User login
Advocating for reality
Our first daughter was born during my last year in medical school, and our second was born as I was finishing my second year in residency. Seeing those two little darlings grow and develop was a critical supplement to my pediatric training. And, watching my wife initially struggle and then succeed with breastfeeding provided a very personal experience and education about lactation that my interactions in the hospital and outpatient clinics didn’t offer.
We considered ourselves lucky because my wife wasn’t facing the additional challenge of returning to an out-of-the-home job. However, our good fortune did not confer immunity against the anxiety, insecurity, discomfort, and sleep deprivation–induced frustrations of breastfeeding. Watching my wife navigate the choppy waters of lactation certainly influenced my approach to counseling new mothers over my subsequent 4 decades of practice. I think I was a more sympathetic and realistic adviser based on my first-hand observations.
In a different survey of American Academy of Pediatrics fellows, more of the 832 pediatricians responding reported having had a personal experience with breastfeeding in 2014 than of the 620 responding in 1995 (68% vs. 42%). However, it is interesting that fewer of the respondents in 2014 felt that any mother can succeed at breastfeeding (predicted value = 70% in 1995, PV = 56% in 2014; P less than .05), and fewer in 2014 believed that the advantages of breastfeeding outweighed the difficulties than among those surveyed in 1995 (PV = 70% in 1995, PV = 50% in 2014; P less than .05) (Pediatrics. 2017 Oct;140[4]. pii: e20171229). These results suggest that, as more pediatricians gained personal experience with breastfeeding, more may have realized that the American Academy of Pediatrics recommendations for breastfeeding are unrealistic and may contribute to the negative experiences of some women, including pediatric trainees.
An implied assumption in the AAP News article is that a pediatrician who has had a negative breastfeeding experience is less likely to be a strong advocate for breastfeeding. I would argue that a pediatrician who has witnessed or personally experienced difficulties is more likely to be a sympathetic and realistic advocate of breastfeeding.
We must walk that fine line between actively advocating for lactation-friendly hospitals and work environments and supporting mothers who, due to circumstances beyond their control, can’t meet the expectations we have created for them.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
Our first daughter was born during my last year in medical school, and our second was born as I was finishing my second year in residency. Seeing those two little darlings grow and develop was a critical supplement to my pediatric training. And, watching my wife initially struggle and then succeed with breastfeeding provided a very personal experience and education about lactation that my interactions in the hospital and outpatient clinics didn’t offer.
We considered ourselves lucky because my wife wasn’t facing the additional challenge of returning to an out-of-the-home job. However, our good fortune did not confer immunity against the anxiety, insecurity, discomfort, and sleep deprivation–induced frustrations of breastfeeding. Watching my wife navigate the choppy waters of lactation certainly influenced my approach to counseling new mothers over my subsequent 4 decades of practice. I think I was a more sympathetic and realistic adviser based on my first-hand observations.
In a different survey of American Academy of Pediatrics fellows, more of the 832 pediatricians responding reported having had a personal experience with breastfeeding in 2014 than of the 620 responding in 1995 (68% vs. 42%). However, it is interesting that fewer of the respondents in 2014 felt that any mother can succeed at breastfeeding (predicted value = 70% in 1995, PV = 56% in 2014; P less than .05), and fewer in 2014 believed that the advantages of breastfeeding outweighed the difficulties than among those surveyed in 1995 (PV = 70% in 1995, PV = 50% in 2014; P less than .05) (Pediatrics. 2017 Oct;140[4]. pii: e20171229). These results suggest that, as more pediatricians gained personal experience with breastfeeding, more may have realized that the American Academy of Pediatrics recommendations for breastfeeding are unrealistic and may contribute to the negative experiences of some women, including pediatric trainees.
An implied assumption in the AAP News article is that a pediatrician who has had a negative breastfeeding experience is less likely to be a strong advocate for breastfeeding. I would argue that a pediatrician who has witnessed or personally experienced difficulties is more likely to be a sympathetic and realistic advocate of breastfeeding.
We must walk that fine line between actively advocating for lactation-friendly hospitals and work environments and supporting mothers who, due to circumstances beyond their control, can’t meet the expectations we have created for them.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
Our first daughter was born during my last year in medical school, and our second was born as I was finishing my second year in residency. Seeing those two little darlings grow and develop was a critical supplement to my pediatric training. And, watching my wife initially struggle and then succeed with breastfeeding provided a very personal experience and education about lactation that my interactions in the hospital and outpatient clinics didn’t offer.
We considered ourselves lucky because my wife wasn’t facing the additional challenge of returning to an out-of-the-home job. However, our good fortune did not confer immunity against the anxiety, insecurity, discomfort, and sleep deprivation–induced frustrations of breastfeeding. Watching my wife navigate the choppy waters of lactation certainly influenced my approach to counseling new mothers over my subsequent 4 decades of practice. I think I was a more sympathetic and realistic adviser based on my first-hand observations.
In a different survey of American Academy of Pediatrics fellows, more of the 832 pediatricians responding reported having had a personal experience with breastfeeding in 2014 than of the 620 responding in 1995 (68% vs. 42%). However, it is interesting that fewer of the respondents in 2014 felt that any mother can succeed at breastfeeding (predicted value = 70% in 1995, PV = 56% in 2014; P less than .05), and fewer in 2014 believed that the advantages of breastfeeding outweighed the difficulties than among those surveyed in 1995 (PV = 70% in 1995, PV = 50% in 2014; P less than .05) (Pediatrics. 2017 Oct;140[4]. pii: e20171229). These results suggest that, as more pediatricians gained personal experience with breastfeeding, more may have realized that the American Academy of Pediatrics recommendations for breastfeeding are unrealistic and may contribute to the negative experiences of some women, including pediatric trainees.
An implied assumption in the AAP News article is that a pediatrician who has had a negative breastfeeding experience is less likely to be a strong advocate for breastfeeding. I would argue that a pediatrician who has witnessed or personally experienced difficulties is more likely to be a sympathetic and realistic advocate of breastfeeding.
We must walk that fine line between actively advocating for lactation-friendly hospitals and work environments and supporting mothers who, due to circumstances beyond their control, can’t meet the expectations we have created for them.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
… What comes naturally
When we were invited to a family gathering to celebrate a 60th birthday, we expected to hear an abundance of news about grandchildren. They are natural, and seldom controversial, topics of discussion. If there is a child still waiting in utero and destined to be the first grandchild on one or both sides of the family, the impending adventure in parenthood will dominate the conversation.
To our great surprise, despite the presence of one very pregnant young woman, who in 6 weeks would be giving birth to the first grandchild in my nephew’s family, my wife and I can recall only one brief dialogue in which I was asked about how one might go about selecting a pediatrician.
I’m not sure why the blessed event to come was being ignored, but I found the oversight unusual and refreshing. It is possible that there had been so much hype about the pregnancy on her side of the family that the couple relished its absence from the birthday party’s topics for discussion.
In the spirit of full disclosure, I must add that, as a result of my frequent claims of ignorance when asked about medically related topics, I am often referred to by the extended family as “Dr. I-Don’t-Know.” It may be that my presence influenced the conversation, but regardless of the reason, I was impressed with the ease at which this couple was approaching the birth of their first child.
I am sure they harbor some anxieties, and I am sure they have listened to some horror stories from their peers about sleep and breastfeeding problems. They are bright people who acknowledge that they are going to encounter some bumps along the road of parenthood. However, they seem to be immune to the epidemic of anxiety that for decades has been sweeping over cohorts of North Americans entering their family-building years.
The young couple my wife and I encountered are just as clueless about what parenthood has in store as their anxiety-driven peers are. The difference is that they are enjoying their pregnancy in blissful ignorance buffered by their refreshing confidence that, however they do it, they will be doing it naturally.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
When we were invited to a family gathering to celebrate a 60th birthday, we expected to hear an abundance of news about grandchildren. They are natural, and seldom controversial, topics of discussion. If there is a child still waiting in utero and destined to be the first grandchild on one or both sides of the family, the impending adventure in parenthood will dominate the conversation.
To our great surprise, despite the presence of one very pregnant young woman, who in 6 weeks would be giving birth to the first grandchild in my nephew’s family, my wife and I can recall only one brief dialogue in which I was asked about how one might go about selecting a pediatrician.
I’m not sure why the blessed event to come was being ignored, but I found the oversight unusual and refreshing. It is possible that there had been so much hype about the pregnancy on her side of the family that the couple relished its absence from the birthday party’s topics for discussion.
In the spirit of full disclosure, I must add that, as a result of my frequent claims of ignorance when asked about medically related topics, I am often referred to by the extended family as “Dr. I-Don’t-Know.” It may be that my presence influenced the conversation, but regardless of the reason, I was impressed with the ease at which this couple was approaching the birth of their first child.
I am sure they harbor some anxieties, and I am sure they have listened to some horror stories from their peers about sleep and breastfeeding problems. They are bright people who acknowledge that they are going to encounter some bumps along the road of parenthood. However, they seem to be immune to the epidemic of anxiety that for decades has been sweeping over cohorts of North Americans entering their family-building years.
The young couple my wife and I encountered are just as clueless about what parenthood has in store as their anxiety-driven peers are. The difference is that they are enjoying their pregnancy in blissful ignorance buffered by their refreshing confidence that, however they do it, they will be doing it naturally.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
When we were invited to a family gathering to celebrate a 60th birthday, we expected to hear an abundance of news about grandchildren. They are natural, and seldom controversial, topics of discussion. If there is a child still waiting in utero and destined to be the first grandchild on one or both sides of the family, the impending adventure in parenthood will dominate the conversation.
To our great surprise, despite the presence of one very pregnant young woman, who in 6 weeks would be giving birth to the first grandchild in my nephew’s family, my wife and I can recall only one brief dialogue in which I was asked about how one might go about selecting a pediatrician.
I’m not sure why the blessed event to come was being ignored, but I found the oversight unusual and refreshing. It is possible that there had been so much hype about the pregnancy on her side of the family that the couple relished its absence from the birthday party’s topics for discussion.
In the spirit of full disclosure, I must add that, as a result of my frequent claims of ignorance when asked about medically related topics, I am often referred to by the extended family as “Dr. I-Don’t-Know.” It may be that my presence influenced the conversation, but regardless of the reason, I was impressed with the ease at which this couple was approaching the birth of their first child.
I am sure they harbor some anxieties, and I am sure they have listened to some horror stories from their peers about sleep and breastfeeding problems. They are bright people who acknowledge that they are going to encounter some bumps along the road of parenthood. However, they seem to be immune to the epidemic of anxiety that for decades has been sweeping over cohorts of North Americans entering their family-building years.
The young couple my wife and I encountered are just as clueless about what parenthood has in store as their anxiety-driven peers are. The difference is that they are enjoying their pregnancy in blissful ignorance buffered by their refreshing confidence that, however they do it, they will be doing it naturally.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
Shades of gray
If you were born in or after the 1970s, it is very likely that you have never watched a television show on a black and white set. Although the roots of its technology extend well back into the early 20th century, the first color broadcast on a national television network didn’t occur until 1954 with NBC’s coverage of the Tournament of Roses Parade.
When we compare the popularization of color television with the rapid pace at which we adopt new technology today, the popularization of color TV was glacial. In large part because of their expense, sales of color sets did not surpass black and white sets until 1972. Our family lagged behind the curve and finally caved in and junked our black and white television around 1977.
The observable change in our viewing behavior was dramatic. While programming in black and white was interesting, the color images were magnetic. We were drawn by the visual excitement and stimulation that color offered, and our family’s viewing standards took a precipitous dip. We seemed to watch anything that was colorful and moved. The quality of the content took a back seat. Viewing in color seemed to require much less cognitive effort. Ironically what attracted our attention allowed us to invest less energy in paying attention.
As a regular reader of Letters From Maine, you know that I am convinced that sleep deprivation is a major contributor to the emergence of the ADHD phenomenon. However, I can make a similar argument that the introduction of color television is an equally potent coconspirator or confounder. The magnetism inherent in a moving color image can tempt even the most health conscious among us to stay well past a brain-friendly bedtime. The invention of the electric light may have gotten the ball rolling, but the ubiquity of moving electronic color images has certainly greased what was already a very slippery slope into an abyss of unhealthy sleep habits.
There are those who argue that smartphones and tablets can open a world of creative opportunities for even very young children. And, it is obvious that parents are struggling to find a balance as they try to decide when, where, and how often to allow their infants and toddlers access to handheld electronic devices.
Recently there has been much finger-pointing at the developers and manufacturers of smartphones and tablets. How can any company with a social conscience sell a product with such dangerous attractive potential for children without providing safeguards? Isn’t it like selling a swimming pool without a gated fence?
Of course the answer to this question goes to the heart of how our society views its responsibility to protect its children. Regardless of who makes the rules and how the responsibility is assigned, it is still the child’s parents who must make sure that the gate is locked.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
If you were born in or after the 1970s, it is very likely that you have never watched a television show on a black and white set. Although the roots of its technology extend well back into the early 20th century, the first color broadcast on a national television network didn’t occur until 1954 with NBC’s coverage of the Tournament of Roses Parade.
When we compare the popularization of color television with the rapid pace at which we adopt new technology today, the popularization of color TV was glacial. In large part because of their expense, sales of color sets did not surpass black and white sets until 1972. Our family lagged behind the curve and finally caved in and junked our black and white television around 1977.
The observable change in our viewing behavior was dramatic. While programming in black and white was interesting, the color images were magnetic. We were drawn by the visual excitement and stimulation that color offered, and our family’s viewing standards took a precipitous dip. We seemed to watch anything that was colorful and moved. The quality of the content took a back seat. Viewing in color seemed to require much less cognitive effort. Ironically what attracted our attention allowed us to invest less energy in paying attention.
As a regular reader of Letters From Maine, you know that I am convinced that sleep deprivation is a major contributor to the emergence of the ADHD phenomenon. However, I can make a similar argument that the introduction of color television is an equally potent coconspirator or confounder. The magnetism inherent in a moving color image can tempt even the most health conscious among us to stay well past a brain-friendly bedtime. The invention of the electric light may have gotten the ball rolling, but the ubiquity of moving electronic color images has certainly greased what was already a very slippery slope into an abyss of unhealthy sleep habits.
There are those who argue that smartphones and tablets can open a world of creative opportunities for even very young children. And, it is obvious that parents are struggling to find a balance as they try to decide when, where, and how often to allow their infants and toddlers access to handheld electronic devices.
Recently there has been much finger-pointing at the developers and manufacturers of smartphones and tablets. How can any company with a social conscience sell a product with such dangerous attractive potential for children without providing safeguards? Isn’t it like selling a swimming pool without a gated fence?
Of course the answer to this question goes to the heart of how our society views its responsibility to protect its children. Regardless of who makes the rules and how the responsibility is assigned, it is still the child’s parents who must make sure that the gate is locked.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
If you were born in or after the 1970s, it is very likely that you have never watched a television show on a black and white set. Although the roots of its technology extend well back into the early 20th century, the first color broadcast on a national television network didn’t occur until 1954 with NBC’s coverage of the Tournament of Roses Parade.
When we compare the popularization of color television with the rapid pace at which we adopt new technology today, the popularization of color TV was glacial. In large part because of their expense, sales of color sets did not surpass black and white sets until 1972. Our family lagged behind the curve and finally caved in and junked our black and white television around 1977.
The observable change in our viewing behavior was dramatic. While programming in black and white was interesting, the color images were magnetic. We were drawn by the visual excitement and stimulation that color offered, and our family’s viewing standards took a precipitous dip. We seemed to watch anything that was colorful and moved. The quality of the content took a back seat. Viewing in color seemed to require much less cognitive effort. Ironically what attracted our attention allowed us to invest less energy in paying attention.
As a regular reader of Letters From Maine, you know that I am convinced that sleep deprivation is a major contributor to the emergence of the ADHD phenomenon. However, I can make a similar argument that the introduction of color television is an equally potent coconspirator or confounder. The magnetism inherent in a moving color image can tempt even the most health conscious among us to stay well past a brain-friendly bedtime. The invention of the electric light may have gotten the ball rolling, but the ubiquity of moving electronic color images has certainly greased what was already a very slippery slope into an abyss of unhealthy sleep habits.
There are those who argue that smartphones and tablets can open a world of creative opportunities for even very young children. And, it is obvious that parents are struggling to find a balance as they try to decide when, where, and how often to allow their infants and toddlers access to handheld electronic devices.
Recently there has been much finger-pointing at the developers and manufacturers of smartphones and tablets. How can any company with a social conscience sell a product with such dangerous attractive potential for children without providing safeguards? Isn’t it like selling a swimming pool without a gated fence?
Of course the answer to this question goes to the heart of how our society views its responsibility to protect its children. Regardless of who makes the rules and how the responsibility is assigned, it is still the child’s parents who must make sure that the gate is locked.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
Commentary—Serotonin Syndrome and Triptans
Serotonin syndrome (SS) is diagnosed by the clinical triad of dysautonomia (fever, mydriasis, diaphoresis, tachycardia), neuromuscular signs (ataxia, hyperreflexia, tremor, myoclonus), and altered mental status (seizures, delirium). Two validated criteria groups are accepted, the Hunter criteria and the Sternbach criteria. These criteria require a menu-like approach of clinical manifestations of the above signs with known addition or increase of a serotonergic medication and the absence of other possible causes, such as neuroleptics.
In 2006, the FDA issued a clinical warning titled “Potentially Life-Threatening Serotonin Syndrome With Combined Use of SSRIs or SNRIs and Triptan Medications.” Subsequently, Randolph W. Evans, MD, and others conducted a close evaluation of the cases used by the FDA as the basis for their warning. They noted that none of the initial cases met Hunter criteria, only 10 of 29 met Sternbach criteria, and a second set of 11 patients also were questionable in terms of the diagnosis of serotonin toxicity. Serotonin (5-HT) toxicity is mediated by excessive activity of 5-HT2A receptors, and triptans have no action at those receptors, only having activity at 5-HT1B, 1D, and 1F receptors.
In 2010, the American Headache Society (AHS) published a position paper on this drug-drug interaction. In it, they stated, “with only Class IV evidence available in the literature and available through the FDA registration of adverse events, …the currently available evidence does not support limiting the use of triptans with SSRIs or SNRIs, or the use of triptan monotherapy, due to concerns for serotonin syndrome (Level U).”
Confirming the lack of evidence for an interaction, Dr. Yulia Orlova from the Graham Headache Center in Boston reported from the Partners Healthcare System Research Patient Data Registry on about 48,000 patients prescribed triptans, of whom about 19,000 were also co-prescribed SSRI or SNRI antidepressants. None of the cases met Hunter and Sternbach criteria and one patient who manifested serotonin toxicity had signs that preceded triptan use. A previous trial of a cohort of 240,268 patients receiving pharmacy benefits reported that the frequency of co-prescription of triptans with SSRIs was about 20%. With the size of these reports, the absence of documented cases fulfilling both sets of criteria, and the lack of receptor plausibility as a cause for serotonin toxicity from triptans, the likelihood of the syndrome from triptan use is low, and the warning inappropriate. The co-occurrence of depression, anxiety, and migraine often makes co-prescription of triptans and antidepressants necessary, and the concern for co-prescription excessive.
—Stewart J. Tepper, MD
Professor of Neurology
Geisel School of Medicine at Dartmouth
Serotonin syndrome (SS) is diagnosed by the clinical triad of dysautonomia (fever, mydriasis, diaphoresis, tachycardia), neuromuscular signs (ataxia, hyperreflexia, tremor, myoclonus), and altered mental status (seizures, delirium). Two validated criteria groups are accepted, the Hunter criteria and the Sternbach criteria. These criteria require a menu-like approach of clinical manifestations of the above signs with known addition or increase of a serotonergic medication and the absence of other possible causes, such as neuroleptics.
In 2006, the FDA issued a clinical warning titled “Potentially Life-Threatening Serotonin Syndrome With Combined Use of SSRIs or SNRIs and Triptan Medications.” Subsequently, Randolph W. Evans, MD, and others conducted a close evaluation of the cases used by the FDA as the basis for their warning. They noted that none of the initial cases met Hunter criteria, only 10 of 29 met Sternbach criteria, and a second set of 11 patients also were questionable in terms of the diagnosis of serotonin toxicity. Serotonin (5-HT) toxicity is mediated by excessive activity of 5-HT2A receptors, and triptans have no action at those receptors, only having activity at 5-HT1B, 1D, and 1F receptors.
In 2010, the American Headache Society (AHS) published a position paper on this drug-drug interaction. In it, they stated, “with only Class IV evidence available in the literature and available through the FDA registration of adverse events, …the currently available evidence does not support limiting the use of triptans with SSRIs or SNRIs, or the use of triptan monotherapy, due to concerns for serotonin syndrome (Level U).”
Confirming the lack of evidence for an interaction, Dr. Yulia Orlova from the Graham Headache Center in Boston reported from the Partners Healthcare System Research Patient Data Registry on about 48,000 patients prescribed triptans, of whom about 19,000 were also co-prescribed SSRI or SNRI antidepressants. None of the cases met Hunter and Sternbach criteria and one patient who manifested serotonin toxicity had signs that preceded triptan use. A previous trial of a cohort of 240,268 patients receiving pharmacy benefits reported that the frequency of co-prescription of triptans with SSRIs was about 20%. With the size of these reports, the absence of documented cases fulfilling both sets of criteria, and the lack of receptor plausibility as a cause for serotonin toxicity from triptans, the likelihood of the syndrome from triptan use is low, and the warning inappropriate. The co-occurrence of depression, anxiety, and migraine often makes co-prescription of triptans and antidepressants necessary, and the concern for co-prescription excessive.
—Stewart J. Tepper, MD
Professor of Neurology
Geisel School of Medicine at Dartmouth
Serotonin syndrome (SS) is diagnosed by the clinical triad of dysautonomia (fever, mydriasis, diaphoresis, tachycardia), neuromuscular signs (ataxia, hyperreflexia, tremor, myoclonus), and altered mental status (seizures, delirium). Two validated criteria groups are accepted, the Hunter criteria and the Sternbach criteria. These criteria require a menu-like approach of clinical manifestations of the above signs with known addition or increase of a serotonergic medication and the absence of other possible causes, such as neuroleptics.
In 2006, the FDA issued a clinical warning titled “Potentially Life-Threatening Serotonin Syndrome With Combined Use of SSRIs or SNRIs and Triptan Medications.” Subsequently, Randolph W. Evans, MD, and others conducted a close evaluation of the cases used by the FDA as the basis for their warning. They noted that none of the initial cases met Hunter criteria, only 10 of 29 met Sternbach criteria, and a second set of 11 patients also were questionable in terms of the diagnosis of serotonin toxicity. Serotonin (5-HT) toxicity is mediated by excessive activity of 5-HT2A receptors, and triptans have no action at those receptors, only having activity at 5-HT1B, 1D, and 1F receptors.
In 2010, the American Headache Society (AHS) published a position paper on this drug-drug interaction. In it, they stated, “with only Class IV evidence available in the literature and available through the FDA registration of adverse events, …the currently available evidence does not support limiting the use of triptans with SSRIs or SNRIs, or the use of triptan monotherapy, due to concerns for serotonin syndrome (Level U).”
Confirming the lack of evidence for an interaction, Dr. Yulia Orlova from the Graham Headache Center in Boston reported from the Partners Healthcare System Research Patient Data Registry on about 48,000 patients prescribed triptans, of whom about 19,000 were also co-prescribed SSRI or SNRI antidepressants. None of the cases met Hunter and Sternbach criteria and one patient who manifested serotonin toxicity had signs that preceded triptan use. A previous trial of a cohort of 240,268 patients receiving pharmacy benefits reported that the frequency of co-prescription of triptans with SSRIs was about 20%. With the size of these reports, the absence of documented cases fulfilling both sets of criteria, and the lack of receptor plausibility as a cause for serotonin toxicity from triptans, the likelihood of the syndrome from triptan use is low, and the warning inappropriate. The co-occurrence of depression, anxiety, and migraine often makes co-prescription of triptans and antidepressants necessary, and the concern for co-prescription excessive.
—Stewart J. Tepper, MD
Professor of Neurology
Geisel School of Medicine at Dartmouth
A New Era for Physician-Patient Communication in Dermatology
The physician-patient relationship is an important component of patient care. In the last few years a new paradigm has emerged of instant communication. Because dermatologic diagnosis is visual, many patients feel that making a correct diagnosis is as easy as taking a quick look. The availability of smartphone photography and easy ways to get in touch with dermatologists have created a new reality in physician-patient communication, which sometimes may be abused. We conducted an email survey to assess the attitudes of Chilean dermatologists regarding new methods of communication with their patients.
A survey of 16 questions was distributed to all 343 members of the Chilean Society of Dermatology and Venerology from July 2016 to August 2016. A total of 147 (42.9%) dermatologists completed the survey. When asked if they use personal and direct communication with their patients outside of an office visit, 39% of respondents said always, 41% said sometimes, 17% said only in some circumstances, and 3% said never. Regarding the method of communication, 79% used personal email, 59% used mobile phones, 35% used corporate email, and 34% used text messages. Among respondents who gave their personal email address and phone number to patients, the primary reason stated was to be available for any kind of emergency (67%), for patient follow-up (57%), and for patients to feel close to their dermatologist (28%).
Sixty-nine percent of respondents said patients occasionally have requested to receive a diagnosis via a mobile messaging application, social networks, and email. Of them, 22% said they were very annoyed by these requests. When dermatologists were asked if these instant types of communication improved their relationship with patients, 30% said it does help and 36% said it does not; 30% said they do not know and 4% did not respond. If patients used personal methods of communication to contact their dermatologist that was considered outside of physician-patient boundaries, 63% of physician respondents said they kindly directed patients to formal ways of communication and 15% did not respond to such requests; 22% responded by informal methods of communication. Eighty-one percent of all respondents felt the limits of formal communication between physicians and patients have been surpassed.
To improve the quality of health care, many clinicians use modern methods of communication with their patients. Today, patients can turn to their physicians for medical advice by mobile phone or email. We attempted to characterize the attitudes of Chilean dermatologists regarding new ways of communicating with patients. Our results are similar to other studies. One analysis of primary care physicians in Geneva, Switzerland (N=372), showed that 72% gave their personal email address and 74% gave their mobile phone number to patients. The latter is higher than what was found in our study (59%), which may be explained by the fact that primary care physicians may need to maintain closer contact with their patients.1
In another study performed in primary care physicians in Israel, physicians preferred to provide their mobile phone number rather than their personal email address because they felt that email communication was more likely to lead miscommunication than a phone call.2 There are few reports on this subject in the international literature, and we believe cultural differences may be important when physicians confront these issues.
In general, patient satisfaction is high when patients can contact their physician by phone or email; however, new immediate forms of communication may lead to physician burnout, as patients expect immediate responses and solutions to their requests and healthy physician-patient boundaries may be surpassed. It is important to educate both patients and physicians on how these new tools may be properly used on both sides. New boundaries must be set.
- Dash J, Haller DM, Sommer J, et al. Use of email, cell phone and text message between patients and primary-care physicians: cross-sectional study in a French-speaking part of Switzerland. BMC Health Serv Res. 2016;16:549.
- Peleg R, Avdalimov A, Freud T. Providing cell phone numbers and email addresses to patients: the physician’s perspective. BMC Res Notes. 2011;4:76.
The physician-patient relationship is an important component of patient care. In the last few years a new paradigm has emerged of instant communication. Because dermatologic diagnosis is visual, many patients feel that making a correct diagnosis is as easy as taking a quick look. The availability of smartphone photography and easy ways to get in touch with dermatologists have created a new reality in physician-patient communication, which sometimes may be abused. We conducted an email survey to assess the attitudes of Chilean dermatologists regarding new methods of communication with their patients.
A survey of 16 questions was distributed to all 343 members of the Chilean Society of Dermatology and Venerology from July 2016 to August 2016. A total of 147 (42.9%) dermatologists completed the survey. When asked if they use personal and direct communication with their patients outside of an office visit, 39% of respondents said always, 41% said sometimes, 17% said only in some circumstances, and 3% said never. Regarding the method of communication, 79% used personal email, 59% used mobile phones, 35% used corporate email, and 34% used text messages. Among respondents who gave their personal email address and phone number to patients, the primary reason stated was to be available for any kind of emergency (67%), for patient follow-up (57%), and for patients to feel close to their dermatologist (28%).
Sixty-nine percent of respondents said patients occasionally have requested to receive a diagnosis via a mobile messaging application, social networks, and email. Of them, 22% said they were very annoyed by these requests. When dermatologists were asked if these instant types of communication improved their relationship with patients, 30% said it does help and 36% said it does not; 30% said they do not know and 4% did not respond. If patients used personal methods of communication to contact their dermatologist that was considered outside of physician-patient boundaries, 63% of physician respondents said they kindly directed patients to formal ways of communication and 15% did not respond to such requests; 22% responded by informal methods of communication. Eighty-one percent of all respondents felt the limits of formal communication between physicians and patients have been surpassed.
To improve the quality of health care, many clinicians use modern methods of communication with their patients. Today, patients can turn to their physicians for medical advice by mobile phone or email. We attempted to characterize the attitudes of Chilean dermatologists regarding new ways of communicating with patients. Our results are similar to other studies. One analysis of primary care physicians in Geneva, Switzerland (N=372), showed that 72% gave their personal email address and 74% gave their mobile phone number to patients. The latter is higher than what was found in our study (59%), which may be explained by the fact that primary care physicians may need to maintain closer contact with their patients.1
In another study performed in primary care physicians in Israel, physicians preferred to provide their mobile phone number rather than their personal email address because they felt that email communication was more likely to lead miscommunication than a phone call.2 There are few reports on this subject in the international literature, and we believe cultural differences may be important when physicians confront these issues.
In general, patient satisfaction is high when patients can contact their physician by phone or email; however, new immediate forms of communication may lead to physician burnout, as patients expect immediate responses and solutions to their requests and healthy physician-patient boundaries may be surpassed. It is important to educate both patients and physicians on how these new tools may be properly used on both sides. New boundaries must be set.
The physician-patient relationship is an important component of patient care. In the last few years a new paradigm has emerged of instant communication. Because dermatologic diagnosis is visual, many patients feel that making a correct diagnosis is as easy as taking a quick look. The availability of smartphone photography and easy ways to get in touch with dermatologists have created a new reality in physician-patient communication, which sometimes may be abused. We conducted an email survey to assess the attitudes of Chilean dermatologists regarding new methods of communication with their patients.
A survey of 16 questions was distributed to all 343 members of the Chilean Society of Dermatology and Venerology from July 2016 to August 2016. A total of 147 (42.9%) dermatologists completed the survey. When asked if they use personal and direct communication with their patients outside of an office visit, 39% of respondents said always, 41% said sometimes, 17% said only in some circumstances, and 3% said never. Regarding the method of communication, 79% used personal email, 59% used mobile phones, 35% used corporate email, and 34% used text messages. Among respondents who gave their personal email address and phone number to patients, the primary reason stated was to be available for any kind of emergency (67%), for patient follow-up (57%), and for patients to feel close to their dermatologist (28%).
Sixty-nine percent of respondents said patients occasionally have requested to receive a diagnosis via a mobile messaging application, social networks, and email. Of them, 22% said they were very annoyed by these requests. When dermatologists were asked if these instant types of communication improved their relationship with patients, 30% said it does help and 36% said it does not; 30% said they do not know and 4% did not respond. If patients used personal methods of communication to contact their dermatologist that was considered outside of physician-patient boundaries, 63% of physician respondents said they kindly directed patients to formal ways of communication and 15% did not respond to such requests; 22% responded by informal methods of communication. Eighty-one percent of all respondents felt the limits of formal communication between physicians and patients have been surpassed.
To improve the quality of health care, many clinicians use modern methods of communication with their patients. Today, patients can turn to their physicians for medical advice by mobile phone or email. We attempted to characterize the attitudes of Chilean dermatologists regarding new ways of communicating with patients. Our results are similar to other studies. One analysis of primary care physicians in Geneva, Switzerland (N=372), showed that 72% gave their personal email address and 74% gave their mobile phone number to patients. The latter is higher than what was found in our study (59%), which may be explained by the fact that primary care physicians may need to maintain closer contact with their patients.1
In another study performed in primary care physicians in Israel, physicians preferred to provide their mobile phone number rather than their personal email address because they felt that email communication was more likely to lead miscommunication than a phone call.2 There are few reports on this subject in the international literature, and we believe cultural differences may be important when physicians confront these issues.
In general, patient satisfaction is high when patients can contact their physician by phone or email; however, new immediate forms of communication may lead to physician burnout, as patients expect immediate responses and solutions to their requests and healthy physician-patient boundaries may be surpassed. It is important to educate both patients and physicians on how these new tools may be properly used on both sides. New boundaries must be set.
- Dash J, Haller DM, Sommer J, et al. Use of email, cell phone and text message between patients and primary-care physicians: cross-sectional study in a French-speaking part of Switzerland. BMC Health Serv Res. 2016;16:549.
- Peleg R, Avdalimov A, Freud T. Providing cell phone numbers and email addresses to patients: the physician’s perspective. BMC Res Notes. 2011;4:76.
- Dash J, Haller DM, Sommer J, et al. Use of email, cell phone and text message between patients and primary-care physicians: cross-sectional study in a French-speaking part of Switzerland. BMC Health Serv Res. 2016;16:549.
- Peleg R, Avdalimov A, Freud T. Providing cell phone numbers and email addresses to patients: the physician’s perspective. BMC Res Notes. 2011;4:76.
Pain-Minimizing Strategies for Nail Surgery
Nail surgery is an important part of dermatologic training and clinical practice, both for diagnosis and treatment of nail disorders as well as benign and malignant nail tumors. Patient comfort is essential prior to the procedure and while administering local anesthetics. Effective anesthesia facilitates nail unit biopsies, excisions, and other surgical nail procedures. Pain management immediately following the procedure and during the postoperative period are equally important.
Patients who undergo nail surgery may experience anxiety due to fear of a cancer diagnosis, pain during the surgery, or disfigurement from the procedure. This anxiety may lead to increased blood pressure, a decreased pain threshold, and mental and physical discomfort.1 A detailed explanation of the procedure itself as well as expectations following the surgery are helpful in diminishing these fears. Administration of a fast-acting benzodiazepine also may be helpful in these patients to decrease anxiety prior to the procedure.2
Attaining adequate anesthesia requires an understanding of digital anatomy, particularly innervation. Innervation of the digits is supplied by the volar and dorsal nerves, which divide into 3 branches at the distal interphalangeal joint, innervating the nail bed, the digital tip, and the pulp.3 Pacinian and Ruffini corpuscles and free-ended nociceptors activate nerve fibers that transmit pain impulses.4,5 Local anesthetics block pain transmission by impeding voltage-gated sodium channels located at free nerve endings. Pain from anesthesia may be due to both needle insertion and fluid infiltration.
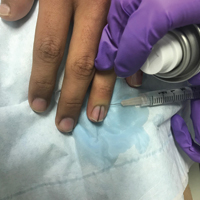
Simple measures can maximize patient comfort during digital anesthesia. Both audiovisual distraction and interpersonal interaction can help to put the patient at ease.6,7 Application of topical anesthetic cream (1–2 hours prior to the procedure under occlusion),8 ice (at least 6 minutes),9 or an ethyl chloride spray can be applied to the nail folds prior to needle insertion to alleviate injection pain, but these methods do little for infiltration pain. Use of an ethyl chloride spray may be the preferred technique due to the rapidity of the analgesic effects (Figure).10 A vibrating massager also can be applied in close proximity to the site of needle insertion.11
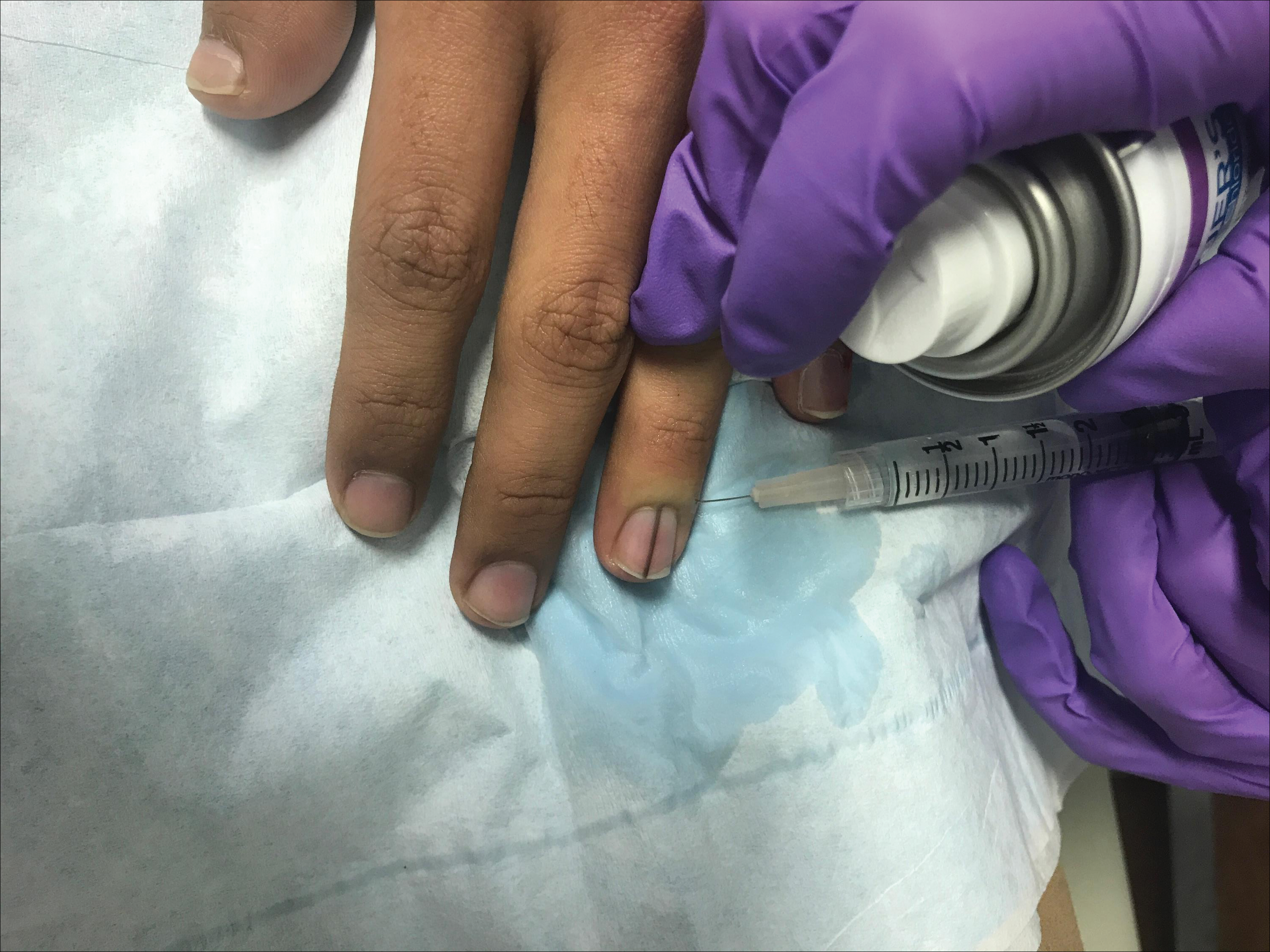
Proper anesthetic preparation and technique also can minimize pain during injection. Because lidocaine 1% is acidic (pH, 6.09), buffering with sodium bicarbonate 8.4% can result in decreased injection pain and faster onset of action.6,12 Warming the anesthetic using a water bath, incubator, or autoclave can decrease pain without degradation of lidocaine or epinephrine.13 At a minimum, 30-gauge needles are preferred to minimize pain from needle insertion. Use of 33-gauge needles has shown benefit for injecting the face and scalp and may prove to be helpful injecting sensitive areas such as the digits.14 A slow injection technique is more comfortable for the patient, as rapid injection causes tissue distention.11
The ideal anesthetic for nail surgery would have a fast onset and a long duration of action, which would allow for shorter operation time as well as alleviation of pain postprocedure and some degree of vasoconstriction to help maintain a bloodless field. Lidocaine has the fastest time of onset (<1–3 minutes) but a short duration of action (30–120 minutes) and a vasodilatory effect. Bupivacaine takes 2 to 5 minutes to take effect and has a long duration of action (120–240 minutes) but a risk for cardiotoxicity. Ropivacaine is the preferred anesthetic by some nail surgeons because of its intermediate time of onset (1–15 minutes), long duration of action (120–360 minutes), and the benefit of some vasoconstriction.5,15 The addition of epinephrine has 2 main advantages: vasoconstriction and prolongation of anesthetic effects; the latter may help to alleviate postoperative pain. If there are no contraindications to its use (ie, severe hypertension, Raynaud phenomenon), it can be used safely in digital anesthesia without risk for ischemia or infarction.11
Digital anesthesia can be achieved by infiltration or using nerve blocks. One major difference between these 2 approaches is the time of onset of anesthesia, with the former being nearly instantaneous and the latter taking up to 15 minutes.16 There also usually is more prolonged pain at the site of needle insertion with nerve blocks compared to infiltration. The type of nail surgery being performed, the digit involved, and surgeon preference will determine the anesthetic method of choice.17
Pain management immediately following the procedure and for several days after is essential. Use of a longer-acting anesthetic, such as bupivacaine or ropivacaine, will provide anesthesia for several hours. A well-padded dressing serves to absorb blood and protect the nail and distal digit from trauma, as even minor trauma can exacerbate pain and bleeding. The patient should be instructed to apply ice to the surgical site and keep the ipsilateral extremity elevated for the next 2 days to reduce edema and pain.15 Written instructions are helpful, as anxiety during and after the procedure may limit the patient’s understanding and recollection of the verbal postoperative instructions. To maximize readability of the information, the National Institutes of Health and American Medical Association recommend that the instructions be written at a fourth- to sixth-grade reading level.18,19
A single dose of ibuprofen (400 mg) or acetaminophen (500 mg to 1 g) immediately before or after the procedure can reduce opioid use and postoperative pain.20 Gabapentin (300–1200 mg) given 1 to 2 hours before surgery may be considered in patients who are at high risk for postsurgical pain.21 Acetaminophen or nonsteroidal anti-inflammatory drugs (eg, ibuprofen [200–400 mg]) administered every 4 to 6 hours provides considerable pain reduction postprocedure. Nonsteroidal anti-inflammatory drugs may be superior to acetaminophen for pain control22 and carry a low risk for postoperative bleeding.23 Additionally, a combination of acetaminophen with a nonsteroidal anti-inflammatory drug for 3 doses may be more effective than either drug alone.24 Some patients may require an opioid combination, such as codeine plus acetaminophen, for a short time (up to 3 days) for pain relief following surgery. Excessive pain or pain lasting than more than 3 days is not normal or expected; in these cases, patients should return to the office to rule out ischemia or infection.
It is important to implement pain-minimizing strategies for nail surgeries. Because many of these approaches are derived from other surgical specialties, well-controlled clinical trials in patients undergoing nail surgery will be necessary to improve outcomes.
- Goktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39.
- Ravitskiy L, Phillips PK, Roenigk RK, et al. The use of oral midazolam for perioperative anxiolysis of healthy patients undergoing Mohs surgery: conclusions from randomized controlled and prospective studies. J Am Acad Dermatol. 2011;64:310-322.
- Richert B. Anesthesia of the nail apparatus. In: Richert B, Di Chiacchio N, Haneke E, eds. Nail Surgery. New York, NY: Informa Healthcare; 2010:24-30.
- Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3:41-49.
- Soriano TT, Beynet DP. Anesthesia and analgesia. In: Robinson J, Hanke CW, Siegel D, et al, eds. Surgery of the Skin. 2nd ed. New York, NY: Elsevier; 2010:43-63.
- Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
- Drahota A, Galloway E, Stores R, et al. Audiovisual distraction as an adjunct to pain and anxiety relief during minor surgery. Foot (Edinb). 2008;18:211-219.
- Browne J, Fung M, Donnelly M, et al. The use of EMLA reduces the pain associated with digital ring block for ingrowing toenail correction. Eur J Anaesthesiol. 2000;17:182-184.
- Hayward SC, Landorf KB, Redmond AC. Ice reduces needle-stick pain associated with a digital nerve block of the hallux. Foot. 2006;16:145-148.
- Kose O, Saylan S, Ediz N, et al. Effects of topical alkane vapocoolant spray on pain intensity prior to digital nerve block for ingrown nail surgery. Foot Ankle Spec. 2010;3:73-75.
- Jellinek NJ, Velez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271.
- Strazar R, Lalonde D. Minimizing injection pain in local anesthesia. CMAJ. 2012;184:2016.
- Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
- Zelickson BR, Goldberg LH, Rubenzik MK, et al. Finer needles reduce pain associated with injection of local anesthetic using a minimal insertion injection technique [published online October 6, 2017]. Dermatol Surg. doi:10.1097/DSS.0000000000001279.
- Haneke E. Nail surgery. Clin Dermatol. 2013;31:516-525.
- Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-51.e5.
- Jellinek NJ. Nail surgery: practical tips and treatment options. Dermatol Ther. 2007;20:68-74.
- How to write easy-to-read health materials. Medline Plus website. https://medlineplus.gov/etr.html. Updated June 28, 2017.
Accessed January 29, 2018. - Weis BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Foundation, American Medical Association; 2003.
- Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg. 2014;134(4 suppl 2):85S-93S.
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12 2010]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008183.pub2.
- Bailey E, Worthington H, Coulthard P. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth, a Cochrane systematic review. Br Dent J. 2014;216:451-455.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560; quiz 561-562.
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013.
Nail surgery is an important part of dermatologic training and clinical practice, both for diagnosis and treatment of nail disorders as well as benign and malignant nail tumors. Patient comfort is essential prior to the procedure and while administering local anesthetics. Effective anesthesia facilitates nail unit biopsies, excisions, and other surgical nail procedures. Pain management immediately following the procedure and during the postoperative period are equally important.
Patients who undergo nail surgery may experience anxiety due to fear of a cancer diagnosis, pain during the surgery, or disfigurement from the procedure. This anxiety may lead to increased blood pressure, a decreased pain threshold, and mental and physical discomfort.1 A detailed explanation of the procedure itself as well as expectations following the surgery are helpful in diminishing these fears. Administration of a fast-acting benzodiazepine also may be helpful in these patients to decrease anxiety prior to the procedure.2
Attaining adequate anesthesia requires an understanding of digital anatomy, particularly innervation. Innervation of the digits is supplied by the volar and dorsal nerves, which divide into 3 branches at the distal interphalangeal joint, innervating the nail bed, the digital tip, and the pulp.3 Pacinian and Ruffini corpuscles and free-ended nociceptors activate nerve fibers that transmit pain impulses.4,5 Local anesthetics block pain transmission by impeding voltage-gated sodium channels located at free nerve endings. Pain from anesthesia may be due to both needle insertion and fluid infiltration.
Simple measures can maximize patient comfort during digital anesthesia. Both audiovisual distraction and interpersonal interaction can help to put the patient at ease.6,7 Application of topical anesthetic cream (1–2 hours prior to the procedure under occlusion),8 ice (at least 6 minutes),9 or an ethyl chloride spray can be applied to the nail folds prior to needle insertion to alleviate injection pain, but these methods do little for infiltration pain. Use of an ethyl chloride spray may be the preferred technique due to the rapidity of the analgesic effects (Figure).10 A vibrating massager also can be applied in close proximity to the site of needle insertion.11

Proper anesthetic preparation and technique also can minimize pain during injection. Because lidocaine 1% is acidic (pH, 6.09), buffering with sodium bicarbonate 8.4% can result in decreased injection pain and faster onset of action.6,12 Warming the anesthetic using a water bath, incubator, or autoclave can decrease pain without degradation of lidocaine or epinephrine.13 At a minimum, 30-gauge needles are preferred to minimize pain from needle insertion. Use of 33-gauge needles has shown benefit for injecting the face and scalp and may prove to be helpful injecting sensitive areas such as the digits.14 A slow injection technique is more comfortable for the patient, as rapid injection causes tissue distention.11
The ideal anesthetic for nail surgery would have a fast onset and a long duration of action, which would allow for shorter operation time as well as alleviation of pain postprocedure and some degree of vasoconstriction to help maintain a bloodless field. Lidocaine has the fastest time of onset (<1–3 minutes) but a short duration of action (30–120 minutes) and a vasodilatory effect. Bupivacaine takes 2 to 5 minutes to take effect and has a long duration of action (120–240 minutes) but a risk for cardiotoxicity. Ropivacaine is the preferred anesthetic by some nail surgeons because of its intermediate time of onset (1–15 minutes), long duration of action (120–360 minutes), and the benefit of some vasoconstriction.5,15 The addition of epinephrine has 2 main advantages: vasoconstriction and prolongation of anesthetic effects; the latter may help to alleviate postoperative pain. If there are no contraindications to its use (ie, severe hypertension, Raynaud phenomenon), it can be used safely in digital anesthesia without risk for ischemia or infarction.11
Digital anesthesia can be achieved by infiltration or using nerve blocks. One major difference between these 2 approaches is the time of onset of anesthesia, with the former being nearly instantaneous and the latter taking up to 15 minutes.16 There also usually is more prolonged pain at the site of needle insertion with nerve blocks compared to infiltration. The type of nail surgery being performed, the digit involved, and surgeon preference will determine the anesthetic method of choice.17
Pain management immediately following the procedure and for several days after is essential. Use of a longer-acting anesthetic, such as bupivacaine or ropivacaine, will provide anesthesia for several hours. A well-padded dressing serves to absorb blood and protect the nail and distal digit from trauma, as even minor trauma can exacerbate pain and bleeding. The patient should be instructed to apply ice to the surgical site and keep the ipsilateral extremity elevated for the next 2 days to reduce edema and pain.15 Written instructions are helpful, as anxiety during and after the procedure may limit the patient’s understanding and recollection of the verbal postoperative instructions. To maximize readability of the information, the National Institutes of Health and American Medical Association recommend that the instructions be written at a fourth- to sixth-grade reading level.18,19
A single dose of ibuprofen (400 mg) or acetaminophen (500 mg to 1 g) immediately before or after the procedure can reduce opioid use and postoperative pain.20 Gabapentin (300–1200 mg) given 1 to 2 hours before surgery may be considered in patients who are at high risk for postsurgical pain.21 Acetaminophen or nonsteroidal anti-inflammatory drugs (eg, ibuprofen [200–400 mg]) administered every 4 to 6 hours provides considerable pain reduction postprocedure. Nonsteroidal anti-inflammatory drugs may be superior to acetaminophen for pain control22 and carry a low risk for postoperative bleeding.23 Additionally, a combination of acetaminophen with a nonsteroidal anti-inflammatory drug for 3 doses may be more effective than either drug alone.24 Some patients may require an opioid combination, such as codeine plus acetaminophen, for a short time (up to 3 days) for pain relief following surgery. Excessive pain or pain lasting than more than 3 days is not normal or expected; in these cases, patients should return to the office to rule out ischemia or infection.
It is important to implement pain-minimizing strategies for nail surgeries. Because many of these approaches are derived from other surgical specialties, well-controlled clinical trials in patients undergoing nail surgery will be necessary to improve outcomes.
Nail surgery is an important part of dermatologic training and clinical practice, both for diagnosis and treatment of nail disorders as well as benign and malignant nail tumors. Patient comfort is essential prior to the procedure and while administering local anesthetics. Effective anesthesia facilitates nail unit biopsies, excisions, and other surgical nail procedures. Pain management immediately following the procedure and during the postoperative period are equally important.
Patients who undergo nail surgery may experience anxiety due to fear of a cancer diagnosis, pain during the surgery, or disfigurement from the procedure. This anxiety may lead to increased blood pressure, a decreased pain threshold, and mental and physical discomfort.1 A detailed explanation of the procedure itself as well as expectations following the surgery are helpful in diminishing these fears. Administration of a fast-acting benzodiazepine also may be helpful in these patients to decrease anxiety prior to the procedure.2
Attaining adequate anesthesia requires an understanding of digital anatomy, particularly innervation. Innervation of the digits is supplied by the volar and dorsal nerves, which divide into 3 branches at the distal interphalangeal joint, innervating the nail bed, the digital tip, and the pulp.3 Pacinian and Ruffini corpuscles and free-ended nociceptors activate nerve fibers that transmit pain impulses.4,5 Local anesthetics block pain transmission by impeding voltage-gated sodium channels located at free nerve endings. Pain from anesthesia may be due to both needle insertion and fluid infiltration.
Simple measures can maximize patient comfort during digital anesthesia. Both audiovisual distraction and interpersonal interaction can help to put the patient at ease.6,7 Application of topical anesthetic cream (1–2 hours prior to the procedure under occlusion),8 ice (at least 6 minutes),9 or an ethyl chloride spray can be applied to the nail folds prior to needle insertion to alleviate injection pain, but these methods do little for infiltration pain. Use of an ethyl chloride spray may be the preferred technique due to the rapidity of the analgesic effects (Figure).10 A vibrating massager also can be applied in close proximity to the site of needle insertion.11

Proper anesthetic preparation and technique also can minimize pain during injection. Because lidocaine 1% is acidic (pH, 6.09), buffering with sodium bicarbonate 8.4% can result in decreased injection pain and faster onset of action.6,12 Warming the anesthetic using a water bath, incubator, or autoclave can decrease pain without degradation of lidocaine or epinephrine.13 At a minimum, 30-gauge needles are preferred to minimize pain from needle insertion. Use of 33-gauge needles has shown benefit for injecting the face and scalp and may prove to be helpful injecting sensitive areas such as the digits.14 A slow injection technique is more comfortable for the patient, as rapid injection causes tissue distention.11
The ideal anesthetic for nail surgery would have a fast onset and a long duration of action, which would allow for shorter operation time as well as alleviation of pain postprocedure and some degree of vasoconstriction to help maintain a bloodless field. Lidocaine has the fastest time of onset (<1–3 minutes) but a short duration of action (30–120 minutes) and a vasodilatory effect. Bupivacaine takes 2 to 5 minutes to take effect and has a long duration of action (120–240 minutes) but a risk for cardiotoxicity. Ropivacaine is the preferred anesthetic by some nail surgeons because of its intermediate time of onset (1–15 minutes), long duration of action (120–360 minutes), and the benefit of some vasoconstriction.5,15 The addition of epinephrine has 2 main advantages: vasoconstriction and prolongation of anesthetic effects; the latter may help to alleviate postoperative pain. If there are no contraindications to its use (ie, severe hypertension, Raynaud phenomenon), it can be used safely in digital anesthesia without risk for ischemia or infarction.11
Digital anesthesia can be achieved by infiltration or using nerve blocks. One major difference between these 2 approaches is the time of onset of anesthesia, with the former being nearly instantaneous and the latter taking up to 15 minutes.16 There also usually is more prolonged pain at the site of needle insertion with nerve blocks compared to infiltration. The type of nail surgery being performed, the digit involved, and surgeon preference will determine the anesthetic method of choice.17
Pain management immediately following the procedure and for several days after is essential. Use of a longer-acting anesthetic, such as bupivacaine or ropivacaine, will provide anesthesia for several hours. A well-padded dressing serves to absorb blood and protect the nail and distal digit from trauma, as even minor trauma can exacerbate pain and bleeding. The patient should be instructed to apply ice to the surgical site and keep the ipsilateral extremity elevated for the next 2 days to reduce edema and pain.15 Written instructions are helpful, as anxiety during and after the procedure may limit the patient’s understanding and recollection of the verbal postoperative instructions. To maximize readability of the information, the National Institutes of Health and American Medical Association recommend that the instructions be written at a fourth- to sixth-grade reading level.18,19
A single dose of ibuprofen (400 mg) or acetaminophen (500 mg to 1 g) immediately before or after the procedure can reduce opioid use and postoperative pain.20 Gabapentin (300–1200 mg) given 1 to 2 hours before surgery may be considered in patients who are at high risk for postsurgical pain.21 Acetaminophen or nonsteroidal anti-inflammatory drugs (eg, ibuprofen [200–400 mg]) administered every 4 to 6 hours provides considerable pain reduction postprocedure. Nonsteroidal anti-inflammatory drugs may be superior to acetaminophen for pain control22 and carry a low risk for postoperative bleeding.23 Additionally, a combination of acetaminophen with a nonsteroidal anti-inflammatory drug for 3 doses may be more effective than either drug alone.24 Some patients may require an opioid combination, such as codeine plus acetaminophen, for a short time (up to 3 days) for pain relief following surgery. Excessive pain or pain lasting than more than 3 days is not normal or expected; in these cases, patients should return to the office to rule out ischemia or infection.
It is important to implement pain-minimizing strategies for nail surgeries. Because many of these approaches are derived from other surgical specialties, well-controlled clinical trials in patients undergoing nail surgery will be necessary to improve outcomes.
- Goktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39.
- Ravitskiy L, Phillips PK, Roenigk RK, et al. The use of oral midazolam for perioperative anxiolysis of healthy patients undergoing Mohs surgery: conclusions from randomized controlled and prospective studies. J Am Acad Dermatol. 2011;64:310-322.
- Richert B. Anesthesia of the nail apparatus. In: Richert B, Di Chiacchio N, Haneke E, eds. Nail Surgery. New York, NY: Informa Healthcare; 2010:24-30.
- Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3:41-49.
- Soriano TT, Beynet DP. Anesthesia and analgesia. In: Robinson J, Hanke CW, Siegel D, et al, eds. Surgery of the Skin. 2nd ed. New York, NY: Elsevier; 2010:43-63.
- Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
- Drahota A, Galloway E, Stores R, et al. Audiovisual distraction as an adjunct to pain and anxiety relief during minor surgery. Foot (Edinb). 2008;18:211-219.
- Browne J, Fung M, Donnelly M, et al. The use of EMLA reduces the pain associated with digital ring block for ingrowing toenail correction. Eur J Anaesthesiol. 2000;17:182-184.
- Hayward SC, Landorf KB, Redmond AC. Ice reduces needle-stick pain associated with a digital nerve block of the hallux. Foot. 2006;16:145-148.
- Kose O, Saylan S, Ediz N, et al. Effects of topical alkane vapocoolant spray on pain intensity prior to digital nerve block for ingrown nail surgery. Foot Ankle Spec. 2010;3:73-75.
- Jellinek NJ, Velez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271.
- Strazar R, Lalonde D. Minimizing injection pain in local anesthesia. CMAJ. 2012;184:2016.
- Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
- Zelickson BR, Goldberg LH, Rubenzik MK, et al. Finer needles reduce pain associated with injection of local anesthetic using a minimal insertion injection technique [published online October 6, 2017]. Dermatol Surg. doi:10.1097/DSS.0000000000001279.
- Haneke E. Nail surgery. Clin Dermatol. 2013;31:516-525.
- Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-51.e5.
- Jellinek NJ. Nail surgery: practical tips and treatment options. Dermatol Ther. 2007;20:68-74.
- How to write easy-to-read health materials. Medline Plus website. https://medlineplus.gov/etr.html. Updated June 28, 2017.
Accessed January 29, 2018. - Weis BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Foundation, American Medical Association; 2003.
- Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg. 2014;134(4 suppl 2):85S-93S.
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12 2010]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008183.pub2.
- Bailey E, Worthington H, Coulthard P. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth, a Cochrane systematic review. Br Dent J. 2014;216:451-455.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560; quiz 561-562.
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013.
- Goktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39.
- Ravitskiy L, Phillips PK, Roenigk RK, et al. The use of oral midazolam for perioperative anxiolysis of healthy patients undergoing Mohs surgery: conclusions from randomized controlled and prospective studies. J Am Acad Dermatol. 2011;64:310-322.
- Richert B. Anesthesia of the nail apparatus. In: Richert B, Di Chiacchio N, Haneke E, eds. Nail Surgery. New York, NY: Informa Healthcare; 2010:24-30.
- Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3:41-49.
- Soriano TT, Beynet DP. Anesthesia and analgesia. In: Robinson J, Hanke CW, Siegel D, et al, eds. Surgery of the Skin. 2nd ed. New York, NY: Elsevier; 2010:43-63.
- Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
- Drahota A, Galloway E, Stores R, et al. Audiovisual distraction as an adjunct to pain and anxiety relief during minor surgery. Foot (Edinb). 2008;18:211-219.
- Browne J, Fung M, Donnelly M, et al. The use of EMLA reduces the pain associated with digital ring block for ingrowing toenail correction. Eur J Anaesthesiol. 2000;17:182-184.
- Hayward SC, Landorf KB, Redmond AC. Ice reduces needle-stick pain associated with a digital nerve block of the hallux. Foot. 2006;16:145-148.
- Kose O, Saylan S, Ediz N, et al. Effects of topical alkane vapocoolant spray on pain intensity prior to digital nerve block for ingrown nail surgery. Foot Ankle Spec. 2010;3:73-75.
- Jellinek NJ, Velez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271.
- Strazar R, Lalonde D. Minimizing injection pain in local anesthesia. CMAJ. 2012;184:2016.
- Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
- Zelickson BR, Goldberg LH, Rubenzik MK, et al. Finer needles reduce pain associated with injection of local anesthetic using a minimal insertion injection technique [published online October 6, 2017]. Dermatol Surg. doi:10.1097/DSS.0000000000001279.
- Haneke E. Nail surgery. Clin Dermatol. 2013;31:516-525.
- Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-51.e5.
- Jellinek NJ. Nail surgery: practical tips and treatment options. Dermatol Ther. 2007;20:68-74.
- How to write easy-to-read health materials. Medline Plus website. https://medlineplus.gov/etr.html. Updated June 28, 2017.
Accessed January 29, 2018. - Weis BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Foundation, American Medical Association; 2003.
- Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg. 2014;134(4 suppl 2):85S-93S.
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12 2010]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008183.pub2.
- Bailey E, Worthington H, Coulthard P. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth, a Cochrane systematic review. Br Dent J. 2014;216:451-455.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560; quiz 561-562.
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013.
MS: Past, Present, and Future
Stuart D. Cook, MD, and Abdul Rahman Alchaki
Dr. Cook is the Ruth Dunietz Kushner and Michael Jay Serwitz Professor of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark. Dr. Alchaki is a resident in the Deptartment of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark.
Disclosure: Stuart Cook has received honoraria for lectures from Bayer HealthCare and Merck Serono. He has served as a consultant for Merck Serono, Bayer HealthCare, Teva, Novartis, Sanofi-Aventis, Biogen Idec, and Actinobac Biomed. He has served on steering committees for the BEYOND and CLARITY Studies and as a member of Advisory Boards for Merck Serono, Bayer HealthCare, Teva, Biogen Idec, Sanofi Aventis, and Actinobac Biomed.
The Initial Years (1838 to 1930s)
The earliest recognition of MS clinical features and pathology was attributed to Jean-Martin Charcot, Robert Carswell, and Jean Cruveilhier in Europe from 1838 to 1868. Beyond those early descriptions, relatively few MS breakthroughs occurred until the 1930s, when Thomas Rivers discovered experimental autoimmune encephalomyelitis (EAE), a demyelinating disease, in animals. His insightful concepts were widely cited and ultimately contributed to undestanding of the immune mechanisms of MS and acute disseminated encephalomyelitis (ADEM).
Advances in Diagnosis (1965 to 1992)
In 1965, Schumacher et al provided the essential clinical criteria for MS diagnosis. Poser et al refined these criteria in 1983. In 2001, McDonald et al added neuroimaging, CSF analysis, and evoked potentials to further complement MS clinical diagnosis. For the first time, the disease could generally be recognized.
Early Treatments
Various treatments for MS were tried over the years, without great success. However, in 1953, a small descriptive trial by Miller and Gibbons reported clinical benefits in patients using intramuscular (IM) adrenocorticotropic hormone (ACTH) for MS and disseminated encephalomyelitis. This was followed in 1970 by a Cooperative Study of IM ACTH versus placebo by Rose et al, which resulted in ACTH, and subsequently oral corticosteroids, being widely used to treat MS, particularly for acute exacerbations of the disease. However, robust evidence of long-term steroids remain limited, even to the present.
High-Dose Steroids
By 1980, the initial descriptive treatment of high-dose intravenous (IV) steroids for demyelinating diseases, including MS and transverse myelitis, by Dowling et al resulted in rapid clinical improvement in some patients. This result was ultimately confirmed by others. High-dose IV steroids became the gold standard for acute attacks, particularly those aggressive in nature. In the mid 1980s, work by Troiano et al, as well as others, showed that the rapid use of high-dose IV as well as oral steroids showed similar effects, with reduction or elimination of CT contrast-enhancing lesions within as few as eight hours, while lower doses or alternative-day treatments were less effective. In addition, descriptive studies of immune modulatory and immunosuppressive drugs, as well as small randomized studies, were published. These agents did not receive FDA approval.
The Golden Age of Therapy (1993 to 2018)
A remarkable era in MS prognosis and treatment began with immunomodulation injections of Betaseron (INFβ-1b), Avonex (INFβ-1a), and Copaxone (glatiramer acetate). This can be attributed, at least in part, to advances in molecular biology, genetics, and neuroimaging, and support by corporate, private, and public funding. Since the initial FDA approval of INFβ-1b, 15 MS therapies have become clinically available, including eight injectables, three orals, and four infusion treatments (see Table 1). In addition, two other drugs have been FDA approved for uses other than MS: rituximab (approved for lymphoma) and cladribine (for hairy cell leukemia), with the latter now approved by the European Medicines Agency for MS. Table 1 depicts characteristics of these therapies approved by US or European agencies (or for other disorders increasingly used off label for MS) in an attempt to compare annual relapse rates (ARR) and decreases in the percent of gadolinium-enhancing MS lesions versus placebo. This information was chosen because ARR has been uniformly selected and defined for such trials, while percent decrease of gadolinium-enhancing lesions on MRI has been the most sensitive barometer available for assessing acute clinical activity. As a result, risk-benefit considerations have been critical in evaluating these drug treatments, with efficacy improving greatly over time, whereas risks have been more variable.
Disease Categories
In 1996, Lublin and Reingold provided a new classification, not specifically for the diagnosis of MS, but rather for the clinical course of the disease. Initially, there were four categories—relapsing-remitting MS, secondary progressive MS, primary progressive MS, and progressive-relapsing MS—that were universally identified. These were thought to be relatively distinct clinical categories, but over time it became clear that the classification did not fully distinguish MS disease activity within these categories. For that reason, it was subsequently recommended, by Lincoln et al in 2009 and Cook et al in 2012, to include MRI, a vastly more sensitive modality, as well as clinical data in assessing disease activity.
On another note, MS and neuromyelitis optica (NMO), although having similar features, were clearly identified as different diseases by Lennon et al in 2004. Differences in pathology, clinical characteristics, immunology, and therapy separate the two disorders.
MRI in MS
Work by Young et al in 1981 established the central role of MRI brain imaging in MS diagnosis and therapeutic considerations. Since then it has become ubiquitous.
An example of a sensitive and highly productive MRI protocol is the BECOME study of MS and clinically isolated syndrome by Cadavid et al from 2009 to 2017. In this study, IFNβ-1b was compared with glatiramer acetate treatment. Cadavid et al used a 3T scanner with triple-dose gadolinium, performed monthly for as long as 24 consecutive months. This unique study brought about a virtual gold mine of valuable research and clinical information. This included proof that gadolinium-enhancing lesions persisted for six months or more, evidence of a 30:1 ratio of new MRI brain lesions to clinical activity, and documentation that 96% of T2 lesions and black holes derive from prior gadolinium-enhancing lesions. It was further noted that 80% to 90% of acute black holes disappeared with treatment and 75% to 80% of patients taking IFNβ-1b or glatiramer acetate had new MRI lesions despite continuing treatment. Perhaps most interestingly, monthly MRIs could predict relapse and disability in a relatively small number of patients, depending upon the frequency and activity of MRI lesions. In 2017, Brown et al documented that magnetization transfer ratio recovery in MS brain lesions occurred more significantly with glatiramer acetate than with IFNβ-1b, whereas more chronic black hole lesions were found with glatiramer acetate. Also in 2017, Maranzano et al found evidence of acute inflammatory leukocortical lesions, which were not as well recognized previously.
In summary, it has become increasingly clear that MRI is the most sen
The Future of MS
While it is not yet a curable disease, there is growing evidence that MS prognosis has improved and will continue to improve. This is based on incremental decreases in acute MS exacerbations, progressive disability, and MRI lesion activity, as well as a combination of the three—no evidence of disease activity (NEDA).
Not only are drug therapies becoming more effective, but patients and physicians now have many more treatment options to carefully consider with regard to efficacy, side effect profiles, treatment frequency, route of administration, cost, and quality of life. Newer drugs with different mechanisms of action such as cladribine, now approved in Europe, fulfill most of these beneficial criteria (see Giovannoni et al, 2010). More promising MS treatments, including long-acting induction therapies, are still being evaluated. As with other complex diseases, multiple therapies are likely to be used as well.
In summary, compared with the time before 1993, MS will be much less likely to be a progressive disease, and quality of life will be much improved. In my opinion, patients will be less fearful about their prognosis than ever before, and with appropriate evaluations and treatments, we may realize that disabling MS will be far less common.
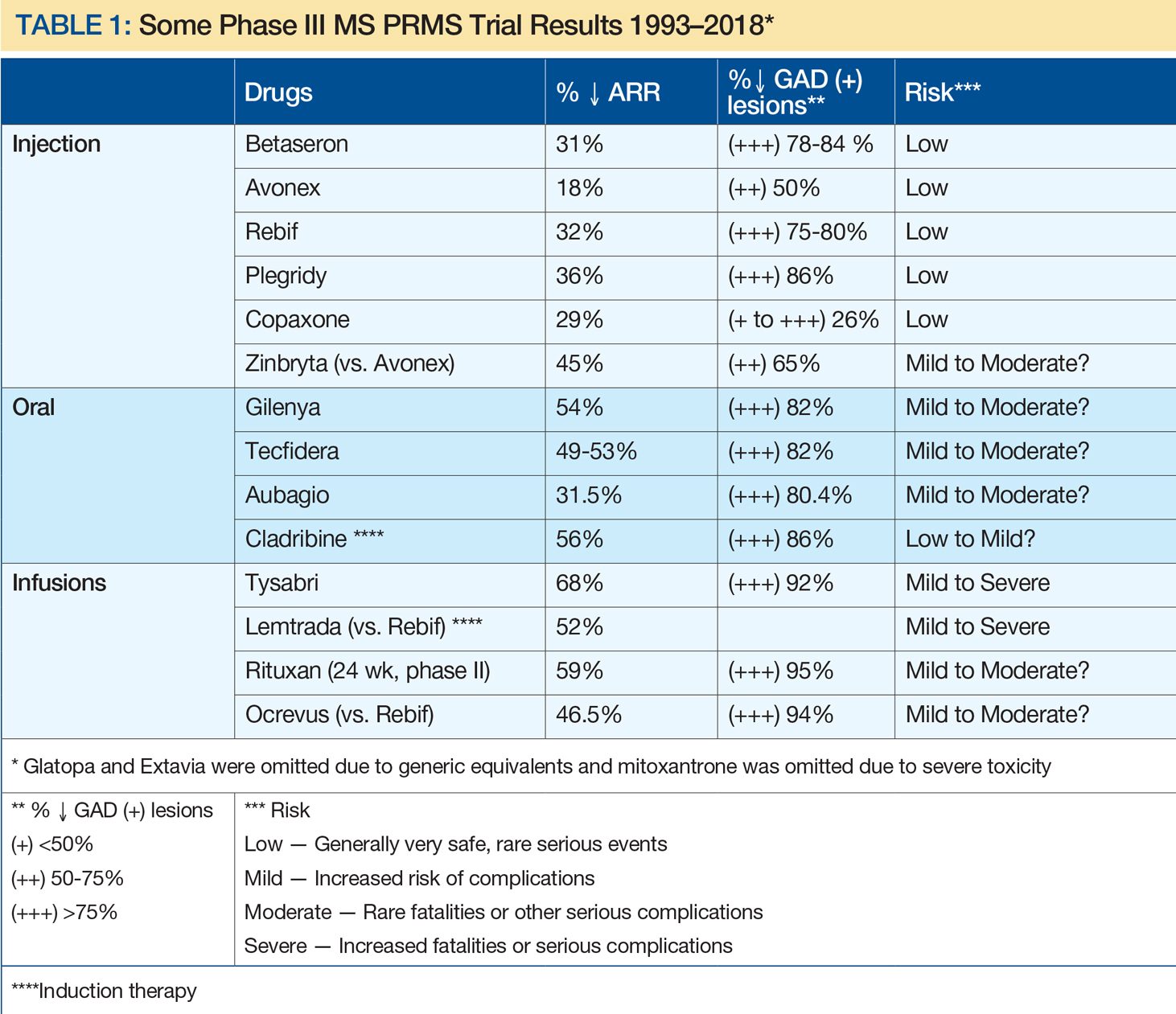
Suggested Reading
Brown JW, Pardini M, Brownlee WJ, et al. An abnormal periventricular magnetization transfer ratio gradient occurs early in multiple sclerosis. Brain. 2017;140(2):387-398.
Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology. 2009;72(23):1976-1983.
Cook SD, Dhib-Jalbut S, Dowling P, et al. Use of magnetic resonance imaging as well as clinical disease activity in the clinical classification of multiple sclerosis and assessment of its course: a report from an international CMSC consensus conference, March 5-7, 2010. Int J MS Care. 2012;14(3):105-114.
Dowling PC, Bosch VV, Cook SD. Possible beneficial effect of high-dose intravenous steroid therapy in acute demyelinating disease and transverse myelitis. Neurology. 1980;30(7 Pt 2):33-36.
Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416-426.
Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106-2112.
Lincoln JA, Cadavid D, Pollard J, et al. We should use magnetic resonance imaging to classify and monitor the course of multiple sclerosis. Arch Neurol. 2009;66(3):412-414.
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907-911.
Maranzano J, Rudko DA, Nakamura K, et al. MRI evidence of acute inflammation in leukocortical lesions of patients with early multiple sclerosis. Neurology. 2017;89(7):714-721.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
Miller HG, Gibbons JL. Acute disseminated encephalomyelitis and acute disseminated sclerosis; results of treatment with A.C.T.H. Br Med J. 1953;2(4850):1345-1348.
Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231.
Rose AS, Kuzma JW, Kurtzke JF, et al. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo--final report. Neurology. 1970;20(5):1-59.
Troiano R, Hafstein M, Ruderman M, et al. Effect of high-dose intravenous steroid administration on contrast-enhancing computed tomographic scan lesions in multiple sclerosis. Ann Neurol. 1984;15(3):257-263.
Troiano RA, Hafstein MP, Zito G, et al. The effect of oral corticosteroid dosage on CT enhancing multiple sclerosis plaques. J Neurol Sci. 1985;70(1):67-72.
Young IR, Hall AS, Pallis A, et al. Nuclear magnetic resonance imaging of the brain in multiple sclerosis. Lancet. 1981;2(8255):1063-1066.
Stuart D. Cook, MD, and Abdul Rahman Alchaki
Dr. Cook is the Ruth Dunietz Kushner and Michael Jay Serwitz Professor of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark. Dr. Alchaki is a resident in the Deptartment of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark.
Disclosure: Stuart Cook has received honoraria for lectures from Bayer HealthCare and Merck Serono. He has served as a consultant for Merck Serono, Bayer HealthCare, Teva, Novartis, Sanofi-Aventis, Biogen Idec, and Actinobac Biomed. He has served on steering committees for the BEYOND and CLARITY Studies and as a member of Advisory Boards for Merck Serono, Bayer HealthCare, Teva, Biogen Idec, Sanofi Aventis, and Actinobac Biomed.
The Initial Years (1838 to 1930s)
The earliest recognition of MS clinical features and pathology was attributed to Jean-Martin Charcot, Robert Carswell, and Jean Cruveilhier in Europe from 1838 to 1868. Beyond those early descriptions, relatively few MS breakthroughs occurred until the 1930s, when Thomas Rivers discovered experimental autoimmune encephalomyelitis (EAE), a demyelinating disease, in animals. His insightful concepts were widely cited and ultimately contributed to undestanding of the immune mechanisms of MS and acute disseminated encephalomyelitis (ADEM).
Advances in Diagnosis (1965 to 1992)
In 1965, Schumacher et al provided the essential clinical criteria for MS diagnosis. Poser et al refined these criteria in 1983. In 2001, McDonald et al added neuroimaging, CSF analysis, and evoked potentials to further complement MS clinical diagnosis. For the first time, the disease could generally be recognized.
Early Treatments
Various treatments for MS were tried over the years, without great success. However, in 1953, a small descriptive trial by Miller and Gibbons reported clinical benefits in patients using intramuscular (IM) adrenocorticotropic hormone (ACTH) for MS and disseminated encephalomyelitis. This was followed in 1970 by a Cooperative Study of IM ACTH versus placebo by Rose et al, which resulted in ACTH, and subsequently oral corticosteroids, being widely used to treat MS, particularly for acute exacerbations of the disease. However, robust evidence of long-term steroids remain limited, even to the present.
High-Dose Steroids
By 1980, the initial descriptive treatment of high-dose intravenous (IV) steroids for demyelinating diseases, including MS and transverse myelitis, by Dowling et al resulted in rapid clinical improvement in some patients. This result was ultimately confirmed by others. High-dose IV steroids became the gold standard for acute attacks, particularly those aggressive in nature. In the mid 1980s, work by Troiano et al, as well as others, showed that the rapid use of high-dose IV as well as oral steroids showed similar effects, with reduction or elimination of CT contrast-enhancing lesions within as few as eight hours, while lower doses or alternative-day treatments were less effective. In addition, descriptive studies of immune modulatory and immunosuppressive drugs, as well as small randomized studies, were published. These agents did not receive FDA approval.
The Golden Age of Therapy (1993 to 2018)
A remarkable era in MS prognosis and treatment began with immunomodulation injections of Betaseron (INFβ-1b), Avonex (INFβ-1a), and Copaxone (glatiramer acetate). This can be attributed, at least in part, to advances in molecular biology, genetics, and neuroimaging, and support by corporate, private, and public funding. Since the initial FDA approval of INFβ-1b, 15 MS therapies have become clinically available, including eight injectables, three orals, and four infusion treatments (see Table 1). In addition, two other drugs have been FDA approved for uses other than MS: rituximab (approved for lymphoma) and cladribine (for hairy cell leukemia), with the latter now approved by the European Medicines Agency for MS. Table 1 depicts characteristics of these therapies approved by US or European agencies (or for other disorders increasingly used off label for MS) in an attempt to compare annual relapse rates (ARR) and decreases in the percent of gadolinium-enhancing MS lesions versus placebo. This information was chosen because ARR has been uniformly selected and defined for such trials, while percent decrease of gadolinium-enhancing lesions on MRI has been the most sensitive barometer available for assessing acute clinical activity. As a result, risk-benefit considerations have been critical in evaluating these drug treatments, with efficacy improving greatly over time, whereas risks have been more variable.
Disease Categories
In 1996, Lublin and Reingold provided a new classification, not specifically for the diagnosis of MS, but rather for the clinical course of the disease. Initially, there were four categories—relapsing-remitting MS, secondary progressive MS, primary progressive MS, and progressive-relapsing MS—that were universally identified. These were thought to be relatively distinct clinical categories, but over time it became clear that the classification did not fully distinguish MS disease activity within these categories. For that reason, it was subsequently recommended, by Lincoln et al in 2009 and Cook et al in 2012, to include MRI, a vastly more sensitive modality, as well as clinical data in assessing disease activity.
On another note, MS and neuromyelitis optica (NMO), although having similar features, were clearly identified as different diseases by Lennon et al in 2004. Differences in pathology, clinical characteristics, immunology, and therapy separate the two disorders.
MRI in MS
Work by Young et al in 1981 established the central role of MRI brain imaging in MS diagnosis and therapeutic considerations. Since then it has become ubiquitous.
An example of a sensitive and highly productive MRI protocol is the BECOME study of MS and clinically isolated syndrome by Cadavid et al from 2009 to 2017. In this study, IFNβ-1b was compared with glatiramer acetate treatment. Cadavid et al used a 3T scanner with triple-dose gadolinium, performed monthly for as long as 24 consecutive months. This unique study brought about a virtual gold mine of valuable research and clinical information. This included proof that gadolinium-enhancing lesions persisted for six months or more, evidence of a 30:1 ratio of new MRI brain lesions to clinical activity, and documentation that 96% of T2 lesions and black holes derive from prior gadolinium-enhancing lesions. It was further noted that 80% to 90% of acute black holes disappeared with treatment and 75% to 80% of patients taking IFNβ-1b or glatiramer acetate had new MRI lesions despite continuing treatment. Perhaps most interestingly, monthly MRIs could predict relapse and disability in a relatively small number of patients, depending upon the frequency and activity of MRI lesions. In 2017, Brown et al documented that magnetization transfer ratio recovery in MS brain lesions occurred more significantly with glatiramer acetate than with IFNβ-1b, whereas more chronic black hole lesions were found with glatiramer acetate. Also in 2017, Maranzano et al found evidence of acute inflammatory leukocortical lesions, which were not as well recognized previously.
In summary, it has become increasingly clear that MRI is the most sen
The Future of MS
While it is not yet a curable disease, there is growing evidence that MS prognosis has improved and will continue to improve. This is based on incremental decreases in acute MS exacerbations, progressive disability, and MRI lesion activity, as well as a combination of the three—no evidence of disease activity (NEDA).
Not only are drug therapies becoming more effective, but patients and physicians now have many more treatment options to carefully consider with regard to efficacy, side effect profiles, treatment frequency, route of administration, cost, and quality of life. Newer drugs with different mechanisms of action such as cladribine, now approved in Europe, fulfill most of these beneficial criteria (see Giovannoni et al, 2010). More promising MS treatments, including long-acting induction therapies, are still being evaluated. As with other complex diseases, multiple therapies are likely to be used as well.
In summary, compared with the time before 1993, MS will be much less likely to be a progressive disease, and quality of life will be much improved. In my opinion, patients will be less fearful about their prognosis than ever before, and with appropriate evaluations and treatments, we may realize that disabling MS will be far less common.

Suggested Reading
Brown JW, Pardini M, Brownlee WJ, et al. An abnormal periventricular magnetization transfer ratio gradient occurs early in multiple sclerosis. Brain. 2017;140(2):387-398.
Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology. 2009;72(23):1976-1983.
Cook SD, Dhib-Jalbut S, Dowling P, et al. Use of magnetic resonance imaging as well as clinical disease activity in the clinical classification of multiple sclerosis and assessment of its course: a report from an international CMSC consensus conference, March 5-7, 2010. Int J MS Care. 2012;14(3):105-114.
Dowling PC, Bosch VV, Cook SD. Possible beneficial effect of high-dose intravenous steroid therapy in acute demyelinating disease and transverse myelitis. Neurology. 1980;30(7 Pt 2):33-36.
Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416-426.
Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106-2112.
Lincoln JA, Cadavid D, Pollard J, et al. We should use magnetic resonance imaging to classify and monitor the course of multiple sclerosis. Arch Neurol. 2009;66(3):412-414.
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907-911.
Maranzano J, Rudko DA, Nakamura K, et al. MRI evidence of acute inflammation in leukocortical lesions of patients with early multiple sclerosis. Neurology. 2017;89(7):714-721.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
Miller HG, Gibbons JL. Acute disseminated encephalomyelitis and acute disseminated sclerosis; results of treatment with A.C.T.H. Br Med J. 1953;2(4850):1345-1348.
Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231.
Rose AS, Kuzma JW, Kurtzke JF, et al. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo--final report. Neurology. 1970;20(5):1-59.
Troiano R, Hafstein M, Ruderman M, et al. Effect of high-dose intravenous steroid administration on contrast-enhancing computed tomographic scan lesions in multiple sclerosis. Ann Neurol. 1984;15(3):257-263.
Troiano RA, Hafstein MP, Zito G, et al. The effect of oral corticosteroid dosage on CT enhancing multiple sclerosis plaques. J Neurol Sci. 1985;70(1):67-72.
Young IR, Hall AS, Pallis A, et al. Nuclear magnetic resonance imaging of the brain in multiple sclerosis. Lancet. 1981;2(8255):1063-1066.
Stuart D. Cook, MD, and Abdul Rahman Alchaki
Dr. Cook is the Ruth Dunietz Kushner and Michael Jay Serwitz Professor of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark. Dr. Alchaki is a resident in the Deptartment of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark.
Disclosure: Stuart Cook has received honoraria for lectures from Bayer HealthCare and Merck Serono. He has served as a consultant for Merck Serono, Bayer HealthCare, Teva, Novartis, Sanofi-Aventis, Biogen Idec, and Actinobac Biomed. He has served on steering committees for the BEYOND and CLARITY Studies and as a member of Advisory Boards for Merck Serono, Bayer HealthCare, Teva, Biogen Idec, Sanofi Aventis, and Actinobac Biomed.
The Initial Years (1838 to 1930s)
The earliest recognition of MS clinical features and pathology was attributed to Jean-Martin Charcot, Robert Carswell, and Jean Cruveilhier in Europe from 1838 to 1868. Beyond those early descriptions, relatively few MS breakthroughs occurred until the 1930s, when Thomas Rivers discovered experimental autoimmune encephalomyelitis (EAE), a demyelinating disease, in animals. His insightful concepts were widely cited and ultimately contributed to undestanding of the immune mechanisms of MS and acute disseminated encephalomyelitis (ADEM).
Advances in Diagnosis (1965 to 1992)
In 1965, Schumacher et al provided the essential clinical criteria for MS diagnosis. Poser et al refined these criteria in 1983. In 2001, McDonald et al added neuroimaging, CSF analysis, and evoked potentials to further complement MS clinical diagnosis. For the first time, the disease could generally be recognized.
Early Treatments
Various treatments for MS were tried over the years, without great success. However, in 1953, a small descriptive trial by Miller and Gibbons reported clinical benefits in patients using intramuscular (IM) adrenocorticotropic hormone (ACTH) for MS and disseminated encephalomyelitis. This was followed in 1970 by a Cooperative Study of IM ACTH versus placebo by Rose et al, which resulted in ACTH, and subsequently oral corticosteroids, being widely used to treat MS, particularly for acute exacerbations of the disease. However, robust evidence of long-term steroids remain limited, even to the present.
High-Dose Steroids
By 1980, the initial descriptive treatment of high-dose intravenous (IV) steroids for demyelinating diseases, including MS and transverse myelitis, by Dowling et al resulted in rapid clinical improvement in some patients. This result was ultimately confirmed by others. High-dose IV steroids became the gold standard for acute attacks, particularly those aggressive in nature. In the mid 1980s, work by Troiano et al, as well as others, showed that the rapid use of high-dose IV as well as oral steroids showed similar effects, with reduction or elimination of CT contrast-enhancing lesions within as few as eight hours, while lower doses or alternative-day treatments were less effective. In addition, descriptive studies of immune modulatory and immunosuppressive drugs, as well as small randomized studies, were published. These agents did not receive FDA approval.
The Golden Age of Therapy (1993 to 2018)
A remarkable era in MS prognosis and treatment began with immunomodulation injections of Betaseron (INFβ-1b), Avonex (INFβ-1a), and Copaxone (glatiramer acetate). This can be attributed, at least in part, to advances in molecular biology, genetics, and neuroimaging, and support by corporate, private, and public funding. Since the initial FDA approval of INFβ-1b, 15 MS therapies have become clinically available, including eight injectables, three orals, and four infusion treatments (see Table 1). In addition, two other drugs have been FDA approved for uses other than MS: rituximab (approved for lymphoma) and cladribine (for hairy cell leukemia), with the latter now approved by the European Medicines Agency for MS. Table 1 depicts characteristics of these therapies approved by US or European agencies (or for other disorders increasingly used off label for MS) in an attempt to compare annual relapse rates (ARR) and decreases in the percent of gadolinium-enhancing MS lesions versus placebo. This information was chosen because ARR has been uniformly selected and defined for such trials, while percent decrease of gadolinium-enhancing lesions on MRI has been the most sensitive barometer available for assessing acute clinical activity. As a result, risk-benefit considerations have been critical in evaluating these drug treatments, with efficacy improving greatly over time, whereas risks have been more variable.
Disease Categories
In 1996, Lublin and Reingold provided a new classification, not specifically for the diagnosis of MS, but rather for the clinical course of the disease. Initially, there were four categories—relapsing-remitting MS, secondary progressive MS, primary progressive MS, and progressive-relapsing MS—that were universally identified. These were thought to be relatively distinct clinical categories, but over time it became clear that the classification did not fully distinguish MS disease activity within these categories. For that reason, it was subsequently recommended, by Lincoln et al in 2009 and Cook et al in 2012, to include MRI, a vastly more sensitive modality, as well as clinical data in assessing disease activity.
On another note, MS and neuromyelitis optica (NMO), although having similar features, were clearly identified as different diseases by Lennon et al in 2004. Differences in pathology, clinical characteristics, immunology, and therapy separate the two disorders.
MRI in MS
Work by Young et al in 1981 established the central role of MRI brain imaging in MS diagnosis and therapeutic considerations. Since then it has become ubiquitous.
An example of a sensitive and highly productive MRI protocol is the BECOME study of MS and clinically isolated syndrome by Cadavid et al from 2009 to 2017. In this study, IFNβ-1b was compared with glatiramer acetate treatment. Cadavid et al used a 3T scanner with triple-dose gadolinium, performed monthly for as long as 24 consecutive months. This unique study brought about a virtual gold mine of valuable research and clinical information. This included proof that gadolinium-enhancing lesions persisted for six months or more, evidence of a 30:1 ratio of new MRI brain lesions to clinical activity, and documentation that 96% of T2 lesions and black holes derive from prior gadolinium-enhancing lesions. It was further noted that 80% to 90% of acute black holes disappeared with treatment and 75% to 80% of patients taking IFNβ-1b or glatiramer acetate had new MRI lesions despite continuing treatment. Perhaps most interestingly, monthly MRIs could predict relapse and disability in a relatively small number of patients, depending upon the frequency and activity of MRI lesions. In 2017, Brown et al documented that magnetization transfer ratio recovery in MS brain lesions occurred more significantly with glatiramer acetate than with IFNβ-1b, whereas more chronic black hole lesions were found with glatiramer acetate. Also in 2017, Maranzano et al found evidence of acute inflammatory leukocortical lesions, which were not as well recognized previously.
In summary, it has become increasingly clear that MRI is the most sen
The Future of MS
While it is not yet a curable disease, there is growing evidence that MS prognosis has improved and will continue to improve. This is based on incremental decreases in acute MS exacerbations, progressive disability, and MRI lesion activity, as well as a combination of the three—no evidence of disease activity (NEDA).
Not only are drug therapies becoming more effective, but patients and physicians now have many more treatment options to carefully consider with regard to efficacy, side effect profiles, treatment frequency, route of administration, cost, and quality of life. Newer drugs with different mechanisms of action such as cladribine, now approved in Europe, fulfill most of these beneficial criteria (see Giovannoni et al, 2010). More promising MS treatments, including long-acting induction therapies, are still being evaluated. As with other complex diseases, multiple therapies are likely to be used as well.
In summary, compared with the time before 1993, MS will be much less likely to be a progressive disease, and quality of life will be much improved. In my opinion, patients will be less fearful about their prognosis than ever before, and with appropriate evaluations and treatments, we may realize that disabling MS will be far less common.

Suggested Reading
Brown JW, Pardini M, Brownlee WJ, et al. An abnormal periventricular magnetization transfer ratio gradient occurs early in multiple sclerosis. Brain. 2017;140(2):387-398.
Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology. 2009;72(23):1976-1983.
Cook SD, Dhib-Jalbut S, Dowling P, et al. Use of magnetic resonance imaging as well as clinical disease activity in the clinical classification of multiple sclerosis and assessment of its course: a report from an international CMSC consensus conference, March 5-7, 2010. Int J MS Care. 2012;14(3):105-114.
Dowling PC, Bosch VV, Cook SD. Possible beneficial effect of high-dose intravenous steroid therapy in acute demyelinating disease and transverse myelitis. Neurology. 1980;30(7 Pt 2):33-36.
Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416-426.
Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106-2112.
Lincoln JA, Cadavid D, Pollard J, et al. We should use magnetic resonance imaging to classify and monitor the course of multiple sclerosis. Arch Neurol. 2009;66(3):412-414.
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907-911.
Maranzano J, Rudko DA, Nakamura K, et al. MRI evidence of acute inflammation in leukocortical lesions of patients with early multiple sclerosis. Neurology. 2017;89(7):714-721.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
Miller HG, Gibbons JL. Acute disseminated encephalomyelitis and acute disseminated sclerosis; results of treatment with A.C.T.H. Br Med J. 1953;2(4850):1345-1348.
Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231.
Rose AS, Kuzma JW, Kurtzke JF, et al. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo--final report. Neurology. 1970;20(5):1-59.
Troiano R, Hafstein M, Ruderman M, et al. Effect of high-dose intravenous steroid administration on contrast-enhancing computed tomographic scan lesions in multiple sclerosis. Ann Neurol. 1984;15(3):257-263.
Troiano RA, Hafstein MP, Zito G, et al. The effect of oral corticosteroid dosage on CT enhancing multiple sclerosis plaques. J Neurol Sci. 1985;70(1):67-72.
Young IR, Hall AS, Pallis A, et al. Nuclear magnetic resonance imaging of the brain in multiple sclerosis. Lancet. 1981;2(8255):1063-1066.
Silent No More: Harassment in the Workplace
Sexual harassment is one of the most insidious and caustic evils in the US workplace—and it knows no bounds, judging by the rampant allegations throughout 2017 and into 2018. While sexual harassment affects both sexes, the majority of cases target women.
In a recent survey, the New York Times asked 615 men about objectionable behavior toward colleagues, including whether they have made uninvited attempts to stroke, fondle, or kiss a coworker. Twenty-five percent admitted to telling crude jokes or sharing inappropriate videos. Two percent said they had pressured people into sexual acts by offering rewards or threatening retaliation—that means 12 men admitted to this.1
Sadly, the health care industry is not immune from this corruption. In fact, sexual harassment is a major problem in health care—one that is particularly prevalent in nursing, according to Fiedler and Hamby.2 More than 50% of female nurses, physicians, and students report experiencing sexual harassment.3 And even that, I believe, is an underreported percentage.
Sexual harassment in the workplace includes any situation in which there is a demand for sexual favors in exchange for a job benefit or where an unwanted condition on any person’s employment is imposed because of that person’s sex. Since 1964, Title VII of the Civil Rights Act (42 USC Sec.2000e-2[a]) has prohibited discrimination in places of employment based on an individual’s sex.4 In 1976, it was acknowledged that this also prohibits sexual harassment as a form of sex discrimination. In 1991, amendments (42 USC Sec.1981 [a][1]) authorized compensatory and punitive damages as well as jury trials.5
The Equal Employment Opportunity Commission (EEOC), the federal agency that enforces Title VII, stipulates that
- Harassment can include unwelcome sexual advances, requests for sexual favors, and other verbal or physical harassment—but it does not have to be sexual in nature (eg, making offensive comments about women in general).
- Both women and men can be victims and harassers—and the victim and harasser can be the same sex.
- Harassment is illegal when it is so frequent or severe that it creates a hostile or offensive work environment, or when it results in an adverse employment decision (eg, the victim being fired or demoted).6
Although the EEOC guidelines are not law, they do guide the judicial interpretation of what constitutes sexual harassment. Sexual harassment is an increasing source of workplace lawsuits that often result in large judgments against employers. Clinicians can file class-action suits against hospitals and other institutions, and juries may render verdicts on harassment complaints to the tune of millions of dollars.
Unfortunately, it is common for workplace harassment to go unreported. According to the EEOC, standard responses to workplace sexual harassment include avoiding the harasser, denying or downplaying the gravity of the situation, or attempting to ignore, forget, or endure the behavior. The least common response is to take formal action—either by reporting it internally or filing a legal complaint. Further, roughly three of four individuals who experience harassment in the workplace never tell a supervisor, manager, or union representative due to fear of disbelief, inaction, blame, or social or professional retaliation.7
How can we, as clinicians, protect ourselves and others from this type of harassment? Establishing a strong zero-tolerance policy on sexual harassment and reporting unacceptable behavior is the only answer. I realize this is no easy issue, but we must be firm in our resolve. Although 2017 was a watershed year with “Silence Breaker” or “#MeToo,” there are still many voices not being heard.8
So, here is my request: If you have been directly affected by, or have observed, objectionable behavior that violates your organization’s policy or code of conduct (or your personal sense of decency), document the incident and report it. You may be hesitant; maybe you think the behavior is an isolated act, or maybe the offender is a well-liked colleague who usually acts professionally. But your role is not to decide whether this behavior is acute or chronic—the human resources department will determine that. Maybe you are reluctant to report harassment because you fear reprisal. This is understandable, but remember that intimidation allows the behavior to persist. If the incident was upsetting and objectionable, it needs to be reported—no matter what.

The process of documenting and reporting varies depending on your institution’s policy but typically involves writing a factual description of the incident, including the time, place, and a list of any witnesses (including patients). Make sure your report is objective, and include any effect the behavior has had on patient care. Document any verbal exchanges verbatim, if possible. File the report as soon as possible, and remember to document everything. Nothing is trivial when it comes to building a case against sexual harassment. After you document and report the incident, continue to act professionally.9
If you are unable to resolve a harassment-related issue through your employer’s internal procedure, you may need to pursue the matter via the EEOC or your state’s human rights or civil rights enforcement agency.10 But know that any time and effort you put into reporting this is worthwhile; predatory behavior often crosses the workplace to other social realms. The other people being affected will be grateful that you came forward.
Some of you may be reading this and thinking, “Easy for you to say, Randy. You are not the one being harassed!” I know. This is not an easy topic for anyone. I have been shocked and appalled by the level and prevalence of sexual harassment in the workplace. I was always taught (by my grandmother—thank you!) to respect women. My hope is that by introducing this sensitive topic, we can open the discussion and encourage everyone to speak up. Please share your thoughts, comments, and ideas with me at [email protected]—let’s start a conversation.
1. Patel JK, Griggs T, Cain Miller C. The UpShot: We asked 615 men about how they conduct themselves at work. The New York Times. December 28, 2017. www.nytimes.com/interactive/2017/12/28/upshot/sexual-harassment-survey-600-men.html. Accessed January 16, 2018.
2. Fiedler A, Hamby E. Sexual harassment in the workplace: nurses’ perceptions. J Nurs Adm. 2000;30(10):497-503.
3. Lockwood W. Sexual harassment in healthcare. www.rn.org/courses/coursematerial-236.pdf. Accessed January 16, 2018.
4. Legal Information Institute. 42 U.S. Code § 2000e–2 - Unlawful employment practices. www.law.cornell.edu/uscode/text/42/2000e-2. Accessed January 16, 2018.
5. US Equal Employment Opportunity Commission (EEOC). The Civil Rights Act of 1991. www.eeoc.gov/laws/statutes/cra-1991.cfm. Accessed January 16, 2018.
6. US EEOC. Sexual harassment. www.eeoc.gov/laws/types/sexual_harassment.cfm. Accessed January 16, 2018.
7. US EEOC. Select task force on the study of harassment in the workplace. June 2016. www.eeoc.gov/eeoc/task_force/harassment/report.cfm. Accessed January 16, 2018.
8. O’Brien SA, Carpenter J. 2017 was the year of (certain) women’s voices. December 28, 2017. www.ozarksfirst.com/news/business/2017-was-the-year-of-certain-womens-voices/889901636. Accessed January 16, 2018.
9. AAUW. Know your rights at work. www.aauw.org/what-we-do/legal-resources/know-your-rights-at-work/workplace-sexual-harassment/. Accessed January 16, 2018.
10. EEOC’s Charge Processing Procedures. http://employment.findlaw.com/employment-discrimination/eeoc-s-charge-processing-procedures.html. Accessed January 16, 2018.
Sexual harassment is one of the most insidious and caustic evils in the US workplace—and it knows no bounds, judging by the rampant allegations throughout 2017 and into 2018. While sexual harassment affects both sexes, the majority of cases target women.
In a recent survey, the New York Times asked 615 men about objectionable behavior toward colleagues, including whether they have made uninvited attempts to stroke, fondle, or kiss a coworker. Twenty-five percent admitted to telling crude jokes or sharing inappropriate videos. Two percent said they had pressured people into sexual acts by offering rewards or threatening retaliation—that means 12 men admitted to this.1
Sadly, the health care industry is not immune from this corruption. In fact, sexual harassment is a major problem in health care—one that is particularly prevalent in nursing, according to Fiedler and Hamby.2 More than 50% of female nurses, physicians, and students report experiencing sexual harassment.3 And even that, I believe, is an underreported percentage.
Sexual harassment in the workplace includes any situation in which there is a demand for sexual favors in exchange for a job benefit or where an unwanted condition on any person’s employment is imposed because of that person’s sex. Since 1964, Title VII of the Civil Rights Act (42 USC Sec.2000e-2[a]) has prohibited discrimination in places of employment based on an individual’s sex.4 In 1976, it was acknowledged that this also prohibits sexual harassment as a form of sex discrimination. In 1991, amendments (42 USC Sec.1981 [a][1]) authorized compensatory and punitive damages as well as jury trials.5
The Equal Employment Opportunity Commission (EEOC), the federal agency that enforces Title VII, stipulates that
- Harassment can include unwelcome sexual advances, requests for sexual favors, and other verbal or physical harassment—but it does not have to be sexual in nature (eg, making offensive comments about women in general).
- Both women and men can be victims and harassers—and the victim and harasser can be the same sex.
- Harassment is illegal when it is so frequent or severe that it creates a hostile or offensive work environment, or when it results in an adverse employment decision (eg, the victim being fired or demoted).6
Although the EEOC guidelines are not law, they do guide the judicial interpretation of what constitutes sexual harassment. Sexual harassment is an increasing source of workplace lawsuits that often result in large judgments against employers. Clinicians can file class-action suits against hospitals and other institutions, and juries may render verdicts on harassment complaints to the tune of millions of dollars.
Unfortunately, it is common for workplace harassment to go unreported. According to the EEOC, standard responses to workplace sexual harassment include avoiding the harasser, denying or downplaying the gravity of the situation, or attempting to ignore, forget, or endure the behavior. The least common response is to take formal action—either by reporting it internally or filing a legal complaint. Further, roughly three of four individuals who experience harassment in the workplace never tell a supervisor, manager, or union representative due to fear of disbelief, inaction, blame, or social or professional retaliation.7
How can we, as clinicians, protect ourselves and others from this type of harassment? Establishing a strong zero-tolerance policy on sexual harassment and reporting unacceptable behavior is the only answer. I realize this is no easy issue, but we must be firm in our resolve. Although 2017 was a watershed year with “Silence Breaker” or “#MeToo,” there are still many voices not being heard.8
So, here is my request: If you have been directly affected by, or have observed, objectionable behavior that violates your organization’s policy or code of conduct (or your personal sense of decency), document the incident and report it. You may be hesitant; maybe you think the behavior is an isolated act, or maybe the offender is a well-liked colleague who usually acts professionally. But your role is not to decide whether this behavior is acute or chronic—the human resources department will determine that. Maybe you are reluctant to report harassment because you fear reprisal. This is understandable, but remember that intimidation allows the behavior to persist. If the incident was upsetting and objectionable, it needs to be reported—no matter what.

The process of documenting and reporting varies depending on your institution’s policy but typically involves writing a factual description of the incident, including the time, place, and a list of any witnesses (including patients). Make sure your report is objective, and include any effect the behavior has had on patient care. Document any verbal exchanges verbatim, if possible. File the report as soon as possible, and remember to document everything. Nothing is trivial when it comes to building a case against sexual harassment. After you document and report the incident, continue to act professionally.9
If you are unable to resolve a harassment-related issue through your employer’s internal procedure, you may need to pursue the matter via the EEOC or your state’s human rights or civil rights enforcement agency.10 But know that any time and effort you put into reporting this is worthwhile; predatory behavior often crosses the workplace to other social realms. The other people being affected will be grateful that you came forward.
Some of you may be reading this and thinking, “Easy for you to say, Randy. You are not the one being harassed!” I know. This is not an easy topic for anyone. I have been shocked and appalled by the level and prevalence of sexual harassment in the workplace. I was always taught (by my grandmother—thank you!) to respect women. My hope is that by introducing this sensitive topic, we can open the discussion and encourage everyone to speak up. Please share your thoughts, comments, and ideas with me at [email protected]—let’s start a conversation.
Sexual harassment is one of the most insidious and caustic evils in the US workplace—and it knows no bounds, judging by the rampant allegations throughout 2017 and into 2018. While sexual harassment affects both sexes, the majority of cases target women.
In a recent survey, the New York Times asked 615 men about objectionable behavior toward colleagues, including whether they have made uninvited attempts to stroke, fondle, or kiss a coworker. Twenty-five percent admitted to telling crude jokes or sharing inappropriate videos. Two percent said they had pressured people into sexual acts by offering rewards or threatening retaliation—that means 12 men admitted to this.1
Sadly, the health care industry is not immune from this corruption. In fact, sexual harassment is a major problem in health care—one that is particularly prevalent in nursing, according to Fiedler and Hamby.2 More than 50% of female nurses, physicians, and students report experiencing sexual harassment.3 And even that, I believe, is an underreported percentage.
Sexual harassment in the workplace includes any situation in which there is a demand for sexual favors in exchange for a job benefit or where an unwanted condition on any person’s employment is imposed because of that person’s sex. Since 1964, Title VII of the Civil Rights Act (42 USC Sec.2000e-2[a]) has prohibited discrimination in places of employment based on an individual’s sex.4 In 1976, it was acknowledged that this also prohibits sexual harassment as a form of sex discrimination. In 1991, amendments (42 USC Sec.1981 [a][1]) authorized compensatory and punitive damages as well as jury trials.5
The Equal Employment Opportunity Commission (EEOC), the federal agency that enforces Title VII, stipulates that
- Harassment can include unwelcome sexual advances, requests for sexual favors, and other verbal or physical harassment—but it does not have to be sexual in nature (eg, making offensive comments about women in general).
- Both women and men can be victims and harassers—and the victim and harasser can be the same sex.
- Harassment is illegal when it is so frequent or severe that it creates a hostile or offensive work environment, or when it results in an adverse employment decision (eg, the victim being fired or demoted).6
Although the EEOC guidelines are not law, they do guide the judicial interpretation of what constitutes sexual harassment. Sexual harassment is an increasing source of workplace lawsuits that often result in large judgments against employers. Clinicians can file class-action suits against hospitals and other institutions, and juries may render verdicts on harassment complaints to the tune of millions of dollars.
Unfortunately, it is common for workplace harassment to go unreported. According to the EEOC, standard responses to workplace sexual harassment include avoiding the harasser, denying or downplaying the gravity of the situation, or attempting to ignore, forget, or endure the behavior. The least common response is to take formal action—either by reporting it internally or filing a legal complaint. Further, roughly three of four individuals who experience harassment in the workplace never tell a supervisor, manager, or union representative due to fear of disbelief, inaction, blame, or social or professional retaliation.7
How can we, as clinicians, protect ourselves and others from this type of harassment? Establishing a strong zero-tolerance policy on sexual harassment and reporting unacceptable behavior is the only answer. I realize this is no easy issue, but we must be firm in our resolve. Although 2017 was a watershed year with “Silence Breaker” or “#MeToo,” there are still many voices not being heard.8
So, here is my request: If you have been directly affected by, or have observed, objectionable behavior that violates your organization’s policy or code of conduct (or your personal sense of decency), document the incident and report it. You may be hesitant; maybe you think the behavior is an isolated act, or maybe the offender is a well-liked colleague who usually acts professionally. But your role is not to decide whether this behavior is acute or chronic—the human resources department will determine that. Maybe you are reluctant to report harassment because you fear reprisal. This is understandable, but remember that intimidation allows the behavior to persist. If the incident was upsetting and objectionable, it needs to be reported—no matter what.

The process of documenting and reporting varies depending on your institution’s policy but typically involves writing a factual description of the incident, including the time, place, and a list of any witnesses (including patients). Make sure your report is objective, and include any effect the behavior has had on patient care. Document any verbal exchanges verbatim, if possible. File the report as soon as possible, and remember to document everything. Nothing is trivial when it comes to building a case against sexual harassment. After you document and report the incident, continue to act professionally.9
If you are unable to resolve a harassment-related issue through your employer’s internal procedure, you may need to pursue the matter via the EEOC or your state’s human rights or civil rights enforcement agency.10 But know that any time and effort you put into reporting this is worthwhile; predatory behavior often crosses the workplace to other social realms. The other people being affected will be grateful that you came forward.
Some of you may be reading this and thinking, “Easy for you to say, Randy. You are not the one being harassed!” I know. This is not an easy topic for anyone. I have been shocked and appalled by the level and prevalence of sexual harassment in the workplace. I was always taught (by my grandmother—thank you!) to respect women. My hope is that by introducing this sensitive topic, we can open the discussion and encourage everyone to speak up. Please share your thoughts, comments, and ideas with me at [email protected]—let’s start a conversation.
1. Patel JK, Griggs T, Cain Miller C. The UpShot: We asked 615 men about how they conduct themselves at work. The New York Times. December 28, 2017. www.nytimes.com/interactive/2017/12/28/upshot/sexual-harassment-survey-600-men.html. Accessed January 16, 2018.
2. Fiedler A, Hamby E. Sexual harassment in the workplace: nurses’ perceptions. J Nurs Adm. 2000;30(10):497-503.
3. Lockwood W. Sexual harassment in healthcare. www.rn.org/courses/coursematerial-236.pdf. Accessed January 16, 2018.
4. Legal Information Institute. 42 U.S. Code § 2000e–2 - Unlawful employment practices. www.law.cornell.edu/uscode/text/42/2000e-2. Accessed January 16, 2018.
5. US Equal Employment Opportunity Commission (EEOC). The Civil Rights Act of 1991. www.eeoc.gov/laws/statutes/cra-1991.cfm. Accessed January 16, 2018.
6. US EEOC. Sexual harassment. www.eeoc.gov/laws/types/sexual_harassment.cfm. Accessed January 16, 2018.
7. US EEOC. Select task force on the study of harassment in the workplace. June 2016. www.eeoc.gov/eeoc/task_force/harassment/report.cfm. Accessed January 16, 2018.
8. O’Brien SA, Carpenter J. 2017 was the year of (certain) women’s voices. December 28, 2017. www.ozarksfirst.com/news/business/2017-was-the-year-of-certain-womens-voices/889901636. Accessed January 16, 2018.
9. AAUW. Know your rights at work. www.aauw.org/what-we-do/legal-resources/know-your-rights-at-work/workplace-sexual-harassment/. Accessed January 16, 2018.
10. EEOC’s Charge Processing Procedures. http://employment.findlaw.com/employment-discrimination/eeoc-s-charge-processing-procedures.html. Accessed January 16, 2018.
1. Patel JK, Griggs T, Cain Miller C. The UpShot: We asked 615 men about how they conduct themselves at work. The New York Times. December 28, 2017. www.nytimes.com/interactive/2017/12/28/upshot/sexual-harassment-survey-600-men.html. Accessed January 16, 2018.
2. Fiedler A, Hamby E. Sexual harassment in the workplace: nurses’ perceptions. J Nurs Adm. 2000;30(10):497-503.
3. Lockwood W. Sexual harassment in healthcare. www.rn.org/courses/coursematerial-236.pdf. Accessed January 16, 2018.
4. Legal Information Institute. 42 U.S. Code § 2000e–2 - Unlawful employment practices. www.law.cornell.edu/uscode/text/42/2000e-2. Accessed January 16, 2018.
5. US Equal Employment Opportunity Commission (EEOC). The Civil Rights Act of 1991. www.eeoc.gov/laws/statutes/cra-1991.cfm. Accessed January 16, 2018.
6. US EEOC. Sexual harassment. www.eeoc.gov/laws/types/sexual_harassment.cfm. Accessed January 16, 2018.
7. US EEOC. Select task force on the study of harassment in the workplace. June 2016. www.eeoc.gov/eeoc/task_force/harassment/report.cfm. Accessed January 16, 2018.
8. O’Brien SA, Carpenter J. 2017 was the year of (certain) women’s voices. December 28, 2017. www.ozarksfirst.com/news/business/2017-was-the-year-of-certain-womens-voices/889901636. Accessed January 16, 2018.
9. AAUW. Know your rights at work. www.aauw.org/what-we-do/legal-resources/know-your-rights-at-work/workplace-sexual-harassment/. Accessed January 16, 2018.
10. EEOC’s Charge Processing Procedures. http://employment.findlaw.com/employment-discrimination/eeoc-s-charge-processing-procedures.html. Accessed January 16, 2018.
Integrating behavioral health into primary care
This is the sixth in a series of articles from the National Center for Excellence in Primary Care Research in the Agency for Healthcare Research and Quality. This series introduces sets of tools and resources designed to help your practice.
Primary care practice is under increasing pressure to evolve. As highlighted in this series, topics such as shared decision making, team-based care, integration of behavioral health into primary care practice, and practice facilitation all offer the potential to enhance your primary care practice. On the other hand, quality of care must remain a top priority during this transformation. While much of the Agency for Healthcare Research and Quality’s (AHRQ) work in quality of care focuses on the inpatient setting, AHRQ offers many tools and resources to evaluate quality of care and to implement quality improvement into your primary care practice.
The NQMC mission is to provide an accessible mechanism for obtaining detailed information on quality measures and to further the dissemination, implementation, and use of these measures to inform health care decisions. NQMC is designed for practitioners, health care providers, health plans, integrated delivery systems, purchasers, and others interested in health care quality measurement. Funding for the NQMC is in question, and the future of this resource is not certain.
Confidential feedback reporting is widely considered to be a precursor to and a foundation for performance improvement. However, to enable change, the clinician responsible for and capable of change must receive, understand, and act on the information. The following publications from the National Center for Excellence in Primary Care Research offer some guidance from the on ways to best do so:
- Confidential Physician Feedback Reports: Designing for Optimal Impact on Performance is a guide that informs developers of feedback reports about evidence-based strategies to consider when they develop or refine a feedback reporting system.
- Will It Work Here? A Decisionmaker’s Guide to Adopting Innovations can help you determine if an innovation would be a good fit – or an appropriate stretch – for your practice or health care organization by asking a series of questions. It links users to actionable Web-based tools and presents case studies that illustrate how other organizations have addressed these questions.
- Improving Your Office Testing Process: A Toolkit for Rapid-Cycle Patient Safety and Quality Improvement provides information and resources to help physicians’ offices, clinics, and other ambulatory care facilities assess and improve the testing process in their offices.
These and other tools can be found at the AHRQ website.
Dr. Ganiats is the director for the National Center for Excellence in Primary Care Research at AHRQ.
This is the sixth in a series of articles from the National Center for Excellence in Primary Care Research in the Agency for Healthcare Research and Quality. This series introduces sets of tools and resources designed to help your practice.
Primary care practice is under increasing pressure to evolve. As highlighted in this series, topics such as shared decision making, team-based care, integration of behavioral health into primary care practice, and practice facilitation all offer the potential to enhance your primary care practice. On the other hand, quality of care must remain a top priority during this transformation. While much of the Agency for Healthcare Research and Quality’s (AHRQ) work in quality of care focuses on the inpatient setting, AHRQ offers many tools and resources to evaluate quality of care and to implement quality improvement into your primary care practice.
The NQMC mission is to provide an accessible mechanism for obtaining detailed information on quality measures and to further the dissemination, implementation, and use of these measures to inform health care decisions. NQMC is designed for practitioners, health care providers, health plans, integrated delivery systems, purchasers, and others interested in health care quality measurement. Funding for the NQMC is in question, and the future of this resource is not certain.
Confidential feedback reporting is widely considered to be a precursor to and a foundation for performance improvement. However, to enable change, the clinician responsible for and capable of change must receive, understand, and act on the information. The following publications from the National Center for Excellence in Primary Care Research offer some guidance from the on ways to best do so:
- Confidential Physician Feedback Reports: Designing for Optimal Impact on Performance is a guide that informs developers of feedback reports about evidence-based strategies to consider when they develop or refine a feedback reporting system.
- Will It Work Here? A Decisionmaker’s Guide to Adopting Innovations can help you determine if an innovation would be a good fit – or an appropriate stretch – for your practice or health care organization by asking a series of questions. It links users to actionable Web-based tools and presents case studies that illustrate how other organizations have addressed these questions.
- Improving Your Office Testing Process: A Toolkit for Rapid-Cycle Patient Safety and Quality Improvement provides information and resources to help physicians’ offices, clinics, and other ambulatory care facilities assess and improve the testing process in their offices.
These and other tools can be found at the AHRQ website.
Dr. Ganiats is the director for the National Center for Excellence in Primary Care Research at AHRQ.
This is the sixth in a series of articles from the National Center for Excellence in Primary Care Research in the Agency for Healthcare Research and Quality. This series introduces sets of tools and resources designed to help your practice.
Primary care practice is under increasing pressure to evolve. As highlighted in this series, topics such as shared decision making, team-based care, integration of behavioral health into primary care practice, and practice facilitation all offer the potential to enhance your primary care practice. On the other hand, quality of care must remain a top priority during this transformation. While much of the Agency for Healthcare Research and Quality’s (AHRQ) work in quality of care focuses on the inpatient setting, AHRQ offers many tools and resources to evaluate quality of care and to implement quality improvement into your primary care practice.
The NQMC mission is to provide an accessible mechanism for obtaining detailed information on quality measures and to further the dissemination, implementation, and use of these measures to inform health care decisions. NQMC is designed for practitioners, health care providers, health plans, integrated delivery systems, purchasers, and others interested in health care quality measurement. Funding for the NQMC is in question, and the future of this resource is not certain.
Confidential feedback reporting is widely considered to be a precursor to and a foundation for performance improvement. However, to enable change, the clinician responsible for and capable of change must receive, understand, and act on the information. The following publications from the National Center for Excellence in Primary Care Research offer some guidance from the on ways to best do so:
- Confidential Physician Feedback Reports: Designing for Optimal Impact on Performance is a guide that informs developers of feedback reports about evidence-based strategies to consider when they develop or refine a feedback reporting system.
- Will It Work Here? A Decisionmaker’s Guide to Adopting Innovations can help you determine if an innovation would be a good fit – or an appropriate stretch – for your practice or health care organization by asking a series of questions. It links users to actionable Web-based tools and presents case studies that illustrate how other organizations have addressed these questions.
- Improving Your Office Testing Process: A Toolkit for Rapid-Cycle Patient Safety and Quality Improvement provides information and resources to help physicians’ offices, clinics, and other ambulatory care facilities assess and improve the testing process in their offices.
These and other tools can be found at the AHRQ website.
Dr. Ganiats is the director for the National Center for Excellence in Primary Care Research at AHRQ.
No improvement in sight for Alzheimer’s drug development
Another one bites the dust.
Yet another investigational agent joins intepirdine, verubecestat, solanezumab, bapineuzumab, latrepirdine, and many others on the scrap pile of research: The complete release of trial data on idalopirdine found the drug wasn’t of clinically significant benefit in Alzheimer’s disease (JAMA. 2018;319[2]:130-42).
The numbers are bad enough that a handful of companies, including the giant Pfizer, have decided to leave Alzheimer’s drug development entirely to focus on more promising fields. And I get that. All of us – on any exhausting, fruitless, task – will reach the point where it’s time to cut our losses and move on. I don’t blame these companies for mostly leaving the field. (Pfizer is planning to form a neuroscience venture fund to support further research.)
Optimists will argue that you still learn things from a negative trial, which is true, but nothing to date is on the immediate horizon to help. The five agents we’ve had available for the past 15-20 years are all old enough to have lost their patents, and their benefits are modest, at best.
And all this going on as the overall human population, including myself, gradually ages and dementia becomes a medical-cost time bomb on the horizon. This isn’t an American problem. Every country in the world is facing it.
Politicians love to promise hope for these things: creating fast-track programs to get drugs to market faster, finding ways to bring down costs so more people can afford them, and improving methods to treat those in need. But none of those things matter if the medications don’t work.
Many of these trials test similar molecules because the evidence to date suggests they’re targeting the cause of Alzheimer’s. But so far they aren’t working. What if, as the Firesign Theatre and others have said, everything you know is wrong?
Perhaps our greatest quality as a species is resilience. We go on because we have to. The planet keeps moving around the sun as it has for almost 5 billion years, and we face tomorrow. Caregivers wake up for another day of doing their best for a faltering parent. I wake up for another day of doing my best to help them. And the researchers go back for another day hoping to find the real answer and treatment. Without trying, no treatment for anything will ever be found. We owe our patients, and ourselves, a better future than that.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Another one bites the dust.
Yet another investigational agent joins intepirdine, verubecestat, solanezumab, bapineuzumab, latrepirdine, and many others on the scrap pile of research: The complete release of trial data on idalopirdine found the drug wasn’t of clinically significant benefit in Alzheimer’s disease (JAMA. 2018;319[2]:130-42).
The numbers are bad enough that a handful of companies, including the giant Pfizer, have decided to leave Alzheimer’s drug development entirely to focus on more promising fields. And I get that. All of us – on any exhausting, fruitless, task – will reach the point where it’s time to cut our losses and move on. I don’t blame these companies for mostly leaving the field. (Pfizer is planning to form a neuroscience venture fund to support further research.)
Optimists will argue that you still learn things from a negative trial, which is true, but nothing to date is on the immediate horizon to help. The five agents we’ve had available for the past 15-20 years are all old enough to have lost their patents, and their benefits are modest, at best.
And all this going on as the overall human population, including myself, gradually ages and dementia becomes a medical-cost time bomb on the horizon. This isn’t an American problem. Every country in the world is facing it.
Politicians love to promise hope for these things: creating fast-track programs to get drugs to market faster, finding ways to bring down costs so more people can afford them, and improving methods to treat those in need. But none of those things matter if the medications don’t work.
Many of these trials test similar molecules because the evidence to date suggests they’re targeting the cause of Alzheimer’s. But so far they aren’t working. What if, as the Firesign Theatre and others have said, everything you know is wrong?
Perhaps our greatest quality as a species is resilience. We go on because we have to. The planet keeps moving around the sun as it has for almost 5 billion years, and we face tomorrow. Caregivers wake up for another day of doing their best for a faltering parent. I wake up for another day of doing my best to help them. And the researchers go back for another day hoping to find the real answer and treatment. Without trying, no treatment for anything will ever be found. We owe our patients, and ourselves, a better future than that.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Another one bites the dust.
Yet another investigational agent joins intepirdine, verubecestat, solanezumab, bapineuzumab, latrepirdine, and many others on the scrap pile of research: The complete release of trial data on idalopirdine found the drug wasn’t of clinically significant benefit in Alzheimer’s disease (JAMA. 2018;319[2]:130-42).
The numbers are bad enough that a handful of companies, including the giant Pfizer, have decided to leave Alzheimer’s drug development entirely to focus on more promising fields. And I get that. All of us – on any exhausting, fruitless, task – will reach the point where it’s time to cut our losses and move on. I don’t blame these companies for mostly leaving the field. (Pfizer is planning to form a neuroscience venture fund to support further research.)
Optimists will argue that you still learn things from a negative trial, which is true, but nothing to date is on the immediate horizon to help. The five agents we’ve had available for the past 15-20 years are all old enough to have lost their patents, and their benefits are modest, at best.
And all this going on as the overall human population, including myself, gradually ages and dementia becomes a medical-cost time bomb on the horizon. This isn’t an American problem. Every country in the world is facing it.
Politicians love to promise hope for these things: creating fast-track programs to get drugs to market faster, finding ways to bring down costs so more people can afford them, and improving methods to treat those in need. But none of those things matter if the medications don’t work.
Many of these trials test similar molecules because the evidence to date suggests they’re targeting the cause of Alzheimer’s. But so far they aren’t working. What if, as the Firesign Theatre and others have said, everything you know is wrong?
Perhaps our greatest quality as a species is resilience. We go on because we have to. The planet keeps moving around the sun as it has for almost 5 billion years, and we face tomorrow. Caregivers wake up for another day of doing their best for a faltering parent. I wake up for another day of doing my best to help them. And the researchers go back for another day hoping to find the real answer and treatment. Without trying, no treatment for anything will ever be found. We owe our patients, and ourselves, a better future than that.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.