User login
Treatment of Femoroacetabular Impingement: Labrum, Cartilage, Osseous Deformity, and Capsule
Take-Home Points
- Repair the labrum when tissue quality is good.
- Avoid overcorrection of acetabulum by measuring center edge angle.
- Cam resection should be comprehensive and restore a smooth head-neck offset to restore the suction seal.
- Chondral débridement for Outerbridge grade 0-3 and microfracture for grade 4.
- Routine capsular closure to prevent postoperative instability.
The surgical approach of femoroacetabular impingement (FAI) pathology should cover the entire hip joint. Both bony and cartilaginous tissue pathology should be adequately addressed. However, treating soft-tissue abnormalities (acetabular labrum and joint capsule) is also crucial. Overall, any surgical intervention should focus on restoring the hip labrum seal mechanism to ensure successful clinical outcomes. This restoration, combined with the use of biological therapies and rehabilitation, will produce the maximum benefit for the patient.
Management of Acetabular Labrum
The final decision regarding how to surgically approach the acetabular labrum is made during the operation. We focus restoring the labrum seal mechanism, which is crucial for proper function and health of the hip joint.1 The intra-articular hydrostatic pressure loss caused by labral deficiency results in abnormal load distribution and joint microinstability, which have detrimental effects on cartilage and periarticular tissues. A biomechanical study highlighted the role of the hip labrum in maintaining intra-articular fluid pressurization and showed that labral reconstruction restores intra-articular fluid pressure to levels similar to those of the intact state.1
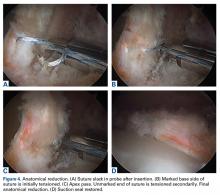
In cases in which the remaining labral tissue is adequate and of good quality (reparable), the labral repair technique is preferred.2 After diagnostic arthroscopy, the labral tear is identified, and a 4.5-mm burr is used to correct (rim-trim) any osseous deformity of the acetabulum to create a “new rim” for labrum reattachment. Suture anchors are placed on the rim about 2 mm to 3 mm below the cartilage surface. Considering the rim angle3 is helpful in avoiding acetabular cartilage damage. Labral sutures can be looped around or pierced through the labrum to secure it to the acetabulum. No difference in clinical outcomes was found between the 2 suture types,4 though biomechanically piercing sutures help restore the labrum seal better.1 When the labrum is deficient and longitudinal fibers remain but are insufficient for seal restoration, the repair can be augmented with adjacent iliotibial band (ITB) tissue. This technique is similar to labral reconstruction but involves placing a graft on top of the remaining labral tissue, and suture around both the native tissue and the graft. The additional tissue gives the labrum the volume it needs to recreate the seal.
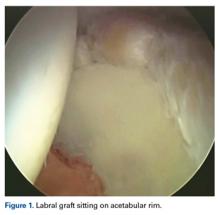
The labral reconstruction technique is indicated when the remaining labrum is irreparable, absent, or severely hypotrophic or deficient, or when an irreparable complex tear or poor-quality tissue is present. Different types of grafts can be used to reconstruct the labrum. ITB, semitendinosus, gracilis, and anterior tibialis grafts and the human acetabular labrum exhibit similar cyclic elongation behavior in response to simulated physiologic forces, though there is variability in both elongation and geometry for all graft types.5 We prefer the ITB autograft technique.6 The graft should be about 30% to 40% longer than the labral defect as measured with arthroscopic probe. With the leg in traction, the graft is inserted through the mid-anterior portal, and a suture anchor is used to secure it against the acetabulum medially.
With proper patient selection, these techniques have excellent clinical outcomes.4,7 Severe osteoarthritis (joint space <2 mm) is a contraindication for these procedures.8
Osseous Deformity
On approaching the bony structures of the hip joint, the surgeon should examine the acetabular rim (pincer lesion), the femoral head and neck shape (cam lesion), and the anterior inferior iliac spine (AIIS). Preoperative imaging and physical examination are important for identifying severe bone deformities that can complicate the procedure.9
The acetabular rim can be directly viewed after labrum detachment, but usually complete detachment is not necessary. Pincer deformity causes focal or global overcoverage of the femoral head. Rim trimming is performed with a 4.5-mm round curved burr. Resection is usually performed to the end of rim chondrosis (about 3-5 mm). Using a simple formula, you can calculate how the lateral center edge will be reduced by the amount of rim resected, maintaining a safe margin.2 A new acetabular “bed” is created where the to-be-attached labral tissue will contribute to the suction seal mechanism of the joint.2Cam lesion correction is challenging, and the amount of bone that should be resected is a matter of disagreement. We perform cam osteochondroplasty2 with a 5.5-mm round burr inserted through the anterolateral portal while the hip is positioned in 45° of flexion, neutral rotation, and adduction/abduction. This position allows an osteoplasty from 6 to 10 o’clock on the head–neck junction. Osteoplasty performed between 10 and 12 o’clock requires hip extension and slight traction. The proximal limit of osteochondroplasty is about 15 mm from the labral edge, while distally the resection stops beneath the zona orbicularis. The lateral epiphyseal vessels and the Weitbrecht ligament constitute the lateral and medial borders, respectively.
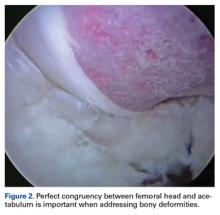
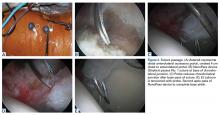
The surgeon should create a smooth head–neck offset that prevents elevation of the labrum during flexion and achieves a nearly perfect anatomical relationship between the femoral head and the acetabular labrum, restoring the hip joint seal (Figure 2).
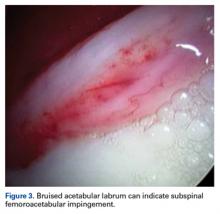
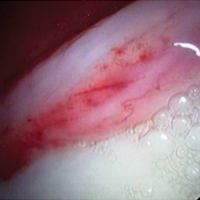
A hypertrophic AIIS can impinge the femur (extra-articular subspinal impingement). Patients present with limited range of motion (especially hip flexion), pain in the AIIS area, and, in some cases, a history of avulsion injury.11 Seeing a bruised labrum (Figure 3) during surgery is common with this pathology.
Treatment of Cartilage Lesions
The indications and contraindications for hip arthroscopy in patients with cartilage lesions are important. Our study’s 5-year outcomes of treating FAI with hip arthroscopy in patients with preserved joint space (>2 mm) were promising, though 86% of patients with limited joint space (≤2 mm) converted to total hip arthroplasty.8 We regard patients with severe osteoarthritis as not being candidates for hip arthroscopy.
As 3 Tesla magnetic resonance imaging has low positive predictive value in identifying severe cartilage damage,13 the cartilage should be examined during surgery to further define the diagnosis. Nearly half of the hip arthroscopy patients in our study had at least 1 Outerbridge grade 3 or 4 cartilage lesion.14 Compared with the femoral head, acetabular cartilage was damaged 3 times more often. More than 90% of acetabular cartilage lesions were in the anterosuperior region.
Grades 0 and 1 cartilage lesions are usually left untreated; no intervention is necessary. Grades 2 and 3 cartilage lesions are reduced by partial débridement and/or thermal shrinkage. With the improved joint microenvironment arising from simple correction of the underlying hip bony abnormalities, these lesions should not produce further symptoms.
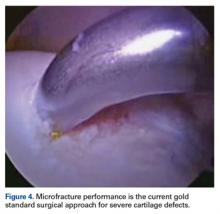
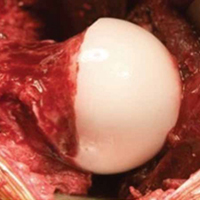
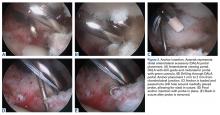
Grade 4 hip cartilage defects are challenging. We prefer microfracture for grade 4 lesions (Figure 4).
A ring curette is used to prepare the defect, and perpendicular borders are created to hold the clot in place. Deep débridement removes the calcified layer while maintaining the integrity of the subchondral plate.15 As a recent study found microfracture performed with small-diameter awls improved cartilage repair more effectively than microfracture with large-diameter awls,16 we prefer making small-diameter holes when establishing the maximum number of holes possible. As it is important to make a perpendicular hole, not a scratch, we use an XL Microfracture Pick (Smith & Nephew) 90° curve, which is suitable for creating a vertical entry point. The 60° curved awl is then used to further deepen the hole. Creation and stability of the marrow clot are ensured by shutting down the infusion pump device and verifying that blood and marrow elements are released from the microfractures.
Capsule Management
The increase in hip arthroscopies performed worldwide has generated interest in proper capsular management and development of iatrogenic microinstability.17 Hip capsulotomy is routinely performed for adequate visualization of the intra-articular compartment. Standard anterosuperior interportal capsulotomy for hip arthroscopic surgery (12 to 3 o’clock) sacrifices the integrity of the iliofemoral ligament (ligament of Bigelow),18 which provides rotational stability. Failure to restore the anatomical and biomechanical properties of the iliofemoral ligament after arthroscopic surgery increases the likelihood of postoperative microinstability or gross instability,19 which can lead to persistent pain and/or sense of an unstable joint, in addition to accelerated cartilage wear.
Capsulotomies are useful in obtaining adequate intraoperative exposure of the central and peripheral compartments. In the past, little attention was given to capsular closure on completion of the procedure. However, concern about postoperative instability from capsular laxity or deficiency made the introduction of capsular repair techniques necessary. Although deciding between capsular closure and plication remains debatable, we routinely perform capsular closure with a Quebec City slider knot.20 Mindful management of the capsule throughout the procedure is important in avoiding irreversible capsular damage, which would complicate capsular closure. Mindful management involves leaving a proximal leaflet of at least 1 cm during the capsulotomy, avoiding capsular thinning during shaver use, and using a cannula to prevent soft-tissue bridging.
Recent evidence suggests that capsule repair restores near native hip joint stability.17 In addition to capsular shift or capsulorrhaphy, 2 to 6 sutures have been used for capsular closure or plication after an interportal or T capsulotomy. Chahla and colleagues21 reported that 2- and 3-suture constructs produced comparable biomechanical failure torques when external rotation forces were applied to conventional hip capsulotomy on cadavers. Three-suture constructs were significantly stronger than 1-suture constructs, but there was no significant difference between 2- and 3-suture constructs. All constructs failed at about 36° of external rotation. Therefore, restricted external rotation is recommended for 3 weeks after surgery.
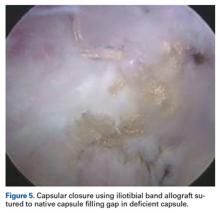
In one study, 35% of revision hip arthroscopy patients had undiagnosed hip instability from iatrogenic injury,22 which can lead to labral and chondral injury.17 Capsular reconstruction is recommended in cases of symptomatic capsular deficiency; capsular deficiency caused by adhesion removal; and pain and range-of-motion limitation caused by capsular adhesions. However, indications need to be further established. We have performed capsular reconstruction with ITB allograft23 (Figure 5).
Biologics
At the end of the procedure, we use platelet-rich plasma and/or bone marrow aspirate injections (individualized to the patient) to potentiate the biological healing of the tissues. Further research is planned to determine how to prepare these biological products to provide the best mix of biological factors for improved healing. Antifibrotic factors are useful in preventing adhesions, and angiotensin II receptor blockers are effective, but clinical studies are needed to establish their use.
Rehabilitation
Immediately after surgery, a postoperative hip brace and antirotational boots are applied to the patient to protect the operative site and reduce pain. The actual postoperative protocol is based on the procedure and individualized to the patient. During microfractures, the patient is kept 20 pounds touch-toe weight-bearing for 4 to 8 weeks. The capsular closure is brace-protected by limiting abduction to 0° to 45° and hip flexion to 0° to 90° while external rotation and extension are prohibited (first 3 weeks). Immediate mobilization with passive rotational movement is crucial in preventing adhesions. Stationary bike exercise and use of a continuous passive motion machine are helpful. Progressive functional and sport-specific rehabilitation help the patient return to full activity, though the decision to return to full activity is based on several factors, both objective (functional tests) and subjective (physician–patient co-decisions).
Conclusion
Although hip arthroscopic techniques have expanded significantly in recent years, our treatment approach is based on restoring the normal anatomy of the hip joint—combining the procedures with biological therapies and a postoperative rehabilitation program that is individualized to the patient’s special needs.
Am J Orthop. 2017;46(1):23-27. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
2. Philippon MJ, Faucet SC, Briggs KK. Arthroscopic hip labral repair. Arthrosc Tech. 2013;2(2):e73-e76.
3. Lertwanich P, Ejnisman L, Torry MR, Giphart JE, Philippon MJ. Defining a safety margin for labral suture anchor insertion using the acetabular rim angle. Am J Sports Med. 2011;39(suppl):111S-116S.
4. Sawyer GA, Briggs KK, Dornan GJ, Ommen ND, Philippon MJ. Clinical outcomes after arthroscopic hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683-1688.
5. Ferro FP, Philippon MJ, Rasmussen MT, Smith SD, LaPrade RF, Wijdicks CA. Tensile properties of the human acetabular labrum and hip labral reconstruction grafts. Am J Sports Med. 2015;43(5):1222-1227.
6. Philippon MJ, Briggs KK, Boykin RE. Results of arthroscopic labral reconstruction of the hip in elite athletes: response. Am J Sports Med. 2014;42(10):NP48.
7. Geyer MR, Philippon MJ, Fagrelius TS, Briggs KK. Acetabular labral reconstruction with an iliotibial band autograft: outcome and survivorship analysis at minimum 3-year follow-up. Am J Sports Med. 2013;41(8):1750-1756.
8. Skendzel JG, Philippon MJ, Briggs KK, Goljan P. The effect of joint space on midterm outcomes after arthroscopic hip surgery for femoroacetabular impingement. Am J Sports Med. 2014;42(5):1127-1133.
9. Yeung M, Kowalczuk M, Simunovic N, Ayeni OR. Hip arthroscopy in the setting of hip dysplasia: a systematic review. Bone Joint Res. 2016;5(6):225-231.
10. Locks R, Chahla J, Mitchell JJ, Soares E, Philippon MJ. Dynamic hip examination for assesment of impingement during hip arthroscopy. Arthroscopy Tech. 2016 Nov 28. http://dx.doi.org/10.1016/j.eats.2016.08.011
11. Nabhan DC, Moreau WJ, McNamara SC, Briggs KK, Philippon MJ. Subspine hip impingement: an unusual cause of hip pain in an elite weightlifter. Curr Sports Med Rep. 2016;15(5):315-319.
12. Philippon MJ, Michalski MP, Campbell KJ, et al. An anatomical study of the acetabulum with clinical applications to hip arthroscopy. J Bone Joint Surg Am. 2014;96(20):1673-1682.
13. Ho CP, Ommen ND, Bhatia S, et al. Predictive value of 3-T magnetic resonance imaging in diagnosing grade 3 and 4 chondral lesions in the hip. Arthroscopy. 2016;32(9):1808-1813.
14. Bhatia S, Nowak DD, Briggs KK, Patterson DC, Philippon MJ. Outerbridge grade IV cartilage lesions in the hip identified at arthroscopy. Arthroscopy. 2016;32(5):814-819.
15. Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34(11):1824-1831.
16. Orth P, Duffner J, Zurakowski D, Cucchiarini M, Madry H. Small-diameter awls improve articular cartilage repair after microfracture treatment in a translational animal model. Am J Sports Med. 2016;44(1):209-219.
17. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
18. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
19. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
20. Menge TJ, Chahla J, Soares E, Mitchell JJ, Philippon MJ. The Quebec City slider: a technique for capsular closure and plication in hip arthroscopy. Arthrosc Tech. 2016;5(5):e971-e974.
21. Chahla J, Mikula JD, Schon JM, et al. Hip capsular closure: a biomechanical analysis of failure torque. Am J Sports Med. doi:10.1177/0363546516666353.
22. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-1921.
23. Trindade CA, Sawyer GA, Fukui K, Briggs KK, Philippon MJ. Arthroscopic capsule reconstruction in the hip using iliotibial band allograft. Arthrosc Tech. 2015;4(1):e71-e74.
Take-Home Points
- Repair the labrum when tissue quality is good.
- Avoid overcorrection of acetabulum by measuring center edge angle.
- Cam resection should be comprehensive and restore a smooth head-neck offset to restore the suction seal.
- Chondral débridement for Outerbridge grade 0-3 and microfracture for grade 4.
- Routine capsular closure to prevent postoperative instability.
The surgical approach of femoroacetabular impingement (FAI) pathology should cover the entire hip joint. Both bony and cartilaginous tissue pathology should be adequately addressed. However, treating soft-tissue abnormalities (acetabular labrum and joint capsule) is also crucial. Overall, any surgical intervention should focus on restoring the hip labrum seal mechanism to ensure successful clinical outcomes. This restoration, combined with the use of biological therapies and rehabilitation, will produce the maximum benefit for the patient.
Management of Acetabular Labrum
The final decision regarding how to surgically approach the acetabular labrum is made during the operation. We focus restoring the labrum seal mechanism, which is crucial for proper function and health of the hip joint.1 The intra-articular hydrostatic pressure loss caused by labral deficiency results in abnormal load distribution and joint microinstability, which have detrimental effects on cartilage and periarticular tissues. A biomechanical study highlighted the role of the hip labrum in maintaining intra-articular fluid pressurization and showed that labral reconstruction restores intra-articular fluid pressure to levels similar to those of the intact state.1
In cases in which the remaining labral tissue is adequate and of good quality (reparable), the labral repair technique is preferred.2 After diagnostic arthroscopy, the labral tear is identified, and a 4.5-mm burr is used to correct (rim-trim) any osseous deformity of the acetabulum to create a “new rim” for labrum reattachment. Suture anchors are placed on the rim about 2 mm to 3 mm below the cartilage surface. Considering the rim angle3 is helpful in avoiding acetabular cartilage damage. Labral sutures can be looped around or pierced through the labrum to secure it to the acetabulum. No difference in clinical outcomes was found between the 2 suture types,4 though biomechanically piercing sutures help restore the labrum seal better.1 When the labrum is deficient and longitudinal fibers remain but are insufficient for seal restoration, the repair can be augmented with adjacent iliotibial band (ITB) tissue. This technique is similar to labral reconstruction but involves placing a graft on top of the remaining labral tissue, and suture around both the native tissue and the graft. The additional tissue gives the labrum the volume it needs to recreate the seal.
The labral reconstruction technique is indicated when the remaining labrum is irreparable, absent, or severely hypotrophic or deficient, or when an irreparable complex tear or poor-quality tissue is present. Different types of grafts can be used to reconstruct the labrum. ITB, semitendinosus, gracilis, and anterior tibialis grafts and the human acetabular labrum exhibit similar cyclic elongation behavior in response to simulated physiologic forces, though there is variability in both elongation and geometry for all graft types.5 We prefer the ITB autograft technique.6 The graft should be about 30% to 40% longer than the labral defect as measured with arthroscopic probe. With the leg in traction, the graft is inserted through the mid-anterior portal, and a suture anchor is used to secure it against the acetabulum medially.
With proper patient selection, these techniques have excellent clinical outcomes.4,7 Severe osteoarthritis (joint space <2 mm) is a contraindication for these procedures.8
Osseous Deformity
On approaching the bony structures of the hip joint, the surgeon should examine the acetabular rim (pincer lesion), the femoral head and neck shape (cam lesion), and the anterior inferior iliac spine (AIIS). Preoperative imaging and physical examination are important for identifying severe bone deformities that can complicate the procedure.9
The acetabular rim can be directly viewed after labrum detachment, but usually complete detachment is not necessary. Pincer deformity causes focal or global overcoverage of the femoral head. Rim trimming is performed with a 4.5-mm round curved burr. Resection is usually performed to the end of rim chondrosis (about 3-5 mm). Using a simple formula, you can calculate how the lateral center edge will be reduced by the amount of rim resected, maintaining a safe margin.2 A new acetabular “bed” is created where the to-be-attached labral tissue will contribute to the suction seal mechanism of the joint.2Cam lesion correction is challenging, and the amount of bone that should be resected is a matter of disagreement. We perform cam osteochondroplasty2 with a 5.5-mm round burr inserted through the anterolateral portal while the hip is positioned in 45° of flexion, neutral rotation, and adduction/abduction. This position allows an osteoplasty from 6 to 10 o’clock on the head–neck junction. Osteoplasty performed between 10 and 12 o’clock requires hip extension and slight traction. The proximal limit of osteochondroplasty is about 15 mm from the labral edge, while distally the resection stops beneath the zona orbicularis. The lateral epiphyseal vessels and the Weitbrecht ligament constitute the lateral and medial borders, respectively.
The surgeon should create a smooth head–neck offset that prevents elevation of the labrum during flexion and achieves a nearly perfect anatomical relationship between the femoral head and the acetabular labrum, restoring the hip joint seal (Figure 2).
A hypertrophic AIIS can impinge the femur (extra-articular subspinal impingement). Patients present with limited range of motion (especially hip flexion), pain in the AIIS area, and, in some cases, a history of avulsion injury.11 Seeing a bruised labrum (Figure 3) during surgery is common with this pathology.
Treatment of Cartilage Lesions
The indications and contraindications for hip arthroscopy in patients with cartilage lesions are important. Our study’s 5-year outcomes of treating FAI with hip arthroscopy in patients with preserved joint space (>2 mm) were promising, though 86% of patients with limited joint space (≤2 mm) converted to total hip arthroplasty.8 We regard patients with severe osteoarthritis as not being candidates for hip arthroscopy.
As 3 Tesla magnetic resonance imaging has low positive predictive value in identifying severe cartilage damage,13 the cartilage should be examined during surgery to further define the diagnosis. Nearly half of the hip arthroscopy patients in our study had at least 1 Outerbridge grade 3 or 4 cartilage lesion.14 Compared with the femoral head, acetabular cartilage was damaged 3 times more often. More than 90% of acetabular cartilage lesions were in the anterosuperior region.
Grades 0 and 1 cartilage lesions are usually left untreated; no intervention is necessary. Grades 2 and 3 cartilage lesions are reduced by partial débridement and/or thermal shrinkage. With the improved joint microenvironment arising from simple correction of the underlying hip bony abnormalities, these lesions should not produce further symptoms.
Grade 4 hip cartilage defects are challenging. We prefer microfracture for grade 4 lesions (Figure 4).
A ring curette is used to prepare the defect, and perpendicular borders are created to hold the clot in place. Deep débridement removes the calcified layer while maintaining the integrity of the subchondral plate.15 As a recent study found microfracture performed with small-diameter awls improved cartilage repair more effectively than microfracture with large-diameter awls,16 we prefer making small-diameter holes when establishing the maximum number of holes possible. As it is important to make a perpendicular hole, not a scratch, we use an XL Microfracture Pick (Smith & Nephew) 90° curve, which is suitable for creating a vertical entry point. The 60° curved awl is then used to further deepen the hole. Creation and stability of the marrow clot are ensured by shutting down the infusion pump device and verifying that blood and marrow elements are released from the microfractures.
Capsule Management
The increase in hip arthroscopies performed worldwide has generated interest in proper capsular management and development of iatrogenic microinstability.17 Hip capsulotomy is routinely performed for adequate visualization of the intra-articular compartment. Standard anterosuperior interportal capsulotomy for hip arthroscopic surgery (12 to 3 o’clock) sacrifices the integrity of the iliofemoral ligament (ligament of Bigelow),18 which provides rotational stability. Failure to restore the anatomical and biomechanical properties of the iliofemoral ligament after arthroscopic surgery increases the likelihood of postoperative microinstability or gross instability,19 which can lead to persistent pain and/or sense of an unstable joint, in addition to accelerated cartilage wear.
Capsulotomies are useful in obtaining adequate intraoperative exposure of the central and peripheral compartments. In the past, little attention was given to capsular closure on completion of the procedure. However, concern about postoperative instability from capsular laxity or deficiency made the introduction of capsular repair techniques necessary. Although deciding between capsular closure and plication remains debatable, we routinely perform capsular closure with a Quebec City slider knot.20 Mindful management of the capsule throughout the procedure is important in avoiding irreversible capsular damage, which would complicate capsular closure. Mindful management involves leaving a proximal leaflet of at least 1 cm during the capsulotomy, avoiding capsular thinning during shaver use, and using a cannula to prevent soft-tissue bridging.
Recent evidence suggests that capsule repair restores near native hip joint stability.17 In addition to capsular shift or capsulorrhaphy, 2 to 6 sutures have been used for capsular closure or plication after an interportal or T capsulotomy. Chahla and colleagues21 reported that 2- and 3-suture constructs produced comparable biomechanical failure torques when external rotation forces were applied to conventional hip capsulotomy on cadavers. Three-suture constructs were significantly stronger than 1-suture constructs, but there was no significant difference between 2- and 3-suture constructs. All constructs failed at about 36° of external rotation. Therefore, restricted external rotation is recommended for 3 weeks after surgery.
In one study, 35% of revision hip arthroscopy patients had undiagnosed hip instability from iatrogenic injury,22 which can lead to labral and chondral injury.17 Capsular reconstruction is recommended in cases of symptomatic capsular deficiency; capsular deficiency caused by adhesion removal; and pain and range-of-motion limitation caused by capsular adhesions. However, indications need to be further established. We have performed capsular reconstruction with ITB allograft23 (Figure 5).
Biologics
At the end of the procedure, we use platelet-rich plasma and/or bone marrow aspirate injections (individualized to the patient) to potentiate the biological healing of the tissues. Further research is planned to determine how to prepare these biological products to provide the best mix of biological factors for improved healing. Antifibrotic factors are useful in preventing adhesions, and angiotensin II receptor blockers are effective, but clinical studies are needed to establish their use.
Rehabilitation
Immediately after surgery, a postoperative hip brace and antirotational boots are applied to the patient to protect the operative site and reduce pain. The actual postoperative protocol is based on the procedure and individualized to the patient. During microfractures, the patient is kept 20 pounds touch-toe weight-bearing for 4 to 8 weeks. The capsular closure is brace-protected by limiting abduction to 0° to 45° and hip flexion to 0° to 90° while external rotation and extension are prohibited (first 3 weeks). Immediate mobilization with passive rotational movement is crucial in preventing adhesions. Stationary bike exercise and use of a continuous passive motion machine are helpful. Progressive functional and sport-specific rehabilitation help the patient return to full activity, though the decision to return to full activity is based on several factors, both objective (functional tests) and subjective (physician–patient co-decisions).
Conclusion
Although hip arthroscopic techniques have expanded significantly in recent years, our treatment approach is based on restoring the normal anatomy of the hip joint—combining the procedures with biological therapies and a postoperative rehabilitation program that is individualized to the patient’s special needs.
Am J Orthop. 2017;46(1):23-27. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Repair the labrum when tissue quality is good.
- Avoid overcorrection of acetabulum by measuring center edge angle.
- Cam resection should be comprehensive and restore a smooth head-neck offset to restore the suction seal.
- Chondral débridement for Outerbridge grade 0-3 and microfracture for grade 4.
- Routine capsular closure to prevent postoperative instability.
The surgical approach of femoroacetabular impingement (FAI) pathology should cover the entire hip joint. Both bony and cartilaginous tissue pathology should be adequately addressed. However, treating soft-tissue abnormalities (acetabular labrum and joint capsule) is also crucial. Overall, any surgical intervention should focus on restoring the hip labrum seal mechanism to ensure successful clinical outcomes. This restoration, combined with the use of biological therapies and rehabilitation, will produce the maximum benefit for the patient.
Management of Acetabular Labrum
The final decision regarding how to surgically approach the acetabular labrum is made during the operation. We focus restoring the labrum seal mechanism, which is crucial for proper function and health of the hip joint.1 The intra-articular hydrostatic pressure loss caused by labral deficiency results in abnormal load distribution and joint microinstability, which have detrimental effects on cartilage and periarticular tissues. A biomechanical study highlighted the role of the hip labrum in maintaining intra-articular fluid pressurization and showed that labral reconstruction restores intra-articular fluid pressure to levels similar to those of the intact state.1
In cases in which the remaining labral tissue is adequate and of good quality (reparable), the labral repair technique is preferred.2 After diagnostic arthroscopy, the labral tear is identified, and a 4.5-mm burr is used to correct (rim-trim) any osseous deformity of the acetabulum to create a “new rim” for labrum reattachment. Suture anchors are placed on the rim about 2 mm to 3 mm below the cartilage surface. Considering the rim angle3 is helpful in avoiding acetabular cartilage damage. Labral sutures can be looped around or pierced through the labrum to secure it to the acetabulum. No difference in clinical outcomes was found between the 2 suture types,4 though biomechanically piercing sutures help restore the labrum seal better.1 When the labrum is deficient and longitudinal fibers remain but are insufficient for seal restoration, the repair can be augmented with adjacent iliotibial band (ITB) tissue. This technique is similar to labral reconstruction but involves placing a graft on top of the remaining labral tissue, and suture around both the native tissue and the graft. The additional tissue gives the labrum the volume it needs to recreate the seal.
The labral reconstruction technique is indicated when the remaining labrum is irreparable, absent, or severely hypotrophic or deficient, or when an irreparable complex tear or poor-quality tissue is present. Different types of grafts can be used to reconstruct the labrum. ITB, semitendinosus, gracilis, and anterior tibialis grafts and the human acetabular labrum exhibit similar cyclic elongation behavior in response to simulated physiologic forces, though there is variability in both elongation and geometry for all graft types.5 We prefer the ITB autograft technique.6 The graft should be about 30% to 40% longer than the labral defect as measured with arthroscopic probe. With the leg in traction, the graft is inserted through the mid-anterior portal, and a suture anchor is used to secure it against the acetabulum medially.
With proper patient selection, these techniques have excellent clinical outcomes.4,7 Severe osteoarthritis (joint space <2 mm) is a contraindication for these procedures.8
Osseous Deformity
On approaching the bony structures of the hip joint, the surgeon should examine the acetabular rim (pincer lesion), the femoral head and neck shape (cam lesion), and the anterior inferior iliac spine (AIIS). Preoperative imaging and physical examination are important for identifying severe bone deformities that can complicate the procedure.9
The acetabular rim can be directly viewed after labrum detachment, but usually complete detachment is not necessary. Pincer deformity causes focal or global overcoverage of the femoral head. Rim trimming is performed with a 4.5-mm round curved burr. Resection is usually performed to the end of rim chondrosis (about 3-5 mm). Using a simple formula, you can calculate how the lateral center edge will be reduced by the amount of rim resected, maintaining a safe margin.2 A new acetabular “bed” is created where the to-be-attached labral tissue will contribute to the suction seal mechanism of the joint.2Cam lesion correction is challenging, and the amount of bone that should be resected is a matter of disagreement. We perform cam osteochondroplasty2 with a 5.5-mm round burr inserted through the anterolateral portal while the hip is positioned in 45° of flexion, neutral rotation, and adduction/abduction. This position allows an osteoplasty from 6 to 10 o’clock on the head–neck junction. Osteoplasty performed between 10 and 12 o’clock requires hip extension and slight traction. The proximal limit of osteochondroplasty is about 15 mm from the labral edge, while distally the resection stops beneath the zona orbicularis. The lateral epiphyseal vessels and the Weitbrecht ligament constitute the lateral and medial borders, respectively.
The surgeon should create a smooth head–neck offset that prevents elevation of the labrum during flexion and achieves a nearly perfect anatomical relationship between the femoral head and the acetabular labrum, restoring the hip joint seal (Figure 2).
A hypertrophic AIIS can impinge the femur (extra-articular subspinal impingement). Patients present with limited range of motion (especially hip flexion), pain in the AIIS area, and, in some cases, a history of avulsion injury.11 Seeing a bruised labrum (Figure 3) during surgery is common with this pathology.
Treatment of Cartilage Lesions
The indications and contraindications for hip arthroscopy in patients with cartilage lesions are important. Our study’s 5-year outcomes of treating FAI with hip arthroscopy in patients with preserved joint space (>2 mm) were promising, though 86% of patients with limited joint space (≤2 mm) converted to total hip arthroplasty.8 We regard patients with severe osteoarthritis as not being candidates for hip arthroscopy.
As 3 Tesla magnetic resonance imaging has low positive predictive value in identifying severe cartilage damage,13 the cartilage should be examined during surgery to further define the diagnosis. Nearly half of the hip arthroscopy patients in our study had at least 1 Outerbridge grade 3 or 4 cartilage lesion.14 Compared with the femoral head, acetabular cartilage was damaged 3 times more often. More than 90% of acetabular cartilage lesions were in the anterosuperior region.
Grades 0 and 1 cartilage lesions are usually left untreated; no intervention is necessary. Grades 2 and 3 cartilage lesions are reduced by partial débridement and/or thermal shrinkage. With the improved joint microenvironment arising from simple correction of the underlying hip bony abnormalities, these lesions should not produce further symptoms.
Grade 4 hip cartilage defects are challenging. We prefer microfracture for grade 4 lesions (Figure 4).
A ring curette is used to prepare the defect, and perpendicular borders are created to hold the clot in place. Deep débridement removes the calcified layer while maintaining the integrity of the subchondral plate.15 As a recent study found microfracture performed with small-diameter awls improved cartilage repair more effectively than microfracture with large-diameter awls,16 we prefer making small-diameter holes when establishing the maximum number of holes possible. As it is important to make a perpendicular hole, not a scratch, we use an XL Microfracture Pick (Smith & Nephew) 90° curve, which is suitable for creating a vertical entry point. The 60° curved awl is then used to further deepen the hole. Creation and stability of the marrow clot are ensured by shutting down the infusion pump device and verifying that blood and marrow elements are released from the microfractures.
Capsule Management
The increase in hip arthroscopies performed worldwide has generated interest in proper capsular management and development of iatrogenic microinstability.17 Hip capsulotomy is routinely performed for adequate visualization of the intra-articular compartment. Standard anterosuperior interportal capsulotomy for hip arthroscopic surgery (12 to 3 o’clock) sacrifices the integrity of the iliofemoral ligament (ligament of Bigelow),18 which provides rotational stability. Failure to restore the anatomical and biomechanical properties of the iliofemoral ligament after arthroscopic surgery increases the likelihood of postoperative microinstability or gross instability,19 which can lead to persistent pain and/or sense of an unstable joint, in addition to accelerated cartilage wear.
Capsulotomies are useful in obtaining adequate intraoperative exposure of the central and peripheral compartments. In the past, little attention was given to capsular closure on completion of the procedure. However, concern about postoperative instability from capsular laxity or deficiency made the introduction of capsular repair techniques necessary. Although deciding between capsular closure and plication remains debatable, we routinely perform capsular closure with a Quebec City slider knot.20 Mindful management of the capsule throughout the procedure is important in avoiding irreversible capsular damage, which would complicate capsular closure. Mindful management involves leaving a proximal leaflet of at least 1 cm during the capsulotomy, avoiding capsular thinning during shaver use, and using a cannula to prevent soft-tissue bridging.
Recent evidence suggests that capsule repair restores near native hip joint stability.17 In addition to capsular shift or capsulorrhaphy, 2 to 6 sutures have been used for capsular closure or plication after an interportal or T capsulotomy. Chahla and colleagues21 reported that 2- and 3-suture constructs produced comparable biomechanical failure torques when external rotation forces were applied to conventional hip capsulotomy on cadavers. Three-suture constructs were significantly stronger than 1-suture constructs, but there was no significant difference between 2- and 3-suture constructs. All constructs failed at about 36° of external rotation. Therefore, restricted external rotation is recommended for 3 weeks after surgery.
In one study, 35% of revision hip arthroscopy patients had undiagnosed hip instability from iatrogenic injury,22 which can lead to labral and chondral injury.17 Capsular reconstruction is recommended in cases of symptomatic capsular deficiency; capsular deficiency caused by adhesion removal; and pain and range-of-motion limitation caused by capsular adhesions. However, indications need to be further established. We have performed capsular reconstruction with ITB allograft23 (Figure 5).
Biologics
At the end of the procedure, we use platelet-rich plasma and/or bone marrow aspirate injections (individualized to the patient) to potentiate the biological healing of the tissues. Further research is planned to determine how to prepare these biological products to provide the best mix of biological factors for improved healing. Antifibrotic factors are useful in preventing adhesions, and angiotensin II receptor blockers are effective, but clinical studies are needed to establish their use.
Rehabilitation
Immediately after surgery, a postoperative hip brace and antirotational boots are applied to the patient to protect the operative site and reduce pain. The actual postoperative protocol is based on the procedure and individualized to the patient. During microfractures, the patient is kept 20 pounds touch-toe weight-bearing for 4 to 8 weeks. The capsular closure is brace-protected by limiting abduction to 0° to 45° and hip flexion to 0° to 90° while external rotation and extension are prohibited (first 3 weeks). Immediate mobilization with passive rotational movement is crucial in preventing adhesions. Stationary bike exercise and use of a continuous passive motion machine are helpful. Progressive functional and sport-specific rehabilitation help the patient return to full activity, though the decision to return to full activity is based on several factors, both objective (functional tests) and subjective (physician–patient co-decisions).
Conclusion
Although hip arthroscopic techniques have expanded significantly in recent years, our treatment approach is based on restoring the normal anatomy of the hip joint—combining the procedures with biological therapies and a postoperative rehabilitation program that is individualized to the patient’s special needs.
Am J Orthop. 2017;46(1):23-27. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
2. Philippon MJ, Faucet SC, Briggs KK. Arthroscopic hip labral repair. Arthrosc Tech. 2013;2(2):e73-e76.
3. Lertwanich P, Ejnisman L, Torry MR, Giphart JE, Philippon MJ. Defining a safety margin for labral suture anchor insertion using the acetabular rim angle. Am J Sports Med. 2011;39(suppl):111S-116S.
4. Sawyer GA, Briggs KK, Dornan GJ, Ommen ND, Philippon MJ. Clinical outcomes after arthroscopic hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683-1688.
5. Ferro FP, Philippon MJ, Rasmussen MT, Smith SD, LaPrade RF, Wijdicks CA. Tensile properties of the human acetabular labrum and hip labral reconstruction grafts. Am J Sports Med. 2015;43(5):1222-1227.
6. Philippon MJ, Briggs KK, Boykin RE. Results of arthroscopic labral reconstruction of the hip in elite athletes: response. Am J Sports Med. 2014;42(10):NP48.
7. Geyer MR, Philippon MJ, Fagrelius TS, Briggs KK. Acetabular labral reconstruction with an iliotibial band autograft: outcome and survivorship analysis at minimum 3-year follow-up. Am J Sports Med. 2013;41(8):1750-1756.
8. Skendzel JG, Philippon MJ, Briggs KK, Goljan P. The effect of joint space on midterm outcomes after arthroscopic hip surgery for femoroacetabular impingement. Am J Sports Med. 2014;42(5):1127-1133.
9. Yeung M, Kowalczuk M, Simunovic N, Ayeni OR. Hip arthroscopy in the setting of hip dysplasia: a systematic review. Bone Joint Res. 2016;5(6):225-231.
10. Locks R, Chahla J, Mitchell JJ, Soares E, Philippon MJ. Dynamic hip examination for assesment of impingement during hip arthroscopy. Arthroscopy Tech. 2016 Nov 28. http://dx.doi.org/10.1016/j.eats.2016.08.011
11. Nabhan DC, Moreau WJ, McNamara SC, Briggs KK, Philippon MJ. Subspine hip impingement: an unusual cause of hip pain in an elite weightlifter. Curr Sports Med Rep. 2016;15(5):315-319.
12. Philippon MJ, Michalski MP, Campbell KJ, et al. An anatomical study of the acetabulum with clinical applications to hip arthroscopy. J Bone Joint Surg Am. 2014;96(20):1673-1682.
13. Ho CP, Ommen ND, Bhatia S, et al. Predictive value of 3-T magnetic resonance imaging in diagnosing grade 3 and 4 chondral lesions in the hip. Arthroscopy. 2016;32(9):1808-1813.
14. Bhatia S, Nowak DD, Briggs KK, Patterson DC, Philippon MJ. Outerbridge grade IV cartilage lesions in the hip identified at arthroscopy. Arthroscopy. 2016;32(5):814-819.
15. Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34(11):1824-1831.
16. Orth P, Duffner J, Zurakowski D, Cucchiarini M, Madry H. Small-diameter awls improve articular cartilage repair after microfracture treatment in a translational animal model. Am J Sports Med. 2016;44(1):209-219.
17. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
18. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
19. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
20. Menge TJ, Chahla J, Soares E, Mitchell JJ, Philippon MJ. The Quebec City slider: a technique for capsular closure and plication in hip arthroscopy. Arthrosc Tech. 2016;5(5):e971-e974.
21. Chahla J, Mikula JD, Schon JM, et al. Hip capsular closure: a biomechanical analysis of failure torque. Am J Sports Med. doi:10.1177/0363546516666353.
22. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-1921.
23. Trindade CA, Sawyer GA, Fukui K, Briggs KK, Philippon MJ. Arthroscopic capsule reconstruction in the hip using iliotibial band allograft. Arthrosc Tech. 2015;4(1):e71-e74.
1. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
2. Philippon MJ, Faucet SC, Briggs KK. Arthroscopic hip labral repair. Arthrosc Tech. 2013;2(2):e73-e76.
3. Lertwanich P, Ejnisman L, Torry MR, Giphart JE, Philippon MJ. Defining a safety margin for labral suture anchor insertion using the acetabular rim angle. Am J Sports Med. 2011;39(suppl):111S-116S.
4. Sawyer GA, Briggs KK, Dornan GJ, Ommen ND, Philippon MJ. Clinical outcomes after arthroscopic hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683-1688.
5. Ferro FP, Philippon MJ, Rasmussen MT, Smith SD, LaPrade RF, Wijdicks CA. Tensile properties of the human acetabular labrum and hip labral reconstruction grafts. Am J Sports Med. 2015;43(5):1222-1227.
6. Philippon MJ, Briggs KK, Boykin RE. Results of arthroscopic labral reconstruction of the hip in elite athletes: response. Am J Sports Med. 2014;42(10):NP48.
7. Geyer MR, Philippon MJ, Fagrelius TS, Briggs KK. Acetabular labral reconstruction with an iliotibial band autograft: outcome and survivorship analysis at minimum 3-year follow-up. Am J Sports Med. 2013;41(8):1750-1756.
8. Skendzel JG, Philippon MJ, Briggs KK, Goljan P. The effect of joint space on midterm outcomes after arthroscopic hip surgery for femoroacetabular impingement. Am J Sports Med. 2014;42(5):1127-1133.
9. Yeung M, Kowalczuk M, Simunovic N, Ayeni OR. Hip arthroscopy in the setting of hip dysplasia: a systematic review. Bone Joint Res. 2016;5(6):225-231.
10. Locks R, Chahla J, Mitchell JJ, Soares E, Philippon MJ. Dynamic hip examination for assesment of impingement during hip arthroscopy. Arthroscopy Tech. 2016 Nov 28. http://dx.doi.org/10.1016/j.eats.2016.08.011
11. Nabhan DC, Moreau WJ, McNamara SC, Briggs KK, Philippon MJ. Subspine hip impingement: an unusual cause of hip pain in an elite weightlifter. Curr Sports Med Rep. 2016;15(5):315-319.
12. Philippon MJ, Michalski MP, Campbell KJ, et al. An anatomical study of the acetabulum with clinical applications to hip arthroscopy. J Bone Joint Surg Am. 2014;96(20):1673-1682.
13. Ho CP, Ommen ND, Bhatia S, et al. Predictive value of 3-T magnetic resonance imaging in diagnosing grade 3 and 4 chondral lesions in the hip. Arthroscopy. 2016;32(9):1808-1813.
14. Bhatia S, Nowak DD, Briggs KK, Patterson DC, Philippon MJ. Outerbridge grade IV cartilage lesions in the hip identified at arthroscopy. Arthroscopy. 2016;32(5):814-819.
15. Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34(11):1824-1831.
16. Orth P, Duffner J, Zurakowski D, Cucchiarini M, Madry H. Small-diameter awls improve articular cartilage repair after microfracture treatment in a translational animal model. Am J Sports Med. 2016;44(1):209-219.
17. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
18. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
19. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
20. Menge TJ, Chahla J, Soares E, Mitchell JJ, Philippon MJ. The Quebec City slider: a technique for capsular closure and plication in hip arthroscopy. Arthrosc Tech. 2016;5(5):e971-e974.
21. Chahla J, Mikula JD, Schon JM, et al. Hip capsular closure: a biomechanical analysis of failure torque. Am J Sports Med. doi:10.1177/0363546516666353.
22. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-1921.
23. Trindade CA, Sawyer GA, Fukui K, Briggs KK, Philippon MJ. Arthroscopic capsule reconstruction in the hip using iliotibial band allograft. Arthrosc Tech. 2015;4(1):e71-e74.
Evolution of Femoroacetabular Impingement Treatment: The ANCHOR Experience
Take-Home Points
- Our understanding of FAI has evolved from cam-type and pincer-type impingement to much more complex disease patterns.
- Most surgeons are performing less aggressive acetabular rim trimming.
- Inadequate osseous correction is still the most common cause of the failed hip arthroscopy.
- Labral preservation is important to maintaining suction seal effect.
- Open surgical techniques have a role for more severe and complex FAI deformities.
Femoroacetabular impingement (FAI) was described by Ganz and colleagues1 in 2003 as a refinement of concepts introduced decades earlier. This description advanced our understanding of FAI as a mechanism for prearthritic hip pain and secondary hip osteoarthritis1 (OA) and allowed for treatment of FAI. The concept of proximal femoral and acetabular/pelvic deformity contributing to OA had been previously speculated by Smith-Petersen,2 Murray,3 Solomon,4 and Stulberg.5 Early cases of overcorrection of dysplasia using the periacetabular osteotomy created iatrogenic FAI, which further stimulated early development of the FAI concept.6 Improved anatomical characterization of the proximal femoral blood supply (medial femoral circumflex artery) allowed for development of the open surgical hip dislocation.7 Through open surgical hip dislocation, an improved understanding of hip pathomechanics by direct visualization helped pave the way for a better understanding of FAI. Open surgical hip dislocation allows for global treatment of labrochondral pathology and deformity of the proximal femoral head–neck junction and/or acetabular rim in FAI.
Hip arthroscopy has further developed and improved our understanding of FAI. Early hip arthroscopy was generally limited to débridement of labral and chondral pathology, and management of the soft-tissue structures. Advances in the understanding of FAI through open techniques allowed for application of similar techniques to hip arthroscopy. Improvements in arthroscopic instrumentation and techniques have allowed for treatment of labrochondral and acetabular-sided rim deformity in the central compartment and cam morphologies in the peripheral compartment through arthroscopic surgery. Appropriate bony correction by arthroscopic techniques has always been a concern, but improved techniques, dynamic assessment, and accurate use of intraoperative imaging have made this feasible and more predictable. Treatment of cam deformities extending adjacent and proximal to the retinacular vessels is possible but more technically demanding. Inadequate bony correction of FAI by arthroscopic means remains one of the most common causes of failure.8-10In 2013, the Academic Network of Conservational Hip Outcome Research (ANCHOR) Study Group reported the characteristics of a FAI cohort of 1130 hips (1076 patients) that underwent surgical treatment of FAI across 8 institutions and 12 surgeons.11 At that time, most ANCHOR surgeons (or surgeon groups) performed both open and arthroscopic surgeries and had significant referral volumes of complex cases that may have overrepresented the proportion of complex FAI cases in the cohort. During the 2008 to 2011 study period, FAI was treated with arthroscopy in 56% of these cases, open surgical hip dislocation in 34%, and reverse periacetabular osteotomy in 9%. FAI was characterized as isolated cam-type in 48%, combined cam–pincer type in 45%, and isolated pincer-type in 8%. Fifty-five percent of the patients were female. Patient-reported outcome studies in this cohort of patients are ongoing.
The FAI Concept
In 2003, after treating more than 600 open surgical hip dislocations over the previous decade, Ganz and colleagues1 coined the term femoroacetabular impingement to describe a “mechanism for the development of early osteoarthritis for most nondysplastic hips.” They reported surgical treatment focused on “improving the clearance for hip motion and alleviation of femoral abutment against the acetabular rim” with the goal of improving pain and possibly of halting progression of the degenerative process. FAI was defined as “abnormal contact between the proximal femur and acetabular rim that occurs during terminal motion of the hip” leading to “lesions of the acetabular labrum and/or the adjacent acetabular cartilage.” Subtle, previously overlooked deformities of the proximal femur and acetabulum were recognized as the cause of FAI, “including the presence of a bony prominence usually in the anterolateral head and neck junction that is seen best on the lateral radiographs, reduced offset of the femoral neck and head junction, and changes on the acetabular rim such as os acetabuli or a double line that is seen with rim ossification.” Ganz and colleagues1 recognized that “normal or near normal” hips could also experience FAI in the setting of excessive or supraphysiologic range of motion. Cam-type and pincer-type FAI deformities were introduced as 2 distinct mechanisms of FAI. By 2003, arthroscopic hip surgery was increasingly being used as a treatment for labral tears but not bony abnormalities. These FAI concepts seemed to explain the prevalence of labral tears at the anterosuperior rim, which had been noted during hip arthroscopy, and paved the way for major changes in arthroscopic hip surgery during the next decade. The ANCHOR group reported the descriptive epidemiology of a cohort of more than 1000 patients with FAI.11
Cam-Type FAI
Cam-type impingement results from femoral-sided deformities. The mechanism was described as inclusion-type impingement in which “jamming of an abnormal femoral head with increasing radius into the acetabulum during forceful motion, especially flexion.”1 This results in outside-in abrasion of the acetabular cartilage of the anterosuperior rim with detachment of the “principally uninvolved labrum”1 and potentially delamination of the adjacent cartilage from the subchondral bone. Ganz and colleagues1 recognized in their initial descriptions of FAI that cam-type FAI could involve decreased femoral version, femoral head–neck junction asphericity, and decreased head–neck offset. The complexity and variability in the topography and geography of the cam morphology have been increasingly recognized. Accurate understanding and characterization of the proximal femoral deformity are important in guiding surgical correction of the cam deformity.
Advances in understanding the prevalence of the cam morphology and the association with OA have been important to our understanding of the pathophysiology of FAI. Several studies12 have established that a cam morphology of the proximal femur (defined by a variety of different metrics) is common among asymptomatic individuals. In light of this fact, a description of the femoral anatomy as a “cam morphology” rather than a cam deformity is now favored. Similarly, FAI is better used to refer to symptomatic individuals and is not equivalent to a cam morphology. The cam morphology seems significantly more common among athletes. Siebenrock and colleagues13 demonstrated the correlation of high-level athletics during late stages of skeletal immaturity and development of a cam morphology. A recent systematic review of 9 studies found that elite male athletes in late skeletal immaturity were 2 to 8 times more likely to develop a cam morphology before skeletal maturity.14Several population-based studies15,16 have quantified the apparent association of the cam morphology with hip OA. However, the studies were limited in their ability to adequately define the presence of cam morphology based on anteroposterior (AP) pelvis radiographs.
In a prospective study, Agricola and colleagues15 found the risk of OA was increased 2.4 times in the setting of moderate cam morphology (α angle, >60°) over a 5-year period. Thomas and colleagues16 found increased risk in a female cohort when the α angle was >65°.
Treatment of cam-type FAI is focused on adequate correction of the abnormal bone morphology. Inadequate or inappropriate bony correction of FAI is a common cause of treatment failure and is more common with arthroscopic techniques.9,10,17 Inadequate bony resection may be the result of surgical inexperience, poor visualization, or lack of understanding of the underlying bony deformity. Modern osteoplasty techniques also focus on gradual bony contour correction that restores the normal concavity–convexity transition of the head–neck junction. Overresection of the cam deformity not only may increase the risk of femoral neck fracture but may result in early disruption of the hip fluid seal from loss of contact between the femoral head and the acetabular labrum earlier in the arc of motion. In addition, high range-of-motion impingement can be seen in various athletic populations (dance, gymnastics, martial arts, hockey goalies), and the regions of impingement tend to be farther away from classically described impingement. Impingement in these situations occurs at the distal femoral neck and subspine regions, adding a level of complexity and unpredictability from a surgical standpoint.
FAI can also occur in the setting of more complex deformities than the typical cam morphology. Complex cases of FAI caused by slipped capital femoral epiphysis (SCFE) and residual Legg-Calvé-Perthes disease are relatively common. Complex deformities may also result in extra-articular impingement of the proximal femur (greater/lesser trochanter, distal femoral neck) on the pelvis (ilium, ischium) in addition to typical FAI. Mild to moderate cases of residual SCFE may be adequately treated with osteoplasty by arthroscopic techniques. In the setting of more severe residual SCFE, presence of underlying femoral retroversion and retrotilt of the femoral epiphysis may prevent adequate deformity correction and motion improvement by arthroscopy. Surgical hip dislocation (with or without relative femoral neck lengthening) and/or proximal femoral flexion derotational osteotomy may be the best means of treatment in these more severe deformities but may be dependent on the chronicity of the deformity and associated compensatory changes occurring on the acetabular side. Similarly, in moderate to severe residual Legg-Calvé-Perthes disease, presence of coxa vara, high greater trochanter, short femoral neck, and ovoid femoral head may be better treated in open techniques to allow comprehensive deformity correction, including correction of acetabular dysplasia in some cases.
Pincer-Type FAI
Pincer-type FAI results from acetabular-sided deformities in which acetabular deformity leads to impaction-type impingement with “linear contact between the acetabular rim and the femoral head–neck junction.”1 Pincer FAI causes primarily labral damage with progressive degeneration and, in some cases, ossification of the acetabular labrum that further worsens the acetabular overcoverage and premature rim impaction. Chondral damage in pincer-type FAI is generally less significant and limited to the peripheral acetabular rim.
Pincer-type FAI may be caused by acetabular retroversion, coxa profunda, or protrusio acetabuli. Our understanding of what defines a pincer morphology has evolved significantly. Through efforts to better define structural features of the acetabular rim that represent abnormalities, we have improved our understanding of how these features may influence OA development. One example of improved understanding involves coxa profunda, classically defined as the medial acetabular fossa touching or projecting medial to the ilioischial line on an AP pelvis radiograph. Several studies have found that this classic definition poorly describes the “overcovered” hip, as it is present in 70% of females and commonly present (41%) in the setting of acetabular dysplasia.18,19 Acetabular retroversion was previously associated with hip OA. Although central acetabular retroversion is relatively uncommon, cranial acetabular retroversion is more common. Presence of a crossover sign on AP pelvis radiographs generally has been viewed as indicative of acetabular retroversion. However, alterations in pelvic tilt on supine or standing AP pelvis radiographs can result in apparent retroversion in the setting of normal acetabular anatomy20 and potentially influence the development of impingement.21 Zaltz and colleagues22 found that abnormal morphology of the anterior inferior iliac spine can also lead to the presence of a crossover sign in an otherwise anteverted acetabulum. Larson and colleagues23 recently found that a crossover sign is present in 11% of asymptomatic hips (19% of males) and may be considered a normal variant. A crossover sign can also be present in the setting of posterior acetabular deficiency with normal anterior acetabular coverage. Ultimately, acetabular retroversion might indicate pincer-type FAI or dysplasia or be a normal variant that does not require treatment. Global acetabular overcoverage, including coxa protrusio, may be associated with OA in population-based studies but is not uniformly demonstrated in all studies.16,24,25 A lateral center edge angle of >40° and a Tönnis angle (acetabular inclination) of <0° are commonly viewed as markers of global overcoverage.
FAI Treatment
Improvements in hip arthroscopy techniques and instrumentation have led to hip arthroscopy becoming the primary surgical technique for the treatment of most cases of FAI. Hip arthroscopy allows for precise visualization and treatment of labral and chondral disease in the central compartment by traction. Larson and colleagues26 reported complication rates for hip arthroscopy in a prospective series of >1600 cases. The overall complication rate was 8.3%, with higher rates noted in female patients and in the setting of traction time longer than 60 minutes. Nonetheless, major complications occurred in 1.1%, with only 0.1% having persistent disability. The most common complications were lateral femoral cutaneous nerve dysesthesias (1.6%), pudendal nerve neuropraxia (1.4%), and iatrogenic labral/chondral damage (2.1%).
The importance of preserving the acetabular labrum is now well accepted from clinical and biomechanical evidence.27-29 As in previous studies in surgical hip dislocation,30 arthroscopic labral repair (vs débridement) results in improved clinical outcomes.31,32 Labral repair techniques currently focus on stable fixation of the labrum while maintaining the normal position of the labrum relative to the femoral head and avoiding labral eversion, which may compromise the hip suction seal. With continued technical advancements and biomechanical support, arthroscopic labral reconstruction is possible in the setting of labral deficiency, often resulting from prior resection. However, the optimal indications, surgical techniques, and long-term outcomes continue to be better defined. Open and arthroscopic techniques have shown similar ability to correct the typical mild to moderate cam morphology in FAI.33 Yet, inadequate femoral bony correction of FAI seems to be the most common cause for revision hip preservation surgery.9,10,17Mild to moderate acetabular rim deformities are commonly treated with hip arthroscopy. As our understanding of pincer-type FAI continues to improve, many surgeons are performing less- aggressive bone resection along the anterior rim. On the other hand, subspinous impingement was recently recognized as a form of extra-articular pincer FAI variant.34 Subspine decompression without true acetabular rim resection has become a more common treatment for pincer lesions and may be a consideration even with restricted range of motion after periacetabular osteotomy. Severe acetabular deformities with global overcoverage or acetabular protrusion are particularly challenging by arthroscopy, even for the most experienced surgeons. Although some improvement in deformity is feasible with arthroscopy, even cases reported in the literature have demonstrated incomplete deformity correction. Open surgical hip dislocation may continue to be the ideal treatment technique for severe pincer impingement.
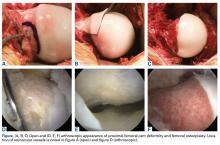
Cam-type FAI is commonly treated with hip arthroscopy (Figures A-F).
Open surgical techniques will continue to have an important role in the treatment of severe and complex FAI deformities in which arthroscopic techniques do not consistently achieve adequate bony correction (Figures A-F). Surgical hip dislocation remains a powerful surgical technique for deformity correction in FAI. Sink and colleagues37 reported rates of complications after open surgical hip dislocation in the ANCHOR study group. In a cohort of 334 hips (302 patients), trochanteric nonunion occurred in 1.8% of cases, and there were no cases of avascular necrosis. Overall major complications were observed in 4.8% of cases, with 0.3% having chronic disability. Excellent outcomes, including high rates of return to sports, have been reported after surgical hip dislocation for FAI.38 Midterm studies from the early phase of surgical treatment of FAI have helped identify factors that may play a major role in optimizing patient outcomes.
Conclusion
Our understanding and treatment of FAI continue to evolve. Both open and arthroscopic techniques have demonstrated excellent outcomes in the treatment of FAI. Most cases of FAI are now amenable to arthroscopic treatment. Inadequate resection and underlying acetabular dysplasia remain common causes of treatment failure. Open surgical hip dislocation continues to play a role in the treatment of severe deformities that are poorly accessible by arthroscopy—including cam lesions with posterior extension, severe global acetabular overcoverage, or extra-articular impingement. The association of FAI with OA is most apparent for cam-type FAI. Future research will define the optimal treatment strategies and determine if they modify disease progression.
Am J Orthop. 2017;46(1):28-34. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):112-120.
2. Smith-Petersen MN. The classic: treatment of malum coxae senilis, old slipped upper femoral epiphysis, intrapelvic protrusion of the acetabulum, and coxa plana by means of acetabuloplasty. 1936. Clin Orthop Relat Res. 2009;467(3):608-615.
3. Murray RO. The aetiology of primary osteoarthritis of the hip. Br J Radiol. 1965;38(455):810-824.
4. Solomon L. Patterns of osteoarthritis of the hip. J Bone Joint Surg Br. 1976;58(2):176-183.
5. Stulberg SD. Unrecognized childhood hip disease: a major cause of idiopathic osteoarthritis of the hip. In: Cordell LD, Harris WH, Ramsey PL, MacEwen GD, eds. The Hip: Proceedings of the Third Open Scientific Meeting of the Hip Society. St. Louis, MO: Mosby; 1975:212-228.
6. Myers SR, Eijer H, Ganz R. Anterior femoroacetabular impingement after periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):93-99.
7. Ganz R, Gill TJ, Gautier E, Ganz K, Krügel N, Berlemann U. Surgical dislocation of the adult hip: a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. J Bone Joint Surg Br. 2001;83(8):1119-1124.
8. Ross JR, Larson CM, Adeoye O, Kelly BT, Bedi A. Residual deformity is the most common reason for revision hip arthroscopy: a three-dimensional CT study. Clin Orthop Relat Res. 2015;473(4):1388-1395.
9. Clohisy JC, Nepple JJ, Larson CM, Zaltz I, Millis M; Academic Network of Conservation Hip Outcome Research (ANCHOR) Members. Persistent structural disease is the most common cause of repeat hip preservation surgery. Clin Orthop Relat Res. 2013;471(12):3788-3794.
10. Heyworth BE, Shindle MK, Voos JE, Rudzki JR, Kelly BT. Radiologic and intraoperative findings in revision hip arthroscopy. Arthroscopy. 2007;23(12):1295-1302.
11. Clohisy JC, Baca G, Beaulé PE, et al; ANCHOR Study Group. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
12. Frank JM, Harris JD, Erickson BJ, et al. Prevalence of femoroacetabular impingement imaging findings in asymptomatic volunteers: a systematic review. Arthroscopy. 2015;31(6):1199-11204.
13. Siebenrock KA, Ferner F, Noble PC, Santore RF, Werlen S, Mamisch TC. The cam-type deformity of the proximal femur arises in childhood in response to vigorous sporting activity. Clin Orthop Relat Res. 2011;469(11):3229-3240.
14. Nepple JJ, Vigdorchik JM, Clohisy JC. What is the association between sports participation and the development of proximal femoral cam deformity? A systematic review and meta-analysis. Am J Sports Med. 2015;43(11):2833-2840.
15. Agricola R, Waarsing JH, Arden NK, et al. Cam impingement of the hip: a risk factor for hip osteoarthritis. Nat Rev Rheumatol. 2013;9(10):630-634.
16. Thomas GE, Palmer AJ, Batra RN, et al. Subclinical deformities of the hip are significant predictors of radiographic osteoarthritis and joint replacement in women. A 20 year longitudinal cohort study. Osteoarthritis Cartilage. 2014;22(10):1504-1510.
17. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-1921.
18. Nepple JJ, Lehmann CL, Ross JR, Schoenecker PL, Clohisy JC. Coxa profunda is not a useful radiographic parameter for diagnosing pincer-type femoroacetabular impingement. J Bone Joint Surg Am. 2013;95(5):417-423.
19. Anderson LA, Kapron AL, Aoki SK, Peters CL. Coxa profunda: is the deep acetabulum overcovered? Clin Orthop Relat Res. 2012;470(12):3375-3382.
20. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop Relat Res. 2003;(407):241-248.
21. Ross JR, Nepple JJ, Philippon MJ, Kelly BT, Larson CM, Bedi A. Effect of changes in pelvic tilt on range of motion to impingement and radiographic parameters of acetabular morphologic characteristics. Am J Sports Med. 2014;42(10):2402-2409.
22. Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471(8):2463-2470.
23. Larson CM, Moreau-Gaudry A, Kelly BT, et al. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473(4):1247-1254.
24. Gosvig KK, Jacobsen S, Sonne-Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am. 2010;92(5):1162-1169.
25. Agricola R, Heijboer MP, Roze RH, et al. Pincer deformity does not lead to osteoarthritis of the hip whereas acetabular dysplasia does: acetabular coverage and development of osteoarthritis in a nationwide prospective cohort study (CHECK). Osteoarthritis Cartilage. 2013;21(10):1514-1521.
26. Larson CM, Clohisy JC, Beaulé PE, et al; ANCHOR Study Group. Intraoperative and early postoperative complications after hip arthroscopic surgery: a prospective multicenter trial utilizing a validated grading scheme. Am J Sports Med. 2016;44(9):2292-2298.
27. Ferguson SJ, Bryant JT, Ganz R, Ito K. An in vitro investigation of the acetabular labral seal in hip joint mechanics. J Biomech. 2003;36(2):171-178.
28. Nepple JJ, Philippon MJ, Campbell KJ, et al. The hip fluid seal—part II: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip stability to distraction. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):730-736.
29. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
30. Espinosa N, Rothenfluh DA, Beck M, Ganz R, Leunig M. Treatment of femoro-acetabular impingement: preliminary results of labral refixation. J Bone Joint Surg Am. 2006;88(5):925-935.
31. Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46-53.
32. Larson CM, Giveans MR. Arthroscopic debridement versus refixation of the acetabular labrum associated with femoroacetabular impingement. Arthroscopy. 2009;25(4):369-376.
33. Bedi A, Zaltz I, De La Torre K, Kelly BT. Radiographic comparison of surgical hip dislocation and hip arthroscopy for treatment of cam deformity in femoroacetabular impingement. Am J Sports Med. 2011;39(suppl):20S–28S.
34. Larson CM, Kelly BT, Stone RM. Making a case for anterior inferior iliac spine/subspine hip impingement: three representative case reports and proposed concept. Arthroscopy. 2011;27(12):1732-1737.
35. Ross JR, Bedi A, Stone RM, et al. Intraoperative fluoroscopic imaging to treat cam deformities: correlation with 3-dimensional computed tomography [published correction appears in Am J Sports Med. 2015;43(8):NP27]. Am J Sports Med. 2014;42(6):1370-1376.
36. Fabricant PD, Fields KG, Taylor SA, Magennis E, Bedi A, Kelly BT. The effect of femoral and acetabular version on clinical outcomes after arthroscopic femoroacetabular impingement surgery. J Bone Joint Surg Am. 2015;97(7):537-543.
37. Sink EL, Beaulé PE, Sucato D, et al. Multicenter study of complications following surgical dislocation of the hip. J Bone Joint Surg Am. 2011;93(12):1132-1136.
38. Naal FD, Miozzari HH, Wyss TF, Nötzli HP. Surgical hip dislocation for the treatment of femoroacetabular impingement in high-level athletes. Am J Sports Med. 2011;39(3):544-550.
Take-Home Points
- Our understanding of FAI has evolved from cam-type and pincer-type impingement to much more complex disease patterns.
- Most surgeons are performing less aggressive acetabular rim trimming.
- Inadequate osseous correction is still the most common cause of the failed hip arthroscopy.
- Labral preservation is important to maintaining suction seal effect.
- Open surgical techniques have a role for more severe and complex FAI deformities.
Femoroacetabular impingement (FAI) was described by Ganz and colleagues1 in 2003 as a refinement of concepts introduced decades earlier. This description advanced our understanding of FAI as a mechanism for prearthritic hip pain and secondary hip osteoarthritis1 (OA) and allowed for treatment of FAI. The concept of proximal femoral and acetabular/pelvic deformity contributing to OA had been previously speculated by Smith-Petersen,2 Murray,3 Solomon,4 and Stulberg.5 Early cases of overcorrection of dysplasia using the periacetabular osteotomy created iatrogenic FAI, which further stimulated early development of the FAI concept.6 Improved anatomical characterization of the proximal femoral blood supply (medial femoral circumflex artery) allowed for development of the open surgical hip dislocation.7 Through open surgical hip dislocation, an improved understanding of hip pathomechanics by direct visualization helped pave the way for a better understanding of FAI. Open surgical hip dislocation allows for global treatment of labrochondral pathology and deformity of the proximal femoral head–neck junction and/or acetabular rim in FAI.
Hip arthroscopy has further developed and improved our understanding of FAI. Early hip arthroscopy was generally limited to débridement of labral and chondral pathology, and management of the soft-tissue structures. Advances in the understanding of FAI through open techniques allowed for application of similar techniques to hip arthroscopy. Improvements in arthroscopic instrumentation and techniques have allowed for treatment of labrochondral and acetabular-sided rim deformity in the central compartment and cam morphologies in the peripheral compartment through arthroscopic surgery. Appropriate bony correction by arthroscopic techniques has always been a concern, but improved techniques, dynamic assessment, and accurate use of intraoperative imaging have made this feasible and more predictable. Treatment of cam deformities extending adjacent and proximal to the retinacular vessels is possible but more technically demanding. Inadequate bony correction of FAI by arthroscopic means remains one of the most common causes of failure.8-10In 2013, the Academic Network of Conservational Hip Outcome Research (ANCHOR) Study Group reported the characteristics of a FAI cohort of 1130 hips (1076 patients) that underwent surgical treatment of FAI across 8 institutions and 12 surgeons.11 At that time, most ANCHOR surgeons (or surgeon groups) performed both open and arthroscopic surgeries and had significant referral volumes of complex cases that may have overrepresented the proportion of complex FAI cases in the cohort. During the 2008 to 2011 study period, FAI was treated with arthroscopy in 56% of these cases, open surgical hip dislocation in 34%, and reverse periacetabular osteotomy in 9%. FAI was characterized as isolated cam-type in 48%, combined cam–pincer type in 45%, and isolated pincer-type in 8%. Fifty-five percent of the patients were female. Patient-reported outcome studies in this cohort of patients are ongoing.
The FAI Concept
In 2003, after treating more than 600 open surgical hip dislocations over the previous decade, Ganz and colleagues1 coined the term femoroacetabular impingement to describe a “mechanism for the development of early osteoarthritis for most nondysplastic hips.” They reported surgical treatment focused on “improving the clearance for hip motion and alleviation of femoral abutment against the acetabular rim” with the goal of improving pain and possibly of halting progression of the degenerative process. FAI was defined as “abnormal contact between the proximal femur and acetabular rim that occurs during terminal motion of the hip” leading to “lesions of the acetabular labrum and/or the adjacent acetabular cartilage.” Subtle, previously overlooked deformities of the proximal femur and acetabulum were recognized as the cause of FAI, “including the presence of a bony prominence usually in the anterolateral head and neck junction that is seen best on the lateral radiographs, reduced offset of the femoral neck and head junction, and changes on the acetabular rim such as os acetabuli or a double line that is seen with rim ossification.” Ganz and colleagues1 recognized that “normal or near normal” hips could also experience FAI in the setting of excessive or supraphysiologic range of motion. Cam-type and pincer-type FAI deformities were introduced as 2 distinct mechanisms of FAI. By 2003, arthroscopic hip surgery was increasingly being used as a treatment for labral tears but not bony abnormalities. These FAI concepts seemed to explain the prevalence of labral tears at the anterosuperior rim, which had been noted during hip arthroscopy, and paved the way for major changes in arthroscopic hip surgery during the next decade. The ANCHOR group reported the descriptive epidemiology of a cohort of more than 1000 patients with FAI.11
Cam-Type FAI
Cam-type impingement results from femoral-sided deformities. The mechanism was described as inclusion-type impingement in which “jamming of an abnormal femoral head with increasing radius into the acetabulum during forceful motion, especially flexion.”1 This results in outside-in abrasion of the acetabular cartilage of the anterosuperior rim with detachment of the “principally uninvolved labrum”1 and potentially delamination of the adjacent cartilage from the subchondral bone. Ganz and colleagues1 recognized in their initial descriptions of FAI that cam-type FAI could involve decreased femoral version, femoral head–neck junction asphericity, and decreased head–neck offset. The complexity and variability in the topography and geography of the cam morphology have been increasingly recognized. Accurate understanding and characterization of the proximal femoral deformity are important in guiding surgical correction of the cam deformity.
Advances in understanding the prevalence of the cam morphology and the association with OA have been important to our understanding of the pathophysiology of FAI. Several studies12 have established that a cam morphology of the proximal femur (defined by a variety of different metrics) is common among asymptomatic individuals. In light of this fact, a description of the femoral anatomy as a “cam morphology” rather than a cam deformity is now favored. Similarly, FAI is better used to refer to symptomatic individuals and is not equivalent to a cam morphology. The cam morphology seems significantly more common among athletes. Siebenrock and colleagues13 demonstrated the correlation of high-level athletics during late stages of skeletal immaturity and development of a cam morphology. A recent systematic review of 9 studies found that elite male athletes in late skeletal immaturity were 2 to 8 times more likely to develop a cam morphology before skeletal maturity.14Several population-based studies15,16 have quantified the apparent association of the cam morphology with hip OA. However, the studies were limited in their ability to adequately define the presence of cam morphology based on anteroposterior (AP) pelvis radiographs.
In a prospective study, Agricola and colleagues15 found the risk of OA was increased 2.4 times in the setting of moderate cam morphology (α angle, >60°) over a 5-year period. Thomas and colleagues16 found increased risk in a female cohort when the α angle was >65°.
Treatment of cam-type FAI is focused on adequate correction of the abnormal bone morphology. Inadequate or inappropriate bony correction of FAI is a common cause of treatment failure and is more common with arthroscopic techniques.9,10,17 Inadequate bony resection may be the result of surgical inexperience, poor visualization, or lack of understanding of the underlying bony deformity. Modern osteoplasty techniques also focus on gradual bony contour correction that restores the normal concavity–convexity transition of the head–neck junction. Overresection of the cam deformity not only may increase the risk of femoral neck fracture but may result in early disruption of the hip fluid seal from loss of contact between the femoral head and the acetabular labrum earlier in the arc of motion. In addition, high range-of-motion impingement can be seen in various athletic populations (dance, gymnastics, martial arts, hockey goalies), and the regions of impingement tend to be farther away from classically described impingement. Impingement in these situations occurs at the distal femoral neck and subspine regions, adding a level of complexity and unpredictability from a surgical standpoint.
FAI can also occur in the setting of more complex deformities than the typical cam morphology. Complex cases of FAI caused by slipped capital femoral epiphysis (SCFE) and residual Legg-Calvé-Perthes disease are relatively common. Complex deformities may also result in extra-articular impingement of the proximal femur (greater/lesser trochanter, distal femoral neck) on the pelvis (ilium, ischium) in addition to typical FAI. Mild to moderate cases of residual SCFE may be adequately treated with osteoplasty by arthroscopic techniques. In the setting of more severe residual SCFE, presence of underlying femoral retroversion and retrotilt of the femoral epiphysis may prevent adequate deformity correction and motion improvement by arthroscopy. Surgical hip dislocation (with or without relative femoral neck lengthening) and/or proximal femoral flexion derotational osteotomy may be the best means of treatment in these more severe deformities but may be dependent on the chronicity of the deformity and associated compensatory changes occurring on the acetabular side. Similarly, in moderate to severe residual Legg-Calvé-Perthes disease, presence of coxa vara, high greater trochanter, short femoral neck, and ovoid femoral head may be better treated in open techniques to allow comprehensive deformity correction, including correction of acetabular dysplasia in some cases.
Pincer-Type FAI
Pincer-type FAI results from acetabular-sided deformities in which acetabular deformity leads to impaction-type impingement with “linear contact between the acetabular rim and the femoral head–neck junction.”1 Pincer FAI causes primarily labral damage with progressive degeneration and, in some cases, ossification of the acetabular labrum that further worsens the acetabular overcoverage and premature rim impaction. Chondral damage in pincer-type FAI is generally less significant and limited to the peripheral acetabular rim.
Pincer-type FAI may be caused by acetabular retroversion, coxa profunda, or protrusio acetabuli. Our understanding of what defines a pincer morphology has evolved significantly. Through efforts to better define structural features of the acetabular rim that represent abnormalities, we have improved our understanding of how these features may influence OA development. One example of improved understanding involves coxa profunda, classically defined as the medial acetabular fossa touching or projecting medial to the ilioischial line on an AP pelvis radiograph. Several studies have found that this classic definition poorly describes the “overcovered” hip, as it is present in 70% of females and commonly present (41%) in the setting of acetabular dysplasia.18,19 Acetabular retroversion was previously associated with hip OA. Although central acetabular retroversion is relatively uncommon, cranial acetabular retroversion is more common. Presence of a crossover sign on AP pelvis radiographs generally has been viewed as indicative of acetabular retroversion. However, alterations in pelvic tilt on supine or standing AP pelvis radiographs can result in apparent retroversion in the setting of normal acetabular anatomy20 and potentially influence the development of impingement.21 Zaltz and colleagues22 found that abnormal morphology of the anterior inferior iliac spine can also lead to the presence of a crossover sign in an otherwise anteverted acetabulum. Larson and colleagues23 recently found that a crossover sign is present in 11% of asymptomatic hips (19% of males) and may be considered a normal variant. A crossover sign can also be present in the setting of posterior acetabular deficiency with normal anterior acetabular coverage. Ultimately, acetabular retroversion might indicate pincer-type FAI or dysplasia or be a normal variant that does not require treatment. Global acetabular overcoverage, including coxa protrusio, may be associated with OA in population-based studies but is not uniformly demonstrated in all studies.16,24,25 A lateral center edge angle of >40° and a Tönnis angle (acetabular inclination) of <0° are commonly viewed as markers of global overcoverage.
FAI Treatment
Improvements in hip arthroscopy techniques and instrumentation have led to hip arthroscopy becoming the primary surgical technique for the treatment of most cases of FAI. Hip arthroscopy allows for precise visualization and treatment of labral and chondral disease in the central compartment by traction. Larson and colleagues26 reported complication rates for hip arthroscopy in a prospective series of >1600 cases. The overall complication rate was 8.3%, with higher rates noted in female patients and in the setting of traction time longer than 60 minutes. Nonetheless, major complications occurred in 1.1%, with only 0.1% having persistent disability. The most common complications were lateral femoral cutaneous nerve dysesthesias (1.6%), pudendal nerve neuropraxia (1.4%), and iatrogenic labral/chondral damage (2.1%).
The importance of preserving the acetabular labrum is now well accepted from clinical and biomechanical evidence.27-29 As in previous studies in surgical hip dislocation,30 arthroscopic labral repair (vs débridement) results in improved clinical outcomes.31,32 Labral repair techniques currently focus on stable fixation of the labrum while maintaining the normal position of the labrum relative to the femoral head and avoiding labral eversion, which may compromise the hip suction seal. With continued technical advancements and biomechanical support, arthroscopic labral reconstruction is possible in the setting of labral deficiency, often resulting from prior resection. However, the optimal indications, surgical techniques, and long-term outcomes continue to be better defined. Open and arthroscopic techniques have shown similar ability to correct the typical mild to moderate cam morphology in FAI.33 Yet, inadequate femoral bony correction of FAI seems to be the most common cause for revision hip preservation surgery.9,10,17Mild to moderate acetabular rim deformities are commonly treated with hip arthroscopy. As our understanding of pincer-type FAI continues to improve, many surgeons are performing less- aggressive bone resection along the anterior rim. On the other hand, subspinous impingement was recently recognized as a form of extra-articular pincer FAI variant.34 Subspine decompression without true acetabular rim resection has become a more common treatment for pincer lesions and may be a consideration even with restricted range of motion after periacetabular osteotomy. Severe acetabular deformities with global overcoverage or acetabular protrusion are particularly challenging by arthroscopy, even for the most experienced surgeons. Although some improvement in deformity is feasible with arthroscopy, even cases reported in the literature have demonstrated incomplete deformity correction. Open surgical hip dislocation may continue to be the ideal treatment technique for severe pincer impingement.
Cam-type FAI is commonly treated with hip arthroscopy (Figures A-F).
Open surgical techniques will continue to have an important role in the treatment of severe and complex FAI deformities in which arthroscopic techniques do not consistently achieve adequate bony correction (Figures A-F). Surgical hip dislocation remains a powerful surgical technique for deformity correction in FAI. Sink and colleagues37 reported rates of complications after open surgical hip dislocation in the ANCHOR study group. In a cohort of 334 hips (302 patients), trochanteric nonunion occurred in 1.8% of cases, and there were no cases of avascular necrosis. Overall major complications were observed in 4.8% of cases, with 0.3% having chronic disability. Excellent outcomes, including high rates of return to sports, have been reported after surgical hip dislocation for FAI.38 Midterm studies from the early phase of surgical treatment of FAI have helped identify factors that may play a major role in optimizing patient outcomes.
Conclusion
Our understanding and treatment of FAI continue to evolve. Both open and arthroscopic techniques have demonstrated excellent outcomes in the treatment of FAI. Most cases of FAI are now amenable to arthroscopic treatment. Inadequate resection and underlying acetabular dysplasia remain common causes of treatment failure. Open surgical hip dislocation continues to play a role in the treatment of severe deformities that are poorly accessible by arthroscopy—including cam lesions with posterior extension, severe global acetabular overcoverage, or extra-articular impingement. The association of FAI with OA is most apparent for cam-type FAI. Future research will define the optimal treatment strategies and determine if they modify disease progression.
Am J Orthop. 2017;46(1):28-34. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Our understanding of FAI has evolved from cam-type and pincer-type impingement to much more complex disease patterns.
- Most surgeons are performing less aggressive acetabular rim trimming.
- Inadequate osseous correction is still the most common cause of the failed hip arthroscopy.
- Labral preservation is important to maintaining suction seal effect.
- Open surgical techniques have a role for more severe and complex FAI deformities.
Femoroacetabular impingement (FAI) was described by Ganz and colleagues1 in 2003 as a refinement of concepts introduced decades earlier. This description advanced our understanding of FAI as a mechanism for prearthritic hip pain and secondary hip osteoarthritis1 (OA) and allowed for treatment of FAI. The concept of proximal femoral and acetabular/pelvic deformity contributing to OA had been previously speculated by Smith-Petersen,2 Murray,3 Solomon,4 and Stulberg.5 Early cases of overcorrection of dysplasia using the periacetabular osteotomy created iatrogenic FAI, which further stimulated early development of the FAI concept.6 Improved anatomical characterization of the proximal femoral blood supply (medial femoral circumflex artery) allowed for development of the open surgical hip dislocation.7 Through open surgical hip dislocation, an improved understanding of hip pathomechanics by direct visualization helped pave the way for a better understanding of FAI. Open surgical hip dislocation allows for global treatment of labrochondral pathology and deformity of the proximal femoral head–neck junction and/or acetabular rim in FAI.
Hip arthroscopy has further developed and improved our understanding of FAI. Early hip arthroscopy was generally limited to débridement of labral and chondral pathology, and management of the soft-tissue structures. Advances in the understanding of FAI through open techniques allowed for application of similar techniques to hip arthroscopy. Improvements in arthroscopic instrumentation and techniques have allowed for treatment of labrochondral and acetabular-sided rim deformity in the central compartment and cam morphologies in the peripheral compartment through arthroscopic surgery. Appropriate bony correction by arthroscopic techniques has always been a concern, but improved techniques, dynamic assessment, and accurate use of intraoperative imaging have made this feasible and more predictable. Treatment of cam deformities extending adjacent and proximal to the retinacular vessels is possible but more technically demanding. Inadequate bony correction of FAI by arthroscopic means remains one of the most common causes of failure.8-10In 2013, the Academic Network of Conservational Hip Outcome Research (ANCHOR) Study Group reported the characteristics of a FAI cohort of 1130 hips (1076 patients) that underwent surgical treatment of FAI across 8 institutions and 12 surgeons.11 At that time, most ANCHOR surgeons (or surgeon groups) performed both open and arthroscopic surgeries and had significant referral volumes of complex cases that may have overrepresented the proportion of complex FAI cases in the cohort. During the 2008 to 2011 study period, FAI was treated with arthroscopy in 56% of these cases, open surgical hip dislocation in 34%, and reverse periacetabular osteotomy in 9%. FAI was characterized as isolated cam-type in 48%, combined cam–pincer type in 45%, and isolated pincer-type in 8%. Fifty-five percent of the patients were female. Patient-reported outcome studies in this cohort of patients are ongoing.
The FAI Concept
In 2003, after treating more than 600 open surgical hip dislocations over the previous decade, Ganz and colleagues1 coined the term femoroacetabular impingement to describe a “mechanism for the development of early osteoarthritis for most nondysplastic hips.” They reported surgical treatment focused on “improving the clearance for hip motion and alleviation of femoral abutment against the acetabular rim” with the goal of improving pain and possibly of halting progression of the degenerative process. FAI was defined as “abnormal contact between the proximal femur and acetabular rim that occurs during terminal motion of the hip” leading to “lesions of the acetabular labrum and/or the adjacent acetabular cartilage.” Subtle, previously overlooked deformities of the proximal femur and acetabulum were recognized as the cause of FAI, “including the presence of a bony prominence usually in the anterolateral head and neck junction that is seen best on the lateral radiographs, reduced offset of the femoral neck and head junction, and changes on the acetabular rim such as os acetabuli or a double line that is seen with rim ossification.” Ganz and colleagues1 recognized that “normal or near normal” hips could also experience FAI in the setting of excessive or supraphysiologic range of motion. Cam-type and pincer-type FAI deformities were introduced as 2 distinct mechanisms of FAI. By 2003, arthroscopic hip surgery was increasingly being used as a treatment for labral tears but not bony abnormalities. These FAI concepts seemed to explain the prevalence of labral tears at the anterosuperior rim, which had been noted during hip arthroscopy, and paved the way for major changes in arthroscopic hip surgery during the next decade. The ANCHOR group reported the descriptive epidemiology of a cohort of more than 1000 patients with FAI.11
Cam-Type FAI
Cam-type impingement results from femoral-sided deformities. The mechanism was described as inclusion-type impingement in which “jamming of an abnormal femoral head with increasing radius into the acetabulum during forceful motion, especially flexion.”1 This results in outside-in abrasion of the acetabular cartilage of the anterosuperior rim with detachment of the “principally uninvolved labrum”1 and potentially delamination of the adjacent cartilage from the subchondral bone. Ganz and colleagues1 recognized in their initial descriptions of FAI that cam-type FAI could involve decreased femoral version, femoral head–neck junction asphericity, and decreased head–neck offset. The complexity and variability in the topography and geography of the cam morphology have been increasingly recognized. Accurate understanding and characterization of the proximal femoral deformity are important in guiding surgical correction of the cam deformity.
Advances in understanding the prevalence of the cam morphology and the association with OA have been important to our understanding of the pathophysiology of FAI. Several studies12 have established that a cam morphology of the proximal femur (defined by a variety of different metrics) is common among asymptomatic individuals. In light of this fact, a description of the femoral anatomy as a “cam morphology” rather than a cam deformity is now favored. Similarly, FAI is better used to refer to symptomatic individuals and is not equivalent to a cam morphology. The cam morphology seems significantly more common among athletes. Siebenrock and colleagues13 demonstrated the correlation of high-level athletics during late stages of skeletal immaturity and development of a cam morphology. A recent systematic review of 9 studies found that elite male athletes in late skeletal immaturity were 2 to 8 times more likely to develop a cam morphology before skeletal maturity.14Several population-based studies15,16 have quantified the apparent association of the cam morphology with hip OA. However, the studies were limited in their ability to adequately define the presence of cam morphology based on anteroposterior (AP) pelvis radiographs.
In a prospective study, Agricola and colleagues15 found the risk of OA was increased 2.4 times in the setting of moderate cam morphology (α angle, >60°) over a 5-year period. Thomas and colleagues16 found increased risk in a female cohort when the α angle was >65°.
Treatment of cam-type FAI is focused on adequate correction of the abnormal bone morphology. Inadequate or inappropriate bony correction of FAI is a common cause of treatment failure and is more common with arthroscopic techniques.9,10,17 Inadequate bony resection may be the result of surgical inexperience, poor visualization, or lack of understanding of the underlying bony deformity. Modern osteoplasty techniques also focus on gradual bony contour correction that restores the normal concavity–convexity transition of the head–neck junction. Overresection of the cam deformity not only may increase the risk of femoral neck fracture but may result in early disruption of the hip fluid seal from loss of contact between the femoral head and the acetabular labrum earlier in the arc of motion. In addition, high range-of-motion impingement can be seen in various athletic populations (dance, gymnastics, martial arts, hockey goalies), and the regions of impingement tend to be farther away from classically described impingement. Impingement in these situations occurs at the distal femoral neck and subspine regions, adding a level of complexity and unpredictability from a surgical standpoint.
FAI can also occur in the setting of more complex deformities than the typical cam morphology. Complex cases of FAI caused by slipped capital femoral epiphysis (SCFE) and residual Legg-Calvé-Perthes disease are relatively common. Complex deformities may also result in extra-articular impingement of the proximal femur (greater/lesser trochanter, distal femoral neck) on the pelvis (ilium, ischium) in addition to typical FAI. Mild to moderate cases of residual SCFE may be adequately treated with osteoplasty by arthroscopic techniques. In the setting of more severe residual SCFE, presence of underlying femoral retroversion and retrotilt of the femoral epiphysis may prevent adequate deformity correction and motion improvement by arthroscopy. Surgical hip dislocation (with or without relative femoral neck lengthening) and/or proximal femoral flexion derotational osteotomy may be the best means of treatment in these more severe deformities but may be dependent on the chronicity of the deformity and associated compensatory changes occurring on the acetabular side. Similarly, in moderate to severe residual Legg-Calvé-Perthes disease, presence of coxa vara, high greater trochanter, short femoral neck, and ovoid femoral head may be better treated in open techniques to allow comprehensive deformity correction, including correction of acetabular dysplasia in some cases.
Pincer-Type FAI
Pincer-type FAI results from acetabular-sided deformities in which acetabular deformity leads to impaction-type impingement with “linear contact between the acetabular rim and the femoral head–neck junction.”1 Pincer FAI causes primarily labral damage with progressive degeneration and, in some cases, ossification of the acetabular labrum that further worsens the acetabular overcoverage and premature rim impaction. Chondral damage in pincer-type FAI is generally less significant and limited to the peripheral acetabular rim.
Pincer-type FAI may be caused by acetabular retroversion, coxa profunda, or protrusio acetabuli. Our understanding of what defines a pincer morphology has evolved significantly. Through efforts to better define structural features of the acetabular rim that represent abnormalities, we have improved our understanding of how these features may influence OA development. One example of improved understanding involves coxa profunda, classically defined as the medial acetabular fossa touching or projecting medial to the ilioischial line on an AP pelvis radiograph. Several studies have found that this classic definition poorly describes the “overcovered” hip, as it is present in 70% of females and commonly present (41%) in the setting of acetabular dysplasia.18,19 Acetabular retroversion was previously associated with hip OA. Although central acetabular retroversion is relatively uncommon, cranial acetabular retroversion is more common. Presence of a crossover sign on AP pelvis radiographs generally has been viewed as indicative of acetabular retroversion. However, alterations in pelvic tilt on supine or standing AP pelvis radiographs can result in apparent retroversion in the setting of normal acetabular anatomy20 and potentially influence the development of impingement.21 Zaltz and colleagues22 found that abnormal morphology of the anterior inferior iliac spine can also lead to the presence of a crossover sign in an otherwise anteverted acetabulum. Larson and colleagues23 recently found that a crossover sign is present in 11% of asymptomatic hips (19% of males) and may be considered a normal variant. A crossover sign can also be present in the setting of posterior acetabular deficiency with normal anterior acetabular coverage. Ultimately, acetabular retroversion might indicate pincer-type FAI or dysplasia or be a normal variant that does not require treatment. Global acetabular overcoverage, including coxa protrusio, may be associated with OA in population-based studies but is not uniformly demonstrated in all studies.16,24,25 A lateral center edge angle of >40° and a Tönnis angle (acetabular inclination) of <0° are commonly viewed as markers of global overcoverage.
FAI Treatment
Improvements in hip arthroscopy techniques and instrumentation have led to hip arthroscopy becoming the primary surgical technique for the treatment of most cases of FAI. Hip arthroscopy allows for precise visualization and treatment of labral and chondral disease in the central compartment by traction. Larson and colleagues26 reported complication rates for hip arthroscopy in a prospective series of >1600 cases. The overall complication rate was 8.3%, with higher rates noted in female patients and in the setting of traction time longer than 60 minutes. Nonetheless, major complications occurred in 1.1%, with only 0.1% having persistent disability. The most common complications were lateral femoral cutaneous nerve dysesthesias (1.6%), pudendal nerve neuropraxia (1.4%), and iatrogenic labral/chondral damage (2.1%).
The importance of preserving the acetabular labrum is now well accepted from clinical and biomechanical evidence.27-29 As in previous studies in surgical hip dislocation,30 arthroscopic labral repair (vs débridement) results in improved clinical outcomes.31,32 Labral repair techniques currently focus on stable fixation of the labrum while maintaining the normal position of the labrum relative to the femoral head and avoiding labral eversion, which may compromise the hip suction seal. With continued technical advancements and biomechanical support, arthroscopic labral reconstruction is possible in the setting of labral deficiency, often resulting from prior resection. However, the optimal indications, surgical techniques, and long-term outcomes continue to be better defined. Open and arthroscopic techniques have shown similar ability to correct the typical mild to moderate cam morphology in FAI.33 Yet, inadequate femoral bony correction of FAI seems to be the most common cause for revision hip preservation surgery.9,10,17Mild to moderate acetabular rim deformities are commonly treated with hip arthroscopy. As our understanding of pincer-type FAI continues to improve, many surgeons are performing less- aggressive bone resection along the anterior rim. On the other hand, subspinous impingement was recently recognized as a form of extra-articular pincer FAI variant.34 Subspine decompression without true acetabular rim resection has become a more common treatment for pincer lesions and may be a consideration even with restricted range of motion after periacetabular osteotomy. Severe acetabular deformities with global overcoverage or acetabular protrusion are particularly challenging by arthroscopy, even for the most experienced surgeons. Although some improvement in deformity is feasible with arthroscopy, even cases reported in the literature have demonstrated incomplete deformity correction. Open surgical hip dislocation may continue to be the ideal treatment technique for severe pincer impingement.
Cam-type FAI is commonly treated with hip arthroscopy (Figures A-F).
Open surgical techniques will continue to have an important role in the treatment of severe and complex FAI deformities in which arthroscopic techniques do not consistently achieve adequate bony correction (Figures A-F). Surgical hip dislocation remains a powerful surgical technique for deformity correction in FAI. Sink and colleagues37 reported rates of complications after open surgical hip dislocation in the ANCHOR study group. In a cohort of 334 hips (302 patients), trochanteric nonunion occurred in 1.8% of cases, and there were no cases of avascular necrosis. Overall major complications were observed in 4.8% of cases, with 0.3% having chronic disability. Excellent outcomes, including high rates of return to sports, have been reported after surgical hip dislocation for FAI.38 Midterm studies from the early phase of surgical treatment of FAI have helped identify factors that may play a major role in optimizing patient outcomes.
Conclusion
Our understanding and treatment of FAI continue to evolve. Both open and arthroscopic techniques have demonstrated excellent outcomes in the treatment of FAI. Most cases of FAI are now amenable to arthroscopic treatment. Inadequate resection and underlying acetabular dysplasia remain common causes of treatment failure. Open surgical hip dislocation continues to play a role in the treatment of severe deformities that are poorly accessible by arthroscopy—including cam lesions with posterior extension, severe global acetabular overcoverage, or extra-articular impingement. The association of FAI with OA is most apparent for cam-type FAI. Future research will define the optimal treatment strategies and determine if they modify disease progression.
Am J Orthop. 2017;46(1):28-34. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):112-120.
2. Smith-Petersen MN. The classic: treatment of malum coxae senilis, old slipped upper femoral epiphysis, intrapelvic protrusion of the acetabulum, and coxa plana by means of acetabuloplasty. 1936. Clin Orthop Relat Res. 2009;467(3):608-615.
3. Murray RO. The aetiology of primary osteoarthritis of the hip. Br J Radiol. 1965;38(455):810-824.
4. Solomon L. Patterns of osteoarthritis of the hip. J Bone Joint Surg Br. 1976;58(2):176-183.
5. Stulberg SD. Unrecognized childhood hip disease: a major cause of idiopathic osteoarthritis of the hip. In: Cordell LD, Harris WH, Ramsey PL, MacEwen GD, eds. The Hip: Proceedings of the Third Open Scientific Meeting of the Hip Society. St. Louis, MO: Mosby; 1975:212-228.
6. Myers SR, Eijer H, Ganz R. Anterior femoroacetabular impingement after periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):93-99.
7. Ganz R, Gill TJ, Gautier E, Ganz K, Krügel N, Berlemann U. Surgical dislocation of the adult hip: a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. J Bone Joint Surg Br. 2001;83(8):1119-1124.
8. Ross JR, Larson CM, Adeoye O, Kelly BT, Bedi A. Residual deformity is the most common reason for revision hip arthroscopy: a three-dimensional CT study. Clin Orthop Relat Res. 2015;473(4):1388-1395.
9. Clohisy JC, Nepple JJ, Larson CM, Zaltz I, Millis M; Academic Network of Conservation Hip Outcome Research (ANCHOR) Members. Persistent structural disease is the most common cause of repeat hip preservation surgery. Clin Orthop Relat Res. 2013;471(12):3788-3794.
10. Heyworth BE, Shindle MK, Voos JE, Rudzki JR, Kelly BT. Radiologic and intraoperative findings in revision hip arthroscopy. Arthroscopy. 2007;23(12):1295-1302.
11. Clohisy JC, Baca G, Beaulé PE, et al; ANCHOR Study Group. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
12. Frank JM, Harris JD, Erickson BJ, et al. Prevalence of femoroacetabular impingement imaging findings in asymptomatic volunteers: a systematic review. Arthroscopy. 2015;31(6):1199-11204.
13. Siebenrock KA, Ferner F, Noble PC, Santore RF, Werlen S, Mamisch TC. The cam-type deformity of the proximal femur arises in childhood in response to vigorous sporting activity. Clin Orthop Relat Res. 2011;469(11):3229-3240.
14. Nepple JJ, Vigdorchik JM, Clohisy JC. What is the association between sports participation and the development of proximal femoral cam deformity? A systematic review and meta-analysis. Am J Sports Med. 2015;43(11):2833-2840.
15. Agricola R, Waarsing JH, Arden NK, et al. Cam impingement of the hip: a risk factor for hip osteoarthritis. Nat Rev Rheumatol. 2013;9(10):630-634.
16. Thomas GE, Palmer AJ, Batra RN, et al. Subclinical deformities of the hip are significant predictors of radiographic osteoarthritis and joint replacement in women. A 20 year longitudinal cohort study. Osteoarthritis Cartilage. 2014;22(10):1504-1510.
17. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-1921.
18. Nepple JJ, Lehmann CL, Ross JR, Schoenecker PL, Clohisy JC. Coxa profunda is not a useful radiographic parameter for diagnosing pincer-type femoroacetabular impingement. J Bone Joint Surg Am. 2013;95(5):417-423.
19. Anderson LA, Kapron AL, Aoki SK, Peters CL. Coxa profunda: is the deep acetabulum overcovered? Clin Orthop Relat Res. 2012;470(12):3375-3382.
20. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop Relat Res. 2003;(407):241-248.
21. Ross JR, Nepple JJ, Philippon MJ, Kelly BT, Larson CM, Bedi A. Effect of changes in pelvic tilt on range of motion to impingement and radiographic parameters of acetabular morphologic characteristics. Am J Sports Med. 2014;42(10):2402-2409.
22. Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471(8):2463-2470.
23. Larson CM, Moreau-Gaudry A, Kelly BT, et al. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473(4):1247-1254.
24. Gosvig KK, Jacobsen S, Sonne-Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am. 2010;92(5):1162-1169.
25. Agricola R, Heijboer MP, Roze RH, et al. Pincer deformity does not lead to osteoarthritis of the hip whereas acetabular dysplasia does: acetabular coverage and development of osteoarthritis in a nationwide prospective cohort study (CHECK). Osteoarthritis Cartilage. 2013;21(10):1514-1521.
26. Larson CM, Clohisy JC, Beaulé PE, et al; ANCHOR Study Group. Intraoperative and early postoperative complications after hip arthroscopic surgery: a prospective multicenter trial utilizing a validated grading scheme. Am J Sports Med. 2016;44(9):2292-2298.
27. Ferguson SJ, Bryant JT, Ganz R, Ito K. An in vitro investigation of the acetabular labral seal in hip joint mechanics. J Biomech. 2003;36(2):171-178.
28. Nepple JJ, Philippon MJ, Campbell KJ, et al. The hip fluid seal—part II: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip stability to distraction. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):730-736.
29. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
30. Espinosa N, Rothenfluh DA, Beck M, Ganz R, Leunig M. Treatment of femoro-acetabular impingement: preliminary results of labral refixation. J Bone Joint Surg Am. 2006;88(5):925-935.
31. Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46-53.
32. Larson CM, Giveans MR. Arthroscopic debridement versus refixation of the acetabular labrum associated with femoroacetabular impingement. Arthroscopy. 2009;25(4):369-376.
33. Bedi A, Zaltz I, De La Torre K, Kelly BT. Radiographic comparison of surgical hip dislocation and hip arthroscopy for treatment of cam deformity in femoroacetabular impingement. Am J Sports Med. 2011;39(suppl):20S–28S.
34. Larson CM, Kelly BT, Stone RM. Making a case for anterior inferior iliac spine/subspine hip impingement: three representative case reports and proposed concept. Arthroscopy. 2011;27(12):1732-1737.
35. Ross JR, Bedi A, Stone RM, et al. Intraoperative fluoroscopic imaging to treat cam deformities: correlation with 3-dimensional computed tomography [published correction appears in Am J Sports Med. 2015;43(8):NP27]. Am J Sports Med. 2014;42(6):1370-1376.
36. Fabricant PD, Fields KG, Taylor SA, Magennis E, Bedi A, Kelly BT. The effect of femoral and acetabular version on clinical outcomes after arthroscopic femoroacetabular impingement surgery. J Bone Joint Surg Am. 2015;97(7):537-543.
37. Sink EL, Beaulé PE, Sucato D, et al. Multicenter study of complications following surgical dislocation of the hip. J Bone Joint Surg Am. 2011;93(12):1132-1136.
38. Naal FD, Miozzari HH, Wyss TF, Nötzli HP. Surgical hip dislocation for the treatment of femoroacetabular impingement in high-level athletes. Am J Sports Med. 2011;39(3):544-550.
1. Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):112-120.
2. Smith-Petersen MN. The classic: treatment of malum coxae senilis, old slipped upper femoral epiphysis, intrapelvic protrusion of the acetabulum, and coxa plana by means of acetabuloplasty. 1936. Clin Orthop Relat Res. 2009;467(3):608-615.
3. Murray RO. The aetiology of primary osteoarthritis of the hip. Br J Radiol. 1965;38(455):810-824.
4. Solomon L. Patterns of osteoarthritis of the hip. J Bone Joint Surg Br. 1976;58(2):176-183.
5. Stulberg SD. Unrecognized childhood hip disease: a major cause of idiopathic osteoarthritis of the hip. In: Cordell LD, Harris WH, Ramsey PL, MacEwen GD, eds. The Hip: Proceedings of the Third Open Scientific Meeting of the Hip Society. St. Louis, MO: Mosby; 1975:212-228.
6. Myers SR, Eijer H, Ganz R. Anterior femoroacetabular impingement after periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):93-99.
7. Ganz R, Gill TJ, Gautier E, Ganz K, Krügel N, Berlemann U. Surgical dislocation of the adult hip: a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. J Bone Joint Surg Br. 2001;83(8):1119-1124.
8. Ross JR, Larson CM, Adeoye O, Kelly BT, Bedi A. Residual deformity is the most common reason for revision hip arthroscopy: a three-dimensional CT study. Clin Orthop Relat Res. 2015;473(4):1388-1395.
9. Clohisy JC, Nepple JJ, Larson CM, Zaltz I, Millis M; Academic Network of Conservation Hip Outcome Research (ANCHOR) Members. Persistent structural disease is the most common cause of repeat hip preservation surgery. Clin Orthop Relat Res. 2013;471(12):3788-3794.
10. Heyworth BE, Shindle MK, Voos JE, Rudzki JR, Kelly BT. Radiologic and intraoperative findings in revision hip arthroscopy. Arthroscopy. 2007;23(12):1295-1302.
11. Clohisy JC, Baca G, Beaulé PE, et al; ANCHOR Study Group. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
12. Frank JM, Harris JD, Erickson BJ, et al. Prevalence of femoroacetabular impingement imaging findings in asymptomatic volunteers: a systematic review. Arthroscopy. 2015;31(6):1199-11204.
13. Siebenrock KA, Ferner F, Noble PC, Santore RF, Werlen S, Mamisch TC. The cam-type deformity of the proximal femur arises in childhood in response to vigorous sporting activity. Clin Orthop Relat Res. 2011;469(11):3229-3240.
14. Nepple JJ, Vigdorchik JM, Clohisy JC. What is the association between sports participation and the development of proximal femoral cam deformity? A systematic review and meta-analysis. Am J Sports Med. 2015;43(11):2833-2840.
15. Agricola R, Waarsing JH, Arden NK, et al. Cam impingement of the hip: a risk factor for hip osteoarthritis. Nat Rev Rheumatol. 2013;9(10):630-634.
16. Thomas GE, Palmer AJ, Batra RN, et al. Subclinical deformities of the hip are significant predictors of radiographic osteoarthritis and joint replacement in women. A 20 year longitudinal cohort study. Osteoarthritis Cartilage. 2014;22(10):1504-1510.
17. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-1921.
18. Nepple JJ, Lehmann CL, Ross JR, Schoenecker PL, Clohisy JC. Coxa profunda is not a useful radiographic parameter for diagnosing pincer-type femoroacetabular impingement. J Bone Joint Surg Am. 2013;95(5):417-423.
19. Anderson LA, Kapron AL, Aoki SK, Peters CL. Coxa profunda: is the deep acetabulum overcovered? Clin Orthop Relat Res. 2012;470(12):3375-3382.
20. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop Relat Res. 2003;(407):241-248.
21. Ross JR, Nepple JJ, Philippon MJ, Kelly BT, Larson CM, Bedi A. Effect of changes in pelvic tilt on range of motion to impingement and radiographic parameters of acetabular morphologic characteristics. Am J Sports Med. 2014;42(10):2402-2409.
22. Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471(8):2463-2470.
23. Larson CM, Moreau-Gaudry A, Kelly BT, et al. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473(4):1247-1254.
24. Gosvig KK, Jacobsen S, Sonne-Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am. 2010;92(5):1162-1169.
25. Agricola R, Heijboer MP, Roze RH, et al. Pincer deformity does not lead to osteoarthritis of the hip whereas acetabular dysplasia does: acetabular coverage and development of osteoarthritis in a nationwide prospective cohort study (CHECK). Osteoarthritis Cartilage. 2013;21(10):1514-1521.
26. Larson CM, Clohisy JC, Beaulé PE, et al; ANCHOR Study Group. Intraoperative and early postoperative complications after hip arthroscopic surgery: a prospective multicenter trial utilizing a validated grading scheme. Am J Sports Med. 2016;44(9):2292-2298.
27. Ferguson SJ, Bryant JT, Ganz R, Ito K. An in vitro investigation of the acetabular labral seal in hip joint mechanics. J Biomech. 2003;36(2):171-178.
28. Nepple JJ, Philippon MJ, Campbell KJ, et al. The hip fluid seal—part II: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip stability to distraction. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):730-736.
29. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
30. Espinosa N, Rothenfluh DA, Beck M, Ganz R, Leunig M. Treatment of femoro-acetabular impingement: preliminary results of labral refixation. J Bone Joint Surg Am. 2006;88(5):925-935.
31. Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46-53.
32. Larson CM, Giveans MR. Arthroscopic debridement versus refixation of the acetabular labrum associated with femoroacetabular impingement. Arthroscopy. 2009;25(4):369-376.
33. Bedi A, Zaltz I, De La Torre K, Kelly BT. Radiographic comparison of surgical hip dislocation and hip arthroscopy for treatment of cam deformity in femoroacetabular impingement. Am J Sports Med. 2011;39(suppl):20S–28S.
34. Larson CM, Kelly BT, Stone RM. Making a case for anterior inferior iliac spine/subspine hip impingement: three representative case reports and proposed concept. Arthroscopy. 2011;27(12):1732-1737.
35. Ross JR, Bedi A, Stone RM, et al. Intraoperative fluoroscopic imaging to treat cam deformities: correlation with 3-dimensional computed tomography [published correction appears in Am J Sports Med. 2015;43(8):NP27]. Am J Sports Med. 2014;42(6):1370-1376.
36. Fabricant PD, Fields KG, Taylor SA, Magennis E, Bedi A, Kelly BT. The effect of femoral and acetabular version on clinical outcomes after arthroscopic femoroacetabular impingement surgery. J Bone Joint Surg Am. 2015;97(7):537-543.
37. Sink EL, Beaulé PE, Sucato D, et al. Multicenter study of complications following surgical dislocation of the hip. J Bone Joint Surg Am. 2011;93(12):1132-1136.
38. Naal FD, Miozzari HH, Wyss TF, Nötzli HP. Surgical hip dislocation for the treatment of femoroacetabular impingement in high-level athletes. Am J Sports Med. 2011;39(3):544-550.
Multicenter Outcomes After Hip Arthroscopy: Epidemiology (MASH Study Group). What Are We Seeing in the Office, and Who Are We Choosing to Treat?
Take-Home Points
- MASH is a multicenter arthroscopic study of the hip that features a large prospective database of 10 separate institutions in the United States.
- The mean patient demographic was age 34.6 years, BMI 25.9 kg/m2, 62.8% females, and 97% white.
- Most patients had anterior or groin pain, but 17.6% had lateral hip pain, 13.8% had posterior hip pain, and 2.9% had low back or sacral pain.
- Patients typically had pain for about 1 year that was worsened with athletic activity as well as sitting.
- The most common surgical procedures that were performed included labral surgery in 64.7%, femoroplasty in 49.9%, acetabuloplasty in 33.3%, and chondroplasty in 31.1%
Arthroscopic surgery of the hip has been growing over the past decade, with drastically increasing rates of arthroscopic hip procedures and increased education and interest in orthopedic trainees.1-3 The rise of this minimally invasive surgical technique may be attributed to expanding knowledge of surgical management of morphologic hip disorders as a means of hip preservation. Many arthroscopic techniques have been developed to treat intra-articular hip joint pathologies, including femoroacetabular impingement (FAI), labral tears, and cartilage damage.4-11 These hip pathologies are widely recognized as painful limitations to activities of daily living and sports as well as early indicators of hip osteoarthritis.12,13 Limited evidence suggests that arthroscopic treatment of these intra-articular hip joint pathologies preserves the hip from osteoarthritis and progression to total hip arthroplasty.13-15
FAI is the most common etiology of pathologies related to arthroscopic surgery of the hip, including both labral tears and cartilage damage.4,7,14 FAI is a morphologic bone disorder characterized by impingement of the femur and the acetabulum on flexion or rotation. The etiology of FAI is not completely understood, but evidence suggests that stress to the proximal femoral physis during skeletal growth increases the risk of developing femoral head and neck deformations leading to cam-type FAI.15-17 Understanding the characteristics of the patient population in which FAI occurs may shed light on the processes of intra-articular damage, such as labral tears and cartilage damage.
In the present study, we collected epidemiologic data, including demographics, pathologic entities treated, patient-reported measures of disease, and surgical treatment preferences, on a hip pathology population that elected to undergo arthroscopic surgery. These data are important in gaining a better understanding of the population and environment in which hip arthroscopy is performed across multiple centers throughout the United States and may help guide clinical practice and research to advance hip arthroscopy.
Methods
The Multicenter Arthroscopic Study of the Hip (MASH) Study Group conducts multicenter clinical studies in arthroscopic hip preservation surgery. Patients are enrolled in this large prospective longitudinal study at 10 sites nationwide by 10 fellowship-trained hip arthroscopists. Institutional Review Board approval was obtained from all institutions before patient enrollment. After enrollment, we collected comprehensive patient data, including demographics, common symptoms and their duration, provocative activities, patient-reported outcome measures (modified Harris Hip Score, International Hip Outcome Tool, 12-item Short Form Health Survey, visual analog scale pain rating, Hip Outcome Score), physical examination findings, imaging findings, diagnoses, surgical findings, and surgical procedures.
All study participants were patients undergoing arthroscopic hip surgery by one of the members of the MASH Study Group. Patients with incomplete preoperative information (needed for data analysis) were excluded. Data analysis was performed with SPSS Statistics Version 21.0 (SPSS Inc.) to obtain descriptive statistics of the quantitative data and frequencies of the nominal data.
Results
Between January 2014 and November 2016, we enrolled 1738 patients (647 male, 1091 female) in the study. Table 1 lists the demographics of the population.
Regarding symptom location, 40.9% of patients described pain in the groin region, 24.2% in the anterior hip region, and 11.3% in a C-sign distribution (Table 2).
Table 3 lists the results of the patient-reported outcome measures.
Of the 1738 patients enrolled, 424 (24.4%) had prior surgery related to current symptoms, 252 (14.5%) had 1 previous surgery, 120 (6.9%) had 2 previous surgeries, and 52 (3%) had 3 previous surgeries. Twenty-six patients (1.5%) had a previous revision hip arthroscopy on the ipsilateral side, and 14 (0.8%) had a previous hip arthroscopy on the contralateral side. Before surgery, 80% of patients received an intra-articular injection of corticosteroid and lidocaine. The peritrochanteric region was injected in 11.5% of patients and the psoas bursa in 2.2% (Table 4).
Of the 1011 patients who had magnetic resonance imaging (MRI) performed, 943 (93.3%) had abnormal acetabular labrum findings, and 163 (17.1%) had acetabular articular damage. According to radiographic evaluation, 953 patients had abnormal hip joint morphology consistent with FAI. Figure 3 shows the FAI classification percentages.
On clinical examination, 1079 patients (62.1%) had a positive anterior impingement sign. The subspine impingement sign was positive in 447 patients (25.7%), and the trochanteric pain sign was positive in 400 (23%). Table 5 lists range-of-motion values for flexion and hip rotation from 90° of flexion.
As seen in Table 6, labral pathology was the most common diagnosis (1426/1738 patients, 82%).
As seen in Table 7, the most common procedure was femoroplasty (867/1738, 49.9%).
Discussion
In this study, we collected epidemiologic data (demographics, pathologic entities treated, patient-reported measures of disease, surgical treatment preferences) from a large multicenter population of hip pathology patients who elected to undergo arthroscopic surgery. Our results showed these patients were most commonly younger to middle-aged white females with pain primarily in the groin region. Most had pain for at least 1 year, and it was commonly exacerbated by sitting and athletics. Patients reported clinically significant pain and functional limitation, which showed evidence of affecting general physical and mental health. It was not uncommon for patients to have undergone another, related surgery and nonoperative treatments, including intra-articular injection and/or physical therapy, before surgery. There was a high incidence of abnormal hip morphology suggestive of a cam lesion, but the incidence of arthritic changes on radiographs was relatively low. Labral tear was the most common diagnosis, and most often it was addressed with repair. Many patients underwent femoroplasty, acetabuloplasty, and chondroplasty in addition to labral repair.
According to patient-reported outcome measures administered before surgery, 40% to 65% of patients seeking hip preservation surgery reported functional deficits and pain—which falls within the range of results from other multicenter studies on the epidemiology of FAI.18,19 There was, however, a high amount of variability in individual scores on the functional and pain measures; some patients rated their functional ability very high. These findings were supported by the general health forms measuring global physical and mental health. Mean Physical Health and Mental Health scores on the 12-item Short Form Health Survey indicated that patients seeking hip preservation surgery thought their hip condition affected their general well-being. This finding is consistent with research on FAI,18 hip arthritis,20 and total hip arthroplasty.19Our results further showed that hip arthroscopists commonly prescribed alternative treatment measures ahead of surgery. Before elective surgery, 80% of patients received an intra-articular injection, underwent physical therapy, or both. This could suggest a high failure rate for patients who chose conservative treatment approaches for hip-related pathology. However, our study was limited in that it may have included patients who had improved significantly with conservative measures and decided to forgo arthroscopic hip surgery. Although conservative treatment often is recommended in an effort to potentially avoid surgery, there is a lack of research evaluating the efficacy of nonoperative care.21,22Analysis of diagnostic imaging and clinical examination findings revealed some unique characteristics of patients undergoing elective hip preservation surgery. MRI showed labral pathology in an overwhelming majority of these patients, but few had evidence of articular damage. Previous research has found a 67% rate of arthritic changes on diagnostic imaging, but our rate was much lower (17%).23 Radiograph evaluation confirmed the pattern: More than 90% of our patients had Tönnis grade 0 osteoarthritis. Tönnis grade 1 or 2 osteoarthritis is a predictor of acetabular cartilage degeneration,23 and long-term studies have related these osteoarthritic changes to poorer hip arthroscopy outcomes.24 Thus, the lower incidence of osteoarthritis in our study population may reflect current evidence-based practice and a contemporary approach to patient selection.
Most of our patients had isolated cam-type FAI as opposed to pincer-type FAI or a combination of cam and pincer—contrary to research findings that combination cam–pincer FAI is most prevalent.25,26 Our results are more consistent with more recent research findings of a higher incidence of isolated cam lesion, particularly in female patients, and combination cam–pincer in male patients.18,27,28 Similar distributions of surgical procedures and diagnoses exist between the present study and other multicenter evaluations of the epidemiologic characteristics of patients with hip pathology.18Our study had several limitations. First, the population consisted entirely of patients who sought evaluation by a hip arthroscopy specialist and underwent elective surgery. Therefore, the data cannot be applied to a more general orthopedic population or to patients who consult other medical specialists. Second, the population, which was 97% white and had small percentages of African-American, Latino, and Asian patients, lacked ethnic diversity. This finding is consistent with recent epidemiologic research in which ethnicity was identified as a factor in patterns of hip disease.13,29,30 Access to specialists, however, was likely affected by multiple other factors. Fourth, the validity and the reliability of the imaging modalities used have been questioned.31-33 There is controversy regarding ideal imaging modalities for assessment of articular cartilage damage31,32 and FAI. However, the modalities that we used to determine diagnoses in this study are well supported26 and represent common practice patterns.
Am J Orthop. 2017;46(1):35-41. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016;32(7):1286-1292.
2. Peters CL, Aoki SK, Erickson JA, Anderson LA, Anderson AE. Early experience with a comprehensive hip preservation service intended to improve clinical care, education, and academic productivity. Clin Orthop Relat Res. 2012;470(12):3446-3452.
3. Siebenrock KA, Peters CL. ABJS Carl T. Brighton workshop on hip preservation surgery: editorial comment. Clin Orthop Relat Res. 2012;470(12):3281-3283.
4. Parvizi J, Leunig M, Ganz R. Femoroacetabular impingement. J Am Acad Orthop Surg. 2007;15(9):561-570.
5. Poh SY, Hube R, Dienst M. Arthroscopic treatment of femoroacetabular pincer impingement. Oper Orthop Traumatol. 2015;27(6):536-552.
6. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
7. Groh MM, Herrera J. A comprehensive review of hip labral tears. Curr Rev Musculoskelet Med. 2009;2(2):105-117.
8. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
9. White BJ, Herzog MM. Labral reconstruction: when to perform and how. Front Surg. 2015;2:27.
10. Yen YM, Kocher MS. Chondral lesions of the hip: microfracture and chondroplasty. Sports Med Arthrosc. 2010;18(2):83-89.
11. Jordan MA, Van Thiel GS, Chahal J, Nho SJ. Operative treatment of chondral defects in the hip joint: a systematic review. Curr Rev Musculoskelet Med. 2012;5(3):244-253.
12. Griffin DW, Kinnard MJ, Formby PM, McCabe MP, Anderson TD. Outcomes of hip arthroscopy in the older adult: a systematic review of the literature [published online October 18, 2016]. Am J Sports Med. doi:10.1177/0363546516667915.
13. Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 2008;466(2):264-272.
14. Kaya M, Suzuki T, Emori M, Yamashita T. Hip morphology influences the pattern of articular cartilage damage. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):2016-2023.
15. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
16. Byrd JT. Hip arthroscopy in athletes. Oper Tech Sports Med. 2005;13(1):24-36.
17. Werner BC, Gaudiani MA, Ranawat AS. The etiology and arthroscopic surgical management of cam lesions. Clin Sports Med. 2016;35(3):391-404.
18. Clohisy JC, Baca G, Beaulé PE, et al; ANCHOR Study Group. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
19. Shia DS, Clohisy JC, Schinsky MF, Martell JM, Maloney WJ. THA with highly cross-linked polyethylene in patients 50 years or younger. Clin Orthop Relat Res. 2009;467(8):2059-2065.
20. Gandhi SK, Salmon JW, Zhao SZ, Lambert BL, Gore PR, Conrad K. Psychometric evaluation of the 12-item Short-Form Health Survey (SF-12) in osteoarthritis and rheumatoid arthritis clinical trials. Clin Ther. 2001;23(7):1080-1098.
21. Loudon JK, Reiman MP. Conservative management of femoroacetabular impingement (FAI) in the long distance runner. Phys Ther Sport. 2014;15(2):82-90.
22. Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418-426.
23. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med. 2011;39(2):296-303.
24. McCormick F, Nwachukwu BU, Alpaugh K, Martin SD. Predictors of hip arthroscopy outcomes for labral tears at minimum 2-year follow-up: the influence of age and arthritis. Arthroscopy. 2012;28(10):1359-1364.
25. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
26. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis—what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552.
27. Kapron AL, Peters CL, Aoki SK, et al. The prevalence of radiographic findings of structural hip deformities in female collegiate athletes. Am J Sports Med. 2015;43(6):1324-1330.
28. Lee WY, Kang C, Hwang DS, Jeon JH, Zheng L. Descriptive epidemiology of symptomatic femoroacetabular impingement in young athlete: single center study. Hip Pelvis. 2016;28(1):29-34.
29. Dudda M, Kim YJ, Zhang Y, et al. Morphologic differences between the hips of Chinese women and white women: could they account for the ethnic difference in the prevalence of hip osteoarthritis? Arthritis Rheum. 2011;63(10):2992-2999.
30. Solomon L, Beighton P. Osteoarthrosis of the hip and its relationship to pre-existing in an African population. J Bone Joint Surg Br. 1973;55(1):216-217.
31. Keeney JA, Peelle MW, Jackson J, Rubin D, Maloney WJ, Clohisy JC. Magnetic resonance arthrography versus arthroscopy in the evaluation of articular hip pathology. Clin Orthop Relat Res. 2004;(429):163-169.
32. Schmid MR, Nötzli HP, Zanetti M, Wyss TF, Hodler J. Cartilage lesions in the hip: diagnostic effectiveness of MR arthrography. Radiology. 2003;226(2):382-386.
33. Chevillotte CJ, Ali MH, Trousdale RT, Pagnano MW. Variability in hip range of motion on clinical examination. J Arthroplasty. 2009;24(5):693-697.
Take-Home Points
- MASH is a multicenter arthroscopic study of the hip that features a large prospective database of 10 separate institutions in the United States.
- The mean patient demographic was age 34.6 years, BMI 25.9 kg/m2, 62.8% females, and 97% white.
- Most patients had anterior or groin pain, but 17.6% had lateral hip pain, 13.8% had posterior hip pain, and 2.9% had low back or sacral pain.
- Patients typically had pain for about 1 year that was worsened with athletic activity as well as sitting.
- The most common surgical procedures that were performed included labral surgery in 64.7%, femoroplasty in 49.9%, acetabuloplasty in 33.3%, and chondroplasty in 31.1%
Arthroscopic surgery of the hip has been growing over the past decade, with drastically increasing rates of arthroscopic hip procedures and increased education and interest in orthopedic trainees.1-3 The rise of this minimally invasive surgical technique may be attributed to expanding knowledge of surgical management of morphologic hip disorders as a means of hip preservation. Many arthroscopic techniques have been developed to treat intra-articular hip joint pathologies, including femoroacetabular impingement (FAI), labral tears, and cartilage damage.4-11 These hip pathologies are widely recognized as painful limitations to activities of daily living and sports as well as early indicators of hip osteoarthritis.12,13 Limited evidence suggests that arthroscopic treatment of these intra-articular hip joint pathologies preserves the hip from osteoarthritis and progression to total hip arthroplasty.13-15
FAI is the most common etiology of pathologies related to arthroscopic surgery of the hip, including both labral tears and cartilage damage.4,7,14 FAI is a morphologic bone disorder characterized by impingement of the femur and the acetabulum on flexion or rotation. The etiology of FAI is not completely understood, but evidence suggests that stress to the proximal femoral physis during skeletal growth increases the risk of developing femoral head and neck deformations leading to cam-type FAI.15-17 Understanding the characteristics of the patient population in which FAI occurs may shed light on the processes of intra-articular damage, such as labral tears and cartilage damage.
In the present study, we collected epidemiologic data, including demographics, pathologic entities treated, patient-reported measures of disease, and surgical treatment preferences, on a hip pathology population that elected to undergo arthroscopic surgery. These data are important in gaining a better understanding of the population and environment in which hip arthroscopy is performed across multiple centers throughout the United States and may help guide clinical practice and research to advance hip arthroscopy.
Methods
The Multicenter Arthroscopic Study of the Hip (MASH) Study Group conducts multicenter clinical studies in arthroscopic hip preservation surgery. Patients are enrolled in this large prospective longitudinal study at 10 sites nationwide by 10 fellowship-trained hip arthroscopists. Institutional Review Board approval was obtained from all institutions before patient enrollment. After enrollment, we collected comprehensive patient data, including demographics, common symptoms and their duration, provocative activities, patient-reported outcome measures (modified Harris Hip Score, International Hip Outcome Tool, 12-item Short Form Health Survey, visual analog scale pain rating, Hip Outcome Score), physical examination findings, imaging findings, diagnoses, surgical findings, and surgical procedures.
All study participants were patients undergoing arthroscopic hip surgery by one of the members of the MASH Study Group. Patients with incomplete preoperative information (needed for data analysis) were excluded. Data analysis was performed with SPSS Statistics Version 21.0 (SPSS Inc.) to obtain descriptive statistics of the quantitative data and frequencies of the nominal data.
Results
Between January 2014 and November 2016, we enrolled 1738 patients (647 male, 1091 female) in the study. Table 1 lists the demographics of the population.
Regarding symptom location, 40.9% of patients described pain in the groin region, 24.2% in the anterior hip region, and 11.3% in a C-sign distribution (Table 2).
Table 3 lists the results of the patient-reported outcome measures.
Of the 1738 patients enrolled, 424 (24.4%) had prior surgery related to current symptoms, 252 (14.5%) had 1 previous surgery, 120 (6.9%) had 2 previous surgeries, and 52 (3%) had 3 previous surgeries. Twenty-six patients (1.5%) had a previous revision hip arthroscopy on the ipsilateral side, and 14 (0.8%) had a previous hip arthroscopy on the contralateral side. Before surgery, 80% of patients received an intra-articular injection of corticosteroid and lidocaine. The peritrochanteric region was injected in 11.5% of patients and the psoas bursa in 2.2% (Table 4).
Of the 1011 patients who had magnetic resonance imaging (MRI) performed, 943 (93.3%) had abnormal acetabular labrum findings, and 163 (17.1%) had acetabular articular damage. According to radiographic evaluation, 953 patients had abnormal hip joint morphology consistent with FAI. Figure 3 shows the FAI classification percentages.
On clinical examination, 1079 patients (62.1%) had a positive anterior impingement sign. The subspine impingement sign was positive in 447 patients (25.7%), and the trochanteric pain sign was positive in 400 (23%). Table 5 lists range-of-motion values for flexion and hip rotation from 90° of flexion.
As seen in Table 6, labral pathology was the most common diagnosis (1426/1738 patients, 82%).
As seen in Table 7, the most common procedure was femoroplasty (867/1738, 49.9%).
Discussion
In this study, we collected epidemiologic data (demographics, pathologic entities treated, patient-reported measures of disease, surgical treatment preferences) from a large multicenter population of hip pathology patients who elected to undergo arthroscopic surgery. Our results showed these patients were most commonly younger to middle-aged white females with pain primarily in the groin region. Most had pain for at least 1 year, and it was commonly exacerbated by sitting and athletics. Patients reported clinically significant pain and functional limitation, which showed evidence of affecting general physical and mental health. It was not uncommon for patients to have undergone another, related surgery and nonoperative treatments, including intra-articular injection and/or physical therapy, before surgery. There was a high incidence of abnormal hip morphology suggestive of a cam lesion, but the incidence of arthritic changes on radiographs was relatively low. Labral tear was the most common diagnosis, and most often it was addressed with repair. Many patients underwent femoroplasty, acetabuloplasty, and chondroplasty in addition to labral repair.
According to patient-reported outcome measures administered before surgery, 40% to 65% of patients seeking hip preservation surgery reported functional deficits and pain—which falls within the range of results from other multicenter studies on the epidemiology of FAI.18,19 There was, however, a high amount of variability in individual scores on the functional and pain measures; some patients rated their functional ability very high. These findings were supported by the general health forms measuring global physical and mental health. Mean Physical Health and Mental Health scores on the 12-item Short Form Health Survey indicated that patients seeking hip preservation surgery thought their hip condition affected their general well-being. This finding is consistent with research on FAI,18 hip arthritis,20 and total hip arthroplasty.19Our results further showed that hip arthroscopists commonly prescribed alternative treatment measures ahead of surgery. Before elective surgery, 80% of patients received an intra-articular injection, underwent physical therapy, or both. This could suggest a high failure rate for patients who chose conservative treatment approaches for hip-related pathology. However, our study was limited in that it may have included patients who had improved significantly with conservative measures and decided to forgo arthroscopic hip surgery. Although conservative treatment often is recommended in an effort to potentially avoid surgery, there is a lack of research evaluating the efficacy of nonoperative care.21,22Analysis of diagnostic imaging and clinical examination findings revealed some unique characteristics of patients undergoing elective hip preservation surgery. MRI showed labral pathology in an overwhelming majority of these patients, but few had evidence of articular damage. Previous research has found a 67% rate of arthritic changes on diagnostic imaging, but our rate was much lower (17%).23 Radiograph evaluation confirmed the pattern: More than 90% of our patients had Tönnis grade 0 osteoarthritis. Tönnis grade 1 or 2 osteoarthritis is a predictor of acetabular cartilage degeneration,23 and long-term studies have related these osteoarthritic changes to poorer hip arthroscopy outcomes.24 Thus, the lower incidence of osteoarthritis in our study population may reflect current evidence-based practice and a contemporary approach to patient selection.
Most of our patients had isolated cam-type FAI as opposed to pincer-type FAI or a combination of cam and pincer—contrary to research findings that combination cam–pincer FAI is most prevalent.25,26 Our results are more consistent with more recent research findings of a higher incidence of isolated cam lesion, particularly in female patients, and combination cam–pincer in male patients.18,27,28 Similar distributions of surgical procedures and diagnoses exist between the present study and other multicenter evaluations of the epidemiologic characteristics of patients with hip pathology.18Our study had several limitations. First, the population consisted entirely of patients who sought evaluation by a hip arthroscopy specialist and underwent elective surgery. Therefore, the data cannot be applied to a more general orthopedic population or to patients who consult other medical specialists. Second, the population, which was 97% white and had small percentages of African-American, Latino, and Asian patients, lacked ethnic diversity. This finding is consistent with recent epidemiologic research in which ethnicity was identified as a factor in patterns of hip disease.13,29,30 Access to specialists, however, was likely affected by multiple other factors. Fourth, the validity and the reliability of the imaging modalities used have been questioned.31-33 There is controversy regarding ideal imaging modalities for assessment of articular cartilage damage31,32 and FAI. However, the modalities that we used to determine diagnoses in this study are well supported26 and represent common practice patterns.
Am J Orthop. 2017;46(1):35-41. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- MASH is a multicenter arthroscopic study of the hip that features a large prospective database of 10 separate institutions in the United States.
- The mean patient demographic was age 34.6 years, BMI 25.9 kg/m2, 62.8% females, and 97% white.
- Most patients had anterior or groin pain, but 17.6% had lateral hip pain, 13.8% had posterior hip pain, and 2.9% had low back or sacral pain.
- Patients typically had pain for about 1 year that was worsened with athletic activity as well as sitting.
- The most common surgical procedures that were performed included labral surgery in 64.7%, femoroplasty in 49.9%, acetabuloplasty in 33.3%, and chondroplasty in 31.1%
Arthroscopic surgery of the hip has been growing over the past decade, with drastically increasing rates of arthroscopic hip procedures and increased education and interest in orthopedic trainees.1-3 The rise of this minimally invasive surgical technique may be attributed to expanding knowledge of surgical management of morphologic hip disorders as a means of hip preservation. Many arthroscopic techniques have been developed to treat intra-articular hip joint pathologies, including femoroacetabular impingement (FAI), labral tears, and cartilage damage.4-11 These hip pathologies are widely recognized as painful limitations to activities of daily living and sports as well as early indicators of hip osteoarthritis.12,13 Limited evidence suggests that arthroscopic treatment of these intra-articular hip joint pathologies preserves the hip from osteoarthritis and progression to total hip arthroplasty.13-15
FAI is the most common etiology of pathologies related to arthroscopic surgery of the hip, including both labral tears and cartilage damage.4,7,14 FAI is a morphologic bone disorder characterized by impingement of the femur and the acetabulum on flexion or rotation. The etiology of FAI is not completely understood, but evidence suggests that stress to the proximal femoral physis during skeletal growth increases the risk of developing femoral head and neck deformations leading to cam-type FAI.15-17 Understanding the characteristics of the patient population in which FAI occurs may shed light on the processes of intra-articular damage, such as labral tears and cartilage damage.
In the present study, we collected epidemiologic data, including demographics, pathologic entities treated, patient-reported measures of disease, and surgical treatment preferences, on a hip pathology population that elected to undergo arthroscopic surgery. These data are important in gaining a better understanding of the population and environment in which hip arthroscopy is performed across multiple centers throughout the United States and may help guide clinical practice and research to advance hip arthroscopy.
Methods
The Multicenter Arthroscopic Study of the Hip (MASH) Study Group conducts multicenter clinical studies in arthroscopic hip preservation surgery. Patients are enrolled in this large prospective longitudinal study at 10 sites nationwide by 10 fellowship-trained hip arthroscopists. Institutional Review Board approval was obtained from all institutions before patient enrollment. After enrollment, we collected comprehensive patient data, including demographics, common symptoms and their duration, provocative activities, patient-reported outcome measures (modified Harris Hip Score, International Hip Outcome Tool, 12-item Short Form Health Survey, visual analog scale pain rating, Hip Outcome Score), physical examination findings, imaging findings, diagnoses, surgical findings, and surgical procedures.
All study participants were patients undergoing arthroscopic hip surgery by one of the members of the MASH Study Group. Patients with incomplete preoperative information (needed for data analysis) were excluded. Data analysis was performed with SPSS Statistics Version 21.0 (SPSS Inc.) to obtain descriptive statistics of the quantitative data and frequencies of the nominal data.
Results
Between January 2014 and November 2016, we enrolled 1738 patients (647 male, 1091 female) in the study. Table 1 lists the demographics of the population.
Regarding symptom location, 40.9% of patients described pain in the groin region, 24.2% in the anterior hip region, and 11.3% in a C-sign distribution (Table 2).
Table 3 lists the results of the patient-reported outcome measures.
Of the 1738 patients enrolled, 424 (24.4%) had prior surgery related to current symptoms, 252 (14.5%) had 1 previous surgery, 120 (6.9%) had 2 previous surgeries, and 52 (3%) had 3 previous surgeries. Twenty-six patients (1.5%) had a previous revision hip arthroscopy on the ipsilateral side, and 14 (0.8%) had a previous hip arthroscopy on the contralateral side. Before surgery, 80% of patients received an intra-articular injection of corticosteroid and lidocaine. The peritrochanteric region was injected in 11.5% of patients and the psoas bursa in 2.2% (Table 4).
Of the 1011 patients who had magnetic resonance imaging (MRI) performed, 943 (93.3%) had abnormal acetabular labrum findings, and 163 (17.1%) had acetabular articular damage. According to radiographic evaluation, 953 patients had abnormal hip joint morphology consistent with FAI. Figure 3 shows the FAI classification percentages.
On clinical examination, 1079 patients (62.1%) had a positive anterior impingement sign. The subspine impingement sign was positive in 447 patients (25.7%), and the trochanteric pain sign was positive in 400 (23%). Table 5 lists range-of-motion values for flexion and hip rotation from 90° of flexion.
As seen in Table 6, labral pathology was the most common diagnosis (1426/1738 patients, 82%).
As seen in Table 7, the most common procedure was femoroplasty (867/1738, 49.9%).
Discussion
In this study, we collected epidemiologic data (demographics, pathologic entities treated, patient-reported measures of disease, surgical treatment preferences) from a large multicenter population of hip pathology patients who elected to undergo arthroscopic surgery. Our results showed these patients were most commonly younger to middle-aged white females with pain primarily in the groin region. Most had pain for at least 1 year, and it was commonly exacerbated by sitting and athletics. Patients reported clinically significant pain and functional limitation, which showed evidence of affecting general physical and mental health. It was not uncommon for patients to have undergone another, related surgery and nonoperative treatments, including intra-articular injection and/or physical therapy, before surgery. There was a high incidence of abnormal hip morphology suggestive of a cam lesion, but the incidence of arthritic changes on radiographs was relatively low. Labral tear was the most common diagnosis, and most often it was addressed with repair. Many patients underwent femoroplasty, acetabuloplasty, and chondroplasty in addition to labral repair.
According to patient-reported outcome measures administered before surgery, 40% to 65% of patients seeking hip preservation surgery reported functional deficits and pain—which falls within the range of results from other multicenter studies on the epidemiology of FAI.18,19 There was, however, a high amount of variability in individual scores on the functional and pain measures; some patients rated their functional ability very high. These findings were supported by the general health forms measuring global physical and mental health. Mean Physical Health and Mental Health scores on the 12-item Short Form Health Survey indicated that patients seeking hip preservation surgery thought their hip condition affected their general well-being. This finding is consistent with research on FAI,18 hip arthritis,20 and total hip arthroplasty.19Our results further showed that hip arthroscopists commonly prescribed alternative treatment measures ahead of surgery. Before elective surgery, 80% of patients received an intra-articular injection, underwent physical therapy, or both. This could suggest a high failure rate for patients who chose conservative treatment approaches for hip-related pathology. However, our study was limited in that it may have included patients who had improved significantly with conservative measures and decided to forgo arthroscopic hip surgery. Although conservative treatment often is recommended in an effort to potentially avoid surgery, there is a lack of research evaluating the efficacy of nonoperative care.21,22Analysis of diagnostic imaging and clinical examination findings revealed some unique characteristics of patients undergoing elective hip preservation surgery. MRI showed labral pathology in an overwhelming majority of these patients, but few had evidence of articular damage. Previous research has found a 67% rate of arthritic changes on diagnostic imaging, but our rate was much lower (17%).23 Radiograph evaluation confirmed the pattern: More than 90% of our patients had Tönnis grade 0 osteoarthritis. Tönnis grade 1 or 2 osteoarthritis is a predictor of acetabular cartilage degeneration,23 and long-term studies have related these osteoarthritic changes to poorer hip arthroscopy outcomes.24 Thus, the lower incidence of osteoarthritis in our study population may reflect current evidence-based practice and a contemporary approach to patient selection.
Most of our patients had isolated cam-type FAI as opposed to pincer-type FAI or a combination of cam and pincer—contrary to research findings that combination cam–pincer FAI is most prevalent.25,26 Our results are more consistent with more recent research findings of a higher incidence of isolated cam lesion, particularly in female patients, and combination cam–pincer in male patients.18,27,28 Similar distributions of surgical procedures and diagnoses exist between the present study and other multicenter evaluations of the epidemiologic characteristics of patients with hip pathology.18Our study had several limitations. First, the population consisted entirely of patients who sought evaluation by a hip arthroscopy specialist and underwent elective surgery. Therefore, the data cannot be applied to a more general orthopedic population or to patients who consult other medical specialists. Second, the population, which was 97% white and had small percentages of African-American, Latino, and Asian patients, lacked ethnic diversity. This finding is consistent with recent epidemiologic research in which ethnicity was identified as a factor in patterns of hip disease.13,29,30 Access to specialists, however, was likely affected by multiple other factors. Fourth, the validity and the reliability of the imaging modalities used have been questioned.31-33 There is controversy regarding ideal imaging modalities for assessment of articular cartilage damage31,32 and FAI. However, the modalities that we used to determine diagnoses in this study are well supported26 and represent common practice patterns.
Am J Orthop. 2017;46(1):35-41. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016;32(7):1286-1292.
2. Peters CL, Aoki SK, Erickson JA, Anderson LA, Anderson AE. Early experience with a comprehensive hip preservation service intended to improve clinical care, education, and academic productivity. Clin Orthop Relat Res. 2012;470(12):3446-3452.
3. Siebenrock KA, Peters CL. ABJS Carl T. Brighton workshop on hip preservation surgery: editorial comment. Clin Orthop Relat Res. 2012;470(12):3281-3283.
4. Parvizi J, Leunig M, Ganz R. Femoroacetabular impingement. J Am Acad Orthop Surg. 2007;15(9):561-570.
5. Poh SY, Hube R, Dienst M. Arthroscopic treatment of femoroacetabular pincer impingement. Oper Orthop Traumatol. 2015;27(6):536-552.
6. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
7. Groh MM, Herrera J. A comprehensive review of hip labral tears. Curr Rev Musculoskelet Med. 2009;2(2):105-117.
8. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
9. White BJ, Herzog MM. Labral reconstruction: when to perform and how. Front Surg. 2015;2:27.
10. Yen YM, Kocher MS. Chondral lesions of the hip: microfracture and chondroplasty. Sports Med Arthrosc. 2010;18(2):83-89.
11. Jordan MA, Van Thiel GS, Chahal J, Nho SJ. Operative treatment of chondral defects in the hip joint: a systematic review. Curr Rev Musculoskelet Med. 2012;5(3):244-253.
12. Griffin DW, Kinnard MJ, Formby PM, McCabe MP, Anderson TD. Outcomes of hip arthroscopy in the older adult: a systematic review of the literature [published online October 18, 2016]. Am J Sports Med. doi:10.1177/0363546516667915.
13. Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 2008;466(2):264-272.
14. Kaya M, Suzuki T, Emori M, Yamashita T. Hip morphology influences the pattern of articular cartilage damage. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):2016-2023.
15. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
16. Byrd JT. Hip arthroscopy in athletes. Oper Tech Sports Med. 2005;13(1):24-36.
17. Werner BC, Gaudiani MA, Ranawat AS. The etiology and arthroscopic surgical management of cam lesions. Clin Sports Med. 2016;35(3):391-404.
18. Clohisy JC, Baca G, Beaulé PE, et al; ANCHOR Study Group. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
19. Shia DS, Clohisy JC, Schinsky MF, Martell JM, Maloney WJ. THA with highly cross-linked polyethylene in patients 50 years or younger. Clin Orthop Relat Res. 2009;467(8):2059-2065.
20. Gandhi SK, Salmon JW, Zhao SZ, Lambert BL, Gore PR, Conrad K. Psychometric evaluation of the 12-item Short-Form Health Survey (SF-12) in osteoarthritis and rheumatoid arthritis clinical trials. Clin Ther. 2001;23(7):1080-1098.
21. Loudon JK, Reiman MP. Conservative management of femoroacetabular impingement (FAI) in the long distance runner. Phys Ther Sport. 2014;15(2):82-90.
22. Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418-426.
23. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med. 2011;39(2):296-303.
24. McCormick F, Nwachukwu BU, Alpaugh K, Martin SD. Predictors of hip arthroscopy outcomes for labral tears at minimum 2-year follow-up: the influence of age and arthritis. Arthroscopy. 2012;28(10):1359-1364.
25. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
26. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis—what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552.
27. Kapron AL, Peters CL, Aoki SK, et al. The prevalence of radiographic findings of structural hip deformities in female collegiate athletes. Am J Sports Med. 2015;43(6):1324-1330.
28. Lee WY, Kang C, Hwang DS, Jeon JH, Zheng L. Descriptive epidemiology of symptomatic femoroacetabular impingement in young athlete: single center study. Hip Pelvis. 2016;28(1):29-34.
29. Dudda M, Kim YJ, Zhang Y, et al. Morphologic differences between the hips of Chinese women and white women: could they account for the ethnic difference in the prevalence of hip osteoarthritis? Arthritis Rheum. 2011;63(10):2992-2999.
30. Solomon L, Beighton P. Osteoarthrosis of the hip and its relationship to pre-existing in an African population. J Bone Joint Surg Br. 1973;55(1):216-217.
31. Keeney JA, Peelle MW, Jackson J, Rubin D, Maloney WJ, Clohisy JC. Magnetic resonance arthrography versus arthroscopy in the evaluation of articular hip pathology. Clin Orthop Relat Res. 2004;(429):163-169.
32. Schmid MR, Nötzli HP, Zanetti M, Wyss TF, Hodler J. Cartilage lesions in the hip: diagnostic effectiveness of MR arthrography. Radiology. 2003;226(2):382-386.
33. Chevillotte CJ, Ali MH, Trousdale RT, Pagnano MW. Variability in hip range of motion on clinical examination. J Arthroplasty. 2009;24(5):693-697.
1. Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016;32(7):1286-1292.
2. Peters CL, Aoki SK, Erickson JA, Anderson LA, Anderson AE. Early experience with a comprehensive hip preservation service intended to improve clinical care, education, and academic productivity. Clin Orthop Relat Res. 2012;470(12):3446-3452.
3. Siebenrock KA, Peters CL. ABJS Carl T. Brighton workshop on hip preservation surgery: editorial comment. Clin Orthop Relat Res. 2012;470(12):3281-3283.
4. Parvizi J, Leunig M, Ganz R. Femoroacetabular impingement. J Am Acad Orthop Surg. 2007;15(9):561-570.
5. Poh SY, Hube R, Dienst M. Arthroscopic treatment of femoroacetabular pincer impingement. Oper Orthop Traumatol. 2015;27(6):536-552.
6. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
7. Groh MM, Herrera J. A comprehensive review of hip labral tears. Curr Rev Musculoskelet Med. 2009;2(2):105-117.
8. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
9. White BJ, Herzog MM. Labral reconstruction: when to perform and how. Front Surg. 2015;2:27.
10. Yen YM, Kocher MS. Chondral lesions of the hip: microfracture and chondroplasty. Sports Med Arthrosc. 2010;18(2):83-89.
11. Jordan MA, Van Thiel GS, Chahal J, Nho SJ. Operative treatment of chondral defects in the hip joint: a systematic review. Curr Rev Musculoskelet Med. 2012;5(3):244-253.
12. Griffin DW, Kinnard MJ, Formby PM, McCabe MP, Anderson TD. Outcomes of hip arthroscopy in the older adult: a systematic review of the literature [published online October 18, 2016]. Am J Sports Med. doi:10.1177/0363546516667915.
13. Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 2008;466(2):264-272.
14. Kaya M, Suzuki T, Emori M, Yamashita T. Hip morphology influences the pattern of articular cartilage damage. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):2016-2023.
15. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
16. Byrd JT. Hip arthroscopy in athletes. Oper Tech Sports Med. 2005;13(1):24-36.
17. Werner BC, Gaudiani MA, Ranawat AS. The etiology and arthroscopic surgical management of cam lesions. Clin Sports Med. 2016;35(3):391-404.
18. Clohisy JC, Baca G, Beaulé PE, et al; ANCHOR Study Group. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
19. Shia DS, Clohisy JC, Schinsky MF, Martell JM, Maloney WJ. THA with highly cross-linked polyethylene in patients 50 years or younger. Clin Orthop Relat Res. 2009;467(8):2059-2065.
20. Gandhi SK, Salmon JW, Zhao SZ, Lambert BL, Gore PR, Conrad K. Psychometric evaluation of the 12-item Short-Form Health Survey (SF-12) in osteoarthritis and rheumatoid arthritis clinical trials. Clin Ther. 2001;23(7):1080-1098.
21. Loudon JK, Reiman MP. Conservative management of femoroacetabular impingement (FAI) in the long distance runner. Phys Ther Sport. 2014;15(2):82-90.
22. Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418-426.
23. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med. 2011;39(2):296-303.
24. McCormick F, Nwachukwu BU, Alpaugh K, Martin SD. Predictors of hip arthroscopy outcomes for labral tears at minimum 2-year follow-up: the influence of age and arthritis. Arthroscopy. 2012;28(10):1359-1364.
25. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
26. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis—what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552.
27. Kapron AL, Peters CL, Aoki SK, et al. The prevalence of radiographic findings of structural hip deformities in female collegiate athletes. Am J Sports Med. 2015;43(6):1324-1330.
28. Lee WY, Kang C, Hwang DS, Jeon JH, Zheng L. Descriptive epidemiology of symptomatic femoroacetabular impingement in young athlete: single center study. Hip Pelvis. 2016;28(1):29-34.
29. Dudda M, Kim YJ, Zhang Y, et al. Morphologic differences between the hips of Chinese women and white women: could they account for the ethnic difference in the prevalence of hip osteoarthritis? Arthritis Rheum. 2011;63(10):2992-2999.
30. Solomon L, Beighton P. Osteoarthrosis of the hip and its relationship to pre-existing in an African population. J Bone Joint Surg Br. 1973;55(1):216-217.
31. Keeney JA, Peelle MW, Jackson J, Rubin D, Maloney WJ, Clohisy JC. Magnetic resonance arthrography versus arthroscopy in the evaluation of articular hip pathology. Clin Orthop Relat Res. 2004;(429):163-169.
32. Schmid MR, Nötzli HP, Zanetti M, Wyss TF, Hodler J. Cartilage lesions in the hip: diagnostic effectiveness of MR arthrography. Radiology. 2003;226(2):382-386.
33. Chevillotte CJ, Ali MH, Trousdale RT, Pagnano MW. Variability in hip range of motion on clinical examination. J Arthroplasty. 2009;24(5):693-697.
Current Concepts in Labral Repair and Refixation: Anatomical Approach to Labral Management
Take-Home Points
- Labral preservation is recommended when possible to ensure restoration of suction seal, stability, and contact pressure of the hip joint.
- Over 95% of labral tears can be addressed with primary repair.
- Consider using an accessory portal (ie, DALA) to allow for more anatomic placement of suture anchor.
- Mattress stitch when labrum >3 mm and looped stitch when labrum <3 mm.
- 10Control labral repair to avoid excessive inversion or eversion.
Arthroscopic labral repair and refixation have garnered much attention over the past several years. Restoration of suction seal and native labral function has been an evolving focus for achieving excellent results in hip preservation surgery.1-6 Given the superior results of labral repair, including level I evidence, repair or refixation should be pursued whenever possible.7 Authors have reported using several labral management techniques: débridement, labralization, looped suture fixation, base stitch fixation, inversion-eversion, and reconstruction.7-13 The optimal technique is yet to be determined. When possible, steps should be taken to repair the labrum to an anatomical position. Absolute indications for labral repair are a confirmed intra-articular diagnosis with symptomatic pain, joint space >2 mm with or without femoroacetabular impingement (FAI), labral tear or instability, and failed conservative management.9,11,12,14,15 More important, the surgeon must have a clear etiology of the pathologic cause of the tear and be aware of the limitations of the procedure. Labral repair is relatively contraindicated in end-stage arthritis and has failed when used alone in undiagnosed dysplasia or hip instability.16 In this article, we discuss indications for labral repair; describe Dr. Mather’s preoperative planning, labral repair technique, and postoperative care; and review published outcomes and future trends in labral repair.
Indications
At our institution, anatomical labral repair is the preferred procedure for most primary and revision hip arthroscopy procedures. We aim to restore the suction seal, re-create the contact of the labrum and the femoral head to facilitate proprioception, and restore normal stability of the labrum. Indications for primary repair are labrum width >3 mm, no more than 2 repairs, and ability to hold a suture. Our indications for reconstruction or débridement are stage 3 irreparable labral tear, calcified/cystic labrum, and multiple failed labral repairs or reconstructions. The decision to perform labral débridement or reconstruction is made on a case-by-case basis but is primarily influenced by the stability of the hip joint and the activity goals of the patient. If preoperative presentation and intraoperative examination suggest labral instability as a major component of the pathology, or if the patient wants to return to high-demand activity, we more strongly favor reconstruction over débridement. In our experience, with the technique described in this article, more than 95% of all primary labral tears can be addressed with repair.
Preoperative Planning
The goals in hip preservation surgery are to identify and address the underlying cause of the labral tear, whether it be FAI syndrome, trauma, labral instability, or all 3, and to re-create the anatomy and biomechanics of the acetabular labrum. For repair, we prefer an inversion-eversion technique with independent control of the labrum. Our initial work-up includes a thorough history and physical examination with baseline patient-reported outcome scores. Standard erect anteroposterior pelvis, Dunn lateral, and false-profile radiographs are obtained. Standard measurements of lateral center edge angle, anterior center edge angle, Tönnis angle, Tönnis grade, lateral joint space, and head extrusion indices are evaluated. Selective in-office ultrasound-guided injections are used to confirm an intra-articular source of pain. At our institution, noncontrast 3.0 Tesla magnetic resonance imaging (MRI) with volumetric interpolated breath-hold examination (VIBE) sequencing and 3-dimensional rendering is obtained for evaluation of labral and FAI morphology.17 All advanced imaging is performed without arthrogram or radiation exposure (Figures 1A-1C).
With use of the radiographs and the MRI scans, we engage the patient in an informed discussion about the labral tear, FAI, and concomitant pathology. We discuss expected outcomes of conservative or operative management given the patient’s expected functional activities, and inform the patient that primary repair is indicated for many others in similar situations. The potential for possible labral reconstruction is discussed if the patient had prior intra-articular hip surgery, has a large calcified labrum or a cystic labrum, is an athlete with failed prior surgery, or is younger than 40 years.
Labral Repair Technique
The patient is taken to the surgical suite, and a general anesthetic is administered. A peripheral nerve block is not routinely used. The patient’s feet are padded, and boots for the traction table are applied. The patient is carefully placed on a Hana table in modified supine position. Balanced traction is used to achieve proper joint distraction. The C-arm is used to verify proper distraction, assess hip stability, and achieve standard anterolateral (AL) portal placement. A midanterior portal (MAP) is created and an interportal capsulotomy is performed. Capsular suspension is performed with the InJector II Capsule Restoration System (Stryker Sports Medicine) and typically 4 or 5 high-strength No. 2 sutures (Zipline; Stryker Sports Medicine).19 Diagnostic arthroscopy is performed to identify the tear type, measure the labral width, determine the impingement area, and identify the intra-articular pathology. After the intra-articular pathology is addressed, a radiofrequency Ambient HIPVAC 50 Coblation Wand (Smith & Nephew) is used to expose the acetabular rim and subspine as indicated. Acetabuloplasty or subspine decompression is performed, and then a primary repair or refixation of the labrum is performed. We do not routinely detach the labrum for acetabular rim trimming. A crucial step here is to expose a bleeding surface to which the labrum can be repaired. If the rim is sclerotic, or the rim cannot be removed because of underlying low acetabular coverage, we prefer to obtain the bleeding surface with a microdrilling device (Stryker) that is routinely used for acetabular microfracture.
Labrum quality is used to determine which repair method to use. A hypertrophic labrum is debulked. The acetabular rim is seldom resected >3 mm, but, when it is, the newly exposed cartilage is removed. We have found that >3 mm of residual cartilage prevents refixation of the labrum directly to the bone and may interfere with anatomical positioning. When a labrum is <3 mm in width or will not hold a base technique, repair stability is the priority, and a looped method is used. A knotless anchor with No. 1 permanent suture designed for hip labral repair (CinchLock; Stryker) is our first-line anchor choice. A distal anterolateral accessory (DALA) portal is created with an outside-in technique, and anchors are drilled through this portal into zones 2 to 4 (Figures 2A-2E).
A 2.4-mm drill guide is advanced through the DALA portal and placed in the appropriate position for drilling. We aim for 1 mm to 2 mm from the chondrolabral junction. Next, the probe is placed intra-articular and medial to the anchor insertion site, and the anchor is loaded and then inserted around the probe (Figures 3A-3E).
The hip is then reduced. If indicated, a T-capsulotomy is performed for femoral osteochondroplasty.
Postoperative Care
Patients are placed in a postoperative hip brace and use a continuous passive motion machine 6 hours a day for 2 weeks, and an ice machine. They maintain 30 lb of foot-flat weight-bearing for 3 weeks, and begin a standard labral repair protocol on postoperative days 3 to 7.
Discussion
Hip labral preservation has evolved over the past 10 years, and current options for labral management include excision, débridement, labralization, repair, and reconstruction.1-13 Labral excision was studied by Miozzari and colleagues,8 who postulated on the basis of animal models that the labrum may regenerate. In their series of 9 patients treated with surgical hip dislocation and labral excision at average 4-year follow-up, repeat magnetic resonance angiography revealed no regeneration of tissue—modified Harris Hip Score was 83. The hip scores were less than those of patients treated with the same procedure with repair, and the authors concluded that defining labral débridement versus excision in the literature, and treating patients with primary repair or reconstruction techniques, may lead to better results. Their study used a small sample and was limited to an open procedure. Arthroscopic labral débridement in isolation was also a poor option for treatment of a labral tear. In a 2-year follow-up of 59 isolated labral débridement procedures, Krych and colleagues9 found 47% combined poor results.
There is level I evidence of the importance of labral repair. In 2013, Krych and colleagues7 conducted a randomized control trial of 38 female patients who underwent hip arthroscopy for FAI. At time of surgery, patients were randomly assigned to either débridement or repair. At 1-year follow-up, activities of daily living and Sports specific Hip Outcome Scores were statistically significantly superior in the repair group. On a subjective scale, 94% vs 78% of patients reported normal or near normal hips in the repair versus débridement groups respectively. Ayeni and colleagues20 performed a systematic review of 6 studies in an attempt to develop labral management recommendations. Five of the studies (N = 490 patients total) had improved results with labral repair over reconstruction. Although the studies had a low level of evidence, they found a trend toward improved results with labral repair. These studies highlight the importance of labral preservation and proper FAI management.
Techniques for labrum repair have advanced as well—from a looped suture technique to a base stitch and knotless independent tensioning.11-13 Restoration of the hip labrum function as a suction seal, fluid circulator and anatomic capsular repair is paramount to excellent results and stresses the importance of performing an anatomic labral repair.1-6 Knotless anchor repair is not novel and has been previously described. Fry and Domb12 reported on a knotless labral repair technique that uses push-lock devices (Arthrex) that do not allow for independent tensioning. Inversion-eversion was introduced to the literature by Moreira and colleagues,13 who described an independent tensioning technique that uses speed-lock anchors (Smith & Nephew). Our technique differs in that it involves a DALA portal; labral reduction and tensioning with a probe assist to ensure the second pass of the base stitch is at the apex of the labrum; and use of No. 1 instead of No. 2 suture. Although seemingly subtle, these differences allow for proper anchor placement nearer the rim, additional support in achieving precise suture placement, and less disruption of small labra. These differences are particularly relevant for smaller labra.
Evaluating repair techniques on the basis of high-evidence literature is challenging. In a matched-cohort study of 220 patients, Jackson and colleagues21 compared 2 techniques: looped and base stitch. At 2-year follow-up, patients in both groups showed improvement, and there was no statistically significant difference in patient-reported outcome measures between the groups. Sawyer and colleagues22 studied the outcomes of 326 consecutive patients who underwent looped, pierced, or combined labral repair at an average 32-month follow-up. The groups’ revision rates were comparable, each group improved in postoperative patient-reported outcomes, and the pierced group had significantly higher preoperative scores on the Western Ontario and McMaster Universities Osteoarthritis Index. These studies described a base or pierce repair that did not differ from a looped repair, though the techniques did not allow for independent tensioning to re-create an anatomical inversion-eversion repair and may have altered the reported outcomes.
Our current technique uses independent tensioning of the repair to allow control of labrum inversion-eversion to give an anatomical repair with restoration of the suction seal. Preoperative planning, addressing the FAI appropriately, proper suture-passing technique, controlling the labrum in inversion-eversion fashion, and anatomical labral repair are the elements of Dr. Mather’s preferred method for preserving the native labrum and allowing it to assume its native function.
Future Directions
As our understanding of FAI and labral function evolves, labral preservation surgery continues to advance. With surgeons continually developing new techniques and following up on previous techniques, the ability to preserve the native hip with lasting procedures evolves as well. Proper identification of the underlying cause of the labral tear and proper anatomical repair are paramount to the success of FAI surgery.
Am J Orthop. 2017;46(1):42-48. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
2. Nepple JJ, Philippon MJ, Campbell KJ, et al. The hip fluid seal—part II: the effect of an acetabular labral tear, repair, resection and reconstruction on hip stability to distraction. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):730-736.
3. Dwyer MK, Jones HL, Hogan MG, Field RE, McCarthy JC, Noble PC. The acetabular labrum regulates fluid circulation of the hip joint during functional activities. Am J Sports Med. 2014;42(4):812-819.
4. Greaves LL, Gilbart MK, Yung AC, Kozlowski, Wilson DR. Effect of acetabular labral tears, repair and resection on hip cartilage strain: a 7T MR study. J Biomech. 2010;43(5):858-863.
5. Freehill MT, Safran MR. The labrum of the hip: diagnosis and rationale for surgical correction. Clin Sports Med. 2011;30(2):293-315.
6. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
7. Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46-53.
8. Miozzari HH, Celia M, Clark JM, Werlen S, Naal FD, Nötzli HP. No regeneration of the human acetabular labrum after excision to bone. Clin Orthop Relat Res. 2015;473(4):1349-1357.
9. Krych AJ, Kuzma SA, Kovachevich R, Hudgens JL, Stuart MJ, Levy BA. Modest mid-term outcomes after isolated arthroscopic debridement of acetabular labral tears. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):763-767.
10. Matsuda DK. Arthroscopic labralization of the hip: an alternative to labral reconstruction. Arthrosc Tech. 2014;3(1):e131-e133.
11. Philippon MJ, Faucet SC, Briggs KK. Arthroscopic hip labral repair. Arthrosc Tech. 2013;2(2):e73-e76.
12. Fry D, Domb B. Labral base refixation in the hip: rationale and technique for an anatomic approach to labral repair. Arthroscopy. 2010;26(9 suppl):S81-S89.
13. Moreira B, Pascual-Garrido C, Chadayamurri V, Mei-Dan O. Eversion-inversion labral repair and reconstruction technique for optimal suction seal. Arthrosc Tech. 2015;4(6):e697-e700.
14. Mook WR, Briggs KK, Philippon MJ. Evidence and approach for management of labral deficiency: the role for labral reconstruction. Sports Med Arthrosc. 2015;23(4):205-212.
15. Gupta A, Suarez-Ahedo C, Redmond JM, et al. Best practices during hip arthroscopy: aggregate recommendations of high-volume surgeons. Arthroscopy. 2015;31(9):1722-1727.
16. Yeung M, Kowalczuk M, Simunovic N, Ayeni OR. Hip arthroscopy in the setting of hip dysplasia: a systematic review. Bone Joint Res. 2016;5(6):225-231.
17. Hash TW. Magnetic resonance imaging of the hip. In: Nho SJ, Leunig M, Larson CM, Bedi A, Kelly BT, eds. Hip Arthroscopy and Hip Joint Preservation Surgery, Vol. 1. New York, NY: Springer; 2015:65-113.
18. Sutter R, Zubler V, Hoffmann A, et al. Hip MRI: how useful is intraarticular contrast material for evaluating surgically proven lesions of the labrum and articular cartilage? AJR Am J Roentgenol. 2014;202(1):160-169.
19. Federer AE, Karas V, Nho S, Coleman SH, Mather RC 3rd. Capsular suspension technique for hip arthroscopy. Arthrosc Tech. 2015;4(4):e317-e322.
20. Ayeni OR, Adamich J, Farrokhyar F, et al. Surgical management of labral tears during femoroacetabular impingement surgery: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):756-762.
21. Jackson TJ, Hammarstedt JE, Vemula SP, Domb BG. Acetabular labral base repair versus circumferential suture repair: a matched-paired comparison of clinical outcomes. Arthroscopy. 2015;31(9):1716-1721.
22. Sawyer GA, Briggs KK, Dornan GJ, Ommen ND, Philippon MJ. Clinical outcomes after arthroscopic hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683-1688.
Take-Home Points
- Labral preservation is recommended when possible to ensure restoration of suction seal, stability, and contact pressure of the hip joint.
- Over 95% of labral tears can be addressed with primary repair.
- Consider using an accessory portal (ie, DALA) to allow for more anatomic placement of suture anchor.
- Mattress stitch when labrum >3 mm and looped stitch when labrum <3 mm.
- 10Control labral repair to avoid excessive inversion or eversion.
Arthroscopic labral repair and refixation have garnered much attention over the past several years. Restoration of suction seal and native labral function has been an evolving focus for achieving excellent results in hip preservation surgery.1-6 Given the superior results of labral repair, including level I evidence, repair or refixation should be pursued whenever possible.7 Authors have reported using several labral management techniques: débridement, labralization, looped suture fixation, base stitch fixation, inversion-eversion, and reconstruction.7-13 The optimal technique is yet to be determined. When possible, steps should be taken to repair the labrum to an anatomical position. Absolute indications for labral repair are a confirmed intra-articular diagnosis with symptomatic pain, joint space >2 mm with or without femoroacetabular impingement (FAI), labral tear or instability, and failed conservative management.9,11,12,14,15 More important, the surgeon must have a clear etiology of the pathologic cause of the tear and be aware of the limitations of the procedure. Labral repair is relatively contraindicated in end-stage arthritis and has failed when used alone in undiagnosed dysplasia or hip instability.16 In this article, we discuss indications for labral repair; describe Dr. Mather’s preoperative planning, labral repair technique, and postoperative care; and review published outcomes and future trends in labral repair.
Indications
At our institution, anatomical labral repair is the preferred procedure for most primary and revision hip arthroscopy procedures. We aim to restore the suction seal, re-create the contact of the labrum and the femoral head to facilitate proprioception, and restore normal stability of the labrum. Indications for primary repair are labrum width >3 mm, no more than 2 repairs, and ability to hold a suture. Our indications for reconstruction or débridement are stage 3 irreparable labral tear, calcified/cystic labrum, and multiple failed labral repairs or reconstructions. The decision to perform labral débridement or reconstruction is made on a case-by-case basis but is primarily influenced by the stability of the hip joint and the activity goals of the patient. If preoperative presentation and intraoperative examination suggest labral instability as a major component of the pathology, or if the patient wants to return to high-demand activity, we more strongly favor reconstruction over débridement. In our experience, with the technique described in this article, more than 95% of all primary labral tears can be addressed with repair.
Preoperative Planning
The goals in hip preservation surgery are to identify and address the underlying cause of the labral tear, whether it be FAI syndrome, trauma, labral instability, or all 3, and to re-create the anatomy and biomechanics of the acetabular labrum. For repair, we prefer an inversion-eversion technique with independent control of the labrum. Our initial work-up includes a thorough history and physical examination with baseline patient-reported outcome scores. Standard erect anteroposterior pelvis, Dunn lateral, and false-profile radiographs are obtained. Standard measurements of lateral center edge angle, anterior center edge angle, Tönnis angle, Tönnis grade, lateral joint space, and head extrusion indices are evaluated. Selective in-office ultrasound-guided injections are used to confirm an intra-articular source of pain. At our institution, noncontrast 3.0 Tesla magnetic resonance imaging (MRI) with volumetric interpolated breath-hold examination (VIBE) sequencing and 3-dimensional rendering is obtained for evaluation of labral and FAI morphology.17 All advanced imaging is performed without arthrogram or radiation exposure (Figures 1A-1C).
With use of the radiographs and the MRI scans, we engage the patient in an informed discussion about the labral tear, FAI, and concomitant pathology. We discuss expected outcomes of conservative or operative management given the patient’s expected functional activities, and inform the patient that primary repair is indicated for many others in similar situations. The potential for possible labral reconstruction is discussed if the patient had prior intra-articular hip surgery, has a large calcified labrum or a cystic labrum, is an athlete with failed prior surgery, or is younger than 40 years.
Labral Repair Technique
The patient is taken to the surgical suite, and a general anesthetic is administered. A peripheral nerve block is not routinely used. The patient’s feet are padded, and boots for the traction table are applied. The patient is carefully placed on a Hana table in modified supine position. Balanced traction is used to achieve proper joint distraction. The C-arm is used to verify proper distraction, assess hip stability, and achieve standard anterolateral (AL) portal placement. A midanterior portal (MAP) is created and an interportal capsulotomy is performed. Capsular suspension is performed with the InJector II Capsule Restoration System (Stryker Sports Medicine) and typically 4 or 5 high-strength No. 2 sutures (Zipline; Stryker Sports Medicine).19 Diagnostic arthroscopy is performed to identify the tear type, measure the labral width, determine the impingement area, and identify the intra-articular pathology. After the intra-articular pathology is addressed, a radiofrequency Ambient HIPVAC 50 Coblation Wand (Smith & Nephew) is used to expose the acetabular rim and subspine as indicated. Acetabuloplasty or subspine decompression is performed, and then a primary repair or refixation of the labrum is performed. We do not routinely detach the labrum for acetabular rim trimming. A crucial step here is to expose a bleeding surface to which the labrum can be repaired. If the rim is sclerotic, or the rim cannot be removed because of underlying low acetabular coverage, we prefer to obtain the bleeding surface with a microdrilling device (Stryker) that is routinely used for acetabular microfracture.
Labrum quality is used to determine which repair method to use. A hypertrophic labrum is debulked. The acetabular rim is seldom resected >3 mm, but, when it is, the newly exposed cartilage is removed. We have found that >3 mm of residual cartilage prevents refixation of the labrum directly to the bone and may interfere with anatomical positioning. When a labrum is <3 mm in width or will not hold a base technique, repair stability is the priority, and a looped method is used. A knotless anchor with No. 1 permanent suture designed for hip labral repair (CinchLock; Stryker) is our first-line anchor choice. A distal anterolateral accessory (DALA) portal is created with an outside-in technique, and anchors are drilled through this portal into zones 2 to 4 (Figures 2A-2E).
A 2.4-mm drill guide is advanced through the DALA portal and placed in the appropriate position for drilling. We aim for 1 mm to 2 mm from the chondrolabral junction. Next, the probe is placed intra-articular and medial to the anchor insertion site, and the anchor is loaded and then inserted around the probe (Figures 3A-3E).
The hip is then reduced. If indicated, a T-capsulotomy is performed for femoral osteochondroplasty.
Postoperative Care
Patients are placed in a postoperative hip brace and use a continuous passive motion machine 6 hours a day for 2 weeks, and an ice machine. They maintain 30 lb of foot-flat weight-bearing for 3 weeks, and begin a standard labral repair protocol on postoperative days 3 to 7.
Discussion
Hip labral preservation has evolved over the past 10 years, and current options for labral management include excision, débridement, labralization, repair, and reconstruction.1-13 Labral excision was studied by Miozzari and colleagues,8 who postulated on the basis of animal models that the labrum may regenerate. In their series of 9 patients treated with surgical hip dislocation and labral excision at average 4-year follow-up, repeat magnetic resonance angiography revealed no regeneration of tissue—modified Harris Hip Score was 83. The hip scores were less than those of patients treated with the same procedure with repair, and the authors concluded that defining labral débridement versus excision in the literature, and treating patients with primary repair or reconstruction techniques, may lead to better results. Their study used a small sample and was limited to an open procedure. Arthroscopic labral débridement in isolation was also a poor option for treatment of a labral tear. In a 2-year follow-up of 59 isolated labral débridement procedures, Krych and colleagues9 found 47% combined poor results.
There is level I evidence of the importance of labral repair. In 2013, Krych and colleagues7 conducted a randomized control trial of 38 female patients who underwent hip arthroscopy for FAI. At time of surgery, patients were randomly assigned to either débridement or repair. At 1-year follow-up, activities of daily living and Sports specific Hip Outcome Scores were statistically significantly superior in the repair group. On a subjective scale, 94% vs 78% of patients reported normal or near normal hips in the repair versus débridement groups respectively. Ayeni and colleagues20 performed a systematic review of 6 studies in an attempt to develop labral management recommendations. Five of the studies (N = 490 patients total) had improved results with labral repair over reconstruction. Although the studies had a low level of evidence, they found a trend toward improved results with labral repair. These studies highlight the importance of labral preservation and proper FAI management.
Techniques for labrum repair have advanced as well—from a looped suture technique to a base stitch and knotless independent tensioning.11-13 Restoration of the hip labrum function as a suction seal, fluid circulator and anatomic capsular repair is paramount to excellent results and stresses the importance of performing an anatomic labral repair.1-6 Knotless anchor repair is not novel and has been previously described. Fry and Domb12 reported on a knotless labral repair technique that uses push-lock devices (Arthrex) that do not allow for independent tensioning. Inversion-eversion was introduced to the literature by Moreira and colleagues,13 who described an independent tensioning technique that uses speed-lock anchors (Smith & Nephew). Our technique differs in that it involves a DALA portal; labral reduction and tensioning with a probe assist to ensure the second pass of the base stitch is at the apex of the labrum; and use of No. 1 instead of No. 2 suture. Although seemingly subtle, these differences allow for proper anchor placement nearer the rim, additional support in achieving precise suture placement, and less disruption of small labra. These differences are particularly relevant for smaller labra.
Evaluating repair techniques on the basis of high-evidence literature is challenging. In a matched-cohort study of 220 patients, Jackson and colleagues21 compared 2 techniques: looped and base stitch. At 2-year follow-up, patients in both groups showed improvement, and there was no statistically significant difference in patient-reported outcome measures between the groups. Sawyer and colleagues22 studied the outcomes of 326 consecutive patients who underwent looped, pierced, or combined labral repair at an average 32-month follow-up. The groups’ revision rates were comparable, each group improved in postoperative patient-reported outcomes, and the pierced group had significantly higher preoperative scores on the Western Ontario and McMaster Universities Osteoarthritis Index. These studies described a base or pierce repair that did not differ from a looped repair, though the techniques did not allow for independent tensioning to re-create an anatomical inversion-eversion repair and may have altered the reported outcomes.
Our current technique uses independent tensioning of the repair to allow control of labrum inversion-eversion to give an anatomical repair with restoration of the suction seal. Preoperative planning, addressing the FAI appropriately, proper suture-passing technique, controlling the labrum in inversion-eversion fashion, and anatomical labral repair are the elements of Dr. Mather’s preferred method for preserving the native labrum and allowing it to assume its native function.
Future Directions
As our understanding of FAI and labral function evolves, labral preservation surgery continues to advance. With surgeons continually developing new techniques and following up on previous techniques, the ability to preserve the native hip with lasting procedures evolves as well. Proper identification of the underlying cause of the labral tear and proper anatomical repair are paramount to the success of FAI surgery.
Am J Orthop. 2017;46(1):42-48. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Labral preservation is recommended when possible to ensure restoration of suction seal, stability, and contact pressure of the hip joint.
- Over 95% of labral tears can be addressed with primary repair.
- Consider using an accessory portal (ie, DALA) to allow for more anatomic placement of suture anchor.
- Mattress stitch when labrum >3 mm and looped stitch when labrum <3 mm.
- 10Control labral repair to avoid excessive inversion or eversion.
Arthroscopic labral repair and refixation have garnered much attention over the past several years. Restoration of suction seal and native labral function has been an evolving focus for achieving excellent results in hip preservation surgery.1-6 Given the superior results of labral repair, including level I evidence, repair or refixation should be pursued whenever possible.7 Authors have reported using several labral management techniques: débridement, labralization, looped suture fixation, base stitch fixation, inversion-eversion, and reconstruction.7-13 The optimal technique is yet to be determined. When possible, steps should be taken to repair the labrum to an anatomical position. Absolute indications for labral repair are a confirmed intra-articular diagnosis with symptomatic pain, joint space >2 mm with or without femoroacetabular impingement (FAI), labral tear or instability, and failed conservative management.9,11,12,14,15 More important, the surgeon must have a clear etiology of the pathologic cause of the tear and be aware of the limitations of the procedure. Labral repair is relatively contraindicated in end-stage arthritis and has failed when used alone in undiagnosed dysplasia or hip instability.16 In this article, we discuss indications for labral repair; describe Dr. Mather’s preoperative planning, labral repair technique, and postoperative care; and review published outcomes and future trends in labral repair.
Indications
At our institution, anatomical labral repair is the preferred procedure for most primary and revision hip arthroscopy procedures. We aim to restore the suction seal, re-create the contact of the labrum and the femoral head to facilitate proprioception, and restore normal stability of the labrum. Indications for primary repair are labrum width >3 mm, no more than 2 repairs, and ability to hold a suture. Our indications for reconstruction or débridement are stage 3 irreparable labral tear, calcified/cystic labrum, and multiple failed labral repairs or reconstructions. The decision to perform labral débridement or reconstruction is made on a case-by-case basis but is primarily influenced by the stability of the hip joint and the activity goals of the patient. If preoperative presentation and intraoperative examination suggest labral instability as a major component of the pathology, or if the patient wants to return to high-demand activity, we more strongly favor reconstruction over débridement. In our experience, with the technique described in this article, more than 95% of all primary labral tears can be addressed with repair.
Preoperative Planning
The goals in hip preservation surgery are to identify and address the underlying cause of the labral tear, whether it be FAI syndrome, trauma, labral instability, or all 3, and to re-create the anatomy and biomechanics of the acetabular labrum. For repair, we prefer an inversion-eversion technique with independent control of the labrum. Our initial work-up includes a thorough history and physical examination with baseline patient-reported outcome scores. Standard erect anteroposterior pelvis, Dunn lateral, and false-profile radiographs are obtained. Standard measurements of lateral center edge angle, anterior center edge angle, Tönnis angle, Tönnis grade, lateral joint space, and head extrusion indices are evaluated. Selective in-office ultrasound-guided injections are used to confirm an intra-articular source of pain. At our institution, noncontrast 3.0 Tesla magnetic resonance imaging (MRI) with volumetric interpolated breath-hold examination (VIBE) sequencing and 3-dimensional rendering is obtained for evaluation of labral and FAI morphology.17 All advanced imaging is performed without arthrogram or radiation exposure (Figures 1A-1C).
With use of the radiographs and the MRI scans, we engage the patient in an informed discussion about the labral tear, FAI, and concomitant pathology. We discuss expected outcomes of conservative or operative management given the patient’s expected functional activities, and inform the patient that primary repair is indicated for many others in similar situations. The potential for possible labral reconstruction is discussed if the patient had prior intra-articular hip surgery, has a large calcified labrum or a cystic labrum, is an athlete with failed prior surgery, or is younger than 40 years.
Labral Repair Technique
The patient is taken to the surgical suite, and a general anesthetic is administered. A peripheral nerve block is not routinely used. The patient’s feet are padded, and boots for the traction table are applied. The patient is carefully placed on a Hana table in modified supine position. Balanced traction is used to achieve proper joint distraction. The C-arm is used to verify proper distraction, assess hip stability, and achieve standard anterolateral (AL) portal placement. A midanterior portal (MAP) is created and an interportal capsulotomy is performed. Capsular suspension is performed with the InJector II Capsule Restoration System (Stryker Sports Medicine) and typically 4 or 5 high-strength No. 2 sutures (Zipline; Stryker Sports Medicine).19 Diagnostic arthroscopy is performed to identify the tear type, measure the labral width, determine the impingement area, and identify the intra-articular pathology. After the intra-articular pathology is addressed, a radiofrequency Ambient HIPVAC 50 Coblation Wand (Smith & Nephew) is used to expose the acetabular rim and subspine as indicated. Acetabuloplasty or subspine decompression is performed, and then a primary repair or refixation of the labrum is performed. We do not routinely detach the labrum for acetabular rim trimming. A crucial step here is to expose a bleeding surface to which the labrum can be repaired. If the rim is sclerotic, or the rim cannot be removed because of underlying low acetabular coverage, we prefer to obtain the bleeding surface with a microdrilling device (Stryker) that is routinely used for acetabular microfracture.
Labrum quality is used to determine which repair method to use. A hypertrophic labrum is debulked. The acetabular rim is seldom resected >3 mm, but, when it is, the newly exposed cartilage is removed. We have found that >3 mm of residual cartilage prevents refixation of the labrum directly to the bone and may interfere with anatomical positioning. When a labrum is <3 mm in width or will not hold a base technique, repair stability is the priority, and a looped method is used. A knotless anchor with No. 1 permanent suture designed for hip labral repair (CinchLock; Stryker) is our first-line anchor choice. A distal anterolateral accessory (DALA) portal is created with an outside-in technique, and anchors are drilled through this portal into zones 2 to 4 (Figures 2A-2E).
A 2.4-mm drill guide is advanced through the DALA portal and placed in the appropriate position for drilling. We aim for 1 mm to 2 mm from the chondrolabral junction. Next, the probe is placed intra-articular and medial to the anchor insertion site, and the anchor is loaded and then inserted around the probe (Figures 3A-3E).
The hip is then reduced. If indicated, a T-capsulotomy is performed for femoral osteochondroplasty.
Postoperative Care
Patients are placed in a postoperative hip brace and use a continuous passive motion machine 6 hours a day for 2 weeks, and an ice machine. They maintain 30 lb of foot-flat weight-bearing for 3 weeks, and begin a standard labral repair protocol on postoperative days 3 to 7.
Discussion
Hip labral preservation has evolved over the past 10 years, and current options for labral management include excision, débridement, labralization, repair, and reconstruction.1-13 Labral excision was studied by Miozzari and colleagues,8 who postulated on the basis of animal models that the labrum may regenerate. In their series of 9 patients treated with surgical hip dislocation and labral excision at average 4-year follow-up, repeat magnetic resonance angiography revealed no regeneration of tissue—modified Harris Hip Score was 83. The hip scores were less than those of patients treated with the same procedure with repair, and the authors concluded that defining labral débridement versus excision in the literature, and treating patients with primary repair or reconstruction techniques, may lead to better results. Their study used a small sample and was limited to an open procedure. Arthroscopic labral débridement in isolation was also a poor option for treatment of a labral tear. In a 2-year follow-up of 59 isolated labral débridement procedures, Krych and colleagues9 found 47% combined poor results.
There is level I evidence of the importance of labral repair. In 2013, Krych and colleagues7 conducted a randomized control trial of 38 female patients who underwent hip arthroscopy for FAI. At time of surgery, patients were randomly assigned to either débridement or repair. At 1-year follow-up, activities of daily living and Sports specific Hip Outcome Scores were statistically significantly superior in the repair group. On a subjective scale, 94% vs 78% of patients reported normal or near normal hips in the repair versus débridement groups respectively. Ayeni and colleagues20 performed a systematic review of 6 studies in an attempt to develop labral management recommendations. Five of the studies (N = 490 patients total) had improved results with labral repair over reconstruction. Although the studies had a low level of evidence, they found a trend toward improved results with labral repair. These studies highlight the importance of labral preservation and proper FAI management.
Techniques for labrum repair have advanced as well—from a looped suture technique to a base stitch and knotless independent tensioning.11-13 Restoration of the hip labrum function as a suction seal, fluid circulator and anatomic capsular repair is paramount to excellent results and stresses the importance of performing an anatomic labral repair.1-6 Knotless anchor repair is not novel and has been previously described. Fry and Domb12 reported on a knotless labral repair technique that uses push-lock devices (Arthrex) that do not allow for independent tensioning. Inversion-eversion was introduced to the literature by Moreira and colleagues,13 who described an independent tensioning technique that uses speed-lock anchors (Smith & Nephew). Our technique differs in that it involves a DALA portal; labral reduction and tensioning with a probe assist to ensure the second pass of the base stitch is at the apex of the labrum; and use of No. 1 instead of No. 2 suture. Although seemingly subtle, these differences allow for proper anchor placement nearer the rim, additional support in achieving precise suture placement, and less disruption of small labra. These differences are particularly relevant for smaller labra.
Evaluating repair techniques on the basis of high-evidence literature is challenging. In a matched-cohort study of 220 patients, Jackson and colleagues21 compared 2 techniques: looped and base stitch. At 2-year follow-up, patients in both groups showed improvement, and there was no statistically significant difference in patient-reported outcome measures between the groups. Sawyer and colleagues22 studied the outcomes of 326 consecutive patients who underwent looped, pierced, or combined labral repair at an average 32-month follow-up. The groups’ revision rates were comparable, each group improved in postoperative patient-reported outcomes, and the pierced group had significantly higher preoperative scores on the Western Ontario and McMaster Universities Osteoarthritis Index. These studies described a base or pierce repair that did not differ from a looped repair, though the techniques did not allow for independent tensioning to re-create an anatomical inversion-eversion repair and may have altered the reported outcomes.
Our current technique uses independent tensioning of the repair to allow control of labrum inversion-eversion to give an anatomical repair with restoration of the suction seal. Preoperative planning, addressing the FAI appropriately, proper suture-passing technique, controlling the labrum in inversion-eversion fashion, and anatomical labral repair are the elements of Dr. Mather’s preferred method for preserving the native labrum and allowing it to assume its native function.
Future Directions
As our understanding of FAI and labral function evolves, labral preservation surgery continues to advance. With surgeons continually developing new techniques and following up on previous techniques, the ability to preserve the native hip with lasting procedures evolves as well. Proper identification of the underlying cause of the labral tear and proper anatomical repair are paramount to the success of FAI surgery.
Am J Orthop. 2017;46(1):42-48. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
2. Nepple JJ, Philippon MJ, Campbell KJ, et al. The hip fluid seal—part II: the effect of an acetabular labral tear, repair, resection and reconstruction on hip stability to distraction. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):730-736.
3. Dwyer MK, Jones HL, Hogan MG, Field RE, McCarthy JC, Noble PC. The acetabular labrum regulates fluid circulation of the hip joint during functional activities. Am J Sports Med. 2014;42(4):812-819.
4. Greaves LL, Gilbart MK, Yung AC, Kozlowski, Wilson DR. Effect of acetabular labral tears, repair and resection on hip cartilage strain: a 7T MR study. J Biomech. 2010;43(5):858-863.
5. Freehill MT, Safran MR. The labrum of the hip: diagnosis and rationale for surgical correction. Clin Sports Med. 2011;30(2):293-315.
6. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
7. Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46-53.
8. Miozzari HH, Celia M, Clark JM, Werlen S, Naal FD, Nötzli HP. No regeneration of the human acetabular labrum after excision to bone. Clin Orthop Relat Res. 2015;473(4):1349-1357.
9. Krych AJ, Kuzma SA, Kovachevich R, Hudgens JL, Stuart MJ, Levy BA. Modest mid-term outcomes after isolated arthroscopic debridement of acetabular labral tears. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):763-767.
10. Matsuda DK. Arthroscopic labralization of the hip: an alternative to labral reconstruction. Arthrosc Tech. 2014;3(1):e131-e133.
11. Philippon MJ, Faucet SC, Briggs KK. Arthroscopic hip labral repair. Arthrosc Tech. 2013;2(2):e73-e76.
12. Fry D, Domb B. Labral base refixation in the hip: rationale and technique for an anatomic approach to labral repair. Arthroscopy. 2010;26(9 suppl):S81-S89.
13. Moreira B, Pascual-Garrido C, Chadayamurri V, Mei-Dan O. Eversion-inversion labral repair and reconstruction technique for optimal suction seal. Arthrosc Tech. 2015;4(6):e697-e700.
14. Mook WR, Briggs KK, Philippon MJ. Evidence and approach for management of labral deficiency: the role for labral reconstruction. Sports Med Arthrosc. 2015;23(4):205-212.
15. Gupta A, Suarez-Ahedo C, Redmond JM, et al. Best practices during hip arthroscopy: aggregate recommendations of high-volume surgeons. Arthroscopy. 2015;31(9):1722-1727.
16. Yeung M, Kowalczuk M, Simunovic N, Ayeni OR. Hip arthroscopy in the setting of hip dysplasia: a systematic review. Bone Joint Res. 2016;5(6):225-231.
17. Hash TW. Magnetic resonance imaging of the hip. In: Nho SJ, Leunig M, Larson CM, Bedi A, Kelly BT, eds. Hip Arthroscopy and Hip Joint Preservation Surgery, Vol. 1. New York, NY: Springer; 2015:65-113.
18. Sutter R, Zubler V, Hoffmann A, et al. Hip MRI: how useful is intraarticular contrast material for evaluating surgically proven lesions of the labrum and articular cartilage? AJR Am J Roentgenol. 2014;202(1):160-169.
19. Federer AE, Karas V, Nho S, Coleman SH, Mather RC 3rd. Capsular suspension technique for hip arthroscopy. Arthrosc Tech. 2015;4(4):e317-e322.
20. Ayeni OR, Adamich J, Farrokhyar F, et al. Surgical management of labral tears during femoroacetabular impingement surgery: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):756-762.
21. Jackson TJ, Hammarstedt JE, Vemula SP, Domb BG. Acetabular labral base repair versus circumferential suture repair: a matched-paired comparison of clinical outcomes. Arthroscopy. 2015;31(9):1716-1721.
22. Sawyer GA, Briggs KK, Dornan GJ, Ommen ND, Philippon MJ. Clinical outcomes after arthroscopic hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683-1688.
1. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
2. Nepple JJ, Philippon MJ, Campbell KJ, et al. The hip fluid seal—part II: the effect of an acetabular labral tear, repair, resection and reconstruction on hip stability to distraction. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):730-736.
3. Dwyer MK, Jones HL, Hogan MG, Field RE, McCarthy JC, Noble PC. The acetabular labrum regulates fluid circulation of the hip joint during functional activities. Am J Sports Med. 2014;42(4):812-819.
4. Greaves LL, Gilbart MK, Yung AC, Kozlowski, Wilson DR. Effect of acetabular labral tears, repair and resection on hip cartilage strain: a 7T MR study. J Biomech. 2010;43(5):858-863.
5. Freehill MT, Safran MR. The labrum of the hip: diagnosis and rationale for surgical correction. Clin Sports Med. 2011;30(2):293-315.
6. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
7. Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46-53.
8. Miozzari HH, Celia M, Clark JM, Werlen S, Naal FD, Nötzli HP. No regeneration of the human acetabular labrum after excision to bone. Clin Orthop Relat Res. 2015;473(4):1349-1357.
9. Krych AJ, Kuzma SA, Kovachevich R, Hudgens JL, Stuart MJ, Levy BA. Modest mid-term outcomes after isolated arthroscopic debridement of acetabular labral tears. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):763-767.
10. Matsuda DK. Arthroscopic labralization of the hip: an alternative to labral reconstruction. Arthrosc Tech. 2014;3(1):e131-e133.
11. Philippon MJ, Faucet SC, Briggs KK. Arthroscopic hip labral repair. Arthrosc Tech. 2013;2(2):e73-e76.
12. Fry D, Domb B. Labral base refixation in the hip: rationale and technique for an anatomic approach to labral repair. Arthroscopy. 2010;26(9 suppl):S81-S89.
13. Moreira B, Pascual-Garrido C, Chadayamurri V, Mei-Dan O. Eversion-inversion labral repair and reconstruction technique for optimal suction seal. Arthrosc Tech. 2015;4(6):e697-e700.
14. Mook WR, Briggs KK, Philippon MJ. Evidence and approach for management of labral deficiency: the role for labral reconstruction. Sports Med Arthrosc. 2015;23(4):205-212.
15. Gupta A, Suarez-Ahedo C, Redmond JM, et al. Best practices during hip arthroscopy: aggregate recommendations of high-volume surgeons. Arthroscopy. 2015;31(9):1722-1727.
16. Yeung M, Kowalczuk M, Simunovic N, Ayeni OR. Hip arthroscopy in the setting of hip dysplasia: a systematic review. Bone Joint Res. 2016;5(6):225-231.
17. Hash TW. Magnetic resonance imaging of the hip. In: Nho SJ, Leunig M, Larson CM, Bedi A, Kelly BT, eds. Hip Arthroscopy and Hip Joint Preservation Surgery, Vol. 1. New York, NY: Springer; 2015:65-113.
18. Sutter R, Zubler V, Hoffmann A, et al. Hip MRI: how useful is intraarticular contrast material for evaluating surgically proven lesions of the labrum and articular cartilage? AJR Am J Roentgenol. 2014;202(1):160-169.
19. Federer AE, Karas V, Nho S, Coleman SH, Mather RC 3rd. Capsular suspension technique for hip arthroscopy. Arthrosc Tech. 2015;4(4):e317-e322.
20. Ayeni OR, Adamich J, Farrokhyar F, et al. Surgical management of labral tears during femoroacetabular impingement surgery: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):756-762.
21. Jackson TJ, Hammarstedt JE, Vemula SP, Domb BG. Acetabular labral base repair versus circumferential suture repair: a matched-paired comparison of clinical outcomes. Arthroscopy. 2015;31(9):1716-1721.
22. Sawyer GA, Briggs KK, Dornan GJ, Ommen ND, Philippon MJ. Clinical outcomes after arthroscopic hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683-1688.
Shoulder Dislocations
IN THIS ARTICLE
- Types of shoulder dislocations
- Schematics of the shoulder with three types of dislocations
- Association with seizures
CASE A 59-year-old man with a remote history of seizures is transported to the emergency department (ED) by ambulance after a witnessed tonic-clonic seizure. At the time of arrival he is postictal and confused, but his vital signs are stable. A left eyebrow laceration indicating a possible fall is observed on physical exam, as is a left shoulder displacement with no obvious signs of neurovascular compromise. The patient is not currently taking anticonvulsant medication, stating that he has been “seizure free” for five years, and therefore chose to discontinue taking phenytoin against medical advice.
An anteroposterior (AP) bilateral shoulder x-ray is obtained in the ED (see Figures 1a and 1b). The image shows the humeral head to be anteriorly dislocated and reveals a large impaction fracture of the posterior superior humeral head. For a more detailed view of the fracture and to further assess any associated deformities, CT of the left shoulder is performed. The fracture has a depth of 11.6 mm and a length of 24.1 mm, with no additional pathology noted (see Figure 1c).
The shoulder is a large joint capable of moving in many directions and therefore is inherently unstable. The glenoid fossa is shallow, and stability of the joint is provided by both the fibrocartilaginous labrum and varying muscles of the rotator cuff. Because the shoulder joint is poorly supported, dislocations are not uncommon (see the illustrations).
The first step in evaluating a suspected shoulder dislocation is to order an AP radiographic view of the shoulder (known as the Grashey view). A transcapular view (known as the scapular “Y” view) is also sufficient.1 While diagnostic studies, such as CT or MRI arthrography, are excellent for evaluating the glenohumeral ligaments and labrum, they generally are not done in an acute setting.1 For patients who present to the ED, some would recommend taking a CT scan, especially if a posterior dislocation is suspected.2
The three types of shoulder dislocations include anterior, posterior, and inferior.
ANTERIOR
Anterior dislocations account for 95% of all presented cases of shoulder dislocation, making them the most common type.3 They may be caused by a fall on an outstretched arm, trauma to the posterior humerus, or—more frequently—trauma to the arm while it is extended, externally rotated, and abducted (eg, blocking a shot in basketball).
A patient with an anterior dislocation will enter the ED with a slightly abducted and externally rotated arm (see illustration) and will resist any movement by the examiner. Typically, the shoulder loses its rounded appearance, and in thin individuals, the acromion may be prominent. A detailed neurovascular examination of the arm must be performed.
Dislocation of the humerus in any direction may compromise the axillary nerve, artery, or both. The axillary nerve and artery run parallel to each other, beneath and in close proximity to the humeral head. The axillary artery is located upstream from the radial artery; compression of the artery may lead to a diminution or complete absence of the radial pulse and/or coolness of the hand.4 The axillary nerve is both a sensory and motor nerve. If injured, a 2- to 3-cm area over the lateral deltoid may have complete sensory loss, which can be tested for with a light touch and pinprick.5 The patient may also have difficulty abducting the arm, but limitations of movement are difficult to measure with a new dislocation and a patient in pain.4
Any patient presenting with an anterior shoulder dislocation should also be screened for two other potential abnormalities. Hill-Sachs lesion, which occurs in up to 40% of anterior dislocations and 90% of all dislocations, is a cortical depression occurring in the humeral head. Bankart lesions, which occur in less than 5% of all dislocations, are avulsed bone fragments that occur when there is a glenoid labrum disruption.6 Both can be seen on plain films, although Bankart lesions are best seen on CT.4
The combination of an anterior dislocation and a humeral fracture, as seen in this case, is rare.7
POSTERIOR
Posterior shoulder dislocations occur far less frequently than anterior dislocations, representing 2% to 5% of all shoulder dislocations.2 They often result from blows to the anterior portion of the shoulder (ie, motor vehicle accidents or sports-related collisions) or violent muscle contractions (eg, electrocution, electroconvulsive therapy, or seizures).
Unable to externally rotate the shoulder, patients with posterior dislocations present with the arm in adduction and internal rotation, making the coracoid process prominent (see illustration).8 This position is sometimes misdiagnosed as a “frozen shoulder.”2
INFERIOR
Inferior dislocation of the shoulder is the rarest type, accounting for only 0.5% of all cases of shoulder dislocation. The mechanism of injury is forceful hyperabduction and extension of the shoulder during a fall.
Patients present with the affected arm hyperadducted, flexed at the elbow, with the hand positioned above or behind the head in fixed abduction: a “hands up” position of the affected arm (see illustration). These dislocations are best identified via the transcapular “Y” radiographs. Inferior dislocations are often associated with neurovascular compromise, and there are often related tears of the infraspinatus, supraspinatus, and teres minor muscles.9
ASSOCIATION WITH SEIZURES
Any patient who has had a seizure is subject to a variety of injuries, including lacerations, contusions, long bone and skull fractures, and dislocations. Seizures with a fall are associated with a 20% chance of injury.10
Shaw et al were the first to note that, during an active convulsion, the patient’s shoulder is in adduction, internal rotation, and flexion. This positioning predisposes to injury: With sustained contraction of the surrounding shoulder girdle muscles, the humeral head is forced superiorly and posteriorly against the acromion andmedially against the glenoid fossa. The glenoid fossa is shallow; therefore, the humeral head is forced posteriorly and dislocates.11
Researchers at the Mayo Clinic followed 247 patients who were diagnosed with seizures over nine years; 16% of the cohort experienced seizure-related injuries. Of the seizures recorded, 82% were tonic-clonic seizures. The singular predictive factor for injury was seizure frequency: Patients who had more seizures were more susceptible to injury.12
In an evaluation of outpatients with epilepsy, 25% of recorded seizures involved a fall. Among those who sustained an orthopedic injury, one injury occurred for every 178.6 generalized tonic-clonic seizures (0.6%)—a number that doubled for generalized tonic-clonic seizure associated with a fall (1.2%).10
The collective evidence from these and other studies suggests that patients who have poorly controlled tonic-clonic seizures have a higher incidence of seizures and, therefore, falls and injuries.10,12 In the absence of known trauma, a posterior shoulder dislocation is almost pathognomonic of a seizure. In high-risk populations (ie, individuals who have poorly controlled diabetes or who are experiencing alcohol or drug withdrawal), suspicion for posterior shoulder dislocation should be elevated.8
After evaluation in the ED, the patient immediately underwent a nonsurgical closed reduction of the shoulder and suturing of the laceration. He was admitted overnight for further evaluation and was started on an anticonvulsant (levetiracetam). An orthopedic consult was obtained; the dislocation/fracture was managed conservatively with a sling for immobilization. No surgical intervention was recommended, since the patient had a manageable fracture without neurovascular compromise. He was discharged home within 36 hours and scheduled for follow-up appointments with both the neurologist and orthopedic surgeon.
CONCLUSION
This patient had a seizure with an associated fall; both the laceration and the anterior shoulder dislocation with a humeral fracture were associated with the fall and not with tonic-clonic activity from the seizure. Because injuries vary widely from soft tissue to joint dislocations, with possible axillary nerve and/or artery damage, clinicians must do a comprehensive examination of patients entering the ED who have had seizures. Each injury must be addressed individually.
1. Omoumi P, Teixeira P, Lecouvet F, Chung CB. Glenohumeral joint instability. J Magn Reson Imaging. 2010;33(1):2-16.
2. Rouleau DM, Hebert-Davies J. Incidence of associated injury in posterior shoulder dislocation: systematic review of the literature. J Orthop Trauma. 2012;26(4):246-251.
3. Sachit M, Shekhar A, Shekhar S, Joban SH. Acute spontaneous atraumatic bilateral anterior dislocation of the shoulder joint with Hill-Sach’s lesions: a rare case. J Orthop Case Rep. 2015;5(1):55-57.
4. Cutts S, Prempeh M, Drew S. Anterior shoulder dislocation. Ann R Coll Surg Engl. 2009;91(1):2-7.
5. Magee DJ. Orthopedic Physical Assessment. 5th ed. St. Louis, MO. Saunders Elsevier; 2008.
6. Greenspan A. Orthopedic Imaging: A Practical Approach. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
7. Karimi-Nasab MH, Shayesteh-Azar M, Sajjadi-Saravi M, Mehdi Daneshpoor SM. Anterior shoulder dislocation and ipsilateral humeral shaft fracture. Iran J Med Sci. 2012; 37(3):202-204.
8. Robinson CM, Aderinto J. Posterior shoulder dislocations and fracture-dislocations. J Bone Joint Surg Am. 2005; 87(3):639-650.
9. Cacioppo E, Waymack JR. Bilateral inferior shoulder dislocation. West J Emerg Med. 2015;16(1):157.
10. Tiamkao S, Shorvon SD. Seizure-related injury in an adult tertiary epilepsy clinic. Hong Kong Med J. 2006;12(4):260-263.
11. Shaw JL. Bilateral posterior fracture-dislocation of the shoulder and other trauma caused by convulsive seizures. J Bone Joint Surg Am. 1971;53(7):1437-1440.
12. Lawn ND, Bamlet WR, Radhakirshnan K, et al. Injuries due to seizures in persons with epilepsy: a population-based study. Neurology. 2004;63(9):1565-1570.
IN THIS ARTICLE
- Types of shoulder dislocations
- Schematics of the shoulder with three types of dislocations
- Association with seizures
CASE A 59-year-old man with a remote history of seizures is transported to the emergency department (ED) by ambulance after a witnessed tonic-clonic seizure. At the time of arrival he is postictal and confused, but his vital signs are stable. A left eyebrow laceration indicating a possible fall is observed on physical exam, as is a left shoulder displacement with no obvious signs of neurovascular compromise. The patient is not currently taking anticonvulsant medication, stating that he has been “seizure free” for five years, and therefore chose to discontinue taking phenytoin against medical advice.
An anteroposterior (AP) bilateral shoulder x-ray is obtained in the ED (see Figures 1a and 1b). The image shows the humeral head to be anteriorly dislocated and reveals a large impaction fracture of the posterior superior humeral head. For a more detailed view of the fracture and to further assess any associated deformities, CT of the left shoulder is performed. The fracture has a depth of 11.6 mm and a length of 24.1 mm, with no additional pathology noted (see Figure 1c).
The shoulder is a large joint capable of moving in many directions and therefore is inherently unstable. The glenoid fossa is shallow, and stability of the joint is provided by both the fibrocartilaginous labrum and varying muscles of the rotator cuff. Because the shoulder joint is poorly supported, dislocations are not uncommon (see the illustrations).
The first step in evaluating a suspected shoulder dislocation is to order an AP radiographic view of the shoulder (known as the Grashey view). A transcapular view (known as the scapular “Y” view) is also sufficient.1 While diagnostic studies, such as CT or MRI arthrography, are excellent for evaluating the glenohumeral ligaments and labrum, they generally are not done in an acute setting.1 For patients who present to the ED, some would recommend taking a CT scan, especially if a posterior dislocation is suspected.2
The three types of shoulder dislocations include anterior, posterior, and inferior.
ANTERIOR
Anterior dislocations account for 95% of all presented cases of shoulder dislocation, making them the most common type.3 They may be caused by a fall on an outstretched arm, trauma to the posterior humerus, or—more frequently—trauma to the arm while it is extended, externally rotated, and abducted (eg, blocking a shot in basketball).
A patient with an anterior dislocation will enter the ED with a slightly abducted and externally rotated arm (see illustration) and will resist any movement by the examiner. Typically, the shoulder loses its rounded appearance, and in thin individuals, the acromion may be prominent. A detailed neurovascular examination of the arm must be performed.
Dislocation of the humerus in any direction may compromise the axillary nerve, artery, or both. The axillary nerve and artery run parallel to each other, beneath and in close proximity to the humeral head. The axillary artery is located upstream from the radial artery; compression of the artery may lead to a diminution or complete absence of the radial pulse and/or coolness of the hand.4 The axillary nerve is both a sensory and motor nerve. If injured, a 2- to 3-cm area over the lateral deltoid may have complete sensory loss, which can be tested for with a light touch and pinprick.5 The patient may also have difficulty abducting the arm, but limitations of movement are difficult to measure with a new dislocation and a patient in pain.4
Any patient presenting with an anterior shoulder dislocation should also be screened for two other potential abnormalities. Hill-Sachs lesion, which occurs in up to 40% of anterior dislocations and 90% of all dislocations, is a cortical depression occurring in the humeral head. Bankart lesions, which occur in less than 5% of all dislocations, are avulsed bone fragments that occur when there is a glenoid labrum disruption.6 Both can be seen on plain films, although Bankart lesions are best seen on CT.4
The combination of an anterior dislocation and a humeral fracture, as seen in this case, is rare.7
POSTERIOR
Posterior shoulder dislocations occur far less frequently than anterior dislocations, representing 2% to 5% of all shoulder dislocations.2 They often result from blows to the anterior portion of the shoulder (ie, motor vehicle accidents or sports-related collisions) or violent muscle contractions (eg, electrocution, electroconvulsive therapy, or seizures).
Unable to externally rotate the shoulder, patients with posterior dislocations present with the arm in adduction and internal rotation, making the coracoid process prominent (see illustration).8 This position is sometimes misdiagnosed as a “frozen shoulder.”2
INFERIOR
Inferior dislocation of the shoulder is the rarest type, accounting for only 0.5% of all cases of shoulder dislocation. The mechanism of injury is forceful hyperabduction and extension of the shoulder during a fall.
Patients present with the affected arm hyperadducted, flexed at the elbow, with the hand positioned above or behind the head in fixed abduction: a “hands up” position of the affected arm (see illustration). These dislocations are best identified via the transcapular “Y” radiographs. Inferior dislocations are often associated with neurovascular compromise, and there are often related tears of the infraspinatus, supraspinatus, and teres minor muscles.9
ASSOCIATION WITH SEIZURES
Any patient who has had a seizure is subject to a variety of injuries, including lacerations, contusions, long bone and skull fractures, and dislocations. Seizures with a fall are associated with a 20% chance of injury.10
Shaw et al were the first to note that, during an active convulsion, the patient’s shoulder is in adduction, internal rotation, and flexion. This positioning predisposes to injury: With sustained contraction of the surrounding shoulder girdle muscles, the humeral head is forced superiorly and posteriorly against the acromion andmedially against the glenoid fossa. The glenoid fossa is shallow; therefore, the humeral head is forced posteriorly and dislocates.11
Researchers at the Mayo Clinic followed 247 patients who were diagnosed with seizures over nine years; 16% of the cohort experienced seizure-related injuries. Of the seizures recorded, 82% were tonic-clonic seizures. The singular predictive factor for injury was seizure frequency: Patients who had more seizures were more susceptible to injury.12
In an evaluation of outpatients with epilepsy, 25% of recorded seizures involved a fall. Among those who sustained an orthopedic injury, one injury occurred for every 178.6 generalized tonic-clonic seizures (0.6%)—a number that doubled for generalized tonic-clonic seizure associated with a fall (1.2%).10
The collective evidence from these and other studies suggests that patients who have poorly controlled tonic-clonic seizures have a higher incidence of seizures and, therefore, falls and injuries.10,12 In the absence of known trauma, a posterior shoulder dislocation is almost pathognomonic of a seizure. In high-risk populations (ie, individuals who have poorly controlled diabetes or who are experiencing alcohol or drug withdrawal), suspicion for posterior shoulder dislocation should be elevated.8
After evaluation in the ED, the patient immediately underwent a nonsurgical closed reduction of the shoulder and suturing of the laceration. He was admitted overnight for further evaluation and was started on an anticonvulsant (levetiracetam). An orthopedic consult was obtained; the dislocation/fracture was managed conservatively with a sling for immobilization. No surgical intervention was recommended, since the patient had a manageable fracture without neurovascular compromise. He was discharged home within 36 hours and scheduled for follow-up appointments with both the neurologist and orthopedic surgeon.
CONCLUSION
This patient had a seizure with an associated fall; both the laceration and the anterior shoulder dislocation with a humeral fracture were associated with the fall and not with tonic-clonic activity from the seizure. Because injuries vary widely from soft tissue to joint dislocations, with possible axillary nerve and/or artery damage, clinicians must do a comprehensive examination of patients entering the ED who have had seizures. Each injury must be addressed individually.
IN THIS ARTICLE
- Types of shoulder dislocations
- Schematics of the shoulder with three types of dislocations
- Association with seizures
CASE A 59-year-old man with a remote history of seizures is transported to the emergency department (ED) by ambulance after a witnessed tonic-clonic seizure. At the time of arrival he is postictal and confused, but his vital signs are stable. A left eyebrow laceration indicating a possible fall is observed on physical exam, as is a left shoulder displacement with no obvious signs of neurovascular compromise. The patient is not currently taking anticonvulsant medication, stating that he has been “seizure free” for five years, and therefore chose to discontinue taking phenytoin against medical advice.
An anteroposterior (AP) bilateral shoulder x-ray is obtained in the ED (see Figures 1a and 1b). The image shows the humeral head to be anteriorly dislocated and reveals a large impaction fracture of the posterior superior humeral head. For a more detailed view of the fracture and to further assess any associated deformities, CT of the left shoulder is performed. The fracture has a depth of 11.6 mm and a length of 24.1 mm, with no additional pathology noted (see Figure 1c).
The shoulder is a large joint capable of moving in many directions and therefore is inherently unstable. The glenoid fossa is shallow, and stability of the joint is provided by both the fibrocartilaginous labrum and varying muscles of the rotator cuff. Because the shoulder joint is poorly supported, dislocations are not uncommon (see the illustrations).
The first step in evaluating a suspected shoulder dislocation is to order an AP radiographic view of the shoulder (known as the Grashey view). A transcapular view (known as the scapular “Y” view) is also sufficient.1 While diagnostic studies, such as CT or MRI arthrography, are excellent for evaluating the glenohumeral ligaments and labrum, they generally are not done in an acute setting.1 For patients who present to the ED, some would recommend taking a CT scan, especially if a posterior dislocation is suspected.2
The three types of shoulder dislocations include anterior, posterior, and inferior.
ANTERIOR
Anterior dislocations account for 95% of all presented cases of shoulder dislocation, making them the most common type.3 They may be caused by a fall on an outstretched arm, trauma to the posterior humerus, or—more frequently—trauma to the arm while it is extended, externally rotated, and abducted (eg, blocking a shot in basketball).
A patient with an anterior dislocation will enter the ED with a slightly abducted and externally rotated arm (see illustration) and will resist any movement by the examiner. Typically, the shoulder loses its rounded appearance, and in thin individuals, the acromion may be prominent. A detailed neurovascular examination of the arm must be performed.
Dislocation of the humerus in any direction may compromise the axillary nerve, artery, or both. The axillary nerve and artery run parallel to each other, beneath and in close proximity to the humeral head. The axillary artery is located upstream from the radial artery; compression of the artery may lead to a diminution or complete absence of the radial pulse and/or coolness of the hand.4 The axillary nerve is both a sensory and motor nerve. If injured, a 2- to 3-cm area over the lateral deltoid may have complete sensory loss, which can be tested for with a light touch and pinprick.5 The patient may also have difficulty abducting the arm, but limitations of movement are difficult to measure with a new dislocation and a patient in pain.4
Any patient presenting with an anterior shoulder dislocation should also be screened for two other potential abnormalities. Hill-Sachs lesion, which occurs in up to 40% of anterior dislocations and 90% of all dislocations, is a cortical depression occurring in the humeral head. Bankart lesions, which occur in less than 5% of all dislocations, are avulsed bone fragments that occur when there is a glenoid labrum disruption.6 Both can be seen on plain films, although Bankart lesions are best seen on CT.4
The combination of an anterior dislocation and a humeral fracture, as seen in this case, is rare.7
POSTERIOR
Posterior shoulder dislocations occur far less frequently than anterior dislocations, representing 2% to 5% of all shoulder dislocations.2 They often result from blows to the anterior portion of the shoulder (ie, motor vehicle accidents or sports-related collisions) or violent muscle contractions (eg, electrocution, electroconvulsive therapy, or seizures).
Unable to externally rotate the shoulder, patients with posterior dislocations present with the arm in adduction and internal rotation, making the coracoid process prominent (see illustration).8 This position is sometimes misdiagnosed as a “frozen shoulder.”2
INFERIOR
Inferior dislocation of the shoulder is the rarest type, accounting for only 0.5% of all cases of shoulder dislocation. The mechanism of injury is forceful hyperabduction and extension of the shoulder during a fall.
Patients present with the affected arm hyperadducted, flexed at the elbow, with the hand positioned above or behind the head in fixed abduction: a “hands up” position of the affected arm (see illustration). These dislocations are best identified via the transcapular “Y” radiographs. Inferior dislocations are often associated with neurovascular compromise, and there are often related tears of the infraspinatus, supraspinatus, and teres minor muscles.9
ASSOCIATION WITH SEIZURES
Any patient who has had a seizure is subject to a variety of injuries, including lacerations, contusions, long bone and skull fractures, and dislocations. Seizures with a fall are associated with a 20% chance of injury.10
Shaw et al were the first to note that, during an active convulsion, the patient’s shoulder is in adduction, internal rotation, and flexion. This positioning predisposes to injury: With sustained contraction of the surrounding shoulder girdle muscles, the humeral head is forced superiorly and posteriorly against the acromion andmedially against the glenoid fossa. The glenoid fossa is shallow; therefore, the humeral head is forced posteriorly and dislocates.11
Researchers at the Mayo Clinic followed 247 patients who were diagnosed with seizures over nine years; 16% of the cohort experienced seizure-related injuries. Of the seizures recorded, 82% were tonic-clonic seizures. The singular predictive factor for injury was seizure frequency: Patients who had more seizures were more susceptible to injury.12
In an evaluation of outpatients with epilepsy, 25% of recorded seizures involved a fall. Among those who sustained an orthopedic injury, one injury occurred for every 178.6 generalized tonic-clonic seizures (0.6%)—a number that doubled for generalized tonic-clonic seizure associated with a fall (1.2%).10
The collective evidence from these and other studies suggests that patients who have poorly controlled tonic-clonic seizures have a higher incidence of seizures and, therefore, falls and injuries.10,12 In the absence of known trauma, a posterior shoulder dislocation is almost pathognomonic of a seizure. In high-risk populations (ie, individuals who have poorly controlled diabetes or who are experiencing alcohol or drug withdrawal), suspicion for posterior shoulder dislocation should be elevated.8
After evaluation in the ED, the patient immediately underwent a nonsurgical closed reduction of the shoulder and suturing of the laceration. He was admitted overnight for further evaluation and was started on an anticonvulsant (levetiracetam). An orthopedic consult was obtained; the dislocation/fracture was managed conservatively with a sling for immobilization. No surgical intervention was recommended, since the patient had a manageable fracture without neurovascular compromise. He was discharged home within 36 hours and scheduled for follow-up appointments with both the neurologist and orthopedic surgeon.
CONCLUSION
This patient had a seizure with an associated fall; both the laceration and the anterior shoulder dislocation with a humeral fracture were associated with the fall and not with tonic-clonic activity from the seizure. Because injuries vary widely from soft tissue to joint dislocations, with possible axillary nerve and/or artery damage, clinicians must do a comprehensive examination of patients entering the ED who have had seizures. Each injury must be addressed individually.
1. Omoumi P, Teixeira P, Lecouvet F, Chung CB. Glenohumeral joint instability. J Magn Reson Imaging. 2010;33(1):2-16.
2. Rouleau DM, Hebert-Davies J. Incidence of associated injury in posterior shoulder dislocation: systematic review of the literature. J Orthop Trauma. 2012;26(4):246-251.
3. Sachit M, Shekhar A, Shekhar S, Joban SH. Acute spontaneous atraumatic bilateral anterior dislocation of the shoulder joint with Hill-Sach’s lesions: a rare case. J Orthop Case Rep. 2015;5(1):55-57.
4. Cutts S, Prempeh M, Drew S. Anterior shoulder dislocation. Ann R Coll Surg Engl. 2009;91(1):2-7.
5. Magee DJ. Orthopedic Physical Assessment. 5th ed. St. Louis, MO. Saunders Elsevier; 2008.
6. Greenspan A. Orthopedic Imaging: A Practical Approach. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
7. Karimi-Nasab MH, Shayesteh-Azar M, Sajjadi-Saravi M, Mehdi Daneshpoor SM. Anterior shoulder dislocation and ipsilateral humeral shaft fracture. Iran J Med Sci. 2012; 37(3):202-204.
8. Robinson CM, Aderinto J. Posterior shoulder dislocations and fracture-dislocations. J Bone Joint Surg Am. 2005; 87(3):639-650.
9. Cacioppo E, Waymack JR. Bilateral inferior shoulder dislocation. West J Emerg Med. 2015;16(1):157.
10. Tiamkao S, Shorvon SD. Seizure-related injury in an adult tertiary epilepsy clinic. Hong Kong Med J. 2006;12(4):260-263.
11. Shaw JL. Bilateral posterior fracture-dislocation of the shoulder and other trauma caused by convulsive seizures. J Bone Joint Surg Am. 1971;53(7):1437-1440.
12. Lawn ND, Bamlet WR, Radhakirshnan K, et al. Injuries due to seizures in persons with epilepsy: a population-based study. Neurology. 2004;63(9):1565-1570.
1. Omoumi P, Teixeira P, Lecouvet F, Chung CB. Glenohumeral joint instability. J Magn Reson Imaging. 2010;33(1):2-16.
2. Rouleau DM, Hebert-Davies J. Incidence of associated injury in posterior shoulder dislocation: systematic review of the literature. J Orthop Trauma. 2012;26(4):246-251.
3. Sachit M, Shekhar A, Shekhar S, Joban SH. Acute spontaneous atraumatic bilateral anterior dislocation of the shoulder joint with Hill-Sach’s lesions: a rare case. J Orthop Case Rep. 2015;5(1):55-57.
4. Cutts S, Prempeh M, Drew S. Anterior shoulder dislocation. Ann R Coll Surg Engl. 2009;91(1):2-7.
5. Magee DJ. Orthopedic Physical Assessment. 5th ed. St. Louis, MO. Saunders Elsevier; 2008.
6. Greenspan A. Orthopedic Imaging: A Practical Approach. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
7. Karimi-Nasab MH, Shayesteh-Azar M, Sajjadi-Saravi M, Mehdi Daneshpoor SM. Anterior shoulder dislocation and ipsilateral humeral shaft fracture. Iran J Med Sci. 2012; 37(3):202-204.
8. Robinson CM, Aderinto J. Posterior shoulder dislocations and fracture-dislocations. J Bone Joint Surg Am. 2005; 87(3):639-650.
9. Cacioppo E, Waymack JR. Bilateral inferior shoulder dislocation. West J Emerg Med. 2015;16(1):157.
10. Tiamkao S, Shorvon SD. Seizure-related injury in an adult tertiary epilepsy clinic. Hong Kong Med J. 2006;12(4):260-263.
11. Shaw JL. Bilateral posterior fracture-dislocation of the shoulder and other trauma caused by convulsive seizures. J Bone Joint Surg Am. 1971;53(7):1437-1440.
12. Lawn ND, Bamlet WR, Radhakirshnan K, et al. Injuries due to seizures in persons with epilepsy: a population-based study. Neurology. 2004;63(9):1565-1570.
Current Therapeutic Approaches to Renal Cell Carcinoma
INTRODUCTION
Renal cell carcinoma (RCC) is the most common malignancy arising in the kidney, comprising 90% of all renal tumors.1 Approximately 55,000 new RCC cases are diagnosed each year.2 Patients with RCC are often asymptomatic, and most cases are discovered as incidental findings on abdominal imaging performed during evaluation of nonrenal complaints. Limited-stage RCC that is found early can be cured surgically, with estimated 5-year survival rates approaching 90%; however, long-term survival for metastatic disease is poor, with rates ranging from 0% to 20%.2 Advanced RCC is resistant to conventional chemotherapy and radiotherapy, and outcomes for patients with metastatic or unresectable RCC remain poor. However, the recent development of new therapeutic modalities that target tumor molecular pathways has expanded the treatment options for these patients and changed the management of RCC.
EPIDEMIOLOGY AND CLASSIFICATION
Median age at diagnosis in the United States is 64 years. Men have a higher incidence of RCC than women, with the highest incidence seen in American Indian and Alaska Native men (30.1 per 100,000 population). Genetic syndromes account for 2% to 4% of all RCCs.2 Risk factors for RCC include smoking, hypertension, obesity, and acquired cystic kidney disease that is associated with end-stage renal failure.3 Longer duration of tobacco use is associated with a more aggressive course.
The 2004 World Health Organization (WHO) classification of renal tumors summarizes the previous classification systems (including the Heidelberg and Mainz classification systems) to describe different categories of RCC based on histologic and molecular genetics characteristics.2 Using the WHO classification criteria, RCC comprises 90% of all renal tumors, with clear cell being the most common type (80%).2 Other types of renal tumors include papillary, chromophobe, oncocytoma, and collecting-duct or Bellini duct tumors. Approximately 3% to 5% of tumors are unclassified. Oncocytomas are generally considered benign, and chromophobe tumors typically have an indolent course and rarely metastasize. Sarcomatoid differentiation can be seen in any histologic type and is associated with a worse prognosis. While different types of tumors may be seen in the kidney (such as transitional cell or lymphomas), the focus of this review is the primary malignancies of the renal parenchyma.
FAMILIAL SYNDROMES
Several genetic syndromes have been identified by studying families with inherited RCC. Among these, von Hippel-Lindau (VHL) gene mutation is the most commonly found inherited genetic defect. Table 1 summarizes the incidence of gene mutations and the corresponding histologic appearance of the most common sporadic and hereditary RCCs.4
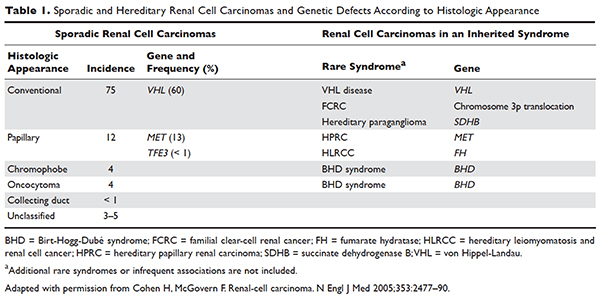
VHL disease is an autosomal dominant familial syndrome. Patients with this mutation are at higher risk for developing RCC (clear cell histology), retinal angiomas, pheochromocytomas, as well as hemangioblastomas of the central nervous system (CNS).4 Of all the genetic mutations seen in RCC, the somatic mutation in the VHL tumor-suppressor gene is by far the most common.5 VHL targets hypoxia–inducible factor-1 alpha (HIF-α) for ubiquitination and subsequent degradation, which has been shown to suppress the growth of clear-cell RCC in mouse models.6–8 HIF expression under hypoxic conditions leads to activation of a number of genes important in blood vessel development, cell proliferation, and glucose metabolism, including vascular endothelial growth factor (VEGF), erythropoietin, platelet-derived growth factor beta (PDGF-β), transforming growth factor alpha (TGF-α), and glucose transporter-1 (GLUT-1). Mutation in the VHL gene prevents degradation of the HIF-α protein, thereby leading to increased expression of these downstream proteins, including MET and Axl. The upregulation of these angiogenic factors is thought to be the underlying process for increased vascularity of CNS hemangioblastomas and clear-cell renal tumors in VHL disease.4–8
Other less common genetic syndromes seen in hereditary RCC include hereditary papillary RCC, hereditary leiomyomatosis, and Birt-Hogg-Dubé (BHD) syndrome.9 In hereditary papillary RCC, the MET gene is mutated. BHD syndrome is a rare, autosomal dominant syndrome characterized by hair follicle hamartomas of the face and neck. About 15% of patients have multiple renal tumors, the majority of which are of the chromophobe or mixed chromophobe-oncocytoma histology. The BHD gene encodes the protein folliculin, which is thought to be a tumor-suppressor gene.
DIAGNOSIS AND STAGING
CASE PRESENTATION
A 74-year-old man who works as an airplane mechanic repairman presents to the emergency department with sudden worsening of chronic right upper arm and shoulder pain after lifting a jug of orange juice. He does not have a significant past medical history and initially thought that his pain was due to a work-related injury. Upon initial evaluation in the emergency department he is found to have a fracture of his right humerus. Given that the fracture appears to be pathologic, further work-up is recommended.
• What are common clinical presentations of RCC?
Most patients are asymptomatic until the disease becomes advanced. The classic triad of flank pain, hematuria, and palpable abdominal mass is seen in approximately 10% of patients with RCC, partly because of earlier detection of renal masses by imaging performed for other purposes.10 Less frequently, patients present with signs or symptoms of metastatic disease such as bone pain or fracture (as seen in the case patient), painful adenopathy, and pulmonary symptoms related to mediastinal masses. Fever, weight loss, anemia, and/or varicocele often occur in young patients (≤ 46 years) and may indicate the presence of a hereditary form of the disease. Patients may present with paraneoplastic syndromes seen as abnormalities on routine blood work. These can include polycythemia or elevated liver function tests (LFTs) without the presence of liver metastases (known as Stauffer syndrome), which can be seen in localized renal tumors. Nearly half (45%) of patients present with localized disease, 25% present with locally advanced disease, and 30% present with metastatic disease.11 Bone is the second most common site of distant metastatic spread (following lung) in patients with advanced RCC.
• What is the approach to initial evaluation for a patient with suspected RCC?
Initial evaluation consists of a physical exam, laboratory tests including complete blood count (CBC) and comprehensive metabolic panel (calcium, serum creatinine, LFTs, lactate dehydrogenase [LDH], and urinalysis), and imaging. Imaging studies include computed tomography (CT) scan with contrast of the abdomen and pelvis or magnetic resonance imaging (MRI) of the abdomen and chest imaging. A chest radiograph may be obtained, although a chest CT is more sensitive for the presence of pulmonary metastases. MRI can be used in patients with renal dysfunction to evaluate the renal vein and inferior vena cava (IVC) for thrombus or to determine the presence of local invasion.12 Although bone and brain are common sites for metastases, routine imaging is not indicated unless the patient is symptomatic. The value of positron emission tomography in RCC remains undetermined at this time.
Staging is done according to the American Joint Committee on Cancer (AJCC) staging classification for RCC; the Figure summarizes the staging and 5-year survival data based on this classification scheme.4,13
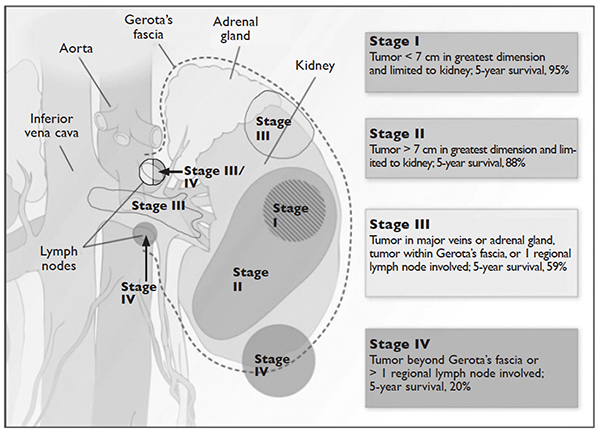
J Med 2005;353:2477–90.)
LIMITED-STAGE DISEASE
• What are the therapeutic options for limited-stage disease?
For patients with nondistant metastases, or limited-stage disease, surgical intervention with curative intent is considered. Convention suggests considering definitive surgery for patients with stage I and II disease, select patients with stage III disease with pathologically enlarged retroperitoneal lymph nodes, patients with IVC and/or cardiac atrium involvement of tumor thrombus, and patients with direct extension of the renal tumor into the ipsilateral adrenal gland if there is no evidence of distant disease. While there may be a role for aggressive surgical intervention in patients with distant metastatic disease, this topic will not be covered in this review.
SURGICAL INTERVENTION
Once patients are determined to be appropriate candidates for surgical removal of a renal tumor, the urologist will perform either a radical nephrectomy or a nephron-sparing nephrectomy, also called a partial nephrectomy. The urologist will evaluate the patient based on his or her body habitus, the location of the tumor, whether multiple tumors in one kidney or bilateral tumors are present, whether the patient has a solitary kidney or otherwise impaired kidney function, and whether the patient has a history of a hereditary syndrome involving kidney cancer as this affects the risk of future kidney tumors.
A radical nephrectomy is surgically preferred in the presence of the following factors: tumor larger than 7 cm in diameter, a more centrally located tumor, suspicion of lymph node involvement, tumor involvement with renal vein or IVC, and/or direct extension of the tumor into the ipsilateral adrenal gland. Nephrectomy involves ligation of the vascular supply (renal artery and vein) followed by removal of the kidney and surrounding Gerota’s fascia. The ipsilateral adrenal gland is removed if there is a high-risk for or presence of invasion of the adrenal gland. Removal of the adrenal gland is not standard since the literature demonstrates there is less than a 10% chance of solitary, ipsilateral adrenal gland involvement of tumor at the time of nephrectomy in the absence of high-risk features, and a recent systematic review suggests that the chance may be as low as 1.8%.14 Preoperative factors that correlated with adrenal involvement included upper pole kidney location, renal vein thrombosis, higher T stage (T3a and greater), multifocal tumors, and evidence for distant metastases or lymph node involvement. Lymphadenectomy previously had been included in radical nephrectomy but now is performed selectively. Radical nephrectomy may be performed as
either an open or laparoscopic procedure, the latter of which may be performed robotically.15 Oncologic outcomes appear to be comparable between the 2 approaches, with equivalent 5-year cancer-specific survival (91% with laparoscopic versus 93% with open approach) and recurrence-free survival (91% with laparoscopic versus 93% with open approach).16 The approach ultimately is selected based on provider- and patient-specific input, though in all cases the goal is to remove the specimen intact.16,17
Conversely, a nephron-sparing approach is preferred for tumors less than 7 cm in diameter, for patients with a solitary kidney or impaired renal function, for patients with multiple small ipsilateral tumors or with bilateral tumors, or for radical nephrectomy candidates with comorbidities for whom a limited intervention is deemed to be a lower-risk procedure. A nephron-sparing procedure may also be performed open or laparoscopically. In nephron-sparing procedures, the tumor is removed along with a small margin of normal parenchyma.15
In summary, the goal of surgical intervention is curative intent with removal of the tumor while maintaining as much residual renal function as possible to limit long-term morbidity of chronic kidney disease and associated cardiovascular events.18 Oncologic outcomes for radical nephrectomy and partial nephrectomy are similar. In one study, overall survival was slightly lower in the partial nephrectomy cohort, but only a small number of the deaths were due to RCC.19
ADJUVANT THERAPY
Adjuvant systemic therapy currently has no role following nephrectomy for RCC because no systemic therapy has been able to reduce the likelihood of relapse. Randomized trials of cytokine therapy (eg, interferon, interleukin 2) or tyrosine kinase inhibitors (TKIs; eg, sorafenib, sunitinib) with observation alone in patients with locally advanced completely resected RCC have shown no delay in time to relapse or improvement of survival with adjuvant therapy.20 Similarly, adjuvant radiation therapy has not shown benefit even in patients with nodal involvement or incomplete resection.21 Therefore, observation remains the standard of care after nephrectomy.
RENAL TUMOR ABLATION
For patients who are deemed not to be surgical candidates due to age, comorbidities, or patient preference and who have tumors less than 4 cm in size (stage I tumors), ablative techniques may be considered. The 2 most well-studied and effective techniques at present are cryoablation and radiofrequency ablation (RFA). Microwave ablation may be an option in some facilities, but the data in RCC are limited. An emerging ablative technique under investigation is irreversible electroporation. At present, the long-term efficacy of all ablative techniques is unknown.
Patient selection is undertaken by urologists and interventional radiologists who evaluate the patient with ultrasound, CT, and/or MRI to determine the location and size of the tumor and the presence or absence of metastatic disease. A pretreatment biopsy is recommended to document the histology of the lesion to confirm a malignancy and to guide future treatment for recurrent or metastatic disease. Contraindications to the procedure include the presence of metastatic disease, a life expectancy of less than 1 year, general medical instability, or uncorrectable coagulopathy due to increased risk of bleeding complications. Tumors in close proximity to the renal hilum or collecting system are a contraindication to the procedure because of the risk for hemorrhage or damage to the collecting system. The location of the tumor in relation to the vasculature is also important to maximize efficacy because the vasculature acts as a “heat sink,” causing dissipation of the thermal energy. Occasionally, stenting of the proximal ureter due to upper tumor location is necessary to prevent thermal injury that could lead to urine leaks.
Selection of the modality to be used primarily depends on operator comfort, which translates to good patient outcomes, such as better cancer control and fewer complications. Cryoablation and RFA have both demonstrated good clinical efficacy and cancer control of 89% and 90%, respectively, with comparable complication rates.22 There have been no studies performed directly comparing the modalities.
Cryoablation
Cryoablation is performed through the insertion of a probe into the tumor, which may be done through a surgical or percutaneous approach. Once the probe is in place, a high- pressure gas (argon, nitrogen) is passed through the probe and upon entering a low pressure region the gas cools. The gas is able to cool to temperatures as low as –185°C. The tissue is then rewarmed through the use of helium, which conversely warms when entering a low pressure area. The process of freezing followed by rewarming subsequently causes cell death/tissue destruction through direct cell injury from cellular dehydration and vascular injury. Clinically, 2 freeze-thaw cycles are used to treat a tumor.23,24
RFA
Radiofrequency ablation, or RFA, targets tumors via an electrode placed within the mass that produces intense frictional heat from medium-frequency alternating current (approximately 500 kHz) produced by a connected generator that is grounded on the patient. The thermal energy created causes coagulative necrosis. Due to the reliance on heat for tumor destruction, central lesions are less amenable to this approach because of the “heat sink” effect from the hilum.24
Microwave Ablation
Microwave ablation, like RFA, relies on the generation of frictional heat to cause cell death by coagulative necrosis. In this case, the friction is created through the activation of water molecules; because of the different thermal kinetics involved with microwave ablation, the “heat sink” effect is minimized when treatment is employed near large vessels, in comparison to RFA.24 The data on this mechanism of ablation are still maturing, with varied outcomes thus far. One study demonstrated outcomes comparable to RFA and cryoablation, with cancer-specific survival of 97.8% at 3 years.25 However, a study by Castle and colleagues26 demonstrated higher recurrence rates. The overarching impediment to widespread adoption of microwave ablation is inconclusive data gleaned from studies with small numbers of patients with limited follow up. The role of this modality will need to be revisited.
Irreversible Electroporation
Irreversible electroporation (IRE) is under investigation. IRE is a non-thermal ablative technique that employs rapid electrical pulses to create pores in cell membranes, leading to cell death. The postulated benefits of IRE include the lack of an effect from “heat sinks” and less collateral damage to the surrounding tissues, when compared with the thermal modalities. In a human phase 1 study of patients undergoing IRE prior to immediate surgical resection, the procedure appeared feasible and safe.27 Significant concerns for this method of ablation possibly inducing cardiac arrhythmias, and the resultant need for sedation with neuromuscular blockade and associated electrocardiography monitoring, may impede its implementation in nonresearch settings.24
ACTIVE SURVEILLANCE
Due to the more frequent use of imaging for various indications, there has been an increase in the discovery of small renal masses (SRM); 85% of RCC that present in an asymptomatic or incidental manner are tumors under 4 cm in diameter.28,29 The role of active surveillance is evolving, but is primarily suggested for patients who are not candidates for more aggressive intervention based on comorbidities. A recent prospective, nonrandomized analysis of data from the Delayed Intervention and Surveillance for Small Renal Masses (DISSRM) registry evaluated outcomes for patients with SRM looking at primary intervention compared with active surveillance.30 The primary intervention selected was at the discretion of the provider; treatments included partial nephrectomy, RFA, and cryoablation, and active surveillance patients were followed with imaging every 6 months. Progression of SRM, with recommendation for delayed intervention, was defined as a growth rate of mass greater than 0.5 cm/year, size greater than 4 cm, or hematuria. Thirty-six of 158 patients on active surveillance met criteria for progression; 21 underwent delayed intervention. Of note, even the patients who progressed but did not undergo delayed intervention did not develop metastatic disease during the follow-up interval. With a median follow-up of 2 years, cancer-specific survival was noted to be 99% and 100% at 5 years for primary intervention and active surveillance, respectively. Overall survival at 2 years for primary intervention was 98% and 96% for active surveillance; at 5 years, the survival rates were 92% and 75% (P = 0.06). Of note, 2 patients in the primary intervention arm died of RCC, while none in the active surveillance arm died. As would be expected, active surveillance patients were older, had a worse performance status, and had more comorbidities. Interestingly, 40% of patients enrolled selected active surveillance as their preferred management for SRM. The DISSRM results were consistent with data from the Renal Cell Consortium of Canada and other retrospective reviews.31–33
• What is the approach to follow-up after treatment of localized RCC?
After a patient undergoes treatment for a localized RCC, the goal is to optimize oncologic outcomes, monitor for treatment sequelae, such as renal failure, and focus on survivorship. At this time, there is no consensus in the literature or across published national and international guidelines with regards to the appropriate schedule for surveillance to achieve these goals. In principle, the greatest risk for recurrence occurs within the first 3 years, so many guidelines focus on this timeframe. Likewise, the route of spread tends to be hematogenous, so patients present with pulmonary, bone, and brain metastases, in addition to local recurrence within the renal bed. Symptomatic recurrences often are seen
with bone and brain metastases, and thus bone scans and brain imaging are not listed as part of routine surveillance protocols in asymptomatic patients. Although there is inconclusive evidence that surveillance protocols improve outcomes in RCC, many professional associations have outlined recommendations based on expert opinion.34 The American Urological Association released guidelines in 2013 and the National Comprehensive Cancer Network (NCCN) released their most recent set of guidelines in 2016.21,35 These guidelines use TNM staging to risk-stratify patients and recommend follow-up.
METASTATIC DISEASE
CASE CONTINUED
CT scan with contrast of the chest, abdomen, and pelvis as well as bone scan are done. CT of the abdomen and pelvis demonstrates a 7.8-cm left renal mass arising from the lower pole of the left kidney. Paraesophageal lymphadenopathy and mesenteric nodules are also noted. CT of the chest demonstrates bilateral pulmonary emboli. Bone scan is significant for increased activity related to the pathological fracture involving the right humerus. The patient undergoes surgery to stabilize the pathologic fracture of his humerus. He is diagnosed with metastatic RCC (clear cell histology) and undergoes palliative debulking nephrectomy.
• How is prognosis defined for metastatic RCC?
PROGNOSTIC MODELS
Limited-stage RCC that is found early can be cured surgically, with estimated 5-year survival rates for stage T1 and T2 disease approaching 90%; however, long-term survival for metastatic disease is poor, with rates ranging from 0% to 20%.13 Approximately 30% of patients have metastatic disease at diagnosis, and about one-third of patients who have undergone treatment for localized disease experience relapse.36,37 Common sites of metastases include lung, lymph nodes, bone, liver, adrenal gland, and brain.
Prognostic scoring systems have been developed to define risk groups and assist with determining appropriate therapy in the metastatic setting. The most widely used validated prognostic factor model is that from the Memorial Sloan-Kettering Cancer Center (MSKCC), which was developed using a multivariate analysis derived from data of patients enrolled in clinical trials and treated with interferon alfa.38 The factors included in the MSKCC model are Karnofsky performance status less than 80, time from diagnosis to treatment with interferon alfa less than 12 months, hemoglobin level less than lower limit of laboratory’s reference range, LDH level greater than 1.5 times the upper limit of laboratory’s reference range, and corrected serum calcium level greater than 10 mg/dL. Risk groups are categorized as favorable (0 risk factors), intermediate (1 to 2 risk factors), and poor (3 or more risk factors).39 Median survival for favorable-, intermediate-, and poor-risk patients was 20, 10, and 4 months, respectively.40
Another prognostic model, the International Metastatic RCC Database Consortium, or Heng, model was developed to evaluate prognosis in patients treated with VEGF-targeted therapy.41 This model was developed from a retrospective study of patients treated with sunitinib, sorafenib, and bevacizumab plus interferon alfa or prior immunotherapy. Prognostic factors in this model include 4 of the 5 MSKCC risk factors (hemoglobin level, corrected serum calcium level, Karnofsky performance status, and time to initial diagnosis). Additionally, this model includes both absolute neutrophil and platelet counts greater than the upper limit of normal. Risk groups are identified as favorable (0 risk factors), intermediate (1 to 2 risk factors), and poor (3 or more risk factors). Median survival for favorable-, intermediate-, and poor-risk patients was not reached, 27 months, and 8.8 months, respectively. The University of California, Los Angeles scoring algorithm to predict survival after nephrectomy and immunotherapy (SANI) in patients with metastatic RCC is another prognostic model that can be used. This simplified scoring system incorporates lymph node status, constitutional symptoms, metastases location, histology, and thyroid stimulating hormone (TSH) level.42
The role of debulking or cytoreductive nephrectomy in treatment of metastatic RCC is well established. Large randomized studies have demonstrated a statistically significant median survival benefit for patients undergoing nephrectomy plus interferon alfa therapy compared with patients treated with interferon alfa alone (13.6 months versus 7.8 months, respectively).43 The role of cytoreductive nephrectomy in combination with antiangiogenic agents is less clear. While a retrospective study investigating outcomes of patients with metastatic RCC receiving anti-VEGF agents showed a prolonged survival with nephrectomy, results of large randomized trials are not yet available.44,45 Patients with lung-only metastases, good prognostic features, and a good performance status are historically the most likely to benefit from cytoreductive surgery.
CASE CONTINUED
Based on the MSKCC prognostic factor model, the patient is considered to be in the intermediate-risk group (Karnofsky performance status of 80, calcium 9.5 mg/dL, LDH 204 U/L, hemoglobin 13.6 g/dL). He is started on treatment for his bilateral pulmonary emboli and recovers well from orthopedic surgery as well as palliative debulking nephrectomy.
• What is the appropriate first-line therapy in managing this patient’s metastatic disease?
Several approaches to systemic therapy for advanced RCC have been taken based on the histologic type of the tumor. Clear-cell is by far the predominant histologic type in RCC. Several options are available as first-line treatment for patients with metastatic clear-cell RCC (Table 2).46–54 These include biologic agents such as high-dose interleukin-2 (IL-2) immune therapy, as well as targeted therapies including TKIs and anti-VEGF antibodies. The mammalian target of rapamycin (mTOR) inhibitor temsirolimus is recommended as first-line therapy in patients with poor prognosis only. Second-line therapies for clear-cell RCC following antiangiogenic therapy include TKIs, mTOR inhibitors, nivolumab (PD-1 inhibitor), and the combination of the TKI lenvatinib and mTOR inhibitor everolimus.55 In addition, after initial cytokine therapy, TKIs, temsirolimus, and the anti-VEGF antibody bevacizumab are other treatment options available to patients. Best supportive care should always be provided along with initial and subsequent therapies. Clinical trials are also an appropriate choice as first-line or subsequent therapies. All of these therapies require periodic monitoring to prevent and quickly treat adverse effects. Table 3 lists recommended monitoring parameters for each of these agents.56
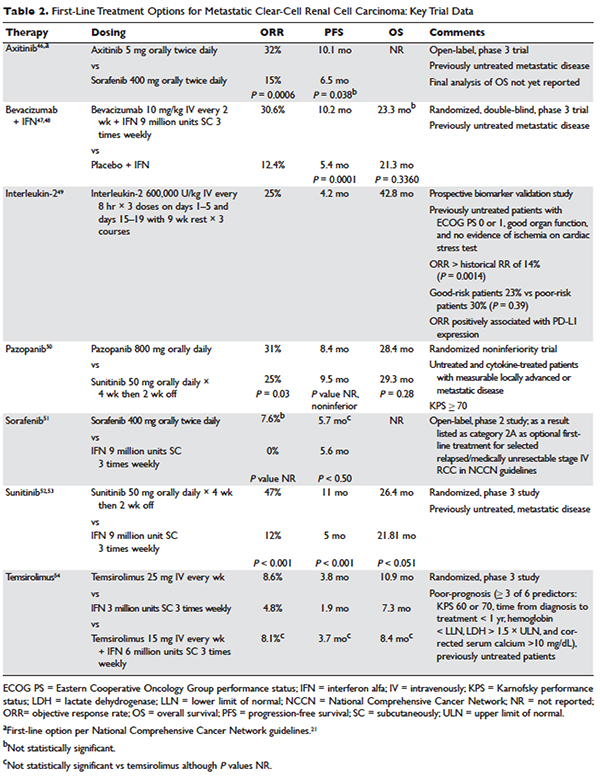
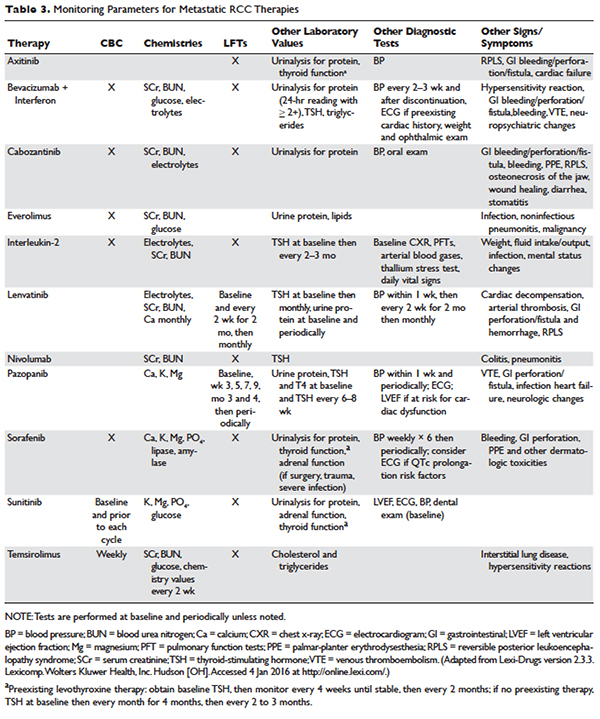
Based on several studies, TKIs seem to be less effective in patients with non–clear-cell type histology.57,58 In these patients, risk factors can guide therapy. In the ASPEN trial, where 108 patients were randomly assigned to everolimus or sunitinib, patients in the good- and intermediate-risk groups had longer overall and median progression-free survival (PFS) on sunitinib (8.3 months versus 5.3 months, respectively). However, those in the poor-risk group had a longer median overall survival with everolimus.59 Given that the role of targeted therapies in non–clear-cell RCCs is less well established, enrollment in clinical trials should be considered as a first-line treatment option.21
Sarcomatoid features can be observed in any of the histologic types of RCC, and RCC with these features has an aggressive course and a poor prognosis. Currently, there is no standard therapy for treatment of patients with metastatic or unresectable RCC with sarcomatoid features.60 Chemotherapeutic regimens used for soft tissue sarcomas, including a trial of ifosfamide and doxorubicin, did not show any objective response.61 A small trial of 10 patients treated with doxorubicin and gemcitabine resulted in complete response in 2 patients and partial response in 1 patient.62
Enrollment in a clinical trial remains a first-line treatment option for these patients. More recently, a phase 2 trial of sunitinib and gemcitabine in patients with sarcomatoid (39 patients) and/or poor-risk (33 patients) metastatic RCC showed overall response rates (ORR) of 26% and 24%, respectively. A higher clinical benefit rate (defined as ORR plus stable disease) was seen in patients with tumors containing more than 10% sarcomatoid histology, as compared with patients whose tumors contained less than 10% sarcomatoid histology. Neutropenia (n = 20), anemia (n = 10), and fatigue (n = 7) were the most common grade 3 toxicities seen in all the patients. Although this was a small study, the results showed a trend towards better efficacy of the combination therapy as compared with the single-agent regimen. Currently, another study is underway to further investigate this in a larger group of patients.63
BIOLOGICS
Cytokine therapy, including high-dose IL-2 and interferon alfa, had long been the only first-line treatment option for patients with metastatic or unresectable RCC. Studies of high-dose IL-2 have shown an ORR of 25% and durable response in up to 11% of patients with clear-cell histology.64 Toxicities were similar to those previously observed with high-dose IL-2 treatment; the most commonly observed grade 3 toxicities were hypotension and capillary leak syndrome. IL-2 requires strict monitoring (Table 3). It is important to note that retrospective studies evaluating the safety and efficacy of using IL-2 as second-line treatment in patients previously treated with TKIs demonstrated significant toxicity without achieving partial or complete response in any of the patients.65
Prior to the advent of TKIs in the treatment of RCC, interferon alfa was a first-line treatment option for those who could not receive high-dose IL-2. It has been shown to produce response rates of approximately 20%, with maximum response seen with a higher dose range of 5 to 20 million units daily in 1 study.66,67 However, with the introduction of TKIs, which produce a higher and more durable response, interferon alfa alone is no longer recommended as a treatment option.
VEGF MONOCLONAL ANTIBODIES
Bevacizumab is a recombinant humanized monoclonal antibody that binds and neutralizes VEGF-A. Given overexpression of VEGF in RCC, the role of bevacizumab both as a single agent and in combination with interferon alfa has been investigated. In a randomized phase 2 study involving patients with cytokine-refractory disease, bevacizumab produced a 10% response rate and PFS of 4.8 months compared to patients treated with placebo.68 In the AVOREN trial, the addition of bevacizumab (10 mg/kg intravenously [IV] every 2 weeks) to interferon alfa (9 million units subcutaneously [SC] 3 times weekly) was shown to significantly increase PFS compared with interferon alfa alone (10.2 months versus 5.4 months; P = 0.0001).47,48 Adverse effects of this combination therapy include fatigue and asthenia. Additionally, hypertension, proteinuria, and bleeding occurred.
TYROSINE KINASE INHIBITORS
TKIs have largely replaced IL-2 as first-line therapy for metastatic RCC. Axitinib, pazopanib, sorafenib, and sunitinib and can be used as first-line therapy. All of the TKIs can be used as subsequent therapy.
Sunitinib
Sunitinib is an orally administered TKI that inhibits VEGF receptor (VEGFR) types 1 and 2, PDGF receptors (PDGFR) α and β, stem cell factor receptor (c-Kit), and FLT-3 and RET kinases. Motzer and colleagues52,53 compared sunitinib 50 mg daily orally for 4 weeks with 2 weeks off to the then standard of care, interferon alfa 9 million units SC 3 times weekly. Sunitinib significantly increased the overall objective response rate (47% versus 12%; P < 0.001), PFS (11 versus 5 months; P < 0.001), and overall survival (26.4 versus 21.8 months; hazard ratio [HR], 0.821). The most common side effects are diarrhea, fatigue, nausea/vomiting, anorexia, hypertension, stomatitis, and hand-foot syndrome, occurring in more than 30% of patients. Often patients will require dose reductions or temporary discontinuations to tolerate therapy. Alternative dosing strategies (eg, 50 mg dose orally daily for 2 weeks alternating with 1-week free interval) have been attempted but not prospectively evaluated for efficacy.69–71
Pazopanib
Pazopanib is an oral multi-kinase inhibitor of VEGFR types 1 and 2, PDGFR, and c-KIT. Results of a phase 3 trial comparing pazopanib (800 mg orally daily) to placebo favored the TKI, with a PFS of 9.2 months versus 4.2 months. A subset of treatment-naïve patients had a longer PFS of 11.1 versus 2.8 months and a response rate of 32% versus 4%.72 This led to a noninferiority phase 3 trial comparing pazopanib with sunitinib as first-line therapy.50 In this study, PFS was similar (8.4 versus 9.5 months; HR 1.05), and overall safety and quality-of-life endpoints favored pazopanib. Much less fatigue, stomatitis, hand-foot syndrome, and thrombocytopenia occurred with pazopanib, whereas hair color changes, weight loss, alopecia, and elevations of LFT enzymes occurred more frequently with pazopanib. Hypertension is common with the administration of pazopanib as well.
Sorafenib
Sorafenib is an orally administered inhibitor of Raf, serine/threonine kinase, VEGFR, PDGFR, FLT-3, c-Kit, and RET. The pivotal phase 3 Treatment Approaches in Renal Cancer Global Evaluation Trial (TARGET) compared sorafenib (400 mg orally twice daily) with placebo in patients who had progressed on prior cytokine-based therapy.73 A final analysis, which excluded patients who were allowed to cross over therapies, found improved overall survival times (14.3 versus 1.8 months, P = 0.029).51 Sorafenib is associated with lower rates of diarrhea, rash, fatigue, hand-foot syndrome, alopecia, hypertension, and nausea than sunitinib, although these agents have not been compared to one another.
Axitinib
Axitinib is an oral inhibitor of VEGFRs 1, 2, and 3. Results of the phase 3 AXIS trial comparing axitinib (5 mg orally twice daily) with sorafenib (400 mg orally twice daily) in patients receiving 1 prior systemic therapy showed axitinib was more active than sorafenib in improving ORR (19% versus 9%; P = 0.001) and PFS (6.7 versus 4.7 months; P < 0.001), although no difference in overall survival times was noted.74 In a subsequent phase 3 trial comparing these drugs in the first-line setting, axitinib showed a nonsignificantly higher response rate and PFS. Despite this, the National Comprehensive Cancer Network guidelines consider axitinib an acceptable first-line therapy because activity with acceptable toxicity was demonstrated (Table 2).46 The most common adverse effects of axitinib are diarrhea, hypertension, fatigue, decreased appetite, dysphonia, hypothyroidism, and upper abdominal pain.
CABOZANTINIB
Given that resistance eventually develops in most patients treated with standard treatments, including bevacizumab and TKIs, the need to evaluate the safety and efficacy of novel agents targeting VEGFR and overcoming this resistance is of vital importance. Cabozantinib is an oral small-molecule inhibitor of VEGFR, Met, and Axl, all tyrosine kinases implicated in metastatic RCC. Overexpression of Met and Axl, which occurs as a result of inactivation of the VHL gene, is associated with a poor prognosis in patients with RCC. In a
randomized, open label, phase 3 trial of cabozantinib versus everolimus in advanced RCC, Choueiri and colleagues75 compared the efficacy of cabozantinib with everolimus in patients with metastatic RCC who had progressed on previous VEGFR-targeted therapies. In this study, 658 patients were randomly assigned to receive cabozantinib (60 mg orally daily) or everolimus (10 mg orally daily). Results of the study found that PFS was longer with cabozantinib in patients who had previously been treated with other TKIs (median PFS of 7.4 months versus 3.8 months; HR 0.58), corresponding to a 42% reduction in the rate of disease progression or death. The most common grade 3 and 4 toxicities seen with cabozantinib were similar to its class effect and consisted of hypertension, diarrhea, and fatigue. In the final analysis of the data, the median overall survival was 21.4 months (95% confidence interval [CI] 18.7–not estimable) with cabozantinib and 16.5 months (95% CI 14.7 to 18.8) with everolimus (HR 0.66 [95% CI 0.53 to 0.83]; P = 0.00026). The median follow-up for overall survival and safety was 18.7 months. These results highlight the importance of cabozantinib as a first line option in treatment of previously treated patients with advanced RCC.76
MTOR INHIBITORS
The mTOR inhibitors, temsirolimus and everolimus, are also approved for the treatment of metastatic or advanced RCC. These drugs block mTOR’s phosphorylation and subsequent translation of mRNA to inhibit cell proliferation, cell growth, and angiogenesis.77 Temsirolimus can be used as first-line therapy for patients with a poor prognosis, and everolimus is appropriate as a subsequent therapy.
Temsirolimus is an intravenous prodrug of rapamycin. It was the first of the class to be approved for metastatic RCC for treatment-naïve patients with a poor prognosis (ie, at least 3 of 6 predictors of poor survival based on MSKCC model).54 The pivotal ARCC trial compared temsirolimus (25 mg IV weekly) alone, interferon alfa (3 million units SC 3 times weekly) alone, or the combination (temsirolimus 15 mg IV weekly plus interferon alfa 6 million units SC 3 times weekly). In this trial, temsirolimus monotherapy produced a significantly longer overall survival time than interferon alfa alone (10.9 versus 7.3 months; P = 0.008) and improved PFS time when administered alone or in combination with interferon alfa (3.8 and 3.7 months, respectively, versus 1.9 months). Because no real efficacy advantage of the combination was demonstrated, temsirolimus is administered alone. The most common adverse effects of temsirolimus are asthenia, rash, anemia, nausea, anorexia, pain, and dyspnea. Additionally, hyperglycemia, hyper-cholesterolemia, and hyperlipidemia occur with these agents. Noninfectious pneumonitis is a rare but often fatal complication.
Everolimus is also an orally administered derivative of rapamycin that is approved for use after failure of VEGF-targeted therapies. The results of the landmark trial RECORD-1 demonstrated that everolimus (10 mg orally daily) is effective at prolonging PFS (4 versus 1.9 months; P < 0.001) when compared with best supportive care, a viable treatment option at the time of approval.78 The most common adverse effects of everolimus are stomatitis, rash, fatigue, asthenia, and diarrhea. As with temsirolimus, elevations in glucose, lipids, and triglycerides and noninfectious pneumonitis can occur.
TKI + MTOR INHIBITOR
Lenvatinib is also a small molecule targeting multiple tyrosine kinases, primarily VEGF2. Combined with the mTOR inhibitor everolimus, it has been shown to be an effective regimen in patients with metastatic RCC who have failed other therapies. In a randomized phase 2 study involving patients with advanced or metastatic clear-cell RCC, patients were randomly assigned to receive either lenvatinib (24 mg/day), everolimus (10 mg/day), or lenvatinib plus everolimus (18 mg/day and 5 mg/day, respectively). Patients received the treatment continuously on a 28-day cycle until progression or inability to tolerate toxicity. Patients in the lenvatinib plus everolimus arm had median PFS of 14.6 months (95% CI 5.9 to 20.1) versus 5.5 months (95% CI 3.5 to 7.1) with everlolimus alone (HR 0.40 [95% CI 0.24 to 0.68]; P = 0.0005). PFS with levantinib alone was 7.4 months (95% CI 5.6 to 10.20; HR 0.66 [95% CI 0.30 to 1.10]; P = 0.12). In addition, PFS with levantinib alone was significantly prolonged in comparison with everolimus alone (HR 0.61 [95% CI 0.38 to 0.98]; P = 0.048). Grade 3 or 4 toxicity were less frequent in the everolimus only arm and the most common grade 3 or 4 toxicity in the lenvatinib plus everolimus arm was diarrhea. The results of this study show that the combination of lenvatinib plus everolimus is an acceptable second-line option for treatment of patients with advanced or metastatic RCC.55
CASE CONTINUED
The patient is initially started on pazopanib and tolerates the medication well, with partial response to the treatment. However, on restaging scans he is noted to have small bowel perforation. Pazopanib is discontinued until the patient has a full recovery. He is then started on everolimus. Restaging scans done 3 months after starting everolimus demonstrate disease progression.
• What is the appropriate next step in treatment?
PD1 BLOCKADE
Programmed death 1 (PD-1) protein is a T-cell inhibitory receptor with 2 ligands, PD-L1 and PD-L2. PD-L1 is expressed on many tumors. Blocking the interaction between PD-1 and PD-L1 by anti-PD-1 humanized antibodies potentiates a robust immune response and has been a breakthrough in the field of cancer immunotherapy.79 Previous studies have demonstrated that overexpression of PD-L1 leads to worse outcomes and poor prognosis in patients with RCC.80 Nivolumab, a fully human IgG4 PD-1 immune checkpoint inhibitor, blocks the interaction between PD-1 and its ligands, PD-L1 and PD-L2. In a randomized, open-label, phase 3 study comparing nivolumab with everolimus in patients with RCC who had previously undergone treatment with other standard therapies, Motzer and colleagues81 demonstrated a longer overall survival time and fewer adverse effects with nivolumab. In this study, 821 patients with clear-cell RCC were randomly assigned to receive nivolumab (3 mg/kg of body weight IV every 2 weeks) or everolimus (10 mg orally once daily). The median overall survival time with nivolumab was 25 months versus 19.6 months with everolimus (P < 0.0148). Nineteen percent of patients receiving nivolumab experienced grade 3 or 4 toxicities, with fatigue being the most common adverse effect. Grade 3 or 4 toxicities were observed in 37% of patients treated with everolimus, with anemia being the most common. Based on the results of this trial, on November 23, 2015, the U.S. Food and Drug Administration approved nivolumab to treat patients with metastatic RCC who have received a prior antiangiogenic therapy.
CASE CONCLUSION
Both TKI and mTOR inhibitor therapy fail, and the patient is eligible for third-line therapy. Because of his previous GI perforation, other TKIs are not an option. The patient opts for enrollment in hospice due to declining performance status. For other patients in this situation with a good performance status, nivolumab would be a reasonable option.
FUTURE DIRECTIONS
With the approval of nivolumab, multiple treatment options are now available for patients with metastatic or unresectable RCC. Development of other PD-1 inhibitors and immunotherapies as well as multi-targeted TKIs will only serve to expand treatment options for these patients. Given the aggressive course and poor prognosis of non-clear cell renal cell tumors and those with sarcomatoid features, evaluation of systemic and targeted therapies for these subtypes should remain active areas of research and investigation.
- Siegel R, Miller, K, Jemal A. Cancer Statistics, 2015. CA Cancer J Clin 2015;65:5–29.
- Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and genetics. Tumors of the urinary system and male genital organs. Lyon: IARC Press; 2004.
- Chow WH, Gridley G, Fraumeni JF Jr, Jarvholm B. Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med 2000;343:1305–11.
- Cohen H, McGovern F. Renal-cell carcinoma. N Engl J Med 2005;353:2477–90.
- Yao M, Yoshida M, Kishida T, et al. VHL tumor suppres sor gene alterations associated with good prognosis in sporadic clear-cell renal carcinoma. J Natl Cancer Inst 2002;94:1569–75.
- Iliopoulos O, Kibel A, Gray S, Kaelin WG Jr. Tumour suppression by the human von Hippel-Lindau gene product. Nat Med 1995;1:822–6
- Chen F, Kishida T, Duh FM, et al. Suppression of growth of renal carcinoma cells by the von Hippel-Lindau tumor suppressor gene. Cancer Res 1995;55:4804–7.
- Iliopoulos O, Levy AP, Jiang C, et al. Negative regulation of hypoxia-inducible genes by the von Hippel Lindau protein. Proc Natl Acad Sci U S A 1996;93:10595–9.
- Nickerson ML, Warren MB, Toro JR, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Bir- Hogg-Dube syndrome. Cancer Cell 2002;2:157–64
- Shuch B, Vorganit S, Ricketts CJ, et al. Defining early-onset kidney cancer: implications for germline and somatic mutation testing and clinical management. J Clin Oncol 2014;32:431–7.
- Bukowski RM. Immunotherapy in renal cell carcinoma. Oncology 1999;13:801–10.
- Mueller-Lisse UG, Mueller-Lisse UL. Imaging of advanced renal cell carcinoma. World J Urol 2010;28: 253–61.
- Edge SB, Byrd DR, Compton CC, et al, eds. AJCC cancer staging manual, 7th ed. New York: Springer Science and Business Media LLC; 2010.
- O’Malley RL, Godoy G, Kanofsky JA, Taneja SS. The necessity of adrenalectomy at the time of radical nephrectomy: a systematic review. J Urol 2009;181:2009–17.
- McDougal S, Wein AJ, Kavoussi LR, et al. Campbell-Walsh Urology. 10th ed. Philadelphia (PA): Saunders; 2012.
- Colombo JR Jr, Haber GP, Kelovsek JE, et al. Seven years after laparoscopic radical nephrectomy: oncologic and renal functional outcomes. Urology 2008:71:1149–54.
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Ca 2013;49: 1374–403.
- Weight CJ, Larson BT, Fergany AF, et al. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol 2010;183:1317–23.
- Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomized EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2011;59:543–52.
- Smaldone MC, Fung C, Uzzo RG, Hass NB. Adjuvant and neoadjuvant therapies in high-risk renal cell carcioma. Hematol Oncol Clin North Am 2011;25:765–91.
- NCCN clinical practice guidelines in oncology. Version 3.2016. www.nccn.org. Accessed July 13, 2016
- El Dib R, Touma NJ, Kapoor A. Cryoablation vs radiofrequency ablation for the treatment of renal cell carcinoma: a meta-amalysis of case series studies. BJU Int 2012;110:510–6.
- Theodorescu D. Cancer cryotherapy: evolution and biology. Rev Urol 2004;6 Suppl 4:S9–S19.
- Khiatani V, Dixon RG. Renal ablation update. Sem Intervent Radiol 2014;31:157–66.
- Yu J, Liang P, Yu XL, et al. US-guided percutaneous microwave ablation of renal cell carcinoma: intermediate-term results. Radiol 2012;263:900–8.
- Castle SM, Salas N, Leveillee RJ. Initial experience using microwave ablation therapy for renal tumor treatment: 18- month follow-up. Urology 2011;77:792–7.
- Pech M, Janitzky A, Wendler JJ, et al. Irreversible electroporation of renal cell carcinoma: a first-in-man phase I clinical study. Cardiovasc Intervent Radiol 2011;34:132–8.
- Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr. Rising incidence of renal cell cancer in the United States. JAMA 1999;281:1628–31.
- Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology 1998;51:203–5.
- Pierorazio PM, Johnson MH, Ball MW, et al. Five-year analysis of a multi-institutional prospective clinical trial of delayed intervention and surveillance for small renal masses: the DISSRM registry. Eur Urol 2015;68:408–15.
- Jewett MA, Mattar K, Basiuk J, et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol 2011;60:39–44.
- Chawla SN, Crispen PL, Hanlon AL, et al. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol 2006;175:425–31.
- Smaldone MC, Kutikov A, Egleston BL, et al. Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer 2012;118:997–1006.
- Williamson TJ, Pearson JR, Ischia J, et al.Guideline of guidelines: follow-up after nephrectomy for renal cell carcinoma. BJU Int 2016;117:555–62.
- Donat S, Diaz M, Bishoff JT, et al. Follow-up for clinically localized renal neoplasms: AUA Guideline. J Urol 2013;190:407–16.
- Janzen NK, Kim HL, Figlin RA, Bell-degrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am 2003:30:843–52.
- Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socio-economic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev 2008;34:193–205.
- Mekhail T, Abou-Jawde R, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering Prognostic Factors Model for Survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 2005;23: 832–41.
- Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002;20:289–96.
- Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530–40.
- Heng DY, Xie W, Regan MM. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794–9.
- Leibovich BC, Han KR, Bui MH, et al. Scoring algorithm to predict survival after nephrectomy and immunotherapy in patients with metastatic renal cell carcinoma: A stratification tool for prospective clinical trials. Cancer 2003;98:2566–77.
- Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol 2004;171:1071–6.
- Choueiri TK, Xie W, Kollmannsberger C, et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol 2011;185:60–6.
- Chapin BF, Delacroix SE Jr, Culp SH, et al. Safety of presurgical targeted therapy in the setting of metastatic renal cell carcinoma. Eur Urol 2011;60:964–71.
- Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomized open-label phase 3 trial. Lancet Oncol 2013;14:1287–94.
- Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metatastic renal cell carcinoma: a randomized, double-blind phase III trial. Lancet 2007;370:2103–11.
- Escudier B, Bellmunt J, Negrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol 2010;28:2144–50.
- McDermott DF, Cheng SC, Signoretti S, et al. The high-dose aldesleukin “select”trial: a trial to prospectively validate predictive models of response to treatment in patients with metastatic renal cell carcinoma. Clin Cancer Res 2015;21:561–8.
- Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369:722–31.
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cell global evaluation trial. J Clin Oncol 2009;27:3312–8.
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115–24.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584–90.
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271–81.
- Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus and the combination in patients with metastatic renal cell carcinoma: a randomized, phase 2, open label, multicenter trial. Lancet Oncology 2015;16:1473–82.
- Lexi-Comp, Inc. (Lexi-Drugs® ). Lexi-Drugs version 2.3.3. Lexicomp. Wolters Kluwer Health, Inc. Hudson, OH.
- Choueiri TK, Plantade A, Elson P, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol 2008;26:127–31.
- Lee JL, Ahn JH, Lim HY, et al. Multicenter phase II study of sunitinib in patients with non-clear cell renal cell carcinoma. Ann Oncol 2012;23:2108–14.
- Armstrong AJ, Broderick S, Eisen T, et al. Final clinical results of a randomized phase II international trial of everolimus vs. sunitinib in patients with metastatic non-clear cell renal cell carcinoma (ASPEN). ASCO Meeting Abstracts 2015;33:4507.
- Chowdhury S, Matrana MR, Tsang C, et al. Systemic therapy for metastatic non-clear-cell renal cell carcinoma: recent progress and future directions. Hematol Oncol Clin North Am 2011;25:853–69.
- Escudier B, Droz JP, Rolland F, et al. Doxorubicin and ifosfamide in patients with metastatic sarcomatoid renal cell carcinoma: a phase II study of the Genitourinary Group of the French Federation of Cancer Centers. J Urol 2002; 168–71
- Nanus DM, Garino A, Milowsky MI, et al. Active chemotherapy for sarcomatoid and rapidly progressing renal cell carcinoma. Cancer 2004;101:1545–51.
- Michaelson MD, McKay RR, Werner L, et al. Phase 2 trial of sunitinib and gemcitabine in patients with sarcomatoid and/or poor-risk metastatic renal cell carcinoma. Cancer 2015;121:3435–43.
- McDermott DF, Cheng SC, Signoretti S, et al. The high-dose aldesleukin “select”trial: a trial to prospectively validate predictive models of response to treatment in patients with metastatic renal cell carcinoma. Clin Cancer Res 2015;21:561–8
- Cho DC, Puzanov I, Regan MM, et al. Retrospective analysis of the safety and efficacy of interleukin-2 after prior VEGF-targeted therapy in patients with advanced renal cell carcinoma. J Immunother 2009;32:181–5.
- Pyrhönen S, Salminen E, Ruutu M, et al. Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. J Clin Oncol 1999;17:2859–67.
- Interferon-alpha and survival in metastatic renal carcinoma: early results of a randomised controlled trial. Medical Research Council Renal Cancer Collaborators. Lancet 1999;353:14–7.
- Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003;349:427–34.
- Atkinson BJ, Kalra S, Wang X, et al. Clinical outcomes for patients with metastatic renal cell carcinoma treated with alternative sunitinib schedules. J Urol 2014;191:611–8.
- Kollmannsberger C, Bjarnason G, Burnett P, et al. Sunitinib in metastatic renal cell carcinoma: recommendations for management of noncardiovascular toxicities. Oncologist 2011;16:543–53.
- Najjar YG, Mittal K, Elson P, et al. A 2 weeks on and 1 week off schedule of sunitinib is associated with decreased toxicity in metastatic renal cell carcinoma. Eur J Cancer 2014;50:1084–9.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061–8.
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125–34
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931–9.
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1814–23.
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR) final results from a randomized, open-label, phase 3 trial. Lancet Oncology 2016;17:917–27.
- Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer 2004;4:335–48.
- Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008;372:449–56.
- Brahmer J, Tykodi S, Chow L, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–65.
- Thomson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow up. Cancer Res 2006;66: 3381–5.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13.
INTRODUCTION
Renal cell carcinoma (RCC) is the most common malignancy arising in the kidney, comprising 90% of all renal tumors.1 Approximately 55,000 new RCC cases are diagnosed each year.2 Patients with RCC are often asymptomatic, and most cases are discovered as incidental findings on abdominal imaging performed during evaluation of nonrenal complaints. Limited-stage RCC that is found early can be cured surgically, with estimated 5-year survival rates approaching 90%; however, long-term survival for metastatic disease is poor, with rates ranging from 0% to 20%.2 Advanced RCC is resistant to conventional chemotherapy and radiotherapy, and outcomes for patients with metastatic or unresectable RCC remain poor. However, the recent development of new therapeutic modalities that target tumor molecular pathways has expanded the treatment options for these patients and changed the management of RCC.
EPIDEMIOLOGY AND CLASSIFICATION
Median age at diagnosis in the United States is 64 years. Men have a higher incidence of RCC than women, with the highest incidence seen in American Indian and Alaska Native men (30.1 per 100,000 population). Genetic syndromes account for 2% to 4% of all RCCs.2 Risk factors for RCC include smoking, hypertension, obesity, and acquired cystic kidney disease that is associated with end-stage renal failure.3 Longer duration of tobacco use is associated with a more aggressive course.
The 2004 World Health Organization (WHO) classification of renal tumors summarizes the previous classification systems (including the Heidelberg and Mainz classification systems) to describe different categories of RCC based on histologic and molecular genetics characteristics.2 Using the WHO classification criteria, RCC comprises 90% of all renal tumors, with clear cell being the most common type (80%).2 Other types of renal tumors include papillary, chromophobe, oncocytoma, and collecting-duct or Bellini duct tumors. Approximately 3% to 5% of tumors are unclassified. Oncocytomas are generally considered benign, and chromophobe tumors typically have an indolent course and rarely metastasize. Sarcomatoid differentiation can be seen in any histologic type and is associated with a worse prognosis. While different types of tumors may be seen in the kidney (such as transitional cell or lymphomas), the focus of this review is the primary malignancies of the renal parenchyma.
FAMILIAL SYNDROMES
Several genetic syndromes have been identified by studying families with inherited RCC. Among these, von Hippel-Lindau (VHL) gene mutation is the most commonly found inherited genetic defect. Table 1 summarizes the incidence of gene mutations and the corresponding histologic appearance of the most common sporadic and hereditary RCCs.4

VHL disease is an autosomal dominant familial syndrome. Patients with this mutation are at higher risk for developing RCC (clear cell histology), retinal angiomas, pheochromocytomas, as well as hemangioblastomas of the central nervous system (CNS).4 Of all the genetic mutations seen in RCC, the somatic mutation in the VHL tumor-suppressor gene is by far the most common.5 VHL targets hypoxia–inducible factor-1 alpha (HIF-α) for ubiquitination and subsequent degradation, which has been shown to suppress the growth of clear-cell RCC in mouse models.6–8 HIF expression under hypoxic conditions leads to activation of a number of genes important in blood vessel development, cell proliferation, and glucose metabolism, including vascular endothelial growth factor (VEGF), erythropoietin, platelet-derived growth factor beta (PDGF-β), transforming growth factor alpha (TGF-α), and glucose transporter-1 (GLUT-1). Mutation in the VHL gene prevents degradation of the HIF-α protein, thereby leading to increased expression of these downstream proteins, including MET and Axl. The upregulation of these angiogenic factors is thought to be the underlying process for increased vascularity of CNS hemangioblastomas and clear-cell renal tumors in VHL disease.4–8
Other less common genetic syndromes seen in hereditary RCC include hereditary papillary RCC, hereditary leiomyomatosis, and Birt-Hogg-Dubé (BHD) syndrome.9 In hereditary papillary RCC, the MET gene is mutated. BHD syndrome is a rare, autosomal dominant syndrome characterized by hair follicle hamartomas of the face and neck. About 15% of patients have multiple renal tumors, the majority of which are of the chromophobe or mixed chromophobe-oncocytoma histology. The BHD gene encodes the protein folliculin, which is thought to be a tumor-suppressor gene.
DIAGNOSIS AND STAGING
CASE PRESENTATION
A 74-year-old man who works as an airplane mechanic repairman presents to the emergency department with sudden worsening of chronic right upper arm and shoulder pain after lifting a jug of orange juice. He does not have a significant past medical history and initially thought that his pain was due to a work-related injury. Upon initial evaluation in the emergency department he is found to have a fracture of his right humerus. Given that the fracture appears to be pathologic, further work-up is recommended.
• What are common clinical presentations of RCC?
Most patients are asymptomatic until the disease becomes advanced. The classic triad of flank pain, hematuria, and palpable abdominal mass is seen in approximately 10% of patients with RCC, partly because of earlier detection of renal masses by imaging performed for other purposes.10 Less frequently, patients present with signs or symptoms of metastatic disease such as bone pain or fracture (as seen in the case patient), painful adenopathy, and pulmonary symptoms related to mediastinal masses. Fever, weight loss, anemia, and/or varicocele often occur in young patients (≤ 46 years) and may indicate the presence of a hereditary form of the disease. Patients may present with paraneoplastic syndromes seen as abnormalities on routine blood work. These can include polycythemia or elevated liver function tests (LFTs) without the presence of liver metastases (known as Stauffer syndrome), which can be seen in localized renal tumors. Nearly half (45%) of patients present with localized disease, 25% present with locally advanced disease, and 30% present with metastatic disease.11 Bone is the second most common site of distant metastatic spread (following lung) in patients with advanced RCC.
• What is the approach to initial evaluation for a patient with suspected RCC?
Initial evaluation consists of a physical exam, laboratory tests including complete blood count (CBC) and comprehensive metabolic panel (calcium, serum creatinine, LFTs, lactate dehydrogenase [LDH], and urinalysis), and imaging. Imaging studies include computed tomography (CT) scan with contrast of the abdomen and pelvis or magnetic resonance imaging (MRI) of the abdomen and chest imaging. A chest radiograph may be obtained, although a chest CT is more sensitive for the presence of pulmonary metastases. MRI can be used in patients with renal dysfunction to evaluate the renal vein and inferior vena cava (IVC) for thrombus or to determine the presence of local invasion.12 Although bone and brain are common sites for metastases, routine imaging is not indicated unless the patient is symptomatic. The value of positron emission tomography in RCC remains undetermined at this time.
Staging is done according to the American Joint Committee on Cancer (AJCC) staging classification for RCC; the Figure summarizes the staging and 5-year survival data based on this classification scheme.4,13

J Med 2005;353:2477–90.)
LIMITED-STAGE DISEASE
• What are the therapeutic options for limited-stage disease?
For patients with nondistant metastases, or limited-stage disease, surgical intervention with curative intent is considered. Convention suggests considering definitive surgery for patients with stage I and II disease, select patients with stage III disease with pathologically enlarged retroperitoneal lymph nodes, patients with IVC and/or cardiac atrium involvement of tumor thrombus, and patients with direct extension of the renal tumor into the ipsilateral adrenal gland if there is no evidence of distant disease. While there may be a role for aggressive surgical intervention in patients with distant metastatic disease, this topic will not be covered in this review.
SURGICAL INTERVENTION
Once patients are determined to be appropriate candidates for surgical removal of a renal tumor, the urologist will perform either a radical nephrectomy or a nephron-sparing nephrectomy, also called a partial nephrectomy. The urologist will evaluate the patient based on his or her body habitus, the location of the tumor, whether multiple tumors in one kidney or bilateral tumors are present, whether the patient has a solitary kidney or otherwise impaired kidney function, and whether the patient has a history of a hereditary syndrome involving kidney cancer as this affects the risk of future kidney tumors.
A radical nephrectomy is surgically preferred in the presence of the following factors: tumor larger than 7 cm in diameter, a more centrally located tumor, suspicion of lymph node involvement, tumor involvement with renal vein or IVC, and/or direct extension of the tumor into the ipsilateral adrenal gland. Nephrectomy involves ligation of the vascular supply (renal artery and vein) followed by removal of the kidney and surrounding Gerota’s fascia. The ipsilateral adrenal gland is removed if there is a high-risk for or presence of invasion of the adrenal gland. Removal of the adrenal gland is not standard since the literature demonstrates there is less than a 10% chance of solitary, ipsilateral adrenal gland involvement of tumor at the time of nephrectomy in the absence of high-risk features, and a recent systematic review suggests that the chance may be as low as 1.8%.14 Preoperative factors that correlated with adrenal involvement included upper pole kidney location, renal vein thrombosis, higher T stage (T3a and greater), multifocal tumors, and evidence for distant metastases or lymph node involvement. Lymphadenectomy previously had been included in radical nephrectomy but now is performed selectively. Radical nephrectomy may be performed as
either an open or laparoscopic procedure, the latter of which may be performed robotically.15 Oncologic outcomes appear to be comparable between the 2 approaches, with equivalent 5-year cancer-specific survival (91% with laparoscopic versus 93% with open approach) and recurrence-free survival (91% with laparoscopic versus 93% with open approach).16 The approach ultimately is selected based on provider- and patient-specific input, though in all cases the goal is to remove the specimen intact.16,17
Conversely, a nephron-sparing approach is preferred for tumors less than 7 cm in diameter, for patients with a solitary kidney or impaired renal function, for patients with multiple small ipsilateral tumors or with bilateral tumors, or for radical nephrectomy candidates with comorbidities for whom a limited intervention is deemed to be a lower-risk procedure. A nephron-sparing procedure may also be performed open or laparoscopically. In nephron-sparing procedures, the tumor is removed along with a small margin of normal parenchyma.15
In summary, the goal of surgical intervention is curative intent with removal of the tumor while maintaining as much residual renal function as possible to limit long-term morbidity of chronic kidney disease and associated cardiovascular events.18 Oncologic outcomes for radical nephrectomy and partial nephrectomy are similar. In one study, overall survival was slightly lower in the partial nephrectomy cohort, but only a small number of the deaths were due to RCC.19
ADJUVANT THERAPY
Adjuvant systemic therapy currently has no role following nephrectomy for RCC because no systemic therapy has been able to reduce the likelihood of relapse. Randomized trials of cytokine therapy (eg, interferon, interleukin 2) or tyrosine kinase inhibitors (TKIs; eg, sorafenib, sunitinib) with observation alone in patients with locally advanced completely resected RCC have shown no delay in time to relapse or improvement of survival with adjuvant therapy.20 Similarly, adjuvant radiation therapy has not shown benefit even in patients with nodal involvement or incomplete resection.21 Therefore, observation remains the standard of care after nephrectomy.
RENAL TUMOR ABLATION
For patients who are deemed not to be surgical candidates due to age, comorbidities, or patient preference and who have tumors less than 4 cm in size (stage I tumors), ablative techniques may be considered. The 2 most well-studied and effective techniques at present are cryoablation and radiofrequency ablation (RFA). Microwave ablation may be an option in some facilities, but the data in RCC are limited. An emerging ablative technique under investigation is irreversible electroporation. At present, the long-term efficacy of all ablative techniques is unknown.
Patient selection is undertaken by urologists and interventional radiologists who evaluate the patient with ultrasound, CT, and/or MRI to determine the location and size of the tumor and the presence or absence of metastatic disease. A pretreatment biopsy is recommended to document the histology of the lesion to confirm a malignancy and to guide future treatment for recurrent or metastatic disease. Contraindications to the procedure include the presence of metastatic disease, a life expectancy of less than 1 year, general medical instability, or uncorrectable coagulopathy due to increased risk of bleeding complications. Tumors in close proximity to the renal hilum or collecting system are a contraindication to the procedure because of the risk for hemorrhage or damage to the collecting system. The location of the tumor in relation to the vasculature is also important to maximize efficacy because the vasculature acts as a “heat sink,” causing dissipation of the thermal energy. Occasionally, stenting of the proximal ureter due to upper tumor location is necessary to prevent thermal injury that could lead to urine leaks.
Selection of the modality to be used primarily depends on operator comfort, which translates to good patient outcomes, such as better cancer control and fewer complications. Cryoablation and RFA have both demonstrated good clinical efficacy and cancer control of 89% and 90%, respectively, with comparable complication rates.22 There have been no studies performed directly comparing the modalities.
Cryoablation
Cryoablation is performed through the insertion of a probe into the tumor, which may be done through a surgical or percutaneous approach. Once the probe is in place, a high- pressure gas (argon, nitrogen) is passed through the probe and upon entering a low pressure region the gas cools. The gas is able to cool to temperatures as low as –185°C. The tissue is then rewarmed through the use of helium, which conversely warms when entering a low pressure area. The process of freezing followed by rewarming subsequently causes cell death/tissue destruction through direct cell injury from cellular dehydration and vascular injury. Clinically, 2 freeze-thaw cycles are used to treat a tumor.23,24
RFA
Radiofrequency ablation, or RFA, targets tumors via an electrode placed within the mass that produces intense frictional heat from medium-frequency alternating current (approximately 500 kHz) produced by a connected generator that is grounded on the patient. The thermal energy created causes coagulative necrosis. Due to the reliance on heat for tumor destruction, central lesions are less amenable to this approach because of the “heat sink” effect from the hilum.24
Microwave Ablation
Microwave ablation, like RFA, relies on the generation of frictional heat to cause cell death by coagulative necrosis. In this case, the friction is created through the activation of water molecules; because of the different thermal kinetics involved with microwave ablation, the “heat sink” effect is minimized when treatment is employed near large vessels, in comparison to RFA.24 The data on this mechanism of ablation are still maturing, with varied outcomes thus far. One study demonstrated outcomes comparable to RFA and cryoablation, with cancer-specific survival of 97.8% at 3 years.25 However, a study by Castle and colleagues26 demonstrated higher recurrence rates. The overarching impediment to widespread adoption of microwave ablation is inconclusive data gleaned from studies with small numbers of patients with limited follow up. The role of this modality will need to be revisited.
Irreversible Electroporation
Irreversible electroporation (IRE) is under investigation. IRE is a non-thermal ablative technique that employs rapid electrical pulses to create pores in cell membranes, leading to cell death. The postulated benefits of IRE include the lack of an effect from “heat sinks” and less collateral damage to the surrounding tissues, when compared with the thermal modalities. In a human phase 1 study of patients undergoing IRE prior to immediate surgical resection, the procedure appeared feasible and safe.27 Significant concerns for this method of ablation possibly inducing cardiac arrhythmias, and the resultant need for sedation with neuromuscular blockade and associated electrocardiography monitoring, may impede its implementation in nonresearch settings.24
ACTIVE SURVEILLANCE
Due to the more frequent use of imaging for various indications, there has been an increase in the discovery of small renal masses (SRM); 85% of RCC that present in an asymptomatic or incidental manner are tumors under 4 cm in diameter.28,29 The role of active surveillance is evolving, but is primarily suggested for patients who are not candidates for more aggressive intervention based on comorbidities. A recent prospective, nonrandomized analysis of data from the Delayed Intervention and Surveillance for Small Renal Masses (DISSRM) registry evaluated outcomes for patients with SRM looking at primary intervention compared with active surveillance.30 The primary intervention selected was at the discretion of the provider; treatments included partial nephrectomy, RFA, and cryoablation, and active surveillance patients were followed with imaging every 6 months. Progression of SRM, with recommendation for delayed intervention, was defined as a growth rate of mass greater than 0.5 cm/year, size greater than 4 cm, or hematuria. Thirty-six of 158 patients on active surveillance met criteria for progression; 21 underwent delayed intervention. Of note, even the patients who progressed but did not undergo delayed intervention did not develop metastatic disease during the follow-up interval. With a median follow-up of 2 years, cancer-specific survival was noted to be 99% and 100% at 5 years for primary intervention and active surveillance, respectively. Overall survival at 2 years for primary intervention was 98% and 96% for active surveillance; at 5 years, the survival rates were 92% and 75% (P = 0.06). Of note, 2 patients in the primary intervention arm died of RCC, while none in the active surveillance arm died. As would be expected, active surveillance patients were older, had a worse performance status, and had more comorbidities. Interestingly, 40% of patients enrolled selected active surveillance as their preferred management for SRM. The DISSRM results were consistent with data from the Renal Cell Consortium of Canada and other retrospective reviews.31–33
• What is the approach to follow-up after treatment of localized RCC?
After a patient undergoes treatment for a localized RCC, the goal is to optimize oncologic outcomes, monitor for treatment sequelae, such as renal failure, and focus on survivorship. At this time, there is no consensus in the literature or across published national and international guidelines with regards to the appropriate schedule for surveillance to achieve these goals. In principle, the greatest risk for recurrence occurs within the first 3 years, so many guidelines focus on this timeframe. Likewise, the route of spread tends to be hematogenous, so patients present with pulmonary, bone, and brain metastases, in addition to local recurrence within the renal bed. Symptomatic recurrences often are seen
with bone and brain metastases, and thus bone scans and brain imaging are not listed as part of routine surveillance protocols in asymptomatic patients. Although there is inconclusive evidence that surveillance protocols improve outcomes in RCC, many professional associations have outlined recommendations based on expert opinion.34 The American Urological Association released guidelines in 2013 and the National Comprehensive Cancer Network (NCCN) released their most recent set of guidelines in 2016.21,35 These guidelines use TNM staging to risk-stratify patients and recommend follow-up.
METASTATIC DISEASE
CASE CONTINUED
CT scan with contrast of the chest, abdomen, and pelvis as well as bone scan are done. CT of the abdomen and pelvis demonstrates a 7.8-cm left renal mass arising from the lower pole of the left kidney. Paraesophageal lymphadenopathy and mesenteric nodules are also noted. CT of the chest demonstrates bilateral pulmonary emboli. Bone scan is significant for increased activity related to the pathological fracture involving the right humerus. The patient undergoes surgery to stabilize the pathologic fracture of his humerus. He is diagnosed with metastatic RCC (clear cell histology) and undergoes palliative debulking nephrectomy.
• How is prognosis defined for metastatic RCC?
PROGNOSTIC MODELS
Limited-stage RCC that is found early can be cured surgically, with estimated 5-year survival rates for stage T1 and T2 disease approaching 90%; however, long-term survival for metastatic disease is poor, with rates ranging from 0% to 20%.13 Approximately 30% of patients have metastatic disease at diagnosis, and about one-third of patients who have undergone treatment for localized disease experience relapse.36,37 Common sites of metastases include lung, lymph nodes, bone, liver, adrenal gland, and brain.
Prognostic scoring systems have been developed to define risk groups and assist with determining appropriate therapy in the metastatic setting. The most widely used validated prognostic factor model is that from the Memorial Sloan-Kettering Cancer Center (MSKCC), which was developed using a multivariate analysis derived from data of patients enrolled in clinical trials and treated with interferon alfa.38 The factors included in the MSKCC model are Karnofsky performance status less than 80, time from diagnosis to treatment with interferon alfa less than 12 months, hemoglobin level less than lower limit of laboratory’s reference range, LDH level greater than 1.5 times the upper limit of laboratory’s reference range, and corrected serum calcium level greater than 10 mg/dL. Risk groups are categorized as favorable (0 risk factors), intermediate (1 to 2 risk factors), and poor (3 or more risk factors).39 Median survival for favorable-, intermediate-, and poor-risk patients was 20, 10, and 4 months, respectively.40
Another prognostic model, the International Metastatic RCC Database Consortium, or Heng, model was developed to evaluate prognosis in patients treated with VEGF-targeted therapy.41 This model was developed from a retrospective study of patients treated with sunitinib, sorafenib, and bevacizumab plus interferon alfa or prior immunotherapy. Prognostic factors in this model include 4 of the 5 MSKCC risk factors (hemoglobin level, corrected serum calcium level, Karnofsky performance status, and time to initial diagnosis). Additionally, this model includes both absolute neutrophil and platelet counts greater than the upper limit of normal. Risk groups are identified as favorable (0 risk factors), intermediate (1 to 2 risk factors), and poor (3 or more risk factors). Median survival for favorable-, intermediate-, and poor-risk patients was not reached, 27 months, and 8.8 months, respectively. The University of California, Los Angeles scoring algorithm to predict survival after nephrectomy and immunotherapy (SANI) in patients with metastatic RCC is another prognostic model that can be used. This simplified scoring system incorporates lymph node status, constitutional symptoms, metastases location, histology, and thyroid stimulating hormone (TSH) level.42
The role of debulking or cytoreductive nephrectomy in treatment of metastatic RCC is well established. Large randomized studies have demonstrated a statistically significant median survival benefit for patients undergoing nephrectomy plus interferon alfa therapy compared with patients treated with interferon alfa alone (13.6 months versus 7.8 months, respectively).43 The role of cytoreductive nephrectomy in combination with antiangiogenic agents is less clear. While a retrospective study investigating outcomes of patients with metastatic RCC receiving anti-VEGF agents showed a prolonged survival with nephrectomy, results of large randomized trials are not yet available.44,45 Patients with lung-only metastases, good prognostic features, and a good performance status are historically the most likely to benefit from cytoreductive surgery.
CASE CONTINUED
Based on the MSKCC prognostic factor model, the patient is considered to be in the intermediate-risk group (Karnofsky performance status of 80, calcium 9.5 mg/dL, LDH 204 U/L, hemoglobin 13.6 g/dL). He is started on treatment for his bilateral pulmonary emboli and recovers well from orthopedic surgery as well as palliative debulking nephrectomy.
• What is the appropriate first-line therapy in managing this patient’s metastatic disease?
Several approaches to systemic therapy for advanced RCC have been taken based on the histologic type of the tumor. Clear-cell is by far the predominant histologic type in RCC. Several options are available as first-line treatment for patients with metastatic clear-cell RCC (Table 2).46–54 These include biologic agents such as high-dose interleukin-2 (IL-2) immune therapy, as well as targeted therapies including TKIs and anti-VEGF antibodies. The mammalian target of rapamycin (mTOR) inhibitor temsirolimus is recommended as first-line therapy in patients with poor prognosis only. Second-line therapies for clear-cell RCC following antiangiogenic therapy include TKIs, mTOR inhibitors, nivolumab (PD-1 inhibitor), and the combination of the TKI lenvatinib and mTOR inhibitor everolimus.55 In addition, after initial cytokine therapy, TKIs, temsirolimus, and the anti-VEGF antibody bevacizumab are other treatment options available to patients. Best supportive care should always be provided along with initial and subsequent therapies. Clinical trials are also an appropriate choice as first-line or subsequent therapies. All of these therapies require periodic monitoring to prevent and quickly treat adverse effects. Table 3 lists recommended monitoring parameters for each of these agents.56


Based on several studies, TKIs seem to be less effective in patients with non–clear-cell type histology.57,58 In these patients, risk factors can guide therapy. In the ASPEN trial, where 108 patients were randomly assigned to everolimus or sunitinib, patients in the good- and intermediate-risk groups had longer overall and median progression-free survival (PFS) on sunitinib (8.3 months versus 5.3 months, respectively). However, those in the poor-risk group had a longer median overall survival with everolimus.59 Given that the role of targeted therapies in non–clear-cell RCCs is less well established, enrollment in clinical trials should be considered as a first-line treatment option.21
Sarcomatoid features can be observed in any of the histologic types of RCC, and RCC with these features has an aggressive course and a poor prognosis. Currently, there is no standard therapy for treatment of patients with metastatic or unresectable RCC with sarcomatoid features.60 Chemotherapeutic regimens used for soft tissue sarcomas, including a trial of ifosfamide and doxorubicin, did not show any objective response.61 A small trial of 10 patients treated with doxorubicin and gemcitabine resulted in complete response in 2 patients and partial response in 1 patient.62
Enrollment in a clinical trial remains a first-line treatment option for these patients. More recently, a phase 2 trial of sunitinib and gemcitabine in patients with sarcomatoid (39 patients) and/or poor-risk (33 patients) metastatic RCC showed overall response rates (ORR) of 26% and 24%, respectively. A higher clinical benefit rate (defined as ORR plus stable disease) was seen in patients with tumors containing more than 10% sarcomatoid histology, as compared with patients whose tumors contained less than 10% sarcomatoid histology. Neutropenia (n = 20), anemia (n = 10), and fatigue (n = 7) were the most common grade 3 toxicities seen in all the patients. Although this was a small study, the results showed a trend towards better efficacy of the combination therapy as compared with the single-agent regimen. Currently, another study is underway to further investigate this in a larger group of patients.63
BIOLOGICS
Cytokine therapy, including high-dose IL-2 and interferon alfa, had long been the only first-line treatment option for patients with metastatic or unresectable RCC. Studies of high-dose IL-2 have shown an ORR of 25% and durable response in up to 11% of patients with clear-cell histology.64 Toxicities were similar to those previously observed with high-dose IL-2 treatment; the most commonly observed grade 3 toxicities were hypotension and capillary leak syndrome. IL-2 requires strict monitoring (Table 3). It is important to note that retrospective studies evaluating the safety and efficacy of using IL-2 as second-line treatment in patients previously treated with TKIs demonstrated significant toxicity without achieving partial or complete response in any of the patients.65
Prior to the advent of TKIs in the treatment of RCC, interferon alfa was a first-line treatment option for those who could not receive high-dose IL-2. It has been shown to produce response rates of approximately 20%, with maximum response seen with a higher dose range of 5 to 20 million units daily in 1 study.66,67 However, with the introduction of TKIs, which produce a higher and more durable response, interferon alfa alone is no longer recommended as a treatment option.
VEGF MONOCLONAL ANTIBODIES
Bevacizumab is a recombinant humanized monoclonal antibody that binds and neutralizes VEGF-A. Given overexpression of VEGF in RCC, the role of bevacizumab both as a single agent and in combination with interferon alfa has been investigated. In a randomized phase 2 study involving patients with cytokine-refractory disease, bevacizumab produced a 10% response rate and PFS of 4.8 months compared to patients treated with placebo.68 In the AVOREN trial, the addition of bevacizumab (10 mg/kg intravenously [IV] every 2 weeks) to interferon alfa (9 million units subcutaneously [SC] 3 times weekly) was shown to significantly increase PFS compared with interferon alfa alone (10.2 months versus 5.4 months; P = 0.0001).47,48 Adverse effects of this combination therapy include fatigue and asthenia. Additionally, hypertension, proteinuria, and bleeding occurred.
TYROSINE KINASE INHIBITORS
TKIs have largely replaced IL-2 as first-line therapy for metastatic RCC. Axitinib, pazopanib, sorafenib, and sunitinib and can be used as first-line therapy. All of the TKIs can be used as subsequent therapy.
Sunitinib
Sunitinib is an orally administered TKI that inhibits VEGF receptor (VEGFR) types 1 and 2, PDGF receptors (PDGFR) α and β, stem cell factor receptor (c-Kit), and FLT-3 and RET kinases. Motzer and colleagues52,53 compared sunitinib 50 mg daily orally for 4 weeks with 2 weeks off to the then standard of care, interferon alfa 9 million units SC 3 times weekly. Sunitinib significantly increased the overall objective response rate (47% versus 12%; P < 0.001), PFS (11 versus 5 months; P < 0.001), and overall survival (26.4 versus 21.8 months; hazard ratio [HR], 0.821). The most common side effects are diarrhea, fatigue, nausea/vomiting, anorexia, hypertension, stomatitis, and hand-foot syndrome, occurring in more than 30% of patients. Often patients will require dose reductions or temporary discontinuations to tolerate therapy. Alternative dosing strategies (eg, 50 mg dose orally daily for 2 weeks alternating with 1-week free interval) have been attempted but not prospectively evaluated for efficacy.69–71
Pazopanib
Pazopanib is an oral multi-kinase inhibitor of VEGFR types 1 and 2, PDGFR, and c-KIT. Results of a phase 3 trial comparing pazopanib (800 mg orally daily) to placebo favored the TKI, with a PFS of 9.2 months versus 4.2 months. A subset of treatment-naïve patients had a longer PFS of 11.1 versus 2.8 months and a response rate of 32% versus 4%.72 This led to a noninferiority phase 3 trial comparing pazopanib with sunitinib as first-line therapy.50 In this study, PFS was similar (8.4 versus 9.5 months; HR 1.05), and overall safety and quality-of-life endpoints favored pazopanib. Much less fatigue, stomatitis, hand-foot syndrome, and thrombocytopenia occurred with pazopanib, whereas hair color changes, weight loss, alopecia, and elevations of LFT enzymes occurred more frequently with pazopanib. Hypertension is common with the administration of pazopanib as well.
Sorafenib
Sorafenib is an orally administered inhibitor of Raf, serine/threonine kinase, VEGFR, PDGFR, FLT-3, c-Kit, and RET. The pivotal phase 3 Treatment Approaches in Renal Cancer Global Evaluation Trial (TARGET) compared sorafenib (400 mg orally twice daily) with placebo in patients who had progressed on prior cytokine-based therapy.73 A final analysis, which excluded patients who were allowed to cross over therapies, found improved overall survival times (14.3 versus 1.8 months, P = 0.029).51 Sorafenib is associated with lower rates of diarrhea, rash, fatigue, hand-foot syndrome, alopecia, hypertension, and nausea than sunitinib, although these agents have not been compared to one another.
Axitinib
Axitinib is an oral inhibitor of VEGFRs 1, 2, and 3. Results of the phase 3 AXIS trial comparing axitinib (5 mg orally twice daily) with sorafenib (400 mg orally twice daily) in patients receiving 1 prior systemic therapy showed axitinib was more active than sorafenib in improving ORR (19% versus 9%; P = 0.001) and PFS (6.7 versus 4.7 months; P < 0.001), although no difference in overall survival times was noted.74 In a subsequent phase 3 trial comparing these drugs in the first-line setting, axitinib showed a nonsignificantly higher response rate and PFS. Despite this, the National Comprehensive Cancer Network guidelines consider axitinib an acceptable first-line therapy because activity with acceptable toxicity was demonstrated (Table 2).46 The most common adverse effects of axitinib are diarrhea, hypertension, fatigue, decreased appetite, dysphonia, hypothyroidism, and upper abdominal pain.
CABOZANTINIB
Given that resistance eventually develops in most patients treated with standard treatments, including bevacizumab and TKIs, the need to evaluate the safety and efficacy of novel agents targeting VEGFR and overcoming this resistance is of vital importance. Cabozantinib is an oral small-molecule inhibitor of VEGFR, Met, and Axl, all tyrosine kinases implicated in metastatic RCC. Overexpression of Met and Axl, which occurs as a result of inactivation of the VHL gene, is associated with a poor prognosis in patients with RCC. In a
randomized, open label, phase 3 trial of cabozantinib versus everolimus in advanced RCC, Choueiri and colleagues75 compared the efficacy of cabozantinib with everolimus in patients with metastatic RCC who had progressed on previous VEGFR-targeted therapies. In this study, 658 patients were randomly assigned to receive cabozantinib (60 mg orally daily) or everolimus (10 mg orally daily). Results of the study found that PFS was longer with cabozantinib in patients who had previously been treated with other TKIs (median PFS of 7.4 months versus 3.8 months; HR 0.58), corresponding to a 42% reduction in the rate of disease progression or death. The most common grade 3 and 4 toxicities seen with cabozantinib were similar to its class effect and consisted of hypertension, diarrhea, and fatigue. In the final analysis of the data, the median overall survival was 21.4 months (95% confidence interval [CI] 18.7–not estimable) with cabozantinib and 16.5 months (95% CI 14.7 to 18.8) with everolimus (HR 0.66 [95% CI 0.53 to 0.83]; P = 0.00026). The median follow-up for overall survival and safety was 18.7 months. These results highlight the importance of cabozantinib as a first line option in treatment of previously treated patients with advanced RCC.76
MTOR INHIBITORS
The mTOR inhibitors, temsirolimus and everolimus, are also approved for the treatment of metastatic or advanced RCC. These drugs block mTOR’s phosphorylation and subsequent translation of mRNA to inhibit cell proliferation, cell growth, and angiogenesis.77 Temsirolimus can be used as first-line therapy for patients with a poor prognosis, and everolimus is appropriate as a subsequent therapy.
Temsirolimus is an intravenous prodrug of rapamycin. It was the first of the class to be approved for metastatic RCC for treatment-naïve patients with a poor prognosis (ie, at least 3 of 6 predictors of poor survival based on MSKCC model).54 The pivotal ARCC trial compared temsirolimus (25 mg IV weekly) alone, interferon alfa (3 million units SC 3 times weekly) alone, or the combination (temsirolimus 15 mg IV weekly plus interferon alfa 6 million units SC 3 times weekly). In this trial, temsirolimus monotherapy produced a significantly longer overall survival time than interferon alfa alone (10.9 versus 7.3 months; P = 0.008) and improved PFS time when administered alone or in combination with interferon alfa (3.8 and 3.7 months, respectively, versus 1.9 months). Because no real efficacy advantage of the combination was demonstrated, temsirolimus is administered alone. The most common adverse effects of temsirolimus are asthenia, rash, anemia, nausea, anorexia, pain, and dyspnea. Additionally, hyperglycemia, hyper-cholesterolemia, and hyperlipidemia occur with these agents. Noninfectious pneumonitis is a rare but often fatal complication.
Everolimus is also an orally administered derivative of rapamycin that is approved for use after failure of VEGF-targeted therapies. The results of the landmark trial RECORD-1 demonstrated that everolimus (10 mg orally daily) is effective at prolonging PFS (4 versus 1.9 months; P < 0.001) when compared with best supportive care, a viable treatment option at the time of approval.78 The most common adverse effects of everolimus are stomatitis, rash, fatigue, asthenia, and diarrhea. As with temsirolimus, elevations in glucose, lipids, and triglycerides and noninfectious pneumonitis can occur.
TKI + MTOR INHIBITOR
Lenvatinib is also a small molecule targeting multiple tyrosine kinases, primarily VEGF2. Combined with the mTOR inhibitor everolimus, it has been shown to be an effective regimen in patients with metastatic RCC who have failed other therapies. In a randomized phase 2 study involving patients with advanced or metastatic clear-cell RCC, patients were randomly assigned to receive either lenvatinib (24 mg/day), everolimus (10 mg/day), or lenvatinib plus everolimus (18 mg/day and 5 mg/day, respectively). Patients received the treatment continuously on a 28-day cycle until progression or inability to tolerate toxicity. Patients in the lenvatinib plus everolimus arm had median PFS of 14.6 months (95% CI 5.9 to 20.1) versus 5.5 months (95% CI 3.5 to 7.1) with everlolimus alone (HR 0.40 [95% CI 0.24 to 0.68]; P = 0.0005). PFS with levantinib alone was 7.4 months (95% CI 5.6 to 10.20; HR 0.66 [95% CI 0.30 to 1.10]; P = 0.12). In addition, PFS with levantinib alone was significantly prolonged in comparison with everolimus alone (HR 0.61 [95% CI 0.38 to 0.98]; P = 0.048). Grade 3 or 4 toxicity were less frequent in the everolimus only arm and the most common grade 3 or 4 toxicity in the lenvatinib plus everolimus arm was diarrhea. The results of this study show that the combination of lenvatinib plus everolimus is an acceptable second-line option for treatment of patients with advanced or metastatic RCC.55
CASE CONTINUED
The patient is initially started on pazopanib and tolerates the medication well, with partial response to the treatment. However, on restaging scans he is noted to have small bowel perforation. Pazopanib is discontinued until the patient has a full recovery. He is then started on everolimus. Restaging scans done 3 months after starting everolimus demonstrate disease progression.
• What is the appropriate next step in treatment?
PD1 BLOCKADE
Programmed death 1 (PD-1) protein is a T-cell inhibitory receptor with 2 ligands, PD-L1 and PD-L2. PD-L1 is expressed on many tumors. Blocking the interaction between PD-1 and PD-L1 by anti-PD-1 humanized antibodies potentiates a robust immune response and has been a breakthrough in the field of cancer immunotherapy.79 Previous studies have demonstrated that overexpression of PD-L1 leads to worse outcomes and poor prognosis in patients with RCC.80 Nivolumab, a fully human IgG4 PD-1 immune checkpoint inhibitor, blocks the interaction between PD-1 and its ligands, PD-L1 and PD-L2. In a randomized, open-label, phase 3 study comparing nivolumab with everolimus in patients with RCC who had previously undergone treatment with other standard therapies, Motzer and colleagues81 demonstrated a longer overall survival time and fewer adverse effects with nivolumab. In this study, 821 patients with clear-cell RCC were randomly assigned to receive nivolumab (3 mg/kg of body weight IV every 2 weeks) or everolimus (10 mg orally once daily). The median overall survival time with nivolumab was 25 months versus 19.6 months with everolimus (P < 0.0148). Nineteen percent of patients receiving nivolumab experienced grade 3 or 4 toxicities, with fatigue being the most common adverse effect. Grade 3 or 4 toxicities were observed in 37% of patients treated with everolimus, with anemia being the most common. Based on the results of this trial, on November 23, 2015, the U.S. Food and Drug Administration approved nivolumab to treat patients with metastatic RCC who have received a prior antiangiogenic therapy.
CASE CONCLUSION
Both TKI and mTOR inhibitor therapy fail, and the patient is eligible for third-line therapy. Because of his previous GI perforation, other TKIs are not an option. The patient opts for enrollment in hospice due to declining performance status. For other patients in this situation with a good performance status, nivolumab would be a reasonable option.
FUTURE DIRECTIONS
With the approval of nivolumab, multiple treatment options are now available for patients with metastatic or unresectable RCC. Development of other PD-1 inhibitors and immunotherapies as well as multi-targeted TKIs will only serve to expand treatment options for these patients. Given the aggressive course and poor prognosis of non-clear cell renal cell tumors and those with sarcomatoid features, evaluation of systemic and targeted therapies for these subtypes should remain active areas of research and investigation.
INTRODUCTION
Renal cell carcinoma (RCC) is the most common malignancy arising in the kidney, comprising 90% of all renal tumors.1 Approximately 55,000 new RCC cases are diagnosed each year.2 Patients with RCC are often asymptomatic, and most cases are discovered as incidental findings on abdominal imaging performed during evaluation of nonrenal complaints. Limited-stage RCC that is found early can be cured surgically, with estimated 5-year survival rates approaching 90%; however, long-term survival for metastatic disease is poor, with rates ranging from 0% to 20%.2 Advanced RCC is resistant to conventional chemotherapy and radiotherapy, and outcomes for patients with metastatic or unresectable RCC remain poor. However, the recent development of new therapeutic modalities that target tumor molecular pathways has expanded the treatment options for these patients and changed the management of RCC.
EPIDEMIOLOGY AND CLASSIFICATION
Median age at diagnosis in the United States is 64 years. Men have a higher incidence of RCC than women, with the highest incidence seen in American Indian and Alaska Native men (30.1 per 100,000 population). Genetic syndromes account for 2% to 4% of all RCCs.2 Risk factors for RCC include smoking, hypertension, obesity, and acquired cystic kidney disease that is associated with end-stage renal failure.3 Longer duration of tobacco use is associated with a more aggressive course.
The 2004 World Health Organization (WHO) classification of renal tumors summarizes the previous classification systems (including the Heidelberg and Mainz classification systems) to describe different categories of RCC based on histologic and molecular genetics characteristics.2 Using the WHO classification criteria, RCC comprises 90% of all renal tumors, with clear cell being the most common type (80%).2 Other types of renal tumors include papillary, chromophobe, oncocytoma, and collecting-duct or Bellini duct tumors. Approximately 3% to 5% of tumors are unclassified. Oncocytomas are generally considered benign, and chromophobe tumors typically have an indolent course and rarely metastasize. Sarcomatoid differentiation can be seen in any histologic type and is associated with a worse prognosis. While different types of tumors may be seen in the kidney (such as transitional cell or lymphomas), the focus of this review is the primary malignancies of the renal parenchyma.
FAMILIAL SYNDROMES
Several genetic syndromes have been identified by studying families with inherited RCC. Among these, von Hippel-Lindau (VHL) gene mutation is the most commonly found inherited genetic defect. Table 1 summarizes the incidence of gene mutations and the corresponding histologic appearance of the most common sporadic and hereditary RCCs.4

VHL disease is an autosomal dominant familial syndrome. Patients with this mutation are at higher risk for developing RCC (clear cell histology), retinal angiomas, pheochromocytomas, as well as hemangioblastomas of the central nervous system (CNS).4 Of all the genetic mutations seen in RCC, the somatic mutation in the VHL tumor-suppressor gene is by far the most common.5 VHL targets hypoxia–inducible factor-1 alpha (HIF-α) for ubiquitination and subsequent degradation, which has been shown to suppress the growth of clear-cell RCC in mouse models.6–8 HIF expression under hypoxic conditions leads to activation of a number of genes important in blood vessel development, cell proliferation, and glucose metabolism, including vascular endothelial growth factor (VEGF), erythropoietin, platelet-derived growth factor beta (PDGF-β), transforming growth factor alpha (TGF-α), and glucose transporter-1 (GLUT-1). Mutation in the VHL gene prevents degradation of the HIF-α protein, thereby leading to increased expression of these downstream proteins, including MET and Axl. The upregulation of these angiogenic factors is thought to be the underlying process for increased vascularity of CNS hemangioblastomas and clear-cell renal tumors in VHL disease.4–8
Other less common genetic syndromes seen in hereditary RCC include hereditary papillary RCC, hereditary leiomyomatosis, and Birt-Hogg-Dubé (BHD) syndrome.9 In hereditary papillary RCC, the MET gene is mutated. BHD syndrome is a rare, autosomal dominant syndrome characterized by hair follicle hamartomas of the face and neck. About 15% of patients have multiple renal tumors, the majority of which are of the chromophobe or mixed chromophobe-oncocytoma histology. The BHD gene encodes the protein folliculin, which is thought to be a tumor-suppressor gene.
DIAGNOSIS AND STAGING
CASE PRESENTATION
A 74-year-old man who works as an airplane mechanic repairman presents to the emergency department with sudden worsening of chronic right upper arm and shoulder pain after lifting a jug of orange juice. He does not have a significant past medical history and initially thought that his pain was due to a work-related injury. Upon initial evaluation in the emergency department he is found to have a fracture of his right humerus. Given that the fracture appears to be pathologic, further work-up is recommended.
• What are common clinical presentations of RCC?
Most patients are asymptomatic until the disease becomes advanced. The classic triad of flank pain, hematuria, and palpable abdominal mass is seen in approximately 10% of patients with RCC, partly because of earlier detection of renal masses by imaging performed for other purposes.10 Less frequently, patients present with signs or symptoms of metastatic disease such as bone pain or fracture (as seen in the case patient), painful adenopathy, and pulmonary symptoms related to mediastinal masses. Fever, weight loss, anemia, and/or varicocele often occur in young patients (≤ 46 years) and may indicate the presence of a hereditary form of the disease. Patients may present with paraneoplastic syndromes seen as abnormalities on routine blood work. These can include polycythemia or elevated liver function tests (LFTs) without the presence of liver metastases (known as Stauffer syndrome), which can be seen in localized renal tumors. Nearly half (45%) of patients present with localized disease, 25% present with locally advanced disease, and 30% present with metastatic disease.11 Bone is the second most common site of distant metastatic spread (following lung) in patients with advanced RCC.
• What is the approach to initial evaluation for a patient with suspected RCC?
Initial evaluation consists of a physical exam, laboratory tests including complete blood count (CBC) and comprehensive metabolic panel (calcium, serum creatinine, LFTs, lactate dehydrogenase [LDH], and urinalysis), and imaging. Imaging studies include computed tomography (CT) scan with contrast of the abdomen and pelvis or magnetic resonance imaging (MRI) of the abdomen and chest imaging. A chest radiograph may be obtained, although a chest CT is more sensitive for the presence of pulmonary metastases. MRI can be used in patients with renal dysfunction to evaluate the renal vein and inferior vena cava (IVC) for thrombus or to determine the presence of local invasion.12 Although bone and brain are common sites for metastases, routine imaging is not indicated unless the patient is symptomatic. The value of positron emission tomography in RCC remains undetermined at this time.
Staging is done according to the American Joint Committee on Cancer (AJCC) staging classification for RCC; the Figure summarizes the staging and 5-year survival data based on this classification scheme.4,13

J Med 2005;353:2477–90.)
LIMITED-STAGE DISEASE
• What are the therapeutic options for limited-stage disease?
For patients with nondistant metastases, or limited-stage disease, surgical intervention with curative intent is considered. Convention suggests considering definitive surgery for patients with stage I and II disease, select patients with stage III disease with pathologically enlarged retroperitoneal lymph nodes, patients with IVC and/or cardiac atrium involvement of tumor thrombus, and patients with direct extension of the renal tumor into the ipsilateral adrenal gland if there is no evidence of distant disease. While there may be a role for aggressive surgical intervention in patients with distant metastatic disease, this topic will not be covered in this review.
SURGICAL INTERVENTION
Once patients are determined to be appropriate candidates for surgical removal of a renal tumor, the urologist will perform either a radical nephrectomy or a nephron-sparing nephrectomy, also called a partial nephrectomy. The urologist will evaluate the patient based on his or her body habitus, the location of the tumor, whether multiple tumors in one kidney or bilateral tumors are present, whether the patient has a solitary kidney or otherwise impaired kidney function, and whether the patient has a history of a hereditary syndrome involving kidney cancer as this affects the risk of future kidney tumors.
A radical nephrectomy is surgically preferred in the presence of the following factors: tumor larger than 7 cm in diameter, a more centrally located tumor, suspicion of lymph node involvement, tumor involvement with renal vein or IVC, and/or direct extension of the tumor into the ipsilateral adrenal gland. Nephrectomy involves ligation of the vascular supply (renal artery and vein) followed by removal of the kidney and surrounding Gerota’s fascia. The ipsilateral adrenal gland is removed if there is a high-risk for or presence of invasion of the adrenal gland. Removal of the adrenal gland is not standard since the literature demonstrates there is less than a 10% chance of solitary, ipsilateral adrenal gland involvement of tumor at the time of nephrectomy in the absence of high-risk features, and a recent systematic review suggests that the chance may be as low as 1.8%.14 Preoperative factors that correlated with adrenal involvement included upper pole kidney location, renal vein thrombosis, higher T stage (T3a and greater), multifocal tumors, and evidence for distant metastases or lymph node involvement. Lymphadenectomy previously had been included in radical nephrectomy but now is performed selectively. Radical nephrectomy may be performed as
either an open or laparoscopic procedure, the latter of which may be performed robotically.15 Oncologic outcomes appear to be comparable between the 2 approaches, with equivalent 5-year cancer-specific survival (91% with laparoscopic versus 93% with open approach) and recurrence-free survival (91% with laparoscopic versus 93% with open approach).16 The approach ultimately is selected based on provider- and patient-specific input, though in all cases the goal is to remove the specimen intact.16,17
Conversely, a nephron-sparing approach is preferred for tumors less than 7 cm in diameter, for patients with a solitary kidney or impaired renal function, for patients with multiple small ipsilateral tumors or with bilateral tumors, or for radical nephrectomy candidates with comorbidities for whom a limited intervention is deemed to be a lower-risk procedure. A nephron-sparing procedure may also be performed open or laparoscopically. In nephron-sparing procedures, the tumor is removed along with a small margin of normal parenchyma.15
In summary, the goal of surgical intervention is curative intent with removal of the tumor while maintaining as much residual renal function as possible to limit long-term morbidity of chronic kidney disease and associated cardiovascular events.18 Oncologic outcomes for radical nephrectomy and partial nephrectomy are similar. In one study, overall survival was slightly lower in the partial nephrectomy cohort, but only a small number of the deaths were due to RCC.19
ADJUVANT THERAPY
Adjuvant systemic therapy currently has no role following nephrectomy for RCC because no systemic therapy has been able to reduce the likelihood of relapse. Randomized trials of cytokine therapy (eg, interferon, interleukin 2) or tyrosine kinase inhibitors (TKIs; eg, sorafenib, sunitinib) with observation alone in patients with locally advanced completely resected RCC have shown no delay in time to relapse or improvement of survival with adjuvant therapy.20 Similarly, adjuvant radiation therapy has not shown benefit even in patients with nodal involvement or incomplete resection.21 Therefore, observation remains the standard of care after nephrectomy.
RENAL TUMOR ABLATION
For patients who are deemed not to be surgical candidates due to age, comorbidities, or patient preference and who have tumors less than 4 cm in size (stage I tumors), ablative techniques may be considered. The 2 most well-studied and effective techniques at present are cryoablation and radiofrequency ablation (RFA). Microwave ablation may be an option in some facilities, but the data in RCC are limited. An emerging ablative technique under investigation is irreversible electroporation. At present, the long-term efficacy of all ablative techniques is unknown.
Patient selection is undertaken by urologists and interventional radiologists who evaluate the patient with ultrasound, CT, and/or MRI to determine the location and size of the tumor and the presence or absence of metastatic disease. A pretreatment biopsy is recommended to document the histology of the lesion to confirm a malignancy and to guide future treatment for recurrent or metastatic disease. Contraindications to the procedure include the presence of metastatic disease, a life expectancy of less than 1 year, general medical instability, or uncorrectable coagulopathy due to increased risk of bleeding complications. Tumors in close proximity to the renal hilum or collecting system are a contraindication to the procedure because of the risk for hemorrhage or damage to the collecting system. The location of the tumor in relation to the vasculature is also important to maximize efficacy because the vasculature acts as a “heat sink,” causing dissipation of the thermal energy. Occasionally, stenting of the proximal ureter due to upper tumor location is necessary to prevent thermal injury that could lead to urine leaks.
Selection of the modality to be used primarily depends on operator comfort, which translates to good patient outcomes, such as better cancer control and fewer complications. Cryoablation and RFA have both demonstrated good clinical efficacy and cancer control of 89% and 90%, respectively, with comparable complication rates.22 There have been no studies performed directly comparing the modalities.
Cryoablation
Cryoablation is performed through the insertion of a probe into the tumor, which may be done through a surgical or percutaneous approach. Once the probe is in place, a high- pressure gas (argon, nitrogen) is passed through the probe and upon entering a low pressure region the gas cools. The gas is able to cool to temperatures as low as –185°C. The tissue is then rewarmed through the use of helium, which conversely warms when entering a low pressure area. The process of freezing followed by rewarming subsequently causes cell death/tissue destruction through direct cell injury from cellular dehydration and vascular injury. Clinically, 2 freeze-thaw cycles are used to treat a tumor.23,24
RFA
Radiofrequency ablation, or RFA, targets tumors via an electrode placed within the mass that produces intense frictional heat from medium-frequency alternating current (approximately 500 kHz) produced by a connected generator that is grounded on the patient. The thermal energy created causes coagulative necrosis. Due to the reliance on heat for tumor destruction, central lesions are less amenable to this approach because of the “heat sink” effect from the hilum.24
Microwave Ablation
Microwave ablation, like RFA, relies on the generation of frictional heat to cause cell death by coagulative necrosis. In this case, the friction is created through the activation of water molecules; because of the different thermal kinetics involved with microwave ablation, the “heat sink” effect is minimized when treatment is employed near large vessels, in comparison to RFA.24 The data on this mechanism of ablation are still maturing, with varied outcomes thus far. One study demonstrated outcomes comparable to RFA and cryoablation, with cancer-specific survival of 97.8% at 3 years.25 However, a study by Castle and colleagues26 demonstrated higher recurrence rates. The overarching impediment to widespread adoption of microwave ablation is inconclusive data gleaned from studies with small numbers of patients with limited follow up. The role of this modality will need to be revisited.
Irreversible Electroporation
Irreversible electroporation (IRE) is under investigation. IRE is a non-thermal ablative technique that employs rapid electrical pulses to create pores in cell membranes, leading to cell death. The postulated benefits of IRE include the lack of an effect from “heat sinks” and less collateral damage to the surrounding tissues, when compared with the thermal modalities. In a human phase 1 study of patients undergoing IRE prior to immediate surgical resection, the procedure appeared feasible and safe.27 Significant concerns for this method of ablation possibly inducing cardiac arrhythmias, and the resultant need for sedation with neuromuscular blockade and associated electrocardiography monitoring, may impede its implementation in nonresearch settings.24
ACTIVE SURVEILLANCE
Due to the more frequent use of imaging for various indications, there has been an increase in the discovery of small renal masses (SRM); 85% of RCC that present in an asymptomatic or incidental manner are tumors under 4 cm in diameter.28,29 The role of active surveillance is evolving, but is primarily suggested for patients who are not candidates for more aggressive intervention based on comorbidities. A recent prospective, nonrandomized analysis of data from the Delayed Intervention and Surveillance for Small Renal Masses (DISSRM) registry evaluated outcomes for patients with SRM looking at primary intervention compared with active surveillance.30 The primary intervention selected was at the discretion of the provider; treatments included partial nephrectomy, RFA, and cryoablation, and active surveillance patients were followed with imaging every 6 months. Progression of SRM, with recommendation for delayed intervention, was defined as a growth rate of mass greater than 0.5 cm/year, size greater than 4 cm, or hematuria. Thirty-six of 158 patients on active surveillance met criteria for progression; 21 underwent delayed intervention. Of note, even the patients who progressed but did not undergo delayed intervention did not develop metastatic disease during the follow-up interval. With a median follow-up of 2 years, cancer-specific survival was noted to be 99% and 100% at 5 years for primary intervention and active surveillance, respectively. Overall survival at 2 years for primary intervention was 98% and 96% for active surveillance; at 5 years, the survival rates were 92% and 75% (P = 0.06). Of note, 2 patients in the primary intervention arm died of RCC, while none in the active surveillance arm died. As would be expected, active surveillance patients were older, had a worse performance status, and had more comorbidities. Interestingly, 40% of patients enrolled selected active surveillance as their preferred management for SRM. The DISSRM results were consistent with data from the Renal Cell Consortium of Canada and other retrospective reviews.31–33
• What is the approach to follow-up after treatment of localized RCC?
After a patient undergoes treatment for a localized RCC, the goal is to optimize oncologic outcomes, monitor for treatment sequelae, such as renal failure, and focus on survivorship. At this time, there is no consensus in the literature or across published national and international guidelines with regards to the appropriate schedule for surveillance to achieve these goals. In principle, the greatest risk for recurrence occurs within the first 3 years, so many guidelines focus on this timeframe. Likewise, the route of spread tends to be hematogenous, so patients present with pulmonary, bone, and brain metastases, in addition to local recurrence within the renal bed. Symptomatic recurrences often are seen
with bone and brain metastases, and thus bone scans and brain imaging are not listed as part of routine surveillance protocols in asymptomatic patients. Although there is inconclusive evidence that surveillance protocols improve outcomes in RCC, many professional associations have outlined recommendations based on expert opinion.34 The American Urological Association released guidelines in 2013 and the National Comprehensive Cancer Network (NCCN) released their most recent set of guidelines in 2016.21,35 These guidelines use TNM staging to risk-stratify patients and recommend follow-up.
METASTATIC DISEASE
CASE CONTINUED
CT scan with contrast of the chest, abdomen, and pelvis as well as bone scan are done. CT of the abdomen and pelvis demonstrates a 7.8-cm left renal mass arising from the lower pole of the left kidney. Paraesophageal lymphadenopathy and mesenteric nodules are also noted. CT of the chest demonstrates bilateral pulmonary emboli. Bone scan is significant for increased activity related to the pathological fracture involving the right humerus. The patient undergoes surgery to stabilize the pathologic fracture of his humerus. He is diagnosed with metastatic RCC (clear cell histology) and undergoes palliative debulking nephrectomy.
• How is prognosis defined for metastatic RCC?
PROGNOSTIC MODELS
Limited-stage RCC that is found early can be cured surgically, with estimated 5-year survival rates for stage T1 and T2 disease approaching 90%; however, long-term survival for metastatic disease is poor, with rates ranging from 0% to 20%.13 Approximately 30% of patients have metastatic disease at diagnosis, and about one-third of patients who have undergone treatment for localized disease experience relapse.36,37 Common sites of metastases include lung, lymph nodes, bone, liver, adrenal gland, and brain.
Prognostic scoring systems have been developed to define risk groups and assist with determining appropriate therapy in the metastatic setting. The most widely used validated prognostic factor model is that from the Memorial Sloan-Kettering Cancer Center (MSKCC), which was developed using a multivariate analysis derived from data of patients enrolled in clinical trials and treated with interferon alfa.38 The factors included in the MSKCC model are Karnofsky performance status less than 80, time from diagnosis to treatment with interferon alfa less than 12 months, hemoglobin level less than lower limit of laboratory’s reference range, LDH level greater than 1.5 times the upper limit of laboratory’s reference range, and corrected serum calcium level greater than 10 mg/dL. Risk groups are categorized as favorable (0 risk factors), intermediate (1 to 2 risk factors), and poor (3 or more risk factors).39 Median survival for favorable-, intermediate-, and poor-risk patients was 20, 10, and 4 months, respectively.40
Another prognostic model, the International Metastatic RCC Database Consortium, or Heng, model was developed to evaluate prognosis in patients treated with VEGF-targeted therapy.41 This model was developed from a retrospective study of patients treated with sunitinib, sorafenib, and bevacizumab plus interferon alfa or prior immunotherapy. Prognostic factors in this model include 4 of the 5 MSKCC risk factors (hemoglobin level, corrected serum calcium level, Karnofsky performance status, and time to initial diagnosis). Additionally, this model includes both absolute neutrophil and platelet counts greater than the upper limit of normal. Risk groups are identified as favorable (0 risk factors), intermediate (1 to 2 risk factors), and poor (3 or more risk factors). Median survival for favorable-, intermediate-, and poor-risk patients was not reached, 27 months, and 8.8 months, respectively. The University of California, Los Angeles scoring algorithm to predict survival after nephrectomy and immunotherapy (SANI) in patients with metastatic RCC is another prognostic model that can be used. This simplified scoring system incorporates lymph node status, constitutional symptoms, metastases location, histology, and thyroid stimulating hormone (TSH) level.42
The role of debulking or cytoreductive nephrectomy in treatment of metastatic RCC is well established. Large randomized studies have demonstrated a statistically significant median survival benefit for patients undergoing nephrectomy plus interferon alfa therapy compared with patients treated with interferon alfa alone (13.6 months versus 7.8 months, respectively).43 The role of cytoreductive nephrectomy in combination with antiangiogenic agents is less clear. While a retrospective study investigating outcomes of patients with metastatic RCC receiving anti-VEGF agents showed a prolonged survival with nephrectomy, results of large randomized trials are not yet available.44,45 Patients with lung-only metastases, good prognostic features, and a good performance status are historically the most likely to benefit from cytoreductive surgery.
CASE CONTINUED
Based on the MSKCC prognostic factor model, the patient is considered to be in the intermediate-risk group (Karnofsky performance status of 80, calcium 9.5 mg/dL, LDH 204 U/L, hemoglobin 13.6 g/dL). He is started on treatment for his bilateral pulmonary emboli and recovers well from orthopedic surgery as well as palliative debulking nephrectomy.
• What is the appropriate first-line therapy in managing this patient’s metastatic disease?
Several approaches to systemic therapy for advanced RCC have been taken based on the histologic type of the tumor. Clear-cell is by far the predominant histologic type in RCC. Several options are available as first-line treatment for patients with metastatic clear-cell RCC (Table 2).46–54 These include biologic agents such as high-dose interleukin-2 (IL-2) immune therapy, as well as targeted therapies including TKIs and anti-VEGF antibodies. The mammalian target of rapamycin (mTOR) inhibitor temsirolimus is recommended as first-line therapy in patients with poor prognosis only. Second-line therapies for clear-cell RCC following antiangiogenic therapy include TKIs, mTOR inhibitors, nivolumab (PD-1 inhibitor), and the combination of the TKI lenvatinib and mTOR inhibitor everolimus.55 In addition, after initial cytokine therapy, TKIs, temsirolimus, and the anti-VEGF antibody bevacizumab are other treatment options available to patients. Best supportive care should always be provided along with initial and subsequent therapies. Clinical trials are also an appropriate choice as first-line or subsequent therapies. All of these therapies require periodic monitoring to prevent and quickly treat adverse effects. Table 3 lists recommended monitoring parameters for each of these agents.56


Based on several studies, TKIs seem to be less effective in patients with non–clear-cell type histology.57,58 In these patients, risk factors can guide therapy. In the ASPEN trial, where 108 patients were randomly assigned to everolimus or sunitinib, patients in the good- and intermediate-risk groups had longer overall and median progression-free survival (PFS) on sunitinib (8.3 months versus 5.3 months, respectively). However, those in the poor-risk group had a longer median overall survival with everolimus.59 Given that the role of targeted therapies in non–clear-cell RCCs is less well established, enrollment in clinical trials should be considered as a first-line treatment option.21
Sarcomatoid features can be observed in any of the histologic types of RCC, and RCC with these features has an aggressive course and a poor prognosis. Currently, there is no standard therapy for treatment of patients with metastatic or unresectable RCC with sarcomatoid features.60 Chemotherapeutic regimens used for soft tissue sarcomas, including a trial of ifosfamide and doxorubicin, did not show any objective response.61 A small trial of 10 patients treated with doxorubicin and gemcitabine resulted in complete response in 2 patients and partial response in 1 patient.62
Enrollment in a clinical trial remains a first-line treatment option for these patients. More recently, a phase 2 trial of sunitinib and gemcitabine in patients with sarcomatoid (39 patients) and/or poor-risk (33 patients) metastatic RCC showed overall response rates (ORR) of 26% and 24%, respectively. A higher clinical benefit rate (defined as ORR plus stable disease) was seen in patients with tumors containing more than 10% sarcomatoid histology, as compared with patients whose tumors contained less than 10% sarcomatoid histology. Neutropenia (n = 20), anemia (n = 10), and fatigue (n = 7) were the most common grade 3 toxicities seen in all the patients. Although this was a small study, the results showed a trend towards better efficacy of the combination therapy as compared with the single-agent regimen. Currently, another study is underway to further investigate this in a larger group of patients.63
BIOLOGICS
Cytokine therapy, including high-dose IL-2 and interferon alfa, had long been the only first-line treatment option for patients with metastatic or unresectable RCC. Studies of high-dose IL-2 have shown an ORR of 25% and durable response in up to 11% of patients with clear-cell histology.64 Toxicities were similar to those previously observed with high-dose IL-2 treatment; the most commonly observed grade 3 toxicities were hypotension and capillary leak syndrome. IL-2 requires strict monitoring (Table 3). It is important to note that retrospective studies evaluating the safety and efficacy of using IL-2 as second-line treatment in patients previously treated with TKIs demonstrated significant toxicity without achieving partial or complete response in any of the patients.65
Prior to the advent of TKIs in the treatment of RCC, interferon alfa was a first-line treatment option for those who could not receive high-dose IL-2. It has been shown to produce response rates of approximately 20%, with maximum response seen with a higher dose range of 5 to 20 million units daily in 1 study.66,67 However, with the introduction of TKIs, which produce a higher and more durable response, interferon alfa alone is no longer recommended as a treatment option.
VEGF MONOCLONAL ANTIBODIES
Bevacizumab is a recombinant humanized monoclonal antibody that binds and neutralizes VEGF-A. Given overexpression of VEGF in RCC, the role of bevacizumab both as a single agent and in combination with interferon alfa has been investigated. In a randomized phase 2 study involving patients with cytokine-refractory disease, bevacizumab produced a 10% response rate and PFS of 4.8 months compared to patients treated with placebo.68 In the AVOREN trial, the addition of bevacizumab (10 mg/kg intravenously [IV] every 2 weeks) to interferon alfa (9 million units subcutaneously [SC] 3 times weekly) was shown to significantly increase PFS compared with interferon alfa alone (10.2 months versus 5.4 months; P = 0.0001).47,48 Adverse effects of this combination therapy include fatigue and asthenia. Additionally, hypertension, proteinuria, and bleeding occurred.
TYROSINE KINASE INHIBITORS
TKIs have largely replaced IL-2 as first-line therapy for metastatic RCC. Axitinib, pazopanib, sorafenib, and sunitinib and can be used as first-line therapy. All of the TKIs can be used as subsequent therapy.
Sunitinib
Sunitinib is an orally administered TKI that inhibits VEGF receptor (VEGFR) types 1 and 2, PDGF receptors (PDGFR) α and β, stem cell factor receptor (c-Kit), and FLT-3 and RET kinases. Motzer and colleagues52,53 compared sunitinib 50 mg daily orally for 4 weeks with 2 weeks off to the then standard of care, interferon alfa 9 million units SC 3 times weekly. Sunitinib significantly increased the overall objective response rate (47% versus 12%; P < 0.001), PFS (11 versus 5 months; P < 0.001), and overall survival (26.4 versus 21.8 months; hazard ratio [HR], 0.821). The most common side effects are diarrhea, fatigue, nausea/vomiting, anorexia, hypertension, stomatitis, and hand-foot syndrome, occurring in more than 30% of patients. Often patients will require dose reductions or temporary discontinuations to tolerate therapy. Alternative dosing strategies (eg, 50 mg dose orally daily for 2 weeks alternating with 1-week free interval) have been attempted but not prospectively evaluated for efficacy.69–71
Pazopanib
Pazopanib is an oral multi-kinase inhibitor of VEGFR types 1 and 2, PDGFR, and c-KIT. Results of a phase 3 trial comparing pazopanib (800 mg orally daily) to placebo favored the TKI, with a PFS of 9.2 months versus 4.2 months. A subset of treatment-naïve patients had a longer PFS of 11.1 versus 2.8 months and a response rate of 32% versus 4%.72 This led to a noninferiority phase 3 trial comparing pazopanib with sunitinib as first-line therapy.50 In this study, PFS was similar (8.4 versus 9.5 months; HR 1.05), and overall safety and quality-of-life endpoints favored pazopanib. Much less fatigue, stomatitis, hand-foot syndrome, and thrombocytopenia occurred with pazopanib, whereas hair color changes, weight loss, alopecia, and elevations of LFT enzymes occurred more frequently with pazopanib. Hypertension is common with the administration of pazopanib as well.
Sorafenib
Sorafenib is an orally administered inhibitor of Raf, serine/threonine kinase, VEGFR, PDGFR, FLT-3, c-Kit, and RET. The pivotal phase 3 Treatment Approaches in Renal Cancer Global Evaluation Trial (TARGET) compared sorafenib (400 mg orally twice daily) with placebo in patients who had progressed on prior cytokine-based therapy.73 A final analysis, which excluded patients who were allowed to cross over therapies, found improved overall survival times (14.3 versus 1.8 months, P = 0.029).51 Sorafenib is associated with lower rates of diarrhea, rash, fatigue, hand-foot syndrome, alopecia, hypertension, and nausea than sunitinib, although these agents have not been compared to one another.
Axitinib
Axitinib is an oral inhibitor of VEGFRs 1, 2, and 3. Results of the phase 3 AXIS trial comparing axitinib (5 mg orally twice daily) with sorafenib (400 mg orally twice daily) in patients receiving 1 prior systemic therapy showed axitinib was more active than sorafenib in improving ORR (19% versus 9%; P = 0.001) and PFS (6.7 versus 4.7 months; P < 0.001), although no difference in overall survival times was noted.74 In a subsequent phase 3 trial comparing these drugs in the first-line setting, axitinib showed a nonsignificantly higher response rate and PFS. Despite this, the National Comprehensive Cancer Network guidelines consider axitinib an acceptable first-line therapy because activity with acceptable toxicity was demonstrated (Table 2).46 The most common adverse effects of axitinib are diarrhea, hypertension, fatigue, decreased appetite, dysphonia, hypothyroidism, and upper abdominal pain.
CABOZANTINIB
Given that resistance eventually develops in most patients treated with standard treatments, including bevacizumab and TKIs, the need to evaluate the safety and efficacy of novel agents targeting VEGFR and overcoming this resistance is of vital importance. Cabozantinib is an oral small-molecule inhibitor of VEGFR, Met, and Axl, all tyrosine kinases implicated in metastatic RCC. Overexpression of Met and Axl, which occurs as a result of inactivation of the VHL gene, is associated with a poor prognosis in patients with RCC. In a
randomized, open label, phase 3 trial of cabozantinib versus everolimus in advanced RCC, Choueiri and colleagues75 compared the efficacy of cabozantinib with everolimus in patients with metastatic RCC who had progressed on previous VEGFR-targeted therapies. In this study, 658 patients were randomly assigned to receive cabozantinib (60 mg orally daily) or everolimus (10 mg orally daily). Results of the study found that PFS was longer with cabozantinib in patients who had previously been treated with other TKIs (median PFS of 7.4 months versus 3.8 months; HR 0.58), corresponding to a 42% reduction in the rate of disease progression or death. The most common grade 3 and 4 toxicities seen with cabozantinib were similar to its class effect and consisted of hypertension, diarrhea, and fatigue. In the final analysis of the data, the median overall survival was 21.4 months (95% confidence interval [CI] 18.7–not estimable) with cabozantinib and 16.5 months (95% CI 14.7 to 18.8) with everolimus (HR 0.66 [95% CI 0.53 to 0.83]; P = 0.00026). The median follow-up for overall survival and safety was 18.7 months. These results highlight the importance of cabozantinib as a first line option in treatment of previously treated patients with advanced RCC.76
MTOR INHIBITORS
The mTOR inhibitors, temsirolimus and everolimus, are also approved for the treatment of metastatic or advanced RCC. These drugs block mTOR’s phosphorylation and subsequent translation of mRNA to inhibit cell proliferation, cell growth, and angiogenesis.77 Temsirolimus can be used as first-line therapy for patients with a poor prognosis, and everolimus is appropriate as a subsequent therapy.
Temsirolimus is an intravenous prodrug of rapamycin. It was the first of the class to be approved for metastatic RCC for treatment-naïve patients with a poor prognosis (ie, at least 3 of 6 predictors of poor survival based on MSKCC model).54 The pivotal ARCC trial compared temsirolimus (25 mg IV weekly) alone, interferon alfa (3 million units SC 3 times weekly) alone, or the combination (temsirolimus 15 mg IV weekly plus interferon alfa 6 million units SC 3 times weekly). In this trial, temsirolimus monotherapy produced a significantly longer overall survival time than interferon alfa alone (10.9 versus 7.3 months; P = 0.008) and improved PFS time when administered alone or in combination with interferon alfa (3.8 and 3.7 months, respectively, versus 1.9 months). Because no real efficacy advantage of the combination was demonstrated, temsirolimus is administered alone. The most common adverse effects of temsirolimus are asthenia, rash, anemia, nausea, anorexia, pain, and dyspnea. Additionally, hyperglycemia, hyper-cholesterolemia, and hyperlipidemia occur with these agents. Noninfectious pneumonitis is a rare but often fatal complication.
Everolimus is also an orally administered derivative of rapamycin that is approved for use after failure of VEGF-targeted therapies. The results of the landmark trial RECORD-1 demonstrated that everolimus (10 mg orally daily) is effective at prolonging PFS (4 versus 1.9 months; P < 0.001) when compared with best supportive care, a viable treatment option at the time of approval.78 The most common adverse effects of everolimus are stomatitis, rash, fatigue, asthenia, and diarrhea. As with temsirolimus, elevations in glucose, lipids, and triglycerides and noninfectious pneumonitis can occur.
TKI + MTOR INHIBITOR
Lenvatinib is also a small molecule targeting multiple tyrosine kinases, primarily VEGF2. Combined with the mTOR inhibitor everolimus, it has been shown to be an effective regimen in patients with metastatic RCC who have failed other therapies. In a randomized phase 2 study involving patients with advanced or metastatic clear-cell RCC, patients were randomly assigned to receive either lenvatinib (24 mg/day), everolimus (10 mg/day), or lenvatinib plus everolimus (18 mg/day and 5 mg/day, respectively). Patients received the treatment continuously on a 28-day cycle until progression or inability to tolerate toxicity. Patients in the lenvatinib plus everolimus arm had median PFS of 14.6 months (95% CI 5.9 to 20.1) versus 5.5 months (95% CI 3.5 to 7.1) with everlolimus alone (HR 0.40 [95% CI 0.24 to 0.68]; P = 0.0005). PFS with levantinib alone was 7.4 months (95% CI 5.6 to 10.20; HR 0.66 [95% CI 0.30 to 1.10]; P = 0.12). In addition, PFS with levantinib alone was significantly prolonged in comparison with everolimus alone (HR 0.61 [95% CI 0.38 to 0.98]; P = 0.048). Grade 3 or 4 toxicity were less frequent in the everolimus only arm and the most common grade 3 or 4 toxicity in the lenvatinib plus everolimus arm was diarrhea. The results of this study show that the combination of lenvatinib plus everolimus is an acceptable second-line option for treatment of patients with advanced or metastatic RCC.55
CASE CONTINUED
The patient is initially started on pazopanib and tolerates the medication well, with partial response to the treatment. However, on restaging scans he is noted to have small bowel perforation. Pazopanib is discontinued until the patient has a full recovery. He is then started on everolimus. Restaging scans done 3 months after starting everolimus demonstrate disease progression.
• What is the appropriate next step in treatment?
PD1 BLOCKADE
Programmed death 1 (PD-1) protein is a T-cell inhibitory receptor with 2 ligands, PD-L1 and PD-L2. PD-L1 is expressed on many tumors. Blocking the interaction between PD-1 and PD-L1 by anti-PD-1 humanized antibodies potentiates a robust immune response and has been a breakthrough in the field of cancer immunotherapy.79 Previous studies have demonstrated that overexpression of PD-L1 leads to worse outcomes and poor prognosis in patients with RCC.80 Nivolumab, a fully human IgG4 PD-1 immune checkpoint inhibitor, blocks the interaction between PD-1 and its ligands, PD-L1 and PD-L2. In a randomized, open-label, phase 3 study comparing nivolumab with everolimus in patients with RCC who had previously undergone treatment with other standard therapies, Motzer and colleagues81 demonstrated a longer overall survival time and fewer adverse effects with nivolumab. In this study, 821 patients with clear-cell RCC were randomly assigned to receive nivolumab (3 mg/kg of body weight IV every 2 weeks) or everolimus (10 mg orally once daily). The median overall survival time with nivolumab was 25 months versus 19.6 months with everolimus (P < 0.0148). Nineteen percent of patients receiving nivolumab experienced grade 3 or 4 toxicities, with fatigue being the most common adverse effect. Grade 3 or 4 toxicities were observed in 37% of patients treated with everolimus, with anemia being the most common. Based on the results of this trial, on November 23, 2015, the U.S. Food and Drug Administration approved nivolumab to treat patients with metastatic RCC who have received a prior antiangiogenic therapy.
CASE CONCLUSION
Both TKI and mTOR inhibitor therapy fail, and the patient is eligible for third-line therapy. Because of his previous GI perforation, other TKIs are not an option. The patient opts for enrollment in hospice due to declining performance status. For other patients in this situation with a good performance status, nivolumab would be a reasonable option.
FUTURE DIRECTIONS
With the approval of nivolumab, multiple treatment options are now available for patients with metastatic or unresectable RCC. Development of other PD-1 inhibitors and immunotherapies as well as multi-targeted TKIs will only serve to expand treatment options for these patients. Given the aggressive course and poor prognosis of non-clear cell renal cell tumors and those with sarcomatoid features, evaluation of systemic and targeted therapies for these subtypes should remain active areas of research and investigation.
- Siegel R, Miller, K, Jemal A. Cancer Statistics, 2015. CA Cancer J Clin 2015;65:5–29.
- Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and genetics. Tumors of the urinary system and male genital organs. Lyon: IARC Press; 2004.
- Chow WH, Gridley G, Fraumeni JF Jr, Jarvholm B. Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med 2000;343:1305–11.
- Cohen H, McGovern F. Renal-cell carcinoma. N Engl J Med 2005;353:2477–90.
- Yao M, Yoshida M, Kishida T, et al. VHL tumor suppres sor gene alterations associated with good prognosis in sporadic clear-cell renal carcinoma. J Natl Cancer Inst 2002;94:1569–75.
- Iliopoulos O, Kibel A, Gray S, Kaelin WG Jr. Tumour suppression by the human von Hippel-Lindau gene product. Nat Med 1995;1:822–6
- Chen F, Kishida T, Duh FM, et al. Suppression of growth of renal carcinoma cells by the von Hippel-Lindau tumor suppressor gene. Cancer Res 1995;55:4804–7.
- Iliopoulos O, Levy AP, Jiang C, et al. Negative regulation of hypoxia-inducible genes by the von Hippel Lindau protein. Proc Natl Acad Sci U S A 1996;93:10595–9.
- Nickerson ML, Warren MB, Toro JR, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Bir- Hogg-Dube syndrome. Cancer Cell 2002;2:157–64
- Shuch B, Vorganit S, Ricketts CJ, et al. Defining early-onset kidney cancer: implications for germline and somatic mutation testing and clinical management. J Clin Oncol 2014;32:431–7.
- Bukowski RM. Immunotherapy in renal cell carcinoma. Oncology 1999;13:801–10.
- Mueller-Lisse UG, Mueller-Lisse UL. Imaging of advanced renal cell carcinoma. World J Urol 2010;28: 253–61.
- Edge SB, Byrd DR, Compton CC, et al, eds. AJCC cancer staging manual, 7th ed. New York: Springer Science and Business Media LLC; 2010.
- O’Malley RL, Godoy G, Kanofsky JA, Taneja SS. The necessity of adrenalectomy at the time of radical nephrectomy: a systematic review. J Urol 2009;181:2009–17.
- McDougal S, Wein AJ, Kavoussi LR, et al. Campbell-Walsh Urology. 10th ed. Philadelphia (PA): Saunders; 2012.
- Colombo JR Jr, Haber GP, Kelovsek JE, et al. Seven years after laparoscopic radical nephrectomy: oncologic and renal functional outcomes. Urology 2008:71:1149–54.
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Ca 2013;49: 1374–403.
- Weight CJ, Larson BT, Fergany AF, et al. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol 2010;183:1317–23.
- Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomized EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2011;59:543–52.
- Smaldone MC, Fung C, Uzzo RG, Hass NB. Adjuvant and neoadjuvant therapies in high-risk renal cell carcioma. Hematol Oncol Clin North Am 2011;25:765–91.
- NCCN clinical practice guidelines in oncology. Version 3.2016. www.nccn.org. Accessed July 13, 2016
- El Dib R, Touma NJ, Kapoor A. Cryoablation vs radiofrequency ablation for the treatment of renal cell carcinoma: a meta-amalysis of case series studies. BJU Int 2012;110:510–6.
- Theodorescu D. Cancer cryotherapy: evolution and biology. Rev Urol 2004;6 Suppl 4:S9–S19.
- Khiatani V, Dixon RG. Renal ablation update. Sem Intervent Radiol 2014;31:157–66.
- Yu J, Liang P, Yu XL, et al. US-guided percutaneous microwave ablation of renal cell carcinoma: intermediate-term results. Radiol 2012;263:900–8.
- Castle SM, Salas N, Leveillee RJ. Initial experience using microwave ablation therapy for renal tumor treatment: 18- month follow-up. Urology 2011;77:792–7.
- Pech M, Janitzky A, Wendler JJ, et al. Irreversible electroporation of renal cell carcinoma: a first-in-man phase I clinical study. Cardiovasc Intervent Radiol 2011;34:132–8.
- Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr. Rising incidence of renal cell cancer in the United States. JAMA 1999;281:1628–31.
- Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology 1998;51:203–5.
- Pierorazio PM, Johnson MH, Ball MW, et al. Five-year analysis of a multi-institutional prospective clinical trial of delayed intervention and surveillance for small renal masses: the DISSRM registry. Eur Urol 2015;68:408–15.
- Jewett MA, Mattar K, Basiuk J, et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol 2011;60:39–44.
- Chawla SN, Crispen PL, Hanlon AL, et al. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol 2006;175:425–31.
- Smaldone MC, Kutikov A, Egleston BL, et al. Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer 2012;118:997–1006.
- Williamson TJ, Pearson JR, Ischia J, et al.Guideline of guidelines: follow-up after nephrectomy for renal cell carcinoma. BJU Int 2016;117:555–62.
- Donat S, Diaz M, Bishoff JT, et al. Follow-up for clinically localized renal neoplasms: AUA Guideline. J Urol 2013;190:407–16.
- Janzen NK, Kim HL, Figlin RA, Bell-degrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am 2003:30:843–52.
- Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socio-economic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev 2008;34:193–205.
- Mekhail T, Abou-Jawde R, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering Prognostic Factors Model for Survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 2005;23: 832–41.
- Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002;20:289–96.
- Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530–40.
- Heng DY, Xie W, Regan MM. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794–9.
- Leibovich BC, Han KR, Bui MH, et al. Scoring algorithm to predict survival after nephrectomy and immunotherapy in patients with metastatic renal cell carcinoma: A stratification tool for prospective clinical trials. Cancer 2003;98:2566–77.
- Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol 2004;171:1071–6.
- Choueiri TK, Xie W, Kollmannsberger C, et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol 2011;185:60–6.
- Chapin BF, Delacroix SE Jr, Culp SH, et al. Safety of presurgical targeted therapy in the setting of metastatic renal cell carcinoma. Eur Urol 2011;60:964–71.
- Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomized open-label phase 3 trial. Lancet Oncol 2013;14:1287–94.
- Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metatastic renal cell carcinoma: a randomized, double-blind phase III trial. Lancet 2007;370:2103–11.
- Escudier B, Bellmunt J, Negrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol 2010;28:2144–50.
- McDermott DF, Cheng SC, Signoretti S, et al. The high-dose aldesleukin “select”trial: a trial to prospectively validate predictive models of response to treatment in patients with metastatic renal cell carcinoma. Clin Cancer Res 2015;21:561–8.
- Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369:722–31.
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cell global evaluation trial. J Clin Oncol 2009;27:3312–8.
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115–24.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584–90.
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271–81.
- Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus and the combination in patients with metastatic renal cell carcinoma: a randomized, phase 2, open label, multicenter trial. Lancet Oncology 2015;16:1473–82.
- Lexi-Comp, Inc. (Lexi-Drugs® ). Lexi-Drugs version 2.3.3. Lexicomp. Wolters Kluwer Health, Inc. Hudson, OH.
- Choueiri TK, Plantade A, Elson P, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol 2008;26:127–31.
- Lee JL, Ahn JH, Lim HY, et al. Multicenter phase II study of sunitinib in patients with non-clear cell renal cell carcinoma. Ann Oncol 2012;23:2108–14.
- Armstrong AJ, Broderick S, Eisen T, et al. Final clinical results of a randomized phase II international trial of everolimus vs. sunitinib in patients with metastatic non-clear cell renal cell carcinoma (ASPEN). ASCO Meeting Abstracts 2015;33:4507.
- Chowdhury S, Matrana MR, Tsang C, et al. Systemic therapy for metastatic non-clear-cell renal cell carcinoma: recent progress and future directions. Hematol Oncol Clin North Am 2011;25:853–69.
- Escudier B, Droz JP, Rolland F, et al. Doxorubicin and ifosfamide in patients with metastatic sarcomatoid renal cell carcinoma: a phase II study of the Genitourinary Group of the French Federation of Cancer Centers. J Urol 2002; 168–71
- Nanus DM, Garino A, Milowsky MI, et al. Active chemotherapy for sarcomatoid and rapidly progressing renal cell carcinoma. Cancer 2004;101:1545–51.
- Michaelson MD, McKay RR, Werner L, et al. Phase 2 trial of sunitinib and gemcitabine in patients with sarcomatoid and/or poor-risk metastatic renal cell carcinoma. Cancer 2015;121:3435–43.
- McDermott DF, Cheng SC, Signoretti S, et al. The high-dose aldesleukin “select”trial: a trial to prospectively validate predictive models of response to treatment in patients with metastatic renal cell carcinoma. Clin Cancer Res 2015;21:561–8
- Cho DC, Puzanov I, Regan MM, et al. Retrospective analysis of the safety and efficacy of interleukin-2 after prior VEGF-targeted therapy in patients with advanced renal cell carcinoma. J Immunother 2009;32:181–5.
- Pyrhönen S, Salminen E, Ruutu M, et al. Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. J Clin Oncol 1999;17:2859–67.
- Interferon-alpha and survival in metastatic renal carcinoma: early results of a randomised controlled trial. Medical Research Council Renal Cancer Collaborators. Lancet 1999;353:14–7.
- Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003;349:427–34.
- Atkinson BJ, Kalra S, Wang X, et al. Clinical outcomes for patients with metastatic renal cell carcinoma treated with alternative sunitinib schedules. J Urol 2014;191:611–8.
- Kollmannsberger C, Bjarnason G, Burnett P, et al. Sunitinib in metastatic renal cell carcinoma: recommendations for management of noncardiovascular toxicities. Oncologist 2011;16:543–53.
- Najjar YG, Mittal K, Elson P, et al. A 2 weeks on and 1 week off schedule of sunitinib is associated with decreased toxicity in metastatic renal cell carcinoma. Eur J Cancer 2014;50:1084–9.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061–8.
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125–34
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931–9.
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1814–23.
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR) final results from a randomized, open-label, phase 3 trial. Lancet Oncology 2016;17:917–27.
- Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer 2004;4:335–48.
- Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008;372:449–56.
- Brahmer J, Tykodi S, Chow L, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–65.
- Thomson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow up. Cancer Res 2006;66: 3381–5.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13.
- Siegel R, Miller, K, Jemal A. Cancer Statistics, 2015. CA Cancer J Clin 2015;65:5–29.
- Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and genetics. Tumors of the urinary system and male genital organs. Lyon: IARC Press; 2004.
- Chow WH, Gridley G, Fraumeni JF Jr, Jarvholm B. Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med 2000;343:1305–11.
- Cohen H, McGovern F. Renal-cell carcinoma. N Engl J Med 2005;353:2477–90.
- Yao M, Yoshida M, Kishida T, et al. VHL tumor suppres sor gene alterations associated with good prognosis in sporadic clear-cell renal carcinoma. J Natl Cancer Inst 2002;94:1569–75.
- Iliopoulos O, Kibel A, Gray S, Kaelin WG Jr. Tumour suppression by the human von Hippel-Lindau gene product. Nat Med 1995;1:822–6
- Chen F, Kishida T, Duh FM, et al. Suppression of growth of renal carcinoma cells by the von Hippel-Lindau tumor suppressor gene. Cancer Res 1995;55:4804–7.
- Iliopoulos O, Levy AP, Jiang C, et al. Negative regulation of hypoxia-inducible genes by the von Hippel Lindau protein. Proc Natl Acad Sci U S A 1996;93:10595–9.
- Nickerson ML, Warren MB, Toro JR, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Bir- Hogg-Dube syndrome. Cancer Cell 2002;2:157–64
- Shuch B, Vorganit S, Ricketts CJ, et al. Defining early-onset kidney cancer: implications for germline and somatic mutation testing and clinical management. J Clin Oncol 2014;32:431–7.
- Bukowski RM. Immunotherapy in renal cell carcinoma. Oncology 1999;13:801–10.
- Mueller-Lisse UG, Mueller-Lisse UL. Imaging of advanced renal cell carcinoma. World J Urol 2010;28: 253–61.
- Edge SB, Byrd DR, Compton CC, et al, eds. AJCC cancer staging manual, 7th ed. New York: Springer Science and Business Media LLC; 2010.
- O’Malley RL, Godoy G, Kanofsky JA, Taneja SS. The necessity of adrenalectomy at the time of radical nephrectomy: a systematic review. J Urol 2009;181:2009–17.
- McDougal S, Wein AJ, Kavoussi LR, et al. Campbell-Walsh Urology. 10th ed. Philadelphia (PA): Saunders; 2012.
- Colombo JR Jr, Haber GP, Kelovsek JE, et al. Seven years after laparoscopic radical nephrectomy: oncologic and renal functional outcomes. Urology 2008:71:1149–54.
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Ca 2013;49: 1374–403.
- Weight CJ, Larson BT, Fergany AF, et al. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol 2010;183:1317–23.
- Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomized EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2011;59:543–52.
- Smaldone MC, Fung C, Uzzo RG, Hass NB. Adjuvant and neoadjuvant therapies in high-risk renal cell carcioma. Hematol Oncol Clin North Am 2011;25:765–91.
- NCCN clinical practice guidelines in oncology. Version 3.2016. www.nccn.org. Accessed July 13, 2016
- El Dib R, Touma NJ, Kapoor A. Cryoablation vs radiofrequency ablation for the treatment of renal cell carcinoma: a meta-amalysis of case series studies. BJU Int 2012;110:510–6.
- Theodorescu D. Cancer cryotherapy: evolution and biology. Rev Urol 2004;6 Suppl 4:S9–S19.
- Khiatani V, Dixon RG. Renal ablation update. Sem Intervent Radiol 2014;31:157–66.
- Yu J, Liang P, Yu XL, et al. US-guided percutaneous microwave ablation of renal cell carcinoma: intermediate-term results. Radiol 2012;263:900–8.
- Castle SM, Salas N, Leveillee RJ. Initial experience using microwave ablation therapy for renal tumor treatment: 18- month follow-up. Urology 2011;77:792–7.
- Pech M, Janitzky A, Wendler JJ, et al. Irreversible electroporation of renal cell carcinoma: a first-in-man phase I clinical study. Cardiovasc Intervent Radiol 2011;34:132–8.
- Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr. Rising incidence of renal cell cancer in the United States. JAMA 1999;281:1628–31.
- Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology 1998;51:203–5.
- Pierorazio PM, Johnson MH, Ball MW, et al. Five-year analysis of a multi-institutional prospective clinical trial of delayed intervention and surveillance for small renal masses: the DISSRM registry. Eur Urol 2015;68:408–15.
- Jewett MA, Mattar K, Basiuk J, et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol 2011;60:39–44.
- Chawla SN, Crispen PL, Hanlon AL, et al. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol 2006;175:425–31.
- Smaldone MC, Kutikov A, Egleston BL, et al. Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer 2012;118:997–1006.
- Williamson TJ, Pearson JR, Ischia J, et al.Guideline of guidelines: follow-up after nephrectomy for renal cell carcinoma. BJU Int 2016;117:555–62.
- Donat S, Diaz M, Bishoff JT, et al. Follow-up for clinically localized renal neoplasms: AUA Guideline. J Urol 2013;190:407–16.
- Janzen NK, Kim HL, Figlin RA, Bell-degrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am 2003:30:843–52.
- Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socio-economic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev 2008;34:193–205.
- Mekhail T, Abou-Jawde R, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering Prognostic Factors Model for Survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 2005;23: 832–41.
- Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002;20:289–96.
- Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530–40.
- Heng DY, Xie W, Regan MM. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794–9.
- Leibovich BC, Han KR, Bui MH, et al. Scoring algorithm to predict survival after nephrectomy and immunotherapy in patients with metastatic renal cell carcinoma: A stratification tool for prospective clinical trials. Cancer 2003;98:2566–77.
- Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol 2004;171:1071–6.
- Choueiri TK, Xie W, Kollmannsberger C, et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol 2011;185:60–6.
- Chapin BF, Delacroix SE Jr, Culp SH, et al. Safety of presurgical targeted therapy in the setting of metastatic renal cell carcinoma. Eur Urol 2011;60:964–71.
- Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomized open-label phase 3 trial. Lancet Oncol 2013;14:1287–94.
- Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metatastic renal cell carcinoma: a randomized, double-blind phase III trial. Lancet 2007;370:2103–11.
- Escudier B, Bellmunt J, Negrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol 2010;28:2144–50.
- McDermott DF, Cheng SC, Signoretti S, et al. The high-dose aldesleukin “select”trial: a trial to prospectively validate predictive models of response to treatment in patients with metastatic renal cell carcinoma. Clin Cancer Res 2015;21:561–8.
- Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369:722–31.
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cell global evaluation trial. J Clin Oncol 2009;27:3312–8.
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115–24.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584–90.
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271–81.
- Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus and the combination in patients with metastatic renal cell carcinoma: a randomized, phase 2, open label, multicenter trial. Lancet Oncology 2015;16:1473–82.
- Lexi-Comp, Inc. (Lexi-Drugs® ). Lexi-Drugs version 2.3.3. Lexicomp. Wolters Kluwer Health, Inc. Hudson, OH.
- Choueiri TK, Plantade A, Elson P, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol 2008;26:127–31.
- Lee JL, Ahn JH, Lim HY, et al. Multicenter phase II study of sunitinib in patients with non-clear cell renal cell carcinoma. Ann Oncol 2012;23:2108–14.
- Armstrong AJ, Broderick S, Eisen T, et al. Final clinical results of a randomized phase II international trial of everolimus vs. sunitinib in patients with metastatic non-clear cell renal cell carcinoma (ASPEN). ASCO Meeting Abstracts 2015;33:4507.
- Chowdhury S, Matrana MR, Tsang C, et al. Systemic therapy for metastatic non-clear-cell renal cell carcinoma: recent progress and future directions. Hematol Oncol Clin North Am 2011;25:853–69.
- Escudier B, Droz JP, Rolland F, et al. Doxorubicin and ifosfamide in patients with metastatic sarcomatoid renal cell carcinoma: a phase II study of the Genitourinary Group of the French Federation of Cancer Centers. J Urol 2002; 168–71
- Nanus DM, Garino A, Milowsky MI, et al. Active chemotherapy for sarcomatoid and rapidly progressing renal cell carcinoma. Cancer 2004;101:1545–51.
- Michaelson MD, McKay RR, Werner L, et al. Phase 2 trial of sunitinib and gemcitabine in patients with sarcomatoid and/or poor-risk metastatic renal cell carcinoma. Cancer 2015;121:3435–43.
- McDermott DF, Cheng SC, Signoretti S, et al. The high-dose aldesleukin “select”trial: a trial to prospectively validate predictive models of response to treatment in patients with metastatic renal cell carcinoma. Clin Cancer Res 2015;21:561–8
- Cho DC, Puzanov I, Regan MM, et al. Retrospective analysis of the safety and efficacy of interleukin-2 after prior VEGF-targeted therapy in patients with advanced renal cell carcinoma. J Immunother 2009;32:181–5.
- Pyrhönen S, Salminen E, Ruutu M, et al. Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. J Clin Oncol 1999;17:2859–67.
- Interferon-alpha and survival in metastatic renal carcinoma: early results of a randomised controlled trial. Medical Research Council Renal Cancer Collaborators. Lancet 1999;353:14–7.
- Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003;349:427–34.
- Atkinson BJ, Kalra S, Wang X, et al. Clinical outcomes for patients with metastatic renal cell carcinoma treated with alternative sunitinib schedules. J Urol 2014;191:611–8.
- Kollmannsberger C, Bjarnason G, Burnett P, et al. Sunitinib in metastatic renal cell carcinoma: recommendations for management of noncardiovascular toxicities. Oncologist 2011;16:543–53.
- Najjar YG, Mittal K, Elson P, et al. A 2 weeks on and 1 week off schedule of sunitinib is associated with decreased toxicity in metastatic renal cell carcinoma. Eur J Cancer 2014;50:1084–9.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061–8.
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125–34
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931–9.
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1814–23.
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR) final results from a randomized, open-label, phase 3 trial. Lancet Oncology 2016;17:917–27.
- Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer 2004;4:335–48.
- Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008;372:449–56.
- Brahmer J, Tykodi S, Chow L, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–65.
- Thomson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow up. Cancer Res 2006;66: 3381–5.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13.
Soft Tissue Sarcoma: Diagnosis and Treatment
INTRODUCTION
Soft tissue sarcomas (STSs) are rare adult tumors, with 3.4 new cases per 100,000 persons or 12,310 expected new cases in 2016.1 Sarcomas are a heterogeneous collection of tumors that affect fat, muscle, nerve, nerve sheath, vascular, and connective tissues. There are more than 50 histological subtypes that comprise this diverse category of tumors. Treatment varies by stage, with limb-sparing surgery representing the mainstay of curative-intent treatment. Radiation and chemotherapy may also be considered depending on the size, grade, and location of the tumor. Survival rates have been stagnant until recently, with a disease-specific survival hovering around 65%.1 Given the complexity of these cases, all patients ideally should be evaluated and treated by a multidisciplinary team at an institution with extensive experience treating STS.2
EPIDEMIOLOGY AND CLASSIFICATION
The most common STS subtypes are gastrointestinal stromal tumor (GIST), undifferentiate pleomorphic sarcoma (previously referred to as malignant fibrous histiocytoma), liposarcoma, leiomyosarcoma, synovial sarcoma, malignant peripheral nerve sheath tumor, rhabdomyosarcoma, and unclassified sarcoma.3 Liposarcoma is one of the most common subtypes, comprising 20% of all STSs; it is subdivided into well-differentiated/dedifferentiated liposarcomas, myxoid/round cell liposarcomas, and pleomorphic liposarcomas. Well-differentiated liposarcomas tend to occur in the retroperitoneum and limbs, while both myxoid and round cell as well as pleomorphic liposarcomas more commonly originate on the limbs. Histology varies based on subtype and ranges from mature-appearing adipocytes and fibroblasts to undifferentiated cells with minimal lipogenic differentiation.4
Leiomyosarcomas are smooth muscle tumors and are usually located in the retroperitoneum, but have also been associated with peripheral soft tissue and vasculature. Typical histology ranges from well-defined areas of spindle-shaped cells to poorly differentiated anaplastic spindle cells.5,6 Synovial sarcomas are a distinct type of STS that can show epithelial differentiation and account for 5% of adult STSs. The extremities are the most common presenting location (90%).7
Rhabdomyosarcomas are skeletal muscle tumors and are further subdivided into embryonal, alveolar, and pleomorphic subtypes. Embryonal histology ranges from primitive mesenchymal-appearing cells to highly differentiated muscle cells. Alveolar rhabdomyosarcoma has the worst prognosis of the subtypes and consists of round cells with high nuclear-to-chromatin ratios that form “glandular-like” or “alveolar” spaces.8 Pleomorphic rhabdomyosarcomas are composed of rhabdomyoblasts that can affect many different locations, but most commonly present on the lower extremities.9
Malignant peripheral nerve sheath tumor (MPNST) comprises 5% to 10% of all STSs. These tumors are associated with neurofibromatosis type 1 (NF-1), with 25% to 50% of tumors occurring in NF-1 patients. Additionally, most patients have a truncating lesion in the NF1 gene on chromosome 17.10 Anghileri et al in their single institution analysis of 205 patients with MPNSTs found the 2 most common presenting sites were the trunk and extremities. Histologically, these tumors have dense fascicles of spindle cells.10
GISTs are the most common STS of the gastrointestinal (GI) tract. Previously, GISTs were classified as smooth muscle tumors and were not accounted for in the literature as a separate entity distinct from leiomyomas, leiomyoblastomas, and leiomyosarcomas.11 GISTs are found throughout the GI tract: the most common sites are the stomach (60%) and small intestine (30%). Less common sites include duodenum (4%–5%), esophagus (1%), rectum (1%–2%), and appendix (< 0.2%).12 GISTs can be spindle cell, epithelioid, or mesenchymal tumors. Immunohistochemically, GISTs are KIT (CD117) positive. Other cell markers that are also commonly positive include CD34 (60%–70%) and smooth muscle actin (SMA) (25%).11 The majority of GISTs (80%) have an activating c-KIT gene mutation. The most common mutation site is exon 11, with less common c-KIT gene mutations also occurring at exon 9 or 13. Not all GISTs have KIT mutations. The second most common mutation is the PDGFRA mutation (5%–10% of GISTs).2 A minority of GISTs are negative for both KIT and PDGFRA mutations. These tumors were previously called wild-type, but as the majority have either a succinate dehydrogenase (SDH) loss of function or loss of SDHB protein expression, they are now referred to as SDH-deficient GISTs.2 GISTs vary in aggressiveness from incidental to aggressive. Typically, small intestine and rectal GISTs are more aggressive than gastric GISTs. Both size and mitotic rate help to predict the metastatic potential of the tumor. Tumors less than 2 cm in size and having a mitotic rate of less than 5 per 50 high-power fields (hpf) have the lowest risk of metastases, while tumors greater than 5 cm and with more than 5 mitoses per 50 hpf have the highest rates of metastases.12
Angiosarcomas are rare tumors comprising 4% of all STSs. Although they can occur in any site, the majority are cutaneous and occur most frequently in the head and neck regions. These tumors are either of vascular or lymphatic origin and are comprised of abnormal, pleomorphic, malignant endothelial cells. The most useful immunohistochemical markers include von Willebrand factor, CD31, and Ulex europaeus agglutinin 1. The majority of these tumors occur sporadically; however, radiation exposure, chronic lymphedema, and certain toxins including vinyl chloride and thorium dioxide are known risk factors.13
Undifferentiated sarcomas have no specific features and typically consist of primitive mesenchymal cells.
CLINICAL EVALUATION
CASE PRESENTATION
Initial Presentation and History
A 55-year-old man presents to his primary care physician with a painless mass in his anterior thigh. The mass has been present for the past 3 months and he believes that it is enlarging. The patient has a history of well-controlled hypertension and hyperlipidemia. His medications include atorvastatin and hydrochlorothiazide. He has no known drug allergies. Family history is notable for diabetes and hypertension. He drinks 4 to 5 alcoholic drinks a week and he is a former smoker. He quit smoking in his 30s and only smoked intermittently prior to quitting. He denies any illicit drug use. He works as a high school principal. Currently, he feels well. His review of systems is otherwise noncontributory.
Physical Examination
On physical exam, he is afebrile with a blood pressure of 132/75 mm Hg, respiratory rate of 10 breaths/min, and oxygen saturation of 99% on room air. He is a well appearing, overweight male. His head and neck exam is unremarkable. Lung exam reveals clear breath sounds, and cardiac exam reveals a regular rate and rhythm. His abdomen is obese, soft, and without hepatosplenomegaly. There is a large, fixed mass on the anterior lateral aspect of his right thigh. He has no appreciable lymphadenopathy. His neurological exam is unremarkable.
• What are risk factors for sarcoma?
There are few known risk factors for sarcoma. Established risks factors include prior radiation therapy, chronic lymphedema, viruses, and genetic cancer syndromes including Li-Fraumeni syndrome, hereditary retinoblastoma, and NF-1. Other environmental exposures include phenoxyacetic acids and chlorophenols.14 The majority of cases are sporadic, with only a minority of patients having one of these known risk factors.15 Up to one third of sarcomas have a specific translocation and are driven by fusion oncogenes (Table 1).
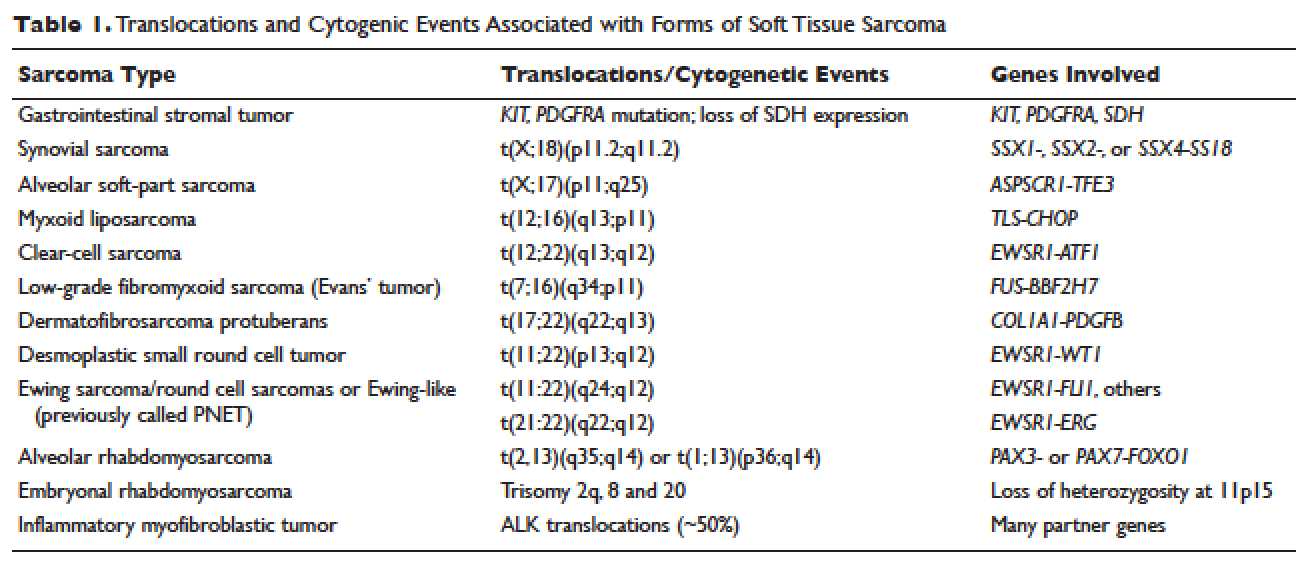
A painless mass is the most typical presenting symptom. Size at presentation varies based on location, with extremity and head and neck locations typically presenting at smaller sizes than retroperitoneal tumors.14 Patients may experience pain and numbness as the mass enlarges and impinges on surrounding structures including nerves and vasculature. The vast majority of patients are without systemic symptoms.
• How is sarcoma staged?
The American Joint Committee on Cancer (AJCC) staging system is the most widely used staging system in the United States. The latest AJCC manual was updated in 2010 to include a 3-tiered grading system where the tumor is classified according to tumor size, lymph node involvement, metastases, and grade at time of diagnosis (Table 2 and Table 3). Additionally, tumor depth in relation to deep fascia is also taken into account, with superficial tumors being assigned a designation of “a” and deep tumors a designation of “b.”
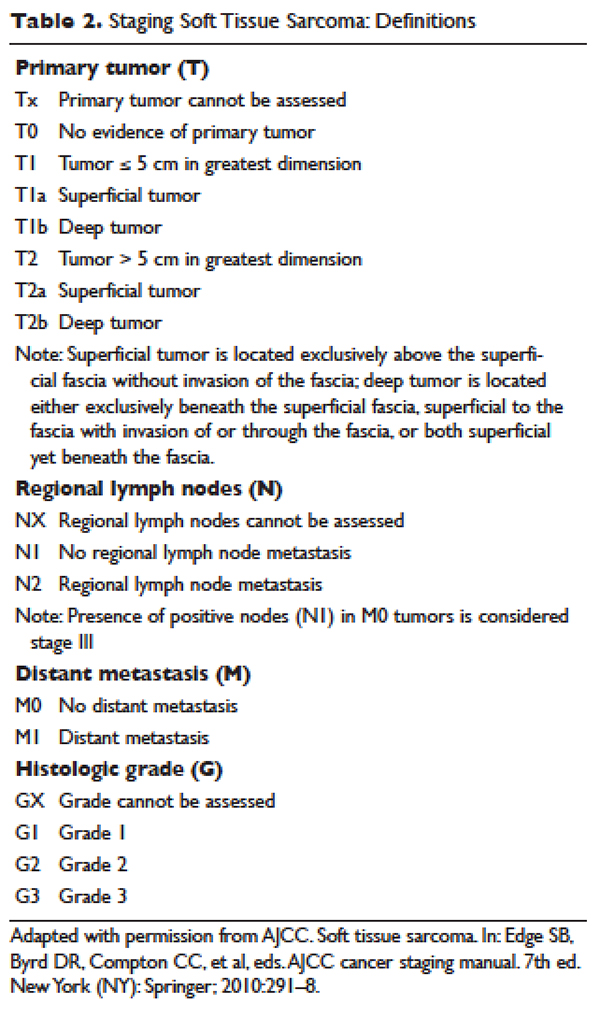
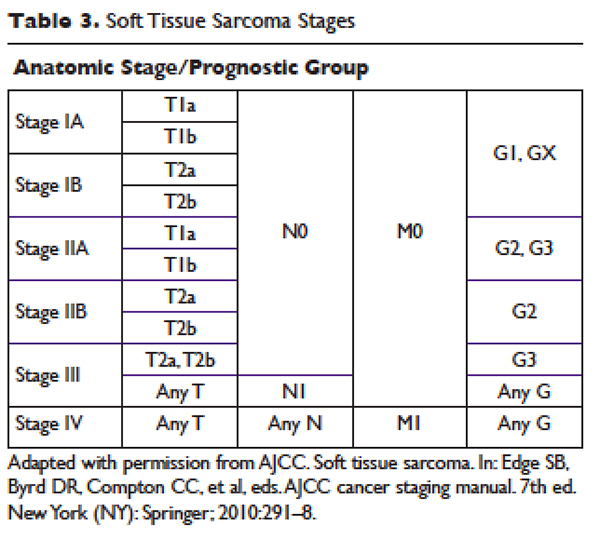
Previously, 2 of the most widely used grading systems were the National Cancer Institute (NCI) and French Federation of Cancer Centers Sarcoma Group (FNCLCC) systems, both 3-tier grading systems. The main components that determine the NCI grade are the tumor’s histologic type and location and the amount of tumor necrosis. The FNCLCC system evaluation focuses on tumor differentiation, mitotic rate, and amount of tumor necrosis. A study that compared the NCI and FNCLCC grading systems found that FNCLCC was a better predictor of mortality and distant metastasis.16 Previously, the AJCC was a 4-tier grading system, but the 2010 version was updated to the 3-tier FNCLCC grading system. Additionally, the AJCC system has reclassified single lymph node disease as stage III as it confers better survival than metastatic disease.17 It is important that pathology be evaluated by a sarcoma specialist as disagreements with regard to histologic subtype and grade are common.18,19
• What are the most important prognostic factors?
Prognostic factors include grade, size, and presence of metastases at presentation. Best survival is associated with low-grade, small tumors with no metastases at time of diagnosis.14
• What imaging should be considered?
Imaging should be undertaken to help differentiate between benign and malignant lesions. Ideally, it should be undertaken before a biopsy is planned as the imaging can be used to plan biopsy as well as provide invaluable prognostic information. There are several imaging modalities that should be considered during the preliminary work-up and staging of STSs. Conventional imaging includes magnetic resonance imaging (MRI) of the original tumor site; computed tomography (CT) to evaluate for pulmonary metastases and, depending on location, liver metastases; and in the case of small, low-grade tumors, chest radiography. MRI is considered the test of choice for soft tissue masses and can help delineate benign masses such as hematomas, lipomas, and hemangiomas from sarcomas.20 It is difficult to compare the accuracy of positron emission tomography (PET)/CT to CT and MRI because most studies have evaluated PET/CT in parallel with CT and MRI.21 Tateishi et al compared the accuracy of conventional imaging, PET/CT, and PET/CT combined with conventional imaging at determining the TNM staging for 117 patients. They found that conventional imaging correctly classified 77% of patients, PET alone correctly classified 70%, PET/CT correctly classified 83%, and PET/CT combined with conventional imaging correctly staged 87%.22
• Which subtypes are most likely to metastasize?
Although the vast majority of sarcomas spread hematogenously, 3 have a propensity to spread lymphogenously: epithelioid sarcoma, rhabdomyosarcoma, and clear-cell sarcoma. Additionally, certain subtypes are more likely to metastasize: leiomyosarcomas, synovial sarcomas, neurogenic sarcomas, rhabdomyosarcomas, and epithelioid sarcomas.23 Sarcomas metastasize to the lungs more frequently than to the liver. The metastatic pattern is defined primarily by sarcoma subtype and site of primary tumor. Sarcomas rarely metastasize to the brain (~1%).
MANAGEMENT
CASE CONTINUED
The patient undergoes an ultrasound to better visualize the mass. Given the heterogeneous character of the mass, he is referred for an MRI to evaluate the mass and a CT scan of the chest, abdomen, and pelvis to evaluate for distant metastases. MRI reveals a 5.1 cm × 4.6 cm heterogeneous mass invading the superficial fascia of the rectus femoris muscle. No suspicious lymph nodes or other masses are identified on imaging. The patient next undergoes an image-guided core needle biopsy. Pathology from that procedure is consistent with a stage III, T2bNxMx, grade 3, dedifferentiated liposarcoma.
• What is the best management approach for this patient?
SURGERY
Surgery is the mainstay of treatment for STS. Patients with the best prognosis are those who undergo complete resection with negative surgical margins.24,25 Goal tumor-free margin is 1 to 3 cm.26 Complete resection confers the best long-term survival. Both local and metastatic recurrence is higher in patients with incomplete resection and positive margins.24,25 In a study that analyzed 2084 localized primary STSs, patients with negative margins had a local recurrence rate of 15% versus a rate of 28% in patients with positive margins. This translated into higher 5-year local recurrence-free survival for patients with negative surgical margins (82%) compared to patients with positive margins (65%).27 Another study similarly found that patients with negative margins at referral to their institution who underwent postoperative radiation had high local control rates of 93% (95% confidence interval [CI] 87% to 97%) at 5, 10, and 15 years.26 Although radiation improves local control, neither preoperative or postoperative radiation has been shown to improve progression-free or overall survival.28 Other factors that are associated with risk of recurrence are tumor location, history of previous recurrence, age of patient, histopathology, tumor grade, and tumor size. Approximately 40% to 50% of patients with high-grade tumors (defined as size > 5 cm, deep location, and high grade) will develop distant metastases.29
Zagars et al found that positive or uncertain resection margin had a relative risk of local recurrence of 2.0 (95% CI 1.3 to 3.1; P = 0.002), and presentation with locally recurrent disease (vs new tumor) had a relative risk of local recurrence of 2.0 (95% CI 1.2 to 3.4; P = 0.013).26 Patients with STS of head and neck and deep trunk have higher recurrence rates than those with superficial trunk and extremity STS. A single-institution retrospective review demonstrated that patients with completely resectable retroperitoneal sarcomas have longer median survival (103 months) compared to patients with incompletely resected abdominal sarcomas (18 months).25
Rosenberg and colleagues compared amputation to limb-sparing surgery and radiation.24 Their prospective analysis of 65 patients found no difference in disease-free and overall survival between the 2 treatment groups.The limb-sparing treatment group had higher rates of local recurrence, which was highly correlated with positive surgical margins on pathology.24 Evidence from this and similar studies has resulted in radical amputations being replaced by conservative limb-sparing procedures and radiation therapy. In those found to have positive margins, re-resection is an option for some. Patients who undergo re-resection have higher local control rates than patients with positive margins who do not undergo re-resection. The 5-year control rate for patients who undergo re-resection is 85% (95% CI 80% to 89%) compared to 78% (95% CI 71% to 83%) for those who do not undergo re-resection. Similarly, patients who undergo re-resection have lower rates of metastases at 5, 10, and 15 years as well as higher 5-, 10-, and 15-year disease-free survival rates.26
CASE CONTINUED
The patient is referred for limb-sparing surgery after presentation at a multidisciplinary tumor board. Prior to undergoing resection of the tumor, he is also referred to radiation-oncology to discuss the risks and benefits of combination radiotherapy and surgery as opposed to surgical resection alone.
• What is the evidence for radiation therapy?
RADIATION THERAPY
Radiation therapy is used in the preoperative, intraoperative, and postoperative settings to reduce the risk of local recurrence. There are several options for radiation, including external beam radiation therapy (EBRT), intraoperative radiation, and brachytherapy. A newer strategy, intensity-modulated radiation therapy (IMRT), utilizes 3-dimensional modeling to reduce radiation dosages. Overall there are no differences in overall survival or local recurrence rates between preoperative and postoperative radiation in STS.28
The rationale behind preoperative radiation is that it reduces seeding of tumor cells, especially at the time of surgery.30 Additionally, for EBRT, preoperative radiation has smaller field sizes and lower radiation doses. It can also help to reduce the size of the tumor prior to resection. Intraoperative radiation is often paired with preoperative radiation as a boost dose given only to the area of residual tumor.
Suit et al reviewed patients treated at a single institution with limb-sparing surgery and different radiation strategies. Local control rates between preoperative and postoperative radiation groups were not statistically significant. Local recurrence was linked to grade and size of the tumor in both groups. The authors did note, however, that the preoperative radiation group tended to have larger tumor sizes at baseline compared to the patients who received postoperative radiation.30 A study that compared 190 patients who received preoperative and postoperative EBRT or brachytherapy (primary end point was wound complications, and local control was a secondary end point) showed a trend towards greater local control with preoperative radiation; however, the preoperative radiation group had significantly more wound complications compared to the postoperative radiation group.31
Yang et al found that postoperative EBRT decreases rates of local recurrence compared to surgery alone in high-grade extremity sarcomas.32 However, there were no differences in rates of distant metastases and overall survival between the 2 treatment groups. Similarly, in patients with low-grade sarcoma, there were fewer local recurrences in those who received EBRT and surgery as compared to surgery alone.32 Another study that evaluated 164 patients who received either adjuvant brachytherapy or no further therapy after complete resection found that brachytherapy reduced local recurrence in high-grade sarcomas. No difference in local recurrence rates was found in patients with low-grade sarcomas, nor was a significant difference found in the rates of distant metastases and overall survival between the 2 treatment groups.33 With regards to IMRT, a single institution cohort experience with 41 patients who received IMRT following limb-sparing surgery had similar local control rates when compared to historical controls.34
CASE CONTINUED
After discussion of the risks and benefits of radiation therapy, the patient opts for preoperative radiation prior to resection of his liposarcoma. He receives 50 Gy of EBRT prior to undergoing resection. Resection results in R1 margin consistent with microscopic disease. He receives 16 Gy of EBRT as a boost after recovery from his resection.2
• What is the evidence for neoadjuvant and adjuvant chemotherapy for stage I tumors?
CHEMOTHERAPY
Localized Sarcoma
For localized sarcoma, limb-sparing resection with or without radiation forms the backbone of treatment. Studies have evaluated chemotherapy in both the neoadjuvant and adjuvant settings, with the vast majority of studies evaluating doxorubicin-based chemotherapy regimens in the adjuvant settings. Due to the rare nature of sarcomas, most studies are not sufficiently powered to detect significant benefit from chemotherapy. Several trials evaluating chemotherapy regimens in the neoadjuvant and adjuvant settings needed to be terminated prematurely due to inadequate enrollment into the study. 35,36
For stage IA (T1a-Tb, N0, M0, low grade) tumors, no additional therapy is recommended after limb-sparing surgery with appropriate surgical margins. For stage IB (T2a-2b, N0, M0, low grade) tumors with insufficient margins, re-resection and radiation therapy should be considered, while for stage IIA (T1a-1b, N0, M0, G2-3) tumors preoperative or postoperative radiation therapy is recommended.2 Studies have not found benefit of adjuvant chemotherapy in these low-grade, stage I tumors in terms of progression-free survival and overall survival.37
• At what stage should chemotherapy be considered?
For stage IIb and stage III tumors, surgery and radiation therapy again form the backbone of therapy; however, neoadjuvant and adjuvant chemotherapy are also recommended as considerations. Anthracycline-based chemotherapy with either single-agent doxorubicin or doxorubicin and ifosfamide in combination are considered first-line chemotherapy agents in locally advanced STS.2,29,37
Evidence regarding the efficacy of both neoadjuvant and adjuvant chemotherapy regimens in the setting of locally advanced high-grade STS has been mixed. The Sarcoma Meta-analysis Collaboration evaluated 14 trials of doxorubicin-based adjuvant chemotherapy and found a trend towards overall survival in the treatment groups that received chemotherapy.37 All trials included in the meta-analysis compared patients with localized resectable soft-tissue sarcomas who were randomized to either adjuvant chemotherapy or no adjuvant chemotherapy after limb-sparing surgery with or without radiation therapy. None of the individual trials showed a significant benefit, and all trials had large confidence intervals; however, the meta-analysis showed significant benefit in the chemotherapy treatment groups with regard to local recurrence, distant recurrence, and progression-free survival. No significant difference in overall survival was found.37 Pervais et al updated the Sarcoma Meta-analysis Collaboration’s 1997 meta-analysis with the inclusion of 4 new trials that evaluated doxorubicin combined with ifosfamide and found that both patients who received doxorubicin-based regimens or doxorubicin with ifosfamide had significant decreases in distant and overall recurrences. Only the trials that utilized doxorubicin and ifosfamide had an improved overall survival that was statistically significant (hazard ratio 0.56 [95% CI 0.36 to 0.85]; P = 0.01).29 Although no significant heterogeneity was found among the trials included in either meta-analysis, a variety of sarcomas were included in each clinical trial evaluated. Given the extremely small number of each sarcoma subtype present in each trial, subgroup analysis is difficult and prone to inaccuracies. As a result, it is not known if certain histological subtypes are more or less responsive to chemotherapy.37–39
One randomized controlled trial evaluated neoadjuvant chemotherapy in high-risk sarcomas defined as tumors greater than 8 cm or grade II/III tumors. This study evaluated doxorubicin and ifosfamide and found no significant difference in disease-free and overall survival in the neoadjuvant therapy group compared to the control group.35 There remains controversy in the literature with regards to adjuvant chemotherapy. Many oncologists offer adjuvant chemotherapy to patients with certain stage III subtypes. Examples of subtypes that may be offered adjuvant therapy include myxoid liposarcomas, synovial sarcomas, and leiomyosarcomas.2 With regards to how many cycles of chemotherapy should be considered, a noninferiority study compared 3 cycles of epirubicin and ifosfamide to 5 cycles of epirubicin and ifosfamide in patients with high-risk locally advanced adult STSs. Three cycles of preoperative epirubicin and ifosfamide was found to be noninferior to 5 cycles with regards to overall survival.38
• What is this patient’s risk for recurrence?
The patient is at intermediate risk for recurrence. Numerous studies have demonstrated that tumor size, grade, and location are the most important factors to determine risk of recurrence, with larger size, higher grades, and deeper locations being associated with higher risk of recurrence. In an analysis of 1041 patients with STS of the extremities, high grade was the most important risk factor for distant metastases.39 The highest risk of recurrence is within the first 2 years. Given that the patient’s initial tumor was located in the extremity, he is more likely to have a distant metastasis as his site of recurrence; individuals with retroperitoneal tumors and visceral tumors are more likely to recur locally.40 For STSs of the extremity, distant metastases determine overall survival, whereas patients with retroperitoneal sarcomas can die from complications of local metastases.41 Once a patient develops distant metastases, the most important prognostic factor is the size of the tumor, with tumors larger than 10 cm having a relative risk of 1.5 (95% CI 1.0 to 2.0).39
• What are the recommendations for surveillance?
Surveillance recommendations are based on the stage of the sarcoma. Stage I tumors are the least likely to recur either locally or distally. As a result, it is recommended that stage I tumors be followed with history and physical exam every 3 to 6 months for the first 2 to 3 years, and then annually after the first 2 to 3 years. Chest x-rays should be considered every 6 to 12 months.2 For stage II–IV tumors, history and physical exam is recommended every 3 to 6 months for the first 2 to 3 years. Chest and distant metastases imaging should also be performed every 3 to 6 months during this time frame. For the next 2 years, history and physical exam and imaging are recommended every 6 months. After the first 4 to 5 years, annual follow-up is recommended.2
A study that followed 141 patients with primary extremity STSs for a median interval of 49 months found that high-grade tumors were most likely to recur during the first 2 years, with 20% of their patients recurring locally and 40% recurring distally. Chest x-rays performed during surveillance follow-up found distant lung metastases in 36 asymptomatic patients and had a positive predictive value of 92%, a negative predictive value of 97%, and a quality-adjusted life-year of $30,000.40,41 No laboratory testing was found to aid in detection of recurrence.
CASE CONTINUED
The patient does well for 1 year. With physical therapy, he regains most of the strength and coordination of the lower extremity. He is followed every 3 months with chest x-rays and a MRI of the thigh for the first year. On his fourth follow-up clinic visit, he describes increased dyspnea on exertion over the previous few weeks and is found to have multiple lung metastases in both lungs on chest x-ray. He undergoes further evaluation for metastases and is not found to have any other metastatic lesions. Bronchoscopy and biopsy of 1 of the lung nodules confirms recurrent dedifferentiated liposarcoma.
• Should this patient undergo metastectomy?
An analysis of 3149 patients with STS treated at Memorial Sloan-Kettering who developed lung metastases found that patients with pulmonary metastases have survival rates of 25%. The most important prognostic factor for survival was complete resection of all metastases.42 For stage IV disease, surgery is used only in certain instances. In instances where tumor is more localized or limited, removal of metastases or metastectomy can play a role in management.2
CASE CONTINUED
Because the patient’s metastases are limited to the lungs, he is referred for metastectomy. He undergoes wedge resection for definitive diagnosis but it is not possible to completely resect all of the metastases. He is thus referred to a medical oncologist to discuss his treatment options.
• What are treatment options for unresectable or metastatic disease?
Metastatic Disease
Unlike local and locally advanced disease, chemotherapy forms the backbone of treatment in stage IV disease. Doxorubicin and olaratumab or doxorubicin and ifosfamide in combination are considered first line in metastatic disease. Response rates for single-agent doxorubicin range from 16% to 27%, while phase 2 and phase 3 studies of doxorubicin and ifosfamide have found response rates ranging from 18% to 36%.43 In addition, the effectiveness of doxorubicin and ifosfamide phase 2 and 3 trials varied. Edmonson et al found a tumor regression rate of 34% for doxorubicin and ifosfamide as compared to 20% for doxorubicin alone.44 In comparison, Santoro et al found a response rate of 21.3% for doxorubicin alone and 25.2% for doxorubicin and ifosfamide.45 Neither study found increased survival benefit for doxorubicin and ifosfamide when compared to doxorubicin alone. In a Cochrane review evaluating randomized trials that compared doxorubicin and combination chemotherapy regimens, response rates varied from 14% for doxorubicin in combination with streptomycin to 34% for doxorubicin and ifosfamide. Most trials did not show a significant benefit for combination therapies when compared to doxorubicin alone.43 Mean survival with doxorubicin or doxorubicin and ifosfamide is 12 months. High rates of recurrence highlight the need for additional chemotherapy regimens.
The newest approved agent is olaratumab, a monoclonal antibody that binds platelet-derived growth factor receptor alpha and prevents receptor activation. A phase 1-b and phase 2 trial evaluated patients with locally advanced and metastatic STS and randomly assigned them to either olaratumab and doxorubicin or doxorubicin alone.46 Progression-free survival for olaratumab/doxorubicin was 6.6 months (95% CI 4.1 to 8.3) compared to 4.1 months (95% CI 2.8 to 5.4) for doxorubicin alone. The objective response rate was 18.2% (95% CI 9.8 to 29.6) for olaratumab/doxorubicin compared to 7.5% (95% CI 2.5 to 6.6) for doxorubicin alone. Furthermore, the median overall survival for olaratumab plus doxorubicin was 26.5 months (95% CI 20.9 to 31.7) compared to 14.7 months for doxorubicin alone (95% CI 5.5 to 26.0). Impressively, this improved response was notable across histological types. Furthermore, patients who had previously been treated with more than 1 regimen and those who were treatment naïve had similar response rates.46
• What are second-line treatment options?
Doxorubicin has been used in combination with several other agents including dacarbazine (DTIC) as well as DTIC and ifosfamide (MAID). Borden et al evaluated patients with metastatic STS and randomly assigned the patients to either doxorubicin or doxorubicin and DTIC. Combination therapy demonstrated better tumor response than doxorubicin alone: 30% complete or partial response for combination therapy and 18% for doxorubicin alone.47 However, Omura et al
found similar rates of efficacy between doxorubicin and combination doxorubicin and DTIC in women with recurrent or nonresectable uterine sarcomas.48 MAID has never been directly compared in a randomized trial to doxorubicin alone. In a study that compared MAID to doxorubicin and DTIC (AD) in patients with unresectable or metastatic sarcomas, MAID had superior response rates (32% versus 17%), but there was no difference with regards to overall survival (mean survival of 12.5 months).49
Several additional regimens have undergone evaluation in metastatic and recurrent STSs. Gemcitabine has been used both as a single agent and as part of combination therapy in many studies. Studies with gemcitabine in combination with either docetaxel or DTIC have been the most efficacious. In a phase 2 trial, patients with metastatic STS were randomly assigned to either gemcitabine alone or gemcitabine and docetaxel. Combination therapy had a higher response rate (16% versus 8%) and longer overall survival (17.9 months versus 11.5 months) than gemcitabine alone.50 Furthermore, a phase 2 trial of gemcitabine and docetaxel in patients with unresectable leiomyosarcoma showed an overall response rate of 56%, with 3 complete and 15 partial responses among the 34 patients enrolled in the study.51
A phase 2 trial randomly assigned patients with unresectable or metastatic STS to either DTIC or combination gemcitabine and DTIC.52 Gemcitabine-DTIC had a superior progression-free survival at 3 months (56% [95% CI 43% to 69%]) as compared to DTIC alone (37% [95% CI 23.5% to 50%]). Furthermore, mean progression-free survival and overall survival were improved in the gemcitabine-DTIC group (4.2 months and 16.8 months) as compared to the DTIC group (2.0 months and 8.2 months).52 DTIC has a single-agent response rate of 16%, but has been shown to be particularly effective in the setting of leiomyosarcomas.49
• Does response to treatment regimens differ by histologic subtype?
The majority of STS trials include many different histologic subtypes. Given the rarity of sarcomas as a whole, many trials have had difficulty recruiting adequate numbers of patients to have sufficient power to definitely determine if the treatment under investigation has clinical benefit. Furthermore, the patients recruited have been heterogeneous with regard to subtype. Many older studies hypothesized that the efficacy of chemotherapeutic agents vary based on histologic subtype; however, for most subtypes the number of individuals included in those trials was too low to evaluate efficacy based on subtype.
Some exceptions exist, however. For example, both gemcitabine-DTIC and gemcitabine-docetaxel have been found to be particularly effective in the treatment of leiomyosarcomas.50,52 Additionally, a retrospective study found a 51% overall response rate for patients with myxoid liposarcomas treated with trabectedin.53 Studies of patients with angiosarcoma treated with paclitaxel have demonstrated response rates of 43% and 53%.54,55
• What are the newest approved and investigational agents?
A recently approved agent is trabectedin, a tris tetrahydroisoquinoline alkaloid isolated from ascidians that binds to the minor groove of DNA and causes disruptions in the cell cycle. Samuels et al reported data from a single-arm, open-label expanded access trial that evaluated patients with advanced metastatic sarcomas.56 In this study, patients with liposarcomas and leiomyosarcomas had an objective response rate of 6.9% (95% CI 4.8 to 9.6) as compared to a rate of 5.9% (95% CI 4.4 to 7.8) for all assessable patients. Median survival was 11.9 months for all patients, with improved median survivals for liposarcoma and leiomyosarcomas of 16.2 months (95% CI 14.1 to 19.5) compared to 8.4 months (95% CI 7.1 to 10.7 months) for other subtypes.56
Schöffski et al evaluated eribulin, a chemotherapeutic agent that affects microtubule dynamics, in a phase 2 trial of patients with progressive or high-grade STS with progression on previous chemotherapy. They found a median progression-free survival of 2.6 months (95% CI 1.7 to 6.2) for adipocytic sarcoma, 2.9 months (95% CI 2.4 to 4.6) for leiomyosarcoma, 2.6 months (95% CI 2.3 to 4.3) for synovial sarcoma, and 2.1 months (95% CI 1.4 to 2.9) for other sarcomas.57
Van der Graaf and colleagues randomly assigned patients with metastatic nonadipocytic STS to pazopanib or placebo in a phase 3 trial. Pazopanib is a small-molecule endothelial growth factor inhibitor with activity against vascular endothelial growth factors 1, 2, and 3 as well as platelet-derived growth factors. Median progression-free survival was 4.6 months (95% CI 3.7 to 4.8) with pazopanib compared to 1.6 months (95% CI 0.9 to 1.8) with placebo.58 Adipocytic sarcomas (liposarcomas) were excluded from the trial because phase 2 trials had found a lower rate of progression-free survival (26%) for them compared to other subtypes.
• What are the most common toxicities associated with the approved and investigational chemotherapeutic agents?
Toxicities were seen with each of the regimens studied and were common in the randomized trials, with higher rates of toxicities in the combination chemotherapy regimens. The most common toxicities are myelosuppression, nausea, and vomiting. In the doxorubicin trials, the most common toxicities were myelosuppression, nausea, and vomiting.44
Ifosfamide both as an individual agent and in combination with doxorubicin has higher rates and higher grades of toxicity than doxorubicin alone. Myelosuppression is the most common toxicity associated with ifosfamide, and the most commonly affected cell line is leukocytes.44 Combination doxorubicin and ifosfamide also had high rates of nausea and vomiting (95%) and alopecia (100%).35
Neutropenia is the most common toxicity associated with gemcitabine and dacarbazine, while their most common nonhematologic toxicities are fatigue and nausea.52,59 Trabectedin’s most common toxicities are nausea (29%), neutropenia (24%), and fatigue (23%). It has also been shown to cause increased alkaline phosphatase (20%) and alanine aminotransferase (19%) levels.56 In a phase 2 study of eribulin, 50% of patients had neutropenia, and other toxicities included fatigue, alopecia, nausea, sensory neuropathy, and thrombocytopenia.57 Pazopanib is generally well tolerated; the most common toxicities are fatigue (65%), diarrhea (58%), nausea (54%), and hypertension (41%).58 Higher rates of neutropenia, mucositis, nausea, vomiting, diarrhea, and transfusion reactions were seen with olaratumab and doxorubicin compared to doxorubicin alone in phase 1b and 2 studies.46
CASE CONCLUSION
Given his poor prognosis with unresectable metastatic undifferentiated liposarcoma, the patient considers a clinical trial prior to undergoing combined therapy with doxorubicin and ifosfamide. He tolerates therapy well with stable disease at 6 months.
CONCLUSION
STSs are a heterogeneous collection of rare tumors. Low-grade, localized tumors have the best prognosis, and patients who undergo complete resection have the best long-term survival. Due to the rarity of STSs, trials often have limited enrollment, and little progress has been made with regards to treatment and survival rates for metastatic and unresectable disease. All patients should be evaluated and treated at specialized sarcoma centers. This case highlights the need for continued research and clinical trials to improve overall survival of patients with sarcoma.
- American Cancer Society. Cancer facts and figures 2016. American Cancer Society Web site. www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed December 20, 2016.
- National Comprehensive Cancer Network. NCCN clinical guidelines in oncology: soft tissue sarcoma. 2016
- Coindre J, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 2001;91:1914–26.
- Dei Tos A. Liposarcoma: new entities and evolving concepts. Ann Diagn Pathol 2000;4:252–66.
- Wile AG, Evans HL, Romsdahl MM. Leiomyosarcoma of soft tissue: a clinicopathologic study. Cancer 1981;48:1022–32.
- Hashimoto H, Daimaru Y, Tsuneyoshi M, Enjoji M. Leiomyosarcoma of the external soft tissues. A clinicopathologic, immunohistochemical, and electron microscopic study. Cancer 1986;57:2077–88
- Fisher C. Synovial sarcoma. Ann Diagn Pathol 1998;2:401–21.
- Newton WA Jr, Gehan EA, Webber BL, et al. Classification of rhabdomyosarcomas and related sarcomas. Pathologic aspects and proposal for a new classification--an Intergroup Rhabdomyosarcoma Study. Cancer 1995;76:1073–85.
- Furlong MA. Pleomorphic rhabdomyosarcoma in adults: a clinicopathologic study of 38 cases with emphasis on morphologic variants and recent skeletal muscle-specific markers. Mod Pathol. 2001;14:595–603.
- Anghileri M, Miceli R, Fiore M. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer 2006;107:1065–74.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors–definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Archive 2001;438:1–12.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70–83.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol 2010;11:983–91.
- Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin 2004;54:94–109.
- Penel N, Grosjean J, Robin YM, et al. Frequency of certain established risk factors in soft tissue sarcomas in adults: a prospective descriptive study of 658 cases. Sarcoma 2008;2008:459386.
- Guillou L, Coindre JM, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 1997;15:350–62.
- Maki RG, Moraco N, Antonescu CR, et al. Toward better soft tissue sarcoma staging: building on American joint committee on cancer staging systems versions 6 and 7. Ann Surg Oncol 2013;20:3377–83.
- Shiraki M, Enterline HT, Brooks JJ, et al. Pathologic analysis of advanced adult soft tissue sarcomas, bone sarcomas, and mesotheliomas. The Eastern Cooperative Oncology Group (ECOG) experience. Cancer 1989;64:484–90.
- Presant CA, Russell WO, Alexander RW, Fu YS. Soft-tissue and bone sarcoma histopathology peer review: The frequency of disagreement in diagnosis and the need for second pathology opinions. The Southeastern Cancer Study Group experience. J Clin Oncol 1986; 4:1658–61.
- Sundaram M, McLeod RA. MR imaging of tumor and tumorlike lesions of bone and soft tissue. AJR Am J Roentgenol 1990;155:817–24.
- Ioannidis JP, Lau J. 18F-FDG PET for the diagnosis and grading of soft-tissue sarcoma: a meta-analysis. J Nucl Med 2003;44:717–24.
- Tateishi U, Yamaguchi U, Seki K, et al. Bone and soft-tissue sarcoma: preoperative staging with fluorine 18 fluorodeoxyglucose PET/CT and conventional imaging. Radiology 2007;245:839–47.
- Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer 2003;97:2530–43
- Rosenberg S, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982;196:305–14.
- Lewis J, Leung D, Woodruff J, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg 1998;288:355–65.
- Zagars GK, Ballo MT, Pisters PW, et al. Surgical margins and reresection in the management of patients with soft tissue sarcoma using conservative surgery and radiation therapy. Cancer 2003;97:2544–53.
- Stojadinovic A, Leung DH, Hoos A. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tisusse sarcomas. Ann Surg 2002;235:424–34.
- O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 2002;359:2235–41.
- Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008;113:573–81.
- Suit HD, Mankin HJ, Wood WC, Proppe KH. Preoperative, intraoperative, and postoperative radiation in the treatment of primary soft tissue sarcoma. Cancer 1985;55:2659–67
- O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 2002;359:2235–41.
- Yang J, Chang A, Baker A, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 1998;16:197–203.
- Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 1996;14:859–68.
- Alektiar KM, Brennan MF, Healey JH, Singer S. Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol 2008;26:3440–5.
- Gortzak E, Azzarelli A, Buesa J, et al. A randomized phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer 2001;37:1096–1103.
- Fakhari N, Ebm C, Kostler WJ, et al. Intensified adjuvant IFADIC chemotherapy in combination with radiotherapy versus radiotherapy alone for soft tissue sarcoma: long-term follow-up of a prospective randomized feasibility trial. Wein Klin Wochenschr 2010;122:614–9.
- Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet 1997;350:1647–54.
- Gronchi A, Frustaci S, Mercuri M, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol 2012;30:850–56.
- Pisters PW, Leung DH, Woodruff J. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 1996;14:1679–89.
- Whooley B, Gibbs J, Mooney M. Primary Extremity Sarcoma: What is the Appropriate Follow-up? Annals of Surg Oncol 2000; 7: 9-14.
- Whooley BP, Mooney MN, Gibbs JF, Graybill WG. Effective follow-up strategies in soft tissue sarcoma. Sem Surg Oncol 1999;17:83–87.
- Billingsley KG, Burt ME, Jara E, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann Surg 1999;229:602–10.
- Bramwell VH, Anderson D, Charette ML; Sarcoma Disease Site Group. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev 2003;(3):CD003293.
- Edmonson J, Ryan L, Blum R. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 1993;11:1269–75.
- Santoro A, Tursz T, Mouridsen H. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 1995;13:1537–45.
- Tap WD, Jones RL, Van Tine B, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet 2016;388:488–97.
- Borden EC, Amato DA, Rosenbaum C, et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol 1987;5:840–50.
- Omura GA, Major FJ, Blessing JA, et al. A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 1983;52:626–32.
- Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol 1993;11:1276–85.
- Maki R, Wathen K, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 2007; 25: 2755–63.
- Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol 2002;12:2824–31.
- Garcia-del-Muro X, Lopez-Pousa A, Maurel J, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on Sarcomas study. J Clin Oncol 2011;29:2528–33.
- Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol 2007;7:595–602.
- Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer 2012;118:3330–6.
- Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve outcome. Ann Oncol 2012;23:517–23.
- Samuels BL, Chawla S, Patel S, et al. Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann Oncol 2013;24:1703–9.
- Schöffski P, Ray-Coquard IL, Cioffi A, et al. Activity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histolical subtypes. Lancet 2011;11:1045–52.
- Van der Graaf W, Blay JY, Chawla S, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomized, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86.
- Dileo P, Morgan JA, Zahrieh D, et al. Gemcitabine and vinorelbine combination chemotherapy for patients with advanced soft tissue sarcomas: results of a phase II trial. Cancer 2007;109:1863–9.
INTRODUCTION
Soft tissue sarcomas (STSs) are rare adult tumors, with 3.4 new cases per 100,000 persons or 12,310 expected new cases in 2016.1 Sarcomas are a heterogeneous collection of tumors that affect fat, muscle, nerve, nerve sheath, vascular, and connective tissues. There are more than 50 histological subtypes that comprise this diverse category of tumors. Treatment varies by stage, with limb-sparing surgery representing the mainstay of curative-intent treatment. Radiation and chemotherapy may also be considered depending on the size, grade, and location of the tumor. Survival rates have been stagnant until recently, with a disease-specific survival hovering around 65%.1 Given the complexity of these cases, all patients ideally should be evaluated and treated by a multidisciplinary team at an institution with extensive experience treating STS.2
EPIDEMIOLOGY AND CLASSIFICATION
The most common STS subtypes are gastrointestinal stromal tumor (GIST), undifferentiate pleomorphic sarcoma (previously referred to as malignant fibrous histiocytoma), liposarcoma, leiomyosarcoma, synovial sarcoma, malignant peripheral nerve sheath tumor, rhabdomyosarcoma, and unclassified sarcoma.3 Liposarcoma is one of the most common subtypes, comprising 20% of all STSs; it is subdivided into well-differentiated/dedifferentiated liposarcomas, myxoid/round cell liposarcomas, and pleomorphic liposarcomas. Well-differentiated liposarcomas tend to occur in the retroperitoneum and limbs, while both myxoid and round cell as well as pleomorphic liposarcomas more commonly originate on the limbs. Histology varies based on subtype and ranges from mature-appearing adipocytes and fibroblasts to undifferentiated cells with minimal lipogenic differentiation.4
Leiomyosarcomas are smooth muscle tumors and are usually located in the retroperitoneum, but have also been associated with peripheral soft tissue and vasculature. Typical histology ranges from well-defined areas of spindle-shaped cells to poorly differentiated anaplastic spindle cells.5,6 Synovial sarcomas are a distinct type of STS that can show epithelial differentiation and account for 5% of adult STSs. The extremities are the most common presenting location (90%).7
Rhabdomyosarcomas are skeletal muscle tumors and are further subdivided into embryonal, alveolar, and pleomorphic subtypes. Embryonal histology ranges from primitive mesenchymal-appearing cells to highly differentiated muscle cells. Alveolar rhabdomyosarcoma has the worst prognosis of the subtypes and consists of round cells with high nuclear-to-chromatin ratios that form “glandular-like” or “alveolar” spaces.8 Pleomorphic rhabdomyosarcomas are composed of rhabdomyoblasts that can affect many different locations, but most commonly present on the lower extremities.9
Malignant peripheral nerve sheath tumor (MPNST) comprises 5% to 10% of all STSs. These tumors are associated with neurofibromatosis type 1 (NF-1), with 25% to 50% of tumors occurring in NF-1 patients. Additionally, most patients have a truncating lesion in the NF1 gene on chromosome 17.10 Anghileri et al in their single institution analysis of 205 patients with MPNSTs found the 2 most common presenting sites were the trunk and extremities. Histologically, these tumors have dense fascicles of spindle cells.10
GISTs are the most common STS of the gastrointestinal (GI) tract. Previously, GISTs were classified as smooth muscle tumors and were not accounted for in the literature as a separate entity distinct from leiomyomas, leiomyoblastomas, and leiomyosarcomas.11 GISTs are found throughout the GI tract: the most common sites are the stomach (60%) and small intestine (30%). Less common sites include duodenum (4%–5%), esophagus (1%), rectum (1%–2%), and appendix (< 0.2%).12 GISTs can be spindle cell, epithelioid, or mesenchymal tumors. Immunohistochemically, GISTs are KIT (CD117) positive. Other cell markers that are also commonly positive include CD34 (60%–70%) and smooth muscle actin (SMA) (25%).11 The majority of GISTs (80%) have an activating c-KIT gene mutation. The most common mutation site is exon 11, with less common c-KIT gene mutations also occurring at exon 9 or 13. Not all GISTs have KIT mutations. The second most common mutation is the PDGFRA mutation (5%–10% of GISTs).2 A minority of GISTs are negative for both KIT and PDGFRA mutations. These tumors were previously called wild-type, but as the majority have either a succinate dehydrogenase (SDH) loss of function or loss of SDHB protein expression, they are now referred to as SDH-deficient GISTs.2 GISTs vary in aggressiveness from incidental to aggressive. Typically, small intestine and rectal GISTs are more aggressive than gastric GISTs. Both size and mitotic rate help to predict the metastatic potential of the tumor. Tumors less than 2 cm in size and having a mitotic rate of less than 5 per 50 high-power fields (hpf) have the lowest risk of metastases, while tumors greater than 5 cm and with more than 5 mitoses per 50 hpf have the highest rates of metastases.12
Angiosarcomas are rare tumors comprising 4% of all STSs. Although they can occur in any site, the majority are cutaneous and occur most frequently in the head and neck regions. These tumors are either of vascular or lymphatic origin and are comprised of abnormal, pleomorphic, malignant endothelial cells. The most useful immunohistochemical markers include von Willebrand factor, CD31, and Ulex europaeus agglutinin 1. The majority of these tumors occur sporadically; however, radiation exposure, chronic lymphedema, and certain toxins including vinyl chloride and thorium dioxide are known risk factors.13
Undifferentiated sarcomas have no specific features and typically consist of primitive mesenchymal cells.
CLINICAL EVALUATION
CASE PRESENTATION
Initial Presentation and History
A 55-year-old man presents to his primary care physician with a painless mass in his anterior thigh. The mass has been present for the past 3 months and he believes that it is enlarging. The patient has a history of well-controlled hypertension and hyperlipidemia. His medications include atorvastatin and hydrochlorothiazide. He has no known drug allergies. Family history is notable for diabetes and hypertension. He drinks 4 to 5 alcoholic drinks a week and he is a former smoker. He quit smoking in his 30s and only smoked intermittently prior to quitting. He denies any illicit drug use. He works as a high school principal. Currently, he feels well. His review of systems is otherwise noncontributory.
Physical Examination
On physical exam, he is afebrile with a blood pressure of 132/75 mm Hg, respiratory rate of 10 breaths/min, and oxygen saturation of 99% on room air. He is a well appearing, overweight male. His head and neck exam is unremarkable. Lung exam reveals clear breath sounds, and cardiac exam reveals a regular rate and rhythm. His abdomen is obese, soft, and without hepatosplenomegaly. There is a large, fixed mass on the anterior lateral aspect of his right thigh. He has no appreciable lymphadenopathy. His neurological exam is unremarkable.
• What are risk factors for sarcoma?
There are few known risk factors for sarcoma. Established risks factors include prior radiation therapy, chronic lymphedema, viruses, and genetic cancer syndromes including Li-Fraumeni syndrome, hereditary retinoblastoma, and NF-1. Other environmental exposures include phenoxyacetic acids and chlorophenols.14 The majority of cases are sporadic, with only a minority of patients having one of these known risk factors.15 Up to one third of sarcomas have a specific translocation and are driven by fusion oncogenes (Table 1).

A painless mass is the most typical presenting symptom. Size at presentation varies based on location, with extremity and head and neck locations typically presenting at smaller sizes than retroperitoneal tumors.14 Patients may experience pain and numbness as the mass enlarges and impinges on surrounding structures including nerves and vasculature. The vast majority of patients are without systemic symptoms.
• How is sarcoma staged?
The American Joint Committee on Cancer (AJCC) staging system is the most widely used staging system in the United States. The latest AJCC manual was updated in 2010 to include a 3-tiered grading system where the tumor is classified according to tumor size, lymph node involvement, metastases, and grade at time of diagnosis (Table 2 and Table 3). Additionally, tumor depth in relation to deep fascia is also taken into account, with superficial tumors being assigned a designation of “a” and deep tumors a designation of “b.”


Previously, 2 of the most widely used grading systems were the National Cancer Institute (NCI) and French Federation of Cancer Centers Sarcoma Group (FNCLCC) systems, both 3-tier grading systems. The main components that determine the NCI grade are the tumor’s histologic type and location and the amount of tumor necrosis. The FNCLCC system evaluation focuses on tumor differentiation, mitotic rate, and amount of tumor necrosis. A study that compared the NCI and FNCLCC grading systems found that FNCLCC was a better predictor of mortality and distant metastasis.16 Previously, the AJCC was a 4-tier grading system, but the 2010 version was updated to the 3-tier FNCLCC grading system. Additionally, the AJCC system has reclassified single lymph node disease as stage III as it confers better survival than metastatic disease.17 It is important that pathology be evaluated by a sarcoma specialist as disagreements with regard to histologic subtype and grade are common.18,19
• What are the most important prognostic factors?
Prognostic factors include grade, size, and presence of metastases at presentation. Best survival is associated with low-grade, small tumors with no metastases at time of diagnosis.14
• What imaging should be considered?
Imaging should be undertaken to help differentiate between benign and malignant lesions. Ideally, it should be undertaken before a biopsy is planned as the imaging can be used to plan biopsy as well as provide invaluable prognostic information. There are several imaging modalities that should be considered during the preliminary work-up and staging of STSs. Conventional imaging includes magnetic resonance imaging (MRI) of the original tumor site; computed tomography (CT) to evaluate for pulmonary metastases and, depending on location, liver metastases; and in the case of small, low-grade tumors, chest radiography. MRI is considered the test of choice for soft tissue masses and can help delineate benign masses such as hematomas, lipomas, and hemangiomas from sarcomas.20 It is difficult to compare the accuracy of positron emission tomography (PET)/CT to CT and MRI because most studies have evaluated PET/CT in parallel with CT and MRI.21 Tateishi et al compared the accuracy of conventional imaging, PET/CT, and PET/CT combined with conventional imaging at determining the TNM staging for 117 patients. They found that conventional imaging correctly classified 77% of patients, PET alone correctly classified 70%, PET/CT correctly classified 83%, and PET/CT combined with conventional imaging correctly staged 87%.22
• Which subtypes are most likely to metastasize?
Although the vast majority of sarcomas spread hematogenously, 3 have a propensity to spread lymphogenously: epithelioid sarcoma, rhabdomyosarcoma, and clear-cell sarcoma. Additionally, certain subtypes are more likely to metastasize: leiomyosarcomas, synovial sarcomas, neurogenic sarcomas, rhabdomyosarcomas, and epithelioid sarcomas.23 Sarcomas metastasize to the lungs more frequently than to the liver. The metastatic pattern is defined primarily by sarcoma subtype and site of primary tumor. Sarcomas rarely metastasize to the brain (~1%).
MANAGEMENT
CASE CONTINUED
The patient undergoes an ultrasound to better visualize the mass. Given the heterogeneous character of the mass, he is referred for an MRI to evaluate the mass and a CT scan of the chest, abdomen, and pelvis to evaluate for distant metastases. MRI reveals a 5.1 cm × 4.6 cm heterogeneous mass invading the superficial fascia of the rectus femoris muscle. No suspicious lymph nodes or other masses are identified on imaging. The patient next undergoes an image-guided core needle biopsy. Pathology from that procedure is consistent with a stage III, T2bNxMx, grade 3, dedifferentiated liposarcoma.
• What is the best management approach for this patient?
SURGERY
Surgery is the mainstay of treatment for STS. Patients with the best prognosis are those who undergo complete resection with negative surgical margins.24,25 Goal tumor-free margin is 1 to 3 cm.26 Complete resection confers the best long-term survival. Both local and metastatic recurrence is higher in patients with incomplete resection and positive margins.24,25 In a study that analyzed 2084 localized primary STSs, patients with negative margins had a local recurrence rate of 15% versus a rate of 28% in patients with positive margins. This translated into higher 5-year local recurrence-free survival for patients with negative surgical margins (82%) compared to patients with positive margins (65%).27 Another study similarly found that patients with negative margins at referral to their institution who underwent postoperative radiation had high local control rates of 93% (95% confidence interval [CI] 87% to 97%) at 5, 10, and 15 years.26 Although radiation improves local control, neither preoperative or postoperative radiation has been shown to improve progression-free or overall survival.28 Other factors that are associated with risk of recurrence are tumor location, history of previous recurrence, age of patient, histopathology, tumor grade, and tumor size. Approximately 40% to 50% of patients with high-grade tumors (defined as size > 5 cm, deep location, and high grade) will develop distant metastases.29
Zagars et al found that positive or uncertain resection margin had a relative risk of local recurrence of 2.0 (95% CI 1.3 to 3.1; P = 0.002), and presentation with locally recurrent disease (vs new tumor) had a relative risk of local recurrence of 2.0 (95% CI 1.2 to 3.4; P = 0.013).26 Patients with STS of head and neck and deep trunk have higher recurrence rates than those with superficial trunk and extremity STS. A single-institution retrospective review demonstrated that patients with completely resectable retroperitoneal sarcomas have longer median survival (103 months) compared to patients with incompletely resected abdominal sarcomas (18 months).25
Rosenberg and colleagues compared amputation to limb-sparing surgery and radiation.24 Their prospective analysis of 65 patients found no difference in disease-free and overall survival between the 2 treatment groups.The limb-sparing treatment group had higher rates of local recurrence, which was highly correlated with positive surgical margins on pathology.24 Evidence from this and similar studies has resulted in radical amputations being replaced by conservative limb-sparing procedures and radiation therapy. In those found to have positive margins, re-resection is an option for some. Patients who undergo re-resection have higher local control rates than patients with positive margins who do not undergo re-resection. The 5-year control rate for patients who undergo re-resection is 85% (95% CI 80% to 89%) compared to 78% (95% CI 71% to 83%) for those who do not undergo re-resection. Similarly, patients who undergo re-resection have lower rates of metastases at 5, 10, and 15 years as well as higher 5-, 10-, and 15-year disease-free survival rates.26
CASE CONTINUED
The patient is referred for limb-sparing surgery after presentation at a multidisciplinary tumor board. Prior to undergoing resection of the tumor, he is also referred to radiation-oncology to discuss the risks and benefits of combination radiotherapy and surgery as opposed to surgical resection alone.
• What is the evidence for radiation therapy?
RADIATION THERAPY
Radiation therapy is used in the preoperative, intraoperative, and postoperative settings to reduce the risk of local recurrence. There are several options for radiation, including external beam radiation therapy (EBRT), intraoperative radiation, and brachytherapy. A newer strategy, intensity-modulated radiation therapy (IMRT), utilizes 3-dimensional modeling to reduce radiation dosages. Overall there are no differences in overall survival or local recurrence rates between preoperative and postoperative radiation in STS.28
The rationale behind preoperative radiation is that it reduces seeding of tumor cells, especially at the time of surgery.30 Additionally, for EBRT, preoperative radiation has smaller field sizes and lower radiation doses. It can also help to reduce the size of the tumor prior to resection. Intraoperative radiation is often paired with preoperative radiation as a boost dose given only to the area of residual tumor.
Suit et al reviewed patients treated at a single institution with limb-sparing surgery and different radiation strategies. Local control rates between preoperative and postoperative radiation groups were not statistically significant. Local recurrence was linked to grade and size of the tumor in both groups. The authors did note, however, that the preoperative radiation group tended to have larger tumor sizes at baseline compared to the patients who received postoperative radiation.30 A study that compared 190 patients who received preoperative and postoperative EBRT or brachytherapy (primary end point was wound complications, and local control was a secondary end point) showed a trend towards greater local control with preoperative radiation; however, the preoperative radiation group had significantly more wound complications compared to the postoperative radiation group.31
Yang et al found that postoperative EBRT decreases rates of local recurrence compared to surgery alone in high-grade extremity sarcomas.32 However, there were no differences in rates of distant metastases and overall survival between the 2 treatment groups. Similarly, in patients with low-grade sarcoma, there were fewer local recurrences in those who received EBRT and surgery as compared to surgery alone.32 Another study that evaluated 164 patients who received either adjuvant brachytherapy or no further therapy after complete resection found that brachytherapy reduced local recurrence in high-grade sarcomas. No difference in local recurrence rates was found in patients with low-grade sarcomas, nor was a significant difference found in the rates of distant metastases and overall survival between the 2 treatment groups.33 With regards to IMRT, a single institution cohort experience with 41 patients who received IMRT following limb-sparing surgery had similar local control rates when compared to historical controls.34
CASE CONTINUED
After discussion of the risks and benefits of radiation therapy, the patient opts for preoperative radiation prior to resection of his liposarcoma. He receives 50 Gy of EBRT prior to undergoing resection. Resection results in R1 margin consistent with microscopic disease. He receives 16 Gy of EBRT as a boost after recovery from his resection.2
• What is the evidence for neoadjuvant and adjuvant chemotherapy for stage I tumors?
CHEMOTHERAPY
Localized Sarcoma
For localized sarcoma, limb-sparing resection with or without radiation forms the backbone of treatment. Studies have evaluated chemotherapy in both the neoadjuvant and adjuvant settings, with the vast majority of studies evaluating doxorubicin-based chemotherapy regimens in the adjuvant settings. Due to the rare nature of sarcomas, most studies are not sufficiently powered to detect significant benefit from chemotherapy. Several trials evaluating chemotherapy regimens in the neoadjuvant and adjuvant settings needed to be terminated prematurely due to inadequate enrollment into the study. 35,36
For stage IA (T1a-Tb, N0, M0, low grade) tumors, no additional therapy is recommended after limb-sparing surgery with appropriate surgical margins. For stage IB (T2a-2b, N0, M0, low grade) tumors with insufficient margins, re-resection and radiation therapy should be considered, while for stage IIA (T1a-1b, N0, M0, G2-3) tumors preoperative or postoperative radiation therapy is recommended.2 Studies have not found benefit of adjuvant chemotherapy in these low-grade, stage I tumors in terms of progression-free survival and overall survival.37
• At what stage should chemotherapy be considered?
For stage IIb and stage III tumors, surgery and radiation therapy again form the backbone of therapy; however, neoadjuvant and adjuvant chemotherapy are also recommended as considerations. Anthracycline-based chemotherapy with either single-agent doxorubicin or doxorubicin and ifosfamide in combination are considered first-line chemotherapy agents in locally advanced STS.2,29,37
Evidence regarding the efficacy of both neoadjuvant and adjuvant chemotherapy regimens in the setting of locally advanced high-grade STS has been mixed. The Sarcoma Meta-analysis Collaboration evaluated 14 trials of doxorubicin-based adjuvant chemotherapy and found a trend towards overall survival in the treatment groups that received chemotherapy.37 All trials included in the meta-analysis compared patients with localized resectable soft-tissue sarcomas who were randomized to either adjuvant chemotherapy or no adjuvant chemotherapy after limb-sparing surgery with or without radiation therapy. None of the individual trials showed a significant benefit, and all trials had large confidence intervals; however, the meta-analysis showed significant benefit in the chemotherapy treatment groups with regard to local recurrence, distant recurrence, and progression-free survival. No significant difference in overall survival was found.37 Pervais et al updated the Sarcoma Meta-analysis Collaboration’s 1997 meta-analysis with the inclusion of 4 new trials that evaluated doxorubicin combined with ifosfamide and found that both patients who received doxorubicin-based regimens or doxorubicin with ifosfamide had significant decreases in distant and overall recurrences. Only the trials that utilized doxorubicin and ifosfamide had an improved overall survival that was statistically significant (hazard ratio 0.56 [95% CI 0.36 to 0.85]; P = 0.01).29 Although no significant heterogeneity was found among the trials included in either meta-analysis, a variety of sarcomas were included in each clinical trial evaluated. Given the extremely small number of each sarcoma subtype present in each trial, subgroup analysis is difficult and prone to inaccuracies. As a result, it is not known if certain histological subtypes are more or less responsive to chemotherapy.37–39
One randomized controlled trial evaluated neoadjuvant chemotherapy in high-risk sarcomas defined as tumors greater than 8 cm or grade II/III tumors. This study evaluated doxorubicin and ifosfamide and found no significant difference in disease-free and overall survival in the neoadjuvant therapy group compared to the control group.35 There remains controversy in the literature with regards to adjuvant chemotherapy. Many oncologists offer adjuvant chemotherapy to patients with certain stage III subtypes. Examples of subtypes that may be offered adjuvant therapy include myxoid liposarcomas, synovial sarcomas, and leiomyosarcomas.2 With regards to how many cycles of chemotherapy should be considered, a noninferiority study compared 3 cycles of epirubicin and ifosfamide to 5 cycles of epirubicin and ifosfamide in patients with high-risk locally advanced adult STSs. Three cycles of preoperative epirubicin and ifosfamide was found to be noninferior to 5 cycles with regards to overall survival.38
• What is this patient’s risk for recurrence?
The patient is at intermediate risk for recurrence. Numerous studies have demonstrated that tumor size, grade, and location are the most important factors to determine risk of recurrence, with larger size, higher grades, and deeper locations being associated with higher risk of recurrence. In an analysis of 1041 patients with STS of the extremities, high grade was the most important risk factor for distant metastases.39 The highest risk of recurrence is within the first 2 years. Given that the patient’s initial tumor was located in the extremity, he is more likely to have a distant metastasis as his site of recurrence; individuals with retroperitoneal tumors and visceral tumors are more likely to recur locally.40 For STSs of the extremity, distant metastases determine overall survival, whereas patients with retroperitoneal sarcomas can die from complications of local metastases.41 Once a patient develops distant metastases, the most important prognostic factor is the size of the tumor, with tumors larger than 10 cm having a relative risk of 1.5 (95% CI 1.0 to 2.0).39
• What are the recommendations for surveillance?
Surveillance recommendations are based on the stage of the sarcoma. Stage I tumors are the least likely to recur either locally or distally. As a result, it is recommended that stage I tumors be followed with history and physical exam every 3 to 6 months for the first 2 to 3 years, and then annually after the first 2 to 3 years. Chest x-rays should be considered every 6 to 12 months.2 For stage II–IV tumors, history and physical exam is recommended every 3 to 6 months for the first 2 to 3 years. Chest and distant metastases imaging should also be performed every 3 to 6 months during this time frame. For the next 2 years, history and physical exam and imaging are recommended every 6 months. After the first 4 to 5 years, annual follow-up is recommended.2
A study that followed 141 patients with primary extremity STSs for a median interval of 49 months found that high-grade tumors were most likely to recur during the first 2 years, with 20% of their patients recurring locally and 40% recurring distally. Chest x-rays performed during surveillance follow-up found distant lung metastases in 36 asymptomatic patients and had a positive predictive value of 92%, a negative predictive value of 97%, and a quality-adjusted life-year of $30,000.40,41 No laboratory testing was found to aid in detection of recurrence.
CASE CONTINUED
The patient does well for 1 year. With physical therapy, he regains most of the strength and coordination of the lower extremity. He is followed every 3 months with chest x-rays and a MRI of the thigh for the first year. On his fourth follow-up clinic visit, he describes increased dyspnea on exertion over the previous few weeks and is found to have multiple lung metastases in both lungs on chest x-ray. He undergoes further evaluation for metastases and is not found to have any other metastatic lesions. Bronchoscopy and biopsy of 1 of the lung nodules confirms recurrent dedifferentiated liposarcoma.
• Should this patient undergo metastectomy?
An analysis of 3149 patients with STS treated at Memorial Sloan-Kettering who developed lung metastases found that patients with pulmonary metastases have survival rates of 25%. The most important prognostic factor for survival was complete resection of all metastases.42 For stage IV disease, surgery is used only in certain instances. In instances where tumor is more localized or limited, removal of metastases or metastectomy can play a role in management.2
CASE CONTINUED
Because the patient’s metastases are limited to the lungs, he is referred for metastectomy. He undergoes wedge resection for definitive diagnosis but it is not possible to completely resect all of the metastases. He is thus referred to a medical oncologist to discuss his treatment options.
• What are treatment options for unresectable or metastatic disease?
Metastatic Disease
Unlike local and locally advanced disease, chemotherapy forms the backbone of treatment in stage IV disease. Doxorubicin and olaratumab or doxorubicin and ifosfamide in combination are considered first line in metastatic disease. Response rates for single-agent doxorubicin range from 16% to 27%, while phase 2 and phase 3 studies of doxorubicin and ifosfamide have found response rates ranging from 18% to 36%.43 In addition, the effectiveness of doxorubicin and ifosfamide phase 2 and 3 trials varied. Edmonson et al found a tumor regression rate of 34% for doxorubicin and ifosfamide as compared to 20% for doxorubicin alone.44 In comparison, Santoro et al found a response rate of 21.3% for doxorubicin alone and 25.2% for doxorubicin and ifosfamide.45 Neither study found increased survival benefit for doxorubicin and ifosfamide when compared to doxorubicin alone. In a Cochrane review evaluating randomized trials that compared doxorubicin and combination chemotherapy regimens, response rates varied from 14% for doxorubicin in combination with streptomycin to 34% for doxorubicin and ifosfamide. Most trials did not show a significant benefit for combination therapies when compared to doxorubicin alone.43 Mean survival with doxorubicin or doxorubicin and ifosfamide is 12 months. High rates of recurrence highlight the need for additional chemotherapy regimens.
The newest approved agent is olaratumab, a monoclonal antibody that binds platelet-derived growth factor receptor alpha and prevents receptor activation. A phase 1-b and phase 2 trial evaluated patients with locally advanced and metastatic STS and randomly assigned them to either olaratumab and doxorubicin or doxorubicin alone.46 Progression-free survival for olaratumab/doxorubicin was 6.6 months (95% CI 4.1 to 8.3) compared to 4.1 months (95% CI 2.8 to 5.4) for doxorubicin alone. The objective response rate was 18.2% (95% CI 9.8 to 29.6) for olaratumab/doxorubicin compared to 7.5% (95% CI 2.5 to 6.6) for doxorubicin alone. Furthermore, the median overall survival for olaratumab plus doxorubicin was 26.5 months (95% CI 20.9 to 31.7) compared to 14.7 months for doxorubicin alone (95% CI 5.5 to 26.0). Impressively, this improved response was notable across histological types. Furthermore, patients who had previously been treated with more than 1 regimen and those who were treatment naïve had similar response rates.46
• What are second-line treatment options?
Doxorubicin has been used in combination with several other agents including dacarbazine (DTIC) as well as DTIC and ifosfamide (MAID). Borden et al evaluated patients with metastatic STS and randomly assigned the patients to either doxorubicin or doxorubicin and DTIC. Combination therapy demonstrated better tumor response than doxorubicin alone: 30% complete or partial response for combination therapy and 18% for doxorubicin alone.47 However, Omura et al
found similar rates of efficacy between doxorubicin and combination doxorubicin and DTIC in women with recurrent or nonresectable uterine sarcomas.48 MAID has never been directly compared in a randomized trial to doxorubicin alone. In a study that compared MAID to doxorubicin and DTIC (AD) in patients with unresectable or metastatic sarcomas, MAID had superior response rates (32% versus 17%), but there was no difference with regards to overall survival (mean survival of 12.5 months).49
Several additional regimens have undergone evaluation in metastatic and recurrent STSs. Gemcitabine has been used both as a single agent and as part of combination therapy in many studies. Studies with gemcitabine in combination with either docetaxel or DTIC have been the most efficacious. In a phase 2 trial, patients with metastatic STS were randomly assigned to either gemcitabine alone or gemcitabine and docetaxel. Combination therapy had a higher response rate (16% versus 8%) and longer overall survival (17.9 months versus 11.5 months) than gemcitabine alone.50 Furthermore, a phase 2 trial of gemcitabine and docetaxel in patients with unresectable leiomyosarcoma showed an overall response rate of 56%, with 3 complete and 15 partial responses among the 34 patients enrolled in the study.51
A phase 2 trial randomly assigned patients with unresectable or metastatic STS to either DTIC or combination gemcitabine and DTIC.52 Gemcitabine-DTIC had a superior progression-free survival at 3 months (56% [95% CI 43% to 69%]) as compared to DTIC alone (37% [95% CI 23.5% to 50%]). Furthermore, mean progression-free survival and overall survival were improved in the gemcitabine-DTIC group (4.2 months and 16.8 months) as compared to the DTIC group (2.0 months and 8.2 months).52 DTIC has a single-agent response rate of 16%, but has been shown to be particularly effective in the setting of leiomyosarcomas.49
• Does response to treatment regimens differ by histologic subtype?
The majority of STS trials include many different histologic subtypes. Given the rarity of sarcomas as a whole, many trials have had difficulty recruiting adequate numbers of patients to have sufficient power to definitely determine if the treatment under investigation has clinical benefit. Furthermore, the patients recruited have been heterogeneous with regard to subtype. Many older studies hypothesized that the efficacy of chemotherapeutic agents vary based on histologic subtype; however, for most subtypes the number of individuals included in those trials was too low to evaluate efficacy based on subtype.
Some exceptions exist, however. For example, both gemcitabine-DTIC and gemcitabine-docetaxel have been found to be particularly effective in the treatment of leiomyosarcomas.50,52 Additionally, a retrospective study found a 51% overall response rate for patients with myxoid liposarcomas treated with trabectedin.53 Studies of patients with angiosarcoma treated with paclitaxel have demonstrated response rates of 43% and 53%.54,55
• What are the newest approved and investigational agents?
A recently approved agent is trabectedin, a tris tetrahydroisoquinoline alkaloid isolated from ascidians that binds to the minor groove of DNA and causes disruptions in the cell cycle. Samuels et al reported data from a single-arm, open-label expanded access trial that evaluated patients with advanced metastatic sarcomas.56 In this study, patients with liposarcomas and leiomyosarcomas had an objective response rate of 6.9% (95% CI 4.8 to 9.6) as compared to a rate of 5.9% (95% CI 4.4 to 7.8) for all assessable patients. Median survival was 11.9 months for all patients, with improved median survivals for liposarcoma and leiomyosarcomas of 16.2 months (95% CI 14.1 to 19.5) compared to 8.4 months (95% CI 7.1 to 10.7 months) for other subtypes.56
Schöffski et al evaluated eribulin, a chemotherapeutic agent that affects microtubule dynamics, in a phase 2 trial of patients with progressive or high-grade STS with progression on previous chemotherapy. They found a median progression-free survival of 2.6 months (95% CI 1.7 to 6.2) for adipocytic sarcoma, 2.9 months (95% CI 2.4 to 4.6) for leiomyosarcoma, 2.6 months (95% CI 2.3 to 4.3) for synovial sarcoma, and 2.1 months (95% CI 1.4 to 2.9) for other sarcomas.57
Van der Graaf and colleagues randomly assigned patients with metastatic nonadipocytic STS to pazopanib or placebo in a phase 3 trial. Pazopanib is a small-molecule endothelial growth factor inhibitor with activity against vascular endothelial growth factors 1, 2, and 3 as well as platelet-derived growth factors. Median progression-free survival was 4.6 months (95% CI 3.7 to 4.8) with pazopanib compared to 1.6 months (95% CI 0.9 to 1.8) with placebo.58 Adipocytic sarcomas (liposarcomas) were excluded from the trial because phase 2 trials had found a lower rate of progression-free survival (26%) for them compared to other subtypes.
• What are the most common toxicities associated with the approved and investigational chemotherapeutic agents?
Toxicities were seen with each of the regimens studied and were common in the randomized trials, with higher rates of toxicities in the combination chemotherapy regimens. The most common toxicities are myelosuppression, nausea, and vomiting. In the doxorubicin trials, the most common toxicities were myelosuppression, nausea, and vomiting.44
Ifosfamide both as an individual agent and in combination with doxorubicin has higher rates and higher grades of toxicity than doxorubicin alone. Myelosuppression is the most common toxicity associated with ifosfamide, and the most commonly affected cell line is leukocytes.44 Combination doxorubicin and ifosfamide also had high rates of nausea and vomiting (95%) and alopecia (100%).35
Neutropenia is the most common toxicity associated with gemcitabine and dacarbazine, while their most common nonhematologic toxicities are fatigue and nausea.52,59 Trabectedin’s most common toxicities are nausea (29%), neutropenia (24%), and fatigue (23%). It has also been shown to cause increased alkaline phosphatase (20%) and alanine aminotransferase (19%) levels.56 In a phase 2 study of eribulin, 50% of patients had neutropenia, and other toxicities included fatigue, alopecia, nausea, sensory neuropathy, and thrombocytopenia.57 Pazopanib is generally well tolerated; the most common toxicities are fatigue (65%), diarrhea (58%), nausea (54%), and hypertension (41%).58 Higher rates of neutropenia, mucositis, nausea, vomiting, diarrhea, and transfusion reactions were seen with olaratumab and doxorubicin compared to doxorubicin alone in phase 1b and 2 studies.46
CASE CONCLUSION
Given his poor prognosis with unresectable metastatic undifferentiated liposarcoma, the patient considers a clinical trial prior to undergoing combined therapy with doxorubicin and ifosfamide. He tolerates therapy well with stable disease at 6 months.
CONCLUSION
STSs are a heterogeneous collection of rare tumors. Low-grade, localized tumors have the best prognosis, and patients who undergo complete resection have the best long-term survival. Due to the rarity of STSs, trials often have limited enrollment, and little progress has been made with regards to treatment and survival rates for metastatic and unresectable disease. All patients should be evaluated and treated at specialized sarcoma centers. This case highlights the need for continued research and clinical trials to improve overall survival of patients with sarcoma.
INTRODUCTION
Soft tissue sarcomas (STSs) are rare adult tumors, with 3.4 new cases per 100,000 persons or 12,310 expected new cases in 2016.1 Sarcomas are a heterogeneous collection of tumors that affect fat, muscle, nerve, nerve sheath, vascular, and connective tissues. There are more than 50 histological subtypes that comprise this diverse category of tumors. Treatment varies by stage, with limb-sparing surgery representing the mainstay of curative-intent treatment. Radiation and chemotherapy may also be considered depending on the size, grade, and location of the tumor. Survival rates have been stagnant until recently, with a disease-specific survival hovering around 65%.1 Given the complexity of these cases, all patients ideally should be evaluated and treated by a multidisciplinary team at an institution with extensive experience treating STS.2
EPIDEMIOLOGY AND CLASSIFICATION
The most common STS subtypes are gastrointestinal stromal tumor (GIST), undifferentiate pleomorphic sarcoma (previously referred to as malignant fibrous histiocytoma), liposarcoma, leiomyosarcoma, synovial sarcoma, malignant peripheral nerve sheath tumor, rhabdomyosarcoma, and unclassified sarcoma.3 Liposarcoma is one of the most common subtypes, comprising 20% of all STSs; it is subdivided into well-differentiated/dedifferentiated liposarcomas, myxoid/round cell liposarcomas, and pleomorphic liposarcomas. Well-differentiated liposarcomas tend to occur in the retroperitoneum and limbs, while both myxoid and round cell as well as pleomorphic liposarcomas more commonly originate on the limbs. Histology varies based on subtype and ranges from mature-appearing adipocytes and fibroblasts to undifferentiated cells with minimal lipogenic differentiation.4
Leiomyosarcomas are smooth muscle tumors and are usually located in the retroperitoneum, but have also been associated with peripheral soft tissue and vasculature. Typical histology ranges from well-defined areas of spindle-shaped cells to poorly differentiated anaplastic spindle cells.5,6 Synovial sarcomas are a distinct type of STS that can show epithelial differentiation and account for 5% of adult STSs. The extremities are the most common presenting location (90%).7
Rhabdomyosarcomas are skeletal muscle tumors and are further subdivided into embryonal, alveolar, and pleomorphic subtypes. Embryonal histology ranges from primitive mesenchymal-appearing cells to highly differentiated muscle cells. Alveolar rhabdomyosarcoma has the worst prognosis of the subtypes and consists of round cells with high nuclear-to-chromatin ratios that form “glandular-like” or “alveolar” spaces.8 Pleomorphic rhabdomyosarcomas are composed of rhabdomyoblasts that can affect many different locations, but most commonly present on the lower extremities.9
Malignant peripheral nerve sheath tumor (MPNST) comprises 5% to 10% of all STSs. These tumors are associated with neurofibromatosis type 1 (NF-1), with 25% to 50% of tumors occurring in NF-1 patients. Additionally, most patients have a truncating lesion in the NF1 gene on chromosome 17.10 Anghileri et al in their single institution analysis of 205 patients with MPNSTs found the 2 most common presenting sites were the trunk and extremities. Histologically, these tumors have dense fascicles of spindle cells.10
GISTs are the most common STS of the gastrointestinal (GI) tract. Previously, GISTs were classified as smooth muscle tumors and were not accounted for in the literature as a separate entity distinct from leiomyomas, leiomyoblastomas, and leiomyosarcomas.11 GISTs are found throughout the GI tract: the most common sites are the stomach (60%) and small intestine (30%). Less common sites include duodenum (4%–5%), esophagus (1%), rectum (1%–2%), and appendix (< 0.2%).12 GISTs can be spindle cell, epithelioid, or mesenchymal tumors. Immunohistochemically, GISTs are KIT (CD117) positive. Other cell markers that are also commonly positive include CD34 (60%–70%) and smooth muscle actin (SMA) (25%).11 The majority of GISTs (80%) have an activating c-KIT gene mutation. The most common mutation site is exon 11, with less common c-KIT gene mutations also occurring at exon 9 or 13. Not all GISTs have KIT mutations. The second most common mutation is the PDGFRA mutation (5%–10% of GISTs).2 A minority of GISTs are negative for both KIT and PDGFRA mutations. These tumors were previously called wild-type, but as the majority have either a succinate dehydrogenase (SDH) loss of function or loss of SDHB protein expression, they are now referred to as SDH-deficient GISTs.2 GISTs vary in aggressiveness from incidental to aggressive. Typically, small intestine and rectal GISTs are more aggressive than gastric GISTs. Both size and mitotic rate help to predict the metastatic potential of the tumor. Tumors less than 2 cm in size and having a mitotic rate of less than 5 per 50 high-power fields (hpf) have the lowest risk of metastases, while tumors greater than 5 cm and with more than 5 mitoses per 50 hpf have the highest rates of metastases.12
Angiosarcomas are rare tumors comprising 4% of all STSs. Although they can occur in any site, the majority are cutaneous and occur most frequently in the head and neck regions. These tumors are either of vascular or lymphatic origin and are comprised of abnormal, pleomorphic, malignant endothelial cells. The most useful immunohistochemical markers include von Willebrand factor, CD31, and Ulex europaeus agglutinin 1. The majority of these tumors occur sporadically; however, radiation exposure, chronic lymphedema, and certain toxins including vinyl chloride and thorium dioxide are known risk factors.13
Undifferentiated sarcomas have no specific features and typically consist of primitive mesenchymal cells.
CLINICAL EVALUATION
CASE PRESENTATION
Initial Presentation and History
A 55-year-old man presents to his primary care physician with a painless mass in his anterior thigh. The mass has been present for the past 3 months and he believes that it is enlarging. The patient has a history of well-controlled hypertension and hyperlipidemia. His medications include atorvastatin and hydrochlorothiazide. He has no known drug allergies. Family history is notable for diabetes and hypertension. He drinks 4 to 5 alcoholic drinks a week and he is a former smoker. He quit smoking in his 30s and only smoked intermittently prior to quitting. He denies any illicit drug use. He works as a high school principal. Currently, he feels well. His review of systems is otherwise noncontributory.
Physical Examination
On physical exam, he is afebrile with a blood pressure of 132/75 mm Hg, respiratory rate of 10 breaths/min, and oxygen saturation of 99% on room air. He is a well appearing, overweight male. His head and neck exam is unremarkable. Lung exam reveals clear breath sounds, and cardiac exam reveals a regular rate and rhythm. His abdomen is obese, soft, and without hepatosplenomegaly. There is a large, fixed mass on the anterior lateral aspect of his right thigh. He has no appreciable lymphadenopathy. His neurological exam is unremarkable.
• What are risk factors for sarcoma?
There are few known risk factors for sarcoma. Established risks factors include prior radiation therapy, chronic lymphedema, viruses, and genetic cancer syndromes including Li-Fraumeni syndrome, hereditary retinoblastoma, and NF-1. Other environmental exposures include phenoxyacetic acids and chlorophenols.14 The majority of cases are sporadic, with only a minority of patients having one of these known risk factors.15 Up to one third of sarcomas have a specific translocation and are driven by fusion oncogenes (Table 1).

A painless mass is the most typical presenting symptom. Size at presentation varies based on location, with extremity and head and neck locations typically presenting at smaller sizes than retroperitoneal tumors.14 Patients may experience pain and numbness as the mass enlarges and impinges on surrounding structures including nerves and vasculature. The vast majority of patients are without systemic symptoms.
• How is sarcoma staged?
The American Joint Committee on Cancer (AJCC) staging system is the most widely used staging system in the United States. The latest AJCC manual was updated in 2010 to include a 3-tiered grading system where the tumor is classified according to tumor size, lymph node involvement, metastases, and grade at time of diagnosis (Table 2 and Table 3). Additionally, tumor depth in relation to deep fascia is also taken into account, with superficial tumors being assigned a designation of “a” and deep tumors a designation of “b.”


Previously, 2 of the most widely used grading systems were the National Cancer Institute (NCI) and French Federation of Cancer Centers Sarcoma Group (FNCLCC) systems, both 3-tier grading systems. The main components that determine the NCI grade are the tumor’s histologic type and location and the amount of tumor necrosis. The FNCLCC system evaluation focuses on tumor differentiation, mitotic rate, and amount of tumor necrosis. A study that compared the NCI and FNCLCC grading systems found that FNCLCC was a better predictor of mortality and distant metastasis.16 Previously, the AJCC was a 4-tier grading system, but the 2010 version was updated to the 3-tier FNCLCC grading system. Additionally, the AJCC system has reclassified single lymph node disease as stage III as it confers better survival than metastatic disease.17 It is important that pathology be evaluated by a sarcoma specialist as disagreements with regard to histologic subtype and grade are common.18,19
• What are the most important prognostic factors?
Prognostic factors include grade, size, and presence of metastases at presentation. Best survival is associated with low-grade, small tumors with no metastases at time of diagnosis.14
• What imaging should be considered?
Imaging should be undertaken to help differentiate between benign and malignant lesions. Ideally, it should be undertaken before a biopsy is planned as the imaging can be used to plan biopsy as well as provide invaluable prognostic information. There are several imaging modalities that should be considered during the preliminary work-up and staging of STSs. Conventional imaging includes magnetic resonance imaging (MRI) of the original tumor site; computed tomography (CT) to evaluate for pulmonary metastases and, depending on location, liver metastases; and in the case of small, low-grade tumors, chest radiography. MRI is considered the test of choice for soft tissue masses and can help delineate benign masses such as hematomas, lipomas, and hemangiomas from sarcomas.20 It is difficult to compare the accuracy of positron emission tomography (PET)/CT to CT and MRI because most studies have evaluated PET/CT in parallel with CT and MRI.21 Tateishi et al compared the accuracy of conventional imaging, PET/CT, and PET/CT combined with conventional imaging at determining the TNM staging for 117 patients. They found that conventional imaging correctly classified 77% of patients, PET alone correctly classified 70%, PET/CT correctly classified 83%, and PET/CT combined with conventional imaging correctly staged 87%.22
• Which subtypes are most likely to metastasize?
Although the vast majority of sarcomas spread hematogenously, 3 have a propensity to spread lymphogenously: epithelioid sarcoma, rhabdomyosarcoma, and clear-cell sarcoma. Additionally, certain subtypes are more likely to metastasize: leiomyosarcomas, synovial sarcomas, neurogenic sarcomas, rhabdomyosarcomas, and epithelioid sarcomas.23 Sarcomas metastasize to the lungs more frequently than to the liver. The metastatic pattern is defined primarily by sarcoma subtype and site of primary tumor. Sarcomas rarely metastasize to the brain (~1%).
MANAGEMENT
CASE CONTINUED
The patient undergoes an ultrasound to better visualize the mass. Given the heterogeneous character of the mass, he is referred for an MRI to evaluate the mass and a CT scan of the chest, abdomen, and pelvis to evaluate for distant metastases. MRI reveals a 5.1 cm × 4.6 cm heterogeneous mass invading the superficial fascia of the rectus femoris muscle. No suspicious lymph nodes or other masses are identified on imaging. The patient next undergoes an image-guided core needle biopsy. Pathology from that procedure is consistent with a stage III, T2bNxMx, grade 3, dedifferentiated liposarcoma.
• What is the best management approach for this patient?
SURGERY
Surgery is the mainstay of treatment for STS. Patients with the best prognosis are those who undergo complete resection with negative surgical margins.24,25 Goal tumor-free margin is 1 to 3 cm.26 Complete resection confers the best long-term survival. Both local and metastatic recurrence is higher in patients with incomplete resection and positive margins.24,25 In a study that analyzed 2084 localized primary STSs, patients with negative margins had a local recurrence rate of 15% versus a rate of 28% in patients with positive margins. This translated into higher 5-year local recurrence-free survival for patients with negative surgical margins (82%) compared to patients with positive margins (65%).27 Another study similarly found that patients with negative margins at referral to their institution who underwent postoperative radiation had high local control rates of 93% (95% confidence interval [CI] 87% to 97%) at 5, 10, and 15 years.26 Although radiation improves local control, neither preoperative or postoperative radiation has been shown to improve progression-free or overall survival.28 Other factors that are associated with risk of recurrence are tumor location, history of previous recurrence, age of patient, histopathology, tumor grade, and tumor size. Approximately 40% to 50% of patients with high-grade tumors (defined as size > 5 cm, deep location, and high grade) will develop distant metastases.29
Zagars et al found that positive or uncertain resection margin had a relative risk of local recurrence of 2.0 (95% CI 1.3 to 3.1; P = 0.002), and presentation with locally recurrent disease (vs new tumor) had a relative risk of local recurrence of 2.0 (95% CI 1.2 to 3.4; P = 0.013).26 Patients with STS of head and neck and deep trunk have higher recurrence rates than those with superficial trunk and extremity STS. A single-institution retrospective review demonstrated that patients with completely resectable retroperitoneal sarcomas have longer median survival (103 months) compared to patients with incompletely resected abdominal sarcomas (18 months).25
Rosenberg and colleagues compared amputation to limb-sparing surgery and radiation.24 Their prospective analysis of 65 patients found no difference in disease-free and overall survival between the 2 treatment groups.The limb-sparing treatment group had higher rates of local recurrence, which was highly correlated with positive surgical margins on pathology.24 Evidence from this and similar studies has resulted in radical amputations being replaced by conservative limb-sparing procedures and radiation therapy. In those found to have positive margins, re-resection is an option for some. Patients who undergo re-resection have higher local control rates than patients with positive margins who do not undergo re-resection. The 5-year control rate for patients who undergo re-resection is 85% (95% CI 80% to 89%) compared to 78% (95% CI 71% to 83%) for those who do not undergo re-resection. Similarly, patients who undergo re-resection have lower rates of metastases at 5, 10, and 15 years as well as higher 5-, 10-, and 15-year disease-free survival rates.26
CASE CONTINUED
The patient is referred for limb-sparing surgery after presentation at a multidisciplinary tumor board. Prior to undergoing resection of the tumor, he is also referred to radiation-oncology to discuss the risks and benefits of combination radiotherapy and surgery as opposed to surgical resection alone.
• What is the evidence for radiation therapy?
RADIATION THERAPY
Radiation therapy is used in the preoperative, intraoperative, and postoperative settings to reduce the risk of local recurrence. There are several options for radiation, including external beam radiation therapy (EBRT), intraoperative radiation, and brachytherapy. A newer strategy, intensity-modulated radiation therapy (IMRT), utilizes 3-dimensional modeling to reduce radiation dosages. Overall there are no differences in overall survival or local recurrence rates between preoperative and postoperative radiation in STS.28
The rationale behind preoperative radiation is that it reduces seeding of tumor cells, especially at the time of surgery.30 Additionally, for EBRT, preoperative radiation has smaller field sizes and lower radiation doses. It can also help to reduce the size of the tumor prior to resection. Intraoperative radiation is often paired with preoperative radiation as a boost dose given only to the area of residual tumor.
Suit et al reviewed patients treated at a single institution with limb-sparing surgery and different radiation strategies. Local control rates between preoperative and postoperative radiation groups were not statistically significant. Local recurrence was linked to grade and size of the tumor in both groups. The authors did note, however, that the preoperative radiation group tended to have larger tumor sizes at baseline compared to the patients who received postoperative radiation.30 A study that compared 190 patients who received preoperative and postoperative EBRT or brachytherapy (primary end point was wound complications, and local control was a secondary end point) showed a trend towards greater local control with preoperative radiation; however, the preoperative radiation group had significantly more wound complications compared to the postoperative radiation group.31
Yang et al found that postoperative EBRT decreases rates of local recurrence compared to surgery alone in high-grade extremity sarcomas.32 However, there were no differences in rates of distant metastases and overall survival between the 2 treatment groups. Similarly, in patients with low-grade sarcoma, there were fewer local recurrences in those who received EBRT and surgery as compared to surgery alone.32 Another study that evaluated 164 patients who received either adjuvant brachytherapy or no further therapy after complete resection found that brachytherapy reduced local recurrence in high-grade sarcomas. No difference in local recurrence rates was found in patients with low-grade sarcomas, nor was a significant difference found in the rates of distant metastases and overall survival between the 2 treatment groups.33 With regards to IMRT, a single institution cohort experience with 41 patients who received IMRT following limb-sparing surgery had similar local control rates when compared to historical controls.34
CASE CONTINUED
After discussion of the risks and benefits of radiation therapy, the patient opts for preoperative radiation prior to resection of his liposarcoma. He receives 50 Gy of EBRT prior to undergoing resection. Resection results in R1 margin consistent with microscopic disease. He receives 16 Gy of EBRT as a boost after recovery from his resection.2
• What is the evidence for neoadjuvant and adjuvant chemotherapy for stage I tumors?
CHEMOTHERAPY
Localized Sarcoma
For localized sarcoma, limb-sparing resection with or without radiation forms the backbone of treatment. Studies have evaluated chemotherapy in both the neoadjuvant and adjuvant settings, with the vast majority of studies evaluating doxorubicin-based chemotherapy regimens in the adjuvant settings. Due to the rare nature of sarcomas, most studies are not sufficiently powered to detect significant benefit from chemotherapy. Several trials evaluating chemotherapy regimens in the neoadjuvant and adjuvant settings needed to be terminated prematurely due to inadequate enrollment into the study. 35,36
For stage IA (T1a-Tb, N0, M0, low grade) tumors, no additional therapy is recommended after limb-sparing surgery with appropriate surgical margins. For stage IB (T2a-2b, N0, M0, low grade) tumors with insufficient margins, re-resection and radiation therapy should be considered, while for stage IIA (T1a-1b, N0, M0, G2-3) tumors preoperative or postoperative radiation therapy is recommended.2 Studies have not found benefit of adjuvant chemotherapy in these low-grade, stage I tumors in terms of progression-free survival and overall survival.37
• At what stage should chemotherapy be considered?
For stage IIb and stage III tumors, surgery and radiation therapy again form the backbone of therapy; however, neoadjuvant and adjuvant chemotherapy are also recommended as considerations. Anthracycline-based chemotherapy with either single-agent doxorubicin or doxorubicin and ifosfamide in combination are considered first-line chemotherapy agents in locally advanced STS.2,29,37
Evidence regarding the efficacy of both neoadjuvant and adjuvant chemotherapy regimens in the setting of locally advanced high-grade STS has been mixed. The Sarcoma Meta-analysis Collaboration evaluated 14 trials of doxorubicin-based adjuvant chemotherapy and found a trend towards overall survival in the treatment groups that received chemotherapy.37 All trials included in the meta-analysis compared patients with localized resectable soft-tissue sarcomas who were randomized to either adjuvant chemotherapy or no adjuvant chemotherapy after limb-sparing surgery with or without radiation therapy. None of the individual trials showed a significant benefit, and all trials had large confidence intervals; however, the meta-analysis showed significant benefit in the chemotherapy treatment groups with regard to local recurrence, distant recurrence, and progression-free survival. No significant difference in overall survival was found.37 Pervais et al updated the Sarcoma Meta-analysis Collaboration’s 1997 meta-analysis with the inclusion of 4 new trials that evaluated doxorubicin combined with ifosfamide and found that both patients who received doxorubicin-based regimens or doxorubicin with ifosfamide had significant decreases in distant and overall recurrences. Only the trials that utilized doxorubicin and ifosfamide had an improved overall survival that was statistically significant (hazard ratio 0.56 [95% CI 0.36 to 0.85]; P = 0.01).29 Although no significant heterogeneity was found among the trials included in either meta-analysis, a variety of sarcomas were included in each clinical trial evaluated. Given the extremely small number of each sarcoma subtype present in each trial, subgroup analysis is difficult and prone to inaccuracies. As a result, it is not known if certain histological subtypes are more or less responsive to chemotherapy.37–39
One randomized controlled trial evaluated neoadjuvant chemotherapy in high-risk sarcomas defined as tumors greater than 8 cm or grade II/III tumors. This study evaluated doxorubicin and ifosfamide and found no significant difference in disease-free and overall survival in the neoadjuvant therapy group compared to the control group.35 There remains controversy in the literature with regards to adjuvant chemotherapy. Many oncologists offer adjuvant chemotherapy to patients with certain stage III subtypes. Examples of subtypes that may be offered adjuvant therapy include myxoid liposarcomas, synovial sarcomas, and leiomyosarcomas.2 With regards to how many cycles of chemotherapy should be considered, a noninferiority study compared 3 cycles of epirubicin and ifosfamide to 5 cycles of epirubicin and ifosfamide in patients with high-risk locally advanced adult STSs. Three cycles of preoperative epirubicin and ifosfamide was found to be noninferior to 5 cycles with regards to overall survival.38
• What is this patient’s risk for recurrence?
The patient is at intermediate risk for recurrence. Numerous studies have demonstrated that tumor size, grade, and location are the most important factors to determine risk of recurrence, with larger size, higher grades, and deeper locations being associated with higher risk of recurrence. In an analysis of 1041 patients with STS of the extremities, high grade was the most important risk factor for distant metastases.39 The highest risk of recurrence is within the first 2 years. Given that the patient’s initial tumor was located in the extremity, he is more likely to have a distant metastasis as his site of recurrence; individuals with retroperitoneal tumors and visceral tumors are more likely to recur locally.40 For STSs of the extremity, distant metastases determine overall survival, whereas patients with retroperitoneal sarcomas can die from complications of local metastases.41 Once a patient develops distant metastases, the most important prognostic factor is the size of the tumor, with tumors larger than 10 cm having a relative risk of 1.5 (95% CI 1.0 to 2.0).39
• What are the recommendations for surveillance?
Surveillance recommendations are based on the stage of the sarcoma. Stage I tumors are the least likely to recur either locally or distally. As a result, it is recommended that stage I tumors be followed with history and physical exam every 3 to 6 months for the first 2 to 3 years, and then annually after the first 2 to 3 years. Chest x-rays should be considered every 6 to 12 months.2 For stage II–IV tumors, history and physical exam is recommended every 3 to 6 months for the first 2 to 3 years. Chest and distant metastases imaging should also be performed every 3 to 6 months during this time frame. For the next 2 years, history and physical exam and imaging are recommended every 6 months. After the first 4 to 5 years, annual follow-up is recommended.2
A study that followed 141 patients with primary extremity STSs for a median interval of 49 months found that high-grade tumors were most likely to recur during the first 2 years, with 20% of their patients recurring locally and 40% recurring distally. Chest x-rays performed during surveillance follow-up found distant lung metastases in 36 asymptomatic patients and had a positive predictive value of 92%, a negative predictive value of 97%, and a quality-adjusted life-year of $30,000.40,41 No laboratory testing was found to aid in detection of recurrence.
CASE CONTINUED
The patient does well for 1 year. With physical therapy, he regains most of the strength and coordination of the lower extremity. He is followed every 3 months with chest x-rays and a MRI of the thigh for the first year. On his fourth follow-up clinic visit, he describes increased dyspnea on exertion over the previous few weeks and is found to have multiple lung metastases in both lungs on chest x-ray. He undergoes further evaluation for metastases and is not found to have any other metastatic lesions. Bronchoscopy and biopsy of 1 of the lung nodules confirms recurrent dedifferentiated liposarcoma.
• Should this patient undergo metastectomy?
An analysis of 3149 patients with STS treated at Memorial Sloan-Kettering who developed lung metastases found that patients with pulmonary metastases have survival rates of 25%. The most important prognostic factor for survival was complete resection of all metastases.42 For stage IV disease, surgery is used only in certain instances. In instances where tumor is more localized or limited, removal of metastases or metastectomy can play a role in management.2
CASE CONTINUED
Because the patient’s metastases are limited to the lungs, he is referred for metastectomy. He undergoes wedge resection for definitive diagnosis but it is not possible to completely resect all of the metastases. He is thus referred to a medical oncologist to discuss his treatment options.
• What are treatment options for unresectable or metastatic disease?
Metastatic Disease
Unlike local and locally advanced disease, chemotherapy forms the backbone of treatment in stage IV disease. Doxorubicin and olaratumab or doxorubicin and ifosfamide in combination are considered first line in metastatic disease. Response rates for single-agent doxorubicin range from 16% to 27%, while phase 2 and phase 3 studies of doxorubicin and ifosfamide have found response rates ranging from 18% to 36%.43 In addition, the effectiveness of doxorubicin and ifosfamide phase 2 and 3 trials varied. Edmonson et al found a tumor regression rate of 34% for doxorubicin and ifosfamide as compared to 20% for doxorubicin alone.44 In comparison, Santoro et al found a response rate of 21.3% for doxorubicin alone and 25.2% for doxorubicin and ifosfamide.45 Neither study found increased survival benefit for doxorubicin and ifosfamide when compared to doxorubicin alone. In a Cochrane review evaluating randomized trials that compared doxorubicin and combination chemotherapy regimens, response rates varied from 14% for doxorubicin in combination with streptomycin to 34% for doxorubicin and ifosfamide. Most trials did not show a significant benefit for combination therapies when compared to doxorubicin alone.43 Mean survival with doxorubicin or doxorubicin and ifosfamide is 12 months. High rates of recurrence highlight the need for additional chemotherapy regimens.
The newest approved agent is olaratumab, a monoclonal antibody that binds platelet-derived growth factor receptor alpha and prevents receptor activation. A phase 1-b and phase 2 trial evaluated patients with locally advanced and metastatic STS and randomly assigned them to either olaratumab and doxorubicin or doxorubicin alone.46 Progression-free survival for olaratumab/doxorubicin was 6.6 months (95% CI 4.1 to 8.3) compared to 4.1 months (95% CI 2.8 to 5.4) for doxorubicin alone. The objective response rate was 18.2% (95% CI 9.8 to 29.6) for olaratumab/doxorubicin compared to 7.5% (95% CI 2.5 to 6.6) for doxorubicin alone. Furthermore, the median overall survival for olaratumab plus doxorubicin was 26.5 months (95% CI 20.9 to 31.7) compared to 14.7 months for doxorubicin alone (95% CI 5.5 to 26.0). Impressively, this improved response was notable across histological types. Furthermore, patients who had previously been treated with more than 1 regimen and those who were treatment naïve had similar response rates.46
• What are second-line treatment options?
Doxorubicin has been used in combination with several other agents including dacarbazine (DTIC) as well as DTIC and ifosfamide (MAID). Borden et al evaluated patients with metastatic STS and randomly assigned the patients to either doxorubicin or doxorubicin and DTIC. Combination therapy demonstrated better tumor response than doxorubicin alone: 30% complete or partial response for combination therapy and 18% for doxorubicin alone.47 However, Omura et al
found similar rates of efficacy between doxorubicin and combination doxorubicin and DTIC in women with recurrent or nonresectable uterine sarcomas.48 MAID has never been directly compared in a randomized trial to doxorubicin alone. In a study that compared MAID to doxorubicin and DTIC (AD) in patients with unresectable or metastatic sarcomas, MAID had superior response rates (32% versus 17%), but there was no difference with regards to overall survival (mean survival of 12.5 months).49
Several additional regimens have undergone evaluation in metastatic and recurrent STSs. Gemcitabine has been used both as a single agent and as part of combination therapy in many studies. Studies with gemcitabine in combination with either docetaxel or DTIC have been the most efficacious. In a phase 2 trial, patients with metastatic STS were randomly assigned to either gemcitabine alone or gemcitabine and docetaxel. Combination therapy had a higher response rate (16% versus 8%) and longer overall survival (17.9 months versus 11.5 months) than gemcitabine alone.50 Furthermore, a phase 2 trial of gemcitabine and docetaxel in patients with unresectable leiomyosarcoma showed an overall response rate of 56%, with 3 complete and 15 partial responses among the 34 patients enrolled in the study.51
A phase 2 trial randomly assigned patients with unresectable or metastatic STS to either DTIC or combination gemcitabine and DTIC.52 Gemcitabine-DTIC had a superior progression-free survival at 3 months (56% [95% CI 43% to 69%]) as compared to DTIC alone (37% [95% CI 23.5% to 50%]). Furthermore, mean progression-free survival and overall survival were improved in the gemcitabine-DTIC group (4.2 months and 16.8 months) as compared to the DTIC group (2.0 months and 8.2 months).52 DTIC has a single-agent response rate of 16%, but has been shown to be particularly effective in the setting of leiomyosarcomas.49
• Does response to treatment regimens differ by histologic subtype?
The majority of STS trials include many different histologic subtypes. Given the rarity of sarcomas as a whole, many trials have had difficulty recruiting adequate numbers of patients to have sufficient power to definitely determine if the treatment under investigation has clinical benefit. Furthermore, the patients recruited have been heterogeneous with regard to subtype. Many older studies hypothesized that the efficacy of chemotherapeutic agents vary based on histologic subtype; however, for most subtypes the number of individuals included in those trials was too low to evaluate efficacy based on subtype.
Some exceptions exist, however. For example, both gemcitabine-DTIC and gemcitabine-docetaxel have been found to be particularly effective in the treatment of leiomyosarcomas.50,52 Additionally, a retrospective study found a 51% overall response rate for patients with myxoid liposarcomas treated with trabectedin.53 Studies of patients with angiosarcoma treated with paclitaxel have demonstrated response rates of 43% and 53%.54,55
• What are the newest approved and investigational agents?
A recently approved agent is trabectedin, a tris tetrahydroisoquinoline alkaloid isolated from ascidians that binds to the minor groove of DNA and causes disruptions in the cell cycle. Samuels et al reported data from a single-arm, open-label expanded access trial that evaluated patients with advanced metastatic sarcomas.56 In this study, patients with liposarcomas and leiomyosarcomas had an objective response rate of 6.9% (95% CI 4.8 to 9.6) as compared to a rate of 5.9% (95% CI 4.4 to 7.8) for all assessable patients. Median survival was 11.9 months for all patients, with improved median survivals for liposarcoma and leiomyosarcomas of 16.2 months (95% CI 14.1 to 19.5) compared to 8.4 months (95% CI 7.1 to 10.7 months) for other subtypes.56
Schöffski et al evaluated eribulin, a chemotherapeutic agent that affects microtubule dynamics, in a phase 2 trial of patients with progressive or high-grade STS with progression on previous chemotherapy. They found a median progression-free survival of 2.6 months (95% CI 1.7 to 6.2) for adipocytic sarcoma, 2.9 months (95% CI 2.4 to 4.6) for leiomyosarcoma, 2.6 months (95% CI 2.3 to 4.3) for synovial sarcoma, and 2.1 months (95% CI 1.4 to 2.9) for other sarcomas.57
Van der Graaf and colleagues randomly assigned patients with metastatic nonadipocytic STS to pazopanib or placebo in a phase 3 trial. Pazopanib is a small-molecule endothelial growth factor inhibitor with activity against vascular endothelial growth factors 1, 2, and 3 as well as platelet-derived growth factors. Median progression-free survival was 4.6 months (95% CI 3.7 to 4.8) with pazopanib compared to 1.6 months (95% CI 0.9 to 1.8) with placebo.58 Adipocytic sarcomas (liposarcomas) were excluded from the trial because phase 2 trials had found a lower rate of progression-free survival (26%) for them compared to other subtypes.
• What are the most common toxicities associated with the approved and investigational chemotherapeutic agents?
Toxicities were seen with each of the regimens studied and were common in the randomized trials, with higher rates of toxicities in the combination chemotherapy regimens. The most common toxicities are myelosuppression, nausea, and vomiting. In the doxorubicin trials, the most common toxicities were myelosuppression, nausea, and vomiting.44
Ifosfamide both as an individual agent and in combination with doxorubicin has higher rates and higher grades of toxicity than doxorubicin alone. Myelosuppression is the most common toxicity associated with ifosfamide, and the most commonly affected cell line is leukocytes.44 Combination doxorubicin and ifosfamide also had high rates of nausea and vomiting (95%) and alopecia (100%).35
Neutropenia is the most common toxicity associated with gemcitabine and dacarbazine, while their most common nonhematologic toxicities are fatigue and nausea.52,59 Trabectedin’s most common toxicities are nausea (29%), neutropenia (24%), and fatigue (23%). It has also been shown to cause increased alkaline phosphatase (20%) and alanine aminotransferase (19%) levels.56 In a phase 2 study of eribulin, 50% of patients had neutropenia, and other toxicities included fatigue, alopecia, nausea, sensory neuropathy, and thrombocytopenia.57 Pazopanib is generally well tolerated; the most common toxicities are fatigue (65%), diarrhea (58%), nausea (54%), and hypertension (41%).58 Higher rates of neutropenia, mucositis, nausea, vomiting, diarrhea, and transfusion reactions were seen with olaratumab and doxorubicin compared to doxorubicin alone in phase 1b and 2 studies.46
CASE CONCLUSION
Given his poor prognosis with unresectable metastatic undifferentiated liposarcoma, the patient considers a clinical trial prior to undergoing combined therapy with doxorubicin and ifosfamide. He tolerates therapy well with stable disease at 6 months.
CONCLUSION
STSs are a heterogeneous collection of rare tumors. Low-grade, localized tumors have the best prognosis, and patients who undergo complete resection have the best long-term survival. Due to the rarity of STSs, trials often have limited enrollment, and little progress has been made with regards to treatment and survival rates for metastatic and unresectable disease. All patients should be evaluated and treated at specialized sarcoma centers. This case highlights the need for continued research and clinical trials to improve overall survival of patients with sarcoma.
- American Cancer Society. Cancer facts and figures 2016. American Cancer Society Web site. www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed December 20, 2016.
- National Comprehensive Cancer Network. NCCN clinical guidelines in oncology: soft tissue sarcoma. 2016
- Coindre J, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 2001;91:1914–26.
- Dei Tos A. Liposarcoma: new entities and evolving concepts. Ann Diagn Pathol 2000;4:252–66.
- Wile AG, Evans HL, Romsdahl MM. Leiomyosarcoma of soft tissue: a clinicopathologic study. Cancer 1981;48:1022–32.
- Hashimoto H, Daimaru Y, Tsuneyoshi M, Enjoji M. Leiomyosarcoma of the external soft tissues. A clinicopathologic, immunohistochemical, and electron microscopic study. Cancer 1986;57:2077–88
- Fisher C. Synovial sarcoma. Ann Diagn Pathol 1998;2:401–21.
- Newton WA Jr, Gehan EA, Webber BL, et al. Classification of rhabdomyosarcomas and related sarcomas. Pathologic aspects and proposal for a new classification--an Intergroup Rhabdomyosarcoma Study. Cancer 1995;76:1073–85.
- Furlong MA. Pleomorphic rhabdomyosarcoma in adults: a clinicopathologic study of 38 cases with emphasis on morphologic variants and recent skeletal muscle-specific markers. Mod Pathol. 2001;14:595–603.
- Anghileri M, Miceli R, Fiore M. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer 2006;107:1065–74.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors–definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Archive 2001;438:1–12.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70–83.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol 2010;11:983–91.
- Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin 2004;54:94–109.
- Penel N, Grosjean J, Robin YM, et al. Frequency of certain established risk factors in soft tissue sarcomas in adults: a prospective descriptive study of 658 cases. Sarcoma 2008;2008:459386.
- Guillou L, Coindre JM, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 1997;15:350–62.
- Maki RG, Moraco N, Antonescu CR, et al. Toward better soft tissue sarcoma staging: building on American joint committee on cancer staging systems versions 6 and 7. Ann Surg Oncol 2013;20:3377–83.
- Shiraki M, Enterline HT, Brooks JJ, et al. Pathologic analysis of advanced adult soft tissue sarcomas, bone sarcomas, and mesotheliomas. The Eastern Cooperative Oncology Group (ECOG) experience. Cancer 1989;64:484–90.
- Presant CA, Russell WO, Alexander RW, Fu YS. Soft-tissue and bone sarcoma histopathology peer review: The frequency of disagreement in diagnosis and the need for second pathology opinions. The Southeastern Cancer Study Group experience. J Clin Oncol 1986; 4:1658–61.
- Sundaram M, McLeod RA. MR imaging of tumor and tumorlike lesions of bone and soft tissue. AJR Am J Roentgenol 1990;155:817–24.
- Ioannidis JP, Lau J. 18F-FDG PET for the diagnosis and grading of soft-tissue sarcoma: a meta-analysis. J Nucl Med 2003;44:717–24.
- Tateishi U, Yamaguchi U, Seki K, et al. Bone and soft-tissue sarcoma: preoperative staging with fluorine 18 fluorodeoxyglucose PET/CT and conventional imaging. Radiology 2007;245:839–47.
- Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer 2003;97:2530–43
- Rosenberg S, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982;196:305–14.
- Lewis J, Leung D, Woodruff J, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg 1998;288:355–65.
- Zagars GK, Ballo MT, Pisters PW, et al. Surgical margins and reresection in the management of patients with soft tissue sarcoma using conservative surgery and radiation therapy. Cancer 2003;97:2544–53.
- Stojadinovic A, Leung DH, Hoos A. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tisusse sarcomas. Ann Surg 2002;235:424–34.
- O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 2002;359:2235–41.
- Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008;113:573–81.
- Suit HD, Mankin HJ, Wood WC, Proppe KH. Preoperative, intraoperative, and postoperative radiation in the treatment of primary soft tissue sarcoma. Cancer 1985;55:2659–67
- O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 2002;359:2235–41.
- Yang J, Chang A, Baker A, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 1998;16:197–203.
- Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 1996;14:859–68.
- Alektiar KM, Brennan MF, Healey JH, Singer S. Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol 2008;26:3440–5.
- Gortzak E, Azzarelli A, Buesa J, et al. A randomized phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer 2001;37:1096–1103.
- Fakhari N, Ebm C, Kostler WJ, et al. Intensified adjuvant IFADIC chemotherapy in combination with radiotherapy versus radiotherapy alone for soft tissue sarcoma: long-term follow-up of a prospective randomized feasibility trial. Wein Klin Wochenschr 2010;122:614–9.
- Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet 1997;350:1647–54.
- Gronchi A, Frustaci S, Mercuri M, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol 2012;30:850–56.
- Pisters PW, Leung DH, Woodruff J. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 1996;14:1679–89.
- Whooley B, Gibbs J, Mooney M. Primary Extremity Sarcoma: What is the Appropriate Follow-up? Annals of Surg Oncol 2000; 7: 9-14.
- Whooley BP, Mooney MN, Gibbs JF, Graybill WG. Effective follow-up strategies in soft tissue sarcoma. Sem Surg Oncol 1999;17:83–87.
- Billingsley KG, Burt ME, Jara E, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann Surg 1999;229:602–10.
- Bramwell VH, Anderson D, Charette ML; Sarcoma Disease Site Group. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev 2003;(3):CD003293.
- Edmonson J, Ryan L, Blum R. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 1993;11:1269–75.
- Santoro A, Tursz T, Mouridsen H. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 1995;13:1537–45.
- Tap WD, Jones RL, Van Tine B, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet 2016;388:488–97.
- Borden EC, Amato DA, Rosenbaum C, et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol 1987;5:840–50.
- Omura GA, Major FJ, Blessing JA, et al. A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 1983;52:626–32.
- Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol 1993;11:1276–85.
- Maki R, Wathen K, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 2007; 25: 2755–63.
- Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol 2002;12:2824–31.
- Garcia-del-Muro X, Lopez-Pousa A, Maurel J, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on Sarcomas study. J Clin Oncol 2011;29:2528–33.
- Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol 2007;7:595–602.
- Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer 2012;118:3330–6.
- Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve outcome. Ann Oncol 2012;23:517–23.
- Samuels BL, Chawla S, Patel S, et al. Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann Oncol 2013;24:1703–9.
- Schöffski P, Ray-Coquard IL, Cioffi A, et al. Activity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histolical subtypes. Lancet 2011;11:1045–52.
- Van der Graaf W, Blay JY, Chawla S, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomized, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86.
- Dileo P, Morgan JA, Zahrieh D, et al. Gemcitabine and vinorelbine combination chemotherapy for patients with advanced soft tissue sarcomas: results of a phase II trial. Cancer 2007;109:1863–9.
- American Cancer Society. Cancer facts and figures 2016. American Cancer Society Web site. www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed December 20, 2016.
- National Comprehensive Cancer Network. NCCN clinical guidelines in oncology: soft tissue sarcoma. 2016
- Coindre J, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 2001;91:1914–26.
- Dei Tos A. Liposarcoma: new entities and evolving concepts. Ann Diagn Pathol 2000;4:252–66.
- Wile AG, Evans HL, Romsdahl MM. Leiomyosarcoma of soft tissue: a clinicopathologic study. Cancer 1981;48:1022–32.
- Hashimoto H, Daimaru Y, Tsuneyoshi M, Enjoji M. Leiomyosarcoma of the external soft tissues. A clinicopathologic, immunohistochemical, and electron microscopic study. Cancer 1986;57:2077–88
- Fisher C. Synovial sarcoma. Ann Diagn Pathol 1998;2:401–21.
- Newton WA Jr, Gehan EA, Webber BL, et al. Classification of rhabdomyosarcomas and related sarcomas. Pathologic aspects and proposal for a new classification--an Intergroup Rhabdomyosarcoma Study. Cancer 1995;76:1073–85.
- Furlong MA. Pleomorphic rhabdomyosarcoma in adults: a clinicopathologic study of 38 cases with emphasis on morphologic variants and recent skeletal muscle-specific markers. Mod Pathol. 2001;14:595–603.
- Anghileri M, Miceli R, Fiore M. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer 2006;107:1065–74.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors–definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Archive 2001;438:1–12.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70–83.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol 2010;11:983–91.
- Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin 2004;54:94–109.
- Penel N, Grosjean J, Robin YM, et al. Frequency of certain established risk factors in soft tissue sarcomas in adults: a prospective descriptive study of 658 cases. Sarcoma 2008;2008:459386.
- Guillou L, Coindre JM, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 1997;15:350–62.
- Maki RG, Moraco N, Antonescu CR, et al. Toward better soft tissue sarcoma staging: building on American joint committee on cancer staging systems versions 6 and 7. Ann Surg Oncol 2013;20:3377–83.
- Shiraki M, Enterline HT, Brooks JJ, et al. Pathologic analysis of advanced adult soft tissue sarcomas, bone sarcomas, and mesotheliomas. The Eastern Cooperative Oncology Group (ECOG) experience. Cancer 1989;64:484–90.
- Presant CA, Russell WO, Alexander RW, Fu YS. Soft-tissue and bone sarcoma histopathology peer review: The frequency of disagreement in diagnosis and the need for second pathology opinions. The Southeastern Cancer Study Group experience. J Clin Oncol 1986; 4:1658–61.
- Sundaram M, McLeod RA. MR imaging of tumor and tumorlike lesions of bone and soft tissue. AJR Am J Roentgenol 1990;155:817–24.
- Ioannidis JP, Lau J. 18F-FDG PET for the diagnosis and grading of soft-tissue sarcoma: a meta-analysis. J Nucl Med 2003;44:717–24.
- Tateishi U, Yamaguchi U, Seki K, et al. Bone and soft-tissue sarcoma: preoperative staging with fluorine 18 fluorodeoxyglucose PET/CT and conventional imaging. Radiology 2007;245:839–47.
- Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer 2003;97:2530–43
- Rosenberg S, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982;196:305–14.
- Lewis J, Leung D, Woodruff J, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg 1998;288:355–65.
- Zagars GK, Ballo MT, Pisters PW, et al. Surgical margins and reresection in the management of patients with soft tissue sarcoma using conservative surgery and radiation therapy. Cancer 2003;97:2544–53.
- Stojadinovic A, Leung DH, Hoos A. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tisusse sarcomas. Ann Surg 2002;235:424–34.
- O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 2002;359:2235–41.
- Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008;113:573–81.
- Suit HD, Mankin HJ, Wood WC, Proppe KH. Preoperative, intraoperative, and postoperative radiation in the treatment of primary soft tissue sarcoma. Cancer 1985;55:2659–67
- O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 2002;359:2235–41.
- Yang J, Chang A, Baker A, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 1998;16:197–203.
- Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 1996;14:859–68.
- Alektiar KM, Brennan MF, Healey JH, Singer S. Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol 2008;26:3440–5.
- Gortzak E, Azzarelli A, Buesa J, et al. A randomized phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer 2001;37:1096–1103.
- Fakhari N, Ebm C, Kostler WJ, et al. Intensified adjuvant IFADIC chemotherapy in combination with radiotherapy versus radiotherapy alone for soft tissue sarcoma: long-term follow-up of a prospective randomized feasibility trial. Wein Klin Wochenschr 2010;122:614–9.
- Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet 1997;350:1647–54.
- Gronchi A, Frustaci S, Mercuri M, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol 2012;30:850–56.
- Pisters PW, Leung DH, Woodruff J. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 1996;14:1679–89.
- Whooley B, Gibbs J, Mooney M. Primary Extremity Sarcoma: What is the Appropriate Follow-up? Annals of Surg Oncol 2000; 7: 9-14.
- Whooley BP, Mooney MN, Gibbs JF, Graybill WG. Effective follow-up strategies in soft tissue sarcoma. Sem Surg Oncol 1999;17:83–87.
- Billingsley KG, Burt ME, Jara E, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann Surg 1999;229:602–10.
- Bramwell VH, Anderson D, Charette ML; Sarcoma Disease Site Group. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev 2003;(3):CD003293.
- Edmonson J, Ryan L, Blum R. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 1993;11:1269–75.
- Santoro A, Tursz T, Mouridsen H. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 1995;13:1537–45.
- Tap WD, Jones RL, Van Tine B, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet 2016;388:488–97.
- Borden EC, Amato DA, Rosenbaum C, et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol 1987;5:840–50.
- Omura GA, Major FJ, Blessing JA, et al. A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 1983;52:626–32.
- Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol 1993;11:1276–85.
- Maki R, Wathen K, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 2007; 25: 2755–63.
- Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol 2002;12:2824–31.
- Garcia-del-Muro X, Lopez-Pousa A, Maurel J, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on Sarcomas study. J Clin Oncol 2011;29:2528–33.
- Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol 2007;7:595–602.
- Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer 2012;118:3330–6.
- Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve outcome. Ann Oncol 2012;23:517–23.
- Samuels BL, Chawla S, Patel S, et al. Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann Oncol 2013;24:1703–9.
- Schöffski P, Ray-Coquard IL, Cioffi A, et al. Activity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histolical subtypes. Lancet 2011;11:1045–52.
- Van der Graaf W, Blay JY, Chawla S, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomized, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86.
- Dileo P, Morgan JA, Zahrieh D, et al. Gemcitabine and vinorelbine combination chemotherapy for patients with advanced soft tissue sarcomas: results of a phase II trial. Cancer 2007;109:1863–9.
Sickle Cell Disease
INTRODUCTION
Sickle cell disease is the most common inherited blood disorder in the world. It affects more than 100,000 individuals in the United States, and millions more worldwide.1 Sickle cell disease is most commonly found in individuals of African heritage, but the disease also occurs in Hispanics and people of Middle Eastern and subcontinent Indian heritage.2 The distribution of the sickle hemoglobin (hemoglobin S [HbS]) allele overlaps with the distribution of malaria; HbS carriers, or individuals with sickle cell trait, have protection against malaria,3 and are not considered to have sickle cell disease.
Sickle cell disease is a severe monogenic disorder marked by significant morbidity and mortality, affecting every organ in the body.4 The term sickle cell disease refers to all genotypes that cause sickling; the most common are the homozygous hemoglobin SS (HbSS) and compound heterozygotes hemoglobin SC (HbSC), hemoglobin S–β0-thalassemia (HbSβ0),and hemoglobin S–β+-thalassemia(HbSβ+), although HbS and several rarer hemoglobin variants such as HbSO(Arab) and HbSD(Punjab) can also cause sickle cell disease.The term sickle cell anemia refers exclusively to the most severe genotypes, HbSS and HbSβ0.5 Common sickling genotypes along with their relative clinical severity are shown in Table 1.6–11
| Table 1. Genotypes of Sickling Syndromes and Their Relative Severities | ||
Genotype | Severity | Characteristics |
HbSS | Severe | Most common form |
HbSβ0 | Severe | Clinically indistinguishable from HbSS6 |
HbSO-Arab | Severe | Relatively rare6 |
HbSD-Punjab | Severe | Mostly in northern India6 |
HbSC-Harlem | Severe | Migrates like HbSC, but rare double β-globin mutation7 |
HbCS-Antilles | Severe | Rare double β-globin mutation8 |
HbSC | Moderate | 25% of SCD9 |
HbSβ+, Mediterranean | Moderate | 5%–16% HbA6 |
HbAS-Oman | Moderate | Dominant rare double β-globin mutation10 |
HbSβ+, African | Mild | 16%–30% HbA6 |
HbSE | Mild | HbE found mostly in Southeast Asia11 |
HbS-HPFH | Very mild | Large deletions in β-globin gene complex; > 30% HbF6 |
| HbA = hemoglobin A; HbE = hemoglobin E; HbF = fetal hemoglobin; HbS-HPFH = HbS and gene deletion HPFH; HbSC = heterozygous hemoglobin SC; HbSS = homozygous hemoglobin SS; HbSβ0 = hemoglobin S-β thalassemia0; HbSβ+ = hemoglobin S-β thalassemia+; SCD = sickle cell disease. | ||
This article reviews the pathophysiology of sickle cell disease, common clinical complications, and available therapies. A complex case which illustrates diagnostic and management challenges is presented as well.
PATHOPHYSIOLOGY
HbS is the result of a substitution of valine for glutamic acid in the sixth amino acid of the β-globin chain.12 The change from a hydrophilic to a hydrophobic amino acid causes the hemoglobin molecules to stack, or polymerize, when deoxygenated. This rigid rod of hemoglobin distorts the cell, producing the characteristic crescent or sickle shape that gives the disease its name.13 Polymerization of hemoglobin within the cell is promoted by dehydration, which increases the concentration of HbS.13,14 Polymerization occurs when hemoglobin is in the deoxygenated state.13
The sickle red blood cell is abnormal; it is rigid and dense, and lacks the deformability needed to navigate the microvasculature.15 Blockages of blood flow result in painful vaso-occlusion that is the hallmark of the disease, and that also can cause damage to the spleen, kidneys, and liver.16 The sickle red cell is also fragile, with a lifespan of only 20 days compared to the 120-day lifespan of a normal red blood cell.13 Frequent hemolysis results in anemia and the release of free hemoglobin, which both scavenges nitric oxide and impairs the production of more nitric oxide, which is essential for vasodilatation.17 This contributes to vascular dysfunction and an increased risk for stroke.18 If untreated, the natural course of sickle cell anemia is mortality in early childhood in most cases.19 Common chronic and acute sickle cell disease–related complications and recommended therapies, based on 2014 National Institutes of Health guidelines, are shown in Table 2 and Table 3.20
| Table 2. Common Adult Sickle Cell Disease Chronic Complications and Recommended Therapies | ||
Chronic Complication | Recommended Therapy | Strength of Recommendation |
Chronic pain | Opioids | Consensus |
Avascular necrosis | Analgesics and physical therapy | Consensus |
Proliferative sickle retinopathy | Laser photocoagulation | Strong |
Leg ulcers | Standard wound care | Moderate |
Recurrent priapism | Consult urology | Moderate |
| Data from Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48. | ||
| Table 3. Common Adult Sickle Cell Disease Acute Complications and Recommended Therapies | ||
Acute Complication | Recommended Therapy | Strength of Recommendation |
Vaso-occlusive crisis | NSAIDs, opioids for severe pain | Moderate-consensus |
ACS | Antibiotics, oxygen | Strong |
Simple transfusiona | Weak | |
Urgent exchange transfusionb | Strong | |
Acute stroke | Exchange transfusion | Strong |
Priapism ≥ 4 hr | Aggressive hydration, pain control, and urology consult | Strong-consensus |
Gallstones, symptomatic | Cholecystectomy, laparoscopic | Strong |
Splenic sequestration | Intravenous fluids, transfuse cautiously, discuss surgical splenectomy | Strong-moderate |
Acute renal failure | Consult nephrologyc | Consensus |
ACS = acute chest syndrome; NSAIDs = nonsteroidal anti-inflammatory drugs. a For symptomatic ACS with hemoglobin > 1 g/dL below baseline but > 9.0 g/dL. b When there is progression of ACS (SpO2 < 90% despite supplemental oxygen, increasing respiratory distress, progressive pulmonary infiltrates despite simple transfusion). c For acute rise in creatinine ≥ 0.3 mg/dL; do not give transfusions unless there are other indications. Data from Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48. | ||
One of the most challenging aspects of sickle cell disease is its clinical variability. While in general, HbSS and HbSβ0 are the most severe genotypes, there are patients with HbSC and HbSb+ who have significant sickle-cell–related complications, and may have a more severe clinical course than a HbSS patient.21 A great deal of this clinical variability cannot be explained, but some can be attributed to endogenous fetal hemoglobin (HbF) levels.22–24 The importance of HbF levels in sickle cell disease was first noted by a pediatrician in the 1940s.25 She observed that sickle cell disease complications in children under the age of 1 were rare, and attributed it to the presence of HbF.25 HbF levels decline more slowly in individuals with hemoglobinopathies, reaching their nadir after the age of 5 rather than within 6 months of birth in individuals without hemoglobinopathies.26 HbF levels remain elevated lifelong in most sickle cell disease patients, especially those with the HbSS and HbSβ0 genotypes. Levels of HbF vary widely between individuals, from zero to 20% to 30%, with a median of 10%.26–28 Individuals who produce more HbF have a milder course, in general.24 An association between the 4 β-globin haplotypes and HbF levels has been reported in the past,27,29 but more sophisticated next-generation sequencing has revealed causal variants in BCL11A and HBS1L-MYB that contribute approximately 50% of the observed variability in HbF levels.30–33
Co-inheritance of α-thalassemia also modifies disease course; less available α-globin chains results in a lower hemoglobin concentration within the cell. Paradoxically, this results in a higher overall hemoglobin level, as there is a reduction in polymerization, and therefore sickling due to lower HbS concentrations in the cell. Patients therefore are less anemic, reducing the risk of stroke in childhood,34,35 but blood viscosity may be higher, resulting in more frequent pain crises and increased risk36 of avascular necrosis.34,35,37 It is often helpful to think of sickle cell patients as falling into 1 of 2 groups: high hemolysis/low hemoglobin and high viscosity/high hemoglobin. Individuals with high rates of hemolysis are at greater risk for stroke, pulmonary hypertension, and acute chest syndrome (ACS). Higher rates of hemolysis result in higher levels of free hemoglobin, which scavenges nitric oxide. This leads to the vascular damage and dysfunction that contributes to the associated clinical complications. This phenotype is most commonly seen in HbSS and HbSβ0.38 High hemoglobin/high viscosity phenotypes are most often found in HbSC patients and in sickle cell anemia with α-thalassemia coinheritance.39–42
TREATMENT OPTIONS
In high-resource countries with newborn screening, the initiation of penicillin prophylaxis has dramatically altered the natural history of the disease, allowing the majority of patients to reach adulthood.43 Penicillin prophylaxis is usually discontinued at age 5 years; however, individuals who have undergone surgical splenectomy or have had pneumococcal sepsis on penicillin prophylaxis may remain on penicillin to age 18 or beyond.20
Another advance in sickle cell care is screening for stroke risk through transcranial Doppler ultrasound (TCD).44–47 This screening tool has reduced the incidence of childhood stroke from 10% by age 11 to 1%. TCDs typically cannot be performed after the age of 16 due to changes in the skull. Individuals found to have abnormal (elevated) TCD velocities are placed on chronic transfusion therapy for primary stroke prevention. They may remain on monthly chronic transfusions, with the goal of suppressing the percentage of HbS to 30% to 50% indefinitely. A clinical trial (STOPII) designed to determine if pediatric sickle cell disease patients on chronic transfusion therapy for primary stroke prevention could be safely taken off transfusion therapy was discontinued early due to an excess of strokes and conversion to abnormal TCD velocities in the untransfused arm.44 Individuals who have experienced an ischemic stroke have a 70% risk of another stroke, and must remain on chronic transfusion therapy indefinitely. Chronic transfusion reduces their stroke risk to 13%.
The only widely used pharmacologic therapy for sickle cell disease is hydroxyurea.12,48–50 A significant portion of the benefit of hydroxyurea stems from its induction of HbF.51 HbF does not sickle, and it interrupts the polymerization of HbS in the cell, if present in high enough concentrations.50 The level of HbF needed to achieve clinical improvement is not known, but in vitro assays suggest 20% HbF is needed to prevent sickling.52,53 However, endogenous and hydroxyurea-induced HbF is not distributed evenly through the red cells, so sickling is possible regardless of the level of HbF induced.54,55 Hydroxyurea likely has other disease-modifying effects as well, including reduction of white blood cell count and reticulocyte count and reduction of red cell adhesion to the endothelium.56–58 Clinical criteria for initiation of hydroxyurea in adult sickle cell disease patients are shown in Table 4.20 Hydroxyurea is given daily and is dosed to maximum tolerated dose for the individual by following the absolute neutrophil count (ANC). The goal ANC is between 2000 and 4000/µL. At times, absolute reticulocyte count (ARC) can be dose-limiting; goal ARC is greater than 70,000/µL.59 Platelet counts may be reduced as well, especially in HbSC patients.60,61
| Table 4. Indications for Hydroxyurea in Adult Patients with Sickle Cell Disease | |
Indication | Strength of Recommendation |
SCA with ≥ 3 pain crises per year | Strong |
SCA with pain that interferes with ADL and QoL | Strong |
History of severe or recurrent ACS | Strong |
Chronic kidney disease on epoetin | Weak |
HbSβ+ and HbSC with pain that interferes with ADL and QoL; consult sickle cell disease expert | Moderate |
| ACS = acute chest syndrome; ADL = activities of daily living; QoL = quality of life; SCA = sickle cell anemia. | |
The only curative therapy for sickle cell disease is hematopoietic stem cell transplant.62 Transplant use is limited by availability of matched sibling donors,62 and even at experienced centers transplant carries a small risk for mortality, graft rejection, and graft-versus-host disease. Furthermore, consensus on disease complications for which transplant is recommended is also lacking.63–65 Clinical trials of gene therapy for sickle cell disease and thalassemia are ongoing.66
COMPLICATIONS AND DISEASE-SPECIFIC THERAPIES
CASE PRESENTATION
A 26-year-old African-American man who works as a school bus driver presents to an academic center’s emergency department complaining of pain in his left leg, similar to prior pain events. He is described as having sickle cell trait, although no hemoglobin profile is available in his chart. He describes the pain as dull and aching, 10/10 in intensity. A complete blood count (CBC) is obtained; it reveals a hemoglobin of 14.5 g/dL, white blood cell (WBC) count of 5600/µL, and platelet count of 344,000/µL. His CBC is also notable for a mean corpuscular volume (MCV) of 72 fL, a mean corpuscular hemoglobin concentration (MCHC) of 37 g/dL, and a red blood cell distribution width (RDW) of 12. Slide review of a peripheral blood smear shows 2+ target cells (Figure).
The patient is given 6 mg of morphine, which provides some relief of his pain, and is discharged with a prescription for hydrocodone bitartrate/acetaminophen 5/325 mg. The diagnosis given is musculoskeletal pain, and he is instructed to follow-up with a primary care physician. His past medical history is significant for 4 or 5 visits to the emergency department per year in the past 4 years. Prior to 4 years ago, he rarely required medical attention.
• What laboratory and clinical features might lead you to question the diagnosis of sickle cell trait in this patient?
The patient’s hemoglobin is within normal range, which is consistent with sickle cell trait; however, he is microcytic, with a normal RDW. It is possible to be mildly microcytic in the early stages of iron deficiency, prior to the development of anemia, but the RDW would typically be elevated, demonstrating the presence of newer, smaller cells produced under conditions of iron deficiency.67 It is also possible that his microcytosis with a normal RDW could represent sickle cell trait with co-inheritance of β-thalassemia. Up to 30% of African Americans have β-thalassemia,2 and 1 in 10 have sickle cell trait.68 However, a high MCHC, indicating the presence of dense cells, and target cells noted on slide review are most consistent with HbSC.9 HbSC patients, especially males, can have hemoglobin levels in the normal range.4 The biggest inconsistency with the diagnosis of sickle cell trait is his history of frequent pain events. Individuals with sickle cell trait rarely present with pain crises, except under extreme conditions of dehydration or high altitude.68 Sickle cell trait is generally regarded as a benign condition, although a study of U.S. military recruits found a 30-fold higher risk of sudden death during basic training in persons with sickle cell trait.69 Additional sickle cell trait–related complications include hematuria, risk of splenic sequestration or infarct under extreme conditions and high altitude, and a rare and usually fatal renal malignancy, renal medullary carcinoma, which is vanishingly rare in individuals without sickle cell trait.70,71 Although the patient reported having sickle cell trait, this diagnosis should have been verified with a hemoglobin panel, given his atypical presentation.20
• What is the approach to managing pain episodes in sickle cell disease?
In sickle cell disease, vaso-occlusive pain events can be common, often beginning in early childhood.17 This disease complication accounts for 95% of all adult sickle cell disease hospitalizations.72 There is a great deal of variability in pain symptoms between individuals, and within individuals at various times in their lives:73 30% have no pain events, 50% have occasional events, and 20% have monthly or more frequent events that require hospitalization.74 The frequency and severity of pain events are modulated by HbF levels, β-thalassemia status, genotypes, therapies like hydroxyurea, or in rare cases, chronic transfusion therapy.23 Personal factors, such as psychosocial stressors, also contribute to the frequency of pain events.75 Pain event triggers include exposure to cold water, windy or cold weather, temperature changes, and extreme temperatures.76–79 Patient age also contributes to pain event frequency. Many patients see an increase in pain event frequency in their late 20s, and a marked decrease in their 40s.23,73 More than 3 pain events per year is associated with reduced life expectancy.23
Acute management of pain episodes involves nonsteroidal anti-inflammatory drugs, oral opioids, and when hospitalization is required, intravenous opioids, often delivered via patient-controlled analgesia (PCA) pumps.79 As sickle cell disease patients become teenagers and young adults, some experience an increased frequency of pain episodes, with fewer pain-free days, or a failure to return to baseline before the next pain crisis occurs.80,81 This is characteristic of emerging chronic pain.82 Chronic pain is a significant problem in adult patients with sickle cell disease, with up to 85% reporting pain on most days.72,80 The development of chronic pain may be reduced by early and aggressive treatment of acute pain events, as well as use of hydroxyurea to reduce the number of pain events. Many adult sickle cell patients with chronic pain are treated with daily opioids.20 Given the significant side effects of chronic opioid use—sedation, respiratory depression, itching, nausea, and impairment of function and quality of life—non-opioid therapies are under investigation.83 Many chronic pain patients have symptoms of neuropathic pain, and may benefit from neuropathic agents like gabapentin, both to reduce opioid use and to more effectively treat chronic neuropathic pain, which is known to respond poorly to opioids.84–86
• Is the patient’s peripheral blood smear consistent with a diagnosis of sickle cell trait?
Several target cells are visible, which is not typical of sickle cell trait, but may be seen in HbSC or thalassemia. The finding of an intracellular crystal is pathognomonic for HbSC or HbCC. HbC polymerizes in high oxygen conditions, opposite of HbS, which polymerizes in low oxygen conditions.9
CASE CONTINUED
The patient’s family history is significant for a sister who died at age 3 from sickle cell–related complications, and a sister with sickle cell trait who had a cholecystectomy for gallstones at age 22. His father died at age 38 due to unknown causes. The sickle cell trait status of his parents is unknown. His mother is alive, and has hypertension.
• Is the medical history of this patient’s family members consistent with sickle cell trait?
It is unlikely that sickle cell trait would result in early death in childhood, or in gallstones at age 22. Gallstones in early adulthood is a common presentation for HbSC patients not diagnosed by newborn screening.87 Any hemolytic condition can lead to the formation of hemoglobin-containing pigmented gallstones, biliary sludge, and obstruction of the gallbladder. In the presence of right-sided abdominal pain, a serum bilirubin level of more than 4 mg/dL should lead to measurement of direct bilirubin; if greater than 10% of total, imaging of the gallbladder should be obtained. In sickle cell disease, 30% of patients will have gallstones by 18 years of age. The low hemolysis/high viscosity phenotype patients are typically older at diagnosis. Co-inheritance of Gilbert syndrome and sickle cell disease is not uncommon, and can result in formation of gallstones at a young age; Gilbert syndrome alone typically results in gallstones in mid-life.88
CASE CONTINUED
Two months later, the patient presents again to the emergency department with the same complaint of leg pain, as well as abdominal pain. His hemoglobin is 12.5 g/dL, and his platelet count is 134,000/µL. His pain is not improved with 3 doses of morphine 6 mg intravenously, and he is admitted to the medicine service. A hemoglobin profile is obtained, revealing 52% HbS, 45% HbC, and 1.5% HbF, consistent with HbSC. In sickle cell trait, the hemoglobin profile is 60% HbA and 40% HbS (available α-globin prefers to pair with a normal β-globin, so the ratio of HbA to HbS is 60:40, not 50:50).
On the second hospital day, the patient’s hemoglobin drops to 7.2 g/dL and his platelet count decreases to 44,000/µL. His abdomen is distended and diffusely tender. The internist transfuses him with 2 units of packed red blood cells (PRBC), after which his hemoglobin increases to 11 g/dL, while his platelet count increases to 112,000/µL. Following the transfusion, his abdominal pain resolves, as does his anemia and thrombocytopenia.
• What caused this patient’s anemia and thrombocytopenia?
High on the differential diagnosis is a splenic sequestration. Acute splenic sequestration occurs when red cells are trapped in the splenic sinuses. Massive splenic enlargement may occur over several hours.89,90 Unrecognized splenic sequestration has a high mortality rate from severe anemia and splenic rupture.90 Splenic sequestration must be ruled out in a sickle cell patient with abdominal pain accompanied by dropping platelet and red cell counts, especially in milder subtypes that often have splenic function preserved into adolescence and adulthood. Sickle cell anemia patients usually become functionally asplenic in early childhood.89,91,92 The rise in hemoglobin, more than would be expected from 2 units of PRBC, plus the improvement in platelet count without a platelet transfusion observed in the case patient strongly supports the diagnosis of splenic sequestration.
Splenic sequestration can occur in any sickle cell patient whose spleen has not fibrosed. Splenic sequestration in adulthood is not uncommon in HbSC patients, who often have preserved splenic function into adulthood.93–95
Clinical signs of splenic sequestration include a rapid drop in hemoglobin, rise in reticulocyte count, a tender, enlarged spleen, and, in severe cases, hypovolemia.89,93 It is treated with prompt blood transfusion, but care must be taken not to overtransfuse the patient, as the spleen can trap several grams of hemoglobin, which may be released upon transfusion, potentially causing life-threatening hyperviscosity.89 Hemoglobin levels must be checked following transfusion in suspected splenic sequestration, and “mini transfusions” of 5 mL/kg are recommended in sickle cell disease patients who are hemodynamically stable.20
Hepatic sequestration may also occur, but it is much less common than splenic sequestration.96 Other conditions on the differential diagnosis include thrombotic thrombocytopenic purpura, which would be unlikely to respond to a transfusion. ACS can cause a drop in hemoglobin, and is treated with simple or exchange transfusions.97 ACS is less likely without respiratory symptoms or oxygen requirement, and usually is not associated with thrombocytopenia. Sepsis may also cause anemia and thrombocytopenia, but again would not likely respond to a simple transfusion. The patient’s response to transfusion is consistent with a sequestering event, not a destructive event as in the case of sepsis.
CASE CONTINUED
Imaging reveals a grossly enlarged spleen, which is having a mass effect on the left kidney. The patient is started on hydroxyurea therapy at 500 mg 3 times daily. Discharge instructions include following up with his primary care physician, continuing hydroxyurea therapy, and receiving yearly dilated eye exams to evaluate for proliferative sickle retinopathy.
• Are these discharge instructions complete?
Splenic sequestration has a 50% recurrence rate.98 In very young children, watchful waiting or chronic transfusion may be implemented to preserve the immunologic function of the spleen and reduce the risk of sepsis.89 Splenectomy after a single episode of sequestration in adults is a matter of debate, with experts advising both watchful waiting99 and splenectomy after recovery from the first sequestering event.100 The patient should have been informed of the risk for recurrence, and the signs and symptoms of splenic sequestration as well as the need for emergency medical attention should have been discussed. Splenic sequestration may be milder in adults than in children, but fatal sequestrations have been reported.95,101–103
Proliferative sickle cell retinopathy is a high viscosity/high hemoglobin complication that may occur more frequently in HbSC than HbSS, with an incidence of 33% in HbSC.42,104 Spontaneous regression of retinopathy occurs in approximately 32% of eyes, and laser or scatter photocoagulation is an effective intervention.105
• Would the patient need to be transfused prior to splenectomy?
Preoperative transfusion therapy is standard of care for HbSS patients undergoing general anesthesia. The TRAP study found that simple “top off” transfusion to a hemoglobin of 10 g/dL was as effective at preventing postoperative sickle cell–related complications as exchange transfusion to HbS of 30% or less, and had fewer transfusion-related complications like alloimmunization.106 There is little data regarding preoperative transfusions in HbSC disease. A retrospective study suggests that HbSC patients undergoing abdominal surgeries should be transfused.107 The higher hemoglobin level of the typical HbSC patient necessitates exchange transfusion to avoid hyperviscosity.
• Is hydroxyurea therapy indicated in this patient?
• Has it been dosed appropriately?
If the patient had the HbSS subtype, hydroxyurea would be clearly indicated, given his frequent pain events.20 HbSC patients may be placed on hydroxyurea on a case-by-case basis, but evidence for its efficacy in this sickle cell subtype is lacking.108 Large clinical trials like the Multi-Center Study of Hydroxyurea (MSH) that established the safety and efficacy of hydroxyurea in sickle cell anemia excluded HbSC and HbSβ+ patients.109 These mild to moderate subtypes produce less HbF at baseline, and typically have a minimal to modest rise in HbF on hydroxyurea.110 In sickle cell anemia, hydroxyurea is titrated to maximum tolerated dose, defined as an ANC of 2000 to 4000/µL and an ARC of 70,000/µL or higher.53 Because of their lower levels of chronic inflammation and lower reticulocyte counts due to higher hemoglobin levels, many HbSC and HbSβ+ patients have values in that range before initiating hydroxyurea therapy.9 Cytopenias, particularly of platelets in HbSC, occur at low doses of hydroxyurea.111
Of note, although the half-life of hydroxyurea would suggest that 3 times daily dosing is indicated, daily dosing has been found to have equal response and is preferred. Another concern is the monitoring of this myelosuppressive medication. This patient has repeatedly failed to obtain a primary care physician or a hematologist, and hydroxyurea requires laboratory monitoring at least every 2 months, especially in a HbSC patient with a very large spleen who is at significant risk for thrombocytopenia and neutropenia.9
CASE CONTINUED
A week after discharge from his admission for abdominal pain diagnosed as splenic sequestration, the patient presents again to the emergency department with abdominal pain which he reports is his typical sickle cell pain. Hemoglobin is 13.8 g/dL, platelet count is 388,000/µL, and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels are both 10 times their prior value. Creatinine is 1.2 mg/dL (0.75 mg/dL on his prior admission), and total bilirubin is 3 mg/dL, with 0.3 mg/dL direct bilirubin. He undergoes an ultrasound exam of his gallbladder, which reveals sludge and a possible gallstone. There is no evidence of cholecystitis. General surgery performs a laparoscopic cholecystectomy.
• Was this cholecystectomy necessary?
In patients with sickle cell disease, symptomatic gallstones and gallbladder sludge should be observed; recurrent abdominal pain without a significant change in bilirubin may not be due to gallstones or sludge, and therefore may not be relieved by cholecystectomy.112,113 In sickle cell disease, 40% of patients with gallbladder sludge do not develop gallstones.87 The patient’s bilirubin level was at baseline, and there was no increase in the direct (conjugated) fraction. Watchful waiting would have been appropriate, with cholecystectomy being performed if he experienced recurrent symptoms associated with fatty foods accompanied by an elevation in direct bilirubin.
More concerning and deserving of investigation was his elevated liver enzymes. Patients with sickle cell disease may experience recurrent ischemia and reperfusion injuries in the liver, which is called right upper quadrant syndrome. On autopsy of 70 sickle cell patients, 91% had hepatomegaly and 34% had focal necrosis.114 AST is often elevated in sickle cell disease, as it is affected by hemolysis. In this patient, both AST and ALT are elevated, consistent with a hepatocellular disorder. His abdominal pain and ALT rise may be a sign of a hepatic crisis.115 Rapid resolution of ALT elevation in a matter of days suggests a vaso-occlusive, inflammatory event that is self- limiting. Prolonged AST elevation requires further investigation, with consideration of autoimmune hepatitis, viral hepatitis, or iron overload. Iron overload is unlikely in this patient given his lifetime history of only 1 transfusion. Hepatic iron overload typically occurs in sickle cell disease after a minimum of 10 transfusions.115
CASE CONTINUED
The patient is discharged on the day after the procedure, with instructions to continue his hydroxyurea.
• Should the patient resume hydroxyurea therapy?
Hydroxyurea is hepatically cleared and thus it should be held until his liver function tests normalize.106
CASE CONTINUED
Two months later, the patient presents to the emergency department with abdominal pain that moves to his left leg. A CBC is obtained, showing a hemoglobin of 11.8 g/dL and a platelet count of 144,000/µL. He is given 2 doses of morphine 6 mg intravenously, and reports that his leg pain is now a 4/10. He is discharged home with a prescription for hydrocodone/acetaminophen.
• Is the emergency department evaluation sufficient?
This patient remains at high risk for splenic sequestration,93 with a hemoglobin 2 g lower than it was 2 months ago and platelets less than half. This decline could be consistent with early splenic sequestration.20 Additionally, he had elevated liver function tests on a recent admission, as well as rising creatinine, without evidence of resolution. It is not appropriate to discharge him without checking a chemistry and liver panel, and abdominal imaging should be considered. The best plan would be to admit him for observation, given his risk for splenic sequestration, and consult surgery for an elective splenectomy if he has a second episode of splenic sequestration 2 months after the first.100 His abdominal pain that migrates to his left leg could be due to his massive splenomegaly compressing his left kidney, as noted on imaging during his recent admission for splenic sequestration
CASE CONTINUED
An hour after discharge from the emergency department, EMS is called to his home for intractable pain. He is found lying on the floor, and reports excruciating left leg pain. He is brought to the closest hospital, a community hospital that he has not visited previously. There, he is admitted for hydration and pain control and placed on hydromorphone 2 mg every 4 hours as needed for pain. His hemoglobin is 10.8 g/dL, and platelets are 121,000/µL. A chemistry panel is remarkable for a creatinine level of 1.5 mg/dL and a potassium level of 3.2 mEq/L. Liver function tests are not obtained. After 3 doses of hydromorphone, he falls asleep. He is not in a monitored bed, and intravenous fluids, while ordered, are not started. At 6:30 AM the day after admission, he cannot be aroused on a routine vital sign check; he has an SpO2 of 60%, a blood pressure of 80/60 mm Hg, and heart rate of 148 beats/min. A rapid response is called, and naloxone is administered along with oxygen by face mask and several fluid boluses. His systolic blood pressure increases to 100 mm Hg from a low of 70 mm Hg. His SpO2 increases to 92%, and he is arousable and alert, although he reports 10/10 leg pain. His abdomen is noted to be distended and tender.
• What may have contributed to his clinical condition?
The patient is opioid tolerant and has received equivalent doses of opioids in the past without excess sedation. He may have liver dysfunction making him unable to metabolize opioids effectively. His hemoglobin and platelets continue to decline, raising concern for splenic sequestration versus sepsis. Failure to place him on a monitor allowed his hypoxia to continue for an unknown amount of time, placing him at high risk for developing ACS. Lack of intravenous hydration while he was too sedated to drink likely exacerbated his sickling.
CASE CONTINUED
At 9:20 AM, a CBC is obtained and reveals a hemoglobin of 4.8 g/dL and a platelet count of 44,000/µL. Two units of stat O negative blood are administered, and preparations are made to administer an exchange transfusion. A liver panel is obtained 3 hours later, which reveals an AST level of 1200 U/L and an ALT level of 1050 U/L. His bilirubin is 10 mg/dL, and his lactate dehydrogenase level is 1800 U/L. His urine is dark and is positive for bilirubin and ketones. He is transferred to the intensive care unit. A chest X-ray shows pulmonary congestion. Hematology/oncology is consulted.
He receives a 7-unit red blood cell exchange, which reduces his HbS to 11%. He continues to be hypotensive, and requires norepinephrine to support his blood pressure. Antibiotic therapy is started. His creatinine concentration rises to 2.3 mg/dL, potassium is 7.8 mEq/L, and bicarbonate is 12 mEq/L. He is placed on hemodialysis.
Computed tomography of the chest and abdomen reveals lower posterior lung infiltrates and a grossly enlarged spleen. He requires intubation. He is given a diagnosis of ACS in addition to kidney failure, liver failure, and “sickle crisis.” He continues to require daily to twice daily transfusions to maintain a hemoglobin of 7 to 9 g/dL, and his abdominal distension increases. As his condition worsens, surgery is consulted to discuss a liver transplant. He is deemed to not be a surgical candidate, and he passes away 6 days after entering the hospital. The immediate cause of death is listed as vaso-occlusive crisis, with ACS and sickle crisis listed as contributors.
• Are the causes of death accurate and complete?
If vaso-occlusive crisis is used to indicate a pain event, it is not an accurate cause of death. Pain is one of the most distressing complications of sickle cell disease, and frequent pain events are associated with early mortality,4,80 but they are not in themselves fatal. ACS is the number one cause of death in sickle cell disease,4 and it likely contributed to this patient’s death. Sickle crisis is a vague term that should not be used in this context. Causes of death should include splenic sequestration and multisystem organ failure. Multisystem organ failure in sickle cell disease often responds to aggressive transfusion therapy, which this patient received.116–118
CONCLUSION
Sickle cell disease is a complex chronic disease that impacts almost every organ system in the body. Clinicians may be inclined to attribute most pain in a patient with sickle cell disease to a simple vaso-occlusive crisis, treat them for this, and not investigate further. As the case presented here demonstrates, failure to identify the actual life-threatening process occurring in a patient with sickle cell disease presenting with pain can result in preventable early mortality. Clinicians must approach a sickle cell patient reporting pain in a thoughtful manner, and consider a complete differential diagnosis, including both sickle cell disease complications and those unrelated to sickle cell disease. Knowledge of the disease courses of the different sickle cell genotypes is essential, and must go beyond a superficial hierarchy of severity, but rather include an understanding of the complications each genotype is most prone to, and at what ages. Complete laboratory assessment, including a comprehensive metabolic panel, should be performed on all admitted patients, not just a complete blood count. Treating pain with high-dose opioids, while appropriate in an uncomplicated pain crisis, can lead to ACS or even respiratory failure in a patient with uninvestigated liver and kidney dysfunction. The most important lesson to remember is that even the sickle cell disease patient who has been given the unfortunate and pejorative label of “frequent flyer” by some providers has the potential for rapid deterioration into multisystem organ failure and death.
- Weatherall D, Hofman K, Rodgers G, Ruffin J, Hrynkow S. A case for developing North-South partnerships for research in sickle cell disease. Blood 2005;105:921–3.
- Flint J, Harding RM, Boyce AJ, Clegg JB. The population genetics of the haemoglobinopathies. Baillieres Clin Haematol 1998;11:1–51.
- Allison AC. The distribution of the sickle-cell trait in East Africa and elsewhere, and its apparent relationship to the incidence of subtertian malaria. Trans R Soc Trop Med Hyg 1954;48:312–8.
- Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med 1994;330:1639–44.
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010;376:2018–31.
- Serjeant GR, Serjeant BE. Sickle cell disease. 3rd ed. New York: Oxford University Press; 2001.
- Moo-Penn W, Bechtel K, Jue D, et al. The presence of hemoglobin S and C Harlem in an individual in the United States. Blood 1975;46:363–7.
- Monplaisir N, Merault G, Poyart C, et al. Hemoglobin S Antilles: a variant with lower solubility than hemoglobin S and producing sickle cell disease in heterozygotes. Proc Natl Acad Sci U S A 1986;83:9363–7.
- Nagel RL, Fabry ME, Steinberg MH. The paradox of hemoglobin SC disease. Blood Rev 2003;17:167–78.
- Nagel RL, Daar S, Romero JR, et al. HbS-oman heterozygote: a new dominant sickle syndrome. Blood 1998;92:4375–82.
- Masiello D, Heeney MM, Adewoye AH, et al. Hemoglobin SE disease: a concise review. Am J Hematol 2007;82:643–9.
- Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med 1997;337:762–9.
- Brittenham GM, Schechter AN, Noguchi CT. Hemoglobin S polymerization: primary determinant of the hemolytic and clinical severity of the sickling syndromes. Blood 1985;65:183–9.
- .Nagel RL, Bookchin RM, Johnson J, et al. Structural bases of the inhibitory effects of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S. Proc Natl Acad Sci U S A 1979;76:670–2.
- .Ballas SK, Dover GJ, Charache S. Effect of hydroxyurea on the rheological properties of sickle erythrocytes in vivo. Am J Hematol 1989;32:104–11.
- Frenette PS. Sickle cell vaso-occlusion: multistep and multicellular paradigm. Curr Opin Hematol 2002;9:101–6.
- Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Med 2002;8:1383–9.
- Nouraie M, Lee JS, Zhang Y, et al. The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica 2013;98:464–72.
- Serjeant GR. The natural history of sickle cell disease. Cold Spring Harb Perspect Med 2013;3:a011783.
- Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48.
- Serjeant GR. Natural history and determinants of clinical severity of sickle cell disease. Curr Opin Hematol 1995;2:103–8.
- Odenheimer DJ, Sarnaik SA, Whitten CF, et al. The relationship between fetal hemoglobin and disease severity in children with sickle cell anemia. Am J Med Genet 1987;27:525–35.
- Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med 1991;325:11–6.
- Falusi AG, Olatunji PO. Effects of alpha thalassaemia and haemoglobin F (HbF) level on the clinical severity of sickle-cell anaemia. Eur J Haematol 1994;52:13–5.
- Watson J. The significance of the paucity of sickle cells in newborn Negro infants. Am J Med Sci 1948;215:419–23.
- Marcus SJ, Ware RE. Physiologic decline in fetal hemoglobin parameters in infants with sickle cell disease: implications for pharmacological intervention. J Pediatr Hematol Oncol 1999;21:407–11.
- Schroeder WA, Powars DR, Kay LM, et al. Beta-cluster haplotypes, alpha-gene status, and hematological data from SS, SC, and S-beta-thalassemia patients in southern California. Hemoglobin 1989;13:325–53.
- Steinberg MH, Voskaridou E, Kutlar A, et al. Concordant fetal hemoglobin response to hydroxyurea in siblings with sickle cell disease. Am J Hematol 2003;72:121–6.
- Ngo D, Bae H, Steinberg MH, et al. Fetal hemoglobin in sickle cell anemia: genetic studies of the Arab-Indian haplotype. Blood Cells Mol Dis 2013;51:22–6.
- Galarneau G, Palmer CD, Sankaran VG, et al. Fine-mapping at three loci known to affect fetal hemoglobin levels explains additional genetic variation. Nat Genet 2010;42:1049–51.
- Lettre G, Sankaran VG, Bezerra MA, et al. DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci U S A 2008;105:11869–74.
- Sankaran VG, Menne TF, Xu J, et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 2008;322:1839–42.
- Uda M, Galanello R, Sanna S, et al. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc Natl Acad Sci U S A 2008;105:1620–5.
- Adams RJ, Kutlar A, McKie V, et al. Alpha thalassemia and stroke risk in sickle cell anemia. Am J Hematol 1994;45:279–82.
- Ballas SK. Effect of alpha-globin genotype on the pathophysiology of sickle cell disease. Pediatr Pathol Mol Med 2001;20:107–21.
- Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med 1986;314:1593–9.
- Ballas SK, Talacki CA, Rao VM, Steiner RM. The prevalence of avascular necrosis in sickle cell anemia: correlation with alpha-thalassemia. Hemoglobin 1989;13:649–55.
- .Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev 2007;21:37–47.
- Murphy JR, Wengard M, Brereton W. Rheological studies of Hb SS blood: influence of hematocrit, hypertonicity, separation of cells, deoxygenation, and mixture with normal cells. J Lab Clin Med 1976;87:475–86.
- Fabry ME, Kaul DK, Raventos-Suarez C, Chang H, Nagel RL. SC erythrocytes have an abnormally high intracellular hemoglobin concentration. Pathophysiological consequences. J Clin Invest 1982;70:1315–9.
- Stuart J, Johnson CS. Rheology of the sickle cell disorders. Baillieres Clin Haematol 1987;1:747–75.
- Lionnet F, Hammoudi N, Stojanovic KS, et al. Hemoglobin sickle cell disease complications: a clinical study of 179 cases. Haematologica 2012;97:1136-41.
- Adamkiewicz TV, Sarnaik S, Buchanan GR, et al. Invasive pneumococcal infections in children with sickle cell disease in the era of penicillin prophylaxis, antibiotic resistance, and 23-valent pneumococcal polysaccharide vaccination. J Pediatr 2003;143:438–44.
- Abboud MR, Yim E, Musallam KM, Adams RJ, Investigators SIS. Discontinuing prophylactic transfusions increases the risk of silent brain infarction in children with sickle cell disease: data from STOP II. Blood 2011;118:894–8.
- Adams RJ. Lessons from the Stroke Prevention Trial in Sickle Cell Anemia (STOP) study. J Child Neurol 2000;15:344–9.
- Adams RJ, Brambilla DJ, Granger S, et al. Stroke and conversion to high risk in children screened with transcranial Doppler ultrasound during the STOP study. Blood 2004;103:3689–94.
- Lee MT, Piomelli S, Granger S, et al. Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results. Blood 2006;108:847–52.
- Charache S, Dover GJ, Moore RD, et al. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood 1992;79:2555–65.
- Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med 1995;332:1317–22.
- Charache S. Mechanism of action of hydroxyurea in the management of sickle cell anemia in adults. Semin Hematol 1997;34:15–21.
- Steinberg MH, McCarthy WF, Castro O, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17.5 year follow-up. Am J Hematol 2010;85:403–8.
- Noguchi CT, Rodgers GP, Serjeant G, Schechter AN. Levels of fetal hemoglobin necessary for treatment of sickle cell disease. N Engl J Med 1988;318:96–9.
- Powars DR, Weiss JN, Chan LS, Schroeder WA. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood 1984;63:921–6.
- Maier-Redelsperger M, de Montalembert M, Flahault A, et al. Fetal hemoglobin and F-cell responses to long-term hydroxyurea treatment in young sickle cell patients. The French Study Group on Sickle Cell Disease. Blood 1998;91:4472–9.
- Steinberg MH, Chui DH, Dover GJ, et al. Fetal hemoglobin in sickle cell anemia: a glass half full? Blood 2014;123:481–5.
- Adragna NC, Fonseca P, Lauf PK. Hydroxyurea affects cell morphology, cation transport, and red blood cell adhesion in cultured vascular endothelial cells. Blood 1994;83:553–60.
- Bridges KR, Barabino GD, Brugnara C, et al. A multiparameter analysis of sickle erythrocytes in patients undergoing hydroxyurea therapy. Blood 1996;88:4701–10.
- Jiang J, Jordan SJ, Barr DP, et al. In vivo production of nitric oxide in rats after administration of hydroxyurea. Mol Pharmacol 1997;52:1081–6.
- Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood 2010;115:5300–11.
- Yates AM, Dedeken L, Smeltzer MP, et al. Hydroxyurea treatment of children with hemoglobin SC disease. Pediatr Blood Cancer 2013;60:323–5.
- Barbosa CG, Aleluia AC, Pacheco AP, et al. Genetic modulation of HbF in Brazilians with HbSC disease and sickle cell anemia. Am J Hematol 2013;88:923–4.
- Hsieh MM, Kang EM, Fitzhugh CD, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med 2009;361:2309–17.
- King A, Shenoy S. Evidence-based focused review of the status of hematopoietic stem cell transplantation as treatment of sickle cell disease and thalassemia. Blood 2014;123:3089–94.
- Oringanje C, Nemecek E, Oniyangi O. Hematopoietic stem cell transplantation for people with sickle cell disease. Cochrane Database Syst Rev 2013;5:CD007001.
- Freed J, Talano J, Small T, et al. Allogeneic cellular and autologous stem cell therapy for sickle cell disease: ‘whom, when and how’. Bone Marrow Transplant 2012;47:1489–98.
- Urbinati F, Hargrove PW, Geiger S, et al. Potentially therapeutic levels of anti-sickling globin gene expression following lentivirus-mediated gene transfer in sickle cell disease bone marrow CD34 cells. Exp Hematol 2015;43:346–51.
- Brugnara C, Mohandas N. Red cell indices in classification and treatment of anemias: from M.M. Wintrobes’s original 1934 classification to the third millennium. Curr Opin Hematol 2013;20:222–30.
- Key NS, Derebail VK. Sickle-cell trait: novel clinical significance. Hematology Am Soc Hematol Educ Program 2010;2010:418–22.
- Kark JA, Posey DM, Schumacher HR, Ruehle CJ. Sickle-cell trait as a risk factor for sudden death in physical training. N Engl J Med 1987;317:781–7.
- Goldsmith JC, Bonham VL, Joiner CH, et al. Framing the research agenda for sickle cell trait: building on the current understanding of clinical events and their potential implications. Am J Hematol 2012;87:340–6.
- Grant AM, Parker CS, Jordan LB, et al. Public health implications of sickle cell trait: a report of the CDC meeting. Am J Prev Med 2011;41:S435–9.
- Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol 2005;79:17–25.
- Serjeant GR, Ceulaer CD, Lethbridge R, et al. The painful crisis of homozygous sickle cell disease: clinical features. Br J Haematol 1994;87:586–91.
- Vichinsky EP, Johnson R, Lubin BH. Multidisciplinary approach to pain management in sickle cell disease. Am J Pediatr Hematol Oncol 1982;4:328–33.
- Gil KM, Carson JW, Porter LS, et al. Daily mood and stress predict pain, health care use, and work activity in African American adults with sickle-cell disease. Health Psychol 2004;23:267–74.
- Amjad H, Bannerman RM, Judisch JM. Letter: Sickling pain and season. Br Med J 1974;2:54.
- Ibrahim AS. Relationship between meteorological changes and occurrence of painful sickle cell crises in Kuwait. Trans R Soc Trop Med Hyg 1980;74:159–61.
- Jones S, Duncan ER, Thomas N, et al. Windy weather and low humidity are associated with an increased number of hospital admissions for acute pain and sickle cell disease in an urban environment with a maritime temperate climate. Br J Haematol 2005;131:530–3.
- Resar LM, Oski FA. Cold water exposure and vaso-occlusive crises in sickle cell anemia. J Pediatr 1991;118:407–9.
- Darbari DS, Ballas SK, Clauw DJ. Thinking beyond sickling to better understand pain in sickle cell disease. Eur J Haematol 2014;93:89–95.
- Darbari DS, Onyekwere O, Nouraie M, et al. Markers of severe vaso-occlusive painful episode frequency in children and adolescents with sickle cell anemia. J Pediatr 2011;160:286–90.
- Hollins M, Stonerock GL, Kisaalita NR, et al. Detecting the emergence of chronic pain in sickle cell disease. J Pain Symptom Manage 2012;43:1082–93.
- Ballas SK, Darbari DS. Neuropathy, neuropathic pain, and sickle cell disease. Am J Hematol 2013;88:927–9.
- Brandow AM, Farley RA, Panepinto JA. Early insights into the neurobiology of pain in sickle cell disease: A systematic review of the literature. Pediatr Blood Cancer 2015 May 13. doi: 10.1002/pbc.25574. [Epub ahead of print].
- Brandow AM, Farley RA, Panepinto JA. Neuropathic pain in patients with sickle cell disease. Pediatr Blood Cancer 2014;61:512–7.
- Brandow AM, Farley RA, Dasgupta M, et al. The use of neuropathic pain drugs in children with sickle cell disease is associated with older age, female sex, and longer length of hospital stay. J Pediatr Hematol Oncol 2015;37:10–5.
- Walker TM, Hambleton IR, Serjeant GR. Gallstones in sickle cell disease: observations from The Jamaican Cohort study. J Pediatr 2000;136:80–5.
- Penner E, Mayr WR, Djawan S, et al. [The genetics of Gilbert syndrome]. Schweiz Med Wochenschr 1976;106:860–2. [German]
- Powell RW, Levine GL, Yang YM, Mankad VN. Acute splenic sequestration crisis in sickle cell disease: early detection and treatment. J Pediatr Surg 1992;27:215–8.
- Al Salem AH, Qaisaruddin S, Nasserullah Z, et al. Splenectomy and acute splenic sequestration crises in sickle cell disease. Pediatr Surg Int 1996;11:26–8.
- Pearson HA, Spencer RP, Cornelius EA. Functional asplenia in sickle-cell anemia. N Engl J Med 1969;281:923–6.
- Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet 2011;377:1663–72.
- Brousse V, Buffet P, Rees D. The spleen and sickle cell disease: the sick(led) spleen. Br J Haematol 2014;166:165–76.
- Orringer EP, Fowler VG Jr, Owens CM, et al. Case report: splenic infarction and acute splenic sequestration in adults with hemoglobin SC disease. Am J Med Sci 1991;302:374–9.
- Michel JB, Hernandez JA, Buchanan GR. A fatal case of acute splenic sequestration in a 53–year-old woman with sickle-hemoglobin C disease. Am J Med 1992;92:97–100.
- Hatton CS, Bunch C, Weatherall DJ. Hepatic sequestration in sickle cell anaemia. Br Med J (Clin Res Ed) 1985;290:744–5.
- Castro O, Brambilla DJ, Thorington B, et al. The acute chest syndrome in sickle cell disease: incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood 1994;84:643–9.
- Gill FM, Sleeper LA, Weiner SJ, et al. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood 1995;86:776–83.
- Owusu-Ofori S, Hirst C. Splenectomy versus conservative management for acute sequestration crises in people with sickle cell disease. Cochrane Database Syst Rev 2013;5:CD003425.
- 00.Al-Salem AH. Splenic complications of sickle cell anemia and the role of splenectomy. ISRN Hematol 2011;2011:864257.
- Sabarense AP, Lima GO, Silva LM, Viana MB. Characterization of mortality in children with sickle cell disease diagnosed through the Newborn Screening Program. J Pediatr (Rio J) 2015;91:242–7.
- Aslam AF, Aslam AK, Dipillo F. Fatal splenic sequestration crisis with multiorgan failure in an adult woman with sickle cell-beta+ thalassemia. Am J Med Sci 2005;329:141–3.
- Berry RA, Odumakinde EA, Lewis JP. Massive splenic infarction in doubly abnormal heterozygous sickling disorders. A new complication of acute splenic sequestration syndrome. The West J Med 1991;155:531–2.
- Bonanomi MT, Lavezzo MM. Sickle cell retinopathy: diagnosis and treatment. Arq Bras de Oftalmol 2013;76:320–7.
- Chen RW, Flynn HW Jr, Lee WH, et al. Vitreoretinal management and surgical outcomes in proliferative sickle retinopathy: a case series. Am J Ophthalmol 2014;157:870–5 e1.
- Howard J, Malfroy M, Llewelyn C, et al. The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study: a randomised, controlled, multicentre clinical trial. Lancet 2013;381:930–8.
- Neumayr L, Koshy M, Haberkern C, et al. Surgery in patients with hemoglobin SC disease. Preoperative Transfusion in Sickle Cell Disease Study Group. Am J Hematol 1998;57:101–8.
- 08. Savage WJ, Buchanan GR, Yawn BP, et al. Evidence gaps in the management of sickle cell disease: A summary of needed research. Am J Hematol 2015;90:273–5.
- Charache S, Barton FB, Moore RD, et al. Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive “switching” agent. The Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Medicine (Baltimore) 1996;75:300–26.
- Steinberg MH, Nagel RL, Brugnara C. Cellular effects of hydroxyurea in Hb SC disease. Br J Haematol 1997;98:838–44.
- Wang W, Brugnara C, Snyder C, et al. The effects of hydroxycarbamide and magnesium on haemoglobin SC disease: results of the multi-centre CHAMPS trial. Br J Haematol 2011;152:771–6.
- Jungst C, Kullak-Ublick GA, Jungst D. Gallstone disease: Microlithiasis and sludge. Best Pract Res Clin Gastroenterol 2006;20:1053–62.
- Walker TM, Serjeant GR. Biliary sludge in sickle cell disease. J Pediatr 1996;129:443–5.
- Bauer TW, Moore GW, Hutchins GM. The liver in sickle cell disease. A clinicopathologic study of 70 patients. Am J Med 1980;69:833–7.
- Ebert EC, Nagar M, Hagspiel KD. Gastrointestinal and hepatic complications of sickle cell disease. Clin Gastroenterol Hepatol 2010;8:483–9.
- Hiran S. Multiorgan dysfunction syndrome in sickle cell disease. J Assoc Physicians India 2005;53:19–22.
- Shao SH, Orringer EP. Case report: splenic sequestration and multiorgan failure as the presenting manifestation of hemoglobin SC disease. Am J Med Sci 1996;311:139–41.
- Hassell KL, Eckman JR, Lane PA. Acute multiorgan failure syndrome: a potentially catastrophic complication of severe sickle cell pain episodes. Am J Med 1994;96:155–62.
INTRODUCTION
Sickle cell disease is the most common inherited blood disorder in the world. It affects more than 100,000 individuals in the United States, and millions more worldwide.1 Sickle cell disease is most commonly found in individuals of African heritage, but the disease also occurs in Hispanics and people of Middle Eastern and subcontinent Indian heritage.2 The distribution of the sickle hemoglobin (hemoglobin S [HbS]) allele overlaps with the distribution of malaria; HbS carriers, or individuals with sickle cell trait, have protection against malaria,3 and are not considered to have sickle cell disease.
Sickle cell disease is a severe monogenic disorder marked by significant morbidity and mortality, affecting every organ in the body.4 The term sickle cell disease refers to all genotypes that cause sickling; the most common are the homozygous hemoglobin SS (HbSS) and compound heterozygotes hemoglobin SC (HbSC), hemoglobin S–β0-thalassemia (HbSβ0),and hemoglobin S–β+-thalassemia(HbSβ+), although HbS and several rarer hemoglobin variants such as HbSO(Arab) and HbSD(Punjab) can also cause sickle cell disease.The term sickle cell anemia refers exclusively to the most severe genotypes, HbSS and HbSβ0.5 Common sickling genotypes along with their relative clinical severity are shown in Table 1.6–11
| Table 1. Genotypes of Sickling Syndromes and Their Relative Severities | ||
Genotype | Severity | Characteristics |
HbSS | Severe | Most common form |
HbSβ0 | Severe | Clinically indistinguishable from HbSS6 |
HbSO-Arab | Severe | Relatively rare6 |
HbSD-Punjab | Severe | Mostly in northern India6 |
HbSC-Harlem | Severe | Migrates like HbSC, but rare double β-globin mutation7 |
HbCS-Antilles | Severe | Rare double β-globin mutation8 |
HbSC | Moderate | 25% of SCD9 |
HbSβ+, Mediterranean | Moderate | 5%–16% HbA6 |
HbAS-Oman | Moderate | Dominant rare double β-globin mutation10 |
HbSβ+, African | Mild | 16%–30% HbA6 |
HbSE | Mild | HbE found mostly in Southeast Asia11 |
HbS-HPFH | Very mild | Large deletions in β-globin gene complex; > 30% HbF6 |
| HbA = hemoglobin A; HbE = hemoglobin E; HbF = fetal hemoglobin; HbS-HPFH = HbS and gene deletion HPFH; HbSC = heterozygous hemoglobin SC; HbSS = homozygous hemoglobin SS; HbSβ0 = hemoglobin S-β thalassemia0; HbSβ+ = hemoglobin S-β thalassemia+; SCD = sickle cell disease. | ||
This article reviews the pathophysiology of sickle cell disease, common clinical complications, and available therapies. A complex case which illustrates diagnostic and management challenges is presented as well.
PATHOPHYSIOLOGY
HbS is the result of a substitution of valine for glutamic acid in the sixth amino acid of the β-globin chain.12 The change from a hydrophilic to a hydrophobic amino acid causes the hemoglobin molecules to stack, or polymerize, when deoxygenated. This rigid rod of hemoglobin distorts the cell, producing the characteristic crescent or sickle shape that gives the disease its name.13 Polymerization of hemoglobin within the cell is promoted by dehydration, which increases the concentration of HbS.13,14 Polymerization occurs when hemoglobin is in the deoxygenated state.13
The sickle red blood cell is abnormal; it is rigid and dense, and lacks the deformability needed to navigate the microvasculature.15 Blockages of blood flow result in painful vaso-occlusion that is the hallmark of the disease, and that also can cause damage to the spleen, kidneys, and liver.16 The sickle red cell is also fragile, with a lifespan of only 20 days compared to the 120-day lifespan of a normal red blood cell.13 Frequent hemolysis results in anemia and the release of free hemoglobin, which both scavenges nitric oxide and impairs the production of more nitric oxide, which is essential for vasodilatation.17 This contributes to vascular dysfunction and an increased risk for stroke.18 If untreated, the natural course of sickle cell anemia is mortality in early childhood in most cases.19 Common chronic and acute sickle cell disease–related complications and recommended therapies, based on 2014 National Institutes of Health guidelines, are shown in Table 2 and Table 3.20
| Table 2. Common Adult Sickle Cell Disease Chronic Complications and Recommended Therapies | ||
Chronic Complication | Recommended Therapy | Strength of Recommendation |
Chronic pain | Opioids | Consensus |
Avascular necrosis | Analgesics and physical therapy | Consensus |
Proliferative sickle retinopathy | Laser photocoagulation | Strong |
Leg ulcers | Standard wound care | Moderate |
Recurrent priapism | Consult urology | Moderate |
| Data from Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48. | ||
| Table 3. Common Adult Sickle Cell Disease Acute Complications and Recommended Therapies | ||
Acute Complication | Recommended Therapy | Strength of Recommendation |
Vaso-occlusive crisis | NSAIDs, opioids for severe pain | Moderate-consensus |
ACS | Antibiotics, oxygen | Strong |
Simple transfusiona | Weak | |
Urgent exchange transfusionb | Strong | |
Acute stroke | Exchange transfusion | Strong |
Priapism ≥ 4 hr | Aggressive hydration, pain control, and urology consult | Strong-consensus |
Gallstones, symptomatic | Cholecystectomy, laparoscopic | Strong |
Splenic sequestration | Intravenous fluids, transfuse cautiously, discuss surgical splenectomy | Strong-moderate |
Acute renal failure | Consult nephrologyc | Consensus |
ACS = acute chest syndrome; NSAIDs = nonsteroidal anti-inflammatory drugs. a For symptomatic ACS with hemoglobin > 1 g/dL below baseline but > 9.0 g/dL. b When there is progression of ACS (SpO2 < 90% despite supplemental oxygen, increasing respiratory distress, progressive pulmonary infiltrates despite simple transfusion). c For acute rise in creatinine ≥ 0.3 mg/dL; do not give transfusions unless there are other indications. Data from Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48. | ||
One of the most challenging aspects of sickle cell disease is its clinical variability. While in general, HbSS and HbSβ0 are the most severe genotypes, there are patients with HbSC and HbSb+ who have significant sickle-cell–related complications, and may have a more severe clinical course than a HbSS patient.21 A great deal of this clinical variability cannot be explained, but some can be attributed to endogenous fetal hemoglobin (HbF) levels.22–24 The importance of HbF levels in sickle cell disease was first noted by a pediatrician in the 1940s.25 She observed that sickle cell disease complications in children under the age of 1 were rare, and attributed it to the presence of HbF.25 HbF levels decline more slowly in individuals with hemoglobinopathies, reaching their nadir after the age of 5 rather than within 6 months of birth in individuals without hemoglobinopathies.26 HbF levels remain elevated lifelong in most sickle cell disease patients, especially those with the HbSS and HbSβ0 genotypes. Levels of HbF vary widely between individuals, from zero to 20% to 30%, with a median of 10%.26–28 Individuals who produce more HbF have a milder course, in general.24 An association between the 4 β-globin haplotypes and HbF levels has been reported in the past,27,29 but more sophisticated next-generation sequencing has revealed causal variants in BCL11A and HBS1L-MYB that contribute approximately 50% of the observed variability in HbF levels.30–33
Co-inheritance of α-thalassemia also modifies disease course; less available α-globin chains results in a lower hemoglobin concentration within the cell. Paradoxically, this results in a higher overall hemoglobin level, as there is a reduction in polymerization, and therefore sickling due to lower HbS concentrations in the cell. Patients therefore are less anemic, reducing the risk of stroke in childhood,34,35 but blood viscosity may be higher, resulting in more frequent pain crises and increased risk36 of avascular necrosis.34,35,37 It is often helpful to think of sickle cell patients as falling into 1 of 2 groups: high hemolysis/low hemoglobin and high viscosity/high hemoglobin. Individuals with high rates of hemolysis are at greater risk for stroke, pulmonary hypertension, and acute chest syndrome (ACS). Higher rates of hemolysis result in higher levels of free hemoglobin, which scavenges nitric oxide. This leads to the vascular damage and dysfunction that contributes to the associated clinical complications. This phenotype is most commonly seen in HbSS and HbSβ0.38 High hemoglobin/high viscosity phenotypes are most often found in HbSC patients and in sickle cell anemia with α-thalassemia coinheritance.39–42
TREATMENT OPTIONS
In high-resource countries with newborn screening, the initiation of penicillin prophylaxis has dramatically altered the natural history of the disease, allowing the majority of patients to reach adulthood.43 Penicillin prophylaxis is usually discontinued at age 5 years; however, individuals who have undergone surgical splenectomy or have had pneumococcal sepsis on penicillin prophylaxis may remain on penicillin to age 18 or beyond.20
Another advance in sickle cell care is screening for stroke risk through transcranial Doppler ultrasound (TCD).44–47 This screening tool has reduced the incidence of childhood stroke from 10% by age 11 to 1%. TCDs typically cannot be performed after the age of 16 due to changes in the skull. Individuals found to have abnormal (elevated) TCD velocities are placed on chronic transfusion therapy for primary stroke prevention. They may remain on monthly chronic transfusions, with the goal of suppressing the percentage of HbS to 30% to 50% indefinitely. A clinical trial (STOPII) designed to determine if pediatric sickle cell disease patients on chronic transfusion therapy for primary stroke prevention could be safely taken off transfusion therapy was discontinued early due to an excess of strokes and conversion to abnormal TCD velocities in the untransfused arm.44 Individuals who have experienced an ischemic stroke have a 70% risk of another stroke, and must remain on chronic transfusion therapy indefinitely. Chronic transfusion reduces their stroke risk to 13%.
The only widely used pharmacologic therapy for sickle cell disease is hydroxyurea.12,48–50 A significant portion of the benefit of hydroxyurea stems from its induction of HbF.51 HbF does not sickle, and it interrupts the polymerization of HbS in the cell, if present in high enough concentrations.50 The level of HbF needed to achieve clinical improvement is not known, but in vitro assays suggest 20% HbF is needed to prevent sickling.52,53 However, endogenous and hydroxyurea-induced HbF is not distributed evenly through the red cells, so sickling is possible regardless of the level of HbF induced.54,55 Hydroxyurea likely has other disease-modifying effects as well, including reduction of white blood cell count and reticulocyte count and reduction of red cell adhesion to the endothelium.56–58 Clinical criteria for initiation of hydroxyurea in adult sickle cell disease patients are shown in Table 4.20 Hydroxyurea is given daily and is dosed to maximum tolerated dose for the individual by following the absolute neutrophil count (ANC). The goal ANC is between 2000 and 4000/µL. At times, absolute reticulocyte count (ARC) can be dose-limiting; goal ARC is greater than 70,000/µL.59 Platelet counts may be reduced as well, especially in HbSC patients.60,61
| Table 4. Indications for Hydroxyurea in Adult Patients with Sickle Cell Disease | |
Indication | Strength of Recommendation |
SCA with ≥ 3 pain crises per year | Strong |
SCA with pain that interferes with ADL and QoL | Strong |
History of severe or recurrent ACS | Strong |
Chronic kidney disease on epoetin | Weak |
HbSβ+ and HbSC with pain that interferes with ADL and QoL; consult sickle cell disease expert | Moderate |
| ACS = acute chest syndrome; ADL = activities of daily living; QoL = quality of life; SCA = sickle cell anemia. | |
The only curative therapy for sickle cell disease is hematopoietic stem cell transplant.62 Transplant use is limited by availability of matched sibling donors,62 and even at experienced centers transplant carries a small risk for mortality, graft rejection, and graft-versus-host disease. Furthermore, consensus on disease complications for which transplant is recommended is also lacking.63–65 Clinical trials of gene therapy for sickle cell disease and thalassemia are ongoing.66
COMPLICATIONS AND DISEASE-SPECIFIC THERAPIES
CASE PRESENTATION
A 26-year-old African-American man who works as a school bus driver presents to an academic center’s emergency department complaining of pain in his left leg, similar to prior pain events. He is described as having sickle cell trait, although no hemoglobin profile is available in his chart. He describes the pain as dull and aching, 10/10 in intensity. A complete blood count (CBC) is obtained; it reveals a hemoglobin of 14.5 g/dL, white blood cell (WBC) count of 5600/µL, and platelet count of 344,000/µL. His CBC is also notable for a mean corpuscular volume (MCV) of 72 fL, a mean corpuscular hemoglobin concentration (MCHC) of 37 g/dL, and a red blood cell distribution width (RDW) of 12. Slide review of a peripheral blood smear shows 2+ target cells (Figure).

The patient is given 6 mg of morphine, which provides some relief of his pain, and is discharged with a prescription for hydrocodone bitartrate/acetaminophen 5/325 mg. The diagnosis given is musculoskeletal pain, and he is instructed to follow-up with a primary care physician. His past medical history is significant for 4 or 5 visits to the emergency department per year in the past 4 years. Prior to 4 years ago, he rarely required medical attention.
• What laboratory and clinical features might lead you to question the diagnosis of sickle cell trait in this patient?
The patient’s hemoglobin is within normal range, which is consistent with sickle cell trait; however, he is microcytic, with a normal RDW. It is possible to be mildly microcytic in the early stages of iron deficiency, prior to the development of anemia, but the RDW would typically be elevated, demonstrating the presence of newer, smaller cells produced under conditions of iron deficiency.67 It is also possible that his microcytosis with a normal RDW could represent sickle cell trait with co-inheritance of β-thalassemia. Up to 30% of African Americans have β-thalassemia,2 and 1 in 10 have sickle cell trait.68 However, a high MCHC, indicating the presence of dense cells, and target cells noted on slide review are most consistent with HbSC.9 HbSC patients, especially males, can have hemoglobin levels in the normal range.4 The biggest inconsistency with the diagnosis of sickle cell trait is his history of frequent pain events. Individuals with sickle cell trait rarely present with pain crises, except under extreme conditions of dehydration or high altitude.68 Sickle cell trait is generally regarded as a benign condition, although a study of U.S. military recruits found a 30-fold higher risk of sudden death during basic training in persons with sickle cell trait.69 Additional sickle cell trait–related complications include hematuria, risk of splenic sequestration or infarct under extreme conditions and high altitude, and a rare and usually fatal renal malignancy, renal medullary carcinoma, which is vanishingly rare in individuals without sickle cell trait.70,71 Although the patient reported having sickle cell trait, this diagnosis should have been verified with a hemoglobin panel, given his atypical presentation.20
• What is the approach to managing pain episodes in sickle cell disease?
In sickle cell disease, vaso-occlusive pain events can be common, often beginning in early childhood.17 This disease complication accounts for 95% of all adult sickle cell disease hospitalizations.72 There is a great deal of variability in pain symptoms between individuals, and within individuals at various times in their lives:73 30% have no pain events, 50% have occasional events, and 20% have monthly or more frequent events that require hospitalization.74 The frequency and severity of pain events are modulated by HbF levels, β-thalassemia status, genotypes, therapies like hydroxyurea, or in rare cases, chronic transfusion therapy.23 Personal factors, such as psychosocial stressors, also contribute to the frequency of pain events.75 Pain event triggers include exposure to cold water, windy or cold weather, temperature changes, and extreme temperatures.76–79 Patient age also contributes to pain event frequency. Many patients see an increase in pain event frequency in their late 20s, and a marked decrease in their 40s.23,73 More than 3 pain events per year is associated with reduced life expectancy.23
Acute management of pain episodes involves nonsteroidal anti-inflammatory drugs, oral opioids, and when hospitalization is required, intravenous opioids, often delivered via patient-controlled analgesia (PCA) pumps.79 As sickle cell disease patients become teenagers and young adults, some experience an increased frequency of pain episodes, with fewer pain-free days, or a failure to return to baseline before the next pain crisis occurs.80,81 This is characteristic of emerging chronic pain.82 Chronic pain is a significant problem in adult patients with sickle cell disease, with up to 85% reporting pain on most days.72,80 The development of chronic pain may be reduced by early and aggressive treatment of acute pain events, as well as use of hydroxyurea to reduce the number of pain events. Many adult sickle cell patients with chronic pain are treated with daily opioids.20 Given the significant side effects of chronic opioid use—sedation, respiratory depression, itching, nausea, and impairment of function and quality of life—non-opioid therapies are under investigation.83 Many chronic pain patients have symptoms of neuropathic pain, and may benefit from neuropathic agents like gabapentin, both to reduce opioid use and to more effectively treat chronic neuropathic pain, which is known to respond poorly to opioids.84–86
• Is the patient’s peripheral blood smear consistent with a diagnosis of sickle cell trait?
Several target cells are visible, which is not typical of sickle cell trait, but may be seen in HbSC or thalassemia. The finding of an intracellular crystal is pathognomonic for HbSC or HbCC. HbC polymerizes in high oxygen conditions, opposite of HbS, which polymerizes in low oxygen conditions.9
CASE CONTINUED
The patient’s family history is significant for a sister who died at age 3 from sickle cell–related complications, and a sister with sickle cell trait who had a cholecystectomy for gallstones at age 22. His father died at age 38 due to unknown causes. The sickle cell trait status of his parents is unknown. His mother is alive, and has hypertension.
• Is the medical history of this patient’s family members consistent with sickle cell trait?
It is unlikely that sickle cell trait would result in early death in childhood, or in gallstones at age 22. Gallstones in early adulthood is a common presentation for HbSC patients not diagnosed by newborn screening.87 Any hemolytic condition can lead to the formation of hemoglobin-containing pigmented gallstones, biliary sludge, and obstruction of the gallbladder. In the presence of right-sided abdominal pain, a serum bilirubin level of more than 4 mg/dL should lead to measurement of direct bilirubin; if greater than 10% of total, imaging of the gallbladder should be obtained. In sickle cell disease, 30% of patients will have gallstones by 18 years of age. The low hemolysis/high viscosity phenotype patients are typically older at diagnosis. Co-inheritance of Gilbert syndrome and sickle cell disease is not uncommon, and can result in formation of gallstones at a young age; Gilbert syndrome alone typically results in gallstones in mid-life.88
CASE CONTINUED
Two months later, the patient presents again to the emergency department with the same complaint of leg pain, as well as abdominal pain. His hemoglobin is 12.5 g/dL, and his platelet count is 134,000/µL. His pain is not improved with 3 doses of morphine 6 mg intravenously, and he is admitted to the medicine service. A hemoglobin profile is obtained, revealing 52% HbS, 45% HbC, and 1.5% HbF, consistent with HbSC. In sickle cell trait, the hemoglobin profile is 60% HbA and 40% HbS (available α-globin prefers to pair with a normal β-globin, so the ratio of HbA to HbS is 60:40, not 50:50).
On the second hospital day, the patient’s hemoglobin drops to 7.2 g/dL and his platelet count decreases to 44,000/µL. His abdomen is distended and diffusely tender. The internist transfuses him with 2 units of packed red blood cells (PRBC), after which his hemoglobin increases to 11 g/dL, while his platelet count increases to 112,000/µL. Following the transfusion, his abdominal pain resolves, as does his anemia and thrombocytopenia.
• What caused this patient’s anemia and thrombocytopenia?
High on the differential diagnosis is a splenic sequestration. Acute splenic sequestration occurs when red cells are trapped in the splenic sinuses. Massive splenic enlargement may occur over several hours.89,90 Unrecognized splenic sequestration has a high mortality rate from severe anemia and splenic rupture.90 Splenic sequestration must be ruled out in a sickle cell patient with abdominal pain accompanied by dropping platelet and red cell counts, especially in milder subtypes that often have splenic function preserved into adolescence and adulthood. Sickle cell anemia patients usually become functionally asplenic in early childhood.89,91,92 The rise in hemoglobin, more than would be expected from 2 units of PRBC, plus the improvement in platelet count without a platelet transfusion observed in the case patient strongly supports the diagnosis of splenic sequestration.
Splenic sequestration can occur in any sickle cell patient whose spleen has not fibrosed. Splenic sequestration in adulthood is not uncommon in HbSC patients, who often have preserved splenic function into adulthood.93–95
Clinical signs of splenic sequestration include a rapid drop in hemoglobin, rise in reticulocyte count, a tender, enlarged spleen, and, in severe cases, hypovolemia.89,93 It is treated with prompt blood transfusion, but care must be taken not to overtransfuse the patient, as the spleen can trap several grams of hemoglobin, which may be released upon transfusion, potentially causing life-threatening hyperviscosity.89 Hemoglobin levels must be checked following transfusion in suspected splenic sequestration, and “mini transfusions” of 5 mL/kg are recommended in sickle cell disease patients who are hemodynamically stable.20
Hepatic sequestration may also occur, but it is much less common than splenic sequestration.96 Other conditions on the differential diagnosis include thrombotic thrombocytopenic purpura, which would be unlikely to respond to a transfusion. ACS can cause a drop in hemoglobin, and is treated with simple or exchange transfusions.97 ACS is less likely without respiratory symptoms or oxygen requirement, and usually is not associated with thrombocytopenia. Sepsis may also cause anemia and thrombocytopenia, but again would not likely respond to a simple transfusion. The patient’s response to transfusion is consistent with a sequestering event, not a destructive event as in the case of sepsis.
CASE CONTINUED
Imaging reveals a grossly enlarged spleen, which is having a mass effect on the left kidney. The patient is started on hydroxyurea therapy at 500 mg 3 times daily. Discharge instructions include following up with his primary care physician, continuing hydroxyurea therapy, and receiving yearly dilated eye exams to evaluate for proliferative sickle retinopathy.
• Are these discharge instructions complete?
Splenic sequestration has a 50% recurrence rate.98 In very young children, watchful waiting or chronic transfusion may be implemented to preserve the immunologic function of the spleen and reduce the risk of sepsis.89 Splenectomy after a single episode of sequestration in adults is a matter of debate, with experts advising both watchful waiting99 and splenectomy after recovery from the first sequestering event.100 The patient should have been informed of the risk for recurrence, and the signs and symptoms of splenic sequestration as well as the need for emergency medical attention should have been discussed. Splenic sequestration may be milder in adults than in children, but fatal sequestrations have been reported.95,101–103
Proliferative sickle cell retinopathy is a high viscosity/high hemoglobin complication that may occur more frequently in HbSC than HbSS, with an incidence of 33% in HbSC.42,104 Spontaneous regression of retinopathy occurs in approximately 32% of eyes, and laser or scatter photocoagulation is an effective intervention.105
• Would the patient need to be transfused prior to splenectomy?
Preoperative transfusion therapy is standard of care for HbSS patients undergoing general anesthesia. The TRAP study found that simple “top off” transfusion to a hemoglobin of 10 g/dL was as effective at preventing postoperative sickle cell–related complications as exchange transfusion to HbS of 30% or less, and had fewer transfusion-related complications like alloimmunization.106 There is little data regarding preoperative transfusions in HbSC disease. A retrospective study suggests that HbSC patients undergoing abdominal surgeries should be transfused.107 The higher hemoglobin level of the typical HbSC patient necessitates exchange transfusion to avoid hyperviscosity.
• Is hydroxyurea therapy indicated in this patient?
• Has it been dosed appropriately?
If the patient had the HbSS subtype, hydroxyurea would be clearly indicated, given his frequent pain events.20 HbSC patients may be placed on hydroxyurea on a case-by-case basis, but evidence for its efficacy in this sickle cell subtype is lacking.108 Large clinical trials like the Multi-Center Study of Hydroxyurea (MSH) that established the safety and efficacy of hydroxyurea in sickle cell anemia excluded HbSC and HbSβ+ patients.109 These mild to moderate subtypes produce less HbF at baseline, and typically have a minimal to modest rise in HbF on hydroxyurea.110 In sickle cell anemia, hydroxyurea is titrated to maximum tolerated dose, defined as an ANC of 2000 to 4000/µL and an ARC of 70,000/µL or higher.53 Because of their lower levels of chronic inflammation and lower reticulocyte counts due to higher hemoglobin levels, many HbSC and HbSβ+ patients have values in that range before initiating hydroxyurea therapy.9 Cytopenias, particularly of platelets in HbSC, occur at low doses of hydroxyurea.111
Of note, although the half-life of hydroxyurea would suggest that 3 times daily dosing is indicated, daily dosing has been found to have equal response and is preferred. Another concern is the monitoring of this myelosuppressive medication. This patient has repeatedly failed to obtain a primary care physician or a hematologist, and hydroxyurea requires laboratory monitoring at least every 2 months, especially in a HbSC patient with a very large spleen who is at significant risk for thrombocytopenia and neutropenia.9
CASE CONTINUED
A week after discharge from his admission for abdominal pain diagnosed as splenic sequestration, the patient presents again to the emergency department with abdominal pain which he reports is his typical sickle cell pain. Hemoglobin is 13.8 g/dL, platelet count is 388,000/µL, and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels are both 10 times their prior value. Creatinine is 1.2 mg/dL (0.75 mg/dL on his prior admission), and total bilirubin is 3 mg/dL, with 0.3 mg/dL direct bilirubin. He undergoes an ultrasound exam of his gallbladder, which reveals sludge and a possible gallstone. There is no evidence of cholecystitis. General surgery performs a laparoscopic cholecystectomy.
• Was this cholecystectomy necessary?
In patients with sickle cell disease, symptomatic gallstones and gallbladder sludge should be observed; recurrent abdominal pain without a significant change in bilirubin may not be due to gallstones or sludge, and therefore may not be relieved by cholecystectomy.112,113 In sickle cell disease, 40% of patients with gallbladder sludge do not develop gallstones.87 The patient’s bilirubin level was at baseline, and there was no increase in the direct (conjugated) fraction. Watchful waiting would have been appropriate, with cholecystectomy being performed if he experienced recurrent symptoms associated with fatty foods accompanied by an elevation in direct bilirubin.
More concerning and deserving of investigation was his elevated liver enzymes. Patients with sickle cell disease may experience recurrent ischemia and reperfusion injuries in the liver, which is called right upper quadrant syndrome. On autopsy of 70 sickle cell patients, 91% had hepatomegaly and 34% had focal necrosis.114 AST is often elevated in sickle cell disease, as it is affected by hemolysis. In this patient, both AST and ALT are elevated, consistent with a hepatocellular disorder. His abdominal pain and ALT rise may be a sign of a hepatic crisis.115 Rapid resolution of ALT elevation in a matter of days suggests a vaso-occlusive, inflammatory event that is self- limiting. Prolonged AST elevation requires further investigation, with consideration of autoimmune hepatitis, viral hepatitis, or iron overload. Iron overload is unlikely in this patient given his lifetime history of only 1 transfusion. Hepatic iron overload typically occurs in sickle cell disease after a minimum of 10 transfusions.115
CASE CONTINUED
The patient is discharged on the day after the procedure, with instructions to continue his hydroxyurea.
• Should the patient resume hydroxyurea therapy?
Hydroxyurea is hepatically cleared and thus it should be held until his liver function tests normalize.106
CASE CONTINUED
Two months later, the patient presents to the emergency department with abdominal pain that moves to his left leg. A CBC is obtained, showing a hemoglobin of 11.8 g/dL and a platelet count of 144,000/µL. He is given 2 doses of morphine 6 mg intravenously, and reports that his leg pain is now a 4/10. He is discharged home with a prescription for hydrocodone/acetaminophen.
• Is the emergency department evaluation sufficient?
This patient remains at high risk for splenic sequestration,93 with a hemoglobin 2 g lower than it was 2 months ago and platelets less than half. This decline could be consistent with early splenic sequestration.20 Additionally, he had elevated liver function tests on a recent admission, as well as rising creatinine, without evidence of resolution. It is not appropriate to discharge him without checking a chemistry and liver panel, and abdominal imaging should be considered. The best plan would be to admit him for observation, given his risk for splenic sequestration, and consult surgery for an elective splenectomy if he has a second episode of splenic sequestration 2 months after the first.100 His abdominal pain that migrates to his left leg could be due to his massive splenomegaly compressing his left kidney, as noted on imaging during his recent admission for splenic sequestration
CASE CONTINUED
An hour after discharge from the emergency department, EMS is called to his home for intractable pain. He is found lying on the floor, and reports excruciating left leg pain. He is brought to the closest hospital, a community hospital that he has not visited previously. There, he is admitted for hydration and pain control and placed on hydromorphone 2 mg every 4 hours as needed for pain. His hemoglobin is 10.8 g/dL, and platelets are 121,000/µL. A chemistry panel is remarkable for a creatinine level of 1.5 mg/dL and a potassium level of 3.2 mEq/L. Liver function tests are not obtained. After 3 doses of hydromorphone, he falls asleep. He is not in a monitored bed, and intravenous fluids, while ordered, are not started. At 6:30 AM the day after admission, he cannot be aroused on a routine vital sign check; he has an SpO2 of 60%, a blood pressure of 80/60 mm Hg, and heart rate of 148 beats/min. A rapid response is called, and naloxone is administered along with oxygen by face mask and several fluid boluses. His systolic blood pressure increases to 100 mm Hg from a low of 70 mm Hg. His SpO2 increases to 92%, and he is arousable and alert, although he reports 10/10 leg pain. His abdomen is noted to be distended and tender.
• What may have contributed to his clinical condition?
The patient is opioid tolerant and has received equivalent doses of opioids in the past without excess sedation. He may have liver dysfunction making him unable to metabolize opioids effectively. His hemoglobin and platelets continue to decline, raising concern for splenic sequestration versus sepsis. Failure to place him on a monitor allowed his hypoxia to continue for an unknown amount of time, placing him at high risk for developing ACS. Lack of intravenous hydration while he was too sedated to drink likely exacerbated his sickling.
CASE CONTINUED
At 9:20 AM, a CBC is obtained and reveals a hemoglobin of 4.8 g/dL and a platelet count of 44,000/µL. Two units of stat O negative blood are administered, and preparations are made to administer an exchange transfusion. A liver panel is obtained 3 hours later, which reveals an AST level of 1200 U/L and an ALT level of 1050 U/L. His bilirubin is 10 mg/dL, and his lactate dehydrogenase level is 1800 U/L. His urine is dark and is positive for bilirubin and ketones. He is transferred to the intensive care unit. A chest X-ray shows pulmonary congestion. Hematology/oncology is consulted.
He receives a 7-unit red blood cell exchange, which reduces his HbS to 11%. He continues to be hypotensive, and requires norepinephrine to support his blood pressure. Antibiotic therapy is started. His creatinine concentration rises to 2.3 mg/dL, potassium is 7.8 mEq/L, and bicarbonate is 12 mEq/L. He is placed on hemodialysis.
Computed tomography of the chest and abdomen reveals lower posterior lung infiltrates and a grossly enlarged spleen. He requires intubation. He is given a diagnosis of ACS in addition to kidney failure, liver failure, and “sickle crisis.” He continues to require daily to twice daily transfusions to maintain a hemoglobin of 7 to 9 g/dL, and his abdominal distension increases. As his condition worsens, surgery is consulted to discuss a liver transplant. He is deemed to not be a surgical candidate, and he passes away 6 days after entering the hospital. The immediate cause of death is listed as vaso-occlusive crisis, with ACS and sickle crisis listed as contributors.
• Are the causes of death accurate and complete?
If vaso-occlusive crisis is used to indicate a pain event, it is not an accurate cause of death. Pain is one of the most distressing complications of sickle cell disease, and frequent pain events are associated with early mortality,4,80 but they are not in themselves fatal. ACS is the number one cause of death in sickle cell disease,4 and it likely contributed to this patient’s death. Sickle crisis is a vague term that should not be used in this context. Causes of death should include splenic sequestration and multisystem organ failure. Multisystem organ failure in sickle cell disease often responds to aggressive transfusion therapy, which this patient received.116–118
CONCLUSION
Sickle cell disease is a complex chronic disease that impacts almost every organ system in the body. Clinicians may be inclined to attribute most pain in a patient with sickle cell disease to a simple vaso-occlusive crisis, treat them for this, and not investigate further. As the case presented here demonstrates, failure to identify the actual life-threatening process occurring in a patient with sickle cell disease presenting with pain can result in preventable early mortality. Clinicians must approach a sickle cell patient reporting pain in a thoughtful manner, and consider a complete differential diagnosis, including both sickle cell disease complications and those unrelated to sickle cell disease. Knowledge of the disease courses of the different sickle cell genotypes is essential, and must go beyond a superficial hierarchy of severity, but rather include an understanding of the complications each genotype is most prone to, and at what ages. Complete laboratory assessment, including a comprehensive metabolic panel, should be performed on all admitted patients, not just a complete blood count. Treating pain with high-dose opioids, while appropriate in an uncomplicated pain crisis, can lead to ACS or even respiratory failure in a patient with uninvestigated liver and kidney dysfunction. The most important lesson to remember is that even the sickle cell disease patient who has been given the unfortunate and pejorative label of “frequent flyer” by some providers has the potential for rapid deterioration into multisystem organ failure and death.
INTRODUCTION
Sickle cell disease is the most common inherited blood disorder in the world. It affects more than 100,000 individuals in the United States, and millions more worldwide.1 Sickle cell disease is most commonly found in individuals of African heritage, but the disease also occurs in Hispanics and people of Middle Eastern and subcontinent Indian heritage.2 The distribution of the sickle hemoglobin (hemoglobin S [HbS]) allele overlaps with the distribution of malaria; HbS carriers, or individuals with sickle cell trait, have protection against malaria,3 and are not considered to have sickle cell disease.
Sickle cell disease is a severe monogenic disorder marked by significant morbidity and mortality, affecting every organ in the body.4 The term sickle cell disease refers to all genotypes that cause sickling; the most common are the homozygous hemoglobin SS (HbSS) and compound heterozygotes hemoglobin SC (HbSC), hemoglobin S–β0-thalassemia (HbSβ0),and hemoglobin S–β+-thalassemia(HbSβ+), although HbS and several rarer hemoglobin variants such as HbSO(Arab) and HbSD(Punjab) can also cause sickle cell disease.The term sickle cell anemia refers exclusively to the most severe genotypes, HbSS and HbSβ0.5 Common sickling genotypes along with their relative clinical severity are shown in Table 1.6–11
| Table 1. Genotypes of Sickling Syndromes and Their Relative Severities | ||
Genotype | Severity | Characteristics |
HbSS | Severe | Most common form |
HbSβ0 | Severe | Clinically indistinguishable from HbSS6 |
HbSO-Arab | Severe | Relatively rare6 |
HbSD-Punjab | Severe | Mostly in northern India6 |
HbSC-Harlem | Severe | Migrates like HbSC, but rare double β-globin mutation7 |
HbCS-Antilles | Severe | Rare double β-globin mutation8 |
HbSC | Moderate | 25% of SCD9 |
HbSβ+, Mediterranean | Moderate | 5%–16% HbA6 |
HbAS-Oman | Moderate | Dominant rare double β-globin mutation10 |
HbSβ+, African | Mild | 16%–30% HbA6 |
HbSE | Mild | HbE found mostly in Southeast Asia11 |
HbS-HPFH | Very mild | Large deletions in β-globin gene complex; > 30% HbF6 |
| HbA = hemoglobin A; HbE = hemoglobin E; HbF = fetal hemoglobin; HbS-HPFH = HbS and gene deletion HPFH; HbSC = heterozygous hemoglobin SC; HbSS = homozygous hemoglobin SS; HbSβ0 = hemoglobin S-β thalassemia0; HbSβ+ = hemoglobin S-β thalassemia+; SCD = sickle cell disease. | ||
This article reviews the pathophysiology of sickle cell disease, common clinical complications, and available therapies. A complex case which illustrates diagnostic and management challenges is presented as well.
PATHOPHYSIOLOGY
HbS is the result of a substitution of valine for glutamic acid in the sixth amino acid of the β-globin chain.12 The change from a hydrophilic to a hydrophobic amino acid causes the hemoglobin molecules to stack, or polymerize, when deoxygenated. This rigid rod of hemoglobin distorts the cell, producing the characteristic crescent or sickle shape that gives the disease its name.13 Polymerization of hemoglobin within the cell is promoted by dehydration, which increases the concentration of HbS.13,14 Polymerization occurs when hemoglobin is in the deoxygenated state.13
The sickle red blood cell is abnormal; it is rigid and dense, and lacks the deformability needed to navigate the microvasculature.15 Blockages of blood flow result in painful vaso-occlusion that is the hallmark of the disease, and that also can cause damage to the spleen, kidneys, and liver.16 The sickle red cell is also fragile, with a lifespan of only 20 days compared to the 120-day lifespan of a normal red blood cell.13 Frequent hemolysis results in anemia and the release of free hemoglobin, which both scavenges nitric oxide and impairs the production of more nitric oxide, which is essential for vasodilatation.17 This contributes to vascular dysfunction and an increased risk for stroke.18 If untreated, the natural course of sickle cell anemia is mortality in early childhood in most cases.19 Common chronic and acute sickle cell disease–related complications and recommended therapies, based on 2014 National Institutes of Health guidelines, are shown in Table 2 and Table 3.20
| Table 2. Common Adult Sickle Cell Disease Chronic Complications and Recommended Therapies | ||
Chronic Complication | Recommended Therapy | Strength of Recommendation |
Chronic pain | Opioids | Consensus |
Avascular necrosis | Analgesics and physical therapy | Consensus |
Proliferative sickle retinopathy | Laser photocoagulation | Strong |
Leg ulcers | Standard wound care | Moderate |
Recurrent priapism | Consult urology | Moderate |
| Data from Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48. | ||
| Table 3. Common Adult Sickle Cell Disease Acute Complications and Recommended Therapies | ||
Acute Complication | Recommended Therapy | Strength of Recommendation |
Vaso-occlusive crisis | NSAIDs, opioids for severe pain | Moderate-consensus |
ACS | Antibiotics, oxygen | Strong |
Simple transfusiona | Weak | |
Urgent exchange transfusionb | Strong | |
Acute stroke | Exchange transfusion | Strong |
Priapism ≥ 4 hr | Aggressive hydration, pain control, and urology consult | Strong-consensus |
Gallstones, symptomatic | Cholecystectomy, laparoscopic | Strong |
Splenic sequestration | Intravenous fluids, transfuse cautiously, discuss surgical splenectomy | Strong-moderate |
Acute renal failure | Consult nephrologyc | Consensus |
ACS = acute chest syndrome; NSAIDs = nonsteroidal anti-inflammatory drugs. a For symptomatic ACS with hemoglobin > 1 g/dL below baseline but > 9.0 g/dL. b When there is progression of ACS (SpO2 < 90% despite supplemental oxygen, increasing respiratory distress, progressive pulmonary infiltrates despite simple transfusion). c For acute rise in creatinine ≥ 0.3 mg/dL; do not give transfusions unless there are other indications. Data from Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48. | ||
One of the most challenging aspects of sickle cell disease is its clinical variability. While in general, HbSS and HbSβ0 are the most severe genotypes, there are patients with HbSC and HbSb+ who have significant sickle-cell–related complications, and may have a more severe clinical course than a HbSS patient.21 A great deal of this clinical variability cannot be explained, but some can be attributed to endogenous fetal hemoglobin (HbF) levels.22–24 The importance of HbF levels in sickle cell disease was first noted by a pediatrician in the 1940s.25 She observed that sickle cell disease complications in children under the age of 1 were rare, and attributed it to the presence of HbF.25 HbF levels decline more slowly in individuals with hemoglobinopathies, reaching their nadir after the age of 5 rather than within 6 months of birth in individuals without hemoglobinopathies.26 HbF levels remain elevated lifelong in most sickle cell disease patients, especially those with the HbSS and HbSβ0 genotypes. Levels of HbF vary widely between individuals, from zero to 20% to 30%, with a median of 10%.26–28 Individuals who produce more HbF have a milder course, in general.24 An association between the 4 β-globin haplotypes and HbF levels has been reported in the past,27,29 but more sophisticated next-generation sequencing has revealed causal variants in BCL11A and HBS1L-MYB that contribute approximately 50% of the observed variability in HbF levels.30–33
Co-inheritance of α-thalassemia also modifies disease course; less available α-globin chains results in a lower hemoglobin concentration within the cell. Paradoxically, this results in a higher overall hemoglobin level, as there is a reduction in polymerization, and therefore sickling due to lower HbS concentrations in the cell. Patients therefore are less anemic, reducing the risk of stroke in childhood,34,35 but blood viscosity may be higher, resulting in more frequent pain crises and increased risk36 of avascular necrosis.34,35,37 It is often helpful to think of sickle cell patients as falling into 1 of 2 groups: high hemolysis/low hemoglobin and high viscosity/high hemoglobin. Individuals with high rates of hemolysis are at greater risk for stroke, pulmonary hypertension, and acute chest syndrome (ACS). Higher rates of hemolysis result in higher levels of free hemoglobin, which scavenges nitric oxide. This leads to the vascular damage and dysfunction that contributes to the associated clinical complications. This phenotype is most commonly seen in HbSS and HbSβ0.38 High hemoglobin/high viscosity phenotypes are most often found in HbSC patients and in sickle cell anemia with α-thalassemia coinheritance.39–42
TREATMENT OPTIONS
In high-resource countries with newborn screening, the initiation of penicillin prophylaxis has dramatically altered the natural history of the disease, allowing the majority of patients to reach adulthood.43 Penicillin prophylaxis is usually discontinued at age 5 years; however, individuals who have undergone surgical splenectomy or have had pneumococcal sepsis on penicillin prophylaxis may remain on penicillin to age 18 or beyond.20
Another advance in sickle cell care is screening for stroke risk through transcranial Doppler ultrasound (TCD).44–47 This screening tool has reduced the incidence of childhood stroke from 10% by age 11 to 1%. TCDs typically cannot be performed after the age of 16 due to changes in the skull. Individuals found to have abnormal (elevated) TCD velocities are placed on chronic transfusion therapy for primary stroke prevention. They may remain on monthly chronic transfusions, with the goal of suppressing the percentage of HbS to 30% to 50% indefinitely. A clinical trial (STOPII) designed to determine if pediatric sickle cell disease patients on chronic transfusion therapy for primary stroke prevention could be safely taken off transfusion therapy was discontinued early due to an excess of strokes and conversion to abnormal TCD velocities in the untransfused arm.44 Individuals who have experienced an ischemic stroke have a 70% risk of another stroke, and must remain on chronic transfusion therapy indefinitely. Chronic transfusion reduces their stroke risk to 13%.
The only widely used pharmacologic therapy for sickle cell disease is hydroxyurea.12,48–50 A significant portion of the benefit of hydroxyurea stems from its induction of HbF.51 HbF does not sickle, and it interrupts the polymerization of HbS in the cell, if present in high enough concentrations.50 The level of HbF needed to achieve clinical improvement is not known, but in vitro assays suggest 20% HbF is needed to prevent sickling.52,53 However, endogenous and hydroxyurea-induced HbF is not distributed evenly through the red cells, so sickling is possible regardless of the level of HbF induced.54,55 Hydroxyurea likely has other disease-modifying effects as well, including reduction of white blood cell count and reticulocyte count and reduction of red cell adhesion to the endothelium.56–58 Clinical criteria for initiation of hydroxyurea in adult sickle cell disease patients are shown in Table 4.20 Hydroxyurea is given daily and is dosed to maximum tolerated dose for the individual by following the absolute neutrophil count (ANC). The goal ANC is between 2000 and 4000/µL. At times, absolute reticulocyte count (ARC) can be dose-limiting; goal ARC is greater than 70,000/µL.59 Platelet counts may be reduced as well, especially in HbSC patients.60,61
| Table 4. Indications for Hydroxyurea in Adult Patients with Sickle Cell Disease | |
Indication | Strength of Recommendation |
SCA with ≥ 3 pain crises per year | Strong |
SCA with pain that interferes with ADL and QoL | Strong |
History of severe or recurrent ACS | Strong |
Chronic kidney disease on epoetin | Weak |
HbSβ+ and HbSC with pain that interferes with ADL and QoL; consult sickle cell disease expert | Moderate |
| ACS = acute chest syndrome; ADL = activities of daily living; QoL = quality of life; SCA = sickle cell anemia. | |
The only curative therapy for sickle cell disease is hematopoietic stem cell transplant.62 Transplant use is limited by availability of matched sibling donors,62 and even at experienced centers transplant carries a small risk for mortality, graft rejection, and graft-versus-host disease. Furthermore, consensus on disease complications for which transplant is recommended is also lacking.63–65 Clinical trials of gene therapy for sickle cell disease and thalassemia are ongoing.66
COMPLICATIONS AND DISEASE-SPECIFIC THERAPIES
CASE PRESENTATION
A 26-year-old African-American man who works as a school bus driver presents to an academic center’s emergency department complaining of pain in his left leg, similar to prior pain events. He is described as having sickle cell trait, although no hemoglobin profile is available in his chart. He describes the pain as dull and aching, 10/10 in intensity. A complete blood count (CBC) is obtained; it reveals a hemoglobin of 14.5 g/dL, white blood cell (WBC) count of 5600/µL, and platelet count of 344,000/µL. His CBC is also notable for a mean corpuscular volume (MCV) of 72 fL, a mean corpuscular hemoglobin concentration (MCHC) of 37 g/dL, and a red blood cell distribution width (RDW) of 12. Slide review of a peripheral blood smear shows 2+ target cells (Figure).

The patient is given 6 mg of morphine, which provides some relief of his pain, and is discharged with a prescription for hydrocodone bitartrate/acetaminophen 5/325 mg. The diagnosis given is musculoskeletal pain, and he is instructed to follow-up with a primary care physician. His past medical history is significant for 4 or 5 visits to the emergency department per year in the past 4 years. Prior to 4 years ago, he rarely required medical attention.
• What laboratory and clinical features might lead you to question the diagnosis of sickle cell trait in this patient?
The patient’s hemoglobin is within normal range, which is consistent with sickle cell trait; however, he is microcytic, with a normal RDW. It is possible to be mildly microcytic in the early stages of iron deficiency, prior to the development of anemia, but the RDW would typically be elevated, demonstrating the presence of newer, smaller cells produced under conditions of iron deficiency.67 It is also possible that his microcytosis with a normal RDW could represent sickle cell trait with co-inheritance of β-thalassemia. Up to 30% of African Americans have β-thalassemia,2 and 1 in 10 have sickle cell trait.68 However, a high MCHC, indicating the presence of dense cells, and target cells noted on slide review are most consistent with HbSC.9 HbSC patients, especially males, can have hemoglobin levels in the normal range.4 The biggest inconsistency with the diagnosis of sickle cell trait is his history of frequent pain events. Individuals with sickle cell trait rarely present with pain crises, except under extreme conditions of dehydration or high altitude.68 Sickle cell trait is generally regarded as a benign condition, although a study of U.S. military recruits found a 30-fold higher risk of sudden death during basic training in persons with sickle cell trait.69 Additional sickle cell trait–related complications include hematuria, risk of splenic sequestration or infarct under extreme conditions and high altitude, and a rare and usually fatal renal malignancy, renal medullary carcinoma, which is vanishingly rare in individuals without sickle cell trait.70,71 Although the patient reported having sickle cell trait, this diagnosis should have been verified with a hemoglobin panel, given his atypical presentation.20
• What is the approach to managing pain episodes in sickle cell disease?
In sickle cell disease, vaso-occlusive pain events can be common, often beginning in early childhood.17 This disease complication accounts for 95% of all adult sickle cell disease hospitalizations.72 There is a great deal of variability in pain symptoms between individuals, and within individuals at various times in their lives:73 30% have no pain events, 50% have occasional events, and 20% have monthly or more frequent events that require hospitalization.74 The frequency and severity of pain events are modulated by HbF levels, β-thalassemia status, genotypes, therapies like hydroxyurea, or in rare cases, chronic transfusion therapy.23 Personal factors, such as psychosocial stressors, also contribute to the frequency of pain events.75 Pain event triggers include exposure to cold water, windy or cold weather, temperature changes, and extreme temperatures.76–79 Patient age also contributes to pain event frequency. Many patients see an increase in pain event frequency in their late 20s, and a marked decrease in their 40s.23,73 More than 3 pain events per year is associated with reduced life expectancy.23
Acute management of pain episodes involves nonsteroidal anti-inflammatory drugs, oral opioids, and when hospitalization is required, intravenous opioids, often delivered via patient-controlled analgesia (PCA) pumps.79 As sickle cell disease patients become teenagers and young adults, some experience an increased frequency of pain episodes, with fewer pain-free days, or a failure to return to baseline before the next pain crisis occurs.80,81 This is characteristic of emerging chronic pain.82 Chronic pain is a significant problem in adult patients with sickle cell disease, with up to 85% reporting pain on most days.72,80 The development of chronic pain may be reduced by early and aggressive treatment of acute pain events, as well as use of hydroxyurea to reduce the number of pain events. Many adult sickle cell patients with chronic pain are treated with daily opioids.20 Given the significant side effects of chronic opioid use—sedation, respiratory depression, itching, nausea, and impairment of function and quality of life—non-opioid therapies are under investigation.83 Many chronic pain patients have symptoms of neuropathic pain, and may benefit from neuropathic agents like gabapentin, both to reduce opioid use and to more effectively treat chronic neuropathic pain, which is known to respond poorly to opioids.84–86
• Is the patient’s peripheral blood smear consistent with a diagnosis of sickle cell trait?
Several target cells are visible, which is not typical of sickle cell trait, but may be seen in HbSC or thalassemia. The finding of an intracellular crystal is pathognomonic for HbSC or HbCC. HbC polymerizes in high oxygen conditions, opposite of HbS, which polymerizes in low oxygen conditions.9
CASE CONTINUED
The patient’s family history is significant for a sister who died at age 3 from sickle cell–related complications, and a sister with sickle cell trait who had a cholecystectomy for gallstones at age 22. His father died at age 38 due to unknown causes. The sickle cell trait status of his parents is unknown. His mother is alive, and has hypertension.
• Is the medical history of this patient’s family members consistent with sickle cell trait?
It is unlikely that sickle cell trait would result in early death in childhood, or in gallstones at age 22. Gallstones in early adulthood is a common presentation for HbSC patients not diagnosed by newborn screening.87 Any hemolytic condition can lead to the formation of hemoglobin-containing pigmented gallstones, biliary sludge, and obstruction of the gallbladder. In the presence of right-sided abdominal pain, a serum bilirubin level of more than 4 mg/dL should lead to measurement of direct bilirubin; if greater than 10% of total, imaging of the gallbladder should be obtained. In sickle cell disease, 30% of patients will have gallstones by 18 years of age. The low hemolysis/high viscosity phenotype patients are typically older at diagnosis. Co-inheritance of Gilbert syndrome and sickle cell disease is not uncommon, and can result in formation of gallstones at a young age; Gilbert syndrome alone typically results in gallstones in mid-life.88
CASE CONTINUED
Two months later, the patient presents again to the emergency department with the same complaint of leg pain, as well as abdominal pain. His hemoglobin is 12.5 g/dL, and his platelet count is 134,000/µL. His pain is not improved with 3 doses of morphine 6 mg intravenously, and he is admitted to the medicine service. A hemoglobin profile is obtained, revealing 52% HbS, 45% HbC, and 1.5% HbF, consistent with HbSC. In sickle cell trait, the hemoglobin profile is 60% HbA and 40% HbS (available α-globin prefers to pair with a normal β-globin, so the ratio of HbA to HbS is 60:40, not 50:50).
On the second hospital day, the patient’s hemoglobin drops to 7.2 g/dL and his platelet count decreases to 44,000/µL. His abdomen is distended and diffusely tender. The internist transfuses him with 2 units of packed red blood cells (PRBC), after which his hemoglobin increases to 11 g/dL, while his platelet count increases to 112,000/µL. Following the transfusion, his abdominal pain resolves, as does his anemia and thrombocytopenia.
• What caused this patient’s anemia and thrombocytopenia?
High on the differential diagnosis is a splenic sequestration. Acute splenic sequestration occurs when red cells are trapped in the splenic sinuses. Massive splenic enlargement may occur over several hours.89,90 Unrecognized splenic sequestration has a high mortality rate from severe anemia and splenic rupture.90 Splenic sequestration must be ruled out in a sickle cell patient with abdominal pain accompanied by dropping platelet and red cell counts, especially in milder subtypes that often have splenic function preserved into adolescence and adulthood. Sickle cell anemia patients usually become functionally asplenic in early childhood.89,91,92 The rise in hemoglobin, more than would be expected from 2 units of PRBC, plus the improvement in platelet count without a platelet transfusion observed in the case patient strongly supports the diagnosis of splenic sequestration.
Splenic sequestration can occur in any sickle cell patient whose spleen has not fibrosed. Splenic sequestration in adulthood is not uncommon in HbSC patients, who often have preserved splenic function into adulthood.93–95
Clinical signs of splenic sequestration include a rapid drop in hemoglobin, rise in reticulocyte count, a tender, enlarged spleen, and, in severe cases, hypovolemia.89,93 It is treated with prompt blood transfusion, but care must be taken not to overtransfuse the patient, as the spleen can trap several grams of hemoglobin, which may be released upon transfusion, potentially causing life-threatening hyperviscosity.89 Hemoglobin levels must be checked following transfusion in suspected splenic sequestration, and “mini transfusions” of 5 mL/kg are recommended in sickle cell disease patients who are hemodynamically stable.20
Hepatic sequestration may also occur, but it is much less common than splenic sequestration.96 Other conditions on the differential diagnosis include thrombotic thrombocytopenic purpura, which would be unlikely to respond to a transfusion. ACS can cause a drop in hemoglobin, and is treated with simple or exchange transfusions.97 ACS is less likely without respiratory symptoms or oxygen requirement, and usually is not associated with thrombocytopenia. Sepsis may also cause anemia and thrombocytopenia, but again would not likely respond to a simple transfusion. The patient’s response to transfusion is consistent with a sequestering event, not a destructive event as in the case of sepsis.
CASE CONTINUED
Imaging reveals a grossly enlarged spleen, which is having a mass effect on the left kidney. The patient is started on hydroxyurea therapy at 500 mg 3 times daily. Discharge instructions include following up with his primary care physician, continuing hydroxyurea therapy, and receiving yearly dilated eye exams to evaluate for proliferative sickle retinopathy.
• Are these discharge instructions complete?
Splenic sequestration has a 50% recurrence rate.98 In very young children, watchful waiting or chronic transfusion may be implemented to preserve the immunologic function of the spleen and reduce the risk of sepsis.89 Splenectomy after a single episode of sequestration in adults is a matter of debate, with experts advising both watchful waiting99 and splenectomy after recovery from the first sequestering event.100 The patient should have been informed of the risk for recurrence, and the signs and symptoms of splenic sequestration as well as the need for emergency medical attention should have been discussed. Splenic sequestration may be milder in adults than in children, but fatal sequestrations have been reported.95,101–103
Proliferative sickle cell retinopathy is a high viscosity/high hemoglobin complication that may occur more frequently in HbSC than HbSS, with an incidence of 33% in HbSC.42,104 Spontaneous regression of retinopathy occurs in approximately 32% of eyes, and laser or scatter photocoagulation is an effective intervention.105
• Would the patient need to be transfused prior to splenectomy?
Preoperative transfusion therapy is standard of care for HbSS patients undergoing general anesthesia. The TRAP study found that simple “top off” transfusion to a hemoglobin of 10 g/dL was as effective at preventing postoperative sickle cell–related complications as exchange transfusion to HbS of 30% or less, and had fewer transfusion-related complications like alloimmunization.106 There is little data regarding preoperative transfusions in HbSC disease. A retrospective study suggests that HbSC patients undergoing abdominal surgeries should be transfused.107 The higher hemoglobin level of the typical HbSC patient necessitates exchange transfusion to avoid hyperviscosity.
• Is hydroxyurea therapy indicated in this patient?
• Has it been dosed appropriately?
If the patient had the HbSS subtype, hydroxyurea would be clearly indicated, given his frequent pain events.20 HbSC patients may be placed on hydroxyurea on a case-by-case basis, but evidence for its efficacy in this sickle cell subtype is lacking.108 Large clinical trials like the Multi-Center Study of Hydroxyurea (MSH) that established the safety and efficacy of hydroxyurea in sickle cell anemia excluded HbSC and HbSβ+ patients.109 These mild to moderate subtypes produce less HbF at baseline, and typically have a minimal to modest rise in HbF on hydroxyurea.110 In sickle cell anemia, hydroxyurea is titrated to maximum tolerated dose, defined as an ANC of 2000 to 4000/µL and an ARC of 70,000/µL or higher.53 Because of their lower levels of chronic inflammation and lower reticulocyte counts due to higher hemoglobin levels, many HbSC and HbSβ+ patients have values in that range before initiating hydroxyurea therapy.9 Cytopenias, particularly of platelets in HbSC, occur at low doses of hydroxyurea.111
Of note, although the half-life of hydroxyurea would suggest that 3 times daily dosing is indicated, daily dosing has been found to have equal response and is preferred. Another concern is the monitoring of this myelosuppressive medication. This patient has repeatedly failed to obtain a primary care physician or a hematologist, and hydroxyurea requires laboratory monitoring at least every 2 months, especially in a HbSC patient with a very large spleen who is at significant risk for thrombocytopenia and neutropenia.9
CASE CONTINUED
A week after discharge from his admission for abdominal pain diagnosed as splenic sequestration, the patient presents again to the emergency department with abdominal pain which he reports is his typical sickle cell pain. Hemoglobin is 13.8 g/dL, platelet count is 388,000/µL, and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels are both 10 times their prior value. Creatinine is 1.2 mg/dL (0.75 mg/dL on his prior admission), and total bilirubin is 3 mg/dL, with 0.3 mg/dL direct bilirubin. He undergoes an ultrasound exam of his gallbladder, which reveals sludge and a possible gallstone. There is no evidence of cholecystitis. General surgery performs a laparoscopic cholecystectomy.
• Was this cholecystectomy necessary?
In patients with sickle cell disease, symptomatic gallstones and gallbladder sludge should be observed; recurrent abdominal pain without a significant change in bilirubin may not be due to gallstones or sludge, and therefore may not be relieved by cholecystectomy.112,113 In sickle cell disease, 40% of patients with gallbladder sludge do not develop gallstones.87 The patient’s bilirubin level was at baseline, and there was no increase in the direct (conjugated) fraction. Watchful waiting would have been appropriate, with cholecystectomy being performed if he experienced recurrent symptoms associated with fatty foods accompanied by an elevation in direct bilirubin.
More concerning and deserving of investigation was his elevated liver enzymes. Patients with sickle cell disease may experience recurrent ischemia and reperfusion injuries in the liver, which is called right upper quadrant syndrome. On autopsy of 70 sickle cell patients, 91% had hepatomegaly and 34% had focal necrosis.114 AST is often elevated in sickle cell disease, as it is affected by hemolysis. In this patient, both AST and ALT are elevated, consistent with a hepatocellular disorder. His abdominal pain and ALT rise may be a sign of a hepatic crisis.115 Rapid resolution of ALT elevation in a matter of days suggests a vaso-occlusive, inflammatory event that is self- limiting. Prolonged AST elevation requires further investigation, with consideration of autoimmune hepatitis, viral hepatitis, or iron overload. Iron overload is unlikely in this patient given his lifetime history of only 1 transfusion. Hepatic iron overload typically occurs in sickle cell disease after a minimum of 10 transfusions.115
CASE CONTINUED
The patient is discharged on the day after the procedure, with instructions to continue his hydroxyurea.
• Should the patient resume hydroxyurea therapy?
Hydroxyurea is hepatically cleared and thus it should be held until his liver function tests normalize.106
CASE CONTINUED
Two months later, the patient presents to the emergency department with abdominal pain that moves to his left leg. A CBC is obtained, showing a hemoglobin of 11.8 g/dL and a platelet count of 144,000/µL. He is given 2 doses of morphine 6 mg intravenously, and reports that his leg pain is now a 4/10. He is discharged home with a prescription for hydrocodone/acetaminophen.
• Is the emergency department evaluation sufficient?
This patient remains at high risk for splenic sequestration,93 with a hemoglobin 2 g lower than it was 2 months ago and platelets less than half. This decline could be consistent with early splenic sequestration.20 Additionally, he had elevated liver function tests on a recent admission, as well as rising creatinine, without evidence of resolution. It is not appropriate to discharge him without checking a chemistry and liver panel, and abdominal imaging should be considered. The best plan would be to admit him for observation, given his risk for splenic sequestration, and consult surgery for an elective splenectomy if he has a second episode of splenic sequestration 2 months after the first.100 His abdominal pain that migrates to his left leg could be due to his massive splenomegaly compressing his left kidney, as noted on imaging during his recent admission for splenic sequestration
CASE CONTINUED
An hour after discharge from the emergency department, EMS is called to his home for intractable pain. He is found lying on the floor, and reports excruciating left leg pain. He is brought to the closest hospital, a community hospital that he has not visited previously. There, he is admitted for hydration and pain control and placed on hydromorphone 2 mg every 4 hours as needed for pain. His hemoglobin is 10.8 g/dL, and platelets are 121,000/µL. A chemistry panel is remarkable for a creatinine level of 1.5 mg/dL and a potassium level of 3.2 mEq/L. Liver function tests are not obtained. After 3 doses of hydromorphone, he falls asleep. He is not in a monitored bed, and intravenous fluids, while ordered, are not started. At 6:30 AM the day after admission, he cannot be aroused on a routine vital sign check; he has an SpO2 of 60%, a blood pressure of 80/60 mm Hg, and heart rate of 148 beats/min. A rapid response is called, and naloxone is administered along with oxygen by face mask and several fluid boluses. His systolic blood pressure increases to 100 mm Hg from a low of 70 mm Hg. His SpO2 increases to 92%, and he is arousable and alert, although he reports 10/10 leg pain. His abdomen is noted to be distended and tender.
• What may have contributed to his clinical condition?
The patient is opioid tolerant and has received equivalent doses of opioids in the past without excess sedation. He may have liver dysfunction making him unable to metabolize opioids effectively. His hemoglobin and platelets continue to decline, raising concern for splenic sequestration versus sepsis. Failure to place him on a monitor allowed his hypoxia to continue for an unknown amount of time, placing him at high risk for developing ACS. Lack of intravenous hydration while he was too sedated to drink likely exacerbated his sickling.
CASE CONTINUED
At 9:20 AM, a CBC is obtained and reveals a hemoglobin of 4.8 g/dL and a platelet count of 44,000/µL. Two units of stat O negative blood are administered, and preparations are made to administer an exchange transfusion. A liver panel is obtained 3 hours later, which reveals an AST level of 1200 U/L and an ALT level of 1050 U/L. His bilirubin is 10 mg/dL, and his lactate dehydrogenase level is 1800 U/L. His urine is dark and is positive for bilirubin and ketones. He is transferred to the intensive care unit. A chest X-ray shows pulmonary congestion. Hematology/oncology is consulted.
He receives a 7-unit red blood cell exchange, which reduces his HbS to 11%. He continues to be hypotensive, and requires norepinephrine to support his blood pressure. Antibiotic therapy is started. His creatinine concentration rises to 2.3 mg/dL, potassium is 7.8 mEq/L, and bicarbonate is 12 mEq/L. He is placed on hemodialysis.
Computed tomography of the chest and abdomen reveals lower posterior lung infiltrates and a grossly enlarged spleen. He requires intubation. He is given a diagnosis of ACS in addition to kidney failure, liver failure, and “sickle crisis.” He continues to require daily to twice daily transfusions to maintain a hemoglobin of 7 to 9 g/dL, and his abdominal distension increases. As his condition worsens, surgery is consulted to discuss a liver transplant. He is deemed to not be a surgical candidate, and he passes away 6 days after entering the hospital. The immediate cause of death is listed as vaso-occlusive crisis, with ACS and sickle crisis listed as contributors.
• Are the causes of death accurate and complete?
If vaso-occlusive crisis is used to indicate a pain event, it is not an accurate cause of death. Pain is one of the most distressing complications of sickle cell disease, and frequent pain events are associated with early mortality,4,80 but they are not in themselves fatal. ACS is the number one cause of death in sickle cell disease,4 and it likely contributed to this patient’s death. Sickle crisis is a vague term that should not be used in this context. Causes of death should include splenic sequestration and multisystem organ failure. Multisystem organ failure in sickle cell disease often responds to aggressive transfusion therapy, which this patient received.116–118
CONCLUSION
Sickle cell disease is a complex chronic disease that impacts almost every organ system in the body. Clinicians may be inclined to attribute most pain in a patient with sickle cell disease to a simple vaso-occlusive crisis, treat them for this, and not investigate further. As the case presented here demonstrates, failure to identify the actual life-threatening process occurring in a patient with sickle cell disease presenting with pain can result in preventable early mortality. Clinicians must approach a sickle cell patient reporting pain in a thoughtful manner, and consider a complete differential diagnosis, including both sickle cell disease complications and those unrelated to sickle cell disease. Knowledge of the disease courses of the different sickle cell genotypes is essential, and must go beyond a superficial hierarchy of severity, but rather include an understanding of the complications each genotype is most prone to, and at what ages. Complete laboratory assessment, including a comprehensive metabolic panel, should be performed on all admitted patients, not just a complete blood count. Treating pain with high-dose opioids, while appropriate in an uncomplicated pain crisis, can lead to ACS or even respiratory failure in a patient with uninvestigated liver and kidney dysfunction. The most important lesson to remember is that even the sickle cell disease patient who has been given the unfortunate and pejorative label of “frequent flyer” by some providers has the potential for rapid deterioration into multisystem organ failure and death.
- Weatherall D, Hofman K, Rodgers G, Ruffin J, Hrynkow S. A case for developing North-South partnerships for research in sickle cell disease. Blood 2005;105:921–3.
- Flint J, Harding RM, Boyce AJ, Clegg JB. The population genetics of the haemoglobinopathies. Baillieres Clin Haematol 1998;11:1–51.
- Allison AC. The distribution of the sickle-cell trait in East Africa and elsewhere, and its apparent relationship to the incidence of subtertian malaria. Trans R Soc Trop Med Hyg 1954;48:312–8.
- Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med 1994;330:1639–44.
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010;376:2018–31.
- Serjeant GR, Serjeant BE. Sickle cell disease. 3rd ed. New York: Oxford University Press; 2001.
- Moo-Penn W, Bechtel K, Jue D, et al. The presence of hemoglobin S and C Harlem in an individual in the United States. Blood 1975;46:363–7.
- Monplaisir N, Merault G, Poyart C, et al. Hemoglobin S Antilles: a variant with lower solubility than hemoglobin S and producing sickle cell disease in heterozygotes. Proc Natl Acad Sci U S A 1986;83:9363–7.
- Nagel RL, Fabry ME, Steinberg MH. The paradox of hemoglobin SC disease. Blood Rev 2003;17:167–78.
- Nagel RL, Daar S, Romero JR, et al. HbS-oman heterozygote: a new dominant sickle syndrome. Blood 1998;92:4375–82.
- Masiello D, Heeney MM, Adewoye AH, et al. Hemoglobin SE disease: a concise review. Am J Hematol 2007;82:643–9.
- Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med 1997;337:762–9.
- Brittenham GM, Schechter AN, Noguchi CT. Hemoglobin S polymerization: primary determinant of the hemolytic and clinical severity of the sickling syndromes. Blood 1985;65:183–9.
- .Nagel RL, Bookchin RM, Johnson J, et al. Structural bases of the inhibitory effects of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S. Proc Natl Acad Sci U S A 1979;76:670–2.
- .Ballas SK, Dover GJ, Charache S. Effect of hydroxyurea on the rheological properties of sickle erythrocytes in vivo. Am J Hematol 1989;32:104–11.
- Frenette PS. Sickle cell vaso-occlusion: multistep and multicellular paradigm. Curr Opin Hematol 2002;9:101–6.
- Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Med 2002;8:1383–9.
- Nouraie M, Lee JS, Zhang Y, et al. The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica 2013;98:464–72.
- Serjeant GR. The natural history of sickle cell disease. Cold Spring Harb Perspect Med 2013;3:a011783.
- Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48.
- Serjeant GR. Natural history and determinants of clinical severity of sickle cell disease. Curr Opin Hematol 1995;2:103–8.
- Odenheimer DJ, Sarnaik SA, Whitten CF, et al. The relationship between fetal hemoglobin and disease severity in children with sickle cell anemia. Am J Med Genet 1987;27:525–35.
- Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med 1991;325:11–6.
- Falusi AG, Olatunji PO. Effects of alpha thalassaemia and haemoglobin F (HbF) level on the clinical severity of sickle-cell anaemia. Eur J Haematol 1994;52:13–5.
- Watson J. The significance of the paucity of sickle cells in newborn Negro infants. Am J Med Sci 1948;215:419–23.
- Marcus SJ, Ware RE. Physiologic decline in fetal hemoglobin parameters in infants with sickle cell disease: implications for pharmacological intervention. J Pediatr Hematol Oncol 1999;21:407–11.
- Schroeder WA, Powars DR, Kay LM, et al. Beta-cluster haplotypes, alpha-gene status, and hematological data from SS, SC, and S-beta-thalassemia patients in southern California. Hemoglobin 1989;13:325–53.
- Steinberg MH, Voskaridou E, Kutlar A, et al. Concordant fetal hemoglobin response to hydroxyurea in siblings with sickle cell disease. Am J Hematol 2003;72:121–6.
- Ngo D, Bae H, Steinberg MH, et al. Fetal hemoglobin in sickle cell anemia: genetic studies of the Arab-Indian haplotype. Blood Cells Mol Dis 2013;51:22–6.
- Galarneau G, Palmer CD, Sankaran VG, et al. Fine-mapping at three loci known to affect fetal hemoglobin levels explains additional genetic variation. Nat Genet 2010;42:1049–51.
- Lettre G, Sankaran VG, Bezerra MA, et al. DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci U S A 2008;105:11869–74.
- Sankaran VG, Menne TF, Xu J, et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 2008;322:1839–42.
- Uda M, Galanello R, Sanna S, et al. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc Natl Acad Sci U S A 2008;105:1620–5.
- Adams RJ, Kutlar A, McKie V, et al. Alpha thalassemia and stroke risk in sickle cell anemia. Am J Hematol 1994;45:279–82.
- Ballas SK. Effect of alpha-globin genotype on the pathophysiology of sickle cell disease. Pediatr Pathol Mol Med 2001;20:107–21.
- Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med 1986;314:1593–9.
- Ballas SK, Talacki CA, Rao VM, Steiner RM. The prevalence of avascular necrosis in sickle cell anemia: correlation with alpha-thalassemia. Hemoglobin 1989;13:649–55.
- .Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev 2007;21:37–47.
- Murphy JR, Wengard M, Brereton W. Rheological studies of Hb SS blood: influence of hematocrit, hypertonicity, separation of cells, deoxygenation, and mixture with normal cells. J Lab Clin Med 1976;87:475–86.
- Fabry ME, Kaul DK, Raventos-Suarez C, Chang H, Nagel RL. SC erythrocytes have an abnormally high intracellular hemoglobin concentration. Pathophysiological consequences. J Clin Invest 1982;70:1315–9.
- Stuart J, Johnson CS. Rheology of the sickle cell disorders. Baillieres Clin Haematol 1987;1:747–75.
- Lionnet F, Hammoudi N, Stojanovic KS, et al. Hemoglobin sickle cell disease complications: a clinical study of 179 cases. Haematologica 2012;97:1136-41.
- Adamkiewicz TV, Sarnaik S, Buchanan GR, et al. Invasive pneumococcal infections in children with sickle cell disease in the era of penicillin prophylaxis, antibiotic resistance, and 23-valent pneumococcal polysaccharide vaccination. J Pediatr 2003;143:438–44.
- Abboud MR, Yim E, Musallam KM, Adams RJ, Investigators SIS. Discontinuing prophylactic transfusions increases the risk of silent brain infarction in children with sickle cell disease: data from STOP II. Blood 2011;118:894–8.
- Adams RJ. Lessons from the Stroke Prevention Trial in Sickle Cell Anemia (STOP) study. J Child Neurol 2000;15:344–9.
- Adams RJ, Brambilla DJ, Granger S, et al. Stroke and conversion to high risk in children screened with transcranial Doppler ultrasound during the STOP study. Blood 2004;103:3689–94.
- Lee MT, Piomelli S, Granger S, et al. Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results. Blood 2006;108:847–52.
- Charache S, Dover GJ, Moore RD, et al. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood 1992;79:2555–65.
- Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med 1995;332:1317–22.
- Charache S. Mechanism of action of hydroxyurea in the management of sickle cell anemia in adults. Semin Hematol 1997;34:15–21.
- Steinberg MH, McCarthy WF, Castro O, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17.5 year follow-up. Am J Hematol 2010;85:403–8.
- Noguchi CT, Rodgers GP, Serjeant G, Schechter AN. Levels of fetal hemoglobin necessary for treatment of sickle cell disease. N Engl J Med 1988;318:96–9.
- Powars DR, Weiss JN, Chan LS, Schroeder WA. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood 1984;63:921–6.
- Maier-Redelsperger M, de Montalembert M, Flahault A, et al. Fetal hemoglobin and F-cell responses to long-term hydroxyurea treatment in young sickle cell patients. The French Study Group on Sickle Cell Disease. Blood 1998;91:4472–9.
- Steinberg MH, Chui DH, Dover GJ, et al. Fetal hemoglobin in sickle cell anemia: a glass half full? Blood 2014;123:481–5.
- Adragna NC, Fonseca P, Lauf PK. Hydroxyurea affects cell morphology, cation transport, and red blood cell adhesion in cultured vascular endothelial cells. Blood 1994;83:553–60.
- Bridges KR, Barabino GD, Brugnara C, et al. A multiparameter analysis of sickle erythrocytes in patients undergoing hydroxyurea therapy. Blood 1996;88:4701–10.
- Jiang J, Jordan SJ, Barr DP, et al. In vivo production of nitric oxide in rats after administration of hydroxyurea. Mol Pharmacol 1997;52:1081–6.
- Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood 2010;115:5300–11.
- Yates AM, Dedeken L, Smeltzer MP, et al. Hydroxyurea treatment of children with hemoglobin SC disease. Pediatr Blood Cancer 2013;60:323–5.
- Barbosa CG, Aleluia AC, Pacheco AP, et al. Genetic modulation of HbF in Brazilians with HbSC disease and sickle cell anemia. Am J Hematol 2013;88:923–4.
- Hsieh MM, Kang EM, Fitzhugh CD, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med 2009;361:2309–17.
- King A, Shenoy S. Evidence-based focused review of the status of hematopoietic stem cell transplantation as treatment of sickle cell disease and thalassemia. Blood 2014;123:3089–94.
- Oringanje C, Nemecek E, Oniyangi O. Hematopoietic stem cell transplantation for people with sickle cell disease. Cochrane Database Syst Rev 2013;5:CD007001.
- Freed J, Talano J, Small T, et al. Allogeneic cellular and autologous stem cell therapy for sickle cell disease: ‘whom, when and how’. Bone Marrow Transplant 2012;47:1489–98.
- Urbinati F, Hargrove PW, Geiger S, et al. Potentially therapeutic levels of anti-sickling globin gene expression following lentivirus-mediated gene transfer in sickle cell disease bone marrow CD34 cells. Exp Hematol 2015;43:346–51.
- Brugnara C, Mohandas N. Red cell indices in classification and treatment of anemias: from M.M. Wintrobes’s original 1934 classification to the third millennium. Curr Opin Hematol 2013;20:222–30.
- Key NS, Derebail VK. Sickle-cell trait: novel clinical significance. Hematology Am Soc Hematol Educ Program 2010;2010:418–22.
- Kark JA, Posey DM, Schumacher HR, Ruehle CJ. Sickle-cell trait as a risk factor for sudden death in physical training. N Engl J Med 1987;317:781–7.
- Goldsmith JC, Bonham VL, Joiner CH, et al. Framing the research agenda for sickle cell trait: building on the current understanding of clinical events and their potential implications. Am J Hematol 2012;87:340–6.
- Grant AM, Parker CS, Jordan LB, et al. Public health implications of sickle cell trait: a report of the CDC meeting. Am J Prev Med 2011;41:S435–9.
- Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol 2005;79:17–25.
- Serjeant GR, Ceulaer CD, Lethbridge R, et al. The painful crisis of homozygous sickle cell disease: clinical features. Br J Haematol 1994;87:586–91.
- Vichinsky EP, Johnson R, Lubin BH. Multidisciplinary approach to pain management in sickle cell disease. Am J Pediatr Hematol Oncol 1982;4:328–33.
- Gil KM, Carson JW, Porter LS, et al. Daily mood and stress predict pain, health care use, and work activity in African American adults with sickle-cell disease. Health Psychol 2004;23:267–74.
- Amjad H, Bannerman RM, Judisch JM. Letter: Sickling pain and season. Br Med J 1974;2:54.
- Ibrahim AS. Relationship between meteorological changes and occurrence of painful sickle cell crises in Kuwait. Trans R Soc Trop Med Hyg 1980;74:159–61.
- Jones S, Duncan ER, Thomas N, et al. Windy weather and low humidity are associated with an increased number of hospital admissions for acute pain and sickle cell disease in an urban environment with a maritime temperate climate. Br J Haematol 2005;131:530–3.
- Resar LM, Oski FA. Cold water exposure and vaso-occlusive crises in sickle cell anemia. J Pediatr 1991;118:407–9.
- Darbari DS, Ballas SK, Clauw DJ. Thinking beyond sickling to better understand pain in sickle cell disease. Eur J Haematol 2014;93:89–95.
- Darbari DS, Onyekwere O, Nouraie M, et al. Markers of severe vaso-occlusive painful episode frequency in children and adolescents with sickle cell anemia. J Pediatr 2011;160:286–90.
- Hollins M, Stonerock GL, Kisaalita NR, et al. Detecting the emergence of chronic pain in sickle cell disease. J Pain Symptom Manage 2012;43:1082–93.
- Ballas SK, Darbari DS. Neuropathy, neuropathic pain, and sickle cell disease. Am J Hematol 2013;88:927–9.
- Brandow AM, Farley RA, Panepinto JA. Early insights into the neurobiology of pain in sickle cell disease: A systematic review of the literature. Pediatr Blood Cancer 2015 May 13. doi: 10.1002/pbc.25574. [Epub ahead of print].
- Brandow AM, Farley RA, Panepinto JA. Neuropathic pain in patients with sickle cell disease. Pediatr Blood Cancer 2014;61:512–7.
- Brandow AM, Farley RA, Dasgupta M, et al. The use of neuropathic pain drugs in children with sickle cell disease is associated with older age, female sex, and longer length of hospital stay. J Pediatr Hematol Oncol 2015;37:10–5.
- Walker TM, Hambleton IR, Serjeant GR. Gallstones in sickle cell disease: observations from The Jamaican Cohort study. J Pediatr 2000;136:80–5.
- Penner E, Mayr WR, Djawan S, et al. [The genetics of Gilbert syndrome]. Schweiz Med Wochenschr 1976;106:860–2. [German]
- Powell RW, Levine GL, Yang YM, Mankad VN. Acute splenic sequestration crisis in sickle cell disease: early detection and treatment. J Pediatr Surg 1992;27:215–8.
- Al Salem AH, Qaisaruddin S, Nasserullah Z, et al. Splenectomy and acute splenic sequestration crises in sickle cell disease. Pediatr Surg Int 1996;11:26–8.
- Pearson HA, Spencer RP, Cornelius EA. Functional asplenia in sickle-cell anemia. N Engl J Med 1969;281:923–6.
- Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet 2011;377:1663–72.
- Brousse V, Buffet P, Rees D. The spleen and sickle cell disease: the sick(led) spleen. Br J Haematol 2014;166:165–76.
- Orringer EP, Fowler VG Jr, Owens CM, et al. Case report: splenic infarction and acute splenic sequestration in adults with hemoglobin SC disease. Am J Med Sci 1991;302:374–9.
- Michel JB, Hernandez JA, Buchanan GR. A fatal case of acute splenic sequestration in a 53–year-old woman with sickle-hemoglobin C disease. Am J Med 1992;92:97–100.
- Hatton CS, Bunch C, Weatherall DJ. Hepatic sequestration in sickle cell anaemia. Br Med J (Clin Res Ed) 1985;290:744–5.
- Castro O, Brambilla DJ, Thorington B, et al. The acute chest syndrome in sickle cell disease: incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood 1994;84:643–9.
- Gill FM, Sleeper LA, Weiner SJ, et al. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood 1995;86:776–83.
- Owusu-Ofori S, Hirst C. Splenectomy versus conservative management for acute sequestration crises in people with sickle cell disease. Cochrane Database Syst Rev 2013;5:CD003425.
- 00.Al-Salem AH. Splenic complications of sickle cell anemia and the role of splenectomy. ISRN Hematol 2011;2011:864257.
- Sabarense AP, Lima GO, Silva LM, Viana MB. Characterization of mortality in children with sickle cell disease diagnosed through the Newborn Screening Program. J Pediatr (Rio J) 2015;91:242–7.
- Aslam AF, Aslam AK, Dipillo F. Fatal splenic sequestration crisis with multiorgan failure in an adult woman with sickle cell-beta+ thalassemia. Am J Med Sci 2005;329:141–3.
- Berry RA, Odumakinde EA, Lewis JP. Massive splenic infarction in doubly abnormal heterozygous sickling disorders. A new complication of acute splenic sequestration syndrome. The West J Med 1991;155:531–2.
- Bonanomi MT, Lavezzo MM. Sickle cell retinopathy: diagnosis and treatment. Arq Bras de Oftalmol 2013;76:320–7.
- Chen RW, Flynn HW Jr, Lee WH, et al. Vitreoretinal management and surgical outcomes in proliferative sickle retinopathy: a case series. Am J Ophthalmol 2014;157:870–5 e1.
- Howard J, Malfroy M, Llewelyn C, et al. The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study: a randomised, controlled, multicentre clinical trial. Lancet 2013;381:930–8.
- Neumayr L, Koshy M, Haberkern C, et al. Surgery in patients with hemoglobin SC disease. Preoperative Transfusion in Sickle Cell Disease Study Group. Am J Hematol 1998;57:101–8.
- 08. Savage WJ, Buchanan GR, Yawn BP, et al. Evidence gaps in the management of sickle cell disease: A summary of needed research. Am J Hematol 2015;90:273–5.
- Charache S, Barton FB, Moore RD, et al. Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive “switching” agent. The Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Medicine (Baltimore) 1996;75:300–26.
- Steinberg MH, Nagel RL, Brugnara C. Cellular effects of hydroxyurea in Hb SC disease. Br J Haematol 1997;98:838–44.
- Wang W, Brugnara C, Snyder C, et al. The effects of hydroxycarbamide and magnesium on haemoglobin SC disease: results of the multi-centre CHAMPS trial. Br J Haematol 2011;152:771–6.
- Jungst C, Kullak-Ublick GA, Jungst D. Gallstone disease: Microlithiasis and sludge. Best Pract Res Clin Gastroenterol 2006;20:1053–62.
- Walker TM, Serjeant GR. Biliary sludge in sickle cell disease. J Pediatr 1996;129:443–5.
- Bauer TW, Moore GW, Hutchins GM. The liver in sickle cell disease. A clinicopathologic study of 70 patients. Am J Med 1980;69:833–7.
- Ebert EC, Nagar M, Hagspiel KD. Gastrointestinal and hepatic complications of sickle cell disease. Clin Gastroenterol Hepatol 2010;8:483–9.
- Hiran S. Multiorgan dysfunction syndrome in sickle cell disease. J Assoc Physicians India 2005;53:19–22.
- Shao SH, Orringer EP. Case report: splenic sequestration and multiorgan failure as the presenting manifestation of hemoglobin SC disease. Am J Med Sci 1996;311:139–41.
- Hassell KL, Eckman JR, Lane PA. Acute multiorgan failure syndrome: a potentially catastrophic complication of severe sickle cell pain episodes. Am J Med 1994;96:155–62.
- Weatherall D, Hofman K, Rodgers G, Ruffin J, Hrynkow S. A case for developing North-South partnerships for research in sickle cell disease. Blood 2005;105:921–3.
- Flint J, Harding RM, Boyce AJ, Clegg JB. The population genetics of the haemoglobinopathies. Baillieres Clin Haematol 1998;11:1–51.
- Allison AC. The distribution of the sickle-cell trait in East Africa and elsewhere, and its apparent relationship to the incidence of subtertian malaria. Trans R Soc Trop Med Hyg 1954;48:312–8.
- Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med 1994;330:1639–44.
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010;376:2018–31.
- Serjeant GR, Serjeant BE. Sickle cell disease. 3rd ed. New York: Oxford University Press; 2001.
- Moo-Penn W, Bechtel K, Jue D, et al. The presence of hemoglobin S and C Harlem in an individual in the United States. Blood 1975;46:363–7.
- Monplaisir N, Merault G, Poyart C, et al. Hemoglobin S Antilles: a variant with lower solubility than hemoglobin S and producing sickle cell disease in heterozygotes. Proc Natl Acad Sci U S A 1986;83:9363–7.
- Nagel RL, Fabry ME, Steinberg MH. The paradox of hemoglobin SC disease. Blood Rev 2003;17:167–78.
- Nagel RL, Daar S, Romero JR, et al. HbS-oman heterozygote: a new dominant sickle syndrome. Blood 1998;92:4375–82.
- Masiello D, Heeney MM, Adewoye AH, et al. Hemoglobin SE disease: a concise review. Am J Hematol 2007;82:643–9.
- Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med 1997;337:762–9.
- Brittenham GM, Schechter AN, Noguchi CT. Hemoglobin S polymerization: primary determinant of the hemolytic and clinical severity of the sickling syndromes. Blood 1985;65:183–9.
- .Nagel RL, Bookchin RM, Johnson J, et al. Structural bases of the inhibitory effects of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S. Proc Natl Acad Sci U S A 1979;76:670–2.
- .Ballas SK, Dover GJ, Charache S. Effect of hydroxyurea on the rheological properties of sickle erythrocytes in vivo. Am J Hematol 1989;32:104–11.
- Frenette PS. Sickle cell vaso-occlusion: multistep and multicellular paradigm. Curr Opin Hematol 2002;9:101–6.
- Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Med 2002;8:1383–9.
- Nouraie M, Lee JS, Zhang Y, et al. The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica 2013;98:464–72.
- Serjeant GR. The natural history of sickle cell disease. Cold Spring Harb Perspect Med 2013;3:a011783.
- Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48.
- Serjeant GR. Natural history and determinants of clinical severity of sickle cell disease. Curr Opin Hematol 1995;2:103–8.
- Odenheimer DJ, Sarnaik SA, Whitten CF, et al. The relationship between fetal hemoglobin and disease severity in children with sickle cell anemia. Am J Med Genet 1987;27:525–35.
- Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med 1991;325:11–6.
- Falusi AG, Olatunji PO. Effects of alpha thalassaemia and haemoglobin F (HbF) level on the clinical severity of sickle-cell anaemia. Eur J Haematol 1994;52:13–5.
- Watson J. The significance of the paucity of sickle cells in newborn Negro infants. Am J Med Sci 1948;215:419–23.
- Marcus SJ, Ware RE. Physiologic decline in fetal hemoglobin parameters in infants with sickle cell disease: implications for pharmacological intervention. J Pediatr Hematol Oncol 1999;21:407–11.
- Schroeder WA, Powars DR, Kay LM, et al. Beta-cluster haplotypes, alpha-gene status, and hematological data from SS, SC, and S-beta-thalassemia patients in southern California. Hemoglobin 1989;13:325–53.
- Steinberg MH, Voskaridou E, Kutlar A, et al. Concordant fetal hemoglobin response to hydroxyurea in siblings with sickle cell disease. Am J Hematol 2003;72:121–6.
- Ngo D, Bae H, Steinberg MH, et al. Fetal hemoglobin in sickle cell anemia: genetic studies of the Arab-Indian haplotype. Blood Cells Mol Dis 2013;51:22–6.
- Galarneau G, Palmer CD, Sankaran VG, et al. Fine-mapping at three loci known to affect fetal hemoglobin levels explains additional genetic variation. Nat Genet 2010;42:1049–51.
- Lettre G, Sankaran VG, Bezerra MA, et al. DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci U S A 2008;105:11869–74.
- Sankaran VG, Menne TF, Xu J, et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 2008;322:1839–42.
- Uda M, Galanello R, Sanna S, et al. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc Natl Acad Sci U S A 2008;105:1620–5.
- Adams RJ, Kutlar A, McKie V, et al. Alpha thalassemia and stroke risk in sickle cell anemia. Am J Hematol 1994;45:279–82.
- Ballas SK. Effect of alpha-globin genotype on the pathophysiology of sickle cell disease. Pediatr Pathol Mol Med 2001;20:107–21.
- Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med 1986;314:1593–9.
- Ballas SK, Talacki CA, Rao VM, Steiner RM. The prevalence of avascular necrosis in sickle cell anemia: correlation with alpha-thalassemia. Hemoglobin 1989;13:649–55.
- .Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev 2007;21:37–47.
- Murphy JR, Wengard M, Brereton W. Rheological studies of Hb SS blood: influence of hematocrit, hypertonicity, separation of cells, deoxygenation, and mixture with normal cells. J Lab Clin Med 1976;87:475–86.
- Fabry ME, Kaul DK, Raventos-Suarez C, Chang H, Nagel RL. SC erythrocytes have an abnormally high intracellular hemoglobin concentration. Pathophysiological consequences. J Clin Invest 1982;70:1315–9.
- Stuart J, Johnson CS. Rheology of the sickle cell disorders. Baillieres Clin Haematol 1987;1:747–75.
- Lionnet F, Hammoudi N, Stojanovic KS, et al. Hemoglobin sickle cell disease complications: a clinical study of 179 cases. Haematologica 2012;97:1136-41.
- Adamkiewicz TV, Sarnaik S, Buchanan GR, et al. Invasive pneumococcal infections in children with sickle cell disease in the era of penicillin prophylaxis, antibiotic resistance, and 23-valent pneumococcal polysaccharide vaccination. J Pediatr 2003;143:438–44.
- Abboud MR, Yim E, Musallam KM, Adams RJ, Investigators SIS. Discontinuing prophylactic transfusions increases the risk of silent brain infarction in children with sickle cell disease: data from STOP II. Blood 2011;118:894–8.
- Adams RJ. Lessons from the Stroke Prevention Trial in Sickle Cell Anemia (STOP) study. J Child Neurol 2000;15:344–9.
- Adams RJ, Brambilla DJ, Granger S, et al. Stroke and conversion to high risk in children screened with transcranial Doppler ultrasound during the STOP study. Blood 2004;103:3689–94.
- Lee MT, Piomelli S, Granger S, et al. Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results. Blood 2006;108:847–52.
- Charache S, Dover GJ, Moore RD, et al. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood 1992;79:2555–65.
- Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med 1995;332:1317–22.
- Charache S. Mechanism of action of hydroxyurea in the management of sickle cell anemia in adults. Semin Hematol 1997;34:15–21.
- Steinberg MH, McCarthy WF, Castro O, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17.5 year follow-up. Am J Hematol 2010;85:403–8.
- Noguchi CT, Rodgers GP, Serjeant G, Schechter AN. Levels of fetal hemoglobin necessary for treatment of sickle cell disease. N Engl J Med 1988;318:96–9.
- Powars DR, Weiss JN, Chan LS, Schroeder WA. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood 1984;63:921–6.
- Maier-Redelsperger M, de Montalembert M, Flahault A, et al. Fetal hemoglobin and F-cell responses to long-term hydroxyurea treatment in young sickle cell patients. The French Study Group on Sickle Cell Disease. Blood 1998;91:4472–9.
- Steinberg MH, Chui DH, Dover GJ, et al. Fetal hemoglobin in sickle cell anemia: a glass half full? Blood 2014;123:481–5.
- Adragna NC, Fonseca P, Lauf PK. Hydroxyurea affects cell morphology, cation transport, and red blood cell adhesion in cultured vascular endothelial cells. Blood 1994;83:553–60.
- Bridges KR, Barabino GD, Brugnara C, et al. A multiparameter analysis of sickle erythrocytes in patients undergoing hydroxyurea therapy. Blood 1996;88:4701–10.
- Jiang J, Jordan SJ, Barr DP, et al. In vivo production of nitric oxide in rats after administration of hydroxyurea. Mol Pharmacol 1997;52:1081–6.
- Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood 2010;115:5300–11.
- Yates AM, Dedeken L, Smeltzer MP, et al. Hydroxyurea treatment of children with hemoglobin SC disease. Pediatr Blood Cancer 2013;60:323–5.
- Barbosa CG, Aleluia AC, Pacheco AP, et al. Genetic modulation of HbF in Brazilians with HbSC disease and sickle cell anemia. Am J Hematol 2013;88:923–4.
- Hsieh MM, Kang EM, Fitzhugh CD, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med 2009;361:2309–17.
- King A, Shenoy S. Evidence-based focused review of the status of hematopoietic stem cell transplantation as treatment of sickle cell disease and thalassemia. Blood 2014;123:3089–94.
- Oringanje C, Nemecek E, Oniyangi O. Hematopoietic stem cell transplantation for people with sickle cell disease. Cochrane Database Syst Rev 2013;5:CD007001.
- Freed J, Talano J, Small T, et al. Allogeneic cellular and autologous stem cell therapy for sickle cell disease: ‘whom, when and how’. Bone Marrow Transplant 2012;47:1489–98.
- Urbinati F, Hargrove PW, Geiger S, et al. Potentially therapeutic levels of anti-sickling globin gene expression following lentivirus-mediated gene transfer in sickle cell disease bone marrow CD34 cells. Exp Hematol 2015;43:346–51.
- Brugnara C, Mohandas N. Red cell indices in classification and treatment of anemias: from M.M. Wintrobes’s original 1934 classification to the third millennium. Curr Opin Hematol 2013;20:222–30.
- Key NS, Derebail VK. Sickle-cell trait: novel clinical significance. Hematology Am Soc Hematol Educ Program 2010;2010:418–22.
- Kark JA, Posey DM, Schumacher HR, Ruehle CJ. Sickle-cell trait as a risk factor for sudden death in physical training. N Engl J Med 1987;317:781–7.
- Goldsmith JC, Bonham VL, Joiner CH, et al. Framing the research agenda for sickle cell trait: building on the current understanding of clinical events and their potential implications. Am J Hematol 2012;87:340–6.
- Grant AM, Parker CS, Jordan LB, et al. Public health implications of sickle cell trait: a report of the CDC meeting. Am J Prev Med 2011;41:S435–9.
- Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol 2005;79:17–25.
- Serjeant GR, Ceulaer CD, Lethbridge R, et al. The painful crisis of homozygous sickle cell disease: clinical features. Br J Haematol 1994;87:586–91.
- Vichinsky EP, Johnson R, Lubin BH. Multidisciplinary approach to pain management in sickle cell disease. Am J Pediatr Hematol Oncol 1982;4:328–33.
- Gil KM, Carson JW, Porter LS, et al. Daily mood and stress predict pain, health care use, and work activity in African American adults with sickle-cell disease. Health Psychol 2004;23:267–74.
- Amjad H, Bannerman RM, Judisch JM. Letter: Sickling pain and season. Br Med J 1974;2:54.
- Ibrahim AS. Relationship between meteorological changes and occurrence of painful sickle cell crises in Kuwait. Trans R Soc Trop Med Hyg 1980;74:159–61.
- Jones S, Duncan ER, Thomas N, et al. Windy weather and low humidity are associated with an increased number of hospital admissions for acute pain and sickle cell disease in an urban environment with a maritime temperate climate. Br J Haematol 2005;131:530–3.
- Resar LM, Oski FA. Cold water exposure and vaso-occlusive crises in sickle cell anemia. J Pediatr 1991;118:407–9.
- Darbari DS, Ballas SK, Clauw DJ. Thinking beyond sickling to better understand pain in sickle cell disease. Eur J Haematol 2014;93:89–95.
- Darbari DS, Onyekwere O, Nouraie M, et al. Markers of severe vaso-occlusive painful episode frequency in children and adolescents with sickle cell anemia. J Pediatr 2011;160:286–90.
- Hollins M, Stonerock GL, Kisaalita NR, et al. Detecting the emergence of chronic pain in sickle cell disease. J Pain Symptom Manage 2012;43:1082–93.
- Ballas SK, Darbari DS. Neuropathy, neuropathic pain, and sickle cell disease. Am J Hematol 2013;88:927–9.
- Brandow AM, Farley RA, Panepinto JA. Early insights into the neurobiology of pain in sickle cell disease: A systematic review of the literature. Pediatr Blood Cancer 2015 May 13. doi: 10.1002/pbc.25574. [Epub ahead of print].
- Brandow AM, Farley RA, Panepinto JA. Neuropathic pain in patients with sickle cell disease. Pediatr Blood Cancer 2014;61:512–7.
- Brandow AM, Farley RA, Dasgupta M, et al. The use of neuropathic pain drugs in children with sickle cell disease is associated with older age, female sex, and longer length of hospital stay. J Pediatr Hematol Oncol 2015;37:10–5.
- Walker TM, Hambleton IR, Serjeant GR. Gallstones in sickle cell disease: observations from The Jamaican Cohort study. J Pediatr 2000;136:80–5.
- Penner E, Mayr WR, Djawan S, et al. [The genetics of Gilbert syndrome]. Schweiz Med Wochenschr 1976;106:860–2. [German]
- Powell RW, Levine GL, Yang YM, Mankad VN. Acute splenic sequestration crisis in sickle cell disease: early detection and treatment. J Pediatr Surg 1992;27:215–8.
- Al Salem AH, Qaisaruddin S, Nasserullah Z, et al. Splenectomy and acute splenic sequestration crises in sickle cell disease. Pediatr Surg Int 1996;11:26–8.
- Pearson HA, Spencer RP, Cornelius EA. Functional asplenia in sickle-cell anemia. N Engl J Med 1969;281:923–6.
- Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet 2011;377:1663–72.
- Brousse V, Buffet P, Rees D. The spleen and sickle cell disease: the sick(led) spleen. Br J Haematol 2014;166:165–76.
- Orringer EP, Fowler VG Jr, Owens CM, et al. Case report: splenic infarction and acute splenic sequestration in adults with hemoglobin SC disease. Am J Med Sci 1991;302:374–9.
- Michel JB, Hernandez JA, Buchanan GR. A fatal case of acute splenic sequestration in a 53–year-old woman with sickle-hemoglobin C disease. Am J Med 1992;92:97–100.
- Hatton CS, Bunch C, Weatherall DJ. Hepatic sequestration in sickle cell anaemia. Br Med J (Clin Res Ed) 1985;290:744–5.
- Castro O, Brambilla DJ, Thorington B, et al. The acute chest syndrome in sickle cell disease: incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood 1994;84:643–9.
- Gill FM, Sleeper LA, Weiner SJ, et al. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood 1995;86:776–83.
- Owusu-Ofori S, Hirst C. Splenectomy versus conservative management for acute sequestration crises in people with sickle cell disease. Cochrane Database Syst Rev 2013;5:CD003425.
- 00.Al-Salem AH. Splenic complications of sickle cell anemia and the role of splenectomy. ISRN Hematol 2011;2011:864257.
- Sabarense AP, Lima GO, Silva LM, Viana MB. Characterization of mortality in children with sickle cell disease diagnosed through the Newborn Screening Program. J Pediatr (Rio J) 2015;91:242–7.
- Aslam AF, Aslam AK, Dipillo F. Fatal splenic sequestration crisis with multiorgan failure in an adult woman with sickle cell-beta+ thalassemia. Am J Med Sci 2005;329:141–3.
- Berry RA, Odumakinde EA, Lewis JP. Massive splenic infarction in doubly abnormal heterozygous sickling disorders. A new complication of acute splenic sequestration syndrome. The West J Med 1991;155:531–2.
- Bonanomi MT, Lavezzo MM. Sickle cell retinopathy: diagnosis and treatment. Arq Bras de Oftalmol 2013;76:320–7.
- Chen RW, Flynn HW Jr, Lee WH, et al. Vitreoretinal management and surgical outcomes in proliferative sickle retinopathy: a case series. Am J Ophthalmol 2014;157:870–5 e1.
- Howard J, Malfroy M, Llewelyn C, et al. The Transfusion Alternatives Preoperatively in Sickle Cell Disease (TAPS) study: a randomised, controlled, multicentre clinical trial. Lancet 2013;381:930–8.
- Neumayr L, Koshy M, Haberkern C, et al. Surgery in patients with hemoglobin SC disease. Preoperative Transfusion in Sickle Cell Disease Study Group. Am J Hematol 1998;57:101–8.
- 08. Savage WJ, Buchanan GR, Yawn BP, et al. Evidence gaps in the management of sickle cell disease: A summary of needed research. Am J Hematol 2015;90:273–5.
- Charache S, Barton FB, Moore RD, et al. Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive “switching” agent. The Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Medicine (Baltimore) 1996;75:300–26.
- Steinberg MH, Nagel RL, Brugnara C. Cellular effects of hydroxyurea in Hb SC disease. Br J Haematol 1997;98:838–44.
- Wang W, Brugnara C, Snyder C, et al. The effects of hydroxycarbamide and magnesium on haemoglobin SC disease: results of the multi-centre CHAMPS trial. Br J Haematol 2011;152:771–6.
- Jungst C, Kullak-Ublick GA, Jungst D. Gallstone disease: Microlithiasis and sludge. Best Pract Res Clin Gastroenterol 2006;20:1053–62.
- Walker TM, Serjeant GR. Biliary sludge in sickle cell disease. J Pediatr 1996;129:443–5.
- Bauer TW, Moore GW, Hutchins GM. The liver in sickle cell disease. A clinicopathologic study of 70 patients. Am J Med 1980;69:833–7.
- Ebert EC, Nagar M, Hagspiel KD. Gastrointestinal and hepatic complications of sickle cell disease. Clin Gastroenterol Hepatol 2010;8:483–9.
- Hiran S. Multiorgan dysfunction syndrome in sickle cell disease. J Assoc Physicians India 2005;53:19–22.
- Shao SH, Orringer EP. Case report: splenic sequestration and multiorgan failure as the presenting manifestation of hemoglobin SC disease. Am J Med Sci 1996;311:139–41.
- Hassell KL, Eckman JR, Lane PA. Acute multiorgan failure syndrome: a potentially catastrophic complication of severe sickle cell pain episodes. Am J Med 1994;96:155–62.
Tenotomy, Tenodesis, Transfer: A Review of Treatment Options for Biceps-Labrum Complex Disease
Pathology of the biceps-labrum complex (BLC) can be an important source of shoulder pain. Discussion of pathoanatomy, imaging, and surgical intervention is facilitated by distinguishing the anatomical zones of the BLC: inside, junction, and bicipital tunnel (extra-articular), parts of which cannot be visualized with standard diagnostic arthroscopy.
The recent literature indicates that bicipital tunnel lesions are common and perhaps overlooked. Systematic reviews suggest improvement in outcomes of BLC operations when the bicipital tunnel is decompressed. Higher-level clinical and basic science studies are needed to fully elucidate the role of the bicipital tunnel, but it is evident that a comprehensive physical examination and an understanding of the limits of advanced imaging are necessary to correctly diagnose and treat BLC-related shoulder pain.
Anatomy of Biceps-Labrum Complex
The long head of the biceps tendon (LHBT) and the glenoid labrum work as an interdependent functional unit, the biceps-labrum complex (BLC). The BLC is divided into 3 distinct anatomical zones: inside, junction, and bicipital tunnel.1,2
Inside
The inside includes the superior labrum and biceps attachment. The LHBT most commonly originates in the superior labrum.3-5 Vangsness and colleagues3 described 4 types of LHBT origins: Type I biceps attaches solely to the posterior labrum, type II predominantly posterior, type III equally to the anterior and posterior labrum, and type IV mostly to the anterior labrum. The LHBT can also originate in the supraglenoid tubercle or the inferior border of the supraspinatus.3,6
Junction
Junction is the intra-articular segment of the LHBT and the biceps pulley. The LHBT traverses the glenohumeral joint en route to the extra-articular bicipital tunnel.2 The LHBT is enveloped in synovium that extends into part of the bicipital tunnel.2 The intra-articular segment of the LHBT is about 25 mm in length7 and has a diameter of 5 mm to 6 mm.8
A cadaveric study found that the average length of the LHBT that can be arthroscopically visualized at rest is 35.6 mm, or only 40% of the total length of the LHBT with respect to the proximal margin of the pectoralis major tendon.1 When the LHBT was pulled into the joint, more tendon (another 14 mm) was visualized.1 Therefore, diagnostic arthroscopy of the glenohumeral joint visualizes about 50% of the LHBT.9The morphology of the LHBT varies by location. The intra-articular portion of the LHBT is wide and flat, whereas the extra-articular portion is round.8 The tendon becomes smoother and more avascular as it exits the joint to promote gliding within its sheath in the bicipital groove.10 The proximal LHBT receives its vascular supply from superior labrum tributaries, and distally the LHBT is supplied by ascending branches of the anterior humeral circumflex artery.4 There is a hypovascular zone, created by this dual blood supply, about 12 mm to 30 mm from the LHBT origin, predisposing the tendon to rupture or fray in this region.11The LHBT makes a 30° turn into the biceps pulley system as it exits the glenohumeral joint. The fibrous pulley system that stabilizes the LHBT in this region has contributions from the coracohumeral ligament, the superior glenohumeral ligament, and the supraspinatus tendon.12-14
Bicipital Tunnel
The bicipital tunnel, the third portion of the BLC, remains largely hidden from standard diagnostic glenohumeral arthroscopy. The bicipital tunnel is an extra-articular, closed space that constrains the LHBT from the articular margin through the subpectoral region.2
Zone 1 is the traditional bicipital groove or “bony groove” that extends from the articular margin to the distal margin of the subscapularis tendon. The floor consists of a deep osseous groove covered by a continuation of subscapularis tendon fibers and periosteum.2Zone 2, “no man’s land,” extends from the distal margin of the subscapularis tendon to the proximal margin of the pectoralis major (PMPM). The LHBT in this zone cannot be visualized during a pull test at arthroscopy, yet lesions commonly occur here.1 Zones 1 and 2 have a similar histology and contain synovium.2Zone 3 is the subpectoral region distal to the PMPM. Fibers of the latissimus dorsi form the flat floor of zone 3, and the pectoralis major inserts lateral to the LHBT on the humerus in this zone. The synovium encapsulating the LHBT in zones 1 and 2 rarely extends past the PMPM. Taylor and colleagues2 found a higher percentage of unoccupied tunnel space in zone 3 than in zones 1 and 2, which results in a “functional bottleneck” between zones 2 and 3 represented by the PMPM.
Pathoanatomy
BLC lesions may occur in isolation or concomitantly across multiple anatomical zones. In a series of 277 chronically symptomatic shoulders that underwent transfer of the LHBT to the conjoint tendon with subdeltoid arthroscopy, Taylor and colleagues1 found 47% incidence of bicipital tunnel lesions, 44% incidence of junctional lesions, and 35% incidence of inside lesions. In their series, 37% of patients had concomitant lesions involving more than 1 anatomical zone.
Inside Lesions
Inside lesions involve the superior labrum, the LHBT origin, or both. Superior labrum anterior-posterior (SLAP) tears are included as inside BLC lesions. Snyder and colleagues16 originally identified 4 broad categories of SLAP tears, but Powell and colleagues17 described up to 10 variations. Type II lesions, which are the most common, destabilize the biceps anchor.
Dynamic incarceration of the biceps between the humeral head and the glenoid labrum is another inside lesion that can be identified during routine diagnostic glenohumeral arthroscopy. The arthroscopic active compression test, as described by Verma and colleagues,18 can be used during surgery to demonstrate incarceration of the biceps tendon.
Medial biceps chondromalacia, attritional chondral wear along the anteromedial aspect of the humeral head, occurs secondary to a windshield wiper effect of the LHBT in the setting of an incarcerating LHBT or may be associated with destabilization of the biceps pulley.
Junctional Lesions
Junctional lesions, which include lesions that affect the intra-articular LHBT, can be visualized during routine glenohumeral arthroscopy. They include partial and complete biceps tears, biceps pulley lesions, and junctional biceps chondromalacia.
Biceps pulley injuries and/or tears of the upper subscapularis tendon can destabilize the biceps as it exits the joint, and this destabilization may result in medial subluxation of the tendon and the aforementioned medial biceps chondromalacia.10,19 Junctional biceps chondromalacia is attritional chondral wear of the humeral head from abnormal tracking of the LHBT deep to the LHBT near the articular margin.
Recently elucidated is the limited ability of diagnostic glenohumeral arthroscopy to fully identify the extent of BLC pathology.1,20-22 Gilmer and colleagues20 found that diagnostic arthroscopy identified only 67% of biceps pathology and underestimated its extent in 56% of patients in their series. Similarly, Moon and colleagues21 found that 79% of proximal LHBT tears propagated distally into the bicipital tunnel and were incompletely visualized with standard arthroscopy.
Bicipital Tunnel Lesions
Recent evidence indicates that the bicipital tunnel is a closed space that often conceals space-occupying lesions, including scar, synovitis, loose bodies, and osteophytes, which can become trapped in the tunnel. The functional bottleneck between zones 2 and 3 of the bicipital tunnel explains the aggregation of loose bodies in this region.2 Similarly, as the percentage of free space within the bicipital tunnel increases, space-occupying lesions (eg scar, loose bodies, osteophytes) may exude a compressive and/or abrasive force within zones 1 and 2, but not as commonly within zone 3.2
Physical Examination of Biceps-Labrum Complex
Accurate diagnosis of BLC disease is crucial in selecting an optimal intervention, but challenging. Beyond identifying biceps pathology, specific examination maneuvers may help distinguish between lesions of the intra-articular BLC and lesions of the extra-articular bicipital tunnel.23
Traditional examination maneuvers for biceps-related shoulder pain include the Speed test, the full can test, and the Yergason test.24,25 For the Speed test, the patient forward-flexes the shoulder to 60° to 90°, extends the arm at the elbow, and supinates the forearm. The clinician applies a downward force as the patient resists. The reported sensitivity of the Speed test ranges from 37% to 63%, and specificity is 60% to 88%.25,26 In the full can test, with the patient’s arm in the plane of the scapula, the shoulder abducted to 90°, and the forearm in neutral rotation, a downward force is applied against resistance. Sensitivity of the full can test is 60% to 67%, and specificity is 76% to 84%.24 The Yergason test is performed with the patient’s arm at his or her side, the elbow flexed to 90°, and the forearm pronated. The patient supinates the forearm against the clinician’s resistance. Sensitivity of the Yergason test is 19% to 32%, and specificity is 70% to 100%.25,26 The Yergason test has a positive predictive value of 92% for bicipital tunnel disease.
O’Brien and colleagues23,26 introduced a “3-pack” physical examination designed to elicit BLC symptoms. In this examination, the LHBT is palpated along its course within the bicipital tunnel. Reproduction of the patient’s pain by palpation had a sensitivity of 98% for bicipital tunnel disease but was less specific (70%). Gill and colleagues27 reported low sensitivity (53%) and low specificity (54%) for biceps palpation, and they used arthroscopy as a gold standard. Since then, multiple studies have demonstrated that glenohumeral arthroscopy fails to identify lesions concealed within the bicipital tunnel.20-22The second part of the 3-pack examination is the active compression test. A downward force is applied as the patient resists with his or her arm forward-flexed to 90° and adducted 10° to 15° with the thumb pointing downward.28 This action is repeated with the humerus externally rotated and the forearm supinated. A positive test is indicated by reproduction of symptoms with the thumb down, and elimination or reduction of symptoms with the palm up. Test sensitivity is 88% to 96%, and specificity is 46% to 64% for BLC lesions, but for bicipital tunnel disease sensitivity is higher (96%), and the negative predictive value is 93%.26The third component of the 3-pack examination is the throwing test. A late-cocking throwing position is re-created with the shoulder externally rotated and abducted to 90° and the elbow flexed to 90°. The patient steps forward with the contralateral leg and moves into the acceleration phase of throwing while the clinician provides isometric resistance. If this maneuver reproduces pain, the test is positive. As Taylor and colleagues26 reported, the throwing test has sensitivity of 73% to 77% and specificity of 65% to 79% for BLC pathology. This test has moderate sensitivity and negative predictive value for bicipital tunnel disease but may be the only positive test on physical examination in the setting of LHBT instability.
Imaging of Biceps-Labrum Complex
Plain anteroposterior, lateral, and axillary radiographs of the shoulder should be obtained for all patients having an orthopedic examination for shoulder pain. Magnetic resonance imaging (MRI) and ultrasound are the advanced modalities most commonly used for diagnostic imaging. These modalities should be considered in conjunction with, not in place of, a comprehensive history and physical examination.
MRI has sensitivity of 9% to 89% for LHBT pathology29-37 and 38% to 98% for SLAP pathology.35,38-41 The wide range of reported sensitivity and specificity might be attributed to the varying criteria for what constitutes a BLC lesion. Some authors include biceps chondromalacia, dynamic incarceration of LHBT, and extra-articular bicipital tunnel lesions, while others historically have included only intra-articular LHBT lesions that can be directly visualized arthroscopically.
In their retrospective review of 277 shoulders with chronic refractory BLC symptoms treated with subdeltoid transfer of the LHBT to the conjoint tendon, Taylor and colleagues30 reported MRI was more sensitive for inside BLC lesions than for junctional or bicipital tunnel lesions (77% vs 43% and 50%, respectively).
Treatment Options for Biceps-Labrum Complex Lesions
A diagnosis of BLC disease warrants a trial of conservative (nonoperative) management for at least 3 months. Many patients improve with activity modification, use of oral anti-inflammatory medication, and structured physical therapy focused on dynamic stabilizers and range of motion. If pain persists, local anesthetic and corticosteroid can be injected under ultrasound guidance into the bicipital tunnel; this injection has the advantage of being both diagnostic and therapeutic. Hashiuchi and colleagues42 found ultrasound-guided injections are 87% successful in achieving intra-sheath placement (injections without ultrasound guidance are only 27% successful).
If the 3-month trial of conservative management fails, surgical intervention should be considered. The goal in treating BLC pain is to maximize clinical function and alleviate pain in a predictable manner while minimizing technical demands and morbidity. A singular solution has not been identified. Furthermore, 3 systematic reviews failed to identify a difference between the most commonly used techniques, biceps tenodesis and tenotomy.43-45 These reviews grouped all tenotomy procedures together and compared them with all tenodesis procedures. A limitation of these systematic reviews is that they did not differentiate tenodesis techniques. We prefer to classify techniques according to whether or not they decompress zones 1 and 2 of the bicipital tunnel.
Bicipital Tunnel Nondecompressing Techniques
Release of the biceps tendon, a biceps tenotomy, is a simple procedure that potentially avoids open surgery and provides patients with a quick return to activity. Disadvantages of tenotomy include cosmetic (Popeye) deformity after surgery, potential cramping and fatigue, and biomechanical changes in the humeral head,46-48 particularly among patients younger than 65 years. High rates of revision after tenotomy have been reported.43,49 Incomplete retraction of the LHBT and/or residual synovium may be responsible for refractory pain following biceps tenotomy.49 We hypothesize that failure of tenotomy may be related to unaddressed bicipital tunnel disease.
Proximal nondecompressing tenodesis techniques may be performed either on soft tissue in the interval or rotator cuff or on bone at the articular margin or within zone 1 of the bicipital tunnel.50-52 These techniques can be performed with standard glenohumeral arthroscopy and generally are fast and well tolerated and have limited operative morbidity. Advantages of these techniques over simple tenotomy are lower rates of cosmetic deformity and lower rates of cramping and fatigue pain, likely resulting from maintenance of the muscle tension relationship of the LHBT. Disadvantages of proximal tenodesis techniques include introduction of hardware for bony fixation, longer postoperative rehabilitation to protect repairs, and failure to address hidden bicipital tunnel disease. Furthermore, the rate of stiffness in patients who undergo proximal tenodesis without decompression of the bicipital tunnel may be as high as 18%.53
Bicipital Tunnel Decompressing Techniques
Surgical techniques that decompress the bicipital tunnel include proximal techniques that release the bicipital sheath within zones 1 and 2 of the bicipital tunnel (to the level of the proximal margin of the pectoralis major tendon) and certain arthroscopic suprapectoral techniques,54 open subpectoral tenodeses,55-57 and arthroscopic transfer of the LHBT to the conjoint tendon.58,59
Open subpectoral tenodesis techniques have the advantage of maintaining the length-tension relationship of the LHBT and preventing Popeye deformity. However, these techniques require making an incision near the axilla, which may introduce an unnecessary source of infection. Furthermore, open subpectoral tenodesis requires drilling the humerus and placing a screw for bony fixation of the LHBT, which can create a risk of neurovascular injury, given the proximity of neurovascular structures,60-62 and humeral shaft fracture, particularly in athletes.63,64Our preferred method is transfer of the LHBT to the conjoint tendon (Figure 3).59
Am J Orthop. 2016;45(7):E503-E511. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Taylor SA, Khair MM, Gulotta LV, et al. Diagnostic glenohumeral arthroscopy fails to fully evaluate the biceps-labral complex. Arthroscopy. 2015;31(2):215-224.
2. Taylor SA, Fabricant PD, Bansal M, et al. The anatomy and histology of the bicipital tunnel of the shoulder. J Shoulder Elbow Surg. 2015;24(4):511-519.
3. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
4. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. an anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
5. Tuoheti Y, Itoi E, Minagawa H, et al. Attachment types of the long head of the biceps tendon to the glenoid labrum and their relationships with the glenohumeral ligaments. Arthroscopy. 2005;21(10):1242-1249.
6. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
7. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length-tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
8. Ahrens PM, Boileau P. The long head of biceps and associated tendinopathy. J Bone Joint Surg Br. 2007;89(8):1001-1009.
9. Hart ND, Golish SR, Dragoo JL. Effects of arm position on maximizing intra-articular visualization of the biceps tendon: a cadaveric study. Arthroscopy. 2012;28(4):481-485.
10. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
11. Cheng NM, Pan WR, Vally F, Le Roux CM, Richardson MD. The arterial supply of the long head of biceps tendon: anatomical study with implications for tendon rupture. Clin Anat. 2010;23(6):683-692.
12. Habermeyer P, Magosch P, Pritsch M, Scheibel MT, Lichtenberg S. Anterosuperior impingement of the shoulder as a result of pulley lesions: a prospective arthroscopic study. J Shoulder Elbow Surg. 2004;13(1):5-12.
13. Gohlke F, Daum P, Bushe C. The stabilizing function of the glenohumeral joint capsule. Current aspects of the biomechanics of instability [in German]. Z Orthop Ihre Grenzgeb. 1994;132(2):112-119.
14. Arai R, Mochizuki T, Yamaguchi K, et al. Functional anatomy of the superior glenohumeral and coracohumeral ligaments and the subscapularis tendon in view of stabilization of the long head of the biceps tendon. J Shoulder Elbow Surg. 2010;19(1):58-64.
15. Busconi BB, DeAngelis N, Guerrero PE. The proximal biceps tendon: tricks and pearls. Sports Med Arthrosc. 2008;16(3):187-194.
16. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
17. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2004;12(2):99-110.
18. Verma NN, Drakos M, O’Brien SJ. The arthroscopic active compression test. Arthroscopy. 2005;21(5):634.
19. Walch G, Nove-Josserand L, Levigne C, Renaud E. Tears of the supraspinatus tendon associated with “hidden” lesions of the rotator interval. J Shoulder Elbow Surg. 1994;3(6):353-360.
20. Gilmer BB, DeMers AM, Guerrero D, Reid JB 3rd, Lubowitz JH, Guttmann D. Arthroscopic versus open comparison of long head of biceps tendon visualization and pathology in patients requiring tenodesis. Arthroscopy. 2015;31(1):29-34.
21. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
22. Festa A, Allert J, Issa K, Tasto JP, Myer JJ. Visualization of the extra-articular portion of the long head of the biceps tendon during intra-articular shoulder arthroscopy. Arthroscopy. 2014;30(11):1413-1417.
23. O’Brien SJ, Newman AM, Taylor SA, et al. The accurate diagnosis of biceps-labral complex lesions with MRI and “3-pack” physical examination: a retrospective analysis with prospective validation. Orthop J Sports Med. 2013;1(4 suppl). doi:10.1177/2325967113S00018.
24. Hegedus EJ, Goode AP, Cook CE, et al. Which physical examination tests provide clinicians with the most value when examining the shoulder? Update of a systematic review with meta-analysis of individual tests. Br J Sports Med. 2012;46(14):964-978.
25. Chen HS, Lin SH, Hsu YH, Chen SC, Kang JH. A comparison of physical examinations with musculoskeletal ultrasound in the diagnosis of biceps long head tendinitis. Ultrasound Med Biol. 2011;37(9):1392-1398.
26. Taylor SA, Newman AM, Dawson C, et al. The “3-Pack” examination is critical for comprehensive evaluation of the biceps-labrum complex and the bicipital tunnel: a prospective study. Arthroscopy. 2016 Jul 20. [Epub ahead of print]
27. Gill HS, El Rassi G, Bahk MS, Castillo RC, McFarland EG. Physical examination for partial tears of the biceps tendon. Am J Sports Med. 2007;35(8):1334-1340.
28. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
29. Zanetti M, Weishaupt D, Gerber C, Hodler J. Tendinopathy and rupture of the tendon of the long head of the biceps brachii muscle: evaluation with MR arthrography. AJR Am J Roentgenol. 1998;170(6):1557-1561.
30. Taylor SA, Newman AM, Nguyen J, et al. Magnetic resonance imaging currently fails to fully evaluate the biceps-labrum complex and bicipital tunnel. Arthroscopy. 2016;32(2):238-244.
31. Malavolta EA, Assunção JH, Guglielmetti CL, de Souza FF, Gracitelli ME, Ferreira Neto AA. Accuracy of preoperative MRI in the diagnosis of disorders of the long head of the biceps tendon. Eur J Radiol. 2015;84(11):2250-2254.
32. Dubrow SA, Streit JJ, Shishani Y, Robbin MR, Gobezie R. Diagnostic accuracy in detecting tears in the proximal biceps tendon using standard nonenhancing shoulder MRI. Open Access J Sports Med. 2014;5:81-87.
33. Nourissat G, Tribot-Laspiere Q, Aim F, Radier C. Contribution of MRI and CT arthrography to the diagnosis of intra-articular tendinopathy of the long head of the biceps. Orthop Traumatol Surg Res. 2014;100(8 suppl):S391-S394.
34. De Maeseneer M, Boulet C, Pouliart N, et al. Assessment of the long head of the biceps tendon of the shoulder with 3T magnetic resonance arthrography and CT arthrography. Eur J Radiol. 2012;81(5):934-939.
35. Houtz CG, Schwartzberg RS, Barry JA, Reuss BL, Papa L. Shoulder MRI accuracy in the community setting. J Shoulder Elbow Surg. 2011;20(4):537-542.
36. Buck FM, Grehn H, Hilbe M, Pfirrmann CW, Manzanell S, Hodler J. Degeneration of the long biceps tendon: comparison of MRI with gross anatomy and histology. AJR Am J Roentgenol. 2009;193(5):1367-1375.
37. Mohtadi NG, Vellet AD, Clark ML, et al. A prospective, double-blind comparison of magnetic resonance imaging and arthroscopy in the evaluation of patients presenting with shoulder pain. J Shoulder Elbow Surg. 2004;13(3):258-265.
38. Sheridan K, Kreulen C, Kim S, Mak W, Lewis K, Marder R. Accuracy of magnetic resonance imaging to diagnose superior labrum anterior-posterior tears. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2645-2650.
39. Connolly KP, Schwartzberg RS, Reuss B, Crumbie D Jr, Homan BM. Sensitivity and specificity of noncontrast magnetic resonance imaging reports in the diagnosis of type-II superior labral anterior-posterior lesions in the community setting. J Bone Joint Surg Am. 2013;95(4):308-313.
40. Reuss BL, Schwartzberg R, Zlatkin MB, Cooperman A, Dixon JR. Magnetic resonance imaging accuracy for the diagnosis of superior labrum anterior-posterior lesions in the community setting: eighty-three arthroscopically confirmed cases. J Shoulder Elbow Surg. 2006;15(5):580-585.
41. Connell DA, Potter HG, Wickiewicz TL, Altchek DW, Warren RF. Noncontrast magnetic resonance imaging of superior labral lesions. 102 cases confirmed at arthroscopic surgery. Am J Sports Med. 1999;27(2):208-213.
42. Hashiuchi T, Sakurai G, Morimoto M, Komei T, Takakura Y, Tanaka Y. Accuracy of the biceps tendon sheath injection: ultrasound-guided or unguided injection? A randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1069-1073.
43. Hsu AR, Ghodadra NS, Provencher MT, Lewis PB, Bach BR. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20(2):326-332.
44. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
45. Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of pathologic lesions of the tendon of the long head of the biceps brachii. Am J Sports Med. 2009;37(4):828-833.
46. Kelly AM, Drakos MC, Fealy S, Taylor SA, O’Brien SJ. Arthroscopic release of the long head of the biceps tendon: functional outcome and clinical results. Am J Sports Med. 2005;33(2):208-213.
47. Berlemann U, Bayley I. Tenodesis of the long head of biceps brachii in the painful shoulder: improving results in the long term. J Shoulder Elbow Surg. 1995;4(6):429-435.
48. Gill TJ, McIrvin E, Mair SD, Hawkins RJ. Results of biceps tenotomy for treatment of pathology of the long head of the biceps brachii. J Shoulder Elbow Surg. 2001;10(3):247-249.
49. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
50. Gartsman GM, Hammerman SM. Arthroscopic biceps tenodesis: operative technique. Arthroscopy. 2000;16(5):550-552.
51. Richards DP, Burkhart SS. Arthroscopic-assisted biceps tenodesis for ruptures of the long head of biceps brachii: the cobra procedure. Arthroscopy. 2004;20(suppl 2):201-207.
52. Klepps S, Hazrati Y, Flatow E. Arthroscopic biceps tenodesis. Arthroscopy. 2002;18(9):1040-1045.
53. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.54. Werner BC, Lyons ML, Evans CL, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of restoration of length-tension and mechanical strength between techniques. Arthroscopy. 2015;31(4):620-627.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
57. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
58. Taylor SA, Fabricant PD, Baret NJ, et al. Midterm clinical outcomes for arthroscopic subdeltoid transfer of the long head of the biceps tendon to the conjoint tendon. Arthroscopy. 2014;30(12):1574-1581.
59. Drakos MC, Verma NN, Gulotta LV, et al. Arthroscopic transfer of the long head of the biceps tendon: functional outcome and clinical results. Arthroscopy. 2008;24(2):217-223.
60. Ding DY, Gupta A, Snir N, Wolfson T, Meislin RJ. Nerve proximity during bicortical drilling for subpectoral biceps tenodesis: a cadaveric study. Arthroscopy. 2014;30(8):942-946.
61. Dickens JF, Kilcoyne KG, Tintle SM, Giuliani J, Schaefer RA, Rue JP. Subpectoral biceps tenodesis: an anatomic study and evaluation of at-risk structures. Am J Sports Med. 2012;40(10):2337-2341.
62. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
63. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
64. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
65. O’Brien SJ, Taylor SA, DiPietro JR, Newman AM, Drakos MC, Voos JE. The arthroscopic “subdeltoid approach” to the anterior shoulder. J Shoulder Elbow Surg. 2013;22(4):e6-e10.
66. Urch E, Taylor SA, Ramkumar PN, et al. Biceps tenodesis: a comparison of tendon-to-bone and tendon-to-tendon healing in a rat model. Paper presented at: Closed Meeting of the American Shoulder and Elbow Surgeons; October 10, 2015; Asheville, NC. Paper 26.
67. Taylor SA, Ramkumar PN, Fabricant PD, et al. The clinical impact of bicipital tunnel decompression during long head of the biceps tendon surgery: a systematic review and meta-analysis. Arthroscopy. 2016;32(6):1155-1164.
Pathology of the biceps-labrum complex (BLC) can be an important source of shoulder pain. Discussion of pathoanatomy, imaging, and surgical intervention is facilitated by distinguishing the anatomical zones of the BLC: inside, junction, and bicipital tunnel (extra-articular), parts of which cannot be visualized with standard diagnostic arthroscopy.
The recent literature indicates that bicipital tunnel lesions are common and perhaps overlooked. Systematic reviews suggest improvement in outcomes of BLC operations when the bicipital tunnel is decompressed. Higher-level clinical and basic science studies are needed to fully elucidate the role of the bicipital tunnel, but it is evident that a comprehensive physical examination and an understanding of the limits of advanced imaging are necessary to correctly diagnose and treat BLC-related shoulder pain.
Anatomy of Biceps-Labrum Complex
The long head of the biceps tendon (LHBT) and the glenoid labrum work as an interdependent functional unit, the biceps-labrum complex (BLC). The BLC is divided into 3 distinct anatomical zones: inside, junction, and bicipital tunnel.1,2
Inside
The inside includes the superior labrum and biceps attachment. The LHBT most commonly originates in the superior labrum.3-5 Vangsness and colleagues3 described 4 types of LHBT origins: Type I biceps attaches solely to the posterior labrum, type II predominantly posterior, type III equally to the anterior and posterior labrum, and type IV mostly to the anterior labrum. The LHBT can also originate in the supraglenoid tubercle or the inferior border of the supraspinatus.3,6
Junction
Junction is the intra-articular segment of the LHBT and the biceps pulley. The LHBT traverses the glenohumeral joint en route to the extra-articular bicipital tunnel.2 The LHBT is enveloped in synovium that extends into part of the bicipital tunnel.2 The intra-articular segment of the LHBT is about 25 mm in length7 and has a diameter of 5 mm to 6 mm.8
A cadaveric study found that the average length of the LHBT that can be arthroscopically visualized at rest is 35.6 mm, or only 40% of the total length of the LHBT with respect to the proximal margin of the pectoralis major tendon.1 When the LHBT was pulled into the joint, more tendon (another 14 mm) was visualized.1 Therefore, diagnostic arthroscopy of the glenohumeral joint visualizes about 50% of the LHBT.9The morphology of the LHBT varies by location. The intra-articular portion of the LHBT is wide and flat, whereas the extra-articular portion is round.8 The tendon becomes smoother and more avascular as it exits the joint to promote gliding within its sheath in the bicipital groove.10 The proximal LHBT receives its vascular supply from superior labrum tributaries, and distally the LHBT is supplied by ascending branches of the anterior humeral circumflex artery.4 There is a hypovascular zone, created by this dual blood supply, about 12 mm to 30 mm from the LHBT origin, predisposing the tendon to rupture or fray in this region.11The LHBT makes a 30° turn into the biceps pulley system as it exits the glenohumeral joint. The fibrous pulley system that stabilizes the LHBT in this region has contributions from the coracohumeral ligament, the superior glenohumeral ligament, and the supraspinatus tendon.12-14
Bicipital Tunnel
The bicipital tunnel, the third portion of the BLC, remains largely hidden from standard diagnostic glenohumeral arthroscopy. The bicipital tunnel is an extra-articular, closed space that constrains the LHBT from the articular margin through the subpectoral region.2
Zone 1 is the traditional bicipital groove or “bony groove” that extends from the articular margin to the distal margin of the subscapularis tendon. The floor consists of a deep osseous groove covered by a continuation of subscapularis tendon fibers and periosteum.2Zone 2, “no man’s land,” extends from the distal margin of the subscapularis tendon to the proximal margin of the pectoralis major (PMPM). The LHBT in this zone cannot be visualized during a pull test at arthroscopy, yet lesions commonly occur here.1 Zones 1 and 2 have a similar histology and contain synovium.2Zone 3 is the subpectoral region distal to the PMPM. Fibers of the latissimus dorsi form the flat floor of zone 3, and the pectoralis major inserts lateral to the LHBT on the humerus in this zone. The synovium encapsulating the LHBT in zones 1 and 2 rarely extends past the PMPM. Taylor and colleagues2 found a higher percentage of unoccupied tunnel space in zone 3 than in zones 1 and 2, which results in a “functional bottleneck” between zones 2 and 3 represented by the PMPM.
Pathoanatomy
BLC lesions may occur in isolation or concomitantly across multiple anatomical zones. In a series of 277 chronically symptomatic shoulders that underwent transfer of the LHBT to the conjoint tendon with subdeltoid arthroscopy, Taylor and colleagues1 found 47% incidence of bicipital tunnel lesions, 44% incidence of junctional lesions, and 35% incidence of inside lesions. In their series, 37% of patients had concomitant lesions involving more than 1 anatomical zone.
Inside Lesions
Inside lesions involve the superior labrum, the LHBT origin, or both. Superior labrum anterior-posterior (SLAP) tears are included as inside BLC lesions. Snyder and colleagues16 originally identified 4 broad categories of SLAP tears, but Powell and colleagues17 described up to 10 variations. Type II lesions, which are the most common, destabilize the biceps anchor.
Dynamic incarceration of the biceps between the humeral head and the glenoid labrum is another inside lesion that can be identified during routine diagnostic glenohumeral arthroscopy. The arthroscopic active compression test, as described by Verma and colleagues,18 can be used during surgery to demonstrate incarceration of the biceps tendon.
Medial biceps chondromalacia, attritional chondral wear along the anteromedial aspect of the humeral head, occurs secondary to a windshield wiper effect of the LHBT in the setting of an incarcerating LHBT or may be associated with destabilization of the biceps pulley.
Junctional Lesions
Junctional lesions, which include lesions that affect the intra-articular LHBT, can be visualized during routine glenohumeral arthroscopy. They include partial and complete biceps tears, biceps pulley lesions, and junctional biceps chondromalacia.
Biceps pulley injuries and/or tears of the upper subscapularis tendon can destabilize the biceps as it exits the joint, and this destabilization may result in medial subluxation of the tendon and the aforementioned medial biceps chondromalacia.10,19 Junctional biceps chondromalacia is attritional chondral wear of the humeral head from abnormal tracking of the LHBT deep to the LHBT near the articular margin.
Recently elucidated is the limited ability of diagnostic glenohumeral arthroscopy to fully identify the extent of BLC pathology.1,20-22 Gilmer and colleagues20 found that diagnostic arthroscopy identified only 67% of biceps pathology and underestimated its extent in 56% of patients in their series. Similarly, Moon and colleagues21 found that 79% of proximal LHBT tears propagated distally into the bicipital tunnel and were incompletely visualized with standard arthroscopy.
Bicipital Tunnel Lesions
Recent evidence indicates that the bicipital tunnel is a closed space that often conceals space-occupying lesions, including scar, synovitis, loose bodies, and osteophytes, which can become trapped in the tunnel. The functional bottleneck between zones 2 and 3 of the bicipital tunnel explains the aggregation of loose bodies in this region.2 Similarly, as the percentage of free space within the bicipital tunnel increases, space-occupying lesions (eg scar, loose bodies, osteophytes) may exude a compressive and/or abrasive force within zones 1 and 2, but not as commonly within zone 3.2
Physical Examination of Biceps-Labrum Complex
Accurate diagnosis of BLC disease is crucial in selecting an optimal intervention, but challenging. Beyond identifying biceps pathology, specific examination maneuvers may help distinguish between lesions of the intra-articular BLC and lesions of the extra-articular bicipital tunnel.23
Traditional examination maneuvers for biceps-related shoulder pain include the Speed test, the full can test, and the Yergason test.24,25 For the Speed test, the patient forward-flexes the shoulder to 60° to 90°, extends the arm at the elbow, and supinates the forearm. The clinician applies a downward force as the patient resists. The reported sensitivity of the Speed test ranges from 37% to 63%, and specificity is 60% to 88%.25,26 In the full can test, with the patient’s arm in the plane of the scapula, the shoulder abducted to 90°, and the forearm in neutral rotation, a downward force is applied against resistance. Sensitivity of the full can test is 60% to 67%, and specificity is 76% to 84%.24 The Yergason test is performed with the patient’s arm at his or her side, the elbow flexed to 90°, and the forearm pronated. The patient supinates the forearm against the clinician’s resistance. Sensitivity of the Yergason test is 19% to 32%, and specificity is 70% to 100%.25,26 The Yergason test has a positive predictive value of 92% for bicipital tunnel disease.
O’Brien and colleagues23,26 introduced a “3-pack” physical examination designed to elicit BLC symptoms. In this examination, the LHBT is palpated along its course within the bicipital tunnel. Reproduction of the patient’s pain by palpation had a sensitivity of 98% for bicipital tunnel disease but was less specific (70%). Gill and colleagues27 reported low sensitivity (53%) and low specificity (54%) for biceps palpation, and they used arthroscopy as a gold standard. Since then, multiple studies have demonstrated that glenohumeral arthroscopy fails to identify lesions concealed within the bicipital tunnel.20-22The second part of the 3-pack examination is the active compression test. A downward force is applied as the patient resists with his or her arm forward-flexed to 90° and adducted 10° to 15° with the thumb pointing downward.28 This action is repeated with the humerus externally rotated and the forearm supinated. A positive test is indicated by reproduction of symptoms with the thumb down, and elimination or reduction of symptoms with the palm up. Test sensitivity is 88% to 96%, and specificity is 46% to 64% for BLC lesions, but for bicipital tunnel disease sensitivity is higher (96%), and the negative predictive value is 93%.26The third component of the 3-pack examination is the throwing test. A late-cocking throwing position is re-created with the shoulder externally rotated and abducted to 90° and the elbow flexed to 90°. The patient steps forward with the contralateral leg and moves into the acceleration phase of throwing while the clinician provides isometric resistance. If this maneuver reproduces pain, the test is positive. As Taylor and colleagues26 reported, the throwing test has sensitivity of 73% to 77% and specificity of 65% to 79% for BLC pathology. This test has moderate sensitivity and negative predictive value for bicipital tunnel disease but may be the only positive test on physical examination in the setting of LHBT instability.
Imaging of Biceps-Labrum Complex
Plain anteroposterior, lateral, and axillary radiographs of the shoulder should be obtained for all patients having an orthopedic examination for shoulder pain. Magnetic resonance imaging (MRI) and ultrasound are the advanced modalities most commonly used for diagnostic imaging. These modalities should be considered in conjunction with, not in place of, a comprehensive history and physical examination.
MRI has sensitivity of 9% to 89% for LHBT pathology29-37 and 38% to 98% for SLAP pathology.35,38-41 The wide range of reported sensitivity and specificity might be attributed to the varying criteria for what constitutes a BLC lesion. Some authors include biceps chondromalacia, dynamic incarceration of LHBT, and extra-articular bicipital tunnel lesions, while others historically have included only intra-articular LHBT lesions that can be directly visualized arthroscopically.
In their retrospective review of 277 shoulders with chronic refractory BLC symptoms treated with subdeltoid transfer of the LHBT to the conjoint tendon, Taylor and colleagues30 reported MRI was more sensitive for inside BLC lesions than for junctional or bicipital tunnel lesions (77% vs 43% and 50%, respectively).
Treatment Options for Biceps-Labrum Complex Lesions
A diagnosis of BLC disease warrants a trial of conservative (nonoperative) management for at least 3 months. Many patients improve with activity modification, use of oral anti-inflammatory medication, and structured physical therapy focused on dynamic stabilizers and range of motion. If pain persists, local anesthetic and corticosteroid can be injected under ultrasound guidance into the bicipital tunnel; this injection has the advantage of being both diagnostic and therapeutic. Hashiuchi and colleagues42 found ultrasound-guided injections are 87% successful in achieving intra-sheath placement (injections without ultrasound guidance are only 27% successful).
If the 3-month trial of conservative management fails, surgical intervention should be considered. The goal in treating BLC pain is to maximize clinical function and alleviate pain in a predictable manner while minimizing technical demands and morbidity. A singular solution has not been identified. Furthermore, 3 systematic reviews failed to identify a difference between the most commonly used techniques, biceps tenodesis and tenotomy.43-45 These reviews grouped all tenotomy procedures together and compared them with all tenodesis procedures. A limitation of these systematic reviews is that they did not differentiate tenodesis techniques. We prefer to classify techniques according to whether or not they decompress zones 1 and 2 of the bicipital tunnel.
Bicipital Tunnel Nondecompressing Techniques
Release of the biceps tendon, a biceps tenotomy, is a simple procedure that potentially avoids open surgery and provides patients with a quick return to activity. Disadvantages of tenotomy include cosmetic (Popeye) deformity after surgery, potential cramping and fatigue, and biomechanical changes in the humeral head,46-48 particularly among patients younger than 65 years. High rates of revision after tenotomy have been reported.43,49 Incomplete retraction of the LHBT and/or residual synovium may be responsible for refractory pain following biceps tenotomy.49 We hypothesize that failure of tenotomy may be related to unaddressed bicipital tunnel disease.
Proximal nondecompressing tenodesis techniques may be performed either on soft tissue in the interval or rotator cuff or on bone at the articular margin or within zone 1 of the bicipital tunnel.50-52 These techniques can be performed with standard glenohumeral arthroscopy and generally are fast and well tolerated and have limited operative morbidity. Advantages of these techniques over simple tenotomy are lower rates of cosmetic deformity and lower rates of cramping and fatigue pain, likely resulting from maintenance of the muscle tension relationship of the LHBT. Disadvantages of proximal tenodesis techniques include introduction of hardware for bony fixation, longer postoperative rehabilitation to protect repairs, and failure to address hidden bicipital tunnel disease. Furthermore, the rate of stiffness in patients who undergo proximal tenodesis without decompression of the bicipital tunnel may be as high as 18%.53
Bicipital Tunnel Decompressing Techniques
Surgical techniques that decompress the bicipital tunnel include proximal techniques that release the bicipital sheath within zones 1 and 2 of the bicipital tunnel (to the level of the proximal margin of the pectoralis major tendon) and certain arthroscopic suprapectoral techniques,54 open subpectoral tenodeses,55-57 and arthroscopic transfer of the LHBT to the conjoint tendon.58,59
Open subpectoral tenodesis techniques have the advantage of maintaining the length-tension relationship of the LHBT and preventing Popeye deformity. However, these techniques require making an incision near the axilla, which may introduce an unnecessary source of infection. Furthermore, open subpectoral tenodesis requires drilling the humerus and placing a screw for bony fixation of the LHBT, which can create a risk of neurovascular injury, given the proximity of neurovascular structures,60-62 and humeral shaft fracture, particularly in athletes.63,64Our preferred method is transfer of the LHBT to the conjoint tendon (Figure 3).59
Am J Orthop. 2016;45(7):E503-E511. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Pathology of the biceps-labrum complex (BLC) can be an important source of shoulder pain. Discussion of pathoanatomy, imaging, and surgical intervention is facilitated by distinguishing the anatomical zones of the BLC: inside, junction, and bicipital tunnel (extra-articular), parts of which cannot be visualized with standard diagnostic arthroscopy.
The recent literature indicates that bicipital tunnel lesions are common and perhaps overlooked. Systematic reviews suggest improvement in outcomes of BLC operations when the bicipital tunnel is decompressed. Higher-level clinical and basic science studies are needed to fully elucidate the role of the bicipital tunnel, but it is evident that a comprehensive physical examination and an understanding of the limits of advanced imaging are necessary to correctly diagnose and treat BLC-related shoulder pain.
Anatomy of Biceps-Labrum Complex
The long head of the biceps tendon (LHBT) and the glenoid labrum work as an interdependent functional unit, the biceps-labrum complex (BLC). The BLC is divided into 3 distinct anatomical zones: inside, junction, and bicipital tunnel.1,2
Inside
The inside includes the superior labrum and biceps attachment. The LHBT most commonly originates in the superior labrum.3-5 Vangsness and colleagues3 described 4 types of LHBT origins: Type I biceps attaches solely to the posterior labrum, type II predominantly posterior, type III equally to the anterior and posterior labrum, and type IV mostly to the anterior labrum. The LHBT can also originate in the supraglenoid tubercle or the inferior border of the supraspinatus.3,6
Junction
Junction is the intra-articular segment of the LHBT and the biceps pulley. The LHBT traverses the glenohumeral joint en route to the extra-articular bicipital tunnel.2 The LHBT is enveloped in synovium that extends into part of the bicipital tunnel.2 The intra-articular segment of the LHBT is about 25 mm in length7 and has a diameter of 5 mm to 6 mm.8
A cadaveric study found that the average length of the LHBT that can be arthroscopically visualized at rest is 35.6 mm, or only 40% of the total length of the LHBT with respect to the proximal margin of the pectoralis major tendon.1 When the LHBT was pulled into the joint, more tendon (another 14 mm) was visualized.1 Therefore, diagnostic arthroscopy of the glenohumeral joint visualizes about 50% of the LHBT.9The morphology of the LHBT varies by location. The intra-articular portion of the LHBT is wide and flat, whereas the extra-articular portion is round.8 The tendon becomes smoother and more avascular as it exits the joint to promote gliding within its sheath in the bicipital groove.10 The proximal LHBT receives its vascular supply from superior labrum tributaries, and distally the LHBT is supplied by ascending branches of the anterior humeral circumflex artery.4 There is a hypovascular zone, created by this dual blood supply, about 12 mm to 30 mm from the LHBT origin, predisposing the tendon to rupture or fray in this region.11The LHBT makes a 30° turn into the biceps pulley system as it exits the glenohumeral joint. The fibrous pulley system that stabilizes the LHBT in this region has contributions from the coracohumeral ligament, the superior glenohumeral ligament, and the supraspinatus tendon.12-14
Bicipital Tunnel
The bicipital tunnel, the third portion of the BLC, remains largely hidden from standard diagnostic glenohumeral arthroscopy. The bicipital tunnel is an extra-articular, closed space that constrains the LHBT from the articular margin through the subpectoral region.2
Zone 1 is the traditional bicipital groove or “bony groove” that extends from the articular margin to the distal margin of the subscapularis tendon. The floor consists of a deep osseous groove covered by a continuation of subscapularis tendon fibers and periosteum.2Zone 2, “no man’s land,” extends from the distal margin of the subscapularis tendon to the proximal margin of the pectoralis major (PMPM). The LHBT in this zone cannot be visualized during a pull test at arthroscopy, yet lesions commonly occur here.1 Zones 1 and 2 have a similar histology and contain synovium.2Zone 3 is the subpectoral region distal to the PMPM. Fibers of the latissimus dorsi form the flat floor of zone 3, and the pectoralis major inserts lateral to the LHBT on the humerus in this zone. The synovium encapsulating the LHBT in zones 1 and 2 rarely extends past the PMPM. Taylor and colleagues2 found a higher percentage of unoccupied tunnel space in zone 3 than in zones 1 and 2, which results in a “functional bottleneck” between zones 2 and 3 represented by the PMPM.
Pathoanatomy
BLC lesions may occur in isolation or concomitantly across multiple anatomical zones. In a series of 277 chronically symptomatic shoulders that underwent transfer of the LHBT to the conjoint tendon with subdeltoid arthroscopy, Taylor and colleagues1 found 47% incidence of bicipital tunnel lesions, 44% incidence of junctional lesions, and 35% incidence of inside lesions. In their series, 37% of patients had concomitant lesions involving more than 1 anatomical zone.
Inside Lesions
Inside lesions involve the superior labrum, the LHBT origin, or both. Superior labrum anterior-posterior (SLAP) tears are included as inside BLC lesions. Snyder and colleagues16 originally identified 4 broad categories of SLAP tears, but Powell and colleagues17 described up to 10 variations. Type II lesions, which are the most common, destabilize the biceps anchor.
Dynamic incarceration of the biceps between the humeral head and the glenoid labrum is another inside lesion that can be identified during routine diagnostic glenohumeral arthroscopy. The arthroscopic active compression test, as described by Verma and colleagues,18 can be used during surgery to demonstrate incarceration of the biceps tendon.
Medial biceps chondromalacia, attritional chondral wear along the anteromedial aspect of the humeral head, occurs secondary to a windshield wiper effect of the LHBT in the setting of an incarcerating LHBT or may be associated with destabilization of the biceps pulley.
Junctional Lesions
Junctional lesions, which include lesions that affect the intra-articular LHBT, can be visualized during routine glenohumeral arthroscopy. They include partial and complete biceps tears, biceps pulley lesions, and junctional biceps chondromalacia.
Biceps pulley injuries and/or tears of the upper subscapularis tendon can destabilize the biceps as it exits the joint, and this destabilization may result in medial subluxation of the tendon and the aforementioned medial biceps chondromalacia.10,19 Junctional biceps chondromalacia is attritional chondral wear of the humeral head from abnormal tracking of the LHBT deep to the LHBT near the articular margin.
Recently elucidated is the limited ability of diagnostic glenohumeral arthroscopy to fully identify the extent of BLC pathology.1,20-22 Gilmer and colleagues20 found that diagnostic arthroscopy identified only 67% of biceps pathology and underestimated its extent in 56% of patients in their series. Similarly, Moon and colleagues21 found that 79% of proximal LHBT tears propagated distally into the bicipital tunnel and were incompletely visualized with standard arthroscopy.
Bicipital Tunnel Lesions
Recent evidence indicates that the bicipital tunnel is a closed space that often conceals space-occupying lesions, including scar, synovitis, loose bodies, and osteophytes, which can become trapped in the tunnel. The functional bottleneck between zones 2 and 3 of the bicipital tunnel explains the aggregation of loose bodies in this region.2 Similarly, as the percentage of free space within the bicipital tunnel increases, space-occupying lesions (eg scar, loose bodies, osteophytes) may exude a compressive and/or abrasive force within zones 1 and 2, but not as commonly within zone 3.2
Physical Examination of Biceps-Labrum Complex
Accurate diagnosis of BLC disease is crucial in selecting an optimal intervention, but challenging. Beyond identifying biceps pathology, specific examination maneuvers may help distinguish between lesions of the intra-articular BLC and lesions of the extra-articular bicipital tunnel.23
Traditional examination maneuvers for biceps-related shoulder pain include the Speed test, the full can test, and the Yergason test.24,25 For the Speed test, the patient forward-flexes the shoulder to 60° to 90°, extends the arm at the elbow, and supinates the forearm. The clinician applies a downward force as the patient resists. The reported sensitivity of the Speed test ranges from 37% to 63%, and specificity is 60% to 88%.25,26 In the full can test, with the patient’s arm in the plane of the scapula, the shoulder abducted to 90°, and the forearm in neutral rotation, a downward force is applied against resistance. Sensitivity of the full can test is 60% to 67%, and specificity is 76% to 84%.24 The Yergason test is performed with the patient’s arm at his or her side, the elbow flexed to 90°, and the forearm pronated. The patient supinates the forearm against the clinician’s resistance. Sensitivity of the Yergason test is 19% to 32%, and specificity is 70% to 100%.25,26 The Yergason test has a positive predictive value of 92% for bicipital tunnel disease.
O’Brien and colleagues23,26 introduced a “3-pack” physical examination designed to elicit BLC symptoms. In this examination, the LHBT is palpated along its course within the bicipital tunnel. Reproduction of the patient’s pain by palpation had a sensitivity of 98% for bicipital tunnel disease but was less specific (70%). Gill and colleagues27 reported low sensitivity (53%) and low specificity (54%) for biceps palpation, and they used arthroscopy as a gold standard. Since then, multiple studies have demonstrated that glenohumeral arthroscopy fails to identify lesions concealed within the bicipital tunnel.20-22The second part of the 3-pack examination is the active compression test. A downward force is applied as the patient resists with his or her arm forward-flexed to 90° and adducted 10° to 15° with the thumb pointing downward.28 This action is repeated with the humerus externally rotated and the forearm supinated. A positive test is indicated by reproduction of symptoms with the thumb down, and elimination or reduction of symptoms with the palm up. Test sensitivity is 88% to 96%, and specificity is 46% to 64% for BLC lesions, but for bicipital tunnel disease sensitivity is higher (96%), and the negative predictive value is 93%.26The third component of the 3-pack examination is the throwing test. A late-cocking throwing position is re-created with the shoulder externally rotated and abducted to 90° and the elbow flexed to 90°. The patient steps forward with the contralateral leg and moves into the acceleration phase of throwing while the clinician provides isometric resistance. If this maneuver reproduces pain, the test is positive. As Taylor and colleagues26 reported, the throwing test has sensitivity of 73% to 77% and specificity of 65% to 79% for BLC pathology. This test has moderate sensitivity and negative predictive value for bicipital tunnel disease but may be the only positive test on physical examination in the setting of LHBT instability.
Imaging of Biceps-Labrum Complex
Plain anteroposterior, lateral, and axillary radiographs of the shoulder should be obtained for all patients having an orthopedic examination for shoulder pain. Magnetic resonance imaging (MRI) and ultrasound are the advanced modalities most commonly used for diagnostic imaging. These modalities should be considered in conjunction with, not in place of, a comprehensive history and physical examination.
MRI has sensitivity of 9% to 89% for LHBT pathology29-37 and 38% to 98% for SLAP pathology.35,38-41 The wide range of reported sensitivity and specificity might be attributed to the varying criteria for what constitutes a BLC lesion. Some authors include biceps chondromalacia, dynamic incarceration of LHBT, and extra-articular bicipital tunnel lesions, while others historically have included only intra-articular LHBT lesions that can be directly visualized arthroscopically.
In their retrospective review of 277 shoulders with chronic refractory BLC symptoms treated with subdeltoid transfer of the LHBT to the conjoint tendon, Taylor and colleagues30 reported MRI was more sensitive for inside BLC lesions than for junctional or bicipital tunnel lesions (77% vs 43% and 50%, respectively).
Treatment Options for Biceps-Labrum Complex Lesions
A diagnosis of BLC disease warrants a trial of conservative (nonoperative) management for at least 3 months. Many patients improve with activity modification, use of oral anti-inflammatory medication, and structured physical therapy focused on dynamic stabilizers and range of motion. If pain persists, local anesthetic and corticosteroid can be injected under ultrasound guidance into the bicipital tunnel; this injection has the advantage of being both diagnostic and therapeutic. Hashiuchi and colleagues42 found ultrasound-guided injections are 87% successful in achieving intra-sheath placement (injections without ultrasound guidance are only 27% successful).
If the 3-month trial of conservative management fails, surgical intervention should be considered. The goal in treating BLC pain is to maximize clinical function and alleviate pain in a predictable manner while minimizing technical demands and morbidity. A singular solution has not been identified. Furthermore, 3 systematic reviews failed to identify a difference between the most commonly used techniques, biceps tenodesis and tenotomy.43-45 These reviews grouped all tenotomy procedures together and compared them with all tenodesis procedures. A limitation of these systematic reviews is that they did not differentiate tenodesis techniques. We prefer to classify techniques according to whether or not they decompress zones 1 and 2 of the bicipital tunnel.
Bicipital Tunnel Nondecompressing Techniques
Release of the biceps tendon, a biceps tenotomy, is a simple procedure that potentially avoids open surgery and provides patients with a quick return to activity. Disadvantages of tenotomy include cosmetic (Popeye) deformity after surgery, potential cramping and fatigue, and biomechanical changes in the humeral head,46-48 particularly among patients younger than 65 years. High rates of revision after tenotomy have been reported.43,49 Incomplete retraction of the LHBT and/or residual synovium may be responsible for refractory pain following biceps tenotomy.49 We hypothesize that failure of tenotomy may be related to unaddressed bicipital tunnel disease.
Proximal nondecompressing tenodesis techniques may be performed either on soft tissue in the interval or rotator cuff or on bone at the articular margin or within zone 1 of the bicipital tunnel.50-52 These techniques can be performed with standard glenohumeral arthroscopy and generally are fast and well tolerated and have limited operative morbidity. Advantages of these techniques over simple tenotomy are lower rates of cosmetic deformity and lower rates of cramping and fatigue pain, likely resulting from maintenance of the muscle tension relationship of the LHBT. Disadvantages of proximal tenodesis techniques include introduction of hardware for bony fixation, longer postoperative rehabilitation to protect repairs, and failure to address hidden bicipital tunnel disease. Furthermore, the rate of stiffness in patients who undergo proximal tenodesis without decompression of the bicipital tunnel may be as high as 18%.53
Bicipital Tunnel Decompressing Techniques
Surgical techniques that decompress the bicipital tunnel include proximal techniques that release the bicipital sheath within zones 1 and 2 of the bicipital tunnel (to the level of the proximal margin of the pectoralis major tendon) and certain arthroscopic suprapectoral techniques,54 open subpectoral tenodeses,55-57 and arthroscopic transfer of the LHBT to the conjoint tendon.58,59
Open subpectoral tenodesis techniques have the advantage of maintaining the length-tension relationship of the LHBT and preventing Popeye deformity. However, these techniques require making an incision near the axilla, which may introduce an unnecessary source of infection. Furthermore, open subpectoral tenodesis requires drilling the humerus and placing a screw for bony fixation of the LHBT, which can create a risk of neurovascular injury, given the proximity of neurovascular structures,60-62 and humeral shaft fracture, particularly in athletes.63,64Our preferred method is transfer of the LHBT to the conjoint tendon (Figure 3).59
Am J Orthop. 2016;45(7):E503-E511. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Taylor SA, Khair MM, Gulotta LV, et al. Diagnostic glenohumeral arthroscopy fails to fully evaluate the biceps-labral complex. Arthroscopy. 2015;31(2):215-224.
2. Taylor SA, Fabricant PD, Bansal M, et al. The anatomy and histology of the bicipital tunnel of the shoulder. J Shoulder Elbow Surg. 2015;24(4):511-519.
3. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
4. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. an anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
5. Tuoheti Y, Itoi E, Minagawa H, et al. Attachment types of the long head of the biceps tendon to the glenoid labrum and their relationships with the glenohumeral ligaments. Arthroscopy. 2005;21(10):1242-1249.
6. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
7. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length-tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
8. Ahrens PM, Boileau P. The long head of biceps and associated tendinopathy. J Bone Joint Surg Br. 2007;89(8):1001-1009.
9. Hart ND, Golish SR, Dragoo JL. Effects of arm position on maximizing intra-articular visualization of the biceps tendon: a cadaveric study. Arthroscopy. 2012;28(4):481-485.
10. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
11. Cheng NM, Pan WR, Vally F, Le Roux CM, Richardson MD. The arterial supply of the long head of biceps tendon: anatomical study with implications for tendon rupture. Clin Anat. 2010;23(6):683-692.
12. Habermeyer P, Magosch P, Pritsch M, Scheibel MT, Lichtenberg S. Anterosuperior impingement of the shoulder as a result of pulley lesions: a prospective arthroscopic study. J Shoulder Elbow Surg. 2004;13(1):5-12.
13. Gohlke F, Daum P, Bushe C. The stabilizing function of the glenohumeral joint capsule. Current aspects of the biomechanics of instability [in German]. Z Orthop Ihre Grenzgeb. 1994;132(2):112-119.
14. Arai R, Mochizuki T, Yamaguchi K, et al. Functional anatomy of the superior glenohumeral and coracohumeral ligaments and the subscapularis tendon in view of stabilization of the long head of the biceps tendon. J Shoulder Elbow Surg. 2010;19(1):58-64.
15. Busconi BB, DeAngelis N, Guerrero PE. The proximal biceps tendon: tricks and pearls. Sports Med Arthrosc. 2008;16(3):187-194.
16. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
17. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2004;12(2):99-110.
18. Verma NN, Drakos M, O’Brien SJ. The arthroscopic active compression test. Arthroscopy. 2005;21(5):634.
19. Walch G, Nove-Josserand L, Levigne C, Renaud E. Tears of the supraspinatus tendon associated with “hidden” lesions of the rotator interval. J Shoulder Elbow Surg. 1994;3(6):353-360.
20. Gilmer BB, DeMers AM, Guerrero D, Reid JB 3rd, Lubowitz JH, Guttmann D. Arthroscopic versus open comparison of long head of biceps tendon visualization and pathology in patients requiring tenodesis. Arthroscopy. 2015;31(1):29-34.
21. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
22. Festa A, Allert J, Issa K, Tasto JP, Myer JJ. Visualization of the extra-articular portion of the long head of the biceps tendon during intra-articular shoulder arthroscopy. Arthroscopy. 2014;30(11):1413-1417.
23. O’Brien SJ, Newman AM, Taylor SA, et al. The accurate diagnosis of biceps-labral complex lesions with MRI and “3-pack” physical examination: a retrospective analysis with prospective validation. Orthop J Sports Med. 2013;1(4 suppl). doi:10.1177/2325967113S00018.
24. Hegedus EJ, Goode AP, Cook CE, et al. Which physical examination tests provide clinicians with the most value when examining the shoulder? Update of a systematic review with meta-analysis of individual tests. Br J Sports Med. 2012;46(14):964-978.
25. Chen HS, Lin SH, Hsu YH, Chen SC, Kang JH. A comparison of physical examinations with musculoskeletal ultrasound in the diagnosis of biceps long head tendinitis. Ultrasound Med Biol. 2011;37(9):1392-1398.
26. Taylor SA, Newman AM, Dawson C, et al. The “3-Pack” examination is critical for comprehensive evaluation of the biceps-labrum complex and the bicipital tunnel: a prospective study. Arthroscopy. 2016 Jul 20. [Epub ahead of print]
27. Gill HS, El Rassi G, Bahk MS, Castillo RC, McFarland EG. Physical examination for partial tears of the biceps tendon. Am J Sports Med. 2007;35(8):1334-1340.
28. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
29. Zanetti M, Weishaupt D, Gerber C, Hodler J. Tendinopathy and rupture of the tendon of the long head of the biceps brachii muscle: evaluation with MR arthrography. AJR Am J Roentgenol. 1998;170(6):1557-1561.
30. Taylor SA, Newman AM, Nguyen J, et al. Magnetic resonance imaging currently fails to fully evaluate the biceps-labrum complex and bicipital tunnel. Arthroscopy. 2016;32(2):238-244.
31. Malavolta EA, Assunção JH, Guglielmetti CL, de Souza FF, Gracitelli ME, Ferreira Neto AA. Accuracy of preoperative MRI in the diagnosis of disorders of the long head of the biceps tendon. Eur J Radiol. 2015;84(11):2250-2254.
32. Dubrow SA, Streit JJ, Shishani Y, Robbin MR, Gobezie R. Diagnostic accuracy in detecting tears in the proximal biceps tendon using standard nonenhancing shoulder MRI. Open Access J Sports Med. 2014;5:81-87.
33. Nourissat G, Tribot-Laspiere Q, Aim F, Radier C. Contribution of MRI and CT arthrography to the diagnosis of intra-articular tendinopathy of the long head of the biceps. Orthop Traumatol Surg Res. 2014;100(8 suppl):S391-S394.
34. De Maeseneer M, Boulet C, Pouliart N, et al. Assessment of the long head of the biceps tendon of the shoulder with 3T magnetic resonance arthrography and CT arthrography. Eur J Radiol. 2012;81(5):934-939.
35. Houtz CG, Schwartzberg RS, Barry JA, Reuss BL, Papa L. Shoulder MRI accuracy in the community setting. J Shoulder Elbow Surg. 2011;20(4):537-542.
36. Buck FM, Grehn H, Hilbe M, Pfirrmann CW, Manzanell S, Hodler J. Degeneration of the long biceps tendon: comparison of MRI with gross anatomy and histology. AJR Am J Roentgenol. 2009;193(5):1367-1375.
37. Mohtadi NG, Vellet AD, Clark ML, et al. A prospective, double-blind comparison of magnetic resonance imaging and arthroscopy in the evaluation of patients presenting with shoulder pain. J Shoulder Elbow Surg. 2004;13(3):258-265.
38. Sheridan K, Kreulen C, Kim S, Mak W, Lewis K, Marder R. Accuracy of magnetic resonance imaging to diagnose superior labrum anterior-posterior tears. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2645-2650.
39. Connolly KP, Schwartzberg RS, Reuss B, Crumbie D Jr, Homan BM. Sensitivity and specificity of noncontrast magnetic resonance imaging reports in the diagnosis of type-II superior labral anterior-posterior lesions in the community setting. J Bone Joint Surg Am. 2013;95(4):308-313.
40. Reuss BL, Schwartzberg R, Zlatkin MB, Cooperman A, Dixon JR. Magnetic resonance imaging accuracy for the diagnosis of superior labrum anterior-posterior lesions in the community setting: eighty-three arthroscopically confirmed cases. J Shoulder Elbow Surg. 2006;15(5):580-585.
41. Connell DA, Potter HG, Wickiewicz TL, Altchek DW, Warren RF. Noncontrast magnetic resonance imaging of superior labral lesions. 102 cases confirmed at arthroscopic surgery. Am J Sports Med. 1999;27(2):208-213.
42. Hashiuchi T, Sakurai G, Morimoto M, Komei T, Takakura Y, Tanaka Y. Accuracy of the biceps tendon sheath injection: ultrasound-guided or unguided injection? A randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1069-1073.
43. Hsu AR, Ghodadra NS, Provencher MT, Lewis PB, Bach BR. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20(2):326-332.
44. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
45. Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of pathologic lesions of the tendon of the long head of the biceps brachii. Am J Sports Med. 2009;37(4):828-833.
46. Kelly AM, Drakos MC, Fealy S, Taylor SA, O’Brien SJ. Arthroscopic release of the long head of the biceps tendon: functional outcome and clinical results. Am J Sports Med. 2005;33(2):208-213.
47. Berlemann U, Bayley I. Tenodesis of the long head of biceps brachii in the painful shoulder: improving results in the long term. J Shoulder Elbow Surg. 1995;4(6):429-435.
48. Gill TJ, McIrvin E, Mair SD, Hawkins RJ. Results of biceps tenotomy for treatment of pathology of the long head of the biceps brachii. J Shoulder Elbow Surg. 2001;10(3):247-249.
49. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
50. Gartsman GM, Hammerman SM. Arthroscopic biceps tenodesis: operative technique. Arthroscopy. 2000;16(5):550-552.
51. Richards DP, Burkhart SS. Arthroscopic-assisted biceps tenodesis for ruptures of the long head of biceps brachii: the cobra procedure. Arthroscopy. 2004;20(suppl 2):201-207.
52. Klepps S, Hazrati Y, Flatow E. Arthroscopic biceps tenodesis. Arthroscopy. 2002;18(9):1040-1045.
53. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.54. Werner BC, Lyons ML, Evans CL, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of restoration of length-tension and mechanical strength between techniques. Arthroscopy. 2015;31(4):620-627.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
57. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
58. Taylor SA, Fabricant PD, Baret NJ, et al. Midterm clinical outcomes for arthroscopic subdeltoid transfer of the long head of the biceps tendon to the conjoint tendon. Arthroscopy. 2014;30(12):1574-1581.
59. Drakos MC, Verma NN, Gulotta LV, et al. Arthroscopic transfer of the long head of the biceps tendon: functional outcome and clinical results. Arthroscopy. 2008;24(2):217-223.
60. Ding DY, Gupta A, Snir N, Wolfson T, Meislin RJ. Nerve proximity during bicortical drilling for subpectoral biceps tenodesis: a cadaveric study. Arthroscopy. 2014;30(8):942-946.
61. Dickens JF, Kilcoyne KG, Tintle SM, Giuliani J, Schaefer RA, Rue JP. Subpectoral biceps tenodesis: an anatomic study and evaluation of at-risk structures. Am J Sports Med. 2012;40(10):2337-2341.
62. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
63. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
64. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
65. O’Brien SJ, Taylor SA, DiPietro JR, Newman AM, Drakos MC, Voos JE. The arthroscopic “subdeltoid approach” to the anterior shoulder. J Shoulder Elbow Surg. 2013;22(4):e6-e10.
66. Urch E, Taylor SA, Ramkumar PN, et al. Biceps tenodesis: a comparison of tendon-to-bone and tendon-to-tendon healing in a rat model. Paper presented at: Closed Meeting of the American Shoulder and Elbow Surgeons; October 10, 2015; Asheville, NC. Paper 26.
67. Taylor SA, Ramkumar PN, Fabricant PD, et al. The clinical impact of bicipital tunnel decompression during long head of the biceps tendon surgery: a systematic review and meta-analysis. Arthroscopy. 2016;32(6):1155-1164.
1. Taylor SA, Khair MM, Gulotta LV, et al. Diagnostic glenohumeral arthroscopy fails to fully evaluate the biceps-labral complex. Arthroscopy. 2015;31(2):215-224.
2. Taylor SA, Fabricant PD, Bansal M, et al. The anatomy and histology of the bicipital tunnel of the shoulder. J Shoulder Elbow Surg. 2015;24(4):511-519.
3. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
4. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. an anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
5. Tuoheti Y, Itoi E, Minagawa H, et al. Attachment types of the long head of the biceps tendon to the glenoid labrum and their relationships with the glenohumeral ligaments. Arthroscopy. 2005;21(10):1242-1249.
6. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
7. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length-tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
8. Ahrens PM, Boileau P. The long head of biceps and associated tendinopathy. J Bone Joint Surg Br. 2007;89(8):1001-1009.
9. Hart ND, Golish SR, Dragoo JL. Effects of arm position on maximizing intra-articular visualization of the biceps tendon: a cadaveric study. Arthroscopy. 2012;28(4):481-485.
10. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27(4):581-592.
11. Cheng NM, Pan WR, Vally F, Le Roux CM, Richardson MD. The arterial supply of the long head of biceps tendon: anatomical study with implications for tendon rupture. Clin Anat. 2010;23(6):683-692.
12. Habermeyer P, Magosch P, Pritsch M, Scheibel MT, Lichtenberg S. Anterosuperior impingement of the shoulder as a result of pulley lesions: a prospective arthroscopic study. J Shoulder Elbow Surg. 2004;13(1):5-12.
13. Gohlke F, Daum P, Bushe C. The stabilizing function of the glenohumeral joint capsule. Current aspects of the biomechanics of instability [in German]. Z Orthop Ihre Grenzgeb. 1994;132(2):112-119.
14. Arai R, Mochizuki T, Yamaguchi K, et al. Functional anatomy of the superior glenohumeral and coracohumeral ligaments and the subscapularis tendon in view of stabilization of the long head of the biceps tendon. J Shoulder Elbow Surg. 2010;19(1):58-64.
15. Busconi BB, DeAngelis N, Guerrero PE. The proximal biceps tendon: tricks and pearls. Sports Med Arthrosc. 2008;16(3):187-194.
16. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
17. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2004;12(2):99-110.
18. Verma NN, Drakos M, O’Brien SJ. The arthroscopic active compression test. Arthroscopy. 2005;21(5):634.
19. Walch G, Nove-Josserand L, Levigne C, Renaud E. Tears of the supraspinatus tendon associated with “hidden” lesions of the rotator interval. J Shoulder Elbow Surg. 1994;3(6):353-360.
20. Gilmer BB, DeMers AM, Guerrero D, Reid JB 3rd, Lubowitz JH, Guttmann D. Arthroscopic versus open comparison of long head of biceps tendon visualization and pathology in patients requiring tenodesis. Arthroscopy. 2015;31(1):29-34.
21. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
22. Festa A, Allert J, Issa K, Tasto JP, Myer JJ. Visualization of the extra-articular portion of the long head of the biceps tendon during intra-articular shoulder arthroscopy. Arthroscopy. 2014;30(11):1413-1417.
23. O’Brien SJ, Newman AM, Taylor SA, et al. The accurate diagnosis of biceps-labral complex lesions with MRI and “3-pack” physical examination: a retrospective analysis with prospective validation. Orthop J Sports Med. 2013;1(4 suppl). doi:10.1177/2325967113S00018.
24. Hegedus EJ, Goode AP, Cook CE, et al. Which physical examination tests provide clinicians with the most value when examining the shoulder? Update of a systematic review with meta-analysis of individual tests. Br J Sports Med. 2012;46(14):964-978.
25. Chen HS, Lin SH, Hsu YH, Chen SC, Kang JH. A comparison of physical examinations with musculoskeletal ultrasound in the diagnosis of biceps long head tendinitis. Ultrasound Med Biol. 2011;37(9):1392-1398.
26. Taylor SA, Newman AM, Dawson C, et al. The “3-Pack” examination is critical for comprehensive evaluation of the biceps-labrum complex and the bicipital tunnel: a prospective study. Arthroscopy. 2016 Jul 20. [Epub ahead of print]
27. Gill HS, El Rassi G, Bahk MS, Castillo RC, McFarland EG. Physical examination for partial tears of the biceps tendon. Am J Sports Med. 2007;35(8):1334-1340.
28. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
29. Zanetti M, Weishaupt D, Gerber C, Hodler J. Tendinopathy and rupture of the tendon of the long head of the biceps brachii muscle: evaluation with MR arthrography. AJR Am J Roentgenol. 1998;170(6):1557-1561.
30. Taylor SA, Newman AM, Nguyen J, et al. Magnetic resonance imaging currently fails to fully evaluate the biceps-labrum complex and bicipital tunnel. Arthroscopy. 2016;32(2):238-244.
31. Malavolta EA, Assunção JH, Guglielmetti CL, de Souza FF, Gracitelli ME, Ferreira Neto AA. Accuracy of preoperative MRI in the diagnosis of disorders of the long head of the biceps tendon. Eur J Radiol. 2015;84(11):2250-2254.
32. Dubrow SA, Streit JJ, Shishani Y, Robbin MR, Gobezie R. Diagnostic accuracy in detecting tears in the proximal biceps tendon using standard nonenhancing shoulder MRI. Open Access J Sports Med. 2014;5:81-87.
33. Nourissat G, Tribot-Laspiere Q, Aim F, Radier C. Contribution of MRI and CT arthrography to the diagnosis of intra-articular tendinopathy of the long head of the biceps. Orthop Traumatol Surg Res. 2014;100(8 suppl):S391-S394.
34. De Maeseneer M, Boulet C, Pouliart N, et al. Assessment of the long head of the biceps tendon of the shoulder with 3T magnetic resonance arthrography and CT arthrography. Eur J Radiol. 2012;81(5):934-939.
35. Houtz CG, Schwartzberg RS, Barry JA, Reuss BL, Papa L. Shoulder MRI accuracy in the community setting. J Shoulder Elbow Surg. 2011;20(4):537-542.
36. Buck FM, Grehn H, Hilbe M, Pfirrmann CW, Manzanell S, Hodler J. Degeneration of the long biceps tendon: comparison of MRI with gross anatomy and histology. AJR Am J Roentgenol. 2009;193(5):1367-1375.
37. Mohtadi NG, Vellet AD, Clark ML, et al. A prospective, double-blind comparison of magnetic resonance imaging and arthroscopy in the evaluation of patients presenting with shoulder pain. J Shoulder Elbow Surg. 2004;13(3):258-265.
38. Sheridan K, Kreulen C, Kim S, Mak W, Lewis K, Marder R. Accuracy of magnetic resonance imaging to diagnose superior labrum anterior-posterior tears. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2645-2650.
39. Connolly KP, Schwartzberg RS, Reuss B, Crumbie D Jr, Homan BM. Sensitivity and specificity of noncontrast magnetic resonance imaging reports in the diagnosis of type-II superior labral anterior-posterior lesions in the community setting. J Bone Joint Surg Am. 2013;95(4):308-313.
40. Reuss BL, Schwartzberg R, Zlatkin MB, Cooperman A, Dixon JR. Magnetic resonance imaging accuracy for the diagnosis of superior labrum anterior-posterior lesions in the community setting: eighty-three arthroscopically confirmed cases. J Shoulder Elbow Surg. 2006;15(5):580-585.
41. Connell DA, Potter HG, Wickiewicz TL, Altchek DW, Warren RF. Noncontrast magnetic resonance imaging of superior labral lesions. 102 cases confirmed at arthroscopic surgery. Am J Sports Med. 1999;27(2):208-213.
42. Hashiuchi T, Sakurai G, Morimoto M, Komei T, Takakura Y, Tanaka Y. Accuracy of the biceps tendon sheath injection: ultrasound-guided or unguided injection? A randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1069-1073.
43. Hsu AR, Ghodadra NS, Provencher MT, Lewis PB, Bach BR. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20(2):326-332.
44. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
45. Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of pathologic lesions of the tendon of the long head of the biceps brachii. Am J Sports Med. 2009;37(4):828-833.
46. Kelly AM, Drakos MC, Fealy S, Taylor SA, O’Brien SJ. Arthroscopic release of the long head of the biceps tendon: functional outcome and clinical results. Am J Sports Med. 2005;33(2):208-213.
47. Berlemann U, Bayley I. Tenodesis of the long head of biceps brachii in the painful shoulder: improving results in the long term. J Shoulder Elbow Surg. 1995;4(6):429-435.
48. Gill TJ, McIrvin E, Mair SD, Hawkins RJ. Results of biceps tenotomy for treatment of pathology of the long head of the biceps brachii. J Shoulder Elbow Surg. 2001;10(3):247-249.
49. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
50. Gartsman GM, Hammerman SM. Arthroscopic biceps tenodesis: operative technique. Arthroscopy. 2000;16(5):550-552.
51. Richards DP, Burkhart SS. Arthroscopic-assisted biceps tenodesis for ruptures of the long head of biceps brachii: the cobra procedure. Arthroscopy. 2004;20(suppl 2):201-207.
52. Klepps S, Hazrati Y, Flatow E. Arthroscopic biceps tenodesis. Arthroscopy. 2002;18(9):1040-1045.
53. Werner BC, Pehlivan HC, Hart JM, et al. Increased incidence of postoperative stiffness after arthroscopic compared with open biceps tenodesis. Arthroscopy. 2014;30(9):1075-1084.54. Werner BC, Lyons ML, Evans CL, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of restoration of length-tension and mechanical strength between techniques. Arthroscopy. 2015;31(4):620-627.
55. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
56. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
57. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
58. Taylor SA, Fabricant PD, Baret NJ, et al. Midterm clinical outcomes for arthroscopic subdeltoid transfer of the long head of the biceps tendon to the conjoint tendon. Arthroscopy. 2014;30(12):1574-1581.
59. Drakos MC, Verma NN, Gulotta LV, et al. Arthroscopic transfer of the long head of the biceps tendon: functional outcome and clinical results. Arthroscopy. 2008;24(2):217-223.
60. Ding DY, Gupta A, Snir N, Wolfson T, Meislin RJ. Nerve proximity during bicortical drilling for subpectoral biceps tenodesis: a cadaveric study. Arthroscopy. 2014;30(8):942-946.
61. Dickens JF, Kilcoyne KG, Tintle SM, Giuliani J, Schaefer RA, Rue JP. Subpectoral biceps tenodesis: an anatomic study and evaluation of at-risk structures. Am J Sports Med. 2012;40(10):2337-2341.
62. Ma H, Van Heest A, Glisson C, Patel S. Musculocutaneous nerve entrapment: an unusual complication after biceps tenodesis. Am J Sports Med. 2009;37(12):2467-2469.
63. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
64. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
65. O’Brien SJ, Taylor SA, DiPietro JR, Newman AM, Drakos MC, Voos JE. The arthroscopic “subdeltoid approach” to the anterior shoulder. J Shoulder Elbow Surg. 2013;22(4):e6-e10.
66. Urch E, Taylor SA, Ramkumar PN, et al. Biceps tenodesis: a comparison of tendon-to-bone and tendon-to-tendon healing in a rat model. Paper presented at: Closed Meeting of the American Shoulder and Elbow Surgeons; October 10, 2015; Asheville, NC. Paper 26.
67. Taylor SA, Ramkumar PN, Fabricant PD, et al. The clinical impact of bicipital tunnel decompression during long head of the biceps tendon surgery: a systematic review and meta-analysis. Arthroscopy. 2016;32(6):1155-1164.