User login
America’s PCPs: Take a Bow
Hi, everyone. I’m Dr. Kenny Lin. I am a family physician and associate director of the Lancaster General Hospital Family Medicine Residency, and I blog at Common Sense Family Doctor.
For the past 4 years, primary care clinicians have labored under a seemingly endless onslaught of bad news. A recent report estimated that there were over 1.3 million excess deaths in the United States from March 2020 to May 2023, including nearly half a million Americans younger than age 65. Social isolation and an ailing economy accelerated preexisting rises in drug overdoses and obesity, while teenage vaping threatened to hook a new generation on tobacco products even as adult smoking plummeted. Meanwhile, more than half of the nation’s physicians now report feelings of burnout, pay for family doctors appears to be stagnating, and our interactions with an increasing number of patients are fraught with suspicions about the value of vaccines— not just against COVID-19 but against flu and other viruses, too — and the medical system as a whole, doctors included.
Now, for the good news.
A year and a half since the end of the pandemic emergency, we are seeing gains on several fronts, and physicians deserve much of the credit. Preliminary data from the Centers for Disease Control and Prevention show that 10,000 fewer people died from drug overdoses than in the previous year. Although multiple factors contributed to this change, the elimination of the X-waiver, which had previously been required for physicians to prescribe buprenorphine for opioid use disorder, in January 2023 has improved access to medications for addiction treatment. In addition, the expansion of state requirements to check prescription drug monitoring programs when opioids or benzodiazepines are prescribed, and to prescribe naloxone to patients taking more than a certain number of morphine milligram equivalents per day, has probably reduced the harms of hazardous drug use.
On the obesity front, recent data from the National Health and Nutrition Examination Survey found that the prevalence of obesity in adults fell for the first time in more than a decade, from 41.9% to 40.3%. To be sure, obesity remains far too common, and this finding could be the result of statistical chance rather than representing a true decline. But the widespread prescribing of GLP-1 receptor agonists by primary care physicians, in particular, could have played a role in the encouraging trend.
Although more research is needed to prove causality, one analysis suggests that these drugs could easily have lowered the body mass index (BMI) of more than enough patients to account for the observed decline. What’s more, the rise in prevalence of BMIs above 40 (from 7.7% to 9.7%) could be explained by the mortality benefit of the drugs: More people remained in this severe obesity category because they didn’t die from complications of their weight. Whether future studies support keeping people on GLP-1s for life or eventually “off-ramping” them to other weight control strategies, family physicians are well positioned to help.
Finally, with little fanfare, the youth smoking rate has fallen precipitously. In 2023, 1.9% of high school students and 1.1% of middle-schoolers reported smoking cigarettes in the past 30 days. And they didn’t simply swap one form of nicotine delivery device for another. The 30-day prevalence of vaping among high school students fell from 27.5% in 2019 to 7.8% this year. Changing social norms and stricter federal regulation of tobacco products are probably more responsible for this positive trend than medical care, though the US Preventive Services Task Force recommends education or brief counseling to prevent initiation of tobacco use among school-aged children and adolescents. Should tobacco use in youth remain at these historically low levels, millions of premature deaths from lung cancer and heart disease will have been prevented.
America’s doctors have earned the right to take a bow. We have much more work to do, but our efforts are making a meaningful difference in three seemingly intractable health problems.
Dr. Lin, Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hi, everyone. I’m Dr. Kenny Lin. I am a family physician and associate director of the Lancaster General Hospital Family Medicine Residency, and I blog at Common Sense Family Doctor.
For the past 4 years, primary care clinicians have labored under a seemingly endless onslaught of bad news. A recent report estimated that there were over 1.3 million excess deaths in the United States from March 2020 to May 2023, including nearly half a million Americans younger than age 65. Social isolation and an ailing economy accelerated preexisting rises in drug overdoses and obesity, while teenage vaping threatened to hook a new generation on tobacco products even as adult smoking plummeted. Meanwhile, more than half of the nation’s physicians now report feelings of burnout, pay for family doctors appears to be stagnating, and our interactions with an increasing number of patients are fraught with suspicions about the value of vaccines— not just against COVID-19 but against flu and other viruses, too — and the medical system as a whole, doctors included.
Now, for the good news.
A year and a half since the end of the pandemic emergency, we are seeing gains on several fronts, and physicians deserve much of the credit. Preliminary data from the Centers for Disease Control and Prevention show that 10,000 fewer people died from drug overdoses than in the previous year. Although multiple factors contributed to this change, the elimination of the X-waiver, which had previously been required for physicians to prescribe buprenorphine for opioid use disorder, in January 2023 has improved access to medications for addiction treatment. In addition, the expansion of state requirements to check prescription drug monitoring programs when opioids or benzodiazepines are prescribed, and to prescribe naloxone to patients taking more than a certain number of morphine milligram equivalents per day, has probably reduced the harms of hazardous drug use.
On the obesity front, recent data from the National Health and Nutrition Examination Survey found that the prevalence of obesity in adults fell for the first time in more than a decade, from 41.9% to 40.3%. To be sure, obesity remains far too common, and this finding could be the result of statistical chance rather than representing a true decline. But the widespread prescribing of GLP-1 receptor agonists by primary care physicians, in particular, could have played a role in the encouraging trend.
Although more research is needed to prove causality, one analysis suggests that these drugs could easily have lowered the body mass index (BMI) of more than enough patients to account for the observed decline. What’s more, the rise in prevalence of BMIs above 40 (from 7.7% to 9.7%) could be explained by the mortality benefit of the drugs: More people remained in this severe obesity category because they didn’t die from complications of their weight. Whether future studies support keeping people on GLP-1s for life or eventually “off-ramping” them to other weight control strategies, family physicians are well positioned to help.
Finally, with little fanfare, the youth smoking rate has fallen precipitously. In 2023, 1.9% of high school students and 1.1% of middle-schoolers reported smoking cigarettes in the past 30 days. And they didn’t simply swap one form of nicotine delivery device for another. The 30-day prevalence of vaping among high school students fell from 27.5% in 2019 to 7.8% this year. Changing social norms and stricter federal regulation of tobacco products are probably more responsible for this positive trend than medical care, though the US Preventive Services Task Force recommends education or brief counseling to prevent initiation of tobacco use among school-aged children and adolescents. Should tobacco use in youth remain at these historically low levels, millions of premature deaths from lung cancer and heart disease will have been prevented.
America’s doctors have earned the right to take a bow. We have much more work to do, but our efforts are making a meaningful difference in three seemingly intractable health problems.
Dr. Lin, Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hi, everyone. I’m Dr. Kenny Lin. I am a family physician and associate director of the Lancaster General Hospital Family Medicine Residency, and I blog at Common Sense Family Doctor.
For the past 4 years, primary care clinicians have labored under a seemingly endless onslaught of bad news. A recent report estimated that there were over 1.3 million excess deaths in the United States from March 2020 to May 2023, including nearly half a million Americans younger than age 65. Social isolation and an ailing economy accelerated preexisting rises in drug overdoses and obesity, while teenage vaping threatened to hook a new generation on tobacco products even as adult smoking plummeted. Meanwhile, more than half of the nation’s physicians now report feelings of burnout, pay for family doctors appears to be stagnating, and our interactions with an increasing number of patients are fraught with suspicions about the value of vaccines— not just against COVID-19 but against flu and other viruses, too — and the medical system as a whole, doctors included.
Now, for the good news.
A year and a half since the end of the pandemic emergency, we are seeing gains on several fronts, and physicians deserve much of the credit. Preliminary data from the Centers for Disease Control and Prevention show that 10,000 fewer people died from drug overdoses than in the previous year. Although multiple factors contributed to this change, the elimination of the X-waiver, which had previously been required for physicians to prescribe buprenorphine for opioid use disorder, in January 2023 has improved access to medications for addiction treatment. In addition, the expansion of state requirements to check prescription drug monitoring programs when opioids or benzodiazepines are prescribed, and to prescribe naloxone to patients taking more than a certain number of morphine milligram equivalents per day, has probably reduced the harms of hazardous drug use.
On the obesity front, recent data from the National Health and Nutrition Examination Survey found that the prevalence of obesity in adults fell for the first time in more than a decade, from 41.9% to 40.3%. To be sure, obesity remains far too common, and this finding could be the result of statistical chance rather than representing a true decline. But the widespread prescribing of GLP-1 receptor agonists by primary care physicians, in particular, could have played a role in the encouraging trend.
Although more research is needed to prove causality, one analysis suggests that these drugs could easily have lowered the body mass index (BMI) of more than enough patients to account for the observed decline. What’s more, the rise in prevalence of BMIs above 40 (from 7.7% to 9.7%) could be explained by the mortality benefit of the drugs: More people remained in this severe obesity category because they didn’t die from complications of their weight. Whether future studies support keeping people on GLP-1s for life or eventually “off-ramping” them to other weight control strategies, family physicians are well positioned to help.
Finally, with little fanfare, the youth smoking rate has fallen precipitously. In 2023, 1.9% of high school students and 1.1% of middle-schoolers reported smoking cigarettes in the past 30 days. And they didn’t simply swap one form of nicotine delivery device for another. The 30-day prevalence of vaping among high school students fell from 27.5% in 2019 to 7.8% this year. Changing social norms and stricter federal regulation of tobacco products are probably more responsible for this positive trend than medical care, though the US Preventive Services Task Force recommends education or brief counseling to prevent initiation of tobacco use among school-aged children and adolescents. Should tobacco use in youth remain at these historically low levels, millions of premature deaths from lung cancer and heart disease will have been prevented.
America’s doctors have earned the right to take a bow. We have much more work to do, but our efforts are making a meaningful difference in three seemingly intractable health problems.
Dr. Lin, Associate Director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
On Second Thought: Aspirin for Primary Prevention — What We Really Know
This transcript has been edited for clarity.
Our recommendations vis-à-vis aspirin have evolved at a dizzying pace. The young’uns watching us right now don’t know what things were like in the 1980s. The Reagan era was a wild, heady time where nuclear war was imminent and we didn’t prescribe aspirin to patients.
That only started in 1988, which was a banner year in human history. Not because a number of doves were incinerated by the lighting of the Olympic torch at the Seoul Olympics — look it up if you don’t know what I’m talking about — but because 1988 saw the publication of the ISIS-2 trial, which first showed a mortality benefit to prescribing aspirin post–myocardial infarction (MI).
Giving patients aspirin during or after a heart attack is not controversial. It’s one of the few things in this business that isn’t, but that’s secondary prevention — treating somebody after they develop a disease. Primary prevention, treating them before they have their incident event, is a very different ballgame. Here, things are messy.
For one thing, the doses used have been very inconsistent. We should point out that the reason for 81 mg of aspirin is very arbitrary and is rooted in the old apothecary system of weights and measurements. A standard dose of aspirin was 5 grains, where 20 grains made 1 scruple, 3 scruples made 1 dram, 8 drams made 1 oz, and 12 oz made 1 lb - because screw you, metric system. Therefore, 5 grains was 325 mg of aspirin, and 1 quarter of the standard dose became 81 mg if you rounded out the decimal.
People have tried all kinds of dosing structures with aspirin prophylaxis. The Physicians’ Health Study used a full-dose aspirin, 325 mg every 2 days, while the Hypertension Optimal Treatment (HOT) trial tested 75 mg daily and the Women’s Health Study tested 100 mg, but every other day.
Ironically, almost no one has studied 81 mg every day, which is weird if you think about it. The bigger problem here is not the variability of doses used, but the discrepancy when you look at older vs newer studies.
Older studies, like the Physicians’ Health Study, did show a benefit, at least in the subgroup of patients over age 50 years, which is probably where the “everybody over 50 should be taking an aspirin” idea comes from, at least as near as I can tell.
More recent studies, like the Women’s Health Study, ASPREE, or ASPIRE, didn’t show a benefit. I know what you’re thinking: Newer stuff is always better. That’s why you should never trust anybody over age 40 years. The context of primary prevention studies has changed. In the ‘80s and ‘90s, people smoked more and we didn’t have the same medications that we have today. We talked about all this in the beta-blocker video to explain why beta-blockers don’t seem to have a benefit post MI.
We have a similar issue here. The magnitude of the benefit with aspirin primary prevention has decreased because we’re all just healthier overall. So, yay! Progress! Here’s where the numbers matter. No one is saying that aspirin doesn’t help. It does.
If we look at the 2019 meta-analysis published in JAMA, there is a cardiovascular benefit. The numbers bear that out. I know you’re all here for the math, so here we go. Aspirin reduced the composite cardiovascular endpoint from 65.2 to 60.2 events per 10,000 patient-years; or to put it more meaningfully in absolute risk reduction terms, because that’s my jam, an absolute risk reduction of 0.41%, which means a number needed to treat of 241, which is okay-ish. It’s not super-great, but it may be justifiable for something that costs next to nothing.
The tradeoff is bleeding. Major bleeding increased from 16.4 to 23.1 bleeds per 10,000 patient-years, or an absolute risk increase of 0.47%, which is a number needed to harm of 210. That’s the problem. Aspirin does prevent heart disease. The benefit is small, for sure, but the real problem is that it’s outweighed by the risk of bleeding, so you’re not really coming out ahead.
The real tragedy here is that the public is locked into this idea of everyone over age 50 years should be taking an aspirin. Even today, even though guidelines have recommended against aspirin for primary prevention for some time, data from the National Health Interview Survey sample found that nearly one in three older adults take aspirin for primary prevention when they shouldn’t be. That’s a large number of people. That’s millions of Americans — and Canadians, but nobody cares about us. It’s fine.
That’s the point. We’re not debunking aspirin. It does work. The benefits are just really small in a primary prevention population and offset by the admittedly also really small risks of bleeding. It’s a tradeoff that doesn’t really work in your favor.
But that’s aspirin for cardiovascular disease. When it comes to cancer or DVT prophylaxis, that’s another really interesting story. We might have to save that for another time. Do I know how to tease a sequel or what?
Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Our recommendations vis-à-vis aspirin have evolved at a dizzying pace. The young’uns watching us right now don’t know what things were like in the 1980s. The Reagan era was a wild, heady time where nuclear war was imminent and we didn’t prescribe aspirin to patients.
That only started in 1988, which was a banner year in human history. Not because a number of doves were incinerated by the lighting of the Olympic torch at the Seoul Olympics — look it up if you don’t know what I’m talking about — but because 1988 saw the publication of the ISIS-2 trial, which first showed a mortality benefit to prescribing aspirin post–myocardial infarction (MI).
Giving patients aspirin during or after a heart attack is not controversial. It’s one of the few things in this business that isn’t, but that’s secondary prevention — treating somebody after they develop a disease. Primary prevention, treating them before they have their incident event, is a very different ballgame. Here, things are messy.
For one thing, the doses used have been very inconsistent. We should point out that the reason for 81 mg of aspirin is very arbitrary and is rooted in the old apothecary system of weights and measurements. A standard dose of aspirin was 5 grains, where 20 grains made 1 scruple, 3 scruples made 1 dram, 8 drams made 1 oz, and 12 oz made 1 lb - because screw you, metric system. Therefore, 5 grains was 325 mg of aspirin, and 1 quarter of the standard dose became 81 mg if you rounded out the decimal.
People have tried all kinds of dosing structures with aspirin prophylaxis. The Physicians’ Health Study used a full-dose aspirin, 325 mg every 2 days, while the Hypertension Optimal Treatment (HOT) trial tested 75 mg daily and the Women’s Health Study tested 100 mg, but every other day.
Ironically, almost no one has studied 81 mg every day, which is weird if you think about it. The bigger problem here is not the variability of doses used, but the discrepancy when you look at older vs newer studies.
Older studies, like the Physicians’ Health Study, did show a benefit, at least in the subgroup of patients over age 50 years, which is probably where the “everybody over 50 should be taking an aspirin” idea comes from, at least as near as I can tell.
More recent studies, like the Women’s Health Study, ASPREE, or ASPIRE, didn’t show a benefit. I know what you’re thinking: Newer stuff is always better. That’s why you should never trust anybody over age 40 years. The context of primary prevention studies has changed. In the ‘80s and ‘90s, people smoked more and we didn’t have the same medications that we have today. We talked about all this in the beta-blocker video to explain why beta-blockers don’t seem to have a benefit post MI.
We have a similar issue here. The magnitude of the benefit with aspirin primary prevention has decreased because we’re all just healthier overall. So, yay! Progress! Here’s where the numbers matter. No one is saying that aspirin doesn’t help. It does.
If we look at the 2019 meta-analysis published in JAMA, there is a cardiovascular benefit. The numbers bear that out. I know you’re all here for the math, so here we go. Aspirin reduced the composite cardiovascular endpoint from 65.2 to 60.2 events per 10,000 patient-years; or to put it more meaningfully in absolute risk reduction terms, because that’s my jam, an absolute risk reduction of 0.41%, which means a number needed to treat of 241, which is okay-ish. It’s not super-great, but it may be justifiable for something that costs next to nothing.
The tradeoff is bleeding. Major bleeding increased from 16.4 to 23.1 bleeds per 10,000 patient-years, or an absolute risk increase of 0.47%, which is a number needed to harm of 210. That’s the problem. Aspirin does prevent heart disease. The benefit is small, for sure, but the real problem is that it’s outweighed by the risk of bleeding, so you’re not really coming out ahead.
The real tragedy here is that the public is locked into this idea of everyone over age 50 years should be taking an aspirin. Even today, even though guidelines have recommended against aspirin for primary prevention for some time, data from the National Health Interview Survey sample found that nearly one in three older adults take aspirin for primary prevention when they shouldn’t be. That’s a large number of people. That’s millions of Americans — and Canadians, but nobody cares about us. It’s fine.
That’s the point. We’re not debunking aspirin. It does work. The benefits are just really small in a primary prevention population and offset by the admittedly also really small risks of bleeding. It’s a tradeoff that doesn’t really work in your favor.
But that’s aspirin for cardiovascular disease. When it comes to cancer or DVT prophylaxis, that’s another really interesting story. We might have to save that for another time. Do I know how to tease a sequel or what?
Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Our recommendations vis-à-vis aspirin have evolved at a dizzying pace. The young’uns watching us right now don’t know what things were like in the 1980s. The Reagan era was a wild, heady time where nuclear war was imminent and we didn’t prescribe aspirin to patients.
That only started in 1988, which was a banner year in human history. Not because a number of doves were incinerated by the lighting of the Olympic torch at the Seoul Olympics — look it up if you don’t know what I’m talking about — but because 1988 saw the publication of the ISIS-2 trial, which first showed a mortality benefit to prescribing aspirin post–myocardial infarction (MI).
Giving patients aspirin during or after a heart attack is not controversial. It’s one of the few things in this business that isn’t, but that’s secondary prevention — treating somebody after they develop a disease. Primary prevention, treating them before they have their incident event, is a very different ballgame. Here, things are messy.
For one thing, the doses used have been very inconsistent. We should point out that the reason for 81 mg of aspirin is very arbitrary and is rooted in the old apothecary system of weights and measurements. A standard dose of aspirin was 5 grains, where 20 grains made 1 scruple, 3 scruples made 1 dram, 8 drams made 1 oz, and 12 oz made 1 lb - because screw you, metric system. Therefore, 5 grains was 325 mg of aspirin, and 1 quarter of the standard dose became 81 mg if you rounded out the decimal.
People have tried all kinds of dosing structures with aspirin prophylaxis. The Physicians’ Health Study used a full-dose aspirin, 325 mg every 2 days, while the Hypertension Optimal Treatment (HOT) trial tested 75 mg daily and the Women’s Health Study tested 100 mg, but every other day.
Ironically, almost no one has studied 81 mg every day, which is weird if you think about it. The bigger problem here is not the variability of doses used, but the discrepancy when you look at older vs newer studies.
Older studies, like the Physicians’ Health Study, did show a benefit, at least in the subgroup of patients over age 50 years, which is probably where the “everybody over 50 should be taking an aspirin” idea comes from, at least as near as I can tell.
More recent studies, like the Women’s Health Study, ASPREE, or ASPIRE, didn’t show a benefit. I know what you’re thinking: Newer stuff is always better. That’s why you should never trust anybody over age 40 years. The context of primary prevention studies has changed. In the ‘80s and ‘90s, people smoked more and we didn’t have the same medications that we have today. We talked about all this in the beta-blocker video to explain why beta-blockers don’t seem to have a benefit post MI.
We have a similar issue here. The magnitude of the benefit with aspirin primary prevention has decreased because we’re all just healthier overall. So, yay! Progress! Here’s where the numbers matter. No one is saying that aspirin doesn’t help. It does.
If we look at the 2019 meta-analysis published in JAMA, there is a cardiovascular benefit. The numbers bear that out. I know you’re all here for the math, so here we go. Aspirin reduced the composite cardiovascular endpoint from 65.2 to 60.2 events per 10,000 patient-years; or to put it more meaningfully in absolute risk reduction terms, because that’s my jam, an absolute risk reduction of 0.41%, which means a number needed to treat of 241, which is okay-ish. It’s not super-great, but it may be justifiable for something that costs next to nothing.
The tradeoff is bleeding. Major bleeding increased from 16.4 to 23.1 bleeds per 10,000 patient-years, or an absolute risk increase of 0.47%, which is a number needed to harm of 210. That’s the problem. Aspirin does prevent heart disease. The benefit is small, for sure, but the real problem is that it’s outweighed by the risk of bleeding, so you’re not really coming out ahead.
The real tragedy here is that the public is locked into this idea of everyone over age 50 years should be taking an aspirin. Even today, even though guidelines have recommended against aspirin for primary prevention for some time, data from the National Health Interview Survey sample found that nearly one in three older adults take aspirin for primary prevention when they shouldn’t be. That’s a large number of people. That’s millions of Americans — and Canadians, but nobody cares about us. It’s fine.
That’s the point. We’re not debunking aspirin. It does work. The benefits are just really small in a primary prevention population and offset by the admittedly also really small risks of bleeding. It’s a tradeoff that doesn’t really work in your favor.
But that’s aspirin for cardiovascular disease. When it comes to cancer or DVT prophylaxis, that’s another really interesting story. We might have to save that for another time. Do I know how to tease a sequel or what?
Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Lifestyle Medicine Trends to Keep an Eye On
Our current healthcare system, which is a costly and unending cycle of merely managing chronic disease symptoms, is failing us. What we truly need is a patient-centered approach that restores health by addressing not just diagnoses but also the physical, emotional, and social needs of each individual. This is the essence of whole-person health, and transformation toward this model of care is already underway.
This shift underscores why clinicians like me support placing lifestyle medicine at the foundation of health and healthcare. Evidence-based lifestyle medicine — which applies interventions in nutrition, physical activity, restorative sleep, stress management, positive social connections, and avoidance of risky substances to prevent, treat, and when used intensively, even reverse lifestyle-related chronic disease — is a medical specialty equipped to successfully address patients’ whole-person health in an effective, high-value clinical care delivery model.
As this transformation continues, here are four key lifestyle medicine trends for 2025.
Lifestyle Medicine Becomes More Ingrained in Primary Care
The 2021 National Academies of Science, Engineering, and Medicine report, “Implementing High-Quality Primary Care” sounded the alarm about the state of primary care and outlined a comprehensive approach to transform it. Lifestyle medicine emerged as a solution as clinicians found innovative ways to integrate lifestyle behavior interventions into existing care models in a financially sustainable, scalable manner. Examples include Blue Zones Health, a new delivery model that aligns lifestyle medicine–certified clinicians with community and payers in California, and the University of Pittsburgh Medical Center lifestyle medicine program, where primary care patients are referred to virtual group coaching, a teaching kitchen, and classes on food as medicine, obesity, type 2 diabetes, and more.
Organizations dedicated to advancing primary care are paying close attention to the potential of lifestyle medicine. Currently, The Primary Care Collaborative has launched a new multi-year initiative on whole-person care and lifestyle medicine. This initiative aims to broaden the primary care community’s understanding of whole health and lifestyle medicine concepts and the evidence behind them, as well as lay the groundwork for future work to promote whole-person primary care and lifestyle medicine among an engaged and committed community of members.
Digital Tools and AI Spark Lifestyle Medicine Innovations
American College of Lifestyle Medicine partner organizations are increasingly utilizing digital tools, such as health apps tailored to lifestyle behavior interventions, to expand access to care and support behavior change. One of the biggest challenges in lifestyle interventions is the limited time during patient encounters. But artificial intelligence (AI) tools can record (with patient permission) and summarize encounters, enabling clinicians to turn away from their keyboards and be more present to learn about the unique living, environmental, and societal factors that impact every individual’s lifestyle choices. AI tools can create individualized whole-food, plant-predominant meal plans or physical activity schedules for patients in just a few seconds. The potential for AI in lifestyle medicine is vast, and its applications were further explored at the American College of Lifestyle Medicine’s annual conference in October.
Behavior Change and Sustainability of the Food-as-Medicine Movement
Significant investments have been made in food as medicine to address diet-related chronic diseases. But merely providing medically tailored meals or produce prescriptions is not enough because once the prescriptions end, so will the health benefits. Clinicians certified in lifestyle medicine are prepared to coach patients into long-term behavior change, supporting them with education and information to shop for and prepare tasty, nutritious, and affordable food. The same applies to the use of glucagon-like peptide 1 drugs. Although the initial weight loss offers motivation, lifestyle changes are necessary to sustain long-term health benefits beyond medications.
Lifestyle Medicine Emerges as a Strategy to Achieve Health Equity
Lifestyle behavior interventions have the unique ability to address health status and social drivers of health. For example, food as medicine affects an individual’s health while also addressing nutrition security. Certainly, no medication can both improve health status and feed someone. The addition of payment for the screening of social drivers of health to the 2024 Medicare Physician Fee Schedule is an important step toward connecting clinicians with community health–based organizations that can address factors that influence patients’ ability to adhere to lifestyle behavior care plans. Lifestyle medicine clinicians are poised to lead this effort because they are already having conversations with patients about their environment, living conditions, and access to nutritious food.
The changes coming to our healthcare system are exciting and long overdue. Lifestyle medicine is positioned to be at the forefront of this transformation now and in the future.
Dr. Patel, president of the American College of Lifestyle Medicine in St. Louis, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Our current healthcare system, which is a costly and unending cycle of merely managing chronic disease symptoms, is failing us. What we truly need is a patient-centered approach that restores health by addressing not just diagnoses but also the physical, emotional, and social needs of each individual. This is the essence of whole-person health, and transformation toward this model of care is already underway.
This shift underscores why clinicians like me support placing lifestyle medicine at the foundation of health and healthcare. Evidence-based lifestyle medicine — which applies interventions in nutrition, physical activity, restorative sleep, stress management, positive social connections, and avoidance of risky substances to prevent, treat, and when used intensively, even reverse lifestyle-related chronic disease — is a medical specialty equipped to successfully address patients’ whole-person health in an effective, high-value clinical care delivery model.
As this transformation continues, here are four key lifestyle medicine trends for 2025.
Lifestyle Medicine Becomes More Ingrained in Primary Care
The 2021 National Academies of Science, Engineering, and Medicine report, “Implementing High-Quality Primary Care” sounded the alarm about the state of primary care and outlined a comprehensive approach to transform it. Lifestyle medicine emerged as a solution as clinicians found innovative ways to integrate lifestyle behavior interventions into existing care models in a financially sustainable, scalable manner. Examples include Blue Zones Health, a new delivery model that aligns lifestyle medicine–certified clinicians with community and payers in California, and the University of Pittsburgh Medical Center lifestyle medicine program, where primary care patients are referred to virtual group coaching, a teaching kitchen, and classes on food as medicine, obesity, type 2 diabetes, and more.
Organizations dedicated to advancing primary care are paying close attention to the potential of lifestyle medicine. Currently, The Primary Care Collaborative has launched a new multi-year initiative on whole-person care and lifestyle medicine. This initiative aims to broaden the primary care community’s understanding of whole health and lifestyle medicine concepts and the evidence behind them, as well as lay the groundwork for future work to promote whole-person primary care and lifestyle medicine among an engaged and committed community of members.
Digital Tools and AI Spark Lifestyle Medicine Innovations
American College of Lifestyle Medicine partner organizations are increasingly utilizing digital tools, such as health apps tailored to lifestyle behavior interventions, to expand access to care and support behavior change. One of the biggest challenges in lifestyle interventions is the limited time during patient encounters. But artificial intelligence (AI) tools can record (with patient permission) and summarize encounters, enabling clinicians to turn away from their keyboards and be more present to learn about the unique living, environmental, and societal factors that impact every individual’s lifestyle choices. AI tools can create individualized whole-food, plant-predominant meal plans or physical activity schedules for patients in just a few seconds. The potential for AI in lifestyle medicine is vast, and its applications were further explored at the American College of Lifestyle Medicine’s annual conference in October.
Behavior Change and Sustainability of the Food-as-Medicine Movement
Significant investments have been made in food as medicine to address diet-related chronic diseases. But merely providing medically tailored meals or produce prescriptions is not enough because once the prescriptions end, so will the health benefits. Clinicians certified in lifestyle medicine are prepared to coach patients into long-term behavior change, supporting them with education and information to shop for and prepare tasty, nutritious, and affordable food. The same applies to the use of glucagon-like peptide 1 drugs. Although the initial weight loss offers motivation, lifestyle changes are necessary to sustain long-term health benefits beyond medications.
Lifestyle Medicine Emerges as a Strategy to Achieve Health Equity
Lifestyle behavior interventions have the unique ability to address health status and social drivers of health. For example, food as medicine affects an individual’s health while also addressing nutrition security. Certainly, no medication can both improve health status and feed someone. The addition of payment for the screening of social drivers of health to the 2024 Medicare Physician Fee Schedule is an important step toward connecting clinicians with community health–based organizations that can address factors that influence patients’ ability to adhere to lifestyle behavior care plans. Lifestyle medicine clinicians are poised to lead this effort because they are already having conversations with patients about their environment, living conditions, and access to nutritious food.
The changes coming to our healthcare system are exciting and long overdue. Lifestyle medicine is positioned to be at the forefront of this transformation now and in the future.
Dr. Patel, president of the American College of Lifestyle Medicine in St. Louis, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Our current healthcare system, which is a costly and unending cycle of merely managing chronic disease symptoms, is failing us. What we truly need is a patient-centered approach that restores health by addressing not just diagnoses but also the physical, emotional, and social needs of each individual. This is the essence of whole-person health, and transformation toward this model of care is already underway.
This shift underscores why clinicians like me support placing lifestyle medicine at the foundation of health and healthcare. Evidence-based lifestyle medicine — which applies interventions in nutrition, physical activity, restorative sleep, stress management, positive social connections, and avoidance of risky substances to prevent, treat, and when used intensively, even reverse lifestyle-related chronic disease — is a medical specialty equipped to successfully address patients’ whole-person health in an effective, high-value clinical care delivery model.
As this transformation continues, here are four key lifestyle medicine trends for 2025.
Lifestyle Medicine Becomes More Ingrained in Primary Care
The 2021 National Academies of Science, Engineering, and Medicine report, “Implementing High-Quality Primary Care” sounded the alarm about the state of primary care and outlined a comprehensive approach to transform it. Lifestyle medicine emerged as a solution as clinicians found innovative ways to integrate lifestyle behavior interventions into existing care models in a financially sustainable, scalable manner. Examples include Blue Zones Health, a new delivery model that aligns lifestyle medicine–certified clinicians with community and payers in California, and the University of Pittsburgh Medical Center lifestyle medicine program, where primary care patients are referred to virtual group coaching, a teaching kitchen, and classes on food as medicine, obesity, type 2 diabetes, and more.
Organizations dedicated to advancing primary care are paying close attention to the potential of lifestyle medicine. Currently, The Primary Care Collaborative has launched a new multi-year initiative on whole-person care and lifestyle medicine. This initiative aims to broaden the primary care community’s understanding of whole health and lifestyle medicine concepts and the evidence behind them, as well as lay the groundwork for future work to promote whole-person primary care and lifestyle medicine among an engaged and committed community of members.
Digital Tools and AI Spark Lifestyle Medicine Innovations
American College of Lifestyle Medicine partner organizations are increasingly utilizing digital tools, such as health apps tailored to lifestyle behavior interventions, to expand access to care and support behavior change. One of the biggest challenges in lifestyle interventions is the limited time during patient encounters. But artificial intelligence (AI) tools can record (with patient permission) and summarize encounters, enabling clinicians to turn away from their keyboards and be more present to learn about the unique living, environmental, and societal factors that impact every individual’s lifestyle choices. AI tools can create individualized whole-food, plant-predominant meal plans or physical activity schedules for patients in just a few seconds. The potential for AI in lifestyle medicine is vast, and its applications were further explored at the American College of Lifestyle Medicine’s annual conference in October.
Behavior Change and Sustainability of the Food-as-Medicine Movement
Significant investments have been made in food as medicine to address diet-related chronic diseases. But merely providing medically tailored meals or produce prescriptions is not enough because once the prescriptions end, so will the health benefits. Clinicians certified in lifestyle medicine are prepared to coach patients into long-term behavior change, supporting them with education and information to shop for and prepare tasty, nutritious, and affordable food. The same applies to the use of glucagon-like peptide 1 drugs. Although the initial weight loss offers motivation, lifestyle changes are necessary to sustain long-term health benefits beyond medications.
Lifestyle Medicine Emerges as a Strategy to Achieve Health Equity
Lifestyle behavior interventions have the unique ability to address health status and social drivers of health. For example, food as medicine affects an individual’s health while also addressing nutrition security. Certainly, no medication can both improve health status and feed someone. The addition of payment for the screening of social drivers of health to the 2024 Medicare Physician Fee Schedule is an important step toward connecting clinicians with community health–based organizations that can address factors that influence patients’ ability to adhere to lifestyle behavior care plans. Lifestyle medicine clinicians are poised to lead this effort because they are already having conversations with patients about their environment, living conditions, and access to nutritious food.
The changes coming to our healthcare system are exciting and long overdue. Lifestyle medicine is positioned to be at the forefront of this transformation now and in the future.
Dr. Patel, president of the American College of Lifestyle Medicine in St. Louis, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Treating Digestive Disease Across the Lifespan
Pediatric gastroenterologists are a vital, yet often overlooked segment of the GI workforce and an important part of AGA’s diverse membership. Per the American Board of Pediatrics, 2,232 pediatricians have been board certified in pediatric gastroenterology since formal certification was first offered in 1990, and AGA Institute Council’s Pediatric Gastroenterology and Developmental Biology Section has nearly 1,900 members.
According to a recently published study in the journal Pediatrics, the pediatric GI workforce is expected to double by 2040, growing at a rate faster than that of most other pediatric subspecialties. This is largely due to the increased scope and complexity of the field driven by scientific advances and the increasing prevalence of digestive and liver diseases in children, including inflammatory bowel and other diseases.
In this month’s Member Spotlight, we highlight Dr. Yoyo Zhang, a pediatric gastroenterologist at Stanford Children’s Health specializing in intestinal and liver transplantation. Her passion for her profession and for improving the lives of her patients shines brightly, and her interview provides fascinating insights into the complexities and rewards of the rapidly expanding field of pediatric gastroenterology.
Also in our November issue, we update you on the FDA’s recent approval of the “next-gen” Cologuard test and query a panel of primary care and GI experts on their thoughts regarding the role that newly FDA-approved (but not yet guideline-recommended) Guardant blood-based CRC screening test should play in CRC screening moving forward.
In our Perspectives feature, we offer expert insights on how to appropriately screen patients for certain rare malignancies. Is it worthwhile screening for pancreatic cancer, and if so, how should it be done? Likewise, diagnosing cholangiocarcinoma is challenging; how best should one evaluate for this in higher-risk populations?
We hope you enjoy all the content in our November issue – as always, thanks for reading!
Megan A. Adams, MD, JD, MSc
Editor in Chief
Pediatric gastroenterologists are a vital, yet often overlooked segment of the GI workforce and an important part of AGA’s diverse membership. Per the American Board of Pediatrics, 2,232 pediatricians have been board certified in pediatric gastroenterology since formal certification was first offered in 1990, and AGA Institute Council’s Pediatric Gastroenterology and Developmental Biology Section has nearly 1,900 members.
According to a recently published study in the journal Pediatrics, the pediatric GI workforce is expected to double by 2040, growing at a rate faster than that of most other pediatric subspecialties. This is largely due to the increased scope and complexity of the field driven by scientific advances and the increasing prevalence of digestive and liver diseases in children, including inflammatory bowel and other diseases.
In this month’s Member Spotlight, we highlight Dr. Yoyo Zhang, a pediatric gastroenterologist at Stanford Children’s Health specializing in intestinal and liver transplantation. Her passion for her profession and for improving the lives of her patients shines brightly, and her interview provides fascinating insights into the complexities and rewards of the rapidly expanding field of pediatric gastroenterology.
Also in our November issue, we update you on the FDA’s recent approval of the “next-gen” Cologuard test and query a panel of primary care and GI experts on their thoughts regarding the role that newly FDA-approved (but not yet guideline-recommended) Guardant blood-based CRC screening test should play in CRC screening moving forward.
In our Perspectives feature, we offer expert insights on how to appropriately screen patients for certain rare malignancies. Is it worthwhile screening for pancreatic cancer, and if so, how should it be done? Likewise, diagnosing cholangiocarcinoma is challenging; how best should one evaluate for this in higher-risk populations?
We hope you enjoy all the content in our November issue – as always, thanks for reading!
Megan A. Adams, MD, JD, MSc
Editor in Chief
Pediatric gastroenterologists are a vital, yet often overlooked segment of the GI workforce and an important part of AGA’s diverse membership. Per the American Board of Pediatrics, 2,232 pediatricians have been board certified in pediatric gastroenterology since formal certification was first offered in 1990, and AGA Institute Council’s Pediatric Gastroenterology and Developmental Biology Section has nearly 1,900 members.
According to a recently published study in the journal Pediatrics, the pediatric GI workforce is expected to double by 2040, growing at a rate faster than that of most other pediatric subspecialties. This is largely due to the increased scope and complexity of the field driven by scientific advances and the increasing prevalence of digestive and liver diseases in children, including inflammatory bowel and other diseases.
In this month’s Member Spotlight, we highlight Dr. Yoyo Zhang, a pediatric gastroenterologist at Stanford Children’s Health specializing in intestinal and liver transplantation. Her passion for her profession and for improving the lives of her patients shines brightly, and her interview provides fascinating insights into the complexities and rewards of the rapidly expanding field of pediatric gastroenterology.
Also in our November issue, we update you on the FDA’s recent approval of the “next-gen” Cologuard test and query a panel of primary care and GI experts on their thoughts regarding the role that newly FDA-approved (but not yet guideline-recommended) Guardant blood-based CRC screening test should play in CRC screening moving forward.
In our Perspectives feature, we offer expert insights on how to appropriately screen patients for certain rare malignancies. Is it worthwhile screening for pancreatic cancer, and if so, how should it be done? Likewise, diagnosing cholangiocarcinoma is challenging; how best should one evaluate for this in higher-risk populations?
We hope you enjoy all the content in our November issue – as always, thanks for reading!
Megan A. Adams, MD, JD, MSc
Editor in Chief
Screening Options for Rare Malignancies
Dear colleagues,
As gastroenterologists and endoscopists, we spend significant time preventing and diagnosing GI malignancies.
For instance, is it worthwhile screening for pancreatic cancer, and, if so, how should this be done? Likewise, diagnosing cholangiocarcinoma is challenging; how best should one evaluate for this in higher risk populations, such as primary sclerosing cholangitis? And what about the costs, financial and otherwise, associated with screening?
In this issue of Perspectives, Dr. Darshan Kothari and Dr. Daniel Bernstein discuss their approach to pancreatic cancer screening, including who is eligible, the preferred screening modalities, and the barriers to screening. In the accompanying perspective, Dr. Aparna Goel and Dr. Judah Kupferman focus on cholangiocarcinoma screening, identifying high-risk populations and discussing some of the concerns with screening, necessitating shared decision-making.
We welcome your thoughts on this issue. Share with us on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
An Approach to Pancreatic Cancer Screening
BY DANIEL A. BERNSTEIN, MD, AND DARSHAN KOTHARI, MD
Pancreatic cancer carries a dismal prognosis, now accounting for the third-most cancer-related mortality in the United States. A small proportion of patients are diagnosed at a local stage of disease, with over half found to have metastatic disease at presentation. Given the low overall incidence and lifetime risk in the general population, population-based screening is not justified.
About 10% of cases of pancreas cancer are associated with germ-line mutations and/or with a strong family history of pancreatic cancer. Several academic societies and expert committees now recommend regular screening for pancreatic cancer in patients who are considered high-risk individuals, as they carry a fivefold relative risk for pancreatic cancer. Moreover, studies suggest that screening has the potential to identify early-stage resectable disease and decrease mortality in this patient population.
Patients who benefit from pancreatic cancer screening are those who carry an increased lifetime risk (in excess of 5%) of pancreatic cancer. High-risk individuals include those with germ-line mutations and/or those with a family history of pancreatic cancer in first-degree relatives. Consensus guidelines by the International Cancer of the Pancreas Screening Consortium and the American Society for Gastrointestinal Endoscopy provide medical centers with detailed recommendations on who and when to start screening.
High-risk individuals fall into three categories:
- Patients with high-risk germline mutations including: familial atypical multiple mole melanoma syndrome (CDKN2A), hereditary breast and ovarian cancer syndromes (BRCA1, BRCA2, and PALB2), Peutz-Jeghers syndrome (STK11), and hereditary pancreatitis (PRSS1 and SPINK1)
- Patients with low- to moderate-risk germ-line mutations with at least one first-degree relative with pancreatic cancer: Lynch Syndrome (particularly MLH1 mutation), ataxia-telangiectasia (ATM), or Li-Fraumeni syndrome (p53)
- Patients with one first-degree relative with pancreatic cancer who in turn has one first-degree relative with pancreatic cancer (eg, a patient’s mother and maternal aunt or a patient’s father and patient’s sister)
Consistent with established guidelines, we recommend screening for high-risk patients beginning at age 50, or 10 years before the youngest age at which pancreas cancer was diagnosed in an affected relative. Screening is recommended earlier in patients with particularly high risk: at age 40 for patients with CDKN2A and STKI11 mutations and age 40 for patients with PRSS1 mutation or 20 years after the first attack of acute pancreatitis. For patients with a strong family history of pancreas cancer, we recommend comprehensive evaluation by a certified genetic counselor at a high-volume cancer center.
In practice, patients at our institution who are identified as high risk based on the above criteria are referred for an initial consultation at our pancreas center. In most cases, this should occur no sooner than 5 years prior to the recommended starting age for screening. All patients who are identified as high risk should be screened annually for diabetes given the growing evidence base supporting an association between new-onset diabetes and pancreatic cancer.
After an initial visit and discussion of the risks and benefits of screening, most screening protocols start with a baseline endoscopic ultrasound (EUS) and contrast-enhanced magnetic resonance abdomen with magnetic resonance cholangiopancreatography (MRI/MRCP), which will be repeated annually or sooner as the clinical condition warrants. A sooner-interval EUS should be considered for patients already undergoing screening who are newly found to have diabetes.
At our institution, we start with an in-person clinic evaluation followed by EUS. Thereafter, patients undergo MRI/MRCP (synchronized with a same-day clinic visit) alternating with EUS every 6 months to ensure patients are seen twice a year, though there is no specific data to support this approach. Non-diabetics also undergo yearly diabetes screening which will trigger an EUS if patients become diabetic.
We engage in shared decision-making with our high-risk individuals undergoing pancreatic cancer screening and at each visit we review their concurrent medical conditions and suitability to continue screening. We consider discontinuing screening after age 75, at the onset of any life-limiting illness, or after a discussion of risks and benefits if comorbidities lead to a substantial deterioration in a patient’s overall health status.
While a growing body of evidence exists to support the application of pancreatic cancer screening in high-risk individuals, this preventive service remains underutilized. Recent analysis of the screening cohort at our institution showed a demographically homogeneous group of mostly highly educated, high-income White females. These findings are consistent with the patient cohorts described in other pancreatic cancer screening programs and represent only a fraction of people who would qualify for pancreatic cancer screening.
A survey of patients undergoing screening at our institution identified cost, travel, and time associated with pancreatic cancer screening to be frequent challenges to participation. Further studies are needed to fully explore the barriers and psychological burden of pancreas cancer screening in high-risk individuals, and to identify ways to enrich the cohort of patients undergoing screening. This may involve novel methods to identify family members of patients with a new diagnosis of pancreas cancer and increasing health literacy around pancreatic cancer screening among patients and providers.
Pancreatic cancer screening has the potential to identify early-stage disease in patients who are at high risk because of germ-line mutations and/or family history. We recommend that patients engage in pancreatic cancer screening at high-volume centers with well-supported oncology, genetics, and research infrastructure.
Dr. Bernstein is a gastroenterology fellow at Duke University School of Medicine, Durham, North Carolina. Dr. Kothari is an associate professor of medicine in gastroenterology and hepatology at Duke University School of Medicine.
Screening for Cholangiocarcinoma
BY JUDAH KUPFERMAN, MD, AND APARNA GOEL, MD
Cholangiocarcinoma is a rare but aggressive cancer of the bile ducts that poses many diagnostic challenges. Approximately 3% of gastrointestinal cancers are attributed to cholangiocarcinoma, and while the annual incidence of disease in the United States is about 1.26 per 100,000 people, the incidence of intrahepatic disease has been rising considerably.1,2 Screening for cholangiocarcinoma is reserved for high-risk individuals — such as those with primary sclerosing cholangitis (PSC), secondary sclerosing cholangitis (SSC), and biliary tract disorders such as choledochal cysts or Caroli’s disease. The goal is to balance the benefits of early diagnosis with the costs and risks associated with screening, particularly given the limitations of available tools like MRI with cholangiopancreatography (MRCP), which has a sensitivity of 70%-85%. In general, we recommend annual cholangiocarcinoma screening for high-risk individuals with MRI and MRCP as well as with cancer antigen (CA) 19-9. .
Screening in Patients with Primary Sclerosing Cholangitis
The lifetime risk of cholangiocarcinoma in patients with PSC is 10%-15% with an annual risk of 0.5%-1.5%. In our experience, this is often the most feared complication for PSC patients, even more so than the risk of liver transplantation. We recommend annual MRI with MRCP in addition to CA 19-9 for patients with PSC in the first decade of their diagnosis, as most cancers are diagnosed during this period. If a patient’s imaging has remained stable for over a decade and there is minimal hepatic fibrosis, we discuss the option of reducing screening frequency to every 2 years to minimize costs and exposure to MRI contrast risks.
If MRI reveals a concerning new large duct stricture, we will evaluate this with an endoscopic retrograde cholangiopancreatography (ERCP), as differentiating benign and malignant strictures is quite challenging with MRI. We generally recommend ERCP with brush cytology and fluorescence in situ hybridization to improve diagnostic yield. Depending on imaging findings and location of the new large duct stricture, we may consider cholangioscopy during ERCP for direct visualization of the bile duct and directed tissue biopsies. Unfortunately, even in young, asymptomatic patients who undergo regular screening, cholangiocarcinoma is frequently diagnosed at an advanced stage.
Screening in Patients with Secondary Sclerosing Cholangitis
Patients with SSC may develop cholangiocarcinoma because of chronic inflammatory and fibrotic processes, such as IgG4-associated cholangiopathy, sarcoidosis, ischemic cholangiopathy, cystic fibrosis, recurrent pyogenic cholangitis, severe sepsis (as recently seen from SARS-CoV-2), surgical complications, or other etiologies. When the condition is reversible, such as with IgG4-associated cholangiopathy, cancer screening may not be necessary. However, when irreversible damage occurs, the cancer risk increases, though it varies by disease type and severity. In most cases, we recommend routine screening for cholangiocarcinoma with MRI and CA 19-9 in this population.
Screening in Patients with Biliary Tract Disorders
Biliary tract disorders such as choledochal cysts and Caroli’s disease also harbor an increased risk of cholangiocarcinoma. Choledochal cysts are congenital cystic dilations of the bile duct that have a 10%-30% lifetime risk of malignant transformation to cholangiocarcinoma. Surgical intervention to remove the cyst is often recommended because of this high risk. However, some patients may be unable or unwilling to undergo this surgery or they may have residual cysts. We recommend ongoing screening with MRI and CA 19-9 for these patients. Similarly, Caroli’s disease is a congenital disease associated with intrahepatic and extrahepatic bile duct cysts and associated with a 5%-15% lifetime risk of cholangiocarcinoma. MRI with MRCP and CA 19-9 should be performed routinely for patients with Caroli’s disease and syndrome.
Risks and Challenges in Cholangiocarcinoma Screening
While MRI with MRCP is the gold standard for cholangiocarcinoma screening, its limitations must be carefully considered. One growing concern is the potential for gadolinium retention in the brain, bones, or skin following repeated MRI scans. Though the long-term effects of gadolinium retention are not fully understood, we factor this into screening decisions, particularly for younger patients who may undergo decades of regular imaging.
MRI is not always feasible for certain patients, including those with metal implants, on hemodialysis, or with severe allergic reactions. In such cases, CT or ultrasound may serve as alternatives, though with lower sensitivity for detecting cholangiocarcinoma. Additionally, claustrophobia during MRI can be addressed with sedation, but this underscores the importance of shared decision-making.
From our perspective, cholangiocarcinoma screening in high-risk patients is crucial but not without challenges. Our current screening methods, while essential, are far from perfect, often missing early cancers or leading to unnecessary interventions. Because of these limitations, the window for treatment of localized disease can easily be missed. In our practice, we tailor screening strategies to each patient’s specific needs, weighing the potential benefits against the risks, costs, and the inherent uncertainty of early detection tools. We believe it is essential to involve patients in this decision-making process to provide a balanced, individualized approach that considers both clinical evidence and the personal preferences of each person.
Dr. Kupferman is a gastroenterology fellow at Stanford University School of Medicine in California. Dr. Goel is a transplant hepatologist and a clinical associate professor in gastroenterology & hepatology at Stanford.
References
1. Vithayathil M and Khan SA. J Hepatol. 2022 Dec. doi: 10.1016/j.jhep.2022.07.022.
2. Patel N and Benipal B. Cureus. 2019 Jan. doi: 10.7759/cureus.3962.
Dear colleagues,
As gastroenterologists and endoscopists, we spend significant time preventing and diagnosing GI malignancies.
For instance, is it worthwhile screening for pancreatic cancer, and, if so, how should this be done? Likewise, diagnosing cholangiocarcinoma is challenging; how best should one evaluate for this in higher risk populations, such as primary sclerosing cholangitis? And what about the costs, financial and otherwise, associated with screening?
In this issue of Perspectives, Dr. Darshan Kothari and Dr. Daniel Bernstein discuss their approach to pancreatic cancer screening, including who is eligible, the preferred screening modalities, and the barriers to screening. In the accompanying perspective, Dr. Aparna Goel and Dr. Judah Kupferman focus on cholangiocarcinoma screening, identifying high-risk populations and discussing some of the concerns with screening, necessitating shared decision-making.
We welcome your thoughts on this issue. Share with us on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
An Approach to Pancreatic Cancer Screening
BY DANIEL A. BERNSTEIN, MD, AND DARSHAN KOTHARI, MD
Pancreatic cancer carries a dismal prognosis, now accounting for the third-most cancer-related mortality in the United States. A small proportion of patients are diagnosed at a local stage of disease, with over half found to have metastatic disease at presentation. Given the low overall incidence and lifetime risk in the general population, population-based screening is not justified.
About 10% of cases of pancreas cancer are associated with germ-line mutations and/or with a strong family history of pancreatic cancer. Several academic societies and expert committees now recommend regular screening for pancreatic cancer in patients who are considered high-risk individuals, as they carry a fivefold relative risk for pancreatic cancer. Moreover, studies suggest that screening has the potential to identify early-stage resectable disease and decrease mortality in this patient population.
Patients who benefit from pancreatic cancer screening are those who carry an increased lifetime risk (in excess of 5%) of pancreatic cancer. High-risk individuals include those with germ-line mutations and/or those with a family history of pancreatic cancer in first-degree relatives. Consensus guidelines by the International Cancer of the Pancreas Screening Consortium and the American Society for Gastrointestinal Endoscopy provide medical centers with detailed recommendations on who and when to start screening.
High-risk individuals fall into three categories:
- Patients with high-risk germline mutations including: familial atypical multiple mole melanoma syndrome (CDKN2A), hereditary breast and ovarian cancer syndromes (BRCA1, BRCA2, and PALB2), Peutz-Jeghers syndrome (STK11), and hereditary pancreatitis (PRSS1 and SPINK1)
- Patients with low- to moderate-risk germ-line mutations with at least one first-degree relative with pancreatic cancer: Lynch Syndrome (particularly MLH1 mutation), ataxia-telangiectasia (ATM), or Li-Fraumeni syndrome (p53)
- Patients with one first-degree relative with pancreatic cancer who in turn has one first-degree relative with pancreatic cancer (eg, a patient’s mother and maternal aunt or a patient’s father and patient’s sister)
Consistent with established guidelines, we recommend screening for high-risk patients beginning at age 50, or 10 years before the youngest age at which pancreas cancer was diagnosed in an affected relative. Screening is recommended earlier in patients with particularly high risk: at age 40 for patients with CDKN2A and STKI11 mutations and age 40 for patients with PRSS1 mutation or 20 years after the first attack of acute pancreatitis. For patients with a strong family history of pancreas cancer, we recommend comprehensive evaluation by a certified genetic counselor at a high-volume cancer center.
In practice, patients at our institution who are identified as high risk based on the above criteria are referred for an initial consultation at our pancreas center. In most cases, this should occur no sooner than 5 years prior to the recommended starting age for screening. All patients who are identified as high risk should be screened annually for diabetes given the growing evidence base supporting an association between new-onset diabetes and pancreatic cancer.
After an initial visit and discussion of the risks and benefits of screening, most screening protocols start with a baseline endoscopic ultrasound (EUS) and contrast-enhanced magnetic resonance abdomen with magnetic resonance cholangiopancreatography (MRI/MRCP), which will be repeated annually or sooner as the clinical condition warrants. A sooner-interval EUS should be considered for patients already undergoing screening who are newly found to have diabetes.
At our institution, we start with an in-person clinic evaluation followed by EUS. Thereafter, patients undergo MRI/MRCP (synchronized with a same-day clinic visit) alternating with EUS every 6 months to ensure patients are seen twice a year, though there is no specific data to support this approach. Non-diabetics also undergo yearly diabetes screening which will trigger an EUS if patients become diabetic.
We engage in shared decision-making with our high-risk individuals undergoing pancreatic cancer screening and at each visit we review their concurrent medical conditions and suitability to continue screening. We consider discontinuing screening after age 75, at the onset of any life-limiting illness, or after a discussion of risks and benefits if comorbidities lead to a substantial deterioration in a patient’s overall health status.
While a growing body of evidence exists to support the application of pancreatic cancer screening in high-risk individuals, this preventive service remains underutilized. Recent analysis of the screening cohort at our institution showed a demographically homogeneous group of mostly highly educated, high-income White females. These findings are consistent with the patient cohorts described in other pancreatic cancer screening programs and represent only a fraction of people who would qualify for pancreatic cancer screening.
A survey of patients undergoing screening at our institution identified cost, travel, and time associated with pancreatic cancer screening to be frequent challenges to participation. Further studies are needed to fully explore the barriers and psychological burden of pancreas cancer screening in high-risk individuals, and to identify ways to enrich the cohort of patients undergoing screening. This may involve novel methods to identify family members of patients with a new diagnosis of pancreas cancer and increasing health literacy around pancreatic cancer screening among patients and providers.
Pancreatic cancer screening has the potential to identify early-stage disease in patients who are at high risk because of germ-line mutations and/or family history. We recommend that patients engage in pancreatic cancer screening at high-volume centers with well-supported oncology, genetics, and research infrastructure.
Dr. Bernstein is a gastroenterology fellow at Duke University School of Medicine, Durham, North Carolina. Dr. Kothari is an associate professor of medicine in gastroenterology and hepatology at Duke University School of Medicine.
Screening for Cholangiocarcinoma
BY JUDAH KUPFERMAN, MD, AND APARNA GOEL, MD
Cholangiocarcinoma is a rare but aggressive cancer of the bile ducts that poses many diagnostic challenges. Approximately 3% of gastrointestinal cancers are attributed to cholangiocarcinoma, and while the annual incidence of disease in the United States is about 1.26 per 100,000 people, the incidence of intrahepatic disease has been rising considerably.1,2 Screening for cholangiocarcinoma is reserved for high-risk individuals — such as those with primary sclerosing cholangitis (PSC), secondary sclerosing cholangitis (SSC), and biliary tract disorders such as choledochal cysts or Caroli’s disease. The goal is to balance the benefits of early diagnosis with the costs and risks associated with screening, particularly given the limitations of available tools like MRI with cholangiopancreatography (MRCP), which has a sensitivity of 70%-85%. In general, we recommend annual cholangiocarcinoma screening for high-risk individuals with MRI and MRCP as well as with cancer antigen (CA) 19-9. .
Screening in Patients with Primary Sclerosing Cholangitis
The lifetime risk of cholangiocarcinoma in patients with PSC is 10%-15% with an annual risk of 0.5%-1.5%. In our experience, this is often the most feared complication for PSC patients, even more so than the risk of liver transplantation. We recommend annual MRI with MRCP in addition to CA 19-9 for patients with PSC in the first decade of their diagnosis, as most cancers are diagnosed during this period. If a patient’s imaging has remained stable for over a decade and there is minimal hepatic fibrosis, we discuss the option of reducing screening frequency to every 2 years to minimize costs and exposure to MRI contrast risks.
If MRI reveals a concerning new large duct stricture, we will evaluate this with an endoscopic retrograde cholangiopancreatography (ERCP), as differentiating benign and malignant strictures is quite challenging with MRI. We generally recommend ERCP with brush cytology and fluorescence in situ hybridization to improve diagnostic yield. Depending on imaging findings and location of the new large duct stricture, we may consider cholangioscopy during ERCP for direct visualization of the bile duct and directed tissue biopsies. Unfortunately, even in young, asymptomatic patients who undergo regular screening, cholangiocarcinoma is frequently diagnosed at an advanced stage.
Screening in Patients with Secondary Sclerosing Cholangitis
Patients with SSC may develop cholangiocarcinoma because of chronic inflammatory and fibrotic processes, such as IgG4-associated cholangiopathy, sarcoidosis, ischemic cholangiopathy, cystic fibrosis, recurrent pyogenic cholangitis, severe sepsis (as recently seen from SARS-CoV-2), surgical complications, or other etiologies. When the condition is reversible, such as with IgG4-associated cholangiopathy, cancer screening may not be necessary. However, when irreversible damage occurs, the cancer risk increases, though it varies by disease type and severity. In most cases, we recommend routine screening for cholangiocarcinoma with MRI and CA 19-9 in this population.
Screening in Patients with Biliary Tract Disorders
Biliary tract disorders such as choledochal cysts and Caroli’s disease also harbor an increased risk of cholangiocarcinoma. Choledochal cysts are congenital cystic dilations of the bile duct that have a 10%-30% lifetime risk of malignant transformation to cholangiocarcinoma. Surgical intervention to remove the cyst is often recommended because of this high risk. However, some patients may be unable or unwilling to undergo this surgery or they may have residual cysts. We recommend ongoing screening with MRI and CA 19-9 for these patients. Similarly, Caroli’s disease is a congenital disease associated with intrahepatic and extrahepatic bile duct cysts and associated with a 5%-15% lifetime risk of cholangiocarcinoma. MRI with MRCP and CA 19-9 should be performed routinely for patients with Caroli’s disease and syndrome.
Risks and Challenges in Cholangiocarcinoma Screening
While MRI with MRCP is the gold standard for cholangiocarcinoma screening, its limitations must be carefully considered. One growing concern is the potential for gadolinium retention in the brain, bones, or skin following repeated MRI scans. Though the long-term effects of gadolinium retention are not fully understood, we factor this into screening decisions, particularly for younger patients who may undergo decades of regular imaging.
MRI is not always feasible for certain patients, including those with metal implants, on hemodialysis, or with severe allergic reactions. In such cases, CT or ultrasound may serve as alternatives, though with lower sensitivity for detecting cholangiocarcinoma. Additionally, claustrophobia during MRI can be addressed with sedation, but this underscores the importance of shared decision-making.
From our perspective, cholangiocarcinoma screening in high-risk patients is crucial but not without challenges. Our current screening methods, while essential, are far from perfect, often missing early cancers or leading to unnecessary interventions. Because of these limitations, the window for treatment of localized disease can easily be missed. In our practice, we tailor screening strategies to each patient’s specific needs, weighing the potential benefits against the risks, costs, and the inherent uncertainty of early detection tools. We believe it is essential to involve patients in this decision-making process to provide a balanced, individualized approach that considers both clinical evidence and the personal preferences of each person.
Dr. Kupferman is a gastroenterology fellow at Stanford University School of Medicine in California. Dr. Goel is a transplant hepatologist and a clinical associate professor in gastroenterology & hepatology at Stanford.
References
1. Vithayathil M and Khan SA. J Hepatol. 2022 Dec. doi: 10.1016/j.jhep.2022.07.022.
2. Patel N and Benipal B. Cureus. 2019 Jan. doi: 10.7759/cureus.3962.
Dear colleagues,
As gastroenterologists and endoscopists, we spend significant time preventing and diagnosing GI malignancies.
For instance, is it worthwhile screening for pancreatic cancer, and, if so, how should this be done? Likewise, diagnosing cholangiocarcinoma is challenging; how best should one evaluate for this in higher risk populations, such as primary sclerosing cholangitis? And what about the costs, financial and otherwise, associated with screening?
In this issue of Perspectives, Dr. Darshan Kothari and Dr. Daniel Bernstein discuss their approach to pancreatic cancer screening, including who is eligible, the preferred screening modalities, and the barriers to screening. In the accompanying perspective, Dr. Aparna Goel and Dr. Judah Kupferman focus on cholangiocarcinoma screening, identifying high-risk populations and discussing some of the concerns with screening, necessitating shared decision-making.
We welcome your thoughts on this issue. Share with us on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
An Approach to Pancreatic Cancer Screening
BY DANIEL A. BERNSTEIN, MD, AND DARSHAN KOTHARI, MD
Pancreatic cancer carries a dismal prognosis, now accounting for the third-most cancer-related mortality in the United States. A small proportion of patients are diagnosed at a local stage of disease, with over half found to have metastatic disease at presentation. Given the low overall incidence and lifetime risk in the general population, population-based screening is not justified.
About 10% of cases of pancreas cancer are associated with germ-line mutations and/or with a strong family history of pancreatic cancer. Several academic societies and expert committees now recommend regular screening for pancreatic cancer in patients who are considered high-risk individuals, as they carry a fivefold relative risk for pancreatic cancer. Moreover, studies suggest that screening has the potential to identify early-stage resectable disease and decrease mortality in this patient population.
Patients who benefit from pancreatic cancer screening are those who carry an increased lifetime risk (in excess of 5%) of pancreatic cancer. High-risk individuals include those with germ-line mutations and/or those with a family history of pancreatic cancer in first-degree relatives. Consensus guidelines by the International Cancer of the Pancreas Screening Consortium and the American Society for Gastrointestinal Endoscopy provide medical centers with detailed recommendations on who and when to start screening.
High-risk individuals fall into three categories:
- Patients with high-risk germline mutations including: familial atypical multiple mole melanoma syndrome (CDKN2A), hereditary breast and ovarian cancer syndromes (BRCA1, BRCA2, and PALB2), Peutz-Jeghers syndrome (STK11), and hereditary pancreatitis (PRSS1 and SPINK1)
- Patients with low- to moderate-risk germ-line mutations with at least one first-degree relative with pancreatic cancer: Lynch Syndrome (particularly MLH1 mutation), ataxia-telangiectasia (ATM), or Li-Fraumeni syndrome (p53)
- Patients with one first-degree relative with pancreatic cancer who in turn has one first-degree relative with pancreatic cancer (eg, a patient’s mother and maternal aunt or a patient’s father and patient’s sister)
Consistent with established guidelines, we recommend screening for high-risk patients beginning at age 50, or 10 years before the youngest age at which pancreas cancer was diagnosed in an affected relative. Screening is recommended earlier in patients with particularly high risk: at age 40 for patients with CDKN2A and STKI11 mutations and age 40 for patients with PRSS1 mutation or 20 years after the first attack of acute pancreatitis. For patients with a strong family history of pancreas cancer, we recommend comprehensive evaluation by a certified genetic counselor at a high-volume cancer center.
In practice, patients at our institution who are identified as high risk based on the above criteria are referred for an initial consultation at our pancreas center. In most cases, this should occur no sooner than 5 years prior to the recommended starting age for screening. All patients who are identified as high risk should be screened annually for diabetes given the growing evidence base supporting an association between new-onset diabetes and pancreatic cancer.
After an initial visit and discussion of the risks and benefits of screening, most screening protocols start with a baseline endoscopic ultrasound (EUS) and contrast-enhanced magnetic resonance abdomen with magnetic resonance cholangiopancreatography (MRI/MRCP), which will be repeated annually or sooner as the clinical condition warrants. A sooner-interval EUS should be considered for patients already undergoing screening who are newly found to have diabetes.
At our institution, we start with an in-person clinic evaluation followed by EUS. Thereafter, patients undergo MRI/MRCP (synchronized with a same-day clinic visit) alternating with EUS every 6 months to ensure patients are seen twice a year, though there is no specific data to support this approach. Non-diabetics also undergo yearly diabetes screening which will trigger an EUS if patients become diabetic.
We engage in shared decision-making with our high-risk individuals undergoing pancreatic cancer screening and at each visit we review their concurrent medical conditions and suitability to continue screening. We consider discontinuing screening after age 75, at the onset of any life-limiting illness, or after a discussion of risks and benefits if comorbidities lead to a substantial deterioration in a patient’s overall health status.
While a growing body of evidence exists to support the application of pancreatic cancer screening in high-risk individuals, this preventive service remains underutilized. Recent analysis of the screening cohort at our institution showed a demographically homogeneous group of mostly highly educated, high-income White females. These findings are consistent with the patient cohorts described in other pancreatic cancer screening programs and represent only a fraction of people who would qualify for pancreatic cancer screening.
A survey of patients undergoing screening at our institution identified cost, travel, and time associated with pancreatic cancer screening to be frequent challenges to participation. Further studies are needed to fully explore the barriers and psychological burden of pancreas cancer screening in high-risk individuals, and to identify ways to enrich the cohort of patients undergoing screening. This may involve novel methods to identify family members of patients with a new diagnosis of pancreas cancer and increasing health literacy around pancreatic cancer screening among patients and providers.
Pancreatic cancer screening has the potential to identify early-stage disease in patients who are at high risk because of germ-line mutations and/or family history. We recommend that patients engage in pancreatic cancer screening at high-volume centers with well-supported oncology, genetics, and research infrastructure.
Dr. Bernstein is a gastroenterology fellow at Duke University School of Medicine, Durham, North Carolina. Dr. Kothari is an associate professor of medicine in gastroenterology and hepatology at Duke University School of Medicine.
Screening for Cholangiocarcinoma
BY JUDAH KUPFERMAN, MD, AND APARNA GOEL, MD
Cholangiocarcinoma is a rare but aggressive cancer of the bile ducts that poses many diagnostic challenges. Approximately 3% of gastrointestinal cancers are attributed to cholangiocarcinoma, and while the annual incidence of disease in the United States is about 1.26 per 100,000 people, the incidence of intrahepatic disease has been rising considerably.1,2 Screening for cholangiocarcinoma is reserved for high-risk individuals — such as those with primary sclerosing cholangitis (PSC), secondary sclerosing cholangitis (SSC), and biliary tract disorders such as choledochal cysts or Caroli’s disease. The goal is to balance the benefits of early diagnosis with the costs and risks associated with screening, particularly given the limitations of available tools like MRI with cholangiopancreatography (MRCP), which has a sensitivity of 70%-85%. In general, we recommend annual cholangiocarcinoma screening for high-risk individuals with MRI and MRCP as well as with cancer antigen (CA) 19-9. .
Screening in Patients with Primary Sclerosing Cholangitis
The lifetime risk of cholangiocarcinoma in patients with PSC is 10%-15% with an annual risk of 0.5%-1.5%. In our experience, this is often the most feared complication for PSC patients, even more so than the risk of liver transplantation. We recommend annual MRI with MRCP in addition to CA 19-9 for patients with PSC in the first decade of their diagnosis, as most cancers are diagnosed during this period. If a patient’s imaging has remained stable for over a decade and there is minimal hepatic fibrosis, we discuss the option of reducing screening frequency to every 2 years to minimize costs and exposure to MRI contrast risks.
If MRI reveals a concerning new large duct stricture, we will evaluate this with an endoscopic retrograde cholangiopancreatography (ERCP), as differentiating benign and malignant strictures is quite challenging with MRI. We generally recommend ERCP with brush cytology and fluorescence in situ hybridization to improve diagnostic yield. Depending on imaging findings and location of the new large duct stricture, we may consider cholangioscopy during ERCP for direct visualization of the bile duct and directed tissue biopsies. Unfortunately, even in young, asymptomatic patients who undergo regular screening, cholangiocarcinoma is frequently diagnosed at an advanced stage.
Screening in Patients with Secondary Sclerosing Cholangitis
Patients with SSC may develop cholangiocarcinoma because of chronic inflammatory and fibrotic processes, such as IgG4-associated cholangiopathy, sarcoidosis, ischemic cholangiopathy, cystic fibrosis, recurrent pyogenic cholangitis, severe sepsis (as recently seen from SARS-CoV-2), surgical complications, or other etiologies. When the condition is reversible, such as with IgG4-associated cholangiopathy, cancer screening may not be necessary. However, when irreversible damage occurs, the cancer risk increases, though it varies by disease type and severity. In most cases, we recommend routine screening for cholangiocarcinoma with MRI and CA 19-9 in this population.
Screening in Patients with Biliary Tract Disorders
Biliary tract disorders such as choledochal cysts and Caroli’s disease also harbor an increased risk of cholangiocarcinoma. Choledochal cysts are congenital cystic dilations of the bile duct that have a 10%-30% lifetime risk of malignant transformation to cholangiocarcinoma. Surgical intervention to remove the cyst is often recommended because of this high risk. However, some patients may be unable or unwilling to undergo this surgery or they may have residual cysts. We recommend ongoing screening with MRI and CA 19-9 for these patients. Similarly, Caroli’s disease is a congenital disease associated with intrahepatic and extrahepatic bile duct cysts and associated with a 5%-15% lifetime risk of cholangiocarcinoma. MRI with MRCP and CA 19-9 should be performed routinely for patients with Caroli’s disease and syndrome.
Risks and Challenges in Cholangiocarcinoma Screening
While MRI with MRCP is the gold standard for cholangiocarcinoma screening, its limitations must be carefully considered. One growing concern is the potential for gadolinium retention in the brain, bones, or skin following repeated MRI scans. Though the long-term effects of gadolinium retention are not fully understood, we factor this into screening decisions, particularly for younger patients who may undergo decades of regular imaging.
MRI is not always feasible for certain patients, including those with metal implants, on hemodialysis, or with severe allergic reactions. In such cases, CT or ultrasound may serve as alternatives, though with lower sensitivity for detecting cholangiocarcinoma. Additionally, claustrophobia during MRI can be addressed with sedation, but this underscores the importance of shared decision-making.
From our perspective, cholangiocarcinoma screening in high-risk patients is crucial but not without challenges. Our current screening methods, while essential, are far from perfect, often missing early cancers or leading to unnecessary interventions. Because of these limitations, the window for treatment of localized disease can easily be missed. In our practice, we tailor screening strategies to each patient’s specific needs, weighing the potential benefits against the risks, costs, and the inherent uncertainty of early detection tools. We believe it is essential to involve patients in this decision-making process to provide a balanced, individualized approach that considers both clinical evidence and the personal preferences of each person.
Dr. Kupferman is a gastroenterology fellow at Stanford University School of Medicine in California. Dr. Goel is a transplant hepatologist and a clinical associate professor in gastroenterology & hepatology at Stanford.
References
1. Vithayathil M and Khan SA. J Hepatol. 2022 Dec. doi: 10.1016/j.jhep.2022.07.022.
2. Patel N and Benipal B. Cureus. 2019 Jan. doi: 10.7759/cureus.3962.
Managing Age-Related Muscle Loss in Primary Care
Scene 1: Exercise Medicine Clinic, Rio de Janeiro, Brazil I just finished one evaluation on physical fitness and health and looked at my schedule. My next patient would be a 65-year-old man. How fit will he be? Will he have evident age-related muscle loss? I gave myself a short break and my mind went to the late 1970s.
Once upon a time, the practice of medicine was based primarily on the skill of your physical examination, previous experiences, and your ability to reason logically and make solid deductions. In 1979, the stethoscope was part of my dress code. After one elective semester as a research fellow at the Ambrose Cardiorespiratory Unit at McMaster University Medical Centre, in Hamilton, Canada, where I was honored to witness the dawn of evidence-based medicine, I graduated from Federal University of Rio de Janeiro. I still remember being introduced to some promising novelties in cardiology, such as M-mode echocardiograms and myocardial scintigraphy. Radiology was primarily centered on x-rays, and lab testing was basic and poorly automatized.
Over the following decades, medical practice changed dramatically with the incorporation of new technologies. Recent advances in diagnostic tools, genetics, artificial intelligence, and sophisticated statistical analyses, along with well-collected scientific data, have molded how clinicians should think and work.
At the same time, clinical profiles also changed. Internists and primary care physicians are regularly managing patients who are, on average, older and have or are on the way to having potentially life-threatening chronic diseases, accompanied by poor lifestyle habits, and, highly important, often some degree of disability, frailty, and loss of independence. Many of them exhibit age-related muscle loss.
Scene 2: Exercise Medicine Clinic, Rio de Janeiro, Brazil
Conscious of the benefits of interrupting my sitting time with activity, I left my office and walked to meet my patient in the waiting room. I called his name and introduced myself. I watched how he listened and reacted to my speech, and how easy or hard it was for him to rise from the chair — readiness, velocity, and number of supports required: none, one, or two hands. I offered my own hand to him, and when we shook, I gauged the strength of his grip.
I invited him into my office and took note of his somatotype and body composition, and whether he had any central obesity. Of course, and I should by no means miss this chance, I carefully observed how he walked in — his gait, speed, balance, posture — how he pulled up the chair, and how he managed to lower himself into his seat. Before I even sat in my own chair, I asked him if he remembered what his body weight was 5 years ago and what it was today. Before we got started in earnest, I had already managed to collect several pieces of relevant information.
Exercise Physiology: Changing Landscape
Muscle activity depends on muscle mass and function, and peaks somewhere between ages 25 and 35 before declining. The drop is slow in the early stages but accelerates rapidly after age 60 or 65.
Two of the most relevant variables in muscle function are strength and power. As a product of force and velocity, muscle power could be a more crucial factor than strength for many daily activities that demand movement against gravity or inertia, such as placing carry-on baggage in the overhead bin of an airplane or rising from the floor or chair.
The association between muscle mass and muscle strength or power is moderate, and physiologic data have indicated that the decline of muscle power with aging is faster and larger than that of muscle strength.
The term “sarcopenia” has become definitively incorporated into the medical glossary. From the Greek (“sark” and “penia”), sarcopenia was defined as reduced muscle mass, but more recently it has encompassed muscle strength in its definition. However, a recent consensus paper from the Global Leadership Initiative in Sarcopenia, using a Delphi approach, rejected the inclusion of muscle power in the concept of sarcopenia. On the other hand, a long time ago, some authors coined and advocated the use the term “dynapenia” to more precisely reflect the reduced levels of muscle strength and power that often accompany aging.
The best available intervention to counteract age-related deterioration of muscle activity is resistance exercise. The types of resistance exercises vary widely — by number of sets and repetitions, intervals between sets, speed of execution of movement, and percentage of maximal weight/load.
We recently proposed that, after an evaluation to identify the main muscle variable requiring attention, the resistance exercise program should be individually tailored and prescribed according to the objective to counteract sarcopenia or dynapenia.
What is more important for autonomy and better daily living conditions in old and very old individuals: muscle mass, muscle strength, or muscle power? More likely the response is muscle power — in practical terms, dynapenia rather than sarcopenia. This short video presents practical tips for obtaining optimal results in fighting dynapenia. The first choice should be power training or high velocity–based training, emphasizing two to three sets of six to eight repetitions performed as fast as possible (on the concentric or shortening phase of muscle contraction) with relatively high loads.
Internists and primary care physicians are most likely satisfied with the information they obtain by simple observation, and already can superficially grade the magnitude of a patient’s age-related muscle loss and its consequences to daily living.
However, those who want more objective information on nonaerobic physical fitness can add one to three simple tests to their consultation: the sitting-rising test (SRT); the 10-second one-legged test (10sOLS test); and the Flexitest. Poor performance on each of these — and particularly all three — is strongly associated with an increased risk for premature death in middle-aged and older individuals. These tests require no extra equipment and can be performed rapidly, and interpreting the results takes only a few moments using published reference values.
Age-related muscle loss profoundly affects our ability to sit and rise from the floor, so if time is limited, the SRT is the best assessment, as it measures all nonaerobic components of physical fitness. For a quick interpretation, consider that SRT scores vary from 0 to 10, do not substantially differ by sex, and that a composite score equal to or greater than 8 will reflect a minimum age-adjusted percentile of 61, most likely indicating relevant age-related muscle loss is not yet occurring.
Dr. Araújo is Professor and Dean of Research and Education, Exercise Medicine Clinic (CLINIMEX), Rio de Janeiro, Brazil. He reported conflicts of interest with INBRAMED.
A version of this article first appeared on Medscape.com.
Scene 1: Exercise Medicine Clinic, Rio de Janeiro, Brazil I just finished one evaluation on physical fitness and health and looked at my schedule. My next patient would be a 65-year-old man. How fit will he be? Will he have evident age-related muscle loss? I gave myself a short break and my mind went to the late 1970s.
Once upon a time, the practice of medicine was based primarily on the skill of your physical examination, previous experiences, and your ability to reason logically and make solid deductions. In 1979, the stethoscope was part of my dress code. After one elective semester as a research fellow at the Ambrose Cardiorespiratory Unit at McMaster University Medical Centre, in Hamilton, Canada, where I was honored to witness the dawn of evidence-based medicine, I graduated from Federal University of Rio de Janeiro. I still remember being introduced to some promising novelties in cardiology, such as M-mode echocardiograms and myocardial scintigraphy. Radiology was primarily centered on x-rays, and lab testing was basic and poorly automatized.
Over the following decades, medical practice changed dramatically with the incorporation of new technologies. Recent advances in diagnostic tools, genetics, artificial intelligence, and sophisticated statistical analyses, along with well-collected scientific data, have molded how clinicians should think and work.
At the same time, clinical profiles also changed. Internists and primary care physicians are regularly managing patients who are, on average, older and have or are on the way to having potentially life-threatening chronic diseases, accompanied by poor lifestyle habits, and, highly important, often some degree of disability, frailty, and loss of independence. Many of them exhibit age-related muscle loss.
Scene 2: Exercise Medicine Clinic, Rio de Janeiro, Brazil
Conscious of the benefits of interrupting my sitting time with activity, I left my office and walked to meet my patient in the waiting room. I called his name and introduced myself. I watched how he listened and reacted to my speech, and how easy or hard it was for him to rise from the chair — readiness, velocity, and number of supports required: none, one, or two hands. I offered my own hand to him, and when we shook, I gauged the strength of his grip.
I invited him into my office and took note of his somatotype and body composition, and whether he had any central obesity. Of course, and I should by no means miss this chance, I carefully observed how he walked in — his gait, speed, balance, posture — how he pulled up the chair, and how he managed to lower himself into his seat. Before I even sat in my own chair, I asked him if he remembered what his body weight was 5 years ago and what it was today. Before we got started in earnest, I had already managed to collect several pieces of relevant information.
Exercise Physiology: Changing Landscape
Muscle activity depends on muscle mass and function, and peaks somewhere between ages 25 and 35 before declining. The drop is slow in the early stages but accelerates rapidly after age 60 or 65.
Two of the most relevant variables in muscle function are strength and power. As a product of force and velocity, muscle power could be a more crucial factor than strength for many daily activities that demand movement against gravity or inertia, such as placing carry-on baggage in the overhead bin of an airplane or rising from the floor or chair.
The association between muscle mass and muscle strength or power is moderate, and physiologic data have indicated that the decline of muscle power with aging is faster and larger than that of muscle strength.
The term “sarcopenia” has become definitively incorporated into the medical glossary. From the Greek (“sark” and “penia”), sarcopenia was defined as reduced muscle mass, but more recently it has encompassed muscle strength in its definition. However, a recent consensus paper from the Global Leadership Initiative in Sarcopenia, using a Delphi approach, rejected the inclusion of muscle power in the concept of sarcopenia. On the other hand, a long time ago, some authors coined and advocated the use the term “dynapenia” to more precisely reflect the reduced levels of muscle strength and power that often accompany aging.
The best available intervention to counteract age-related deterioration of muscle activity is resistance exercise. The types of resistance exercises vary widely — by number of sets and repetitions, intervals between sets, speed of execution of movement, and percentage of maximal weight/load.
We recently proposed that, after an evaluation to identify the main muscle variable requiring attention, the resistance exercise program should be individually tailored and prescribed according to the objective to counteract sarcopenia or dynapenia.
What is more important for autonomy and better daily living conditions in old and very old individuals: muscle mass, muscle strength, or muscle power? More likely the response is muscle power — in practical terms, dynapenia rather than sarcopenia. This short video presents practical tips for obtaining optimal results in fighting dynapenia. The first choice should be power training or high velocity–based training, emphasizing two to three sets of six to eight repetitions performed as fast as possible (on the concentric or shortening phase of muscle contraction) with relatively high loads.
Internists and primary care physicians are most likely satisfied with the information they obtain by simple observation, and already can superficially grade the magnitude of a patient’s age-related muscle loss and its consequences to daily living.
However, those who want more objective information on nonaerobic physical fitness can add one to three simple tests to their consultation: the sitting-rising test (SRT); the 10-second one-legged test (10sOLS test); and the Flexitest. Poor performance on each of these — and particularly all three — is strongly associated with an increased risk for premature death in middle-aged and older individuals. These tests require no extra equipment and can be performed rapidly, and interpreting the results takes only a few moments using published reference values.
Age-related muscle loss profoundly affects our ability to sit and rise from the floor, so if time is limited, the SRT is the best assessment, as it measures all nonaerobic components of physical fitness. For a quick interpretation, consider that SRT scores vary from 0 to 10, do not substantially differ by sex, and that a composite score equal to or greater than 8 will reflect a minimum age-adjusted percentile of 61, most likely indicating relevant age-related muscle loss is not yet occurring.
Dr. Araújo is Professor and Dean of Research and Education, Exercise Medicine Clinic (CLINIMEX), Rio de Janeiro, Brazil. He reported conflicts of interest with INBRAMED.
A version of this article first appeared on Medscape.com.
Scene 1: Exercise Medicine Clinic, Rio de Janeiro, Brazil I just finished one evaluation on physical fitness and health and looked at my schedule. My next patient would be a 65-year-old man. How fit will he be? Will he have evident age-related muscle loss? I gave myself a short break and my mind went to the late 1970s.
Once upon a time, the practice of medicine was based primarily on the skill of your physical examination, previous experiences, and your ability to reason logically and make solid deductions. In 1979, the stethoscope was part of my dress code. After one elective semester as a research fellow at the Ambrose Cardiorespiratory Unit at McMaster University Medical Centre, in Hamilton, Canada, where I was honored to witness the dawn of evidence-based medicine, I graduated from Federal University of Rio de Janeiro. I still remember being introduced to some promising novelties in cardiology, such as M-mode echocardiograms and myocardial scintigraphy. Radiology was primarily centered on x-rays, and lab testing was basic and poorly automatized.
Over the following decades, medical practice changed dramatically with the incorporation of new technologies. Recent advances in diagnostic tools, genetics, artificial intelligence, and sophisticated statistical analyses, along with well-collected scientific data, have molded how clinicians should think and work.
At the same time, clinical profiles also changed. Internists and primary care physicians are regularly managing patients who are, on average, older and have or are on the way to having potentially life-threatening chronic diseases, accompanied by poor lifestyle habits, and, highly important, often some degree of disability, frailty, and loss of independence. Many of them exhibit age-related muscle loss.
Scene 2: Exercise Medicine Clinic, Rio de Janeiro, Brazil
Conscious of the benefits of interrupting my sitting time with activity, I left my office and walked to meet my patient in the waiting room. I called his name and introduced myself. I watched how he listened and reacted to my speech, and how easy or hard it was for him to rise from the chair — readiness, velocity, and number of supports required: none, one, or two hands. I offered my own hand to him, and when we shook, I gauged the strength of his grip.
I invited him into my office and took note of his somatotype and body composition, and whether he had any central obesity. Of course, and I should by no means miss this chance, I carefully observed how he walked in — his gait, speed, balance, posture — how he pulled up the chair, and how he managed to lower himself into his seat. Before I even sat in my own chair, I asked him if he remembered what his body weight was 5 years ago and what it was today. Before we got started in earnest, I had already managed to collect several pieces of relevant information.
Exercise Physiology: Changing Landscape
Muscle activity depends on muscle mass and function, and peaks somewhere between ages 25 and 35 before declining. The drop is slow in the early stages but accelerates rapidly after age 60 or 65.
Two of the most relevant variables in muscle function are strength and power. As a product of force and velocity, muscle power could be a more crucial factor than strength for many daily activities that demand movement against gravity or inertia, such as placing carry-on baggage in the overhead bin of an airplane or rising from the floor or chair.
The association between muscle mass and muscle strength or power is moderate, and physiologic data have indicated that the decline of muscle power with aging is faster and larger than that of muscle strength.
The term “sarcopenia” has become definitively incorporated into the medical glossary. From the Greek (“sark” and “penia”), sarcopenia was defined as reduced muscle mass, but more recently it has encompassed muscle strength in its definition. However, a recent consensus paper from the Global Leadership Initiative in Sarcopenia, using a Delphi approach, rejected the inclusion of muscle power in the concept of sarcopenia. On the other hand, a long time ago, some authors coined and advocated the use the term “dynapenia” to more precisely reflect the reduced levels of muscle strength and power that often accompany aging.
The best available intervention to counteract age-related deterioration of muscle activity is resistance exercise. The types of resistance exercises vary widely — by number of sets and repetitions, intervals between sets, speed of execution of movement, and percentage of maximal weight/load.
We recently proposed that, after an evaluation to identify the main muscle variable requiring attention, the resistance exercise program should be individually tailored and prescribed according to the objective to counteract sarcopenia or dynapenia.
What is more important for autonomy and better daily living conditions in old and very old individuals: muscle mass, muscle strength, or muscle power? More likely the response is muscle power — in practical terms, dynapenia rather than sarcopenia. This short video presents practical tips for obtaining optimal results in fighting dynapenia. The first choice should be power training or high velocity–based training, emphasizing two to three sets of six to eight repetitions performed as fast as possible (on the concentric or shortening phase of muscle contraction) with relatively high loads.
Internists and primary care physicians are most likely satisfied with the information they obtain by simple observation, and already can superficially grade the magnitude of a patient’s age-related muscle loss and its consequences to daily living.
However, those who want more objective information on nonaerobic physical fitness can add one to three simple tests to their consultation: the sitting-rising test (SRT); the 10-second one-legged test (10sOLS test); and the Flexitest. Poor performance on each of these — and particularly all three — is strongly associated with an increased risk for premature death in middle-aged and older individuals. These tests require no extra equipment and can be performed rapidly, and interpreting the results takes only a few moments using published reference values.
Age-related muscle loss profoundly affects our ability to sit and rise from the floor, so if time is limited, the SRT is the best assessment, as it measures all nonaerobic components of physical fitness. For a quick interpretation, consider that SRT scores vary from 0 to 10, do not substantially differ by sex, and that a composite score equal to or greater than 8 will reflect a minimum age-adjusted percentile of 61, most likely indicating relevant age-related muscle loss is not yet occurring.
Dr. Araújo is Professor and Dean of Research and Education, Exercise Medicine Clinic (CLINIMEX), Rio de Janeiro, Brazil. He reported conflicts of interest with INBRAMED.
A version of this article first appeared on Medscape.com.
Obesity: A Social Vulnerability
Sometime in the last year or 2 I wrote that, despite my considerable reservations, I had finally come to the conclusion that the American Medical Association’s decision to designate obesity as a disease was appropriate. My rationalization was that the disease label would open more opportunities for funding obesity treatments. However, the explosive growth and popularity of glucagon-like peptide 1 (GLP-1) agonists over the last year has had me rethinking my decision to suppress my long-held reservations about the disease designation.
So, if it’s not a disease, then what should we call it? How do we explain its surge in high-income countries that began in the 1980s? While there are still some folks who see obesity as a character flaw, I think you and I as healthcare providers have difficulty explaining the increase prevalence of obesity as either global breakdown of willpower or a widespread genetic shift as the result of burst of radiation from solar flares.
However, if we want to continue our search and finger-pointing we need to have a better definition of exactly what obesity is. If we’re going to continue calling it a disease we have done a pretty sloppy job of creating diagnostic criteria. To be honest, we aren’t doing such a hot job with “long COVID” either.
A recent article in the New York Times makes it clear that I’m not the only physician who is feeling uncomfortable with this lack of diagnostic specificity.
We know that using body mass index (BMI) as a criteria is imprecise. There are healthy individuals with elevated BMIs and there are others who are carrying an unhealthy amount of fat who have normal BMIs. And, there are individuals who have what might appear to be an excess amount of fat who are fit and healthy by other criteria.
Some investigators feel that a set of measurements that includes a waist and/or hip measurement may be a more accurate way of determining visceral adipose tissue. However, this body roundness index (BRI) currently relies on a tape measurement. Until the technique can be preformed by an inexpensive and readily available scanner, the BRI cannot be considered a practical tool for determining obesity.
Dr. Francisco Rubino, the chair of metabolic and bariatric surgery at Kings College in London, England, has been quoted as saying that, “if one defines a disease inaccurately, everything that stems from that – from diagnosis to treatment to policies – will be distorted and biased.”
Denmark has been forced to relabel obesity as a risk factor because the disease designation was stressing the financial viability of their healthcare system as more and more patients were being prescribe GLP-1 agonists, sometimes off label. A rationing strategy was resulting in suboptimal treatment of a significant portion of the obese population.
Spearheaded by Dr. Rubino, a Lancet Commission composed of physicians has tasked itself to define an “evidence-based diagnosis for obesity. Instead of relying on a single metric such as the BMI or BRI, diagnosing “clinical obesity” would involve a broad array of observations including a history, physical examination, standard laboratory and additional testing, “naming signs and symptoms, organ by organ, tissue by tissue, with plausible mechanisms for each one.” In other words, treating each patient as an individual using evidence-based criteria to make a diagnosis. While likely to be time consuming, this strategy feels like a more scientific approach. I suspect once clinical obesity is more rigorously defined it could be divided into several subtypes. For example, there would be a few conditions that were genetic; Prader-Willi syndrome being the best known.
However, I think the Lancet Commission’s strategy will find that the majority of individuals who make up this half-century global surge have become clinically obese because they have been unable to adapt to the obeseogenic forces in our society, which include diet, autocentricity, and attractive sedentary forms of entertainment, to name just three.
In some cases these unfortunate individuals are more vulnerable because there were born into an economically disadvantaged situation. In other scenarios a lack of foresight and/or political will may have left individuals with no other choice but to rely on automobiles to get around. Still others may find themselves living in a nutritional desert because all of the grocery stores have closed.
I recently encountered a descriptor in a story about the Federal Emergency Management Agency which could easily be adapted to describe this large and growing subtype of individuals with clinical obesity. “Social vulnerability” is measure of how well a community can withstand external stressors that impact human health. For example, the emergency management folks are thinking in terms of natural disaster such as hurricanes, floods, and tornadoes and are asking how well a given community can meet the challenges one would create.
But, the term social vulnerability can easily be applied to individuals living in a society in which unhealthy food is abundant, an infrastructure that discourages or outright prevents non-motorized travel, and the temptation of sedentary entertainment options is unavoidable. Fortunately, not every citizen living in an obesogenic society becomes obese. What factors have protected the non-obese individuals from these obeseogenic stressors? What are the characteristics of the unfortunate “vulnerables” living in the same society who end up being obese?
It is time to shift our focus away from a poorly defined disease model to one in which we begin looking at our society to find out why we have so many socially vulnerable individuals. The toll of obesity as it is currently defined is many order of magnitudes greater than any natural disaster. We have become communities that can no longer withstand the its obesogenic stressors many of which we have created and/or allowed to accumulate over the last century.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Sometime in the last year or 2 I wrote that, despite my considerable reservations, I had finally come to the conclusion that the American Medical Association’s decision to designate obesity as a disease was appropriate. My rationalization was that the disease label would open more opportunities for funding obesity treatments. However, the explosive growth and popularity of glucagon-like peptide 1 (GLP-1) agonists over the last year has had me rethinking my decision to suppress my long-held reservations about the disease designation.
So, if it’s not a disease, then what should we call it? How do we explain its surge in high-income countries that began in the 1980s? While there are still some folks who see obesity as a character flaw, I think you and I as healthcare providers have difficulty explaining the increase prevalence of obesity as either global breakdown of willpower or a widespread genetic shift as the result of burst of radiation from solar flares.
However, if we want to continue our search and finger-pointing we need to have a better definition of exactly what obesity is. If we’re going to continue calling it a disease we have done a pretty sloppy job of creating diagnostic criteria. To be honest, we aren’t doing such a hot job with “long COVID” either.
A recent article in the New York Times makes it clear that I’m not the only physician who is feeling uncomfortable with this lack of diagnostic specificity.
We know that using body mass index (BMI) as a criteria is imprecise. There are healthy individuals with elevated BMIs and there are others who are carrying an unhealthy amount of fat who have normal BMIs. And, there are individuals who have what might appear to be an excess amount of fat who are fit and healthy by other criteria.
Some investigators feel that a set of measurements that includes a waist and/or hip measurement may be a more accurate way of determining visceral adipose tissue. However, this body roundness index (BRI) currently relies on a tape measurement. Until the technique can be preformed by an inexpensive and readily available scanner, the BRI cannot be considered a practical tool for determining obesity.
Dr. Francisco Rubino, the chair of metabolic and bariatric surgery at Kings College in London, England, has been quoted as saying that, “if one defines a disease inaccurately, everything that stems from that – from diagnosis to treatment to policies – will be distorted and biased.”
Denmark has been forced to relabel obesity as a risk factor because the disease designation was stressing the financial viability of their healthcare system as more and more patients were being prescribe GLP-1 agonists, sometimes off label. A rationing strategy was resulting in suboptimal treatment of a significant portion of the obese population.
Spearheaded by Dr. Rubino, a Lancet Commission composed of physicians has tasked itself to define an “evidence-based diagnosis for obesity. Instead of relying on a single metric such as the BMI or BRI, diagnosing “clinical obesity” would involve a broad array of observations including a history, physical examination, standard laboratory and additional testing, “naming signs and symptoms, organ by organ, tissue by tissue, with plausible mechanisms for each one.” In other words, treating each patient as an individual using evidence-based criteria to make a diagnosis. While likely to be time consuming, this strategy feels like a more scientific approach. I suspect once clinical obesity is more rigorously defined it could be divided into several subtypes. For example, there would be a few conditions that were genetic; Prader-Willi syndrome being the best known.
However, I think the Lancet Commission’s strategy will find that the majority of individuals who make up this half-century global surge have become clinically obese because they have been unable to adapt to the obeseogenic forces in our society, which include diet, autocentricity, and attractive sedentary forms of entertainment, to name just three.
In some cases these unfortunate individuals are more vulnerable because there were born into an economically disadvantaged situation. In other scenarios a lack of foresight and/or political will may have left individuals with no other choice but to rely on automobiles to get around. Still others may find themselves living in a nutritional desert because all of the grocery stores have closed.
I recently encountered a descriptor in a story about the Federal Emergency Management Agency which could easily be adapted to describe this large and growing subtype of individuals with clinical obesity. “Social vulnerability” is measure of how well a community can withstand external stressors that impact human health. For example, the emergency management folks are thinking in terms of natural disaster such as hurricanes, floods, and tornadoes and are asking how well a given community can meet the challenges one would create.
But, the term social vulnerability can easily be applied to individuals living in a society in which unhealthy food is abundant, an infrastructure that discourages or outright prevents non-motorized travel, and the temptation of sedentary entertainment options is unavoidable. Fortunately, not every citizen living in an obesogenic society becomes obese. What factors have protected the non-obese individuals from these obeseogenic stressors? What are the characteristics of the unfortunate “vulnerables” living in the same society who end up being obese?
It is time to shift our focus away from a poorly defined disease model to one in which we begin looking at our society to find out why we have so many socially vulnerable individuals. The toll of obesity as it is currently defined is many order of magnitudes greater than any natural disaster. We have become communities that can no longer withstand the its obesogenic stressors many of which we have created and/or allowed to accumulate over the last century.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Sometime in the last year or 2 I wrote that, despite my considerable reservations, I had finally come to the conclusion that the American Medical Association’s decision to designate obesity as a disease was appropriate. My rationalization was that the disease label would open more opportunities for funding obesity treatments. However, the explosive growth and popularity of glucagon-like peptide 1 (GLP-1) agonists over the last year has had me rethinking my decision to suppress my long-held reservations about the disease designation.
So, if it’s not a disease, then what should we call it? How do we explain its surge in high-income countries that began in the 1980s? While there are still some folks who see obesity as a character flaw, I think you and I as healthcare providers have difficulty explaining the increase prevalence of obesity as either global breakdown of willpower or a widespread genetic shift as the result of burst of radiation from solar flares.
However, if we want to continue our search and finger-pointing we need to have a better definition of exactly what obesity is. If we’re going to continue calling it a disease we have done a pretty sloppy job of creating diagnostic criteria. To be honest, we aren’t doing such a hot job with “long COVID” either.
A recent article in the New York Times makes it clear that I’m not the only physician who is feeling uncomfortable with this lack of diagnostic specificity.
We know that using body mass index (BMI) as a criteria is imprecise. There are healthy individuals with elevated BMIs and there are others who are carrying an unhealthy amount of fat who have normal BMIs. And, there are individuals who have what might appear to be an excess amount of fat who are fit and healthy by other criteria.
Some investigators feel that a set of measurements that includes a waist and/or hip measurement may be a more accurate way of determining visceral adipose tissue. However, this body roundness index (BRI) currently relies on a tape measurement. Until the technique can be preformed by an inexpensive and readily available scanner, the BRI cannot be considered a practical tool for determining obesity.
Dr. Francisco Rubino, the chair of metabolic and bariatric surgery at Kings College in London, England, has been quoted as saying that, “if one defines a disease inaccurately, everything that stems from that – from diagnosis to treatment to policies – will be distorted and biased.”
Denmark has been forced to relabel obesity as a risk factor because the disease designation was stressing the financial viability of their healthcare system as more and more patients were being prescribe GLP-1 agonists, sometimes off label. A rationing strategy was resulting in suboptimal treatment of a significant portion of the obese population.
Spearheaded by Dr. Rubino, a Lancet Commission composed of physicians has tasked itself to define an “evidence-based diagnosis for obesity. Instead of relying on a single metric such as the BMI or BRI, diagnosing “clinical obesity” would involve a broad array of observations including a history, physical examination, standard laboratory and additional testing, “naming signs and symptoms, organ by organ, tissue by tissue, with plausible mechanisms for each one.” In other words, treating each patient as an individual using evidence-based criteria to make a diagnosis. While likely to be time consuming, this strategy feels like a more scientific approach. I suspect once clinical obesity is more rigorously defined it could be divided into several subtypes. For example, there would be a few conditions that were genetic; Prader-Willi syndrome being the best known.
However, I think the Lancet Commission’s strategy will find that the majority of individuals who make up this half-century global surge have become clinically obese because they have been unable to adapt to the obeseogenic forces in our society, which include diet, autocentricity, and attractive sedentary forms of entertainment, to name just three.
In some cases these unfortunate individuals are more vulnerable because there were born into an economically disadvantaged situation. In other scenarios a lack of foresight and/or political will may have left individuals with no other choice but to rely on automobiles to get around. Still others may find themselves living in a nutritional desert because all of the grocery stores have closed.
I recently encountered a descriptor in a story about the Federal Emergency Management Agency which could easily be adapted to describe this large and growing subtype of individuals with clinical obesity. “Social vulnerability” is measure of how well a community can withstand external stressors that impact human health. For example, the emergency management folks are thinking in terms of natural disaster such as hurricanes, floods, and tornadoes and are asking how well a given community can meet the challenges one would create.
But, the term social vulnerability can easily be applied to individuals living in a society in which unhealthy food is abundant, an infrastructure that discourages or outright prevents non-motorized travel, and the temptation of sedentary entertainment options is unavoidable. Fortunately, not every citizen living in an obesogenic society becomes obese. What factors have protected the non-obese individuals from these obeseogenic stressors? What are the characteristics of the unfortunate “vulnerables” living in the same society who end up being obese?
It is time to shift our focus away from a poorly defined disease model to one in which we begin looking at our society to find out why we have so many socially vulnerable individuals. The toll of obesity as it is currently defined is many order of magnitudes greater than any natural disaster. We have become communities that can no longer withstand the its obesogenic stressors many of which we have created and/or allowed to accumulate over the last century.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Is Being ‘Manly’ a Threat to a Man’s Health?
When my normally adorable cat Biscuit bit my ankle in a playful stalking exercise gone wrong, I washed it with soap and some rubbing alcohol, slapped on a Band-Aid, and went about my day.
The next morning, when it was swollen, I told myself it was probably just a hematoma and went about my day.
The next day, when the swelling had increased and red lines started creeping up my leg, I called my doctor. Long story short, I ended up hospitalized for intravenous antibiotics.
This is all to say that, yes, I’m sort of an idiot, but also to introduce the idea that maybe I minimized my very obvious lymphangitis because I am a man.
This week, we have empirical evidence that men downplay their medical symptoms — and that manlier men downplay them even more.
I’m going to talk about a study that links manliness (or, scientifically speaking, “male gender expressivity”) to medical diagnoses that are based on hard evidence and medical diagnoses that are based on self-report. You see where this is going but I want to walk you through the methods here because they are fairly interesting.
This study used data from the US National Longitudinal Study of Adolescent to Adult Health. This study enrolled 20,000 adolescents who were in grades 7-12 in the 1994-1995 school year and has been following them ever since — about 30 years so far.
The authors wanted to link early gender roles to long-term outcomes, so they cut that 20,000 number down to the 4230 males in the group who had complete follow-up.
Now comes the first interesting question. How do you quantify the “male gender expressivity” of boys in 7th-12th grade? There was no survey item that asked them how masculine or manly they felt. What the authors did was look at the surveys that were administered and identify the questions on those surveys where boys and girls gave the most disparate answers. I have some examples here. 
Some of these questions make sense when it comes to gender expressivity: “How often do you cry?” for example, has a lot of validity for the social construct that is gender. But some questions where boys and girls gave very different answers — like “How often do you exercise?” — don’t quite fit that mold. Regardless, this structure allowed the researchers to take individual kids’ responses to these questions and combine them into what amounts to a manliness score — how much their answers aligned with the typical male answer.
The score was established in adolescence — which is interesting because I’m sure some of this stuff may change over time — but notable because adolescence is where many gender roles develop.
Now we can fast-forward 30 years and see how these manliness scores link to various outcomes. The authors were interested in fairly common diseases: diabetes, hypertension, and hyperlipidemia.
Let’s start simply. Are males with higher gender expressivity in adolescence more or less likely to have these diseases in the future?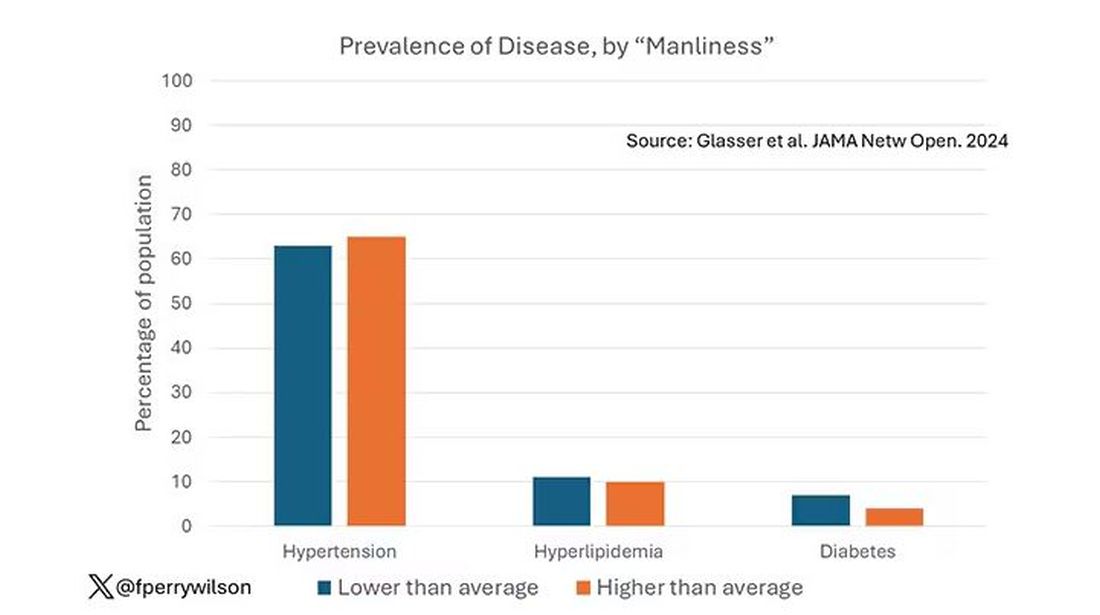
Not really. Those above the average in male gender expressivity had similar rates of hypertension and hyperlipidemia as those below the median. They were actually a bit less likely to have diabetes.
But that’s not what’s really interesting here.
I told you that there was no difference in the rate of hypertension among those with high vs low male gender expressivity. But there was a significant difference in their answer to the question “Do you have hypertension?” The same was seen for hyperlipidemia. In other words, those with higher manliness scores are less likely to admit (or perhaps know) that they have a particular disease.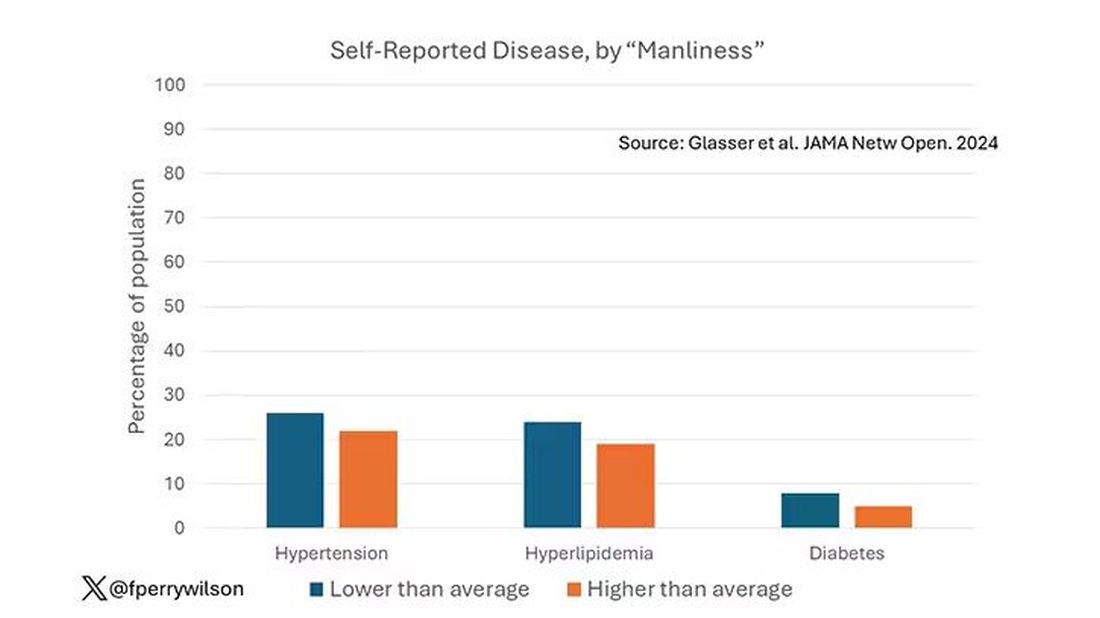
You can see the relationship across the manliness spectrum here in a series of adjusted models. The x-axis is the male gender expressivity score, and the y-axis is the percentage of people who report having the disease that we know they have based on the actual laboratory tests or vital sign measurements. As manliness increases, the self-report of a given disease decreases.
There are some important consequences of this systematic denial. Specifically, men with the diseases of interest who have higher male gender expressivity are less likely to get treatment. And, as we all know, the lack of treatment of something like hypertension puts people at risk for bad downstream outcomes.
Putting this all together, I’m not that surprised. Society trains boys from a young age to behave in certain ways: to hide emotions, to eschew vulnerability, to not complain when we are hurt. And those lessons can persist into later life. Whether the disease that strikes is hypertension or Pasteurella multocida from a slightly psychotic house cat, men are more likely to ignore it, to their detriment.
So, gents, be brave. Get your blood tests and check your blood pressure. If there’s something wrong, admit it, and fix it. After all, fixing problems — that’s a manly thing, right?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
When my normally adorable cat Biscuit bit my ankle in a playful stalking exercise gone wrong, I washed it with soap and some rubbing alcohol, slapped on a Band-Aid, and went about my day.
The next morning, when it was swollen, I told myself it was probably just a hematoma and went about my day.
The next day, when the swelling had increased and red lines started creeping up my leg, I called my doctor. Long story short, I ended up hospitalized for intravenous antibiotics.
This is all to say that, yes, I’m sort of an idiot, but also to introduce the idea that maybe I minimized my very obvious lymphangitis because I am a man.
This week, we have empirical evidence that men downplay their medical symptoms — and that manlier men downplay them even more.
I’m going to talk about a study that links manliness (or, scientifically speaking, “male gender expressivity”) to medical diagnoses that are based on hard evidence and medical diagnoses that are based on self-report. You see where this is going but I want to walk you through the methods here because they are fairly interesting.
This study used data from the US National Longitudinal Study of Adolescent to Adult Health. This study enrolled 20,000 adolescents who were in grades 7-12 in the 1994-1995 school year and has been following them ever since — about 30 years so far.
The authors wanted to link early gender roles to long-term outcomes, so they cut that 20,000 number down to the 4230 males in the group who had complete follow-up.
Now comes the first interesting question. How do you quantify the “male gender expressivity” of boys in 7th-12th grade? There was no survey item that asked them how masculine or manly they felt. What the authors did was look at the surveys that were administered and identify the questions on those surveys where boys and girls gave the most disparate answers. I have some examples here. 
Some of these questions make sense when it comes to gender expressivity: “How often do you cry?” for example, has a lot of validity for the social construct that is gender. But some questions where boys and girls gave very different answers — like “How often do you exercise?” — don’t quite fit that mold. Regardless, this structure allowed the researchers to take individual kids’ responses to these questions and combine them into what amounts to a manliness score — how much their answers aligned with the typical male answer.
The score was established in adolescence — which is interesting because I’m sure some of this stuff may change over time — but notable because adolescence is where many gender roles develop.
Now we can fast-forward 30 years and see how these manliness scores link to various outcomes. The authors were interested in fairly common diseases: diabetes, hypertension, and hyperlipidemia.
Let’s start simply. Are males with higher gender expressivity in adolescence more or less likely to have these diseases in the future?
Not really. Those above the average in male gender expressivity had similar rates of hypertension and hyperlipidemia as those below the median. They were actually a bit less likely to have diabetes.
But that’s not what’s really interesting here.
I told you that there was no difference in the rate of hypertension among those with high vs low male gender expressivity. But there was a significant difference in their answer to the question “Do you have hypertension?” The same was seen for hyperlipidemia. In other words, those with higher manliness scores are less likely to admit (or perhaps know) that they have a particular disease.
You can see the relationship across the manliness spectrum here in a series of adjusted models. The x-axis is the male gender expressivity score, and the y-axis is the percentage of people who report having the disease that we know they have based on the actual laboratory tests or vital sign measurements. As manliness increases, the self-report of a given disease decreases.
There are some important consequences of this systematic denial. Specifically, men with the diseases of interest who have higher male gender expressivity are less likely to get treatment. And, as we all know, the lack of treatment of something like hypertension puts people at risk for bad downstream outcomes.
Putting this all together, I’m not that surprised. Society trains boys from a young age to behave in certain ways: to hide emotions, to eschew vulnerability, to not complain when we are hurt. And those lessons can persist into later life. Whether the disease that strikes is hypertension or Pasteurella multocida from a slightly psychotic house cat, men are more likely to ignore it, to their detriment.
So, gents, be brave. Get your blood tests and check your blood pressure. If there’s something wrong, admit it, and fix it. After all, fixing problems — that’s a manly thing, right?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
When my normally adorable cat Biscuit bit my ankle in a playful stalking exercise gone wrong, I washed it with soap and some rubbing alcohol, slapped on a Band-Aid, and went about my day.
The next morning, when it was swollen, I told myself it was probably just a hematoma and went about my day.
The next day, when the swelling had increased and red lines started creeping up my leg, I called my doctor. Long story short, I ended up hospitalized for intravenous antibiotics.
This is all to say that, yes, I’m sort of an idiot, but also to introduce the idea that maybe I minimized my very obvious lymphangitis because I am a man.
This week, we have empirical evidence that men downplay their medical symptoms — and that manlier men downplay them even more.
I’m going to talk about a study that links manliness (or, scientifically speaking, “male gender expressivity”) to medical diagnoses that are based on hard evidence and medical diagnoses that are based on self-report. You see where this is going but I want to walk you through the methods here because they are fairly interesting.
This study used data from the US National Longitudinal Study of Adolescent to Adult Health. This study enrolled 20,000 adolescents who were in grades 7-12 in the 1994-1995 school year and has been following them ever since — about 30 years so far.
The authors wanted to link early gender roles to long-term outcomes, so they cut that 20,000 number down to the 4230 males in the group who had complete follow-up.
Now comes the first interesting question. How do you quantify the “male gender expressivity” of boys in 7th-12th grade? There was no survey item that asked them how masculine or manly they felt. What the authors did was look at the surveys that were administered and identify the questions on those surveys where boys and girls gave the most disparate answers. I have some examples here. 
Some of these questions make sense when it comes to gender expressivity: “How often do you cry?” for example, has a lot of validity for the social construct that is gender. But some questions where boys and girls gave very different answers — like “How often do you exercise?” — don’t quite fit that mold. Regardless, this structure allowed the researchers to take individual kids’ responses to these questions and combine them into what amounts to a manliness score — how much their answers aligned with the typical male answer.
The score was established in adolescence — which is interesting because I’m sure some of this stuff may change over time — but notable because adolescence is where many gender roles develop.
Now we can fast-forward 30 years and see how these manliness scores link to various outcomes. The authors were interested in fairly common diseases: diabetes, hypertension, and hyperlipidemia.
Let’s start simply. Are males with higher gender expressivity in adolescence more or less likely to have these diseases in the future?
Not really. Those above the average in male gender expressivity had similar rates of hypertension and hyperlipidemia as those below the median. They were actually a bit less likely to have diabetes.
But that’s not what’s really interesting here.
I told you that there was no difference in the rate of hypertension among those with high vs low male gender expressivity. But there was a significant difference in their answer to the question “Do you have hypertension?” The same was seen for hyperlipidemia. In other words, those with higher manliness scores are less likely to admit (or perhaps know) that they have a particular disease.
You can see the relationship across the manliness spectrum here in a series of adjusted models. The x-axis is the male gender expressivity score, and the y-axis is the percentage of people who report having the disease that we know they have based on the actual laboratory tests or vital sign measurements. As manliness increases, the self-report of a given disease decreases.
There are some important consequences of this systematic denial. Specifically, men with the diseases of interest who have higher male gender expressivity are less likely to get treatment. And, as we all know, the lack of treatment of something like hypertension puts people at risk for bad downstream outcomes.
Putting this all together, I’m not that surprised. Society trains boys from a young age to behave in certain ways: to hide emotions, to eschew vulnerability, to not complain when we are hurt. And those lessons can persist into later life. Whether the disease that strikes is hypertension or Pasteurella multocida from a slightly psychotic house cat, men are more likely to ignore it, to their detriment.
So, gents, be brave. Get your blood tests and check your blood pressure. If there’s something wrong, admit it, and fix it. After all, fixing problems — that’s a manly thing, right?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Cardiovascular Disease 2050: No, GLP-1s Won’t Save the Day
This transcript has been edited for clarity .
Robert A. Harrington, MD: I’m here in London at the European Society of Cardiology meetings, at theheart.org | Medscape Cardiology booth, using the meetings as an opportunity to meet with colleagues to talk about recent things that they’ve been writing about.
Today I’m joined by a good friend and colleague, Dr. Dhruv Kazi from Beth Israel Deaconess in Boston. Thanks for joining us.
Dhruv S. Kazi, MD, MS: Thank you for having me.
Harrington: Dr. Kazi is an associate professor of medicine at Harvard Medical School. He’s also the associate director of the Smith Center, which is an outcomes research center at the Beth Israel Deaconess. Thanks for joining us.
Kazi: Excited to be here.
Harrington: The topic I think you know that I want to discuss is a really important paper. There are two papers. They’re part of the American Heart Association’s 100th anniversary celebration, if you will. Many of the papers looked back at where science taken us.
With your coauthor, Karen Joynt Maddox, your papers are looking forward. They’re about the burden of cardiovascular disease in 2050. One paper really focused on what I would call the clinical and public health issues. Yours is focused on the economics. Is that a good description?
Kazi: Perfect.
Harrington: Tell us what you, Karen, and the other writers set out to do. What were you asked to do?
Kazi: As you know, the American Heart Association is entering its second century. Part of this was an exercise to say, where will the country be in 2050, which is a long enough time horizon for us to start planning for the future.
We looked back and said, if prior trends remain the same, where will we be in 2050, accounting for changes in demographics, changes in the composition of the population, and knowing that some of the cardiovascular risk factors are getting worse?
Harrington: For me, what was really striking is that, when I first saw the title and read “2050,” I thought, Oh, that’s a long way away. Then as I started reading it, I realized that this is not so far away.
Kazi: Absolutely.
Harrington: If we’re going to make a difference, it might take us 25 years.
Kazi: Especially if we set ourselves ambitious goals, we›re going to have to dig deep. Business-as-usual is not going to get us there.
Harrington: No. What I think has happened is we›ve spent so much time taking care of acute illness. Case fatality rates are fantastic. I was actually making the comment yesterday to a colleague that when I was an intern, the 30-day death rate from acute myocardial infarction was about 20%.
Kazi: Oh, wow.
Harrington: Now it’s 5%. That’s a big difference in a career.
Trends in the Wrong Direction
Kazi: There are fundamental trends. The decline in case fatalities is a really positive development, and I would hope that, going forward, that would continue. Those are risk-adjusted death rates and what is happening is that risk is going up. This is a function of the fact that the US population is aging; 2030 will be the first year that all the baby boomers will be over the age of 65.
By the mid-2030s, we’ll have more adults over the age of 65 than kids. That aging of the population is going to increase risk. The second is — and this is a positive development — we are a more diverse population, but the populations that are minoritized have higher cardiovascular risk, for a variety of reasons.
As the population of Asian Americans increases and doubles, in fact, as the population of Hispanic Americans doubles, we’re going to see an increase in risk related to cardiovascular disease. The third is that, over the past decade, there are some risk factors that are going in the wrong direction.
Harrington: Let’s talk about that because that’s humbling. I’m involved, as you know, with the American Heart Association, as are you. Despite all the work on Life’s Simple 7 and now Life’s Essential 8, we still have some issues.
Kazi: The big ones that come to mind are hypertension, diabetes, and obesity, all of which are trending in the wrong direction. Hypertension, we were gaining traction; and then over the past decade, we’ve slipped again. As you know, national blood pressure control rates have declined in many populations.
Harrington: Rather substantially.
Kazi: Substantially so, which has implications, in particular, for stroke rates in the future and stroke rates in young adults in the future. Obesity is a problem that we have very little control over. We’re already at 40% on average, which means that some populations are already in the 60% range.
Harrington: We also have obesity in kids — the burden, I’ll call it, of obesity. It’s not that you become obese in your thirties or your forties; you›re becoming obese as a teenager or even younger.
Kazi: Exactly. Since the 1990s, obesity in US adults has doubled, but obesity in US children has quadrupled. It’s starting from a lower base, but it’s very much an escalating problem.
Harrington: Diabetes is tightly linked to it but not totally explained.
Kazi: Exactly. The increase in diabetes is largely driven by obesity, but it›s probably also driven by changes in diet and lifestyle that don›t go through obesity.
Harrington: Yeah, it’s interesting. I think I have this figure correctly. It used to be rare that you saw a child with type 2 diabetes or what we call type 2 diabetes.
Kazi: Yeah.
Harrington: Now, the vast majority of kids with diabetes have type 2 diabetes.
Kazi: In the adolescents/young adults age group, most of it is type 2.
Harrington: Diabetes going up, obesity up, hypertension not well controlled, smoking combustible cigarettes way down.
Kazi: Yeah.
Harrington: Cholesterol levels. I was surprised. Cholesterol looked better. You said — because I was at a meeting where somebody asked you — that’s not explained by treatment.
Kazi: No, it’s not, at least going back to the ‘70s, but likely even sooner. I think that can only be attributed to substantial dietary changes. We are consuming less fat and less trans-fat. It’s possible that those collectively are improving our cholesterol levels, possibly at the expense of our glucose levels, because we basically substituted fats in our diet with more carbs at a population level.
Cigarettes and Vaping
Harrington: Some things certainly trend in the right direction but others in a really difficult direction. It’s going to lead to pretty large changes in risk for coronary disease, atrial fibrillation, and heart failure.
Kazi: I want to go back to the tobacco point. There are definitely marked declines in tobacco, still tightly related to income in the country. You see much higher prevalence of tobacco use in lower-income populations, but it’s unclear to me where it’s going in kids. We know that combustible tobacco use is going down but e-cigarettes went up. What that leads to over the next 30 years is unclear to me.
Harrington: That is a really important comment that’s worth sidebarring. The vaping use has been a terrible epidemic among our high schoolers. What is that going to lead to? Is it going to lead to the use of combustible cigarettes and we’re going to see that go back up? It remains to be seen.
Kazi: Yes, it remains to be seen. Going back to your point about this change in risk factors and this change in demographics, both aging and becoming a more diverse population means that we have large increases in some healthcare conditions.
Coronary heart disease goes up some, there›s a big jump in stroke — nearly a doubling in stroke — which is related to hypertension, obesity, an aging population, and a more diverse population. There are changes in stroke in the young, and atrial fibrillation related to, again, hypertension. We’re seeing these projections, and with them come these pretty large projections in changes in healthcare spending.
Healthcare Spending Not Sustainable
Harrington: Big. I mean, it’s not sustainable. Give the audience the number — it’s pretty frightening.
Kazi: We’re talking about a quadrupling of healthcare costs related to cardiovascular disease over 25 years. We’ve gotten used to the narrative that healthcare in the US is expensive and drugs are expensive, but this is an enormous problem — an unsustainable problem, like you called it.
It’s a doubling as a proportion of the economy. I was looking this up this morning. If the US healthcare economy were its own economy, it would be the fourth largest economy in the world.
Harrington: Healthcare as it is today, is it 21% of our economy?
Kazi: It’s 17% now. If it were its own economy, it would be the fourth largest in the world. We are spending more on healthcare than all but two other countries’ total economies. It’s kind of crazy.
Harrington: We’re talking about a quadrupling.
Kazi: Within that, the cardiovascular piece is a big piece, and we›re talking about a quadrupling.
Harrington: That’s both direct and indirect costs.
Kazi: The quadrupling of costs is just the direct costs. Indirect costs, for the listeners, refer to costs unrelated to healthcare but changes in productivity, either because people are disabled and unable to participate fully in the workforce or they die early.
The productivity costs are also increased substantially as a result. If you look at both healthcare and productivity, that goes up threefold. These are very large changes.
Harrington: Let’s now get to what we can do about it. I made the comment to you when I first read the papers that I was very depressed. Then, after I went through my Kübler-Ross stages of depression, death, and dying, I came to acceptance.
What are we going to do about it? This is a focus on policy, but also a focus on how we deliver healthcare, how we think about healthcare, and how we develop drugs and devices.
The drug question is going to be the one the audience is thinking about. They say, well, what about GLP-1 agonists? Aren’t those going to save the day?
Kazi: Yes and no. I’ll say that, early in my career, I used to be very attracted to simple solutions to complex problems. I’ve come to realize that simple solutions are elegant, attractive, and wrong. We›re dealing with a very complex issue and I think we’re going to need a multipronged approach.
The way I think about it is that there was a group of people who are at very high risk today. How do we help those individuals? Then how do we help the future generation so that they’re not dealing with the projections that we’re talking about.
My colleague, Karen Joynt Maddox, who led one of the papers, as you mentioned, has an elegant line in the paper where she says projections are not destiny. These are things we can change.
Harrington: If nothing changes, this is what it’s going to look like.
Kazi: This is where we’re headed.
Harrington: We can change. We’ve got some time to change, but we don’t have forever.
Kazi: Yes, exactly. We picked the 25-year timeline instead of a “let’s plan for the next century” timeline because we want something concrete and actionable. It’s close enough to be meaningful but far enough to give us the runway we need to act.
Harrington: Give me two things from the policy perspective, because it’s mostly policy.
Kazi: There are policy and clinical interventions. From the policy perspective, if I had to list two things, one is expansion of access to care. As we talk about this big increase in the burden of disease and risk factors, if you have a large proportion of your population that has hypertension or diabetes, you’re going to have to expand access to care to ensure that people get treated so they can get access to this care before they develop the complications that we worry about, like stroke and heart disease, that are very expensive to treat downstream.
The second, more broadly related to access to care, is the access to medications that are effective. You bring up GLP-1s. I think we need a real strategy for how we can give people access to GLP-1s at a price that is affordable to individuals but also affordable to the health system, and to help them stay on the drugs.
GLP-1s are transformative in what they do for weight loss and for diabetes, but more than 50% of people who start one are off it at 12 months. There’s something fundamentally wrong about how we’re delivering GLP-1s today. It’s not just about the cost of the drugs but the support system people need to stay on.
Harrington: I’ve made the comment, in many forms now, that we know the drugs work. We have to figure out how to use them.
Kazi: Exactly, yes.
Harrington: Using them includes chronicity. This is a chronic condition. Some people can come off the drugs, but many can’t. We’re going to have to figure this out, and maybe the newer generations of drugs will help us address what people call the off-ramping. How are we going to do that? I think you’re spot-on. Those are critically important questions.
Kazi: As we looked at this modeling, I’ll tell you — I had a come-to-Jesus moment where I was like, there is no way to fix cardiovascular disease in the US without going through obesity and diabetes. We have to address obesity in the US. We can’t just treat our way out of it. Obesity is fundamentally a food problem and we’ve got to engage again with food policy in a meaningful way.
Harrington: As you know, with the American Heart Association, we›re doing a large amount of work now on food as medicine and food is medicine. We are trying to figure out what the levers are that we can pull to actually help people eat healthier diets.
Kazi: Yes. Rather than framing it as an individual choice that people are eating poorly, it’s, how do we make healthy diets the default in the environment?
Harrington: This is where you get to the children as well.
Kazi: Exactly.
Harrington: I could talk about this all day. I’ve had the benefit of reading the papers now a few times and talking to you on several occasions. Thank you for joining us.
Kazi: Thank you.
Dr. Harrington, Stephen and Suzanne Weiss Dean, Weill Cornell Medicine; Provost for Medical Affairs, Cornell University, New York, NY, disclosed ties with Baim Institute (DSMB); CSL (RCT Executive Committee); Janssen (RCT Char), NHLBI (RCT Executive Committee, DSMB Chair); PCORI (RCT Co-Chair); DCRI, Atropos Health; Bitterroot Bio; Bristol Myers Squibb; BridgeBio; Element Science; Edwards Lifesciences; Foresite Labs; Medscape/WebMD Board of Directors for: American Heart Association; College of the Holy Cross; and Cytokinetics. Dr. Kazi, Associate Director, Smith Center for Outcomes Research, Associate Professor, Department of Medicine (Cardiology), Harvard Medical School, Director, Department of Cardiac Critical Care Unit, Beth Israel Deaconess Medical Center, Boston, Massachusetts, has disclosed receiving a research grant from Boston Scientific (grant to examine the economics of stroke prevention).
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity .
Robert A. Harrington, MD: I’m here in London at the European Society of Cardiology meetings, at theheart.org | Medscape Cardiology booth, using the meetings as an opportunity to meet with colleagues to talk about recent things that they’ve been writing about.
Today I’m joined by a good friend and colleague, Dr. Dhruv Kazi from Beth Israel Deaconess in Boston. Thanks for joining us.
Dhruv S. Kazi, MD, MS: Thank you for having me.
Harrington: Dr. Kazi is an associate professor of medicine at Harvard Medical School. He’s also the associate director of the Smith Center, which is an outcomes research center at the Beth Israel Deaconess. Thanks for joining us.
Kazi: Excited to be here.
Harrington: The topic I think you know that I want to discuss is a really important paper. There are two papers. They’re part of the American Heart Association’s 100th anniversary celebration, if you will. Many of the papers looked back at where science taken us.
With your coauthor, Karen Joynt Maddox, your papers are looking forward. They’re about the burden of cardiovascular disease in 2050. One paper really focused on what I would call the clinical and public health issues. Yours is focused on the economics. Is that a good description?
Kazi: Perfect.
Harrington: Tell us what you, Karen, and the other writers set out to do. What were you asked to do?
Kazi: As you know, the American Heart Association is entering its second century. Part of this was an exercise to say, where will the country be in 2050, which is a long enough time horizon for us to start planning for the future.
We looked back and said, if prior trends remain the same, where will we be in 2050, accounting for changes in demographics, changes in the composition of the population, and knowing that some of the cardiovascular risk factors are getting worse?
Harrington: For me, what was really striking is that, when I first saw the title and read “2050,” I thought, Oh, that’s a long way away. Then as I started reading it, I realized that this is not so far away.
Kazi: Absolutely.
Harrington: If we’re going to make a difference, it might take us 25 years.
Kazi: Especially if we set ourselves ambitious goals, we›re going to have to dig deep. Business-as-usual is not going to get us there.
Harrington: No. What I think has happened is we›ve spent so much time taking care of acute illness. Case fatality rates are fantastic. I was actually making the comment yesterday to a colleague that when I was an intern, the 30-day death rate from acute myocardial infarction was about 20%.
Kazi: Oh, wow.
Harrington: Now it’s 5%. That’s a big difference in a career.
Trends in the Wrong Direction
Kazi: There are fundamental trends. The decline in case fatalities is a really positive development, and I would hope that, going forward, that would continue. Those are risk-adjusted death rates and what is happening is that risk is going up. This is a function of the fact that the US population is aging; 2030 will be the first year that all the baby boomers will be over the age of 65.
By the mid-2030s, we’ll have more adults over the age of 65 than kids. That aging of the population is going to increase risk. The second is — and this is a positive development — we are a more diverse population, but the populations that are minoritized have higher cardiovascular risk, for a variety of reasons.
As the population of Asian Americans increases and doubles, in fact, as the population of Hispanic Americans doubles, we’re going to see an increase in risk related to cardiovascular disease. The third is that, over the past decade, there are some risk factors that are going in the wrong direction.
Harrington: Let’s talk about that because that’s humbling. I’m involved, as you know, with the American Heart Association, as are you. Despite all the work on Life’s Simple 7 and now Life’s Essential 8, we still have some issues.
Kazi: The big ones that come to mind are hypertension, diabetes, and obesity, all of which are trending in the wrong direction. Hypertension, we were gaining traction; and then over the past decade, we’ve slipped again. As you know, national blood pressure control rates have declined in many populations.
Harrington: Rather substantially.
Kazi: Substantially so, which has implications, in particular, for stroke rates in the future and stroke rates in young adults in the future. Obesity is a problem that we have very little control over. We’re already at 40% on average, which means that some populations are already in the 60% range.
Harrington: We also have obesity in kids — the burden, I’ll call it, of obesity. It’s not that you become obese in your thirties or your forties; you›re becoming obese as a teenager or even younger.
Kazi: Exactly. Since the 1990s, obesity in US adults has doubled, but obesity in US children has quadrupled. It’s starting from a lower base, but it’s very much an escalating problem.
Harrington: Diabetes is tightly linked to it but not totally explained.
Kazi: Exactly. The increase in diabetes is largely driven by obesity, but it›s probably also driven by changes in diet and lifestyle that don›t go through obesity.
Harrington: Yeah, it’s interesting. I think I have this figure correctly. It used to be rare that you saw a child with type 2 diabetes or what we call type 2 diabetes.
Kazi: Yeah.
Harrington: Now, the vast majority of kids with diabetes have type 2 diabetes.
Kazi: In the adolescents/young adults age group, most of it is type 2.
Harrington: Diabetes going up, obesity up, hypertension not well controlled, smoking combustible cigarettes way down.
Kazi: Yeah.
Harrington: Cholesterol levels. I was surprised. Cholesterol looked better. You said — because I was at a meeting where somebody asked you — that’s not explained by treatment.
Kazi: No, it’s not, at least going back to the ‘70s, but likely even sooner. I think that can only be attributed to substantial dietary changes. We are consuming less fat and less trans-fat. It’s possible that those collectively are improving our cholesterol levels, possibly at the expense of our glucose levels, because we basically substituted fats in our diet with more carbs at a population level.
Cigarettes and Vaping
Harrington: Some things certainly trend in the right direction but others in a really difficult direction. It’s going to lead to pretty large changes in risk for coronary disease, atrial fibrillation, and heart failure.
Kazi: I want to go back to the tobacco point. There are definitely marked declines in tobacco, still tightly related to income in the country. You see much higher prevalence of tobacco use in lower-income populations, but it’s unclear to me where it’s going in kids. We know that combustible tobacco use is going down but e-cigarettes went up. What that leads to over the next 30 years is unclear to me.
Harrington: That is a really important comment that’s worth sidebarring. The vaping use has been a terrible epidemic among our high schoolers. What is that going to lead to? Is it going to lead to the use of combustible cigarettes and we’re going to see that go back up? It remains to be seen.
Kazi: Yes, it remains to be seen. Going back to your point about this change in risk factors and this change in demographics, both aging and becoming a more diverse population means that we have large increases in some healthcare conditions.
Coronary heart disease goes up some, there›s a big jump in stroke — nearly a doubling in stroke — which is related to hypertension, obesity, an aging population, and a more diverse population. There are changes in stroke in the young, and atrial fibrillation related to, again, hypertension. We’re seeing these projections, and with them come these pretty large projections in changes in healthcare spending.
Healthcare Spending Not Sustainable
Harrington: Big. I mean, it’s not sustainable. Give the audience the number — it’s pretty frightening.
Kazi: We’re talking about a quadrupling of healthcare costs related to cardiovascular disease over 25 years. We’ve gotten used to the narrative that healthcare in the US is expensive and drugs are expensive, but this is an enormous problem — an unsustainable problem, like you called it.
It’s a doubling as a proportion of the economy. I was looking this up this morning. If the US healthcare economy were its own economy, it would be the fourth largest economy in the world.
Harrington: Healthcare as it is today, is it 21% of our economy?
Kazi: It’s 17% now. If it were its own economy, it would be the fourth largest in the world. We are spending more on healthcare than all but two other countries’ total economies. It’s kind of crazy.
Harrington: We’re talking about a quadrupling.
Kazi: Within that, the cardiovascular piece is a big piece, and we›re talking about a quadrupling.
Harrington: That’s both direct and indirect costs.
Kazi: The quadrupling of costs is just the direct costs. Indirect costs, for the listeners, refer to costs unrelated to healthcare but changes in productivity, either because people are disabled and unable to participate fully in the workforce or they die early.
The productivity costs are also increased substantially as a result. If you look at both healthcare and productivity, that goes up threefold. These are very large changes.
Harrington: Let’s now get to what we can do about it. I made the comment to you when I first read the papers that I was very depressed. Then, after I went through my Kübler-Ross stages of depression, death, and dying, I came to acceptance.
What are we going to do about it? This is a focus on policy, but also a focus on how we deliver healthcare, how we think about healthcare, and how we develop drugs and devices.
The drug question is going to be the one the audience is thinking about. They say, well, what about GLP-1 agonists? Aren’t those going to save the day?
Kazi: Yes and no. I’ll say that, early in my career, I used to be very attracted to simple solutions to complex problems. I’ve come to realize that simple solutions are elegant, attractive, and wrong. We›re dealing with a very complex issue and I think we’re going to need a multipronged approach.
The way I think about it is that there was a group of people who are at very high risk today. How do we help those individuals? Then how do we help the future generation so that they’re not dealing with the projections that we’re talking about.
My colleague, Karen Joynt Maddox, who led one of the papers, as you mentioned, has an elegant line in the paper where she says projections are not destiny. These are things we can change.
Harrington: If nothing changes, this is what it’s going to look like.
Kazi: This is where we’re headed.
Harrington: We can change. We’ve got some time to change, but we don’t have forever.
Kazi: Yes, exactly. We picked the 25-year timeline instead of a “let’s plan for the next century” timeline because we want something concrete and actionable. It’s close enough to be meaningful but far enough to give us the runway we need to act.
Harrington: Give me two things from the policy perspective, because it’s mostly policy.
Kazi: There are policy and clinical interventions. From the policy perspective, if I had to list two things, one is expansion of access to care. As we talk about this big increase in the burden of disease and risk factors, if you have a large proportion of your population that has hypertension or diabetes, you’re going to have to expand access to care to ensure that people get treated so they can get access to this care before they develop the complications that we worry about, like stroke and heart disease, that are very expensive to treat downstream.
The second, more broadly related to access to care, is the access to medications that are effective. You bring up GLP-1s. I think we need a real strategy for how we can give people access to GLP-1s at a price that is affordable to individuals but also affordable to the health system, and to help them stay on the drugs.
GLP-1s are transformative in what they do for weight loss and for diabetes, but more than 50% of people who start one are off it at 12 months. There’s something fundamentally wrong about how we’re delivering GLP-1s today. It’s not just about the cost of the drugs but the support system people need to stay on.
Harrington: I’ve made the comment, in many forms now, that we know the drugs work. We have to figure out how to use them.
Kazi: Exactly, yes.
Harrington: Using them includes chronicity. This is a chronic condition. Some people can come off the drugs, but many can’t. We’re going to have to figure this out, and maybe the newer generations of drugs will help us address what people call the off-ramping. How are we going to do that? I think you’re spot-on. Those are critically important questions.
Kazi: As we looked at this modeling, I’ll tell you — I had a come-to-Jesus moment where I was like, there is no way to fix cardiovascular disease in the US without going through obesity and diabetes. We have to address obesity in the US. We can’t just treat our way out of it. Obesity is fundamentally a food problem and we’ve got to engage again with food policy in a meaningful way.
Harrington: As you know, with the American Heart Association, we›re doing a large amount of work now on food as medicine and food is medicine. We are trying to figure out what the levers are that we can pull to actually help people eat healthier diets.
Kazi: Yes. Rather than framing it as an individual choice that people are eating poorly, it’s, how do we make healthy diets the default in the environment?
Harrington: This is where you get to the children as well.
Kazi: Exactly.
Harrington: I could talk about this all day. I’ve had the benefit of reading the papers now a few times and talking to you on several occasions. Thank you for joining us.
Kazi: Thank you.
Dr. Harrington, Stephen and Suzanne Weiss Dean, Weill Cornell Medicine; Provost for Medical Affairs, Cornell University, New York, NY, disclosed ties with Baim Institute (DSMB); CSL (RCT Executive Committee); Janssen (RCT Char), NHLBI (RCT Executive Committee, DSMB Chair); PCORI (RCT Co-Chair); DCRI, Atropos Health; Bitterroot Bio; Bristol Myers Squibb; BridgeBio; Element Science; Edwards Lifesciences; Foresite Labs; Medscape/WebMD Board of Directors for: American Heart Association; College of the Holy Cross; and Cytokinetics. Dr. Kazi, Associate Director, Smith Center for Outcomes Research, Associate Professor, Department of Medicine (Cardiology), Harvard Medical School, Director, Department of Cardiac Critical Care Unit, Beth Israel Deaconess Medical Center, Boston, Massachusetts, has disclosed receiving a research grant from Boston Scientific (grant to examine the economics of stroke prevention).
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity .
Robert A. Harrington, MD: I’m here in London at the European Society of Cardiology meetings, at theheart.org | Medscape Cardiology booth, using the meetings as an opportunity to meet with colleagues to talk about recent things that they’ve been writing about.
Today I’m joined by a good friend and colleague, Dr. Dhruv Kazi from Beth Israel Deaconess in Boston. Thanks for joining us.
Dhruv S. Kazi, MD, MS: Thank you for having me.
Harrington: Dr. Kazi is an associate professor of medicine at Harvard Medical School. He’s also the associate director of the Smith Center, which is an outcomes research center at the Beth Israel Deaconess. Thanks for joining us.
Kazi: Excited to be here.
Harrington: The topic I think you know that I want to discuss is a really important paper. There are two papers. They’re part of the American Heart Association’s 100th anniversary celebration, if you will. Many of the papers looked back at where science taken us.
With your coauthor, Karen Joynt Maddox, your papers are looking forward. They’re about the burden of cardiovascular disease in 2050. One paper really focused on what I would call the clinical and public health issues. Yours is focused on the economics. Is that a good description?
Kazi: Perfect.
Harrington: Tell us what you, Karen, and the other writers set out to do. What were you asked to do?
Kazi: As you know, the American Heart Association is entering its second century. Part of this was an exercise to say, where will the country be in 2050, which is a long enough time horizon for us to start planning for the future.
We looked back and said, if prior trends remain the same, where will we be in 2050, accounting for changes in demographics, changes in the composition of the population, and knowing that some of the cardiovascular risk factors are getting worse?
Harrington: For me, what was really striking is that, when I first saw the title and read “2050,” I thought, Oh, that’s a long way away. Then as I started reading it, I realized that this is not so far away.
Kazi: Absolutely.
Harrington: If we’re going to make a difference, it might take us 25 years.
Kazi: Especially if we set ourselves ambitious goals, we›re going to have to dig deep. Business-as-usual is not going to get us there.
Harrington: No. What I think has happened is we›ve spent so much time taking care of acute illness. Case fatality rates are fantastic. I was actually making the comment yesterday to a colleague that when I was an intern, the 30-day death rate from acute myocardial infarction was about 20%.
Kazi: Oh, wow.
Harrington: Now it’s 5%. That’s a big difference in a career.
Trends in the Wrong Direction
Kazi: There are fundamental trends. The decline in case fatalities is a really positive development, and I would hope that, going forward, that would continue. Those are risk-adjusted death rates and what is happening is that risk is going up. This is a function of the fact that the US population is aging; 2030 will be the first year that all the baby boomers will be over the age of 65.
By the mid-2030s, we’ll have more adults over the age of 65 than kids. That aging of the population is going to increase risk. The second is — and this is a positive development — we are a more diverse population, but the populations that are minoritized have higher cardiovascular risk, for a variety of reasons.
As the population of Asian Americans increases and doubles, in fact, as the population of Hispanic Americans doubles, we’re going to see an increase in risk related to cardiovascular disease. The third is that, over the past decade, there are some risk factors that are going in the wrong direction.
Harrington: Let’s talk about that because that’s humbling. I’m involved, as you know, with the American Heart Association, as are you. Despite all the work on Life’s Simple 7 and now Life’s Essential 8, we still have some issues.
Kazi: The big ones that come to mind are hypertension, diabetes, and obesity, all of which are trending in the wrong direction. Hypertension, we were gaining traction; and then over the past decade, we’ve slipped again. As you know, national blood pressure control rates have declined in many populations.
Harrington: Rather substantially.
Kazi: Substantially so, which has implications, in particular, for stroke rates in the future and stroke rates in young adults in the future. Obesity is a problem that we have very little control over. We’re already at 40% on average, which means that some populations are already in the 60% range.
Harrington: We also have obesity in kids — the burden, I’ll call it, of obesity. It’s not that you become obese in your thirties or your forties; you›re becoming obese as a teenager or even younger.
Kazi: Exactly. Since the 1990s, obesity in US adults has doubled, but obesity in US children has quadrupled. It’s starting from a lower base, but it’s very much an escalating problem.
Harrington: Diabetes is tightly linked to it but not totally explained.
Kazi: Exactly. The increase in diabetes is largely driven by obesity, but it›s probably also driven by changes in diet and lifestyle that don›t go through obesity.
Harrington: Yeah, it’s interesting. I think I have this figure correctly. It used to be rare that you saw a child with type 2 diabetes or what we call type 2 diabetes.
Kazi: Yeah.
Harrington: Now, the vast majority of kids with diabetes have type 2 diabetes.
Kazi: In the adolescents/young adults age group, most of it is type 2.
Harrington: Diabetes going up, obesity up, hypertension not well controlled, smoking combustible cigarettes way down.
Kazi: Yeah.
Harrington: Cholesterol levels. I was surprised. Cholesterol looked better. You said — because I was at a meeting where somebody asked you — that’s not explained by treatment.
Kazi: No, it’s not, at least going back to the ‘70s, but likely even sooner. I think that can only be attributed to substantial dietary changes. We are consuming less fat and less trans-fat. It’s possible that those collectively are improving our cholesterol levels, possibly at the expense of our glucose levels, because we basically substituted fats in our diet with more carbs at a population level.
Cigarettes and Vaping
Harrington: Some things certainly trend in the right direction but others in a really difficult direction. It’s going to lead to pretty large changes in risk for coronary disease, atrial fibrillation, and heart failure.
Kazi: I want to go back to the tobacco point. There are definitely marked declines in tobacco, still tightly related to income in the country. You see much higher prevalence of tobacco use in lower-income populations, but it’s unclear to me where it’s going in kids. We know that combustible tobacco use is going down but e-cigarettes went up. What that leads to over the next 30 years is unclear to me.
Harrington: That is a really important comment that’s worth sidebarring. The vaping use has been a terrible epidemic among our high schoolers. What is that going to lead to? Is it going to lead to the use of combustible cigarettes and we’re going to see that go back up? It remains to be seen.
Kazi: Yes, it remains to be seen. Going back to your point about this change in risk factors and this change in demographics, both aging and becoming a more diverse population means that we have large increases in some healthcare conditions.
Coronary heart disease goes up some, there›s a big jump in stroke — nearly a doubling in stroke — which is related to hypertension, obesity, an aging population, and a more diverse population. There are changes in stroke in the young, and atrial fibrillation related to, again, hypertension. We’re seeing these projections, and with them come these pretty large projections in changes in healthcare spending.
Healthcare Spending Not Sustainable
Harrington: Big. I mean, it’s not sustainable. Give the audience the number — it’s pretty frightening.
Kazi: We’re talking about a quadrupling of healthcare costs related to cardiovascular disease over 25 years. We’ve gotten used to the narrative that healthcare in the US is expensive and drugs are expensive, but this is an enormous problem — an unsustainable problem, like you called it.
It’s a doubling as a proportion of the economy. I was looking this up this morning. If the US healthcare economy were its own economy, it would be the fourth largest economy in the world.
Harrington: Healthcare as it is today, is it 21% of our economy?
Kazi: It’s 17% now. If it were its own economy, it would be the fourth largest in the world. We are spending more on healthcare than all but two other countries’ total economies. It’s kind of crazy.
Harrington: We’re talking about a quadrupling.
Kazi: Within that, the cardiovascular piece is a big piece, and we›re talking about a quadrupling.
Harrington: That’s both direct and indirect costs.
Kazi: The quadrupling of costs is just the direct costs. Indirect costs, for the listeners, refer to costs unrelated to healthcare but changes in productivity, either because people are disabled and unable to participate fully in the workforce or they die early.
The productivity costs are also increased substantially as a result. If you look at both healthcare and productivity, that goes up threefold. These are very large changes.
Harrington: Let’s now get to what we can do about it. I made the comment to you when I first read the papers that I was very depressed. Then, after I went through my Kübler-Ross stages of depression, death, and dying, I came to acceptance.
What are we going to do about it? This is a focus on policy, but also a focus on how we deliver healthcare, how we think about healthcare, and how we develop drugs and devices.
The drug question is going to be the one the audience is thinking about. They say, well, what about GLP-1 agonists? Aren’t those going to save the day?
Kazi: Yes and no. I’ll say that, early in my career, I used to be very attracted to simple solutions to complex problems. I’ve come to realize that simple solutions are elegant, attractive, and wrong. We›re dealing with a very complex issue and I think we’re going to need a multipronged approach.
The way I think about it is that there was a group of people who are at very high risk today. How do we help those individuals? Then how do we help the future generation so that they’re not dealing with the projections that we’re talking about.
My colleague, Karen Joynt Maddox, who led one of the papers, as you mentioned, has an elegant line in the paper where she says projections are not destiny. These are things we can change.
Harrington: If nothing changes, this is what it’s going to look like.
Kazi: This is where we’re headed.
Harrington: We can change. We’ve got some time to change, but we don’t have forever.
Kazi: Yes, exactly. We picked the 25-year timeline instead of a “let’s plan for the next century” timeline because we want something concrete and actionable. It’s close enough to be meaningful but far enough to give us the runway we need to act.
Harrington: Give me two things from the policy perspective, because it’s mostly policy.
Kazi: There are policy and clinical interventions. From the policy perspective, if I had to list two things, one is expansion of access to care. As we talk about this big increase in the burden of disease and risk factors, if you have a large proportion of your population that has hypertension or diabetes, you’re going to have to expand access to care to ensure that people get treated so they can get access to this care before they develop the complications that we worry about, like stroke and heart disease, that are very expensive to treat downstream.
The second, more broadly related to access to care, is the access to medications that are effective. You bring up GLP-1s. I think we need a real strategy for how we can give people access to GLP-1s at a price that is affordable to individuals but also affordable to the health system, and to help them stay on the drugs.
GLP-1s are transformative in what they do for weight loss and for diabetes, but more than 50% of people who start one are off it at 12 months. There’s something fundamentally wrong about how we’re delivering GLP-1s today. It’s not just about the cost of the drugs but the support system people need to stay on.
Harrington: I’ve made the comment, in many forms now, that we know the drugs work. We have to figure out how to use them.
Kazi: Exactly, yes.
Harrington: Using them includes chronicity. This is a chronic condition. Some people can come off the drugs, but many can’t. We’re going to have to figure this out, and maybe the newer generations of drugs will help us address what people call the off-ramping. How are we going to do that? I think you’re spot-on. Those are critically important questions.
Kazi: As we looked at this modeling, I’ll tell you — I had a come-to-Jesus moment where I was like, there is no way to fix cardiovascular disease in the US without going through obesity and diabetes. We have to address obesity in the US. We can’t just treat our way out of it. Obesity is fundamentally a food problem and we’ve got to engage again with food policy in a meaningful way.
Harrington: As you know, with the American Heart Association, we›re doing a large amount of work now on food as medicine and food is medicine. We are trying to figure out what the levers are that we can pull to actually help people eat healthier diets.
Kazi: Yes. Rather than framing it as an individual choice that people are eating poorly, it’s, how do we make healthy diets the default in the environment?
Harrington: This is where you get to the children as well.
Kazi: Exactly.
Harrington: I could talk about this all day. I’ve had the benefit of reading the papers now a few times and talking to you on several occasions. Thank you for joining us.
Kazi: Thank you.
Dr. Harrington, Stephen and Suzanne Weiss Dean, Weill Cornell Medicine; Provost for Medical Affairs, Cornell University, New York, NY, disclosed ties with Baim Institute (DSMB); CSL (RCT Executive Committee); Janssen (RCT Char), NHLBI (RCT Executive Committee, DSMB Chair); PCORI (RCT Co-Chair); DCRI, Atropos Health; Bitterroot Bio; Bristol Myers Squibb; BridgeBio; Element Science; Edwards Lifesciences; Foresite Labs; Medscape/WebMD Board of Directors for: American Heart Association; College of the Holy Cross; and Cytokinetics. Dr. Kazi, Associate Director, Smith Center for Outcomes Research, Associate Professor, Department of Medicine (Cardiology), Harvard Medical School, Director, Department of Cardiac Critical Care Unit, Beth Israel Deaconess Medical Center, Boston, Massachusetts, has disclosed receiving a research grant from Boston Scientific (grant to examine the economics of stroke prevention).
A version of this article appeared on Medscape.com.
Six Tips for Media Interviews
As a physician, you might be contacted by the media to provide your professional opinion and advice. Or you might be looking for media interview opportunities to market your practice or side project. And if you do research, media interviews can be an effective way to spread the word. It’s important to prepare for a media interview so that you achieve the outcome you are looking for.
Keep your message simple. When you are a subject expert, you might think that the basics are obvious or even boring, and that the nuances are more important. However, most of the audience is looking for big-picture information that they can apply to their lives. Consider a few key takeaways, keeping in mind that your interview is likely to be edited to short sound bites or a few quotes. It may help to jot down notes so that you cover the fundamentals clearly. You could even write and rehearse a script beforehand. If there is something complicated or subtle that you want to convey, you can preface it by saying, “This is confusing but very important …” to let the audience know to give extra consideration to what you are about to say.
Avoid extremes and hyperbole. Sometimes, exaggerated statements make their way into medical discussions. Statements such as “it doesn’t matter how many calories you consume — it’s all about the quality” are common oversimplifications. But you might be upset to see your name next to a comment like this because it is not actually correct. Check the phrasing of your key takeaways to avoid being stuck defending or explaining an inaccurate statement when your patients ask you about it later.
Ask the interviewers what they are looking for. Many medical topics have some controversial element, so it is good to know what you’re getting into. Find out the purpose of the article or interview before you decide whether it is right for you. It could be about another doctor in town who is being sued; if you don’t want to be associated with that story, it might be best to decline the interview.
Explain your goals. You might accept or pursue an interview to raise awareness about an underrecognized condition. You might want the public to identify and get help for early symptoms, or you might want to create empathy for people coping with a disease you treat. Consider why you are participating in an interview, and communicate that to the interviewer to ensure that your objective can be part of the final product.
Know whom you’re dealing with. It is good to learn about the publication/media channel before you agree to participate. It may have a political bias, or perhaps the interview is intended to promote a specific product. If you agree with and support their purposes, then you may be happy to lend your opinion. But learning about the “voice” of the publication in advance allows you to make an informed decision about whether you want to be identified with a particular political ideology or product endorsement.
Ask to see your quotes before publication. It’s good to have the opportunity to make corrections in case you are accidentally misquoted or misunderstood. It is best to ask to see quotes before you agree to the interview. Some reporters may agree to (or even prefer) a written question-and-answer format so that they can directly quote your responses without rephrasing your words. You could suggest this, especially if you are too busy for a call or live meeting.
As a physician, your insights and advice can be highly beneficial to others. You can also use media interviews to propel your career forward. Doing your homework can ensure that you will be pleased with the final product and how your words were used.
Dr. Moawad, Clinical Assistant Professor, Department of Medical Education, Case Western Reserve University School of Medicine, Cleveland, Ohio, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
As a physician, you might be contacted by the media to provide your professional opinion and advice. Or you might be looking for media interview opportunities to market your practice or side project. And if you do research, media interviews can be an effective way to spread the word. It’s important to prepare for a media interview so that you achieve the outcome you are looking for.
Keep your message simple. When you are a subject expert, you might think that the basics are obvious or even boring, and that the nuances are more important. However, most of the audience is looking for big-picture information that they can apply to their lives. Consider a few key takeaways, keeping in mind that your interview is likely to be edited to short sound bites or a few quotes. It may help to jot down notes so that you cover the fundamentals clearly. You could even write and rehearse a script beforehand. If there is something complicated or subtle that you want to convey, you can preface it by saying, “This is confusing but very important …” to let the audience know to give extra consideration to what you are about to say.
Avoid extremes and hyperbole. Sometimes, exaggerated statements make their way into medical discussions. Statements such as “it doesn’t matter how many calories you consume — it’s all about the quality” are common oversimplifications. But you might be upset to see your name next to a comment like this because it is not actually correct. Check the phrasing of your key takeaways to avoid being stuck defending or explaining an inaccurate statement when your patients ask you about it later.
Ask the interviewers what they are looking for. Many medical topics have some controversial element, so it is good to know what you’re getting into. Find out the purpose of the article or interview before you decide whether it is right for you. It could be about another doctor in town who is being sued; if you don’t want to be associated with that story, it might be best to decline the interview.
Explain your goals. You might accept or pursue an interview to raise awareness about an underrecognized condition. You might want the public to identify and get help for early symptoms, or you might want to create empathy for people coping with a disease you treat. Consider why you are participating in an interview, and communicate that to the interviewer to ensure that your objective can be part of the final product.
Know whom you’re dealing with. It is good to learn about the publication/media channel before you agree to participate. It may have a political bias, or perhaps the interview is intended to promote a specific product. If you agree with and support their purposes, then you may be happy to lend your opinion. But learning about the “voice” of the publication in advance allows you to make an informed decision about whether you want to be identified with a particular political ideology or product endorsement.
Ask to see your quotes before publication. It’s good to have the opportunity to make corrections in case you are accidentally misquoted or misunderstood. It is best to ask to see quotes before you agree to the interview. Some reporters may agree to (or even prefer) a written question-and-answer format so that they can directly quote your responses without rephrasing your words. You could suggest this, especially if you are too busy for a call or live meeting.
As a physician, your insights and advice can be highly beneficial to others. You can also use media interviews to propel your career forward. Doing your homework can ensure that you will be pleased with the final product and how your words were used.
Dr. Moawad, Clinical Assistant Professor, Department of Medical Education, Case Western Reserve University School of Medicine, Cleveland, Ohio, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
As a physician, you might be contacted by the media to provide your professional opinion and advice. Or you might be looking for media interview opportunities to market your practice or side project. And if you do research, media interviews can be an effective way to spread the word. It’s important to prepare for a media interview so that you achieve the outcome you are looking for.
Keep your message simple. When you are a subject expert, you might think that the basics are obvious or even boring, and that the nuances are more important. However, most of the audience is looking for big-picture information that they can apply to their lives. Consider a few key takeaways, keeping in mind that your interview is likely to be edited to short sound bites or a few quotes. It may help to jot down notes so that you cover the fundamentals clearly. You could even write and rehearse a script beforehand. If there is something complicated or subtle that you want to convey, you can preface it by saying, “This is confusing but very important …” to let the audience know to give extra consideration to what you are about to say.
Avoid extremes and hyperbole. Sometimes, exaggerated statements make their way into medical discussions. Statements such as “it doesn’t matter how many calories you consume — it’s all about the quality” are common oversimplifications. But you might be upset to see your name next to a comment like this because it is not actually correct. Check the phrasing of your key takeaways to avoid being stuck defending or explaining an inaccurate statement when your patients ask you about it later.
Ask the interviewers what they are looking for. Many medical topics have some controversial element, so it is good to know what you’re getting into. Find out the purpose of the article or interview before you decide whether it is right for you. It could be about another doctor in town who is being sued; if you don’t want to be associated with that story, it might be best to decline the interview.
Explain your goals. You might accept or pursue an interview to raise awareness about an underrecognized condition. You might want the public to identify and get help for early symptoms, or you might want to create empathy for people coping with a disease you treat. Consider why you are participating in an interview, and communicate that to the interviewer to ensure that your objective can be part of the final product.
Know whom you’re dealing with. It is good to learn about the publication/media channel before you agree to participate. It may have a political bias, or perhaps the interview is intended to promote a specific product. If you agree with and support their purposes, then you may be happy to lend your opinion. But learning about the “voice” of the publication in advance allows you to make an informed decision about whether you want to be identified with a particular political ideology or product endorsement.
Ask to see your quotes before publication. It’s good to have the opportunity to make corrections in case you are accidentally misquoted or misunderstood. It is best to ask to see quotes before you agree to the interview. Some reporters may agree to (or even prefer) a written question-and-answer format so that they can directly quote your responses without rephrasing your words. You could suggest this, especially if you are too busy for a call or live meeting.
As a physician, your insights and advice can be highly beneficial to others. You can also use media interviews to propel your career forward. Doing your homework can ensure that you will be pleased with the final product and how your words were used.
Dr. Moawad, Clinical Assistant Professor, Department of Medical Education, Case Western Reserve University School of Medicine, Cleveland, Ohio, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.