User login
Comprehensive and Equitable Care for Vulnerable Veterans With Integrated Palliative, Psychology, and Oncology Care
Veterans living with cancer need comprehensive assessment that includes supportive psychosocial care. The National Comprehensive Cancer Network (NCCN) and American College of Surgeons Commission on Cancer require accredited cancer centers to evaluate psychosocial distress and provide appropriate triage and treatment for all patients.1-3 Implementing psychosocial distress screening can be difficult because of procedural barriers and time constraints, clinic and supportive care resources, and lack of knowledge about how to access supportive services.
Distress screening protocols must be designed to address the specific needs of each population. To improve screening for cancer-related distress, deliver effective supportive services, and gain agreement on distress screening standards of care, the Coleman Foundation supported development of the Coleman Supportive Oncology Collaborative (CSOC), a project of 135 interdisciplinary health care professionals from 25 Chicago-area cancer care institutions.4
The Jesse Brown US Department of Veterans Affairs (VA) Medical Center (JBVAMC) was chosen to assess cancer-related concerns among veterans using the CSOC screening tool and to improve access to supportive oncology. JBVAMC provides care to approximately 49,000 veterans in Chicago, Illinois, and northwestern Indiana. The JBVAMC patient population includes a large number of veterans with dual diagnoses (co-occurring substance use and mental health disorders) and veterans experiencing homelessness.
Delivering integrated screening and oncologic care that is culture and age appropriate is particularly important for veterans given their unique risk factors. The veteran population is considered vulnerable in terms of health status, psychological functioning, and social context. Veterans who use the VA health system as a principal source of care have poorer health, greater comorbid medical conditions, and an increased risk of mortality and suicide compared with the general population.5,6 Poorer health status in veterans also may relate to old age, low income, poor education, psychological health, and minority race.7-9
Past studies point to unique risk factors for cancer and poor cancer adjustment among veterans, which may complicate cancer treatment and end-of-life/survivorship care. Veteran-specific risk factors include military-related exposures, particularly Agent Orange and morbidity/mortality secondary to comorbid medical and psychiatric conditions (eg, chronic obstructive pulmonary disease, diabetes mellitus, and posttraumatic stress disorder [PTSD]).10-12 Moreover, the geriatric veteran population continues to grow,with increasing rates of cancer that require unique considerations for effective cancer care.13,14 Despite this, there are minimal data to inform best practices and supportive care approaches for veterans with cancer. Lack of guidelines specific to veterans and other populations with increased psychosocial challenges may impede successful cancer care, making distress screening procedures particularly important. This is especially the case for the JBVAMC, which serves primarily African American urban-dwelling veterans who experience high rates of cancer disparities, including increased rates of mortality and increased levels of psychosocial distress.15,16
The goals of this program were to (1) examine levels of psychological, physical, financial, and treatment-related distress in a large sample of urban-dwelling veterans; (2) create a streamlined, sustainable process to screen a large number of veterans receiving cancer care in the outpatient setting and connect them with available supportive services; and (3) educate oncology physicians, nurses, and other staff about cancer-related distress and concerns using in-service trainings and interpersonal interactions to improve patient care. Our program was based on a Primary Care Mental Health Integration (PCMHI) model that embeds health psychologists in general medical clinics to better reach veterans dealing with mental health issues. We tailored for palliative care involvement.
Studies of this model have shown that mental health integration improves access to mental health services and mental health treatment outcomes and has higher patient and provider satisfaction.17 We were also influenced by the construct of the patient aligned care team (PACT) social worker who, in this veteran-centered approach, often functions as a care coordinator. Social work responsibilities include assessment of patients’ stressors including adjusting to the medical conditions, identifying untreated or undertreated mental health or substance abuse issues, economic instability, legal problems, and inadequate housing and transportation, which can often be exacerbated during cancer treatment.18
We screened for distress-related needs that included mental health concerns, physical needs including uncontrolled symptoms or adverse effects of cancer treatment, physical function complaints (eg, pain and fatigue), nutrition concerns, treatment or care related concerns, family and caregiver needs, along with financial challenges (housing and food) and insurance-related support. The goal of this article is to describe the development and implementation of this VA-specific distress screening program and reflect on the lessons learned for the application of streamlined distress screening and triage in similar settings throughout the VA health system and other similar settings.
Methods
This institutional review board at JBVAMC reviewed and exempted this quality improvement program using the SQUIRE framework.19 It was led by a group of palliative care clinicians, psychologists, and administrators who have worked with the oncology service for many years, primarily in the care of hospitalized patients. Common palliative care services include providing care for patients with serious illness diagnosis through the illness trajectory.
Setting
At the start of this program, we assessed the current clinic workflow to determine how to best screen and assist veterans experiencing distress. We met with team members individually to identify the best method of clinic integration, including attending medical oncologists, medical oncology fellows, psychology interns, oncology nursing staff, the oncology nurse coordinator, and clinic clerks.
The JBVAMC provides cancer care through 4 half-day medical hematology-oncology clinics that serve about 50 patients per half-day clinic. The clinics are staffed by hematology-oncology fellows supervised by hematology-oncology attending physicians, who are affiliated with 2 academic medical centers. These clinics are staffed by 3 registered nurses (RNs) and a licensed practical nurse (LPN) and are adjacent to a chemotherapy infusion clinic with unique nursing staff. The JBVAMC also provides a variety of supportive care services, including extensive mental health and substance use treatment, physical and occupational therapy, acupuncture, nutrition, social work, and housing services. Following our assessment, it was evident that there were a low number of referrals from oncology clinics to supportive care services, mostly due to lack of knowledge of resources and unclear referral procedures.
Based on clinical volume, we determined that our screening program could best be implemented through a stepped approach beginning in one clinic and expanding thereafter. We began by having a palliative care physician and health psychology intern embedded in 1 weekly half-day clinic and a health psychology intern embedded in a second weekly half-day clinic. Our program included 2 health psychology interns (for each academic year of the program) who were supervised by a JBVA health psychologist.
About 15 months after successful integration within the first 2 half-day clinics, we expanded the screening program to staff an additional half-day medical oncology clinic with a palliative care APRN. This allowed us to expand the screening tool distribution and collection to 3 of 4 of the weekly half-day oncology clinics as well as to meet individually with veterans experiencing high levels of distress. Veterans were flagged as having high distress levels by either the results of their completed screening tool or by referral from a medical oncology physician. We initially established screening in clinics that were sufficiently staffed to ensure that screens were appropriately distributed and reviewed. Patients seen in nonparticipating clinics were referred to outpatient social work, mental health and/or outpatient palliative care according to oncology fellows’ clinical assessments of the patient. All oncology fellows received education about distress screening and methods for referring to supportive care. Our clinic screening program extended from February 2017 through January 2020.
Screening
Program staff screened patients with new cancer diagnoses, then identified patients for follow-up screens. This tracking allowed staff to identify patients with oncology appointments that day and cross-reference patients needing a follow-up screen.
Following feedback from the clinic nurses, we determined that nurses would provide the distress tool to patients in paper form after they completed their assessment of vitals and waited to be seen by their medical oncologist. The patient would then deliver their completed form to the nurse who would combine it with the patient’s clinic notes for the oncologist to review.
Veterans referred for supportive care services were contacted by the relevant clinical administrator by phone to schedule an intake; for social work referrals, patients were either seen in a walk-in office located in a colocated building or contacted by a social worker by phone.
Our screening tool was the Coleman Foundation Supportive Oncology Collaborative Screening Tool, compiled from validated instruments. Patients completed this screening tool, which includes the PHQ-4, NCCN problem list concerns, adapted Mini Nutrition Assessment and PROMIS Pain and Fatigue measure (eAppendix B available at doi:10.12788/fp.0158).20-22
We also worked with the VA Computerized Patient Record System (CPRS) to create an electronic template for the screening tool. Completed screening tools were manually entered by the physician, psychologists, or APRN into the CPRS chart.
We analyzed the different supportive care services available at the JBVAMC and noticed that many supportive services were available, yet these services were often separated. Therefore, we created a consult flowsheet to assist oncologists in placing referrals. These supportive care services include mental health services, a cancer support group, home health care, social services, nutrition, physical medicine and rehabilitation, and other specialty services.
Patient Education
The psychology and nursing staff created a patient information bulletin board where patients could access information about supportive services available at JBVAMC. This board required frequent replenishment of handouts because patients consulted the board regularly. Handouts and folders about common clinical issues also were placed in the clinic treatment rooms. We partnered with 2 local cancer support centers, Gilda’s Club and the Cancer Support Center, to make referrals for family members and/or caregivers who would benefit from additional support.
We provided in-service trainings for oncology fellows, including trainings on PTSD and substance abuse and their relationship to cancer care at the VA. These topics were chosen based on the feedback program staff received about perceived knowledge gaps from the oncology fellows. This program allowed for multiple informal conversations between that program staff and oncology fellows about overall patient care. We held trainings with the cancer coordinator and clinical nursing staff on strategies to identify and follow-up on cancer-related distress, and with oncology fellows to review the importance of distress screening and to instruct fellows on instructions for the consult flowsheet.
Funding
This program was funded by the Chicago-based Coleman Foundation as part of the CSOC. Funding was used to support a portion of time for administrative and clinical work of program staff, as well as data collection and analysis.
Results
We established 3 half-day integrated clinics where patients were screened and referred for services based on supportive oncology needs. In addition to our primary activities to screen and refer veterans, we held multiple educational sessions for colleagues, developed a workflow template, and integrated patient education materials into the clinics.
Screening
Veterans completed 1010 distress screens in 3 of 4 half-day oncology clinics over the 2.5-year project period. Veterans were screened at initial diagnosis and every 3 months, or during changes in their clinical care or disease status. As a result, 579 patients completed screening, with some patients doing several follow-up screens during their care. Integration of palliative care providers and health psychologists was instrumental in facilitating screening in these busy general medical oncology clinics. Most veterans were receptive to completing surveys with few refusing to fill out the survey.23 Medical oncology fellows often used the completed screener to inform their review of systems (by reviewing the Coleman screener Physical and Other Concerns section) and connect with the supportive care staff present in clinic for patient’s identifying severe needs (ie, mental health distress or complex psychosocial needs). Veterans’ rates of distress needs and successfuloutcomes of integration with mental health and social work services have been reported elsewhere.23
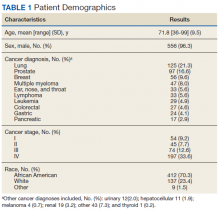
The mean (SD) age for veterans in this cohort was 72 (9.5) years. Participants were primarily African American veterans (70%), with mostly advanced disease (Table 1). Participants endorsed elevated distress needs compared with other patient populations screened in Chicago through the CSOC for depressed mood, pain, housing, transportation, and physical, nutrition, and treatment concerns.23 Elevated presence of needs was especially prominent for food, housing and insurance/medical needs; physical concerns; nutrition, and treatment- or care-related concerns. Veterans in this cohort reported extensive financial and housing concerns: 10.4% reported food and housing concerns, 18.6% reported transportation concerns, and 9.0% reported issues paying for medical care or medications (Table 2).20 Anecdotally, many experienced job loss or strain with their cancer diagnosis or were living at the poverty level before their diagnosis.
Social work referrals were often triggered due to transportation barriers to appointments/medication access, and food and/or housing insecurity. Social workers assisted with referrals for housing, transportation, financial reimbursement, on-site or community-based food banks, home health support, familial support, and hospice services. Social work consults increased 166% from 2016 (the year before the program start date) to the end of 2019.
Based on this increased volume of referrals for social work in our oncology clinics, an oncology-specific social worker was hired at the completion of our program to be based in all 4 half-day oncology clinics in response to results of our quality improvement intervention. The social worker currently sees all patients with a new cancer diagnosis and supports oncology fellows to identify veterans needing a palliative care referral or referrals to other supportive services.
Throughout program implementation, traditional areas of palliative care focus were particularly important as veterans reported significant concerns with understanding their illness (67.4%), wanting to understand their prognosis (71.3%), and having questions about their treatment options (55.1%).20 The palliative care providers spent time educating patients about their disease, coordinating goals of care conversations, promoting patients’ engagement in decision making, and making a large number of referrals to hospice and home health to support veterans at home.
Discussion
This project created a successful program to screen veterans for psychosocial distress and triage them to appropriate services. During the project, patients in VA-outpatient oncology clinics reported significant cancer-related distress due to baseline psychosocial needs, changes in emotional and physical functioning, logistical and financial challenges of receiving cancer care, and lack of instrumental support.23
Staff education supported successful buy-in, development and implementation of supportive oncology programs. We used a combination of in-service trainings, online trainings, and handouts to provide evidence for distress screening.24 Highlighting the evidence-base that demonstrates how cancer-related distress screening improves cancer and quality of life outcomes helped to address physician reluctance to accept the additional requirements needed to address veterans’ psychosocial needs and care concerns. To increase buy-in and collaboration among team members and foster heightened understanding between providers and patients, we recommend creating accessible education for all staff levels.
One specific area of education we focused on was primary palliative care, which includes the core competencies of communication and symptom management recommended for generalists and specialists of all disciplines.25 Program staff supported oncology fellows in developing their primary palliative care skills by being available to discuss basic symptom management and communication issues. VA cancer care programs could benefit from ongoing palliative care education of oncology staff to facilitate primary palliative care as well as earlier integration of secondary palliative care when needed.26 Secondary palliative care or care provided directly by the palliative care team assists with complex symptom management or communication issues. For these needs, oncology fellows were encouraged to refer to either the palliative care staff available in one of the half-day clinics or to the outpatient palliative care clinic. As a unique strength, the VA allows veterans to receive concurrent cancer-directed therapy and hospice care, which enables earlier referrals to hospice care and higher quality end-of-life care and emphasizes the need for primary palliative care in oncology.27,28
Integrating supportive oncology team members, such as licensed clinical social worker and psychology interns, was successful. This was modeled on the VA PACT, which focuses on prevention, health promotion, coordination and chronic disease management.29 Social determinants of health have a major impact on health outcomes especially in veteran-specific and African American populations, making screening for distress critical.30-32 The VA Office of Health Equity actively addresses health inequities by supporting initiation of screening programs for social determinants of health, including education, employment, exposure to abuse and violence, food insecurity, housing instability, legal needs, social isolation, transportation needs, and utility needs. This is especially needed for African-American individuals who are not only more likely to experience cancer, but also more likely to be negatively impacted by the consequences of cancer diagnosis/treatment, such as complications related to one’s job security, access to care, adverse effects, and other highly distressing needs.33,34
Our program found that veterans with cancer often had concerns associated with food and housing insecurity, transportation and paying for medication or medical care, and screening allowed health care providers to detect and address these social determinants of health through referrals to VA and community-specific programs. Social workers integrated
Our ability to roll out distress screening was scaffolded by technological integration into existing VA systems (eg, screening results in CPRS and electronic referrals). Screening procedures could have been even more efficient with improved technology (Table 3). For example, technological limitations made it challenging to easily identify patients due for screening, requiring a cumbersome process of tracking, collecting and entering patients’ paper forms. Health care providers seeking to develop a distress screening program should consider investing in technology that allows for identification of patients requiring screening at a predetermined interval, completion of screening via tablet or personal device, integration of screening responses into the electronic health record, and automatic generation of notifications to the treating physician and appropriate support services.
We also established partnerships with community cancer support groups to offer both referral pathways and in-house programming. Veterans’ cancer care programs could benefit from identifying and securing community partnerships to capitalize on readily available low-cost or no-cost options for supportive oncology in the community. Further, as was the case in our program, cancer support centers may be willing to collaborate with VA hospitals to provide services on site (eg, support groups, art therapy). This would extend the reach of these supportive services while allowing VA employees to address the extensive psychosocial needs of individual veterans.
Conclusions
Veterans with cancer benefited from enhanced screening and psychosocial service availability, similar to a PCMHI model. Robust screening programs helped advocate for veterans dealing with the effects of poverty through identification of need and referral to existing VA programs and services quickly and efficiently. Providing comprehensive care within ambulatory cancer clinics can address cancer-related distress and any potential barriers to care in real time. VA hospitals typically offer an array of supportive services to address veterans’ psychosocial needs, yet these services tend to be siloed. Integrated referrals can help to resolve such access barriers. Since many veterans with burdensome cancers are not able to see their VA primary care physician regularly, offering comprehensive care within medical oncology ensures complete and integrated care that includes psychosocial screening.
We believe that this program is an example of a mechanism for oncologists and palliative care clinicians to integrate their care in a way that identifies needs and triages services for vulnerable veterans. As colleagues have written, “it is fundamental to our commitment to veterans that we ensure comparable, high quality care regardless of a veteran’s gender, race, or where they live.”34 Health care providers may underestimate the extensive change a cancer diagnosis can have on a patient’s quality of life. Cancer diagnosis and treatment have a large impact on all individuals, but this impact may be greater for individuals in poverty due to inability to work from home, inflexible work hours, and limited support structures. By creating screening programs with psychosocial integration in oncology clinics such as we have described, we hope to improve access to more equitable care for vulnerable veterans.
1. National Comprehensive Cancer Network. NCCN guidelines distress management. Version 2.2021. Updated January 5, 2021. Accessed July 8, 2021. http://www.nccn.org/professionals/physician_gls/pdf/distress.pdf
2. American College of Surgeons, Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care. Version 1.2.1. Published 2021. Accessed July 8, 2021. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx
3. Jacobsen PB, Ransom S. Implementation of NCCN distress management guidelines by member institutions. J Natl Compr Canc Netw. 2007;5(1):99-103. doi:10.6004/jnccn.2007.0010
4. The Coleman Supportive Oncology Collaborative. Training tools. Accessed July 14, 2021. https://www.supportiveoncologycollaborative.org/training-tools
5. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
6. Bullman T, Schneiderman A, Gradus JL. Relative importance of posttraumatic stress disorder and depression in predicting risk of suicide among a cohort of Vietnam veterans. Suicide Life Threat Behav. 2019;49(3):838-845. doi:10.1111/sltb.12482
7. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
8. O’Toole BI, Marshall RP, Grayson DA, et al. The Australian Vietnam Veterans Health Study: III. Psychological health of Australian Vietnam veterans and its relationship to combat. Int J Epidemiol. 1996;25(2):331-340. doi:10.1093/ije/25.2.331
9. Vincent C, Chamberlain K, Long N. Mental and physical health status in a community sample of New Zealand Vietnam War veterans. Aust J Public Health. 1994;18(1):58-62. doi:10.1111/j.1753-6405.1994.tb00196.x
10. US Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Updated June 16, 2021. Accessed July 8, 2021. http://www.publichealth.va.gov/exposures/agentorange/diseases.asp#veterans
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in Veterans with hepatocellular carcinoma. HBP (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Saha S, Freeman M, Toure J, Tippens KM, Weeks C, Ibrahim S. Racial and ethnic disparities in the VA health care system: a systematic review. J Gen Intern Med. 2008;23(5):654-671. doi:10.1007/s11606-008-0521-4
13. Amaral EFL, Pollard MS, Mendelsohn J, Cefalu M. Current and future demographics of the veteran population, 2014-2024. Popul Rev. 2018;57(1):28-60. doi:10.1353/prv.2018.0002
14. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
15. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212-236. doi:10.3322/caac.20121
16. Cimino T, Said K, Safier L, Harris H, Kinderman A. Psychosocial distress among oncology patients in the safety net. Psychooncology. 2020;29(11):1927-1935. doi:10.1002/pon.5525
17. Molander R, Hodgkins K, Johnson C, White A, Frazier E, Krahn D. Interprofessional education in patient aligned care team primary care-mental health integration. Fed Pract. 2017;34(6):40-48.
18. Parikh DA, Ragavan M, Dutta R, et al. Financial toxicity of cancer care: an analysis of financial burden in three distinct health care systems [published online ahead of print, 2021 Apr 7]. JCO Oncol Pract. 2021;OP2000890. doi:10.1200/OP.20.00890
19. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
20. Weldon CB, Gerhart JI, Penedo FJ, et al. Correlates of distress for cancer patients: results from multi-institution use of holistic patient-reported screening tool. J Clin Oncol. 2019;37(15)(suppl):11587-11587. doi:10.1200/JCO.2019.37.15_suppl.11587
21. Kroenke K, Spitzer RL, Williams JB, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359. doi:10.1016/j.genhosppsych.2010.03.006
22. Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782-788. doi:10.1007/s12603-009-0214-7
23. Azizoddin DR, Lakin JR, Hauser J, et al. Meeting the guidelines: implementing a distress screening intervention for veterans with cancer. Psychooncology. 2020;29(12):2067-2074. doi:10.1002/pon.5565
24. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177. doi:10.1200/JCO.2011.39.5509
25. Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. doi:10.1056/NEJMp1215620
26. Weissman DE, Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17-23. doi:10.1089/jpm.2010.0347
27. Kumar P, Wright AA, Hatfield LA, Temel JS, Keating NL. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35(4):432-439. doi:10.1200/JCO.2016.68.9257
28. Mor V, Joyce NR, Coté DL, et al. The rise of concurrent care for veterans with advanced cancer at the end of life. Cancer. 2016;122(5):782-790. doi:10.1002/cncr.29827
29. US Department of Veterans Affairs. Patient care services: Patient aligned care team (PACT). Updated November 5, 2020. Accessed July 8, 2021. https://www.patientcare.va.gov/primarycare/PACT.asp
30. US Department of Veterans Affairs, Veterans Health Administration. VHA health equity action plan. Published September 27, 2019. Accessed July 8, 2021. https://www.va.gov/HEALTHEQUITY/docs/Health_Equity_Action_Plan_Final_022020.pdf
31. Alcaraz KI, Wiedt TL, Daniels EC, Yabroff KR, Guerra CE, Wender RC. Understanding and addressing social determinants to advance cancer health equity in the United States: a blueprint for practice, research, and policy. CA Cancer J Clin. 2020;70(1):31-46. doi:10.3322/caac.21586
32. Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we’ve come far but aren’t there yet. Am J Public Health. 2014;104(suppl 4):S525-526. doi:10.2105/AJPH.2014.302216
33. American Cancer Society. Cancer Facts & Figures for African Americans 2019-2021. Atlanta: American Cancer Society; 2019.
34. Hastert TA, Kirchhoff AC, Banegas MP, et al. Work changes and individual, cancer-related, and work-related predictors of decreased work participation among African American cancer survivors. Cancer Med. 2020;9(23):9168-9177. doi:10.1002/cam4.3512
35. Bekelman DB, Nowels CT, Allen LA, Shakar S, Kutner JS, Matlock DD. Outpatient palliative care for chronic heart failure: a case series. J Palliat Med. 2011;14(7):815-821. doi:10.1089/jpm.2010.050
Veterans living with cancer need comprehensive assessment that includes supportive psychosocial care. The National Comprehensive Cancer Network (NCCN) and American College of Surgeons Commission on Cancer require accredited cancer centers to evaluate psychosocial distress and provide appropriate triage and treatment for all patients.1-3 Implementing psychosocial distress screening can be difficult because of procedural barriers and time constraints, clinic and supportive care resources, and lack of knowledge about how to access supportive services.
Distress screening protocols must be designed to address the specific needs of each population. To improve screening for cancer-related distress, deliver effective supportive services, and gain agreement on distress screening standards of care, the Coleman Foundation supported development of the Coleman Supportive Oncology Collaborative (CSOC), a project of 135 interdisciplinary health care professionals from 25 Chicago-area cancer care institutions.4
The Jesse Brown US Department of Veterans Affairs (VA) Medical Center (JBVAMC) was chosen to assess cancer-related concerns among veterans using the CSOC screening tool and to improve access to supportive oncology. JBVAMC provides care to approximately 49,000 veterans in Chicago, Illinois, and northwestern Indiana. The JBVAMC patient population includes a large number of veterans with dual diagnoses (co-occurring substance use and mental health disorders) and veterans experiencing homelessness.
Delivering integrated screening and oncologic care that is culture and age appropriate is particularly important for veterans given their unique risk factors. The veteran population is considered vulnerable in terms of health status, psychological functioning, and social context. Veterans who use the VA health system as a principal source of care have poorer health, greater comorbid medical conditions, and an increased risk of mortality and suicide compared with the general population.5,6 Poorer health status in veterans also may relate to old age, low income, poor education, psychological health, and minority race.7-9
Past studies point to unique risk factors for cancer and poor cancer adjustment among veterans, which may complicate cancer treatment and end-of-life/survivorship care. Veteran-specific risk factors include military-related exposures, particularly Agent Orange and morbidity/mortality secondary to comorbid medical and psychiatric conditions (eg, chronic obstructive pulmonary disease, diabetes mellitus, and posttraumatic stress disorder [PTSD]).10-12 Moreover, the geriatric veteran population continues to grow,with increasing rates of cancer that require unique considerations for effective cancer care.13,14 Despite this, there are minimal data to inform best practices and supportive care approaches for veterans with cancer. Lack of guidelines specific to veterans and other populations with increased psychosocial challenges may impede successful cancer care, making distress screening procedures particularly important. This is especially the case for the JBVAMC, which serves primarily African American urban-dwelling veterans who experience high rates of cancer disparities, including increased rates of mortality and increased levels of psychosocial distress.15,16
The goals of this program were to (1) examine levels of psychological, physical, financial, and treatment-related distress in a large sample of urban-dwelling veterans; (2) create a streamlined, sustainable process to screen a large number of veterans receiving cancer care in the outpatient setting and connect them with available supportive services; and (3) educate oncology physicians, nurses, and other staff about cancer-related distress and concerns using in-service trainings and interpersonal interactions to improve patient care. Our program was based on a Primary Care Mental Health Integration (PCMHI) model that embeds health psychologists in general medical clinics to better reach veterans dealing with mental health issues. We tailored for palliative care involvement.
Studies of this model have shown that mental health integration improves access to mental health services and mental health treatment outcomes and has higher patient and provider satisfaction.17 We were also influenced by the construct of the patient aligned care team (PACT) social worker who, in this veteran-centered approach, often functions as a care coordinator. Social work responsibilities include assessment of patients’ stressors including adjusting to the medical conditions, identifying untreated or undertreated mental health or substance abuse issues, economic instability, legal problems, and inadequate housing and transportation, which can often be exacerbated during cancer treatment.18
We screened for distress-related needs that included mental health concerns, physical needs including uncontrolled symptoms or adverse effects of cancer treatment, physical function complaints (eg, pain and fatigue), nutrition concerns, treatment or care related concerns, family and caregiver needs, along with financial challenges (housing and food) and insurance-related support. The goal of this article is to describe the development and implementation of this VA-specific distress screening program and reflect on the lessons learned for the application of streamlined distress screening and triage in similar settings throughout the VA health system and other similar settings.
Methods
This institutional review board at JBVAMC reviewed and exempted this quality improvement program using the SQUIRE framework.19 It was led by a group of palliative care clinicians, psychologists, and administrators who have worked with the oncology service for many years, primarily in the care of hospitalized patients. Common palliative care services include providing care for patients with serious illness diagnosis through the illness trajectory.
Setting
At the start of this program, we assessed the current clinic workflow to determine how to best screen and assist veterans experiencing distress. We met with team members individually to identify the best method of clinic integration, including attending medical oncologists, medical oncology fellows, psychology interns, oncology nursing staff, the oncology nurse coordinator, and clinic clerks.
The JBVAMC provides cancer care through 4 half-day medical hematology-oncology clinics that serve about 50 patients per half-day clinic. The clinics are staffed by hematology-oncology fellows supervised by hematology-oncology attending physicians, who are affiliated with 2 academic medical centers. These clinics are staffed by 3 registered nurses (RNs) and a licensed practical nurse (LPN) and are adjacent to a chemotherapy infusion clinic with unique nursing staff. The JBVAMC also provides a variety of supportive care services, including extensive mental health and substance use treatment, physical and occupational therapy, acupuncture, nutrition, social work, and housing services. Following our assessment, it was evident that there were a low number of referrals from oncology clinics to supportive care services, mostly due to lack of knowledge of resources and unclear referral procedures.
Based on clinical volume, we determined that our screening program could best be implemented through a stepped approach beginning in one clinic and expanding thereafter. We began by having a palliative care physician and health psychology intern embedded in 1 weekly half-day clinic and a health psychology intern embedded in a second weekly half-day clinic. Our program included 2 health psychology interns (for each academic year of the program) who were supervised by a JBVA health psychologist.
About 15 months after successful integration within the first 2 half-day clinics, we expanded the screening program to staff an additional half-day medical oncology clinic with a palliative care APRN. This allowed us to expand the screening tool distribution and collection to 3 of 4 of the weekly half-day oncology clinics as well as to meet individually with veterans experiencing high levels of distress. Veterans were flagged as having high distress levels by either the results of their completed screening tool or by referral from a medical oncology physician. We initially established screening in clinics that were sufficiently staffed to ensure that screens were appropriately distributed and reviewed. Patients seen in nonparticipating clinics were referred to outpatient social work, mental health and/or outpatient palliative care according to oncology fellows’ clinical assessments of the patient. All oncology fellows received education about distress screening and methods for referring to supportive care. Our clinic screening program extended from February 2017 through January 2020.
Screening
Program staff screened patients with new cancer diagnoses, then identified patients for follow-up screens. This tracking allowed staff to identify patients with oncology appointments that day and cross-reference patients needing a follow-up screen.
Following feedback from the clinic nurses, we determined that nurses would provide the distress tool to patients in paper form after they completed their assessment of vitals and waited to be seen by their medical oncologist. The patient would then deliver their completed form to the nurse who would combine it with the patient’s clinic notes for the oncologist to review.
Veterans referred for supportive care services were contacted by the relevant clinical administrator by phone to schedule an intake; for social work referrals, patients were either seen in a walk-in office located in a colocated building or contacted by a social worker by phone.
Our screening tool was the Coleman Foundation Supportive Oncology Collaborative Screening Tool, compiled from validated instruments. Patients completed this screening tool, which includes the PHQ-4, NCCN problem list concerns, adapted Mini Nutrition Assessment and PROMIS Pain and Fatigue measure (eAppendix B available at doi:10.12788/fp.0158).20-22
We also worked with the VA Computerized Patient Record System (CPRS) to create an electronic template for the screening tool. Completed screening tools were manually entered by the physician, psychologists, or APRN into the CPRS chart.
We analyzed the different supportive care services available at the JBVAMC and noticed that many supportive services were available, yet these services were often separated. Therefore, we created a consult flowsheet to assist oncologists in placing referrals. These supportive care services include mental health services, a cancer support group, home health care, social services, nutrition, physical medicine and rehabilitation, and other specialty services.
Patient Education
The psychology and nursing staff created a patient information bulletin board where patients could access information about supportive services available at JBVAMC. This board required frequent replenishment of handouts because patients consulted the board regularly. Handouts and folders about common clinical issues also were placed in the clinic treatment rooms. We partnered with 2 local cancer support centers, Gilda’s Club and the Cancer Support Center, to make referrals for family members and/or caregivers who would benefit from additional support.
We provided in-service trainings for oncology fellows, including trainings on PTSD and substance abuse and their relationship to cancer care at the VA. These topics were chosen based on the feedback program staff received about perceived knowledge gaps from the oncology fellows. This program allowed for multiple informal conversations between that program staff and oncology fellows about overall patient care. We held trainings with the cancer coordinator and clinical nursing staff on strategies to identify and follow-up on cancer-related distress, and with oncology fellows to review the importance of distress screening and to instruct fellows on instructions for the consult flowsheet.
Funding
This program was funded by the Chicago-based Coleman Foundation as part of the CSOC. Funding was used to support a portion of time for administrative and clinical work of program staff, as well as data collection and analysis.
Results
We established 3 half-day integrated clinics where patients were screened and referred for services based on supportive oncology needs. In addition to our primary activities to screen and refer veterans, we held multiple educational sessions for colleagues, developed a workflow template, and integrated patient education materials into the clinics.
Screening
Veterans completed 1010 distress screens in 3 of 4 half-day oncology clinics over the 2.5-year project period. Veterans were screened at initial diagnosis and every 3 months, or during changes in their clinical care or disease status. As a result, 579 patients completed screening, with some patients doing several follow-up screens during their care. Integration of palliative care providers and health psychologists was instrumental in facilitating screening in these busy general medical oncology clinics. Most veterans were receptive to completing surveys with few refusing to fill out the survey.23 Medical oncology fellows often used the completed screener to inform their review of systems (by reviewing the Coleman screener Physical and Other Concerns section) and connect with the supportive care staff present in clinic for patient’s identifying severe needs (ie, mental health distress or complex psychosocial needs). Veterans’ rates of distress needs and successfuloutcomes of integration with mental health and social work services have been reported elsewhere.23
The mean (SD) age for veterans in this cohort was 72 (9.5) years. Participants were primarily African American veterans (70%), with mostly advanced disease (Table 1). Participants endorsed elevated distress needs compared with other patient populations screened in Chicago through the CSOC for depressed mood, pain, housing, transportation, and physical, nutrition, and treatment concerns.23 Elevated presence of needs was especially prominent for food, housing and insurance/medical needs; physical concerns; nutrition, and treatment- or care-related concerns. Veterans in this cohort reported extensive financial and housing concerns: 10.4% reported food and housing concerns, 18.6% reported transportation concerns, and 9.0% reported issues paying for medical care or medications (Table 2).20 Anecdotally, many experienced job loss or strain with their cancer diagnosis or were living at the poverty level before their diagnosis.
Social work referrals were often triggered due to transportation barriers to appointments/medication access, and food and/or housing insecurity. Social workers assisted with referrals for housing, transportation, financial reimbursement, on-site or community-based food banks, home health support, familial support, and hospice services. Social work consults increased 166% from 2016 (the year before the program start date) to the end of 2019.
Based on this increased volume of referrals for social work in our oncology clinics, an oncology-specific social worker was hired at the completion of our program to be based in all 4 half-day oncology clinics in response to results of our quality improvement intervention. The social worker currently sees all patients with a new cancer diagnosis and supports oncology fellows to identify veterans needing a palliative care referral or referrals to other supportive services.
Throughout program implementation, traditional areas of palliative care focus were particularly important as veterans reported significant concerns with understanding their illness (67.4%), wanting to understand their prognosis (71.3%), and having questions about their treatment options (55.1%).20 The palliative care providers spent time educating patients about their disease, coordinating goals of care conversations, promoting patients’ engagement in decision making, and making a large number of referrals to hospice and home health to support veterans at home.
Discussion
This project created a successful program to screen veterans for psychosocial distress and triage them to appropriate services. During the project, patients in VA-outpatient oncology clinics reported significant cancer-related distress due to baseline psychosocial needs, changes in emotional and physical functioning, logistical and financial challenges of receiving cancer care, and lack of instrumental support.23
Staff education supported successful buy-in, development and implementation of supportive oncology programs. We used a combination of in-service trainings, online trainings, and handouts to provide evidence for distress screening.24 Highlighting the evidence-base that demonstrates how cancer-related distress screening improves cancer and quality of life outcomes helped to address physician reluctance to accept the additional requirements needed to address veterans’ psychosocial needs and care concerns. To increase buy-in and collaboration among team members and foster heightened understanding between providers and patients, we recommend creating accessible education for all staff levels.
One specific area of education we focused on was primary palliative care, which includes the core competencies of communication and symptom management recommended for generalists and specialists of all disciplines.25 Program staff supported oncology fellows in developing their primary palliative care skills by being available to discuss basic symptom management and communication issues. VA cancer care programs could benefit from ongoing palliative care education of oncology staff to facilitate primary palliative care as well as earlier integration of secondary palliative care when needed.26 Secondary palliative care or care provided directly by the palliative care team assists with complex symptom management or communication issues. For these needs, oncology fellows were encouraged to refer to either the palliative care staff available in one of the half-day clinics or to the outpatient palliative care clinic. As a unique strength, the VA allows veterans to receive concurrent cancer-directed therapy and hospice care, which enables earlier referrals to hospice care and higher quality end-of-life care and emphasizes the need for primary palliative care in oncology.27,28
Integrating supportive oncology team members, such as licensed clinical social worker and psychology interns, was successful. This was modeled on the VA PACT, which focuses on prevention, health promotion, coordination and chronic disease management.29 Social determinants of health have a major impact on health outcomes especially in veteran-specific and African American populations, making screening for distress critical.30-32 The VA Office of Health Equity actively addresses health inequities by supporting initiation of screening programs for social determinants of health, including education, employment, exposure to abuse and violence, food insecurity, housing instability, legal needs, social isolation, transportation needs, and utility needs. This is especially needed for African-American individuals who are not only more likely to experience cancer, but also more likely to be negatively impacted by the consequences of cancer diagnosis/treatment, such as complications related to one’s job security, access to care, adverse effects, and other highly distressing needs.33,34
Our program found that veterans with cancer often had concerns associated with food and housing insecurity, transportation and paying for medication or medical care, and screening allowed health care providers to detect and address these social determinants of health through referrals to VA and community-specific programs. Social workers integrated
Our ability to roll out distress screening was scaffolded by technological integration into existing VA systems (eg, screening results in CPRS and electronic referrals). Screening procedures could have been even more efficient with improved technology (Table 3). For example, technological limitations made it challenging to easily identify patients due for screening, requiring a cumbersome process of tracking, collecting and entering patients’ paper forms. Health care providers seeking to develop a distress screening program should consider investing in technology that allows for identification of patients requiring screening at a predetermined interval, completion of screening via tablet or personal device, integration of screening responses into the electronic health record, and automatic generation of notifications to the treating physician and appropriate support services.
We also established partnerships with community cancer support groups to offer both referral pathways and in-house programming. Veterans’ cancer care programs could benefit from identifying and securing community partnerships to capitalize on readily available low-cost or no-cost options for supportive oncology in the community. Further, as was the case in our program, cancer support centers may be willing to collaborate with VA hospitals to provide services on site (eg, support groups, art therapy). This would extend the reach of these supportive services while allowing VA employees to address the extensive psychosocial needs of individual veterans.
Conclusions
Veterans with cancer benefited from enhanced screening and psychosocial service availability, similar to a PCMHI model. Robust screening programs helped advocate for veterans dealing with the effects of poverty through identification of need and referral to existing VA programs and services quickly and efficiently. Providing comprehensive care within ambulatory cancer clinics can address cancer-related distress and any potential barriers to care in real time. VA hospitals typically offer an array of supportive services to address veterans’ psychosocial needs, yet these services tend to be siloed. Integrated referrals can help to resolve such access barriers. Since many veterans with burdensome cancers are not able to see their VA primary care physician regularly, offering comprehensive care within medical oncology ensures complete and integrated care that includes psychosocial screening.
We believe that this program is an example of a mechanism for oncologists and palliative care clinicians to integrate their care in a way that identifies needs and triages services for vulnerable veterans. As colleagues have written, “it is fundamental to our commitment to veterans that we ensure comparable, high quality care regardless of a veteran’s gender, race, or where they live.”34 Health care providers may underestimate the extensive change a cancer diagnosis can have on a patient’s quality of life. Cancer diagnosis and treatment have a large impact on all individuals, but this impact may be greater for individuals in poverty due to inability to work from home, inflexible work hours, and limited support structures. By creating screening programs with psychosocial integration in oncology clinics such as we have described, we hope to improve access to more equitable care for vulnerable veterans.
Veterans living with cancer need comprehensive assessment that includes supportive psychosocial care. The National Comprehensive Cancer Network (NCCN) and American College of Surgeons Commission on Cancer require accredited cancer centers to evaluate psychosocial distress and provide appropriate triage and treatment for all patients.1-3 Implementing psychosocial distress screening can be difficult because of procedural barriers and time constraints, clinic and supportive care resources, and lack of knowledge about how to access supportive services.
Distress screening protocols must be designed to address the specific needs of each population. To improve screening for cancer-related distress, deliver effective supportive services, and gain agreement on distress screening standards of care, the Coleman Foundation supported development of the Coleman Supportive Oncology Collaborative (CSOC), a project of 135 interdisciplinary health care professionals from 25 Chicago-area cancer care institutions.4
The Jesse Brown US Department of Veterans Affairs (VA) Medical Center (JBVAMC) was chosen to assess cancer-related concerns among veterans using the CSOC screening tool and to improve access to supportive oncology. JBVAMC provides care to approximately 49,000 veterans in Chicago, Illinois, and northwestern Indiana. The JBVAMC patient population includes a large number of veterans with dual diagnoses (co-occurring substance use and mental health disorders) and veterans experiencing homelessness.
Delivering integrated screening and oncologic care that is culture and age appropriate is particularly important for veterans given their unique risk factors. The veteran population is considered vulnerable in terms of health status, psychological functioning, and social context. Veterans who use the VA health system as a principal source of care have poorer health, greater comorbid medical conditions, and an increased risk of mortality and suicide compared with the general population.5,6 Poorer health status in veterans also may relate to old age, low income, poor education, psychological health, and minority race.7-9
Past studies point to unique risk factors for cancer and poor cancer adjustment among veterans, which may complicate cancer treatment and end-of-life/survivorship care. Veteran-specific risk factors include military-related exposures, particularly Agent Orange and morbidity/mortality secondary to comorbid medical and psychiatric conditions (eg, chronic obstructive pulmonary disease, diabetes mellitus, and posttraumatic stress disorder [PTSD]).10-12 Moreover, the geriatric veteran population continues to grow,with increasing rates of cancer that require unique considerations for effective cancer care.13,14 Despite this, there are minimal data to inform best practices and supportive care approaches for veterans with cancer. Lack of guidelines specific to veterans and other populations with increased psychosocial challenges may impede successful cancer care, making distress screening procedures particularly important. This is especially the case for the JBVAMC, which serves primarily African American urban-dwelling veterans who experience high rates of cancer disparities, including increased rates of mortality and increased levels of psychosocial distress.15,16
The goals of this program were to (1) examine levels of psychological, physical, financial, and treatment-related distress in a large sample of urban-dwelling veterans; (2) create a streamlined, sustainable process to screen a large number of veterans receiving cancer care in the outpatient setting and connect them with available supportive services; and (3) educate oncology physicians, nurses, and other staff about cancer-related distress and concerns using in-service trainings and interpersonal interactions to improve patient care. Our program was based on a Primary Care Mental Health Integration (PCMHI) model that embeds health psychologists in general medical clinics to better reach veterans dealing with mental health issues. We tailored for palliative care involvement.
Studies of this model have shown that mental health integration improves access to mental health services and mental health treatment outcomes and has higher patient and provider satisfaction.17 We were also influenced by the construct of the patient aligned care team (PACT) social worker who, in this veteran-centered approach, often functions as a care coordinator. Social work responsibilities include assessment of patients’ stressors including adjusting to the medical conditions, identifying untreated or undertreated mental health or substance abuse issues, economic instability, legal problems, and inadequate housing and transportation, which can often be exacerbated during cancer treatment.18
We screened for distress-related needs that included mental health concerns, physical needs including uncontrolled symptoms or adverse effects of cancer treatment, physical function complaints (eg, pain and fatigue), nutrition concerns, treatment or care related concerns, family and caregiver needs, along with financial challenges (housing and food) and insurance-related support. The goal of this article is to describe the development and implementation of this VA-specific distress screening program and reflect on the lessons learned for the application of streamlined distress screening and triage in similar settings throughout the VA health system and other similar settings.
Methods
This institutional review board at JBVAMC reviewed and exempted this quality improvement program using the SQUIRE framework.19 It was led by a group of palliative care clinicians, psychologists, and administrators who have worked with the oncology service for many years, primarily in the care of hospitalized patients. Common palliative care services include providing care for patients with serious illness diagnosis through the illness trajectory.
Setting
At the start of this program, we assessed the current clinic workflow to determine how to best screen and assist veterans experiencing distress. We met with team members individually to identify the best method of clinic integration, including attending medical oncologists, medical oncology fellows, psychology interns, oncology nursing staff, the oncology nurse coordinator, and clinic clerks.
The JBVAMC provides cancer care through 4 half-day medical hematology-oncology clinics that serve about 50 patients per half-day clinic. The clinics are staffed by hematology-oncology fellows supervised by hematology-oncology attending physicians, who are affiliated with 2 academic medical centers. These clinics are staffed by 3 registered nurses (RNs) and a licensed practical nurse (LPN) and are adjacent to a chemotherapy infusion clinic with unique nursing staff. The JBVAMC also provides a variety of supportive care services, including extensive mental health and substance use treatment, physical and occupational therapy, acupuncture, nutrition, social work, and housing services. Following our assessment, it was evident that there were a low number of referrals from oncology clinics to supportive care services, mostly due to lack of knowledge of resources and unclear referral procedures.
Based on clinical volume, we determined that our screening program could best be implemented through a stepped approach beginning in one clinic and expanding thereafter. We began by having a palliative care physician and health psychology intern embedded in 1 weekly half-day clinic and a health psychology intern embedded in a second weekly half-day clinic. Our program included 2 health psychology interns (for each academic year of the program) who were supervised by a JBVA health psychologist.
About 15 months after successful integration within the first 2 half-day clinics, we expanded the screening program to staff an additional half-day medical oncology clinic with a palliative care APRN. This allowed us to expand the screening tool distribution and collection to 3 of 4 of the weekly half-day oncology clinics as well as to meet individually with veterans experiencing high levels of distress. Veterans were flagged as having high distress levels by either the results of their completed screening tool or by referral from a medical oncology physician. We initially established screening in clinics that were sufficiently staffed to ensure that screens were appropriately distributed and reviewed. Patients seen in nonparticipating clinics were referred to outpatient social work, mental health and/or outpatient palliative care according to oncology fellows’ clinical assessments of the patient. All oncology fellows received education about distress screening and methods for referring to supportive care. Our clinic screening program extended from February 2017 through January 2020.
Screening
Program staff screened patients with new cancer diagnoses, then identified patients for follow-up screens. This tracking allowed staff to identify patients with oncology appointments that day and cross-reference patients needing a follow-up screen.
Following feedback from the clinic nurses, we determined that nurses would provide the distress tool to patients in paper form after they completed their assessment of vitals and waited to be seen by their medical oncologist. The patient would then deliver their completed form to the nurse who would combine it with the patient’s clinic notes for the oncologist to review.
Veterans referred for supportive care services were contacted by the relevant clinical administrator by phone to schedule an intake; for social work referrals, patients were either seen in a walk-in office located in a colocated building or contacted by a social worker by phone.
Our screening tool was the Coleman Foundation Supportive Oncology Collaborative Screening Tool, compiled from validated instruments. Patients completed this screening tool, which includes the PHQ-4, NCCN problem list concerns, adapted Mini Nutrition Assessment and PROMIS Pain and Fatigue measure (eAppendix B available at doi:10.12788/fp.0158).20-22
We also worked with the VA Computerized Patient Record System (CPRS) to create an electronic template for the screening tool. Completed screening tools were manually entered by the physician, psychologists, or APRN into the CPRS chart.
We analyzed the different supportive care services available at the JBVAMC and noticed that many supportive services were available, yet these services were often separated. Therefore, we created a consult flowsheet to assist oncologists in placing referrals. These supportive care services include mental health services, a cancer support group, home health care, social services, nutrition, physical medicine and rehabilitation, and other specialty services.
Patient Education
The psychology and nursing staff created a patient information bulletin board where patients could access information about supportive services available at JBVAMC. This board required frequent replenishment of handouts because patients consulted the board regularly. Handouts and folders about common clinical issues also were placed in the clinic treatment rooms. We partnered with 2 local cancer support centers, Gilda’s Club and the Cancer Support Center, to make referrals for family members and/or caregivers who would benefit from additional support.
We provided in-service trainings for oncology fellows, including trainings on PTSD and substance abuse and their relationship to cancer care at the VA. These topics were chosen based on the feedback program staff received about perceived knowledge gaps from the oncology fellows. This program allowed for multiple informal conversations between that program staff and oncology fellows about overall patient care. We held trainings with the cancer coordinator and clinical nursing staff on strategies to identify and follow-up on cancer-related distress, and with oncology fellows to review the importance of distress screening and to instruct fellows on instructions for the consult flowsheet.
Funding
This program was funded by the Chicago-based Coleman Foundation as part of the CSOC. Funding was used to support a portion of time for administrative and clinical work of program staff, as well as data collection and analysis.
Results
We established 3 half-day integrated clinics where patients were screened and referred for services based on supportive oncology needs. In addition to our primary activities to screen and refer veterans, we held multiple educational sessions for colleagues, developed a workflow template, and integrated patient education materials into the clinics.
Screening
Veterans completed 1010 distress screens in 3 of 4 half-day oncology clinics over the 2.5-year project period. Veterans were screened at initial diagnosis and every 3 months, or during changes in their clinical care or disease status. As a result, 579 patients completed screening, with some patients doing several follow-up screens during their care. Integration of palliative care providers and health psychologists was instrumental in facilitating screening in these busy general medical oncology clinics. Most veterans were receptive to completing surveys with few refusing to fill out the survey.23 Medical oncology fellows often used the completed screener to inform their review of systems (by reviewing the Coleman screener Physical and Other Concerns section) and connect with the supportive care staff present in clinic for patient’s identifying severe needs (ie, mental health distress or complex psychosocial needs). Veterans’ rates of distress needs and successfuloutcomes of integration with mental health and social work services have been reported elsewhere.23
The mean (SD) age for veterans in this cohort was 72 (9.5) years. Participants were primarily African American veterans (70%), with mostly advanced disease (Table 1). Participants endorsed elevated distress needs compared with other patient populations screened in Chicago through the CSOC for depressed mood, pain, housing, transportation, and physical, nutrition, and treatment concerns.23 Elevated presence of needs was especially prominent for food, housing and insurance/medical needs; physical concerns; nutrition, and treatment- or care-related concerns. Veterans in this cohort reported extensive financial and housing concerns: 10.4% reported food and housing concerns, 18.6% reported transportation concerns, and 9.0% reported issues paying for medical care or medications (Table 2).20 Anecdotally, many experienced job loss or strain with their cancer diagnosis or were living at the poverty level before their diagnosis.
Social work referrals were often triggered due to transportation barriers to appointments/medication access, and food and/or housing insecurity. Social workers assisted with referrals for housing, transportation, financial reimbursement, on-site or community-based food banks, home health support, familial support, and hospice services. Social work consults increased 166% from 2016 (the year before the program start date) to the end of 2019.
Based on this increased volume of referrals for social work in our oncology clinics, an oncology-specific social worker was hired at the completion of our program to be based in all 4 half-day oncology clinics in response to results of our quality improvement intervention. The social worker currently sees all patients with a new cancer diagnosis and supports oncology fellows to identify veterans needing a palliative care referral or referrals to other supportive services.
Throughout program implementation, traditional areas of palliative care focus were particularly important as veterans reported significant concerns with understanding their illness (67.4%), wanting to understand their prognosis (71.3%), and having questions about their treatment options (55.1%).20 The palliative care providers spent time educating patients about their disease, coordinating goals of care conversations, promoting patients’ engagement in decision making, and making a large number of referrals to hospice and home health to support veterans at home.
Discussion
This project created a successful program to screen veterans for psychosocial distress and triage them to appropriate services. During the project, patients in VA-outpatient oncology clinics reported significant cancer-related distress due to baseline psychosocial needs, changes in emotional and physical functioning, logistical and financial challenges of receiving cancer care, and lack of instrumental support.23
Staff education supported successful buy-in, development and implementation of supportive oncology programs. We used a combination of in-service trainings, online trainings, and handouts to provide evidence for distress screening.24 Highlighting the evidence-base that demonstrates how cancer-related distress screening improves cancer and quality of life outcomes helped to address physician reluctance to accept the additional requirements needed to address veterans’ psychosocial needs and care concerns. To increase buy-in and collaboration among team members and foster heightened understanding between providers and patients, we recommend creating accessible education for all staff levels.
One specific area of education we focused on was primary palliative care, which includes the core competencies of communication and symptom management recommended for generalists and specialists of all disciplines.25 Program staff supported oncology fellows in developing their primary palliative care skills by being available to discuss basic symptom management and communication issues. VA cancer care programs could benefit from ongoing palliative care education of oncology staff to facilitate primary palliative care as well as earlier integration of secondary palliative care when needed.26 Secondary palliative care or care provided directly by the palliative care team assists with complex symptom management or communication issues. For these needs, oncology fellows were encouraged to refer to either the palliative care staff available in one of the half-day clinics or to the outpatient palliative care clinic. As a unique strength, the VA allows veterans to receive concurrent cancer-directed therapy and hospice care, which enables earlier referrals to hospice care and higher quality end-of-life care and emphasizes the need for primary palliative care in oncology.27,28
Integrating supportive oncology team members, such as licensed clinical social worker and psychology interns, was successful. This was modeled on the VA PACT, which focuses on prevention, health promotion, coordination and chronic disease management.29 Social determinants of health have a major impact on health outcomes especially in veteran-specific and African American populations, making screening for distress critical.30-32 The VA Office of Health Equity actively addresses health inequities by supporting initiation of screening programs for social determinants of health, including education, employment, exposure to abuse and violence, food insecurity, housing instability, legal needs, social isolation, transportation needs, and utility needs. This is especially needed for African-American individuals who are not only more likely to experience cancer, but also more likely to be negatively impacted by the consequences of cancer diagnosis/treatment, such as complications related to one’s job security, access to care, adverse effects, and other highly distressing needs.33,34
Our program found that veterans with cancer often had concerns associated with food and housing insecurity, transportation and paying for medication or medical care, and screening allowed health care providers to detect and address these social determinants of health through referrals to VA and community-specific programs. Social workers integrated
Our ability to roll out distress screening was scaffolded by technological integration into existing VA systems (eg, screening results in CPRS and electronic referrals). Screening procedures could have been even more efficient with improved technology (Table 3). For example, technological limitations made it challenging to easily identify patients due for screening, requiring a cumbersome process of tracking, collecting and entering patients’ paper forms. Health care providers seeking to develop a distress screening program should consider investing in technology that allows for identification of patients requiring screening at a predetermined interval, completion of screening via tablet or personal device, integration of screening responses into the electronic health record, and automatic generation of notifications to the treating physician and appropriate support services.
We also established partnerships with community cancer support groups to offer both referral pathways and in-house programming. Veterans’ cancer care programs could benefit from identifying and securing community partnerships to capitalize on readily available low-cost or no-cost options for supportive oncology in the community. Further, as was the case in our program, cancer support centers may be willing to collaborate with VA hospitals to provide services on site (eg, support groups, art therapy). This would extend the reach of these supportive services while allowing VA employees to address the extensive psychosocial needs of individual veterans.
Conclusions
Veterans with cancer benefited from enhanced screening and psychosocial service availability, similar to a PCMHI model. Robust screening programs helped advocate for veterans dealing with the effects of poverty through identification of need and referral to existing VA programs and services quickly and efficiently. Providing comprehensive care within ambulatory cancer clinics can address cancer-related distress and any potential barriers to care in real time. VA hospitals typically offer an array of supportive services to address veterans’ psychosocial needs, yet these services tend to be siloed. Integrated referrals can help to resolve such access barriers. Since many veterans with burdensome cancers are not able to see their VA primary care physician regularly, offering comprehensive care within medical oncology ensures complete and integrated care that includes psychosocial screening.
We believe that this program is an example of a mechanism for oncologists and palliative care clinicians to integrate their care in a way that identifies needs and triages services for vulnerable veterans. As colleagues have written, “it is fundamental to our commitment to veterans that we ensure comparable, high quality care regardless of a veteran’s gender, race, or where they live.”34 Health care providers may underestimate the extensive change a cancer diagnosis can have on a patient’s quality of life. Cancer diagnosis and treatment have a large impact on all individuals, but this impact may be greater for individuals in poverty due to inability to work from home, inflexible work hours, and limited support structures. By creating screening programs with psychosocial integration in oncology clinics such as we have described, we hope to improve access to more equitable care for vulnerable veterans.
1. National Comprehensive Cancer Network. NCCN guidelines distress management. Version 2.2021. Updated January 5, 2021. Accessed July 8, 2021. http://www.nccn.org/professionals/physician_gls/pdf/distress.pdf
2. American College of Surgeons, Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care. Version 1.2.1. Published 2021. Accessed July 8, 2021. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx
3. Jacobsen PB, Ransom S. Implementation of NCCN distress management guidelines by member institutions. J Natl Compr Canc Netw. 2007;5(1):99-103. doi:10.6004/jnccn.2007.0010
4. The Coleman Supportive Oncology Collaborative. Training tools. Accessed July 14, 2021. https://www.supportiveoncologycollaborative.org/training-tools
5. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
6. Bullman T, Schneiderman A, Gradus JL. Relative importance of posttraumatic stress disorder and depression in predicting risk of suicide among a cohort of Vietnam veterans. Suicide Life Threat Behav. 2019;49(3):838-845. doi:10.1111/sltb.12482
7. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
8. O’Toole BI, Marshall RP, Grayson DA, et al. The Australian Vietnam Veterans Health Study: III. Psychological health of Australian Vietnam veterans and its relationship to combat. Int J Epidemiol. 1996;25(2):331-340. doi:10.1093/ije/25.2.331
9. Vincent C, Chamberlain K, Long N. Mental and physical health status in a community sample of New Zealand Vietnam War veterans. Aust J Public Health. 1994;18(1):58-62. doi:10.1111/j.1753-6405.1994.tb00196.x
10. US Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Updated June 16, 2021. Accessed July 8, 2021. http://www.publichealth.va.gov/exposures/agentorange/diseases.asp#veterans
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in Veterans with hepatocellular carcinoma. HBP (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Saha S, Freeman M, Toure J, Tippens KM, Weeks C, Ibrahim S. Racial and ethnic disparities in the VA health care system: a systematic review. J Gen Intern Med. 2008;23(5):654-671. doi:10.1007/s11606-008-0521-4
13. Amaral EFL, Pollard MS, Mendelsohn J, Cefalu M. Current and future demographics of the veteran population, 2014-2024. Popul Rev. 2018;57(1):28-60. doi:10.1353/prv.2018.0002
14. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
15. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212-236. doi:10.3322/caac.20121
16. Cimino T, Said K, Safier L, Harris H, Kinderman A. Psychosocial distress among oncology patients in the safety net. Psychooncology. 2020;29(11):1927-1935. doi:10.1002/pon.5525
17. Molander R, Hodgkins K, Johnson C, White A, Frazier E, Krahn D. Interprofessional education in patient aligned care team primary care-mental health integration. Fed Pract. 2017;34(6):40-48.
18. Parikh DA, Ragavan M, Dutta R, et al. Financial toxicity of cancer care: an analysis of financial burden in three distinct health care systems [published online ahead of print, 2021 Apr 7]. JCO Oncol Pract. 2021;OP2000890. doi:10.1200/OP.20.00890
19. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
20. Weldon CB, Gerhart JI, Penedo FJ, et al. Correlates of distress for cancer patients: results from multi-institution use of holistic patient-reported screening tool. J Clin Oncol. 2019;37(15)(suppl):11587-11587. doi:10.1200/JCO.2019.37.15_suppl.11587
21. Kroenke K, Spitzer RL, Williams JB, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359. doi:10.1016/j.genhosppsych.2010.03.006
22. Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782-788. doi:10.1007/s12603-009-0214-7
23. Azizoddin DR, Lakin JR, Hauser J, et al. Meeting the guidelines: implementing a distress screening intervention for veterans with cancer. Psychooncology. 2020;29(12):2067-2074. doi:10.1002/pon.5565
24. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177. doi:10.1200/JCO.2011.39.5509
25. Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. doi:10.1056/NEJMp1215620
26. Weissman DE, Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17-23. doi:10.1089/jpm.2010.0347
27. Kumar P, Wright AA, Hatfield LA, Temel JS, Keating NL. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35(4):432-439. doi:10.1200/JCO.2016.68.9257
28. Mor V, Joyce NR, Coté DL, et al. The rise of concurrent care for veterans with advanced cancer at the end of life. Cancer. 2016;122(5):782-790. doi:10.1002/cncr.29827
29. US Department of Veterans Affairs. Patient care services: Patient aligned care team (PACT). Updated November 5, 2020. Accessed July 8, 2021. https://www.patientcare.va.gov/primarycare/PACT.asp
30. US Department of Veterans Affairs, Veterans Health Administration. VHA health equity action plan. Published September 27, 2019. Accessed July 8, 2021. https://www.va.gov/HEALTHEQUITY/docs/Health_Equity_Action_Plan_Final_022020.pdf
31. Alcaraz KI, Wiedt TL, Daniels EC, Yabroff KR, Guerra CE, Wender RC. Understanding and addressing social determinants to advance cancer health equity in the United States: a blueprint for practice, research, and policy. CA Cancer J Clin. 2020;70(1):31-46. doi:10.3322/caac.21586
32. Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we’ve come far but aren’t there yet. Am J Public Health. 2014;104(suppl 4):S525-526. doi:10.2105/AJPH.2014.302216
33. American Cancer Society. Cancer Facts & Figures for African Americans 2019-2021. Atlanta: American Cancer Society; 2019.
34. Hastert TA, Kirchhoff AC, Banegas MP, et al. Work changes and individual, cancer-related, and work-related predictors of decreased work participation among African American cancer survivors. Cancer Med. 2020;9(23):9168-9177. doi:10.1002/cam4.3512
35. Bekelman DB, Nowels CT, Allen LA, Shakar S, Kutner JS, Matlock DD. Outpatient palliative care for chronic heart failure: a case series. J Palliat Med. 2011;14(7):815-821. doi:10.1089/jpm.2010.050
1. National Comprehensive Cancer Network. NCCN guidelines distress management. Version 2.2021. Updated January 5, 2021. Accessed July 8, 2021. http://www.nccn.org/professionals/physician_gls/pdf/distress.pdf
2. American College of Surgeons, Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care. Version 1.2.1. Published 2021. Accessed July 8, 2021. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx
3. Jacobsen PB, Ransom S. Implementation of NCCN distress management guidelines by member institutions. J Natl Compr Canc Netw. 2007;5(1):99-103. doi:10.6004/jnccn.2007.0010
4. The Coleman Supportive Oncology Collaborative. Training tools. Accessed July 14, 2021. https://www.supportiveoncologycollaborative.org/training-tools
5. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
6. Bullman T, Schneiderman A, Gradus JL. Relative importance of posttraumatic stress disorder and depression in predicting risk of suicide among a cohort of Vietnam veterans. Suicide Life Threat Behav. 2019;49(3):838-845. doi:10.1111/sltb.12482
7. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
8. O’Toole BI, Marshall RP, Grayson DA, et al. The Australian Vietnam Veterans Health Study: III. Psychological health of Australian Vietnam veterans and its relationship to combat. Int J Epidemiol. 1996;25(2):331-340. doi:10.1093/ije/25.2.331
9. Vincent C, Chamberlain K, Long N. Mental and physical health status in a community sample of New Zealand Vietnam War veterans. Aust J Public Health. 1994;18(1):58-62. doi:10.1111/j.1753-6405.1994.tb00196.x
10. US Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Updated June 16, 2021. Accessed July 8, 2021. http://www.publichealth.va.gov/exposures/agentorange/diseases.asp#veterans
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in Veterans with hepatocellular carcinoma. HBP (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Saha S, Freeman M, Toure J, Tippens KM, Weeks C, Ibrahim S. Racial and ethnic disparities in the VA health care system: a systematic review. J Gen Intern Med. 2008;23(5):654-671. doi:10.1007/s11606-008-0521-4
13. Amaral EFL, Pollard MS, Mendelsohn J, Cefalu M. Current and future demographics of the veteran population, 2014-2024. Popul Rev. 2018;57(1):28-60. doi:10.1353/prv.2018.0002
14. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
15. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212-236. doi:10.3322/caac.20121
16. Cimino T, Said K, Safier L, Harris H, Kinderman A. Psychosocial distress among oncology patients in the safety net. Psychooncology. 2020;29(11):1927-1935. doi:10.1002/pon.5525
17. Molander R, Hodgkins K, Johnson C, White A, Frazier E, Krahn D. Interprofessional education in patient aligned care team primary care-mental health integration. Fed Pract. 2017;34(6):40-48.
18. Parikh DA, Ragavan M, Dutta R, et al. Financial toxicity of cancer care: an analysis of financial burden in three distinct health care systems [published online ahead of print, 2021 Apr 7]. JCO Oncol Pract. 2021;OP2000890. doi:10.1200/OP.20.00890
19. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
20. Weldon CB, Gerhart JI, Penedo FJ, et al. Correlates of distress for cancer patients: results from multi-institution use of holistic patient-reported screening tool. J Clin Oncol. 2019;37(15)(suppl):11587-11587. doi:10.1200/JCO.2019.37.15_suppl.11587
21. Kroenke K, Spitzer RL, Williams JB, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359. doi:10.1016/j.genhosppsych.2010.03.006
22. Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782-788. doi:10.1007/s12603-009-0214-7
23. Azizoddin DR, Lakin JR, Hauser J, et al. Meeting the guidelines: implementing a distress screening intervention for veterans with cancer. Psychooncology. 2020;29(12):2067-2074. doi:10.1002/pon.5565
24. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177. doi:10.1200/JCO.2011.39.5509
25. Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. doi:10.1056/NEJMp1215620
26. Weissman DE, Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17-23. doi:10.1089/jpm.2010.0347
27. Kumar P, Wright AA, Hatfield LA, Temel JS, Keating NL. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35(4):432-439. doi:10.1200/JCO.2016.68.9257
28. Mor V, Joyce NR, Coté DL, et al. The rise of concurrent care for veterans with advanced cancer at the end of life. Cancer. 2016;122(5):782-790. doi:10.1002/cncr.29827
29. US Department of Veterans Affairs. Patient care services: Patient aligned care team (PACT). Updated November 5, 2020. Accessed July 8, 2021. https://www.patientcare.va.gov/primarycare/PACT.asp
30. US Department of Veterans Affairs, Veterans Health Administration. VHA health equity action plan. Published September 27, 2019. Accessed July 8, 2021. https://www.va.gov/HEALTHEQUITY/docs/Health_Equity_Action_Plan_Final_022020.pdf
31. Alcaraz KI, Wiedt TL, Daniels EC, Yabroff KR, Guerra CE, Wender RC. Understanding and addressing social determinants to advance cancer health equity in the United States: a blueprint for practice, research, and policy. CA Cancer J Clin. 2020;70(1):31-46. doi:10.3322/caac.21586
32. Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we’ve come far but aren’t there yet. Am J Public Health. 2014;104(suppl 4):S525-526. doi:10.2105/AJPH.2014.302216
33. American Cancer Society. Cancer Facts & Figures for African Americans 2019-2021. Atlanta: American Cancer Society; 2019.
34. Hastert TA, Kirchhoff AC, Banegas MP, et al. Work changes and individual, cancer-related, and work-related predictors of decreased work participation among African American cancer survivors. Cancer Med. 2020;9(23):9168-9177. doi:10.1002/cam4.3512
35. Bekelman DB, Nowels CT, Allen LA, Shakar S, Kutner JS, Matlock DD. Outpatient palliative care for chronic heart failure: a case series. J Palliat Med. 2011;14(7):815-821. doi:10.1089/jpm.2010.050
Three Primary Cancers in a Veteran With Agent Orange and Agent Blue Exposures
A Vietnam War veteran’s exposures likely contributed to his cancer diagnoses, but these associations are confounded by his substance use, particularly cigarette smoking.
Known as the “6 rainbow herbicides,” based on their identifying color on storage containers, the United States widely deployed the herbicides agents orange, green, pink, purple, white, and blue during the Vietnam War to deny the enemy cover and destroy crops.1 Unfortunately, all these herbicides were found to have contained some form of carcinogen. Agent Blue’s active ingredient consisted of sodium cacodylate trihydrate (C2H6AsNaO2), a compound that is metabolized into the organic form of the carcinogen arsenic before eventually converting into its relatively less toxic inorganic form.2 Agent Orange’s defoliating agent is 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). All rainbow herbicides except Agent Blue were unintentionally contaminated with carcinogenic dioxins. Agent Blue contained the carcinogen cacodylic acid, an organoarsenic acid. Today, herbicides no longer contain polychlorinated dibenzo-p-dioxins such as TCDD or arsenic due to strict manufacturing restrictions.2,3 In the treatment of veteran populations, knowledge of the 6 rainbow herbicides’ carcinogenic potential is important.
Between 1962 and 1971, the United States sprayed more than 45 million liters of Agent Orange on Vietnam and at least 366 kg of TCDD on South Vietnam.1,4 However, because Agent Orange was not a known carcinogen during the Vietnam War, records of exposure are poor. Additionally, individuals in Vietnam during this period were not the only ones exposed to this carcinogen as Agent Orange also was sprayed in Thailand and Korea.5 Even today there are still locations in Vietnam where Agent Orange concentrations exceed internationally acceptable levels. The Da Nang, Bien Hoa, and Phu Cat airports in Vietnam have been found to have dioxin levels exceeding 1000 ppt (parts of dioxin per trillion parts of lipid) toxicity equivalence in the soil. Although the Vietnam government is working toward decontaminating these and many other dioxin hotspots, residents in these locations are exposed to higher than internationally acceptable levels of dioxin.6
Despite receiving less media attention, Vietnam War veterans and Vietnamese soldiers and civilians were exposed to significant amounts of arsenic-based Agent Blue. Arsenic is a compound which has no environmental half-life and is carcinogenic humans if inhaled or ingested.2 Between 1962 and 1971, the United States distributed 7.8 million liters of Agent Blue containing 1,232,400 kg of arsenic across 300,000 hectares of rice paddies, 100,000 hectares of forest, and perimeters of all military bases during the Vietnam War.2,5 According to a review by Saha and colleagues, lower levels of arsenic exposure are associated with acute and chronic diseases, including cancers, of all organ systems.7
The following case presentation involves a Vietnam War veteran aged 70 years who was exposed to Agent Orange and developed 3 primary cancers, including cutaneous large B-cell non-Hodgkin lymphoma (NHL), high-grade urothelial carcinoma, and anal carcinoma in situ. Epidemiologically, this is an uncommon occurrence as only 8% of cancer survivors in the United States have been diagnosed with > 1 cancer.8
With no family history of cancer, the development of multiple malignancies raises concern for a history of toxin exposure. This report of a Vietnam War veteran with multiple conditions found to be associated with Agent Orange exposure provides an opportunity to discuss the role this exposure may have on the development of a comprehensive list of medical conditions as described by the literature. Additionally, the potential contributions of other confounding toxin exposures such as cigarette smoking, excessive alcohol use, and potential Agent Blue exposure on our patients’ health will be discussed.
Case Presentation
A male aged 70 years with Stage IV primary cutaneous large B-cell NHL, incompletely resected high-grade urothelial cancer, carcinoma in situ of the anal canal, and peripheral arterial disease (PAD) presented to the primary care clinic at the Washington DC Veterans Affairs Medical Center (DCVAMC) with concern for left leg ischemia. He also reported 2 large telangiectasias on his back for 6 months accompanied by lymphadenopathy and intermittent night sweats.
He was last seen at the DCVAMC 15 months prior after his twelfth dose of rituximab treatment for NHL. However, the patient failed to return for completion of his treatment due to frustration with the lengthy chemotherapy and follow-up process. Additionally, the patient's history included 3 failed arterial stents with complete nonadherence to the prescribed clopidogrel, resulting in the failure of 3 more subsequent graft placements. On presentation, the patient continued to report nonadherence with the clopidogrel.
The patient’s medical history included coronary artery disease (CAD) status after 2 stents in the left anterior descending artery and 1 stent in the proximal circumflex artery placed 4 years prior. He also had a history of hypertension, type 2 diabetes mellitus (T2DM), amyloid light-chain (AL) amyloidosis, aortic aneurysm, cataracts, obesity, treated hepatitis B and C, and posttraumatic stress disorder. He had no family history of cancer or AL amyloidosis; however, he noted that he was estranged from his family.
His social history was notable for active cigarette smoking up to 3 packs per day for 40 years and consuming large quantities of alcohol—at one point as many as 20 beers per day over a period of 4.5 years. He had a distant history of cocaine use but no current use, which was supported with negative urinary toxicology screens for illicit drugs over the past year.
Our patient also reported a history of Agent Orange exposure. As an artilleryman in the US Army III Corps, he was deployed for about 1 year in the most heavily sprayed regions of Vietnam, including Bien Hua, Long Binh, Xuan Loc, and Camp Zion for about 2 to 4 months at each location.
Hospital Course
The patient was treated on an inpatient basis for expedited workup and treatment for his urothelial carcinoma, NHL, and ischemic limb. His urothelial carcinoma was successfully resected, and the telangiectasias on his back were biopsied and found to be consistent with his known cutaneous large B-cell NHL, for which plans to resume outpatient chemotherapy were made. The patient’s 3 arterial grafts in his left leg were confirmed to have failed, and the patient was counseled that he would soon likely require an amputation of his ischemic leg.
Discussion
We must rely on our patient’s historical recall as there are no widely available laboratory tests or physical examination findings to confirm and/or determine the magnitude of TCDD or arsenic exposure.9-11
Exposures
The patient was stationed in Bien Hoa, the second highest dioxin-contaminated air base in Vietnam (Figure).6 Dioxin also is known to be a particularly persistent environmental pollutant, such that in January 2018, Bien Hoa was found to still have dioxin levels higher than what is considered internationally acceptable. In fact, these levels were deemed significant enough to lead the United States and Vietnamese government to sign a memorandum of intent to begin cleanup of this airport.6 TCDD is known to have a half-life of about 7.6 years, and its long half-life is mainly attributed to its slow elimination process from its stores within the liver and fat, consisting of passive excretion through the gut wall and slow metabolism by the liver.12,13 Thus, as an artilleryman mainly operating 105 howitzers within the foliage of Vietnam, our patient was exposed not only to high levels of this persistent environmental pollutant on a daily basis, but this toxin likely remained within his system for many years after his return from Vietnam.
Our patient also had a convincing history for potential Agent Blue exposure through both inhalation and ingestion of contaminated food and water. Additionally, his description of deforestations occurring within a matter of days increased the level of suspicion for Agent Blue exposure. This is because Agent Blue was the herbicide of choice for missions requiring rapid deforestation, achieving defoliation as quickly as 1 to 2 days.14 Additionally, our patient was stationed within cities in southern Vietnam near Agent Blue hot spots, such as Da Nang and Saigon, and Agent Blue was sprayed along the perimeter of all military bases.2
Levels of Evidence
Using the Veterans and Agent Orange Update in 2018 as our guide, we reviewed the quality of evidence suggesting an association between many of our patient’s comorbidities to Agent Orange exposure.5 This publication categorizes the level of evidence for association between health conditions and Agent Orange exposure in 4 main categories (Table 1).
In the Veterans and Agent Orange Update, NHL notably has a sufficient level of evidence of association with Agent Orange exposure.5 Although our patient’s extensive history of polysubstance use confounds the effect Agent Orange may have had on his health, cutaneous large B-cell NHL is an interesting exception as literature does not support even a correlative link between smoking and excessive alcohol use with primary cutaneous large B-cell NHL. Several case-control studies have found little to no association with cigarette smoking and the large B-cell subtype of NHL.15,16 Moreover, several studies have found that moderate- to-heavy alcohol use, especially beer, may have a protective effect against the development of NHL.17 Of note, our patient’s alcoholic beverage of choice was beer. Regarding our patient’s distant history of cocaine use, it has been reported that cocaine use, in the absence of an HIV infection, has not been found to increase the risk of developing NHL.18 Similarly, arsenic exposure has not been associated with NHL in the literature.19,20
The 2018 update also upgraded bladder carcinoma from having inadequate or insufficient to a limited or suggestive level of evidence for association.5 However, our patient’s most significant risk factor for bladder cancer was smoking, with a meta-analysis of 430,000 patients reporting a risk ratio (RR) of 3.14 for current cigarette smokers.21 The patient’s arsenic exposure from Agent Blue also increased his risk of developing bladder cancer. Several studies suggest a strong association between environmental arsenic exposure and bladder cancer.22-26 A 30-year meta-analysis of 40 studies by Saint-Jacques and colleagues reported that the incidence of bladder cancer was found to increase in a dose-dependent manner, with higher concentrations of arsenic contaminated wate, with incidence rising from 2.7 to 5.8 times as the amount of arsenic contamination water increased from 10 to 150 mg/L.
Our patient’s history is concerning for higher than average Agent Blue exposure compared with that of most Vietnam War veterans. Given the dose-dependent effect of arsenic on bladder cancer risk, both our patient’s history of smoking and Agent Blue exposure are risk factors in the development of his bladder cancer.22 These likely played a more significant role in his development of bladder cancer than did his Agent Orange exposure.
Finally, smoking is the most significant risk factor in our patient’s development of anal carcinoma in situ. The 2018 Agent Orange update does report limited/suggested evidence of no association between Agent Orange and anal carcinoma.5 It also is unknown whether Agent Blue exposure is a contributing cause to his development of anal carcinoma in situ.27 However, current smokers are at significant risk of developing anal cancer independent of age.28-30 Given our patient’s extensive smoking history, this is the most likely contributing factor.
Our patient also had several noncancer-related comorbidities with correlative associations with Agent Orange exposure of varying degrees (Table 2). Somewhat surprising, the development of our patient’s hypertension and T2DM may be associated in some way with his history of Agent Orange exposure. Hypertension had been recategorized from having limited or suggestive evidence to sufficient evidence in this committee’s most recent publication, and the committee is undecided on whether T2DM has a sufficient vs limited level of evidence for association with Agent Orange exposure.5 On the other hand, the committee continues to classify both ischemic heart disease and AL amyloidosis as having a limited or suggestive level of evidence that links Agent Orange exposure to these conditions.5
Arsenic may be another risk factor for our patient’s development of CAD and arterial insufficiency. Arsenic exposure is theorized to cause a direct toxic effect on coronary arteries, and arsenic exposure has been linked to PAD, CAD, and hypertension.31-34 Other significant and compelling risk factors for cardiovascular disease in our patient included his extensive history of heavy cigarette smoking, poorly controlled T2DM, obesity, and hypertension.35-37 AL amyloidosis is a rare disorder with an incidence of only 9 to 14 cases per million person-years.38,39 This disorder has not been linked to smoking or arsenic exposure in the literature. As our patient does not have a history of plasma dyscrasias or a family history of AL amyloidosis, the only known risk factors for AL amyloidosis that apply to our patient included NHL and Agent Orange exposure—NHL being a condition that is noted to be strongly correlated with Agent Orange exposure as discussed previously.5,36,40,41
Conclusions
This case describes a Vietnam War veteran with significant exposure to rainbow herbicides and considerable polysubstance who developed 3 primary cancers and several chronic medical conditions. His exposure to Agents Orange and Blue likely contributed to his medical problems, but these associations are confounded by his substance use, particularly cigarette smoking. Of all his comorbidities, our patient’s NHL is the condition most likely to be associated with his history of Agent Orange exposure. His Agent Blue exposure also increased his risk for developing bladder cancer, cardiovascular disease, and PAD.
This case also highlights the importance of evaluating Vietnam War veterans for rainbow herbicide exposure and the complexity associated with attributing diseases to these exposures. All veterans who served in the inland waterways of Vietnam between 1962 and 1975; in the Korean Demilitarized Zone between April 1, 1968 and August 31, 1971; or in Thailand between February 28, 1961 and May 7, 1975 were at risk of rainbow herbicide exposure. These veterans may not only be eligible for disability compensation but also should be screened for associated comorbidities as outlined by current research.42 We hope that this report will serve as an aid in achieving this mission.
1. Stellman JM, Stellman SD, Christian R, Weber T, Tomasallo C. The extent and patterns of usage of Agent Orange and other herbicides in Vietnam. Nature. 2003;422(6933):681-687. doi:10.1038/nature01537
2. Olson K, Cihacek L. The fate of Agent Blue, the arsenic based herbicide, used in South Vietnam during the Vietnam War. Open J Soil Sci. 2020;10:518-577. doi:10.4236/ojss.2020.1011027
3. Lee Chang A, Dym AA, Venegas-Borsellino C, et al. Comparison between simulation-based training and lecture-based education in teaching situation awareness. a randomized controlled study. Ann Am Thorac Soc. 2017;14(4):529-535. doi:10.1513/AnnalsATS.201612-950OC
4. Stellman SD. Agent Orange during the Vietnam War: the lingering issue of its civilian and military health impact. Am J Public Health. 2018;108(6):726-728. doi:10.2105/AJPH.2018.304426
5. National Academies of Sciences, Engineering, and Medicine. Veterans and Agent Orange: Update 11 (2018). The National Academies Press; 2018. doi:10.17226/25137
6. Martin MF. US Agent Orange/dioxin assistance to Vietnam. Updated January 15, 2021. Accessed June 17, 2021. https://fas.org/sgp/crs/row/R44268.pdf
7. Saha JC, Dikshit AK, Bandyopadhyay M, Saha KC. A review of arsenic poisoning and its effects on human health. Crit Rev Environ Sci Technol. 1999;29(3):281-313. doi:10.1080/10643389991259227
8. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551
9. American Cancer Society. Agent Orange and cancer risk. Updated June 9, 2020. Accessed June 17, 2021. https://www.cancer.org/cancer/cancer-causes/agent-orange-and-cancer.html
10. US Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Updated June 16, 2021. Accessed June 17, 2021. https://www.publichealth.va.gov/exposures/agentorange/conditions/index.asp
11. Katz SA. On the use of hair analysis for assessing arsenic intoxication. Int J Environ Res Public Health. 2019;16(6):977. Published 2019 Mar 18. doi:10.3390/ijerph16060977
12. Chang ET, Boffetta P, Adami HO, Mandel JS. A critical review of the epidemiology of Agent Orange or 2,3,7,8-tetrachlorodibenzo-p-dioxin and lymphoid malignancies. Ann Epidemiol. 2015;25(4):275-292.e30. doi:10.1016/j.annepidem.2015.01.002
13. Kramárová E, Kogevinas M, Anh CT, et al. Exposure to Agent Orange and occurrence of soft-tissue sarcomas or non-Hodgkin lymphomas: an ongoing study in Vietnam. Environ Health Perspect. 1998;106 Suppl 2(suppl 2):671-678. doi:10.1289/ehp.106-1533419
14. Institute of Medicine (US) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides. Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam. National Academies Press US; 1994.
15. Morton LM, Hartge P, Holford TR, et al. Cigarette smoking and risk of non-Hodgkin lymphoma: a pooled analysis from the International Lymphoma Epidemiology Consortium (interlymph). Cancer Epidemiol Biomarkers Prev. 2005;14(4):925-933. doi:10.1158/1055-9965.EPI-04-0693
16. Schöllkopf C, Smedby KE, Hjalgrim H, et al. Cigarette smoking and risk of non-Hodgkin’s lymphoma--a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1791-1796. doi:10.1158/1055-9965.EPI-05-0077
17. Psaltopoulou T, Sergentanis TN, Ntanasis-Stathopoulos I, Tzanninis IG, Tsilimigras DI, Dimopoulos MA. Alcohol consumption and risk of hematological malignancies: a meta-analysis of prospective studies. Int J Cancer. 2018;143(3):486-495. doi:10.1002/ijc.31330
18. Aujla AS, Lee SH. Association between cocaine use and hematological malignancies. J Clin Oncol. 2016;34(15_suppl):e19072-e19072. doi:10.1200/JCO.2016.34.15_suppl.e19072
19. Mao Y, Hu J, Ugnat AM, White K. Non-Hodgkin’s lymphoma and occupational exposure to chemicals in Canada. Canadian Cancer Registries Epidemiology Research Group. Ann Oncol. 2000;11 (suppl 1):69-73. doi:10.1093/annonc/11.suppl_1.S69
20. Kelekci KH, Bilgin I, Ermete M. Arsenical keratoses and non-Hodgkin’s lymphoma: arsenic-induced or coincidental conditions? J Pakistan Assoc Dermatol. 2012;22(4):366-369.
21. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96-108. doi:10.1016/j.eururo.2016.06.010
22. Saint-Jacques N, Parker L, Brown P, Dummer TJ. Arsenic in drinking water and urinary tract cancers: a systematic review of 30 years of epidemiological evidence. Environ Health. 2014;13:44. Published 2014 Jun 2. doi:10.1186/1476-069X-13-44
23. Radosavljevic′ V, Jakovljevic′ B. Arsenic and bladder cancer: observations and suggestions. J Environ Health. 2008;71(3):40-42.
24. Marsit CJ, Karagas MR, Schned A, Kelsey KT. Carcinogen exposure and epigenetic silencing in bladder cancer. Ann N Y Acad Sci. 2006;1076(1):810-821. doi:10.1196/annals.1371.031
25. Mendez WM Jr, Eftim S, Cohen J, et al. Relationships between arsenic concentrations in drinking water and lung and bladder cancer incidence in U.S. counties. J Expo Sci Environ Epidemiol. 2017;27(3):235-243. doi:10.1038/jes.2016.58
26. Pal DK, Agrawal A, Ghosh S, Ghosh A. Association of arsenic with recurrence of urinary bladder cancer. Trop Doct. 2020;50(4):325-330. doi:10.1177/0049475520930155
27. García-Esquinas E, Pollán M, Umans JG, et al. Arsenic exposure and cancer mortality in a US-based prospective cohort: the strong heart study [published correction appears in Cancer Epidemiol Biomarkers Prev. 2013;22(8):1479]. Cancer Epidemiol Biomarkers Prev. 2013;22(11):1944-1953. doi:10.1158/1055-9965.EPI-13-0234-T
28. Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101(2):270-280. doi:10.1002/cncr.20365
29. Bertisch B, Franceschi S, Lise M, et al; Swiss HIV Cohort Study Investigators. Risk factors for anal cancer in persons infected with HIV: a nested case-control study in the Swiss HIV Cohort Study. Am J Epidemiol. 2013;178(6):877-884. doi:10.1093/aje/kwt153
30. Rabkin CS, Biggar RJ, Melbye M, Curtis RE. Second primary cancers following anal and cervical carcinoma: evidence of shared etiologic factors. Am J Epidemiol. 1992;136(1):54-58. doi:10.1093/oxfordjournals.aje.a116420

31. Newman JD, Navas-Acien A, Kuo CC, et al. Peripheral arterial disease and its association with arsenic exposure and metabolism in the Strong Heart Study. Am J Epidemiol. 2016;184(11):806-817. doi:10.1093/aje/kww002
32. Moon KA, Guallar E, Umans JG, et al. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. A prospective cohort study. Ann Intern Med. 2013;159(10):649-659. doi:10.7326/0003-4819-159-10-201311190-00719
33. Moon K, Guallar E, Navas-Acien A. Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep. 2012;14(6):542-555. doi:10.1007/s11883-012-0280-x
34. Stea F, Bianchi F, Cori L, Sicari R. Cardiovascular effects of arsenic: clinical and epidemiological findings. Environ Sci Pollut Res Int. 2014;21(1):244-251. doi:10.1007/s11356-013-2113-z
35. Burns DM. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis. 2003;46(1):11-29. doi:10.1016/s0033-0620(03)00079-3
36. Merlini G, Dispenzieri A, Sanchorawala V, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4(1):38. Published 2018 Oct 25. doi:10.1038/s41572-018-0034-3
37. Dokken BB. The pathophysiology of cardiovascular disease and diabetes: beyond blood pressure and lipids. Diabetes Spectrum. 2008;21(3):160-165. doi:10.2337/diaspect.21.3.160
38. Vaxman I, Gertz M. Recent advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019;141(2):93-106. doi:10.1159/000495455
39. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046-1053. doi:10.1182/bloodadvances.2018016402
40. Basset M, Defrancesco I, Milani P, et al. Nonlymphoplasmacytic lymphomas associated with light-chain amyloidosis. Blood. 2020;135(4):293-296. doi:10.1182/blood.2019002762
41. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564-569. doi:10.1056/NEJMoa01133202
42. US Department of Veterans Affairs. Agent Orange registry health exam for veterans. Updated May 28, 2021. Accessed June 17, 2021. https://www.publichealth.va.gov/exposures/agentorange/benefits/registry-exam.asp
A Vietnam War veteran’s exposures likely contributed to his cancer diagnoses, but these associations are confounded by his substance use, particularly cigarette smoking.
A Vietnam War veteran’s exposures likely contributed to his cancer diagnoses, but these associations are confounded by his substance use, particularly cigarette smoking.
Known as the “6 rainbow herbicides,” based on their identifying color on storage containers, the United States widely deployed the herbicides agents orange, green, pink, purple, white, and blue during the Vietnam War to deny the enemy cover and destroy crops.1 Unfortunately, all these herbicides were found to have contained some form of carcinogen. Agent Blue’s active ingredient consisted of sodium cacodylate trihydrate (C2H6AsNaO2), a compound that is metabolized into the organic form of the carcinogen arsenic before eventually converting into its relatively less toxic inorganic form.2 Agent Orange’s defoliating agent is 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). All rainbow herbicides except Agent Blue were unintentionally contaminated with carcinogenic dioxins. Agent Blue contained the carcinogen cacodylic acid, an organoarsenic acid. Today, herbicides no longer contain polychlorinated dibenzo-p-dioxins such as TCDD or arsenic due to strict manufacturing restrictions.2,3 In the treatment of veteran populations, knowledge of the 6 rainbow herbicides’ carcinogenic potential is important.
Between 1962 and 1971, the United States sprayed more than 45 million liters of Agent Orange on Vietnam and at least 366 kg of TCDD on South Vietnam.1,4 However, because Agent Orange was not a known carcinogen during the Vietnam War, records of exposure are poor. Additionally, individuals in Vietnam during this period were not the only ones exposed to this carcinogen as Agent Orange also was sprayed in Thailand and Korea.5 Even today there are still locations in Vietnam where Agent Orange concentrations exceed internationally acceptable levels. The Da Nang, Bien Hoa, and Phu Cat airports in Vietnam have been found to have dioxin levels exceeding 1000 ppt (parts of dioxin per trillion parts of lipid) toxicity equivalence in the soil. Although the Vietnam government is working toward decontaminating these and many other dioxin hotspots, residents in these locations are exposed to higher than internationally acceptable levels of dioxin.6
Despite receiving less media attention, Vietnam War veterans and Vietnamese soldiers and civilians were exposed to significant amounts of arsenic-based Agent Blue. Arsenic is a compound which has no environmental half-life and is carcinogenic humans if inhaled or ingested.2 Between 1962 and 1971, the United States distributed 7.8 million liters of Agent Blue containing 1,232,400 kg of arsenic across 300,000 hectares of rice paddies, 100,000 hectares of forest, and perimeters of all military bases during the Vietnam War.2,5 According to a review by Saha and colleagues, lower levels of arsenic exposure are associated with acute and chronic diseases, including cancers, of all organ systems.7
The following case presentation involves a Vietnam War veteran aged 70 years who was exposed to Agent Orange and developed 3 primary cancers, including cutaneous large B-cell non-Hodgkin lymphoma (NHL), high-grade urothelial carcinoma, and anal carcinoma in situ. Epidemiologically, this is an uncommon occurrence as only 8% of cancer survivors in the United States have been diagnosed with > 1 cancer.8
With no family history of cancer, the development of multiple malignancies raises concern for a history of toxin exposure. This report of a Vietnam War veteran with multiple conditions found to be associated with Agent Orange exposure provides an opportunity to discuss the role this exposure may have on the development of a comprehensive list of medical conditions as described by the literature. Additionally, the potential contributions of other confounding toxin exposures such as cigarette smoking, excessive alcohol use, and potential Agent Blue exposure on our patients’ health will be discussed.
Case Presentation
A male aged 70 years with Stage IV primary cutaneous large B-cell NHL, incompletely resected high-grade urothelial cancer, carcinoma in situ of the anal canal, and peripheral arterial disease (PAD) presented to the primary care clinic at the Washington DC Veterans Affairs Medical Center (DCVAMC) with concern for left leg ischemia. He also reported 2 large telangiectasias on his back for 6 months accompanied by lymphadenopathy and intermittent night sweats.
He was last seen at the DCVAMC 15 months prior after his twelfth dose of rituximab treatment for NHL. However, the patient failed to return for completion of his treatment due to frustration with the lengthy chemotherapy and follow-up process. Additionally, the patient's history included 3 failed arterial stents with complete nonadherence to the prescribed clopidogrel, resulting in the failure of 3 more subsequent graft placements. On presentation, the patient continued to report nonadherence with the clopidogrel.
The patient’s medical history included coronary artery disease (CAD) status after 2 stents in the left anterior descending artery and 1 stent in the proximal circumflex artery placed 4 years prior. He also had a history of hypertension, type 2 diabetes mellitus (T2DM), amyloid light-chain (AL) amyloidosis, aortic aneurysm, cataracts, obesity, treated hepatitis B and C, and posttraumatic stress disorder. He had no family history of cancer or AL amyloidosis; however, he noted that he was estranged from his family.
His social history was notable for active cigarette smoking up to 3 packs per day for 40 years and consuming large quantities of alcohol—at one point as many as 20 beers per day over a period of 4.5 years. He had a distant history of cocaine use but no current use, which was supported with negative urinary toxicology screens for illicit drugs over the past year.
Our patient also reported a history of Agent Orange exposure. As an artilleryman in the US Army III Corps, he was deployed for about 1 year in the most heavily sprayed regions of Vietnam, including Bien Hua, Long Binh, Xuan Loc, and Camp Zion for about 2 to 4 months at each location.
Hospital Course
The patient was treated on an inpatient basis for expedited workup and treatment for his urothelial carcinoma, NHL, and ischemic limb. His urothelial carcinoma was successfully resected, and the telangiectasias on his back were biopsied and found to be consistent with his known cutaneous large B-cell NHL, for which plans to resume outpatient chemotherapy were made. The patient’s 3 arterial grafts in his left leg were confirmed to have failed, and the patient was counseled that he would soon likely require an amputation of his ischemic leg.
Discussion
We must rely on our patient’s historical recall as there are no widely available laboratory tests or physical examination findings to confirm and/or determine the magnitude of TCDD or arsenic exposure.9-11
Exposures
The patient was stationed in Bien Hoa, the second highest dioxin-contaminated air base in Vietnam (Figure).6 Dioxin also is known to be a particularly persistent environmental pollutant, such that in January 2018, Bien Hoa was found to still have dioxin levels higher than what is considered internationally acceptable. In fact, these levels were deemed significant enough to lead the United States and Vietnamese government to sign a memorandum of intent to begin cleanup of this airport.6 TCDD is known to have a half-life of about 7.6 years, and its long half-life is mainly attributed to its slow elimination process from its stores within the liver and fat, consisting of passive excretion through the gut wall and slow metabolism by the liver.12,13 Thus, as an artilleryman mainly operating 105 howitzers within the foliage of Vietnam, our patient was exposed not only to high levels of this persistent environmental pollutant on a daily basis, but this toxin likely remained within his system for many years after his return from Vietnam.
Our patient also had a convincing history for potential Agent Blue exposure through both inhalation and ingestion of contaminated food and water. Additionally, his description of deforestations occurring within a matter of days increased the level of suspicion for Agent Blue exposure. This is because Agent Blue was the herbicide of choice for missions requiring rapid deforestation, achieving defoliation as quickly as 1 to 2 days.14 Additionally, our patient was stationed within cities in southern Vietnam near Agent Blue hot spots, such as Da Nang and Saigon, and Agent Blue was sprayed along the perimeter of all military bases.2
Levels of Evidence
Using the Veterans and Agent Orange Update in 2018 as our guide, we reviewed the quality of evidence suggesting an association between many of our patient’s comorbidities to Agent Orange exposure.5 This publication categorizes the level of evidence for association between health conditions and Agent Orange exposure in 4 main categories (Table 1).
In the Veterans and Agent Orange Update, NHL notably has a sufficient level of evidence of association with Agent Orange exposure.5 Although our patient’s extensive history of polysubstance use confounds the effect Agent Orange may have had on his health, cutaneous large B-cell NHL is an interesting exception as literature does not support even a correlative link between smoking and excessive alcohol use with primary cutaneous large B-cell NHL. Several case-control studies have found little to no association with cigarette smoking and the large B-cell subtype of NHL.15,16 Moreover, several studies have found that moderate- to-heavy alcohol use, especially beer, may have a protective effect against the development of NHL.17 Of note, our patient’s alcoholic beverage of choice was beer. Regarding our patient’s distant history of cocaine use, it has been reported that cocaine use, in the absence of an HIV infection, has not been found to increase the risk of developing NHL.18 Similarly, arsenic exposure has not been associated with NHL in the literature.19,20
The 2018 update also upgraded bladder carcinoma from having inadequate or insufficient to a limited or suggestive level of evidence for association.5 However, our patient’s most significant risk factor for bladder cancer was smoking, with a meta-analysis of 430,000 patients reporting a risk ratio (RR) of 3.14 for current cigarette smokers.21 The patient’s arsenic exposure from Agent Blue also increased his risk of developing bladder cancer. Several studies suggest a strong association between environmental arsenic exposure and bladder cancer.22-26 A 30-year meta-analysis of 40 studies by Saint-Jacques and colleagues reported that the incidence of bladder cancer was found to increase in a dose-dependent manner, with higher concentrations of arsenic contaminated wate, with incidence rising from 2.7 to 5.8 times as the amount of arsenic contamination water increased from 10 to 150 mg/L.
Our patient’s history is concerning for higher than average Agent Blue exposure compared with that of most Vietnam War veterans. Given the dose-dependent effect of arsenic on bladder cancer risk, both our patient’s history of smoking and Agent Blue exposure are risk factors in the development of his bladder cancer.22 These likely played a more significant role in his development of bladder cancer than did his Agent Orange exposure.
Finally, smoking is the most significant risk factor in our patient’s development of anal carcinoma in situ. The 2018 Agent Orange update does report limited/suggested evidence of no association between Agent Orange and anal carcinoma.5 It also is unknown whether Agent Blue exposure is a contributing cause to his development of anal carcinoma in situ.27 However, current smokers are at significant risk of developing anal cancer independent of age.28-30 Given our patient’s extensive smoking history, this is the most likely contributing factor.
Our patient also had several noncancer-related comorbidities with correlative associations with Agent Orange exposure of varying degrees (Table 2). Somewhat surprising, the development of our patient’s hypertension and T2DM may be associated in some way with his history of Agent Orange exposure. Hypertension had been recategorized from having limited or suggestive evidence to sufficient evidence in this committee’s most recent publication, and the committee is undecided on whether T2DM has a sufficient vs limited level of evidence for association with Agent Orange exposure.5 On the other hand, the committee continues to classify both ischemic heart disease and AL amyloidosis as having a limited or suggestive level of evidence that links Agent Orange exposure to these conditions.5
Arsenic may be another risk factor for our patient’s development of CAD and arterial insufficiency. Arsenic exposure is theorized to cause a direct toxic effect on coronary arteries, and arsenic exposure has been linked to PAD, CAD, and hypertension.31-34 Other significant and compelling risk factors for cardiovascular disease in our patient included his extensive history of heavy cigarette smoking, poorly controlled T2DM, obesity, and hypertension.35-37 AL amyloidosis is a rare disorder with an incidence of only 9 to 14 cases per million person-years.38,39 This disorder has not been linked to smoking or arsenic exposure in the literature. As our patient does not have a history of plasma dyscrasias or a family history of AL amyloidosis, the only known risk factors for AL amyloidosis that apply to our patient included NHL and Agent Orange exposure—NHL being a condition that is noted to be strongly correlated with Agent Orange exposure as discussed previously.5,36,40,41
Conclusions
This case describes a Vietnam War veteran with significant exposure to rainbow herbicides and considerable polysubstance who developed 3 primary cancers and several chronic medical conditions. His exposure to Agents Orange and Blue likely contributed to his medical problems, but these associations are confounded by his substance use, particularly cigarette smoking. Of all his comorbidities, our patient’s NHL is the condition most likely to be associated with his history of Agent Orange exposure. His Agent Blue exposure also increased his risk for developing bladder cancer, cardiovascular disease, and PAD.
This case also highlights the importance of evaluating Vietnam War veterans for rainbow herbicide exposure and the complexity associated with attributing diseases to these exposures. All veterans who served in the inland waterways of Vietnam between 1962 and 1975; in the Korean Demilitarized Zone between April 1, 1968 and August 31, 1971; or in Thailand between February 28, 1961 and May 7, 1975 were at risk of rainbow herbicide exposure. These veterans may not only be eligible for disability compensation but also should be screened for associated comorbidities as outlined by current research.42 We hope that this report will serve as an aid in achieving this mission.
Known as the “6 rainbow herbicides,” based on their identifying color on storage containers, the United States widely deployed the herbicides agents orange, green, pink, purple, white, and blue during the Vietnam War to deny the enemy cover and destroy crops.1 Unfortunately, all these herbicides were found to have contained some form of carcinogen. Agent Blue’s active ingredient consisted of sodium cacodylate trihydrate (C2H6AsNaO2), a compound that is metabolized into the organic form of the carcinogen arsenic before eventually converting into its relatively less toxic inorganic form.2 Agent Orange’s defoliating agent is 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). All rainbow herbicides except Agent Blue were unintentionally contaminated with carcinogenic dioxins. Agent Blue contained the carcinogen cacodylic acid, an organoarsenic acid. Today, herbicides no longer contain polychlorinated dibenzo-p-dioxins such as TCDD or arsenic due to strict manufacturing restrictions.2,3 In the treatment of veteran populations, knowledge of the 6 rainbow herbicides’ carcinogenic potential is important.
Between 1962 and 1971, the United States sprayed more than 45 million liters of Agent Orange on Vietnam and at least 366 kg of TCDD on South Vietnam.1,4 However, because Agent Orange was not a known carcinogen during the Vietnam War, records of exposure are poor. Additionally, individuals in Vietnam during this period were not the only ones exposed to this carcinogen as Agent Orange also was sprayed in Thailand and Korea.5 Even today there are still locations in Vietnam where Agent Orange concentrations exceed internationally acceptable levels. The Da Nang, Bien Hoa, and Phu Cat airports in Vietnam have been found to have dioxin levels exceeding 1000 ppt (parts of dioxin per trillion parts of lipid) toxicity equivalence in the soil. Although the Vietnam government is working toward decontaminating these and many other dioxin hotspots, residents in these locations are exposed to higher than internationally acceptable levels of dioxin.6
Despite receiving less media attention, Vietnam War veterans and Vietnamese soldiers and civilians were exposed to significant amounts of arsenic-based Agent Blue. Arsenic is a compound which has no environmental half-life and is carcinogenic humans if inhaled or ingested.2 Between 1962 and 1971, the United States distributed 7.8 million liters of Agent Blue containing 1,232,400 kg of arsenic across 300,000 hectares of rice paddies, 100,000 hectares of forest, and perimeters of all military bases during the Vietnam War.2,5 According to a review by Saha and colleagues, lower levels of arsenic exposure are associated with acute and chronic diseases, including cancers, of all organ systems.7
The following case presentation involves a Vietnam War veteran aged 70 years who was exposed to Agent Orange and developed 3 primary cancers, including cutaneous large B-cell non-Hodgkin lymphoma (NHL), high-grade urothelial carcinoma, and anal carcinoma in situ. Epidemiologically, this is an uncommon occurrence as only 8% of cancer survivors in the United States have been diagnosed with > 1 cancer.8
With no family history of cancer, the development of multiple malignancies raises concern for a history of toxin exposure. This report of a Vietnam War veteran with multiple conditions found to be associated with Agent Orange exposure provides an opportunity to discuss the role this exposure may have on the development of a comprehensive list of medical conditions as described by the literature. Additionally, the potential contributions of other confounding toxin exposures such as cigarette smoking, excessive alcohol use, and potential Agent Blue exposure on our patients’ health will be discussed.
Case Presentation
A male aged 70 years with Stage IV primary cutaneous large B-cell NHL, incompletely resected high-grade urothelial cancer, carcinoma in situ of the anal canal, and peripheral arterial disease (PAD) presented to the primary care clinic at the Washington DC Veterans Affairs Medical Center (DCVAMC) with concern for left leg ischemia. He also reported 2 large telangiectasias on his back for 6 months accompanied by lymphadenopathy and intermittent night sweats.
He was last seen at the DCVAMC 15 months prior after his twelfth dose of rituximab treatment for NHL. However, the patient failed to return for completion of his treatment due to frustration with the lengthy chemotherapy and follow-up process. Additionally, the patient's history included 3 failed arterial stents with complete nonadherence to the prescribed clopidogrel, resulting in the failure of 3 more subsequent graft placements. On presentation, the patient continued to report nonadherence with the clopidogrel.
The patient’s medical history included coronary artery disease (CAD) status after 2 stents in the left anterior descending artery and 1 stent in the proximal circumflex artery placed 4 years prior. He also had a history of hypertension, type 2 diabetes mellitus (T2DM), amyloid light-chain (AL) amyloidosis, aortic aneurysm, cataracts, obesity, treated hepatitis B and C, and posttraumatic stress disorder. He had no family history of cancer or AL amyloidosis; however, he noted that he was estranged from his family.
His social history was notable for active cigarette smoking up to 3 packs per day for 40 years and consuming large quantities of alcohol—at one point as many as 20 beers per day over a period of 4.5 years. He had a distant history of cocaine use but no current use, which was supported with negative urinary toxicology screens for illicit drugs over the past year.
Our patient also reported a history of Agent Orange exposure. As an artilleryman in the US Army III Corps, he was deployed for about 1 year in the most heavily sprayed regions of Vietnam, including Bien Hua, Long Binh, Xuan Loc, and Camp Zion for about 2 to 4 months at each location.
Hospital Course
The patient was treated on an inpatient basis for expedited workup and treatment for his urothelial carcinoma, NHL, and ischemic limb. His urothelial carcinoma was successfully resected, and the telangiectasias on his back were biopsied and found to be consistent with his known cutaneous large B-cell NHL, for which plans to resume outpatient chemotherapy were made. The patient’s 3 arterial grafts in his left leg were confirmed to have failed, and the patient was counseled that he would soon likely require an amputation of his ischemic leg.
Discussion
We must rely on our patient’s historical recall as there are no widely available laboratory tests or physical examination findings to confirm and/or determine the magnitude of TCDD or arsenic exposure.9-11
Exposures
The patient was stationed in Bien Hoa, the second highest dioxin-contaminated air base in Vietnam (Figure).6 Dioxin also is known to be a particularly persistent environmental pollutant, such that in January 2018, Bien Hoa was found to still have dioxin levels higher than what is considered internationally acceptable. In fact, these levels were deemed significant enough to lead the United States and Vietnamese government to sign a memorandum of intent to begin cleanup of this airport.6 TCDD is known to have a half-life of about 7.6 years, and its long half-life is mainly attributed to its slow elimination process from its stores within the liver and fat, consisting of passive excretion through the gut wall and slow metabolism by the liver.12,13 Thus, as an artilleryman mainly operating 105 howitzers within the foliage of Vietnam, our patient was exposed not only to high levels of this persistent environmental pollutant on a daily basis, but this toxin likely remained within his system for many years after his return from Vietnam.
Our patient also had a convincing history for potential Agent Blue exposure through both inhalation and ingestion of contaminated food and water. Additionally, his description of deforestations occurring within a matter of days increased the level of suspicion for Agent Blue exposure. This is because Agent Blue was the herbicide of choice for missions requiring rapid deforestation, achieving defoliation as quickly as 1 to 2 days.14 Additionally, our patient was stationed within cities in southern Vietnam near Agent Blue hot spots, such as Da Nang and Saigon, and Agent Blue was sprayed along the perimeter of all military bases.2
Levels of Evidence
Using the Veterans and Agent Orange Update in 2018 as our guide, we reviewed the quality of evidence suggesting an association between many of our patient’s comorbidities to Agent Orange exposure.5 This publication categorizes the level of evidence for association between health conditions and Agent Orange exposure in 4 main categories (Table 1).
In the Veterans and Agent Orange Update, NHL notably has a sufficient level of evidence of association with Agent Orange exposure.5 Although our patient’s extensive history of polysubstance use confounds the effect Agent Orange may have had on his health, cutaneous large B-cell NHL is an interesting exception as literature does not support even a correlative link between smoking and excessive alcohol use with primary cutaneous large B-cell NHL. Several case-control studies have found little to no association with cigarette smoking and the large B-cell subtype of NHL.15,16 Moreover, several studies have found that moderate- to-heavy alcohol use, especially beer, may have a protective effect against the development of NHL.17 Of note, our patient’s alcoholic beverage of choice was beer. Regarding our patient’s distant history of cocaine use, it has been reported that cocaine use, in the absence of an HIV infection, has not been found to increase the risk of developing NHL.18 Similarly, arsenic exposure has not been associated with NHL in the literature.19,20
The 2018 update also upgraded bladder carcinoma from having inadequate or insufficient to a limited or suggestive level of evidence for association.5 However, our patient’s most significant risk factor for bladder cancer was smoking, with a meta-analysis of 430,000 patients reporting a risk ratio (RR) of 3.14 for current cigarette smokers.21 The patient’s arsenic exposure from Agent Blue also increased his risk of developing bladder cancer. Several studies suggest a strong association between environmental arsenic exposure and bladder cancer.22-26 A 30-year meta-analysis of 40 studies by Saint-Jacques and colleagues reported that the incidence of bladder cancer was found to increase in a dose-dependent manner, with higher concentrations of arsenic contaminated wate, with incidence rising from 2.7 to 5.8 times as the amount of arsenic contamination water increased from 10 to 150 mg/L.
Our patient’s history is concerning for higher than average Agent Blue exposure compared with that of most Vietnam War veterans. Given the dose-dependent effect of arsenic on bladder cancer risk, both our patient’s history of smoking and Agent Blue exposure are risk factors in the development of his bladder cancer.22 These likely played a more significant role in his development of bladder cancer than did his Agent Orange exposure.
Finally, smoking is the most significant risk factor in our patient’s development of anal carcinoma in situ. The 2018 Agent Orange update does report limited/suggested evidence of no association between Agent Orange and anal carcinoma.5 It also is unknown whether Agent Blue exposure is a contributing cause to his development of anal carcinoma in situ.27 However, current smokers are at significant risk of developing anal cancer independent of age.28-30 Given our patient’s extensive smoking history, this is the most likely contributing factor.
Our patient also had several noncancer-related comorbidities with correlative associations with Agent Orange exposure of varying degrees (Table 2). Somewhat surprising, the development of our patient’s hypertension and T2DM may be associated in some way with his history of Agent Orange exposure. Hypertension had been recategorized from having limited or suggestive evidence to sufficient evidence in this committee’s most recent publication, and the committee is undecided on whether T2DM has a sufficient vs limited level of evidence for association with Agent Orange exposure.5 On the other hand, the committee continues to classify both ischemic heart disease and AL amyloidosis as having a limited or suggestive level of evidence that links Agent Orange exposure to these conditions.5
Arsenic may be another risk factor for our patient’s development of CAD and arterial insufficiency. Arsenic exposure is theorized to cause a direct toxic effect on coronary arteries, and arsenic exposure has been linked to PAD, CAD, and hypertension.31-34 Other significant and compelling risk factors for cardiovascular disease in our patient included his extensive history of heavy cigarette smoking, poorly controlled T2DM, obesity, and hypertension.35-37 AL amyloidosis is a rare disorder with an incidence of only 9 to 14 cases per million person-years.38,39 This disorder has not been linked to smoking or arsenic exposure in the literature. As our patient does not have a history of plasma dyscrasias or a family history of AL amyloidosis, the only known risk factors for AL amyloidosis that apply to our patient included NHL and Agent Orange exposure—NHL being a condition that is noted to be strongly correlated with Agent Orange exposure as discussed previously.5,36,40,41
Conclusions
This case describes a Vietnam War veteran with significant exposure to rainbow herbicides and considerable polysubstance who developed 3 primary cancers and several chronic medical conditions. His exposure to Agents Orange and Blue likely contributed to his medical problems, but these associations are confounded by his substance use, particularly cigarette smoking. Of all his comorbidities, our patient’s NHL is the condition most likely to be associated with his history of Agent Orange exposure. His Agent Blue exposure also increased his risk for developing bladder cancer, cardiovascular disease, and PAD.
This case also highlights the importance of evaluating Vietnam War veterans for rainbow herbicide exposure and the complexity associated with attributing diseases to these exposures. All veterans who served in the inland waterways of Vietnam between 1962 and 1975; in the Korean Demilitarized Zone between April 1, 1968 and August 31, 1971; or in Thailand between February 28, 1961 and May 7, 1975 were at risk of rainbow herbicide exposure. These veterans may not only be eligible for disability compensation but also should be screened for associated comorbidities as outlined by current research.42 We hope that this report will serve as an aid in achieving this mission.
1. Stellman JM, Stellman SD, Christian R, Weber T, Tomasallo C. The extent and patterns of usage of Agent Orange and other herbicides in Vietnam. Nature. 2003;422(6933):681-687. doi:10.1038/nature01537
2. Olson K, Cihacek L. The fate of Agent Blue, the arsenic based herbicide, used in South Vietnam during the Vietnam War. Open J Soil Sci. 2020;10:518-577. doi:10.4236/ojss.2020.1011027
3. Lee Chang A, Dym AA, Venegas-Borsellino C, et al. Comparison between simulation-based training and lecture-based education in teaching situation awareness. a randomized controlled study. Ann Am Thorac Soc. 2017;14(4):529-535. doi:10.1513/AnnalsATS.201612-950OC
4. Stellman SD. Agent Orange during the Vietnam War: the lingering issue of its civilian and military health impact. Am J Public Health. 2018;108(6):726-728. doi:10.2105/AJPH.2018.304426
5. National Academies of Sciences, Engineering, and Medicine. Veterans and Agent Orange: Update 11 (2018). The National Academies Press; 2018. doi:10.17226/25137
6. Martin MF. US Agent Orange/dioxin assistance to Vietnam. Updated January 15, 2021. Accessed June 17, 2021. https://fas.org/sgp/crs/row/R44268.pdf
7. Saha JC, Dikshit AK, Bandyopadhyay M, Saha KC. A review of arsenic poisoning and its effects on human health. Crit Rev Environ Sci Technol. 1999;29(3):281-313. doi:10.1080/10643389991259227
8. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551
9. American Cancer Society. Agent Orange and cancer risk. Updated June 9, 2020. Accessed June 17, 2021. https://www.cancer.org/cancer/cancer-causes/agent-orange-and-cancer.html
10. US Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Updated June 16, 2021. Accessed June 17, 2021. https://www.publichealth.va.gov/exposures/agentorange/conditions/index.asp
11. Katz SA. On the use of hair analysis for assessing arsenic intoxication. Int J Environ Res Public Health. 2019;16(6):977. Published 2019 Mar 18. doi:10.3390/ijerph16060977
12. Chang ET, Boffetta P, Adami HO, Mandel JS. A critical review of the epidemiology of Agent Orange or 2,3,7,8-tetrachlorodibenzo-p-dioxin and lymphoid malignancies. Ann Epidemiol. 2015;25(4):275-292.e30. doi:10.1016/j.annepidem.2015.01.002
13. Kramárová E, Kogevinas M, Anh CT, et al. Exposure to Agent Orange and occurrence of soft-tissue sarcomas or non-Hodgkin lymphomas: an ongoing study in Vietnam. Environ Health Perspect. 1998;106 Suppl 2(suppl 2):671-678. doi:10.1289/ehp.106-1533419
14. Institute of Medicine (US) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides. Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam. National Academies Press US; 1994.
15. Morton LM, Hartge P, Holford TR, et al. Cigarette smoking and risk of non-Hodgkin lymphoma: a pooled analysis from the International Lymphoma Epidemiology Consortium (interlymph). Cancer Epidemiol Biomarkers Prev. 2005;14(4):925-933. doi:10.1158/1055-9965.EPI-04-0693
16. Schöllkopf C, Smedby KE, Hjalgrim H, et al. Cigarette smoking and risk of non-Hodgkin’s lymphoma--a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1791-1796. doi:10.1158/1055-9965.EPI-05-0077
17. Psaltopoulou T, Sergentanis TN, Ntanasis-Stathopoulos I, Tzanninis IG, Tsilimigras DI, Dimopoulos MA. Alcohol consumption and risk of hematological malignancies: a meta-analysis of prospective studies. Int J Cancer. 2018;143(3):486-495. doi:10.1002/ijc.31330
18. Aujla AS, Lee SH. Association between cocaine use and hematological malignancies. J Clin Oncol. 2016;34(15_suppl):e19072-e19072. doi:10.1200/JCO.2016.34.15_suppl.e19072
19. Mao Y, Hu J, Ugnat AM, White K. Non-Hodgkin’s lymphoma and occupational exposure to chemicals in Canada. Canadian Cancer Registries Epidemiology Research Group. Ann Oncol. 2000;11 (suppl 1):69-73. doi:10.1093/annonc/11.suppl_1.S69
20. Kelekci KH, Bilgin I, Ermete M. Arsenical keratoses and non-Hodgkin’s lymphoma: arsenic-induced or coincidental conditions? J Pakistan Assoc Dermatol. 2012;22(4):366-369.
21. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96-108. doi:10.1016/j.eururo.2016.06.010
22. Saint-Jacques N, Parker L, Brown P, Dummer TJ. Arsenic in drinking water and urinary tract cancers: a systematic review of 30 years of epidemiological evidence. Environ Health. 2014;13:44. Published 2014 Jun 2. doi:10.1186/1476-069X-13-44
23. Radosavljevic′ V, Jakovljevic′ B. Arsenic and bladder cancer: observations and suggestions. J Environ Health. 2008;71(3):40-42.
24. Marsit CJ, Karagas MR, Schned A, Kelsey KT. Carcinogen exposure and epigenetic silencing in bladder cancer. Ann N Y Acad Sci. 2006;1076(1):810-821. doi:10.1196/annals.1371.031
25. Mendez WM Jr, Eftim S, Cohen J, et al. Relationships between arsenic concentrations in drinking water and lung and bladder cancer incidence in U.S. counties. J Expo Sci Environ Epidemiol. 2017;27(3):235-243. doi:10.1038/jes.2016.58
26. Pal DK, Agrawal A, Ghosh S, Ghosh A. Association of arsenic with recurrence of urinary bladder cancer. Trop Doct. 2020;50(4):325-330. doi:10.1177/0049475520930155
27. García-Esquinas E, Pollán M, Umans JG, et al. Arsenic exposure and cancer mortality in a US-based prospective cohort: the strong heart study [published correction appears in Cancer Epidemiol Biomarkers Prev. 2013;22(8):1479]. Cancer Epidemiol Biomarkers Prev. 2013;22(11):1944-1953. doi:10.1158/1055-9965.EPI-13-0234-T
28. Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101(2):270-280. doi:10.1002/cncr.20365
29. Bertisch B, Franceschi S, Lise M, et al; Swiss HIV Cohort Study Investigators. Risk factors for anal cancer in persons infected with HIV: a nested case-control study in the Swiss HIV Cohort Study. Am J Epidemiol. 2013;178(6):877-884. doi:10.1093/aje/kwt153
30. Rabkin CS, Biggar RJ, Melbye M, Curtis RE. Second primary cancers following anal and cervical carcinoma: evidence of shared etiologic factors. Am J Epidemiol. 1992;136(1):54-58. doi:10.1093/oxfordjournals.aje.a116420

31. Newman JD, Navas-Acien A, Kuo CC, et al. Peripheral arterial disease and its association with arsenic exposure and metabolism in the Strong Heart Study. Am J Epidemiol. 2016;184(11):806-817. doi:10.1093/aje/kww002
32. Moon KA, Guallar E, Umans JG, et al. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. A prospective cohort study. Ann Intern Med. 2013;159(10):649-659. doi:10.7326/0003-4819-159-10-201311190-00719
33. Moon K, Guallar E, Navas-Acien A. Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep. 2012;14(6):542-555. doi:10.1007/s11883-012-0280-x
34. Stea F, Bianchi F, Cori L, Sicari R. Cardiovascular effects of arsenic: clinical and epidemiological findings. Environ Sci Pollut Res Int. 2014;21(1):244-251. doi:10.1007/s11356-013-2113-z
35. Burns DM. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis. 2003;46(1):11-29. doi:10.1016/s0033-0620(03)00079-3
36. Merlini G, Dispenzieri A, Sanchorawala V, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4(1):38. Published 2018 Oct 25. doi:10.1038/s41572-018-0034-3
37. Dokken BB. The pathophysiology of cardiovascular disease and diabetes: beyond blood pressure and lipids. Diabetes Spectrum. 2008;21(3):160-165. doi:10.2337/diaspect.21.3.160
38. Vaxman I, Gertz M. Recent advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019;141(2):93-106. doi:10.1159/000495455
39. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046-1053. doi:10.1182/bloodadvances.2018016402
40. Basset M, Defrancesco I, Milani P, et al. Nonlymphoplasmacytic lymphomas associated with light-chain amyloidosis. Blood. 2020;135(4):293-296. doi:10.1182/blood.2019002762
41. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564-569. doi:10.1056/NEJMoa01133202
42. US Department of Veterans Affairs. Agent Orange registry health exam for veterans. Updated May 28, 2021. Accessed June 17, 2021. https://www.publichealth.va.gov/exposures/agentorange/benefits/registry-exam.asp
1. Stellman JM, Stellman SD, Christian R, Weber T, Tomasallo C. The extent and patterns of usage of Agent Orange and other herbicides in Vietnam. Nature. 2003;422(6933):681-687. doi:10.1038/nature01537
2. Olson K, Cihacek L. The fate of Agent Blue, the arsenic based herbicide, used in South Vietnam during the Vietnam War. Open J Soil Sci. 2020;10:518-577. doi:10.4236/ojss.2020.1011027
3. Lee Chang A, Dym AA, Venegas-Borsellino C, et al. Comparison between simulation-based training and lecture-based education in teaching situation awareness. a randomized controlled study. Ann Am Thorac Soc. 2017;14(4):529-535. doi:10.1513/AnnalsATS.201612-950OC
4. Stellman SD. Agent Orange during the Vietnam War: the lingering issue of its civilian and military health impact. Am J Public Health. 2018;108(6):726-728. doi:10.2105/AJPH.2018.304426
5. National Academies of Sciences, Engineering, and Medicine. Veterans and Agent Orange: Update 11 (2018). The National Academies Press; 2018. doi:10.17226/25137
6. Martin MF. US Agent Orange/dioxin assistance to Vietnam. Updated January 15, 2021. Accessed June 17, 2021. https://fas.org/sgp/crs/row/R44268.pdf
7. Saha JC, Dikshit AK, Bandyopadhyay M, Saha KC. A review of arsenic poisoning and its effects on human health. Crit Rev Environ Sci Technol. 1999;29(3):281-313. doi:10.1080/10643389991259227
8. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551
9. American Cancer Society. Agent Orange and cancer risk. Updated June 9, 2020. Accessed June 17, 2021. https://www.cancer.org/cancer/cancer-causes/agent-orange-and-cancer.html
10. US Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Updated June 16, 2021. Accessed June 17, 2021. https://www.publichealth.va.gov/exposures/agentorange/conditions/index.asp
11. Katz SA. On the use of hair analysis for assessing arsenic intoxication. Int J Environ Res Public Health. 2019;16(6):977. Published 2019 Mar 18. doi:10.3390/ijerph16060977
12. Chang ET, Boffetta P, Adami HO, Mandel JS. A critical review of the epidemiology of Agent Orange or 2,3,7,8-tetrachlorodibenzo-p-dioxin and lymphoid malignancies. Ann Epidemiol. 2015;25(4):275-292.e30. doi:10.1016/j.annepidem.2015.01.002
13. Kramárová E, Kogevinas M, Anh CT, et al. Exposure to Agent Orange and occurrence of soft-tissue sarcomas or non-Hodgkin lymphomas: an ongoing study in Vietnam. Environ Health Perspect. 1998;106 Suppl 2(suppl 2):671-678. doi:10.1289/ehp.106-1533419
14. Institute of Medicine (US) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides. Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam. National Academies Press US; 1994.
15. Morton LM, Hartge P, Holford TR, et al. Cigarette smoking and risk of non-Hodgkin lymphoma: a pooled analysis from the International Lymphoma Epidemiology Consortium (interlymph). Cancer Epidemiol Biomarkers Prev. 2005;14(4):925-933. doi:10.1158/1055-9965.EPI-04-0693
16. Schöllkopf C, Smedby KE, Hjalgrim H, et al. Cigarette smoking and risk of non-Hodgkin’s lymphoma--a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1791-1796. doi:10.1158/1055-9965.EPI-05-0077
17. Psaltopoulou T, Sergentanis TN, Ntanasis-Stathopoulos I, Tzanninis IG, Tsilimigras DI, Dimopoulos MA. Alcohol consumption and risk of hematological malignancies: a meta-analysis of prospective studies. Int J Cancer. 2018;143(3):486-495. doi:10.1002/ijc.31330
18. Aujla AS, Lee SH. Association between cocaine use and hematological malignancies. J Clin Oncol. 2016;34(15_suppl):e19072-e19072. doi:10.1200/JCO.2016.34.15_suppl.e19072
19. Mao Y, Hu J, Ugnat AM, White K. Non-Hodgkin’s lymphoma and occupational exposure to chemicals in Canada. Canadian Cancer Registries Epidemiology Research Group. Ann Oncol. 2000;11 (suppl 1):69-73. doi:10.1093/annonc/11.suppl_1.S69
20. Kelekci KH, Bilgin I, Ermete M. Arsenical keratoses and non-Hodgkin’s lymphoma: arsenic-induced or coincidental conditions? J Pakistan Assoc Dermatol. 2012;22(4):366-369.
21. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96-108. doi:10.1016/j.eururo.2016.06.010
22. Saint-Jacques N, Parker L, Brown P, Dummer TJ. Arsenic in drinking water and urinary tract cancers: a systematic review of 30 years of epidemiological evidence. Environ Health. 2014;13:44. Published 2014 Jun 2. doi:10.1186/1476-069X-13-44
23. Radosavljevic′ V, Jakovljevic′ B. Arsenic and bladder cancer: observations and suggestions. J Environ Health. 2008;71(3):40-42.
24. Marsit CJ, Karagas MR, Schned A, Kelsey KT. Carcinogen exposure and epigenetic silencing in bladder cancer. Ann N Y Acad Sci. 2006;1076(1):810-821. doi:10.1196/annals.1371.031
25. Mendez WM Jr, Eftim S, Cohen J, et al. Relationships between arsenic concentrations in drinking water and lung and bladder cancer incidence in U.S. counties. J Expo Sci Environ Epidemiol. 2017;27(3):235-243. doi:10.1038/jes.2016.58
26. Pal DK, Agrawal A, Ghosh S, Ghosh A. Association of arsenic with recurrence of urinary bladder cancer. Trop Doct. 2020;50(4):325-330. doi:10.1177/0049475520930155
27. García-Esquinas E, Pollán M, Umans JG, et al. Arsenic exposure and cancer mortality in a US-based prospective cohort: the strong heart study [published correction appears in Cancer Epidemiol Biomarkers Prev. 2013;22(8):1479]. Cancer Epidemiol Biomarkers Prev. 2013;22(11):1944-1953. doi:10.1158/1055-9965.EPI-13-0234-T
28. Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101(2):270-280. doi:10.1002/cncr.20365
29. Bertisch B, Franceschi S, Lise M, et al; Swiss HIV Cohort Study Investigators. Risk factors for anal cancer in persons infected with HIV: a nested case-control study in the Swiss HIV Cohort Study. Am J Epidemiol. 2013;178(6):877-884. doi:10.1093/aje/kwt153
30. Rabkin CS, Biggar RJ, Melbye M, Curtis RE. Second primary cancers following anal and cervical carcinoma: evidence of shared etiologic factors. Am J Epidemiol. 1992;136(1):54-58. doi:10.1093/oxfordjournals.aje.a116420

31. Newman JD, Navas-Acien A, Kuo CC, et al. Peripheral arterial disease and its association with arsenic exposure and metabolism in the Strong Heart Study. Am J Epidemiol. 2016;184(11):806-817. doi:10.1093/aje/kww002
32. Moon KA, Guallar E, Umans JG, et al. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. A prospective cohort study. Ann Intern Med. 2013;159(10):649-659. doi:10.7326/0003-4819-159-10-201311190-00719
33. Moon K, Guallar E, Navas-Acien A. Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep. 2012;14(6):542-555. doi:10.1007/s11883-012-0280-x
34. Stea F, Bianchi F, Cori L, Sicari R. Cardiovascular effects of arsenic: clinical and epidemiological findings. Environ Sci Pollut Res Int. 2014;21(1):244-251. doi:10.1007/s11356-013-2113-z
35. Burns DM. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis. 2003;46(1):11-29. doi:10.1016/s0033-0620(03)00079-3
36. Merlini G, Dispenzieri A, Sanchorawala V, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4(1):38. Published 2018 Oct 25. doi:10.1038/s41572-018-0034-3
37. Dokken BB. The pathophysiology of cardiovascular disease and diabetes: beyond blood pressure and lipids. Diabetes Spectrum. 2008;21(3):160-165. doi:10.2337/diaspect.21.3.160
38. Vaxman I, Gertz M. Recent advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019;141(2):93-106. doi:10.1159/000495455
39. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046-1053. doi:10.1182/bloodadvances.2018016402
40. Basset M, Defrancesco I, Milani P, et al. Nonlymphoplasmacytic lymphomas associated with light-chain amyloidosis. Blood. 2020;135(4):293-296. doi:10.1182/blood.2019002762
41. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564-569. doi:10.1056/NEJMoa01133202
42. US Department of Veterans Affairs. Agent Orange registry health exam for veterans. Updated May 28, 2021. Accessed June 17, 2021. https://www.publichealth.va.gov/exposures/agentorange/benefits/registry-exam.asp
Secretary of Defense Seeks Approval To Make COVID Vaccines Mandatory For DoD Employees
New policy hopes to be in line with full FDA approval expected in September. When the largest employer in the world makes any significant decision, everyone sits up and takes notice.
That’s what happened when Secretary of Defense Lloyd Austin III sent out a memo to all US Department of Defense (DoD) employees saying he was seeking President Biden’s approval to make COVID-19 vaccines mandatory. His decision affects not only the 3.2 million employees on the payroll, but their families, communities, and states. Florida, for instance, where approximately 40% of the population remains unvaccinated has about 55,000 active duty service members and 36,000 reservists.
Vaccination rates in the military have lagged behind other populations, especially among Black and Hispanic service members. An April study published in Medical Surveillance Monthly Report found that “non-Hispanic Blacks, as well as those who were female, younger, of lower rank, with lower education levels, and those serving in the Army were less likely to initiate COVID-19 vaccination after adjusting for other factors.”
The decision had been in the offing for some time but when cases of the Delta variant of the virus began to spike in July, President Biden asked Sec. Austin to consider how and when the COVID vaccine could be added to the list of required vaccines for service members. It’s a long list already: Depending on their location, service members can get as many as 17 vaccines. It also folllows on the heals of the decision by the US Department of Veterans Affairs to require vaccinations for frontline health care workers.
Austin promised to “not let grass grow.” He consulted with Army Gen. Mark Milley, the Joint Chiefs of Staff, service chiefs, service secretaries, and medical professionals. Based on those discussions, he decided to ask for approval to make the vaccines mandatory no later than mid-September or immediately upon FDA licensure, whichever comes first.
However, he added, “[t]o defend this Nation, we need a healthy and ready force. I strongly encourage all DoD military and civilian personnel—as well as contractor personnel—to get vaccinated now and for military Service members to not wait for the mandate.” Currently, 73% of active-duty personnel have had at least one dose of the vaccine.
Leaping upon the news—and based on the wording in the memo—some in the media were reporting that it meant all troops have to be vaccinated by mid-September. “He’ll make the request by mid-September, unless or until FDA licensure occurs before that time, at which point the Secretary has the authority he needs…to make whatever vaccine is then given that license mandatory.” That’s not the case, said Pentagon press secretary John Kirby in a briefing. Some voices also have called on the DoD to do more to dispel vaccine hesitancy among the troops.
In the meantime, Kirby said, “[T]wo things are going to happen. One, the services are going to be tasked to come back to the Secretary with implementation plans for how they’re going to get this moving.” Noting that mid-September isn’t far away, he pointed out that the services have a “fair but limited amount of time” to arrange their implementation plans. “I have every confidence that service leadership and your commanders will implement this new vaccination program with professionalism, skill, and compassion,” Austin wrote in his memo.
The second thing, Kirby said, was that DoD would be developing policies that comply with the President’s direction that the unvaccinated will have to be subjected to “certain requirements and restrictions.” The Delta variant is hitting the unvaccinated hardest. Austin said the DoD will keep a close eye on infection rates “and the impact these rates might have on our readiness. I will not hesitate to act sooner or recommend a different course to the President if I feel the need to do so.”
Kirby said he didn’t have all the details for that yet, but the department is “working hard” on a policy directive that will clarify what those requirements and restrictions might be.
President Biden replied almost immediately to Austin’s message. “I strongly support Secretary Austin’s message to the force today…. Secretary Austin and I share an unshakeable commitment to making sure our troops have every tool they need to do their jobs as safely as possible. These vaccines will save lives. Period.”
“All FDA-authorized COVID-19 vaccines are safe and highly effective,” Austin said in the close to his memo. “They will protect you and your family. They will protect your unit, your ship, and your co-workers. …Get the shot. Stay healthy. Stay ready.”
New policy hopes to be in line with full FDA approval expected in September. When the largest employer in the world makes any significant decision, everyone sits up and takes notice.
That’s what happened when Secretary of Defense Lloyd Austin III sent out a memo to all US Department of Defense (DoD) employees saying he was seeking President Biden’s approval to make COVID-19 vaccines mandatory. His decision affects not only the 3.2 million employees on the payroll, but their families, communities, and states. Florida, for instance, where approximately 40% of the population remains unvaccinated has about 55,000 active duty service members and 36,000 reservists.
Vaccination rates in the military have lagged behind other populations, especially among Black and Hispanic service members. An April study published in Medical Surveillance Monthly Report found that “non-Hispanic Blacks, as well as those who were female, younger, of lower rank, with lower education levels, and those serving in the Army were less likely to initiate COVID-19 vaccination after adjusting for other factors.”
The decision had been in the offing for some time but when cases of the Delta variant of the virus began to spike in July, President Biden asked Sec. Austin to consider how and when the COVID vaccine could be added to the list of required vaccines for service members. It’s a long list already: Depending on their location, service members can get as many as 17 vaccines. It also folllows on the heals of the decision by the US Department of Veterans Affairs to require vaccinations for frontline health care workers.
Austin promised to “not let grass grow.” He consulted with Army Gen. Mark Milley, the Joint Chiefs of Staff, service chiefs, service secretaries, and medical professionals. Based on those discussions, he decided to ask for approval to make the vaccines mandatory no later than mid-September or immediately upon FDA licensure, whichever comes first.
However, he added, “[t]o defend this Nation, we need a healthy and ready force. I strongly encourage all DoD military and civilian personnel—as well as contractor personnel—to get vaccinated now and for military Service members to not wait for the mandate.” Currently, 73% of active-duty personnel have had at least one dose of the vaccine.
Leaping upon the news—and based on the wording in the memo—some in the media were reporting that it meant all troops have to be vaccinated by mid-September. “He’ll make the request by mid-September, unless or until FDA licensure occurs before that time, at which point the Secretary has the authority he needs…to make whatever vaccine is then given that license mandatory.” That’s not the case, said Pentagon press secretary John Kirby in a briefing. Some voices also have called on the DoD to do more to dispel vaccine hesitancy among the troops.
In the meantime, Kirby said, “[T]wo things are going to happen. One, the services are going to be tasked to come back to the Secretary with implementation plans for how they’re going to get this moving.” Noting that mid-September isn’t far away, he pointed out that the services have a “fair but limited amount of time” to arrange their implementation plans. “I have every confidence that service leadership and your commanders will implement this new vaccination program with professionalism, skill, and compassion,” Austin wrote in his memo.
The second thing, Kirby said, was that DoD would be developing policies that comply with the President’s direction that the unvaccinated will have to be subjected to “certain requirements and restrictions.” The Delta variant is hitting the unvaccinated hardest. Austin said the DoD will keep a close eye on infection rates “and the impact these rates might have on our readiness. I will not hesitate to act sooner or recommend a different course to the President if I feel the need to do so.”
Kirby said he didn’t have all the details for that yet, but the department is “working hard” on a policy directive that will clarify what those requirements and restrictions might be.
President Biden replied almost immediately to Austin’s message. “I strongly support Secretary Austin’s message to the force today…. Secretary Austin and I share an unshakeable commitment to making sure our troops have every tool they need to do their jobs as safely as possible. These vaccines will save lives. Period.”
“All FDA-authorized COVID-19 vaccines are safe and highly effective,” Austin said in the close to his memo. “They will protect you and your family. They will protect your unit, your ship, and your co-workers. …Get the shot. Stay healthy. Stay ready.”
New policy hopes to be in line with full FDA approval expected in September. When the largest employer in the world makes any significant decision, everyone sits up and takes notice.
That’s what happened when Secretary of Defense Lloyd Austin III sent out a memo to all US Department of Defense (DoD) employees saying he was seeking President Biden’s approval to make COVID-19 vaccines mandatory. His decision affects not only the 3.2 million employees on the payroll, but their families, communities, and states. Florida, for instance, where approximately 40% of the population remains unvaccinated has about 55,000 active duty service members and 36,000 reservists.
Vaccination rates in the military have lagged behind other populations, especially among Black and Hispanic service members. An April study published in Medical Surveillance Monthly Report found that “non-Hispanic Blacks, as well as those who were female, younger, of lower rank, with lower education levels, and those serving in the Army were less likely to initiate COVID-19 vaccination after adjusting for other factors.”
The decision had been in the offing for some time but when cases of the Delta variant of the virus began to spike in July, President Biden asked Sec. Austin to consider how and when the COVID vaccine could be added to the list of required vaccines for service members. It’s a long list already: Depending on their location, service members can get as many as 17 vaccines. It also folllows on the heals of the decision by the US Department of Veterans Affairs to require vaccinations for frontline health care workers.
Austin promised to “not let grass grow.” He consulted with Army Gen. Mark Milley, the Joint Chiefs of Staff, service chiefs, service secretaries, and medical professionals. Based on those discussions, he decided to ask for approval to make the vaccines mandatory no later than mid-September or immediately upon FDA licensure, whichever comes first.
However, he added, “[t]o defend this Nation, we need a healthy and ready force. I strongly encourage all DoD military and civilian personnel—as well as contractor personnel—to get vaccinated now and for military Service members to not wait for the mandate.” Currently, 73% of active-duty personnel have had at least one dose of the vaccine.
Leaping upon the news—and based on the wording in the memo—some in the media were reporting that it meant all troops have to be vaccinated by mid-September. “He’ll make the request by mid-September, unless or until FDA licensure occurs before that time, at which point the Secretary has the authority he needs…to make whatever vaccine is then given that license mandatory.” That’s not the case, said Pentagon press secretary John Kirby in a briefing. Some voices also have called on the DoD to do more to dispel vaccine hesitancy among the troops.
In the meantime, Kirby said, “[T]wo things are going to happen. One, the services are going to be tasked to come back to the Secretary with implementation plans for how they’re going to get this moving.” Noting that mid-September isn’t far away, he pointed out that the services have a “fair but limited amount of time” to arrange their implementation plans. “I have every confidence that service leadership and your commanders will implement this new vaccination program with professionalism, skill, and compassion,” Austin wrote in his memo.
The second thing, Kirby said, was that DoD would be developing policies that comply with the President’s direction that the unvaccinated will have to be subjected to “certain requirements and restrictions.” The Delta variant is hitting the unvaccinated hardest. Austin said the DoD will keep a close eye on infection rates “and the impact these rates might have on our readiness. I will not hesitate to act sooner or recommend a different course to the President if I feel the need to do so.”
Kirby said he didn’t have all the details for that yet, but the department is “working hard” on a policy directive that will clarify what those requirements and restrictions might be.
President Biden replied almost immediately to Austin’s message. “I strongly support Secretary Austin’s message to the force today…. Secretary Austin and I share an unshakeable commitment to making sure our troops have every tool they need to do their jobs as safely as possible. These vaccines will save lives. Period.”
“All FDA-authorized COVID-19 vaccines are safe and highly effective,” Austin said in the close to his memo. “They will protect you and your family. They will protect your unit, your ship, and your co-workers. …Get the shot. Stay healthy. Stay ready.”
CDC officially endorses third dose of mRNA vaccines for immunocompromised
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, has officially signed off on a recommendation by an independent panel of 11 experts to allow people with weakened immune function to get a third dose of certain COVID-19 vaccines.
The decision follows a unanimous vote by the CDC’s Advisory Committee on Immunization Practices (ACIP), which in turn came hours after the U.S. Food and Drug Administration updated its Emergency Use Authorization (EUA) for the Pfizer and Moderna mRNA vaccines.
About 7 million adults in the United States have moderately to severely impaired immune function because of a medical condition they live with or a medication they take to manage a health condition.
People who fall into this category are at higher risk of being hospitalized or dying if they get COVID-19. They are also more likely to transmit the infection. About 40% of vaccinated patients who are hospitalized with breakthrough cases are immunocompromised.
Recent studies have shown that between one-third and one-half of immunocompromised people who didn’t develop antibodies after two doses of a vaccine do get some level of protection after a third dose.
Even then, however, the protection immunocompromised people get from vaccines is not as robust as someone who has healthy immune function, and some panel members were concerned that a third dose might come with a false sense of security.
“My only concern with adding a third dose for the immunocompromised is the impression that our immunocompromised population [will] then be safe,” said ACIP member Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn.
“I think the reality is they’ll be safer but still at incredibly high risk for severe disease and death,” she said.
In updating its EUA, the FDA stressed that, even after a third dose, people who are immunocompromised will still need to wear a mask indoors, socially distance, and avoid large crowds. In addition, family members and other close contacts should be fully vaccinated to protect these vulnerable individuals.
Johnson & Johnson not in the mix
The boosters will be available to children as young as 12 years of age who’ve had a Pfizer vaccine or those ages 18 and older who’ve gotten the Moderna vaccine.
For now, people who’ve had the one-dose Johnson & Johnson vaccine have not been cleared to get a second dose of any vaccine.
FDA experts acknowledged the gap but said that people who had received the Johnson & Johnson vaccine represented a small slice of vaccinated Americans, and said they couldn’t act before the FDA had updated its authorization for that vaccine, which the agency is actively exploring.
“We had to do what we’re doing based on the data we have in hand,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA, the division of the agency that regulates vaccines.
“We think at least there is a solution here for the very large majority of immunocompromised individuals, and we believe we will probably have a solution for the remainder in the not-too-distant future,” Dr. Marks said.
In its updated EUA, the FDA said that the third shots were intended for people who had undergone solid organ transplants or have an “equivalent level of immunocompromise.”
The details
Clinical experts on the CDC panel spent a good deal of time trying to suss out exactly what conditions might fall under the FDA’s umbrella for a third dose.
In a presentation to the committee, Neela Goswami, MD, PhD, an assistant professor of infectious diseases at Emory University School of Medicine and of epidemiology at the Emory Rollins School of Public Health, Atlanta, stressed that the shots are intended for patients who are moderately or severely immunocompromised, in close consultation with their doctors, but that people who should qualify would include those:
- Receiving treatment for solid tumors or blood cancers
- Taking immunosuppressing medications after a solid organ transplant
- Within 2 years of receiving CAR-T therapy or a stem cell transplant
- Who have primary immunodeficiencies – rare genetic disorders that prevent the immune system from working properly
- With advanced or untreated
- Taking high-dose corticosteroids (more than 20 milligrams of or its equivalent daily), alkylating agents, antimetabolites, chemotherapy, TNF blockers, or other immunomodulating or immunosuppressing biologics
- With certain chronic medical conditions, such as or asplenia – living without a spleen
- Receiving dialysis
In discussion, CDC experts clarified that these third doses were not intended for people whose immune function had waned with age, such as elderly residents of long-term care facilities or people with chronic diseases like diabetes.
The idea is to try to get a third dose of the vaccine they’ve already had – Moderna or Pfizer – but if that’s not feasible, it’s fine for the third dose to be different from what someone has had before. The third dose should be given at least 28 days after a second dose, and, ideally, before the initiation of immunosuppressive therapy.
Participants in the meeting said that the CDC would post updated materials on its website to help guide physicians on exactly who should receive third doses.
Ultimately, however, the extra doses will be given on an honor system; no prescriptions or other kinds of clinical documentation will be required for people to get a third dose of these shots.
Tests to measure neutralizing antibodies are also not recommended before the shots are given because of differences in the types of tests used to measure these antibodies and the difficulty in interpreting them. It’s unclear right now what level of neutralizing antibodies is needed for protection.
‘Peace of mind’
In public testimony, Heather Braaten, a 44-year-old being treated for ovarian cancer, said she was grateful to have gotten two shots of the Pfizer vaccine last winter, in between rounds of chemotherapy, but she knew she was probably not well protected. She said she’d become obsessive over the past few months reading medical studies and trying to understand her risk.
“I have felt distraught over the situation. My prognosis is poor. I most likely have about two to three years left to live, so everything counts,” Ms. Braaten said.
She said her life ambitions were humble. She wants to visit with friends and family and not have to worry that she’ll be a breakthrough case. She wants to go grocery shopping again and “not panic and leave the store after five minutes.” She’d love to feel free to travel, she said.
“While I understand I still need to be cautious, I am hopeful for the peace of mind and greater freedom a third shot can provide,” Ms. Braaten said.
More boosters on the way?
In the second half of the meeting, the CDC also signaled that it was considering the use of boosters for people whose immunity might have waned in the months since they had completed their vaccine series, particularly seniors. About 75% of people hospitalized with vaccine breakthrough cases are over age 65, according to CDC data.
Those considerations are becoming more urgent as the Delta variant continues to pummel less vaccinated states and counties.
In its presentation to the ACIP, Heather Scobie, PhD, MPH, a member of the CDC’s COVID Response Team, highlighted data from Canada, Israel, Qatar, and the United Kingdom showing that, while the Pfizer vaccine was still highly effective at preventing hospitalizations and death, it’s far less likely when faced with Delta to prevent an infection that causes symptoms.
In Israel, Pfizer’s vaccine prevented symptoms an average of 41% of the time. In Qatar, which is also using the Moderna vaccine, Pfizer’s prevented symptomatic infections with Delta about 54% of the time compared with 85% with Moderna’s.
Dr. Scobie noted that Pfizer’s waning efficacy may have something to do with the fact that it uses a lower dosage than Moderna’s. Pfizer’s recommended dosing interval is also shorter – 3 weeks compared with 4 weeks for Moderna’s. Stretching the time between shots has been shown to boost vaccine effectiveness, she said.
New data from the Mayo clinic, published ahead of peer review, also suggest that Pfizer’s protection may be fading more quickly than Moderna’s.
In February, both shots were nearly 100% effective at preventing the SARS-CoV-2 infection, but by July, against Delta, Pfizer’s efficacy had dropped to somewhere between 13% and 62%, while Moderna’s was still effective at preventing infection between 58% and 87% of the time.
In July, Pfizer’s was between 24% and 94% effective at preventing hospitalization with a COVID-19 infection and Moderna’s was between 33% and 96% effective at preventing hospitalization.
While that may sound like cause for concern, Dr. Scobie noted that, as of August 2, severe COVD-19 outcomes after vaccination are still very rare. Among 164 million fully vaccinated people in the United States there have been about 7,000 hospitalizations and 1,500 deaths; nearly three out of four of these have been in people over the age of 65.
The ACIP will next meet on August 24 to focus solely on the COVID-19 vaccines.
A version of this article first appeared on Medscape.com.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, has officially signed off on a recommendation by an independent panel of 11 experts to allow people with weakened immune function to get a third dose of certain COVID-19 vaccines.
The decision follows a unanimous vote by the CDC’s Advisory Committee on Immunization Practices (ACIP), which in turn came hours after the U.S. Food and Drug Administration updated its Emergency Use Authorization (EUA) for the Pfizer and Moderna mRNA vaccines.
About 7 million adults in the United States have moderately to severely impaired immune function because of a medical condition they live with or a medication they take to manage a health condition.
People who fall into this category are at higher risk of being hospitalized or dying if they get COVID-19. They are also more likely to transmit the infection. About 40% of vaccinated patients who are hospitalized with breakthrough cases are immunocompromised.
Recent studies have shown that between one-third and one-half of immunocompromised people who didn’t develop antibodies after two doses of a vaccine do get some level of protection after a third dose.
Even then, however, the protection immunocompromised people get from vaccines is not as robust as someone who has healthy immune function, and some panel members were concerned that a third dose might come with a false sense of security.
“My only concern with adding a third dose for the immunocompromised is the impression that our immunocompromised population [will] then be safe,” said ACIP member Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn.
“I think the reality is they’ll be safer but still at incredibly high risk for severe disease and death,” she said.
In updating its EUA, the FDA stressed that, even after a third dose, people who are immunocompromised will still need to wear a mask indoors, socially distance, and avoid large crowds. In addition, family members and other close contacts should be fully vaccinated to protect these vulnerable individuals.
Johnson & Johnson not in the mix
The boosters will be available to children as young as 12 years of age who’ve had a Pfizer vaccine or those ages 18 and older who’ve gotten the Moderna vaccine.
For now, people who’ve had the one-dose Johnson & Johnson vaccine have not been cleared to get a second dose of any vaccine.
FDA experts acknowledged the gap but said that people who had received the Johnson & Johnson vaccine represented a small slice of vaccinated Americans, and said they couldn’t act before the FDA had updated its authorization for that vaccine, which the agency is actively exploring.
“We had to do what we’re doing based on the data we have in hand,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA, the division of the agency that regulates vaccines.
“We think at least there is a solution here for the very large majority of immunocompromised individuals, and we believe we will probably have a solution for the remainder in the not-too-distant future,” Dr. Marks said.
In its updated EUA, the FDA said that the third shots were intended for people who had undergone solid organ transplants or have an “equivalent level of immunocompromise.”
The details
Clinical experts on the CDC panel spent a good deal of time trying to suss out exactly what conditions might fall under the FDA’s umbrella for a third dose.
In a presentation to the committee, Neela Goswami, MD, PhD, an assistant professor of infectious diseases at Emory University School of Medicine and of epidemiology at the Emory Rollins School of Public Health, Atlanta, stressed that the shots are intended for patients who are moderately or severely immunocompromised, in close consultation with their doctors, but that people who should qualify would include those:
- Receiving treatment for solid tumors or blood cancers
- Taking immunosuppressing medications after a solid organ transplant
- Within 2 years of receiving CAR-T therapy or a stem cell transplant
- Who have primary immunodeficiencies – rare genetic disorders that prevent the immune system from working properly
- With advanced or untreated
- Taking high-dose corticosteroids (more than 20 milligrams of or its equivalent daily), alkylating agents, antimetabolites, chemotherapy, TNF blockers, or other immunomodulating or immunosuppressing biologics
- With certain chronic medical conditions, such as or asplenia – living without a spleen
- Receiving dialysis
In discussion, CDC experts clarified that these third doses were not intended for people whose immune function had waned with age, such as elderly residents of long-term care facilities or people with chronic diseases like diabetes.
The idea is to try to get a third dose of the vaccine they’ve already had – Moderna or Pfizer – but if that’s not feasible, it’s fine for the third dose to be different from what someone has had before. The third dose should be given at least 28 days after a second dose, and, ideally, before the initiation of immunosuppressive therapy.
Participants in the meeting said that the CDC would post updated materials on its website to help guide physicians on exactly who should receive third doses.
Ultimately, however, the extra doses will be given on an honor system; no prescriptions or other kinds of clinical documentation will be required for people to get a third dose of these shots.
Tests to measure neutralizing antibodies are also not recommended before the shots are given because of differences in the types of tests used to measure these antibodies and the difficulty in interpreting them. It’s unclear right now what level of neutralizing antibodies is needed for protection.
‘Peace of mind’
In public testimony, Heather Braaten, a 44-year-old being treated for ovarian cancer, said she was grateful to have gotten two shots of the Pfizer vaccine last winter, in between rounds of chemotherapy, but she knew she was probably not well protected. She said she’d become obsessive over the past few months reading medical studies and trying to understand her risk.
“I have felt distraught over the situation. My prognosis is poor. I most likely have about two to three years left to live, so everything counts,” Ms. Braaten said.
She said her life ambitions were humble. She wants to visit with friends and family and not have to worry that she’ll be a breakthrough case. She wants to go grocery shopping again and “not panic and leave the store after five minutes.” She’d love to feel free to travel, she said.
“While I understand I still need to be cautious, I am hopeful for the peace of mind and greater freedom a third shot can provide,” Ms. Braaten said.
More boosters on the way?
In the second half of the meeting, the CDC also signaled that it was considering the use of boosters for people whose immunity might have waned in the months since they had completed their vaccine series, particularly seniors. About 75% of people hospitalized with vaccine breakthrough cases are over age 65, according to CDC data.
Those considerations are becoming more urgent as the Delta variant continues to pummel less vaccinated states and counties.
In its presentation to the ACIP, Heather Scobie, PhD, MPH, a member of the CDC’s COVID Response Team, highlighted data from Canada, Israel, Qatar, and the United Kingdom showing that, while the Pfizer vaccine was still highly effective at preventing hospitalizations and death, it’s far less likely when faced with Delta to prevent an infection that causes symptoms.
In Israel, Pfizer’s vaccine prevented symptoms an average of 41% of the time. In Qatar, which is also using the Moderna vaccine, Pfizer’s prevented symptomatic infections with Delta about 54% of the time compared with 85% with Moderna’s.
Dr. Scobie noted that Pfizer’s waning efficacy may have something to do with the fact that it uses a lower dosage than Moderna’s. Pfizer’s recommended dosing interval is also shorter – 3 weeks compared with 4 weeks for Moderna’s. Stretching the time between shots has been shown to boost vaccine effectiveness, she said.
New data from the Mayo clinic, published ahead of peer review, also suggest that Pfizer’s protection may be fading more quickly than Moderna’s.
In February, both shots were nearly 100% effective at preventing the SARS-CoV-2 infection, but by July, against Delta, Pfizer’s efficacy had dropped to somewhere between 13% and 62%, while Moderna’s was still effective at preventing infection between 58% and 87% of the time.
In July, Pfizer’s was between 24% and 94% effective at preventing hospitalization with a COVID-19 infection and Moderna’s was between 33% and 96% effective at preventing hospitalization.
While that may sound like cause for concern, Dr. Scobie noted that, as of August 2, severe COVD-19 outcomes after vaccination are still very rare. Among 164 million fully vaccinated people in the United States there have been about 7,000 hospitalizations and 1,500 deaths; nearly three out of four of these have been in people over the age of 65.
The ACIP will next meet on August 24 to focus solely on the COVID-19 vaccines.
A version of this article first appeared on Medscape.com.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, has officially signed off on a recommendation by an independent panel of 11 experts to allow people with weakened immune function to get a third dose of certain COVID-19 vaccines.
The decision follows a unanimous vote by the CDC’s Advisory Committee on Immunization Practices (ACIP), which in turn came hours after the U.S. Food and Drug Administration updated its Emergency Use Authorization (EUA) for the Pfizer and Moderna mRNA vaccines.
About 7 million adults in the United States have moderately to severely impaired immune function because of a medical condition they live with or a medication they take to manage a health condition.
People who fall into this category are at higher risk of being hospitalized or dying if they get COVID-19. They are also more likely to transmit the infection. About 40% of vaccinated patients who are hospitalized with breakthrough cases are immunocompromised.
Recent studies have shown that between one-third and one-half of immunocompromised people who didn’t develop antibodies after two doses of a vaccine do get some level of protection after a third dose.
Even then, however, the protection immunocompromised people get from vaccines is not as robust as someone who has healthy immune function, and some panel members were concerned that a third dose might come with a false sense of security.
“My only concern with adding a third dose for the immunocompromised is the impression that our immunocompromised population [will] then be safe,” said ACIP member Helen Talbot, MD, MPH, an associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn.
“I think the reality is they’ll be safer but still at incredibly high risk for severe disease and death,” she said.
In updating its EUA, the FDA stressed that, even after a third dose, people who are immunocompromised will still need to wear a mask indoors, socially distance, and avoid large crowds. In addition, family members and other close contacts should be fully vaccinated to protect these vulnerable individuals.
Johnson & Johnson not in the mix
The boosters will be available to children as young as 12 years of age who’ve had a Pfizer vaccine or those ages 18 and older who’ve gotten the Moderna vaccine.
For now, people who’ve had the one-dose Johnson & Johnson vaccine have not been cleared to get a second dose of any vaccine.
FDA experts acknowledged the gap but said that people who had received the Johnson & Johnson vaccine represented a small slice of vaccinated Americans, and said they couldn’t act before the FDA had updated its authorization for that vaccine, which the agency is actively exploring.
“We had to do what we’re doing based on the data we have in hand,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA, the division of the agency that regulates vaccines.
“We think at least there is a solution here for the very large majority of immunocompromised individuals, and we believe we will probably have a solution for the remainder in the not-too-distant future,” Dr. Marks said.
In its updated EUA, the FDA said that the third shots were intended for people who had undergone solid organ transplants or have an “equivalent level of immunocompromise.”
The details
Clinical experts on the CDC panel spent a good deal of time trying to suss out exactly what conditions might fall under the FDA’s umbrella for a third dose.
In a presentation to the committee, Neela Goswami, MD, PhD, an assistant professor of infectious diseases at Emory University School of Medicine and of epidemiology at the Emory Rollins School of Public Health, Atlanta, stressed that the shots are intended for patients who are moderately or severely immunocompromised, in close consultation with their doctors, but that people who should qualify would include those:
- Receiving treatment for solid tumors or blood cancers
- Taking immunosuppressing medications after a solid organ transplant
- Within 2 years of receiving CAR-T therapy or a stem cell transplant
- Who have primary immunodeficiencies – rare genetic disorders that prevent the immune system from working properly
- With advanced or untreated
- Taking high-dose corticosteroids (more than 20 milligrams of or its equivalent daily), alkylating agents, antimetabolites, chemotherapy, TNF blockers, or other immunomodulating or immunosuppressing biologics
- With certain chronic medical conditions, such as or asplenia – living without a spleen
- Receiving dialysis
In discussion, CDC experts clarified that these third doses were not intended for people whose immune function had waned with age, such as elderly residents of long-term care facilities or people with chronic diseases like diabetes.
The idea is to try to get a third dose of the vaccine they’ve already had – Moderna or Pfizer – but if that’s not feasible, it’s fine for the third dose to be different from what someone has had before. The third dose should be given at least 28 days after a second dose, and, ideally, before the initiation of immunosuppressive therapy.
Participants in the meeting said that the CDC would post updated materials on its website to help guide physicians on exactly who should receive third doses.
Ultimately, however, the extra doses will be given on an honor system; no prescriptions or other kinds of clinical documentation will be required for people to get a third dose of these shots.
Tests to measure neutralizing antibodies are also not recommended before the shots are given because of differences in the types of tests used to measure these antibodies and the difficulty in interpreting them. It’s unclear right now what level of neutralizing antibodies is needed for protection.
‘Peace of mind’
In public testimony, Heather Braaten, a 44-year-old being treated for ovarian cancer, said she was grateful to have gotten two shots of the Pfizer vaccine last winter, in between rounds of chemotherapy, but she knew she was probably not well protected. She said she’d become obsessive over the past few months reading medical studies and trying to understand her risk.
“I have felt distraught over the situation. My prognosis is poor. I most likely have about two to three years left to live, so everything counts,” Ms. Braaten said.
She said her life ambitions were humble. She wants to visit with friends and family and not have to worry that she’ll be a breakthrough case. She wants to go grocery shopping again and “not panic and leave the store after five minutes.” She’d love to feel free to travel, she said.
“While I understand I still need to be cautious, I am hopeful for the peace of mind and greater freedom a third shot can provide,” Ms. Braaten said.
More boosters on the way?
In the second half of the meeting, the CDC also signaled that it was considering the use of boosters for people whose immunity might have waned in the months since they had completed their vaccine series, particularly seniors. About 75% of people hospitalized with vaccine breakthrough cases are over age 65, according to CDC data.
Those considerations are becoming more urgent as the Delta variant continues to pummel less vaccinated states and counties.
In its presentation to the ACIP, Heather Scobie, PhD, MPH, a member of the CDC’s COVID Response Team, highlighted data from Canada, Israel, Qatar, and the United Kingdom showing that, while the Pfizer vaccine was still highly effective at preventing hospitalizations and death, it’s far less likely when faced with Delta to prevent an infection that causes symptoms.
In Israel, Pfizer’s vaccine prevented symptoms an average of 41% of the time. In Qatar, which is also using the Moderna vaccine, Pfizer’s prevented symptomatic infections with Delta about 54% of the time compared with 85% with Moderna’s.
Dr. Scobie noted that Pfizer’s waning efficacy may have something to do with the fact that it uses a lower dosage than Moderna’s. Pfizer’s recommended dosing interval is also shorter – 3 weeks compared with 4 weeks for Moderna’s. Stretching the time between shots has been shown to boost vaccine effectiveness, she said.
New data from the Mayo clinic, published ahead of peer review, also suggest that Pfizer’s protection may be fading more quickly than Moderna’s.
In February, both shots were nearly 100% effective at preventing the SARS-CoV-2 infection, but by July, against Delta, Pfizer’s efficacy had dropped to somewhere between 13% and 62%, while Moderna’s was still effective at preventing infection between 58% and 87% of the time.
In July, Pfizer’s was between 24% and 94% effective at preventing hospitalization with a COVID-19 infection and Moderna’s was between 33% and 96% effective at preventing hospitalization.
While that may sound like cause for concern, Dr. Scobie noted that, as of August 2, severe COVD-19 outcomes after vaccination are still very rare. Among 164 million fully vaccinated people in the United States there have been about 7,000 hospitalizations and 1,500 deaths; nearly three out of four of these have been in people over the age of 65.
The ACIP will next meet on August 24 to focus solely on the COVID-19 vaccines.
A version of this article first appeared on Medscape.com.
25% of patients with cancer lack immunity against measles
Before the onslaught of COVID-19, researchers at the Fred Hutchinson Cancer Research Center in Seattle had another infectious disease worry: an “unprecedented” outbreak of measles.
“In 2019, we saw the most measles cases in any year since the 1990s,” said Sara Marquis, MPH, a clinical research coordinator at the center. The worry, she says, was that various oncology treatments, such as bone marrow transplantations and assorted biologics, “may leave cancer patients severely immunosuppressed” and thus vulnerable to infectious diseases.
Measles-related illness is typically not severe but can lead to pneumonia, deafness, and death, even in immunocompetent people, Ms. Marquis added.
So in 2019, a team at Fred Hutchinson initiated a study to get a sense of immunity to measles among patients with cancer.
They now report that of a group of 900-plus patients, 25% lacked protective antibodies for measles. That’s “significantly more” than the general population, in which about 8% of people lack these antibodies, Ms. Marquis said.
The study, published online in JAMA Network Open, also found that 38% lacked protection against the less-worrisome infectious disease of mumps, which is more than the 13% found in the general population.
“The scary thing about measles is that it is one of the most contagious diseases known,” Ms. Marquis told this news organization, adding that it is about twice as contagious as the COVID-19 Delta variant.
And it’s not just in the state of Washington. “We’re seeing it more and more in the community,” as various outbreaks continue to happen, she said.
“Deficits in protective antibodies underscore patients’ increased risk during outbreaks and emphasize the need for community-based efforts to increase herd immunity to protect this population,” the study authors conclude.
In short, administration of the measles-mumps-rubella (MMR) vaccine, introduced in 1963, must continue universally, they said
“We’ve had so many incredible advances in cancer treatment in recent years. … it would be devastating to see something like measles, which is a vaccine-preventable disease, come through and negate those efforts,” said study coauthor Elizabeth Krantz, MS, a biostatistician at Fred Hutchinson.
The health care teams and family caregivers of patients with cancer should also make sure they are vaccinated, said Ms. Marquis. However, some patients may not be able to get a measles booster vaccine because it is a live vaccine or because they cannot generate enough antibodies for it to be protective, she explained.
Three subgroups more likely to have deficits
The new study, which is one of the first to measure measles and mumps seroprevalence among patients with cancer in the modern era of cancer treatment, also identified three subgroups that more commonly had immunity deficits: those aged 30-59 years; those with hematologic malignant neoplasms, and those who had received a hematopoietic cell transplant.
In the study, residual clinical plasma samples were obtained from 959 consecutive patients with cancer at Seattle Cancer Care Alliance and Fred Hutchinson in August 2019. These samples were tested for measles and mumps IgG by using a commercial enzyme-linked immunosorbent assay. In all, 60% of patients had a solid tumor and 40% had a blood cancer.
As noted above, the seroprevalence of measles antibodies was 0.75 and the seroprevalence of mumps antibodies was 0.62.
A study author explained why the study included mumps, a less threatening infection.
“We assessed mumps in this study out of interest to compare response in the MMR vaccine component – particularly as we could assess a potent vaccine (measles) versus one that has a weaker immunologic response (mumps). We remain worried about outbreaks of mumps as MMR vaccination rates drop across the U.S.,” wrote Steven Pergam, MD, MPH, infectious disease specialist at Fred Hutchinson, in an email.
Vaccination vigilance is one of the study’s messages. “We all need to do our part to make sure we are up to date with our vaccinations so we can make sure we protect those who are vulnerable,” said Ms. Krantz.
The study was funded by the National Cancer Institute and Seattle Cancer Care Alliance. Multiple study authors have ties to pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Before the onslaught of COVID-19, researchers at the Fred Hutchinson Cancer Research Center in Seattle had another infectious disease worry: an “unprecedented” outbreak of measles.
“In 2019, we saw the most measles cases in any year since the 1990s,” said Sara Marquis, MPH, a clinical research coordinator at the center. The worry, she says, was that various oncology treatments, such as bone marrow transplantations and assorted biologics, “may leave cancer patients severely immunosuppressed” and thus vulnerable to infectious diseases.
Measles-related illness is typically not severe but can lead to pneumonia, deafness, and death, even in immunocompetent people, Ms. Marquis added.
So in 2019, a team at Fred Hutchinson initiated a study to get a sense of immunity to measles among patients with cancer.
They now report that of a group of 900-plus patients, 25% lacked protective antibodies for measles. That’s “significantly more” than the general population, in which about 8% of people lack these antibodies, Ms. Marquis said.
The study, published online in JAMA Network Open, also found that 38% lacked protection against the less-worrisome infectious disease of mumps, which is more than the 13% found in the general population.
“The scary thing about measles is that it is one of the most contagious diseases known,” Ms. Marquis told this news organization, adding that it is about twice as contagious as the COVID-19 Delta variant.
And it’s not just in the state of Washington. “We’re seeing it more and more in the community,” as various outbreaks continue to happen, she said.
“Deficits in protective antibodies underscore patients’ increased risk during outbreaks and emphasize the need for community-based efforts to increase herd immunity to protect this population,” the study authors conclude.
In short, administration of the measles-mumps-rubella (MMR) vaccine, introduced in 1963, must continue universally, they said
“We’ve had so many incredible advances in cancer treatment in recent years. … it would be devastating to see something like measles, which is a vaccine-preventable disease, come through and negate those efforts,” said study coauthor Elizabeth Krantz, MS, a biostatistician at Fred Hutchinson.
The health care teams and family caregivers of patients with cancer should also make sure they are vaccinated, said Ms. Marquis. However, some patients may not be able to get a measles booster vaccine because it is a live vaccine or because they cannot generate enough antibodies for it to be protective, she explained.
Three subgroups more likely to have deficits
The new study, which is one of the first to measure measles and mumps seroprevalence among patients with cancer in the modern era of cancer treatment, also identified three subgroups that more commonly had immunity deficits: those aged 30-59 years; those with hematologic malignant neoplasms, and those who had received a hematopoietic cell transplant.
In the study, residual clinical plasma samples were obtained from 959 consecutive patients with cancer at Seattle Cancer Care Alliance and Fred Hutchinson in August 2019. These samples were tested for measles and mumps IgG by using a commercial enzyme-linked immunosorbent assay. In all, 60% of patients had a solid tumor and 40% had a blood cancer.
As noted above, the seroprevalence of measles antibodies was 0.75 and the seroprevalence of mumps antibodies was 0.62.
A study author explained why the study included mumps, a less threatening infection.
“We assessed mumps in this study out of interest to compare response in the MMR vaccine component – particularly as we could assess a potent vaccine (measles) versus one that has a weaker immunologic response (mumps). We remain worried about outbreaks of mumps as MMR vaccination rates drop across the U.S.,” wrote Steven Pergam, MD, MPH, infectious disease specialist at Fred Hutchinson, in an email.
Vaccination vigilance is one of the study’s messages. “We all need to do our part to make sure we are up to date with our vaccinations so we can make sure we protect those who are vulnerable,” said Ms. Krantz.
The study was funded by the National Cancer Institute and Seattle Cancer Care Alliance. Multiple study authors have ties to pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Before the onslaught of COVID-19, researchers at the Fred Hutchinson Cancer Research Center in Seattle had another infectious disease worry: an “unprecedented” outbreak of measles.
“In 2019, we saw the most measles cases in any year since the 1990s,” said Sara Marquis, MPH, a clinical research coordinator at the center. The worry, she says, was that various oncology treatments, such as bone marrow transplantations and assorted biologics, “may leave cancer patients severely immunosuppressed” and thus vulnerable to infectious diseases.
Measles-related illness is typically not severe but can lead to pneumonia, deafness, and death, even in immunocompetent people, Ms. Marquis added.
So in 2019, a team at Fred Hutchinson initiated a study to get a sense of immunity to measles among patients with cancer.
They now report that of a group of 900-plus patients, 25% lacked protective antibodies for measles. That’s “significantly more” than the general population, in which about 8% of people lack these antibodies, Ms. Marquis said.
The study, published online in JAMA Network Open, also found that 38% lacked protection against the less-worrisome infectious disease of mumps, which is more than the 13% found in the general population.
“The scary thing about measles is that it is one of the most contagious diseases known,” Ms. Marquis told this news organization, adding that it is about twice as contagious as the COVID-19 Delta variant.
And it’s not just in the state of Washington. “We’re seeing it more and more in the community,” as various outbreaks continue to happen, she said.
“Deficits in protective antibodies underscore patients’ increased risk during outbreaks and emphasize the need for community-based efforts to increase herd immunity to protect this population,” the study authors conclude.
In short, administration of the measles-mumps-rubella (MMR) vaccine, introduced in 1963, must continue universally, they said
“We’ve had so many incredible advances in cancer treatment in recent years. … it would be devastating to see something like measles, which is a vaccine-preventable disease, come through and negate those efforts,” said study coauthor Elizabeth Krantz, MS, a biostatistician at Fred Hutchinson.
The health care teams and family caregivers of patients with cancer should also make sure they are vaccinated, said Ms. Marquis. However, some patients may not be able to get a measles booster vaccine because it is a live vaccine or because they cannot generate enough antibodies for it to be protective, she explained.
Three subgroups more likely to have deficits
The new study, which is one of the first to measure measles and mumps seroprevalence among patients with cancer in the modern era of cancer treatment, also identified three subgroups that more commonly had immunity deficits: those aged 30-59 years; those with hematologic malignant neoplasms, and those who had received a hematopoietic cell transplant.
In the study, residual clinical plasma samples were obtained from 959 consecutive patients with cancer at Seattle Cancer Care Alliance and Fred Hutchinson in August 2019. These samples were tested for measles and mumps IgG by using a commercial enzyme-linked immunosorbent assay. In all, 60% of patients had a solid tumor and 40% had a blood cancer.
As noted above, the seroprevalence of measles antibodies was 0.75 and the seroprevalence of mumps antibodies was 0.62.
A study author explained why the study included mumps, a less threatening infection.
“We assessed mumps in this study out of interest to compare response in the MMR vaccine component – particularly as we could assess a potent vaccine (measles) versus one that has a weaker immunologic response (mumps). We remain worried about outbreaks of mumps as MMR vaccination rates drop across the U.S.,” wrote Steven Pergam, MD, MPH, infectious disease specialist at Fred Hutchinson, in an email.
Vaccination vigilance is one of the study’s messages. “We all need to do our part to make sure we are up to date with our vaccinations so we can make sure we protect those who are vulnerable,” said Ms. Krantz.
The study was funded by the National Cancer Institute and Seattle Cancer Care Alliance. Multiple study authors have ties to pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Age, distance from dermatology clinic <p>predict number of melanomas diagnosed
Among patients from a single dermatology practice who were diagnosed with two or more melanomas over an 8-year period, 45% lived more than 20 miles away from the practice, and almost 60% were 70 years of age and older, results from single-center study showed.
“Dermatologists have known that many people are underdiagnosed for melanoma, but now our research supports that the problem is especially concentrated among older patients living in remote areas,” corresponding author Rose Parisi, MBA, said in an interview. “With this information, dermatologists should consider identifying and reaching out to their patients in this at-risk subpopulation, increasing the frequency of full-body skin exams, and collaborating with primary care physicians to educate them about melanoma’s dangers.”
In a study published online Aug. 3 in the Journal of the American Academy of Dermatology, Ms. Parisi of Albany Medical College, New York, and colleagues drew from the electronic medical records of a single-specialty private dermatology practice that serves urban, suburban, and rural patient populations to identify 346 melanoma pathology reports from patients cared for between 2012 and 2020. They limited their investigation to those diagnosed with biopsy-confirmed melanoma and analyzed the number of melanomas, Breslow depth, follow-up full-body skin exams, family history of melanoma, gender, insurance, and age (categorized as younger than 70 years and 70 years or older). To determine patient travel distance, they calculated the miles between the ZIP codes of the patient’s residence and the dermatology practice.
Regression analysis revealed that the . Specifically, among patients diagnosed with two or more melanomas, 45.0% lived more than 20 miles away and 21.3% lived less than 15 miles away; 59.6% were age 70 and older, while 40.4% were younger than age 70 (P less than .01).
No statistically significant association was observed between travel distance and Breslow depth or follow-up full-body skin exams within 1 year following diagnosis.
In other findings, among patients who lived more than 20 miles from the practice, those aged 70 and older were diagnosed with 0.56 more melanomas than patients between the ages of 58 and 70 (P = .00003), and 0.31 more melanomas than patients who lived 15-20 miles away (P = .014). No statistically significant differences in the number of melanomas diagnosed were observed between patients in either age group who lived fewer than 15 miles from the office.
“We were surprised that the combination of age and patient distance to diagnosing dermatology provider was such a powerful predictor of the number of diagnosed melanomas,” Ms. Parisi said. “It’s probably due to less mobility among older patients living in more remote areas, and it puts them at higher risk of multiple melanomas. This was something we haven’t seen in the dermatology literature.”
She and her coauthors acknowledged that the limited sampling of patients from a single practice “may not generalize across all urban and rural settings, and results must be considered preliminary,” they wrote. However, “our findings reveal an important vulnerability among older patients in nonurban areas, and efforts to improve access to melanoma diagnosis should be concentrated on this geodemographic segment.”
Nikolai Klebanov, MD, of the department of dermatology at Massachusetts General Hospital, Boston, who was asked to comment on the study, described what was addressed in the study as a “timely and an important topic.”
In an interview, he said, “there is less access to dermatologists and other medical specialists outside of large metropolitan and suburban areas,” and there are other health disparities affecting people living in rural or more underserved areas, which, he added, “also became exacerbated by the COVID-19 pandemic.”
For future studies on this topic, Dr. Klebanov said that he would be interested to see diagnoses measured per person-year rather than the total number of melanomas diagnosed. “More elderly patients may also be those who have ‘stuck with the practice’ for longer, and had a longer follow-up that gives more time to catch more melanomas,” he said.
“Adjusting for median income using ZIP codes could also help adjust for socioeconomic status, which would help with external validity of the study. Income relationships to geography are not the same in all cities; some have wealthy suburbs within 20 miles, while some have more underserved and rural areas at that distance.”
Neither the researchers nor Dr. Klebanov reported having financial disclosures.
Among patients from a single dermatology practice who were diagnosed with two or more melanomas over an 8-year period, 45% lived more than 20 miles away from the practice, and almost 60% were 70 years of age and older, results from single-center study showed.
“Dermatologists have known that many people are underdiagnosed for melanoma, but now our research supports that the problem is especially concentrated among older patients living in remote areas,” corresponding author Rose Parisi, MBA, said in an interview. “With this information, dermatologists should consider identifying and reaching out to their patients in this at-risk subpopulation, increasing the frequency of full-body skin exams, and collaborating with primary care physicians to educate them about melanoma’s dangers.”
In a study published online Aug. 3 in the Journal of the American Academy of Dermatology, Ms. Parisi of Albany Medical College, New York, and colleagues drew from the electronic medical records of a single-specialty private dermatology practice that serves urban, suburban, and rural patient populations to identify 346 melanoma pathology reports from patients cared for between 2012 and 2020. They limited their investigation to those diagnosed with biopsy-confirmed melanoma and analyzed the number of melanomas, Breslow depth, follow-up full-body skin exams, family history of melanoma, gender, insurance, and age (categorized as younger than 70 years and 70 years or older). To determine patient travel distance, they calculated the miles between the ZIP codes of the patient’s residence and the dermatology practice.
Regression analysis revealed that the . Specifically, among patients diagnosed with two or more melanomas, 45.0% lived more than 20 miles away and 21.3% lived less than 15 miles away; 59.6% were age 70 and older, while 40.4% were younger than age 70 (P less than .01).
No statistically significant association was observed between travel distance and Breslow depth or follow-up full-body skin exams within 1 year following diagnosis.
In other findings, among patients who lived more than 20 miles from the practice, those aged 70 and older were diagnosed with 0.56 more melanomas than patients between the ages of 58 and 70 (P = .00003), and 0.31 more melanomas than patients who lived 15-20 miles away (P = .014). No statistically significant differences in the number of melanomas diagnosed were observed between patients in either age group who lived fewer than 15 miles from the office.
“We were surprised that the combination of age and patient distance to diagnosing dermatology provider was such a powerful predictor of the number of diagnosed melanomas,” Ms. Parisi said. “It’s probably due to less mobility among older patients living in more remote areas, and it puts them at higher risk of multiple melanomas. This was something we haven’t seen in the dermatology literature.”
She and her coauthors acknowledged that the limited sampling of patients from a single practice “may not generalize across all urban and rural settings, and results must be considered preliminary,” they wrote. However, “our findings reveal an important vulnerability among older patients in nonurban areas, and efforts to improve access to melanoma diagnosis should be concentrated on this geodemographic segment.”
Nikolai Klebanov, MD, of the department of dermatology at Massachusetts General Hospital, Boston, who was asked to comment on the study, described what was addressed in the study as a “timely and an important topic.”
In an interview, he said, “there is less access to dermatologists and other medical specialists outside of large metropolitan and suburban areas,” and there are other health disparities affecting people living in rural or more underserved areas, which, he added, “also became exacerbated by the COVID-19 pandemic.”
For future studies on this topic, Dr. Klebanov said that he would be interested to see diagnoses measured per person-year rather than the total number of melanomas diagnosed. “More elderly patients may also be those who have ‘stuck with the practice’ for longer, and had a longer follow-up that gives more time to catch more melanomas,” he said.
“Adjusting for median income using ZIP codes could also help adjust for socioeconomic status, which would help with external validity of the study. Income relationships to geography are not the same in all cities; some have wealthy suburbs within 20 miles, while some have more underserved and rural areas at that distance.”
Neither the researchers nor Dr. Klebanov reported having financial disclosures.
Among patients from a single dermatology practice who were diagnosed with two or more melanomas over an 8-year period, 45% lived more than 20 miles away from the practice, and almost 60% were 70 years of age and older, results from single-center study showed.
“Dermatologists have known that many people are underdiagnosed for melanoma, but now our research supports that the problem is especially concentrated among older patients living in remote areas,” corresponding author Rose Parisi, MBA, said in an interview. “With this information, dermatologists should consider identifying and reaching out to their patients in this at-risk subpopulation, increasing the frequency of full-body skin exams, and collaborating with primary care physicians to educate them about melanoma’s dangers.”
In a study published online Aug. 3 in the Journal of the American Academy of Dermatology, Ms. Parisi of Albany Medical College, New York, and colleagues drew from the electronic medical records of a single-specialty private dermatology practice that serves urban, suburban, and rural patient populations to identify 346 melanoma pathology reports from patients cared for between 2012 and 2020. They limited their investigation to those diagnosed with biopsy-confirmed melanoma and analyzed the number of melanomas, Breslow depth, follow-up full-body skin exams, family history of melanoma, gender, insurance, and age (categorized as younger than 70 years and 70 years or older). To determine patient travel distance, they calculated the miles between the ZIP codes of the patient’s residence and the dermatology practice.
Regression analysis revealed that the . Specifically, among patients diagnosed with two or more melanomas, 45.0% lived more than 20 miles away and 21.3% lived less than 15 miles away; 59.6% were age 70 and older, while 40.4% were younger than age 70 (P less than .01).
No statistically significant association was observed between travel distance and Breslow depth or follow-up full-body skin exams within 1 year following diagnosis.
In other findings, among patients who lived more than 20 miles from the practice, those aged 70 and older were diagnosed with 0.56 more melanomas than patients between the ages of 58 and 70 (P = .00003), and 0.31 more melanomas than patients who lived 15-20 miles away (P = .014). No statistically significant differences in the number of melanomas diagnosed were observed between patients in either age group who lived fewer than 15 miles from the office.
“We were surprised that the combination of age and patient distance to diagnosing dermatology provider was such a powerful predictor of the number of diagnosed melanomas,” Ms. Parisi said. “It’s probably due to less mobility among older patients living in more remote areas, and it puts them at higher risk of multiple melanomas. This was something we haven’t seen in the dermatology literature.”
She and her coauthors acknowledged that the limited sampling of patients from a single practice “may not generalize across all urban and rural settings, and results must be considered preliminary,” they wrote. However, “our findings reveal an important vulnerability among older patients in nonurban areas, and efforts to improve access to melanoma diagnosis should be concentrated on this geodemographic segment.”
Nikolai Klebanov, MD, of the department of dermatology at Massachusetts General Hospital, Boston, who was asked to comment on the study, described what was addressed in the study as a “timely and an important topic.”
In an interview, he said, “there is less access to dermatologists and other medical specialists outside of large metropolitan and suburban areas,” and there are other health disparities affecting people living in rural or more underserved areas, which, he added, “also became exacerbated by the COVID-19 pandemic.”
For future studies on this topic, Dr. Klebanov said that he would be interested to see diagnoses measured per person-year rather than the total number of melanomas diagnosed. “More elderly patients may also be those who have ‘stuck with the practice’ for longer, and had a longer follow-up that gives more time to catch more melanomas,” he said.
“Adjusting for median income using ZIP codes could also help adjust for socioeconomic status, which would help with external validity of the study. Income relationships to geography are not the same in all cities; some have wealthy suburbs within 20 miles, while some have more underserved and rural areas at that distance.”
Neither the researchers nor Dr. Klebanov reported having financial disclosures.
FROM JAMA DERMATOLOGY
‘Routine’ use of focal therapy for prostate cancer in next 5 years
They maintain that focal therapy (FT) offers a “middle ground” between two extremes: Treating the whole gland with radical prostatectomy or radiotherapy and not treating immediately via active surveillance or watchful waiting.
Focal therapy typically treats the primary lesion within the prostate, while leaving the rest of the gland intact. Most often performed with cryoablation or high-intensity focused ultrasound (HIFU), it can also be carried out with a variety of technologies, including transurethral ultrasound ablation and focal laser ablation.
The shift to focal therapy will coincide with maturing, long-term data from studies with various technologies, predict the authors, led by Amir Lebastchi, MD, a urologist at the University of Southern California.
“Standard adoption of focal therapy is ultimately dependent on the availability of robust level I evidence, which in turn will drive medical societies and payees,” the authors also write.
But payees are already making changes, even without such data, they add.
For example, in January the American Medical Association announced a new code for high-intensity focal ultrasound (HIFU): This approach now has a Current Procedural Terminology (CPT) code from the U.S. Centers for Medicare & Medicaid Services
This news organization reached out to Matthew Cooperberg, MD, MPH, a urologist at the University of California, San Francisco (UCSF), for comments about the essay’s optimism; he has questioned focal therapy in the past because of a lack of strong supporting evidence.
“While ‘routine’ is a bit of a vague term, now that HIFU has a CPT code, I do expect its use will in fact increase in the next 5 years,” Dr. Cooperberg wrote in an email. “The question is whether its use will increase appropriately.”
The challenge with focal therapy – regardless of energy modality – remains patient selection and accurate ablation zone definition, he added.
Notably, UCSF has launched a new HIFU program – and Dr. Cooperberg has referred selected patients. “I’m both enthusiastic and cautious about the future, and we need to track our outcomes very closely across various practice settings,” he said.
While waiting for CHRONOS, select wisely
The goal of focal therapy is to treat only the area with the most aggressive tumor, known as the index tumor, while leaving the remaining gland and its surrounding structures alone, according to Derek Lomas, MD, PharmD, a urologist at the Mayo Clinic in Rochester, Minn., in an explanatory article. “This approach is widely accepted in other types of cancer. For example, we commonly treat kidney cancers by removing or ablating only the tumor while leaving the rest of the kidney intact.”
However, some focal therapies also include approaches known as hemiablations, in which a full half of the prostate is destroyed, and approaches that leave very little of the gland behind.
Each of the modalities used for focal therapy has “unique indications, risks, and benefits and uses a different energy source for ablation,” Dr. Lebastchi and colleagues write in their essay.
They assert that focal therapy can provide oncological efficacy similar to radical prostatectomy or radiotherapy “while considerably reducing or even eliminating functional morbidities, such as incontinence and erectile dysfunction.”
Overall, they say focal therapy offers an opportunity for improved care because there is “an increasing body of emerging evidence demonstrating a favorable adverse effect profile with oncological control similar to whole-gland treatment options.”
What is that evidence?
In the essay, Dr. Lebastchi and colleagues point to a number of single-arm studies with encouraging efficacy and safety results. They also highlight a phase 3, randomized trial that they were involved in: This compared focal therapy (partial gland ablation with vascular-targeted photodynamic therapy) with active surveillance in early-stage disease and uniformly showed better post-treatment biopsy (disease/no disease) and conversion-to-prostatectomy results with the focal therapy out to 4 years (J Urol. 2018;200:786-793).
However, that study did not have an active treatment comparator. For that gold standard, there is now anticipation for results from the CHRONOS trial in the United Kingdom, especially part A of the trial, which compares radical therapy to focal therapy (HIFU or cryotherapy), with 5-year progression-free survival as the primary outcome. That trial is slated for completion in 2027.
Until then, the lack of prospective randomized clinical trials and long-term follow-up “hinders acceptance [of focal therapy] in the urology community,” the essay authors comment.
Meanwhile, careful patient selection is very important, they say.
The latest relevant guidelines state that appropriate candidates are men with a solitary, well-defined index lesion; patients with bilateral multifocal lesions; or very advanced tumors that are not appropriate for the focal approach.
A multidisciplinary international expert panel recently convened to establish guidance for clinicians offering focal therapies and then published a consensus statement to advise practitioners and researchers.
UCSF’s Dr. Cooperberg sees plenty of room for improvement among focal therapy practitioners and investigators. “From an outcomes standpoint, follow-up protocols and definitions of success remain inconsistent. I believe we’re making progress in all these areas, but we’re not there yet,” he says.
To date, some patients have been managed poorly, Dr. Cooperberg added. “We certainly see many patients who have been inadequately counseled as to HIFU’s advantages and disadvantages, with sometimes disastrous results.”
Some of those unfortunate results may have arisen from the U.S. Food and Drug Administration’s initial approval of HIFU in 2015, which was for use in ablating prostate tissue in general and not cancer specifically. This approval generated confusion, one expert commented at the time: “The FDA doesn’t specify whether it’s for benign or malignant disease; it’s a bit vague, like saying you can drive this car, but we’re not going to tell you how to drive it,” said Manoj Monga, MD, from the Cleveland Clinic.
Dr. Lebastchi has disclosed no relevant financial relationships; co-author Inderbir Gill, MD, is an unpaid consultant for Steba Biotech, and co-author Andre Luis Abreu, MD, is a consultant for Koelis and was a proctor in training for Steba Biotech. Dr. Cooperberg is a consultant for Alessa Therapeutics.
A version of this article first appeared on Medscape.com.
They maintain that focal therapy (FT) offers a “middle ground” between two extremes: Treating the whole gland with radical prostatectomy or radiotherapy and not treating immediately via active surveillance or watchful waiting.
Focal therapy typically treats the primary lesion within the prostate, while leaving the rest of the gland intact. Most often performed with cryoablation or high-intensity focused ultrasound (HIFU), it can also be carried out with a variety of technologies, including transurethral ultrasound ablation and focal laser ablation.
The shift to focal therapy will coincide with maturing, long-term data from studies with various technologies, predict the authors, led by Amir Lebastchi, MD, a urologist at the University of Southern California.
“Standard adoption of focal therapy is ultimately dependent on the availability of robust level I evidence, which in turn will drive medical societies and payees,” the authors also write.
But payees are already making changes, even without such data, they add.
For example, in January the American Medical Association announced a new code for high-intensity focal ultrasound (HIFU): This approach now has a Current Procedural Terminology (CPT) code from the U.S. Centers for Medicare & Medicaid Services
This news organization reached out to Matthew Cooperberg, MD, MPH, a urologist at the University of California, San Francisco (UCSF), for comments about the essay’s optimism; he has questioned focal therapy in the past because of a lack of strong supporting evidence.
“While ‘routine’ is a bit of a vague term, now that HIFU has a CPT code, I do expect its use will in fact increase in the next 5 years,” Dr. Cooperberg wrote in an email. “The question is whether its use will increase appropriately.”
The challenge with focal therapy – regardless of energy modality – remains patient selection and accurate ablation zone definition, he added.
Notably, UCSF has launched a new HIFU program – and Dr. Cooperberg has referred selected patients. “I’m both enthusiastic and cautious about the future, and we need to track our outcomes very closely across various practice settings,” he said.
While waiting for CHRONOS, select wisely
The goal of focal therapy is to treat only the area with the most aggressive tumor, known as the index tumor, while leaving the remaining gland and its surrounding structures alone, according to Derek Lomas, MD, PharmD, a urologist at the Mayo Clinic in Rochester, Minn., in an explanatory article. “This approach is widely accepted in other types of cancer. For example, we commonly treat kidney cancers by removing or ablating only the tumor while leaving the rest of the kidney intact.”
However, some focal therapies also include approaches known as hemiablations, in which a full half of the prostate is destroyed, and approaches that leave very little of the gland behind.
Each of the modalities used for focal therapy has “unique indications, risks, and benefits and uses a different energy source for ablation,” Dr. Lebastchi and colleagues write in their essay.
They assert that focal therapy can provide oncological efficacy similar to radical prostatectomy or radiotherapy “while considerably reducing or even eliminating functional morbidities, such as incontinence and erectile dysfunction.”
Overall, they say focal therapy offers an opportunity for improved care because there is “an increasing body of emerging evidence demonstrating a favorable adverse effect profile with oncological control similar to whole-gland treatment options.”
What is that evidence?
In the essay, Dr. Lebastchi and colleagues point to a number of single-arm studies with encouraging efficacy and safety results. They also highlight a phase 3, randomized trial that they were involved in: This compared focal therapy (partial gland ablation with vascular-targeted photodynamic therapy) with active surveillance in early-stage disease and uniformly showed better post-treatment biopsy (disease/no disease) and conversion-to-prostatectomy results with the focal therapy out to 4 years (J Urol. 2018;200:786-793).
However, that study did not have an active treatment comparator. For that gold standard, there is now anticipation for results from the CHRONOS trial in the United Kingdom, especially part A of the trial, which compares radical therapy to focal therapy (HIFU or cryotherapy), with 5-year progression-free survival as the primary outcome. That trial is slated for completion in 2027.
Until then, the lack of prospective randomized clinical trials and long-term follow-up “hinders acceptance [of focal therapy] in the urology community,” the essay authors comment.
Meanwhile, careful patient selection is very important, they say.
The latest relevant guidelines state that appropriate candidates are men with a solitary, well-defined index lesion; patients with bilateral multifocal lesions; or very advanced tumors that are not appropriate for the focal approach.
A multidisciplinary international expert panel recently convened to establish guidance for clinicians offering focal therapies and then published a consensus statement to advise practitioners and researchers.
UCSF’s Dr. Cooperberg sees plenty of room for improvement among focal therapy practitioners and investigators. “From an outcomes standpoint, follow-up protocols and definitions of success remain inconsistent. I believe we’re making progress in all these areas, but we’re not there yet,” he says.
To date, some patients have been managed poorly, Dr. Cooperberg added. “We certainly see many patients who have been inadequately counseled as to HIFU’s advantages and disadvantages, with sometimes disastrous results.”
Some of those unfortunate results may have arisen from the U.S. Food and Drug Administration’s initial approval of HIFU in 2015, which was for use in ablating prostate tissue in general and not cancer specifically. This approval generated confusion, one expert commented at the time: “The FDA doesn’t specify whether it’s for benign or malignant disease; it’s a bit vague, like saying you can drive this car, but we’re not going to tell you how to drive it,” said Manoj Monga, MD, from the Cleveland Clinic.
Dr. Lebastchi has disclosed no relevant financial relationships; co-author Inderbir Gill, MD, is an unpaid consultant for Steba Biotech, and co-author Andre Luis Abreu, MD, is a consultant for Koelis and was a proctor in training for Steba Biotech. Dr. Cooperberg is a consultant for Alessa Therapeutics.
A version of this article first appeared on Medscape.com.
They maintain that focal therapy (FT) offers a “middle ground” between two extremes: Treating the whole gland with radical prostatectomy or radiotherapy and not treating immediately via active surveillance or watchful waiting.
Focal therapy typically treats the primary lesion within the prostate, while leaving the rest of the gland intact. Most often performed with cryoablation or high-intensity focused ultrasound (HIFU), it can also be carried out with a variety of technologies, including transurethral ultrasound ablation and focal laser ablation.
The shift to focal therapy will coincide with maturing, long-term data from studies with various technologies, predict the authors, led by Amir Lebastchi, MD, a urologist at the University of Southern California.
“Standard adoption of focal therapy is ultimately dependent on the availability of robust level I evidence, which in turn will drive medical societies and payees,” the authors also write.
But payees are already making changes, even without such data, they add.
For example, in January the American Medical Association announced a new code for high-intensity focal ultrasound (HIFU): This approach now has a Current Procedural Terminology (CPT) code from the U.S. Centers for Medicare & Medicaid Services
This news organization reached out to Matthew Cooperberg, MD, MPH, a urologist at the University of California, San Francisco (UCSF), for comments about the essay’s optimism; he has questioned focal therapy in the past because of a lack of strong supporting evidence.
“While ‘routine’ is a bit of a vague term, now that HIFU has a CPT code, I do expect its use will in fact increase in the next 5 years,” Dr. Cooperberg wrote in an email. “The question is whether its use will increase appropriately.”
The challenge with focal therapy – regardless of energy modality – remains patient selection and accurate ablation zone definition, he added.
Notably, UCSF has launched a new HIFU program – and Dr. Cooperberg has referred selected patients. “I’m both enthusiastic and cautious about the future, and we need to track our outcomes very closely across various practice settings,” he said.
While waiting for CHRONOS, select wisely
The goal of focal therapy is to treat only the area with the most aggressive tumor, known as the index tumor, while leaving the remaining gland and its surrounding structures alone, according to Derek Lomas, MD, PharmD, a urologist at the Mayo Clinic in Rochester, Minn., in an explanatory article. “This approach is widely accepted in other types of cancer. For example, we commonly treat kidney cancers by removing or ablating only the tumor while leaving the rest of the kidney intact.”
However, some focal therapies also include approaches known as hemiablations, in which a full half of the prostate is destroyed, and approaches that leave very little of the gland behind.
Each of the modalities used for focal therapy has “unique indications, risks, and benefits and uses a different energy source for ablation,” Dr. Lebastchi and colleagues write in their essay.
They assert that focal therapy can provide oncological efficacy similar to radical prostatectomy or radiotherapy “while considerably reducing or even eliminating functional morbidities, such as incontinence and erectile dysfunction.”
Overall, they say focal therapy offers an opportunity for improved care because there is “an increasing body of emerging evidence demonstrating a favorable adverse effect profile with oncological control similar to whole-gland treatment options.”
What is that evidence?
In the essay, Dr. Lebastchi and colleagues point to a number of single-arm studies with encouraging efficacy and safety results. They also highlight a phase 3, randomized trial that they were involved in: This compared focal therapy (partial gland ablation with vascular-targeted photodynamic therapy) with active surveillance in early-stage disease and uniformly showed better post-treatment biopsy (disease/no disease) and conversion-to-prostatectomy results with the focal therapy out to 4 years (J Urol. 2018;200:786-793).
However, that study did not have an active treatment comparator. For that gold standard, there is now anticipation for results from the CHRONOS trial in the United Kingdom, especially part A of the trial, which compares radical therapy to focal therapy (HIFU or cryotherapy), with 5-year progression-free survival as the primary outcome. That trial is slated for completion in 2027.
Until then, the lack of prospective randomized clinical trials and long-term follow-up “hinders acceptance [of focal therapy] in the urology community,” the essay authors comment.
Meanwhile, careful patient selection is very important, they say.
The latest relevant guidelines state that appropriate candidates are men with a solitary, well-defined index lesion; patients with bilateral multifocal lesions; or very advanced tumors that are not appropriate for the focal approach.
A multidisciplinary international expert panel recently convened to establish guidance for clinicians offering focal therapies and then published a consensus statement to advise practitioners and researchers.
UCSF’s Dr. Cooperberg sees plenty of room for improvement among focal therapy practitioners and investigators. “From an outcomes standpoint, follow-up protocols and definitions of success remain inconsistent. I believe we’re making progress in all these areas, but we’re not there yet,” he says.
To date, some patients have been managed poorly, Dr. Cooperberg added. “We certainly see many patients who have been inadequately counseled as to HIFU’s advantages and disadvantages, with sometimes disastrous results.”
Some of those unfortunate results may have arisen from the U.S. Food and Drug Administration’s initial approval of HIFU in 2015, which was for use in ablating prostate tissue in general and not cancer specifically. This approval generated confusion, one expert commented at the time: “The FDA doesn’t specify whether it’s for benign or malignant disease; it’s a bit vague, like saying you can drive this car, but we’re not going to tell you how to drive it,” said Manoj Monga, MD, from the Cleveland Clinic.
Dr. Lebastchi has disclosed no relevant financial relationships; co-author Inderbir Gill, MD, is an unpaid consultant for Steba Biotech, and co-author Andre Luis Abreu, MD, is a consultant for Koelis and was a proctor in training for Steba Biotech. Dr. Cooperberg is a consultant for Alessa Therapeutics.
A version of this article first appeared on Medscape.com.
Infusion shown effective for acquired von Willebrand disease
Acquired von Willebrand disease (aVWD) is a rare and serious condition associated with lymphoproliferative disorders, malignancy, autoimmune disorders, and cardiovascular disease. It is most commonly caused by monoclonal gammopathy of undetermined significance (MGUS), which acts to clear von Willebrand factor from the patient’s bloodstream. However, a continuous-infusion of plasma-derived von Willebrand factor (VWF) concentrate provided adequate hemostasis in aVWD resulting from MGUS, according to Kathryn E. Dane, PharmD, of Johns Hopkins University, Baltimore, and colleagues.
The infusion rapidly achieved target ristocetin cofactor activity with or without intravenous immunoglobulin in three patients, as detailed in the report published online in Blood Advances.
The three consecutive patients with aVWD were treated with plasma-derived VWF concentrate administered for periprocedural optimization (patient 1, an 85-year old woman) or to treat bleeding episodes (patient 2, an 88-year-old man; and patient 3, a 53-year-old woman). Factor VIII activity was measured via a 1-stage clotting test and von Willebrand factor activity was measured with a ristocetin cofactor assay.
Promising results
All three patients demonstrated increased VWF ristocetin cofactor and factor VIII activities within hours of initiation of the continuous infusion concentrate, according to the report.
“We hypothesize that the efficacy of CI VWF concentrate in aVWD may be related to continuous provision of VWF, allowing binding and neutralization of anti-VWF IgG antibodies, and providing adequate circulating unbound VWF for appropriate hemostatic efficacy,” the researchers concluded.
The authors reported that they had no competing financial interests.
Acquired von Willebrand disease (aVWD) is a rare and serious condition associated with lymphoproliferative disorders, malignancy, autoimmune disorders, and cardiovascular disease. It is most commonly caused by monoclonal gammopathy of undetermined significance (MGUS), which acts to clear von Willebrand factor from the patient’s bloodstream. However, a continuous-infusion of plasma-derived von Willebrand factor (VWF) concentrate provided adequate hemostasis in aVWD resulting from MGUS, according to Kathryn E. Dane, PharmD, of Johns Hopkins University, Baltimore, and colleagues.
The infusion rapidly achieved target ristocetin cofactor activity with or without intravenous immunoglobulin in three patients, as detailed in the report published online in Blood Advances.
The three consecutive patients with aVWD were treated with plasma-derived VWF concentrate administered for periprocedural optimization (patient 1, an 85-year old woman) or to treat bleeding episodes (patient 2, an 88-year-old man; and patient 3, a 53-year-old woman). Factor VIII activity was measured via a 1-stage clotting test and von Willebrand factor activity was measured with a ristocetin cofactor assay.
Promising results
All three patients demonstrated increased VWF ristocetin cofactor and factor VIII activities within hours of initiation of the continuous infusion concentrate, according to the report.
“We hypothesize that the efficacy of CI VWF concentrate in aVWD may be related to continuous provision of VWF, allowing binding and neutralization of anti-VWF IgG antibodies, and providing adequate circulating unbound VWF for appropriate hemostatic efficacy,” the researchers concluded.
The authors reported that they had no competing financial interests.
Acquired von Willebrand disease (aVWD) is a rare and serious condition associated with lymphoproliferative disorders, malignancy, autoimmune disorders, and cardiovascular disease. It is most commonly caused by monoclonal gammopathy of undetermined significance (MGUS), which acts to clear von Willebrand factor from the patient’s bloodstream. However, a continuous-infusion of plasma-derived von Willebrand factor (VWF) concentrate provided adequate hemostasis in aVWD resulting from MGUS, according to Kathryn E. Dane, PharmD, of Johns Hopkins University, Baltimore, and colleagues.
The infusion rapidly achieved target ristocetin cofactor activity with or without intravenous immunoglobulin in three patients, as detailed in the report published online in Blood Advances.
The three consecutive patients with aVWD were treated with plasma-derived VWF concentrate administered for periprocedural optimization (patient 1, an 85-year old woman) or to treat bleeding episodes (patient 2, an 88-year-old man; and patient 3, a 53-year-old woman). Factor VIII activity was measured via a 1-stage clotting test and von Willebrand factor activity was measured with a ristocetin cofactor assay.
Promising results
All three patients demonstrated increased VWF ristocetin cofactor and factor VIII activities within hours of initiation of the continuous infusion concentrate, according to the report.
“We hypothesize that the efficacy of CI VWF concentrate in aVWD may be related to continuous provision of VWF, allowing binding and neutralization of anti-VWF IgG antibodies, and providing adequate circulating unbound VWF for appropriate hemostatic efficacy,” the researchers concluded.
The authors reported that they had no competing financial interests.
FROM BLOOD ADVANCES
Myasthenic Crisis After Recurrent COVID-19 Infection
A patient with myasthenia gravis who survived 2 COVID-19 infections required plasmapheresis to recover from an acute crisis.
COVID-19 is still in the early stages of understanding, although it is known to be complicated by individual patient comorbidities. The management and treatment of COVID-19 continues to quickly evolve as more is discovered regarding the virus. Multiple treatments have been preliminarily tested and used under a Food and Drug Administration emergency use authorization (EUA) determination. The long-term success of these therapies, however, is yet to be determined. Additionally, if a patient has a second clinical presentation for COVID-19, it is not known whether this represents latency with subsequent reactivation from the previous infection or a second de novo infection. The uncertainty calls into question the duration of immunity, if any, following a primary infection.
COVID-19 management becomes more complicated when patients have complex medical conditions, such as myasthenia gravis (MG). This autoimmune neuromuscular disorder can present with varying weakness, and many patients are on immunomodulator medications. The weakness can worsen into a myasthenic crisis (MC), resulting in profound weakness of the respiratory muscles. Therefore, patients with MG are at increased risk for COVID-19 and may have a more complicated course when infected.
Our patient with MG presented for severe COVID-19 symptoms twice and later developed MC. He received 2 treatment modalities available under an EUA (remdesivir and convalescent plasma) for COVID-19, resulting in symptom resolution and a negative polymerize chain reaction (PCR) test result for the virus. However, after receiving his typical maintenance therapy of IV immunoglobulin (IVIG) for his MG, he again developed symptoms consistent with COVID-19 and tested positive. After recovering from the second episode of COVID-19, the patient went into MC requiring plasmapheresis.
Case Presentation
A 56-year-old male, US Army veteran presented to Carl R. Darnall Army Medical Center emergency department (ED) 6 days after testing positive for COVID-19, with worsening sputum, cough, congestion, dyspnea, and fever. Due to his MG, the patient had a home oxygen monitor and reported that his oxygenation saturation dropped below 90% with minimal exertion. His medical history was significant for MG, status postthymectomy and radiation treatment, left hemidiaphragm paralysis secondary to phrenic nerve injury, and corticosteroid-induced insulin-dependent diabetes mellitus. His current home medications included pyridostigmine 60 mg 3 times a day, mycophenolate (MMF) 1500 mg twice daily, IV immunoglobulin (IVIG) every 3 weeks, insulin aspart up to 16 U per meal, insulin glargine 30 U twice a day, dulaglutide 0.75 mg every week, and metformin 1000 mg twice daily.
On initial examination, the patient’s heart rate (HR) was 111 beats/min, respiratory rate (RR), 22 breaths/min, blood pressure (BP), 138/88 mm Hg, temperature, 100.9 oF, and his initial pulse oximetry, 91% on room air. On physical examination, the patient was tachypneic, though without other signs of respiratory distress. Lung auscultation revealed no adventitial lung sounds. His cardiac examination was notable only for tachycardia. His neurologic examination demonstrated intact cranial nerves, with 5 out of 5 (scale 1 to 5) strength throughout the upper and lower extremities, sensation was intact to light touch, and he had normal cerebellar function. The rest of the examination was normal.
Initial laboratory investigation was notable for a white blood cell count of 14.15x103 cells/mcL with 84% neutrophils, and 6% lymphocytes. Additional tests revealed a C-reactive protein (CRP) level, 17.97 mg/dL (reference range, 0-0.5 mg/dL), ferritin level, 647 ng/mL (reference range, 22-274 ng/mL), d-dimer, 0.64 mcg/mL (reference range, 0-0.47mcg/mL), and a repeated positive COVID-19 PCR test. A portable chest X-ray showed bibasilar opacities (Figure 1).
The patient was diagnosed with COVID-19 and admitted to the intensive care unit (ICU). In the ICU, the patient received 1 U of convalescent plasma (CP) and started on a course of IV remdesivir 100 mg/d consistent with the EUA. He also received a 5-day course of ceftriaxone and azithromycin for possible community acquired pneumonia (CAP). As part of the patient’s MG maintenance medications, he received IVIG 4 g while in the ICU. Throughout his ICU stay, he required supplemental nasal cannula oxygenation to maintain his oxygen saturation > 93%. After 8 days in the ICU, his oxygen requirements decreased, and the patient was transferred out of the ICU and remdesivir was discontinued. On hospital day 10, a repeat COVID-19 PCR test was negative, inflammatory markers returned to within normal limits, and a repeat chest X-ray showed improvement from admission (Figure 2). Having recovered significantly, he was discharged home.
Three weeks later, the patient again presented to the MTF with 3 days of dyspnea, cough, fever, nausea, and vomiting. One day before symptom onset, he had received his maintenance IVIG infusion. The patient reported that his home oxygen saturation was 82% with minimal exertion. On ED presentation his HR was 107 beats/min, RR, 28 breaths/min, temperature, 98.1 oF, BP 118/71 mm Hg, and oxygen saturation, 92% on 2L nasal cannula. His examination was most notable for tachypnea with accessory muscle use. At this time, his neurologic examination was unchanged from prior admission with grossly intact cranial nerves and symmetric 5 of 5 motor strength in all extremities.
At this second ED visit, laboratory results demonstrated a CRP of 3.44 mg/dL, ferritin 2019 ng/mL, d-dimer, 3.39 mcg/mL, and a positive COVID-19 PCR result. His chest X-ray demonstrated new peripheral opacities compared with the X-ray at discharge (Figure 3). He required ICU admission again for his COVID-19 symptoms.
During his ICU course he continued to require supplemental oxygen by nasal cannula, though never required intubation. This second admission, he was again treated empirically for CAP with levofloxacin 750 mg daily for 5 days. He was discharged after 14 days with symptom resolution and down trending of inflammatory markers, though he was not retested for COVID-19.
Four days after his second discharge, he presented to the ED for a third time with diffuse weakness, dysphagia, and dysarthria of 1 day. His HR was 87/beats/min; RR, 17 breaths/min; temperature, 98.7 oF; BP, 144/81 mm Hg; and oxygen saturation, 98% on room air. His examination was significant for slurred speech, bilateral ptosis, 3 of 5 strength in bilateral finger flexion/abduction, wrist extension, knee and ankle flexion/extension; 4 of 5 strength in bilateral proximal muscle testing of deltoid, and hip; normal sensation, cerebellar function and reflexes. His negative inspiratory force (NIF) maximal effort was −30 cmH2O. He was determined to be in MC without evidence of COIVD-19 symptoms, and laboratory results were within normal limits, including a negative COVID-19 PCR. As he received IVIG as maintenance therapy, plasmapheresis was recommended to treat his MC, which required transfer to an outside civilian facility.
At the outside hospital, the patient underwent 5 rounds of plasmapheresis over 10 days. By the third treatment his strength had returned with resolution of the bulbar symptoms and no supplemental oxygen requirements. The patient was discharged and continued his original dosages of MMF and pyridostigmine. At 3 months, he remained asymptomatic from a COVID-19 standpoint and stable from a MG standpoint.
Discussion
Reinfection with the COVID-19 has been continuously debated with alternative explanations suggested for a positive test after a previous negative PCR test in the setting of symptom resolution.1,2 Proposed causes include dynamic PCR results due to prolonged viral shedding and inaccurate or poorly sensitive tests. The repeat positive cases in these scenarios, however, occurred in asymptomatic patients.1,2 COVID-19 shedding averages 20 to 22 days after symptom onset but has been seen up to 36 days after symptom resolution.2,3 This would suggest that fluctuating results during the immediate postsymptom period may be due to variations in viral shedding load and or sampling error—especially in asymptomatic patients. On the other hand, patients who experience return of symptoms days to weeks after previous convalescence leave clinicians wondering whether this represents clinical latency with reactivation or COVID-19 reinfection. A separate case of initial COVID-19 in a patient that had subsequent clinical recovery with a negative PCR developed recurrent respiratory symptoms and had a positive PCR test only 10 days later, further highlighting the reinfection vs reactivation issue of COVID-19.2 Further understanding of this issue may have implications on the extent of natural immunity following primary infection; potential vaccine dosage schedules; and global public health policies.
Although reactivation may be plausible given his immunomodulatory therapy, our patient’s second COVID-19 symptoms started 40 days after the initial symptoms, and 26 days after the initial course resolution; previous cases of return of severe symptoms occurred between 3 and 6 days.1 Given our patient’s time course between resolution and return of symptoms, if latency is the mechanism at play, this case demonstrates an exceptionally longer latency period than the ones that have been reported. Additionally, if latency is an issue in COVID-19, using remdesivir as a treatment further complicates the understanding of this disease.
Remdesivir, a nucleoside analogue antiviral, was shown to benefit recovery in patients with severe symptoms in the Adaptive COVID-19 Treatment Trial-1 study.4 Our patient had originally been placed on a 10-day course; however, on treatment day 8, his symptoms resolved and the remdesivir was discontinued. This is a similar finding to half the patients in the 10-day arm of the study by McCreary and colleagues.5 Although our patient was asymptomatic 4 weeks after the start of remdesivir, consistent with the majority of patients in the McCreary 10-day study arm, further comparison of the presented patient is limited due to study length and follow-up considerations.5 No previous data exist on reactivation, reinfection, or long-term mortality after being treated with remdesivir for COVID-19 infection.
IVIG is being studied in the treatment of COVID-19 and bears consideration as it relates to our patient. There is no evidence that IVIG used in the treatment of autoimmune diseases increases the risk of infection compared with that of other medications used in the treatment of such diseases. Furthermore, the current guidance from the MG expert panel does not suggest that IVIG increases the risk of contracting COVID-19 aside from the risks of exposure to hospital infrastructure.6 Yet the guidance does not discuss the use of IVIG for MG in patients who are already symptomatic from COVID-19 or for patients recovering from the clinical disease or does it discuss a possible compounding risk of thromboembolic events associated with IVIG and COVID-19.6,7 Our patient received his maintenance IVIG during his first admission without any worsening of symptoms or increased oxygen requirements. The day following our patient’s next scheduled IVIG infusion—while asymptomatic—he again developed respiratory symptoms; this could suggest that IVIG did not contribute to his second clinical course nor protect against.
CP is a treatment modality that has been used and studied in previous infectious outbreaks such as the first severe acute respiratory syndrome, and the H1N1 influenza virus.8 Current data on CP for COVID-19 are limited, but early descriptive studies have shown a benefit in improvement of symptoms 5 days sooner in those requiring supplemental oxygen, but no benefit for those requiring mechanical ventilation.9 Like patients that benefitted in these studies, our patient received CP early, 6 days after first testing positive and onset of symptoms. This patient’s reinfection or return of symptoms draws into question the hindrance or even prevention of long-term immunity from administration of CP.
COVID-19 presents many challenges when managing this patient’s coexisting MG, especially as the patient was already being treated with immunosuppressing therapies. The guidance does recommend continuation of standard MG therapies during hospitalizations, including immunosuppression medications such as MMF.6 Immunosuppression is associated with worsened severity of COVID-19 symptoms, although no relation exists to degree of immunosuppression and severity.7,10 To the best of our knowledge there has been no case report of reinfection or reactivation of COVID-19 associated with immunosuppressive agents used in the treatment of MG.
Our patient also was taking pyridostigmine for the treatment of his MG. There is no evidence this medication increases the risk of infection; but the cholinergic activity can increase bronchial secretions, which could theoretically worsen the COVID-19 respiratory symptoms.6,11 During both ICU admissions, our patient continued pyridostigmine use, observing complete return to baseline after discharge. Given the possible association with worsened respiratory outcomes after the second ICU admission, the balance between managing MG symptoms and COVID-19 symptoms needs further examination.
The patient was in MC during his third presentation to the ED. Although respiratory symptoms may be difficult to differentiate from COVID-19, the additional neurologic symptoms seen in this patient allowed for quick determination of the need for MC treatment. There are many potential etiologies contributing to the development of the MC presented here, and it was likely due to multifactorial precipitants. A common cause of MC is viral upper respiratory infections, further challenging the care of these patients during this pandemic.12 Many medications have been cited as causing a MC, 2 of which our patient received during admission for COVID-19: azithromycin and levoquin.12 Although the patient did not receive hydroxychloroquine, which was still being considered as an appropriate COVID-19 treatment at the time, it also is a drug known for precipitating MC and its use scrutinized in patients with MG.12
A key aspect to diagnosing and guiding therapies in myasthenic crisis in addition to the clinical symptoms of acute weakness is respiratory assessment through the nonaerosolizing NIF test.12 Our patient’s NIF measured < 30 cmH2O when in MC, while the reference range is < 75 cmH2O, and for mechanical ventilation is recommended at 20 cmH2O. Although the patient was maintaining O2 saturation > 95%, his NIF value was concerning, and preparations were made in case of precipitous decline. Compounding the NIF assessment in this patient is his history of left phrenic nerve palsy. Without a documented baseline NIF, results were limited in determining his diaphragm strength.13 Treatment for MC includes IVIG or plasmapheresis, since this patient had failed his maintenance therapy IVIG, plasmapheresis was coordinated for definitive therapy.
Conclusions
Federal facilities have seen an increase in the amount of respiratory complaints over the past months. Although COVID-19 is a concerning diagnosis, it is crucial to consider comorbidities in the diagnostic workup of each, even with a previous recent diagnosis of COVID-19. As treatment recommendations for COVID-19 continue to fluctuate coupled with the limitations and difficulties associated with MG patients, so too treatment and evaluation must be carefully considered at each presentation.
1. Gousseff M, Penot P, Gallay L, et al. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020;81(5):816-846. doi:10.1016/j.jinf.2020.06.073
2. Duggan NM, Ludy SM, Shannon BC, Reisner AT, Wilcox SR. Is novel coronavirus 2019 reinfection possible? Interpreting dynamic SARS-CoV-2 test results. Am J Emerg Med. 2021;39:256.e1-256.e3. doi:10.1016/j.ajem.2020.06.079
3. Li J, Zhang L, Liu B, Song D. Case report: viral shedding for 60 days in a woman with COVID-19. Am J Trop Med Hyg. 2020;102(6):1210-1213. doi:10.4269/ajtmh.20-0275
4. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 - preliminary report. Reply. N Engl J Med. 2020;383(10):994. doi:10.1056/NEJMc2022236
5. McCreary EK, Angus DC. Efficacy of remdesivir in COVID-19. JAMA. 2020;324(11):1041-1042. doi:10.1001/jama.2020.16337
6. International MG/COVID-19 Working Group; Jacob S, Muppidi S, Gordon A, et al. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci. 2020;412:116803. doi:10.1016/j.jns.2020.116803
7. Anand P, Slama MCC, Kaku M, et al. COVID-19 in patients with myasthenia gravis. Muscle Nerve. 2020;62(2):254-258. doi:10.1002/mus.26918
8. Wooding DJ, Bach H. Treatment of COVID-19 with convalescent plasma: lessons from past coronavirus outbreaks. Clin Microbiol Infect. 2020;26(10):1436-1446. doi:10.1016/j.cmi.2020.08.005
9. Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (covid-19) patients with convalescent plasma. Am J Pathol. 2020;190(8):1680-1690. doi:10.1016/j.ajpath.2020.05.014
10. Ryan C, Minc A, Caceres J, et al. Predicting severe outcomes in Covid-19 related illness using only patient demographics, comorbidities and symptoms [published online ahead of print, 2020 Sep 9]. Am J Emerg Med. 2020;S0735-6757(20)30809-3. doi:10.1016/j.ajem.2020.09.017
11. Singh S, Govindarajan R. COVID-19 and generalized myasthenia gravis exacerbation: a case report. Clin Neurol Neurosurg. 2020;196:106045. doi:10.1016/j.clineuro.2020.106045
12. Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist. 2011;1(1):16-22. doi:10.1177/1941875210382918
13. Dubé BP, Dres M. Diaphragm dysfunction: diagnostic approaches and management strategies. J Clin Med. 2016;5(12):113. Published 2016 Dec 5. doi:10.3390/jcm5120113
A patient with myasthenia gravis who survived 2 COVID-19 infections required plasmapheresis to recover from an acute crisis.
A patient with myasthenia gravis who survived 2 COVID-19 infections required plasmapheresis to recover from an acute crisis.
COVID-19 is still in the early stages of understanding, although it is known to be complicated by individual patient comorbidities. The management and treatment of COVID-19 continues to quickly evolve as more is discovered regarding the virus. Multiple treatments have been preliminarily tested and used under a Food and Drug Administration emergency use authorization (EUA) determination. The long-term success of these therapies, however, is yet to be determined. Additionally, if a patient has a second clinical presentation for COVID-19, it is not known whether this represents latency with subsequent reactivation from the previous infection or a second de novo infection. The uncertainty calls into question the duration of immunity, if any, following a primary infection.
COVID-19 management becomes more complicated when patients have complex medical conditions, such as myasthenia gravis (MG). This autoimmune neuromuscular disorder can present with varying weakness, and many patients are on immunomodulator medications. The weakness can worsen into a myasthenic crisis (MC), resulting in profound weakness of the respiratory muscles. Therefore, patients with MG are at increased risk for COVID-19 and may have a more complicated course when infected.
Our patient with MG presented for severe COVID-19 symptoms twice and later developed MC. He received 2 treatment modalities available under an EUA (remdesivir and convalescent plasma) for COVID-19, resulting in symptom resolution and a negative polymerize chain reaction (PCR) test result for the virus. However, after receiving his typical maintenance therapy of IV immunoglobulin (IVIG) for his MG, he again developed symptoms consistent with COVID-19 and tested positive. After recovering from the second episode of COVID-19, the patient went into MC requiring plasmapheresis.
Case Presentation
A 56-year-old male, US Army veteran presented to Carl R. Darnall Army Medical Center emergency department (ED) 6 days after testing positive for COVID-19, with worsening sputum, cough, congestion, dyspnea, and fever. Due to his MG, the patient had a home oxygen monitor and reported that his oxygenation saturation dropped below 90% with minimal exertion. His medical history was significant for MG, status postthymectomy and radiation treatment, left hemidiaphragm paralysis secondary to phrenic nerve injury, and corticosteroid-induced insulin-dependent diabetes mellitus. His current home medications included pyridostigmine 60 mg 3 times a day, mycophenolate (MMF) 1500 mg twice daily, IV immunoglobulin (IVIG) every 3 weeks, insulin aspart up to 16 U per meal, insulin glargine 30 U twice a day, dulaglutide 0.75 mg every week, and metformin 1000 mg twice daily.
On initial examination, the patient’s heart rate (HR) was 111 beats/min, respiratory rate (RR), 22 breaths/min, blood pressure (BP), 138/88 mm Hg, temperature, 100.9 oF, and his initial pulse oximetry, 91% on room air. On physical examination, the patient was tachypneic, though without other signs of respiratory distress. Lung auscultation revealed no adventitial lung sounds. His cardiac examination was notable only for tachycardia. His neurologic examination demonstrated intact cranial nerves, with 5 out of 5 (scale 1 to 5) strength throughout the upper and lower extremities, sensation was intact to light touch, and he had normal cerebellar function. The rest of the examination was normal.
Initial laboratory investigation was notable for a white blood cell count of 14.15x103 cells/mcL with 84% neutrophils, and 6% lymphocytes. Additional tests revealed a C-reactive protein (CRP) level, 17.97 mg/dL (reference range, 0-0.5 mg/dL), ferritin level, 647 ng/mL (reference range, 22-274 ng/mL), d-dimer, 0.64 mcg/mL (reference range, 0-0.47mcg/mL), and a repeated positive COVID-19 PCR test. A portable chest X-ray showed bibasilar opacities (Figure 1).
The patient was diagnosed with COVID-19 and admitted to the intensive care unit (ICU). In the ICU, the patient received 1 U of convalescent plasma (CP) and started on a course of IV remdesivir 100 mg/d consistent with the EUA. He also received a 5-day course of ceftriaxone and azithromycin for possible community acquired pneumonia (CAP). As part of the patient’s MG maintenance medications, he received IVIG 4 g while in the ICU. Throughout his ICU stay, he required supplemental nasal cannula oxygenation to maintain his oxygen saturation > 93%. After 8 days in the ICU, his oxygen requirements decreased, and the patient was transferred out of the ICU and remdesivir was discontinued. On hospital day 10, a repeat COVID-19 PCR test was negative, inflammatory markers returned to within normal limits, and a repeat chest X-ray showed improvement from admission (Figure 2). Having recovered significantly, he was discharged home.
Three weeks later, the patient again presented to the MTF with 3 days of dyspnea, cough, fever, nausea, and vomiting. One day before symptom onset, he had received his maintenance IVIG infusion. The patient reported that his home oxygen saturation was 82% with minimal exertion. On ED presentation his HR was 107 beats/min, RR, 28 breaths/min, temperature, 98.1 oF, BP 118/71 mm Hg, and oxygen saturation, 92% on 2L nasal cannula. His examination was most notable for tachypnea with accessory muscle use. At this time, his neurologic examination was unchanged from prior admission with grossly intact cranial nerves and symmetric 5 of 5 motor strength in all extremities.
At this second ED visit, laboratory results demonstrated a CRP of 3.44 mg/dL, ferritin 2019 ng/mL, d-dimer, 3.39 mcg/mL, and a positive COVID-19 PCR result. His chest X-ray demonstrated new peripheral opacities compared with the X-ray at discharge (Figure 3). He required ICU admission again for his COVID-19 symptoms.
During his ICU course he continued to require supplemental oxygen by nasal cannula, though never required intubation. This second admission, he was again treated empirically for CAP with levofloxacin 750 mg daily for 5 days. He was discharged after 14 days with symptom resolution and down trending of inflammatory markers, though he was not retested for COVID-19.
Four days after his second discharge, he presented to the ED for a third time with diffuse weakness, dysphagia, and dysarthria of 1 day. His HR was 87/beats/min; RR, 17 breaths/min; temperature, 98.7 oF; BP, 144/81 mm Hg; and oxygen saturation, 98% on room air. His examination was significant for slurred speech, bilateral ptosis, 3 of 5 strength in bilateral finger flexion/abduction, wrist extension, knee and ankle flexion/extension; 4 of 5 strength in bilateral proximal muscle testing of deltoid, and hip; normal sensation, cerebellar function and reflexes. His negative inspiratory force (NIF) maximal effort was −30 cmH2O. He was determined to be in MC without evidence of COIVD-19 symptoms, and laboratory results were within normal limits, including a negative COVID-19 PCR. As he received IVIG as maintenance therapy, plasmapheresis was recommended to treat his MC, which required transfer to an outside civilian facility.
At the outside hospital, the patient underwent 5 rounds of plasmapheresis over 10 days. By the third treatment his strength had returned with resolution of the bulbar symptoms and no supplemental oxygen requirements. The patient was discharged and continued his original dosages of MMF and pyridostigmine. At 3 months, he remained asymptomatic from a COVID-19 standpoint and stable from a MG standpoint.
Discussion
Reinfection with the COVID-19 has been continuously debated with alternative explanations suggested for a positive test after a previous negative PCR test in the setting of symptom resolution.1,2 Proposed causes include dynamic PCR results due to prolonged viral shedding and inaccurate or poorly sensitive tests. The repeat positive cases in these scenarios, however, occurred in asymptomatic patients.1,2 COVID-19 shedding averages 20 to 22 days after symptom onset but has been seen up to 36 days after symptom resolution.2,3 This would suggest that fluctuating results during the immediate postsymptom period may be due to variations in viral shedding load and or sampling error—especially in asymptomatic patients. On the other hand, patients who experience return of symptoms days to weeks after previous convalescence leave clinicians wondering whether this represents clinical latency with reactivation or COVID-19 reinfection. A separate case of initial COVID-19 in a patient that had subsequent clinical recovery with a negative PCR developed recurrent respiratory symptoms and had a positive PCR test only 10 days later, further highlighting the reinfection vs reactivation issue of COVID-19.2 Further understanding of this issue may have implications on the extent of natural immunity following primary infection; potential vaccine dosage schedules; and global public health policies.
Although reactivation may be plausible given his immunomodulatory therapy, our patient’s second COVID-19 symptoms started 40 days after the initial symptoms, and 26 days after the initial course resolution; previous cases of return of severe symptoms occurred between 3 and 6 days.1 Given our patient’s time course between resolution and return of symptoms, if latency is the mechanism at play, this case demonstrates an exceptionally longer latency period than the ones that have been reported. Additionally, if latency is an issue in COVID-19, using remdesivir as a treatment further complicates the understanding of this disease.
Remdesivir, a nucleoside analogue antiviral, was shown to benefit recovery in patients with severe symptoms in the Adaptive COVID-19 Treatment Trial-1 study.4 Our patient had originally been placed on a 10-day course; however, on treatment day 8, his symptoms resolved and the remdesivir was discontinued. This is a similar finding to half the patients in the 10-day arm of the study by McCreary and colleagues.5 Although our patient was asymptomatic 4 weeks after the start of remdesivir, consistent with the majority of patients in the McCreary 10-day study arm, further comparison of the presented patient is limited due to study length and follow-up considerations.5 No previous data exist on reactivation, reinfection, or long-term mortality after being treated with remdesivir for COVID-19 infection.
IVIG is being studied in the treatment of COVID-19 and bears consideration as it relates to our patient. There is no evidence that IVIG used in the treatment of autoimmune diseases increases the risk of infection compared with that of other medications used in the treatment of such diseases. Furthermore, the current guidance from the MG expert panel does not suggest that IVIG increases the risk of contracting COVID-19 aside from the risks of exposure to hospital infrastructure.6 Yet the guidance does not discuss the use of IVIG for MG in patients who are already symptomatic from COVID-19 or for patients recovering from the clinical disease or does it discuss a possible compounding risk of thromboembolic events associated with IVIG and COVID-19.6,7 Our patient received his maintenance IVIG during his first admission without any worsening of symptoms or increased oxygen requirements. The day following our patient’s next scheduled IVIG infusion—while asymptomatic—he again developed respiratory symptoms; this could suggest that IVIG did not contribute to his second clinical course nor protect against.
CP is a treatment modality that has been used and studied in previous infectious outbreaks such as the first severe acute respiratory syndrome, and the H1N1 influenza virus.8 Current data on CP for COVID-19 are limited, but early descriptive studies have shown a benefit in improvement of symptoms 5 days sooner in those requiring supplemental oxygen, but no benefit for those requiring mechanical ventilation.9 Like patients that benefitted in these studies, our patient received CP early, 6 days after first testing positive and onset of symptoms. This patient’s reinfection or return of symptoms draws into question the hindrance or even prevention of long-term immunity from administration of CP.
COVID-19 presents many challenges when managing this patient’s coexisting MG, especially as the patient was already being treated with immunosuppressing therapies. The guidance does recommend continuation of standard MG therapies during hospitalizations, including immunosuppression medications such as MMF.6 Immunosuppression is associated with worsened severity of COVID-19 symptoms, although no relation exists to degree of immunosuppression and severity.7,10 To the best of our knowledge there has been no case report of reinfection or reactivation of COVID-19 associated with immunosuppressive agents used in the treatment of MG.
Our patient also was taking pyridostigmine for the treatment of his MG. There is no evidence this medication increases the risk of infection; but the cholinergic activity can increase bronchial secretions, which could theoretically worsen the COVID-19 respiratory symptoms.6,11 During both ICU admissions, our patient continued pyridostigmine use, observing complete return to baseline after discharge. Given the possible association with worsened respiratory outcomes after the second ICU admission, the balance between managing MG symptoms and COVID-19 symptoms needs further examination.
The patient was in MC during his third presentation to the ED. Although respiratory symptoms may be difficult to differentiate from COVID-19, the additional neurologic symptoms seen in this patient allowed for quick determination of the need for MC treatment. There are many potential etiologies contributing to the development of the MC presented here, and it was likely due to multifactorial precipitants. A common cause of MC is viral upper respiratory infections, further challenging the care of these patients during this pandemic.12 Many medications have been cited as causing a MC, 2 of which our patient received during admission for COVID-19: azithromycin and levoquin.12 Although the patient did not receive hydroxychloroquine, which was still being considered as an appropriate COVID-19 treatment at the time, it also is a drug known for precipitating MC and its use scrutinized in patients with MG.12
A key aspect to diagnosing and guiding therapies in myasthenic crisis in addition to the clinical symptoms of acute weakness is respiratory assessment through the nonaerosolizing NIF test.12 Our patient’s NIF measured < 30 cmH2O when in MC, while the reference range is < 75 cmH2O, and for mechanical ventilation is recommended at 20 cmH2O. Although the patient was maintaining O2 saturation > 95%, his NIF value was concerning, and preparations were made in case of precipitous decline. Compounding the NIF assessment in this patient is his history of left phrenic nerve palsy. Without a documented baseline NIF, results were limited in determining his diaphragm strength.13 Treatment for MC includes IVIG or plasmapheresis, since this patient had failed his maintenance therapy IVIG, plasmapheresis was coordinated for definitive therapy.
Conclusions
Federal facilities have seen an increase in the amount of respiratory complaints over the past months. Although COVID-19 is a concerning diagnosis, it is crucial to consider comorbidities in the diagnostic workup of each, even with a previous recent diagnosis of COVID-19. As treatment recommendations for COVID-19 continue to fluctuate coupled with the limitations and difficulties associated with MG patients, so too treatment and evaluation must be carefully considered at each presentation.
COVID-19 is still in the early stages of understanding, although it is known to be complicated by individual patient comorbidities. The management and treatment of COVID-19 continues to quickly evolve as more is discovered regarding the virus. Multiple treatments have been preliminarily tested and used under a Food and Drug Administration emergency use authorization (EUA) determination. The long-term success of these therapies, however, is yet to be determined. Additionally, if a patient has a second clinical presentation for COVID-19, it is not known whether this represents latency with subsequent reactivation from the previous infection or a second de novo infection. The uncertainty calls into question the duration of immunity, if any, following a primary infection.
COVID-19 management becomes more complicated when patients have complex medical conditions, such as myasthenia gravis (MG). This autoimmune neuromuscular disorder can present with varying weakness, and many patients are on immunomodulator medications. The weakness can worsen into a myasthenic crisis (MC), resulting in profound weakness of the respiratory muscles. Therefore, patients with MG are at increased risk for COVID-19 and may have a more complicated course when infected.
Our patient with MG presented for severe COVID-19 symptoms twice and later developed MC. He received 2 treatment modalities available under an EUA (remdesivir and convalescent plasma) for COVID-19, resulting in symptom resolution and a negative polymerize chain reaction (PCR) test result for the virus. However, after receiving his typical maintenance therapy of IV immunoglobulin (IVIG) for his MG, he again developed symptoms consistent with COVID-19 and tested positive. After recovering from the second episode of COVID-19, the patient went into MC requiring plasmapheresis.
Case Presentation
A 56-year-old male, US Army veteran presented to Carl R. Darnall Army Medical Center emergency department (ED) 6 days after testing positive for COVID-19, with worsening sputum, cough, congestion, dyspnea, and fever. Due to his MG, the patient had a home oxygen monitor and reported that his oxygenation saturation dropped below 90% with minimal exertion. His medical history was significant for MG, status postthymectomy and radiation treatment, left hemidiaphragm paralysis secondary to phrenic nerve injury, and corticosteroid-induced insulin-dependent diabetes mellitus. His current home medications included pyridostigmine 60 mg 3 times a day, mycophenolate (MMF) 1500 mg twice daily, IV immunoglobulin (IVIG) every 3 weeks, insulin aspart up to 16 U per meal, insulin glargine 30 U twice a day, dulaglutide 0.75 mg every week, and metformin 1000 mg twice daily.
On initial examination, the patient’s heart rate (HR) was 111 beats/min, respiratory rate (RR), 22 breaths/min, blood pressure (BP), 138/88 mm Hg, temperature, 100.9 oF, and his initial pulse oximetry, 91% on room air. On physical examination, the patient was tachypneic, though without other signs of respiratory distress. Lung auscultation revealed no adventitial lung sounds. His cardiac examination was notable only for tachycardia. His neurologic examination demonstrated intact cranial nerves, with 5 out of 5 (scale 1 to 5) strength throughout the upper and lower extremities, sensation was intact to light touch, and he had normal cerebellar function. The rest of the examination was normal.
Initial laboratory investigation was notable for a white blood cell count of 14.15x103 cells/mcL with 84% neutrophils, and 6% lymphocytes. Additional tests revealed a C-reactive protein (CRP) level, 17.97 mg/dL (reference range, 0-0.5 mg/dL), ferritin level, 647 ng/mL (reference range, 22-274 ng/mL), d-dimer, 0.64 mcg/mL (reference range, 0-0.47mcg/mL), and a repeated positive COVID-19 PCR test. A portable chest X-ray showed bibasilar opacities (Figure 1).
The patient was diagnosed with COVID-19 and admitted to the intensive care unit (ICU). In the ICU, the patient received 1 U of convalescent plasma (CP) and started on a course of IV remdesivir 100 mg/d consistent with the EUA. He also received a 5-day course of ceftriaxone and azithromycin for possible community acquired pneumonia (CAP). As part of the patient’s MG maintenance medications, he received IVIG 4 g while in the ICU. Throughout his ICU stay, he required supplemental nasal cannula oxygenation to maintain his oxygen saturation > 93%. After 8 days in the ICU, his oxygen requirements decreased, and the patient was transferred out of the ICU and remdesivir was discontinued. On hospital day 10, a repeat COVID-19 PCR test was negative, inflammatory markers returned to within normal limits, and a repeat chest X-ray showed improvement from admission (Figure 2). Having recovered significantly, he was discharged home.
Three weeks later, the patient again presented to the MTF with 3 days of dyspnea, cough, fever, nausea, and vomiting. One day before symptom onset, he had received his maintenance IVIG infusion. The patient reported that his home oxygen saturation was 82% with minimal exertion. On ED presentation his HR was 107 beats/min, RR, 28 breaths/min, temperature, 98.1 oF, BP 118/71 mm Hg, and oxygen saturation, 92% on 2L nasal cannula. His examination was most notable for tachypnea with accessory muscle use. At this time, his neurologic examination was unchanged from prior admission with grossly intact cranial nerves and symmetric 5 of 5 motor strength in all extremities.
At this second ED visit, laboratory results demonstrated a CRP of 3.44 mg/dL, ferritin 2019 ng/mL, d-dimer, 3.39 mcg/mL, and a positive COVID-19 PCR result. His chest X-ray demonstrated new peripheral opacities compared with the X-ray at discharge (Figure 3). He required ICU admission again for his COVID-19 symptoms.
During his ICU course he continued to require supplemental oxygen by nasal cannula, though never required intubation. This second admission, he was again treated empirically for CAP with levofloxacin 750 mg daily for 5 days. He was discharged after 14 days with symptom resolution and down trending of inflammatory markers, though he was not retested for COVID-19.
Four days after his second discharge, he presented to the ED for a third time with diffuse weakness, dysphagia, and dysarthria of 1 day. His HR was 87/beats/min; RR, 17 breaths/min; temperature, 98.7 oF; BP, 144/81 mm Hg; and oxygen saturation, 98% on room air. His examination was significant for slurred speech, bilateral ptosis, 3 of 5 strength in bilateral finger flexion/abduction, wrist extension, knee and ankle flexion/extension; 4 of 5 strength in bilateral proximal muscle testing of deltoid, and hip; normal sensation, cerebellar function and reflexes. His negative inspiratory force (NIF) maximal effort was −30 cmH2O. He was determined to be in MC without evidence of COIVD-19 symptoms, and laboratory results were within normal limits, including a negative COVID-19 PCR. As he received IVIG as maintenance therapy, plasmapheresis was recommended to treat his MC, which required transfer to an outside civilian facility.
At the outside hospital, the patient underwent 5 rounds of plasmapheresis over 10 days. By the third treatment his strength had returned with resolution of the bulbar symptoms and no supplemental oxygen requirements. The patient was discharged and continued his original dosages of MMF and pyridostigmine. At 3 months, he remained asymptomatic from a COVID-19 standpoint and stable from a MG standpoint.
Discussion
Reinfection with the COVID-19 has been continuously debated with alternative explanations suggested for a positive test after a previous negative PCR test in the setting of symptom resolution.1,2 Proposed causes include dynamic PCR results due to prolonged viral shedding and inaccurate or poorly sensitive tests. The repeat positive cases in these scenarios, however, occurred in asymptomatic patients.1,2 COVID-19 shedding averages 20 to 22 days after symptom onset but has been seen up to 36 days after symptom resolution.2,3 This would suggest that fluctuating results during the immediate postsymptom period may be due to variations in viral shedding load and or sampling error—especially in asymptomatic patients. On the other hand, patients who experience return of symptoms days to weeks after previous convalescence leave clinicians wondering whether this represents clinical latency with reactivation or COVID-19 reinfection. A separate case of initial COVID-19 in a patient that had subsequent clinical recovery with a negative PCR developed recurrent respiratory symptoms and had a positive PCR test only 10 days later, further highlighting the reinfection vs reactivation issue of COVID-19.2 Further understanding of this issue may have implications on the extent of natural immunity following primary infection; potential vaccine dosage schedules; and global public health policies.
Although reactivation may be plausible given his immunomodulatory therapy, our patient’s second COVID-19 symptoms started 40 days after the initial symptoms, and 26 days after the initial course resolution; previous cases of return of severe symptoms occurred between 3 and 6 days.1 Given our patient’s time course between resolution and return of symptoms, if latency is the mechanism at play, this case demonstrates an exceptionally longer latency period than the ones that have been reported. Additionally, if latency is an issue in COVID-19, using remdesivir as a treatment further complicates the understanding of this disease.
Remdesivir, a nucleoside analogue antiviral, was shown to benefit recovery in patients with severe symptoms in the Adaptive COVID-19 Treatment Trial-1 study.4 Our patient had originally been placed on a 10-day course; however, on treatment day 8, his symptoms resolved and the remdesivir was discontinued. This is a similar finding to half the patients in the 10-day arm of the study by McCreary and colleagues.5 Although our patient was asymptomatic 4 weeks after the start of remdesivir, consistent with the majority of patients in the McCreary 10-day study arm, further comparison of the presented patient is limited due to study length and follow-up considerations.5 No previous data exist on reactivation, reinfection, or long-term mortality after being treated with remdesivir for COVID-19 infection.
IVIG is being studied in the treatment of COVID-19 and bears consideration as it relates to our patient. There is no evidence that IVIG used in the treatment of autoimmune diseases increases the risk of infection compared with that of other medications used in the treatment of such diseases. Furthermore, the current guidance from the MG expert panel does not suggest that IVIG increases the risk of contracting COVID-19 aside from the risks of exposure to hospital infrastructure.6 Yet the guidance does not discuss the use of IVIG for MG in patients who are already symptomatic from COVID-19 or for patients recovering from the clinical disease or does it discuss a possible compounding risk of thromboembolic events associated with IVIG and COVID-19.6,7 Our patient received his maintenance IVIG during his first admission without any worsening of symptoms or increased oxygen requirements. The day following our patient’s next scheduled IVIG infusion—while asymptomatic—he again developed respiratory symptoms; this could suggest that IVIG did not contribute to his second clinical course nor protect against.
CP is a treatment modality that has been used and studied in previous infectious outbreaks such as the first severe acute respiratory syndrome, and the H1N1 influenza virus.8 Current data on CP for COVID-19 are limited, but early descriptive studies have shown a benefit in improvement of symptoms 5 days sooner in those requiring supplemental oxygen, but no benefit for those requiring mechanical ventilation.9 Like patients that benefitted in these studies, our patient received CP early, 6 days after first testing positive and onset of symptoms. This patient’s reinfection or return of symptoms draws into question the hindrance or even prevention of long-term immunity from administration of CP.
COVID-19 presents many challenges when managing this patient’s coexisting MG, especially as the patient was already being treated with immunosuppressing therapies. The guidance does recommend continuation of standard MG therapies during hospitalizations, including immunosuppression medications such as MMF.6 Immunosuppression is associated with worsened severity of COVID-19 symptoms, although no relation exists to degree of immunosuppression and severity.7,10 To the best of our knowledge there has been no case report of reinfection or reactivation of COVID-19 associated with immunosuppressive agents used in the treatment of MG.
Our patient also was taking pyridostigmine for the treatment of his MG. There is no evidence this medication increases the risk of infection; but the cholinergic activity can increase bronchial secretions, which could theoretically worsen the COVID-19 respiratory symptoms.6,11 During both ICU admissions, our patient continued pyridostigmine use, observing complete return to baseline after discharge. Given the possible association with worsened respiratory outcomes after the second ICU admission, the balance between managing MG symptoms and COVID-19 symptoms needs further examination.
The patient was in MC during his third presentation to the ED. Although respiratory symptoms may be difficult to differentiate from COVID-19, the additional neurologic symptoms seen in this patient allowed for quick determination of the need for MC treatment. There are many potential etiologies contributing to the development of the MC presented here, and it was likely due to multifactorial precipitants. A common cause of MC is viral upper respiratory infections, further challenging the care of these patients during this pandemic.12 Many medications have been cited as causing a MC, 2 of which our patient received during admission for COVID-19: azithromycin and levoquin.12 Although the patient did not receive hydroxychloroquine, which was still being considered as an appropriate COVID-19 treatment at the time, it also is a drug known for precipitating MC and its use scrutinized in patients with MG.12
A key aspect to diagnosing and guiding therapies in myasthenic crisis in addition to the clinical symptoms of acute weakness is respiratory assessment through the nonaerosolizing NIF test.12 Our patient’s NIF measured < 30 cmH2O when in MC, while the reference range is < 75 cmH2O, and for mechanical ventilation is recommended at 20 cmH2O. Although the patient was maintaining O2 saturation > 95%, his NIF value was concerning, and preparations were made in case of precipitous decline. Compounding the NIF assessment in this patient is his history of left phrenic nerve palsy. Without a documented baseline NIF, results were limited in determining his diaphragm strength.13 Treatment for MC includes IVIG or plasmapheresis, since this patient had failed his maintenance therapy IVIG, plasmapheresis was coordinated for definitive therapy.
Conclusions
Federal facilities have seen an increase in the amount of respiratory complaints over the past months. Although COVID-19 is a concerning diagnosis, it is crucial to consider comorbidities in the diagnostic workup of each, even with a previous recent diagnosis of COVID-19. As treatment recommendations for COVID-19 continue to fluctuate coupled with the limitations and difficulties associated with MG patients, so too treatment and evaluation must be carefully considered at each presentation.
1. Gousseff M, Penot P, Gallay L, et al. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020;81(5):816-846. doi:10.1016/j.jinf.2020.06.073
2. Duggan NM, Ludy SM, Shannon BC, Reisner AT, Wilcox SR. Is novel coronavirus 2019 reinfection possible? Interpreting dynamic SARS-CoV-2 test results. Am J Emerg Med. 2021;39:256.e1-256.e3. doi:10.1016/j.ajem.2020.06.079
3. Li J, Zhang L, Liu B, Song D. Case report: viral shedding for 60 days in a woman with COVID-19. Am J Trop Med Hyg. 2020;102(6):1210-1213. doi:10.4269/ajtmh.20-0275
4. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 - preliminary report. Reply. N Engl J Med. 2020;383(10):994. doi:10.1056/NEJMc2022236
5. McCreary EK, Angus DC. Efficacy of remdesivir in COVID-19. JAMA. 2020;324(11):1041-1042. doi:10.1001/jama.2020.16337
6. International MG/COVID-19 Working Group; Jacob S, Muppidi S, Gordon A, et al. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci. 2020;412:116803. doi:10.1016/j.jns.2020.116803
7. Anand P, Slama MCC, Kaku M, et al. COVID-19 in patients with myasthenia gravis. Muscle Nerve. 2020;62(2):254-258. doi:10.1002/mus.26918
8. Wooding DJ, Bach H. Treatment of COVID-19 with convalescent plasma: lessons from past coronavirus outbreaks. Clin Microbiol Infect. 2020;26(10):1436-1446. doi:10.1016/j.cmi.2020.08.005
9. Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (covid-19) patients with convalescent plasma. Am J Pathol. 2020;190(8):1680-1690. doi:10.1016/j.ajpath.2020.05.014
10. Ryan C, Minc A, Caceres J, et al. Predicting severe outcomes in Covid-19 related illness using only patient demographics, comorbidities and symptoms [published online ahead of print, 2020 Sep 9]. Am J Emerg Med. 2020;S0735-6757(20)30809-3. doi:10.1016/j.ajem.2020.09.017
11. Singh S, Govindarajan R. COVID-19 and generalized myasthenia gravis exacerbation: a case report. Clin Neurol Neurosurg. 2020;196:106045. doi:10.1016/j.clineuro.2020.106045
12. Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist. 2011;1(1):16-22. doi:10.1177/1941875210382918
13. Dubé BP, Dres M. Diaphragm dysfunction: diagnostic approaches and management strategies. J Clin Med. 2016;5(12):113. Published 2016 Dec 5. doi:10.3390/jcm5120113
1. Gousseff M, Penot P, Gallay L, et al. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020;81(5):816-846. doi:10.1016/j.jinf.2020.06.073
2. Duggan NM, Ludy SM, Shannon BC, Reisner AT, Wilcox SR. Is novel coronavirus 2019 reinfection possible? Interpreting dynamic SARS-CoV-2 test results. Am J Emerg Med. 2021;39:256.e1-256.e3. doi:10.1016/j.ajem.2020.06.079
3. Li J, Zhang L, Liu B, Song D. Case report: viral shedding for 60 days in a woman with COVID-19. Am J Trop Med Hyg. 2020;102(6):1210-1213. doi:10.4269/ajtmh.20-0275
4. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 - preliminary report. Reply. N Engl J Med. 2020;383(10):994. doi:10.1056/NEJMc2022236
5. McCreary EK, Angus DC. Efficacy of remdesivir in COVID-19. JAMA. 2020;324(11):1041-1042. doi:10.1001/jama.2020.16337
6. International MG/COVID-19 Working Group; Jacob S, Muppidi S, Gordon A, et al. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci. 2020;412:116803. doi:10.1016/j.jns.2020.116803
7. Anand P, Slama MCC, Kaku M, et al. COVID-19 in patients with myasthenia gravis. Muscle Nerve. 2020;62(2):254-258. doi:10.1002/mus.26918
8. Wooding DJ, Bach H. Treatment of COVID-19 with convalescent plasma: lessons from past coronavirus outbreaks. Clin Microbiol Infect. 2020;26(10):1436-1446. doi:10.1016/j.cmi.2020.08.005
9. Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (covid-19) patients with convalescent plasma. Am J Pathol. 2020;190(8):1680-1690. doi:10.1016/j.ajpath.2020.05.014
10. Ryan C, Minc A, Caceres J, et al. Predicting severe outcomes in Covid-19 related illness using only patient demographics, comorbidities and symptoms [published online ahead of print, 2020 Sep 9]. Am J Emerg Med. 2020;S0735-6757(20)30809-3. doi:10.1016/j.ajem.2020.09.017
11. Singh S, Govindarajan R. COVID-19 and generalized myasthenia gravis exacerbation: a case report. Clin Neurol Neurosurg. 2020;196:106045. doi:10.1016/j.clineuro.2020.106045
12. Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist. 2011;1(1):16-22. doi:10.1177/1941875210382918
13. Dubé BP, Dres M. Diaphragm dysfunction: diagnostic approaches and management strategies. J Clin Med. 2016;5(12):113. Published 2016 Dec 5. doi:10.3390/jcm5120113
Fulminant Hemorrhagic Bullae of the Upper Extremities Arising in the Setting of IV Placement During Severe COVID-19 Infection: Observations From a Major Consultative Practice
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.
A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.

Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.


A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.


Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.


A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.


Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
Practice Points
- Hemorrhagic bullae are an uncommon cutaneous manifestation of COVID-19 infection in hospitalized individuals.
- Although there is no reported treatment for COVID-19–associated hemorrhagic bullae, we recommend supportive care and management of underlying etiology.