, and it is not always in the financial best interest of a pharmaceutical company to upgrade their facilities.
1. Gundlapalli A. Presentation at: IDWeek 2016. October 26-30, 2016. New Orleans, LA.
Hospitalizations for Opioid Poisoning Tripled in Preschool Children
Richard Franki
FRONTLINE MEDICAL NEWS
From 1997 to 2012, the annual number of hospitalizations for opioid poisoning rose 178% among children aged 1 to 19 years, according to data from 13,052 discharges in the Agency for Healthcare Research and Quality’s Kids’ Inpatient Database.
In 2012, there were 2,918 hospitalizations for opioid poisoning among children aged 1 to 19 years, compared with 1,049 in 1997, reported Julie R. Gaither, PhD, MPH, RN, and her associates at Yale University in New Haven, Connecticut.
The greatest change occurred among the youngest children, as the number of those aged 1 to 4 years rose from 133 in 1997 to 421 in 2012—an increase of 217%. For those aged 15 to 19 years, the annual number of hospitalizations went from 715 to 2,171 (204%) over that time period, which included a slight drop from 2009 to 2012, according to the investigators,
The increase in hospitalizations for prescription opioid poisoning in children aged 10 to 14 years was 58% from 1997 to 2012 (rising from 171 to 272), while estimates for 5- to 9-year-old children did not meet the criteria for statistical reliability and were not included in the analysis, Dr Gaither and her associates said.
1. Gaither JR, Leventhal JM, Ryan SA, Camenga DR. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997 to 2012. JAMA Pediatr. 2016 Oct 31. Epub ahead of print. doi:10.1001/jamapediatrics.2016.2154.
Pelvic Fracture Pattern Predicts the Need for Hemorrhage Control
Doug Brunk
FRONTLINE MEDICAL NEWS
Blunt trauma patients admitted in shock with anterior posterior compression III or vertical shear fracture patterns, or patients with open pelvic fracture are at greatest risk of severe bleeding requiring pelvic hemorrhage-control intervention, results from a multicenter trial demonstrated.
Thirty years ago, researchers defined a classification of pelvic fracture based on a pattern of force applied to the pelvis, Todd W. Costantini, MD, said at the annual meeting of the American Association for the Surgery of Trauma (AAST). They identified three main force patterns: lateral compression, anterior posterior compression, and vertical shear.
“They were able to show that certain pelvic fractures were associated with soft-tissue injury and pelvic hemorrhage,” said Dr Costantini, of the division of trauma, surgical critical care, burns and acute care surgery at the University of California, San Diego. “Since then, several single-center studies have been conducted in an attempt to correlate fracture pattern with the risk of pelvic hemorrhage. A majority of these studies evaluated angiogram [and embolization] as the endpoint for hemorrhage control. Modern trauma care has evolved to include multiple modalities to control hemorrhage, which include pelvic external fixator placement, pelvic angiography and embolization, preperitoneal pelvic packing, and the use of the REBOA [Resuscitative Endovascular Balloon Occlusion of the Aorta] catheter as an adjunct to hemorrhage control.”
In a recently published study, Dr Costantini and his associates found wide variability in the use of pelvic hemorrhage-control methods.1 “While angioembolization alone and external fixator placement alone were the most common methods used, there were various combinations of these methods used at different times by different institutions,” he said.
These results prompted the researchers to prospectively evaluate the correlation between pelvic fracture pattern and modern care of pelvic hemorrhage control at 11 Level 1 trauma centers over a 2-year period.2 Inclusion criteria for the study, which was sponsored by the AAST Multi-institutional Trials Committee, were patients over age 18 years, blunt mechanism of injury, and shock on admission defined as “...systolic blood pressure <90 mm Hg or heart rate >120 beats per minute or base deficit <-5.”1 Exclusion criteria included isolated hip fracture, pregnancy, and lack of pelvic imaging.
The researchers evaluated the pelvic fracture pattern for each patient in the study. “Each pelvic image was evaluated by a trauma surgeon, orthopedic surgeon, or radiologist and classified using the Young-Burgess Classification system,” Dr Costantini said. Next, they used univariate and multivariate logistic regression analyses to examine predictors for hemorrhage control intervention and mortality. The objective was to determine whether pelvic fracture pattern would predict the need for a hemorrhage control intervention.
Of the 46,716 trauma patients admitted over the 2-year period, 1,339 sustained a pelvic fracture. Of these, 178 met criteria for shock. The researchers excluded 15 patients due to lack of pelvic imaging, which left 163 patients in the final analysis. Their mean age was 44 years and 58% were male. On admission, their mean systolic BP was 93 mm Hg, their mean HR was 117 beats/min, and their median Injury Severity Score was 28. The mean hospital length of stay was 12 days and the mortality rate was 30%. The three most common mechanisms of injury were motor vehicle crash (42%), followed by pedestrian vs auto (23%), and falls (18%).
Compared with patients who did not require hemorrhage-control intervention, those who did received more transfusion of packed red blood cells (13 vs 7 units, respectively; P < .01) and fresh frozen plasma (10 U vs 5 U; P = .01). In addition, 67% of patients with open pelvic fracture required a hemorrhage control intervention. The rate of mortality was similar between the patients who required a pelvic hemorrhage control intervention and those who did not (34% vs 28%; P = .47).
The three most common types of pelvic fracture patterns were lateral compression I (36%) and II (23%), followed by vertical shear (13%). Patients with lateral compression I and II fractures were least likely to require hemorrhage-control intervention (22% and 19%, respectively). However, on univariate analysis, patients with anterior posterior compression III fractures and those with vertical shear fractures were more likely to require a pelvic hemorrhage control intervention, compared with those who sustained other types of pelvic fractures (83% and 55%, respectively).
On multivariate analysis, the three main independent predictors of need for a hemorrhagic control intervention were anterior posterior compression III fracture (OR, 109.43; P < .001), open pelvic fracture (OR, 7.36; P = .014), and vertical shear fracture (OR, 6.99; P = .002). Pelvic fracture pattern did not predict mortality on multivariate analysis.
The invited discussant, Joseph M. Galante, MD, trauma medical director for the University of California, Davis Health System, characterized the study as important “because it examines all forms of hemorrhage control, not just arterioembolism in the treatment of pelvic fractures,” he said. “The ability to predict who will need hemorrhage control allows for earlier mobilization to resources, both in the operating room or interventional suite and in the resuscitation bay.”
1. Costantini TW, Coimbra R, Holcomb JB, et al. Current management of hemorrhage from severe pelvic fractures: Results of an American Association for the Surgery of Trauma multi-institutional trial. J Trauma Acute Care Surg. 2016;80(5):717-723; discussion 723-725. doi:10.1097/TA.0000000000001034.2. Costantini TW. Presentation at: 75th Annual Meeting of American Association for the Surgery of Trauma (AAST) and Clinical Congress of Acute Care Surgery. September 14-17, 2016. Waikoloa, Hawaii.
Holiday Poisonings
The holiday season, a time of warmth, joy, and good cheer, is upon us. Yet with this most wonderful time of the year comes the possibility of poisoning and hazards in the home. As emergency physicians (EPs), we must ask ourselves: Which holiday items are potentially toxic to our patients? How do we evaluate and manage poisonings that result from exposure to these items? In this article, we review several plants and decorations that are unique to the holiday season. We discuss recommendations for evaluation and management of holiday poisonings that will avoid inappropriate work-ups and interventions while increasing recognition of truly dangerous ingestions, thus help keeping the season safe and merry.
Plants
Plant exposures are the fourth most common cause for calls to poison centers.1 In 2012, US Poison Control Centers reported more than 30,000 toxic plant exposures in children younger than age 5 years.2 Not surprisingly, toxic plant ingestions occur most commonly in early childhood. The highest rate of mortality, however, takes place during the teenaged years, when suicide attempts are common.2 The most common plant ingestions reported in the United States include peace lily, holly, philodendron, and poinsettia.3 These and the other frequently ingested potentially poisonous plants produce very little, if any, toxic effects. Approximately 95% of unintentional potentially toxic plant ingestions reported in the United States are managed safely at home.3
Poinsettia
The poinsettia is a large, prominent plant that was introduced to the United States in 1825 by Joel Poinsett, the US ambassador to Mexico. The poinsettia is one of the most commonly researched plants, and studies show the plant is not actually toxic.4,5 The myth of poinsettia toxicity is a widely held, yet false, belief. The legend involves a young child of an army officer stationed in Hawaii in 1919 who reportedly died after eating poinsettia leaves.4 However, the reality is that the poinsettia plant was not actually involved in the child’s death. In fact, the wild plant involved in this case probably had little resemblance to the popular plant cultivated domestically in North America today.6
A majority of poinsettia exposures will be asymptomatic, or involve simple nausea and vomiting. Krenzelok et al5 reviewed 22,793 cases of poinsettia exposures from 1985 to 1992. Almost all of these exposures (98.9%) were accidental poisonings.5 Not surprisingly, 93.3% of poinsettia exposures involved children; most importantly, 96.1% of these patients did not require treatment at a health care facility,and there were no fatalities.5 Another study could not identify an LD50 (lethal dose, 50%—ie, the amount of an ingested substance that kills 50% of the test sample) in rats.4
The majority of patients presenting to the ED with symptoms from poinsettia exposure will have gastrointestinal (GI) upset. Most patients require only symptomatic care. Those who do present to the ED do not require gastric emptying.
Interestingly, there is a crossreactivity of poinsettia sap in latex-allergic vulnerable patients.6 Poinsettia is part of the same plant family as natural rubber latex, and patients can present with symptoms of contact dermatitis, especially if they have a latex allergy.4 Washing the area thoroughly with soap and water and avoiding future contact is all that is required for most patients with contact dermatitis.
Holly
Holly exposure accounted for the third highest rate of genus-specific human plant exposure calls to poison centers in 2010.4 In the United States, there are two common forms of holly: English holly and American holly. The berries of both varieties contain saponin, a toxin that can cause erythrocyte hemolysis and changes in the permeability of small intestinal mucosal cells.4 Most holly berry ingestions cause minor or no symptoms. The prickly leaves of the holly plant are nontoxic but consumption may result in minor injury. When symptoms do occur, they can include nausea, vomiting, abdominal cramping, and possible dermatitis.5 Mydriasis, hyperthermia, and drowsiness are rare but possible symptoms.4
For symptoms to develop, children need to have eaten only five berries, while adults reportedly must consume at least 20 to 30 berries.4 A study by Wax et al7 done at the University of Rochester reviewed 103 cases of toxic berry ingestion in children aged 9 months to 5 years, with children who swallowed six or fewer berries of holly, yew, or nightshade. Investigators compared home observation alone versus ipecac administration with home observation. Every patient treated with ipecac had emesis at home with increased sedation and diarrhea, while there was no emesis in the group with home observation alone.7 These results suggest the symptoms were due to the ipecac rather than plant toxicity.7 Thus, ipecac is not recommended, and patients should be treated symptomatically.
Bittersweet and Jerusalem Cherry
Bittersweet, also known as the woody nightshade, and Jerusalem cherry, or Christmas orange, are the most dangerous of the holiday plants. While there is little evidence to support serious toxicity to adults, ingestion may be dangerous to children. Bittersweet has purple and yellow flowers, spreading petals, and red, ovoid berries.4 Both plants are part of the genus Solanum. In both plants, the immature fruit is more poisonous than ripened fruit due to the glycoalkaloid solanine via hypothetical alteration of mitochondrial potassium and calcium transport.4 Case reports document the rare anticholinergic effects of these plants, likely due to dulcamarine.4
The largest case series included 319 ingestions of bittersweet or Jerusalem cherry.4 Of these, 295 patients were under age 10 years, and only nine experienced solanine-related symptoms; none required hospitalization.4 The symptoms of ingestion were primarily nausea and vomiting and abdominal cramping, possibly due to anticholinergic effects. Symptoms typically occur several hours after ingestion and may last for days.
Historically, induced emesis was recommended for ingestion in children, but this is no longer recommended. Prolonged observation may be necessary for children in the setting of high likelihood of ingestion. Management includes rehydration with intravenous (IV) fluids, antiemetics, and physostigmine if clinically warranted.4
Mistletoe
Mistletoe, a perennial with white or translucent berries, has traditionally been associated with kissing, fertility, and vitality. The American mistletoe is known as Phoradendron serotinum and the European mistletoe as Viscum album. Both the American and European mistletoe contain the toxalbumins phoratoxin and viscotoxin, which are associated with inhibiting cellular synthesis, thereby affecting cells with rapid turnover, including the GI mucosa.4
After several hours, clinical effects are primarily GI upset with potential sloughing of portions of the intestinal tract.4 Bradycardia, delirium, and hepatic, central nervous system, kidney, and adrenal gland toxicities can also occur.8 The American species has a lower toxicity compared to the European species. Cases involving death likely related to P serotinum usually occur due to excessive, concentrated herbal use, such as brewing mistletoe in tea.9 Placing the plant in hot water may result in larger amounts of ingested toxin. The only two reported deaths from ingestion of mistletoe were in patients who consumed brewed teas.4
A case review of 14 patients with American mistletoe leaf or berry ingestions failed to find any toxic symptoms.4 Krenzelok et al10 compiled the largest case review of 1,754 exposures from 1985 to 1992. In this review, patient outcomes were good. There were no fatalities, and 99% of patients experienced no morbidity. Outcomes were not influenced by GI decontamination.10
Another study by Spiller et al11 described 92 American mistletoe exposures involving ingestions of up to 20 berries and five leaves. In cases where five or more berries were consumed, none of the patients had symptoms.11 Three of the 11 patients (27%) who swallowed one to five leaves developed GI upset. One child had a seizure, likely not related to the mistletoe. The study concluded that severe toxic symptoms are uncommon.11
Management in the ED should involve supportive care for dehydration and vomiting, typically IV rehydration with normal saline or Ringer’s lactate and IV antiemetics. According to multiple case reviews, GI decontamination is not believed to alter patient outcome and is not recommended.4 An observation period of 6 hours is reasonable.4,10,11
Christmas Cactus
Christmas cactus is an old-time favorite. It is made of arching, drooping branches and spineless joints. Christmas cactus is essentially nontoxic, and patients and family can be reassured of its safety.
Holiday Decorations
Artificial Snow
Fake snow sprays, powders, and granules are popular decorative additions used in holiday games and celebrations. The “snow” typically consists of a polymer of sodium polyacrylate, both of which can cause injury to the eyes. Repeatedly inhaling the aerosol spray can cause breathing problems, especially in patients who have asthma or other underlying bronchospastic disease.
Devastating outcomes may occur from ocular alkaline injury. When mixed with water, the fake snow absorbs the water and expands as a gel material that may stick to the ocular surface, resulting in a change in pH and osmolarity.12 A case report by Al-Amry and Al-Ghadeer12 recently described a 7-year-old boy with corneal epitheliopathy due to a chemical burn injury following ocular contact with fake snow.The case was later managed with multiple debridements over 3 days, topical antibiotics, and bandage contact lenses. The child had complete resolution at 1 week follow-up.12
Some fake snow-product sprays contain acetone or methylene chloride, which is harmful when inhaled and can cause nausea, lightheadedness, and headache.13 Methylene chloride can be metabolized to carbon monoxide, but the quantity required for such an exposure is unknown and has not been reported in this context. Emergency physicians should consider ordering carboxyhemoglobin levels in symptomatic patients.
Tinsel
Tinsel, which gets its name from the Old French word “estincele,” translated as sparkle, used to be made of actual silver, and was affordable only for wealthy individuals. However, in the early 1900s, manufacturers began to make tinsel from metals such as aluminum and copper. These materials did not tarnish and could be reused annually. However, during World War I, copper became difficult to buy, while aluminum proved to be flammable and dangerous. Thus, manufacturers began to produce tinsel from lead. Tinsel was made with lead until the 1970s, when the US Food and Drug Administration realized the toxic risks of lead exposure, especially in young children. Today, tinsel is made of plastic; though a poor imitation of the previous tinsel, it is relatively harmless.14
Angel Hair
Angel hair is finely spun glass that can be irritating to the skin, eyes, and throat, especially if swallowed.13 The greatest danger is airway obstruction if a patient attempts to eat the angel hair and it becomes lodged in the oropharynx. For contact irritation, thoroughly washing and irrigating affected areas are recommended.
Snow Globes
Snow globes are popular holiday decorations that are available in a range of sizes. While the majority of globes made in the United States are filled with water, those manufactured overseas many contain a small amount of ethylene glycol (EG) (ie, antifreeze) to prevent freezing and breakage during shipping. Fortunately, the amount of EG is not usually sufficient to cause symptoms if ingested. For globes made in the United States, the water can be contaminated with bacteria, and drinking it can cause GI upset. The snow in these globes is typically made of inert material and does not cause toxicity. If a child does exhibit symptoms after ingesting any portion of a snow globe, parents are advised to call their local poison center.
Ethanol
While alcohol is not unique to the holiday season, its availability and use are more pronounced during this time of year, and the incidence of alcohol poisoning increases during the holiday season. Some traditional holiday drinks containing alcohol, such as egg nog, can entice young children. Children may often imitate adults and drink from partially filled leftover glasses.
Therefore, families with young children must ensure that all alcoholic beverages are placed out of children’s reach.
A common presentation of alcohol poisoning is seen in the child who is brought to the ED by parents concerned because their child is acting strangely. On examination, the child may appear dazed and have tachycardia, tachypnea, and hypotension, depending on the amount of alcohol ingested. Hypoglycemia in an alcohol-intoxicated pediatric patient is a concern, but it appears the effects of alcohol on glucose regulation in infants is unpredictable.15
Intravenous access should be obtained in any patient presenting with altered mental status, and rapid blood glucose level determined. Blood samples should be sent to assess ethanol concentration. Other laboratory and imaging studies should be obtained as clinically indicated, including electrolytes, serum osmolality, acetaminophen level, urine drug screen, X-ray, and computed tomography scan of the head. Treatment of respiratory depression, hypoglycemia, hypovolemia, and hypothermia are the key interventions to ensure good outcomes.16 Supportive care is the mainstay of therapy for pediatric patients, who rarely require thiamine supplementation.16 Medical evaluation is recommended for all symptomatic children; hourly observation for 6 hours is recommended for asymptomatic children.17
Alcohol is also associated with cardiac arrhythmias. Alcohol-induced atrial arrhythmias, most commonly atrial fibrillation (AF), are referred to as “holiday heart syndrome.” This should be considered early in the differential diagnosis of new-onset AF in young adults. Consuming massive quantities of alcohol or binge drinking can also result in metabolic and electrolyte alterations. Treatment includes rehydration with IV fluids, electrolyte replacement, and IV diltiazem or cardioversion for AF with rapid ventricular response.18
Conclusion
During the holiday season, it is easy to overlook the fact that some of the most unsuspecting items in the home can pose real hazards (Table). In addition, many holiday plants are used as table decorations, which can confuse small children, who may assume the colorful berries must be edible if they are on the dining room table.
It is vital that patients, parents, and physicians know what to do when someone ingests a potential toxin. Parents often try to induce vomiting, but ipecac and other forms of gastric emptying are no longer recommended.6 Instead, the recommended action is to separate the patient from the plant, remove plant material that may cause a sensitivity reaction, and consult a poison control center, which can save unnecessary interventions—including an ED visit.6
Fortunately, most holiday toxicities are relatively nonthreatening. Holiday-related toxic ingestions primarily occur in children, and most are asymptomatic, innocuous, and treated with symptomatic care as necessary. The most poisonous holiday-related toxins are bittersweet and Jerusalem cherry. Work-ups for holiday-plant ingestions are usually limited to severe gastroenteritis, which may require IV fluids and evaluation of electrolytes.
Holiday decorations, such as artificial snow and angel hair, present hazards that should be treated on a case-by-case basis. Finally, alcohol intoxication should be considered in the differential diagnosis for pediatric patients presenting with altered mental status, or the otherwise healthy binge drinker who presents with palpitations and new-onset AF.
1. Krenzelok EP, Jacobsen TD, Aronis J. Those pesky berries...are they a source of concern? Vet Hum Toxicol. 1998;40(2):101-103.
2. Martínez Monseny A, Martínez Sánchez L, Margarit Soler A, Trenchs Sainz de la Maza V, Luaces Cubells C. [Poisonous plants: An ongoing problem]. An Pediatr (Barc). 2015;82(5):347-353. doi:10.1016/j.anpedi.2014.08.008.
3. Krenzelok EP, Mrvos R. Friends and foes in the plant world: a profile of plant ingestions and fatalities. Clin Toxicol (Phila). 2011;49(3):142-149. doi:10.3109/15563650.2011.568945.
4. Evens ZN, Stellpflug SJ. Holiday plants with toxic misconceptions. West J Emerg Med. 2012;13(6):538-542. doi:10.5811/westjem.2012.8.12572.
5. Krenzelok E, Jacobsen TD, Aronis JM. Poinsettia exposures have good outcomes…just as we thought. Am J Emerg Med. 1996;14(7):671-674. doi:10.1016/S0735-6757(96)90086-90088.
6. Courtemanche J, Peterson, RG. Beware the mistletoe. CMAJ. 2006;175(12):1523-1524. doi:10.1503/cmaj.061432.
7. Wax PM, Cobaugh DJ, Lawrence RA. Should home ipecac-induced emesis be routinely recommended in the management of toxic berry ingestions? Vet Hum Toxicol. 1999;41(6):394-397.
8. Palmer ME, Betz JM. Plants. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:1537-1560.
9. Bruneton J. Toxic plants dangerous to humans and animals. Paris, France: Lavoisier Publishing; 1999.10. Krenzelok EP, Jacobsen TD, Aronis J. American mistletoe exposures. Am J Emerg Med. 1997;15(5):516-520.
11. Spiller HA, Willias DB, Gorman SE, Sanftleban J. Retrospective study of mistletoe ingestion. J Toxicol Clin Toxicol. 1996;34(4):405-408.
12. Al-Amry MA, Al-Ghadeer HA. Corneal epithliopathy after trauma by fake snow powder in a 7-year-old child. Middle East Afr J Ophthalmol. 2016;23(3):274-276. doi:10.4103/0974-9233.186157.
13. California Poison Control Center. Winter holiday safety & poison prevention tips. http://www.calpoison.org/public/winter-holidays.html. Accessed October 16, 2016.
14. Romm C. Don’t lick the tinsel. The Atlantic. December 21, 2015. http://www.theatlantic.com/health/archive/2015/12/dont-lick-the-tinsel/421506/. Accessed October 16, 2016.
15. Minera G, Robinson E. Accidental acute alcohol intoxication in infants: review and case report. J Emerg Med. 2014;47(5):524-526.
16. Baum CR. Ethanol intoxication in children: clinical features, evaluation, and management. UpToDate. http://www.uptodate.com/contents/ethanol-intoxication-in-children-clinical-features-evaluation-and-management. Accessed October 16, 2016.
17. Vogel C, Caraccio T, Mofenson H, Hart S. Alcohol intoxication in young children. J Toxicol Clin Toxicol. 1995;33(1):25-33.
18. Carey MG, Al-Zaiti SS, Kozik TM, Pelter M. Holiday heart syndrome. Am J Crit Care. 2014;23(2):171-172.
The holiday season, a time of warmth, joy, and good cheer, is upon us. Yet with this most wonderful time of the year comes the possibility of poisoning and hazards in the home. As emergency physicians (EPs), we must ask ourselves: Which holiday items are potentially toxic to our patients? How do we evaluate and manage poisonings that result from exposure to these items? In this article, we review several plants and decorations that are unique to the holiday season. We discuss recommendations for evaluation and management of holiday poisonings that will avoid inappropriate work-ups and interventions while increasing recognition of truly dangerous ingestions, thus help keeping the season safe and merry.
Plants
Plant exposures are the fourth most common cause for calls to poison centers.1 In 2012, US Poison Control Centers reported more than 30,000 toxic plant exposures in children younger than age 5 years.2 Not surprisingly, toxic plant ingestions occur most commonly in early childhood. The highest rate of mortality, however, takes place during the teenaged years, when suicide attempts are common.2 The most common plant ingestions reported in the United States include peace lily, holly, philodendron, and poinsettia.3 These and the other frequently ingested potentially poisonous plants produce very little, if any, toxic effects. Approximately 95% of unintentional potentially toxic plant ingestions reported in the United States are managed safely at home.3
Poinsettia
The poinsettia is a large, prominent plant that was introduced to the United States in 1825 by Joel Poinsett, the US ambassador to Mexico. The poinsettia is one of the most commonly researched plants, and studies show the plant is not actually toxic.4,5 The myth of poinsettia toxicity is a widely held, yet false, belief. The legend involves a young child of an army officer stationed in Hawaii in 1919 who reportedly died after eating poinsettia leaves.4 However, the reality is that the poinsettia plant was not actually involved in the child’s death. In fact, the wild plant involved in this case probably had little resemblance to the popular plant cultivated domestically in North America today.6
A majority of poinsettia exposures will be asymptomatic, or involve simple nausea and vomiting. Krenzelok et al5 reviewed 22,793 cases of poinsettia exposures from 1985 to 1992. Almost all of these exposures (98.9%) were accidental poisonings.5 Not surprisingly, 93.3% of poinsettia exposures involved children; most importantly, 96.1% of these patients did not require treatment at a health care facility,and there were no fatalities.5 Another study could not identify an LD50 (lethal dose, 50%—ie, the amount of an ingested substance that kills 50% of the test sample) in rats.4
The majority of patients presenting to the ED with symptoms from poinsettia exposure will have gastrointestinal (GI) upset. Most patients require only symptomatic care. Those who do present to the ED do not require gastric emptying.
Interestingly, there is a crossreactivity of poinsettia sap in latex-allergic vulnerable patients.6 Poinsettia is part of the same plant family as natural rubber latex, and patients can present with symptoms of contact dermatitis, especially if they have a latex allergy.4 Washing the area thoroughly with soap and water and avoiding future contact is all that is required for most patients with contact dermatitis.
Holly
Holly exposure accounted for the third highest rate of genus-specific human plant exposure calls to poison centers in 2010.4 In the United States, there are two common forms of holly: English holly and American holly. The berries of both varieties contain saponin, a toxin that can cause erythrocyte hemolysis and changes in the permeability of small intestinal mucosal cells.4 Most holly berry ingestions cause minor or no symptoms. The prickly leaves of the holly plant are nontoxic but consumption may result in minor injury. When symptoms do occur, they can include nausea, vomiting, abdominal cramping, and possible dermatitis.5 Mydriasis, hyperthermia, and drowsiness are rare but possible symptoms.4
For symptoms to develop, children need to have eaten only five berries, while adults reportedly must consume at least 20 to 30 berries.4 A study by Wax et al7 done at the University of Rochester reviewed 103 cases of toxic berry ingestion in children aged 9 months to 5 years, with children who swallowed six or fewer berries of holly, yew, or nightshade. Investigators compared home observation alone versus ipecac administration with home observation. Every patient treated with ipecac had emesis at home with increased sedation and diarrhea, while there was no emesis in the group with home observation alone.7 These results suggest the symptoms were due to the ipecac rather than plant toxicity.7 Thus, ipecac is not recommended, and patients should be treated symptomatically.
Bittersweet and Jerusalem Cherry
Bittersweet, also known as the woody nightshade, and Jerusalem cherry, or Christmas orange, are the most dangerous of the holiday plants. While there is little evidence to support serious toxicity to adults, ingestion may be dangerous to children. Bittersweet has purple and yellow flowers, spreading petals, and red, ovoid berries.4 Both plants are part of the genus Solanum. In both plants, the immature fruit is more poisonous than ripened fruit due to the glycoalkaloid solanine via hypothetical alteration of mitochondrial potassium and calcium transport.4 Case reports document the rare anticholinergic effects of these plants, likely due to dulcamarine.4
The largest case series included 319 ingestions of bittersweet or Jerusalem cherry.4 Of these, 295 patients were under age 10 years, and only nine experienced solanine-related symptoms; none required hospitalization.4 The symptoms of ingestion were primarily nausea and vomiting and abdominal cramping, possibly due to anticholinergic effects. Symptoms typically occur several hours after ingestion and may last for days.
Historically, induced emesis was recommended for ingestion in children, but this is no longer recommended. Prolonged observation may be necessary for children in the setting of high likelihood of ingestion. Management includes rehydration with intravenous (IV) fluids, antiemetics, and physostigmine if clinically warranted.4
Mistletoe
Mistletoe, a perennial with white or translucent berries, has traditionally been associated with kissing, fertility, and vitality. The American mistletoe is known as Phoradendron serotinum and the European mistletoe as Viscum album. Both the American and European mistletoe contain the toxalbumins phoratoxin and viscotoxin, which are associated with inhibiting cellular synthesis, thereby affecting cells with rapid turnover, including the GI mucosa.4
After several hours, clinical effects are primarily GI upset with potential sloughing of portions of the intestinal tract.4 Bradycardia, delirium, and hepatic, central nervous system, kidney, and adrenal gland toxicities can also occur.8 The American species has a lower toxicity compared to the European species. Cases involving death likely related to P serotinum usually occur due to excessive, concentrated herbal use, such as brewing mistletoe in tea.9 Placing the plant in hot water may result in larger amounts of ingested toxin. The only two reported deaths from ingestion of mistletoe were in patients who consumed brewed teas.4
A case review of 14 patients with American mistletoe leaf or berry ingestions failed to find any toxic symptoms.4 Krenzelok et al10 compiled the largest case review of 1,754 exposures from 1985 to 1992. In this review, patient outcomes were good. There were no fatalities, and 99% of patients experienced no morbidity. Outcomes were not influenced by GI decontamination.10
Another study by Spiller et al11 described 92 American mistletoe exposures involving ingestions of up to 20 berries and five leaves. In cases where five or more berries were consumed, none of the patients had symptoms.11 Three of the 11 patients (27%) who swallowed one to five leaves developed GI upset. One child had a seizure, likely not related to the mistletoe. The study concluded that severe toxic symptoms are uncommon.11
Management in the ED should involve supportive care for dehydration and vomiting, typically IV rehydration with normal saline or Ringer’s lactate and IV antiemetics. According to multiple case reviews, GI decontamination is not believed to alter patient outcome and is not recommended.4 An observation period of 6 hours is reasonable.4,10,11
Christmas Cactus
Christmas cactus is an old-time favorite. It is made of arching, drooping branches and spineless joints. Christmas cactus is essentially nontoxic, and patients and family can be reassured of its safety.
Holiday Decorations
Artificial Snow
Fake snow sprays, powders, and granules are popular decorative additions used in holiday games and celebrations. The “snow” typically consists of a polymer of sodium polyacrylate, both of which can cause injury to the eyes. Repeatedly inhaling the aerosol spray can cause breathing problems, especially in patients who have asthma or other underlying bronchospastic disease.
Devastating outcomes may occur from ocular alkaline injury. When mixed with water, the fake snow absorbs the water and expands as a gel material that may stick to the ocular surface, resulting in a change in pH and osmolarity.12 A case report by Al-Amry and Al-Ghadeer12 recently described a 7-year-old boy with corneal epitheliopathy due to a chemical burn injury following ocular contact with fake snow.The case was later managed with multiple debridements over 3 days, topical antibiotics, and bandage contact lenses. The child had complete resolution at 1 week follow-up.12
Some fake snow-product sprays contain acetone or methylene chloride, which is harmful when inhaled and can cause nausea, lightheadedness, and headache.13 Methylene chloride can be metabolized to carbon monoxide, but the quantity required for such an exposure is unknown and has not been reported in this context. Emergency physicians should consider ordering carboxyhemoglobin levels in symptomatic patients.
Tinsel
Tinsel, which gets its name from the Old French word “estincele,” translated as sparkle, used to be made of actual silver, and was affordable only for wealthy individuals. However, in the early 1900s, manufacturers began to make tinsel from metals such as aluminum and copper. These materials did not tarnish and could be reused annually. However, during World War I, copper became difficult to buy, while aluminum proved to be flammable and dangerous. Thus, manufacturers began to produce tinsel from lead. Tinsel was made with lead until the 1970s, when the US Food and Drug Administration realized the toxic risks of lead exposure, especially in young children. Today, tinsel is made of plastic; though a poor imitation of the previous tinsel, it is relatively harmless.14
Angel Hair
Angel hair is finely spun glass that can be irritating to the skin, eyes, and throat, especially if swallowed.13 The greatest danger is airway obstruction if a patient attempts to eat the angel hair and it becomes lodged in the oropharynx. For contact irritation, thoroughly washing and irrigating affected areas are recommended.
Snow Globes
Snow globes are popular holiday decorations that are available in a range of sizes. While the majority of globes made in the United States are filled with water, those manufactured overseas many contain a small amount of ethylene glycol (EG) (ie, antifreeze) to prevent freezing and breakage during shipping. Fortunately, the amount of EG is not usually sufficient to cause symptoms if ingested. For globes made in the United States, the water can be contaminated with bacteria, and drinking it can cause GI upset. The snow in these globes is typically made of inert material and does not cause toxicity. If a child does exhibit symptoms after ingesting any portion of a snow globe, parents are advised to call their local poison center.
Ethanol
While alcohol is not unique to the holiday season, its availability and use are more pronounced during this time of year, and the incidence of alcohol poisoning increases during the holiday season. Some traditional holiday drinks containing alcohol, such as egg nog, can entice young children. Children may often imitate adults and drink from partially filled leftover glasses.
Therefore, families with young children must ensure that all alcoholic beverages are placed out of children’s reach.
A common presentation of alcohol poisoning is seen in the child who is brought to the ED by parents concerned because their child is acting strangely. On examination, the child may appear dazed and have tachycardia, tachypnea, and hypotension, depending on the amount of alcohol ingested. Hypoglycemia in an alcohol-intoxicated pediatric patient is a concern, but it appears the effects of alcohol on glucose regulation in infants is unpredictable.15
Intravenous access should be obtained in any patient presenting with altered mental status, and rapid blood glucose level determined. Blood samples should be sent to assess ethanol concentration. Other laboratory and imaging studies should be obtained as clinically indicated, including electrolytes, serum osmolality, acetaminophen level, urine drug screen, X-ray, and computed tomography scan of the head. Treatment of respiratory depression, hypoglycemia, hypovolemia, and hypothermia are the key interventions to ensure good outcomes.16 Supportive care is the mainstay of therapy for pediatric patients, who rarely require thiamine supplementation.16 Medical evaluation is recommended for all symptomatic children; hourly observation for 6 hours is recommended for asymptomatic children.17
Alcohol is also associated with cardiac arrhythmias. Alcohol-induced atrial arrhythmias, most commonly atrial fibrillation (AF), are referred to as “holiday heart syndrome.” This should be considered early in the differential diagnosis of new-onset AF in young adults. Consuming massive quantities of alcohol or binge drinking can also result in metabolic and electrolyte alterations. Treatment includes rehydration with IV fluids, electrolyte replacement, and IV diltiazem or cardioversion for AF with rapid ventricular response.18
Conclusion
During the holiday season, it is easy to overlook the fact that some of the most unsuspecting items in the home can pose real hazards (Table). In addition, many holiday plants are used as table decorations, which can confuse small children, who may assume the colorful berries must be edible if they are on the dining room table.
It is vital that patients, parents, and physicians know what to do when someone ingests a potential toxin. Parents often try to induce vomiting, but ipecac and other forms of gastric emptying are no longer recommended.6 Instead, the recommended action is to separate the patient from the plant, remove plant material that may cause a sensitivity reaction, and consult a poison control center, which can save unnecessary interventions—including an ED visit.6
Fortunately, most holiday toxicities are relatively nonthreatening. Holiday-related toxic ingestions primarily occur in children, and most are asymptomatic, innocuous, and treated with symptomatic care as necessary. The most poisonous holiday-related toxins are bittersweet and Jerusalem cherry. Work-ups for holiday-plant ingestions are usually limited to severe gastroenteritis, which may require IV fluids and evaluation of electrolytes.
Holiday decorations, such as artificial snow and angel hair, present hazards that should be treated on a case-by-case basis. Finally, alcohol intoxication should be considered in the differential diagnosis for pediatric patients presenting with altered mental status, or the otherwise healthy binge drinker who presents with palpitations and new-onset AF.
The holiday season, a time of warmth, joy, and good cheer, is upon us. Yet with this most wonderful time of the year comes the possibility of poisoning and hazards in the home. As emergency physicians (EPs), we must ask ourselves: Which holiday items are potentially toxic to our patients? How do we evaluate and manage poisonings that result from exposure to these items? In this article, we review several plants and decorations that are unique to the holiday season. We discuss recommendations for evaluation and management of holiday poisonings that will avoid inappropriate work-ups and interventions while increasing recognition of truly dangerous ingestions, thus help keeping the season safe and merry.
Plants
Plant exposures are the fourth most common cause for calls to poison centers.1 In 2012, US Poison Control Centers reported more than 30,000 toxic plant exposures in children younger than age 5 years.2 Not surprisingly, toxic plant ingestions occur most commonly in early childhood. The highest rate of mortality, however, takes place during the teenaged years, when suicide attempts are common.2 The most common plant ingestions reported in the United States include peace lily, holly, philodendron, and poinsettia.3 These and the other frequently ingested potentially poisonous plants produce very little, if any, toxic effects. Approximately 95% of unintentional potentially toxic plant ingestions reported in the United States are managed safely at home.3
Poinsettia
The poinsettia is a large, prominent plant that was introduced to the United States in 1825 by Joel Poinsett, the US ambassador to Mexico. The poinsettia is one of the most commonly researched plants, and studies show the plant is not actually toxic.4,5 The myth of poinsettia toxicity is a widely held, yet false, belief. The legend involves a young child of an army officer stationed in Hawaii in 1919 who reportedly died after eating poinsettia leaves.4 However, the reality is that the poinsettia plant was not actually involved in the child’s death. In fact, the wild plant involved in this case probably had little resemblance to the popular plant cultivated domestically in North America today.6
A majority of poinsettia exposures will be asymptomatic, or involve simple nausea and vomiting. Krenzelok et al5 reviewed 22,793 cases of poinsettia exposures from 1985 to 1992. Almost all of these exposures (98.9%) were accidental poisonings.5 Not surprisingly, 93.3% of poinsettia exposures involved children; most importantly, 96.1% of these patients did not require treatment at a health care facility,and there were no fatalities.5 Another study could not identify an LD50 (lethal dose, 50%—ie, the amount of an ingested substance that kills 50% of the test sample) in rats.4
The majority of patients presenting to the ED with symptoms from poinsettia exposure will have gastrointestinal (GI) upset. Most patients require only symptomatic care. Those who do present to the ED do not require gastric emptying.
Interestingly, there is a crossreactivity of poinsettia sap in latex-allergic vulnerable patients.6 Poinsettia is part of the same plant family as natural rubber latex, and patients can present with symptoms of contact dermatitis, especially if they have a latex allergy.4 Washing the area thoroughly with soap and water and avoiding future contact is all that is required for most patients with contact dermatitis.
Holly
Holly exposure accounted for the third highest rate of genus-specific human plant exposure calls to poison centers in 2010.4 In the United States, there are two common forms of holly: English holly and American holly. The berries of both varieties contain saponin, a toxin that can cause erythrocyte hemolysis and changes in the permeability of small intestinal mucosal cells.4 Most holly berry ingestions cause minor or no symptoms. The prickly leaves of the holly plant are nontoxic but consumption may result in minor injury. When symptoms do occur, they can include nausea, vomiting, abdominal cramping, and possible dermatitis.5 Mydriasis, hyperthermia, and drowsiness are rare but possible symptoms.4
For symptoms to develop, children need to have eaten only five berries, while adults reportedly must consume at least 20 to 30 berries.4 A study by Wax et al7 done at the University of Rochester reviewed 103 cases of toxic berry ingestion in children aged 9 months to 5 years, with children who swallowed six or fewer berries of holly, yew, or nightshade. Investigators compared home observation alone versus ipecac administration with home observation. Every patient treated with ipecac had emesis at home with increased sedation and diarrhea, while there was no emesis in the group with home observation alone.7 These results suggest the symptoms were due to the ipecac rather than plant toxicity.7 Thus, ipecac is not recommended, and patients should be treated symptomatically.
Bittersweet and Jerusalem Cherry
Bittersweet, also known as the woody nightshade, and Jerusalem cherry, or Christmas orange, are the most dangerous of the holiday plants. While there is little evidence to support serious toxicity to adults, ingestion may be dangerous to children. Bittersweet has purple and yellow flowers, spreading petals, and red, ovoid berries.4 Both plants are part of the genus Solanum. In both plants, the immature fruit is more poisonous than ripened fruit due to the glycoalkaloid solanine via hypothetical alteration of mitochondrial potassium and calcium transport.4 Case reports document the rare anticholinergic effects of these plants, likely due to dulcamarine.4
The largest case series included 319 ingestions of bittersweet or Jerusalem cherry.4 Of these, 295 patients were under age 10 years, and only nine experienced solanine-related symptoms; none required hospitalization.4 The symptoms of ingestion were primarily nausea and vomiting and abdominal cramping, possibly due to anticholinergic effects. Symptoms typically occur several hours after ingestion and may last for days.
Historically, induced emesis was recommended for ingestion in children, but this is no longer recommended. Prolonged observation may be necessary for children in the setting of high likelihood of ingestion. Management includes rehydration with intravenous (IV) fluids, antiemetics, and physostigmine if clinically warranted.4
Mistletoe
Mistletoe, a perennial with white or translucent berries, has traditionally been associated with kissing, fertility, and vitality. The American mistletoe is known as Phoradendron serotinum and the European mistletoe as Viscum album. Both the American and European mistletoe contain the toxalbumins phoratoxin and viscotoxin, which are associated with inhibiting cellular synthesis, thereby affecting cells with rapid turnover, including the GI mucosa.4
After several hours, clinical effects are primarily GI upset with potential sloughing of portions of the intestinal tract.4 Bradycardia, delirium, and hepatic, central nervous system, kidney, and adrenal gland toxicities can also occur.8 The American species has a lower toxicity compared to the European species. Cases involving death likely related to P serotinum usually occur due to excessive, concentrated herbal use, such as brewing mistletoe in tea.9 Placing the plant in hot water may result in larger amounts of ingested toxin. The only two reported deaths from ingestion of mistletoe were in patients who consumed brewed teas.4
A case review of 14 patients with American mistletoe leaf or berry ingestions failed to find any toxic symptoms.4 Krenzelok et al10 compiled the largest case review of 1,754 exposures from 1985 to 1992. In this review, patient outcomes were good. There were no fatalities, and 99% of patients experienced no morbidity. Outcomes were not influenced by GI decontamination.10
Another study by Spiller et al11 described 92 American mistletoe exposures involving ingestions of up to 20 berries and five leaves. In cases where five or more berries were consumed, none of the patients had symptoms.11 Three of the 11 patients (27%) who swallowed one to five leaves developed GI upset. One child had a seizure, likely not related to the mistletoe. The study concluded that severe toxic symptoms are uncommon.11
Management in the ED should involve supportive care for dehydration and vomiting, typically IV rehydration with normal saline or Ringer’s lactate and IV antiemetics. According to multiple case reviews, GI decontamination is not believed to alter patient outcome and is not recommended.4 An observation period of 6 hours is reasonable.4,10,11
Christmas Cactus
Christmas cactus is an old-time favorite. It is made of arching, drooping branches and spineless joints. Christmas cactus is essentially nontoxic, and patients and family can be reassured of its safety.
Holiday Decorations
Artificial Snow
Fake snow sprays, powders, and granules are popular decorative additions used in holiday games and celebrations. The “snow” typically consists of a polymer of sodium polyacrylate, both of which can cause injury to the eyes. Repeatedly inhaling the aerosol spray can cause breathing problems, especially in patients who have asthma or other underlying bronchospastic disease.
Devastating outcomes may occur from ocular alkaline injury. When mixed with water, the fake snow absorbs the water and expands as a gel material that may stick to the ocular surface, resulting in a change in pH and osmolarity.12 A case report by Al-Amry and Al-Ghadeer12 recently described a 7-year-old boy with corneal epitheliopathy due to a chemical burn injury following ocular contact with fake snow.The case was later managed with multiple debridements over 3 days, topical antibiotics, and bandage contact lenses. The child had complete resolution at 1 week follow-up.12
Some fake snow-product sprays contain acetone or methylene chloride, which is harmful when inhaled and can cause nausea, lightheadedness, and headache.13 Methylene chloride can be metabolized to carbon monoxide, but the quantity required for such an exposure is unknown and has not been reported in this context. Emergency physicians should consider ordering carboxyhemoglobin levels in symptomatic patients.
Tinsel
Tinsel, which gets its name from the Old French word “estincele,” translated as sparkle, used to be made of actual silver, and was affordable only for wealthy individuals. However, in the early 1900s, manufacturers began to make tinsel from metals such as aluminum and copper. These materials did not tarnish and could be reused annually. However, during World War I, copper became difficult to buy, while aluminum proved to be flammable and dangerous. Thus, manufacturers began to produce tinsel from lead. Tinsel was made with lead until the 1970s, when the US Food and Drug Administration realized the toxic risks of lead exposure, especially in young children. Today, tinsel is made of plastic; though a poor imitation of the previous tinsel, it is relatively harmless.14
Angel Hair
Angel hair is finely spun glass that can be irritating to the skin, eyes, and throat, especially if swallowed.13 The greatest danger is airway obstruction if a patient attempts to eat the angel hair and it becomes lodged in the oropharynx. For contact irritation, thoroughly washing and irrigating affected areas are recommended.
Snow Globes
Snow globes are popular holiday decorations that are available in a range of sizes. While the majority of globes made in the United States are filled with water, those manufactured overseas many contain a small amount of ethylene glycol (EG) (ie, antifreeze) to prevent freezing and breakage during shipping. Fortunately, the amount of EG is not usually sufficient to cause symptoms if ingested. For globes made in the United States, the water can be contaminated with bacteria, and drinking it can cause GI upset. The snow in these globes is typically made of inert material and does not cause toxicity. If a child does exhibit symptoms after ingesting any portion of a snow globe, parents are advised to call their local poison center.
Ethanol
While alcohol is not unique to the holiday season, its availability and use are more pronounced during this time of year, and the incidence of alcohol poisoning increases during the holiday season. Some traditional holiday drinks containing alcohol, such as egg nog, can entice young children. Children may often imitate adults and drink from partially filled leftover glasses.
Therefore, families with young children must ensure that all alcoholic beverages are placed out of children’s reach.
A common presentation of alcohol poisoning is seen in the child who is brought to the ED by parents concerned because their child is acting strangely. On examination, the child may appear dazed and have tachycardia, tachypnea, and hypotension, depending on the amount of alcohol ingested. Hypoglycemia in an alcohol-intoxicated pediatric patient is a concern, but it appears the effects of alcohol on glucose regulation in infants is unpredictable.15
Intravenous access should be obtained in any patient presenting with altered mental status, and rapid blood glucose level determined. Blood samples should be sent to assess ethanol concentration. Other laboratory and imaging studies should be obtained as clinically indicated, including electrolytes, serum osmolality, acetaminophen level, urine drug screen, X-ray, and computed tomography scan of the head. Treatment of respiratory depression, hypoglycemia, hypovolemia, and hypothermia are the key interventions to ensure good outcomes.16 Supportive care is the mainstay of therapy for pediatric patients, who rarely require thiamine supplementation.16 Medical evaluation is recommended for all symptomatic children; hourly observation for 6 hours is recommended for asymptomatic children.17
Alcohol is also associated with cardiac arrhythmias. Alcohol-induced atrial arrhythmias, most commonly atrial fibrillation (AF), are referred to as “holiday heart syndrome.” This should be considered early in the differential diagnosis of new-onset AF in young adults. Consuming massive quantities of alcohol or binge drinking can also result in metabolic and electrolyte alterations. Treatment includes rehydration with IV fluids, electrolyte replacement, and IV diltiazem or cardioversion for AF with rapid ventricular response.18
Conclusion
During the holiday season, it is easy to overlook the fact that some of the most unsuspecting items in the home can pose real hazards (Table). In addition, many holiday plants are used as table decorations, which can confuse small children, who may assume the colorful berries must be edible if they are on the dining room table.
It is vital that patients, parents, and physicians know what to do when someone ingests a potential toxin. Parents often try to induce vomiting, but ipecac and other forms of gastric emptying are no longer recommended.6 Instead, the recommended action is to separate the patient from the plant, remove plant material that may cause a sensitivity reaction, and consult a poison control center, which can save unnecessary interventions—including an ED visit.6
Fortunately, most holiday toxicities are relatively nonthreatening. Holiday-related toxic ingestions primarily occur in children, and most are asymptomatic, innocuous, and treated with symptomatic care as necessary. The most poisonous holiday-related toxins are bittersweet and Jerusalem cherry. Work-ups for holiday-plant ingestions are usually limited to severe gastroenteritis, which may require IV fluids and evaluation of electrolytes.
Holiday decorations, such as artificial snow and angel hair, present hazards that should be treated on a case-by-case basis. Finally, alcohol intoxication should be considered in the differential diagnosis for pediatric patients presenting with altered mental status, or the otherwise healthy binge drinker who presents with palpitations and new-onset AF.
1. Krenzelok EP, Jacobsen TD, Aronis J. Those pesky berries...are they a source of concern? Vet Hum Toxicol. 1998;40(2):101-103.
2. Martínez Monseny A, Martínez Sánchez L, Margarit Soler A, Trenchs Sainz de la Maza V, Luaces Cubells C. [Poisonous plants: An ongoing problem]. An Pediatr (Barc). 2015;82(5):347-353. doi:10.1016/j.anpedi.2014.08.008.
3. Krenzelok EP, Mrvos R. Friends and foes in the plant world: a profile of plant ingestions and fatalities. Clin Toxicol (Phila). 2011;49(3):142-149. doi:10.3109/15563650.2011.568945.
4. Evens ZN, Stellpflug SJ. Holiday plants with toxic misconceptions. West J Emerg Med. 2012;13(6):538-542. doi:10.5811/westjem.2012.8.12572.
5. Krenzelok E, Jacobsen TD, Aronis JM. Poinsettia exposures have good outcomes…just as we thought. Am J Emerg Med. 1996;14(7):671-674. doi:10.1016/S0735-6757(96)90086-90088.
6. Courtemanche J, Peterson, RG. Beware the mistletoe. CMAJ. 2006;175(12):1523-1524. doi:10.1503/cmaj.061432.
7. Wax PM, Cobaugh DJ, Lawrence RA. Should home ipecac-induced emesis be routinely recommended in the management of toxic berry ingestions? Vet Hum Toxicol. 1999;41(6):394-397.
8. Palmer ME, Betz JM. Plants. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:1537-1560.
9. Bruneton J. Toxic plants dangerous to humans and animals. Paris, France: Lavoisier Publishing; 1999.10. Krenzelok EP, Jacobsen TD, Aronis J. American mistletoe exposures. Am J Emerg Med. 1997;15(5):516-520.
11. Spiller HA, Willias DB, Gorman SE, Sanftleban J. Retrospective study of mistletoe ingestion. J Toxicol Clin Toxicol. 1996;34(4):405-408.
12. Al-Amry MA, Al-Ghadeer HA. Corneal epithliopathy after trauma by fake snow powder in a 7-year-old child. Middle East Afr J Ophthalmol. 2016;23(3):274-276. doi:10.4103/0974-9233.186157.
13. California Poison Control Center. Winter holiday safety & poison prevention tips. http://www.calpoison.org/public/winter-holidays.html. Accessed October 16, 2016.
14. Romm C. Don’t lick the tinsel. The Atlantic. December 21, 2015. http://www.theatlantic.com/health/archive/2015/12/dont-lick-the-tinsel/421506/. Accessed October 16, 2016.
15. Minera G, Robinson E. Accidental acute alcohol intoxication in infants: review and case report. J Emerg Med. 2014;47(5):524-526.
16. Baum CR. Ethanol intoxication in children: clinical features, evaluation, and management. UpToDate. http://www.uptodate.com/contents/ethanol-intoxication-in-children-clinical-features-evaluation-and-management. Accessed October 16, 2016.
17. Vogel C, Caraccio T, Mofenson H, Hart S. Alcohol intoxication in young children. J Toxicol Clin Toxicol. 1995;33(1):25-33.
18. Carey MG, Al-Zaiti SS, Kozik TM, Pelter M. Holiday heart syndrome. Am J Crit Care. 2014;23(2):171-172.
1. Krenzelok EP, Jacobsen TD, Aronis J. Those pesky berries...are they a source of concern? Vet Hum Toxicol. 1998;40(2):101-103.
2. Martínez Monseny A, Martínez Sánchez L, Margarit Soler A, Trenchs Sainz de la Maza V, Luaces Cubells C. [Poisonous plants: An ongoing problem]. An Pediatr (Barc). 2015;82(5):347-353. doi:10.1016/j.anpedi.2014.08.008.
3. Krenzelok EP, Mrvos R. Friends and foes in the plant world: a profile of plant ingestions and fatalities. Clin Toxicol (Phila). 2011;49(3):142-149. doi:10.3109/15563650.2011.568945.
4. Evens ZN, Stellpflug SJ. Holiday plants with toxic misconceptions. West J Emerg Med. 2012;13(6):538-542. doi:10.5811/westjem.2012.8.12572.
5. Krenzelok E, Jacobsen TD, Aronis JM. Poinsettia exposures have good outcomes…just as we thought. Am J Emerg Med. 1996;14(7):671-674. doi:10.1016/S0735-6757(96)90086-90088.
6. Courtemanche J, Peterson, RG. Beware the mistletoe. CMAJ. 2006;175(12):1523-1524. doi:10.1503/cmaj.061432.
7. Wax PM, Cobaugh DJ, Lawrence RA. Should home ipecac-induced emesis be routinely recommended in the management of toxic berry ingestions? Vet Hum Toxicol. 1999;41(6):394-397.
8. Palmer ME, Betz JM. Plants. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:1537-1560.
9. Bruneton J. Toxic plants dangerous to humans and animals. Paris, France: Lavoisier Publishing; 1999.10. Krenzelok EP, Jacobsen TD, Aronis J. American mistletoe exposures. Am J Emerg Med. 1997;15(5):516-520.
11. Spiller HA, Willias DB, Gorman SE, Sanftleban J. Retrospective study of mistletoe ingestion. J Toxicol Clin Toxicol. 1996;34(4):405-408.
12. Al-Amry MA, Al-Ghadeer HA. Corneal epithliopathy after trauma by fake snow powder in a 7-year-old child. Middle East Afr J Ophthalmol. 2016;23(3):274-276. doi:10.4103/0974-9233.186157.
13. California Poison Control Center. Winter holiday safety & poison prevention tips. http://www.calpoison.org/public/winter-holidays.html. Accessed October 16, 2016.
14. Romm C. Don’t lick the tinsel. The Atlantic. December 21, 2015. http://www.theatlantic.com/health/archive/2015/12/dont-lick-the-tinsel/421506/. Accessed October 16, 2016.
15. Minera G, Robinson E. Accidental acute alcohol intoxication in infants: review and case report. J Emerg Med. 2014;47(5):524-526.
16. Baum CR. Ethanol intoxication in children: clinical features, evaluation, and management. UpToDate. http://www.uptodate.com/contents/ethanol-intoxication-in-children-clinical-features-evaluation-and-management. Accessed October 16, 2016.
17. Vogel C, Caraccio T, Mofenson H, Hart S. Alcohol intoxication in young children. J Toxicol Clin Toxicol. 1995;33(1):25-33.
18. Carey MG, Al-Zaiti SS, Kozik TM, Pelter M. Holiday heart syndrome. Am J Crit Care. 2014;23(2):171-172.
Over-the-counter and Natural Remedies for Onychomycosis: Do They Really Work?
Onychomycosis is a fungal infection of the nail unit by dermatophytes, yeasts, and nondermatophyte molds. It is characterized by a white or yellow discoloration of the nail plate; hyperkeratosis of the nail bed; distal detachment of the nail plate from its bed (onycholysis); and nail plate dystrophy, including thickening, crumbling, and ridging. Onychomycosis is an important problem, representing 30% of all superficial fungal infections and an estimated 50% of all nail diseases.1 Reported prevalence rates of onychomycosis in the United States and worldwide are varied, but the mean prevalence based on population-based studies in Europe and North America is estimated to be 4.3%.2 It is more common in older individuals, with an incidence rate of 20% in those older than 60 years and 50% in those older than 70 years.3 Onychomycosis is more common in patients with diabetes and 1.9 to 2.8 times higher than the general population.4 Dermatophytes are responsible for the majority of cases of onychomycosis, particularly Trichophyton rubrum and Trichophyton mentagrophytes.5
Onychomycosis is divided into different subtypes based on clinical presentation, which in turn are characterized by varying infecting organisms and prognoses. The subtypes of onychomycosis are distal and lateral subungual (DLSO), proximal subungual, superficial, endonyx, mixed pattern, total dystrophic, and secondary. Distal and lateral subungual onychomycosis are by far the most common presentation and begins when the infecting organism invades the hyponychium and distal or lateral nail bed. Trichophyton rubrum is the most common organism and T mentagrophytes is second, but Candida parapsilosis and Candida albicans also are possibilities. Proximal subungual onychomycosis is far less frequent than DLSO and is usually caused by T rubrum. The fungus invades the proximal nail folds and penetrates the newly growing nail plate.6 This pattern is more common in immunosuppressed patients and should prompt testing for human immunodeficiency virus.7 Total dystrophic onychomycosis is the end stage of fungal nail plate invasion, may follow DLSO or proximal subungual onychomycosis, and is difficult to treat.6
Onychomycosis causes pain, paresthesia, and difficulty with ambulation.8 In patients with peripheral neuropathy and vascular problems, including diabetes, onychomycosis can increase the risk for foot ulcers, with amputation in severe cases.9 Patients also may present with aesthetic concerns that may impact their quality of life.10
Given the effect on quality of life along with medical risks associated with onychomycosis, a safe and successful treatment modality with a low risk of recurrence is desirable. Unfortunately, treatment of nail fungus is quite challenging for a number of reasons. First, the thickness of the nail and/or the fungal mass may be a barrier to the delivery of topical and systemic drugs at the source of the infection. In addition, the nail plate does not have intrinsic immunity. Also, recurrence after treatment is common due to residual hyphae or spores that were not previously eliminated.11 Finally, many topical medications require long treatment courses, which may limit patient compliance, especially in patients who want to use nail polish for cosmesis or camouflage.
Currently Approved Therapies for Onychomycosis
Several definitions are needed to better interpret the results of onychomycosis clinical trials. Complete cure is defined as a negative potassium hydroxide preparation and negative fungal culture with a completely normal appearance of the nail. Mycological cure is defined as potassium hydroxide microscopy and fungal culture negative. Clinical cure is stated as 0% nail plate involvement but at times is reported as less than 5% and less than 10% involvement.
Terbinafine and itraconazole are the only US Food and Drug Administration (FDA)–approved systemic therapies, and ciclopirox, efinaconazole, and tavaborole are the only FDA-approved topicals. Advantages of systemic agents generally are higher cure rates and shorter treatment courses, thus better compliance. Disadvantages include greater incidence of systemic side effects and drug-drug interactions as well as the need for laboratory monitoring. Pros of topical therapies are low potential for adverse effects, no drug-drug interactions, and no monitoring of blood work. Cons include lower efficacy, long treatment courses, and poor patient compliance.
Terbinafine, an allylamine, taken orally once daily (250 mg) for 12 weeks for toenails and 6 weeks for fingernails currently is the preferred systemic treatment of onychomycosis, with complete cure rates of 38% and 59% and mycological cure rates of 70% and 79% for toenails and fingernails, respectively.12 Itraconazole, an azole, is dosed orally at 200 mg daily for 3 months for toenails, with a complete cure rate of 14% and mycological cure rate of 54%.13 For fingernail onychomycosis only, itraconazole is dosed at 200 mg twice daily for 1 week, followed by a treatment-free period of 3 weeks, and then another 1-week course at thesame dose. The complete cure rate is 47% and the mycological cure is 61% for this pulse regimen.13
Ciclopirox is a hydroxypyridone and the 8% nail lacquer formulation was approved in 1999, making it the first topical medication to gain FDA approval for the treatment of toenail onychomycosis. Based on 2 clinical trials, complete cure rates for toenails are 5.5% and 8.5% and mycological cure rates are 29% and 36% at 48 weeks with removal of residual lacquer and debridement.14Efinaconazole is an azole and the 10% solution was FDA approved for the treatment of toenail onychomycosis in 2014.15 In 2 clinical trials, complete cure rates were 17.8% and 15.2% and mycological cure rates were 55.2% and 53.4% with once daily toenail application for 48 weeks.16 Tavaborole is a benzoxaborole and the 5% solution also was approved for the treatment of toenail onychomycosis in 2014.17 Two clinical trials reported complete cure rates of 6.5% and 9.1% and mycological cure rates of 31.1% and 35.9% with once daily toenail application for 48 weeks.18
Given the poor efficacy, systemic side effects, potential for drug-drug interactions, long-term treatment courses, and cost associated with current systemic and/or topical treatments, there has been a renewed interest in natural remedies and over-the-counter (OTC) therapies for onychomycosis. This review summarizes the in vitro and in vivo data, mechanisms of action, and clinical efficacy of various natural and OTC agents for the treatment of onychomycosis. Specifically, we summarize the data on tea tree oil (TTO), a popular topical cough suppressant (TCS), natural coniferous resin (NCR) lacquer, Ageratina pichinchensis (AP) extract, and ozonized sunflower oil.
Tea Tree Oil
Background
Tea tree oil is a volatile oil whose medicinal use dates back to the early 20th century when the Bundjabung aborigines of North and New South Wales extracted TTO from the dried leaves of the Melaleuca alternifolia plant and used it to treat superficial wounds.19 Tea tree oil has been shown to be an effective treatment of tinea pedis,20 and it is widely used in Australia as well as in Europe and North America.21 Tea tree oil also has been investigated as an antifungal agent for the treatment of onychomycosis, both in vitro22-28 and in clinical trials.29,30
In Vitro Data
Because TTO is composed of more than 100 active components,23 the antifungal activity of these individual components was investigated against 14 fungal isolates, including C albicans, T mentagrophytes, and Aspergillus species. The minimum inhibitory concentration (MIC) for α-pinene was less than 0.004% for T mentagrophytes and the components with the greatest MIC and minimum fungicidal concentration for the fungi tested were terpinen-4-ol and α-terpineol, respectively.22 The antifungal activity of TTO also was tested using disk diffusion assay experiments with 58 clinical isolates of fungi including C albicans, T rubrum, T mentagrophytes, and Aspergillus niger.24 Tea tree oil was most effective at inhibiting T rubrum followed by T mentagrophytes,24 which are the 2 most common etiologies of onychomycosis.5 In another report, the authors determined the MIC of TTO utilizing 4 different experiments with T rubrum as the infecting organism. Because TTO inhibited the growth of T rubrum at all concentrations greater than 0.1%, they found that the MIC was 0.1%.25 Given the lack of adequate nail penetration of most topical therapies, TTO in nanocapsules (TTO-NC), TTO nanoemulsions, and normal emulsions were tested in vitro for their ability to inhibit the growth of T rubrum inoculated into nail shavings. Colony growth decreased significantly within the first week of treatment, with TTO-NC showing maximum efficacy (P<.001). This study showed that TTO, particularly TTO-NC, was effective in inhibiting the growth of T rubrum in vitro and that using nanocapsule technology may increase nail penetration and bioavailability.31
Much of what we know about TTO’s antifungal mechanism of action comes from experiments involving C albicans. To date, it has not been studied in T rubrum or T mentagrophytes, the 2 most common etiologies of onychomycosis.5 In C albicans, TTO causes altered permeability of plasma membranes,32 dose-dependent alteration of respiration,33 decreased glucose-induced acidification of media surrounding fungi,32 and reversible inhibition of germ tube formation.19,34
Clinical Trials
A randomized, double-blind, multicenter trial was performed on 117 patients with culture-proven DLSO who were randomized to receive TTO 100% or clotrimazole solution 1% applied twice daily to affected toenails for 6 months.29 Primary outcome measures were mycologic cure, clinical assessment, and patient subjective assessment (Table 1). There were no statistical differences between the 2 treatment groups. Erythema and irritation were the most common adverse reactions occurring in 7.8% (5/64) of the TTO group.29
Another study was a double-blind, placebo-controlled trial involving 60 patients with clinical and mycologic evidence of DLSO who were randomized to treatment with a cream containing butenafine hydrochloride 2% and TTO 5% (n=40) or a control cream containing only TTO (n=20), with active treatment for 8 weeks and final follow-up at 36 weeks.30 Patients were instructed to apply the cream 3 times daily under occlusion for 8 weeks and the nail was debrided between weeks 4 and 6 if feasible. If the nail could not be debrided after 8 weeks, it was considered resistant to treatment. At the end of the study, the complete cure rate was 80% in the active group compared to 0% in the placebo group (P<.0001), and the mean time to complete healing with progressive nail growth was 29 weeks. There were no adverse effects in the placebo group, but 4 patients in the active group had mild skin inflammation.30
Topical Cough Suppressant
Background
Topical cough suppressants, which are made up of several natural ingredients, are OTC ointments for adults and children 2 years and older that are indicated as cough suppressants when applied to the chest and throat and as relief of mild muscle and joint pains.35 The active ingredients are camphor 4.8%, eucalyptus oil 1.2%, and menthol 2.6%, while the inactive ingredients are cedarleaf oil, nutmeg oil, petrolatum, thymol, and turpentine oil.35 Some of the active and inactive ingredients in TCSs have shown efficacy against dermatophytes in vitro,36-38 and although they are not specifically indicated for onychomycosis, they have been popularized as home remedies for fungal nail infections.36,39 A TCS has been evaluated for its efficacy for the treatment of onychomycosis in one clinical trial.40
In Vitro Data
An in vitro study was performed to evaluate the antifungal activity of the individual and combined components of TCS on 16 different dermatophytes, nondermatophytes, and molds. The zones of inhibition against these organisms were greatest for camphor, menthol, thymol, and eucalyptus oil. Interestingly, there were large zones of inhibition and a synergistic effect when a mixture of components was used against T rubrum and T mentagrophytes.36 The in vitro activity of thymol, a component of TCS, was tested against Candida species.37 The essential oil subtypes Thymus vulgaris and Thymus zygis (subspecies zygis) showed similar antifungal activity, which was superior to Thymus mastichina, and all 3 compounds had similar MIC and minimal lethal concentration values. The authors showed that the antifungal mechanism was due to cell membrane damage and inhibition of germ tube formation.37 It should be noted that Candida species are less common causes of onychomycosis, and it is not known whether this data is applicable to T rubrum. In another study, the authors investigated the antifungal activity of Thymus pulegioides and found that MIC ranged from 0.16 to 0.32 μL/mL for dermatophytes and Aspergillus strains and 0.32 to 0.64 μL/mL for Candida species. When an essential oil concentration of 0.08 μL/mL was used against T rubrum, ergosterol content decreased by 70 %, indicating that T pulegioides inhibits ergosterol biosynthesis in T rubrum.38
Clinical Observations and Clinical Trial
There is one report documenting the clinical observations on a group of patients with a clinical diagnosis of onychomycosis who were instructed to apply TCS to affected nail(s) once daily.36 Eighty-five charts were reviewed (mean age, 77 years), and although follow-up was not complete or standardized, the following data were reported: 32 (38%) cleared their fungal infection, 21 (25%) had no record of change but also no record of compliance, 19 (22%) had only 1 documented follow-up visit, 9 (11%) reported they did not use the treatment, and 4 (5%) did not return for a follow-up visit. Of the 32 patients whose nails were cured, 3 (9%) had clearance within 5 months, 8 (25%) within 7 months, 11 (34%) within 9 months, 4 (13%) within 11 months, and 6 (19%) within 16 months.36
A small pilot study was performed to evaluate the efficacy of daily application of TCS in the treatment of onychomycosis in patients 18 years and older with at least 1 great toenail affected.40 The primary end points were mycologic cure at 48 weeks and clinical cure at the end of the study graded as complete, partial, or no change. The secondary end point was patient satisfaction with the appearance of the affected nail at 48 weeks. Eighteen participants completed the study; 55% (10/18) were male, with an average age of 51 years (age range, 30–85 years). The mean initial amount of affected nail was 62% (range, 16%–100%), and cultures included dermatophytes, nondermatophytes, and molds. With TCS treatment, 27.8% (5/18) showed mycologic cure of which 4 (22.2%) had a complete clinical cure. Ten participants (55.6%) had partial clinical cure and 3 (16.7%) had no clinical improvement. Interestingly, the 4 participants who had complete clinical cure had baseline cultures positive for either T mentagrophytes or C parapsilosis. Most patients were content with the treatment, as 9 participants stated that they were very satisfied and 9 stated that they were satisfied. The average ratio of affected to total nail area declined from 63% at screening to 41% at the end of the study (P<.001). No adverse effects were reported with study drug.40
NCR Lacquer
Background
Resins are natural products derived from coniferous trees and are believed to protect trees against insects and microbial pathogens.41 Natural coniferous resin derived from the Norway spruce tree (Picea abies) mixed with boiled animal fat or butter has been used topically for centuries in Finland and Sweden to treat infections and wounds.42-44 The activity of NCR has been studied against a wide range of microbes, demonstrating broad-spectrum antimicrobial activity against both gram-positive bacteria and fungi.45-48 There are 2 published clinical trials evaluating NCR in the treatment of onychomycosis.49,50
In Vitro Data
Natural coniferous resin has shown antifungal activity against T mentagrophytes, Trichophyton tonsurans, and T rubrum in vitro, which was demonstrated using medicated disks of resin on petri dishes inoculated with these organisms.46 In another study, the authors evaluated the antifungal activity of NCR against human pathogenic fungi and yeasts using agar plate diffusion tests and showed that the resin had antifungal activity against Trichophyton species but not against Fusarium and most Candida species. Electron microscopy of T mentagrophytes exposed to NCR showed that all cells were dead inside the inhibition zone, with striking changes seen in the hyphal cell walls, while fungal cells outside the inhibition zone were morphologically normal.47 In another report, utilizing the European Pharmacopoeia challenge test, NCR was highly effective against gram-positive and gram-negative bacteria as well as C albicans.42
Clinical Trials
In one preliminary observational and prospective clinical trial, 15 participants with clinical and mycologic evidence of onychomycosis were instructed to apply NCR lacquer once daily for 9 months with a 4-week washout period, with the primary outcome measures being clinical and mycologic cure.49 Thirteen (87%) enrolled participants were male and the average age was 65 years (age range, 37–80 years). The DLSO subtype was present in 9 (60%) participants. The mycologic cure rate at the end of the study was 65% (95% CI, 42%-87%), and none achieved clinical cure, but 6 participants showed some improvement in the appearance of the nail.49
The second trial was a prospective, controlled, investigator-blinded study of 73 patients with clinical and mycologic evidence of toenail onychomycosis who were randomized to receive NCR 30%, amorolfine lacquer 5%, or 250 mg oral terbinafine.50 The primary end point was mycologic cure at 10 months, and secondary end points were clinical efficacy, cost-effectiveness, and patient compliance. Clinical efficacy was based on the proximal linear growth of healthy nail and was classified as unchanged, partial, or complete. Partial responses were described as substantial decreases in onycholysis, subungual hyperkeratosis, and streaks. A complete response was defined as a fully normal appearance of the toenail. Most patients were male in the NCR (91% [21/23]), amorolfine (80% [20/25]), and terbinafine (68% [17/25]) groups; the average ages were 64, 63, and 64 years, respectively. Trichophyton rubrum was cultured most often in all 3 groups: NCR, 87% (20/23); amorolfine, 96% (24/25); and terbinafine, 84% (21/25). The remaining cases were from T mentagrophytes. A summary of the results is shown in Table 2. Patient compliance was 100% in all except 1 patient in the amorolfine treatment group with moderate compliance. There were no adverse events, except for 2 in the terbinafine group: diarrhea and rash.50
AP Extract
Background
Ageratina pichinchensis, a member of the Asteraceae family, has been used historically in Mexico for fungal infections of the skin.51,52 Fresh or dried leaves were extracted with alcohol and the product was administered topically onto damaged skin without considerable skin irritation.53 Multiple studies have demonstrated that AP extract has in vitro antifungal activity along with other members of the Asteraceae family.54-56 There also is evidence from clinical trials that AP extract is effective against superficial dermatophyte infections such as tinea pedis.57 Given the positive antifungal in vitro data, the potential use of this agent was investigated for onychomycosis treatment.53,58
In Vitro Data
The antifungal properties of the Asteraceae family have been tested in several in vitro experiments. Eupatorium aschenbornianum, described as synonymous with A pichinchensis,59 was found to be most active against the dermatophytes T rubrum and T mentagrophytes with MICs of 0.3 and 0.03 mg/mL, respectively.54 It is thought that the primary antimycotic activity is due to encecalin, an acetylchromene compound that was identified in other plants from the Asteraceae family and has activity against dermatophytes.55 In another study, Ageratum houstanianum Mill, a comparable member of the Asteraceae family, had fungitoxic activity against T rubrum and C albicans isolated from nail infections.56
Clinical Trials
A double-blind controlled trial was performed on 110 patients with clinical and mycologic evidence of mild to moderate toenail onychomycosis randomized to treatment with AP lacquer or ciclopirox lacquer 8% (control).58 Primary end points were clinical effectiveness (completely normal nails) and mycologic cure. Patients were instructed to apply the lacquer once every third day during the first month, twice a week for the second month, and once a week for 16 weeks, with removal of the lacquer weekly. Demographics were similar between the AP lacquer and control groups, with mean ages of 44.6 and 46.5 years, respectively; women made up 74.5% and 67.2%, respectively, of each treatment group, with most patients having a 2- to 5-year history of disease (41.8% and 40.1%, respectively).58 A summary of the data is shown in Table 3. No severe side effects were documented, but minimal nail fold skin pain was reported in 3 patients in the control group in the first week, resolving later in the trial.58
A follow-up study was performed to determine the optimal concentration of AP lacquer for the treatment of onychomycosis.53 One hundred twenty-two patients aged 19 to 65 years with clinical and mycologic evidence of mild to moderate DLSO were randomized to receive 12.6% or 16.8% AP lacquer applied once daily to the affected nails for 6 months. The nails were graded as healthy, mild, or moderately affected before and after treatment. There were no significant differences in demographics between the 2 treatment groups, and 77% of patients were women with a median age of 47 years. There were no significant side effects from either concentration of AP lacquer.53
Ozonized Sunflower Oil
Background
Ozonized sunflower oil is derived by reacting ozone (O3) with sunflower plant (Helianthus annuus) oil to form a petroleum jelly–like material.60 It was originally shown to have antibacterial properties in vitro,61 and further studies have confirmed these findings and demonstrated anti-inflammatory, wound healing, and antifungal properties.62-64 A formulation of ozonized sunflower oil used in Cuba is clinically indicated for the treatment of tinea pedis and impetigo.65 The clinical efficacy of this product has been evaluated in a clinical trial for the treatment of onychomycosis.65
In Vitro Data
A compound made up of 30% ozonized sunflower oil with 0.5% of α-lipoic acid was found to have antifungal activity against C albicans using the disk diffusion method, in addition to other bacterial organisms. The MIC values ranged from 2.0 to 3.5 mg/mL.62 Another study was designed to evaluate the in vitro antifungal activity of this formulation on samples cultured from patients with onychomycosis using the disk diffusion method. They found inhibition of growth of C albicans, C parapsilosis, and Candida tropicalis, which was inferior to amphotericin B, ketoconazole, fluconazole, and itraconazole.64
Clinical Trial
A single-blind, controlled, phase 3 study was performed on 400 patients with clinical and mycologic evidence of onychomycosis. Patients were randomized to treatment with an ozonized sunflower oil solution or ketoconazole cream 2% applied to affected nails twice daily for 3 months, with filing and massage of the affected nails upon application of treatment.65 Cured was defined as mycologic cure in addition to a healthy appearing nail, improved as an increase in healthy appearing nail in addition to a decrease in symptoms (ie, paresthesia, pain, itching) but positive mycological testing, same as no clinical change in appearance with positive mycological findings, and worse as increasing diseased nail involvement in the presence of positive mycological findings. Demographics were similar between groups with a mean age of 35 years. Men accounted for 80% of the study population, and 65% of the study population was white. The mean duration of disease was 30 months. They also reported on a 1-year follow-up, with 2.8% of patients in the ozonized sunflower oil solution group and 37.0% of patients in the ketoconazole group describing relapses. Trichophyton rubrum and C albicans were cultured from these patients.65
Comment
Due to the poor efficacy, long-term treatment courses, inability to use nail polish, and high cost associated with many FDA-approved topical treatments, along with the systemic side effects, potential for drug-drug interactions, and cost associated with many oral therapies approved for onychomycosis, there has been a renewed interest in natural remedies and OTC treatments. Overall, TTO, TCS, NCR, AP extract, and ozonized sunflower oil have shown efficacy in vitro against some dermatophytes, nondermatophytes, and molds responsible for onychomycosis. One or more clinical trials were performed with each of these agents for the treatment of onychomycosis. They were mostly small pilot studies, and due to differences in trial design, the results cannot be compared with each other or with currently FDA-approved treatments. We can conclude that because adverse events were rare with all of these therapies—most commonly skin irritation or mild skin pain—they exhibit good safety.
For TTO, there was no statistical difference between the clotrimazole and TTO treatment groups in mycologic cure, clinical assessment, or patient subjective assessment of the nails.29 Although there was an 80% complete cure in the butenafine and TTO group, it was 0% in the TTO group at week 36.30 Trial design, longer treatment periods, incorporation into nanocapsules, or combination treatment with other antifungal agents may influence our future use of TTO for onychomycosis, but based on the present data we cannot recommend this treatment in clinical practice.
With TCS, 27.8% of participants had a mycologic cure and 22.2% had complete clinical cure.40 Although it is difficult to draw firm conclusions from this small pilot study, there may be some benefit to treating toenail onychomycosis due to T mentagrophytes or C parapsilosis with TCS but no benefit in treating onychomycosis due to T rubrum, the more common cause of onychomycosis. Limitations of this study were lack of a placebo group, small sample size, wide variety of represented pathogens that may not be representative of the true population, and lack of stratification by baseline severity or involvement of nail. A larger randomized controlled clinical trial would be necessary to confirm the results of this small study and make formal recommendations.
In one clinical trial with NCR, mycologic cure was 65% at the end of the study.49 No participants achieved clinical cure, but 6 participants showed some improvement in the appearance of the nail. Because this study was small (N=15), it is difficult to draw firm conclusions.49 In another study with NCR, mycologic cure rates with NCR, amorolfine, and terbinafine were 13%, 8%, and 56%, respectively. Based on these results, NCR has similar antifungal efficacy to amorolfine but was inferior to oral terbinafine.50 A larger randomized controlled clinical trial with more homogenous and less severely affected patients and longer treatment periods would be necessary to confirm the results of these small studies and make formal recommendations.
Because there were no significant differences in clinical effectiveness of mycologic cure rates between AP lacquer 10% and ciclopirox lacquer 8% in one clinical trial,58 AP does not seem to be more effective than at least one of the current FDA-approved topical treatments; however, because AP lacquer 16.8% was shown to be more effective than AP lacquer 12.6% in one onychomycosis clinical trial, using higher concentrations of AP may yield better results in future trials.53
One trial comparing ozonized sunflower oil to ketoconazole cream 2% showed 90.5% and 13.5% cure rates, respectively.65 Although there is good in vitro antifungal activity and a clinical trial showing efficacy using ozonized sunflower oil for the treatment of onychomycosis, confirmatory studies are necessary before we can recommend this OTC treatment to our patients. Specifically, we will get the most data from large randomized controlled trials with strict inclusion/exclusion and efficacy criteria.
Conclusion
Over-the-counter and natural remedies may be an emerging area of research in the treatment of onychomycosis. This review summarizes the laboratory data and clinical trials on several of these agents and, when available, compares their clinical and mycologic efficacy with FDA-approved therapies. Shortcomings of some of these studies include a small study population, lack of adequate controls, nonstandardized mycologic testing, and abbreviated posttreatment evaluation times. It may be concluded that these products have varying degrees of efficacy and appear to be safe in the studies cited; however, at present, we cannot recommend any of them to our patients until there are larger randomized clinical trials with appropriate controls demonstrating their efficacy.
- Scher RK, Daniel CR. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005.
- Sigurgeirsson B, Baran R. The prevalence of onychomycosis in the global population: a literature study. J Eur Acad Dermatol Venereol. 2014;28:1480-1491.
- Thomas J, Jacobson GA, Narkowicz CK, et al. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther. 2010;35:497-519.
- Mayser P, Freund V, Budihardja D. Toenail onychomycosis in diabetic patients: issues and management. Am J Clin Dermatol. 2009;10:211-220.
- Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641-648.
- Hay RJ, Baran R. Onychomycosis: a proposed revision of the clinical classification J Am Acad Dermatol. 2011;65:1219-1227.
- Elewski B. Clinical pearl: proximal white subungual onychomycosis in AIDS. J Am Acad Dermatol. 1993;29:631-632.
- Scher RK. Onychomycosis is more than a cosmetic problem. Br J Dermatol. 1994;130(suppl 43):15.
- Boyko EJ, Ahroni JH, Cohen V, et al. Prediction of diabetic foot ulcer occurrence using commonly available clinical information: the Seattle Diabetic Foot Study. Diabetes Care. 2006;29:1202-1207.
- Szepietowski JC, Reich A, Pacan P, et al. Evaluation of quality of life in patients with toenail onychomycosis by Polish version of an international onychomycosis-specific questionnaire. J Eur Acad Dermatol Venereol. 2007;21:491-496.
- Scher RK, Baron R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(suppl 65):5-9.
- Lamisil [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2011.
- Sporanox [package insert]. Raritan, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2001
- Penlac [package insert]. Bridgewater, NJ: Dermik Laboratories; 2006.
- Jublia [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC; 2014.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Kerydin [package insert]. Palo Alto, CA: Anacor Pharmaceuticals, Inc; 2014
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies [published online May 5, 2015]. J Am Acad Dermatol. 2015;73:62-69.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Satchell AC, Saurajen A, Bell C, et al. Treatment of interdigital tinea pedis with 25% and 50% tea tree oil solution: a randomized, placebo-controlled, blinded study. Australas J Dermatol. 2002;43:175-178.
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50-62.
- Hammer KA, Carson CF, Riley TV. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J Appl Microbiol. 2003;95:853-860.
- Brophy JJ, Davies NW, Southwell IA, et al. Gas chromatographic quality control for oil of Melaleuca terpinen-4-ol type (Australian tea tree). J Agric Food Chem. 1989;37:1330-1335.
- Concha JM, Moore LS, Holloway WJ. 1998 William J. Stickel Bronze Award. Antifungal activity of Melaleuca alternifolia (tea-tree) oil against various pathogenic organisms. J Am Podiatr Med Assoc. 1998;88:489-492.
- Benger S, Townsend P, Ashford RL, et al. An in vitro study to determine the minimum inhibitory concentration of Melaleuca alternifolia against the dermatophyte Trichophyton rubrum. Foot. 2004;14:86-91.
- Hammer KA, Carson CF, Riley TV. In-vitro activity of essential oils, in particular Melaleuca alternifolia (tea tree) oil and tea tree oil products, against Candida spp. J Antimicrob Chemother. 1998;42:591-595.
- Altman P. Australian tea tree oil. Aust J Pharm. 1998;69:276-278.
- Guterres SS, Alves MP, Pohlmann AR. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights. 2007;2:147-157.
- Buck DS, Nidorf DM, Addino JG. Comparison of two topical preparations for the treatment of onychomycosis: Melaleuca alternifolia (tea tree) oil and clotrimazole. J Fam Pract. 1994;38:601-605.
- Syed TA, Qureshi ZA, Ali SM, et al. Treatment of toenail onychomycosis with 2% butenafine and 5% Melaleuca alternifolia (tea tree) oil in cream. Tropical Med Int Health. 1999;4:284-287.
- Flores FC, de Lima JA, Ribeiro RF, et al. Antifungal activity of nanocapsule suspensions containing tea tree oil on the growth of Trichophyton rubrum. Mycopathologia. 2013;175:281-286.
- Hammer KA, Carson CF, Riley TV. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J Antimicrob Chemother. 2004;53:1081-1085.
- Cox SD, Mann CM, Markham JL, et al. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J Appl Microbiol. 2000;88:170-175.
- Hammer KA, Carson CF, Riley TV. Melaleuca alternifolia (tea tree) oil inhibits germ tube formation by Candida albicans. Med Mycol. 2000;38:355-362.
- Vicks VapoRub [package insert]. Gross-Gerau, Germany: Proctor & Gamble; 2010.
- Ramsewak RS, Nair MG, Stommel M, et al. In vitro antagonistic activity of monoterpenes and their mixtures against ‘toe nail fungus’ pathogens. Phytother Res. 2003;17:376-379.
- Pina-Vaz C, Gonçalves Rodrigues A, Pinto E, et al. Antifungal activity of Thymus oils and their major compounds. J Eur Acad Dermatol Venereol. 2004;18:73-78.
- Pinto E, Pina-Vaz C, Salgueiro L, et al. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J Med Microbiol. 2006;55:1367-1373.
- Vicks VapoRub might help fight toenail fungus. Consumer Reports. 2006;71:49.
- Derby R, Rohal P, Jackson C, et al. Novel treatment of onychomycosis using over-the-counter mentholated ointment: a clinical case series. J Am Board Fam Med. 2011;24:69-74.
- Trapp S, Croteau R. Defensive resin biosynthesis in conifers. Ann Rev Plant Physiol Plant Mol Biol. 2001;52:689-724.
- Sipponen A, Laitinen K. Antimicrobial properties of natural coniferous rosin in the European Pharmacopoeia challenge test. APMIS. 2011;119:720-724.
- Sipponen A, Lohi J. Lappish gum care “new” treatment of pressure ulcers? People’s improvement at it’s best. Eng Med J. 2003;58:2775-2776.
- Benedictus O. Een Nyttigh Läkare. Malmö: Kroon; 1938.
- Rautio M, Sipponen A, Peltola R, et al. Antibacterial effects of home-made resin salve from Norway spruce (Picea abies). APMIS. 2007;115:335-340.
- Laitinen K, Sipponen A, Jokinen JJ, et al. Resin salve from Norway spruce is antifungal against dermatophytes causing nail infections. EWMA. 2009;56:289-296.
- Rautio M, Sipponen A, Lohi J, et al. In vitro fungistatic effects of natural coniferous resin from Norway spruce (Picea abies). Eur J Clin Microbiol Infect Dis. 2012;31:1783-1789.
- Sipponen A, Peltola R, Jokinen JJ, et al. Effects of Norway spruce (Picea abies) resin on cell wall and cell membrane of Staphylococcus aureus. Ultrastruct Pathol. 2009;33:128-135.
- Sipponen P, Sipponen A, Lohi J, et al. Natural coniferous resin lacquer in treatment of toenail onychomycosis: an observational study. Mycoses. 2013;56:289-296.
- Auvinen T, Tiihonen R, Soini M, et al. Efficacy of topical resin lacquer, amorolfine, and oral terbinafine for treating toenail onychomycosis: a prospective, randomized, controlled, investigator-blinded, parallel-group clinical trial. Br J Dermatol. 2015;173:940-948.
- Argueta A, Cano L, Rodarte M. Atlas de las Plantas de la Medicina Tradicional Mexicana. Vol 3. Mexico City, Mexico: Instituto Nacional Indigenista; 1994:72-680.
- Avilés M, Suárez G. Catálogo de Plantas Medicinales del Jardín Etnobotánico. Peru: Instituto Nacional de Antropología e Historia; 1994.
- Romero-Cerecero O, Roman-Ramos R, Zamilpa A, et al. Clinical trial to compare the effectiveness of two concentrations of the Ageratina pichinchensis extract in the topical treatment of onychomycosis. J Ethnopharmacol. 2009;126:74-78.
- Navarro Garcia VM, Gonzalez A, Fuentes M, et al. Antifungal activities of nine traditional Mexican medicinal plants. J Ethnopharmacol. 2003;87:85-88.
- Castañeda P, Gómez L, Mata R, et al. Phytogrowth-inhibitory and antifungal constituents of Helianthella quinquenervis. J Nat Prod. 1996;59:323-326.
- Kumar N. Inhibition of nail infecting fungi of peoples of North Eastern UP causing Tinea unguium through leaf essential oil of Ageratum houstonianum Mill. IOSR J Pharm. June 2014;4:36-42.
- Romero-Cerecero O, Rojas G, Navarro V, et al. Effectiveness and tolerability of a standardized extract from Ageratina pichinchensis on patients with tinea pedis: an explorative pilot study controlled with ketoconazole. Planta Med. 2006;72:1257-1261.
- Romero-Cerecero O, Zamilpa A, Jimenez-Ferrer JE, et al. Double-blind clinical trial for evaluating the effectiveness and tolerability of Ageratina pichinchensis extract on patients with mild to moderate onychomycosis. a comparative study with ciclopirox. Planta Med. 2008;74:1430-1435.
- Rzedowski J, De Rzedowski GC. Flora Fanerogámica del Valle de México. Mexico City, Mexico: Instituto de Ecología Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional; 1985.
- Bocci V. Biological and clinical effects of ozone. has ozone therapy a future in medicine? Br J Biomed Sci. 1999;56:270-279.
- Sechi LA, Lezcano I, Nunez N, et al. Antibacterial activity of ozonized sunflower oil (Oleozon). J Appl Microbiol. 2001;90:279-284.
- Rodrigues KL, Cardoso CC, Caputo LR, et al. Cicatrizing and antimicrobial properties of an ozonised oil from sunflower seeds. Inflammopharmacology. 2004;12:261-270.
- Daud FV, Ueda SMY, Navarini A, et al. The use of ozonized oil in the treatment of dermatophitosis caused by Microsporum canis in rabbits. Braz J Microbiol. 2011;42:274-281.
- Guerrer LV, Cunha KC, Nogueira MC, et al. “In vitro” antifungal activity of ozonized sunflower oil on yeasts from onychomycosis. Braz J Microbiol. 2012;43:1315-1318.
- Menéndez S, Falcón L, Maqueira Y. Therapeutic efficacy of topical OLEOZON in patients suffering from onychomycosis. Mycoses. 2011;54:E272-E277.
Onychomycosis is a fungal infection of the nail unit by dermatophytes, yeasts, and nondermatophyte molds. It is characterized by a white or yellow discoloration of the nail plate; hyperkeratosis of the nail bed; distal detachment of the nail plate from its bed (onycholysis); and nail plate dystrophy, including thickening, crumbling, and ridging. Onychomycosis is an important problem, representing 30% of all superficial fungal infections and an estimated 50% of all nail diseases.1 Reported prevalence rates of onychomycosis in the United States and worldwide are varied, but the mean prevalence based on population-based studies in Europe and North America is estimated to be 4.3%.2 It is more common in older individuals, with an incidence rate of 20% in those older than 60 years and 50% in those older than 70 years.3 Onychomycosis is more common in patients with diabetes and 1.9 to 2.8 times higher than the general population.4 Dermatophytes are responsible for the majority of cases of onychomycosis, particularly Trichophyton rubrum and Trichophyton mentagrophytes.5
Onychomycosis is divided into different subtypes based on clinical presentation, which in turn are characterized by varying infecting organisms and prognoses. The subtypes of onychomycosis are distal and lateral subungual (DLSO), proximal subungual, superficial, endonyx, mixed pattern, total dystrophic, and secondary. Distal and lateral subungual onychomycosis are by far the most common presentation and begins when the infecting organism invades the hyponychium and distal or lateral nail bed. Trichophyton rubrum is the most common organism and T mentagrophytes is second, but Candida parapsilosis and Candida albicans also are possibilities. Proximal subungual onychomycosis is far less frequent than DLSO and is usually caused by T rubrum. The fungus invades the proximal nail folds and penetrates the newly growing nail plate.6 This pattern is more common in immunosuppressed patients and should prompt testing for human immunodeficiency virus.7 Total dystrophic onychomycosis is the end stage of fungal nail plate invasion, may follow DLSO or proximal subungual onychomycosis, and is difficult to treat.6
Onychomycosis causes pain, paresthesia, and difficulty with ambulation.8 In patients with peripheral neuropathy and vascular problems, including diabetes, onychomycosis can increase the risk for foot ulcers, with amputation in severe cases.9 Patients also may present with aesthetic concerns that may impact their quality of life.10
Given the effect on quality of life along with medical risks associated with onychomycosis, a safe and successful treatment modality with a low risk of recurrence is desirable. Unfortunately, treatment of nail fungus is quite challenging for a number of reasons. First, the thickness of the nail and/or the fungal mass may be a barrier to the delivery of topical and systemic drugs at the source of the infection. In addition, the nail plate does not have intrinsic immunity. Also, recurrence after treatment is common due to residual hyphae or spores that were not previously eliminated.11 Finally, many topical medications require long treatment courses, which may limit patient compliance, especially in patients who want to use nail polish for cosmesis or camouflage.
Currently Approved Therapies for Onychomycosis
Several definitions are needed to better interpret the results of onychomycosis clinical trials. Complete cure is defined as a negative potassium hydroxide preparation and negative fungal culture with a completely normal appearance of the nail. Mycological cure is defined as potassium hydroxide microscopy and fungal culture negative. Clinical cure is stated as 0% nail plate involvement but at times is reported as less than 5% and less than 10% involvement.
Terbinafine and itraconazole are the only US Food and Drug Administration (FDA)–approved systemic therapies, and ciclopirox, efinaconazole, and tavaborole are the only FDA-approved topicals. Advantages of systemic agents generally are higher cure rates and shorter treatment courses, thus better compliance. Disadvantages include greater incidence of systemic side effects and drug-drug interactions as well as the need for laboratory monitoring. Pros of topical therapies are low potential for adverse effects, no drug-drug interactions, and no monitoring of blood work. Cons include lower efficacy, long treatment courses, and poor patient compliance.
Terbinafine, an allylamine, taken orally once daily (250 mg) for 12 weeks for toenails and 6 weeks for fingernails currently is the preferred systemic treatment of onychomycosis, with complete cure rates of 38% and 59% and mycological cure rates of 70% and 79% for toenails and fingernails, respectively.12 Itraconazole, an azole, is dosed orally at 200 mg daily for 3 months for toenails, with a complete cure rate of 14% and mycological cure rate of 54%.13 For fingernail onychomycosis only, itraconazole is dosed at 200 mg twice daily for 1 week, followed by a treatment-free period of 3 weeks, and then another 1-week course at thesame dose. The complete cure rate is 47% and the mycological cure is 61% for this pulse regimen.13
Ciclopirox is a hydroxypyridone and the 8% nail lacquer formulation was approved in 1999, making it the first topical medication to gain FDA approval for the treatment of toenail onychomycosis. Based on 2 clinical trials, complete cure rates for toenails are 5.5% and 8.5% and mycological cure rates are 29% and 36% at 48 weeks with removal of residual lacquer and debridement.14Efinaconazole is an azole and the 10% solution was FDA approved for the treatment of toenail onychomycosis in 2014.15 In 2 clinical trials, complete cure rates were 17.8% and 15.2% and mycological cure rates were 55.2% and 53.4% with once daily toenail application for 48 weeks.16 Tavaborole is a benzoxaborole and the 5% solution also was approved for the treatment of toenail onychomycosis in 2014.17 Two clinical trials reported complete cure rates of 6.5% and 9.1% and mycological cure rates of 31.1% and 35.9% with once daily toenail application for 48 weeks.18
Given the poor efficacy, systemic side effects, potential for drug-drug interactions, long-term treatment courses, and cost associated with current systemic and/or topical treatments, there has been a renewed interest in natural remedies and over-the-counter (OTC) therapies for onychomycosis. This review summarizes the in vitro and in vivo data, mechanisms of action, and clinical efficacy of various natural and OTC agents for the treatment of onychomycosis. Specifically, we summarize the data on tea tree oil (TTO), a popular topical cough suppressant (TCS), natural coniferous resin (NCR) lacquer, Ageratina pichinchensis (AP) extract, and ozonized sunflower oil.
Tea Tree Oil
Background
Tea tree oil is a volatile oil whose medicinal use dates back to the early 20th century when the Bundjabung aborigines of North and New South Wales extracted TTO from the dried leaves of the Melaleuca alternifolia plant and used it to treat superficial wounds.19 Tea tree oil has been shown to be an effective treatment of tinea pedis,20 and it is widely used in Australia as well as in Europe and North America.21 Tea tree oil also has been investigated as an antifungal agent for the treatment of onychomycosis, both in vitro22-28 and in clinical trials.29,30
In Vitro Data
Because TTO is composed of more than 100 active components,23 the antifungal activity of these individual components was investigated against 14 fungal isolates, including C albicans, T mentagrophytes, and Aspergillus species. The minimum inhibitory concentration (MIC) for α-pinene was less than 0.004% for T mentagrophytes and the components with the greatest MIC and minimum fungicidal concentration for the fungi tested were terpinen-4-ol and α-terpineol, respectively.22 The antifungal activity of TTO also was tested using disk diffusion assay experiments with 58 clinical isolates of fungi including C albicans, T rubrum, T mentagrophytes, and Aspergillus niger.24 Tea tree oil was most effective at inhibiting T rubrum followed by T mentagrophytes,24 which are the 2 most common etiologies of onychomycosis.5 In another report, the authors determined the MIC of TTO utilizing 4 different experiments with T rubrum as the infecting organism. Because TTO inhibited the growth of T rubrum at all concentrations greater than 0.1%, they found that the MIC was 0.1%.25 Given the lack of adequate nail penetration of most topical therapies, TTO in nanocapsules (TTO-NC), TTO nanoemulsions, and normal emulsions were tested in vitro for their ability to inhibit the growth of T rubrum inoculated into nail shavings. Colony growth decreased significantly within the first week of treatment, with TTO-NC showing maximum efficacy (P<.001). This study showed that TTO, particularly TTO-NC, was effective in inhibiting the growth of T rubrum in vitro and that using nanocapsule technology may increase nail penetration and bioavailability.31
Much of what we know about TTO’s antifungal mechanism of action comes from experiments involving C albicans. To date, it has not been studied in T rubrum or T mentagrophytes, the 2 most common etiologies of onychomycosis.5 In C albicans, TTO causes altered permeability of plasma membranes,32 dose-dependent alteration of respiration,33 decreased glucose-induced acidification of media surrounding fungi,32 and reversible inhibition of germ tube formation.19,34
Clinical Trials
A randomized, double-blind, multicenter trial was performed on 117 patients with culture-proven DLSO who were randomized to receive TTO 100% or clotrimazole solution 1% applied twice daily to affected toenails for 6 months.29 Primary outcome measures were mycologic cure, clinical assessment, and patient subjective assessment (Table 1). There were no statistical differences between the 2 treatment groups. Erythema and irritation were the most common adverse reactions occurring in 7.8% (5/64) of the TTO group.29
Another study was a double-blind, placebo-controlled trial involving 60 patients with clinical and mycologic evidence of DLSO who were randomized to treatment with a cream containing butenafine hydrochloride 2% and TTO 5% (n=40) or a control cream containing only TTO (n=20), with active treatment for 8 weeks and final follow-up at 36 weeks.30 Patients were instructed to apply the cream 3 times daily under occlusion for 8 weeks and the nail was debrided between weeks 4 and 6 if feasible. If the nail could not be debrided after 8 weeks, it was considered resistant to treatment. At the end of the study, the complete cure rate was 80% in the active group compared to 0% in the placebo group (P<.0001), and the mean time to complete healing with progressive nail growth was 29 weeks. There were no adverse effects in the placebo group, but 4 patients in the active group had mild skin inflammation.30
Topical Cough Suppressant
Background
Topical cough suppressants, which are made up of several natural ingredients, are OTC ointments for adults and children 2 years and older that are indicated as cough suppressants when applied to the chest and throat and as relief of mild muscle and joint pains.35 The active ingredients are camphor 4.8%, eucalyptus oil 1.2%, and menthol 2.6%, while the inactive ingredients are cedarleaf oil, nutmeg oil, petrolatum, thymol, and turpentine oil.35 Some of the active and inactive ingredients in TCSs have shown efficacy against dermatophytes in vitro,36-38 and although they are not specifically indicated for onychomycosis, they have been popularized as home remedies for fungal nail infections.36,39 A TCS has been evaluated for its efficacy for the treatment of onychomycosis in one clinical trial.40
In Vitro Data
An in vitro study was performed to evaluate the antifungal activity of the individual and combined components of TCS on 16 different dermatophytes, nondermatophytes, and molds. The zones of inhibition against these organisms were greatest for camphor, menthol, thymol, and eucalyptus oil. Interestingly, there were large zones of inhibition and a synergistic effect when a mixture of components was used against T rubrum and T mentagrophytes.36 The in vitro activity of thymol, a component of TCS, was tested against Candida species.37 The essential oil subtypes Thymus vulgaris and Thymus zygis (subspecies zygis) showed similar antifungal activity, which was superior to Thymus mastichina, and all 3 compounds had similar MIC and minimal lethal concentration values. The authors showed that the antifungal mechanism was due to cell membrane damage and inhibition of germ tube formation.37 It should be noted that Candida species are less common causes of onychomycosis, and it is not known whether this data is applicable to T rubrum. In another study, the authors investigated the antifungal activity of Thymus pulegioides and found that MIC ranged from 0.16 to 0.32 μL/mL for dermatophytes and Aspergillus strains and 0.32 to 0.64 μL/mL for Candida species. When an essential oil concentration of 0.08 μL/mL was used against T rubrum, ergosterol content decreased by 70 %, indicating that T pulegioides inhibits ergosterol biosynthesis in T rubrum.38
Clinical Observations and Clinical Trial
There is one report documenting the clinical observations on a group of patients with a clinical diagnosis of onychomycosis who were instructed to apply TCS to affected nail(s) once daily.36 Eighty-five charts were reviewed (mean age, 77 years), and although follow-up was not complete or standardized, the following data were reported: 32 (38%) cleared their fungal infection, 21 (25%) had no record of change but also no record of compliance, 19 (22%) had only 1 documented follow-up visit, 9 (11%) reported they did not use the treatment, and 4 (5%) did not return for a follow-up visit. Of the 32 patients whose nails were cured, 3 (9%) had clearance within 5 months, 8 (25%) within 7 months, 11 (34%) within 9 months, 4 (13%) within 11 months, and 6 (19%) within 16 months.36
A small pilot study was performed to evaluate the efficacy of daily application of TCS in the treatment of onychomycosis in patients 18 years and older with at least 1 great toenail affected.40 The primary end points were mycologic cure at 48 weeks and clinical cure at the end of the study graded as complete, partial, or no change. The secondary end point was patient satisfaction with the appearance of the affected nail at 48 weeks. Eighteen participants completed the study; 55% (10/18) were male, with an average age of 51 years (age range, 30–85 years). The mean initial amount of affected nail was 62% (range, 16%–100%), and cultures included dermatophytes, nondermatophytes, and molds. With TCS treatment, 27.8% (5/18) showed mycologic cure of which 4 (22.2%) had a complete clinical cure. Ten participants (55.6%) had partial clinical cure and 3 (16.7%) had no clinical improvement. Interestingly, the 4 participants who had complete clinical cure had baseline cultures positive for either T mentagrophytes or C parapsilosis. Most patients were content with the treatment, as 9 participants stated that they were very satisfied and 9 stated that they were satisfied. The average ratio of affected to total nail area declined from 63% at screening to 41% at the end of the study (P<.001). No adverse effects were reported with study drug.40
NCR Lacquer
Background
Resins are natural products derived from coniferous trees and are believed to protect trees against insects and microbial pathogens.41 Natural coniferous resin derived from the Norway spruce tree (Picea abies) mixed with boiled animal fat or butter has been used topically for centuries in Finland and Sweden to treat infections and wounds.42-44 The activity of NCR has been studied against a wide range of microbes, demonstrating broad-spectrum antimicrobial activity against both gram-positive bacteria and fungi.45-48 There are 2 published clinical trials evaluating NCR in the treatment of onychomycosis.49,50
In Vitro Data
Natural coniferous resin has shown antifungal activity against T mentagrophytes, Trichophyton tonsurans, and T rubrum in vitro, which was demonstrated using medicated disks of resin on petri dishes inoculated with these organisms.46 In another study, the authors evaluated the antifungal activity of NCR against human pathogenic fungi and yeasts using agar plate diffusion tests and showed that the resin had antifungal activity against Trichophyton species but not against Fusarium and most Candida species. Electron microscopy of T mentagrophytes exposed to NCR showed that all cells were dead inside the inhibition zone, with striking changes seen in the hyphal cell walls, while fungal cells outside the inhibition zone were morphologically normal.47 In another report, utilizing the European Pharmacopoeia challenge test, NCR was highly effective against gram-positive and gram-negative bacteria as well as C albicans.42
Clinical Trials
In one preliminary observational and prospective clinical trial, 15 participants with clinical and mycologic evidence of onychomycosis were instructed to apply NCR lacquer once daily for 9 months with a 4-week washout period, with the primary outcome measures being clinical and mycologic cure.49 Thirteen (87%) enrolled participants were male and the average age was 65 years (age range, 37–80 years). The DLSO subtype was present in 9 (60%) participants. The mycologic cure rate at the end of the study was 65% (95% CI, 42%-87%), and none achieved clinical cure, but 6 participants showed some improvement in the appearance of the nail.49
The second trial was a prospective, controlled, investigator-blinded study of 73 patients with clinical and mycologic evidence of toenail onychomycosis who were randomized to receive NCR 30%, amorolfine lacquer 5%, or 250 mg oral terbinafine.50 The primary end point was mycologic cure at 10 months, and secondary end points were clinical efficacy, cost-effectiveness, and patient compliance. Clinical efficacy was based on the proximal linear growth of healthy nail and was classified as unchanged, partial, or complete. Partial responses were described as substantial decreases in onycholysis, subungual hyperkeratosis, and streaks. A complete response was defined as a fully normal appearance of the toenail. Most patients were male in the NCR (91% [21/23]), amorolfine (80% [20/25]), and terbinafine (68% [17/25]) groups; the average ages were 64, 63, and 64 years, respectively. Trichophyton rubrum was cultured most often in all 3 groups: NCR, 87% (20/23); amorolfine, 96% (24/25); and terbinafine, 84% (21/25). The remaining cases were from T mentagrophytes. A summary of the results is shown in Table 2. Patient compliance was 100% in all except 1 patient in the amorolfine treatment group with moderate compliance. There were no adverse events, except for 2 in the terbinafine group: diarrhea and rash.50
AP Extract
Background
Ageratina pichinchensis, a member of the Asteraceae family, has been used historically in Mexico for fungal infections of the skin.51,52 Fresh or dried leaves were extracted with alcohol and the product was administered topically onto damaged skin without considerable skin irritation.53 Multiple studies have demonstrated that AP extract has in vitro antifungal activity along with other members of the Asteraceae family.54-56 There also is evidence from clinical trials that AP extract is effective against superficial dermatophyte infections such as tinea pedis.57 Given the positive antifungal in vitro data, the potential use of this agent was investigated for onychomycosis treatment.53,58
In Vitro Data
The antifungal properties of the Asteraceae family have been tested in several in vitro experiments. Eupatorium aschenbornianum, described as synonymous with A pichinchensis,59 was found to be most active against the dermatophytes T rubrum and T mentagrophytes with MICs of 0.3 and 0.03 mg/mL, respectively.54 It is thought that the primary antimycotic activity is due to encecalin, an acetylchromene compound that was identified in other plants from the Asteraceae family and has activity against dermatophytes.55 In another study, Ageratum houstanianum Mill, a comparable member of the Asteraceae family, had fungitoxic activity against T rubrum and C albicans isolated from nail infections.56
Clinical Trials
A double-blind controlled trial was performed on 110 patients with clinical and mycologic evidence of mild to moderate toenail onychomycosis randomized to treatment with AP lacquer or ciclopirox lacquer 8% (control).58 Primary end points were clinical effectiveness (completely normal nails) and mycologic cure. Patients were instructed to apply the lacquer once every third day during the first month, twice a week for the second month, and once a week for 16 weeks, with removal of the lacquer weekly. Demographics were similar between the AP lacquer and control groups, with mean ages of 44.6 and 46.5 years, respectively; women made up 74.5% and 67.2%, respectively, of each treatment group, with most patients having a 2- to 5-year history of disease (41.8% and 40.1%, respectively).58 A summary of the data is shown in Table 3. No severe side effects were documented, but minimal nail fold skin pain was reported in 3 patients in the control group in the first week, resolving later in the trial.58
A follow-up study was performed to determine the optimal concentration of AP lacquer for the treatment of onychomycosis.53 One hundred twenty-two patients aged 19 to 65 years with clinical and mycologic evidence of mild to moderate DLSO were randomized to receive 12.6% or 16.8% AP lacquer applied once daily to the affected nails for 6 months. The nails were graded as healthy, mild, or moderately affected before and after treatment. There were no significant differences in demographics between the 2 treatment groups, and 77% of patients were women with a median age of 47 years. There were no significant side effects from either concentration of AP lacquer.53
Ozonized Sunflower Oil
Background
Ozonized sunflower oil is derived by reacting ozone (O3) with sunflower plant (Helianthus annuus) oil to form a petroleum jelly–like material.60 It was originally shown to have antibacterial properties in vitro,61 and further studies have confirmed these findings and demonstrated anti-inflammatory, wound healing, and antifungal properties.62-64 A formulation of ozonized sunflower oil used in Cuba is clinically indicated for the treatment of tinea pedis and impetigo.65 The clinical efficacy of this product has been evaluated in a clinical trial for the treatment of onychomycosis.65
In Vitro Data
A compound made up of 30% ozonized sunflower oil with 0.5% of α-lipoic acid was found to have antifungal activity against C albicans using the disk diffusion method, in addition to other bacterial organisms. The MIC values ranged from 2.0 to 3.5 mg/mL.62 Another study was designed to evaluate the in vitro antifungal activity of this formulation on samples cultured from patients with onychomycosis using the disk diffusion method. They found inhibition of growth of C albicans, C parapsilosis, and Candida tropicalis, which was inferior to amphotericin B, ketoconazole, fluconazole, and itraconazole.64
Clinical Trial
A single-blind, controlled, phase 3 study was performed on 400 patients with clinical and mycologic evidence of onychomycosis. Patients were randomized to treatment with an ozonized sunflower oil solution or ketoconazole cream 2% applied to affected nails twice daily for 3 months, with filing and massage of the affected nails upon application of treatment.65 Cured was defined as mycologic cure in addition to a healthy appearing nail, improved as an increase in healthy appearing nail in addition to a decrease in symptoms (ie, paresthesia, pain, itching) but positive mycological testing, same as no clinical change in appearance with positive mycological findings, and worse as increasing diseased nail involvement in the presence of positive mycological findings. Demographics were similar between groups with a mean age of 35 years. Men accounted for 80% of the study population, and 65% of the study population was white. The mean duration of disease was 30 months. They also reported on a 1-year follow-up, with 2.8% of patients in the ozonized sunflower oil solution group and 37.0% of patients in the ketoconazole group describing relapses. Trichophyton rubrum and C albicans were cultured from these patients.65
Comment
Due to the poor efficacy, long-term treatment courses, inability to use nail polish, and high cost associated with many FDA-approved topical treatments, along with the systemic side effects, potential for drug-drug interactions, and cost associated with many oral therapies approved for onychomycosis, there has been a renewed interest in natural remedies and OTC treatments. Overall, TTO, TCS, NCR, AP extract, and ozonized sunflower oil have shown efficacy in vitro against some dermatophytes, nondermatophytes, and molds responsible for onychomycosis. One or more clinical trials were performed with each of these agents for the treatment of onychomycosis. They were mostly small pilot studies, and due to differences in trial design, the results cannot be compared with each other or with currently FDA-approved treatments. We can conclude that because adverse events were rare with all of these therapies—most commonly skin irritation or mild skin pain—they exhibit good safety.
For TTO, there was no statistical difference between the clotrimazole and TTO treatment groups in mycologic cure, clinical assessment, or patient subjective assessment of the nails.29 Although there was an 80% complete cure in the butenafine and TTO group, it was 0% in the TTO group at week 36.30 Trial design, longer treatment periods, incorporation into nanocapsules, or combination treatment with other antifungal agents may influence our future use of TTO for onychomycosis, but based on the present data we cannot recommend this treatment in clinical practice.
With TCS, 27.8% of participants had a mycologic cure and 22.2% had complete clinical cure.40 Although it is difficult to draw firm conclusions from this small pilot study, there may be some benefit to treating toenail onychomycosis due to T mentagrophytes or C parapsilosis with TCS but no benefit in treating onychomycosis due to T rubrum, the more common cause of onychomycosis. Limitations of this study were lack of a placebo group, small sample size, wide variety of represented pathogens that may not be representative of the true population, and lack of stratification by baseline severity or involvement of nail. A larger randomized controlled clinical trial would be necessary to confirm the results of this small study and make formal recommendations.
In one clinical trial with NCR, mycologic cure was 65% at the end of the study.49 No participants achieved clinical cure, but 6 participants showed some improvement in the appearance of the nail. Because this study was small (N=15), it is difficult to draw firm conclusions.49 In another study with NCR, mycologic cure rates with NCR, amorolfine, and terbinafine were 13%, 8%, and 56%, respectively. Based on these results, NCR has similar antifungal efficacy to amorolfine but was inferior to oral terbinafine.50 A larger randomized controlled clinical trial with more homogenous and less severely affected patients and longer treatment periods would be necessary to confirm the results of these small studies and make formal recommendations.
Because there were no significant differences in clinical effectiveness of mycologic cure rates between AP lacquer 10% and ciclopirox lacquer 8% in one clinical trial,58 AP does not seem to be more effective than at least one of the current FDA-approved topical treatments; however, because AP lacquer 16.8% was shown to be more effective than AP lacquer 12.6% in one onychomycosis clinical trial, using higher concentrations of AP may yield better results in future trials.53
One trial comparing ozonized sunflower oil to ketoconazole cream 2% showed 90.5% and 13.5% cure rates, respectively.65 Although there is good in vitro antifungal activity and a clinical trial showing efficacy using ozonized sunflower oil for the treatment of onychomycosis, confirmatory studies are necessary before we can recommend this OTC treatment to our patients. Specifically, we will get the most data from large randomized controlled trials with strict inclusion/exclusion and efficacy criteria.
Conclusion
Over-the-counter and natural remedies may be an emerging area of research in the treatment of onychomycosis. This review summarizes the laboratory data and clinical trials on several of these agents and, when available, compares their clinical and mycologic efficacy with FDA-approved therapies. Shortcomings of some of these studies include a small study population, lack of adequate controls, nonstandardized mycologic testing, and abbreviated posttreatment evaluation times. It may be concluded that these products have varying degrees of efficacy and appear to be safe in the studies cited; however, at present, we cannot recommend any of them to our patients until there are larger randomized clinical trials with appropriate controls demonstrating their efficacy.
Onychomycosis is a fungal infection of the nail unit by dermatophytes, yeasts, and nondermatophyte molds. It is characterized by a white or yellow discoloration of the nail plate; hyperkeratosis of the nail bed; distal detachment of the nail plate from its bed (onycholysis); and nail plate dystrophy, including thickening, crumbling, and ridging. Onychomycosis is an important problem, representing 30% of all superficial fungal infections and an estimated 50% of all nail diseases.1 Reported prevalence rates of onychomycosis in the United States and worldwide are varied, but the mean prevalence based on population-based studies in Europe and North America is estimated to be 4.3%.2 It is more common in older individuals, with an incidence rate of 20% in those older than 60 years and 50% in those older than 70 years.3 Onychomycosis is more common in patients with diabetes and 1.9 to 2.8 times higher than the general population.4 Dermatophytes are responsible for the majority of cases of onychomycosis, particularly Trichophyton rubrum and Trichophyton mentagrophytes.5
Onychomycosis is divided into different subtypes based on clinical presentation, which in turn are characterized by varying infecting organisms and prognoses. The subtypes of onychomycosis are distal and lateral subungual (DLSO), proximal subungual, superficial, endonyx, mixed pattern, total dystrophic, and secondary. Distal and lateral subungual onychomycosis are by far the most common presentation and begins when the infecting organism invades the hyponychium and distal or lateral nail bed. Trichophyton rubrum is the most common organism and T mentagrophytes is second, but Candida parapsilosis and Candida albicans also are possibilities. Proximal subungual onychomycosis is far less frequent than DLSO and is usually caused by T rubrum. The fungus invades the proximal nail folds and penetrates the newly growing nail plate.6 This pattern is more common in immunosuppressed patients and should prompt testing for human immunodeficiency virus.7 Total dystrophic onychomycosis is the end stage of fungal nail plate invasion, may follow DLSO or proximal subungual onychomycosis, and is difficult to treat.6
Onychomycosis causes pain, paresthesia, and difficulty with ambulation.8 In patients with peripheral neuropathy and vascular problems, including diabetes, onychomycosis can increase the risk for foot ulcers, with amputation in severe cases.9 Patients also may present with aesthetic concerns that may impact their quality of life.10
Given the effect on quality of life along with medical risks associated with onychomycosis, a safe and successful treatment modality with a low risk of recurrence is desirable. Unfortunately, treatment of nail fungus is quite challenging for a number of reasons. First, the thickness of the nail and/or the fungal mass may be a barrier to the delivery of topical and systemic drugs at the source of the infection. In addition, the nail plate does not have intrinsic immunity. Also, recurrence after treatment is common due to residual hyphae or spores that were not previously eliminated.11 Finally, many topical medications require long treatment courses, which may limit patient compliance, especially in patients who want to use nail polish for cosmesis or camouflage.
Currently Approved Therapies for Onychomycosis
Several definitions are needed to better interpret the results of onychomycosis clinical trials. Complete cure is defined as a negative potassium hydroxide preparation and negative fungal culture with a completely normal appearance of the nail. Mycological cure is defined as potassium hydroxide microscopy and fungal culture negative. Clinical cure is stated as 0% nail plate involvement but at times is reported as less than 5% and less than 10% involvement.
Terbinafine and itraconazole are the only US Food and Drug Administration (FDA)–approved systemic therapies, and ciclopirox, efinaconazole, and tavaborole are the only FDA-approved topicals. Advantages of systemic agents generally are higher cure rates and shorter treatment courses, thus better compliance. Disadvantages include greater incidence of systemic side effects and drug-drug interactions as well as the need for laboratory monitoring. Pros of topical therapies are low potential for adverse effects, no drug-drug interactions, and no monitoring of blood work. Cons include lower efficacy, long treatment courses, and poor patient compliance.
Terbinafine, an allylamine, taken orally once daily (250 mg) for 12 weeks for toenails and 6 weeks for fingernails currently is the preferred systemic treatment of onychomycosis, with complete cure rates of 38% and 59% and mycological cure rates of 70% and 79% for toenails and fingernails, respectively.12 Itraconazole, an azole, is dosed orally at 200 mg daily for 3 months for toenails, with a complete cure rate of 14% and mycological cure rate of 54%.13 For fingernail onychomycosis only, itraconazole is dosed at 200 mg twice daily for 1 week, followed by a treatment-free period of 3 weeks, and then another 1-week course at thesame dose. The complete cure rate is 47% and the mycological cure is 61% for this pulse regimen.13
Ciclopirox is a hydroxypyridone and the 8% nail lacquer formulation was approved in 1999, making it the first topical medication to gain FDA approval for the treatment of toenail onychomycosis. Based on 2 clinical trials, complete cure rates for toenails are 5.5% and 8.5% and mycological cure rates are 29% and 36% at 48 weeks with removal of residual lacquer and debridement.14Efinaconazole is an azole and the 10% solution was FDA approved for the treatment of toenail onychomycosis in 2014.15 In 2 clinical trials, complete cure rates were 17.8% and 15.2% and mycological cure rates were 55.2% and 53.4% with once daily toenail application for 48 weeks.16 Tavaborole is a benzoxaborole and the 5% solution also was approved for the treatment of toenail onychomycosis in 2014.17 Two clinical trials reported complete cure rates of 6.5% and 9.1% and mycological cure rates of 31.1% and 35.9% with once daily toenail application for 48 weeks.18
Given the poor efficacy, systemic side effects, potential for drug-drug interactions, long-term treatment courses, and cost associated with current systemic and/or topical treatments, there has been a renewed interest in natural remedies and over-the-counter (OTC) therapies for onychomycosis. This review summarizes the in vitro and in vivo data, mechanisms of action, and clinical efficacy of various natural and OTC agents for the treatment of onychomycosis. Specifically, we summarize the data on tea tree oil (TTO), a popular topical cough suppressant (TCS), natural coniferous resin (NCR) lacquer, Ageratina pichinchensis (AP) extract, and ozonized sunflower oil.
Tea Tree Oil
Background
Tea tree oil is a volatile oil whose medicinal use dates back to the early 20th century when the Bundjabung aborigines of North and New South Wales extracted TTO from the dried leaves of the Melaleuca alternifolia plant and used it to treat superficial wounds.19 Tea tree oil has been shown to be an effective treatment of tinea pedis,20 and it is widely used in Australia as well as in Europe and North America.21 Tea tree oil also has been investigated as an antifungal agent for the treatment of onychomycosis, both in vitro22-28 and in clinical trials.29,30
In Vitro Data
Because TTO is composed of more than 100 active components,23 the antifungal activity of these individual components was investigated against 14 fungal isolates, including C albicans, T mentagrophytes, and Aspergillus species. The minimum inhibitory concentration (MIC) for α-pinene was less than 0.004% for T mentagrophytes and the components with the greatest MIC and minimum fungicidal concentration for the fungi tested were terpinen-4-ol and α-terpineol, respectively.22 The antifungal activity of TTO also was tested using disk diffusion assay experiments with 58 clinical isolates of fungi including C albicans, T rubrum, T mentagrophytes, and Aspergillus niger.24 Tea tree oil was most effective at inhibiting T rubrum followed by T mentagrophytes,24 which are the 2 most common etiologies of onychomycosis.5 In another report, the authors determined the MIC of TTO utilizing 4 different experiments with T rubrum as the infecting organism. Because TTO inhibited the growth of T rubrum at all concentrations greater than 0.1%, they found that the MIC was 0.1%.25 Given the lack of adequate nail penetration of most topical therapies, TTO in nanocapsules (TTO-NC), TTO nanoemulsions, and normal emulsions were tested in vitro for their ability to inhibit the growth of T rubrum inoculated into nail shavings. Colony growth decreased significantly within the first week of treatment, with TTO-NC showing maximum efficacy (P<.001). This study showed that TTO, particularly TTO-NC, was effective in inhibiting the growth of T rubrum in vitro and that using nanocapsule technology may increase nail penetration and bioavailability.31
Much of what we know about TTO’s antifungal mechanism of action comes from experiments involving C albicans. To date, it has not been studied in T rubrum or T mentagrophytes, the 2 most common etiologies of onychomycosis.5 In C albicans, TTO causes altered permeability of plasma membranes,32 dose-dependent alteration of respiration,33 decreased glucose-induced acidification of media surrounding fungi,32 and reversible inhibition of germ tube formation.19,34
Clinical Trials
A randomized, double-blind, multicenter trial was performed on 117 patients with culture-proven DLSO who were randomized to receive TTO 100% or clotrimazole solution 1% applied twice daily to affected toenails for 6 months.29 Primary outcome measures were mycologic cure, clinical assessment, and patient subjective assessment (Table 1). There were no statistical differences between the 2 treatment groups. Erythema and irritation were the most common adverse reactions occurring in 7.8% (5/64) of the TTO group.29
Another study was a double-blind, placebo-controlled trial involving 60 patients with clinical and mycologic evidence of DLSO who were randomized to treatment with a cream containing butenafine hydrochloride 2% and TTO 5% (n=40) or a control cream containing only TTO (n=20), with active treatment for 8 weeks and final follow-up at 36 weeks.30 Patients were instructed to apply the cream 3 times daily under occlusion for 8 weeks and the nail was debrided between weeks 4 and 6 if feasible. If the nail could not be debrided after 8 weeks, it was considered resistant to treatment. At the end of the study, the complete cure rate was 80% in the active group compared to 0% in the placebo group (P<.0001), and the mean time to complete healing with progressive nail growth was 29 weeks. There were no adverse effects in the placebo group, but 4 patients in the active group had mild skin inflammation.30
Topical Cough Suppressant
Background
Topical cough suppressants, which are made up of several natural ingredients, are OTC ointments for adults and children 2 years and older that are indicated as cough suppressants when applied to the chest and throat and as relief of mild muscle and joint pains.35 The active ingredients are camphor 4.8%, eucalyptus oil 1.2%, and menthol 2.6%, while the inactive ingredients are cedarleaf oil, nutmeg oil, petrolatum, thymol, and turpentine oil.35 Some of the active and inactive ingredients in TCSs have shown efficacy against dermatophytes in vitro,36-38 and although they are not specifically indicated for onychomycosis, they have been popularized as home remedies for fungal nail infections.36,39 A TCS has been evaluated for its efficacy for the treatment of onychomycosis in one clinical trial.40
In Vitro Data
An in vitro study was performed to evaluate the antifungal activity of the individual and combined components of TCS on 16 different dermatophytes, nondermatophytes, and molds. The zones of inhibition against these organisms were greatest for camphor, menthol, thymol, and eucalyptus oil. Interestingly, there were large zones of inhibition and a synergistic effect when a mixture of components was used against T rubrum and T mentagrophytes.36 The in vitro activity of thymol, a component of TCS, was tested against Candida species.37 The essential oil subtypes Thymus vulgaris and Thymus zygis (subspecies zygis) showed similar antifungal activity, which was superior to Thymus mastichina, and all 3 compounds had similar MIC and minimal lethal concentration values. The authors showed that the antifungal mechanism was due to cell membrane damage and inhibition of germ tube formation.37 It should be noted that Candida species are less common causes of onychomycosis, and it is not known whether this data is applicable to T rubrum. In another study, the authors investigated the antifungal activity of Thymus pulegioides and found that MIC ranged from 0.16 to 0.32 μL/mL for dermatophytes and Aspergillus strains and 0.32 to 0.64 μL/mL for Candida species. When an essential oil concentration of 0.08 μL/mL was used against T rubrum, ergosterol content decreased by 70 %, indicating that T pulegioides inhibits ergosterol biosynthesis in T rubrum.38
Clinical Observations and Clinical Trial
There is one report documenting the clinical observations on a group of patients with a clinical diagnosis of onychomycosis who were instructed to apply TCS to affected nail(s) once daily.36 Eighty-five charts were reviewed (mean age, 77 years), and although follow-up was not complete or standardized, the following data were reported: 32 (38%) cleared their fungal infection, 21 (25%) had no record of change but also no record of compliance, 19 (22%) had only 1 documented follow-up visit, 9 (11%) reported they did not use the treatment, and 4 (5%) did not return for a follow-up visit. Of the 32 patients whose nails were cured, 3 (9%) had clearance within 5 months, 8 (25%) within 7 months, 11 (34%) within 9 months, 4 (13%) within 11 months, and 6 (19%) within 16 months.36
A small pilot study was performed to evaluate the efficacy of daily application of TCS in the treatment of onychomycosis in patients 18 years and older with at least 1 great toenail affected.40 The primary end points were mycologic cure at 48 weeks and clinical cure at the end of the study graded as complete, partial, or no change. The secondary end point was patient satisfaction with the appearance of the affected nail at 48 weeks. Eighteen participants completed the study; 55% (10/18) were male, with an average age of 51 years (age range, 30–85 years). The mean initial amount of affected nail was 62% (range, 16%–100%), and cultures included dermatophytes, nondermatophytes, and molds. With TCS treatment, 27.8% (5/18) showed mycologic cure of which 4 (22.2%) had a complete clinical cure. Ten participants (55.6%) had partial clinical cure and 3 (16.7%) had no clinical improvement. Interestingly, the 4 participants who had complete clinical cure had baseline cultures positive for either T mentagrophytes or C parapsilosis. Most patients were content with the treatment, as 9 participants stated that they were very satisfied and 9 stated that they were satisfied. The average ratio of affected to total nail area declined from 63% at screening to 41% at the end of the study (P<.001). No adverse effects were reported with study drug.40
NCR Lacquer
Background
Resins are natural products derived from coniferous trees and are believed to protect trees against insects and microbial pathogens.41 Natural coniferous resin derived from the Norway spruce tree (Picea abies) mixed with boiled animal fat or butter has been used topically for centuries in Finland and Sweden to treat infections and wounds.42-44 The activity of NCR has been studied against a wide range of microbes, demonstrating broad-spectrum antimicrobial activity against both gram-positive bacteria and fungi.45-48 There are 2 published clinical trials evaluating NCR in the treatment of onychomycosis.49,50
In Vitro Data
Natural coniferous resin has shown antifungal activity against T mentagrophytes, Trichophyton tonsurans, and T rubrum in vitro, which was demonstrated using medicated disks of resin on petri dishes inoculated with these organisms.46 In another study, the authors evaluated the antifungal activity of NCR against human pathogenic fungi and yeasts using agar plate diffusion tests and showed that the resin had antifungal activity against Trichophyton species but not against Fusarium and most Candida species. Electron microscopy of T mentagrophytes exposed to NCR showed that all cells were dead inside the inhibition zone, with striking changes seen in the hyphal cell walls, while fungal cells outside the inhibition zone were morphologically normal.47 In another report, utilizing the European Pharmacopoeia challenge test, NCR was highly effective against gram-positive and gram-negative bacteria as well as C albicans.42
Clinical Trials
In one preliminary observational and prospective clinical trial, 15 participants with clinical and mycologic evidence of onychomycosis were instructed to apply NCR lacquer once daily for 9 months with a 4-week washout period, with the primary outcome measures being clinical and mycologic cure.49 Thirteen (87%) enrolled participants were male and the average age was 65 years (age range, 37–80 years). The DLSO subtype was present in 9 (60%) participants. The mycologic cure rate at the end of the study was 65% (95% CI, 42%-87%), and none achieved clinical cure, but 6 participants showed some improvement in the appearance of the nail.49
The second trial was a prospective, controlled, investigator-blinded study of 73 patients with clinical and mycologic evidence of toenail onychomycosis who were randomized to receive NCR 30%, amorolfine lacquer 5%, or 250 mg oral terbinafine.50 The primary end point was mycologic cure at 10 months, and secondary end points were clinical efficacy, cost-effectiveness, and patient compliance. Clinical efficacy was based on the proximal linear growth of healthy nail and was classified as unchanged, partial, or complete. Partial responses were described as substantial decreases in onycholysis, subungual hyperkeratosis, and streaks. A complete response was defined as a fully normal appearance of the toenail. Most patients were male in the NCR (91% [21/23]), amorolfine (80% [20/25]), and terbinafine (68% [17/25]) groups; the average ages were 64, 63, and 64 years, respectively. Trichophyton rubrum was cultured most often in all 3 groups: NCR, 87% (20/23); amorolfine, 96% (24/25); and terbinafine, 84% (21/25). The remaining cases were from T mentagrophytes. A summary of the results is shown in Table 2. Patient compliance was 100% in all except 1 patient in the amorolfine treatment group with moderate compliance. There were no adverse events, except for 2 in the terbinafine group: diarrhea and rash.50
AP Extract
Background
Ageratina pichinchensis, a member of the Asteraceae family, has been used historically in Mexico for fungal infections of the skin.51,52 Fresh or dried leaves were extracted with alcohol and the product was administered topically onto damaged skin without considerable skin irritation.53 Multiple studies have demonstrated that AP extract has in vitro antifungal activity along with other members of the Asteraceae family.54-56 There also is evidence from clinical trials that AP extract is effective against superficial dermatophyte infections such as tinea pedis.57 Given the positive antifungal in vitro data, the potential use of this agent was investigated for onychomycosis treatment.53,58
In Vitro Data
The antifungal properties of the Asteraceae family have been tested in several in vitro experiments. Eupatorium aschenbornianum, described as synonymous with A pichinchensis,59 was found to be most active against the dermatophytes T rubrum and T mentagrophytes with MICs of 0.3 and 0.03 mg/mL, respectively.54 It is thought that the primary antimycotic activity is due to encecalin, an acetylchromene compound that was identified in other plants from the Asteraceae family and has activity against dermatophytes.55 In another study, Ageratum houstanianum Mill, a comparable member of the Asteraceae family, had fungitoxic activity against T rubrum and C albicans isolated from nail infections.56
Clinical Trials
A double-blind controlled trial was performed on 110 patients with clinical and mycologic evidence of mild to moderate toenail onychomycosis randomized to treatment with AP lacquer or ciclopirox lacquer 8% (control).58 Primary end points were clinical effectiveness (completely normal nails) and mycologic cure. Patients were instructed to apply the lacquer once every third day during the first month, twice a week for the second month, and once a week for 16 weeks, with removal of the lacquer weekly. Demographics were similar between the AP lacquer and control groups, with mean ages of 44.6 and 46.5 years, respectively; women made up 74.5% and 67.2%, respectively, of each treatment group, with most patients having a 2- to 5-year history of disease (41.8% and 40.1%, respectively).58 A summary of the data is shown in Table 3. No severe side effects were documented, but minimal nail fold skin pain was reported in 3 patients in the control group in the first week, resolving later in the trial.58
A follow-up study was performed to determine the optimal concentration of AP lacquer for the treatment of onychomycosis.53 One hundred twenty-two patients aged 19 to 65 years with clinical and mycologic evidence of mild to moderate DLSO were randomized to receive 12.6% or 16.8% AP lacquer applied once daily to the affected nails for 6 months. The nails were graded as healthy, mild, or moderately affected before and after treatment. There were no significant differences in demographics between the 2 treatment groups, and 77% of patients were women with a median age of 47 years. There were no significant side effects from either concentration of AP lacquer.53
Ozonized Sunflower Oil
Background
Ozonized sunflower oil is derived by reacting ozone (O3) with sunflower plant (Helianthus annuus) oil to form a petroleum jelly–like material.60 It was originally shown to have antibacterial properties in vitro,61 and further studies have confirmed these findings and demonstrated anti-inflammatory, wound healing, and antifungal properties.62-64 A formulation of ozonized sunflower oil used in Cuba is clinically indicated for the treatment of tinea pedis and impetigo.65 The clinical efficacy of this product has been evaluated in a clinical trial for the treatment of onychomycosis.65
In Vitro Data
A compound made up of 30% ozonized sunflower oil with 0.5% of α-lipoic acid was found to have antifungal activity against C albicans using the disk diffusion method, in addition to other bacterial organisms. The MIC values ranged from 2.0 to 3.5 mg/mL.62 Another study was designed to evaluate the in vitro antifungal activity of this formulation on samples cultured from patients with onychomycosis using the disk diffusion method. They found inhibition of growth of C albicans, C parapsilosis, and Candida tropicalis, which was inferior to amphotericin B, ketoconazole, fluconazole, and itraconazole.64
Clinical Trial
A single-blind, controlled, phase 3 study was performed on 400 patients with clinical and mycologic evidence of onychomycosis. Patients were randomized to treatment with an ozonized sunflower oil solution or ketoconazole cream 2% applied to affected nails twice daily for 3 months, with filing and massage of the affected nails upon application of treatment.65 Cured was defined as mycologic cure in addition to a healthy appearing nail, improved as an increase in healthy appearing nail in addition to a decrease in symptoms (ie, paresthesia, pain, itching) but positive mycological testing, same as no clinical change in appearance with positive mycological findings, and worse as increasing diseased nail involvement in the presence of positive mycological findings. Demographics were similar between groups with a mean age of 35 years. Men accounted for 80% of the study population, and 65% of the study population was white. The mean duration of disease was 30 months. They also reported on a 1-year follow-up, with 2.8% of patients in the ozonized sunflower oil solution group and 37.0% of patients in the ketoconazole group describing relapses. Trichophyton rubrum and C albicans were cultured from these patients.65
Comment
Due to the poor efficacy, long-term treatment courses, inability to use nail polish, and high cost associated with many FDA-approved topical treatments, along with the systemic side effects, potential for drug-drug interactions, and cost associated with many oral therapies approved for onychomycosis, there has been a renewed interest in natural remedies and OTC treatments. Overall, TTO, TCS, NCR, AP extract, and ozonized sunflower oil have shown efficacy in vitro against some dermatophytes, nondermatophytes, and molds responsible for onychomycosis. One or more clinical trials were performed with each of these agents for the treatment of onychomycosis. They were mostly small pilot studies, and due to differences in trial design, the results cannot be compared with each other or with currently FDA-approved treatments. We can conclude that because adverse events were rare with all of these therapies—most commonly skin irritation or mild skin pain—they exhibit good safety.
For TTO, there was no statistical difference between the clotrimazole and TTO treatment groups in mycologic cure, clinical assessment, or patient subjective assessment of the nails.29 Although there was an 80% complete cure in the butenafine and TTO group, it was 0% in the TTO group at week 36.30 Trial design, longer treatment periods, incorporation into nanocapsules, or combination treatment with other antifungal agents may influence our future use of TTO for onychomycosis, but based on the present data we cannot recommend this treatment in clinical practice.
With TCS, 27.8% of participants had a mycologic cure and 22.2% had complete clinical cure.40 Although it is difficult to draw firm conclusions from this small pilot study, there may be some benefit to treating toenail onychomycosis due to T mentagrophytes or C parapsilosis with TCS but no benefit in treating onychomycosis due to T rubrum, the more common cause of onychomycosis. Limitations of this study were lack of a placebo group, small sample size, wide variety of represented pathogens that may not be representative of the true population, and lack of stratification by baseline severity or involvement of nail. A larger randomized controlled clinical trial would be necessary to confirm the results of this small study and make formal recommendations.
In one clinical trial with NCR, mycologic cure was 65% at the end of the study.49 No participants achieved clinical cure, but 6 participants showed some improvement in the appearance of the nail. Because this study was small (N=15), it is difficult to draw firm conclusions.49 In another study with NCR, mycologic cure rates with NCR, amorolfine, and terbinafine were 13%, 8%, and 56%, respectively. Based on these results, NCR has similar antifungal efficacy to amorolfine but was inferior to oral terbinafine.50 A larger randomized controlled clinical trial with more homogenous and less severely affected patients and longer treatment periods would be necessary to confirm the results of these small studies and make formal recommendations.
Because there were no significant differences in clinical effectiveness of mycologic cure rates between AP lacquer 10% and ciclopirox lacquer 8% in one clinical trial,58 AP does not seem to be more effective than at least one of the current FDA-approved topical treatments; however, because AP lacquer 16.8% was shown to be more effective than AP lacquer 12.6% in one onychomycosis clinical trial, using higher concentrations of AP may yield better results in future trials.53
One trial comparing ozonized sunflower oil to ketoconazole cream 2% showed 90.5% and 13.5% cure rates, respectively.65 Although there is good in vitro antifungal activity and a clinical trial showing efficacy using ozonized sunflower oil for the treatment of onychomycosis, confirmatory studies are necessary before we can recommend this OTC treatment to our patients. Specifically, we will get the most data from large randomized controlled trials with strict inclusion/exclusion and efficacy criteria.
Conclusion
Over-the-counter and natural remedies may be an emerging area of research in the treatment of onychomycosis. This review summarizes the laboratory data and clinical trials on several of these agents and, when available, compares their clinical and mycologic efficacy with FDA-approved therapies. Shortcomings of some of these studies include a small study population, lack of adequate controls, nonstandardized mycologic testing, and abbreviated posttreatment evaluation times. It may be concluded that these products have varying degrees of efficacy and appear to be safe in the studies cited; however, at present, we cannot recommend any of them to our patients until there are larger randomized clinical trials with appropriate controls demonstrating their efficacy.
- Scher RK, Daniel CR. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005.
- Sigurgeirsson B, Baran R. The prevalence of onychomycosis in the global population: a literature study. J Eur Acad Dermatol Venereol. 2014;28:1480-1491.
- Thomas J, Jacobson GA, Narkowicz CK, et al. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther. 2010;35:497-519.
- Mayser P, Freund V, Budihardja D. Toenail onychomycosis in diabetic patients: issues and management. Am J Clin Dermatol. 2009;10:211-220.
- Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641-648.
- Hay RJ, Baran R. Onychomycosis: a proposed revision of the clinical classification J Am Acad Dermatol. 2011;65:1219-1227.
- Elewski B. Clinical pearl: proximal white subungual onychomycosis in AIDS. J Am Acad Dermatol. 1993;29:631-632.
- Scher RK. Onychomycosis is more than a cosmetic problem. Br J Dermatol. 1994;130(suppl 43):15.
- Boyko EJ, Ahroni JH, Cohen V, et al. Prediction of diabetic foot ulcer occurrence using commonly available clinical information: the Seattle Diabetic Foot Study. Diabetes Care. 2006;29:1202-1207.
- Szepietowski JC, Reich A, Pacan P, et al. Evaluation of quality of life in patients with toenail onychomycosis by Polish version of an international onychomycosis-specific questionnaire. J Eur Acad Dermatol Venereol. 2007;21:491-496.
- Scher RK, Baron R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(suppl 65):5-9.
- Lamisil [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2011.
- Sporanox [package insert]. Raritan, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2001
- Penlac [package insert]. Bridgewater, NJ: Dermik Laboratories; 2006.
- Jublia [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC; 2014.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Kerydin [package insert]. Palo Alto, CA: Anacor Pharmaceuticals, Inc; 2014
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies [published online May 5, 2015]. J Am Acad Dermatol. 2015;73:62-69.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Satchell AC, Saurajen A, Bell C, et al. Treatment of interdigital tinea pedis with 25% and 50% tea tree oil solution: a randomized, placebo-controlled, blinded study. Australas J Dermatol. 2002;43:175-178.
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50-62.
- Hammer KA, Carson CF, Riley TV. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J Appl Microbiol. 2003;95:853-860.
- Brophy JJ, Davies NW, Southwell IA, et al. Gas chromatographic quality control for oil of Melaleuca terpinen-4-ol type (Australian tea tree). J Agric Food Chem. 1989;37:1330-1335.
- Concha JM, Moore LS, Holloway WJ. 1998 William J. Stickel Bronze Award. Antifungal activity of Melaleuca alternifolia (tea-tree) oil against various pathogenic organisms. J Am Podiatr Med Assoc. 1998;88:489-492.
- Benger S, Townsend P, Ashford RL, et al. An in vitro study to determine the minimum inhibitory concentration of Melaleuca alternifolia against the dermatophyte Trichophyton rubrum. Foot. 2004;14:86-91.
- Hammer KA, Carson CF, Riley TV. In-vitro activity of essential oils, in particular Melaleuca alternifolia (tea tree) oil and tea tree oil products, against Candida spp. J Antimicrob Chemother. 1998;42:591-595.
- Altman P. Australian tea tree oil. Aust J Pharm. 1998;69:276-278.
- Guterres SS, Alves MP, Pohlmann AR. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights. 2007;2:147-157.
- Buck DS, Nidorf DM, Addino JG. Comparison of two topical preparations for the treatment of onychomycosis: Melaleuca alternifolia (tea tree) oil and clotrimazole. J Fam Pract. 1994;38:601-605.
- Syed TA, Qureshi ZA, Ali SM, et al. Treatment of toenail onychomycosis with 2% butenafine and 5% Melaleuca alternifolia (tea tree) oil in cream. Tropical Med Int Health. 1999;4:284-287.
- Flores FC, de Lima JA, Ribeiro RF, et al. Antifungal activity of nanocapsule suspensions containing tea tree oil on the growth of Trichophyton rubrum. Mycopathologia. 2013;175:281-286.
- Hammer KA, Carson CF, Riley TV. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J Antimicrob Chemother. 2004;53:1081-1085.
- Cox SD, Mann CM, Markham JL, et al. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J Appl Microbiol. 2000;88:170-175.
- Hammer KA, Carson CF, Riley TV. Melaleuca alternifolia (tea tree) oil inhibits germ tube formation by Candida albicans. Med Mycol. 2000;38:355-362.
- Vicks VapoRub [package insert]. Gross-Gerau, Germany: Proctor & Gamble; 2010.
- Ramsewak RS, Nair MG, Stommel M, et al. In vitro antagonistic activity of monoterpenes and their mixtures against ‘toe nail fungus’ pathogens. Phytother Res. 2003;17:376-379.
- Pina-Vaz C, Gonçalves Rodrigues A, Pinto E, et al. Antifungal activity of Thymus oils and their major compounds. J Eur Acad Dermatol Venereol. 2004;18:73-78.
- Pinto E, Pina-Vaz C, Salgueiro L, et al. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J Med Microbiol. 2006;55:1367-1373.
- Vicks VapoRub might help fight toenail fungus. Consumer Reports. 2006;71:49.
- Derby R, Rohal P, Jackson C, et al. Novel treatment of onychomycosis using over-the-counter mentholated ointment: a clinical case series. J Am Board Fam Med. 2011;24:69-74.
- Trapp S, Croteau R. Defensive resin biosynthesis in conifers. Ann Rev Plant Physiol Plant Mol Biol. 2001;52:689-724.
- Sipponen A, Laitinen K. Antimicrobial properties of natural coniferous rosin in the European Pharmacopoeia challenge test. APMIS. 2011;119:720-724.
- Sipponen A, Lohi J. Lappish gum care “new” treatment of pressure ulcers? People’s improvement at it’s best. Eng Med J. 2003;58:2775-2776.
- Benedictus O. Een Nyttigh Läkare. Malmö: Kroon; 1938.
- Rautio M, Sipponen A, Peltola R, et al. Antibacterial effects of home-made resin salve from Norway spruce (Picea abies). APMIS. 2007;115:335-340.
- Laitinen K, Sipponen A, Jokinen JJ, et al. Resin salve from Norway spruce is antifungal against dermatophytes causing nail infections. EWMA. 2009;56:289-296.
- Rautio M, Sipponen A, Lohi J, et al. In vitro fungistatic effects of natural coniferous resin from Norway spruce (Picea abies). Eur J Clin Microbiol Infect Dis. 2012;31:1783-1789.
- Sipponen A, Peltola R, Jokinen JJ, et al. Effects of Norway spruce (Picea abies) resin on cell wall and cell membrane of Staphylococcus aureus. Ultrastruct Pathol. 2009;33:128-135.
- Sipponen P, Sipponen A, Lohi J, et al. Natural coniferous resin lacquer in treatment of toenail onychomycosis: an observational study. Mycoses. 2013;56:289-296.
- Auvinen T, Tiihonen R, Soini M, et al. Efficacy of topical resin lacquer, amorolfine, and oral terbinafine for treating toenail onychomycosis: a prospective, randomized, controlled, investigator-blinded, parallel-group clinical trial. Br J Dermatol. 2015;173:940-948.
- Argueta A, Cano L, Rodarte M. Atlas de las Plantas de la Medicina Tradicional Mexicana. Vol 3. Mexico City, Mexico: Instituto Nacional Indigenista; 1994:72-680.
- Avilés M, Suárez G. Catálogo de Plantas Medicinales del Jardín Etnobotánico. Peru: Instituto Nacional de Antropología e Historia; 1994.
- Romero-Cerecero O, Roman-Ramos R, Zamilpa A, et al. Clinical trial to compare the effectiveness of two concentrations of the Ageratina pichinchensis extract in the topical treatment of onychomycosis. J Ethnopharmacol. 2009;126:74-78.
- Navarro Garcia VM, Gonzalez A, Fuentes M, et al. Antifungal activities of nine traditional Mexican medicinal plants. J Ethnopharmacol. 2003;87:85-88.
- Castañeda P, Gómez L, Mata R, et al. Phytogrowth-inhibitory and antifungal constituents of Helianthella quinquenervis. J Nat Prod. 1996;59:323-326.
- Kumar N. Inhibition of nail infecting fungi of peoples of North Eastern UP causing Tinea unguium through leaf essential oil of Ageratum houstonianum Mill. IOSR J Pharm. June 2014;4:36-42.
- Romero-Cerecero O, Rojas G, Navarro V, et al. Effectiveness and tolerability of a standardized extract from Ageratina pichinchensis on patients with tinea pedis: an explorative pilot study controlled with ketoconazole. Planta Med. 2006;72:1257-1261.
- Romero-Cerecero O, Zamilpa A, Jimenez-Ferrer JE, et al. Double-blind clinical trial for evaluating the effectiveness and tolerability of Ageratina pichinchensis extract on patients with mild to moderate onychomycosis. a comparative study with ciclopirox. Planta Med. 2008;74:1430-1435.
- Rzedowski J, De Rzedowski GC. Flora Fanerogámica del Valle de México. Mexico City, Mexico: Instituto de Ecología Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional; 1985.
- Bocci V. Biological and clinical effects of ozone. has ozone therapy a future in medicine? Br J Biomed Sci. 1999;56:270-279.
- Sechi LA, Lezcano I, Nunez N, et al. Antibacterial activity of ozonized sunflower oil (Oleozon). J Appl Microbiol. 2001;90:279-284.
- Rodrigues KL, Cardoso CC, Caputo LR, et al. Cicatrizing and antimicrobial properties of an ozonised oil from sunflower seeds. Inflammopharmacology. 2004;12:261-270.
- Daud FV, Ueda SMY, Navarini A, et al. The use of ozonized oil in the treatment of dermatophitosis caused by Microsporum canis in rabbits. Braz J Microbiol. 2011;42:274-281.
- Guerrer LV, Cunha KC, Nogueira MC, et al. “In vitro” antifungal activity of ozonized sunflower oil on yeasts from onychomycosis. Braz J Microbiol. 2012;43:1315-1318.
- Menéndez S, Falcón L, Maqueira Y. Therapeutic efficacy of topical OLEOZON in patients suffering from onychomycosis. Mycoses. 2011;54:E272-E277.
- Scher RK, Daniel CR. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005.
- Sigurgeirsson B, Baran R. The prevalence of onychomycosis in the global population: a literature study. J Eur Acad Dermatol Venereol. 2014;28:1480-1491.
- Thomas J, Jacobson GA, Narkowicz CK, et al. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther. 2010;35:497-519.
- Mayser P, Freund V, Budihardja D. Toenail onychomycosis in diabetic patients: issues and management. Am J Clin Dermatol. 2009;10:211-220.
- Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641-648.
- Hay RJ, Baran R. Onychomycosis: a proposed revision of the clinical classification J Am Acad Dermatol. 2011;65:1219-1227.
- Elewski B. Clinical pearl: proximal white subungual onychomycosis in AIDS. J Am Acad Dermatol. 1993;29:631-632.
- Scher RK. Onychomycosis is more than a cosmetic problem. Br J Dermatol. 1994;130(suppl 43):15.
- Boyko EJ, Ahroni JH, Cohen V, et al. Prediction of diabetic foot ulcer occurrence using commonly available clinical information: the Seattle Diabetic Foot Study. Diabetes Care. 2006;29:1202-1207.
- Szepietowski JC, Reich A, Pacan P, et al. Evaluation of quality of life in patients with toenail onychomycosis by Polish version of an international onychomycosis-specific questionnaire. J Eur Acad Dermatol Venereol. 2007;21:491-496.
- Scher RK, Baron R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(suppl 65):5-9.
- Lamisil [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2011.
- Sporanox [package insert]. Raritan, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2001
- Penlac [package insert]. Bridgewater, NJ: Dermik Laboratories; 2006.
- Jublia [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC; 2014.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Kerydin [package insert]. Palo Alto, CA: Anacor Pharmaceuticals, Inc; 2014
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies [published online May 5, 2015]. J Am Acad Dermatol. 2015;73:62-69.
- D’Auria FD, Laino L, Strippoli V, et al. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J Chemother. 2001;13:377-383.
- Satchell AC, Saurajen A, Bell C, et al. Treatment of interdigital tinea pedis with 25% and 50% tea tree oil solution: a randomized, placebo-controlled, blinded study. Australas J Dermatol. 2002;43:175-178.
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50-62.
- Hammer KA, Carson CF, Riley TV. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J Appl Microbiol. 2003;95:853-860.
- Brophy JJ, Davies NW, Southwell IA, et al. Gas chromatographic quality control for oil of Melaleuca terpinen-4-ol type (Australian tea tree). J Agric Food Chem. 1989;37:1330-1335.
- Concha JM, Moore LS, Holloway WJ. 1998 William J. Stickel Bronze Award. Antifungal activity of Melaleuca alternifolia (tea-tree) oil against various pathogenic organisms. J Am Podiatr Med Assoc. 1998;88:489-492.
- Benger S, Townsend P, Ashford RL, et al. An in vitro study to determine the minimum inhibitory concentration of Melaleuca alternifolia against the dermatophyte Trichophyton rubrum. Foot. 2004;14:86-91.
- Hammer KA, Carson CF, Riley TV. In-vitro activity of essential oils, in particular Melaleuca alternifolia (tea tree) oil and tea tree oil products, against Candida spp. J Antimicrob Chemother. 1998;42:591-595.
- Altman P. Australian tea tree oil. Aust J Pharm. 1998;69:276-278.
- Guterres SS, Alves MP, Pohlmann AR. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights. 2007;2:147-157.
- Buck DS, Nidorf DM, Addino JG. Comparison of two topical preparations for the treatment of onychomycosis: Melaleuca alternifolia (tea tree) oil and clotrimazole. J Fam Pract. 1994;38:601-605.
- Syed TA, Qureshi ZA, Ali SM, et al. Treatment of toenail onychomycosis with 2% butenafine and 5% Melaleuca alternifolia (tea tree) oil in cream. Tropical Med Int Health. 1999;4:284-287.
- Flores FC, de Lima JA, Ribeiro RF, et al. Antifungal activity of nanocapsule suspensions containing tea tree oil on the growth of Trichophyton rubrum. Mycopathologia. 2013;175:281-286.
- Hammer KA, Carson CF, Riley TV. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J Antimicrob Chemother. 2004;53:1081-1085.
- Cox SD, Mann CM, Markham JL, et al. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J Appl Microbiol. 2000;88:170-175.
- Hammer KA, Carson CF, Riley TV. Melaleuca alternifolia (tea tree) oil inhibits germ tube formation by Candida albicans. Med Mycol. 2000;38:355-362.
- Vicks VapoRub [package insert]. Gross-Gerau, Germany: Proctor & Gamble; 2010.
- Ramsewak RS, Nair MG, Stommel M, et al. In vitro antagonistic activity of monoterpenes and their mixtures against ‘toe nail fungus’ pathogens. Phytother Res. 2003;17:376-379.
- Pina-Vaz C, Gonçalves Rodrigues A, Pinto E, et al. Antifungal activity of Thymus oils and their major compounds. J Eur Acad Dermatol Venereol. 2004;18:73-78.
- Pinto E, Pina-Vaz C, Salgueiro L, et al. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J Med Microbiol. 2006;55:1367-1373.
- Vicks VapoRub might help fight toenail fungus. Consumer Reports. 2006;71:49.
- Derby R, Rohal P, Jackson C, et al. Novel treatment of onychomycosis using over-the-counter mentholated ointment: a clinical case series. J Am Board Fam Med. 2011;24:69-74.
- Trapp S, Croteau R. Defensive resin biosynthesis in conifers. Ann Rev Plant Physiol Plant Mol Biol. 2001;52:689-724.
- Sipponen A, Laitinen K. Antimicrobial properties of natural coniferous rosin in the European Pharmacopoeia challenge test. APMIS. 2011;119:720-724.
- Sipponen A, Lohi J. Lappish gum care “new” treatment of pressure ulcers? People’s improvement at it’s best. Eng Med J. 2003;58:2775-2776.
- Benedictus O. Een Nyttigh Läkare. Malmö: Kroon; 1938.
- Rautio M, Sipponen A, Peltola R, et al. Antibacterial effects of home-made resin salve from Norway spruce (Picea abies). APMIS. 2007;115:335-340.
- Laitinen K, Sipponen A, Jokinen JJ, et al. Resin salve from Norway spruce is antifungal against dermatophytes causing nail infections. EWMA. 2009;56:289-296.
- Rautio M, Sipponen A, Lohi J, et al. In vitro fungistatic effects of natural coniferous resin from Norway spruce (Picea abies). Eur J Clin Microbiol Infect Dis. 2012;31:1783-1789.
- Sipponen A, Peltola R, Jokinen JJ, et al. Effects of Norway spruce (Picea abies) resin on cell wall and cell membrane of Staphylococcus aureus. Ultrastruct Pathol. 2009;33:128-135.
- Sipponen P, Sipponen A, Lohi J, et al. Natural coniferous resin lacquer in treatment of toenail onychomycosis: an observational study. Mycoses. 2013;56:289-296.
- Auvinen T, Tiihonen R, Soini M, et al. Efficacy of topical resin lacquer, amorolfine, and oral terbinafine for treating toenail onychomycosis: a prospective, randomized, controlled, investigator-blinded, parallel-group clinical trial. Br J Dermatol. 2015;173:940-948.
- Argueta A, Cano L, Rodarte M. Atlas de las Plantas de la Medicina Tradicional Mexicana. Vol 3. Mexico City, Mexico: Instituto Nacional Indigenista; 1994:72-680.
- Avilés M, Suárez G. Catálogo de Plantas Medicinales del Jardín Etnobotánico. Peru: Instituto Nacional de Antropología e Historia; 1994.
- Romero-Cerecero O, Roman-Ramos R, Zamilpa A, et al. Clinical trial to compare the effectiveness of two concentrations of the Ageratina pichinchensis extract in the topical treatment of onychomycosis. J Ethnopharmacol. 2009;126:74-78.
- Navarro Garcia VM, Gonzalez A, Fuentes M, et al. Antifungal activities of nine traditional Mexican medicinal plants. J Ethnopharmacol. 2003;87:85-88.
- Castañeda P, Gómez L, Mata R, et al. Phytogrowth-inhibitory and antifungal constituents of Helianthella quinquenervis. J Nat Prod. 1996;59:323-326.
- Kumar N. Inhibition of nail infecting fungi of peoples of North Eastern UP causing Tinea unguium through leaf essential oil of Ageratum houstonianum Mill. IOSR J Pharm. June 2014;4:36-42.
- Romero-Cerecero O, Rojas G, Navarro V, et al. Effectiveness and tolerability of a standardized extract from Ageratina pichinchensis on patients with tinea pedis: an explorative pilot study controlled with ketoconazole. Planta Med. 2006;72:1257-1261.
- Romero-Cerecero O, Zamilpa A, Jimenez-Ferrer JE, et al. Double-blind clinical trial for evaluating the effectiveness and tolerability of Ageratina pichinchensis extract on patients with mild to moderate onychomycosis. a comparative study with ciclopirox. Planta Med. 2008;74:1430-1435.
- Rzedowski J, De Rzedowski GC. Flora Fanerogámica del Valle de México. Mexico City, Mexico: Instituto de Ecología Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional; 1985.
- Bocci V. Biological and clinical effects of ozone. has ozone therapy a future in medicine? Br J Biomed Sci. 1999;56:270-279.
- Sechi LA, Lezcano I, Nunez N, et al. Antibacterial activity of ozonized sunflower oil (Oleozon). J Appl Microbiol. 2001;90:279-284.
- Rodrigues KL, Cardoso CC, Caputo LR, et al. Cicatrizing and antimicrobial properties of an ozonised oil from sunflower seeds. Inflammopharmacology. 2004;12:261-270.
- Daud FV, Ueda SMY, Navarini A, et al. The use of ozonized oil in the treatment of dermatophitosis caused by Microsporum canis in rabbits. Braz J Microbiol. 2011;42:274-281.
- Guerrer LV, Cunha KC, Nogueira MC, et al. “In vitro” antifungal activity of ozonized sunflower oil on yeasts from onychomycosis. Braz J Microbiol. 2012;43:1315-1318.
- Menéndez S, Falcón L, Maqueira Y. Therapeutic efficacy of topical OLEOZON in patients suffering from onychomycosis. Mycoses. 2011;54:E272-E277.
Practice Points
- Natural remedies, including tea tree oil, natural topical cough suppressants, natural coniferous resin lacquer, Ageratina pichinchensis extract, and ozonized sunflower oil, have shown antifungal activities in in vitro studies.
- Some of these products have efficacy and appear to be safe in clinical studies.
- Larger randomized clinical trials demonstrating efficacy are required before we can recommend these products to our patients.
2016 Update on bone health
Prioritize bone health, as osteoporotic fracture is a major source of morbidity and mortality among women. In this article: fracture risk with OC use in perimenopause, data that inform calcium’s role in cardiovascular disease, sarcopenia management, and an emerging treatment.
Most women’s health care providers are aware of recent changes and controversies regarding cervical cancer screening, mammography frequency, and whether a pelvic bimanual exam should be part of our annual well woman evaluation.1 However, I believe one of the most important things we as clinicians can do is be frontline in promoting bone health. Osteoporotic fracture is a major source of morbidity and mortality.2,3 Thus, promoting the maintenance of bone health is a priority in my own practice. It is also one of my many academic interests.
What follows is an update on bone health. In past years, this update has been entitled, “Update on osteoporosis,” but what we are trying to accomplish is fracture reduction. Thus, priorities for bone health consist of recognition of risk, lifestyle and dietary counseling, as well as the use of pharmacologic agents when appropriate. Certain research stands out as informative for your practice:
- a recent study on the risk of fracture with oral contraceptive (OC) use in perimenopause
- 3 just-published studies that inform our understanding of calcium’s role in cardiovascular health
- a review on sarcopenia management
- new data on romosozumab.
Oral contraceptive use in perimenopause
Scholes D, LaCroix AZ, Hubbard RA, et al. Oral contraceptive use and fracture risk around the menopausal transition. Menopause. 2016;23(2):166-174.
The use of OCs in women of older reproductive age has increased ever since the cutoff age of 35 years was eliminated.4 Lower doses have continued to be utilized in these "older" women with excellent control of irregular bleeding due to ovulatory dysfunction (and reduction in psychosocial symptoms as well).5
The effect of OC use on risk of fracture remains unclear, and use during later reproductive life may be increasing. To determine the association between OC use during later reproductive life and risk of fracture across the menopausal transition, Scholes and colleagues conducted a population-based case-controlled study in a Pacific Northwest HMO, Group Health Cooperative.
Details of the study
Scholes and colleagues enrolled 1,204 case women aged 45 to 59 years with incident fractures, and 2,275 control women. Potential cases with fracture codes in automated data were adjudicated by electronic health record review. Potential control women without fracture codes were selected concurrently, sampling based on age. Participants received a structured study interview. Using logistic regression, associations between OC use and fracture risk were calculated as odds ratios (ORs) and 95% confidence intervals (CIs).
Participation was 69% for cases and 64% for controls. The study sample was 82% white; mean age was 54 years. The most common fracture site for cases was the wrist/forearm (32%). Adjusted fracture risk did not differ between cases and controls for OC use:
- in the 10 years before menopause (OR, 0.90; 95% CI, 0.74-1.11)
- after age 38 years (OR, 0.94; 95% CI, 0.78-1.14)
- over the duration, or
- for other OC exposures.
Related article:
2016 Update on female sexual dysfunction
Association between fractures and OC use near menopause
The current study does not show an association between fractures near the menopausal transition and OC use in the decade before menopause or after age 38 years. For women considering OC use at these times, fracture risk does not seem to be either reduced or increased.
These results, looking at fracture, seem to be further supported by trials conducted by Gambacciani and colleagues,6 in which researchers randomly assigned irregularly cycling perimenopausal women (aged 40-49 years) to 20 μg ethinyl estradiol OCs or calcium/placebo. Results showed that this low-dose OC use significantly increased bone density at the femoral neck, spine, and other sites relative to control women after 24 months.
In the current Scholes study, the use of OCs in the decade before menopause or after age 38 did not reduce fracture risk in the years around the time of menopause. It is reassuring that their use was not associated with any increased fracture risk.
These findings provide additional clarity and guidance to women and their clinicians at a time of increasing public health concern about fractures. For women who may choose to use OCs during late premenopause (around age 38-48 years), fracture risk around the menopausal transition will not differ from women not choosing this option.
Calcium and calcium supplements: The data continue to grow
Anderson JJ, Kruszka B, Delaney JA, et al. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the Multi-Ethnic Study of Atherosclerosis (MESA) [published online ahead of print October 11, 2016]. J Am Heart Assoc. pii: e003815.
Billington EO, Bristow SM, Gamble GD, et al. Acute effects of calcium supplements on blood pressure: randomised, crossover trial in postmenopausal women [published online ahead of print August 20, 2016]. Osteoporos Int. doi:10.1007/s00198-016-3744-y.
Crandall CJ, Aragaki AK, LeBoff MS, et al. Calcium plus vitamin D supplementation and height loss: findings from the Women's Health Initiative Calcium and Vitamin D clinical trial [published online ahead of print August 1, 2016]. Menopause. doi:10.1097 /GME.0000000000000704.
In 2001, a National Institutes of Health (NIH) Consensus Development Panel on osteoporosis concluded that calcium intake is crucial to maintain bone mass and should be maintained at 1,000-1,500 mg/day in older adults. The panel acknowledged that the majority of older adults did not meet the recommended intake from dietary sources alone, and therefore would require calcium supplementation. Calcium supplements are one of the most commonly used dietary supplements, and population-based surveys have shown that they are used by the majority of older men and women in the United States.7
More recently results from large randomized controlled trials (RCTs) of calcium supplements have been reported, leading to concerns about calcium efficacy for fracture risk and safety. Bolland and colleagues8 reported that calcium supplements increased the rate of cardiovascular events in healthy older women and suggested that their role in osteoporosis management be reconsidered. More recently, the US Preventive Services Task Force recommended against calcium supplements for the primary prevention of fractures in noninstitutionalized postmenopausal women.9
The association between calcium intake and CVD events
Anderson and colleagues acknowledged that recent randomized data suggest that calcium supplements may be associated with increased risk of cardiovascular disease (CVD) events. Using a longitudinal cohort study, they assessed the association between calcium intake, from both foods and supplements, and atherosclerosis, as measured by coronary artery calcification (CAC).
Details of the study by Anderson and colleagues
The authors studied 5,448 adults free of clinically diagnosed CVD (52% female; age range, 45-84 years) from the Multi-Ethnic Study of Atherosclerosis. Baseline total calcium intake was assessed from diet (using a food frequency questionnaire) and calcium supplements (by a medication inventory) and categorized into quintiles based on overall population distribution. Baseline CAC was measured by computed tomography (CT) scan, and CAC measurements were repeated in 2,742 participants approximately 10 years later. Women had higher calcium intakes than men.
After adjustment for potential confounders, among 1,567 participants without baseline CAC, the relative risk (RR) of developing incident CAC over 10 years, by quintile 1 to 5 of calcium intake is included in the TABLE. After accounting for total calcium intake, calcium supplement use was associated with increased risk for incident CAC (RR, 1.22; 95% CI, 1.07-1.39). No relation was found between baseline calcium intake and 10-year changes in CAC among those participants with baseline CAC less than zero.
They concluded that high total calcium intake was associated with a decreased risk of incident atherosclerosis over long-term follow-up, particularly if achieved without supplement use. However, calcium supplement use may increase the risk for incident CAC.
Related article:
Does the discontinuation of menopausal hormone therapy affect a woman’s cardiovascular risk?
Calcium supplements and blood pressure
Billington and colleagues acknowledged that calcium supplements appear to increase cardiovascular risk but that the mechanism is unknown. They had previously reported that blood pressure declines over the course of the day in older women.10
Details of the study by Billington and colleagues
In this new study the investigators examined the acute effects of calcium supplements on blood pressure in a randomized controlled crossover trial in 40 healthy postmenopausal women (mean age, 71 years; body mass index [BMI], 27.2 kg/m2). Women attended on 2 occasions, with visits separated by 7 or more days. At each visit, they received either 1 g of calcium as citrate or placebo. Blood pressure and serum calcium concentrations were measured immediately before and 2, 4, and 6 hours after each intervention.
Ionized and total calcium concentrations increased after calcium (P<.0001 vs placebo). Systolic blood pressure (SBP) measurements decreased after both calcium and placebo but significantly less so after calcium (P=.02). The reduction in SBP from baseline was smaller after calcium compared with placebo by 6 mm Hg at 4 hours (P=.036) and by 9 mm Hg at 6 hours (P=.002). The reduction in diastolic blood pressure was similar after calcium and placebo.
These findings indicate that the use of calcium supplements in postmenopausal women attenuates the postbreakfast reduction in SBP by 6 to 9 mm Hg. Whether these changes in blood pressure influence cardiovascular risk requires further study.
Association between calcium, vitamin D, and height loss
Crandall and colleagues looked at the association between calcium and vitamin D supplementation and height loss in 36,282 participants of the Women's Health Initiative Calcium and Vitamin D trial.
Details of the study by Crandall and colleagues
The authors performed a post hoc analysis of data from a double-blind randomized controlled trial of 1,000 mg of elemental calcium as calcium carbonate with 400 IU of vitamin D3 daily (CaD) or placebo in postmenopausal women at 40 US clinical centers. Height was measured annually (mean follow-up, 5.9 years) with a stadiometer.
Average height loss was 1.28 mm/yr among participants assigned to CaD, versus 1.26 mm/yr for women assigned to placebo (P=.35). A strong association (P<.001) was observed between age group and height loss. The study authors concluded that, compared with placebo, calcium and vitamin D supplementation used in this trial did not prevent height loss in healthy postmenopausal women.
Adequate calcium is necessary for bone health. While calcium supplementation may not be adequate to prevent fractures, it is also not involved in the inevitable loss of overall height seen in postmenopausal women. Calcium supplementation has been implicated in an increase in CVD. These data seem to indicate that, while calcium supplementation results in higher systolic blood pressure during the day, as well as higher coronary artery calcium scores, greater dietary calcium actually may decrease the incidence of atherosclerosis.
Sarcopenia: Still important, clinical approaches to easily detect it
Beaudart C, McCloskey E, Bruyére O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16(1):170.
In last year's update, I reviewed the article by He and colleagues11 on the relationship between sarcopenia and body composition with osteoporosis. Sarcopenia, which is the age-related loss of muscle mass and strength, is important to address in patients. Body composition and muscle strength are directly correlated with bone density, and this is not surprising since bone and muscle share some common hormonal, genetic, nutritional, and lifestyle determinants.12,13 Sarcopenia can be diagnosed via dual-energy x-ray absorptiometry (DXA) scan looking at lean muscle mass.
The term sarcopenia was first coined by Rosenberg and colleagues in 198914 as a progressive loss of skeletal muscle mass with advancing age. Since then, the definition has expanded to incorporate the notion of impaired muscle strength or physical performance. Sarcopenia is associated with morbidity and mortality from linked physical disability, falls, fractures, poor quality of life, depression, and hospitalization.15
Current research is focusing on nutritional exercise/activity-based and other novel interventions for improving the quality and quantity of skeletal muscle in older people. Some studies demonstrated that resistance training combined with nutritional supplements can improve muscle function.16
Details of the study
Beaudart and colleagues propose some user-friendly and inexpensive methods that can be utilized to assess sarcopenia in real life settings. They acknowledge that in research settings or even specialist clinical settings, DXA or computed tomography (CT) scans are the best assessment of muscle mass.
Anthropometric measurements. In a primary care setting, anthropometric measurement, especially calf circumference and mid-upper arm muscle circumference, correlate with overall muscle mass and reflect both health and nutritional status and predict performance, health, and survival in older people.
However, with advancing age, changes in the distribution of fat and loss of skin elasticity are such that circumference incurs a loss of accuracy and precision in older people. Some studies suggest that an adjustment of anthropometric measurements for age, sex, or BMI results in a better correlation with DXA-measured lean mass.17 Anthropometric measurements are simple clinical prediction tools that can be easily applied for sarcopenia since they offer the most portable, commonly applicable, inexpensive, and noninvasive technique for assessing size, proportions, and composition of the human body. However, their validity is limited when applied to individuals because cutoff points to identify low muscle mass still need to be defined. Still, serial measurements in a patient over time may be valuable.
Related article:
2014 Update on osteoporosis
Handgrip strength, as measured with a dynamometer, appears to be the most widely used method for the measurement of muscle strength. In general, isometric handgrip strength shows a good correlation with leg strength and also with lower extremity power, and calf cross-sectional muscle area. The measurement is easy to perform, inexpensive and does not require a specialist-trained staff.
Standardized conditions for the test include seating the patient in a standard chair with her forearms resting flat on the chair arms. Clinicians should demonstrate the use of the dynamometer and show that gripping very tightly registers the best score. Six measurements should be taken, 3 with each arm. Ideally, patients should be encouraged to squeeze as hard and tightly as possible during 3 to 5 seconds for each of the 6 trials; usually the highest reading of the 6 measurements is reported as the final result. The Jamar dynamometer, or similar hydraulic dynamometer, is the gold standard for this measurement.
Gait speed measurement. The most widely used tool in clinical practice for the assessment of physical performance is the gait speed measurement. The test is highly acceptable for participants and health professionals in clinical settings. No special equipment is required; it needs only a flat floor devoid of obstacles. In the 4-meter gait speed test, men and women with a gait speed of less than 0.8 meters/sec are described as having a poor physical performance. The average extra time added to the consultation by measuring the 4-meter gait speed was only 95 seconds (SD, 20 seconds).
Loss of muscle mass correlates with loss of bone mass as our patients age. In addition, such sarcopenia increases the risk of falls, a significant component of the rising rate of fragility fractures. Anthropometric measures, grip strength, and gait speed are easy, low-cost measures that can identify patients at increased risk.
Romosozumab: An interesting new agent to look forward to
Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532-1543.
Romosozumab is a monoclonal antibody that binds sclerostin, increasing bone formation and decreasing bone resorption. Cosman and colleagues enrolled 7,180 postmenopausal women with a T score of -2.5 to -3.5 at the total hip or femoral neck. Participants were randomly assigned to receive subcutaneous injections of romosozumab 210 mg or placebo monthly for 12 months. Thereafter, women in each group received subcutaneous denosumab 60 mg for 12 months--administered every 6 months. The coprimary end points were the cumulative incidences of new vertebral fractures at 12 and 24 months. Secondary end points included clinical and nonvertebral fractures.
Details of the study
At 12 months, new vertebral fractures had occurred in 16 of 3,321 women (0.5%) in the romosozumab group, as compared with 59 of 3,322 (1.8%) in the placebo group (representing a 73% lower risk of fracture with romosozumab; P<.001). Clinical fractures had occurred in 58 of 3,589 women (1.6%) in the romosozumab group, as compared with 90 of 3,591 (2.5%) in the placebo group (a 36% lower fracture risk with romosozumab; P = .008). Nonvertebral fractures had occurred in 56 of 3,589 women (1.6%) in the romosozumab group and in 75 of 3,591 (2.1%) in the placebo group (P = .10).
At 24 months, the rates of vertebral fractures were significantly lower in the romosozumab group than in the placebo group after each group made the transition to denosumab (0.6% [21 of 3,325 women] in the romosozumab group vs 2.5% [84 of 3,327 women] in the placebo group, a 75% lower risk with romosozumab; P<.001). Adverse events, including cardiovascular events, osteoarthritis, and cancer, appeared to be balanced between the groups. One atypical femoral fracture and 2 cases of osteonecrosis of the jaw were observed in the romosozumab group.
Lower risk of fracture
Thus, in postmenopausal women with osteoporosis, romosozumab was associated with a lower risk of vertebral fracture than placebo at 12 months and, after the transition to denosumab, at 24 months. The lower risk of clinical fracture that was seen with romosozumab was evident at 1 year.
Of note, the effect of romosozumab on the risk of vertebral fracture was rapid, with only 2 additional vertebral fractures (of a total of 16 such fractures in the romosozumab group) occurring in the second 6 months of the first year of therapy. Because vertebral and clinical fractures are associated with increased morbidity and considerable health care costs, a treatment that would reduce this risk rapidly could offer appropriate patients an important benefit.
Romosozumab is a new agent. Though not yet available, it is extremely interesting because it not only decreases bone resorption but also increases bone formation. The results of this large prospective trial show that such an agent reduces both vertebral and clinical fracture and reduces that fracture risk quite rapidly within the first 6 months of therapy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- MacLaughlin KL, Faubion SS, Long ME, Pruthi S, Casey PM. Should the annual pelvic examination go the way of annual cervical cytology? Womens Health (Lond). 2014;10(4):373–384.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King AB, Tosterson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–475.
- Kaunitz AM. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262–1270.
- Kaunitz AM. Oral contraceptive use in perimenopause. Am J Obstet Gynecol. 2001;185(2 suppl):S32–S37.
- Gambacciani M, Cappagli B, Lazzarini V, Ciaponi M, Fruzzetti F, Genazzani AR. Longitudinal evaluation of perimenopausal bone loss: effects of different low dose oral contraceptive preparations on bone mineral density. Maturitas. 2006;54(2):176–180.
- Bailey R, Dodd K, Goldman J, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140(4):817–822.
- Bolland MJ, Grey A, Reid IR. Calcium supplements and cardiovascular risk: 5 years on. Ther Adv Drug Saf. 2013;4(5):199–210.
- Moyer VA; U.S. Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;158(9):691–696.
- Bristow SM, Gamble GD, Stewart A, Horne AM, Reid IR. Acute effects of calcium supplements on blood pressure and blood coagulation: secondary analysis of a randomised controlled trial in post-menopausal women. Br J Nutr. 2015;114(11):1868–1874.
- He H, Liu Y, Tian Q, Papasian CJ, Hu T, Deng HW. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int. 2016;27(2):473–482.
- Coin A, Perissinotto E, Enzi G, et al. Predictors of low bone mineral density in the elderly: the role of dietary intake, nutritional status and sarcopenia. Eur J Clin Nutr. 2008;62(6):802–809.
- Taaffe DR, Cauley JA, Danielson M, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2001;16(7):1343–1352.
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 suppl):990S–991S.
- Beaudart C, Rizzoli R, Bruyere O, Reginster JY, Biver E. Sarcopenia: Burden and challenges for Public Health. Arch Public Health. 2014;72(1):45.
- Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748–759.
- Kulkarni B, Kuper H, Taylor A, et al. Development and validation of anthropometric prediction equations for estimation of lean body mass and appendicular lean soft tissue in Indian men and women. J Appl Physiol. 2013;115(8):1156–1162.
Prioritize bone health, as osteoporotic fracture is a major source of morbidity and mortality among women. In this article: fracture risk with OC use in perimenopause, data that inform calcium’s role in cardiovascular disease, sarcopenia management, and an emerging treatment.
Most women’s health care providers are aware of recent changes and controversies regarding cervical cancer screening, mammography frequency, and whether a pelvic bimanual exam should be part of our annual well woman evaluation.1 However, I believe one of the most important things we as clinicians can do is be frontline in promoting bone health. Osteoporotic fracture is a major source of morbidity and mortality.2,3 Thus, promoting the maintenance of bone health is a priority in my own practice. It is also one of my many academic interests.
What follows is an update on bone health. In past years, this update has been entitled, “Update on osteoporosis,” but what we are trying to accomplish is fracture reduction. Thus, priorities for bone health consist of recognition of risk, lifestyle and dietary counseling, as well as the use of pharmacologic agents when appropriate. Certain research stands out as informative for your practice:
- a recent study on the risk of fracture with oral contraceptive (OC) use in perimenopause
- 3 just-published studies that inform our understanding of calcium’s role in cardiovascular health
- a review on sarcopenia management
- new data on romosozumab.
Oral contraceptive use in perimenopause
Scholes D, LaCroix AZ, Hubbard RA, et al. Oral contraceptive use and fracture risk around the menopausal transition. Menopause. 2016;23(2):166-174.
The use of OCs in women of older reproductive age has increased ever since the cutoff age of 35 years was eliminated.4 Lower doses have continued to be utilized in these "older" women with excellent control of irregular bleeding due to ovulatory dysfunction (and reduction in psychosocial symptoms as well).5
The effect of OC use on risk of fracture remains unclear, and use during later reproductive life may be increasing. To determine the association between OC use during later reproductive life and risk of fracture across the menopausal transition, Scholes and colleagues conducted a population-based case-controlled study in a Pacific Northwest HMO, Group Health Cooperative.
Details of the study
Scholes and colleagues enrolled 1,204 case women aged 45 to 59 years with incident fractures, and 2,275 control women. Potential cases with fracture codes in automated data were adjudicated by electronic health record review. Potential control women without fracture codes were selected concurrently, sampling based on age. Participants received a structured study interview. Using logistic regression, associations between OC use and fracture risk were calculated as odds ratios (ORs) and 95% confidence intervals (CIs).
Participation was 69% for cases and 64% for controls. The study sample was 82% white; mean age was 54 years. The most common fracture site for cases was the wrist/forearm (32%). Adjusted fracture risk did not differ between cases and controls for OC use:
- in the 10 years before menopause (OR, 0.90; 95% CI, 0.74-1.11)
- after age 38 years (OR, 0.94; 95% CI, 0.78-1.14)
- over the duration, or
- for other OC exposures.
Related article:
2016 Update on female sexual dysfunction
Association between fractures and OC use near menopause
The current study does not show an association between fractures near the menopausal transition and OC use in the decade before menopause or after age 38 years. For women considering OC use at these times, fracture risk does not seem to be either reduced or increased.
These results, looking at fracture, seem to be further supported by trials conducted by Gambacciani and colleagues,6 in which researchers randomly assigned irregularly cycling perimenopausal women (aged 40-49 years) to 20 μg ethinyl estradiol OCs or calcium/placebo. Results showed that this low-dose OC use significantly increased bone density at the femoral neck, spine, and other sites relative to control women after 24 months.
In the current Scholes study, the use of OCs in the decade before menopause or after age 38 did not reduce fracture risk in the years around the time of menopause. It is reassuring that their use was not associated with any increased fracture risk.
These findings provide additional clarity and guidance to women and their clinicians at a time of increasing public health concern about fractures. For women who may choose to use OCs during late premenopause (around age 38-48 years), fracture risk around the menopausal transition will not differ from women not choosing this option.
Calcium and calcium supplements: The data continue to grow
Anderson JJ, Kruszka B, Delaney JA, et al. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the Multi-Ethnic Study of Atherosclerosis (MESA) [published online ahead of print October 11, 2016]. J Am Heart Assoc. pii: e003815.
Billington EO, Bristow SM, Gamble GD, et al. Acute effects of calcium supplements on blood pressure: randomised, crossover trial in postmenopausal women [published online ahead of print August 20, 2016]. Osteoporos Int. doi:10.1007/s00198-016-3744-y.
Crandall CJ, Aragaki AK, LeBoff MS, et al. Calcium plus vitamin D supplementation and height loss: findings from the Women's Health Initiative Calcium and Vitamin D clinical trial [published online ahead of print August 1, 2016]. Menopause. doi:10.1097 /GME.0000000000000704.
In 2001, a National Institutes of Health (NIH) Consensus Development Panel on osteoporosis concluded that calcium intake is crucial to maintain bone mass and should be maintained at 1,000-1,500 mg/day in older adults. The panel acknowledged that the majority of older adults did not meet the recommended intake from dietary sources alone, and therefore would require calcium supplementation. Calcium supplements are one of the most commonly used dietary supplements, and population-based surveys have shown that they are used by the majority of older men and women in the United States.7
More recently results from large randomized controlled trials (RCTs) of calcium supplements have been reported, leading to concerns about calcium efficacy for fracture risk and safety. Bolland and colleagues8 reported that calcium supplements increased the rate of cardiovascular events in healthy older women and suggested that their role in osteoporosis management be reconsidered. More recently, the US Preventive Services Task Force recommended against calcium supplements for the primary prevention of fractures in noninstitutionalized postmenopausal women.9
The association between calcium intake and CVD events
Anderson and colleagues acknowledged that recent randomized data suggest that calcium supplements may be associated with increased risk of cardiovascular disease (CVD) events. Using a longitudinal cohort study, they assessed the association between calcium intake, from both foods and supplements, and atherosclerosis, as measured by coronary artery calcification (CAC).
Details of the study by Anderson and colleagues
The authors studied 5,448 adults free of clinically diagnosed CVD (52% female; age range, 45-84 years) from the Multi-Ethnic Study of Atherosclerosis. Baseline total calcium intake was assessed from diet (using a food frequency questionnaire) and calcium supplements (by a medication inventory) and categorized into quintiles based on overall population distribution. Baseline CAC was measured by computed tomography (CT) scan, and CAC measurements were repeated in 2,742 participants approximately 10 years later. Women had higher calcium intakes than men.
After adjustment for potential confounders, among 1,567 participants without baseline CAC, the relative risk (RR) of developing incident CAC over 10 years, by quintile 1 to 5 of calcium intake is included in the TABLE. After accounting for total calcium intake, calcium supplement use was associated with increased risk for incident CAC (RR, 1.22; 95% CI, 1.07-1.39). No relation was found between baseline calcium intake and 10-year changes in CAC among those participants with baseline CAC less than zero.
They concluded that high total calcium intake was associated with a decreased risk of incident atherosclerosis over long-term follow-up, particularly if achieved without supplement use. However, calcium supplement use may increase the risk for incident CAC.
Related article:
Does the discontinuation of menopausal hormone therapy affect a woman’s cardiovascular risk?
Calcium supplements and blood pressure
Billington and colleagues acknowledged that calcium supplements appear to increase cardiovascular risk but that the mechanism is unknown. They had previously reported that blood pressure declines over the course of the day in older women.10
Details of the study by Billington and colleagues
In this new study the investigators examined the acute effects of calcium supplements on blood pressure in a randomized controlled crossover trial in 40 healthy postmenopausal women (mean age, 71 years; body mass index [BMI], 27.2 kg/m2). Women attended on 2 occasions, with visits separated by 7 or more days. At each visit, they received either 1 g of calcium as citrate or placebo. Blood pressure and serum calcium concentrations were measured immediately before and 2, 4, and 6 hours after each intervention.
Ionized and total calcium concentrations increased after calcium (P<.0001 vs placebo). Systolic blood pressure (SBP) measurements decreased after both calcium and placebo but significantly less so after calcium (P=.02). The reduction in SBP from baseline was smaller after calcium compared with placebo by 6 mm Hg at 4 hours (P=.036) and by 9 mm Hg at 6 hours (P=.002). The reduction in diastolic blood pressure was similar after calcium and placebo.
These findings indicate that the use of calcium supplements in postmenopausal women attenuates the postbreakfast reduction in SBP by 6 to 9 mm Hg. Whether these changes in blood pressure influence cardiovascular risk requires further study.
Association between calcium, vitamin D, and height loss
Crandall and colleagues looked at the association between calcium and vitamin D supplementation and height loss in 36,282 participants of the Women's Health Initiative Calcium and Vitamin D trial.
Details of the study by Crandall and colleagues
The authors performed a post hoc analysis of data from a double-blind randomized controlled trial of 1,000 mg of elemental calcium as calcium carbonate with 400 IU of vitamin D3 daily (CaD) or placebo in postmenopausal women at 40 US clinical centers. Height was measured annually (mean follow-up, 5.9 years) with a stadiometer.
Average height loss was 1.28 mm/yr among participants assigned to CaD, versus 1.26 mm/yr for women assigned to placebo (P=.35). A strong association (P<.001) was observed between age group and height loss. The study authors concluded that, compared with placebo, calcium and vitamin D supplementation used in this trial did not prevent height loss in healthy postmenopausal women.
Adequate calcium is necessary for bone health. While calcium supplementation may not be adequate to prevent fractures, it is also not involved in the inevitable loss of overall height seen in postmenopausal women. Calcium supplementation has been implicated in an increase in CVD. These data seem to indicate that, while calcium supplementation results in higher systolic blood pressure during the day, as well as higher coronary artery calcium scores, greater dietary calcium actually may decrease the incidence of atherosclerosis.
Sarcopenia: Still important, clinical approaches to easily detect it
Beaudart C, McCloskey E, Bruyére O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16(1):170.
In last year's update, I reviewed the article by He and colleagues11 on the relationship between sarcopenia and body composition with osteoporosis. Sarcopenia, which is the age-related loss of muscle mass and strength, is important to address in patients. Body composition and muscle strength are directly correlated with bone density, and this is not surprising since bone and muscle share some common hormonal, genetic, nutritional, and lifestyle determinants.12,13 Sarcopenia can be diagnosed via dual-energy x-ray absorptiometry (DXA) scan looking at lean muscle mass.
The term sarcopenia was first coined by Rosenberg and colleagues in 198914 as a progressive loss of skeletal muscle mass with advancing age. Since then, the definition has expanded to incorporate the notion of impaired muscle strength or physical performance. Sarcopenia is associated with morbidity and mortality from linked physical disability, falls, fractures, poor quality of life, depression, and hospitalization.15
Current research is focusing on nutritional exercise/activity-based and other novel interventions for improving the quality and quantity of skeletal muscle in older people. Some studies demonstrated that resistance training combined with nutritional supplements can improve muscle function.16
Details of the study
Beaudart and colleagues propose some user-friendly and inexpensive methods that can be utilized to assess sarcopenia in real life settings. They acknowledge that in research settings or even specialist clinical settings, DXA or computed tomography (CT) scans are the best assessment of muscle mass.
Anthropometric measurements. In a primary care setting, anthropometric measurement, especially calf circumference and mid-upper arm muscle circumference, correlate with overall muscle mass and reflect both health and nutritional status and predict performance, health, and survival in older people.
However, with advancing age, changes in the distribution of fat and loss of skin elasticity are such that circumference incurs a loss of accuracy and precision in older people. Some studies suggest that an adjustment of anthropometric measurements for age, sex, or BMI results in a better correlation with DXA-measured lean mass.17 Anthropometric measurements are simple clinical prediction tools that can be easily applied for sarcopenia since they offer the most portable, commonly applicable, inexpensive, and noninvasive technique for assessing size, proportions, and composition of the human body. However, their validity is limited when applied to individuals because cutoff points to identify low muscle mass still need to be defined. Still, serial measurements in a patient over time may be valuable.
Related article:
2014 Update on osteoporosis
Handgrip strength, as measured with a dynamometer, appears to be the most widely used method for the measurement of muscle strength. In general, isometric handgrip strength shows a good correlation with leg strength and also with lower extremity power, and calf cross-sectional muscle area. The measurement is easy to perform, inexpensive and does not require a specialist-trained staff.
Standardized conditions for the test include seating the patient in a standard chair with her forearms resting flat on the chair arms. Clinicians should demonstrate the use of the dynamometer and show that gripping very tightly registers the best score. Six measurements should be taken, 3 with each arm. Ideally, patients should be encouraged to squeeze as hard and tightly as possible during 3 to 5 seconds for each of the 6 trials; usually the highest reading of the 6 measurements is reported as the final result. The Jamar dynamometer, or similar hydraulic dynamometer, is the gold standard for this measurement.
Gait speed measurement. The most widely used tool in clinical practice for the assessment of physical performance is the gait speed measurement. The test is highly acceptable for participants and health professionals in clinical settings. No special equipment is required; it needs only a flat floor devoid of obstacles. In the 4-meter gait speed test, men and women with a gait speed of less than 0.8 meters/sec are described as having a poor physical performance. The average extra time added to the consultation by measuring the 4-meter gait speed was only 95 seconds (SD, 20 seconds).
Loss of muscle mass correlates with loss of bone mass as our patients age. In addition, such sarcopenia increases the risk of falls, a significant component of the rising rate of fragility fractures. Anthropometric measures, grip strength, and gait speed are easy, low-cost measures that can identify patients at increased risk.
Romosozumab: An interesting new agent to look forward to
Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532-1543.
Romosozumab is a monoclonal antibody that binds sclerostin, increasing bone formation and decreasing bone resorption. Cosman and colleagues enrolled 7,180 postmenopausal women with a T score of -2.5 to -3.5 at the total hip or femoral neck. Participants were randomly assigned to receive subcutaneous injections of romosozumab 210 mg or placebo monthly for 12 months. Thereafter, women in each group received subcutaneous denosumab 60 mg for 12 months--administered every 6 months. The coprimary end points were the cumulative incidences of new vertebral fractures at 12 and 24 months. Secondary end points included clinical and nonvertebral fractures.
Details of the study
At 12 months, new vertebral fractures had occurred in 16 of 3,321 women (0.5%) in the romosozumab group, as compared with 59 of 3,322 (1.8%) in the placebo group (representing a 73% lower risk of fracture with romosozumab; P<.001). Clinical fractures had occurred in 58 of 3,589 women (1.6%) in the romosozumab group, as compared with 90 of 3,591 (2.5%) in the placebo group (a 36% lower fracture risk with romosozumab; P = .008). Nonvertebral fractures had occurred in 56 of 3,589 women (1.6%) in the romosozumab group and in 75 of 3,591 (2.1%) in the placebo group (P = .10).
At 24 months, the rates of vertebral fractures were significantly lower in the romosozumab group than in the placebo group after each group made the transition to denosumab (0.6% [21 of 3,325 women] in the romosozumab group vs 2.5% [84 of 3,327 women] in the placebo group, a 75% lower risk with romosozumab; P<.001). Adverse events, including cardiovascular events, osteoarthritis, and cancer, appeared to be balanced between the groups. One atypical femoral fracture and 2 cases of osteonecrosis of the jaw were observed in the romosozumab group.
Lower risk of fracture
Thus, in postmenopausal women with osteoporosis, romosozumab was associated with a lower risk of vertebral fracture than placebo at 12 months and, after the transition to denosumab, at 24 months. The lower risk of clinical fracture that was seen with romosozumab was evident at 1 year.
Of note, the effect of romosozumab on the risk of vertebral fracture was rapid, with only 2 additional vertebral fractures (of a total of 16 such fractures in the romosozumab group) occurring in the second 6 months of the first year of therapy. Because vertebral and clinical fractures are associated with increased morbidity and considerable health care costs, a treatment that would reduce this risk rapidly could offer appropriate patients an important benefit.
Romosozumab is a new agent. Though not yet available, it is extremely interesting because it not only decreases bone resorption but also increases bone formation. The results of this large prospective trial show that such an agent reduces both vertebral and clinical fracture and reduces that fracture risk quite rapidly within the first 6 months of therapy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Prioritize bone health, as osteoporotic fracture is a major source of morbidity and mortality among women. In this article: fracture risk with OC use in perimenopause, data that inform calcium’s role in cardiovascular disease, sarcopenia management, and an emerging treatment.
Most women’s health care providers are aware of recent changes and controversies regarding cervical cancer screening, mammography frequency, and whether a pelvic bimanual exam should be part of our annual well woman evaluation.1 However, I believe one of the most important things we as clinicians can do is be frontline in promoting bone health. Osteoporotic fracture is a major source of morbidity and mortality.2,3 Thus, promoting the maintenance of bone health is a priority in my own practice. It is also one of my many academic interests.
What follows is an update on bone health. In past years, this update has been entitled, “Update on osteoporosis,” but what we are trying to accomplish is fracture reduction. Thus, priorities for bone health consist of recognition of risk, lifestyle and dietary counseling, as well as the use of pharmacologic agents when appropriate. Certain research stands out as informative for your practice:
- a recent study on the risk of fracture with oral contraceptive (OC) use in perimenopause
- 3 just-published studies that inform our understanding of calcium’s role in cardiovascular health
- a review on sarcopenia management
- new data on romosozumab.
Oral contraceptive use in perimenopause
Scholes D, LaCroix AZ, Hubbard RA, et al. Oral contraceptive use and fracture risk around the menopausal transition. Menopause. 2016;23(2):166-174.
The use of OCs in women of older reproductive age has increased ever since the cutoff age of 35 years was eliminated.4 Lower doses have continued to be utilized in these "older" women with excellent control of irregular bleeding due to ovulatory dysfunction (and reduction in psychosocial symptoms as well).5
The effect of OC use on risk of fracture remains unclear, and use during later reproductive life may be increasing. To determine the association between OC use during later reproductive life and risk of fracture across the menopausal transition, Scholes and colleagues conducted a population-based case-controlled study in a Pacific Northwest HMO, Group Health Cooperative.
Details of the study
Scholes and colleagues enrolled 1,204 case women aged 45 to 59 years with incident fractures, and 2,275 control women. Potential cases with fracture codes in automated data were adjudicated by electronic health record review. Potential control women without fracture codes were selected concurrently, sampling based on age. Participants received a structured study interview. Using logistic regression, associations between OC use and fracture risk were calculated as odds ratios (ORs) and 95% confidence intervals (CIs).
Participation was 69% for cases and 64% for controls. The study sample was 82% white; mean age was 54 years. The most common fracture site for cases was the wrist/forearm (32%). Adjusted fracture risk did not differ between cases and controls for OC use:
- in the 10 years before menopause (OR, 0.90; 95% CI, 0.74-1.11)
- after age 38 years (OR, 0.94; 95% CI, 0.78-1.14)
- over the duration, or
- for other OC exposures.
Related article:
2016 Update on female sexual dysfunction
Association between fractures and OC use near menopause
The current study does not show an association between fractures near the menopausal transition and OC use in the decade before menopause or after age 38 years. For women considering OC use at these times, fracture risk does not seem to be either reduced or increased.
These results, looking at fracture, seem to be further supported by trials conducted by Gambacciani and colleagues,6 in which researchers randomly assigned irregularly cycling perimenopausal women (aged 40-49 years) to 20 μg ethinyl estradiol OCs or calcium/placebo. Results showed that this low-dose OC use significantly increased bone density at the femoral neck, spine, and other sites relative to control women after 24 months.
In the current Scholes study, the use of OCs in the decade before menopause or after age 38 did not reduce fracture risk in the years around the time of menopause. It is reassuring that their use was not associated with any increased fracture risk.
These findings provide additional clarity and guidance to women and their clinicians at a time of increasing public health concern about fractures. For women who may choose to use OCs during late premenopause (around age 38-48 years), fracture risk around the menopausal transition will not differ from women not choosing this option.
Calcium and calcium supplements: The data continue to grow
Anderson JJ, Kruszka B, Delaney JA, et al. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the Multi-Ethnic Study of Atherosclerosis (MESA) [published online ahead of print October 11, 2016]. J Am Heart Assoc. pii: e003815.
Billington EO, Bristow SM, Gamble GD, et al. Acute effects of calcium supplements on blood pressure: randomised, crossover trial in postmenopausal women [published online ahead of print August 20, 2016]. Osteoporos Int. doi:10.1007/s00198-016-3744-y.
Crandall CJ, Aragaki AK, LeBoff MS, et al. Calcium plus vitamin D supplementation and height loss: findings from the Women's Health Initiative Calcium and Vitamin D clinical trial [published online ahead of print August 1, 2016]. Menopause. doi:10.1097 /GME.0000000000000704.
In 2001, a National Institutes of Health (NIH) Consensus Development Panel on osteoporosis concluded that calcium intake is crucial to maintain bone mass and should be maintained at 1,000-1,500 mg/day in older adults. The panel acknowledged that the majority of older adults did not meet the recommended intake from dietary sources alone, and therefore would require calcium supplementation. Calcium supplements are one of the most commonly used dietary supplements, and population-based surveys have shown that they are used by the majority of older men and women in the United States.7
More recently results from large randomized controlled trials (RCTs) of calcium supplements have been reported, leading to concerns about calcium efficacy for fracture risk and safety. Bolland and colleagues8 reported that calcium supplements increased the rate of cardiovascular events in healthy older women and suggested that their role in osteoporosis management be reconsidered. More recently, the US Preventive Services Task Force recommended against calcium supplements for the primary prevention of fractures in noninstitutionalized postmenopausal women.9
The association between calcium intake and CVD events
Anderson and colleagues acknowledged that recent randomized data suggest that calcium supplements may be associated with increased risk of cardiovascular disease (CVD) events. Using a longitudinal cohort study, they assessed the association between calcium intake, from both foods and supplements, and atherosclerosis, as measured by coronary artery calcification (CAC).
Details of the study by Anderson and colleagues
The authors studied 5,448 adults free of clinically diagnosed CVD (52% female; age range, 45-84 years) from the Multi-Ethnic Study of Atherosclerosis. Baseline total calcium intake was assessed from diet (using a food frequency questionnaire) and calcium supplements (by a medication inventory) and categorized into quintiles based on overall population distribution. Baseline CAC was measured by computed tomography (CT) scan, and CAC measurements were repeated in 2,742 participants approximately 10 years later. Women had higher calcium intakes than men.
After adjustment for potential confounders, among 1,567 participants without baseline CAC, the relative risk (RR) of developing incident CAC over 10 years, by quintile 1 to 5 of calcium intake is included in the TABLE. After accounting for total calcium intake, calcium supplement use was associated with increased risk for incident CAC (RR, 1.22; 95% CI, 1.07-1.39). No relation was found between baseline calcium intake and 10-year changes in CAC among those participants with baseline CAC less than zero.
They concluded that high total calcium intake was associated with a decreased risk of incident atherosclerosis over long-term follow-up, particularly if achieved without supplement use. However, calcium supplement use may increase the risk for incident CAC.
Related article:
Does the discontinuation of menopausal hormone therapy affect a woman’s cardiovascular risk?
Calcium supplements and blood pressure
Billington and colleagues acknowledged that calcium supplements appear to increase cardiovascular risk but that the mechanism is unknown. They had previously reported that blood pressure declines over the course of the day in older women.10
Details of the study by Billington and colleagues
In this new study the investigators examined the acute effects of calcium supplements on blood pressure in a randomized controlled crossover trial in 40 healthy postmenopausal women (mean age, 71 years; body mass index [BMI], 27.2 kg/m2). Women attended on 2 occasions, with visits separated by 7 or more days. At each visit, they received either 1 g of calcium as citrate or placebo. Blood pressure and serum calcium concentrations were measured immediately before and 2, 4, and 6 hours after each intervention.
Ionized and total calcium concentrations increased after calcium (P<.0001 vs placebo). Systolic blood pressure (SBP) measurements decreased after both calcium and placebo but significantly less so after calcium (P=.02). The reduction in SBP from baseline was smaller after calcium compared with placebo by 6 mm Hg at 4 hours (P=.036) and by 9 mm Hg at 6 hours (P=.002). The reduction in diastolic blood pressure was similar after calcium and placebo.
These findings indicate that the use of calcium supplements in postmenopausal women attenuates the postbreakfast reduction in SBP by 6 to 9 mm Hg. Whether these changes in blood pressure influence cardiovascular risk requires further study.
Association between calcium, vitamin D, and height loss
Crandall and colleagues looked at the association between calcium and vitamin D supplementation and height loss in 36,282 participants of the Women's Health Initiative Calcium and Vitamin D trial.
Details of the study by Crandall and colleagues
The authors performed a post hoc analysis of data from a double-blind randomized controlled trial of 1,000 mg of elemental calcium as calcium carbonate with 400 IU of vitamin D3 daily (CaD) or placebo in postmenopausal women at 40 US clinical centers. Height was measured annually (mean follow-up, 5.9 years) with a stadiometer.
Average height loss was 1.28 mm/yr among participants assigned to CaD, versus 1.26 mm/yr for women assigned to placebo (P=.35). A strong association (P<.001) was observed between age group and height loss. The study authors concluded that, compared with placebo, calcium and vitamin D supplementation used in this trial did not prevent height loss in healthy postmenopausal women.
Adequate calcium is necessary for bone health. While calcium supplementation may not be adequate to prevent fractures, it is also not involved in the inevitable loss of overall height seen in postmenopausal women. Calcium supplementation has been implicated in an increase in CVD. These data seem to indicate that, while calcium supplementation results in higher systolic blood pressure during the day, as well as higher coronary artery calcium scores, greater dietary calcium actually may decrease the incidence of atherosclerosis.
Sarcopenia: Still important, clinical approaches to easily detect it
Beaudart C, McCloskey E, Bruyére O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16(1):170.
In last year's update, I reviewed the article by He and colleagues11 on the relationship between sarcopenia and body composition with osteoporosis. Sarcopenia, which is the age-related loss of muscle mass and strength, is important to address in patients. Body composition and muscle strength are directly correlated with bone density, and this is not surprising since bone and muscle share some common hormonal, genetic, nutritional, and lifestyle determinants.12,13 Sarcopenia can be diagnosed via dual-energy x-ray absorptiometry (DXA) scan looking at lean muscle mass.
The term sarcopenia was first coined by Rosenberg and colleagues in 198914 as a progressive loss of skeletal muscle mass with advancing age. Since then, the definition has expanded to incorporate the notion of impaired muscle strength or physical performance. Sarcopenia is associated with morbidity and mortality from linked physical disability, falls, fractures, poor quality of life, depression, and hospitalization.15
Current research is focusing on nutritional exercise/activity-based and other novel interventions for improving the quality and quantity of skeletal muscle in older people. Some studies demonstrated that resistance training combined with nutritional supplements can improve muscle function.16
Details of the study
Beaudart and colleagues propose some user-friendly and inexpensive methods that can be utilized to assess sarcopenia in real life settings. They acknowledge that in research settings or even specialist clinical settings, DXA or computed tomography (CT) scans are the best assessment of muscle mass.
Anthropometric measurements. In a primary care setting, anthropometric measurement, especially calf circumference and mid-upper arm muscle circumference, correlate with overall muscle mass and reflect both health and nutritional status and predict performance, health, and survival in older people.
However, with advancing age, changes in the distribution of fat and loss of skin elasticity are such that circumference incurs a loss of accuracy and precision in older people. Some studies suggest that an adjustment of anthropometric measurements for age, sex, or BMI results in a better correlation with DXA-measured lean mass.17 Anthropometric measurements are simple clinical prediction tools that can be easily applied for sarcopenia since they offer the most portable, commonly applicable, inexpensive, and noninvasive technique for assessing size, proportions, and composition of the human body. However, their validity is limited when applied to individuals because cutoff points to identify low muscle mass still need to be defined. Still, serial measurements in a patient over time may be valuable.
Related article:
2014 Update on osteoporosis
Handgrip strength, as measured with a dynamometer, appears to be the most widely used method for the measurement of muscle strength. In general, isometric handgrip strength shows a good correlation with leg strength and also with lower extremity power, and calf cross-sectional muscle area. The measurement is easy to perform, inexpensive and does not require a specialist-trained staff.
Standardized conditions for the test include seating the patient in a standard chair with her forearms resting flat on the chair arms. Clinicians should demonstrate the use of the dynamometer and show that gripping very tightly registers the best score. Six measurements should be taken, 3 with each arm. Ideally, patients should be encouraged to squeeze as hard and tightly as possible during 3 to 5 seconds for each of the 6 trials; usually the highest reading of the 6 measurements is reported as the final result. The Jamar dynamometer, or similar hydraulic dynamometer, is the gold standard for this measurement.
Gait speed measurement. The most widely used tool in clinical practice for the assessment of physical performance is the gait speed measurement. The test is highly acceptable for participants and health professionals in clinical settings. No special equipment is required; it needs only a flat floor devoid of obstacles. In the 4-meter gait speed test, men and women with a gait speed of less than 0.8 meters/sec are described as having a poor physical performance. The average extra time added to the consultation by measuring the 4-meter gait speed was only 95 seconds (SD, 20 seconds).
Loss of muscle mass correlates with loss of bone mass as our patients age. In addition, such sarcopenia increases the risk of falls, a significant component of the rising rate of fragility fractures. Anthropometric measures, grip strength, and gait speed are easy, low-cost measures that can identify patients at increased risk.
Romosozumab: An interesting new agent to look forward to
Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532-1543.
Romosozumab is a monoclonal antibody that binds sclerostin, increasing bone formation and decreasing bone resorption. Cosman and colleagues enrolled 7,180 postmenopausal women with a T score of -2.5 to -3.5 at the total hip or femoral neck. Participants were randomly assigned to receive subcutaneous injections of romosozumab 210 mg or placebo monthly for 12 months. Thereafter, women in each group received subcutaneous denosumab 60 mg for 12 months--administered every 6 months. The coprimary end points were the cumulative incidences of new vertebral fractures at 12 and 24 months. Secondary end points included clinical and nonvertebral fractures.
Details of the study
At 12 months, new vertebral fractures had occurred in 16 of 3,321 women (0.5%) in the romosozumab group, as compared with 59 of 3,322 (1.8%) in the placebo group (representing a 73% lower risk of fracture with romosozumab; P<.001). Clinical fractures had occurred in 58 of 3,589 women (1.6%) in the romosozumab group, as compared with 90 of 3,591 (2.5%) in the placebo group (a 36% lower fracture risk with romosozumab; P = .008). Nonvertebral fractures had occurred in 56 of 3,589 women (1.6%) in the romosozumab group and in 75 of 3,591 (2.1%) in the placebo group (P = .10).
At 24 months, the rates of vertebral fractures were significantly lower in the romosozumab group than in the placebo group after each group made the transition to denosumab (0.6% [21 of 3,325 women] in the romosozumab group vs 2.5% [84 of 3,327 women] in the placebo group, a 75% lower risk with romosozumab; P<.001). Adverse events, including cardiovascular events, osteoarthritis, and cancer, appeared to be balanced between the groups. One atypical femoral fracture and 2 cases of osteonecrosis of the jaw were observed in the romosozumab group.
Lower risk of fracture
Thus, in postmenopausal women with osteoporosis, romosozumab was associated with a lower risk of vertebral fracture than placebo at 12 months and, after the transition to denosumab, at 24 months. The lower risk of clinical fracture that was seen with romosozumab was evident at 1 year.
Of note, the effect of romosozumab on the risk of vertebral fracture was rapid, with only 2 additional vertebral fractures (of a total of 16 such fractures in the romosozumab group) occurring in the second 6 months of the first year of therapy. Because vertebral and clinical fractures are associated with increased morbidity and considerable health care costs, a treatment that would reduce this risk rapidly could offer appropriate patients an important benefit.
Romosozumab is a new agent. Though not yet available, it is extremely interesting because it not only decreases bone resorption but also increases bone formation. The results of this large prospective trial show that such an agent reduces both vertebral and clinical fracture and reduces that fracture risk quite rapidly within the first 6 months of therapy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- MacLaughlin KL, Faubion SS, Long ME, Pruthi S, Casey PM. Should the annual pelvic examination go the way of annual cervical cytology? Womens Health (Lond). 2014;10(4):373–384.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King AB, Tosterson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–475.
- Kaunitz AM. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262–1270.
- Kaunitz AM. Oral contraceptive use in perimenopause. Am J Obstet Gynecol. 2001;185(2 suppl):S32–S37.
- Gambacciani M, Cappagli B, Lazzarini V, Ciaponi M, Fruzzetti F, Genazzani AR. Longitudinal evaluation of perimenopausal bone loss: effects of different low dose oral contraceptive preparations on bone mineral density. Maturitas. 2006;54(2):176–180.
- Bailey R, Dodd K, Goldman J, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140(4):817–822.
- Bolland MJ, Grey A, Reid IR. Calcium supplements and cardiovascular risk: 5 years on. Ther Adv Drug Saf. 2013;4(5):199–210.
- Moyer VA; U.S. Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;158(9):691–696.
- Bristow SM, Gamble GD, Stewart A, Horne AM, Reid IR. Acute effects of calcium supplements on blood pressure and blood coagulation: secondary analysis of a randomised controlled trial in post-menopausal women. Br J Nutr. 2015;114(11):1868–1874.
- He H, Liu Y, Tian Q, Papasian CJ, Hu T, Deng HW. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int. 2016;27(2):473–482.
- Coin A, Perissinotto E, Enzi G, et al. Predictors of low bone mineral density in the elderly: the role of dietary intake, nutritional status and sarcopenia. Eur J Clin Nutr. 2008;62(6):802–809.
- Taaffe DR, Cauley JA, Danielson M, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2001;16(7):1343–1352.
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 suppl):990S–991S.
- Beaudart C, Rizzoli R, Bruyere O, Reginster JY, Biver E. Sarcopenia: Burden and challenges for Public Health. Arch Public Health. 2014;72(1):45.
- Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748–759.
- Kulkarni B, Kuper H, Taylor A, et al. Development and validation of anthropometric prediction equations for estimation of lean body mass and appendicular lean soft tissue in Indian men and women. J Appl Physiol. 2013;115(8):1156–1162.
- MacLaughlin KL, Faubion SS, Long ME, Pruthi S, Casey PM. Should the annual pelvic examination go the way of annual cervical cytology? Womens Health (Lond). 2014;10(4):373–384.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King AB, Tosterson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–475.
- Kaunitz AM. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262–1270.
- Kaunitz AM. Oral contraceptive use in perimenopause. Am J Obstet Gynecol. 2001;185(2 suppl):S32–S37.
- Gambacciani M, Cappagli B, Lazzarini V, Ciaponi M, Fruzzetti F, Genazzani AR. Longitudinal evaluation of perimenopausal bone loss: effects of different low dose oral contraceptive preparations on bone mineral density. Maturitas. 2006;54(2):176–180.
- Bailey R, Dodd K, Goldman J, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140(4):817–822.
- Bolland MJ, Grey A, Reid IR. Calcium supplements and cardiovascular risk: 5 years on. Ther Adv Drug Saf. 2013;4(5):199–210.
- Moyer VA; U.S. Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;158(9):691–696.
- Bristow SM, Gamble GD, Stewart A, Horne AM, Reid IR. Acute effects of calcium supplements on blood pressure and blood coagulation: secondary analysis of a randomised controlled trial in post-menopausal women. Br J Nutr. 2015;114(11):1868–1874.
- He H, Liu Y, Tian Q, Papasian CJ, Hu T, Deng HW. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int. 2016;27(2):473–482.
- Coin A, Perissinotto E, Enzi G, et al. Predictors of low bone mineral density in the elderly: the role of dietary intake, nutritional status and sarcopenia. Eur J Clin Nutr. 2008;62(6):802–809.
- Taaffe DR, Cauley JA, Danielson M, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2001;16(7):1343–1352.
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 suppl):990S–991S.
- Beaudart C, Rizzoli R, Bruyere O, Reginster JY, Biver E. Sarcopenia: Burden and challenges for Public Health. Arch Public Health. 2014;72(1):45.
- Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748–759.
- Kulkarni B, Kuper H, Taylor A, et al. Development and validation of anthropometric prediction equations for estimation of lean body mass and appendicular lean soft tissue in Indian men and women. J Appl Physiol. 2013;115(8):1156–1162.
Preventing infection after cesarean delivery: 5 more evidence-based measures to consider
In part 1 of our review on preventing postcesarean infection, we critically evaluated methods of skin preparation and administration of prophylactic antibiotics. In part 2, we address preoperative cleansing of the vagina with an antiseptic solution, preoperative bathing with an antiseptic solution, methods of placental extraction, closure of the deep subcutaneous layer of the abdomen, and closure of the skin.
Related article:
Preventing infection after cesarean delivery: Evidence-based guidance
CASE: Should vaginal cleansing be performed prior to cesarean delivery?
An 18-year-old primigravid woman at 41 weeks’ gestation has been in labor for 16 hours, and now has an arrest of descent at 0 station. An intrauterine pressure catheter and scalp electrode have been in place for the same length of time. The patient has had 9 internal examinations during the period of membrane rupture. As you are preparing to scrub the patient’s abdomen, the third-year medical student asks, “When I was on the Gynecology Service, I saw the doctors wash the vagina with an antiseptic solution before they performed a vaginal hysterectomy. Should we also do that before we operate on this patient?”
Preoperative vaginal cleansing
A preoperative antiseptic vaginal scrub is often used as an additional step to help reduce postcesarean infection.
Does cleansing the vagina with povidone-iodine before surgery further reduce the risk of endometritis and wound infection?
Multiple studies have sought to determine if cleansing the vagina with an antiseptic solution further reduces the incidence of postcesarean infection beyond what can be achieved with systemic antibiotic prophylaxis. These studies typically have focused on 3 specific outcomes: endometritis, wound (surgical site) infection, and febrile morbidity. The term febrile morbidity is defined as a temperature ≥100.4°F (38°C) on any 2 postoperative days excluding the first 24 hours. However, many patients who meet the standard definition of febrile morbidity may not have a proven infection and will not require treatment with antibiotics. The more precise measures of outcome are distinctly symptomatic infections, such as endometritis and wound infection, although, as noted in the review of published studies below, some authors continue to use the term febrile morbidity as one measure of postoperative complications.
In a randomized, placebo-controlled trial (RCT) of 308 women having a nonemergent cesarean delivery, Starr and colleagues reported a decreased incidence of postoperative endometritis in women who received a 30-second vaginal scrub with povidone-iodine compared with women who received only an abdominal scrub (7.0% vs 14.5%, P<.05).1 The groups did not differ in the frequency of wound infection (0.7% vs 1.2%, P = .4) or febrile morbidity (23.9% vs 28.3%, P = .4).1
In another RCT, Haas and colleagues found that preoperative vaginal cleansing with povidone-iodine compared with an abdominal scrub alone was associated with a decreased incidence of a composite measure of postoperative morbidity (6.5% vs 11.7%; relative risk [RR], 0.55; 95% confidence interval [CI], 0.26–1.11; P = .11).2 The postoperative composite included fever, endometritis, sepsis, readmission, and wound infection.
Subsequently, Asghania and associates conducted a double-blind, nonrandomized study of 568 women having cesarean delivery who received an abdominal scrub plus a 30-second vaginal scrub with povidone-iodine or received an abdominal scrub alone.3 They documented a decreased incidence of postoperative endometritis in the women who received the combined scrub (1.4% vs 2.5%; P = .03, adjusted odds ratio [AOR], 0.03; 95% CI, 0.008–0.7). The authors observed no significant difference in febrile morbidity (4.9% vs 6.0%; P = .73) or wound infection (3.5% vs 3.2%; P = .5).3
Yildirim and colleagues conducted an RCT comparing rates of infection in 334 women who received an abdominal scrub plus vaginal cleansing with povidone-iodine and 336 patients who had only a standard abdominal scrub.4 They documented a decreased incidence of endometritis in women who received the vaginal scrub (6.9% vs 11.6%; P = .04; RR for infection in the control group, 1.69; 95% CI, 1.03–2.76.) The authors found no difference in febrile morbidity (16.5% vs 18.2%; P = .61) or wound infection (1.8% vs 2.7%; P = .60). Of note, in excluding from the analysis women who had ruptured membranes or who were in labor, the investigators found no differences in outcome, indicating that the greatest impact of vaginal cleansing was in the highest risk patients.
In 2014, Haas and associates published a Cochrane review evaluating the effectiveness of preoperative vaginal cleansing with povidone-iodine.5 The authors reviewed 7 studies that analyzed outcomes in 2,635 women. They concluded that vaginal preparation with povidone-iodine at the time of cesarean delivery significantly decreased postoperative endometritis when compared with the control group (4.3% vs 8.3%; RR, 0.45; 95% CI, 0.25–0.81). They also noted that the most profound impact of vaginal cleansing was in women who were in labor before delivery (7.4% vs 13.0%; RR, 0.56; 95% CI, 0.34–0.95) and in women with ruptured membranes at the time of delivery (4.3% vs 17.9%; RR, 0.24; 95% CI, 0.10–0.55). The authors did not find a significant difference in postoperative wound infection or frequency of fever in women who received the vaginal scrub.
Related article:
STOP using instruments to assist with delivery of the head at cesarean
A notable exception to the beneficial outcomes reported above was the study by Reid et al.6 These authors randomly assigned 247 women having cesarean delivery to an abdominal scrub plus vaginal scrub with povidone-iodine and assigned 251 women to only an abdominal scrub. The authors were unable to document any significant difference between the groups with respect to frequency of fever, endometritis, and wound infection.
Other methods of vaginal preparation also have been studied. For example, Pitt and colleagues conducted a double-blind RCT of 224 women having cesarean delivery and compared preoperative metronidazole vaginal gel with placebo.7 Most of the patients in this trial also received systemic antibiotic prophylaxis after the umbilical cord was clamped. The authors demonstrated a decreased incidence of postcesarean endometritis in women who received the intravaginal antibiotic gel (7% vs 17%; RR, 0.42; 95% CI, 0.19–0.92). There was no difference in febrile morbidity (13% vs 19%; P = .28) or wound infection (4% vs 3%, P = .50).
What the evidence says
Consider vaginal preparation with povidone-iodine at the time of cesarean delivery to reduce the risk of postpartum endometritis. Do not expect this intervention to significantly reduce the frequency of wound infection. Vaginal cleansing is of most benefit to women who have ruptured membranes or are in labor at the time of delivery (Level I Evidence, Level A Recommendation; TABLE). Whether vaginal preparation with chlorhexidine with 4% alcohol would have the same beneficial effect has not been studied in a systematic manner.
Placenta extraction, closure techniques
Evidence suggests that employing certain intraoperative approaches helps reduce the incidence of postcesarean infection.
What other measures help prevent infection following cesarean surgery?
One other measure known to decrease the risk of postcesarean endometritis is removing the placenta by exerting traction on the umbilical cord rather than extracting it manually. In one of the first descriptions of this intervention, Lasley and associates showed that, in high-risk patients who also received intravenous antibiotic prophylaxis after cord clamping, the rate of postoperative endometritis was 15% in the group that had spontaneous delivery of the placenta compared with 27% in women who had manual extraction (RR, 0.6; 95% CI, 0.3–0.9; P = .02).8 A recent Cochrane review that included multiple subsequent reports confirmed this observation (Level I Evidence, Level A Recommendation; TABLE, page 2).9
Abdominal wall closure. Two other interventions are valuable in decreasing the frequency of deep and superficial wound infection. In patients whose subcutaneous layer is >2 cm thick, closure of the deep subcutaneous tissue significantly reduces the risk of wound seroma, hematoma, and infection.10 In addition, closure of the skin edges with a subcuticular suture, as opposed to surgical staples, significantly reduces the frequency of superficial wound complications (Level I Evidence, Level A Recommendation; TABLE, page 2).11 Poliglecaprone 25, polyglactin 910, and polyglycolic acid suture, 3-0 or 4-0 gauge, are excellent suture choices for this closure.
Related article:
Does one particular cesarean technique confer better maternal and neonatal outcomes?
CASE
Planned cesarean delivery: Is preoperative antiseptic bathing warranted?
A 33-year-old woman (G2P1001) at 39 weeks’ gestation is scheduled for a repeat low transverse cesarean delivery. In addition to planning to implement the measures discussed above, her clinician is considering whether to recommend that the patient bathe with an antiseptic solution, such as chlorhexidine, the day before the procedure.
Preoperative antiseptic bathing
The concept of bathing with an antiseptic solution before surgery to prevent surgical site infections (SSIs) has been considered for many years. Intuitively, if the body’s resident and transient skin flora are decreased preoperatively with whole-body antiseptic washing, then the overall pathogen burden should be decreased and the risk of SSI also should be reduced. Historically, chlorhexidine preparations have been used as preoperative antiseptic solutions because they are so effective in reducing colony counts of skin flora, especially staphylococci.12 Although preoperative antiseptic washing definitely reduces the concentration of skin bacteria, the data regarding reduction in SSI are inconsistent. Of particular note, there are no studies investigating the impact of preoperative antiseptic bathing in women having cesarean delivery.
Does preop bathing with an antiseptic reduce infection risk?
One of the first studies evaluating preoperative antiseptic washing was published by Cruse and Foord in 1980.13 In this 10-year prospective investigation, the authors demonstrated that patients who underwent preoperative washing with a hexachlorophene solution had fewer SSIs compared with those who washed with a nonmedicated soap and those who did not wash at all. Subsequent studies by Brady et al in 1990,14 Wilcox et al in 2003,15 and Colling et al in 201516 all showed a decrease in the rate of SSIs with preoperative antiseptic washing, and the authors strongly supported this intervention. However, care must be taken when interpreting the results of these cohort investigations because in some cases antiseptic washing was not the only preoperative intervention. Thus, it is difficult to ascertain the true benefit of antiseptic washing alone.14,15 Moreover, in one study, preoperative antiseptic washing did not decrease the overall incidence of SSIs, just those caused by Staphylococcus aureus and methicillin-resistant S aureus (MRSA).16
Authors of 3 recent reviews have assessed the relationship between preoperative antiseptic washing and SSIs. Webster and Osborne analyzed 7 RCTs in a Cochrane review.17 All trials used 4% chlorhexidine gluconate as the antiseptic, and they included a total of 10,157 patients. The authors concluded that bathing with chlorhexidine did not significantly reduce SSIs compared with either placebo (RR, 0.91; 95% CI, 0.8–1.04) or bar soap (RR, 1.02; 95% CI, 0.57–1.84). Three additional studies in this review compared chlorhexidine bathing with no washing. One study showed a significant reduction of SSIs after the patients bathed with chlorhexidine (RR, 0.36; 95% CI, 0.17–0.79); the other 2 studies demonstrated no significant difference in outcome.
Kamel and colleagues conducted a recent systematic review that included 20 randomized and nonrandomized studies (n = 9,520); while the authors concluded that showering with an antiseptic solution reduced skin flora, they could not confirm that it produced a significant reduction in infection.18 Finally, in a meta-analysis that included 16 randomized and nonrandomized studies with 17,932 patients, Chlebicki and associates concluded that there was no significant reduction in SSIs with whole-body bathing with chlorhexidine compared with bathing with soap or placebo or with no bathing (RR, 0.90; 95% CI, 0.77–1.05; P = .19).19 A recent report from the World Health Organization confirmed these observations, although the report did not specifically focus on patients who had had a cesarean delivery.20
What the evidence says
Although chlorhexidine bathing reduces skin flora, especially in the number of staphylococcal species, this effect does not necessarily translate into a reduction of SSIs. Therefore, we recommend against routine chlorhexidine bathing before cesarean delivery, although we acknowledge that there is no apparent harm associated with this practice, assuming that the patient is not allergic to the medicated soap (Level II Evidence, Level C Recommendation; TABLE, page 2).
Did you read Part 1 of this series?
Preventing infection after cesarean delivery: Evidence-based guidance, Part 1
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2005;105(5 pt 1):1024–1029.
- Haas DM, Pazouki F, Smith RR, et al. Vaginal cleansing before cesarean delivery to reduce postoperative infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2010;202(3):310.e1–e6.
- Asghania M, Mirblouk F, Shakiba M, Faraji R. Preoperative vaginal preparation with povidone-iodine on post-caesarean infectious morbidity. J Obstet Gynaecol. 2011;31(5):400–403.
- Yildirim G, Güngördük K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to caesarean delivery reduce the risk of endometritis? A randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25(11):2316–2321.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Sys Rev. 2014;(12):CD007892.
- Reid VC, Hartmann KE, McMahon M, Fry EP. Vaginal preparation with povidone iodine and postcesarean infectious morbidity: a randomized controlled trial. Obstet Gynecol. 2001;97(1):147–152.
- Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745–750.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Methods of delivering the placenta at caesarean section [comment]. Obstet Gynecol. 2008;112(5):1173–1174.
- Chelmow D, Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103(5 pt 1):974–980.
- Mackeen AD, Schuster M, Berghella V. Suture versus staples for skin closure after cesarean: a metaanalysis. Am J Obstet Gynecol. 2015;212(5):621.e1–e10.
- , , , . Influence of preoperative showers on staphylococcal skin colonization: a comparative trial of antiseptic skin cleansers . Ann Thorac Surg. 1988 ; 45(1) : 35 –3 8 .
- , . The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds . Surg Clin North Am. 1980 ; 60 ( 1 ): 27 – 40 .
- , , , Harkness JL. Successful control of endemic MRSA in a cardiothoracic surgical unit . Med J Aust. 1990 ; 152(5) : 240 –24 5 .
- , , , et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003 ; 54(3) : 196 – 201 .
- , , , Banton K, Bellman G. Pre-operative antiseptic shower and bath policy decreases the rate of S aureus and methicillin-resistant S aureus surgical site infections in patients undergoing joint arthroplasty . Surg Infect. 2015 ; 16(2):124–132.
- Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. 2012;(9):CD004985.
- , , , Mierzwinski-Urban M, Embil JM. Preoperative skin antiseptic preparations for preventing surgical site infections: a systematic review . Infect Control Hosp Epidemiol. 2012 ; 33(6) : 608 – 617 .
- , , , Maki DG. Preoperative chlorhexidine shower or bath for prevention of surgical site infection: a meta-analysis . Am J Infect Control. 2013 ; 41(2) : 167 –1 73 .
- Global guidelines for the prevention of surgical site infection. Geneva, Switzerland: World Health Organization; November 2016. http://www.who.int/gpsc/global-guidelines-web.pdf?ua=1. Accessed November 9, 2016.
In part 1 of our review on preventing postcesarean infection, we critically evaluated methods of skin preparation and administration of prophylactic antibiotics. In part 2, we address preoperative cleansing of the vagina with an antiseptic solution, preoperative bathing with an antiseptic solution, methods of placental extraction, closure of the deep subcutaneous layer of the abdomen, and closure of the skin.
Related article:
Preventing infection after cesarean delivery: Evidence-based guidance
CASE: Should vaginal cleansing be performed prior to cesarean delivery?
An 18-year-old primigravid woman at 41 weeks’ gestation has been in labor for 16 hours, and now has an arrest of descent at 0 station. An intrauterine pressure catheter and scalp electrode have been in place for the same length of time. The patient has had 9 internal examinations during the period of membrane rupture. As you are preparing to scrub the patient’s abdomen, the third-year medical student asks, “When I was on the Gynecology Service, I saw the doctors wash the vagina with an antiseptic solution before they performed a vaginal hysterectomy. Should we also do that before we operate on this patient?”
Preoperative vaginal cleansing
A preoperative antiseptic vaginal scrub is often used as an additional step to help reduce postcesarean infection.
Does cleansing the vagina with povidone-iodine before surgery further reduce the risk of endometritis and wound infection?
Multiple studies have sought to determine if cleansing the vagina with an antiseptic solution further reduces the incidence of postcesarean infection beyond what can be achieved with systemic antibiotic prophylaxis. These studies typically have focused on 3 specific outcomes: endometritis, wound (surgical site) infection, and febrile morbidity. The term febrile morbidity is defined as a temperature ≥100.4°F (38°C) on any 2 postoperative days excluding the first 24 hours. However, many patients who meet the standard definition of febrile morbidity may not have a proven infection and will not require treatment with antibiotics. The more precise measures of outcome are distinctly symptomatic infections, such as endometritis and wound infection, although, as noted in the review of published studies below, some authors continue to use the term febrile morbidity as one measure of postoperative complications.
In a randomized, placebo-controlled trial (RCT) of 308 women having a nonemergent cesarean delivery, Starr and colleagues reported a decreased incidence of postoperative endometritis in women who received a 30-second vaginal scrub with povidone-iodine compared with women who received only an abdominal scrub (7.0% vs 14.5%, P<.05).1 The groups did not differ in the frequency of wound infection (0.7% vs 1.2%, P = .4) or febrile morbidity (23.9% vs 28.3%, P = .4).1
In another RCT, Haas and colleagues found that preoperative vaginal cleansing with povidone-iodine compared with an abdominal scrub alone was associated with a decreased incidence of a composite measure of postoperative morbidity (6.5% vs 11.7%; relative risk [RR], 0.55; 95% confidence interval [CI], 0.26–1.11; P = .11).2 The postoperative composite included fever, endometritis, sepsis, readmission, and wound infection.
Subsequently, Asghania and associates conducted a double-blind, nonrandomized study of 568 women having cesarean delivery who received an abdominal scrub plus a 30-second vaginal scrub with povidone-iodine or received an abdominal scrub alone.3 They documented a decreased incidence of postoperative endometritis in the women who received the combined scrub (1.4% vs 2.5%; P = .03, adjusted odds ratio [AOR], 0.03; 95% CI, 0.008–0.7). The authors observed no significant difference in febrile morbidity (4.9% vs 6.0%; P = .73) or wound infection (3.5% vs 3.2%; P = .5).3
Yildirim and colleagues conducted an RCT comparing rates of infection in 334 women who received an abdominal scrub plus vaginal cleansing with povidone-iodine and 336 patients who had only a standard abdominal scrub.4 They documented a decreased incidence of endometritis in women who received the vaginal scrub (6.9% vs 11.6%; P = .04; RR for infection in the control group, 1.69; 95% CI, 1.03–2.76.) The authors found no difference in febrile morbidity (16.5% vs 18.2%; P = .61) or wound infection (1.8% vs 2.7%; P = .60). Of note, in excluding from the analysis women who had ruptured membranes or who were in labor, the investigators found no differences in outcome, indicating that the greatest impact of vaginal cleansing was in the highest risk patients.
In 2014, Haas and associates published a Cochrane review evaluating the effectiveness of preoperative vaginal cleansing with povidone-iodine.5 The authors reviewed 7 studies that analyzed outcomes in 2,635 women. They concluded that vaginal preparation with povidone-iodine at the time of cesarean delivery significantly decreased postoperative endometritis when compared with the control group (4.3% vs 8.3%; RR, 0.45; 95% CI, 0.25–0.81). They also noted that the most profound impact of vaginal cleansing was in women who were in labor before delivery (7.4% vs 13.0%; RR, 0.56; 95% CI, 0.34–0.95) and in women with ruptured membranes at the time of delivery (4.3% vs 17.9%; RR, 0.24; 95% CI, 0.10–0.55). The authors did not find a significant difference in postoperative wound infection or frequency of fever in women who received the vaginal scrub.
Related article:
STOP using instruments to assist with delivery of the head at cesarean
A notable exception to the beneficial outcomes reported above was the study by Reid et al.6 These authors randomly assigned 247 women having cesarean delivery to an abdominal scrub plus vaginal scrub with povidone-iodine and assigned 251 women to only an abdominal scrub. The authors were unable to document any significant difference between the groups with respect to frequency of fever, endometritis, and wound infection.
Other methods of vaginal preparation also have been studied. For example, Pitt and colleagues conducted a double-blind RCT of 224 women having cesarean delivery and compared preoperative metronidazole vaginal gel with placebo.7 Most of the patients in this trial also received systemic antibiotic prophylaxis after the umbilical cord was clamped. The authors demonstrated a decreased incidence of postcesarean endometritis in women who received the intravaginal antibiotic gel (7% vs 17%; RR, 0.42; 95% CI, 0.19–0.92). There was no difference in febrile morbidity (13% vs 19%; P = .28) or wound infection (4% vs 3%, P = .50).
What the evidence says
Consider vaginal preparation with povidone-iodine at the time of cesarean delivery to reduce the risk of postpartum endometritis. Do not expect this intervention to significantly reduce the frequency of wound infection. Vaginal cleansing is of most benefit to women who have ruptured membranes or are in labor at the time of delivery (Level I Evidence, Level A Recommendation; TABLE). Whether vaginal preparation with chlorhexidine with 4% alcohol would have the same beneficial effect has not been studied in a systematic manner.
Placenta extraction, closure techniques
Evidence suggests that employing certain intraoperative approaches helps reduce the incidence of postcesarean infection.
What other measures help prevent infection following cesarean surgery?
One other measure known to decrease the risk of postcesarean endometritis is removing the placenta by exerting traction on the umbilical cord rather than extracting it manually. In one of the first descriptions of this intervention, Lasley and associates showed that, in high-risk patients who also received intravenous antibiotic prophylaxis after cord clamping, the rate of postoperative endometritis was 15% in the group that had spontaneous delivery of the placenta compared with 27% in women who had manual extraction (RR, 0.6; 95% CI, 0.3–0.9; P = .02).8 A recent Cochrane review that included multiple subsequent reports confirmed this observation (Level I Evidence, Level A Recommendation; TABLE, page 2).9
Abdominal wall closure. Two other interventions are valuable in decreasing the frequency of deep and superficial wound infection. In patients whose subcutaneous layer is >2 cm thick, closure of the deep subcutaneous tissue significantly reduces the risk of wound seroma, hematoma, and infection.10 In addition, closure of the skin edges with a subcuticular suture, as opposed to surgical staples, significantly reduces the frequency of superficial wound complications (Level I Evidence, Level A Recommendation; TABLE, page 2).11 Poliglecaprone 25, polyglactin 910, and polyglycolic acid suture, 3-0 or 4-0 gauge, are excellent suture choices for this closure.
Related article:
Does one particular cesarean technique confer better maternal and neonatal outcomes?
CASE
Planned cesarean delivery: Is preoperative antiseptic bathing warranted?
A 33-year-old woman (G2P1001) at 39 weeks’ gestation is scheduled for a repeat low transverse cesarean delivery. In addition to planning to implement the measures discussed above, her clinician is considering whether to recommend that the patient bathe with an antiseptic solution, such as chlorhexidine, the day before the procedure.
Preoperative antiseptic bathing
The concept of bathing with an antiseptic solution before surgery to prevent surgical site infections (SSIs) has been considered for many years. Intuitively, if the body’s resident and transient skin flora are decreased preoperatively with whole-body antiseptic washing, then the overall pathogen burden should be decreased and the risk of SSI also should be reduced. Historically, chlorhexidine preparations have been used as preoperative antiseptic solutions because they are so effective in reducing colony counts of skin flora, especially staphylococci.12 Although preoperative antiseptic washing definitely reduces the concentration of skin bacteria, the data regarding reduction in SSI are inconsistent. Of particular note, there are no studies investigating the impact of preoperative antiseptic bathing in women having cesarean delivery.
Does preop bathing with an antiseptic reduce infection risk?
One of the first studies evaluating preoperative antiseptic washing was published by Cruse and Foord in 1980.13 In this 10-year prospective investigation, the authors demonstrated that patients who underwent preoperative washing with a hexachlorophene solution had fewer SSIs compared with those who washed with a nonmedicated soap and those who did not wash at all. Subsequent studies by Brady et al in 1990,14 Wilcox et al in 2003,15 and Colling et al in 201516 all showed a decrease in the rate of SSIs with preoperative antiseptic washing, and the authors strongly supported this intervention. However, care must be taken when interpreting the results of these cohort investigations because in some cases antiseptic washing was not the only preoperative intervention. Thus, it is difficult to ascertain the true benefit of antiseptic washing alone.14,15 Moreover, in one study, preoperative antiseptic washing did not decrease the overall incidence of SSIs, just those caused by Staphylococcus aureus and methicillin-resistant S aureus (MRSA).16
Authors of 3 recent reviews have assessed the relationship between preoperative antiseptic washing and SSIs. Webster and Osborne analyzed 7 RCTs in a Cochrane review.17 All trials used 4% chlorhexidine gluconate as the antiseptic, and they included a total of 10,157 patients. The authors concluded that bathing with chlorhexidine did not significantly reduce SSIs compared with either placebo (RR, 0.91; 95% CI, 0.8–1.04) or bar soap (RR, 1.02; 95% CI, 0.57–1.84). Three additional studies in this review compared chlorhexidine bathing with no washing. One study showed a significant reduction of SSIs after the patients bathed with chlorhexidine (RR, 0.36; 95% CI, 0.17–0.79); the other 2 studies demonstrated no significant difference in outcome.
Kamel and colleagues conducted a recent systematic review that included 20 randomized and nonrandomized studies (n = 9,520); while the authors concluded that showering with an antiseptic solution reduced skin flora, they could not confirm that it produced a significant reduction in infection.18 Finally, in a meta-analysis that included 16 randomized and nonrandomized studies with 17,932 patients, Chlebicki and associates concluded that there was no significant reduction in SSIs with whole-body bathing with chlorhexidine compared with bathing with soap or placebo or with no bathing (RR, 0.90; 95% CI, 0.77–1.05; P = .19).19 A recent report from the World Health Organization confirmed these observations, although the report did not specifically focus on patients who had had a cesarean delivery.20
What the evidence says
Although chlorhexidine bathing reduces skin flora, especially in the number of staphylococcal species, this effect does not necessarily translate into a reduction of SSIs. Therefore, we recommend against routine chlorhexidine bathing before cesarean delivery, although we acknowledge that there is no apparent harm associated with this practice, assuming that the patient is not allergic to the medicated soap (Level II Evidence, Level C Recommendation; TABLE, page 2).
Did you read Part 1 of this series?
Preventing infection after cesarean delivery: Evidence-based guidance, Part 1
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In part 1 of our review on preventing postcesarean infection, we critically evaluated methods of skin preparation and administration of prophylactic antibiotics. In part 2, we address preoperative cleansing of the vagina with an antiseptic solution, preoperative bathing with an antiseptic solution, methods of placental extraction, closure of the deep subcutaneous layer of the abdomen, and closure of the skin.
Related article:
Preventing infection after cesarean delivery: Evidence-based guidance
CASE: Should vaginal cleansing be performed prior to cesarean delivery?
An 18-year-old primigravid woman at 41 weeks’ gestation has been in labor for 16 hours, and now has an arrest of descent at 0 station. An intrauterine pressure catheter and scalp electrode have been in place for the same length of time. The patient has had 9 internal examinations during the period of membrane rupture. As you are preparing to scrub the patient’s abdomen, the third-year medical student asks, “When I was on the Gynecology Service, I saw the doctors wash the vagina with an antiseptic solution before they performed a vaginal hysterectomy. Should we also do that before we operate on this patient?”
Preoperative vaginal cleansing
A preoperative antiseptic vaginal scrub is often used as an additional step to help reduce postcesarean infection.
Does cleansing the vagina with povidone-iodine before surgery further reduce the risk of endometritis and wound infection?
Multiple studies have sought to determine if cleansing the vagina with an antiseptic solution further reduces the incidence of postcesarean infection beyond what can be achieved with systemic antibiotic prophylaxis. These studies typically have focused on 3 specific outcomes: endometritis, wound (surgical site) infection, and febrile morbidity. The term febrile morbidity is defined as a temperature ≥100.4°F (38°C) on any 2 postoperative days excluding the first 24 hours. However, many patients who meet the standard definition of febrile morbidity may not have a proven infection and will not require treatment with antibiotics. The more precise measures of outcome are distinctly symptomatic infections, such as endometritis and wound infection, although, as noted in the review of published studies below, some authors continue to use the term febrile morbidity as one measure of postoperative complications.
In a randomized, placebo-controlled trial (RCT) of 308 women having a nonemergent cesarean delivery, Starr and colleagues reported a decreased incidence of postoperative endometritis in women who received a 30-second vaginal scrub with povidone-iodine compared with women who received only an abdominal scrub (7.0% vs 14.5%, P<.05).1 The groups did not differ in the frequency of wound infection (0.7% vs 1.2%, P = .4) or febrile morbidity (23.9% vs 28.3%, P = .4).1
In another RCT, Haas and colleagues found that preoperative vaginal cleansing with povidone-iodine compared with an abdominal scrub alone was associated with a decreased incidence of a composite measure of postoperative morbidity (6.5% vs 11.7%; relative risk [RR], 0.55; 95% confidence interval [CI], 0.26–1.11; P = .11).2 The postoperative composite included fever, endometritis, sepsis, readmission, and wound infection.
Subsequently, Asghania and associates conducted a double-blind, nonrandomized study of 568 women having cesarean delivery who received an abdominal scrub plus a 30-second vaginal scrub with povidone-iodine or received an abdominal scrub alone.3 They documented a decreased incidence of postoperative endometritis in the women who received the combined scrub (1.4% vs 2.5%; P = .03, adjusted odds ratio [AOR], 0.03; 95% CI, 0.008–0.7). The authors observed no significant difference in febrile morbidity (4.9% vs 6.0%; P = .73) or wound infection (3.5% vs 3.2%; P = .5).3
Yildirim and colleagues conducted an RCT comparing rates of infection in 334 women who received an abdominal scrub plus vaginal cleansing with povidone-iodine and 336 patients who had only a standard abdominal scrub.4 They documented a decreased incidence of endometritis in women who received the vaginal scrub (6.9% vs 11.6%; P = .04; RR for infection in the control group, 1.69; 95% CI, 1.03–2.76.) The authors found no difference in febrile morbidity (16.5% vs 18.2%; P = .61) or wound infection (1.8% vs 2.7%; P = .60). Of note, in excluding from the analysis women who had ruptured membranes or who were in labor, the investigators found no differences in outcome, indicating that the greatest impact of vaginal cleansing was in the highest risk patients.
In 2014, Haas and associates published a Cochrane review evaluating the effectiveness of preoperative vaginal cleansing with povidone-iodine.5 The authors reviewed 7 studies that analyzed outcomes in 2,635 women. They concluded that vaginal preparation with povidone-iodine at the time of cesarean delivery significantly decreased postoperative endometritis when compared with the control group (4.3% vs 8.3%; RR, 0.45; 95% CI, 0.25–0.81). They also noted that the most profound impact of vaginal cleansing was in women who were in labor before delivery (7.4% vs 13.0%; RR, 0.56; 95% CI, 0.34–0.95) and in women with ruptured membranes at the time of delivery (4.3% vs 17.9%; RR, 0.24; 95% CI, 0.10–0.55). The authors did not find a significant difference in postoperative wound infection or frequency of fever in women who received the vaginal scrub.
Related article:
STOP using instruments to assist with delivery of the head at cesarean
A notable exception to the beneficial outcomes reported above was the study by Reid et al.6 These authors randomly assigned 247 women having cesarean delivery to an abdominal scrub plus vaginal scrub with povidone-iodine and assigned 251 women to only an abdominal scrub. The authors were unable to document any significant difference between the groups with respect to frequency of fever, endometritis, and wound infection.
Other methods of vaginal preparation also have been studied. For example, Pitt and colleagues conducted a double-blind RCT of 224 women having cesarean delivery and compared preoperative metronidazole vaginal gel with placebo.7 Most of the patients in this trial also received systemic antibiotic prophylaxis after the umbilical cord was clamped. The authors demonstrated a decreased incidence of postcesarean endometritis in women who received the intravaginal antibiotic gel (7% vs 17%; RR, 0.42; 95% CI, 0.19–0.92). There was no difference in febrile morbidity (13% vs 19%; P = .28) or wound infection (4% vs 3%, P = .50).
What the evidence says
Consider vaginal preparation with povidone-iodine at the time of cesarean delivery to reduce the risk of postpartum endometritis. Do not expect this intervention to significantly reduce the frequency of wound infection. Vaginal cleansing is of most benefit to women who have ruptured membranes or are in labor at the time of delivery (Level I Evidence, Level A Recommendation; TABLE). Whether vaginal preparation with chlorhexidine with 4% alcohol would have the same beneficial effect has not been studied in a systematic manner.
Placenta extraction, closure techniques
Evidence suggests that employing certain intraoperative approaches helps reduce the incidence of postcesarean infection.
What other measures help prevent infection following cesarean surgery?
One other measure known to decrease the risk of postcesarean endometritis is removing the placenta by exerting traction on the umbilical cord rather than extracting it manually. In one of the first descriptions of this intervention, Lasley and associates showed that, in high-risk patients who also received intravenous antibiotic prophylaxis after cord clamping, the rate of postoperative endometritis was 15% in the group that had spontaneous delivery of the placenta compared with 27% in women who had manual extraction (RR, 0.6; 95% CI, 0.3–0.9; P = .02).8 A recent Cochrane review that included multiple subsequent reports confirmed this observation (Level I Evidence, Level A Recommendation; TABLE, page 2).9
Abdominal wall closure. Two other interventions are valuable in decreasing the frequency of deep and superficial wound infection. In patients whose subcutaneous layer is >2 cm thick, closure of the deep subcutaneous tissue significantly reduces the risk of wound seroma, hematoma, and infection.10 In addition, closure of the skin edges with a subcuticular suture, as opposed to surgical staples, significantly reduces the frequency of superficial wound complications (Level I Evidence, Level A Recommendation; TABLE, page 2).11 Poliglecaprone 25, polyglactin 910, and polyglycolic acid suture, 3-0 or 4-0 gauge, are excellent suture choices for this closure.
Related article:
Does one particular cesarean technique confer better maternal and neonatal outcomes?
CASE
Planned cesarean delivery: Is preoperative antiseptic bathing warranted?
A 33-year-old woman (G2P1001) at 39 weeks’ gestation is scheduled for a repeat low transverse cesarean delivery. In addition to planning to implement the measures discussed above, her clinician is considering whether to recommend that the patient bathe with an antiseptic solution, such as chlorhexidine, the day before the procedure.
Preoperative antiseptic bathing
The concept of bathing with an antiseptic solution before surgery to prevent surgical site infections (SSIs) has been considered for many years. Intuitively, if the body’s resident and transient skin flora are decreased preoperatively with whole-body antiseptic washing, then the overall pathogen burden should be decreased and the risk of SSI also should be reduced. Historically, chlorhexidine preparations have been used as preoperative antiseptic solutions because they are so effective in reducing colony counts of skin flora, especially staphylococci.12 Although preoperative antiseptic washing definitely reduces the concentration of skin bacteria, the data regarding reduction in SSI are inconsistent. Of particular note, there are no studies investigating the impact of preoperative antiseptic bathing in women having cesarean delivery.
Does preop bathing with an antiseptic reduce infection risk?
One of the first studies evaluating preoperative antiseptic washing was published by Cruse and Foord in 1980.13 In this 10-year prospective investigation, the authors demonstrated that patients who underwent preoperative washing with a hexachlorophene solution had fewer SSIs compared with those who washed with a nonmedicated soap and those who did not wash at all. Subsequent studies by Brady et al in 1990,14 Wilcox et al in 2003,15 and Colling et al in 201516 all showed a decrease in the rate of SSIs with preoperative antiseptic washing, and the authors strongly supported this intervention. However, care must be taken when interpreting the results of these cohort investigations because in some cases antiseptic washing was not the only preoperative intervention. Thus, it is difficult to ascertain the true benefit of antiseptic washing alone.14,15 Moreover, in one study, preoperative antiseptic washing did not decrease the overall incidence of SSIs, just those caused by Staphylococcus aureus and methicillin-resistant S aureus (MRSA).16
Authors of 3 recent reviews have assessed the relationship between preoperative antiseptic washing and SSIs. Webster and Osborne analyzed 7 RCTs in a Cochrane review.17 All trials used 4% chlorhexidine gluconate as the antiseptic, and they included a total of 10,157 patients. The authors concluded that bathing with chlorhexidine did not significantly reduce SSIs compared with either placebo (RR, 0.91; 95% CI, 0.8–1.04) or bar soap (RR, 1.02; 95% CI, 0.57–1.84). Three additional studies in this review compared chlorhexidine bathing with no washing. One study showed a significant reduction of SSIs after the patients bathed with chlorhexidine (RR, 0.36; 95% CI, 0.17–0.79); the other 2 studies demonstrated no significant difference in outcome.
Kamel and colleagues conducted a recent systematic review that included 20 randomized and nonrandomized studies (n = 9,520); while the authors concluded that showering with an antiseptic solution reduced skin flora, they could not confirm that it produced a significant reduction in infection.18 Finally, in a meta-analysis that included 16 randomized and nonrandomized studies with 17,932 patients, Chlebicki and associates concluded that there was no significant reduction in SSIs with whole-body bathing with chlorhexidine compared with bathing with soap or placebo or with no bathing (RR, 0.90; 95% CI, 0.77–1.05; P = .19).19 A recent report from the World Health Organization confirmed these observations, although the report did not specifically focus on patients who had had a cesarean delivery.20
What the evidence says
Although chlorhexidine bathing reduces skin flora, especially in the number of staphylococcal species, this effect does not necessarily translate into a reduction of SSIs. Therefore, we recommend against routine chlorhexidine bathing before cesarean delivery, although we acknowledge that there is no apparent harm associated with this practice, assuming that the patient is not allergic to the medicated soap (Level II Evidence, Level C Recommendation; TABLE, page 2).
Did you read Part 1 of this series?
Preventing infection after cesarean delivery: Evidence-based guidance, Part 1
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2005;105(5 pt 1):1024–1029.
- Haas DM, Pazouki F, Smith RR, et al. Vaginal cleansing before cesarean delivery to reduce postoperative infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2010;202(3):310.e1–e6.
- Asghania M, Mirblouk F, Shakiba M, Faraji R. Preoperative vaginal preparation with povidone-iodine on post-caesarean infectious morbidity. J Obstet Gynaecol. 2011;31(5):400–403.
- Yildirim G, Güngördük K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to caesarean delivery reduce the risk of endometritis? A randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25(11):2316–2321.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Sys Rev. 2014;(12):CD007892.
- Reid VC, Hartmann KE, McMahon M, Fry EP. Vaginal preparation with povidone iodine and postcesarean infectious morbidity: a randomized controlled trial. Obstet Gynecol. 2001;97(1):147–152.
- Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745–750.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Methods of delivering the placenta at caesarean section [comment]. Obstet Gynecol. 2008;112(5):1173–1174.
- Chelmow D, Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103(5 pt 1):974–980.
- Mackeen AD, Schuster M, Berghella V. Suture versus staples for skin closure after cesarean: a metaanalysis. Am J Obstet Gynecol. 2015;212(5):621.e1–e10.
- , , , . Influence of preoperative showers on staphylococcal skin colonization: a comparative trial of antiseptic skin cleansers . Ann Thorac Surg. 1988 ; 45(1) : 35 –3 8 .
- , . The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds . Surg Clin North Am. 1980 ; 60 ( 1 ): 27 – 40 .
- , , , Harkness JL. Successful control of endemic MRSA in a cardiothoracic surgical unit . Med J Aust. 1990 ; 152(5) : 240 –24 5 .
- , , , et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003 ; 54(3) : 196 – 201 .
- , , , Banton K, Bellman G. Pre-operative antiseptic shower and bath policy decreases the rate of S aureus and methicillin-resistant S aureus surgical site infections in patients undergoing joint arthroplasty . Surg Infect. 2015 ; 16(2):124–132.
- Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. 2012;(9):CD004985.
- , , , Mierzwinski-Urban M, Embil JM. Preoperative skin antiseptic preparations for preventing surgical site infections: a systematic review . Infect Control Hosp Epidemiol. 2012 ; 33(6) : 608 – 617 .
- , , , Maki DG. Preoperative chlorhexidine shower or bath for prevention of surgical site infection: a meta-analysis . Am J Infect Control. 2013 ; 41(2) : 167 –1 73 .
- Global guidelines for the prevention of surgical site infection. Geneva, Switzerland: World Health Organization; November 2016. http://www.who.int/gpsc/global-guidelines-web.pdf?ua=1. Accessed November 9, 2016.
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2005;105(5 pt 1):1024–1029.
- Haas DM, Pazouki F, Smith RR, et al. Vaginal cleansing before cesarean delivery to reduce postoperative infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2010;202(3):310.e1–e6.
- Asghania M, Mirblouk F, Shakiba M, Faraji R. Preoperative vaginal preparation with povidone-iodine on post-caesarean infectious morbidity. J Obstet Gynaecol. 2011;31(5):400–403.
- Yildirim G, Güngördük K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to caesarean delivery reduce the risk of endometritis? A randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25(11):2316–2321.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Sys Rev. 2014;(12):CD007892.
- Reid VC, Hartmann KE, McMahon M, Fry EP. Vaginal preparation with povidone iodine and postcesarean infectious morbidity: a randomized controlled trial. Obstet Gynecol. 2001;97(1):147–152.
- Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745–750.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Methods of delivering the placenta at caesarean section [comment]. Obstet Gynecol. 2008;112(5):1173–1174.
- Chelmow D, Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103(5 pt 1):974–980.
- Mackeen AD, Schuster M, Berghella V. Suture versus staples for skin closure after cesarean: a metaanalysis. Am J Obstet Gynecol. 2015;212(5):621.e1–e10.
- , , , . Influence of preoperative showers on staphylococcal skin colonization: a comparative trial of antiseptic skin cleansers . Ann Thorac Surg. 1988 ; 45(1) : 35 –3 8 .
- , . The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds . Surg Clin North Am. 1980 ; 60 ( 1 ): 27 – 40 .
- , , , Harkness JL. Successful control of endemic MRSA in a cardiothoracic surgical unit . Med J Aust. 1990 ; 152(5) : 240 –24 5 .
- , , , et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003 ; 54(3) : 196 – 201 .
- , , , Banton K, Bellman G. Pre-operative antiseptic shower and bath policy decreases the rate of S aureus and methicillin-resistant S aureus surgical site infections in patients undergoing joint arthroplasty . Surg Infect. 2015 ; 16(2):124–132.
- Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. 2012;(9):CD004985.
- , , , Mierzwinski-Urban M, Embil JM. Preoperative skin antiseptic preparations for preventing surgical site infections: a systematic review . Infect Control Hosp Epidemiol. 2012 ; 33(6) : 608 – 617 .
- , , , Maki DG. Preoperative chlorhexidine shower or bath for prevention of surgical site infection: a meta-analysis . Am J Infect Control. 2013 ; 41(2) : 167 –1 73 .
- Global guidelines for the prevention of surgical site infection. Geneva, Switzerland: World Health Organization; November 2016. http://www.who.int/gpsc/global-guidelines-web.pdf?ua=1. Accessed November 9, 2016.
An Overview of the History of Orthopedic Surgery
The modern term orthopedics stems from the older word orthopedia, which was the title of a book published in 1741 by Nicholas Andry, a professor of medicine at the University of Paris.1 The term orthopedia is a composite of 2 Greek words: orthos, meaning “straight and free from deformity,” and paidios, meaning “child.” Together, orthopedics literally means straight child, suggesting the importance of pediatric injuries and deformities in the development of this field. Interestingly, Andry’s book also depicted a crooked young tree attached to a straight and strong staff, which has become the universal symbol of orthopedic surgery and underscores the focus on correcting deformities in the young (Figure).1
Orthopedic surgery is a rapidly advancing medical field with several recent advances noted within orthopedic subspecialties,2-4 basic science,5 and clinical research.6 It is important to recognize the role of history with regards to innovation and research, especially for young trainees and medical students interested in a particular medical specialty. More specifically, it is important to understand the successes and failures of the past in order to advance research and practice, and ultimately improve patient care and outcomes.
In the recent literature, there is no concise yet comprehensive article focusing on the history of orthopedic surgery. The goal of this review is to provide an overview of the history and development of orthopedic surgery from ancient practices to the modern era.
Ancient Orthopedics
While the evidence is limited, the practice of orthopedics dates back to the primitive man.7 Fossil evidence suggests that the orthopedic pathology of today, such as fractures and traumatic amputations, existed in primitive times.8 The union of fractures in fair alignment has also been observed, which emphasizes the efficacy of nonoperative orthopedics and suggests the early use of splints and rehabilitation practices.8,9 Since procedures such as trepanation and crude amputations occurred during the New Stone Age, it is feasible that sophisticated techniques had also been developed for the treatment of injuries.7-9 However, evidence continues to remain limited.7
Later civilizations also developed creative ways to manage orthopedic injuries. For example, the Shoshone Indians, who were known to exist around 700-2000 BCE, made a splint of fresh rawhide that had been soaked in water.9,10 Similarly, some South Australian tribes made splints of clay, which when dried were as good as plaster of Paris.9 Furthermore, bone-setting or reductions was practiced as a profession in many tribes, underscoring the importance of orthopedic injuries in early civilizations.8,9
Ancient Egypt
The ancient Egyptians seemed to have carried on the practices of splinting. For example, 2 splinted specimens were discovered during the Hearst Egyptian Expedition in 1903.7 More specifically, these specimens included a femur and forearm and dated to approximately 300 BCE.7 Other examples of splints made of bamboo and reed padded with linen have been found on mummies as well.8 Similarly, crutches were also used by this civilization, as depicted on a carving made on an Egyptian tomb in 2830 BCE.8
One of the earliest and most significant documents on medicine was discovered in 1862, known as the Edwin Smith papyrus. This document is thought to have been composed by Imhotep, a prominent Egyptian physician, astrologer, architect, and politician, and it specifically categorizes diseases and treatments. Many scholars recognize this medical document as the oldest surgical textbook.11,12 With regards to orthopedic conditions, this document describes the reduction of a dislocated mandible, signs of spinal or vertebral injuries, description of torticollis, and the treatment of fractures such as clavicle fractures.8 This document also discusses ryt, which refers to the purulent discharge from osteomyelitis.8 The following is an excerpt from this ancient document:9
“Instructions on erring a break in his upper arm…Thou shouldst spread out with his two shoulders in order to stretch apart his upper arm until that break falls into its place. Thou shouldst make for him two splints of linen, and thou shouldst apply for him one of them both on the inside of his arm, and the other of them both on the underside of his arm.”
This account illustrates the methodical and meticulous nature of this textbook, and it highlights some of the essentials of medical practice from diagnosis to medical decision-making to treatment.
There are various other contributions to the field of medicine from the Far East; however, many of these pertain to the fields of plastic surgery and general surgery.9
Greeks and Romans
The Greeks are considered to be the first to systematically employ the scientific approach to medicine.8 In the period between 430 BCE to 330 BCE, the Corpus Hippocrates was compiled, which is a Greek text on medicine. It is named for Hippocrates (460 BCE-370 BCE), the father of medicine, and it contains text that applies specifically to the field of orthopedic surgery. For example, this text discuses shoulder dislocations and describes various reduction maneuvers. Hippocrates had a keen understanding of the principles of traction and countertraction, especially as it pertains to the musculoskeletal system.8 In fact, the Hippocratic method is still used for reducing anterior shoulder dislocations, and its description can be found in several modern orthopedic texts, including recent articles.13 The Corpus Hippocrates also describes the correction of clubfoot deformity, and the treatment of infected open fractures with pitch cerate and wine compresses.8
Hippocrates also described the treatment of fractures, the principles of traction, and the implications of malunions. For example, Hippocrates wrote, “For the arm, when shortened, might be concealed and the mistake will not be great, but a shortened thigh bone will leave a man maimed.”1 In addition, spinal deformities were recognized by the Greeks, and Hippocrates devised an extension bench for the correction of such deformities.1 From their contributions to anatomy and surgical practice, the Greeks have made significant contributions to the field of surgery.9
During the Roman period, another Greek surgeon by the name of Galen described the musculoskeletal and nervous systems. He served as a gladiatorial surgeon in Rome, and today, he is considered to be the father of sports medicine.8 He is also credited with coining the terms scoliosis, kyphosis, and lordosis to denote the spinal deformities that were first described by Hippocrates.1 In the Roman period, amputations were also performed, and primitive prostheses were developed.9
The Middle Ages
There was relatively little progress in the study of medicine for a thousand years after the fall of the Roman Empire.9 This stagnation was predominantly due to the early Christian Church inhibiting freedom of thought and observation, as well as prohibiting human dissection and the study of anatomy. The first medical school in Europe was established in Salerno, Italy, during the ninth century. This school provided primarily pedantic teaching to its students and perpetuated the theories of the elements and humors. Later on, the University of Bologna became one of the first academic institutions to offer hands-on surgical training.9 One of the most famous surgeons of the Middle Ages was Guy de Chuauliac, who studied at Montpellier and Bologna. He was a leader in the ethical principles of surgery as well as the practice of surgery, and wrote the following with regards to femur fractures:9
“After the application of splints, I attach to the foot a mass of lead as a weight, taking care to pass the cord which supports the weight over a small pulley in such a manner that it shall pull on the leg in a horizontal direction.”
This description is strikingly similar to the modern-day nonoperative management of femur fractures, and underscores the importance of traction, which as mentioned above, was first described by Hippocrates.
Eventually, medicine began to separate from the Church, most likely due to an increase in the complexity of medical theories, the rise of secular universities, and an increase in medical knowledge from Eastern and Middle-Eastern groups.9
The Renaissance and the Foundations of Modern Orthopedics
Until the 16th century, the majority of medical theories were heavily influenced by the work of Hippocrates.8 The scientific study of anatomy gained prominence during this time, especially due to the work done by great artists, such as Leonardo Di Vinci.9 The Table
After a period of rapid expansion of the field of orthopedics, and following the Renaissance, many hospitals were built focusing on the sick and disabled, which solidified orthopedics’ position as a major medical specialty.1 For example, in 1863, James Knight founded the Hospital for the Ruptured and Crippled in New York City. This hospital became the oldest orthopedic hospital in the United States, and it later became known as the Hospital for Special Surgery.14,15 Several additional orthopedic institutions were formed, including the New York Orthopedic Dispensary in 1886 and Hospital for Deformities and Joint Diseases in 1917. Orthopedic surgery residency programs also began to be developed in the late 1800s.14 More specifically, Virgil Gibney at Hospital for the Ruptured and Crippled began the first orthopedic training program in the United States in 1888. Young doctors in this program trained for 1 year as junior assistant, senior assistant, and house surgeon, and began to be known as resident doctors.14
The Modern Era
In the 20th century, rapid development continued to better control infections as well as develop and introduce novel technology. For example, the invention of x-ray in 1895 by Wilhelm Conrad Röntgen improved our ability to diagnose and manage orthopedic conditions ranging from fractures to avascular necrosis of the femoral head to osteoarthritis.8,14 Spinal surgery also developed rapidly with Russell Hibbs describing a technique for spinal fusion at the New York Orthopedic Hospital.8 Similarly, the World Wars served as a catalyst in the development of the subspecialty of orthopedic trauma, with increasing attention placed on open wounds and proficiency with amputations, internal fixation, and wound care. In 1942, Austin Moore performed the first metal hip arthroplasty, and the field of joint replacement was subsequently advanced by the work of Sir John Charnley in the 1960s.8
Conclusion
Despite its relatively recent specialization, orthopedic surgery has a rich history rooted in ancient practices dating back to the primitive man. Over time, there has been significant development in the field in terms of surgical and nonsurgical treatment of orthopedic pathology and disease. Various cultures have played an instrumental role in developing this field, and it is remarkable to see that several practices have persisted since the time of these ancient civilizations. During the Renaissance, there was a considerable emphasis placed on pediatric deformity, but orthopedic surgeons have now branched out to subspecialty practice ranging from orthopedic trauma to joint replacement to oncology.1 For students of medicine and orthopedics, it is important to learn about the origins of this field and to appreciate its gradual development. Orthopedic surgery is a diverse and fascinating field that will most likely continue to develop with increased subspecialization and improved research at the molecular and population level. With a growing emphasis placed on outcomes and healthcare cost by today’s society, it will be fascinating to see how this field continues to evolve in the future.
Am J Orthop. 2016;45(7):E434-E438. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Ponseti IV. History of orthopedic surgery. Iowa Orthop J. 1991;11:59-64.
2. Ninomiya JT, Dean JC, Incavo SJ. What’s new in hip replacement. J Bone Joint Surg Am. 2015;97(18):1543-1551.
3. Sabharwal S, Nelson SC, Sontich JK. What’s new in limb lengthening and deformity correction. J Bone Joint Surg Am. 2015;97(16):1375-1384.
4. Ricci WM, Black JC, McAndrew CM, Gardner MJ. What’s new in orthopedic trauma. J Bone Joint Surg Am. 2015;97(14):1200-1207.
5. Rodeo SA, Sugiguchi F, Fortier LA, Cunningham ME, Maher S. What’s new in orthopedic research. J Bone Joint Surg Am. 2014;96(23):2015-2019.
6. Pugley AJ, Martin CT, Harwood J, Ong KL, Bozic KJ, Callaghan JJ. Database and registry research in orthopedic surgery. Part 1: Claims-based data. J Bone Joint Surg Am. 2015;97(15):1278-1287.
7. Colton CL. The history of fracture treatment. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma: Basic Science, Management, and Reconstruction. 4th ed. Philadelphia, PA: Saunders Elsevier; 2009:3-32.
8. Brakoulias,V. History of orthopaedics. WorldOrtho Web site. http://pioa.net/documents/Historyoforthopaedics.pdf. Accessed October 6, 2016.
9. Bishop WJ. The Early History of Surgery. New York, NY: Barnes & Noble Books; 1995.
10. Watson T. Wyoming site reveals more prehistoric mountain villages. USA Today. October 20, 2013. http://www.usatoday.com/story/news/nation/2013/10/20/wyoming-prehistoric-villages/2965263. Accessed October 6, 2016.
11. Minagar A, Ragheb J, Kelley RE. The Edwin Smith surgical papyrus: description and analysis of the earliest case of aphasia. J Med Biogr. 2003;11(2):114-117.
12. Atta HM. Edwin Smith Surgical Papyrus: the oldest known surgical treatise. Am Surg. 1999;65(12):1190-1192.
13. Sayegh FE, Kenanidis EI, Papavasiliou KA, Potoupnis ME, Kirkos JM, Kapetanos GA. Reduction of acute anterior dislocations: a prospective randomized study comparing a new technique with the Hippocratic and Kocher methods. J Bone Joint Surg Am. 2009;91(12):2775-2782.
14. Levine DB. Anatomy of a Hospital: Hospital for Special Surgery 1863-2013. New York, NY: Print Mattes; 2013.
15. Wilson PD, Levine DB. Hospital for special surgery. A brief review of its development and current position. Clin Orthop Relat Res. 2000;(374):90-106.
The modern term orthopedics stems from the older word orthopedia, which was the title of a book published in 1741 by Nicholas Andry, a professor of medicine at the University of Paris.1 The term orthopedia is a composite of 2 Greek words: orthos, meaning “straight and free from deformity,” and paidios, meaning “child.” Together, orthopedics literally means straight child, suggesting the importance of pediatric injuries and deformities in the development of this field. Interestingly, Andry’s book also depicted a crooked young tree attached to a straight and strong staff, which has become the universal symbol of orthopedic surgery and underscores the focus on correcting deformities in the young (Figure).1
Orthopedic surgery is a rapidly advancing medical field with several recent advances noted within orthopedic subspecialties,2-4 basic science,5 and clinical research.6 It is important to recognize the role of history with regards to innovation and research, especially for young trainees and medical students interested in a particular medical specialty. More specifically, it is important to understand the successes and failures of the past in order to advance research and practice, and ultimately improve patient care and outcomes.
In the recent literature, there is no concise yet comprehensive article focusing on the history of orthopedic surgery. The goal of this review is to provide an overview of the history and development of orthopedic surgery from ancient practices to the modern era.
Ancient Orthopedics
While the evidence is limited, the practice of orthopedics dates back to the primitive man.7 Fossil evidence suggests that the orthopedic pathology of today, such as fractures and traumatic amputations, existed in primitive times.8 The union of fractures in fair alignment has also been observed, which emphasizes the efficacy of nonoperative orthopedics and suggests the early use of splints and rehabilitation practices.8,9 Since procedures such as trepanation and crude amputations occurred during the New Stone Age, it is feasible that sophisticated techniques had also been developed for the treatment of injuries.7-9 However, evidence continues to remain limited.7
Later civilizations also developed creative ways to manage orthopedic injuries. For example, the Shoshone Indians, who were known to exist around 700-2000 BCE, made a splint of fresh rawhide that had been soaked in water.9,10 Similarly, some South Australian tribes made splints of clay, which when dried were as good as plaster of Paris.9 Furthermore, bone-setting or reductions was practiced as a profession in many tribes, underscoring the importance of orthopedic injuries in early civilizations.8,9
Ancient Egypt
The ancient Egyptians seemed to have carried on the practices of splinting. For example, 2 splinted specimens were discovered during the Hearst Egyptian Expedition in 1903.7 More specifically, these specimens included a femur and forearm and dated to approximately 300 BCE.7 Other examples of splints made of bamboo and reed padded with linen have been found on mummies as well.8 Similarly, crutches were also used by this civilization, as depicted on a carving made on an Egyptian tomb in 2830 BCE.8
One of the earliest and most significant documents on medicine was discovered in 1862, known as the Edwin Smith papyrus. This document is thought to have been composed by Imhotep, a prominent Egyptian physician, astrologer, architect, and politician, and it specifically categorizes diseases and treatments. Many scholars recognize this medical document as the oldest surgical textbook.11,12 With regards to orthopedic conditions, this document describes the reduction of a dislocated mandible, signs of spinal or vertebral injuries, description of torticollis, and the treatment of fractures such as clavicle fractures.8 This document also discusses ryt, which refers to the purulent discharge from osteomyelitis.8 The following is an excerpt from this ancient document:9
“Instructions on erring a break in his upper arm…Thou shouldst spread out with his two shoulders in order to stretch apart his upper arm until that break falls into its place. Thou shouldst make for him two splints of linen, and thou shouldst apply for him one of them both on the inside of his arm, and the other of them both on the underside of his arm.”
This account illustrates the methodical and meticulous nature of this textbook, and it highlights some of the essentials of medical practice from diagnosis to medical decision-making to treatment.
There are various other contributions to the field of medicine from the Far East; however, many of these pertain to the fields of plastic surgery and general surgery.9
Greeks and Romans
The Greeks are considered to be the first to systematically employ the scientific approach to medicine.8 In the period between 430 BCE to 330 BCE, the Corpus Hippocrates was compiled, which is a Greek text on medicine. It is named for Hippocrates (460 BCE-370 BCE), the father of medicine, and it contains text that applies specifically to the field of orthopedic surgery. For example, this text discuses shoulder dislocations and describes various reduction maneuvers. Hippocrates had a keen understanding of the principles of traction and countertraction, especially as it pertains to the musculoskeletal system.8 In fact, the Hippocratic method is still used for reducing anterior shoulder dislocations, and its description can be found in several modern orthopedic texts, including recent articles.13 The Corpus Hippocrates also describes the correction of clubfoot deformity, and the treatment of infected open fractures with pitch cerate and wine compresses.8
Hippocrates also described the treatment of fractures, the principles of traction, and the implications of malunions. For example, Hippocrates wrote, “For the arm, when shortened, might be concealed and the mistake will not be great, but a shortened thigh bone will leave a man maimed.”1 In addition, spinal deformities were recognized by the Greeks, and Hippocrates devised an extension bench for the correction of such deformities.1 From their contributions to anatomy and surgical practice, the Greeks have made significant contributions to the field of surgery.9
During the Roman period, another Greek surgeon by the name of Galen described the musculoskeletal and nervous systems. He served as a gladiatorial surgeon in Rome, and today, he is considered to be the father of sports medicine.8 He is also credited with coining the terms scoliosis, kyphosis, and lordosis to denote the spinal deformities that were first described by Hippocrates.1 In the Roman period, amputations were also performed, and primitive prostheses were developed.9
The Middle Ages
There was relatively little progress in the study of medicine for a thousand years after the fall of the Roman Empire.9 This stagnation was predominantly due to the early Christian Church inhibiting freedom of thought and observation, as well as prohibiting human dissection and the study of anatomy. The first medical school in Europe was established in Salerno, Italy, during the ninth century. This school provided primarily pedantic teaching to its students and perpetuated the theories of the elements and humors. Later on, the University of Bologna became one of the first academic institutions to offer hands-on surgical training.9 One of the most famous surgeons of the Middle Ages was Guy de Chuauliac, who studied at Montpellier and Bologna. He was a leader in the ethical principles of surgery as well as the practice of surgery, and wrote the following with regards to femur fractures:9
“After the application of splints, I attach to the foot a mass of lead as a weight, taking care to pass the cord which supports the weight over a small pulley in such a manner that it shall pull on the leg in a horizontal direction.”
This description is strikingly similar to the modern-day nonoperative management of femur fractures, and underscores the importance of traction, which as mentioned above, was first described by Hippocrates.
Eventually, medicine began to separate from the Church, most likely due to an increase in the complexity of medical theories, the rise of secular universities, and an increase in medical knowledge from Eastern and Middle-Eastern groups.9
The Renaissance and the Foundations of Modern Orthopedics
Until the 16th century, the majority of medical theories were heavily influenced by the work of Hippocrates.8 The scientific study of anatomy gained prominence during this time, especially due to the work done by great artists, such as Leonardo Di Vinci.9 The Table
After a period of rapid expansion of the field of orthopedics, and following the Renaissance, many hospitals were built focusing on the sick and disabled, which solidified orthopedics’ position as a major medical specialty.1 For example, in 1863, James Knight founded the Hospital for the Ruptured and Crippled in New York City. This hospital became the oldest orthopedic hospital in the United States, and it later became known as the Hospital for Special Surgery.14,15 Several additional orthopedic institutions were formed, including the New York Orthopedic Dispensary in 1886 and Hospital for Deformities and Joint Diseases in 1917. Orthopedic surgery residency programs also began to be developed in the late 1800s.14 More specifically, Virgil Gibney at Hospital for the Ruptured and Crippled began the first orthopedic training program in the United States in 1888. Young doctors in this program trained for 1 year as junior assistant, senior assistant, and house surgeon, and began to be known as resident doctors.14
The Modern Era
In the 20th century, rapid development continued to better control infections as well as develop and introduce novel technology. For example, the invention of x-ray in 1895 by Wilhelm Conrad Röntgen improved our ability to diagnose and manage orthopedic conditions ranging from fractures to avascular necrosis of the femoral head to osteoarthritis.8,14 Spinal surgery also developed rapidly with Russell Hibbs describing a technique for spinal fusion at the New York Orthopedic Hospital.8 Similarly, the World Wars served as a catalyst in the development of the subspecialty of orthopedic trauma, with increasing attention placed on open wounds and proficiency with amputations, internal fixation, and wound care. In 1942, Austin Moore performed the first metal hip arthroplasty, and the field of joint replacement was subsequently advanced by the work of Sir John Charnley in the 1960s.8
Conclusion
Despite its relatively recent specialization, orthopedic surgery has a rich history rooted in ancient practices dating back to the primitive man. Over time, there has been significant development in the field in terms of surgical and nonsurgical treatment of orthopedic pathology and disease. Various cultures have played an instrumental role in developing this field, and it is remarkable to see that several practices have persisted since the time of these ancient civilizations. During the Renaissance, there was a considerable emphasis placed on pediatric deformity, but orthopedic surgeons have now branched out to subspecialty practice ranging from orthopedic trauma to joint replacement to oncology.1 For students of medicine and orthopedics, it is important to learn about the origins of this field and to appreciate its gradual development. Orthopedic surgery is a diverse and fascinating field that will most likely continue to develop with increased subspecialization and improved research at the molecular and population level. With a growing emphasis placed on outcomes and healthcare cost by today’s society, it will be fascinating to see how this field continues to evolve in the future.
Am J Orthop. 2016;45(7):E434-E438. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
The modern term orthopedics stems from the older word orthopedia, which was the title of a book published in 1741 by Nicholas Andry, a professor of medicine at the University of Paris.1 The term orthopedia is a composite of 2 Greek words: orthos, meaning “straight and free from deformity,” and paidios, meaning “child.” Together, orthopedics literally means straight child, suggesting the importance of pediatric injuries and deformities in the development of this field. Interestingly, Andry’s book also depicted a crooked young tree attached to a straight and strong staff, which has become the universal symbol of orthopedic surgery and underscores the focus on correcting deformities in the young (Figure).1
Orthopedic surgery is a rapidly advancing medical field with several recent advances noted within orthopedic subspecialties,2-4 basic science,5 and clinical research.6 It is important to recognize the role of history with regards to innovation and research, especially for young trainees and medical students interested in a particular medical specialty. More specifically, it is important to understand the successes and failures of the past in order to advance research and practice, and ultimately improve patient care and outcomes.
In the recent literature, there is no concise yet comprehensive article focusing on the history of orthopedic surgery. The goal of this review is to provide an overview of the history and development of orthopedic surgery from ancient practices to the modern era.
Ancient Orthopedics
While the evidence is limited, the practice of orthopedics dates back to the primitive man.7 Fossil evidence suggests that the orthopedic pathology of today, such as fractures and traumatic amputations, existed in primitive times.8 The union of fractures in fair alignment has also been observed, which emphasizes the efficacy of nonoperative orthopedics and suggests the early use of splints and rehabilitation practices.8,9 Since procedures such as trepanation and crude amputations occurred during the New Stone Age, it is feasible that sophisticated techniques had also been developed for the treatment of injuries.7-9 However, evidence continues to remain limited.7
Later civilizations also developed creative ways to manage orthopedic injuries. For example, the Shoshone Indians, who were known to exist around 700-2000 BCE, made a splint of fresh rawhide that had been soaked in water.9,10 Similarly, some South Australian tribes made splints of clay, which when dried were as good as plaster of Paris.9 Furthermore, bone-setting or reductions was practiced as a profession in many tribes, underscoring the importance of orthopedic injuries in early civilizations.8,9
Ancient Egypt
The ancient Egyptians seemed to have carried on the practices of splinting. For example, 2 splinted specimens were discovered during the Hearst Egyptian Expedition in 1903.7 More specifically, these specimens included a femur and forearm and dated to approximately 300 BCE.7 Other examples of splints made of bamboo and reed padded with linen have been found on mummies as well.8 Similarly, crutches were also used by this civilization, as depicted on a carving made on an Egyptian tomb in 2830 BCE.8
One of the earliest and most significant documents on medicine was discovered in 1862, known as the Edwin Smith papyrus. This document is thought to have been composed by Imhotep, a prominent Egyptian physician, astrologer, architect, and politician, and it specifically categorizes diseases and treatments. Many scholars recognize this medical document as the oldest surgical textbook.11,12 With regards to orthopedic conditions, this document describes the reduction of a dislocated mandible, signs of spinal or vertebral injuries, description of torticollis, and the treatment of fractures such as clavicle fractures.8 This document also discusses ryt, which refers to the purulent discharge from osteomyelitis.8 The following is an excerpt from this ancient document:9
“Instructions on erring a break in his upper arm…Thou shouldst spread out with his two shoulders in order to stretch apart his upper arm until that break falls into its place. Thou shouldst make for him two splints of linen, and thou shouldst apply for him one of them both on the inside of his arm, and the other of them both on the underside of his arm.”
This account illustrates the methodical and meticulous nature of this textbook, and it highlights some of the essentials of medical practice from diagnosis to medical decision-making to treatment.
There are various other contributions to the field of medicine from the Far East; however, many of these pertain to the fields of plastic surgery and general surgery.9
Greeks and Romans
The Greeks are considered to be the first to systematically employ the scientific approach to medicine.8 In the period between 430 BCE to 330 BCE, the Corpus Hippocrates was compiled, which is a Greek text on medicine. It is named for Hippocrates (460 BCE-370 BCE), the father of medicine, and it contains text that applies specifically to the field of orthopedic surgery. For example, this text discuses shoulder dislocations and describes various reduction maneuvers. Hippocrates had a keen understanding of the principles of traction and countertraction, especially as it pertains to the musculoskeletal system.8 In fact, the Hippocratic method is still used for reducing anterior shoulder dislocations, and its description can be found in several modern orthopedic texts, including recent articles.13 The Corpus Hippocrates also describes the correction of clubfoot deformity, and the treatment of infected open fractures with pitch cerate and wine compresses.8
Hippocrates also described the treatment of fractures, the principles of traction, and the implications of malunions. For example, Hippocrates wrote, “For the arm, when shortened, might be concealed and the mistake will not be great, but a shortened thigh bone will leave a man maimed.”1 In addition, spinal deformities were recognized by the Greeks, and Hippocrates devised an extension bench for the correction of such deformities.1 From their contributions to anatomy and surgical practice, the Greeks have made significant contributions to the field of surgery.9
During the Roman period, another Greek surgeon by the name of Galen described the musculoskeletal and nervous systems. He served as a gladiatorial surgeon in Rome, and today, he is considered to be the father of sports medicine.8 He is also credited with coining the terms scoliosis, kyphosis, and lordosis to denote the spinal deformities that were first described by Hippocrates.1 In the Roman period, amputations were also performed, and primitive prostheses were developed.9
The Middle Ages
There was relatively little progress in the study of medicine for a thousand years after the fall of the Roman Empire.9 This stagnation was predominantly due to the early Christian Church inhibiting freedom of thought and observation, as well as prohibiting human dissection and the study of anatomy. The first medical school in Europe was established in Salerno, Italy, during the ninth century. This school provided primarily pedantic teaching to its students and perpetuated the theories of the elements and humors. Later on, the University of Bologna became one of the first academic institutions to offer hands-on surgical training.9 One of the most famous surgeons of the Middle Ages was Guy de Chuauliac, who studied at Montpellier and Bologna. He was a leader in the ethical principles of surgery as well as the practice of surgery, and wrote the following with regards to femur fractures:9
“After the application of splints, I attach to the foot a mass of lead as a weight, taking care to pass the cord which supports the weight over a small pulley in such a manner that it shall pull on the leg in a horizontal direction.”
This description is strikingly similar to the modern-day nonoperative management of femur fractures, and underscores the importance of traction, which as mentioned above, was first described by Hippocrates.
Eventually, medicine began to separate from the Church, most likely due to an increase in the complexity of medical theories, the rise of secular universities, and an increase in medical knowledge from Eastern and Middle-Eastern groups.9
The Renaissance and the Foundations of Modern Orthopedics
Until the 16th century, the majority of medical theories were heavily influenced by the work of Hippocrates.8 The scientific study of anatomy gained prominence during this time, especially due to the work done by great artists, such as Leonardo Di Vinci.9 The Table
After a period of rapid expansion of the field of orthopedics, and following the Renaissance, many hospitals were built focusing on the sick and disabled, which solidified orthopedics’ position as a major medical specialty.1 For example, in 1863, James Knight founded the Hospital for the Ruptured and Crippled in New York City. This hospital became the oldest orthopedic hospital in the United States, and it later became known as the Hospital for Special Surgery.14,15 Several additional orthopedic institutions were formed, including the New York Orthopedic Dispensary in 1886 and Hospital for Deformities and Joint Diseases in 1917. Orthopedic surgery residency programs also began to be developed in the late 1800s.14 More specifically, Virgil Gibney at Hospital for the Ruptured and Crippled began the first orthopedic training program in the United States in 1888. Young doctors in this program trained for 1 year as junior assistant, senior assistant, and house surgeon, and began to be known as resident doctors.14
The Modern Era
In the 20th century, rapid development continued to better control infections as well as develop and introduce novel technology. For example, the invention of x-ray in 1895 by Wilhelm Conrad Röntgen improved our ability to diagnose and manage orthopedic conditions ranging from fractures to avascular necrosis of the femoral head to osteoarthritis.8,14 Spinal surgery also developed rapidly with Russell Hibbs describing a technique for spinal fusion at the New York Orthopedic Hospital.8 Similarly, the World Wars served as a catalyst in the development of the subspecialty of orthopedic trauma, with increasing attention placed on open wounds and proficiency with amputations, internal fixation, and wound care. In 1942, Austin Moore performed the first metal hip arthroplasty, and the field of joint replacement was subsequently advanced by the work of Sir John Charnley in the 1960s.8
Conclusion
Despite its relatively recent specialization, orthopedic surgery has a rich history rooted in ancient practices dating back to the primitive man. Over time, there has been significant development in the field in terms of surgical and nonsurgical treatment of orthopedic pathology and disease. Various cultures have played an instrumental role in developing this field, and it is remarkable to see that several practices have persisted since the time of these ancient civilizations. During the Renaissance, there was a considerable emphasis placed on pediatric deformity, but orthopedic surgeons have now branched out to subspecialty practice ranging from orthopedic trauma to joint replacement to oncology.1 For students of medicine and orthopedics, it is important to learn about the origins of this field and to appreciate its gradual development. Orthopedic surgery is a diverse and fascinating field that will most likely continue to develop with increased subspecialization and improved research at the molecular and population level. With a growing emphasis placed on outcomes and healthcare cost by today’s society, it will be fascinating to see how this field continues to evolve in the future.
Am J Orthop. 2016;45(7):E434-E438. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Ponseti IV. History of orthopedic surgery. Iowa Orthop J. 1991;11:59-64.
2. Ninomiya JT, Dean JC, Incavo SJ. What’s new in hip replacement. J Bone Joint Surg Am. 2015;97(18):1543-1551.
3. Sabharwal S, Nelson SC, Sontich JK. What’s new in limb lengthening and deformity correction. J Bone Joint Surg Am. 2015;97(16):1375-1384.
4. Ricci WM, Black JC, McAndrew CM, Gardner MJ. What’s new in orthopedic trauma. J Bone Joint Surg Am. 2015;97(14):1200-1207.
5. Rodeo SA, Sugiguchi F, Fortier LA, Cunningham ME, Maher S. What’s new in orthopedic research. J Bone Joint Surg Am. 2014;96(23):2015-2019.
6. Pugley AJ, Martin CT, Harwood J, Ong KL, Bozic KJ, Callaghan JJ. Database and registry research in orthopedic surgery. Part 1: Claims-based data. J Bone Joint Surg Am. 2015;97(15):1278-1287.
7. Colton CL. The history of fracture treatment. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma: Basic Science, Management, and Reconstruction. 4th ed. Philadelphia, PA: Saunders Elsevier; 2009:3-32.
8. Brakoulias,V. History of orthopaedics. WorldOrtho Web site. http://pioa.net/documents/Historyoforthopaedics.pdf. Accessed October 6, 2016.
9. Bishop WJ. The Early History of Surgery. New York, NY: Barnes & Noble Books; 1995.
10. Watson T. Wyoming site reveals more prehistoric mountain villages. USA Today. October 20, 2013. http://www.usatoday.com/story/news/nation/2013/10/20/wyoming-prehistoric-villages/2965263. Accessed October 6, 2016.
11. Minagar A, Ragheb J, Kelley RE. The Edwin Smith surgical papyrus: description and analysis of the earliest case of aphasia. J Med Biogr. 2003;11(2):114-117.
12. Atta HM. Edwin Smith Surgical Papyrus: the oldest known surgical treatise. Am Surg. 1999;65(12):1190-1192.
13. Sayegh FE, Kenanidis EI, Papavasiliou KA, Potoupnis ME, Kirkos JM, Kapetanos GA. Reduction of acute anterior dislocations: a prospective randomized study comparing a new technique with the Hippocratic and Kocher methods. J Bone Joint Surg Am. 2009;91(12):2775-2782.
14. Levine DB. Anatomy of a Hospital: Hospital for Special Surgery 1863-2013. New York, NY: Print Mattes; 2013.
15. Wilson PD, Levine DB. Hospital for special surgery. A brief review of its development and current position. Clin Orthop Relat Res. 2000;(374):90-106.
1. Ponseti IV. History of orthopedic surgery. Iowa Orthop J. 1991;11:59-64.
2. Ninomiya JT, Dean JC, Incavo SJ. What’s new in hip replacement. J Bone Joint Surg Am. 2015;97(18):1543-1551.
3. Sabharwal S, Nelson SC, Sontich JK. What’s new in limb lengthening and deformity correction. J Bone Joint Surg Am. 2015;97(16):1375-1384.
4. Ricci WM, Black JC, McAndrew CM, Gardner MJ. What’s new in orthopedic trauma. J Bone Joint Surg Am. 2015;97(14):1200-1207.
5. Rodeo SA, Sugiguchi F, Fortier LA, Cunningham ME, Maher S. What’s new in orthopedic research. J Bone Joint Surg Am. 2014;96(23):2015-2019.
6. Pugley AJ, Martin CT, Harwood J, Ong KL, Bozic KJ, Callaghan JJ. Database and registry research in orthopedic surgery. Part 1: Claims-based data. J Bone Joint Surg Am. 2015;97(15):1278-1287.
7. Colton CL. The history of fracture treatment. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, eds. Skeletal Trauma: Basic Science, Management, and Reconstruction. 4th ed. Philadelphia, PA: Saunders Elsevier; 2009:3-32.
8. Brakoulias,V. History of orthopaedics. WorldOrtho Web site. http://pioa.net/documents/Historyoforthopaedics.pdf. Accessed October 6, 2016.
9. Bishop WJ. The Early History of Surgery. New York, NY: Barnes & Noble Books; 1995.
10. Watson T. Wyoming site reveals more prehistoric mountain villages. USA Today. October 20, 2013. http://www.usatoday.com/story/news/nation/2013/10/20/wyoming-prehistoric-villages/2965263. Accessed October 6, 2016.
11. Minagar A, Ragheb J, Kelley RE. The Edwin Smith surgical papyrus: description and analysis of the earliest case of aphasia. J Med Biogr. 2003;11(2):114-117.
12. Atta HM. Edwin Smith Surgical Papyrus: the oldest known surgical treatise. Am Surg. 1999;65(12):1190-1192.
13. Sayegh FE, Kenanidis EI, Papavasiliou KA, Potoupnis ME, Kirkos JM, Kapetanos GA. Reduction of acute anterior dislocations: a prospective randomized study comparing a new technique with the Hippocratic and Kocher methods. J Bone Joint Surg Am. 2009;91(12):2775-2782.
14. Levine DB. Anatomy of a Hospital: Hospital for Special Surgery 1863-2013. New York, NY: Print Mattes; 2013.
15. Wilson PD, Levine DB. Hospital for special surgery. A brief review of its development and current position. Clin Orthop Relat Res. 2000;(374):90-106.
When Losing Weight Leads to Gaining Weight
Just what someone with diabetes who’s successfully losing weight doesn’t want to hear: Losing weight may boost appetite significantly.
NIH researchers analyzed data from a year-long study of 242 people, of whom 153 were taking canagliflozin, a drug that increases the amount of glucose excreted in urine. That calorie loss led to a gradual weight loss that averaged 8 pounds. The patients were not “directly aware” of the calorie loss, the researchers say.
Related: A VA-Based, Multidisciplinary Weight Management Program
The participants could eat and drink without restriction. Using a math model, the researchers calculated the changes in the amount of calories consumed during the study. They found no long-term calorie intake changes in the 89 people on placebo. By contrast, for every pound of lost weight, the canagliflozin patients consumed about 50 calories more per day than they were eating before the study. After about 6 months, the extra appetite-fueled calories led to a plateau in weight loss.
The findings didn’t entirely surprise the researchers. In an earlier study of participants in a weight loss program not involving canagliflozin, something similar happened. Despite the dieters’ consistent efforts, increased appetite led to a calorie intake 3 times stronger than the changes in caloric expenditure that typically accompany weight loss, the researchers say—and a plateau.
Related: Keeping Diabetes at Bay
The study provides the “first quantification of the homeostatic control of energy intake in free-living humans,” the researchers say. While energy expenditure adaptations often are thought to be the main reasons for slowing of weight loss and subsequent regain, feedback control of energy intake plays an even larger role and helps explain why long-term maintenance of a reduced body weight is so difficult, the researchers say.
The conclusion? “Persistent effort is required to avoid weight gain,” the NIH report notes. Unfortunately, “weight regain is typical in the absence of heroic and vigilant efforts to maintain behavior changes in the face of an omnipresent obesogenic environment.”
Related: A Call to Action: Intensive Lifestyle Intervention Against Diabesity
Just what someone with diabetes who’s successfully losing weight doesn’t want to hear: Losing weight may boost appetite significantly.
NIH researchers analyzed data from a year-long study of 242 people, of whom 153 were taking canagliflozin, a drug that increases the amount of glucose excreted in urine. That calorie loss led to a gradual weight loss that averaged 8 pounds. The patients were not “directly aware” of the calorie loss, the researchers say.
Related: A VA-Based, Multidisciplinary Weight Management Program
The participants could eat and drink without restriction. Using a math model, the researchers calculated the changes in the amount of calories consumed during the study. They found no long-term calorie intake changes in the 89 people on placebo. By contrast, for every pound of lost weight, the canagliflozin patients consumed about 50 calories more per day than they were eating before the study. After about 6 months, the extra appetite-fueled calories led to a plateau in weight loss.
The findings didn’t entirely surprise the researchers. In an earlier study of participants in a weight loss program not involving canagliflozin, something similar happened. Despite the dieters’ consistent efforts, increased appetite led to a calorie intake 3 times stronger than the changes in caloric expenditure that typically accompany weight loss, the researchers say—and a plateau.
Related: Keeping Diabetes at Bay
The study provides the “first quantification of the homeostatic control of energy intake in free-living humans,” the researchers say. While energy expenditure adaptations often are thought to be the main reasons for slowing of weight loss and subsequent regain, feedback control of energy intake plays an even larger role and helps explain why long-term maintenance of a reduced body weight is so difficult, the researchers say.
The conclusion? “Persistent effort is required to avoid weight gain,” the NIH report notes. Unfortunately, “weight regain is typical in the absence of heroic and vigilant efforts to maintain behavior changes in the face of an omnipresent obesogenic environment.”
Related: A Call to Action: Intensive Lifestyle Intervention Against Diabesity
Just what someone with diabetes who’s successfully losing weight doesn’t want to hear: Losing weight may boost appetite significantly.
NIH researchers analyzed data from a year-long study of 242 people, of whom 153 were taking canagliflozin, a drug that increases the amount of glucose excreted in urine. That calorie loss led to a gradual weight loss that averaged 8 pounds. The patients were not “directly aware” of the calorie loss, the researchers say.
Related: A VA-Based, Multidisciplinary Weight Management Program
The participants could eat and drink without restriction. Using a math model, the researchers calculated the changes in the amount of calories consumed during the study. They found no long-term calorie intake changes in the 89 people on placebo. By contrast, for every pound of lost weight, the canagliflozin patients consumed about 50 calories more per day than they were eating before the study. After about 6 months, the extra appetite-fueled calories led to a plateau in weight loss.
The findings didn’t entirely surprise the researchers. In an earlier study of participants in a weight loss program not involving canagliflozin, something similar happened. Despite the dieters’ consistent efforts, increased appetite led to a calorie intake 3 times stronger than the changes in caloric expenditure that typically accompany weight loss, the researchers say—and a plateau.
Related: Keeping Diabetes at Bay
The study provides the “first quantification of the homeostatic control of energy intake in free-living humans,” the researchers say. While energy expenditure adaptations often are thought to be the main reasons for slowing of weight loss and subsequent regain, feedback control of energy intake plays an even larger role and helps explain why long-term maintenance of a reduced body weight is so difficult, the researchers say.
The conclusion? “Persistent effort is required to avoid weight gain,” the NIH report notes. Unfortunately, “weight regain is typical in the absence of heroic and vigilant efforts to maintain behavior changes in the face of an omnipresent obesogenic environment.”
Related: A Call to Action: Intensive Lifestyle Intervention Against Diabesity
Application of Amniotic Tissue in Orthopedic Surgery
The amniotic membrane is a multilayer tissue forming the innermost layer of the amniotic sac that surrounds the developing fetus. It is comprised of 5 layers, from the inside out: a single layer of epithelial cells, a thick basement membrane, a compact layer, a fibroblast layer, and a spongy layer that abuts the surrounding chorion (Figure 1).1
Amniotic epithelial cells are derived from the pluripotent epiblast at approximately day 8 of gestation. This is well before gastrulation occurs at days 15 to 17, considered the “tipping point” when pluripotent cells differentiate into ectoderm, mesoderm, and endoderm.3 These cells express Oct-4 and Nanog, 2 molecular markers that are indicative of pluripotency.3 Two cell types have been identified in amniotic tissues that possess stem cell-like characteristics: human amniotic epithelial cells and human amniotic mesenchymal stromal cells.4 Both of these cell types have demonstrated the ability to differentiate into various cell lineages, including endothelial cells, adipocytes, myogenic cells, neurogenic cells, chondrocytes, tenocytes, and osteogenic cells.5-7 These previously reported findings indicate that amniotic cells and tissue have the capability to generate mesenchymal tissues.
FDA Classification and Available Forms
The US Food and Drug Administration (FDA) classifies amnion as an allograft tissue under Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) 361. To meet criteria, the tissue needs to be minimally manipulated. It is to be for homologous use and cannot be combined with other cells or tissues. There can be no systemic effect or dependence on the metabolic activity of living cells to achieve its primary function. The tissue has to have a localized effect in vivo. Therefore, amnion allograft tissue can be commercialized, provided it is not marketed as a stem cell product or to contain viable cells.
Amniotic tissue is commercially available in several forms.
Safety
Amniotic tissue has been used for over 100 years in burn, ophthalmology, and chronic wound patients with favorable outcomes and no adverse effects reported in the literature. Unlike embryonic stem cells, which may be tumorigenic,8 amniotic cells do not possess any known tumorigenicity.9 In one study, 50 immunodeficient mice were injected with 1 to 2 million amniotic epithelial cells and observed for a maximum of 516 days with no tumorigenicity observed in any of the animals.10 In another study, amniotic epithelial cells were implanted into the forearms of healthy volunteers and no immunologic response was observed in any of the recipients.11 Furthermore, viable amniotic cells were recovered via biopsy 7 weeks following transplantation, demonstrating viability of the transplanted cells.11 The lack of tumorigenicity and immunologic response in hosts is due in part to the fact that amniotic cells do not express human leukocyte antigen class II antigens and only express class I antigens in small amounts.3
Advantages of Amnion Tissue
Amniotic tissue is readily available, as it is often discarded after childbirth. The use of this tissue poses no added risk to the fetus or mother, eliminating the ethical concerns associated with obtaining embryonic stem cells. Amniotic tissue is comprised of an extracellular matrix, which acts as a natural scaffold for cellular attachment and structural support for cells as well as collagen types I, III, IV, V, and VI, hyaluronic acid, and a host of growth factors.12 In addition, it possesses antimicrobial properties, including beta-defensins.13
Amniotic tissue has been shown to exert an anti-inflammatory effect by inhibiting the inflammatory cascade. Specifically, it has been shown to inhibit cytokines such as tumor necrosis factor-alpha in the presence of dendritic cells,14 as well as inhibiting transforming growth factor-beta, interleukin-8, and fibroblast proliferation.15 These findings indicate that amniotic tissue has the ability to dampen the “cytokine storm” that occurs after an injury in an adult, which would lead to beneficial impacts on healing and scar formation in patients.16
Basic Science and Animal Studies
Several studies have demonstrated promising outcomes for orthopedic applications in vitro. A comparison of osteogenic potential found that amniotic fluid-derived cells were able to produce approximately 5 times more mineralized matrix than bone marrow-derived mesenchymal stem cells.17 More recently, Si and colleagues18 compared the osteogenic potential of human amniotic epithelial cells, amniotic cells, and human bone marrow-derived mesenchymal stem cells. They found that all 3 cell lines were osteogenic, though the amniotic epithelial cells had better immunomodulatory properties and marginally less osteogenic potential than the other 2 cell types. Furthermore, several in vivo animal studies have demonstrated the ability of human amniotic cells to stimulate bone growth in rats,19,20 rabbits,21 and sheep.22
Amniotic tissue also possesses potential for chondrogenesis. Cryopreserved human amniotic membrane cells used for in vitro human osteoarthritis tissue scaffolds did not differentiate in culture, and they integrated and repaired damaged articular cartilage.23 Various in vitro24,25 and animal in vivo26,27 studies have reported similar supportive findings. Kunisaki and colleagues28 used sheep amniotic fluid mesenchymal stem cells to reconstruct lamb tracheal cartilage in utero, concluding that cells obtained from the amniotic fluid possess chondrogenic capabilities. Further in utero lamb studies of cartilage artificial defects, given 7 days to settle before adding a hypocellular matrix as a scaffold, showed chondrocyte density and cell architecture was restored at the defect site after 28 days without the formation of an inflammatory response or scar tissue.29
Amniotic tissue has had similar success in tendon repair studies in vivo.9,30,31 Barboni and colleagues32 implanted amniotic epithelial cells (AECs) into artificially created sheep Achilles tendon defects in situ, inducing superior structural and mechanical recovery in the defects at a faster rate compared to controls not receiving AECs. Healing via AECs started at the healthy tissue around the borders of the defect and progressed centrally, suggesting recruitment of native progenitor cells to the lesion.32 Kueckelhaus and colleagues33 investigated the role of amnion-derived cellular cytokine solution in the healing of transections of rat Achilles tendons, reporting improved mechanical properties of healing tendons at early time points compared to controls. Beredjiklian and colleagues34 compared the healing of transected extensor tendons of pregnant ewes and of their fetus in utero, reporting a reparative form of healing with scar formation in adult subjects and regenerative form of healing without scar formation or inflammation in fetal subjects.
Amniotic tissue has properties that prevent adhesion formation around tendons following injury and reconstruction.35 Ozgenel36 investigated the effects of hyaluronic acid and amniotic membrane alone and in combination on the presence of adhesions and the rate of healing following chicken flexor tendon repair. The study found amniotic membrane wrapped around the repaired tendon was superior in preventing adhesion formation. Kim and colleagues37 report a similar reduction in fibrosis and adhesion following application of a human amniotic membrane wrap to rabbit ulnar neurorrhaphy sites.
This barrier function of amniotic tissue has also been investigated in the prevention of surgical scarring and peridural fibrosis in animal models following spinal discectomy. A study in canine models showed a reduction of scarring following the application of cross-linked amniotic membrane compared to freeze dried amniotic membrane.38 Similar reductions in scarring in rat models with the application of freeze-dried amniotic membrane compared to negative controls have been reported.39
Human Studies
A randomized trial investigated the outcomes of prenatal vs postnatal repair of myelomeningocele in humans, finding a reduced need for implanted shunts and improved functional outcomes at 30 months of life in the prenatal intervention group compared to the postnatal group.40 This study was concluded early due to the efficacy of prenatal surgery and the benefit of nervous system repair in utero in the presence of amniotic growth factors.
Vines and colleagues41 performed a 6-patient feasibility study using amnion injections to treat symptomatic knee osteoarthritis. Each patient received a single intra-articular cryopreserved amniotic suspension allograft (ASA) injection and was followed for 1 year. No adverse outcomes were reported, with the only abnormal finding being a small increase in serum immunoglobulin G and immunoglobulin E levels. Intra-articular ASA injection was found to be safe, but a large-scale trial investigating symptomatic relief was recommended.41
Most of the human studies using amnion pertain to foot and ankle surgery. Its use as a treatment for diabetic foot ulcers and recalcitrant plantar fasciitis was one of the early-recognized successes.42-45 Zelen and colleagues46 investigated the applications of injectable micronized dehydrated human amniotic/chorionic membrane as an alternative to surgical intervention in the treatment of refractory plantar fasciitis. This prospective, randomized trial with 45 patients showed significant improvement in plantar fasciitis symptoms at 8 weeks compared to controls (saline injections). A similar study compared the use of cryopreserved human amniotic membrane (c-hAM) injections to corticosteroid injections in plantar fasciitis patients.47 The results indicated that c-hAM is safe and comparable to corticosteroids, with the authors noting that pain improvement was greatest in patients receiving 2 injections of c-hAM at 18 weeks.
Tendon wrapping, in which the amniotic membrane is laid over a tendon repair, has been reported with success. Amniotic membrane is superior to collagen for tendon wrapping as it actively contributes to healing while minimizing adhesions, which collagen alone cannot do.48 The membrane serves as a protective sheath around repaired tendons with anti-inflammatory, anti-adhesive, immunomodulatory, and antimicrobial benefits. A 124-patient study demonstrated the safety of using amnion in this manner, and the authors reported a decreased rate of complication compared to previously published data.49 Another study of 14 patients undergoing foot and ankle surgery with tendon wrapping reported clinical improvement with reduced pain and greater functional outcomes postoperatively compared to preoperative measurements.50
Conclusion
Amniotic membrane-derived tissues are safe and non-tumorigenic, producing an abundance of growth factors that have shown promise as tissue scaffolds and as aids in the regeneration of human bone and soft tissues. Amnion applications in orthopedic surgery may be numerous, but development is ongoing. Given the vast array of in vitro and in vivo animal data supporting the benefits of amnion in tissue regeneration, orthopedic surgeons and researchers should place emphasis on conducting clinical studies to validate the safety and efficacy of amniotic cells in the treatment of orthopedic conditions.
Am J Orthop. 2016;45(7):E421-E425. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Benirschke K, Kaufman P. Anatomy and pathology of the placental membranes. In: Pathology of the Human Placent., 4th ed. New York, NY: Springer-Verlag; 2000:281-334.
2. Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349(2):447-458.
3. Miki T, Strom SC. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006;2(2):133-142.
4. Parolini O, Alviano F, Bagnara GP, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008;26(2):300-311.
5. Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77(3):577-588.
6. Alviano F, Fossati V, Marchionni C, et al. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol. 2007;7:11.
7. Barboni B, Curini V, Russo V, et al. Indirect co-culture with tendons or tenocytes can program amniotic epithelial cells towards stepwise tenogenic differentiation. PLoS One. 2012;7(2):e30974.
8. Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nature Reviews Cancer. 2011;11(4):268-277.
9. Lange-Consiglio A, Rossi D, Tassan S, Perego R, Cremonesi F, Parolini O. Conditioned medium from horse amniotic membrane-derived multipotent progenitor cells: immunomodulatory activity in vitro and first clinical application in tendon and ligament injuries in vivo. Stem Cells Dev. 2013;22(22):3015-3024.
10. Miki T. Amnion-derived stem cells: in quest of clinical applications. Stem Cell Res Ther. 2011;2(3):25.
11. Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2(8254):1003-1035.
12. Gupta A, Kedige SD, Jain K. Amnion and chorion membranes: potential stem cell reservoir with wide applications in periodontics. Int J Biomater. 2015;2015:274082.
13. Buhimschi IA, Jabr M, Buhimschi CS, Petkova AP, Weiner CP, Saed GM. The novel antimicrobial peptide beta3-defensin is produced by the amnion: a possible role of the fetal membranes in innate immunity of the amniotic cavity. Am J Obstet Gynecol. 2004;191(5):1678-1687.
14. Magatti M, De Munari S, Vertua E, et al. Amniotic mesenchymal tissue cells inhibit dendritic cell differentiation of peripheral blood and amnion resident monocytes. Cell Transplant. 2009;18(8):899-914.
15. Solomon A, Wajngarten M, Alviano F, et al. Suppression of inflammatory and fibrotic responses in allergic inflammation by the amniotic membrane stromal matrix. Clin Exp Allergy. 2005;35(7):941-948.
16. Silini A, Parolini O, Huppertz B, Lang I. Soluble factors of amnion-derived cells in treatment of inflammatory and fibrotic pathologies. Curr Stem Cell Res Ther. 2013;8(1):6-14.
17. Peister A, Woodruff MA, Prince JJ, Gray DP, Hutmacher DW, Guldberg RE. Cell sourcing for bone tissue engineering: amniotic fluid stem cells have a delayed, robust differentiation compared to mesenchymal stem cells. Stem Cell Res. 2011;7(1):17-27.
18. Si J, Dai J, Zhang J, et al. Comparative investigation of human amniotic epithelial cells and mesenchymal stem cells for application in bone tissue engineering. Stem Cells Int. 2015;2015:565732.
19. Starecki M, Schwartz JA, Grande DA. Evaluation of amniotic-derived membrane biomaterial as an adjunct for repair of critical sized bone defects. Advances in Orthopedic Surgery. 2014;2014:572586.
20. Kerimoglu S, Livaoglu M, Sönmez B, et al. Effects of human amniotic fluid on fracture healing in rat tibia. J Surg Res. 2009;152(2):281-287.
21. Karaçal N, Kocucu P, Cobanglu U, Kutlu N. Effect of human amniotic fluid on bone healing. J Surg Res. 2005;129(2):283-287.
22. Barboni B, Mangano C, Valbonetti L, et al. Synthetic bone substitute engineered with amniotic epithelial cells enhances bone regeneration after maxillary sinus augmentation. PLoS One. 2013;8(5):e63256.
23. Díaz-Prado S, Rendal-Vázquez ME, Muiños-Lopez E, et al. Potential use of the human amniotic membrane as a scaffold in human articular cartilage repair. Cell Tissue Bank. 2010;11(2):183-195.
24. Krishnamurithy G, Shilpa PN, Ahmad RE, Sulaiman S, Ng CL, Kamarul T. Human amniotic membrane as a chondrocyte carrier vehicle/substrate: in vitro study. J Biomed Mater Res A. 2011;99(3):500-506.
25. Tan SL, Sulaiman S, Pingguan-Murphy B, Selvaratnam L, Tai CC, Kamarul T. Human amnion as a novel cell delivery vehicle for chondrogenic mesenchymal stem cells. Cell Tissue Bank. 2011;12(1):59-70.
26. Jin CZ, Park SR, Choi BH, Lee KY, Kang CK, Min BH. Human amniotic membrane as a delivery matrix for articular cartilage repair. Tissue Eng. 2007;13(4):693-702.
27. Garcia D, Longo UG, Vaquero J, et al. Amniotic membrane transplant for articular cartilage repair: an experimental study in sheep. Curr Stem Cell Res Ther. 2014;10(1):77-83.
28. Kunisaki SM, Freedman DA, Fauza DO. Fetal tracheal reconstruction with cartilaginous grafts engineered from mesenchymal amniocytes. J Pediatr Surg. 2006;41(4):675-682.
29. Namba RS, Meuli M, Sullivan KM, Le AX, Adzick NS. Spontaneous repair of superficial defects in articular cartilage in a fetal lamb model. J Bone Joint Surg Am. 1998;80(1):4-10.
30. Philip J, Hackl F, Canseco JA, et al. Amnion-derived multipotent progenitor cells improve achilles tendon repair in rats. Eplasty. 2013;13:e31.
31. Lange-Consiglio A, Tassan S, Corradetti B, et al. Investigating the efficacy of amnion-derived compared with bone marrow–derived mesenchymal stromal cells in equine tendon and ligament injuries. Cytotherapy. 2013;15(8):1011-1020.
32. Barboni B, Russo V, Curini V, et al. Achilles tendon regeneration can be improved by amniotic epithelial cell allotransplantation. Cell Transplant. 2012;21(11):2377-2395.
33. Kueckelhaus M, Philip J, Kamel RA, et al. Sustained release of amnion-derived cellular cytokine solution facilitates achilles tendon healing in rats. Eplasty. 2014;14:e29.
34. Beredjiklian PK, Favata M, Cartmell JS, Flanagan CL, Crombleholme TM, Soslowski LJ. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31(10):1143-1152.
35. Demirkan F, Colakoglu N, Herek O, Erkula G. The use of amniotic membrane in flexor tendon repair: an experimental model. Arch Orthop Trauma Surg. 2002;122(7):396-369.
36. Ozgenel GY. The effects of a combination of hyaluronic and amniotic membrane on the formation of peritendinous adhesions after flexor tendon surgery in chickens. J Bone Joint Surg Br. 2004;86(2):301-307.
37. Kim SS, Sohn SK, Lee KY, Lee MJ, Roh MS, Kim CH. Use of human amniotic membrane wrap in reducing perineural adhesions in a rabbit model of ulnar nerve neurorrhaphy. J Hand Surg Eur Vol. 2010;35(3):214-219.
38. Tao H, Fan H. Implantation of amniotic membrane to reduce postlaminectomy epidural adhesions. Eur Spine J. 2009;18(8):1202-1212.
39. Choi HJ, Kim KB, Kwon YM. Effect of amniotic membrane to reduce postlaminectomy epidural adhesion on a rat model. J Korean Neurosurg Soc. 2011;49(6):323-328.
40. Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364(11):993-1004.
41. Vines JB, Aliprantis AO, Gomoll AH, Farr J. Cryopreserved amniotic suspension for the treatment of knee osteoarthritis. J Knee Surg. 2016;29(6):443-450.
42. Zelen CM. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J Wound Care. 2013;22(7):347-348,350-351.
43. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502-507.
44. Zelen CM, Serena TE, Snyder RJ. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J. 2014;11(2):122-128.
45. Zelen CM, Snyder RJ, Serena TE, Li WW. The use of human amnion/chorion membrane in the clinical setting for lower extremity repair: a review. Clin Podiatr Med Surg. 2015;32(1):135-146.
46. Zelen CM, Poka A, Andrews J. Prospective, randomized, blinded, comparative study of injectable micronized dehydrated amniotic/chorionic membrane allograft for plantar fasciitis: a feasibility study. Foot Ankle Int. 2013;34(10):1332-1339.
47. Hanselman AE, Tidwell JE, Santrock RD. Cryopreserved human amniotic membrane injection for plantar fasciitis: a randomized, controlled, double-blind pilot study. Foot Ankle Int. 2015;36(2):151-158.
48. Jay RM. Initial clinical experience with the use of human amniotic membrane tissue during repair of posterior tibial and achilles tendons. 2009. http://encompassbiologics.com/wp-content/uploads/2015/07/DrJayClinicalExperience.pdf. Accessed September 29, 2016.
49. DeMill SL, Granata JD, McAlister JE, Berlet GC, Hyer CF. Safety analysis of cryopreserved amniotic membrane/umbilical cord tissue in foot and ankle surgery: a consecutive case series of 124 patients. Surg Technol Int. 2014;25:257-261.
50. Warner M, Lasyone L. An open-label, single-center, retrospective study of cryopreserved amniotic membrane and umbilical cord tissue as an adjunct for foot and ankle surgery. Surg Technol Int. 2014;25:251-255.
The amniotic membrane is a multilayer tissue forming the innermost layer of the amniotic sac that surrounds the developing fetus. It is comprised of 5 layers, from the inside out: a single layer of epithelial cells, a thick basement membrane, a compact layer, a fibroblast layer, and a spongy layer that abuts the surrounding chorion (Figure 1).1
Amniotic epithelial cells are derived from the pluripotent epiblast at approximately day 8 of gestation. This is well before gastrulation occurs at days 15 to 17, considered the “tipping point” when pluripotent cells differentiate into ectoderm, mesoderm, and endoderm.3 These cells express Oct-4 and Nanog, 2 molecular markers that are indicative of pluripotency.3 Two cell types have been identified in amniotic tissues that possess stem cell-like characteristics: human amniotic epithelial cells and human amniotic mesenchymal stromal cells.4 Both of these cell types have demonstrated the ability to differentiate into various cell lineages, including endothelial cells, adipocytes, myogenic cells, neurogenic cells, chondrocytes, tenocytes, and osteogenic cells.5-7 These previously reported findings indicate that amniotic cells and tissue have the capability to generate mesenchymal tissues.
FDA Classification and Available Forms
The US Food and Drug Administration (FDA) classifies amnion as an allograft tissue under Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) 361. To meet criteria, the tissue needs to be minimally manipulated. It is to be for homologous use and cannot be combined with other cells or tissues. There can be no systemic effect or dependence on the metabolic activity of living cells to achieve its primary function. The tissue has to have a localized effect in vivo. Therefore, amnion allograft tissue can be commercialized, provided it is not marketed as a stem cell product or to contain viable cells.
Amniotic tissue is commercially available in several forms.
Safety
Amniotic tissue has been used for over 100 years in burn, ophthalmology, and chronic wound patients with favorable outcomes and no adverse effects reported in the literature. Unlike embryonic stem cells, which may be tumorigenic,8 amniotic cells do not possess any known tumorigenicity.9 In one study, 50 immunodeficient mice were injected with 1 to 2 million amniotic epithelial cells and observed for a maximum of 516 days with no tumorigenicity observed in any of the animals.10 In another study, amniotic epithelial cells were implanted into the forearms of healthy volunteers and no immunologic response was observed in any of the recipients.11 Furthermore, viable amniotic cells were recovered via biopsy 7 weeks following transplantation, demonstrating viability of the transplanted cells.11 The lack of tumorigenicity and immunologic response in hosts is due in part to the fact that amniotic cells do not express human leukocyte antigen class II antigens and only express class I antigens in small amounts.3
Advantages of Amnion Tissue
Amniotic tissue is readily available, as it is often discarded after childbirth. The use of this tissue poses no added risk to the fetus or mother, eliminating the ethical concerns associated with obtaining embryonic stem cells. Amniotic tissue is comprised of an extracellular matrix, which acts as a natural scaffold for cellular attachment and structural support for cells as well as collagen types I, III, IV, V, and VI, hyaluronic acid, and a host of growth factors.12 In addition, it possesses antimicrobial properties, including beta-defensins.13
Amniotic tissue has been shown to exert an anti-inflammatory effect by inhibiting the inflammatory cascade. Specifically, it has been shown to inhibit cytokines such as tumor necrosis factor-alpha in the presence of dendritic cells,14 as well as inhibiting transforming growth factor-beta, interleukin-8, and fibroblast proliferation.15 These findings indicate that amniotic tissue has the ability to dampen the “cytokine storm” that occurs after an injury in an adult, which would lead to beneficial impacts on healing and scar formation in patients.16
Basic Science and Animal Studies
Several studies have demonstrated promising outcomes for orthopedic applications in vitro. A comparison of osteogenic potential found that amniotic fluid-derived cells were able to produce approximately 5 times more mineralized matrix than bone marrow-derived mesenchymal stem cells.17 More recently, Si and colleagues18 compared the osteogenic potential of human amniotic epithelial cells, amniotic cells, and human bone marrow-derived mesenchymal stem cells. They found that all 3 cell lines were osteogenic, though the amniotic epithelial cells had better immunomodulatory properties and marginally less osteogenic potential than the other 2 cell types. Furthermore, several in vivo animal studies have demonstrated the ability of human amniotic cells to stimulate bone growth in rats,19,20 rabbits,21 and sheep.22
Amniotic tissue also possesses potential for chondrogenesis. Cryopreserved human amniotic membrane cells used for in vitro human osteoarthritis tissue scaffolds did not differentiate in culture, and they integrated and repaired damaged articular cartilage.23 Various in vitro24,25 and animal in vivo26,27 studies have reported similar supportive findings. Kunisaki and colleagues28 used sheep amniotic fluid mesenchymal stem cells to reconstruct lamb tracheal cartilage in utero, concluding that cells obtained from the amniotic fluid possess chondrogenic capabilities. Further in utero lamb studies of cartilage artificial defects, given 7 days to settle before adding a hypocellular matrix as a scaffold, showed chondrocyte density and cell architecture was restored at the defect site after 28 days without the formation of an inflammatory response or scar tissue.29
Amniotic tissue has had similar success in tendon repair studies in vivo.9,30,31 Barboni and colleagues32 implanted amniotic epithelial cells (AECs) into artificially created sheep Achilles tendon defects in situ, inducing superior structural and mechanical recovery in the defects at a faster rate compared to controls not receiving AECs. Healing via AECs started at the healthy tissue around the borders of the defect and progressed centrally, suggesting recruitment of native progenitor cells to the lesion.32 Kueckelhaus and colleagues33 investigated the role of amnion-derived cellular cytokine solution in the healing of transections of rat Achilles tendons, reporting improved mechanical properties of healing tendons at early time points compared to controls. Beredjiklian and colleagues34 compared the healing of transected extensor tendons of pregnant ewes and of their fetus in utero, reporting a reparative form of healing with scar formation in adult subjects and regenerative form of healing without scar formation or inflammation in fetal subjects.
Amniotic tissue has properties that prevent adhesion formation around tendons following injury and reconstruction.35 Ozgenel36 investigated the effects of hyaluronic acid and amniotic membrane alone and in combination on the presence of adhesions and the rate of healing following chicken flexor tendon repair. The study found amniotic membrane wrapped around the repaired tendon was superior in preventing adhesion formation. Kim and colleagues37 report a similar reduction in fibrosis and adhesion following application of a human amniotic membrane wrap to rabbit ulnar neurorrhaphy sites.
This barrier function of amniotic tissue has also been investigated in the prevention of surgical scarring and peridural fibrosis in animal models following spinal discectomy. A study in canine models showed a reduction of scarring following the application of cross-linked amniotic membrane compared to freeze dried amniotic membrane.38 Similar reductions in scarring in rat models with the application of freeze-dried amniotic membrane compared to negative controls have been reported.39
Human Studies
A randomized trial investigated the outcomes of prenatal vs postnatal repair of myelomeningocele in humans, finding a reduced need for implanted shunts and improved functional outcomes at 30 months of life in the prenatal intervention group compared to the postnatal group.40 This study was concluded early due to the efficacy of prenatal surgery and the benefit of nervous system repair in utero in the presence of amniotic growth factors.
Vines and colleagues41 performed a 6-patient feasibility study using amnion injections to treat symptomatic knee osteoarthritis. Each patient received a single intra-articular cryopreserved amniotic suspension allograft (ASA) injection and was followed for 1 year. No adverse outcomes were reported, with the only abnormal finding being a small increase in serum immunoglobulin G and immunoglobulin E levels. Intra-articular ASA injection was found to be safe, but a large-scale trial investigating symptomatic relief was recommended.41
Most of the human studies using amnion pertain to foot and ankle surgery. Its use as a treatment for diabetic foot ulcers and recalcitrant plantar fasciitis was one of the early-recognized successes.42-45 Zelen and colleagues46 investigated the applications of injectable micronized dehydrated human amniotic/chorionic membrane as an alternative to surgical intervention in the treatment of refractory plantar fasciitis. This prospective, randomized trial with 45 patients showed significant improvement in plantar fasciitis symptoms at 8 weeks compared to controls (saline injections). A similar study compared the use of cryopreserved human amniotic membrane (c-hAM) injections to corticosteroid injections in plantar fasciitis patients.47 The results indicated that c-hAM is safe and comparable to corticosteroids, with the authors noting that pain improvement was greatest in patients receiving 2 injections of c-hAM at 18 weeks.
Tendon wrapping, in which the amniotic membrane is laid over a tendon repair, has been reported with success. Amniotic membrane is superior to collagen for tendon wrapping as it actively contributes to healing while minimizing adhesions, which collagen alone cannot do.48 The membrane serves as a protective sheath around repaired tendons with anti-inflammatory, anti-adhesive, immunomodulatory, and antimicrobial benefits. A 124-patient study demonstrated the safety of using amnion in this manner, and the authors reported a decreased rate of complication compared to previously published data.49 Another study of 14 patients undergoing foot and ankle surgery with tendon wrapping reported clinical improvement with reduced pain and greater functional outcomes postoperatively compared to preoperative measurements.50
Conclusion
Amniotic membrane-derived tissues are safe and non-tumorigenic, producing an abundance of growth factors that have shown promise as tissue scaffolds and as aids in the regeneration of human bone and soft tissues. Amnion applications in orthopedic surgery may be numerous, but development is ongoing. Given the vast array of in vitro and in vivo animal data supporting the benefits of amnion in tissue regeneration, orthopedic surgeons and researchers should place emphasis on conducting clinical studies to validate the safety and efficacy of amniotic cells in the treatment of orthopedic conditions.
Am J Orthop. 2016;45(7):E421-E425. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
The amniotic membrane is a multilayer tissue forming the innermost layer of the amniotic sac that surrounds the developing fetus. It is comprised of 5 layers, from the inside out: a single layer of epithelial cells, a thick basement membrane, a compact layer, a fibroblast layer, and a spongy layer that abuts the surrounding chorion (Figure 1).1
Amniotic epithelial cells are derived from the pluripotent epiblast at approximately day 8 of gestation. This is well before gastrulation occurs at days 15 to 17, considered the “tipping point” when pluripotent cells differentiate into ectoderm, mesoderm, and endoderm.3 These cells express Oct-4 and Nanog, 2 molecular markers that are indicative of pluripotency.3 Two cell types have been identified in amniotic tissues that possess stem cell-like characteristics: human amniotic epithelial cells and human amniotic mesenchymal stromal cells.4 Both of these cell types have demonstrated the ability to differentiate into various cell lineages, including endothelial cells, adipocytes, myogenic cells, neurogenic cells, chondrocytes, tenocytes, and osteogenic cells.5-7 These previously reported findings indicate that amniotic cells and tissue have the capability to generate mesenchymal tissues.
FDA Classification and Available Forms
The US Food and Drug Administration (FDA) classifies amnion as an allograft tissue under Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) 361. To meet criteria, the tissue needs to be minimally manipulated. It is to be for homologous use and cannot be combined with other cells or tissues. There can be no systemic effect or dependence on the metabolic activity of living cells to achieve its primary function. The tissue has to have a localized effect in vivo. Therefore, amnion allograft tissue can be commercialized, provided it is not marketed as a stem cell product or to contain viable cells.
Amniotic tissue is commercially available in several forms.
Safety
Amniotic tissue has been used for over 100 years in burn, ophthalmology, and chronic wound patients with favorable outcomes and no adverse effects reported in the literature. Unlike embryonic stem cells, which may be tumorigenic,8 amniotic cells do not possess any known tumorigenicity.9 In one study, 50 immunodeficient mice were injected with 1 to 2 million amniotic epithelial cells and observed for a maximum of 516 days with no tumorigenicity observed in any of the animals.10 In another study, amniotic epithelial cells were implanted into the forearms of healthy volunteers and no immunologic response was observed in any of the recipients.11 Furthermore, viable amniotic cells were recovered via biopsy 7 weeks following transplantation, demonstrating viability of the transplanted cells.11 The lack of tumorigenicity and immunologic response in hosts is due in part to the fact that amniotic cells do not express human leukocyte antigen class II antigens and only express class I antigens in small amounts.3
Advantages of Amnion Tissue
Amniotic tissue is readily available, as it is often discarded after childbirth. The use of this tissue poses no added risk to the fetus or mother, eliminating the ethical concerns associated with obtaining embryonic stem cells. Amniotic tissue is comprised of an extracellular matrix, which acts as a natural scaffold for cellular attachment and structural support for cells as well as collagen types I, III, IV, V, and VI, hyaluronic acid, and a host of growth factors.12 In addition, it possesses antimicrobial properties, including beta-defensins.13
Amniotic tissue has been shown to exert an anti-inflammatory effect by inhibiting the inflammatory cascade. Specifically, it has been shown to inhibit cytokines such as tumor necrosis factor-alpha in the presence of dendritic cells,14 as well as inhibiting transforming growth factor-beta, interleukin-8, and fibroblast proliferation.15 These findings indicate that amniotic tissue has the ability to dampen the “cytokine storm” that occurs after an injury in an adult, which would lead to beneficial impacts on healing and scar formation in patients.16
Basic Science and Animal Studies
Several studies have demonstrated promising outcomes for orthopedic applications in vitro. A comparison of osteogenic potential found that amniotic fluid-derived cells were able to produce approximately 5 times more mineralized matrix than bone marrow-derived mesenchymal stem cells.17 More recently, Si and colleagues18 compared the osteogenic potential of human amniotic epithelial cells, amniotic cells, and human bone marrow-derived mesenchymal stem cells. They found that all 3 cell lines were osteogenic, though the amniotic epithelial cells had better immunomodulatory properties and marginally less osteogenic potential than the other 2 cell types. Furthermore, several in vivo animal studies have demonstrated the ability of human amniotic cells to stimulate bone growth in rats,19,20 rabbits,21 and sheep.22
Amniotic tissue also possesses potential for chondrogenesis. Cryopreserved human amniotic membrane cells used for in vitro human osteoarthritis tissue scaffolds did not differentiate in culture, and they integrated and repaired damaged articular cartilage.23 Various in vitro24,25 and animal in vivo26,27 studies have reported similar supportive findings. Kunisaki and colleagues28 used sheep amniotic fluid mesenchymal stem cells to reconstruct lamb tracheal cartilage in utero, concluding that cells obtained from the amniotic fluid possess chondrogenic capabilities. Further in utero lamb studies of cartilage artificial defects, given 7 days to settle before adding a hypocellular matrix as a scaffold, showed chondrocyte density and cell architecture was restored at the defect site after 28 days without the formation of an inflammatory response or scar tissue.29
Amniotic tissue has had similar success in tendon repair studies in vivo.9,30,31 Barboni and colleagues32 implanted amniotic epithelial cells (AECs) into artificially created sheep Achilles tendon defects in situ, inducing superior structural and mechanical recovery in the defects at a faster rate compared to controls not receiving AECs. Healing via AECs started at the healthy tissue around the borders of the defect and progressed centrally, suggesting recruitment of native progenitor cells to the lesion.32 Kueckelhaus and colleagues33 investigated the role of amnion-derived cellular cytokine solution in the healing of transections of rat Achilles tendons, reporting improved mechanical properties of healing tendons at early time points compared to controls. Beredjiklian and colleagues34 compared the healing of transected extensor tendons of pregnant ewes and of their fetus in utero, reporting a reparative form of healing with scar formation in adult subjects and regenerative form of healing without scar formation or inflammation in fetal subjects.
Amniotic tissue has properties that prevent adhesion formation around tendons following injury and reconstruction.35 Ozgenel36 investigated the effects of hyaluronic acid and amniotic membrane alone and in combination on the presence of adhesions and the rate of healing following chicken flexor tendon repair. The study found amniotic membrane wrapped around the repaired tendon was superior in preventing adhesion formation. Kim and colleagues37 report a similar reduction in fibrosis and adhesion following application of a human amniotic membrane wrap to rabbit ulnar neurorrhaphy sites.
This barrier function of amniotic tissue has also been investigated in the prevention of surgical scarring and peridural fibrosis in animal models following spinal discectomy. A study in canine models showed a reduction of scarring following the application of cross-linked amniotic membrane compared to freeze dried amniotic membrane.38 Similar reductions in scarring in rat models with the application of freeze-dried amniotic membrane compared to negative controls have been reported.39
Human Studies
A randomized trial investigated the outcomes of prenatal vs postnatal repair of myelomeningocele in humans, finding a reduced need for implanted shunts and improved functional outcomes at 30 months of life in the prenatal intervention group compared to the postnatal group.40 This study was concluded early due to the efficacy of prenatal surgery and the benefit of nervous system repair in utero in the presence of amniotic growth factors.
Vines and colleagues41 performed a 6-patient feasibility study using amnion injections to treat symptomatic knee osteoarthritis. Each patient received a single intra-articular cryopreserved amniotic suspension allograft (ASA) injection and was followed for 1 year. No adverse outcomes were reported, with the only abnormal finding being a small increase in serum immunoglobulin G and immunoglobulin E levels. Intra-articular ASA injection was found to be safe, but a large-scale trial investigating symptomatic relief was recommended.41
Most of the human studies using amnion pertain to foot and ankle surgery. Its use as a treatment for diabetic foot ulcers and recalcitrant plantar fasciitis was one of the early-recognized successes.42-45 Zelen and colleagues46 investigated the applications of injectable micronized dehydrated human amniotic/chorionic membrane as an alternative to surgical intervention in the treatment of refractory plantar fasciitis. This prospective, randomized trial with 45 patients showed significant improvement in plantar fasciitis symptoms at 8 weeks compared to controls (saline injections). A similar study compared the use of cryopreserved human amniotic membrane (c-hAM) injections to corticosteroid injections in plantar fasciitis patients.47 The results indicated that c-hAM is safe and comparable to corticosteroids, with the authors noting that pain improvement was greatest in patients receiving 2 injections of c-hAM at 18 weeks.
Tendon wrapping, in which the amniotic membrane is laid over a tendon repair, has been reported with success. Amniotic membrane is superior to collagen for tendon wrapping as it actively contributes to healing while minimizing adhesions, which collagen alone cannot do.48 The membrane serves as a protective sheath around repaired tendons with anti-inflammatory, anti-adhesive, immunomodulatory, and antimicrobial benefits. A 124-patient study demonstrated the safety of using amnion in this manner, and the authors reported a decreased rate of complication compared to previously published data.49 Another study of 14 patients undergoing foot and ankle surgery with tendon wrapping reported clinical improvement with reduced pain and greater functional outcomes postoperatively compared to preoperative measurements.50
Conclusion
Amniotic membrane-derived tissues are safe and non-tumorigenic, producing an abundance of growth factors that have shown promise as tissue scaffolds and as aids in the regeneration of human bone and soft tissues. Amnion applications in orthopedic surgery may be numerous, but development is ongoing. Given the vast array of in vitro and in vivo animal data supporting the benefits of amnion in tissue regeneration, orthopedic surgeons and researchers should place emphasis on conducting clinical studies to validate the safety and efficacy of amniotic cells in the treatment of orthopedic conditions.
Am J Orthop. 2016;45(7):E421-E425. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Benirschke K, Kaufman P. Anatomy and pathology of the placental membranes. In: Pathology of the Human Placent., 4th ed. New York, NY: Springer-Verlag; 2000:281-334.
2. Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349(2):447-458.
3. Miki T, Strom SC. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006;2(2):133-142.
4. Parolini O, Alviano F, Bagnara GP, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008;26(2):300-311.
5. Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77(3):577-588.
6. Alviano F, Fossati V, Marchionni C, et al. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol. 2007;7:11.
7. Barboni B, Curini V, Russo V, et al. Indirect co-culture with tendons or tenocytes can program amniotic epithelial cells towards stepwise tenogenic differentiation. PLoS One. 2012;7(2):e30974.
8. Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nature Reviews Cancer. 2011;11(4):268-277.
9. Lange-Consiglio A, Rossi D, Tassan S, Perego R, Cremonesi F, Parolini O. Conditioned medium from horse amniotic membrane-derived multipotent progenitor cells: immunomodulatory activity in vitro and first clinical application in tendon and ligament injuries in vivo. Stem Cells Dev. 2013;22(22):3015-3024.
10. Miki T. Amnion-derived stem cells: in quest of clinical applications. Stem Cell Res Ther. 2011;2(3):25.
11. Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2(8254):1003-1035.
12. Gupta A, Kedige SD, Jain K. Amnion and chorion membranes: potential stem cell reservoir with wide applications in periodontics. Int J Biomater. 2015;2015:274082.
13. Buhimschi IA, Jabr M, Buhimschi CS, Petkova AP, Weiner CP, Saed GM. The novel antimicrobial peptide beta3-defensin is produced by the amnion: a possible role of the fetal membranes in innate immunity of the amniotic cavity. Am J Obstet Gynecol. 2004;191(5):1678-1687.
14. Magatti M, De Munari S, Vertua E, et al. Amniotic mesenchymal tissue cells inhibit dendritic cell differentiation of peripheral blood and amnion resident monocytes. Cell Transplant. 2009;18(8):899-914.
15. Solomon A, Wajngarten M, Alviano F, et al. Suppression of inflammatory and fibrotic responses in allergic inflammation by the amniotic membrane stromal matrix. Clin Exp Allergy. 2005;35(7):941-948.
16. Silini A, Parolini O, Huppertz B, Lang I. Soluble factors of amnion-derived cells in treatment of inflammatory and fibrotic pathologies. Curr Stem Cell Res Ther. 2013;8(1):6-14.
17. Peister A, Woodruff MA, Prince JJ, Gray DP, Hutmacher DW, Guldberg RE. Cell sourcing for bone tissue engineering: amniotic fluid stem cells have a delayed, robust differentiation compared to mesenchymal stem cells. Stem Cell Res. 2011;7(1):17-27.
18. Si J, Dai J, Zhang J, et al. Comparative investigation of human amniotic epithelial cells and mesenchymal stem cells for application in bone tissue engineering. Stem Cells Int. 2015;2015:565732.
19. Starecki M, Schwartz JA, Grande DA. Evaluation of amniotic-derived membrane biomaterial as an adjunct for repair of critical sized bone defects. Advances in Orthopedic Surgery. 2014;2014:572586.
20. Kerimoglu S, Livaoglu M, Sönmez B, et al. Effects of human amniotic fluid on fracture healing in rat tibia. J Surg Res. 2009;152(2):281-287.
21. Karaçal N, Kocucu P, Cobanglu U, Kutlu N. Effect of human amniotic fluid on bone healing. J Surg Res. 2005;129(2):283-287.
22. Barboni B, Mangano C, Valbonetti L, et al. Synthetic bone substitute engineered with amniotic epithelial cells enhances bone regeneration after maxillary sinus augmentation. PLoS One. 2013;8(5):e63256.
23. Díaz-Prado S, Rendal-Vázquez ME, Muiños-Lopez E, et al. Potential use of the human amniotic membrane as a scaffold in human articular cartilage repair. Cell Tissue Bank. 2010;11(2):183-195.
24. Krishnamurithy G, Shilpa PN, Ahmad RE, Sulaiman S, Ng CL, Kamarul T. Human amniotic membrane as a chondrocyte carrier vehicle/substrate: in vitro study. J Biomed Mater Res A. 2011;99(3):500-506.
25. Tan SL, Sulaiman S, Pingguan-Murphy B, Selvaratnam L, Tai CC, Kamarul T. Human amnion as a novel cell delivery vehicle for chondrogenic mesenchymal stem cells. Cell Tissue Bank. 2011;12(1):59-70.
26. Jin CZ, Park SR, Choi BH, Lee KY, Kang CK, Min BH. Human amniotic membrane as a delivery matrix for articular cartilage repair. Tissue Eng. 2007;13(4):693-702.
27. Garcia D, Longo UG, Vaquero J, et al. Amniotic membrane transplant for articular cartilage repair: an experimental study in sheep. Curr Stem Cell Res Ther. 2014;10(1):77-83.
28. Kunisaki SM, Freedman DA, Fauza DO. Fetal tracheal reconstruction with cartilaginous grafts engineered from mesenchymal amniocytes. J Pediatr Surg. 2006;41(4):675-682.
29. Namba RS, Meuli M, Sullivan KM, Le AX, Adzick NS. Spontaneous repair of superficial defects in articular cartilage in a fetal lamb model. J Bone Joint Surg Am. 1998;80(1):4-10.
30. Philip J, Hackl F, Canseco JA, et al. Amnion-derived multipotent progenitor cells improve achilles tendon repair in rats. Eplasty. 2013;13:e31.
31. Lange-Consiglio A, Tassan S, Corradetti B, et al. Investigating the efficacy of amnion-derived compared with bone marrow–derived mesenchymal stromal cells in equine tendon and ligament injuries. Cytotherapy. 2013;15(8):1011-1020.
32. Barboni B, Russo V, Curini V, et al. Achilles tendon regeneration can be improved by amniotic epithelial cell allotransplantation. Cell Transplant. 2012;21(11):2377-2395.
33. Kueckelhaus M, Philip J, Kamel RA, et al. Sustained release of amnion-derived cellular cytokine solution facilitates achilles tendon healing in rats. Eplasty. 2014;14:e29.
34. Beredjiklian PK, Favata M, Cartmell JS, Flanagan CL, Crombleholme TM, Soslowski LJ. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31(10):1143-1152.
35. Demirkan F, Colakoglu N, Herek O, Erkula G. The use of amniotic membrane in flexor tendon repair: an experimental model. Arch Orthop Trauma Surg. 2002;122(7):396-369.
36. Ozgenel GY. The effects of a combination of hyaluronic and amniotic membrane on the formation of peritendinous adhesions after flexor tendon surgery in chickens. J Bone Joint Surg Br. 2004;86(2):301-307.
37. Kim SS, Sohn SK, Lee KY, Lee MJ, Roh MS, Kim CH. Use of human amniotic membrane wrap in reducing perineural adhesions in a rabbit model of ulnar nerve neurorrhaphy. J Hand Surg Eur Vol. 2010;35(3):214-219.
38. Tao H, Fan H. Implantation of amniotic membrane to reduce postlaminectomy epidural adhesions. Eur Spine J. 2009;18(8):1202-1212.
39. Choi HJ, Kim KB, Kwon YM. Effect of amniotic membrane to reduce postlaminectomy epidural adhesion on a rat model. J Korean Neurosurg Soc. 2011;49(6):323-328.
40. Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364(11):993-1004.
41. Vines JB, Aliprantis AO, Gomoll AH, Farr J. Cryopreserved amniotic suspension for the treatment of knee osteoarthritis. J Knee Surg. 2016;29(6):443-450.
42. Zelen CM. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J Wound Care. 2013;22(7):347-348,350-351.
43. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502-507.
44. Zelen CM, Serena TE, Snyder RJ. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J. 2014;11(2):122-128.
45. Zelen CM, Snyder RJ, Serena TE, Li WW. The use of human amnion/chorion membrane in the clinical setting for lower extremity repair: a review. Clin Podiatr Med Surg. 2015;32(1):135-146.
46. Zelen CM, Poka A, Andrews J. Prospective, randomized, blinded, comparative study of injectable micronized dehydrated amniotic/chorionic membrane allograft for plantar fasciitis: a feasibility study. Foot Ankle Int. 2013;34(10):1332-1339.
47. Hanselman AE, Tidwell JE, Santrock RD. Cryopreserved human amniotic membrane injection for plantar fasciitis: a randomized, controlled, double-blind pilot study. Foot Ankle Int. 2015;36(2):151-158.
48. Jay RM. Initial clinical experience with the use of human amniotic membrane tissue during repair of posterior tibial and achilles tendons. 2009. http://encompassbiologics.com/wp-content/uploads/2015/07/DrJayClinicalExperience.pdf. Accessed September 29, 2016.
49. DeMill SL, Granata JD, McAlister JE, Berlet GC, Hyer CF. Safety analysis of cryopreserved amniotic membrane/umbilical cord tissue in foot and ankle surgery: a consecutive case series of 124 patients. Surg Technol Int. 2014;25:257-261.
50. Warner M, Lasyone L. An open-label, single-center, retrospective study of cryopreserved amniotic membrane and umbilical cord tissue as an adjunct for foot and ankle surgery. Surg Technol Int. 2014;25:251-255.
1. Benirschke K, Kaufman P. Anatomy and pathology of the placental membranes. In: Pathology of the Human Placent., 4th ed. New York, NY: Springer-Verlag; 2000:281-334.
2. Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349(2):447-458.
3. Miki T, Strom SC. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006;2(2):133-142.
4. Parolini O, Alviano F, Bagnara GP, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008;26(2):300-311.
5. Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77(3):577-588.
6. Alviano F, Fossati V, Marchionni C, et al. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol. 2007;7:11.
7. Barboni B, Curini V, Russo V, et al. Indirect co-culture with tendons or tenocytes can program amniotic epithelial cells towards stepwise tenogenic differentiation. PLoS One. 2012;7(2):e30974.
8. Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nature Reviews Cancer. 2011;11(4):268-277.
9. Lange-Consiglio A, Rossi D, Tassan S, Perego R, Cremonesi F, Parolini O. Conditioned medium from horse amniotic membrane-derived multipotent progenitor cells: immunomodulatory activity in vitro and first clinical application in tendon and ligament injuries in vivo. Stem Cells Dev. 2013;22(22):3015-3024.
10. Miki T. Amnion-derived stem cells: in quest of clinical applications. Stem Cell Res Ther. 2011;2(3):25.
11. Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2(8254):1003-1035.
12. Gupta A, Kedige SD, Jain K. Amnion and chorion membranes: potential stem cell reservoir with wide applications in periodontics. Int J Biomater. 2015;2015:274082.
13. Buhimschi IA, Jabr M, Buhimschi CS, Petkova AP, Weiner CP, Saed GM. The novel antimicrobial peptide beta3-defensin is produced by the amnion: a possible role of the fetal membranes in innate immunity of the amniotic cavity. Am J Obstet Gynecol. 2004;191(5):1678-1687.
14. Magatti M, De Munari S, Vertua E, et al. Amniotic mesenchymal tissue cells inhibit dendritic cell differentiation of peripheral blood and amnion resident monocytes. Cell Transplant. 2009;18(8):899-914.
15. Solomon A, Wajngarten M, Alviano F, et al. Suppression of inflammatory and fibrotic responses in allergic inflammation by the amniotic membrane stromal matrix. Clin Exp Allergy. 2005;35(7):941-948.
16. Silini A, Parolini O, Huppertz B, Lang I. Soluble factors of amnion-derived cells in treatment of inflammatory and fibrotic pathologies. Curr Stem Cell Res Ther. 2013;8(1):6-14.
17. Peister A, Woodruff MA, Prince JJ, Gray DP, Hutmacher DW, Guldberg RE. Cell sourcing for bone tissue engineering: amniotic fluid stem cells have a delayed, robust differentiation compared to mesenchymal stem cells. Stem Cell Res. 2011;7(1):17-27.
18. Si J, Dai J, Zhang J, et al. Comparative investigation of human amniotic epithelial cells and mesenchymal stem cells for application in bone tissue engineering. Stem Cells Int. 2015;2015:565732.
19. Starecki M, Schwartz JA, Grande DA. Evaluation of amniotic-derived membrane biomaterial as an adjunct for repair of critical sized bone defects. Advances in Orthopedic Surgery. 2014;2014:572586.
20. Kerimoglu S, Livaoglu M, Sönmez B, et al. Effects of human amniotic fluid on fracture healing in rat tibia. J Surg Res. 2009;152(2):281-287.
21. Karaçal N, Kocucu P, Cobanglu U, Kutlu N. Effect of human amniotic fluid on bone healing. J Surg Res. 2005;129(2):283-287.
22. Barboni B, Mangano C, Valbonetti L, et al. Synthetic bone substitute engineered with amniotic epithelial cells enhances bone regeneration after maxillary sinus augmentation. PLoS One. 2013;8(5):e63256.
23. Díaz-Prado S, Rendal-Vázquez ME, Muiños-Lopez E, et al. Potential use of the human amniotic membrane as a scaffold in human articular cartilage repair. Cell Tissue Bank. 2010;11(2):183-195.
24. Krishnamurithy G, Shilpa PN, Ahmad RE, Sulaiman S, Ng CL, Kamarul T. Human amniotic membrane as a chondrocyte carrier vehicle/substrate: in vitro study. J Biomed Mater Res A. 2011;99(3):500-506.
25. Tan SL, Sulaiman S, Pingguan-Murphy B, Selvaratnam L, Tai CC, Kamarul T. Human amnion as a novel cell delivery vehicle for chondrogenic mesenchymal stem cells. Cell Tissue Bank. 2011;12(1):59-70.
26. Jin CZ, Park SR, Choi BH, Lee KY, Kang CK, Min BH. Human amniotic membrane as a delivery matrix for articular cartilage repair. Tissue Eng. 2007;13(4):693-702.
27. Garcia D, Longo UG, Vaquero J, et al. Amniotic membrane transplant for articular cartilage repair: an experimental study in sheep. Curr Stem Cell Res Ther. 2014;10(1):77-83.
28. Kunisaki SM, Freedman DA, Fauza DO. Fetal tracheal reconstruction with cartilaginous grafts engineered from mesenchymal amniocytes. J Pediatr Surg. 2006;41(4):675-682.
29. Namba RS, Meuli M, Sullivan KM, Le AX, Adzick NS. Spontaneous repair of superficial defects in articular cartilage in a fetal lamb model. J Bone Joint Surg Am. 1998;80(1):4-10.
30. Philip J, Hackl F, Canseco JA, et al. Amnion-derived multipotent progenitor cells improve achilles tendon repair in rats. Eplasty. 2013;13:e31.
31. Lange-Consiglio A, Tassan S, Corradetti B, et al. Investigating the efficacy of amnion-derived compared with bone marrow–derived mesenchymal stromal cells in equine tendon and ligament injuries. Cytotherapy. 2013;15(8):1011-1020.
32. Barboni B, Russo V, Curini V, et al. Achilles tendon regeneration can be improved by amniotic epithelial cell allotransplantation. Cell Transplant. 2012;21(11):2377-2395.
33. Kueckelhaus M, Philip J, Kamel RA, et al. Sustained release of amnion-derived cellular cytokine solution facilitates achilles tendon healing in rats. Eplasty. 2014;14:e29.
34. Beredjiklian PK, Favata M, Cartmell JS, Flanagan CL, Crombleholme TM, Soslowski LJ. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31(10):1143-1152.
35. Demirkan F, Colakoglu N, Herek O, Erkula G. The use of amniotic membrane in flexor tendon repair: an experimental model. Arch Orthop Trauma Surg. 2002;122(7):396-369.
36. Ozgenel GY. The effects of a combination of hyaluronic and amniotic membrane on the formation of peritendinous adhesions after flexor tendon surgery in chickens. J Bone Joint Surg Br. 2004;86(2):301-307.
37. Kim SS, Sohn SK, Lee KY, Lee MJ, Roh MS, Kim CH. Use of human amniotic membrane wrap in reducing perineural adhesions in a rabbit model of ulnar nerve neurorrhaphy. J Hand Surg Eur Vol. 2010;35(3):214-219.
38. Tao H, Fan H. Implantation of amniotic membrane to reduce postlaminectomy epidural adhesions. Eur Spine J. 2009;18(8):1202-1212.
39. Choi HJ, Kim KB, Kwon YM. Effect of amniotic membrane to reduce postlaminectomy epidural adhesion on a rat model. J Korean Neurosurg Soc. 2011;49(6):323-328.
40. Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364(11):993-1004.
41. Vines JB, Aliprantis AO, Gomoll AH, Farr J. Cryopreserved amniotic suspension for the treatment of knee osteoarthritis. J Knee Surg. 2016;29(6):443-450.
42. Zelen CM. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J Wound Care. 2013;22(7):347-348,350-351.
43. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502-507.
44. Zelen CM, Serena TE, Snyder RJ. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J. 2014;11(2):122-128.
45. Zelen CM, Snyder RJ, Serena TE, Li WW. The use of human amnion/chorion membrane in the clinical setting for lower extremity repair: a review. Clin Podiatr Med Surg. 2015;32(1):135-146.
46. Zelen CM, Poka A, Andrews J. Prospective, randomized, blinded, comparative study of injectable micronized dehydrated amniotic/chorionic membrane allograft for plantar fasciitis: a feasibility study. Foot Ankle Int. 2013;34(10):1332-1339.
47. Hanselman AE, Tidwell JE, Santrock RD. Cryopreserved human amniotic membrane injection for plantar fasciitis: a randomized, controlled, double-blind pilot study. Foot Ankle Int. 2015;36(2):151-158.
48. Jay RM. Initial clinical experience with the use of human amniotic membrane tissue during repair of posterior tibial and achilles tendons. 2009. http://encompassbiologics.com/wp-content/uploads/2015/07/DrJayClinicalExperience.pdf. Accessed September 29, 2016.
49. DeMill SL, Granata JD, McAlister JE, Berlet GC, Hyer CF. Safety analysis of cryopreserved amniotic membrane/umbilical cord tissue in foot and ankle surgery: a consecutive case series of 124 patients. Surg Technol Int. 2014;25:257-261.
50. Warner M, Lasyone L. An open-label, single-center, retrospective study of cryopreserved amniotic membrane and umbilical cord tissue as an adjunct for foot and ankle surgery. Surg Technol Int. 2014;25:251-255.
Allografts for Ligament Reconstruction: Where Are We Now?
Musculoskeletal allografts are becoming increasingly accepted as a viable alternative to autografts in a variety of orthopedic procedures. A 2006 American Orthopaedic Society for Sports Medicine (AOSSM) survey indicated that 86% of the participating 365 orthopedic surgeons use allografts in their practice.1 Although the overwhelming majority of orthopedic surgeons use allografts, they share common concerns, including safety, tissue integrity, and biologic incorporation. It is essential for the orthopedic surgeon to understand the current standards of tissue banking, risks and benefits related to the use of allografts, and common indications for safe use in clinical practice. This article reviews the current status of musculoskeletal allografts, including tissue procurement and processing, infections, complications, and specific uses tailored to ligament reconstruction.
Donor Bank, Processing, Sterilization, and Regulation
In the United States, the American Association of Tissue Banks (AATB) is responsible for establishing the standards for more than 100 accredited tissue banks. These tissue banks recover tissue from approximately 30,000 donors annually and account for an estimated 90% of the available musculoskeletal allografts used in the United States. While not all tissue banks are accredited by the AATB, all are required to register with the Food and Drug Administration (FDA), which allows for unannounced inspections of any facility. Facilities are required to abide by the FDA-implemented Current Good Tissue Practices (CGTP), which encompasses regulations on all donor tissue collected after May 2005 to help prevent the transmission of communicable diseases. The FDA released an updated draft in January 2009 that emphasizes safe practices and regulations spanning from environmental control to specific equipment.2
The safety of a transplanted allograft tissue begins within the tissue bank. Donor screening and testing is the first step in reducing the risk of transmission. Screening consists of collecting medical and social history from the family and any healthcare resources to assess the eligibility of the donor. If prior blood donations or autopsy information is available, that information is scrutinized. Donor tissue undergoes nucleic acid testing (NAT), which is required by both the AATB and FDA. All donor tissue must be screened for both types of human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), treponema pallidum, and human transmissible spongiform encephalopathies.3 NAT of donor tissue effectively reduces the risk of viral transmission. Additionally, routine preprocessing swabs for bacterial and fungal cultures are performed, although the sensitivity of these cultures ranges from 78% to 92%.4
After donor screening and testing, allograft tissues are usually obtained under aseptic conditions, though this is not FDA-required.5 Once procured, the tissue undergoes sterilization. Currently, there is no standard method ubiquitous to all tissue banks, nor does the FDA require a specific method. Rather, the FDA and AATB require tissue banks to validate their sterilization process and provide supporting data. The goal of sterilization is to inactivate viruses and eradicate bacteria while maintaining the biological and mechanical properties of the tissue. The AATB requires a Sterility Assurance Level (SAL) of 10-6, meaning there is no more than one in a million chance that a nonviral viable microbe exists on or within the tissue. Sterilization techniques may include both radiation and a variety of chemical reagents. Gamma irradiation is a commonly used method of sterilizing soft tissue allografts, although some studies indicate that it is detrimental to tissue biology.6 Newer methods of sterilization are being tested, one of which includes carbon dioxide in combination with antioxidants and irradiation. Bui and colleagues7 directly compared the biomechanical and histological properties of allograft tissue after either the standard 25 kGy gamma irradiation or supercritical carbon dioxide techniques. Although there is no histological difference, the samples treated with supercritical carbon dioxide had less biomechanical damage.7 Finally, the terminally sterilized allograft tissue is frozen to temperatures between -40°C and -80°C.5
Infections
One major concern of allografts is the risk of disease transmission. While numerous studies have investigated the incidence of bacterial infection following transplantation of allograft tissue, there are challenges associated with differentiating common postoperative infections from ones directly associated with the transmission of bacteria within the graft. There is a wide array of reported incidences of infection in the literature, from the Tomford and colleagues8 1981 study that reported a 6.9% rateto the 2001 study by Munting and colleagues,9 who reported 0% in their series. Multiple confounding variables exist, such as possible contamination during handling of an otherwise noncontaminated or properly sterilized allograft with inappropriate inclusion of all postoperative infections. In contrast, recognizing viral transmission has been somewhat easier, although reporting of these incidences has been variable in the past. In either case, there is no accredited reporting system for infections related to allografts.
Bacterial Transmission
Clostridium species. Clostridium species are commonly found among intestinal flora. There is a general consensus that between 24 to 48 hours after death intestinal flora transmigrates into the surrounding tissue and blood. Therefore, a commonly accepted recommendation is that cadaveric tissue needs to be excised prior to 24 hours postmortem.10
In 2001, a 23-year-old man underwent reconstructive knee surgery with a femoral condyle allograft. A few days after surgery, he became septic and ultimately died from the infection. Clostridium sordellii was cultured from the tissue. Several days later, a 17-year-old boy underwent reconstructive knee surgery with a fresh femoral condyle and frozen meniscus from the same donor. Twenty-four hours after surgery, he developed a fever and was readmitted a week later for presumed infection and treated effectively with penicillin and ampicillin/sulbactam. Tissue from the same cadaveric donor had been transplanted into 7 other patients without reports of infection. In a 2002 Centers for Disease Control and Prevention (CDC) update report,11 there were 26 total bacterial cases from allografts and 13 cases were attributed to Clostridium. Malinin and colleagues10 reviewed 795 consecutive cadaveric donors and found that 64 (8.1%) had positive cultures for Clostridia. Of all the positive cultures for Clostridia, 81.3% had positive blood cultures, 57.8% had positive bone marrow aspirate cultures, and 46.9% had positive tissue cultures. They concluded that multiple cultures are required for cadaveric tissue donors in order to reach a higher sensitivity for Clostridial contamination, and these should be done routinely to guide the sterilization process.
Strep species. In 2003, a 17-year-old boy underwent anterior cruciate ligament (ACL) reconstruction with a patellar tendon allograft.12 About 1 week later, he was admitted for signs of infection and received intravenous antibiotics. He required surgical debridement, and intraoperative cultures grew Group A Streptococcus (GAS) that was also identified in the postmortem donor cultures. The tissues underwent processing in an antimicrobial solution and postprocessing cultures were negative for bacteria, but they were not sterilized. Tissues from this donor had been implanted in 5 other patients without report of infection. Following this event, recommendations have been made for prompt rejection of tissue with cultures positive for GAS, unless a sterilizing procedure is used.
Other bacteria. According to the 2002 CDC update, 11 of the 26 cases of bacterial infection reported to the agency were a combination of gram-negative bacilli, polymicrobial flora, or culture negative.11
Viral Transmission
The most effective way to prevent transmission of a viral disease from allografts is thorough donor screening. Since the AATB implemented NAT in 2005 for HIV and HCV, there have been no reported cases of transmission.3 Even prior to this, regular blood screening along with social questionnaires completed by donors or donor families eliminated high-risk donors and significantly decreased the rate of transmission.
Human Immunodeficiency Virus. The first reported case of HIV transmission via implantation of allograft was in 1988. Further investigation revealed that there were 8 transmissions between 1984 and 1986, when routine screening of donors had not yet been implemented. The last reported case of HIV transmission occurred in 1996 with an untested donor.13Hepatitis C Virus. There are several reported cases of HCV transmission that occurred where the donors initially tested negative for HCV. In one case, 40 allografts from the same donor were transplanted over a period of nearly 2 years. This resulted in at least 8 patients being infected with HCV.14 Another case of HCV transmission was reported in 2005 after a patient developed acute HCV 6 weeks after transplantation of a patellar tendon allograft. Further investigation revealed that there had been 3 additional cases over a year from the same donor. Researchers determined that if the initial case had been reported, at least 3 transmissions could have been prevented.15Human T-cell Lymphotropic Virus (HTLV).The first reported transmission of HTLV was in 1991. This was reported in an asymptomatic patient who received a femoral head allograft from a donor who had been previously infected via a blood transfusion.16Zika virus. With recent outbreaks of the Zika virus, the FDA recently released recommendations regarding the screening and deferral of donors, mainly for blood transfusion. Orthopedists should take into consideration the potential for transmission through allografts. The FDA states that all potential donors should be screened for Zika virus using questionnaires and whole blood tests. Symptomatic donors are deferred at least 4 weeks following resolution of symptoms. While this is a recent recommendation from the FDA, orthopedists must be cognizant of the potential harms from this unfamiliar and evolving situation.17
Graft Specifics
Anterior Cruciate Ligament
ACL reconstruction is one of the most commonly performed surgeries by orthopedic surgeons, with an estimated 200,000 reconstructions per year.18Despite the popularity of this surgery, controversies remain regarding the optimal graft for reconstruction.19,20 One would provide adequate strength, be readily available, not elicit an immunologic response from the host, rapidly incorporate, elicit low morbidity, and vascularize early. Current options include both autografts and allografts. Common autograft options include patellar bone-tendon-bone (PBTB), hamstrings tendon, quadriceps tendon, and iliotibial band. PBTB autograft remains a common choice among orthopedic surgeons, as it allows early incorporation of the graft into bone and eliminates immune rejection. However, donor site morbidity, including anterior knee pain, weakness of knee extension, joint stiffness, increased postoperative pain, and iatrogenic patella fractures, have been reported in the literature.21 Commonly used allograft options include donor bone-patellar tendon-bone, quadriceps tendon, Achilles tendon, anterior and posterior tibialis tendons, hamstring tendons, and iliotibial band. Allografts provide the advantage of avoiding donor site morbidity, being readily available, allowing for shorter operative times, and providing lower postoperative pain compared to autografts, although they carry the risk of disease transmission, rejection, and slower incorporation into bone.22-27
Autograft donor site morbidities. One of the general disadvantages of autografts is the donor site morbidity associated with harvesting the grafts. In specific, PBTB grafts allow for bony blocks on both ends of the graft to incorporate into the host bone. However, this technique comes with the risk of disrupting the extensor mechanism.28,29 Milankov and colleagues30 published a retrospective review of over 2000 ACLs using autologous PBTB graft. They noted a 0.45% incidence of patella fracture and 0.18% patellar tendon rupture.30 Others have reported that intraoperative repair of the patellar tendon after tendon harvesting can increase infrapatellar fibrosis, thus increasing the risk for stiffness.31-33
Hamstring autografts include the semitendinosus and the gracilis tendons. The harvesting process is technically demanding and can be complicated by inadvertent amputation of the tendons, making the graft unsuitable for reconstructive purposes.34 Additionally, several reports have identified persistent numbness and hyperesthesia following hamstring harvesting due to iatrogenic injury to the prepatellar branches of the saphenous nerve.35,36A comprehensive review by Slone and colleagues37 reported comparable functional outcomes with quadriceps tendon autograft compared to PBTB; however, this comes with the risk of postoperative hematoma formation and the potential for thigh compartment syndrome.
Biology and Biomechanics of Allografts
One of the major disadvantages of allografts is the reduced ability to incorporate into the host tissue. Several in vitro and animal studies have suggested that allografts incorporate in the host slower than autografts.24,26,38 Early studies by Jackson and colleagues24 on goat models demonstrated that allografts and autografts have similar structural and biological properties initially, but allografts display significantly slower incorporation into the host tissue at 6 months. Histologically, allografts demonstrated lower revascularization, a smaller cross-sectional area, and a prolonged inflammatory response at 6 months postoperatively.24,39,40 Muramatsu and colleagues41 further showed through the use of magnetic resonance imaging a slower rate of revascularization of allografts over 2 years post-reconstruction.
Acknowledging these limitations, one should use caution when choosing to use an allograft or starting aggressive early rehabilitation after an allograft reconstruction, especially in athletes and young patients.
Clinical Outcomes
Although in vitro studies demonstrate inferior strength and delayed incorporation of allografts in the early postoperative period, there is still controversy surrounding the clinical and functional outcomes. Numerous studies have identified allografts as a viable option for ACL reconstruction, with similar reported patient satisfaction scores compared to autografts.43,44
The MOON Consortium recently published a prospective study of nearly 2500 subjects looking to identify risk factors for failure of ACL reconstruction. The study found that allografts had an odds ratio for failure 5.2 times that of PBTB autografts, correlating this factor to an increased re-tear rate of 6.9% in the allograft group compared to 3.2% in the PBTB group (P < .01).45 The elevated risk is more prevalent in younger patients, especially athletic teenagers. This issue has been reiterated in multiple studies.45-50A meta-analysis by Hu and colleagues23 identified 9 studies, either randomized control trials or prospective cohort studies, that looked at clinical outcomes between the different graft choices. They showed there was no significant difference between graft options in terms of instrumental laxity (P = .59), Lachman test (P = .41), pivot shift test (P = .88), and multiple functional outcome scores, including the International Knee Documentation Committee (IKDC), Lysholm, and Tegner scores.23,51-59Processing and sterilization techniques are thought to play a role in allograft failure. Guo and other researchers have demonstrated a significantly higher rate of failure for patients who received gamma-irradiated allografts compared to fresh frozen allografts.23,58-64 With improved sterilization techniques and a strict selection process of donors, gamma radiation has fallen out of favor to protect the biological characteristics of the tissue graft.5,65,66Several factors need to be considered when selecting between allograft or autograft tissue for ligamentous reconstruction. The selection must be balanced between the surgeon’s experience, patient and surgeon preferences, age of the patient, level of physical activity, primary or revision surgical setting, multiligamentous failure, geographical availability of donor grafts, and economical factors.
Medial Patellofemoral Ligament Reconstruction
Another relatively recent application for allografts has been described for the reconstruction of the medial patellofemoral ligament (MPFL) in recurrent lateral patellar dislocations.67-74
Typically, MPFL reconstructions make use of autografts, including quadriceps tendon, patellar tendon, and hamstring ligaments. However, allografts have the potential to limit postoperative donor site morbidity and to allow a faster rehabilitation.75,76 Allografts include semitendinosus, gracilis, anterior tibialis, posterior tibialis, and quadriceps tendons.
Calvo Rodríguez and colleagues76 performed a retrospective review in 2015 comparing allografts to autografts for MPFL reconstruction with respect to postoperative knee function and re-dislocation rates. Among the collective 28 patients, there was no difference in overall functional scores or dislocation rates between the grafts. Although this was a retrospective review and had a small number of subjects, the findings identify allografts as a reliable graft option for MPFL reconstruction.76While there has been a surge of interest in techniques for MPFL reconstruction, there is limited research available regarding the superiority of allografts compared to autografts. For this specific application, it seems that clinical outcomes correlate more to adequate stabilization of the patellofemoral joint than to the type of graft used.77,78 Future research should be dedicated to prospective randomized control trials to delineate any disadvantages to using allografts for MPFL reconstruction.
Discussion
Musculoskeletal allografts are gaining popularity for ligamentous reconstruction as their safety and efficacy continue to improve. With the great majority of tissue banks being accredited by the AATB and specific regulations such as NAT screening becoming common practice, infection rates and transmission of diseases have become incredibly rare. However, a thorough consideration needs to be taken into account when choosing between autograft and allograft on a case-by-case basis (Table).
Am J Orthop. 2016;45(7):446-453. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. The American Orthopaedic Society for Sports Medicine. Allografts for ACL Reconstruction Survey Report. 2013. http://www.sportsmed.org/AOSSMIMIS/members/downloads/research/AllograftACLReconstructionSurveyReport.pdf. Accessed October 21, 2016.
2. US Department of Health and Human Services, Food and Drug Administration. Guidance for industry: Current good tissue practice (CGTP) and additional requirements for manufacturers of human cells, tissues, and cellular and tissue-based products (HCT/Ps). http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM285223.pdf. Published December 2011. Accessed August 17, 2015.
3. Vaishnav S, Thomas Vangsness C Jr, Dellamaggiora R. New techniques in allograft tissue processing. Clin Sports Med. 2009;28(1):127-141.
4. Veen MR, Bloem RM, Petit PL. Sensitivity and negative predictive value of swab cultures in musculoskeletal allograft procurement. Clin Orthop Relat Res. 1994;(300):259-263.
5. McAllister DR, Joyce MJ, Mann BJ, Vangsness CT Jr. Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2007;35(12):2148-2158.
6. Mickiewicz P, Binkowski M, Bursig H, Wróbel Z. Preservation and sterilization methods of the meniscal allografts: literature review. Cell Tissue Bank. 2014;15(3):307-317.
7. Bui D, Lovric V, Oliver R, Bertollo N, Broe D, Walsh WR. Meniscal allograft sterilisation: effect on biomechanical and histological properties. Cell Tissue Bank. 2015;16(3):467-475.
8. Tomford WW, Starkweather RJ, Goldman MH. A study of the clinical incidence of infection in the use of banked allograft bone. J Bone Joint Surg Am. 1981;63(2):244-248.
9. Munting E, Faundez A, Manche E. Vertebral reconstruction with cortical allograft: long-term evaluation. Eur Spine J. 2001;10 Suppl 2:S153-S157.
10. Malinin TI, Buck BE, Temple HT, Martinez OV, Fox WP. Incidence of clostridial contamination in donors’ musculoskeletal tissue. J Bone Joint Surg Br. 2003;85(7):1051-1054.
11. Centers for Disease Control and Prevention (CDC). Update: allograft-associated bacterial infections--United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51(10):207-210.
12. Centers for Disease Control and Prevention (CDC). Invasive Streptococcus pyogenes after allograft implantation--Colorado, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(48):1174-1176.
13. Hinsenkamp M, Muylle L, Eastlund T, Fehily D, Noël L, Strong DM. Adverse reactions and events related to musculoskeletal allografts: reviewed by the World Health Organisation project NOTIFY. Int Orthop. 2012;36(3):633-641.
14. Schratt HE, Regel G, Kiesewetter B, Tscherne H. HIV infection caused by cold preserved bone transplants. Unfallchirurg. 1996;99(9):679-684.
15. Tugwell BD, Patel PR, Williams IT, et al. Transmission of hepatitis C virus to several organ and tissue recipients from an antibody-negative donor. Ann Intern Med. 2005;143(9):648-654.
16. Sanzén L, Carlsson A. Transmission of human T-cell lymphotrophic virus type 1 by a deep-frozen bone allograft. Acta Orthop Scand. 1997;68(1):72-74.
17. US Department of Health and Human Services, Food and Drug Administration. Recommendations for donor screening, deferral, and product management to reduce the risk of transfusion-transmission of Zika virus. Guidance for industry. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM486360.pdf. Published February 2016. Accessed August 10, 2016.
18. Gottlob CA, Baker CL Jr, Pellissier JM, Colvin L. Cost effectiveness of anterior cruciate ligament reconstruction in young adults. Clin Orthop Relat Res. 1999;(367):272-282.
19. Fu F, Christel P, Miller MD, Johnson DL. Graft selection for anterior cruciate ligament reconstruction. Instr Course Lect. 2009;58:337-354.
20. Chechik O, Amar E, Khashan M, Lador R, Eyal G, Gold A. An international survey on anterior cruciate ligament reconstruction practices. Int Orthop. 2013;37(2):201-206.
21. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
22. Harner CD, Irrgang JJ, Paul J, Dearwater S, Fu FH. Loss of motion after anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):499-506.
23. Hu J, Qu J, Xu D, Zhou J, Lu H. Allograft versus autograft for anterior cruciate ligament reconstruction: an up-to-date meta-analysis of prospective studies. Int Orthop. 2013;37(2):311-320.
24. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
25. Mroz TE, Joyce MJ, Steinmetz MP, Lieberman IH, Wang JC. Musculoskeletal allograft risks and recalls in the United States. J Am Acad Orthop Surg. 2008;16(10):559-565.
26. Malinin TI, Levitt RL, Bashore C, Temple HT, Mnaymneh W. A study of retrieved allografts used to replace anterior cruciate ligaments. Arthroscopy. 2002;18(2):163-170.
27. Foster TE, Wolfe BL, Ryan S, Silvestri L, Kaye EK. Does the graft source really matter in the outcome of patients undergoing anterior cruciate ligament reconstruction? An evaluation of autograft versus allograft reconstruction results: a systematic review. Am J Sports Med. 2010;38(1):189-199.
28. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
29. Marumoto JM, Mitsunaga MM, Richardson AB, Medoff RJ, Mayfield GW. Late patellar tendon ruptures after removal of the central third for anterior cruciate ligament reconstruction. A report of two cases. Am J Sports Med. 1996;24(5):698-701.
30 Milankov M, Kecojević V, Rasović P, Kovacević N, Gvozdenović N, Obradović M. Disruption of the knee extensor apparatus complicating anterior cruciate ligament reconstruction. Acta Chir Iugosl. 2013;60(2):13-21.
31. Atkinson TS, Atkinson PJ, Mendenhall HV, Haut RC. Patellar tendon and infrapatellar fat pad healing after harvest of an ACL graft. J Surg Res. 1998;79(1):25-30.
32. Tang G, Niitsu M, Ikeda K, Endo H, Itai Y. Fibrous scar in the infrapatellar fat pad after arthroscopy: MR imaging. Radiat Med. 2000;18(1):1-5.
33. Unterhauser FN, Bosch U, Zeichen J, Weiler A. Alpha-smooth muscle actin containing contractile fibroblastic cells in human knee arthrofibrosis tissue. Winner of the AGA-DonJoy Award 2003. Arch Orthop Trauma Surg. 2004;124(9):585-591.
34. Prodromos CC, Fu FH, Howell SM, Johnson DH, Lawhorn K. Controversies in soft-tissue anterior cruciate ligament reconstruction: grafts, bundles, tunnels, fixation, and harvest. J Am Acad Orthop Surg. 2008;16(7):376-384.
35. Sabat D, Kumar V. Nerve injury during hamstring graft harvest: a prospective comparative study of three different incisions. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2089-2095.
36. Kjaergaard J, Faunø LZ, Faunø P. Sensibility loss after ACL reconstruction with hamstring graft. Int J Sports Med. 2008;29(6):507-511.
37. Slone HS, Romine SE, Premkumar A, Xerogeanes JW. Quadriceps tendon autograft for anterior cruciate ligament reconstruction: a comprehensive review of current literature and systematic review of clinical results. Arthroscopy. 2015;31(3):541-554.
38. Nikolaou PK, Seaber AV, Glisson RR, Ribbeck BM, Bassett FH 3rd. Anterior cruciate ligament allograft transplantation. Long-term function, histology, revascularization, and operative technique. Am J Sports Med. 1986;14(5):348-360.
39. Arnoczky SP, Warren RF, Ashlock MA. Replacement of the anterior cruciate ligament using a patellar tendon allograft. An experimental study. J Bone Joint Surg Am. 1986;68(3):376-385.
40. Scheffler SU, Schmidt T, Gangéy I, Dustmann M, Unterhauser F, Weiler A. Fresh-frozen free-tendon allografts versus autografts in anterior cruciate ligament reconstruction: delayed remodeling and inferior mechanical function during long-term healing in sheep. Arthroscopy. 2008;24(4):448-458.
41. Muramatsu K, Hachiya Y, Izawa H. Serial evaluation of human anterior cruciate ligament grafts by contrast-enhanced magnetic resonance imaging: comparison of allografts and autografts. Arthroscopy. 2008;24(9):1038-1044.
42. Jackson DW, Grood ES, Arnoczky SP, Butler DL, Simon TM. Freeze dried anterior cruciate ligament allografts. Preliminary studies in a goat model. Am J Sports Med. 1987;15(4):295-303.
43. Chang SK, Egami DK, Shaieb MD, Kan DM, Richardson AB. Anterior cruciate ligament reconstruction: allograft versus autograft. Arthroscopy. 2003;19(5):453-462.
44. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
45. Kaeding CC, Pedroza AD, Reinke EK, Huston LJ; MOON Consortium, Spindler KP. Risk factors and predictors of subsequent ACL injury in either knee after ACL reconstruction: prospective analysis of 2488 primary ACL reconstructions from the MOON cohort. Am J Sports Med. 2015;43(7):1583-1590.
46. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73-81.
47. Lynch TS, Parker RD, Patel RM, et al. The impact of the Multicenter Orthopaedic Outcomes Network (MOON) research on anterior cruciate ligament reconstruction and orthopaedic practice. J Am Acad Orthop Surg. 2015;23(3):154-163.
48. Hettrich CM, Dunn WR, Reinke EK; MOON Group, Spindler KP. The rate of subsequent surgery and predictors after anterior cruciate ligament reconstruction: two- and 6-year follow-up results from a multicenter cohort. Am J Sports Med. 2013;41(7):1534-1540.
49. Steadman JR, Matheny LM, Hurst JM, Briggs KK. Patient-centered outcomes and revision rate in patients undergoing ACL reconstruction using bone-patellar tendon-bone autograft compared with bone-patellar tendon-bone allograft: a matched case-control study. Arthroscopy. 2015;31(12):2320-2326.
50. Lenehan EA, Payne WB, Askam BM, Grana WA, Farrow LD. Long-term outcomes of allograft reconstruction of the anterior cruciate ligament. Am J Orthop. 2015;44(5):217-222.
51. Noh JH, Yi SR, Song SJ, Kim SW, Kim W. Comparison between hamstring autograft and free tendon achilles allograft: minimum 2-year follow-up after anterior cruciate ligament reconstruction using EndoButton and Intrafix. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):816-822.
52. Victor J, Bellemans J, Witvrouw E, Govaers K, Fabry G. Graft selection in anterior cruciate ligament reconstruction--prospective analysis of patellar tendon autografts compared with allografts. Int Orthop. 1997;21(2):93-97.
53. Kleipool AE, Zijl JA, Willems WJ. Arthroscopic anterior cruciate ligament reconstruction with bone-patellar tendon-bone allograft or autograft. A prospective study with an average follow up of 4 years. Knee Surg Sports Traumatol Arthrosc. 1998;6(4):224-230.
54. Peterson RK, Shelton WR, Bomboy AL. Allograft versus autograft patellar tendon anterior cruciate ligament reconstruction: a 5-year follow-up. Arthroscopy. 2001;17(1):9-13.
55. Edgar CM, Zimmer S, Kakar S, Jones H, Schepsis AA. Prospective comparison of auto and allograft hamstring tendon constructs for ACL reconstruction. Clin Orthop Relat Res. 2008;466(9):2238-2246.
56. Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):464-474.
57. Leal-Blanquet J, Alentorn-Geli E, Tuneu J, Valentí JR, Maestro A. Anterior cruciate ligament reconstruction: a multicenter prospective cohort study evaluating 3 different grafts using same bone drilling method. Clin J Sport Med. 2011;21(4):294-300.
58. Sun K, Zhang J, Wang Y, et al. Arthroscopic reconstruction of the anterior cruciate ligament with hamstring tendon autograft and fresh-frozen allograft: a prospective, randomized controlled study. Am J Sports Med. 2011;39(7):1430-1438.
59. Lawhorn KW, Howell SM, Traina SM, Gottlieb JE, Meade TD, Freedberg HI. The effect of graft tissue on anterior cruciate ligament outcomes: a multicenter, prospective, randomized controlled trial comparing autograft hamstrings with fresh-frozen anterior tibialis allograft. Arthroscopy. 2012;28(8):1079-1086.
60. Guo L, Yang L, Duan XJ, et al. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft: comparison of autograft, fresh-frozen allograft, and γ-irradiated allograft. Arthroscopy. 2012;28(2):211-217.
61. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
62. Mayr HO, Willkomm D, Stoehr A, et al. Revision of anterior cruciate ligament reconstruction with patellar tendon allograft and autograft: 2- and 5-year results. Arch Orthop Trauma Surg. 2012;132(6):867-874.
63. Mariscalco MW, Magnussen RA, Mehta D, Hewett TE, Flanigan DC, Kaeding CC. Autograft versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: a systematic review. Am J Sports Med. 2014;42(2):492-499.
64. Mehta VM, Mandala C, Foster D, Petsche TS. Comparison of revision rates in bone-patella tendon-bone autograft and allograft anterior cruciate ligament reconstruction. Orthopedics. 2010;33(1):12.
65. Vangsness CT Jr, Garcia IA, Mills CR, Kainer MA, Roberts MR, Moore TM. Allograft transplantation in the knee: tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2003;31(3):474-481.
66. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
67. Reagan J, Kullar R, Burks R. MPFL reconstruction: technique and results. Clin Sports Med. 2014;33(3):501-516.
68. Christiansen SE, Jacobsen BW, Lund B, Lind M. Reconstruction of the medial patellofemoral ligament with gracilis tendon autograft in transverse patellar drill holes. Arthroscopy. 2008;24(1):82-87.
69. Schöttle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):516-521.
70. Deie M, Ochi M, Sumen Y, Adachi N, Kobayashi K, Yasumoto M. A long-term follow-up study after medial patellofemoral ligament reconstruction using the transferred semitendinosus tendon for patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):522-528.
71. Nomura E, Inoue M. Hybrid medial patellofemoral ligament reconstruction using the semitendinous tendon for recurrent patellar dislocation: minimum 3 years’ follow-up. Arthroscopy. 2006;22(7):787-793.
72. Nomura E, Inoue M. Surgical technique and rationale for medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Arthroscopy. 2003;19(5):E47.
73. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
74. Drez D Jr, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthroscopy. 2001;17(3):298-306.
75. Fink C, Veselko M, Herbort M, Hoser C. MPFL reconstruction using a quadriceps tendon graft: part 2: operative technique and short term clinical results. Knee. 2014;21(6):1175-1179.
76. Calvo Rodríguez R, Figueroa Poblete D, Anastasiadis Le Roy Z, Etchegaray Bascur F, Vaisman Burucker A, Calvo Mena R. Reconstruction of the medial patellofemoral ligament: evaluation of the clinical results of autografts versus allografts. Rev Esp Cir Ortop Traumatol. 2015;59(5):348-353.
77. Becher C, Kley K, Lobenhoffer P, Ezechieli M, Smith T, Ostermeier S. Dynamic versus static reconstruction of the medial patellofemoral ligament for recurrent lateral patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2452-2457.
78. Gomes JE. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24(4):430-435.
Musculoskeletal allografts are becoming increasingly accepted as a viable alternative to autografts in a variety of orthopedic procedures. A 2006 American Orthopaedic Society for Sports Medicine (AOSSM) survey indicated that 86% of the participating 365 orthopedic surgeons use allografts in their practice.1 Although the overwhelming majority of orthopedic surgeons use allografts, they share common concerns, including safety, tissue integrity, and biologic incorporation. It is essential for the orthopedic surgeon to understand the current standards of tissue banking, risks and benefits related to the use of allografts, and common indications for safe use in clinical practice. This article reviews the current status of musculoskeletal allografts, including tissue procurement and processing, infections, complications, and specific uses tailored to ligament reconstruction.
Donor Bank, Processing, Sterilization, and Regulation
In the United States, the American Association of Tissue Banks (AATB) is responsible for establishing the standards for more than 100 accredited tissue banks. These tissue banks recover tissue from approximately 30,000 donors annually and account for an estimated 90% of the available musculoskeletal allografts used in the United States. While not all tissue banks are accredited by the AATB, all are required to register with the Food and Drug Administration (FDA), which allows for unannounced inspections of any facility. Facilities are required to abide by the FDA-implemented Current Good Tissue Practices (CGTP), which encompasses regulations on all donor tissue collected after May 2005 to help prevent the transmission of communicable diseases. The FDA released an updated draft in January 2009 that emphasizes safe practices and regulations spanning from environmental control to specific equipment.2
The safety of a transplanted allograft tissue begins within the tissue bank. Donor screening and testing is the first step in reducing the risk of transmission. Screening consists of collecting medical and social history from the family and any healthcare resources to assess the eligibility of the donor. If prior blood donations or autopsy information is available, that information is scrutinized. Donor tissue undergoes nucleic acid testing (NAT), which is required by both the AATB and FDA. All donor tissue must be screened for both types of human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), treponema pallidum, and human transmissible spongiform encephalopathies.3 NAT of donor tissue effectively reduces the risk of viral transmission. Additionally, routine preprocessing swabs for bacterial and fungal cultures are performed, although the sensitivity of these cultures ranges from 78% to 92%.4
After donor screening and testing, allograft tissues are usually obtained under aseptic conditions, though this is not FDA-required.5 Once procured, the tissue undergoes sterilization. Currently, there is no standard method ubiquitous to all tissue banks, nor does the FDA require a specific method. Rather, the FDA and AATB require tissue banks to validate their sterilization process and provide supporting data. The goal of sterilization is to inactivate viruses and eradicate bacteria while maintaining the biological and mechanical properties of the tissue. The AATB requires a Sterility Assurance Level (SAL) of 10-6, meaning there is no more than one in a million chance that a nonviral viable microbe exists on or within the tissue. Sterilization techniques may include both radiation and a variety of chemical reagents. Gamma irradiation is a commonly used method of sterilizing soft tissue allografts, although some studies indicate that it is detrimental to tissue biology.6 Newer methods of sterilization are being tested, one of which includes carbon dioxide in combination with antioxidants and irradiation. Bui and colleagues7 directly compared the biomechanical and histological properties of allograft tissue after either the standard 25 kGy gamma irradiation or supercritical carbon dioxide techniques. Although there is no histological difference, the samples treated with supercritical carbon dioxide had less biomechanical damage.7 Finally, the terminally sterilized allograft tissue is frozen to temperatures between -40°C and -80°C.5
Infections
One major concern of allografts is the risk of disease transmission. While numerous studies have investigated the incidence of bacterial infection following transplantation of allograft tissue, there are challenges associated with differentiating common postoperative infections from ones directly associated with the transmission of bacteria within the graft. There is a wide array of reported incidences of infection in the literature, from the Tomford and colleagues8 1981 study that reported a 6.9% rateto the 2001 study by Munting and colleagues,9 who reported 0% in their series. Multiple confounding variables exist, such as possible contamination during handling of an otherwise noncontaminated or properly sterilized allograft with inappropriate inclusion of all postoperative infections. In contrast, recognizing viral transmission has been somewhat easier, although reporting of these incidences has been variable in the past. In either case, there is no accredited reporting system for infections related to allografts.
Bacterial Transmission
Clostridium species. Clostridium species are commonly found among intestinal flora. There is a general consensus that between 24 to 48 hours after death intestinal flora transmigrates into the surrounding tissue and blood. Therefore, a commonly accepted recommendation is that cadaveric tissue needs to be excised prior to 24 hours postmortem.10
In 2001, a 23-year-old man underwent reconstructive knee surgery with a femoral condyle allograft. A few days after surgery, he became septic and ultimately died from the infection. Clostridium sordellii was cultured from the tissue. Several days later, a 17-year-old boy underwent reconstructive knee surgery with a fresh femoral condyle and frozen meniscus from the same donor. Twenty-four hours after surgery, he developed a fever and was readmitted a week later for presumed infection and treated effectively with penicillin and ampicillin/sulbactam. Tissue from the same cadaveric donor had been transplanted into 7 other patients without reports of infection. In a 2002 Centers for Disease Control and Prevention (CDC) update report,11 there were 26 total bacterial cases from allografts and 13 cases were attributed to Clostridium. Malinin and colleagues10 reviewed 795 consecutive cadaveric donors and found that 64 (8.1%) had positive cultures for Clostridia. Of all the positive cultures for Clostridia, 81.3% had positive blood cultures, 57.8% had positive bone marrow aspirate cultures, and 46.9% had positive tissue cultures. They concluded that multiple cultures are required for cadaveric tissue donors in order to reach a higher sensitivity for Clostridial contamination, and these should be done routinely to guide the sterilization process.
Strep species. In 2003, a 17-year-old boy underwent anterior cruciate ligament (ACL) reconstruction with a patellar tendon allograft.12 About 1 week later, he was admitted for signs of infection and received intravenous antibiotics. He required surgical debridement, and intraoperative cultures grew Group A Streptococcus (GAS) that was also identified in the postmortem donor cultures. The tissues underwent processing in an antimicrobial solution and postprocessing cultures were negative for bacteria, but they were not sterilized. Tissues from this donor had been implanted in 5 other patients without report of infection. Following this event, recommendations have been made for prompt rejection of tissue with cultures positive for GAS, unless a sterilizing procedure is used.
Other bacteria. According to the 2002 CDC update, 11 of the 26 cases of bacterial infection reported to the agency were a combination of gram-negative bacilli, polymicrobial flora, or culture negative.11
Viral Transmission
The most effective way to prevent transmission of a viral disease from allografts is thorough donor screening. Since the AATB implemented NAT in 2005 for HIV and HCV, there have been no reported cases of transmission.3 Even prior to this, regular blood screening along with social questionnaires completed by donors or donor families eliminated high-risk donors and significantly decreased the rate of transmission.
Human Immunodeficiency Virus. The first reported case of HIV transmission via implantation of allograft was in 1988. Further investigation revealed that there were 8 transmissions between 1984 and 1986, when routine screening of donors had not yet been implemented. The last reported case of HIV transmission occurred in 1996 with an untested donor.13Hepatitis C Virus. There are several reported cases of HCV transmission that occurred where the donors initially tested negative for HCV. In one case, 40 allografts from the same donor were transplanted over a period of nearly 2 years. This resulted in at least 8 patients being infected with HCV.14 Another case of HCV transmission was reported in 2005 after a patient developed acute HCV 6 weeks after transplantation of a patellar tendon allograft. Further investigation revealed that there had been 3 additional cases over a year from the same donor. Researchers determined that if the initial case had been reported, at least 3 transmissions could have been prevented.15Human T-cell Lymphotropic Virus (HTLV).The first reported transmission of HTLV was in 1991. This was reported in an asymptomatic patient who received a femoral head allograft from a donor who had been previously infected via a blood transfusion.16Zika virus. With recent outbreaks of the Zika virus, the FDA recently released recommendations regarding the screening and deferral of donors, mainly for blood transfusion. Orthopedists should take into consideration the potential for transmission through allografts. The FDA states that all potential donors should be screened for Zika virus using questionnaires and whole blood tests. Symptomatic donors are deferred at least 4 weeks following resolution of symptoms. While this is a recent recommendation from the FDA, orthopedists must be cognizant of the potential harms from this unfamiliar and evolving situation.17
Graft Specifics
Anterior Cruciate Ligament
ACL reconstruction is one of the most commonly performed surgeries by orthopedic surgeons, with an estimated 200,000 reconstructions per year.18Despite the popularity of this surgery, controversies remain regarding the optimal graft for reconstruction.19,20 One would provide adequate strength, be readily available, not elicit an immunologic response from the host, rapidly incorporate, elicit low morbidity, and vascularize early. Current options include both autografts and allografts. Common autograft options include patellar bone-tendon-bone (PBTB), hamstrings tendon, quadriceps tendon, and iliotibial band. PBTB autograft remains a common choice among orthopedic surgeons, as it allows early incorporation of the graft into bone and eliminates immune rejection. However, donor site morbidity, including anterior knee pain, weakness of knee extension, joint stiffness, increased postoperative pain, and iatrogenic patella fractures, have been reported in the literature.21 Commonly used allograft options include donor bone-patellar tendon-bone, quadriceps tendon, Achilles tendon, anterior and posterior tibialis tendons, hamstring tendons, and iliotibial band. Allografts provide the advantage of avoiding donor site morbidity, being readily available, allowing for shorter operative times, and providing lower postoperative pain compared to autografts, although they carry the risk of disease transmission, rejection, and slower incorporation into bone.22-27
Autograft donor site morbidities. One of the general disadvantages of autografts is the donor site morbidity associated with harvesting the grafts. In specific, PBTB grafts allow for bony blocks on both ends of the graft to incorporate into the host bone. However, this technique comes with the risk of disrupting the extensor mechanism.28,29 Milankov and colleagues30 published a retrospective review of over 2000 ACLs using autologous PBTB graft. They noted a 0.45% incidence of patella fracture and 0.18% patellar tendon rupture.30 Others have reported that intraoperative repair of the patellar tendon after tendon harvesting can increase infrapatellar fibrosis, thus increasing the risk for stiffness.31-33
Hamstring autografts include the semitendinosus and the gracilis tendons. The harvesting process is technically demanding and can be complicated by inadvertent amputation of the tendons, making the graft unsuitable for reconstructive purposes.34 Additionally, several reports have identified persistent numbness and hyperesthesia following hamstring harvesting due to iatrogenic injury to the prepatellar branches of the saphenous nerve.35,36A comprehensive review by Slone and colleagues37 reported comparable functional outcomes with quadriceps tendon autograft compared to PBTB; however, this comes with the risk of postoperative hematoma formation and the potential for thigh compartment syndrome.
Biology and Biomechanics of Allografts
One of the major disadvantages of allografts is the reduced ability to incorporate into the host tissue. Several in vitro and animal studies have suggested that allografts incorporate in the host slower than autografts.24,26,38 Early studies by Jackson and colleagues24 on goat models demonstrated that allografts and autografts have similar structural and biological properties initially, but allografts display significantly slower incorporation into the host tissue at 6 months. Histologically, allografts demonstrated lower revascularization, a smaller cross-sectional area, and a prolonged inflammatory response at 6 months postoperatively.24,39,40 Muramatsu and colleagues41 further showed through the use of magnetic resonance imaging a slower rate of revascularization of allografts over 2 years post-reconstruction.
Acknowledging these limitations, one should use caution when choosing to use an allograft or starting aggressive early rehabilitation after an allograft reconstruction, especially in athletes and young patients.
Clinical Outcomes
Although in vitro studies demonstrate inferior strength and delayed incorporation of allografts in the early postoperative period, there is still controversy surrounding the clinical and functional outcomes. Numerous studies have identified allografts as a viable option for ACL reconstruction, with similar reported patient satisfaction scores compared to autografts.43,44
The MOON Consortium recently published a prospective study of nearly 2500 subjects looking to identify risk factors for failure of ACL reconstruction. The study found that allografts had an odds ratio for failure 5.2 times that of PBTB autografts, correlating this factor to an increased re-tear rate of 6.9% in the allograft group compared to 3.2% in the PBTB group (P < .01).45 The elevated risk is more prevalent in younger patients, especially athletic teenagers. This issue has been reiterated in multiple studies.45-50A meta-analysis by Hu and colleagues23 identified 9 studies, either randomized control trials or prospective cohort studies, that looked at clinical outcomes between the different graft choices. They showed there was no significant difference between graft options in terms of instrumental laxity (P = .59), Lachman test (P = .41), pivot shift test (P = .88), and multiple functional outcome scores, including the International Knee Documentation Committee (IKDC), Lysholm, and Tegner scores.23,51-59Processing and sterilization techniques are thought to play a role in allograft failure. Guo and other researchers have demonstrated a significantly higher rate of failure for patients who received gamma-irradiated allografts compared to fresh frozen allografts.23,58-64 With improved sterilization techniques and a strict selection process of donors, gamma radiation has fallen out of favor to protect the biological characteristics of the tissue graft.5,65,66Several factors need to be considered when selecting between allograft or autograft tissue for ligamentous reconstruction. The selection must be balanced between the surgeon’s experience, patient and surgeon preferences, age of the patient, level of physical activity, primary or revision surgical setting, multiligamentous failure, geographical availability of donor grafts, and economical factors.
Medial Patellofemoral Ligament Reconstruction
Another relatively recent application for allografts has been described for the reconstruction of the medial patellofemoral ligament (MPFL) in recurrent lateral patellar dislocations.67-74
Typically, MPFL reconstructions make use of autografts, including quadriceps tendon, patellar tendon, and hamstring ligaments. However, allografts have the potential to limit postoperative donor site morbidity and to allow a faster rehabilitation.75,76 Allografts include semitendinosus, gracilis, anterior tibialis, posterior tibialis, and quadriceps tendons.
Calvo Rodríguez and colleagues76 performed a retrospective review in 2015 comparing allografts to autografts for MPFL reconstruction with respect to postoperative knee function and re-dislocation rates. Among the collective 28 patients, there was no difference in overall functional scores or dislocation rates between the grafts. Although this was a retrospective review and had a small number of subjects, the findings identify allografts as a reliable graft option for MPFL reconstruction.76While there has been a surge of interest in techniques for MPFL reconstruction, there is limited research available regarding the superiority of allografts compared to autografts. For this specific application, it seems that clinical outcomes correlate more to adequate stabilization of the patellofemoral joint than to the type of graft used.77,78 Future research should be dedicated to prospective randomized control trials to delineate any disadvantages to using allografts for MPFL reconstruction.
Discussion
Musculoskeletal allografts are gaining popularity for ligamentous reconstruction as their safety and efficacy continue to improve. With the great majority of tissue banks being accredited by the AATB and specific regulations such as NAT screening becoming common practice, infection rates and transmission of diseases have become incredibly rare. However, a thorough consideration needs to be taken into account when choosing between autograft and allograft on a case-by-case basis (Table).
Am J Orthop. 2016;45(7):446-453. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Musculoskeletal allografts are becoming increasingly accepted as a viable alternative to autografts in a variety of orthopedic procedures. A 2006 American Orthopaedic Society for Sports Medicine (AOSSM) survey indicated that 86% of the participating 365 orthopedic surgeons use allografts in their practice.1 Although the overwhelming majority of orthopedic surgeons use allografts, they share common concerns, including safety, tissue integrity, and biologic incorporation. It is essential for the orthopedic surgeon to understand the current standards of tissue banking, risks and benefits related to the use of allografts, and common indications for safe use in clinical practice. This article reviews the current status of musculoskeletal allografts, including tissue procurement and processing, infections, complications, and specific uses tailored to ligament reconstruction.
Donor Bank, Processing, Sterilization, and Regulation
In the United States, the American Association of Tissue Banks (AATB) is responsible for establishing the standards for more than 100 accredited tissue banks. These tissue banks recover tissue from approximately 30,000 donors annually and account for an estimated 90% of the available musculoskeletal allografts used in the United States. While not all tissue banks are accredited by the AATB, all are required to register with the Food and Drug Administration (FDA), which allows for unannounced inspections of any facility. Facilities are required to abide by the FDA-implemented Current Good Tissue Practices (CGTP), which encompasses regulations on all donor tissue collected after May 2005 to help prevent the transmission of communicable diseases. The FDA released an updated draft in January 2009 that emphasizes safe practices and regulations spanning from environmental control to specific equipment.2
The safety of a transplanted allograft tissue begins within the tissue bank. Donor screening and testing is the first step in reducing the risk of transmission. Screening consists of collecting medical and social history from the family and any healthcare resources to assess the eligibility of the donor. If prior blood donations or autopsy information is available, that information is scrutinized. Donor tissue undergoes nucleic acid testing (NAT), which is required by both the AATB and FDA. All donor tissue must be screened for both types of human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), treponema pallidum, and human transmissible spongiform encephalopathies.3 NAT of donor tissue effectively reduces the risk of viral transmission. Additionally, routine preprocessing swabs for bacterial and fungal cultures are performed, although the sensitivity of these cultures ranges from 78% to 92%.4
After donor screening and testing, allograft tissues are usually obtained under aseptic conditions, though this is not FDA-required.5 Once procured, the tissue undergoes sterilization. Currently, there is no standard method ubiquitous to all tissue banks, nor does the FDA require a specific method. Rather, the FDA and AATB require tissue banks to validate their sterilization process and provide supporting data. The goal of sterilization is to inactivate viruses and eradicate bacteria while maintaining the biological and mechanical properties of the tissue. The AATB requires a Sterility Assurance Level (SAL) of 10-6, meaning there is no more than one in a million chance that a nonviral viable microbe exists on or within the tissue. Sterilization techniques may include both radiation and a variety of chemical reagents. Gamma irradiation is a commonly used method of sterilizing soft tissue allografts, although some studies indicate that it is detrimental to tissue biology.6 Newer methods of sterilization are being tested, one of which includes carbon dioxide in combination with antioxidants and irradiation. Bui and colleagues7 directly compared the biomechanical and histological properties of allograft tissue after either the standard 25 kGy gamma irradiation or supercritical carbon dioxide techniques. Although there is no histological difference, the samples treated with supercritical carbon dioxide had less biomechanical damage.7 Finally, the terminally sterilized allograft tissue is frozen to temperatures between -40°C and -80°C.5
Infections
One major concern of allografts is the risk of disease transmission. While numerous studies have investigated the incidence of bacterial infection following transplantation of allograft tissue, there are challenges associated with differentiating common postoperative infections from ones directly associated with the transmission of bacteria within the graft. There is a wide array of reported incidences of infection in the literature, from the Tomford and colleagues8 1981 study that reported a 6.9% rateto the 2001 study by Munting and colleagues,9 who reported 0% in their series. Multiple confounding variables exist, such as possible contamination during handling of an otherwise noncontaminated or properly sterilized allograft with inappropriate inclusion of all postoperative infections. In contrast, recognizing viral transmission has been somewhat easier, although reporting of these incidences has been variable in the past. In either case, there is no accredited reporting system for infections related to allografts.
Bacterial Transmission
Clostridium species. Clostridium species are commonly found among intestinal flora. There is a general consensus that between 24 to 48 hours after death intestinal flora transmigrates into the surrounding tissue and blood. Therefore, a commonly accepted recommendation is that cadaveric tissue needs to be excised prior to 24 hours postmortem.10
In 2001, a 23-year-old man underwent reconstructive knee surgery with a femoral condyle allograft. A few days after surgery, he became septic and ultimately died from the infection. Clostridium sordellii was cultured from the tissue. Several days later, a 17-year-old boy underwent reconstructive knee surgery with a fresh femoral condyle and frozen meniscus from the same donor. Twenty-four hours after surgery, he developed a fever and was readmitted a week later for presumed infection and treated effectively with penicillin and ampicillin/sulbactam. Tissue from the same cadaveric donor had been transplanted into 7 other patients without reports of infection. In a 2002 Centers for Disease Control and Prevention (CDC) update report,11 there were 26 total bacterial cases from allografts and 13 cases were attributed to Clostridium. Malinin and colleagues10 reviewed 795 consecutive cadaveric donors and found that 64 (8.1%) had positive cultures for Clostridia. Of all the positive cultures for Clostridia, 81.3% had positive blood cultures, 57.8% had positive bone marrow aspirate cultures, and 46.9% had positive tissue cultures. They concluded that multiple cultures are required for cadaveric tissue donors in order to reach a higher sensitivity for Clostridial contamination, and these should be done routinely to guide the sterilization process.
Strep species. In 2003, a 17-year-old boy underwent anterior cruciate ligament (ACL) reconstruction with a patellar tendon allograft.12 About 1 week later, he was admitted for signs of infection and received intravenous antibiotics. He required surgical debridement, and intraoperative cultures grew Group A Streptococcus (GAS) that was also identified in the postmortem donor cultures. The tissues underwent processing in an antimicrobial solution and postprocessing cultures were negative for bacteria, but they were not sterilized. Tissues from this donor had been implanted in 5 other patients without report of infection. Following this event, recommendations have been made for prompt rejection of tissue with cultures positive for GAS, unless a sterilizing procedure is used.
Other bacteria. According to the 2002 CDC update, 11 of the 26 cases of bacterial infection reported to the agency were a combination of gram-negative bacilli, polymicrobial flora, or culture negative.11
Viral Transmission
The most effective way to prevent transmission of a viral disease from allografts is thorough donor screening. Since the AATB implemented NAT in 2005 for HIV and HCV, there have been no reported cases of transmission.3 Even prior to this, regular blood screening along with social questionnaires completed by donors or donor families eliminated high-risk donors and significantly decreased the rate of transmission.
Human Immunodeficiency Virus. The first reported case of HIV transmission via implantation of allograft was in 1988. Further investigation revealed that there were 8 transmissions between 1984 and 1986, when routine screening of donors had not yet been implemented. The last reported case of HIV transmission occurred in 1996 with an untested donor.13Hepatitis C Virus. There are several reported cases of HCV transmission that occurred where the donors initially tested negative for HCV. In one case, 40 allografts from the same donor were transplanted over a period of nearly 2 years. This resulted in at least 8 patients being infected with HCV.14 Another case of HCV transmission was reported in 2005 after a patient developed acute HCV 6 weeks after transplantation of a patellar tendon allograft. Further investigation revealed that there had been 3 additional cases over a year from the same donor. Researchers determined that if the initial case had been reported, at least 3 transmissions could have been prevented.15Human T-cell Lymphotropic Virus (HTLV).The first reported transmission of HTLV was in 1991. This was reported in an asymptomatic patient who received a femoral head allograft from a donor who had been previously infected via a blood transfusion.16Zika virus. With recent outbreaks of the Zika virus, the FDA recently released recommendations regarding the screening and deferral of donors, mainly for blood transfusion. Orthopedists should take into consideration the potential for transmission through allografts. The FDA states that all potential donors should be screened for Zika virus using questionnaires and whole blood tests. Symptomatic donors are deferred at least 4 weeks following resolution of symptoms. While this is a recent recommendation from the FDA, orthopedists must be cognizant of the potential harms from this unfamiliar and evolving situation.17
Graft Specifics
Anterior Cruciate Ligament
ACL reconstruction is one of the most commonly performed surgeries by orthopedic surgeons, with an estimated 200,000 reconstructions per year.18Despite the popularity of this surgery, controversies remain regarding the optimal graft for reconstruction.19,20 One would provide adequate strength, be readily available, not elicit an immunologic response from the host, rapidly incorporate, elicit low morbidity, and vascularize early. Current options include both autografts and allografts. Common autograft options include patellar bone-tendon-bone (PBTB), hamstrings tendon, quadriceps tendon, and iliotibial band. PBTB autograft remains a common choice among orthopedic surgeons, as it allows early incorporation of the graft into bone and eliminates immune rejection. However, donor site morbidity, including anterior knee pain, weakness of knee extension, joint stiffness, increased postoperative pain, and iatrogenic patella fractures, have been reported in the literature.21 Commonly used allograft options include donor bone-patellar tendon-bone, quadriceps tendon, Achilles tendon, anterior and posterior tibialis tendons, hamstring tendons, and iliotibial band. Allografts provide the advantage of avoiding donor site morbidity, being readily available, allowing for shorter operative times, and providing lower postoperative pain compared to autografts, although they carry the risk of disease transmission, rejection, and slower incorporation into bone.22-27
Autograft donor site morbidities. One of the general disadvantages of autografts is the donor site morbidity associated with harvesting the grafts. In specific, PBTB grafts allow for bony blocks on both ends of the graft to incorporate into the host bone. However, this technique comes with the risk of disrupting the extensor mechanism.28,29 Milankov and colleagues30 published a retrospective review of over 2000 ACLs using autologous PBTB graft. They noted a 0.45% incidence of patella fracture and 0.18% patellar tendon rupture.30 Others have reported that intraoperative repair of the patellar tendon after tendon harvesting can increase infrapatellar fibrosis, thus increasing the risk for stiffness.31-33
Hamstring autografts include the semitendinosus and the gracilis tendons. The harvesting process is technically demanding and can be complicated by inadvertent amputation of the tendons, making the graft unsuitable for reconstructive purposes.34 Additionally, several reports have identified persistent numbness and hyperesthesia following hamstring harvesting due to iatrogenic injury to the prepatellar branches of the saphenous nerve.35,36A comprehensive review by Slone and colleagues37 reported comparable functional outcomes with quadriceps tendon autograft compared to PBTB; however, this comes with the risk of postoperative hematoma formation and the potential for thigh compartment syndrome.
Biology and Biomechanics of Allografts
One of the major disadvantages of allografts is the reduced ability to incorporate into the host tissue. Several in vitro and animal studies have suggested that allografts incorporate in the host slower than autografts.24,26,38 Early studies by Jackson and colleagues24 on goat models demonstrated that allografts and autografts have similar structural and biological properties initially, but allografts display significantly slower incorporation into the host tissue at 6 months. Histologically, allografts demonstrated lower revascularization, a smaller cross-sectional area, and a prolonged inflammatory response at 6 months postoperatively.24,39,40 Muramatsu and colleagues41 further showed through the use of magnetic resonance imaging a slower rate of revascularization of allografts over 2 years post-reconstruction.
Acknowledging these limitations, one should use caution when choosing to use an allograft or starting aggressive early rehabilitation after an allograft reconstruction, especially in athletes and young patients.
Clinical Outcomes
Although in vitro studies demonstrate inferior strength and delayed incorporation of allografts in the early postoperative period, there is still controversy surrounding the clinical and functional outcomes. Numerous studies have identified allografts as a viable option for ACL reconstruction, with similar reported patient satisfaction scores compared to autografts.43,44
The MOON Consortium recently published a prospective study of nearly 2500 subjects looking to identify risk factors for failure of ACL reconstruction. The study found that allografts had an odds ratio for failure 5.2 times that of PBTB autografts, correlating this factor to an increased re-tear rate of 6.9% in the allograft group compared to 3.2% in the PBTB group (P < .01).45 The elevated risk is more prevalent in younger patients, especially athletic teenagers. This issue has been reiterated in multiple studies.45-50A meta-analysis by Hu and colleagues23 identified 9 studies, either randomized control trials or prospective cohort studies, that looked at clinical outcomes between the different graft choices. They showed there was no significant difference between graft options in terms of instrumental laxity (P = .59), Lachman test (P = .41), pivot shift test (P = .88), and multiple functional outcome scores, including the International Knee Documentation Committee (IKDC), Lysholm, and Tegner scores.23,51-59Processing and sterilization techniques are thought to play a role in allograft failure. Guo and other researchers have demonstrated a significantly higher rate of failure for patients who received gamma-irradiated allografts compared to fresh frozen allografts.23,58-64 With improved sterilization techniques and a strict selection process of donors, gamma radiation has fallen out of favor to protect the biological characteristics of the tissue graft.5,65,66Several factors need to be considered when selecting between allograft or autograft tissue for ligamentous reconstruction. The selection must be balanced between the surgeon’s experience, patient and surgeon preferences, age of the patient, level of physical activity, primary or revision surgical setting, multiligamentous failure, geographical availability of donor grafts, and economical factors.
Medial Patellofemoral Ligament Reconstruction
Another relatively recent application for allografts has been described for the reconstruction of the medial patellofemoral ligament (MPFL) in recurrent lateral patellar dislocations.67-74
Typically, MPFL reconstructions make use of autografts, including quadriceps tendon, patellar tendon, and hamstring ligaments. However, allografts have the potential to limit postoperative donor site morbidity and to allow a faster rehabilitation.75,76 Allografts include semitendinosus, gracilis, anterior tibialis, posterior tibialis, and quadriceps tendons.
Calvo Rodríguez and colleagues76 performed a retrospective review in 2015 comparing allografts to autografts for MPFL reconstruction with respect to postoperative knee function and re-dislocation rates. Among the collective 28 patients, there was no difference in overall functional scores or dislocation rates between the grafts. Although this was a retrospective review and had a small number of subjects, the findings identify allografts as a reliable graft option for MPFL reconstruction.76While there has been a surge of interest in techniques for MPFL reconstruction, there is limited research available regarding the superiority of allografts compared to autografts. For this specific application, it seems that clinical outcomes correlate more to adequate stabilization of the patellofemoral joint than to the type of graft used.77,78 Future research should be dedicated to prospective randomized control trials to delineate any disadvantages to using allografts for MPFL reconstruction.
Discussion
Musculoskeletal allografts are gaining popularity for ligamentous reconstruction as their safety and efficacy continue to improve. With the great majority of tissue banks being accredited by the AATB and specific regulations such as NAT screening becoming common practice, infection rates and transmission of diseases have become incredibly rare. However, a thorough consideration needs to be taken into account when choosing between autograft and allograft on a case-by-case basis (Table).
Am J Orthop. 2016;45(7):446-453. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. The American Orthopaedic Society for Sports Medicine. Allografts for ACL Reconstruction Survey Report. 2013. http://www.sportsmed.org/AOSSMIMIS/members/downloads/research/AllograftACLReconstructionSurveyReport.pdf. Accessed October 21, 2016.
2. US Department of Health and Human Services, Food and Drug Administration. Guidance for industry: Current good tissue practice (CGTP) and additional requirements for manufacturers of human cells, tissues, and cellular and tissue-based products (HCT/Ps). http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM285223.pdf. Published December 2011. Accessed August 17, 2015.
3. Vaishnav S, Thomas Vangsness C Jr, Dellamaggiora R. New techniques in allograft tissue processing. Clin Sports Med. 2009;28(1):127-141.
4. Veen MR, Bloem RM, Petit PL. Sensitivity and negative predictive value of swab cultures in musculoskeletal allograft procurement. Clin Orthop Relat Res. 1994;(300):259-263.
5. McAllister DR, Joyce MJ, Mann BJ, Vangsness CT Jr. Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2007;35(12):2148-2158.
6. Mickiewicz P, Binkowski M, Bursig H, Wróbel Z. Preservation and sterilization methods of the meniscal allografts: literature review. Cell Tissue Bank. 2014;15(3):307-317.
7. Bui D, Lovric V, Oliver R, Bertollo N, Broe D, Walsh WR. Meniscal allograft sterilisation: effect on biomechanical and histological properties. Cell Tissue Bank. 2015;16(3):467-475.
8. Tomford WW, Starkweather RJ, Goldman MH. A study of the clinical incidence of infection in the use of banked allograft bone. J Bone Joint Surg Am. 1981;63(2):244-248.
9. Munting E, Faundez A, Manche E. Vertebral reconstruction with cortical allograft: long-term evaluation. Eur Spine J. 2001;10 Suppl 2:S153-S157.
10. Malinin TI, Buck BE, Temple HT, Martinez OV, Fox WP. Incidence of clostridial contamination in donors’ musculoskeletal tissue. J Bone Joint Surg Br. 2003;85(7):1051-1054.
11. Centers for Disease Control and Prevention (CDC). Update: allograft-associated bacterial infections--United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51(10):207-210.
12. Centers for Disease Control and Prevention (CDC). Invasive Streptococcus pyogenes after allograft implantation--Colorado, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(48):1174-1176.
13. Hinsenkamp M, Muylle L, Eastlund T, Fehily D, Noël L, Strong DM. Adverse reactions and events related to musculoskeletal allografts: reviewed by the World Health Organisation project NOTIFY. Int Orthop. 2012;36(3):633-641.
14. Schratt HE, Regel G, Kiesewetter B, Tscherne H. HIV infection caused by cold preserved bone transplants. Unfallchirurg. 1996;99(9):679-684.
15. Tugwell BD, Patel PR, Williams IT, et al. Transmission of hepatitis C virus to several organ and tissue recipients from an antibody-negative donor. Ann Intern Med. 2005;143(9):648-654.
16. Sanzén L, Carlsson A. Transmission of human T-cell lymphotrophic virus type 1 by a deep-frozen bone allograft. Acta Orthop Scand. 1997;68(1):72-74.
17. US Department of Health and Human Services, Food and Drug Administration. Recommendations for donor screening, deferral, and product management to reduce the risk of transfusion-transmission of Zika virus. Guidance for industry. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM486360.pdf. Published February 2016. Accessed August 10, 2016.
18. Gottlob CA, Baker CL Jr, Pellissier JM, Colvin L. Cost effectiveness of anterior cruciate ligament reconstruction in young adults. Clin Orthop Relat Res. 1999;(367):272-282.
19. Fu F, Christel P, Miller MD, Johnson DL. Graft selection for anterior cruciate ligament reconstruction. Instr Course Lect. 2009;58:337-354.
20. Chechik O, Amar E, Khashan M, Lador R, Eyal G, Gold A. An international survey on anterior cruciate ligament reconstruction practices. Int Orthop. 2013;37(2):201-206.
21. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
22. Harner CD, Irrgang JJ, Paul J, Dearwater S, Fu FH. Loss of motion after anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):499-506.
23. Hu J, Qu J, Xu D, Zhou J, Lu H. Allograft versus autograft for anterior cruciate ligament reconstruction: an up-to-date meta-analysis of prospective studies. Int Orthop. 2013;37(2):311-320.
24. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
25. Mroz TE, Joyce MJ, Steinmetz MP, Lieberman IH, Wang JC. Musculoskeletal allograft risks and recalls in the United States. J Am Acad Orthop Surg. 2008;16(10):559-565.
26. Malinin TI, Levitt RL, Bashore C, Temple HT, Mnaymneh W. A study of retrieved allografts used to replace anterior cruciate ligaments. Arthroscopy. 2002;18(2):163-170.
27. Foster TE, Wolfe BL, Ryan S, Silvestri L, Kaye EK. Does the graft source really matter in the outcome of patients undergoing anterior cruciate ligament reconstruction? An evaluation of autograft versus allograft reconstruction results: a systematic review. Am J Sports Med. 2010;38(1):189-199.
28. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
29. Marumoto JM, Mitsunaga MM, Richardson AB, Medoff RJ, Mayfield GW. Late patellar tendon ruptures after removal of the central third for anterior cruciate ligament reconstruction. A report of two cases. Am J Sports Med. 1996;24(5):698-701.
30 Milankov M, Kecojević V, Rasović P, Kovacević N, Gvozdenović N, Obradović M. Disruption of the knee extensor apparatus complicating anterior cruciate ligament reconstruction. Acta Chir Iugosl. 2013;60(2):13-21.
31. Atkinson TS, Atkinson PJ, Mendenhall HV, Haut RC. Patellar tendon and infrapatellar fat pad healing after harvest of an ACL graft. J Surg Res. 1998;79(1):25-30.
32. Tang G, Niitsu M, Ikeda K, Endo H, Itai Y. Fibrous scar in the infrapatellar fat pad after arthroscopy: MR imaging. Radiat Med. 2000;18(1):1-5.
33. Unterhauser FN, Bosch U, Zeichen J, Weiler A. Alpha-smooth muscle actin containing contractile fibroblastic cells in human knee arthrofibrosis tissue. Winner of the AGA-DonJoy Award 2003. Arch Orthop Trauma Surg. 2004;124(9):585-591.
34. Prodromos CC, Fu FH, Howell SM, Johnson DH, Lawhorn K. Controversies in soft-tissue anterior cruciate ligament reconstruction: grafts, bundles, tunnels, fixation, and harvest. J Am Acad Orthop Surg. 2008;16(7):376-384.
35. Sabat D, Kumar V. Nerve injury during hamstring graft harvest: a prospective comparative study of three different incisions. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2089-2095.
36. Kjaergaard J, Faunø LZ, Faunø P. Sensibility loss after ACL reconstruction with hamstring graft. Int J Sports Med. 2008;29(6):507-511.
37. Slone HS, Romine SE, Premkumar A, Xerogeanes JW. Quadriceps tendon autograft for anterior cruciate ligament reconstruction: a comprehensive review of current literature and systematic review of clinical results. Arthroscopy. 2015;31(3):541-554.
38. Nikolaou PK, Seaber AV, Glisson RR, Ribbeck BM, Bassett FH 3rd. Anterior cruciate ligament allograft transplantation. Long-term function, histology, revascularization, and operative technique. Am J Sports Med. 1986;14(5):348-360.
39. Arnoczky SP, Warren RF, Ashlock MA. Replacement of the anterior cruciate ligament using a patellar tendon allograft. An experimental study. J Bone Joint Surg Am. 1986;68(3):376-385.
40. Scheffler SU, Schmidt T, Gangéy I, Dustmann M, Unterhauser F, Weiler A. Fresh-frozen free-tendon allografts versus autografts in anterior cruciate ligament reconstruction: delayed remodeling and inferior mechanical function during long-term healing in sheep. Arthroscopy. 2008;24(4):448-458.
41. Muramatsu K, Hachiya Y, Izawa H. Serial evaluation of human anterior cruciate ligament grafts by contrast-enhanced magnetic resonance imaging: comparison of allografts and autografts. Arthroscopy. 2008;24(9):1038-1044.
42. Jackson DW, Grood ES, Arnoczky SP, Butler DL, Simon TM. Freeze dried anterior cruciate ligament allografts. Preliminary studies in a goat model. Am J Sports Med. 1987;15(4):295-303.
43. Chang SK, Egami DK, Shaieb MD, Kan DM, Richardson AB. Anterior cruciate ligament reconstruction: allograft versus autograft. Arthroscopy. 2003;19(5):453-462.
44. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
45. Kaeding CC, Pedroza AD, Reinke EK, Huston LJ; MOON Consortium, Spindler KP. Risk factors and predictors of subsequent ACL injury in either knee after ACL reconstruction: prospective analysis of 2488 primary ACL reconstructions from the MOON cohort. Am J Sports Med. 2015;43(7):1583-1590.
46. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73-81.
47. Lynch TS, Parker RD, Patel RM, et al. The impact of the Multicenter Orthopaedic Outcomes Network (MOON) research on anterior cruciate ligament reconstruction and orthopaedic practice. J Am Acad Orthop Surg. 2015;23(3):154-163.
48. Hettrich CM, Dunn WR, Reinke EK; MOON Group, Spindler KP. The rate of subsequent surgery and predictors after anterior cruciate ligament reconstruction: two- and 6-year follow-up results from a multicenter cohort. Am J Sports Med. 2013;41(7):1534-1540.
49. Steadman JR, Matheny LM, Hurst JM, Briggs KK. Patient-centered outcomes and revision rate in patients undergoing ACL reconstruction using bone-patellar tendon-bone autograft compared with bone-patellar tendon-bone allograft: a matched case-control study. Arthroscopy. 2015;31(12):2320-2326.
50. Lenehan EA, Payne WB, Askam BM, Grana WA, Farrow LD. Long-term outcomes of allograft reconstruction of the anterior cruciate ligament. Am J Orthop. 2015;44(5):217-222.
51. Noh JH, Yi SR, Song SJ, Kim SW, Kim W. Comparison between hamstring autograft and free tendon achilles allograft: minimum 2-year follow-up after anterior cruciate ligament reconstruction using EndoButton and Intrafix. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):816-822.
52. Victor J, Bellemans J, Witvrouw E, Govaers K, Fabry G. Graft selection in anterior cruciate ligament reconstruction--prospective analysis of patellar tendon autografts compared with allografts. Int Orthop. 1997;21(2):93-97.
53. Kleipool AE, Zijl JA, Willems WJ. Arthroscopic anterior cruciate ligament reconstruction with bone-patellar tendon-bone allograft or autograft. A prospective study with an average follow up of 4 years. Knee Surg Sports Traumatol Arthrosc. 1998;6(4):224-230.
54. Peterson RK, Shelton WR, Bomboy AL. Allograft versus autograft patellar tendon anterior cruciate ligament reconstruction: a 5-year follow-up. Arthroscopy. 2001;17(1):9-13.
55. Edgar CM, Zimmer S, Kakar S, Jones H, Schepsis AA. Prospective comparison of auto and allograft hamstring tendon constructs for ACL reconstruction. Clin Orthop Relat Res. 2008;466(9):2238-2246.
56. Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):464-474.
57. Leal-Blanquet J, Alentorn-Geli E, Tuneu J, Valentí JR, Maestro A. Anterior cruciate ligament reconstruction: a multicenter prospective cohort study evaluating 3 different grafts using same bone drilling method. Clin J Sport Med. 2011;21(4):294-300.
58. Sun K, Zhang J, Wang Y, et al. Arthroscopic reconstruction of the anterior cruciate ligament with hamstring tendon autograft and fresh-frozen allograft: a prospective, randomized controlled study. Am J Sports Med. 2011;39(7):1430-1438.
59. Lawhorn KW, Howell SM, Traina SM, Gottlieb JE, Meade TD, Freedberg HI. The effect of graft tissue on anterior cruciate ligament outcomes: a multicenter, prospective, randomized controlled trial comparing autograft hamstrings with fresh-frozen anterior tibialis allograft. Arthroscopy. 2012;28(8):1079-1086.
60. Guo L, Yang L, Duan XJ, et al. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft: comparison of autograft, fresh-frozen allograft, and γ-irradiated allograft. Arthroscopy. 2012;28(2):211-217.
61. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
62. Mayr HO, Willkomm D, Stoehr A, et al. Revision of anterior cruciate ligament reconstruction with patellar tendon allograft and autograft: 2- and 5-year results. Arch Orthop Trauma Surg. 2012;132(6):867-874.
63. Mariscalco MW, Magnussen RA, Mehta D, Hewett TE, Flanigan DC, Kaeding CC. Autograft versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: a systematic review. Am J Sports Med. 2014;42(2):492-499.
64. Mehta VM, Mandala C, Foster D, Petsche TS. Comparison of revision rates in bone-patella tendon-bone autograft and allograft anterior cruciate ligament reconstruction. Orthopedics. 2010;33(1):12.
65. Vangsness CT Jr, Garcia IA, Mills CR, Kainer MA, Roberts MR, Moore TM. Allograft transplantation in the knee: tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2003;31(3):474-481.
66. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
67. Reagan J, Kullar R, Burks R. MPFL reconstruction: technique and results. Clin Sports Med. 2014;33(3):501-516.
68. Christiansen SE, Jacobsen BW, Lund B, Lind M. Reconstruction of the medial patellofemoral ligament with gracilis tendon autograft in transverse patellar drill holes. Arthroscopy. 2008;24(1):82-87.
69. Schöttle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):516-521.
70. Deie M, Ochi M, Sumen Y, Adachi N, Kobayashi K, Yasumoto M. A long-term follow-up study after medial patellofemoral ligament reconstruction using the transferred semitendinosus tendon for patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):522-528.
71. Nomura E, Inoue M. Hybrid medial patellofemoral ligament reconstruction using the semitendinous tendon for recurrent patellar dislocation: minimum 3 years’ follow-up. Arthroscopy. 2006;22(7):787-793.
72. Nomura E, Inoue M. Surgical technique and rationale for medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Arthroscopy. 2003;19(5):E47.
73. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
74. Drez D Jr, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthroscopy. 2001;17(3):298-306.
75. Fink C, Veselko M, Herbort M, Hoser C. MPFL reconstruction using a quadriceps tendon graft: part 2: operative technique and short term clinical results. Knee. 2014;21(6):1175-1179.
76. Calvo Rodríguez R, Figueroa Poblete D, Anastasiadis Le Roy Z, Etchegaray Bascur F, Vaisman Burucker A, Calvo Mena R. Reconstruction of the medial patellofemoral ligament: evaluation of the clinical results of autografts versus allografts. Rev Esp Cir Ortop Traumatol. 2015;59(5):348-353.
77. Becher C, Kley K, Lobenhoffer P, Ezechieli M, Smith T, Ostermeier S. Dynamic versus static reconstruction of the medial patellofemoral ligament for recurrent lateral patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2452-2457.
78. Gomes JE. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24(4):430-435.
1. The American Orthopaedic Society for Sports Medicine. Allografts for ACL Reconstruction Survey Report. 2013. http://www.sportsmed.org/AOSSMIMIS/members/downloads/research/AllograftACLReconstructionSurveyReport.pdf. Accessed October 21, 2016.
2. US Department of Health and Human Services, Food and Drug Administration. Guidance for industry: Current good tissue practice (CGTP) and additional requirements for manufacturers of human cells, tissues, and cellular and tissue-based products (HCT/Ps). http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM285223.pdf. Published December 2011. Accessed August 17, 2015.
3. Vaishnav S, Thomas Vangsness C Jr, Dellamaggiora R. New techniques in allograft tissue processing. Clin Sports Med. 2009;28(1):127-141.
4. Veen MR, Bloem RM, Petit PL. Sensitivity and negative predictive value of swab cultures in musculoskeletal allograft procurement. Clin Orthop Relat Res. 1994;(300):259-263.
5. McAllister DR, Joyce MJ, Mann BJ, Vangsness CT Jr. Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2007;35(12):2148-2158.
6. Mickiewicz P, Binkowski M, Bursig H, Wróbel Z. Preservation and sterilization methods of the meniscal allografts: literature review. Cell Tissue Bank. 2014;15(3):307-317.
7. Bui D, Lovric V, Oliver R, Bertollo N, Broe D, Walsh WR. Meniscal allograft sterilisation: effect on biomechanical and histological properties. Cell Tissue Bank. 2015;16(3):467-475.
8. Tomford WW, Starkweather RJ, Goldman MH. A study of the clinical incidence of infection in the use of banked allograft bone. J Bone Joint Surg Am. 1981;63(2):244-248.
9. Munting E, Faundez A, Manche E. Vertebral reconstruction with cortical allograft: long-term evaluation. Eur Spine J. 2001;10 Suppl 2:S153-S157.
10. Malinin TI, Buck BE, Temple HT, Martinez OV, Fox WP. Incidence of clostridial contamination in donors’ musculoskeletal tissue. J Bone Joint Surg Br. 2003;85(7):1051-1054.
11. Centers for Disease Control and Prevention (CDC). Update: allograft-associated bacterial infections--United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51(10):207-210.
12. Centers for Disease Control and Prevention (CDC). Invasive Streptococcus pyogenes after allograft implantation--Colorado, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(48):1174-1176.
13. Hinsenkamp M, Muylle L, Eastlund T, Fehily D, Noël L, Strong DM. Adverse reactions and events related to musculoskeletal allografts: reviewed by the World Health Organisation project NOTIFY. Int Orthop. 2012;36(3):633-641.
14. Schratt HE, Regel G, Kiesewetter B, Tscherne H. HIV infection caused by cold preserved bone transplants. Unfallchirurg. 1996;99(9):679-684.
15. Tugwell BD, Patel PR, Williams IT, et al. Transmission of hepatitis C virus to several organ and tissue recipients from an antibody-negative donor. Ann Intern Med. 2005;143(9):648-654.
16. Sanzén L, Carlsson A. Transmission of human T-cell lymphotrophic virus type 1 by a deep-frozen bone allograft. Acta Orthop Scand. 1997;68(1):72-74.
17. US Department of Health and Human Services, Food and Drug Administration. Recommendations for donor screening, deferral, and product management to reduce the risk of transfusion-transmission of Zika virus. Guidance for industry. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM486360.pdf. Published February 2016. Accessed August 10, 2016.
18. Gottlob CA, Baker CL Jr, Pellissier JM, Colvin L. Cost effectiveness of anterior cruciate ligament reconstruction in young adults. Clin Orthop Relat Res. 1999;(367):272-282.
19. Fu F, Christel P, Miller MD, Johnson DL. Graft selection for anterior cruciate ligament reconstruction. Instr Course Lect. 2009;58:337-354.
20. Chechik O, Amar E, Khashan M, Lador R, Eyal G, Gold A. An international survey on anterior cruciate ligament reconstruction practices. Int Orthop. 2013;37(2):201-206.
21. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
22. Harner CD, Irrgang JJ, Paul J, Dearwater S, Fu FH. Loss of motion after anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):499-506.
23. Hu J, Qu J, Xu D, Zhou J, Lu H. Allograft versus autograft for anterior cruciate ligament reconstruction: an up-to-date meta-analysis of prospective studies. Int Orthop. 2013;37(2):311-320.
24. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
25. Mroz TE, Joyce MJ, Steinmetz MP, Lieberman IH, Wang JC. Musculoskeletal allograft risks and recalls in the United States. J Am Acad Orthop Surg. 2008;16(10):559-565.
26. Malinin TI, Levitt RL, Bashore C, Temple HT, Mnaymneh W. A study of retrieved allografts used to replace anterior cruciate ligaments. Arthroscopy. 2002;18(2):163-170.
27. Foster TE, Wolfe BL, Ryan S, Silvestri L, Kaye EK. Does the graft source really matter in the outcome of patients undergoing anterior cruciate ligament reconstruction? An evaluation of autograft versus allograft reconstruction results: a systematic review. Am J Sports Med. 2010;38(1):189-199.
28. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
29. Marumoto JM, Mitsunaga MM, Richardson AB, Medoff RJ, Mayfield GW. Late patellar tendon ruptures after removal of the central third for anterior cruciate ligament reconstruction. A report of two cases. Am J Sports Med. 1996;24(5):698-701.
30 Milankov M, Kecojević V, Rasović P, Kovacević N, Gvozdenović N, Obradović M. Disruption of the knee extensor apparatus complicating anterior cruciate ligament reconstruction. Acta Chir Iugosl. 2013;60(2):13-21.
31. Atkinson TS, Atkinson PJ, Mendenhall HV, Haut RC. Patellar tendon and infrapatellar fat pad healing after harvest of an ACL graft. J Surg Res. 1998;79(1):25-30.
32. Tang G, Niitsu M, Ikeda K, Endo H, Itai Y. Fibrous scar in the infrapatellar fat pad after arthroscopy: MR imaging. Radiat Med. 2000;18(1):1-5.
33. Unterhauser FN, Bosch U, Zeichen J, Weiler A. Alpha-smooth muscle actin containing contractile fibroblastic cells in human knee arthrofibrosis tissue. Winner of the AGA-DonJoy Award 2003. Arch Orthop Trauma Surg. 2004;124(9):585-591.
34. Prodromos CC, Fu FH, Howell SM, Johnson DH, Lawhorn K. Controversies in soft-tissue anterior cruciate ligament reconstruction: grafts, bundles, tunnels, fixation, and harvest. J Am Acad Orthop Surg. 2008;16(7):376-384.
35. Sabat D, Kumar V. Nerve injury during hamstring graft harvest: a prospective comparative study of three different incisions. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2089-2095.
36. Kjaergaard J, Faunø LZ, Faunø P. Sensibility loss after ACL reconstruction with hamstring graft. Int J Sports Med. 2008;29(6):507-511.
37. Slone HS, Romine SE, Premkumar A, Xerogeanes JW. Quadriceps tendon autograft for anterior cruciate ligament reconstruction: a comprehensive review of current literature and systematic review of clinical results. Arthroscopy. 2015;31(3):541-554.
38. Nikolaou PK, Seaber AV, Glisson RR, Ribbeck BM, Bassett FH 3rd. Anterior cruciate ligament allograft transplantation. Long-term function, histology, revascularization, and operative technique. Am J Sports Med. 1986;14(5):348-360.
39. Arnoczky SP, Warren RF, Ashlock MA. Replacement of the anterior cruciate ligament using a patellar tendon allograft. An experimental study. J Bone Joint Surg Am. 1986;68(3):376-385.
40. Scheffler SU, Schmidt T, Gangéy I, Dustmann M, Unterhauser F, Weiler A. Fresh-frozen free-tendon allografts versus autografts in anterior cruciate ligament reconstruction: delayed remodeling and inferior mechanical function during long-term healing in sheep. Arthroscopy. 2008;24(4):448-458.
41. Muramatsu K, Hachiya Y, Izawa H. Serial evaluation of human anterior cruciate ligament grafts by contrast-enhanced magnetic resonance imaging: comparison of allografts and autografts. Arthroscopy. 2008;24(9):1038-1044.
42. Jackson DW, Grood ES, Arnoczky SP, Butler DL, Simon TM. Freeze dried anterior cruciate ligament allografts. Preliminary studies in a goat model. Am J Sports Med. 1987;15(4):295-303.
43. Chang SK, Egami DK, Shaieb MD, Kan DM, Richardson AB. Anterior cruciate ligament reconstruction: allograft versus autograft. Arthroscopy. 2003;19(5):453-462.
44. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
45. Kaeding CC, Pedroza AD, Reinke EK, Huston LJ; MOON Consortium, Spindler KP. Risk factors and predictors of subsequent ACL injury in either knee after ACL reconstruction: prospective analysis of 2488 primary ACL reconstructions from the MOON cohort. Am J Sports Med. 2015;43(7):1583-1590.
46. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73-81.
47. Lynch TS, Parker RD, Patel RM, et al. The impact of the Multicenter Orthopaedic Outcomes Network (MOON) research on anterior cruciate ligament reconstruction and orthopaedic practice. J Am Acad Orthop Surg. 2015;23(3):154-163.
48. Hettrich CM, Dunn WR, Reinke EK; MOON Group, Spindler KP. The rate of subsequent surgery and predictors after anterior cruciate ligament reconstruction: two- and 6-year follow-up results from a multicenter cohort. Am J Sports Med. 2013;41(7):1534-1540.
49. Steadman JR, Matheny LM, Hurst JM, Briggs KK. Patient-centered outcomes and revision rate in patients undergoing ACL reconstruction using bone-patellar tendon-bone autograft compared with bone-patellar tendon-bone allograft: a matched case-control study. Arthroscopy. 2015;31(12):2320-2326.
50. Lenehan EA, Payne WB, Askam BM, Grana WA, Farrow LD. Long-term outcomes of allograft reconstruction of the anterior cruciate ligament. Am J Orthop. 2015;44(5):217-222.
51. Noh JH, Yi SR, Song SJ, Kim SW, Kim W. Comparison between hamstring autograft and free tendon achilles allograft: minimum 2-year follow-up after anterior cruciate ligament reconstruction using EndoButton and Intrafix. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):816-822.
52. Victor J, Bellemans J, Witvrouw E, Govaers K, Fabry G. Graft selection in anterior cruciate ligament reconstruction--prospective analysis of patellar tendon autografts compared with allografts. Int Orthop. 1997;21(2):93-97.
53. Kleipool AE, Zijl JA, Willems WJ. Arthroscopic anterior cruciate ligament reconstruction with bone-patellar tendon-bone allograft or autograft. A prospective study with an average follow up of 4 years. Knee Surg Sports Traumatol Arthrosc. 1998;6(4):224-230.
54. Peterson RK, Shelton WR, Bomboy AL. Allograft versus autograft patellar tendon anterior cruciate ligament reconstruction: a 5-year follow-up. Arthroscopy. 2001;17(1):9-13.
55. Edgar CM, Zimmer S, Kakar S, Jones H, Schepsis AA. Prospective comparison of auto and allograft hamstring tendon constructs for ACL reconstruction. Clin Orthop Relat Res. 2008;466(9):2238-2246.
56. Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):464-474.
57. Leal-Blanquet J, Alentorn-Geli E, Tuneu J, Valentí JR, Maestro A. Anterior cruciate ligament reconstruction: a multicenter prospective cohort study evaluating 3 different grafts using same bone drilling method. Clin J Sport Med. 2011;21(4):294-300.
58. Sun K, Zhang J, Wang Y, et al. Arthroscopic reconstruction of the anterior cruciate ligament with hamstring tendon autograft and fresh-frozen allograft: a prospective, randomized controlled study. Am J Sports Med. 2011;39(7):1430-1438.
59. Lawhorn KW, Howell SM, Traina SM, Gottlieb JE, Meade TD, Freedberg HI. The effect of graft tissue on anterior cruciate ligament outcomes: a multicenter, prospective, randomized controlled trial comparing autograft hamstrings with fresh-frozen anterior tibialis allograft. Arthroscopy. 2012;28(8):1079-1086.
60. Guo L, Yang L, Duan XJ, et al. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft: comparison of autograft, fresh-frozen allograft, and γ-irradiated allograft. Arthroscopy. 2012;28(2):211-217.
61. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
62. Mayr HO, Willkomm D, Stoehr A, et al. Revision of anterior cruciate ligament reconstruction with patellar tendon allograft and autograft: 2- and 5-year results. Arch Orthop Trauma Surg. 2012;132(6):867-874.
63. Mariscalco MW, Magnussen RA, Mehta D, Hewett TE, Flanigan DC, Kaeding CC. Autograft versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: a systematic review. Am J Sports Med. 2014;42(2):492-499.
64. Mehta VM, Mandala C, Foster D, Petsche TS. Comparison of revision rates in bone-patella tendon-bone autograft and allograft anterior cruciate ligament reconstruction. Orthopedics. 2010;33(1):12.
65. Vangsness CT Jr, Garcia IA, Mills CR, Kainer MA, Roberts MR, Moore TM. Allograft transplantation in the knee: tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2003;31(3):474-481.
66. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
67. Reagan J, Kullar R, Burks R. MPFL reconstruction: technique and results. Clin Sports Med. 2014;33(3):501-516.
68. Christiansen SE, Jacobsen BW, Lund B, Lind M. Reconstruction of the medial patellofemoral ligament with gracilis tendon autograft in transverse patellar drill holes. Arthroscopy. 2008;24(1):82-87.
69. Schöttle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):516-521.
70. Deie M, Ochi M, Sumen Y, Adachi N, Kobayashi K, Yasumoto M. A long-term follow-up study after medial patellofemoral ligament reconstruction using the transferred semitendinosus tendon for patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):522-528.
71. Nomura E, Inoue M. Hybrid medial patellofemoral ligament reconstruction using the semitendinous tendon for recurrent patellar dislocation: minimum 3 years’ follow-up. Arthroscopy. 2006;22(7):787-793.
72. Nomura E, Inoue M. Surgical technique and rationale for medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Arthroscopy. 2003;19(5):E47.
73. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
74. Drez D Jr, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthroscopy. 2001;17(3):298-306.
75. Fink C, Veselko M, Herbort M, Hoser C. MPFL reconstruction using a quadriceps tendon graft: part 2: operative technique and short term clinical results. Knee. 2014;21(6):1175-1179.
76. Calvo Rodríguez R, Figueroa Poblete D, Anastasiadis Le Roy Z, Etchegaray Bascur F, Vaisman Burucker A, Calvo Mena R. Reconstruction of the medial patellofemoral ligament: evaluation of the clinical results of autografts versus allografts. Rev Esp Cir Ortop Traumatol. 2015;59(5):348-353.
77. Becher C, Kley K, Lobenhoffer P, Ezechieli M, Smith T, Ostermeier S. Dynamic versus static reconstruction of the medial patellofemoral ligament for recurrent lateral patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2452-2457.
78. Gomes JE. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24(4):430-435.