User login
Intermittent Fasting + HIIT: Fitness Fad or Fix?
Let’s be honest: Although as physicians we have the power of the prescription pad, so much of health, in the end, comes down to lifestyle. Of course, taking a pill is often way easier than changing your longstanding habits. And what’s worse, doesn’t it always seem like the lifestyle stuff that is good for your health is unpleasant?
Two recent lifestyle interventions that I have tried and find really not enjoyable are time-restricted eating (also known as intermittent fasting) and high-intensity interval training, or HIIT. The former leaves me hangry for half the day; the latter is, well, it’s just really hard compared with my usual jog.
But given the rule of unpleasant lifestyle changes, I knew as soon as I saw this recent study what the result would be. What if we combined time-restricted eating with high-intensity interval training?
I’m referring to this study, appearing in PLOS ONE from Ranya Ameur and colleagues, which is a small trial that enrolled otherwise healthy women with a BMI > 30 and randomized them to one of three conditions.
First was time-restricted eating. Women in this group could eat whatever they wanted, but only from 8 a.m. to 4 p.m. daily.
Second: high-intensity functional training. This is a variant of high-intensity interval training which focuses a bit more on resistance exercise than on pure cardiovascular stuff but has the same vibe of doing brief bursts of intensive activity followed by a cool-down period.
Third: a combination of the two. Sounds rough to me.
The study was otherwise straightforward. At baseline, researchers collected data on body composition and dietary intake, and measured blood pressure, glucose, insulin, and lipid biomarkers. A 12-week intervention period followed, after which all of this stuff was measured again.
Now, you may have noticed that there is no control group in this study. We’ll come back to that — a few times.
Let me walk you through some of the outcomes here.
First off, body composition metrics. All three groups lost weight — on average, around 10% of body weight which, for a 12-week intervention, is fairly impressive. BMI and waist circumference went down as well, and, interestingly, much of the weight loss here was in fat mass, not fat-free mass.
Most interventions that lead to weight loss — and I’m including some of the newer drugs here — lead to both fat and muscle loss. That might not be as bad as it sounds; the truth is that muscle mass increases as fat increases because of the simple fact that if you’re carrying more weight when you walk around, your leg muscles get bigger. But to preserve muscle mass in the face of fat loss is sort of a Goldilocks finding, and, based on these results, there’s a suggestion that the high-intensity functional training helps to do just that.
The dietary intake findings were really surprising to me. Across the board, caloric intake decreased. It’s no surprise that time-restricted eating reduces calorie intake. That has been shown many times before and is probably the main reason it induces weight loss — less time to eat means you eat less.
But why would high-intensity functional training lead to lower caloric intake? Most people, myself included, get hungry after they exercise. In fact, one of the reasons it’s hard to lose weight with exercise alone is that we end up eating more calories to make up for what we lost during the exercise. This calorie reduction could be a unique effect of this type of exercise, but honestly this could also be something called the Hawthorne effect. Women in the study kept a food diary to track their intake, and the act of doing that might actually make you eat less. It makes it a little more annoying to snack a bit if you know you have to write it down. This is a situation where I would kill for a control group.
The lipid findings are also pretty striking, with around a 40% reduction in LDL across the board, and evidence of synergistic effects of combined TRE and high-intensity training on total cholesterol and triglycerides. This is like a statin level of effect — pretty impressive. Again, my heart pines for a control group, though.
Same story with glucose and insulin measures: an impressive reduction in fasting glucose and good evidence that the combination of time-restricted eating and high-intensity functional training reduces insulin levels and HOMA-IR as well.
Really the only thing that wasn’t very impressive was the change in blood pressure, with only modest decreases across the board.
Okay, so let’s take a breath after this high-intensity cerebral workout and put this all together. This was a small study, lacking a control group, but with large effect sizes in very relevant clinical areas. It confirms what we know about time-restricted eating — that it makes you eat less calories — and introduces the potential that vigorous exercise can not only magnify the benefits of time-restricted eating but maybe even mitigate some of the risks, like the risk for muscle loss. And of course, it comports with my central hypothesis, which is that the more unpleasant a lifestyle intervention is, the better it is for you. No pain, no gain, right?
Of course, I am being overly dogmatic. There are plenty of caveats. Wrestling bears is quite unpleasant and almost certainly bad for you. And there are even some pleasant things that are pretty good for you — like coffee and sex. And there are even people who find time-restricted eating and high-intensity training pleasurable. They are called masochists.
I’m joking. The truth is that Or, at least, much less painful. The trick is getting over the hump of change. If only there were a pill for that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Let’s be honest: Although as physicians we have the power of the prescription pad, so much of health, in the end, comes down to lifestyle. Of course, taking a pill is often way easier than changing your longstanding habits. And what’s worse, doesn’t it always seem like the lifestyle stuff that is good for your health is unpleasant?
Two recent lifestyle interventions that I have tried and find really not enjoyable are time-restricted eating (also known as intermittent fasting) and high-intensity interval training, or HIIT. The former leaves me hangry for half the day; the latter is, well, it’s just really hard compared with my usual jog.
But given the rule of unpleasant lifestyle changes, I knew as soon as I saw this recent study what the result would be. What if we combined time-restricted eating with high-intensity interval training?
I’m referring to this study, appearing in PLOS ONE from Ranya Ameur and colleagues, which is a small trial that enrolled otherwise healthy women with a BMI > 30 and randomized them to one of three conditions.
First was time-restricted eating. Women in this group could eat whatever they wanted, but only from 8 a.m. to 4 p.m. daily.
Second: high-intensity functional training. This is a variant of high-intensity interval training which focuses a bit more on resistance exercise than on pure cardiovascular stuff but has the same vibe of doing brief bursts of intensive activity followed by a cool-down period.
Third: a combination of the two. Sounds rough to me.
The study was otherwise straightforward. At baseline, researchers collected data on body composition and dietary intake, and measured blood pressure, glucose, insulin, and lipid biomarkers. A 12-week intervention period followed, after which all of this stuff was measured again.
Now, you may have noticed that there is no control group in this study. We’ll come back to that — a few times.
Let me walk you through some of the outcomes here.
First off, body composition metrics. All three groups lost weight — on average, around 10% of body weight which, for a 12-week intervention, is fairly impressive. BMI and waist circumference went down as well, and, interestingly, much of the weight loss here was in fat mass, not fat-free mass.
Most interventions that lead to weight loss — and I’m including some of the newer drugs here — lead to both fat and muscle loss. That might not be as bad as it sounds; the truth is that muscle mass increases as fat increases because of the simple fact that if you’re carrying more weight when you walk around, your leg muscles get bigger. But to preserve muscle mass in the face of fat loss is sort of a Goldilocks finding, and, based on these results, there’s a suggestion that the high-intensity functional training helps to do just that.
The dietary intake findings were really surprising to me. Across the board, caloric intake decreased. It’s no surprise that time-restricted eating reduces calorie intake. That has been shown many times before and is probably the main reason it induces weight loss — less time to eat means you eat less.
But why would high-intensity functional training lead to lower caloric intake? Most people, myself included, get hungry after they exercise. In fact, one of the reasons it’s hard to lose weight with exercise alone is that we end up eating more calories to make up for what we lost during the exercise. This calorie reduction could be a unique effect of this type of exercise, but honestly this could also be something called the Hawthorne effect. Women in the study kept a food diary to track their intake, and the act of doing that might actually make you eat less. It makes it a little more annoying to snack a bit if you know you have to write it down. This is a situation where I would kill for a control group.
The lipid findings are also pretty striking, with around a 40% reduction in LDL across the board, and evidence of synergistic effects of combined TRE and high-intensity training on total cholesterol and triglycerides. This is like a statin level of effect — pretty impressive. Again, my heart pines for a control group, though.
Same story with glucose and insulin measures: an impressive reduction in fasting glucose and good evidence that the combination of time-restricted eating and high-intensity functional training reduces insulin levels and HOMA-IR as well.
Really the only thing that wasn’t very impressive was the change in blood pressure, with only modest decreases across the board.
Okay, so let’s take a breath after this high-intensity cerebral workout and put this all together. This was a small study, lacking a control group, but with large effect sizes in very relevant clinical areas. It confirms what we know about time-restricted eating — that it makes you eat less calories — and introduces the potential that vigorous exercise can not only magnify the benefits of time-restricted eating but maybe even mitigate some of the risks, like the risk for muscle loss. And of course, it comports with my central hypothesis, which is that the more unpleasant a lifestyle intervention is, the better it is for you. No pain, no gain, right?
Of course, I am being overly dogmatic. There are plenty of caveats. Wrestling bears is quite unpleasant and almost certainly bad for you. And there are even some pleasant things that are pretty good for you — like coffee and sex. And there are even people who find time-restricted eating and high-intensity training pleasurable. They are called masochists.
I’m joking. The truth is that Or, at least, much less painful. The trick is getting over the hump of change. If only there were a pill for that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Let’s be honest: Although as physicians we have the power of the prescription pad, so much of health, in the end, comes down to lifestyle. Of course, taking a pill is often way easier than changing your longstanding habits. And what’s worse, doesn’t it always seem like the lifestyle stuff that is good for your health is unpleasant?
Two recent lifestyle interventions that I have tried and find really not enjoyable are time-restricted eating (also known as intermittent fasting) and high-intensity interval training, or HIIT. The former leaves me hangry for half the day; the latter is, well, it’s just really hard compared with my usual jog.
But given the rule of unpleasant lifestyle changes, I knew as soon as I saw this recent study what the result would be. What if we combined time-restricted eating with high-intensity interval training?
I’m referring to this study, appearing in PLOS ONE from Ranya Ameur and colleagues, which is a small trial that enrolled otherwise healthy women with a BMI > 30 and randomized them to one of three conditions.
First was time-restricted eating. Women in this group could eat whatever they wanted, but only from 8 a.m. to 4 p.m. daily.
Second: high-intensity functional training. This is a variant of high-intensity interval training which focuses a bit more on resistance exercise than on pure cardiovascular stuff but has the same vibe of doing brief bursts of intensive activity followed by a cool-down period.
Third: a combination of the two. Sounds rough to me.
The study was otherwise straightforward. At baseline, researchers collected data on body composition and dietary intake, and measured blood pressure, glucose, insulin, and lipid biomarkers. A 12-week intervention period followed, after which all of this stuff was measured again.
Now, you may have noticed that there is no control group in this study. We’ll come back to that — a few times.
Let me walk you through some of the outcomes here.
First off, body composition metrics. All three groups lost weight — on average, around 10% of body weight which, for a 12-week intervention, is fairly impressive. BMI and waist circumference went down as well, and, interestingly, much of the weight loss here was in fat mass, not fat-free mass.
Most interventions that lead to weight loss — and I’m including some of the newer drugs here — lead to both fat and muscle loss. That might not be as bad as it sounds; the truth is that muscle mass increases as fat increases because of the simple fact that if you’re carrying more weight when you walk around, your leg muscles get bigger. But to preserve muscle mass in the face of fat loss is sort of a Goldilocks finding, and, based on these results, there’s a suggestion that the high-intensity functional training helps to do just that.
The dietary intake findings were really surprising to me. Across the board, caloric intake decreased. It’s no surprise that time-restricted eating reduces calorie intake. That has been shown many times before and is probably the main reason it induces weight loss — less time to eat means you eat less.
But why would high-intensity functional training lead to lower caloric intake? Most people, myself included, get hungry after they exercise. In fact, one of the reasons it’s hard to lose weight with exercise alone is that we end up eating more calories to make up for what we lost during the exercise. This calorie reduction could be a unique effect of this type of exercise, but honestly this could also be something called the Hawthorne effect. Women in the study kept a food diary to track their intake, and the act of doing that might actually make you eat less. It makes it a little more annoying to snack a bit if you know you have to write it down. This is a situation where I would kill for a control group.
The lipid findings are also pretty striking, with around a 40% reduction in LDL across the board, and evidence of synergistic effects of combined TRE and high-intensity training on total cholesterol and triglycerides. This is like a statin level of effect — pretty impressive. Again, my heart pines for a control group, though.
Same story with glucose and insulin measures: an impressive reduction in fasting glucose and good evidence that the combination of time-restricted eating and high-intensity functional training reduces insulin levels and HOMA-IR as well.
Really the only thing that wasn’t very impressive was the change in blood pressure, with only modest decreases across the board.
Okay, so let’s take a breath after this high-intensity cerebral workout and put this all together. This was a small study, lacking a control group, but with large effect sizes in very relevant clinical areas. It confirms what we know about time-restricted eating — that it makes you eat less calories — and introduces the potential that vigorous exercise can not only magnify the benefits of time-restricted eating but maybe even mitigate some of the risks, like the risk for muscle loss. And of course, it comports with my central hypothesis, which is that the more unpleasant a lifestyle intervention is, the better it is for you. No pain, no gain, right?
Of course, I am being overly dogmatic. There are plenty of caveats. Wrestling bears is quite unpleasant and almost certainly bad for you. And there are even some pleasant things that are pretty good for you — like coffee and sex. And there are even people who find time-restricted eating and high-intensity training pleasurable. They are called masochists.
I’m joking. The truth is that Or, at least, much less painful. The trick is getting over the hump of change. If only there were a pill for that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Remembering the Dead in Unity and Peace
Soldiers’ graves are the greatest preachers of peace.
Albert Schweitzer 1
From the window of my room in the house where I grew up, I could see the American flag flying over Fort Sam Houston National Cemetery. I would ride my bicycle around the paths that divided the grassy sections of graves to the blocks where my father and grandfather were buried. I would stand before the gravesites in a state combining prayer, processing, and remembrance. Carved into my grandfather’s headstone were the 2 world wars he fought in and on my father’s, the 3 conflicts in which he served. I would walk up to their headstones and trace the emblems of belief: the engraved Star of David that marked my grandfather’s grave and the simple cross for my father.
My visits and writing about them may strike some readers as morbid. However, for me, the experience and memories are calming and peaceful, like the cemetery. There was something incredibly comforting about the uniformity of the headstones standing out for miles, mirroring the ranks of soldiers in the wars they commemorated. Yet, as with the men and women who fought each conflict, every grave told a succinct Hemingway-like story of their military career etched in stone. I know now that discrimination in the military segregated even the burial of service members.2 It appeared to my younger self that at least compared to civilian cemeteries with their massive monuments to the wealthy and powerful, there was an egalitarian effect: my master sergeant grandfather’s plot was indistinguishable from that of my colonel father.
Memorial Day and military cemeteries have a shared history. While Veterans Day honors all who have worn the uniform, living and dead, Memorial Day, as its name suggests, remembers those who have died in a broadly conceived line of duty. To emphasize the more solemn character of the holiday, the original name, Decoration Day, was changed to emphasize the reverence of remembrance.3 The first widespread observance of Memorial Day was to commemorate those who perished in the Civil War, which remains the conflict with the highest number of casualties in American history. The first national commemoration occurred at Arlington National Cemetery when 5000 volunteers decorated 20,000 Union and Confederate graves in an act of solidarity and reconciliation. The practice struck a chord in a country beleaguered by war and division.2
National cemeteries also emerged from the grief and gratitude that marked the Civil War. President Abraham Lincoln, who gave us the famous US Department of Veterans Affairs (VA) mission motto, also inaugurated national cemeteries. At the beginning of the Civil War, only Union soldiers who sacrificed their lives to end slavery were entitled to burial. Reflective of the rift that divided the country, Confederate soldiers contended that such divisiveness should not continue unto death and were granted the right to be buried beside those they fought against, united in death and memory.4
Today, the country is more divided than ever: more than a few observers of American culture, including the new popular film Civil War, believe we are on the brink of another civil war.5 While we take their warning seriously, there are still signs of unity amongst the people, like those who followed the war between the states. Recently, in that same national cemetery where I first contemplated these themes, justice, delayed too long, was not entirely denied. A ceremony was held to dedicate 17 headstones to honor the memories of Black World War I Army soldiers who were court-martialed and hanged in the wake of the Houston riots of 1917. As a sign of their dishonor, their headstones listed only their dates and names—nothing of their military service. At the urging of their descendants, the US Army reopened the files and found the verdict to have been racially motivated. They set aside their convictions, gave them honorable discharges for their service in life, and replaced their gravesites with ones that enshrined that respect in death.6
Some reading this column may, like me, have had the profound privilege of participating in a burial at a national cemetery. We recall the stirring mix of pride and loss when the honor guard hands the perfectly folded flag to the bereaved family member and bids farewell to their comrade with a salute. Yet, not all families have this privilege. One of the saddest experiences I recall is when I was in a leadership position at a VA facility and unable to help impoverished families who were denied VA burial benefits or payments to transport their deceased veteran closer to home. That sorrow often turned to thankful relief when a veterans service organization or other community group offered to pay the funerary expenses. Fortunately, like eligibility for VA health care, the criteria for burial benefits have steadily expanded to encompass spouses, adult children, and others who served.7
In a similar display of altruism this Memorial Day, veterans service organizations, Boy Scouts, and volunteers will place a flag on every grave to show that some memories are stronger than death. If you have never seen it, I encourage you to visit a VA or a national cemetery this holiday or, even better, volunteer to place flags. Either way, spend a few moments thankfully remembering that we can all engage in those uniquely American Memorial Day pastimes of barbecues and baseball games because so many served and died to protect our way of life. The epigraph at the beginning of this column is attributed to Albert Schweitzer, the physician-theologian of reverence for life. The news today is full of war and rumors of war.8 Let us all hope that the message is heard around the world so there is no need to build more national cemeteries to remember our veterans.
1. Cohen R. On Omaha Beach today, where’s the comradeship? The New York Times. June 5, 2024. Accessed April 26, 2024. https://www.nytimes.com/2004/06/05/world/on-omaha-beach-today-where-s-the-comradeship.html
2. Stillwell B. ‘How decoration day’ became memorial day. Military.com. Published May 12, 2020. Accessed April 26, 2024. https://www.military.com/holidays/memorial-day/how-decoration-day-became-memorial-day.html
3. The history of Memorial Day. PBS. Accessed April 26, 2024. https://www.pbs.org/national-memorial-day-concert/memorial-day/history/
4. US Department of Veterans Affairs, National Cemetery Administration. Facts: NCA history and development. Updated October 18, 2023. Accessed April 26, 2024. https://www.cem.va.gov/facts/NCA_History_and_Development_1.asp
5. Lerer L. How the movie ‘civil war’ echoes real political anxieties. The New York Times. April 21, 2024. Accessed April 26, 2024. https://www.nytimes.com/2024/04/21/us/politics/civil-war-movie-politics.html
6. VA’s national cemetery administration dedicates new headstones to honor black soldiers, correcting 1917 injustice. News release. US Department of Veterans Affairs. Published February 22, 2024. Accessed April 26, 2024. https://news.va.gov/press-room/va-headstones-black-soldiers-1917-injustice/
7. US Department of Veterans Affairs, National Cemetery Administration. Burial benefits. Updated September 27, 2023. Accessed April 26, 2024. https://www.cem.va.gov/burial_benefits/
8. Racker M. Why so many politicians are talking about world war III. Time. November 20, 2023. Accessed April 29, 2024. https://time.com/6336897/israel-war-gaza-world-war-iii/
Soldiers’ graves are the greatest preachers of peace.
Albert Schweitzer 1
From the window of my room in the house where I grew up, I could see the American flag flying over Fort Sam Houston National Cemetery. I would ride my bicycle around the paths that divided the grassy sections of graves to the blocks where my father and grandfather were buried. I would stand before the gravesites in a state combining prayer, processing, and remembrance. Carved into my grandfather’s headstone were the 2 world wars he fought in and on my father’s, the 3 conflicts in which he served. I would walk up to their headstones and trace the emblems of belief: the engraved Star of David that marked my grandfather’s grave and the simple cross for my father.
My visits and writing about them may strike some readers as morbid. However, for me, the experience and memories are calming and peaceful, like the cemetery. There was something incredibly comforting about the uniformity of the headstones standing out for miles, mirroring the ranks of soldiers in the wars they commemorated. Yet, as with the men and women who fought each conflict, every grave told a succinct Hemingway-like story of their military career etched in stone. I know now that discrimination in the military segregated even the burial of service members.2 It appeared to my younger self that at least compared to civilian cemeteries with their massive monuments to the wealthy and powerful, there was an egalitarian effect: my master sergeant grandfather’s plot was indistinguishable from that of my colonel father.
Memorial Day and military cemeteries have a shared history. While Veterans Day honors all who have worn the uniform, living and dead, Memorial Day, as its name suggests, remembers those who have died in a broadly conceived line of duty. To emphasize the more solemn character of the holiday, the original name, Decoration Day, was changed to emphasize the reverence of remembrance.3 The first widespread observance of Memorial Day was to commemorate those who perished in the Civil War, which remains the conflict with the highest number of casualties in American history. The first national commemoration occurred at Arlington National Cemetery when 5000 volunteers decorated 20,000 Union and Confederate graves in an act of solidarity and reconciliation. The practice struck a chord in a country beleaguered by war and division.2
National cemeteries also emerged from the grief and gratitude that marked the Civil War. President Abraham Lincoln, who gave us the famous US Department of Veterans Affairs (VA) mission motto, also inaugurated national cemeteries. At the beginning of the Civil War, only Union soldiers who sacrificed their lives to end slavery were entitled to burial. Reflective of the rift that divided the country, Confederate soldiers contended that such divisiveness should not continue unto death and were granted the right to be buried beside those they fought against, united in death and memory.4
Today, the country is more divided than ever: more than a few observers of American culture, including the new popular film Civil War, believe we are on the brink of another civil war.5 While we take their warning seriously, there are still signs of unity amongst the people, like those who followed the war between the states. Recently, in that same national cemetery where I first contemplated these themes, justice, delayed too long, was not entirely denied. A ceremony was held to dedicate 17 headstones to honor the memories of Black World War I Army soldiers who were court-martialed and hanged in the wake of the Houston riots of 1917. As a sign of their dishonor, their headstones listed only their dates and names—nothing of their military service. At the urging of their descendants, the US Army reopened the files and found the verdict to have been racially motivated. They set aside their convictions, gave them honorable discharges for their service in life, and replaced their gravesites with ones that enshrined that respect in death.6
Some reading this column may, like me, have had the profound privilege of participating in a burial at a national cemetery. We recall the stirring mix of pride and loss when the honor guard hands the perfectly folded flag to the bereaved family member and bids farewell to their comrade with a salute. Yet, not all families have this privilege. One of the saddest experiences I recall is when I was in a leadership position at a VA facility and unable to help impoverished families who were denied VA burial benefits or payments to transport their deceased veteran closer to home. That sorrow often turned to thankful relief when a veterans service organization or other community group offered to pay the funerary expenses. Fortunately, like eligibility for VA health care, the criteria for burial benefits have steadily expanded to encompass spouses, adult children, and others who served.7
In a similar display of altruism this Memorial Day, veterans service organizations, Boy Scouts, and volunteers will place a flag on every grave to show that some memories are stronger than death. If you have never seen it, I encourage you to visit a VA or a national cemetery this holiday or, even better, volunteer to place flags. Either way, spend a few moments thankfully remembering that we can all engage in those uniquely American Memorial Day pastimes of barbecues and baseball games because so many served and died to protect our way of life. The epigraph at the beginning of this column is attributed to Albert Schweitzer, the physician-theologian of reverence for life. The news today is full of war and rumors of war.8 Let us all hope that the message is heard around the world so there is no need to build more national cemeteries to remember our veterans.
Soldiers’ graves are the greatest preachers of peace.
Albert Schweitzer 1
From the window of my room in the house where I grew up, I could see the American flag flying over Fort Sam Houston National Cemetery. I would ride my bicycle around the paths that divided the grassy sections of graves to the blocks where my father and grandfather were buried. I would stand before the gravesites in a state combining prayer, processing, and remembrance. Carved into my grandfather’s headstone were the 2 world wars he fought in and on my father’s, the 3 conflicts in which he served. I would walk up to their headstones and trace the emblems of belief: the engraved Star of David that marked my grandfather’s grave and the simple cross for my father.
My visits and writing about them may strike some readers as morbid. However, for me, the experience and memories are calming and peaceful, like the cemetery. There was something incredibly comforting about the uniformity of the headstones standing out for miles, mirroring the ranks of soldiers in the wars they commemorated. Yet, as with the men and women who fought each conflict, every grave told a succinct Hemingway-like story of their military career etched in stone. I know now that discrimination in the military segregated even the burial of service members.2 It appeared to my younger self that at least compared to civilian cemeteries with their massive monuments to the wealthy and powerful, there was an egalitarian effect: my master sergeant grandfather’s plot was indistinguishable from that of my colonel father.
Memorial Day and military cemeteries have a shared history. While Veterans Day honors all who have worn the uniform, living and dead, Memorial Day, as its name suggests, remembers those who have died in a broadly conceived line of duty. To emphasize the more solemn character of the holiday, the original name, Decoration Day, was changed to emphasize the reverence of remembrance.3 The first widespread observance of Memorial Day was to commemorate those who perished in the Civil War, which remains the conflict with the highest number of casualties in American history. The first national commemoration occurred at Arlington National Cemetery when 5000 volunteers decorated 20,000 Union and Confederate graves in an act of solidarity and reconciliation. The practice struck a chord in a country beleaguered by war and division.2
National cemeteries also emerged from the grief and gratitude that marked the Civil War. President Abraham Lincoln, who gave us the famous US Department of Veterans Affairs (VA) mission motto, also inaugurated national cemeteries. At the beginning of the Civil War, only Union soldiers who sacrificed their lives to end slavery were entitled to burial. Reflective of the rift that divided the country, Confederate soldiers contended that such divisiveness should not continue unto death and were granted the right to be buried beside those they fought against, united in death and memory.4
Today, the country is more divided than ever: more than a few observers of American culture, including the new popular film Civil War, believe we are on the brink of another civil war.5 While we take their warning seriously, there are still signs of unity amongst the people, like those who followed the war between the states. Recently, in that same national cemetery where I first contemplated these themes, justice, delayed too long, was not entirely denied. A ceremony was held to dedicate 17 headstones to honor the memories of Black World War I Army soldiers who were court-martialed and hanged in the wake of the Houston riots of 1917. As a sign of their dishonor, their headstones listed only their dates and names—nothing of their military service. At the urging of their descendants, the US Army reopened the files and found the verdict to have been racially motivated. They set aside their convictions, gave them honorable discharges for their service in life, and replaced their gravesites with ones that enshrined that respect in death.6
Some reading this column may, like me, have had the profound privilege of participating in a burial at a national cemetery. We recall the stirring mix of pride and loss when the honor guard hands the perfectly folded flag to the bereaved family member and bids farewell to their comrade with a salute. Yet, not all families have this privilege. One of the saddest experiences I recall is when I was in a leadership position at a VA facility and unable to help impoverished families who were denied VA burial benefits or payments to transport their deceased veteran closer to home. That sorrow often turned to thankful relief when a veterans service organization or other community group offered to pay the funerary expenses. Fortunately, like eligibility for VA health care, the criteria for burial benefits have steadily expanded to encompass spouses, adult children, and others who served.7
In a similar display of altruism this Memorial Day, veterans service organizations, Boy Scouts, and volunteers will place a flag on every grave to show that some memories are stronger than death. If you have never seen it, I encourage you to visit a VA or a national cemetery this holiday or, even better, volunteer to place flags. Either way, spend a few moments thankfully remembering that we can all engage in those uniquely American Memorial Day pastimes of barbecues and baseball games because so many served and died to protect our way of life. The epigraph at the beginning of this column is attributed to Albert Schweitzer, the physician-theologian of reverence for life. The news today is full of war and rumors of war.8 Let us all hope that the message is heard around the world so there is no need to build more national cemeteries to remember our veterans.
1. Cohen R. On Omaha Beach today, where’s the comradeship? The New York Times. June 5, 2024. Accessed April 26, 2024. https://www.nytimes.com/2004/06/05/world/on-omaha-beach-today-where-s-the-comradeship.html
2. Stillwell B. ‘How decoration day’ became memorial day. Military.com. Published May 12, 2020. Accessed April 26, 2024. https://www.military.com/holidays/memorial-day/how-decoration-day-became-memorial-day.html
3. The history of Memorial Day. PBS. Accessed April 26, 2024. https://www.pbs.org/national-memorial-day-concert/memorial-day/history/
4. US Department of Veterans Affairs, National Cemetery Administration. Facts: NCA history and development. Updated October 18, 2023. Accessed April 26, 2024. https://www.cem.va.gov/facts/NCA_History_and_Development_1.asp
5. Lerer L. How the movie ‘civil war’ echoes real political anxieties. The New York Times. April 21, 2024. Accessed April 26, 2024. https://www.nytimes.com/2024/04/21/us/politics/civil-war-movie-politics.html
6. VA’s national cemetery administration dedicates new headstones to honor black soldiers, correcting 1917 injustice. News release. US Department of Veterans Affairs. Published February 22, 2024. Accessed April 26, 2024. https://news.va.gov/press-room/va-headstones-black-soldiers-1917-injustice/
7. US Department of Veterans Affairs, National Cemetery Administration. Burial benefits. Updated September 27, 2023. Accessed April 26, 2024. https://www.cem.va.gov/burial_benefits/
8. Racker M. Why so many politicians are talking about world war III. Time. November 20, 2023. Accessed April 29, 2024. https://time.com/6336897/israel-war-gaza-world-war-iii/
1. Cohen R. On Omaha Beach today, where’s the comradeship? The New York Times. June 5, 2024. Accessed April 26, 2024. https://www.nytimes.com/2004/06/05/world/on-omaha-beach-today-where-s-the-comradeship.html
2. Stillwell B. ‘How decoration day’ became memorial day. Military.com. Published May 12, 2020. Accessed April 26, 2024. https://www.military.com/holidays/memorial-day/how-decoration-day-became-memorial-day.html
3. The history of Memorial Day. PBS. Accessed April 26, 2024. https://www.pbs.org/national-memorial-day-concert/memorial-day/history/
4. US Department of Veterans Affairs, National Cemetery Administration. Facts: NCA history and development. Updated October 18, 2023. Accessed April 26, 2024. https://www.cem.va.gov/facts/NCA_History_and_Development_1.asp
5. Lerer L. How the movie ‘civil war’ echoes real political anxieties. The New York Times. April 21, 2024. Accessed April 26, 2024. https://www.nytimes.com/2024/04/21/us/politics/civil-war-movie-politics.html
6. VA’s national cemetery administration dedicates new headstones to honor black soldiers, correcting 1917 injustice. News release. US Department of Veterans Affairs. Published February 22, 2024. Accessed April 26, 2024. https://news.va.gov/press-room/va-headstones-black-soldiers-1917-injustice/
7. US Department of Veterans Affairs, National Cemetery Administration. Burial benefits. Updated September 27, 2023. Accessed April 26, 2024. https://www.cem.va.gov/burial_benefits/
8. Racker M. Why so many politicians are talking about world war III. Time. November 20, 2023. Accessed April 29, 2024. https://time.com/6336897/israel-war-gaza-world-war-iii/
Comment on “Skin Cancer Screening: The Paradox of Melanoma and Improved All-Cause Mortality”
To the Editor:
I was unsurprised and gratified by the information presented in the Viewpoint on skin cancer screening by Ngo1 (Cutis. 2024;113:94-96). In my 30 years as a community dermatologist, I have observed that patients who opt to have periodic full-body skin examinations usually are more health literate, more likely to have a primary care physician (PCP) who has encouraged them to do so (ie, a conscientious practitioner directing their preventive care), more likely to have a strong will to live, and less likely to have multiple stressors that preclude self-care (eg, may be less likely to have a spouse for whom they are a caregiver) compared to those who do not get screened.
Findings on a full-body skin examination may impact patients in many ways, not only by the detection of skin cancers. I have discovered the following:
- evidence of diabetes/insulin resistance in the form of acanthosis nigricans, tinea corporis, erythrasma;
- evidence of rosacea associated with excessive alcohol intake;
- evidence of smoking-related issues such as psoriasis or hidradenitis suppurativa;
- cutaneous evidence of other systemic diseases (eg, autoimmune disease, cancer);
- elucidation of other chronic health problems (eg, psoriasis of the skin as a clue for undiagnosed psoriatic arthritis); and
- detection of parasites on the skin (eg, ticks) or signs of infection that may have notable ramifications (eg, interdigital maceration of a diabetic patient with tinea pedis).
I even saw a patient who had been sent for magnetic resonance imaging for back pain by her internist without any physical examination when she actually had an erosion over the sacrum from a rug burn!
When conducting full-body skin examinations, dermatologists should not underestimate these principles:
- The “magic” of using a relatively noninvasive and sensitive screening tool—comfort and stress reduction for the patient from a thorough visual, tactile, olfactory, and auditory examination.
- Human interaction—especially when the patient is seen annually or even more frequently over a period of years or decades, and especially when an excellent patient-physician rapport has been established.
- The impact of improving a patient’s appearance on their overall sense of well-being (eg, by controlling rosacea).
- The opportunity to introduce concepts (ie, educate patients) such as alcohol avoidance, smoking cessation, weight reduction, hygiene, diet, and exercise in a more tangential way than a PCP, as well as to consider with patients the idea that lifestyle modification may be an adjunct, if not a replacement, for prescription treatments.
- The stress reduction that ensues when a variety of self-identified health issues are addressed, for which the only treatment may be reassurance.
I would add to Dr. Ngo’s argument that stratifying patients into skin cancer risk categories may be a useful measure if the only goal of periodic dermatologic evaluation is skin cancer detection. One size rarely fits all when it comes to health recommendations.
In sum, I believe that periodic full-body skin examination is absolutely beneficial to patient care, and I am not at all surprised that all-cause mortality was lower in patients who have those examinations. Furthermore, when I offer my healthy, low-risk patients the option to return in 2 years rather than 1, the vast majority insist on 1 year. My mother used to say, “It’s better to be looked over than to be overlooked,” and I tell my patients that, too—but it seems they already know that instinctively.
- Ngo BT. Skin cancer screening: the paradox of melanoma and improved all-cause mortality. Cutis. 2024;113:94-96. doi:10.12788/cutis.0948
To the Editor:
I was unsurprised and gratified by the information presented in the Viewpoint on skin cancer screening by Ngo1 (Cutis. 2024;113:94-96). In my 30 years as a community dermatologist, I have observed that patients who opt to have periodic full-body skin examinations usually are more health literate, more likely to have a primary care physician (PCP) who has encouraged them to do so (ie, a conscientious practitioner directing their preventive care), more likely to have a strong will to live, and less likely to have multiple stressors that preclude self-care (eg, may be less likely to have a spouse for whom they are a caregiver) compared to those who do not get screened.
Findings on a full-body skin examination may impact patients in many ways, not only by the detection of skin cancers. I have discovered the following:
- evidence of diabetes/insulin resistance in the form of acanthosis nigricans, tinea corporis, erythrasma;
- evidence of rosacea associated with excessive alcohol intake;
- evidence of smoking-related issues such as psoriasis or hidradenitis suppurativa;
- cutaneous evidence of other systemic diseases (eg, autoimmune disease, cancer);
- elucidation of other chronic health problems (eg, psoriasis of the skin as a clue for undiagnosed psoriatic arthritis); and
- detection of parasites on the skin (eg, ticks) or signs of infection that may have notable ramifications (eg, interdigital maceration of a diabetic patient with tinea pedis).
I even saw a patient who had been sent for magnetic resonance imaging for back pain by her internist without any physical examination when she actually had an erosion over the sacrum from a rug burn!
When conducting full-body skin examinations, dermatologists should not underestimate these principles:
- The “magic” of using a relatively noninvasive and sensitive screening tool—comfort and stress reduction for the patient from a thorough visual, tactile, olfactory, and auditory examination.
- Human interaction—especially when the patient is seen annually or even more frequently over a period of years or decades, and especially when an excellent patient-physician rapport has been established.
- The impact of improving a patient’s appearance on their overall sense of well-being (eg, by controlling rosacea).
- The opportunity to introduce concepts (ie, educate patients) such as alcohol avoidance, smoking cessation, weight reduction, hygiene, diet, and exercise in a more tangential way than a PCP, as well as to consider with patients the idea that lifestyle modification may be an adjunct, if not a replacement, for prescription treatments.
- The stress reduction that ensues when a variety of self-identified health issues are addressed, for which the only treatment may be reassurance.
I would add to Dr. Ngo’s argument that stratifying patients into skin cancer risk categories may be a useful measure if the only goal of periodic dermatologic evaluation is skin cancer detection. One size rarely fits all when it comes to health recommendations.
In sum, I believe that periodic full-body skin examination is absolutely beneficial to patient care, and I am not at all surprised that all-cause mortality was lower in patients who have those examinations. Furthermore, when I offer my healthy, low-risk patients the option to return in 2 years rather than 1, the vast majority insist on 1 year. My mother used to say, “It’s better to be looked over than to be overlooked,” and I tell my patients that, too—but it seems they already know that instinctively.
To the Editor:
I was unsurprised and gratified by the information presented in the Viewpoint on skin cancer screening by Ngo1 (Cutis. 2024;113:94-96). In my 30 years as a community dermatologist, I have observed that patients who opt to have periodic full-body skin examinations usually are more health literate, more likely to have a primary care physician (PCP) who has encouraged them to do so (ie, a conscientious practitioner directing their preventive care), more likely to have a strong will to live, and less likely to have multiple stressors that preclude self-care (eg, may be less likely to have a spouse for whom they are a caregiver) compared to those who do not get screened.
Findings on a full-body skin examination may impact patients in many ways, not only by the detection of skin cancers. I have discovered the following:
- evidence of diabetes/insulin resistance in the form of acanthosis nigricans, tinea corporis, erythrasma;
- evidence of rosacea associated with excessive alcohol intake;
- evidence of smoking-related issues such as psoriasis or hidradenitis suppurativa;
- cutaneous evidence of other systemic diseases (eg, autoimmune disease, cancer);
- elucidation of other chronic health problems (eg, psoriasis of the skin as a clue for undiagnosed psoriatic arthritis); and
- detection of parasites on the skin (eg, ticks) or signs of infection that may have notable ramifications (eg, interdigital maceration of a diabetic patient with tinea pedis).
I even saw a patient who had been sent for magnetic resonance imaging for back pain by her internist without any physical examination when she actually had an erosion over the sacrum from a rug burn!
When conducting full-body skin examinations, dermatologists should not underestimate these principles:
- The “magic” of using a relatively noninvasive and sensitive screening tool—comfort and stress reduction for the patient from a thorough visual, tactile, olfactory, and auditory examination.
- Human interaction—especially when the patient is seen annually or even more frequently over a period of years or decades, and especially when an excellent patient-physician rapport has been established.
- The impact of improving a patient’s appearance on their overall sense of well-being (eg, by controlling rosacea).
- The opportunity to introduce concepts (ie, educate patients) such as alcohol avoidance, smoking cessation, weight reduction, hygiene, diet, and exercise in a more tangential way than a PCP, as well as to consider with patients the idea that lifestyle modification may be an adjunct, if not a replacement, for prescription treatments.
- The stress reduction that ensues when a variety of self-identified health issues are addressed, for which the only treatment may be reassurance.
I would add to Dr. Ngo’s argument that stratifying patients into skin cancer risk categories may be a useful measure if the only goal of periodic dermatologic evaluation is skin cancer detection. One size rarely fits all when it comes to health recommendations.
In sum, I believe that periodic full-body skin examination is absolutely beneficial to patient care, and I am not at all surprised that all-cause mortality was lower in patients who have those examinations. Furthermore, when I offer my healthy, low-risk patients the option to return in 2 years rather than 1, the vast majority insist on 1 year. My mother used to say, “It’s better to be looked over than to be overlooked,” and I tell my patients that, too—but it seems they already know that instinctively.
- Ngo BT. Skin cancer screening: the paradox of melanoma and improved all-cause mortality. Cutis. 2024;113:94-96. doi:10.12788/cutis.0948
- Ngo BT. Skin cancer screening: the paradox of melanoma and improved all-cause mortality. Cutis. 2024;113:94-96. doi:10.12788/cutis.0948
Hereditary Amyloidosis: 5 Things to Know
Amyloidosis is a condition marked by the accumulation of insoluble beta-sheet fibrillar protein aggregates in tissues that can be acquired or hereditary. Hereditary amyloidogenic transthyretin (hATTR) amyloidosis is an autosomal-dominant disease caused by pathogenic variants in the TTR gene. The TTR protein is essential for transporting thyroxine and retinol-binding protein and is primarily synthesized in the liver, becoming unstable as a result of the pathogenic mutations. Inherited pathogenic variants lead to the protein’s misfolding, aggregation, and deposition as amyloid fibrils in different organs, resulting in progressive multisystem dysfunction. hATTR amyloidosis is a heterogenous disease, characterized by a wide range of clinical manifestations affecting the peripheral (both somatic and autonomic) nervous system, heart, kidneys, and central nervous system (CNS); however, the heart and peripheral nerves appear to be the main targets of the TTR-related pathologic process. Without treatment, the prognosis is poor, with an average life expectancy of 7-11 years; however, in recent years, the development of new therapeutics has brought new hope to patients.
Here are five things to know about hereditary amyloidosis.
1. Diagnosis of hereditary amyloidosis requires a high level of suspicion.
The diagnosis of hATTR amyloidosis presents a significant challenge, particularly in nonendemic regions where a lack of family history and heterogeneity of clinical presentation can delay diagnosis by 4-5 years. A timely diagnosis requires clinicians to maintain a high index of suspicion, especially when evaluating patients with neuropathic symptoms. Early diagnosis is crucial to begin patients on recently available disease-modifying therapies that can slow the disease course. Failure to recognize is the major barrier to improved patient outcomes.
Confirming the diagnosis involves detecting amyloid deposits in tissue biopsy specimens from various possible sites, including the skin, nerves, myocardium, and others. However, the diagnosis can be challenging owing to the uneven distribution of amyloid fibrils, sometimes requiring multiple biopsies or alternative diagnostic approaches, such as TTR gene sequencing, to confirm the presence of an amyloidogenic pathogenic variant. Biopsy for hATTR amyloidosis is not required if imaging of the clinical phenotype and genetic testing are consistent.
Once diagnosed, the assessment of organ involvement is essential, using nerve conduction studies, cardiac investigations (eg, echocardiography, ECG, scintigraphy), ophthalmologic assessments, and complete renal function evaluations to fully understand the extent of disease impact.
2. Hereditary amyloidosis diseases are classified into two primary categories.
Hereditary amyloidosis represents a group of diseases caused by inherited gene mutations and is classified into two main types: ATTR (transthyretin-related) and non-TTR. Most cases of hereditary amyloidosis are associated with the TTR gene. Mutations in this protein lead to different forms of ATTR amyloidosis, categorized on the basis of the specific mutation involved, such as hATTR50M (genotype Val50Met), which is the most prevalent form.
ATTR mutations result in a variety of health issues, manifesting in three primary forms:
- Neuropathic ATTR (genotype Val50Met): Early symptoms include sensorimotor polyneuropathy of the legs, carpal tunnel syndrome, autonomic dysfunction, constipation/diarrhea, and impotence; late symptoms include cardiomyopathy, vitreous opacities, glaucoma, nephropathy, and CNS symptoms.
- Cardiac ATTR (genotype Val142Ile): This type is characterized by cardiomegaly, conduction block, arrhythmia, anginal pain, congestive heart failure, and sudden death.
- Leptomeningeal ATTR (genotype Asp38Gly): This is characterized by transient focal neurologic episodes, intracerebral and/or subarachnoid hemorrhages, dementia, ataxia, and psychosis.
Non-TTR amyloidoses are rarer than are ATTR variations and involve mutations in different genes that also have significant health impacts. These include proteins such as apolipoprotein AI, fibrinogen A alpha, lysozyme, apolipoprotein AII, gelsolin, and cystatin C. Each type contributes to a range of symptoms and requires individualized management approaches.
3. Heightened disease awareness has increased the recognized prevalence of hereditary amyloidosis.
hATTR amyloidosis has historically been recognized as a rare disease, with significant clusters in Portugal, Brazil, Sweden, and Japan and alongside smaller foci in regions such as Cyprus and Majorca. This disease›s variable incidence across Europe is now perceived to be on the rise. It is attributed to heightened disease awareness among healthcare providers and the broader availability of genetic testing, extending its recognized impact to at least 29 countries globally. The genetic landscape of hATTR amyloidosis is diverse, with over 140 mutations identified in the TTR gene. Among these, the Val50Met mutation is particularly notable for its association with large patient clusters in the endemic regions.
Morbidity and mortality associated with hATTR amyloidosis are significant, with an average lifespan of 7-11 years post diagnosis; however, survival rates can vary widely depending on the specific genetic variant and organ involvement. Early diagnosis can substantially improve outcomes; yet, for many, the prognosis remains poor, especially in cases dominated by cardiomyopathy. Genetics play a central role in the disease›s transmission, with autosomal-dominant inheritance patterns and high penetrance among carriers of pathogenic mutations. Research continues to uncover the broad spectrum of genetic variations contributing to hATTR amyloidosis, with ongoing studies poised to expand our understanding of its molecular underpinnings and potential treatment options.
4. The effect on quality of life is significant both in patients living with hATTR amyloidosis and their caregivers.
hATTR amyloidosis imposes a multifaceted burden on patients and their caregivers as the disease progresses. Symptoms range from sensorimotor impairment and gastrointestinal or autonomic dysfunction to heart failure, leading to significant health-related quality-of-life deficits. The systemic nature of hATTR amyloidosis significantly affects patients› lifestyles, daily activities, and general well-being, especially because it typically manifests in adulthood — a crucial time for occupational changes. The progression of hATTR amyloidosis exacerbates the challenges in maintaining employment and managing household chores, with symptomatic patients often unable to work and experiencing difficulties with absenteeism and presenteeism when they are able to work.
hATTR amyloidosis leads to physical, mental, occupational, and social limitations for patients, and it also places a considerable strain on their families and caregivers, who report poor mental health, work impairment, and a high time commitment (mean, 45.9 h/wk) to providing care.
5. There have been significant advancements in therapeutic options for early-stage hATTR amyloidosis.
After diagnosis, prompt initiation of treatment is recommended to delay the progression of hATTR amyloidosis; a multidisciplinary approach is essential, incorporating anti-amyloid therapy to inhibit further production and/or deposition of amyloid aggregates. Treatment strategies also include addressing symptomatic therapy and managing cardiac, renal, and ocular involvement. Although many therapies have been developed, especially for the early stages of hATTR amyloidosis, therapeutic benefits for patients with advanced disease remain limited.
Recent advancements in the treatment of hATTR amyloidosis have introduced RNA-targeted therapies including patisiran, vutrisiran, and eplontersen, which have shown efficacy in reducing hepatic TTR synthesis and the aggregation of misfolded monomers into amyloid deposits. These therapies, ranging from small interfering RNA formulations to antisense oligonucleotides, offer benefits in managing both cardiomyopathy and neuropathy associated with hATTR amyloidosis , administered through various methods, including intravenous infusions and subcutaneous injections. In addition, the stabilization of TTR tetramers with the use of drugs such as tafamidis and diflunisal has effectively prevented the formation of amyloidogenic monomers. Moreover, other investigational agents, including TTR stabilizers like acoramidis and tolcapone, as well as novel compounds that inhibit amyloid formation and disrupt fibrils, are expanding the therapeutic landscape for hATTR amyloidosis , providing hope for improved management of this complex condition.
Dr. Gertz is a professor and consultant in the Department of Hematology, Mayo Clinic, Rochester, Minnesota. He has disclosed the following relevant financial relationships: Received income in an amount equal to or greater than $250 from AstraZeneca, Ionis, and Alnylym.
A version of this article appeared on Medscape.com.
Amyloidosis is a condition marked by the accumulation of insoluble beta-sheet fibrillar protein aggregates in tissues that can be acquired or hereditary. Hereditary amyloidogenic transthyretin (hATTR) amyloidosis is an autosomal-dominant disease caused by pathogenic variants in the TTR gene. The TTR protein is essential for transporting thyroxine and retinol-binding protein and is primarily synthesized in the liver, becoming unstable as a result of the pathogenic mutations. Inherited pathogenic variants lead to the protein’s misfolding, aggregation, and deposition as amyloid fibrils in different organs, resulting in progressive multisystem dysfunction. hATTR amyloidosis is a heterogenous disease, characterized by a wide range of clinical manifestations affecting the peripheral (both somatic and autonomic) nervous system, heart, kidneys, and central nervous system (CNS); however, the heart and peripheral nerves appear to be the main targets of the TTR-related pathologic process. Without treatment, the prognosis is poor, with an average life expectancy of 7-11 years; however, in recent years, the development of new therapeutics has brought new hope to patients.
Here are five things to know about hereditary amyloidosis.
1. Diagnosis of hereditary amyloidosis requires a high level of suspicion.
The diagnosis of hATTR amyloidosis presents a significant challenge, particularly in nonendemic regions where a lack of family history and heterogeneity of clinical presentation can delay diagnosis by 4-5 years. A timely diagnosis requires clinicians to maintain a high index of suspicion, especially when evaluating patients with neuropathic symptoms. Early diagnosis is crucial to begin patients on recently available disease-modifying therapies that can slow the disease course. Failure to recognize is the major barrier to improved patient outcomes.
Confirming the diagnosis involves detecting amyloid deposits in tissue biopsy specimens from various possible sites, including the skin, nerves, myocardium, and others. However, the diagnosis can be challenging owing to the uneven distribution of amyloid fibrils, sometimes requiring multiple biopsies or alternative diagnostic approaches, such as TTR gene sequencing, to confirm the presence of an amyloidogenic pathogenic variant. Biopsy for hATTR amyloidosis is not required if imaging of the clinical phenotype and genetic testing are consistent.
Once diagnosed, the assessment of organ involvement is essential, using nerve conduction studies, cardiac investigations (eg, echocardiography, ECG, scintigraphy), ophthalmologic assessments, and complete renal function evaluations to fully understand the extent of disease impact.
2. Hereditary amyloidosis diseases are classified into two primary categories.
Hereditary amyloidosis represents a group of diseases caused by inherited gene mutations and is classified into two main types: ATTR (transthyretin-related) and non-TTR. Most cases of hereditary amyloidosis are associated with the TTR gene. Mutations in this protein lead to different forms of ATTR amyloidosis, categorized on the basis of the specific mutation involved, such as hATTR50M (genotype Val50Met), which is the most prevalent form.
ATTR mutations result in a variety of health issues, manifesting in three primary forms:
- Neuropathic ATTR (genotype Val50Met): Early symptoms include sensorimotor polyneuropathy of the legs, carpal tunnel syndrome, autonomic dysfunction, constipation/diarrhea, and impotence; late symptoms include cardiomyopathy, vitreous opacities, glaucoma, nephropathy, and CNS symptoms.
- Cardiac ATTR (genotype Val142Ile): This type is characterized by cardiomegaly, conduction block, arrhythmia, anginal pain, congestive heart failure, and sudden death.
- Leptomeningeal ATTR (genotype Asp38Gly): This is characterized by transient focal neurologic episodes, intracerebral and/or subarachnoid hemorrhages, dementia, ataxia, and psychosis.
Non-TTR amyloidoses are rarer than are ATTR variations and involve mutations in different genes that also have significant health impacts. These include proteins such as apolipoprotein AI, fibrinogen A alpha, lysozyme, apolipoprotein AII, gelsolin, and cystatin C. Each type contributes to a range of symptoms and requires individualized management approaches.
3. Heightened disease awareness has increased the recognized prevalence of hereditary amyloidosis.
hATTR amyloidosis has historically been recognized as a rare disease, with significant clusters in Portugal, Brazil, Sweden, and Japan and alongside smaller foci in regions such as Cyprus and Majorca. This disease›s variable incidence across Europe is now perceived to be on the rise. It is attributed to heightened disease awareness among healthcare providers and the broader availability of genetic testing, extending its recognized impact to at least 29 countries globally. The genetic landscape of hATTR amyloidosis is diverse, with over 140 mutations identified in the TTR gene. Among these, the Val50Met mutation is particularly notable for its association with large patient clusters in the endemic regions.
Morbidity and mortality associated with hATTR amyloidosis are significant, with an average lifespan of 7-11 years post diagnosis; however, survival rates can vary widely depending on the specific genetic variant and organ involvement. Early diagnosis can substantially improve outcomes; yet, for many, the prognosis remains poor, especially in cases dominated by cardiomyopathy. Genetics play a central role in the disease›s transmission, with autosomal-dominant inheritance patterns and high penetrance among carriers of pathogenic mutations. Research continues to uncover the broad spectrum of genetic variations contributing to hATTR amyloidosis, with ongoing studies poised to expand our understanding of its molecular underpinnings and potential treatment options.
4. The effect on quality of life is significant both in patients living with hATTR amyloidosis and their caregivers.
hATTR amyloidosis imposes a multifaceted burden on patients and their caregivers as the disease progresses. Symptoms range from sensorimotor impairment and gastrointestinal or autonomic dysfunction to heart failure, leading to significant health-related quality-of-life deficits. The systemic nature of hATTR amyloidosis significantly affects patients› lifestyles, daily activities, and general well-being, especially because it typically manifests in adulthood — a crucial time for occupational changes. The progression of hATTR amyloidosis exacerbates the challenges in maintaining employment and managing household chores, with symptomatic patients often unable to work and experiencing difficulties with absenteeism and presenteeism when they are able to work.
hATTR amyloidosis leads to physical, mental, occupational, and social limitations for patients, and it also places a considerable strain on their families and caregivers, who report poor mental health, work impairment, and a high time commitment (mean, 45.9 h/wk) to providing care.
5. There have been significant advancements in therapeutic options for early-stage hATTR amyloidosis.
After diagnosis, prompt initiation of treatment is recommended to delay the progression of hATTR amyloidosis; a multidisciplinary approach is essential, incorporating anti-amyloid therapy to inhibit further production and/or deposition of amyloid aggregates. Treatment strategies also include addressing symptomatic therapy and managing cardiac, renal, and ocular involvement. Although many therapies have been developed, especially for the early stages of hATTR amyloidosis, therapeutic benefits for patients with advanced disease remain limited.
Recent advancements in the treatment of hATTR amyloidosis have introduced RNA-targeted therapies including patisiran, vutrisiran, and eplontersen, which have shown efficacy in reducing hepatic TTR synthesis and the aggregation of misfolded monomers into amyloid deposits. These therapies, ranging from small interfering RNA formulations to antisense oligonucleotides, offer benefits in managing both cardiomyopathy and neuropathy associated with hATTR amyloidosis , administered through various methods, including intravenous infusions and subcutaneous injections. In addition, the stabilization of TTR tetramers with the use of drugs such as tafamidis and diflunisal has effectively prevented the formation of amyloidogenic monomers. Moreover, other investigational agents, including TTR stabilizers like acoramidis and tolcapone, as well as novel compounds that inhibit amyloid formation and disrupt fibrils, are expanding the therapeutic landscape for hATTR amyloidosis , providing hope for improved management of this complex condition.
Dr. Gertz is a professor and consultant in the Department of Hematology, Mayo Clinic, Rochester, Minnesota. He has disclosed the following relevant financial relationships: Received income in an amount equal to or greater than $250 from AstraZeneca, Ionis, and Alnylym.
A version of this article appeared on Medscape.com.
Amyloidosis is a condition marked by the accumulation of insoluble beta-sheet fibrillar protein aggregates in tissues that can be acquired or hereditary. Hereditary amyloidogenic transthyretin (hATTR) amyloidosis is an autosomal-dominant disease caused by pathogenic variants in the TTR gene. The TTR protein is essential for transporting thyroxine and retinol-binding protein and is primarily synthesized in the liver, becoming unstable as a result of the pathogenic mutations. Inherited pathogenic variants lead to the protein’s misfolding, aggregation, and deposition as amyloid fibrils in different organs, resulting in progressive multisystem dysfunction. hATTR amyloidosis is a heterogenous disease, characterized by a wide range of clinical manifestations affecting the peripheral (both somatic and autonomic) nervous system, heart, kidneys, and central nervous system (CNS); however, the heart and peripheral nerves appear to be the main targets of the TTR-related pathologic process. Without treatment, the prognosis is poor, with an average life expectancy of 7-11 years; however, in recent years, the development of new therapeutics has brought new hope to patients.
Here are five things to know about hereditary amyloidosis.
1. Diagnosis of hereditary amyloidosis requires a high level of suspicion.
The diagnosis of hATTR amyloidosis presents a significant challenge, particularly in nonendemic regions where a lack of family history and heterogeneity of clinical presentation can delay diagnosis by 4-5 years. A timely diagnosis requires clinicians to maintain a high index of suspicion, especially when evaluating patients with neuropathic symptoms. Early diagnosis is crucial to begin patients on recently available disease-modifying therapies that can slow the disease course. Failure to recognize is the major barrier to improved patient outcomes.
Confirming the diagnosis involves detecting amyloid deposits in tissue biopsy specimens from various possible sites, including the skin, nerves, myocardium, and others. However, the diagnosis can be challenging owing to the uneven distribution of amyloid fibrils, sometimes requiring multiple biopsies or alternative diagnostic approaches, such as TTR gene sequencing, to confirm the presence of an amyloidogenic pathogenic variant. Biopsy for hATTR amyloidosis is not required if imaging of the clinical phenotype and genetic testing are consistent.
Once diagnosed, the assessment of organ involvement is essential, using nerve conduction studies, cardiac investigations (eg, echocardiography, ECG, scintigraphy), ophthalmologic assessments, and complete renal function evaluations to fully understand the extent of disease impact.
2. Hereditary amyloidosis diseases are classified into two primary categories.
Hereditary amyloidosis represents a group of diseases caused by inherited gene mutations and is classified into two main types: ATTR (transthyretin-related) and non-TTR. Most cases of hereditary amyloidosis are associated with the TTR gene. Mutations in this protein lead to different forms of ATTR amyloidosis, categorized on the basis of the specific mutation involved, such as hATTR50M (genotype Val50Met), which is the most prevalent form.
ATTR mutations result in a variety of health issues, manifesting in three primary forms:
- Neuropathic ATTR (genotype Val50Met): Early symptoms include sensorimotor polyneuropathy of the legs, carpal tunnel syndrome, autonomic dysfunction, constipation/diarrhea, and impotence; late symptoms include cardiomyopathy, vitreous opacities, glaucoma, nephropathy, and CNS symptoms.
- Cardiac ATTR (genotype Val142Ile): This type is characterized by cardiomegaly, conduction block, arrhythmia, anginal pain, congestive heart failure, and sudden death.
- Leptomeningeal ATTR (genotype Asp38Gly): This is characterized by transient focal neurologic episodes, intracerebral and/or subarachnoid hemorrhages, dementia, ataxia, and psychosis.
Non-TTR amyloidoses are rarer than are ATTR variations and involve mutations in different genes that also have significant health impacts. These include proteins such as apolipoprotein AI, fibrinogen A alpha, lysozyme, apolipoprotein AII, gelsolin, and cystatin C. Each type contributes to a range of symptoms and requires individualized management approaches.
3. Heightened disease awareness has increased the recognized prevalence of hereditary amyloidosis.
hATTR amyloidosis has historically been recognized as a rare disease, with significant clusters in Portugal, Brazil, Sweden, and Japan and alongside smaller foci in regions such as Cyprus and Majorca. This disease›s variable incidence across Europe is now perceived to be on the rise. It is attributed to heightened disease awareness among healthcare providers and the broader availability of genetic testing, extending its recognized impact to at least 29 countries globally. The genetic landscape of hATTR amyloidosis is diverse, with over 140 mutations identified in the TTR gene. Among these, the Val50Met mutation is particularly notable for its association with large patient clusters in the endemic regions.
Morbidity and mortality associated with hATTR amyloidosis are significant, with an average lifespan of 7-11 years post diagnosis; however, survival rates can vary widely depending on the specific genetic variant and organ involvement. Early diagnosis can substantially improve outcomes; yet, for many, the prognosis remains poor, especially in cases dominated by cardiomyopathy. Genetics play a central role in the disease›s transmission, with autosomal-dominant inheritance patterns and high penetrance among carriers of pathogenic mutations. Research continues to uncover the broad spectrum of genetic variations contributing to hATTR amyloidosis, with ongoing studies poised to expand our understanding of its molecular underpinnings and potential treatment options.
4. The effect on quality of life is significant both in patients living with hATTR amyloidosis and their caregivers.
hATTR amyloidosis imposes a multifaceted burden on patients and their caregivers as the disease progresses. Symptoms range from sensorimotor impairment and gastrointestinal or autonomic dysfunction to heart failure, leading to significant health-related quality-of-life deficits. The systemic nature of hATTR amyloidosis significantly affects patients› lifestyles, daily activities, and general well-being, especially because it typically manifests in adulthood — a crucial time for occupational changes. The progression of hATTR amyloidosis exacerbates the challenges in maintaining employment and managing household chores, with symptomatic patients often unable to work and experiencing difficulties with absenteeism and presenteeism when they are able to work.
hATTR amyloidosis leads to physical, mental, occupational, and social limitations for patients, and it also places a considerable strain on their families and caregivers, who report poor mental health, work impairment, and a high time commitment (mean, 45.9 h/wk) to providing care.
5. There have been significant advancements in therapeutic options for early-stage hATTR amyloidosis.
After diagnosis, prompt initiation of treatment is recommended to delay the progression of hATTR amyloidosis; a multidisciplinary approach is essential, incorporating anti-amyloid therapy to inhibit further production and/or deposition of amyloid aggregates. Treatment strategies also include addressing symptomatic therapy and managing cardiac, renal, and ocular involvement. Although many therapies have been developed, especially for the early stages of hATTR amyloidosis, therapeutic benefits for patients with advanced disease remain limited.
Recent advancements in the treatment of hATTR amyloidosis have introduced RNA-targeted therapies including patisiran, vutrisiran, and eplontersen, which have shown efficacy in reducing hepatic TTR synthesis and the aggregation of misfolded monomers into amyloid deposits. These therapies, ranging from small interfering RNA formulations to antisense oligonucleotides, offer benefits in managing both cardiomyopathy and neuropathy associated with hATTR amyloidosis , administered through various methods, including intravenous infusions and subcutaneous injections. In addition, the stabilization of TTR tetramers with the use of drugs such as tafamidis and diflunisal has effectively prevented the formation of amyloidogenic monomers. Moreover, other investigational agents, including TTR stabilizers like acoramidis and tolcapone, as well as novel compounds that inhibit amyloid formation and disrupt fibrils, are expanding the therapeutic landscape for hATTR amyloidosis , providing hope for improved management of this complex condition.
Dr. Gertz is a professor and consultant in the Department of Hematology, Mayo Clinic, Rochester, Minnesota. He has disclosed the following relevant financial relationships: Received income in an amount equal to or greater than $250 from AstraZeneca, Ionis, and Alnylym.
A version of this article appeared on Medscape.com.
Sunscreen Safety: 2024 Updates
Sunscreen is a cornerstone of skin cancer prevention. The first commercial sunscreen was developed nearly 100 years ago,1 yet questions and concerns about the safety of these essential topical photoprotective agents continue to occupy our minds. This article serves as an update on some of the big sunscreen questions, as informed by the available evidence.
Are sunscreens safe?
The story of sunscreen regulation in the United States is long and dry. The major pain point is that sunscreens are regulated by the US Food and Drug Administration (FDA) as over-the-counter drugs rather than cosmetics (as in Europe).2 Regulatory hurdles created a situation wherein no new active sunscreen ingredient has been approved by the FDA since 1999, except ecamsule for use in one product line. There is hope that changes enacted under the CARES Act will streamline and expedite the sunscreen approval process in the future.3
Amid the ongoing regulatory slog, the FDA became interested in learning more about sunscreen safety. Specifically, they sought to determine the GRASE (generally regarded as safe and effective) status of the active ingredients in sunscreens. In 2019, only the inorganic (physical/mineral) UV filters zinc oxide and titanium dioxide were considered GRASE.4 Trolamine salicylate and para-aminobenzoic acid were not GRASE, but they currently are not used in sunscreens in the United States. For all the remaining organic (chemical) filters, additional safety data were required to establish GRASE status.4 In 2024, the situation remains largely unchanged. Industry is working with the FDA on testing requirements.5
Why the focus on safety? After all, sunscreens have been used widely for decades without any major safety signals; their only well-established adverse effects are contact dermatitis and staining of clothing.6 Although preclinical studies raised concerns that chemical sunscreens could be associated with endocrine, reproductive, and neurologic toxicities, to date there are no high-quality human studies demonstrating negative effects.7,8
However, exposure patterns have evolved. Sunscreen is recommended to be applied (and reapplied) daily. Also, chemical UV filters are used in many nonsunscreen products such as cosmetics, shampoos, fragrances, and plastics. In the United States, exposure to chemical sunscreens is ubiquitous; according to data from the National Health and Nutrition Examination Survey 2003-2004, oxybenzone was detected in 97% of more than 2500 urine samples, implying systemic absorption but not harm.9
The FDA confirmed the implication of systemic absorption via 2 maximal usage trials published in 2019 and 2020.10,11 In both studies, several chemical sunscreens were applied at the recommended density of 2 mg/cm2 to 75% of the body surface area multiple times over 4 days. For all tested organic UV filters, blood levels exceeded the predetermined FDA cutoff (0.5 ng/mL), even after one application.10,11 What’s the takeaway? Simply that the FDA now requires additional safety data for chemical sunscreen filters5; the findings in no way imply any associated harm. Two potential mitigating factors are that no one applies sunscreen at 2 mg/cm2, and the FDA’s blood level cutoff was a general estimate not specific to sunscreens.4,12
Nevertheless, a good long-term safety record for sunscreens does not negate the need for enhanced safety data when there is clear evidence of systemic absorption. In the meantime, concerned patients should be counseled that the physical/mineral sunscreens containing zinc oxide and titanium dioxide are considered GRASE by the FDA; even in nanoparticle form, they generally have not been found to penetrate beneath the stratum corneum.7,13
Does sunscreen cause frontal fibrosing alopecia?
Dermatologists are confronting the conundrum of rising cases of frontal fibrosing alopecia (FFA). Several theories on the pathogenesis of this idiopathic scarring alopecia have been raised, one of which involves increased use of sunscreen. Proposed explanations for sunscreen’s role in FFA include a lichenoid reaction inducing hair follicle autoimmunity through an unclear mechanism; a T cell–mediated allergic reaction, which is unlikely according to contact dermatitis experts14; reactive oxygen species production by titanium nanoparticles, yet titanium has been detected in hair follicles of both patients with FFA and controls15; and endocrine disruption following systemic absorption, which has not been supported by any high-quality human studies.7
An association between facial sunscreen use and FFA has been reported in case-control studies16; however, they have been criticized due to methodologic issues and biases, and they provide no evidence of causality.17,18 The jury remains out on the controversial association between sunscreen and FFA, with a need for more convincing data.
Does sunscreen impact coral reef health?
Coral reefs—crucial sources of aquatic biodiversity—are under attack from several different directions including climate change and pollution. As much as 14,000 tons of sunscreen enter coral reefs each year, and chemical sunscreen filters are detectable in waterways throughout the world—even in the Arctic.19,20 Thus, sunscreen has come under scrutiny as a potential environmental threat, particularly with coral bleaching.
Bleaching is a process in which corals exposed to an environmental stressor expel their symbiotic photosynthetic algae and turn white; if conditions fail to improve, the corals are vulnerable to death. In a highly cited 2016 study, coral larvae exposed to oxybenzone in artificial laboratory conditions displayed concentration-dependent mortality and decreased chlorophyll fluorescence, which suggested bleaching.19 These findings influenced legislation in Hawaii and other localities banning sunscreens containing oxybenzone. Problematically, the study has been criticized for acutely exposing the most susceptible coral life-forms to unrealistic oxybenzone concentrations; more broadly, there is no standardized approach to coral toxicity testing.21
The bigger picture (and elephant in the room) is that the primary cause of coral bleaching is undoubtedly climate change/ocean warming.7 More recent studies suggest that oxybenzone probably adds insult to injury for corals already debilitated by ocean warming.22,23
It has been posited that a narrow focus on sunscreens detracts attention from the climate issue.24 Individuals can take a number of actions to reduce their carbon footprint in an effort to preserve our environment, specifically coral reefs.25 Concerned patients should be counseled to use sunscreens containing the physical/mineral UV filters zinc oxide and titanium dioxide, which are unlikely to contribute to coral bleaching as commercially formulated.7
Ongoing Questions
A lot of unknowns about sunscreen safety remain, and much hubbub has been made over studies that often are preliminary at best. At the time of this writing, absent a crystal ball, this author continues to wear chemical sunscreens; spends a lot more time worrying about their carbon footprint than what type of sunscreen to use at the beach; and believes the association of FFA with sunscreen is unlikely to be causal. Hopefully much-needed rigorous evidence will guide our future approach to sunscreen formulation and use.
- Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044-1049.
- Pantelic MN, Wong N, Kwa M, et al. Ultraviolet filters in the United States and European Union: a review of safety and implications for the future of US sunscreens. J Am Acad Dermatol. 2023;88:632-646.
- Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509-511.
- Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regist. 2019;84:6204-6275.
- Lim HW, Mohammad TF, Wang SQ. Food and Drug Administration’s proposed sunscreen final administrative order: how does it affect sunscreens in the United States? J Am Acad Dermatol. 2022;86:E83-E84.
- Ekstein SF, Hylwa S. Sunscreens: a review of UV filters and their allergic potential. Dermatitis. 2023;34:176-190.
- Adler BL, DeLeo VA. Sunscreen safety: a review of recent studies on humans and the environment. Curr Dermatol Rep. 2020;9:1-9.
- Suh S, Pham C, Smith J, et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033-1042.
- Calafat AM, Wong LY, Ye X, et al. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893-897.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30:96-101.
- Mohammed YH, Holmes A, Haridass IN, et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol. 2019;139:308-315.
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482.
- Thompson CT, Chen ZQ, Kolivras A, et al. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181:216-217.
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396.
- Seegobin SD, Tziotzios C, Stefanato CM, et al. Frontal fibrosing alopecia:there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407-1408.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Regarding methodologic concerns in clinical studies on frontal fibrosing alopecia. J Am Acad Dermatol. 2021;84:E207-E208.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- National Academies of Sciences, Engineering, and Medicine. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health. The National Academies Press; 2022.
- Mitchelmore CL, Burns EE, Conway A, et al. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967-988.
- Vuckovic D, Tinoco AI, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals. Science. 2022;376:644-648.
- Wijgerde T, van Ballegooijen M, Nijland R, et al. Adding insult to injury: effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci Total Environ. 2020;733:139030.
- Sirois J. Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211-212.
- United Nations. Actions for a healthy planet. Accessed April 15, 2024. https://www.un.org/en/actnow/ten-actions
Sunscreen is a cornerstone of skin cancer prevention. The first commercial sunscreen was developed nearly 100 years ago,1 yet questions and concerns about the safety of these essential topical photoprotective agents continue to occupy our minds. This article serves as an update on some of the big sunscreen questions, as informed by the available evidence.
Are sunscreens safe?
The story of sunscreen regulation in the United States is long and dry. The major pain point is that sunscreens are regulated by the US Food and Drug Administration (FDA) as over-the-counter drugs rather than cosmetics (as in Europe).2 Regulatory hurdles created a situation wherein no new active sunscreen ingredient has been approved by the FDA since 1999, except ecamsule for use in one product line. There is hope that changes enacted under the CARES Act will streamline and expedite the sunscreen approval process in the future.3
Amid the ongoing regulatory slog, the FDA became interested in learning more about sunscreen safety. Specifically, they sought to determine the GRASE (generally regarded as safe and effective) status of the active ingredients in sunscreens. In 2019, only the inorganic (physical/mineral) UV filters zinc oxide and titanium dioxide were considered GRASE.4 Trolamine salicylate and para-aminobenzoic acid were not GRASE, but they currently are not used in sunscreens in the United States. For all the remaining organic (chemical) filters, additional safety data were required to establish GRASE status.4 In 2024, the situation remains largely unchanged. Industry is working with the FDA on testing requirements.5
Why the focus on safety? After all, sunscreens have been used widely for decades without any major safety signals; their only well-established adverse effects are contact dermatitis and staining of clothing.6 Although preclinical studies raised concerns that chemical sunscreens could be associated with endocrine, reproductive, and neurologic toxicities, to date there are no high-quality human studies demonstrating negative effects.7,8
However, exposure patterns have evolved. Sunscreen is recommended to be applied (and reapplied) daily. Also, chemical UV filters are used in many nonsunscreen products such as cosmetics, shampoos, fragrances, and plastics. In the United States, exposure to chemical sunscreens is ubiquitous; according to data from the National Health and Nutrition Examination Survey 2003-2004, oxybenzone was detected in 97% of more than 2500 urine samples, implying systemic absorption but not harm.9
The FDA confirmed the implication of systemic absorption via 2 maximal usage trials published in 2019 and 2020.10,11 In both studies, several chemical sunscreens were applied at the recommended density of 2 mg/cm2 to 75% of the body surface area multiple times over 4 days. For all tested organic UV filters, blood levels exceeded the predetermined FDA cutoff (0.5 ng/mL), even after one application.10,11 What’s the takeaway? Simply that the FDA now requires additional safety data for chemical sunscreen filters5; the findings in no way imply any associated harm. Two potential mitigating factors are that no one applies sunscreen at 2 mg/cm2, and the FDA’s blood level cutoff was a general estimate not specific to sunscreens.4,12
Nevertheless, a good long-term safety record for sunscreens does not negate the need for enhanced safety data when there is clear evidence of systemic absorption. In the meantime, concerned patients should be counseled that the physical/mineral sunscreens containing zinc oxide and titanium dioxide are considered GRASE by the FDA; even in nanoparticle form, they generally have not been found to penetrate beneath the stratum corneum.7,13
Does sunscreen cause frontal fibrosing alopecia?
Dermatologists are confronting the conundrum of rising cases of frontal fibrosing alopecia (FFA). Several theories on the pathogenesis of this idiopathic scarring alopecia have been raised, one of which involves increased use of sunscreen. Proposed explanations for sunscreen’s role in FFA include a lichenoid reaction inducing hair follicle autoimmunity through an unclear mechanism; a T cell–mediated allergic reaction, which is unlikely according to contact dermatitis experts14; reactive oxygen species production by titanium nanoparticles, yet titanium has been detected in hair follicles of both patients with FFA and controls15; and endocrine disruption following systemic absorption, which has not been supported by any high-quality human studies.7
An association between facial sunscreen use and FFA has been reported in case-control studies16; however, they have been criticized due to methodologic issues and biases, and they provide no evidence of causality.17,18 The jury remains out on the controversial association between sunscreen and FFA, with a need for more convincing data.
Does sunscreen impact coral reef health?
Coral reefs—crucial sources of aquatic biodiversity—are under attack from several different directions including climate change and pollution. As much as 14,000 tons of sunscreen enter coral reefs each year, and chemical sunscreen filters are detectable in waterways throughout the world—even in the Arctic.19,20 Thus, sunscreen has come under scrutiny as a potential environmental threat, particularly with coral bleaching.
Bleaching is a process in which corals exposed to an environmental stressor expel their symbiotic photosynthetic algae and turn white; if conditions fail to improve, the corals are vulnerable to death. In a highly cited 2016 study, coral larvae exposed to oxybenzone in artificial laboratory conditions displayed concentration-dependent mortality and decreased chlorophyll fluorescence, which suggested bleaching.19 These findings influenced legislation in Hawaii and other localities banning sunscreens containing oxybenzone. Problematically, the study has been criticized for acutely exposing the most susceptible coral life-forms to unrealistic oxybenzone concentrations; more broadly, there is no standardized approach to coral toxicity testing.21
The bigger picture (and elephant in the room) is that the primary cause of coral bleaching is undoubtedly climate change/ocean warming.7 More recent studies suggest that oxybenzone probably adds insult to injury for corals already debilitated by ocean warming.22,23
It has been posited that a narrow focus on sunscreens detracts attention from the climate issue.24 Individuals can take a number of actions to reduce their carbon footprint in an effort to preserve our environment, specifically coral reefs.25 Concerned patients should be counseled to use sunscreens containing the physical/mineral UV filters zinc oxide and titanium dioxide, which are unlikely to contribute to coral bleaching as commercially formulated.7
Ongoing Questions
A lot of unknowns about sunscreen safety remain, and much hubbub has been made over studies that often are preliminary at best. At the time of this writing, absent a crystal ball, this author continues to wear chemical sunscreens; spends a lot more time worrying about their carbon footprint than what type of sunscreen to use at the beach; and believes the association of FFA with sunscreen is unlikely to be causal. Hopefully much-needed rigorous evidence will guide our future approach to sunscreen formulation and use.
Sunscreen is a cornerstone of skin cancer prevention. The first commercial sunscreen was developed nearly 100 years ago,1 yet questions and concerns about the safety of these essential topical photoprotective agents continue to occupy our minds. This article serves as an update on some of the big sunscreen questions, as informed by the available evidence.
Are sunscreens safe?
The story of sunscreen regulation in the United States is long and dry. The major pain point is that sunscreens are regulated by the US Food and Drug Administration (FDA) as over-the-counter drugs rather than cosmetics (as in Europe).2 Regulatory hurdles created a situation wherein no new active sunscreen ingredient has been approved by the FDA since 1999, except ecamsule for use in one product line. There is hope that changes enacted under the CARES Act will streamline and expedite the sunscreen approval process in the future.3
Amid the ongoing regulatory slog, the FDA became interested in learning more about sunscreen safety. Specifically, they sought to determine the GRASE (generally regarded as safe and effective) status of the active ingredients in sunscreens. In 2019, only the inorganic (physical/mineral) UV filters zinc oxide and titanium dioxide were considered GRASE.4 Trolamine salicylate and para-aminobenzoic acid were not GRASE, but they currently are not used in sunscreens in the United States. For all the remaining organic (chemical) filters, additional safety data were required to establish GRASE status.4 In 2024, the situation remains largely unchanged. Industry is working with the FDA on testing requirements.5
Why the focus on safety? After all, sunscreens have been used widely for decades without any major safety signals; their only well-established adverse effects are contact dermatitis and staining of clothing.6 Although preclinical studies raised concerns that chemical sunscreens could be associated with endocrine, reproductive, and neurologic toxicities, to date there are no high-quality human studies demonstrating negative effects.7,8
However, exposure patterns have evolved. Sunscreen is recommended to be applied (and reapplied) daily. Also, chemical UV filters are used in many nonsunscreen products such as cosmetics, shampoos, fragrances, and plastics. In the United States, exposure to chemical sunscreens is ubiquitous; according to data from the National Health and Nutrition Examination Survey 2003-2004, oxybenzone was detected in 97% of more than 2500 urine samples, implying systemic absorption but not harm.9
The FDA confirmed the implication of systemic absorption via 2 maximal usage trials published in 2019 and 2020.10,11 In both studies, several chemical sunscreens were applied at the recommended density of 2 mg/cm2 to 75% of the body surface area multiple times over 4 days. For all tested organic UV filters, blood levels exceeded the predetermined FDA cutoff (0.5 ng/mL), even after one application.10,11 What’s the takeaway? Simply that the FDA now requires additional safety data for chemical sunscreen filters5; the findings in no way imply any associated harm. Two potential mitigating factors are that no one applies sunscreen at 2 mg/cm2, and the FDA’s blood level cutoff was a general estimate not specific to sunscreens.4,12
Nevertheless, a good long-term safety record for sunscreens does not negate the need for enhanced safety data when there is clear evidence of systemic absorption. In the meantime, concerned patients should be counseled that the physical/mineral sunscreens containing zinc oxide and titanium dioxide are considered GRASE by the FDA; even in nanoparticle form, they generally have not been found to penetrate beneath the stratum corneum.7,13
Does sunscreen cause frontal fibrosing alopecia?
Dermatologists are confronting the conundrum of rising cases of frontal fibrosing alopecia (FFA). Several theories on the pathogenesis of this idiopathic scarring alopecia have been raised, one of which involves increased use of sunscreen. Proposed explanations for sunscreen’s role in FFA include a lichenoid reaction inducing hair follicle autoimmunity through an unclear mechanism; a T cell–mediated allergic reaction, which is unlikely according to contact dermatitis experts14; reactive oxygen species production by titanium nanoparticles, yet titanium has been detected in hair follicles of both patients with FFA and controls15; and endocrine disruption following systemic absorption, which has not been supported by any high-quality human studies.7
An association between facial sunscreen use and FFA has been reported in case-control studies16; however, they have been criticized due to methodologic issues and biases, and they provide no evidence of causality.17,18 The jury remains out on the controversial association between sunscreen and FFA, with a need for more convincing data.
Does sunscreen impact coral reef health?
Coral reefs—crucial sources of aquatic biodiversity—are under attack from several different directions including climate change and pollution. As much as 14,000 tons of sunscreen enter coral reefs each year, and chemical sunscreen filters are detectable in waterways throughout the world—even in the Arctic.19,20 Thus, sunscreen has come under scrutiny as a potential environmental threat, particularly with coral bleaching.
Bleaching is a process in which corals exposed to an environmental stressor expel their symbiotic photosynthetic algae and turn white; if conditions fail to improve, the corals are vulnerable to death. In a highly cited 2016 study, coral larvae exposed to oxybenzone in artificial laboratory conditions displayed concentration-dependent mortality and decreased chlorophyll fluorescence, which suggested bleaching.19 These findings influenced legislation in Hawaii and other localities banning sunscreens containing oxybenzone. Problematically, the study has been criticized for acutely exposing the most susceptible coral life-forms to unrealistic oxybenzone concentrations; more broadly, there is no standardized approach to coral toxicity testing.21
The bigger picture (and elephant in the room) is that the primary cause of coral bleaching is undoubtedly climate change/ocean warming.7 More recent studies suggest that oxybenzone probably adds insult to injury for corals already debilitated by ocean warming.22,23
It has been posited that a narrow focus on sunscreens detracts attention from the climate issue.24 Individuals can take a number of actions to reduce their carbon footprint in an effort to preserve our environment, specifically coral reefs.25 Concerned patients should be counseled to use sunscreens containing the physical/mineral UV filters zinc oxide and titanium dioxide, which are unlikely to contribute to coral bleaching as commercially formulated.7
Ongoing Questions
A lot of unknowns about sunscreen safety remain, and much hubbub has been made over studies that often are preliminary at best. At the time of this writing, absent a crystal ball, this author continues to wear chemical sunscreens; spends a lot more time worrying about their carbon footprint than what type of sunscreen to use at the beach; and believes the association of FFA with sunscreen is unlikely to be causal. Hopefully much-needed rigorous evidence will guide our future approach to sunscreen formulation and use.
- Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044-1049.
- Pantelic MN, Wong N, Kwa M, et al. Ultraviolet filters in the United States and European Union: a review of safety and implications for the future of US sunscreens. J Am Acad Dermatol. 2023;88:632-646.
- Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509-511.
- Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regist. 2019;84:6204-6275.
- Lim HW, Mohammad TF, Wang SQ. Food and Drug Administration’s proposed sunscreen final administrative order: how does it affect sunscreens in the United States? J Am Acad Dermatol. 2022;86:E83-E84.
- Ekstein SF, Hylwa S. Sunscreens: a review of UV filters and their allergic potential. Dermatitis. 2023;34:176-190.
- Adler BL, DeLeo VA. Sunscreen safety: a review of recent studies on humans and the environment. Curr Dermatol Rep. 2020;9:1-9.
- Suh S, Pham C, Smith J, et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033-1042.
- Calafat AM, Wong LY, Ye X, et al. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893-897.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30:96-101.
- Mohammed YH, Holmes A, Haridass IN, et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol. 2019;139:308-315.
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482.
- Thompson CT, Chen ZQ, Kolivras A, et al. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181:216-217.
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396.
- Seegobin SD, Tziotzios C, Stefanato CM, et al. Frontal fibrosing alopecia:there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407-1408.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Regarding methodologic concerns in clinical studies on frontal fibrosing alopecia. J Am Acad Dermatol. 2021;84:E207-E208.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- National Academies of Sciences, Engineering, and Medicine. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health. The National Academies Press; 2022.
- Mitchelmore CL, Burns EE, Conway A, et al. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967-988.
- Vuckovic D, Tinoco AI, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals. Science. 2022;376:644-648.
- Wijgerde T, van Ballegooijen M, Nijland R, et al. Adding insult to injury: effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci Total Environ. 2020;733:139030.
- Sirois J. Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211-212.
- United Nations. Actions for a healthy planet. Accessed April 15, 2024. https://www.un.org/en/actnow/ten-actions
- Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044-1049.
- Pantelic MN, Wong N, Kwa M, et al. Ultraviolet filters in the United States and European Union: a review of safety and implications for the future of US sunscreens. J Am Acad Dermatol. 2023;88:632-646.
- Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509-511.
- Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regist. 2019;84:6204-6275.
- Lim HW, Mohammad TF, Wang SQ. Food and Drug Administration’s proposed sunscreen final administrative order: how does it affect sunscreens in the United States? J Am Acad Dermatol. 2022;86:E83-E84.
- Ekstein SF, Hylwa S. Sunscreens: a review of UV filters and their allergic potential. Dermatitis. 2023;34:176-190.
- Adler BL, DeLeo VA. Sunscreen safety: a review of recent studies on humans and the environment. Curr Dermatol Rep. 2020;9:1-9.
- Suh S, Pham C, Smith J, et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033-1042.
- Calafat AM, Wong LY, Ye X, et al. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893-897.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30:96-101.
- Mohammed YH, Holmes A, Haridass IN, et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol. 2019;139:308-315.
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482.
- Thompson CT, Chen ZQ, Kolivras A, et al. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181:216-217.
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396.
- Seegobin SD, Tziotzios C, Stefanato CM, et al. Frontal fibrosing alopecia:there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407-1408.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Regarding methodologic concerns in clinical studies on frontal fibrosing alopecia. J Am Acad Dermatol. 2021;84:E207-E208.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- National Academies of Sciences, Engineering, and Medicine. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health. The National Academies Press; 2022.
- Mitchelmore CL, Burns EE, Conway A, et al. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967-988.
- Vuckovic D, Tinoco AI, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals. Science. 2022;376:644-648.
- Wijgerde T, van Ballegooijen M, Nijland R, et al. Adding insult to injury: effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci Total Environ. 2020;733:139030.
- Sirois J. Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211-212.
- United Nations. Actions for a healthy planet. Accessed April 15, 2024. https://www.un.org/en/actnow/ten-actions
Artificial Intelligence in GI and Hepatology
Dear colleagues,
Since our prior Perspectives piece on artificial intelligence (AI) in GI and Hepatology in 2022, the field has seen almost exponential growth. Expectations are high that AI will revolutionize our field and significantly improve patient care. But as the global discussion on AI has shown, there are real challenges with adoption, including issues with accuracy, reliability, and privacy.
In this issue, Dr. Nabil M. Mansour and Dr. Thomas R. McCarty explore the current and future impact of AI on gastroenterology, while Dr. Basile Njei and Yazan A. Al Ajlouni assess its role in hepatology. We hope these pieces will help your discussions in incorporating or researching AI for use in your own practices. We welcome your thoughts on this issue on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Artificial Intelligence in Gastrointestinal Endoscopy
BY THOMAS R. MCCARTY, MD, MPH; NABIL M. MANSOUR, MD
The last few decades have seen an exponential increase and interest in the role of artificial intelligence (AI) and adoption of deep learning algorithms within healthcare and patient care services. The field of gastroenterology and endoscopy has similarly seen a tremendous uptake in acceptance and implementation of AI for a variety of gastrointestinal conditions. The spectrum of AI-based applications includes detection or diagnostic-based as well as therapeutic assistance tools. From the first US Food and Drug Administration (FDA)-approved device that uses machine learning to assist clinicians in detecting lesions during colonoscopy, to other more innovative machine learning techniques for small bowel, esophageal, and hepatobiliary conditions, AI has dramatically changed the landscape of gastrointestinal endoscopy.
Approved applications for colorectal cancer
In an attempt to improve colorectal cancer screening and outcomes related to screening and surveillance, efforts have been focused on procedural performance metrics, quality indicators, and tools to aid in lesion detection and improve quality of care. One such tool has been computer-aided detection (CADe), with early randomized controlled trial (RCT) data showing significantly increased adenoma detection rate (ADR) and adenomas per colonoscopy (APC).1-3
Ultimately, this data led to FDA approval of the CADe system GI Genius (Medtronic, Dublin, Ireland) in 2021.4 Additional systems have since been FDA approved or 510(k) cleared including Endoscreener (Wision AI, Shanghai, China), SKOUT (Iterative Health, Cambridge, Massachusetts), MAGENTIQ-COLO (MAGENTIQ-EYE LTD, Haifa, Israel), and CAD EYE (Fujifilm, Tokyo), all of which have shown increased ADR and/or increased APC and/or reduced adenoma miss rates in randomized trials.5
Yet despite the promise of improved quality and subsequent translation to better patient outcomes, there has been a noticeable disconnect between RCT data and more real-world literature.6 In a recent study, no improvement was seen in ADR after implementation of a CADe system for colorectal cancer screening — including both higher and lower-ADR performers. Looking at change over time after implementation, CADe had no positive effect in any group over time, divergent from early RCT data. In a more recent multicenter, community-based RCT study, again CADe did not result in a statistically significant difference in the number of adenomas detected.7 The differences between some of these more recent “real-world” studies vs the majority of data from RCTs raise important questions regarding the potential of bias (due to unblinding) in prospective trials, as well as the role of the human-AI interaction.
Importantly for RCT data, both cohorts in these studies met adequate ADR benchmarks, though it remains unclear whether a truly increased ADR necessitates better patient outcomes — is higher always better? In addition, an important consideration with evaluating any AI/CADe system is that they often undergo frequent updates, each promising improved accuracy, sensitivity, and specificity. This is an interesting dilemma and raises questions about the enduring relevance of studies conducted using an outdated version of a CADe system.
Additional unanswered questions regarding an ideal ADR for implementation, preferred patient populations for screening (especially for younger individuals), and the role and adoption of computer-aided polyp diagnosis/characterization (CADx) within the United States remain. Furthermore, questions regarding procedural withdrawal time, impact on sessile serrated lesion detection, cost-effectiveness, and preferred adoption strategies have begun to be explored, though require more data to better define a best practice approach. Ultimately, answers to some of these unknowns may explain the discordant results and help guide future implementation measures.
Innovative applications for alternative gastrointestinal conditions
Given the fervor and excitement, as well as the outcomes associated with AI-based colorectal screening, it is not surprising these techniques have been expanded to other gastrointestinal conditions. At this time, all of these are fledgling, mostly single-center tools, not yet ready for widespread adoption. Nonetheless, these represent a potentially important step forward for difficult-to-manage gastrointestinal diseases.
Machine learning CADe systems have been developed to help identify early Barrett’s neoplasia, depth and invasion of gastric cancer, as well as lesion detection in small bowel video capsule endoscopy.8-10 Endoscopic retrograde cholangiopancreatography (ERCP)-based applications for cholangiocarcinoma and indeterminate stricture diagnosis have also been studied.11 Additional AI-based algorithms have been employed for complex procedures such as endoscopic submucosal dissection (ESD) or peroral endoscopic myotomy (POEM) to delineate vessels, better define tissue planes for dissection, and visualize landmark structures.12,13 Furthermore, AI-based scope guidance/manipulation, bleeding detection, landmark identification, and lesion detection have the potential to revolutionize endoscopic training and education. The impact that generative AI can potentially have on clinical practice is also an exciting prospect that warrants further investigation.
Artificial intelligence adoption in clinical practice
Clinical practice with regard to AI and colorectal cancer screening largely mirrors the disconnect in the current literature, with “believers” and “non-believers” as well as innovators and early adopters alongside laggards. In our own academic practices, we continue to struggle with the adoption and standardized implementation of AI-based colorectal cancer CADe systems, despite the RCT data showing positive results. It is likely that AI uptake will follow the technology predictions of Amara’s Law — i.e., individuals tend to overestimate the short-term impact of new technologies while underestimating long-term effects. In the end, more widespread adoption in community practice and larger scale real-world clinical outcomes studies are likely to determine the true impact of these exciting technologies. For other, less established AI-based tools, more data are currently required.
Conclusions
Ultimately, AI-based algorithms are likely here to stay, with continued improvement and evolution to occur based on provider feedback and patient care needs. Current tools, while not all-encompassing, have the potential to dramatically change the landscape of endoscopic training, diagnostic evaluation, and therapeutic care. It is critically important that relevant stakeholders, both endoscopists and patients, be involved in future applications and design to improve efficiency and quality outcomes overall.
Dr. McCarty is based in the Lynda K. and David M. Underwood Center for Digestive Disorders, Houston Methodist Hospital. Dr. Mansour is based in the section of gastroenterology, Baylor College of Medicine, Houston. Dr. McCarty reports no conflicts of interest. Dr. Mansour reports having been a consultant for Iterative Health.
References
1. Repici A, et al. Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Gastroenterology. 2020 Aug. doi: 10.1053/j.gastro.2020.04.062.
2. Repici A, et al. Artificial intelligence and colonoscopy experience: Lessons from two randomised trials. Gut. Apr 2022. doi: 10.1136/gutjnl-2021-324471.
3. Wallace MB, et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology 2022 Jul. doi: 10.1053/j.gastro.2022.03.007.
4. United States Food and Drug Administration (FDA). GI Genius FDA Approval [April 9, 2021]. Accessed January 5, 2022. Available at: www.accessdata.fda.gov/cdrh_docs/pdf21/K211951.pdf.
5. Maas MHJ, et al. A computer-aided polyp detection system in screening and surveillance colonoscopy: An international, multicentre, randomised, tandem trial. Lancet Digit Health. 2024 Mar. doi: 10.1016/S2589-7500(23)00242-X.
6. Ladabaum U, et al. Computer-aided detection of polyps does not improve colonoscopist performance in a pragmatic implementation trial. Gastroenterology. 2023 Mar. doi: 10.1053/j.gastro.2022.12.004.
7. Wei MT, et al. Evaluation of computer-aided detection during colonoscopy in the community (AI-SEE): A multicenter randomized clinical trial. Am J Gastroenterol. 2023 Oct. doi: 10.14309/ajg.0000000000002239.
8. de Groof J, et al. The Argos project: The development of a computer-aided detection system to improve detection of Barrett’s neoplasia on white light endoscopy. United European Gastroenterol J. 2019 May. doi: 10.1177/2050640619837443.
9. Kanesaka T, et al. Computer-aided diagnosis for identifying and delineating early gastric cancers in magnifying narrow-band imaging. Gastrointest Endosc. 2018 May. doi: 10.1016/j.gie.2017.11.029.
10. Sahafi A, et al. Edge artificial intelligence wireless video capsule endoscopy. Sci Rep. 2022 Aug. doi: 10.1038/s41598-022-17502-7.
11. Njei B, et al. Artificial intelligence in endoscopic imaging for detection of malignant biliary strictures and cholangiocarcinoma: A systematic review. Ann Gastroenterol. 2023 Mar-Apr. doi: 10.20524/aog.2023.0779.
12. Ebigbo A, et al. Vessel and tissue recognition during third-space endoscopy using a deep learning algorithm. Gut. 2022 Dec. doi: 10.1136/gutjnl-2021-326470.
13. Cao J, et al. Intelligent surgical workflow recognition for endoscopic submucosal dissection with real-time animal study. Nat Commun. 2023 Oct. doi: 10.1038/s41467-023-42451-8.
The Promise and Challenges of AI in Hepatology
BY BASILE NJEI, MD, MPH, PHD; YAZAN A. AL-AJLOUNI, MPHIL
In the dynamic realm of medicine, artificial intelligence (AI) emerges as a transformative force, notably within hepatology. The discipline of hepatology, dedicated to liver and related organ diseases, is ripe for AI’s promise to revolutionize diagnostics and treatment, pushing toward a future of precision medicine. Yet, the path to fully realizing AI’s potential in hepatology is laced with data, ethical, and integration challenges.
The application of AI, particularly in histopathology, significantly enhances disease diagnosis and staging in hepatology. AI-driven approaches remedy traditional histopathological challenges, such as interpretative variability, providing more consistent and accurate disease analyses. This is especially evident in conditions like metabolic dysfunction-associated steatohepatitis (MASH) and hepatocellular carcinoma (HCC), where AI aids in identifying critical gene signatures, thereby refining therapy selection.
Similarly, deep learning (DL), a branch of AI, has attracted significant interest globally, particularly in image recognition. AI’s incorporation into medical imaging marks a significant advancement, enabling early detection of malignancies like HCC and improving diagnostics in steatotic liver disease through enhanced imaging analyses using convolutional neural networks (CNN). The abundance of imaging data alongside clinical outcomes has catalyzed AI’s integration into radiology, leading to the swift growth of radiomics as a novel domain in medical research.
AI has also been shown to identify nuanced alterations in electrocardiograms (EKGs) associated with liver conditions, potentially detecting the progression of liver diseases at an earlier stage than currently possible. By leveraging complex algorithms and machine learning, AI can analyze EKG patterns with a precision and depth unattainable through traditional manual interpretation. Given that liver diseases, such as cirrhosis or hepatitis, can induce subtle cardiac changes long before other clinical symptoms manifest, early detection through AI-enhanced EKG analysis could lead to timely interventions, potentially halting or reversing disease progression. This approach further enriches our understanding of the intricate interplay between liver function and cardiac health, highlighting the potential for AI to transform not just liver disease diagnostics but also to foster a more integrated approach to patient care.
Beyond diagnostics, the burgeoning field of generative AI introduces groundbreaking possibilities in treatment planning and patient education, particularly for chronic conditions like cirrhosis. Generative AI produces original content, including text, visuals, and music, by identifying and learning patterns from its training data. When it leverages large language models (LLMs), it entails training on vast collections of textual data and using AI models characterized by many parameters. A notable instance of generative AI employing LLMs is ChatGPT (General Pretrained Transformers). By simulating disease progression and treatment outcomes, generative AI can foster personalized treatment strategies and empower patients with knowledge about their health trajectories. Yet, realizing these potential demands requires overcoming data quality and interpretability challenges, and ensuring AI outputs are accessible and actionable for clinicians and patients.
Despite these advancements, leveraging AI in hepatology is not devoid of hurdles. The development and training of AI models require extensive and diverse datasets, raising concerns about data privacy and ethical use. Addressing these concerns is paramount for successfully integrating AI into clinical hepatology practice, necessitating transparent algorithmic processes and stringent ethical standards. Ethical considerations are central to AI’s integration into hepatology. Algorithmic biases, patient privacy, and the impact of AI-driven decisions underscore the need for cautious AI deployment. Developing transparent, understandable algorithms and establishing ethical guidelines for AI use are critical steps towards ethically leveraging AI in patient care.
In conclusion, AI’s integration into hepatology holds tremendous promise for advancing patient care through enhanced diagnostics, treatment planning, and patient education. Overcoming the associated challenges, including ethical concerns, data diversity, and algorithm interpretability, is crucial. As the hepatology community navigates this technological evolution, a balanced approach that marries technological advancements with ethical stewardship will be key to harnessing AI’s full potential, ensuring it serves the best interests of patients and propels the field of hepatology into the future.
We predict a trajectory of increased use and adoption of AI in hepatology. AI in hepatology is likely to meet the test of pervasiveness, improvement, and innovation. The adoption of AI in routine hepatology diagnosis and management will likely follow Amara’s law and the five stages of the hype cycle. We believe that we are still in the infant stages of adopting AI technology in hepatology, and this phase may last 5 years before there is a peak of inflated expectations. The trough of disillusionment and slopes of enlightenment may only be observed in the next decades.
Dr. Njei is based in the Section of Digestive Diseases, Yale School of Medicine, New Haven, Conn. Mr. Al-Ajlouni is a senior medical student at New York Medical College School of Medicine, Valhalla, N.Y. They have no conflicts of interest to declare.
Sources
Taylor-Weiner A, et al. A Machine Learning Approach Enables Quantitative Measurement of Liver Histology and Disease Monitoring in NASH. Hepatology. 2021 Jul. doi: 10.1002/hep.31750.
Zeng Q, et al. Artificial intelligence predicts immune and inflammatory gene signatures directly from hepatocellular carcinoma histology. J Hepatol. 2022 Jul. doi: 10.1016/j.jhep.2022.01.018.
Ahn JC, et al. Development of the AI-Cirrhosis-ECG Score: An Electrocardiogram-Based Deep Learning Model in Cirrhosis. Am J Gastroenterol. 2022 Mar. doi: 10.14309/ajg.0000000000001617.
Nduma BN, et al. The Application of Artificial Intelligence (AI)-Based Ultrasound for the Diagnosis of Fatty Liver Disease: A Systematic Review. Cureus. 2023 Dec 15. doi: 10.7759/cureus.50601.
Dear colleagues,
Since our prior Perspectives piece on artificial intelligence (AI) in GI and Hepatology in 2022, the field has seen almost exponential growth. Expectations are high that AI will revolutionize our field and significantly improve patient care. But as the global discussion on AI has shown, there are real challenges with adoption, including issues with accuracy, reliability, and privacy.
In this issue, Dr. Nabil M. Mansour and Dr. Thomas R. McCarty explore the current and future impact of AI on gastroenterology, while Dr. Basile Njei and Yazan A. Al Ajlouni assess its role in hepatology. We hope these pieces will help your discussions in incorporating or researching AI for use in your own practices. We welcome your thoughts on this issue on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Artificial Intelligence in Gastrointestinal Endoscopy
BY THOMAS R. MCCARTY, MD, MPH; NABIL M. MANSOUR, MD
The last few decades have seen an exponential increase and interest in the role of artificial intelligence (AI) and adoption of deep learning algorithms within healthcare and patient care services. The field of gastroenterology and endoscopy has similarly seen a tremendous uptake in acceptance and implementation of AI for a variety of gastrointestinal conditions. The spectrum of AI-based applications includes detection or diagnostic-based as well as therapeutic assistance tools. From the first US Food and Drug Administration (FDA)-approved device that uses machine learning to assist clinicians in detecting lesions during colonoscopy, to other more innovative machine learning techniques for small bowel, esophageal, and hepatobiliary conditions, AI has dramatically changed the landscape of gastrointestinal endoscopy.
Approved applications for colorectal cancer
In an attempt to improve colorectal cancer screening and outcomes related to screening and surveillance, efforts have been focused on procedural performance metrics, quality indicators, and tools to aid in lesion detection and improve quality of care. One such tool has been computer-aided detection (CADe), with early randomized controlled trial (RCT) data showing significantly increased adenoma detection rate (ADR) and adenomas per colonoscopy (APC).1-3
Ultimately, this data led to FDA approval of the CADe system GI Genius (Medtronic, Dublin, Ireland) in 2021.4 Additional systems have since been FDA approved or 510(k) cleared including Endoscreener (Wision AI, Shanghai, China), SKOUT (Iterative Health, Cambridge, Massachusetts), MAGENTIQ-COLO (MAGENTIQ-EYE LTD, Haifa, Israel), and CAD EYE (Fujifilm, Tokyo), all of which have shown increased ADR and/or increased APC and/or reduced adenoma miss rates in randomized trials.5
Yet despite the promise of improved quality and subsequent translation to better patient outcomes, there has been a noticeable disconnect between RCT data and more real-world literature.6 In a recent study, no improvement was seen in ADR after implementation of a CADe system for colorectal cancer screening — including both higher and lower-ADR performers. Looking at change over time after implementation, CADe had no positive effect in any group over time, divergent from early RCT data. In a more recent multicenter, community-based RCT study, again CADe did not result in a statistically significant difference in the number of adenomas detected.7 The differences between some of these more recent “real-world” studies vs the majority of data from RCTs raise important questions regarding the potential of bias (due to unblinding) in prospective trials, as well as the role of the human-AI interaction.
Importantly for RCT data, both cohorts in these studies met adequate ADR benchmarks, though it remains unclear whether a truly increased ADR necessitates better patient outcomes — is higher always better? In addition, an important consideration with evaluating any AI/CADe system is that they often undergo frequent updates, each promising improved accuracy, sensitivity, and specificity. This is an interesting dilemma and raises questions about the enduring relevance of studies conducted using an outdated version of a CADe system.
Additional unanswered questions regarding an ideal ADR for implementation, preferred patient populations for screening (especially for younger individuals), and the role and adoption of computer-aided polyp diagnosis/characterization (CADx) within the United States remain. Furthermore, questions regarding procedural withdrawal time, impact on sessile serrated lesion detection, cost-effectiveness, and preferred adoption strategies have begun to be explored, though require more data to better define a best practice approach. Ultimately, answers to some of these unknowns may explain the discordant results and help guide future implementation measures.
Innovative applications for alternative gastrointestinal conditions
Given the fervor and excitement, as well as the outcomes associated with AI-based colorectal screening, it is not surprising these techniques have been expanded to other gastrointestinal conditions. At this time, all of these are fledgling, mostly single-center tools, not yet ready for widespread adoption. Nonetheless, these represent a potentially important step forward for difficult-to-manage gastrointestinal diseases.
Machine learning CADe systems have been developed to help identify early Barrett’s neoplasia, depth and invasion of gastric cancer, as well as lesion detection in small bowel video capsule endoscopy.8-10 Endoscopic retrograde cholangiopancreatography (ERCP)-based applications for cholangiocarcinoma and indeterminate stricture diagnosis have also been studied.11 Additional AI-based algorithms have been employed for complex procedures such as endoscopic submucosal dissection (ESD) or peroral endoscopic myotomy (POEM) to delineate vessels, better define tissue planes for dissection, and visualize landmark structures.12,13 Furthermore, AI-based scope guidance/manipulation, bleeding detection, landmark identification, and lesion detection have the potential to revolutionize endoscopic training and education. The impact that generative AI can potentially have on clinical practice is also an exciting prospect that warrants further investigation.
Artificial intelligence adoption in clinical practice
Clinical practice with regard to AI and colorectal cancer screening largely mirrors the disconnect in the current literature, with “believers” and “non-believers” as well as innovators and early adopters alongside laggards. In our own academic practices, we continue to struggle with the adoption and standardized implementation of AI-based colorectal cancer CADe systems, despite the RCT data showing positive results. It is likely that AI uptake will follow the technology predictions of Amara’s Law — i.e., individuals tend to overestimate the short-term impact of new technologies while underestimating long-term effects. In the end, more widespread adoption in community practice and larger scale real-world clinical outcomes studies are likely to determine the true impact of these exciting technologies. For other, less established AI-based tools, more data are currently required.
Conclusions
Ultimately, AI-based algorithms are likely here to stay, with continued improvement and evolution to occur based on provider feedback and patient care needs. Current tools, while not all-encompassing, have the potential to dramatically change the landscape of endoscopic training, diagnostic evaluation, and therapeutic care. It is critically important that relevant stakeholders, both endoscopists and patients, be involved in future applications and design to improve efficiency and quality outcomes overall.
Dr. McCarty is based in the Lynda K. and David M. Underwood Center for Digestive Disorders, Houston Methodist Hospital. Dr. Mansour is based in the section of gastroenterology, Baylor College of Medicine, Houston. Dr. McCarty reports no conflicts of interest. Dr. Mansour reports having been a consultant for Iterative Health.
References
1. Repici A, et al. Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Gastroenterology. 2020 Aug. doi: 10.1053/j.gastro.2020.04.062.
2. Repici A, et al. Artificial intelligence and colonoscopy experience: Lessons from two randomised trials. Gut. Apr 2022. doi: 10.1136/gutjnl-2021-324471.
3. Wallace MB, et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology 2022 Jul. doi: 10.1053/j.gastro.2022.03.007.
4. United States Food and Drug Administration (FDA). GI Genius FDA Approval [April 9, 2021]. Accessed January 5, 2022. Available at: www.accessdata.fda.gov/cdrh_docs/pdf21/K211951.pdf.
5. Maas MHJ, et al. A computer-aided polyp detection system in screening and surveillance colonoscopy: An international, multicentre, randomised, tandem trial. Lancet Digit Health. 2024 Mar. doi: 10.1016/S2589-7500(23)00242-X.
6. Ladabaum U, et al. Computer-aided detection of polyps does not improve colonoscopist performance in a pragmatic implementation trial. Gastroenterology. 2023 Mar. doi: 10.1053/j.gastro.2022.12.004.
7. Wei MT, et al. Evaluation of computer-aided detection during colonoscopy in the community (AI-SEE): A multicenter randomized clinical trial. Am J Gastroenterol. 2023 Oct. doi: 10.14309/ajg.0000000000002239.
8. de Groof J, et al. The Argos project: The development of a computer-aided detection system to improve detection of Barrett’s neoplasia on white light endoscopy. United European Gastroenterol J. 2019 May. doi: 10.1177/2050640619837443.
9. Kanesaka T, et al. Computer-aided diagnosis for identifying and delineating early gastric cancers in magnifying narrow-band imaging. Gastrointest Endosc. 2018 May. doi: 10.1016/j.gie.2017.11.029.
10. Sahafi A, et al. Edge artificial intelligence wireless video capsule endoscopy. Sci Rep. 2022 Aug. doi: 10.1038/s41598-022-17502-7.
11. Njei B, et al. Artificial intelligence in endoscopic imaging for detection of malignant biliary strictures and cholangiocarcinoma: A systematic review. Ann Gastroenterol. 2023 Mar-Apr. doi: 10.20524/aog.2023.0779.
12. Ebigbo A, et al. Vessel and tissue recognition during third-space endoscopy using a deep learning algorithm. Gut. 2022 Dec. doi: 10.1136/gutjnl-2021-326470.
13. Cao J, et al. Intelligent surgical workflow recognition for endoscopic submucosal dissection with real-time animal study. Nat Commun. 2023 Oct. doi: 10.1038/s41467-023-42451-8.
The Promise and Challenges of AI in Hepatology
BY BASILE NJEI, MD, MPH, PHD; YAZAN A. AL-AJLOUNI, MPHIL
In the dynamic realm of medicine, artificial intelligence (AI) emerges as a transformative force, notably within hepatology. The discipline of hepatology, dedicated to liver and related organ diseases, is ripe for AI’s promise to revolutionize diagnostics and treatment, pushing toward a future of precision medicine. Yet, the path to fully realizing AI’s potential in hepatology is laced with data, ethical, and integration challenges.
The application of AI, particularly in histopathology, significantly enhances disease diagnosis and staging in hepatology. AI-driven approaches remedy traditional histopathological challenges, such as interpretative variability, providing more consistent and accurate disease analyses. This is especially evident in conditions like metabolic dysfunction-associated steatohepatitis (MASH) and hepatocellular carcinoma (HCC), where AI aids in identifying critical gene signatures, thereby refining therapy selection.
Similarly, deep learning (DL), a branch of AI, has attracted significant interest globally, particularly in image recognition. AI’s incorporation into medical imaging marks a significant advancement, enabling early detection of malignancies like HCC and improving diagnostics in steatotic liver disease through enhanced imaging analyses using convolutional neural networks (CNN). The abundance of imaging data alongside clinical outcomes has catalyzed AI’s integration into radiology, leading to the swift growth of radiomics as a novel domain in medical research.
AI has also been shown to identify nuanced alterations in electrocardiograms (EKGs) associated with liver conditions, potentially detecting the progression of liver diseases at an earlier stage than currently possible. By leveraging complex algorithms and machine learning, AI can analyze EKG patterns with a precision and depth unattainable through traditional manual interpretation. Given that liver diseases, such as cirrhosis or hepatitis, can induce subtle cardiac changes long before other clinical symptoms manifest, early detection through AI-enhanced EKG analysis could lead to timely interventions, potentially halting or reversing disease progression. This approach further enriches our understanding of the intricate interplay between liver function and cardiac health, highlighting the potential for AI to transform not just liver disease diagnostics but also to foster a more integrated approach to patient care.
Beyond diagnostics, the burgeoning field of generative AI introduces groundbreaking possibilities in treatment planning and patient education, particularly for chronic conditions like cirrhosis. Generative AI produces original content, including text, visuals, and music, by identifying and learning patterns from its training data. When it leverages large language models (LLMs), it entails training on vast collections of textual data and using AI models characterized by many parameters. A notable instance of generative AI employing LLMs is ChatGPT (General Pretrained Transformers). By simulating disease progression and treatment outcomes, generative AI can foster personalized treatment strategies and empower patients with knowledge about their health trajectories. Yet, realizing these potential demands requires overcoming data quality and interpretability challenges, and ensuring AI outputs are accessible and actionable for clinicians and patients.
Despite these advancements, leveraging AI in hepatology is not devoid of hurdles. The development and training of AI models require extensive and diverse datasets, raising concerns about data privacy and ethical use. Addressing these concerns is paramount for successfully integrating AI into clinical hepatology practice, necessitating transparent algorithmic processes and stringent ethical standards. Ethical considerations are central to AI’s integration into hepatology. Algorithmic biases, patient privacy, and the impact of AI-driven decisions underscore the need for cautious AI deployment. Developing transparent, understandable algorithms and establishing ethical guidelines for AI use are critical steps towards ethically leveraging AI in patient care.
In conclusion, AI’s integration into hepatology holds tremendous promise for advancing patient care through enhanced diagnostics, treatment planning, and patient education. Overcoming the associated challenges, including ethical concerns, data diversity, and algorithm interpretability, is crucial. As the hepatology community navigates this technological evolution, a balanced approach that marries technological advancements with ethical stewardship will be key to harnessing AI’s full potential, ensuring it serves the best interests of patients and propels the field of hepatology into the future.
We predict a trajectory of increased use and adoption of AI in hepatology. AI in hepatology is likely to meet the test of pervasiveness, improvement, and innovation. The adoption of AI in routine hepatology diagnosis and management will likely follow Amara’s law and the five stages of the hype cycle. We believe that we are still in the infant stages of adopting AI technology in hepatology, and this phase may last 5 years before there is a peak of inflated expectations. The trough of disillusionment and slopes of enlightenment may only be observed in the next decades.
Dr. Njei is based in the Section of Digestive Diseases, Yale School of Medicine, New Haven, Conn. Mr. Al-Ajlouni is a senior medical student at New York Medical College School of Medicine, Valhalla, N.Y. They have no conflicts of interest to declare.
Sources
Taylor-Weiner A, et al. A Machine Learning Approach Enables Quantitative Measurement of Liver Histology and Disease Monitoring in NASH. Hepatology. 2021 Jul. doi: 10.1002/hep.31750.
Zeng Q, et al. Artificial intelligence predicts immune and inflammatory gene signatures directly from hepatocellular carcinoma histology. J Hepatol. 2022 Jul. doi: 10.1016/j.jhep.2022.01.018.
Ahn JC, et al. Development of the AI-Cirrhosis-ECG Score: An Electrocardiogram-Based Deep Learning Model in Cirrhosis. Am J Gastroenterol. 2022 Mar. doi: 10.14309/ajg.0000000000001617.
Nduma BN, et al. The Application of Artificial Intelligence (AI)-Based Ultrasound for the Diagnosis of Fatty Liver Disease: A Systematic Review. Cureus. 2023 Dec 15. doi: 10.7759/cureus.50601.
Dear colleagues,
Since our prior Perspectives piece on artificial intelligence (AI) in GI and Hepatology in 2022, the field has seen almost exponential growth. Expectations are high that AI will revolutionize our field and significantly improve patient care. But as the global discussion on AI has shown, there are real challenges with adoption, including issues with accuracy, reliability, and privacy.
In this issue, Dr. Nabil M. Mansour and Dr. Thomas R. McCarty explore the current and future impact of AI on gastroenterology, while Dr. Basile Njei and Yazan A. Al Ajlouni assess its role in hepatology. We hope these pieces will help your discussions in incorporating or researching AI for use in your own practices. We welcome your thoughts on this issue on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Artificial Intelligence in Gastrointestinal Endoscopy
BY THOMAS R. MCCARTY, MD, MPH; NABIL M. MANSOUR, MD
The last few decades have seen an exponential increase and interest in the role of artificial intelligence (AI) and adoption of deep learning algorithms within healthcare and patient care services. The field of gastroenterology and endoscopy has similarly seen a tremendous uptake in acceptance and implementation of AI for a variety of gastrointestinal conditions. The spectrum of AI-based applications includes detection or diagnostic-based as well as therapeutic assistance tools. From the first US Food and Drug Administration (FDA)-approved device that uses machine learning to assist clinicians in detecting lesions during colonoscopy, to other more innovative machine learning techniques for small bowel, esophageal, and hepatobiliary conditions, AI has dramatically changed the landscape of gastrointestinal endoscopy.
Approved applications for colorectal cancer
In an attempt to improve colorectal cancer screening and outcomes related to screening and surveillance, efforts have been focused on procedural performance metrics, quality indicators, and tools to aid in lesion detection and improve quality of care. One such tool has been computer-aided detection (CADe), with early randomized controlled trial (RCT) data showing significantly increased adenoma detection rate (ADR) and adenomas per colonoscopy (APC).1-3
Ultimately, this data led to FDA approval of the CADe system GI Genius (Medtronic, Dublin, Ireland) in 2021.4 Additional systems have since been FDA approved or 510(k) cleared including Endoscreener (Wision AI, Shanghai, China), SKOUT (Iterative Health, Cambridge, Massachusetts), MAGENTIQ-COLO (MAGENTIQ-EYE LTD, Haifa, Israel), and CAD EYE (Fujifilm, Tokyo), all of which have shown increased ADR and/or increased APC and/or reduced adenoma miss rates in randomized trials.5
Yet despite the promise of improved quality and subsequent translation to better patient outcomes, there has been a noticeable disconnect between RCT data and more real-world literature.6 In a recent study, no improvement was seen in ADR after implementation of a CADe system for colorectal cancer screening — including both higher and lower-ADR performers. Looking at change over time after implementation, CADe had no positive effect in any group over time, divergent from early RCT data. In a more recent multicenter, community-based RCT study, again CADe did not result in a statistically significant difference in the number of adenomas detected.7 The differences between some of these more recent “real-world” studies vs the majority of data from RCTs raise important questions regarding the potential of bias (due to unblinding) in prospective trials, as well as the role of the human-AI interaction.
Importantly for RCT data, both cohorts in these studies met adequate ADR benchmarks, though it remains unclear whether a truly increased ADR necessitates better patient outcomes — is higher always better? In addition, an important consideration with evaluating any AI/CADe system is that they often undergo frequent updates, each promising improved accuracy, sensitivity, and specificity. This is an interesting dilemma and raises questions about the enduring relevance of studies conducted using an outdated version of a CADe system.
Additional unanswered questions regarding an ideal ADR for implementation, preferred patient populations for screening (especially for younger individuals), and the role and adoption of computer-aided polyp diagnosis/characterization (CADx) within the United States remain. Furthermore, questions regarding procedural withdrawal time, impact on sessile serrated lesion detection, cost-effectiveness, and preferred adoption strategies have begun to be explored, though require more data to better define a best practice approach. Ultimately, answers to some of these unknowns may explain the discordant results and help guide future implementation measures.
Innovative applications for alternative gastrointestinal conditions
Given the fervor and excitement, as well as the outcomes associated with AI-based colorectal screening, it is not surprising these techniques have been expanded to other gastrointestinal conditions. At this time, all of these are fledgling, mostly single-center tools, not yet ready for widespread adoption. Nonetheless, these represent a potentially important step forward for difficult-to-manage gastrointestinal diseases.
Machine learning CADe systems have been developed to help identify early Barrett’s neoplasia, depth and invasion of gastric cancer, as well as lesion detection in small bowel video capsule endoscopy.8-10 Endoscopic retrograde cholangiopancreatography (ERCP)-based applications for cholangiocarcinoma and indeterminate stricture diagnosis have also been studied.11 Additional AI-based algorithms have been employed for complex procedures such as endoscopic submucosal dissection (ESD) or peroral endoscopic myotomy (POEM) to delineate vessels, better define tissue planes for dissection, and visualize landmark structures.12,13 Furthermore, AI-based scope guidance/manipulation, bleeding detection, landmark identification, and lesion detection have the potential to revolutionize endoscopic training and education. The impact that generative AI can potentially have on clinical practice is also an exciting prospect that warrants further investigation.
Artificial intelligence adoption in clinical practice
Clinical practice with regard to AI and colorectal cancer screening largely mirrors the disconnect in the current literature, with “believers” and “non-believers” as well as innovators and early adopters alongside laggards. In our own academic practices, we continue to struggle with the adoption and standardized implementation of AI-based colorectal cancer CADe systems, despite the RCT data showing positive results. It is likely that AI uptake will follow the technology predictions of Amara’s Law — i.e., individuals tend to overestimate the short-term impact of new technologies while underestimating long-term effects. In the end, more widespread adoption in community practice and larger scale real-world clinical outcomes studies are likely to determine the true impact of these exciting technologies. For other, less established AI-based tools, more data are currently required.
Conclusions
Ultimately, AI-based algorithms are likely here to stay, with continued improvement and evolution to occur based on provider feedback and patient care needs. Current tools, while not all-encompassing, have the potential to dramatically change the landscape of endoscopic training, diagnostic evaluation, and therapeutic care. It is critically important that relevant stakeholders, both endoscopists and patients, be involved in future applications and design to improve efficiency and quality outcomes overall.
Dr. McCarty is based in the Lynda K. and David M. Underwood Center for Digestive Disorders, Houston Methodist Hospital. Dr. Mansour is based in the section of gastroenterology, Baylor College of Medicine, Houston. Dr. McCarty reports no conflicts of interest. Dr. Mansour reports having been a consultant for Iterative Health.
References
1. Repici A, et al. Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Gastroenterology. 2020 Aug. doi: 10.1053/j.gastro.2020.04.062.
2. Repici A, et al. Artificial intelligence and colonoscopy experience: Lessons from two randomised trials. Gut. Apr 2022. doi: 10.1136/gutjnl-2021-324471.
3. Wallace MB, et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology 2022 Jul. doi: 10.1053/j.gastro.2022.03.007.
4. United States Food and Drug Administration (FDA). GI Genius FDA Approval [April 9, 2021]. Accessed January 5, 2022. Available at: www.accessdata.fda.gov/cdrh_docs/pdf21/K211951.pdf.
5. Maas MHJ, et al. A computer-aided polyp detection system in screening and surveillance colonoscopy: An international, multicentre, randomised, tandem trial. Lancet Digit Health. 2024 Mar. doi: 10.1016/S2589-7500(23)00242-X.
6. Ladabaum U, et al. Computer-aided detection of polyps does not improve colonoscopist performance in a pragmatic implementation trial. Gastroenterology. 2023 Mar. doi: 10.1053/j.gastro.2022.12.004.
7. Wei MT, et al. Evaluation of computer-aided detection during colonoscopy in the community (AI-SEE): A multicenter randomized clinical trial. Am J Gastroenterol. 2023 Oct. doi: 10.14309/ajg.0000000000002239.
8. de Groof J, et al. The Argos project: The development of a computer-aided detection system to improve detection of Barrett’s neoplasia on white light endoscopy. United European Gastroenterol J. 2019 May. doi: 10.1177/2050640619837443.
9. Kanesaka T, et al. Computer-aided diagnosis for identifying and delineating early gastric cancers in magnifying narrow-band imaging. Gastrointest Endosc. 2018 May. doi: 10.1016/j.gie.2017.11.029.
10. Sahafi A, et al. Edge artificial intelligence wireless video capsule endoscopy. Sci Rep. 2022 Aug. doi: 10.1038/s41598-022-17502-7.
11. Njei B, et al. Artificial intelligence in endoscopic imaging for detection of malignant biliary strictures and cholangiocarcinoma: A systematic review. Ann Gastroenterol. 2023 Mar-Apr. doi: 10.20524/aog.2023.0779.
12. Ebigbo A, et al. Vessel and tissue recognition during third-space endoscopy using a deep learning algorithm. Gut. 2022 Dec. doi: 10.1136/gutjnl-2021-326470.
13. Cao J, et al. Intelligent surgical workflow recognition for endoscopic submucosal dissection with real-time animal study. Nat Commun. 2023 Oct. doi: 10.1038/s41467-023-42451-8.
The Promise and Challenges of AI in Hepatology
BY BASILE NJEI, MD, MPH, PHD; YAZAN A. AL-AJLOUNI, MPHIL
In the dynamic realm of medicine, artificial intelligence (AI) emerges as a transformative force, notably within hepatology. The discipline of hepatology, dedicated to liver and related organ diseases, is ripe for AI’s promise to revolutionize diagnostics and treatment, pushing toward a future of precision medicine. Yet, the path to fully realizing AI’s potential in hepatology is laced with data, ethical, and integration challenges.
The application of AI, particularly in histopathology, significantly enhances disease diagnosis and staging in hepatology. AI-driven approaches remedy traditional histopathological challenges, such as interpretative variability, providing more consistent and accurate disease analyses. This is especially evident in conditions like metabolic dysfunction-associated steatohepatitis (MASH) and hepatocellular carcinoma (HCC), where AI aids in identifying critical gene signatures, thereby refining therapy selection.
Similarly, deep learning (DL), a branch of AI, has attracted significant interest globally, particularly in image recognition. AI’s incorporation into medical imaging marks a significant advancement, enabling early detection of malignancies like HCC and improving diagnostics in steatotic liver disease through enhanced imaging analyses using convolutional neural networks (CNN). The abundance of imaging data alongside clinical outcomes has catalyzed AI’s integration into radiology, leading to the swift growth of radiomics as a novel domain in medical research.
AI has also been shown to identify nuanced alterations in electrocardiograms (EKGs) associated with liver conditions, potentially detecting the progression of liver diseases at an earlier stage than currently possible. By leveraging complex algorithms and machine learning, AI can analyze EKG patterns with a precision and depth unattainable through traditional manual interpretation. Given that liver diseases, such as cirrhosis or hepatitis, can induce subtle cardiac changes long before other clinical symptoms manifest, early detection through AI-enhanced EKG analysis could lead to timely interventions, potentially halting or reversing disease progression. This approach further enriches our understanding of the intricate interplay between liver function and cardiac health, highlighting the potential for AI to transform not just liver disease diagnostics but also to foster a more integrated approach to patient care.
Beyond diagnostics, the burgeoning field of generative AI introduces groundbreaking possibilities in treatment planning and patient education, particularly for chronic conditions like cirrhosis. Generative AI produces original content, including text, visuals, and music, by identifying and learning patterns from its training data. When it leverages large language models (LLMs), it entails training on vast collections of textual data and using AI models characterized by many parameters. A notable instance of generative AI employing LLMs is ChatGPT (General Pretrained Transformers). By simulating disease progression and treatment outcomes, generative AI can foster personalized treatment strategies and empower patients with knowledge about their health trajectories. Yet, realizing these potential demands requires overcoming data quality and interpretability challenges, and ensuring AI outputs are accessible and actionable for clinicians and patients.
Despite these advancements, leveraging AI in hepatology is not devoid of hurdles. The development and training of AI models require extensive and diverse datasets, raising concerns about data privacy and ethical use. Addressing these concerns is paramount for successfully integrating AI into clinical hepatology practice, necessitating transparent algorithmic processes and stringent ethical standards. Ethical considerations are central to AI’s integration into hepatology. Algorithmic biases, patient privacy, and the impact of AI-driven decisions underscore the need for cautious AI deployment. Developing transparent, understandable algorithms and establishing ethical guidelines for AI use are critical steps towards ethically leveraging AI in patient care.
In conclusion, AI’s integration into hepatology holds tremendous promise for advancing patient care through enhanced diagnostics, treatment planning, and patient education. Overcoming the associated challenges, including ethical concerns, data diversity, and algorithm interpretability, is crucial. As the hepatology community navigates this technological evolution, a balanced approach that marries technological advancements with ethical stewardship will be key to harnessing AI’s full potential, ensuring it serves the best interests of patients and propels the field of hepatology into the future.
We predict a trajectory of increased use and adoption of AI in hepatology. AI in hepatology is likely to meet the test of pervasiveness, improvement, and innovation. The adoption of AI in routine hepatology diagnosis and management will likely follow Amara’s law and the five stages of the hype cycle. We believe that we are still in the infant stages of adopting AI technology in hepatology, and this phase may last 5 years before there is a peak of inflated expectations. The trough of disillusionment and slopes of enlightenment may only be observed in the next decades.
Dr. Njei is based in the Section of Digestive Diseases, Yale School of Medicine, New Haven, Conn. Mr. Al-Ajlouni is a senior medical student at New York Medical College School of Medicine, Valhalla, N.Y. They have no conflicts of interest to declare.
Sources
Taylor-Weiner A, et al. A Machine Learning Approach Enables Quantitative Measurement of Liver Histology and Disease Monitoring in NASH. Hepatology. 2021 Jul. doi: 10.1002/hep.31750.
Zeng Q, et al. Artificial intelligence predicts immune and inflammatory gene signatures directly from hepatocellular carcinoma histology. J Hepatol. 2022 Jul. doi: 10.1016/j.jhep.2022.01.018.
Ahn JC, et al. Development of the AI-Cirrhosis-ECG Score: An Electrocardiogram-Based Deep Learning Model in Cirrhosis. Am J Gastroenterol. 2022 Mar. doi: 10.14309/ajg.0000000000001617.
Nduma BN, et al. The Application of Artificial Intelligence (AI)-Based Ultrasound for the Diagnosis of Fatty Liver Disease: A Systematic Review. Cureus. 2023 Dec 15. doi: 10.7759/cureus.50601.
Navigating the Search for a Financial Adviser
As gastroenterologists, we spend innumerable years in medical training with an abrupt and significant increase in our earning potential upon beginning practice. The majority of us also carry a sizeable amount of student loan debt. This combination results in a unique situation that can make us hesitant about how best to set ourselves up financially while also making us vulnerable to potentially predatory financial practices.
Although your initial steps to achieve financial wellness and build wealth can be obtained on your own with some education, a financial adviser becomes indispensable when you have significant assets, a high income, complex finances, and/or are experiencing a major life change. Additionally, as there are so many avenues to invest and grow your capital, a financial adviser can assist in designing a portfolio to best accomplish specific monetary goals. Studies have demonstrated that those working with a financial adviser reduce their single-stock risk and have more significant increase in portfolio value, reducing the total cost associated with their investments’ management.1 Those working with a financial adviser will also net up to a 3% larger annual return, compared with a standard baseline investment plan.2,3
Based on this information, it may appear that working with a personal financial adviser would be a no-brainer. Unfortunately, there is a caveat: There is no legal regulation regarding who can use the title “financial adviser.” It is therefore crucial to be aware of common practices and terminology to best help you identify a reputable financial adviser and reduce your risk of excessive fees or financial loss. This is also a highly personal decision and your search should first begin with understanding why you are looking for an adviser, as this will determine the appropriate type of service to look for.
Types of Advisers
A certified financial planner (CFP) is an expert in estate planning, taxes, retirement saving, and financial planning who has a formal designation by the Certified Financial Planner Board of Standards Inc.4 They must undergo stringent licensing examinations following a 3-year course with required continuing education to maintain their credentials. CFPs are fiduciaries, meaning they must make financial decisions in your best interest, even if they may make less money with that product or investment strategy. In other words, they are beholden to give honest, impartial recommendations to their clients, and may face sanctions by the CFP Board if found to violate its Code of Ethics and Standards of Conduct, which includes failure to act in a fiduciary duty.5
CFPs evaluate your total financial picture, such as investments, insurance policies, and overall current financial position, to develop a comprehensive strategy that will successfully guide you to your financial goal. There are many individuals who may refer to themselves as financial planners without having the CFP designation; while they may offer similar services as above, they will not be required to act as a fiduciary. Hence, it is important to do your due diligence and verify they hold this certification via the CFP Board website: www.cfp.net/verify-a-cfp-professional.
An investment adviser is a legal term from the U.S. Securities and Exchange Commission (SEC) and the Financial Industry Regulatory Authority (FINRA) referring to an individual who provides recommendations and analyses for financial securities such as stock. Both of these agencies ensure investment advisers adhere to regulatory requirements designed to protect client investers. Similar to CFPs, they are held to a fiduciary standard, and their firm is required to register with the SEC or the state of practice based on the amount of assets under management.6
An individual investment adviser must also register with their state as an Investment Adviser Representative (IAR), the distinctive term referring to an individual as opposed to an investment advising firm. Investment advisers are required to pass the extensive Series 65, Uniform Investment Advisor Law Exam, or equivalent, by states requiring licensure.7 They can guide you on the selection of particular investments and portfolio management based on a discussion with you regarding your current financial standing and what fiscal ambitions you wish to achieve.
A financial adviser provides direction on a multitude of financially related topics such as investing, tax laws, and life insurance with the goal to help you reach specific financial objectives. However, this term is often used quite ubiquitously given the lack of formal regulation of the title. Essentially, those with varying types of educational background can give themselves the title of financial adviser.
If a financial adviser buys or sells financial securities such as stocks or bonds, then they must be registered as a licensed broker with the SEC and IAR and pass the Series 6 or Series 7 exam. Unlike CFPs and investment advisers, a financial adviser (if also a licensed broker) is not required to be a fiduciary, and instead works under the suitability standard.8 Suitability requires that financial recommendations made by the adviser are appropriate but not necessarily the best for the client. In fact, these recommendations do not even have to be the most suitable. This is where conflicts of interest can arise with the adviser recommending products and securities that best compensate them while not serving the best return on investment for you.
Making the search for a financial adviser more complex, an individual can be a combination of any of the above, pending the appropriate licensing. For example, a CFP can also be an asset manager and thus hold the title of a financial adviser and/or IAR. A financial adviser may also not directly manage your assets if they have a partnership with a third party or another licensed individual. Questions to ask of your potential financial adviser should therefore include the following:
- What licensure and related education do you have?
- What is your particular area of expertise?
- How long have you been in practice?
- How will you be managing my assets?
Financial Adviser Fee Schedules
Prior to working with a financial adviser, you must also inquire about their fee structure. There are two kinds of fee schedules used by financial advisers: fee-only and fee-based.
Fee-only advisers receive payment solely for the services they provide. They do not collect commissions from third parties providing the recommended products. There is variability in how this type of payment schedule is structured, encompassing flat fees, hourly rates, or the adviser charging a retainer. The Table below compares the types of fee-only structures and range of charges based on 2023 rates.9 Of note, fee-only advisers serve as fiduciaries.10
Fee-based financial advisers receive payment for services but may also receive commission on specific products they sell to you.9 Most, if not all, financial experts recommend avoiding advisers using commission-based charges given the potential conflict of interest: How can one be absolutely sure this recommended financial product is best for you, knowing your adviser has a financial stake in said item?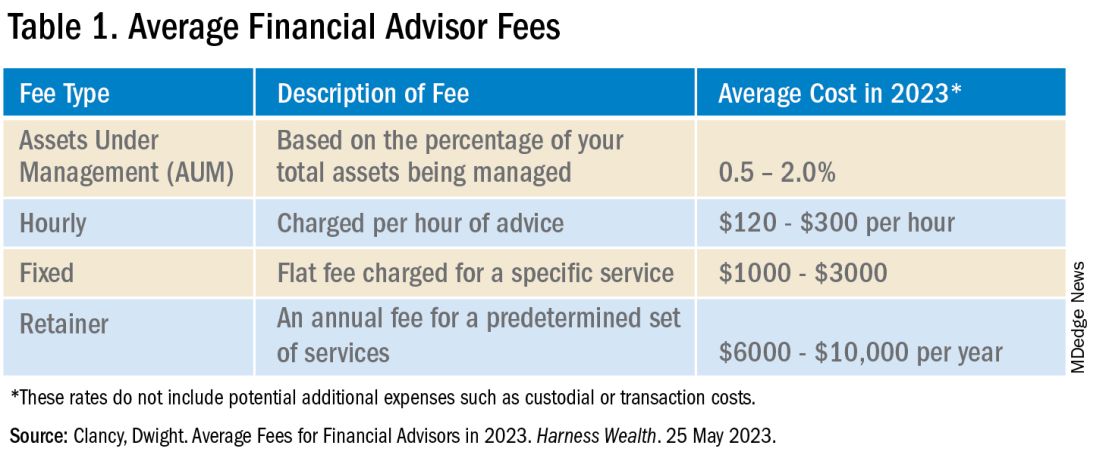
In addition to charging the fees above, your financial adviser, if they are actively managing your investment portfolio, will also charge an assets under management (AUM) fee. This is a percentage of the dollar amount within your portfolio. For example, if your adviser charges a 1% AUM rate for your account totaling $100,000, this equates to a $1,000 fee in that calendar year. AUM fees typically decrease as the size of your portfolio increases. As seen in the Table, there is a wide range of the average AUM rate (0.5%–2%); however, an AUM fee approaching 2% is unnecessarily high and consumes a significant portion of your portfolio. Thus, it is recommended to look for a money manager with an approximate 1% AUM fee.
Many of us delay or avoid working with a financial adviser due to the potential perceived risks of having poor portfolio management from an adviser not working in our best interest, along with the concern for excessive fees. In many ways, it is how we counsel our patients. While they can seek medical information on their own, their best care is under the guidance of an expert: a healthcare professional. That being said, personal finance is indeed personal, so I hope this guide helps facilitate your search and increase your financial wellness.
Dr. Luthra is a therapeutic endoscopist at Moffitt Cancer Center, Tampa, Florida, and the founder of The Scope of Finance, a financial wellness education and coaching company focused on physicians. Her interest in financial well-being is thanks to the teachings of her father, an entrepreneur and former Certified Financial Planner (CFP). She can be found on Instagram (thescopeoffinance) and X (@ScopeofFinance). She reports no financial disclosures relevant to this article.
References
1. Pagliaro CA and Utkus SP. Assessing the value of advice. Vanguard. 2019 Sept.
2. Kinniry Jr. FM et al. Putting a value on your value: Quantifying Vanguard Advisor’s Alpha. Vanguard. 2022 July.
3. Horan S. What Are the Benefits of Working with a Financial Advisor? – 2021 Study. Smart Asset. 2023 July 27.
4. Kagan J. Certified Financial PlannerTM(CFP): What It Is and How to Become One. Investopedia. 2023 Aug 3.
5. CFP Board. Our Commitment to Ethical Standards. CFP Board. 2024.
6. Staff of the Investment Adviser Regulation Office Division of Investment Management, U.S. Securities and Exchange Commission. Regulation of Investment Advisers by the U.S. Securities and Exchange Commission. 2013 Mar.
7. Hicks C. Investment Advisor vs. Financial Advisor: There is a Difference. US News & World Report. 2019 June 13.
8. Roberts K. Financial advisor vs. financial planner: What is the difference? Bankrate. 2023 Nov 21.
9. Clancy D. Average Fees for Financial Advisors in 2023. Harness Wealth. 2023 May 25.
10. Palmer B. Fee- vs. Commission-Based Advisor: What’s the Difference? Investopedia. 2023 June 20.
As gastroenterologists, we spend innumerable years in medical training with an abrupt and significant increase in our earning potential upon beginning practice. The majority of us also carry a sizeable amount of student loan debt. This combination results in a unique situation that can make us hesitant about how best to set ourselves up financially while also making us vulnerable to potentially predatory financial practices.
Although your initial steps to achieve financial wellness and build wealth can be obtained on your own with some education, a financial adviser becomes indispensable when you have significant assets, a high income, complex finances, and/or are experiencing a major life change. Additionally, as there are so many avenues to invest and grow your capital, a financial adviser can assist in designing a portfolio to best accomplish specific monetary goals. Studies have demonstrated that those working with a financial adviser reduce their single-stock risk and have more significant increase in portfolio value, reducing the total cost associated with their investments’ management.1 Those working with a financial adviser will also net up to a 3% larger annual return, compared with a standard baseline investment plan.2,3
Based on this information, it may appear that working with a personal financial adviser would be a no-brainer. Unfortunately, there is a caveat: There is no legal regulation regarding who can use the title “financial adviser.” It is therefore crucial to be aware of common practices and terminology to best help you identify a reputable financial adviser and reduce your risk of excessive fees or financial loss. This is also a highly personal decision and your search should first begin with understanding why you are looking for an adviser, as this will determine the appropriate type of service to look for.
Types of Advisers
A certified financial planner (CFP) is an expert in estate planning, taxes, retirement saving, and financial planning who has a formal designation by the Certified Financial Planner Board of Standards Inc.4 They must undergo stringent licensing examinations following a 3-year course with required continuing education to maintain their credentials. CFPs are fiduciaries, meaning they must make financial decisions in your best interest, even if they may make less money with that product or investment strategy. In other words, they are beholden to give honest, impartial recommendations to their clients, and may face sanctions by the CFP Board if found to violate its Code of Ethics and Standards of Conduct, which includes failure to act in a fiduciary duty.5
CFPs evaluate your total financial picture, such as investments, insurance policies, and overall current financial position, to develop a comprehensive strategy that will successfully guide you to your financial goal. There are many individuals who may refer to themselves as financial planners without having the CFP designation; while they may offer similar services as above, they will not be required to act as a fiduciary. Hence, it is important to do your due diligence and verify they hold this certification via the CFP Board website: www.cfp.net/verify-a-cfp-professional.
An investment adviser is a legal term from the U.S. Securities and Exchange Commission (SEC) and the Financial Industry Regulatory Authority (FINRA) referring to an individual who provides recommendations and analyses for financial securities such as stock. Both of these agencies ensure investment advisers adhere to regulatory requirements designed to protect client investers. Similar to CFPs, they are held to a fiduciary standard, and their firm is required to register with the SEC or the state of practice based on the amount of assets under management.6
An individual investment adviser must also register with their state as an Investment Adviser Representative (IAR), the distinctive term referring to an individual as opposed to an investment advising firm. Investment advisers are required to pass the extensive Series 65, Uniform Investment Advisor Law Exam, or equivalent, by states requiring licensure.7 They can guide you on the selection of particular investments and portfolio management based on a discussion with you regarding your current financial standing and what fiscal ambitions you wish to achieve.
A financial adviser provides direction on a multitude of financially related topics such as investing, tax laws, and life insurance with the goal to help you reach specific financial objectives. However, this term is often used quite ubiquitously given the lack of formal regulation of the title. Essentially, those with varying types of educational background can give themselves the title of financial adviser.
If a financial adviser buys or sells financial securities such as stocks or bonds, then they must be registered as a licensed broker with the SEC and IAR and pass the Series 6 or Series 7 exam. Unlike CFPs and investment advisers, a financial adviser (if also a licensed broker) is not required to be a fiduciary, and instead works under the suitability standard.8 Suitability requires that financial recommendations made by the adviser are appropriate but not necessarily the best for the client. In fact, these recommendations do not even have to be the most suitable. This is where conflicts of interest can arise with the adviser recommending products and securities that best compensate them while not serving the best return on investment for you.
Making the search for a financial adviser more complex, an individual can be a combination of any of the above, pending the appropriate licensing. For example, a CFP can also be an asset manager and thus hold the title of a financial adviser and/or IAR. A financial adviser may also not directly manage your assets if they have a partnership with a third party or another licensed individual. Questions to ask of your potential financial adviser should therefore include the following:
- What licensure and related education do you have?
- What is your particular area of expertise?
- How long have you been in practice?
- How will you be managing my assets?
Financial Adviser Fee Schedules
Prior to working with a financial adviser, you must also inquire about their fee structure. There are two kinds of fee schedules used by financial advisers: fee-only and fee-based.
Fee-only advisers receive payment solely for the services they provide. They do not collect commissions from third parties providing the recommended products. There is variability in how this type of payment schedule is structured, encompassing flat fees, hourly rates, or the adviser charging a retainer. The Table below compares the types of fee-only structures and range of charges based on 2023 rates.9 Of note, fee-only advisers serve as fiduciaries.10
Fee-based financial advisers receive payment for services but may also receive commission on specific products they sell to you.9 Most, if not all, financial experts recommend avoiding advisers using commission-based charges given the potential conflict of interest: How can one be absolutely sure this recommended financial product is best for you, knowing your adviser has a financial stake in said item?
In addition to charging the fees above, your financial adviser, if they are actively managing your investment portfolio, will also charge an assets under management (AUM) fee. This is a percentage of the dollar amount within your portfolio. For example, if your adviser charges a 1% AUM rate for your account totaling $100,000, this equates to a $1,000 fee in that calendar year. AUM fees typically decrease as the size of your portfolio increases. As seen in the Table, there is a wide range of the average AUM rate (0.5%–2%); however, an AUM fee approaching 2% is unnecessarily high and consumes a significant portion of your portfolio. Thus, it is recommended to look for a money manager with an approximate 1% AUM fee.
Many of us delay or avoid working with a financial adviser due to the potential perceived risks of having poor portfolio management from an adviser not working in our best interest, along with the concern for excessive fees. In many ways, it is how we counsel our patients. While they can seek medical information on their own, their best care is under the guidance of an expert: a healthcare professional. That being said, personal finance is indeed personal, so I hope this guide helps facilitate your search and increase your financial wellness.
Dr. Luthra is a therapeutic endoscopist at Moffitt Cancer Center, Tampa, Florida, and the founder of The Scope of Finance, a financial wellness education and coaching company focused on physicians. Her interest in financial well-being is thanks to the teachings of her father, an entrepreneur and former Certified Financial Planner (CFP). She can be found on Instagram (thescopeoffinance) and X (@ScopeofFinance). She reports no financial disclosures relevant to this article.
References
1. Pagliaro CA and Utkus SP. Assessing the value of advice. Vanguard. 2019 Sept.
2. Kinniry Jr. FM et al. Putting a value on your value: Quantifying Vanguard Advisor’s Alpha. Vanguard. 2022 July.
3. Horan S. What Are the Benefits of Working with a Financial Advisor? – 2021 Study. Smart Asset. 2023 July 27.
4. Kagan J. Certified Financial PlannerTM(CFP): What It Is and How to Become One. Investopedia. 2023 Aug 3.
5. CFP Board. Our Commitment to Ethical Standards. CFP Board. 2024.
6. Staff of the Investment Adviser Regulation Office Division of Investment Management, U.S. Securities and Exchange Commission. Regulation of Investment Advisers by the U.S. Securities and Exchange Commission. 2013 Mar.
7. Hicks C. Investment Advisor vs. Financial Advisor: There is a Difference. US News & World Report. 2019 June 13.
8. Roberts K. Financial advisor vs. financial planner: What is the difference? Bankrate. 2023 Nov 21.
9. Clancy D. Average Fees for Financial Advisors in 2023. Harness Wealth. 2023 May 25.
10. Palmer B. Fee- vs. Commission-Based Advisor: What’s the Difference? Investopedia. 2023 June 20.
As gastroenterologists, we spend innumerable years in medical training with an abrupt and significant increase in our earning potential upon beginning practice. The majority of us also carry a sizeable amount of student loan debt. This combination results in a unique situation that can make us hesitant about how best to set ourselves up financially while also making us vulnerable to potentially predatory financial practices.
Although your initial steps to achieve financial wellness and build wealth can be obtained on your own with some education, a financial adviser becomes indispensable when you have significant assets, a high income, complex finances, and/or are experiencing a major life change. Additionally, as there are so many avenues to invest and grow your capital, a financial adviser can assist in designing a portfolio to best accomplish specific monetary goals. Studies have demonstrated that those working with a financial adviser reduce their single-stock risk and have more significant increase in portfolio value, reducing the total cost associated with their investments’ management.1 Those working with a financial adviser will also net up to a 3% larger annual return, compared with a standard baseline investment plan.2,3
Based on this information, it may appear that working with a personal financial adviser would be a no-brainer. Unfortunately, there is a caveat: There is no legal regulation regarding who can use the title “financial adviser.” It is therefore crucial to be aware of common practices and terminology to best help you identify a reputable financial adviser and reduce your risk of excessive fees or financial loss. This is also a highly personal decision and your search should first begin with understanding why you are looking for an adviser, as this will determine the appropriate type of service to look for.
Types of Advisers
A certified financial planner (CFP) is an expert in estate planning, taxes, retirement saving, and financial planning who has a formal designation by the Certified Financial Planner Board of Standards Inc.4 They must undergo stringent licensing examinations following a 3-year course with required continuing education to maintain their credentials. CFPs are fiduciaries, meaning they must make financial decisions in your best interest, even if they may make less money with that product or investment strategy. In other words, they are beholden to give honest, impartial recommendations to their clients, and may face sanctions by the CFP Board if found to violate its Code of Ethics and Standards of Conduct, which includes failure to act in a fiduciary duty.5
CFPs evaluate your total financial picture, such as investments, insurance policies, and overall current financial position, to develop a comprehensive strategy that will successfully guide you to your financial goal. There are many individuals who may refer to themselves as financial planners without having the CFP designation; while they may offer similar services as above, they will not be required to act as a fiduciary. Hence, it is important to do your due diligence and verify they hold this certification via the CFP Board website: www.cfp.net/verify-a-cfp-professional.
An investment adviser is a legal term from the U.S. Securities and Exchange Commission (SEC) and the Financial Industry Regulatory Authority (FINRA) referring to an individual who provides recommendations and analyses for financial securities such as stock. Both of these agencies ensure investment advisers adhere to regulatory requirements designed to protect client investers. Similar to CFPs, they are held to a fiduciary standard, and their firm is required to register with the SEC or the state of practice based on the amount of assets under management.6
An individual investment adviser must also register with their state as an Investment Adviser Representative (IAR), the distinctive term referring to an individual as opposed to an investment advising firm. Investment advisers are required to pass the extensive Series 65, Uniform Investment Advisor Law Exam, or equivalent, by states requiring licensure.7 They can guide you on the selection of particular investments and portfolio management based on a discussion with you regarding your current financial standing and what fiscal ambitions you wish to achieve.
A financial adviser provides direction on a multitude of financially related topics such as investing, tax laws, and life insurance with the goal to help you reach specific financial objectives. However, this term is often used quite ubiquitously given the lack of formal regulation of the title. Essentially, those with varying types of educational background can give themselves the title of financial adviser.
If a financial adviser buys or sells financial securities such as stocks or bonds, then they must be registered as a licensed broker with the SEC and IAR and pass the Series 6 or Series 7 exam. Unlike CFPs and investment advisers, a financial adviser (if also a licensed broker) is not required to be a fiduciary, and instead works under the suitability standard.8 Suitability requires that financial recommendations made by the adviser are appropriate but not necessarily the best for the client. In fact, these recommendations do not even have to be the most suitable. This is where conflicts of interest can arise with the adviser recommending products and securities that best compensate them while not serving the best return on investment for you.
Making the search for a financial adviser more complex, an individual can be a combination of any of the above, pending the appropriate licensing. For example, a CFP can also be an asset manager and thus hold the title of a financial adviser and/or IAR. A financial adviser may also not directly manage your assets if they have a partnership with a third party or another licensed individual. Questions to ask of your potential financial adviser should therefore include the following:
- What licensure and related education do you have?
- What is your particular area of expertise?
- How long have you been in practice?
- How will you be managing my assets?
Financial Adviser Fee Schedules
Prior to working with a financial adviser, you must also inquire about their fee structure. There are two kinds of fee schedules used by financial advisers: fee-only and fee-based.
Fee-only advisers receive payment solely for the services they provide. They do not collect commissions from third parties providing the recommended products. There is variability in how this type of payment schedule is structured, encompassing flat fees, hourly rates, or the adviser charging a retainer. The Table below compares the types of fee-only structures and range of charges based on 2023 rates.9 Of note, fee-only advisers serve as fiduciaries.10
Fee-based financial advisers receive payment for services but may also receive commission on specific products they sell to you.9 Most, if not all, financial experts recommend avoiding advisers using commission-based charges given the potential conflict of interest: How can one be absolutely sure this recommended financial product is best for you, knowing your adviser has a financial stake in said item?
In addition to charging the fees above, your financial adviser, if they are actively managing your investment portfolio, will also charge an assets under management (AUM) fee. This is a percentage of the dollar amount within your portfolio. For example, if your adviser charges a 1% AUM rate for your account totaling $100,000, this equates to a $1,000 fee in that calendar year. AUM fees typically decrease as the size of your portfolio increases. As seen in the Table, there is a wide range of the average AUM rate (0.5%–2%); however, an AUM fee approaching 2% is unnecessarily high and consumes a significant portion of your portfolio. Thus, it is recommended to look for a money manager with an approximate 1% AUM fee.
Many of us delay or avoid working with a financial adviser due to the potential perceived risks of having poor portfolio management from an adviser not working in our best interest, along with the concern for excessive fees. In many ways, it is how we counsel our patients. While they can seek medical information on their own, their best care is under the guidance of an expert: a healthcare professional. That being said, personal finance is indeed personal, so I hope this guide helps facilitate your search and increase your financial wellness.
Dr. Luthra is a therapeutic endoscopist at Moffitt Cancer Center, Tampa, Florida, and the founder of The Scope of Finance, a financial wellness education and coaching company focused on physicians. Her interest in financial well-being is thanks to the teachings of her father, an entrepreneur and former Certified Financial Planner (CFP). She can be found on Instagram (thescopeoffinance) and X (@ScopeofFinance). She reports no financial disclosures relevant to this article.
References
1. Pagliaro CA and Utkus SP. Assessing the value of advice. Vanguard. 2019 Sept.
2. Kinniry Jr. FM et al. Putting a value on your value: Quantifying Vanguard Advisor’s Alpha. Vanguard. 2022 July.
3. Horan S. What Are the Benefits of Working with a Financial Advisor? – 2021 Study. Smart Asset. 2023 July 27.
4. Kagan J. Certified Financial PlannerTM(CFP): What It Is and How to Become One. Investopedia. 2023 Aug 3.
5. CFP Board. Our Commitment to Ethical Standards. CFP Board. 2024.
6. Staff of the Investment Adviser Regulation Office Division of Investment Management, U.S. Securities and Exchange Commission. Regulation of Investment Advisers by the U.S. Securities and Exchange Commission. 2013 Mar.
7. Hicks C. Investment Advisor vs. Financial Advisor: There is a Difference. US News & World Report. 2019 June 13.
8. Roberts K. Financial advisor vs. financial planner: What is the difference? Bankrate. 2023 Nov 21.
9. Clancy D. Average Fees for Financial Advisors in 2023. Harness Wealth. 2023 May 25.
10. Palmer B. Fee- vs. Commission-Based Advisor: What’s the Difference? Investopedia. 2023 June 20.
Achieving Promotion for Junior Faculty in Academic Medicine: An Interview With Experts
Academic medicine plays a crucial role at the crossroads of medical practice, education, and research, influencing the future landscape of healthcare. Many physicians aspire to pursue and sustain a career in academic medicine to contribute to the advancement of medical knowledge, enhance patient care, and influence the trajectory of the medical field. Opting for a career in academic medicine can offer benefits such as increased autonomy and scheduling flexibility, which can significantly improve the quality of life. In addition, engagement in scholarly activities and working in a dynamic environment with continuous learning opportunities can help mitigate burnout.
However, embarking on an academic career can be daunting for junior faculty members who face the challenge of providing clinical care while excelling in research and dedicating time to mentorship and teaching trainees. According to a report by the Association of American Medical Colleges, 38% of physicians leave academic medicine within a decade of obtaining a faculty position. Barriers to promotion and retention within academic medicine include ineffective mentorship, unclear or inconsistent promotion criteria, and disparities in gender/ethnic representation.
In this article, we interview two accomplished physicians in academic medicine who have attained the rank of professors.
Interview with Sophie Balzora, MD
Dr. Balzora is a professor of medicine at NYU Grossman School of Medicine and a practicing gastroenterologist specializing in the care of patients with inflammatory bowel disease at NYU Langone Health. She serves as the American College of Gastroenterology’s Diversity, Equity, and Inclusion Committee Chair, on the Advisory Board of ACG’s Leadership, Ethics, and Equity (LE&E) Center, and is president and cofounder of the Association of Black Gastroenterologists and Hepatologists (ABGH). Dr. Balzora was promoted to full professor 11 years after graduating from fellowship.
What would you identify as some of the most important factors that led to your success in achieving a promotion to professor of medicine?
Surround yourself with individuals whose professional and personal priorities align with yours. To achieve this, it is essential to gain an understanding of what is important to you, what you envision your success to look like, and establish a timeline to achieve it. The concept of personal success and how to best achieve it will absolutely change as you grow, and that is okay and expected. Connecting with those outside of your clinical interests, at other institutions, and even outside of the medical field, can help you achieve these goals and better shape how you see your career unfolding and how you want it to look.
Historically, the proportion of physicians who achieve professorship is lower among women compared with men. What do you believe are some of the barriers involved in this, and how would you counsel women who are interested in pursuing the rank of professor?
Systemic gender bias and discrimination, over-mentorship and under-sponsorship, inconsistent parental leave, and delayed parenthood are a few of the factors that contribute to the observed disparities in academic rank. Predictably, for women from underrepresented backgrounds in medicine, the chasm grows.
What has helped me most is to keep my eyes on the prize, and to recognize that the prize is different for everyone. It’s important not to make direct comparisons to any other individual, because they are not you. Harness what makes you different and drown out the naysayers — the “we’ve never seen this done before” camp, the “it’s too soon [for someone like you] to go up for promotion” folks. While these voices are sometimes well intentioned, they can distract you from your goals and ambitions because they are rooted in bias and adherence to traditional expectations. To do something new, and to change the game, requires going against the grain and utilizing your skills and talents to achieve what you want to achieve in a way that works for you.
What are some practical tips you have for junior gastroenterologists to track their promotion in academia?
- Keep your curriculum vitae (CV) up to date and formatted to your institutional guidelines. Ensure that you document your academic activities, even if it doesn’t seem important in the moment. When it’s time to submit that promotion portfolio, you want to be ready and organized.
- Remember: “No” is a full sentence, and saying it takes practice and time and confidence. It is a skill I still struggle to adopt at times, but it’s important to recognize the power of no, for it opens opportunities to say yes to other things.
- Lift as you climb — a critical part of changing the status quo is fostering the future of those underrepresented in medicine. A professional goal of mine that keeps me steady and passionate is to create supporting and enriching systemic and institutional changes that work to dismantle the obstacles perpetuating disparities in academic rank for women and those underrepresented in medicine. Discovering your “why” is a complex, difficult, and rewarding journey.
Interview with Mark Schattner, MD, AGAF
Dr. Schattner is a professor of clinical medicine at Weill Cornell College of Medicine and chief of the gastroenterology, hepatology, and nutrition service at Memorial Sloan Kettering Cancer Center, both in New York. He is a former president of the New York Society for Gastrointestinal Endoscopy and a fellow of the AGA and ASGE.
In your role as chief, you serve as a mentor for early career gastroenterologists for pursuing career promotion. What advice do you have for achieving this?
Promoting junior faculty is one of the prime responsibilities of a service chief. Generally, the early steps of promotion are straightforward, with criteria becoming more stringent as you progress. I think it is critical to understand the criteria used by promotion committees and to be aware of the various available tracks. I believe every meeting a junior faculty member has with their service chief should include, at the least, a brief check-in on where they are in the promotion process and plans (both short term and long term) to move forward. Successful promotion is facilitated when done upon a solid foundation of production and accomplishment. It is very challenging or even impossible when trying to piece together a package from discordant activities.
Most institutions require or encourage academic involvement at both national and international levels for career promotion. Do you have advice for junior faculty about how to achieve this type of recognition or experience?
The easiest place to start is with regional professional societies. Active involvement in these local societies fosters valuable networking and lays the groundwork for involvement at the national or international level. I would strongly encourage junior faculty to seek opportunities for a leadership position at any level in these societies and move up the ladder as their career matures. This is also a very good avenue to network and get invited to join collaborative research projects, which can be a fruitful means to enhance your academic productivity.
In your opinion, what factors are likely to hinder or delay an individual’s promotion?
I think it is crucial to consider the career track you are on. If you are very clinically productive and love to teach, that is completely appropriate, and most institutions will recognize the value of that and promote you along a clinical-educator tract. On the other hand, if you have a passion for research and can successfully lead research and compete for grants, then you would move along a traditional tenure track. It is also critical to think ahead, know the criteria on which you will be judged, and incorporate that into your practice early. Trying to scramble to enhance your CV in a short time just for promotion will likely prove ineffective.
Do you have advice for junior faculty who have families about how to manage career goals but also prioritize time with family?
There is no one-size-fits-all approach to this. I think this requires a lot of shared decision-making with your family. Compromise will undoubtedly be required. For example, I always chose to live in close proximity to my workplace, eliminating any commuting time. This choice really allowed me spend time with my family.
In conclusion, a career in academic medicine presents both opportunities and challenges. A successful academic career, and achieving promotion to the rank of professor of medicine, requires a combination of factors including understanding institution-specific criteria for promotion, proactive engagement at the regional and national level, and envisioning your career goals and creating a timeline to achieve them. There are challenges to promotion, including navigating systemic biases and balancing career goals with family commitments, which also requires consideration and open communication. Ultimately, we hope these insights provide valuable guidance and advice for junior faculty who are navigating this complex environment of academic medicine and are motivated toward achieving professional fulfillment and satisfaction in their careers.
Dr. Rolston is based in the Department of Gastroenterology, Hepatology, and Nutrition, Memorial Sloan Kettering Cancer Center, New York. She reports no conflicts in relation this article. Dr. Balzora and Dr. Schattner are based in the Division of Gastroenterology and Hepatology, New York University Langone Health, New York. Dr. Schattner is a consultant for Boston Scientific and Novo Nordisk. Dr. Balzora reports no conflicts in relation to this article.
References
Campbell KM. Mitigating the isolation of minoritized faculty in academic medicine. J Gen Intern Med. 2023 May. doi: 10.1007/s11606-022-07982-8.
Howard-Anderson JR et al. Strategies for developing a successful career in academic medicine. Am J Med Sci. 2024 Apr. doi: 10.1016/j.amjms.2023.12.010.
Murphy M et al. Women’s experiences of promotion and tenure in academic medicine and potential implications for gender disparities in career advancement: A qualitative analysis. JAMA Netw Open. 2021 Sep 1. doi: 10.1001/jamanetworkopen.2021.25843.
Sambunjak D et al. Mentoring in academic medicine: A systematic review. JAMA. 2006 Sep 6. doi: 10.1001/jama.296.9.1103.
Shen MR et al. Impact of mentoring on academic career success for women in medicine: A systematic review. Acad Med. 2022 Mar 1. doi: 10.1097/ACM.0000000000004563.
Academic medicine plays a crucial role at the crossroads of medical practice, education, and research, influencing the future landscape of healthcare. Many physicians aspire to pursue and sustain a career in academic medicine to contribute to the advancement of medical knowledge, enhance patient care, and influence the trajectory of the medical field. Opting for a career in academic medicine can offer benefits such as increased autonomy and scheduling flexibility, which can significantly improve the quality of life. In addition, engagement in scholarly activities and working in a dynamic environment with continuous learning opportunities can help mitigate burnout.
However, embarking on an academic career can be daunting for junior faculty members who face the challenge of providing clinical care while excelling in research and dedicating time to mentorship and teaching trainees. According to a report by the Association of American Medical Colleges, 38% of physicians leave academic medicine within a decade of obtaining a faculty position. Barriers to promotion and retention within academic medicine include ineffective mentorship, unclear or inconsistent promotion criteria, and disparities in gender/ethnic representation.
In this article, we interview two accomplished physicians in academic medicine who have attained the rank of professors.
Interview with Sophie Balzora, MD
Dr. Balzora is a professor of medicine at NYU Grossman School of Medicine and a practicing gastroenterologist specializing in the care of patients with inflammatory bowel disease at NYU Langone Health. She serves as the American College of Gastroenterology’s Diversity, Equity, and Inclusion Committee Chair, on the Advisory Board of ACG’s Leadership, Ethics, and Equity (LE&E) Center, and is president and cofounder of the Association of Black Gastroenterologists and Hepatologists (ABGH). Dr. Balzora was promoted to full professor 11 years after graduating from fellowship.
What would you identify as some of the most important factors that led to your success in achieving a promotion to professor of medicine?
Surround yourself with individuals whose professional and personal priorities align with yours. To achieve this, it is essential to gain an understanding of what is important to you, what you envision your success to look like, and establish a timeline to achieve it. The concept of personal success and how to best achieve it will absolutely change as you grow, and that is okay and expected. Connecting with those outside of your clinical interests, at other institutions, and even outside of the medical field, can help you achieve these goals and better shape how you see your career unfolding and how you want it to look.
Historically, the proportion of physicians who achieve professorship is lower among women compared with men. What do you believe are some of the barriers involved in this, and how would you counsel women who are interested in pursuing the rank of professor?
Systemic gender bias and discrimination, over-mentorship and under-sponsorship, inconsistent parental leave, and delayed parenthood are a few of the factors that contribute to the observed disparities in academic rank. Predictably, for women from underrepresented backgrounds in medicine, the chasm grows.
What has helped me most is to keep my eyes on the prize, and to recognize that the prize is different for everyone. It’s important not to make direct comparisons to any other individual, because they are not you. Harness what makes you different and drown out the naysayers — the “we’ve never seen this done before” camp, the “it’s too soon [for someone like you] to go up for promotion” folks. While these voices are sometimes well intentioned, they can distract you from your goals and ambitions because they are rooted in bias and adherence to traditional expectations. To do something new, and to change the game, requires going against the grain and utilizing your skills and talents to achieve what you want to achieve in a way that works for you.
What are some practical tips you have for junior gastroenterologists to track their promotion in academia?
- Keep your curriculum vitae (CV) up to date and formatted to your institutional guidelines. Ensure that you document your academic activities, even if it doesn’t seem important in the moment. When it’s time to submit that promotion portfolio, you want to be ready and organized.
- Remember: “No” is a full sentence, and saying it takes practice and time and confidence. It is a skill I still struggle to adopt at times, but it’s important to recognize the power of no, for it opens opportunities to say yes to other things.
- Lift as you climb — a critical part of changing the status quo is fostering the future of those underrepresented in medicine. A professional goal of mine that keeps me steady and passionate is to create supporting and enriching systemic and institutional changes that work to dismantle the obstacles perpetuating disparities in academic rank for women and those underrepresented in medicine. Discovering your “why” is a complex, difficult, and rewarding journey.
Interview with Mark Schattner, MD, AGAF
Dr. Schattner is a professor of clinical medicine at Weill Cornell College of Medicine and chief of the gastroenterology, hepatology, and nutrition service at Memorial Sloan Kettering Cancer Center, both in New York. He is a former president of the New York Society for Gastrointestinal Endoscopy and a fellow of the AGA and ASGE.
In your role as chief, you serve as a mentor for early career gastroenterologists for pursuing career promotion. What advice do you have for achieving this?
Promoting junior faculty is one of the prime responsibilities of a service chief. Generally, the early steps of promotion are straightforward, with criteria becoming more stringent as you progress. I think it is critical to understand the criteria used by promotion committees and to be aware of the various available tracks. I believe every meeting a junior faculty member has with their service chief should include, at the least, a brief check-in on where they are in the promotion process and plans (both short term and long term) to move forward. Successful promotion is facilitated when done upon a solid foundation of production and accomplishment. It is very challenging or even impossible when trying to piece together a package from discordant activities.
Most institutions require or encourage academic involvement at both national and international levels for career promotion. Do you have advice for junior faculty about how to achieve this type of recognition or experience?
The easiest place to start is with regional professional societies. Active involvement in these local societies fosters valuable networking and lays the groundwork for involvement at the national or international level. I would strongly encourage junior faculty to seek opportunities for a leadership position at any level in these societies and move up the ladder as their career matures. This is also a very good avenue to network and get invited to join collaborative research projects, which can be a fruitful means to enhance your academic productivity.
In your opinion, what factors are likely to hinder or delay an individual’s promotion?
I think it is crucial to consider the career track you are on. If you are very clinically productive and love to teach, that is completely appropriate, and most institutions will recognize the value of that and promote you along a clinical-educator tract. On the other hand, if you have a passion for research and can successfully lead research and compete for grants, then you would move along a traditional tenure track. It is also critical to think ahead, know the criteria on which you will be judged, and incorporate that into your practice early. Trying to scramble to enhance your CV in a short time just for promotion will likely prove ineffective.
Do you have advice for junior faculty who have families about how to manage career goals but also prioritize time with family?
There is no one-size-fits-all approach to this. I think this requires a lot of shared decision-making with your family. Compromise will undoubtedly be required. For example, I always chose to live in close proximity to my workplace, eliminating any commuting time. This choice really allowed me spend time with my family.
In conclusion, a career in academic medicine presents both opportunities and challenges. A successful academic career, and achieving promotion to the rank of professor of medicine, requires a combination of factors including understanding institution-specific criteria for promotion, proactive engagement at the regional and national level, and envisioning your career goals and creating a timeline to achieve them. There are challenges to promotion, including navigating systemic biases and balancing career goals with family commitments, which also requires consideration and open communication. Ultimately, we hope these insights provide valuable guidance and advice for junior faculty who are navigating this complex environment of academic medicine and are motivated toward achieving professional fulfillment and satisfaction in their careers.
Dr. Rolston is based in the Department of Gastroenterology, Hepatology, and Nutrition, Memorial Sloan Kettering Cancer Center, New York. She reports no conflicts in relation this article. Dr. Balzora and Dr. Schattner are based in the Division of Gastroenterology and Hepatology, New York University Langone Health, New York. Dr. Schattner is a consultant for Boston Scientific and Novo Nordisk. Dr. Balzora reports no conflicts in relation to this article.
References
Campbell KM. Mitigating the isolation of minoritized faculty in academic medicine. J Gen Intern Med. 2023 May. doi: 10.1007/s11606-022-07982-8.
Howard-Anderson JR et al. Strategies for developing a successful career in academic medicine. Am J Med Sci. 2024 Apr. doi: 10.1016/j.amjms.2023.12.010.
Murphy M et al. Women’s experiences of promotion and tenure in academic medicine and potential implications for gender disparities in career advancement: A qualitative analysis. JAMA Netw Open. 2021 Sep 1. doi: 10.1001/jamanetworkopen.2021.25843.
Sambunjak D et al. Mentoring in academic medicine: A systematic review. JAMA. 2006 Sep 6. doi: 10.1001/jama.296.9.1103.
Shen MR et al. Impact of mentoring on academic career success for women in medicine: A systematic review. Acad Med. 2022 Mar 1. doi: 10.1097/ACM.0000000000004563.
Academic medicine plays a crucial role at the crossroads of medical practice, education, and research, influencing the future landscape of healthcare. Many physicians aspire to pursue and sustain a career in academic medicine to contribute to the advancement of medical knowledge, enhance patient care, and influence the trajectory of the medical field. Opting for a career in academic medicine can offer benefits such as increased autonomy and scheduling flexibility, which can significantly improve the quality of life. In addition, engagement in scholarly activities and working in a dynamic environment with continuous learning opportunities can help mitigate burnout.
However, embarking on an academic career can be daunting for junior faculty members who face the challenge of providing clinical care while excelling in research and dedicating time to mentorship and teaching trainees. According to a report by the Association of American Medical Colleges, 38% of physicians leave academic medicine within a decade of obtaining a faculty position. Barriers to promotion and retention within academic medicine include ineffective mentorship, unclear or inconsistent promotion criteria, and disparities in gender/ethnic representation.
In this article, we interview two accomplished physicians in academic medicine who have attained the rank of professors.
Interview with Sophie Balzora, MD
Dr. Balzora is a professor of medicine at NYU Grossman School of Medicine and a practicing gastroenterologist specializing in the care of patients with inflammatory bowel disease at NYU Langone Health. She serves as the American College of Gastroenterology’s Diversity, Equity, and Inclusion Committee Chair, on the Advisory Board of ACG’s Leadership, Ethics, and Equity (LE&E) Center, and is president and cofounder of the Association of Black Gastroenterologists and Hepatologists (ABGH). Dr. Balzora was promoted to full professor 11 years after graduating from fellowship.
What would you identify as some of the most important factors that led to your success in achieving a promotion to professor of medicine?
Surround yourself with individuals whose professional and personal priorities align with yours. To achieve this, it is essential to gain an understanding of what is important to you, what you envision your success to look like, and establish a timeline to achieve it. The concept of personal success and how to best achieve it will absolutely change as you grow, and that is okay and expected. Connecting with those outside of your clinical interests, at other institutions, and even outside of the medical field, can help you achieve these goals and better shape how you see your career unfolding and how you want it to look.
Historically, the proportion of physicians who achieve professorship is lower among women compared with men. What do you believe are some of the barriers involved in this, and how would you counsel women who are interested in pursuing the rank of professor?
Systemic gender bias and discrimination, over-mentorship and under-sponsorship, inconsistent parental leave, and delayed parenthood are a few of the factors that contribute to the observed disparities in academic rank. Predictably, for women from underrepresented backgrounds in medicine, the chasm grows.
What has helped me most is to keep my eyes on the prize, and to recognize that the prize is different for everyone. It’s important not to make direct comparisons to any other individual, because they are not you. Harness what makes you different and drown out the naysayers — the “we’ve never seen this done before” camp, the “it’s too soon [for someone like you] to go up for promotion” folks. While these voices are sometimes well intentioned, they can distract you from your goals and ambitions because they are rooted in bias and adherence to traditional expectations. To do something new, and to change the game, requires going against the grain and utilizing your skills and talents to achieve what you want to achieve in a way that works for you.
What are some practical tips you have for junior gastroenterologists to track their promotion in academia?
- Keep your curriculum vitae (CV) up to date and formatted to your institutional guidelines. Ensure that you document your academic activities, even if it doesn’t seem important in the moment. When it’s time to submit that promotion portfolio, you want to be ready and organized.
- Remember: “No” is a full sentence, and saying it takes practice and time and confidence. It is a skill I still struggle to adopt at times, but it’s important to recognize the power of no, for it opens opportunities to say yes to other things.
- Lift as you climb — a critical part of changing the status quo is fostering the future of those underrepresented in medicine. A professional goal of mine that keeps me steady and passionate is to create supporting and enriching systemic and institutional changes that work to dismantle the obstacles perpetuating disparities in academic rank for women and those underrepresented in medicine. Discovering your “why” is a complex, difficult, and rewarding journey.
Interview with Mark Schattner, MD, AGAF
Dr. Schattner is a professor of clinical medicine at Weill Cornell College of Medicine and chief of the gastroenterology, hepatology, and nutrition service at Memorial Sloan Kettering Cancer Center, both in New York. He is a former president of the New York Society for Gastrointestinal Endoscopy and a fellow of the AGA and ASGE.
In your role as chief, you serve as a mentor for early career gastroenterologists for pursuing career promotion. What advice do you have for achieving this?
Promoting junior faculty is one of the prime responsibilities of a service chief. Generally, the early steps of promotion are straightforward, with criteria becoming more stringent as you progress. I think it is critical to understand the criteria used by promotion committees and to be aware of the various available tracks. I believe every meeting a junior faculty member has with their service chief should include, at the least, a brief check-in on where they are in the promotion process and plans (both short term and long term) to move forward. Successful promotion is facilitated when done upon a solid foundation of production and accomplishment. It is very challenging or even impossible when trying to piece together a package from discordant activities.
Most institutions require or encourage academic involvement at both national and international levels for career promotion. Do you have advice for junior faculty about how to achieve this type of recognition or experience?
The easiest place to start is with regional professional societies. Active involvement in these local societies fosters valuable networking and lays the groundwork for involvement at the national or international level. I would strongly encourage junior faculty to seek opportunities for a leadership position at any level in these societies and move up the ladder as their career matures. This is also a very good avenue to network and get invited to join collaborative research projects, which can be a fruitful means to enhance your academic productivity.
In your opinion, what factors are likely to hinder or delay an individual’s promotion?
I think it is crucial to consider the career track you are on. If you are very clinically productive and love to teach, that is completely appropriate, and most institutions will recognize the value of that and promote you along a clinical-educator tract. On the other hand, if you have a passion for research and can successfully lead research and compete for grants, then you would move along a traditional tenure track. It is also critical to think ahead, know the criteria on which you will be judged, and incorporate that into your practice early. Trying to scramble to enhance your CV in a short time just for promotion will likely prove ineffective.
Do you have advice for junior faculty who have families about how to manage career goals but also prioritize time with family?
There is no one-size-fits-all approach to this. I think this requires a lot of shared decision-making with your family. Compromise will undoubtedly be required. For example, I always chose to live in close proximity to my workplace, eliminating any commuting time. This choice really allowed me spend time with my family.
In conclusion, a career in academic medicine presents both opportunities and challenges. A successful academic career, and achieving promotion to the rank of professor of medicine, requires a combination of factors including understanding institution-specific criteria for promotion, proactive engagement at the regional and national level, and envisioning your career goals and creating a timeline to achieve them. There are challenges to promotion, including navigating systemic biases and balancing career goals with family commitments, which also requires consideration and open communication. Ultimately, we hope these insights provide valuable guidance and advice for junior faculty who are navigating this complex environment of academic medicine and are motivated toward achieving professional fulfillment and satisfaction in their careers.
Dr. Rolston is based in the Department of Gastroenterology, Hepatology, and Nutrition, Memorial Sloan Kettering Cancer Center, New York. She reports no conflicts in relation this article. Dr. Balzora and Dr. Schattner are based in the Division of Gastroenterology and Hepatology, New York University Langone Health, New York. Dr. Schattner is a consultant for Boston Scientific and Novo Nordisk. Dr. Balzora reports no conflicts in relation to this article.
References
Campbell KM. Mitigating the isolation of minoritized faculty in academic medicine. J Gen Intern Med. 2023 May. doi: 10.1007/s11606-022-07982-8.
Howard-Anderson JR et al. Strategies for developing a successful career in academic medicine. Am J Med Sci. 2024 Apr. doi: 10.1016/j.amjms.2023.12.010.
Murphy M et al. Women’s experiences of promotion and tenure in academic medicine and potential implications for gender disparities in career advancement: A qualitative analysis. JAMA Netw Open. 2021 Sep 1. doi: 10.1001/jamanetworkopen.2021.25843.
Sambunjak D et al. Mentoring in academic medicine: A systematic review. JAMA. 2006 Sep 6. doi: 10.1001/jama.296.9.1103.
Shen MR et al. Impact of mentoring on academic career success for women in medicine: A systematic review. Acad Med. 2022 Mar 1. doi: 10.1097/ACM.0000000000004563.
Converging on Our Nation’s Capital
Release of our May issue coincides with our annual pilgrimage to Digestive Disease Week® (DDW), this year held in our nation’s capital of Washington, D.C.
As we peruse the preliminary program in planning our meeting coverage, I am always amazed at the breadth and depth of programming offered as part of a relatively brief, 4-day meeting — this is a testament to the hard work of the AGA Council and DDW organizing committees, who have the gargantuan task of ensuring an engaging, seamless meeting each year.
This year’s conference features over 400 original scientific sessions and 4,300 oral abstract and poster presentations, in addition to the always well-attended AGA Postgraduate Course. This year’s AGA Presidential Plenary, which will feature a series of thought-provoking panel discussions on the future of GI healthcare and innovations in how we treat, disseminate, and teach, also is not to be missed. Beyond DDW, I hope you will join me in taking advantage of some of D.C.’s amazing cultural offerings, including the Smithsonian museums, National Gallery, Kennedy Center for the Performing Arts, and many others.
In this month’s issue of GIHN, we highlight an important AGA expert consensus commentary published in Clinical Gastroenterology and Hepatology examining the role of blood-based tests (“liquid biopsy”) in colorectal cancer screening. This guidance, which recognizes the promise of such tests but also urges caution in their adoption, is particularly important considering recently published data from the ECLIPSE study (also covered in this issue) evaluating the performance of Guardant’s ctDNA liquid biopsy compared to a screening colonoscopy. Also relevant to CRC screening, we highlight data on the performance of the “next gen” Cologuard test compared with FIT, which was recently published in NEJM. In our May Member Spotlight, we feature gastroenterologist Adjoa Anyane-Yeboa, MD, MPH, who shares her passion for addressing barriers to CRC screening for Black patients. Finally, GIHN Associate Editor Dr. Avi Ketwaroo introduces our quarterly Perspectives column highlighting emerging applications of AI in GI endoscopy and hepatology. We hope you enjoy all the exciting content featured in this issue and look forward to seeing you in Washington, D.C. (or virtually) for DDW.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Release of our May issue coincides with our annual pilgrimage to Digestive Disease Week® (DDW), this year held in our nation’s capital of Washington, D.C.
As we peruse the preliminary program in planning our meeting coverage, I am always amazed at the breadth and depth of programming offered as part of a relatively brief, 4-day meeting — this is a testament to the hard work of the AGA Council and DDW organizing committees, who have the gargantuan task of ensuring an engaging, seamless meeting each year.
This year’s conference features over 400 original scientific sessions and 4,300 oral abstract and poster presentations, in addition to the always well-attended AGA Postgraduate Course. This year’s AGA Presidential Plenary, which will feature a series of thought-provoking panel discussions on the future of GI healthcare and innovations in how we treat, disseminate, and teach, also is not to be missed. Beyond DDW, I hope you will join me in taking advantage of some of D.C.’s amazing cultural offerings, including the Smithsonian museums, National Gallery, Kennedy Center for the Performing Arts, and many others.
In this month’s issue of GIHN, we highlight an important AGA expert consensus commentary published in Clinical Gastroenterology and Hepatology examining the role of blood-based tests (“liquid biopsy”) in colorectal cancer screening. This guidance, which recognizes the promise of such tests but also urges caution in their adoption, is particularly important considering recently published data from the ECLIPSE study (also covered in this issue) evaluating the performance of Guardant’s ctDNA liquid biopsy compared to a screening colonoscopy. Also relevant to CRC screening, we highlight data on the performance of the “next gen” Cologuard test compared with FIT, which was recently published in NEJM. In our May Member Spotlight, we feature gastroenterologist Adjoa Anyane-Yeboa, MD, MPH, who shares her passion for addressing barriers to CRC screening for Black patients. Finally, GIHN Associate Editor Dr. Avi Ketwaroo introduces our quarterly Perspectives column highlighting emerging applications of AI in GI endoscopy and hepatology. We hope you enjoy all the exciting content featured in this issue and look forward to seeing you in Washington, D.C. (or virtually) for DDW.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Release of our May issue coincides with our annual pilgrimage to Digestive Disease Week® (DDW), this year held in our nation’s capital of Washington, D.C.
As we peruse the preliminary program in planning our meeting coverage, I am always amazed at the breadth and depth of programming offered as part of a relatively brief, 4-day meeting — this is a testament to the hard work of the AGA Council and DDW organizing committees, who have the gargantuan task of ensuring an engaging, seamless meeting each year.
This year’s conference features over 400 original scientific sessions and 4,300 oral abstract and poster presentations, in addition to the always well-attended AGA Postgraduate Course. This year’s AGA Presidential Plenary, which will feature a series of thought-provoking panel discussions on the future of GI healthcare and innovations in how we treat, disseminate, and teach, also is not to be missed. Beyond DDW, I hope you will join me in taking advantage of some of D.C.’s amazing cultural offerings, including the Smithsonian museums, National Gallery, Kennedy Center for the Performing Arts, and many others.
In this month’s issue of GIHN, we highlight an important AGA expert consensus commentary published in Clinical Gastroenterology and Hepatology examining the role of blood-based tests (“liquid biopsy”) in colorectal cancer screening. This guidance, which recognizes the promise of such tests but also urges caution in their adoption, is particularly important considering recently published data from the ECLIPSE study (also covered in this issue) evaluating the performance of Guardant’s ctDNA liquid biopsy compared to a screening colonoscopy. Also relevant to CRC screening, we highlight data on the performance of the “next gen” Cologuard test compared with FIT, which was recently published in NEJM. In our May Member Spotlight, we feature gastroenterologist Adjoa Anyane-Yeboa, MD, MPH, who shares her passion for addressing barriers to CRC screening for Black patients. Finally, GIHN Associate Editor Dr. Avi Ketwaroo introduces our quarterly Perspectives column highlighting emerging applications of AI in GI endoscopy and hepatology. We hope you enjoy all the exciting content featured in this issue and look forward to seeing you in Washington, D.C. (or virtually) for DDW.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Do No Harm: What Smoldering Myeloma Teaches Us
My approach to treating SMM takes into account what its history can teach us about 1) how advancements in imaging and diagnostic reclassifications can revise the entire natural history of a disease, and 2) how evidence generated by even the best of studies may have an expiration date.
Much of what we know about SMM today dates to a pivotal study by Robert A. Kyle, MD, and colleagues, published in 2007. That inspirational team of investigators followed people diagnosed with SMM from 1970 to 1995 and established the first natural history of the condition. Their monumental effort and the data and conclusions it generated (eg,10% risk annually of SMM becoming MM for the first 5 years) are still cited today in references, papers, and slide sets.
Despite the seminal importance of this work, from today’s perspective the 2007 study might just as well have been describing a different disease. Back then people were diagnosed with SMM if their blood work detected a monoclonal protein and a follow-up bone marrow biopsy found at least 10% plasma cells (or a monoclonal protein exceeding 3g/dL). If there were no signs of end-organ damage (ie, no anemia or kidney problems) and an x-ray showed no fractures or lesions in the bones, the diagnosis was determined to be SMM.
What’s different in 2024? First and foremost: advanced, highly sensitive imaging techniques. MRIs can pick up small lytic lesions (and even the precursor to lytic lesions) that would not appear on an x-ray. In fact, relying solely on x-rays risks missing half of the lytic lesions.
Therefore, using the same criteria, many people who in the past were diagnosed with SMM would today be diagnosed with MM. Furthermore, in 2014 a diagnostic change reclassified people’s diagnosis from the highest risk category of SMM to the category of active MM.
Due to these scientific advances and classification changes, I believe that the natural history of SMM is unknown. Risk stratification models for SMM derived from data sets of people who had not undergone rigorous advanced imaging likely are skewed by data from people who had MM. In addition, current risk stratification models have very poor concordance with each other. I routinely see people whose 2-year risk according to different models varies by more than 30%-40%.
All this information tells us that SMM today is more indolent than the SMM of the past. Paradoxically, however, our therapies keep getting more and more aggressive, exposing this vulnerable group of people to intense treatment regimens that they may not require. Therapies tested on people diagnosed with SMM include an aggressive three-drug regimen, autologous stem cell transplant, and 2 years of additional therapy, as well as, more recently CAR T-cell therapy which so far has at least a 4%-5% treatment-related mortality risk in people with myeloma and a strong signal for secondary cancer risk. Other trials are testing bispecific therapies such as talquetamab, a drug which in my experience causes horrendous skin toxicity, profound weight loss, and one’s nails to fall off.
Doctors routinely keep showing slides from Kyle’s pivotal work to describe the natural history of SMM and to justify the need for treatment, and trials continue to use outdated progression prediction models. In my opinion, as people with MM keep living longer and treatments for MM keep getting better, the threshold for intervening with asymptomatic, healthy people with SMM should be getting higher, not lower.
I strongly believe that the current landscape of SMM treatment exemplifies good intentions leading to bad outcomes. A routine blood test in a completely healthy person that finds elevated total protein in the blood could culminate in well-intentioned but aggressive therapies that can lead to many serious side effects. (I repeat: Secondary cancers and deaths from infections have all occurred in SMM trials.)
With no control arm, we simply don’t know how well these people might have fared without any therapy. For all we know, treatment may have shortened their lives due to complications up to and including death — all because of a blood test often conducted for reasons that have no evidentiary basis.
For example, plasma cell diseases are not linked to low bone density or auto-immune diseases, yet these labs are sent routinely as part of a workup for those conditions, leading to increasing anxiety and costs.
So, what is my approach? When treating people with SMM, I hold nuanced discussions of this data to help prioritize and reach informed decisions. After our honest conversation about the limitations of SMM models, older data, and the limitations of prospective data studying pharmacological treatment, almost no one signs up for treatment.
I want these people to stay safe, and I’m proud to be a part of a trial (SPOTLIGHT, NCT06212323) that aims to show prospectively that these people can be watched off treatment with monitoring via advanced imaging modalities.
In conclusion: SMM teaches us how, even in the absence of pharmacological interventions, the natural history of a disease can change over time, simply via better imaging techniques and changes in diagnostic classifications. Unfortunately, SMM also illustrates how good intentions can lead to harm.
Dr. Mohyuddin is assistant professor in the multiple myeloma program at the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
My approach to treating SMM takes into account what its history can teach us about 1) how advancements in imaging and diagnostic reclassifications can revise the entire natural history of a disease, and 2) how evidence generated by even the best of studies may have an expiration date.
Much of what we know about SMM today dates to a pivotal study by Robert A. Kyle, MD, and colleagues, published in 2007. That inspirational team of investigators followed people diagnosed with SMM from 1970 to 1995 and established the first natural history of the condition. Their monumental effort and the data and conclusions it generated (eg,10% risk annually of SMM becoming MM for the first 5 years) are still cited today in references, papers, and slide sets.
Despite the seminal importance of this work, from today’s perspective the 2007 study might just as well have been describing a different disease. Back then people were diagnosed with SMM if their blood work detected a monoclonal protein and a follow-up bone marrow biopsy found at least 10% plasma cells (or a monoclonal protein exceeding 3g/dL). If there were no signs of end-organ damage (ie, no anemia or kidney problems) and an x-ray showed no fractures or lesions in the bones, the diagnosis was determined to be SMM.
What’s different in 2024? First and foremost: advanced, highly sensitive imaging techniques. MRIs can pick up small lytic lesions (and even the precursor to lytic lesions) that would not appear on an x-ray. In fact, relying solely on x-rays risks missing half of the lytic lesions.
Therefore, using the same criteria, many people who in the past were diagnosed with SMM would today be diagnosed with MM. Furthermore, in 2014 a diagnostic change reclassified people’s diagnosis from the highest risk category of SMM to the category of active MM.
Due to these scientific advances and classification changes, I believe that the natural history of SMM is unknown. Risk stratification models for SMM derived from data sets of people who had not undergone rigorous advanced imaging likely are skewed by data from people who had MM. In addition, current risk stratification models have very poor concordance with each other. I routinely see people whose 2-year risk according to different models varies by more than 30%-40%.
All this information tells us that SMM today is more indolent than the SMM of the past. Paradoxically, however, our therapies keep getting more and more aggressive, exposing this vulnerable group of people to intense treatment regimens that they may not require. Therapies tested on people diagnosed with SMM include an aggressive three-drug regimen, autologous stem cell transplant, and 2 years of additional therapy, as well as, more recently CAR T-cell therapy which so far has at least a 4%-5% treatment-related mortality risk in people with myeloma and a strong signal for secondary cancer risk. Other trials are testing bispecific therapies such as talquetamab, a drug which in my experience causes horrendous skin toxicity, profound weight loss, and one’s nails to fall off.
Doctors routinely keep showing slides from Kyle’s pivotal work to describe the natural history of SMM and to justify the need for treatment, and trials continue to use outdated progression prediction models. In my opinion, as people with MM keep living longer and treatments for MM keep getting better, the threshold for intervening with asymptomatic, healthy people with SMM should be getting higher, not lower.
I strongly believe that the current landscape of SMM treatment exemplifies good intentions leading to bad outcomes. A routine blood test in a completely healthy person that finds elevated total protein in the blood could culminate in well-intentioned but aggressive therapies that can lead to many serious side effects. (I repeat: Secondary cancers and deaths from infections have all occurred in SMM trials.)
With no control arm, we simply don’t know how well these people might have fared without any therapy. For all we know, treatment may have shortened their lives due to complications up to and including death — all because of a blood test often conducted for reasons that have no evidentiary basis.
For example, plasma cell diseases are not linked to low bone density or auto-immune diseases, yet these labs are sent routinely as part of a workup for those conditions, leading to increasing anxiety and costs.
So, what is my approach? When treating people with SMM, I hold nuanced discussions of this data to help prioritize and reach informed decisions. After our honest conversation about the limitations of SMM models, older data, and the limitations of prospective data studying pharmacological treatment, almost no one signs up for treatment.
I want these people to stay safe, and I’m proud to be a part of a trial (SPOTLIGHT, NCT06212323) that aims to show prospectively that these people can be watched off treatment with monitoring via advanced imaging modalities.
In conclusion: SMM teaches us how, even in the absence of pharmacological interventions, the natural history of a disease can change over time, simply via better imaging techniques and changes in diagnostic classifications. Unfortunately, SMM also illustrates how good intentions can lead to harm.
Dr. Mohyuddin is assistant professor in the multiple myeloma program at the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
My approach to treating SMM takes into account what its history can teach us about 1) how advancements in imaging and diagnostic reclassifications can revise the entire natural history of a disease, and 2) how evidence generated by even the best of studies may have an expiration date.
Much of what we know about SMM today dates to a pivotal study by Robert A. Kyle, MD, and colleagues, published in 2007. That inspirational team of investigators followed people diagnosed with SMM from 1970 to 1995 and established the first natural history of the condition. Their monumental effort and the data and conclusions it generated (eg,10% risk annually of SMM becoming MM for the first 5 years) are still cited today in references, papers, and slide sets.
Despite the seminal importance of this work, from today’s perspective the 2007 study might just as well have been describing a different disease. Back then people were diagnosed with SMM if their blood work detected a monoclonal protein and a follow-up bone marrow biopsy found at least 10% plasma cells (or a monoclonal protein exceeding 3g/dL). If there were no signs of end-organ damage (ie, no anemia or kidney problems) and an x-ray showed no fractures or lesions in the bones, the diagnosis was determined to be SMM.
What’s different in 2024? First and foremost: advanced, highly sensitive imaging techniques. MRIs can pick up small lytic lesions (and even the precursor to lytic lesions) that would not appear on an x-ray. In fact, relying solely on x-rays risks missing half of the lytic lesions.
Therefore, using the same criteria, many people who in the past were diagnosed with SMM would today be diagnosed with MM. Furthermore, in 2014 a diagnostic change reclassified people’s diagnosis from the highest risk category of SMM to the category of active MM.
Due to these scientific advances and classification changes, I believe that the natural history of SMM is unknown. Risk stratification models for SMM derived from data sets of people who had not undergone rigorous advanced imaging likely are skewed by data from people who had MM. In addition, current risk stratification models have very poor concordance with each other. I routinely see people whose 2-year risk according to different models varies by more than 30%-40%.
All this information tells us that SMM today is more indolent than the SMM of the past. Paradoxically, however, our therapies keep getting more and more aggressive, exposing this vulnerable group of people to intense treatment regimens that they may not require. Therapies tested on people diagnosed with SMM include an aggressive three-drug regimen, autologous stem cell transplant, and 2 years of additional therapy, as well as, more recently CAR T-cell therapy which so far has at least a 4%-5% treatment-related mortality risk in people with myeloma and a strong signal for secondary cancer risk. Other trials are testing bispecific therapies such as talquetamab, a drug which in my experience causes horrendous skin toxicity, profound weight loss, and one’s nails to fall off.
Doctors routinely keep showing slides from Kyle’s pivotal work to describe the natural history of SMM and to justify the need for treatment, and trials continue to use outdated progression prediction models. In my opinion, as people with MM keep living longer and treatments for MM keep getting better, the threshold for intervening with asymptomatic, healthy people with SMM should be getting higher, not lower.
I strongly believe that the current landscape of SMM treatment exemplifies good intentions leading to bad outcomes. A routine blood test in a completely healthy person that finds elevated total protein in the blood could culminate in well-intentioned but aggressive therapies that can lead to many serious side effects. (I repeat: Secondary cancers and deaths from infections have all occurred in SMM trials.)
With no control arm, we simply don’t know how well these people might have fared without any therapy. For all we know, treatment may have shortened their lives due to complications up to and including death — all because of a blood test often conducted for reasons that have no evidentiary basis.
For example, plasma cell diseases are not linked to low bone density or auto-immune diseases, yet these labs are sent routinely as part of a workup for those conditions, leading to increasing anxiety and costs.
So, what is my approach? When treating people with SMM, I hold nuanced discussions of this data to help prioritize and reach informed decisions. After our honest conversation about the limitations of SMM models, older data, and the limitations of prospective data studying pharmacological treatment, almost no one signs up for treatment.
I want these people to stay safe, and I’m proud to be a part of a trial (SPOTLIGHT, NCT06212323) that aims to show prospectively that these people can be watched off treatment with monitoring via advanced imaging modalities.
In conclusion: SMM teaches us how, even in the absence of pharmacological interventions, the natural history of a disease can change over time, simply via better imaging techniques and changes in diagnostic classifications. Unfortunately, SMM also illustrates how good intentions can lead to harm.
Dr. Mohyuddin is assistant professor in the multiple myeloma program at the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
















