User login
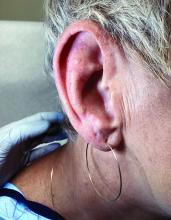
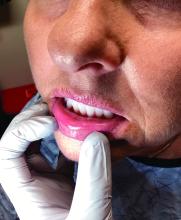
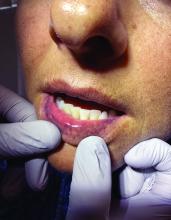
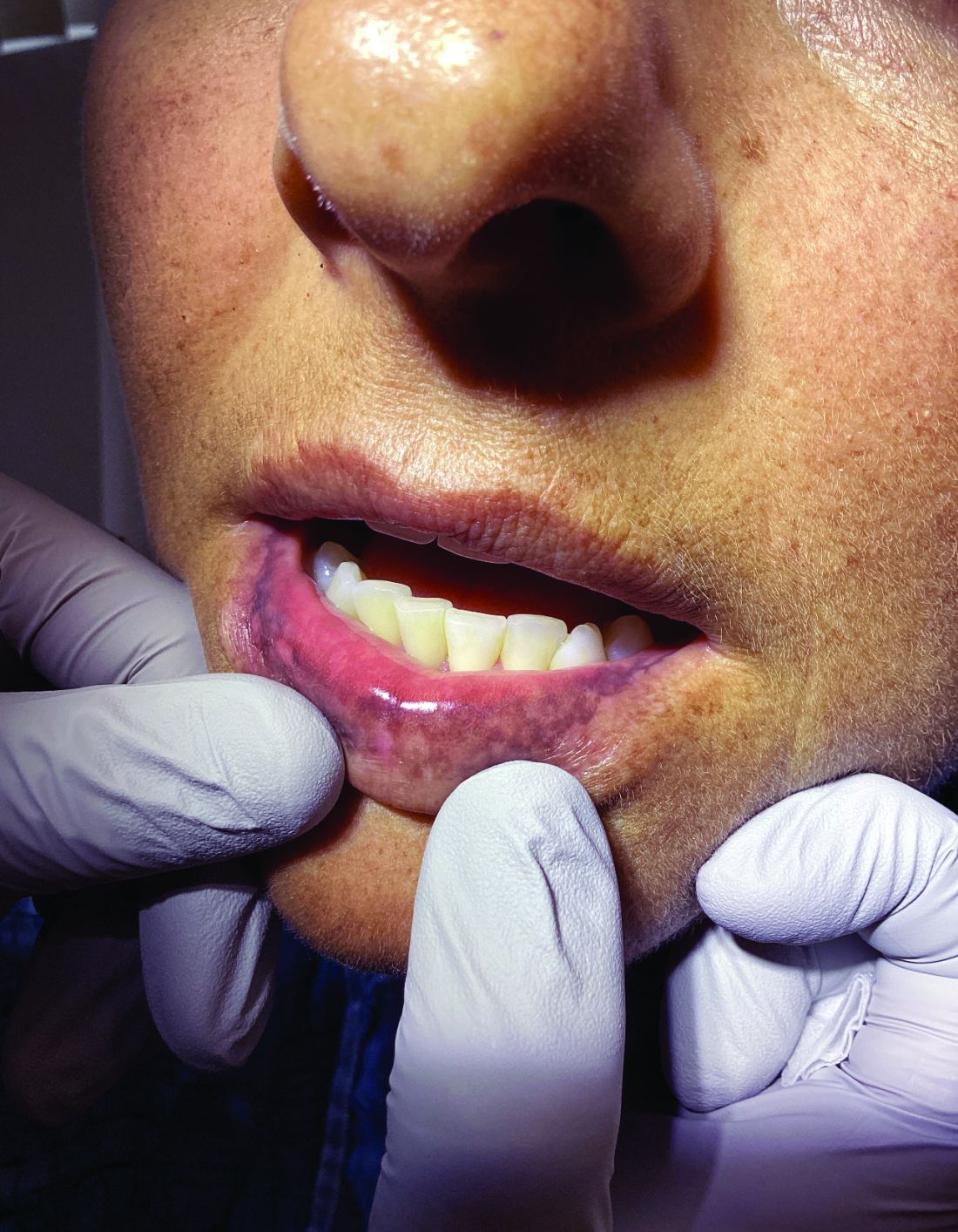
A 51-year-old woman presented for a routine full body skin exam after vacationing in Hawaii.
Primary adrenal insufficiency (Addison’s disease) results from a dysfunction of the adrenal glands, which may be secondary to autoimmune diseases, genetic conditions, infections, and vasculopathies,or may be drug-induced (e.g. checkpoint inhibitors), among others . In contrast, secondary adrenal insufficiency results from pituitary dysfunction of low adrenocorticotropic hormone (ACTH). The most common cause of primary adrenal insufficiency in developed countries is autoimmune adrenalitis, which accounts for upwards of 90% of cases. Typically, 21-hydroxylase autoantibodies are identified and account for destruction of the adrenal cortex through cell-mediated and humoral immune responses.
Palmar creases, subungual surfaces, sites of trauma, and joint spaces (including the knees, spine, elbows, and shoulders) are commonly affected. Hair depletes in the pubic area and axillary vaults. Nevi may also appear darker. In patients with autoimmune adrenalitis, vitiligo may be seen secondary to autoimmune destruction of melanocytes.
Diagnosis may be difficult in the early stages, but historical findings of fatigue and clinical findings of hyperpigmentation in classic areas may prompt appropriate lab screening workup. It is essential to determine whether adrenal insufficiency is primary or secondary. Evaluation of decreased cortisol production, determination of whether production is ACTH-dependent or -independent, and evaluation for the underlying causes of adrenal dysfunction are important. Lab screening includes morning serum cortisol, morning ACTH (cosyntropin) stimulation test, fasting CBC with differential, and CMP to evaluate for normocytic normochromic anemia, hyponatremia, hyperkalemia, hypoglycemia, plasma renin/aldosterone ratio, and 21-hydroxylase autoantibodies.
Management strategies of primary adrenal insufficiency require corticosteroid supplementation and multidisciplinary collaboration with endocrinology. If untreated, primary adrenal insufficiency can be fatal. Adrenal crisis is a critical condition following a precipitating event, such as GI infection, fever, acute stress, and/or untreated adrenal or pituitary disorders. Clinical findings include acute shock with hypotension, nausea, vomiting, abdominal pain, back or leg pain, and a change in mental status. In this scenario, increasing the dose of corticosteroid supplementation is essential for reducing mortality.
Upon examining this patient’s new skin findings of hyperpigmentation and discussing her fatigue, primary adrenal insufficiency was suspected. With further prompting, the patient reported an ICU hospitalization several months prior because of sepsis originating from a peritonsillar abscess. With these clinical and historical findings, preliminary workup was conducted by dermatology, which included morning cortisol level, ACTH, CBC with differential, CMP, plasma renin-aldosterone ratio, and 21-hydroxylase autoantibodies. Work up demonstrated a low morning cortisol level of 1.3 mcg/dL, an elevated ACTH of 2,739 pg/mL, and positive 21-hydroxylase autoantibodies. The patient was urgently referred to endocrinology and started on oral hydrocortisone. Her fatigue immediately improved, and at 1-year follow-up with dermatology, her mucocutaneous hyperpigmentation had subsided dramatically.
Dermatologists can play a major role in the early diagnosis of primary adrenal insufficiency, which is essential for reducing patient morbidity and mortality. Skin findings on full body skin exams can clue in dermatologists for ordering preliminary workup to expedite care for these patients.
The case and photos were submitted by Dr. Akhiyat, Scripps Clinic Medical Group, La Jolla, California. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
J Am Acad Dermatol. 2014 May;70(5):Supplement 1AB118. doi: 10.1016/j.jaad.2014.01.491.
Michels A, Michels N. Am Fam Physician. 2014 Apr 1;89(7):563-568.
Kauzman A et al. J Can Dent Assoc. 2004 Nov;70(10):682-683.
Primary adrenal insufficiency (Addison’s disease) results from a dysfunction of the adrenal glands, which may be secondary to autoimmune diseases, genetic conditions, infections, and vasculopathies,or may be drug-induced (e.g. checkpoint inhibitors), among others . In contrast, secondary adrenal insufficiency results from pituitary dysfunction of low adrenocorticotropic hormone (ACTH). The most common cause of primary adrenal insufficiency in developed countries is autoimmune adrenalitis, which accounts for upwards of 90% of cases. Typically, 21-hydroxylase autoantibodies are identified and account for destruction of the adrenal cortex through cell-mediated and humoral immune responses.
Palmar creases, subungual surfaces, sites of trauma, and joint spaces (including the knees, spine, elbows, and shoulders) are commonly affected. Hair depletes in the pubic area and axillary vaults. Nevi may also appear darker. In patients with autoimmune adrenalitis, vitiligo may be seen secondary to autoimmune destruction of melanocytes.
Diagnosis may be difficult in the early stages, but historical findings of fatigue and clinical findings of hyperpigmentation in classic areas may prompt appropriate lab screening workup. It is essential to determine whether adrenal insufficiency is primary or secondary. Evaluation of decreased cortisol production, determination of whether production is ACTH-dependent or -independent, and evaluation for the underlying causes of adrenal dysfunction are important. Lab screening includes morning serum cortisol, morning ACTH (cosyntropin) stimulation test, fasting CBC with differential, and CMP to evaluate for normocytic normochromic anemia, hyponatremia, hyperkalemia, hypoglycemia, plasma renin/aldosterone ratio, and 21-hydroxylase autoantibodies.
Management strategies of primary adrenal insufficiency require corticosteroid supplementation and multidisciplinary collaboration with endocrinology. If untreated, primary adrenal insufficiency can be fatal. Adrenal crisis is a critical condition following a precipitating event, such as GI infection, fever, acute stress, and/or untreated adrenal or pituitary disorders. Clinical findings include acute shock with hypotension, nausea, vomiting, abdominal pain, back or leg pain, and a change in mental status. In this scenario, increasing the dose of corticosteroid supplementation is essential for reducing mortality.
Upon examining this patient’s new skin findings of hyperpigmentation and discussing her fatigue, primary adrenal insufficiency was suspected. With further prompting, the patient reported an ICU hospitalization several months prior because of sepsis originating from a peritonsillar abscess. With these clinical and historical findings, preliminary workup was conducted by dermatology, which included morning cortisol level, ACTH, CBC with differential, CMP, plasma renin-aldosterone ratio, and 21-hydroxylase autoantibodies. Work up demonstrated a low morning cortisol level of 1.3 mcg/dL, an elevated ACTH of 2,739 pg/mL, and positive 21-hydroxylase autoantibodies. The patient was urgently referred to endocrinology and started on oral hydrocortisone. Her fatigue immediately improved, and at 1-year follow-up with dermatology, her mucocutaneous hyperpigmentation had subsided dramatically.
Dermatologists can play a major role in the early diagnosis of primary adrenal insufficiency, which is essential for reducing patient morbidity and mortality. Skin findings on full body skin exams can clue in dermatologists for ordering preliminary workup to expedite care for these patients.
The case and photos were submitted by Dr. Akhiyat, Scripps Clinic Medical Group, La Jolla, California. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
J Am Acad Dermatol. 2014 May;70(5):Supplement 1AB118. doi: 10.1016/j.jaad.2014.01.491.
Michels A, Michels N. Am Fam Physician. 2014 Apr 1;89(7):563-568.
Kauzman A et al. J Can Dent Assoc. 2004 Nov;70(10):682-683.
Primary adrenal insufficiency (Addison’s disease) results from a dysfunction of the adrenal glands, which may be secondary to autoimmune diseases, genetic conditions, infections, and vasculopathies,or may be drug-induced (e.g. checkpoint inhibitors), among others . In contrast, secondary adrenal insufficiency results from pituitary dysfunction of low adrenocorticotropic hormone (ACTH). The most common cause of primary adrenal insufficiency in developed countries is autoimmune adrenalitis, which accounts for upwards of 90% of cases. Typically, 21-hydroxylase autoantibodies are identified and account for destruction of the adrenal cortex through cell-mediated and humoral immune responses.
Palmar creases, subungual surfaces, sites of trauma, and joint spaces (including the knees, spine, elbows, and shoulders) are commonly affected. Hair depletes in the pubic area and axillary vaults. Nevi may also appear darker. In patients with autoimmune adrenalitis, vitiligo may be seen secondary to autoimmune destruction of melanocytes.
Diagnosis may be difficult in the early stages, but historical findings of fatigue and clinical findings of hyperpigmentation in classic areas may prompt appropriate lab screening workup. It is essential to determine whether adrenal insufficiency is primary or secondary. Evaluation of decreased cortisol production, determination of whether production is ACTH-dependent or -independent, and evaluation for the underlying causes of adrenal dysfunction are important. Lab screening includes morning serum cortisol, morning ACTH (cosyntropin) stimulation test, fasting CBC with differential, and CMP to evaluate for normocytic normochromic anemia, hyponatremia, hyperkalemia, hypoglycemia, plasma renin/aldosterone ratio, and 21-hydroxylase autoantibodies.
Management strategies of primary adrenal insufficiency require corticosteroid supplementation and multidisciplinary collaboration with endocrinology. If untreated, primary adrenal insufficiency can be fatal. Adrenal crisis is a critical condition following a precipitating event, such as GI infection, fever, acute stress, and/or untreated adrenal or pituitary disorders. Clinical findings include acute shock with hypotension, nausea, vomiting, abdominal pain, back or leg pain, and a change in mental status. In this scenario, increasing the dose of corticosteroid supplementation is essential for reducing mortality.
Upon examining this patient’s new skin findings of hyperpigmentation and discussing her fatigue, primary adrenal insufficiency was suspected. With further prompting, the patient reported an ICU hospitalization several months prior because of sepsis originating from a peritonsillar abscess. With these clinical and historical findings, preliminary workup was conducted by dermatology, which included morning cortisol level, ACTH, CBC with differential, CMP, plasma renin-aldosterone ratio, and 21-hydroxylase autoantibodies. Work up demonstrated a low morning cortisol level of 1.3 mcg/dL, an elevated ACTH of 2,739 pg/mL, and positive 21-hydroxylase autoantibodies. The patient was urgently referred to endocrinology and started on oral hydrocortisone. Her fatigue immediately improved, and at 1-year follow-up with dermatology, her mucocutaneous hyperpigmentation had subsided dramatically.
Dermatologists can play a major role in the early diagnosis of primary adrenal insufficiency, which is essential for reducing patient morbidity and mortality. Skin findings on full body skin exams can clue in dermatologists for ordering preliminary workup to expedite care for these patients.
The case and photos were submitted by Dr. Akhiyat, Scripps Clinic Medical Group, La Jolla, California. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
J Am Acad Dermatol. 2014 May;70(5):Supplement 1AB118. doi: 10.1016/j.jaad.2014.01.491.
Michels A, Michels N. Am Fam Physician. 2014 Apr 1;89(7):563-568.
Kauzman A et al. J Can Dent Assoc. 2004 Nov;70(10):682-683.
How Old Are You? Stand on One Leg and I’ll Tell You
This transcript has been edited for clarity.
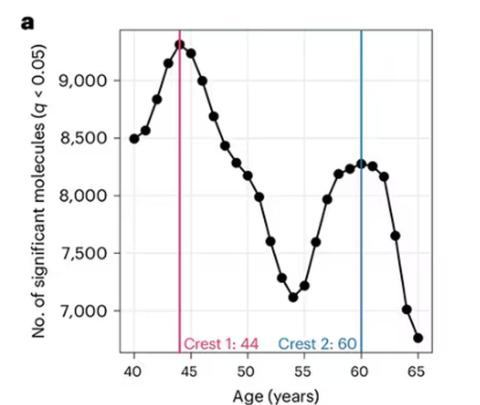
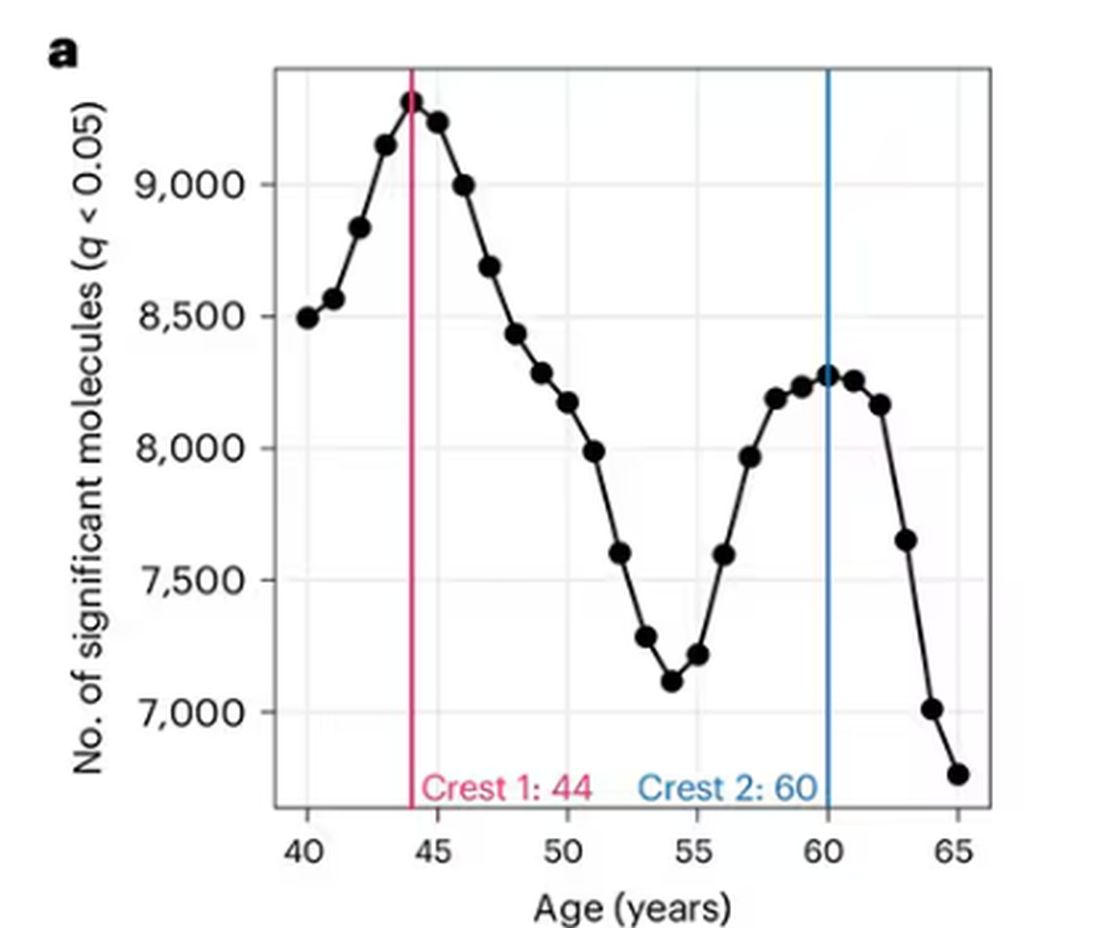
So I was lying in bed the other night, trying to read my phone, and started complaining to my wife about how my vision keeps getting worse, and then how stiff I feel when I wake up in the morning, and how a recent injury is taking too long to heal, and she said, “Well, yeah. You’re 44. That’s when things start to head downhill.”
And I was like, “Forty-four? That seems very specific. I thought 50 was what people complain about.” And she said, “No, it’s a thing — 44 years old and 60 years old. There’s a drop-off there.”
And you know what? She was right.
A study, “Nonlinear dynamics of multi-omics profiles during human aging,” published in Nature Aging in August 2024, analyzed a ton of proteins and metabolites in people of various ages and found, when you put it all together, that I should know better than to doubt my brilliant spouse.
But deep down, I believe the cliché that age is just a number. I don’t particularly care about being 44, or turning 50 or 60. I care about how my body and brain are aging. If I can be a happy, healthy, 80-year-old in full command of my faculties, I would consider that a major win no matter what the calendar says.
So I’m always interested in ways to quantify how my body is aging, independent of how many birthdays I have passed. And, according to a new study, there’s actually a really easy way to do this: Just stand on one leg.
The surprising results come from “Age-related changes in gait, balance, and strength parameters: A cross-sectional study,” appearing in PLOS One, which analyzed 40 individuals — half under age 65 and half over age 65 — across a variety of domains of strength, balance, and gait. The conceit of the study? We all know that things like strength and balance worsen over time, but what worsens fastest? What might be the best metric to tell us how our bodies are aging?
To that end, you have a variety of correlations between various metrics and calendar age.
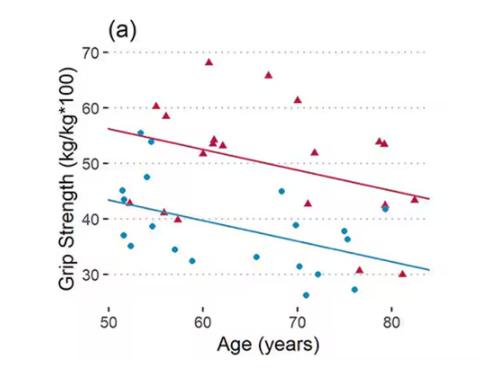
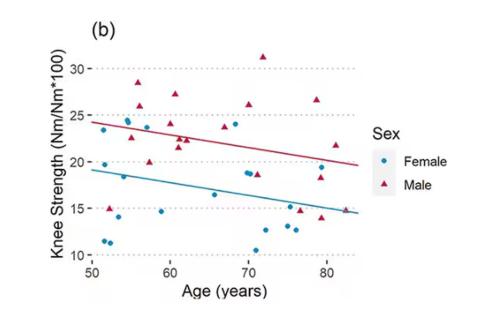
As age increases, grip strength goes down. Men (inexplicably in pink) have higher grip strength overall, and women (confusingly in blue) lower. Somewhat less strong correlations were seen for knee strength.
What about balance?
To assess this, the researchers had the participants stand on a pressure plate. In one scenario, they did this with eyes open, and the next with eyes closed. They then measured how much the pressure varied around the center of the individual on the plate — basically, how much the person swayed while they were standing there.
Sway increased as age increased. Sway increased a bit more with eyes closed than with eyes open.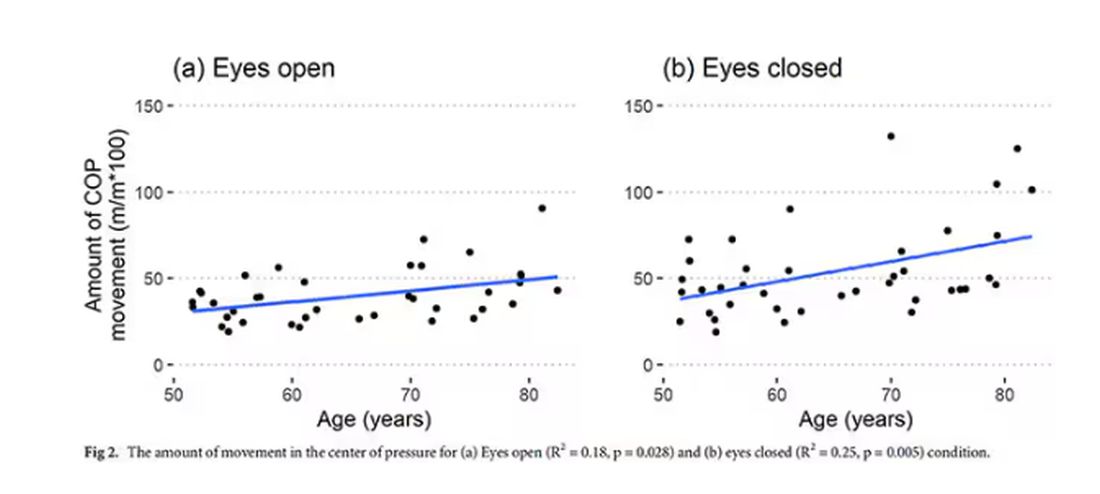
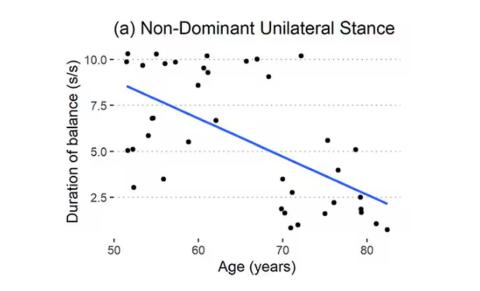
But the strongest correlation between any of these metrics and age was a simple one: How long can you stand on one leg?
Particularly for the nondominant leg, what you see here is a pretty dramatic drop-off in balance time around age 65, with younger people able to do 10 seconds with ease and some older people barely being able to make it to 2.
Of course, I had to try this for myself. And as I was standing around on one leg, it became clear to me exactly why this might be a good metric. It really integrates balance and strength in a way that the other tests don’t: balance, clearly, since you have to stay vertical over a relatively small base; but strength as well, because, well, one leg is holding up all the rest of you. You do feel it after a while.
So this metric passes the smell test to me, at least as a potential proxy for age-related physical decline.
But I should be careful to note that this was a cross-sectional study; the researchers looked at various people who were all different ages, not the same people over time to watch how these things change as they aged.
Also, the use of the correlation coefficient in graphs like this implies a certain linear relationship between age and standing-on-one-foot time. The raw data — the points on this graph — don’t appear that linear to me. As I mentioned above, it seems like there might be a bit of a sharp drop-off somewhere in the mid-60s. That means that we may not be able to use this as a sensitive test for aging that slowly changes as your body gets older. It might be that you’re able to essentially stand on one leg as long as you want until, one day, you can’t. That gives us less warning and less to act on.
And finally, we don’t know that changing this metric will change your health for the better. I’m sure a good physiatrist or physical therapist could design some exercises to increase any of our standing-on-one leg times. And no doubt, with practice, you could get your numbers way up. But that doesn’t necessarily mean you’re healthier. It’s like “teaching to the test”; you might score better on the standardized exam but you didn’t really learn the material.
So I am not adding one-leg standing to my daily exercise routine. But I won’t lie and tell you that, from time to time, and certainly on my 60th birthday, you may find me standing like a flamingo with a stopwatch in my hand.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
So I was lying in bed the other night, trying to read my phone, and started complaining to my wife about how my vision keeps getting worse, and then how stiff I feel when I wake up in the morning, and how a recent injury is taking too long to heal, and she said, “Well, yeah. You’re 44. That’s when things start to head downhill.”
And I was like, “Forty-four? That seems very specific. I thought 50 was what people complain about.” And she said, “No, it’s a thing — 44 years old and 60 years old. There’s a drop-off there.”
And you know what? She was right.
A study, “Nonlinear dynamics of multi-omics profiles during human aging,” published in Nature Aging in August 2024, analyzed a ton of proteins and metabolites in people of various ages and found, when you put it all together, that I should know better than to doubt my brilliant spouse.
But deep down, I believe the cliché that age is just a number. I don’t particularly care about being 44, or turning 50 or 60. I care about how my body and brain are aging. If I can be a happy, healthy, 80-year-old in full command of my faculties, I would consider that a major win no matter what the calendar says.
So I’m always interested in ways to quantify how my body is aging, independent of how many birthdays I have passed. And, according to a new study, there’s actually a really easy way to do this: Just stand on one leg.
The surprising results come from “Age-related changes in gait, balance, and strength parameters: A cross-sectional study,” appearing in PLOS One, which analyzed 40 individuals — half under age 65 and half over age 65 — across a variety of domains of strength, balance, and gait. The conceit of the study? We all know that things like strength and balance worsen over time, but what worsens fastest? What might be the best metric to tell us how our bodies are aging?
To that end, you have a variety of correlations between various metrics and calendar age.
As age increases, grip strength goes down. Men (inexplicably in pink) have higher grip strength overall, and women (confusingly in blue) lower. Somewhat less strong correlations were seen for knee strength.
What about balance?
To assess this, the researchers had the participants stand on a pressure plate. In one scenario, they did this with eyes open, and the next with eyes closed. They then measured how much the pressure varied around the center of the individual on the plate — basically, how much the person swayed while they were standing there.
Sway increased as age increased. Sway increased a bit more with eyes closed than with eyes open.
But the strongest correlation between any of these metrics and age was a simple one: How long can you stand on one leg?
Particularly for the nondominant leg, what you see here is a pretty dramatic drop-off in balance time around age 65, with younger people able to do 10 seconds with ease and some older people barely being able to make it to 2.
Of course, I had to try this for myself. And as I was standing around on one leg, it became clear to me exactly why this might be a good metric. It really integrates balance and strength in a way that the other tests don’t: balance, clearly, since you have to stay vertical over a relatively small base; but strength as well, because, well, one leg is holding up all the rest of you. You do feel it after a while.
So this metric passes the smell test to me, at least as a potential proxy for age-related physical decline.
But I should be careful to note that this was a cross-sectional study; the researchers looked at various people who were all different ages, not the same people over time to watch how these things change as they aged.
Also, the use of the correlation coefficient in graphs like this implies a certain linear relationship between age and standing-on-one-foot time. The raw data — the points on this graph — don’t appear that linear to me. As I mentioned above, it seems like there might be a bit of a sharp drop-off somewhere in the mid-60s. That means that we may not be able to use this as a sensitive test for aging that slowly changes as your body gets older. It might be that you’re able to essentially stand on one leg as long as you want until, one day, you can’t. That gives us less warning and less to act on.
And finally, we don’t know that changing this metric will change your health for the better. I’m sure a good physiatrist or physical therapist could design some exercises to increase any of our standing-on-one leg times. And no doubt, with practice, you could get your numbers way up. But that doesn’t necessarily mean you’re healthier. It’s like “teaching to the test”; you might score better on the standardized exam but you didn’t really learn the material.
So I am not adding one-leg standing to my daily exercise routine. But I won’t lie and tell you that, from time to time, and certainly on my 60th birthday, you may find me standing like a flamingo with a stopwatch in my hand.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
So I was lying in bed the other night, trying to read my phone, and started complaining to my wife about how my vision keeps getting worse, and then how stiff I feel when I wake up in the morning, and how a recent injury is taking too long to heal, and she said, “Well, yeah. You’re 44. That’s when things start to head downhill.”
And I was like, “Forty-four? That seems very specific. I thought 50 was what people complain about.” And she said, “No, it’s a thing — 44 years old and 60 years old. There’s a drop-off there.”
And you know what? She was right.
A study, “Nonlinear dynamics of multi-omics profiles during human aging,” published in Nature Aging in August 2024, analyzed a ton of proteins and metabolites in people of various ages and found, when you put it all together, that I should know better than to doubt my brilliant spouse.
But deep down, I believe the cliché that age is just a number. I don’t particularly care about being 44, or turning 50 or 60. I care about how my body and brain are aging. If I can be a happy, healthy, 80-year-old in full command of my faculties, I would consider that a major win no matter what the calendar says.
So I’m always interested in ways to quantify how my body is aging, independent of how many birthdays I have passed. And, according to a new study, there’s actually a really easy way to do this: Just stand on one leg.
The surprising results come from “Age-related changes in gait, balance, and strength parameters: A cross-sectional study,” appearing in PLOS One, which analyzed 40 individuals — half under age 65 and half over age 65 — across a variety of domains of strength, balance, and gait. The conceit of the study? We all know that things like strength and balance worsen over time, but what worsens fastest? What might be the best metric to tell us how our bodies are aging?
To that end, you have a variety of correlations between various metrics and calendar age.
As age increases, grip strength goes down. Men (inexplicably in pink) have higher grip strength overall, and women (confusingly in blue) lower. Somewhat less strong correlations were seen for knee strength.
What about balance?
To assess this, the researchers had the participants stand on a pressure plate. In one scenario, they did this with eyes open, and the next with eyes closed. They then measured how much the pressure varied around the center of the individual on the plate — basically, how much the person swayed while they were standing there.
Sway increased as age increased. Sway increased a bit more with eyes closed than with eyes open.
But the strongest correlation between any of these metrics and age was a simple one: How long can you stand on one leg?
Particularly for the nondominant leg, what you see here is a pretty dramatic drop-off in balance time around age 65, with younger people able to do 10 seconds with ease and some older people barely being able to make it to 2.
Of course, I had to try this for myself. And as I was standing around on one leg, it became clear to me exactly why this might be a good metric. It really integrates balance and strength in a way that the other tests don’t: balance, clearly, since you have to stay vertical over a relatively small base; but strength as well, because, well, one leg is holding up all the rest of you. You do feel it after a while.
So this metric passes the smell test to me, at least as a potential proxy for age-related physical decline.
But I should be careful to note that this was a cross-sectional study; the researchers looked at various people who were all different ages, not the same people over time to watch how these things change as they aged.
Also, the use of the correlation coefficient in graphs like this implies a certain linear relationship between age and standing-on-one-foot time. The raw data — the points on this graph — don’t appear that linear to me. As I mentioned above, it seems like there might be a bit of a sharp drop-off somewhere in the mid-60s. That means that we may not be able to use this as a sensitive test for aging that slowly changes as your body gets older. It might be that you’re able to essentially stand on one leg as long as you want until, one day, you can’t. That gives us less warning and less to act on.
And finally, we don’t know that changing this metric will change your health for the better. I’m sure a good physiatrist or physical therapist could design some exercises to increase any of our standing-on-one leg times. And no doubt, with practice, you could get your numbers way up. But that doesn’t necessarily mean you’re healthier. It’s like “teaching to the test”; you might score better on the standardized exam but you didn’t really learn the material.
So I am not adding one-leg standing to my daily exercise routine. But I won’t lie and tell you that, from time to time, and certainly on my 60th birthday, you may find me standing like a flamingo with a stopwatch in my hand.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
H pylori: ACG Guideline Advises New Approaches to Treatment
Helicobacter pylori is one of the most common human bacterial chronic infections globally. Its prevalence has actually decreased in North America in recent years, although its current range of approximately 30%-40% remains substantial given the potential clinical implications of infection.
Standards have changed considerably regarding the testing, treatment, and follow-up of H pylori. This is made clear by the just-published clinical practice guideline from the American College of Gastroenterology (ACG), which provides several new recommendations based on recent scientific evidence that should change your clinical approach to managing this common infection.
This discussion aims to synthesize and highlight key concepts from the ACG’s comprehensive publication.
Who Should Be Tested and Treated?
The cardinal diseases caused by H pylori have traditionally included peptic ulcer disease, marginal zone B-cell lymphoma, gastric adenocarcinoma, and dyspepsia.
Additional associations have been made with idiopathic thrombocytopenic purpura and otherwise unexplained iron deficiency.
New evidence suggests that patients taking long-term nonsteroidal anti-inflammatory drugs, including low-dose aspirin, are relatively more susceptible to infection.
The ACG’s guideline also recommends testing persons at an increased risk for gastric adenocarcinoma (eg, those with autoimmune gastritis, current or history of premalignant conditions, or first-degree relative with gastric cancer), as well as household members of patients with a positive nonserologic test for H pylori.
The authors note that those with an indication for testing should be offered treatment if determined to have an infection. These patients should also undergo a posttreatment test-of-cure, which should occur at least 4 weeks afterwards using a urea breath test, fecal antigen test, or gastric biopsy.
Caveats to Treatment
Patients with H pylori infections are advised to undergo treatment for a duration of 14 days. Some of the commercial prepackaged H pylori treatment options (eg, Pylera, which contains bismuth subcitrate/metronidazole/tetracycline) are dispensed in regimens lasting only 10 days and currently are viewed as inadequate.
In the United States, the patterns of antibiotic resistance for the previously used standard drugs in the treatment of H pylori have increased considerably. They range from 32% for clarithromycin, 38% for levofloxacin, and 42% for metronidazole, in contrast to 3% for amoxicillin, 1% for tetracycline, and 0% for rifabutin.
Clarithromycin- and levofloxacin-containing treatments should be avoided in treatment-naive patients unless specifically directed following the results of susceptibility tests with either a phenotypic method (culture-based) or a molecular method (polymerase chain reaction or next-generation sequencing). Notably, the mutations responsible for both clarithromycin and levofloxacin resistance may be detectable by stool-based testing.
Maintenance of intragastric acid suppression is key to H pylori eradication, as elevated intragastric pH promotes active replication of H pylori and makes it more susceptible to bactericidal antibiotics.
Therefore, the use of histamine-2 receptors is not recommended, as they are inadequate for achieving acid suppression. Instead, a dual-based therapy of either the potassium-competitive acid blocker (PCAB) vonoprazan (20 mg) or a high-dose proton pump inhibitor (PPI) and amoxicillin, administered twice daily, is effective, although this finding is based on limited evidence.
Treatment-Naive Patients
In treatment-naive patients without penicillin allergy and for whom antibiotic susceptibility testing has not been obtained, the guideline offers its strongest recommendation for bismuth quadruple therapy. This therapy typically consists of a PPI, bismuth subcitrate or subsalicylate, tetracycline, and metronidazole.
Among those with a penicillin allergy, bismuth quadruple therapy is also the primary treatment choice. The authors suggest that patients with a suspected allergy are referred to an allergist for possible penicillin desensitization, given that less than 1% of the population is thought to present with a “true” allergy.
The guideline also presented conditional recommendations, based on low- to moderate-quality evidence, for using a rifabutin-based triple regimen of omeprazole, amoxicillin, and rifabutin (Talicia); a PCAB-based dual regimen of vonoprazan and amoxicillin (Voquezna Dual Pak); and a PCAB-based triple regimen of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak). In patients with unknown clarithromycin susceptibility, the PCAB-based triple therapy is preferred over PPI-clarithromycin triple therapy.
Although probiotics have been suggested to possibly lead to increased effectiveness or tolerability for H pylori eradication, this was based on studies with significant heterogeneity in their designs. At present, no high-quality data support probiotic therapy.
Clinicians may substitute doxycycline for tetracycline due to availability or cost, and also may prescribe metronidazole at a lower dose than recommended (1.5-2 g/d) to limit side effects. Both modifications have been associated with lower rates of H pylori eradication and are not recommended.
Treatment-Experienced Patients
Quadruple bismuth therapy is the optimal approach among treatment-experienced patients with persistent H pylori infection who have not previously received this therapy. However, this recommendation was rated as conditional, given that it was based on a low quality of evidence.
The guideline offered other recommendations for treatment-experienced patients with persistent infection who had received bismuth quadruple therapy — also conditionally based on a low quality of evidence.
In such patients, it is recommended to consider the use of a rifabutin-based triple therapy (ie, a PPI standard to double dose, amoxicillin, and rifabutin) and a levofloxacin-based triple therapy (ie, a PPI standard dose, levofloxacin, and amoxicillin or metronidazole).
Although significant evidence gaps prevented the authors from providing formal recommendations, they included a PCAB-based triple therapy of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak) and a high-dose dual therapy of either vonoprazan (20 mg) or PPI (double dose) and amoxicillin among their suggested salvage regimens for these patients.
A New Standard
We must recognize, however, that there are still substantial evidence gaps, particularly around the use of a PCAB-based regimen and its relative advantages over a standard or high-dose PPI-based regimen. This may be of particular importance based on the variable prevalence of cytochrome P450 2C19 (CYP2C19) polymorphisms in the specific patient populations, as PCABs are not metabolized by CYP2C19.
Reviewing the entirety of the ACG’s clinical guideline is encouraged for additional details about the management of H pylori beyond what is highlighted herein.
Dr. Johnson, Professor of Medicine, Chief of Gastroenterology, Eastern Virginia Medical School, Norfolk, Virginia, disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Helicobacter pylori is one of the most common human bacterial chronic infections globally. Its prevalence has actually decreased in North America in recent years, although its current range of approximately 30%-40% remains substantial given the potential clinical implications of infection.
Standards have changed considerably regarding the testing, treatment, and follow-up of H pylori. This is made clear by the just-published clinical practice guideline from the American College of Gastroenterology (ACG), which provides several new recommendations based on recent scientific evidence that should change your clinical approach to managing this common infection.
This discussion aims to synthesize and highlight key concepts from the ACG’s comprehensive publication.
Who Should Be Tested and Treated?
The cardinal diseases caused by H pylori have traditionally included peptic ulcer disease, marginal zone B-cell lymphoma, gastric adenocarcinoma, and dyspepsia.
Additional associations have been made with idiopathic thrombocytopenic purpura and otherwise unexplained iron deficiency.
New evidence suggests that patients taking long-term nonsteroidal anti-inflammatory drugs, including low-dose aspirin, are relatively more susceptible to infection.
The ACG’s guideline also recommends testing persons at an increased risk for gastric adenocarcinoma (eg, those with autoimmune gastritis, current or history of premalignant conditions, or first-degree relative with gastric cancer), as well as household members of patients with a positive nonserologic test for H pylori.
The authors note that those with an indication for testing should be offered treatment if determined to have an infection. These patients should also undergo a posttreatment test-of-cure, which should occur at least 4 weeks afterwards using a urea breath test, fecal antigen test, or gastric biopsy.
Caveats to Treatment
Patients with H pylori infections are advised to undergo treatment for a duration of 14 days. Some of the commercial prepackaged H pylori treatment options (eg, Pylera, which contains bismuth subcitrate/metronidazole/tetracycline) are dispensed in regimens lasting only 10 days and currently are viewed as inadequate.
In the United States, the patterns of antibiotic resistance for the previously used standard drugs in the treatment of H pylori have increased considerably. They range from 32% for clarithromycin, 38% for levofloxacin, and 42% for metronidazole, in contrast to 3% for amoxicillin, 1% for tetracycline, and 0% for rifabutin.
Clarithromycin- and levofloxacin-containing treatments should be avoided in treatment-naive patients unless specifically directed following the results of susceptibility tests with either a phenotypic method (culture-based) or a molecular method (polymerase chain reaction or next-generation sequencing). Notably, the mutations responsible for both clarithromycin and levofloxacin resistance may be detectable by stool-based testing.
Maintenance of intragastric acid suppression is key to H pylori eradication, as elevated intragastric pH promotes active replication of H pylori and makes it more susceptible to bactericidal antibiotics.
Therefore, the use of histamine-2 receptors is not recommended, as they are inadequate for achieving acid suppression. Instead, a dual-based therapy of either the potassium-competitive acid blocker (PCAB) vonoprazan (20 mg) or a high-dose proton pump inhibitor (PPI) and amoxicillin, administered twice daily, is effective, although this finding is based on limited evidence.
Treatment-Naive Patients
In treatment-naive patients without penicillin allergy and for whom antibiotic susceptibility testing has not been obtained, the guideline offers its strongest recommendation for bismuth quadruple therapy. This therapy typically consists of a PPI, bismuth subcitrate or subsalicylate, tetracycline, and metronidazole.
Among those with a penicillin allergy, bismuth quadruple therapy is also the primary treatment choice. The authors suggest that patients with a suspected allergy are referred to an allergist for possible penicillin desensitization, given that less than 1% of the population is thought to present with a “true” allergy.
The guideline also presented conditional recommendations, based on low- to moderate-quality evidence, for using a rifabutin-based triple regimen of omeprazole, amoxicillin, and rifabutin (Talicia); a PCAB-based dual regimen of vonoprazan and amoxicillin (Voquezna Dual Pak); and a PCAB-based triple regimen of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak). In patients with unknown clarithromycin susceptibility, the PCAB-based triple therapy is preferred over PPI-clarithromycin triple therapy.
Although probiotics have been suggested to possibly lead to increased effectiveness or tolerability for H pylori eradication, this was based on studies with significant heterogeneity in their designs. At present, no high-quality data support probiotic therapy.
Clinicians may substitute doxycycline for tetracycline due to availability or cost, and also may prescribe metronidazole at a lower dose than recommended (1.5-2 g/d) to limit side effects. Both modifications have been associated with lower rates of H pylori eradication and are not recommended.
Treatment-Experienced Patients
Quadruple bismuth therapy is the optimal approach among treatment-experienced patients with persistent H pylori infection who have not previously received this therapy. However, this recommendation was rated as conditional, given that it was based on a low quality of evidence.
The guideline offered other recommendations for treatment-experienced patients with persistent infection who had received bismuth quadruple therapy — also conditionally based on a low quality of evidence.
In such patients, it is recommended to consider the use of a rifabutin-based triple therapy (ie, a PPI standard to double dose, amoxicillin, and rifabutin) and a levofloxacin-based triple therapy (ie, a PPI standard dose, levofloxacin, and amoxicillin or metronidazole).
Although significant evidence gaps prevented the authors from providing formal recommendations, they included a PCAB-based triple therapy of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak) and a high-dose dual therapy of either vonoprazan (20 mg) or PPI (double dose) and amoxicillin among their suggested salvage regimens for these patients.
A New Standard
We must recognize, however, that there are still substantial evidence gaps, particularly around the use of a PCAB-based regimen and its relative advantages over a standard or high-dose PPI-based regimen. This may be of particular importance based on the variable prevalence of cytochrome P450 2C19 (CYP2C19) polymorphisms in the specific patient populations, as PCABs are not metabolized by CYP2C19.
Reviewing the entirety of the ACG’s clinical guideline is encouraged for additional details about the management of H pylori beyond what is highlighted herein.
Dr. Johnson, Professor of Medicine, Chief of Gastroenterology, Eastern Virginia Medical School, Norfolk, Virginia, disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Helicobacter pylori is one of the most common human bacterial chronic infections globally. Its prevalence has actually decreased in North America in recent years, although its current range of approximately 30%-40% remains substantial given the potential clinical implications of infection.
Standards have changed considerably regarding the testing, treatment, and follow-up of H pylori. This is made clear by the just-published clinical practice guideline from the American College of Gastroenterology (ACG), which provides several new recommendations based on recent scientific evidence that should change your clinical approach to managing this common infection.
This discussion aims to synthesize and highlight key concepts from the ACG’s comprehensive publication.
Who Should Be Tested and Treated?
The cardinal diseases caused by H pylori have traditionally included peptic ulcer disease, marginal zone B-cell lymphoma, gastric adenocarcinoma, and dyspepsia.
Additional associations have been made with idiopathic thrombocytopenic purpura and otherwise unexplained iron deficiency.
New evidence suggests that patients taking long-term nonsteroidal anti-inflammatory drugs, including low-dose aspirin, are relatively more susceptible to infection.
The ACG’s guideline also recommends testing persons at an increased risk for gastric adenocarcinoma (eg, those with autoimmune gastritis, current or history of premalignant conditions, or first-degree relative with gastric cancer), as well as household members of patients with a positive nonserologic test for H pylori.
The authors note that those with an indication for testing should be offered treatment if determined to have an infection. These patients should also undergo a posttreatment test-of-cure, which should occur at least 4 weeks afterwards using a urea breath test, fecal antigen test, or gastric biopsy.
Caveats to Treatment
Patients with H pylori infections are advised to undergo treatment for a duration of 14 days. Some of the commercial prepackaged H pylori treatment options (eg, Pylera, which contains bismuth subcitrate/metronidazole/tetracycline) are dispensed in regimens lasting only 10 days and currently are viewed as inadequate.
In the United States, the patterns of antibiotic resistance for the previously used standard drugs in the treatment of H pylori have increased considerably. They range from 32% for clarithromycin, 38% for levofloxacin, and 42% for metronidazole, in contrast to 3% for amoxicillin, 1% for tetracycline, and 0% for rifabutin.
Clarithromycin- and levofloxacin-containing treatments should be avoided in treatment-naive patients unless specifically directed following the results of susceptibility tests with either a phenotypic method (culture-based) or a molecular method (polymerase chain reaction or next-generation sequencing). Notably, the mutations responsible for both clarithromycin and levofloxacin resistance may be detectable by stool-based testing.
Maintenance of intragastric acid suppression is key to H pylori eradication, as elevated intragastric pH promotes active replication of H pylori and makes it more susceptible to bactericidal antibiotics.
Therefore, the use of histamine-2 receptors is not recommended, as they are inadequate for achieving acid suppression. Instead, a dual-based therapy of either the potassium-competitive acid blocker (PCAB) vonoprazan (20 mg) or a high-dose proton pump inhibitor (PPI) and amoxicillin, administered twice daily, is effective, although this finding is based on limited evidence.
Treatment-Naive Patients
In treatment-naive patients without penicillin allergy and for whom antibiotic susceptibility testing has not been obtained, the guideline offers its strongest recommendation for bismuth quadruple therapy. This therapy typically consists of a PPI, bismuth subcitrate or subsalicylate, tetracycline, and metronidazole.
Among those with a penicillin allergy, bismuth quadruple therapy is also the primary treatment choice. The authors suggest that patients with a suspected allergy are referred to an allergist for possible penicillin desensitization, given that less than 1% of the population is thought to present with a “true” allergy.
The guideline also presented conditional recommendations, based on low- to moderate-quality evidence, for using a rifabutin-based triple regimen of omeprazole, amoxicillin, and rifabutin (Talicia); a PCAB-based dual regimen of vonoprazan and amoxicillin (Voquezna Dual Pak); and a PCAB-based triple regimen of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak). In patients with unknown clarithromycin susceptibility, the PCAB-based triple therapy is preferred over PPI-clarithromycin triple therapy.
Although probiotics have been suggested to possibly lead to increased effectiveness or tolerability for H pylori eradication, this was based on studies with significant heterogeneity in their designs. At present, no high-quality data support probiotic therapy.
Clinicians may substitute doxycycline for tetracycline due to availability or cost, and also may prescribe metronidazole at a lower dose than recommended (1.5-2 g/d) to limit side effects. Both modifications have been associated with lower rates of H pylori eradication and are not recommended.
Treatment-Experienced Patients
Quadruple bismuth therapy is the optimal approach among treatment-experienced patients with persistent H pylori infection who have not previously received this therapy. However, this recommendation was rated as conditional, given that it was based on a low quality of evidence.
The guideline offered other recommendations for treatment-experienced patients with persistent infection who had received bismuth quadruple therapy — also conditionally based on a low quality of evidence.
In such patients, it is recommended to consider the use of a rifabutin-based triple therapy (ie, a PPI standard to double dose, amoxicillin, and rifabutin) and a levofloxacin-based triple therapy (ie, a PPI standard dose, levofloxacin, and amoxicillin or metronidazole).
Although significant evidence gaps prevented the authors from providing formal recommendations, they included a PCAB-based triple therapy of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak) and a high-dose dual therapy of either vonoprazan (20 mg) or PPI (double dose) and amoxicillin among their suggested salvage regimens for these patients.
A New Standard
We must recognize, however, that there are still substantial evidence gaps, particularly around the use of a PCAB-based regimen and its relative advantages over a standard or high-dose PPI-based regimen. This may be of particular importance based on the variable prevalence of cytochrome P450 2C19 (CYP2C19) polymorphisms in the specific patient populations, as PCABs are not metabolized by CYP2C19.
Reviewing the entirety of the ACG’s clinical guideline is encouraged for additional details about the management of H pylori beyond what is highlighted herein.
Dr. Johnson, Professor of Medicine, Chief of Gastroenterology, Eastern Virginia Medical School, Norfolk, Virginia, disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Help Your Patients Reap the Benefits of Plant-Based Diets
Research pooled from nearly 100 studies has indicated that people who adhere to a vegan diet (ie, completely devoid of animal products) or a vegetarian diet (ie, devoid of meat, but may include dairy and eggs) are able to ward off some chronic diseases, such as cardiovascular disease, optimize glycemic control, and decrease their risk for cancer compared with those who consume omnivorous diets.
Vegan and vegetarian diets, or flexitarian diets — which are less reliant on animal protein than the standard US diet but do not completely exclude meat, fish, eggs, or dairy — may promote homeostasis and decrease inflammation by providing more fiber, antioxidants, and unsaturated fatty acids than the typical Western diet.
Inflammation and Obesity
Adipose tissue is a major producer of pro-inflammatory cytokines like interleukin (IL)-6, whose presence then triggers a rush of acute-phase reactants such as C-reactive protein (CRP) by the liver. This process develops into chronic low-grade inflammation that can increase a person’s chances of developing diabetes, cardiovascular disease, kidney disease, metabolic syndrome, and related complications.
Adopting a plant-based diet can improve markers of chronic low-grade inflammation that can lead to chronic disease and worsen existent chronic disease. A meta-analysis of 29 studies encompassing nearly 2700 participants found that initiation of a plant-based diet showed significant improvement in CRP, IL-6, and soluble intercellular adhesion molecule 1.
If we want to prevent these inflammatory disease states and their complications, the obvious response is to counsel patients to avoid excessive weight gain or to lose weight if obesity is their baseline. This can be tough for some patients, but it is nonetheless an important step in chronic disease prevention and management.
Plant-Based Diet for Type 2 Diabetes
According to a review of nine studies of patients living with type 2 diabetes who adhered to a plant-based diet, all but one found that this approach led to significantly lower A1c values than those seen in control groups. Six of the included studies reported that participants were able to decrease or discontinue medications for the management of diabetes. Researchers across all included studies also noted a decrease in total cholesterol, low-density lipoprotein cholesterol, and triglycerides, as well as increased weight loss in participants in each intervention group.
Such improvements are probably the result of the increase in fiber intake that occurs with a plant-based diet. A high-fiber diet is known to promote improved glucose and lipid metabolism as well as weight loss.
It is also worth noting that participants in the intervention groups also experienced improvements in depression and less chronic pain than did those in the control groups.
Plant-Based Diet for Chronic Kidney Disease (CKD)
Although the use of a plant-based diet in the prevention of CKD is well documented, adopting such diets for the treatment of CKD may intimidate both patients and practitioners owing to the high potassium and phosphorus content of many fruits and vegetables.
However, research indicates that the bioavailability of both potassium and phosphorus is lower in plant-based, whole foods than in preservatives and the highly processed food items that incorporate them. This makes a plant-based diet more viable than previously thought.
Diets rich in vegetables, whole grains, nuts, and legumes have been shown to decrease dietary acid load, both preventing and treating metabolic acidosis. Such diets have also been shown to decrease blood pressure and the risk for a decline in estimated glomerular filtration rate. This type of diet would also prioritize the unsaturated fatty acids and fiber-rich proteins such as avocados, beans, and nuts shown to improve dyslipidemia, which may occur alongside CKD.
Realistic Options for Patients on Medical Diets
There is one question that I always seem to get from when recommending a plant-based diet: “These patients already have so many restrictions. Why would you add more?” And my answer is also always the same: I don’t.
I rarely, if ever, recommend completely cutting out any food item or food group. Instead, I ask the patient to increase their intake of plant-based foods and only limit highly processed foods and fatty meats. By shifting a patient’s focus to beans; nuts; and low-carbohydrate, high-fiber fruits and vegetables, I am often opening up a whole new world of possibilities.
Instead of a sandwich with low-sodium turkey and cheese on white bread with a side of unsalted pretzels, I recommend a caprese salad with blueberries and almonds or a Southwest salad with black beans, corn, and avocado. I don’t encourage my patients to skip the foods that they love, but instead to only think about all the delicious plant-based options that will provide them with more than just calories.
Meat, dairy, seafood, and eggs can certainly be a part of a healthy diet, but what if our chronically ill patients, especially those with diabetes, had more options than just grilled chicken and green beans for every meal? What if we focus on decreasing dietary restrictions, incorporating a variety of nourishing foods, and educating our patients, instead of on portion control and moderation?
This is how I choose to incorporate plant-based diets into my practice to treat and prevent these chronic inflammatory conditions and promote sustainable, realistic change in my clients’ health.
Brandy Winfree Root, a renal dietitian in private practice in Mary Esther, Florida, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Research pooled from nearly 100 studies has indicated that people who adhere to a vegan diet (ie, completely devoid of animal products) or a vegetarian diet (ie, devoid of meat, but may include dairy and eggs) are able to ward off some chronic diseases, such as cardiovascular disease, optimize glycemic control, and decrease their risk for cancer compared with those who consume omnivorous diets.
Vegan and vegetarian diets, or flexitarian diets — which are less reliant on animal protein than the standard US diet but do not completely exclude meat, fish, eggs, or dairy — may promote homeostasis and decrease inflammation by providing more fiber, antioxidants, and unsaturated fatty acids than the typical Western diet.
Inflammation and Obesity
Adipose tissue is a major producer of pro-inflammatory cytokines like interleukin (IL)-6, whose presence then triggers a rush of acute-phase reactants such as C-reactive protein (CRP) by the liver. This process develops into chronic low-grade inflammation that can increase a person’s chances of developing diabetes, cardiovascular disease, kidney disease, metabolic syndrome, and related complications.
Adopting a plant-based diet can improve markers of chronic low-grade inflammation that can lead to chronic disease and worsen existent chronic disease. A meta-analysis of 29 studies encompassing nearly 2700 participants found that initiation of a plant-based diet showed significant improvement in CRP, IL-6, and soluble intercellular adhesion molecule 1.
If we want to prevent these inflammatory disease states and their complications, the obvious response is to counsel patients to avoid excessive weight gain or to lose weight if obesity is their baseline. This can be tough for some patients, but it is nonetheless an important step in chronic disease prevention and management.
Plant-Based Diet for Type 2 Diabetes
According to a review of nine studies of patients living with type 2 diabetes who adhered to a plant-based diet, all but one found that this approach led to significantly lower A1c values than those seen in control groups. Six of the included studies reported that participants were able to decrease or discontinue medications for the management of diabetes. Researchers across all included studies also noted a decrease in total cholesterol, low-density lipoprotein cholesterol, and triglycerides, as well as increased weight loss in participants in each intervention group.
Such improvements are probably the result of the increase in fiber intake that occurs with a plant-based diet. A high-fiber diet is known to promote improved glucose and lipid metabolism as well as weight loss.
It is also worth noting that participants in the intervention groups also experienced improvements in depression and less chronic pain than did those in the control groups.
Plant-Based Diet for Chronic Kidney Disease (CKD)
Although the use of a plant-based diet in the prevention of CKD is well documented, adopting such diets for the treatment of CKD may intimidate both patients and practitioners owing to the high potassium and phosphorus content of many fruits and vegetables.
However, research indicates that the bioavailability of both potassium and phosphorus is lower in plant-based, whole foods than in preservatives and the highly processed food items that incorporate them. This makes a plant-based diet more viable than previously thought.
Diets rich in vegetables, whole grains, nuts, and legumes have been shown to decrease dietary acid load, both preventing and treating metabolic acidosis. Such diets have also been shown to decrease blood pressure and the risk for a decline in estimated glomerular filtration rate. This type of diet would also prioritize the unsaturated fatty acids and fiber-rich proteins such as avocados, beans, and nuts shown to improve dyslipidemia, which may occur alongside CKD.
Realistic Options for Patients on Medical Diets
There is one question that I always seem to get from when recommending a plant-based diet: “These patients already have so many restrictions. Why would you add more?” And my answer is also always the same: I don’t.
I rarely, if ever, recommend completely cutting out any food item or food group. Instead, I ask the patient to increase their intake of plant-based foods and only limit highly processed foods and fatty meats. By shifting a patient’s focus to beans; nuts; and low-carbohydrate, high-fiber fruits and vegetables, I am often opening up a whole new world of possibilities.
Instead of a sandwich with low-sodium turkey and cheese on white bread with a side of unsalted pretzels, I recommend a caprese salad with blueberries and almonds or a Southwest salad with black beans, corn, and avocado. I don’t encourage my patients to skip the foods that they love, but instead to only think about all the delicious plant-based options that will provide them with more than just calories.
Meat, dairy, seafood, and eggs can certainly be a part of a healthy diet, but what if our chronically ill patients, especially those with diabetes, had more options than just grilled chicken and green beans for every meal? What if we focus on decreasing dietary restrictions, incorporating a variety of nourishing foods, and educating our patients, instead of on portion control and moderation?
This is how I choose to incorporate plant-based diets into my practice to treat and prevent these chronic inflammatory conditions and promote sustainable, realistic change in my clients’ health.
Brandy Winfree Root, a renal dietitian in private practice in Mary Esther, Florida, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Research pooled from nearly 100 studies has indicated that people who adhere to a vegan diet (ie, completely devoid of animal products) or a vegetarian diet (ie, devoid of meat, but may include dairy and eggs) are able to ward off some chronic diseases, such as cardiovascular disease, optimize glycemic control, and decrease their risk for cancer compared with those who consume omnivorous diets.
Vegan and vegetarian diets, or flexitarian diets — which are less reliant on animal protein than the standard US diet but do not completely exclude meat, fish, eggs, or dairy — may promote homeostasis and decrease inflammation by providing more fiber, antioxidants, and unsaturated fatty acids than the typical Western diet.
Inflammation and Obesity
Adipose tissue is a major producer of pro-inflammatory cytokines like interleukin (IL)-6, whose presence then triggers a rush of acute-phase reactants such as C-reactive protein (CRP) by the liver. This process develops into chronic low-grade inflammation that can increase a person’s chances of developing diabetes, cardiovascular disease, kidney disease, metabolic syndrome, and related complications.
Adopting a plant-based diet can improve markers of chronic low-grade inflammation that can lead to chronic disease and worsen existent chronic disease. A meta-analysis of 29 studies encompassing nearly 2700 participants found that initiation of a plant-based diet showed significant improvement in CRP, IL-6, and soluble intercellular adhesion molecule 1.
If we want to prevent these inflammatory disease states and their complications, the obvious response is to counsel patients to avoid excessive weight gain or to lose weight if obesity is their baseline. This can be tough for some patients, but it is nonetheless an important step in chronic disease prevention and management.
Plant-Based Diet for Type 2 Diabetes
According to a review of nine studies of patients living with type 2 diabetes who adhered to a plant-based diet, all but one found that this approach led to significantly lower A1c values than those seen in control groups. Six of the included studies reported that participants were able to decrease or discontinue medications for the management of diabetes. Researchers across all included studies also noted a decrease in total cholesterol, low-density lipoprotein cholesterol, and triglycerides, as well as increased weight loss in participants in each intervention group.
Such improvements are probably the result of the increase in fiber intake that occurs with a plant-based diet. A high-fiber diet is known to promote improved glucose and lipid metabolism as well as weight loss.
It is also worth noting that participants in the intervention groups also experienced improvements in depression and less chronic pain than did those in the control groups.
Plant-Based Diet for Chronic Kidney Disease (CKD)
Although the use of a plant-based diet in the prevention of CKD is well documented, adopting such diets for the treatment of CKD may intimidate both patients and practitioners owing to the high potassium and phosphorus content of many fruits and vegetables.
However, research indicates that the bioavailability of both potassium and phosphorus is lower in plant-based, whole foods than in preservatives and the highly processed food items that incorporate them. This makes a plant-based diet more viable than previously thought.
Diets rich in vegetables, whole grains, nuts, and legumes have been shown to decrease dietary acid load, both preventing and treating metabolic acidosis. Such diets have also been shown to decrease blood pressure and the risk for a decline in estimated glomerular filtration rate. This type of diet would also prioritize the unsaturated fatty acids and fiber-rich proteins such as avocados, beans, and nuts shown to improve dyslipidemia, which may occur alongside CKD.
Realistic Options for Patients on Medical Diets
There is one question that I always seem to get from when recommending a plant-based diet: “These patients already have so many restrictions. Why would you add more?” And my answer is also always the same: I don’t.
I rarely, if ever, recommend completely cutting out any food item or food group. Instead, I ask the patient to increase their intake of plant-based foods and only limit highly processed foods and fatty meats. By shifting a patient’s focus to beans; nuts; and low-carbohydrate, high-fiber fruits and vegetables, I am often opening up a whole new world of possibilities.
Instead of a sandwich with low-sodium turkey and cheese on white bread with a side of unsalted pretzels, I recommend a caprese salad with blueberries and almonds or a Southwest salad with black beans, corn, and avocado. I don’t encourage my patients to skip the foods that they love, but instead to only think about all the delicious plant-based options that will provide them with more than just calories.
Meat, dairy, seafood, and eggs can certainly be a part of a healthy diet, but what if our chronically ill patients, especially those with diabetes, had more options than just grilled chicken and green beans for every meal? What if we focus on decreasing dietary restrictions, incorporating a variety of nourishing foods, and educating our patients, instead of on portion control and moderation?
This is how I choose to incorporate plant-based diets into my practice to treat and prevent these chronic inflammatory conditions and promote sustainable, realistic change in my clients’ health.
Brandy Winfree Root, a renal dietitian in private practice in Mary Esther, Florida, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The Game We Play Every Day
Words do have power. Names have power. Words are events, they do things, change things. They transform both speaker and hearer ... They feed understanding or emotion back and forth and amplify it. — Ursula K. Le Guin
Every medical student should have a class in linguistics. I’m just unsure what it might replace. Maybe physiology? (When was the last time you used Fick’s or Fourier’s Laws anyway?). Even if we don’t supplant any core curriculum, it’s worth noting that we spend more time in our daily work calculating how to communicate things than calculating cardiac outputs. That we can convey so much so consistently and without specific training is a marvel. Making the diagnosis or a plan is often the easy part.
Linguistics is a broad field. At its essence, it studies how we communicate. It’s fascinating how we use tone, word choice, gestures, syntax, and grammar to explain, reassure, instruct or implore patients. Medical appointments are sometimes high stakes and occur within a huge variety of circumstances. In a single day of clinic, I had a patient with dementia, and one pursuing a PhD in P-Chem. I had English speakers, second language English speakers, and a Vietnamese patient who knew no English. In just one day, I explained things to toddlers and adults, a Black woman from Oklahoma and a Jewish woman from New York. For a brief few minutes, each of them was my partner in a game of medical charades. For each one, I had to figure out how to get them to know what I’m thinking.
I learned of this game of charades concept from a podcast featuring Morten Christiansen, professor of psychology at Cornell University, and professor in Cognitive Science of Language, at Aarhus University, Denmark. The idea is that language can be thought of as a game where speakers constantly improvise based on the topic, each one’s expertise, and the shared understanding. I found this intriguing. In his explanation, grammar and definitions are less important than the mutual understanding of what is being communicated. It helps explain the wide variations of speech even among those speaking the same language. It also flips the idea that brains are designed for language, a concept proposed by linguistic greats such as Noam Chomsky and Steven Pinker. Rather, what we call language is just the best solution our brains could create to convey information.
I thought about how each of us instinctively varies the complexity of sentences and tone of voice based on the ability of each patient to understand. Gestures, storytelling and analogies are linguistic tools we use without thinking about them. We’ve a unique communications conundrum in that we often need patients to understand a complex idea, but only have minutes to get them there. We don’t want them to panic. We also don’t want them to be so dispassionate as to not act. To speed things up, we often use a technique known as chunking, short phrases that capture an idea in one bite. For example, “soak and smear” to get atopic patients to moisturize or “scrape and burn” to describe a curettage and electrodesiccation of a basal cell carcinoma or “a stick and a burn” before injecting them (I never liked that one). These are pithy, efficient. But they don’t always work.
One afternoon I had a 93-year-old woman with glossodynia. She had dementia and her 96-year-old husband was helping. When I explained how she’d “swish and spit” her magic mouthwash, he looked perplexed. Is she swishing a wand or something? I shook my head, “No” and gestured with my hands palms down, waving back and forth. It is just a mouthwash. She should rinse, then spit it out. I lost that round.
Then a 64-year-old woman whom I had to advise that the pink bump on her arm was a cutaneous neuroendocrine tumor. Do I call it a Merkel cell carcinoma? Do I say, “You know, like the one Jimmy Buffett had?” (Nope, not a good use of storytelling). She wanted to know how she got it. Sun exposure, we think. Or, perhaps a virus. Just how does one explain a virus called MCPyV that is ubiquitous but somehow caused cancer just for you? How do you convey, “This is serious, but you might not die like Jimmy Buffett?” I had to use all my language skills to get this right.
Then there is the Henderson-Hasselbalch problem of linguistics: communicating through a translator. When doing so, I’m cognizant of choosing short, simple sentences. Subject, verb, object. First this, then that. This mitigates what’s lost in translation and reduces waiting for translations (especially when your patient is storytelling in paragraphs). But try doing this with an emotionally wrought condition like alopecia. Finding the fewest words to convey that your FSH and estrogen levels are irrelevant to your telogen effluvium to a Vietnamese speaker is tricky. “Yes, I see your primary care physician ordered these tests. No, the numbers do not matter.” Did that translate as they are normal? Or that they don’t matter because she is 54? Or that they don’t matter to me because I didn’t order them?
When you find yourself exhausted at the day’s end, perhaps you’ll better appreciate how it was not only the graduate level medicine you did today; you’ve practically got a PhD in linguistics as well. You just didn’t realize it.
Dr. Benabio is chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
Words do have power. Names have power. Words are events, they do things, change things. They transform both speaker and hearer ... They feed understanding or emotion back and forth and amplify it. — Ursula K. Le Guin
Every medical student should have a class in linguistics. I’m just unsure what it might replace. Maybe physiology? (When was the last time you used Fick’s or Fourier’s Laws anyway?). Even if we don’t supplant any core curriculum, it’s worth noting that we spend more time in our daily work calculating how to communicate things than calculating cardiac outputs. That we can convey so much so consistently and without specific training is a marvel. Making the diagnosis or a plan is often the easy part.
Linguistics is a broad field. At its essence, it studies how we communicate. It’s fascinating how we use tone, word choice, gestures, syntax, and grammar to explain, reassure, instruct or implore patients. Medical appointments are sometimes high stakes and occur within a huge variety of circumstances. In a single day of clinic, I had a patient with dementia, and one pursuing a PhD in P-Chem. I had English speakers, second language English speakers, and a Vietnamese patient who knew no English. In just one day, I explained things to toddlers and adults, a Black woman from Oklahoma and a Jewish woman from New York. For a brief few minutes, each of them was my partner in a game of medical charades. For each one, I had to figure out how to get them to know what I’m thinking.
I learned of this game of charades concept from a podcast featuring Morten Christiansen, professor of psychology at Cornell University, and professor in Cognitive Science of Language, at Aarhus University, Denmark. The idea is that language can be thought of as a game where speakers constantly improvise based on the topic, each one’s expertise, and the shared understanding. I found this intriguing. In his explanation, grammar and definitions are less important than the mutual understanding of what is being communicated. It helps explain the wide variations of speech even among those speaking the same language. It also flips the idea that brains are designed for language, a concept proposed by linguistic greats such as Noam Chomsky and Steven Pinker. Rather, what we call language is just the best solution our brains could create to convey information.
I thought about how each of us instinctively varies the complexity of sentences and tone of voice based on the ability of each patient to understand. Gestures, storytelling and analogies are linguistic tools we use without thinking about them. We’ve a unique communications conundrum in that we often need patients to understand a complex idea, but only have minutes to get them there. We don’t want them to panic. We also don’t want them to be so dispassionate as to not act. To speed things up, we often use a technique known as chunking, short phrases that capture an idea in one bite. For example, “soak and smear” to get atopic patients to moisturize or “scrape and burn” to describe a curettage and electrodesiccation of a basal cell carcinoma or “a stick and a burn” before injecting them (I never liked that one). These are pithy, efficient. But they don’t always work.
One afternoon I had a 93-year-old woman with glossodynia. She had dementia and her 96-year-old husband was helping. When I explained how she’d “swish and spit” her magic mouthwash, he looked perplexed. Is she swishing a wand or something? I shook my head, “No” and gestured with my hands palms down, waving back and forth. It is just a mouthwash. She should rinse, then spit it out. I lost that round.
Then a 64-year-old woman whom I had to advise that the pink bump on her arm was a cutaneous neuroendocrine tumor. Do I call it a Merkel cell carcinoma? Do I say, “You know, like the one Jimmy Buffett had?” (Nope, not a good use of storytelling). She wanted to know how she got it. Sun exposure, we think. Or, perhaps a virus. Just how does one explain a virus called MCPyV that is ubiquitous but somehow caused cancer just for you? How do you convey, “This is serious, but you might not die like Jimmy Buffett?” I had to use all my language skills to get this right.
Then there is the Henderson-Hasselbalch problem of linguistics: communicating through a translator. When doing so, I’m cognizant of choosing short, simple sentences. Subject, verb, object. First this, then that. This mitigates what’s lost in translation and reduces waiting for translations (especially when your patient is storytelling in paragraphs). But try doing this with an emotionally wrought condition like alopecia. Finding the fewest words to convey that your FSH and estrogen levels are irrelevant to your telogen effluvium to a Vietnamese speaker is tricky. “Yes, I see your primary care physician ordered these tests. No, the numbers do not matter.” Did that translate as they are normal? Or that they don’t matter because she is 54? Or that they don’t matter to me because I didn’t order them?
When you find yourself exhausted at the day’s end, perhaps you’ll better appreciate how it was not only the graduate level medicine you did today; you’ve practically got a PhD in linguistics as well. You just didn’t realize it.
Dr. Benabio is chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
Words do have power. Names have power. Words are events, they do things, change things. They transform both speaker and hearer ... They feed understanding or emotion back and forth and amplify it. — Ursula K. Le Guin
Every medical student should have a class in linguistics. I’m just unsure what it might replace. Maybe physiology? (When was the last time you used Fick’s or Fourier’s Laws anyway?). Even if we don’t supplant any core curriculum, it’s worth noting that we spend more time in our daily work calculating how to communicate things than calculating cardiac outputs. That we can convey so much so consistently and without specific training is a marvel. Making the diagnosis or a plan is often the easy part.
Linguistics is a broad field. At its essence, it studies how we communicate. It’s fascinating how we use tone, word choice, gestures, syntax, and grammar to explain, reassure, instruct or implore patients. Medical appointments are sometimes high stakes and occur within a huge variety of circumstances. In a single day of clinic, I had a patient with dementia, and one pursuing a PhD in P-Chem. I had English speakers, second language English speakers, and a Vietnamese patient who knew no English. In just one day, I explained things to toddlers and adults, a Black woman from Oklahoma and a Jewish woman from New York. For a brief few minutes, each of them was my partner in a game of medical charades. For each one, I had to figure out how to get them to know what I’m thinking.
I learned of this game of charades concept from a podcast featuring Morten Christiansen, professor of psychology at Cornell University, and professor in Cognitive Science of Language, at Aarhus University, Denmark. The idea is that language can be thought of as a game where speakers constantly improvise based on the topic, each one’s expertise, and the shared understanding. I found this intriguing. In his explanation, grammar and definitions are less important than the mutual understanding of what is being communicated. It helps explain the wide variations of speech even among those speaking the same language. It also flips the idea that brains are designed for language, a concept proposed by linguistic greats such as Noam Chomsky and Steven Pinker. Rather, what we call language is just the best solution our brains could create to convey information.
I thought about how each of us instinctively varies the complexity of sentences and tone of voice based on the ability of each patient to understand. Gestures, storytelling and analogies are linguistic tools we use without thinking about them. We’ve a unique communications conundrum in that we often need patients to understand a complex idea, but only have minutes to get them there. We don’t want them to panic. We also don’t want them to be so dispassionate as to not act. To speed things up, we often use a technique known as chunking, short phrases that capture an idea in one bite. For example, “soak and smear” to get atopic patients to moisturize or “scrape and burn” to describe a curettage and electrodesiccation of a basal cell carcinoma or “a stick and a burn” before injecting them (I never liked that one). These are pithy, efficient. But they don’t always work.
One afternoon I had a 93-year-old woman with glossodynia. She had dementia and her 96-year-old husband was helping. When I explained how she’d “swish and spit” her magic mouthwash, he looked perplexed. Is she swishing a wand or something? I shook my head, “No” and gestured with my hands palms down, waving back and forth. It is just a mouthwash. She should rinse, then spit it out. I lost that round.
Then a 64-year-old woman whom I had to advise that the pink bump on her arm was a cutaneous neuroendocrine tumor. Do I call it a Merkel cell carcinoma? Do I say, “You know, like the one Jimmy Buffett had?” (Nope, not a good use of storytelling). She wanted to know how she got it. Sun exposure, we think. Or, perhaps a virus. Just how does one explain a virus called MCPyV that is ubiquitous but somehow caused cancer just for you? How do you convey, “This is serious, but you might not die like Jimmy Buffett?” I had to use all my language skills to get this right.
Then there is the Henderson-Hasselbalch problem of linguistics: communicating through a translator. When doing so, I’m cognizant of choosing short, simple sentences. Subject, verb, object. First this, then that. This mitigates what’s lost in translation and reduces waiting for translations (especially when your patient is storytelling in paragraphs). But try doing this with an emotionally wrought condition like alopecia. Finding the fewest words to convey that your FSH and estrogen levels are irrelevant to your telogen effluvium to a Vietnamese speaker is tricky. “Yes, I see your primary care physician ordered these tests. No, the numbers do not matter.” Did that translate as they are normal? Or that they don’t matter because she is 54? Or that they don’t matter to me because I didn’t order them?
When you find yourself exhausted at the day’s end, perhaps you’ll better appreciate how it was not only the graduate level medicine you did today; you’ve practically got a PhD in linguistics as well. You just didn’t realize it.
Dr. Benabio is chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
A Doctor Gets the Save When a Little League Umpire Collapses
Emergencies happen anywhere, anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a Medscape Medical News series telling these stories.
I sincerely believe that what goes around comes around. Good things come to good people. And sometimes that saves lives.
My 10-year-old son was in the semifinals of the Little League district championship. And we were losing. My son is an excellent pitcher, and he had started the game. But that night, he was struggling. He just couldn’t find where to throw the ball. Needless to say, he was frustrated.
He was changed to shortstop in the second inning, and the home plate umpire walked over to him. This umpire is well known in the area for his kindness and commitment, how he encourages the kids and helps make baseball fun even when it’s stressful.
We didn’t know him well, but he was really supportive of my kid in that moment, talking to him about how baseball is a team sport and we’re here to have fun. Just being really positive.
As the game continued, I saw the umpire suddenly walk to the side of the field. I hadn’t seen it, but he had been hit by a wild pitch on the side of his neck. He was wearing protective gear, but the ball managed to bounce up the side and caught bare neck. I knew something wasn’t right.
I went down to talk to him, and my medical assistant (MA), who was also at the game, came with me. I could tell the umpire was injured, but he didn’t want to leave the game. I suggested going to the hospital, but he wouldn’t consider it. So I sat there with my arms crossed, watching him.
His symptoms got worse. I could see he was in pain, and it was getting harder for him to speak.
Again, I strongly urged him to go to the hospital, but again, he said no.
In the sixth inning, things got bad enough that the umpire finally agreed to leave the game. As I was figuring out how to get him to the hospital, he disappeared on me. He had walked up to the second floor of the snack shack. My MA and I got him back downstairs and sat him on a bench behind home plate.
We were in the process of calling 911 ... when he arrested.
Luckily, when he lost vital signs, my MA and I were standing right next to him. We were able to activate ACLS protocol and start CPR within seconds.
Many times in these critical situations — especially if people are scared or have never seen an emergency like this — there’s the potential for chaos. Well, that was the polar opposite of what happened.
As soon as I started to run the code, there was this sense of order. People were keeping their composure and following directions. My MA and I would say, “this is what we need,” and the task would immediately be assigned to someone. It was quiet. There was no yelling. Everyone trusted me, even though some of them had never met me before. It was so surprising. I remember thinking, we’re running an arrest, but it’s so calm.
We were an organized team, and it really worked like clockwork, which was remarkable given where we were. It’s one thing to be in the hospital for an event like that. But to be on a baseball field where you have nothing is a completely different scenario.
Meanwhile, the game went on.
I had requested that all the kids be placed in the dugout when they weren’t on the field. So they saw the umpire walk off, but none of them saw him arrest. Some parents were really helpful with making sure the kids were okay.
The president of Oxford Little League ran across the street to a fire station to get an AED. But the fire department personnel were out on a call. He had to break down the door.
By the time he got back, the umpire’s vital signs were returning. And then EMS arrived.
They loaded him in the ambulance, and I called ahead to the trauma team, so they knew exactly what was happening.
I was pretty worried. My hypothesis was that there was probably compression on the vasculature, which had caused him to lose his vital signs. I thought he probably had an impending airway loss. I wasn’t sure if he was going to make it through the night.
What I didn’t know was that while I was giving CPR, my son stole home, and we won the game. As the ambulance was leaving, the celebration was going on in the outfield.
The umpire was in the hospital for several days. Early on, I got permission from his family to visit him. The first time I saw him, I felt this incredible gratitude and peace.
My dad was an ER doctor, and growing up, it seemed like every time we went on a family vacation, there was an emergency. We would be near a car accident or something, and my father would fly in and save the day. I remember being on the Autobahn somewhere in Europe, and there was a devastating accident between a car and a motorcycle. My father stabilized the guy, had him airlifted out, and apparently, he did fine. I grew up watching things like this and thinking, wow, that’s incredible.
Fast forward to 2 years ago, my father was diagnosed with a lung cancer he never should have had. He never smoked. As a cancer surgeon, I know we did everything in our power to save him. But it didn’t happen. He passed away.
I realize this is superstitious, but seeing the umpire alive, I had this feeling that somehow my dad was there. It was bittersweet but also a joyful moment — like I could breathe again.
I met the umpire’s family that first time, and it was like meeting family that you didn’t know you had but now you have forever. Even though the event was traumatic — I’m still trying not to be on high alert every time I go to a game — it felt like a gift to be part of this journey with them.
Little League’s mission is to teach kids about teamwork, leadership, and making good choices so communities are stronger. Our umpire is a guy who does that every day. He’s not a Little League umpire because he makes any money. He shows up at every single game to support these kids and engage them, to model respect, gratitude, and kindness.
I think our obligation as people is to live with intentionality. We all need to make sure we leave the world a better place, even when we are called upon to do uncomfortable things. Our umpire showed our kids what that looks like, and in that moment when he could have died, we were able to do the same for him.
Jennifer LaFemina, MD, is a surgical oncologist at UMass Memorial Medical Center in Massachusetts.
Are you a medical professional with a dramatic story outside the clinic? Medscape Medical News would love to consider your story for Is There a Doctor in the House? Please email your contact information and a short summary to [email protected].
A version of this article appeared on Medscape.com.
Emergencies happen anywhere, anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a Medscape Medical News series telling these stories.
I sincerely believe that what goes around comes around. Good things come to good people. And sometimes that saves lives.
My 10-year-old son was in the semifinals of the Little League district championship. And we were losing. My son is an excellent pitcher, and he had started the game. But that night, he was struggling. He just couldn’t find where to throw the ball. Needless to say, he was frustrated.
He was changed to shortstop in the second inning, and the home plate umpire walked over to him. This umpire is well known in the area for his kindness and commitment, how he encourages the kids and helps make baseball fun even when it’s stressful.
We didn’t know him well, but he was really supportive of my kid in that moment, talking to him about how baseball is a team sport and we’re here to have fun. Just being really positive.
As the game continued, I saw the umpire suddenly walk to the side of the field. I hadn’t seen it, but he had been hit by a wild pitch on the side of his neck. He was wearing protective gear, but the ball managed to bounce up the side and caught bare neck. I knew something wasn’t right.
I went down to talk to him, and my medical assistant (MA), who was also at the game, came with me. I could tell the umpire was injured, but he didn’t want to leave the game. I suggested going to the hospital, but he wouldn’t consider it. So I sat there with my arms crossed, watching him.
His symptoms got worse. I could see he was in pain, and it was getting harder for him to speak.
Again, I strongly urged him to go to the hospital, but again, he said no.
In the sixth inning, things got bad enough that the umpire finally agreed to leave the game. As I was figuring out how to get him to the hospital, he disappeared on me. He had walked up to the second floor of the snack shack. My MA and I got him back downstairs and sat him on a bench behind home plate.
We were in the process of calling 911 ... when he arrested.
Luckily, when he lost vital signs, my MA and I were standing right next to him. We were able to activate ACLS protocol and start CPR within seconds.
Many times in these critical situations — especially if people are scared or have never seen an emergency like this — there’s the potential for chaos. Well, that was the polar opposite of what happened.
As soon as I started to run the code, there was this sense of order. People were keeping their composure and following directions. My MA and I would say, “this is what we need,” and the task would immediately be assigned to someone. It was quiet. There was no yelling. Everyone trusted me, even though some of them had never met me before. It was so surprising. I remember thinking, we’re running an arrest, but it’s so calm.
We were an organized team, and it really worked like clockwork, which was remarkable given where we were. It’s one thing to be in the hospital for an event like that. But to be on a baseball field where you have nothing is a completely different scenario.
Meanwhile, the game went on.
I had requested that all the kids be placed in the dugout when they weren’t on the field. So they saw the umpire walk off, but none of them saw him arrest. Some parents were really helpful with making sure the kids were okay.
The president of Oxford Little League ran across the street to a fire station to get an AED. But the fire department personnel were out on a call. He had to break down the door.
By the time he got back, the umpire’s vital signs were returning. And then EMS arrived.
They loaded him in the ambulance, and I called ahead to the trauma team, so they knew exactly what was happening.
I was pretty worried. My hypothesis was that there was probably compression on the vasculature, which had caused him to lose his vital signs. I thought he probably had an impending airway loss. I wasn’t sure if he was going to make it through the night.
What I didn’t know was that while I was giving CPR, my son stole home, and we won the game. As the ambulance was leaving, the celebration was going on in the outfield.
The umpire was in the hospital for several days. Early on, I got permission from his family to visit him. The first time I saw him, I felt this incredible gratitude and peace.
My dad was an ER doctor, and growing up, it seemed like every time we went on a family vacation, there was an emergency. We would be near a car accident or something, and my father would fly in and save the day. I remember being on the Autobahn somewhere in Europe, and there was a devastating accident between a car and a motorcycle. My father stabilized the guy, had him airlifted out, and apparently, he did fine. I grew up watching things like this and thinking, wow, that’s incredible.
Fast forward to 2 years ago, my father was diagnosed with a lung cancer he never should have had. He never smoked. As a cancer surgeon, I know we did everything in our power to save him. But it didn’t happen. He passed away.
I realize this is superstitious, but seeing the umpire alive, I had this feeling that somehow my dad was there. It was bittersweet but also a joyful moment — like I could breathe again.
I met the umpire’s family that first time, and it was like meeting family that you didn’t know you had but now you have forever. Even though the event was traumatic — I’m still trying not to be on high alert every time I go to a game — it felt like a gift to be part of this journey with them.
Little League’s mission is to teach kids about teamwork, leadership, and making good choices so communities are stronger. Our umpire is a guy who does that every day. He’s not a Little League umpire because he makes any money. He shows up at every single game to support these kids and engage them, to model respect, gratitude, and kindness.
I think our obligation as people is to live with intentionality. We all need to make sure we leave the world a better place, even when we are called upon to do uncomfortable things. Our umpire showed our kids what that looks like, and in that moment when he could have died, we were able to do the same for him.
Jennifer LaFemina, MD, is a surgical oncologist at UMass Memorial Medical Center in Massachusetts.
Are you a medical professional with a dramatic story outside the clinic? Medscape Medical News would love to consider your story for Is There a Doctor in the House? Please email your contact information and a short summary to [email protected].
A version of this article appeared on Medscape.com.
Emergencies happen anywhere, anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a Medscape Medical News series telling these stories.
I sincerely believe that what goes around comes around. Good things come to good people. And sometimes that saves lives.
My 10-year-old son was in the semifinals of the Little League district championship. And we were losing. My son is an excellent pitcher, and he had started the game. But that night, he was struggling. He just couldn’t find where to throw the ball. Needless to say, he was frustrated.
He was changed to shortstop in the second inning, and the home plate umpire walked over to him. This umpire is well known in the area for his kindness and commitment, how he encourages the kids and helps make baseball fun even when it’s stressful.
We didn’t know him well, but he was really supportive of my kid in that moment, talking to him about how baseball is a team sport and we’re here to have fun. Just being really positive.
As the game continued, I saw the umpire suddenly walk to the side of the field. I hadn’t seen it, but he had been hit by a wild pitch on the side of his neck. He was wearing protective gear, but the ball managed to bounce up the side and caught bare neck. I knew something wasn’t right.
I went down to talk to him, and my medical assistant (MA), who was also at the game, came with me. I could tell the umpire was injured, but he didn’t want to leave the game. I suggested going to the hospital, but he wouldn’t consider it. So I sat there with my arms crossed, watching him.
His symptoms got worse. I could see he was in pain, and it was getting harder for him to speak.
Again, I strongly urged him to go to the hospital, but again, he said no.
In the sixth inning, things got bad enough that the umpire finally agreed to leave the game. As I was figuring out how to get him to the hospital, he disappeared on me. He had walked up to the second floor of the snack shack. My MA and I got him back downstairs and sat him on a bench behind home plate.
We were in the process of calling 911 ... when he arrested.
Luckily, when he lost vital signs, my MA and I were standing right next to him. We were able to activate ACLS protocol and start CPR within seconds.
Many times in these critical situations — especially if people are scared or have never seen an emergency like this — there’s the potential for chaos. Well, that was the polar opposite of what happened.
As soon as I started to run the code, there was this sense of order. People were keeping their composure and following directions. My MA and I would say, “this is what we need,” and the task would immediately be assigned to someone. It was quiet. There was no yelling. Everyone trusted me, even though some of them had never met me before. It was so surprising. I remember thinking, we’re running an arrest, but it’s so calm.
We were an organized team, and it really worked like clockwork, which was remarkable given where we were. It’s one thing to be in the hospital for an event like that. But to be on a baseball field where you have nothing is a completely different scenario.
Meanwhile, the game went on.
I had requested that all the kids be placed in the dugout when they weren’t on the field. So they saw the umpire walk off, but none of them saw him arrest. Some parents were really helpful with making sure the kids were okay.
The president of Oxford Little League ran across the street to a fire station to get an AED. But the fire department personnel were out on a call. He had to break down the door.
By the time he got back, the umpire’s vital signs were returning. And then EMS arrived.
They loaded him in the ambulance, and I called ahead to the trauma team, so they knew exactly what was happening.
I was pretty worried. My hypothesis was that there was probably compression on the vasculature, which had caused him to lose his vital signs. I thought he probably had an impending airway loss. I wasn’t sure if he was going to make it through the night.
What I didn’t know was that while I was giving CPR, my son stole home, and we won the game. As the ambulance was leaving, the celebration was going on in the outfield.
The umpire was in the hospital for several days. Early on, I got permission from his family to visit him. The first time I saw him, I felt this incredible gratitude and peace.
My dad was an ER doctor, and growing up, it seemed like every time we went on a family vacation, there was an emergency. We would be near a car accident or something, and my father would fly in and save the day. I remember being on the Autobahn somewhere in Europe, and there was a devastating accident between a car and a motorcycle. My father stabilized the guy, had him airlifted out, and apparently, he did fine. I grew up watching things like this and thinking, wow, that’s incredible.
Fast forward to 2 years ago, my father was diagnosed with a lung cancer he never should have had. He never smoked. As a cancer surgeon, I know we did everything in our power to save him. But it didn’t happen. He passed away.
I realize this is superstitious, but seeing the umpire alive, I had this feeling that somehow my dad was there. It was bittersweet but also a joyful moment — like I could breathe again.
I met the umpire’s family that first time, and it was like meeting family that you didn’t know you had but now you have forever. Even though the event was traumatic — I’m still trying not to be on high alert every time I go to a game — it felt like a gift to be part of this journey with them.
Little League’s mission is to teach kids about teamwork, leadership, and making good choices so communities are stronger. Our umpire is a guy who does that every day. He’s not a Little League umpire because he makes any money. He shows up at every single game to support these kids and engage them, to model respect, gratitude, and kindness.
I think our obligation as people is to live with intentionality. We all need to make sure we leave the world a better place, even when we are called upon to do uncomfortable things. Our umpire showed our kids what that looks like, and in that moment when he could have died, we were able to do the same for him.
Jennifer LaFemina, MD, is a surgical oncologist at UMass Memorial Medical Center in Massachusetts.
Are you a medical professional with a dramatic story outside the clinic? Medscape Medical News would love to consider your story for Is There a Doctor in the House? Please email your contact information and a short summary to [email protected].
A version of this article appeared on Medscape.com.
Is CGM the New CBT?
Lauren is a 45-year-old corporate lawyer who managed to excel in every aspect of her life, including parenting her three children while working full-time as a corporate lawyer. A math major at Harvard, she loves data.
Suffice it to say, given that I was treating her for a thyroid condition rather than diabetes, I was a little surprised when she requested I prescribe her a FreeStyle Libre (Abbott) monitor. She explained she was struggling to lose 10 pounds, and she thought continuous glucose monitoring (CGM) would help her determine which foods were impeding her weight loss journey.
While I didn’t see much downside to acquiescing, I felt she had probably been spending too much time on Reddit. What information could CGM give someone without diabetes that couldn’t be gleaned from a food label? Nevertheless, Lauren filled the prescription and began her foray into this relatively uncharted world. When she returned for a follow-up visit several months later, I was shocked to see that she had lost her intended weight. With my tail between my legs, I decided to review the theories and science behind the use of CGM in patients without insulin resistance.
Although it’s not rocket science, CGM can help patients through a “carrot and stick” approach to dieting. Lean proteins, nonstarchy vegetables, and monounsaturated fats such as nuts and avocado all support weight loss and tend to keep blood glucose levels stable. In contrast, foods known to cause weight gain (eg, sugary foods, refined starches, and processed foods) cause sugar spikes in real time. Similarly, large portion sizes are more likely to result in sugar spikes, and pairing proteins with carbohydrates minimizes blood glucose excursions.
Though all of this is basic common sense, . And because blood glucose is influenced by myriad factors including stress, genetics and metabolism, CGM can also potentially help create personal guidance for food choices.
In addition, CGM can reveal the effect of poor sleep and stress on blood glucose levels, thereby encouraging healthier lifestyle choices. The data collected also may provide information on how different modalities of physical activity affect blood glucose levels. A recent study compared the effect of high-intensity interval training (HIIT) and continuous moderate-intensity exercise on postmeal blood glucose in overweight individuals without diabetes. CGM revealed that HIIT is more advantageous for preventing postmeal spikes.
Although CGM appears to be a sophisticated form of cognitive-behavioral therapy, I do worry that the incessant stream of information can lead to worsening anxiety, obsessive compulsive behaviors, or restrictive eating tendencies. Still, thanks to Lauren, I now believe that real-time CGM may lead to behavior modification in food selection and physical activity.
Dr. Messer, Clinical Assistant Professor, Mount Sinai School of Medicine; Associate Professor, Hofstra School of Medicine, New York, NY, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Lauren is a 45-year-old corporate lawyer who managed to excel in every aspect of her life, including parenting her three children while working full-time as a corporate lawyer. A math major at Harvard, she loves data.
Suffice it to say, given that I was treating her for a thyroid condition rather than diabetes, I was a little surprised when she requested I prescribe her a FreeStyle Libre (Abbott) monitor. She explained she was struggling to lose 10 pounds, and she thought continuous glucose monitoring (CGM) would help her determine which foods were impeding her weight loss journey.
While I didn’t see much downside to acquiescing, I felt she had probably been spending too much time on Reddit. What information could CGM give someone without diabetes that couldn’t be gleaned from a food label? Nevertheless, Lauren filled the prescription and began her foray into this relatively uncharted world. When she returned for a follow-up visit several months later, I was shocked to see that she had lost her intended weight. With my tail between my legs, I decided to review the theories and science behind the use of CGM in patients without insulin resistance.
Although it’s not rocket science, CGM can help patients through a “carrot and stick” approach to dieting. Lean proteins, nonstarchy vegetables, and monounsaturated fats such as nuts and avocado all support weight loss and tend to keep blood glucose levels stable. In contrast, foods known to cause weight gain (eg, sugary foods, refined starches, and processed foods) cause sugar spikes in real time. Similarly, large portion sizes are more likely to result in sugar spikes, and pairing proteins with carbohydrates minimizes blood glucose excursions.
Though all of this is basic common sense, . And because blood glucose is influenced by myriad factors including stress, genetics and metabolism, CGM can also potentially help create personal guidance for food choices.
In addition, CGM can reveal the effect of poor sleep and stress on blood glucose levels, thereby encouraging healthier lifestyle choices. The data collected also may provide information on how different modalities of physical activity affect blood glucose levels. A recent study compared the effect of high-intensity interval training (HIIT) and continuous moderate-intensity exercise on postmeal blood glucose in overweight individuals without diabetes. CGM revealed that HIIT is more advantageous for preventing postmeal spikes.
Although CGM appears to be a sophisticated form of cognitive-behavioral therapy, I do worry that the incessant stream of information can lead to worsening anxiety, obsessive compulsive behaviors, or restrictive eating tendencies. Still, thanks to Lauren, I now believe that real-time CGM may lead to behavior modification in food selection and physical activity.
Dr. Messer, Clinical Assistant Professor, Mount Sinai School of Medicine; Associate Professor, Hofstra School of Medicine, New York, NY, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Lauren is a 45-year-old corporate lawyer who managed to excel in every aspect of her life, including parenting her three children while working full-time as a corporate lawyer. A math major at Harvard, she loves data.
Suffice it to say, given that I was treating her for a thyroid condition rather than diabetes, I was a little surprised when she requested I prescribe her a FreeStyle Libre (Abbott) monitor. She explained she was struggling to lose 10 pounds, and she thought continuous glucose monitoring (CGM) would help her determine which foods were impeding her weight loss journey.
While I didn’t see much downside to acquiescing, I felt she had probably been spending too much time on Reddit. What information could CGM give someone without diabetes that couldn’t be gleaned from a food label? Nevertheless, Lauren filled the prescription and began her foray into this relatively uncharted world. When she returned for a follow-up visit several months later, I was shocked to see that she had lost her intended weight. With my tail between my legs, I decided to review the theories and science behind the use of CGM in patients without insulin resistance.
Although it’s not rocket science, CGM can help patients through a “carrot and stick” approach to dieting. Lean proteins, nonstarchy vegetables, and monounsaturated fats such as nuts and avocado all support weight loss and tend to keep blood glucose levels stable. In contrast, foods known to cause weight gain (eg, sugary foods, refined starches, and processed foods) cause sugar spikes in real time. Similarly, large portion sizes are more likely to result in sugar spikes, and pairing proteins with carbohydrates minimizes blood glucose excursions.
Though all of this is basic common sense, . And because blood glucose is influenced by myriad factors including stress, genetics and metabolism, CGM can also potentially help create personal guidance for food choices.
In addition, CGM can reveal the effect of poor sleep and stress on blood glucose levels, thereby encouraging healthier lifestyle choices. The data collected also may provide information on how different modalities of physical activity affect blood glucose levels. A recent study compared the effect of high-intensity interval training (HIIT) and continuous moderate-intensity exercise on postmeal blood glucose in overweight individuals without diabetes. CGM revealed that HIIT is more advantageous for preventing postmeal spikes.
Although CGM appears to be a sophisticated form of cognitive-behavioral therapy, I do worry that the incessant stream of information can lead to worsening anxiety, obsessive compulsive behaviors, or restrictive eating tendencies. Still, thanks to Lauren, I now believe that real-time CGM may lead to behavior modification in food selection and physical activity.
Dr. Messer, Clinical Assistant Professor, Mount Sinai School of Medicine; Associate Professor, Hofstra School of Medicine, New York, NY, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Home HPV Testing: A New Frontier in Primary Care
Human papillomavirus (HPV) is one of the most common sexually transmitted infections and persistent infection with high-risk strains is the leading cause of cervical cancer. Fortunately, vaccines are available to prevent many HPV-related diseases, but they haven’t fully eliminated the risks. Cervical cancer screening remains essential for early detection and prevention.
The US Preventive Services Task Force (USPSTF) currently recommends regular cervical cancer screenings for women aged 21-65. These screenings can include a Pap test every 3 years, a combination of HPV testing and Pap smear every 5 years, or high-risk HPV testing alone every 5 years, depending on age and individual risk factors.
Although these guidelines are currently under review, routine screenings have been instrumental in reducing cervical cancer rates. However, many patients still face barriers that prevent them from accessing these services. Common challenges include discomfort with pelvic exams, lack of time, and limited access to healthcare services. In recent years, advancements in home-based diagnostic testing have opened new avenues for preventative care.
Home HPV testing is one such advancement, offering an alternative to traditional in-office screening methods. While the US Food and Drug Administration (FDA) has not yet approved home HPV testing, self-collection in clinical settings is available and gaining traction. Primary care physicians can integrate this self-collection method into their practices, helping to close the screening gap, especially for underserved populations.
If approved, home HPV testing could be a game-changer for patients who have difficulty attending in-person visits. Geographical barriers, transportation issues, and personal discomfort with in-office exams can prevent patients from receiving the care they need. Home testing eliminates many of these hurdles, enabling patients to perform the test in the comfort of their own homes at a time that works for them. This flexibility is particularly beneficial for rural and underserved populations, where access to healthcare is limited.
Similarly, in-office self-collection offers a comfortable alternative for those who find traditional pelvic exams uncomfortable or distressing. Self-administered HPV tests allow patients to take control of their cervical cancer screening, fostering empowerment and personal responsibility for their health. By reducing the discomfort and inconvenience of traditional screening, self-collection can improve adherence to screening guidelines, leading to earlier detection and prevention of cervical cancer.
Primary care physicians may soon offer both in-office and at-home testing options, tailoring the approach to each patient’s unique needs. Virtual appointments provide an excellent opportunity to educate patients about the importance of cervical cancer screening and offer guidance on using home HPV testing kits. This personalized care ensures patients feel supported even without in-person visits. If home testing becomes FDA approved, patients could receive test kits by mail, perform the test, and send it back to the lab for analysis. For those with positive results, primary care physicians can ensure timely follow-up, including Pap smears or colposcopies, to further evaluate cervical health.
Although home HPV testing offers many benefits, there are valid concerns about accuracy and follow-up care. Studies show that self-collected samples for HPV testing are highly accurate, with sensitivity and specificity comparable with clinician-collected samples, echoing the success of self-swabbing for other sexually transmitted infections.
It is crucial, however, that patients receive clear instructions on proper sample collection to maintain this accuracy. Follow-up care is another essential aspect of the screening process. While many HPV infections resolve on their own, high-risk strains require closer monitoring to prevent progression to cervical cancer. Primary care physicians must establish clear protocols for notifying patients of their results and ensuring appropriate follow-up appointments.
Additionally, there may be concerns about the cost and insurance coverage of home HPV tests. However, home testing could prove more cost-effective than multiple in-office visits, especially when factoring in travel expenses and missed work. Physicians should work to make home testing accessible to all patients, including those in low-income and rural communities.
Should these options become more widely available, it will be important to communicate that this does not fully eliminate the need for pelvic exams. As primary care physicians, we will still need to advise patients that they should bring up concerns of vaginal bleeding, vaginal discharge, and other symptoms. Pelvic exams will still be necessary for diagnosis when symptoms are present. Home HPV tests also will not replace in-office clinician collected exams for those who do not feel comfortable with self-collection.
Home and in-office self-collection for HPV testing are promising tools for improving cervical cancer screening rates and patient satisfaction. By offering a convenient, private, and accessible option, primary care physicians can help more patients stay on track with their preventive care and reduce their risk of cervical cancer. As this technology continues to evolve, embracing both in-office and home HPV testing will be essential to ensuring all patients benefit from these innovations.
Dr. Wheat is Vice Chair of Diversity, Equity, and Inclusion, Department of Family and Community Medicine and Associate Professor, Family and Community Medicine Feinberg School of Medicine, Northwestern University, Chicago. She serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
Daponte N et al. HPV-Based Self-Sampling in Cervical Cancer Screening: An Updated Review of the Current Evidence in the Literature. Cancers (Basel). 2023 Mar 8;15(6):1669.
Di Gennaro G et al. Does self-sampling for human papilloma virus testing have the potential to increase cervical cancer screening? An updated meta-analysis of observational studies and randomized clinical trials. Front Public Health. 2022 Dec 8;10:1003461.
US Preventive Services Task Force. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(7):674-686.
Human papillomavirus (HPV) is one of the most common sexually transmitted infections and persistent infection with high-risk strains is the leading cause of cervical cancer. Fortunately, vaccines are available to prevent many HPV-related diseases, but they haven’t fully eliminated the risks. Cervical cancer screening remains essential for early detection and prevention.
The US Preventive Services Task Force (USPSTF) currently recommends regular cervical cancer screenings for women aged 21-65. These screenings can include a Pap test every 3 years, a combination of HPV testing and Pap smear every 5 years, or high-risk HPV testing alone every 5 years, depending on age and individual risk factors.
Although these guidelines are currently under review, routine screenings have been instrumental in reducing cervical cancer rates. However, many patients still face barriers that prevent them from accessing these services. Common challenges include discomfort with pelvic exams, lack of time, and limited access to healthcare services. In recent years, advancements in home-based diagnostic testing have opened new avenues for preventative care.
Home HPV testing is one such advancement, offering an alternative to traditional in-office screening methods. While the US Food and Drug Administration (FDA) has not yet approved home HPV testing, self-collection in clinical settings is available and gaining traction. Primary care physicians can integrate this self-collection method into their practices, helping to close the screening gap, especially for underserved populations.
If approved, home HPV testing could be a game-changer for patients who have difficulty attending in-person visits. Geographical barriers, transportation issues, and personal discomfort with in-office exams can prevent patients from receiving the care they need. Home testing eliminates many of these hurdles, enabling patients to perform the test in the comfort of their own homes at a time that works for them. This flexibility is particularly beneficial for rural and underserved populations, where access to healthcare is limited.
Similarly, in-office self-collection offers a comfortable alternative for those who find traditional pelvic exams uncomfortable or distressing. Self-administered HPV tests allow patients to take control of their cervical cancer screening, fostering empowerment and personal responsibility for their health. By reducing the discomfort and inconvenience of traditional screening, self-collection can improve adherence to screening guidelines, leading to earlier detection and prevention of cervical cancer.
Primary care physicians may soon offer both in-office and at-home testing options, tailoring the approach to each patient’s unique needs. Virtual appointments provide an excellent opportunity to educate patients about the importance of cervical cancer screening and offer guidance on using home HPV testing kits. This personalized care ensures patients feel supported even without in-person visits. If home testing becomes FDA approved, patients could receive test kits by mail, perform the test, and send it back to the lab for analysis. For those with positive results, primary care physicians can ensure timely follow-up, including Pap smears or colposcopies, to further evaluate cervical health.
Although home HPV testing offers many benefits, there are valid concerns about accuracy and follow-up care. Studies show that self-collected samples for HPV testing are highly accurate, with sensitivity and specificity comparable with clinician-collected samples, echoing the success of self-swabbing for other sexually transmitted infections.
It is crucial, however, that patients receive clear instructions on proper sample collection to maintain this accuracy. Follow-up care is another essential aspect of the screening process. While many HPV infections resolve on their own, high-risk strains require closer monitoring to prevent progression to cervical cancer. Primary care physicians must establish clear protocols for notifying patients of their results and ensuring appropriate follow-up appointments.
Additionally, there may be concerns about the cost and insurance coverage of home HPV tests. However, home testing could prove more cost-effective than multiple in-office visits, especially when factoring in travel expenses and missed work. Physicians should work to make home testing accessible to all patients, including those in low-income and rural communities.
Should these options become more widely available, it will be important to communicate that this does not fully eliminate the need for pelvic exams. As primary care physicians, we will still need to advise patients that they should bring up concerns of vaginal bleeding, vaginal discharge, and other symptoms. Pelvic exams will still be necessary for diagnosis when symptoms are present. Home HPV tests also will not replace in-office clinician collected exams for those who do not feel comfortable with self-collection.
Home and in-office self-collection for HPV testing are promising tools for improving cervical cancer screening rates and patient satisfaction. By offering a convenient, private, and accessible option, primary care physicians can help more patients stay on track with their preventive care and reduce their risk of cervical cancer. As this technology continues to evolve, embracing both in-office and home HPV testing will be essential to ensuring all patients benefit from these innovations.
Dr. Wheat is Vice Chair of Diversity, Equity, and Inclusion, Department of Family and Community Medicine and Associate Professor, Family and Community Medicine Feinberg School of Medicine, Northwestern University, Chicago. She serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
Daponte N et al. HPV-Based Self-Sampling in Cervical Cancer Screening: An Updated Review of the Current Evidence in the Literature. Cancers (Basel). 2023 Mar 8;15(6):1669.
Di Gennaro G et al. Does self-sampling for human papilloma virus testing have the potential to increase cervical cancer screening? An updated meta-analysis of observational studies and randomized clinical trials. Front Public Health. 2022 Dec 8;10:1003461.
US Preventive Services Task Force. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(7):674-686.
Human papillomavirus (HPV) is one of the most common sexually transmitted infections and persistent infection with high-risk strains is the leading cause of cervical cancer. Fortunately, vaccines are available to prevent many HPV-related diseases, but they haven’t fully eliminated the risks. Cervical cancer screening remains essential for early detection and prevention.
The US Preventive Services Task Force (USPSTF) currently recommends regular cervical cancer screenings for women aged 21-65. These screenings can include a Pap test every 3 years, a combination of HPV testing and Pap smear every 5 years, or high-risk HPV testing alone every 5 years, depending on age and individual risk factors.
Although these guidelines are currently under review, routine screenings have been instrumental in reducing cervical cancer rates. However, many patients still face barriers that prevent them from accessing these services. Common challenges include discomfort with pelvic exams, lack of time, and limited access to healthcare services. In recent years, advancements in home-based diagnostic testing have opened new avenues for preventative care.
Home HPV testing is one such advancement, offering an alternative to traditional in-office screening methods. While the US Food and Drug Administration (FDA) has not yet approved home HPV testing, self-collection in clinical settings is available and gaining traction. Primary care physicians can integrate this self-collection method into their practices, helping to close the screening gap, especially for underserved populations.
If approved, home HPV testing could be a game-changer for patients who have difficulty attending in-person visits. Geographical barriers, transportation issues, and personal discomfort with in-office exams can prevent patients from receiving the care they need. Home testing eliminates many of these hurdles, enabling patients to perform the test in the comfort of their own homes at a time that works for them. This flexibility is particularly beneficial for rural and underserved populations, where access to healthcare is limited.
Similarly, in-office self-collection offers a comfortable alternative for those who find traditional pelvic exams uncomfortable or distressing. Self-administered HPV tests allow patients to take control of their cervical cancer screening, fostering empowerment and personal responsibility for their health. By reducing the discomfort and inconvenience of traditional screening, self-collection can improve adherence to screening guidelines, leading to earlier detection and prevention of cervical cancer.
Primary care physicians may soon offer both in-office and at-home testing options, tailoring the approach to each patient’s unique needs. Virtual appointments provide an excellent opportunity to educate patients about the importance of cervical cancer screening and offer guidance on using home HPV testing kits. This personalized care ensures patients feel supported even without in-person visits. If home testing becomes FDA approved, patients could receive test kits by mail, perform the test, and send it back to the lab for analysis. For those with positive results, primary care physicians can ensure timely follow-up, including Pap smears or colposcopies, to further evaluate cervical health.
Although home HPV testing offers many benefits, there are valid concerns about accuracy and follow-up care. Studies show that self-collected samples for HPV testing are highly accurate, with sensitivity and specificity comparable with clinician-collected samples, echoing the success of self-swabbing for other sexually transmitted infections.
It is crucial, however, that patients receive clear instructions on proper sample collection to maintain this accuracy. Follow-up care is another essential aspect of the screening process. While many HPV infections resolve on their own, high-risk strains require closer monitoring to prevent progression to cervical cancer. Primary care physicians must establish clear protocols for notifying patients of their results and ensuring appropriate follow-up appointments.
Additionally, there may be concerns about the cost and insurance coverage of home HPV tests. However, home testing could prove more cost-effective than multiple in-office visits, especially when factoring in travel expenses and missed work. Physicians should work to make home testing accessible to all patients, including those in low-income and rural communities.
Should these options become more widely available, it will be important to communicate that this does not fully eliminate the need for pelvic exams. As primary care physicians, we will still need to advise patients that they should bring up concerns of vaginal bleeding, vaginal discharge, and other symptoms. Pelvic exams will still be necessary for diagnosis when symptoms are present. Home HPV tests also will not replace in-office clinician collected exams for those who do not feel comfortable with self-collection.
Home and in-office self-collection for HPV testing are promising tools for improving cervical cancer screening rates and patient satisfaction. By offering a convenient, private, and accessible option, primary care physicians can help more patients stay on track with their preventive care and reduce their risk of cervical cancer. As this technology continues to evolve, embracing both in-office and home HPV testing will be essential to ensuring all patients benefit from these innovations.
Dr. Wheat is Vice Chair of Diversity, Equity, and Inclusion, Department of Family and Community Medicine and Associate Professor, Family and Community Medicine Feinberg School of Medicine, Northwestern University, Chicago. She serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
Daponte N et al. HPV-Based Self-Sampling in Cervical Cancer Screening: An Updated Review of the Current Evidence in the Literature. Cancers (Basel). 2023 Mar 8;15(6):1669.
Di Gennaro G et al. Does self-sampling for human papilloma virus testing have the potential to increase cervical cancer screening? An updated meta-analysis of observational studies and randomized clinical trials. Front Public Health. 2022 Dec 8;10:1003461.
US Preventive Services Task Force. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(7):674-686.
Sex After Pregnancy: Why It Matters and How to Start the Conversation
Sarah, a new mom who’s thrilled about her 3-month-old baby, is struggling with her sex life. Her once vibrant physical relationship with her partner has dwindled, and she’s dealing with painful intercourse and a notable drop in desire.
Pregnancy and childbirth are transformative experiences that affect every facet of a person’s life, including their sexual well-being. Despite this fact, clinicians frequently ignore sexual well-being, beyond contraception, in prenatal and postpartum care. In peripartum care, anticipatory guidance is recognized to be crucial to the well-being of new parents and their babies. Why should sexual well-being get the shaft?
Why Talk About Sex?
Sex is a fundamental aspect of many people’s lives and relationships and can significantly affect overall well-being. Despite cultural narratives that often exclude the sexual function and pleasure aspect of sexual health from peripartum discussions, many new parents face sexual challenges that can worsen their physical and emotional health.
While up to 88% of new parents report problems with sexual well-being, less than 30% report receiving anticipatory guidance about sexual function changes. One study found only 15% of postpartum women reported discussing sexual concerns with their medical providers. And, when new parents receive more information about sexual health, they tend to report improved sexual well-being. Clearly, a gap needs bridging.
Sexual health doesn’t just affect individual well-being; it intertwines with relationship satisfaction. Attending to satisfaction in one’s relationship may be an important component of child health as well.
Declines in the frequency of sexual engagement and desire are common after childbirth. Changes in arousal, orgasm, and sexual pleasure, often accompanied by pain, are also reported by many women. Some birthing parents report changes to the sensation of their genitals that are thought to be related to stretch of the pudendal nerve during parturition. Most experience resolution of these concerns within the first year after childbirth, although some women report persistent problems, including up to 33% with persistent sexual pain.
Many factors can contribute to postpartum sexual issues, including hormonal changes, body image concerns, and mental health conditions. Breastfeeding, for instance, can lead to vaginal dryness and reduced sexual arousal due to hormonal shifts. Body image issues and mood disorders like depression and anxiety can also adversely affect sexual function. Women with postpartum depression are more likely to experience sexual concerns, and the relationship between sexual difficulties and depression can be bidirectional.
Empowerment and Expectations
One commonly cited recommendation is to wait until 6 weeks postpartum before resuming penetrative intercourse after a normal vaginal delivery. However, this guideline lacks robust scientific backing. Many people might feel ready for sexual intercourse much sooner or, conversely, might not feel comfortable at the 6-week mark. As clinicians, we must empower our patients to trust their own bodies and make decisions based on their comfort and readiness.
The 6-week advice can sometimes unintentionally convey to women that they are not experts on their own bodies, or that any kind of sex is risky. Acknowledging the recovery timeline for every person is unique and various forms of sexual expression are safe can help foster a healthier approach to resuming sexual activities. In one study of postpartum sexual behavior, in the first 6 weeks after delivery, the most common kinds of sexual play included giving oral sex to a partner and solo sex. Between 80% and 90% have resumed vaginal-receiving sexual play (including intercourse) by 3 months.
While recognizing that changes to sexual experience occur, we need to reinforce that gradual recovery is expected. And if women express distress about a sexual change, or if those changes persist, primary care providers should be prepared to help them with their concerns.
Parents need to know experiencing pain is not something they should “just deal with” or ignore. Attempting to repeatedly endure sexual pain can cause new issues, such as high-tone dysfunction of the pelvic floor or an understandable decrease in willingness or receptiveness to sexual play of any kind. Encouraging open communication about these issues can help couples navigate these changes more smoothly.
Partners, especially new fathers, also experience sexual and emotional challenges. They can feel blindsided by the changes in their relationship and might struggle with feelings of jealousy or inadequacy. Understanding partners also face difficulties can help in providing a more comprehensive approach to sexual health care.
Starting the Conversation
So, how can we initiate these important conversations with new parents? Start by providing permission. As healthcare providers, we need to create an environment where discussing sexual health is normalized and welcomed. Simple, nonjudgmental statements can open the door to these discussions. For example, saying, “Many people notice changes in their sexual desire or pleasure after childbirth. Has anything like this happened to you or your partner?” can encourage patients to share their concerns.
Assessing the importance to patients of sexual problems can help direct the need for intervention. Follow up on these concerns and offer support, whether through counseling, pelvic floor physical therapy, or a referral to a sexual medicine specialist, a sex therapist, or other appropriate resource.
Let’s return to Sarah. In the ideal world, at 3 months postpartum she will already have had a handful of clinical conversations about her sexual well-being with her healthcare team — at prenatal visits, at well-baby visits, and at her postpartum checkups. Several of these conversations included her partner. They both understand the transition to parenthood could be rocky for their sex lives. They’ve set aside time to connect and stay physically close. She’s listened to her body and only engaged in sexual play for which she feels ready. Now, noting that some aspects of sexual play are persistently uncomfortable, she knows it’s time to follow up. Without shame or anxiety, she books an appointment with you, knowing that you understand how important this issue is for her, her partner, and her baby.
If you’re working with new parents, remember: Open dialogue about sexual health is not just beneficial — it’s essential. Let’s bridge the gap in care by embracing these conversations and offering the support new parents truly need.
Dr. Kranz, Clinical Assistant Professor of Obstetrics/Gynecology and Family Medicine, University of Rochester Medical Center, Rochester, New York, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sarah, a new mom who’s thrilled about her 3-month-old baby, is struggling with her sex life. Her once vibrant physical relationship with her partner has dwindled, and she’s dealing with painful intercourse and a notable drop in desire.
Pregnancy and childbirth are transformative experiences that affect every facet of a person’s life, including their sexual well-being. Despite this fact, clinicians frequently ignore sexual well-being, beyond contraception, in prenatal and postpartum care. In peripartum care, anticipatory guidance is recognized to be crucial to the well-being of new parents and their babies. Why should sexual well-being get the shaft?
Why Talk About Sex?
Sex is a fundamental aspect of many people’s lives and relationships and can significantly affect overall well-being. Despite cultural narratives that often exclude the sexual function and pleasure aspect of sexual health from peripartum discussions, many new parents face sexual challenges that can worsen their physical and emotional health.
While up to 88% of new parents report problems with sexual well-being, less than 30% report receiving anticipatory guidance about sexual function changes. One study found only 15% of postpartum women reported discussing sexual concerns with their medical providers. And, when new parents receive more information about sexual health, they tend to report improved sexual well-being. Clearly, a gap needs bridging.
Sexual health doesn’t just affect individual well-being; it intertwines with relationship satisfaction. Attending to satisfaction in one’s relationship may be an important component of child health as well.
Declines in the frequency of sexual engagement and desire are common after childbirth. Changes in arousal, orgasm, and sexual pleasure, often accompanied by pain, are also reported by many women. Some birthing parents report changes to the sensation of their genitals that are thought to be related to stretch of the pudendal nerve during parturition. Most experience resolution of these concerns within the first year after childbirth, although some women report persistent problems, including up to 33% with persistent sexual pain.
Many factors can contribute to postpartum sexual issues, including hormonal changes, body image concerns, and mental health conditions. Breastfeeding, for instance, can lead to vaginal dryness and reduced sexual arousal due to hormonal shifts. Body image issues and mood disorders like depression and anxiety can also adversely affect sexual function. Women with postpartum depression are more likely to experience sexual concerns, and the relationship between sexual difficulties and depression can be bidirectional.
Empowerment and Expectations
One commonly cited recommendation is to wait until 6 weeks postpartum before resuming penetrative intercourse after a normal vaginal delivery. However, this guideline lacks robust scientific backing. Many people might feel ready for sexual intercourse much sooner or, conversely, might not feel comfortable at the 6-week mark. As clinicians, we must empower our patients to trust their own bodies and make decisions based on their comfort and readiness.
The 6-week advice can sometimes unintentionally convey to women that they are not experts on their own bodies, or that any kind of sex is risky. Acknowledging the recovery timeline for every person is unique and various forms of sexual expression are safe can help foster a healthier approach to resuming sexual activities. In one study of postpartum sexual behavior, in the first 6 weeks after delivery, the most common kinds of sexual play included giving oral sex to a partner and solo sex. Between 80% and 90% have resumed vaginal-receiving sexual play (including intercourse) by 3 months.
While recognizing that changes to sexual experience occur, we need to reinforce that gradual recovery is expected. And if women express distress about a sexual change, or if those changes persist, primary care providers should be prepared to help them with their concerns.
Parents need to know experiencing pain is not something they should “just deal with” or ignore. Attempting to repeatedly endure sexual pain can cause new issues, such as high-tone dysfunction of the pelvic floor or an understandable decrease in willingness or receptiveness to sexual play of any kind. Encouraging open communication about these issues can help couples navigate these changes more smoothly.
Partners, especially new fathers, also experience sexual and emotional challenges. They can feel blindsided by the changes in their relationship and might struggle with feelings of jealousy or inadequacy. Understanding partners also face difficulties can help in providing a more comprehensive approach to sexual health care.
Starting the Conversation
So, how can we initiate these important conversations with new parents? Start by providing permission. As healthcare providers, we need to create an environment where discussing sexual health is normalized and welcomed. Simple, nonjudgmental statements can open the door to these discussions. For example, saying, “Many people notice changes in their sexual desire or pleasure after childbirth. Has anything like this happened to you or your partner?” can encourage patients to share their concerns.
Assessing the importance to patients of sexual problems can help direct the need for intervention. Follow up on these concerns and offer support, whether through counseling, pelvic floor physical therapy, or a referral to a sexual medicine specialist, a sex therapist, or other appropriate resource.
Let’s return to Sarah. In the ideal world, at 3 months postpartum she will already have had a handful of clinical conversations about her sexual well-being with her healthcare team — at prenatal visits, at well-baby visits, and at her postpartum checkups. Several of these conversations included her partner. They both understand the transition to parenthood could be rocky for their sex lives. They’ve set aside time to connect and stay physically close. She’s listened to her body and only engaged in sexual play for which she feels ready. Now, noting that some aspects of sexual play are persistently uncomfortable, she knows it’s time to follow up. Without shame or anxiety, she books an appointment with you, knowing that you understand how important this issue is for her, her partner, and her baby.
If you’re working with new parents, remember: Open dialogue about sexual health is not just beneficial — it’s essential. Let’s bridge the gap in care by embracing these conversations and offering the support new parents truly need.
Dr. Kranz, Clinical Assistant Professor of Obstetrics/Gynecology and Family Medicine, University of Rochester Medical Center, Rochester, New York, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sarah, a new mom who’s thrilled about her 3-month-old baby, is struggling with her sex life. Her once vibrant physical relationship with her partner has dwindled, and she’s dealing with painful intercourse and a notable drop in desire.
Pregnancy and childbirth are transformative experiences that affect every facet of a person’s life, including their sexual well-being. Despite this fact, clinicians frequently ignore sexual well-being, beyond contraception, in prenatal and postpartum care. In peripartum care, anticipatory guidance is recognized to be crucial to the well-being of new parents and their babies. Why should sexual well-being get the shaft?
Why Talk About Sex?
Sex is a fundamental aspect of many people’s lives and relationships and can significantly affect overall well-being. Despite cultural narratives that often exclude the sexual function and pleasure aspect of sexual health from peripartum discussions, many new parents face sexual challenges that can worsen their physical and emotional health.
While up to 88% of new parents report problems with sexual well-being, less than 30% report receiving anticipatory guidance about sexual function changes. One study found only 15% of postpartum women reported discussing sexual concerns with their medical providers. And, when new parents receive more information about sexual health, they tend to report improved sexual well-being. Clearly, a gap needs bridging.
Sexual health doesn’t just affect individual well-being; it intertwines with relationship satisfaction. Attending to satisfaction in one’s relationship may be an important component of child health as well.
Declines in the frequency of sexual engagement and desire are common after childbirth. Changes in arousal, orgasm, and sexual pleasure, often accompanied by pain, are also reported by many women. Some birthing parents report changes to the sensation of their genitals that are thought to be related to stretch of the pudendal nerve during parturition. Most experience resolution of these concerns within the first year after childbirth, although some women report persistent problems, including up to 33% with persistent sexual pain.
Many factors can contribute to postpartum sexual issues, including hormonal changes, body image concerns, and mental health conditions. Breastfeeding, for instance, can lead to vaginal dryness and reduced sexual arousal due to hormonal shifts. Body image issues and mood disorders like depression and anxiety can also adversely affect sexual function. Women with postpartum depression are more likely to experience sexual concerns, and the relationship between sexual difficulties and depression can be bidirectional.
Empowerment and Expectations
One commonly cited recommendation is to wait until 6 weeks postpartum before resuming penetrative intercourse after a normal vaginal delivery. However, this guideline lacks robust scientific backing. Many people might feel ready for sexual intercourse much sooner or, conversely, might not feel comfortable at the 6-week mark. As clinicians, we must empower our patients to trust their own bodies and make decisions based on their comfort and readiness.
The 6-week advice can sometimes unintentionally convey to women that they are not experts on their own bodies, or that any kind of sex is risky. Acknowledging the recovery timeline for every person is unique and various forms of sexual expression are safe can help foster a healthier approach to resuming sexual activities. In one study of postpartum sexual behavior, in the first 6 weeks after delivery, the most common kinds of sexual play included giving oral sex to a partner and solo sex. Between 80% and 90% have resumed vaginal-receiving sexual play (including intercourse) by 3 months.
While recognizing that changes to sexual experience occur, we need to reinforce that gradual recovery is expected. And if women express distress about a sexual change, or if those changes persist, primary care providers should be prepared to help them with their concerns.
Parents need to know experiencing pain is not something they should “just deal with” or ignore. Attempting to repeatedly endure sexual pain can cause new issues, such as high-tone dysfunction of the pelvic floor or an understandable decrease in willingness or receptiveness to sexual play of any kind. Encouraging open communication about these issues can help couples navigate these changes more smoothly.
Partners, especially new fathers, also experience sexual and emotional challenges. They can feel blindsided by the changes in their relationship and might struggle with feelings of jealousy or inadequacy. Understanding partners also face difficulties can help in providing a more comprehensive approach to sexual health care.
Starting the Conversation
So, how can we initiate these important conversations with new parents? Start by providing permission. As healthcare providers, we need to create an environment where discussing sexual health is normalized and welcomed. Simple, nonjudgmental statements can open the door to these discussions. For example, saying, “Many people notice changes in their sexual desire or pleasure after childbirth. Has anything like this happened to you or your partner?” can encourage patients to share their concerns.
Assessing the importance to patients of sexual problems can help direct the need for intervention. Follow up on these concerns and offer support, whether through counseling, pelvic floor physical therapy, or a referral to a sexual medicine specialist, a sex therapist, or other appropriate resource.
Let’s return to Sarah. In the ideal world, at 3 months postpartum she will already have had a handful of clinical conversations about her sexual well-being with her healthcare team — at prenatal visits, at well-baby visits, and at her postpartum checkups. Several of these conversations included her partner. They both understand the transition to parenthood could be rocky for their sex lives. They’ve set aside time to connect and stay physically close. She’s listened to her body and only engaged in sexual play for which she feels ready. Now, noting that some aspects of sexual play are persistently uncomfortable, she knows it’s time to follow up. Without shame or anxiety, she books an appointment with you, knowing that you understand how important this issue is for her, her partner, and her baby.
If you’re working with new parents, remember: Open dialogue about sexual health is not just beneficial — it’s essential. Let’s bridge the gap in care by embracing these conversations and offering the support new parents truly need.
Dr. Kranz, Clinical Assistant Professor of Obstetrics/Gynecology and Family Medicine, University of Rochester Medical Center, Rochester, New York, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Genitals Are a Window Into Health: Sex as a Vital Sign
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I’m Dr. Rachel Rubin, a urologist and sexual medicine specialist in the Washington, DC, area. And I am so thrilled because my co-fellow, the brilliant and famous Dr. Ashley Winter, a board-certified urologist and a certified menopause practitioner, who sees patients in our practice from Los Angeles, is joining us today to talk about sex as a vital sign.
Ashley Winter, MD: To have the best sexual function, you need many different systems to work. You need your hormones to be in the right place. You need your blood vessels to dilate when you want them to. You need your nerves to connect to your genitalia to make them responsive. The way people say, “The eyes are the window into the soul” — well, the genitals are the window into the cardiovascular system, the peripheral nervous system, and the hormonal system. It’s so dynamic. Patients can understand how this reflects their health. We just need healthcare providers to hammer home how those things connect.
Rubin: If you’re a primary care doctor seeing a patient and you want to educate them on diabetes or high blood pressure, how can you “ ‘sell it with ‘sex”? How can you use sex to educate them about these important medical conditions?
Winter: I hate using it as a fear tactic, but sometimes you have to. Time and again, I’ve seen men with severe profound erectile dysfunction at a young age, with chronically uncontrolled diabetes.
Diabetes can impair the peripheral nerves, resulting in peripheral neuropathy. The same way that it can affect the fingers and toes, diabetes can affect the penis, even before those other areas. Diabetes can also lead to other conditions such as low testosterone, which also affects the function of the penis.
I’m being brutally honest when I tell patients that diabetes control is critical to having a wonderful sexspan — the duration of your life where you’re able to be sexually active and have great sex and do it in the way that you want.
Chronic conditions such as high cholesterol or hypertension can affect your ability to become erect or aroused whether you have a penis or a vulva, and even your ability to have an orgasm.
Rubin: None of my doctors has ever asked me about these issues. But we have to bring them up with patients because they›re not going to bring them up to us. I always say in the review of systems, we shouldn›t just ask, “Do you have any sexual problems?” (which nobody ever does) and move past the question about men, women or both. We should be asking, “Do you have any issues with libido? Do you want to talk about it? Any issues with erection, arousal, orgasm, or sexual pain?”
When you can talk about those things, you can treat the patient from a whole physiologic perspective. For example, how does their sciatica affect their sexual pain? How does their antidepressant cause a delayed orgasm? How does their low testosterone level affect their energy level, their libido, and their desire?
We see so much shame and guilt in sexual health, to the extent that patients feel broken. We can help them understand the anatomy and physiology and explain that they aren’t broken. Instead, it’s “You need this medicine for your crippling anxiety, and that’s why your orgasm is delayed, and so can we augment it or add or subtract something to help you with it.”
Winter: In a primary care setting, where we are considering the patient›s overall health, we strive for medication compliance, but a huge part of medication noncompliance is sexual side effects, whether it›s antidepressants, beta-blockers, birth control, or this new world of GLP-1 agonists.
Rubin: I would add breast cancer treatments. Many patients go off their anastrozole or their tamoxifen because of the sexual side effects.
Winter: This is where we get to the crux of this discussion about sex being a vital sign — something you need to check routinely. We need to become comfortable with it, because then we are unlocking the ability to treat every patient like a whole person, give them better outcomes, improve their compliance, and have a really powerful tool for education.
Rubin: We have a growing toolbox for all genders when it comes to sexual health. We have FDA- approved medications for low libido in women. We use testosterone in men in an evidence-based way to safely improve libido. We use medications to help with the genitourinary syndrome of menopause. Orgasm is a challenging one, but we have devices that can help with those reflexes. And working with people who specialize in sexual pain can be extremely helpful for patients.
Dr. Winter, having practiced in different settings, what would you tell the primary care doctors who don’t want to talk about libido or who minimize sexual complaints because they don’t know how to navigate them?
Winter: I do not envy the challenge of being a primary care provider in the healthcare world we are living in. I think it is the hardest job. The ultimate takeaway is to just normalize the conversation and be able to validate what is happening. Have a few basic tools, and then have referrals. It›s not that you have to have all the time in the world or you have to treat every condition, but you have to start the conversation, be comfortable with it, and then get patients hooked up with the right resources.
Rubin: Every doctor of every kind can connect with patients and try to understand what they care about. What are their goals? What do they want for their families, for their relationships, for their quality of life? And how can we work collaboratively as a team to help them with those things?
Sex is a huge part of people’s lives. If we don’t ask about it; if we don’t look into it; and if we don’t admit that our physiology, our medications, and our surgeries can affect sexual health and functioning, how can we improve people’s lives? We can do so much as a team when we consider sex as a true vital sign.
Dr. Rubin, Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, DC, has disclosed ties with Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I’m Dr. Rachel Rubin, a urologist and sexual medicine specialist in the Washington, DC, area. And I am so thrilled because my co-fellow, the brilliant and famous Dr. Ashley Winter, a board-certified urologist and a certified menopause practitioner, who sees patients in our practice from Los Angeles, is joining us today to talk about sex as a vital sign.
Ashley Winter, MD: To have the best sexual function, you need many different systems to work. You need your hormones to be in the right place. You need your blood vessels to dilate when you want them to. You need your nerves to connect to your genitalia to make them responsive. The way people say, “The eyes are the window into the soul” — well, the genitals are the window into the cardiovascular system, the peripheral nervous system, and the hormonal system. It’s so dynamic. Patients can understand how this reflects their health. We just need healthcare providers to hammer home how those things connect.
Rubin: If you’re a primary care doctor seeing a patient and you want to educate them on diabetes or high blood pressure, how can you “ ‘sell it with ‘sex”? How can you use sex to educate them about these important medical conditions?
Winter: I hate using it as a fear tactic, but sometimes you have to. Time and again, I’ve seen men with severe profound erectile dysfunction at a young age, with chronically uncontrolled diabetes.
Diabetes can impair the peripheral nerves, resulting in peripheral neuropathy. The same way that it can affect the fingers and toes, diabetes can affect the penis, even before those other areas. Diabetes can also lead to other conditions such as low testosterone, which also affects the function of the penis.
I’m being brutally honest when I tell patients that diabetes control is critical to having a wonderful sexspan — the duration of your life where you’re able to be sexually active and have great sex and do it in the way that you want.
Chronic conditions such as high cholesterol or hypertension can affect your ability to become erect or aroused whether you have a penis or a vulva, and even your ability to have an orgasm.
Rubin: None of my doctors has ever asked me about these issues. But we have to bring them up with patients because they›re not going to bring them up to us. I always say in the review of systems, we shouldn›t just ask, “Do you have any sexual problems?” (which nobody ever does) and move past the question about men, women or both. We should be asking, “Do you have any issues with libido? Do you want to talk about it? Any issues with erection, arousal, orgasm, or sexual pain?”
When you can talk about those things, you can treat the patient from a whole physiologic perspective. For example, how does their sciatica affect their sexual pain? How does their antidepressant cause a delayed orgasm? How does their low testosterone level affect their energy level, their libido, and their desire?
We see so much shame and guilt in sexual health, to the extent that patients feel broken. We can help them understand the anatomy and physiology and explain that they aren’t broken. Instead, it’s “You need this medicine for your crippling anxiety, and that’s why your orgasm is delayed, and so can we augment it or add or subtract something to help you with it.”
Winter: In a primary care setting, where we are considering the patient›s overall health, we strive for medication compliance, but a huge part of medication noncompliance is sexual side effects, whether it›s antidepressants, beta-blockers, birth control, or this new world of GLP-1 agonists.
Rubin: I would add breast cancer treatments. Many patients go off their anastrozole or their tamoxifen because of the sexual side effects.
Winter: This is where we get to the crux of this discussion about sex being a vital sign — something you need to check routinely. We need to become comfortable with it, because then we are unlocking the ability to treat every patient like a whole person, give them better outcomes, improve their compliance, and have a really powerful tool for education.
Rubin: We have a growing toolbox for all genders when it comes to sexual health. We have FDA- approved medications for low libido in women. We use testosterone in men in an evidence-based way to safely improve libido. We use medications to help with the genitourinary syndrome of menopause. Orgasm is a challenging one, but we have devices that can help with those reflexes. And working with people who specialize in sexual pain can be extremely helpful for patients.
Dr. Winter, having practiced in different settings, what would you tell the primary care doctors who don’t want to talk about libido or who minimize sexual complaints because they don’t know how to navigate them?
Winter: I do not envy the challenge of being a primary care provider in the healthcare world we are living in. I think it is the hardest job. The ultimate takeaway is to just normalize the conversation and be able to validate what is happening. Have a few basic tools, and then have referrals. It›s not that you have to have all the time in the world or you have to treat every condition, but you have to start the conversation, be comfortable with it, and then get patients hooked up with the right resources.
Rubin: Every doctor of every kind can connect with patients and try to understand what they care about. What are their goals? What do they want for their families, for their relationships, for their quality of life? And how can we work collaboratively as a team to help them with those things?
Sex is a huge part of people’s lives. If we don’t ask about it; if we don’t look into it; and if we don’t admit that our physiology, our medications, and our surgeries can affect sexual health and functioning, how can we improve people’s lives? We can do so much as a team when we consider sex as a true vital sign.
Dr. Rubin, Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, DC, has disclosed ties with Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I’m Dr. Rachel Rubin, a urologist and sexual medicine specialist in the Washington, DC, area. And I am so thrilled because my co-fellow, the brilliant and famous Dr. Ashley Winter, a board-certified urologist and a certified menopause practitioner, who sees patients in our practice from Los Angeles, is joining us today to talk about sex as a vital sign.
Ashley Winter, MD: To have the best sexual function, you need many different systems to work. You need your hormones to be in the right place. You need your blood vessels to dilate when you want them to. You need your nerves to connect to your genitalia to make them responsive. The way people say, “The eyes are the window into the soul” — well, the genitals are the window into the cardiovascular system, the peripheral nervous system, and the hormonal system. It’s so dynamic. Patients can understand how this reflects their health. We just need healthcare providers to hammer home how those things connect.
Rubin: If you’re a primary care doctor seeing a patient and you want to educate them on diabetes or high blood pressure, how can you “ ‘sell it with ‘sex”? How can you use sex to educate them about these important medical conditions?
Winter: I hate using it as a fear tactic, but sometimes you have to. Time and again, I’ve seen men with severe profound erectile dysfunction at a young age, with chronically uncontrolled diabetes.
Diabetes can impair the peripheral nerves, resulting in peripheral neuropathy. The same way that it can affect the fingers and toes, diabetes can affect the penis, even before those other areas. Diabetes can also lead to other conditions such as low testosterone, which also affects the function of the penis.
I’m being brutally honest when I tell patients that diabetes control is critical to having a wonderful sexspan — the duration of your life where you’re able to be sexually active and have great sex and do it in the way that you want.
Chronic conditions such as high cholesterol or hypertension can affect your ability to become erect or aroused whether you have a penis or a vulva, and even your ability to have an orgasm.
Rubin: None of my doctors has ever asked me about these issues. But we have to bring them up with patients because they›re not going to bring them up to us. I always say in the review of systems, we shouldn›t just ask, “Do you have any sexual problems?” (which nobody ever does) and move past the question about men, women or both. We should be asking, “Do you have any issues with libido? Do you want to talk about it? Any issues with erection, arousal, orgasm, or sexual pain?”
When you can talk about those things, you can treat the patient from a whole physiologic perspective. For example, how does their sciatica affect their sexual pain? How does their antidepressant cause a delayed orgasm? How does their low testosterone level affect their energy level, their libido, and their desire?
We see so much shame and guilt in sexual health, to the extent that patients feel broken. We can help them understand the anatomy and physiology and explain that they aren’t broken. Instead, it’s “You need this medicine for your crippling anxiety, and that’s why your orgasm is delayed, and so can we augment it or add or subtract something to help you with it.”
Winter: In a primary care setting, where we are considering the patient›s overall health, we strive for medication compliance, but a huge part of medication noncompliance is sexual side effects, whether it›s antidepressants, beta-blockers, birth control, or this new world of GLP-1 agonists.
Rubin: I would add breast cancer treatments. Many patients go off their anastrozole or their tamoxifen because of the sexual side effects.
Winter: This is where we get to the crux of this discussion about sex being a vital sign — something you need to check routinely. We need to become comfortable with it, because then we are unlocking the ability to treat every patient like a whole person, give them better outcomes, improve their compliance, and have a really powerful tool for education.
Rubin: We have a growing toolbox for all genders when it comes to sexual health. We have FDA- approved medications for low libido in women. We use testosterone in men in an evidence-based way to safely improve libido. We use medications to help with the genitourinary syndrome of menopause. Orgasm is a challenging one, but we have devices that can help with those reflexes. And working with people who specialize in sexual pain can be extremely helpful for patients.
Dr. Winter, having practiced in different settings, what would you tell the primary care doctors who don’t want to talk about libido or who minimize sexual complaints because they don’t know how to navigate them?
Winter: I do not envy the challenge of being a primary care provider in the healthcare world we are living in. I think it is the hardest job. The ultimate takeaway is to just normalize the conversation and be able to validate what is happening. Have a few basic tools, and then have referrals. It›s not that you have to have all the time in the world or you have to treat every condition, but you have to start the conversation, be comfortable with it, and then get patients hooked up with the right resources.
Rubin: Every doctor of every kind can connect with patients and try to understand what they care about. What are their goals? What do they want for their families, for their relationships, for their quality of life? And how can we work collaboratively as a team to help them with those things?
Sex is a huge part of people’s lives. If we don’t ask about it; if we don’t look into it; and if we don’t admit that our physiology, our medications, and our surgeries can affect sexual health and functioning, how can we improve people’s lives? We can do so much as a team when we consider sex as a true vital sign.
Dr. Rubin, Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, DC, has disclosed ties with Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.