User login
COVID-19 quarantine: Managing pediatric behavioral issues
We are living through unprecedented challenges, faced with profound uncertainties about the public health, the economy, the safety of our workplaces, the risks of gathering with friends and family, and even about the rhythm of the school year. Parents always have sought guidance from their pediatric providers when they are uncertain about their children’s health, behavior, and development. We want to share some guidance with you about several of the most common questions we have been hearing in the past few months, in the hope that it may prove useful in your conversations with patients and families.
What happens when we are so busy at home that our 2-year-old is ignored for much of the day?
If they are fortunate enough to be able to work from home, but have lost their child care, many parents are suddenly facing the sustained challenge of parenting while working. Even older children will have a tough time remembering that home is now a workplace, and they can’t interrupt their parents during a Zoom meeting. But older children will understand. Younger children (preschoolers) simply will not be able to understand that their parents are in sight but not fully available to them. They are exquisitely sensitive to their parents’ attention. If they are consistently ignored, behavioral problems can emerge. If both parents are at home, they should try to arrange a schedule taking turns so that one of them could turn their full attention to their kids if need be. If a working parent can be out of sight (i.e., in another room), it makes the situation easier for everyone.
If there is only one parent at home, that mom or dad should consider arranging a babysitter or sharing child care with a friend, with some reasonable safety provisions in place. The small risk of exposure to the virus is balanced by the risk of sustained invalidation in a developing child. Help parents set reasonable expectations for how productive they can be at home. If possible, they can manage their employer’s expectations, so that they do not find themselves in the impossible bind of choosing between a crying child and a crucial deadline. If they can work near the child (and be prepared for interruptions) when reading emails or writing, that may be enough availability for the child. And parents should not be discouraged when they have to repeatedly remind their children that they adore them, but also have to work while they are at home right now. Using age-appropriate screen time as a babysitter for a few hours each day is a perfectly acceptable part of a plan. Simply planning regular breaks when their children can have their attention will make the day easier for everyone at home.
What can I do about my 13-year-old who is lying around the house all day?
This is a time to pick your battles. If children can keep their regular sleep schedule, get their schoolwork done, and do some physical exercise every day, they are doing great. And if parents are continuously complaining that they are being lazy, it will probably cease to mean much to them. Instead, focus on clear, simple expectations, and parents should live by them, too. If parents can exercise with them, or try a new activity, that is a wonderful way to model self-care and trying new things. It is important to remember that the developmental task for a 13-year-old is to establish new avenues of independence that they will drive down further with each passing year. Give them some leeway to experiment and figure out their own way of handling this challenge, although it is bound to create some tension. Parents should always acknowledge how hard it is to stick with schoolwork without school, exercise without a team, practice music without a band, or do your work without an office!
What do we do about our 16-year-old who is staying up all night and sleeping until the late afternoon?
Adolescents naturally have their sleep cycle shift, so they are sleepy later and sleep longer. But staying up all night is usually about texting with friends or playing video games. The problem is that their sleep schedule can flip. They will not be able to participate in online class or enjoy exercise in the sun, and they rarely get enough sleep during the daytime, making them more irritable, anxious, inattentive, and tired. This will only make managing their schoolwork harder and increase the chances of conflict at home. So it is important to preserve rules around sleep. You might extend bedtime by an hour or so, but preserve rules and bedtime routines. Sleep is essential to health, well-being, and resilience, and all are critical during times of uncertainty and change.
We think our 17-year-old is using marijuana, and it might be a problem.
When parents think their children may have a problem with drugs, the children almost certainly do, as parents are typically the last to know about the extent of their use. Sheltering in place together may make their drug use much more apparent, and offer an opportunity for parents to respond. Talk with them about it. Let them know what you have noticed. See if they can tell you honestly about their drug use. Kids who are only experimenting socially are unlikely to be using drugs at home under quarantine. If you are truly calm and curious, they are more likely to be honest, and it could be a relief for them to discuss it with you. Find out what they think it helps, and what – if anything – they are worried about. Then share your concerns about marijuana use and the developing brain, and the risk of addiction. If they think it is “medical” use, remind them that anxiety or mood symptoms get better with therapy, whereas drugs (including marijuana) and alcohol actually worsen those problems. It is also a time to establish home rules, explain them, and enforce them. They will have your support while stopping and may learn that they are actually sleeping and feeling better after a few weeks without marijuana.
Parents should not hesitate to reach out to pediatric providers for guidance on local resources for assessment and treatment for substance abuse and addiction. These are medical problems, and they can become serious if untreated.
My 12-year-old perfectionist is very stressed about getting her work done well now that she is home schooling. How do I help her relax?
Some children, especially our anxious perfectionists, may respond to the switch to home school with great effort and organization. These kids usually are not the ones parents worry about. But they are very prone to expanding anxiety without the regular support and feedback of teachers. The school environment naturally encourages their taking chances and normalizes the setbacks and failures that are an essential part of learning something new. At home, parents are inclined to let these kids work independently. But they benefit from regular check-ins that are not focused on work completion or scores. Instead, ask about what they are doing that is hardest, and let them teach you about it. Model how you approach a new challenge, and how you regroup and try again when you don’t get it right. Finally, this is a good age to start discussing “reasonable expectations.” No one can “do their best” all the time; not parents, not professional athletes, not even machines can sustain long bursts of maximum speed without problems. Help them to start experimenting with different speeds and levels of effort, and see how it feels.
My 10-year-old is very anxious about catching coronavirus or one of us catching it. How do I help ease her anxiety when there is no certainty about how to prevent it?
Anxiety is a normal response to a situation with as much uncertainty as this one. But some are prone to more profound anxiety, and parents may find they are doing a lot of reassuring throughout the day. For especially anxious children (and adults), accommodating the anxiety by avoiding the stressful situation is a common response that provides temporary relief. But accommodation and avoidance actually fuel anxiety, and make it harder and harder to manage. It is important to talk about the “accommodations” we all are doing, how masks are recommended to protect others (not ourselves) and to slow down the spread of a new illness so our hospitals aren’t overwhelmed. It can seem counterintuitive, but rather than jumping to reassurance or dismissing their sense of risk, ask your children to play the full movie of what they are most worried about. What happens if they get sick? If you get sick? If they are worried about dying, go ahead and ask what they think happens then. You are demonstrating that you have confidence they can handle these feelings, and you are modeling curiosity – not avoidance – yourself. Correct any misunderstandings, check on facts together, acknowledge uncertainty. It also is very important for parents to assess whether their own anxiety level makes this task especially hard or may even be contributing to their children’s level of worry. Each of us is managing anxiety right now, and this moment presents an opportunity for all of us to learn about how we can face and bear it, learn to manage, and even master it.
We are all getting cabin fever at home and snapping at each other constantly. How do we keep the peace without just hiding in our rooms all day?
Cabin fever seems inevitable when a family is suddenly at home together all day every day with no end in sight. But if we establish some simple and realistic routines and preserve some structure without being rigid, it can go a long way to helping each member of a family to find their equilibrium in this new normal. Structure can be about preserving normal sleep and meal times. Ensuring everyone is getting adequate, restful sleep and is not hungry is probably the most powerful way to keep irritability and conflict low. It is also helpful to establish some new routines. These should be simple enough to be memorable and should be realistic. You might identify predictable blocks of time that are dedicated to school (or work), exercise, creative time, and family time. While much of the day may find each family member doing some independent activity, it helps when these “blocks” are the same for everybody. Try to consistently do one or two things together, like a walk after the family dinner or family game time. And also remember that everyone needs some alone time. Respect their need for this, and it will help you to explain when you need it. If someone wants to sit out the family Yahtzee tournament, don’t shame or punish them. Just invite them again the next night!
What are going to be the consequences of all this screen time?
The great majority of kids (and parents) will not suffer any adverse consequences from the increased amount of time spent in front of screens when these activities are varied and serve a useful purpose – including distraction, senseless fun, and social time. Beyond letter or email writing, screen and phone time are the only ways to stay socially connected while physically distant. But parents are the experts on their kids. Youth who are depressed and have in the past wanted to escape into long hours of video games or YouTube videos should not be allowed to do that now. Youth with attentional issues who have a hard time stopping video games will still have that difficulty. If they are getting adequate sleep and regular exercise, and are doing most of their school work and staying socially connected, screens are not dangerous. They are proving to be a wonderful tool to help us visit libraries and museums, take dance classes, learn new languages, follow the news, order groceries, or enjoy a movie together. If we stay connected to those we care about and to the world, then this time – although marked by profound suffering and loss – may prove to be a time when we were able to slow down and remember what truly matters in our lives.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. They have no relevant financial disclosures. Email them at [email protected].
We are living through unprecedented challenges, faced with profound uncertainties about the public health, the economy, the safety of our workplaces, the risks of gathering with friends and family, and even about the rhythm of the school year. Parents always have sought guidance from their pediatric providers when they are uncertain about their children’s health, behavior, and development. We want to share some guidance with you about several of the most common questions we have been hearing in the past few months, in the hope that it may prove useful in your conversations with patients and families.
What happens when we are so busy at home that our 2-year-old is ignored for much of the day?
If they are fortunate enough to be able to work from home, but have lost their child care, many parents are suddenly facing the sustained challenge of parenting while working. Even older children will have a tough time remembering that home is now a workplace, and they can’t interrupt their parents during a Zoom meeting. But older children will understand. Younger children (preschoolers) simply will not be able to understand that their parents are in sight but not fully available to them. They are exquisitely sensitive to their parents’ attention. If they are consistently ignored, behavioral problems can emerge. If both parents are at home, they should try to arrange a schedule taking turns so that one of them could turn their full attention to their kids if need be. If a working parent can be out of sight (i.e., in another room), it makes the situation easier for everyone.
If there is only one parent at home, that mom or dad should consider arranging a babysitter or sharing child care with a friend, with some reasonable safety provisions in place. The small risk of exposure to the virus is balanced by the risk of sustained invalidation in a developing child. Help parents set reasonable expectations for how productive they can be at home. If possible, they can manage their employer’s expectations, so that they do not find themselves in the impossible bind of choosing between a crying child and a crucial deadline. If they can work near the child (and be prepared for interruptions) when reading emails or writing, that may be enough availability for the child. And parents should not be discouraged when they have to repeatedly remind their children that they adore them, but also have to work while they are at home right now. Using age-appropriate screen time as a babysitter for a few hours each day is a perfectly acceptable part of a plan. Simply planning regular breaks when their children can have their attention will make the day easier for everyone at home.
What can I do about my 13-year-old who is lying around the house all day?
This is a time to pick your battles. If children can keep their regular sleep schedule, get their schoolwork done, and do some physical exercise every day, they are doing great. And if parents are continuously complaining that they are being lazy, it will probably cease to mean much to them. Instead, focus on clear, simple expectations, and parents should live by them, too. If parents can exercise with them, or try a new activity, that is a wonderful way to model self-care and trying new things. It is important to remember that the developmental task for a 13-year-old is to establish new avenues of independence that they will drive down further with each passing year. Give them some leeway to experiment and figure out their own way of handling this challenge, although it is bound to create some tension. Parents should always acknowledge how hard it is to stick with schoolwork without school, exercise without a team, practice music without a band, or do your work without an office!
What do we do about our 16-year-old who is staying up all night and sleeping until the late afternoon?
Adolescents naturally have their sleep cycle shift, so they are sleepy later and sleep longer. But staying up all night is usually about texting with friends or playing video games. The problem is that their sleep schedule can flip. They will not be able to participate in online class or enjoy exercise in the sun, and they rarely get enough sleep during the daytime, making them more irritable, anxious, inattentive, and tired. This will only make managing their schoolwork harder and increase the chances of conflict at home. So it is important to preserve rules around sleep. You might extend bedtime by an hour or so, but preserve rules and bedtime routines. Sleep is essential to health, well-being, and resilience, and all are critical during times of uncertainty and change.
We think our 17-year-old is using marijuana, and it might be a problem.
When parents think their children may have a problem with drugs, the children almost certainly do, as parents are typically the last to know about the extent of their use. Sheltering in place together may make their drug use much more apparent, and offer an opportunity for parents to respond. Talk with them about it. Let them know what you have noticed. See if they can tell you honestly about their drug use. Kids who are only experimenting socially are unlikely to be using drugs at home under quarantine. If you are truly calm and curious, they are more likely to be honest, and it could be a relief for them to discuss it with you. Find out what they think it helps, and what – if anything – they are worried about. Then share your concerns about marijuana use and the developing brain, and the risk of addiction. If they think it is “medical” use, remind them that anxiety or mood symptoms get better with therapy, whereas drugs (including marijuana) and alcohol actually worsen those problems. It is also a time to establish home rules, explain them, and enforce them. They will have your support while stopping and may learn that they are actually sleeping and feeling better after a few weeks without marijuana.
Parents should not hesitate to reach out to pediatric providers for guidance on local resources for assessment and treatment for substance abuse and addiction. These are medical problems, and they can become serious if untreated.
My 12-year-old perfectionist is very stressed about getting her work done well now that she is home schooling. How do I help her relax?
Some children, especially our anxious perfectionists, may respond to the switch to home school with great effort and organization. These kids usually are not the ones parents worry about. But they are very prone to expanding anxiety without the regular support and feedback of teachers. The school environment naturally encourages their taking chances and normalizes the setbacks and failures that are an essential part of learning something new. At home, parents are inclined to let these kids work independently. But they benefit from regular check-ins that are not focused on work completion or scores. Instead, ask about what they are doing that is hardest, and let them teach you about it. Model how you approach a new challenge, and how you regroup and try again when you don’t get it right. Finally, this is a good age to start discussing “reasonable expectations.” No one can “do their best” all the time; not parents, not professional athletes, not even machines can sustain long bursts of maximum speed without problems. Help them to start experimenting with different speeds and levels of effort, and see how it feels.
My 10-year-old is very anxious about catching coronavirus or one of us catching it. How do I help ease her anxiety when there is no certainty about how to prevent it?
Anxiety is a normal response to a situation with as much uncertainty as this one. But some are prone to more profound anxiety, and parents may find they are doing a lot of reassuring throughout the day. For especially anxious children (and adults), accommodating the anxiety by avoiding the stressful situation is a common response that provides temporary relief. But accommodation and avoidance actually fuel anxiety, and make it harder and harder to manage. It is important to talk about the “accommodations” we all are doing, how masks are recommended to protect others (not ourselves) and to slow down the spread of a new illness so our hospitals aren’t overwhelmed. It can seem counterintuitive, but rather than jumping to reassurance or dismissing their sense of risk, ask your children to play the full movie of what they are most worried about. What happens if they get sick? If you get sick? If they are worried about dying, go ahead and ask what they think happens then. You are demonstrating that you have confidence they can handle these feelings, and you are modeling curiosity – not avoidance – yourself. Correct any misunderstandings, check on facts together, acknowledge uncertainty. It also is very important for parents to assess whether their own anxiety level makes this task especially hard or may even be contributing to their children’s level of worry. Each of us is managing anxiety right now, and this moment presents an opportunity for all of us to learn about how we can face and bear it, learn to manage, and even master it.
We are all getting cabin fever at home and snapping at each other constantly. How do we keep the peace without just hiding in our rooms all day?
Cabin fever seems inevitable when a family is suddenly at home together all day every day with no end in sight. But if we establish some simple and realistic routines and preserve some structure without being rigid, it can go a long way to helping each member of a family to find their equilibrium in this new normal. Structure can be about preserving normal sleep and meal times. Ensuring everyone is getting adequate, restful sleep and is not hungry is probably the most powerful way to keep irritability and conflict low. It is also helpful to establish some new routines. These should be simple enough to be memorable and should be realistic. You might identify predictable blocks of time that are dedicated to school (or work), exercise, creative time, and family time. While much of the day may find each family member doing some independent activity, it helps when these “blocks” are the same for everybody. Try to consistently do one or two things together, like a walk after the family dinner or family game time. And also remember that everyone needs some alone time. Respect their need for this, and it will help you to explain when you need it. If someone wants to sit out the family Yahtzee tournament, don’t shame or punish them. Just invite them again the next night!
What are going to be the consequences of all this screen time?
The great majority of kids (and parents) will not suffer any adverse consequences from the increased amount of time spent in front of screens when these activities are varied and serve a useful purpose – including distraction, senseless fun, and social time. Beyond letter or email writing, screen and phone time are the only ways to stay socially connected while physically distant. But parents are the experts on their kids. Youth who are depressed and have in the past wanted to escape into long hours of video games or YouTube videos should not be allowed to do that now. Youth with attentional issues who have a hard time stopping video games will still have that difficulty. If they are getting adequate sleep and regular exercise, and are doing most of their school work and staying socially connected, screens are not dangerous. They are proving to be a wonderful tool to help us visit libraries and museums, take dance classes, learn new languages, follow the news, order groceries, or enjoy a movie together. If we stay connected to those we care about and to the world, then this time – although marked by profound suffering and loss – may prove to be a time when we were able to slow down and remember what truly matters in our lives.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. They have no relevant financial disclosures. Email them at [email protected].
We are living through unprecedented challenges, faced with profound uncertainties about the public health, the economy, the safety of our workplaces, the risks of gathering with friends and family, and even about the rhythm of the school year. Parents always have sought guidance from their pediatric providers when they are uncertain about their children’s health, behavior, and development. We want to share some guidance with you about several of the most common questions we have been hearing in the past few months, in the hope that it may prove useful in your conversations with patients and families.
What happens when we are so busy at home that our 2-year-old is ignored for much of the day?
If they are fortunate enough to be able to work from home, but have lost their child care, many parents are suddenly facing the sustained challenge of parenting while working. Even older children will have a tough time remembering that home is now a workplace, and they can’t interrupt their parents during a Zoom meeting. But older children will understand. Younger children (preschoolers) simply will not be able to understand that their parents are in sight but not fully available to them. They are exquisitely sensitive to their parents’ attention. If they are consistently ignored, behavioral problems can emerge. If both parents are at home, they should try to arrange a schedule taking turns so that one of them could turn their full attention to their kids if need be. If a working parent can be out of sight (i.e., in another room), it makes the situation easier for everyone.
If there is only one parent at home, that mom or dad should consider arranging a babysitter or sharing child care with a friend, with some reasonable safety provisions in place. The small risk of exposure to the virus is balanced by the risk of sustained invalidation in a developing child. Help parents set reasonable expectations for how productive they can be at home. If possible, they can manage their employer’s expectations, so that they do not find themselves in the impossible bind of choosing between a crying child and a crucial deadline. If they can work near the child (and be prepared for interruptions) when reading emails or writing, that may be enough availability for the child. And parents should not be discouraged when they have to repeatedly remind their children that they adore them, but also have to work while they are at home right now. Using age-appropriate screen time as a babysitter for a few hours each day is a perfectly acceptable part of a plan. Simply planning regular breaks when their children can have their attention will make the day easier for everyone at home.
What can I do about my 13-year-old who is lying around the house all day?
This is a time to pick your battles. If children can keep their regular sleep schedule, get their schoolwork done, and do some physical exercise every day, they are doing great. And if parents are continuously complaining that they are being lazy, it will probably cease to mean much to them. Instead, focus on clear, simple expectations, and parents should live by them, too. If parents can exercise with them, or try a new activity, that is a wonderful way to model self-care and trying new things. It is important to remember that the developmental task for a 13-year-old is to establish new avenues of independence that they will drive down further with each passing year. Give them some leeway to experiment and figure out their own way of handling this challenge, although it is bound to create some tension. Parents should always acknowledge how hard it is to stick with schoolwork without school, exercise without a team, practice music without a band, or do your work without an office!
What do we do about our 16-year-old who is staying up all night and sleeping until the late afternoon?
Adolescents naturally have their sleep cycle shift, so they are sleepy later and sleep longer. But staying up all night is usually about texting with friends or playing video games. The problem is that their sleep schedule can flip. They will not be able to participate in online class or enjoy exercise in the sun, and they rarely get enough sleep during the daytime, making them more irritable, anxious, inattentive, and tired. This will only make managing their schoolwork harder and increase the chances of conflict at home. So it is important to preserve rules around sleep. You might extend bedtime by an hour or so, but preserve rules and bedtime routines. Sleep is essential to health, well-being, and resilience, and all are critical during times of uncertainty and change.
We think our 17-year-old is using marijuana, and it might be a problem.
When parents think their children may have a problem with drugs, the children almost certainly do, as parents are typically the last to know about the extent of their use. Sheltering in place together may make their drug use much more apparent, and offer an opportunity for parents to respond. Talk with them about it. Let them know what you have noticed. See if they can tell you honestly about their drug use. Kids who are only experimenting socially are unlikely to be using drugs at home under quarantine. If you are truly calm and curious, they are more likely to be honest, and it could be a relief for them to discuss it with you. Find out what they think it helps, and what – if anything – they are worried about. Then share your concerns about marijuana use and the developing brain, and the risk of addiction. If they think it is “medical” use, remind them that anxiety or mood symptoms get better with therapy, whereas drugs (including marijuana) and alcohol actually worsen those problems. It is also a time to establish home rules, explain them, and enforce them. They will have your support while stopping and may learn that they are actually sleeping and feeling better after a few weeks without marijuana.
Parents should not hesitate to reach out to pediatric providers for guidance on local resources for assessment and treatment for substance abuse and addiction. These are medical problems, and they can become serious if untreated.
My 12-year-old perfectionist is very stressed about getting her work done well now that she is home schooling. How do I help her relax?
Some children, especially our anxious perfectionists, may respond to the switch to home school with great effort and organization. These kids usually are not the ones parents worry about. But they are very prone to expanding anxiety without the regular support and feedback of teachers. The school environment naturally encourages their taking chances and normalizes the setbacks and failures that are an essential part of learning something new. At home, parents are inclined to let these kids work independently. But they benefit from regular check-ins that are not focused on work completion or scores. Instead, ask about what they are doing that is hardest, and let them teach you about it. Model how you approach a new challenge, and how you regroup and try again when you don’t get it right. Finally, this is a good age to start discussing “reasonable expectations.” No one can “do their best” all the time; not parents, not professional athletes, not even machines can sustain long bursts of maximum speed without problems. Help them to start experimenting with different speeds and levels of effort, and see how it feels.
My 10-year-old is very anxious about catching coronavirus or one of us catching it. How do I help ease her anxiety when there is no certainty about how to prevent it?
Anxiety is a normal response to a situation with as much uncertainty as this one. But some are prone to more profound anxiety, and parents may find they are doing a lot of reassuring throughout the day. For especially anxious children (and adults), accommodating the anxiety by avoiding the stressful situation is a common response that provides temporary relief. But accommodation and avoidance actually fuel anxiety, and make it harder and harder to manage. It is important to talk about the “accommodations” we all are doing, how masks are recommended to protect others (not ourselves) and to slow down the spread of a new illness so our hospitals aren’t overwhelmed. It can seem counterintuitive, but rather than jumping to reassurance or dismissing their sense of risk, ask your children to play the full movie of what they are most worried about. What happens if they get sick? If you get sick? If they are worried about dying, go ahead and ask what they think happens then. You are demonstrating that you have confidence they can handle these feelings, and you are modeling curiosity – not avoidance – yourself. Correct any misunderstandings, check on facts together, acknowledge uncertainty. It also is very important for parents to assess whether their own anxiety level makes this task especially hard or may even be contributing to their children’s level of worry. Each of us is managing anxiety right now, and this moment presents an opportunity for all of us to learn about how we can face and bear it, learn to manage, and even master it.
We are all getting cabin fever at home and snapping at each other constantly. How do we keep the peace without just hiding in our rooms all day?
Cabin fever seems inevitable when a family is suddenly at home together all day every day with no end in sight. But if we establish some simple and realistic routines and preserve some structure without being rigid, it can go a long way to helping each member of a family to find their equilibrium in this new normal. Structure can be about preserving normal sleep and meal times. Ensuring everyone is getting adequate, restful sleep and is not hungry is probably the most powerful way to keep irritability and conflict low. It is also helpful to establish some new routines. These should be simple enough to be memorable and should be realistic. You might identify predictable blocks of time that are dedicated to school (or work), exercise, creative time, and family time. While much of the day may find each family member doing some independent activity, it helps when these “blocks” are the same for everybody. Try to consistently do one or two things together, like a walk after the family dinner or family game time. And also remember that everyone needs some alone time. Respect their need for this, and it will help you to explain when you need it. If someone wants to sit out the family Yahtzee tournament, don’t shame or punish them. Just invite them again the next night!
What are going to be the consequences of all this screen time?
The great majority of kids (and parents) will not suffer any adverse consequences from the increased amount of time spent in front of screens when these activities are varied and serve a useful purpose – including distraction, senseless fun, and social time. Beyond letter or email writing, screen and phone time are the only ways to stay socially connected while physically distant. But parents are the experts on their kids. Youth who are depressed and have in the past wanted to escape into long hours of video games or YouTube videos should not be allowed to do that now. Youth with attentional issues who have a hard time stopping video games will still have that difficulty. If they are getting adequate sleep and regular exercise, and are doing most of their school work and staying socially connected, screens are not dangerous. They are proving to be a wonderful tool to help us visit libraries and museums, take dance classes, learn new languages, follow the news, order groceries, or enjoy a movie together. If we stay connected to those we care about and to the world, then this time – although marked by profound suffering and loss – may prove to be a time when we were able to slow down and remember what truly matters in our lives.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. They have no relevant financial disclosures. Email them at [email protected].
Consider COVID-19–associated multisystem hyperinflammatory syndrome
A 21-year-old young adult presented to the ED with a 1-week history of high fever, vomiting, diarrhea, and abdominal pain. His mother was SARS-CoV-2 positive by polymerase chain reaction approximately 3 weeks prior; his PCR was negative for SARS-CoV-2.
Following admission, he became hypotensive and tachycardic with evidence of myocarditis. His chest x-ray was normal and his O2 saturation was 100% on room air. His clinical presentation was initially suggestive of toxic shock syndrome without a rash, but despite aggressive fluid resuscitation and broad-spectrum antibiotics, he continued to clinically deteriorate with persistent high fever and increasing cardiac stress. Echocardiography revealed biventricular dysfunction. His laboratory abnormalities included rising inflammatory markers and troponin I and B-type natriuretic peptide (BNP). A repeat PCR for SARS-CoV-2 was negative on day 2 of illness. He was diagnosed as likely having macrophage-activation syndrome (MAS) despite the atypical features (myocarditis), and he received Anakinra with no apparent response. He also was given intravenous immunoglobulin (IVIg) for his myocarditis and subsequently high-dose steroids. He became afebrile, his blood pressure stabilized, his inflammatory markers declined, and over several days he returned to normal. His COVID-19 antibody test IgG was positive on day 4 of illness.
This case challenged us for several reasons. First, the PCR from his nasopharynx was negative on two occasions, which raises the issue of how sensitive and accurate these PCR tests are for SARS-CoV-2 or are patients with COVID-19–associated hyperinflammatory syndrome still PCR positive? Second, although we have seen many adult cases with a cytokine storm picture similar to this patient, nearly all of the prior cases had chest x-ray abnormalities and hypoxia. Third, the severity of the myocardial dysfunction and rising troponin and BNP also was unusual in our experience with COVID-19 infection. Lastly, the use of antibody detection to SARS-CoV-2 enabled us to confirm recent COIVD-19 disease and see his illness as part of the likely spectrum of clinical syndromes seen with this virus.
The Lancet reported eight children, aged 4-14 years, with a hyperinflammatory shock-like syndrome in early May.1 The cases had features similar to atypical Kawasaki disease, KD shock syndrome, and toxic shock syndrome. Each case had high fever for multiple days; diarrhea and abdominal pain was present in even children; elevated ferritin, C-reactive protein, d-dimer, increased troponins, and ventricular dysfunction also was present in seven. Most patients had no pulmonary involvement, and most tested negative for SARS-CoV-2 despite four of the eight having direct contact with a COVID-positive family member. All received IVIg and antibiotics; six received aspirin. Seven of the eight made a full recovery; one child died from a large cerebrovascular infarct.
Also in early May, the New York Times described a “mysterious” hyperinflammatory syndrome in children thought to be linked to COVID-19. A total of 76 suspected cases in children had been reported in New York state, three of whom died. The syndrome has been given the name pediatric multisystem inflammatory syndrome. The syndrome can resemble KD shock syndrome with rash; fever; conjunctivitis; hypotension; and redness in the lips, tongue and mucous membranes . It also can resemble toxic shock syndrome with abdominal pain, vomiting, and diarrhea. However, the degree of cardiac inflammation and dysfunction is substantial in many cases and usually beyond that seen in KD or toxic shock.
The syndrome is not limited to the United States. The Royal College of Pediatrics and Child Health has created a case definition:2
- A child presenting with persistent fever, inflammation (elevated C-reactive protein, neutrophilia, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurologic) with additional features.
- Exclusion of any other microbial causes such as bacterial sepsis or staphylococcal or streptococcal shock syndromes, infections known to be associated with myocarditis (such as enterovirus).
- SARS-CoV-2 testing may or may not be positive.
As with our young adult, treatment is supportive, nonspecific, and aimed at quieting the inflammatory response. The current thinking is the syndrome is seen as antibody to SARS-CoV-2 appears and frequently the nasopharyngeal PCR is negative. It is hypothesized that the syndrome occurs in genetically predisposed hosts and potentially is a late-onset inflammatory process or potentially an antibody-triggered inflammatory process. The negative PCR from nasopharyngeal specimens reflects that the onset is later in the course of disease; whether fecal samples would be COVID positive is unknown. As with our case, antibody testing for IgG against SARS-CoV-2 is appropriate to confirm COVID-19 disease and may be positive as early as day 7.
The approach needs to be team oriented and include cardiology, rheumatology, infectious diseases, and intensive care specialists working collaboratively. Such cases should be considered COVID positive despite negative PCR tests, and full personal protective equipment should be used as we do not as yet know if live virus could be found in stool. We initiated treatment with Anakinra (an interleukin-1 type-1 receptor inhibitor) as part of our treatment protocol for MAS; we did not appreciate a response. He then received IVIg and high-dose steroids, and he recovered over several days with improved cardiac function and stable blood pressure.
What is the pathogenesis? Is SARS-CoV-2 causative or just an associated finding? Who are the at-risk children, adolescents, and adults? Is there a genetic predisposition? What therapies work best? The eight cases described in London all received IVIg, as did our case, and all but one improved and survived. In adults we have seen substantial inflammation with elevated C-reactive protein (often as high as 300), ferritin, lactate dehydrogenase, triglycerides, fibrinogen, and d-dimers, but nearly all have extensive pulmonary disease, hypoxia, and are SARS-CoV-2 positive by PCR. Influenza is also associated with a cytokine storm syndrome in adolescents and young adults.3 The mechanisms influenza virus uses to initiate a cytokine storm and strategies for immunomodulatory treatment may provide insights into COVID-19–associated multisystem hyperinflammatory syndrome.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician in pediatric infectious diseases at Boston Medical Center. Dr. Camelo is a senior fellow in pediatric infectious diseases at Boston Medical Center. They have no relevant financial disclosures. Email them at [email protected].
References
1. Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19.
3. Liu Q et al.Cell Mol Immunol. 2016 Jan;13(1):3-10.
A 21-year-old young adult presented to the ED with a 1-week history of high fever, vomiting, diarrhea, and abdominal pain. His mother was SARS-CoV-2 positive by polymerase chain reaction approximately 3 weeks prior; his PCR was negative for SARS-CoV-2.
Following admission, he became hypotensive and tachycardic with evidence of myocarditis. His chest x-ray was normal and his O2 saturation was 100% on room air. His clinical presentation was initially suggestive of toxic shock syndrome without a rash, but despite aggressive fluid resuscitation and broad-spectrum antibiotics, he continued to clinically deteriorate with persistent high fever and increasing cardiac stress. Echocardiography revealed biventricular dysfunction. His laboratory abnormalities included rising inflammatory markers and troponin I and B-type natriuretic peptide (BNP). A repeat PCR for SARS-CoV-2 was negative on day 2 of illness. He was diagnosed as likely having macrophage-activation syndrome (MAS) despite the atypical features (myocarditis), and he received Anakinra with no apparent response. He also was given intravenous immunoglobulin (IVIg) for his myocarditis and subsequently high-dose steroids. He became afebrile, his blood pressure stabilized, his inflammatory markers declined, and over several days he returned to normal. His COVID-19 antibody test IgG was positive on day 4 of illness.
This case challenged us for several reasons. First, the PCR from his nasopharynx was negative on two occasions, which raises the issue of how sensitive and accurate these PCR tests are for SARS-CoV-2 or are patients with COVID-19–associated hyperinflammatory syndrome still PCR positive? Second, although we have seen many adult cases with a cytokine storm picture similar to this patient, nearly all of the prior cases had chest x-ray abnormalities and hypoxia. Third, the severity of the myocardial dysfunction and rising troponin and BNP also was unusual in our experience with COVID-19 infection. Lastly, the use of antibody detection to SARS-CoV-2 enabled us to confirm recent COIVD-19 disease and see his illness as part of the likely spectrum of clinical syndromes seen with this virus.
The Lancet reported eight children, aged 4-14 years, with a hyperinflammatory shock-like syndrome in early May.1 The cases had features similar to atypical Kawasaki disease, KD shock syndrome, and toxic shock syndrome. Each case had high fever for multiple days; diarrhea and abdominal pain was present in even children; elevated ferritin, C-reactive protein, d-dimer, increased troponins, and ventricular dysfunction also was present in seven. Most patients had no pulmonary involvement, and most tested negative for SARS-CoV-2 despite four of the eight having direct contact with a COVID-positive family member. All received IVIg and antibiotics; six received aspirin. Seven of the eight made a full recovery; one child died from a large cerebrovascular infarct.
Also in early May, the New York Times described a “mysterious” hyperinflammatory syndrome in children thought to be linked to COVID-19. A total of 76 suspected cases in children had been reported in New York state, three of whom died. The syndrome has been given the name pediatric multisystem inflammatory syndrome. The syndrome can resemble KD shock syndrome with rash; fever; conjunctivitis; hypotension; and redness in the lips, tongue and mucous membranes . It also can resemble toxic shock syndrome with abdominal pain, vomiting, and diarrhea. However, the degree of cardiac inflammation and dysfunction is substantial in many cases and usually beyond that seen in KD or toxic shock.
The syndrome is not limited to the United States. The Royal College of Pediatrics and Child Health has created a case definition:2
- A child presenting with persistent fever, inflammation (elevated C-reactive protein, neutrophilia, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurologic) with additional features.
- Exclusion of any other microbial causes such as bacterial sepsis or staphylococcal or streptococcal shock syndromes, infections known to be associated with myocarditis (such as enterovirus).
- SARS-CoV-2 testing may or may not be positive.
As with our young adult, treatment is supportive, nonspecific, and aimed at quieting the inflammatory response. The current thinking is the syndrome is seen as antibody to SARS-CoV-2 appears and frequently the nasopharyngeal PCR is negative. It is hypothesized that the syndrome occurs in genetically predisposed hosts and potentially is a late-onset inflammatory process or potentially an antibody-triggered inflammatory process. The negative PCR from nasopharyngeal specimens reflects that the onset is later in the course of disease; whether fecal samples would be COVID positive is unknown. As with our case, antibody testing for IgG against SARS-CoV-2 is appropriate to confirm COVID-19 disease and may be positive as early as day 7.
The approach needs to be team oriented and include cardiology, rheumatology, infectious diseases, and intensive care specialists working collaboratively. Such cases should be considered COVID positive despite negative PCR tests, and full personal protective equipment should be used as we do not as yet know if live virus could be found in stool. We initiated treatment with Anakinra (an interleukin-1 type-1 receptor inhibitor) as part of our treatment protocol for MAS; we did not appreciate a response. He then received IVIg and high-dose steroids, and he recovered over several days with improved cardiac function and stable blood pressure.
What is the pathogenesis? Is SARS-CoV-2 causative or just an associated finding? Who are the at-risk children, adolescents, and adults? Is there a genetic predisposition? What therapies work best? The eight cases described in London all received IVIg, as did our case, and all but one improved and survived. In adults we have seen substantial inflammation with elevated C-reactive protein (often as high as 300), ferritin, lactate dehydrogenase, triglycerides, fibrinogen, and d-dimers, but nearly all have extensive pulmonary disease, hypoxia, and are SARS-CoV-2 positive by PCR. Influenza is also associated with a cytokine storm syndrome in adolescents and young adults.3 The mechanisms influenza virus uses to initiate a cytokine storm and strategies for immunomodulatory treatment may provide insights into COVID-19–associated multisystem hyperinflammatory syndrome.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician in pediatric infectious diseases at Boston Medical Center. Dr. Camelo is a senior fellow in pediatric infectious diseases at Boston Medical Center. They have no relevant financial disclosures. Email them at [email protected].
References
1. Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19.
3. Liu Q et al.Cell Mol Immunol. 2016 Jan;13(1):3-10.
A 21-year-old young adult presented to the ED with a 1-week history of high fever, vomiting, diarrhea, and abdominal pain. His mother was SARS-CoV-2 positive by polymerase chain reaction approximately 3 weeks prior; his PCR was negative for SARS-CoV-2.
Following admission, he became hypotensive and tachycardic with evidence of myocarditis. His chest x-ray was normal and his O2 saturation was 100% on room air. His clinical presentation was initially suggestive of toxic shock syndrome without a rash, but despite aggressive fluid resuscitation and broad-spectrum antibiotics, he continued to clinically deteriorate with persistent high fever and increasing cardiac stress. Echocardiography revealed biventricular dysfunction. His laboratory abnormalities included rising inflammatory markers and troponin I and B-type natriuretic peptide (BNP). A repeat PCR for SARS-CoV-2 was negative on day 2 of illness. He was diagnosed as likely having macrophage-activation syndrome (MAS) despite the atypical features (myocarditis), and he received Anakinra with no apparent response. He also was given intravenous immunoglobulin (IVIg) for his myocarditis and subsequently high-dose steroids. He became afebrile, his blood pressure stabilized, his inflammatory markers declined, and over several days he returned to normal. His COVID-19 antibody test IgG was positive on day 4 of illness.
This case challenged us for several reasons. First, the PCR from his nasopharynx was negative on two occasions, which raises the issue of how sensitive and accurate these PCR tests are for SARS-CoV-2 or are patients with COVID-19–associated hyperinflammatory syndrome still PCR positive? Second, although we have seen many adult cases with a cytokine storm picture similar to this patient, nearly all of the prior cases had chest x-ray abnormalities and hypoxia. Third, the severity of the myocardial dysfunction and rising troponin and BNP also was unusual in our experience with COVID-19 infection. Lastly, the use of antibody detection to SARS-CoV-2 enabled us to confirm recent COIVD-19 disease and see his illness as part of the likely spectrum of clinical syndromes seen with this virus.
The Lancet reported eight children, aged 4-14 years, with a hyperinflammatory shock-like syndrome in early May.1 The cases had features similar to atypical Kawasaki disease, KD shock syndrome, and toxic shock syndrome. Each case had high fever for multiple days; diarrhea and abdominal pain was present in even children; elevated ferritin, C-reactive protein, d-dimer, increased troponins, and ventricular dysfunction also was present in seven. Most patients had no pulmonary involvement, and most tested negative for SARS-CoV-2 despite four of the eight having direct contact with a COVID-positive family member. All received IVIg and antibiotics; six received aspirin. Seven of the eight made a full recovery; one child died from a large cerebrovascular infarct.
Also in early May, the New York Times described a “mysterious” hyperinflammatory syndrome in children thought to be linked to COVID-19. A total of 76 suspected cases in children had been reported in New York state, three of whom died. The syndrome has been given the name pediatric multisystem inflammatory syndrome. The syndrome can resemble KD shock syndrome with rash; fever; conjunctivitis; hypotension; and redness in the lips, tongue and mucous membranes . It also can resemble toxic shock syndrome with abdominal pain, vomiting, and diarrhea. However, the degree of cardiac inflammation and dysfunction is substantial in many cases and usually beyond that seen in KD or toxic shock.
The syndrome is not limited to the United States. The Royal College of Pediatrics and Child Health has created a case definition:2
- A child presenting with persistent fever, inflammation (elevated C-reactive protein, neutrophilia, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurologic) with additional features.
- Exclusion of any other microbial causes such as bacterial sepsis or staphylococcal or streptococcal shock syndromes, infections known to be associated with myocarditis (such as enterovirus).
- SARS-CoV-2 testing may or may not be positive.
As with our young adult, treatment is supportive, nonspecific, and aimed at quieting the inflammatory response. The current thinking is the syndrome is seen as antibody to SARS-CoV-2 appears and frequently the nasopharyngeal PCR is negative. It is hypothesized that the syndrome occurs in genetically predisposed hosts and potentially is a late-onset inflammatory process or potentially an antibody-triggered inflammatory process. The negative PCR from nasopharyngeal specimens reflects that the onset is later in the course of disease; whether fecal samples would be COVID positive is unknown. As with our case, antibody testing for IgG against SARS-CoV-2 is appropriate to confirm COVID-19 disease and may be positive as early as day 7.
The approach needs to be team oriented and include cardiology, rheumatology, infectious diseases, and intensive care specialists working collaboratively. Such cases should be considered COVID positive despite negative PCR tests, and full personal protective equipment should be used as we do not as yet know if live virus could be found in stool. We initiated treatment with Anakinra (an interleukin-1 type-1 receptor inhibitor) as part of our treatment protocol for MAS; we did not appreciate a response. He then received IVIg and high-dose steroids, and he recovered over several days with improved cardiac function and stable blood pressure.
What is the pathogenesis? Is SARS-CoV-2 causative or just an associated finding? Who are the at-risk children, adolescents, and adults? Is there a genetic predisposition? What therapies work best? The eight cases described in London all received IVIg, as did our case, and all but one improved and survived. In adults we have seen substantial inflammation with elevated C-reactive protein (often as high as 300), ferritin, lactate dehydrogenase, triglycerides, fibrinogen, and d-dimers, but nearly all have extensive pulmonary disease, hypoxia, and are SARS-CoV-2 positive by PCR. Influenza is also associated with a cytokine storm syndrome in adolescents and young adults.3 The mechanisms influenza virus uses to initiate a cytokine storm and strategies for immunomodulatory treatment may provide insights into COVID-19–associated multisystem hyperinflammatory syndrome.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician in pediatric infectious diseases at Boston Medical Center. Dr. Camelo is a senior fellow in pediatric infectious diseases at Boston Medical Center. They have no relevant financial disclosures. Email them at [email protected].
References
1. Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19.
3. Liu Q et al.Cell Mol Immunol. 2016 Jan;13(1):3-10.
Pediatric OCD: A case for vigilance
Max is an 8-year-old boy in the third grade, and you have been his pediatrician since birth. Described as “emotional” and “particular” since his early years, Max is prone to prolonged tantrums that have not improved with age. Parents have described a tic that involves repeatedly touching his ear, but this has not been observed in the office setting. Max has struggled with some attention issues at school, and often needs help finishing assignments. The family is feeling increasingly desperate for ways to manage his near-daily meltdowns at home, and parenting strategies you’ve discussed thus far don’t seem to be helping much. Should obsessive-compulsive disorder be in your differential? And at what point do you seek outside evaluation?
OCD is a condition characterized by recurrent, intrusive, and unwanted thoughts, images, and urges (obsessions), and repetitive behaviors or mental acts performed in a particular way to reduce anxiety (compulsions). It affects 1%-3% of children, and onset can be as early as age 3-4 years. While the average age of onset in children is approximately 10 years old, average age of diagnosis is at least several years later.1 A primary care physician’s ability to recognize OCD symptoms in children, perform an initial assessment, and connect the child to appropriate clinical care is key to reducing the years of difficulty that children and families often endure prior to beginning treatment.
Common obsessions in children include contamination, fear of harm to self or others, symmetry, and the belief that bad things will occur if rituals are performed incorrectly. Common compulsions include checking, washing, ordering, and mental acts such as praying or counting to one’s self.1,2 In addition to the fact that OCD presentations are highly heterogeneous, early diagnosis is challenging due to significant overlap of OCD symptoms with developmentally normal behaviors. For example, magical or superstitious thinking is common among school-age children who avoid stepping on cracks or utilize lucky numbers. What differentiates OCD is the presence of obsessions and/or compulsions that are time consuming and cause subjective distress or functional impairment. Children often are adept at keeping OCD symptoms secret. At time of diagnosis, the child may have a complex array of discreet behaviors to manage distress and minimize shame. Children may not have insight into the irrationality of their thoughts or behaviors, but they are certainly aware of how terrible and confused they feel inside, and how it affects their relationship with their parents. Rituals, such as those that delay bedtime or cause school tardiness, may look like oppositional behaviors and cause immense frustration for parents.
Comorbidities are common and include ADHD, oppositional defiant disorder, depression, and Tourette syndrome.3 Nearly 60% of children with OCD meet criteria for a tic disorder at some point in their lifetime.4 Compulsions designed to ease a feeling of internal discomfort, such as touching or tapping, are particularly typical of patients with OCD and comorbid tics. Often these children will express a need for things to be “just right,” with lasting relief from such a feeling rarely found. While sensory intolerances are not part of OCD’s diagnostic criteria, clinical experiences and growing research point to a high prevalence in affected children.5,6 Sensory intolerances may even be the primary presenting problem. Examples include clothing feeling uncomfortable, or inability to tolerate certain smells or innocuous sounds.
The preferred method for assessment of OCD in children is the Children’s Yale–Brown Obsessive Compulsive Scale (CY-BOCS), a semi-structured, clinician-rated interview designed to elicit symptoms, severity, and distress. While time constraints may prevent use of the CY-BOCS in the primary care setting, a handful of screening questions instead can go a long way. These might include:
- Do you have to do things in a certain way, such as washing or making things “just right?”
- What happens if you can’t do things in a certain way?
- Do you have unwanted thoughts that keep coming back and are hard to get rid of?
Equally as important as understanding a child’s OCD symptoms is understanding how the family has, often unwittingly, become intertwined in a web of OCD-driven behaviors. In an effort to soothe the child, prevent emotional outbursts, or simply get through the day, parents may find themselves accommodating behaviors that seem irrational. Despite parents’ best intentions, this is likely reinforcing OCD patterns. Parents may be asked by the child to repeat a reassuring phrase in a certain way, arrange furniture “just so,” or drive a certain route to school. In the case of contamination fears, a child may be taking several showers per day, using two bottles of shampoo per week, and demanding that his or her clothes be washed separately before a parent begins to realize the cumulative impact of these unusual behaviors on the household. In addition to exploring concerns, primary care physicians can provide a sounding board for exhausted parents wondering if other families face the same thing. While connecting the family to treatment, they also can provide reassurances that treatment can dramatically shift the trajectory of the illness.
Treatment of pediatric OCD begins with a specific form of cognitive behavioral therapy (CBT) called Exposure and Response Prevention therapy (ERP). ERP requires a skilled therapist, and a strong alliance with a child and family because the child will be asked to gradually challenge compulsions head-on and tolerate the accompanied distress. CBT/ERP is associated with a 40%-65% reduction in symptoms, but combination with SSRI therapy improves outcomes in more severe cases.3 Despite limited mental health resources and long wait lists in many parts of the country, connection to OCD-specific treatment is increasingly feasible in virtual format via online support groups and telemedicine.
“Max” may experience any number of OCD-related symptoms that a primary care physician could deftly uncover. He may become “stuck” at school because his handwriting accidentally strayed below the line. He may have hours-long meltdowns because his hair never feels right. He may touch his ear to prevent tragic harm coming to his mother. Whatever further exploration reveals, Max and his family stand to benefit immensely from early detection and intervention.
Dr. McGowan is assistant professor of psychiatry and pediatrics at the Vermont Center for Children, Youth, and Families, University of Vermont Medical Center, Burlington. She had no relevant financial disclosures. Email Dr. McGowan at [email protected].
Resources for providers and families*
UNSTUCK: An OCD Kids Movie. Featuring a 23-minute documentary film about children living with OCD, this website also is rich in OCD-related resources.
International OCD Foundation. Has information for families about OCD. Also has a resource directory for therapists, clinics, support groups, and other organizations specializing in OCD and related disorders in different geographic areas.
*Of note, both resources above include COVID-19-specific resources for those struggling with worsening OCD symptoms as a result of the pandemic.
References
1. Lewis’s Child and Adolescent Psychiatry: A Comprehensive Textbook, 4th ed. (Baltimore: Lippincott Williams & Wilkins, 2020, pp. 518-27).
2. J Amer Acad Child Adol Psychiatry. 2012;51(1):98-113.
3. J Clin. Invest. 2009;119(4):737-46.
4. Arch Dis Child. 2015;100(5):495-9.
5. J Develop Behav Pediatr. 2019 Jun;40(5):377-82.
6. Ann Clin Psychiatry. 2008 Oct-Dec;20(4):199-203.
Max is an 8-year-old boy in the third grade, and you have been his pediatrician since birth. Described as “emotional” and “particular” since his early years, Max is prone to prolonged tantrums that have not improved with age. Parents have described a tic that involves repeatedly touching his ear, but this has not been observed in the office setting. Max has struggled with some attention issues at school, and often needs help finishing assignments. The family is feeling increasingly desperate for ways to manage his near-daily meltdowns at home, and parenting strategies you’ve discussed thus far don’t seem to be helping much. Should obsessive-compulsive disorder be in your differential? And at what point do you seek outside evaluation?
OCD is a condition characterized by recurrent, intrusive, and unwanted thoughts, images, and urges (obsessions), and repetitive behaviors or mental acts performed in a particular way to reduce anxiety (compulsions). It affects 1%-3% of children, and onset can be as early as age 3-4 years. While the average age of onset in children is approximately 10 years old, average age of diagnosis is at least several years later.1 A primary care physician’s ability to recognize OCD symptoms in children, perform an initial assessment, and connect the child to appropriate clinical care is key to reducing the years of difficulty that children and families often endure prior to beginning treatment.
Common obsessions in children include contamination, fear of harm to self or others, symmetry, and the belief that bad things will occur if rituals are performed incorrectly. Common compulsions include checking, washing, ordering, and mental acts such as praying or counting to one’s self.1,2 In addition to the fact that OCD presentations are highly heterogeneous, early diagnosis is challenging due to significant overlap of OCD symptoms with developmentally normal behaviors. For example, magical or superstitious thinking is common among school-age children who avoid stepping on cracks or utilize lucky numbers. What differentiates OCD is the presence of obsessions and/or compulsions that are time consuming and cause subjective distress or functional impairment. Children often are adept at keeping OCD symptoms secret. At time of diagnosis, the child may have a complex array of discreet behaviors to manage distress and minimize shame. Children may not have insight into the irrationality of their thoughts or behaviors, but they are certainly aware of how terrible and confused they feel inside, and how it affects their relationship with their parents. Rituals, such as those that delay bedtime or cause school tardiness, may look like oppositional behaviors and cause immense frustration for parents.
Comorbidities are common and include ADHD, oppositional defiant disorder, depression, and Tourette syndrome.3 Nearly 60% of children with OCD meet criteria for a tic disorder at some point in their lifetime.4 Compulsions designed to ease a feeling of internal discomfort, such as touching or tapping, are particularly typical of patients with OCD and comorbid tics. Often these children will express a need for things to be “just right,” with lasting relief from such a feeling rarely found. While sensory intolerances are not part of OCD’s diagnostic criteria, clinical experiences and growing research point to a high prevalence in affected children.5,6 Sensory intolerances may even be the primary presenting problem. Examples include clothing feeling uncomfortable, or inability to tolerate certain smells or innocuous sounds.
The preferred method for assessment of OCD in children is the Children’s Yale–Brown Obsessive Compulsive Scale (CY-BOCS), a semi-structured, clinician-rated interview designed to elicit symptoms, severity, and distress. While time constraints may prevent use of the CY-BOCS in the primary care setting, a handful of screening questions instead can go a long way. These might include:
- Do you have to do things in a certain way, such as washing or making things “just right?”
- What happens if you can’t do things in a certain way?
- Do you have unwanted thoughts that keep coming back and are hard to get rid of?
Equally as important as understanding a child’s OCD symptoms is understanding how the family has, often unwittingly, become intertwined in a web of OCD-driven behaviors. In an effort to soothe the child, prevent emotional outbursts, or simply get through the day, parents may find themselves accommodating behaviors that seem irrational. Despite parents’ best intentions, this is likely reinforcing OCD patterns. Parents may be asked by the child to repeat a reassuring phrase in a certain way, arrange furniture “just so,” or drive a certain route to school. In the case of contamination fears, a child may be taking several showers per day, using two bottles of shampoo per week, and demanding that his or her clothes be washed separately before a parent begins to realize the cumulative impact of these unusual behaviors on the household. In addition to exploring concerns, primary care physicians can provide a sounding board for exhausted parents wondering if other families face the same thing. While connecting the family to treatment, they also can provide reassurances that treatment can dramatically shift the trajectory of the illness.
Treatment of pediatric OCD begins with a specific form of cognitive behavioral therapy (CBT) called Exposure and Response Prevention therapy (ERP). ERP requires a skilled therapist, and a strong alliance with a child and family because the child will be asked to gradually challenge compulsions head-on and tolerate the accompanied distress. CBT/ERP is associated with a 40%-65% reduction in symptoms, but combination with SSRI therapy improves outcomes in more severe cases.3 Despite limited mental health resources and long wait lists in many parts of the country, connection to OCD-specific treatment is increasingly feasible in virtual format via online support groups and telemedicine.
“Max” may experience any number of OCD-related symptoms that a primary care physician could deftly uncover. He may become “stuck” at school because his handwriting accidentally strayed below the line. He may have hours-long meltdowns because his hair never feels right. He may touch his ear to prevent tragic harm coming to his mother. Whatever further exploration reveals, Max and his family stand to benefit immensely from early detection and intervention.
Dr. McGowan is assistant professor of psychiatry and pediatrics at the Vermont Center for Children, Youth, and Families, University of Vermont Medical Center, Burlington. She had no relevant financial disclosures. Email Dr. McGowan at [email protected].
Resources for providers and families*
UNSTUCK: An OCD Kids Movie. Featuring a 23-minute documentary film about children living with OCD, this website also is rich in OCD-related resources.
International OCD Foundation. Has information for families about OCD. Also has a resource directory for therapists, clinics, support groups, and other organizations specializing in OCD and related disorders in different geographic areas.
*Of note, both resources above include COVID-19-specific resources for those struggling with worsening OCD symptoms as a result of the pandemic.
References
1. Lewis’s Child and Adolescent Psychiatry: A Comprehensive Textbook, 4th ed. (Baltimore: Lippincott Williams & Wilkins, 2020, pp. 518-27).
2. J Amer Acad Child Adol Psychiatry. 2012;51(1):98-113.
3. J Clin. Invest. 2009;119(4):737-46.
4. Arch Dis Child. 2015;100(5):495-9.
5. J Develop Behav Pediatr. 2019 Jun;40(5):377-82.
6. Ann Clin Psychiatry. 2008 Oct-Dec;20(4):199-203.
Max is an 8-year-old boy in the third grade, and you have been his pediatrician since birth. Described as “emotional” and “particular” since his early years, Max is prone to prolonged tantrums that have not improved with age. Parents have described a tic that involves repeatedly touching his ear, but this has not been observed in the office setting. Max has struggled with some attention issues at school, and often needs help finishing assignments. The family is feeling increasingly desperate for ways to manage his near-daily meltdowns at home, and parenting strategies you’ve discussed thus far don’t seem to be helping much. Should obsessive-compulsive disorder be in your differential? And at what point do you seek outside evaluation?
OCD is a condition characterized by recurrent, intrusive, and unwanted thoughts, images, and urges (obsessions), and repetitive behaviors or mental acts performed in a particular way to reduce anxiety (compulsions). It affects 1%-3% of children, and onset can be as early as age 3-4 years. While the average age of onset in children is approximately 10 years old, average age of diagnosis is at least several years later.1 A primary care physician’s ability to recognize OCD symptoms in children, perform an initial assessment, and connect the child to appropriate clinical care is key to reducing the years of difficulty that children and families often endure prior to beginning treatment.
Common obsessions in children include contamination, fear of harm to self or others, symmetry, and the belief that bad things will occur if rituals are performed incorrectly. Common compulsions include checking, washing, ordering, and mental acts such as praying or counting to one’s self.1,2 In addition to the fact that OCD presentations are highly heterogeneous, early diagnosis is challenging due to significant overlap of OCD symptoms with developmentally normal behaviors. For example, magical or superstitious thinking is common among school-age children who avoid stepping on cracks or utilize lucky numbers. What differentiates OCD is the presence of obsessions and/or compulsions that are time consuming and cause subjective distress or functional impairment. Children often are adept at keeping OCD symptoms secret. At time of diagnosis, the child may have a complex array of discreet behaviors to manage distress and minimize shame. Children may not have insight into the irrationality of their thoughts or behaviors, but they are certainly aware of how terrible and confused they feel inside, and how it affects their relationship with their parents. Rituals, such as those that delay bedtime or cause school tardiness, may look like oppositional behaviors and cause immense frustration for parents.
Comorbidities are common and include ADHD, oppositional defiant disorder, depression, and Tourette syndrome.3 Nearly 60% of children with OCD meet criteria for a tic disorder at some point in their lifetime.4 Compulsions designed to ease a feeling of internal discomfort, such as touching or tapping, are particularly typical of patients with OCD and comorbid tics. Often these children will express a need for things to be “just right,” with lasting relief from such a feeling rarely found. While sensory intolerances are not part of OCD’s diagnostic criteria, clinical experiences and growing research point to a high prevalence in affected children.5,6 Sensory intolerances may even be the primary presenting problem. Examples include clothing feeling uncomfortable, or inability to tolerate certain smells or innocuous sounds.
The preferred method for assessment of OCD in children is the Children’s Yale–Brown Obsessive Compulsive Scale (CY-BOCS), a semi-structured, clinician-rated interview designed to elicit symptoms, severity, and distress. While time constraints may prevent use of the CY-BOCS in the primary care setting, a handful of screening questions instead can go a long way. These might include:
- Do you have to do things in a certain way, such as washing or making things “just right?”
- What happens if you can’t do things in a certain way?
- Do you have unwanted thoughts that keep coming back and are hard to get rid of?
Equally as important as understanding a child’s OCD symptoms is understanding how the family has, often unwittingly, become intertwined in a web of OCD-driven behaviors. In an effort to soothe the child, prevent emotional outbursts, or simply get through the day, parents may find themselves accommodating behaviors that seem irrational. Despite parents’ best intentions, this is likely reinforcing OCD patterns. Parents may be asked by the child to repeat a reassuring phrase in a certain way, arrange furniture “just so,” or drive a certain route to school. In the case of contamination fears, a child may be taking several showers per day, using two bottles of shampoo per week, and demanding that his or her clothes be washed separately before a parent begins to realize the cumulative impact of these unusual behaviors on the household. In addition to exploring concerns, primary care physicians can provide a sounding board for exhausted parents wondering if other families face the same thing. While connecting the family to treatment, they also can provide reassurances that treatment can dramatically shift the trajectory of the illness.
Treatment of pediatric OCD begins with a specific form of cognitive behavioral therapy (CBT) called Exposure and Response Prevention therapy (ERP). ERP requires a skilled therapist, and a strong alliance with a child and family because the child will be asked to gradually challenge compulsions head-on and tolerate the accompanied distress. CBT/ERP is associated with a 40%-65% reduction in symptoms, but combination with SSRI therapy improves outcomes in more severe cases.3 Despite limited mental health resources and long wait lists in many parts of the country, connection to OCD-specific treatment is increasingly feasible in virtual format via online support groups and telemedicine.
“Max” may experience any number of OCD-related symptoms that a primary care physician could deftly uncover. He may become “stuck” at school because his handwriting accidentally strayed below the line. He may have hours-long meltdowns because his hair never feels right. He may touch his ear to prevent tragic harm coming to his mother. Whatever further exploration reveals, Max and his family stand to benefit immensely from early detection and intervention.
Dr. McGowan is assistant professor of psychiatry and pediatrics at the Vermont Center for Children, Youth, and Families, University of Vermont Medical Center, Burlington. She had no relevant financial disclosures. Email Dr. McGowan at [email protected].
Resources for providers and families*
UNSTUCK: An OCD Kids Movie. Featuring a 23-minute documentary film about children living with OCD, this website also is rich in OCD-related resources.
International OCD Foundation. Has information for families about OCD. Also has a resource directory for therapists, clinics, support groups, and other organizations specializing in OCD and related disorders in different geographic areas.
*Of note, both resources above include COVID-19-specific resources for those struggling with worsening OCD symptoms as a result of the pandemic.
References
1. Lewis’s Child and Adolescent Psychiatry: A Comprehensive Textbook, 4th ed. (Baltimore: Lippincott Williams & Wilkins, 2020, pp. 518-27).
2. J Amer Acad Child Adol Psychiatry. 2012;51(1):98-113.
3. J Clin. Invest. 2009;119(4):737-46.
4. Arch Dis Child. 2015;100(5):495-9.
5. J Develop Behav Pediatr. 2019 Jun;40(5):377-82.
6. Ann Clin Psychiatry. 2008 Oct-Dec;20(4):199-203.
The third surge: Are we prepared for the non-COVID crisis?
Over the last several weeks, hospitals and health systems have focused on the COVID-19 epidemic, preparing and expanding bed capacities for the surge of admissions both in intensive care and medical units. An indirect impact of this has been the reduction in outpatient staffing and resources, with the shifting of staff for inpatient care. Many areas seem to have passed the peak in the number of cases and are now seeing a plateau or downward trend in the admissions to acute care facilities.
During this period, there has been a noticeable downtrend in patients being evaluated in the ED, or admitted for decompensation of chronic conditions like heart failure, COPD and diabetes mellitus, or such acute conditions as stroke and MI. Studies from Italy and Spain, and closer to home from Atlanta and Boston, point to a significant decrease in numbers of ST-elevation myocardial infarction (STEMI) admissions.1 Duke Health saw a decrease in stroke admissions in their hospitals by 34%.2
One could argue that these patients are in fact presenting with COVID-19 or similar symptoms as is evidenced by the studies linking the severity of SARS-Co-V2 infection to chronic conditions like diabetes mellitus and obesity.2 On the other hand, the message of social isolation and avoidance of nonurgent visits could lead to delays in care resulting in patients presenting sicker and in advanced stages.3 Also, this has not been limited to the adult population. For example, reports indicate that visits to WakeMed’s pediatric emergency rooms in Wake County, N.C., were down by 60%.2
We could well be seeing a calm before the storm. While it is anticipated that there may be a second surge of COVID-19 cases, health systems would do well to be prepared for the “third surge,” consisting of patients coming in with chronic medical conditions for which they have been, so far, avoiding follow-up and managing at home, and acute medical conditions with delayed diagnoses. The impact could likely be more in the subset of patients with limited access to health care, including medications and follow-up, resulting in a disproportionate burden on safety-net hospitals.
Compounding this issue would be the economic impact of the current crisis on health systems, their staffing, and resources. Several major organizations have already proposed budget cuts and reduction of the workforce, raising significant concerns about the future of health care workers who put their lives at risk during this pandemic.4 There is no guarantee that the federal funding provided by the stimulus packages will save jobs in the health care industry. This problem needs new leadership thinking, and every organization that puts employees over profits margins will have a long-term impact on communities.
Another area of concern is a shift in resources and workflow from ambulatory to inpatient settings for the COVID-19 pandemic, and the need for revamping the ambulatory services with reshifting the workforce. As COVID-19 cases plateau, the resurgence of non-COVID–related admissions will require additional help in inpatient settings. Prioritizing the ambulatory services based on financial benefits versus patient outcomes is also a major challenge to leadership.5
Lastly, the current health care crisis has led to significant stress, both emotional and physical, among frontline caregivers, increasing the risk of burnout.6 How leadership helps health care workers to cope with these stressors, and the resources they provide, is going to play a key role in long term retention of their talent, and will reflect on the organizational culture. Though it might seem trivial, posttraumatic stress disorder related to this is already obvious, and health care leadership needs to put every effort in providing the resources to help prevent burnout, in partnership with national organizations like the Society of Hospital Medicine and the American College of Physicians.
The expansion of telemedicine has provided a unique opportunity to address several of these issues while maintaining the nonpharmacologic interventions to fight the epidemic, and keeping the cost curve as low as possible.7 Extension of these services to all ambulatory service lines, including home health and therapy, is the next big step in the new health care era. Virtual check-ins by physicians, advance practice clinicians, and home care nurses could help alleviate the concerns regarding delays in care of patients with chronic conditions, and help identify those at risk. This would also be of help with staffing shortages, and possibly provide much needed support to frontline providers.
Dr. Prasad is currently medical director of care management and a hospitalist at Advocate Aurora Health in Milwaukee. He was previously quality and utilization officer and chief of the medical staff at Aurora Sinai Medical Center. Dr. Prasad is cochair of SHM’s IT Special Interest Group, sits on the HQPS Committee, and is president of SHM’s Wisconsin Chapter. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Wood S. TCTMD. 2020 Apr 2. “The mystery of the missing STEMIs during the COVID-19 pandemic.”
2. Stradling R. The News & Observer. 2020 Apr 21. “Fewer people are going to Triangle [N.C.] emergency rooms, and that could be a bad thing.”
3. Kasanagottu K. USA Today. 2020 Apr 15. “Don’t delay care for chronic illness over coronavirus. It’s bad for you and for hospitals.”
4. Snowbeck C. The Star Tribune. 2020 Apr 11. “Mayo Clinic cutting pay for more than 20,000 workers.”
5. LaPointe J. RevCycle Intelligence. 2020 Mar 31. “How much will the COVID-19 pandemic cost hospitals?”
6. Gavidia M. AJMC. 2020 Mar 31. “Sleep, physician burnout linked amid COVID-19 pandemic.”
7. Hollander JE and Carr BG. N Engl J Med. 2020 Apr 30;382(18):1679-81. “Virtually perfect? Telemedicine for COVID-19.”
Over the last several weeks, hospitals and health systems have focused on the COVID-19 epidemic, preparing and expanding bed capacities for the surge of admissions both in intensive care and medical units. An indirect impact of this has been the reduction in outpatient staffing and resources, with the shifting of staff for inpatient care. Many areas seem to have passed the peak in the number of cases and are now seeing a plateau or downward trend in the admissions to acute care facilities.
During this period, there has been a noticeable downtrend in patients being evaluated in the ED, or admitted for decompensation of chronic conditions like heart failure, COPD and diabetes mellitus, or such acute conditions as stroke and MI. Studies from Italy and Spain, and closer to home from Atlanta and Boston, point to a significant decrease in numbers of ST-elevation myocardial infarction (STEMI) admissions.1 Duke Health saw a decrease in stroke admissions in their hospitals by 34%.2
One could argue that these patients are in fact presenting with COVID-19 or similar symptoms as is evidenced by the studies linking the severity of SARS-Co-V2 infection to chronic conditions like diabetes mellitus and obesity.2 On the other hand, the message of social isolation and avoidance of nonurgent visits could lead to delays in care resulting in patients presenting sicker and in advanced stages.3 Also, this has not been limited to the adult population. For example, reports indicate that visits to WakeMed’s pediatric emergency rooms in Wake County, N.C., were down by 60%.2
We could well be seeing a calm before the storm. While it is anticipated that there may be a second surge of COVID-19 cases, health systems would do well to be prepared for the “third surge,” consisting of patients coming in with chronic medical conditions for which they have been, so far, avoiding follow-up and managing at home, and acute medical conditions with delayed diagnoses. The impact could likely be more in the subset of patients with limited access to health care, including medications and follow-up, resulting in a disproportionate burden on safety-net hospitals.
Compounding this issue would be the economic impact of the current crisis on health systems, their staffing, and resources. Several major organizations have already proposed budget cuts and reduction of the workforce, raising significant concerns about the future of health care workers who put their lives at risk during this pandemic.4 There is no guarantee that the federal funding provided by the stimulus packages will save jobs in the health care industry. This problem needs new leadership thinking, and every organization that puts employees over profits margins will have a long-term impact on communities.
Another area of concern is a shift in resources and workflow from ambulatory to inpatient settings for the COVID-19 pandemic, and the need for revamping the ambulatory services with reshifting the workforce. As COVID-19 cases plateau, the resurgence of non-COVID–related admissions will require additional help in inpatient settings. Prioritizing the ambulatory services based on financial benefits versus patient outcomes is also a major challenge to leadership.5
Lastly, the current health care crisis has led to significant stress, both emotional and physical, among frontline caregivers, increasing the risk of burnout.6 How leadership helps health care workers to cope with these stressors, and the resources they provide, is going to play a key role in long term retention of their talent, and will reflect on the organizational culture. Though it might seem trivial, posttraumatic stress disorder related to this is already obvious, and health care leadership needs to put every effort in providing the resources to help prevent burnout, in partnership with national organizations like the Society of Hospital Medicine and the American College of Physicians.
The expansion of telemedicine has provided a unique opportunity to address several of these issues while maintaining the nonpharmacologic interventions to fight the epidemic, and keeping the cost curve as low as possible.7 Extension of these services to all ambulatory service lines, including home health and therapy, is the next big step in the new health care era. Virtual check-ins by physicians, advance practice clinicians, and home care nurses could help alleviate the concerns regarding delays in care of patients with chronic conditions, and help identify those at risk. This would also be of help with staffing shortages, and possibly provide much needed support to frontline providers.
Dr. Prasad is currently medical director of care management and a hospitalist at Advocate Aurora Health in Milwaukee. He was previously quality and utilization officer and chief of the medical staff at Aurora Sinai Medical Center. Dr. Prasad is cochair of SHM’s IT Special Interest Group, sits on the HQPS Committee, and is president of SHM’s Wisconsin Chapter. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Wood S. TCTMD. 2020 Apr 2. “The mystery of the missing STEMIs during the COVID-19 pandemic.”
2. Stradling R. The News & Observer. 2020 Apr 21. “Fewer people are going to Triangle [N.C.] emergency rooms, and that could be a bad thing.”
3. Kasanagottu K. USA Today. 2020 Apr 15. “Don’t delay care for chronic illness over coronavirus. It’s bad for you and for hospitals.”
4. Snowbeck C. The Star Tribune. 2020 Apr 11. “Mayo Clinic cutting pay for more than 20,000 workers.”
5. LaPointe J. RevCycle Intelligence. 2020 Mar 31. “How much will the COVID-19 pandemic cost hospitals?”
6. Gavidia M. AJMC. 2020 Mar 31. “Sleep, physician burnout linked amid COVID-19 pandemic.”
7. Hollander JE and Carr BG. N Engl J Med. 2020 Apr 30;382(18):1679-81. “Virtually perfect? Telemedicine for COVID-19.”
Over the last several weeks, hospitals and health systems have focused on the COVID-19 epidemic, preparing and expanding bed capacities for the surge of admissions both in intensive care and medical units. An indirect impact of this has been the reduction in outpatient staffing and resources, with the shifting of staff for inpatient care. Many areas seem to have passed the peak in the number of cases and are now seeing a plateau or downward trend in the admissions to acute care facilities.
During this period, there has been a noticeable downtrend in patients being evaluated in the ED, or admitted for decompensation of chronic conditions like heart failure, COPD and diabetes mellitus, or such acute conditions as stroke and MI. Studies from Italy and Spain, and closer to home from Atlanta and Boston, point to a significant decrease in numbers of ST-elevation myocardial infarction (STEMI) admissions.1 Duke Health saw a decrease in stroke admissions in their hospitals by 34%.2
One could argue that these patients are in fact presenting with COVID-19 or similar symptoms as is evidenced by the studies linking the severity of SARS-Co-V2 infection to chronic conditions like diabetes mellitus and obesity.2 On the other hand, the message of social isolation and avoidance of nonurgent visits could lead to delays in care resulting in patients presenting sicker and in advanced stages.3 Also, this has not been limited to the adult population. For example, reports indicate that visits to WakeMed’s pediatric emergency rooms in Wake County, N.C., were down by 60%.2
We could well be seeing a calm before the storm. While it is anticipated that there may be a second surge of COVID-19 cases, health systems would do well to be prepared for the “third surge,” consisting of patients coming in with chronic medical conditions for which they have been, so far, avoiding follow-up and managing at home, and acute medical conditions with delayed diagnoses. The impact could likely be more in the subset of patients with limited access to health care, including medications and follow-up, resulting in a disproportionate burden on safety-net hospitals.
Compounding this issue would be the economic impact of the current crisis on health systems, their staffing, and resources. Several major organizations have already proposed budget cuts and reduction of the workforce, raising significant concerns about the future of health care workers who put their lives at risk during this pandemic.4 There is no guarantee that the federal funding provided by the stimulus packages will save jobs in the health care industry. This problem needs new leadership thinking, and every organization that puts employees over profits margins will have a long-term impact on communities.
Another area of concern is a shift in resources and workflow from ambulatory to inpatient settings for the COVID-19 pandemic, and the need for revamping the ambulatory services with reshifting the workforce. As COVID-19 cases plateau, the resurgence of non-COVID–related admissions will require additional help in inpatient settings. Prioritizing the ambulatory services based on financial benefits versus patient outcomes is also a major challenge to leadership.5
Lastly, the current health care crisis has led to significant stress, both emotional and physical, among frontline caregivers, increasing the risk of burnout.6 How leadership helps health care workers to cope with these stressors, and the resources they provide, is going to play a key role in long term retention of their talent, and will reflect on the organizational culture. Though it might seem trivial, posttraumatic stress disorder related to this is already obvious, and health care leadership needs to put every effort in providing the resources to help prevent burnout, in partnership with national organizations like the Society of Hospital Medicine and the American College of Physicians.
The expansion of telemedicine has provided a unique opportunity to address several of these issues while maintaining the nonpharmacologic interventions to fight the epidemic, and keeping the cost curve as low as possible.7 Extension of these services to all ambulatory service lines, including home health and therapy, is the next big step in the new health care era. Virtual check-ins by physicians, advance practice clinicians, and home care nurses could help alleviate the concerns regarding delays in care of patients with chronic conditions, and help identify those at risk. This would also be of help with staffing shortages, and possibly provide much needed support to frontline providers.
Dr. Prasad is currently medical director of care management and a hospitalist at Advocate Aurora Health in Milwaukee. He was previously quality and utilization officer and chief of the medical staff at Aurora Sinai Medical Center. Dr. Prasad is cochair of SHM’s IT Special Interest Group, sits on the HQPS Committee, and is president of SHM’s Wisconsin Chapter. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Wood S. TCTMD. 2020 Apr 2. “The mystery of the missing STEMIs during the COVID-19 pandemic.”
2. Stradling R. The News & Observer. 2020 Apr 21. “Fewer people are going to Triangle [N.C.] emergency rooms, and that could be a bad thing.”
3. Kasanagottu K. USA Today. 2020 Apr 15. “Don’t delay care for chronic illness over coronavirus. It’s bad for you and for hospitals.”
4. Snowbeck C. The Star Tribune. 2020 Apr 11. “Mayo Clinic cutting pay for more than 20,000 workers.”
5. LaPointe J. RevCycle Intelligence. 2020 Mar 31. “How much will the COVID-19 pandemic cost hospitals?”
6. Gavidia M. AJMC. 2020 Mar 31. “Sleep, physician burnout linked amid COVID-19 pandemic.”
7. Hollander JE and Carr BG. N Engl J Med. 2020 Apr 30;382(18):1679-81. “Virtually perfect? Telemedicine for COVID-19.”
For Indigenous communities, climate crisis could prove calamitous
Drought, fires, and pandemics lead to anxiety, depression, trauma
Kind wishes and donations worldwide came to help Australian communities and wildlife affected by the extreme drought and uncontrollable bushfires. Indeed, Australians have become a warning beacon for the planet to recognize how factors associated with global warming can morph rapidly into runaway national emergencies.
Little attention, however, has addressed the extreme vulnerability of Australia’s First Nations people, the Aboriginal & Torres Strait Islander communities, to the climate crisis. U.N. reports conclude that “Indigenous people with close emotional and ancestral ties to the land are also likely to be disproportionately affected by environmental change and extreme weather events.”1
In fact, Indigenous peoples, whether living traditionally or assimilated, are among the first to be adversely affected by climate change. This is because, in part, of extreme poverty, inadequate housing, unemployment and other social determinants, transgenerational cultural losses of life and culture, dislocations, traumatic experiences of child removal, overrepresentation in the prison system, and chronic diseases already leading to dramatic disparities in life expectancy and other health outcomes.
Research confirms that rural and remote Aboriginal communities will be Australia’s first mass climate refugees. “Without action to stop climate change, people will be forced to leave their country and leave behind much of what makes them Aboriginal.”2 This is because of hotter temperatures, poorly built and unstable homes more vulnerable to heat, and longer and drier droughts. Their communities, in fire-prone townships, are running out of water. Abject poverty severely limits their options, aggravated by government inaction because of ideological climate change denialism. And now we have the overlay of COVID-19 threatening these communities.3
Human pandemics are potentially more likely to occur with climate change. Pandemics also are more apt to be associated with population growth, human settlement encroaching on forests, increasing wild animal or intermediary vector contact, and growth in global travel.
Subsequently, Spatial separation is difficult in overcrowded, multigenerational households. It is hard to keep your hands washed with soap where reliable water supply is sometimes only communal. Their health workers’ access to protective and lifesaving ICU equipment and expertise may be extremely limited or erratic.
Much of the population is classified as highly vulnerable to COVID-19 because of chronic health disorders (for example, cardiovascular, respiratory, and renal issues; diabetes, and suicidality) and preexisting much shorter life expectancies. Their health workers’ access to protective and lifesaving ICU equipment and expertise is extremely limited. There are fears that, if COVID-19 gains a foothold, they may lose a whole generation of revered elders, who often are also the last fluent tribal language speakers and carriers of life-enhancing cultural stories, traditions, and rites. More urban-living Indigenous families may yet have a rough time avoiding these ravages.
In Australia, COVID-19 has been largely held at bay so far by state and territory governments that have closed borders, restricted nonessential travel, and discouraged or excluded outsiders from visiting remote Indigenous communities wherever possible. There have been complaints that such restrictions occasionally had been applied in these communities in a heavy-handed way by police and other authorities, and may be resisted if enforced unilaterally. They will work only if applied with cultural sensitivity, full Indigenous community consultation, and collaboration. So far, COVID-19 infection rates have been kept very low, with no Indigenous deaths. In Brazil, by contrast, infections and deaths are more than double the national average, itinerant missionaries have only just been excluded from Amazonian tribal lands so far by independent judicial intervention, while loggers and miners come and go freely, as sources of contagion.4 Some Indigenous peoples in the United States have experienced among the highest COVID-19 infection and death rates in the country (for example, the Navajo Nation in New Mexico, Arizona, and Utah), amounting to catastrophic loss and grief.
"Black Lives Matter" marches protesting the filmed police killing of George Floyd in the USA have spread worldwide, in the wake of ultra-high rates of police brutality and killings with impunity of non-white individuals.
Many Australians, including considerable numbers of Indigenous people, marched here in sympathy, despite their infective risk and vulnerabilities. They were also protesting the excessive rates of Aboriginal imprisonment, deaths in custody, and police killings without consequences. Both internationally and here, there was an apparent sense of release of pent-up anger and frustrations at both these injustices and the extra susceptibility of poor and non-white people to severe illness, death and dire economic consequences because of the pandemic. It is a deceptive myth that "we are all in this together." So it is encouraging that there is also forming a widespread sense of collective purpose and determination to get governments to address these iniquities and inequities at last.*
I have worked as a community psychiatrist in Barkinje Aboriginal tribal lands of the Far West region of New South Wales (NSW) regularly for 35 years, much of this time while also leading Royal North Shore University General Hospital & Community Mental Health Services in Sydney. Barkinje translates as “River People,” but local media mainly talk about the impact of prolonged drought on farmers and ranchers, who certainly are deeply affected by it. However, the media rarely mention the calamitous impacts on Aboriginal communities. The drought effects are exacerbated by multinational corporate irrigators that divert and allegedly steal river water with tacit encouragement from ostensibly responsible government ministers. The rivers dry up into algal ponds with millions of bloated, rotting dead fish, and entire communities’ water supplies fail.
Researchers have reported on the mental health impacts of prolonged drought and diversion of river water on rural and remote indigenous communities throughout the state of NSW.5 We have heard Barkinje and neighboring Wiradjuri people say, “if the land is sick, we are sick,” and, “if the river dries up, there’s nowhere to meet.” Fishing, a popular recreational activity and source of nutrition is now denied to these communities. Unlike farmers, they receive no governmental exceptional circumstance compensation payments during droughts. Instead, they lose their farming jobs, so there is no disposable income and loss of capacity to travel to connect to their extended kinship system and cultural roots (e.g., for funerals or football matches) in other remote townships. Such droughts exacerbate wildfires, loss of fish and birdlife, some of which are sacred spiritual totems; dying of traditional “lifeblood” rivers, decimating precious ancient red-river gumtrees that line the shores; and irreversible damage to other sacred sites (e.g., melting ancient rock art).
So, loss of sustainable food sources, meaningful livelihood, and cultural and leisure pursuits could create an existential threat to Aboriginal identity. However, rural Indigenous communities also told us “whatever you do to us, we will survive and persist, as we have done in the past.”6 This is comparable with the tenacity and resilience of other ancient cultures that have suffered genocidal persecution and discrimination in the past, and have stubbornly regrown and persisted and regrown into the future.
They yearn to care for their lands, rivers, and seas of their traditions and upbringing, whether as “saltwater” coastal or “freshwater” inland peoples. They value their extended families, honor their elders and their collective wisdom, while also living in “two worlds.” They often encourage their children to get educated and pursue individualistic aspirations to help their communities by training as tradespeople and professionals who may be better trusted to look after their own. As Charles Perkins, a most celebrated Aboriginal role model for living in both worlds, famously said: “We know we can’t live in the past, but the past lives in us.”
As anxiety and depression, psychological trauma, drug and alcohol misuse, family and communal violence, ecological grief,and suicidal vulnerability are precipitated or exacerbated by the stress of extreme environmental adversity, significant investment in ameliorating these harms is essential, not just for farmers, town businesspeople and their families, but for all those affected, especially these most vulnerable members of the community.7 We must provide more essential community services controlled by Aboriginal community members themselves. We must also train and support more Aboriginal mental health workers, healers, mental health educators, peer workers and Aboriginal liaison officers, to work alongside other mental health, and health and social service professionals. Aboriginal people need stable local employment opportunities in their communities. There is a huge opportunity to synergize traditional indigenous fire management with Western techniques, creating and consolidating more valued jobs and respected land management roles for Aboriginal rangers, vital for the future of both Aboriginal and wider communities. Pilot programs are emerging.
Aboriginal communities also need a more preventive, whole-of-life approach to social determinants, lifestyle factors, trauma, and political decisions associated with compromised neurodevelopment, and increased subsequent incidence and severity of mental illnesses in their communities.7
As Alexander Solzhenitsyn observed: “On our crowded planet there are no longer any ‘internal affairs.’ ”8 Climate change is the ultimate form of globalization: What we each do about it affects all others’ lives. We can only insist that, alongside adequate resourcing of our most evidence-based methods of fire, water, and climate control, our governments consult and listen to our Indigenous elders about applying climate management methods. These have been demonstrated to be sustainable and effective, possibly over 60,000 years – which is the longest established record of continuous Indigenous culture worldwide.
References
1. Ten impacts of the Australian bushfires. U.N. Environment Programme. 2020 Jan 20.
2. Allam L, Evershed N. “Too hot for humans? First Nations people fear becoming Australia’s first climate refugees.” The Guardian. 2019 Dec 17.
3. National Indigenous Australians Agency. “Coronavirus (COVID-19).”
4. Phillips D. “Brazil: Judge bans missionaries from Indigenous reserve over COVID-19 fears.” The Guardian. 2020 Apr 17.
5. Rigby CW et al. Aust J Rural Health. 2011 Oct;19(5):249-54.
6. Cunsolo A, Ellis NR. Nature Clim Change. 2018 Apr 3;8:275-81.
7. Gynther B et al. EClinicalMedicine. 2019 Apr 26;10:68-77.
8. Solzhenitsyn A. “Warning to the West,” speech delivered 30 Jun 1975. New York: Fararr, Straux & Girous, 1976.
Dr. Rosen, an officer of the Order of Australia and a Fellow of the Royal Australian and New Zealand College of Psychiatrists, is affiliated with the Brain & Mind Centre, University of Sydney, and the Institute of Mental Health at the University of Wollongong (Australia). He also is a community psychiatrist in a remote region of New South Wales. Dr. Rosen has no conflicts of interest.
*This article was updated 6/16/2020.
Drought, fires, and pandemics lead to anxiety, depression, trauma
Drought, fires, and pandemics lead to anxiety, depression, trauma
Kind wishes and donations worldwide came to help Australian communities and wildlife affected by the extreme drought and uncontrollable bushfires. Indeed, Australians have become a warning beacon for the planet to recognize how factors associated with global warming can morph rapidly into runaway national emergencies.
Little attention, however, has addressed the extreme vulnerability of Australia’s First Nations people, the Aboriginal & Torres Strait Islander communities, to the climate crisis. U.N. reports conclude that “Indigenous people with close emotional and ancestral ties to the land are also likely to be disproportionately affected by environmental change and extreme weather events.”1
In fact, Indigenous peoples, whether living traditionally or assimilated, are among the first to be adversely affected by climate change. This is because, in part, of extreme poverty, inadequate housing, unemployment and other social determinants, transgenerational cultural losses of life and culture, dislocations, traumatic experiences of child removal, overrepresentation in the prison system, and chronic diseases already leading to dramatic disparities in life expectancy and other health outcomes.
Research confirms that rural and remote Aboriginal communities will be Australia’s first mass climate refugees. “Without action to stop climate change, people will be forced to leave their country and leave behind much of what makes them Aboriginal.”2 This is because of hotter temperatures, poorly built and unstable homes more vulnerable to heat, and longer and drier droughts. Their communities, in fire-prone townships, are running out of water. Abject poverty severely limits their options, aggravated by government inaction because of ideological climate change denialism. And now we have the overlay of COVID-19 threatening these communities.3
Human pandemics are potentially more likely to occur with climate change. Pandemics also are more apt to be associated with population growth, human settlement encroaching on forests, increasing wild animal or intermediary vector contact, and growth in global travel.
Subsequently, Spatial separation is difficult in overcrowded, multigenerational households. It is hard to keep your hands washed with soap where reliable water supply is sometimes only communal. Their health workers’ access to protective and lifesaving ICU equipment and expertise may be extremely limited or erratic.
Much of the population is classified as highly vulnerable to COVID-19 because of chronic health disorders (for example, cardiovascular, respiratory, and renal issues; diabetes, and suicidality) and preexisting much shorter life expectancies. Their health workers’ access to protective and lifesaving ICU equipment and expertise is extremely limited. There are fears that, if COVID-19 gains a foothold, they may lose a whole generation of revered elders, who often are also the last fluent tribal language speakers and carriers of life-enhancing cultural stories, traditions, and rites. More urban-living Indigenous families may yet have a rough time avoiding these ravages.
In Australia, COVID-19 has been largely held at bay so far by state and territory governments that have closed borders, restricted nonessential travel, and discouraged or excluded outsiders from visiting remote Indigenous communities wherever possible. There have been complaints that such restrictions occasionally had been applied in these communities in a heavy-handed way by police and other authorities, and may be resisted if enforced unilaterally. They will work only if applied with cultural sensitivity, full Indigenous community consultation, and collaboration. So far, COVID-19 infection rates have been kept very low, with no Indigenous deaths. In Brazil, by contrast, infections and deaths are more than double the national average, itinerant missionaries have only just been excluded from Amazonian tribal lands so far by independent judicial intervention, while loggers and miners come and go freely, as sources of contagion.4 Some Indigenous peoples in the United States have experienced among the highest COVID-19 infection and death rates in the country (for example, the Navajo Nation in New Mexico, Arizona, and Utah), amounting to catastrophic loss and grief.
"Black Lives Matter" marches protesting the filmed police killing of George Floyd in the USA have spread worldwide, in the wake of ultra-high rates of police brutality and killings with impunity of non-white individuals.
Many Australians, including considerable numbers of Indigenous people, marched here in sympathy, despite their infective risk and vulnerabilities. They were also protesting the excessive rates of Aboriginal imprisonment, deaths in custody, and police killings without consequences. Both internationally and here, there was an apparent sense of release of pent-up anger and frustrations at both these injustices and the extra susceptibility of poor and non-white people to severe illness, death and dire economic consequences because of the pandemic. It is a deceptive myth that "we are all in this together." So it is encouraging that there is also forming a widespread sense of collective purpose and determination to get governments to address these iniquities and inequities at last.*
I have worked as a community psychiatrist in Barkinje Aboriginal tribal lands of the Far West region of New South Wales (NSW) regularly for 35 years, much of this time while also leading Royal North Shore University General Hospital & Community Mental Health Services in Sydney. Barkinje translates as “River People,” but local media mainly talk about the impact of prolonged drought on farmers and ranchers, who certainly are deeply affected by it. However, the media rarely mention the calamitous impacts on Aboriginal communities. The drought effects are exacerbated by multinational corporate irrigators that divert and allegedly steal river water with tacit encouragement from ostensibly responsible government ministers. The rivers dry up into algal ponds with millions of bloated, rotting dead fish, and entire communities’ water supplies fail.
Researchers have reported on the mental health impacts of prolonged drought and diversion of river water on rural and remote indigenous communities throughout the state of NSW.5 We have heard Barkinje and neighboring Wiradjuri people say, “if the land is sick, we are sick,” and, “if the river dries up, there’s nowhere to meet.” Fishing, a popular recreational activity and source of nutrition is now denied to these communities. Unlike farmers, they receive no governmental exceptional circumstance compensation payments during droughts. Instead, they lose their farming jobs, so there is no disposable income and loss of capacity to travel to connect to their extended kinship system and cultural roots (e.g., for funerals or football matches) in other remote townships. Such droughts exacerbate wildfires, loss of fish and birdlife, some of which are sacred spiritual totems; dying of traditional “lifeblood” rivers, decimating precious ancient red-river gumtrees that line the shores; and irreversible damage to other sacred sites (e.g., melting ancient rock art).
So, loss of sustainable food sources, meaningful livelihood, and cultural and leisure pursuits could create an existential threat to Aboriginal identity. However, rural Indigenous communities also told us “whatever you do to us, we will survive and persist, as we have done in the past.”6 This is comparable with the tenacity and resilience of other ancient cultures that have suffered genocidal persecution and discrimination in the past, and have stubbornly regrown and persisted and regrown into the future.
They yearn to care for their lands, rivers, and seas of their traditions and upbringing, whether as “saltwater” coastal or “freshwater” inland peoples. They value their extended families, honor their elders and their collective wisdom, while also living in “two worlds.” They often encourage their children to get educated and pursue individualistic aspirations to help their communities by training as tradespeople and professionals who may be better trusted to look after their own. As Charles Perkins, a most celebrated Aboriginal role model for living in both worlds, famously said: “We know we can’t live in the past, but the past lives in us.”
As anxiety and depression, psychological trauma, drug and alcohol misuse, family and communal violence, ecological grief,and suicidal vulnerability are precipitated or exacerbated by the stress of extreme environmental adversity, significant investment in ameliorating these harms is essential, not just for farmers, town businesspeople and their families, but for all those affected, especially these most vulnerable members of the community.7 We must provide more essential community services controlled by Aboriginal community members themselves. We must also train and support more Aboriginal mental health workers, healers, mental health educators, peer workers and Aboriginal liaison officers, to work alongside other mental health, and health and social service professionals. Aboriginal people need stable local employment opportunities in their communities. There is a huge opportunity to synergize traditional indigenous fire management with Western techniques, creating and consolidating more valued jobs and respected land management roles for Aboriginal rangers, vital for the future of both Aboriginal and wider communities. Pilot programs are emerging.
Aboriginal communities also need a more preventive, whole-of-life approach to social determinants, lifestyle factors, trauma, and political decisions associated with compromised neurodevelopment, and increased subsequent incidence and severity of mental illnesses in their communities.7
As Alexander Solzhenitsyn observed: “On our crowded planet there are no longer any ‘internal affairs.’ ”8 Climate change is the ultimate form of globalization: What we each do about it affects all others’ lives. We can only insist that, alongside adequate resourcing of our most evidence-based methods of fire, water, and climate control, our governments consult and listen to our Indigenous elders about applying climate management methods. These have been demonstrated to be sustainable and effective, possibly over 60,000 years – which is the longest established record of continuous Indigenous culture worldwide.
References
1. Ten impacts of the Australian bushfires. U.N. Environment Programme. 2020 Jan 20.
2. Allam L, Evershed N. “Too hot for humans? First Nations people fear becoming Australia’s first climate refugees.” The Guardian. 2019 Dec 17.
3. National Indigenous Australians Agency. “Coronavirus (COVID-19).”
4. Phillips D. “Brazil: Judge bans missionaries from Indigenous reserve over COVID-19 fears.” The Guardian. 2020 Apr 17.
5. Rigby CW et al. Aust J Rural Health. 2011 Oct;19(5):249-54.
6. Cunsolo A, Ellis NR. Nature Clim Change. 2018 Apr 3;8:275-81.
7. Gynther B et al. EClinicalMedicine. 2019 Apr 26;10:68-77.
8. Solzhenitsyn A. “Warning to the West,” speech delivered 30 Jun 1975. New York: Fararr, Straux & Girous, 1976.
Dr. Rosen, an officer of the Order of Australia and a Fellow of the Royal Australian and New Zealand College of Psychiatrists, is affiliated with the Brain & Mind Centre, University of Sydney, and the Institute of Mental Health at the University of Wollongong (Australia). He also is a community psychiatrist in a remote region of New South Wales. Dr. Rosen has no conflicts of interest.
*This article was updated 6/16/2020.
Kind wishes and donations worldwide came to help Australian communities and wildlife affected by the extreme drought and uncontrollable bushfires. Indeed, Australians have become a warning beacon for the planet to recognize how factors associated with global warming can morph rapidly into runaway national emergencies.
Little attention, however, has addressed the extreme vulnerability of Australia’s First Nations people, the Aboriginal & Torres Strait Islander communities, to the climate crisis. U.N. reports conclude that “Indigenous people with close emotional and ancestral ties to the land are also likely to be disproportionately affected by environmental change and extreme weather events.”1
In fact, Indigenous peoples, whether living traditionally or assimilated, are among the first to be adversely affected by climate change. This is because, in part, of extreme poverty, inadequate housing, unemployment and other social determinants, transgenerational cultural losses of life and culture, dislocations, traumatic experiences of child removal, overrepresentation in the prison system, and chronic diseases already leading to dramatic disparities in life expectancy and other health outcomes.
Research confirms that rural and remote Aboriginal communities will be Australia’s first mass climate refugees. “Without action to stop climate change, people will be forced to leave their country and leave behind much of what makes them Aboriginal.”2 This is because of hotter temperatures, poorly built and unstable homes more vulnerable to heat, and longer and drier droughts. Their communities, in fire-prone townships, are running out of water. Abject poverty severely limits their options, aggravated by government inaction because of ideological climate change denialism. And now we have the overlay of COVID-19 threatening these communities.3
Human pandemics are potentially more likely to occur with climate change. Pandemics also are more apt to be associated with population growth, human settlement encroaching on forests, increasing wild animal or intermediary vector contact, and growth in global travel.
Subsequently, Spatial separation is difficult in overcrowded, multigenerational households. It is hard to keep your hands washed with soap where reliable water supply is sometimes only communal. Their health workers’ access to protective and lifesaving ICU equipment and expertise may be extremely limited or erratic.
Much of the population is classified as highly vulnerable to COVID-19 because of chronic health disorders (for example, cardiovascular, respiratory, and renal issues; diabetes, and suicidality) and preexisting much shorter life expectancies. Their health workers’ access to protective and lifesaving ICU equipment and expertise is extremely limited. There are fears that, if COVID-19 gains a foothold, they may lose a whole generation of revered elders, who often are also the last fluent tribal language speakers and carriers of life-enhancing cultural stories, traditions, and rites. More urban-living Indigenous families may yet have a rough time avoiding these ravages.
In Australia, COVID-19 has been largely held at bay so far by state and territory governments that have closed borders, restricted nonessential travel, and discouraged or excluded outsiders from visiting remote Indigenous communities wherever possible. There have been complaints that such restrictions occasionally had been applied in these communities in a heavy-handed way by police and other authorities, and may be resisted if enforced unilaterally. They will work only if applied with cultural sensitivity, full Indigenous community consultation, and collaboration. So far, COVID-19 infection rates have been kept very low, with no Indigenous deaths. In Brazil, by contrast, infections and deaths are more than double the national average, itinerant missionaries have only just been excluded from Amazonian tribal lands so far by independent judicial intervention, while loggers and miners come and go freely, as sources of contagion.4 Some Indigenous peoples in the United States have experienced among the highest COVID-19 infection and death rates in the country (for example, the Navajo Nation in New Mexico, Arizona, and Utah), amounting to catastrophic loss and grief.
"Black Lives Matter" marches protesting the filmed police killing of George Floyd in the USA have spread worldwide, in the wake of ultra-high rates of police brutality and killings with impunity of non-white individuals.
Many Australians, including considerable numbers of Indigenous people, marched here in sympathy, despite their infective risk and vulnerabilities. They were also protesting the excessive rates of Aboriginal imprisonment, deaths in custody, and police killings without consequences. Both internationally and here, there was an apparent sense of release of pent-up anger and frustrations at both these injustices and the extra susceptibility of poor and non-white people to severe illness, death and dire economic consequences because of the pandemic. It is a deceptive myth that "we are all in this together." So it is encouraging that there is also forming a widespread sense of collective purpose and determination to get governments to address these iniquities and inequities at last.*
I have worked as a community psychiatrist in Barkinje Aboriginal tribal lands of the Far West region of New South Wales (NSW) regularly for 35 years, much of this time while also leading Royal North Shore University General Hospital & Community Mental Health Services in Sydney. Barkinje translates as “River People,” but local media mainly talk about the impact of prolonged drought on farmers and ranchers, who certainly are deeply affected by it. However, the media rarely mention the calamitous impacts on Aboriginal communities. The drought effects are exacerbated by multinational corporate irrigators that divert and allegedly steal river water with tacit encouragement from ostensibly responsible government ministers. The rivers dry up into algal ponds with millions of bloated, rotting dead fish, and entire communities’ water supplies fail.
Researchers have reported on the mental health impacts of prolonged drought and diversion of river water on rural and remote indigenous communities throughout the state of NSW.5 We have heard Barkinje and neighboring Wiradjuri people say, “if the land is sick, we are sick,” and, “if the river dries up, there’s nowhere to meet.” Fishing, a popular recreational activity and source of nutrition is now denied to these communities. Unlike farmers, they receive no governmental exceptional circumstance compensation payments during droughts. Instead, they lose their farming jobs, so there is no disposable income and loss of capacity to travel to connect to their extended kinship system and cultural roots (e.g., for funerals or football matches) in other remote townships. Such droughts exacerbate wildfires, loss of fish and birdlife, some of which are sacred spiritual totems; dying of traditional “lifeblood” rivers, decimating precious ancient red-river gumtrees that line the shores; and irreversible damage to other sacred sites (e.g., melting ancient rock art).
So, loss of sustainable food sources, meaningful livelihood, and cultural and leisure pursuits could create an existential threat to Aboriginal identity. However, rural Indigenous communities also told us “whatever you do to us, we will survive and persist, as we have done in the past.”6 This is comparable with the tenacity and resilience of other ancient cultures that have suffered genocidal persecution and discrimination in the past, and have stubbornly regrown and persisted and regrown into the future.
They yearn to care for their lands, rivers, and seas of their traditions and upbringing, whether as “saltwater” coastal or “freshwater” inland peoples. They value their extended families, honor their elders and their collective wisdom, while also living in “two worlds.” They often encourage their children to get educated and pursue individualistic aspirations to help their communities by training as tradespeople and professionals who may be better trusted to look after their own. As Charles Perkins, a most celebrated Aboriginal role model for living in both worlds, famously said: “We know we can’t live in the past, but the past lives in us.”
As anxiety and depression, psychological trauma, drug and alcohol misuse, family and communal violence, ecological grief,and suicidal vulnerability are precipitated or exacerbated by the stress of extreme environmental adversity, significant investment in ameliorating these harms is essential, not just for farmers, town businesspeople and their families, but for all those affected, especially these most vulnerable members of the community.7 We must provide more essential community services controlled by Aboriginal community members themselves. We must also train and support more Aboriginal mental health workers, healers, mental health educators, peer workers and Aboriginal liaison officers, to work alongside other mental health, and health and social service professionals. Aboriginal people need stable local employment opportunities in their communities. There is a huge opportunity to synergize traditional indigenous fire management with Western techniques, creating and consolidating more valued jobs and respected land management roles for Aboriginal rangers, vital for the future of both Aboriginal and wider communities. Pilot programs are emerging.
Aboriginal communities also need a more preventive, whole-of-life approach to social determinants, lifestyle factors, trauma, and political decisions associated with compromised neurodevelopment, and increased subsequent incidence and severity of mental illnesses in their communities.7
As Alexander Solzhenitsyn observed: “On our crowded planet there are no longer any ‘internal affairs.’ ”8 Climate change is the ultimate form of globalization: What we each do about it affects all others’ lives. We can only insist that, alongside adequate resourcing of our most evidence-based methods of fire, water, and climate control, our governments consult and listen to our Indigenous elders about applying climate management methods. These have been demonstrated to be sustainable and effective, possibly over 60,000 years – which is the longest established record of continuous Indigenous culture worldwide.
References
1. Ten impacts of the Australian bushfires. U.N. Environment Programme. 2020 Jan 20.
2. Allam L, Evershed N. “Too hot for humans? First Nations people fear becoming Australia’s first climate refugees.” The Guardian. 2019 Dec 17.
3. National Indigenous Australians Agency. “Coronavirus (COVID-19).”
4. Phillips D. “Brazil: Judge bans missionaries from Indigenous reserve over COVID-19 fears.” The Guardian. 2020 Apr 17.
5. Rigby CW et al. Aust J Rural Health. 2011 Oct;19(5):249-54.
6. Cunsolo A, Ellis NR. Nature Clim Change. 2018 Apr 3;8:275-81.
7. Gynther B et al. EClinicalMedicine. 2019 Apr 26;10:68-77.
8. Solzhenitsyn A. “Warning to the West,” speech delivered 30 Jun 1975. New York: Fararr, Straux & Girous, 1976.
Dr. Rosen, an officer of the Order of Australia and a Fellow of the Royal Australian and New Zealand College of Psychiatrists, is affiliated with the Brain & Mind Centre, University of Sydney, and the Institute of Mental Health at the University of Wollongong (Australia). He also is a community psychiatrist in a remote region of New South Wales. Dr. Rosen has no conflicts of interest.
*This article was updated 6/16/2020.
COVID-19 experiences from the pediatrician front line
As the COVID-19 pandemic continues to spread across the United States, several members of the Pediatric News Editorial Advisory Board shared how practices have been adapting to the pandemic, especially in terms of immunization.
Karalyn Kinsella, MD, a member of a four-pediatrician private practice in Cheshire, Conn., said in an interview that “we have been seeing only children under age 2 years for their well visits to keep them up to date on their vaccinations” as recommended by infectious disease departments at nearby hospitals such as Connecticut Children’s Medical Center. “We also are seeing the 4- and 5-year-old children for vaccinations.”
Dr. Kinsella explained that, in case parents don’t want to bring their children into the office, her staff is offering to give the vaccinations in the parking lot. But most families are coming into the office.
“We are only seeing well babies and take the parent and child back to a room as soon as they come in the office to avoid having patients sit in the waiting room. At this point, both parents and office staff are wearing masks; we are cleaning the rooms between patients,” Dr. Kinsella said.
“Most of our patients are coming in for their vaccines, so I don’t anticipate a lot of kids being behind. However, we will have a surge of all the physicals that need to be done prior to school in the fall. We have thought about opening up for the weekends for physicals to accommodate this. We also may need to start the day earlier and end later. I have heard some schools may be postponing the date the physicals are due.”
Because of a lack of full personal protective equipment, the practice has not been seeing sick visits in the office, but they have been doing a lot of telehealth visits. “We have been using doxy.me for that, which is free, incredibly easy to use, and Health Insurance Portability and Accountability Act (HIPAA)–compliant,” she said. “I am finding some visits, such as ADHD follow-ups and mental health follow-ups, very amenable to telehealth.”
“The hardest part – as I am sure is for most pediatricians – is the financial strain to a small business,” Dr. Kinsella noted. “We are down about 70% in revenue from this time last year. We have had to lay-off half our staff, and those who are working have much-reduced hours. We did not get the first round of funding for the paycheck protection program loan from the government and are waiting on the second round. We are trying to recoup some business by doing telehealth, but [the insurance companies] are only paying about 75%-80%. We also are charging for phone calls over 5 minutes. It will take a long time once we are up and running to recoup the losses.
“When this is all over, I’m hoping that we will be able to continue to incorporate telehealth into our schedules as I think it is convenient for families. I also am hoping that pediatricians continue to bill for phone calls as we have been giving out a lot of free care prior to this. I hope the American Academy of Pediatrics and all pediatricians work together to advocate for payment of these modalities,” she said.
J. Howard Smart, MD, who is chairman of the department of pediatrics at Sharp Rees-Stealy Medical Group in San Diego, said in an interview, “We have been bringing all of the infants and toddlers in for checkups and vaccines up to age 18 months.” These visits are scheduled in the morning, and sick patients are scheduled in the afternoon. “Well-child visits for older ages are being done by video, and the kindergarten and adolescent vaccines can be done by quick nurse visits. We will have some catching up to do once restrictions are lifted.”
“A fair amount of discussion went into these decisions. Is a video checkup better than no checkup? There is no clear-cut answer. Important things can be addressed by video: lifestyle, diet, exercise, family coping with stay-at-home orders, maintaining healthy childhood relationships, Internet use, ongoing education, among others. We know that we may miss things that can only be picked up by physical examination: hypertension, heart murmurs, abnormal growth, sexual development, abdominal masses, subtle strabismus. This is why we need to bring these children back for the physical exam later,” Dr. Smart emphasized.
“One possible negative result of doing the ‘well-child check’ by video would be if the parent assumed that the ‘checkup’ was done, never brought the child back for the exam, and something was missed that needed intervention. It will be important to get the message across that the return visit is needed. The American Academy of Pediatrics made this a part of their recommendations. It is going to be important for payers to realize that we need to do both visits – and to pay accordingly,” he concluded.
Francis E. Rushton Jr., MD, of Birmingham, Ala., described in an interview how the pediatricians in his former practice are looking for new ways to encourage shot administration in a timely manner during the COVID-19 pandemic, as well as exploring ways to partner with home visitors in encouraging timely infant and toddler vaccinations.
At South Carolina’s Beaufort Pediatrics, Joseph Floyd, MD, described a multipronged initiative. The practice’s well-child visit reminder system is being reprogrammed to check for lapses in vaccinations rather than just well-child visit attendance. For the most part, Dr. Floyd stated parents appreciate the reminders and accept the need for vaccination: “In the absence of immunizations for coronavirus, families seem to be more cognizant of the value of the vaccines we do have.” Beaufort Pediatrics is also partnering with their local hospital on a publicity campaign stressing the importance of staying up to date with currently available and recommended vaccines.
Other child-service organizations are concerned as well. Dr. Francis E. Rushton Jr., as faculty with the Education Development Center’s Health Resources and Services Administration–funded home-visiting quality improvement collaborative (HV CoIIN 2.0), described efforts with home visitors in Alabama and other states. “Home visitors understand the importance of immunizations to the health and welfare of the infants they care for. They’re looking for opportunities to improve compliance with vaccination regimens.” Some of these home-visiting agencies are employing quality improvement technique to improve compliance. One idea they are working on is documenting annual training on updated vaccines for the home visitors. They are working on protocols for linking their clients with primary health care providers, referral relations, and relationship development with local pediatric offices. Motivational interviewing techniques for home visitors focused on immunizations are being considered. For families who are hesitant, home visitors are considering accompanying the family when they come to the doctor’s office while paying attention to COVID-19 social distancing policies at medical facilities.
As the COVID-19 pandemic continues to spread across the United States, several members of the Pediatric News Editorial Advisory Board shared how practices have been adapting to the pandemic, especially in terms of immunization.
Karalyn Kinsella, MD, a member of a four-pediatrician private practice in Cheshire, Conn., said in an interview that “we have been seeing only children under age 2 years for their well visits to keep them up to date on their vaccinations” as recommended by infectious disease departments at nearby hospitals such as Connecticut Children’s Medical Center. “We also are seeing the 4- and 5-year-old children for vaccinations.”
Dr. Kinsella explained that, in case parents don’t want to bring their children into the office, her staff is offering to give the vaccinations in the parking lot. But most families are coming into the office.
“We are only seeing well babies and take the parent and child back to a room as soon as they come in the office to avoid having patients sit in the waiting room. At this point, both parents and office staff are wearing masks; we are cleaning the rooms between patients,” Dr. Kinsella said.
“Most of our patients are coming in for their vaccines, so I don’t anticipate a lot of kids being behind. However, we will have a surge of all the physicals that need to be done prior to school in the fall. We have thought about opening up for the weekends for physicals to accommodate this. We also may need to start the day earlier and end later. I have heard some schools may be postponing the date the physicals are due.”
Because of a lack of full personal protective equipment, the practice has not been seeing sick visits in the office, but they have been doing a lot of telehealth visits. “We have been using doxy.me for that, which is free, incredibly easy to use, and Health Insurance Portability and Accountability Act (HIPAA)–compliant,” she said. “I am finding some visits, such as ADHD follow-ups and mental health follow-ups, very amenable to telehealth.”
“The hardest part – as I am sure is for most pediatricians – is the financial strain to a small business,” Dr. Kinsella noted. “We are down about 70% in revenue from this time last year. We have had to lay-off half our staff, and those who are working have much-reduced hours. We did not get the first round of funding for the paycheck protection program loan from the government and are waiting on the second round. We are trying to recoup some business by doing telehealth, but [the insurance companies] are only paying about 75%-80%. We also are charging for phone calls over 5 minutes. It will take a long time once we are up and running to recoup the losses.
“When this is all over, I’m hoping that we will be able to continue to incorporate telehealth into our schedules as I think it is convenient for families. I also am hoping that pediatricians continue to bill for phone calls as we have been giving out a lot of free care prior to this. I hope the American Academy of Pediatrics and all pediatricians work together to advocate for payment of these modalities,” she said.
J. Howard Smart, MD, who is chairman of the department of pediatrics at Sharp Rees-Stealy Medical Group in San Diego, said in an interview, “We have been bringing all of the infants and toddlers in for checkups and vaccines up to age 18 months.” These visits are scheduled in the morning, and sick patients are scheduled in the afternoon. “Well-child visits for older ages are being done by video, and the kindergarten and adolescent vaccines can be done by quick nurse visits. We will have some catching up to do once restrictions are lifted.”
“A fair amount of discussion went into these decisions. Is a video checkup better than no checkup? There is no clear-cut answer. Important things can be addressed by video: lifestyle, diet, exercise, family coping with stay-at-home orders, maintaining healthy childhood relationships, Internet use, ongoing education, among others. We know that we may miss things that can only be picked up by physical examination: hypertension, heart murmurs, abnormal growth, sexual development, abdominal masses, subtle strabismus. This is why we need to bring these children back for the physical exam later,” Dr. Smart emphasized.
“One possible negative result of doing the ‘well-child check’ by video would be if the parent assumed that the ‘checkup’ was done, never brought the child back for the exam, and something was missed that needed intervention. It will be important to get the message across that the return visit is needed. The American Academy of Pediatrics made this a part of their recommendations. It is going to be important for payers to realize that we need to do both visits – and to pay accordingly,” he concluded.
Francis E. Rushton Jr., MD, of Birmingham, Ala., described in an interview how the pediatricians in his former practice are looking for new ways to encourage shot administration in a timely manner during the COVID-19 pandemic, as well as exploring ways to partner with home visitors in encouraging timely infant and toddler vaccinations.
At South Carolina’s Beaufort Pediatrics, Joseph Floyd, MD, described a multipronged initiative. The practice’s well-child visit reminder system is being reprogrammed to check for lapses in vaccinations rather than just well-child visit attendance. For the most part, Dr. Floyd stated parents appreciate the reminders and accept the need for vaccination: “In the absence of immunizations for coronavirus, families seem to be more cognizant of the value of the vaccines we do have.” Beaufort Pediatrics is also partnering with their local hospital on a publicity campaign stressing the importance of staying up to date with currently available and recommended vaccines.
Other child-service organizations are concerned as well. Dr. Francis E. Rushton Jr., as faculty with the Education Development Center’s Health Resources and Services Administration–funded home-visiting quality improvement collaborative (HV CoIIN 2.0), described efforts with home visitors in Alabama and other states. “Home visitors understand the importance of immunizations to the health and welfare of the infants they care for. They’re looking for opportunities to improve compliance with vaccination regimens.” Some of these home-visiting agencies are employing quality improvement technique to improve compliance. One idea they are working on is documenting annual training on updated vaccines for the home visitors. They are working on protocols for linking their clients with primary health care providers, referral relations, and relationship development with local pediatric offices. Motivational interviewing techniques for home visitors focused on immunizations are being considered. For families who are hesitant, home visitors are considering accompanying the family when they come to the doctor’s office while paying attention to COVID-19 social distancing policies at medical facilities.
As the COVID-19 pandemic continues to spread across the United States, several members of the Pediatric News Editorial Advisory Board shared how practices have been adapting to the pandemic, especially in terms of immunization.
Karalyn Kinsella, MD, a member of a four-pediatrician private practice in Cheshire, Conn., said in an interview that “we have been seeing only children under age 2 years for their well visits to keep them up to date on their vaccinations” as recommended by infectious disease departments at nearby hospitals such as Connecticut Children’s Medical Center. “We also are seeing the 4- and 5-year-old children for vaccinations.”
Dr. Kinsella explained that, in case parents don’t want to bring their children into the office, her staff is offering to give the vaccinations in the parking lot. But most families are coming into the office.
“We are only seeing well babies and take the parent and child back to a room as soon as they come in the office to avoid having patients sit in the waiting room. At this point, both parents and office staff are wearing masks; we are cleaning the rooms between patients,” Dr. Kinsella said.
“Most of our patients are coming in for their vaccines, so I don’t anticipate a lot of kids being behind. However, we will have a surge of all the physicals that need to be done prior to school in the fall. We have thought about opening up for the weekends for physicals to accommodate this. We also may need to start the day earlier and end later. I have heard some schools may be postponing the date the physicals are due.”
Because of a lack of full personal protective equipment, the practice has not been seeing sick visits in the office, but they have been doing a lot of telehealth visits. “We have been using doxy.me for that, which is free, incredibly easy to use, and Health Insurance Portability and Accountability Act (HIPAA)–compliant,” she said. “I am finding some visits, such as ADHD follow-ups and mental health follow-ups, very amenable to telehealth.”
“The hardest part – as I am sure is for most pediatricians – is the financial strain to a small business,” Dr. Kinsella noted. “We are down about 70% in revenue from this time last year. We have had to lay-off half our staff, and those who are working have much-reduced hours. We did not get the first round of funding for the paycheck protection program loan from the government and are waiting on the second round. We are trying to recoup some business by doing telehealth, but [the insurance companies] are only paying about 75%-80%. We also are charging for phone calls over 5 minutes. It will take a long time once we are up and running to recoup the losses.
“When this is all over, I’m hoping that we will be able to continue to incorporate telehealth into our schedules as I think it is convenient for families. I also am hoping that pediatricians continue to bill for phone calls as we have been giving out a lot of free care prior to this. I hope the American Academy of Pediatrics and all pediatricians work together to advocate for payment of these modalities,” she said.
J. Howard Smart, MD, who is chairman of the department of pediatrics at Sharp Rees-Stealy Medical Group in San Diego, said in an interview, “We have been bringing all of the infants and toddlers in for checkups and vaccines up to age 18 months.” These visits are scheduled in the morning, and sick patients are scheduled in the afternoon. “Well-child visits for older ages are being done by video, and the kindergarten and adolescent vaccines can be done by quick nurse visits. We will have some catching up to do once restrictions are lifted.”
“A fair amount of discussion went into these decisions. Is a video checkup better than no checkup? There is no clear-cut answer. Important things can be addressed by video: lifestyle, diet, exercise, family coping with stay-at-home orders, maintaining healthy childhood relationships, Internet use, ongoing education, among others. We know that we may miss things that can only be picked up by physical examination: hypertension, heart murmurs, abnormal growth, sexual development, abdominal masses, subtle strabismus. This is why we need to bring these children back for the physical exam later,” Dr. Smart emphasized.
“One possible negative result of doing the ‘well-child check’ by video would be if the parent assumed that the ‘checkup’ was done, never brought the child back for the exam, and something was missed that needed intervention. It will be important to get the message across that the return visit is needed. The American Academy of Pediatrics made this a part of their recommendations. It is going to be important for payers to realize that we need to do both visits – and to pay accordingly,” he concluded.
Francis E. Rushton Jr., MD, of Birmingham, Ala., described in an interview how the pediatricians in his former practice are looking for new ways to encourage shot administration in a timely manner during the COVID-19 pandemic, as well as exploring ways to partner with home visitors in encouraging timely infant and toddler vaccinations.
At South Carolina’s Beaufort Pediatrics, Joseph Floyd, MD, described a multipronged initiative. The practice’s well-child visit reminder system is being reprogrammed to check for lapses in vaccinations rather than just well-child visit attendance. For the most part, Dr. Floyd stated parents appreciate the reminders and accept the need for vaccination: “In the absence of immunizations for coronavirus, families seem to be more cognizant of the value of the vaccines we do have.” Beaufort Pediatrics is also partnering with their local hospital on a publicity campaign stressing the importance of staying up to date with currently available and recommended vaccines.
Other child-service organizations are concerned as well. Dr. Francis E. Rushton Jr., as faculty with the Education Development Center’s Health Resources and Services Administration–funded home-visiting quality improvement collaborative (HV CoIIN 2.0), described efforts with home visitors in Alabama and other states. “Home visitors understand the importance of immunizations to the health and welfare of the infants they care for. They’re looking for opportunities to improve compliance with vaccination regimens.” Some of these home-visiting agencies are employing quality improvement technique to improve compliance. One idea they are working on is documenting annual training on updated vaccines for the home visitors. They are working on protocols for linking their clients with primary health care providers, referral relations, and relationship development with local pediatric offices. Motivational interviewing techniques for home visitors focused on immunizations are being considered. For families who are hesitant, home visitors are considering accompanying the family when they come to the doctor’s office while paying attention to COVID-19 social distancing policies at medical facilities.
Advice on treating rheumatic diseases from a COVID-19 epicenter
The COVID-19 pandemic continues to pose an unprecedented challenge to health care systems worldwide. In addition to the direct impact of the disease itself, there is a growing concern related to ensuring adequate health care utilization and addressing the needs of vulnerable populations, such as those with chronic illness.
Emanuel et al. have advocated a framework of fair allocation of resources, led by the principles of equity, maximizing benefits, and prioritizing the vulnerable. In these uncertain times, patients with rheumatic diseases represent a vulnerable population whose health and wellness are particularly threatened, not only by the risk of COVID-19, but also by reduced access to usual medical care (e.g., in-person clinic visits), potential treatment interruptions (e.g., planned infusion therapies), and the ongoing shortage of hydroxychloroquine, to name a few.
As rheumatologists, we are now tasked with the development of best practices for caring for patients with rheumatic conditions in this uncertain, evolving, and nearly data-free landscape. We also must maintain an active role as advocates for our patients to help them navigate this pandemic. Herein, we discuss our approach to caring for patients with rheumatic diseases within our practice in New York City, an epicenter of the COVID-19 pandemic.
Communication with patients
Maintaining an open line of communication with our patients (by phone, patient portal, telemedicine, and so on) has become more essential than ever. It is through these communications that we best understand our patients’ concerns and provide support and personalized treatment decisions. The most common questions we have received during recent weeks are:
- Should I stop my medication to lower my risk for infection?
- Are my current symptoms caused by coronavirus, and what should I do next?
- Where can I fill my hydroxychloroquine prescription?
The American College of Rheumatology has deployed a number of task forces aimed at advocating for rheumatologists and patients with rheumatic diseases and is doing an exemplary job guiding us. For patients, several other organizations (e.g., CreakyJoints, Arthritis Foundation, Lupus Research Alliance, Vasculitis Foundation, and Scleroderma Foundation) are also providing accurate information regarding hygiene practices, social distancing, management of medications, and other guidance related to specific rheumatic diseases. In line with ACR recommendations, we encourage a personalized, shared decision-making process with each of our patients.
Patients with rheumatic disease at risk for COVID-19 infection
First, for rheumatology patients who have no COVID-19 symptoms, our management approach is individualized. For patients who are able to maintain social distancing, we have not routinely stopped immunosuppressive medications, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents. However, we discuss the risks and benefits of continuing immunosuppressive therapy during this time with all of our patients.
In certain cases of stable, non–life-threatening disease, we may consider spacing or temporarily interrupting immunosuppressive therapy, using individualized, shared decision making. Yet, it is important to recognize that, for some patients, achieving adequate disease control can require a substantial amount of time.
Furthermore, it is important to acknowledge that disease flares requiring steroid therapy may increase the risk for infection even more, keeping in mind that, in some rheumatic diseases, high disease activity itself can increase infection risk. We advise patients who are continuing therapy to maintain at least a 1-month supply of their medications.
Decisions regarding infusions in the hospital and outpatient settings are similarly made on an individual basis, weighing the risk for virus exposure against that of disease flare. The more limited availability of appropriately distanced infusion chairs in some already overburdened systems must be considered in this discussion. We agree with the ACR, whose infusion guidance recommends that “possible changes might include temporary interruption of therapy, temporary initiation of a bridge therapy such as a less potent anti-inflammatory or immune-modulating agent, or temporary change to an alternative therapy.”
We also reinforce recommended behaviors for preventing infection, including social distancing, frequent handwashing, and avoiding touching one’s face.
Patients with rheumatic disease and confirmed or suspected COVID-19 infection
With the worldwide spread of COVID-19, patients with rheumatic diseases will undoubtedly be among those exposed and infected. Though current data are limited, within a cohort from China, 1% had an autoimmune disease. Testing recommendations to confirm COVID-19 and decision guidelines for outpatient versus inpatient management are evolving, and we consult the most up-to-date, local information regarding testing as individual potential cases arise.
For patients who develop COVID-19 and are currently taking DMARDs and biologics, we recommend that they discontinue these medications, with the exception of hydroxychloroquine (HCQ). HCQ may be continued because its mechanism is not expected to worsen infection, and it plays a key role in the management of patients with systemic lupus erythematosus (SLE). In addition, in vitro antiviral effects have been reported and there is growing interest for its use in the management of COVID-19. However, there are conflicting data and methodological concerns about the nonrandomized human studies that suggest a benefit of HCQ against COVID-19.
The decision regarding management of glucocorticoids in the setting of new COVID-19 infection is challenging and should be individualized. At present, expert panels recommend against the use of glucocorticoids among individuals with COVID-19 who do not have acute respiratory distress syndrome. However, adrenal insufficiency must be considered among patients with COVID-19 who are treated with chronic glucocorticoids. Again, these decisions should be made on an individual, case-by-case basis.
Implications of a hydroxychloroquine shortage
The use of HCQ in rheumatology is supported by years of research. Particularly in SLE, HCQ has been shown to reduce disease activity and damage and to improve survival. Furthermore, for pregnant patients with SLE, numerous studies have demonstrated the safety and benefit of HCQ for both the mother and fetus; thus, it is strongly recommended. By contrast, despite the growing interest for HCQ in patients with COVID-19, the evidence is inconclusive and limited.
The ACR suggests that decisions regarding HCQ dose reductions to extend individual patients supplies should be tailored to each patient’s need and risk in the unfortunate setting of medication shortages. Even in patients with stable SLE, however, disease flares at 6 months are more common among individuals who discontinue HCQ. Of note, these flares may incorporate novel and severe disease manifestations.
Unfortunately, other therapeutic options for SLE are associated with more adverse effects (including increased susceptibility to infection) or are largely unavailable (e.g., quinacrine). Thus, we strive to continue standard dosing of HCQ for patients who are currently flaring or recently flared, and we make shared, individualized decisions for those patients with stable disease as the HCQ shortage evolves.
Future research on COVID-19 and rheumatic disease
While we might expect that an underlying rheumatic disease and associated treatments may predispose individuals to developing COVID-19, current data do not indicate which, if any, rheumatic diseases and associated therapies convey the greatest risk.
To address this uncertainty, the rheumatology community created the COVID-19 Global Rheumatology Alliance, an international effort to initiate and maintain a deidentified patient registry for individuals with rheumatic disease who develop COVID-19. These efforts will allow us to gain essential insights regarding which patient demographics, underlying diseases, and medications are most common among patients who develop COVID-19.
This alliance encourages rheumatologists and those caring for patients with rheumatic diseases to report their patient cases to this registry. As we are confronted with making management decisions with a scarcity of supporting data, efforts like these will improve our ability to make individualized treatment recommendations.
The COVID-19 pandemic has presented us all with unprecedented challenges. As rheumatologists, it is our duty to lead our patients through this uncharted territory with close communication, information, advocacy, and personalized treatment decisions. Each of these is central to the management of rheumatology patients during the COVID-19 pandemic.
With the growing interest in immunomodulatory therapies for the complications of this infection, we have the unique opportunity to share our expertise, recommendations, and caution with our colleagues. As clinicians and scientists, we must advocate for data collection and studies that will allow us to develop novel, data-driven disease management approaches while providing the best care possible for our patients.
Stephen Paget, MD, is physician in chief emeritus for the Center for Rheumatology at Hospital for Special Surgery in New York. Kimberly Showalter, MD, is a third-year rheumatology fellow at Hospital for Special Surgery. Sebastian E. Sattui, MD, is a third-year rheumatology and 1-year vasculitis fellow at Hospital for Special Surgery.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic continues to pose an unprecedented challenge to health care systems worldwide. In addition to the direct impact of the disease itself, there is a growing concern related to ensuring adequate health care utilization and addressing the needs of vulnerable populations, such as those with chronic illness.
Emanuel et al. have advocated a framework of fair allocation of resources, led by the principles of equity, maximizing benefits, and prioritizing the vulnerable. In these uncertain times, patients with rheumatic diseases represent a vulnerable population whose health and wellness are particularly threatened, not only by the risk of COVID-19, but also by reduced access to usual medical care (e.g., in-person clinic visits), potential treatment interruptions (e.g., planned infusion therapies), and the ongoing shortage of hydroxychloroquine, to name a few.
As rheumatologists, we are now tasked with the development of best practices for caring for patients with rheumatic conditions in this uncertain, evolving, and nearly data-free landscape. We also must maintain an active role as advocates for our patients to help them navigate this pandemic. Herein, we discuss our approach to caring for patients with rheumatic diseases within our practice in New York City, an epicenter of the COVID-19 pandemic.
Communication with patients
Maintaining an open line of communication with our patients (by phone, patient portal, telemedicine, and so on) has become more essential than ever. It is through these communications that we best understand our patients’ concerns and provide support and personalized treatment decisions. The most common questions we have received during recent weeks are:
- Should I stop my medication to lower my risk for infection?
- Are my current symptoms caused by coronavirus, and what should I do next?
- Where can I fill my hydroxychloroquine prescription?
The American College of Rheumatology has deployed a number of task forces aimed at advocating for rheumatologists and patients with rheumatic diseases and is doing an exemplary job guiding us. For patients, several other organizations (e.g., CreakyJoints, Arthritis Foundation, Lupus Research Alliance, Vasculitis Foundation, and Scleroderma Foundation) are also providing accurate information regarding hygiene practices, social distancing, management of medications, and other guidance related to specific rheumatic diseases. In line with ACR recommendations, we encourage a personalized, shared decision-making process with each of our patients.
Patients with rheumatic disease at risk for COVID-19 infection
First, for rheumatology patients who have no COVID-19 symptoms, our management approach is individualized. For patients who are able to maintain social distancing, we have not routinely stopped immunosuppressive medications, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents. However, we discuss the risks and benefits of continuing immunosuppressive therapy during this time with all of our patients.
In certain cases of stable, non–life-threatening disease, we may consider spacing or temporarily interrupting immunosuppressive therapy, using individualized, shared decision making. Yet, it is important to recognize that, for some patients, achieving adequate disease control can require a substantial amount of time.
Furthermore, it is important to acknowledge that disease flares requiring steroid therapy may increase the risk for infection even more, keeping in mind that, in some rheumatic diseases, high disease activity itself can increase infection risk. We advise patients who are continuing therapy to maintain at least a 1-month supply of their medications.
Decisions regarding infusions in the hospital and outpatient settings are similarly made on an individual basis, weighing the risk for virus exposure against that of disease flare. The more limited availability of appropriately distanced infusion chairs in some already overburdened systems must be considered in this discussion. We agree with the ACR, whose infusion guidance recommends that “possible changes might include temporary interruption of therapy, temporary initiation of a bridge therapy such as a less potent anti-inflammatory or immune-modulating agent, or temporary change to an alternative therapy.”
We also reinforce recommended behaviors for preventing infection, including social distancing, frequent handwashing, and avoiding touching one’s face.
Patients with rheumatic disease and confirmed or suspected COVID-19 infection
With the worldwide spread of COVID-19, patients with rheumatic diseases will undoubtedly be among those exposed and infected. Though current data are limited, within a cohort from China, 1% had an autoimmune disease. Testing recommendations to confirm COVID-19 and decision guidelines for outpatient versus inpatient management are evolving, and we consult the most up-to-date, local information regarding testing as individual potential cases arise.
For patients who develop COVID-19 and are currently taking DMARDs and biologics, we recommend that they discontinue these medications, with the exception of hydroxychloroquine (HCQ). HCQ may be continued because its mechanism is not expected to worsen infection, and it plays a key role in the management of patients with systemic lupus erythematosus (SLE). In addition, in vitro antiviral effects have been reported and there is growing interest for its use in the management of COVID-19. However, there are conflicting data and methodological concerns about the nonrandomized human studies that suggest a benefit of HCQ against COVID-19.
The decision regarding management of glucocorticoids in the setting of new COVID-19 infection is challenging and should be individualized. At present, expert panels recommend against the use of glucocorticoids among individuals with COVID-19 who do not have acute respiratory distress syndrome. However, adrenal insufficiency must be considered among patients with COVID-19 who are treated with chronic glucocorticoids. Again, these decisions should be made on an individual, case-by-case basis.
Implications of a hydroxychloroquine shortage
The use of HCQ in rheumatology is supported by years of research. Particularly in SLE, HCQ has been shown to reduce disease activity and damage and to improve survival. Furthermore, for pregnant patients with SLE, numerous studies have demonstrated the safety and benefit of HCQ for both the mother and fetus; thus, it is strongly recommended. By contrast, despite the growing interest for HCQ in patients with COVID-19, the evidence is inconclusive and limited.
The ACR suggests that decisions regarding HCQ dose reductions to extend individual patients supplies should be tailored to each patient’s need and risk in the unfortunate setting of medication shortages. Even in patients with stable SLE, however, disease flares at 6 months are more common among individuals who discontinue HCQ. Of note, these flares may incorporate novel and severe disease manifestations.
Unfortunately, other therapeutic options for SLE are associated with more adverse effects (including increased susceptibility to infection) or are largely unavailable (e.g., quinacrine). Thus, we strive to continue standard dosing of HCQ for patients who are currently flaring or recently flared, and we make shared, individualized decisions for those patients with stable disease as the HCQ shortage evolves.
Future research on COVID-19 and rheumatic disease
While we might expect that an underlying rheumatic disease and associated treatments may predispose individuals to developing COVID-19, current data do not indicate which, if any, rheumatic diseases and associated therapies convey the greatest risk.
To address this uncertainty, the rheumatology community created the COVID-19 Global Rheumatology Alliance, an international effort to initiate and maintain a deidentified patient registry for individuals with rheumatic disease who develop COVID-19. These efforts will allow us to gain essential insights regarding which patient demographics, underlying diseases, and medications are most common among patients who develop COVID-19.
This alliance encourages rheumatologists and those caring for patients with rheumatic diseases to report their patient cases to this registry. As we are confronted with making management decisions with a scarcity of supporting data, efforts like these will improve our ability to make individualized treatment recommendations.
The COVID-19 pandemic has presented us all with unprecedented challenges. As rheumatologists, it is our duty to lead our patients through this uncharted territory with close communication, information, advocacy, and personalized treatment decisions. Each of these is central to the management of rheumatology patients during the COVID-19 pandemic.
With the growing interest in immunomodulatory therapies for the complications of this infection, we have the unique opportunity to share our expertise, recommendations, and caution with our colleagues. As clinicians and scientists, we must advocate for data collection and studies that will allow us to develop novel, data-driven disease management approaches while providing the best care possible for our patients.
Stephen Paget, MD, is physician in chief emeritus for the Center for Rheumatology at Hospital for Special Surgery in New York. Kimberly Showalter, MD, is a third-year rheumatology fellow at Hospital for Special Surgery. Sebastian E. Sattui, MD, is a third-year rheumatology and 1-year vasculitis fellow at Hospital for Special Surgery.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic continues to pose an unprecedented challenge to health care systems worldwide. In addition to the direct impact of the disease itself, there is a growing concern related to ensuring adequate health care utilization and addressing the needs of vulnerable populations, such as those with chronic illness.
Emanuel et al. have advocated a framework of fair allocation of resources, led by the principles of equity, maximizing benefits, and prioritizing the vulnerable. In these uncertain times, patients with rheumatic diseases represent a vulnerable population whose health and wellness are particularly threatened, not only by the risk of COVID-19, but also by reduced access to usual medical care (e.g., in-person clinic visits), potential treatment interruptions (e.g., planned infusion therapies), and the ongoing shortage of hydroxychloroquine, to name a few.
As rheumatologists, we are now tasked with the development of best practices for caring for patients with rheumatic conditions in this uncertain, evolving, and nearly data-free landscape. We also must maintain an active role as advocates for our patients to help them navigate this pandemic. Herein, we discuss our approach to caring for patients with rheumatic diseases within our practice in New York City, an epicenter of the COVID-19 pandemic.
Communication with patients
Maintaining an open line of communication with our patients (by phone, patient portal, telemedicine, and so on) has become more essential than ever. It is through these communications that we best understand our patients’ concerns and provide support and personalized treatment decisions. The most common questions we have received during recent weeks are:
- Should I stop my medication to lower my risk for infection?
- Are my current symptoms caused by coronavirus, and what should I do next?
- Where can I fill my hydroxychloroquine prescription?
The American College of Rheumatology has deployed a number of task forces aimed at advocating for rheumatologists and patients with rheumatic diseases and is doing an exemplary job guiding us. For patients, several other organizations (e.g., CreakyJoints, Arthritis Foundation, Lupus Research Alliance, Vasculitis Foundation, and Scleroderma Foundation) are also providing accurate information regarding hygiene practices, social distancing, management of medications, and other guidance related to specific rheumatic diseases. In line with ACR recommendations, we encourage a personalized, shared decision-making process with each of our patients.
Patients with rheumatic disease at risk for COVID-19 infection
First, for rheumatology patients who have no COVID-19 symptoms, our management approach is individualized. For patients who are able to maintain social distancing, we have not routinely stopped immunosuppressive medications, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents. However, we discuss the risks and benefits of continuing immunosuppressive therapy during this time with all of our patients.
In certain cases of stable, non–life-threatening disease, we may consider spacing or temporarily interrupting immunosuppressive therapy, using individualized, shared decision making. Yet, it is important to recognize that, for some patients, achieving adequate disease control can require a substantial amount of time.
Furthermore, it is important to acknowledge that disease flares requiring steroid therapy may increase the risk for infection even more, keeping in mind that, in some rheumatic diseases, high disease activity itself can increase infection risk. We advise patients who are continuing therapy to maintain at least a 1-month supply of their medications.
Decisions regarding infusions in the hospital and outpatient settings are similarly made on an individual basis, weighing the risk for virus exposure against that of disease flare. The more limited availability of appropriately distanced infusion chairs in some already overburdened systems must be considered in this discussion. We agree with the ACR, whose infusion guidance recommends that “possible changes might include temporary interruption of therapy, temporary initiation of a bridge therapy such as a less potent anti-inflammatory or immune-modulating agent, or temporary change to an alternative therapy.”
We also reinforce recommended behaviors for preventing infection, including social distancing, frequent handwashing, and avoiding touching one’s face.
Patients with rheumatic disease and confirmed or suspected COVID-19 infection
With the worldwide spread of COVID-19, patients with rheumatic diseases will undoubtedly be among those exposed and infected. Though current data are limited, within a cohort from China, 1% had an autoimmune disease. Testing recommendations to confirm COVID-19 and decision guidelines for outpatient versus inpatient management are evolving, and we consult the most up-to-date, local information regarding testing as individual potential cases arise.
For patients who develop COVID-19 and are currently taking DMARDs and biologics, we recommend that they discontinue these medications, with the exception of hydroxychloroquine (HCQ). HCQ may be continued because its mechanism is not expected to worsen infection, and it plays a key role in the management of patients with systemic lupus erythematosus (SLE). In addition, in vitro antiviral effects have been reported and there is growing interest for its use in the management of COVID-19. However, there are conflicting data and methodological concerns about the nonrandomized human studies that suggest a benefit of HCQ against COVID-19.
The decision regarding management of glucocorticoids in the setting of new COVID-19 infection is challenging and should be individualized. At present, expert panels recommend against the use of glucocorticoids among individuals with COVID-19 who do not have acute respiratory distress syndrome. However, adrenal insufficiency must be considered among patients with COVID-19 who are treated with chronic glucocorticoids. Again, these decisions should be made on an individual, case-by-case basis.
Implications of a hydroxychloroquine shortage
The use of HCQ in rheumatology is supported by years of research. Particularly in SLE, HCQ has been shown to reduce disease activity and damage and to improve survival. Furthermore, for pregnant patients with SLE, numerous studies have demonstrated the safety and benefit of HCQ for both the mother and fetus; thus, it is strongly recommended. By contrast, despite the growing interest for HCQ in patients with COVID-19, the evidence is inconclusive and limited.
The ACR suggests that decisions regarding HCQ dose reductions to extend individual patients supplies should be tailored to each patient’s need and risk in the unfortunate setting of medication shortages. Even in patients with stable SLE, however, disease flares at 6 months are more common among individuals who discontinue HCQ. Of note, these flares may incorporate novel and severe disease manifestations.
Unfortunately, other therapeutic options for SLE are associated with more adverse effects (including increased susceptibility to infection) or are largely unavailable (e.g., quinacrine). Thus, we strive to continue standard dosing of HCQ for patients who are currently flaring or recently flared, and we make shared, individualized decisions for those patients with stable disease as the HCQ shortage evolves.
Future research on COVID-19 and rheumatic disease
While we might expect that an underlying rheumatic disease and associated treatments may predispose individuals to developing COVID-19, current data do not indicate which, if any, rheumatic diseases and associated therapies convey the greatest risk.
To address this uncertainty, the rheumatology community created the COVID-19 Global Rheumatology Alliance, an international effort to initiate and maintain a deidentified patient registry for individuals with rheumatic disease who develop COVID-19. These efforts will allow us to gain essential insights regarding which patient demographics, underlying diseases, and medications are most common among patients who develop COVID-19.
This alliance encourages rheumatologists and those caring for patients with rheumatic diseases to report their patient cases to this registry. As we are confronted with making management decisions with a scarcity of supporting data, efforts like these will improve our ability to make individualized treatment recommendations.
The COVID-19 pandemic has presented us all with unprecedented challenges. As rheumatologists, it is our duty to lead our patients through this uncharted territory with close communication, information, advocacy, and personalized treatment decisions. Each of these is central to the management of rheumatology patients during the COVID-19 pandemic.
With the growing interest in immunomodulatory therapies for the complications of this infection, we have the unique opportunity to share our expertise, recommendations, and caution with our colleagues. As clinicians and scientists, we must advocate for data collection and studies that will allow us to develop novel, data-driven disease management approaches while providing the best care possible for our patients.
Stephen Paget, MD, is physician in chief emeritus for the Center for Rheumatology at Hospital for Special Surgery in New York. Kimberly Showalter, MD, is a third-year rheumatology fellow at Hospital for Special Surgery. Sebastian E. Sattui, MD, is a third-year rheumatology and 1-year vasculitis fellow at Hospital for Special Surgery.
A version of this article originally appeared on Medscape.com.
COVID fatigue is setting in
The slow-moving game of viral roulette is wearing on everyone. Eventually, we may all become fatigued and say, “well, let’s just take our chances,” the isolation being worse than the disease. I must say, however, the sight of the local funeral director loading lumber into his van at the hardware store last week made me snug up my mask a bit. We have had a surge of COVID-19 deaths in local nursing homes and I heard refrigerated space is tight. Who knows, though, maybe he just needed more shelf space in his garage.
The most exasperating thing is not knowing who has had the virus and who hasn’t, and what medicine might or might not work. My son, quartered in the sardine-tin bunks of an aircraft carrier has “it,” as do all his mates, is in total isolation except for fever checks once a day, and is having a tough time. His eagerness to receive our phone calls was sweet at first, but is now starting to worry me. Today, I received a letter from him, which I dutifully steam-microwaved for 5 minutes and am letting dry in the sun. He is asymptomatic by the way. This was not the case for one of my buddies in New York. He suffered through 10 days of shaking chills so bad he thought he had chipped his teeth, and weeks later he still has no sense of smell.
My practice has been completely disrupted, but we are open a couple of days a week. I have kept all my employees, doing busy things mostly. There will be long hours for everyone because of widely spaced appointments and a certain amount of friction with patients who miss appointments. My fellow is going to take a long trip in July. Who knows when he will have a month off again? I wonder where he plans to go.
We have rearranged the waiting room furniture, so everyone is 6 feet apart, though I am not confident this makes a difference. We all have masks, and use alcohol gel before and after patient encounters, and spritz all fixtures and handles with alcohol after encounters. I have a large exhaust fan in the lab that creates a negative pressure gradient in the office. Somehow, I don’t think it is quite the same as in the hospital.
One slick trick we’ve enacted is running an ozone generator in the office at night, which will kill all things on all surfaces and in the air. It also is probably eroding the insides of my computers, but hey, the insects and burglars hate it too.
We heard the fighter jets fly over today saluting the frontline health care workers, but did not go out and wave. I feel a little guilt about this. Treating cancer is important, but we are not in the ICU or ED immersed in virus. That is who the jets are for.
My daughter, a high school senior, is taking the loss of graduation, prom, and pomp and circumstance quite well. I am relieved I don’t have to worry about the after-prom parties. She is gearing up for college, I just hope they allow classes to start.
The future is cloudy and uncertain, despite this beautiful spring day as I write this column. Surely the way we practice medicine is going to change, and for a long while. I am thinking of taking a part-time job out of town for a year or so, and my wife is considering closing her practice altogether. If we were a few years older, there is little doubt we would just move it down the line and retire.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. He has no disclosures. Write to him at [email protected].
The slow-moving game of viral roulette is wearing on everyone. Eventually, we may all become fatigued and say, “well, let’s just take our chances,” the isolation being worse than the disease. I must say, however, the sight of the local funeral director loading lumber into his van at the hardware store last week made me snug up my mask a bit. We have had a surge of COVID-19 deaths in local nursing homes and I heard refrigerated space is tight. Who knows, though, maybe he just needed more shelf space in his garage.
The most exasperating thing is not knowing who has had the virus and who hasn’t, and what medicine might or might not work. My son, quartered in the sardine-tin bunks of an aircraft carrier has “it,” as do all his mates, is in total isolation except for fever checks once a day, and is having a tough time. His eagerness to receive our phone calls was sweet at first, but is now starting to worry me. Today, I received a letter from him, which I dutifully steam-microwaved for 5 minutes and am letting dry in the sun. He is asymptomatic by the way. This was not the case for one of my buddies in New York. He suffered through 10 days of shaking chills so bad he thought he had chipped his teeth, and weeks later he still has no sense of smell.
My practice has been completely disrupted, but we are open a couple of days a week. I have kept all my employees, doing busy things mostly. There will be long hours for everyone because of widely spaced appointments and a certain amount of friction with patients who miss appointments. My fellow is going to take a long trip in July. Who knows when he will have a month off again? I wonder where he plans to go.
We have rearranged the waiting room furniture, so everyone is 6 feet apart, though I am not confident this makes a difference. We all have masks, and use alcohol gel before and after patient encounters, and spritz all fixtures and handles with alcohol after encounters. I have a large exhaust fan in the lab that creates a negative pressure gradient in the office. Somehow, I don’t think it is quite the same as in the hospital.
One slick trick we’ve enacted is running an ozone generator in the office at night, which will kill all things on all surfaces and in the air. It also is probably eroding the insides of my computers, but hey, the insects and burglars hate it too.
We heard the fighter jets fly over today saluting the frontline health care workers, but did not go out and wave. I feel a little guilt about this. Treating cancer is important, but we are not in the ICU or ED immersed in virus. That is who the jets are for.
My daughter, a high school senior, is taking the loss of graduation, prom, and pomp and circumstance quite well. I am relieved I don’t have to worry about the after-prom parties. She is gearing up for college, I just hope they allow classes to start.
The future is cloudy and uncertain, despite this beautiful spring day as I write this column. Surely the way we practice medicine is going to change, and for a long while. I am thinking of taking a part-time job out of town for a year or so, and my wife is considering closing her practice altogether. If we were a few years older, there is little doubt we would just move it down the line and retire.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. He has no disclosures. Write to him at [email protected].
The slow-moving game of viral roulette is wearing on everyone. Eventually, we may all become fatigued and say, “well, let’s just take our chances,” the isolation being worse than the disease. I must say, however, the sight of the local funeral director loading lumber into his van at the hardware store last week made me snug up my mask a bit. We have had a surge of COVID-19 deaths in local nursing homes and I heard refrigerated space is tight. Who knows, though, maybe he just needed more shelf space in his garage.
The most exasperating thing is not knowing who has had the virus and who hasn’t, and what medicine might or might not work. My son, quartered in the sardine-tin bunks of an aircraft carrier has “it,” as do all his mates, is in total isolation except for fever checks once a day, and is having a tough time. His eagerness to receive our phone calls was sweet at first, but is now starting to worry me. Today, I received a letter from him, which I dutifully steam-microwaved for 5 minutes and am letting dry in the sun. He is asymptomatic by the way. This was not the case for one of my buddies in New York. He suffered through 10 days of shaking chills so bad he thought he had chipped his teeth, and weeks later he still has no sense of smell.
My practice has been completely disrupted, but we are open a couple of days a week. I have kept all my employees, doing busy things mostly. There will be long hours for everyone because of widely spaced appointments and a certain amount of friction with patients who miss appointments. My fellow is going to take a long trip in July. Who knows when he will have a month off again? I wonder where he plans to go.
We have rearranged the waiting room furniture, so everyone is 6 feet apart, though I am not confident this makes a difference. We all have masks, and use alcohol gel before and after patient encounters, and spritz all fixtures and handles with alcohol after encounters. I have a large exhaust fan in the lab that creates a negative pressure gradient in the office. Somehow, I don’t think it is quite the same as in the hospital.
One slick trick we’ve enacted is running an ozone generator in the office at night, which will kill all things on all surfaces and in the air. It also is probably eroding the insides of my computers, but hey, the insects and burglars hate it too.
We heard the fighter jets fly over today saluting the frontline health care workers, but did not go out and wave. I feel a little guilt about this. Treating cancer is important, but we are not in the ICU or ED immersed in virus. That is who the jets are for.
My daughter, a high school senior, is taking the loss of graduation, prom, and pomp and circumstance quite well. I am relieved I don’t have to worry about the after-prom parties. She is gearing up for college, I just hope they allow classes to start.
The future is cloudy and uncertain, despite this beautiful spring day as I write this column. Surely the way we practice medicine is going to change, and for a long while. I am thinking of taking a part-time job out of town for a year or so, and my wife is considering closing her practice altogether. If we were a few years older, there is little doubt we would just move it down the line and retire.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. He has no disclosures. Write to him at [email protected].
A surge in PTSD may be the ‘new normal’
The prolonged and unique stresses imparted by the COVID-19 pandemic has many predicting a significant rise in mental health issues in the weeks, months, and years ahead.
To understand how health care workers can best get ahead of this emerging crisis within a crisis, Medscape Psychiatry editorial director Bret Stetka, MD, spoke with Sheila Rauch, PhD, who’s with the Department of Psychiatry and Behavioral Sciences at the Emory University, Atlanta. The director of Mental Health Research and Program Evaluation at the Atlanta VA Medical Center, Dr. Rauch has studied the effects of and best treatments for posttraumatic stress disorder (PTSD) and anxiety disorders over the past 20 years.
Are we going to see a PTSD or anxiety epidemic as a result of the pandemic?
First, I think it’s really important that we prepare for the worst but hope for the best. But I would expect that, given the high levels of stress, the impact on resources, and other factors, we are going to see a pretty significant mental health impact over time. This could be the new normal for a while. Some of that will be PTSD, but there will also be other things. I would suspect that the resulting increase in rates of depression, traumatic grief, and loss is probably going to be a significant issue for years to come.
What will the anxiety we see as a result of COVID-19 look like compared with that seen in past disasters, like 9/11?
Most disasters in recent history, like 9/11, are single incidents. Something horrible happened, it impacted people at different levels, and we were able to start putting the pieces back together right away. The prolonged nature of this pandemic makes it even more variable given that the impact is going to be extended over time.
We’re also going to see a lot more people with compound impact – people who’ve lost their jobs, loved ones, maybe even their homes. All of those financial and resource losses put people in a higher risk category for negative mental health outcomes.
Is this analogous to the prolonged trauma that can occur with military service during war?
There is some similarity there. Combat is kind of an overarching context in which people experience trauma and, much like this pandemic, may or may not have traumatic exposures during it.
We’re asking health care workers to actually be in a role similar to what we ask of our military: going into danger, sometimes even without proper protective equipment, in order to save the lives of others. That’s also something we need to be factoring in as we plan to support those people and their families.
This is an ongoing incident, but is there a time window we need to be particularly worried about for seeing spikes in anxiety and PTSD?
I think we’re going to see variability on that. PTSD is a disorder that’s related to a specific incident or a couple of incidents that are similar. It’s a memory that’s haunting you.
For instance, typically if you have a combat veteran who has PTSD, they’ve been exposed to the overarching context of combat but then they have specific memories that are stuck. If they don’t have PTSD about 3-6 months after those incidents happen, then we would expect that they will not develop it, or it’s much less common that they would.
Depression has a very different course. It’s more prolonged and tends to grow with time.
Are you already seeing increased symptoms in your patients?
This is pretty similar to what we see in combat veterans. They’ll often be unhappy with the leadership decisions that were made as they were being deployed.
We’re also seeing lots more anger, sadness, and isolation now. Especially over the past couple of weeks, we’ve seen a rise in things like people reaching out for help in our intakes because we’re still open and doing phone assessments and telehealth with veterans and the veterans program.
In terms of interventions for this, what should psychiatrists, psychologists, and other clinicians be thinking about?
Right now, the best thing that we can do as mental health providers for people affected by the trauma is provide crisis intervention for those saying they are a danger to themselves and others. That means providing coping strategies and support. It also means making sure people are taking breaks and taking care of themselves, taking that little bit of time off so that they can go back, fully recharged, to their jobs and really stay there.
As we move forward, it will be clearer whether people are going to naturally recover, which most people will. For those who are going to have ongoing problems with time, we need to be getting ready as a system and as a country for those long-term mental health issues that are going to be coming up. And when I say long-term, it means the next 1-3 months. We want to be providing preventive interventions, versions of prolonged exposure, and other things that have shown some help in preventing PTSD. Psychological first aid is helpful.
There’s also an app called COVID Coach that the National Center for PTSD has created. That features a lot of positive coping resources together in one source.
Then when we get to the middle of that point and beyond it, we need to be ready to provide those evidence-based interventions for PTSD, depression, panic disorder, and other issues that are going to come out of this current situation.
But we were already short-staffed as far as mental health resources in general across the country, and especially in rural areas. So that means finding ways to efficiently use what we have through potentially briefer versions of interventions, through primary care, mental health, and other staff.
In what ways can primary care providers help?
There are versions of prolonged exposure therapy for primary care. That’s one of my big areas of research – increasing access. That would be something that we need to be building, by training and embedding mental health providers in primary care settings so that they can help to accommodate the increased need for access that’s going to be showing up for the next, I would suspect, several years with the pandemic.
Is there evidence that a prior episode of PTSD or traumatic experience like combat influences a subsequent reaction to a trauma like this?
It depends on how they manage. Research suggests that veterans or other people who have experienced trauma and naturally recovered, or who have gotten good treatment and remitted from that issue, are probably at no higher risk. But people who have subsyndromal PTSD or depression, or who are still experiencing symptoms from a history of trauma exposure, are maybe at a higher risk of having problems over time.
Do you have any guidance for healthcare providers on how to approach the pandemic with their patients, and also on how they can look after their own mental health?
In talking to patients, make sure that they have what they need. Ask if they’ve thought through how they’re going to cope if things get harder for them.
For people who have preexisting mental health issues, I’m talking with them about whether things have gotten worse. If they’re at high risk for suicide, I’m checking in to make sure that they’ve got new plans and ways to connect with people to reduce isolation, keeping in mind the social distancing that we’re asked to engage in so that they can do that safely.
It’s important to check and see if they have had any losses, whether it’s a financial loss or a personal loss of people that they care about. Also have them think through ways to stay entertained, which tends to help manage their own anxiety.
Every coping strategy we outline for patients also applies to mental health professionals. However, you would add to it the real need to take time to recharge, to take breaks, time off. It can feel overwhelming and like you need to just keep going. But the more that you get stuck in that mode of overdoing it, the less effective you’re going to be in helping people and also the more likely that you’ll be at risk of perhaps being one of the people that needs help.
It’s also important to make sure you’re staying connected with family and friends virtually, in whatever ways you can safely do that with social distancing.
So take a break to watch some Netflix now and then?
Yes!
A version of this article originally appeared on Medscape.com.
The prolonged and unique stresses imparted by the COVID-19 pandemic has many predicting a significant rise in mental health issues in the weeks, months, and years ahead.
To understand how health care workers can best get ahead of this emerging crisis within a crisis, Medscape Psychiatry editorial director Bret Stetka, MD, spoke with Sheila Rauch, PhD, who’s with the Department of Psychiatry and Behavioral Sciences at the Emory University, Atlanta. The director of Mental Health Research and Program Evaluation at the Atlanta VA Medical Center, Dr. Rauch has studied the effects of and best treatments for posttraumatic stress disorder (PTSD) and anxiety disorders over the past 20 years.
Are we going to see a PTSD or anxiety epidemic as a result of the pandemic?
First, I think it’s really important that we prepare for the worst but hope for the best. But I would expect that, given the high levels of stress, the impact on resources, and other factors, we are going to see a pretty significant mental health impact over time. This could be the new normal for a while. Some of that will be PTSD, but there will also be other things. I would suspect that the resulting increase in rates of depression, traumatic grief, and loss is probably going to be a significant issue for years to come.
What will the anxiety we see as a result of COVID-19 look like compared with that seen in past disasters, like 9/11?
Most disasters in recent history, like 9/11, are single incidents. Something horrible happened, it impacted people at different levels, and we were able to start putting the pieces back together right away. The prolonged nature of this pandemic makes it even more variable given that the impact is going to be extended over time.
We’re also going to see a lot more people with compound impact – people who’ve lost their jobs, loved ones, maybe even their homes. All of those financial and resource losses put people in a higher risk category for negative mental health outcomes.
Is this analogous to the prolonged trauma that can occur with military service during war?
There is some similarity there. Combat is kind of an overarching context in which people experience trauma and, much like this pandemic, may or may not have traumatic exposures during it.
We’re asking health care workers to actually be in a role similar to what we ask of our military: going into danger, sometimes even without proper protective equipment, in order to save the lives of others. That’s also something we need to be factoring in as we plan to support those people and their families.
This is an ongoing incident, but is there a time window we need to be particularly worried about for seeing spikes in anxiety and PTSD?
I think we’re going to see variability on that. PTSD is a disorder that’s related to a specific incident or a couple of incidents that are similar. It’s a memory that’s haunting you.
For instance, typically if you have a combat veteran who has PTSD, they’ve been exposed to the overarching context of combat but then they have specific memories that are stuck. If they don’t have PTSD about 3-6 months after those incidents happen, then we would expect that they will not develop it, or it’s much less common that they would.
Depression has a very different course. It’s more prolonged and tends to grow with time.
Are you already seeing increased symptoms in your patients?
This is pretty similar to what we see in combat veterans. They’ll often be unhappy with the leadership decisions that were made as they were being deployed.
We’re also seeing lots more anger, sadness, and isolation now. Especially over the past couple of weeks, we’ve seen a rise in things like people reaching out for help in our intakes because we’re still open and doing phone assessments and telehealth with veterans and the veterans program.
In terms of interventions for this, what should psychiatrists, psychologists, and other clinicians be thinking about?
Right now, the best thing that we can do as mental health providers for people affected by the trauma is provide crisis intervention for those saying they are a danger to themselves and others. That means providing coping strategies and support. It also means making sure people are taking breaks and taking care of themselves, taking that little bit of time off so that they can go back, fully recharged, to their jobs and really stay there.
As we move forward, it will be clearer whether people are going to naturally recover, which most people will. For those who are going to have ongoing problems with time, we need to be getting ready as a system and as a country for those long-term mental health issues that are going to be coming up. And when I say long-term, it means the next 1-3 months. We want to be providing preventive interventions, versions of prolonged exposure, and other things that have shown some help in preventing PTSD. Psychological first aid is helpful.
There’s also an app called COVID Coach that the National Center for PTSD has created. That features a lot of positive coping resources together in one source.
Then when we get to the middle of that point and beyond it, we need to be ready to provide those evidence-based interventions for PTSD, depression, panic disorder, and other issues that are going to come out of this current situation.
But we were already short-staffed as far as mental health resources in general across the country, and especially in rural areas. So that means finding ways to efficiently use what we have through potentially briefer versions of interventions, through primary care, mental health, and other staff.
In what ways can primary care providers help?
There are versions of prolonged exposure therapy for primary care. That’s one of my big areas of research – increasing access. That would be something that we need to be building, by training and embedding mental health providers in primary care settings so that they can help to accommodate the increased need for access that’s going to be showing up for the next, I would suspect, several years with the pandemic.
Is there evidence that a prior episode of PTSD or traumatic experience like combat influences a subsequent reaction to a trauma like this?
It depends on how they manage. Research suggests that veterans or other people who have experienced trauma and naturally recovered, or who have gotten good treatment and remitted from that issue, are probably at no higher risk. But people who have subsyndromal PTSD or depression, or who are still experiencing symptoms from a history of trauma exposure, are maybe at a higher risk of having problems over time.
Do you have any guidance for healthcare providers on how to approach the pandemic with their patients, and also on how they can look after their own mental health?
In talking to patients, make sure that they have what they need. Ask if they’ve thought through how they’re going to cope if things get harder for them.
For people who have preexisting mental health issues, I’m talking with them about whether things have gotten worse. If they’re at high risk for suicide, I’m checking in to make sure that they’ve got new plans and ways to connect with people to reduce isolation, keeping in mind the social distancing that we’re asked to engage in so that they can do that safely.
It’s important to check and see if they have had any losses, whether it’s a financial loss or a personal loss of people that they care about. Also have them think through ways to stay entertained, which tends to help manage their own anxiety.
Every coping strategy we outline for patients also applies to mental health professionals. However, you would add to it the real need to take time to recharge, to take breaks, time off. It can feel overwhelming and like you need to just keep going. But the more that you get stuck in that mode of overdoing it, the less effective you’re going to be in helping people and also the more likely that you’ll be at risk of perhaps being one of the people that needs help.
It’s also important to make sure you’re staying connected with family and friends virtually, in whatever ways you can safely do that with social distancing.
So take a break to watch some Netflix now and then?
Yes!
A version of this article originally appeared on Medscape.com.
The prolonged and unique stresses imparted by the COVID-19 pandemic has many predicting a significant rise in mental health issues in the weeks, months, and years ahead.
To understand how health care workers can best get ahead of this emerging crisis within a crisis, Medscape Psychiatry editorial director Bret Stetka, MD, spoke with Sheila Rauch, PhD, who’s with the Department of Psychiatry and Behavioral Sciences at the Emory University, Atlanta. The director of Mental Health Research and Program Evaluation at the Atlanta VA Medical Center, Dr. Rauch has studied the effects of and best treatments for posttraumatic stress disorder (PTSD) and anxiety disorders over the past 20 years.
Are we going to see a PTSD or anxiety epidemic as a result of the pandemic?
First, I think it’s really important that we prepare for the worst but hope for the best. But I would expect that, given the high levels of stress, the impact on resources, and other factors, we are going to see a pretty significant mental health impact over time. This could be the new normal for a while. Some of that will be PTSD, but there will also be other things. I would suspect that the resulting increase in rates of depression, traumatic grief, and loss is probably going to be a significant issue for years to come.
What will the anxiety we see as a result of COVID-19 look like compared with that seen in past disasters, like 9/11?
Most disasters in recent history, like 9/11, are single incidents. Something horrible happened, it impacted people at different levels, and we were able to start putting the pieces back together right away. The prolonged nature of this pandemic makes it even more variable given that the impact is going to be extended over time.
We’re also going to see a lot more people with compound impact – people who’ve lost their jobs, loved ones, maybe even their homes. All of those financial and resource losses put people in a higher risk category for negative mental health outcomes.
Is this analogous to the prolonged trauma that can occur with military service during war?
There is some similarity there. Combat is kind of an overarching context in which people experience trauma and, much like this pandemic, may or may not have traumatic exposures during it.
We’re asking health care workers to actually be in a role similar to what we ask of our military: going into danger, sometimes even without proper protective equipment, in order to save the lives of others. That’s also something we need to be factoring in as we plan to support those people and their families.
This is an ongoing incident, but is there a time window we need to be particularly worried about for seeing spikes in anxiety and PTSD?
I think we’re going to see variability on that. PTSD is a disorder that’s related to a specific incident or a couple of incidents that are similar. It’s a memory that’s haunting you.
For instance, typically if you have a combat veteran who has PTSD, they’ve been exposed to the overarching context of combat but then they have specific memories that are stuck. If they don’t have PTSD about 3-6 months after those incidents happen, then we would expect that they will not develop it, or it’s much less common that they would.
Depression has a very different course. It’s more prolonged and tends to grow with time.
Are you already seeing increased symptoms in your patients?
This is pretty similar to what we see in combat veterans. They’ll often be unhappy with the leadership decisions that were made as they were being deployed.
We’re also seeing lots more anger, sadness, and isolation now. Especially over the past couple of weeks, we’ve seen a rise in things like people reaching out for help in our intakes because we’re still open and doing phone assessments and telehealth with veterans and the veterans program.
In terms of interventions for this, what should psychiatrists, psychologists, and other clinicians be thinking about?
Right now, the best thing that we can do as mental health providers for people affected by the trauma is provide crisis intervention for those saying they are a danger to themselves and others. That means providing coping strategies and support. It also means making sure people are taking breaks and taking care of themselves, taking that little bit of time off so that they can go back, fully recharged, to their jobs and really stay there.
As we move forward, it will be clearer whether people are going to naturally recover, which most people will. For those who are going to have ongoing problems with time, we need to be getting ready as a system and as a country for those long-term mental health issues that are going to be coming up. And when I say long-term, it means the next 1-3 months. We want to be providing preventive interventions, versions of prolonged exposure, and other things that have shown some help in preventing PTSD. Psychological first aid is helpful.
There’s also an app called COVID Coach that the National Center for PTSD has created. That features a lot of positive coping resources together in one source.
Then when we get to the middle of that point and beyond it, we need to be ready to provide those evidence-based interventions for PTSD, depression, panic disorder, and other issues that are going to come out of this current situation.
But we were already short-staffed as far as mental health resources in general across the country, and especially in rural areas. So that means finding ways to efficiently use what we have through potentially briefer versions of interventions, through primary care, mental health, and other staff.
In what ways can primary care providers help?
There are versions of prolonged exposure therapy for primary care. That’s one of my big areas of research – increasing access. That would be something that we need to be building, by training and embedding mental health providers in primary care settings so that they can help to accommodate the increased need for access that’s going to be showing up for the next, I would suspect, several years with the pandemic.
Is there evidence that a prior episode of PTSD or traumatic experience like combat influences a subsequent reaction to a trauma like this?
It depends on how they manage. Research suggests that veterans or other people who have experienced trauma and naturally recovered, or who have gotten good treatment and remitted from that issue, are probably at no higher risk. But people who have subsyndromal PTSD or depression, or who are still experiencing symptoms from a history of trauma exposure, are maybe at a higher risk of having problems over time.
Do you have any guidance for healthcare providers on how to approach the pandemic with their patients, and also on how they can look after their own mental health?
In talking to patients, make sure that they have what they need. Ask if they’ve thought through how they’re going to cope if things get harder for them.
For people who have preexisting mental health issues, I’m talking with them about whether things have gotten worse. If they’re at high risk for suicide, I’m checking in to make sure that they’ve got new plans and ways to connect with people to reduce isolation, keeping in mind the social distancing that we’re asked to engage in so that they can do that safely.
It’s important to check and see if they have had any losses, whether it’s a financial loss or a personal loss of people that they care about. Also have them think through ways to stay entertained, which tends to help manage their own anxiety.
Every coping strategy we outline for patients also applies to mental health professionals. However, you would add to it the real need to take time to recharge, to take breaks, time off. It can feel overwhelming and like you need to just keep going. But the more that you get stuck in that mode of overdoing it, the less effective you’re going to be in helping people and also the more likely that you’ll be at risk of perhaps being one of the people that needs help.
It’s also important to make sure you’re staying connected with family and friends virtually, in whatever ways you can safely do that with social distancing.
So take a break to watch some Netflix now and then?
Yes!
A version of this article originally appeared on Medscape.com.
COVID-19: Telehealth at the forefront of the pandemic
On Jan. 20, 2020, the first confirmed case of the 2019 novel coronavirus in the United States was admitted to Providence Regional Medical Center in Everett, Wash. Less than 3 months later, the COVID-19 pandemic has put enormous stress on the U.S. health care system, which is confronting acute resource shortage because of the surge of acute and critically ill patients, health care provider safety and burnout, and an ongoing need for managing vulnerable populations while minimizing the infection spread.
With the onset of these unprecedented challenges, telehealth has emerged as a powerful new resource for health care providers, hospitals, and health care systems across the country. This article offers a summary of government regulations that enabled telehealth expansion, and provides an overview of how two health care organizations, Providence St. Joseph Health and Sound Physicians, are employing telehealth services to combat the COVID-19 health care crisis.
The government response: Telehealth expansion
In response to the pandemic, the Centers for Medicare and Medicaid Services (CMS) have significantly increased access to telehealth services for Medicare and Medicaid beneficiaries. CMS swiftly put measures in place such as:
- Expanding telehealth beyond rural areas.
- Adding 80 services that can be provided in all settings, including patient homes
- Allowing providers to bill for telehealth visits at the same rate as in-person visits.
The U.S. Department of Health and Human Services also aided this effort by:
- Waiving requirements that physicians or other health care professionals must have licenses in the state in which they provide services, if they have an equivalent license from another state.
- Waving penalties for HIPAA violations against health care providers that serve patients in good faith through everyday communications technologies, such as FaceTime or Skype
Without prior regulatory and reimbursement restrictions, telehealth rapidly became a powerful tool in helping to solve some of the problems brought about by the COVID-19 pandemic.
Providence Telehealth for COVID-19
Providence St. Joseph Health is a not-for-profit health care system operating 51 hospitals and 1,085 clinics across Alaska, California, Montana, New Mexico, Oregon, Texas, and Washington. Providence has developed an enterprise telemedicine network with more than 100 virtual programs. Several of these services – including Telestroke, Telepsychiatry, TeleICU, and Telehospitalist – have been scaled across several states as a clinical cloud. More than 400 telemedicine endpoints are deployed, such as robotic carts and fixed InTouch TVs. In fact, the first U.S. COVID-19 patient was treated at Providence Regional Medical Center in Everett, Wash., using the telemedical robot Vici from InTouch Health.
According to Todd Czartoski, MD, chief medical technology officer at Providence, “while telehealth has been around for many years, COVID-19 opened a lot of people’s eyes to the value of virtual care delivery.”
Providence’s telehealth response to COVID-19 has encompassed five main areas: COVID-19 home care, COVID-19 acute care, ambulatory virtual visits, behavioral health concierge (BHC) expansion, and additional support for outside partnerships.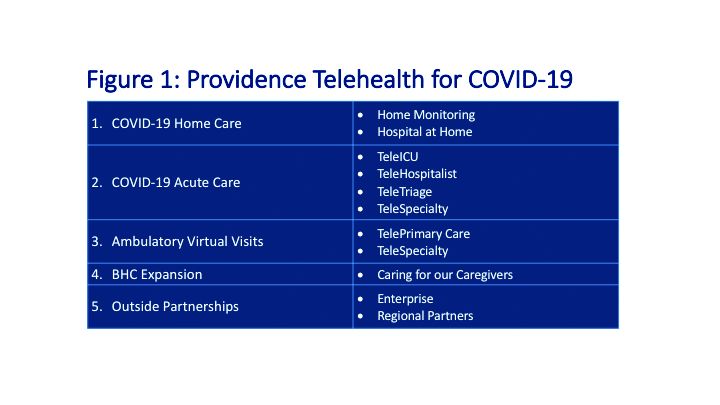
COVID-19 Home Care
Providence rapidly deployed home monitoring for nearly 2,000 positive or presumptive COVID-19 patients. Those symptomatic, clinically stable patients are given a thermometer and a pulse oximeter, and are monitored from home by a central team of nurses and physicians using the Xealth and Twistle programs.
Providence is evaluating expansion of home monitoring to other diagnoses, including higher acuity conditions.
COVID-19 Acute Care
TeleTriage expedites the triage of suspected COVID-19 patients and reduces the use of personal protective equipment (PPE) by 50% per patient per day. To date, TeleTriage has resulted in the conservation of more than 90,000 PPE units.
TeleHospitalist services expanded from traditional night coverage to caring for patients in COVID-19 units around the clock. Currently, there are 25 telehospitalists who practice both in-person and virtual medicine.
TeleICU offers remote management of more than 180 ICU beds across 17 hospitals from two central command centers in Washington state and Alaska. The services include night-time intensivist and ICU nurse coverage, including medication and ventilator management, and family conferences. COVID-19 increased the demand for TeleICU, with anticipated expansion to more than 300 beds.
Core TeleSpecialty services include TeleStroke and TelePsychiatry across 135 remote sites.
Ambulatory Virtual Visits
Providence launched the COVID-19 hub microsite to help educate patients by providing accurate and timely information. A chatbot named Grace helps screen patients who are worried about COVID-19. Grace also suggests next steps, such as a video visit with a patient’s primary care provider or a visit using Express Care/Virtual team, a direct-to-consumer service available to patients within and outside of the health care system.
In less than 2 weeks, Providence enabled virtual visits for more than 7,000 outpatient providers, with more than 14,000 alternative visits now occurring daily. This has allowed primary and specialty providers to continue to manage their patient panels remotely. The number of Express Care/Virtual visits increased from 60 to more than 1,000 per day.
BHC Expansion
In the effort to improve care for its caregivers, Providence launched a behavioral health concierge (BHC) service that offers employees and their dependents virtual access to licensed mental health professionals. Over the last half of 2019, BHC provided more than 1,000 phone and virtual visits, depending on the individual preference of patients. Notably, 21% percent of users were physicians; 65% of users were seen the same day and 100% of users were seen within 48 hours.
COVID-19 increased demand for services that initially started in Seattle and rapidly expanded to Montana, Oregon, and California.
Outside Partnerships
Providence has established partnerships with outside facilities by providing services to 135 sites across eight states. COVID-19 accelerated the employment of new services, including TeleICU.
Telemedicine at Sound Physicians
Sound Physicians is a national physician-founded and -led organization that provides emergency medicine, critical care, hospital medicine, population health, and physician advisory services. Five years ago, Sound launched a telemedicine service line. I spoke with Brian Carpenter, MD, national medical director for TeleHospitalist Services at Sound, to learn about his experience implementing Telehospitalist programs across 22 hospitals and 22 skilled nursing facilities.
Prior to COVID-19, Sound offered a spectrum of telemedicine services including night-time telephonic cross coverage, as well as video-assisted admissions, transfers, and rapid responses. In 2019, Sound Telehospitalists received 88,000 connect requests, including 6,400 video-assisted new admissions and 82 rapid responses. Typically, one physician covers four to eight hospitals with back-up available for surges. The team uses a predictive model for staffing and developed an acuity-based algorithm to ensure that patients in distress are evaluated immediately, new stable admissions on average are seen within 12 minutes, and order clarifications are provided within 30 minutes.
The COVID-19 pandemic created an urgent demand for providers to support an overwhelmed health care system. Without the traditional barriers to implementation – such as lack of acceptance by medical staff, nurses and patients, strict state licensing and technology requirements, lack of reimbursement, and delays in hospital credentialing – Sound was able to develop a rapid implementation model for telemedicine services. Currently, four new hospitals are in the active implementation phase, with 40 more hospitals in the pipeline.
Implementing a telemedicine program at your hospital
In order to successfully launch a telemedicine program, Dr. Carpenter outlined the following critical implementation steps:
- In collaboration with local leadership, define the problem you are trying to solve, which helps inform the scope of the telemedicine practice and technology requirements (for example, night-time cross-coverage vs. full telemedicine service).
- Complete a discovery process (for example, existing workflow for patient admission and transfer) with the end-goal of developing a workflow and rules of engagement.
- Obtain hospital credentialing/privileges and EMR access.
- Train end-users, including physicians and nurse telepresenters.
Dr. Carpenter offered this advice to those considering a telemedicine program: “Telemedicine is not just about technology; a true telemedicine program encompasses change management, workflow development, end-user training, compliance, and mechanisms for continuous process improvement. We want to make things better for the physicians, nurses, and patients.”
Telehealth is offering support to health care providers on the front lines, patients in need of care, and health care systems managing the unprecedented surges in volume.
Dr. Farah is a hospitalist, physician adviser, and Lean Six Sigma Black Belt. She is a performance improvement consultant based in Corvallis, Ore., and a member of The Hospitalist’s editorial advisory board.
On Jan. 20, 2020, the first confirmed case of the 2019 novel coronavirus in the United States was admitted to Providence Regional Medical Center in Everett, Wash. Less than 3 months later, the COVID-19 pandemic has put enormous stress on the U.S. health care system, which is confronting acute resource shortage because of the surge of acute and critically ill patients, health care provider safety and burnout, and an ongoing need for managing vulnerable populations while minimizing the infection spread.
With the onset of these unprecedented challenges, telehealth has emerged as a powerful new resource for health care providers, hospitals, and health care systems across the country. This article offers a summary of government regulations that enabled telehealth expansion, and provides an overview of how two health care organizations, Providence St. Joseph Health and Sound Physicians, are employing telehealth services to combat the COVID-19 health care crisis.
The government response: Telehealth expansion
In response to the pandemic, the Centers for Medicare and Medicaid Services (CMS) have significantly increased access to telehealth services for Medicare and Medicaid beneficiaries. CMS swiftly put measures in place such as:
- Expanding telehealth beyond rural areas.
- Adding 80 services that can be provided in all settings, including patient homes
- Allowing providers to bill for telehealth visits at the same rate as in-person visits.
The U.S. Department of Health and Human Services also aided this effort by:
- Waiving requirements that physicians or other health care professionals must have licenses in the state in which they provide services, if they have an equivalent license from another state.
- Waving penalties for HIPAA violations against health care providers that serve patients in good faith through everyday communications technologies, such as FaceTime or Skype
Without prior regulatory and reimbursement restrictions, telehealth rapidly became a powerful tool in helping to solve some of the problems brought about by the COVID-19 pandemic.
Providence Telehealth for COVID-19
Providence St. Joseph Health is a not-for-profit health care system operating 51 hospitals and 1,085 clinics across Alaska, California, Montana, New Mexico, Oregon, Texas, and Washington. Providence has developed an enterprise telemedicine network with more than 100 virtual programs. Several of these services – including Telestroke, Telepsychiatry, TeleICU, and Telehospitalist – have been scaled across several states as a clinical cloud. More than 400 telemedicine endpoints are deployed, such as robotic carts and fixed InTouch TVs. In fact, the first U.S. COVID-19 patient was treated at Providence Regional Medical Center in Everett, Wash., using the telemedical robot Vici from InTouch Health.
According to Todd Czartoski, MD, chief medical technology officer at Providence, “while telehealth has been around for many years, COVID-19 opened a lot of people’s eyes to the value of virtual care delivery.”
Providence’s telehealth response to COVID-19 has encompassed five main areas: COVID-19 home care, COVID-19 acute care, ambulatory virtual visits, behavioral health concierge (BHC) expansion, and additional support for outside partnerships.
COVID-19 Home Care
Providence rapidly deployed home monitoring for nearly 2,000 positive or presumptive COVID-19 patients. Those symptomatic, clinically stable patients are given a thermometer and a pulse oximeter, and are monitored from home by a central team of nurses and physicians using the Xealth and Twistle programs.
Providence is evaluating expansion of home monitoring to other diagnoses, including higher acuity conditions.
COVID-19 Acute Care
TeleTriage expedites the triage of suspected COVID-19 patients and reduces the use of personal protective equipment (PPE) by 50% per patient per day. To date, TeleTriage has resulted in the conservation of more than 90,000 PPE units.
TeleHospitalist services expanded from traditional night coverage to caring for patients in COVID-19 units around the clock. Currently, there are 25 telehospitalists who practice both in-person and virtual medicine.
TeleICU offers remote management of more than 180 ICU beds across 17 hospitals from two central command centers in Washington state and Alaska. The services include night-time intensivist and ICU nurse coverage, including medication and ventilator management, and family conferences. COVID-19 increased the demand for TeleICU, with anticipated expansion to more than 300 beds.
Core TeleSpecialty services include TeleStroke and TelePsychiatry across 135 remote sites.
Ambulatory Virtual Visits
Providence launched the COVID-19 hub microsite to help educate patients by providing accurate and timely information. A chatbot named Grace helps screen patients who are worried about COVID-19. Grace also suggests next steps, such as a video visit with a patient’s primary care provider or a visit using Express Care/Virtual team, a direct-to-consumer service available to patients within and outside of the health care system.
In less than 2 weeks, Providence enabled virtual visits for more than 7,000 outpatient providers, with more than 14,000 alternative visits now occurring daily. This has allowed primary and specialty providers to continue to manage their patient panels remotely. The number of Express Care/Virtual visits increased from 60 to more than 1,000 per day.
BHC Expansion
In the effort to improve care for its caregivers, Providence launched a behavioral health concierge (BHC) service that offers employees and their dependents virtual access to licensed mental health professionals. Over the last half of 2019, BHC provided more than 1,000 phone and virtual visits, depending on the individual preference of patients. Notably, 21% percent of users were physicians; 65% of users were seen the same day and 100% of users were seen within 48 hours.
COVID-19 increased demand for services that initially started in Seattle and rapidly expanded to Montana, Oregon, and California.
Outside Partnerships
Providence has established partnerships with outside facilities by providing services to 135 sites across eight states. COVID-19 accelerated the employment of new services, including TeleICU.
Telemedicine at Sound Physicians
Sound Physicians is a national physician-founded and -led organization that provides emergency medicine, critical care, hospital medicine, population health, and physician advisory services. Five years ago, Sound launched a telemedicine service line. I spoke with Brian Carpenter, MD, national medical director for TeleHospitalist Services at Sound, to learn about his experience implementing Telehospitalist programs across 22 hospitals and 22 skilled nursing facilities.
Prior to COVID-19, Sound offered a spectrum of telemedicine services including night-time telephonic cross coverage, as well as video-assisted admissions, transfers, and rapid responses. In 2019, Sound Telehospitalists received 88,000 connect requests, including 6,400 video-assisted new admissions and 82 rapid responses. Typically, one physician covers four to eight hospitals with back-up available for surges. The team uses a predictive model for staffing and developed an acuity-based algorithm to ensure that patients in distress are evaluated immediately, new stable admissions on average are seen within 12 minutes, and order clarifications are provided within 30 minutes.
The COVID-19 pandemic created an urgent demand for providers to support an overwhelmed health care system. Without the traditional barriers to implementation – such as lack of acceptance by medical staff, nurses and patients, strict state licensing and technology requirements, lack of reimbursement, and delays in hospital credentialing – Sound was able to develop a rapid implementation model for telemedicine services. Currently, four new hospitals are in the active implementation phase, with 40 more hospitals in the pipeline.
Implementing a telemedicine program at your hospital
In order to successfully launch a telemedicine program, Dr. Carpenter outlined the following critical implementation steps:
- In collaboration with local leadership, define the problem you are trying to solve, which helps inform the scope of the telemedicine practice and technology requirements (for example, night-time cross-coverage vs. full telemedicine service).
- Complete a discovery process (for example, existing workflow for patient admission and transfer) with the end-goal of developing a workflow and rules of engagement.
- Obtain hospital credentialing/privileges and EMR access.
- Train end-users, including physicians and nurse telepresenters.
Dr. Carpenter offered this advice to those considering a telemedicine program: “Telemedicine is not just about technology; a true telemedicine program encompasses change management, workflow development, end-user training, compliance, and mechanisms for continuous process improvement. We want to make things better for the physicians, nurses, and patients.”
Telehealth is offering support to health care providers on the front lines, patients in need of care, and health care systems managing the unprecedented surges in volume.
Dr. Farah is a hospitalist, physician adviser, and Lean Six Sigma Black Belt. She is a performance improvement consultant based in Corvallis, Ore., and a member of The Hospitalist’s editorial advisory board.
On Jan. 20, 2020, the first confirmed case of the 2019 novel coronavirus in the United States was admitted to Providence Regional Medical Center in Everett, Wash. Less than 3 months later, the COVID-19 pandemic has put enormous stress on the U.S. health care system, which is confronting acute resource shortage because of the surge of acute and critically ill patients, health care provider safety and burnout, and an ongoing need for managing vulnerable populations while minimizing the infection spread.
With the onset of these unprecedented challenges, telehealth has emerged as a powerful new resource for health care providers, hospitals, and health care systems across the country. This article offers a summary of government regulations that enabled telehealth expansion, and provides an overview of how two health care organizations, Providence St. Joseph Health and Sound Physicians, are employing telehealth services to combat the COVID-19 health care crisis.
The government response: Telehealth expansion
In response to the pandemic, the Centers for Medicare and Medicaid Services (CMS) have significantly increased access to telehealth services for Medicare and Medicaid beneficiaries. CMS swiftly put measures in place such as:
- Expanding telehealth beyond rural areas.
- Adding 80 services that can be provided in all settings, including patient homes
- Allowing providers to bill for telehealth visits at the same rate as in-person visits.
The U.S. Department of Health and Human Services also aided this effort by:
- Waiving requirements that physicians or other health care professionals must have licenses in the state in which they provide services, if they have an equivalent license from another state.
- Waving penalties for HIPAA violations against health care providers that serve patients in good faith through everyday communications technologies, such as FaceTime or Skype
Without prior regulatory and reimbursement restrictions, telehealth rapidly became a powerful tool in helping to solve some of the problems brought about by the COVID-19 pandemic.
Providence Telehealth for COVID-19
Providence St. Joseph Health is a not-for-profit health care system operating 51 hospitals and 1,085 clinics across Alaska, California, Montana, New Mexico, Oregon, Texas, and Washington. Providence has developed an enterprise telemedicine network with more than 100 virtual programs. Several of these services – including Telestroke, Telepsychiatry, TeleICU, and Telehospitalist – have been scaled across several states as a clinical cloud. More than 400 telemedicine endpoints are deployed, such as robotic carts and fixed InTouch TVs. In fact, the first U.S. COVID-19 patient was treated at Providence Regional Medical Center in Everett, Wash., using the telemedical robot Vici from InTouch Health.
According to Todd Czartoski, MD, chief medical technology officer at Providence, “while telehealth has been around for many years, COVID-19 opened a lot of people’s eyes to the value of virtual care delivery.”
Providence’s telehealth response to COVID-19 has encompassed five main areas: COVID-19 home care, COVID-19 acute care, ambulatory virtual visits, behavioral health concierge (BHC) expansion, and additional support for outside partnerships.
COVID-19 Home Care
Providence rapidly deployed home monitoring for nearly 2,000 positive or presumptive COVID-19 patients. Those symptomatic, clinically stable patients are given a thermometer and a pulse oximeter, and are monitored from home by a central team of nurses and physicians using the Xealth and Twistle programs.
Providence is evaluating expansion of home monitoring to other diagnoses, including higher acuity conditions.
COVID-19 Acute Care
TeleTriage expedites the triage of suspected COVID-19 patients and reduces the use of personal protective equipment (PPE) by 50% per patient per day. To date, TeleTriage has resulted in the conservation of more than 90,000 PPE units.
TeleHospitalist services expanded from traditional night coverage to caring for patients in COVID-19 units around the clock. Currently, there are 25 telehospitalists who practice both in-person and virtual medicine.
TeleICU offers remote management of more than 180 ICU beds across 17 hospitals from two central command centers in Washington state and Alaska. The services include night-time intensivist and ICU nurse coverage, including medication and ventilator management, and family conferences. COVID-19 increased the demand for TeleICU, with anticipated expansion to more than 300 beds.
Core TeleSpecialty services include TeleStroke and TelePsychiatry across 135 remote sites.
Ambulatory Virtual Visits
Providence launched the COVID-19 hub microsite to help educate patients by providing accurate and timely information. A chatbot named Grace helps screen patients who are worried about COVID-19. Grace also suggests next steps, such as a video visit with a patient’s primary care provider or a visit using Express Care/Virtual team, a direct-to-consumer service available to patients within and outside of the health care system.
In less than 2 weeks, Providence enabled virtual visits for more than 7,000 outpatient providers, with more than 14,000 alternative visits now occurring daily. This has allowed primary and specialty providers to continue to manage their patient panels remotely. The number of Express Care/Virtual visits increased from 60 to more than 1,000 per day.
BHC Expansion
In the effort to improve care for its caregivers, Providence launched a behavioral health concierge (BHC) service that offers employees and their dependents virtual access to licensed mental health professionals. Over the last half of 2019, BHC provided more than 1,000 phone and virtual visits, depending on the individual preference of patients. Notably, 21% percent of users were physicians; 65% of users were seen the same day and 100% of users were seen within 48 hours.
COVID-19 increased demand for services that initially started in Seattle and rapidly expanded to Montana, Oregon, and California.
Outside Partnerships
Providence has established partnerships with outside facilities by providing services to 135 sites across eight states. COVID-19 accelerated the employment of new services, including TeleICU.
Telemedicine at Sound Physicians
Sound Physicians is a national physician-founded and -led organization that provides emergency medicine, critical care, hospital medicine, population health, and physician advisory services. Five years ago, Sound launched a telemedicine service line. I spoke with Brian Carpenter, MD, national medical director for TeleHospitalist Services at Sound, to learn about his experience implementing Telehospitalist programs across 22 hospitals and 22 skilled nursing facilities.
Prior to COVID-19, Sound offered a spectrum of telemedicine services including night-time telephonic cross coverage, as well as video-assisted admissions, transfers, and rapid responses. In 2019, Sound Telehospitalists received 88,000 connect requests, including 6,400 video-assisted new admissions and 82 rapid responses. Typically, one physician covers four to eight hospitals with back-up available for surges. The team uses a predictive model for staffing and developed an acuity-based algorithm to ensure that patients in distress are evaluated immediately, new stable admissions on average are seen within 12 minutes, and order clarifications are provided within 30 minutes.
The COVID-19 pandemic created an urgent demand for providers to support an overwhelmed health care system. Without the traditional barriers to implementation – such as lack of acceptance by medical staff, nurses and patients, strict state licensing and technology requirements, lack of reimbursement, and delays in hospital credentialing – Sound was able to develop a rapid implementation model for telemedicine services. Currently, four new hospitals are in the active implementation phase, with 40 more hospitals in the pipeline.
Implementing a telemedicine program at your hospital
In order to successfully launch a telemedicine program, Dr. Carpenter outlined the following critical implementation steps:
- In collaboration with local leadership, define the problem you are trying to solve, which helps inform the scope of the telemedicine practice and technology requirements (for example, night-time cross-coverage vs. full telemedicine service).
- Complete a discovery process (for example, existing workflow for patient admission and transfer) with the end-goal of developing a workflow and rules of engagement.
- Obtain hospital credentialing/privileges and EMR access.
- Train end-users, including physicians and nurse telepresenters.
Dr. Carpenter offered this advice to those considering a telemedicine program: “Telemedicine is not just about technology; a true telemedicine program encompasses change management, workflow development, end-user training, compliance, and mechanisms for continuous process improvement. We want to make things better for the physicians, nurses, and patients.”
Telehealth is offering support to health care providers on the front lines, patients in need of care, and health care systems managing the unprecedented surges in volume.
Dr. Farah is a hospitalist, physician adviser, and Lean Six Sigma Black Belt. She is a performance improvement consultant based in Corvallis, Ore., and a member of The Hospitalist’s editorial advisory board.

























