User login
Defending the Home Planet
Like me, some of you may have been following the agonizing news about the unprecedented brushfires in Australia that have devastated human, animal, and vegetative life in that country so culturally akin to our own.1 For many people who believe the overwhelming majority of scientific reports on climate change, these apocalyptic fires are an empirical demonstration of the truth of the dire prophecies for the future of our planet. Scientists have demonstrated that although climate change may not have caused the worst fires in Australia’s history, they may have contributed to the conditions that enabled them to spread so far and wide and reach such a destructive intensity.2The heartbreaking pictures of singed koalas and displaced people and the helpless feeling that all I can do from here is donate money set me to thinking about the relationship between the military, health, and climate change, which is the subject of this column.
As I write this in mid-January of a new decade and glance at the weather headlines, I read about an earthquake in Puerto Rico and tornadoes in the southern US. This makes it quite plausible that our comfortable lifestyle and technological civilization could in the coming decades go the way of the dinosaurs, also victims of climate change.
Initially, my first thought about this relationship is a negative one—images of scorched earth policies that stretch back to ancient wars jump to mind. Reflection and research on the topic though suggest that the relationship may be more complicated and conflicted. Alas, I can only touch on a few of the themes in this brief format.
It may not be as obvious that climate change also threatens the military, which is the guardian of that civilization. In 2018, for example, Hurricane Michael caused nearly $5 billion in damages to Tyndall Air Force Base in Florida.3 A year later, the US Department of Defense (DoD) released a report on the effects of climate change as mandated by Congress.4 Even though some congressional critics expressed concern about the report’s lack of depth and detail,5 the report asserted that, “The effects of a changing climate are a national security issue with potential impacts to Department of Defense (DoD or the Department) missions, operational plans, and installations.”4
The US Department of Veterans Affairs (VA) is not immune either. Natural disasters have already disrupted the delivery of health care at its many aging facilities. Climate change was called the “engine”6 driving Hurricane Maria, which in 2017 slammed into Puerto Rico, including its VA medical center, and resulted in shortages of supplies, staff, and basic utilities.7 The facility and the island are still trying to rebuild. In response to weather-exposed vulnerability in VA infrastructure, Senator and presidential candidate Elizabeth Warren (D-MA) and Senator Brian Schatz (D-HI), the ranking member of the Subcommittee on Military Construction, sent a letter to VA leadership arguing that “Strengthening VA’s resilience to climate change is consistent with the agency’s mission to deliver timely, high-quality care and benefits to America’s veterans.”8
It has been reported that the current administration has countered initiatives to prepare for the challenges of providing health care to service members and veterans in a climate changed world.9 Sadly, but predictably, in the politicized federal health care arena, the safety of our service members and, in turn, the domestic and national security and peace that depend on them are caught in the partisan debate over global warming, though it is not likely Congress or federal agency leaders will abandon planning to safeguard service members who will see duty and combat in a radically altered ecology and veterans and who will need to have VA continue to be the reliable safety net despite an increasingly erratic environment.10
Climate change is a divisive political issue; there is a proud tradition of conservatism and self-reliance in military members, active duty and veteran alike. That was why I was surprised and impressed when I saw the results of a recent survey on climate change. In January 2019, 293 active-duty service members and veterans were surveyed.
Participants were selected to reflect the ethnic makeup, educational level, and political allegiance of the military population, which enhanced the validity of the findings.11Participants were asked to indicate whether they believed that the earth was warming secondary to human or natural processes; not growing warmer at all; or whether they were unsure. Similar to the general population, 46% agreed that climate change is anthropogenic.11 More than three-fourths believed it was likely climate change would adversely affect the places they worked, like military installations; 61% thought it likely that global warming could lead to armed conflict over resources. Seven in 10 respondents believed that climate is changing vs 46% who did not. Of respondents who believe climate change is real, 87% see it as a threat to military bases compared with 60% who do not accept the science that the earth is warming.11
This survey, though, is only a small study, and the military and VA are big tents under which a wide range of political persuasions and diverse beliefs co-exist. There are many readers of Federal Practitioner who will no doubt reject nearly every word I have written, in what I know is a controversial column. But it matters that the military and veteran constituency are thinking and speaking about the issue of climate change.11 Why? The answer takes us back to the disaster in Australia. When the fires and the devastation they wrought escalated beyond the powers of the civil authorities to handle, it was the military whose technical skill, coordinated readiness, and personal courage and dedication that was called on to rescue thousands of civilians from the inferno.12 So it will be in our country and around the world when disasters—manmade, natural, or both—threaten to engulf life in all its wondrous variety. Those who battle extreme weather will have unique health needs, and their valiant sacrifices deserve to have health care systems ready and able to treat them.
1. Thompson A. Australia’s bushfires have likely devastated wildlife–and the impact will only get worse. Scientific American. https://www.scientificamerican.com/article/australias-bushfires-have-likely-devastated-wildlife-and-the-impact-will-only-get-worse. Published January 8, 2020. Accessed January 16, 2020.
2. Gibbens S. Intense ‘firestorms’ forming from Australia’s deadly wildfires. https://www.nationalgeographic.com/science/2020/01/australian-wildfires-cause-firestorms. Published January 9, 2020. Accessed January 15, 2020.
3. Shapiro A. Tyndall Air Force Base still faces challenges in recovering from Hurricane Michael. https://www.npr.org/2019/05/31/728754872/tyndall-air-force-base-still-faces-challenges-in-recovering-from-hurricane-micha. Published May 31, 2019. Accessed January 16, 2020.
4. US Department of Defense, Office of the Undersecretary for Acquisition and Sustainment. Report on effects of a changing climate to the Department of Defense. https://www.documentcloud.org/documents/5689153-DoD-Final-Climate-Report.html. Published January 2019. Accessed January 16, 2020.
5. Maucione S. DoD justifies climate change report, says response was mission-centric. https://federalnewsnetwork.com/defense-main/2019/03/dod-justifies-climate-change-report-says-response-was-mission-centric. Published March 28, 2019. Accessed January 16, 2020.
6. Shane L 3rd. Puerto Rico’s VA hospital weathers Maria, but challenges loom. https://www.armytimes.com/veterans/2017/09/22/puerto-ricos-va-hospital-weathers-hurricane-maria-but-challenges-loom. Published September 22, 2017. Accessed January 16, 2020.
7. Hersher R. Climate change was the engine that powered Hurricane Maria’s devastating rains. https://www.npr.org/2019/04/17/714098828/climate-change-was-the-engine-that-powered-hurricane-marias-devastating-rains. Published April 17, 2019. Accessed January 16, 2020.
8. Senators Warren and Schatz request an update from the Department of Veterans Affairs on efforts to build resilience to climate change [press release]. https://www.warren.senate.gov/oversight/letters/senators-warren-and-schatz-request-an-update-from-the-department-of-veterans-affairs-on-efforts-to-build-resilience-to-climate-change. Published October 1, 2019. Accessed January 16, 2020.
9. Simkins JD. Navy quietly ends climate change task force, reversing Obama initiative. https://www.navytimes.com/off-duty/military-culture/2019/08/26/navy-quietly-ends-climate-change-task-force-reversing-obama-initiative. Published August 26, 2019. Accessed January 16, 2020.
10. Eilperin J, Dennis B, Ryan M. As White House questions climate change, U.S. military is planning for it. https://www.washingtonpost.com/national/health-science/as-white-house-questions-climate-change-us-military-is-planning-for-it/2019/04/08/78142546-57c0-11e9-814f-e2f46684196e_story.html. Published April 8, 2019. Accessed January 16, 2020.
11. Motta M, Spindel J, Ralston R. Veterans are concerned about climate change and that matters. http://theconversation.com/veterans-are-concerned-about-climate-change-and-that-matters-110685. Published March 8, 2019. Accessed January 16, 2020.
12. Albeck-Ripka L, Kwai I, Fuller T, Tarabay J. ‘It’s an atomic bomb’: Australia deploys military as fires spread. https://www.nytimes.com/2020/01/04/world/australia/fires-military.html. Updated January 5, 2020. Accessed January 18, 2020.
Like me, some of you may have been following the agonizing news about the unprecedented brushfires in Australia that have devastated human, animal, and vegetative life in that country so culturally akin to our own.1 For many people who believe the overwhelming majority of scientific reports on climate change, these apocalyptic fires are an empirical demonstration of the truth of the dire prophecies for the future of our planet. Scientists have demonstrated that although climate change may not have caused the worst fires in Australia’s history, they may have contributed to the conditions that enabled them to spread so far and wide and reach such a destructive intensity.2The heartbreaking pictures of singed koalas and displaced people and the helpless feeling that all I can do from here is donate money set me to thinking about the relationship between the military, health, and climate change, which is the subject of this column.
As I write this in mid-January of a new decade and glance at the weather headlines, I read about an earthquake in Puerto Rico and tornadoes in the southern US. This makes it quite plausible that our comfortable lifestyle and technological civilization could in the coming decades go the way of the dinosaurs, also victims of climate change.
Initially, my first thought about this relationship is a negative one—images of scorched earth policies that stretch back to ancient wars jump to mind. Reflection and research on the topic though suggest that the relationship may be more complicated and conflicted. Alas, I can only touch on a few of the themes in this brief format.
It may not be as obvious that climate change also threatens the military, which is the guardian of that civilization. In 2018, for example, Hurricane Michael caused nearly $5 billion in damages to Tyndall Air Force Base in Florida.3 A year later, the US Department of Defense (DoD) released a report on the effects of climate change as mandated by Congress.4 Even though some congressional critics expressed concern about the report’s lack of depth and detail,5 the report asserted that, “The effects of a changing climate are a national security issue with potential impacts to Department of Defense (DoD or the Department) missions, operational plans, and installations.”4
The US Department of Veterans Affairs (VA) is not immune either. Natural disasters have already disrupted the delivery of health care at its many aging facilities. Climate change was called the “engine”6 driving Hurricane Maria, which in 2017 slammed into Puerto Rico, including its VA medical center, and resulted in shortages of supplies, staff, and basic utilities.7 The facility and the island are still trying to rebuild. In response to weather-exposed vulnerability in VA infrastructure, Senator and presidential candidate Elizabeth Warren (D-MA) and Senator Brian Schatz (D-HI), the ranking member of the Subcommittee on Military Construction, sent a letter to VA leadership arguing that “Strengthening VA’s resilience to climate change is consistent with the agency’s mission to deliver timely, high-quality care and benefits to America’s veterans.”8
It has been reported that the current administration has countered initiatives to prepare for the challenges of providing health care to service members and veterans in a climate changed world.9 Sadly, but predictably, in the politicized federal health care arena, the safety of our service members and, in turn, the domestic and national security and peace that depend on them are caught in the partisan debate over global warming, though it is not likely Congress or federal agency leaders will abandon planning to safeguard service members who will see duty and combat in a radically altered ecology and veterans and who will need to have VA continue to be the reliable safety net despite an increasingly erratic environment.10
Climate change is a divisive political issue; there is a proud tradition of conservatism and self-reliance in military members, active duty and veteran alike. That was why I was surprised and impressed when I saw the results of a recent survey on climate change. In January 2019, 293 active-duty service members and veterans were surveyed.
Participants were selected to reflect the ethnic makeup, educational level, and political allegiance of the military population, which enhanced the validity of the findings.11Participants were asked to indicate whether they believed that the earth was warming secondary to human or natural processes; not growing warmer at all; or whether they were unsure. Similar to the general population, 46% agreed that climate change is anthropogenic.11 More than three-fourths believed it was likely climate change would adversely affect the places they worked, like military installations; 61% thought it likely that global warming could lead to armed conflict over resources. Seven in 10 respondents believed that climate is changing vs 46% who did not. Of respondents who believe climate change is real, 87% see it as a threat to military bases compared with 60% who do not accept the science that the earth is warming.11
This survey, though, is only a small study, and the military and VA are big tents under which a wide range of political persuasions and diverse beliefs co-exist. There are many readers of Federal Practitioner who will no doubt reject nearly every word I have written, in what I know is a controversial column. But it matters that the military and veteran constituency are thinking and speaking about the issue of climate change.11 Why? The answer takes us back to the disaster in Australia. When the fires and the devastation they wrought escalated beyond the powers of the civil authorities to handle, it was the military whose technical skill, coordinated readiness, and personal courage and dedication that was called on to rescue thousands of civilians from the inferno.12 So it will be in our country and around the world when disasters—manmade, natural, or both—threaten to engulf life in all its wondrous variety. Those who battle extreme weather will have unique health needs, and their valiant sacrifices deserve to have health care systems ready and able to treat them.
Like me, some of you may have been following the agonizing news about the unprecedented brushfires in Australia that have devastated human, animal, and vegetative life in that country so culturally akin to our own.1 For many people who believe the overwhelming majority of scientific reports on climate change, these apocalyptic fires are an empirical demonstration of the truth of the dire prophecies for the future of our planet. Scientists have demonstrated that although climate change may not have caused the worst fires in Australia’s history, they may have contributed to the conditions that enabled them to spread so far and wide and reach such a destructive intensity.2The heartbreaking pictures of singed koalas and displaced people and the helpless feeling that all I can do from here is donate money set me to thinking about the relationship between the military, health, and climate change, which is the subject of this column.
As I write this in mid-January of a new decade and glance at the weather headlines, I read about an earthquake in Puerto Rico and tornadoes in the southern US. This makes it quite plausible that our comfortable lifestyle and technological civilization could in the coming decades go the way of the dinosaurs, also victims of climate change.
Initially, my first thought about this relationship is a negative one—images of scorched earth policies that stretch back to ancient wars jump to mind. Reflection and research on the topic though suggest that the relationship may be more complicated and conflicted. Alas, I can only touch on a few of the themes in this brief format.
It may not be as obvious that climate change also threatens the military, which is the guardian of that civilization. In 2018, for example, Hurricane Michael caused nearly $5 billion in damages to Tyndall Air Force Base in Florida.3 A year later, the US Department of Defense (DoD) released a report on the effects of climate change as mandated by Congress.4 Even though some congressional critics expressed concern about the report’s lack of depth and detail,5 the report asserted that, “The effects of a changing climate are a national security issue with potential impacts to Department of Defense (DoD or the Department) missions, operational plans, and installations.”4
The US Department of Veterans Affairs (VA) is not immune either. Natural disasters have already disrupted the delivery of health care at its many aging facilities. Climate change was called the “engine”6 driving Hurricane Maria, which in 2017 slammed into Puerto Rico, including its VA medical center, and resulted in shortages of supplies, staff, and basic utilities.7 The facility and the island are still trying to rebuild. In response to weather-exposed vulnerability in VA infrastructure, Senator and presidential candidate Elizabeth Warren (D-MA) and Senator Brian Schatz (D-HI), the ranking member of the Subcommittee on Military Construction, sent a letter to VA leadership arguing that “Strengthening VA’s resilience to climate change is consistent with the agency’s mission to deliver timely, high-quality care and benefits to America’s veterans.”8
It has been reported that the current administration has countered initiatives to prepare for the challenges of providing health care to service members and veterans in a climate changed world.9 Sadly, but predictably, in the politicized federal health care arena, the safety of our service members and, in turn, the domestic and national security and peace that depend on them are caught in the partisan debate over global warming, though it is not likely Congress or federal agency leaders will abandon planning to safeguard service members who will see duty and combat in a radically altered ecology and veterans and who will need to have VA continue to be the reliable safety net despite an increasingly erratic environment.10
Climate change is a divisive political issue; there is a proud tradition of conservatism and self-reliance in military members, active duty and veteran alike. That was why I was surprised and impressed when I saw the results of a recent survey on climate change. In January 2019, 293 active-duty service members and veterans were surveyed.
Participants were selected to reflect the ethnic makeup, educational level, and political allegiance of the military population, which enhanced the validity of the findings.11Participants were asked to indicate whether they believed that the earth was warming secondary to human or natural processes; not growing warmer at all; or whether they were unsure. Similar to the general population, 46% agreed that climate change is anthropogenic.11 More than three-fourths believed it was likely climate change would adversely affect the places they worked, like military installations; 61% thought it likely that global warming could lead to armed conflict over resources. Seven in 10 respondents believed that climate is changing vs 46% who did not. Of respondents who believe climate change is real, 87% see it as a threat to military bases compared with 60% who do not accept the science that the earth is warming.11
This survey, though, is only a small study, and the military and VA are big tents under which a wide range of political persuasions and diverse beliefs co-exist. There are many readers of Federal Practitioner who will no doubt reject nearly every word I have written, in what I know is a controversial column. But it matters that the military and veteran constituency are thinking and speaking about the issue of climate change.11 Why? The answer takes us back to the disaster in Australia. When the fires and the devastation they wrought escalated beyond the powers of the civil authorities to handle, it was the military whose technical skill, coordinated readiness, and personal courage and dedication that was called on to rescue thousands of civilians from the inferno.12 So it will be in our country and around the world when disasters—manmade, natural, or both—threaten to engulf life in all its wondrous variety. Those who battle extreme weather will have unique health needs, and their valiant sacrifices deserve to have health care systems ready and able to treat them.
1. Thompson A. Australia’s bushfires have likely devastated wildlife–and the impact will only get worse. Scientific American. https://www.scientificamerican.com/article/australias-bushfires-have-likely-devastated-wildlife-and-the-impact-will-only-get-worse. Published January 8, 2020. Accessed January 16, 2020.
2. Gibbens S. Intense ‘firestorms’ forming from Australia’s deadly wildfires. https://www.nationalgeographic.com/science/2020/01/australian-wildfires-cause-firestorms. Published January 9, 2020. Accessed January 15, 2020.
3. Shapiro A. Tyndall Air Force Base still faces challenges in recovering from Hurricane Michael. https://www.npr.org/2019/05/31/728754872/tyndall-air-force-base-still-faces-challenges-in-recovering-from-hurricane-micha. Published May 31, 2019. Accessed January 16, 2020.
4. US Department of Defense, Office of the Undersecretary for Acquisition and Sustainment. Report on effects of a changing climate to the Department of Defense. https://www.documentcloud.org/documents/5689153-DoD-Final-Climate-Report.html. Published January 2019. Accessed January 16, 2020.
5. Maucione S. DoD justifies climate change report, says response was mission-centric. https://federalnewsnetwork.com/defense-main/2019/03/dod-justifies-climate-change-report-says-response-was-mission-centric. Published March 28, 2019. Accessed January 16, 2020.
6. Shane L 3rd. Puerto Rico’s VA hospital weathers Maria, but challenges loom. https://www.armytimes.com/veterans/2017/09/22/puerto-ricos-va-hospital-weathers-hurricane-maria-but-challenges-loom. Published September 22, 2017. Accessed January 16, 2020.
7. Hersher R. Climate change was the engine that powered Hurricane Maria’s devastating rains. https://www.npr.org/2019/04/17/714098828/climate-change-was-the-engine-that-powered-hurricane-marias-devastating-rains. Published April 17, 2019. Accessed January 16, 2020.
8. Senators Warren and Schatz request an update from the Department of Veterans Affairs on efforts to build resilience to climate change [press release]. https://www.warren.senate.gov/oversight/letters/senators-warren-and-schatz-request-an-update-from-the-department-of-veterans-affairs-on-efforts-to-build-resilience-to-climate-change. Published October 1, 2019. Accessed January 16, 2020.
9. Simkins JD. Navy quietly ends climate change task force, reversing Obama initiative. https://www.navytimes.com/off-duty/military-culture/2019/08/26/navy-quietly-ends-climate-change-task-force-reversing-obama-initiative. Published August 26, 2019. Accessed January 16, 2020.
10. Eilperin J, Dennis B, Ryan M. As White House questions climate change, U.S. military is planning for it. https://www.washingtonpost.com/national/health-science/as-white-house-questions-climate-change-us-military-is-planning-for-it/2019/04/08/78142546-57c0-11e9-814f-e2f46684196e_story.html. Published April 8, 2019. Accessed January 16, 2020.
11. Motta M, Spindel J, Ralston R. Veterans are concerned about climate change and that matters. http://theconversation.com/veterans-are-concerned-about-climate-change-and-that-matters-110685. Published March 8, 2019. Accessed January 16, 2020.
12. Albeck-Ripka L, Kwai I, Fuller T, Tarabay J. ‘It’s an atomic bomb’: Australia deploys military as fires spread. https://www.nytimes.com/2020/01/04/world/australia/fires-military.html. Updated January 5, 2020. Accessed January 18, 2020.
1. Thompson A. Australia’s bushfires have likely devastated wildlife–and the impact will only get worse. Scientific American. https://www.scientificamerican.com/article/australias-bushfires-have-likely-devastated-wildlife-and-the-impact-will-only-get-worse. Published January 8, 2020. Accessed January 16, 2020.
2. Gibbens S. Intense ‘firestorms’ forming from Australia’s deadly wildfires. https://www.nationalgeographic.com/science/2020/01/australian-wildfires-cause-firestorms. Published January 9, 2020. Accessed January 15, 2020.
3. Shapiro A. Tyndall Air Force Base still faces challenges in recovering from Hurricane Michael. https://www.npr.org/2019/05/31/728754872/tyndall-air-force-base-still-faces-challenges-in-recovering-from-hurricane-micha. Published May 31, 2019. Accessed January 16, 2020.
4. US Department of Defense, Office of the Undersecretary for Acquisition and Sustainment. Report on effects of a changing climate to the Department of Defense. https://www.documentcloud.org/documents/5689153-DoD-Final-Climate-Report.html. Published January 2019. Accessed January 16, 2020.
5. Maucione S. DoD justifies climate change report, says response was mission-centric. https://federalnewsnetwork.com/defense-main/2019/03/dod-justifies-climate-change-report-says-response-was-mission-centric. Published March 28, 2019. Accessed January 16, 2020.
6. Shane L 3rd. Puerto Rico’s VA hospital weathers Maria, but challenges loom. https://www.armytimes.com/veterans/2017/09/22/puerto-ricos-va-hospital-weathers-hurricane-maria-but-challenges-loom. Published September 22, 2017. Accessed January 16, 2020.
7. Hersher R. Climate change was the engine that powered Hurricane Maria’s devastating rains. https://www.npr.org/2019/04/17/714098828/climate-change-was-the-engine-that-powered-hurricane-marias-devastating-rains. Published April 17, 2019. Accessed January 16, 2020.
8. Senators Warren and Schatz request an update from the Department of Veterans Affairs on efforts to build resilience to climate change [press release]. https://www.warren.senate.gov/oversight/letters/senators-warren-and-schatz-request-an-update-from-the-department-of-veterans-affairs-on-efforts-to-build-resilience-to-climate-change. Published October 1, 2019. Accessed January 16, 2020.
9. Simkins JD. Navy quietly ends climate change task force, reversing Obama initiative. https://www.navytimes.com/off-duty/military-culture/2019/08/26/navy-quietly-ends-climate-change-task-force-reversing-obama-initiative. Published August 26, 2019. Accessed January 16, 2020.
10. Eilperin J, Dennis B, Ryan M. As White House questions climate change, U.S. military is planning for it. https://www.washingtonpost.com/national/health-science/as-white-house-questions-climate-change-us-military-is-planning-for-it/2019/04/08/78142546-57c0-11e9-814f-e2f46684196e_story.html. Published April 8, 2019. Accessed January 16, 2020.
11. Motta M, Spindel J, Ralston R. Veterans are concerned about climate change and that matters. http://theconversation.com/veterans-are-concerned-about-climate-change-and-that-matters-110685. Published March 8, 2019. Accessed January 16, 2020.
12. Albeck-Ripka L, Kwai I, Fuller T, Tarabay J. ‘It’s an atomic bomb’: Australia deploys military as fires spread. https://www.nytimes.com/2020/01/04/world/australia/fires-military.html. Updated January 5, 2020. Accessed January 18, 2020.
Understanding postpartum psychosis: From course to treatment
Although the last decade has brought appropriate increased interest in the diagnosis and treatment of postpartum depression, with screening initiatives across more than 40 states in place and even new medications being brought to market for treatment, far less attention has been given to diagnosis and treatment of a particularly serious psychiatric illness: postpartum psychosis.
Clinically, women can experience rapid mood changes, most often with the presentation that is consistent with a manic-like psychosis, with associated symptoms of delusional thinking, hallucinations, paranoia and either depression or elation, or an amalgam of these so-called “mixed symptoms.” Onset of symptoms typically is early, within 72 hours as is classically described, but may have a somewhat later time of onset in some women.
Many investigators have studied risk factors for postpartum psychosis, and it has been well established that a history of mood disorder, particularly bipolar disorder, is one of the strongest predictors of risk for postpartum psychosis. Women with histories of postpartum psychosis are at very high risk of recurrence, with as many as 70%-90% of women experiencing recurrence if not prophylaxed with an appropriate agent. From a clinical point of view, women with postpartum psychosis typically are hospitalized, given that this is both a psychiatric and potential obstetrical emergency. In fact, the data would suggest that although postpartum suicide and infanticide are not common, they can be a tragic concomitant of postpartum psychosis (Am J Psychiatry. 2016 Dec 1;173[12]:1179-88).
A great amount of interest has been placed on the etiology of postpartum psychosis, as it’s a dramatic presentation with very rapid onset in the acute postpartum period. A rich evidence base with respect to an algorithm of treatment that maximizes likelihood of full recovery or sustaining of euthymia after recovery is limited. Few studies have looked systematically at the optimum way to treat postpartum psychosis. Clinical wisdom has dictated that, given the dramatic symptoms with which these patients present, most patients are treated with lithium and an antipsychotic medication as if they have a manic-like psychosis. It may take brief or extended periods of time for patients to stabilize. Once they are stabilized, one of the most challenging questions for clinicians is how long to treat. Again, an evidence base clearly informing this question is lacking.
Over the years, many clinicians have treated patients with postpartum psychosis as if they have bipolar disorder, given the index presentation of the illness, so some of these patients are treated with antimanic drugs indefinitely. However, clinical experience from several centers that treat women with postpartum psychosis suggests that in remitted patients, a proportion of them may be able to taper and discontinue treatment, then sustain well-being for protracted periods.
One obstacle with respect to treatment of postpartum psychosis derives from the short length of stay after delivery for many women. Some women who present with symptoms of postpartum psychosis in the first 24-48 hours frequently are managed with direct admission to an inpatient psychiatric service. But others may not develop symptoms until they are home, which may place both mother and newborn at risk.
Given that the risk for recurrent postpartum psychosis is so great (70%-90%), women with histories of postpartum psychosis invariably are prophylaxed with mood stabilizer prior to delivery in a subsequent pregnancy. In our own center, we have published on the value of such prophylactic intervention, not just in women with postpartum psychosis, but in women with bipolar disorder, who are, as noted, at great risk for developing postpartum psychotic symptoms (Am J Psychiatry. 1995 Nov;152[11]:1641-5.)
Although postpartum psychosis may be rare, over the last 3 decades we have seen a substantial number of women with postpartum psychosis and have been fascinated with the spectrum of symptoms with which some women with postpartum psychotic illness present. We also have been impressed with the time required for some women to recompensate from their illness and the course of their disorder after they have seemingly remitted. Some women appear to be able to discontinue treatment as noted above; others, particularly if there is any history of bipolar disorder, need to be maintained on treatment with mood stabilizer indefinitely.
To better understand the phenomenology of postpartum psychosis, as well as the longitudinal course of the illness, in 2019, the Mass General Hospital Postpartum Psychosis Project (MGHP3) was established. The project is conducted as a hospital-based registry where women with histories of postpartum psychosis over the last decade are invited to participate in an in-depth interview to understand both symptoms and course of underlying illness. This is complemented by obtaining a sample of saliva, which is used for genetic testing to try to identify a genetic underpinning associated with postpartum psychosis, as the question of genetic etiology of postpartum psychosis is still an open one.
As part of the MGHP3 project, clinicians across the country are able to contact perinatal psychiatrists in our center with expertise in the treatment of postpartum psychosis. Our psychiatrists also can counsel clinicians on issues regarding long-term management of postpartum psychosis because for many, knowledge of precisely how to manage this disorder or the follow-up treatment may be incomplete.
From a clinical point of view, the relevant questions really include not only acute treatment, which has already been outlined, but also the issue of duration of treatment. While some patients may be able to taper and discontinue treatment after, for example, a year of being totally well, to date we are unable to know who those patients are. We tend to be more conservative in our own center and treat patients with puerperal psychosis for a more protracted period of time, usually over several years. We also ask women about their family history of bipolar disorder or postpartum psychosis. Depending on the clinical course (if the patient really has sustained euthymia), we consider slow taper and ultimate discontinuation. As always, treatment decisions are tailored to individual clinical history, course, and patient wishes.
Postpartum psychosis remains one of the most serious illnesses that we find in reproductive psychiatry, and incomplete attention has been given to this devastating illness, which we read about periodically in newspapers and magazines. Greater understanding of postpartum psychosis will lead to a more precision-like psychiatric approach, tailoring treatment to the invariable heterogeneity of this illness.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Although the last decade has brought appropriate increased interest in the diagnosis and treatment of postpartum depression, with screening initiatives across more than 40 states in place and even new medications being brought to market for treatment, far less attention has been given to diagnosis and treatment of a particularly serious psychiatric illness: postpartum psychosis.
Clinically, women can experience rapid mood changes, most often with the presentation that is consistent with a manic-like psychosis, with associated symptoms of delusional thinking, hallucinations, paranoia and either depression or elation, or an amalgam of these so-called “mixed symptoms.” Onset of symptoms typically is early, within 72 hours as is classically described, but may have a somewhat later time of onset in some women.
Many investigators have studied risk factors for postpartum psychosis, and it has been well established that a history of mood disorder, particularly bipolar disorder, is one of the strongest predictors of risk for postpartum psychosis. Women with histories of postpartum psychosis are at very high risk of recurrence, with as many as 70%-90% of women experiencing recurrence if not prophylaxed with an appropriate agent. From a clinical point of view, women with postpartum psychosis typically are hospitalized, given that this is both a psychiatric and potential obstetrical emergency. In fact, the data would suggest that although postpartum suicide and infanticide are not common, they can be a tragic concomitant of postpartum psychosis (Am J Psychiatry. 2016 Dec 1;173[12]:1179-88).
A great amount of interest has been placed on the etiology of postpartum psychosis, as it’s a dramatic presentation with very rapid onset in the acute postpartum period. A rich evidence base with respect to an algorithm of treatment that maximizes likelihood of full recovery or sustaining of euthymia after recovery is limited. Few studies have looked systematically at the optimum way to treat postpartum psychosis. Clinical wisdom has dictated that, given the dramatic symptoms with which these patients present, most patients are treated with lithium and an antipsychotic medication as if they have a manic-like psychosis. It may take brief or extended periods of time for patients to stabilize. Once they are stabilized, one of the most challenging questions for clinicians is how long to treat. Again, an evidence base clearly informing this question is lacking.
Over the years, many clinicians have treated patients with postpartum psychosis as if they have bipolar disorder, given the index presentation of the illness, so some of these patients are treated with antimanic drugs indefinitely. However, clinical experience from several centers that treat women with postpartum psychosis suggests that in remitted patients, a proportion of them may be able to taper and discontinue treatment, then sustain well-being for protracted periods.
One obstacle with respect to treatment of postpartum psychosis derives from the short length of stay after delivery for many women. Some women who present with symptoms of postpartum psychosis in the first 24-48 hours frequently are managed with direct admission to an inpatient psychiatric service. But others may not develop symptoms until they are home, which may place both mother and newborn at risk.
Given that the risk for recurrent postpartum psychosis is so great (70%-90%), women with histories of postpartum psychosis invariably are prophylaxed with mood stabilizer prior to delivery in a subsequent pregnancy. In our own center, we have published on the value of such prophylactic intervention, not just in women with postpartum psychosis, but in women with bipolar disorder, who are, as noted, at great risk for developing postpartum psychotic symptoms (Am J Psychiatry. 1995 Nov;152[11]:1641-5.)
Although postpartum psychosis may be rare, over the last 3 decades we have seen a substantial number of women with postpartum psychosis and have been fascinated with the spectrum of symptoms with which some women with postpartum psychotic illness present. We also have been impressed with the time required for some women to recompensate from their illness and the course of their disorder after they have seemingly remitted. Some women appear to be able to discontinue treatment as noted above; others, particularly if there is any history of bipolar disorder, need to be maintained on treatment with mood stabilizer indefinitely.
To better understand the phenomenology of postpartum psychosis, as well as the longitudinal course of the illness, in 2019, the Mass General Hospital Postpartum Psychosis Project (MGHP3) was established. The project is conducted as a hospital-based registry where women with histories of postpartum psychosis over the last decade are invited to participate in an in-depth interview to understand both symptoms and course of underlying illness. This is complemented by obtaining a sample of saliva, which is used for genetic testing to try to identify a genetic underpinning associated with postpartum psychosis, as the question of genetic etiology of postpartum psychosis is still an open one.
As part of the MGHP3 project, clinicians across the country are able to contact perinatal psychiatrists in our center with expertise in the treatment of postpartum psychosis. Our psychiatrists also can counsel clinicians on issues regarding long-term management of postpartum psychosis because for many, knowledge of precisely how to manage this disorder or the follow-up treatment may be incomplete.
From a clinical point of view, the relevant questions really include not only acute treatment, which has already been outlined, but also the issue of duration of treatment. While some patients may be able to taper and discontinue treatment after, for example, a year of being totally well, to date we are unable to know who those patients are. We tend to be more conservative in our own center and treat patients with puerperal psychosis for a more protracted period of time, usually over several years. We also ask women about their family history of bipolar disorder or postpartum psychosis. Depending on the clinical course (if the patient really has sustained euthymia), we consider slow taper and ultimate discontinuation. As always, treatment decisions are tailored to individual clinical history, course, and patient wishes.
Postpartum psychosis remains one of the most serious illnesses that we find in reproductive psychiatry, and incomplete attention has been given to this devastating illness, which we read about periodically in newspapers and magazines. Greater understanding of postpartum psychosis will lead to a more precision-like psychiatric approach, tailoring treatment to the invariable heterogeneity of this illness.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Although the last decade has brought appropriate increased interest in the diagnosis and treatment of postpartum depression, with screening initiatives across more than 40 states in place and even new medications being brought to market for treatment, far less attention has been given to diagnosis and treatment of a particularly serious psychiatric illness: postpartum psychosis.
Clinically, women can experience rapid mood changes, most often with the presentation that is consistent with a manic-like psychosis, with associated symptoms of delusional thinking, hallucinations, paranoia and either depression or elation, or an amalgam of these so-called “mixed symptoms.” Onset of symptoms typically is early, within 72 hours as is classically described, but may have a somewhat later time of onset in some women.
Many investigators have studied risk factors for postpartum psychosis, and it has been well established that a history of mood disorder, particularly bipolar disorder, is one of the strongest predictors of risk for postpartum psychosis. Women with histories of postpartum psychosis are at very high risk of recurrence, with as many as 70%-90% of women experiencing recurrence if not prophylaxed with an appropriate agent. From a clinical point of view, women with postpartum psychosis typically are hospitalized, given that this is both a psychiatric and potential obstetrical emergency. In fact, the data would suggest that although postpartum suicide and infanticide are not common, they can be a tragic concomitant of postpartum psychosis (Am J Psychiatry. 2016 Dec 1;173[12]:1179-88).
A great amount of interest has been placed on the etiology of postpartum psychosis, as it’s a dramatic presentation with very rapid onset in the acute postpartum period. A rich evidence base with respect to an algorithm of treatment that maximizes likelihood of full recovery or sustaining of euthymia after recovery is limited. Few studies have looked systematically at the optimum way to treat postpartum psychosis. Clinical wisdom has dictated that, given the dramatic symptoms with which these patients present, most patients are treated with lithium and an antipsychotic medication as if they have a manic-like psychosis. It may take brief or extended periods of time for patients to stabilize. Once they are stabilized, one of the most challenging questions for clinicians is how long to treat. Again, an evidence base clearly informing this question is lacking.
Over the years, many clinicians have treated patients with postpartum psychosis as if they have bipolar disorder, given the index presentation of the illness, so some of these patients are treated with antimanic drugs indefinitely. However, clinical experience from several centers that treat women with postpartum psychosis suggests that in remitted patients, a proportion of them may be able to taper and discontinue treatment, then sustain well-being for protracted periods.
One obstacle with respect to treatment of postpartum psychosis derives from the short length of stay after delivery for many women. Some women who present with symptoms of postpartum psychosis in the first 24-48 hours frequently are managed with direct admission to an inpatient psychiatric service. But others may not develop symptoms until they are home, which may place both mother and newborn at risk.
Given that the risk for recurrent postpartum psychosis is so great (70%-90%), women with histories of postpartum psychosis invariably are prophylaxed with mood stabilizer prior to delivery in a subsequent pregnancy. In our own center, we have published on the value of such prophylactic intervention, not just in women with postpartum psychosis, but in women with bipolar disorder, who are, as noted, at great risk for developing postpartum psychotic symptoms (Am J Psychiatry. 1995 Nov;152[11]:1641-5.)
Although postpartum psychosis may be rare, over the last 3 decades we have seen a substantial number of women with postpartum psychosis and have been fascinated with the spectrum of symptoms with which some women with postpartum psychotic illness present. We also have been impressed with the time required for some women to recompensate from their illness and the course of their disorder after they have seemingly remitted. Some women appear to be able to discontinue treatment as noted above; others, particularly if there is any history of bipolar disorder, need to be maintained on treatment with mood stabilizer indefinitely.
To better understand the phenomenology of postpartum psychosis, as well as the longitudinal course of the illness, in 2019, the Mass General Hospital Postpartum Psychosis Project (MGHP3) was established. The project is conducted as a hospital-based registry where women with histories of postpartum psychosis over the last decade are invited to participate in an in-depth interview to understand both symptoms and course of underlying illness. This is complemented by obtaining a sample of saliva, which is used for genetic testing to try to identify a genetic underpinning associated with postpartum psychosis, as the question of genetic etiology of postpartum psychosis is still an open one.
As part of the MGHP3 project, clinicians across the country are able to contact perinatal psychiatrists in our center with expertise in the treatment of postpartum psychosis. Our psychiatrists also can counsel clinicians on issues regarding long-term management of postpartum psychosis because for many, knowledge of precisely how to manage this disorder or the follow-up treatment may be incomplete.
From a clinical point of view, the relevant questions really include not only acute treatment, which has already been outlined, but also the issue of duration of treatment. While some patients may be able to taper and discontinue treatment after, for example, a year of being totally well, to date we are unable to know who those patients are. We tend to be more conservative in our own center and treat patients with puerperal psychosis for a more protracted period of time, usually over several years. We also ask women about their family history of bipolar disorder or postpartum psychosis. Depending on the clinical course (if the patient really has sustained euthymia), we consider slow taper and ultimate discontinuation. As always, treatment decisions are tailored to individual clinical history, course, and patient wishes.
Postpartum psychosis remains one of the most serious illnesses that we find in reproductive psychiatry, and incomplete attention has been given to this devastating illness, which we read about periodically in newspapers and magazines. Greater understanding of postpartum psychosis will lead to a more precision-like psychiatric approach, tailoring treatment to the invariable heterogeneity of this illness.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Docs weigh pulling out of MIPS over paltry payments
If you’ve knocked yourself out to earn a Merit-Based Incentive Payment System (MIPS) bonus payment, it’s pretty safe to say that getting a 1.68% payment boost probably didn’t feel like a “win” that was worth the effort.
And although it saved you from having a negative 5% payment adjustment, many physicians don’t feel that it was worth the effort.
On Jan. 6, the Centers for Medicare & Medicaid Services announced the 2020 payouts for MIPS.
Based on 2018 participation, the bonus for those who scored a perfect 100 is only a 1.68% boost in Medicare reimbursement, slightly lower than last year’s 1.88%. This decline comes as no surprise as the agency leader admits: “As the program matures, we expect that the increases in the performance thresholds in future program years will create a smaller distribution of positive payment adjustments.” Overall, more than 97% of participants avoided having a negative 5% payment adjustment.
Indeed, these bonus monies are based on a short-term appropriation of extra funds from Congress. After these temporary funds are no longer available, there will be little, if any, monies to distribute as the program is based on a “losers-feed-the-winners” construct.
It may be very tempting for many physicians to decide to ignore MIPS, with the rationale that 1.68% is not worth the effort. But don’t let your foot off the gas pedal yet, since the penalty for not participating in 2020 is a substantial 9%.
However, it is certainly time to reconsider efforts to participate at the highest level.
Should you or shouldn’t you bother with MIPS?
Let’s say you have $75,000 in revenue from Medicare Part B per year. Depending on the services you offer in your practice, that equates to 500-750 encounters with Medicare beneficiaries per year. (A reminder that MIPS affects only Part B; Medicare Advantage plans do not partake in the program.)
The recent announcement reveals that perfection would equate to an additional $1,260 per year. That’s only if you received the full 100 points; if you were simply an “exceptional performer,” the government will allot an additional $157. That’s less than you get paid for a single office visit.
The difference between perfection and compliance is approximately $1,000. Failure to participate, however, knocks $6,750 off your bottom line. Clearly, that’s a substantial financial loss that would affect most practices. Obviously, the numbers change if you have higher – or lower – Medicare revenue, but it’s important to do the math.
Why? Physicians are spending a significant amount of money to comply with the program requirements. This includes substantial payments to registries – typically $200 to >$1,000 per year – to report the quality measures for the program; electronic health record (EHR) systems, many of which require additional funding for the “upgrade” to a MIPS-compatible system, are also a sizable investment.
These hard costs pale in comparison with the time spent on understanding the ever-changing requirements of the program and the process by which your practice will implement them. Take, for example, something as innocuous as the required “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
You first must understand the elements of the measure: What is a “referral loop?” When do we need to generate one? To whom shall it be sent? What needs to be included in “health information?” What is the electronic address to which we should route the information? How do we obtain that address? Then you must determine how your EHR system captures and reports it.
Only then comes the hard part: How are we going to implement this? That’s only one of more than a dozen required elements: six quality measures, two (to four) improvement activities, and four promoting interoperability requirements. Each one of these elements has a host of requirements, all listed on multipage specification sheets.
The government does not seem to be listening. John Cullen, MD, president of the American Academy of Family Physicians, testified at the Senate Finance Committee in May 2019 that MIPS “has created a burdensome and extremely complex program that has increased practice costs ... ” Yet, later that year, CMS issued another hefty ruling that outlines significant changes to the program, despite the fact that it’s in its fourth performance year.
Turning frustration into action
Frustration or even anger may be one reaction, but now is an opportune time to determine your investment in the program. At a minimum, it’s vital to understand and meet the threshold to avoid the penalty. It’s been shifting to date, but it’s now set at 9% for perpetuity.
First, it’s crucial to check on your participation status. CMS revealed that the participation database was recently corrected for so-called inconsistencies, so it pays to double-check. It only takes seconds: Insert your NPI in the QPP Participation Status Tool to determine your eligibility for 2020.
In 2020, the threshold to avoid the penalty is 45 points. To get the 45 points, practices must participate in two improvement activities, which is not difficult as there are 118 options. That will garner 15 points. Then there are 45 points available from the quality category; you need at least 30 to reach the 45-point threshold for penalty avoidance.
Smart MIPS hacks that can help you
To obtain the additional 30 points, turn your attention to the quality category. There are 268 quality measures; choose at least six to measure. If you report directly from your EHR system, you’ll get a bonus point for each reported measure, plus one just for trying. (There are a few other opportunities for bonus points, such as improving your scores over last year.) Those bonus points give you a base with which to work, but getting to 45 will require effort to report successfully on at least a couple of the measures.
The quality category has a total of 100 points available, which are converted to 45 toward your composite score. Since you need 30 to reach that magical 45 (if 15 were attained from improvement activities), that means you must come up with 75 points in the quality category. Between the bonus points and measuring a handful of measures successfully through the year, you’ll achieve this threshold.
There are two other categories in the program: promoting interoperability (PI) and cost. The PI category mirrors the old “meaningful use” program; however, it has become increasingly difficult over the years. If you think that you can meet the required elements, you can pick up 25 more points toward your composite score.
Cost is a bit of an unknown, as the scoring is based on a retrospective review of your claims. You’ll likely pick up a few more points on this 15-point category, but there’s no method to determine performance until after the reporting period. Therefore, be cautious about relying on this category.
The best MIPS hack, however, is if you are a small practice. CMS – remarkably – defines a “small practice” as 15 or fewer eligible professionals. If you qualify under this paradigm, you have multiple options to ease compliance:
Apply for a “hardship exemption” simply on the basis of being small; the exemption relates to the promoting operability category, shifting those points to the quality category.
Gain three points per quality measure, regardless of data completeness; this compares to just one point for other physicians.
Capture all of the points available from the Improvement Activities category by confirming participation with just a single activity. (This also applies to all physicians in rural or Health Professional Shortage Areas.)
In the event that you don’t qualify as a “small practice” or you’re still falling short of the requirements, CMS allows for the ultimate “out”: You can apply for exemption on the basis of an “extreme and uncontrollable circumstance.” The applications for these exceptions open this summer.
Unless you qualify for the program exemption, it’s important to keep pace with the program to ensure that you reach the 45-point threshold. It may not, however, be worthwhile to gear up for all 100 points unless your estimate of the potential return – and what it costs you to get there – reveals otherwise. MIPS is not going anywhere; the program is written into the law.
But that doesn’t mean that CMS can’t make tweaks and updates. Hopefully, the revisions won’t create even more administrative burden as the program is quickly turning into a big stick with only a small carrot at the end.
Elizabeth Woodcock is president of Woodcock & Associates in Atlanta. She has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
If you’ve knocked yourself out to earn a Merit-Based Incentive Payment System (MIPS) bonus payment, it’s pretty safe to say that getting a 1.68% payment boost probably didn’t feel like a “win” that was worth the effort.
And although it saved you from having a negative 5% payment adjustment, many physicians don’t feel that it was worth the effort.
On Jan. 6, the Centers for Medicare & Medicaid Services announced the 2020 payouts for MIPS.
Based on 2018 participation, the bonus for those who scored a perfect 100 is only a 1.68% boost in Medicare reimbursement, slightly lower than last year’s 1.88%. This decline comes as no surprise as the agency leader admits: “As the program matures, we expect that the increases in the performance thresholds in future program years will create a smaller distribution of positive payment adjustments.” Overall, more than 97% of participants avoided having a negative 5% payment adjustment.
Indeed, these bonus monies are based on a short-term appropriation of extra funds from Congress. After these temporary funds are no longer available, there will be little, if any, monies to distribute as the program is based on a “losers-feed-the-winners” construct.
It may be very tempting for many physicians to decide to ignore MIPS, with the rationale that 1.68% is not worth the effort. But don’t let your foot off the gas pedal yet, since the penalty for not participating in 2020 is a substantial 9%.
However, it is certainly time to reconsider efforts to participate at the highest level.
Should you or shouldn’t you bother with MIPS?
Let’s say you have $75,000 in revenue from Medicare Part B per year. Depending on the services you offer in your practice, that equates to 500-750 encounters with Medicare beneficiaries per year. (A reminder that MIPS affects only Part B; Medicare Advantage plans do not partake in the program.)
The recent announcement reveals that perfection would equate to an additional $1,260 per year. That’s only if you received the full 100 points; if you were simply an “exceptional performer,” the government will allot an additional $157. That’s less than you get paid for a single office visit.
The difference between perfection and compliance is approximately $1,000. Failure to participate, however, knocks $6,750 off your bottom line. Clearly, that’s a substantial financial loss that would affect most practices. Obviously, the numbers change if you have higher – or lower – Medicare revenue, but it’s important to do the math.
Why? Physicians are spending a significant amount of money to comply with the program requirements. This includes substantial payments to registries – typically $200 to >$1,000 per year – to report the quality measures for the program; electronic health record (EHR) systems, many of which require additional funding for the “upgrade” to a MIPS-compatible system, are also a sizable investment.
These hard costs pale in comparison with the time spent on understanding the ever-changing requirements of the program and the process by which your practice will implement them. Take, for example, something as innocuous as the required “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
You first must understand the elements of the measure: What is a “referral loop?” When do we need to generate one? To whom shall it be sent? What needs to be included in “health information?” What is the electronic address to which we should route the information? How do we obtain that address? Then you must determine how your EHR system captures and reports it.
Only then comes the hard part: How are we going to implement this? That’s only one of more than a dozen required elements: six quality measures, two (to four) improvement activities, and four promoting interoperability requirements. Each one of these elements has a host of requirements, all listed on multipage specification sheets.
The government does not seem to be listening. John Cullen, MD, president of the American Academy of Family Physicians, testified at the Senate Finance Committee in May 2019 that MIPS “has created a burdensome and extremely complex program that has increased practice costs ... ” Yet, later that year, CMS issued another hefty ruling that outlines significant changes to the program, despite the fact that it’s in its fourth performance year.
Turning frustration into action
Frustration or even anger may be one reaction, but now is an opportune time to determine your investment in the program. At a minimum, it’s vital to understand and meet the threshold to avoid the penalty. It’s been shifting to date, but it’s now set at 9% for perpetuity.
First, it’s crucial to check on your participation status. CMS revealed that the participation database was recently corrected for so-called inconsistencies, so it pays to double-check. It only takes seconds: Insert your NPI in the QPP Participation Status Tool to determine your eligibility for 2020.
In 2020, the threshold to avoid the penalty is 45 points. To get the 45 points, practices must participate in two improvement activities, which is not difficult as there are 118 options. That will garner 15 points. Then there are 45 points available from the quality category; you need at least 30 to reach the 45-point threshold for penalty avoidance.
Smart MIPS hacks that can help you
To obtain the additional 30 points, turn your attention to the quality category. There are 268 quality measures; choose at least six to measure. If you report directly from your EHR system, you’ll get a bonus point for each reported measure, plus one just for trying. (There are a few other opportunities for bonus points, such as improving your scores over last year.) Those bonus points give you a base with which to work, but getting to 45 will require effort to report successfully on at least a couple of the measures.
The quality category has a total of 100 points available, which are converted to 45 toward your composite score. Since you need 30 to reach that magical 45 (if 15 were attained from improvement activities), that means you must come up with 75 points in the quality category. Between the bonus points and measuring a handful of measures successfully through the year, you’ll achieve this threshold.
There are two other categories in the program: promoting interoperability (PI) and cost. The PI category mirrors the old “meaningful use” program; however, it has become increasingly difficult over the years. If you think that you can meet the required elements, you can pick up 25 more points toward your composite score.
Cost is a bit of an unknown, as the scoring is based on a retrospective review of your claims. You’ll likely pick up a few more points on this 15-point category, but there’s no method to determine performance until after the reporting period. Therefore, be cautious about relying on this category.
The best MIPS hack, however, is if you are a small practice. CMS – remarkably – defines a “small practice” as 15 or fewer eligible professionals. If you qualify under this paradigm, you have multiple options to ease compliance:
Apply for a “hardship exemption” simply on the basis of being small; the exemption relates to the promoting operability category, shifting those points to the quality category.
Gain three points per quality measure, regardless of data completeness; this compares to just one point for other physicians.
Capture all of the points available from the Improvement Activities category by confirming participation with just a single activity. (This also applies to all physicians in rural or Health Professional Shortage Areas.)
In the event that you don’t qualify as a “small practice” or you’re still falling short of the requirements, CMS allows for the ultimate “out”: You can apply for exemption on the basis of an “extreme and uncontrollable circumstance.” The applications for these exceptions open this summer.
Unless you qualify for the program exemption, it’s important to keep pace with the program to ensure that you reach the 45-point threshold. It may not, however, be worthwhile to gear up for all 100 points unless your estimate of the potential return – and what it costs you to get there – reveals otherwise. MIPS is not going anywhere; the program is written into the law.
But that doesn’t mean that CMS can’t make tweaks and updates. Hopefully, the revisions won’t create even more administrative burden as the program is quickly turning into a big stick with only a small carrot at the end.
Elizabeth Woodcock is president of Woodcock & Associates in Atlanta. She has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
If you’ve knocked yourself out to earn a Merit-Based Incentive Payment System (MIPS) bonus payment, it’s pretty safe to say that getting a 1.68% payment boost probably didn’t feel like a “win” that was worth the effort.
And although it saved you from having a negative 5% payment adjustment, many physicians don’t feel that it was worth the effort.
On Jan. 6, the Centers for Medicare & Medicaid Services announced the 2020 payouts for MIPS.
Based on 2018 participation, the bonus for those who scored a perfect 100 is only a 1.68% boost in Medicare reimbursement, slightly lower than last year’s 1.88%. This decline comes as no surprise as the agency leader admits: “As the program matures, we expect that the increases in the performance thresholds in future program years will create a smaller distribution of positive payment adjustments.” Overall, more than 97% of participants avoided having a negative 5% payment adjustment.
Indeed, these bonus monies are based on a short-term appropriation of extra funds from Congress. After these temporary funds are no longer available, there will be little, if any, monies to distribute as the program is based on a “losers-feed-the-winners” construct.
It may be very tempting for many physicians to decide to ignore MIPS, with the rationale that 1.68% is not worth the effort. But don’t let your foot off the gas pedal yet, since the penalty for not participating in 2020 is a substantial 9%.
However, it is certainly time to reconsider efforts to participate at the highest level.
Should you or shouldn’t you bother with MIPS?
Let’s say you have $75,000 in revenue from Medicare Part B per year. Depending on the services you offer in your practice, that equates to 500-750 encounters with Medicare beneficiaries per year. (A reminder that MIPS affects only Part B; Medicare Advantage plans do not partake in the program.)
The recent announcement reveals that perfection would equate to an additional $1,260 per year. That’s only if you received the full 100 points; if you were simply an “exceptional performer,” the government will allot an additional $157. That’s less than you get paid for a single office visit.
The difference between perfection and compliance is approximately $1,000. Failure to participate, however, knocks $6,750 off your bottom line. Clearly, that’s a substantial financial loss that would affect most practices. Obviously, the numbers change if you have higher – or lower – Medicare revenue, but it’s important to do the math.
Why? Physicians are spending a significant amount of money to comply with the program requirements. This includes substantial payments to registries – typically $200 to >$1,000 per year – to report the quality measures for the program; electronic health record (EHR) systems, many of which require additional funding for the “upgrade” to a MIPS-compatible system, are also a sizable investment.
These hard costs pale in comparison with the time spent on understanding the ever-changing requirements of the program and the process by which your practice will implement them. Take, for example, something as innocuous as the required “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
You first must understand the elements of the measure: What is a “referral loop?” When do we need to generate one? To whom shall it be sent? What needs to be included in “health information?” What is the electronic address to which we should route the information? How do we obtain that address? Then you must determine how your EHR system captures and reports it.
Only then comes the hard part: How are we going to implement this? That’s only one of more than a dozen required elements: six quality measures, two (to four) improvement activities, and four promoting interoperability requirements. Each one of these elements has a host of requirements, all listed on multipage specification sheets.
The government does not seem to be listening. John Cullen, MD, president of the American Academy of Family Physicians, testified at the Senate Finance Committee in May 2019 that MIPS “has created a burdensome and extremely complex program that has increased practice costs ... ” Yet, later that year, CMS issued another hefty ruling that outlines significant changes to the program, despite the fact that it’s in its fourth performance year.
Turning frustration into action
Frustration or even anger may be one reaction, but now is an opportune time to determine your investment in the program. At a minimum, it’s vital to understand and meet the threshold to avoid the penalty. It’s been shifting to date, but it’s now set at 9% for perpetuity.
First, it’s crucial to check on your participation status. CMS revealed that the participation database was recently corrected for so-called inconsistencies, so it pays to double-check. It only takes seconds: Insert your NPI in the QPP Participation Status Tool to determine your eligibility for 2020.
In 2020, the threshold to avoid the penalty is 45 points. To get the 45 points, practices must participate in two improvement activities, which is not difficult as there are 118 options. That will garner 15 points. Then there are 45 points available from the quality category; you need at least 30 to reach the 45-point threshold for penalty avoidance.
Smart MIPS hacks that can help you
To obtain the additional 30 points, turn your attention to the quality category. There are 268 quality measures; choose at least six to measure. If you report directly from your EHR system, you’ll get a bonus point for each reported measure, plus one just for trying. (There are a few other opportunities for bonus points, such as improving your scores over last year.) Those bonus points give you a base with which to work, but getting to 45 will require effort to report successfully on at least a couple of the measures.
The quality category has a total of 100 points available, which are converted to 45 toward your composite score. Since you need 30 to reach that magical 45 (if 15 were attained from improvement activities), that means you must come up with 75 points in the quality category. Between the bonus points and measuring a handful of measures successfully through the year, you’ll achieve this threshold.
There are two other categories in the program: promoting interoperability (PI) and cost. The PI category mirrors the old “meaningful use” program; however, it has become increasingly difficult over the years. If you think that you can meet the required elements, you can pick up 25 more points toward your composite score.
Cost is a bit of an unknown, as the scoring is based on a retrospective review of your claims. You’ll likely pick up a few more points on this 15-point category, but there’s no method to determine performance until after the reporting period. Therefore, be cautious about relying on this category.
The best MIPS hack, however, is if you are a small practice. CMS – remarkably – defines a “small practice” as 15 or fewer eligible professionals. If you qualify under this paradigm, you have multiple options to ease compliance:
Apply for a “hardship exemption” simply on the basis of being small; the exemption relates to the promoting operability category, shifting those points to the quality category.
Gain three points per quality measure, regardless of data completeness; this compares to just one point for other physicians.
Capture all of the points available from the Improvement Activities category by confirming participation with just a single activity. (This also applies to all physicians in rural or Health Professional Shortage Areas.)
In the event that you don’t qualify as a “small practice” or you’re still falling short of the requirements, CMS allows for the ultimate “out”: You can apply for exemption on the basis of an “extreme and uncontrollable circumstance.” The applications for these exceptions open this summer.
Unless you qualify for the program exemption, it’s important to keep pace with the program to ensure that you reach the 45-point threshold. It may not, however, be worthwhile to gear up for all 100 points unless your estimate of the potential return – and what it costs you to get there – reveals otherwise. MIPS is not going anywhere; the program is written into the law.
But that doesn’t mean that CMS can’t make tweaks and updates. Hopefully, the revisions won’t create even more administrative burden as the program is quickly turning into a big stick with only a small carrot at the end.
Elizabeth Woodcock is president of Woodcock & Associates in Atlanta. She has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Are unmatched residency graduates a solution for ‘shrinking shrinks’?
‘Physician associates’ could be used to expand the reach of psychiatry
For many years now, we have been lamenting the shortage of psychiatrists practicing in the United States. At this point, we must identify possible solutions.1,2 Currently, the shortage of practicing psychiatrists in the United States could be as high as 45,000.3 The major problem is that the number of psychiatry residency positions will not increase in the foreseeable future, thus generating more psychiatrists is not an option.
Medicare pays about $150,000 per residency slot per year. To solve the mental health access problem, $27 billion (45,000 x $150,000 x 4 years)* would be required from Medicare, which is not feasible.4 The national average starting salary for psychiatrists from 2018-2019 was about $273,000 (much lower in academic institutions), according to Merritt Hawkins, the physician recruiting firm. That salary is modest, compared with those offered in other medical specialties. For this reason, many graduates choose other lucrative specialties. And we know that increasing the salaries of psychiatrists alone would not lead more people to choose psychiatry. On paper, it may say they work a 40-hour week, but they end up working 60 hours a week.
To make matters worse, family medicine and internal medicine doctors generally would rather not deal with people with mental illness and do “cherry-picking and lemon-dropping.” While many patients present to primary care with mental health issues, lack of time and education in psychiatric disorders and treatment hinder these physicians. In short, the mental health field cannot count on primary care physicians.
Meanwhile, there are thousands of unmatched residency graduates. In light of those realities, perhaps psychiatry residency programs could provide these unmatched graduates with 6 months of training and use them to supplement the workforce. These medical doctors, or “physician associates,” could be paired with a few psychiatrists to do clinical and administrative work. With one in four individuals having mental health issues, and more and more people seeking help because of increasing awareness and the benefits that accompanied the Affordable Care Act (ACA), physician associates might ease the workload of psychiatrists so that they can deliver better care to more people. We must take advantage of these two trends: The surge in unmatched graduates and “shrinking shrinks,” or the decline in the psychiatric workforce pool. (The Royal College of Physicians has established a category of clinicians called physician associates,5 but they are comparable to physician assistants in the United States. As you will see, the construct I am proposing is different.)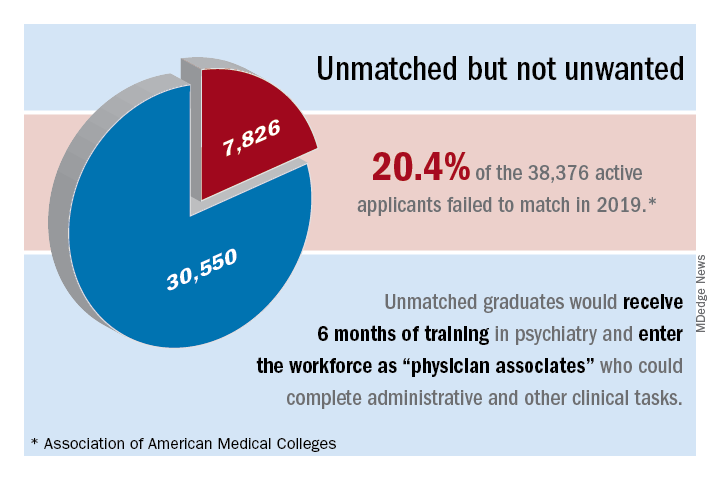
The current landscape
Currently, psychiatrists are under a lot of pressure to see a certain number of patients. Patients consistently complain that psychiatrists spend a maximum of 15 minutes with them, that the visits are interrupted by phone calls, and that they are not being heard and helped. Burnout, a silent epidemic among physicians, is relatively prevalent in psychiatry.6 Hence, some psychiatrists are reducing their hours and retiring early. Psychiatry has the third-oldest workforce, with 59% of current psychiatrists aged 55 years or older.7 A better pay/work ratio and work/life balance would enable psychiatrists to enjoy more fulfilling careers.
Many psychiatrists are spending a lot of their time in research, administration, and the classroom. In addition to those issues, the United States currently has a broken mental health care system.8 Finally, the medical practice landscape has changed dramatically in recent years, and those changes undermine both the effectiveness and well-being of clinicians.
The historical landscape
Some people proudly refer to the deinstitutionalization of mental asylums and state mental hospitals in the United States. But where have these patients gone? According to a U.S. Justice Department report, 2,220,300 adults were incarcerated in U.S. federal and state prisons and county jails in 2013.9 In addition, 4,751,400 adults in 2013 were on probation or parole. The percentages of inmates in state and federal prisons and local jails with a psychiatric diagnosis were 56%, 45%, and 64%, respectively.
I work at the Maryland correctional institutions, part of the Maryland Department of Public Safety and Correctional Services. One thing that I consistently hear from several correctional officers is “had these inmates received timely help and care, they wouldn’t have ended up behind bars.” Because of the criminalization of mental illness, in 44 states, the number of people with mental illness is higher in a jail or prison than in the largest state psychiatric hospital, according to the Treatment Advocacy Center. We have to be responsible for many of the inmates currently in correctional facilities for committing crimes related to mental health problems. In Maryland, a small state, there are 30,000 inmates in jails, and state and federal prison. The average cost of a meal is $1.36, thus $1.36 x 3 meals x 30,000 inmates = $122,400.00 for food alone for 1 day – this average does not take other expenses into account. By using money and manpower wisely and taking care of individuals’ mental health problems before they commit crimes, better outcomes could be achieved.
I used to work for MedOptions Inc. doing psychiatry consults at nursing homes and assisted-living facilities. Because of the shortage of psychiatrists and nurse practitioners, especially in the suburbs and rural areas, those patients could not be seen in a timely manner even for their 3-month routine follow-ups. As my colleagues and I have written previously, many elderly individuals with major neurocognitive disorders are not on the Food and Drug Administration–approved cognitive enhancers, such as donepezil, galantamine, and memantine.10 Instead, those patients are on benzodiazepines, which are associated with cognitive impairments, and increased risk of pneumonia and falls. Benzodiazepines also can cause and/or worsen disinhibited behavior. Also, in those settings, crisis situations often are addressed days to weeks later because of the doctor shortage. This situation is going to get worse, because this patient population is growing.
Child and geriatric psychiatry shortages
Child and geriatric psychiatrist shortages are even higher than those in general psychiatry.11 Many years of training and low salaries are a few of the reasons some choose not to do a fellowship. These residency graduates would rather join a practice at an attending salary than at a fellow’s salary, which requires an additional 1 to 2 years of training. Student loans of $100,000–$500,000 after residency also discourage some from pursuing fellowship opportunities. We need to consider models such as 2 years of residency with 2 years of a child psychiatry fellowship or 3 years of residency with 1 year of geriatric psychiatry fellowship. Working as an adult attending physician (50% of the time) and concurrently doing a fellowship (50% of the time) while receiving an attending salary might motivate more people to complete a fellowship.
In specialties such as radiology, international medical graduates (IMGs) who have completed residency training in radiology in other countries can complete a radiology fellowship in a particular area for several years and can practice in the United States as board-eligible certified MDs. Likewise, in line with the model proposed here, we could provide unmatched graduates who have no residency training with 3 to 4 years of child psychiatry and geriatric psychiatry training in addition to some adult psychiatry training.
Implementation of such a model might take care of the shortage of child and geriatric psychiatrists. In 2015, there were 56 geriatric psychiatry fellowship programs; 54 positions were filled, and 51 fellows completed training.12 “It appears that a reasonable percentage of IMGs who obtain a fellowship in geriatric psychiatry do not have an intent of pursuing a career in the field,” Marc H. Zisselman, MD, former geriatric psychiatry fellowship director and currently with the Einstein Medical Center in Philadelphia, told me in 2016. These numbers are not at all sufficient to take care of the nation’s unmet need. Hence, implementing alternate strategies is imperative.
Administrative tasks and care
What consumes a psychiatrist’s time and leads to burnout? The answer has to do with administrative tasks at work. Administrative tasks are not an effective use of time for an MD who has spent more than a decade in medical school, residency, and fellowship training. Although electronic medical record (EMR) systems are considered a major advancement, engaging in the process throughout the day is associated with exhaustion.
Many physicians feel that EMRs have slowed them down, and some are not well-equipped to use them in quick and efficient ways. EMRs also have led to physicians making minimal eye contact in interviews with patients. Patients often complain: “I am talking, and the doctor is looking at the computer and typing.” Patients consider this behavior to be unprofessional and rude. In a survey of 57 U.S. physicians in family medicine, internal medicine, cardiology, and orthopedics, results showed that during the work day, 27% of their time was spent on direct clinical face time with patients and 49.2% was spent on EMR and desk work. While in the examination room with patients, physicians spent 52.9% of their time on direct clinical face time and 37.0% on EMR and desk work. Outside office hours, physicians spend up to 2 hours of personal time each night doing additional computer and other clerical work.13
Several EMR software systems, such as CareLogic, Cerner, Epic,NextGen, PointClickCare, and Sunrise, are used in the United States. The U.S. Veterans Affairs Medical Centers (VAMCs) use the computerized patient record system (CPRS) across the country. VA clinicians find CPRS extremely useful when they move from one VAMC to another. Likewise, hospitals and universities may use one software system such as the CPRS and thus, when clinicians change jobs, they find it hard to adapt to the new system.
Because psychiatrists are wasting a lot of time doing administrative tasks, they might be unable to do a good job with regard to making the right diagnoses and prescribing the best treatments.When I ask patients what are they diagnosed with, they tell me: “It depends on who you ask,” or “I’ve been diagnosed with everything.” This shows that we are not doing a good job or something is not right.
Currently, psychiatrists do not have the time and/or interest to make the right diagnoses and provide adequate psychoeducation for their patients. This also could be attributable to a variety of factors, including, but not limited to, time constraints, cynicism, and apathy. Time constraints also lead to the gross underutilization14 of relapse prevention strategies such as long-acting injectables and medications that can prevent suicide, such as lithium and clozapine.15
Other factors that undermine good care include not participating in continuing medical education (CME) and not staying up to date with the literature. For example, haloperidol continues to be one of the most frequently prescribed (probably, the most common) antipsychotic, although it is clearly neurotoxic16,17 and other safer options are available.18 Board certification and maintenance of certification (MOC) are not synonymous with good clinical practice. Many physicians are finding it hard to complete daily documentation, let alone time for MOC. For a variety of reasons, many are not maintaining certification, and this number is likely to increase. Think about how much time is devoted to the one-to-one interview with the patient and direct patient care during the 15-minute medical check appointment and the hour-long new evaluation. In some clinics, psychiatrists are asked to see more than 25 patients in 4 hours. Some U.S.-based psychiatrists see 65 inpatients and initiate 10 new evaluations in a single day. Under those kinds of time constraints, how can we provide quality care?
A model that would address the shortage
Overall, 7,826 PGY-1 applicants were unmatched in 2019, according to data from the 2019 Main Residency Match.19 Psychiatry residency programs could give these unmatched graduates 6 months of training (arbitrary duration) in psychiatry, which is not at all difficult with the program modules that are available.20 We could use them as physician associates as a major contributor to our workforce to complete administrative and other clinical tasks.
Administrative tasks are not necessarily negative, as all psychiatrists have done administrative tasks as medical students, residents, and fellows. However, at this point, administrative tasks are not an effective use of a psychiatrist’s time. Those physician associates could be paired with two to three psychiatrists to do administrative tasks (for making daytime and overnight phone calls; handling prescriptions, prior authorizations, and medication orders, especially over-the-counter and comfort medications in the inpatient units; doing chart reviews; ordering and checking laboratory tests; collecting collateral information from previous clinicians and records; printing medication education pamphlets; faxing; corresponding with insurance companies/utilization review; performing documentation; billing; and taking care of other clinical and administrative paperwork).
In addition, physician associates could collect information using rating scales such as the 9-item Patient Health Questionnaire for measurement-based care21 and Geriatric Depression Scale, both of which are currently not used in psychiatric practice because of time constraints and lack of manpower. Keep in mind that these individuals are medical doctors and could do a good job with these kinds of tasks. Most of them already have clinical experience in the United States and know the health care system. These MDs could conduct an initial interview (what medical students, residents, and fellows do) and present to the attending psychiatrist. Psychiatrists could then focus on the follow-up interview; diagnoses and treatment; major medical decision making, including shared decision making (patients feel that they are not part of the treatment plan); and seeing more patients, which is a more effective use of their time. This training would give these physician associates a chance to work as doctors and make a living. These MDs have completed medical school training after passing Medical College Admission Test – equivalent exams in their countries. They have passed all steps of the U.S. Medical Licensing Examination and have received Educational Commission for Foreign Medical Graduates certification. Some have even completed residency programs in their home countries.
Some U.S. states already have implemented these kinds of programs. In Arkansas, Kansas, and Missouri,22,23 legislators have passed laws allowing unmatched graduates who have not completed a residency program to work in medically underserved areas with a collaborating physician. These physicians must directly supervise the new doctors for at least a month before they can see patients on their own. Another proposal that has been suggested to address the psychiatrist shortage is employing physician assistants to provide care.24-26
The model proposed here is comparable to postdoctoral fellow-principal investigator and resident-attending collaborative work. At hospitals, a certified nurse assistant helps patients with health care needs under the supervision of a nurse. Similarly, a physician associate could help a psychiatrist under his or her supervision. In the Sheppard Pratt Health System in Baltimore, where I worked previously, for example, nurses dictate and prepare discharge summaries for the attending physician with whom they work. These are the kinds of tasks that physician associates could do as well.
The wait time to get a new evaluation with a psychiatrist is enormous. The policy is that a new patient admitted to an inpatient unit must be seen within 24 hours. With this model, the physician associates could see patients within a few hours, take care of their most immediate needs, take a history and conduct a physical, and write an admission note for the attending psychiatrist to sign. Currently, the outpatient practice is so busy that psychiatrists do not have the time to read the discharge summaries of patients referred to them after a recent hospitalization, which often leads to poor aftercare. The physician associates could read the discharge summaries and provide pertinent information to the attending psychiatrists.
In the inpatient units and emergency departments, nurses and social workers see patients before the attending physician, present patient information to the attending psychiatrist, and document their findings. It is redundant for the physician to write the same narrative again. Rather, the physician could add an addendum to the nurse’s or social worker’s notes and sign off. This would save a lot of time.
Numerous well-designed studies support the adoption of collaborative care models as one means of providing quality psychiatric care to larger populations.27,28 The American Psychiatric Association (APA) is currently training 3,500 psychiatrists in collaborative care through the Centers for Medicare and Medicaid Services’ Transforming Clinical Practice Initiative.29,30 Despite this training and the services provided by the nurse practitioners and physician assistants, the shortage of psychiatrists has not been adequately addressed. Hence, we need to think outside the box to find other potential pragmatic solutions.
Simply increasing the hours of work or the number of nurse practitioners or physician assistants already in practice is not going to solve the problem completely. The model proposed here and previously31 is likely to improve the quality of care that we now provide. This model should not be seen as exploiting these unmatched medical graduates and setting up a two-tiered health care system. The salary for these physicians would be a small percentage (5%-10%; these are arbitrary percentages) from the reimbursement of the attending psychiatrist. This model would not affect the salary of the attending psychiatrists; with this model, they would be able to see 25%-50% more patients (again, arbitrary percentages) with the help and support from these physician associates.
Potential barriers to implementation
There could be inherent barriers and complications to implementation of this model that are difficult to foresee at this point. Nurse practitioners (222,000 plus) and physician assistants (83,000 plus) have a fixed and structured curriculum, have national examining boards and national organizations with recertification requirements, and are licensed as independent practitioners, at least as far as CME is concerned.
Physician associates would need a standardized curriculum and examinations to validate what they have studied and learned. This process might be an important part of the credentialing of these individuals, as well as evaluation of cultural competency. If this model is to successfully lead to formation of a specific clinical group, it might need its own specific identity, national organization, national standards of competency, national certification and recertification processes, and national conference and CME or at least a subsection in a national behavioral and medical health organization, such as the APA or the American Academy of Child and Adolescent Psychiatry.
It would be desirable to “field test” the physician associate concept to clarify implementation difficulties, including the ones described above, that could arise. The cost of implementation of this program should not be of much concern; the 6-month training could be on a volunteer basis, or a small stipend might be paid by graduate medical education funding. This model could prove to be rewarding long term, save trillions of health care dollars, and allow us to provide exceptional and timely care.
Conclusion
The 2020 Mental Health America annual State of Mental Health in America report found that more than 70% of youth with severe major depressive disorder were in need of treatment in 2017. The percentage of adults with any mental illness who did not receive treatment stood at about 57.2%.32 Meanwhile, from 1999 through 2014, the age-adjusted suicide rate in the United States increased 24%.33 More individuals are seeking help because of increased awareness.34,35 In light of the access to services afforded by the ACA, physician associates might ease the workload of psychiatrists and enable them to deliver better care to more people. We would not necessarily have to use the term “physician associate” and could generate better terminologies later. In short, let’s tap into the pools of unmatched graduates and shrinking shrinks! If this model is successful, it could be used in other specialties and countries. The stakes for our patients have never been higher.
References
1. Bishop TF et al. Health Aff. 2016;35(7):1271-7.
2. National Council Medical Director Institute. The psychiatric shortage: Causes and solutions. 2017. Washington: National Council for Behavioral Health.
3. Satiani A et al. Psychiatric Serv. 2018;69:710-3.
4. Carlat D. Psychiatric Times. 2010 Aug 3;27(8).
5. McCartney M. BMJ. 2017;359:j5022.
6. Maslach C and Leiter MP. World Psychiatry. 2016 Jun 5;15:103-11.
7. Merritt Hawkins. “The silent shortage: A white paper examining supply, demand and recruitment trends in psychiatry.” 2018.
8. Sederer LI and Sharfstein SS. JAMA. 2014 Sep 24;312:1195-6.
9. James DJ and Glaze LE. Mental health problems of prison and jail inmates. 2006 Sep. U.S. Justice Department, Bureau of Justice Statistics Special Report.
10. Koola MM et al. J Geriatr Care Res. 2018;5(2):57-67.
11. Buckley PF and Nasrallah HA. Curr Psychiatr. 2016;15:23-4.
12. American Medical Association Database. Open Residency and Fellowship Positions.
13. Sinsky C et al. Ann Intern Med. 2016;165:753-60.
14. Koola MM. Curr Psychiatr. 2017 Mar. 16(3):19-20,47,e1.
15. Koola MM and Sebastian J. HSOA J Psychiatry Depress Anxiety. 2016;(2):1-11.
16. Nasrallah HA and Chen AT. Ann Clin Psychiatry. 2017 Aug;29(3):195-202.
17. Nasrallah HA. Curr Psychiatr. 2013 Jul;7-8.
18. Chen AT and Nasrallah HA. Schizophr Res. 2019 Jun;208:1-7.
19. National Resident Matching Program, Results and Data: 2019 Main Residency Match. National Resident Matching Program, Washington, 2019.
20. Masters KJ. J Physician Assist Educ. 2015 Sep;26(3):136-43.
21. Koola MM et al. J Nerv Ment Dis. 2011;199(12):989-90.
22. “New Missouri licensing offers ‘Band-Aid’ for physician shortages.” Kansas City Business Journal. Updated 2017 May 16.
23. “After earning an MD, she’s headed back to school – to become a nurse.” STAT. 2016 Nov 8.
24. Keizer TB and Trangle MA. Acad Psychiatry. 2015 Dec;39(6):691-4.
25. Miller JG and Peterson DJ. Acad Psychiatry. 2015 Dec;39(6):685-6.
26. Smith MS. Curr Psychiatr. 2019 Sep;18(9):17-24.
27. Osofsky HJ et al. Acad Psychiatry. 2016 Oct;40(5):747-54.
28. Dreier-Wolfgramm A et al. Z Gerontol Geriatr. 2017 May;50(Suppl 2):68-77.
29. Huang H and Barkil-Oteo A. Psychosomatics. 2015 Nov-Dec;56(6):658-61.
30. Raney L et al. Fam Syst Health. 2014 Jun;32(2):147-8.
31. Koola MM. Curr Psychiatr. 2016 Dec. 15(12):33-4.
32. Mental Health America. State of Mental Health in America 2020.
33. Curtin SC et al. NCHS Data Brief. 2016 Apr;(241):1-8.
34. Kelly DL et al. Ann Intern Med. 2020;172(2):167-8.
35. Miller JP and Nasrallah HA. Curr Psychiatr. 2015;14(12):45-6.
Dr. Koola is an associate professor in the department of psychiatry and behavioral health at Stony Brook (N.Y.) University. His main area of interest is novel therapeutic discovery in the treatment of schizophrenia. He has a particular interest in improving the health care delivery system for people with psychiatric illness. Dr. Koola declared no conflicts of interest. He can be reached at [email protected].
*This commentary was updated 2/2/2020.
‘Physician associates’ could be used to expand the reach of psychiatry
‘Physician associates’ could be used to expand the reach of psychiatry
For many years now, we have been lamenting the shortage of psychiatrists practicing in the United States. At this point, we must identify possible solutions.1,2 Currently, the shortage of practicing psychiatrists in the United States could be as high as 45,000.3 The major problem is that the number of psychiatry residency positions will not increase in the foreseeable future, thus generating more psychiatrists is not an option.
Medicare pays about $150,000 per residency slot per year. To solve the mental health access problem, $27 billion (45,000 x $150,000 x 4 years)* would be required from Medicare, which is not feasible.4 The national average starting salary for psychiatrists from 2018-2019 was about $273,000 (much lower in academic institutions), according to Merritt Hawkins, the physician recruiting firm. That salary is modest, compared with those offered in other medical specialties. For this reason, many graduates choose other lucrative specialties. And we know that increasing the salaries of psychiatrists alone would not lead more people to choose psychiatry. On paper, it may say they work a 40-hour week, but they end up working 60 hours a week.
To make matters worse, family medicine and internal medicine doctors generally would rather not deal with people with mental illness and do “cherry-picking and lemon-dropping.” While many patients present to primary care with mental health issues, lack of time and education in psychiatric disorders and treatment hinder these physicians. In short, the mental health field cannot count on primary care physicians.
Meanwhile, there are thousands of unmatched residency graduates. In light of those realities, perhaps psychiatry residency programs could provide these unmatched graduates with 6 months of training and use them to supplement the workforce. These medical doctors, or “physician associates,” could be paired with a few psychiatrists to do clinical and administrative work. With one in four individuals having mental health issues, and more and more people seeking help because of increasing awareness and the benefits that accompanied the Affordable Care Act (ACA), physician associates might ease the workload of psychiatrists so that they can deliver better care to more people. We must take advantage of these two trends: The surge in unmatched graduates and “shrinking shrinks,” or the decline in the psychiatric workforce pool. (The Royal College of Physicians has established a category of clinicians called physician associates,5 but they are comparable to physician assistants in the United States. As you will see, the construct I am proposing is different.)
The current landscape
Currently, psychiatrists are under a lot of pressure to see a certain number of patients. Patients consistently complain that psychiatrists spend a maximum of 15 minutes with them, that the visits are interrupted by phone calls, and that they are not being heard and helped. Burnout, a silent epidemic among physicians, is relatively prevalent in psychiatry.6 Hence, some psychiatrists are reducing their hours and retiring early. Psychiatry has the third-oldest workforce, with 59% of current psychiatrists aged 55 years or older.7 A better pay/work ratio and work/life balance would enable psychiatrists to enjoy more fulfilling careers.
Many psychiatrists are spending a lot of their time in research, administration, and the classroom. In addition to those issues, the United States currently has a broken mental health care system.8 Finally, the medical practice landscape has changed dramatically in recent years, and those changes undermine both the effectiveness and well-being of clinicians.
The historical landscape
Some people proudly refer to the deinstitutionalization of mental asylums and state mental hospitals in the United States. But where have these patients gone? According to a U.S. Justice Department report, 2,220,300 adults were incarcerated in U.S. federal and state prisons and county jails in 2013.9 In addition, 4,751,400 adults in 2013 were on probation or parole. The percentages of inmates in state and federal prisons and local jails with a psychiatric diagnosis were 56%, 45%, and 64%, respectively.
I work at the Maryland correctional institutions, part of the Maryland Department of Public Safety and Correctional Services. One thing that I consistently hear from several correctional officers is “had these inmates received timely help and care, they wouldn’t have ended up behind bars.” Because of the criminalization of mental illness, in 44 states, the number of people with mental illness is higher in a jail or prison than in the largest state psychiatric hospital, according to the Treatment Advocacy Center. We have to be responsible for many of the inmates currently in correctional facilities for committing crimes related to mental health problems. In Maryland, a small state, there are 30,000 inmates in jails, and state and federal prison. The average cost of a meal is $1.36, thus $1.36 x 3 meals x 30,000 inmates = $122,400.00 for food alone for 1 day – this average does not take other expenses into account. By using money and manpower wisely and taking care of individuals’ mental health problems before they commit crimes, better outcomes could be achieved.
I used to work for MedOptions Inc. doing psychiatry consults at nursing homes and assisted-living facilities. Because of the shortage of psychiatrists and nurse practitioners, especially in the suburbs and rural areas, those patients could not be seen in a timely manner even for their 3-month routine follow-ups. As my colleagues and I have written previously, many elderly individuals with major neurocognitive disorders are not on the Food and Drug Administration–approved cognitive enhancers, such as donepezil, galantamine, and memantine.10 Instead, those patients are on benzodiazepines, which are associated with cognitive impairments, and increased risk of pneumonia and falls. Benzodiazepines also can cause and/or worsen disinhibited behavior. Also, in those settings, crisis situations often are addressed days to weeks later because of the doctor shortage. This situation is going to get worse, because this patient population is growing.
Child and geriatric psychiatry shortages
Child and geriatric psychiatrist shortages are even higher than those in general psychiatry.11 Many years of training and low salaries are a few of the reasons some choose not to do a fellowship. These residency graduates would rather join a practice at an attending salary than at a fellow’s salary, which requires an additional 1 to 2 years of training. Student loans of $100,000–$500,000 after residency also discourage some from pursuing fellowship opportunities. We need to consider models such as 2 years of residency with 2 years of a child psychiatry fellowship or 3 years of residency with 1 year of geriatric psychiatry fellowship. Working as an adult attending physician (50% of the time) and concurrently doing a fellowship (50% of the time) while receiving an attending salary might motivate more people to complete a fellowship.
In specialties such as radiology, international medical graduates (IMGs) who have completed residency training in radiology in other countries can complete a radiology fellowship in a particular area for several years and can practice in the United States as board-eligible certified MDs. Likewise, in line with the model proposed here, we could provide unmatched graduates who have no residency training with 3 to 4 years of child psychiatry and geriatric psychiatry training in addition to some adult psychiatry training.
Implementation of such a model might take care of the shortage of child and geriatric psychiatrists. In 2015, there were 56 geriatric psychiatry fellowship programs; 54 positions were filled, and 51 fellows completed training.12 “It appears that a reasonable percentage of IMGs who obtain a fellowship in geriatric psychiatry do not have an intent of pursuing a career in the field,” Marc H. Zisselman, MD, former geriatric psychiatry fellowship director and currently with the Einstein Medical Center in Philadelphia, told me in 2016. These numbers are not at all sufficient to take care of the nation’s unmet need. Hence, implementing alternate strategies is imperative.
Administrative tasks and care
What consumes a psychiatrist’s time and leads to burnout? The answer has to do with administrative tasks at work. Administrative tasks are not an effective use of time for an MD who has spent more than a decade in medical school, residency, and fellowship training. Although electronic medical record (EMR) systems are considered a major advancement, engaging in the process throughout the day is associated with exhaustion.
Many physicians feel that EMRs have slowed them down, and some are not well-equipped to use them in quick and efficient ways. EMRs also have led to physicians making minimal eye contact in interviews with patients. Patients often complain: “I am talking, and the doctor is looking at the computer and typing.” Patients consider this behavior to be unprofessional and rude. In a survey of 57 U.S. physicians in family medicine, internal medicine, cardiology, and orthopedics, results showed that during the work day, 27% of their time was spent on direct clinical face time with patients and 49.2% was spent on EMR and desk work. While in the examination room with patients, physicians spent 52.9% of their time on direct clinical face time and 37.0% on EMR and desk work. Outside office hours, physicians spend up to 2 hours of personal time each night doing additional computer and other clerical work.13
Several EMR software systems, such as CareLogic, Cerner, Epic,NextGen, PointClickCare, and Sunrise, are used in the United States. The U.S. Veterans Affairs Medical Centers (VAMCs) use the computerized patient record system (CPRS) across the country. VA clinicians find CPRS extremely useful when they move from one VAMC to another. Likewise, hospitals and universities may use one software system such as the CPRS and thus, when clinicians change jobs, they find it hard to adapt to the new system.
Because psychiatrists are wasting a lot of time doing administrative tasks, they might be unable to do a good job with regard to making the right diagnoses and prescribing the best treatments.When I ask patients what are they diagnosed with, they tell me: “It depends on who you ask,” or “I’ve been diagnosed with everything.” This shows that we are not doing a good job or something is not right.
Currently, psychiatrists do not have the time and/or interest to make the right diagnoses and provide adequate psychoeducation for their patients. This also could be attributable to a variety of factors, including, but not limited to, time constraints, cynicism, and apathy. Time constraints also lead to the gross underutilization14 of relapse prevention strategies such as long-acting injectables and medications that can prevent suicide, such as lithium and clozapine.15
Other factors that undermine good care include not participating in continuing medical education (CME) and not staying up to date with the literature. For example, haloperidol continues to be one of the most frequently prescribed (probably, the most common) antipsychotic, although it is clearly neurotoxic16,17 and other safer options are available.18 Board certification and maintenance of certification (MOC) are not synonymous with good clinical practice. Many physicians are finding it hard to complete daily documentation, let alone time for MOC. For a variety of reasons, many are not maintaining certification, and this number is likely to increase. Think about how much time is devoted to the one-to-one interview with the patient and direct patient care during the 15-minute medical check appointment and the hour-long new evaluation. In some clinics, psychiatrists are asked to see more than 25 patients in 4 hours. Some U.S.-based psychiatrists see 65 inpatients and initiate 10 new evaluations in a single day. Under those kinds of time constraints, how can we provide quality care?
A model that would address the shortage
Overall, 7,826 PGY-1 applicants were unmatched in 2019, according to data from the 2019 Main Residency Match.19 Psychiatry residency programs could give these unmatched graduates 6 months of training (arbitrary duration) in psychiatry, which is not at all difficult with the program modules that are available.20 We could use them as physician associates as a major contributor to our workforce to complete administrative and other clinical tasks.
Administrative tasks are not necessarily negative, as all psychiatrists have done administrative tasks as medical students, residents, and fellows. However, at this point, administrative tasks are not an effective use of a psychiatrist’s time. Those physician associates could be paired with two to three psychiatrists to do administrative tasks (for making daytime and overnight phone calls; handling prescriptions, prior authorizations, and medication orders, especially over-the-counter and comfort medications in the inpatient units; doing chart reviews; ordering and checking laboratory tests; collecting collateral information from previous clinicians and records; printing medication education pamphlets; faxing; corresponding with insurance companies/utilization review; performing documentation; billing; and taking care of other clinical and administrative paperwork).
In addition, physician associates could collect information using rating scales such as the 9-item Patient Health Questionnaire for measurement-based care21 and Geriatric Depression Scale, both of which are currently not used in psychiatric practice because of time constraints and lack of manpower. Keep in mind that these individuals are medical doctors and could do a good job with these kinds of tasks. Most of them already have clinical experience in the United States and know the health care system. These MDs could conduct an initial interview (what medical students, residents, and fellows do) and present to the attending psychiatrist. Psychiatrists could then focus on the follow-up interview; diagnoses and treatment; major medical decision making, including shared decision making (patients feel that they are not part of the treatment plan); and seeing more patients, which is a more effective use of their time. This training would give these physician associates a chance to work as doctors and make a living. These MDs have completed medical school training after passing Medical College Admission Test – equivalent exams in their countries. They have passed all steps of the U.S. Medical Licensing Examination and have received Educational Commission for Foreign Medical Graduates certification. Some have even completed residency programs in their home countries.
Some U.S. states already have implemented these kinds of programs. In Arkansas, Kansas, and Missouri,22,23 legislators have passed laws allowing unmatched graduates who have not completed a residency program to work in medically underserved areas with a collaborating physician. These physicians must directly supervise the new doctors for at least a month before they can see patients on their own. Another proposal that has been suggested to address the psychiatrist shortage is employing physician assistants to provide care.24-26
The model proposed here is comparable to postdoctoral fellow-principal investigator and resident-attending collaborative work. At hospitals, a certified nurse assistant helps patients with health care needs under the supervision of a nurse. Similarly, a physician associate could help a psychiatrist under his or her supervision. In the Sheppard Pratt Health System in Baltimore, where I worked previously, for example, nurses dictate and prepare discharge summaries for the attending physician with whom they work. These are the kinds of tasks that physician associates could do as well.
The wait time to get a new evaluation with a psychiatrist is enormous. The policy is that a new patient admitted to an inpatient unit must be seen within 24 hours. With this model, the physician associates could see patients within a few hours, take care of their most immediate needs, take a history and conduct a physical, and write an admission note for the attending psychiatrist to sign. Currently, the outpatient practice is so busy that psychiatrists do not have the time to read the discharge summaries of patients referred to them after a recent hospitalization, which often leads to poor aftercare. The physician associates could read the discharge summaries and provide pertinent information to the attending psychiatrists.
In the inpatient units and emergency departments, nurses and social workers see patients before the attending physician, present patient information to the attending psychiatrist, and document their findings. It is redundant for the physician to write the same narrative again. Rather, the physician could add an addendum to the nurse’s or social worker’s notes and sign off. This would save a lot of time.
Numerous well-designed studies support the adoption of collaborative care models as one means of providing quality psychiatric care to larger populations.27,28 The American Psychiatric Association (APA) is currently training 3,500 psychiatrists in collaborative care through the Centers for Medicare and Medicaid Services’ Transforming Clinical Practice Initiative.29,30 Despite this training and the services provided by the nurse practitioners and physician assistants, the shortage of psychiatrists has not been adequately addressed. Hence, we need to think outside the box to find other potential pragmatic solutions.
Simply increasing the hours of work or the number of nurse practitioners or physician assistants already in practice is not going to solve the problem completely. The model proposed here and previously31 is likely to improve the quality of care that we now provide. This model should not be seen as exploiting these unmatched medical graduates and setting up a two-tiered health care system. The salary for these physicians would be a small percentage (5%-10%; these are arbitrary percentages) from the reimbursement of the attending psychiatrist. This model would not affect the salary of the attending psychiatrists; with this model, they would be able to see 25%-50% more patients (again, arbitrary percentages) with the help and support from these physician associates.
Potential barriers to implementation
There could be inherent barriers and complications to implementation of this model that are difficult to foresee at this point. Nurse practitioners (222,000 plus) and physician assistants (83,000 plus) have a fixed and structured curriculum, have national examining boards and national organizations with recertification requirements, and are licensed as independent practitioners, at least as far as CME is concerned.
Physician associates would need a standardized curriculum and examinations to validate what they have studied and learned. This process might be an important part of the credentialing of these individuals, as well as evaluation of cultural competency. If this model is to successfully lead to formation of a specific clinical group, it might need its own specific identity, national organization, national standards of competency, national certification and recertification processes, and national conference and CME or at least a subsection in a national behavioral and medical health organization, such as the APA or the American Academy of Child and Adolescent Psychiatry.
It would be desirable to “field test” the physician associate concept to clarify implementation difficulties, including the ones described above, that could arise. The cost of implementation of this program should not be of much concern; the 6-month training could be on a volunteer basis, or a small stipend might be paid by graduate medical education funding. This model could prove to be rewarding long term, save trillions of health care dollars, and allow us to provide exceptional and timely care.
Conclusion
The 2020 Mental Health America annual State of Mental Health in America report found that more than 70% of youth with severe major depressive disorder were in need of treatment in 2017. The percentage of adults with any mental illness who did not receive treatment stood at about 57.2%.32 Meanwhile, from 1999 through 2014, the age-adjusted suicide rate in the United States increased 24%.33 More individuals are seeking help because of increased awareness.34,35 In light of the access to services afforded by the ACA, physician associates might ease the workload of psychiatrists and enable them to deliver better care to more people. We would not necessarily have to use the term “physician associate” and could generate better terminologies later. In short, let’s tap into the pools of unmatched graduates and shrinking shrinks! If this model is successful, it could be used in other specialties and countries. The stakes for our patients have never been higher.
References
1. Bishop TF et al. Health Aff. 2016;35(7):1271-7.
2. National Council Medical Director Institute. The psychiatric shortage: Causes and solutions. 2017. Washington: National Council for Behavioral Health.
3. Satiani A et al. Psychiatric Serv. 2018;69:710-3.
4. Carlat D. Psychiatric Times. 2010 Aug 3;27(8).
5. McCartney M. BMJ. 2017;359:j5022.
6. Maslach C and Leiter MP. World Psychiatry. 2016 Jun 5;15:103-11.
7. Merritt Hawkins. “The silent shortage: A white paper examining supply, demand and recruitment trends in psychiatry.” 2018.
8. Sederer LI and Sharfstein SS. JAMA. 2014 Sep 24;312:1195-6.
9. James DJ and Glaze LE. Mental health problems of prison and jail inmates. 2006 Sep. U.S. Justice Department, Bureau of Justice Statistics Special Report.
10. Koola MM et al. J Geriatr Care Res. 2018;5(2):57-67.
11. Buckley PF and Nasrallah HA. Curr Psychiatr. 2016;15:23-4.
12. American Medical Association Database. Open Residency and Fellowship Positions.
13. Sinsky C et al. Ann Intern Med. 2016;165:753-60.
14. Koola MM. Curr Psychiatr. 2017 Mar. 16(3):19-20,47,e1.
15. Koola MM and Sebastian J. HSOA J Psychiatry Depress Anxiety. 2016;(2):1-11.
16. Nasrallah HA and Chen AT. Ann Clin Psychiatry. 2017 Aug;29(3):195-202.
17. Nasrallah HA. Curr Psychiatr. 2013 Jul;7-8.
18. Chen AT and Nasrallah HA. Schizophr Res. 2019 Jun;208:1-7.
19. National Resident Matching Program, Results and Data: 2019 Main Residency Match. National Resident Matching Program, Washington, 2019.
20. Masters KJ. J Physician Assist Educ. 2015 Sep;26(3):136-43.
21. Koola MM et al. J Nerv Ment Dis. 2011;199(12):989-90.
22. “New Missouri licensing offers ‘Band-Aid’ for physician shortages.” Kansas City Business Journal. Updated 2017 May 16.
23. “After earning an MD, she’s headed back to school – to become a nurse.” STAT. 2016 Nov 8.
24. Keizer TB and Trangle MA. Acad Psychiatry. 2015 Dec;39(6):691-4.
25. Miller JG and Peterson DJ. Acad Psychiatry. 2015 Dec;39(6):685-6.
26. Smith MS. Curr Psychiatr. 2019 Sep;18(9):17-24.
27. Osofsky HJ et al. Acad Psychiatry. 2016 Oct;40(5):747-54.
28. Dreier-Wolfgramm A et al. Z Gerontol Geriatr. 2017 May;50(Suppl 2):68-77.
29. Huang H and Barkil-Oteo A. Psychosomatics. 2015 Nov-Dec;56(6):658-61.
30. Raney L et al. Fam Syst Health. 2014 Jun;32(2):147-8.
31. Koola MM. Curr Psychiatr. 2016 Dec. 15(12):33-4.
32. Mental Health America. State of Mental Health in America 2020.
33. Curtin SC et al. NCHS Data Brief. 2016 Apr;(241):1-8.
34. Kelly DL et al. Ann Intern Med. 2020;172(2):167-8.
35. Miller JP and Nasrallah HA. Curr Psychiatr. 2015;14(12):45-6.
Dr. Koola is an associate professor in the department of psychiatry and behavioral health at Stony Brook (N.Y.) University. His main area of interest is novel therapeutic discovery in the treatment of schizophrenia. He has a particular interest in improving the health care delivery system for people with psychiatric illness. Dr. Koola declared no conflicts of interest. He can be reached at [email protected].
*This commentary was updated 2/2/2020.
For many years now, we have been lamenting the shortage of psychiatrists practicing in the United States. At this point, we must identify possible solutions.1,2 Currently, the shortage of practicing psychiatrists in the United States could be as high as 45,000.3 The major problem is that the number of psychiatry residency positions will not increase in the foreseeable future, thus generating more psychiatrists is not an option.
Medicare pays about $150,000 per residency slot per year. To solve the mental health access problem, $27 billion (45,000 x $150,000 x 4 years)* would be required from Medicare, which is not feasible.4 The national average starting salary for psychiatrists from 2018-2019 was about $273,000 (much lower in academic institutions), according to Merritt Hawkins, the physician recruiting firm. That salary is modest, compared with those offered in other medical specialties. For this reason, many graduates choose other lucrative specialties. And we know that increasing the salaries of psychiatrists alone would not lead more people to choose psychiatry. On paper, it may say they work a 40-hour week, but they end up working 60 hours a week.
To make matters worse, family medicine and internal medicine doctors generally would rather not deal with people with mental illness and do “cherry-picking and lemon-dropping.” While many patients present to primary care with mental health issues, lack of time and education in psychiatric disorders and treatment hinder these physicians. In short, the mental health field cannot count on primary care physicians.
Meanwhile, there are thousands of unmatched residency graduates. In light of those realities, perhaps psychiatry residency programs could provide these unmatched graduates with 6 months of training and use them to supplement the workforce. These medical doctors, or “physician associates,” could be paired with a few psychiatrists to do clinical and administrative work. With one in four individuals having mental health issues, and more and more people seeking help because of increasing awareness and the benefits that accompanied the Affordable Care Act (ACA), physician associates might ease the workload of psychiatrists so that they can deliver better care to more people. We must take advantage of these two trends: The surge in unmatched graduates and “shrinking shrinks,” or the decline in the psychiatric workforce pool. (The Royal College of Physicians has established a category of clinicians called physician associates,5 but they are comparable to physician assistants in the United States. As you will see, the construct I am proposing is different.)
The current landscape
Currently, psychiatrists are under a lot of pressure to see a certain number of patients. Patients consistently complain that psychiatrists spend a maximum of 15 minutes with them, that the visits are interrupted by phone calls, and that they are not being heard and helped. Burnout, a silent epidemic among physicians, is relatively prevalent in psychiatry.6 Hence, some psychiatrists are reducing their hours and retiring early. Psychiatry has the third-oldest workforce, with 59% of current psychiatrists aged 55 years or older.7 A better pay/work ratio and work/life balance would enable psychiatrists to enjoy more fulfilling careers.
Many psychiatrists are spending a lot of their time in research, administration, and the classroom. In addition to those issues, the United States currently has a broken mental health care system.8 Finally, the medical practice landscape has changed dramatically in recent years, and those changes undermine both the effectiveness and well-being of clinicians.
The historical landscape
Some people proudly refer to the deinstitutionalization of mental asylums and state mental hospitals in the United States. But where have these patients gone? According to a U.S. Justice Department report, 2,220,300 adults were incarcerated in U.S. federal and state prisons and county jails in 2013.9 In addition, 4,751,400 adults in 2013 were on probation or parole. The percentages of inmates in state and federal prisons and local jails with a psychiatric diagnosis were 56%, 45%, and 64%, respectively.
I work at the Maryland correctional institutions, part of the Maryland Department of Public Safety and Correctional Services. One thing that I consistently hear from several correctional officers is “had these inmates received timely help and care, they wouldn’t have ended up behind bars.” Because of the criminalization of mental illness, in 44 states, the number of people with mental illness is higher in a jail or prison than in the largest state psychiatric hospital, according to the Treatment Advocacy Center. We have to be responsible for many of the inmates currently in correctional facilities for committing crimes related to mental health problems. In Maryland, a small state, there are 30,000 inmates in jails, and state and federal prison. The average cost of a meal is $1.36, thus $1.36 x 3 meals x 30,000 inmates = $122,400.00 for food alone for 1 day – this average does not take other expenses into account. By using money and manpower wisely and taking care of individuals’ mental health problems before they commit crimes, better outcomes could be achieved.
I used to work for MedOptions Inc. doing psychiatry consults at nursing homes and assisted-living facilities. Because of the shortage of psychiatrists and nurse practitioners, especially in the suburbs and rural areas, those patients could not be seen in a timely manner even for their 3-month routine follow-ups. As my colleagues and I have written previously, many elderly individuals with major neurocognitive disorders are not on the Food and Drug Administration–approved cognitive enhancers, such as donepezil, galantamine, and memantine.10 Instead, those patients are on benzodiazepines, which are associated with cognitive impairments, and increased risk of pneumonia and falls. Benzodiazepines also can cause and/or worsen disinhibited behavior. Also, in those settings, crisis situations often are addressed days to weeks later because of the doctor shortage. This situation is going to get worse, because this patient population is growing.
Child and geriatric psychiatry shortages
Child and geriatric psychiatrist shortages are even higher than those in general psychiatry.11 Many years of training and low salaries are a few of the reasons some choose not to do a fellowship. These residency graduates would rather join a practice at an attending salary than at a fellow’s salary, which requires an additional 1 to 2 years of training. Student loans of $100,000–$500,000 after residency also discourage some from pursuing fellowship opportunities. We need to consider models such as 2 years of residency with 2 years of a child psychiatry fellowship or 3 years of residency with 1 year of geriatric psychiatry fellowship. Working as an adult attending physician (50% of the time) and concurrently doing a fellowship (50% of the time) while receiving an attending salary might motivate more people to complete a fellowship.
In specialties such as radiology, international medical graduates (IMGs) who have completed residency training in radiology in other countries can complete a radiology fellowship in a particular area for several years and can practice in the United States as board-eligible certified MDs. Likewise, in line with the model proposed here, we could provide unmatched graduates who have no residency training with 3 to 4 years of child psychiatry and geriatric psychiatry training in addition to some adult psychiatry training.
Implementation of such a model might take care of the shortage of child and geriatric psychiatrists. In 2015, there were 56 geriatric psychiatry fellowship programs; 54 positions were filled, and 51 fellows completed training.12 “It appears that a reasonable percentage of IMGs who obtain a fellowship in geriatric psychiatry do not have an intent of pursuing a career in the field,” Marc H. Zisselman, MD, former geriatric psychiatry fellowship director and currently with the Einstein Medical Center in Philadelphia, told me in 2016. These numbers are not at all sufficient to take care of the nation’s unmet need. Hence, implementing alternate strategies is imperative.
Administrative tasks and care
What consumes a psychiatrist’s time and leads to burnout? The answer has to do with administrative tasks at work. Administrative tasks are not an effective use of time for an MD who has spent more than a decade in medical school, residency, and fellowship training. Although electronic medical record (EMR) systems are considered a major advancement, engaging in the process throughout the day is associated with exhaustion.
Many physicians feel that EMRs have slowed them down, and some are not well-equipped to use them in quick and efficient ways. EMRs also have led to physicians making minimal eye contact in interviews with patients. Patients often complain: “I am talking, and the doctor is looking at the computer and typing.” Patients consider this behavior to be unprofessional and rude. In a survey of 57 U.S. physicians in family medicine, internal medicine, cardiology, and orthopedics, results showed that during the work day, 27% of their time was spent on direct clinical face time with patients and 49.2% was spent on EMR and desk work. While in the examination room with patients, physicians spent 52.9% of their time on direct clinical face time and 37.0% on EMR and desk work. Outside office hours, physicians spend up to 2 hours of personal time each night doing additional computer and other clerical work.13
Several EMR software systems, such as CareLogic, Cerner, Epic,NextGen, PointClickCare, and Sunrise, are used in the United States. The U.S. Veterans Affairs Medical Centers (VAMCs) use the computerized patient record system (CPRS) across the country. VA clinicians find CPRS extremely useful when they move from one VAMC to another. Likewise, hospitals and universities may use one software system such as the CPRS and thus, when clinicians change jobs, they find it hard to adapt to the new system.
Because psychiatrists are wasting a lot of time doing administrative tasks, they might be unable to do a good job with regard to making the right diagnoses and prescribing the best treatments.When I ask patients what are they diagnosed with, they tell me: “It depends on who you ask,” or “I’ve been diagnosed with everything.” This shows that we are not doing a good job or something is not right.
Currently, psychiatrists do not have the time and/or interest to make the right diagnoses and provide adequate psychoeducation for their patients. This also could be attributable to a variety of factors, including, but not limited to, time constraints, cynicism, and apathy. Time constraints also lead to the gross underutilization14 of relapse prevention strategies such as long-acting injectables and medications that can prevent suicide, such as lithium and clozapine.15
Other factors that undermine good care include not participating in continuing medical education (CME) and not staying up to date with the literature. For example, haloperidol continues to be one of the most frequently prescribed (probably, the most common) antipsychotic, although it is clearly neurotoxic16,17 and other safer options are available.18 Board certification and maintenance of certification (MOC) are not synonymous with good clinical practice. Many physicians are finding it hard to complete daily documentation, let alone time for MOC. For a variety of reasons, many are not maintaining certification, and this number is likely to increase. Think about how much time is devoted to the one-to-one interview with the patient and direct patient care during the 15-minute medical check appointment and the hour-long new evaluation. In some clinics, psychiatrists are asked to see more than 25 patients in 4 hours. Some U.S.-based psychiatrists see 65 inpatients and initiate 10 new evaluations in a single day. Under those kinds of time constraints, how can we provide quality care?
A model that would address the shortage
Overall, 7,826 PGY-1 applicants were unmatched in 2019, according to data from the 2019 Main Residency Match.19 Psychiatry residency programs could give these unmatched graduates 6 months of training (arbitrary duration) in psychiatry, which is not at all difficult with the program modules that are available.20 We could use them as physician associates as a major contributor to our workforce to complete administrative and other clinical tasks.
Administrative tasks are not necessarily negative, as all psychiatrists have done administrative tasks as medical students, residents, and fellows. However, at this point, administrative tasks are not an effective use of a psychiatrist’s time. Those physician associates could be paired with two to three psychiatrists to do administrative tasks (for making daytime and overnight phone calls; handling prescriptions, prior authorizations, and medication orders, especially over-the-counter and comfort medications in the inpatient units; doing chart reviews; ordering and checking laboratory tests; collecting collateral information from previous clinicians and records; printing medication education pamphlets; faxing; corresponding with insurance companies/utilization review; performing documentation; billing; and taking care of other clinical and administrative paperwork).
In addition, physician associates could collect information using rating scales such as the 9-item Patient Health Questionnaire for measurement-based care21 and Geriatric Depression Scale, both of which are currently not used in psychiatric practice because of time constraints and lack of manpower. Keep in mind that these individuals are medical doctors and could do a good job with these kinds of tasks. Most of them already have clinical experience in the United States and know the health care system. These MDs could conduct an initial interview (what medical students, residents, and fellows do) and present to the attending psychiatrist. Psychiatrists could then focus on the follow-up interview; diagnoses and treatment; major medical decision making, including shared decision making (patients feel that they are not part of the treatment plan); and seeing more patients, which is a more effective use of their time. This training would give these physician associates a chance to work as doctors and make a living. These MDs have completed medical school training after passing Medical College Admission Test – equivalent exams in their countries. They have passed all steps of the U.S. Medical Licensing Examination and have received Educational Commission for Foreign Medical Graduates certification. Some have even completed residency programs in their home countries.
Some U.S. states already have implemented these kinds of programs. In Arkansas, Kansas, and Missouri,22,23 legislators have passed laws allowing unmatched graduates who have not completed a residency program to work in medically underserved areas with a collaborating physician. These physicians must directly supervise the new doctors for at least a month before they can see patients on their own. Another proposal that has been suggested to address the psychiatrist shortage is employing physician assistants to provide care.24-26
The model proposed here is comparable to postdoctoral fellow-principal investigator and resident-attending collaborative work. At hospitals, a certified nurse assistant helps patients with health care needs under the supervision of a nurse. Similarly, a physician associate could help a psychiatrist under his or her supervision. In the Sheppard Pratt Health System in Baltimore, where I worked previously, for example, nurses dictate and prepare discharge summaries for the attending physician with whom they work. These are the kinds of tasks that physician associates could do as well.
The wait time to get a new evaluation with a psychiatrist is enormous. The policy is that a new patient admitted to an inpatient unit must be seen within 24 hours. With this model, the physician associates could see patients within a few hours, take care of their most immediate needs, take a history and conduct a physical, and write an admission note for the attending psychiatrist to sign. Currently, the outpatient practice is so busy that psychiatrists do not have the time to read the discharge summaries of patients referred to them after a recent hospitalization, which often leads to poor aftercare. The physician associates could read the discharge summaries and provide pertinent information to the attending psychiatrists.
In the inpatient units and emergency departments, nurses and social workers see patients before the attending physician, present patient information to the attending psychiatrist, and document their findings. It is redundant for the physician to write the same narrative again. Rather, the physician could add an addendum to the nurse’s or social worker’s notes and sign off. This would save a lot of time.
Numerous well-designed studies support the adoption of collaborative care models as one means of providing quality psychiatric care to larger populations.27,28 The American Psychiatric Association (APA) is currently training 3,500 psychiatrists in collaborative care through the Centers for Medicare and Medicaid Services’ Transforming Clinical Practice Initiative.29,30 Despite this training and the services provided by the nurse practitioners and physician assistants, the shortage of psychiatrists has not been adequately addressed. Hence, we need to think outside the box to find other potential pragmatic solutions.
Simply increasing the hours of work or the number of nurse practitioners or physician assistants already in practice is not going to solve the problem completely. The model proposed here and previously31 is likely to improve the quality of care that we now provide. This model should not be seen as exploiting these unmatched medical graduates and setting up a two-tiered health care system. The salary for these physicians would be a small percentage (5%-10%; these are arbitrary percentages) from the reimbursement of the attending psychiatrist. This model would not affect the salary of the attending psychiatrists; with this model, they would be able to see 25%-50% more patients (again, arbitrary percentages) with the help and support from these physician associates.
Potential barriers to implementation
There could be inherent barriers and complications to implementation of this model that are difficult to foresee at this point. Nurse practitioners (222,000 plus) and physician assistants (83,000 plus) have a fixed and structured curriculum, have national examining boards and national organizations with recertification requirements, and are licensed as independent practitioners, at least as far as CME is concerned.
Physician associates would need a standardized curriculum and examinations to validate what they have studied and learned. This process might be an important part of the credentialing of these individuals, as well as evaluation of cultural competency. If this model is to successfully lead to formation of a specific clinical group, it might need its own specific identity, national organization, national standards of competency, national certification and recertification processes, and national conference and CME or at least a subsection in a national behavioral and medical health organization, such as the APA or the American Academy of Child and Adolescent Psychiatry.
It would be desirable to “field test” the physician associate concept to clarify implementation difficulties, including the ones described above, that could arise. The cost of implementation of this program should not be of much concern; the 6-month training could be on a volunteer basis, or a small stipend might be paid by graduate medical education funding. This model could prove to be rewarding long term, save trillions of health care dollars, and allow us to provide exceptional and timely care.
Conclusion
The 2020 Mental Health America annual State of Mental Health in America report found that more than 70% of youth with severe major depressive disorder were in need of treatment in 2017. The percentage of adults with any mental illness who did not receive treatment stood at about 57.2%.32 Meanwhile, from 1999 through 2014, the age-adjusted suicide rate in the United States increased 24%.33 More individuals are seeking help because of increased awareness.34,35 In light of the access to services afforded by the ACA, physician associates might ease the workload of psychiatrists and enable them to deliver better care to more people. We would not necessarily have to use the term “physician associate” and could generate better terminologies later. In short, let’s tap into the pools of unmatched graduates and shrinking shrinks! If this model is successful, it could be used in other specialties and countries. The stakes for our patients have never been higher.
References
1. Bishop TF et al. Health Aff. 2016;35(7):1271-7.
2. National Council Medical Director Institute. The psychiatric shortage: Causes and solutions. 2017. Washington: National Council for Behavioral Health.
3. Satiani A et al. Psychiatric Serv. 2018;69:710-3.
4. Carlat D. Psychiatric Times. 2010 Aug 3;27(8).
5. McCartney M. BMJ. 2017;359:j5022.
6. Maslach C and Leiter MP. World Psychiatry. 2016 Jun 5;15:103-11.
7. Merritt Hawkins. “The silent shortage: A white paper examining supply, demand and recruitment trends in psychiatry.” 2018.
8. Sederer LI and Sharfstein SS. JAMA. 2014 Sep 24;312:1195-6.
9. James DJ and Glaze LE. Mental health problems of prison and jail inmates. 2006 Sep. U.S. Justice Department, Bureau of Justice Statistics Special Report.
10. Koola MM et al. J Geriatr Care Res. 2018;5(2):57-67.
11. Buckley PF and Nasrallah HA. Curr Psychiatr. 2016;15:23-4.
12. American Medical Association Database. Open Residency and Fellowship Positions.
13. Sinsky C et al. Ann Intern Med. 2016;165:753-60.
14. Koola MM. Curr Psychiatr. 2017 Mar. 16(3):19-20,47,e1.
15. Koola MM and Sebastian J. HSOA J Psychiatry Depress Anxiety. 2016;(2):1-11.
16. Nasrallah HA and Chen AT. Ann Clin Psychiatry. 2017 Aug;29(3):195-202.
17. Nasrallah HA. Curr Psychiatr. 2013 Jul;7-8.
18. Chen AT and Nasrallah HA. Schizophr Res. 2019 Jun;208:1-7.
19. National Resident Matching Program, Results and Data: 2019 Main Residency Match. National Resident Matching Program, Washington, 2019.
20. Masters KJ. J Physician Assist Educ. 2015 Sep;26(3):136-43.
21. Koola MM et al. J Nerv Ment Dis. 2011;199(12):989-90.
22. “New Missouri licensing offers ‘Band-Aid’ for physician shortages.” Kansas City Business Journal. Updated 2017 May 16.
23. “After earning an MD, she’s headed back to school – to become a nurse.” STAT. 2016 Nov 8.
24. Keizer TB and Trangle MA. Acad Psychiatry. 2015 Dec;39(6):691-4.
25. Miller JG and Peterson DJ. Acad Psychiatry. 2015 Dec;39(6):685-6.
26. Smith MS. Curr Psychiatr. 2019 Sep;18(9):17-24.
27. Osofsky HJ et al. Acad Psychiatry. 2016 Oct;40(5):747-54.
28. Dreier-Wolfgramm A et al. Z Gerontol Geriatr. 2017 May;50(Suppl 2):68-77.
29. Huang H and Barkil-Oteo A. Psychosomatics. 2015 Nov-Dec;56(6):658-61.
30. Raney L et al. Fam Syst Health. 2014 Jun;32(2):147-8.
31. Koola MM. Curr Psychiatr. 2016 Dec. 15(12):33-4.
32. Mental Health America. State of Mental Health in America 2020.
33. Curtin SC et al. NCHS Data Brief. 2016 Apr;(241):1-8.
34. Kelly DL et al. Ann Intern Med. 2020;172(2):167-8.
35. Miller JP and Nasrallah HA. Curr Psychiatr. 2015;14(12):45-6.
Dr. Koola is an associate professor in the department of psychiatry and behavioral health at Stony Brook (N.Y.) University. His main area of interest is novel therapeutic discovery in the treatment of schizophrenia. He has a particular interest in improving the health care delivery system for people with psychiatric illness. Dr. Koola declared no conflicts of interest. He can be reached at [email protected].
*This commentary was updated 2/2/2020.
ID Blog: Wuhan coronavirus – just a stop on the zoonotic highway
Emerging viruses that spread to humans from an animal host are commonplace and represent some of the deadliest diseases known. Given the details of the Wuhan coronavirus (2019-nCoV) outbreak, including the genetic profile of the disease agent, the hypothesis of a snake origin was the first raised in the peer-reviewed literature.
It is a highly controversial origin story, however, given that mammals have been the sources of all other such zoonotic coronaviruses, as well as a host of other zoonotic diseases.
An animal source for emerging infections such as the 2019-nCoV is the default hypothesis, because “around 60% of all infectious diseases in humans are zoonotic, as are 75% of all emerging infectious diseases,” according to a United Nations report. The report goes on to say that, “on average, one new infectious disease emerges in humans every 4 months.”
To appreciate the emergence and nature of 2019-nCoV, it is important to examine the history of zoonotic outbreaks of other such diseases, especially with regard to the “mixing-vessel” phenomenon, which has been noted in closely related coronaviruses, including SARS and MERS, as well as the widely disparate HIV, Ebola, and influenza viruses.
Mutants in the mixing vessel
The mixing-vessel phenomenon is conceptually easy but molecularly complex. A single animal is coinfected with two related viruses; the virus genomes recombine together (virus “sex”) in that animal to form a new variant of virus. Such new mutant viruses can be more or less infective, more or less deadly, and more or less able to jump the species or even genus barrier. An emerging viral zoonosis can occur when a human being is exposed to one of these new viruses (either from the origin species or another species intermediate) that is capable of also infecting a human cell. Such exposure can occur from close proximity to animal waste or body fluids, as in the farm environment, or from wildlife pets or the capturing and slaughtering of wildlife for food, as is proposed in the case of the Wuhan seafood market scenario. In fact, the scientists who postulated a snake intermediary as the potential mixing vessel also stated that 2019‐nCoV appears to be a recombinant virus between a bat coronavirus and an origin‐unknown coronavirus.
Coronaviruses in particular have a history of moving from animal to human hosts (and even back again), and their detailed genetic pattern and taxonomy can reveal the animal origin of these diseases.
Going batty
Bats, in particular, have been shown to be a reservoir species for both alphacoronaviruses and betacoronaviruses. Given their ecology and behavior, they have been found to play a key role in transmitting coronaviruses between species. A highly pertinent example of this is the SARS coronavirus, which was shown to have likely originated in Chinese horseshoe bats. The SARS virus, which is genetically closely related to the new Wuhan coronavirus, first infected humans in the Guangdong province of southern China in 2002.
Scientists speculate that the virus was then either transmitted directly to humans from bats, or passed through an intermediate host species, with SARS-like viruses isolated from Himalayan palm civets found in a live-animal market in Guangdong. The virus infection was also detected in other animals (including a raccoon dog, Nyctereutes procyonoides) and in humans working at the market.
The MERS coronavirus is a betacoronavirus that was first reported in Saudi Arabia in 2012. It turned out to be far more deadly than either SARS or the Wuhan virus (at least as far as current estimates of the new coronavirus’s behavior). The MERS genotype was found to be closely related to MERS-like viruses in bats in Saudi Arabia, Africa, Europe, and Asia. Studies done on the cell receptor for MERS showed an apparently conserved viral receptor in both bats and humans. And an identical strain of MERS was found in bats in a nearby cave and near the workplace of the first known human patient.
However, in many of the other locations of the outbreak in the Middle East, there appeared to be limited contact between bats and humans, so scientists looked for another vector species, perhaps one that was acting as an intermediate. A high seroprevalence of MERS-CoV or a closely related virus was found in camels across the Arabian Peninsula and parts of eastern and northern Africa, while tests for MERS antibodies were negative in the most-likely other species of livestock or pet animals, including chickens, cows, goats, horses, and sheep.
In addition, the MERS-related CoV carried by camels was genetically highly similar to that detected in humans, as demonstrated in one particular outbreak on a farm in Qatar where the genetic sequences of MERS-CoV in the nasal swabs from 3 of 14 seropositive camels were similar to those of 2 human cases on the same farm. Similar genomic results were found in MERS-CoV from nasal swabs from camels in Saudi Arabia.
Other mixing-vessel zoonoses
HIV, the viral cause of AIDS, provides an almost-textbook origin story of the rise of a zoonotic supervillain. The virus was genetically traced to have a chimpanzee-to-human origin, but it was found to be more complicated than that. The virus first emerged in the 1920s in Africa in what is now the Democratic Republic of the Congo, well before its rise to a global pandemic in the 1980s.
Researchers believe the chimpanzee virus is a hybrid of the simian immunodeficiency viruses (SIVs) naturally infecting two different monkey species: the red-capped mangabey (Cercocebus torquatus) and the greater spot-nosed monkey (Cercopithecus nictitans). Chimpanzees kill and eat monkeys, which is likely how they acquired the monkey viruses. The viruses hybridized in a chimpanzee; the hybrid virus then spread through the chimpanzee population and was later transmitted to humans who captured and slaughtered chimps for meat (becoming exposed to their blood). This was the most likely origin of HIV-1.
HIV-1 also shows one of the major risks of zoonotic infections. They can continue to mutate in its human host, increasing the risk of greater virulence, but also interfering with the production of a universally effective vaccine. Since its transmission to humans, for example, many subtypes of the HIV-1 strain have developed, with genetic differences even in the same subtypes found to be up to 20%.
Ebolavirus, first detected in 1976, is another case of bats being the potential culprit. Genetic analysis has shown that African fruit bats are likely involved in the spread of the virus and may be its reservoir host. Further evidence of this was found in the most recent human-infecting Bombali variant of the virus, which was identified in samples from bats collected from Sierra Leone.
It was also found that pigs can also become infected with Zaire ebolavirus, leading to the fear that pigs could serve as a mixing vessel for it and other filoviruses. Pigs have their own forms of Ebola-like disease viruses, which are not currently transmissible to humans, but could provide a potential mixing-vessel reservoir.
Emergent influenzas
The Western world has been most affected by these highly mutable, multispecies zoonotic viruses. The 1957 and 1968 flu pandemics contained a mixture of gene segments from human and avian influenza viruses. “What is clear from genetic analysis of the viruses that caused these past pandemics is that reassortment (gene swapping) occurred to produce novel influenza viruses that caused the pandemics. In both of these cases, the new viruses that emerged showed major differences from the parent viruses,” according to the Centers for Disease Control and Prevention.
Influenza is, however, a good example that all zoonoses are not the result of a mixing-vessel phenomenon, with evidence showing that the origin of the catastrophic 1918 virus pandemic likely resulted from a bird influenza virus directly infecting humans and pigs at about the same time without reassortment, according to the CDC.
Building a protective infrastructure
The first 2 decades of the 21st century saw a huge increase in efforts to develop an infrastructure to monitor and potentially prevent the spread of new zoonoses. As part of a global effort led by the United Nations, the U.S. Agency for International AID developed the PREDICT program in 2009 “to strengthen global capacity for detection and discovery of zoonotic viruses with pandemic potential. Those include coronaviruses, the family to which SARS and MERS belong; paramyxoviruses, like Nipah virus; influenza viruses; and filoviruses, like the ebolavirus.”
PREDICT funding to the EcoHealth Alliance led to discovery of the likely bat origins of the Zaire ebolavirus during the 2013-2016 outbreak. And throughout the existence of PREDICT, more than 145,000 animals and people were surveyed in areas of likely zoonotic outbreaks, leading to the detection of more than “1,100 unique viruses, including zoonotic diseases of public health concern such as Bombali ebolavirus, Zaire ebolavirus, Marburg virus, and MERS- and SARS-like coronaviruses,” according to PREDICT partner, the University of California, Davis.
PREDICT-2 was launched in 2014 with the continuing goals of “identifying and better characterizing pathogens of known epidemic and unknown pandemic potential; recognizing animal reservoirs and amplification hosts of human-infectious viruses; and efficiently targeting intervention action at human behaviors which amplify disease transmission at critical animal-animal and animal-human interfaces in hotspots of viral evolution, spillover, amplification, and spread.”
However, in October 2019, the Trump administration cut all funding to the PREDICT program, leading to its shutdown. In a New York Times interview, Peter Daszak, president of the EcoHealth Alliance, stated: “PREDICT was an approach to heading off pandemics, instead of sitting there waiting for them to emerge and then mobilizing.”
Ultimately, in addition to its human cost, the current Wuhan coronavirus outbreak can be looked at an object lesson – a test of the pandemic surveillance and control systems currently in place, and a practice run for the next and potentially deadlier zoonotic outbreaks to come. Perhaps it is also a reminder that cutting resources to detect zoonoses at their source in their animal hosts – before they enter the human chain– is perhaps not the most prudent of ideas.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & celluar biology at Georgetown University, Washington.
Emerging viruses that spread to humans from an animal host are commonplace and represent some of the deadliest diseases known. Given the details of the Wuhan coronavirus (2019-nCoV) outbreak, including the genetic profile of the disease agent, the hypothesis of a snake origin was the first raised in the peer-reviewed literature.
It is a highly controversial origin story, however, given that mammals have been the sources of all other such zoonotic coronaviruses, as well as a host of other zoonotic diseases.
An animal source for emerging infections such as the 2019-nCoV is the default hypothesis, because “around 60% of all infectious diseases in humans are zoonotic, as are 75% of all emerging infectious diseases,” according to a United Nations report. The report goes on to say that, “on average, one new infectious disease emerges in humans every 4 months.”
To appreciate the emergence and nature of 2019-nCoV, it is important to examine the history of zoonotic outbreaks of other such diseases, especially with regard to the “mixing-vessel” phenomenon, which has been noted in closely related coronaviruses, including SARS and MERS, as well as the widely disparate HIV, Ebola, and influenza viruses.
Mutants in the mixing vessel
The mixing-vessel phenomenon is conceptually easy but molecularly complex. A single animal is coinfected with two related viruses; the virus genomes recombine together (virus “sex”) in that animal to form a new variant of virus. Such new mutant viruses can be more or less infective, more or less deadly, and more or less able to jump the species or even genus barrier. An emerging viral zoonosis can occur when a human being is exposed to one of these new viruses (either from the origin species or another species intermediate) that is capable of also infecting a human cell. Such exposure can occur from close proximity to animal waste or body fluids, as in the farm environment, or from wildlife pets or the capturing and slaughtering of wildlife for food, as is proposed in the case of the Wuhan seafood market scenario. In fact, the scientists who postulated a snake intermediary as the potential mixing vessel also stated that 2019‐nCoV appears to be a recombinant virus between a bat coronavirus and an origin‐unknown coronavirus.
Coronaviruses in particular have a history of moving from animal to human hosts (and even back again), and their detailed genetic pattern and taxonomy can reveal the animal origin of these diseases.
Going batty
Bats, in particular, have been shown to be a reservoir species for both alphacoronaviruses and betacoronaviruses. Given their ecology and behavior, they have been found to play a key role in transmitting coronaviruses between species. A highly pertinent example of this is the SARS coronavirus, which was shown to have likely originated in Chinese horseshoe bats. The SARS virus, which is genetically closely related to the new Wuhan coronavirus, first infected humans in the Guangdong province of southern China in 2002.
Scientists speculate that the virus was then either transmitted directly to humans from bats, or passed through an intermediate host species, with SARS-like viruses isolated from Himalayan palm civets found in a live-animal market in Guangdong. The virus infection was also detected in other animals (including a raccoon dog, Nyctereutes procyonoides) and in humans working at the market.
The MERS coronavirus is a betacoronavirus that was first reported in Saudi Arabia in 2012. It turned out to be far more deadly than either SARS or the Wuhan virus (at least as far as current estimates of the new coronavirus’s behavior). The MERS genotype was found to be closely related to MERS-like viruses in bats in Saudi Arabia, Africa, Europe, and Asia. Studies done on the cell receptor for MERS showed an apparently conserved viral receptor in both bats and humans. And an identical strain of MERS was found in bats in a nearby cave and near the workplace of the first known human patient.
However, in many of the other locations of the outbreak in the Middle East, there appeared to be limited contact between bats and humans, so scientists looked for another vector species, perhaps one that was acting as an intermediate. A high seroprevalence of MERS-CoV or a closely related virus was found in camels across the Arabian Peninsula and parts of eastern and northern Africa, while tests for MERS antibodies were negative in the most-likely other species of livestock or pet animals, including chickens, cows, goats, horses, and sheep.
In addition, the MERS-related CoV carried by camels was genetically highly similar to that detected in humans, as demonstrated in one particular outbreak on a farm in Qatar where the genetic sequences of MERS-CoV in the nasal swabs from 3 of 14 seropositive camels were similar to those of 2 human cases on the same farm. Similar genomic results were found in MERS-CoV from nasal swabs from camels in Saudi Arabia.
Other mixing-vessel zoonoses
HIV, the viral cause of AIDS, provides an almost-textbook origin story of the rise of a zoonotic supervillain. The virus was genetically traced to have a chimpanzee-to-human origin, but it was found to be more complicated than that. The virus first emerged in the 1920s in Africa in what is now the Democratic Republic of the Congo, well before its rise to a global pandemic in the 1980s.
Researchers believe the chimpanzee virus is a hybrid of the simian immunodeficiency viruses (SIVs) naturally infecting two different monkey species: the red-capped mangabey (Cercocebus torquatus) and the greater spot-nosed monkey (Cercopithecus nictitans). Chimpanzees kill and eat monkeys, which is likely how they acquired the monkey viruses. The viruses hybridized in a chimpanzee; the hybrid virus then spread through the chimpanzee population and was later transmitted to humans who captured and slaughtered chimps for meat (becoming exposed to their blood). This was the most likely origin of HIV-1.
HIV-1 also shows one of the major risks of zoonotic infections. They can continue to mutate in its human host, increasing the risk of greater virulence, but also interfering with the production of a universally effective vaccine. Since its transmission to humans, for example, many subtypes of the HIV-1 strain have developed, with genetic differences even in the same subtypes found to be up to 20%.
Ebolavirus, first detected in 1976, is another case of bats being the potential culprit. Genetic analysis has shown that African fruit bats are likely involved in the spread of the virus and may be its reservoir host. Further evidence of this was found in the most recent human-infecting Bombali variant of the virus, which was identified in samples from bats collected from Sierra Leone.
It was also found that pigs can also become infected with Zaire ebolavirus, leading to the fear that pigs could serve as a mixing vessel for it and other filoviruses. Pigs have their own forms of Ebola-like disease viruses, which are not currently transmissible to humans, but could provide a potential mixing-vessel reservoir.
Emergent influenzas
The Western world has been most affected by these highly mutable, multispecies zoonotic viruses. The 1957 and 1968 flu pandemics contained a mixture of gene segments from human and avian influenza viruses. “What is clear from genetic analysis of the viruses that caused these past pandemics is that reassortment (gene swapping) occurred to produce novel influenza viruses that caused the pandemics. In both of these cases, the new viruses that emerged showed major differences from the parent viruses,” according to the Centers for Disease Control and Prevention.
Influenza is, however, a good example that all zoonoses are not the result of a mixing-vessel phenomenon, with evidence showing that the origin of the catastrophic 1918 virus pandemic likely resulted from a bird influenza virus directly infecting humans and pigs at about the same time without reassortment, according to the CDC.
Building a protective infrastructure
The first 2 decades of the 21st century saw a huge increase in efforts to develop an infrastructure to monitor and potentially prevent the spread of new zoonoses. As part of a global effort led by the United Nations, the U.S. Agency for International AID developed the PREDICT program in 2009 “to strengthen global capacity for detection and discovery of zoonotic viruses with pandemic potential. Those include coronaviruses, the family to which SARS and MERS belong; paramyxoviruses, like Nipah virus; influenza viruses; and filoviruses, like the ebolavirus.”
PREDICT funding to the EcoHealth Alliance led to discovery of the likely bat origins of the Zaire ebolavirus during the 2013-2016 outbreak. And throughout the existence of PREDICT, more than 145,000 animals and people were surveyed in areas of likely zoonotic outbreaks, leading to the detection of more than “1,100 unique viruses, including zoonotic diseases of public health concern such as Bombali ebolavirus, Zaire ebolavirus, Marburg virus, and MERS- and SARS-like coronaviruses,” according to PREDICT partner, the University of California, Davis.
PREDICT-2 was launched in 2014 with the continuing goals of “identifying and better characterizing pathogens of known epidemic and unknown pandemic potential; recognizing animal reservoirs and amplification hosts of human-infectious viruses; and efficiently targeting intervention action at human behaviors which amplify disease transmission at critical animal-animal and animal-human interfaces in hotspots of viral evolution, spillover, amplification, and spread.”
However, in October 2019, the Trump administration cut all funding to the PREDICT program, leading to its shutdown. In a New York Times interview, Peter Daszak, president of the EcoHealth Alliance, stated: “PREDICT was an approach to heading off pandemics, instead of sitting there waiting for them to emerge and then mobilizing.”
Ultimately, in addition to its human cost, the current Wuhan coronavirus outbreak can be looked at an object lesson – a test of the pandemic surveillance and control systems currently in place, and a practice run for the next and potentially deadlier zoonotic outbreaks to come. Perhaps it is also a reminder that cutting resources to detect zoonoses at their source in their animal hosts – before they enter the human chain– is perhaps not the most prudent of ideas.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & celluar biology at Georgetown University, Washington.
Emerging viruses that spread to humans from an animal host are commonplace and represent some of the deadliest diseases known. Given the details of the Wuhan coronavirus (2019-nCoV) outbreak, including the genetic profile of the disease agent, the hypothesis of a snake origin was the first raised in the peer-reviewed literature.
It is a highly controversial origin story, however, given that mammals have been the sources of all other such zoonotic coronaviruses, as well as a host of other zoonotic diseases.
An animal source for emerging infections such as the 2019-nCoV is the default hypothesis, because “around 60% of all infectious diseases in humans are zoonotic, as are 75% of all emerging infectious diseases,” according to a United Nations report. The report goes on to say that, “on average, one new infectious disease emerges in humans every 4 months.”
To appreciate the emergence and nature of 2019-nCoV, it is important to examine the history of zoonotic outbreaks of other such diseases, especially with regard to the “mixing-vessel” phenomenon, which has been noted in closely related coronaviruses, including SARS and MERS, as well as the widely disparate HIV, Ebola, and influenza viruses.
Mutants in the mixing vessel
The mixing-vessel phenomenon is conceptually easy but molecularly complex. A single animal is coinfected with two related viruses; the virus genomes recombine together (virus “sex”) in that animal to form a new variant of virus. Such new mutant viruses can be more or less infective, more or less deadly, and more or less able to jump the species or even genus barrier. An emerging viral zoonosis can occur when a human being is exposed to one of these new viruses (either from the origin species or another species intermediate) that is capable of also infecting a human cell. Such exposure can occur from close proximity to animal waste or body fluids, as in the farm environment, or from wildlife pets or the capturing and slaughtering of wildlife for food, as is proposed in the case of the Wuhan seafood market scenario. In fact, the scientists who postulated a snake intermediary as the potential mixing vessel also stated that 2019‐nCoV appears to be a recombinant virus between a bat coronavirus and an origin‐unknown coronavirus.
Coronaviruses in particular have a history of moving from animal to human hosts (and even back again), and their detailed genetic pattern and taxonomy can reveal the animal origin of these diseases.
Going batty
Bats, in particular, have been shown to be a reservoir species for both alphacoronaviruses and betacoronaviruses. Given their ecology and behavior, they have been found to play a key role in transmitting coronaviruses between species. A highly pertinent example of this is the SARS coronavirus, which was shown to have likely originated in Chinese horseshoe bats. The SARS virus, which is genetically closely related to the new Wuhan coronavirus, first infected humans in the Guangdong province of southern China in 2002.
Scientists speculate that the virus was then either transmitted directly to humans from bats, or passed through an intermediate host species, with SARS-like viruses isolated from Himalayan palm civets found in a live-animal market in Guangdong. The virus infection was also detected in other animals (including a raccoon dog, Nyctereutes procyonoides) and in humans working at the market.
The MERS coronavirus is a betacoronavirus that was first reported in Saudi Arabia in 2012. It turned out to be far more deadly than either SARS or the Wuhan virus (at least as far as current estimates of the new coronavirus’s behavior). The MERS genotype was found to be closely related to MERS-like viruses in bats in Saudi Arabia, Africa, Europe, and Asia. Studies done on the cell receptor for MERS showed an apparently conserved viral receptor in both bats and humans. And an identical strain of MERS was found in bats in a nearby cave and near the workplace of the first known human patient.
However, in many of the other locations of the outbreak in the Middle East, there appeared to be limited contact between bats and humans, so scientists looked for another vector species, perhaps one that was acting as an intermediate. A high seroprevalence of MERS-CoV or a closely related virus was found in camels across the Arabian Peninsula and parts of eastern and northern Africa, while tests for MERS antibodies were negative in the most-likely other species of livestock or pet animals, including chickens, cows, goats, horses, and sheep.
In addition, the MERS-related CoV carried by camels was genetically highly similar to that detected in humans, as demonstrated in one particular outbreak on a farm in Qatar where the genetic sequences of MERS-CoV in the nasal swabs from 3 of 14 seropositive camels were similar to those of 2 human cases on the same farm. Similar genomic results were found in MERS-CoV from nasal swabs from camels in Saudi Arabia.
Other mixing-vessel zoonoses
HIV, the viral cause of AIDS, provides an almost-textbook origin story of the rise of a zoonotic supervillain. The virus was genetically traced to have a chimpanzee-to-human origin, but it was found to be more complicated than that. The virus first emerged in the 1920s in Africa in what is now the Democratic Republic of the Congo, well before its rise to a global pandemic in the 1980s.
Researchers believe the chimpanzee virus is a hybrid of the simian immunodeficiency viruses (SIVs) naturally infecting two different monkey species: the red-capped mangabey (Cercocebus torquatus) and the greater spot-nosed monkey (Cercopithecus nictitans). Chimpanzees kill and eat monkeys, which is likely how they acquired the monkey viruses. The viruses hybridized in a chimpanzee; the hybrid virus then spread through the chimpanzee population and was later transmitted to humans who captured and slaughtered chimps for meat (becoming exposed to their blood). This was the most likely origin of HIV-1.
HIV-1 also shows one of the major risks of zoonotic infections. They can continue to mutate in its human host, increasing the risk of greater virulence, but also interfering with the production of a universally effective vaccine. Since its transmission to humans, for example, many subtypes of the HIV-1 strain have developed, with genetic differences even in the same subtypes found to be up to 20%.
Ebolavirus, first detected in 1976, is another case of bats being the potential culprit. Genetic analysis has shown that African fruit bats are likely involved in the spread of the virus and may be its reservoir host. Further evidence of this was found in the most recent human-infecting Bombali variant of the virus, which was identified in samples from bats collected from Sierra Leone.
It was also found that pigs can also become infected with Zaire ebolavirus, leading to the fear that pigs could serve as a mixing vessel for it and other filoviruses. Pigs have their own forms of Ebola-like disease viruses, which are not currently transmissible to humans, but could provide a potential mixing-vessel reservoir.
Emergent influenzas
The Western world has been most affected by these highly mutable, multispecies zoonotic viruses. The 1957 and 1968 flu pandemics contained a mixture of gene segments from human and avian influenza viruses. “What is clear from genetic analysis of the viruses that caused these past pandemics is that reassortment (gene swapping) occurred to produce novel influenza viruses that caused the pandemics. In both of these cases, the new viruses that emerged showed major differences from the parent viruses,” according to the Centers for Disease Control and Prevention.
Influenza is, however, a good example that all zoonoses are not the result of a mixing-vessel phenomenon, with evidence showing that the origin of the catastrophic 1918 virus pandemic likely resulted from a bird influenza virus directly infecting humans and pigs at about the same time without reassortment, according to the CDC.
Building a protective infrastructure
The first 2 decades of the 21st century saw a huge increase in efforts to develop an infrastructure to monitor and potentially prevent the spread of new zoonoses. As part of a global effort led by the United Nations, the U.S. Agency for International AID developed the PREDICT program in 2009 “to strengthen global capacity for detection and discovery of zoonotic viruses with pandemic potential. Those include coronaviruses, the family to which SARS and MERS belong; paramyxoviruses, like Nipah virus; influenza viruses; and filoviruses, like the ebolavirus.”
PREDICT funding to the EcoHealth Alliance led to discovery of the likely bat origins of the Zaire ebolavirus during the 2013-2016 outbreak. And throughout the existence of PREDICT, more than 145,000 animals and people were surveyed in areas of likely zoonotic outbreaks, leading to the detection of more than “1,100 unique viruses, including zoonotic diseases of public health concern such as Bombali ebolavirus, Zaire ebolavirus, Marburg virus, and MERS- and SARS-like coronaviruses,” according to PREDICT partner, the University of California, Davis.
PREDICT-2 was launched in 2014 with the continuing goals of “identifying and better characterizing pathogens of known epidemic and unknown pandemic potential; recognizing animal reservoirs and amplification hosts of human-infectious viruses; and efficiently targeting intervention action at human behaviors which amplify disease transmission at critical animal-animal and animal-human interfaces in hotspots of viral evolution, spillover, amplification, and spread.”
However, in October 2019, the Trump administration cut all funding to the PREDICT program, leading to its shutdown. In a New York Times interview, Peter Daszak, president of the EcoHealth Alliance, stated: “PREDICT was an approach to heading off pandemics, instead of sitting there waiting for them to emerge and then mobilizing.”
Ultimately, in addition to its human cost, the current Wuhan coronavirus outbreak can be looked at an object lesson – a test of the pandemic surveillance and control systems currently in place, and a practice run for the next and potentially deadlier zoonotic outbreaks to come. Perhaps it is also a reminder that cutting resources to detect zoonoses at their source in their animal hosts – before they enter the human chain– is perhaps not the most prudent of ideas.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & celluar biology at Georgetown University, Washington.
Performing gender-reaffirming surgery: Guidelines for the general ob.gyn.
According to the DSM-V, gender dysphoria in adolescents and adults “involves a difference between one’s experienced/expressed gender and assigned gender, and significant distress or problems functioning. It lasts at least 6 months,” and several other criteria must be met.1 Many patients with gender dysphoria also identify as transgender. A “transition” or “transitioning” is a process by which individuals come to inhabit their gender identity.2 A gender transition may take many forms, and only some people will choose to include medical assistance in their transition process. Although the scope of this article will not address these concerns, it should be noted that many people in the transgender and gender nonconforming community would object to the concepts of gender dysphoria and gender transition because they rely on a binary model of gender that may exclude individuals that see themselves as something other than “man or woman.”
There are both medical and surgical options for medical assistance in a gender transition. This article will focus on the surgical care of patients assigned female at birth who are seeking masculinizing surgical therapy. Many writers will discuss “gender-affirming” surgery, but we will use the term “gender-reaffirming” surgery because transgender patients have already affirmed their own genders and do not require surgery to inhabit this affirmation. Surgical options might include bilateral mastectomy, hysterectomy, bilateral salpingo-oophorectomy (BSO), metoidioplasty (surgical formation of a neophallus with existing genital tissue), or phalloplasty. There currently is no single surgical subspecialty that encompasses training in all forms of gender-reaffirming surgical therapies. In some areas of the country, centers of excellence have given rise to multidisciplinary teams that combine the skill sets of surgical subspecialists to provide a streamlined approach to gender-reaffirming surgery. Because of the scarcity of these integrated centers, most patients seeking gender-reaffirming surgeries will need to find individual subspecialists whose surgical training focuses on one area of the body. For example, patients seeking all possible surgical options may need a breast surgeon to perform their mastectomy, an ob.gyn. to perform their hysterectomy and BSO, a urologist to perform their metoidioplasty, and a plastic surgeon to perform their phalloplasty. In these scenarios,
There are many reasons why transgender men might desire hysterectomy/BSO as part of their transition. Removal of the uterus and cervix eliminates concerns surrounding menstruation, pregnancy, and cervical cancer screening, all of which may add to their experience of gender dysphoria. Furthermore, removal of the ovaries may simplify long-term hormonal therapy with testosterone by eliminating the need for estrogen suppression. Lastly, a hysterectomy/BSO is a lower-risk and more cost-effective masculinizing surgery, compared with metoidioplasty or phalloplasty.
While the technical aspect of performing a hysterectomy/BSO certainly is within the scope of training for a general ob.gyn., there are several nuances of which providers should be aware when planning gender-reaffirming surgery for a transgender man. During the preoperative planning phase, it is of utmost importance to provide an environment of safety so that the focus of the preop visit is not clouded by communication mishaps between office staff and the patient. These barriers can be avoided by implementing office intake forms that give patients the opportunity to inform the health care team of their chosen name and personal pronouns upon registration for the visit.
A pelvic exam is commonly performed by ob.gyns. to determine surgical approach for a hysterectomy/BSO. When approaching transgender male patients for preoperative pelvic exams, it is important to be mindful of the fact that this type of exam may trigger gender dysphoria. While pelvic exams should be handled in sensitive fashion regardless of a patient’s gender identity, a patient who is a transgender man may benefit from some added steps in discussing the pelvic exam. One approach is to acknowledge that these exams/discussions may be especially triggering of gender dysphoria, and ask if the patient would prefer certain words to be used or not used in reference to their anatomy. As with any patient, the provider should explain the purpose of the examination and offer opportunities for the patient to have some control in the exam such as by assisting with insertion of the speculum or designating a “safe word” that would signal the provider to stop or pause the exam. In some cases, patients may not be able to tolerate the pelvic exam while awake because of the degree of gender dysphoria that the exam would induce. Providers might consider noninvasive imaging studies to help with surgical planning if they find they need more information before scheduling the operation, or they may offer a staged procedure with exam under anesthesia prior to the definitive surgery.
In conclusion, performing a gender-reaffirming hysterectomy/BSO requires thoughtful preparation to ensure a safe surgical environment for this vulnerable population. Care should be taken to plan the operation with a culturally sensitive approach.
Dr. Joyner is an assistant professor at Emory University, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital, both in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner and Dr. Bahng reported no relevant financial disclosures.
References
1. American Psychiatric Association. What is Gender Dysphoria? https://www.psychiatry.org/patients-families/gender-dysphoria/what-is-gender-dysphoria
2. UCSF Transgender Care. Transition Roadmap. https://transcare.ucsf.edu/transition-roadmap
According to the DSM-V, gender dysphoria in adolescents and adults “involves a difference between one’s experienced/expressed gender and assigned gender, and significant distress or problems functioning. It lasts at least 6 months,” and several other criteria must be met.1 Many patients with gender dysphoria also identify as transgender. A “transition” or “transitioning” is a process by which individuals come to inhabit their gender identity.2 A gender transition may take many forms, and only some people will choose to include medical assistance in their transition process. Although the scope of this article will not address these concerns, it should be noted that many people in the transgender and gender nonconforming community would object to the concepts of gender dysphoria and gender transition because they rely on a binary model of gender that may exclude individuals that see themselves as something other than “man or woman.”
There are both medical and surgical options for medical assistance in a gender transition. This article will focus on the surgical care of patients assigned female at birth who are seeking masculinizing surgical therapy. Many writers will discuss “gender-affirming” surgery, but we will use the term “gender-reaffirming” surgery because transgender patients have already affirmed their own genders and do not require surgery to inhabit this affirmation. Surgical options might include bilateral mastectomy, hysterectomy, bilateral salpingo-oophorectomy (BSO), metoidioplasty (surgical formation of a neophallus with existing genital tissue), or phalloplasty. There currently is no single surgical subspecialty that encompasses training in all forms of gender-reaffirming surgical therapies. In some areas of the country, centers of excellence have given rise to multidisciplinary teams that combine the skill sets of surgical subspecialists to provide a streamlined approach to gender-reaffirming surgery. Because of the scarcity of these integrated centers, most patients seeking gender-reaffirming surgeries will need to find individual subspecialists whose surgical training focuses on one area of the body. For example, patients seeking all possible surgical options may need a breast surgeon to perform their mastectomy, an ob.gyn. to perform their hysterectomy and BSO, a urologist to perform their metoidioplasty, and a plastic surgeon to perform their phalloplasty. In these scenarios,
There are many reasons why transgender men might desire hysterectomy/BSO as part of their transition. Removal of the uterus and cervix eliminates concerns surrounding menstruation, pregnancy, and cervical cancer screening, all of which may add to their experience of gender dysphoria. Furthermore, removal of the ovaries may simplify long-term hormonal therapy with testosterone by eliminating the need for estrogen suppression. Lastly, a hysterectomy/BSO is a lower-risk and more cost-effective masculinizing surgery, compared with metoidioplasty or phalloplasty.
While the technical aspect of performing a hysterectomy/BSO certainly is within the scope of training for a general ob.gyn., there are several nuances of which providers should be aware when planning gender-reaffirming surgery for a transgender man. During the preoperative planning phase, it is of utmost importance to provide an environment of safety so that the focus of the preop visit is not clouded by communication mishaps between office staff and the patient. These barriers can be avoided by implementing office intake forms that give patients the opportunity to inform the health care team of their chosen name and personal pronouns upon registration for the visit.
A pelvic exam is commonly performed by ob.gyns. to determine surgical approach for a hysterectomy/BSO. When approaching transgender male patients for preoperative pelvic exams, it is important to be mindful of the fact that this type of exam may trigger gender dysphoria. While pelvic exams should be handled in sensitive fashion regardless of a patient’s gender identity, a patient who is a transgender man may benefit from some added steps in discussing the pelvic exam. One approach is to acknowledge that these exams/discussions may be especially triggering of gender dysphoria, and ask if the patient would prefer certain words to be used or not used in reference to their anatomy. As with any patient, the provider should explain the purpose of the examination and offer opportunities for the patient to have some control in the exam such as by assisting with insertion of the speculum or designating a “safe word” that would signal the provider to stop or pause the exam. In some cases, patients may not be able to tolerate the pelvic exam while awake because of the degree of gender dysphoria that the exam would induce. Providers might consider noninvasive imaging studies to help with surgical planning if they find they need more information before scheduling the operation, or they may offer a staged procedure with exam under anesthesia prior to the definitive surgery.
In conclusion, performing a gender-reaffirming hysterectomy/BSO requires thoughtful preparation to ensure a safe surgical environment for this vulnerable population. Care should be taken to plan the operation with a culturally sensitive approach.
Dr. Joyner is an assistant professor at Emory University, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital, both in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner and Dr. Bahng reported no relevant financial disclosures.
References
1. American Psychiatric Association. What is Gender Dysphoria? https://www.psychiatry.org/patients-families/gender-dysphoria/what-is-gender-dysphoria
2. UCSF Transgender Care. Transition Roadmap. https://transcare.ucsf.edu/transition-roadmap
According to the DSM-V, gender dysphoria in adolescents and adults “involves a difference between one’s experienced/expressed gender and assigned gender, and significant distress or problems functioning. It lasts at least 6 months,” and several other criteria must be met.1 Many patients with gender dysphoria also identify as transgender. A “transition” or “transitioning” is a process by which individuals come to inhabit their gender identity.2 A gender transition may take many forms, and only some people will choose to include medical assistance in their transition process. Although the scope of this article will not address these concerns, it should be noted that many people in the transgender and gender nonconforming community would object to the concepts of gender dysphoria and gender transition because they rely on a binary model of gender that may exclude individuals that see themselves as something other than “man or woman.”
There are both medical and surgical options for medical assistance in a gender transition. This article will focus on the surgical care of patients assigned female at birth who are seeking masculinizing surgical therapy. Many writers will discuss “gender-affirming” surgery, but we will use the term “gender-reaffirming” surgery because transgender patients have already affirmed their own genders and do not require surgery to inhabit this affirmation. Surgical options might include bilateral mastectomy, hysterectomy, bilateral salpingo-oophorectomy (BSO), metoidioplasty (surgical formation of a neophallus with existing genital tissue), or phalloplasty. There currently is no single surgical subspecialty that encompasses training in all forms of gender-reaffirming surgical therapies. In some areas of the country, centers of excellence have given rise to multidisciplinary teams that combine the skill sets of surgical subspecialists to provide a streamlined approach to gender-reaffirming surgery. Because of the scarcity of these integrated centers, most patients seeking gender-reaffirming surgeries will need to find individual subspecialists whose surgical training focuses on one area of the body. For example, patients seeking all possible surgical options may need a breast surgeon to perform their mastectomy, an ob.gyn. to perform their hysterectomy and BSO, a urologist to perform their metoidioplasty, and a plastic surgeon to perform their phalloplasty. In these scenarios,
There are many reasons why transgender men might desire hysterectomy/BSO as part of their transition. Removal of the uterus and cervix eliminates concerns surrounding menstruation, pregnancy, and cervical cancer screening, all of which may add to their experience of gender dysphoria. Furthermore, removal of the ovaries may simplify long-term hormonal therapy with testosterone by eliminating the need for estrogen suppression. Lastly, a hysterectomy/BSO is a lower-risk and more cost-effective masculinizing surgery, compared with metoidioplasty or phalloplasty.
While the technical aspect of performing a hysterectomy/BSO certainly is within the scope of training for a general ob.gyn., there are several nuances of which providers should be aware when planning gender-reaffirming surgery for a transgender man. During the preoperative planning phase, it is of utmost importance to provide an environment of safety so that the focus of the preop visit is not clouded by communication mishaps between office staff and the patient. These barriers can be avoided by implementing office intake forms that give patients the opportunity to inform the health care team of their chosen name and personal pronouns upon registration for the visit.
A pelvic exam is commonly performed by ob.gyns. to determine surgical approach for a hysterectomy/BSO. When approaching transgender male patients for preoperative pelvic exams, it is important to be mindful of the fact that this type of exam may trigger gender dysphoria. While pelvic exams should be handled in sensitive fashion regardless of a patient’s gender identity, a patient who is a transgender man may benefit from some added steps in discussing the pelvic exam. One approach is to acknowledge that these exams/discussions may be especially triggering of gender dysphoria, and ask if the patient would prefer certain words to be used or not used in reference to their anatomy. As with any patient, the provider should explain the purpose of the examination and offer opportunities for the patient to have some control in the exam such as by assisting with insertion of the speculum or designating a “safe word” that would signal the provider to stop or pause the exam. In some cases, patients may not be able to tolerate the pelvic exam while awake because of the degree of gender dysphoria that the exam would induce. Providers might consider noninvasive imaging studies to help with surgical planning if they find they need more information before scheduling the operation, or they may offer a staged procedure with exam under anesthesia prior to the definitive surgery.
In conclusion, performing a gender-reaffirming hysterectomy/BSO requires thoughtful preparation to ensure a safe surgical environment for this vulnerable population. Care should be taken to plan the operation with a culturally sensitive approach.
Dr. Joyner is an assistant professor at Emory University, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital, both in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner and Dr. Bahng reported no relevant financial disclosures.
References
1. American Psychiatric Association. What is Gender Dysphoria? https://www.psychiatry.org/patients-families/gender-dysphoria/what-is-gender-dysphoria
2. UCSF Transgender Care. Transition Roadmap. https://transcare.ucsf.edu/transition-roadmap
Molar pregnancy: The next steps after diagnosis
Molar pregnancy is an uncommon but serious condition that affects young women of reproductive age. The diagnosis and management of molar pregnancy is familiar to most gynecologists. However, in the days and weeks following evacuation of molar pregnancy, clinicians face a critical time period in which they must be vigilant for the development of postmolar gestational trophoblastic neoplasia (GTN). If recognized early and treated appropriately, it almost always can be cured; however, errors or delays in the management of this condition can have catastrophic consequences for patients, including decreasing the likelihood of cure. Here we will review some of the steps and actions that can be taken immediately following the diagnosis of a molar pregnancy to expeditiously identify postmolar GTN and ensure patients are appropriately prepared for further consultation and intervention.
Postmolar GTN includes the diagnoses of invasive mole and choriocarcinoma that contain highly atypical trophoblasts with the capacity for local invasion and metastasis. Typically, the diagnosis is made clinically and not distinguished with histology. While molar pregnancies are a benign condition, invasive moles and choriocarcinoma are malignant conditions in which the molar tissue infiltrates the uterine myometrium, vasculature, and frequently is associated with hematogenous spread with distant metastases. It is a highly chemosensitive disease, and cure with chemotherapy typically is achieved with the ability to preserve fertility if desired even in advanced stage disease.1
After evacuation of a molar pregnancy, gynecologists should be on alert for the development of postmolar GTN if the following known risk factors are present: a history of a prior GTN diagnosis, complete mole on pathology (as opposed to partial mole), serum human chorionic gonadotropin (hCG) levels greater than 100,000 mIU/mL, age greater than 40 years, an enlarged uterus or large ovarian theca lutein cysts, and slow to normalize (more than 2 months) hCG. Symptoms for the development of postmolar GTN include persistent vaginal bleeding after evacuation, a persistently enlarged or enlarging uterine size, and adnexal masses. Ultimately, the diagnosis is made through plateaued or rising serum hCG assessments.2 (See graphic.)
Following the evacuation of a molar pregnancy, hCG levels should be drawn at the same laboratory every 1-2 weeks until normalization and then three consecutive normal values. Once this has been achieved, hCG levels should be tested once at 3 months and again at 6 months. During this 6 month period, patients should use reliable contraception, ideally, and through oral contraceptive pills that suppress the secretion of pituitary hCG if not contraindicated. Should a woman become pregnant during this 6-month surveillance, it becomes impossible to rule out occult postmolar GTN.
Typically after evacuation of a molar pregnancy, there is rapid fall in hCG levels, but this does not occur when the molar pregnancy has become invasive or is associated with choriocarcinoma. In these cases, after an initial drop in hCG levels, there is an observed rise or plateau in levels (as defined in the accompanying table), and this establishes the diagnosis of postmolar GTN. It is common for hCG to fall in fits and starts, rather than have a smooth, consistent diminution, and this can be worrying for gynecologists; however, provided there is a consistent reduction in values in accordance with the stated definitions, observation can continue.
Another source of confusion and concern is an HCG level that fails to completely normalize during observation, yet reaches a very low level. If this is observed, clinicians should consider the diagnosis of quiescent hCG, pituitary hCG, or phantom hCG.3 These can be difficult to distinguish from postmolar GTN, and consultation with a gynecologic oncologist with experience in the diagnosis and management of these rare tumors is helpful to determine if the persistent low levels in hCG require intervention.
Once a clinician has observed a plateau or rise in hCG levels, a gynecologic examination should be performed because the lower genital tract is a common site for metastatic postmolar GTN. If during this evaluation, a suspicious lesion is identified (typically a blue-black, slightly raised, hemorrhagic-appearing lesion), it should not be biopsied, but rather assumed to be a metastatic site. The vasculature of metastatic sites is extremely fragile, and biopsy or disruption can result in catastrophic hemorrhage, even from very small lesions.
In addition to physical examination, several diagnostic studies should be performed which may expedite the triage and management of the case. A pelvic ultrasound should evaluate the endometrial cavity for a new viable pregnancy, and residual molar tissue; sometimes, myometrial invasion consistent with an invasive mole can be appreciated. Chest x-ray or CT scan should be ordered to evaluate for pulmonary metastatic lesions. Additionally, CT scans of the abdomen and pelvis should be ordered, and if lung metastases are present, brain imaging with either MRI or CT scan also should be obtained. These imaging studies will provide the necessary information to stage the GTN (as metastatic or not).
Treatment for postmolar GTN is determined based on further prognostic categorization (“high risk” or “low risk”) in accordance with the WHO classification, which is derived using several prognostic clinical variables including age, antecedent pregnancy, interval from index pregnancy, pretreatment hCG, largest tumor size, sites and number of metastases, and response to previous chemotherapy.4 These assignments are necessary to determine whether single-agent or multiagent chemotherapy should be prescribed.
Laboratory studies are helpful to obtain at this time and include metabolic panels (which can ensure that renal and hepatic function are within normal limits in anticipation of future chemotherapy), and complete blood count ,which can establish viable bone marrow function prior to chemotherapy.
Once postmolar GTN has been diagnosed, it is most appropriate to refer the patient to a gynecologic oncologist with experience in the treatment of these relatively rare malignancies. At that point, the patient will be formally staged, and offered treatment based on these staging results.
Among women with low-risk, nonmetastatic GTN who desire future fertility it is appropriate to offer a repeat dilation and curettage (D&C) procedure rather than immediately proceeding with chemotherapy. Approximately two-thirds of women with low risk disease can avoid chemotherapy with repeat curettage.5 Risk factors for needing chemotherapy after repeat D&C include the presence of trophoblastic disease in the pathology specimen and urinary hCG levels greater than 1,500 mIU/mL at the time of curettage. In my experience, many women appreciate this option to potentially avoid toxic chemotherapy.
For women with low-risk, nonmetastatic postmolar GTN who do not desire future fertility, and hope to avoid chemotherapy, hysterectomy also is a reasonable first option. This can be performed via either minimally invasive, laparotomy, or vaginal route. If performing a minimally invasive procedure in the setting of GTN, there should be caution or avoidance of use of a uterine manipulator because the uterine wall typically is soft and prone to perforation, and bleeding can be significant secondary to disruption of the tumor.
If repeat D&C or hysterectomy are adopted instead of chemotherapy, it is important that patients are very closely monitored post operatively to ensure normalization of their hCG levels (as described above). If it fails to normalize, restaging scans and examinations should be performed, and referral for the appropriate chemotherapy regimen should be initiated without delay.
Postmolar GTN is a serious condition that usually can be cured with chemotherapy or, if appropriate, surgery. and refer to a gynecologic oncologist when criteria are met to ensure that overtreatment is avoided and essential therapy is ensured.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Lancet Oncol. 2007 Aug;8(8):715-24.
2. J Natl Compr Canc Netw. 2019 Nov 1;17(11):1374-91.
3. Gynecol Oncol. 2009 Mar;112(3):663-72.
4. World Health Organ Tech Rep Ser. 1983;692:7-81.
5. Obstet Gynecol. 2016;128(3):535-42.
Molar pregnancy is an uncommon but serious condition that affects young women of reproductive age. The diagnosis and management of molar pregnancy is familiar to most gynecologists. However, in the days and weeks following evacuation of molar pregnancy, clinicians face a critical time period in which they must be vigilant for the development of postmolar gestational trophoblastic neoplasia (GTN). If recognized early and treated appropriately, it almost always can be cured; however, errors or delays in the management of this condition can have catastrophic consequences for patients, including decreasing the likelihood of cure. Here we will review some of the steps and actions that can be taken immediately following the diagnosis of a molar pregnancy to expeditiously identify postmolar GTN and ensure patients are appropriately prepared for further consultation and intervention.
Postmolar GTN includes the diagnoses of invasive mole and choriocarcinoma that contain highly atypical trophoblasts with the capacity for local invasion and metastasis. Typically, the diagnosis is made clinically and not distinguished with histology. While molar pregnancies are a benign condition, invasive moles and choriocarcinoma are malignant conditions in which the molar tissue infiltrates the uterine myometrium, vasculature, and frequently is associated with hematogenous spread with distant metastases. It is a highly chemosensitive disease, and cure with chemotherapy typically is achieved with the ability to preserve fertility if desired even in advanced stage disease.1
After evacuation of a molar pregnancy, gynecologists should be on alert for the development of postmolar GTN if the following known risk factors are present: a history of a prior GTN diagnosis, complete mole on pathology (as opposed to partial mole), serum human chorionic gonadotropin (hCG) levels greater than 100,000 mIU/mL, age greater than 40 years, an enlarged uterus or large ovarian theca lutein cysts, and slow to normalize (more than 2 months) hCG. Symptoms for the development of postmolar GTN include persistent vaginal bleeding after evacuation, a persistently enlarged or enlarging uterine size, and adnexal masses. Ultimately, the diagnosis is made through plateaued or rising serum hCG assessments.2 (See graphic.)
Following the evacuation of a molar pregnancy, hCG levels should be drawn at the same laboratory every 1-2 weeks until normalization and then three consecutive normal values. Once this has been achieved, hCG levels should be tested once at 3 months and again at 6 months. During this 6 month period, patients should use reliable contraception, ideally, and through oral contraceptive pills that suppress the secretion of pituitary hCG if not contraindicated. Should a woman become pregnant during this 6-month surveillance, it becomes impossible to rule out occult postmolar GTN.
Typically after evacuation of a molar pregnancy, there is rapid fall in hCG levels, but this does not occur when the molar pregnancy has become invasive or is associated with choriocarcinoma. In these cases, after an initial drop in hCG levels, there is an observed rise or plateau in levels (as defined in the accompanying table), and this establishes the diagnosis of postmolar GTN. It is common for hCG to fall in fits and starts, rather than have a smooth, consistent diminution, and this can be worrying for gynecologists; however, provided there is a consistent reduction in values in accordance with the stated definitions, observation can continue.
Another source of confusion and concern is an HCG level that fails to completely normalize during observation, yet reaches a very low level. If this is observed, clinicians should consider the diagnosis of quiescent hCG, pituitary hCG, or phantom hCG.3 These can be difficult to distinguish from postmolar GTN, and consultation with a gynecologic oncologist with experience in the diagnosis and management of these rare tumors is helpful to determine if the persistent low levels in hCG require intervention.
Once a clinician has observed a plateau or rise in hCG levels, a gynecologic examination should be performed because the lower genital tract is a common site for metastatic postmolar GTN. If during this evaluation, a suspicious lesion is identified (typically a blue-black, slightly raised, hemorrhagic-appearing lesion), it should not be biopsied, but rather assumed to be a metastatic site. The vasculature of metastatic sites is extremely fragile, and biopsy or disruption can result in catastrophic hemorrhage, even from very small lesions.
In addition to physical examination, several diagnostic studies should be performed which may expedite the triage and management of the case. A pelvic ultrasound should evaluate the endometrial cavity for a new viable pregnancy, and residual molar tissue; sometimes, myometrial invasion consistent with an invasive mole can be appreciated. Chest x-ray or CT scan should be ordered to evaluate for pulmonary metastatic lesions. Additionally, CT scans of the abdomen and pelvis should be ordered, and if lung metastases are present, brain imaging with either MRI or CT scan also should be obtained. These imaging studies will provide the necessary information to stage the GTN (as metastatic or not).
Treatment for postmolar GTN is determined based on further prognostic categorization (“high risk” or “low risk”) in accordance with the WHO classification, which is derived using several prognostic clinical variables including age, antecedent pregnancy, interval from index pregnancy, pretreatment hCG, largest tumor size, sites and number of metastases, and response to previous chemotherapy.4 These assignments are necessary to determine whether single-agent or multiagent chemotherapy should be prescribed.
Laboratory studies are helpful to obtain at this time and include metabolic panels (which can ensure that renal and hepatic function are within normal limits in anticipation of future chemotherapy), and complete blood count ,which can establish viable bone marrow function prior to chemotherapy.
Once postmolar GTN has been diagnosed, it is most appropriate to refer the patient to a gynecologic oncologist with experience in the treatment of these relatively rare malignancies. At that point, the patient will be formally staged, and offered treatment based on these staging results.
Among women with low-risk, nonmetastatic GTN who desire future fertility it is appropriate to offer a repeat dilation and curettage (D&C) procedure rather than immediately proceeding with chemotherapy. Approximately two-thirds of women with low risk disease can avoid chemotherapy with repeat curettage.5 Risk factors for needing chemotherapy after repeat D&C include the presence of trophoblastic disease in the pathology specimen and urinary hCG levels greater than 1,500 mIU/mL at the time of curettage. In my experience, many women appreciate this option to potentially avoid toxic chemotherapy.
For women with low-risk, nonmetastatic postmolar GTN who do not desire future fertility, and hope to avoid chemotherapy, hysterectomy also is a reasonable first option. This can be performed via either minimally invasive, laparotomy, or vaginal route. If performing a minimally invasive procedure in the setting of GTN, there should be caution or avoidance of use of a uterine manipulator because the uterine wall typically is soft and prone to perforation, and bleeding can be significant secondary to disruption of the tumor.
If repeat D&C or hysterectomy are adopted instead of chemotherapy, it is important that patients are very closely monitored post operatively to ensure normalization of their hCG levels (as described above). If it fails to normalize, restaging scans and examinations should be performed, and referral for the appropriate chemotherapy regimen should be initiated without delay.
Postmolar GTN is a serious condition that usually can be cured with chemotherapy or, if appropriate, surgery. and refer to a gynecologic oncologist when criteria are met to ensure that overtreatment is avoided and essential therapy is ensured.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Lancet Oncol. 2007 Aug;8(8):715-24.
2. J Natl Compr Canc Netw. 2019 Nov 1;17(11):1374-91.
3. Gynecol Oncol. 2009 Mar;112(3):663-72.
4. World Health Organ Tech Rep Ser. 1983;692:7-81.
5. Obstet Gynecol. 2016;128(3):535-42.
Molar pregnancy is an uncommon but serious condition that affects young women of reproductive age. The diagnosis and management of molar pregnancy is familiar to most gynecologists. However, in the days and weeks following evacuation of molar pregnancy, clinicians face a critical time period in which they must be vigilant for the development of postmolar gestational trophoblastic neoplasia (GTN). If recognized early and treated appropriately, it almost always can be cured; however, errors or delays in the management of this condition can have catastrophic consequences for patients, including decreasing the likelihood of cure. Here we will review some of the steps and actions that can be taken immediately following the diagnosis of a molar pregnancy to expeditiously identify postmolar GTN and ensure patients are appropriately prepared for further consultation and intervention.
Postmolar GTN includes the diagnoses of invasive mole and choriocarcinoma that contain highly atypical trophoblasts with the capacity for local invasion and metastasis. Typically, the diagnosis is made clinically and not distinguished with histology. While molar pregnancies are a benign condition, invasive moles and choriocarcinoma are malignant conditions in which the molar tissue infiltrates the uterine myometrium, vasculature, and frequently is associated with hematogenous spread with distant metastases. It is a highly chemosensitive disease, and cure with chemotherapy typically is achieved with the ability to preserve fertility if desired even in advanced stage disease.1
After evacuation of a molar pregnancy, gynecologists should be on alert for the development of postmolar GTN if the following known risk factors are present: a history of a prior GTN diagnosis, complete mole on pathology (as opposed to partial mole), serum human chorionic gonadotropin (hCG) levels greater than 100,000 mIU/mL, age greater than 40 years, an enlarged uterus or large ovarian theca lutein cysts, and slow to normalize (more than 2 months) hCG. Symptoms for the development of postmolar GTN include persistent vaginal bleeding after evacuation, a persistently enlarged or enlarging uterine size, and adnexal masses. Ultimately, the diagnosis is made through plateaued or rising serum hCG assessments.2 (See graphic.)
Following the evacuation of a molar pregnancy, hCG levels should be drawn at the same laboratory every 1-2 weeks until normalization and then three consecutive normal values. Once this has been achieved, hCG levels should be tested once at 3 months and again at 6 months. During this 6 month period, patients should use reliable contraception, ideally, and through oral contraceptive pills that suppress the secretion of pituitary hCG if not contraindicated. Should a woman become pregnant during this 6-month surveillance, it becomes impossible to rule out occult postmolar GTN.
Typically after evacuation of a molar pregnancy, there is rapid fall in hCG levels, but this does not occur when the molar pregnancy has become invasive or is associated with choriocarcinoma. In these cases, after an initial drop in hCG levels, there is an observed rise or plateau in levels (as defined in the accompanying table), and this establishes the diagnosis of postmolar GTN. It is common for hCG to fall in fits and starts, rather than have a smooth, consistent diminution, and this can be worrying for gynecologists; however, provided there is a consistent reduction in values in accordance with the stated definitions, observation can continue.
Another source of confusion and concern is an HCG level that fails to completely normalize during observation, yet reaches a very low level. If this is observed, clinicians should consider the diagnosis of quiescent hCG, pituitary hCG, or phantom hCG.3 These can be difficult to distinguish from postmolar GTN, and consultation with a gynecologic oncologist with experience in the diagnosis and management of these rare tumors is helpful to determine if the persistent low levels in hCG require intervention.
Once a clinician has observed a plateau or rise in hCG levels, a gynecologic examination should be performed because the lower genital tract is a common site for metastatic postmolar GTN. If during this evaluation, a suspicious lesion is identified (typically a blue-black, slightly raised, hemorrhagic-appearing lesion), it should not be biopsied, but rather assumed to be a metastatic site. The vasculature of metastatic sites is extremely fragile, and biopsy or disruption can result in catastrophic hemorrhage, even from very small lesions.
In addition to physical examination, several diagnostic studies should be performed which may expedite the triage and management of the case. A pelvic ultrasound should evaluate the endometrial cavity for a new viable pregnancy, and residual molar tissue; sometimes, myometrial invasion consistent with an invasive mole can be appreciated. Chest x-ray or CT scan should be ordered to evaluate for pulmonary metastatic lesions. Additionally, CT scans of the abdomen and pelvis should be ordered, and if lung metastases are present, brain imaging with either MRI or CT scan also should be obtained. These imaging studies will provide the necessary information to stage the GTN (as metastatic or not).
Treatment for postmolar GTN is determined based on further prognostic categorization (“high risk” or “low risk”) in accordance with the WHO classification, which is derived using several prognostic clinical variables including age, antecedent pregnancy, interval from index pregnancy, pretreatment hCG, largest tumor size, sites and number of metastases, and response to previous chemotherapy.4 These assignments are necessary to determine whether single-agent or multiagent chemotherapy should be prescribed.
Laboratory studies are helpful to obtain at this time and include metabolic panels (which can ensure that renal and hepatic function are within normal limits in anticipation of future chemotherapy), and complete blood count ,which can establish viable bone marrow function prior to chemotherapy.
Once postmolar GTN has been diagnosed, it is most appropriate to refer the patient to a gynecologic oncologist with experience in the treatment of these relatively rare malignancies. At that point, the patient will be formally staged, and offered treatment based on these staging results.
Among women with low-risk, nonmetastatic GTN who desire future fertility it is appropriate to offer a repeat dilation and curettage (D&C) procedure rather than immediately proceeding with chemotherapy. Approximately two-thirds of women with low risk disease can avoid chemotherapy with repeat curettage.5 Risk factors for needing chemotherapy after repeat D&C include the presence of trophoblastic disease in the pathology specimen and urinary hCG levels greater than 1,500 mIU/mL at the time of curettage. In my experience, many women appreciate this option to potentially avoid toxic chemotherapy.
For women with low-risk, nonmetastatic postmolar GTN who do not desire future fertility, and hope to avoid chemotherapy, hysterectomy also is a reasonable first option. This can be performed via either minimally invasive, laparotomy, or vaginal route. If performing a minimally invasive procedure in the setting of GTN, there should be caution or avoidance of use of a uterine manipulator because the uterine wall typically is soft and prone to perforation, and bleeding can be significant secondary to disruption of the tumor.
If repeat D&C or hysterectomy are adopted instead of chemotherapy, it is important that patients are very closely monitored post operatively to ensure normalization of their hCG levels (as described above). If it fails to normalize, restaging scans and examinations should be performed, and referral for the appropriate chemotherapy regimen should be initiated without delay.
Postmolar GTN is a serious condition that usually can be cured with chemotherapy or, if appropriate, surgery. and refer to a gynecologic oncologist when criteria are met to ensure that overtreatment is avoided and essential therapy is ensured.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Lancet Oncol. 2007 Aug;8(8):715-24.
2. J Natl Compr Canc Netw. 2019 Nov 1;17(11):1374-91.
3. Gynecol Oncol. 2009 Mar;112(3):663-72.
4. World Health Organ Tech Rep Ser. 1983;692:7-81.
5. Obstet Gynecol. 2016;128(3):535-42.
How to help patients become successful diabetes self-managers
Through the years, I have had the privilege of educating clinicians about scientific advances in diabetes care. Prior to displaying the first slide of my presentation, I ask the audience, “How many of you have seen a ‘noncompliant’ patient with diabetes within the past month?” Without fail, 99% of the attendees will raise their hand and start laughing, as if to say, “Well, of course they’re noncompliant. They just don’t get it! How incompetent can these people be?”
Blaming patients for failing to achieve metabolic control is inappropriate and misguided. How many physicians would be able or willing to monitor their blood glucose levels 4 times a day? Based on the premeal glucose level, how many physicians know how much insulin to inject in order to keep the postprandial excursions below 180 mg/dL? How many would remember to take 8 other medications daily without missing a dose? And how many physicians exercise daily; have actually looked at their feet in the past month; and have gained no weight in the past year?
Diabetes self-management is time-consuming and difficult for many patients, especially those with health illiteracy, financial restraints, or social barriers. Any patient who presents to the doctor is, in fact, “compliant.” These individuals expect to receive the safest and most effective treatments for their diabetes while learning as much as possible about lifestyle and behavioral interventions.
I challenge each of you to ask your patients: “What concerns you the most about having diabetes?” Initially, patients will express guilt and remorse about having diabetes. They will hang their heads in shame, admit to not going to the gym as often as they could, and promise to eat smaller portions. They will then look you in the eye and say, “I am worried about losing my eye, my leg, and my kidney to this life-threatening disease. I’m scared I won’t be able to see my daughter walk down the aisle at her wedding or my son graduate from college.”
These patients are terrified because they are unfamiliar with the advances in clinical science that aid our ability to improve the lives of all patients with diabetes. After hearing patients’ concerns about dying prematurely or losing an extremity to diabetes, I assure them that, “Nothing is going to happen to you on my watch. You are safe with me, and I will always have your back.” This level of trust is vitally important to the patient as well as to the treating physician. We all want our patients to achieve treatment success, just as any teacher would want their students to excel and graduate to the next grade level. Diabetes cannot be cured, yet we, as physicians, are able to heal with reassurance and expert guidance.
So, how do we help our patients achieve better adherence to their chronic disease state interventions? Here are 9 techniques that I have learned in my years helping patients manage their diabetes (all of which are more broadly applicable to any chronic disease state):
- Explain the disease state you are comanaging with the patient to the best of your ability. The more the patient understands, the easier your job as the “drug police” becomes.
- Remind the patient that he/she is the captain of the disease management team. You, the physician, serve as the personal coach. You can help the patient win the game, but he/she is ultimately responsible for achieving successful metabolic targets.
- Explain the risks, benefits, and any expected adverse effects that are likely to occur. Do this prior to initiating any medication.
- Discuss when metabolic change might be expected after initiating a given medication. Patients who observe rapid improvement in their glucose levels will be encouraged to adhere to their prescribed treatment regimen.
- Make certain that any and all screening tests are performed prior to initiating a medication. For example, renal function should be assessed prior to beginning most diabetes medications.
- Use shared decision-making to negotiate acceptable metabolic targets with each patient. Discuss the urgency and importance of achieving these goals.
- Assess the A1C reduction from baseline at 4 weeks after therapy initiation, rather than at 3 months. About 50% of the total A1C is reflective of the preceding 4 weeks of treatment.1 Thus, if the baseline A1C drops from 8.2% to 7.8%, the patient is moving in the right direction. However, if the A1C increases from 8.2% to 8.5%, the patient is not taking the prescribed medications.
- Recommend that the patient use a continuous glucose monitor; this newer technology is now readily available for patients who are covered by private insurance or Medicare. These devices allow patients to observe their glucose levels every 5 minutes of every day without fingersticks and actually cost less than self-monitoring one’s blood glucose.
- Find a reason to praise the patient at each visit. Patients who receive a powerful compliment, such as “I am so very proud of your efforts at improving your diabetes control,” will do their absolute best to remain adherent to their prescribed medication regimen. The response might be different if a patient is told, “Once again, your blood sugars are too high. At this rate, you are probably going to die, just like your father did 20 years ago. Now come back in 6 months and show me what you’re really made of!”
With more than 30 million Americans living with diabetes and another 84 million with prediabetes, the burden of preventive and intensive care lies squarely with family physicians.2 Rather than complain about our patients’ lack of metabolic control, we should provide them with the knowledge, skills, tools, and encouragement that they need to become successful diabetes self-managers.
1. Berard LD, Siemens R, Woo V; Diabetes Canada Clinical Practice Guidelines Expert Committee. Monitoring glycemic control. Can J Diabetes. 2018;42(suppl 1):S47-S325.
2. Centers for Disease Control and Prevention. New CDC report: More than 100 million Americans have diabetes or prediabetes. July 18, 2017. www.cdc.gov/media/releases/2017/p0718-diabetes-report.html. Accessed January 15, 2020.
Through the years, I have had the privilege of educating clinicians about scientific advances in diabetes care. Prior to displaying the first slide of my presentation, I ask the audience, “How many of you have seen a ‘noncompliant’ patient with diabetes within the past month?” Without fail, 99% of the attendees will raise their hand and start laughing, as if to say, “Well, of course they’re noncompliant. They just don’t get it! How incompetent can these people be?”
Blaming patients for failing to achieve metabolic control is inappropriate and misguided. How many physicians would be able or willing to monitor their blood glucose levels 4 times a day? Based on the premeal glucose level, how many physicians know how much insulin to inject in order to keep the postprandial excursions below 180 mg/dL? How many would remember to take 8 other medications daily without missing a dose? And how many physicians exercise daily; have actually looked at their feet in the past month; and have gained no weight in the past year?
Diabetes self-management is time-consuming and difficult for many patients, especially those with health illiteracy, financial restraints, or social barriers. Any patient who presents to the doctor is, in fact, “compliant.” These individuals expect to receive the safest and most effective treatments for their diabetes while learning as much as possible about lifestyle and behavioral interventions.
I challenge each of you to ask your patients: “What concerns you the most about having diabetes?” Initially, patients will express guilt and remorse about having diabetes. They will hang their heads in shame, admit to not going to the gym as often as they could, and promise to eat smaller portions. They will then look you in the eye and say, “I am worried about losing my eye, my leg, and my kidney to this life-threatening disease. I’m scared I won’t be able to see my daughter walk down the aisle at her wedding or my son graduate from college.”
These patients are terrified because they are unfamiliar with the advances in clinical science that aid our ability to improve the lives of all patients with diabetes. After hearing patients’ concerns about dying prematurely or losing an extremity to diabetes, I assure them that, “Nothing is going to happen to you on my watch. You are safe with me, and I will always have your back.” This level of trust is vitally important to the patient as well as to the treating physician. We all want our patients to achieve treatment success, just as any teacher would want their students to excel and graduate to the next grade level. Diabetes cannot be cured, yet we, as physicians, are able to heal with reassurance and expert guidance.
So, how do we help our patients achieve better adherence to their chronic disease state interventions? Here are 9 techniques that I have learned in my years helping patients manage their diabetes (all of which are more broadly applicable to any chronic disease state):
- Explain the disease state you are comanaging with the patient to the best of your ability. The more the patient understands, the easier your job as the “drug police” becomes.
- Remind the patient that he/she is the captain of the disease management team. You, the physician, serve as the personal coach. You can help the patient win the game, but he/she is ultimately responsible for achieving successful metabolic targets.
- Explain the risks, benefits, and any expected adverse effects that are likely to occur. Do this prior to initiating any medication.
- Discuss when metabolic change might be expected after initiating a given medication. Patients who observe rapid improvement in their glucose levels will be encouraged to adhere to their prescribed treatment regimen.
- Make certain that any and all screening tests are performed prior to initiating a medication. For example, renal function should be assessed prior to beginning most diabetes medications.
- Use shared decision-making to negotiate acceptable metabolic targets with each patient. Discuss the urgency and importance of achieving these goals.
- Assess the A1C reduction from baseline at 4 weeks after therapy initiation, rather than at 3 months. About 50% of the total A1C is reflective of the preceding 4 weeks of treatment.1 Thus, if the baseline A1C drops from 8.2% to 7.8%, the patient is moving in the right direction. However, if the A1C increases from 8.2% to 8.5%, the patient is not taking the prescribed medications.
- Recommend that the patient use a continuous glucose monitor; this newer technology is now readily available for patients who are covered by private insurance or Medicare. These devices allow patients to observe their glucose levels every 5 minutes of every day without fingersticks and actually cost less than self-monitoring one’s blood glucose.
- Find a reason to praise the patient at each visit. Patients who receive a powerful compliment, such as “I am so very proud of your efforts at improving your diabetes control,” will do their absolute best to remain adherent to their prescribed medication regimen. The response might be different if a patient is told, “Once again, your blood sugars are too high. At this rate, you are probably going to die, just like your father did 20 years ago. Now come back in 6 months and show me what you’re really made of!”
With more than 30 million Americans living with diabetes and another 84 million with prediabetes, the burden of preventive and intensive care lies squarely with family physicians.2 Rather than complain about our patients’ lack of metabolic control, we should provide them with the knowledge, skills, tools, and encouragement that they need to become successful diabetes self-managers.
Through the years, I have had the privilege of educating clinicians about scientific advances in diabetes care. Prior to displaying the first slide of my presentation, I ask the audience, “How many of you have seen a ‘noncompliant’ patient with diabetes within the past month?” Without fail, 99% of the attendees will raise their hand and start laughing, as if to say, “Well, of course they’re noncompliant. They just don’t get it! How incompetent can these people be?”
Blaming patients for failing to achieve metabolic control is inappropriate and misguided. How many physicians would be able or willing to monitor their blood glucose levels 4 times a day? Based on the premeal glucose level, how many physicians know how much insulin to inject in order to keep the postprandial excursions below 180 mg/dL? How many would remember to take 8 other medications daily without missing a dose? And how many physicians exercise daily; have actually looked at their feet in the past month; and have gained no weight in the past year?
Diabetes self-management is time-consuming and difficult for many patients, especially those with health illiteracy, financial restraints, or social barriers. Any patient who presents to the doctor is, in fact, “compliant.” These individuals expect to receive the safest and most effective treatments for their diabetes while learning as much as possible about lifestyle and behavioral interventions.
I challenge each of you to ask your patients: “What concerns you the most about having diabetes?” Initially, patients will express guilt and remorse about having diabetes. They will hang their heads in shame, admit to not going to the gym as often as they could, and promise to eat smaller portions. They will then look you in the eye and say, “I am worried about losing my eye, my leg, and my kidney to this life-threatening disease. I’m scared I won’t be able to see my daughter walk down the aisle at her wedding or my son graduate from college.”
These patients are terrified because they are unfamiliar with the advances in clinical science that aid our ability to improve the lives of all patients with diabetes. After hearing patients’ concerns about dying prematurely or losing an extremity to diabetes, I assure them that, “Nothing is going to happen to you on my watch. You are safe with me, and I will always have your back.” This level of trust is vitally important to the patient as well as to the treating physician. We all want our patients to achieve treatment success, just as any teacher would want their students to excel and graduate to the next grade level. Diabetes cannot be cured, yet we, as physicians, are able to heal with reassurance and expert guidance.
So, how do we help our patients achieve better adherence to their chronic disease state interventions? Here are 9 techniques that I have learned in my years helping patients manage their diabetes (all of which are more broadly applicable to any chronic disease state):
- Explain the disease state you are comanaging with the patient to the best of your ability. The more the patient understands, the easier your job as the “drug police” becomes.
- Remind the patient that he/she is the captain of the disease management team. You, the physician, serve as the personal coach. You can help the patient win the game, but he/she is ultimately responsible for achieving successful metabolic targets.
- Explain the risks, benefits, and any expected adverse effects that are likely to occur. Do this prior to initiating any medication.
- Discuss when metabolic change might be expected after initiating a given medication. Patients who observe rapid improvement in their glucose levels will be encouraged to adhere to their prescribed treatment regimen.
- Make certain that any and all screening tests are performed prior to initiating a medication. For example, renal function should be assessed prior to beginning most diabetes medications.
- Use shared decision-making to negotiate acceptable metabolic targets with each patient. Discuss the urgency and importance of achieving these goals.
- Assess the A1C reduction from baseline at 4 weeks after therapy initiation, rather than at 3 months. About 50% of the total A1C is reflective of the preceding 4 weeks of treatment.1 Thus, if the baseline A1C drops from 8.2% to 7.8%, the patient is moving in the right direction. However, if the A1C increases from 8.2% to 8.5%, the patient is not taking the prescribed medications.
- Recommend that the patient use a continuous glucose monitor; this newer technology is now readily available for patients who are covered by private insurance or Medicare. These devices allow patients to observe their glucose levels every 5 minutes of every day without fingersticks and actually cost less than self-monitoring one’s blood glucose.
- Find a reason to praise the patient at each visit. Patients who receive a powerful compliment, such as “I am so very proud of your efforts at improving your diabetes control,” will do their absolute best to remain adherent to their prescribed medication regimen. The response might be different if a patient is told, “Once again, your blood sugars are too high. At this rate, you are probably going to die, just like your father did 20 years ago. Now come back in 6 months and show me what you’re really made of!”
With more than 30 million Americans living with diabetes and another 84 million with prediabetes, the burden of preventive and intensive care lies squarely with family physicians.2 Rather than complain about our patients’ lack of metabolic control, we should provide them with the knowledge, skills, tools, and encouragement that they need to become successful diabetes self-managers.
1. Berard LD, Siemens R, Woo V; Diabetes Canada Clinical Practice Guidelines Expert Committee. Monitoring glycemic control. Can J Diabetes. 2018;42(suppl 1):S47-S325.
2. Centers for Disease Control and Prevention. New CDC report: More than 100 million Americans have diabetes or prediabetes. July 18, 2017. www.cdc.gov/media/releases/2017/p0718-diabetes-report.html. Accessed January 15, 2020.
1. Berard LD, Siemens R, Woo V; Diabetes Canada Clinical Practice Guidelines Expert Committee. Monitoring glycemic control. Can J Diabetes. 2018;42(suppl 1):S47-S325.
2. Centers for Disease Control and Prevention. New CDC report: More than 100 million Americans have diabetes or prediabetes. July 18, 2017. www.cdc.gov/media/releases/2017/p0718-diabetes-report.html. Accessed January 15, 2020.
Depression after miscarriage: Follow-up care is key
A Washington Post article on depression after miscarriage is a reminder that, although couples can suffer deeply from such a loss, there still are ways to provide them with meaningful support (“After miscarriage, I was rocked by depression. Like many other women, I didn’t get follow-up care for this loss,” by Katie C. Reilly, Nov 30, 2019).
Psychiatrists who focus on reproductive psychiatry and collaborative care are trying to change the current therapeutic landscape and improve practitioner awareness and treatment. Ob.gyns. managing patients who have experienced reproductive loss, especially early-term loss, may not immediately refer couples to a therapist or psychiatrist, but we can change this. Practitioners who focus on reproductive health – both physical and mental – are trying to better understand such couples’ experiences, increase their access to care, develop preventative care strategies, and improve provider education.
At the outset, providers who treat patients who have experienced a perinatal loss must recognize that not all individuals will feel that a loss is tragic. Instead, patient reactions occur along a spectrum, and there is no “correct” way to process a loss. A couple’s reaction may depend on a variety of factors, including how late or early in pregnancy the loss occurs, whether the pregnancy is planned or unplanned, and what other psychosocial stressors, such as unstable housing, limited income, and few social supports, may exist. Not every patient experiencing grief, even profoundly, will shed tears; we need to be open to all potential reactions and be mindful when a person may need additional support.
According to the Washington Post article, even though 50% of miscarriages are due to chromosomal abnormalities, women still feel ultimately responsible for the loss. As a society we are bombarded with “experts” in the media telling us the best way, the right way, the healthiest way to live. This barrage of advice distorts our views of what it really means to be a good parent and subtly conveys the idea that mothers are solely responsible for any bad pregnancy outcomes. I remember being fearful of causing unintentional harm to my unborn baby during my own pregnancy. What if I accidentally ate something that would affect her development? Is exposure to second-hand smoke as I walk down the street harming her? How bad would it be if I just had one cup of coffee? My doubts caused quite a bit of distress for me, which is a mild form of the distress I see when counseling couples after their miscarriages.
The article’s author also expressed concern about the emotional sterility of the environment in which miscarriages usually occur: a hospital ED. EDs are designed to promote a level of detachment and to quell any stress for the clinicians so that they can calmly handle unexpected health crises. EDs are not primarily designed to provide patients with emotional support, nor should they be. However, we still can make some improvements to existing ED design to better address couples’ emotional needs. For example, some EDs have placed mental health clinicians on staff, others call patients post discharge to address concerns, and some EDs even provide patients access to mental health trauma teams. Such services are not found in all EDs, and even those that exist may just scratch the surface of what is needed, but they are a step in the right direction. Providing this level of auxiliary care directly from the ED increases patients’ ability to access mental health support in the place where miscarriages are most likely to be first diagnosed and managed.
The American College of Obstetricians and Gynecologists already is trying to fill in the missing pieces when it comes to identifying mood symptoms following miscarriage. One of the key recommendations from the May 2018 Committee Opinion on Redefining the Postpartum Visit is that every woman who has experienced a miscarriage, stillbirth, or neonatal death should receive follow-up care. Mental health is a suggested component of the postpartum care plan. Some outpatient ob.gyn. practices and inpatient units are using screening tools to identify postpartum depression. For example, the Edinburgh Postnatal Depression Scale can be utilized following a miscarriage to help providers identify symptoms of depression and anxiety.
However, The trend in psychiatry over the past decade has been toward collaborative care, models that embed psychiatrists and other mental health clinicians in ob.gyn. practices to help guide the diagnosis and treatment of mental health problems. Some psychiatrists practice a co-located model in which they see patients alongside their ob.gyn. colleagues, whereas other psychiatrists treat a larger number of patients by using chart reviews for medication management while relying on behavioral health care managers for counseling and monitoring. Using this model of mental health care, more patients have access to services that are provided in a location familiar to them.
Another step in the right direction is the October 2019 launch of The National Curriculum in Reproductive Psychiatry (NCRP), which provides free educational material for psychiatry faculty and residents to enhance education on topics related to reproductive psychiatry, including miscarriage, loss, and development of trauma disorders. NCRP aspires to develop educational materials for ob.gyn. residents.
In the past we may have missed the mark in recognizing and treating the trauma that prenatal loss can cause, but we are trying to improve our approaches. More and more couples are sharing their experiences and advocating for themselves and others, often creating change in medical practice, and doctors are starting to listen. As any clinician knows, changes to standards of care can take several years to disseminate into general practice, but this gap between knowledge and treatment is now in the forefront of our minds. I am hopeful that we will continue to make advances and provide better care to our patients who have endured the loss of a pregnancy.
Dr. Latorre is an assistant professor in the department of psychiatry at the University of Maryland School of Medicine. She has reported no relevant financial disclosures. Email her at [email protected].
A Washington Post article on depression after miscarriage is a reminder that, although couples can suffer deeply from such a loss, there still are ways to provide them with meaningful support (“After miscarriage, I was rocked by depression. Like many other women, I didn’t get follow-up care for this loss,” by Katie C. Reilly, Nov 30, 2019).
Psychiatrists who focus on reproductive psychiatry and collaborative care are trying to change the current therapeutic landscape and improve practitioner awareness and treatment. Ob.gyns. managing patients who have experienced reproductive loss, especially early-term loss, may not immediately refer couples to a therapist or psychiatrist, but we can change this. Practitioners who focus on reproductive health – both physical and mental – are trying to better understand such couples’ experiences, increase their access to care, develop preventative care strategies, and improve provider education.
At the outset, providers who treat patients who have experienced a perinatal loss must recognize that not all individuals will feel that a loss is tragic. Instead, patient reactions occur along a spectrum, and there is no “correct” way to process a loss. A couple’s reaction may depend on a variety of factors, including how late or early in pregnancy the loss occurs, whether the pregnancy is planned or unplanned, and what other psychosocial stressors, such as unstable housing, limited income, and few social supports, may exist. Not every patient experiencing grief, even profoundly, will shed tears; we need to be open to all potential reactions and be mindful when a person may need additional support.
According to the Washington Post article, even though 50% of miscarriages are due to chromosomal abnormalities, women still feel ultimately responsible for the loss. As a society we are bombarded with “experts” in the media telling us the best way, the right way, the healthiest way to live. This barrage of advice distorts our views of what it really means to be a good parent and subtly conveys the idea that mothers are solely responsible for any bad pregnancy outcomes. I remember being fearful of causing unintentional harm to my unborn baby during my own pregnancy. What if I accidentally ate something that would affect her development? Is exposure to second-hand smoke as I walk down the street harming her? How bad would it be if I just had one cup of coffee? My doubts caused quite a bit of distress for me, which is a mild form of the distress I see when counseling couples after their miscarriages.
The article’s author also expressed concern about the emotional sterility of the environment in which miscarriages usually occur: a hospital ED. EDs are designed to promote a level of detachment and to quell any stress for the clinicians so that they can calmly handle unexpected health crises. EDs are not primarily designed to provide patients with emotional support, nor should they be. However, we still can make some improvements to existing ED design to better address couples’ emotional needs. For example, some EDs have placed mental health clinicians on staff, others call patients post discharge to address concerns, and some EDs even provide patients access to mental health trauma teams. Such services are not found in all EDs, and even those that exist may just scratch the surface of what is needed, but they are a step in the right direction. Providing this level of auxiliary care directly from the ED increases patients’ ability to access mental health support in the place where miscarriages are most likely to be first diagnosed and managed.
The American College of Obstetricians and Gynecologists already is trying to fill in the missing pieces when it comes to identifying mood symptoms following miscarriage. One of the key recommendations from the May 2018 Committee Opinion on Redefining the Postpartum Visit is that every woman who has experienced a miscarriage, stillbirth, or neonatal death should receive follow-up care. Mental health is a suggested component of the postpartum care plan. Some outpatient ob.gyn. practices and inpatient units are using screening tools to identify postpartum depression. For example, the Edinburgh Postnatal Depression Scale can be utilized following a miscarriage to help providers identify symptoms of depression and anxiety.
However, The trend in psychiatry over the past decade has been toward collaborative care, models that embed psychiatrists and other mental health clinicians in ob.gyn. practices to help guide the diagnosis and treatment of mental health problems. Some psychiatrists practice a co-located model in which they see patients alongside their ob.gyn. colleagues, whereas other psychiatrists treat a larger number of patients by using chart reviews for medication management while relying on behavioral health care managers for counseling and monitoring. Using this model of mental health care, more patients have access to services that are provided in a location familiar to them.
Another step in the right direction is the October 2019 launch of The National Curriculum in Reproductive Psychiatry (NCRP), which provides free educational material for psychiatry faculty and residents to enhance education on topics related to reproductive psychiatry, including miscarriage, loss, and development of trauma disorders. NCRP aspires to develop educational materials for ob.gyn. residents.
In the past we may have missed the mark in recognizing and treating the trauma that prenatal loss can cause, but we are trying to improve our approaches. More and more couples are sharing their experiences and advocating for themselves and others, often creating change in medical practice, and doctors are starting to listen. As any clinician knows, changes to standards of care can take several years to disseminate into general practice, but this gap between knowledge and treatment is now in the forefront of our minds. I am hopeful that we will continue to make advances and provide better care to our patients who have endured the loss of a pregnancy.
Dr. Latorre is an assistant professor in the department of psychiatry at the University of Maryland School of Medicine. She has reported no relevant financial disclosures. Email her at [email protected].
A Washington Post article on depression after miscarriage is a reminder that, although couples can suffer deeply from such a loss, there still are ways to provide them with meaningful support (“After miscarriage, I was rocked by depression. Like many other women, I didn’t get follow-up care for this loss,” by Katie C. Reilly, Nov 30, 2019).
Psychiatrists who focus on reproductive psychiatry and collaborative care are trying to change the current therapeutic landscape and improve practitioner awareness and treatment. Ob.gyns. managing patients who have experienced reproductive loss, especially early-term loss, may not immediately refer couples to a therapist or psychiatrist, but we can change this. Practitioners who focus on reproductive health – both physical and mental – are trying to better understand such couples’ experiences, increase their access to care, develop preventative care strategies, and improve provider education.
At the outset, providers who treat patients who have experienced a perinatal loss must recognize that not all individuals will feel that a loss is tragic. Instead, patient reactions occur along a spectrum, and there is no “correct” way to process a loss. A couple’s reaction may depend on a variety of factors, including how late or early in pregnancy the loss occurs, whether the pregnancy is planned or unplanned, and what other psychosocial stressors, such as unstable housing, limited income, and few social supports, may exist. Not every patient experiencing grief, even profoundly, will shed tears; we need to be open to all potential reactions and be mindful when a person may need additional support.
According to the Washington Post article, even though 50% of miscarriages are due to chromosomal abnormalities, women still feel ultimately responsible for the loss. As a society we are bombarded with “experts” in the media telling us the best way, the right way, the healthiest way to live. This barrage of advice distorts our views of what it really means to be a good parent and subtly conveys the idea that mothers are solely responsible for any bad pregnancy outcomes. I remember being fearful of causing unintentional harm to my unborn baby during my own pregnancy. What if I accidentally ate something that would affect her development? Is exposure to second-hand smoke as I walk down the street harming her? How bad would it be if I just had one cup of coffee? My doubts caused quite a bit of distress for me, which is a mild form of the distress I see when counseling couples after their miscarriages.
The article’s author also expressed concern about the emotional sterility of the environment in which miscarriages usually occur: a hospital ED. EDs are designed to promote a level of detachment and to quell any stress for the clinicians so that they can calmly handle unexpected health crises. EDs are not primarily designed to provide patients with emotional support, nor should they be. However, we still can make some improvements to existing ED design to better address couples’ emotional needs. For example, some EDs have placed mental health clinicians on staff, others call patients post discharge to address concerns, and some EDs even provide patients access to mental health trauma teams. Such services are not found in all EDs, and even those that exist may just scratch the surface of what is needed, but they are a step in the right direction. Providing this level of auxiliary care directly from the ED increases patients’ ability to access mental health support in the place where miscarriages are most likely to be first diagnosed and managed.
The American College of Obstetricians and Gynecologists already is trying to fill in the missing pieces when it comes to identifying mood symptoms following miscarriage. One of the key recommendations from the May 2018 Committee Opinion on Redefining the Postpartum Visit is that every woman who has experienced a miscarriage, stillbirth, or neonatal death should receive follow-up care. Mental health is a suggested component of the postpartum care plan. Some outpatient ob.gyn. practices and inpatient units are using screening tools to identify postpartum depression. For example, the Edinburgh Postnatal Depression Scale can be utilized following a miscarriage to help providers identify symptoms of depression and anxiety.
However, The trend in psychiatry over the past decade has been toward collaborative care, models that embed psychiatrists and other mental health clinicians in ob.gyn. practices to help guide the diagnosis and treatment of mental health problems. Some psychiatrists practice a co-located model in which they see patients alongside their ob.gyn. colleagues, whereas other psychiatrists treat a larger number of patients by using chart reviews for medication management while relying on behavioral health care managers for counseling and monitoring. Using this model of mental health care, more patients have access to services that are provided in a location familiar to them.
Another step in the right direction is the October 2019 launch of The National Curriculum in Reproductive Psychiatry (NCRP), which provides free educational material for psychiatry faculty and residents to enhance education on topics related to reproductive psychiatry, including miscarriage, loss, and development of trauma disorders. NCRP aspires to develop educational materials for ob.gyn. residents.
In the past we may have missed the mark in recognizing and treating the trauma that prenatal loss can cause, but we are trying to improve our approaches. More and more couples are sharing their experiences and advocating for themselves and others, often creating change in medical practice, and doctors are starting to listen. As any clinician knows, changes to standards of care can take several years to disseminate into general practice, but this gap between knowledge and treatment is now in the forefront of our minds. I am hopeful that we will continue to make advances and provide better care to our patients who have endured the loss of a pregnancy.
Dr. Latorre is an assistant professor in the department of psychiatry at the University of Maryland School of Medicine. She has reported no relevant financial disclosures. Email her at [email protected].