User login
Unvaccinated people 20 times more likely to die from COVID: Texas study
During the month of September, , according to a new study from the Texas Department of State Health Services.
The data also showed that unvaccinated people were 13 times more likely to test positive for COVID-19 than people who were fully vaccinated.
“This analysis quantifies what we’ve known for months,” Jennifer Shuford, MD, the state’s chief epidemiologist, told The Dallas Morning News.
“The COVID-19 vaccines are doing an excellent job of protecting people from getting sick and from dying from COVID-19,” she said. “Vaccination remains the best way to keep yourself and the people close to you safe from this deadly disease.”
As part of the study, researchers analyzed electronic lab reports, death certificates, and state immunization records, with a particular focus on September when the contagious Delta variant surged across Texas. The research marks the state’s first statistical analysis of COVID-19 vaccinations in Texas and the effects, the newspaper reported.
The protective effect of vaccination was most noticeable among younger groups. During September, the risk of COVID-19 death was 23 times higher in unvaccinated people in their 30s and 55 times higher for unvaccinated people in their 40s.
In addition, there were fewer than 10 COVID-19 deaths in September among fully vaccinated people between ages 18-29, as compared with 339 deaths among unvaccinated people in the same age group.
Then, looking at a longer time period -- from Jan. 15 to Oct. 1 -- the researchers found that unvaccinated people were 45 times more likely to contract COVID-19 than fully vaccinated people. The protective effect of vaccination against infection was strong across all adult age groups but greatest among ages 12-17.
“All authorized COVID-19 vaccines in the United States are highly effective at protecting people from getting sick or severely ill with COVID-19, including those infected with Delta and other known variants,” the study authors wrote. “Real world data from Texas clearly shows these benefits.”
About 15.6 million people in Texas have been fully vaccinated against COVID-19 in a state of about 29 million residents, according to state data. About 66% of the population has received at least one dose, while 58% is fully vaccinated.
A version of this article first appeared on WebMD.com.
During the month of September, , according to a new study from the Texas Department of State Health Services.
The data also showed that unvaccinated people were 13 times more likely to test positive for COVID-19 than people who were fully vaccinated.
“This analysis quantifies what we’ve known for months,” Jennifer Shuford, MD, the state’s chief epidemiologist, told The Dallas Morning News.
“The COVID-19 vaccines are doing an excellent job of protecting people from getting sick and from dying from COVID-19,” she said. “Vaccination remains the best way to keep yourself and the people close to you safe from this deadly disease.”
As part of the study, researchers analyzed electronic lab reports, death certificates, and state immunization records, with a particular focus on September when the contagious Delta variant surged across Texas. The research marks the state’s first statistical analysis of COVID-19 vaccinations in Texas and the effects, the newspaper reported.
The protective effect of vaccination was most noticeable among younger groups. During September, the risk of COVID-19 death was 23 times higher in unvaccinated people in their 30s and 55 times higher for unvaccinated people in their 40s.
In addition, there were fewer than 10 COVID-19 deaths in September among fully vaccinated people between ages 18-29, as compared with 339 deaths among unvaccinated people in the same age group.
Then, looking at a longer time period -- from Jan. 15 to Oct. 1 -- the researchers found that unvaccinated people were 45 times more likely to contract COVID-19 than fully vaccinated people. The protective effect of vaccination against infection was strong across all adult age groups but greatest among ages 12-17.
“All authorized COVID-19 vaccines in the United States are highly effective at protecting people from getting sick or severely ill with COVID-19, including those infected with Delta and other known variants,” the study authors wrote. “Real world data from Texas clearly shows these benefits.”
About 15.6 million people in Texas have been fully vaccinated against COVID-19 in a state of about 29 million residents, according to state data. About 66% of the population has received at least one dose, while 58% is fully vaccinated.
A version of this article first appeared on WebMD.com.
During the month of September, , according to a new study from the Texas Department of State Health Services.
The data also showed that unvaccinated people were 13 times more likely to test positive for COVID-19 than people who were fully vaccinated.
“This analysis quantifies what we’ve known for months,” Jennifer Shuford, MD, the state’s chief epidemiologist, told The Dallas Morning News.
“The COVID-19 vaccines are doing an excellent job of protecting people from getting sick and from dying from COVID-19,” she said. “Vaccination remains the best way to keep yourself and the people close to you safe from this deadly disease.”
As part of the study, researchers analyzed electronic lab reports, death certificates, and state immunization records, with a particular focus on September when the contagious Delta variant surged across Texas. The research marks the state’s first statistical analysis of COVID-19 vaccinations in Texas and the effects, the newspaper reported.
The protective effect of vaccination was most noticeable among younger groups. During September, the risk of COVID-19 death was 23 times higher in unvaccinated people in their 30s and 55 times higher for unvaccinated people in their 40s.
In addition, there were fewer than 10 COVID-19 deaths in September among fully vaccinated people between ages 18-29, as compared with 339 deaths among unvaccinated people in the same age group.
Then, looking at a longer time period -- from Jan. 15 to Oct. 1 -- the researchers found that unvaccinated people were 45 times more likely to contract COVID-19 than fully vaccinated people. The protective effect of vaccination against infection was strong across all adult age groups but greatest among ages 12-17.
“All authorized COVID-19 vaccines in the United States are highly effective at protecting people from getting sick or severely ill with COVID-19, including those infected with Delta and other known variants,” the study authors wrote. “Real world data from Texas clearly shows these benefits.”
About 15.6 million people in Texas have been fully vaccinated against COVID-19 in a state of about 29 million residents, according to state data. About 66% of the population has received at least one dose, while 58% is fully vaccinated.
A version of this article first appeared on WebMD.com.
Children and COVID: New cases up again after dropping for 8 weeks
As children aged 5-11 years began to receive the first officially approved doses of COVID-19 vaccine, new pediatric cases increased after 8 consecutive weeks of declines, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Weekly cases peaked at almost 252,000 in early September and then dropped for 8 straight weeks before this latest rise, the AAP and the CHA said in their weekly COVID report, which is based on data reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.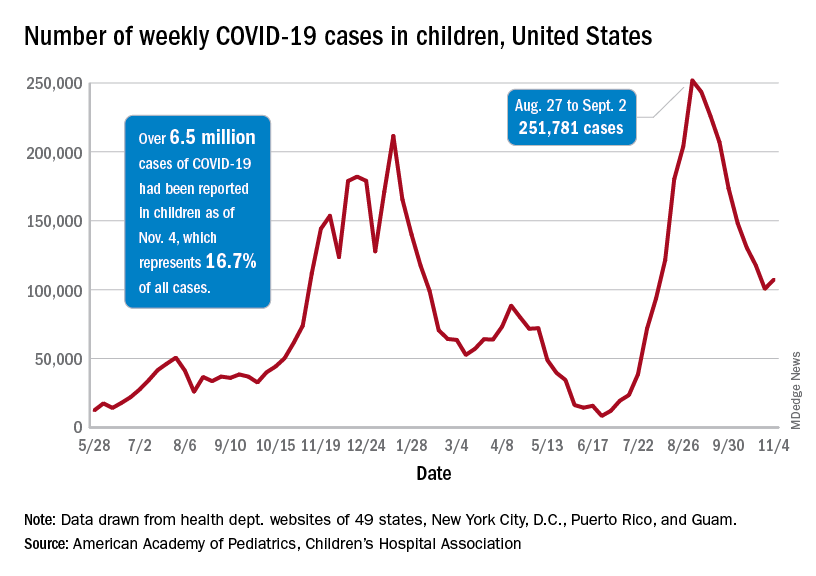
The end of that 8-week drop, unfortunately, allowed another streak to continue: New cases have been above 100,000 for 13 consecutive weeks, the AAP and CHA noted.
The cumulative COVID count in children as of Nov. 4 was 6.5 million, the AAP/CHA said, although that figure does not fully cover Alabama, Nebraska, and Texas, which stopped public reporting over the summer. The Centers for Disease Control and Prevention, with input from all states and territories, puts the total through Nov. 8 at almost 5.7 million cases in children under 18 years of age, while most states define a child as someone aged 0-19 years.
As for the newest group of vaccinees, the CDC said that “updated vaccination data for 5-11 year-olds will be added to COVID Data Tracker later this week,” meaning the week of Nov. 7-13. Currently available data, however, show that almost 157,000 children under age 12 initiated vaccination in the 14 days ending Nov. 8, which was more than those aged 12-15 and 16-17 years combined (127,000).
Among those older groups, the CDC reports that 57.1% of 12- to 15-year-olds have received at least one dose and 47.9% are fully vaccinated, while 64.0% of those aged 16-17 have gotten at least one dose and 55.2% are fully vaccinated. Altogether, about 13.9 million children under age 18 have gotten at least one dose and almost 11.6 million are fully vaccinated, according to the CDC.
As children aged 5-11 years began to receive the first officially approved doses of COVID-19 vaccine, new pediatric cases increased after 8 consecutive weeks of declines, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Weekly cases peaked at almost 252,000 in early September and then dropped for 8 straight weeks before this latest rise, the AAP and the CHA said in their weekly COVID report, which is based on data reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The end of that 8-week drop, unfortunately, allowed another streak to continue: New cases have been above 100,000 for 13 consecutive weeks, the AAP and CHA noted.
The cumulative COVID count in children as of Nov. 4 was 6.5 million, the AAP/CHA said, although that figure does not fully cover Alabama, Nebraska, and Texas, which stopped public reporting over the summer. The Centers for Disease Control and Prevention, with input from all states and territories, puts the total through Nov. 8 at almost 5.7 million cases in children under 18 years of age, while most states define a child as someone aged 0-19 years.
As for the newest group of vaccinees, the CDC said that “updated vaccination data for 5-11 year-olds will be added to COVID Data Tracker later this week,” meaning the week of Nov. 7-13. Currently available data, however, show that almost 157,000 children under age 12 initiated vaccination in the 14 days ending Nov. 8, which was more than those aged 12-15 and 16-17 years combined (127,000).
Among those older groups, the CDC reports that 57.1% of 12- to 15-year-olds have received at least one dose and 47.9% are fully vaccinated, while 64.0% of those aged 16-17 have gotten at least one dose and 55.2% are fully vaccinated. Altogether, about 13.9 million children under age 18 have gotten at least one dose and almost 11.6 million are fully vaccinated, according to the CDC.
As children aged 5-11 years began to receive the first officially approved doses of COVID-19 vaccine, new pediatric cases increased after 8 consecutive weeks of declines, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Weekly cases peaked at almost 252,000 in early September and then dropped for 8 straight weeks before this latest rise, the AAP and the CHA said in their weekly COVID report, which is based on data reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The end of that 8-week drop, unfortunately, allowed another streak to continue: New cases have been above 100,000 for 13 consecutive weeks, the AAP and CHA noted.
The cumulative COVID count in children as of Nov. 4 was 6.5 million, the AAP/CHA said, although that figure does not fully cover Alabama, Nebraska, and Texas, which stopped public reporting over the summer. The Centers for Disease Control and Prevention, with input from all states and territories, puts the total through Nov. 8 at almost 5.7 million cases in children under 18 years of age, while most states define a child as someone aged 0-19 years.
As for the newest group of vaccinees, the CDC said that “updated vaccination data for 5-11 year-olds will be added to COVID Data Tracker later this week,” meaning the week of Nov. 7-13. Currently available data, however, show that almost 157,000 children under age 12 initiated vaccination in the 14 days ending Nov. 8, which was more than those aged 12-15 and 16-17 years combined (127,000).
Among those older groups, the CDC reports that 57.1% of 12- to 15-year-olds have received at least one dose and 47.9% are fully vaccinated, while 64.0% of those aged 16-17 have gotten at least one dose and 55.2% are fully vaccinated. Altogether, about 13.9 million children under age 18 have gotten at least one dose and almost 11.6 million are fully vaccinated, according to the CDC.
Severe COVID two times higher for cancer patients
A new systematic review and meta-analysis finds that unvaccinated cancer patients who contracted COVID-19 last year, were more than two times more likely – than people without cancer – to develop a case of COVID-19 so severe it required hospitalization in an intensive care unit.
“Our study provides the most precise measure to date of the effect of COVID-19 in cancer patients,” wrote researchers who were led by Paolo Boffetta, MD, MPH, a specialist in population science with the Stony Brook Cancer Center in New York.
Dr. Boffetta and colleagues also found that patients with hematologic neoplasms had a higher mortality rate from COVID-19 comparable to that of all cancers combined.
Cancer patients have long been considered to be among those patients who are at high risk of developing COVID-19, and if they contract the disease, they are at high risk of having poor outcomes. Other high-risk patients include those with hypertension, diabetes, chronic kidney disease, or COPD, or the elderly. But how high the risk of developing severe COVID-19 disease is for cancer patients hasn’t yet been documented on a wide scale.
The study, which was made available as a preprint on medRxiv on Oct. 23, is based on an analysis of COVID-19 cases that were documented in 35 reviews, meta-analyses, case reports, and studies indexed in PubMed from authors in North America, Europe, and Asia.
In this study, the pooled odds ratio for mortality for all patients with any cancer was 2.32 (95% confidence interval, 1.82-2.94; 24 studies). For ICU admission, the odds ratio was 2.39 (95% CI, 1.90-3.02; I2 0.0%; 5 studies). And, for disease severity or hospitalization, it was 2.08 (95% CI, 1.60-2.72; I2 92.1%; 15 studies). The pooled mortality odds ratio for hematologic neoplasms was 2.14 (95% CI, 1.87-2.44; I2 20.8%; 8 studies).
Their findings, which have not yet been peer reviewed, confirmed the results of a similar analysis from China published as a preprint in May 2020. The analysis included 181,323 patients (23,736 cancer patients) from 26 studies reported an odds ratio of 2.54 (95% CI, 1.47-4.42). “Cancer patients with COVID-19 have an increased likelihood of death compared to non-cancer COVID-19 patients,” Venkatesulu et al. wrote. And a systematic review and meta-analysis of five studies of 2,619 patients published in October 2020 in Medicine also found a significantly higher risk of death from COVID-19 among cancer patients (odds ratio, 2.63; 95% confidence interval, 1.14-6.06; P = .023; I2 = 26.4%).
Fakih et al., writing in the journal Hematology/Oncology and Stem Cell Therapy conducted a meta-analysis early last year finding a threefold increase for admission to the intensive care unit, an almost fourfold increase for a severe SARS-CoV-2 infection, and a fivefold increase for being intubated.
The three studies show that mortality rates were higher early in the pandemic “when diagnosis and treatment for SARS-CoV-2 might have been delayed, resulting in higher death rate,” Boffetta et al. wrote, adding that their analysis showed only a twofold increase most likely because it was a year-long analysis.
“Future studies will be able to better analyze this association for the different subtypes of cancer. Furthermore, they will eventually be able to evaluate whether the difference among vaccinated population is reduced,” Boffetta et al. wrote.
The authors noted several limitations for the study, including the fact that many of the studies included in the analysis did not include sex, age, comorbidities, and therapy. Nor were the authors able to analyze specific cancers other than hematologic neoplasms.
The authors declared no conflicts of interest.
A new systematic review and meta-analysis finds that unvaccinated cancer patients who contracted COVID-19 last year, were more than two times more likely – than people without cancer – to develop a case of COVID-19 so severe it required hospitalization in an intensive care unit.
“Our study provides the most precise measure to date of the effect of COVID-19 in cancer patients,” wrote researchers who were led by Paolo Boffetta, MD, MPH, a specialist in population science with the Stony Brook Cancer Center in New York.
Dr. Boffetta and colleagues also found that patients with hematologic neoplasms had a higher mortality rate from COVID-19 comparable to that of all cancers combined.
Cancer patients have long been considered to be among those patients who are at high risk of developing COVID-19, and if they contract the disease, they are at high risk of having poor outcomes. Other high-risk patients include those with hypertension, diabetes, chronic kidney disease, or COPD, or the elderly. But how high the risk of developing severe COVID-19 disease is for cancer patients hasn’t yet been documented on a wide scale.
The study, which was made available as a preprint on medRxiv on Oct. 23, is based on an analysis of COVID-19 cases that were documented in 35 reviews, meta-analyses, case reports, and studies indexed in PubMed from authors in North America, Europe, and Asia.
In this study, the pooled odds ratio for mortality for all patients with any cancer was 2.32 (95% confidence interval, 1.82-2.94; 24 studies). For ICU admission, the odds ratio was 2.39 (95% CI, 1.90-3.02; I2 0.0%; 5 studies). And, for disease severity or hospitalization, it was 2.08 (95% CI, 1.60-2.72; I2 92.1%; 15 studies). The pooled mortality odds ratio for hematologic neoplasms was 2.14 (95% CI, 1.87-2.44; I2 20.8%; 8 studies).
Their findings, which have not yet been peer reviewed, confirmed the results of a similar analysis from China published as a preprint in May 2020. The analysis included 181,323 patients (23,736 cancer patients) from 26 studies reported an odds ratio of 2.54 (95% CI, 1.47-4.42). “Cancer patients with COVID-19 have an increased likelihood of death compared to non-cancer COVID-19 patients,” Venkatesulu et al. wrote. And a systematic review and meta-analysis of five studies of 2,619 patients published in October 2020 in Medicine also found a significantly higher risk of death from COVID-19 among cancer patients (odds ratio, 2.63; 95% confidence interval, 1.14-6.06; P = .023; I2 = 26.4%).
Fakih et al., writing in the journal Hematology/Oncology and Stem Cell Therapy conducted a meta-analysis early last year finding a threefold increase for admission to the intensive care unit, an almost fourfold increase for a severe SARS-CoV-2 infection, and a fivefold increase for being intubated.
The three studies show that mortality rates were higher early in the pandemic “when diagnosis and treatment for SARS-CoV-2 might have been delayed, resulting in higher death rate,” Boffetta et al. wrote, adding that their analysis showed only a twofold increase most likely because it was a year-long analysis.
“Future studies will be able to better analyze this association for the different subtypes of cancer. Furthermore, they will eventually be able to evaluate whether the difference among vaccinated population is reduced,” Boffetta et al. wrote.
The authors noted several limitations for the study, including the fact that many of the studies included in the analysis did not include sex, age, comorbidities, and therapy. Nor were the authors able to analyze specific cancers other than hematologic neoplasms.
The authors declared no conflicts of interest.
A new systematic review and meta-analysis finds that unvaccinated cancer patients who contracted COVID-19 last year, were more than two times more likely – than people without cancer – to develop a case of COVID-19 so severe it required hospitalization in an intensive care unit.
“Our study provides the most precise measure to date of the effect of COVID-19 in cancer patients,” wrote researchers who were led by Paolo Boffetta, MD, MPH, a specialist in population science with the Stony Brook Cancer Center in New York.
Dr. Boffetta and colleagues also found that patients with hematologic neoplasms had a higher mortality rate from COVID-19 comparable to that of all cancers combined.
Cancer patients have long been considered to be among those patients who are at high risk of developing COVID-19, and if they contract the disease, they are at high risk of having poor outcomes. Other high-risk patients include those with hypertension, diabetes, chronic kidney disease, or COPD, or the elderly. But how high the risk of developing severe COVID-19 disease is for cancer patients hasn’t yet been documented on a wide scale.
The study, which was made available as a preprint on medRxiv on Oct. 23, is based on an analysis of COVID-19 cases that were documented in 35 reviews, meta-analyses, case reports, and studies indexed in PubMed from authors in North America, Europe, and Asia.
In this study, the pooled odds ratio for mortality for all patients with any cancer was 2.32 (95% confidence interval, 1.82-2.94; 24 studies). For ICU admission, the odds ratio was 2.39 (95% CI, 1.90-3.02; I2 0.0%; 5 studies). And, for disease severity or hospitalization, it was 2.08 (95% CI, 1.60-2.72; I2 92.1%; 15 studies). The pooled mortality odds ratio for hematologic neoplasms was 2.14 (95% CI, 1.87-2.44; I2 20.8%; 8 studies).
Their findings, which have not yet been peer reviewed, confirmed the results of a similar analysis from China published as a preprint in May 2020. The analysis included 181,323 patients (23,736 cancer patients) from 26 studies reported an odds ratio of 2.54 (95% CI, 1.47-4.42). “Cancer patients with COVID-19 have an increased likelihood of death compared to non-cancer COVID-19 patients,” Venkatesulu et al. wrote. And a systematic review and meta-analysis of five studies of 2,619 patients published in October 2020 in Medicine also found a significantly higher risk of death from COVID-19 among cancer patients (odds ratio, 2.63; 95% confidence interval, 1.14-6.06; P = .023; I2 = 26.4%).
Fakih et al., writing in the journal Hematology/Oncology and Stem Cell Therapy conducted a meta-analysis early last year finding a threefold increase for admission to the intensive care unit, an almost fourfold increase for a severe SARS-CoV-2 infection, and a fivefold increase for being intubated.
The three studies show that mortality rates were higher early in the pandemic “when diagnosis and treatment for SARS-CoV-2 might have been delayed, resulting in higher death rate,” Boffetta et al. wrote, adding that their analysis showed only a twofold increase most likely because it was a year-long analysis.
“Future studies will be able to better analyze this association for the different subtypes of cancer. Furthermore, they will eventually be able to evaluate whether the difference among vaccinated population is reduced,” Boffetta et al. wrote.
The authors noted several limitations for the study, including the fact that many of the studies included in the analysis did not include sex, age, comorbidities, and therapy. Nor were the authors able to analyze specific cancers other than hematologic neoplasms.
The authors declared no conflicts of interest.
FROM MEDRXIV
New transmission information should motivate hospitals to reexamine aerosol procedures, researchers say
Two studies published in Thorax have found that the use of continuous positive airways pressure (CPAP) or high-flow nasal oxygen (HFNO) to treat moderate to severe COVID-19 is not linked to a heightened risk of infection, as currently thought. Researchers say hospitals should use this information to re-examine aerosol procedures in regard to risk of transmission of SARS-CoV-2.
CPAP and HFNO have been thought to generate virus particles capable of contaminating the air and surfaces, necessitating additional infection control precautions such as segregating patients. However, this research demonstrates that both methods produced little measurable air or surface viral contamination. The amount of contamination was no more than with the use of supplemental oxygen and less than that produced when breathing, speaking, or coughing.
In one study, led by a team from the North Bristol NHS Trust, 25 healthy volunteers and eight hospitalized patients with COVID-19 were recruited and asked to breathe, speak, and cough in ultra-clean, laminar flow theaters followed by use of CPAP and HFNO. Aerosol emission was measured via two methodologies, simultaneously. Hospitalized patients with COVID-19 had cough recorded via the same methodology on the infectious diseases ward.
CPAP (with exhalation port filter) was found to produce less aerosol than breathing, speaking, and coughing, even with large > 50 L/min face mask leaks. Coughing was associated with the highest aerosol emissions of any recorded activity.
HFNO was associated with aerosol emission from the machine. Generated particles were small (< 1 mcm), passing from the machine through the patient and to the detector without coalescence with respiratory aerosol, and, consequently, would be unlikely to carry viral particles.
More aerosol was generated in cough from patients with COVID-19 (n = 8) than from volunteers.
In the second study, 30 hospitalized patients with COVID-19 requiring supplemental oxygen were prospectively enrolled. In this observational environmental sampling study, participants received either supplemental oxygen, CPAP, or HFNO (n = 10 in each group). A nasopharyngeal swab, three air, and three surface samples were collected from each participant and the clinical environment.
Overall, 21 of the 30 participants tested positive for SARS-CoV-2 RNA in the nasopharynx. In contrast, 4 out of 90 air samples and 6 of 90 surface samples tested positive for viral RNA, although there were an additional 10 suspected-positive samples in both air and surfaces samples.
Neither the use of CPAP nor HFNO nor coughing were associated with significantly more environmental contamination than supplemental oxygen use. Of the total positive or suspected-positive samples by viral PCR detection, only one nasopharynx sample from an HFNO patient was biologically viable in cell culture assay.
“Our findings show that the noninvasive breathing support methods do not pose a higher risk of transmitting infection, which has significant implications for the management of the patients,” said coauthor Danny McAuley, MD.
“If there isn’t a higher risk of infection transmission, current practices may be overcautious measures for certain settings, for example preventing relatives visiting the sickest patients, whilst underestimating the risk in other settings, such as coughing patients with early infection on general wards.”
Although both studies are small, the results do suggest that there is a need for an evidence-based reassessment of infection prevention and control measures for noninvasive respiratory support treatments that are currently considered aerosol generating procedures.
A version of this article first appeared on Univadis.com.
Two studies published in Thorax have found that the use of continuous positive airways pressure (CPAP) or high-flow nasal oxygen (HFNO) to treat moderate to severe COVID-19 is not linked to a heightened risk of infection, as currently thought. Researchers say hospitals should use this information to re-examine aerosol procedures in regard to risk of transmission of SARS-CoV-2.
CPAP and HFNO have been thought to generate virus particles capable of contaminating the air and surfaces, necessitating additional infection control precautions such as segregating patients. However, this research demonstrates that both methods produced little measurable air or surface viral contamination. The amount of contamination was no more than with the use of supplemental oxygen and less than that produced when breathing, speaking, or coughing.
In one study, led by a team from the North Bristol NHS Trust, 25 healthy volunteers and eight hospitalized patients with COVID-19 were recruited and asked to breathe, speak, and cough in ultra-clean, laminar flow theaters followed by use of CPAP and HFNO. Aerosol emission was measured via two methodologies, simultaneously. Hospitalized patients with COVID-19 had cough recorded via the same methodology on the infectious diseases ward.
CPAP (with exhalation port filter) was found to produce less aerosol than breathing, speaking, and coughing, even with large > 50 L/min face mask leaks. Coughing was associated with the highest aerosol emissions of any recorded activity.
HFNO was associated with aerosol emission from the machine. Generated particles were small (< 1 mcm), passing from the machine through the patient and to the detector without coalescence with respiratory aerosol, and, consequently, would be unlikely to carry viral particles.
More aerosol was generated in cough from patients with COVID-19 (n = 8) than from volunteers.
In the second study, 30 hospitalized patients with COVID-19 requiring supplemental oxygen were prospectively enrolled. In this observational environmental sampling study, participants received either supplemental oxygen, CPAP, or HFNO (n = 10 in each group). A nasopharyngeal swab, three air, and three surface samples were collected from each participant and the clinical environment.
Overall, 21 of the 30 participants tested positive for SARS-CoV-2 RNA in the nasopharynx. In contrast, 4 out of 90 air samples and 6 of 90 surface samples tested positive for viral RNA, although there were an additional 10 suspected-positive samples in both air and surfaces samples.
Neither the use of CPAP nor HFNO nor coughing were associated with significantly more environmental contamination than supplemental oxygen use. Of the total positive or suspected-positive samples by viral PCR detection, only one nasopharynx sample from an HFNO patient was biologically viable in cell culture assay.
“Our findings show that the noninvasive breathing support methods do not pose a higher risk of transmitting infection, which has significant implications for the management of the patients,” said coauthor Danny McAuley, MD.
“If there isn’t a higher risk of infection transmission, current practices may be overcautious measures for certain settings, for example preventing relatives visiting the sickest patients, whilst underestimating the risk in other settings, such as coughing patients with early infection on general wards.”
Although both studies are small, the results do suggest that there is a need for an evidence-based reassessment of infection prevention and control measures for noninvasive respiratory support treatments that are currently considered aerosol generating procedures.
A version of this article first appeared on Univadis.com.
Two studies published in Thorax have found that the use of continuous positive airways pressure (CPAP) or high-flow nasal oxygen (HFNO) to treat moderate to severe COVID-19 is not linked to a heightened risk of infection, as currently thought. Researchers say hospitals should use this information to re-examine aerosol procedures in regard to risk of transmission of SARS-CoV-2.
CPAP and HFNO have been thought to generate virus particles capable of contaminating the air and surfaces, necessitating additional infection control precautions such as segregating patients. However, this research demonstrates that both methods produced little measurable air or surface viral contamination. The amount of contamination was no more than with the use of supplemental oxygen and less than that produced when breathing, speaking, or coughing.
In one study, led by a team from the North Bristol NHS Trust, 25 healthy volunteers and eight hospitalized patients with COVID-19 were recruited and asked to breathe, speak, and cough in ultra-clean, laminar flow theaters followed by use of CPAP and HFNO. Aerosol emission was measured via two methodologies, simultaneously. Hospitalized patients with COVID-19 had cough recorded via the same methodology on the infectious diseases ward.
CPAP (with exhalation port filter) was found to produce less aerosol than breathing, speaking, and coughing, even with large > 50 L/min face mask leaks. Coughing was associated with the highest aerosol emissions of any recorded activity.
HFNO was associated with aerosol emission from the machine. Generated particles were small (< 1 mcm), passing from the machine through the patient and to the detector without coalescence with respiratory aerosol, and, consequently, would be unlikely to carry viral particles.
More aerosol was generated in cough from patients with COVID-19 (n = 8) than from volunteers.
In the second study, 30 hospitalized patients with COVID-19 requiring supplemental oxygen were prospectively enrolled. In this observational environmental sampling study, participants received either supplemental oxygen, CPAP, or HFNO (n = 10 in each group). A nasopharyngeal swab, three air, and three surface samples were collected from each participant and the clinical environment.
Overall, 21 of the 30 participants tested positive for SARS-CoV-2 RNA in the nasopharynx. In contrast, 4 out of 90 air samples and 6 of 90 surface samples tested positive for viral RNA, although there were an additional 10 suspected-positive samples in both air and surfaces samples.
Neither the use of CPAP nor HFNO nor coughing were associated with significantly more environmental contamination than supplemental oxygen use. Of the total positive or suspected-positive samples by viral PCR detection, only one nasopharynx sample from an HFNO patient was biologically viable in cell culture assay.
“Our findings show that the noninvasive breathing support methods do not pose a higher risk of transmitting infection, which has significant implications for the management of the patients,” said coauthor Danny McAuley, MD.
“If there isn’t a higher risk of infection transmission, current practices may be overcautious measures for certain settings, for example preventing relatives visiting the sickest patients, whilst underestimating the risk in other settings, such as coughing patients with early infection on general wards.”
Although both studies are small, the results do suggest that there is a need for an evidence-based reassessment of infection prevention and control measures for noninvasive respiratory support treatments that are currently considered aerosol generating procedures.
A version of this article first appeared on Univadis.com.
FROM THORAX
Not COVID Toes: Pool Palms and Feet in Pediatric Patients
Practice Gap
Frictional, symmetric, asymptomatic, erythematous macules of the hands and feet can be mistaken for perniolike lesions associated with COVID-19, commonly known as COVID toes. However, in a low-risk setting without other associated symptoms or concerning findings on examination, consider and inquire about frequent use of a swimming pool. This activity can lead to localized pressure- and friction-induced erythema on palmar and plantar surfaces, called “pool palms and feet,” expanding on the already-named lesion “pool palms”—an entity that is distinct from COVID toes.
Technique for Diagnosis
We evaluated 4 patients in the outpatient setting who presented with localized, patterned, erythematous lesions of the hands or feet, or both, during the COVID-19 pandemic. The parents of our patients were concerned that the rash represented “COVID fingers and toes,” which are perniolike lesions seen in patients with suspected or confirmed current or prior COVID-19.1
Pernio, also known as chilblains, is a superficial inflammatory vascular response, usually in the setting of exposure to cold.2 This phenomenon usually appears as erythematous or violaceous macules and papules on acral skin, particularly on the dorsum and sides of the fingers and toes, with edema, vesiculation, and ulceration in more severe cases. Initially, it is pruritic and painful at times.
With COVID toes, there often is a delayed presentation of perniolike lesions after the onset of other COVID-19 symptoms, such as fever, cough, headache, and sore throat.2,3 It has been described more often in younger patients and those with milder disease. However, because our patients had no known exposure to SARS-CoV-2 or other associated symptoms, our suspicion was low.
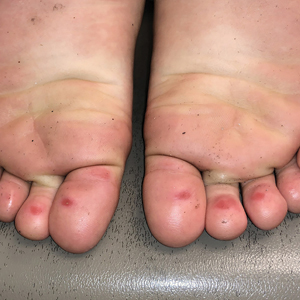
The 4 patients we evaluated—aged 4 to 12 years and in their usual good health—had blanchable erythema of the palmar fingers, palmar eminences of both hands, and plantar surfaces of both feet (Figure). There was no swelling or tenderness, and the lesions had no violaceous coloration, vesiculation, or ulceration. There was no associated pruritus or pain. One patient reported rough texture and mild peeling of the hands.
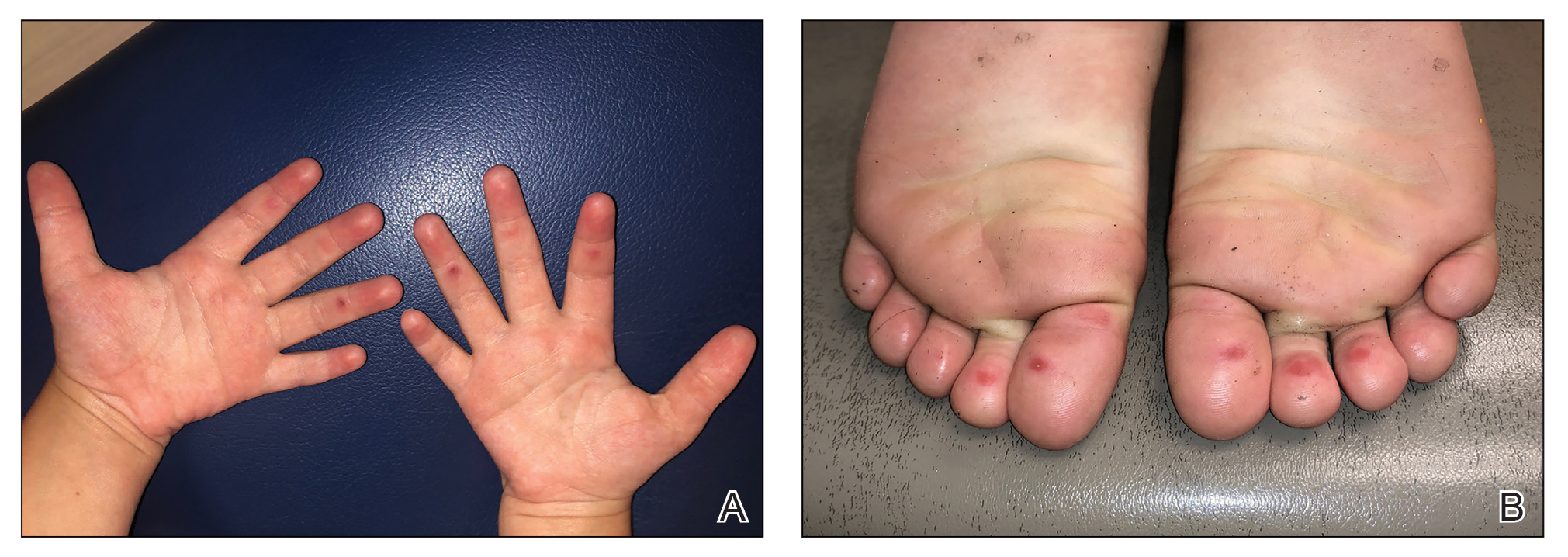
Upon further inquiry, the patients reported a history of extended time spent in home swimming pools, including holding on to the edge of the pool, due to limitation of activities because of COVID restrictions. One parent noted that the pool that caused the rash had a rough nonslip surface, whereas other pools that the children used, which had a smoother surface, caused no problems.
The morphology of symmetric blanching erythema in areas of pressure and friction, in the absence of a notable medical history, signs, or symptoms, was consistent with a diagnosis of pool palms, which has been described in the medical literature.4-9 Pool palms can affect the palms and soles, which are subject to substantial friction, especially when a person is getting in and out of the pool. There is a general consensus that pool palms is a frictional dermatitis affecting children because the greater fragility of their skin is exacerbated by immersion in water.4-9
Pool palms and feet is benign. Only supportive care, with cessation of swimming and application of emollients, is necessary.
Apart from COVID-19, other conditions to consider in a patient with erythematous lesions of the palms and soles include eczematous dermatitis; neutrophilic eccrine hidradenitis; and, if lesions are vesicular, hand-foot-and-mouth disease. Juvenile plantar dermatosis, which is thought to be due to moisture with occlusion in shoes, also might be considered but is distinguished by more scales and fissures that can be painful.
Location of the lesions is a critical variable. The patients we evaluated had lesions primarily on palmar and plantar surfaces where contact with pool surfaces was greatest, such as at bony prominences, which supported a diagnosis of frictional dermatitis, such as pool palms and feet. A thorough history and physical examination are helpful in determining the diagnosis.
Practical Implications
It is important to consider and recognize this localized pressure phenomenon of pool palms and feet, thus obviating an unnecessary workup or therapeutic interventions. Specifically, a finding of erythematous asymptomatic macules, with or without scaling, on bony prominences of the palms and soles is more consistent with pool palms and feet.
Pernio and COVID toes both present as erythematous to violaceous papules and macules, with edema, vesiculation, and ulceration in severe cases, often on the dorsum and sides of fingers and toes; typically the conditions are pruritic and painful at times.
Explaining the diagnosis of pool palms and feet and sharing one’s experience with similar cases might help alleviate parental fear and anxiety during the COVID-19 pandemic.
- de Masson A, Bouaziz J-D, Sulimovic L, et al; SNDV (French National Union of Dermatologists–Venereologists). Chilblains is a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83:667-670. doi:10.1016/j.jaad.2020.04.161
- Freeman EE, McMahon DE, Lipoff JB, et al; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Blauvelt A, Duarte AM, Schachner LA. Pool palms. J Am Acad Dermatol. 1992;27:111. doi:10.1016/s0190-9622(08)80819-5
- Wong L-C, Rogers M. Pool palms. Pediatr Dermatol. 2007;24:95. doi:10.1111/j.1525-1470.2007.00347.x
- Novoa A, Klear S. Pool palms. Arch Dis Child. 2016;101:41. doi:10.1136/archdischild-2015-309633
- Morgado-Carasco D, Feola H, Vargas-Mora P. Pool palms. Dermatol Pract Concept. 2020;10:e2020009. doi:10.5826/dpc.1001a09
- Cutrone M, Valerio E, Grimalt R. Pool palms: a case report. Dermatol Case Rep. 2019;4:1000154.
- Martína JM, Ricart JM. Erythematous–violaceous lesions on the palms. Actas Dermosifiliogr. 2009;100:507-508.
Practice Gap
Frictional, symmetric, asymptomatic, erythematous macules of the hands and feet can be mistaken for perniolike lesions associated with COVID-19, commonly known as COVID toes. However, in a low-risk setting without other associated symptoms or concerning findings on examination, consider and inquire about frequent use of a swimming pool. This activity can lead to localized pressure- and friction-induced erythema on palmar and plantar surfaces, called “pool palms and feet,” expanding on the already-named lesion “pool palms”—an entity that is distinct from COVID toes.
Technique for Diagnosis
We evaluated 4 patients in the outpatient setting who presented with localized, patterned, erythematous lesions of the hands or feet, or both, during the COVID-19 pandemic. The parents of our patients were concerned that the rash represented “COVID fingers and toes,” which are perniolike lesions seen in patients with suspected or confirmed current or prior COVID-19.1
Pernio, also known as chilblains, is a superficial inflammatory vascular response, usually in the setting of exposure to cold.2 This phenomenon usually appears as erythematous or violaceous macules and papules on acral skin, particularly on the dorsum and sides of the fingers and toes, with edema, vesiculation, and ulceration in more severe cases. Initially, it is pruritic and painful at times.
With COVID toes, there often is a delayed presentation of perniolike lesions after the onset of other COVID-19 symptoms, such as fever, cough, headache, and sore throat.2,3 It has been described more often in younger patients and those with milder disease. However, because our patients had no known exposure to SARS-CoV-2 or other associated symptoms, our suspicion was low.
The 4 patients we evaluated—aged 4 to 12 years and in their usual good health—had blanchable erythema of the palmar fingers, palmar eminences of both hands, and plantar surfaces of both feet (Figure). There was no swelling or tenderness, and the lesions had no violaceous coloration, vesiculation, or ulceration. There was no associated pruritus or pain. One patient reported rough texture and mild peeling of the hands.

Upon further inquiry, the patients reported a history of extended time spent in home swimming pools, including holding on to the edge of the pool, due to limitation of activities because of COVID restrictions. One parent noted that the pool that caused the rash had a rough nonslip surface, whereas other pools that the children used, which had a smoother surface, caused no problems.
The morphology of symmetric blanching erythema in areas of pressure and friction, in the absence of a notable medical history, signs, or symptoms, was consistent with a diagnosis of pool palms, which has been described in the medical literature.4-9 Pool palms can affect the palms and soles, which are subject to substantial friction, especially when a person is getting in and out of the pool. There is a general consensus that pool palms is a frictional dermatitis affecting children because the greater fragility of their skin is exacerbated by immersion in water.4-9
Pool palms and feet is benign. Only supportive care, with cessation of swimming and application of emollients, is necessary.
Apart from COVID-19, other conditions to consider in a patient with erythematous lesions of the palms and soles include eczematous dermatitis; neutrophilic eccrine hidradenitis; and, if lesions are vesicular, hand-foot-and-mouth disease. Juvenile plantar dermatosis, which is thought to be due to moisture with occlusion in shoes, also might be considered but is distinguished by more scales and fissures that can be painful.
Location of the lesions is a critical variable. The patients we evaluated had lesions primarily on palmar and plantar surfaces where contact with pool surfaces was greatest, such as at bony prominences, which supported a diagnosis of frictional dermatitis, such as pool palms and feet. A thorough history and physical examination are helpful in determining the diagnosis.
Practical Implications
It is important to consider and recognize this localized pressure phenomenon of pool palms and feet, thus obviating an unnecessary workup or therapeutic interventions. Specifically, a finding of erythematous asymptomatic macules, with or without scaling, on bony prominences of the palms and soles is more consistent with pool palms and feet.
Pernio and COVID toes both present as erythematous to violaceous papules and macules, with edema, vesiculation, and ulceration in severe cases, often on the dorsum and sides of fingers and toes; typically the conditions are pruritic and painful at times.
Explaining the diagnosis of pool palms and feet and sharing one’s experience with similar cases might help alleviate parental fear and anxiety during the COVID-19 pandemic.
Practice Gap
Frictional, symmetric, asymptomatic, erythematous macules of the hands and feet can be mistaken for perniolike lesions associated with COVID-19, commonly known as COVID toes. However, in a low-risk setting without other associated symptoms or concerning findings on examination, consider and inquire about frequent use of a swimming pool. This activity can lead to localized pressure- and friction-induced erythema on palmar and plantar surfaces, called “pool palms and feet,” expanding on the already-named lesion “pool palms”—an entity that is distinct from COVID toes.
Technique for Diagnosis
We evaluated 4 patients in the outpatient setting who presented with localized, patterned, erythematous lesions of the hands or feet, or both, during the COVID-19 pandemic. The parents of our patients were concerned that the rash represented “COVID fingers and toes,” which are perniolike lesions seen in patients with suspected or confirmed current or prior COVID-19.1
Pernio, also known as chilblains, is a superficial inflammatory vascular response, usually in the setting of exposure to cold.2 This phenomenon usually appears as erythematous or violaceous macules and papules on acral skin, particularly on the dorsum and sides of the fingers and toes, with edema, vesiculation, and ulceration in more severe cases. Initially, it is pruritic and painful at times.
With COVID toes, there often is a delayed presentation of perniolike lesions after the onset of other COVID-19 symptoms, such as fever, cough, headache, and sore throat.2,3 It has been described more often in younger patients and those with milder disease. However, because our patients had no known exposure to SARS-CoV-2 or other associated symptoms, our suspicion was low.
The 4 patients we evaluated—aged 4 to 12 years and in their usual good health—had blanchable erythema of the palmar fingers, palmar eminences of both hands, and plantar surfaces of both feet (Figure). There was no swelling or tenderness, and the lesions had no violaceous coloration, vesiculation, or ulceration. There was no associated pruritus or pain. One patient reported rough texture and mild peeling of the hands.

Upon further inquiry, the patients reported a history of extended time spent in home swimming pools, including holding on to the edge of the pool, due to limitation of activities because of COVID restrictions. One parent noted that the pool that caused the rash had a rough nonslip surface, whereas other pools that the children used, which had a smoother surface, caused no problems.
The morphology of symmetric blanching erythema in areas of pressure and friction, in the absence of a notable medical history, signs, or symptoms, was consistent with a diagnosis of pool palms, which has been described in the medical literature.4-9 Pool palms can affect the palms and soles, which are subject to substantial friction, especially when a person is getting in and out of the pool. There is a general consensus that pool palms is a frictional dermatitis affecting children because the greater fragility of their skin is exacerbated by immersion in water.4-9
Pool palms and feet is benign. Only supportive care, with cessation of swimming and application of emollients, is necessary.
Apart from COVID-19, other conditions to consider in a patient with erythematous lesions of the palms and soles include eczematous dermatitis; neutrophilic eccrine hidradenitis; and, if lesions are vesicular, hand-foot-and-mouth disease. Juvenile plantar dermatosis, which is thought to be due to moisture with occlusion in shoes, also might be considered but is distinguished by more scales and fissures that can be painful.
Location of the lesions is a critical variable. The patients we evaluated had lesions primarily on palmar and plantar surfaces where contact with pool surfaces was greatest, such as at bony prominences, which supported a diagnosis of frictional dermatitis, such as pool palms and feet. A thorough history and physical examination are helpful in determining the diagnosis.
Practical Implications
It is important to consider and recognize this localized pressure phenomenon of pool palms and feet, thus obviating an unnecessary workup or therapeutic interventions. Specifically, a finding of erythematous asymptomatic macules, with or without scaling, on bony prominences of the palms and soles is more consistent with pool palms and feet.
Pernio and COVID toes both present as erythematous to violaceous papules and macules, with edema, vesiculation, and ulceration in severe cases, often on the dorsum and sides of fingers and toes; typically the conditions are pruritic and painful at times.
Explaining the diagnosis of pool palms and feet and sharing one’s experience with similar cases might help alleviate parental fear and anxiety during the COVID-19 pandemic.
- de Masson A, Bouaziz J-D, Sulimovic L, et al; SNDV (French National Union of Dermatologists–Venereologists). Chilblains is a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83:667-670. doi:10.1016/j.jaad.2020.04.161
- Freeman EE, McMahon DE, Lipoff JB, et al; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Blauvelt A, Duarte AM, Schachner LA. Pool palms. J Am Acad Dermatol. 1992;27:111. doi:10.1016/s0190-9622(08)80819-5
- Wong L-C, Rogers M. Pool palms. Pediatr Dermatol. 2007;24:95. doi:10.1111/j.1525-1470.2007.00347.x
- Novoa A, Klear S. Pool palms. Arch Dis Child. 2016;101:41. doi:10.1136/archdischild-2015-309633
- Morgado-Carasco D, Feola H, Vargas-Mora P. Pool palms. Dermatol Pract Concept. 2020;10:e2020009. doi:10.5826/dpc.1001a09
- Cutrone M, Valerio E, Grimalt R. Pool palms: a case report. Dermatol Case Rep. 2019;4:1000154.
- Martína JM, Ricart JM. Erythematous–violaceous lesions on the palms. Actas Dermosifiliogr. 2009;100:507-508.
- de Masson A, Bouaziz J-D, Sulimovic L, et al; SNDV (French National Union of Dermatologists–Venereologists). Chilblains is a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83:667-670. doi:10.1016/j.jaad.2020.04.161
- Freeman EE, McMahon DE, Lipoff JB, et al; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Blauvelt A, Duarte AM, Schachner LA. Pool palms. J Am Acad Dermatol. 1992;27:111. doi:10.1016/s0190-9622(08)80819-5
- Wong L-C, Rogers M. Pool palms. Pediatr Dermatol. 2007;24:95. doi:10.1111/j.1525-1470.2007.00347.x
- Novoa A, Klear S. Pool palms. Arch Dis Child. 2016;101:41. doi:10.1136/archdischild-2015-309633
- Morgado-Carasco D, Feola H, Vargas-Mora P. Pool palms. Dermatol Pract Concept. 2020;10:e2020009. doi:10.5826/dpc.1001a09
- Cutrone M, Valerio E, Grimalt R. Pool palms: a case report. Dermatol Case Rep. 2019;4:1000154.
- Martína JM, Ricart JM. Erythematous–violaceous lesions on the palms. Actas Dermosifiliogr. 2009;100:507-508.
Feds launch COVID-19 worker vaccine mandates
The Biden administration on Nov. 4 unveiled its rule to require most of the country’s larger employers to mandate workers be fully vaccinated against COVID-19, but set a Jan. 4 deadline, avoiding the busy holiday season.
The White House also shifted the time lines for earlier mandates applying to federal workers and contractors to Jan. 4. And the same deadline applies to a new separate rule for health care workers.
The new rules are meant to preempt “any inconsistent state or local laws,” including bans and limits on employers’ authority to require vaccination, masks, or testing, the White House said in a statement.
The rule on employers from the Occupational Safety and Health Administration will apply to organizations with 100 or more employees. These employers will need to make sure each worker is fully vaccinated or tests for COVID-19 on at least a weekly basis. The OSHA rule will also require that employers provide paid time for employees to get vaccinated and ensure that all unvaccinated workers wear a face mask in the workplace. This rule will cover 84 million employees. The OSHA rule will not apply to workplaces covered by either the Centers for Medicare & Medicaid Services rule or the federal contractor vaccination requirement
“The virus will not go away by itself, or because we wish it away: We have to act,” President Joe Biden said in a statement. “Vaccination is the single best pathway out of this pandemic.”
Mandates were not the preferred route to managing the pandemic, he said.
“Too many people remain unvaccinated for us to get out of this pandemic for good,” he said. “So I instituted requirements – and they are working.”
The White House said 70% percent of U.S. adults are now fully vaccinated – up from less than 1% when Mr. Biden took office in January.
The CMS vaccine rule is meant to cover more than 17 million workers and about 76,000 medical care sites, including hospitals, ambulatory surgery centers, nursing homes, dialysis facilities, home health agencies, and long-term care facilities. The rule will apply to employees whether their positions involve patient care or not.
Unlike the OSHA mandate, the one for health care workers will not offer the option of frequent COVID-19 testing instead of vaccination. There is a “higher bar” for health care workers, given their role in treating patients, so the mandate allows only for vaccination or limited exemptions, a senior administration official said on Nov. 3 during a call with reporters.
The CMS rule includes a “range of remedies,” including penalties and denial of payment for health care facilities that fail to meet the vaccine mandate. CMS could theoretically cut off hospitals and other medical organizations for failure to comply, but that would be a “last resort,” a senior administration official said. CMS will instead work with health care facilities to help them comply with the federal rule on vaccination of medical workers.
The new CMS rules apply only to Medicare- and Medicaid-certified centers and organizations. The rule does not directly apply to other health care entities, such as doctor’s offices, that are not regulated by CMS.
“Most states have separate licensing requirements for health care staff and health care providers that would be applicable to physician office staff and other staff in small health care entities that are not subject to vaccination requirements under this IFC,” CMS said in the rule.
A version of this article first appeared on WebMD.com.
The Biden administration on Nov. 4 unveiled its rule to require most of the country’s larger employers to mandate workers be fully vaccinated against COVID-19, but set a Jan. 4 deadline, avoiding the busy holiday season.
The White House also shifted the time lines for earlier mandates applying to federal workers and contractors to Jan. 4. And the same deadline applies to a new separate rule for health care workers.
The new rules are meant to preempt “any inconsistent state or local laws,” including bans and limits on employers’ authority to require vaccination, masks, or testing, the White House said in a statement.
The rule on employers from the Occupational Safety and Health Administration will apply to organizations with 100 or more employees. These employers will need to make sure each worker is fully vaccinated or tests for COVID-19 on at least a weekly basis. The OSHA rule will also require that employers provide paid time for employees to get vaccinated and ensure that all unvaccinated workers wear a face mask in the workplace. This rule will cover 84 million employees. The OSHA rule will not apply to workplaces covered by either the Centers for Medicare & Medicaid Services rule or the federal contractor vaccination requirement
“The virus will not go away by itself, or because we wish it away: We have to act,” President Joe Biden said in a statement. “Vaccination is the single best pathway out of this pandemic.”
Mandates were not the preferred route to managing the pandemic, he said.
“Too many people remain unvaccinated for us to get out of this pandemic for good,” he said. “So I instituted requirements – and they are working.”
The White House said 70% percent of U.S. adults are now fully vaccinated – up from less than 1% when Mr. Biden took office in January.
The CMS vaccine rule is meant to cover more than 17 million workers and about 76,000 medical care sites, including hospitals, ambulatory surgery centers, nursing homes, dialysis facilities, home health agencies, and long-term care facilities. The rule will apply to employees whether their positions involve patient care or not.
Unlike the OSHA mandate, the one for health care workers will not offer the option of frequent COVID-19 testing instead of vaccination. There is a “higher bar” for health care workers, given their role in treating patients, so the mandate allows only for vaccination or limited exemptions, a senior administration official said on Nov. 3 during a call with reporters.
The CMS rule includes a “range of remedies,” including penalties and denial of payment for health care facilities that fail to meet the vaccine mandate. CMS could theoretically cut off hospitals and other medical organizations for failure to comply, but that would be a “last resort,” a senior administration official said. CMS will instead work with health care facilities to help them comply with the federal rule on vaccination of medical workers.
The new CMS rules apply only to Medicare- and Medicaid-certified centers and organizations. The rule does not directly apply to other health care entities, such as doctor’s offices, that are not regulated by CMS.
“Most states have separate licensing requirements for health care staff and health care providers that would be applicable to physician office staff and other staff in small health care entities that are not subject to vaccination requirements under this IFC,” CMS said in the rule.
A version of this article first appeared on WebMD.com.
The Biden administration on Nov. 4 unveiled its rule to require most of the country’s larger employers to mandate workers be fully vaccinated against COVID-19, but set a Jan. 4 deadline, avoiding the busy holiday season.
The White House also shifted the time lines for earlier mandates applying to federal workers and contractors to Jan. 4. And the same deadline applies to a new separate rule for health care workers.
The new rules are meant to preempt “any inconsistent state or local laws,” including bans and limits on employers’ authority to require vaccination, masks, or testing, the White House said in a statement.
The rule on employers from the Occupational Safety and Health Administration will apply to organizations with 100 or more employees. These employers will need to make sure each worker is fully vaccinated or tests for COVID-19 on at least a weekly basis. The OSHA rule will also require that employers provide paid time for employees to get vaccinated and ensure that all unvaccinated workers wear a face mask in the workplace. This rule will cover 84 million employees. The OSHA rule will not apply to workplaces covered by either the Centers for Medicare & Medicaid Services rule or the federal contractor vaccination requirement
“The virus will not go away by itself, or because we wish it away: We have to act,” President Joe Biden said in a statement. “Vaccination is the single best pathway out of this pandemic.”
Mandates were not the preferred route to managing the pandemic, he said.
“Too many people remain unvaccinated for us to get out of this pandemic for good,” he said. “So I instituted requirements – and they are working.”
The White House said 70% percent of U.S. adults are now fully vaccinated – up from less than 1% when Mr. Biden took office in January.
The CMS vaccine rule is meant to cover more than 17 million workers and about 76,000 medical care sites, including hospitals, ambulatory surgery centers, nursing homes, dialysis facilities, home health agencies, and long-term care facilities. The rule will apply to employees whether their positions involve patient care or not.
Unlike the OSHA mandate, the one for health care workers will not offer the option of frequent COVID-19 testing instead of vaccination. There is a “higher bar” for health care workers, given their role in treating patients, so the mandate allows only for vaccination or limited exemptions, a senior administration official said on Nov. 3 during a call with reporters.
The CMS rule includes a “range of remedies,” including penalties and denial of payment for health care facilities that fail to meet the vaccine mandate. CMS could theoretically cut off hospitals and other medical organizations for failure to comply, but that would be a “last resort,” a senior administration official said. CMS will instead work with health care facilities to help them comply with the federal rule on vaccination of medical workers.
The new CMS rules apply only to Medicare- and Medicaid-certified centers and organizations. The rule does not directly apply to other health care entities, such as doctor’s offices, that are not regulated by CMS.
“Most states have separate licensing requirements for health care staff and health care providers that would be applicable to physician office staff and other staff in small health care entities that are not subject to vaccination requirements under this IFC,” CMS said in the rule.
A version of this article first appeared on WebMD.com.
Rural hospitalists confront COVID-19
Unique demands of patient care in small hospitals
In 2018, Atashi Mandal, MD, a hospitalist residing in Orange County, Calif., was recruited along with several other doctors to fill hospitalist positions in rural Bishop, Calif. She has since driven 600 miles round trip every month for a week of hospital medicine shifts at Northern Inyo Hospital.
Dr. Mandal said she has really enjoyed her time at the small rural hospital and found it professionally fulfilling to participate so fully in the health of its local community. She was building personal bonds and calling the experience the pinnacle of her career when the COVID-19 pandemic swept across America and the world, even reaching up into Bishop, population 3,760, in the isolated Owens Valley.
The 25-bed hospital has seen at least 100 COVID patients in the past year and some months. Responsibility for taking care of these patients has been both humbling and gratifying, Dr. Mandal said. The facility’s hospitalists made a commitment to keep working through the pandemic. “We were able to come together (around COVID) as a team and our teamwork really made a difference,” she said.
“One of the advantages in a smaller hospital is you can have greater cohesiveness and your communication can be tighter. That played a big role in how we were able to accomplish so much with fewer resources as a rural hospital.” But staffing shortages, recruitment, and retention remain a perennial challenge for rural hospitals. “And COVID only exacerbated the problems,” she said. “I’ve had my challenges trying to make proper treatment plans without access to specialists.”
It was also difficult to witness so many patients severely ill or dying from COVID, Dr. Mandal said, especially since patients were not allowed family visitors – even though that was for a good reason, to minimize the virus’s spread.
HM in rural communities
Hospital medicine continues to extend into rural communities and small rural hospitals. In 2018, 35.7% of all rural counties in America had hospitals staffed with hospitalists, and 63.3% of rural hospitals had hospitalist programs (compared with 79.2% of urban hospitals). These numbers come from Medicare resources files from the Department of Health & Human Services, analyzed by Peiyin Hung, PhD, assistant professor of health services management and policy at the University of South Carolina, Columbia.1 Hospitalist penetration rates rose steadily from 2011 to 2017, with a slight dip in 2018, Dr. Hung said in an interview.
A total of 138 rural hospitals have closed since 2010, according to the Cecil G. Sheps Center for Health Services Research in Chapel Hill, N.C. Nineteen rural hospitals closed in 2020 alone, although many of those were caused by factors predating the pandemic. Only one has closed so far in 2021. But financial pressures, including low patient volumes and loss of revenue from canceled routine services like elective surgeries during the pandemic, have added to hospitals’ difficulties. Pandemic relief funding may have helped some hospitals stay open, but that support eventually will go away.
Experts emphasize the diversity of rural America and its health care systems. Rural economies are volatile and more diverse than is often appreciated. The hospital may be a cornerstone of the local economy; when one closes, it can devastate the community. Workforce is one of the chief components of a hospital’s ability to meet its strategic vision, and hospitalists are a big part in that. But while hospitalists are valued and appreciated, if the hospital is suffering severe financial problems, that will impact its doctors’ jobs and livelihoods.
“Bandwidth” varies widely for rural hospitalists and their hospitalist groups, said Ken Simone, DO, SFHM, executive chair of SHM’s Rural Special Interest Group and founder and principal of KGS Consultants, a Hospital Medicine and Primary Care Practice Management Consulting company. They may face scarce resources, scarce clinical staffing, lack of support staff to help operations run smoothly, lack of access to specialists locally, and lack of technology. While practicing in a rural setting presents various challenges, it can be rewarding for those clinicians who embrace its autonomy and broad scope of services, Dr. Simone said.
SHM’s Rural SIG focuses on the unique needs of rural hospitalists, providing them with an opportunity to share their concerns, challenges and solutions through roundtable discussions every other month and a special interest forum held in conjunction with the SHM Converge annual conference, Dr. Simone said. (The next SHM Converge will be April 7-10, 2022, in Nashville, Tenn.) The Rural SIG also collaborates with other hospital medicine SIGs and committees and is working on a white paper, “Key Principles and Characteristics of an Effective Rural Hospital Medicine Group.” It is also looking to develop a rural mentorship exchange program.
COVID reaches rural America
Early COVID caseloads tended to be in urban areas, but subsequent surges of infections have spread to many rural areas. Some rural settings became epicenters for the pandemic in November and December 2020. More recent troubling rises in COVID cases, particularly in areas with lower vaccination rates – suggest that the challenges of the pandemic are still not behind us.
“By no means is the crisis done in rural America,” said Alan Morgan, CEO of the National Rural Health Association, in a Virtual Rural Health Journalism workshop on rural health care sponsored by the Association of Health Care Journalists.2
Mr. Morgan’s colleague, Brock Slabach, NRHA’s chief operations officer, said in an interview that, while 453 of the 1,800 hospitals in rural areas fit NRHA’s criteria as being vulnerable to closure, the rest are not, and are fulfilling their missions for their communities. Hospitalists are becoming more common in these hospitals, he said, and rural hospitalists can be an important asset in attracting primary care physicians – who might not appreciate being perpetually on call for their hospitalized patients – to rural communities.
In many cases, traveling doctors like Dr. Mandal or telemedicine backup, particularly for after-hours coverage or ICU beds, are important pieces of the puzzle for smaller hospitals. There are different ways to use the spectrum of telemedicine services to interact with a hospital’s daytime and night routines. In some isolated locations, nurse practitioners or physician assistants provide on-the-ground coverage with virtual backup. Rural hospitals often affiliate with telemedicine networks within health systems – or else contract with independent specialized providers of telemedicine consultation.
Mr. Slabach said another alternative for staffing hospitals with smaller ED and inpatient volumes is to have one doctor on duty who can cover both departments simultaneously. Meanwhile, the new federal Rural Emergency Hospital Program proposes to allow rural hospitals to become essentially freestanding EDs – starting Jan. 1, 2023 – that can manage patients for a maximum of 24 hours.3
Community connections and proactive staffing
Lisa Kaufmann, MD, works as a hospitalist for a two-hospital system in North Carolina, Appalachian Regional Health Care. She practices at Watauga Medical Center, with 100 licensed beds in Boone, and at Cannon Memorial Hospital, a critical access hospital in unincorporated Linville. “We are proud of what we have been able to accomplish during the pandemic,” she said.
A former critical care unit at Watauga had been shut down, but its wiring remained intact. “We turned it into a COVID unit in three days. Then we opened another COVID unit with 18 beds, but that still wasn’t enough. We converted half of our med/surg capacity into a COVID unit. At one point almost half of all of our acute beds were for COVID patients. We made plans for what we would do if it got worse, since we had almost run out of beds,” she said. Demand peaked at the end of January 2021.
“The biggest barrier for us was if someone needed to be transferred, for example, if they needed ECMO [extracorporeal membrane oxygenation], and we couldn’t find another hospital to provide that technology.” In ARHC’s mountainous region – known as the “High Country” – weather can also make it difficult to transport patients. “Sometimes the ambulance can’t make it off the mountain, and half of the time the medical helicopter can’t fly. So we have to be prepared to keep people who we might think ought to be transferred,” she said.
Like many rural communities, the High Country is tightly knit, and its hospitals are really connected to their communities, Dr. Kaufmann said. The health system already had a lot of community connections beyond acute care, and that meant the pandemic wasn’t experienced as severely as it was in some other rural communities. “But without hospitalists in our hospitals, it would have been much more difficult.”
Proactive supply fulfillment meant that her hospitals never ran out of personal protective equipment. “Staffing was a challenge, but we were proactive in getting traveling doctors to come here. We also utilized extra doctors from the local community,” she said. Another key was well-established disaster planning, with regular drills, and a robust incident command structure, which just needed to be activated in the crisis. “Small hospitals need to be prepared for disaster,” Dr. Kaufmann said.
For Dale Wiersma, MD, a hospitalist with Spectrum Health, a 14-hospital system in western Michigan, telemedicine services are coordinated across 8 rural regional hospitals. “We don’t tend to use it for direct hospitalist work during daytime hours, unless a facility is swamped, in which case we can cross-cover. We do more telemedicine at night. But during daytime hours we have access to stroke neurology, cardiology, psychiatry, critical care and infectious disease specialists who are able to offer virtual consults,” Dr. Wiersma said. A virtual critical care team of doctor and nurse is often the only intensivist service covering Spectrum’s rural hospitals.
“In our system, the pandemic accelerated the adoption of telemedicine,” Dr. Wiersma said. “We had been working on the tele-ICU program, trying to get it rolled out. When the pandemic hit, we launched it in just 6 weeks.”
There have been several COVID surges in Michigan, he said. “We were stretched pretty close to our limit several times, but never to the breaking point. For our physicians, it was the protracted nature of the pandemic that was fatiguing for everyone involved. Our system worked hard to staff up as well as it could, to make sure our people didn’t go over the edge.” It was also hard for hospitals that typically might see one or two deaths in a month to suddenly have five in a week.
Another Spectrum hospitalist, Christopher Skinner, MD, works at two rural Michigan hospitals 15 minutes apart in Big Rapids and Reed City. “I prefer working in rural areas. I’ve never had an ambition to be a top dog. I like the style of practice where you don’t have all of the medical subspecialties on site. It frees you up to use all your skills,” Dr. Skinner said.
But that approach was put to the test by the pandemic, since it was harder to transfer those patients who normally would not have stayed at these rural hospitals. “We had to make do,” he said, although virtual backup and second opinions from Spectrum’s virtual critical care team helped.
“It was a great collaboration, which helped us to handle critical care cases that we hadn’t had to manage pre-COVID. We’ve gotten used to it, with the backup, so I expect we’ll still be taking care of these kind of sick ventilator patients even after the pandemic ends,” Dr. Skinner said. “We’ve gotten pretty good at it.”
Sukhbir Pannu, MD, a hospitalist in Denver and CEO and founder of Rural Physicians Group, said the pandemic was highly impactful, operationally and logistically, for his firm, which contracts with 54 hospitals to provide their hospitalist staffing. “There was no preparation. Everything had to be done on the fly. Initially, it was felt that rural areas weren’t at as great a risk for COVID, but that proved not to be true. Many experienced a sudden increase in very sick patients. We set up a task force to manage daily census in all of our contracted facilities.”
How did Rural Physicians Group manage through the crisis? “The short answer is telemedicine,” he said. “We had physicians on the ground in these hospitals. But we needed intensivists at the other end of the line to support them.” A lot of conversations about telemedicine were already going on in the company, but the pandemic provided the impetus to launch its network, which has grown to include rheumatologists, pulmonologists, cardiologists, infection medicine, neurology, and psychiatry, all reachable through a central command structure.
Telemedicine is not a cure-all, Dr. Pannu said. It doesn’t work in a vacuum. It requires both a provider on the ground and specialists available remotely. “But it can be a massive multiplier.”
Critical medicine
Other hospitals, including small and rural ones, have reported taking on the challenge of covering critical care with nonintensivist physicians because the pandemic demanded it. David Aymond, MD, a hospitalist at 60-bed Byrd Regional Hospital in Leesville, La., population 6,612, has advocated for years for expanded training and credentialing opportunities in intensive care medicine beyond the traditional path of becoming a board-certified intensivist. Some rural hospitalists were already experienced in providing critical care for ICU patients even before the pandemic hit.
“What COVID did was to highlight the problem that there aren’t enough intensivists in this country, particular for smaller hospitals,” Dr. Aymond said. Some hospitalists who stepped into crisis roles in ICUs during COVID surges showed that they could take care of COVID patients very well.
Dr. Aymond, who is a fellowship-trained hospitalist with primary training in family medicine, has used his ICU experience in both fellowship and practice to make a thorough study of critical care medicine, which he put to good use when the seven-bed ICU at Byrd Memorial filled with COVID patients. “Early on, we were managing multiple ventilators throughout the hospital,” he said. “But we were having good outcomes. Our COVID patients were surviving.” That led to Dr. Aymond being interviewed by local news media, which led to other patients across the state asking to be transferred to “the COVID specialist who practices at Byrd.”
Dr. Aymond would like to see opportunities for abbreviated 1-year critical care fellowships for hospitalists who have amassed enough ICU experience in practice or in residency, and to make room for family medicine physicians in such programs. He is also working through SHM with the Society of Critical Care Medicine to generate educational ICU content. SHM now has a critical care lecture series at: www.hospitalmedicine.org/clinical-topics/critical-care/.
Dr. Mandal, who also works as a pediatric hospitalist, said that experience gave her more familiarity with using noninvasive methods for delivering respiratory therapies like high-flow oxygen. “When I saw a COVID patient who had hypoxia but was still able to talk, I didn’t hesitate to deliver oxygen through noninvasive means.” Eventually hospital practice generally for COVID caught up with this approach.
But she ran into personal difficulties because N95 face masks didn’t fit her face. Instead, she had to wear a portable respirator, which made it hard to hear what her patients were saying. “I formulated a lot of workarounds, such as interviewing the patient over the phone before going into the room for the physical exam.”
Throughout the pandemic, she never wavered in her commitment to rural hospital medicine and its opportunities for working in a small and wonderful community, where she could practice at the top of her license, with a degree of autonomy not granted in other settings. For doctors who want that kind of practice, she said, “the rewards will be paid back in spades. That’s been my experience.”
For more information on SHM’s Rural SIG and its supports for rural hospitalists, contact its executive chair, Kenneth Simone, DO, at [email protected].
References
1. Personal communication from Peiyin Hung, June 2021.
2. Association of Health Care Journalists. Rural Health Journalism Workshop 2021. June 21, 2021. https://healthjournalism.org/calendar-details.php?id=2369.
3. Congress Establishes New Medicare Provider Category and Reimbursement for Rural Emergency Hospitals. National Law Review. Jan. 5, 2021. https://www.natlawreview.com/article/congress-establishes-new-medicare-provider-category-and-reimbursement-rural.
Unique demands of patient care in small hospitals
Unique demands of patient care in small hospitals
In 2018, Atashi Mandal, MD, a hospitalist residing in Orange County, Calif., was recruited along with several other doctors to fill hospitalist positions in rural Bishop, Calif. She has since driven 600 miles round trip every month for a week of hospital medicine shifts at Northern Inyo Hospital.
Dr. Mandal said she has really enjoyed her time at the small rural hospital and found it professionally fulfilling to participate so fully in the health of its local community. She was building personal bonds and calling the experience the pinnacle of her career when the COVID-19 pandemic swept across America and the world, even reaching up into Bishop, population 3,760, in the isolated Owens Valley.
The 25-bed hospital has seen at least 100 COVID patients in the past year and some months. Responsibility for taking care of these patients has been both humbling and gratifying, Dr. Mandal said. The facility’s hospitalists made a commitment to keep working through the pandemic. “We were able to come together (around COVID) as a team and our teamwork really made a difference,” she said.
“One of the advantages in a smaller hospital is you can have greater cohesiveness and your communication can be tighter. That played a big role in how we were able to accomplish so much with fewer resources as a rural hospital.” But staffing shortages, recruitment, and retention remain a perennial challenge for rural hospitals. “And COVID only exacerbated the problems,” she said. “I’ve had my challenges trying to make proper treatment plans without access to specialists.”
It was also difficult to witness so many patients severely ill or dying from COVID, Dr. Mandal said, especially since patients were not allowed family visitors – even though that was for a good reason, to minimize the virus’s spread.
HM in rural communities
Hospital medicine continues to extend into rural communities and small rural hospitals. In 2018, 35.7% of all rural counties in America had hospitals staffed with hospitalists, and 63.3% of rural hospitals had hospitalist programs (compared with 79.2% of urban hospitals). These numbers come from Medicare resources files from the Department of Health & Human Services, analyzed by Peiyin Hung, PhD, assistant professor of health services management and policy at the University of South Carolina, Columbia.1 Hospitalist penetration rates rose steadily from 2011 to 2017, with a slight dip in 2018, Dr. Hung said in an interview.
A total of 138 rural hospitals have closed since 2010, according to the Cecil G. Sheps Center for Health Services Research in Chapel Hill, N.C. Nineteen rural hospitals closed in 2020 alone, although many of those were caused by factors predating the pandemic. Only one has closed so far in 2021. But financial pressures, including low patient volumes and loss of revenue from canceled routine services like elective surgeries during the pandemic, have added to hospitals’ difficulties. Pandemic relief funding may have helped some hospitals stay open, but that support eventually will go away.
Experts emphasize the diversity of rural America and its health care systems. Rural economies are volatile and more diverse than is often appreciated. The hospital may be a cornerstone of the local economy; when one closes, it can devastate the community. Workforce is one of the chief components of a hospital’s ability to meet its strategic vision, and hospitalists are a big part in that. But while hospitalists are valued and appreciated, if the hospital is suffering severe financial problems, that will impact its doctors’ jobs and livelihoods.
“Bandwidth” varies widely for rural hospitalists and their hospitalist groups, said Ken Simone, DO, SFHM, executive chair of SHM’s Rural Special Interest Group and founder and principal of KGS Consultants, a Hospital Medicine and Primary Care Practice Management Consulting company. They may face scarce resources, scarce clinical staffing, lack of support staff to help operations run smoothly, lack of access to specialists locally, and lack of technology. While practicing in a rural setting presents various challenges, it can be rewarding for those clinicians who embrace its autonomy and broad scope of services, Dr. Simone said.
SHM’s Rural SIG focuses on the unique needs of rural hospitalists, providing them with an opportunity to share their concerns, challenges and solutions through roundtable discussions every other month and a special interest forum held in conjunction with the SHM Converge annual conference, Dr. Simone said. (The next SHM Converge will be April 7-10, 2022, in Nashville, Tenn.) The Rural SIG also collaborates with other hospital medicine SIGs and committees and is working on a white paper, “Key Principles and Characteristics of an Effective Rural Hospital Medicine Group.” It is also looking to develop a rural mentorship exchange program.
COVID reaches rural America
Early COVID caseloads tended to be in urban areas, but subsequent surges of infections have spread to many rural areas. Some rural settings became epicenters for the pandemic in November and December 2020. More recent troubling rises in COVID cases, particularly in areas with lower vaccination rates – suggest that the challenges of the pandemic are still not behind us.
“By no means is the crisis done in rural America,” said Alan Morgan, CEO of the National Rural Health Association, in a Virtual Rural Health Journalism workshop on rural health care sponsored by the Association of Health Care Journalists.2
Mr. Morgan’s colleague, Brock Slabach, NRHA’s chief operations officer, said in an interview that, while 453 of the 1,800 hospitals in rural areas fit NRHA’s criteria as being vulnerable to closure, the rest are not, and are fulfilling their missions for their communities. Hospitalists are becoming more common in these hospitals, he said, and rural hospitalists can be an important asset in attracting primary care physicians – who might not appreciate being perpetually on call for their hospitalized patients – to rural communities.
In many cases, traveling doctors like Dr. Mandal or telemedicine backup, particularly for after-hours coverage or ICU beds, are important pieces of the puzzle for smaller hospitals. There are different ways to use the spectrum of telemedicine services to interact with a hospital’s daytime and night routines. In some isolated locations, nurse practitioners or physician assistants provide on-the-ground coverage with virtual backup. Rural hospitals often affiliate with telemedicine networks within health systems – or else contract with independent specialized providers of telemedicine consultation.
Mr. Slabach said another alternative for staffing hospitals with smaller ED and inpatient volumes is to have one doctor on duty who can cover both departments simultaneously. Meanwhile, the new federal Rural Emergency Hospital Program proposes to allow rural hospitals to become essentially freestanding EDs – starting Jan. 1, 2023 – that can manage patients for a maximum of 24 hours.3
Community connections and proactive staffing
Lisa Kaufmann, MD, works as a hospitalist for a two-hospital system in North Carolina, Appalachian Regional Health Care. She practices at Watauga Medical Center, with 100 licensed beds in Boone, and at Cannon Memorial Hospital, a critical access hospital in unincorporated Linville. “We are proud of what we have been able to accomplish during the pandemic,” she said.
A former critical care unit at Watauga had been shut down, but its wiring remained intact. “We turned it into a COVID unit in three days. Then we opened another COVID unit with 18 beds, but that still wasn’t enough. We converted half of our med/surg capacity into a COVID unit. At one point almost half of all of our acute beds were for COVID patients. We made plans for what we would do if it got worse, since we had almost run out of beds,” she said. Demand peaked at the end of January 2021.
“The biggest barrier for us was if someone needed to be transferred, for example, if they needed ECMO [extracorporeal membrane oxygenation], and we couldn’t find another hospital to provide that technology.” In ARHC’s mountainous region – known as the “High Country” – weather can also make it difficult to transport patients. “Sometimes the ambulance can’t make it off the mountain, and half of the time the medical helicopter can’t fly. So we have to be prepared to keep people who we might think ought to be transferred,” she said.
Like many rural communities, the High Country is tightly knit, and its hospitals are really connected to their communities, Dr. Kaufmann said. The health system already had a lot of community connections beyond acute care, and that meant the pandemic wasn’t experienced as severely as it was in some other rural communities. “But without hospitalists in our hospitals, it would have been much more difficult.”
Proactive supply fulfillment meant that her hospitals never ran out of personal protective equipment. “Staffing was a challenge, but we were proactive in getting traveling doctors to come here. We also utilized extra doctors from the local community,” she said. Another key was well-established disaster planning, with regular drills, and a robust incident command structure, which just needed to be activated in the crisis. “Small hospitals need to be prepared for disaster,” Dr. Kaufmann said.
For Dale Wiersma, MD, a hospitalist with Spectrum Health, a 14-hospital system in western Michigan, telemedicine services are coordinated across 8 rural regional hospitals. “We don’t tend to use it for direct hospitalist work during daytime hours, unless a facility is swamped, in which case we can cross-cover. We do more telemedicine at night. But during daytime hours we have access to stroke neurology, cardiology, psychiatry, critical care and infectious disease specialists who are able to offer virtual consults,” Dr. Wiersma said. A virtual critical care team of doctor and nurse is often the only intensivist service covering Spectrum’s rural hospitals.
“In our system, the pandemic accelerated the adoption of telemedicine,” Dr. Wiersma said. “We had been working on the tele-ICU program, trying to get it rolled out. When the pandemic hit, we launched it in just 6 weeks.”
There have been several COVID surges in Michigan, he said. “We were stretched pretty close to our limit several times, but never to the breaking point. For our physicians, it was the protracted nature of the pandemic that was fatiguing for everyone involved. Our system worked hard to staff up as well as it could, to make sure our people didn’t go over the edge.” It was also hard for hospitals that typically might see one or two deaths in a month to suddenly have five in a week.
Another Spectrum hospitalist, Christopher Skinner, MD, works at two rural Michigan hospitals 15 minutes apart in Big Rapids and Reed City. “I prefer working in rural areas. I’ve never had an ambition to be a top dog. I like the style of practice where you don’t have all of the medical subspecialties on site. It frees you up to use all your skills,” Dr. Skinner said.
But that approach was put to the test by the pandemic, since it was harder to transfer those patients who normally would not have stayed at these rural hospitals. “We had to make do,” he said, although virtual backup and second opinions from Spectrum’s virtual critical care team helped.
“It was a great collaboration, which helped us to handle critical care cases that we hadn’t had to manage pre-COVID. We’ve gotten used to it, with the backup, so I expect we’ll still be taking care of these kind of sick ventilator patients even after the pandemic ends,” Dr. Skinner said. “We’ve gotten pretty good at it.”
Sukhbir Pannu, MD, a hospitalist in Denver and CEO and founder of Rural Physicians Group, said the pandemic was highly impactful, operationally and logistically, for his firm, which contracts with 54 hospitals to provide their hospitalist staffing. “There was no preparation. Everything had to be done on the fly. Initially, it was felt that rural areas weren’t at as great a risk for COVID, but that proved not to be true. Many experienced a sudden increase in very sick patients. We set up a task force to manage daily census in all of our contracted facilities.”
How did Rural Physicians Group manage through the crisis? “The short answer is telemedicine,” he said. “We had physicians on the ground in these hospitals. But we needed intensivists at the other end of the line to support them.” A lot of conversations about telemedicine were already going on in the company, but the pandemic provided the impetus to launch its network, which has grown to include rheumatologists, pulmonologists, cardiologists, infection medicine, neurology, and psychiatry, all reachable through a central command structure.
Telemedicine is not a cure-all, Dr. Pannu said. It doesn’t work in a vacuum. It requires both a provider on the ground and specialists available remotely. “But it can be a massive multiplier.”
Critical medicine
Other hospitals, including small and rural ones, have reported taking on the challenge of covering critical care with nonintensivist physicians because the pandemic demanded it. David Aymond, MD, a hospitalist at 60-bed Byrd Regional Hospital in Leesville, La., population 6,612, has advocated for years for expanded training and credentialing opportunities in intensive care medicine beyond the traditional path of becoming a board-certified intensivist. Some rural hospitalists were already experienced in providing critical care for ICU patients even before the pandemic hit.
“What COVID did was to highlight the problem that there aren’t enough intensivists in this country, particular for smaller hospitals,” Dr. Aymond said. Some hospitalists who stepped into crisis roles in ICUs during COVID surges showed that they could take care of COVID patients very well.
Dr. Aymond, who is a fellowship-trained hospitalist with primary training in family medicine, has used his ICU experience in both fellowship and practice to make a thorough study of critical care medicine, which he put to good use when the seven-bed ICU at Byrd Memorial filled with COVID patients. “Early on, we were managing multiple ventilators throughout the hospital,” he said. “But we were having good outcomes. Our COVID patients were surviving.” That led to Dr. Aymond being interviewed by local news media, which led to other patients across the state asking to be transferred to “the COVID specialist who practices at Byrd.”
Dr. Aymond would like to see opportunities for abbreviated 1-year critical care fellowships for hospitalists who have amassed enough ICU experience in practice or in residency, and to make room for family medicine physicians in such programs. He is also working through SHM with the Society of Critical Care Medicine to generate educational ICU content. SHM now has a critical care lecture series at: www.hospitalmedicine.org/clinical-topics/critical-care/.
Dr. Mandal, who also works as a pediatric hospitalist, said that experience gave her more familiarity with using noninvasive methods for delivering respiratory therapies like high-flow oxygen. “When I saw a COVID patient who had hypoxia but was still able to talk, I didn’t hesitate to deliver oxygen through noninvasive means.” Eventually hospital practice generally for COVID caught up with this approach.
But she ran into personal difficulties because N95 face masks didn’t fit her face. Instead, she had to wear a portable respirator, which made it hard to hear what her patients were saying. “I formulated a lot of workarounds, such as interviewing the patient over the phone before going into the room for the physical exam.”
Throughout the pandemic, she never wavered in her commitment to rural hospital medicine and its opportunities for working in a small and wonderful community, where she could practice at the top of her license, with a degree of autonomy not granted in other settings. For doctors who want that kind of practice, she said, “the rewards will be paid back in spades. That’s been my experience.”
For more information on SHM’s Rural SIG and its supports for rural hospitalists, contact its executive chair, Kenneth Simone, DO, at [email protected].
References
1. Personal communication from Peiyin Hung, June 2021.
2. Association of Health Care Journalists. Rural Health Journalism Workshop 2021. June 21, 2021. https://healthjournalism.org/calendar-details.php?id=2369.
3. Congress Establishes New Medicare Provider Category and Reimbursement for Rural Emergency Hospitals. National Law Review. Jan. 5, 2021. https://www.natlawreview.com/article/congress-establishes-new-medicare-provider-category-and-reimbursement-rural.
In 2018, Atashi Mandal, MD, a hospitalist residing in Orange County, Calif., was recruited along with several other doctors to fill hospitalist positions in rural Bishop, Calif. She has since driven 600 miles round trip every month for a week of hospital medicine shifts at Northern Inyo Hospital.
Dr. Mandal said she has really enjoyed her time at the small rural hospital and found it professionally fulfilling to participate so fully in the health of its local community. She was building personal bonds and calling the experience the pinnacle of her career when the COVID-19 pandemic swept across America and the world, even reaching up into Bishop, population 3,760, in the isolated Owens Valley.
The 25-bed hospital has seen at least 100 COVID patients in the past year and some months. Responsibility for taking care of these patients has been both humbling and gratifying, Dr. Mandal said. The facility’s hospitalists made a commitment to keep working through the pandemic. “We were able to come together (around COVID) as a team and our teamwork really made a difference,” she said.
“One of the advantages in a smaller hospital is you can have greater cohesiveness and your communication can be tighter. That played a big role in how we were able to accomplish so much with fewer resources as a rural hospital.” But staffing shortages, recruitment, and retention remain a perennial challenge for rural hospitals. “And COVID only exacerbated the problems,” she said. “I’ve had my challenges trying to make proper treatment plans without access to specialists.”
It was also difficult to witness so many patients severely ill or dying from COVID, Dr. Mandal said, especially since patients were not allowed family visitors – even though that was for a good reason, to minimize the virus’s spread.
HM in rural communities
Hospital medicine continues to extend into rural communities and small rural hospitals. In 2018, 35.7% of all rural counties in America had hospitals staffed with hospitalists, and 63.3% of rural hospitals had hospitalist programs (compared with 79.2% of urban hospitals). These numbers come from Medicare resources files from the Department of Health & Human Services, analyzed by Peiyin Hung, PhD, assistant professor of health services management and policy at the University of South Carolina, Columbia.1 Hospitalist penetration rates rose steadily from 2011 to 2017, with a slight dip in 2018, Dr. Hung said in an interview.
A total of 138 rural hospitals have closed since 2010, according to the Cecil G. Sheps Center for Health Services Research in Chapel Hill, N.C. Nineteen rural hospitals closed in 2020 alone, although many of those were caused by factors predating the pandemic. Only one has closed so far in 2021. But financial pressures, including low patient volumes and loss of revenue from canceled routine services like elective surgeries during the pandemic, have added to hospitals’ difficulties. Pandemic relief funding may have helped some hospitals stay open, but that support eventually will go away.
Experts emphasize the diversity of rural America and its health care systems. Rural economies are volatile and more diverse than is often appreciated. The hospital may be a cornerstone of the local economy; when one closes, it can devastate the community. Workforce is one of the chief components of a hospital’s ability to meet its strategic vision, and hospitalists are a big part in that. But while hospitalists are valued and appreciated, if the hospital is suffering severe financial problems, that will impact its doctors’ jobs and livelihoods.
“Bandwidth” varies widely for rural hospitalists and their hospitalist groups, said Ken Simone, DO, SFHM, executive chair of SHM’s Rural Special Interest Group and founder and principal of KGS Consultants, a Hospital Medicine and Primary Care Practice Management Consulting company. They may face scarce resources, scarce clinical staffing, lack of support staff to help operations run smoothly, lack of access to specialists locally, and lack of technology. While practicing in a rural setting presents various challenges, it can be rewarding for those clinicians who embrace its autonomy and broad scope of services, Dr. Simone said.
SHM’s Rural SIG focuses on the unique needs of rural hospitalists, providing them with an opportunity to share their concerns, challenges and solutions through roundtable discussions every other month and a special interest forum held in conjunction with the SHM Converge annual conference, Dr. Simone said. (The next SHM Converge will be April 7-10, 2022, in Nashville, Tenn.) The Rural SIG also collaborates with other hospital medicine SIGs and committees and is working on a white paper, “Key Principles and Characteristics of an Effective Rural Hospital Medicine Group.” It is also looking to develop a rural mentorship exchange program.
COVID reaches rural America
Early COVID caseloads tended to be in urban areas, but subsequent surges of infections have spread to many rural areas. Some rural settings became epicenters for the pandemic in November and December 2020. More recent troubling rises in COVID cases, particularly in areas with lower vaccination rates – suggest that the challenges of the pandemic are still not behind us.
“By no means is the crisis done in rural America,” said Alan Morgan, CEO of the National Rural Health Association, in a Virtual Rural Health Journalism workshop on rural health care sponsored by the Association of Health Care Journalists.2
Mr. Morgan’s colleague, Brock Slabach, NRHA’s chief operations officer, said in an interview that, while 453 of the 1,800 hospitals in rural areas fit NRHA’s criteria as being vulnerable to closure, the rest are not, and are fulfilling their missions for their communities. Hospitalists are becoming more common in these hospitals, he said, and rural hospitalists can be an important asset in attracting primary care physicians – who might not appreciate being perpetually on call for their hospitalized patients – to rural communities.
In many cases, traveling doctors like Dr. Mandal or telemedicine backup, particularly for after-hours coverage or ICU beds, are important pieces of the puzzle for smaller hospitals. There are different ways to use the spectrum of telemedicine services to interact with a hospital’s daytime and night routines. In some isolated locations, nurse practitioners or physician assistants provide on-the-ground coverage with virtual backup. Rural hospitals often affiliate with telemedicine networks within health systems – or else contract with independent specialized providers of telemedicine consultation.
Mr. Slabach said another alternative for staffing hospitals with smaller ED and inpatient volumes is to have one doctor on duty who can cover both departments simultaneously. Meanwhile, the new federal Rural Emergency Hospital Program proposes to allow rural hospitals to become essentially freestanding EDs – starting Jan. 1, 2023 – that can manage patients for a maximum of 24 hours.3
Community connections and proactive staffing
Lisa Kaufmann, MD, works as a hospitalist for a two-hospital system in North Carolina, Appalachian Regional Health Care. She practices at Watauga Medical Center, with 100 licensed beds in Boone, and at Cannon Memorial Hospital, a critical access hospital in unincorporated Linville. “We are proud of what we have been able to accomplish during the pandemic,” she said.
A former critical care unit at Watauga had been shut down, but its wiring remained intact. “We turned it into a COVID unit in three days. Then we opened another COVID unit with 18 beds, but that still wasn’t enough. We converted half of our med/surg capacity into a COVID unit. At one point almost half of all of our acute beds were for COVID patients. We made plans for what we would do if it got worse, since we had almost run out of beds,” she said. Demand peaked at the end of January 2021.
“The biggest barrier for us was if someone needed to be transferred, for example, if they needed ECMO [extracorporeal membrane oxygenation], and we couldn’t find another hospital to provide that technology.” In ARHC’s mountainous region – known as the “High Country” – weather can also make it difficult to transport patients. “Sometimes the ambulance can’t make it off the mountain, and half of the time the medical helicopter can’t fly. So we have to be prepared to keep people who we might think ought to be transferred,” she said.
Like many rural communities, the High Country is tightly knit, and its hospitals are really connected to their communities, Dr. Kaufmann said. The health system already had a lot of community connections beyond acute care, and that meant the pandemic wasn’t experienced as severely as it was in some other rural communities. “But without hospitalists in our hospitals, it would have been much more difficult.”
Proactive supply fulfillment meant that her hospitals never ran out of personal protective equipment. “Staffing was a challenge, but we were proactive in getting traveling doctors to come here. We also utilized extra doctors from the local community,” she said. Another key was well-established disaster planning, with regular drills, and a robust incident command structure, which just needed to be activated in the crisis. “Small hospitals need to be prepared for disaster,” Dr. Kaufmann said.
For Dale Wiersma, MD, a hospitalist with Spectrum Health, a 14-hospital system in western Michigan, telemedicine services are coordinated across 8 rural regional hospitals. “We don’t tend to use it for direct hospitalist work during daytime hours, unless a facility is swamped, in which case we can cross-cover. We do more telemedicine at night. But during daytime hours we have access to stroke neurology, cardiology, psychiatry, critical care and infectious disease specialists who are able to offer virtual consults,” Dr. Wiersma said. A virtual critical care team of doctor and nurse is often the only intensivist service covering Spectrum’s rural hospitals.
“In our system, the pandemic accelerated the adoption of telemedicine,” Dr. Wiersma said. “We had been working on the tele-ICU program, trying to get it rolled out. When the pandemic hit, we launched it in just 6 weeks.”
There have been several COVID surges in Michigan, he said. “We were stretched pretty close to our limit several times, but never to the breaking point. For our physicians, it was the protracted nature of the pandemic that was fatiguing for everyone involved. Our system worked hard to staff up as well as it could, to make sure our people didn’t go over the edge.” It was also hard for hospitals that typically might see one or two deaths in a month to suddenly have five in a week.
Another Spectrum hospitalist, Christopher Skinner, MD, works at two rural Michigan hospitals 15 minutes apart in Big Rapids and Reed City. “I prefer working in rural areas. I’ve never had an ambition to be a top dog. I like the style of practice where you don’t have all of the medical subspecialties on site. It frees you up to use all your skills,” Dr. Skinner said.
But that approach was put to the test by the pandemic, since it was harder to transfer those patients who normally would not have stayed at these rural hospitals. “We had to make do,” he said, although virtual backup and second opinions from Spectrum’s virtual critical care team helped.
“It was a great collaboration, which helped us to handle critical care cases that we hadn’t had to manage pre-COVID. We’ve gotten used to it, with the backup, so I expect we’ll still be taking care of these kind of sick ventilator patients even after the pandemic ends,” Dr. Skinner said. “We’ve gotten pretty good at it.”
Sukhbir Pannu, MD, a hospitalist in Denver and CEO and founder of Rural Physicians Group, said the pandemic was highly impactful, operationally and logistically, for his firm, which contracts with 54 hospitals to provide their hospitalist staffing. “There was no preparation. Everything had to be done on the fly. Initially, it was felt that rural areas weren’t at as great a risk for COVID, but that proved not to be true. Many experienced a sudden increase in very sick patients. We set up a task force to manage daily census in all of our contracted facilities.”
How did Rural Physicians Group manage through the crisis? “The short answer is telemedicine,” he said. “We had physicians on the ground in these hospitals. But we needed intensivists at the other end of the line to support them.” A lot of conversations about telemedicine were already going on in the company, but the pandemic provided the impetus to launch its network, which has grown to include rheumatologists, pulmonologists, cardiologists, infection medicine, neurology, and psychiatry, all reachable through a central command structure.
Telemedicine is not a cure-all, Dr. Pannu said. It doesn’t work in a vacuum. It requires both a provider on the ground and specialists available remotely. “But it can be a massive multiplier.”
Critical medicine
Other hospitals, including small and rural ones, have reported taking on the challenge of covering critical care with nonintensivist physicians because the pandemic demanded it. David Aymond, MD, a hospitalist at 60-bed Byrd Regional Hospital in Leesville, La., population 6,612, has advocated for years for expanded training and credentialing opportunities in intensive care medicine beyond the traditional path of becoming a board-certified intensivist. Some rural hospitalists were already experienced in providing critical care for ICU patients even before the pandemic hit.
“What COVID did was to highlight the problem that there aren’t enough intensivists in this country, particular for smaller hospitals,” Dr. Aymond said. Some hospitalists who stepped into crisis roles in ICUs during COVID surges showed that they could take care of COVID patients very well.
Dr. Aymond, who is a fellowship-trained hospitalist with primary training in family medicine, has used his ICU experience in both fellowship and practice to make a thorough study of critical care medicine, which he put to good use when the seven-bed ICU at Byrd Memorial filled with COVID patients. “Early on, we were managing multiple ventilators throughout the hospital,” he said. “But we were having good outcomes. Our COVID patients were surviving.” That led to Dr. Aymond being interviewed by local news media, which led to other patients across the state asking to be transferred to “the COVID specialist who practices at Byrd.”
Dr. Aymond would like to see opportunities for abbreviated 1-year critical care fellowships for hospitalists who have amassed enough ICU experience in practice or in residency, and to make room for family medicine physicians in such programs. He is also working through SHM with the Society of Critical Care Medicine to generate educational ICU content. SHM now has a critical care lecture series at: www.hospitalmedicine.org/clinical-topics/critical-care/.
Dr. Mandal, who also works as a pediatric hospitalist, said that experience gave her more familiarity with using noninvasive methods for delivering respiratory therapies like high-flow oxygen. “When I saw a COVID patient who had hypoxia but was still able to talk, I didn’t hesitate to deliver oxygen through noninvasive means.” Eventually hospital practice generally for COVID caught up with this approach.
But she ran into personal difficulties because N95 face masks didn’t fit her face. Instead, she had to wear a portable respirator, which made it hard to hear what her patients were saying. “I formulated a lot of workarounds, such as interviewing the patient over the phone before going into the room for the physical exam.”
Throughout the pandemic, she never wavered in her commitment to rural hospital medicine and its opportunities for working in a small and wonderful community, where she could practice at the top of her license, with a degree of autonomy not granted in other settings. For doctors who want that kind of practice, she said, “the rewards will be paid back in spades. That’s been my experience.”
For more information on SHM’s Rural SIG and its supports for rural hospitalists, contact its executive chair, Kenneth Simone, DO, at [email protected].
References
1. Personal communication from Peiyin Hung, June 2021.
2. Association of Health Care Journalists. Rural Health Journalism Workshop 2021. June 21, 2021. https://healthjournalism.org/calendar-details.php?id=2369.
3. Congress Establishes New Medicare Provider Category and Reimbursement for Rural Emergency Hospitals. National Law Review. Jan. 5, 2021. https://www.natlawreview.com/article/congress-establishes-new-medicare-provider-category-and-reimbursement-rural.
Droperidol/midazolam combo curbs agitation in ED patients
a study involving 86 adult patients at a single tertiary medical care center.
Patients with acute agitation present significant safety concerns in the emergency department, according to Jessica Javed, MD, of the University of Louisville (Ky.) and colleagues.
A combination of haloperidol and lorazepam has been widely used to curb agitation in these patients, but droperidol and midazolam could be more effective, owing to faster onset of action, Dr. Javed noted in a presentation at the annual meeting of the American College of Emergency Physicians.
Dr. Javed and colleagues conducted a prospective study to compare time to adequate sedation in agitated patients in the ED. In the trial, 43 patients received droperidol 5 mg plus midazolam 5 mg, and 43 patients received haloperidol plus lorazepam 2 mg. The average age of the patients in the droperidol/midazolam group was 34 years; the average age of the patients in the haloperidol/lorazepam group was 38 years. Baseline demographics, including height, weight, body mass index, and baseline Sedation Assessment Tool (SAT) scores, were similar between the groups.
The SAT score scale ranges from +3 (combative, violent, or out of control) to –3 (no response to stimulation); zero indicates being awake and calm/cooperative. The median baseline SAT score was 3 for both treatment groups.
The primary outcome was the proportion of patients with adequate sedation (defined as SAT scores of ≤0) 10 min after treatment.
Significantly more patients in the droperidol/midazolam group met this outcome, compared with the patients in the haloperidol/lorazepam group (51.2% vs. 7%). Also, significantly more patients in the droperidol/midazolam group achieved adequate sedation at 5, 10, 15, and 30 min than in the haloperidol/lorazepam group.
Fewer patients in the haloperidol/lorazepam group required supplemental oxygen, compared with the droperidol/midazolam group (9.3% vs. 25.6%). However, none of the droperidol/midazolam patients required rescue sedation, compared with 16.3% of the haloperidol/lorazepam patients, Dr. Javed noted. None of the patients required endotracheal intubation or experienced extrapyramidal symptoms, she said.
The study was limited by the small sample size and inclusion of data from only a single center.
The results suggest that droperidol/midazolam is superior to intramuscular haloperidol/lorazepam for producing adequate sedation after 10 min in agitated patients, Dr. Javed concluded.
The study received no outside funding. The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a study involving 86 adult patients at a single tertiary medical care center.
Patients with acute agitation present significant safety concerns in the emergency department, according to Jessica Javed, MD, of the University of Louisville (Ky.) and colleagues.
A combination of haloperidol and lorazepam has been widely used to curb agitation in these patients, but droperidol and midazolam could be more effective, owing to faster onset of action, Dr. Javed noted in a presentation at the annual meeting of the American College of Emergency Physicians.
Dr. Javed and colleagues conducted a prospective study to compare time to adequate sedation in agitated patients in the ED. In the trial, 43 patients received droperidol 5 mg plus midazolam 5 mg, and 43 patients received haloperidol plus lorazepam 2 mg. The average age of the patients in the droperidol/midazolam group was 34 years; the average age of the patients in the haloperidol/lorazepam group was 38 years. Baseline demographics, including height, weight, body mass index, and baseline Sedation Assessment Tool (SAT) scores, were similar between the groups.
The SAT score scale ranges from +3 (combative, violent, or out of control) to –3 (no response to stimulation); zero indicates being awake and calm/cooperative. The median baseline SAT score was 3 for both treatment groups.
The primary outcome was the proportion of patients with adequate sedation (defined as SAT scores of ≤0) 10 min after treatment.
Significantly more patients in the droperidol/midazolam group met this outcome, compared with the patients in the haloperidol/lorazepam group (51.2% vs. 7%). Also, significantly more patients in the droperidol/midazolam group achieved adequate sedation at 5, 10, 15, and 30 min than in the haloperidol/lorazepam group.
Fewer patients in the haloperidol/lorazepam group required supplemental oxygen, compared with the droperidol/midazolam group (9.3% vs. 25.6%). However, none of the droperidol/midazolam patients required rescue sedation, compared with 16.3% of the haloperidol/lorazepam patients, Dr. Javed noted. None of the patients required endotracheal intubation or experienced extrapyramidal symptoms, she said.
The study was limited by the small sample size and inclusion of data from only a single center.
The results suggest that droperidol/midazolam is superior to intramuscular haloperidol/lorazepam for producing adequate sedation after 10 min in agitated patients, Dr. Javed concluded.
The study received no outside funding. The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a study involving 86 adult patients at a single tertiary medical care center.
Patients with acute agitation present significant safety concerns in the emergency department, according to Jessica Javed, MD, of the University of Louisville (Ky.) and colleagues.
A combination of haloperidol and lorazepam has been widely used to curb agitation in these patients, but droperidol and midazolam could be more effective, owing to faster onset of action, Dr. Javed noted in a presentation at the annual meeting of the American College of Emergency Physicians.
Dr. Javed and colleagues conducted a prospective study to compare time to adequate sedation in agitated patients in the ED. In the trial, 43 patients received droperidol 5 mg plus midazolam 5 mg, and 43 patients received haloperidol plus lorazepam 2 mg. The average age of the patients in the droperidol/midazolam group was 34 years; the average age of the patients in the haloperidol/lorazepam group was 38 years. Baseline demographics, including height, weight, body mass index, and baseline Sedation Assessment Tool (SAT) scores, were similar between the groups.
The SAT score scale ranges from +3 (combative, violent, or out of control) to –3 (no response to stimulation); zero indicates being awake and calm/cooperative. The median baseline SAT score was 3 for both treatment groups.
The primary outcome was the proportion of patients with adequate sedation (defined as SAT scores of ≤0) 10 min after treatment.
Significantly more patients in the droperidol/midazolam group met this outcome, compared with the patients in the haloperidol/lorazepam group (51.2% vs. 7%). Also, significantly more patients in the droperidol/midazolam group achieved adequate sedation at 5, 10, 15, and 30 min than in the haloperidol/lorazepam group.
Fewer patients in the haloperidol/lorazepam group required supplemental oxygen, compared with the droperidol/midazolam group (9.3% vs. 25.6%). However, none of the droperidol/midazolam patients required rescue sedation, compared with 16.3% of the haloperidol/lorazepam patients, Dr. Javed noted. None of the patients required endotracheal intubation or experienced extrapyramidal symptoms, she said.
The study was limited by the small sample size and inclusion of data from only a single center.
The results suggest that droperidol/midazolam is superior to intramuscular haloperidol/lorazepam for producing adequate sedation after 10 min in agitated patients, Dr. Javed concluded.
The study received no outside funding. The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ASNC rejects new chest pain guideline it helped create
It was Oct. 28 when the two big North American cardiology societies issued a joint practice guideline on evaluating and managing chest pain that was endorsed by five other subspecialty groups. The next day, another group that had taken part in the document’s genesis explained why it wasn’t one of those five.
Although the American Society of Nuclear Cardiology (ASNC) was “actively engaged at every stage of the guideline-writing and review process,” the society “could not endorse the guideline,” the society announced in a statement released to clinicians and the media. The most prominent cited reason: It doesn’t adequately “support the principle of Patient First Imaging.”
The guideline was published in Circulation and the Journal of the American College of Cardiology, flagship journals of the American Heart Association and American College of Cardiology, respectively.
The document notes at least two clinicians represented ASNC as peer reviewers, and another was on the writing committee, but the organization does not appear in the list of societies endorsing the document.
“We believe that the document fails to provide unbiased guidance to health care professionals on the optimal evaluation of patients with chest pain,” contends an editorial ASNC board members have scheduled for the Jan. 10 issue of the Journal of Nuclear Medicine but is available now on an open-access preprint server.
“Despite the many important and helpful recommendations in the new guideline, there are several recommendations that we could not support,” it states.
“The ASNC board of directors reviewed the document twice during the endorsement process,” and the society “offered substantive comments after the first endorsement review, several of which were addressed,” Randall C. Thompson, MD, St. Luke’s Mid America Heart Institute and University of Missouri–Kansas City, said in an interview.
“However, some of the board’s concerns went unresolved. It was after the board’s second review, when the document had been declared finalized, that they voted not to endorse,” said Dr. Thompson, who is ASNC president.
“When we gather multiple organizations together to review and summarize the evidence, we work collaboratively to interpret the extensive catalog of peer-reviewed, published literature and create clinical practice recommendations,” Guideline Writing Committee Chair Martha Gulati, MD, University of Arizona, Phoenix, told this news organization in a prepared statement.
“The ASNC had a representative on the writing committee who is a coauthor on the paper and actively participated throughout the writing process the past 4 years,” she said. “The final guideline reflects the latest evidence-based recommendations for the evaluation and diagnosis of chest pain, as agreed by the seven endorsing organizations.”
The document does not clearly note that an ASNC representative was on the writing committee. However, ASNC confirmed that Renee Bullock-Palmer, MD, Deborah Heart and Lung Center, Browns Mills, N.J., is a fellow of the ASNC and had represented the group as one of the coauthors. Two “official reviewers” of the document, however, are listed as ASNC representatives.
Points of contention
“The decision about which test to order can be a nuanced one, and cardiac imaging tests tend to be complementary,” elaborates the editorial on the issue of patient-centered management.
Careful patient selection for different tests is important, “and physician and technical local expertise, availability, quality of equipment, and patient preference are extremely important factors to consider. There is not enough emphasis on this important point,” contend the authors. “This is an important limitation of the guideline.”
Other issues of concern include “lack of balance in the document’s presentation of the science on FFR-CT [fractional flow reserve assessment with computed tomography] and its inappropriately prominent endorsement,” the editorial states.
The U.S. Food and Drug Administration–recognized “limitations and contraindications” to FFR-CT tend to be glossed over in the document, Dr. Thompson said. And most ASNC board members were “concerned with the prominent location of the recommendations for FFR-CT in various tables – especially since there was minimal-to-no discussion of the fact that it is currently provided by only one company, that it is not widely available nor covered routinely by health insurance carriers, and [that] the accuracy in the most relevant population is disputed.”
In other concerns, the document “inadequately discusses the benefit” of combining coronary artery calcium (CAC) scores with functional testing, which ASNC said it supports. For example, adding CAC scores to myocardial perfusion imaging improves its diagnostic accuracy and prognostic power.
Functional vs. anatomic testing?
Moreover, “it is no longer appropriate to bundle all types of stress testing together. All stress imaging tests have their unique advantages and limitations.” Yet, “the concept of the dichotomy of functional testing versus anatomic testing is a common theme in the guideline in many important patient groups,” the editorial states. That could overemphasize CT angiography and thus “blur distinction between different types of functional tests.”
Such concerns about “imbalance” in the portrayals of the two kinds of tests were “amplified by the problem of health insurance companies and radiologic benefits managers inappropriately substituting a test that was ordered by a physician with a different test,” Dr. Thompson elaborated. “There is the impression that some of them ‘cherry-pick’ certain guidelines and that this practice is harmful to patients.”
The ASNC currently does not plan its own corresponding guideline, he said. But the editorial says that “over the coming weeks and months ASNC will offer a series of webinars and other programs that address specific patient populations and dilemmas.” Also, “we will enhance our focus on programs to address quality and efficiency to support a patient-first approach to imaging.”
The five subspecialty groups that have endorsed the document are the American Society of Echocardiography, American College of Chest Physicians, Society for Academic Emergency Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance.
Dr. Thompson has reported no relevant financial relationships. Statements of disclosure for the other editorial writers are listed in the publication.
A version of this article first appeared on Medscape.com.
It was Oct. 28 when the two big North American cardiology societies issued a joint practice guideline on evaluating and managing chest pain that was endorsed by five other subspecialty groups. The next day, another group that had taken part in the document’s genesis explained why it wasn’t one of those five.
Although the American Society of Nuclear Cardiology (ASNC) was “actively engaged at every stage of the guideline-writing and review process,” the society “could not endorse the guideline,” the society announced in a statement released to clinicians and the media. The most prominent cited reason: It doesn’t adequately “support the principle of Patient First Imaging.”
The guideline was published in Circulation and the Journal of the American College of Cardiology, flagship journals of the American Heart Association and American College of Cardiology, respectively.
The document notes at least two clinicians represented ASNC as peer reviewers, and another was on the writing committee, but the organization does not appear in the list of societies endorsing the document.
“We believe that the document fails to provide unbiased guidance to health care professionals on the optimal evaluation of patients with chest pain,” contends an editorial ASNC board members have scheduled for the Jan. 10 issue of the Journal of Nuclear Medicine but is available now on an open-access preprint server.
“Despite the many important and helpful recommendations in the new guideline, there are several recommendations that we could not support,” it states.
“The ASNC board of directors reviewed the document twice during the endorsement process,” and the society “offered substantive comments after the first endorsement review, several of which were addressed,” Randall C. Thompson, MD, St. Luke’s Mid America Heart Institute and University of Missouri–Kansas City, said in an interview.
“However, some of the board’s concerns went unresolved. It was after the board’s second review, when the document had been declared finalized, that they voted not to endorse,” said Dr. Thompson, who is ASNC president.
“When we gather multiple organizations together to review and summarize the evidence, we work collaboratively to interpret the extensive catalog of peer-reviewed, published literature and create clinical practice recommendations,” Guideline Writing Committee Chair Martha Gulati, MD, University of Arizona, Phoenix, told this news organization in a prepared statement.
“The ASNC had a representative on the writing committee who is a coauthor on the paper and actively participated throughout the writing process the past 4 years,” she said. “The final guideline reflects the latest evidence-based recommendations for the evaluation and diagnosis of chest pain, as agreed by the seven endorsing organizations.”
The document does not clearly note that an ASNC representative was on the writing committee. However, ASNC confirmed that Renee Bullock-Palmer, MD, Deborah Heart and Lung Center, Browns Mills, N.J., is a fellow of the ASNC and had represented the group as one of the coauthors. Two “official reviewers” of the document, however, are listed as ASNC representatives.
Points of contention
“The decision about which test to order can be a nuanced one, and cardiac imaging tests tend to be complementary,” elaborates the editorial on the issue of patient-centered management.
Careful patient selection for different tests is important, “and physician and technical local expertise, availability, quality of equipment, and patient preference are extremely important factors to consider. There is not enough emphasis on this important point,” contend the authors. “This is an important limitation of the guideline.”
Other issues of concern include “lack of balance in the document’s presentation of the science on FFR-CT [fractional flow reserve assessment with computed tomography] and its inappropriately prominent endorsement,” the editorial states.
The U.S. Food and Drug Administration–recognized “limitations and contraindications” to FFR-CT tend to be glossed over in the document, Dr. Thompson said. And most ASNC board members were “concerned with the prominent location of the recommendations for FFR-CT in various tables – especially since there was minimal-to-no discussion of the fact that it is currently provided by only one company, that it is not widely available nor covered routinely by health insurance carriers, and [that] the accuracy in the most relevant population is disputed.”
In other concerns, the document “inadequately discusses the benefit” of combining coronary artery calcium (CAC) scores with functional testing, which ASNC said it supports. For example, adding CAC scores to myocardial perfusion imaging improves its diagnostic accuracy and prognostic power.
Functional vs. anatomic testing?
Moreover, “it is no longer appropriate to bundle all types of stress testing together. All stress imaging tests have their unique advantages and limitations.” Yet, “the concept of the dichotomy of functional testing versus anatomic testing is a common theme in the guideline in many important patient groups,” the editorial states. That could overemphasize CT angiography and thus “blur distinction between different types of functional tests.”
Such concerns about “imbalance” in the portrayals of the two kinds of tests were “amplified by the problem of health insurance companies and radiologic benefits managers inappropriately substituting a test that was ordered by a physician with a different test,” Dr. Thompson elaborated. “There is the impression that some of them ‘cherry-pick’ certain guidelines and that this practice is harmful to patients.”
The ASNC currently does not plan its own corresponding guideline, he said. But the editorial says that “over the coming weeks and months ASNC will offer a series of webinars and other programs that address specific patient populations and dilemmas.” Also, “we will enhance our focus on programs to address quality and efficiency to support a patient-first approach to imaging.”
The five subspecialty groups that have endorsed the document are the American Society of Echocardiography, American College of Chest Physicians, Society for Academic Emergency Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance.
Dr. Thompson has reported no relevant financial relationships. Statements of disclosure for the other editorial writers are listed in the publication.
A version of this article first appeared on Medscape.com.
It was Oct. 28 when the two big North American cardiology societies issued a joint practice guideline on evaluating and managing chest pain that was endorsed by five other subspecialty groups. The next day, another group that had taken part in the document’s genesis explained why it wasn’t one of those five.
Although the American Society of Nuclear Cardiology (ASNC) was “actively engaged at every stage of the guideline-writing and review process,” the society “could not endorse the guideline,” the society announced in a statement released to clinicians and the media. The most prominent cited reason: It doesn’t adequately “support the principle of Patient First Imaging.”
The guideline was published in Circulation and the Journal of the American College of Cardiology, flagship journals of the American Heart Association and American College of Cardiology, respectively.
The document notes at least two clinicians represented ASNC as peer reviewers, and another was on the writing committee, but the organization does not appear in the list of societies endorsing the document.
“We believe that the document fails to provide unbiased guidance to health care professionals on the optimal evaluation of patients with chest pain,” contends an editorial ASNC board members have scheduled for the Jan. 10 issue of the Journal of Nuclear Medicine but is available now on an open-access preprint server.
“Despite the many important and helpful recommendations in the new guideline, there are several recommendations that we could not support,” it states.
“The ASNC board of directors reviewed the document twice during the endorsement process,” and the society “offered substantive comments after the first endorsement review, several of which were addressed,” Randall C. Thompson, MD, St. Luke’s Mid America Heart Institute and University of Missouri–Kansas City, said in an interview.
“However, some of the board’s concerns went unresolved. It was after the board’s second review, when the document had been declared finalized, that they voted not to endorse,” said Dr. Thompson, who is ASNC president.
“When we gather multiple organizations together to review and summarize the evidence, we work collaboratively to interpret the extensive catalog of peer-reviewed, published literature and create clinical practice recommendations,” Guideline Writing Committee Chair Martha Gulati, MD, University of Arizona, Phoenix, told this news organization in a prepared statement.
“The ASNC had a representative on the writing committee who is a coauthor on the paper and actively participated throughout the writing process the past 4 years,” she said. “The final guideline reflects the latest evidence-based recommendations for the evaluation and diagnosis of chest pain, as agreed by the seven endorsing organizations.”
The document does not clearly note that an ASNC representative was on the writing committee. However, ASNC confirmed that Renee Bullock-Palmer, MD, Deborah Heart and Lung Center, Browns Mills, N.J., is a fellow of the ASNC and had represented the group as one of the coauthors. Two “official reviewers” of the document, however, are listed as ASNC representatives.
Points of contention
“The decision about which test to order can be a nuanced one, and cardiac imaging tests tend to be complementary,” elaborates the editorial on the issue of patient-centered management.
Careful patient selection for different tests is important, “and physician and technical local expertise, availability, quality of equipment, and patient preference are extremely important factors to consider. There is not enough emphasis on this important point,” contend the authors. “This is an important limitation of the guideline.”
Other issues of concern include “lack of balance in the document’s presentation of the science on FFR-CT [fractional flow reserve assessment with computed tomography] and its inappropriately prominent endorsement,” the editorial states.
The U.S. Food and Drug Administration–recognized “limitations and contraindications” to FFR-CT tend to be glossed over in the document, Dr. Thompson said. And most ASNC board members were “concerned with the prominent location of the recommendations for FFR-CT in various tables – especially since there was minimal-to-no discussion of the fact that it is currently provided by only one company, that it is not widely available nor covered routinely by health insurance carriers, and [that] the accuracy in the most relevant population is disputed.”
In other concerns, the document “inadequately discusses the benefit” of combining coronary artery calcium (CAC) scores with functional testing, which ASNC said it supports. For example, adding CAC scores to myocardial perfusion imaging improves its diagnostic accuracy and prognostic power.
Functional vs. anatomic testing?
Moreover, “it is no longer appropriate to bundle all types of stress testing together. All stress imaging tests have their unique advantages and limitations.” Yet, “the concept of the dichotomy of functional testing versus anatomic testing is a common theme in the guideline in many important patient groups,” the editorial states. That could overemphasize CT angiography and thus “blur distinction between different types of functional tests.”
Such concerns about “imbalance” in the portrayals of the two kinds of tests were “amplified by the problem of health insurance companies and radiologic benefits managers inappropriately substituting a test that was ordered by a physician with a different test,” Dr. Thompson elaborated. “There is the impression that some of them ‘cherry-pick’ certain guidelines and that this practice is harmful to patients.”
The ASNC currently does not plan its own corresponding guideline, he said. But the editorial says that “over the coming weeks and months ASNC will offer a series of webinars and other programs that address specific patient populations and dilemmas.” Also, “we will enhance our focus on programs to address quality and efficiency to support a patient-first approach to imaging.”
The five subspecialty groups that have endorsed the document are the American Society of Echocardiography, American College of Chest Physicians, Society for Academic Emergency Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance.
Dr. Thompson has reported no relevant financial relationships. Statements of disclosure for the other editorial writers are listed in the publication.
A version of this article first appeared on Medscape.com.
COVID-19 vaccines provide 5 times the protection of natural immunity, CDC study says
, according to a new study published recently in the CDC’s Morbidity and Mortality Weekly Report.
The research team concluded that vaccination can provide a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least six months.
“We now have additional evidence that reaffirms the importance of COVID-19 vaccines, even if you have had prior infection,” Rochelle Walensky, MD, director of the CDC, said in a statement.
“This study adds more to the body of knowledge demonstrating the protection of vaccines against severe disease from COVID-19,” she said. “The best way to stop COVID-19, including the emergence of variants, is with widespread COVID-19 vaccination and with disease prevention actions such as mask wearing, washing hands often, physical distancing and staying home when sick.”
Researchers looked at data from the VISION Network, which included more than 201,000 hospitalizations for COVID-like illness at 187 hospitals across nine states between Jan. 1 to Sept. 2. Among those, more than 94,000 had rapid testing for the coronavirus, and 7,300 had a lab-confirmed test for COVID-19.
The research team found that unvaccinated people with a prior infection within 3 to 6 months were about 5-1/2 times more likely to have laboratory-confirmed COVID-19 than those who were fully vaccinated within 3 to 6 months with the Pfizer or Moderna shots. They found similar results when looking at the months that the Delta variant was the dominant strain of the coronavirus.
Protection from the Moderna vaccine “appeared to be higher” than for the Pfizer vaccine, the study authors wrote. The boost in protection also “trended higher” among older adults, as compared to those under age 65.
Importantly, the research team noted, these estimates may change over time as immunity wanes. Future studies should consider infection-induced and vaccine-induced immunity as time passes during the pandemic, they wrote.
Additional research is also needed for the Johnson & Johnson vaccine, they wrote. Those who have received the Johnson & Johnson vaccine are currently recommended to receive a booster shot at least two months after the first shot.
Overall, “all eligible persons should be vaccinated against COVID-19 as soon as possible, including unvaccinated persons previously infected,” the research team concluded.
A version of this article first appeared on WebMD.com.
, according to a new study published recently in the CDC’s Morbidity and Mortality Weekly Report.
The research team concluded that vaccination can provide a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least six months.
“We now have additional evidence that reaffirms the importance of COVID-19 vaccines, even if you have had prior infection,” Rochelle Walensky, MD, director of the CDC, said in a statement.
“This study adds more to the body of knowledge demonstrating the protection of vaccines against severe disease from COVID-19,” she said. “The best way to stop COVID-19, including the emergence of variants, is with widespread COVID-19 vaccination and with disease prevention actions such as mask wearing, washing hands often, physical distancing and staying home when sick.”
Researchers looked at data from the VISION Network, which included more than 201,000 hospitalizations for COVID-like illness at 187 hospitals across nine states between Jan. 1 to Sept. 2. Among those, more than 94,000 had rapid testing for the coronavirus, and 7,300 had a lab-confirmed test for COVID-19.
The research team found that unvaccinated people with a prior infection within 3 to 6 months were about 5-1/2 times more likely to have laboratory-confirmed COVID-19 than those who were fully vaccinated within 3 to 6 months with the Pfizer or Moderna shots. They found similar results when looking at the months that the Delta variant was the dominant strain of the coronavirus.
Protection from the Moderna vaccine “appeared to be higher” than for the Pfizer vaccine, the study authors wrote. The boost in protection also “trended higher” among older adults, as compared to those under age 65.
Importantly, the research team noted, these estimates may change over time as immunity wanes. Future studies should consider infection-induced and vaccine-induced immunity as time passes during the pandemic, they wrote.
Additional research is also needed for the Johnson & Johnson vaccine, they wrote. Those who have received the Johnson & Johnson vaccine are currently recommended to receive a booster shot at least two months after the first shot.
Overall, “all eligible persons should be vaccinated against COVID-19 as soon as possible, including unvaccinated persons previously infected,” the research team concluded.
A version of this article first appeared on WebMD.com.
, according to a new study published recently in the CDC’s Morbidity and Mortality Weekly Report.
The research team concluded that vaccination can provide a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least six months.
“We now have additional evidence that reaffirms the importance of COVID-19 vaccines, even if you have had prior infection,” Rochelle Walensky, MD, director of the CDC, said in a statement.
“This study adds more to the body of knowledge demonstrating the protection of vaccines against severe disease from COVID-19,” she said. “The best way to stop COVID-19, including the emergence of variants, is with widespread COVID-19 vaccination and with disease prevention actions such as mask wearing, washing hands often, physical distancing and staying home when sick.”
Researchers looked at data from the VISION Network, which included more than 201,000 hospitalizations for COVID-like illness at 187 hospitals across nine states between Jan. 1 to Sept. 2. Among those, more than 94,000 had rapid testing for the coronavirus, and 7,300 had a lab-confirmed test for COVID-19.
The research team found that unvaccinated people with a prior infection within 3 to 6 months were about 5-1/2 times more likely to have laboratory-confirmed COVID-19 than those who were fully vaccinated within 3 to 6 months with the Pfizer or Moderna shots. They found similar results when looking at the months that the Delta variant was the dominant strain of the coronavirus.
Protection from the Moderna vaccine “appeared to be higher” than for the Pfizer vaccine, the study authors wrote. The boost in protection also “trended higher” among older adults, as compared to those under age 65.
Importantly, the research team noted, these estimates may change over time as immunity wanes. Future studies should consider infection-induced and vaccine-induced immunity as time passes during the pandemic, they wrote.
Additional research is also needed for the Johnson & Johnson vaccine, they wrote. Those who have received the Johnson & Johnson vaccine are currently recommended to receive a booster shot at least two months after the first shot.
Overall, “all eligible persons should be vaccinated against COVID-19 as soon as possible, including unvaccinated persons previously infected,” the research team concluded.
A version of this article first appeared on WebMD.com.