User login
The power and promise of person-generated health data – part 1
The time shared during clinical encounters provides small peeks into patients’ lives that get documented as episodic snapshots in electronic health records. But there is little information about how patients are doing outside of the office. With increasing emphasis on filling out mandatory parts of the EHR, there is less time available for in-depth, in-office conversations and phone follow-ups.
At the same time, it has become clear that it is not just the medicines we prescribe that affect our patients’ lives. Their behaviors outside of the office – being physically active, eating well, getting a good night’s rest, and adhering to medications – also impact their health outcomes.
The explosion of technology and personal data in our increasingly connected world provides powerful new sources of health and behavior information that generate new understanding of patients’ lives in their everyday settings.
The ubiquity and remarkable technological progress of personal computing devices – including wearables, smartphones, and tablets – along with the multitude of sensor modalities embedded within these devices, has enabled us to establish a continuous connection with people who want to share information about their behavior and daily life.
Such rich, longitudinal information, known as person-generated health data (PGHD), can be searched for physiological and behavioral signatures that can be used in combination with traditional clinical information to predict, diagnose, and treat disease. It can also be used to understand the safety and effectiveness of medical interventions.
PGHD is defined as wellness and/or health-related data created, recorded, or gathered by individuals. It reflects events and interactions that occur during an person’s everyday life. Systematically gathering this information and organizing it to better understand patients’ approach to their health or their unique experience living with disease provides meaningful insights that complement the data traditionally collected as part of clinical trials or periodic office visits.
PGHD can produce a rich picture of a person’s health or symptom burden with disease. It allows the opportunity to measure the real human burden of a patient’s disease and how it changes over time, with an opportunity to detect changes in symptoms in real time.
PGHD can also enable participation in health research.
An example would be the work of Evidation Health in San Mateo, Calif. Evidation provides a platform to run research studies utilizing technology and systems to measure health in everyday life. Its app, Achievement, collects continuous behavior-related data from smartphones, wearables, connected devices, and apps. That provides opportunities for participants to join research studies that develop novel measures designed to quantify health outcomes in a way that more accurately reflects an individual’s day-to-day activities and experience. All data collected are at the direction of and with the permission of the individual.
“Achievers” are given points for taking health-related actions such as tracking steps or their sleep, which convert to cash that can be kept or donated to their favorite charities. Achievement’s 3.5 million diverse participants also receive offers to join research studies. This paradigm shift dramatically expands access to research to increase diversity, shortens the time to first data through rapid recruitment, and enhances retention rates by making it easier to engage. To date, more than 1 million users have chosen to participate in research studies. The technology is bringing new data and insights to health research; it supports important questions about quality of life, medical products’ real-world effectiveness, and the development of hyperpersonalized health care services.
This new type of data is transforming medical research by creating real-world studies of unprecedented size, such as the Apple Heart Study – a virtual study with more than 400,000 enrolled participants – which was designed to test the accuracy of Apple Watches in safely identifying atrial fibrillation. The FDA has cleared two features on the Apple Watch: the device’s ability to detect and notify the user of an irregular heart rhythm, and the ability to take a single-lead EKG feature that can provide a rhythm strip for a clinician to review.
The FDA clearance letters specify that the apps are “not intended to replace traditional methods of diagnosis or treatment.” They provide extra information, and that information might be helpful – but the apps won’t replace a doctor’s visit. It remains to be seen how these data will be used, but they have the potential to identify atrial fibrillation early, leading to treatment that may prevent devastating strokes.
Another example of home-generated health data is a tool that has obtained FDA clearance as a diagnostic device with insurance reimbursement: WatchPAT, a portable sleep apnea diagnostic device. WatchPAT is worn like a simple wristwatch, with no need for belts, wires, or nasal cannulas.
Over time, in-home tests like these that are of minimal inconvenience to the patient and reflect a real-world experience may eclipse traditional sleep studies that require patients to spend the night in a clinic while attached to wires and monitors.
Health data generated by connected populations will yield novel insights that may help us better predict, diagnose, and treat disease. These are examples of innovations that can extend clinicians’ abilities to remotely monitor or diagnose health conditions, and we can expect that more will continue to be integrated into the clinical and research settings in the near future.
In part 2 of this series, we will discuss novel digital measures and studies utilizing PGHD to impact population health.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director, family medicine residency program, Abington (Pa.) Jefferson Health. Dr. Foschini is cofounder and chief data scientist at Evidation Health in San Mateo, Calif. Bray Patrick-Lake is a patient thought leader and director, strategic partnerships, at Evidation Health.
References
Determining real-world data’s fitness for use and the role of reliability, September 2019. Duke-Margolis Center for Health Policy.
N Engl J Med. 2019 Nov 14;381(20):1909-17.
The time shared during clinical encounters provides small peeks into patients’ lives that get documented as episodic snapshots in electronic health records. But there is little information about how patients are doing outside of the office. With increasing emphasis on filling out mandatory parts of the EHR, there is less time available for in-depth, in-office conversations and phone follow-ups.
At the same time, it has become clear that it is not just the medicines we prescribe that affect our patients’ lives. Their behaviors outside of the office – being physically active, eating well, getting a good night’s rest, and adhering to medications – also impact their health outcomes.
The explosion of technology and personal data in our increasingly connected world provides powerful new sources of health and behavior information that generate new understanding of patients’ lives in their everyday settings.
The ubiquity and remarkable technological progress of personal computing devices – including wearables, smartphones, and tablets – along with the multitude of sensor modalities embedded within these devices, has enabled us to establish a continuous connection with people who want to share information about their behavior and daily life.
Such rich, longitudinal information, known as person-generated health data (PGHD), can be searched for physiological and behavioral signatures that can be used in combination with traditional clinical information to predict, diagnose, and treat disease. It can also be used to understand the safety and effectiveness of medical interventions.
PGHD is defined as wellness and/or health-related data created, recorded, or gathered by individuals. It reflects events and interactions that occur during an person’s everyday life. Systematically gathering this information and organizing it to better understand patients’ approach to their health or their unique experience living with disease provides meaningful insights that complement the data traditionally collected as part of clinical trials or periodic office visits.
PGHD can produce a rich picture of a person’s health or symptom burden with disease. It allows the opportunity to measure the real human burden of a patient’s disease and how it changes over time, with an opportunity to detect changes in symptoms in real time.
PGHD can also enable participation in health research.
An example would be the work of Evidation Health in San Mateo, Calif. Evidation provides a platform to run research studies utilizing technology and systems to measure health in everyday life. Its app, Achievement, collects continuous behavior-related data from smartphones, wearables, connected devices, and apps. That provides opportunities for participants to join research studies that develop novel measures designed to quantify health outcomes in a way that more accurately reflects an individual’s day-to-day activities and experience. All data collected are at the direction of and with the permission of the individual.
“Achievers” are given points for taking health-related actions such as tracking steps or their sleep, which convert to cash that can be kept or donated to their favorite charities. Achievement’s 3.5 million diverse participants also receive offers to join research studies. This paradigm shift dramatically expands access to research to increase diversity, shortens the time to first data through rapid recruitment, and enhances retention rates by making it easier to engage. To date, more than 1 million users have chosen to participate in research studies. The technology is bringing new data and insights to health research; it supports important questions about quality of life, medical products’ real-world effectiveness, and the development of hyperpersonalized health care services.
This new type of data is transforming medical research by creating real-world studies of unprecedented size, such as the Apple Heart Study – a virtual study with more than 400,000 enrolled participants – which was designed to test the accuracy of Apple Watches in safely identifying atrial fibrillation. The FDA has cleared two features on the Apple Watch: the device’s ability to detect and notify the user of an irregular heart rhythm, and the ability to take a single-lead EKG feature that can provide a rhythm strip for a clinician to review.
The FDA clearance letters specify that the apps are “not intended to replace traditional methods of diagnosis or treatment.” They provide extra information, and that information might be helpful – but the apps won’t replace a doctor’s visit. It remains to be seen how these data will be used, but they have the potential to identify atrial fibrillation early, leading to treatment that may prevent devastating strokes.
Another example of home-generated health data is a tool that has obtained FDA clearance as a diagnostic device with insurance reimbursement: WatchPAT, a portable sleep apnea diagnostic device. WatchPAT is worn like a simple wristwatch, with no need for belts, wires, or nasal cannulas.
Over time, in-home tests like these that are of minimal inconvenience to the patient and reflect a real-world experience may eclipse traditional sleep studies that require patients to spend the night in a clinic while attached to wires and monitors.
Health data generated by connected populations will yield novel insights that may help us better predict, diagnose, and treat disease. These are examples of innovations that can extend clinicians’ abilities to remotely monitor or diagnose health conditions, and we can expect that more will continue to be integrated into the clinical and research settings in the near future.
In part 2 of this series, we will discuss novel digital measures and studies utilizing PGHD to impact population health.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director, family medicine residency program, Abington (Pa.) Jefferson Health. Dr. Foschini is cofounder and chief data scientist at Evidation Health in San Mateo, Calif. Bray Patrick-Lake is a patient thought leader and director, strategic partnerships, at Evidation Health.
References
Determining real-world data’s fitness for use and the role of reliability, September 2019. Duke-Margolis Center for Health Policy.
N Engl J Med. 2019 Nov 14;381(20):1909-17.
The time shared during clinical encounters provides small peeks into patients’ lives that get documented as episodic snapshots in electronic health records. But there is little information about how patients are doing outside of the office. With increasing emphasis on filling out mandatory parts of the EHR, there is less time available for in-depth, in-office conversations and phone follow-ups.
At the same time, it has become clear that it is not just the medicines we prescribe that affect our patients’ lives. Their behaviors outside of the office – being physically active, eating well, getting a good night’s rest, and adhering to medications – also impact their health outcomes.
The explosion of technology and personal data in our increasingly connected world provides powerful new sources of health and behavior information that generate new understanding of patients’ lives in their everyday settings.
The ubiquity and remarkable technological progress of personal computing devices – including wearables, smartphones, and tablets – along with the multitude of sensor modalities embedded within these devices, has enabled us to establish a continuous connection with people who want to share information about their behavior and daily life.
Such rich, longitudinal information, known as person-generated health data (PGHD), can be searched for physiological and behavioral signatures that can be used in combination with traditional clinical information to predict, diagnose, and treat disease. It can also be used to understand the safety and effectiveness of medical interventions.
PGHD is defined as wellness and/or health-related data created, recorded, or gathered by individuals. It reflects events and interactions that occur during an person’s everyday life. Systematically gathering this information and organizing it to better understand patients’ approach to their health or their unique experience living with disease provides meaningful insights that complement the data traditionally collected as part of clinical trials or periodic office visits.
PGHD can produce a rich picture of a person’s health or symptom burden with disease. It allows the opportunity to measure the real human burden of a patient’s disease and how it changes over time, with an opportunity to detect changes in symptoms in real time.
PGHD can also enable participation in health research.
An example would be the work of Evidation Health in San Mateo, Calif. Evidation provides a platform to run research studies utilizing technology and systems to measure health in everyday life. Its app, Achievement, collects continuous behavior-related data from smartphones, wearables, connected devices, and apps. That provides opportunities for participants to join research studies that develop novel measures designed to quantify health outcomes in a way that more accurately reflects an individual’s day-to-day activities and experience. All data collected are at the direction of and with the permission of the individual.
“Achievers” are given points for taking health-related actions such as tracking steps or their sleep, which convert to cash that can be kept or donated to their favorite charities. Achievement’s 3.5 million diverse participants also receive offers to join research studies. This paradigm shift dramatically expands access to research to increase diversity, shortens the time to first data through rapid recruitment, and enhances retention rates by making it easier to engage. To date, more than 1 million users have chosen to participate in research studies. The technology is bringing new data and insights to health research; it supports important questions about quality of life, medical products’ real-world effectiveness, and the development of hyperpersonalized health care services.
This new type of data is transforming medical research by creating real-world studies of unprecedented size, such as the Apple Heart Study – a virtual study with more than 400,000 enrolled participants – which was designed to test the accuracy of Apple Watches in safely identifying atrial fibrillation. The FDA has cleared two features on the Apple Watch: the device’s ability to detect and notify the user of an irregular heart rhythm, and the ability to take a single-lead EKG feature that can provide a rhythm strip for a clinician to review.
The FDA clearance letters specify that the apps are “not intended to replace traditional methods of diagnosis or treatment.” They provide extra information, and that information might be helpful – but the apps won’t replace a doctor’s visit. It remains to be seen how these data will be used, but they have the potential to identify atrial fibrillation early, leading to treatment that may prevent devastating strokes.
Another example of home-generated health data is a tool that has obtained FDA clearance as a diagnostic device with insurance reimbursement: WatchPAT, a portable sleep apnea diagnostic device. WatchPAT is worn like a simple wristwatch, with no need for belts, wires, or nasal cannulas.
Over time, in-home tests like these that are of minimal inconvenience to the patient and reflect a real-world experience may eclipse traditional sleep studies that require patients to spend the night in a clinic while attached to wires and monitors.
Health data generated by connected populations will yield novel insights that may help us better predict, diagnose, and treat disease. These are examples of innovations that can extend clinicians’ abilities to remotely monitor or diagnose health conditions, and we can expect that more will continue to be integrated into the clinical and research settings in the near future.
In part 2 of this series, we will discuss novel digital measures and studies utilizing PGHD to impact population health.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director, family medicine residency program, Abington (Pa.) Jefferson Health. Dr. Foschini is cofounder and chief data scientist at Evidation Health in San Mateo, Calif. Bray Patrick-Lake is a patient thought leader and director, strategic partnerships, at Evidation Health.
References
Determining real-world data’s fitness for use and the role of reliability, September 2019. Duke-Margolis Center for Health Policy.
N Engl J Med. 2019 Nov 14;381(20):1909-17.
Rash on hands and feet
Lichenoid dermatoses are a heterogeneous group of diseases with varying clinical presentations. The term “lichenoid” refers to the popular lesions of certain skin disorders of which lichen planus (LP) is the prototype. The papules are shiny, flat topped, polygonal, of different sizes, and occur in clusters creating a pattern that resembles lichen growing on a rock. Lichenoid eruptions are quite common in children and can result from many different origins. In most instances the precise mechanism of disease is not known, although it is usually believed to be immunologic in nature. Certain disorders are common in children, whereas others more often affect the adult population.
Lichen striatus, lichen nitidus (LN), and lichen spinulosus are lichenoid lesions that are more common in children than adults.
LN – as seen in the patient described here – is an uncommon benign inflammatory skin disease, primarily of children. Individual lesions are sharply demarcated, pinpoint to pinhead sized, round or polygonal, and strikingly monomorphous in nature. The papules are usually flesh colored, however, the color varies from yellow and brown to violet hues depending on the background color of the patient’s skin. This variation in color is in contrast with LP which is characteristically violaceous. The surfaces of the papules are flat, shiny, and slightly elevated. They may have a fine scale or a hyperkeratotic plug. The lesions tend to occur in groups, primarily on the abdomen, chest, glans penis, and upper extremities. The Koebner phenomenon is observed and is a hallmark for the disorder. LN is generally asymptomatic, unlike LP, which is exceedingly pruritic.
The cause of LN is unknown; however, it has been proposed that LN, in particular generalized LN, may be associated with immune alterations in the patient. The course of LN is slowly progressive with a tendency toward remission. The lesions can remain stationary for years; however, they sometimes disappear spontaneously and completely.
The differential diagnosis of LN beyond the entities discussed above includes frictional lichenoid eruption, lichenoid drug eruption, LP, and keratosis pilaris.
LP is the classic lichenoid eruption. It is rare in children and occurs most frequently in individuals aged 30-60 years. LP usually manifests as an extremely pruritic eruption of flat-topped polygonal and violaceous papules that often have fine linear white scales known as Wickham striae. The distribution is usually bilateral and symmetric with most of the papules and plaques located on the legs, flexor wrists, neck, and genitalia. The lesions may exhibit the Koebner phenomenon, appearing in a linear pattern along the site of a scratch. Generally, in childhood cases there is reported itching, and oral and nail lesions are less common.
Frictional lichenoid eruption occurs in childhood. The lesions consist of lichenoid papules with regular borders 1-2 mm in diameter that generally are asymptomatic, although they may be mildly pruritic. The papules are found in a very characteristic distribution with almost exclusive involvement of the backs of the hands, fingers, elbows, and knees with occasional involvement of the extensor forearms and cheeks. This disorder occurs in predisposed children who have been exposed to significant frictional force during play, and typically resolves spontaneously after removal of the stimulus.
Keratosis pilaris is a rash that usually is found on the outer areas of the upper arms, upper thighs, buttocks, and cheeks. It consists of small bumps that are flesh colored to red. The bumps generally don’t hurt or itch.
The lack of symptoms and spontaneous healing have rendered treatment unnecessary in most cases. LN generally is self-limiting, thus treatment may not be necessary. However, topical treatment with mid- to high-potency corticosteroids has hastened resolution of lesions in some children, as have topical dinitrochlorobenzene and systemic treatment with psoralens, astemizole, etretinate, and psoralen-UVA.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Neither Dr. Eichenfield nor Dr. Bhatti has any relevant financial disclosures. Email them at [email protected].
References
Pickert A. Cutis. 2012 Sep;90(3):E1-3. https://mdedge-files-live.s3.us-east-2.amazonaws.com/files/s3fs-public/Document/September-2017/0900300E1.pdf Tziotzios C et al. J Am Acad Dermatol. 2018 Nov;79(5):789-804. Tilly JJ et al. J Am Acad Dermatol. 2004 Oct;51(4):606-24.
Lichenoid dermatoses are a heterogeneous group of diseases with varying clinical presentations. The term “lichenoid” refers to the popular lesions of certain skin disorders of which lichen planus (LP) is the prototype. The papules are shiny, flat topped, polygonal, of different sizes, and occur in clusters creating a pattern that resembles lichen growing on a rock. Lichenoid eruptions are quite common in children and can result from many different origins. In most instances the precise mechanism of disease is not known, although it is usually believed to be immunologic in nature. Certain disorders are common in children, whereas others more often affect the adult population.
Lichen striatus, lichen nitidus (LN), and lichen spinulosus are lichenoid lesions that are more common in children than adults.
LN – as seen in the patient described here – is an uncommon benign inflammatory skin disease, primarily of children. Individual lesions are sharply demarcated, pinpoint to pinhead sized, round or polygonal, and strikingly monomorphous in nature. The papules are usually flesh colored, however, the color varies from yellow and brown to violet hues depending on the background color of the patient’s skin. This variation in color is in contrast with LP which is characteristically violaceous. The surfaces of the papules are flat, shiny, and slightly elevated. They may have a fine scale or a hyperkeratotic plug. The lesions tend to occur in groups, primarily on the abdomen, chest, glans penis, and upper extremities. The Koebner phenomenon is observed and is a hallmark for the disorder. LN is generally asymptomatic, unlike LP, which is exceedingly pruritic.
The cause of LN is unknown; however, it has been proposed that LN, in particular generalized LN, may be associated with immune alterations in the patient. The course of LN is slowly progressive with a tendency toward remission. The lesions can remain stationary for years; however, they sometimes disappear spontaneously and completely.
The differential diagnosis of LN beyond the entities discussed above includes frictional lichenoid eruption, lichenoid drug eruption, LP, and keratosis pilaris.
LP is the classic lichenoid eruption. It is rare in children and occurs most frequently in individuals aged 30-60 years. LP usually manifests as an extremely pruritic eruption of flat-topped polygonal and violaceous papules that often have fine linear white scales known as Wickham striae. The distribution is usually bilateral and symmetric with most of the papules and plaques located on the legs, flexor wrists, neck, and genitalia. The lesions may exhibit the Koebner phenomenon, appearing in a linear pattern along the site of a scratch. Generally, in childhood cases there is reported itching, and oral and nail lesions are less common.
Frictional lichenoid eruption occurs in childhood. The lesions consist of lichenoid papules with regular borders 1-2 mm in diameter that generally are asymptomatic, although they may be mildly pruritic. The papules are found in a very characteristic distribution with almost exclusive involvement of the backs of the hands, fingers, elbows, and knees with occasional involvement of the extensor forearms and cheeks. This disorder occurs in predisposed children who have been exposed to significant frictional force during play, and typically resolves spontaneously after removal of the stimulus.
Keratosis pilaris is a rash that usually is found on the outer areas of the upper arms, upper thighs, buttocks, and cheeks. It consists of small bumps that are flesh colored to red. The bumps generally don’t hurt or itch.
The lack of symptoms and spontaneous healing have rendered treatment unnecessary in most cases. LN generally is self-limiting, thus treatment may not be necessary. However, topical treatment with mid- to high-potency corticosteroids has hastened resolution of lesions in some children, as have topical dinitrochlorobenzene and systemic treatment with psoralens, astemizole, etretinate, and psoralen-UVA.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Neither Dr. Eichenfield nor Dr. Bhatti has any relevant financial disclosures. Email them at [email protected].
References
Pickert A. Cutis. 2012 Sep;90(3):E1-3. https://mdedge-files-live.s3.us-east-2.amazonaws.com/files/s3fs-public/Document/September-2017/0900300E1.pdf Tziotzios C et al. J Am Acad Dermatol. 2018 Nov;79(5):789-804. Tilly JJ et al. J Am Acad Dermatol. 2004 Oct;51(4):606-24.
Lichenoid dermatoses are a heterogeneous group of diseases with varying clinical presentations. The term “lichenoid” refers to the popular lesions of certain skin disorders of which lichen planus (LP) is the prototype. The papules are shiny, flat topped, polygonal, of different sizes, and occur in clusters creating a pattern that resembles lichen growing on a rock. Lichenoid eruptions are quite common in children and can result from many different origins. In most instances the precise mechanism of disease is not known, although it is usually believed to be immunologic in nature. Certain disorders are common in children, whereas others more often affect the adult population.
Lichen striatus, lichen nitidus (LN), and lichen spinulosus are lichenoid lesions that are more common in children than adults.
LN – as seen in the patient described here – is an uncommon benign inflammatory skin disease, primarily of children. Individual lesions are sharply demarcated, pinpoint to pinhead sized, round or polygonal, and strikingly monomorphous in nature. The papules are usually flesh colored, however, the color varies from yellow and brown to violet hues depending on the background color of the patient’s skin. This variation in color is in contrast with LP which is characteristically violaceous. The surfaces of the papules are flat, shiny, and slightly elevated. They may have a fine scale or a hyperkeratotic plug. The lesions tend to occur in groups, primarily on the abdomen, chest, glans penis, and upper extremities. The Koebner phenomenon is observed and is a hallmark for the disorder. LN is generally asymptomatic, unlike LP, which is exceedingly pruritic.
The cause of LN is unknown; however, it has been proposed that LN, in particular generalized LN, may be associated with immune alterations in the patient. The course of LN is slowly progressive with a tendency toward remission. The lesions can remain stationary for years; however, they sometimes disappear spontaneously and completely.
The differential diagnosis of LN beyond the entities discussed above includes frictional lichenoid eruption, lichenoid drug eruption, LP, and keratosis pilaris.
LP is the classic lichenoid eruption. It is rare in children and occurs most frequently in individuals aged 30-60 years. LP usually manifests as an extremely pruritic eruption of flat-topped polygonal and violaceous papules that often have fine linear white scales known as Wickham striae. The distribution is usually bilateral and symmetric with most of the papules and plaques located on the legs, flexor wrists, neck, and genitalia. The lesions may exhibit the Koebner phenomenon, appearing in a linear pattern along the site of a scratch. Generally, in childhood cases there is reported itching, and oral and nail lesions are less common.
Frictional lichenoid eruption occurs in childhood. The lesions consist of lichenoid papules with regular borders 1-2 mm in diameter that generally are asymptomatic, although they may be mildly pruritic. The papules are found in a very characteristic distribution with almost exclusive involvement of the backs of the hands, fingers, elbows, and knees with occasional involvement of the extensor forearms and cheeks. This disorder occurs in predisposed children who have been exposed to significant frictional force during play, and typically resolves spontaneously after removal of the stimulus.
Keratosis pilaris is a rash that usually is found on the outer areas of the upper arms, upper thighs, buttocks, and cheeks. It consists of small bumps that are flesh colored to red. The bumps generally don’t hurt or itch.
The lack of symptoms and spontaneous healing have rendered treatment unnecessary in most cases. LN generally is self-limiting, thus treatment may not be necessary. However, topical treatment with mid- to high-potency corticosteroids has hastened resolution of lesions in some children, as have topical dinitrochlorobenzene and systemic treatment with psoralens, astemizole, etretinate, and psoralen-UVA.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Neither Dr. Eichenfield nor Dr. Bhatti has any relevant financial disclosures. Email them at [email protected].
References
Pickert A. Cutis. 2012 Sep;90(3):E1-3. https://mdedge-files-live.s3.us-east-2.amazonaws.com/files/s3fs-public/Document/September-2017/0900300E1.pdf Tziotzios C et al. J Am Acad Dermatol. 2018 Nov;79(5):789-804. Tilly JJ et al. J Am Acad Dermatol. 2004 Oct;51(4):606-24.
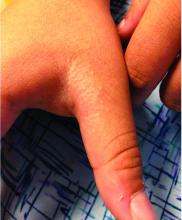
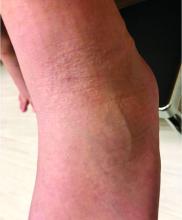
A 9-year-old healthy Kuwaiti male with no significant past medical history presents with a rash on his hands and feet that has been present for 3 years.
His mother reports that he has been seen by dermatologists in various countries and was last seen by a dermatologist in Kuwait 3 years ago. At that time, he was told that it was dryness and advised to not shower daily. Since then he has been taking showers three times weekly and using Cetaphil once weekly without improvement. He was seen by his pediatrician 6 months ago, diagnosed with xerosis, and was given hydrocortisone 2.5% to use twice daily, again without any improvement.
The rash is not itchy, and he has no oral lesions or nail involvement. Exam revealed lichenoid papules on bilateral dorsal hands and feet, bilateral upper arms, bilateral axilla, lower abdomen, and left upper chest.
Community pediatric care is diminishing
The mantra of community hospital administrators is that pediatric care does not pay. Neonatal intensive care pays. For pediatrics, it is similar to how football programs (Medicare patients) support minor sports (pediatrics and obstetrics) at colleges. However, fewer even mildly sick newborns are cared for at community hospitals, which has led to a centralization of neonatal and pediatric care and a loss of pediatric expertise at the affected hospitals.
Pediatric hospitalists are hired to cover the pediatric floor, the emergency department, and labor and delivery, then fired over empty pediatric beds. The rationale expressed is that pediatricians have done such a good job in preventive care that children rarely need hospitalization, so why have a pediatric inpatient unit? It is true that preventive care has been an integral part of primary care for children. Significantly less that 1% of child office visits result in hospitalization.
Advocate Health Care has closed inpatient pediatric units at Illinois Masonic, on Chicago’s North Side, Good Samaritan in Downers Grove, and Good Shepherd in Barrington. Units also have been closed at Mount Sinai in North Lawndale, Norwegian American on Chicago’s West Side, Little Company of Mary in Evergreen Park, and Alexian Brothers in Elk Grove.
As a Chicago-area pediatrician for more than 30 years, I have learned several things about community-based pediatric care:
1. Pediatrics is a geographic specialty. Parents will travel to shop, but would rather walk or have a short ride to their children’s medical providers. Secondary care should be community based, and hospitalization, if necessary, should be close by as well.
2. Hospitals that ceased delivering pediatric inpatient care lost their child-friendliness and pediatric competence, becoming uncomfortable delivering almost any care for children (e.g., sedated MRIs and EEGs, x-rays and ultrasounds, ECGs and echocardiograms, and emergency care).
3. In almost all hospitals, after pediatrics was gone eventually so passed obstetrics (another less remunerative specialty). Sick newborns need immediate, competent care. Most pediatric hospitalizations are short term, often overnight. Delaying newborn care is a medicolegal nightmare. and exposes the child and his or her family to a potentially dangerous drive or helicopter ride.
4. As pediatric subspecialty care becomes more centralized, parents are asked to travel for hours to see a pediatric specialist. There are times when that is necessary (e.g., cardiovascular surgery). Pediatric subspecialists, such as pediatric otolaryngologists, then leave community hospitals, forcing even minor surgeries (e.g., ear ventilation tubes) to be done at a center. In rural areas, this could mean hours of travel, lost work days, and family disruption.
5. Children’s hospitals get uninsured and publicly insured children sent hundreds of miles, because there were no subspecialists in the community who would care for these children.
What is the solution, in our profit-focused health care system?
1. Hospitals’ Certificates of Need could include a mandate for pediatric care.
2. Children’s hospitals could be made responsible for community-based care within their geographic catchment areas.
3. The state or the federal government could mandate and financially support community-based hospital care.
4. Deciding what level of care might be appropriate for each community could depend upon closeness to a pediatric hospital, health problems in the community, and the availability of pediatric specialists.
5. A condition for medical licensure might be that a community-based pediatric subspecialist is required to care for a proportion of the uninsured or publicly insured children in his or her area.
6. Reimbursements for pediatric care need to rise enough to make caring for children worth it.
The major decision point regarding care for children cannot be financial, but must instead embrace the needs of each affected community. If quality health care is a right, and not a privilege, then it is time to stop closing pediatric inpatient units, and, instead, look for creative ways to better care for our children.
This process has led to pediatric care being available only in designated centers. The centralization of pediatric care has progressed from 30 years ago, when most community hospitals had inpatient pediatric units, to the search for innovative ways to fill pediatric beds in the mid-90s (sick day care, flex- or shared pediatric units), to the wholesale closure of community pediatric inpatient beds, from 2000 to the present. I have, unfortunately, seen this firsthand, watching the rise of pediatric mega-hospitals and the demise of community pediatrics. It is a simple financial argument. Care for children simply does not pay nearly as well as does care for adults, especially Medicaid patients. Pediatricians are the poorest paid practicing doctors (public health doctors are paid less).
It is true that pediatricians always have been at the forefront of preventive medicine, and that pediatric patients almost always get better, in spite of our best-intentioned interventions. So community-based pediatricians admit very few patients.
With the loss of pediatric units, community hospitals lose their comfort caring for children. This includes phlebotomy, x-ray, trauma, surgery, and behavioral health. And eroding community hospital pediatric expertise has catastrophic implications for rural hospitals, where parents may have to drive for hours to find a child-friendly emergency department.
Is there an answer?
1. Hospitals are responsible for the patients they serve, including children. Why should a hospital be able to close pediatric services so easily?
2. Every hospital that sees children, through the emergency department, needs to have a pediatrician available to evaluate a child, 24/7.
3. There needs to be an observation unit for children, with pediatric staffing, for overnight stays.
4. Pediatric hospitalists should be staffing community hospitals.
5. Pediatric behavioral health resources need to be available, e.g., inpatient psychiatry, partial hospitalization programs, intensive outpatient programs.
6. Telehealth communication is not adequate to address acute care problems, because the hospital caring for the child has to have the proper equipment and adequate expertise to carry out the recommendations of the teleconsultant.
If we accept that our children will shape the future, we must allow them to survive and thrive. Is health care a right or a privilege, and is it just for adults or for children, too?
Dr. Ochs is in private practice at Ravenswood Pediatrics in Chicago. He said he had no relevant financial disclosures. Email him at [email protected].
The mantra of community hospital administrators is that pediatric care does not pay. Neonatal intensive care pays. For pediatrics, it is similar to how football programs (Medicare patients) support minor sports (pediatrics and obstetrics) at colleges. However, fewer even mildly sick newborns are cared for at community hospitals, which has led to a centralization of neonatal and pediatric care and a loss of pediatric expertise at the affected hospitals.
Pediatric hospitalists are hired to cover the pediatric floor, the emergency department, and labor and delivery, then fired over empty pediatric beds. The rationale expressed is that pediatricians have done such a good job in preventive care that children rarely need hospitalization, so why have a pediatric inpatient unit? It is true that preventive care has been an integral part of primary care for children. Significantly less that 1% of child office visits result in hospitalization.
Advocate Health Care has closed inpatient pediatric units at Illinois Masonic, on Chicago’s North Side, Good Samaritan in Downers Grove, and Good Shepherd in Barrington. Units also have been closed at Mount Sinai in North Lawndale, Norwegian American on Chicago’s West Side, Little Company of Mary in Evergreen Park, and Alexian Brothers in Elk Grove.
As a Chicago-area pediatrician for more than 30 years, I have learned several things about community-based pediatric care:
1. Pediatrics is a geographic specialty. Parents will travel to shop, but would rather walk or have a short ride to their children’s medical providers. Secondary care should be community based, and hospitalization, if necessary, should be close by as well.
2. Hospitals that ceased delivering pediatric inpatient care lost their child-friendliness and pediatric competence, becoming uncomfortable delivering almost any care for children (e.g., sedated MRIs and EEGs, x-rays and ultrasounds, ECGs and echocardiograms, and emergency care).
3. In almost all hospitals, after pediatrics was gone eventually so passed obstetrics (another less remunerative specialty). Sick newborns need immediate, competent care. Most pediatric hospitalizations are short term, often overnight. Delaying newborn care is a medicolegal nightmare. and exposes the child and his or her family to a potentially dangerous drive or helicopter ride.
4. As pediatric subspecialty care becomes more centralized, parents are asked to travel for hours to see a pediatric specialist. There are times when that is necessary (e.g., cardiovascular surgery). Pediatric subspecialists, such as pediatric otolaryngologists, then leave community hospitals, forcing even minor surgeries (e.g., ear ventilation tubes) to be done at a center. In rural areas, this could mean hours of travel, lost work days, and family disruption.
5. Children’s hospitals get uninsured and publicly insured children sent hundreds of miles, because there were no subspecialists in the community who would care for these children.
What is the solution, in our profit-focused health care system?
1. Hospitals’ Certificates of Need could include a mandate for pediatric care.
2. Children’s hospitals could be made responsible for community-based care within their geographic catchment areas.
3. The state or the federal government could mandate and financially support community-based hospital care.
4. Deciding what level of care might be appropriate for each community could depend upon closeness to a pediatric hospital, health problems in the community, and the availability of pediatric specialists.
5. A condition for medical licensure might be that a community-based pediatric subspecialist is required to care for a proportion of the uninsured or publicly insured children in his or her area.
6. Reimbursements for pediatric care need to rise enough to make caring for children worth it.
The major decision point regarding care for children cannot be financial, but must instead embrace the needs of each affected community. If quality health care is a right, and not a privilege, then it is time to stop closing pediatric inpatient units, and, instead, look for creative ways to better care for our children.
This process has led to pediatric care being available only in designated centers. The centralization of pediatric care has progressed from 30 years ago, when most community hospitals had inpatient pediatric units, to the search for innovative ways to fill pediatric beds in the mid-90s (sick day care, flex- or shared pediatric units), to the wholesale closure of community pediatric inpatient beds, from 2000 to the present. I have, unfortunately, seen this firsthand, watching the rise of pediatric mega-hospitals and the demise of community pediatrics. It is a simple financial argument. Care for children simply does not pay nearly as well as does care for adults, especially Medicaid patients. Pediatricians are the poorest paid practicing doctors (public health doctors are paid less).
It is true that pediatricians always have been at the forefront of preventive medicine, and that pediatric patients almost always get better, in spite of our best-intentioned interventions. So community-based pediatricians admit very few patients.
With the loss of pediatric units, community hospitals lose their comfort caring for children. This includes phlebotomy, x-ray, trauma, surgery, and behavioral health. And eroding community hospital pediatric expertise has catastrophic implications for rural hospitals, where parents may have to drive for hours to find a child-friendly emergency department.
Is there an answer?
1. Hospitals are responsible for the patients they serve, including children. Why should a hospital be able to close pediatric services so easily?
2. Every hospital that sees children, through the emergency department, needs to have a pediatrician available to evaluate a child, 24/7.
3. There needs to be an observation unit for children, with pediatric staffing, for overnight stays.
4. Pediatric hospitalists should be staffing community hospitals.
5. Pediatric behavioral health resources need to be available, e.g., inpatient psychiatry, partial hospitalization programs, intensive outpatient programs.
6. Telehealth communication is not adequate to address acute care problems, because the hospital caring for the child has to have the proper equipment and adequate expertise to carry out the recommendations of the teleconsultant.
If we accept that our children will shape the future, we must allow them to survive and thrive. Is health care a right or a privilege, and is it just for adults or for children, too?
Dr. Ochs is in private practice at Ravenswood Pediatrics in Chicago. He said he had no relevant financial disclosures. Email him at [email protected].
The mantra of community hospital administrators is that pediatric care does not pay. Neonatal intensive care pays. For pediatrics, it is similar to how football programs (Medicare patients) support minor sports (pediatrics and obstetrics) at colleges. However, fewer even mildly sick newborns are cared for at community hospitals, which has led to a centralization of neonatal and pediatric care and a loss of pediatric expertise at the affected hospitals.
Pediatric hospitalists are hired to cover the pediatric floor, the emergency department, and labor and delivery, then fired over empty pediatric beds. The rationale expressed is that pediatricians have done such a good job in preventive care that children rarely need hospitalization, so why have a pediatric inpatient unit? It is true that preventive care has been an integral part of primary care for children. Significantly less that 1% of child office visits result in hospitalization.
Advocate Health Care has closed inpatient pediatric units at Illinois Masonic, on Chicago’s North Side, Good Samaritan in Downers Grove, and Good Shepherd in Barrington. Units also have been closed at Mount Sinai in North Lawndale, Norwegian American on Chicago’s West Side, Little Company of Mary in Evergreen Park, and Alexian Brothers in Elk Grove.
As a Chicago-area pediatrician for more than 30 years, I have learned several things about community-based pediatric care:
1. Pediatrics is a geographic specialty. Parents will travel to shop, but would rather walk or have a short ride to their children’s medical providers. Secondary care should be community based, and hospitalization, if necessary, should be close by as well.
2. Hospitals that ceased delivering pediatric inpatient care lost their child-friendliness and pediatric competence, becoming uncomfortable delivering almost any care for children (e.g., sedated MRIs and EEGs, x-rays and ultrasounds, ECGs and echocardiograms, and emergency care).
3. In almost all hospitals, after pediatrics was gone eventually so passed obstetrics (another less remunerative specialty). Sick newborns need immediate, competent care. Most pediatric hospitalizations are short term, often overnight. Delaying newborn care is a medicolegal nightmare. and exposes the child and his or her family to a potentially dangerous drive or helicopter ride.
4. As pediatric subspecialty care becomes more centralized, parents are asked to travel for hours to see a pediatric specialist. There are times when that is necessary (e.g., cardiovascular surgery). Pediatric subspecialists, such as pediatric otolaryngologists, then leave community hospitals, forcing even minor surgeries (e.g., ear ventilation tubes) to be done at a center. In rural areas, this could mean hours of travel, lost work days, and family disruption.
5. Children’s hospitals get uninsured and publicly insured children sent hundreds of miles, because there were no subspecialists in the community who would care for these children.
What is the solution, in our profit-focused health care system?
1. Hospitals’ Certificates of Need could include a mandate for pediatric care.
2. Children’s hospitals could be made responsible for community-based care within their geographic catchment areas.
3. The state or the federal government could mandate and financially support community-based hospital care.
4. Deciding what level of care might be appropriate for each community could depend upon closeness to a pediatric hospital, health problems in the community, and the availability of pediatric specialists.
5. A condition for medical licensure might be that a community-based pediatric subspecialist is required to care for a proportion of the uninsured or publicly insured children in his or her area.
6. Reimbursements for pediatric care need to rise enough to make caring for children worth it.
The major decision point regarding care for children cannot be financial, but must instead embrace the needs of each affected community. If quality health care is a right, and not a privilege, then it is time to stop closing pediatric inpatient units, and, instead, look for creative ways to better care for our children.
This process has led to pediatric care being available only in designated centers. The centralization of pediatric care has progressed from 30 years ago, when most community hospitals had inpatient pediatric units, to the search for innovative ways to fill pediatric beds in the mid-90s (sick day care, flex- or shared pediatric units), to the wholesale closure of community pediatric inpatient beds, from 2000 to the present. I have, unfortunately, seen this firsthand, watching the rise of pediatric mega-hospitals and the demise of community pediatrics. It is a simple financial argument. Care for children simply does not pay nearly as well as does care for adults, especially Medicaid patients. Pediatricians are the poorest paid practicing doctors (public health doctors are paid less).
It is true that pediatricians always have been at the forefront of preventive medicine, and that pediatric patients almost always get better, in spite of our best-intentioned interventions. So community-based pediatricians admit very few patients.
With the loss of pediatric units, community hospitals lose their comfort caring for children. This includes phlebotomy, x-ray, trauma, surgery, and behavioral health. And eroding community hospital pediatric expertise has catastrophic implications for rural hospitals, where parents may have to drive for hours to find a child-friendly emergency department.
Is there an answer?
1. Hospitals are responsible for the patients they serve, including children. Why should a hospital be able to close pediatric services so easily?
2. Every hospital that sees children, through the emergency department, needs to have a pediatrician available to evaluate a child, 24/7.
3. There needs to be an observation unit for children, with pediatric staffing, for overnight stays.
4. Pediatric hospitalists should be staffing community hospitals.
5. Pediatric behavioral health resources need to be available, e.g., inpatient psychiatry, partial hospitalization programs, intensive outpatient programs.
6. Telehealth communication is not adequate to address acute care problems, because the hospital caring for the child has to have the proper equipment and adequate expertise to carry out the recommendations of the teleconsultant.
If we accept that our children will shape the future, we must allow them to survive and thrive. Is health care a right or a privilege, and is it just for adults or for children, too?
Dr. Ochs is in private practice at Ravenswood Pediatrics in Chicago. He said he had no relevant financial disclosures. Email him at [email protected].
Why is AOM frequency decreasing in the pneumococcal conjugate vaccine era?
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at [email protected].
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at [email protected].
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at [email protected].
Letter to the Editor
Editor’s note: This is one of the many emails readers sent to Dermatology News in response to Dr. Alan Rockoff’s last “Under My Skin” column in the December issue.
Dr. Rockoff,
I was deeply heartbroken to read that this would be your last column in Dermatology News today. I am a medical dermatologist with 19 years of experience in a small town in North Carolina and have always looked forward to reading your columns. No matter what the topic for the article you chose, I would always glean something worthwhile from it, be it a poignant insight, a relevant practice tip on treatment or patient management, and nearly always a hearty laugh.
Being somewhat rural and without a lot of competition, my very busy practice consists mainly of salt of the earth patients needing straight dermatologic care, mostly skin cancer, psoriasis, acne, the general stuff. Your ability to capture the essence of what it is like to be one of us in the trenches, to expose and eloquently define the most common and frustrating issues we face, has been a source of pleasure for years. On more than one occasion, I put down the magazine and attempted to write a thank you letter for one article or another you wrote, to say that you are doing a great job and please continue to enlighten us with your insight and that your efforts are greatly valued. I am embarrassed to admit, I never could complete those emails, thinking “Why bother the man? He is clearly as busy as me, and why would he want to hear from me anyway?”
Well, at the risk of bothering you, sir, please do accept my apologies for not writing you before you retired your article, and please know that your articles have personally given me years of immense happiness. They will be sorely missed, and likely not ever replaced.
With gratitude,
Jeff Suchniak, MD
Rocky Mount, N.C.
Editor’s note: This is one of the many emails readers sent to Dermatology News in response to Dr. Alan Rockoff’s last “Under My Skin” column in the December issue.
Dr. Rockoff,
I was deeply heartbroken to read that this would be your last column in Dermatology News today. I am a medical dermatologist with 19 years of experience in a small town in North Carolina and have always looked forward to reading your columns. No matter what the topic for the article you chose, I would always glean something worthwhile from it, be it a poignant insight, a relevant practice tip on treatment or patient management, and nearly always a hearty laugh.
Being somewhat rural and without a lot of competition, my very busy practice consists mainly of salt of the earth patients needing straight dermatologic care, mostly skin cancer, psoriasis, acne, the general stuff. Your ability to capture the essence of what it is like to be one of us in the trenches, to expose and eloquently define the most common and frustrating issues we face, has been a source of pleasure for years. On more than one occasion, I put down the magazine and attempted to write a thank you letter for one article or another you wrote, to say that you are doing a great job and please continue to enlighten us with your insight and that your efforts are greatly valued. I am embarrassed to admit, I never could complete those emails, thinking “Why bother the man? He is clearly as busy as me, and why would he want to hear from me anyway?”
Well, at the risk of bothering you, sir, please do accept my apologies for not writing you before you retired your article, and please know that your articles have personally given me years of immense happiness. They will be sorely missed, and likely not ever replaced.
With gratitude,
Jeff Suchniak, MD
Rocky Mount, N.C.
Editor’s note: This is one of the many emails readers sent to Dermatology News in response to Dr. Alan Rockoff’s last “Under My Skin” column in the December issue.
Dr. Rockoff,
I was deeply heartbroken to read that this would be your last column in Dermatology News today. I am a medical dermatologist with 19 years of experience in a small town in North Carolina and have always looked forward to reading your columns. No matter what the topic for the article you chose, I would always glean something worthwhile from it, be it a poignant insight, a relevant practice tip on treatment or patient management, and nearly always a hearty laugh.
Being somewhat rural and without a lot of competition, my very busy practice consists mainly of salt of the earth patients needing straight dermatologic care, mostly skin cancer, psoriasis, acne, the general stuff. Your ability to capture the essence of what it is like to be one of us in the trenches, to expose and eloquently define the most common and frustrating issues we face, has been a source of pleasure for years. On more than one occasion, I put down the magazine and attempted to write a thank you letter for one article or another you wrote, to say that you are doing a great job and please continue to enlighten us with your insight and that your efforts are greatly valued. I am embarrassed to admit, I never could complete those emails, thinking “Why bother the man? He is clearly as busy as me, and why would he want to hear from me anyway?”
Well, at the risk of bothering you, sir, please do accept my apologies for not writing you before you retired your article, and please know that your articles have personally given me years of immense happiness. They will be sorely missed, and likely not ever replaced.
With gratitude,
Jeff Suchniak, MD
Rocky Mount, N.C.
Provide appropriate sexual, reproductive health care for transgender patients
I recently was on a panel of experts discussing how to prevent HIV among transgender youth. Preventing HIV among transgender youth, especially transgender youth of color, remains a challenge for multiple reasons – racism, poverty, stigma, marginalization, and discrimination play a role in the HIV epidemic. A barrier to preventing HIV infections among transgender youth is a lack of knowledge on how to provide them with comprehensive sexual and reproductive health care. Here are some tips and resources that can help you ensure that transgender youth are safe and healthy.
One of the challenges of obtaining a sexual history is asking the right questions For example, if you have a transgender male assigned female at birth, ask whether their partners produce sperm instead of asking about the sex of their partners. A transgender male’s partner may identify as female but is assigned male at birth and uses her penis during sex. Furthermore, a transgender male may be on testosterone, but he still can get pregnant. Asking how they use their organs is just as important. A transgender male who has condomless penile-vaginal sex with multiple partners is at a higher risk for HIV infection than is a transgender male who shares sex toys with his only partner.
Normalizing that you ask a comprehensive sexual history to all your patients regardless of gender identity may put the patient at ease. Many transgender people are reluctant to disclose their gender identity to their provider because they are afraid that the provider may fixate on their sexuality once they do. Stating that you ask sexual health questions to all your patients may prevent the transgender patient from feeling singled out.
Finally, you don’t have to ask a sexual history with every transgender patient, just as you wouldn’t for your cisgender patients. If a patient is complaining of a sprained ankle, a sexual history may not be helpful, compared with obtaining one when a patient comes in with pelvic pain. Many transgender patients avoid care because they are frequently asked about their sexual history or gender identity when these are not relevant to their chief complaint.
Here are some helpful questions to ask when taking a sexual history, according to the University of California, San Francisco, Transgender Care & Treatment Guidelines.1
- Are you having sex? How many sex partners have you had in the past year?
- Who are you having sex with? What types of sex are you having? What parts of your anatomy do you use for sex?
- How do you protect yourself from STIs?
- What STIs have you had in the past, if any? When were you last tested for STIs?
- Has your partner(s) ever been diagnosed with any STIs?
- Do you use alcohol or any drugs when you have sex?
- Do you exchange sex for money, drugs, or a place to stay?
Also, use a trauma-informed approach when working with transgender patients. Many have been victims of sexual trauma. Always have a chaperone accompany you during the exam, explain to the patient what you plan to do and why it is necessary, and allow them to decline (and document their declining the physical exam). Also consider having your patient self-swab for STI screening if appropriate.1
Like obtaining a sexual history, routine screenings for certain types of cancers will be based on the organs the patient has. For example, a transgender woman assigned male at birth will not need a cervical cancer screening, but a transgender man assigned female at birth may need one – if the patient still has a cervix. Cervical cancer screening guidelines are similar for transgender men as it is for nontransgender women, and one should use the same guidelines endorsed by the American Cancer Society, American Society of Colposcopy and Cervical Pathology, American Society of Clinical Pathologists, U.S. Preventive Services Task Force, and the World Health Organization.2-4
Cervical screenings should never be a requirement for testosterone therapy, and no transgender male under the age of 21 years will need cervical screening. The University of California guidelines offers tips on how to make transgender men more comfortable during cervical cancer screening.5
Contraception and menstrual management also are important for transgender patients. Testosterone can induce amenorrhea for transgender men, but it is not good birth control. If a transgender male patient has sex with partners that produce sperm, then the physician should discuss effective birth control options. There is no ideal birth control option for transgender men. One must consider multiple factors including the patient’s desire for pregnancy, desire to cease periods, ease of administration, and risk for thrombosis.
Most transgender men may balk at the idea of taking estrogen-containing contraception, but it is more effective than oral progestin-only pills. Intrauterine devices are highly effective in pregnancy prevention and can achieve amenorrhea in 50% of users within 1 year,but some transmen may become dysphoric with the procedure. 6 The etonogestrel implants also are highly effective birth control, but irregular periods are common, leading to discontinuation. Depot medroxyprogesterone is highly effective in preventing pregnancy and can induce amenorrhea in 70% of users within 1 year and 80% of users in 2 years, but also is associated with weight gain in one-third of users.7 Finally, pubertal blockers can rapidly stop periods for transmen who are severely dysphoric from their menses; however, before achieving amenorrhea, a flare bleed can occur 4-6 weeks after administration.8 Support from a mental health therapist during this time is critical. Pubertal blockers, nevertheless, are not suitable birth control.
When providing affirming sexual and reproductive health care for transgender patients, key principles include focusing on organs and activities over identity. Additionally, screening for certain types of cancers also is dependent on organs. Finally, do not neglect the importance of contraception among transgender men. Taking these principles in consideration will help you provide excellent care for transgender youth.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at the Children’s Hospital of Pittsburgh. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Transgender people and sexually transmitted infections (https://transcare.ucsf.edu/guidelines/stis).
2. CA Cancer J Clin. 2012 May-Jun;62(3):147-72.
3. Ann Intern Med. 2012;156(12):880-91.
4. Cervical cancer screening in developing countries: Report of a WHO consultation. 2002. World Health Organization, Geneva.
5. Screening for cervical cancer for transgender men (https://transcare.ucsf.edu/guidelines/cervical-cancer).
6. Contraception. 2002 Feb;65(2):129-32.
7. Rev Endocr Metab Disord. 2011 Jun;12(2):93-106.
8. Int J Womens Health. 2014 Jun 23;6:631-7.
Resources
Breast cancer screening in transgender men. (https://transcare.ucsf.edu/guidelines/breast-cancer-men).
Screening for breast cancer in transgender women. (https://transcare.ucsf.edu/guidelines/breast-cancer-women).
Transgender health and HIV (https://transcare.ucsf.edu/guidelines/hiv).
Centers for Disease Control and Prevention: HIV and Transgender People (https://www.cdc.gov/hiv/group/gender/transgender/index.html).
I recently was on a panel of experts discussing how to prevent HIV among transgender youth. Preventing HIV among transgender youth, especially transgender youth of color, remains a challenge for multiple reasons – racism, poverty, stigma, marginalization, and discrimination play a role in the HIV epidemic. A barrier to preventing HIV infections among transgender youth is a lack of knowledge on how to provide them with comprehensive sexual and reproductive health care. Here are some tips and resources that can help you ensure that transgender youth are safe and healthy.
One of the challenges of obtaining a sexual history is asking the right questions For example, if you have a transgender male assigned female at birth, ask whether their partners produce sperm instead of asking about the sex of their partners. A transgender male’s partner may identify as female but is assigned male at birth and uses her penis during sex. Furthermore, a transgender male may be on testosterone, but he still can get pregnant. Asking how they use their organs is just as important. A transgender male who has condomless penile-vaginal sex with multiple partners is at a higher risk for HIV infection than is a transgender male who shares sex toys with his only partner.
Normalizing that you ask a comprehensive sexual history to all your patients regardless of gender identity may put the patient at ease. Many transgender people are reluctant to disclose their gender identity to their provider because they are afraid that the provider may fixate on their sexuality once they do. Stating that you ask sexual health questions to all your patients may prevent the transgender patient from feeling singled out.
Finally, you don’t have to ask a sexual history with every transgender patient, just as you wouldn’t for your cisgender patients. If a patient is complaining of a sprained ankle, a sexual history may not be helpful, compared with obtaining one when a patient comes in with pelvic pain. Many transgender patients avoid care because they are frequently asked about their sexual history or gender identity when these are not relevant to their chief complaint.
Here are some helpful questions to ask when taking a sexual history, according to the University of California, San Francisco, Transgender Care & Treatment Guidelines.1
- Are you having sex? How many sex partners have you had in the past year?
- Who are you having sex with? What types of sex are you having? What parts of your anatomy do you use for sex?
- How do you protect yourself from STIs?
- What STIs have you had in the past, if any? When were you last tested for STIs?
- Has your partner(s) ever been diagnosed with any STIs?
- Do you use alcohol or any drugs when you have sex?
- Do you exchange sex for money, drugs, or a place to stay?
Also, use a trauma-informed approach when working with transgender patients. Many have been victims of sexual trauma. Always have a chaperone accompany you during the exam, explain to the patient what you plan to do and why it is necessary, and allow them to decline (and document their declining the physical exam). Also consider having your patient self-swab for STI screening if appropriate.1
Like obtaining a sexual history, routine screenings for certain types of cancers will be based on the organs the patient has. For example, a transgender woman assigned male at birth will not need a cervical cancer screening, but a transgender man assigned female at birth may need one – if the patient still has a cervix. Cervical cancer screening guidelines are similar for transgender men as it is for nontransgender women, and one should use the same guidelines endorsed by the American Cancer Society, American Society of Colposcopy and Cervical Pathology, American Society of Clinical Pathologists, U.S. Preventive Services Task Force, and the World Health Organization.2-4
Cervical screenings should never be a requirement for testosterone therapy, and no transgender male under the age of 21 years will need cervical screening. The University of California guidelines offers tips on how to make transgender men more comfortable during cervical cancer screening.5
Contraception and menstrual management also are important for transgender patients. Testosterone can induce amenorrhea for transgender men, but it is not good birth control. If a transgender male patient has sex with partners that produce sperm, then the physician should discuss effective birth control options. There is no ideal birth control option for transgender men. One must consider multiple factors including the patient’s desire for pregnancy, desire to cease periods, ease of administration, and risk for thrombosis.
Most transgender men may balk at the idea of taking estrogen-containing contraception, but it is more effective than oral progestin-only pills. Intrauterine devices are highly effective in pregnancy prevention and can achieve amenorrhea in 50% of users within 1 year,but some transmen may become dysphoric with the procedure. 6 The etonogestrel implants also are highly effective birth control, but irregular periods are common, leading to discontinuation. Depot medroxyprogesterone is highly effective in preventing pregnancy and can induce amenorrhea in 70% of users within 1 year and 80% of users in 2 years, but also is associated with weight gain in one-third of users.7 Finally, pubertal blockers can rapidly stop periods for transmen who are severely dysphoric from their menses; however, before achieving amenorrhea, a flare bleed can occur 4-6 weeks after administration.8 Support from a mental health therapist during this time is critical. Pubertal blockers, nevertheless, are not suitable birth control.
When providing affirming sexual and reproductive health care for transgender patients, key principles include focusing on organs and activities over identity. Additionally, screening for certain types of cancers also is dependent on organs. Finally, do not neglect the importance of contraception among transgender men. Taking these principles in consideration will help you provide excellent care for transgender youth.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at the Children’s Hospital of Pittsburgh. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Transgender people and sexually transmitted infections (https://transcare.ucsf.edu/guidelines/stis).
2. CA Cancer J Clin. 2012 May-Jun;62(3):147-72.
3. Ann Intern Med. 2012;156(12):880-91.
4. Cervical cancer screening in developing countries: Report of a WHO consultation. 2002. World Health Organization, Geneva.
5. Screening for cervical cancer for transgender men (https://transcare.ucsf.edu/guidelines/cervical-cancer).
6. Contraception. 2002 Feb;65(2):129-32.
7. Rev Endocr Metab Disord. 2011 Jun;12(2):93-106.
8. Int J Womens Health. 2014 Jun 23;6:631-7.
Resources
Breast cancer screening in transgender men. (https://transcare.ucsf.edu/guidelines/breast-cancer-men).
Screening for breast cancer in transgender women. (https://transcare.ucsf.edu/guidelines/breast-cancer-women).
Transgender health and HIV (https://transcare.ucsf.edu/guidelines/hiv).
Centers for Disease Control and Prevention: HIV and Transgender People (https://www.cdc.gov/hiv/group/gender/transgender/index.html).
I recently was on a panel of experts discussing how to prevent HIV among transgender youth. Preventing HIV among transgender youth, especially transgender youth of color, remains a challenge for multiple reasons – racism, poverty, stigma, marginalization, and discrimination play a role in the HIV epidemic. A barrier to preventing HIV infections among transgender youth is a lack of knowledge on how to provide them with comprehensive sexual and reproductive health care. Here are some tips and resources that can help you ensure that transgender youth are safe and healthy.
One of the challenges of obtaining a sexual history is asking the right questions For example, if you have a transgender male assigned female at birth, ask whether their partners produce sperm instead of asking about the sex of their partners. A transgender male’s partner may identify as female but is assigned male at birth and uses her penis during sex. Furthermore, a transgender male may be on testosterone, but he still can get pregnant. Asking how they use their organs is just as important. A transgender male who has condomless penile-vaginal sex with multiple partners is at a higher risk for HIV infection than is a transgender male who shares sex toys with his only partner.
Normalizing that you ask a comprehensive sexual history to all your patients regardless of gender identity may put the patient at ease. Many transgender people are reluctant to disclose their gender identity to their provider because they are afraid that the provider may fixate on their sexuality once they do. Stating that you ask sexual health questions to all your patients may prevent the transgender patient from feeling singled out.
Finally, you don’t have to ask a sexual history with every transgender patient, just as you wouldn’t for your cisgender patients. If a patient is complaining of a sprained ankle, a sexual history may not be helpful, compared with obtaining one when a patient comes in with pelvic pain. Many transgender patients avoid care because they are frequently asked about their sexual history or gender identity when these are not relevant to their chief complaint.
Here are some helpful questions to ask when taking a sexual history, according to the University of California, San Francisco, Transgender Care & Treatment Guidelines.1
- Are you having sex? How many sex partners have you had in the past year?
- Who are you having sex with? What types of sex are you having? What parts of your anatomy do you use for sex?
- How do you protect yourself from STIs?
- What STIs have you had in the past, if any? When were you last tested for STIs?
- Has your partner(s) ever been diagnosed with any STIs?
- Do you use alcohol or any drugs when you have sex?
- Do you exchange sex for money, drugs, or a place to stay?
Also, use a trauma-informed approach when working with transgender patients. Many have been victims of sexual trauma. Always have a chaperone accompany you during the exam, explain to the patient what you plan to do and why it is necessary, and allow them to decline (and document their declining the physical exam). Also consider having your patient self-swab for STI screening if appropriate.1
Like obtaining a sexual history, routine screenings for certain types of cancers will be based on the organs the patient has. For example, a transgender woman assigned male at birth will not need a cervical cancer screening, but a transgender man assigned female at birth may need one – if the patient still has a cervix. Cervical cancer screening guidelines are similar for transgender men as it is for nontransgender women, and one should use the same guidelines endorsed by the American Cancer Society, American Society of Colposcopy and Cervical Pathology, American Society of Clinical Pathologists, U.S. Preventive Services Task Force, and the World Health Organization.2-4
Cervical screenings should never be a requirement for testosterone therapy, and no transgender male under the age of 21 years will need cervical screening. The University of California guidelines offers tips on how to make transgender men more comfortable during cervical cancer screening.5
Contraception and menstrual management also are important for transgender patients. Testosterone can induce amenorrhea for transgender men, but it is not good birth control. If a transgender male patient has sex with partners that produce sperm, then the physician should discuss effective birth control options. There is no ideal birth control option for transgender men. One must consider multiple factors including the patient’s desire for pregnancy, desire to cease periods, ease of administration, and risk for thrombosis.
Most transgender men may balk at the idea of taking estrogen-containing contraception, but it is more effective than oral progestin-only pills. Intrauterine devices are highly effective in pregnancy prevention and can achieve amenorrhea in 50% of users within 1 year,but some transmen may become dysphoric with the procedure. 6 The etonogestrel implants also are highly effective birth control, but irregular periods are common, leading to discontinuation. Depot medroxyprogesterone is highly effective in preventing pregnancy and can induce amenorrhea in 70% of users within 1 year and 80% of users in 2 years, but also is associated with weight gain in one-third of users.7 Finally, pubertal blockers can rapidly stop periods for transmen who are severely dysphoric from their menses; however, before achieving amenorrhea, a flare bleed can occur 4-6 weeks after administration.8 Support from a mental health therapist during this time is critical. Pubertal blockers, nevertheless, are not suitable birth control.
When providing affirming sexual and reproductive health care for transgender patients, key principles include focusing on organs and activities over identity. Additionally, screening for certain types of cancers also is dependent on organs. Finally, do not neglect the importance of contraception among transgender men. Taking these principles in consideration will help you provide excellent care for transgender youth.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at the Children’s Hospital of Pittsburgh. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Transgender people and sexually transmitted infections (https://transcare.ucsf.edu/guidelines/stis).
2. CA Cancer J Clin. 2012 May-Jun;62(3):147-72.
3. Ann Intern Med. 2012;156(12):880-91.
4. Cervical cancer screening in developing countries: Report of a WHO consultation. 2002. World Health Organization, Geneva.
5. Screening for cervical cancer for transgender men (https://transcare.ucsf.edu/guidelines/cervical-cancer).
6. Contraception. 2002 Feb;65(2):129-32.
7. Rev Endocr Metab Disord. 2011 Jun;12(2):93-106.
8. Int J Womens Health. 2014 Jun 23;6:631-7.
Resources
Breast cancer screening in transgender men. (https://transcare.ucsf.edu/guidelines/breast-cancer-men).
Screening for breast cancer in transgender women. (https://transcare.ucsf.edu/guidelines/breast-cancer-women).
Transgender health and HIV (https://transcare.ucsf.edu/guidelines/hiv).
Centers for Disease Control and Prevention: HIV and Transgender People (https://www.cdc.gov/hiv/group/gender/transgender/index.html).
Should secondary cytoreduction be performed for platinum-sensitive recurrent ovarian cancer?
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
EXPERT COMMENTARY
Ovarian cancer represents the most lethal gynecologic cancer, with an estimated 14,000 deaths in 2019.1 While the incidence of this disease is low in comparison to uterine cancer, the advanced stage at diagnosis portends poor prognosis. While stage is an independent risk factor for death, it is also a risk for recurrence, with more than 80% of women developing recurrent disease.2-4 Secondary cytoreduction remains an option for patients in which disease recurs; up until now this management option was driven by retrospective data.5
Details of the study
Coleman and colleagues conducted the Gynecologic Oncology Group (GOG) 0213 trial—a phase 3, multicenter, randomized clinical trial that included 485 women with recurrent ovarian cancer. The surgical objective of the trial was to determine whether secondary cytoreduction in operable, platinum-sensitive (PS) patients improved overall survival (OS).
Patients were eligible to participate in the surgical portion of the trial if they had PS measurable disease and had the intention to achieve complete gross resection. Women with ascites, evidence of extraabdominal disease, and “diffuse carcinomatosis” were excluded. The primary and secondary end points were OS and progression-free survival (PFS), respectively.
Results. There were no statistical differences between the surgery and no surgery groups with regard to median OS (50.6 months vs 64.7 months, respectively; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.97–1.72) or median PFS (18.9 months vs 16.2 months; HR, 0.82; 95% CI, 0.66 to 1.01). When comparing patients in which complete gross resection was achieved (150 patients vs 245 who did not receive surgery), there was only a statistical difference in PFS in favor of the surgical group (22.4 months vs 16.2 months; HR, 0.62; 95% CI, 0.48–0.80).
Of note, 67% of the patients who received surgery (63% intention-to-treat) were debulked to complete gross resection. There were 33% more patients with extraabdominal disease (10% vs 7% of total patients in each group) and 15% more patients with upper abdominal disease (40% vs 33% of total patients in each group) included in the surgical group. Finally, the median time to chemotherapy was 40 days in the surgery group versus 7 days in the no surgery group.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
The authors deserve to be commended for this well-designed and laborious trial, which is the first of its kind. The strength of the study is its randomized design producing level I data.
Study weaknesses include lack of reporting of BRCA status and the impact of receiving targeted therapies after the trial was over. It is well established that BRCA-mutated patients have an independent survival advantage, even when taking into account platinum sensitivity.6-8BRCA status of the study population is not specifically addressed in this paper. The authors noted in the first GOG 0213 trial publication, which assessed bevacizumab in the recurrent setting, that BRCA status has an impact on patient outcomes. Subsequently, they state that they do not report BRCA status because “…its independent effect on response to an anti-angiogenesis agent was unknown,” but it clearly would affect survival analysis if unbalanced between groups.9
Similarly, in the introduction to their study, Coleman and colleagues list availability of maintenance therapy, for instance poly ADP (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, as rationale for conducting their trial. They subsequently cite this as a possible reason that the median overall survival was 3 times longer than expected. However, they provide no data on which patients received maintenance therapy, which again could have drastically affected survival outcomes.10 They do report in the supplementary information that, when stratifying those receiving bevacizumab adjuvantly during the trial, the median OS was comparable between the surgical and nonsurgical groups (58.5 months vs 61.7 months).
The authors discuss the presence of patient selection bias as a weakness in the study. Selection bias is evident in this trial (as in many surgical trials) because patients with a limited volume of disease were selected to participate over those with large-volume disease. It is reasonable to conclude that this study is likely selecting patients with less aggressive tumor biology, not only evident by low-volume disease at recurrence but also by the 20.4-month median platinum-free interval in the surgical group, which certainly affects the trial’s validity. Despite being considered PS, the disease biology in a patient with a platinum-free interval of 20.4 months is surely different from the disease biology in a patient with a 6.4-month platinum-free interval; therefore, it is difficult to generalize these data to all PS recurrent ovarian cancer patients. Similarly, other research has suggested strict selection criteria, which was not apparent in this study’s methodology.11 While the number of metastatic sites were relatively equal between the surgery and no surgery groups, there were more patients in the surgical group with extraabdominal disease, which the authors used as an exclusion criterion.
Lastly, the time to treatment commencement in each arm, which was 40 days for the surgical arm and 7 days in the nonsurgical arm, could represent a flaw in this trial. While we expect a difference in duration to account for recovery time, many centers start chemotherapy as soon as 21 days after surgery, which is almost half of the median interval in the surgical group in this trial. While the authors address this by stating that they completed a landmark analysis, no data or information about what time points they used for the analysis are provided. They simply report an interquartile range of 28 to 51 days. It is hard to know what effect this may have had on the outcome.
This is the first randomized clinical trial conducted to assess whether secondary surgical cytoreduction is beneficial in PS recurrent ovarian cancer patients. It provides compelling evidence to critically evaluate whether surgical cytoreduction is appropriate in a similar patient population. However, we would recommend using caution applying these data to patients who have different platinum-free intervals or low-volume disease limited to the pelvis.
The trial is not without flaws, as the authors point out in their discussion, but currently, it is the best evidence afforded to gynecologic oncologists. There are multiple trials currently ongoing, including DESTOP-III, which had similar PFS results as GOG 0213. If consensus is reached with these 2 trials, we believe that secondary cytoreduction will be utilized far less often in patients with recurrent ovarian cancer and a long platinum-free interval, thereby changing the current treatment paradigm for these patients.
MICHAEL D. TOBONI, MD, MPH, AND DAVID G. MUTCH, MD
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505-515.
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. 2019;152:416-425.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 18, 2019.
- Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187-2195.
- Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127-1132.
- Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
EXPERT COMMENTARY
Ovarian cancer represents the most lethal gynecologic cancer, with an estimated 14,000 deaths in 2019.1 While the incidence of this disease is low in comparison to uterine cancer, the advanced stage at diagnosis portends poor prognosis. While stage is an independent risk factor for death, it is also a risk for recurrence, with more than 80% of women developing recurrent disease.2-4 Secondary cytoreduction remains an option for patients in which disease recurs; up until now this management option was driven by retrospective data.5
Details of the study
Coleman and colleagues conducted the Gynecologic Oncology Group (GOG) 0213 trial—a phase 3, multicenter, randomized clinical trial that included 485 women with recurrent ovarian cancer. The surgical objective of the trial was to determine whether secondary cytoreduction in operable, platinum-sensitive (PS) patients improved overall survival (OS).
Patients were eligible to participate in the surgical portion of the trial if they had PS measurable disease and had the intention to achieve complete gross resection. Women with ascites, evidence of extraabdominal disease, and “diffuse carcinomatosis” were excluded. The primary and secondary end points were OS and progression-free survival (PFS), respectively.
Results. There were no statistical differences between the surgery and no surgery groups with regard to median OS (50.6 months vs 64.7 months, respectively; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.97–1.72) or median PFS (18.9 months vs 16.2 months; HR, 0.82; 95% CI, 0.66 to 1.01). When comparing patients in which complete gross resection was achieved (150 patients vs 245 who did not receive surgery), there was only a statistical difference in PFS in favor of the surgical group (22.4 months vs 16.2 months; HR, 0.62; 95% CI, 0.48–0.80).
Of note, 67% of the patients who received surgery (63% intention-to-treat) were debulked to complete gross resection. There were 33% more patients with extraabdominal disease (10% vs 7% of total patients in each group) and 15% more patients with upper abdominal disease (40% vs 33% of total patients in each group) included in the surgical group. Finally, the median time to chemotherapy was 40 days in the surgery group versus 7 days in the no surgery group.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
The authors deserve to be commended for this well-designed and laborious trial, which is the first of its kind. The strength of the study is its randomized design producing level I data.
Study weaknesses include lack of reporting of BRCA status and the impact of receiving targeted therapies after the trial was over. It is well established that BRCA-mutated patients have an independent survival advantage, even when taking into account platinum sensitivity.6-8BRCA status of the study population is not specifically addressed in this paper. The authors noted in the first GOG 0213 trial publication, which assessed bevacizumab in the recurrent setting, that BRCA status has an impact on patient outcomes. Subsequently, they state that they do not report BRCA status because “…its independent effect on response to an anti-angiogenesis agent was unknown,” but it clearly would affect survival analysis if unbalanced between groups.9
Similarly, in the introduction to their study, Coleman and colleagues list availability of maintenance therapy, for instance poly ADP (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, as rationale for conducting their trial. They subsequently cite this as a possible reason that the median overall survival was 3 times longer than expected. However, they provide no data on which patients received maintenance therapy, which again could have drastically affected survival outcomes.10 They do report in the supplementary information that, when stratifying those receiving bevacizumab adjuvantly during the trial, the median OS was comparable between the surgical and nonsurgical groups (58.5 months vs 61.7 months).
The authors discuss the presence of patient selection bias as a weakness in the study. Selection bias is evident in this trial (as in many surgical trials) because patients with a limited volume of disease were selected to participate over those with large-volume disease. It is reasonable to conclude that this study is likely selecting patients with less aggressive tumor biology, not only evident by low-volume disease at recurrence but also by the 20.4-month median platinum-free interval in the surgical group, which certainly affects the trial’s validity. Despite being considered PS, the disease biology in a patient with a platinum-free interval of 20.4 months is surely different from the disease biology in a patient with a 6.4-month platinum-free interval; therefore, it is difficult to generalize these data to all PS recurrent ovarian cancer patients. Similarly, other research has suggested strict selection criteria, which was not apparent in this study’s methodology.11 While the number of metastatic sites were relatively equal between the surgery and no surgery groups, there were more patients in the surgical group with extraabdominal disease, which the authors used as an exclusion criterion.
Lastly, the time to treatment commencement in each arm, which was 40 days for the surgical arm and 7 days in the nonsurgical arm, could represent a flaw in this trial. While we expect a difference in duration to account for recovery time, many centers start chemotherapy as soon as 21 days after surgery, which is almost half of the median interval in the surgical group in this trial. While the authors address this by stating that they completed a landmark analysis, no data or information about what time points they used for the analysis are provided. They simply report an interquartile range of 28 to 51 days. It is hard to know what effect this may have had on the outcome.
This is the first randomized clinical trial conducted to assess whether secondary surgical cytoreduction is beneficial in PS recurrent ovarian cancer patients. It provides compelling evidence to critically evaluate whether surgical cytoreduction is appropriate in a similar patient population. However, we would recommend using caution applying these data to patients who have different platinum-free intervals or low-volume disease limited to the pelvis.
The trial is not without flaws, as the authors point out in their discussion, but currently, it is the best evidence afforded to gynecologic oncologists. There are multiple trials currently ongoing, including DESTOP-III, which had similar PFS results as GOG 0213. If consensus is reached with these 2 trials, we believe that secondary cytoreduction will be utilized far less often in patients with recurrent ovarian cancer and a long platinum-free interval, thereby changing the current treatment paradigm for these patients.
MICHAEL D. TOBONI, MD, MPH, AND DAVID G. MUTCH, MD
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
EXPERT COMMENTARY
Ovarian cancer represents the most lethal gynecologic cancer, with an estimated 14,000 deaths in 2019.1 While the incidence of this disease is low in comparison to uterine cancer, the advanced stage at diagnosis portends poor prognosis. While stage is an independent risk factor for death, it is also a risk for recurrence, with more than 80% of women developing recurrent disease.2-4 Secondary cytoreduction remains an option for patients in which disease recurs; up until now this management option was driven by retrospective data.5
Details of the study
Coleman and colleagues conducted the Gynecologic Oncology Group (GOG) 0213 trial—a phase 3, multicenter, randomized clinical trial that included 485 women with recurrent ovarian cancer. The surgical objective of the trial was to determine whether secondary cytoreduction in operable, platinum-sensitive (PS) patients improved overall survival (OS).
Patients were eligible to participate in the surgical portion of the trial if they had PS measurable disease and had the intention to achieve complete gross resection. Women with ascites, evidence of extraabdominal disease, and “diffuse carcinomatosis” were excluded. The primary and secondary end points were OS and progression-free survival (PFS), respectively.
Results. There were no statistical differences between the surgery and no surgery groups with regard to median OS (50.6 months vs 64.7 months, respectively; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.97–1.72) or median PFS (18.9 months vs 16.2 months; HR, 0.82; 95% CI, 0.66 to 1.01). When comparing patients in which complete gross resection was achieved (150 patients vs 245 who did not receive surgery), there was only a statistical difference in PFS in favor of the surgical group (22.4 months vs 16.2 months; HR, 0.62; 95% CI, 0.48–0.80).
Of note, 67% of the patients who received surgery (63% intention-to-treat) were debulked to complete gross resection. There were 33% more patients with extraabdominal disease (10% vs 7% of total patients in each group) and 15% more patients with upper abdominal disease (40% vs 33% of total patients in each group) included in the surgical group. Finally, the median time to chemotherapy was 40 days in the surgery group versus 7 days in the no surgery group.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
The authors deserve to be commended for this well-designed and laborious trial, which is the first of its kind. The strength of the study is its randomized design producing level I data.
Study weaknesses include lack of reporting of BRCA status and the impact of receiving targeted therapies after the trial was over. It is well established that BRCA-mutated patients have an independent survival advantage, even when taking into account platinum sensitivity.6-8BRCA status of the study population is not specifically addressed in this paper. The authors noted in the first GOG 0213 trial publication, which assessed bevacizumab in the recurrent setting, that BRCA status has an impact on patient outcomes. Subsequently, they state that they do not report BRCA status because “…its independent effect on response to an anti-angiogenesis agent was unknown,” but it clearly would affect survival analysis if unbalanced between groups.9
Similarly, in the introduction to their study, Coleman and colleagues list availability of maintenance therapy, for instance poly ADP (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, as rationale for conducting their trial. They subsequently cite this as a possible reason that the median overall survival was 3 times longer than expected. However, they provide no data on which patients received maintenance therapy, which again could have drastically affected survival outcomes.10 They do report in the supplementary information that, when stratifying those receiving bevacizumab adjuvantly during the trial, the median OS was comparable between the surgical and nonsurgical groups (58.5 months vs 61.7 months).
The authors discuss the presence of patient selection bias as a weakness in the study. Selection bias is evident in this trial (as in many surgical trials) because patients with a limited volume of disease were selected to participate over those with large-volume disease. It is reasonable to conclude that this study is likely selecting patients with less aggressive tumor biology, not only evident by low-volume disease at recurrence but also by the 20.4-month median platinum-free interval in the surgical group, which certainly affects the trial’s validity. Despite being considered PS, the disease biology in a patient with a platinum-free interval of 20.4 months is surely different from the disease biology in a patient with a 6.4-month platinum-free interval; therefore, it is difficult to generalize these data to all PS recurrent ovarian cancer patients. Similarly, other research has suggested strict selection criteria, which was not apparent in this study’s methodology.11 While the number of metastatic sites were relatively equal between the surgery and no surgery groups, there were more patients in the surgical group with extraabdominal disease, which the authors used as an exclusion criterion.
Lastly, the time to treatment commencement in each arm, which was 40 days for the surgical arm and 7 days in the nonsurgical arm, could represent a flaw in this trial. While we expect a difference in duration to account for recovery time, many centers start chemotherapy as soon as 21 days after surgery, which is almost half of the median interval in the surgical group in this trial. While the authors address this by stating that they completed a landmark analysis, no data or information about what time points they used for the analysis are provided. They simply report an interquartile range of 28 to 51 days. It is hard to know what effect this may have had on the outcome.
This is the first randomized clinical trial conducted to assess whether secondary surgical cytoreduction is beneficial in PS recurrent ovarian cancer patients. It provides compelling evidence to critically evaluate whether surgical cytoreduction is appropriate in a similar patient population. However, we would recommend using caution applying these data to patients who have different platinum-free intervals or low-volume disease limited to the pelvis.
The trial is not without flaws, as the authors point out in their discussion, but currently, it is the best evidence afforded to gynecologic oncologists. There are multiple trials currently ongoing, including DESTOP-III, which had similar PFS results as GOG 0213. If consensus is reached with these 2 trials, we believe that secondary cytoreduction will be utilized far less often in patients with recurrent ovarian cancer and a long platinum-free interval, thereby changing the current treatment paradigm for these patients.
MICHAEL D. TOBONI, MD, MPH, AND DAVID G. MUTCH, MD
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505-515.
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. 2019;152:416-425.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 18, 2019.
- Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187-2195.
- Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127-1132.
- Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505-515.
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. 2019;152:416-425.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 18, 2019.
- Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187-2195.
- Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127-1132.
- Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
Can the office visit interval for routine pessary care be extended safely?
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2019 Dec 5. Doi: 10.1097/AOG.0000000000003580.
EXPERT COMMENTARY
Vaginal pessaries are a common and effective approach for managing pelvic organ prolapse (POP) as well as stress urinary incontinence (SUI). Vaginal mucosal erosions, however, may complicate pessary use. The risk for erosions may be associated with the frequency of pessary change, which involves removing the pessary, washing it, and replacing it in the vagina. Existing data do not address the frequency of pessary change. Recently, however, investigators conducted a randomized noninferiority trial to evaluate the effect of pessary visit intervals on the development of vaginal epithelial abnormalities.
Details of the study
At a single US hospital, Propst and colleagues randomly assigned women who used pessaries for POP, SUI, or both to routine pessary care (offices visits every 12 weeks) or to extended interval pessary care (office visits every 24 weeks). The women used ring, incontinence dish, or Gelhorn pessaries, did not change their pessaries on their own, and had no vaginal mucosal abnormalities.
A total of 130 women were randomly assigned, 64 to the routine care group and 66 to the extended interval care group. The mean age was 79 years and 90% were white, 4.6% were black, and 4% were Hispanic. Approximately 74% of the women used vaginal estrogen.
The primary outcome was the rate of vaginal epithelial abnormalities, including epithelial breaks or erosions. The predetermined noninferiority margin was set at 7.5%.
Results. At the 48-week follow-up, the rate of epithelial erosion was 7.4% in the routine care group and 1.7% in the extended interval care group, thus meeting the prespecified criteria for noninferiority of extended interval pessary care.
Women in each care group reported a similar amount of bothersome vaginal discharge. This was reported on a 5-point scale, with higher numbers indicating greater degree of bother. The mean scores were 1.39 in the routine care group and 1.34 in the extended interval care group. No other pessary-related adverse events occurred in either care group.
Study strengths and limitations
This trial provides good evidence that the timing of office pessary care can be extended to 24 weeks without compromising outcomes. However, since nearly three-quarters of the study participants used vaginal estrogen, the results may not be applicable to pessary users who do not use vaginal estrogen.
Many women change their pessary at home as often as weekly or daily. For women who rely on office visits for pessary care, however, the trial by Propst and colleagues provides good quality evidence that pessaries can be changed as infrequently as every 24 weeks without compromising outcomes. An important limitation of these data is that since most study participants used vaginal estrogen, the findings may not apply to pessary use among women who do not use vaginal estrogen.
ANDREW M. KAUNITZ, MD, NCMP
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2019 Dec 5. Doi: 10.1097/AOG.0000000000003580.
EXPERT COMMENTARY
Vaginal pessaries are a common and effective approach for managing pelvic organ prolapse (POP) as well as stress urinary incontinence (SUI). Vaginal mucosal erosions, however, may complicate pessary use. The risk for erosions may be associated with the frequency of pessary change, which involves removing the pessary, washing it, and replacing it in the vagina. Existing data do not address the frequency of pessary change. Recently, however, investigators conducted a randomized noninferiority trial to evaluate the effect of pessary visit intervals on the development of vaginal epithelial abnormalities.
Details of the study
At a single US hospital, Propst and colleagues randomly assigned women who used pessaries for POP, SUI, or both to routine pessary care (offices visits every 12 weeks) or to extended interval pessary care (office visits every 24 weeks). The women used ring, incontinence dish, or Gelhorn pessaries, did not change their pessaries on their own, and had no vaginal mucosal abnormalities.
A total of 130 women were randomly assigned, 64 to the routine care group and 66 to the extended interval care group. The mean age was 79 years and 90% were white, 4.6% were black, and 4% were Hispanic. Approximately 74% of the women used vaginal estrogen.
The primary outcome was the rate of vaginal epithelial abnormalities, including epithelial breaks or erosions. The predetermined noninferiority margin was set at 7.5%.
Results. At the 48-week follow-up, the rate of epithelial erosion was 7.4% in the routine care group and 1.7% in the extended interval care group, thus meeting the prespecified criteria for noninferiority of extended interval pessary care.
Women in each care group reported a similar amount of bothersome vaginal discharge. This was reported on a 5-point scale, with higher numbers indicating greater degree of bother. The mean scores were 1.39 in the routine care group and 1.34 in the extended interval care group. No other pessary-related adverse events occurred in either care group.
Study strengths and limitations
This trial provides good evidence that the timing of office pessary care can be extended to 24 weeks without compromising outcomes. However, since nearly three-quarters of the study participants used vaginal estrogen, the results may not be applicable to pessary users who do not use vaginal estrogen.
Many women change their pessary at home as often as weekly or daily. For women who rely on office visits for pessary care, however, the trial by Propst and colleagues provides good quality evidence that pessaries can be changed as infrequently as every 24 weeks without compromising outcomes. An important limitation of these data is that since most study participants used vaginal estrogen, the findings may not apply to pessary use among women who do not use vaginal estrogen.
ANDREW M. KAUNITZ, MD, NCMP
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2019 Dec 5. Doi: 10.1097/AOG.0000000000003580.
EXPERT COMMENTARY
Vaginal pessaries are a common and effective approach for managing pelvic organ prolapse (POP) as well as stress urinary incontinence (SUI). Vaginal mucosal erosions, however, may complicate pessary use. The risk for erosions may be associated with the frequency of pessary change, which involves removing the pessary, washing it, and replacing it in the vagina. Existing data do not address the frequency of pessary change. Recently, however, investigators conducted a randomized noninferiority trial to evaluate the effect of pessary visit intervals on the development of vaginal epithelial abnormalities.
Details of the study
At a single US hospital, Propst and colleagues randomly assigned women who used pessaries for POP, SUI, or both to routine pessary care (offices visits every 12 weeks) or to extended interval pessary care (office visits every 24 weeks). The women used ring, incontinence dish, or Gelhorn pessaries, did not change their pessaries on their own, and had no vaginal mucosal abnormalities.
A total of 130 women were randomly assigned, 64 to the routine care group and 66 to the extended interval care group. The mean age was 79 years and 90% were white, 4.6% were black, and 4% were Hispanic. Approximately 74% of the women used vaginal estrogen.
The primary outcome was the rate of vaginal epithelial abnormalities, including epithelial breaks or erosions. The predetermined noninferiority margin was set at 7.5%.
Results. At the 48-week follow-up, the rate of epithelial erosion was 7.4% in the routine care group and 1.7% in the extended interval care group, thus meeting the prespecified criteria for noninferiority of extended interval pessary care.
Women in each care group reported a similar amount of bothersome vaginal discharge. This was reported on a 5-point scale, with higher numbers indicating greater degree of bother. The mean scores were 1.39 in the routine care group and 1.34 in the extended interval care group. No other pessary-related adverse events occurred in either care group.
Study strengths and limitations
This trial provides good evidence that the timing of office pessary care can be extended to 24 weeks without compromising outcomes. However, since nearly three-quarters of the study participants used vaginal estrogen, the results may not be applicable to pessary users who do not use vaginal estrogen.
Many women change their pessary at home as often as weekly or daily. For women who rely on office visits for pessary care, however, the trial by Propst and colleagues provides good quality evidence that pessaries can be changed as infrequently as every 24 weeks without compromising outcomes. An important limitation of these data is that since most study participants used vaginal estrogen, the findings may not apply to pessary use among women who do not use vaginal estrogen.
ANDREW M. KAUNITZ, MD, NCMP
Dermatology Continuing Certification Changes for the Better
Major changes in continuing board certification are occurring across medical specialties. On January 6, 2020, the American Board of Dermatology (ABD) launches its new web-based longitudinal assessment program called CertLink (https://abderm.mycertlink.org/).1 This new platform is designed to eventually replace the sit-down, high-stakes, once-every-10-year medical knowledge examination that dermatologists take to remain board certified. With this alternative, every participating dermatologist will receive a batch of 13 web-based questions every quarter that he/she may answer at a convenient time and place. Questions are answered one at a time or in batches, depending on the test taker’s preference, and can be completed on home or office computers (and eventually on smartphones). Participating in this type of testing does not require shutting down practice, traveling to a test center, or paying for expensive board review courses. CertLink is designed to be convenient, affordable, and relevant to an individual’s practice.
How did the ABD arrive at CertLink?
The ABD launched its original Maintenance of Certification (MOC) program in 2006. Since then, newly board-certified dermatologists, recertifying dermatologists with time-limited certificates, and time-unlimited dermatologists who volunteered to participate in MOC have experienced the dermatology MOC program. In its first 10 years, the program was met with very mixed reviews. The program was designed to assess and promote competence in a 4-part framework, including professionalism; commitment to lifelong learning and self-assessment; demonstration of knowledge, judgment, and skills; and improvement in medical practice. All 4 are areas of rational pursuit for medical professionals seeking to perform and maintain the highest quality patient care possible. But there were problems. First iterations are rarely perfect, and dermatology MOC was no exception.
At the onset, the ABD chose to oversee the MOC requirements and remained hands off in the delivery of education, relying instead on other organizations to fulfill the ABD’s requirements. Unfortunately, with limited educational offerings available, many diplomates paid notable registration fees for each qualifying MOC activity. Quality improvement activities were a relatively new experience for dermatologists and were time consuming. Required medical record reviews were onerous, often requiring more than 35 data points to be collected per medical record reviewed. The limited number and limited diversity of educational offerings also created circumstances in which the material covered was not maximally relevant to many participants. When paying to answer questions about patient populations or procedure types never encountered by the dermatologist who purchased the particular MOC activity, many asked the question “How does this make me a better doctor?” They were right to ask.
Cost, time commitment to participate in MOC, and relevance to practice were 3 key areas of concern for many dermatologists. In response to internal and external MOC feedback, in 2015 the ABD took a hard look at its 10-year experience with MOC. While contemplating its next strategies, the ABD temporarily put its component 4—practice improvement—requirements on hold. After much review, the ABD decided to take over a notable portion of the education delivery. Its goal was to provide education that would fulfill MOC requirements in a more affordable, relevant, quicker, and easier manner.
First, the ABD made the decision to assume a more notable role as educator, in part to offer qualifying activities at no additional cost to diplomates. By taking on the role as educator, 3 major changes resulted: the way ABD approached quality improvement activities, partnership to initiate a question-of-the-week self-assessment program, and initiation of a longitudinal assessment strategy that resulted in this month’s launch of CertLink.
The ABD revolutionized its quality improvement requirements with the launch of its practice improvement modules made available through its website.2 These modules utilize recently published clinical practice gaps in 5 dermatology subspecialty domains to fulfill the practice improvement requirements. Participants read a brief synopsis of the supporting literature explaining practice improvement recommendations found in the module. Next, they find 5 patients in their practice with the condition, medication, or process in question and review whether they provided the care supported by the best available evidence. No module requires more than 5 medical records to review, and no more than 3 questions are answered per medical record review. If review confirms that the care was appropriate, no further action is needed. If a care gap is identified, then participants implement changes and later remeasure practice to detect any change. This certification activity was incredibly popular with the thousands of diplomates who have participated thus far; more than 97% stated the modules were relevant to practice, 98% stated they would recommend the modules to fellow dermatologists, and nearly 25% reported the module helped to change their practice for the better (unpublished data, July 2019). Relevance had been restored.
The ABD worked closely with the American Academy of Dermatology (AAD) to develop new education for weekly self-assessment. The ABD created the content and delivered to the AAD the first year of material for what would become the most successful and popular dermatology CME activity in history: the AAD Question of the Week (QOW). Thousands of dermatologists are registered to receive the QOW, with very active weekly participation. Participants receive 1 self-assessment point and 0.25 CME credits for each attempted question, right or wrong. This quizzing tool also was educational, with explanation of right answers and wrong choices included. The average amount of time spent answering each question was approximately 40 seconds. American Academy of Dermatology members can participate in its QOW as a member benefit. Self-assessment is no longer a time- consuming or costly process.
The third major change was the ABD initiation of the longitudinal assessment strategy called CertLink, a web-based testing platform operated by the American Board of Medical Specialties. Longitudinal assessment differs from traditional certification and recertification assessment. It allows the test taker to answer the certification test questions over time instead of all at once. Longitudinal assessment not only provides a greater level of convenience to the test taker but also allows boards a more continuous set of touch points in the assessment of diplomates over the course of the continuing certification period.
What will be part of CertLink?
In addition to standard multiple-choice questions, there are many interesting elements to the CertLink program, such as article-based questions. At the beginning of each year, dermatologists select 8 articles from a list of those hosted by CertLink. These are recently published articles, chosen for their meaningfulness to practicing dermatologists. Each subsequent quarter, 2 of these articles are issued to the diplomate to read at his/her leisure. Once ready, participants launch and answer 2 questions about the key points of each article. The article-based questions were designed to help the practicing dermatologist stay up-to-date and relevant in personally chosen areas.
Diplomates are offered a chance to learn from any question that was missed, with explanations or resources provided to help them understand why the correct answer is correct. In this new learn-to-competence model, diplomates are not penalized the first time they answer a particular question incorrectly. Each is provided an opportunity to learn through the explanations given, and then in a future quarter, the dermatologist is given a second chance to answer a similarly themed question, with only that second chance counting toward his/her overall score.
Another unique aspect of CertLink is the allowance of time off from assessment. The ABD recognizes that life happens, and that intermittent time off from career-long assessment will be necessary to accommodate life events, including but not limited to maternity leave, other medical leave, or mental health breaks. Diplomates may take off up to 1 quarter of testing each year to accommodate such life events. Those who need extra time (beyond 1 quarter per year) would need to communicate directly with ABD to request. Those who continue to answer questions throughout the year will have their lowest-performing quarter dropped, to maximize fairness to all. Only the top 3 quarters of CertLink test performance will be counted each year when making certification status decisions. Those who take 1 quarter off will have their other 3 quarters counted toward their scoring.
How will CertLink measure performance?
At the onset of CertLink, there is no predetermined passing score. It will take a few years for the ABD psychometricians to determine an acceptable performance. Questions are written not to be tricky but rather to assess patient issues the dermatologist is likely to encounter in practice. Article-based questions are designed to assess the key points of important recent articles to advance the dermatologist’s practice.
Final Thoughts
In the end, the ABD approach to the new area of continuing certification centers on strategies to be relevant, inexpensive, and minimally disruptive to practice, and to teach to competence and advance practice by bringing forward articles that address key recent literature. We think it is a much better approach to dermatology continuing certification.
- ABD announces CertLink launch in 2020 [news release]. Newton, MA: American Board of Dermatology; 2019. https://www.abderm.org/public/announcements/certlink-2020.aspx. Accessed December 17, 2019.
- American Board of Dermatology. Focused practice improvement modules. https://www.abderm.org/diplomates/fulfilling-moc-requirements/abd-focused-pi-modules-for-moc.aspx. Accessed December 18, 2019.
Major changes in continuing board certification are occurring across medical specialties. On January 6, 2020, the American Board of Dermatology (ABD) launches its new web-based longitudinal assessment program called CertLink (https://abderm.mycertlink.org/).1 This new platform is designed to eventually replace the sit-down, high-stakes, once-every-10-year medical knowledge examination that dermatologists take to remain board certified. With this alternative, every participating dermatologist will receive a batch of 13 web-based questions every quarter that he/she may answer at a convenient time and place. Questions are answered one at a time or in batches, depending on the test taker’s preference, and can be completed on home or office computers (and eventually on smartphones). Participating in this type of testing does not require shutting down practice, traveling to a test center, or paying for expensive board review courses. CertLink is designed to be convenient, affordable, and relevant to an individual’s practice.
How did the ABD arrive at CertLink?
The ABD launched its original Maintenance of Certification (MOC) program in 2006. Since then, newly board-certified dermatologists, recertifying dermatologists with time-limited certificates, and time-unlimited dermatologists who volunteered to participate in MOC have experienced the dermatology MOC program. In its first 10 years, the program was met with very mixed reviews. The program was designed to assess and promote competence in a 4-part framework, including professionalism; commitment to lifelong learning and self-assessment; demonstration of knowledge, judgment, and skills; and improvement in medical practice. All 4 are areas of rational pursuit for medical professionals seeking to perform and maintain the highest quality patient care possible. But there were problems. First iterations are rarely perfect, and dermatology MOC was no exception.
At the onset, the ABD chose to oversee the MOC requirements and remained hands off in the delivery of education, relying instead on other organizations to fulfill the ABD’s requirements. Unfortunately, with limited educational offerings available, many diplomates paid notable registration fees for each qualifying MOC activity. Quality improvement activities were a relatively new experience for dermatologists and were time consuming. Required medical record reviews were onerous, often requiring more than 35 data points to be collected per medical record reviewed. The limited number and limited diversity of educational offerings also created circumstances in which the material covered was not maximally relevant to many participants. When paying to answer questions about patient populations or procedure types never encountered by the dermatologist who purchased the particular MOC activity, many asked the question “How does this make me a better doctor?” They were right to ask.
Cost, time commitment to participate in MOC, and relevance to practice were 3 key areas of concern for many dermatologists. In response to internal and external MOC feedback, in 2015 the ABD took a hard look at its 10-year experience with MOC. While contemplating its next strategies, the ABD temporarily put its component 4—practice improvement—requirements on hold. After much review, the ABD decided to take over a notable portion of the education delivery. Its goal was to provide education that would fulfill MOC requirements in a more affordable, relevant, quicker, and easier manner.
First, the ABD made the decision to assume a more notable role as educator, in part to offer qualifying activities at no additional cost to diplomates. By taking on the role as educator, 3 major changes resulted: the way ABD approached quality improvement activities, partnership to initiate a question-of-the-week self-assessment program, and initiation of a longitudinal assessment strategy that resulted in this month’s launch of CertLink.
The ABD revolutionized its quality improvement requirements with the launch of its practice improvement modules made available through its website.2 These modules utilize recently published clinical practice gaps in 5 dermatology subspecialty domains to fulfill the practice improvement requirements. Participants read a brief synopsis of the supporting literature explaining practice improvement recommendations found in the module. Next, they find 5 patients in their practice with the condition, medication, or process in question and review whether they provided the care supported by the best available evidence. No module requires more than 5 medical records to review, and no more than 3 questions are answered per medical record review. If review confirms that the care was appropriate, no further action is needed. If a care gap is identified, then participants implement changes and later remeasure practice to detect any change. This certification activity was incredibly popular with the thousands of diplomates who have participated thus far; more than 97% stated the modules were relevant to practice, 98% stated they would recommend the modules to fellow dermatologists, and nearly 25% reported the module helped to change their practice for the better (unpublished data, July 2019). Relevance had been restored.
The ABD worked closely with the American Academy of Dermatology (AAD) to develop new education for weekly self-assessment. The ABD created the content and delivered to the AAD the first year of material for what would become the most successful and popular dermatology CME activity in history: the AAD Question of the Week (QOW). Thousands of dermatologists are registered to receive the QOW, with very active weekly participation. Participants receive 1 self-assessment point and 0.25 CME credits for each attempted question, right or wrong. This quizzing tool also was educational, with explanation of right answers and wrong choices included. The average amount of time spent answering each question was approximately 40 seconds. American Academy of Dermatology members can participate in its QOW as a member benefit. Self-assessment is no longer a time- consuming or costly process.
The third major change was the ABD initiation of the longitudinal assessment strategy called CertLink, a web-based testing platform operated by the American Board of Medical Specialties. Longitudinal assessment differs from traditional certification and recertification assessment. It allows the test taker to answer the certification test questions over time instead of all at once. Longitudinal assessment not only provides a greater level of convenience to the test taker but also allows boards a more continuous set of touch points in the assessment of diplomates over the course of the continuing certification period.
What will be part of CertLink?
In addition to standard multiple-choice questions, there are many interesting elements to the CertLink program, such as article-based questions. At the beginning of each year, dermatologists select 8 articles from a list of those hosted by CertLink. These are recently published articles, chosen for their meaningfulness to practicing dermatologists. Each subsequent quarter, 2 of these articles are issued to the diplomate to read at his/her leisure. Once ready, participants launch and answer 2 questions about the key points of each article. The article-based questions were designed to help the practicing dermatologist stay up-to-date and relevant in personally chosen areas.
Diplomates are offered a chance to learn from any question that was missed, with explanations or resources provided to help them understand why the correct answer is correct. In this new learn-to-competence model, diplomates are not penalized the first time they answer a particular question incorrectly. Each is provided an opportunity to learn through the explanations given, and then in a future quarter, the dermatologist is given a second chance to answer a similarly themed question, with only that second chance counting toward his/her overall score.
Another unique aspect of CertLink is the allowance of time off from assessment. The ABD recognizes that life happens, and that intermittent time off from career-long assessment will be necessary to accommodate life events, including but not limited to maternity leave, other medical leave, or mental health breaks. Diplomates may take off up to 1 quarter of testing each year to accommodate such life events. Those who need extra time (beyond 1 quarter per year) would need to communicate directly with ABD to request. Those who continue to answer questions throughout the year will have their lowest-performing quarter dropped, to maximize fairness to all. Only the top 3 quarters of CertLink test performance will be counted each year when making certification status decisions. Those who take 1 quarter off will have their other 3 quarters counted toward their scoring.
How will CertLink measure performance?
At the onset of CertLink, there is no predetermined passing score. It will take a few years for the ABD psychometricians to determine an acceptable performance. Questions are written not to be tricky but rather to assess patient issues the dermatologist is likely to encounter in practice. Article-based questions are designed to assess the key points of important recent articles to advance the dermatologist’s practice.
Final Thoughts
In the end, the ABD approach to the new area of continuing certification centers on strategies to be relevant, inexpensive, and minimally disruptive to practice, and to teach to competence and advance practice by bringing forward articles that address key recent literature. We think it is a much better approach to dermatology continuing certification.
Major changes in continuing board certification are occurring across medical specialties. On January 6, 2020, the American Board of Dermatology (ABD) launches its new web-based longitudinal assessment program called CertLink (https://abderm.mycertlink.org/).1 This new platform is designed to eventually replace the sit-down, high-stakes, once-every-10-year medical knowledge examination that dermatologists take to remain board certified. With this alternative, every participating dermatologist will receive a batch of 13 web-based questions every quarter that he/she may answer at a convenient time and place. Questions are answered one at a time or in batches, depending on the test taker’s preference, and can be completed on home or office computers (and eventually on smartphones). Participating in this type of testing does not require shutting down practice, traveling to a test center, or paying for expensive board review courses. CertLink is designed to be convenient, affordable, and relevant to an individual’s practice.
How did the ABD arrive at CertLink?
The ABD launched its original Maintenance of Certification (MOC) program in 2006. Since then, newly board-certified dermatologists, recertifying dermatologists with time-limited certificates, and time-unlimited dermatologists who volunteered to participate in MOC have experienced the dermatology MOC program. In its first 10 years, the program was met with very mixed reviews. The program was designed to assess and promote competence in a 4-part framework, including professionalism; commitment to lifelong learning and self-assessment; demonstration of knowledge, judgment, and skills; and improvement in medical practice. All 4 are areas of rational pursuit for medical professionals seeking to perform and maintain the highest quality patient care possible. But there were problems. First iterations are rarely perfect, and dermatology MOC was no exception.
At the onset, the ABD chose to oversee the MOC requirements and remained hands off in the delivery of education, relying instead on other organizations to fulfill the ABD’s requirements. Unfortunately, with limited educational offerings available, many diplomates paid notable registration fees for each qualifying MOC activity. Quality improvement activities were a relatively new experience for dermatologists and were time consuming. Required medical record reviews were onerous, often requiring more than 35 data points to be collected per medical record reviewed. The limited number and limited diversity of educational offerings also created circumstances in which the material covered was not maximally relevant to many participants. When paying to answer questions about patient populations or procedure types never encountered by the dermatologist who purchased the particular MOC activity, many asked the question “How does this make me a better doctor?” They were right to ask.
Cost, time commitment to participate in MOC, and relevance to practice were 3 key areas of concern for many dermatologists. In response to internal and external MOC feedback, in 2015 the ABD took a hard look at its 10-year experience with MOC. While contemplating its next strategies, the ABD temporarily put its component 4—practice improvement—requirements on hold. After much review, the ABD decided to take over a notable portion of the education delivery. Its goal was to provide education that would fulfill MOC requirements in a more affordable, relevant, quicker, and easier manner.
First, the ABD made the decision to assume a more notable role as educator, in part to offer qualifying activities at no additional cost to diplomates. By taking on the role as educator, 3 major changes resulted: the way ABD approached quality improvement activities, partnership to initiate a question-of-the-week self-assessment program, and initiation of a longitudinal assessment strategy that resulted in this month’s launch of CertLink.
The ABD revolutionized its quality improvement requirements with the launch of its practice improvement modules made available through its website.2 These modules utilize recently published clinical practice gaps in 5 dermatology subspecialty domains to fulfill the practice improvement requirements. Participants read a brief synopsis of the supporting literature explaining practice improvement recommendations found in the module. Next, they find 5 patients in their practice with the condition, medication, or process in question and review whether they provided the care supported by the best available evidence. No module requires more than 5 medical records to review, and no more than 3 questions are answered per medical record review. If review confirms that the care was appropriate, no further action is needed. If a care gap is identified, then participants implement changes and later remeasure practice to detect any change. This certification activity was incredibly popular with the thousands of diplomates who have participated thus far; more than 97% stated the modules were relevant to practice, 98% stated they would recommend the modules to fellow dermatologists, and nearly 25% reported the module helped to change their practice for the better (unpublished data, July 2019). Relevance had been restored.
The ABD worked closely with the American Academy of Dermatology (AAD) to develop new education for weekly self-assessment. The ABD created the content and delivered to the AAD the first year of material for what would become the most successful and popular dermatology CME activity in history: the AAD Question of the Week (QOW). Thousands of dermatologists are registered to receive the QOW, with very active weekly participation. Participants receive 1 self-assessment point and 0.25 CME credits for each attempted question, right or wrong. This quizzing tool also was educational, with explanation of right answers and wrong choices included. The average amount of time spent answering each question was approximately 40 seconds. American Academy of Dermatology members can participate in its QOW as a member benefit. Self-assessment is no longer a time- consuming or costly process.
The third major change was the ABD initiation of the longitudinal assessment strategy called CertLink, a web-based testing platform operated by the American Board of Medical Specialties. Longitudinal assessment differs from traditional certification and recertification assessment. It allows the test taker to answer the certification test questions over time instead of all at once. Longitudinal assessment not only provides a greater level of convenience to the test taker but also allows boards a more continuous set of touch points in the assessment of diplomates over the course of the continuing certification period.
What will be part of CertLink?
In addition to standard multiple-choice questions, there are many interesting elements to the CertLink program, such as article-based questions. At the beginning of each year, dermatologists select 8 articles from a list of those hosted by CertLink. These are recently published articles, chosen for their meaningfulness to practicing dermatologists. Each subsequent quarter, 2 of these articles are issued to the diplomate to read at his/her leisure. Once ready, participants launch and answer 2 questions about the key points of each article. The article-based questions were designed to help the practicing dermatologist stay up-to-date and relevant in personally chosen areas.
Diplomates are offered a chance to learn from any question that was missed, with explanations or resources provided to help them understand why the correct answer is correct. In this new learn-to-competence model, diplomates are not penalized the first time they answer a particular question incorrectly. Each is provided an opportunity to learn through the explanations given, and then in a future quarter, the dermatologist is given a second chance to answer a similarly themed question, with only that second chance counting toward his/her overall score.
Another unique aspect of CertLink is the allowance of time off from assessment. The ABD recognizes that life happens, and that intermittent time off from career-long assessment will be necessary to accommodate life events, including but not limited to maternity leave, other medical leave, or mental health breaks. Diplomates may take off up to 1 quarter of testing each year to accommodate such life events. Those who need extra time (beyond 1 quarter per year) would need to communicate directly with ABD to request. Those who continue to answer questions throughout the year will have their lowest-performing quarter dropped, to maximize fairness to all. Only the top 3 quarters of CertLink test performance will be counted each year when making certification status decisions. Those who take 1 quarter off will have their other 3 quarters counted toward their scoring.
How will CertLink measure performance?
At the onset of CertLink, there is no predetermined passing score. It will take a few years for the ABD psychometricians to determine an acceptable performance. Questions are written not to be tricky but rather to assess patient issues the dermatologist is likely to encounter in practice. Article-based questions are designed to assess the key points of important recent articles to advance the dermatologist’s practice.
Final Thoughts
In the end, the ABD approach to the new area of continuing certification centers on strategies to be relevant, inexpensive, and minimally disruptive to practice, and to teach to competence and advance practice by bringing forward articles that address key recent literature. We think it is a much better approach to dermatology continuing certification.
- ABD announces CertLink launch in 2020 [news release]. Newton, MA: American Board of Dermatology; 2019. https://www.abderm.org/public/announcements/certlink-2020.aspx. Accessed December 17, 2019.
- American Board of Dermatology. Focused practice improvement modules. https://www.abderm.org/diplomates/fulfilling-moc-requirements/abd-focused-pi-modules-for-moc.aspx. Accessed December 18, 2019.
- ABD announces CertLink launch in 2020 [news release]. Newton, MA: American Board of Dermatology; 2019. https://www.abderm.org/public/announcements/certlink-2020.aspx. Accessed December 17, 2019.
- American Board of Dermatology. Focused practice improvement modules. https://www.abderm.org/diplomates/fulfilling-moc-requirements/abd-focused-pi-modules-for-moc.aspx. Accessed December 18, 2019.
Military Health Care at a Crossroads
The certainty that federal health care will be different, and the equal uncertainty about when and how the systems will evolve, were major topics at the recent AMSUS annual meeting. The Veterans Health Administration (VHA) and Military Health System (MHS) are in the midst of major transformations, although they are at very different points in the process and the final outcomes are yet unknown. This editorial, written at the end of 2019, will review some of the highlights of a discussion that is sure to continue in 2020 and beyond.
Almost everyone in the VA and many of the public can pinpoint the exact place (and time) the VHA’s upheaval began: Phoenix, Arizona, in 2014. “The attack on our system,” as VHA Executive in Charge Richard A. Stone, MD, described it at AMSUS, happened because “we were just too slow a bureaucracy,” he explained.1 “We can debate how many veterans died while waiting for care, but the answer is that 1 was too many and it had to be fixed. We had to become a more agile organization.”
The US Department of Veterans Affairs (VA) response to the media firestorm and congressional outrage was uncharacteristically swift and sweeping. Both the VA Secretary and Deputy Under Secretary of Health were removed, as were many others in leadership at Phoenix and elsewhere. The VA faced an existential crisis as many loud voices called for dismantling the entire system in the wake of its perceived inability or unwillingness to care for those it was legally mandated to serve.2 The Veterans’ Access to Care through Choice, Accountability, and Transparency Act of 2014 and its successor the VA Mission Act of 2018 dramatically expanded veterans’ access to covered health care from non-VA health care providers (HCPs).
Debate continues in the veteran community and the wider society about whether this expansion constitutes an abandonment of a health care system dedicated to veterans and their unique health problems or a commitment to deliver the most efficient and high-quality care to veterans that can be obtained.3-5 Many see this as a crossroads for the VA. Still, even if the VA will continue to exist, the question remains: in what form?
The increased use of private sector HCPs has wrought significant and long-lasting modifications to the traditional VA organization. In fiscal year (FY) 2017, the VA paid for care that non-VA HCPs provided for 24% of patients.6 Veterans with higher service-connected disability ratings and aged > 65 years were more likely to rely on the VA for care than were less disabled and younger patients.6 The Mission Act is expected to increase the VA expenditures by nearly $19 billion between FY 2019 and FY 2023, with the bulk of the patients still going to the VHA for their care.6 Stakeholders from unions to politicians are concerned that every dollar spent on community care is one less they can spend in VA institutions. It is unclear to what degree this concern will be actualized, as smaller hospitals and those in rural areas have always had contact with the private sector to obtain the specialty care veterans needed that the VA could not provide.
Compounding these trends is the VA’s ongoing staffing challenges. To meet the demand and eliminate wait times between September 2014 and September 2018, the VHA grew its workforce by > 40,000 individuals, a 13% growth rate. In FY 2019 alone, the VHA hired 28,000 new employees. And yet despite the rapid growth, a lower than average turnover rate, and relatively high employee satisfaction measures (at least when compared with those of other federal employees), the VHA still has 43,000 vacancies.7,8
Which brings us to the very different set of challenges facing the Defense Health Agency (DHA). In an era of ballooning military budgets the DHA is being asked to “transform the MHS into an integrated readiness and health system, eliminate redundancies, and create a common high-quality experience for our beneficiaries.”9 The seeds of change were tucked into the National Defense Authorization Act (NDAA) of 2017, and their ramifications are only now becoming apparent. Among the most consequential of these changes are transfer of the management of hundreds of MHS hospitals and clinics from the medical services of the Army, Navy, and Air Force to the DHA.
“If we don’t shape our future, others will step in and do it for us,” Tom McCaffrey, Assistant Secretary of Defense for Health Affairs explained at AMSUS.10 In October 2019, DoD transitioned the first group of facilities to the DHA, and the remainder will change management by the end of 2022. In the next step of the process, facilities will be combined—along with TRICARE providers—in 21 geographically based “markets” to streamline management and avoid “redundancies.”
Lost in the bland language, though, is the scale of the contemplated changes. Although the exact shape of the changes have not been finalized, up to 18,000 MHS health care providers—civilian or uniformed—may be eliminated as DHA relies more heavily on TRICARE providers.11 Not even the future of the Uniformed Service University for the Health Sciences and its leadership training and health care research are guaranteed.12 The ominous possibility that the nation could lose its only military medical school has raised alarm among medical educators. They fear that the country may sacrifice its ability to train physicians with the highly skilled specialities needed on the battlefield and the familiarity with military culture that enables doctors in uniform to relate to the problems of active-duty families and retired service members.12VHA and MHS colleagues are undergoing a similar organizational transition with all the trepidation and expectation that accompanies the turning of an enormous ship in stormy seas. In the midst of these major institutional transformations, VHA and MHS need to band together if the unique specialty of military and VA medicine is to survive. Unless these unprecedented changes can establish a new spirit of solidarity to 2 often separate partners in one mission to care for those who serve, we may well be asking in the next few years, “Where have all the federal practitioners gone?”
1. Stone R. Plenary session. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
2. Lane C. Why don’t we just abolish the VA? Washington Post. April 22, 2015. https://www.washingtonpost.com/opinions/caring-for-veterans-is-our-national-responsibility/2015/04/22/ae61eb88-e929-11e4-aae1-d642717d8afa_story.html. Accessed December 18, 2019.
3. Lemle RB. Choice Program expansion jeopardizes high-quality VHA mental health services. Fed Pract. 2018;35(3):18-24.
4. Shulkin D. Implications for Veterans’ health care: the danger becomes clearer. JAMA Intern Med. 2019;10.1001/jamainternmed.2019.2996. [Published online ahead of print, 2019 Jul 22.]
5. Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a blueprint for helping veterans make the most of new choices. J Gen Intern Med. 2019;10.1007/s11606-019-05404-w. [Published online ahead of print, 2019 Oct 24.]
6. Statement of Merideth Randles, FSA, MAAA Principal and Consulting Actuary, Milliman, Inc. For Presentation Before the Senate Committee on Veterans’ Affairs. VA Mission Act: Implementing the Veterans Community Care Program. https://www.veterans.senate.gov/imo/media/doc/04.10.19%20Milliman%20Testimony.pdf. Submitted April 10, 2019. Accessed December 18, 2019.
7. Sitterly DR. Statement of Daniel R. Sitterly, Assistant Secretary, Office of Human Resources and Administration/Operations Security, and Preparedness, on behalf of U.S. Department of Veterans Affairs Before the House Committee on Veterans Affairs, September 18, 2019. https://docs.house.gov/meetings/VR/VR00/20190918/109925/HHRG-116-VR00-Wstate-SitterlyD-20190918.pdf. Published September 18, 2019. Accessed December 22, 2019.
8. US Office of Personnel Management, FedScope. Federal workforce data. https://www.fedscope.opm.gov. Accessed December 22, 2019.
9. US Department of Defense. Defense Health Program Fiscal Year (FY) 2020 President’s Budget Operation and Maintenance Introductory Statement. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2020/budget_justification/pdfs/09_Defense_Health_Program/Vol_I_Sec_1_PBA-19_Introductory_Statement_DHP_PB20.pdf. Accessed December 23, 2019.
10. McCaffery T. MHS vision. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
11. Sternberg S. Military Health System in the crosshairs. https://www.usnews.com/news/health-news/articles/2019-12-11/military-health-system-in-the-crosshairs. Published December 11, 2019. Accessed December 23, 2019.
12. Novak D. Officials warn Pentagon cuts could force closing of Bethesda military medical university. https://cnsmaryland.org/2019/11/20/officials-warn-pentagon-cuts-could-force-closing-of-bethesda-military-medical-university. Published November 20, 2019. Accessed December 23, 2019.
The certainty that federal health care will be different, and the equal uncertainty about when and how the systems will evolve, were major topics at the recent AMSUS annual meeting. The Veterans Health Administration (VHA) and Military Health System (MHS) are in the midst of major transformations, although they are at very different points in the process and the final outcomes are yet unknown. This editorial, written at the end of 2019, will review some of the highlights of a discussion that is sure to continue in 2020 and beyond.
Almost everyone in the VA and many of the public can pinpoint the exact place (and time) the VHA’s upheaval began: Phoenix, Arizona, in 2014. “The attack on our system,” as VHA Executive in Charge Richard A. Stone, MD, described it at AMSUS, happened because “we were just too slow a bureaucracy,” he explained.1 “We can debate how many veterans died while waiting for care, but the answer is that 1 was too many and it had to be fixed. We had to become a more agile organization.”
The US Department of Veterans Affairs (VA) response to the media firestorm and congressional outrage was uncharacteristically swift and sweeping. Both the VA Secretary and Deputy Under Secretary of Health were removed, as were many others in leadership at Phoenix and elsewhere. The VA faced an existential crisis as many loud voices called for dismantling the entire system in the wake of its perceived inability or unwillingness to care for those it was legally mandated to serve.2 The Veterans’ Access to Care through Choice, Accountability, and Transparency Act of 2014 and its successor the VA Mission Act of 2018 dramatically expanded veterans’ access to covered health care from non-VA health care providers (HCPs).
Debate continues in the veteran community and the wider society about whether this expansion constitutes an abandonment of a health care system dedicated to veterans and their unique health problems or a commitment to deliver the most efficient and high-quality care to veterans that can be obtained.3-5 Many see this as a crossroads for the VA. Still, even if the VA will continue to exist, the question remains: in what form?
The increased use of private sector HCPs has wrought significant and long-lasting modifications to the traditional VA organization. In fiscal year (FY) 2017, the VA paid for care that non-VA HCPs provided for 24% of patients.6 Veterans with higher service-connected disability ratings and aged > 65 years were more likely to rely on the VA for care than were less disabled and younger patients.6 The Mission Act is expected to increase the VA expenditures by nearly $19 billion between FY 2019 and FY 2023, with the bulk of the patients still going to the VHA for their care.6 Stakeholders from unions to politicians are concerned that every dollar spent on community care is one less they can spend in VA institutions. It is unclear to what degree this concern will be actualized, as smaller hospitals and those in rural areas have always had contact with the private sector to obtain the specialty care veterans needed that the VA could not provide.
Compounding these trends is the VA’s ongoing staffing challenges. To meet the demand and eliminate wait times between September 2014 and September 2018, the VHA grew its workforce by > 40,000 individuals, a 13% growth rate. In FY 2019 alone, the VHA hired 28,000 new employees. And yet despite the rapid growth, a lower than average turnover rate, and relatively high employee satisfaction measures (at least when compared with those of other federal employees), the VHA still has 43,000 vacancies.7,8
Which brings us to the very different set of challenges facing the Defense Health Agency (DHA). In an era of ballooning military budgets the DHA is being asked to “transform the MHS into an integrated readiness and health system, eliminate redundancies, and create a common high-quality experience for our beneficiaries.”9 The seeds of change were tucked into the National Defense Authorization Act (NDAA) of 2017, and their ramifications are only now becoming apparent. Among the most consequential of these changes are transfer of the management of hundreds of MHS hospitals and clinics from the medical services of the Army, Navy, and Air Force to the DHA.
“If we don’t shape our future, others will step in and do it for us,” Tom McCaffrey, Assistant Secretary of Defense for Health Affairs explained at AMSUS.10 In October 2019, DoD transitioned the first group of facilities to the DHA, and the remainder will change management by the end of 2022. In the next step of the process, facilities will be combined—along with TRICARE providers—in 21 geographically based “markets” to streamline management and avoid “redundancies.”
Lost in the bland language, though, is the scale of the contemplated changes. Although the exact shape of the changes have not been finalized, up to 18,000 MHS health care providers—civilian or uniformed—may be eliminated as DHA relies more heavily on TRICARE providers.11 Not even the future of the Uniformed Service University for the Health Sciences and its leadership training and health care research are guaranteed.12 The ominous possibility that the nation could lose its only military medical school has raised alarm among medical educators. They fear that the country may sacrifice its ability to train physicians with the highly skilled specialities needed on the battlefield and the familiarity with military culture that enables doctors in uniform to relate to the problems of active-duty families and retired service members.12VHA and MHS colleagues are undergoing a similar organizational transition with all the trepidation and expectation that accompanies the turning of an enormous ship in stormy seas. In the midst of these major institutional transformations, VHA and MHS need to band together if the unique specialty of military and VA medicine is to survive. Unless these unprecedented changes can establish a new spirit of solidarity to 2 often separate partners in one mission to care for those who serve, we may well be asking in the next few years, “Where have all the federal practitioners gone?”
The certainty that federal health care will be different, and the equal uncertainty about when and how the systems will evolve, were major topics at the recent AMSUS annual meeting. The Veterans Health Administration (VHA) and Military Health System (MHS) are in the midst of major transformations, although they are at very different points in the process and the final outcomes are yet unknown. This editorial, written at the end of 2019, will review some of the highlights of a discussion that is sure to continue in 2020 and beyond.
Almost everyone in the VA and many of the public can pinpoint the exact place (and time) the VHA’s upheaval began: Phoenix, Arizona, in 2014. “The attack on our system,” as VHA Executive in Charge Richard A. Stone, MD, described it at AMSUS, happened because “we were just too slow a bureaucracy,” he explained.1 “We can debate how many veterans died while waiting for care, but the answer is that 1 was too many and it had to be fixed. We had to become a more agile organization.”
The US Department of Veterans Affairs (VA) response to the media firestorm and congressional outrage was uncharacteristically swift and sweeping. Both the VA Secretary and Deputy Under Secretary of Health were removed, as were many others in leadership at Phoenix and elsewhere. The VA faced an existential crisis as many loud voices called for dismantling the entire system in the wake of its perceived inability or unwillingness to care for those it was legally mandated to serve.2 The Veterans’ Access to Care through Choice, Accountability, and Transparency Act of 2014 and its successor the VA Mission Act of 2018 dramatically expanded veterans’ access to covered health care from non-VA health care providers (HCPs).
Debate continues in the veteran community and the wider society about whether this expansion constitutes an abandonment of a health care system dedicated to veterans and their unique health problems or a commitment to deliver the most efficient and high-quality care to veterans that can be obtained.3-5 Many see this as a crossroads for the VA. Still, even if the VA will continue to exist, the question remains: in what form?
The increased use of private sector HCPs has wrought significant and long-lasting modifications to the traditional VA organization. In fiscal year (FY) 2017, the VA paid for care that non-VA HCPs provided for 24% of patients.6 Veterans with higher service-connected disability ratings and aged > 65 years were more likely to rely on the VA for care than were less disabled and younger patients.6 The Mission Act is expected to increase the VA expenditures by nearly $19 billion between FY 2019 and FY 2023, with the bulk of the patients still going to the VHA for their care.6 Stakeholders from unions to politicians are concerned that every dollar spent on community care is one less they can spend in VA institutions. It is unclear to what degree this concern will be actualized, as smaller hospitals and those in rural areas have always had contact with the private sector to obtain the specialty care veterans needed that the VA could not provide.
Compounding these trends is the VA’s ongoing staffing challenges. To meet the demand and eliminate wait times between September 2014 and September 2018, the VHA grew its workforce by > 40,000 individuals, a 13% growth rate. In FY 2019 alone, the VHA hired 28,000 new employees. And yet despite the rapid growth, a lower than average turnover rate, and relatively high employee satisfaction measures (at least when compared with those of other federal employees), the VHA still has 43,000 vacancies.7,8
Which brings us to the very different set of challenges facing the Defense Health Agency (DHA). In an era of ballooning military budgets the DHA is being asked to “transform the MHS into an integrated readiness and health system, eliminate redundancies, and create a common high-quality experience for our beneficiaries.”9 The seeds of change were tucked into the National Defense Authorization Act (NDAA) of 2017, and their ramifications are only now becoming apparent. Among the most consequential of these changes are transfer of the management of hundreds of MHS hospitals and clinics from the medical services of the Army, Navy, and Air Force to the DHA.
“If we don’t shape our future, others will step in and do it for us,” Tom McCaffrey, Assistant Secretary of Defense for Health Affairs explained at AMSUS.10 In October 2019, DoD transitioned the first group of facilities to the DHA, and the remainder will change management by the end of 2022. In the next step of the process, facilities will be combined—along with TRICARE providers—in 21 geographically based “markets” to streamline management and avoid “redundancies.”
Lost in the bland language, though, is the scale of the contemplated changes. Although the exact shape of the changes have not been finalized, up to 18,000 MHS health care providers—civilian or uniformed—may be eliminated as DHA relies more heavily on TRICARE providers.11 Not even the future of the Uniformed Service University for the Health Sciences and its leadership training and health care research are guaranteed.12 The ominous possibility that the nation could lose its only military medical school has raised alarm among medical educators. They fear that the country may sacrifice its ability to train physicians with the highly skilled specialities needed on the battlefield and the familiarity with military culture that enables doctors in uniform to relate to the problems of active-duty families and retired service members.12VHA and MHS colleagues are undergoing a similar organizational transition with all the trepidation and expectation that accompanies the turning of an enormous ship in stormy seas. In the midst of these major institutional transformations, VHA and MHS need to band together if the unique specialty of military and VA medicine is to survive. Unless these unprecedented changes can establish a new spirit of solidarity to 2 often separate partners in one mission to care for those who serve, we may well be asking in the next few years, “Where have all the federal practitioners gone?”
1. Stone R. Plenary session. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
2. Lane C. Why don’t we just abolish the VA? Washington Post. April 22, 2015. https://www.washingtonpost.com/opinions/caring-for-veterans-is-our-national-responsibility/2015/04/22/ae61eb88-e929-11e4-aae1-d642717d8afa_story.html. Accessed December 18, 2019.
3. Lemle RB. Choice Program expansion jeopardizes high-quality VHA mental health services. Fed Pract. 2018;35(3):18-24.
4. Shulkin D. Implications for Veterans’ health care: the danger becomes clearer. JAMA Intern Med. 2019;10.1001/jamainternmed.2019.2996. [Published online ahead of print, 2019 Jul 22.]
5. Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a blueprint for helping veterans make the most of new choices. J Gen Intern Med. 2019;10.1007/s11606-019-05404-w. [Published online ahead of print, 2019 Oct 24.]
6. Statement of Merideth Randles, FSA, MAAA Principal and Consulting Actuary, Milliman, Inc. For Presentation Before the Senate Committee on Veterans’ Affairs. VA Mission Act: Implementing the Veterans Community Care Program. https://www.veterans.senate.gov/imo/media/doc/04.10.19%20Milliman%20Testimony.pdf. Submitted April 10, 2019. Accessed December 18, 2019.
7. Sitterly DR. Statement of Daniel R. Sitterly, Assistant Secretary, Office of Human Resources and Administration/Operations Security, and Preparedness, on behalf of U.S. Department of Veterans Affairs Before the House Committee on Veterans Affairs, September 18, 2019. https://docs.house.gov/meetings/VR/VR00/20190918/109925/HHRG-116-VR00-Wstate-SitterlyD-20190918.pdf. Published September 18, 2019. Accessed December 22, 2019.
8. US Office of Personnel Management, FedScope. Federal workforce data. https://www.fedscope.opm.gov. Accessed December 22, 2019.
9. US Department of Defense. Defense Health Program Fiscal Year (FY) 2020 President’s Budget Operation and Maintenance Introductory Statement. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2020/budget_justification/pdfs/09_Defense_Health_Program/Vol_I_Sec_1_PBA-19_Introductory_Statement_DHP_PB20.pdf. Accessed December 23, 2019.
10. McCaffery T. MHS vision. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
11. Sternberg S. Military Health System in the crosshairs. https://www.usnews.com/news/health-news/articles/2019-12-11/military-health-system-in-the-crosshairs. Published December 11, 2019. Accessed December 23, 2019.
12. Novak D. Officials warn Pentagon cuts could force closing of Bethesda military medical university. https://cnsmaryland.org/2019/11/20/officials-warn-pentagon-cuts-could-force-closing-of-bethesda-military-medical-university. Published November 20, 2019. Accessed December 23, 2019.
1. Stone R. Plenary session. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
2. Lane C. Why don’t we just abolish the VA? Washington Post. April 22, 2015. https://www.washingtonpost.com/opinions/caring-for-veterans-is-our-national-responsibility/2015/04/22/ae61eb88-e929-11e4-aae1-d642717d8afa_story.html. Accessed December 18, 2019.
3. Lemle RB. Choice Program expansion jeopardizes high-quality VHA mental health services. Fed Pract. 2018;35(3):18-24.
4. Shulkin D. Implications for Veterans’ health care: the danger becomes clearer. JAMA Intern Med. 2019;10.1001/jamainternmed.2019.2996. [Published online ahead of print, 2019 Jul 22.]
5. Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a blueprint for helping veterans make the most of new choices. J Gen Intern Med. 2019;10.1007/s11606-019-05404-w. [Published online ahead of print, 2019 Oct 24.]
6. Statement of Merideth Randles, FSA, MAAA Principal and Consulting Actuary, Milliman, Inc. For Presentation Before the Senate Committee on Veterans’ Affairs. VA Mission Act: Implementing the Veterans Community Care Program. https://www.veterans.senate.gov/imo/media/doc/04.10.19%20Milliman%20Testimony.pdf. Submitted April 10, 2019. Accessed December 18, 2019.
7. Sitterly DR. Statement of Daniel R. Sitterly, Assistant Secretary, Office of Human Resources and Administration/Operations Security, and Preparedness, on behalf of U.S. Department of Veterans Affairs Before the House Committee on Veterans Affairs, September 18, 2019. https://docs.house.gov/meetings/VR/VR00/20190918/109925/HHRG-116-VR00-Wstate-SitterlyD-20190918.pdf. Published September 18, 2019. Accessed December 22, 2019.
8. US Office of Personnel Management, FedScope. Federal workforce data. https://www.fedscope.opm.gov. Accessed December 22, 2019.
9. US Department of Defense. Defense Health Program Fiscal Year (FY) 2020 President’s Budget Operation and Maintenance Introductory Statement. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2020/budget_justification/pdfs/09_Defense_Health_Program/Vol_I_Sec_1_PBA-19_Introductory_Statement_DHP_PB20.pdf. Accessed December 23, 2019.
10. McCaffery T. MHS vision. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
11. Sternberg S. Military Health System in the crosshairs. https://www.usnews.com/news/health-news/articles/2019-12-11/military-health-system-in-the-crosshairs. Published December 11, 2019. Accessed December 23, 2019.
12. Novak D. Officials warn Pentagon cuts could force closing of Bethesda military medical university. https://cnsmaryland.org/2019/11/20/officials-warn-pentagon-cuts-could-force-closing-of-bethesda-military-medical-university. Published November 20, 2019. Accessed December 23, 2019.