User login
Cannabis use in pregnancy and lactation: A changing landscape
National survey data from 2007-2012 of more than 93,000 pregnant women suggest that around 7% of pregnant respondents reported any cannabis use in the last 2-12 months; of those, 16% reported daily or almost daily use. Among pregnant past-year users in the same survey, 70% perceived slight or no risk of harm from cannabis use 1-2 times a week in pregnancy.1
Data from the Kaiser Northern California health plan involving more than 279,000 pregnancies followed during 2009-2016 suggest that there has been a significant upward trend in use of cannabis during pregnancy, from 4% to 7%, as reported by the mother and/or identified by routine urine screening. The highest prevalence in that study was seen among 18- to 24-year-old pregnant women, increasing from 13% to 22% over the 7-year study period. Importantly, more than 50% of cannabis users in the sample were identified by toxicology screening alone.2,3 Common reasons given for use of cannabis in pregnancy include anxiety, pain, and nausea and vomiting of pregnancy.4
With respect to adverse perinatal outcomes, several case-control studies have examined risks for major birth defects with maternal self-report of cannabis use. Some have noted very modest increased risks for selected major birth defects (odds ratios less than 2); however, data still are very limited.5,6
A number of prospective studies have addressed risks of preterm birth and growth restriction, accounting for mother’s concomitant tobacco use.7-11 Some of these studies have suggested about a twofold to threefold increased risk for preterm delivery and an increased risk for reduced birth weight – particularly with heavier or regular cannabis use – but study findings have not been entirely consistent.
Given its psychoactive properties, there has been high interest in understanding whether there are any short- or long-term neurodevelopmental effects on children prenatally exposed to cannabis. These outcomes have been studied in two small older cohorts in the United States and Canada and one more recent cohort in the Netherlands.12-15 Deficits in several measures of cognition and behavior were noted in follow-up of those children from birth to adulthood. However, it is unclear to what extent these findings may have been influenced by heredity, environment, or other factors.
There have been limitations in almost all studies published to date, including small sample sizes, no biomarker validation of maternal report of dose and gestational timing of cannabis use, and lack of detailed data on common coexposures, such as alcohol, tobacco, and other drugs. In addition, newer studies of pregnancy outcomes in women who use currently available cannabis products are needed, given the substantial increase in the potency of cannabis used today, compared with that of 20 years ago. For example, the tetrahydrocannabinol (THC) concentration in commonly cultivated marijuana plants has increased threefold from 4% to 12% between 1995 and 2014.16
There are very limited data on the presence of cannabis in breast milk and the potential effects of exposure to THC and other metabolites for breastfed infants. However, two recent studies have demonstrated there are low but measurable levels of some cannabis metabolites in breast milk.17-18 Further work is needed to determine if these metabolites accumulate in milk and if at a given dose and age of the breastfed infant, there are any growth, neurodevelopmental, or other clinically important adverse effects.
Related questions, such as potential differences in the effects of exposure during pregnancy or lactation based on the route of administration (edible vs. inhaled) and the use of cannabidiol (CBD) products, have not been studied.
At the present time, the American College of Obstetricians and Gynecologists recommends that women who are pregnant or contemplating pregnancy be encouraged to discontinue marijuana use. With respect to lactation and breastfeeding, ACOG concludes there are insufficient data to evaluate the effects on infants, and in the absence of such data, marijuana use is discouraged. Similarly, the American Academy of Pediatrics recommends women of childbearing age abstain from marijuana use while pregnant or breastfeeding because of potential adverse consequences to the fetus, infant, or child.
In August 2019, the U.S. Surgeon General issued an advisory regarding potential harm to developing brains from the use of marijuana during pregnancy and lactation. The Food and Drug Administration issued a similar statement in October 2019 strongly advising against the use of CBD, THC, and marijuana in any form during pregnancy or while breastfeeding.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, president of the Organization of Teratology Information Specialists, and past president of the Teratology Society.
References
1. Am J Obstet Gynecol. 2015 Aug;213(2):201.e1-10.
2. JAMA. 2017 Dec 26;318(24):2490-1.
3. JAMA. 2017 Jan 10;317(2):207-9.
4. Complement Ther Clin Pract. 2009 Nov;15(4)242-6.
5. Paediatr Perinat Epidemiol. 2014 Sep; 28(5): 424-33.
6. J Toxicol Environ Health A. 2007 Jan;70(1):7-18.
7. Am J Obstet Gynecol. 1983 Aug 15;146(8):992-4.
8. Clin Perinatol. 1991 Mar;18(1):77-91.
9. Am J Epidemiol. 1986 Dec;124(6):986-93.
10. Pediatr Res. 2012 Feb;71(2):215-9.
11. Reprod Toxicol. 2016;62:77-86.
12. Neurotoxicol Teratol. 1987 Jan-Feb;9(1):1-7.
13. Neurotoxicol Teratol. 1994 Mar-Apr;16(2):169-75.
14. Biol Psychiatry. 2016 Jun 15;79(12):971-9.
15. Pharmacol Ther. 2018 Feb;182:133-51.
16. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
17. Obstet Gynecol. 2018 May;131(5):783-8.
18. Pediatrics. 2018 Sep;142(3):e20181076.
National survey data from 2007-2012 of more than 93,000 pregnant women suggest that around 7% of pregnant respondents reported any cannabis use in the last 2-12 months; of those, 16% reported daily or almost daily use. Among pregnant past-year users in the same survey, 70% perceived slight or no risk of harm from cannabis use 1-2 times a week in pregnancy.1
Data from the Kaiser Northern California health plan involving more than 279,000 pregnancies followed during 2009-2016 suggest that there has been a significant upward trend in use of cannabis during pregnancy, from 4% to 7%, as reported by the mother and/or identified by routine urine screening. The highest prevalence in that study was seen among 18- to 24-year-old pregnant women, increasing from 13% to 22% over the 7-year study period. Importantly, more than 50% of cannabis users in the sample were identified by toxicology screening alone.2,3 Common reasons given for use of cannabis in pregnancy include anxiety, pain, and nausea and vomiting of pregnancy.4
With respect to adverse perinatal outcomes, several case-control studies have examined risks for major birth defects with maternal self-report of cannabis use. Some have noted very modest increased risks for selected major birth defects (odds ratios less than 2); however, data still are very limited.5,6
A number of prospective studies have addressed risks of preterm birth and growth restriction, accounting for mother’s concomitant tobacco use.7-11 Some of these studies have suggested about a twofold to threefold increased risk for preterm delivery and an increased risk for reduced birth weight – particularly with heavier or regular cannabis use – but study findings have not been entirely consistent.
Given its psychoactive properties, there has been high interest in understanding whether there are any short- or long-term neurodevelopmental effects on children prenatally exposed to cannabis. These outcomes have been studied in two small older cohorts in the United States and Canada and one more recent cohort in the Netherlands.12-15 Deficits in several measures of cognition and behavior were noted in follow-up of those children from birth to adulthood. However, it is unclear to what extent these findings may have been influenced by heredity, environment, or other factors.
There have been limitations in almost all studies published to date, including small sample sizes, no biomarker validation of maternal report of dose and gestational timing of cannabis use, and lack of detailed data on common coexposures, such as alcohol, tobacco, and other drugs. In addition, newer studies of pregnancy outcomes in women who use currently available cannabis products are needed, given the substantial increase in the potency of cannabis used today, compared with that of 20 years ago. For example, the tetrahydrocannabinol (THC) concentration in commonly cultivated marijuana plants has increased threefold from 4% to 12% between 1995 and 2014.16
There are very limited data on the presence of cannabis in breast milk and the potential effects of exposure to THC and other metabolites for breastfed infants. However, two recent studies have demonstrated there are low but measurable levels of some cannabis metabolites in breast milk.17-18 Further work is needed to determine if these metabolites accumulate in milk and if at a given dose and age of the breastfed infant, there are any growth, neurodevelopmental, or other clinically important adverse effects.
Related questions, such as potential differences in the effects of exposure during pregnancy or lactation based on the route of administration (edible vs. inhaled) and the use of cannabidiol (CBD) products, have not been studied.
At the present time, the American College of Obstetricians and Gynecologists recommends that women who are pregnant or contemplating pregnancy be encouraged to discontinue marijuana use. With respect to lactation and breastfeeding, ACOG concludes there are insufficient data to evaluate the effects on infants, and in the absence of such data, marijuana use is discouraged. Similarly, the American Academy of Pediatrics recommends women of childbearing age abstain from marijuana use while pregnant or breastfeeding because of potential adverse consequences to the fetus, infant, or child.
In August 2019, the U.S. Surgeon General issued an advisory regarding potential harm to developing brains from the use of marijuana during pregnancy and lactation. The Food and Drug Administration issued a similar statement in October 2019 strongly advising against the use of CBD, THC, and marijuana in any form during pregnancy or while breastfeeding.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, president of the Organization of Teratology Information Specialists, and past president of the Teratology Society.
References
1. Am J Obstet Gynecol. 2015 Aug;213(2):201.e1-10.
2. JAMA. 2017 Dec 26;318(24):2490-1.
3. JAMA. 2017 Jan 10;317(2):207-9.
4. Complement Ther Clin Pract. 2009 Nov;15(4)242-6.
5. Paediatr Perinat Epidemiol. 2014 Sep; 28(5): 424-33.
6. J Toxicol Environ Health A. 2007 Jan;70(1):7-18.
7. Am J Obstet Gynecol. 1983 Aug 15;146(8):992-4.
8. Clin Perinatol. 1991 Mar;18(1):77-91.
9. Am J Epidemiol. 1986 Dec;124(6):986-93.
10. Pediatr Res. 2012 Feb;71(2):215-9.
11. Reprod Toxicol. 2016;62:77-86.
12. Neurotoxicol Teratol. 1987 Jan-Feb;9(1):1-7.
13. Neurotoxicol Teratol. 1994 Mar-Apr;16(2):169-75.
14. Biol Psychiatry. 2016 Jun 15;79(12):971-9.
15. Pharmacol Ther. 2018 Feb;182:133-51.
16. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
17. Obstet Gynecol. 2018 May;131(5):783-8.
18. Pediatrics. 2018 Sep;142(3):e20181076.
National survey data from 2007-2012 of more than 93,000 pregnant women suggest that around 7% of pregnant respondents reported any cannabis use in the last 2-12 months; of those, 16% reported daily or almost daily use. Among pregnant past-year users in the same survey, 70% perceived slight or no risk of harm from cannabis use 1-2 times a week in pregnancy.1
Data from the Kaiser Northern California health plan involving more than 279,000 pregnancies followed during 2009-2016 suggest that there has been a significant upward trend in use of cannabis during pregnancy, from 4% to 7%, as reported by the mother and/or identified by routine urine screening. The highest prevalence in that study was seen among 18- to 24-year-old pregnant women, increasing from 13% to 22% over the 7-year study period. Importantly, more than 50% of cannabis users in the sample were identified by toxicology screening alone.2,3 Common reasons given for use of cannabis in pregnancy include anxiety, pain, and nausea and vomiting of pregnancy.4
With respect to adverse perinatal outcomes, several case-control studies have examined risks for major birth defects with maternal self-report of cannabis use. Some have noted very modest increased risks for selected major birth defects (odds ratios less than 2); however, data still are very limited.5,6
A number of prospective studies have addressed risks of preterm birth and growth restriction, accounting for mother’s concomitant tobacco use.7-11 Some of these studies have suggested about a twofold to threefold increased risk for preterm delivery and an increased risk for reduced birth weight – particularly with heavier or regular cannabis use – but study findings have not been entirely consistent.
Given its psychoactive properties, there has been high interest in understanding whether there are any short- or long-term neurodevelopmental effects on children prenatally exposed to cannabis. These outcomes have been studied in two small older cohorts in the United States and Canada and one more recent cohort in the Netherlands.12-15 Deficits in several measures of cognition and behavior were noted in follow-up of those children from birth to adulthood. However, it is unclear to what extent these findings may have been influenced by heredity, environment, or other factors.
There have been limitations in almost all studies published to date, including small sample sizes, no biomarker validation of maternal report of dose and gestational timing of cannabis use, and lack of detailed data on common coexposures, such as alcohol, tobacco, and other drugs. In addition, newer studies of pregnancy outcomes in women who use currently available cannabis products are needed, given the substantial increase in the potency of cannabis used today, compared with that of 20 years ago. For example, the tetrahydrocannabinol (THC) concentration in commonly cultivated marijuana plants has increased threefold from 4% to 12% between 1995 and 2014.16
There are very limited data on the presence of cannabis in breast milk and the potential effects of exposure to THC and other metabolites for breastfed infants. However, two recent studies have demonstrated there are low but measurable levels of some cannabis metabolites in breast milk.17-18 Further work is needed to determine if these metabolites accumulate in milk and if at a given dose and age of the breastfed infant, there are any growth, neurodevelopmental, or other clinically important adverse effects.
Related questions, such as potential differences in the effects of exposure during pregnancy or lactation based on the route of administration (edible vs. inhaled) and the use of cannabidiol (CBD) products, have not been studied.
At the present time, the American College of Obstetricians and Gynecologists recommends that women who are pregnant or contemplating pregnancy be encouraged to discontinue marijuana use. With respect to lactation and breastfeeding, ACOG concludes there are insufficient data to evaluate the effects on infants, and in the absence of such data, marijuana use is discouraged. Similarly, the American Academy of Pediatrics recommends women of childbearing age abstain from marijuana use while pregnant or breastfeeding because of potential adverse consequences to the fetus, infant, or child.
In August 2019, the U.S. Surgeon General issued an advisory regarding potential harm to developing brains from the use of marijuana during pregnancy and lactation. The Food and Drug Administration issued a similar statement in October 2019 strongly advising against the use of CBD, THC, and marijuana in any form during pregnancy or while breastfeeding.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, president of the Organization of Teratology Information Specialists, and past president of the Teratology Society.
References
1. Am J Obstet Gynecol. 2015 Aug;213(2):201.e1-10.
2. JAMA. 2017 Dec 26;318(24):2490-1.
3. JAMA. 2017 Jan 10;317(2):207-9.
4. Complement Ther Clin Pract. 2009 Nov;15(4)242-6.
5. Paediatr Perinat Epidemiol. 2014 Sep; 28(5): 424-33.
6. J Toxicol Environ Health A. 2007 Jan;70(1):7-18.
7. Am J Obstet Gynecol. 1983 Aug 15;146(8):992-4.
8. Clin Perinatol. 1991 Mar;18(1):77-91.
9. Am J Epidemiol. 1986 Dec;124(6):986-93.
10. Pediatr Res. 2012 Feb;71(2):215-9.
11. Reprod Toxicol. 2016;62:77-86.
12. Neurotoxicol Teratol. 1987 Jan-Feb;9(1):1-7.
13. Neurotoxicol Teratol. 1994 Mar-Apr;16(2):169-75.
14. Biol Psychiatry. 2016 Jun 15;79(12):971-9.
15. Pharmacol Ther. 2018 Feb;182:133-51.
16. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
17. Obstet Gynecol. 2018 May;131(5):783-8.
18. Pediatrics. 2018 Sep;142(3):e20181076.
The suicide wars
Topic of suicide prevention causing divisions within psychiatry
At every swipe through my social media feeds, I’m greeted with another topic that has advocates clustered at the extremes. People align, and they align quickly in our strangely polarized world in which anyone who might sit in the middle lies low.
It seems we’re divided: On the left you are a CNN fan or you’re one of those soulless monsters who tunes in to Fox News. You’re pro-life or you’re a baby killer, advocating for late-term abortions or even the execution of live infants. When it comes to firearm regulation, one side says you’re a threat to the Constitution, while the other says that those opposed are responsible for the death of every person who was ever the victim of a discharged firearm. And those who feel strongly about a given topic often justify their attacks on those who disagree. Psychiatry is no stranger to this thinking, and we are the only medical specialty with organized “antipsychiatry” groups who oppose our work. I have been a bit surprised, however, that the topic of suicide prevention is one that has us divided within our own specialty.
Amy Barnhorst, MD, is a psychiatrist at the University of California, Davis, and the author of “The empty promise of suicide prevention: Many of the problems that lead people to kill themselves cannot be fixed with a little serotonin,” an op-ed piece that appeared in the New York Times on April 26, 2019. Dr. Barnhorst began her essay with the story of a patient who was hospitalized after a relative realized she was planning her suicide. That story had an ending that psychiatrists relish: A person with previously unrecognized and untreated bipolar disorder received care, including medication, and got better. This suicide was preventable, a life was saved, and this story followed a model we all hope is being replicated over and over.
Dr. Barnhorst went on to say that this was an outlier in her career, that most of the suicidal patients she sees are impoverished, homeless, addicted, and she wrote about how little the treatment setting has to offer: The idea that a pill would fix these problems is almost laughable. She suggests that there is more to suicide prevention than identifying prospective patients and getting them acute psychiatric care.
The decision to stop living is one that people arrive at by different paths, some over months, but many in a matter of minutes. Those people won’t be intercepted by the mental health system. We certainly need more psychiatric services and more research into better, faster-acting treatments for severe depression and suicidal thoughts, but that will never be enough.
We need to address the root causes of our nation’s suicide problem – poverty, homelessness, and the accompanying exposure to trauma, crime, and drugs. That means better alcohol and drug treatment, family counseling, low-income housing resources, job training, and individual therapy. And for those at risk who still slip past all the checkpoints, we need to make sure they don’t have access to guns and lethal medications.
Psychological autopsies done after suicides have indicated that more than 90% of people who die from suicide suffered from a mental illness, yet 54% of those who ended their own lives had never received a psychiatric diagnosis. There is a hopefulness that, if only we had more – more services, more therapy, more medication – then we could prevent suicide. Unfortunately, this line of thinking, with a “Zero Suicide” initiative, points a finger at those who survive: Suicide is preventable, so someone is to blame, if not a family member for missing the warning signs then the clinician who offered treatment that wasn’t good enough.
Along this line, the New York Times printed another opinion piece on Jan. 6 by Richard A. Friedman, MD, titled, “Why are young Americans killing themselves?” Dr. Friedman’s conclusion was more along the psychiatrist party line: “The good news is that we don’t have to wait for all the answers to know what to do. We know that various psychotherapies and medication are highly effective in treating depression. We just need to do a better job of identifying, reaching out to and providing resources for at-risk youths.”
Dr. Friedman goes on to propose universal screening at school, among other measures to identify those at risk. It is no surprise that Dr. Friedman’s article had more than 1,700 comments before commenting was closed by the Times. I have written about the pros and cons of screening adolescents for depression in a primary care setting, so putting the responsibility of identifying suicidal teenagers on school teachers seems like an ominous responsibility to add to a teacher’s obligations.
I did not read Dr. Barnhorst’s earlier op-ed piece as a condemnation of psychiatric care, but rather as a call to action and a reality check on the idea that psychiatry is the only answer to our suicide epidemic. More people than ever get treatment – from psychiatrists, from primary care doctors, from nonphysician prescribing clinicians, and from so many varieties of psychotherapists, and yet our suicide rates continue to rise.
In a post on the Psychology Today website, Sara Gorman, PhD, and Jack M. Gorman, MD, discussed Dr. Barnhorst’s article. “In the process of making her point, Barnhorst also manages to seriously trivialize the role of antidepressant medication in the treatment of depression and to imply that, given societal woes, there isn’t much we can do to try to prevent suicides – aside from limiting access to lethal means,” they wrote.
The Gormans were not alone in their objections; the day after the op-ed appeared in the New York Times, a well-respected psychiatry department chairman took on not just the content of the op-ed, but also the author, in his Twitter feed. He wrote, “@amybarnhorst doesn’t read scientific literature or skipped training. this article is wrong. #suicide is largely preventable, if proper measures taken n Rx provided. @nytimes please vet authors better @APAPsychiatric.” Dr. Barnhorst, also a voice on Twitter, added the wry response, “I skipped training.” When Twitter users responded that initial Twitter comment conveyed a lack of civility toward a colleague, the original Tweeter – I’m withholding his name with the hope that even writing about these interactions won’t put me on anyone’s enemy list – like many others sitting on the poles of these contentious topics, responded with the following, “All for civility except in the case of misinformation that puts lives at risk, especially when purveyed by a professional who wears the patina of credibility.”
If it’s not yet obvious, I don’t believe there is a simple answer to our suicide problem, nor do I think it puts lives at risk to point out that, so far, our treatments have not lowered suicide rates. The issue is complex and we have no perfect explanation as to why countries differ so greatly with regard to suicide. There are impoverished, war-torn countries with remarkably lower suicide rates, and nations with much stricter gun laws that have higher statistics. Honduras, deemed “the murder capital of the world,” has an enviable suicide rate of only 2.9 per 100,000.
If the solution were as simple as making medications more accessible, the answer might be an easy one (or at least worth trying) – make antidepressants available over-the-counter, a move that would both increase access and decrease stigma.
Some people are determined to end their own lives. They aren’t looking to see psychiatrists or to call hotlines, and they may well resort to an alternate method if any given one is not readily available. For these individuals, suicide may not be preventable, and we may be left to say that this tragic phenomena with its diverse causes should also lead us to explore the root causes of human misery and our cultural features that lead some people to end their own lives while others endure.
Clearly, there are those who have untreated psychiatric illnesses and who make impulsive and lethal decisions – access to care and means restrictions certainly save some lives. And while it is obvious to us as psychiatrists that anyone who is depressed or is having suicidal thoughts is deserving of a psychiatric evaluation and intervention, the truth remains that access to treatment in this country is limited by finances, by the availability of mental health professionals, and by stigma and shame. In the end, The one thing I am certain of is that our efforts to prevent suicide should unite, and not fracture, our profession.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Topic of suicide prevention causing divisions within psychiatry
Topic of suicide prevention causing divisions within psychiatry
At every swipe through my social media feeds, I’m greeted with another topic that has advocates clustered at the extremes. People align, and they align quickly in our strangely polarized world in which anyone who might sit in the middle lies low.
It seems we’re divided: On the left you are a CNN fan or you’re one of those soulless monsters who tunes in to Fox News. You’re pro-life or you’re a baby killer, advocating for late-term abortions or even the execution of live infants. When it comes to firearm regulation, one side says you’re a threat to the Constitution, while the other says that those opposed are responsible for the death of every person who was ever the victim of a discharged firearm. And those who feel strongly about a given topic often justify their attacks on those who disagree. Psychiatry is no stranger to this thinking, and we are the only medical specialty with organized “antipsychiatry” groups who oppose our work. I have been a bit surprised, however, that the topic of suicide prevention is one that has us divided within our own specialty.
Amy Barnhorst, MD, is a psychiatrist at the University of California, Davis, and the author of “The empty promise of suicide prevention: Many of the problems that lead people to kill themselves cannot be fixed with a little serotonin,” an op-ed piece that appeared in the New York Times on April 26, 2019. Dr. Barnhorst began her essay with the story of a patient who was hospitalized after a relative realized she was planning her suicide. That story had an ending that psychiatrists relish: A person with previously unrecognized and untreated bipolar disorder received care, including medication, and got better. This suicide was preventable, a life was saved, and this story followed a model we all hope is being replicated over and over.
Dr. Barnhorst went on to say that this was an outlier in her career, that most of the suicidal patients she sees are impoverished, homeless, addicted, and she wrote about how little the treatment setting has to offer: The idea that a pill would fix these problems is almost laughable. She suggests that there is more to suicide prevention than identifying prospective patients and getting them acute psychiatric care.
The decision to stop living is one that people arrive at by different paths, some over months, but many in a matter of minutes. Those people won’t be intercepted by the mental health system. We certainly need more psychiatric services and more research into better, faster-acting treatments for severe depression and suicidal thoughts, but that will never be enough.
We need to address the root causes of our nation’s suicide problem – poverty, homelessness, and the accompanying exposure to trauma, crime, and drugs. That means better alcohol and drug treatment, family counseling, low-income housing resources, job training, and individual therapy. And for those at risk who still slip past all the checkpoints, we need to make sure they don’t have access to guns and lethal medications.
Psychological autopsies done after suicides have indicated that more than 90% of people who die from suicide suffered from a mental illness, yet 54% of those who ended their own lives had never received a psychiatric diagnosis. There is a hopefulness that, if only we had more – more services, more therapy, more medication – then we could prevent suicide. Unfortunately, this line of thinking, with a “Zero Suicide” initiative, points a finger at those who survive: Suicide is preventable, so someone is to blame, if not a family member for missing the warning signs then the clinician who offered treatment that wasn’t good enough.
Along this line, the New York Times printed another opinion piece on Jan. 6 by Richard A. Friedman, MD, titled, “Why are young Americans killing themselves?” Dr. Friedman’s conclusion was more along the psychiatrist party line: “The good news is that we don’t have to wait for all the answers to know what to do. We know that various psychotherapies and medication are highly effective in treating depression. We just need to do a better job of identifying, reaching out to and providing resources for at-risk youths.”
Dr. Friedman goes on to propose universal screening at school, among other measures to identify those at risk. It is no surprise that Dr. Friedman’s article had more than 1,700 comments before commenting was closed by the Times. I have written about the pros and cons of screening adolescents for depression in a primary care setting, so putting the responsibility of identifying suicidal teenagers on school teachers seems like an ominous responsibility to add to a teacher’s obligations.
I did not read Dr. Barnhorst’s earlier op-ed piece as a condemnation of psychiatric care, but rather as a call to action and a reality check on the idea that psychiatry is the only answer to our suicide epidemic. More people than ever get treatment – from psychiatrists, from primary care doctors, from nonphysician prescribing clinicians, and from so many varieties of psychotherapists, and yet our suicide rates continue to rise.
In a post on the Psychology Today website, Sara Gorman, PhD, and Jack M. Gorman, MD, discussed Dr. Barnhorst’s article. “In the process of making her point, Barnhorst also manages to seriously trivialize the role of antidepressant medication in the treatment of depression and to imply that, given societal woes, there isn’t much we can do to try to prevent suicides – aside from limiting access to lethal means,” they wrote.
The Gormans were not alone in their objections; the day after the op-ed appeared in the New York Times, a well-respected psychiatry department chairman took on not just the content of the op-ed, but also the author, in his Twitter feed. He wrote, “@amybarnhorst doesn’t read scientific literature or skipped training. this article is wrong. #suicide is largely preventable, if proper measures taken n Rx provided. @nytimes please vet authors better @APAPsychiatric.” Dr. Barnhorst, also a voice on Twitter, added the wry response, “I skipped training.” When Twitter users responded that initial Twitter comment conveyed a lack of civility toward a colleague, the original Tweeter – I’m withholding his name with the hope that even writing about these interactions won’t put me on anyone’s enemy list – like many others sitting on the poles of these contentious topics, responded with the following, “All for civility except in the case of misinformation that puts lives at risk, especially when purveyed by a professional who wears the patina of credibility.”
If it’s not yet obvious, I don’t believe there is a simple answer to our suicide problem, nor do I think it puts lives at risk to point out that, so far, our treatments have not lowered suicide rates. The issue is complex and we have no perfect explanation as to why countries differ so greatly with regard to suicide. There are impoverished, war-torn countries with remarkably lower suicide rates, and nations with much stricter gun laws that have higher statistics. Honduras, deemed “the murder capital of the world,” has an enviable suicide rate of only 2.9 per 100,000.
If the solution were as simple as making medications more accessible, the answer might be an easy one (or at least worth trying) – make antidepressants available over-the-counter, a move that would both increase access and decrease stigma.
Some people are determined to end their own lives. They aren’t looking to see psychiatrists or to call hotlines, and they may well resort to an alternate method if any given one is not readily available. For these individuals, suicide may not be preventable, and we may be left to say that this tragic phenomena with its diverse causes should also lead us to explore the root causes of human misery and our cultural features that lead some people to end their own lives while others endure.
Clearly, there are those who have untreated psychiatric illnesses and who make impulsive and lethal decisions – access to care and means restrictions certainly save some lives. And while it is obvious to us as psychiatrists that anyone who is depressed or is having suicidal thoughts is deserving of a psychiatric evaluation and intervention, the truth remains that access to treatment in this country is limited by finances, by the availability of mental health professionals, and by stigma and shame. In the end, The one thing I am certain of is that our efforts to prevent suicide should unite, and not fracture, our profession.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
At every swipe through my social media feeds, I’m greeted with another topic that has advocates clustered at the extremes. People align, and they align quickly in our strangely polarized world in which anyone who might sit in the middle lies low.
It seems we’re divided: On the left you are a CNN fan or you’re one of those soulless monsters who tunes in to Fox News. You’re pro-life or you’re a baby killer, advocating for late-term abortions or even the execution of live infants. When it comes to firearm regulation, one side says you’re a threat to the Constitution, while the other says that those opposed are responsible for the death of every person who was ever the victim of a discharged firearm. And those who feel strongly about a given topic often justify their attacks on those who disagree. Psychiatry is no stranger to this thinking, and we are the only medical specialty with organized “antipsychiatry” groups who oppose our work. I have been a bit surprised, however, that the topic of suicide prevention is one that has us divided within our own specialty.
Amy Barnhorst, MD, is a psychiatrist at the University of California, Davis, and the author of “The empty promise of suicide prevention: Many of the problems that lead people to kill themselves cannot be fixed with a little serotonin,” an op-ed piece that appeared in the New York Times on April 26, 2019. Dr. Barnhorst began her essay with the story of a patient who was hospitalized after a relative realized she was planning her suicide. That story had an ending that psychiatrists relish: A person with previously unrecognized and untreated bipolar disorder received care, including medication, and got better. This suicide was preventable, a life was saved, and this story followed a model we all hope is being replicated over and over.
Dr. Barnhorst went on to say that this was an outlier in her career, that most of the suicidal patients she sees are impoverished, homeless, addicted, and she wrote about how little the treatment setting has to offer: The idea that a pill would fix these problems is almost laughable. She suggests that there is more to suicide prevention than identifying prospective patients and getting them acute psychiatric care.
The decision to stop living is one that people arrive at by different paths, some over months, but many in a matter of minutes. Those people won’t be intercepted by the mental health system. We certainly need more psychiatric services and more research into better, faster-acting treatments for severe depression and suicidal thoughts, but that will never be enough.
We need to address the root causes of our nation’s suicide problem – poverty, homelessness, and the accompanying exposure to trauma, crime, and drugs. That means better alcohol and drug treatment, family counseling, low-income housing resources, job training, and individual therapy. And for those at risk who still slip past all the checkpoints, we need to make sure they don’t have access to guns and lethal medications.
Psychological autopsies done after suicides have indicated that more than 90% of people who die from suicide suffered from a mental illness, yet 54% of those who ended their own lives had never received a psychiatric diagnosis. There is a hopefulness that, if only we had more – more services, more therapy, more medication – then we could prevent suicide. Unfortunately, this line of thinking, with a “Zero Suicide” initiative, points a finger at those who survive: Suicide is preventable, so someone is to blame, if not a family member for missing the warning signs then the clinician who offered treatment that wasn’t good enough.
Along this line, the New York Times printed another opinion piece on Jan. 6 by Richard A. Friedman, MD, titled, “Why are young Americans killing themselves?” Dr. Friedman’s conclusion was more along the psychiatrist party line: “The good news is that we don’t have to wait for all the answers to know what to do. We know that various psychotherapies and medication are highly effective in treating depression. We just need to do a better job of identifying, reaching out to and providing resources for at-risk youths.”
Dr. Friedman goes on to propose universal screening at school, among other measures to identify those at risk. It is no surprise that Dr. Friedman’s article had more than 1,700 comments before commenting was closed by the Times. I have written about the pros and cons of screening adolescents for depression in a primary care setting, so putting the responsibility of identifying suicidal teenagers on school teachers seems like an ominous responsibility to add to a teacher’s obligations.
I did not read Dr. Barnhorst’s earlier op-ed piece as a condemnation of psychiatric care, but rather as a call to action and a reality check on the idea that psychiatry is the only answer to our suicide epidemic. More people than ever get treatment – from psychiatrists, from primary care doctors, from nonphysician prescribing clinicians, and from so many varieties of psychotherapists, and yet our suicide rates continue to rise.
In a post on the Psychology Today website, Sara Gorman, PhD, and Jack M. Gorman, MD, discussed Dr. Barnhorst’s article. “In the process of making her point, Barnhorst also manages to seriously trivialize the role of antidepressant medication in the treatment of depression and to imply that, given societal woes, there isn’t much we can do to try to prevent suicides – aside from limiting access to lethal means,” they wrote.
The Gormans were not alone in their objections; the day after the op-ed appeared in the New York Times, a well-respected psychiatry department chairman took on not just the content of the op-ed, but also the author, in his Twitter feed. He wrote, “@amybarnhorst doesn’t read scientific literature or skipped training. this article is wrong. #suicide is largely preventable, if proper measures taken n Rx provided. @nytimes please vet authors better @APAPsychiatric.” Dr. Barnhorst, also a voice on Twitter, added the wry response, “I skipped training.” When Twitter users responded that initial Twitter comment conveyed a lack of civility toward a colleague, the original Tweeter – I’m withholding his name with the hope that even writing about these interactions won’t put me on anyone’s enemy list – like many others sitting on the poles of these contentious topics, responded with the following, “All for civility except in the case of misinformation that puts lives at risk, especially when purveyed by a professional who wears the patina of credibility.”
If it’s not yet obvious, I don’t believe there is a simple answer to our suicide problem, nor do I think it puts lives at risk to point out that, so far, our treatments have not lowered suicide rates. The issue is complex and we have no perfect explanation as to why countries differ so greatly with regard to suicide. There are impoverished, war-torn countries with remarkably lower suicide rates, and nations with much stricter gun laws that have higher statistics. Honduras, deemed “the murder capital of the world,” has an enviable suicide rate of only 2.9 per 100,000.
If the solution were as simple as making medications more accessible, the answer might be an easy one (or at least worth trying) – make antidepressants available over-the-counter, a move that would both increase access and decrease stigma.
Some people are determined to end their own lives. They aren’t looking to see psychiatrists or to call hotlines, and they may well resort to an alternate method if any given one is not readily available. For these individuals, suicide may not be preventable, and we may be left to say that this tragic phenomena with its diverse causes should also lead us to explore the root causes of human misery and our cultural features that lead some people to end their own lives while others endure.
Clearly, there are those who have untreated psychiatric illnesses and who make impulsive and lethal decisions – access to care and means restrictions certainly save some lives. And while it is obvious to us as psychiatrists that anyone who is depressed or is having suicidal thoughts is deserving of a psychiatric evaluation and intervention, the truth remains that access to treatment in this country is limited by finances, by the availability of mental health professionals, and by stigma and shame. In the end, The one thing I am certain of is that our efforts to prevent suicide should unite, and not fracture, our profession.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
February 2020
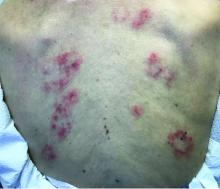
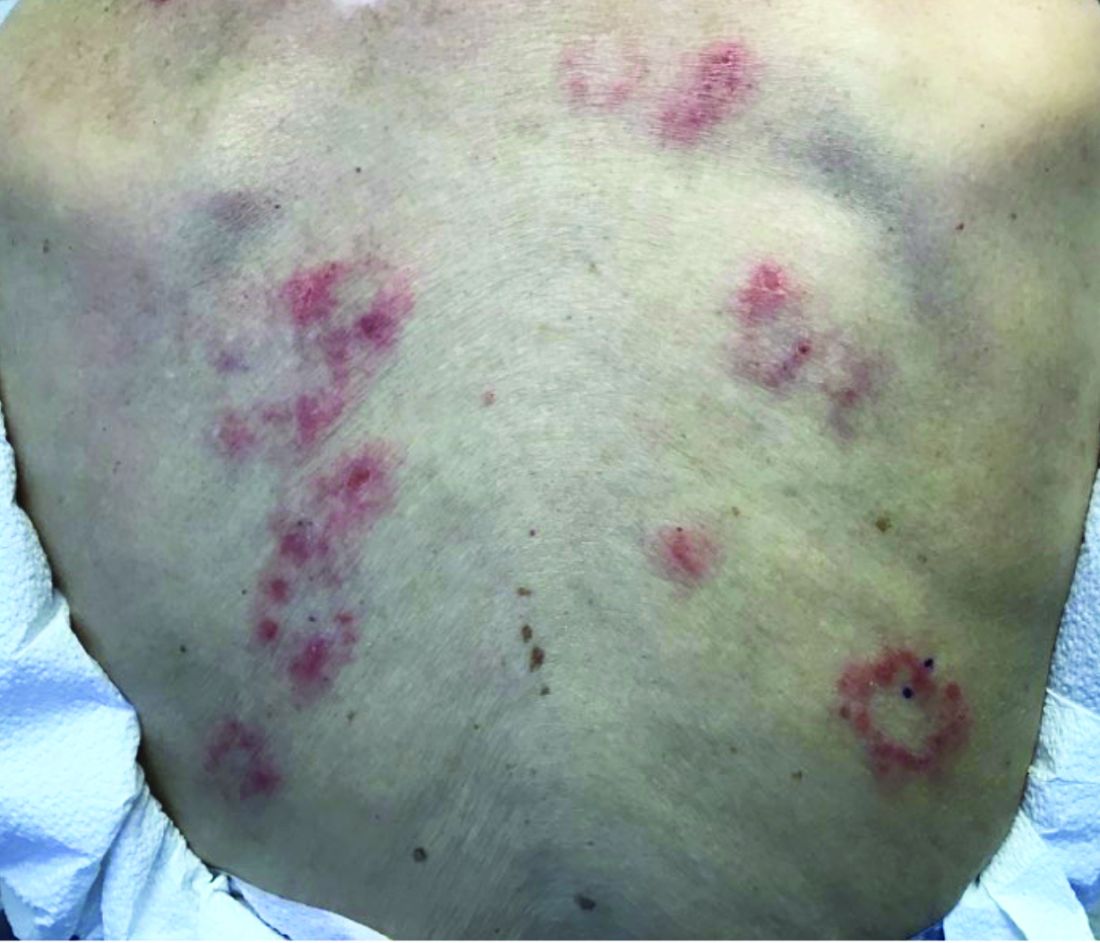
Subacute cutaneous lupus erythematosus
Subacute cutaneous lupus erythematosus (SCLE) is a type of cutaneous lupus erythematosus that may occur independently of or in combination with systemic lupus erythematosus. About 10%-15% of patients with SCLE will develop systemic lupus erythematosus. White females are more typically affected.
SCLE lesions often present as scaly, annular, or polycyclic scaly patches and plaques with central clearing. They may appear psoriasiform. They heal without atrophy or scarring but may leave dyspigmentation. Follicular plugging is absent. Lesions generally occur on sun exposed areas such as the neck, V of the chest, and upper extremities. Up to 75% of patients may exhibit associated symptoms such as photosensitivity, oral ulcers, and arthritis. Less than 20% of patients will develop internal disease, including nephritis and pulmonary disease. Symptoms of Sjögren’s syndrome and SCLE may overlap in some patients, and will portend higher risk for internal disease.
The differential diagnosis includes eczema, psoriasis, dermatophytosis, granuloma annulare, and erythema annulare centrifugum. Histology reveals epidermal atrophy and keratinocyte apoptosis, with a superficial and perivascular lymphohistiocytic infiltrate in the upper dermis. Interface changes at the dermal-epidermal junction can be seen. Direct immunofluorescence of lesional skin is positive in one-third of cases, often revealing granular deposits of IgG and IgM at the dermal-epidermal junction and around hair follicles (called the lupus-band test). Serology in SCLE may reveal a positive antinuclear antigen test, as well as positive Ro/SSA antigen. Other lupus serologies such as La/SSB, dsDNA, antihistone, and Sm antibodies may be positive, but are less commonly seen.
Several drugs may cause SCLE, such as hydrochlorothiazide, terbinafine, ACE inhibitors, NSAIDs, calcium-channel blockers, interferons, anticonvulsants, griseofulvin, penicillamine, spironolactone, tumor necrosis factor–alpha inhibitors, and statins. Discontinuing the offending medications may clear the lesions, but not always.
Treatment includes sunscreen and avoidance of sun exposure. Potent topical corticosteroids are helpful. If systemic treatment is indicated, antimalarials are first line.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Subacute cutaneous lupus erythematosus
Subacute cutaneous lupus erythematosus (SCLE) is a type of cutaneous lupus erythematosus that may occur independently of or in combination with systemic lupus erythematosus. About 10%-15% of patients with SCLE will develop systemic lupus erythematosus. White females are more typically affected.
SCLE lesions often present as scaly, annular, or polycyclic scaly patches and plaques with central clearing. They may appear psoriasiform. They heal without atrophy or scarring but may leave dyspigmentation. Follicular plugging is absent. Lesions generally occur on sun exposed areas such as the neck, V of the chest, and upper extremities. Up to 75% of patients may exhibit associated symptoms such as photosensitivity, oral ulcers, and arthritis. Less than 20% of patients will develop internal disease, including nephritis and pulmonary disease. Symptoms of Sjögren’s syndrome and SCLE may overlap in some patients, and will portend higher risk for internal disease.
The differential diagnosis includes eczema, psoriasis, dermatophytosis, granuloma annulare, and erythema annulare centrifugum. Histology reveals epidermal atrophy and keratinocyte apoptosis, with a superficial and perivascular lymphohistiocytic infiltrate in the upper dermis. Interface changes at the dermal-epidermal junction can be seen. Direct immunofluorescence of lesional skin is positive in one-third of cases, often revealing granular deposits of IgG and IgM at the dermal-epidermal junction and around hair follicles (called the lupus-band test). Serology in SCLE may reveal a positive antinuclear antigen test, as well as positive Ro/SSA antigen. Other lupus serologies such as La/SSB, dsDNA, antihistone, and Sm antibodies may be positive, but are less commonly seen.
Several drugs may cause SCLE, such as hydrochlorothiazide, terbinafine, ACE inhibitors, NSAIDs, calcium-channel blockers, interferons, anticonvulsants, griseofulvin, penicillamine, spironolactone, tumor necrosis factor–alpha inhibitors, and statins. Discontinuing the offending medications may clear the lesions, but not always.
Treatment includes sunscreen and avoidance of sun exposure. Potent topical corticosteroids are helpful. If systemic treatment is indicated, antimalarials are first line.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Subacute cutaneous lupus erythematosus
Subacute cutaneous lupus erythematosus (SCLE) is a type of cutaneous lupus erythematosus that may occur independently of or in combination with systemic lupus erythematosus. About 10%-15% of patients with SCLE will develop systemic lupus erythematosus. White females are more typically affected.
SCLE lesions often present as scaly, annular, or polycyclic scaly patches and plaques with central clearing. They may appear psoriasiform. They heal without atrophy or scarring but may leave dyspigmentation. Follicular plugging is absent. Lesions generally occur on sun exposed areas such as the neck, V of the chest, and upper extremities. Up to 75% of patients may exhibit associated symptoms such as photosensitivity, oral ulcers, and arthritis. Less than 20% of patients will develop internal disease, including nephritis and pulmonary disease. Symptoms of Sjögren’s syndrome and SCLE may overlap in some patients, and will portend higher risk for internal disease.
The differential diagnosis includes eczema, psoriasis, dermatophytosis, granuloma annulare, and erythema annulare centrifugum. Histology reveals epidermal atrophy and keratinocyte apoptosis, with a superficial and perivascular lymphohistiocytic infiltrate in the upper dermis. Interface changes at the dermal-epidermal junction can be seen. Direct immunofluorescence of lesional skin is positive in one-third of cases, often revealing granular deposits of IgG and IgM at the dermal-epidermal junction and around hair follicles (called the lupus-band test). Serology in SCLE may reveal a positive antinuclear antigen test, as well as positive Ro/SSA antigen. Other lupus serologies such as La/SSB, dsDNA, antihistone, and Sm antibodies may be positive, but are less commonly seen.
Several drugs may cause SCLE, such as hydrochlorothiazide, terbinafine, ACE inhibitors, NSAIDs, calcium-channel blockers, interferons, anticonvulsants, griseofulvin, penicillamine, spironolactone, tumor necrosis factor–alpha inhibitors, and statins. Discontinuing the offending medications may clear the lesions, but not always.
Treatment includes sunscreen and avoidance of sun exposure. Potent topical corticosteroids are helpful. If systemic treatment is indicated, antimalarials are first line.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
New year, old you
This column should arrive just in time. By this time in February, eighty percent of us will abort what we resolved to do this year. If this was you, it could be considered a catastrophic failure because not only is it a new year, it is a new decade. That’s right, the opportunity to fix the 10-year-imperfect you won’t come again until 2030!
I’m among you. I intended to read fiction daily (starting with “The Great Gatsby,” not “Moby Dick” – I thought I would give myself a fighting chance, but alas ...), to workout at least 5 days every week (I tore my left triangular fibrocartilage complex, so there’s that), to write at least 500 words daily (I’m typing this one-handed: I’m lucky to get 500 letters a day). So I’m out.
If you resolved to do something this year, chances are it was to make a better you: a self-improvement goal such as losing weight, saving more money, or exercising more. According to a Marist Poll, these were the most popular resolutions for 2020. At the bottom of the most-likely-resolutions list were things like “worry less” or “be kinder to others.” These are important goals we’d agree, but we don’t deem them resolution-worthy. Why?
And why do we have New Year’s resolutions in the first place? When I looked into this further, I was surprised by some of the history I discovered.
As far back as the Babylonians, once a year, we’ve tried our best to get better. At the feast of Akitu, the Babylonian new year festival (about March on our modern calendar), people resolved to do a better job of paying debts and returning favors – spin had not been invented, and yoga hadn’t caught on in the Middle East yet. This fundamental desire to be a better human seems hardwired, and long before Bullet Journals we seem to have loved “fresh start” days on the calendar. Yet, we’re doomed to fail, over and over, at least for the last 5,000 or so attempts.
We know so much more now. Put your Nike Renue Fusion shoes next to your bed so you get up and run first thing. Set SMART goals. Sign up for automatic retirement contribution and for automatic, plant-based meal delivery from Blue Apron. (I’ve no conflict of interest in these products).
Good ideas all, but I’m suggesting a different approach: Resolve to do something else this year.
Rather than try the same things we’ve attempted, how about selecting something from the bottom of the Marist Poll list – such as resolving to be more humble. Admit when you don’t know something or don’t understand what’s being discussed. Recognize and acknowledge when you’ve screwed up. Or resolve to be more selfless. Add on someone else’s patient, an extra call without expecting a favor in return, or do what you can to help a curbside consult, even if there is no reward or even a small risk to you. Repay the debt you owe your friends, family, colleagues, staff, and patients.
These things are a little trickier to track, but you can find a way to keep yourself accountable. Add a box to your weekly planner that says “Be humble and kind” and check it off for the next 42 weeks. Good news, March 1 falls on a Sunday this year – let’s call it the feast of Akitu.
Happy New Year! And good luck!
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
This column should arrive just in time. By this time in February, eighty percent of us will abort what we resolved to do this year. If this was you, it could be considered a catastrophic failure because not only is it a new year, it is a new decade. That’s right, the opportunity to fix the 10-year-imperfect you won’t come again until 2030!
I’m among you. I intended to read fiction daily (starting with “The Great Gatsby,” not “Moby Dick” – I thought I would give myself a fighting chance, but alas ...), to workout at least 5 days every week (I tore my left triangular fibrocartilage complex, so there’s that), to write at least 500 words daily (I’m typing this one-handed: I’m lucky to get 500 letters a day). So I’m out.
If you resolved to do something this year, chances are it was to make a better you: a self-improvement goal such as losing weight, saving more money, or exercising more. According to a Marist Poll, these were the most popular resolutions for 2020. At the bottom of the most-likely-resolutions list were things like “worry less” or “be kinder to others.” These are important goals we’d agree, but we don’t deem them resolution-worthy. Why?
And why do we have New Year’s resolutions in the first place? When I looked into this further, I was surprised by some of the history I discovered.
As far back as the Babylonians, once a year, we’ve tried our best to get better. At the feast of Akitu, the Babylonian new year festival (about March on our modern calendar), people resolved to do a better job of paying debts and returning favors – spin had not been invented, and yoga hadn’t caught on in the Middle East yet. This fundamental desire to be a better human seems hardwired, and long before Bullet Journals we seem to have loved “fresh start” days on the calendar. Yet, we’re doomed to fail, over and over, at least for the last 5,000 or so attempts.
We know so much more now. Put your Nike Renue Fusion shoes next to your bed so you get up and run first thing. Set SMART goals. Sign up for automatic retirement contribution and for automatic, plant-based meal delivery from Blue Apron. (I’ve no conflict of interest in these products).
Good ideas all, but I’m suggesting a different approach: Resolve to do something else this year.
Rather than try the same things we’ve attempted, how about selecting something from the bottom of the Marist Poll list – such as resolving to be more humble. Admit when you don’t know something or don’t understand what’s being discussed. Recognize and acknowledge when you’ve screwed up. Or resolve to be more selfless. Add on someone else’s patient, an extra call without expecting a favor in return, or do what you can to help a curbside consult, even if there is no reward or even a small risk to you. Repay the debt you owe your friends, family, colleagues, staff, and patients.
These things are a little trickier to track, but you can find a way to keep yourself accountable. Add a box to your weekly planner that says “Be humble and kind” and check it off for the next 42 weeks. Good news, March 1 falls on a Sunday this year – let’s call it the feast of Akitu.
Happy New Year! And good luck!
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
This column should arrive just in time. By this time in February, eighty percent of us will abort what we resolved to do this year. If this was you, it could be considered a catastrophic failure because not only is it a new year, it is a new decade. That’s right, the opportunity to fix the 10-year-imperfect you won’t come again until 2030!
I’m among you. I intended to read fiction daily (starting with “The Great Gatsby,” not “Moby Dick” – I thought I would give myself a fighting chance, but alas ...), to workout at least 5 days every week (I tore my left triangular fibrocartilage complex, so there’s that), to write at least 500 words daily (I’m typing this one-handed: I’m lucky to get 500 letters a day). So I’m out.
If you resolved to do something this year, chances are it was to make a better you: a self-improvement goal such as losing weight, saving more money, or exercising more. According to a Marist Poll, these were the most popular resolutions for 2020. At the bottom of the most-likely-resolutions list were things like “worry less” or “be kinder to others.” These are important goals we’d agree, but we don’t deem them resolution-worthy. Why?
And why do we have New Year’s resolutions in the first place? When I looked into this further, I was surprised by some of the history I discovered.
As far back as the Babylonians, once a year, we’ve tried our best to get better. At the feast of Akitu, the Babylonian new year festival (about March on our modern calendar), people resolved to do a better job of paying debts and returning favors – spin had not been invented, and yoga hadn’t caught on in the Middle East yet. This fundamental desire to be a better human seems hardwired, and long before Bullet Journals we seem to have loved “fresh start” days on the calendar. Yet, we’re doomed to fail, over and over, at least for the last 5,000 or so attempts.
We know so much more now. Put your Nike Renue Fusion shoes next to your bed so you get up and run first thing. Set SMART goals. Sign up for automatic retirement contribution and for automatic, plant-based meal delivery from Blue Apron. (I’ve no conflict of interest in these products).
Good ideas all, but I’m suggesting a different approach: Resolve to do something else this year.
Rather than try the same things we’ve attempted, how about selecting something from the bottom of the Marist Poll list – such as resolving to be more humble. Admit when you don’t know something or don’t understand what’s being discussed. Recognize and acknowledge when you’ve screwed up. Or resolve to be more selfless. Add on someone else’s patient, an extra call without expecting a favor in return, or do what you can to help a curbside consult, even if there is no reward or even a small risk to you. Repay the debt you owe your friends, family, colleagues, staff, and patients.
These things are a little trickier to track, but you can find a way to keep yourself accountable. Add a box to your weekly planner that says “Be humble and kind” and check it off for the next 42 weeks. Good news, March 1 falls on a Sunday this year – let’s call it the feast of Akitu.
Happy New Year! And good luck!
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Common drug with lots of surprising side effects
A 55-year-old woman comes to clinic for follow-up. She reports her family is worried that she isn’t getting enough sleep and is more tired than usual. The patient reports she is sleeping 8 hours a night and wakes up feeling rested, but she has noticed she has been yawning much more frequently than she remembers in the past.
Past medical history: gastroesophageal reflux disease, hypertension, generalized anxiety disorder, hypothyroidism, and osteoporosis. Medications: amlodipine, lansoprazole, irbesartan, escitalopram, levothyroxine, and alendronate. Physical examination: blood pressure 110/70 mm Hg, pulse 60 bpm. Lower extremities: 1+ edema.
What is the likely cause of her increased yawning?
A. Amlodipine.
B. Alendronate.
C. Irbesartan.
D. Escitalopram.
E. Lansoprazole.
The correct answer here is escitalopram. Selective serotonin reuptake inhibitors in general are well tolerated. Given how commonly these drugs are used, however, there are a number of lesser-known side effects that you are likely to see.
In the above case, this patient has yawning caused by her SSRI. Roncero et al. described a case of yawning in a patient on escitalopram that resolved when the dose of escitalopram was reduced.1 Paroxetine has been reported to cause yawning at both low and high doses.2
In a review of drug-induced yawning, SSRIs as a class were most frequently involved, and sertraline and fluoxetine were implicated in addition to paroxetine.3 The serotonin-norepinephrine reuptake inhibitors duloxetine and venlafaxine have also been associated with yawning.4,5
Hyperhydrosis has also been linked to SSRIs and SNRIs, and both yawning and hyperhidrosis may occur because of an underlying thermoregulatory dysfunction.6
SSRIs have been linked to increased bleeding risk, especially increased risk of upper gastrointestinal hemorrhage. Laporte and colleagues showed an association of SSRI use and risk of bleeding in a meta-analysis of 42 observational studies, with an odds ratio of 1.41 (95% confidence interval, 1.27-1.57; P less than .0001).7 The risk of upper gastrointestinal (UGI) bleeding is further increased if patients are also taking NSAIDs.
Anglin et al. looked at 15 case-control studies and 4 cohort studies and found an OR of 1.66 for UGI bleeding with SSRI use, and an OR of 4.25 for UGI bleeding if SSRI use was combined with NSAID use.8 The number needed to harm is 3,177 for NSAID use in populations at low risk for GI bleeding, but it is much lower (881) in higher-risk populations.8 Make sure to think about patients’ bleeding risks when starting SSRIs.
An issue that comes up frequently is: What is the risk of bleeding in patients on SSRIs who are also on anticoagulants? Dr. Quinn and colleagues looked at the bleeding risk of anticoagulated patients also taking SSRIs in the ROCKET AF trial.9 They found 737 patients who received SSRIs and matched them with other patients not on SSRIs in the trial. All patients in the trial were either receiving rivaroxaban or warfarin for stroke prophylaxis. They found no significant increase risk in bleeding in the patients on SSRIs and anticoagulants.
Take-home points:
- Yawning and hyperhidrosis are interesting side effects of SSRIs.
- Bleeding risk is increased in patients on SSRIs, especially when combined with NSAIDs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Neurologia. 2013 Nov-Dec;28(9):589-90.
2. Psychiatry Clin Neurosci. 2006 Apr;60(2):260.
3. Presse Med. 2014 Oct;43(10 Pt 1):1135-6.
4. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Jun 15;33(4):747.
5. Ann Pharmacother. 2011 Oct;45(10):1297-301.
6. Depress Anxiety. 2017 Dec;34(12):1134-46.
7. Pharmacol Res. 2017 Apr;118:19-32.
8. Am J Gastroenterol. 2014 Jun;109(6):811-9.
9. J Am Heart Assoc. 2018 Aug 7;7(15):e008755.
A 55-year-old woman comes to clinic for follow-up. She reports her family is worried that she isn’t getting enough sleep and is more tired than usual. The patient reports she is sleeping 8 hours a night and wakes up feeling rested, but she has noticed she has been yawning much more frequently than she remembers in the past.
Past medical history: gastroesophageal reflux disease, hypertension, generalized anxiety disorder, hypothyroidism, and osteoporosis. Medications: amlodipine, lansoprazole, irbesartan, escitalopram, levothyroxine, and alendronate. Physical examination: blood pressure 110/70 mm Hg, pulse 60 bpm. Lower extremities: 1+ edema.
What is the likely cause of her increased yawning?
A. Amlodipine.
B. Alendronate.
C. Irbesartan.
D. Escitalopram.
E. Lansoprazole.
The correct answer here is escitalopram. Selective serotonin reuptake inhibitors in general are well tolerated. Given how commonly these drugs are used, however, there are a number of lesser-known side effects that you are likely to see.
In the above case, this patient has yawning caused by her SSRI. Roncero et al. described a case of yawning in a patient on escitalopram that resolved when the dose of escitalopram was reduced.1 Paroxetine has been reported to cause yawning at both low and high doses.2
In a review of drug-induced yawning, SSRIs as a class were most frequently involved, and sertraline and fluoxetine were implicated in addition to paroxetine.3 The serotonin-norepinephrine reuptake inhibitors duloxetine and venlafaxine have also been associated with yawning.4,5
Hyperhydrosis has also been linked to SSRIs and SNRIs, and both yawning and hyperhidrosis may occur because of an underlying thermoregulatory dysfunction.6
SSRIs have been linked to increased bleeding risk, especially increased risk of upper gastrointestinal hemorrhage. Laporte and colleagues showed an association of SSRI use and risk of bleeding in a meta-analysis of 42 observational studies, with an odds ratio of 1.41 (95% confidence interval, 1.27-1.57; P less than .0001).7 The risk of upper gastrointestinal (UGI) bleeding is further increased if patients are also taking NSAIDs.
Anglin et al. looked at 15 case-control studies and 4 cohort studies and found an OR of 1.66 for UGI bleeding with SSRI use, and an OR of 4.25 for UGI bleeding if SSRI use was combined with NSAID use.8 The number needed to harm is 3,177 for NSAID use in populations at low risk for GI bleeding, but it is much lower (881) in higher-risk populations.8 Make sure to think about patients’ bleeding risks when starting SSRIs.
An issue that comes up frequently is: What is the risk of bleeding in patients on SSRIs who are also on anticoagulants? Dr. Quinn and colleagues looked at the bleeding risk of anticoagulated patients also taking SSRIs in the ROCKET AF trial.9 They found 737 patients who received SSRIs and matched them with other patients not on SSRIs in the trial. All patients in the trial were either receiving rivaroxaban or warfarin for stroke prophylaxis. They found no significant increase risk in bleeding in the patients on SSRIs and anticoagulants.
Take-home points:
- Yawning and hyperhidrosis are interesting side effects of SSRIs.
- Bleeding risk is increased in patients on SSRIs, especially when combined with NSAIDs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Neurologia. 2013 Nov-Dec;28(9):589-90.
2. Psychiatry Clin Neurosci. 2006 Apr;60(2):260.
3. Presse Med. 2014 Oct;43(10 Pt 1):1135-6.
4. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Jun 15;33(4):747.
5. Ann Pharmacother. 2011 Oct;45(10):1297-301.
6. Depress Anxiety. 2017 Dec;34(12):1134-46.
7. Pharmacol Res. 2017 Apr;118:19-32.
8. Am J Gastroenterol. 2014 Jun;109(6):811-9.
9. J Am Heart Assoc. 2018 Aug 7;7(15):e008755.
A 55-year-old woman comes to clinic for follow-up. She reports her family is worried that she isn’t getting enough sleep and is more tired than usual. The patient reports she is sleeping 8 hours a night and wakes up feeling rested, but she has noticed she has been yawning much more frequently than she remembers in the past.
Past medical history: gastroesophageal reflux disease, hypertension, generalized anxiety disorder, hypothyroidism, and osteoporosis. Medications: amlodipine, lansoprazole, irbesartan, escitalopram, levothyroxine, and alendronate. Physical examination: blood pressure 110/70 mm Hg, pulse 60 bpm. Lower extremities: 1+ edema.
What is the likely cause of her increased yawning?
A. Amlodipine.
B. Alendronate.
C. Irbesartan.
D. Escitalopram.
E. Lansoprazole.
The correct answer here is escitalopram. Selective serotonin reuptake inhibitors in general are well tolerated. Given how commonly these drugs are used, however, there are a number of lesser-known side effects that you are likely to see.
In the above case, this patient has yawning caused by her SSRI. Roncero et al. described a case of yawning in a patient on escitalopram that resolved when the dose of escitalopram was reduced.1 Paroxetine has been reported to cause yawning at both low and high doses.2
In a review of drug-induced yawning, SSRIs as a class were most frequently involved, and sertraline and fluoxetine were implicated in addition to paroxetine.3 The serotonin-norepinephrine reuptake inhibitors duloxetine and venlafaxine have also been associated with yawning.4,5
Hyperhydrosis has also been linked to SSRIs and SNRIs, and both yawning and hyperhidrosis may occur because of an underlying thermoregulatory dysfunction.6
SSRIs have been linked to increased bleeding risk, especially increased risk of upper gastrointestinal hemorrhage. Laporte and colleagues showed an association of SSRI use and risk of bleeding in a meta-analysis of 42 observational studies, with an odds ratio of 1.41 (95% confidence interval, 1.27-1.57; P less than .0001).7 The risk of upper gastrointestinal (UGI) bleeding is further increased if patients are also taking NSAIDs.
Anglin et al. looked at 15 case-control studies and 4 cohort studies and found an OR of 1.66 for UGI bleeding with SSRI use, and an OR of 4.25 for UGI bleeding if SSRI use was combined with NSAID use.8 The number needed to harm is 3,177 for NSAID use in populations at low risk for GI bleeding, but it is much lower (881) in higher-risk populations.8 Make sure to think about patients’ bleeding risks when starting SSRIs.
An issue that comes up frequently is: What is the risk of bleeding in patients on SSRIs who are also on anticoagulants? Dr. Quinn and colleagues looked at the bleeding risk of anticoagulated patients also taking SSRIs in the ROCKET AF trial.9 They found 737 patients who received SSRIs and matched them with other patients not on SSRIs in the trial. All patients in the trial were either receiving rivaroxaban or warfarin for stroke prophylaxis. They found no significant increase risk in bleeding in the patients on SSRIs and anticoagulants.
Take-home points:
- Yawning and hyperhidrosis are interesting side effects of SSRIs.
- Bleeding risk is increased in patients on SSRIs, especially when combined with NSAIDs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Neurologia. 2013 Nov-Dec;28(9):589-90.
2. Psychiatry Clin Neurosci. 2006 Apr;60(2):260.
3. Presse Med. 2014 Oct;43(10 Pt 1):1135-6.
4. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Jun 15;33(4):747.
5. Ann Pharmacother. 2011 Oct;45(10):1297-301.
6. Depress Anxiety. 2017 Dec;34(12):1134-46.
7. Pharmacol Res. 2017 Apr;118:19-32.
8. Am J Gastroenterol. 2014 Jun;109(6):811-9.
9. J Am Heart Assoc. 2018 Aug 7;7(15):e008755.
CD1a and cosmetic-related contact dermatitis
As industries develop more chemical extraction techniques for synthetic or purified botanical ingredients to include in cosmetic and personal care products, the incidence of contact dermatitis is rising. Contact dermatitis (irritant or allergic) is the most common occupational skin disease, with current lifetime incidence exceeding 50%. For allergic contact dermatitis, type IV hypersensitivity (or delayed-type hypersensitivity) is thought to be the immunologic mediated pathway in which a T cell–mediated response occurs approximately 72 hours after exposure to the contact allergen. Diagnosis currently is predominately made clinically, after identifying the potential allergen or via patch testing. Treatment typically involves topical steroids or anti-inflammatories should a rash occur, and avoidance of the identified allergen.
In delayed-type hypersensitivity, most T-cell receptors recognize a peptide antigen bound to major histocompatibility complex (MHC) I or MHC II proteins, which stimulates a subsequent inflammatory immune response. However, in a recently published study, the authors wrote that “most known contact allergens are nonpeptidic small molecules, cations, or metals that are typically delivered to skin as drugs, oils, cosmetics, skin creams, or fragrances.” The chemical nature and structure of contact allergens “does not match the chemical structures of most antigens commonly recognized within the TCR-peptide-MHC axis,” they added. Thus, the mechanism by which nonpeptide molecules found in cosmetics cause a T cell–mediated hypersensitivity is poorly understood.
In that study, investigators from Brigham and Women’s Hospital, Boston; Columbia University, New York; and Monash University, Melbourne, looked at whether a protein found in immune cells – CD1a – could be involved in these allergic reactions. In a press release describing the results, cosenior author D. Branch Moody, MD, a principal investigator and physician in Brigham and Women’s division of rheumatology, inflammation, and immunity, noted that they “questioned the prevailing paradigm that T cell–mediated allergic reaction is only triggered when T cells respond to proteins or peptide antigens,” and found “a mechanism through which fragrance can initiate a T-cell response through a protein called CD1a.”
In their study, CD1a was identified as the and personal care products. Specifically, balsam of Peru (a tree oil commonly found in cosmetics and toothpaste), benzyl benzoate, benzyl cinnamate, and farnesol (often present in “fragrance”) after positive patch tests were found to elicit a CD1a-mediated immune response. Their findings suggest that, for these hydrophobic contact allergens, in forming CD1a-farnesol (or other) complexes, displacement of self-lipids normally bound to CD1a occurs, exposing T cell–stimulatory surface regions of CD1a that are normally hidden, thereby eliciting T cell–mediated hypersensitivity reactions.
The authors note that having a better understanding of how these ingredients elicit an immune response on a molecular level can help us potentially identify other molecules that can potentially block this response in humans, thereby treating or potentially mitigating allergic skin disease from these ingredients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Resource
Nicolai S et al. Sci Immunol. 2020 Jan 3. doi: 10.1126/sciimmunol.aax5430.
As industries develop more chemical extraction techniques for synthetic or purified botanical ingredients to include in cosmetic and personal care products, the incidence of contact dermatitis is rising. Contact dermatitis (irritant or allergic) is the most common occupational skin disease, with current lifetime incidence exceeding 50%. For allergic contact dermatitis, type IV hypersensitivity (or delayed-type hypersensitivity) is thought to be the immunologic mediated pathway in which a T cell–mediated response occurs approximately 72 hours after exposure to the contact allergen. Diagnosis currently is predominately made clinically, after identifying the potential allergen or via patch testing. Treatment typically involves topical steroids or anti-inflammatories should a rash occur, and avoidance of the identified allergen.
In delayed-type hypersensitivity, most T-cell receptors recognize a peptide antigen bound to major histocompatibility complex (MHC) I or MHC II proteins, which stimulates a subsequent inflammatory immune response. However, in a recently published study, the authors wrote that “most known contact allergens are nonpeptidic small molecules, cations, or metals that are typically delivered to skin as drugs, oils, cosmetics, skin creams, or fragrances.” The chemical nature and structure of contact allergens “does not match the chemical structures of most antigens commonly recognized within the TCR-peptide-MHC axis,” they added. Thus, the mechanism by which nonpeptide molecules found in cosmetics cause a T cell–mediated hypersensitivity is poorly understood.
In that study, investigators from Brigham and Women’s Hospital, Boston; Columbia University, New York; and Monash University, Melbourne, looked at whether a protein found in immune cells – CD1a – could be involved in these allergic reactions. In a press release describing the results, cosenior author D. Branch Moody, MD, a principal investigator and physician in Brigham and Women’s division of rheumatology, inflammation, and immunity, noted that they “questioned the prevailing paradigm that T cell–mediated allergic reaction is only triggered when T cells respond to proteins or peptide antigens,” and found “a mechanism through which fragrance can initiate a T-cell response through a protein called CD1a.”
In their study, CD1a was identified as the and personal care products. Specifically, balsam of Peru (a tree oil commonly found in cosmetics and toothpaste), benzyl benzoate, benzyl cinnamate, and farnesol (often present in “fragrance”) after positive patch tests were found to elicit a CD1a-mediated immune response. Their findings suggest that, for these hydrophobic contact allergens, in forming CD1a-farnesol (or other) complexes, displacement of self-lipids normally bound to CD1a occurs, exposing T cell–stimulatory surface regions of CD1a that are normally hidden, thereby eliciting T cell–mediated hypersensitivity reactions.
The authors note that having a better understanding of how these ingredients elicit an immune response on a molecular level can help us potentially identify other molecules that can potentially block this response in humans, thereby treating or potentially mitigating allergic skin disease from these ingredients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Resource
Nicolai S et al. Sci Immunol. 2020 Jan 3. doi: 10.1126/sciimmunol.aax5430.
As industries develop more chemical extraction techniques for synthetic or purified botanical ingredients to include in cosmetic and personal care products, the incidence of contact dermatitis is rising. Contact dermatitis (irritant or allergic) is the most common occupational skin disease, with current lifetime incidence exceeding 50%. For allergic contact dermatitis, type IV hypersensitivity (or delayed-type hypersensitivity) is thought to be the immunologic mediated pathway in which a T cell–mediated response occurs approximately 72 hours after exposure to the contact allergen. Diagnosis currently is predominately made clinically, after identifying the potential allergen or via patch testing. Treatment typically involves topical steroids or anti-inflammatories should a rash occur, and avoidance of the identified allergen.
In delayed-type hypersensitivity, most T-cell receptors recognize a peptide antigen bound to major histocompatibility complex (MHC) I or MHC II proteins, which stimulates a subsequent inflammatory immune response. However, in a recently published study, the authors wrote that “most known contact allergens are nonpeptidic small molecules, cations, or metals that are typically delivered to skin as drugs, oils, cosmetics, skin creams, or fragrances.” The chemical nature and structure of contact allergens “does not match the chemical structures of most antigens commonly recognized within the TCR-peptide-MHC axis,” they added. Thus, the mechanism by which nonpeptide molecules found in cosmetics cause a T cell–mediated hypersensitivity is poorly understood.
In that study, investigators from Brigham and Women’s Hospital, Boston; Columbia University, New York; and Monash University, Melbourne, looked at whether a protein found in immune cells – CD1a – could be involved in these allergic reactions. In a press release describing the results, cosenior author D. Branch Moody, MD, a principal investigator and physician in Brigham and Women’s division of rheumatology, inflammation, and immunity, noted that they “questioned the prevailing paradigm that T cell–mediated allergic reaction is only triggered when T cells respond to proteins or peptide antigens,” and found “a mechanism through which fragrance can initiate a T-cell response through a protein called CD1a.”
In their study, CD1a was identified as the and personal care products. Specifically, balsam of Peru (a tree oil commonly found in cosmetics and toothpaste), benzyl benzoate, benzyl cinnamate, and farnesol (often present in “fragrance”) after positive patch tests were found to elicit a CD1a-mediated immune response. Their findings suggest that, for these hydrophobic contact allergens, in forming CD1a-farnesol (or other) complexes, displacement of self-lipids normally bound to CD1a occurs, exposing T cell–stimulatory surface regions of CD1a that are normally hidden, thereby eliciting T cell–mediated hypersensitivity reactions.
The authors note that having a better understanding of how these ingredients elicit an immune response on a molecular level can help us potentially identify other molecules that can potentially block this response in humans, thereby treating or potentially mitigating allergic skin disease from these ingredients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Resource
Nicolai S et al. Sci Immunol. 2020 Jan 3. doi: 10.1126/sciimmunol.aax5430.
The evolving landscape of complement inhibition therapy
The introduction of eculizumab, a monoclonal antibody targeting C5 of the complement cascade, revolutionized the treatment of paroxysmal nocturnal hemoglobinuria (PNH), a rare hematologic disorder characterized by complement-mediated intravascular hemolysis, bone marrow failure, and thrombophilia. Treatment options for PNH were limited before eculizumab was approved by the Food and Drug Administration in 2007.
Its use resulted in the inhibition of intravascular hemolysis, hemoglobin stabilization, and substantial reductions in transfusion requirements. Moreover, eculizumab had the unexpected effect of reducing the risk of thromboembolic complications, the most severe complication of PNH. Patients treated with eculizumab experienced fewer thrombotic events (4%), compared with historical cohorts (27%). Importantly, 5-year overall survival rates for patients with PNH taking eculizumab improved more than 90%, compared wity the 80% reported historically.
More than 10 years later, we are tasked with assessing the impact of this drug. Unquestionably, eculizumab has done more for PNH than we could have hoped for. However, 10 years of additional data reveal the limitations of this groundbreaking therapy. Despite the overall sustained response and survival benefit, hematologic response remains variable. Complete normalization of hemoglobin occurs in less than one-third of patients. Transfusion requirements persist in many patients. Residual anemia during eculizumab therapy is at least partly attributed to bone marrow failure, a feature the complement inhibition does not address. Still, pharmacokinetic limitations of the drug also contribute to the lack of complete responses. There is residual intravascular hemolysis because of insufficient inhibition of C5 and the emergence of C3-mediated extravascular hemolysis constitutes an unanticipated mechanistic complication of all C5-mediated therapies.
The last few years have seen a surge in novel anticomplement agents, which improve upon the already well-established inhibition of C5 but also explore the efficacy of targeting earlier aspects of the complement pathway. During the American Society of Hematology (ASH) annual meeting, we had exciting updates on some of the promising new kids on the block.
Ravulizumab, the newest C5 monoclonal antibody approved by the FDA for PNH, displays more robust C5 inhibition, thereby reducing the breakthrough hemolysis still seen with eculizumab use. Crovalimab, also an anti-C5 humanized antibody, is engineered with Sequential Monoclonal Antibody Recycling Technology that improves the half-life of the drug and facilitates subcutaneous dosing while still achieving complete C5 inhibition. Some of the most exciting data is on danicopan, a small-molecule factor D inhibitor that targets the alternative pathway thereby inhibiting C3 convertase and blocking extravascular hemolysis. It has shown promise as a stand-alone agent, as well as with combined C5 inhibition, while promising safety, a reasonable concern as we explore the long-term risks of targeting the proximal complement pathway.
I was recently asked to comment on how the new complement inhibitors are addressing unmet needs in PNH. While the recent presentations at ASH demonstrate an improvement on the efficacy of C5 inhibition, pharmacokinetics, and drug delivery – all which translate to improved hemoglobin and reduced breakthrough hemolysis for PNH patients – I am most excited at the promise this new generation of drugs holds for other diseases. Since its approval for PNH, eculizumab has also been approved for use in atypical hemolytic uremic syndrome (aHUS), myasthenia gravis, and neuromyelitis optica spectrum disorder.
Perhaps the greatest potential I envision for the new generation of drugs is in aHUS, a chronic disease characterized by hemolytic anemia, thrombocytopenia, and end-stage renal disease that cannot be cured with renal transplantation. The pathophysiology involves dysregulation of complement activation because of genetic mutations or autoantibodies to key proteins in the complement cascade. Though we have experienced some success with eculizumab, responses can be incomplete, particularly in patients with C5 mutations. The newer drugs offer the opportunity to inhibit complement activation at both proximal and distal aspects of the cascade, which may prove ideal in a disease in which the affected protein is not consistent. Moreover, preclinical and clinical trials have shown promise for these novel complement inhibitors in other autoimmune diseases: antibody-mediated vasculitis, C3 glomerulopathy, catastrophic antibody syndrome, membranous nephropathy, and lupus nephritis.
The surge of new complement inhibitors could revolutionize our strategy for treatment of autoimmune-mediated diseases, in which downstream complement activation can manifest with life-threatening tissue injury. Inhibition of complement offers a promising strategy for blocking downstream immune-mediated effector mechanisms of injury common in several autoimmune diseases.
As the results from various clinical trials come to fruition, it will be exciting to determine how to best use this new generation of drugs to target new diseases and whether the next decade is poised to eclipse the progress in complement therapy already established by eculizumab.
Dr. Sosa is a benign hematologist at Fox Chase Cancer Center in Philadelphia. Her research interests are in thromboembolic disease, with a focus in racial and gender disparities.
The introduction of eculizumab, a monoclonal antibody targeting C5 of the complement cascade, revolutionized the treatment of paroxysmal nocturnal hemoglobinuria (PNH), a rare hematologic disorder characterized by complement-mediated intravascular hemolysis, bone marrow failure, and thrombophilia. Treatment options for PNH were limited before eculizumab was approved by the Food and Drug Administration in 2007.
Its use resulted in the inhibition of intravascular hemolysis, hemoglobin stabilization, and substantial reductions in transfusion requirements. Moreover, eculizumab had the unexpected effect of reducing the risk of thromboembolic complications, the most severe complication of PNH. Patients treated with eculizumab experienced fewer thrombotic events (4%), compared with historical cohorts (27%). Importantly, 5-year overall survival rates for patients with PNH taking eculizumab improved more than 90%, compared wity the 80% reported historically.
More than 10 years later, we are tasked with assessing the impact of this drug. Unquestionably, eculizumab has done more for PNH than we could have hoped for. However, 10 years of additional data reveal the limitations of this groundbreaking therapy. Despite the overall sustained response and survival benefit, hematologic response remains variable. Complete normalization of hemoglobin occurs in less than one-third of patients. Transfusion requirements persist in many patients. Residual anemia during eculizumab therapy is at least partly attributed to bone marrow failure, a feature the complement inhibition does not address. Still, pharmacokinetic limitations of the drug also contribute to the lack of complete responses. There is residual intravascular hemolysis because of insufficient inhibition of C5 and the emergence of C3-mediated extravascular hemolysis constitutes an unanticipated mechanistic complication of all C5-mediated therapies.
The last few years have seen a surge in novel anticomplement agents, which improve upon the already well-established inhibition of C5 but also explore the efficacy of targeting earlier aspects of the complement pathway. During the American Society of Hematology (ASH) annual meeting, we had exciting updates on some of the promising new kids on the block.
Ravulizumab, the newest C5 monoclonal antibody approved by the FDA for PNH, displays more robust C5 inhibition, thereby reducing the breakthrough hemolysis still seen with eculizumab use. Crovalimab, also an anti-C5 humanized antibody, is engineered with Sequential Monoclonal Antibody Recycling Technology that improves the half-life of the drug and facilitates subcutaneous dosing while still achieving complete C5 inhibition. Some of the most exciting data is on danicopan, a small-molecule factor D inhibitor that targets the alternative pathway thereby inhibiting C3 convertase and blocking extravascular hemolysis. It has shown promise as a stand-alone agent, as well as with combined C5 inhibition, while promising safety, a reasonable concern as we explore the long-term risks of targeting the proximal complement pathway.
I was recently asked to comment on how the new complement inhibitors are addressing unmet needs in PNH. While the recent presentations at ASH demonstrate an improvement on the efficacy of C5 inhibition, pharmacokinetics, and drug delivery – all which translate to improved hemoglobin and reduced breakthrough hemolysis for PNH patients – I am most excited at the promise this new generation of drugs holds for other diseases. Since its approval for PNH, eculizumab has also been approved for use in atypical hemolytic uremic syndrome (aHUS), myasthenia gravis, and neuromyelitis optica spectrum disorder.
Perhaps the greatest potential I envision for the new generation of drugs is in aHUS, a chronic disease characterized by hemolytic anemia, thrombocytopenia, and end-stage renal disease that cannot be cured with renal transplantation. The pathophysiology involves dysregulation of complement activation because of genetic mutations or autoantibodies to key proteins in the complement cascade. Though we have experienced some success with eculizumab, responses can be incomplete, particularly in patients with C5 mutations. The newer drugs offer the opportunity to inhibit complement activation at both proximal and distal aspects of the cascade, which may prove ideal in a disease in which the affected protein is not consistent. Moreover, preclinical and clinical trials have shown promise for these novel complement inhibitors in other autoimmune diseases: antibody-mediated vasculitis, C3 glomerulopathy, catastrophic antibody syndrome, membranous nephropathy, and lupus nephritis.
The surge of new complement inhibitors could revolutionize our strategy for treatment of autoimmune-mediated diseases, in which downstream complement activation can manifest with life-threatening tissue injury. Inhibition of complement offers a promising strategy for blocking downstream immune-mediated effector mechanisms of injury common in several autoimmune diseases.
As the results from various clinical trials come to fruition, it will be exciting to determine how to best use this new generation of drugs to target new diseases and whether the next decade is poised to eclipse the progress in complement therapy already established by eculizumab.
Dr. Sosa is a benign hematologist at Fox Chase Cancer Center in Philadelphia. Her research interests are in thromboembolic disease, with a focus in racial and gender disparities.
The introduction of eculizumab, a monoclonal antibody targeting C5 of the complement cascade, revolutionized the treatment of paroxysmal nocturnal hemoglobinuria (PNH), a rare hematologic disorder characterized by complement-mediated intravascular hemolysis, bone marrow failure, and thrombophilia. Treatment options for PNH were limited before eculizumab was approved by the Food and Drug Administration in 2007.
Its use resulted in the inhibition of intravascular hemolysis, hemoglobin stabilization, and substantial reductions in transfusion requirements. Moreover, eculizumab had the unexpected effect of reducing the risk of thromboembolic complications, the most severe complication of PNH. Patients treated with eculizumab experienced fewer thrombotic events (4%), compared with historical cohorts (27%). Importantly, 5-year overall survival rates for patients with PNH taking eculizumab improved more than 90%, compared wity the 80% reported historically.
More than 10 years later, we are tasked with assessing the impact of this drug. Unquestionably, eculizumab has done more for PNH than we could have hoped for. However, 10 years of additional data reveal the limitations of this groundbreaking therapy. Despite the overall sustained response and survival benefit, hematologic response remains variable. Complete normalization of hemoglobin occurs in less than one-third of patients. Transfusion requirements persist in many patients. Residual anemia during eculizumab therapy is at least partly attributed to bone marrow failure, a feature the complement inhibition does not address. Still, pharmacokinetic limitations of the drug also contribute to the lack of complete responses. There is residual intravascular hemolysis because of insufficient inhibition of C5 and the emergence of C3-mediated extravascular hemolysis constitutes an unanticipated mechanistic complication of all C5-mediated therapies.
The last few years have seen a surge in novel anticomplement agents, which improve upon the already well-established inhibition of C5 but also explore the efficacy of targeting earlier aspects of the complement pathway. During the American Society of Hematology (ASH) annual meeting, we had exciting updates on some of the promising new kids on the block.
Ravulizumab, the newest C5 monoclonal antibody approved by the FDA for PNH, displays more robust C5 inhibition, thereby reducing the breakthrough hemolysis still seen with eculizumab use. Crovalimab, also an anti-C5 humanized antibody, is engineered with Sequential Monoclonal Antibody Recycling Technology that improves the half-life of the drug and facilitates subcutaneous dosing while still achieving complete C5 inhibition. Some of the most exciting data is on danicopan, a small-molecule factor D inhibitor that targets the alternative pathway thereby inhibiting C3 convertase and blocking extravascular hemolysis. It has shown promise as a stand-alone agent, as well as with combined C5 inhibition, while promising safety, a reasonable concern as we explore the long-term risks of targeting the proximal complement pathway.
I was recently asked to comment on how the new complement inhibitors are addressing unmet needs in PNH. While the recent presentations at ASH demonstrate an improvement on the efficacy of C5 inhibition, pharmacokinetics, and drug delivery – all which translate to improved hemoglobin and reduced breakthrough hemolysis for PNH patients – I am most excited at the promise this new generation of drugs holds for other diseases. Since its approval for PNH, eculizumab has also been approved for use in atypical hemolytic uremic syndrome (aHUS), myasthenia gravis, and neuromyelitis optica spectrum disorder.
Perhaps the greatest potential I envision for the new generation of drugs is in aHUS, a chronic disease characterized by hemolytic anemia, thrombocytopenia, and end-stage renal disease that cannot be cured with renal transplantation. The pathophysiology involves dysregulation of complement activation because of genetic mutations or autoantibodies to key proteins in the complement cascade. Though we have experienced some success with eculizumab, responses can be incomplete, particularly in patients with C5 mutations. The newer drugs offer the opportunity to inhibit complement activation at both proximal and distal aspects of the cascade, which may prove ideal in a disease in which the affected protein is not consistent. Moreover, preclinical and clinical trials have shown promise for these novel complement inhibitors in other autoimmune diseases: antibody-mediated vasculitis, C3 glomerulopathy, catastrophic antibody syndrome, membranous nephropathy, and lupus nephritis.
The surge of new complement inhibitors could revolutionize our strategy for treatment of autoimmune-mediated diseases, in which downstream complement activation can manifest with life-threatening tissue injury. Inhibition of complement offers a promising strategy for blocking downstream immune-mediated effector mechanisms of injury common in several autoimmune diseases.
As the results from various clinical trials come to fruition, it will be exciting to determine how to best use this new generation of drugs to target new diseases and whether the next decade is poised to eclipse the progress in complement therapy already established by eculizumab.
Dr. Sosa is a benign hematologist at Fox Chase Cancer Center in Philadelphia. Her research interests are in thromboembolic disease, with a focus in racial and gender disparities.
Elizabeth Wurtzel helped clear the air on stigma
When Elizabeth Wurtzel wrote “Prozac Nation,” an autobiographical account published in 1994 of her experience with depression and psychiatric medications, she helped shift the public dialogue about mental illness, and in doing so chipped away at the stigma that continues to haunt many of our patients.
Ms. Wurtzel, who died recently at age 52, wrote about depression passionately and matter-of-factly.
As she stated in “Prozac Nation”: “Depression was the loneliest &*%$ thing on earth. There were no halfway houses for depressives, no Depression Anonymous meetings that I knew of. Yes, of course, there were mental hospitals like McLean and Bellevue and Payne Whitney and the Menninger Clinic, but I couldn’t hope to end up in one of those places unless I made a suicide attempt serious enough to warrant oxygen or stitches or a stomach pump.
“I used to wish – to pray to God for the courage and strength – that I’d have the guts not to get better, but to slit my wrists and get a whole lot worse so I could land in some mental ward, where real help might have been possible.”
Think of where the public consciousness was in 1994. Peter D. Kramer, MD, had started the conversation on Prozac a year earlier with his book, “Listening to Prozac,” Kurt Cobain died by suicide in 1994, and 2 years later President Bill Clinton signed the Personal Responsibility and Work Opportunity Reconciliation Act, a law that “reformed” welfare and some say made it more difficult for low-income Americans to secure psychiatric and addiction services (Milbank Q. 2005 Mar;83[1]:65-99).
Prozac, as we know, was the first SSRI on the market in the late 1980s and was hailed as a major medication breakthrough in the treatment of depression. It lacked the side effects of the tricyclic antidepressants of previous years and did not have the potentially dangerous food restrictions associated with monoamine oxidase inhibitors.
Interestingly, the major reviews of Ms. Wurtzel’s book, mainly written by men, were negative. Those reviews focused more on the lifestyle of Ms. Wurtzel, her introspection, and how difficult life was for her, rather than the importance of the book. To me, her writing skills were exceptional, as was her willingness to put her lifestyle and suffering on the line.
The literary critics failed to recognize the book’s importance in unmasking the massive denial of mental illnesses and what Ms. Wurtzel was trying to get across. There have been many successful male writers over the years whose lives were difficult and replete with emotional pain and suffering, and their work was lauded. Regardless of the reviews’ negativity, readers found her book open and enlightening, making it a bestseller – thus paving the way for better and more-open discussion of mental disorders. It also became a touchstone in discussions of antidepressants in the psychiatric literature (Lancet. 1998 Sep 26. doi: 10.1016/S0140-6736(98)08418-9; Lancet. 2015 Oct 1. doi: 10.1016/S2215-0366[15]00430-7; and Biol Psychiatry. 2018 Dec 1;84[11]:e73-5).
However, unfortunately, the stigma still exists on many levels, often starting with the medical profession itself. In my experience over the years in teaching and supervising medical students, many of those not interested in becoming a psychiatrist all too often could not wait for their psych rotation to be over. Generally, they did not take the rotation seriously. I’ve even heard students making light of the delusions and paranoia seen in the suffering of acutely ill patients.
We can take this even further within the profession. I have had many referrals from far too many extremely competent physicians, across many medical specialties, who would refer to their patient as “sort of crazy.” Those physicians want the best for their patients, clearly, in making the referral, but they need to change their thinking and, therefore, their vocabulary about mental disorders. I’d like to see these physicians be more respectful of our patients – just as I would be if I were referring a patient complaining of fatigue and joint pain to a general internist or rheumatologist.
I once knew a brilliant orthopedic surgeon who, when he made a referral, would sit down with the patient and clearly explain why they were not crazy but had an anxiety or a mood problem that he didn’t treat but had a person to refer to who could help. Likewise, I know an ophthalmologist who tells his patients with some emotional symptoms that they are experiencing a difficult situation and would benefit from help that he is not able to provide but could be resolved with another type of specialist who works with their “difficulties.” We clearly need more docs like this who go out of their specialty to explain what patients might need – despite the administrative burden exacted by EMRs on doctors’ time and energy.
As we grow more tolerant in our culture and eliminate distasteful words about people and groups, maybe we should try and avoid the word crazy – even in our general vocabulary. Furthermore, in social situations, while out to dinner with friends, at the gym, or even while in the workplace, just as we may refer to our primary care doc as the best or report we have the best cardiologist or dermatologist, we rarely hear someone being open about the best psychiatrist, psychologist, or therapist in the same manner.
“If ‘Prozac Nation’ has any particular purpose,” she wrote in the afterword, “it would be to come out and say that clinical depression is a real problem, that it ruins lives, that it ends lives, that it very nearly ended my life, that it afflicts many, many people, many very bright and worthy and thoughtful and caring people, people who could probably save the world or at the very least do it some real good.” Those people are our patients, and medicine should take the lead in working further to destigmatize mental illness.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
When Elizabeth Wurtzel wrote “Prozac Nation,” an autobiographical account published in 1994 of her experience with depression and psychiatric medications, she helped shift the public dialogue about mental illness, and in doing so chipped away at the stigma that continues to haunt many of our patients.
Ms. Wurtzel, who died recently at age 52, wrote about depression passionately and matter-of-factly.
As she stated in “Prozac Nation”: “Depression was the loneliest &*%$ thing on earth. There were no halfway houses for depressives, no Depression Anonymous meetings that I knew of. Yes, of course, there were mental hospitals like McLean and Bellevue and Payne Whitney and the Menninger Clinic, but I couldn’t hope to end up in one of those places unless I made a suicide attempt serious enough to warrant oxygen or stitches or a stomach pump.
“I used to wish – to pray to God for the courage and strength – that I’d have the guts not to get better, but to slit my wrists and get a whole lot worse so I could land in some mental ward, where real help might have been possible.”
Think of where the public consciousness was in 1994. Peter D. Kramer, MD, had started the conversation on Prozac a year earlier with his book, “Listening to Prozac,” Kurt Cobain died by suicide in 1994, and 2 years later President Bill Clinton signed the Personal Responsibility and Work Opportunity Reconciliation Act, a law that “reformed” welfare and some say made it more difficult for low-income Americans to secure psychiatric and addiction services (Milbank Q. 2005 Mar;83[1]:65-99).
Prozac, as we know, was the first SSRI on the market in the late 1980s and was hailed as a major medication breakthrough in the treatment of depression. It lacked the side effects of the tricyclic antidepressants of previous years and did not have the potentially dangerous food restrictions associated with monoamine oxidase inhibitors.
Interestingly, the major reviews of Ms. Wurtzel’s book, mainly written by men, were negative. Those reviews focused more on the lifestyle of Ms. Wurtzel, her introspection, and how difficult life was for her, rather than the importance of the book. To me, her writing skills were exceptional, as was her willingness to put her lifestyle and suffering on the line.
The literary critics failed to recognize the book’s importance in unmasking the massive denial of mental illnesses and what Ms. Wurtzel was trying to get across. There have been many successful male writers over the years whose lives were difficult and replete with emotional pain and suffering, and their work was lauded. Regardless of the reviews’ negativity, readers found her book open and enlightening, making it a bestseller – thus paving the way for better and more-open discussion of mental disorders. It also became a touchstone in discussions of antidepressants in the psychiatric literature (Lancet. 1998 Sep 26. doi: 10.1016/S0140-6736(98)08418-9; Lancet. 2015 Oct 1. doi: 10.1016/S2215-0366[15]00430-7; and Biol Psychiatry. 2018 Dec 1;84[11]:e73-5).
However, unfortunately, the stigma still exists on many levels, often starting with the medical profession itself. In my experience over the years in teaching and supervising medical students, many of those not interested in becoming a psychiatrist all too often could not wait for their psych rotation to be over. Generally, they did not take the rotation seriously. I’ve even heard students making light of the delusions and paranoia seen in the suffering of acutely ill patients.
We can take this even further within the profession. I have had many referrals from far too many extremely competent physicians, across many medical specialties, who would refer to their patient as “sort of crazy.” Those physicians want the best for their patients, clearly, in making the referral, but they need to change their thinking and, therefore, their vocabulary about mental disorders. I’d like to see these physicians be more respectful of our patients – just as I would be if I were referring a patient complaining of fatigue and joint pain to a general internist or rheumatologist.
I once knew a brilliant orthopedic surgeon who, when he made a referral, would sit down with the patient and clearly explain why they were not crazy but had an anxiety or a mood problem that he didn’t treat but had a person to refer to who could help. Likewise, I know an ophthalmologist who tells his patients with some emotional symptoms that they are experiencing a difficult situation and would benefit from help that he is not able to provide but could be resolved with another type of specialist who works with their “difficulties.” We clearly need more docs like this who go out of their specialty to explain what patients might need – despite the administrative burden exacted by EMRs on doctors’ time and energy.
As we grow more tolerant in our culture and eliminate distasteful words about people and groups, maybe we should try and avoid the word crazy – even in our general vocabulary. Furthermore, in social situations, while out to dinner with friends, at the gym, or even while in the workplace, just as we may refer to our primary care doc as the best or report we have the best cardiologist or dermatologist, we rarely hear someone being open about the best psychiatrist, psychologist, or therapist in the same manner.
“If ‘Prozac Nation’ has any particular purpose,” she wrote in the afterword, “it would be to come out and say that clinical depression is a real problem, that it ruins lives, that it ends lives, that it very nearly ended my life, that it afflicts many, many people, many very bright and worthy and thoughtful and caring people, people who could probably save the world or at the very least do it some real good.” Those people are our patients, and medicine should take the lead in working further to destigmatize mental illness.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
When Elizabeth Wurtzel wrote “Prozac Nation,” an autobiographical account published in 1994 of her experience with depression and psychiatric medications, she helped shift the public dialogue about mental illness, and in doing so chipped away at the stigma that continues to haunt many of our patients.
Ms. Wurtzel, who died recently at age 52, wrote about depression passionately and matter-of-factly.
As she stated in “Prozac Nation”: “Depression was the loneliest &*%$ thing on earth. There were no halfway houses for depressives, no Depression Anonymous meetings that I knew of. Yes, of course, there were mental hospitals like McLean and Bellevue and Payne Whitney and the Menninger Clinic, but I couldn’t hope to end up in one of those places unless I made a suicide attempt serious enough to warrant oxygen or stitches or a stomach pump.
“I used to wish – to pray to God for the courage and strength – that I’d have the guts not to get better, but to slit my wrists and get a whole lot worse so I could land in some mental ward, where real help might have been possible.”
Think of where the public consciousness was in 1994. Peter D. Kramer, MD, had started the conversation on Prozac a year earlier with his book, “Listening to Prozac,” Kurt Cobain died by suicide in 1994, and 2 years later President Bill Clinton signed the Personal Responsibility and Work Opportunity Reconciliation Act, a law that “reformed” welfare and some say made it more difficult for low-income Americans to secure psychiatric and addiction services (Milbank Q. 2005 Mar;83[1]:65-99).
Prozac, as we know, was the first SSRI on the market in the late 1980s and was hailed as a major medication breakthrough in the treatment of depression. It lacked the side effects of the tricyclic antidepressants of previous years and did not have the potentially dangerous food restrictions associated with monoamine oxidase inhibitors.
Interestingly, the major reviews of Ms. Wurtzel’s book, mainly written by men, were negative. Those reviews focused more on the lifestyle of Ms. Wurtzel, her introspection, and how difficult life was for her, rather than the importance of the book. To me, her writing skills were exceptional, as was her willingness to put her lifestyle and suffering on the line.
The literary critics failed to recognize the book’s importance in unmasking the massive denial of mental illnesses and what Ms. Wurtzel was trying to get across. There have been many successful male writers over the years whose lives were difficult and replete with emotional pain and suffering, and their work was lauded. Regardless of the reviews’ negativity, readers found her book open and enlightening, making it a bestseller – thus paving the way for better and more-open discussion of mental disorders. It also became a touchstone in discussions of antidepressants in the psychiatric literature (Lancet. 1998 Sep 26. doi: 10.1016/S0140-6736(98)08418-9; Lancet. 2015 Oct 1. doi: 10.1016/S2215-0366[15]00430-7; and Biol Psychiatry. 2018 Dec 1;84[11]:e73-5).
However, unfortunately, the stigma still exists on many levels, often starting with the medical profession itself. In my experience over the years in teaching and supervising medical students, many of those not interested in becoming a psychiatrist all too often could not wait for their psych rotation to be over. Generally, they did not take the rotation seriously. I’ve even heard students making light of the delusions and paranoia seen in the suffering of acutely ill patients.
We can take this even further within the profession. I have had many referrals from far too many extremely competent physicians, across many medical specialties, who would refer to their patient as “sort of crazy.” Those physicians want the best for their patients, clearly, in making the referral, but they need to change their thinking and, therefore, their vocabulary about mental disorders. I’d like to see these physicians be more respectful of our patients – just as I would be if I were referring a patient complaining of fatigue and joint pain to a general internist or rheumatologist.
I once knew a brilliant orthopedic surgeon who, when he made a referral, would sit down with the patient and clearly explain why they were not crazy but had an anxiety or a mood problem that he didn’t treat but had a person to refer to who could help. Likewise, I know an ophthalmologist who tells his patients with some emotional symptoms that they are experiencing a difficult situation and would benefit from help that he is not able to provide but could be resolved with another type of specialist who works with their “difficulties.” We clearly need more docs like this who go out of their specialty to explain what patients might need – despite the administrative burden exacted by EMRs on doctors’ time and energy.
As we grow more tolerant in our culture and eliminate distasteful words about people and groups, maybe we should try and avoid the word crazy – even in our general vocabulary. Furthermore, in social situations, while out to dinner with friends, at the gym, or even while in the workplace, just as we may refer to our primary care doc as the best or report we have the best cardiologist or dermatologist, we rarely hear someone being open about the best psychiatrist, psychologist, or therapist in the same manner.
“If ‘Prozac Nation’ has any particular purpose,” she wrote in the afterword, “it would be to come out and say that clinical depression is a real problem, that it ruins lives, that it ends lives, that it very nearly ended my life, that it afflicts many, many people, many very bright and worthy and thoughtful and caring people, people who could probably save the world or at the very least do it some real good.” Those people are our patients, and medicine should take the lead in working further to destigmatize mental illness.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Seasonality
Did you notice that your practice slows down in February? In fact, if you plot your patient census over a few years, you may find that it dips every February. And you will discover other slow periods, perhaps in December, and busy months during other parts of the year.
Seasonality is yet another of those basic business concepts that most physicians have never heard of, because of the conspicuous lack of business training in medical schools. . Why are people more or less willing to spend money at certain times of the year? Analysts usually blame slow business during January or February on reluctance to buy products or services after the holiday season. They attribute summer peaks to everything from warm weather to an increased propensity to buy when students are out of school, and summer slumps to vacationing customers. It is not always easy – or necessary – to explain seasonality. The point is that such behavior patterns do exist.
It would seem that this behavior would be easy to change, by running some ads, or doing an e-mail blast; but unfortunately, altering a seasonal pattern is not an option for a small private practice. It can be done, but it is a deep pockets game requiring long, expensive campaigns that are only practical for large corporations.
For example, soup was traditionally consumed during the winter months since time immemorial. After years of pervasive advertising extolling its nutritional virtues (remember “Soup is Good Food”?), the soup industry succeeded in convincing the public to use their product year-round. Obviously, that kind of large-scale behavior modification is not practical for a local medical practice.
Does that mean there is nothing we can do about our practices’ seasonal variations? Not at all; but we must work within the realities of our patients’ seasonal behavior, rather than attempting to change that behavior outright.
First, you need to know what that behavior is, because it varies from practice to practice, even within the same state or city. Plotting your seasonality is easy; you can make a graph on Excel in a few minutes. Ask your office manager or accountant for month-by-month billing figures for the last 2 or 3 years. (Make sure it’s the amount billed, not collected, since the latter lags the former by several weeks at least.) Plot those figures on the vertical arm and time (in months) on the horizontal. Alternatively you can plot patient visits per month, if you wish; I do both.
Once you know your seasonality, review your options. Modify your own habits when necessary. If you typically take a vacation in August, for example, that’s not a great idea if August is one of your busiest months; consider vacationing during predictable slow periods instead.
Though I have said that you can’t change most seasonal behavior, it is possible to “retrain” some of your long-time, loyal patients to come in during your slower periods for at least some of their care. Use insurance company rules as a financial incentive, where possible. Many of my patients are on Medicare, so I send a notice to all of them in early November each year, urging them to come in during December (one of my light months) before their deductible has to be paid again.
If you advertise your services, do the bulk of it during your busiest months. That might seem counterintuitive; why not advertise during slow periods to fill those empty slots? But once again, you cannot change seasonal behavior with a low-budget, local advertising campaign; physicians who attempt it invariably get a poor response to their ads. So don’t try to move the mountain to Mohammed. Advertise during your busy periods, when seasonal patterns predict that potential patients are more willing to spend money and are more likely to respond to your message.
In short, then, try to “flatten” your seasonal dips by persuading as many existing patients as possible to return during slower seasons. You can then encourage new patients to make appointments when they are receptive to purchasing new services, your seasonal peaks. Once in your practice, some of them can then be shifted into your slower periods, especially for predictable, periodic care.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Did you notice that your practice slows down in February? In fact, if you plot your patient census over a few years, you may find that it dips every February. And you will discover other slow periods, perhaps in December, and busy months during other parts of the year.
Seasonality is yet another of those basic business concepts that most physicians have never heard of, because of the conspicuous lack of business training in medical schools. . Why are people more or less willing to spend money at certain times of the year? Analysts usually blame slow business during January or February on reluctance to buy products or services after the holiday season. They attribute summer peaks to everything from warm weather to an increased propensity to buy when students are out of school, and summer slumps to vacationing customers. It is not always easy – or necessary – to explain seasonality. The point is that such behavior patterns do exist.
It would seem that this behavior would be easy to change, by running some ads, or doing an e-mail blast; but unfortunately, altering a seasonal pattern is not an option for a small private practice. It can be done, but it is a deep pockets game requiring long, expensive campaigns that are only practical for large corporations.
For example, soup was traditionally consumed during the winter months since time immemorial. After years of pervasive advertising extolling its nutritional virtues (remember “Soup is Good Food”?), the soup industry succeeded in convincing the public to use their product year-round. Obviously, that kind of large-scale behavior modification is not practical for a local medical practice.
Does that mean there is nothing we can do about our practices’ seasonal variations? Not at all; but we must work within the realities of our patients’ seasonal behavior, rather than attempting to change that behavior outright.
First, you need to know what that behavior is, because it varies from practice to practice, even within the same state or city. Plotting your seasonality is easy; you can make a graph on Excel in a few minutes. Ask your office manager or accountant for month-by-month billing figures for the last 2 or 3 years. (Make sure it’s the amount billed, not collected, since the latter lags the former by several weeks at least.) Plot those figures on the vertical arm and time (in months) on the horizontal. Alternatively you can plot patient visits per month, if you wish; I do both.
Once you know your seasonality, review your options. Modify your own habits when necessary. If you typically take a vacation in August, for example, that’s not a great idea if August is one of your busiest months; consider vacationing during predictable slow periods instead.
Though I have said that you can’t change most seasonal behavior, it is possible to “retrain” some of your long-time, loyal patients to come in during your slower periods for at least some of their care. Use insurance company rules as a financial incentive, where possible. Many of my patients are on Medicare, so I send a notice to all of them in early November each year, urging them to come in during December (one of my light months) before their deductible has to be paid again.
If you advertise your services, do the bulk of it during your busiest months. That might seem counterintuitive; why not advertise during slow periods to fill those empty slots? But once again, you cannot change seasonal behavior with a low-budget, local advertising campaign; physicians who attempt it invariably get a poor response to their ads. So don’t try to move the mountain to Mohammed. Advertise during your busy periods, when seasonal patterns predict that potential patients are more willing to spend money and are more likely to respond to your message.
In short, then, try to “flatten” your seasonal dips by persuading as many existing patients as possible to return during slower seasons. You can then encourage new patients to make appointments when they are receptive to purchasing new services, your seasonal peaks. Once in your practice, some of them can then be shifted into your slower periods, especially for predictable, periodic care.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Did you notice that your practice slows down in February? In fact, if you plot your patient census over a few years, you may find that it dips every February. And you will discover other slow periods, perhaps in December, and busy months during other parts of the year.
Seasonality is yet another of those basic business concepts that most physicians have never heard of, because of the conspicuous lack of business training in medical schools. . Why are people more or less willing to spend money at certain times of the year? Analysts usually blame slow business during January or February on reluctance to buy products or services after the holiday season. They attribute summer peaks to everything from warm weather to an increased propensity to buy when students are out of school, and summer slumps to vacationing customers. It is not always easy – or necessary – to explain seasonality. The point is that such behavior patterns do exist.
It would seem that this behavior would be easy to change, by running some ads, or doing an e-mail blast; but unfortunately, altering a seasonal pattern is not an option for a small private practice. It can be done, but it is a deep pockets game requiring long, expensive campaigns that are only practical for large corporations.
For example, soup was traditionally consumed during the winter months since time immemorial. After years of pervasive advertising extolling its nutritional virtues (remember “Soup is Good Food”?), the soup industry succeeded in convincing the public to use their product year-round. Obviously, that kind of large-scale behavior modification is not practical for a local medical practice.
Does that mean there is nothing we can do about our practices’ seasonal variations? Not at all; but we must work within the realities of our patients’ seasonal behavior, rather than attempting to change that behavior outright.
First, you need to know what that behavior is, because it varies from practice to practice, even within the same state or city. Plotting your seasonality is easy; you can make a graph on Excel in a few minutes. Ask your office manager or accountant for month-by-month billing figures for the last 2 or 3 years. (Make sure it’s the amount billed, not collected, since the latter lags the former by several weeks at least.) Plot those figures on the vertical arm and time (in months) on the horizontal. Alternatively you can plot patient visits per month, if you wish; I do both.
Once you know your seasonality, review your options. Modify your own habits when necessary. If you typically take a vacation in August, for example, that’s not a great idea if August is one of your busiest months; consider vacationing during predictable slow periods instead.
Though I have said that you can’t change most seasonal behavior, it is possible to “retrain” some of your long-time, loyal patients to come in during your slower periods for at least some of their care. Use insurance company rules as a financial incentive, where possible. Many of my patients are on Medicare, so I send a notice to all of them in early November each year, urging them to come in during December (one of my light months) before their deductible has to be paid again.
If you advertise your services, do the bulk of it during your busiest months. That might seem counterintuitive; why not advertise during slow periods to fill those empty slots? But once again, you cannot change seasonal behavior with a low-budget, local advertising campaign; physicians who attempt it invariably get a poor response to their ads. So don’t try to move the mountain to Mohammed. Advertise during your busy periods, when seasonal patterns predict that potential patients are more willing to spend money and are more likely to respond to your message.
In short, then, try to “flatten” your seasonal dips by persuading as many existing patients as possible to return during slower seasons. You can then encourage new patients to make appointments when they are receptive to purchasing new services, your seasonal peaks. Once in your practice, some of them can then be shifted into your slower periods, especially for predictable, periodic care.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].