User login
Pediatric Dermatology Consult - January 2018
Morphea, also known as localized scleroderma, is a rare fibrosing disorder of the skin and the underlying tissue that encompasses a variety of distinct subtypes classified by pattern and depth of lesion involvement. It may involve fat, fascia, muscle, and bone, and rarely, the central nervous system. Morphea is easily differentiated from systemic sclerosis by its primarily cutaneous involvement, although a minority of patients may have associated extracutaneous findings. Systemic sclerosis describes a well-defined disorder of skin sclerosis with a specific pattern of internal organ involvement.
Classification of the different subtypes of morphea are based on clinical presentation of the lesions. The most widely used system characterizes morphea into linear, circumscribed, generalized, pansclerotic, and mixed morphea subtypes.1 Mixed morphea describes the presence of two or more patterns of disease and affects 15% of patients. Morphea lesions initially present as erythematous to violaceous patches and plaques that eventually become white and sclerotic, with resulting destruction of the surrounding structures.
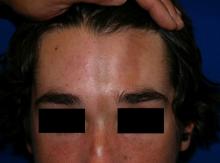
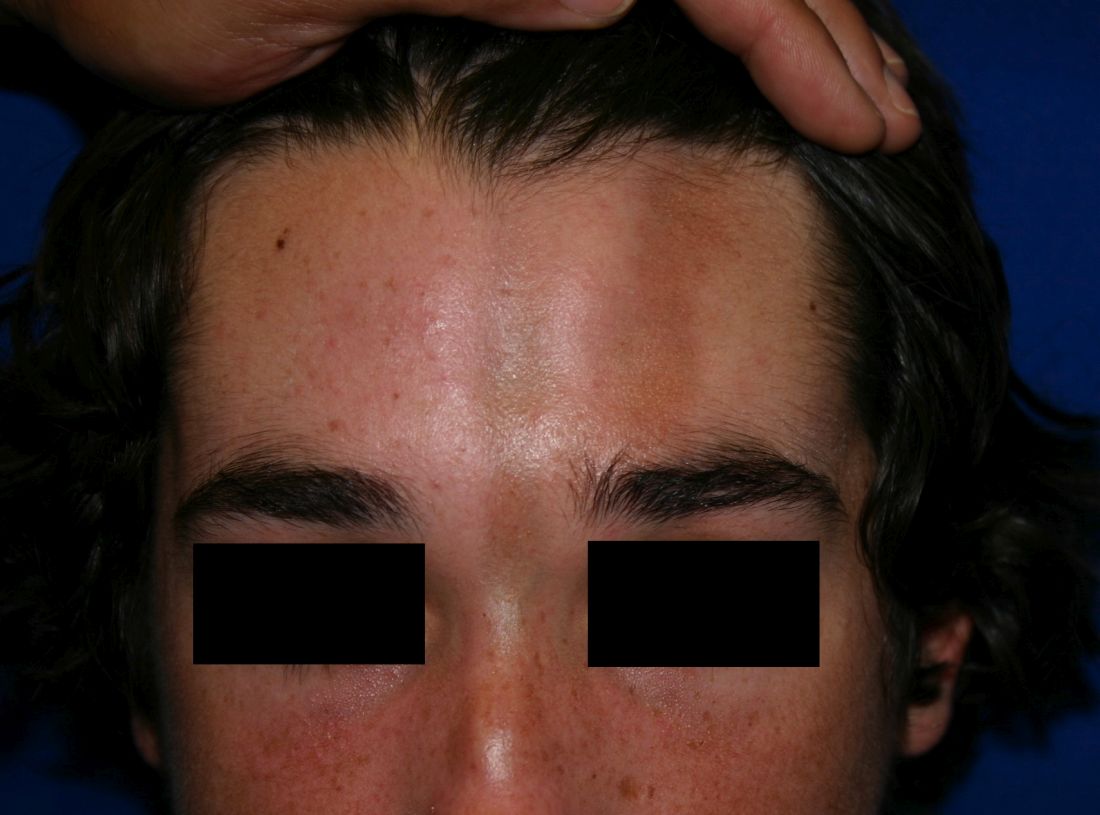
Linear scleroderma is the most common subtype of morphea in children and adolescents, affecting 42%-67% of children with morphea.1 It is characterized by linear plaques, often on the extremities, face, or scalp, that tend to follow Blaschko lines.4 These lesions may extend past the dermis to the subcutaneous tissue, muscle, and even bone, resulting in significant deformities. When on the scalp or face, particularly the forehead, the linear lesion may be referred to as the en coup de sabre variant. Ocular and CNS involvement should be of concern in these patients. When subcutaneous atrophy on the unilateral face is present with unaffected overlaying skin, this is known as the Parry-Romberg syndrome or progressive hemifacial atrophy. Involvement of the extremities is common, and unfortunately, may lead to muscle atrophy of the affected limb, contractures in areas overlying joint spaces, and occasionally limb length discrepancies.
Circumscribed morphea describes three or fewer discrete, oval, or round indurated plaques, with central whitening and a violaceous periphery. They generally are found on the trunk. When lesions have deeper involvement, delving past the dermis to involve the underlying fascia and muscle, the patient may experience a “bound down” sensation. Most lesions soften over 3-5 years.
Generalized morphea is used to describe the presence of at least four plaques, larger than 3 cm, that become confluent in at least two different locations on the body. Patients with generalized morphea have higher rates of systemic symptoms such as arthritis and fatigue.
Pansclerotic morphea, the most severe subtype, is characterized by significant body surface area involvement coupled with deep depth of involvement, often to the bone. The widespread blistering associated with pansclerotic morphea may lead to chronic ulceration and, later on, a higher risk of squamous cell carcinoma development. Despite its extensive distribution, pansclerotic morphea does not cause the severe organ and vascular fibrosis that is characteristically seen in systemic sclerosis. Raynaud’s phenomenon, abnormal nailfold capillaries, and sclerodactyly also will be absent in pansclerotic morphea.
Extracutaneous findings are present in up to 22% of patients with morphea.5 Arthritis is the most common associated finding, and often is correlated with a positive rheumatoid factor. Neurologic involvement most frequently is seen in patients with facial morphea and may present as seizures, as in this patient. MRI abnormalities such as calcifications and white matter changes may be seen. Other common extracutaneous features include fatigue, vascular abnormalities, and ocular findings, such as uveitis.
Morphea and systemic sclerosis appear similar on histology. In early morphea, lymphocytic perivascular infiltrates may be seen in the reticular dermis. In late morphea, the inflammatory cells are replaced by an abundance of collagen bundles infiltrating the dermis.
although the instigating factor activating this pathway is unknown. Multiple factors have been associated with the development of morphea, including autoimmunity, trauma, Borrelia and cytomegalovirus infections, radiation, and certain medications in case reports. Patients with morphea have higher rates of concomitant autoimmune diseases than that found in the general population6 and also have higher rates of autoantibody positivity. In a 750-patient, multicenter study of children with morphea, 42% of patients had positive antinuclear antibodies.7
Diagnosis
Morphea is diagnosed clinically, based on the characteristic appearance of the lesions. A biopsy may be helpful if the presentation is atypical. Although patients with morphea have higher rates of autoantibody positivity, there are no specific laboratory tests that consistently or reliably offer diagnostic value.8 Imaging modalities such as MRI may be utilized to view depth of involvement. Other noninvasive measures, such as thermography and ultrasonography, may be used to determine disease activity.9
Treatment
Treatment for morphea often is multidisciplinary and depends on the severity of involvement and extent to which it impedes functionality and quality of life. Localized plaques that do not restrict movement may be treated with topical corticosteroids, calcipotriene, and tacrolimus. However, topical corticosteroids should be discontinued if there are no signs of improvement in 2-3 months.
For patients with deforming or functionally significant disease, systemic treatment is advised. Methotrexate with or without systemic corticosteroids has been frequently studied, and is the most commonly recommended systemic therapy.11 Some experts have recommended treatment for at least 2-3 years, with at least 1 year of disease inactivity, before discontinuing treatment. Despite this duration of treatment, up to one-quarter of patients, especially those with linear morphea, will still experience recurrence of disease. Management of morphea may be aided by rheumatology and/or dermatology consultation.
Ms. Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and a professor of dermatology and pediatrics at the University of California, San Diego. Ms. Han and Dr. Eichenfield had no conflicts of interest or financial disclosures.
References
1. Fett N et al. J Am Acad Dermatol. 2011 Feb;64(2):217-28.
2. Condie D et al. Arthritis Rheumatol. 2014 Dec;66(12):3496-504.
3. Zulian F et al. J Pediatr. 2006 Aug;149(2):248-51.
4. Weibel L et al. Br J Dermatol. 2008 Jul;159(1):175-81.
5. Zulian F et al. Arthritis Rheum. 2005 Sep;52(9):2873-81.
6. Leitenberger JJ et al. Arch Dermatol. 2009 May;145(5):545-50.
7. Zulian F et al. Rheumatology (Oxford). 2006 May;45(5):614-20.
8. Dharamsi JW et al. JAMA Dermatol. 2013 Oct;149(10):1159-65.
9. Zulian F et al. Curr Opin Rheumatol. 2013 Sep;25(5):643-50.
10. Pope E et al. Pediatr Clin North Am. 2014 Apr;61(2):309-19.
11. Strickland N et al. Am Acad Dermatol. 2015 Apr; 72(4): 727-8.
12. Schoch JJ et al. Pediatr Dermatol. 2018. 35(1): 43-6.
Morphea, also known as localized scleroderma, is a rare fibrosing disorder of the skin and the underlying tissue that encompasses a variety of distinct subtypes classified by pattern and depth of lesion involvement. It may involve fat, fascia, muscle, and bone, and rarely, the central nervous system. Morphea is easily differentiated from systemic sclerosis by its primarily cutaneous involvement, although a minority of patients may have associated extracutaneous findings. Systemic sclerosis describes a well-defined disorder of skin sclerosis with a specific pattern of internal organ involvement.
Classification of the different subtypes of morphea are based on clinical presentation of the lesions. The most widely used system characterizes morphea into linear, circumscribed, generalized, pansclerotic, and mixed morphea subtypes.1 Mixed morphea describes the presence of two or more patterns of disease and affects 15% of patients. Morphea lesions initially present as erythematous to violaceous patches and plaques that eventually become white and sclerotic, with resulting destruction of the surrounding structures.
Linear scleroderma is the most common subtype of morphea in children and adolescents, affecting 42%-67% of children with morphea.1 It is characterized by linear plaques, often on the extremities, face, or scalp, that tend to follow Blaschko lines.4 These lesions may extend past the dermis to the subcutaneous tissue, muscle, and even bone, resulting in significant deformities. When on the scalp or face, particularly the forehead, the linear lesion may be referred to as the en coup de sabre variant. Ocular and CNS involvement should be of concern in these patients. When subcutaneous atrophy on the unilateral face is present with unaffected overlaying skin, this is known as the Parry-Romberg syndrome or progressive hemifacial atrophy. Involvement of the extremities is common, and unfortunately, may lead to muscle atrophy of the affected limb, contractures in areas overlying joint spaces, and occasionally limb length discrepancies.
Circumscribed morphea describes three or fewer discrete, oval, or round indurated plaques, with central whitening and a violaceous periphery. They generally are found on the trunk. When lesions have deeper involvement, delving past the dermis to involve the underlying fascia and muscle, the patient may experience a “bound down” sensation. Most lesions soften over 3-5 years.
Generalized morphea is used to describe the presence of at least four plaques, larger than 3 cm, that become confluent in at least two different locations on the body. Patients with generalized morphea have higher rates of systemic symptoms such as arthritis and fatigue.
Pansclerotic morphea, the most severe subtype, is characterized by significant body surface area involvement coupled with deep depth of involvement, often to the bone. The widespread blistering associated with pansclerotic morphea may lead to chronic ulceration and, later on, a higher risk of squamous cell carcinoma development. Despite its extensive distribution, pansclerotic morphea does not cause the severe organ and vascular fibrosis that is characteristically seen in systemic sclerosis. Raynaud’s phenomenon, abnormal nailfold capillaries, and sclerodactyly also will be absent in pansclerotic morphea.
Extracutaneous findings are present in up to 22% of patients with morphea.5 Arthritis is the most common associated finding, and often is correlated with a positive rheumatoid factor. Neurologic involvement most frequently is seen in patients with facial morphea and may present as seizures, as in this patient. MRI abnormalities such as calcifications and white matter changes may be seen. Other common extracutaneous features include fatigue, vascular abnormalities, and ocular findings, such as uveitis.
Morphea and systemic sclerosis appear similar on histology. In early morphea, lymphocytic perivascular infiltrates may be seen in the reticular dermis. In late morphea, the inflammatory cells are replaced by an abundance of collagen bundles infiltrating the dermis.
although the instigating factor activating this pathway is unknown. Multiple factors have been associated with the development of morphea, including autoimmunity, trauma, Borrelia and cytomegalovirus infections, radiation, and certain medications in case reports. Patients with morphea have higher rates of concomitant autoimmune diseases than that found in the general population6 and also have higher rates of autoantibody positivity. In a 750-patient, multicenter study of children with morphea, 42% of patients had positive antinuclear antibodies.7
Diagnosis
Morphea is diagnosed clinically, based on the characteristic appearance of the lesions. A biopsy may be helpful if the presentation is atypical. Although patients with morphea have higher rates of autoantibody positivity, there are no specific laboratory tests that consistently or reliably offer diagnostic value.8 Imaging modalities such as MRI may be utilized to view depth of involvement. Other noninvasive measures, such as thermography and ultrasonography, may be used to determine disease activity.9
Treatment
Treatment for morphea often is multidisciplinary and depends on the severity of involvement and extent to which it impedes functionality and quality of life. Localized plaques that do not restrict movement may be treated with topical corticosteroids, calcipotriene, and tacrolimus. However, topical corticosteroids should be discontinued if there are no signs of improvement in 2-3 months.
For patients with deforming or functionally significant disease, systemic treatment is advised. Methotrexate with or without systemic corticosteroids has been frequently studied, and is the most commonly recommended systemic therapy.11 Some experts have recommended treatment for at least 2-3 years, with at least 1 year of disease inactivity, before discontinuing treatment. Despite this duration of treatment, up to one-quarter of patients, especially those with linear morphea, will still experience recurrence of disease. Management of morphea may be aided by rheumatology and/or dermatology consultation.
Ms. Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and a professor of dermatology and pediatrics at the University of California, San Diego. Ms. Han and Dr. Eichenfield had no conflicts of interest or financial disclosures.
References
1. Fett N et al. J Am Acad Dermatol. 2011 Feb;64(2):217-28.
2. Condie D et al. Arthritis Rheumatol. 2014 Dec;66(12):3496-504.
3. Zulian F et al. J Pediatr. 2006 Aug;149(2):248-51.
4. Weibel L et al. Br J Dermatol. 2008 Jul;159(1):175-81.
5. Zulian F et al. Arthritis Rheum. 2005 Sep;52(9):2873-81.
6. Leitenberger JJ et al. Arch Dermatol. 2009 May;145(5):545-50.
7. Zulian F et al. Rheumatology (Oxford). 2006 May;45(5):614-20.
8. Dharamsi JW et al. JAMA Dermatol. 2013 Oct;149(10):1159-65.
9. Zulian F et al. Curr Opin Rheumatol. 2013 Sep;25(5):643-50.
10. Pope E et al. Pediatr Clin North Am. 2014 Apr;61(2):309-19.
11. Strickland N et al. Am Acad Dermatol. 2015 Apr; 72(4): 727-8.
12. Schoch JJ et al. Pediatr Dermatol. 2018. 35(1): 43-6.
Morphea, also known as localized scleroderma, is a rare fibrosing disorder of the skin and the underlying tissue that encompasses a variety of distinct subtypes classified by pattern and depth of lesion involvement. It may involve fat, fascia, muscle, and bone, and rarely, the central nervous system. Morphea is easily differentiated from systemic sclerosis by its primarily cutaneous involvement, although a minority of patients may have associated extracutaneous findings. Systemic sclerosis describes a well-defined disorder of skin sclerosis with a specific pattern of internal organ involvement.
Classification of the different subtypes of morphea are based on clinical presentation of the lesions. The most widely used system characterizes morphea into linear, circumscribed, generalized, pansclerotic, and mixed morphea subtypes.1 Mixed morphea describes the presence of two or more patterns of disease and affects 15% of patients. Morphea lesions initially present as erythematous to violaceous patches and plaques that eventually become white and sclerotic, with resulting destruction of the surrounding structures.
Linear scleroderma is the most common subtype of morphea in children and adolescents, affecting 42%-67% of children with morphea.1 It is characterized by linear plaques, often on the extremities, face, or scalp, that tend to follow Blaschko lines.4 These lesions may extend past the dermis to the subcutaneous tissue, muscle, and even bone, resulting in significant deformities. When on the scalp or face, particularly the forehead, the linear lesion may be referred to as the en coup de sabre variant. Ocular and CNS involvement should be of concern in these patients. When subcutaneous atrophy on the unilateral face is present with unaffected overlaying skin, this is known as the Parry-Romberg syndrome or progressive hemifacial atrophy. Involvement of the extremities is common, and unfortunately, may lead to muscle atrophy of the affected limb, contractures in areas overlying joint spaces, and occasionally limb length discrepancies.
Circumscribed morphea describes three or fewer discrete, oval, or round indurated plaques, with central whitening and a violaceous periphery. They generally are found on the trunk. When lesions have deeper involvement, delving past the dermis to involve the underlying fascia and muscle, the patient may experience a “bound down” sensation. Most lesions soften over 3-5 years.
Generalized morphea is used to describe the presence of at least four plaques, larger than 3 cm, that become confluent in at least two different locations on the body. Patients with generalized morphea have higher rates of systemic symptoms such as arthritis and fatigue.
Pansclerotic morphea, the most severe subtype, is characterized by significant body surface area involvement coupled with deep depth of involvement, often to the bone. The widespread blistering associated with pansclerotic morphea may lead to chronic ulceration and, later on, a higher risk of squamous cell carcinoma development. Despite its extensive distribution, pansclerotic morphea does not cause the severe organ and vascular fibrosis that is characteristically seen in systemic sclerosis. Raynaud’s phenomenon, abnormal nailfold capillaries, and sclerodactyly also will be absent in pansclerotic morphea.
Extracutaneous findings are present in up to 22% of patients with morphea.5 Arthritis is the most common associated finding, and often is correlated with a positive rheumatoid factor. Neurologic involvement most frequently is seen in patients with facial morphea and may present as seizures, as in this patient. MRI abnormalities such as calcifications and white matter changes may be seen. Other common extracutaneous features include fatigue, vascular abnormalities, and ocular findings, such as uveitis.
Morphea and systemic sclerosis appear similar on histology. In early morphea, lymphocytic perivascular infiltrates may be seen in the reticular dermis. In late morphea, the inflammatory cells are replaced by an abundance of collagen bundles infiltrating the dermis.
although the instigating factor activating this pathway is unknown. Multiple factors have been associated with the development of morphea, including autoimmunity, trauma, Borrelia and cytomegalovirus infections, radiation, and certain medications in case reports. Patients with morphea have higher rates of concomitant autoimmune diseases than that found in the general population6 and also have higher rates of autoantibody positivity. In a 750-patient, multicenter study of children with morphea, 42% of patients had positive antinuclear antibodies.7
Diagnosis
Morphea is diagnosed clinically, based on the characteristic appearance of the lesions. A biopsy may be helpful if the presentation is atypical. Although patients with morphea have higher rates of autoantibody positivity, there are no specific laboratory tests that consistently or reliably offer diagnostic value.8 Imaging modalities such as MRI may be utilized to view depth of involvement. Other noninvasive measures, such as thermography and ultrasonography, may be used to determine disease activity.9
Treatment
Treatment for morphea often is multidisciplinary and depends on the severity of involvement and extent to which it impedes functionality and quality of life. Localized plaques that do not restrict movement may be treated with topical corticosteroids, calcipotriene, and tacrolimus. However, topical corticosteroids should be discontinued if there are no signs of improvement in 2-3 months.
For patients with deforming or functionally significant disease, systemic treatment is advised. Methotrexate with or without systemic corticosteroids has been frequently studied, and is the most commonly recommended systemic therapy.11 Some experts have recommended treatment for at least 2-3 years, with at least 1 year of disease inactivity, before discontinuing treatment. Despite this duration of treatment, up to one-quarter of patients, especially those with linear morphea, will still experience recurrence of disease. Management of morphea may be aided by rheumatology and/or dermatology consultation.
Ms. Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and a professor of dermatology and pediatrics at the University of California, San Diego. Ms. Han and Dr. Eichenfield had no conflicts of interest or financial disclosures.
References
1. Fett N et al. J Am Acad Dermatol. 2011 Feb;64(2):217-28.
2. Condie D et al. Arthritis Rheumatol. 2014 Dec;66(12):3496-504.
3. Zulian F et al. J Pediatr. 2006 Aug;149(2):248-51.
4. Weibel L et al. Br J Dermatol. 2008 Jul;159(1):175-81.
5. Zulian F et al. Arthritis Rheum. 2005 Sep;52(9):2873-81.
6. Leitenberger JJ et al. Arch Dermatol. 2009 May;145(5):545-50.
7. Zulian F et al. Rheumatology (Oxford). 2006 May;45(5):614-20.
8. Dharamsi JW et al. JAMA Dermatol. 2013 Oct;149(10):1159-65.
9. Zulian F et al. Curr Opin Rheumatol. 2013 Sep;25(5):643-50.
10. Pope E et al. Pediatr Clin North Am. 2014 Apr;61(2):309-19.
11. Strickland N et al. Am Acad Dermatol. 2015 Apr; 72(4): 727-8.
12. Schoch JJ et al. Pediatr Dermatol. 2018. 35(1): 43-6.
A 14-year-old patient presents to a dermatology clinic for a depression on his forehead, which has been there for about 2 years. A few years ago, he used to have a pruritic pink lesion on the forehead where the depression is now. He denies any symptoms.
Anxiety in teens
It seems that every week there is a new headline about the rising rates of anxiety in today’s adolescents. Schools often are asked to address high levels of stress and anxiety in their students, and the pediatrician’s office is often the first place worried parents will call. We will try to help you differentiate between what is normal – even healthy – adolescent stress, and what might represent treatable psychiatric problems. And we will review how to approach stress management with your patients and their parents. For all adolescents, even those with psychiatric diagnoses, learning to manage stress and anxiety is critical to their healthiest development into capable, confident, resilient adults.
Stress is the mental or emotional strain resulting from demanding or adverse circumstances. Anxiety is a feeling of unease about an imminent event with an uncertain outcome. An anxiety disorder is a psychiatric illness characterized by a state of excessive unease leading to functional impairment. These distinctions are critical, as both stress and anxiety are normal-but-uncomfortable parts of the adolescent experience. When all of a teenager’s stress and anxiety is medicalized, it promotes avoidance, which in turn may worsen your patient’s functional impairment rather than improving it.
This is not to suggest that there are not real (and common) psychiatric illnesses that can affect the levels of anxiety in your patients. Anxiety disorders start the earliest, with separation anxiety disorder, specific phobia, and social phobia all having a mean onset before puberty. Anxiety disorders are the most prevalent psychiatric disorders in youth (30% of youth psychiatric illness), and anxiety also may be related to substance use disorders (25%), disruptive behavior disorders (20%), and mood disorders (17%). Despite the excited news coverage, there is no evidence of a statistically significant increase in the incidence of anxiety or mood disorders in young people over the past decade.
It is not difficult to imagine that the challenges facing adolescents are considerable. Of course, adolescence is a time of major change starting with puberty, in which young people actively develop independence, identity, and a rich array of deep relationships beyond their families. Typically, this is a 5- to 10-year process of risk-taking, new experiences, setbacks, delight, heartbreak, and triumphs all alongside growing autonomy.
These forces may make their parents even more stressed than the adolescents themselves, but there is one dramatically different feature of adolescent life today: the constant presence of smartphones. While these devices can improve connectedness to school, family, and friends, use of smartphones also means that today’s teenagers often have little downtime cognitively or socially. Use of smartphones can facilitate both supportive affirmation from friends and relentless social pressures, and the feeling of being excluded or bullied. Smartphone use can interfere with restful sleep, and some virtual activities may compete with the genuine experimentation and exploration where teenagers discover their interests and abilities and develop meaningful confidence and independence.
Several factors might impair an adolescent’s ability to cope with challenge and stress. Those teenagers who have not had the opportunity to face and manage modest setbacks, difficulties, and discomforts during their elementary and middle school years may be overwhelmed by starting with the higher-stakes strains of adolescence. This can happen when young children have not explored many new activities, have been shielded from the consequences of failures, or have tried only activities that came easily to them. Certainly, teenagers who are managing a depressive or anxiety disorder as well as those with learning disabilities may have limited ability to cope with routine stress, although those who have a well-treated disorder often have robust coping skills.
Perhaps obvious, but still very important, chronic sleep deprivation can leave adolescents irritable, impatient, and distractible, all of which make coping with a challenge very difficult. Likewise, substance use can directly impair coping skills, and can create the habit of trying to escape stress rather than manage it.
So what does this mean for you? If your patient has an anxiety, depressive, or substance use disorder, refer for appropriate therapy. For both those who screen in and those who do not, your next task is to help them improve their coping skills. What specifically has them so stressed?
Are there family stressors or unrealistic expectations that can be addressed? Can they see their situation as a challenge and focus on what is within their control to do in response? Remind your patients that challenges are uncomfortable. Mastery comes with practice and, inevitably, some setbacks and failures. Have they identified personal goals or a transcendent purpose? This can improve motivation and keep a challenge in perspective. They might focus on learning about their coping style: Do they do better with a slow, steady, methodical approach or intense bursts of effort? Talk with them about self-care. Adequate sleep, regular exercise, putting effort into relaxation as well as work, and spending time with their actual (not just virtual) friends all are essential to keeping their batteries charged while doing the intense work of normal adolescence.
For those patients who do not meet criteria for depression or anxiety disorders, there are circumstances in which a referral for therapy can be helpful. If they are noticeably disconnected from their parents or their parents seem to be more reactive to the stress and pressures than they are, an outside therapist can be a meaningful support as they build skills. Those patients who are socially isolated and stressed, are using substances regularly, are withdrawing from other interests to manage their source of stress, or are having difficulty telling facts from feelings are at risk for failing to adequately manage their stress and for the development of psychiatric problems. Starting early, helping them to build autonomy as preadolescents, experiencing successes and failures, begins the cultivation of resilience and meaningful confidence they will need during adolescence. Your attention and guidance can help all of your adolescent patients improve their coping and lower both their stress and their anxiety.
It seems that every week there is a new headline about the rising rates of anxiety in today’s adolescents. Schools often are asked to address high levels of stress and anxiety in their students, and the pediatrician’s office is often the first place worried parents will call. We will try to help you differentiate between what is normal – even healthy – adolescent stress, and what might represent treatable psychiatric problems. And we will review how to approach stress management with your patients and their parents. For all adolescents, even those with psychiatric diagnoses, learning to manage stress and anxiety is critical to their healthiest development into capable, confident, resilient adults.
Stress is the mental or emotional strain resulting from demanding or adverse circumstances. Anxiety is a feeling of unease about an imminent event with an uncertain outcome. An anxiety disorder is a psychiatric illness characterized by a state of excessive unease leading to functional impairment. These distinctions are critical, as both stress and anxiety are normal-but-uncomfortable parts of the adolescent experience. When all of a teenager’s stress and anxiety is medicalized, it promotes avoidance, which in turn may worsen your patient’s functional impairment rather than improving it.
This is not to suggest that there are not real (and common) psychiatric illnesses that can affect the levels of anxiety in your patients. Anxiety disorders start the earliest, with separation anxiety disorder, specific phobia, and social phobia all having a mean onset before puberty. Anxiety disorders are the most prevalent psychiatric disorders in youth (30% of youth psychiatric illness), and anxiety also may be related to substance use disorders (25%), disruptive behavior disorders (20%), and mood disorders (17%). Despite the excited news coverage, there is no evidence of a statistically significant increase in the incidence of anxiety or mood disorders in young people over the past decade.
It is not difficult to imagine that the challenges facing adolescents are considerable. Of course, adolescence is a time of major change starting with puberty, in which young people actively develop independence, identity, and a rich array of deep relationships beyond their families. Typically, this is a 5- to 10-year process of risk-taking, new experiences, setbacks, delight, heartbreak, and triumphs all alongside growing autonomy.
These forces may make their parents even more stressed than the adolescents themselves, but there is one dramatically different feature of adolescent life today: the constant presence of smartphones. While these devices can improve connectedness to school, family, and friends, use of smartphones also means that today’s teenagers often have little downtime cognitively or socially. Use of smartphones can facilitate both supportive affirmation from friends and relentless social pressures, and the feeling of being excluded or bullied. Smartphone use can interfere with restful sleep, and some virtual activities may compete with the genuine experimentation and exploration where teenagers discover their interests and abilities and develop meaningful confidence and independence.
Several factors might impair an adolescent’s ability to cope with challenge and stress. Those teenagers who have not had the opportunity to face and manage modest setbacks, difficulties, and discomforts during their elementary and middle school years may be overwhelmed by starting with the higher-stakes strains of adolescence. This can happen when young children have not explored many new activities, have been shielded from the consequences of failures, or have tried only activities that came easily to them. Certainly, teenagers who are managing a depressive or anxiety disorder as well as those with learning disabilities may have limited ability to cope with routine stress, although those who have a well-treated disorder often have robust coping skills.
Perhaps obvious, but still very important, chronic sleep deprivation can leave adolescents irritable, impatient, and distractible, all of which make coping with a challenge very difficult. Likewise, substance use can directly impair coping skills, and can create the habit of trying to escape stress rather than manage it.
So what does this mean for you? If your patient has an anxiety, depressive, or substance use disorder, refer for appropriate therapy. For both those who screen in and those who do not, your next task is to help them improve their coping skills. What specifically has them so stressed?
Are there family stressors or unrealistic expectations that can be addressed? Can they see their situation as a challenge and focus on what is within their control to do in response? Remind your patients that challenges are uncomfortable. Mastery comes with practice and, inevitably, some setbacks and failures. Have they identified personal goals or a transcendent purpose? This can improve motivation and keep a challenge in perspective. They might focus on learning about their coping style: Do they do better with a slow, steady, methodical approach or intense bursts of effort? Talk with them about self-care. Adequate sleep, regular exercise, putting effort into relaxation as well as work, and spending time with their actual (not just virtual) friends all are essential to keeping their batteries charged while doing the intense work of normal adolescence.
For those patients who do not meet criteria for depression or anxiety disorders, there are circumstances in which a referral for therapy can be helpful. If they are noticeably disconnected from their parents or their parents seem to be more reactive to the stress and pressures than they are, an outside therapist can be a meaningful support as they build skills. Those patients who are socially isolated and stressed, are using substances regularly, are withdrawing from other interests to manage their source of stress, or are having difficulty telling facts from feelings are at risk for failing to adequately manage their stress and for the development of psychiatric problems. Starting early, helping them to build autonomy as preadolescents, experiencing successes and failures, begins the cultivation of resilience and meaningful confidence they will need during adolescence. Your attention and guidance can help all of your adolescent patients improve their coping and lower both their stress and their anxiety.
It seems that every week there is a new headline about the rising rates of anxiety in today’s adolescents. Schools often are asked to address high levels of stress and anxiety in their students, and the pediatrician’s office is often the first place worried parents will call. We will try to help you differentiate between what is normal – even healthy – adolescent stress, and what might represent treatable psychiatric problems. And we will review how to approach stress management with your patients and their parents. For all adolescents, even those with psychiatric diagnoses, learning to manage stress and anxiety is critical to their healthiest development into capable, confident, resilient adults.
Stress is the mental or emotional strain resulting from demanding or adverse circumstances. Anxiety is a feeling of unease about an imminent event with an uncertain outcome. An anxiety disorder is a psychiatric illness characterized by a state of excessive unease leading to functional impairment. These distinctions are critical, as both stress and anxiety are normal-but-uncomfortable parts of the adolescent experience. When all of a teenager’s stress and anxiety is medicalized, it promotes avoidance, which in turn may worsen your patient’s functional impairment rather than improving it.
This is not to suggest that there are not real (and common) psychiatric illnesses that can affect the levels of anxiety in your patients. Anxiety disorders start the earliest, with separation anxiety disorder, specific phobia, and social phobia all having a mean onset before puberty. Anxiety disorders are the most prevalent psychiatric disorders in youth (30% of youth psychiatric illness), and anxiety also may be related to substance use disorders (25%), disruptive behavior disorders (20%), and mood disorders (17%). Despite the excited news coverage, there is no evidence of a statistically significant increase in the incidence of anxiety or mood disorders in young people over the past decade.
It is not difficult to imagine that the challenges facing adolescents are considerable. Of course, adolescence is a time of major change starting with puberty, in which young people actively develop independence, identity, and a rich array of deep relationships beyond their families. Typically, this is a 5- to 10-year process of risk-taking, new experiences, setbacks, delight, heartbreak, and triumphs all alongside growing autonomy.
These forces may make their parents even more stressed than the adolescents themselves, but there is one dramatically different feature of adolescent life today: the constant presence of smartphones. While these devices can improve connectedness to school, family, and friends, use of smartphones also means that today’s teenagers often have little downtime cognitively or socially. Use of smartphones can facilitate both supportive affirmation from friends and relentless social pressures, and the feeling of being excluded or bullied. Smartphone use can interfere with restful sleep, and some virtual activities may compete with the genuine experimentation and exploration where teenagers discover their interests and abilities and develop meaningful confidence and independence.
Several factors might impair an adolescent’s ability to cope with challenge and stress. Those teenagers who have not had the opportunity to face and manage modest setbacks, difficulties, and discomforts during their elementary and middle school years may be overwhelmed by starting with the higher-stakes strains of adolescence. This can happen when young children have not explored many new activities, have been shielded from the consequences of failures, or have tried only activities that came easily to them. Certainly, teenagers who are managing a depressive or anxiety disorder as well as those with learning disabilities may have limited ability to cope with routine stress, although those who have a well-treated disorder often have robust coping skills.
Perhaps obvious, but still very important, chronic sleep deprivation can leave adolescents irritable, impatient, and distractible, all of which make coping with a challenge very difficult. Likewise, substance use can directly impair coping skills, and can create the habit of trying to escape stress rather than manage it.
So what does this mean for you? If your patient has an anxiety, depressive, or substance use disorder, refer for appropriate therapy. For both those who screen in and those who do not, your next task is to help them improve their coping skills. What specifically has them so stressed?
Are there family stressors or unrealistic expectations that can be addressed? Can they see their situation as a challenge and focus on what is within their control to do in response? Remind your patients that challenges are uncomfortable. Mastery comes with practice and, inevitably, some setbacks and failures. Have they identified personal goals or a transcendent purpose? This can improve motivation and keep a challenge in perspective. They might focus on learning about their coping style: Do they do better with a slow, steady, methodical approach or intense bursts of effort? Talk with them about self-care. Adequate sleep, regular exercise, putting effort into relaxation as well as work, and spending time with their actual (not just virtual) friends all are essential to keeping their batteries charged while doing the intense work of normal adolescence.
For those patients who do not meet criteria for depression or anxiety disorders, there are circumstances in which a referral for therapy can be helpful. If they are noticeably disconnected from their parents or their parents seem to be more reactive to the stress and pressures than they are, an outside therapist can be a meaningful support as they build skills. Those patients who are socially isolated and stressed, are using substances regularly, are withdrawing from other interests to manage their source of stress, or are having difficulty telling facts from feelings are at risk for failing to adequately manage their stress and for the development of psychiatric problems. Starting early, helping them to build autonomy as preadolescents, experiencing successes and failures, begins the cultivation of resilience and meaningful confidence they will need during adolescence. Your attention and guidance can help all of your adolescent patients improve their coping and lower both their stress and their anxiety.
Anxiety disorders: Psychopharmacologic treatment update
Anxiety disorders, including separation anxiety disorder, social anxiety disorder, and generalized anxiety disorder, are some of the most common psychiatric conditions of childhood and adolescence, affecting up to 20% of youth.1 Patients commonly present with a mix of symptoms that often span multiple anxiety disorder diagnoses. While this pattern can present somewhat of a diagnostic conundrum, it can be reassuring to know that such constellations of symptoms are the rule rather than the exception. Further, given that both the pharmacologic and nonpharmacologic treatment strategies don’t change much among the various anxiety disorders, the lack of a definitive single diagnosis should not delay intervention. Be alert to the possibility that anxiety and anxiety disorders can be the engine that drives what on the surface appears to be more disruptive and oppositional behavior.
Although medications can be a useful part of treatment, they are not recommended as a stand-alone intervention. Nonpharmacologic treatments generally should be tried before medications are considered. Among the different types of psychotherapy, cognitive-behavioral therapy (CBT) has the most empirical support from research trials, although other modalities such as mindfulness-based treatments show some promise. As anxiety disorders often run in families, it also can be very useful to explore the possibility that one or more parents also struggle with an anxiety disorder, which, if untreated, might complicate the child’s course.
With regard to medications, it is being increasingly appreciated that, despite SSRIs being most popularly known as antidepressants, these medications actually may be as efficacious or even more efficacious in the management of anxiety disorders. This class remains the cornerstone of medication treatment, and a brief review of current options follows.
SSRIs and SNRIs
A 2015 meta-analysis that examined nine randomized controlled trials of SSRIs and serotonin and norepinephrine reuptake inhibitors (SNRIs) for pediatric anxiety disorders concluded that these agents provided a benefit of modest effect size. No significant increase in treatment-emergent suicidality was found, and the medications were generally well tolerated.2 This analysis also found some evidence that greater efficacy was related to a medication with more specific serotonergic properties, suggesting improved response with “true” SSRIs versus SNRIs such as venlafaxine and duloxetine. One major study using sertraline found that, at least in the short term, combined use of sertraline with CBT resulted in better efficacy than either treatment alone.3 Dosing of SSRIs should start low: A general rule is to begin at half of the smallest dosage made, depending on the age and size of the patient. One question that often comes up after a successful trial is how long to continue the medications. A recent meta-analysis in adults concluded that there was evidence that stopping medication prior to 1 year resulted in an increased risk of relapse with little to guide clinicians after that 1-year mark.4
Benzodiazepines
Even though benzodiazepines have been around for a long time, data supporting their efficacy and safety in pediatric populations remain extremely limited, and what has been reported has not been particularly positive. Thus, most experts do not suggest using benzodiazepines for anxiety disorders, with the exception of helping children through single or rare events, such as medical procedures or enabling an adolescent who has been fearful of attending school to get to the building on the first day back after a long absence.
Guanfacine
In a recent exploratory trial of guanfacine for children with mixed anxiety disorders,5 the medication was well tolerated overall but did not result in statistically significant improvement relative to placebo on primary anxiety rating scales. However, a higher number of children were rated as improved on a clinician-rated scale. This medication is usually started at 0.5 mg/day and increased as tolerated, while checking vital signs, to a maximum of 4 mg/day.
Atomoxetine
A randomized control trial of pediatric patients with both ADHD and an anxiety disorder showed reductions in both symptom domains with atomoxetine dosed at an average of 1.3 mg/kg per day.6 There is little evidence to suggest its use in primary anxiety disorders without comorbid ADHD.
Buspirone
This 5-hydroxytryptamine 1a agonist has Food and Drug Administration approval for generalized anxiety disorder in adults and is generally well tolerated. Unfortunately, two randomized controlled studies in children and adolescents did not find statistically significant improvement relative to placebo, although some methodological problems may have played a role.7
Antipsychotics
Although sometimes used to augment an SSRI in adult anxiety disorders, there are little data to support the use of antipsychotics in pediatric populations, especially given the antecedent risks of the drugs.
Summary
Pharmacotherapy for anxiety disorders often includes the advice that, if medications are indicated in conjunction with psychotherapy, to start with an SSRI; and if that is not effective to try a different one.7 An SNRI such as venlafaxine or duloxetine may then be a third-line alternative, although for youth with comorbid ADHD, consideration of either atomoxetine or guanfacine is also reasonable. Beyond that point, there unfortunately are little systematic data to guide pharmacologic decision making, and increased potential risks of other classes of medications suggest the need for caution and consultation.
Looking for more mental health training? Attend the 12th annual Child Psychiatry in Primary Care conference in Burlington, on May 4, 2018,organized by the University of Vermont with Dr. Rettew as course director. Go to http://www.med.uvm.edu/cme/conferences.
References
1. Merikangas KR et al. J Am Acad Child Adolesc Psychiatry. 2010 Oct;49(10):980-9.
2. Strawn JR et al. Depress Anxiety. 2015 Mar;32(3):149-57.
3. Walkup J et al. N Engl J Med. 2008 Dec 25;359(26):2753-66.
4. Batelaan N et al. BMJ. 2017 Sep 13;358:j3927.
5. Strawn JR et al. J Child Adolesc Psychopharm. 2017 Feb;27(1): 29-37..
6. Geller D et al. J Am Acad Child Adolesc Psychiatry. 2007 Sep;46(9):1119-27.
7. Strawn JR et al. J Child Adolesc Psychopharm. 2017 Feb;28(1): 2-9.
Anxiety disorders, including separation anxiety disorder, social anxiety disorder, and generalized anxiety disorder, are some of the most common psychiatric conditions of childhood and adolescence, affecting up to 20% of youth.1 Patients commonly present with a mix of symptoms that often span multiple anxiety disorder diagnoses. While this pattern can present somewhat of a diagnostic conundrum, it can be reassuring to know that such constellations of symptoms are the rule rather than the exception. Further, given that both the pharmacologic and nonpharmacologic treatment strategies don’t change much among the various anxiety disorders, the lack of a definitive single diagnosis should not delay intervention. Be alert to the possibility that anxiety and anxiety disorders can be the engine that drives what on the surface appears to be more disruptive and oppositional behavior.
Although medications can be a useful part of treatment, they are not recommended as a stand-alone intervention. Nonpharmacologic treatments generally should be tried before medications are considered. Among the different types of psychotherapy, cognitive-behavioral therapy (CBT) has the most empirical support from research trials, although other modalities such as mindfulness-based treatments show some promise. As anxiety disorders often run in families, it also can be very useful to explore the possibility that one or more parents also struggle with an anxiety disorder, which, if untreated, might complicate the child’s course.
With regard to medications, it is being increasingly appreciated that, despite SSRIs being most popularly known as antidepressants, these medications actually may be as efficacious or even more efficacious in the management of anxiety disorders. This class remains the cornerstone of medication treatment, and a brief review of current options follows.
SSRIs and SNRIs
A 2015 meta-analysis that examined nine randomized controlled trials of SSRIs and serotonin and norepinephrine reuptake inhibitors (SNRIs) for pediatric anxiety disorders concluded that these agents provided a benefit of modest effect size. No significant increase in treatment-emergent suicidality was found, and the medications were generally well tolerated.2 This analysis also found some evidence that greater efficacy was related to a medication with more specific serotonergic properties, suggesting improved response with “true” SSRIs versus SNRIs such as venlafaxine and duloxetine. One major study using sertraline found that, at least in the short term, combined use of sertraline with CBT resulted in better efficacy than either treatment alone.3 Dosing of SSRIs should start low: A general rule is to begin at half of the smallest dosage made, depending on the age and size of the patient. One question that often comes up after a successful trial is how long to continue the medications. A recent meta-analysis in adults concluded that there was evidence that stopping medication prior to 1 year resulted in an increased risk of relapse with little to guide clinicians after that 1-year mark.4
Benzodiazepines
Even though benzodiazepines have been around for a long time, data supporting their efficacy and safety in pediatric populations remain extremely limited, and what has been reported has not been particularly positive. Thus, most experts do not suggest using benzodiazepines for anxiety disorders, with the exception of helping children through single or rare events, such as medical procedures or enabling an adolescent who has been fearful of attending school to get to the building on the first day back after a long absence.
Guanfacine
In a recent exploratory trial of guanfacine for children with mixed anxiety disorders,5 the medication was well tolerated overall but did not result in statistically significant improvement relative to placebo on primary anxiety rating scales. However, a higher number of children were rated as improved on a clinician-rated scale. This medication is usually started at 0.5 mg/day and increased as tolerated, while checking vital signs, to a maximum of 4 mg/day.
Atomoxetine
A randomized control trial of pediatric patients with both ADHD and an anxiety disorder showed reductions in both symptom domains with atomoxetine dosed at an average of 1.3 mg/kg per day.6 There is little evidence to suggest its use in primary anxiety disorders without comorbid ADHD.
Buspirone
This 5-hydroxytryptamine 1a agonist has Food and Drug Administration approval for generalized anxiety disorder in adults and is generally well tolerated. Unfortunately, two randomized controlled studies in children and adolescents did not find statistically significant improvement relative to placebo, although some methodological problems may have played a role.7
Antipsychotics
Although sometimes used to augment an SSRI in adult anxiety disorders, there are little data to support the use of antipsychotics in pediatric populations, especially given the antecedent risks of the drugs.
Summary
Pharmacotherapy for anxiety disorders often includes the advice that, if medications are indicated in conjunction with psychotherapy, to start with an SSRI; and if that is not effective to try a different one.7 An SNRI such as venlafaxine or duloxetine may then be a third-line alternative, although for youth with comorbid ADHD, consideration of either atomoxetine or guanfacine is also reasonable. Beyond that point, there unfortunately are little systematic data to guide pharmacologic decision making, and increased potential risks of other classes of medications suggest the need for caution and consultation.
Looking for more mental health training? Attend the 12th annual Child Psychiatry in Primary Care conference in Burlington, on May 4, 2018,organized by the University of Vermont with Dr. Rettew as course director. Go to http://www.med.uvm.edu/cme/conferences.
References
1. Merikangas KR et al. J Am Acad Child Adolesc Psychiatry. 2010 Oct;49(10):980-9.
2. Strawn JR et al. Depress Anxiety. 2015 Mar;32(3):149-57.
3. Walkup J et al. N Engl J Med. 2008 Dec 25;359(26):2753-66.
4. Batelaan N et al. BMJ. 2017 Sep 13;358:j3927.
5. Strawn JR et al. J Child Adolesc Psychopharm. 2017 Feb;27(1): 29-37..
6. Geller D et al. J Am Acad Child Adolesc Psychiatry. 2007 Sep;46(9):1119-27.
7. Strawn JR et al. J Child Adolesc Psychopharm. 2017 Feb;28(1): 2-9.
Anxiety disorders, including separation anxiety disorder, social anxiety disorder, and generalized anxiety disorder, are some of the most common psychiatric conditions of childhood and adolescence, affecting up to 20% of youth.1 Patients commonly present with a mix of symptoms that often span multiple anxiety disorder diagnoses. While this pattern can present somewhat of a diagnostic conundrum, it can be reassuring to know that such constellations of symptoms are the rule rather than the exception. Further, given that both the pharmacologic and nonpharmacologic treatment strategies don’t change much among the various anxiety disorders, the lack of a definitive single diagnosis should not delay intervention. Be alert to the possibility that anxiety and anxiety disorders can be the engine that drives what on the surface appears to be more disruptive and oppositional behavior.
Although medications can be a useful part of treatment, they are not recommended as a stand-alone intervention. Nonpharmacologic treatments generally should be tried before medications are considered. Among the different types of psychotherapy, cognitive-behavioral therapy (CBT) has the most empirical support from research trials, although other modalities such as mindfulness-based treatments show some promise. As anxiety disorders often run in families, it also can be very useful to explore the possibility that one or more parents also struggle with an anxiety disorder, which, if untreated, might complicate the child’s course.
With regard to medications, it is being increasingly appreciated that, despite SSRIs being most popularly known as antidepressants, these medications actually may be as efficacious or even more efficacious in the management of anxiety disorders. This class remains the cornerstone of medication treatment, and a brief review of current options follows.
SSRIs and SNRIs
A 2015 meta-analysis that examined nine randomized controlled trials of SSRIs and serotonin and norepinephrine reuptake inhibitors (SNRIs) for pediatric anxiety disorders concluded that these agents provided a benefit of modest effect size. No significant increase in treatment-emergent suicidality was found, and the medications were generally well tolerated.2 This analysis also found some evidence that greater efficacy was related to a medication with more specific serotonergic properties, suggesting improved response with “true” SSRIs versus SNRIs such as venlafaxine and duloxetine. One major study using sertraline found that, at least in the short term, combined use of sertraline with CBT resulted in better efficacy than either treatment alone.3 Dosing of SSRIs should start low: A general rule is to begin at half of the smallest dosage made, depending on the age and size of the patient. One question that often comes up after a successful trial is how long to continue the medications. A recent meta-analysis in adults concluded that there was evidence that stopping medication prior to 1 year resulted in an increased risk of relapse with little to guide clinicians after that 1-year mark.4
Benzodiazepines
Even though benzodiazepines have been around for a long time, data supporting their efficacy and safety in pediatric populations remain extremely limited, and what has been reported has not been particularly positive. Thus, most experts do not suggest using benzodiazepines for anxiety disorders, with the exception of helping children through single or rare events, such as medical procedures or enabling an adolescent who has been fearful of attending school to get to the building on the first day back after a long absence.
Guanfacine
In a recent exploratory trial of guanfacine for children with mixed anxiety disorders,5 the medication was well tolerated overall but did not result in statistically significant improvement relative to placebo on primary anxiety rating scales. However, a higher number of children were rated as improved on a clinician-rated scale. This medication is usually started at 0.5 mg/day and increased as tolerated, while checking vital signs, to a maximum of 4 mg/day.
Atomoxetine
A randomized control trial of pediatric patients with both ADHD and an anxiety disorder showed reductions in both symptom domains with atomoxetine dosed at an average of 1.3 mg/kg per day.6 There is little evidence to suggest its use in primary anxiety disorders without comorbid ADHD.
Buspirone
This 5-hydroxytryptamine 1a agonist has Food and Drug Administration approval for generalized anxiety disorder in adults and is generally well tolerated. Unfortunately, two randomized controlled studies in children and adolescents did not find statistically significant improvement relative to placebo, although some methodological problems may have played a role.7
Antipsychotics
Although sometimes used to augment an SSRI in adult anxiety disorders, there are little data to support the use of antipsychotics in pediatric populations, especially given the antecedent risks of the drugs.
Summary
Pharmacotherapy for anxiety disorders often includes the advice that, if medications are indicated in conjunction with psychotherapy, to start with an SSRI; and if that is not effective to try a different one.7 An SNRI such as venlafaxine or duloxetine may then be a third-line alternative, although for youth with comorbid ADHD, consideration of either atomoxetine or guanfacine is also reasonable. Beyond that point, there unfortunately are little systematic data to guide pharmacologic decision making, and increased potential risks of other classes of medications suggest the need for caution and consultation.
Looking for more mental health training? Attend the 12th annual Child Psychiatry in Primary Care conference in Burlington, on May 4, 2018,organized by the University of Vermont with Dr. Rettew as course director. Go to http://www.med.uvm.edu/cme/conferences.
References
1. Merikangas KR et al. J Am Acad Child Adolesc Psychiatry. 2010 Oct;49(10):980-9.
2. Strawn JR et al. Depress Anxiety. 2015 Mar;32(3):149-57.
3. Walkup J et al. N Engl J Med. 2008 Dec 25;359(26):2753-66.
4. Batelaan N et al. BMJ. 2017 Sep 13;358:j3927.
5. Strawn JR et al. J Child Adolesc Psychopharm. 2017 Feb;27(1): 29-37..
6. Geller D et al. J Am Acad Child Adolesc Psychiatry. 2007 Sep;46(9):1119-27.
7. Strawn JR et al. J Child Adolesc Psychopharm. 2017 Feb;28(1): 2-9.
How to help children process, overcome horrific traumas
Unfathomable. Unspeakable.
These are among the terms used to describe children’s extreme traumatic experiences such as severe abuse and neglect. It is often most shocking when these acts are perpetrated by the children’s parents – the very ones that children should be able to depend on for their protection and safety.
Many believe that, in addition to the cumulative and serious nature of repetitive interpersonal traumas themselves, this betrayal of trust will result in irreparable psychological damage. Fortunately, this does not have to be the case. Children are more resilient than we realize; with safety, support, and effective treatment, they can recover from even the most extreme traumas and live healthy, productive lives.
and allow them to live in safe, stable, supportive settings while minimizing traumatic separation from siblings or further disruptions in their living situation. Acute medical problems need to be stabilized, and a thorough mental health assessment should be conducted.
Evidence-based trauma-focused psychotherapy is the first-line treatment for addressing pediatric posttraumatic stress disorder (J Am Acad Child Adolesc Psychiatry. 2010 Apr;49[4]:414-30). Several treatments are currently available. A few examples are trauma-focused cognitive-behavioral therapy, or TF-CBT, for children aged 3-18 years; child-parent psychotherapy (CPP) for children aged 0-6 years; a group school-based model, cognitive behavioral interventions for trauma in schools (CBITS); and Trauma Affect Regulation: Guide for Education and Therapy for teens (TARGET).
Common elements of evidence-based trauma-focused treatments are: 1) nonperpetrating caregivers are included in therapy to enhance support and understanding of the child’s trauma responses, and to address trauma-related behavioral problems; 2) skills are provided to the youth and caregiver for coping with negative trauma-related thoughts, feelings, and behaviors; and 3) children are supported to directly talk about and make meaning of their trauma experiences.
Through trauma-focused treatment, children become able to cope with their traumatic experiences and memories – make sense out of them. These traumas are no longer “unfathomable or “unspeakable,” but rather, manageable memories of bad experiences that the children have had the courage to face and master.
Children can recover from even extreme trauma experiences when they receive effective trauma-focused treatment in the context of a supportive environment. More information about evidence-based treatments is available from the National Child Traumatic Stress Network at www.nctsn.org/resources/topics/treatments-that-work/promising-practices.
Unfathomable. Unspeakable.
These are among the terms used to describe children’s extreme traumatic experiences such as severe abuse and neglect. It is often most shocking when these acts are perpetrated by the children’s parents – the very ones that children should be able to depend on for their protection and safety.
Many believe that, in addition to the cumulative and serious nature of repetitive interpersonal traumas themselves, this betrayal of trust will result in irreparable psychological damage. Fortunately, this does not have to be the case. Children are more resilient than we realize; with safety, support, and effective treatment, they can recover from even the most extreme traumas and live healthy, productive lives.
and allow them to live in safe, stable, supportive settings while minimizing traumatic separation from siblings or further disruptions in their living situation. Acute medical problems need to be stabilized, and a thorough mental health assessment should be conducted.
Evidence-based trauma-focused psychotherapy is the first-line treatment for addressing pediatric posttraumatic stress disorder (J Am Acad Child Adolesc Psychiatry. 2010 Apr;49[4]:414-30). Several treatments are currently available. A few examples are trauma-focused cognitive-behavioral therapy, or TF-CBT, for children aged 3-18 years; child-parent psychotherapy (CPP) for children aged 0-6 years; a group school-based model, cognitive behavioral interventions for trauma in schools (CBITS); and Trauma Affect Regulation: Guide for Education and Therapy for teens (TARGET).
Common elements of evidence-based trauma-focused treatments are: 1) nonperpetrating caregivers are included in therapy to enhance support and understanding of the child’s trauma responses, and to address trauma-related behavioral problems; 2) skills are provided to the youth and caregiver for coping with negative trauma-related thoughts, feelings, and behaviors; and 3) children are supported to directly talk about and make meaning of their trauma experiences.
Through trauma-focused treatment, children become able to cope with their traumatic experiences and memories – make sense out of them. These traumas are no longer “unfathomable or “unspeakable,” but rather, manageable memories of bad experiences that the children have had the courage to face and master.
Children can recover from even extreme trauma experiences when they receive effective trauma-focused treatment in the context of a supportive environment. More information about evidence-based treatments is available from the National Child Traumatic Stress Network at www.nctsn.org/resources/topics/treatments-that-work/promising-practices.
Unfathomable. Unspeakable.
These are among the terms used to describe children’s extreme traumatic experiences such as severe abuse and neglect. It is often most shocking when these acts are perpetrated by the children’s parents – the very ones that children should be able to depend on for their protection and safety.
Many believe that, in addition to the cumulative and serious nature of repetitive interpersonal traumas themselves, this betrayal of trust will result in irreparable psychological damage. Fortunately, this does not have to be the case. Children are more resilient than we realize; with safety, support, and effective treatment, they can recover from even the most extreme traumas and live healthy, productive lives.
and allow them to live in safe, stable, supportive settings while minimizing traumatic separation from siblings or further disruptions in their living situation. Acute medical problems need to be stabilized, and a thorough mental health assessment should be conducted.
Evidence-based trauma-focused psychotherapy is the first-line treatment for addressing pediatric posttraumatic stress disorder (J Am Acad Child Adolesc Psychiatry. 2010 Apr;49[4]:414-30). Several treatments are currently available. A few examples are trauma-focused cognitive-behavioral therapy, or TF-CBT, for children aged 3-18 years; child-parent psychotherapy (CPP) for children aged 0-6 years; a group school-based model, cognitive behavioral interventions for trauma in schools (CBITS); and Trauma Affect Regulation: Guide for Education and Therapy for teens (TARGET).
Common elements of evidence-based trauma-focused treatments are: 1) nonperpetrating caregivers are included in therapy to enhance support and understanding of the child’s trauma responses, and to address trauma-related behavioral problems; 2) skills are provided to the youth and caregiver for coping with negative trauma-related thoughts, feelings, and behaviors; and 3) children are supported to directly talk about and make meaning of their trauma experiences.
Through trauma-focused treatment, children become able to cope with their traumatic experiences and memories – make sense out of them. These traumas are no longer “unfathomable or “unspeakable,” but rather, manageable memories of bad experiences that the children have had the courage to face and master.
Children can recover from even extreme trauma experiences when they receive effective trauma-focused treatment in the context of a supportive environment. More information about evidence-based treatments is available from the National Child Traumatic Stress Network at www.nctsn.org/resources/topics/treatments-that-work/promising-practices.
Antibiotic choice for acute otitis media 2018
It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains. 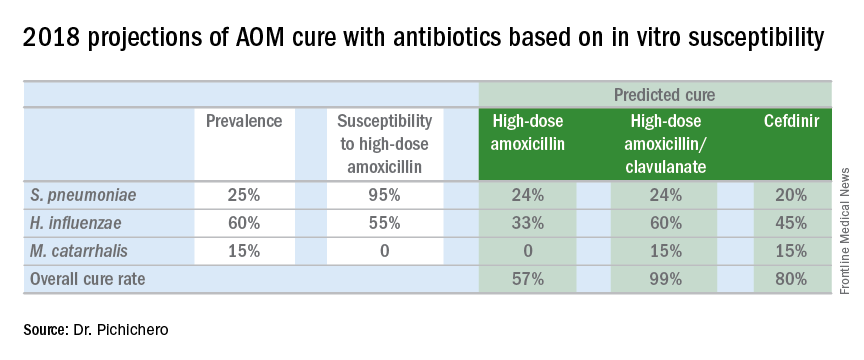
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.
It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains. 
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.
It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains. 
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.
The times they are a-changin’ —Bob Dylan, 1964
The beginning of a new year is always associated with changes, accompanied by new challenges and opportunities. This year is no different and, in fact, begins with some significant changes. First, I am incredibly honored, and humbled, to be named your new editor-in-chief. By way of background and introduction, I am residency-trained and board-certified in emergency medicine (EM). I founded the first academic department of EM in Virginia in 1992, and continue to serve in the role of chair. From 1990 to 2010, I served as the program director of our 3-year EM residency program, which I still consider the best job in EM. Most importantly, I continue to see and care for patients in the ED primarily, in addition to supervising and teaching EM residents and fellows in the delivery of care in the clinical arena. I know first-hand the needs of practicing emergency physicians (EPs).
I feel very fortunate to have been associated with Emergency Medicine (EM) since 1988, the year the journal published my very first manuscript. I served on the editorial board from 1999 to 2006, and for the past 11 years, have served as the associate editor-in-chief. I hold a very special regard and respect for this journal, and its role in our specialty. My goal is to continue to publish high-quality content and ensure we consistently provide timely and clinically useful information to the practicing EP. We will invite the very best in our specialty to share their knowledge and clinical tips. We will of course continue some of your favorite sections, like “Emergency Ultrasound,” “Diagnosis at a Glance,” and “Case Studies in Toxicology.” We will also encourage our readers to submit interesting and informative case reports, review articles, and interesting images. While I plan to write a few editorials each year, I will invite thought leaders in EM to write on their area(s) of expertise.
Come writers and critics who prophesize with your pen.
Another major change has to do with the journal itself. This will be the last paper copy of EM (so think about keeping this one for posterity, or eBay). Starting with the February issue, all future issues will be digital and online-only. This decision was not an easy one, and has been in the making for some time. Thanks to the growth in our Web site traffic, it is clear that many of you have already become “digital-first” readers. This fact, combined with the added financial challenge of publishing a large-circulation journal within an environment of declining print advertising, convinced us that this is the right time to make the leap to the digital-only format. While some of you (including myself), will miss physically holding and reading a hard copy of EM, you may simply continue to access the journal as you have for years, on your desktop, laptop, or iPad, and never further away than your cell phone. This change has the advantage of providing opportunities to deliver valuable clinical content in new ways, through increased use of audio and video, as well as text. To ensure that you receive your copy, please e-mail our Editor, Kellie DeSantis ([email protected]) to make sure we have your correct and preferred e-mail address. While our goal is to push each issue out to you via e-mail, you will always be able to access the most recent articles by going to our Web site, www.emed-journal.com.
And don’t criticize
What you can’t understand.
Finally, 2018 promises to be a very interesting year, with the unknown implications of tax reform, the repeal of the individual mandate for health insurance, the opioid crisis, and the curious mergers within the health insurance industry (ie, CVS and Aetna). It is too soon for anyone to say how these changes will affect EM on the national stage. What will not change however, is that EPs will continue to provide outstanding care to any and every patient who presents to the ED. Emergency physicians will ensure that all patients receive the care they need (but not necessarily the care they want) and will do so without regard to gender, religion, national origin, race, age, sexual preference, or insurance status.
I wish each and every one of you a happy and healthy 2018.
The beginning of a new year is always associated with changes, accompanied by new challenges and opportunities. This year is no different and, in fact, begins with some significant changes. First, I am incredibly honored, and humbled, to be named your new editor-in-chief. By way of background and introduction, I am residency-trained and board-certified in emergency medicine (EM). I founded the first academic department of EM in Virginia in 1992, and continue to serve in the role of chair. From 1990 to 2010, I served as the program director of our 3-year EM residency program, which I still consider the best job in EM. Most importantly, I continue to see and care for patients in the ED primarily, in addition to supervising and teaching EM residents and fellows in the delivery of care in the clinical arena. I know first-hand the needs of practicing emergency physicians (EPs).
I feel very fortunate to have been associated with Emergency Medicine (EM) since 1988, the year the journal published my very first manuscript. I served on the editorial board from 1999 to 2006, and for the past 11 years, have served as the associate editor-in-chief. I hold a very special regard and respect for this journal, and its role in our specialty. My goal is to continue to publish high-quality content and ensure we consistently provide timely and clinically useful information to the practicing EP. We will invite the very best in our specialty to share their knowledge and clinical tips. We will of course continue some of your favorite sections, like “Emergency Ultrasound,” “Diagnosis at a Glance,” and “Case Studies in Toxicology.” We will also encourage our readers to submit interesting and informative case reports, review articles, and interesting images. While I plan to write a few editorials each year, I will invite thought leaders in EM to write on their area(s) of expertise.
Come writers and critics who prophesize with your pen.
Another major change has to do with the journal itself. This will be the last paper copy of EM (so think about keeping this one for posterity, or eBay). Starting with the February issue, all future issues will be digital and online-only. This decision was not an easy one, and has been in the making for some time. Thanks to the growth in our Web site traffic, it is clear that many of you have already become “digital-first” readers. This fact, combined with the added financial challenge of publishing a large-circulation journal within an environment of declining print advertising, convinced us that this is the right time to make the leap to the digital-only format. While some of you (including myself), will miss physically holding and reading a hard copy of EM, you may simply continue to access the journal as you have for years, on your desktop, laptop, or iPad, and never further away than your cell phone. This change has the advantage of providing opportunities to deliver valuable clinical content in new ways, through increased use of audio and video, as well as text. To ensure that you receive your copy, please e-mail our Editor, Kellie DeSantis ([email protected]) to make sure we have your correct and preferred e-mail address. While our goal is to push each issue out to you via e-mail, you will always be able to access the most recent articles by going to our Web site, www.emed-journal.com.
And don’t criticize
What you can’t understand.
Finally, 2018 promises to be a very interesting year, with the unknown implications of tax reform, the repeal of the individual mandate for health insurance, the opioid crisis, and the curious mergers within the health insurance industry (ie, CVS and Aetna). It is too soon for anyone to say how these changes will affect EM on the national stage. What will not change however, is that EPs will continue to provide outstanding care to any and every patient who presents to the ED. Emergency physicians will ensure that all patients receive the care they need (but not necessarily the care they want) and will do so without regard to gender, religion, national origin, race, age, sexual preference, or insurance status.
I wish each and every one of you a happy and healthy 2018.
The beginning of a new year is always associated with changes, accompanied by new challenges and opportunities. This year is no different and, in fact, begins with some significant changes. First, I am incredibly honored, and humbled, to be named your new editor-in-chief. By way of background and introduction, I am residency-trained and board-certified in emergency medicine (EM). I founded the first academic department of EM in Virginia in 1992, and continue to serve in the role of chair. From 1990 to 2010, I served as the program director of our 3-year EM residency program, which I still consider the best job in EM. Most importantly, I continue to see and care for patients in the ED primarily, in addition to supervising and teaching EM residents and fellows in the delivery of care in the clinical arena. I know first-hand the needs of practicing emergency physicians (EPs).
I feel very fortunate to have been associated with Emergency Medicine (EM) since 1988, the year the journal published my very first manuscript. I served on the editorial board from 1999 to 2006, and for the past 11 years, have served as the associate editor-in-chief. I hold a very special regard and respect for this journal, and its role in our specialty. My goal is to continue to publish high-quality content and ensure we consistently provide timely and clinically useful information to the practicing EP. We will invite the very best in our specialty to share their knowledge and clinical tips. We will of course continue some of your favorite sections, like “Emergency Ultrasound,” “Diagnosis at a Glance,” and “Case Studies in Toxicology.” We will also encourage our readers to submit interesting and informative case reports, review articles, and interesting images. While I plan to write a few editorials each year, I will invite thought leaders in EM to write on their area(s) of expertise.
Come writers and critics who prophesize with your pen.
Another major change has to do with the journal itself. This will be the last paper copy of EM (so think about keeping this one for posterity, or eBay). Starting with the February issue, all future issues will be digital and online-only. This decision was not an easy one, and has been in the making for some time. Thanks to the growth in our Web site traffic, it is clear that many of you have already become “digital-first” readers. This fact, combined with the added financial challenge of publishing a large-circulation journal within an environment of declining print advertising, convinced us that this is the right time to make the leap to the digital-only format. While some of you (including myself), will miss physically holding and reading a hard copy of EM, you may simply continue to access the journal as you have for years, on your desktop, laptop, or iPad, and never further away than your cell phone. This change has the advantage of providing opportunities to deliver valuable clinical content in new ways, through increased use of audio and video, as well as text. To ensure that you receive your copy, please e-mail our Editor, Kellie DeSantis ([email protected]) to make sure we have your correct and preferred e-mail address. While our goal is to push each issue out to you via e-mail, you will always be able to access the most recent articles by going to our Web site, www.emed-journal.com.
And don’t criticize
What you can’t understand.
Finally, 2018 promises to be a very interesting year, with the unknown implications of tax reform, the repeal of the individual mandate for health insurance, the opioid crisis, and the curious mergers within the health insurance industry (ie, CVS and Aetna). It is too soon for anyone to say how these changes will affect EM on the national stage. What will not change however, is that EPs will continue to provide outstanding care to any and every patient who presents to the ED. Emergency physicians will ensure that all patients receive the care they need (but not necessarily the care they want) and will do so without regard to gender, religion, national origin, race, age, sexual preference, or insurance status.
I wish each and every one of you a happy and healthy 2018.
Hungry or what?
“She will eat when she is hungry.” That in so many words is the mantra of grandparents blessed with experience and common sense and of most pediatricians when consulting parents challenged with a picky eater. From birth, children understand the simple equation that to survive they must eat. With rare exception, the motivating power of hunger can be leveraged for success even with infants who have spent their first months relying on enteral feedings. I have written an entire book based solely on the premise that if you present a young child food she will eat it ... eventually (“Coping With a Picky Eater: A Guide for the Perplexed Parent” New York: Simon and Schuster, 1998).
But if we reverse the words to read, “When she is eating, she is hungry,” do we have an equally valid observation? I think we have ample evidence that it is not.
The result was a year-long odyssey of pumping that included consultations with five different lactation consultants in the first frustrating month and a half. She eventually received some comforting advice from a pediatrician who reassured her that there was little research to guide her and to “just feed him; trust your instincts.”
While it is unfortunately true that there is very little good science we can fall back on when counseling women who are struggling with breastfeeding, I wonder about the wisdom of telling this mother to trust her instincts. I guess my hesitancy is based on 40 years of primary care pediatrics in which I could generally count on the instincts of young children, but their parents’ not so much. While maternal intuition is generally superior to the paternal version, I am hesitant to rely totally on either when facing a clinical dilemma such as defining hunger.
What about the fussy baby who is comforted by just a pacifier? What is the difference? There are several explanations, but it will require introducing the concept of nutrition deficiency.
Most babies who are satisfied with just a nipple, be it silicone or flesh, simply find sucking a comfort measure. A few, and I am sure you have seen some of them, are overly patient. They seem to be saying, “I need the calories, but you’re a good mom and I enjoy sucking. I can wait. Some day, you may make more milk or give me a bottle.” In the worst-case scenarios, their patience leaves these babies so nutritionally deficient that they can slip into apathy and die.
There are no easy answers. As pediatricians, our job is to sort out those fussy “hungry” babies whose behavior means they are overtired from those who are nutritionally deficient, from those with a dysfunctional satiety center. Making the differentiation is difficult but much easier than helping parents ignore one of their instincts.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
“She will eat when she is hungry.” That in so many words is the mantra of grandparents blessed with experience and common sense and of most pediatricians when consulting parents challenged with a picky eater. From birth, children understand the simple equation that to survive they must eat. With rare exception, the motivating power of hunger can be leveraged for success even with infants who have spent their first months relying on enteral feedings. I have written an entire book based solely on the premise that if you present a young child food she will eat it ... eventually (“Coping With a Picky Eater: A Guide for the Perplexed Parent” New York: Simon and Schuster, 1998).
But if we reverse the words to read, “When she is eating, she is hungry,” do we have an equally valid observation? I think we have ample evidence that it is not.
The result was a year-long odyssey of pumping that included consultations with five different lactation consultants in the first frustrating month and a half. She eventually received some comforting advice from a pediatrician who reassured her that there was little research to guide her and to “just feed him; trust your instincts.”
While it is unfortunately true that there is very little good science we can fall back on when counseling women who are struggling with breastfeeding, I wonder about the wisdom of telling this mother to trust her instincts. I guess my hesitancy is based on 40 years of primary care pediatrics in which I could generally count on the instincts of young children, but their parents’ not so much. While maternal intuition is generally superior to the paternal version, I am hesitant to rely totally on either when facing a clinical dilemma such as defining hunger.
What about the fussy baby who is comforted by just a pacifier? What is the difference? There are several explanations, but it will require introducing the concept of nutrition deficiency.
Most babies who are satisfied with just a nipple, be it silicone or flesh, simply find sucking a comfort measure. A few, and I am sure you have seen some of them, are overly patient. They seem to be saying, “I need the calories, but you’re a good mom and I enjoy sucking. I can wait. Some day, you may make more milk or give me a bottle.” In the worst-case scenarios, their patience leaves these babies so nutritionally deficient that they can slip into apathy and die.
There are no easy answers. As pediatricians, our job is to sort out those fussy “hungry” babies whose behavior means they are overtired from those who are nutritionally deficient, from those with a dysfunctional satiety center. Making the differentiation is difficult but much easier than helping parents ignore one of their instincts.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
“She will eat when she is hungry.” That in so many words is the mantra of grandparents blessed with experience and common sense and of most pediatricians when consulting parents challenged with a picky eater. From birth, children understand the simple equation that to survive they must eat. With rare exception, the motivating power of hunger can be leveraged for success even with infants who have spent their first months relying on enteral feedings. I have written an entire book based solely on the premise that if you present a young child food she will eat it ... eventually (“Coping With a Picky Eater: A Guide for the Perplexed Parent” New York: Simon and Schuster, 1998).
But if we reverse the words to read, “When she is eating, she is hungry,” do we have an equally valid observation? I think we have ample evidence that it is not.
The result was a year-long odyssey of pumping that included consultations with five different lactation consultants in the first frustrating month and a half. She eventually received some comforting advice from a pediatrician who reassured her that there was little research to guide her and to “just feed him; trust your instincts.”
While it is unfortunately true that there is very little good science we can fall back on when counseling women who are struggling with breastfeeding, I wonder about the wisdom of telling this mother to trust her instincts. I guess my hesitancy is based on 40 years of primary care pediatrics in which I could generally count on the instincts of young children, but their parents’ not so much. While maternal intuition is generally superior to the paternal version, I am hesitant to rely totally on either when facing a clinical dilemma such as defining hunger.
What about the fussy baby who is comforted by just a pacifier? What is the difference? There are several explanations, but it will require introducing the concept of nutrition deficiency.
Most babies who are satisfied with just a nipple, be it silicone or flesh, simply find sucking a comfort measure. A few, and I am sure you have seen some of them, are overly patient. They seem to be saying, “I need the calories, but you’re a good mom and I enjoy sucking. I can wait. Some day, you may make more milk or give me a bottle.” In the worst-case scenarios, their patience leaves these babies so nutritionally deficient that they can slip into apathy and die.
There are no easy answers. As pediatricians, our job is to sort out those fussy “hungry” babies whose behavior means they are overtired from those who are nutritionally deficient, from those with a dysfunctional satiety center. Making the differentiation is difficult but much easier than helping parents ignore one of their instincts.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Beware the COPD exacerbation
A 70-year-old man with chronic obstructive pulmonary disease (COPD) is admitted with increased shortness of breath. His O2 saturation levels are usually 90%, but they’re now running 84%-88%. He has had increasing symptoms for the past 3 days.

What would be your next step?
A) Begin a 5-day course of corticosteroids.
B) Begin a 14-day course of corticosteroids.
C) Begin azithromycin.
D) Start BiPAP.
E) Obtain D-dimer.
This is a situation we face frequently. COPD exacerbations are a clinical diagnosis that is often jumped to as the diagnosis in patients with COPD who have increasing dyspnea. This diagnosis is frequently correct – but not always.
Patients with COPD also may be at risk for or have heart failure, which can present with identical symptoms, including widespread wheezing. We are currently in a severe influenza epidemic, and influenza can mimic a COPD exacerbation or be the trigger.
About 20 years ago, I was out of the country when one of my patients with COPD was admitted to the hospital with a COPD exacerbation. I saw him in follow-up a week after his hospitalization. He was very dyspneic and had a room air oxygen saturation of 75%. He told me his dyspnea started a few days after he had injured his leg on a wood pile in his yard.
On exam, his right leg had 3+ edema; left leg, no edema. He reported to me that he was treated for 5 days with steroids and nebulizers, with minimal change in his dyspnea. I reviewed the chart, and five physicians had seen him while he was in the hospital. Starting with the emergency department, the diagnosis was COPD exacerbation, with no differential diagnosis in any note.
The patient had multiple pulmonary emboli, and he eventually improved with anticoagulation.
In 2009, Jacques Rizkallah, MD, and his colleagues published a systematic review and meta-analysis of articles looking at the prevalence of pulmonary emboli (PE) in patients diagnosed/treated for a COPD exacerbation.1 They found five articles comprising a total of 550 patients who met inclusion criteria. The prevalence was 19.9% (P = .014). The prevalence was much higher (24.7%) for hospitalized patients than it was for outpatients (3.3%). A very important finding in this study: There was no difference in symptoms between patients who did and did not have a pulmonary embolus.
Evrim Eylem Akpinar, MD, and colleagues studied all admissions for acute exacerbations of COPD at one hospital in Turkey over a 2-year period.2 A total of 172 patients admitted for COPD exacerbations were studied. The prevalence of pulmonary embolus was 29%.
In this study, patients who were obese or immobile were more likely to have pulmonary emboli. Pleuritic chest pain and lower-limb asymmetry were signs and symptoms more commonly found in patients who had PE. Obesity was the highest independent predictor (odds ratio, 4.97) for pulmonary embolus.
Floor Aleva, MD, and colleagues recently completed a systematic review and meta-analysis on prevalence and localization of pulmonary embolus in patients with acute exacerbations of COPD.3 They found similar numbers to the previous meta-analysis (16.1%) in a total of 880 patients. They also looked at location in the lungs of the emboli and found that two-thirds of the patients had pulmonary emboli in locations that had clear indication for anticoagulation treatment.
This is important, because criticisms of earlier studies were that clinically insignificant pulmonary emboli might be being found in the studies and that they had little to do with the patients’ symptoms.
In the case presented, I think that getting a D-dimer test would be the next best step. Acute exacerbation of COPD still is the most likely diagnosis, but PE is a plausible diagnosis that should be evaluated. If the D-dimer is normal, workup for PE would be complete. If elevated, then given the 20% prevalence of PE, a CT angiography would be warranted.
Key pearl: Among patients hospitalized for COPD exacerbations, 16%-24% have pulmonary embolism.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Chest. 2009 Mar;135(3):786-93.
2. J Bras Pneumol. 2014 Jan-Feb;40(1):38-45.
3. Chest. 2017 Mar;151(3):544-54.
A 70-year-old man with chronic obstructive pulmonary disease (COPD) is admitted with increased shortness of breath. His O2 saturation levels are usually 90%, but they’re now running 84%-88%. He has had increasing symptoms for the past 3 days.

What would be your next step?
A) Begin a 5-day course of corticosteroids.
B) Begin a 14-day course of corticosteroids.
C) Begin azithromycin.
D) Start BiPAP.
E) Obtain D-dimer.
This is a situation we face frequently. COPD exacerbations are a clinical diagnosis that is often jumped to as the diagnosis in patients with COPD who have increasing dyspnea. This diagnosis is frequently correct – but not always.
Patients with COPD also may be at risk for or have heart failure, which can present with identical symptoms, including widespread wheezing. We are currently in a severe influenza epidemic, and influenza can mimic a COPD exacerbation or be the trigger.
About 20 years ago, I was out of the country when one of my patients with COPD was admitted to the hospital with a COPD exacerbation. I saw him in follow-up a week after his hospitalization. He was very dyspneic and had a room air oxygen saturation of 75%. He told me his dyspnea started a few days after he had injured his leg on a wood pile in his yard.
On exam, his right leg had 3+ edema; left leg, no edema. He reported to me that he was treated for 5 days with steroids and nebulizers, with minimal change in his dyspnea. I reviewed the chart, and five physicians had seen him while he was in the hospital. Starting with the emergency department, the diagnosis was COPD exacerbation, with no differential diagnosis in any note.
The patient had multiple pulmonary emboli, and he eventually improved with anticoagulation.
In 2009, Jacques Rizkallah, MD, and his colleagues published a systematic review and meta-analysis of articles looking at the prevalence of pulmonary emboli (PE) in patients diagnosed/treated for a COPD exacerbation.1 They found five articles comprising a total of 550 patients who met inclusion criteria. The prevalence was 19.9% (P = .014). The prevalence was much higher (24.7%) for hospitalized patients than it was for outpatients (3.3%). A very important finding in this study: There was no difference in symptoms between patients who did and did not have a pulmonary embolus.
Evrim Eylem Akpinar, MD, and colleagues studied all admissions for acute exacerbations of COPD at one hospital in Turkey over a 2-year period.2 A total of 172 patients admitted for COPD exacerbations were studied. The prevalence of pulmonary embolus was 29%.
In this study, patients who were obese or immobile were more likely to have pulmonary emboli. Pleuritic chest pain and lower-limb asymmetry were signs and symptoms more commonly found in patients who had PE. Obesity was the highest independent predictor (odds ratio, 4.97) for pulmonary embolus.
Floor Aleva, MD, and colleagues recently completed a systematic review and meta-analysis on prevalence and localization of pulmonary embolus in patients with acute exacerbations of COPD.3 They found similar numbers to the previous meta-analysis (16.1%) in a total of 880 patients. They also looked at location in the lungs of the emboli and found that two-thirds of the patients had pulmonary emboli in locations that had clear indication for anticoagulation treatment.
This is important, because criticisms of earlier studies were that clinically insignificant pulmonary emboli might be being found in the studies and that they had little to do with the patients’ symptoms.
In the case presented, I think that getting a D-dimer test would be the next best step. Acute exacerbation of COPD still is the most likely diagnosis, but PE is a plausible diagnosis that should be evaluated. If the D-dimer is normal, workup for PE would be complete. If elevated, then given the 20% prevalence of PE, a CT angiography would be warranted.
Key pearl: Among patients hospitalized for COPD exacerbations, 16%-24% have pulmonary embolism.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Chest. 2009 Mar;135(3):786-93.
2. J Bras Pneumol. 2014 Jan-Feb;40(1):38-45.
3. Chest. 2017 Mar;151(3):544-54.
A 70-year-old man with chronic obstructive pulmonary disease (COPD) is admitted with increased shortness of breath. His O2 saturation levels are usually 90%, but they’re now running 84%-88%. He has had increasing symptoms for the past 3 days.

What would be your next step?
A) Begin a 5-day course of corticosteroids.
B) Begin a 14-day course of corticosteroids.
C) Begin azithromycin.
D) Start BiPAP.
E) Obtain D-dimer.
This is a situation we face frequently. COPD exacerbations are a clinical diagnosis that is often jumped to as the diagnosis in patients with COPD who have increasing dyspnea. This diagnosis is frequently correct – but not always.
Patients with COPD also may be at risk for or have heart failure, which can present with identical symptoms, including widespread wheezing. We are currently in a severe influenza epidemic, and influenza can mimic a COPD exacerbation or be the trigger.
About 20 years ago, I was out of the country when one of my patients with COPD was admitted to the hospital with a COPD exacerbation. I saw him in follow-up a week after his hospitalization. He was very dyspneic and had a room air oxygen saturation of 75%. He told me his dyspnea started a few days after he had injured his leg on a wood pile in his yard.
On exam, his right leg had 3+ edema; left leg, no edema. He reported to me that he was treated for 5 days with steroids and nebulizers, with minimal change in his dyspnea. I reviewed the chart, and five physicians had seen him while he was in the hospital. Starting with the emergency department, the diagnosis was COPD exacerbation, with no differential diagnosis in any note.
The patient had multiple pulmonary emboli, and he eventually improved with anticoagulation.
In 2009, Jacques Rizkallah, MD, and his colleagues published a systematic review and meta-analysis of articles looking at the prevalence of pulmonary emboli (PE) in patients diagnosed/treated for a COPD exacerbation.1 They found five articles comprising a total of 550 patients who met inclusion criteria. The prevalence was 19.9% (P = .014). The prevalence was much higher (24.7%) for hospitalized patients than it was for outpatients (3.3%). A very important finding in this study: There was no difference in symptoms between patients who did and did not have a pulmonary embolus.
Evrim Eylem Akpinar, MD, and colleagues studied all admissions for acute exacerbations of COPD at one hospital in Turkey over a 2-year period.2 A total of 172 patients admitted for COPD exacerbations were studied. The prevalence of pulmonary embolus was 29%.
In this study, patients who were obese or immobile were more likely to have pulmonary emboli. Pleuritic chest pain and lower-limb asymmetry were signs and symptoms more commonly found in patients who had PE. Obesity was the highest independent predictor (odds ratio, 4.97) for pulmonary embolus.
Floor Aleva, MD, and colleagues recently completed a systematic review and meta-analysis on prevalence and localization of pulmonary embolus in patients with acute exacerbations of COPD.3 They found similar numbers to the previous meta-analysis (16.1%) in a total of 880 patients. They also looked at location in the lungs of the emboli and found that two-thirds of the patients had pulmonary emboli in locations that had clear indication for anticoagulation treatment.
This is important, because criticisms of earlier studies were that clinically insignificant pulmonary emboli might be being found in the studies and that they had little to do with the patients’ symptoms.
In the case presented, I think that getting a D-dimer test would be the next best step. Acute exacerbation of COPD still is the most likely diagnosis, but PE is a plausible diagnosis that should be evaluated. If the D-dimer is normal, workup for PE would be complete. If elevated, then given the 20% prevalence of PE, a CT angiography would be warranted.
Key pearl: Among patients hospitalized for COPD exacerbations, 16%-24% have pulmonary embolism.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Chest. 2009 Mar;135(3):786-93.
2. J Bras Pneumol. 2014 Jan-Feb;40(1):38-45.
3. Chest. 2017 Mar;151(3):544-54.
Psychiatry Innovation Lab nurtures young businesses
The future of mental health is in our own hands. As psychiatrists, we have a wealth of experience to fuel transformation in our field and improve care for our patients. What we lack is the space in which to share our ideas and turn them into tangible solutions like sustainable businesses, diagnostic devices, mobile apps, or prevention campaigns.
The Psychiatry Innovation Lab provides just that – it is an incubator nurturing early-stage ideas and ventures by investing in them through mentorship, education, funding, and collaboration opportunities within a community of mental health innovators.
The lab’s main event is on Sunday, May 6, 2018, when leaders in psychiatry will gather alongside industry leaders at the Jacob Javits Convention Center in New York City during the American Psychiatric Association meeting for this fourth annual live event . At the event, finalists will pitch ideas “Shark Tank” style to a panel of expert judges. Finalists will work closely with industry leaders and audience members to fine-tune their ideas before pitching their revisions to the judges as well as to a live, voting audience, who together select the winners of cash awards and other prizes.
The Psychiatry Innovation Lab was founded in 2015 by Nina Vasan, MD, a Stanford (Calif.) University psychiatrist and author of Do Good Well: Your Guide to Leadership, Action, and Social Innovation (San Francisco: Jossey-Bass, 2013), with the purpose of catalyzing more entrepreneurship and innovation in mental health.
“The most meaningful part of the lab has been the impact that these ventures have had on the patients that we serve,” said Dr. Vasan, chair of the Psychiatry Innovation Lab and director of Brainstorm, the Stanford Laboratory for Brain Health Innovation and Entrepreneurship. “Year after year, psychiatrists come back and tell us that they are looking at their own patient care differently now. They’re looking for ways to be innovative, and we’re giving them the tools necessary to turn their clinical expertise into successful ventures.”
Applications are currently open online to pitch an idea in this year’s lab. Applications consist of brief answers to questions and a short YouTube video. The deadline for submissions is Friday, March 9, 2018. Anyone with innovative ideas, regardless of the stage, is invited to apply. The APA Workgroup on Psychiatry Innovation, which oversees the lab, welcomes ideas from people pitching for the first time to those working on their ideas full time. They noted that past finalists have come from all experience levels and a wide range of backgrounds, including students, CEOs, engineers, and psychiatrists.
Past winners and participants have come from a range of stages, from early start-ups to established companies, including NeuroLex Labs, which uses voice samples to detect behavioral health illness; Spring, which uses machine learning to determine the best depression treatment; Muse, which creates an EEG sensing headband to aid mindfulness practice; and Overdose Recovery Bracelet, which builds a wristband to deliver naloxone to people who have overdosed on opiates.
The diversity of ideas has been remarkable; ideas in the past have ranged from traditional for-profit businesses to nonprofits, educational and public health programs, and proposals for government reform. They address problems at all parts of the health care spectrum: prevention, diagnosis, treatment, systems, EMRs, and more.
“Many past ideas have revolved around technology such as artificial intelligence, social networking, or telemedicine,” Dr. Vasan said. “. Those ideas are especially important in solving the most pressing problems in our field today.”
In addition to those competing, anyone registered to attend the APA annual meeting can attend the event as an audience member or join a team and help as an innovation leader during the coaching portion of the program. Last year alone, more than 70% of the participants in the lab said that it was better than other educational events they have attended, 95% of participants found it useful, and participants made an average of six new contacts through the event.
“The Psychiatry Innovation Lab connected me to people who continue to inspire and educate me toward making a real impact with my own endeavors,” said Cody Rall, MD, founder of the YouTube channel Techforpsych and a 2016 semifinalist.
“The lab has grown dramatically. When we started we worked hard to find just one sponsor; last year we had 10 sponsors and over $25,000 in cash and prizes.” Dr. Vasan said. “We also had our first international finalist from Pakistan. The commitment to solving problems in mental health is taking off, and we want to ensure that psychiatrists are front and center in leading the change.”
For more information about joining the innovation lab or other ways to be involved, including sponsorship and judging, visit https://www.psychiatry.org/innovation or email Nina Taylor, APA deputy director of education, at [email protected].
This article was updated January 23, 2018.
Dr. Chaudhary is a board member of the APA Psychiatry Innovation Lab and the APA Workgroup on Psychiatry Innovation; chief resident in child and adolescent psychiatry at Massachusetts General Hospital/McLean and clinical fellow at Harvard Medical School, both in Boston; and founding partner of Brainstorm: Stanford Laboratory for Brain Health Innovation and Entrepreneurship. Dr. Ghomi is a board member of the APA Psychiatry Innovation Lab and the APA Workgroup on Psychiatry Innovation; resident in psychiatry at the University of Washington, Seattle; chief medical officer at NeuroLex; and founding partner of Brainstorm: Stanford Laboratory for Brain Health Innovation and Entrepreneurship.
The future of mental health is in our own hands. As psychiatrists, we have a wealth of experience to fuel transformation in our field and improve care for our patients. What we lack is the space in which to share our ideas and turn them into tangible solutions like sustainable businesses, diagnostic devices, mobile apps, or prevention campaigns.
The Psychiatry Innovation Lab provides just that – it is an incubator nurturing early-stage ideas and ventures by investing in them through mentorship, education, funding, and collaboration opportunities within a community of mental health innovators.
The lab’s main event is on Sunday, May 6, 2018, when leaders in psychiatry will gather alongside industry leaders at the Jacob Javits Convention Center in New York City during the American Psychiatric Association meeting for this fourth annual live event . At the event, finalists will pitch ideas “Shark Tank” style to a panel of expert judges. Finalists will work closely with industry leaders and audience members to fine-tune their ideas before pitching their revisions to the judges as well as to a live, voting audience, who together select the winners of cash awards and other prizes.
The Psychiatry Innovation Lab was founded in 2015 by Nina Vasan, MD, a Stanford (Calif.) University psychiatrist and author of Do Good Well: Your Guide to Leadership, Action, and Social Innovation (San Francisco: Jossey-Bass, 2013), with the purpose of catalyzing more entrepreneurship and innovation in mental health.
“The most meaningful part of the lab has been the impact that these ventures have had on the patients that we serve,” said Dr. Vasan, chair of the Psychiatry Innovation Lab and director of Brainstorm, the Stanford Laboratory for Brain Health Innovation and Entrepreneurship. “Year after year, psychiatrists come back and tell us that they are looking at their own patient care differently now. They’re looking for ways to be innovative, and we’re giving them the tools necessary to turn their clinical expertise into successful ventures.”
Applications are currently open online to pitch an idea in this year’s lab. Applications consist of brief answers to questions and a short YouTube video. The deadline for submissions is Friday, March 9, 2018. Anyone with innovative ideas, regardless of the stage, is invited to apply. The APA Workgroup on Psychiatry Innovation, which oversees the lab, welcomes ideas from people pitching for the first time to those working on their ideas full time. They noted that past finalists have come from all experience levels and a wide range of backgrounds, including students, CEOs, engineers, and psychiatrists.
Past winners and participants have come from a range of stages, from early start-ups to established companies, including NeuroLex Labs, which uses voice samples to detect behavioral health illness; Spring, which uses machine learning to determine the best depression treatment; Muse, which creates an EEG sensing headband to aid mindfulness practice; and Overdose Recovery Bracelet, which builds a wristband to deliver naloxone to people who have overdosed on opiates.
The diversity of ideas has been remarkable; ideas in the past have ranged from traditional for-profit businesses to nonprofits, educational and public health programs, and proposals for government reform. They address problems at all parts of the health care spectrum: prevention, diagnosis, treatment, systems, EMRs, and more.
“Many past ideas have revolved around technology such as artificial intelligence, social networking, or telemedicine,” Dr. Vasan said. “. Those ideas are especially important in solving the most pressing problems in our field today.”
In addition to those competing, anyone registered to attend the APA annual meeting can attend the event as an audience member or join a team and help as an innovation leader during the coaching portion of the program. Last year alone, more than 70% of the participants in the lab said that it was better than other educational events they have attended, 95% of participants found it useful, and participants made an average of six new contacts through the event.
“The Psychiatry Innovation Lab connected me to people who continue to inspire and educate me toward making a real impact with my own endeavors,” said Cody Rall, MD, founder of the YouTube channel Techforpsych and a 2016 semifinalist.
“The lab has grown dramatically. When we started we worked hard to find just one sponsor; last year we had 10 sponsors and over $25,000 in cash and prizes.” Dr. Vasan said. “We also had our first international finalist from Pakistan. The commitment to solving problems in mental health is taking off, and we want to ensure that psychiatrists are front and center in leading the change.”
For more information about joining the innovation lab or other ways to be involved, including sponsorship and judging, visit https://www.psychiatry.org/innovation or email Nina Taylor, APA deputy director of education, at [email protected].
This article was updated January 23, 2018.
Dr. Chaudhary is a board member of the APA Psychiatry Innovation Lab and the APA Workgroup on Psychiatry Innovation; chief resident in child and adolescent psychiatry at Massachusetts General Hospital/McLean and clinical fellow at Harvard Medical School, both in Boston; and founding partner of Brainstorm: Stanford Laboratory for Brain Health Innovation and Entrepreneurship. Dr. Ghomi is a board member of the APA Psychiatry Innovation Lab and the APA Workgroup on Psychiatry Innovation; resident in psychiatry at the University of Washington, Seattle; chief medical officer at NeuroLex; and founding partner of Brainstorm: Stanford Laboratory for Brain Health Innovation and Entrepreneurship.
The future of mental health is in our own hands. As psychiatrists, we have a wealth of experience to fuel transformation in our field and improve care for our patients. What we lack is the space in which to share our ideas and turn them into tangible solutions like sustainable businesses, diagnostic devices, mobile apps, or prevention campaigns.
The Psychiatry Innovation Lab provides just that – it is an incubator nurturing early-stage ideas and ventures by investing in them through mentorship, education, funding, and collaboration opportunities within a community of mental health innovators.
The lab’s main event is on Sunday, May 6, 2018, when leaders in psychiatry will gather alongside industry leaders at the Jacob Javits Convention Center in New York City during the American Psychiatric Association meeting for this fourth annual live event . At the event, finalists will pitch ideas “Shark Tank” style to a panel of expert judges. Finalists will work closely with industry leaders and audience members to fine-tune their ideas before pitching their revisions to the judges as well as to a live, voting audience, who together select the winners of cash awards and other prizes.
The Psychiatry Innovation Lab was founded in 2015 by Nina Vasan, MD, a Stanford (Calif.) University psychiatrist and author of Do Good Well: Your Guide to Leadership, Action, and Social Innovation (San Francisco: Jossey-Bass, 2013), with the purpose of catalyzing more entrepreneurship and innovation in mental health.
“The most meaningful part of the lab has been the impact that these ventures have had on the patients that we serve,” said Dr. Vasan, chair of the Psychiatry Innovation Lab and director of Brainstorm, the Stanford Laboratory for Brain Health Innovation and Entrepreneurship. “Year after year, psychiatrists come back and tell us that they are looking at their own patient care differently now. They’re looking for ways to be innovative, and we’re giving them the tools necessary to turn their clinical expertise into successful ventures.”
Applications are currently open online to pitch an idea in this year’s lab. Applications consist of brief answers to questions and a short YouTube video. The deadline for submissions is Friday, March 9, 2018. Anyone with innovative ideas, regardless of the stage, is invited to apply. The APA Workgroup on Psychiatry Innovation, which oversees the lab, welcomes ideas from people pitching for the first time to those working on their ideas full time. They noted that past finalists have come from all experience levels and a wide range of backgrounds, including students, CEOs, engineers, and psychiatrists.
Past winners and participants have come from a range of stages, from early start-ups to established companies, including NeuroLex Labs, which uses voice samples to detect behavioral health illness; Spring, which uses machine learning to determine the best depression treatment; Muse, which creates an EEG sensing headband to aid mindfulness practice; and Overdose Recovery Bracelet, which builds a wristband to deliver naloxone to people who have overdosed on opiates.
The diversity of ideas has been remarkable; ideas in the past have ranged from traditional for-profit businesses to nonprofits, educational and public health programs, and proposals for government reform. They address problems at all parts of the health care spectrum: prevention, diagnosis, treatment, systems, EMRs, and more.
“Many past ideas have revolved around technology such as artificial intelligence, social networking, or telemedicine,” Dr. Vasan said. “. Those ideas are especially important in solving the most pressing problems in our field today.”
In addition to those competing, anyone registered to attend the APA annual meeting can attend the event as an audience member or join a team and help as an innovation leader during the coaching portion of the program. Last year alone, more than 70% of the participants in the lab said that it was better than other educational events they have attended, 95% of participants found it useful, and participants made an average of six new contacts through the event.
“The Psychiatry Innovation Lab connected me to people who continue to inspire and educate me toward making a real impact with my own endeavors,” said Cody Rall, MD, founder of the YouTube channel Techforpsych and a 2016 semifinalist.
“The lab has grown dramatically. When we started we worked hard to find just one sponsor; last year we had 10 sponsors and over $25,000 in cash and prizes.” Dr. Vasan said. “We also had our first international finalist from Pakistan. The commitment to solving problems in mental health is taking off, and we want to ensure that psychiatrists are front and center in leading the change.”
For more information about joining the innovation lab or other ways to be involved, including sponsorship and judging, visit https://www.psychiatry.org/innovation or email Nina Taylor, APA deputy director of education, at [email protected].
This article was updated January 23, 2018.
Dr. Chaudhary is a board member of the APA Psychiatry Innovation Lab and the APA Workgroup on Psychiatry Innovation; chief resident in child and adolescent psychiatry at Massachusetts General Hospital/McLean and clinical fellow at Harvard Medical School, both in Boston; and founding partner of Brainstorm: Stanford Laboratory for Brain Health Innovation and Entrepreneurship. Dr. Ghomi is a board member of the APA Psychiatry Innovation Lab and the APA Workgroup on Psychiatry Innovation; resident in psychiatry at the University of Washington, Seattle; chief medical officer at NeuroLex; and founding partner of Brainstorm: Stanford Laboratory for Brain Health Innovation and Entrepreneurship.