User login
Armed conflict disproportionately affects children
I was asked recently about the trauma of 9/11 by a teen patient who was too young to remember the terrorist attacks. I was surprised that even today I become teary eyed thinking about it. My son was in his first week of college at Georgetown, and in the early confusion about what was going on I was panicked at reports of bombings in Washington. Fortunately, for me and my family at least, none of us were physically harmed. But the mental trauma still is with us. It was a momentary panic for me, but it’s not so fleeting for many families around the world today.
I can’t imagine what it is like today to be a parent in an armed conflict zone, or even in an area with very high levels of criminal violence. At a meeting of the International Society for Social Pediatrics (ISSOP), I learned that an estimated 1.5 billion residents of Earth, or about one in five inhabitants, lived in war zones or in areas of tremendous violence, according to the World Bank’s 2011 World Development Report. And I learned that children are disproportionately affected – physically, mentally, and developmentally – from Stella Tsitoura, MD, of the Network for Children’s Rights, Athens, and others at the ISSOP meeting in Beirut, Lebanon, where pediatricians from around the world gathered in Oct. 2019 to consider what we as a profession should be doing in the context of armed conflict’s impact on so many children. The meeting was held in the Middle East because it is an especially hot conflict area, but children in South Asia, central Africa, South America, Central America, even rough inner-city areas in the United States are also affected.
Child soldiers in some parts of the world particularly are affected, sometimes being forced to commit violence on neighbors and kin. One country that I worked in years ago, Yemen, is a horrifying example of the complex impact of war on children and families. In 2017, over 2,100 children had been recruited as soldiers during the 3-year conflict in Yemen, a UNICEF representative reported. The death toll in Yemen was over 17,500 by Nov. 2018, according to a Human Rights Watch report.
Samuel Perlo-Freeman, PhD, an expert on the economics of arms trade, noted at the ISSOP meeting that two-thirds of civilian casualties in Yemen are caused by Coalition air strikes, whose members include Bahrain, Egypt, Jordan, Kuwait, Saudi Arabia, Sudan, and United Arab Emirates. He also said rebel groups in Yemen and elsewhere acquire their arms by capture, by smuggling, or through the aid of foreign backers. Six countries – the United States, Russia, and several western European countries – account for the majority of war tools used in armed conflict zones. In June 2019, courts in Great Britain ruled that British arms sales to the parties involved in Yemen were illegal without an assessment as to whether any violation of internal humanitarian law had taken place.
Many of us feel impotent when facing the magnitude of this problem, and the lives of despair that affected children lead are sometimes too heart breaking to dwell on. ISSOP, the American Academy of Pediatrics, and the International Pediatric Association (IPA) are teaching us differently: Standing up for the human rights of children living in areas of conflict or refugees from those areas is our responsibility, both as individuals and as members of our pediatric associations. At a basic level we need to witness – we need to share what we see with our patients who have immigrated legally or illegally in our practices, our hospitals, and our communities. It’s important to be knowledgeable of current standards of clinical care outlined by both ISSOP and the AAP to ensure our patients affected by conflict and violence get appropriate treatment.
Some of the most lasting health impacts for children in conflict are their mental health needs; the World Health Organization prevalence estimates of mental disorders in conflict settings is 22%, according to a 2019 report in the Lancet (2019 Jun;394[10194]:240-8). Not only do mental health conditions last throughout a lifetime, the impact of war can affect generations through epigenetic forces.
Arms manufacturers should be held accountable as the courts are doing in Great Britain. Too often American-made armaments are falling into the wrong hands. More can be done to limit the sale of weapons that go into conflict zones. Chemical weapons, cluster bombs, and biologic weapons are banned by international agreement; shouldn’t we do the same with nuclear weapons?
Some pediatric health care facilities are impacted by the needs of traumatized children more than others. Countries at the front line of conflict – like Lebanon, Jordon, Turkey, Greece, Italy, and Mexico – should be supported in their efforts on behalf of child refugees. We need to share the burden and support entities such as Doctors Without Borders, Save the Children, and the Red Cross/Crescent as they present themselves in crisis zones. We recognize that the fundamental human rights of children are being ignored by warring parties. especially Goal 16, which asks the world community to make real progress in promoting peace and justice by 2030. Pediatricians may not be experts in armed conflict, but we are experts in what warfare does to the health and well-being of young children. It’s time to speak out and act.
I was asked recently about the trauma of 9/11 by a teen patient who was too young to remember the terrorist attacks. I was surprised that even today I become teary eyed thinking about it. My son was in his first week of college at Georgetown, and in the early confusion about what was going on I was panicked at reports of bombings in Washington. Fortunately, for me and my family at least, none of us were physically harmed. But the mental trauma still is with us. It was a momentary panic for me, but it’s not so fleeting for many families around the world today.
I can’t imagine what it is like today to be a parent in an armed conflict zone, or even in an area with very high levels of criminal violence. At a meeting of the International Society for Social Pediatrics (ISSOP), I learned that an estimated 1.5 billion residents of Earth, or about one in five inhabitants, lived in war zones or in areas of tremendous violence, according to the World Bank’s 2011 World Development Report. And I learned that children are disproportionately affected – physically, mentally, and developmentally – from Stella Tsitoura, MD, of the Network for Children’s Rights, Athens, and others at the ISSOP meeting in Beirut, Lebanon, where pediatricians from around the world gathered in Oct. 2019 to consider what we as a profession should be doing in the context of armed conflict’s impact on so many children. The meeting was held in the Middle East because it is an especially hot conflict area, but children in South Asia, central Africa, South America, Central America, even rough inner-city areas in the United States are also affected.
Child soldiers in some parts of the world particularly are affected, sometimes being forced to commit violence on neighbors and kin. One country that I worked in years ago, Yemen, is a horrifying example of the complex impact of war on children and families. In 2017, over 2,100 children had been recruited as soldiers during the 3-year conflict in Yemen, a UNICEF representative reported. The death toll in Yemen was over 17,500 by Nov. 2018, according to a Human Rights Watch report.
Samuel Perlo-Freeman, PhD, an expert on the economics of arms trade, noted at the ISSOP meeting that two-thirds of civilian casualties in Yemen are caused by Coalition air strikes, whose members include Bahrain, Egypt, Jordan, Kuwait, Saudi Arabia, Sudan, and United Arab Emirates. He also said rebel groups in Yemen and elsewhere acquire their arms by capture, by smuggling, or through the aid of foreign backers. Six countries – the United States, Russia, and several western European countries – account for the majority of war tools used in armed conflict zones. In June 2019, courts in Great Britain ruled that British arms sales to the parties involved in Yemen were illegal without an assessment as to whether any violation of internal humanitarian law had taken place.
Many of us feel impotent when facing the magnitude of this problem, and the lives of despair that affected children lead are sometimes too heart breaking to dwell on. ISSOP, the American Academy of Pediatrics, and the International Pediatric Association (IPA) are teaching us differently: Standing up for the human rights of children living in areas of conflict or refugees from those areas is our responsibility, both as individuals and as members of our pediatric associations. At a basic level we need to witness – we need to share what we see with our patients who have immigrated legally or illegally in our practices, our hospitals, and our communities. It’s important to be knowledgeable of current standards of clinical care outlined by both ISSOP and the AAP to ensure our patients affected by conflict and violence get appropriate treatment.
Some of the most lasting health impacts for children in conflict are their mental health needs; the World Health Organization prevalence estimates of mental disorders in conflict settings is 22%, according to a 2019 report in the Lancet (2019 Jun;394[10194]:240-8). Not only do mental health conditions last throughout a lifetime, the impact of war can affect generations through epigenetic forces.
Arms manufacturers should be held accountable as the courts are doing in Great Britain. Too often American-made armaments are falling into the wrong hands. More can be done to limit the sale of weapons that go into conflict zones. Chemical weapons, cluster bombs, and biologic weapons are banned by international agreement; shouldn’t we do the same with nuclear weapons?
Some pediatric health care facilities are impacted by the needs of traumatized children more than others. Countries at the front line of conflict – like Lebanon, Jordon, Turkey, Greece, Italy, and Mexico – should be supported in their efforts on behalf of child refugees. We need to share the burden and support entities such as Doctors Without Borders, Save the Children, and the Red Cross/Crescent as they present themselves in crisis zones. We recognize that the fundamental human rights of children are being ignored by warring parties. especially Goal 16, which asks the world community to make real progress in promoting peace and justice by 2030. Pediatricians may not be experts in armed conflict, but we are experts in what warfare does to the health and well-being of young children. It’s time to speak out and act.
I was asked recently about the trauma of 9/11 by a teen patient who was too young to remember the terrorist attacks. I was surprised that even today I become teary eyed thinking about it. My son was in his first week of college at Georgetown, and in the early confusion about what was going on I was panicked at reports of bombings in Washington. Fortunately, for me and my family at least, none of us were physically harmed. But the mental trauma still is with us. It was a momentary panic for me, but it’s not so fleeting for many families around the world today.
I can’t imagine what it is like today to be a parent in an armed conflict zone, or even in an area with very high levels of criminal violence. At a meeting of the International Society for Social Pediatrics (ISSOP), I learned that an estimated 1.5 billion residents of Earth, or about one in five inhabitants, lived in war zones or in areas of tremendous violence, according to the World Bank’s 2011 World Development Report. And I learned that children are disproportionately affected – physically, mentally, and developmentally – from Stella Tsitoura, MD, of the Network for Children’s Rights, Athens, and others at the ISSOP meeting in Beirut, Lebanon, where pediatricians from around the world gathered in Oct. 2019 to consider what we as a profession should be doing in the context of armed conflict’s impact on so many children. The meeting was held in the Middle East because it is an especially hot conflict area, but children in South Asia, central Africa, South America, Central America, even rough inner-city areas in the United States are also affected.
Child soldiers in some parts of the world particularly are affected, sometimes being forced to commit violence on neighbors and kin. One country that I worked in years ago, Yemen, is a horrifying example of the complex impact of war on children and families. In 2017, over 2,100 children had been recruited as soldiers during the 3-year conflict in Yemen, a UNICEF representative reported. The death toll in Yemen was over 17,500 by Nov. 2018, according to a Human Rights Watch report.
Samuel Perlo-Freeman, PhD, an expert on the economics of arms trade, noted at the ISSOP meeting that two-thirds of civilian casualties in Yemen are caused by Coalition air strikes, whose members include Bahrain, Egypt, Jordan, Kuwait, Saudi Arabia, Sudan, and United Arab Emirates. He also said rebel groups in Yemen and elsewhere acquire their arms by capture, by smuggling, or through the aid of foreign backers. Six countries – the United States, Russia, and several western European countries – account for the majority of war tools used in armed conflict zones. In June 2019, courts in Great Britain ruled that British arms sales to the parties involved in Yemen were illegal without an assessment as to whether any violation of internal humanitarian law had taken place.
Many of us feel impotent when facing the magnitude of this problem, and the lives of despair that affected children lead are sometimes too heart breaking to dwell on. ISSOP, the American Academy of Pediatrics, and the International Pediatric Association (IPA) are teaching us differently: Standing up for the human rights of children living in areas of conflict or refugees from those areas is our responsibility, both as individuals and as members of our pediatric associations. At a basic level we need to witness – we need to share what we see with our patients who have immigrated legally or illegally in our practices, our hospitals, and our communities. It’s important to be knowledgeable of current standards of clinical care outlined by both ISSOP and the AAP to ensure our patients affected by conflict and violence get appropriate treatment.
Some of the most lasting health impacts for children in conflict are their mental health needs; the World Health Organization prevalence estimates of mental disorders in conflict settings is 22%, according to a 2019 report in the Lancet (2019 Jun;394[10194]:240-8). Not only do mental health conditions last throughout a lifetime, the impact of war can affect generations through epigenetic forces.
Arms manufacturers should be held accountable as the courts are doing in Great Britain. Too often American-made armaments are falling into the wrong hands. More can be done to limit the sale of weapons that go into conflict zones. Chemical weapons, cluster bombs, and biologic weapons are banned by international agreement; shouldn’t we do the same with nuclear weapons?
Some pediatric health care facilities are impacted by the needs of traumatized children more than others. Countries at the front line of conflict – like Lebanon, Jordon, Turkey, Greece, Italy, and Mexico – should be supported in their efforts on behalf of child refugees. We need to share the burden and support entities such as Doctors Without Borders, Save the Children, and the Red Cross/Crescent as they present themselves in crisis zones. We recognize that the fundamental human rights of children are being ignored by warring parties. especially Goal 16, which asks the world community to make real progress in promoting peace and justice by 2030. Pediatricians may not be experts in armed conflict, but we are experts in what warfare does to the health and well-being of young children. It’s time to speak out and act.
Psychiatrists urged to look beyond the ‘monoamine island’
Now that 2019 has passed us by, it is a time for reflection for most of us. We can think about the state of our important relationships, perhaps the growth of our children or other loved ones, the trajectory of our practices, and ideas for the future. Most commonly, I would wager, we likely think about how we might do things differently in the new year.
Applying this lens to our chosen profession, the practice of clinical psychiatry, I hope 2020 brings real, or at least, incremental change. Our profession has evolved markedly over the last several decades, from psychoanalysis to the psychopharmacology revolution, to a now largely multimodal approach. Our treatment of psychiatric illness has evolved and, for the most part, improved the lives of millions across the country and around the world.
However, in so doing, we have, perhaps inadvertently, or maybe out of necessity, found ourselves on an allegorical island that we, as clinical, everyday psychiatrists defend to the death. Surrounded by an ocean of psychiatric disorders, illnesses, and symptomatology, we wave our prescription pads (or e-pads), like wands, hoping to calm the torrents of the psychiatric sea with yet another prescription. However, I’m not writing this to bemoan modern psychopharmacology, for it has saved and improved lives and led to a fruitful practice and livelihood for me and most of my colleagues. As the torrents of illness continue to flare around us, I’m not advocating that we put down our wands and become strictly therapists. What I am advocating for is the use of different wands, as it were.
Our chosen wand is undoubtedly largely composed of monoamine-based remedies – most involving the holy trinity of serotonin, dopamine, and norepinephrine. As we stand on our island, trying to quell hurricane-force winds, shark-infested waters, or a tidal wave, we wave the same wand – hoping that a dash of monoamine will slow down winds, scare away sharks, and reduce the destructive capacity of water.
We, more than those in any other medical profession, use the same basic treatments for heterogeneous disorders, whose true underlying physiology, despite important progress over the years, remains elusive and only partly understood. We see improvement and even resolution sometimes, but, for the most part, our treatments keep our patients going, so they can continue to sail, just avoiding being capsized by the psychiatric torrents beneath the surface. When the torrents flare, we wave the same wand again, hoping that another dash of monoamine modulation, this time, maybe in a new wrapper, or with a new name, will keep the ocean calm for a bit longer. Now, this has worked for decades to keep many ships from being capsized and our island still largely habitable, but this strategy is akin to building a shelter out of twigs and leaves and grasses, and never advancing to permanent construction techniques, and just replacing broken branches and leaves that will, undoubtedly, break again.
As we stand on our island, I say, we use the branches, leaves, and twigs to build a bridge to another island, and, there, we can make new wands.
In 2020, the materials for these new wands are readily available, but we have to be willing to trust these new wands, and yet not completely discard the old wands we have used for so long. These new wands are the nonmonoamine-based treatments, which have shown remarkable efficacy and safety in patients across the country and the world. We must accept that we, as a species, are remarkably complex creatures, and the disorders we try to treat have their origins in the most complex part of our being: the brain. Therefore, considering the complexity, it is only reasonable to think that there is more to our illnesses than modulating monoamines.
In 2020, I challenge every day, clinical psychiatrists to embrace these new treatments and wave these new wands. As someone who has been fortunate enough to prescribe ketamine infusion and nasal sprays in the clinic, I can say we must gravitate toward these new treatments when clinically appropriate. While ketamine treatment is no panacea, its use, and the adoption of other nonmonoamine-based treatments, hopefully will fuel the development, use, and perhaps, most importantly, novel thinking about new biological treatment of psychiatric illness.
Dr. Shroff is board-certified in psychiatry in sleep medicine, and practices in Smyrna, Ga. He is a fellow of the American Psychiatric Association.
Now that 2019 has passed us by, it is a time for reflection for most of us. We can think about the state of our important relationships, perhaps the growth of our children or other loved ones, the trajectory of our practices, and ideas for the future. Most commonly, I would wager, we likely think about how we might do things differently in the new year.
Applying this lens to our chosen profession, the practice of clinical psychiatry, I hope 2020 brings real, or at least, incremental change. Our profession has evolved markedly over the last several decades, from psychoanalysis to the psychopharmacology revolution, to a now largely multimodal approach. Our treatment of psychiatric illness has evolved and, for the most part, improved the lives of millions across the country and around the world.
However, in so doing, we have, perhaps inadvertently, or maybe out of necessity, found ourselves on an allegorical island that we, as clinical, everyday psychiatrists defend to the death. Surrounded by an ocean of psychiatric disorders, illnesses, and symptomatology, we wave our prescription pads (or e-pads), like wands, hoping to calm the torrents of the psychiatric sea with yet another prescription. However, I’m not writing this to bemoan modern psychopharmacology, for it has saved and improved lives and led to a fruitful practice and livelihood for me and most of my colleagues. As the torrents of illness continue to flare around us, I’m not advocating that we put down our wands and become strictly therapists. What I am advocating for is the use of different wands, as it were.
Our chosen wand is undoubtedly largely composed of monoamine-based remedies – most involving the holy trinity of serotonin, dopamine, and norepinephrine. As we stand on our island, trying to quell hurricane-force winds, shark-infested waters, or a tidal wave, we wave the same wand – hoping that a dash of monoamine will slow down winds, scare away sharks, and reduce the destructive capacity of water.
We, more than those in any other medical profession, use the same basic treatments for heterogeneous disorders, whose true underlying physiology, despite important progress over the years, remains elusive and only partly understood. We see improvement and even resolution sometimes, but, for the most part, our treatments keep our patients going, so they can continue to sail, just avoiding being capsized by the psychiatric torrents beneath the surface. When the torrents flare, we wave the same wand again, hoping that another dash of monoamine modulation, this time, maybe in a new wrapper, or with a new name, will keep the ocean calm for a bit longer. Now, this has worked for decades to keep many ships from being capsized and our island still largely habitable, but this strategy is akin to building a shelter out of twigs and leaves and grasses, and never advancing to permanent construction techniques, and just replacing broken branches and leaves that will, undoubtedly, break again.
As we stand on our island, I say, we use the branches, leaves, and twigs to build a bridge to another island, and, there, we can make new wands.
In 2020, the materials for these new wands are readily available, but we have to be willing to trust these new wands, and yet not completely discard the old wands we have used for so long. These new wands are the nonmonoamine-based treatments, which have shown remarkable efficacy and safety in patients across the country and the world. We must accept that we, as a species, are remarkably complex creatures, and the disorders we try to treat have their origins in the most complex part of our being: the brain. Therefore, considering the complexity, it is only reasonable to think that there is more to our illnesses than modulating monoamines.
In 2020, I challenge every day, clinical psychiatrists to embrace these new treatments and wave these new wands. As someone who has been fortunate enough to prescribe ketamine infusion and nasal sprays in the clinic, I can say we must gravitate toward these new treatments when clinically appropriate. While ketamine treatment is no panacea, its use, and the adoption of other nonmonoamine-based treatments, hopefully will fuel the development, use, and perhaps, most importantly, novel thinking about new biological treatment of psychiatric illness.
Dr. Shroff is board-certified in psychiatry in sleep medicine, and practices in Smyrna, Ga. He is a fellow of the American Psychiatric Association.
Now that 2019 has passed us by, it is a time for reflection for most of us. We can think about the state of our important relationships, perhaps the growth of our children or other loved ones, the trajectory of our practices, and ideas for the future. Most commonly, I would wager, we likely think about how we might do things differently in the new year.
Applying this lens to our chosen profession, the practice of clinical psychiatry, I hope 2020 brings real, or at least, incremental change. Our profession has evolved markedly over the last several decades, from psychoanalysis to the psychopharmacology revolution, to a now largely multimodal approach. Our treatment of psychiatric illness has evolved and, for the most part, improved the lives of millions across the country and around the world.
However, in so doing, we have, perhaps inadvertently, or maybe out of necessity, found ourselves on an allegorical island that we, as clinical, everyday psychiatrists defend to the death. Surrounded by an ocean of psychiatric disorders, illnesses, and symptomatology, we wave our prescription pads (or e-pads), like wands, hoping to calm the torrents of the psychiatric sea with yet another prescription. However, I’m not writing this to bemoan modern psychopharmacology, for it has saved and improved lives and led to a fruitful practice and livelihood for me and most of my colleagues. As the torrents of illness continue to flare around us, I’m not advocating that we put down our wands and become strictly therapists. What I am advocating for is the use of different wands, as it were.
Our chosen wand is undoubtedly largely composed of monoamine-based remedies – most involving the holy trinity of serotonin, dopamine, and norepinephrine. As we stand on our island, trying to quell hurricane-force winds, shark-infested waters, or a tidal wave, we wave the same wand – hoping that a dash of monoamine will slow down winds, scare away sharks, and reduce the destructive capacity of water.
We, more than those in any other medical profession, use the same basic treatments for heterogeneous disorders, whose true underlying physiology, despite important progress over the years, remains elusive and only partly understood. We see improvement and even resolution sometimes, but, for the most part, our treatments keep our patients going, so they can continue to sail, just avoiding being capsized by the psychiatric torrents beneath the surface. When the torrents flare, we wave the same wand again, hoping that another dash of monoamine modulation, this time, maybe in a new wrapper, or with a new name, will keep the ocean calm for a bit longer. Now, this has worked for decades to keep many ships from being capsized and our island still largely habitable, but this strategy is akin to building a shelter out of twigs and leaves and grasses, and never advancing to permanent construction techniques, and just replacing broken branches and leaves that will, undoubtedly, break again.
As we stand on our island, I say, we use the branches, leaves, and twigs to build a bridge to another island, and, there, we can make new wands.
In 2020, the materials for these new wands are readily available, but we have to be willing to trust these new wands, and yet not completely discard the old wands we have used for so long. These new wands are the nonmonoamine-based treatments, which have shown remarkable efficacy and safety in patients across the country and the world. We must accept that we, as a species, are remarkably complex creatures, and the disorders we try to treat have their origins in the most complex part of our being: the brain. Therefore, considering the complexity, it is only reasonable to think that there is more to our illnesses than modulating monoamines.
In 2020, I challenge every day, clinical psychiatrists to embrace these new treatments and wave these new wands. As someone who has been fortunate enough to prescribe ketamine infusion and nasal sprays in the clinic, I can say we must gravitate toward these new treatments when clinically appropriate. While ketamine treatment is no panacea, its use, and the adoption of other nonmonoamine-based treatments, hopefully will fuel the development, use, and perhaps, most importantly, novel thinking about new biological treatment of psychiatric illness.
Dr. Shroff is board-certified in psychiatry in sleep medicine, and practices in Smyrna, Ga. He is a fellow of the American Psychiatric Association.
Caution about ‘miracle cures’; more
Caution about ‘miracle cures’
I thank Drs. Katherine Epstein and Helen Farrell for the balanced approach in their article “‘Miracle cures’ in psychiatry?” (Psychiatry 2.0,
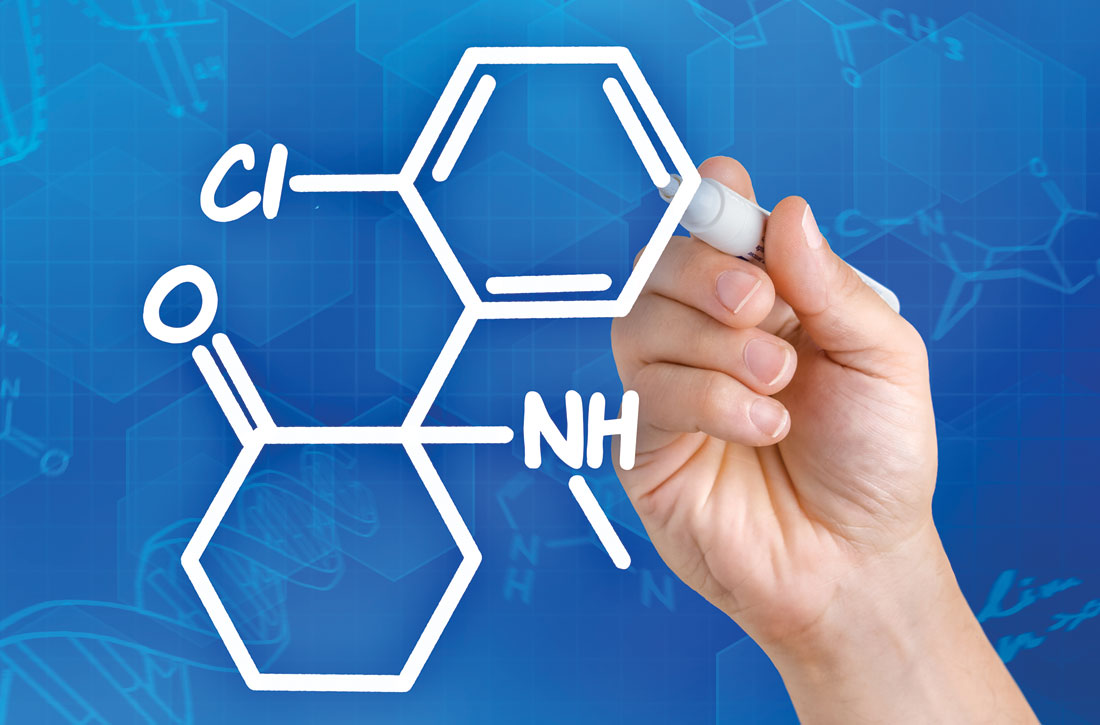
We need to pay serious attention to the small sample sizes and limited criteria for patient selection in trials of ketamine and MDMA, as well as to what sort of “psychotherapy” follows treatment with these agents. Many of us in psychiatric practice for the past 40 years have been humbled by patients’ idiosyncratic reactions to standard medications, let alone novel ones. Those of us who practiced psychiatry in the heyday of “party drugs” have seen many idiosyncratic reactions. Most early research with cannabinoids and lysergic acid diethylamide (and even Strassman’s trials with N,N-dimethyltryptamine [DMT]1-5) highlighted the significance of response by drug-naïve patients vs drug-savvy individuals. Apart from Veterans Affairs trials for posttraumatic stress disorder, many trials of these drugs for treatment-resistant depression or end-of-life care have attracted non-naïve participants.6-8 Private use of entheogens is quite different from medicalizing their use. This requires our best scrutiny. Our earnest interest in improving outcomes must not be influenced by the promise of a quick fix, let alone a miracle cure.
Sara Hartley, MD
Clinical Faculty
Interim Head of Admissions
UC Berkley/UCSF Joint Medical Program
Berkeley, California
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Strassman RJ. Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res. 1996;73(1-2):121-124.
2. Strassman RJ. DMT: the spirit molecule. A doctor’s revolutionary research into the biology of near-death and mystical experiences. Rochester, VT: Park Street Press; 2001.
3. Strassman RJ, Qualls CR. Dose-response study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Arch Gen Psychiatry. 1994;51(2):85-97.
4. Strassman RJ, Qualls CR, Berg LM. Differential tolerance to biological and subjective effects of four closely spaced doses of N,N-dimethyltryptamine in humans. Biol Psychiatry. 1996;39(9):784-795.
5. Strassman RJ, Qualls CR, Uhlenhuth EH, et al. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51(2):98-108.
6. Albott CS, et al. Improvement in suicidal ideation after repeated ketamine infusions: Relationship to reductions in symptoms of posttraumatic stress disorder, depression, and pain. Presented at: The Anxiety and Depression Association of America Annual Conference; Mar. 28-31, 2019; Chicago.
7. Abdallah CG, Sanacora G, Duman RS, et al. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509-523.
8. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-Methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
Continue to: Physician assistants and the psychiatrist shortage
Physician assistants and the psychiatrist shortage
J. Michael Smith’s article “Physician assistants in psychiatry: Helping to meet America’s mental health needs” (Commentary,
There needs to be a multifocal approach to incentivize medical students to choose psychiatry as a specialty. Several factors have discouraged medical students from going into psychiatry. The low reimbursement rates by insurance companies force psychiatrists to not accept insurances or to work for hospital or clinic organizations, where they become a part of the “medication management industry.” This scenario was created by the pharmaceutical industry and often leaves psychotherapy to other types of clinicians. In the not-too-distant future, advances in both neuroscience and artificial intelligence technologies will further reduce the role of medically trained psychiatrists, and might lead to them being replaced by other emerging professions (eg, psychiatric PAs) that are concentrated in urban settings where they are most profitable.
What can possibly be left for the future of the medically trained psychiatrist if a PA can diagnose and treat psychiatric patients? Why would we need more psychiatrists?
Marco T. Carpio, MD
Psychiatrist, private practice
Lynbrook, New York
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
The author responds
I appreciate Dr. Carpio’s comments, and I agree that the shortage of psychiatrists will not be addressed solely by the addition of other types of clinicians, such as PAs and nurse practitioners. However, the use of well-trained health care providers such as PAs will go a long way towards helping patients receive timely and appropriate access to care. Unfortunately, no single plan or method will be adequate to solve the shortage of psychiatrists in the United States, but that does not negate the need for utilizing all available options to improve access to quality mental health care. Physician assistants are well-trained to support this endeavor.
J. Michael Smith, DHSc, MPAS, PA-C, CAQ-Psychiatry
Post-Graduate PA Mental Health Residency Training Director
Physician Assistant, ACCESS Clinic, GMHC
Michael E. DeBakey VA Medical Center
Houston, Texas
Continue to: Additional anathemas in psychiatry
Additional anathemas in psychiatry
While reading Dr. Nasrallah’s “Anathemas of psychiatric practice” (From the Editor,
- Cash-only suboxone clinics. Suboxone was never intended to be used in “suboxone clinics”; it was meant to be part of an integrated treatment provided in an office-based practice. Nevertheless, this treatment has been used as such in this country. As part of this trend, an anathema has grown: cash-only suboxone clinics. Patients with severe substance use disorders can be found in every socioeconomic layer of our society, but many struggle with significant psychosocial adversity and outright poverty. Cash-only suboxone clinics put many patients in a bind. Patients spend their last dollars on a needed treatment or sell these medications to maintain their addiction, or even to purchase food.
- “Medical” marijuana. There is no credible evidence based upon methodologically sound research that cannabis has benefit for treating any mental illness. In fact, there is evidence to the contrary.1 Yet, in many states, physicians—including psychiatrists—are supporting the approval of medical marijuana. I remember taking my Hippocratic Oath when I graduated from medical school, pledging to continue educating myself and my patients about evidenced-based medical science that benefits us all. I have not yet found credible evidence supporting medical marijuana.
Greed in general is a strong anathema in medicine.
Leo Bastiaens, MD
Clinical Associate Professor of Psychiatry
University of Pittsburgh School of Medicine
Pittsburgh, Pennsylvania
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Reference
1. Radhakrishnan R, Ranganathan M, D'Souza DC. Medical marijuana: what physicians need to know. J Clin Psychiatry. 2019;80(5):45-47.
Caution about ‘miracle cures’
I thank Drs. Katherine Epstein and Helen Farrell for the balanced approach in their article “‘Miracle cures’ in psychiatry?” (Psychiatry 2.0,
We need to pay serious attention to the small sample sizes and limited criteria for patient selection in trials of ketamine and MDMA, as well as to what sort of “psychotherapy” follows treatment with these agents. Many of us in psychiatric practice for the past 40 years have been humbled by patients’ idiosyncratic reactions to standard medications, let alone novel ones. Those of us who practiced psychiatry in the heyday of “party drugs” have seen many idiosyncratic reactions. Most early research with cannabinoids and lysergic acid diethylamide (and even Strassman’s trials with N,N-dimethyltryptamine [DMT]1-5) highlighted the significance of response by drug-naïve patients vs drug-savvy individuals. Apart from Veterans Affairs trials for posttraumatic stress disorder, many trials of these drugs for treatment-resistant depression or end-of-life care have attracted non-naïve participants.6-8 Private use of entheogens is quite different from medicalizing their use. This requires our best scrutiny. Our earnest interest in improving outcomes must not be influenced by the promise of a quick fix, let alone a miracle cure.
Sara Hartley, MD
Clinical Faculty
Interim Head of Admissions
UC Berkley/UCSF Joint Medical Program
Berkeley, California
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Strassman RJ. Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res. 1996;73(1-2):121-124.
2. Strassman RJ. DMT: the spirit molecule. A doctor’s revolutionary research into the biology of near-death and mystical experiences. Rochester, VT: Park Street Press; 2001.
3. Strassman RJ, Qualls CR. Dose-response study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Arch Gen Psychiatry. 1994;51(2):85-97.
4. Strassman RJ, Qualls CR, Berg LM. Differential tolerance to biological and subjective effects of four closely spaced doses of N,N-dimethyltryptamine in humans. Biol Psychiatry. 1996;39(9):784-795.
5. Strassman RJ, Qualls CR, Uhlenhuth EH, et al. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51(2):98-108.
6. Albott CS, et al. Improvement in suicidal ideation after repeated ketamine infusions: Relationship to reductions in symptoms of posttraumatic stress disorder, depression, and pain. Presented at: The Anxiety and Depression Association of America Annual Conference; Mar. 28-31, 2019; Chicago.
7. Abdallah CG, Sanacora G, Duman RS, et al. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509-523.
8. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-Methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
Continue to: Physician assistants and the psychiatrist shortage
Physician assistants and the psychiatrist shortage
J. Michael Smith’s article “Physician assistants in psychiatry: Helping to meet America’s mental health needs” (Commentary,
There needs to be a multifocal approach to incentivize medical students to choose psychiatry as a specialty. Several factors have discouraged medical students from going into psychiatry. The low reimbursement rates by insurance companies force psychiatrists to not accept insurances or to work for hospital or clinic organizations, where they become a part of the “medication management industry.” This scenario was created by the pharmaceutical industry and often leaves psychotherapy to other types of clinicians. In the not-too-distant future, advances in both neuroscience and artificial intelligence technologies will further reduce the role of medically trained psychiatrists, and might lead to them being replaced by other emerging professions (eg, psychiatric PAs) that are concentrated in urban settings where they are most profitable.
What can possibly be left for the future of the medically trained psychiatrist if a PA can diagnose and treat psychiatric patients? Why would we need more psychiatrists?
Marco T. Carpio, MD
Psychiatrist, private practice
Lynbrook, New York
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
The author responds
I appreciate Dr. Carpio’s comments, and I agree that the shortage of psychiatrists will not be addressed solely by the addition of other types of clinicians, such as PAs and nurse practitioners. However, the use of well-trained health care providers such as PAs will go a long way towards helping patients receive timely and appropriate access to care. Unfortunately, no single plan or method will be adequate to solve the shortage of psychiatrists in the United States, but that does not negate the need for utilizing all available options to improve access to quality mental health care. Physician assistants are well-trained to support this endeavor.
J. Michael Smith, DHSc, MPAS, PA-C, CAQ-Psychiatry
Post-Graduate PA Mental Health Residency Training Director
Physician Assistant, ACCESS Clinic, GMHC
Michael E. DeBakey VA Medical Center
Houston, Texas
Continue to: Additional anathemas in psychiatry
Additional anathemas in psychiatry
While reading Dr. Nasrallah’s “Anathemas of psychiatric practice” (From the Editor,
- Cash-only suboxone clinics. Suboxone was never intended to be used in “suboxone clinics”; it was meant to be part of an integrated treatment provided in an office-based practice. Nevertheless, this treatment has been used as such in this country. As part of this trend, an anathema has grown: cash-only suboxone clinics. Patients with severe substance use disorders can be found in every socioeconomic layer of our society, but many struggle with significant psychosocial adversity and outright poverty. Cash-only suboxone clinics put many patients in a bind. Patients spend their last dollars on a needed treatment or sell these medications to maintain their addiction, or even to purchase food.
- “Medical” marijuana. There is no credible evidence based upon methodologically sound research that cannabis has benefit for treating any mental illness. In fact, there is evidence to the contrary.1 Yet, in many states, physicians—including psychiatrists—are supporting the approval of medical marijuana. I remember taking my Hippocratic Oath when I graduated from medical school, pledging to continue educating myself and my patients about evidenced-based medical science that benefits us all. I have not yet found credible evidence supporting medical marijuana.
Greed in general is a strong anathema in medicine.
Leo Bastiaens, MD
Clinical Associate Professor of Psychiatry
University of Pittsburgh School of Medicine
Pittsburgh, Pennsylvania
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Reference
1. Radhakrishnan R, Ranganathan M, D'Souza DC. Medical marijuana: what physicians need to know. J Clin Psychiatry. 2019;80(5):45-47.
Caution about ‘miracle cures’
I thank Drs. Katherine Epstein and Helen Farrell for the balanced approach in their article “‘Miracle cures’ in psychiatry?” (Psychiatry 2.0,
We need to pay serious attention to the small sample sizes and limited criteria for patient selection in trials of ketamine and MDMA, as well as to what sort of “psychotherapy” follows treatment with these agents. Many of us in psychiatric practice for the past 40 years have been humbled by patients’ idiosyncratic reactions to standard medications, let alone novel ones. Those of us who practiced psychiatry in the heyday of “party drugs” have seen many idiosyncratic reactions. Most early research with cannabinoids and lysergic acid diethylamide (and even Strassman’s trials with N,N-dimethyltryptamine [DMT]1-5) highlighted the significance of response by drug-naïve patients vs drug-savvy individuals. Apart from Veterans Affairs trials for posttraumatic stress disorder, many trials of these drugs for treatment-resistant depression or end-of-life care have attracted non-naïve participants.6-8 Private use of entheogens is quite different from medicalizing their use. This requires our best scrutiny. Our earnest interest in improving outcomes must not be influenced by the promise of a quick fix, let alone a miracle cure.
Sara Hartley, MD
Clinical Faculty
Interim Head of Admissions
UC Berkley/UCSF Joint Medical Program
Berkeley, California
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Strassman RJ. Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res. 1996;73(1-2):121-124.
2. Strassman RJ. DMT: the spirit molecule. A doctor’s revolutionary research into the biology of near-death and mystical experiences. Rochester, VT: Park Street Press; 2001.
3. Strassman RJ, Qualls CR. Dose-response study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Arch Gen Psychiatry. 1994;51(2):85-97.
4. Strassman RJ, Qualls CR, Berg LM. Differential tolerance to biological and subjective effects of four closely spaced doses of N,N-dimethyltryptamine in humans. Biol Psychiatry. 1996;39(9):784-795.
5. Strassman RJ, Qualls CR, Uhlenhuth EH, et al. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51(2):98-108.
6. Albott CS, et al. Improvement in suicidal ideation after repeated ketamine infusions: Relationship to reductions in symptoms of posttraumatic stress disorder, depression, and pain. Presented at: The Anxiety and Depression Association of America Annual Conference; Mar. 28-31, 2019; Chicago.
7. Abdallah CG, Sanacora G, Duman RS, et al. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509-523.
8. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-Methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
Continue to: Physician assistants and the psychiatrist shortage
Physician assistants and the psychiatrist shortage
J. Michael Smith’s article “Physician assistants in psychiatry: Helping to meet America’s mental health needs” (Commentary,
There needs to be a multifocal approach to incentivize medical students to choose psychiatry as a specialty. Several factors have discouraged medical students from going into psychiatry. The low reimbursement rates by insurance companies force psychiatrists to not accept insurances or to work for hospital or clinic organizations, where they become a part of the “medication management industry.” This scenario was created by the pharmaceutical industry and often leaves psychotherapy to other types of clinicians. In the not-too-distant future, advances in both neuroscience and artificial intelligence technologies will further reduce the role of medically trained psychiatrists, and might lead to them being replaced by other emerging professions (eg, psychiatric PAs) that are concentrated in urban settings where they are most profitable.
What can possibly be left for the future of the medically trained psychiatrist if a PA can diagnose and treat psychiatric patients? Why would we need more psychiatrists?
Marco T. Carpio, MD
Psychiatrist, private practice
Lynbrook, New York
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
The author responds
I appreciate Dr. Carpio’s comments, and I agree that the shortage of psychiatrists will not be addressed solely by the addition of other types of clinicians, such as PAs and nurse practitioners. However, the use of well-trained health care providers such as PAs will go a long way towards helping patients receive timely and appropriate access to care. Unfortunately, no single plan or method will be adequate to solve the shortage of psychiatrists in the United States, but that does not negate the need for utilizing all available options to improve access to quality mental health care. Physician assistants are well-trained to support this endeavor.
J. Michael Smith, DHSc, MPAS, PA-C, CAQ-Psychiatry
Post-Graduate PA Mental Health Residency Training Director
Physician Assistant, ACCESS Clinic, GMHC
Michael E. DeBakey VA Medical Center
Houston, Texas
Continue to: Additional anathemas in psychiatry
Additional anathemas in psychiatry
While reading Dr. Nasrallah’s “Anathemas of psychiatric practice” (From the Editor,
- Cash-only suboxone clinics. Suboxone was never intended to be used in “suboxone clinics”; it was meant to be part of an integrated treatment provided in an office-based practice. Nevertheless, this treatment has been used as such in this country. As part of this trend, an anathema has grown: cash-only suboxone clinics. Patients with severe substance use disorders can be found in every socioeconomic layer of our society, but many struggle with significant psychosocial adversity and outright poverty. Cash-only suboxone clinics put many patients in a bind. Patients spend their last dollars on a needed treatment or sell these medications to maintain their addiction, or even to purchase food.
- “Medical” marijuana. There is no credible evidence based upon methodologically sound research that cannabis has benefit for treating any mental illness. In fact, there is evidence to the contrary.1 Yet, in many states, physicians—including psychiatrists—are supporting the approval of medical marijuana. I remember taking my Hippocratic Oath when I graduated from medical school, pledging to continue educating myself and my patients about evidenced-based medical science that benefits us all. I have not yet found credible evidence supporting medical marijuana.
Greed in general is a strong anathema in medicine.
Leo Bastiaens, MD
Clinical Associate Professor of Psychiatry
University of Pittsburgh School of Medicine
Pittsburgh, Pennsylvania
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Reference
1. Radhakrishnan R, Ranganathan M, D'Souza DC. Medical marijuana: what physicians need to know. J Clin Psychiatry. 2019;80(5):45-47.
Tectonic shift
Every community practice gastroenterologist knows that private equity is making an aggressive push into our specialty. This is a tectonic shift in GI practice and the implications for private practice, academic training programs, and our GI societies are substantial. Gastro Health (Florida), Atlanta Gastro (Georgia), and GI Alliance (Texas) all closed deals with private equity in 2018 and there are reported to be 16-20 deals completed or in process currently. The three “first movers” formed practice management companies that now have acquired numerous large and small practices around the country. We have one practice with more than 200 physicians and we will see single groups of 500-1000 in the near future.
Imagine what a digestive health multi-state practice of 500 physicians (gastroenterologists, pathologists, surgeons), 200 advance practice providers (APPs) plus other ancillary professionals (psychology, nutrition) could accomplish. Gross revenues could top $1 billion. All back-office operations would be consolidated and managed professionally. Each provider would work top of license so much routine care would be shifted away from MDs. Negotiating power with payers, vendors, hospital systems, and referring providers would be immense (care would be taken to avoid the appearance of a monopoly, but Department of Justice scrutiny has already been evident). Referral sources (CVS, Optum, health systems, and a few remaining independent practices) would be secured by contract or favored reimbursement rates. Academic health systems will find competition challenging save for high tertiary and quaternary care, but even these complex procedures often will have been consolidated (and contained within risk-bundles) to a half dozen health systems by direct-to-employer contracting. Current society offerings such as meetings, journals, and clinical guidelines will become obsolete because of practice-distributed virtual education, open publishing, and internal outcomes measurement. The vast provider network will be on a single data platform so it can generate true outcomes based on a payer’s patient base (not guideline-restricted process measures), and these outcomes will be used for negotiating restricted networks.
I hope these trends will be a clarion call for our societies and training programs to awaken to a new world order and adapt their efforts to meet demands from our patients and the critical (and changing) needs of current and future digestive health professionals.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Every community practice gastroenterologist knows that private equity is making an aggressive push into our specialty. This is a tectonic shift in GI practice and the implications for private practice, academic training programs, and our GI societies are substantial. Gastro Health (Florida), Atlanta Gastro (Georgia), and GI Alliance (Texas) all closed deals with private equity in 2018 and there are reported to be 16-20 deals completed or in process currently. The three “first movers” formed practice management companies that now have acquired numerous large and small practices around the country. We have one practice with more than 200 physicians and we will see single groups of 500-1000 in the near future.
Imagine what a digestive health multi-state practice of 500 physicians (gastroenterologists, pathologists, surgeons), 200 advance practice providers (APPs) plus other ancillary professionals (psychology, nutrition) could accomplish. Gross revenues could top $1 billion. All back-office operations would be consolidated and managed professionally. Each provider would work top of license so much routine care would be shifted away from MDs. Negotiating power with payers, vendors, hospital systems, and referring providers would be immense (care would be taken to avoid the appearance of a monopoly, but Department of Justice scrutiny has already been evident). Referral sources (CVS, Optum, health systems, and a few remaining independent practices) would be secured by contract or favored reimbursement rates. Academic health systems will find competition challenging save for high tertiary and quaternary care, but even these complex procedures often will have been consolidated (and contained within risk-bundles) to a half dozen health systems by direct-to-employer contracting. Current society offerings such as meetings, journals, and clinical guidelines will become obsolete because of practice-distributed virtual education, open publishing, and internal outcomes measurement. The vast provider network will be on a single data platform so it can generate true outcomes based on a payer’s patient base (not guideline-restricted process measures), and these outcomes will be used for negotiating restricted networks.
I hope these trends will be a clarion call for our societies and training programs to awaken to a new world order and adapt their efforts to meet demands from our patients and the critical (and changing) needs of current and future digestive health professionals.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Every community practice gastroenterologist knows that private equity is making an aggressive push into our specialty. This is a tectonic shift in GI practice and the implications for private practice, academic training programs, and our GI societies are substantial. Gastro Health (Florida), Atlanta Gastro (Georgia), and GI Alliance (Texas) all closed deals with private equity in 2018 and there are reported to be 16-20 deals completed or in process currently. The three “first movers” formed practice management companies that now have acquired numerous large and small practices around the country. We have one practice with more than 200 physicians and we will see single groups of 500-1000 in the near future.
Imagine what a digestive health multi-state practice of 500 physicians (gastroenterologists, pathologists, surgeons), 200 advance practice providers (APPs) plus other ancillary professionals (psychology, nutrition) could accomplish. Gross revenues could top $1 billion. All back-office operations would be consolidated and managed professionally. Each provider would work top of license so much routine care would be shifted away from MDs. Negotiating power with payers, vendors, hospital systems, and referring providers would be immense (care would be taken to avoid the appearance of a monopoly, but Department of Justice scrutiny has already been evident). Referral sources (CVS, Optum, health systems, and a few remaining independent practices) would be secured by contract or favored reimbursement rates. Academic health systems will find competition challenging save for high tertiary and quaternary care, but even these complex procedures often will have been consolidated (and contained within risk-bundles) to a half dozen health systems by direct-to-employer contracting. Current society offerings such as meetings, journals, and clinical guidelines will become obsolete because of practice-distributed virtual education, open publishing, and internal outcomes measurement. The vast provider network will be on a single data platform so it can generate true outcomes based on a payer’s patient base (not guideline-restricted process measures), and these outcomes will be used for negotiating restricted networks.
I hope these trends will be a clarion call for our societies and training programs to awaken to a new world order and adapt their efforts to meet demands from our patients and the critical (and changing) needs of current and future digestive health professionals.
John I. Allen, MD, MBA, AGAF
Editor in Chief
20 Reasons to celebrate our APA membership in 2020
The American Psychiatric Association (APA) is the largest psychiatric organization in the world, with >38,500 members across 100 countries. At 175 yea
I am truly honored to be nominated as the next APA President-Elect (Note: Dr. Nasrallah has withdrawn his candidacy for APA President-Elect. For a statement of explanation, click here), which prompted me to delve into the history of this great association that unifies us, empowers us, and gives us a loud voice to advocate for our patients, for our noble medical profession, and for advancing the mental health of society at large.
Our APA was established by 13 superintendents of the “Insane Asylums and Hospitals” in 1844. Its first name was a mouthful—the Association of Medical Superintendents of American Institutions of the Insane, a term now regarded as pejorative and unscientific. Thankfully, the name was changed almost 50 years later (in 1893) to the American Medico-Psychological Association, which was refined 28 years later in 1921 to the American Psychiatric Association, a name that has lasted for the past 99 years. If I am fortunate enough to be elected by my peers this month as President-Elect, and assume the APA Presidency in May 2021, a full century after the name of APA was adopted in 1921 (the era of Kraepelin, Bleuler, and Freud), I will propose and ask the APA members to approve inserting “physicians” in the APA name so it will become the American Psychiatric Physicians Association, or APPA. This will clearly reflect our medical training and identity, and underscore the remarkable progress achieved by the inspiring and diligent work of countless psychiatric physicians over the past century.
By the way, per a Google search, the term “physician” came about in the 13th century, when the Anglo-Normans used the French term “physique” or remedy, to coin the English word “physic” or medicine. Science historian Howard Markel discussed how “physic” became “physician.” As for the term “psychiatrist,” it was coined in 1808 by the German physician Johann Christian Reil, and it essentially means “medical treatment of the soul.”
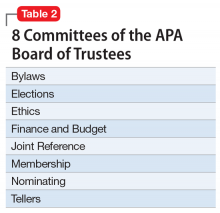
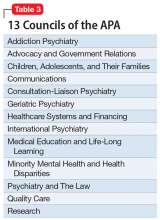
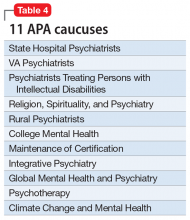
The APA has an amazing structure that is very democratic, enabling members to elect their leaders as well as their representatives on the Assembly. It has a Board of Trustees (Table 1) comprised of 22 members, 7 of whom comprise the Executive Committee, plus 3 attendees. Eight standing committees (Table 2) report to the Board. There are also 13 councils (Table 3), 11 caucuses (Table 4), and 7 minority and underrepresented caucuses (Table 5). The APA has a national network of 76 District Branches (DBs), each usually representing one state, except for large states that have several DBs (California has 5, and New York has 13). The District of Columbia, Puerto Rico, Western Canada, and Quebec/Eastern Canada each have DBs as well. The DBs have their own bylaws, governance structures, and annual dues, and within them, they may have local “societies” in large cities. Finally, each DB elects representatives to the Assembly, which is comprised of 7 Areas, each of which contains several states.
I am glad to have been a member of the APA for more than 4 decades, since my residency days. Although most psychiatrists in the United States and Canada belong to the APA, some do not, either because they never joined, or they dropped out because they think the dues are high (although dues are less than half of 1% of the average psychiatrist’s annual income, which is a great bargain). So, for my colleagues who do belong, and especially for those who do not, I provide 20 reasons why being an APA member offers so many advantages, professionally and personally, and has a tremendous benefit to us individually and collectively:
1. It makes eminent sense to unify as members of a medical profession to enable us to be strong and influential, to overcome our challenges, and to achieve our goals.
Continue to: #2
2. The APA’s main objectives are to advocate for our patients, for member psychiatrists, and for the growth and success of the discipline of psychiatric medicine.
3. Being an APA member helps fight the hurtful stigma and disparity of parity, which we must all strive for together every day for our psychiatric patients.
4. A strong APA will fight for us to eliminate practice hassles such as outrageous pre-authorizations, complicated maintenance of certification process, cumbersome and time-consuming electronic medical records, and medico-legal constraints.
5. Unity affords our Association moral authority and social gravitas so that we become more credible when we educate the public to dispel the many myths and misconceptions about mental illness.
6. The APA provides us with the necessary political power and influence because medical care can be significantly impacted by good or bad legislation.
Continue to: #7
7. Our economic welfare needs a strong APA to which we all belong.
8. The antipsychiatry movement is a malignant antiscientific ideology that must be countered by all of us through a robust APA to which we all must belong.
9. The APA provides an enormous array of services and resources to all of us, individually or as groups. Many members don’t know that because they never ask.
10. While it is good to have subspecialty societies within the APA, we are all psychiatric physicians who have the same medical and psychiatric training and share the same core values. By joining the APA as our Mother Organization, we avoid Balkanization of our profession, which weakens all of us if we are divided into smaller groups.
11. The APA helps cultivate and recruit more medical students to choose psychiatry as a career. This is vital for the health of our field.
Continue to: #12
12. Mentoring residents about the professional issues of our specialty and involving them in committees is one of the priorities of the APA, which extends into the post-residency phase (early career psychiatrists).
13. The APA provides a “Big Tent” of diverse groups of colleagues across a rich mosaic of racial and ethnic groups, genders, national origins, sexual orientations, and practice settings. Our patients are diverse, and so are we.
14. Education is a top priority for the APA, providing its members with a wide array of opportunities for ongoing and life-long learning. This includes the spectacular annual meeting with its cornucopia of educational offers and newsletters, as well as many initiatives throughout the year.
15. The APA journals, especially its flagship American Journal of Psychiatry (AJP), are among the most cited publications in the world. We get them for free, even though the cost of a personal subscription to the AJP alone for non-APA members is equivalent to the entire annual dues!
16. The APA has many top researchers among its members, spread across more than 150 medical schools. Those members generate new knowledge that continuously advances the field of psychiatry and provides new evidence-based tools for psychiatric practitioners.
Continue to: #17
17. The APA is our community, an ecosystem that sustains us as psychiatrists, and connects us in many gratifying ways that keep us rejuvenated and helps us avoid burnout that may occur in absence of a supportive network of supportive peers.
18. The APA provides us discounts on malpractice insurance and other products.
19. Opportunities for personal and professional growth are available within the APA. This includes leadership skills via participation in the DBs or at the national level via committees, councils, caucuses, and the Assembly.
20. Last but not least, the APA represents all of us in The House of Medicine. It has very productive partnerships and collaborations with many other medical organizations that support us and help us achieve our cherished mission. Besides adding “Physicians” to the APA name, working closely with other physicians across many specialties (especially primary care) will consolidate our medical identity and lead to better outcomes for our patients through collaborative care initiatives.
I thank all my colleagues who are APA members or Fellows, and urge all the readers of
PS. Please VOTE in this month’s APA election! It’s our sacred duty.
The American Psychiatric Association (APA) is the largest psychiatric organization in the world, with >38,500 members across 100 countries. At 175 yea
I am truly honored to be nominated as the next APA President-Elect (Note: Dr. Nasrallah has withdrawn his candidacy for APA President-Elect. For a statement of explanation, click here), which prompted me to delve into the history of this great association that unifies us, empowers us, and gives us a loud voice to advocate for our patients, for our noble medical profession, and for advancing the mental health of society at large.
Our APA was established by 13 superintendents of the “Insane Asylums and Hospitals” in 1844. Its first name was a mouthful—the Association of Medical Superintendents of American Institutions of the Insane, a term now regarded as pejorative and unscientific. Thankfully, the name was changed almost 50 years later (in 1893) to the American Medico-Psychological Association, which was refined 28 years later in 1921 to the American Psychiatric Association, a name that has lasted for the past 99 years. If I am fortunate enough to be elected by my peers this month as President-Elect, and assume the APA Presidency in May 2021, a full century after the name of APA was adopted in 1921 (the era of Kraepelin, Bleuler, and Freud), I will propose and ask the APA members to approve inserting “physicians” in the APA name so it will become the American Psychiatric Physicians Association, or APPA. This will clearly reflect our medical training and identity, and underscore the remarkable progress achieved by the inspiring and diligent work of countless psychiatric physicians over the past century.
By the way, per a Google search, the term “physician” came about in the 13th century, when the Anglo-Normans used the French term “physique” or remedy, to coin the English word “physic” or medicine. Science historian Howard Markel discussed how “physic” became “physician.” As for the term “psychiatrist,” it was coined in 1808 by the German physician Johann Christian Reil, and it essentially means “medical treatment of the soul.”
The APA has an amazing structure that is very democratic, enabling members to elect their leaders as well as their representatives on the Assembly. It has a Board of Trustees (Table 1) comprised of 22 members, 7 of whom comprise the Executive Committee, plus 3 attendees. Eight standing committees (Table 2) report to the Board. There are also 13 councils (Table 3), 11 caucuses (Table 4), and 7 minority and underrepresented caucuses (Table 5). The APA has a national network of 76 District Branches (DBs), each usually representing one state, except for large states that have several DBs (California has 5, and New York has 13). The District of Columbia, Puerto Rico, Western Canada, and Quebec/Eastern Canada each have DBs as well. The DBs have their own bylaws, governance structures, and annual dues, and within them, they may have local “societies” in large cities. Finally, each DB elects representatives to the Assembly, which is comprised of 7 Areas, each of which contains several states.
I am glad to have been a member of the APA for more than 4 decades, since my residency days. Although most psychiatrists in the United States and Canada belong to the APA, some do not, either because they never joined, or they dropped out because they think the dues are high (although dues are less than half of 1% of the average psychiatrist’s annual income, which is a great bargain). So, for my colleagues who do belong, and especially for those who do not, I provide 20 reasons why being an APA member offers so many advantages, professionally and personally, and has a tremendous benefit to us individually and collectively:
1. It makes eminent sense to unify as members of a medical profession to enable us to be strong and influential, to overcome our challenges, and to achieve our goals.
Continue to: #2
2. The APA’s main objectives are to advocate for our patients, for member psychiatrists, and for the growth and success of the discipline of psychiatric medicine.
3. Being an APA member helps fight the hurtful stigma and disparity of parity, which we must all strive for together every day for our psychiatric patients.
4. A strong APA will fight for us to eliminate practice hassles such as outrageous pre-authorizations, complicated maintenance of certification process, cumbersome and time-consuming electronic medical records, and medico-legal constraints.
5. Unity affords our Association moral authority and social gravitas so that we become more credible when we educate the public to dispel the many myths and misconceptions about mental illness.
6. The APA provides us with the necessary political power and influence because medical care can be significantly impacted by good or bad legislation.
Continue to: #7
7. Our economic welfare needs a strong APA to which we all belong.
8. The antipsychiatry movement is a malignant antiscientific ideology that must be countered by all of us through a robust APA to which we all must belong.
9. The APA provides an enormous array of services and resources to all of us, individually or as groups. Many members don’t know that because they never ask.
10. While it is good to have subspecialty societies within the APA, we are all psychiatric physicians who have the same medical and psychiatric training and share the same core values. By joining the APA as our Mother Organization, we avoid Balkanization of our profession, which weakens all of us if we are divided into smaller groups.
11. The APA helps cultivate and recruit more medical students to choose psychiatry as a career. This is vital for the health of our field.
Continue to: #12
12. Mentoring residents about the professional issues of our specialty and involving them in committees is one of the priorities of the APA, which extends into the post-residency phase (early career psychiatrists).
13. The APA provides a “Big Tent” of diverse groups of colleagues across a rich mosaic of racial and ethnic groups, genders, national origins, sexual orientations, and practice settings. Our patients are diverse, and so are we.
14. Education is a top priority for the APA, providing its members with a wide array of opportunities for ongoing and life-long learning. This includes the spectacular annual meeting with its cornucopia of educational offers and newsletters, as well as many initiatives throughout the year.
15. The APA journals, especially its flagship American Journal of Psychiatry (AJP), are among the most cited publications in the world. We get them for free, even though the cost of a personal subscription to the AJP alone for non-APA members is equivalent to the entire annual dues!
16. The APA has many top researchers among its members, spread across more than 150 medical schools. Those members generate new knowledge that continuously advances the field of psychiatry and provides new evidence-based tools for psychiatric practitioners.
Continue to: #17
17. The APA is our community, an ecosystem that sustains us as psychiatrists, and connects us in many gratifying ways that keep us rejuvenated and helps us avoid burnout that may occur in absence of a supportive network of supportive peers.
18. The APA provides us discounts on malpractice insurance and other products.
19. Opportunities for personal and professional growth are available within the APA. This includes leadership skills via participation in the DBs or at the national level via committees, councils, caucuses, and the Assembly.
20. Last but not least, the APA represents all of us in The House of Medicine. It has very productive partnerships and collaborations with many other medical organizations that support us and help us achieve our cherished mission. Besides adding “Physicians” to the APA name, working closely with other physicians across many specialties (especially primary care) will consolidate our medical identity and lead to better outcomes for our patients through collaborative care initiatives.
I thank all my colleagues who are APA members or Fellows, and urge all the readers of
PS. Please VOTE in this month’s APA election! It’s our sacred duty.
The American Psychiatric Association (APA) is the largest psychiatric organization in the world, with >38,500 members across 100 countries. At 175 yea
I am truly honored to be nominated as the next APA President-Elect (Note: Dr. Nasrallah has withdrawn his candidacy for APA President-Elect. For a statement of explanation, click here), which prompted me to delve into the history of this great association that unifies us, empowers us, and gives us a loud voice to advocate for our patients, for our noble medical profession, and for advancing the mental health of society at large.
Our APA was established by 13 superintendents of the “Insane Asylums and Hospitals” in 1844. Its first name was a mouthful—the Association of Medical Superintendents of American Institutions of the Insane, a term now regarded as pejorative and unscientific. Thankfully, the name was changed almost 50 years later (in 1893) to the American Medico-Psychological Association, which was refined 28 years later in 1921 to the American Psychiatric Association, a name that has lasted for the past 99 years. If I am fortunate enough to be elected by my peers this month as President-Elect, and assume the APA Presidency in May 2021, a full century after the name of APA was adopted in 1921 (the era of Kraepelin, Bleuler, and Freud), I will propose and ask the APA members to approve inserting “physicians” in the APA name so it will become the American Psychiatric Physicians Association, or APPA. This will clearly reflect our medical training and identity, and underscore the remarkable progress achieved by the inspiring and diligent work of countless psychiatric physicians over the past century.
By the way, per a Google search, the term “physician” came about in the 13th century, when the Anglo-Normans used the French term “physique” or remedy, to coin the English word “physic” or medicine. Science historian Howard Markel discussed how “physic” became “physician.” As for the term “psychiatrist,” it was coined in 1808 by the German physician Johann Christian Reil, and it essentially means “medical treatment of the soul.”
The APA has an amazing structure that is very democratic, enabling members to elect their leaders as well as their representatives on the Assembly. It has a Board of Trustees (Table 1) comprised of 22 members, 7 of whom comprise the Executive Committee, plus 3 attendees. Eight standing committees (Table 2) report to the Board. There are also 13 councils (Table 3), 11 caucuses (Table 4), and 7 minority and underrepresented caucuses (Table 5). The APA has a national network of 76 District Branches (DBs), each usually representing one state, except for large states that have several DBs (California has 5, and New York has 13). The District of Columbia, Puerto Rico, Western Canada, and Quebec/Eastern Canada each have DBs as well. The DBs have their own bylaws, governance structures, and annual dues, and within them, they may have local “societies” in large cities. Finally, each DB elects representatives to the Assembly, which is comprised of 7 Areas, each of which contains several states.
I am glad to have been a member of the APA for more than 4 decades, since my residency days. Although most psychiatrists in the United States and Canada belong to the APA, some do not, either because they never joined, or they dropped out because they think the dues are high (although dues are less than half of 1% of the average psychiatrist’s annual income, which is a great bargain). So, for my colleagues who do belong, and especially for those who do not, I provide 20 reasons why being an APA member offers so many advantages, professionally and personally, and has a tremendous benefit to us individually and collectively:
1. It makes eminent sense to unify as members of a medical profession to enable us to be strong and influential, to overcome our challenges, and to achieve our goals.
Continue to: #2
2. The APA’s main objectives are to advocate for our patients, for member psychiatrists, and for the growth and success of the discipline of psychiatric medicine.
3. Being an APA member helps fight the hurtful stigma and disparity of parity, which we must all strive for together every day for our psychiatric patients.
4. A strong APA will fight for us to eliminate practice hassles such as outrageous pre-authorizations, complicated maintenance of certification process, cumbersome and time-consuming electronic medical records, and medico-legal constraints.
5. Unity affords our Association moral authority and social gravitas so that we become more credible when we educate the public to dispel the many myths and misconceptions about mental illness.
6. The APA provides us with the necessary political power and influence because medical care can be significantly impacted by good or bad legislation.
Continue to: #7
7. Our economic welfare needs a strong APA to which we all belong.
8. The antipsychiatry movement is a malignant antiscientific ideology that must be countered by all of us through a robust APA to which we all must belong.
9. The APA provides an enormous array of services and resources to all of us, individually or as groups. Many members don’t know that because they never ask.
10. While it is good to have subspecialty societies within the APA, we are all psychiatric physicians who have the same medical and psychiatric training and share the same core values. By joining the APA as our Mother Organization, we avoid Balkanization of our profession, which weakens all of us if we are divided into smaller groups.
11. The APA helps cultivate and recruit more medical students to choose psychiatry as a career. This is vital for the health of our field.
Continue to: #12
12. Mentoring residents about the professional issues of our specialty and involving them in committees is one of the priorities of the APA, which extends into the post-residency phase (early career psychiatrists).
13. The APA provides a “Big Tent” of diverse groups of colleagues across a rich mosaic of racial and ethnic groups, genders, national origins, sexual orientations, and practice settings. Our patients are diverse, and so are we.
14. Education is a top priority for the APA, providing its members with a wide array of opportunities for ongoing and life-long learning. This includes the spectacular annual meeting with its cornucopia of educational offers and newsletters, as well as many initiatives throughout the year.
15. The APA journals, especially its flagship American Journal of Psychiatry (AJP), are among the most cited publications in the world. We get them for free, even though the cost of a personal subscription to the AJP alone for non-APA members is equivalent to the entire annual dues!
16. The APA has many top researchers among its members, spread across more than 150 medical schools. Those members generate new knowledge that continuously advances the field of psychiatry and provides new evidence-based tools for psychiatric practitioners.
Continue to: #17
17. The APA is our community, an ecosystem that sustains us as psychiatrists, and connects us in many gratifying ways that keep us rejuvenated and helps us avoid burnout that may occur in absence of a supportive network of supportive peers.
18. The APA provides us discounts on malpractice insurance and other products.
19. Opportunities for personal and professional growth are available within the APA. This includes leadership skills via participation in the DBs or at the national level via committees, councils, caucuses, and the Assembly.
20. Last but not least, the APA represents all of us in The House of Medicine. It has very productive partnerships and collaborations with many other medical organizations that support us and help us achieve our cherished mission. Besides adding “Physicians” to the APA name, working closely with other physicians across many specialties (especially primary care) will consolidate our medical identity and lead to better outcomes for our patients through collaborative care initiatives.
I thank all my colleagues who are APA members or Fellows, and urge all the readers of
PS. Please VOTE in this month’s APA election! It’s our sacred duty.
Ketamine/esketamine: Putative mechanisms of action
Since the FDA approved intranasal esketamine, there has understandably been significant dialogue, debate, and discussion about the possible mechanisms of action of its antidepressant effects. Ketamine, the racemate of esketamine and arketamine, has been used off-label since the late 1990s. The first study of IV ketamine’s rapid antidepressant activity was published in 2000.1 In that study, 7 patients with major depressive disorder (MDD) were treated in a double-blind/placebo-controlled manner with IV ketamine or placebo. Researchers found a significant antidepressant effect within 72 hours with the administration of IV ketamine.
There is a tremendous number of publications related to ketamine, which creates a large reservoir of information to review in an attempt to piece together what we currently know about the mechanisms of action of ketamine/esketamine (K/ESK). A search of PubMed using the search word “ketamine” (October 8, 2019; www.ncbi.nlm.nih.gov/pubmed) produced a list of 4,869 articles just in the last 5 years; and the search words “ketamine and depression” produced a list of 1,221 publications over the same time period.
The FDA approval of intranasal esketamine in March 2019 was based on 5 phase III clinical studies (albeit not all were positive studies) and >9 years of intensive preclinical and clinical research on the efficacy and safety of intranasal esketamine in treatment-resistant depression (TRD). At the time the FDA approved it, esketamine had been studied in 1,700 patients with TRD, with 1-year safety data on approximately 800 patients. Despite this established data portfolio, critics of K/ESK continue to opine that we do not have enough long-term experience with these drugs, and some key opinion leaders continue to voice caution about the clinical use of K/ESK until we obtain more information and experience.
An article in the September 2019 issue
Of greater concern to me is the authors’ simplistic and flawed description of the mechanism of action of ketamine. They state “based on available research, ketamine’s long-lasting effects seem to come from 2 mechanisms… activation of endogenous opioid receptors… [and] blockade of N-methyl-
Ketamine: A plethora of studies
An impressive body of literature is attempting to piece together the complex and multidimensional neurophysiological mechanisms that result in ketamine’s rapid-acting antidepressant (RAAD) effect, which occurs as soon as 4 hours post-dose. A plethora of pre-clinical and clinical studies, including functional connectivity MRI scans in individuals with MDD, have provided a rough outline, albeit incomplete, of ketamine’s mechanisms of action. Ketamine was discovered in 1962 by chemist Calvin L. Stevens, who was experimenting with novel molecular structures to find a replacement for phencyclidine as a safer dissociative anesthetic. After successful experiments in human prisoners in 1964, ketamine was further studied and became FDA-approved in 1970 as a dissociative anesthetic. Lacking respiratory depression and hypotension, which were common adverse effects of other anesthetics, ketamine became commonly used on the battlefield in the Vietnam War, and continues to be used as a dissociative anesthetic.
Following the publication of the Berman article1 in 2000 that demonstrated apparent RAAD activity of IV ketamine, interest in ketamine’s use for TRD—a huge unmet need in psychiatry—skyrocketed. Since the FDA approval of iproniazid (a monoamine oxidase inhibitor) as the first medication approved to treat major depression in 1958, and the FDA approval of imipramine in 1959, all subsequent FDA-approved antidepressants have shared iproniazid/imipramine’s properties of modulating the monoamines serotonin, dopamine, and norepinephrine. The infamous Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial concluded that only 37% of patients with a major depressive episode achieve remission with their first antidepressant trial, and only 49% respond (50% improvement in symptoms).3 Ketamine/esketamine offered a novel mechanism of action, presumed to be related to the glutamate system, that demonstrated a clinical improvement in depressive symptoms in as few as 4 hours, with benefits that lasted up to 1 week after a single dose.
Continue to: A model of how ketamine works
A model of how ketamine works
Numerous publications from preclinical and clinical research collectively have woven a putative model of how K/ESK may rapidly improve depression by ultimately increasing synaptogenesis in the human prefrontal cortex—a part of the brain known to atrophy in states of chronic stress and depression.4 What is well established is the noncompetitive antagonism of K/ESK at the N-methyl-
A significant body of evidence supports agonism of the glutamate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor as an important step in the cascade of events that ultimately increases levels of the mammalian target of rapamycin (mTOR), which unleashes protein synthesis in synapses facilitating synaptogenesis. Pretreatment with AMPA receptor antagonists blocks the downstream effect of synaptogenesis.6,7 In support of this putative mechanism, hydroxynorketamine, a metabolite of racemic ketamine that has also demonstrated RAAD activity in a ketamine-like manner, is dependent upon AMPA glutamate receptor upregulation and activation, while not requiring activity at the NMDA-glutamate receptor.8,9
A comprehensive model on the putative molecular cascade of events contributing to the antidepressant effect of ketamine has recently been published10 and mirrors the excellent previous review by Abdallah et al.11 Hirota and Lambert10 propose that antagonism of interneuronal NMDA-glutamate receptors on GABAergic interneurons may result in a prefrontal cortex surge of glutamate, which increases agonism of the AMPA-glutamate receptor. This AMPA-glutamate receptor agonism has been shown to increase expression of brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF),12 both of which converge on increasing levels of mTOR, and the subsequent activation of mTOR, which putatively plays a role in increased production of scaffolding proteins and increased synaptogenesis, especially in the prefrontal cortex. In support of this model, during infusion and at 24 hours after a single ketamine infusion in individuals with MDD, functional connectivity MRI demonstrated an increase in global brain connectivity in the prefrontal cortex.13,14 The demonstration of increased global connectivity in the prefrontal cortex of patients with MDD, both during ketamine infusion and at 24 hours post-infusion, supports the clinical observations in clinics treating patients with K/ESK.
Opioid receptors and ketamine
During the past year, there has been significant discussion in psychiatry about the possible role of the mu opioid receptor and opioid system activation in ketamine’s RAAD effect. Remarkably, the literature supporting this hypothesis in humans is based on a single study by Williams et al.15 The authors’ claim: “We now present the first evidence in humans that opioid receptors are necessary for ketamine’s acute antidepressant effect.” In fact, in my opinion, this single study, which has not been replicated, is highly flawed. It included 30 adults with TRD, but only 12 of the 14 participants who qualified for the planned interim analysis completed the double-blind crossover. The population studied was quite treatment-refractory; the average duration of MDD was 24.1 years, the average age at onset was 17.3 years, and the duration of the current depressive episode at the time of the study was 8.6 years. Most significant to me was the reason the study was terminated: “At the interim analysis, given the finding that the combination of ketamine and naltrexone was not only ineffective but also noxious for many participants, we decided to stop enrolling patients in the study.” A distinct possibility is that the noxious adverse effects from the naltrexone impacted the participants’ experience in a negative manner, dampening down any antidepressant effect from ketamine.
In the August 2019 issue of Molecular Psychiatry, these same authors published a second article16 with conclusions based solely on “a secondary analysis of” the data from the same 12 participants in their first publication. Williams et al16 concluded that naltrexone also decreases the anti-suicidality effects of ketamine. Without any additional data or clinical research, these same authors extrapolated their hypothesized opioid receptor activity of ketamine to include it being responsible for ketamine’s established anti-suicidal effects.
Continue to: Mathew and Rivas-Grajales...
Mathew and Rivas-Grajales17 recently published a thoughtful critique and analysis of the study design and conclusions of the original Williams paper.15 They concluded that insufficient evidence exists to answer the question of how ketamine may interface with the opioid system, and they encourage further research into this important topic.
Two additional recent publications18,19 reported that naltrexone pretreatment did not attenuate the antidepressant effects of ketamine in their participants. Additionally, a recent publication in the anesthesiology literature20 concluded that esketamine reversed respiratory depression that was induced by remifentanil. From a clinical perspective, the most compelling argument against a direct mu opioid receptor mechanism for K/ESK is the lack of any craving, tolerance, or withdrawal in patients with TRD treated with K/ESK in numerous clinical publications comparing K/ESK with placebo. In the case of esketamine, during the 5 phase III clinical trials—including both short- and long-term studies—there was no signal for an opioid-like pharmacology. Significantly, both K/ESK are rapidly metabolized by the human body, and the typical dosing is 2 doses/week for the first month, then 1 dose/week for the next month, then 1 dose every week or less for the remainder of treatment.
Curiously, in the May 2019 issue of the American Journal of Psychiatry, Schatzberg21 (one of the co-authors of the prior 2 studies opining that ketamine has direct opioid system activation) wrote a “Reviews and Overviews” article in which he misrepresents the conclusions of an elegant study by Abdallah et al22 published in December 2018.
Abdallah et al22 added rapamycin, an immunosuppressant and a known inhibitor of mTOR, as a pretreatment to patients in a major depressive episode prior to infusion with IV ketamine. Their hypothesis was to see if the rapamycin decreased ketamine’s rapid antidepressant response—putatively by inhibiting the effect of mTOR. Rather than decreasing ketamine’s antidepressant effect, and in contrast to the placebo pretreatment group, at 2 weeks post IV ketamine infusion, patients treated with rapamycin-ketamine had a longer duration/greater improvement in their depressive symptoms compared with the patients receiving placebo-ketamine (improvement of 41% vs 13%, respectively, P = .04). Abdallah et al22 hypothesized that the pretreatment with rapamycin provides anti-inflammatory benefits to the synaptogenesis resulting from ketamine, which protects the newly formed synapses and prolongs ketamine’s antidepressant effect. Schatzberg21 came to a different conclusion than Abdallah et al,22 opining that because the rapamycin “failed to decrease ketamine response,” this result debunks the role of mTOR as a mediator in the antidepressant effect of ketamine through synaptogenesis.
Much more to learn
We still have a great deal to learn about the mechanism of action of K/ESK. However, clinics that are augmenting antidepressants with K/ESK in patients with TRD report significant and rapid symptom improvement in some patients (personal communications). We still do not understand the actual mechanisms of action of antidepressants and antipsychotics, but this does not curtail their use and clinical benefits to our patients. Ketamine has been extensively studied. In the current appropriate climate of concern about the pervasive and lethal opioid epidemic in the United States, we must remain on solid scientific ground before attributing an opioid mechanism to a novel treatment that has already benefitted many of our most depressed and refractory patients.
Continue to: Looking at the extensive...
Looking at the extensive published data over the past 20 years, a consistent model has emerged whereby glutamate agonism of the AMPA-glutamate receptor, both with and without antagonism of the NMDA-glutamate receptor, appears to set in motion a molecular cascade involving BDNF and VEGF, and ultimately increasing the activity of mTOR, with resulting synaptogenicity that increases global brain connectivity in the human prefrontal cortex. As we continue to understand the complexities and additional circuitries that are involved in the RAAD effect of K/ESK, the hope is that novel molecular targets for future drug development will emerge.
Bottom Line
Extensive published data over the past 20 years has produced a consistent model to explain the putative mechanisms of action for the rapid antidepressant effects of ketamine and esketamine. We must remain on solid scientific ground before attributing an opioid mechanism to a novel treatment that has already benefitted many of our patients with treatment-resistant depression.
Related Resources
- Mattingly GW, Anderson RH. Intranasal esketamine. Current Psychiatry. 2019;18(5):31-38.
- Thase M. Ketamine and esketamine for treating unipolar depression in adults: Administration, efficacy, and adverse effects. UpToDate. www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects.
Drug Brand Names
Esketamine nasal spray • Spravato
Imipramine • Tofranil
Ketamine • Ketalar
Naltrexone • Vivitrol, ReVia
Rapamycin • Rapamune
Remifentanil • Ultiva
1. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351-354.
2. Epstein K, Farrell HM. ‘Miracle cures’ in psychiatry? Current Psychiatry. 2019;18(9):13-16.
3. Valenstein M. Keeping our eyes on STAR*D. Am J Psychiatry. 2006;163:1484-1486.
4. Abdallah CG, Sanacora G, Duman RS, et al. The neurobiology of depression, ketamine and rapid-acting antidepressants: is it glutamate inhibition or activation? Pharmacol Ther. 2018;190:148-158.
5. Moghaddam B, Adams B, Verma A, et al. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921-2927.
6. Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959-964.
7. Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory, and disease. Trends Neurosci. 2010;33(2):67-75.
8. Zanos P, Moaddel R, Morris PJ, et al. NMDA inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481-486.
9. Collo G, Cavalleri L, Chiamulera C, et al. (2R,6R)-Hydroxynorketamine promotes dendrite outgrowth in human inducible pluripotent stem cell-derived neurons through AMPA receptor with timing and exposure compatible with ketamine infusion pharmacokinetics in humans. Neuroreport. 2018;29(16):1425-1430.
10. Hirota K, Lambert DG. Ketamine and depression. Br J Anaesth. 2018;121(6):1198-1202.
11. Abdallah CG, Adams TG, Kelmendi B, et al. Ketamine’s mechanism of action: a path to rapid-acting antidepressants. Depress Anxiety. 2016;33(8):689-697.
12. Deyama S, Bang E, Wohleb ES, et al. Role of neuronal VEGF signaling in the prefrontal cortex in the rapid antidepressant effects of ketamine. Am J Psychiatry. 2019;176(5):388-400.
13. Abdallah CG, Dutta A, Averill CL, et al. Ketamine, but not the NMDAR antagonist lanicemine, increases prefrontal global connectivity in depressed patients. Chronic Stress (Thousand Oaks). 2018;2. doi: 10.1177/2470547018796102.
14. Abdallah CG, Averill LA, Collins KA, et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology. 2017;42(6):1210-1219.
15. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175:1205-1215.
16. Williams NR, Heifets BD, Bentzley BS, et al. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol Psychiatry. 2019;24(12):1779-1786.
17. Mathew SJ, Rivas-Grajales AM. “Does the opioid system block or enhance the antidepressant effects of ketamine?” Chronic Stress. (Thousand Oaks). 2019;3. doi: 10.1177/2470547019852073.
18. Yoon G, Petrakis IL, Krystal JH. Association of combined naltrexone and ketamine with depressive symptoms in a case series of patients with depression and alcohol use disorder. JAMA Psychiatry. 2019;76:337-338.
19. Marton T, Barnes DE, Wallace A, et al. Concurrent use of buprenorphine, methadone, or naltrexone does not inhibit ketamine’s antidepressant activity. Biol Psychiatry. 2019;85(12):e75-e76.
20. Jonkman K, van Rijnsoever E, Olofsen E, et al. Esketamine counters opioid-induced respiratory depression. Br J Anaesth. 2018;120(5):1117-1127.
21. Schatzberg AF. Scientific issues relevant to improving the diagnosis, risk assessment, and treatment of major depression. Am J Psychiatry. 2019;176(5):342-347.
22. Abdallah C, Averill LA, Gueorgueiva R, et al. Rapamycin, an immunosuppressant and mTORC1 inhibitor, triples the antidepressant response rate of ketamine at 2 weeks following treatment: a double blind, placebo-controlled, cross-over, randomized clinical trial. bioRxiv. December 19, 2018. https://www.biorxiv.org/content/10.1101/500959v1. Accessed December 5, 2019.
Since the FDA approved intranasal esketamine, there has understandably been significant dialogue, debate, and discussion about the possible mechanisms of action of its antidepressant effects. Ketamine, the racemate of esketamine and arketamine, has been used off-label since the late 1990s. The first study of IV ketamine’s rapid antidepressant activity was published in 2000.1 In that study, 7 patients with major depressive disorder (MDD) were treated in a double-blind/placebo-controlled manner with IV ketamine or placebo. Researchers found a significant antidepressant effect within 72 hours with the administration of IV ketamine.
There is a tremendous number of publications related to ketamine, which creates a large reservoir of information to review in an attempt to piece together what we currently know about the mechanisms of action of ketamine/esketamine (K/ESK). A search of PubMed using the search word “ketamine” (October 8, 2019; www.ncbi.nlm.nih.gov/pubmed) produced a list of 4,869 articles just in the last 5 years; and the search words “ketamine and depression” produced a list of 1,221 publications over the same time period.
The FDA approval of intranasal esketamine in March 2019 was based on 5 phase III clinical studies (albeit not all were positive studies) and >9 years of intensive preclinical and clinical research on the efficacy and safety of intranasal esketamine in treatment-resistant depression (TRD). At the time the FDA approved it, esketamine had been studied in 1,700 patients with TRD, with 1-year safety data on approximately 800 patients. Despite this established data portfolio, critics of K/ESK continue to opine that we do not have enough long-term experience with these drugs, and some key opinion leaders continue to voice caution about the clinical use of K/ESK until we obtain more information and experience.
An article in the September 2019 issue
Of greater concern to me is the authors’ simplistic and flawed description of the mechanism of action of ketamine. They state “based on available research, ketamine’s long-lasting effects seem to come from 2 mechanisms… activation of endogenous opioid receptors… [and] blockade of N-methyl-
Ketamine: A plethora of studies
An impressive body of literature is attempting to piece together the complex and multidimensional neurophysiological mechanisms that result in ketamine’s rapid-acting antidepressant (RAAD) effect, which occurs as soon as 4 hours post-dose. A plethora of pre-clinical and clinical studies, including functional connectivity MRI scans in individuals with MDD, have provided a rough outline, albeit incomplete, of ketamine’s mechanisms of action. Ketamine was discovered in 1962 by chemist Calvin L. Stevens, who was experimenting with novel molecular structures to find a replacement for phencyclidine as a safer dissociative anesthetic. After successful experiments in human prisoners in 1964, ketamine was further studied and became FDA-approved in 1970 as a dissociative anesthetic. Lacking respiratory depression and hypotension, which were common adverse effects of other anesthetics, ketamine became commonly used on the battlefield in the Vietnam War, and continues to be used as a dissociative anesthetic.
Following the publication of the Berman article1 in 2000 that demonstrated apparent RAAD activity of IV ketamine, interest in ketamine’s use for TRD—a huge unmet need in psychiatry—skyrocketed. Since the FDA approval of iproniazid (a monoamine oxidase inhibitor) as the first medication approved to treat major depression in 1958, and the FDA approval of imipramine in 1959, all subsequent FDA-approved antidepressants have shared iproniazid/imipramine’s properties of modulating the monoamines serotonin, dopamine, and norepinephrine. The infamous Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial concluded that only 37% of patients with a major depressive episode achieve remission with their first antidepressant trial, and only 49% respond (50% improvement in symptoms).3 Ketamine/esketamine offered a novel mechanism of action, presumed to be related to the glutamate system, that demonstrated a clinical improvement in depressive symptoms in as few as 4 hours, with benefits that lasted up to 1 week after a single dose.
Continue to: A model of how ketamine works
A model of how ketamine works
Numerous publications from preclinical and clinical research collectively have woven a putative model of how K/ESK may rapidly improve depression by ultimately increasing synaptogenesis in the human prefrontal cortex—a part of the brain known to atrophy in states of chronic stress and depression.4 What is well established is the noncompetitive antagonism of K/ESK at the N-methyl-
A significant body of evidence supports agonism of the glutamate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor as an important step in the cascade of events that ultimately increases levels of the mammalian target of rapamycin (mTOR), which unleashes protein synthesis in synapses facilitating synaptogenesis. Pretreatment with AMPA receptor antagonists blocks the downstream effect of synaptogenesis.6,7 In support of this putative mechanism, hydroxynorketamine, a metabolite of racemic ketamine that has also demonstrated RAAD activity in a ketamine-like manner, is dependent upon AMPA glutamate receptor upregulation and activation, while not requiring activity at the NMDA-glutamate receptor.8,9
A comprehensive model on the putative molecular cascade of events contributing to the antidepressant effect of ketamine has recently been published10 and mirrors the excellent previous review by Abdallah et al.11 Hirota and Lambert10 propose that antagonism of interneuronal NMDA-glutamate receptors on GABAergic interneurons may result in a prefrontal cortex surge of glutamate, which increases agonism of the AMPA-glutamate receptor. This AMPA-glutamate receptor agonism has been shown to increase expression of brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF),12 both of which converge on increasing levels of mTOR, and the subsequent activation of mTOR, which putatively plays a role in increased production of scaffolding proteins and increased synaptogenesis, especially in the prefrontal cortex. In support of this model, during infusion and at 24 hours after a single ketamine infusion in individuals with MDD, functional connectivity MRI demonstrated an increase in global brain connectivity in the prefrontal cortex.13,14 The demonstration of increased global connectivity in the prefrontal cortex of patients with MDD, both during ketamine infusion and at 24 hours post-infusion, supports the clinical observations in clinics treating patients with K/ESK.
Opioid receptors and ketamine
During the past year, there has been significant discussion in psychiatry about the possible role of the mu opioid receptor and opioid system activation in ketamine’s RAAD effect. Remarkably, the literature supporting this hypothesis in humans is based on a single study by Williams et al.15 The authors’ claim: “We now present the first evidence in humans that opioid receptors are necessary for ketamine’s acute antidepressant effect.” In fact, in my opinion, this single study, which has not been replicated, is highly flawed. It included 30 adults with TRD, but only 12 of the 14 participants who qualified for the planned interim analysis completed the double-blind crossover. The population studied was quite treatment-refractory; the average duration of MDD was 24.1 years, the average age at onset was 17.3 years, and the duration of the current depressive episode at the time of the study was 8.6 years. Most significant to me was the reason the study was terminated: “At the interim analysis, given the finding that the combination of ketamine and naltrexone was not only ineffective but also noxious for many participants, we decided to stop enrolling patients in the study.” A distinct possibility is that the noxious adverse effects from the naltrexone impacted the participants’ experience in a negative manner, dampening down any antidepressant effect from ketamine.
In the August 2019 issue of Molecular Psychiatry, these same authors published a second article16 with conclusions based solely on “a secondary analysis of” the data from the same 12 participants in their first publication. Williams et al16 concluded that naltrexone also decreases the anti-suicidality effects of ketamine. Without any additional data or clinical research, these same authors extrapolated their hypothesized opioid receptor activity of ketamine to include it being responsible for ketamine’s established anti-suicidal effects.
Continue to: Mathew and Rivas-Grajales...
Mathew and Rivas-Grajales17 recently published a thoughtful critique and analysis of the study design and conclusions of the original Williams paper.15 They concluded that insufficient evidence exists to answer the question of how ketamine may interface with the opioid system, and they encourage further research into this important topic.
Two additional recent publications18,19 reported that naltrexone pretreatment did not attenuate the antidepressant effects of ketamine in their participants. Additionally, a recent publication in the anesthesiology literature20 concluded that esketamine reversed respiratory depression that was induced by remifentanil. From a clinical perspective, the most compelling argument against a direct mu opioid receptor mechanism for K/ESK is the lack of any craving, tolerance, or withdrawal in patients with TRD treated with K/ESK in numerous clinical publications comparing K/ESK with placebo. In the case of esketamine, during the 5 phase III clinical trials—including both short- and long-term studies—there was no signal for an opioid-like pharmacology. Significantly, both K/ESK are rapidly metabolized by the human body, and the typical dosing is 2 doses/week for the first month, then 1 dose/week for the next month, then 1 dose every week or less for the remainder of treatment.
Curiously, in the May 2019 issue of the American Journal of Psychiatry, Schatzberg21 (one of the co-authors of the prior 2 studies opining that ketamine has direct opioid system activation) wrote a “Reviews and Overviews” article in which he misrepresents the conclusions of an elegant study by Abdallah et al22 published in December 2018.
Abdallah et al22 added rapamycin, an immunosuppressant and a known inhibitor of mTOR, as a pretreatment to patients in a major depressive episode prior to infusion with IV ketamine. Their hypothesis was to see if the rapamycin decreased ketamine’s rapid antidepressant response—putatively by inhibiting the effect of mTOR. Rather than decreasing ketamine’s antidepressant effect, and in contrast to the placebo pretreatment group, at 2 weeks post IV ketamine infusion, patients treated with rapamycin-ketamine had a longer duration/greater improvement in their depressive symptoms compared with the patients receiving placebo-ketamine (improvement of 41% vs 13%, respectively, P = .04). Abdallah et al22 hypothesized that the pretreatment with rapamycin provides anti-inflammatory benefits to the synaptogenesis resulting from ketamine, which protects the newly formed synapses and prolongs ketamine’s antidepressant effect. Schatzberg21 came to a different conclusion than Abdallah et al,22 opining that because the rapamycin “failed to decrease ketamine response,” this result debunks the role of mTOR as a mediator in the antidepressant effect of ketamine through synaptogenesis.
Much more to learn
We still have a great deal to learn about the mechanism of action of K/ESK. However, clinics that are augmenting antidepressants with K/ESK in patients with TRD report significant and rapid symptom improvement in some patients (personal communications). We still do not understand the actual mechanisms of action of antidepressants and antipsychotics, but this does not curtail their use and clinical benefits to our patients. Ketamine has been extensively studied. In the current appropriate climate of concern about the pervasive and lethal opioid epidemic in the United States, we must remain on solid scientific ground before attributing an opioid mechanism to a novel treatment that has already benefitted many of our most depressed and refractory patients.
Continue to: Looking at the extensive...
Looking at the extensive published data over the past 20 years, a consistent model has emerged whereby glutamate agonism of the AMPA-glutamate receptor, both with and without antagonism of the NMDA-glutamate receptor, appears to set in motion a molecular cascade involving BDNF and VEGF, and ultimately increasing the activity of mTOR, with resulting synaptogenicity that increases global brain connectivity in the human prefrontal cortex. As we continue to understand the complexities and additional circuitries that are involved in the RAAD effect of K/ESK, the hope is that novel molecular targets for future drug development will emerge.
Bottom Line
Extensive published data over the past 20 years has produced a consistent model to explain the putative mechanisms of action for the rapid antidepressant effects of ketamine and esketamine. We must remain on solid scientific ground before attributing an opioid mechanism to a novel treatment that has already benefitted many of our patients with treatment-resistant depression.
Related Resources
- Mattingly GW, Anderson RH. Intranasal esketamine. Current Psychiatry. 2019;18(5):31-38.
- Thase M. Ketamine and esketamine for treating unipolar depression in adults: Administration, efficacy, and adverse effects. UpToDate. www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects.
Drug Brand Names
Esketamine nasal spray • Spravato
Imipramine • Tofranil
Ketamine • Ketalar
Naltrexone • Vivitrol, ReVia
Rapamycin • Rapamune
Remifentanil • Ultiva
Since the FDA approved intranasal esketamine, there has understandably been significant dialogue, debate, and discussion about the possible mechanisms of action of its antidepressant effects. Ketamine, the racemate of esketamine and arketamine, has been used off-label since the late 1990s. The first study of IV ketamine’s rapid antidepressant activity was published in 2000.1 In that study, 7 patients with major depressive disorder (MDD) were treated in a double-blind/placebo-controlled manner with IV ketamine or placebo. Researchers found a significant antidepressant effect within 72 hours with the administration of IV ketamine.
There is a tremendous number of publications related to ketamine, which creates a large reservoir of information to review in an attempt to piece together what we currently know about the mechanisms of action of ketamine/esketamine (K/ESK). A search of PubMed using the search word “ketamine” (October 8, 2019; www.ncbi.nlm.nih.gov/pubmed) produced a list of 4,869 articles just in the last 5 years; and the search words “ketamine and depression” produced a list of 1,221 publications over the same time period.
The FDA approval of intranasal esketamine in March 2019 was based on 5 phase III clinical studies (albeit not all were positive studies) and >9 years of intensive preclinical and clinical research on the efficacy and safety of intranasal esketamine in treatment-resistant depression (TRD). At the time the FDA approved it, esketamine had been studied in 1,700 patients with TRD, with 1-year safety data on approximately 800 patients. Despite this established data portfolio, critics of K/ESK continue to opine that we do not have enough long-term experience with these drugs, and some key opinion leaders continue to voice caution about the clinical use of K/ESK until we obtain more information and experience.
An article in the September 2019 issue
Of greater concern to me is the authors’ simplistic and flawed description of the mechanism of action of ketamine. They state “based on available research, ketamine’s long-lasting effects seem to come from 2 mechanisms… activation of endogenous opioid receptors… [and] blockade of N-methyl-
Ketamine: A plethora of studies
An impressive body of literature is attempting to piece together the complex and multidimensional neurophysiological mechanisms that result in ketamine’s rapid-acting antidepressant (RAAD) effect, which occurs as soon as 4 hours post-dose. A plethora of pre-clinical and clinical studies, including functional connectivity MRI scans in individuals with MDD, have provided a rough outline, albeit incomplete, of ketamine’s mechanisms of action. Ketamine was discovered in 1962 by chemist Calvin L. Stevens, who was experimenting with novel molecular structures to find a replacement for phencyclidine as a safer dissociative anesthetic. After successful experiments in human prisoners in 1964, ketamine was further studied and became FDA-approved in 1970 as a dissociative anesthetic. Lacking respiratory depression and hypotension, which were common adverse effects of other anesthetics, ketamine became commonly used on the battlefield in the Vietnam War, and continues to be used as a dissociative anesthetic.
Following the publication of the Berman article1 in 2000 that demonstrated apparent RAAD activity of IV ketamine, interest in ketamine’s use for TRD—a huge unmet need in psychiatry—skyrocketed. Since the FDA approval of iproniazid (a monoamine oxidase inhibitor) as the first medication approved to treat major depression in 1958, and the FDA approval of imipramine in 1959, all subsequent FDA-approved antidepressants have shared iproniazid/imipramine’s properties of modulating the monoamines serotonin, dopamine, and norepinephrine. The infamous Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial concluded that only 37% of patients with a major depressive episode achieve remission with their first antidepressant trial, and only 49% respond (50% improvement in symptoms).3 Ketamine/esketamine offered a novel mechanism of action, presumed to be related to the glutamate system, that demonstrated a clinical improvement in depressive symptoms in as few as 4 hours, with benefits that lasted up to 1 week after a single dose.
Continue to: A model of how ketamine works
A model of how ketamine works
Numerous publications from preclinical and clinical research collectively have woven a putative model of how K/ESK may rapidly improve depression by ultimately increasing synaptogenesis in the human prefrontal cortex—a part of the brain known to atrophy in states of chronic stress and depression.4 What is well established is the noncompetitive antagonism of K/ESK at the N-methyl-
A significant body of evidence supports agonism of the glutamate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor as an important step in the cascade of events that ultimately increases levels of the mammalian target of rapamycin (mTOR), which unleashes protein synthesis in synapses facilitating synaptogenesis. Pretreatment with AMPA receptor antagonists blocks the downstream effect of synaptogenesis.6,7 In support of this putative mechanism, hydroxynorketamine, a metabolite of racemic ketamine that has also demonstrated RAAD activity in a ketamine-like manner, is dependent upon AMPA glutamate receptor upregulation and activation, while not requiring activity at the NMDA-glutamate receptor.8,9
A comprehensive model on the putative molecular cascade of events contributing to the antidepressant effect of ketamine has recently been published10 and mirrors the excellent previous review by Abdallah et al.11 Hirota and Lambert10 propose that antagonism of interneuronal NMDA-glutamate receptors on GABAergic interneurons may result in a prefrontal cortex surge of glutamate, which increases agonism of the AMPA-glutamate receptor. This AMPA-glutamate receptor agonism has been shown to increase expression of brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF),12 both of which converge on increasing levels of mTOR, and the subsequent activation of mTOR, which putatively plays a role in increased production of scaffolding proteins and increased synaptogenesis, especially in the prefrontal cortex. In support of this model, during infusion and at 24 hours after a single ketamine infusion in individuals with MDD, functional connectivity MRI demonstrated an increase in global brain connectivity in the prefrontal cortex.13,14 The demonstration of increased global connectivity in the prefrontal cortex of patients with MDD, both during ketamine infusion and at 24 hours post-infusion, supports the clinical observations in clinics treating patients with K/ESK.
Opioid receptors and ketamine
During the past year, there has been significant discussion in psychiatry about the possible role of the mu opioid receptor and opioid system activation in ketamine’s RAAD effect. Remarkably, the literature supporting this hypothesis in humans is based on a single study by Williams et al.15 The authors’ claim: “We now present the first evidence in humans that opioid receptors are necessary for ketamine’s acute antidepressant effect.” In fact, in my opinion, this single study, which has not been replicated, is highly flawed. It included 30 adults with TRD, but only 12 of the 14 participants who qualified for the planned interim analysis completed the double-blind crossover. The population studied was quite treatment-refractory; the average duration of MDD was 24.1 years, the average age at onset was 17.3 years, and the duration of the current depressive episode at the time of the study was 8.6 years. Most significant to me was the reason the study was terminated: “At the interim analysis, given the finding that the combination of ketamine and naltrexone was not only ineffective but also noxious for many participants, we decided to stop enrolling patients in the study.” A distinct possibility is that the noxious adverse effects from the naltrexone impacted the participants’ experience in a negative manner, dampening down any antidepressant effect from ketamine.
In the August 2019 issue of Molecular Psychiatry, these same authors published a second article16 with conclusions based solely on “a secondary analysis of” the data from the same 12 participants in their first publication. Williams et al16 concluded that naltrexone also decreases the anti-suicidality effects of ketamine. Without any additional data or clinical research, these same authors extrapolated their hypothesized opioid receptor activity of ketamine to include it being responsible for ketamine’s established anti-suicidal effects.
Continue to: Mathew and Rivas-Grajales...
Mathew and Rivas-Grajales17 recently published a thoughtful critique and analysis of the study design and conclusions of the original Williams paper.15 They concluded that insufficient evidence exists to answer the question of how ketamine may interface with the opioid system, and they encourage further research into this important topic.
Two additional recent publications18,19 reported that naltrexone pretreatment did not attenuate the antidepressant effects of ketamine in their participants. Additionally, a recent publication in the anesthesiology literature20 concluded that esketamine reversed respiratory depression that was induced by remifentanil. From a clinical perspective, the most compelling argument against a direct mu opioid receptor mechanism for K/ESK is the lack of any craving, tolerance, or withdrawal in patients with TRD treated with K/ESK in numerous clinical publications comparing K/ESK with placebo. In the case of esketamine, during the 5 phase III clinical trials—including both short- and long-term studies—there was no signal for an opioid-like pharmacology. Significantly, both K/ESK are rapidly metabolized by the human body, and the typical dosing is 2 doses/week for the first month, then 1 dose/week for the next month, then 1 dose every week or less for the remainder of treatment.
Curiously, in the May 2019 issue of the American Journal of Psychiatry, Schatzberg21 (one of the co-authors of the prior 2 studies opining that ketamine has direct opioid system activation) wrote a “Reviews and Overviews” article in which he misrepresents the conclusions of an elegant study by Abdallah et al22 published in December 2018.
Abdallah et al22 added rapamycin, an immunosuppressant and a known inhibitor of mTOR, as a pretreatment to patients in a major depressive episode prior to infusion with IV ketamine. Their hypothesis was to see if the rapamycin decreased ketamine’s rapid antidepressant response—putatively by inhibiting the effect of mTOR. Rather than decreasing ketamine’s antidepressant effect, and in contrast to the placebo pretreatment group, at 2 weeks post IV ketamine infusion, patients treated with rapamycin-ketamine had a longer duration/greater improvement in their depressive symptoms compared with the patients receiving placebo-ketamine (improvement of 41% vs 13%, respectively, P = .04). Abdallah et al22 hypothesized that the pretreatment with rapamycin provides anti-inflammatory benefits to the synaptogenesis resulting from ketamine, which protects the newly formed synapses and prolongs ketamine’s antidepressant effect. Schatzberg21 came to a different conclusion than Abdallah et al,22 opining that because the rapamycin “failed to decrease ketamine response,” this result debunks the role of mTOR as a mediator in the antidepressant effect of ketamine through synaptogenesis.
Much more to learn
We still have a great deal to learn about the mechanism of action of K/ESK. However, clinics that are augmenting antidepressants with K/ESK in patients with TRD report significant and rapid symptom improvement in some patients (personal communications). We still do not understand the actual mechanisms of action of antidepressants and antipsychotics, but this does not curtail their use and clinical benefits to our patients. Ketamine has been extensively studied. In the current appropriate climate of concern about the pervasive and lethal opioid epidemic in the United States, we must remain on solid scientific ground before attributing an opioid mechanism to a novel treatment that has already benefitted many of our most depressed and refractory patients.
Continue to: Looking at the extensive...
Looking at the extensive published data over the past 20 years, a consistent model has emerged whereby glutamate agonism of the AMPA-glutamate receptor, both with and without antagonism of the NMDA-glutamate receptor, appears to set in motion a molecular cascade involving BDNF and VEGF, and ultimately increasing the activity of mTOR, with resulting synaptogenicity that increases global brain connectivity in the human prefrontal cortex. As we continue to understand the complexities and additional circuitries that are involved in the RAAD effect of K/ESK, the hope is that novel molecular targets for future drug development will emerge.
Bottom Line
Extensive published data over the past 20 years has produced a consistent model to explain the putative mechanisms of action for the rapid antidepressant effects of ketamine and esketamine. We must remain on solid scientific ground before attributing an opioid mechanism to a novel treatment that has already benefitted many of our patients with treatment-resistant depression.
Related Resources
- Mattingly GW, Anderson RH. Intranasal esketamine. Current Psychiatry. 2019;18(5):31-38.
- Thase M. Ketamine and esketamine for treating unipolar depression in adults: Administration, efficacy, and adverse effects. UpToDate. www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects.
Drug Brand Names
Esketamine nasal spray • Spravato
Imipramine • Tofranil
Ketamine • Ketalar
Naltrexone • Vivitrol, ReVia
Rapamycin • Rapamune
Remifentanil • Ultiva
1. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351-354.
2. Epstein K, Farrell HM. ‘Miracle cures’ in psychiatry? Current Psychiatry. 2019;18(9):13-16.
3. Valenstein M. Keeping our eyes on STAR*D. Am J Psychiatry. 2006;163:1484-1486.
4. Abdallah CG, Sanacora G, Duman RS, et al. The neurobiology of depression, ketamine and rapid-acting antidepressants: is it glutamate inhibition or activation? Pharmacol Ther. 2018;190:148-158.
5. Moghaddam B, Adams B, Verma A, et al. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921-2927.
6. Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959-964.
7. Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory, and disease. Trends Neurosci. 2010;33(2):67-75.
8. Zanos P, Moaddel R, Morris PJ, et al. NMDA inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481-486.
9. Collo G, Cavalleri L, Chiamulera C, et al. (2R,6R)-Hydroxynorketamine promotes dendrite outgrowth in human inducible pluripotent stem cell-derived neurons through AMPA receptor with timing and exposure compatible with ketamine infusion pharmacokinetics in humans. Neuroreport. 2018;29(16):1425-1430.
10. Hirota K, Lambert DG. Ketamine and depression. Br J Anaesth. 2018;121(6):1198-1202.
11. Abdallah CG, Adams TG, Kelmendi B, et al. Ketamine’s mechanism of action: a path to rapid-acting antidepressants. Depress Anxiety. 2016;33(8):689-697.
12. Deyama S, Bang E, Wohleb ES, et al. Role of neuronal VEGF signaling in the prefrontal cortex in the rapid antidepressant effects of ketamine. Am J Psychiatry. 2019;176(5):388-400.
13. Abdallah CG, Dutta A, Averill CL, et al. Ketamine, but not the NMDAR antagonist lanicemine, increases prefrontal global connectivity in depressed patients. Chronic Stress (Thousand Oaks). 2018;2. doi: 10.1177/2470547018796102.
14. Abdallah CG, Averill LA, Collins KA, et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology. 2017;42(6):1210-1219.
15. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175:1205-1215.
16. Williams NR, Heifets BD, Bentzley BS, et al. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol Psychiatry. 2019;24(12):1779-1786.
17. Mathew SJ, Rivas-Grajales AM. “Does the opioid system block or enhance the antidepressant effects of ketamine?” Chronic Stress. (Thousand Oaks). 2019;3. doi: 10.1177/2470547019852073.
18. Yoon G, Petrakis IL, Krystal JH. Association of combined naltrexone and ketamine with depressive symptoms in a case series of patients with depression and alcohol use disorder. JAMA Psychiatry. 2019;76:337-338.
19. Marton T, Barnes DE, Wallace A, et al. Concurrent use of buprenorphine, methadone, or naltrexone does not inhibit ketamine’s antidepressant activity. Biol Psychiatry. 2019;85(12):e75-e76.
20. Jonkman K, van Rijnsoever E, Olofsen E, et al. Esketamine counters opioid-induced respiratory depression. Br J Anaesth. 2018;120(5):1117-1127.
21. Schatzberg AF. Scientific issues relevant to improving the diagnosis, risk assessment, and treatment of major depression. Am J Psychiatry. 2019;176(5):342-347.
22. Abdallah C, Averill LA, Gueorgueiva R, et al. Rapamycin, an immunosuppressant and mTORC1 inhibitor, triples the antidepressant response rate of ketamine at 2 weeks following treatment: a double blind, placebo-controlled, cross-over, randomized clinical trial. bioRxiv. December 19, 2018. https://www.biorxiv.org/content/10.1101/500959v1. Accessed December 5, 2019.
1. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351-354.
2. Epstein K, Farrell HM. ‘Miracle cures’ in psychiatry? Current Psychiatry. 2019;18(9):13-16.
3. Valenstein M. Keeping our eyes on STAR*D. Am J Psychiatry. 2006;163:1484-1486.
4. Abdallah CG, Sanacora G, Duman RS, et al. The neurobiology of depression, ketamine and rapid-acting antidepressants: is it glutamate inhibition or activation? Pharmacol Ther. 2018;190:148-158.
5. Moghaddam B, Adams B, Verma A, et al. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921-2927.
6. Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959-964.
7. Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory, and disease. Trends Neurosci. 2010;33(2):67-75.
8. Zanos P, Moaddel R, Morris PJ, et al. NMDA inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481-486.
9. Collo G, Cavalleri L, Chiamulera C, et al. (2R,6R)-Hydroxynorketamine promotes dendrite outgrowth in human inducible pluripotent stem cell-derived neurons through AMPA receptor with timing and exposure compatible with ketamine infusion pharmacokinetics in humans. Neuroreport. 2018;29(16):1425-1430.
10. Hirota K, Lambert DG. Ketamine and depression. Br J Anaesth. 2018;121(6):1198-1202.
11. Abdallah CG, Adams TG, Kelmendi B, et al. Ketamine’s mechanism of action: a path to rapid-acting antidepressants. Depress Anxiety. 2016;33(8):689-697.
12. Deyama S, Bang E, Wohleb ES, et al. Role of neuronal VEGF signaling in the prefrontal cortex in the rapid antidepressant effects of ketamine. Am J Psychiatry. 2019;176(5):388-400.
13. Abdallah CG, Dutta A, Averill CL, et al. Ketamine, but not the NMDAR antagonist lanicemine, increases prefrontal global connectivity in depressed patients. Chronic Stress (Thousand Oaks). 2018;2. doi: 10.1177/2470547018796102.
14. Abdallah CG, Averill LA, Collins KA, et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology. 2017;42(6):1210-1219.
15. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175:1205-1215.
16. Williams NR, Heifets BD, Bentzley BS, et al. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol Psychiatry. 2019;24(12):1779-1786.
17. Mathew SJ, Rivas-Grajales AM. “Does the opioid system block or enhance the antidepressant effects of ketamine?” Chronic Stress. (Thousand Oaks). 2019;3. doi: 10.1177/2470547019852073.
18. Yoon G, Petrakis IL, Krystal JH. Association of combined naltrexone and ketamine with depressive symptoms in a case series of patients with depression and alcohol use disorder. JAMA Psychiatry. 2019;76:337-338.
19. Marton T, Barnes DE, Wallace A, et al. Concurrent use of buprenorphine, methadone, or naltrexone does not inhibit ketamine’s antidepressant activity. Biol Psychiatry. 2019;85(12):e75-e76.
20. Jonkman K, van Rijnsoever E, Olofsen E, et al. Esketamine counters opioid-induced respiratory depression. Br J Anaesth. 2018;120(5):1117-1127.
21. Schatzberg AF. Scientific issues relevant to improving the diagnosis, risk assessment, and treatment of major depression. Am J Psychiatry. 2019;176(5):342-347.
22. Abdallah C, Averill LA, Gueorgueiva R, et al. Rapamycin, an immunosuppressant and mTORC1 inhibitor, triples the antidepressant response rate of ketamine at 2 weeks following treatment: a double blind, placebo-controlled, cross-over, randomized clinical trial. bioRxiv. December 19, 2018. https://www.biorxiv.org/content/10.1101/500959v1. Accessed December 5, 2019.
Book review: New understanding offered of personality development
Rarely does someone come along who has new insight into behavior, someone who conceptualizes with such clarity that we wonder why we never saw it before.
Homer B. Martin, MD, was such a man. Over the course of 40 years’ psychodynamic psychotherapy work as a psychiatrist, he pieced together a concept of how we are emotionally conditioned in the first 3 years of life and how this conditioning affects us throughout our lives. Conditioning forces us to live on autopilot, creating inappropriate knee-jerk emotional responses to those closest to us.
Dr. Martin’s protégé, child and adolescent psychiatrist Christine B.L. Adams, MD, contributed her own 40 years of clinical practice as a psychodynamic psychotherapist to Dr. Martin’s new concept of emotional conditioning. Their findings are published in the award-winning book “Living on Automatic: How Emotional Conditioning Shapes our Lives and Relationships” (Praeger, 2018).
The authors aim to help both therapists and patients out of the quagmire of conflicted relationships and emotional illnesses that result from emotional conditioning. They propose a new understanding of personality development and subsequent relationship conflict, which incorporates work of Pavlov, Skinner, and Lorenz, along with techniques of Freud.
Dr. Martin and Dr. Adams discovered that we are conditioned into one of two roles – omnipotent and impotent. Those roles become the bedrock of our personalities. We display those roles in marriages, with our children, friends, and colleagues, without regard to gender.
Each role exists on a continuum, from mild to severe, determined by upbringing in the family. Once you acquire a role in childhood, the role is reinforced by both family and society at large – peers, teachers, and friends.
The authors unveil a new conceptualization of how the mind works for each role – thinking style, ways of elaborating emotions, attitudes, personal standards, value systems, reality testing mode, quality of thought, and mode of commitment.
The book has three sections. “Part One, Understanding Emotional Conditioning” describes the basic concepts, the effects of conditioning, and the two personality types. “Part Two, Relationship Struggles: Miscommunications and Marriages” examines marriage conflict, divorce, and living single. “Part Three, Solutions: Psychotherapy and Deconditioning” presents steps we can take to decondition ourselves, as well as the process of deconditioning psychotherapy.
To escape automatic living, Dr. Martin and Dr. Adams endorse the use of deconditioning psychotherapy, which helps people lessen their emotional conditioning. The cornerstone of deconditioning treatment is helping people turn off automatic responses through replacing emotional conditioning with thinking.
In undergoing deconditioning you discover how you were emotionally conditioned as a child and how you skew participation in your relationships. You learn to slow down and dissect the automatic responding that you and others do. You discover how to evaluate what the situation calls for with the involved people. Who needs what, how much, and from whom?
This book is written for both general readers and psychotherapists. Its novel approach for alleviating emotional illnesses in “ordinary” people is a welcome addition to the armamentarium of any therapist.
The book is extraordinarily well written. It offers valuable case vignettes, tables, and self-inquiry questions to assist in understanding the characteristics associated with each emotionally conditioned role.
Dr. Martin and Dr. Adams have made the book very digestible, intriguing and practical. And it is a marvelous tribute to the value of a 30-year mentorship.
Judith R. Milner, MD, MEd, SpecEd, is a general, child, and adolescent psychiatrist in private practice in Everett, Wash. She has traveled with various groups over the years in an effort to alleviate some of the suffering caused by war and natural disaster. She has worked with Step Up Rwanda Women and Pygmy Survival Alliance, as well as on the Committee for Women at the American Psychiatric Association and the Consumer Issues Committee, the Committee on Diversity and Culture, and the Membership Committee for the American Academy of Child and Adolescent Psychiatry.
Rarely does someone come along who has new insight into behavior, someone who conceptualizes with such clarity that we wonder why we never saw it before.
Homer B. Martin, MD, was such a man. Over the course of 40 years’ psychodynamic psychotherapy work as a psychiatrist, he pieced together a concept of how we are emotionally conditioned in the first 3 years of life and how this conditioning affects us throughout our lives. Conditioning forces us to live on autopilot, creating inappropriate knee-jerk emotional responses to those closest to us.
Dr. Martin’s protégé, child and adolescent psychiatrist Christine B.L. Adams, MD, contributed her own 40 years of clinical practice as a psychodynamic psychotherapist to Dr. Martin’s new concept of emotional conditioning. Their findings are published in the award-winning book “Living on Automatic: How Emotional Conditioning Shapes our Lives and Relationships” (Praeger, 2018).
The authors aim to help both therapists and patients out of the quagmire of conflicted relationships and emotional illnesses that result from emotional conditioning. They propose a new understanding of personality development and subsequent relationship conflict, which incorporates work of Pavlov, Skinner, and Lorenz, along with techniques of Freud.
Dr. Martin and Dr. Adams discovered that we are conditioned into one of two roles – omnipotent and impotent. Those roles become the bedrock of our personalities. We display those roles in marriages, with our children, friends, and colleagues, without regard to gender.
Each role exists on a continuum, from mild to severe, determined by upbringing in the family. Once you acquire a role in childhood, the role is reinforced by both family and society at large – peers, teachers, and friends.
The authors unveil a new conceptualization of how the mind works for each role – thinking style, ways of elaborating emotions, attitudes, personal standards, value systems, reality testing mode, quality of thought, and mode of commitment.
The book has three sections. “Part One, Understanding Emotional Conditioning” describes the basic concepts, the effects of conditioning, and the two personality types. “Part Two, Relationship Struggles: Miscommunications and Marriages” examines marriage conflict, divorce, and living single. “Part Three, Solutions: Psychotherapy and Deconditioning” presents steps we can take to decondition ourselves, as well as the process of deconditioning psychotherapy.
To escape automatic living, Dr. Martin and Dr. Adams endorse the use of deconditioning psychotherapy, which helps people lessen their emotional conditioning. The cornerstone of deconditioning treatment is helping people turn off automatic responses through replacing emotional conditioning with thinking.
In undergoing deconditioning you discover how you were emotionally conditioned as a child and how you skew participation in your relationships. You learn to slow down and dissect the automatic responding that you and others do. You discover how to evaluate what the situation calls for with the involved people. Who needs what, how much, and from whom?
This book is written for both general readers and psychotherapists. Its novel approach for alleviating emotional illnesses in “ordinary” people is a welcome addition to the armamentarium of any therapist.
The book is extraordinarily well written. It offers valuable case vignettes, tables, and self-inquiry questions to assist in understanding the characteristics associated with each emotionally conditioned role.
Dr. Martin and Dr. Adams have made the book very digestible, intriguing and practical. And it is a marvelous tribute to the value of a 30-year mentorship.
Judith R. Milner, MD, MEd, SpecEd, is a general, child, and adolescent psychiatrist in private practice in Everett, Wash. She has traveled with various groups over the years in an effort to alleviate some of the suffering caused by war and natural disaster. She has worked with Step Up Rwanda Women and Pygmy Survival Alliance, as well as on the Committee for Women at the American Psychiatric Association and the Consumer Issues Committee, the Committee on Diversity and Culture, and the Membership Committee for the American Academy of Child and Adolescent Psychiatry.
Rarely does someone come along who has new insight into behavior, someone who conceptualizes with such clarity that we wonder why we never saw it before.
Homer B. Martin, MD, was such a man. Over the course of 40 years’ psychodynamic psychotherapy work as a psychiatrist, he pieced together a concept of how we are emotionally conditioned in the first 3 years of life and how this conditioning affects us throughout our lives. Conditioning forces us to live on autopilot, creating inappropriate knee-jerk emotional responses to those closest to us.
Dr. Martin’s protégé, child and adolescent psychiatrist Christine B.L. Adams, MD, contributed her own 40 years of clinical practice as a psychodynamic psychotherapist to Dr. Martin’s new concept of emotional conditioning. Their findings are published in the award-winning book “Living on Automatic: How Emotional Conditioning Shapes our Lives and Relationships” (Praeger, 2018).
The authors aim to help both therapists and patients out of the quagmire of conflicted relationships and emotional illnesses that result from emotional conditioning. They propose a new understanding of personality development and subsequent relationship conflict, which incorporates work of Pavlov, Skinner, and Lorenz, along with techniques of Freud.
Dr. Martin and Dr. Adams discovered that we are conditioned into one of two roles – omnipotent and impotent. Those roles become the bedrock of our personalities. We display those roles in marriages, with our children, friends, and colleagues, without regard to gender.
Each role exists on a continuum, from mild to severe, determined by upbringing in the family. Once you acquire a role in childhood, the role is reinforced by both family and society at large – peers, teachers, and friends.
The authors unveil a new conceptualization of how the mind works for each role – thinking style, ways of elaborating emotions, attitudes, personal standards, value systems, reality testing mode, quality of thought, and mode of commitment.
The book has three sections. “Part One, Understanding Emotional Conditioning” describes the basic concepts, the effects of conditioning, and the two personality types. “Part Two, Relationship Struggles: Miscommunications and Marriages” examines marriage conflict, divorce, and living single. “Part Three, Solutions: Psychotherapy and Deconditioning” presents steps we can take to decondition ourselves, as well as the process of deconditioning psychotherapy.
To escape automatic living, Dr. Martin and Dr. Adams endorse the use of deconditioning psychotherapy, which helps people lessen their emotional conditioning. The cornerstone of deconditioning treatment is helping people turn off automatic responses through replacing emotional conditioning with thinking.
In undergoing deconditioning you discover how you were emotionally conditioned as a child and how you skew participation in your relationships. You learn to slow down and dissect the automatic responding that you and others do. You discover how to evaluate what the situation calls for with the involved people. Who needs what, how much, and from whom?
This book is written for both general readers and psychotherapists. Its novel approach for alleviating emotional illnesses in “ordinary” people is a welcome addition to the armamentarium of any therapist.
The book is extraordinarily well written. It offers valuable case vignettes, tables, and self-inquiry questions to assist in understanding the characteristics associated with each emotionally conditioned role.
Dr. Martin and Dr. Adams have made the book very digestible, intriguing and practical. And it is a marvelous tribute to the value of a 30-year mentorship.
Judith R. Milner, MD, MEd, SpecEd, is a general, child, and adolescent psychiatrist in private practice in Everett, Wash. She has traveled with various groups over the years in an effort to alleviate some of the suffering caused by war and natural disaster. She has worked with Step Up Rwanda Women and Pygmy Survival Alliance, as well as on the Committee for Women at the American Psychiatric Association and the Consumer Issues Committee, the Committee on Diversity and Culture, and the Membership Committee for the American Academy of Child and Adolescent Psychiatry.
SABCS research changes practice
In this edition of “How I Will Treat My Next Patient,” I highlight two presentations from the recent San Antonio Breast Cancer Symposium (SABCS) that will likely change practice for some breast cancer patients – even before the ball drops in Times Square on New Year’s Eve.
Residual cancer burden
Researchers reported a multi-institutional analysis of individual patient-level data on 5,160 patients who had received neoadjuvant chemotherapy (NAC) for localized breast cancer at 11 different centers. They found that residual cancer burden (RCB) was significantly associated with event-free (EFS) and distant recurrence-free survival. The value of calculating RCB was seen across all breast cancer tumor phenotypes (SABCS 2019, Abstract GS5-01).
RCB is calculated by analyzing the residual disease after NAC for the primary tumor bed, the number of positive axillary nodes, and the size of largest node metastasis. It is graded from RCB-0 (pCR) to RCB-III (extensive residual disease).
Both EFS and distant recurrence-free survival were strongly associated with RCB for the overall population and for each breast cancer subtype. For hormone receptor–positive/HER2-negative disease there was a slight hiccup in that RCB-0 was associated with a 10-year EFS of 81%, but EFS was 86% for RCB-I. Fortunately, the prognostic reliability of RCB was clear for RCB-II (69%) and RCB-III (52%). The presenter, W. Fraser Symmans, MD, commented that RCB is most prognostic when higher levels of residual disease are present. RCB remained prognostic in multivariate models adjusting for age, grade, and clinical T and N stage at diagnosis.
How these results influence practice
After neoadjuvant chemotherapy in patients with localized breast cancer, we struggle when patients ask: “So, doctor, how am I likely to do?” We piece together a complicated – and inconsistent – answer, based on breast cancer subtype, original stage of disease, whether pCR was attained, and other factors.
For patients with triple-negative breast cancer, we have the option of adding capecitabine and/or participation in ongoing clinical trials, for patients with residual disease after NAC. Among the HER2-positive patients, we have data from the KATHERINE, ExtaNET, and APHINITY trials that provide options for additional treatment in those patients, as well. For patients with potentially hormonally responsive, HER2-negative disease, we can emphasize the importance of postoperative adjuvant endocrine therapy, the mandate for continued adherence to oral therapy, and the lower likelihood of (and prognostic value of) pCR in that breast cancer subtype.
Where we previously had a complicated, nuanced discussion, we now have data from a multi-institutional, meticulous analysis to guide further treatment, candidacy for clinical trials, and our expectations of what would be meaningful results from additional therapy. This meets my definition of “practice-changing” research.
APHINITY follow-up
APHINITY was a randomized, multicenter, double-blind, placebo-controlled trial that previously demonstrated that pertuzumab added to standard chemotherapy plus 1 year of trastuzumab in operable HER2-positive breast cancer was associated with modest, but statistically significant, improvement in invasive disease–free survival (IDFS), compared with placebo and chemotherapy plus trastuzumab (hazard ratio, 0.81; P = .04). When the initial results – with a median follow-up of 45.4 months – were published, the authors promised an update at 6 years. Martine Piccart, MD, PhD, provided that update at the 2019 SABCS (Abstract GS1-04).
In the updated analysis, overall survival was 94.8% among patients on the pertuzumab arm and 93.9% among patients taking placebo (HR, 0.85). IDFS rates were 90.6% versus 87.8% in the intent-to-treat population. The investigator commented that this difference was caused mainly by a reduction in distant and loco-regional recurrences.
Central nervous system metastases, contralateral invasive breast cancers, and death without a prior event were no different between the two treatment groups.
Improvement in IDFS (the primary endpoint) from pertuzumab was most impressive in the node-positive cohort, 87.9% versus 83.4% for placebo (HR, 0.72). There was no benefit in the node-negative population (95.0% vs. 94.9%; HR, 1.02).
In contrast to the 3-year analysis, the benefits in IDFS were seen regardless of hormone receptor status. Additionally, no new safety concerns emerged. The rate of severe cardiac events was below 1% in both groups.
How these results influence practice
“Patience is a virtue” appeared for the first time in the English language around 1360, in William Langland’s poem “Piers Plowman.” They are true words, indeed.
When the initial results of APHINITY were published in 2017, they led to the approval of pertuzumab as an addition to chemotherapy plus trastuzumab for high-risk, early, HER2-positive breast cancer patients. Still, the difference in IDFS curves was visually unimpressive (absolute difference 1.7% in the intent-to-treat and 3.2% in the node-positive patients) and pertuzumab was associated with more grade 3 toxicities (primarily diarrhea) and treatment discontinuations.
An optimist would have observed that the IDFS curves in 2017 did not start to diverge until 24 months and would have noted that the divergence increased with time. That virtuously patient person would have expected that the second interim analysis would show larger absolute benefits, and that the hormone receptor–positive patients (64% of the total) would not yet have relapsed by the first interim analysis and that pertuzumab’s benefit in that group would emerge late.
That appears to be what is happening. It provides hope that an overall survival benefit will become more evident with time. If the target P value for an overall survival difference (.0012) is met by the time of the third interim analysis, our patience (and the FDA’s decision to grant approval for pertuzumab for this indication) will have been amply rewarded. For selected HER2-posiitve patients with high RCB, especially those who tolerated neoadjuvant trastuzumab plus pertuzumab regimens, postoperative adjuvant pertuzumab may be an appealing option.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I highlight two presentations from the recent San Antonio Breast Cancer Symposium (SABCS) that will likely change practice for some breast cancer patients – even before the ball drops in Times Square on New Year’s Eve.
Residual cancer burden
Researchers reported a multi-institutional analysis of individual patient-level data on 5,160 patients who had received neoadjuvant chemotherapy (NAC) for localized breast cancer at 11 different centers. They found that residual cancer burden (RCB) was significantly associated with event-free (EFS) and distant recurrence-free survival. The value of calculating RCB was seen across all breast cancer tumor phenotypes (SABCS 2019, Abstract GS5-01).
RCB is calculated by analyzing the residual disease after NAC for the primary tumor bed, the number of positive axillary nodes, and the size of largest node metastasis. It is graded from RCB-0 (pCR) to RCB-III (extensive residual disease).
Both EFS and distant recurrence-free survival were strongly associated with RCB for the overall population and for each breast cancer subtype. For hormone receptor–positive/HER2-negative disease there was a slight hiccup in that RCB-0 was associated with a 10-year EFS of 81%, but EFS was 86% for RCB-I. Fortunately, the prognostic reliability of RCB was clear for RCB-II (69%) and RCB-III (52%). The presenter, W. Fraser Symmans, MD, commented that RCB is most prognostic when higher levels of residual disease are present. RCB remained prognostic in multivariate models adjusting for age, grade, and clinical T and N stage at diagnosis.
How these results influence practice
After neoadjuvant chemotherapy in patients with localized breast cancer, we struggle when patients ask: “So, doctor, how am I likely to do?” We piece together a complicated – and inconsistent – answer, based on breast cancer subtype, original stage of disease, whether pCR was attained, and other factors.
For patients with triple-negative breast cancer, we have the option of adding capecitabine and/or participation in ongoing clinical trials, for patients with residual disease after NAC. Among the HER2-positive patients, we have data from the KATHERINE, ExtaNET, and APHINITY trials that provide options for additional treatment in those patients, as well. For patients with potentially hormonally responsive, HER2-negative disease, we can emphasize the importance of postoperative adjuvant endocrine therapy, the mandate for continued adherence to oral therapy, and the lower likelihood of (and prognostic value of) pCR in that breast cancer subtype.
Where we previously had a complicated, nuanced discussion, we now have data from a multi-institutional, meticulous analysis to guide further treatment, candidacy for clinical trials, and our expectations of what would be meaningful results from additional therapy. This meets my definition of “practice-changing” research.
APHINITY follow-up
APHINITY was a randomized, multicenter, double-blind, placebo-controlled trial that previously demonstrated that pertuzumab added to standard chemotherapy plus 1 year of trastuzumab in operable HER2-positive breast cancer was associated with modest, but statistically significant, improvement in invasive disease–free survival (IDFS), compared with placebo and chemotherapy plus trastuzumab (hazard ratio, 0.81; P = .04). When the initial results – with a median follow-up of 45.4 months – were published, the authors promised an update at 6 years. Martine Piccart, MD, PhD, provided that update at the 2019 SABCS (Abstract GS1-04).
In the updated analysis, overall survival was 94.8% among patients on the pertuzumab arm and 93.9% among patients taking placebo (HR, 0.85). IDFS rates were 90.6% versus 87.8% in the intent-to-treat population. The investigator commented that this difference was caused mainly by a reduction in distant and loco-regional recurrences.
Central nervous system metastases, contralateral invasive breast cancers, and death without a prior event were no different between the two treatment groups.
Improvement in IDFS (the primary endpoint) from pertuzumab was most impressive in the node-positive cohort, 87.9% versus 83.4% for placebo (HR, 0.72). There was no benefit in the node-negative population (95.0% vs. 94.9%; HR, 1.02).
In contrast to the 3-year analysis, the benefits in IDFS were seen regardless of hormone receptor status. Additionally, no new safety concerns emerged. The rate of severe cardiac events was below 1% in both groups.
How these results influence practice
“Patience is a virtue” appeared for the first time in the English language around 1360, in William Langland’s poem “Piers Plowman.” They are true words, indeed.
When the initial results of APHINITY were published in 2017, they led to the approval of pertuzumab as an addition to chemotherapy plus trastuzumab for high-risk, early, HER2-positive breast cancer patients. Still, the difference in IDFS curves was visually unimpressive (absolute difference 1.7% in the intent-to-treat and 3.2% in the node-positive patients) and pertuzumab was associated with more grade 3 toxicities (primarily diarrhea) and treatment discontinuations.
An optimist would have observed that the IDFS curves in 2017 did not start to diverge until 24 months and would have noted that the divergence increased with time. That virtuously patient person would have expected that the second interim analysis would show larger absolute benefits, and that the hormone receptor–positive patients (64% of the total) would not yet have relapsed by the first interim analysis and that pertuzumab’s benefit in that group would emerge late.
That appears to be what is happening. It provides hope that an overall survival benefit will become more evident with time. If the target P value for an overall survival difference (.0012) is met by the time of the third interim analysis, our patience (and the FDA’s decision to grant approval for pertuzumab for this indication) will have been amply rewarded. For selected HER2-posiitve patients with high RCB, especially those who tolerated neoadjuvant trastuzumab plus pertuzumab regimens, postoperative adjuvant pertuzumab may be an appealing option.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I highlight two presentations from the recent San Antonio Breast Cancer Symposium (SABCS) that will likely change practice for some breast cancer patients – even before the ball drops in Times Square on New Year’s Eve.
Residual cancer burden
Researchers reported a multi-institutional analysis of individual patient-level data on 5,160 patients who had received neoadjuvant chemotherapy (NAC) for localized breast cancer at 11 different centers. They found that residual cancer burden (RCB) was significantly associated with event-free (EFS) and distant recurrence-free survival. The value of calculating RCB was seen across all breast cancer tumor phenotypes (SABCS 2019, Abstract GS5-01).
RCB is calculated by analyzing the residual disease after NAC for the primary tumor bed, the number of positive axillary nodes, and the size of largest node metastasis. It is graded from RCB-0 (pCR) to RCB-III (extensive residual disease).
Both EFS and distant recurrence-free survival were strongly associated with RCB for the overall population and for each breast cancer subtype. For hormone receptor–positive/HER2-negative disease there was a slight hiccup in that RCB-0 was associated with a 10-year EFS of 81%, but EFS was 86% for RCB-I. Fortunately, the prognostic reliability of RCB was clear for RCB-II (69%) and RCB-III (52%). The presenter, W. Fraser Symmans, MD, commented that RCB is most prognostic when higher levels of residual disease are present. RCB remained prognostic in multivariate models adjusting for age, grade, and clinical T and N stage at diagnosis.
How these results influence practice
After neoadjuvant chemotherapy in patients with localized breast cancer, we struggle when patients ask: “So, doctor, how am I likely to do?” We piece together a complicated – and inconsistent – answer, based on breast cancer subtype, original stage of disease, whether pCR was attained, and other factors.
For patients with triple-negative breast cancer, we have the option of adding capecitabine and/or participation in ongoing clinical trials, for patients with residual disease after NAC. Among the HER2-positive patients, we have data from the KATHERINE, ExtaNET, and APHINITY trials that provide options for additional treatment in those patients, as well. For patients with potentially hormonally responsive, HER2-negative disease, we can emphasize the importance of postoperative adjuvant endocrine therapy, the mandate for continued adherence to oral therapy, and the lower likelihood of (and prognostic value of) pCR in that breast cancer subtype.
Where we previously had a complicated, nuanced discussion, we now have data from a multi-institutional, meticulous analysis to guide further treatment, candidacy for clinical trials, and our expectations of what would be meaningful results from additional therapy. This meets my definition of “practice-changing” research.
APHINITY follow-up
APHINITY was a randomized, multicenter, double-blind, placebo-controlled trial that previously demonstrated that pertuzumab added to standard chemotherapy plus 1 year of trastuzumab in operable HER2-positive breast cancer was associated with modest, but statistically significant, improvement in invasive disease–free survival (IDFS), compared with placebo and chemotherapy plus trastuzumab (hazard ratio, 0.81; P = .04). When the initial results – with a median follow-up of 45.4 months – were published, the authors promised an update at 6 years. Martine Piccart, MD, PhD, provided that update at the 2019 SABCS (Abstract GS1-04).
In the updated analysis, overall survival was 94.8% among patients on the pertuzumab arm and 93.9% among patients taking placebo (HR, 0.85). IDFS rates were 90.6% versus 87.8% in the intent-to-treat population. The investigator commented that this difference was caused mainly by a reduction in distant and loco-regional recurrences.
Central nervous system metastases, contralateral invasive breast cancers, and death without a prior event were no different between the two treatment groups.
Improvement in IDFS (the primary endpoint) from pertuzumab was most impressive in the node-positive cohort, 87.9% versus 83.4% for placebo (HR, 0.72). There was no benefit in the node-negative population (95.0% vs. 94.9%; HR, 1.02).
In contrast to the 3-year analysis, the benefits in IDFS were seen regardless of hormone receptor status. Additionally, no new safety concerns emerged. The rate of severe cardiac events was below 1% in both groups.
How these results influence practice
“Patience is a virtue” appeared for the first time in the English language around 1360, in William Langland’s poem “Piers Plowman.” They are true words, indeed.
When the initial results of APHINITY were published in 2017, they led to the approval of pertuzumab as an addition to chemotherapy plus trastuzumab for high-risk, early, HER2-positive breast cancer patients. Still, the difference in IDFS curves was visually unimpressive (absolute difference 1.7% in the intent-to-treat and 3.2% in the node-positive patients) and pertuzumab was associated with more grade 3 toxicities (primarily diarrhea) and treatment discontinuations.
An optimist would have observed that the IDFS curves in 2017 did not start to diverge until 24 months and would have noted that the divergence increased with time. That virtuously patient person would have expected that the second interim analysis would show larger absolute benefits, and that the hormone receptor–positive patients (64% of the total) would not yet have relapsed by the first interim analysis and that pertuzumab’s benefit in that group would emerge late.
That appears to be what is happening. It provides hope that an overall survival benefit will become more evident with time. If the target P value for an overall survival difference (.0012) is met by the time of the third interim analysis, our patience (and the FDA’s decision to grant approval for pertuzumab for this indication) will have been amply rewarded. For selected HER2-posiitve patients with high RCB, especially those who tolerated neoadjuvant trastuzumab plus pertuzumab regimens, postoperative adjuvant pertuzumab may be an appealing option.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
Jingle All the Way
Editor's note: This article was originally published in the January/February 2003 issue of Clinician News. It placed first in the journal's Most Unusual Patient Contest.
Late one morning, a woman and her 13-year-old daughter came into our office. “Mrs. Smith” reported that “Lisa” had a retained tampon. Lisa looked scared as her mother did all of the talking.
One of the male doctors saw Lisa first. After about 10 minutes, he emerged from the examination room looking perplexed. He said he wasn’t sure what was going on. He asked me to go in and try to get Lisa to allow a more thorough pelvic examination.
My exam of Lisa proved only slightly easier. I was able to visualize something curved and firm that sounded like plastic when touched with the ring forceps. I could pull the object as far as the vaginal introitus, but it would always slide back. “This is no tampon,” I repeated several times. Each time I pulled down, Lisa became more agitated. After several tries, I gave Lisa a rest and left the room to consult with a doctor.
The doctor suggested a trip to same-day surgery for evaluation under anesthesia but decided to give it one more try before heading for the operating room.
Continue to: Together, we entered the exam room...
Together, we entered the exam room. “Well, we’ve had some true confessions,” Mrs. Smith said dryly. “Lisa has a Christmas bell in her vagina—obviously the work of a curious 13-year-old!”
The doctor and I were taken by surprise, but we proceeded with the challenge of the day. Thinking we were dealing with a standard Christmas bell, we attempted to break the suction of the bell on Lisa’s cervix. This proved to be more difficult than we anticipated. After getting a closer look at the bell, we realized that it was a “jingle” bell rather than a standard bell shape.
The crosshair opening of the jingle bell had slipped over Lisa’s cervix. The cervix had become edematous and necrotic. The doctor gave one final pull, and the bell—with cervical tissue inside—flew out.
We needed pliers to open the bell so we could send a tissue sample to the pathology department for evaluation. Lisa received high-dose antibiotics and after several follow-up visits, she was declared healed.
This patient encounter, which also falls under the categories of unbelievable, unconventional, and amazing, was definitely unforgettable to me.
Editor's note: This article was originally published in the January/February 2003 issue of Clinician News. It placed first in the journal's Most Unusual Patient Contest.
Late one morning, a woman and her 13-year-old daughter came into our office. “Mrs. Smith” reported that “Lisa” had a retained tampon. Lisa looked scared as her mother did all of the talking.
One of the male doctors saw Lisa first. After about 10 minutes, he emerged from the examination room looking perplexed. He said he wasn’t sure what was going on. He asked me to go in and try to get Lisa to allow a more thorough pelvic examination.
My exam of Lisa proved only slightly easier. I was able to visualize something curved and firm that sounded like plastic when touched with the ring forceps. I could pull the object as far as the vaginal introitus, but it would always slide back. “This is no tampon,” I repeated several times. Each time I pulled down, Lisa became more agitated. After several tries, I gave Lisa a rest and left the room to consult with a doctor.
The doctor suggested a trip to same-day surgery for evaluation under anesthesia but decided to give it one more try before heading for the operating room.
Continue to: Together, we entered the exam room...
Together, we entered the exam room. “Well, we’ve had some true confessions,” Mrs. Smith said dryly. “Lisa has a Christmas bell in her vagina—obviously the work of a curious 13-year-old!”
The doctor and I were taken by surprise, but we proceeded with the challenge of the day. Thinking we were dealing with a standard Christmas bell, we attempted to break the suction of the bell on Lisa’s cervix. This proved to be more difficult than we anticipated. After getting a closer look at the bell, we realized that it was a “jingle” bell rather than a standard bell shape.
The crosshair opening of the jingle bell had slipped over Lisa’s cervix. The cervix had become edematous and necrotic. The doctor gave one final pull, and the bell—with cervical tissue inside—flew out.
We needed pliers to open the bell so we could send a tissue sample to the pathology department for evaluation. Lisa received high-dose antibiotics and after several follow-up visits, she was declared healed.
This patient encounter, which also falls under the categories of unbelievable, unconventional, and amazing, was definitely unforgettable to me.
Editor's note: This article was originally published in the January/February 2003 issue of Clinician News. It placed first in the journal's Most Unusual Patient Contest.
Late one morning, a woman and her 13-year-old daughter came into our office. “Mrs. Smith” reported that “Lisa” had a retained tampon. Lisa looked scared as her mother did all of the talking.
One of the male doctors saw Lisa first. After about 10 minutes, he emerged from the examination room looking perplexed. He said he wasn’t sure what was going on. He asked me to go in and try to get Lisa to allow a more thorough pelvic examination.
My exam of Lisa proved only slightly easier. I was able to visualize something curved and firm that sounded like plastic when touched with the ring forceps. I could pull the object as far as the vaginal introitus, but it would always slide back. “This is no tampon,” I repeated several times. Each time I pulled down, Lisa became more agitated. After several tries, I gave Lisa a rest and left the room to consult with a doctor.
The doctor suggested a trip to same-day surgery for evaluation under anesthesia but decided to give it one more try before heading for the operating room.
Continue to: Together, we entered the exam room...
Together, we entered the exam room. “Well, we’ve had some true confessions,” Mrs. Smith said dryly. “Lisa has a Christmas bell in her vagina—obviously the work of a curious 13-year-old!”
The doctor and I were taken by surprise, but we proceeded with the challenge of the day. Thinking we were dealing with a standard Christmas bell, we attempted to break the suction of the bell on Lisa’s cervix. This proved to be more difficult than we anticipated. After getting a closer look at the bell, we realized that it was a “jingle” bell rather than a standard bell shape.
The crosshair opening of the jingle bell had slipped over Lisa’s cervix. The cervix had become edematous and necrotic. The doctor gave one final pull, and the bell—with cervical tissue inside—flew out.
We needed pliers to open the bell so we could send a tissue sample to the pathology department for evaluation. Lisa received high-dose antibiotics and after several follow-up visits, she was declared healed.
This patient encounter, which also falls under the categories of unbelievable, unconventional, and amazing, was definitely unforgettable to me.