User login
CPAP Underperforms: The Sequel
A few months ago, I posted a column on continuous positive airway pressure (CPAP) with the title, “CPAP Oversells and Underperforms.” To date, it has 299 likes and 90 comments, which are almost all negative. I’m glad to see that it’s generated interest, and I’d like to address some of the themes expressed in the posts.
Most comments were personal testimonies to the miracles of CPAP. These are important, and the point deserves emphasis. CPAP can provide significant improvements in daytime sleepiness and quality of life. I closed the original piece by acknowledging this important fact. Readers can be forgiven for missing it given that the title and text were otherwise disparaging of CPAP.
But several comments warrant a more in-depth discussion. The original piece focuses on CPAP and cardiovascular (CV) outcomes but made no mention of atrial fibrillation (AF) or ejection fraction (EF). The effects of CPAP on each are touted by cardiologists and PAP-pushers alike and are drivers of frequent referrals. It›s my fault for omitting them from the discussion.
AF is easy. The data is identical to all other things CPAP and CV. Based on biologic plausibility alone, the likelihood of a relationship between AF and obstructive sleep apnea (OSA) is similar to the odds that the Celtics raise an 18th banner come June. There’s hypoxia, intrathoracic pressure swings, sympathetic surges, and sleep state disruptions. It’s easy to get from there to arrhythmogenesis. There’s lots of observational noise, too, but no randomized proof that CPAP alters this relationship.
I found four randomized controlled trials (RCTs) that tested CPAP’s effect on AF. I’ll save you the suspense; they were all negative. One even found a signal for more adverse events in the CPAP group. These studies have several positive qualities: They enrolled patients with moderate to severe sleep apnea and high oxygen desaturation indices, adherence averaged more than 4 hours across all groups in all trials, and the methods for assessing the AF outcomes differed slightly. There’s also a lot not to like: The sample sizes were small, only one trial enrolled “sleepy” patients (as assessed by the Epworth Sleepiness Score), and follow-up was short.
To paraphrase Carl Sagan, “absence of evidence does not equal evidence of absence.” As a statistician would say, type II error cannot be excluded by these RCTs. In medicine, however, the burden of proof falls on demonstrating efficacy. If we treat before concluding that a therapy works, we risk wasting time, money, medical resources, and the most precious of patient commodities: the energy required for behavior change. In their response to letters to the editor, the authors of the third RCT summarize the CPAP, AF, and CV disease data far better than I ever could. They sound the same words of caution and come out against screening patients with AF for OSA.
The story for CPAP’s effects on EF is similar though muddier. The American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for heart failure cite a meta-analysis showing that CPAP improves left ventricular EF. In 2019, the American Academy of Sleep Medicine (AASM) CPAP guidelines included a systematic review and meta-analysis that found that CPAP has no effect on left ventricular EF in patients with or without heart failure.
There are a million reasons why two systematic reviews on the same topic might come to different conclusions. In this case, the included studies only partially overlap, and broadly speaking, it appears the authors made trade-offs. The review cited by the ACC/AHA had broader inclusion and significantly more patients and paid for it in heterogeneity (I2 in the 80%-90% range). The AASM analysis achieved 0% heterogeneity but limited inclusion to fewer than 100 patients. Across both, the improvement in EF was 2%- 5% at a minimally clinically important difference of 4%. Hardly convincing.
In summary, the road to negative trials and patient harm has always been paved with observational signal and biologic plausibility. Throw in some intellectual and academic bias, and you’ve created the perfect storm of therapeutic overconfidence.
Dr. Holley is a professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a physician at Pulmonary/Sleep and Critical Care Medicine, MedStar Washington Hospital Center, Washington. He disclosed ties to Metapharm Inc., CHEST College, and WebMD.
A version of this article appeared on Medscape.com .
A few months ago, I posted a column on continuous positive airway pressure (CPAP) with the title, “CPAP Oversells and Underperforms.” To date, it has 299 likes and 90 comments, which are almost all negative. I’m glad to see that it’s generated interest, and I’d like to address some of the themes expressed in the posts.
Most comments were personal testimonies to the miracles of CPAP. These are important, and the point deserves emphasis. CPAP can provide significant improvements in daytime sleepiness and quality of life. I closed the original piece by acknowledging this important fact. Readers can be forgiven for missing it given that the title and text were otherwise disparaging of CPAP.
But several comments warrant a more in-depth discussion. The original piece focuses on CPAP and cardiovascular (CV) outcomes but made no mention of atrial fibrillation (AF) or ejection fraction (EF). The effects of CPAP on each are touted by cardiologists and PAP-pushers alike and are drivers of frequent referrals. It›s my fault for omitting them from the discussion.
AF is easy. The data is identical to all other things CPAP and CV. Based on biologic plausibility alone, the likelihood of a relationship between AF and obstructive sleep apnea (OSA) is similar to the odds that the Celtics raise an 18th banner come June. There’s hypoxia, intrathoracic pressure swings, sympathetic surges, and sleep state disruptions. It’s easy to get from there to arrhythmogenesis. There’s lots of observational noise, too, but no randomized proof that CPAP alters this relationship.
I found four randomized controlled trials (RCTs) that tested CPAP’s effect on AF. I’ll save you the suspense; they were all negative. One even found a signal for more adverse events in the CPAP group. These studies have several positive qualities: They enrolled patients with moderate to severe sleep apnea and high oxygen desaturation indices, adherence averaged more than 4 hours across all groups in all trials, and the methods for assessing the AF outcomes differed slightly. There’s also a lot not to like: The sample sizes were small, only one trial enrolled “sleepy” patients (as assessed by the Epworth Sleepiness Score), and follow-up was short.
To paraphrase Carl Sagan, “absence of evidence does not equal evidence of absence.” As a statistician would say, type II error cannot be excluded by these RCTs. In medicine, however, the burden of proof falls on demonstrating efficacy. If we treat before concluding that a therapy works, we risk wasting time, money, medical resources, and the most precious of patient commodities: the energy required for behavior change. In their response to letters to the editor, the authors of the third RCT summarize the CPAP, AF, and CV disease data far better than I ever could. They sound the same words of caution and come out against screening patients with AF for OSA.
The story for CPAP’s effects on EF is similar though muddier. The American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for heart failure cite a meta-analysis showing that CPAP improves left ventricular EF. In 2019, the American Academy of Sleep Medicine (AASM) CPAP guidelines included a systematic review and meta-analysis that found that CPAP has no effect on left ventricular EF in patients with or without heart failure.
There are a million reasons why two systematic reviews on the same topic might come to different conclusions. In this case, the included studies only partially overlap, and broadly speaking, it appears the authors made trade-offs. The review cited by the ACC/AHA had broader inclusion and significantly more patients and paid for it in heterogeneity (I2 in the 80%-90% range). The AASM analysis achieved 0% heterogeneity but limited inclusion to fewer than 100 patients. Across both, the improvement in EF was 2%- 5% at a minimally clinically important difference of 4%. Hardly convincing.
In summary, the road to negative trials and patient harm has always been paved with observational signal and biologic plausibility. Throw in some intellectual and academic bias, and you’ve created the perfect storm of therapeutic overconfidence.
Dr. Holley is a professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a physician at Pulmonary/Sleep and Critical Care Medicine, MedStar Washington Hospital Center, Washington. He disclosed ties to Metapharm Inc., CHEST College, and WebMD.
A version of this article appeared on Medscape.com .
A few months ago, I posted a column on continuous positive airway pressure (CPAP) with the title, “CPAP Oversells and Underperforms.” To date, it has 299 likes and 90 comments, which are almost all negative. I’m glad to see that it’s generated interest, and I’d like to address some of the themes expressed in the posts.
Most comments were personal testimonies to the miracles of CPAP. These are important, and the point deserves emphasis. CPAP can provide significant improvements in daytime sleepiness and quality of life. I closed the original piece by acknowledging this important fact. Readers can be forgiven for missing it given that the title and text were otherwise disparaging of CPAP.
But several comments warrant a more in-depth discussion. The original piece focuses on CPAP and cardiovascular (CV) outcomes but made no mention of atrial fibrillation (AF) or ejection fraction (EF). The effects of CPAP on each are touted by cardiologists and PAP-pushers alike and are drivers of frequent referrals. It›s my fault for omitting them from the discussion.
AF is easy. The data is identical to all other things CPAP and CV. Based on biologic plausibility alone, the likelihood of a relationship between AF and obstructive sleep apnea (OSA) is similar to the odds that the Celtics raise an 18th banner come June. There’s hypoxia, intrathoracic pressure swings, sympathetic surges, and sleep state disruptions. It’s easy to get from there to arrhythmogenesis. There’s lots of observational noise, too, but no randomized proof that CPAP alters this relationship.
I found four randomized controlled trials (RCTs) that tested CPAP’s effect on AF. I’ll save you the suspense; they were all negative. One even found a signal for more adverse events in the CPAP group. These studies have several positive qualities: They enrolled patients with moderate to severe sleep apnea and high oxygen desaturation indices, adherence averaged more than 4 hours across all groups in all trials, and the methods for assessing the AF outcomes differed slightly. There’s also a lot not to like: The sample sizes were small, only one trial enrolled “sleepy” patients (as assessed by the Epworth Sleepiness Score), and follow-up was short.
To paraphrase Carl Sagan, “absence of evidence does not equal evidence of absence.” As a statistician would say, type II error cannot be excluded by these RCTs. In medicine, however, the burden of proof falls on demonstrating efficacy. If we treat before concluding that a therapy works, we risk wasting time, money, medical resources, and the most precious of patient commodities: the energy required for behavior change. In their response to letters to the editor, the authors of the third RCT summarize the CPAP, AF, and CV disease data far better than I ever could. They sound the same words of caution and come out against screening patients with AF for OSA.
The story for CPAP’s effects on EF is similar though muddier. The American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for heart failure cite a meta-analysis showing that CPAP improves left ventricular EF. In 2019, the American Academy of Sleep Medicine (AASM) CPAP guidelines included a systematic review and meta-analysis that found that CPAP has no effect on left ventricular EF in patients with or without heart failure.
There are a million reasons why two systematic reviews on the same topic might come to different conclusions. In this case, the included studies only partially overlap, and broadly speaking, it appears the authors made trade-offs. The review cited by the ACC/AHA had broader inclusion and significantly more patients and paid for it in heterogeneity (I2 in the 80%-90% range). The AASM analysis achieved 0% heterogeneity but limited inclusion to fewer than 100 patients. Across both, the improvement in EF was 2%- 5% at a minimally clinically important difference of 4%. Hardly convincing.
In summary, the road to negative trials and patient harm has always been paved with observational signal and biologic plausibility. Throw in some intellectual and academic bias, and you’ve created the perfect storm of therapeutic overconfidence.
Dr. Holley is a professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a physician at Pulmonary/Sleep and Critical Care Medicine, MedStar Washington Hospital Center, Washington. He disclosed ties to Metapharm Inc., CHEST College, and WebMD.
A version of this article appeared on Medscape.com .
Unplanned Pregnancy With Weight Loss Drugs: Fact or Fiction?
Claudia* was a charming 27-year-old newlywed. She and her husband wanted to start a family — with one small catch. She had recently gained 30 pounds. During COVID, she and her husband spent 18 months camped out in her parents’ guest room in upstate New York and had eaten their emotions with abandon. They ate when they were happy and ate more when they were sad. They ate when they felt isolated and again when they felt anxious. It didn’t help that her mother was a Culinary Institute–trained amateur chef. They both worked from home and logged long hours on Zoom calls. Because there was no home gym, they replaced their usual fitness club workouts in the city with leisurely strolls around the local lake. When I met her, Claudia categorically refused to entertain the notion of pregnancy until she reached her pre-COVID weight.
At the time, this all seemed quite reasonable to me. We outlined a plan including semaglutide (Wegovy) until she reached her target weight and then a minimum of 2 months off Wegovy prior to conception. We also lined up sessions with a dietitian and trainer and renewed her birth control pill. There was one detail I failed to mention to her: Birth control pills are less effective while on incretin hormones like semaglutide. The reason for my omission is that the medical community at large wasn’t yet aware of this issue.
About 12 weeks into treatment, Claudia had lost 20 of the 30 pounds. She had canceled several appointments with the trainer and dietitian due to work conflicts. She messaged me over the weekend in a panic. Her period was late, and her pregnancy test was positive.
She had three pressing questions for me:
Q: How had this happened while she had taken the birth control pills faithfully?
A: I answered that the scientific reasons for the decrease in efficacy of birth control pills while on semaglutide medications are threefold:
- Weight loss can improve menstrual cycle irregularities and improve fertility. In fact, I have been using semaglutide-like medications to treat polycystic ovary syndrome for decades, well before these medications became mainstream.
- The delayed gastric emptying inherent to incretins leads to decreased absorption of birth control pills.
- Finally, while this did not apply to Claudia, no medicine is particularly efficacious if vomited up shortly after taking. Wegovy is known to cause nausea and vomiting in a sizable percentage of patients.
Q: Would she have a healthy pregnancy given the lingering effects of Wegovy?
A: The short answer is: most likely yes. A review of the package insert revealed something fascinating. It was not strictly contraindicated. It advised doctors to weigh the risks and benefits of the medication during pregnancy. Animal studies have shown that semaglutide increases the risk for fetal death, birth defects, and growth issues, but this is probably due to restrictive eating patterns rather than a direct effect of the medication. A recent study of health records of more than 50,000 women with diabetes who had been inadvertently taking these medications in early pregnancy showed no increase in birth defects when compared with women who took insulin.
Q: What would happen to her weight loss efforts?
A: To address her third concern, I tried to offset the risk for rebound weight gain by stopping Wegovy and giving her metformin in the second and third trimesters. Considered a safe medication in pregnancy, metformin is thought to support weight loss, but it proved to be ineffective against the rebound weight gain from stopping Wegovy. Claudia had not resumed regular exercise and quickly fell into the age-old eating-for-two trap. She gained nearly 50 pounds over the course of her pregnancy.
After a short and unfulfilling attempt at nursing, Claudia restarted Wegovy, this time in conjunction with a Mediterranean meal plan and regular sessions at a fitness club. After losing the pregnancy weight, she has been able to successfully maintain her ideal body weight for the past year, and her baby is perfectly healthy and beautiful.
*Patient’s name changed.
A version of this article appeared on Medscape.com.
Claudia* was a charming 27-year-old newlywed. She and her husband wanted to start a family — with one small catch. She had recently gained 30 pounds. During COVID, she and her husband spent 18 months camped out in her parents’ guest room in upstate New York and had eaten their emotions with abandon. They ate when they were happy and ate more when they were sad. They ate when they felt isolated and again when they felt anxious. It didn’t help that her mother was a Culinary Institute–trained amateur chef. They both worked from home and logged long hours on Zoom calls. Because there was no home gym, they replaced their usual fitness club workouts in the city with leisurely strolls around the local lake. When I met her, Claudia categorically refused to entertain the notion of pregnancy until she reached her pre-COVID weight.
At the time, this all seemed quite reasonable to me. We outlined a plan including semaglutide (Wegovy) until she reached her target weight and then a minimum of 2 months off Wegovy prior to conception. We also lined up sessions with a dietitian and trainer and renewed her birth control pill. There was one detail I failed to mention to her: Birth control pills are less effective while on incretin hormones like semaglutide. The reason for my omission is that the medical community at large wasn’t yet aware of this issue.
About 12 weeks into treatment, Claudia had lost 20 of the 30 pounds. She had canceled several appointments with the trainer and dietitian due to work conflicts. She messaged me over the weekend in a panic. Her period was late, and her pregnancy test was positive.
She had three pressing questions for me:
Q: How had this happened while she had taken the birth control pills faithfully?
A: I answered that the scientific reasons for the decrease in efficacy of birth control pills while on semaglutide medications are threefold:
- Weight loss can improve menstrual cycle irregularities and improve fertility. In fact, I have been using semaglutide-like medications to treat polycystic ovary syndrome for decades, well before these medications became mainstream.
- The delayed gastric emptying inherent to incretins leads to decreased absorption of birth control pills.
- Finally, while this did not apply to Claudia, no medicine is particularly efficacious if vomited up shortly after taking. Wegovy is known to cause nausea and vomiting in a sizable percentage of patients.
Q: Would she have a healthy pregnancy given the lingering effects of Wegovy?
A: The short answer is: most likely yes. A review of the package insert revealed something fascinating. It was not strictly contraindicated. It advised doctors to weigh the risks and benefits of the medication during pregnancy. Animal studies have shown that semaglutide increases the risk for fetal death, birth defects, and growth issues, but this is probably due to restrictive eating patterns rather than a direct effect of the medication. A recent study of health records of more than 50,000 women with diabetes who had been inadvertently taking these medications in early pregnancy showed no increase in birth defects when compared with women who took insulin.
Q: What would happen to her weight loss efforts?
A: To address her third concern, I tried to offset the risk for rebound weight gain by stopping Wegovy and giving her metformin in the second and third trimesters. Considered a safe medication in pregnancy, metformin is thought to support weight loss, but it proved to be ineffective against the rebound weight gain from stopping Wegovy. Claudia had not resumed regular exercise and quickly fell into the age-old eating-for-two trap. She gained nearly 50 pounds over the course of her pregnancy.
After a short and unfulfilling attempt at nursing, Claudia restarted Wegovy, this time in conjunction with a Mediterranean meal plan and regular sessions at a fitness club. After losing the pregnancy weight, she has been able to successfully maintain her ideal body weight for the past year, and her baby is perfectly healthy and beautiful.
*Patient’s name changed.
A version of this article appeared on Medscape.com.
Claudia* was a charming 27-year-old newlywed. She and her husband wanted to start a family — with one small catch. She had recently gained 30 pounds. During COVID, she and her husband spent 18 months camped out in her parents’ guest room in upstate New York and had eaten their emotions with abandon. They ate when they were happy and ate more when they were sad. They ate when they felt isolated and again when they felt anxious. It didn’t help that her mother was a Culinary Institute–trained amateur chef. They both worked from home and logged long hours on Zoom calls. Because there was no home gym, they replaced their usual fitness club workouts in the city with leisurely strolls around the local lake. When I met her, Claudia categorically refused to entertain the notion of pregnancy until she reached her pre-COVID weight.
At the time, this all seemed quite reasonable to me. We outlined a plan including semaglutide (Wegovy) until she reached her target weight and then a minimum of 2 months off Wegovy prior to conception. We also lined up sessions with a dietitian and trainer and renewed her birth control pill. There was one detail I failed to mention to her: Birth control pills are less effective while on incretin hormones like semaglutide. The reason for my omission is that the medical community at large wasn’t yet aware of this issue.
About 12 weeks into treatment, Claudia had lost 20 of the 30 pounds. She had canceled several appointments with the trainer and dietitian due to work conflicts. She messaged me over the weekend in a panic. Her period was late, and her pregnancy test was positive.
She had three pressing questions for me:
Q: How had this happened while she had taken the birth control pills faithfully?
A: I answered that the scientific reasons for the decrease in efficacy of birth control pills while on semaglutide medications are threefold:
- Weight loss can improve menstrual cycle irregularities and improve fertility. In fact, I have been using semaglutide-like medications to treat polycystic ovary syndrome for decades, well before these medications became mainstream.
- The delayed gastric emptying inherent to incretins leads to decreased absorption of birth control pills.
- Finally, while this did not apply to Claudia, no medicine is particularly efficacious if vomited up shortly after taking. Wegovy is known to cause nausea and vomiting in a sizable percentage of patients.
Q: Would she have a healthy pregnancy given the lingering effects of Wegovy?
A: The short answer is: most likely yes. A review of the package insert revealed something fascinating. It was not strictly contraindicated. It advised doctors to weigh the risks and benefits of the medication during pregnancy. Animal studies have shown that semaglutide increases the risk for fetal death, birth defects, and growth issues, but this is probably due to restrictive eating patterns rather than a direct effect of the medication. A recent study of health records of more than 50,000 women with diabetes who had been inadvertently taking these medications in early pregnancy showed no increase in birth defects when compared with women who took insulin.
Q: What would happen to her weight loss efforts?
A: To address her third concern, I tried to offset the risk for rebound weight gain by stopping Wegovy and giving her metformin in the second and third trimesters. Considered a safe medication in pregnancy, metformin is thought to support weight loss, but it proved to be ineffective against the rebound weight gain from stopping Wegovy. Claudia had not resumed regular exercise and quickly fell into the age-old eating-for-two trap. She gained nearly 50 pounds over the course of her pregnancy.
After a short and unfulfilling attempt at nursing, Claudia restarted Wegovy, this time in conjunction with a Mediterranean meal plan and regular sessions at a fitness club. After losing the pregnancy weight, she has been able to successfully maintain her ideal body weight for the past year, and her baby is perfectly healthy and beautiful.
*Patient’s name changed.
A version of this article appeared on Medscape.com.
Why Cardiac Biomarkers Don’t Help Predict Heart Disease
This transcript has been edited for clarity.
It’s the counterintuitive stuff in epidemiology that always really interests me. One intuition many of us have is that if a risk factor is significantly associated with an outcome, knowledge of that risk factor would help to predict that outcome. Makes sense. Feels right.
But it’s not right. Not always.
Here’s a fake example to illustrate my point. Let’s say we have 10,000 individuals who we follow for 10 years and 2000 of them die. (It’s been a rough decade.) At baseline, I measured a novel biomarker, the Perry Factor, in everyone. To keep it simple, the Perry Factor has only two values: 0 or 1.
I then do a standard associational analysis and find that individuals who are positive for the Perry Factor have a 40-fold higher odds of death than those who are negative for it. I am beginning to reconsider ascribing my good name to this biomarker. This is a highly statistically significant result — a P value <.001.
Clearly, knowledge of the Perry Factor should help me predict who will die in the cohort. I evaluate predictive power using a metric called the area under the receiver operating characteristic curve (AUC, referred to as the C-statistic in time-to-event studies). It tells you, given two people — one who dies and one who doesn’t — how frequently you “pick” the right person, given the knowledge of their Perry Factor.
A C-statistic of 0.5, or 50%, would mean the Perry Factor gives you no better results than a coin flip; it’s chance. A C-statistic of 1 is perfect prediction. So, what will the C-statistic be, given the incredibly strong association of the Perry Factor with outcomes? 0.9? 0.95?
0.5024. Almost useless.

Let’s figure out why strength of association and usefulness for prediction are not always the same thing.
I constructed my fake Perry Factor dataset quite carefully to illustrate this point. Let me show you what happened. What you see here is a breakdown of the patients in my fake study. You can see that just 11 of them were Perry Factor positive, but 10 of those 11 ended up dying.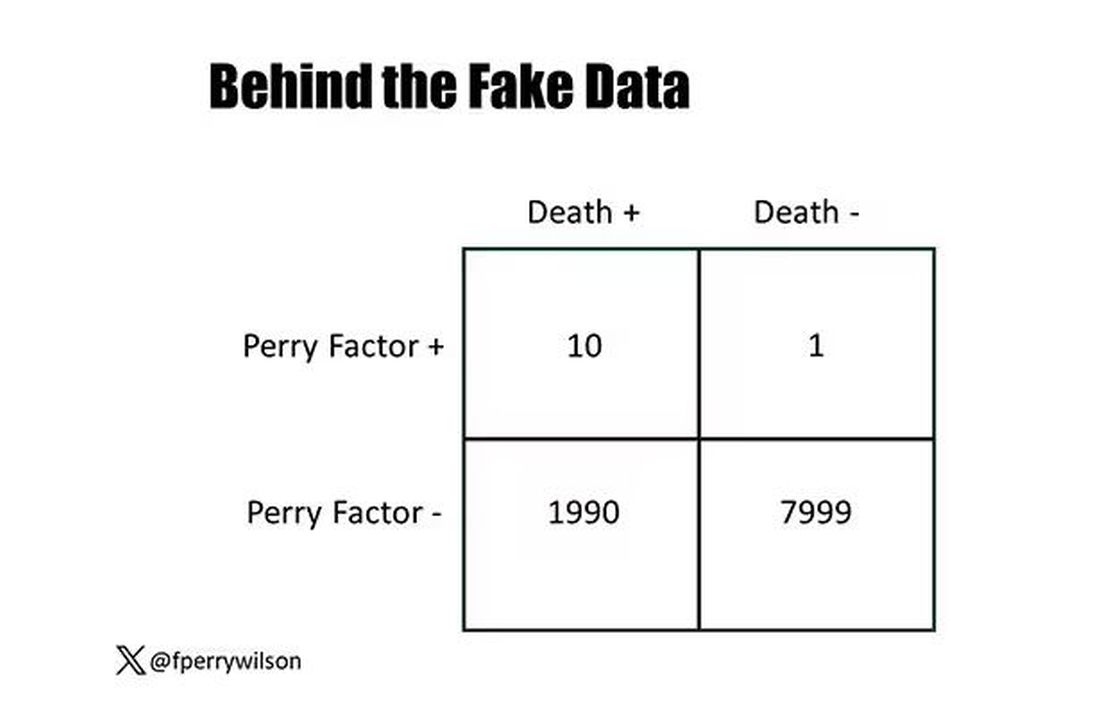
That’s quite unlikely by chance alone. It really does appear that if you have Perry Factor, your risk for death is much higher. But the reason that Perry Factor is a bad predictor is because it is so rare in the population. Sure, you can use it to correctly predict the outcome of 10 of the 11 people who have it, but the vast majority of people don’t have Perry Factor. It’s useless to distinguish who will die vs who will live in that population.
Why have I spent so much time trying to reverse our intuition that strength of association and strength of predictive power must be related? Because it helps to explain this paper, “Prognostic Value of Cardiovascular Biomarkers in the Population,” appearing in JAMA, which is a very nice piece of work trying to help us better predict cardiovascular disease.
I don’t need to tell you that cardiovascular disease is the number-one killer in this country and most of the world. I don’t need to tell you that we have really good preventive therapies and lifestyle interventions that can reduce the risk. But it would be nice to know in whom, specifically, we should use those interventions.
Cardiovascular risk scores, to date, are pretty simple. The most common one in use in the United States, the pooled cohort risk equation, has nine variables, two of which require a cholesterol panel and one a blood pressure test. It’s easy and it’s pretty accurate.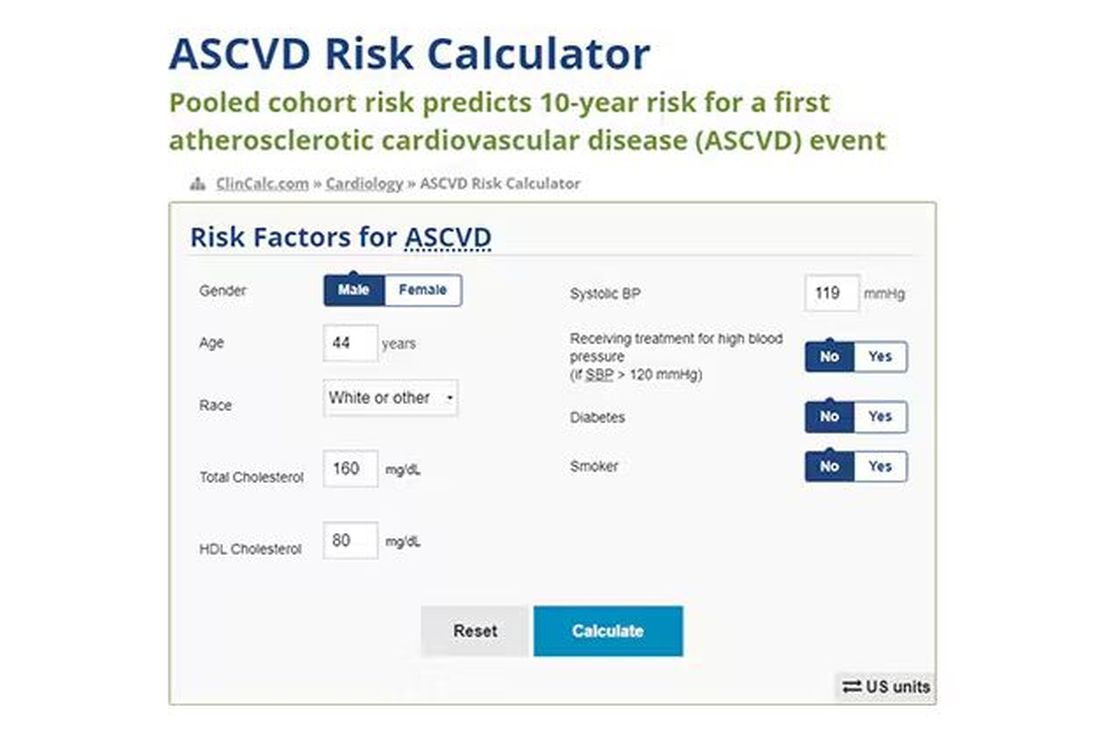
Using the score from the pooled cohort risk calculator, you get a C-statistic as high as 0.82 when applied to Black women, a low of 0.71 when applied to Black men. Non-Black individuals are in the middle. Not bad. But, clearly, not perfect.
And aren’t we in the era of big data, the era of personalized medicine? We have dozens, maybe hundreds, of quantifiable biomarkers that are associated with subsequent heart disease. Surely, by adding these biomarkers into the risk equation, we can improve prediction. Right?
The JAMA study includes 164,054 patients pooled from 28 cohort studies from 12 countries. All the studies measured various key biomarkers at baseline and followed their participants for cardiovascular events like heart attack, stroke, coronary revascularization, and so on.
The biomarkers in question are really the big guns in this space: troponin, a marker of stress on the heart muscle; NT-proBNP, a marker of stretch on the heart muscle; and C-reactive protein, a marker of inflammation. In every case, higher levels of these markers at baseline were associated with a higher risk for cardiovascular disease in the future.
Troponin T, shown here, has a basically linear risk with subsequent cardiovascular disease.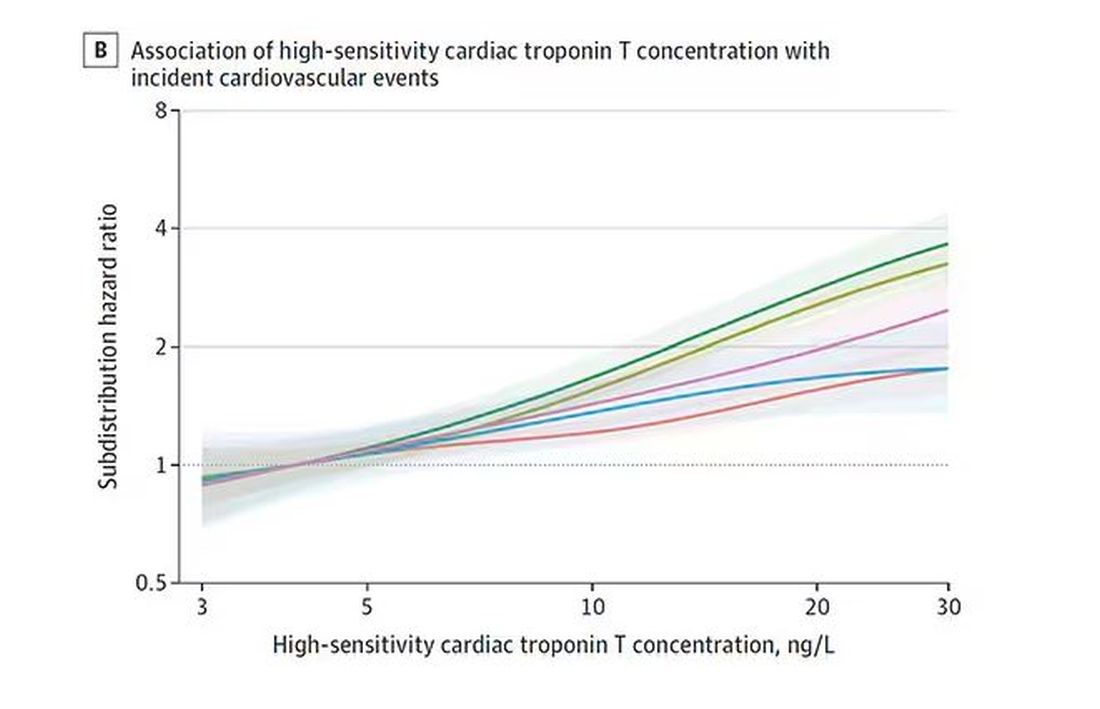
BNP seems to demonstrate more of a threshold effect, where levels above 60 start to associate with problems.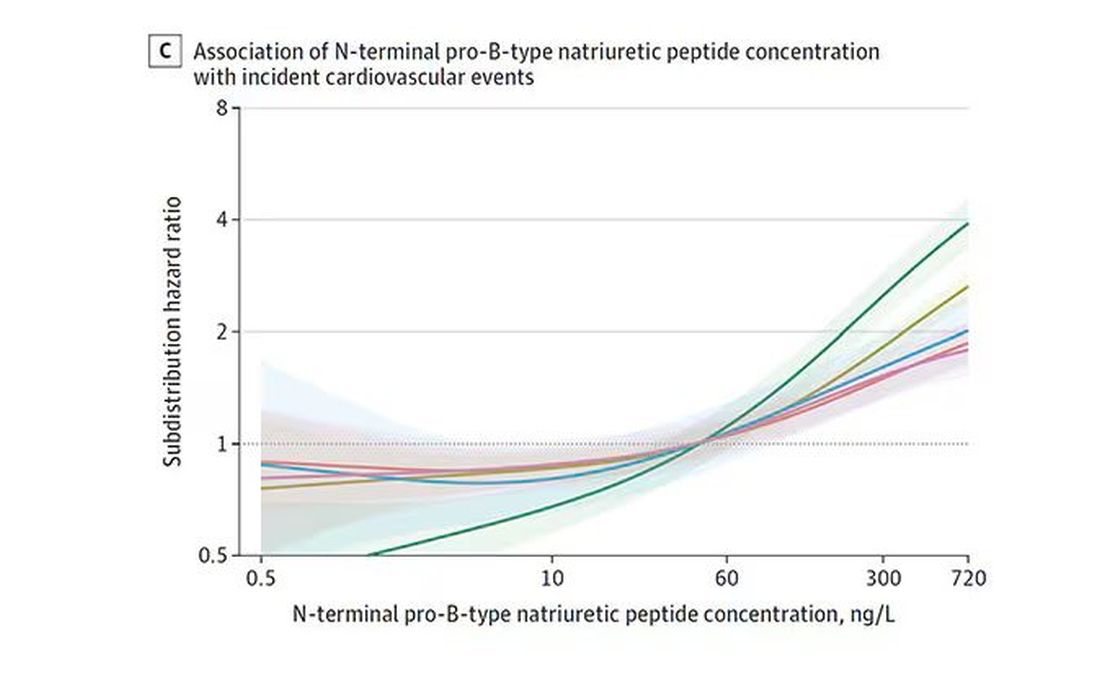
And CRP does a similar thing, with levels above 1.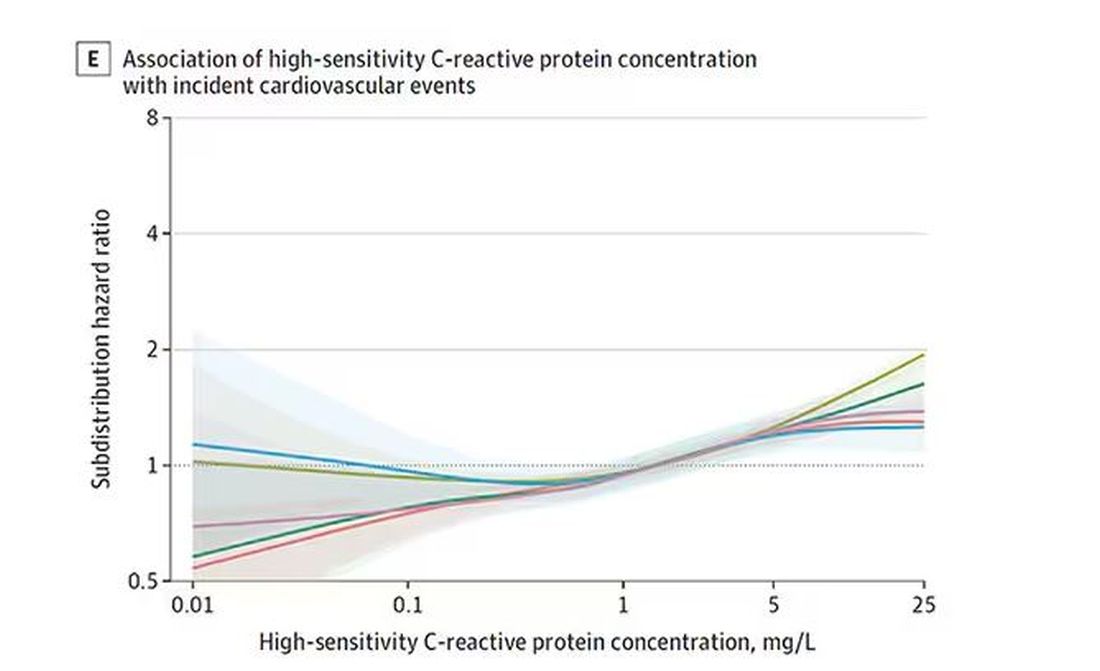
All of these findings were statistically significant. If you have higher levels of one or more of these biomarkers, you are more likely to have cardiovascular disease in the future.
Of course, our old friend the pooled cohort risk equation is still here — in the background — requiring just that one blood test and measurement of blood pressure. Let’s talk about predictive power.
The pooled cohort risk equation score, in this study, had a C-statistic of 0.812.
By adding troponin, BNP, and CRP to the equation, the new C-statistic is 0.819. Barely any change.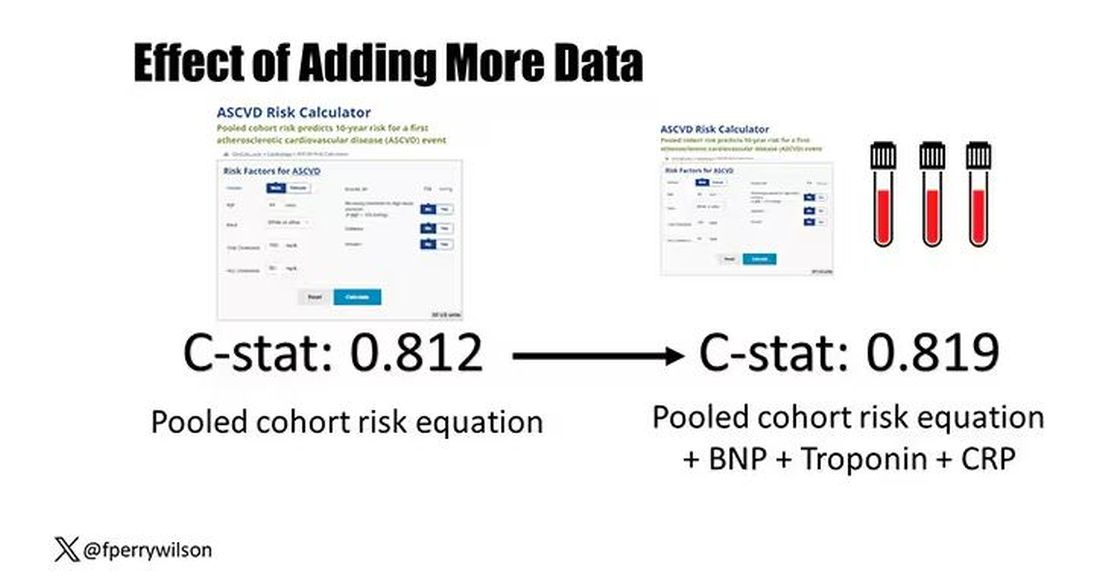
Now, the authors looked at different types of prediction here. The greatest improvement in the AUC was seen when they tried to predict heart failure within 1 year of measurement; there the AUC improved by 0.04. But the presence of BNP as a biomarker and the short time window of 1 year makes me wonder whether this is really prediction at all or whether they were essentially just diagnosing people with existing heart failure.
Why does this happen? Why do these promising biomarkers, clearly associated with bad outcomes, fail to improve our ability to predict the future? I already gave one example, which has to do with how the markers are distributed in the population. But even more relevant here is that the new markers will only improve prediction insofar as they are not already represented in the old predictive model.
Of course, BNP, for example, wasn’t in the old model. But smoking was. Diabetes was. Blood pressure was. All of that data might actually tell you something about the patient’s BNP through their mutual correlation. And improvement in prediction requires new information.
This is actually why I consider this a really successful study. We need to do studies like this to help us find what those new sources of information might be.
We will never get to a C-statistic of 1. Perfect prediction is the domain of palm readers and astrophysicists. But better prediction is always possible through data. The big question, of course, is which data?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s the counterintuitive stuff in epidemiology that always really interests me. One intuition many of us have is that if a risk factor is significantly associated with an outcome, knowledge of that risk factor would help to predict that outcome. Makes sense. Feels right.
But it’s not right. Not always.
Here’s a fake example to illustrate my point. Let’s say we have 10,000 individuals who we follow for 10 years and 2000 of them die. (It’s been a rough decade.) At baseline, I measured a novel biomarker, the Perry Factor, in everyone. To keep it simple, the Perry Factor has only two values: 0 or 1.
I then do a standard associational analysis and find that individuals who are positive for the Perry Factor have a 40-fold higher odds of death than those who are negative for it. I am beginning to reconsider ascribing my good name to this biomarker. This is a highly statistically significant result — a P value <.001.
Clearly, knowledge of the Perry Factor should help me predict who will die in the cohort. I evaluate predictive power using a metric called the area under the receiver operating characteristic curve (AUC, referred to as the C-statistic in time-to-event studies). It tells you, given two people — one who dies and one who doesn’t — how frequently you “pick” the right person, given the knowledge of their Perry Factor.
A C-statistic of 0.5, or 50%, would mean the Perry Factor gives you no better results than a coin flip; it’s chance. A C-statistic of 1 is perfect prediction. So, what will the C-statistic be, given the incredibly strong association of the Perry Factor with outcomes? 0.9? 0.95?
0.5024. Almost useless.

Let’s figure out why strength of association and usefulness for prediction are not always the same thing.
I constructed my fake Perry Factor dataset quite carefully to illustrate this point. Let me show you what happened. What you see here is a breakdown of the patients in my fake study. You can see that just 11 of them were Perry Factor positive, but 10 of those 11 ended up dying.
That’s quite unlikely by chance alone. It really does appear that if you have Perry Factor, your risk for death is much higher. But the reason that Perry Factor is a bad predictor is because it is so rare in the population. Sure, you can use it to correctly predict the outcome of 10 of the 11 people who have it, but the vast majority of people don’t have Perry Factor. It’s useless to distinguish who will die vs who will live in that population.
Why have I spent so much time trying to reverse our intuition that strength of association and strength of predictive power must be related? Because it helps to explain this paper, “Prognostic Value of Cardiovascular Biomarkers in the Population,” appearing in JAMA, which is a very nice piece of work trying to help us better predict cardiovascular disease.
I don’t need to tell you that cardiovascular disease is the number-one killer in this country and most of the world. I don’t need to tell you that we have really good preventive therapies and lifestyle interventions that can reduce the risk. But it would be nice to know in whom, specifically, we should use those interventions.
Cardiovascular risk scores, to date, are pretty simple. The most common one in use in the United States, the pooled cohort risk equation, has nine variables, two of which require a cholesterol panel and one a blood pressure test. It’s easy and it’s pretty accurate.
Using the score from the pooled cohort risk calculator, you get a C-statistic as high as 0.82 when applied to Black women, a low of 0.71 when applied to Black men. Non-Black individuals are in the middle. Not bad. But, clearly, not perfect.
And aren’t we in the era of big data, the era of personalized medicine? We have dozens, maybe hundreds, of quantifiable biomarkers that are associated with subsequent heart disease. Surely, by adding these biomarkers into the risk equation, we can improve prediction. Right?
The JAMA study includes 164,054 patients pooled from 28 cohort studies from 12 countries. All the studies measured various key biomarkers at baseline and followed their participants for cardiovascular events like heart attack, stroke, coronary revascularization, and so on.
The biomarkers in question are really the big guns in this space: troponin, a marker of stress on the heart muscle; NT-proBNP, a marker of stretch on the heart muscle; and C-reactive protein, a marker of inflammation. In every case, higher levels of these markers at baseline were associated with a higher risk for cardiovascular disease in the future.
Troponin T, shown here, has a basically linear risk with subsequent cardiovascular disease.
BNP seems to demonstrate more of a threshold effect, where levels above 60 start to associate with problems.
And CRP does a similar thing, with levels above 1.
All of these findings were statistically significant. If you have higher levels of one or more of these biomarkers, you are more likely to have cardiovascular disease in the future.
Of course, our old friend the pooled cohort risk equation is still here — in the background — requiring just that one blood test and measurement of blood pressure. Let’s talk about predictive power.
The pooled cohort risk equation score, in this study, had a C-statistic of 0.812.
By adding troponin, BNP, and CRP to the equation, the new C-statistic is 0.819. Barely any change.
Now, the authors looked at different types of prediction here. The greatest improvement in the AUC was seen when they tried to predict heart failure within 1 year of measurement; there the AUC improved by 0.04. But the presence of BNP as a biomarker and the short time window of 1 year makes me wonder whether this is really prediction at all or whether they were essentially just diagnosing people with existing heart failure.
Why does this happen? Why do these promising biomarkers, clearly associated with bad outcomes, fail to improve our ability to predict the future? I already gave one example, which has to do with how the markers are distributed in the population. But even more relevant here is that the new markers will only improve prediction insofar as they are not already represented in the old predictive model.
Of course, BNP, for example, wasn’t in the old model. But smoking was. Diabetes was. Blood pressure was. All of that data might actually tell you something about the patient’s BNP through their mutual correlation. And improvement in prediction requires new information.
This is actually why I consider this a really successful study. We need to do studies like this to help us find what those new sources of information might be.
We will never get to a C-statistic of 1. Perfect prediction is the domain of palm readers and astrophysicists. But better prediction is always possible through data. The big question, of course, is which data?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s the counterintuitive stuff in epidemiology that always really interests me. One intuition many of us have is that if a risk factor is significantly associated with an outcome, knowledge of that risk factor would help to predict that outcome. Makes sense. Feels right.
But it’s not right. Not always.
Here’s a fake example to illustrate my point. Let’s say we have 10,000 individuals who we follow for 10 years and 2000 of them die. (It’s been a rough decade.) At baseline, I measured a novel biomarker, the Perry Factor, in everyone. To keep it simple, the Perry Factor has only two values: 0 or 1.
I then do a standard associational analysis and find that individuals who are positive for the Perry Factor have a 40-fold higher odds of death than those who are negative for it. I am beginning to reconsider ascribing my good name to this biomarker. This is a highly statistically significant result — a P value <.001.
Clearly, knowledge of the Perry Factor should help me predict who will die in the cohort. I evaluate predictive power using a metric called the area under the receiver operating characteristic curve (AUC, referred to as the C-statistic in time-to-event studies). It tells you, given two people — one who dies and one who doesn’t — how frequently you “pick” the right person, given the knowledge of their Perry Factor.
A C-statistic of 0.5, or 50%, would mean the Perry Factor gives you no better results than a coin flip; it’s chance. A C-statistic of 1 is perfect prediction. So, what will the C-statistic be, given the incredibly strong association of the Perry Factor with outcomes? 0.9? 0.95?
0.5024. Almost useless.

Let’s figure out why strength of association and usefulness for prediction are not always the same thing.
I constructed my fake Perry Factor dataset quite carefully to illustrate this point. Let me show you what happened. What you see here is a breakdown of the patients in my fake study. You can see that just 11 of them were Perry Factor positive, but 10 of those 11 ended up dying.
That’s quite unlikely by chance alone. It really does appear that if you have Perry Factor, your risk for death is much higher. But the reason that Perry Factor is a bad predictor is because it is so rare in the population. Sure, you can use it to correctly predict the outcome of 10 of the 11 people who have it, but the vast majority of people don’t have Perry Factor. It’s useless to distinguish who will die vs who will live in that population.
Why have I spent so much time trying to reverse our intuition that strength of association and strength of predictive power must be related? Because it helps to explain this paper, “Prognostic Value of Cardiovascular Biomarkers in the Population,” appearing in JAMA, which is a very nice piece of work trying to help us better predict cardiovascular disease.
I don’t need to tell you that cardiovascular disease is the number-one killer in this country and most of the world. I don’t need to tell you that we have really good preventive therapies and lifestyle interventions that can reduce the risk. But it would be nice to know in whom, specifically, we should use those interventions.
Cardiovascular risk scores, to date, are pretty simple. The most common one in use in the United States, the pooled cohort risk equation, has nine variables, two of which require a cholesterol panel and one a blood pressure test. It’s easy and it’s pretty accurate.
Using the score from the pooled cohort risk calculator, you get a C-statistic as high as 0.82 when applied to Black women, a low of 0.71 when applied to Black men. Non-Black individuals are in the middle. Not bad. But, clearly, not perfect.
And aren’t we in the era of big data, the era of personalized medicine? We have dozens, maybe hundreds, of quantifiable biomarkers that are associated with subsequent heart disease. Surely, by adding these biomarkers into the risk equation, we can improve prediction. Right?
The JAMA study includes 164,054 patients pooled from 28 cohort studies from 12 countries. All the studies measured various key biomarkers at baseline and followed their participants for cardiovascular events like heart attack, stroke, coronary revascularization, and so on.
The biomarkers in question are really the big guns in this space: troponin, a marker of stress on the heart muscle; NT-proBNP, a marker of stretch on the heart muscle; and C-reactive protein, a marker of inflammation. In every case, higher levels of these markers at baseline were associated with a higher risk for cardiovascular disease in the future.
Troponin T, shown here, has a basically linear risk with subsequent cardiovascular disease.
BNP seems to demonstrate more of a threshold effect, where levels above 60 start to associate with problems.
And CRP does a similar thing, with levels above 1.
All of these findings were statistically significant. If you have higher levels of one or more of these biomarkers, you are more likely to have cardiovascular disease in the future.
Of course, our old friend the pooled cohort risk equation is still here — in the background — requiring just that one blood test and measurement of blood pressure. Let’s talk about predictive power.
The pooled cohort risk equation score, in this study, had a C-statistic of 0.812.
By adding troponin, BNP, and CRP to the equation, the new C-statistic is 0.819. Barely any change.
Now, the authors looked at different types of prediction here. The greatest improvement in the AUC was seen when they tried to predict heart failure within 1 year of measurement; there the AUC improved by 0.04. But the presence of BNP as a biomarker and the short time window of 1 year makes me wonder whether this is really prediction at all or whether they were essentially just diagnosing people with existing heart failure.
Why does this happen? Why do these promising biomarkers, clearly associated with bad outcomes, fail to improve our ability to predict the future? I already gave one example, which has to do with how the markers are distributed in the population. But even more relevant here is that the new markers will only improve prediction insofar as they are not already represented in the old predictive model.
Of course, BNP, for example, wasn’t in the old model. But smoking was. Diabetes was. Blood pressure was. All of that data might actually tell you something about the patient’s BNP through their mutual correlation. And improvement in prediction requires new information.
This is actually why I consider this a really successful study. We need to do studies like this to help us find what those new sources of information might be.
We will never get to a C-statistic of 1. Perfect prediction is the domain of palm readers and astrophysicists. But better prediction is always possible through data. The big question, of course, is which data?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Vacationing Doctors Fight to Revive a Drowned Child
Emergencies happen anywhere, anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
Jennifer Suders, DO: We were in Florida with our 1-year-old daughter visiting my parents. They moved to an area called Hallandale Beach and live in a high-rise community with a few different pools and spas.
Dan and I were in the spa area at the gym. He was getting me to hurry up because we were supposed to meet my parents who were with our daughter. I was sort of moseying and taking my time.
We were walking by one of the pool decks to get into the building when I heard what sounded like a slap. My first thought was that maybe somebody was choking and someone was hitting their back. Choking has always been my biggest fear with our daughter.
I turned and saw some people who seemed frantic. I looked at Dan and started to ask, “Do you think they need help?” I don’t even think I got the whole sentence out before this mom whipped her head around. I’ll never forget her dark brown hair flying. She screamed, “HELP!”
Dan and I just ran. I let go of my backpack and iPad and water bottle. They scattered across the pool deck. I instantly had my phone in my hand dialing 911.
Daniel Suders, DO: That’s what they teach us, to call 911 first. I didn’t think of it in the moment, but Jenny did.
Jennifer Suders:
Dan and I got down on either side of the boy and checked for a pulse. We couldn’t feel anything. Dan started chest compressions. I was talking to the 911 operator, and then I gave two rescue breaths. We did a sternal rub.
I was kind of yelling in the boy’s face, trying to get him to respond. I tried English and Russian because there’s a big Russian community there, and my family speaks Russian. The grandma asked us if we knew what we were doing.
Daniel Suders: I think she asked if Jenny was a nurse.
Jennifer Suders: Common misconception. Suddenly, the boy started vomiting, and so much water poured out. We turned him on his side, and he had two or three more episodes of spitting up the water. After that, we could see the color start to come back into his face. His eyes started fluttering.
We thought he was probably coming back. But we were too scared to say that in case we were wrong, and he went back under. So, we just held him steady. We didn’t know what had happened, if he might have hit his head, so we needed to keep him still.
Daniel Suders: It was amazing when those eyes opened, and he started to wake up.
Jennifer Suders: It felt like my heart had stopped while I was waiting for his to start.
Daniel Suders: He was clutching his chest like it hurt and started calling for his mom. He was crying and wanting to get in his mom’s arms. We had to keep him from standing up and walking.
Jennifer Suders: He was clearly scared. There were all these strange faces around him. I kept looking at my phone, anxiously waiting for EMS to come. They got there about 8 or 9 minutes later.
At some point, the father walked in with their daughter, a baby under a year old. He was in shock, not knowing what was going on. The grandma explained that the boy had been jumping into the pool over and over with his brother. All of a sudden, they looked over, and he was just lying there, floating, face down. They were right there; they were watching him. It was just that quick.
Daniel Suders: They pulled him out right away, and that was a big thing on his side that it was caught so quickly. He didn’t have to wait long to start resuscitation.
Jennifer Suders: Once EMS got there and assessed him, they put him and his mom on the stretcher. I remember watching them wheel it through the double doors to get to the elevator. As soon as they were gone, I just turned around and broke down. I had been in doctor mode if you will. Straight to the point. No nonsense. Suddenly, I went back into civilian mode, and my emotions just bubbled up.
After we left, we went to meet my parents who had our kid. Dan just beelined toward her and scooped her up and wouldn’t let her go.
For the rest of the day, it was all I could think about. It took me a while to fall asleep that night, and it was the first thing I thought when I woke up the next morning. We were hopeful that the boy was going to be okay, but you never know. We didn’t call the hospital because with HIPAA, I didn’t know if they could tell us anything.
And then the next day — there they were. The family was back at the pool. The little boy was running around like nothing had happened. We were a little surprised. But I would hate for him to be scared of the pool for the rest of his life. His family was watching him like a hawk.
They told us that the boy and his mom had stayed overnight in the ER, but only as a precaution. He didn’t have any more vomiting. He was absolutely fine. They were incredibly grateful.
We got their names and exchanged numbers and took a picture. That’s all I wanted — a photo to remember them.
A day or so later, we saw them again at a nearby park. The boy was climbing trees and seemed completely normal. It was the best outcome we could have hoped for.
Daniel Suders: My biggest worry was any harm to his chest from the resuscitation, or of course how long he was without oxygen. But everyone says that kids are really resilient. I work with adults, so I don’t have a lot of experience.
As a hospitalist, we don’t always see a lot of success with CPR. It’s often an elderly person who just doesn’t have much of a chance. That same week before our vacation, I had lost a 90-year-old in the hospital. It was such a juxtaposition — a 3-year-old with their whole life in front of them. We were able to preserve that, and it was incredible.
Jennifer Suders: I’m a nephrologist, so my field is pretty calm. No big emergencies. We have patients on the floor, but if a code gets called, there’s a team that comes in from the intensive care unit. I always kind of wondered what I would do if I was presented with a scenario like this.
Daniel Suders: We have a lot of friends that do ER medicine, and I felt like those were the guys that really understood when we told them the story. One friend said to me, “By the time they get to us, they’re either in bad shape or they’re better already.” A lot depends on what happens in the field.
Jennifer Suders: I’m even more vigilant about pool safety now. I want to make sure parents know that drowning doesn›t look like flailing theatrics. It can be soundless. Three adults were right next to this little boy and didn›t realize until they looked down and saw him.
If we hadn’t been there, I don’t know if anyone would’ve been able to step in. No one else was medically trained. But I think the message is — you don’t have to be. Anyone can take a CPR class.
When I told my parents, my dad said, “Oh my gosh, I would’ve laid right down there next to that kid and passed out.” Without any training, it’s petrifying to see something like that.
I think about how we could have stayed in the gym longer and been too late. Or we could have gotten on the elevator earlier and been gone. Two minutes, and it would’ve been a story we heard later, not one we were a part of. It feels like we were at a true crossroads in that moment where that boy could have lived or died. And the stars aligned perfectly.
We had no medicine, no monitors, nothing but our hands and our breaths. And we helped a family continue their vacation rather than plan a funeral.
Jennifer Suders, DO, is a nephrologist at West Virginia University Medicine Wheeling Clinic. Daniel Suders, DO, is a hospitalist at West Virginia University Medicine Reynolds Memorial Hospital.
A version of this article appeared on Medscape.com .
Emergencies happen anywhere, anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
Jennifer Suders, DO: We were in Florida with our 1-year-old daughter visiting my parents. They moved to an area called Hallandale Beach and live in a high-rise community with a few different pools and spas.
Dan and I were in the spa area at the gym. He was getting me to hurry up because we were supposed to meet my parents who were with our daughter. I was sort of moseying and taking my time.
We were walking by one of the pool decks to get into the building when I heard what sounded like a slap. My first thought was that maybe somebody was choking and someone was hitting their back. Choking has always been my biggest fear with our daughter.
I turned and saw some people who seemed frantic. I looked at Dan and started to ask, “Do you think they need help?” I don’t even think I got the whole sentence out before this mom whipped her head around. I’ll never forget her dark brown hair flying. She screamed, “HELP!”
Dan and I just ran. I let go of my backpack and iPad and water bottle. They scattered across the pool deck. I instantly had my phone in my hand dialing 911.
Daniel Suders, DO: That’s what they teach us, to call 911 first. I didn’t think of it in the moment, but Jenny did.
Jennifer Suders:
Dan and I got down on either side of the boy and checked for a pulse. We couldn’t feel anything. Dan started chest compressions. I was talking to the 911 operator, and then I gave two rescue breaths. We did a sternal rub.
I was kind of yelling in the boy’s face, trying to get him to respond. I tried English and Russian because there’s a big Russian community there, and my family speaks Russian. The grandma asked us if we knew what we were doing.
Daniel Suders: I think she asked if Jenny was a nurse.
Jennifer Suders: Common misconception. Suddenly, the boy started vomiting, and so much water poured out. We turned him on his side, and he had two or three more episodes of spitting up the water. After that, we could see the color start to come back into his face. His eyes started fluttering.
We thought he was probably coming back. But we were too scared to say that in case we were wrong, and he went back under. So, we just held him steady. We didn’t know what had happened, if he might have hit his head, so we needed to keep him still.
Daniel Suders: It was amazing when those eyes opened, and he started to wake up.
Jennifer Suders: It felt like my heart had stopped while I was waiting for his to start.
Daniel Suders: He was clutching his chest like it hurt and started calling for his mom. He was crying and wanting to get in his mom’s arms. We had to keep him from standing up and walking.
Jennifer Suders: He was clearly scared. There were all these strange faces around him. I kept looking at my phone, anxiously waiting for EMS to come. They got there about 8 or 9 minutes later.
At some point, the father walked in with their daughter, a baby under a year old. He was in shock, not knowing what was going on. The grandma explained that the boy had been jumping into the pool over and over with his brother. All of a sudden, they looked over, and he was just lying there, floating, face down. They were right there; they were watching him. It was just that quick.
Daniel Suders: They pulled him out right away, and that was a big thing on his side that it was caught so quickly. He didn’t have to wait long to start resuscitation.
Jennifer Suders: Once EMS got there and assessed him, they put him and his mom on the stretcher. I remember watching them wheel it through the double doors to get to the elevator. As soon as they were gone, I just turned around and broke down. I had been in doctor mode if you will. Straight to the point. No nonsense. Suddenly, I went back into civilian mode, and my emotions just bubbled up.
After we left, we went to meet my parents who had our kid. Dan just beelined toward her and scooped her up and wouldn’t let her go.
For the rest of the day, it was all I could think about. It took me a while to fall asleep that night, and it was the first thing I thought when I woke up the next morning. We were hopeful that the boy was going to be okay, but you never know. We didn’t call the hospital because with HIPAA, I didn’t know if they could tell us anything.
And then the next day — there they were. The family was back at the pool. The little boy was running around like nothing had happened. We were a little surprised. But I would hate for him to be scared of the pool for the rest of his life. His family was watching him like a hawk.
They told us that the boy and his mom had stayed overnight in the ER, but only as a precaution. He didn’t have any more vomiting. He was absolutely fine. They were incredibly grateful.
We got their names and exchanged numbers and took a picture. That’s all I wanted — a photo to remember them.
A day or so later, we saw them again at a nearby park. The boy was climbing trees and seemed completely normal. It was the best outcome we could have hoped for.
Daniel Suders: My biggest worry was any harm to his chest from the resuscitation, or of course how long he was without oxygen. But everyone says that kids are really resilient. I work with adults, so I don’t have a lot of experience.
As a hospitalist, we don’t always see a lot of success with CPR. It’s often an elderly person who just doesn’t have much of a chance. That same week before our vacation, I had lost a 90-year-old in the hospital. It was such a juxtaposition — a 3-year-old with their whole life in front of them. We were able to preserve that, and it was incredible.
Jennifer Suders: I’m a nephrologist, so my field is pretty calm. No big emergencies. We have patients on the floor, but if a code gets called, there’s a team that comes in from the intensive care unit. I always kind of wondered what I would do if I was presented with a scenario like this.
Daniel Suders: We have a lot of friends that do ER medicine, and I felt like those were the guys that really understood when we told them the story. One friend said to me, “By the time they get to us, they’re either in bad shape or they’re better already.” A lot depends on what happens in the field.
Jennifer Suders: I’m even more vigilant about pool safety now. I want to make sure parents know that drowning doesn›t look like flailing theatrics. It can be soundless. Three adults were right next to this little boy and didn›t realize until they looked down and saw him.
If we hadn’t been there, I don’t know if anyone would’ve been able to step in. No one else was medically trained. But I think the message is — you don’t have to be. Anyone can take a CPR class.
When I told my parents, my dad said, “Oh my gosh, I would’ve laid right down there next to that kid and passed out.” Without any training, it’s petrifying to see something like that.
I think about how we could have stayed in the gym longer and been too late. Or we could have gotten on the elevator earlier and been gone. Two minutes, and it would’ve been a story we heard later, not one we were a part of. It feels like we were at a true crossroads in that moment where that boy could have lived or died. And the stars aligned perfectly.
We had no medicine, no monitors, nothing but our hands and our breaths. And we helped a family continue their vacation rather than plan a funeral.
Jennifer Suders, DO, is a nephrologist at West Virginia University Medicine Wheeling Clinic. Daniel Suders, DO, is a hospitalist at West Virginia University Medicine Reynolds Memorial Hospital.
A version of this article appeared on Medscape.com .
Emergencies happen anywhere, anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
Jennifer Suders, DO: We were in Florida with our 1-year-old daughter visiting my parents. They moved to an area called Hallandale Beach and live in a high-rise community with a few different pools and spas.
Dan and I were in the spa area at the gym. He was getting me to hurry up because we were supposed to meet my parents who were with our daughter. I was sort of moseying and taking my time.
We were walking by one of the pool decks to get into the building when I heard what sounded like a slap. My first thought was that maybe somebody was choking and someone was hitting their back. Choking has always been my biggest fear with our daughter.
I turned and saw some people who seemed frantic. I looked at Dan and started to ask, “Do you think they need help?” I don’t even think I got the whole sentence out before this mom whipped her head around. I’ll never forget her dark brown hair flying. She screamed, “HELP!”
Dan and I just ran. I let go of my backpack and iPad and water bottle. They scattered across the pool deck. I instantly had my phone in my hand dialing 911.
Daniel Suders, DO: That’s what they teach us, to call 911 first. I didn’t think of it in the moment, but Jenny did.
Jennifer Suders:
Dan and I got down on either side of the boy and checked for a pulse. We couldn’t feel anything. Dan started chest compressions. I was talking to the 911 operator, and then I gave two rescue breaths. We did a sternal rub.
I was kind of yelling in the boy’s face, trying to get him to respond. I tried English and Russian because there’s a big Russian community there, and my family speaks Russian. The grandma asked us if we knew what we were doing.
Daniel Suders: I think she asked if Jenny was a nurse.
Jennifer Suders: Common misconception. Suddenly, the boy started vomiting, and so much water poured out. We turned him on his side, and he had two or three more episodes of spitting up the water. After that, we could see the color start to come back into his face. His eyes started fluttering.
We thought he was probably coming back. But we were too scared to say that in case we were wrong, and he went back under. So, we just held him steady. We didn’t know what had happened, if he might have hit his head, so we needed to keep him still.
Daniel Suders: It was amazing when those eyes opened, and he started to wake up.
Jennifer Suders: It felt like my heart had stopped while I was waiting for his to start.
Daniel Suders: He was clutching his chest like it hurt and started calling for his mom. He was crying and wanting to get in his mom’s arms. We had to keep him from standing up and walking.
Jennifer Suders: He was clearly scared. There were all these strange faces around him. I kept looking at my phone, anxiously waiting for EMS to come. They got there about 8 or 9 minutes later.
At some point, the father walked in with their daughter, a baby under a year old. He was in shock, not knowing what was going on. The grandma explained that the boy had been jumping into the pool over and over with his brother. All of a sudden, they looked over, and he was just lying there, floating, face down. They were right there; they were watching him. It was just that quick.
Daniel Suders: They pulled him out right away, and that was a big thing on his side that it was caught so quickly. He didn’t have to wait long to start resuscitation.
Jennifer Suders: Once EMS got there and assessed him, they put him and his mom on the stretcher. I remember watching them wheel it through the double doors to get to the elevator. As soon as they were gone, I just turned around and broke down. I had been in doctor mode if you will. Straight to the point. No nonsense. Suddenly, I went back into civilian mode, and my emotions just bubbled up.
After we left, we went to meet my parents who had our kid. Dan just beelined toward her and scooped her up and wouldn’t let her go.
For the rest of the day, it was all I could think about. It took me a while to fall asleep that night, and it was the first thing I thought when I woke up the next morning. We were hopeful that the boy was going to be okay, but you never know. We didn’t call the hospital because with HIPAA, I didn’t know if they could tell us anything.
And then the next day — there they were. The family was back at the pool. The little boy was running around like nothing had happened. We were a little surprised. But I would hate for him to be scared of the pool for the rest of his life. His family was watching him like a hawk.
They told us that the boy and his mom had stayed overnight in the ER, but only as a precaution. He didn’t have any more vomiting. He was absolutely fine. They were incredibly grateful.
We got their names and exchanged numbers and took a picture. That’s all I wanted — a photo to remember them.
A day or so later, we saw them again at a nearby park. The boy was climbing trees and seemed completely normal. It was the best outcome we could have hoped for.
Daniel Suders: My biggest worry was any harm to his chest from the resuscitation, or of course how long he was without oxygen. But everyone says that kids are really resilient. I work with adults, so I don’t have a lot of experience.
As a hospitalist, we don’t always see a lot of success with CPR. It’s often an elderly person who just doesn’t have much of a chance. That same week before our vacation, I had lost a 90-year-old in the hospital. It was such a juxtaposition — a 3-year-old with their whole life in front of them. We were able to preserve that, and it was incredible.
Jennifer Suders: I’m a nephrologist, so my field is pretty calm. No big emergencies. We have patients on the floor, but if a code gets called, there’s a team that comes in from the intensive care unit. I always kind of wondered what I would do if I was presented with a scenario like this.
Daniel Suders: We have a lot of friends that do ER medicine, and I felt like those were the guys that really understood when we told them the story. One friend said to me, “By the time they get to us, they’re either in bad shape or they’re better already.” A lot depends on what happens in the field.
Jennifer Suders: I’m even more vigilant about pool safety now. I want to make sure parents know that drowning doesn›t look like flailing theatrics. It can be soundless. Three adults were right next to this little boy and didn›t realize until they looked down and saw him.
If we hadn’t been there, I don’t know if anyone would’ve been able to step in. No one else was medically trained. But I think the message is — you don’t have to be. Anyone can take a CPR class.
When I told my parents, my dad said, “Oh my gosh, I would’ve laid right down there next to that kid and passed out.” Without any training, it’s petrifying to see something like that.
I think about how we could have stayed in the gym longer and been too late. Or we could have gotten on the elevator earlier and been gone. Two minutes, and it would’ve been a story we heard later, not one we were a part of. It feels like we were at a true crossroads in that moment where that boy could have lived or died. And the stars aligned perfectly.
We had no medicine, no monitors, nothing but our hands and our breaths. And we helped a family continue their vacation rather than plan a funeral.
Jennifer Suders, DO, is a nephrologist at West Virginia University Medicine Wheeling Clinic. Daniel Suders, DO, is a hospitalist at West Virginia University Medicine Reynolds Memorial Hospital.
A version of this article appeared on Medscape.com .
Nocturnal Hot Flashes and Alzheimer’s Risk
In a recent article in the American Journal of Obstetrics & Gynecology, Rebecca C. Thurston, PhD, and Pauline Maki, PhD, leading scientists in the area of menopause’s impact on brain function, presented data from their assessment of 248 late perimenopausal and postmenopausal women who reported hot flashes, also known as vasomotor symptoms (VMS).
Hot flashes are known to be associated with changes in brain white matter, carotid atherosclerosis, brain function, and memory. Dr. Thurston and colleagues objectively measured VMS over 24 hours, using skin conductance monitoring. Plasma concentrations of Alzheimer’s disease biomarkers, including the amyloid beta 42–to–amyloid beta 40 ratio, were assessed. The mean age of study participants was 59 years, and they experienced a mean of five objective VMS daily.
A key finding was that VMS, particularly those occurring during sleep, were associated with a significantly lower amyloid beta 42–to–beta 40 ratio. This finding suggests that nighttime VMS may be a marker of risk for Alzheimer’s disease.
Previous research has found that menopausal hormone therapy is associated with favorable changes in Alzheimer’s disease biomarkers. Likewise, large observational studies have shown a lower incidence of Alzheimer’s disease among women who initiate hormone therapy in their late perimenopausal or early postmenopausal years and continue such therapy long term.
The findings of this important study by Thurston and colleagues provide further evidence to support the tantalizing possibility that agents that reduce nighttime hot flashes (including hormone therapy) may lower the subsequent incidence of Alzheimer’s disease in high-risk women.
Dr. Kaunitz is a tenured professor and associate chair in the department of obstetrics and gynecology at the University of Florida College of Medicine–Jacksonville, and medical director and director of menopause and gynecologic ultrasound services at the University of Florida Southside Women’s Health, Jacksonville. He disclosed ties to Sumitomo Pharma America, Mithra, Viatris, Bayer, Merck, Mylan (Viatris), and UpToDate.
A version of this article appeared on Medscape.com.
In a recent article in the American Journal of Obstetrics & Gynecology, Rebecca C. Thurston, PhD, and Pauline Maki, PhD, leading scientists in the area of menopause’s impact on brain function, presented data from their assessment of 248 late perimenopausal and postmenopausal women who reported hot flashes, also known as vasomotor symptoms (VMS).
Hot flashes are known to be associated with changes in brain white matter, carotid atherosclerosis, brain function, and memory. Dr. Thurston and colleagues objectively measured VMS over 24 hours, using skin conductance monitoring. Plasma concentrations of Alzheimer’s disease biomarkers, including the amyloid beta 42–to–amyloid beta 40 ratio, were assessed. The mean age of study participants was 59 years, and they experienced a mean of five objective VMS daily.
A key finding was that VMS, particularly those occurring during sleep, were associated with a significantly lower amyloid beta 42–to–beta 40 ratio. This finding suggests that nighttime VMS may be a marker of risk for Alzheimer’s disease.
Previous research has found that menopausal hormone therapy is associated with favorable changes in Alzheimer’s disease biomarkers. Likewise, large observational studies have shown a lower incidence of Alzheimer’s disease among women who initiate hormone therapy in their late perimenopausal or early postmenopausal years and continue such therapy long term.
The findings of this important study by Thurston and colleagues provide further evidence to support the tantalizing possibility that agents that reduce nighttime hot flashes (including hormone therapy) may lower the subsequent incidence of Alzheimer’s disease in high-risk women.
Dr. Kaunitz is a tenured professor and associate chair in the department of obstetrics and gynecology at the University of Florida College of Medicine–Jacksonville, and medical director and director of menopause and gynecologic ultrasound services at the University of Florida Southside Women’s Health, Jacksonville. He disclosed ties to Sumitomo Pharma America, Mithra, Viatris, Bayer, Merck, Mylan (Viatris), and UpToDate.
A version of this article appeared on Medscape.com.
In a recent article in the American Journal of Obstetrics & Gynecology, Rebecca C. Thurston, PhD, and Pauline Maki, PhD, leading scientists in the area of menopause’s impact on brain function, presented data from their assessment of 248 late perimenopausal and postmenopausal women who reported hot flashes, also known as vasomotor symptoms (VMS).
Hot flashes are known to be associated with changes in brain white matter, carotid atherosclerosis, brain function, and memory. Dr. Thurston and colleagues objectively measured VMS over 24 hours, using skin conductance monitoring. Plasma concentrations of Alzheimer’s disease biomarkers, including the amyloid beta 42–to–amyloid beta 40 ratio, were assessed. The mean age of study participants was 59 years, and they experienced a mean of five objective VMS daily.
A key finding was that VMS, particularly those occurring during sleep, were associated with a significantly lower amyloid beta 42–to–beta 40 ratio. This finding suggests that nighttime VMS may be a marker of risk for Alzheimer’s disease.
Previous research has found that menopausal hormone therapy is associated with favorable changes in Alzheimer’s disease biomarkers. Likewise, large observational studies have shown a lower incidence of Alzheimer’s disease among women who initiate hormone therapy in their late perimenopausal or early postmenopausal years and continue such therapy long term.
The findings of this important study by Thurston and colleagues provide further evidence to support the tantalizing possibility that agents that reduce nighttime hot flashes (including hormone therapy) may lower the subsequent incidence of Alzheimer’s disease in high-risk women.
Dr. Kaunitz is a tenured professor and associate chair in the department of obstetrics and gynecology at the University of Florida College of Medicine–Jacksonville, and medical director and director of menopause and gynecologic ultrasound services at the University of Florida Southside Women’s Health, Jacksonville. He disclosed ties to Sumitomo Pharma America, Mithra, Viatris, Bayer, Merck, Mylan (Viatris), and UpToDate.
A version of this article appeared on Medscape.com.
Molecular Classification of Endometrial Carcinomas
Historically, endometrial cancer has been classified as type I or type II. Type I endometrial cancers are typically estrogen driven, low grade, with endometrioid histology, and have a more favorable prognosis. In contrast, type II endometrial cancers are typically high grade, have more aggressive histologies (eg, serous or clear cell), and have a poorer prognosis.1
While this system provides a helpful schema for understanding endometrial cancers, it fails to represent the immense variation of biologic behavior and outcomes in endometrial cancers and oversimplifies what has come to be understood as a complex and molecularly diverse disease.
In 2013, The Cancer Genome Atlas (TCGA) performed genomic, transcriptomic, and proteomic characterization of 373 endometrial carcinomas. They identified four categories with distinct genetic profiles corresponding to clinical outcomes: 1) DNA polymerase epsilon (POLE) mutated; 2) mismatch repair deficient; 3) copy number high/p53 abnormal; and 4) copy number low/no specific molecular profile.2 By providing both predictive and prognostic information, these molecular features may be incorporated into treatment planning decisions in the future.
The POLE-mutated subtype are endometrial cancers with recurrent mutations in the POLE gene, which is involved in DNA replication and repair. POLE mutations occur in about 5%-10% of endometrial cancers. Despite some more aggressive histopathologic findings (eg, higher grade, deeper myometrial invasion, positive lymphovascular space invasion), recurrences rarely occur, and patients with POLE mutations have the best prognosis of the four molecular subtypes, with a 5-year recurrence-free survival of 92%-100%.3
The mismatch repair–deficient (MMRd) subtype are endometrial cancers with abnormalities in any of the mismatch repair proteins (MLH1, PMS2, MSH2, MSH6). These alterations may result from hereditary or somatic mutations in any of the MMR genes or epigenetic changes in the MLH1 promoter. Germ-line mutations are associated with Lynch syndrome; thus, patients found to have a germ-line mutation in any of the MMR genes necessitate a genetics referral. The MMRd subtype accounts for about 20%-30% of endometrial cancers, and patients with MMRd tumors have an intermediate prognosis, with a 5-year recurrence-free survival of about 70%.3. These tumors are more responsive to the use of immunotherapy checkpoint inhibitors. Two recent landmark trials showed improved outcomes in patients with advanced MMRd endometrial cancer treated with immune checkpoint inhibitors in addition to standard chemotherapy.4,5
The worst prognosis belongs to the copy number high subgroup, which accounts for approximately 10% of endometrial cancers. Five-year recurrence-free survival is ~50%.3 These tumors often contain TP53 mutations and are composed of aggressive histologies, such as serous, clear cell, high-grade endometrioid, and carcinosarcomas. Recent data suggests that human epidermal growth factor receptor 2 (HER2) amplification may also be prevalent in this subgroup.6
Endometrial cancers that lack any of the above alterations fall into the no specific molecular profile (NSMP) or copy number low subgroup. Mutations in other genes, such as PTEN, PIK3CA, CTNNB1, KRAS, and ARID1A, are often present in these tumors. As the most common subtype, this heterogeneous group accounts for about 50% of all endometrial cancers. These tumors frequently comprise endometrioid histology with estrogen and progesterone receptor positivity, high rates of response to hormonal therapy, and an overall intermediate to favorable prognosis, with a 5-year recurrence-free survival of ~75%.3
The use of whole-genome sequencing in TCGA limits the clinical applicability of testing because of the cost and complex methodologies involved. Multiple algorithms have been developed in the interim that approximate TCGA subtypes using relatively less expensive and more widely available testing methods, such as immunohistochemistry and next-generation sequencing. In the ProMisE algorithm, immunohistochemistry for p53 and MMR proteins is used as a surrogate for copy number high and MMRd tumors, respectively, and targeted sequencing is used to identify POLE mutations.7
Full molecular classification of endometrial tumors provides important prognostic information and allows for incorporation into treatment planning. To this end, the new 2023 International Federation of Gynecology and Obstetrics (FIGO) endometrial cancer staging incorporates an option for the addition of molecular subtype, with the stance that it allows for better prognostic prediction.8 While complete molecular classification is not required, it is encouraged. Furthermore, several clinical trials are currently investigating different treatment regimens based on these distinct molecular profiles.
Dr. Haag is a gynecologic oncology fellow in the Department of Obstetrics and Gynecology, University of North Carolina Hospitals, Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill. They have no conflicts of interest.
References
1. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecologic Oncology. 1983;15(1):10-17.
2. Kandoth C et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
3. León-Castillo A et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: Impact on prognosis and benefit from adjuvant therapy. J Clin Oncology. 2020;38(29):3388-3397.
4. Mirza MR et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med. 2023;388(23):2145-2158.
5. Eskander RN et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med. 2023;388(23):2159-2170.
6. Talia KL et al. The role of HER2 as a therapeutic biomarker in gynaecological malignancy: Potential for use beyond uterine serous carcinoma. Pathology. 2023;55(1):8-18.
7. Kommoss S et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Annals Oncology. 2018;29(5):1180-1188.
8. Berek JS et al. FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet. 2023;162(2):383-394.
Historically, endometrial cancer has been classified as type I or type II. Type I endometrial cancers are typically estrogen driven, low grade, with endometrioid histology, and have a more favorable prognosis. In contrast, type II endometrial cancers are typically high grade, have more aggressive histologies (eg, serous or clear cell), and have a poorer prognosis.1
While this system provides a helpful schema for understanding endometrial cancers, it fails to represent the immense variation of biologic behavior and outcomes in endometrial cancers and oversimplifies what has come to be understood as a complex and molecularly diverse disease.
In 2013, The Cancer Genome Atlas (TCGA) performed genomic, transcriptomic, and proteomic characterization of 373 endometrial carcinomas. They identified four categories with distinct genetic profiles corresponding to clinical outcomes: 1) DNA polymerase epsilon (POLE) mutated; 2) mismatch repair deficient; 3) copy number high/p53 abnormal; and 4) copy number low/no specific molecular profile.2 By providing both predictive and prognostic information, these molecular features may be incorporated into treatment planning decisions in the future.
The POLE-mutated subtype are endometrial cancers with recurrent mutations in the POLE gene, which is involved in DNA replication and repair. POLE mutations occur in about 5%-10% of endometrial cancers. Despite some more aggressive histopathologic findings (eg, higher grade, deeper myometrial invasion, positive lymphovascular space invasion), recurrences rarely occur, and patients with POLE mutations have the best prognosis of the four molecular subtypes, with a 5-year recurrence-free survival of 92%-100%.3
The mismatch repair–deficient (MMRd) subtype are endometrial cancers with abnormalities in any of the mismatch repair proteins (MLH1, PMS2, MSH2, MSH6). These alterations may result from hereditary or somatic mutations in any of the MMR genes or epigenetic changes in the MLH1 promoter. Germ-line mutations are associated with Lynch syndrome; thus, patients found to have a germ-line mutation in any of the MMR genes necessitate a genetics referral. The MMRd subtype accounts for about 20%-30% of endometrial cancers, and patients with MMRd tumors have an intermediate prognosis, with a 5-year recurrence-free survival of about 70%.3. These tumors are more responsive to the use of immunotherapy checkpoint inhibitors. Two recent landmark trials showed improved outcomes in patients with advanced MMRd endometrial cancer treated with immune checkpoint inhibitors in addition to standard chemotherapy.4,5
The worst prognosis belongs to the copy number high subgroup, which accounts for approximately 10% of endometrial cancers. Five-year recurrence-free survival is ~50%.3 These tumors often contain TP53 mutations and are composed of aggressive histologies, such as serous, clear cell, high-grade endometrioid, and carcinosarcomas. Recent data suggests that human epidermal growth factor receptor 2 (HER2) amplification may also be prevalent in this subgroup.6
Endometrial cancers that lack any of the above alterations fall into the no specific molecular profile (NSMP) or copy number low subgroup. Mutations in other genes, such as PTEN, PIK3CA, CTNNB1, KRAS, and ARID1A, are often present in these tumors. As the most common subtype, this heterogeneous group accounts for about 50% of all endometrial cancers. These tumors frequently comprise endometrioid histology with estrogen and progesterone receptor positivity, high rates of response to hormonal therapy, and an overall intermediate to favorable prognosis, with a 5-year recurrence-free survival of ~75%.3
The use of whole-genome sequencing in TCGA limits the clinical applicability of testing because of the cost and complex methodologies involved. Multiple algorithms have been developed in the interim that approximate TCGA subtypes using relatively less expensive and more widely available testing methods, such as immunohistochemistry and next-generation sequencing. In the ProMisE algorithm, immunohistochemistry for p53 and MMR proteins is used as a surrogate for copy number high and MMRd tumors, respectively, and targeted sequencing is used to identify POLE mutations.7
Full molecular classification of endometrial tumors provides important prognostic information and allows for incorporation into treatment planning. To this end, the new 2023 International Federation of Gynecology and Obstetrics (FIGO) endometrial cancer staging incorporates an option for the addition of molecular subtype, with the stance that it allows for better prognostic prediction.8 While complete molecular classification is not required, it is encouraged. Furthermore, several clinical trials are currently investigating different treatment regimens based on these distinct molecular profiles.
Dr. Haag is a gynecologic oncology fellow in the Department of Obstetrics and Gynecology, University of North Carolina Hospitals, Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill. They have no conflicts of interest.
References
1. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecologic Oncology. 1983;15(1):10-17.
2. Kandoth C et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
3. León-Castillo A et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: Impact on prognosis and benefit from adjuvant therapy. J Clin Oncology. 2020;38(29):3388-3397.
4. Mirza MR et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med. 2023;388(23):2145-2158.
5. Eskander RN et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med. 2023;388(23):2159-2170.
6. Talia KL et al. The role of HER2 as a therapeutic biomarker in gynaecological malignancy: Potential for use beyond uterine serous carcinoma. Pathology. 2023;55(1):8-18.
7. Kommoss S et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Annals Oncology. 2018;29(5):1180-1188.
8. Berek JS et al. FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet. 2023;162(2):383-394.
Historically, endometrial cancer has been classified as type I or type II. Type I endometrial cancers are typically estrogen driven, low grade, with endometrioid histology, and have a more favorable prognosis. In contrast, type II endometrial cancers are typically high grade, have more aggressive histologies (eg, serous or clear cell), and have a poorer prognosis.1
While this system provides a helpful schema for understanding endometrial cancers, it fails to represent the immense variation of biologic behavior and outcomes in endometrial cancers and oversimplifies what has come to be understood as a complex and molecularly diverse disease.
In 2013, The Cancer Genome Atlas (TCGA) performed genomic, transcriptomic, and proteomic characterization of 373 endometrial carcinomas. They identified four categories with distinct genetic profiles corresponding to clinical outcomes: 1) DNA polymerase epsilon (POLE) mutated; 2) mismatch repair deficient; 3) copy number high/p53 abnormal; and 4) copy number low/no specific molecular profile.2 By providing both predictive and prognostic information, these molecular features may be incorporated into treatment planning decisions in the future.
The POLE-mutated subtype are endometrial cancers with recurrent mutations in the POLE gene, which is involved in DNA replication and repair. POLE mutations occur in about 5%-10% of endometrial cancers. Despite some more aggressive histopathologic findings (eg, higher grade, deeper myometrial invasion, positive lymphovascular space invasion), recurrences rarely occur, and patients with POLE mutations have the best prognosis of the four molecular subtypes, with a 5-year recurrence-free survival of 92%-100%.3
The mismatch repair–deficient (MMRd) subtype are endometrial cancers with abnormalities in any of the mismatch repair proteins (MLH1, PMS2, MSH2, MSH6). These alterations may result from hereditary or somatic mutations in any of the MMR genes or epigenetic changes in the MLH1 promoter. Germ-line mutations are associated with Lynch syndrome; thus, patients found to have a germ-line mutation in any of the MMR genes necessitate a genetics referral. The MMRd subtype accounts for about 20%-30% of endometrial cancers, and patients with MMRd tumors have an intermediate prognosis, with a 5-year recurrence-free survival of about 70%.3. These tumors are more responsive to the use of immunotherapy checkpoint inhibitors. Two recent landmark trials showed improved outcomes in patients with advanced MMRd endometrial cancer treated with immune checkpoint inhibitors in addition to standard chemotherapy.4,5
The worst prognosis belongs to the copy number high subgroup, which accounts for approximately 10% of endometrial cancers. Five-year recurrence-free survival is ~50%.3 These tumors often contain TP53 mutations and are composed of aggressive histologies, such as serous, clear cell, high-grade endometrioid, and carcinosarcomas. Recent data suggests that human epidermal growth factor receptor 2 (HER2) amplification may also be prevalent in this subgroup.6
Endometrial cancers that lack any of the above alterations fall into the no specific molecular profile (NSMP) or copy number low subgroup. Mutations in other genes, such as PTEN, PIK3CA, CTNNB1, KRAS, and ARID1A, are often present in these tumors. As the most common subtype, this heterogeneous group accounts for about 50% of all endometrial cancers. These tumors frequently comprise endometrioid histology with estrogen and progesterone receptor positivity, high rates of response to hormonal therapy, and an overall intermediate to favorable prognosis, with a 5-year recurrence-free survival of ~75%.3
The use of whole-genome sequencing in TCGA limits the clinical applicability of testing because of the cost and complex methodologies involved. Multiple algorithms have been developed in the interim that approximate TCGA subtypes using relatively less expensive and more widely available testing methods, such as immunohistochemistry and next-generation sequencing. In the ProMisE algorithm, immunohistochemistry for p53 and MMR proteins is used as a surrogate for copy number high and MMRd tumors, respectively, and targeted sequencing is used to identify POLE mutations.7
Full molecular classification of endometrial tumors provides important prognostic information and allows for incorporation into treatment planning. To this end, the new 2023 International Federation of Gynecology and Obstetrics (FIGO) endometrial cancer staging incorporates an option for the addition of molecular subtype, with the stance that it allows for better prognostic prediction.8 While complete molecular classification is not required, it is encouraged. Furthermore, several clinical trials are currently investigating different treatment regimens based on these distinct molecular profiles.
Dr. Haag is a gynecologic oncology fellow in the Department of Obstetrics and Gynecology, University of North Carolina Hospitals, Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill. They have no conflicts of interest.
References
1. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecologic Oncology. 1983;15(1):10-17.
2. Kandoth C et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
3. León-Castillo A et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: Impact on prognosis and benefit from adjuvant therapy. J Clin Oncology. 2020;38(29):3388-3397.
4. Mirza MR et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med. 2023;388(23):2145-2158.
5. Eskander RN et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med. 2023;388(23):2159-2170.
6. Talia KL et al. The role of HER2 as a therapeutic biomarker in gynaecological malignancy: Potential for use beyond uterine serous carcinoma. Pathology. 2023;55(1):8-18.
7. Kommoss S et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Annals Oncology. 2018;29(5):1180-1188.
8. Berek JS et al. FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet. 2023;162(2):383-394.
Celebrating Excellence
As we settle back into our daily routines following another fantastic DDW, I’d like to take a moment to congratulate this year’s AGA’s Recognition Award recipients, who have made outstanding contributions to the organization and to our field, including through excellence in clinical practice, research, mentorship, and DEI.
This month’s Member Spotlight column highlights one of these remarkable individuals, Dr. Scott Ketover, president and CEO of MNGI Digestive Health, who is the recipient of this year’s AGA Distinguished Clinician Award in Private Practice. We hope you enjoy learning more about Scott, as well as the other award recipients who were recognized at a special ceremony in DC last month.
Also highlighted in our June issue is the FDA’s recent approval of subcutaneous vedolizumab for Crohn’s maintenance therapy, an exciting development that will provide us with more flexible treatment options for our patients. We also report on the 2024 AGA Tech Summit (Chicago, IL), and introduce the winners (survivors?) of its annual Shark Tank competition, Dr. Renu Dhanasekaran and Dr. Venthan Elango. Their company, Arithmedics, which developed technology that harnesses generative AI and data intelligence to streamline medical billing, was identified as the most promising among a robust field of entrants.
We also present some of the best clinically oriented content from our GI journals, including an observational study from Gastroenterology evaluating the effect of longitudinal alcohol use on risk of cirrhosis among patients with steatotic liver disease, and summarize recently released AGA Clinical Practice Updates on performance of high-quality upper endoscopy and treatment of cannabinoid hyperemesis syndrome. We hope you enjoy all the exciting content featured in this issue and take some well-deserved time to rest and recharge this summer!
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
As we settle back into our daily routines following another fantastic DDW, I’d like to take a moment to congratulate this year’s AGA’s Recognition Award recipients, who have made outstanding contributions to the organization and to our field, including through excellence in clinical practice, research, mentorship, and DEI.
This month’s Member Spotlight column highlights one of these remarkable individuals, Dr. Scott Ketover, president and CEO of MNGI Digestive Health, who is the recipient of this year’s AGA Distinguished Clinician Award in Private Practice. We hope you enjoy learning more about Scott, as well as the other award recipients who were recognized at a special ceremony in DC last month.
Also highlighted in our June issue is the FDA’s recent approval of subcutaneous vedolizumab for Crohn’s maintenance therapy, an exciting development that will provide us with more flexible treatment options for our patients. We also report on the 2024 AGA Tech Summit (Chicago, IL), and introduce the winners (survivors?) of its annual Shark Tank competition, Dr. Renu Dhanasekaran and Dr. Venthan Elango. Their company, Arithmedics, which developed technology that harnesses generative AI and data intelligence to streamline medical billing, was identified as the most promising among a robust field of entrants.
We also present some of the best clinically oriented content from our GI journals, including an observational study from Gastroenterology evaluating the effect of longitudinal alcohol use on risk of cirrhosis among patients with steatotic liver disease, and summarize recently released AGA Clinical Practice Updates on performance of high-quality upper endoscopy and treatment of cannabinoid hyperemesis syndrome. We hope you enjoy all the exciting content featured in this issue and take some well-deserved time to rest and recharge this summer!
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
As we settle back into our daily routines following another fantastic DDW, I’d like to take a moment to congratulate this year’s AGA’s Recognition Award recipients, who have made outstanding contributions to the organization and to our field, including through excellence in clinical practice, research, mentorship, and DEI.
This month’s Member Spotlight column highlights one of these remarkable individuals, Dr. Scott Ketover, president and CEO of MNGI Digestive Health, who is the recipient of this year’s AGA Distinguished Clinician Award in Private Practice. We hope you enjoy learning more about Scott, as well as the other award recipients who were recognized at a special ceremony in DC last month.
Also highlighted in our June issue is the FDA’s recent approval of subcutaneous vedolizumab for Crohn’s maintenance therapy, an exciting development that will provide us with more flexible treatment options for our patients. We also report on the 2024 AGA Tech Summit (Chicago, IL), and introduce the winners (survivors?) of its annual Shark Tank competition, Dr. Renu Dhanasekaran and Dr. Venthan Elango. Their company, Arithmedics, which developed technology that harnesses generative AI and data intelligence to streamline medical billing, was identified as the most promising among a robust field of entrants.
We also present some of the best clinically oriented content from our GI journals, including an observational study from Gastroenterology evaluating the effect of longitudinal alcohol use on risk of cirrhosis among patients with steatotic liver disease, and summarize recently released AGA Clinical Practice Updates on performance of high-quality upper endoscopy and treatment of cannabinoid hyperemesis syndrome. We hope you enjoy all the exciting content featured in this issue and take some well-deserved time to rest and recharge this summer!
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Is Red Meat Healthy? Multiverse Analysis Has Lessons Beyond Meat
Observational studies on red meat consumption and lifespan are prime examples of attempts to find signal in a sea of noise.
Randomized controlled trials are the best way to sort cause from mere correlation. But these are not possible in most matters of food consumption. So, we look back and observe groups with different exposures.
My most frequent complaint about these nonrandom comparison studies has been the chance that the two groups differ in important ways, and it’s these differences — not the food in question — that account for the disparate outcomes.
But selection biases are only one issue. There is also the matter of analytic flexibility. Observational studies are born from large databases. Researchers have many choices in how to analyze all these data.
A few years ago, Brian Nosek, PhD, and colleagues elegantly showed that analytic choices can affect results. His Many Analysts, One Data Set study had little uptake in the medical community, perhaps because he studied a social science question.
Multiple Ways to Slice the Data
Recently, a group from McMaster University, led by Dena Zeraatkar, PhD, has confirmed the analytic choices problem, using the question of red meat consumption and mortality.
Their idea was simple: Because there are many plausible and defensible ways to analyze a dataset, we should not choose one method; rather, we should choose thousands, combine the results, and see where the truth lies.
You might wonder how there could be thousands of ways to analyze a dataset. I surely did.
The answer stems from the choices that researchers face. For instance, there is the selection of eligible participants, the choice of analytic model (logistic, Poisson, etc.), and covariates for which to adjust. Think exponents when combining possible choices.
Dr. Zeraatkar and colleagues are research methodologists, so, sadly, they are comfortable with the clunky name of this approach: specification curve analysis. Don’t be deterred. It means that they analyze the data in thousands of ways using computers. Each way is a specification. In the end, the specifications give rise to a curve of hazard ratios for red meat and mortality. Another name for this approach is multiverse analysis.
For their paper in the Journal of Clinical Epidemiology, aptly named “Grilling the Data,” they didn’t just conjure up the many analytic ways to study the red meat–mortality question. Instead, they used a published systematic review of 15 studies on unprocessed red meat and early mortality. The studies included in this review reported 70 unique ways to analyze the association.
Is Red Meat Good or Bad?
Their first finding was that this analysis yielded widely disparate effect estimates, from 0.63 (reduced risk for early death) to 2.31 (a higher risk). The median hazard ratio was 1.14 with an interquartile range (IQR) of 1.02-1.23. One might conclude from this that eating red meat is associated with a slightly higher risk for early mortality.
Their second step was to calculate how many ways (specifications) there were to analyze the data by totaling all possible combinations of choices in the 70 ways found in the systematic review.
They calculated a total of 10 quadrillion possible unique analyses. A quadrillion is 1 with 15 zeros. Computing power cannot handle that amount of analyses yet. So, they generated 20 random unique combinations of covariates, which narrowed the number of analyses to about 1400. About 200 of these were excluded due to implausibly wide confidence intervals.
Voilà. They now had about 1200 different ways to analyze a dataset; they chose an NHANES longitudinal cohort study from 2007-2014. They deemed each of the more than 1200 approaches plausible because they were derived from peer-reviewed papers written by experts in epidemiology.
Specification Curve Analyses Results
Each analysis (or specification) yielded a hazard ratio for red meat exposure and death.
- The median HR was 0.94 (IQR, 0.83-1.05) for the effect of red meat on all-cause mortality — ie, not significant.
- The range of hazard ratios was large. They went from 0.51 — a 49% reduced risk for early mortality — to 1.75: a 75% increase in early mortality.
- Among all analyses, 36% yielded hazard ratios above 1.0 and 64% less than 1.0.
- As for statistical significance, defined as P ≤.05, only 4% (or 48 specifications) met this threshold. Zeraatkar reminded me that this is what you’d expect if unprocessed red meat has no effect on longevity.
- Of the 48 analyses deemed statistically significant, 40 indicated that red meat consumption reduced early death and eight indicated that eating red meat led to higher mortality.
- Nearly half the analyses yielded unexciting point estimates, with hazard ratios between 0.90 and 1.10.
Paradigm Changing
As a user of evidence, I find this a potentially paradigm-changing study. Observational studies far outnumber randomized trials. For many medical questions, observational data are all we have.
Now think about every observational study published. The authors tell you — post hoc — which method they used to analyze the data. The key point is that it is one method.
Dr. Zeraatkar and colleagues have shown that there are thousands of plausible ways to analyze the data, and this can lead to very different findings. In the specific question of red meat and mortality, their many analyses yielded a null result.
Now imagine other cases where the researchers did many analyses of a dataset and chose to publish only the significant ones. Observational studies are rarely preregistered, so a reader cannot know how a result would vary depending on analytic choices. A specification curve analysis of a dataset provides a much broader picture. In the case of red meat, you see some significant results, but the vast majority hover around null.
What about the difficulty in analyzing a dataset 1000 different ways? Dr. Zeraatkar told me that it is harder than just choosing one method, but it’s not impossible.
The main barrier to adopting this multiverse approach to data, she noted, was not the extra work but the entrenched belief among researchers that there is a best way to analyze data.
I hope you read this paper and think about it every time you read an observational study that finds a positive or negative association between two things. Ask: What if the researchers were as careful as Dr. Zeraatkar and colleagues and did multiple different analyses? Would the finding hold up to a series of plausible analytic choices?
Nutritional epidemiology would benefit greatly from this approach. But so would any observational study of an exposure and outcome. I suspect that the number of “positive” associations would diminish. And that would not be a bad thing.
Dr. Mandrola, a clinical electrophysiologist at Baptist Medical Associates, Louisville, Kentucky, disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Observational studies on red meat consumption and lifespan are prime examples of attempts to find signal in a sea of noise.
Randomized controlled trials are the best way to sort cause from mere correlation. But these are not possible in most matters of food consumption. So, we look back and observe groups with different exposures.
My most frequent complaint about these nonrandom comparison studies has been the chance that the two groups differ in important ways, and it’s these differences — not the food in question — that account for the disparate outcomes.
But selection biases are only one issue. There is also the matter of analytic flexibility. Observational studies are born from large databases. Researchers have many choices in how to analyze all these data.
A few years ago, Brian Nosek, PhD, and colleagues elegantly showed that analytic choices can affect results. His Many Analysts, One Data Set study had little uptake in the medical community, perhaps because he studied a social science question.
Multiple Ways to Slice the Data
Recently, a group from McMaster University, led by Dena Zeraatkar, PhD, has confirmed the analytic choices problem, using the question of red meat consumption and mortality.
Their idea was simple: Because there are many plausible and defensible ways to analyze a dataset, we should not choose one method; rather, we should choose thousands, combine the results, and see where the truth lies.
You might wonder how there could be thousands of ways to analyze a dataset. I surely did.
The answer stems from the choices that researchers face. For instance, there is the selection of eligible participants, the choice of analytic model (logistic, Poisson, etc.), and covariates for which to adjust. Think exponents when combining possible choices.
Dr. Zeraatkar and colleagues are research methodologists, so, sadly, they are comfortable with the clunky name of this approach: specification curve analysis. Don’t be deterred. It means that they analyze the data in thousands of ways using computers. Each way is a specification. In the end, the specifications give rise to a curve of hazard ratios for red meat and mortality. Another name for this approach is multiverse analysis.
For their paper in the Journal of Clinical Epidemiology, aptly named “Grilling the Data,” they didn’t just conjure up the many analytic ways to study the red meat–mortality question. Instead, they used a published systematic review of 15 studies on unprocessed red meat and early mortality. The studies included in this review reported 70 unique ways to analyze the association.
Is Red Meat Good or Bad?
Their first finding was that this analysis yielded widely disparate effect estimates, from 0.63 (reduced risk for early death) to 2.31 (a higher risk). The median hazard ratio was 1.14 with an interquartile range (IQR) of 1.02-1.23. One might conclude from this that eating red meat is associated with a slightly higher risk for early mortality.
Their second step was to calculate how many ways (specifications) there were to analyze the data by totaling all possible combinations of choices in the 70 ways found in the systematic review.
They calculated a total of 10 quadrillion possible unique analyses. A quadrillion is 1 with 15 zeros. Computing power cannot handle that amount of analyses yet. So, they generated 20 random unique combinations of covariates, which narrowed the number of analyses to about 1400. About 200 of these were excluded due to implausibly wide confidence intervals.
Voilà. They now had about 1200 different ways to analyze a dataset; they chose an NHANES longitudinal cohort study from 2007-2014. They deemed each of the more than 1200 approaches plausible because they were derived from peer-reviewed papers written by experts in epidemiology.
Specification Curve Analyses Results
Each analysis (or specification) yielded a hazard ratio for red meat exposure and death.
- The median HR was 0.94 (IQR, 0.83-1.05) for the effect of red meat on all-cause mortality — ie, not significant.
- The range of hazard ratios was large. They went from 0.51 — a 49% reduced risk for early mortality — to 1.75: a 75% increase in early mortality.
- Among all analyses, 36% yielded hazard ratios above 1.0 and 64% less than 1.0.
- As for statistical significance, defined as P ≤.05, only 4% (or 48 specifications) met this threshold. Zeraatkar reminded me that this is what you’d expect if unprocessed red meat has no effect on longevity.
- Of the 48 analyses deemed statistically significant, 40 indicated that red meat consumption reduced early death and eight indicated that eating red meat led to higher mortality.
- Nearly half the analyses yielded unexciting point estimates, with hazard ratios between 0.90 and 1.10.
Paradigm Changing
As a user of evidence, I find this a potentially paradigm-changing study. Observational studies far outnumber randomized trials. For many medical questions, observational data are all we have.
Now think about every observational study published. The authors tell you — post hoc — which method they used to analyze the data. The key point is that it is one method.
Dr. Zeraatkar and colleagues have shown that there are thousands of plausible ways to analyze the data, and this can lead to very different findings. In the specific question of red meat and mortality, their many analyses yielded a null result.
Now imagine other cases where the researchers did many analyses of a dataset and chose to publish only the significant ones. Observational studies are rarely preregistered, so a reader cannot know how a result would vary depending on analytic choices. A specification curve analysis of a dataset provides a much broader picture. In the case of red meat, you see some significant results, but the vast majority hover around null.
What about the difficulty in analyzing a dataset 1000 different ways? Dr. Zeraatkar told me that it is harder than just choosing one method, but it’s not impossible.
The main barrier to adopting this multiverse approach to data, she noted, was not the extra work but the entrenched belief among researchers that there is a best way to analyze data.
I hope you read this paper and think about it every time you read an observational study that finds a positive or negative association between two things. Ask: What if the researchers were as careful as Dr. Zeraatkar and colleagues and did multiple different analyses? Would the finding hold up to a series of plausible analytic choices?
Nutritional epidemiology would benefit greatly from this approach. But so would any observational study of an exposure and outcome. I suspect that the number of “positive” associations would diminish. And that would not be a bad thing.
Dr. Mandrola, a clinical electrophysiologist at Baptist Medical Associates, Louisville, Kentucky, disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Observational studies on red meat consumption and lifespan are prime examples of attempts to find signal in a sea of noise.
Randomized controlled trials are the best way to sort cause from mere correlation. But these are not possible in most matters of food consumption. So, we look back and observe groups with different exposures.
My most frequent complaint about these nonrandom comparison studies has been the chance that the two groups differ in important ways, and it’s these differences — not the food in question — that account for the disparate outcomes.
But selection biases are only one issue. There is also the matter of analytic flexibility. Observational studies are born from large databases. Researchers have many choices in how to analyze all these data.
A few years ago, Brian Nosek, PhD, and colleagues elegantly showed that analytic choices can affect results. His Many Analysts, One Data Set study had little uptake in the medical community, perhaps because he studied a social science question.
Multiple Ways to Slice the Data
Recently, a group from McMaster University, led by Dena Zeraatkar, PhD, has confirmed the analytic choices problem, using the question of red meat consumption and mortality.
Their idea was simple: Because there are many plausible and defensible ways to analyze a dataset, we should not choose one method; rather, we should choose thousands, combine the results, and see where the truth lies.
You might wonder how there could be thousands of ways to analyze a dataset. I surely did.
The answer stems from the choices that researchers face. For instance, there is the selection of eligible participants, the choice of analytic model (logistic, Poisson, etc.), and covariates for which to adjust. Think exponents when combining possible choices.
Dr. Zeraatkar and colleagues are research methodologists, so, sadly, they are comfortable with the clunky name of this approach: specification curve analysis. Don’t be deterred. It means that they analyze the data in thousands of ways using computers. Each way is a specification. In the end, the specifications give rise to a curve of hazard ratios for red meat and mortality. Another name for this approach is multiverse analysis.
For their paper in the Journal of Clinical Epidemiology, aptly named “Grilling the Data,” they didn’t just conjure up the many analytic ways to study the red meat–mortality question. Instead, they used a published systematic review of 15 studies on unprocessed red meat and early mortality. The studies included in this review reported 70 unique ways to analyze the association.
Is Red Meat Good or Bad?
Their first finding was that this analysis yielded widely disparate effect estimates, from 0.63 (reduced risk for early death) to 2.31 (a higher risk). The median hazard ratio was 1.14 with an interquartile range (IQR) of 1.02-1.23. One might conclude from this that eating red meat is associated with a slightly higher risk for early mortality.
Their second step was to calculate how many ways (specifications) there were to analyze the data by totaling all possible combinations of choices in the 70 ways found in the systematic review.
They calculated a total of 10 quadrillion possible unique analyses. A quadrillion is 1 with 15 zeros. Computing power cannot handle that amount of analyses yet. So, they generated 20 random unique combinations of covariates, which narrowed the number of analyses to about 1400. About 200 of these were excluded due to implausibly wide confidence intervals.
Voilà. They now had about 1200 different ways to analyze a dataset; they chose an NHANES longitudinal cohort study from 2007-2014. They deemed each of the more than 1200 approaches plausible because they were derived from peer-reviewed papers written by experts in epidemiology.
Specification Curve Analyses Results
Each analysis (or specification) yielded a hazard ratio for red meat exposure and death.
- The median HR was 0.94 (IQR, 0.83-1.05) for the effect of red meat on all-cause mortality — ie, not significant.
- The range of hazard ratios was large. They went from 0.51 — a 49% reduced risk for early mortality — to 1.75: a 75% increase in early mortality.
- Among all analyses, 36% yielded hazard ratios above 1.0 and 64% less than 1.0.
- As for statistical significance, defined as P ≤.05, only 4% (or 48 specifications) met this threshold. Zeraatkar reminded me that this is what you’d expect if unprocessed red meat has no effect on longevity.
- Of the 48 analyses deemed statistically significant, 40 indicated that red meat consumption reduced early death and eight indicated that eating red meat led to higher mortality.
- Nearly half the analyses yielded unexciting point estimates, with hazard ratios between 0.90 and 1.10.
Paradigm Changing
As a user of evidence, I find this a potentially paradigm-changing study. Observational studies far outnumber randomized trials. For many medical questions, observational data are all we have.
Now think about every observational study published. The authors tell you — post hoc — which method they used to analyze the data. The key point is that it is one method.
Dr. Zeraatkar and colleagues have shown that there are thousands of plausible ways to analyze the data, and this can lead to very different findings. In the specific question of red meat and mortality, their many analyses yielded a null result.
Now imagine other cases where the researchers did many analyses of a dataset and chose to publish only the significant ones. Observational studies are rarely preregistered, so a reader cannot know how a result would vary depending on analytic choices. A specification curve analysis of a dataset provides a much broader picture. In the case of red meat, you see some significant results, but the vast majority hover around null.
What about the difficulty in analyzing a dataset 1000 different ways? Dr. Zeraatkar told me that it is harder than just choosing one method, but it’s not impossible.
The main barrier to adopting this multiverse approach to data, she noted, was not the extra work but the entrenched belief among researchers that there is a best way to analyze data.
I hope you read this paper and think about it every time you read an observational study that finds a positive or negative association between two things. Ask: What if the researchers were as careful as Dr. Zeraatkar and colleagues and did multiple different analyses? Would the finding hold up to a series of plausible analytic choices?
Nutritional epidemiology would benefit greatly from this approach. But so would any observational study of an exposure and outcome. I suspect that the number of “positive” associations would diminish. And that would not be a bad thing.
Dr. Mandrola, a clinical electrophysiologist at Baptist Medical Associates, Louisville, Kentucky, disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Myth of the Month: Is Contrast-Induced Acute Kidney Injury Real?
A 59-year-old man presents with abdominal pain. He has a history of small bowel obstruction and diverticulitis. His medical history includes chronic kidney disease (CKD; baseline creatinine, 1.8 mg/dL), hypertension, type 2 diabetes, and depression. He had a colectomy 6 years ago for colon cancer.
He takes the following medications: Semaglutide (1 mg weekly), amlodipine (5 mg once daily), and escitalopram (10 mg once daily). On physical exam his blood pressure is 130/80 mm Hg, his pulse is 90, and his temperature is 37.2 degrees C. He has normal bowel sounds but guarding in the right lower quadrant.
His hemoglobin is 14 g/dL, his blood sodium is 136 mEq/L, his blood potassium is 4.0 mmol/L, his BUN is 26 mg/dL, and his creatinine is 1.9 mg/dL. His kidney, ureter, bladder x-ray is unremarkable.
What imaging would you recommend?
A) CT without contrast
B) CT with contrast
C) MRI
D) Abdominal ultrasound
This patient has several potential causes for his abdominal pain that imaging may clarify. I think a contrast CT scan would be the most likely to provide helpful information. It is likely that if it were ordered, there may be hesitation by the radiologist to perform the scan with contrast because of the patient’s CKD.
Concern for contrast-induced kidney injury has limited diagnostic testing for many years. How strong is the evidence for contrast-induced kidney injury, and should we avoid testing that requires contrast in patients with CKD? McDonald and colleagues performed a meta-analysis with 13 studies meeting inclusion criteria, involving 25,950 patients.1 They found no increased risk of acute kidney injury (AKI) in patients who received contrast medium compared with those who did not receive contrast; relative risk of AKI for those receiving contrast was 0.79 (confidence interval: 0.62-1.02). Importantly, there was no difference in AKI in patients with diabetes or CKD.
Ehmann et al. looked at renal outcomes in patients who received IV contrast when they presented to an emergency department with AKI.2 They found that in patients with AKI, receiving contrast was not associated with persistent AKI at hospital discharge. Hinson and colleagues looked at patients arriving at the emergency department and needing imaging.3 They did a retrospective, cohort analysis of 17,934 patients who had CT with contrast, CT with no contrast, or no CT. Contrast administration was not associated with increased incidence of AKI (odds ratio, 0.96, CI: 0.85-1.08).
Aycock et al. did a meta-analysis of AKI after CT scanning, including 28 studies involving 107,335 patients.4 They found that compared with noncontrast CT, CT scanning with contrast was not associated with AKI (OR, 0.94, CI: 0.83-1.07). Elias and Aronson looked at the risk of AKI after contrast in patients receiving CT scans compared with those who received ventilation/perfusion scans to evaluate for pulmonary embolism.5 There were 44 AKI events (4.5%) in patients exposed to contrast media and 33 events (3.4%) in patients not exposed to contrast media (risk difference: 1.1%, 95% CI: -0.6% to 2.9%; OR, 1.39, CI: 0.86-2.26; P = .18).
Despite multiple studies showing no increased risk, there is still a concern that contrast can cause AKI.6 Animal models have shown iodinated contrast can have a deleterious effect on mitochondria and membrane function.6 Criticisms of the retrospective nature of many of the studies I have shared, and the lack of randomized, controlled trials are that there may be bias in these studies, as the highest-risk patients are the ones most likely not to receive contrast. In a joint guideline from the American College of Radiology and the National Kidney Foundation, this statement was made: “The risk of acute kidney injury developing in patients with reduced kidney function following exposure to intravenous iodinated contrast media has been overstated.”7 Their recommendation was to give contrast if needed in patients with glomerular filtration rates (GFRs) greater than 30.
Myth: Contrast-induced renal injury is a concern.
Clinical impact: For CT scanning, it is OK to give contrast when needed. A conservative cutoff for contrast use would be a GFR less than 30.
Dr. Paauw is professor of medicine in the Division of General Internal Medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. McDonald JS et al. Radiology. 2013:267:119-128.
2. Ehmann MR et al. Intensive Care Med. 2023:49(2):205-215.
3. Hinson JS et al. Ann Emerg Med. 2017;69(5):577-586.
4. Aycock RD et al. Ann Emerg Med. 2018 Jan;71(1):44-53.
5. Elias A, Aronson D. Thromb Haemost. 2021 Jun;121(6):800-807.
6. Weisbord SD, du Cheryon D. Intensive Care Med. 2018;44(1):107-109.
7. Davenport MS et al. Radiology. 2020;294(3):660-668.
A 59-year-old man presents with abdominal pain. He has a history of small bowel obstruction and diverticulitis. His medical history includes chronic kidney disease (CKD; baseline creatinine, 1.8 mg/dL), hypertension, type 2 diabetes, and depression. He had a colectomy 6 years ago for colon cancer.
He takes the following medications: Semaglutide (1 mg weekly), amlodipine (5 mg once daily), and escitalopram (10 mg once daily). On physical exam his blood pressure is 130/80 mm Hg, his pulse is 90, and his temperature is 37.2 degrees C. He has normal bowel sounds but guarding in the right lower quadrant.
His hemoglobin is 14 g/dL, his blood sodium is 136 mEq/L, his blood potassium is 4.0 mmol/L, his BUN is 26 mg/dL, and his creatinine is 1.9 mg/dL. His kidney, ureter, bladder x-ray is unremarkable.
What imaging would you recommend?
A) CT without contrast
B) CT with contrast
C) MRI
D) Abdominal ultrasound
This patient has several potential causes for his abdominal pain that imaging may clarify. I think a contrast CT scan would be the most likely to provide helpful information. It is likely that if it were ordered, there may be hesitation by the radiologist to perform the scan with contrast because of the patient’s CKD.
Concern for contrast-induced kidney injury has limited diagnostic testing for many years. How strong is the evidence for contrast-induced kidney injury, and should we avoid testing that requires contrast in patients with CKD? McDonald and colleagues performed a meta-analysis with 13 studies meeting inclusion criteria, involving 25,950 patients.1 They found no increased risk of acute kidney injury (AKI) in patients who received contrast medium compared with those who did not receive contrast; relative risk of AKI for those receiving contrast was 0.79 (confidence interval: 0.62-1.02). Importantly, there was no difference in AKI in patients with diabetes or CKD.
Ehmann et al. looked at renal outcomes in patients who received IV contrast when they presented to an emergency department with AKI.2 They found that in patients with AKI, receiving contrast was not associated with persistent AKI at hospital discharge. Hinson and colleagues looked at patients arriving at the emergency department and needing imaging.3 They did a retrospective, cohort analysis of 17,934 patients who had CT with contrast, CT with no contrast, or no CT. Contrast administration was not associated with increased incidence of AKI (odds ratio, 0.96, CI: 0.85-1.08).
Aycock et al. did a meta-analysis of AKI after CT scanning, including 28 studies involving 107,335 patients.4 They found that compared with noncontrast CT, CT scanning with contrast was not associated with AKI (OR, 0.94, CI: 0.83-1.07). Elias and Aronson looked at the risk of AKI after contrast in patients receiving CT scans compared with those who received ventilation/perfusion scans to evaluate for pulmonary embolism.5 There were 44 AKI events (4.5%) in patients exposed to contrast media and 33 events (3.4%) in patients not exposed to contrast media (risk difference: 1.1%, 95% CI: -0.6% to 2.9%; OR, 1.39, CI: 0.86-2.26; P = .18).
Despite multiple studies showing no increased risk, there is still a concern that contrast can cause AKI.6 Animal models have shown iodinated contrast can have a deleterious effect on mitochondria and membrane function.6 Criticisms of the retrospective nature of many of the studies I have shared, and the lack of randomized, controlled trials are that there may be bias in these studies, as the highest-risk patients are the ones most likely not to receive contrast. In a joint guideline from the American College of Radiology and the National Kidney Foundation, this statement was made: “The risk of acute kidney injury developing in patients with reduced kidney function following exposure to intravenous iodinated contrast media has been overstated.”7 Their recommendation was to give contrast if needed in patients with glomerular filtration rates (GFRs) greater than 30.
Myth: Contrast-induced renal injury is a concern.
Clinical impact: For CT scanning, it is OK to give contrast when needed. A conservative cutoff for contrast use would be a GFR less than 30.
Dr. Paauw is professor of medicine in the Division of General Internal Medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. McDonald JS et al. Radiology. 2013:267:119-128.
2. Ehmann MR et al. Intensive Care Med. 2023:49(2):205-215.
3. Hinson JS et al. Ann Emerg Med. 2017;69(5):577-586.
4. Aycock RD et al. Ann Emerg Med. 2018 Jan;71(1):44-53.
5. Elias A, Aronson D. Thromb Haemost. 2021 Jun;121(6):800-807.
6. Weisbord SD, du Cheryon D. Intensive Care Med. 2018;44(1):107-109.
7. Davenport MS et al. Radiology. 2020;294(3):660-668.
A 59-year-old man presents with abdominal pain. He has a history of small bowel obstruction and diverticulitis. His medical history includes chronic kidney disease (CKD; baseline creatinine, 1.8 mg/dL), hypertension, type 2 diabetes, and depression. He had a colectomy 6 years ago for colon cancer.
He takes the following medications: Semaglutide (1 mg weekly), amlodipine (5 mg once daily), and escitalopram (10 mg once daily). On physical exam his blood pressure is 130/80 mm Hg, his pulse is 90, and his temperature is 37.2 degrees C. He has normal bowel sounds but guarding in the right lower quadrant.
His hemoglobin is 14 g/dL, his blood sodium is 136 mEq/L, his blood potassium is 4.0 mmol/L, his BUN is 26 mg/dL, and his creatinine is 1.9 mg/dL. His kidney, ureter, bladder x-ray is unremarkable.
What imaging would you recommend?
A) CT without contrast
B) CT with contrast
C) MRI
D) Abdominal ultrasound
This patient has several potential causes for his abdominal pain that imaging may clarify. I think a contrast CT scan would be the most likely to provide helpful information. It is likely that if it were ordered, there may be hesitation by the radiologist to perform the scan with contrast because of the patient’s CKD.
Concern for contrast-induced kidney injury has limited diagnostic testing for many years. How strong is the evidence for contrast-induced kidney injury, and should we avoid testing that requires contrast in patients with CKD? McDonald and colleagues performed a meta-analysis with 13 studies meeting inclusion criteria, involving 25,950 patients.1 They found no increased risk of acute kidney injury (AKI) in patients who received contrast medium compared with those who did not receive contrast; relative risk of AKI for those receiving contrast was 0.79 (confidence interval: 0.62-1.02). Importantly, there was no difference in AKI in patients with diabetes or CKD.
Ehmann et al. looked at renal outcomes in patients who received IV contrast when they presented to an emergency department with AKI.2 They found that in patients with AKI, receiving contrast was not associated with persistent AKI at hospital discharge. Hinson and colleagues looked at patients arriving at the emergency department and needing imaging.3 They did a retrospective, cohort analysis of 17,934 patients who had CT with contrast, CT with no contrast, or no CT. Contrast administration was not associated with increased incidence of AKI (odds ratio, 0.96, CI: 0.85-1.08).
Aycock et al. did a meta-analysis of AKI after CT scanning, including 28 studies involving 107,335 patients.4 They found that compared with noncontrast CT, CT scanning with contrast was not associated with AKI (OR, 0.94, CI: 0.83-1.07). Elias and Aronson looked at the risk of AKI after contrast in patients receiving CT scans compared with those who received ventilation/perfusion scans to evaluate for pulmonary embolism.5 There were 44 AKI events (4.5%) in patients exposed to contrast media and 33 events (3.4%) in patients not exposed to contrast media (risk difference: 1.1%, 95% CI: -0.6% to 2.9%; OR, 1.39, CI: 0.86-2.26; P = .18).
Despite multiple studies showing no increased risk, there is still a concern that contrast can cause AKI.6 Animal models have shown iodinated contrast can have a deleterious effect on mitochondria and membrane function.6 Criticisms of the retrospective nature of many of the studies I have shared, and the lack of randomized, controlled trials are that there may be bias in these studies, as the highest-risk patients are the ones most likely not to receive contrast. In a joint guideline from the American College of Radiology and the National Kidney Foundation, this statement was made: “The risk of acute kidney injury developing in patients with reduced kidney function following exposure to intravenous iodinated contrast media has been overstated.”7 Their recommendation was to give contrast if needed in patients with glomerular filtration rates (GFRs) greater than 30.
Myth: Contrast-induced renal injury is a concern.
Clinical impact: For CT scanning, it is OK to give contrast when needed. A conservative cutoff for contrast use would be a GFR less than 30.
Dr. Paauw is professor of medicine in the Division of General Internal Medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. McDonald JS et al. Radiology. 2013:267:119-128.
2. Ehmann MR et al. Intensive Care Med. 2023:49(2):205-215.
3. Hinson JS et al. Ann Emerg Med. 2017;69(5):577-586.
4. Aycock RD et al. Ann Emerg Med. 2018 Jan;71(1):44-53.
5. Elias A, Aronson D. Thromb Haemost. 2021 Jun;121(6):800-807.
6. Weisbord SD, du Cheryon D. Intensive Care Med. 2018;44(1):107-109.
7. Davenport MS et al. Radiology. 2020;294(3):660-668.
‘Green Whistle’ Provides Pain Relief -- But Not in the US
This discussion was recorded on March 29, 2024. The transcript has been edited for clarity.
Robert D. Glatter, MD: Joining me today to discuss the use of methoxyflurane (Penthrox), an inhaled nonopioid analgesic for the relief of acute pain, is Dr. William Kenneth (Ken) Milne, an emergency physician at Strathroy Middlesex General Hospital in Ontario, Canada, and the founder of the well-known podcast The Skeptics’ Guide to Emergency Medicine (SGEM).
Also joining me is Dr. Sergey Motov, an emergency physician and research director at Maimonides Medical Center in Brooklyn, New York, and an expert in pain management. I want to welcome both of you and thank you for joining me.
RAMPED Trial: Evaluating the Efficacy of Methoxyflurane
Dr. Glatter: Ken, your recent post on Twitter [now X] regarding the utility of Penthrox in the RAMPED trial really caught my attention. While the trial was from 2021, it really is relevant regarding the prehospital management of pain in the practice of emergency medicine, and certainly in-hospital practice. I was hoping you could review the study design but also get into the rationale behind the use of this novel agent.
William Kenneth (Ken) Milne, MD, MSc: Sure. I’d be happy to kick this episode off with talking about a study that was published in 2020 in Academic Emergency Medicine. It was an Australian study by Brichko et al., and they were doing a randomized controlled trial looking at methoxyflurane vs standard care.
They selected out a population of adults, which they defined as 18-75 years of age. They were in the prehospital setting and they had a pain score of greater than 8. They gave the participants methoxyflurane, which is also called the “green whistle.” They had the subjects take that for their prehospital pain, and they compared that with whatever your standard analgesic in the prehospital setting would be.
Their primary outcome was how many patients had at least 50% reduction in their pain score within 30 minutes. They recruited about 120 people, and they found that there was no statistical difference in the primary outcome between methoxyflurane and standard care. Again, that primary outcome was a reduction in pain score by greater than 50% at 30 minutes, and there wasn’t a statistical difference between the two.
There are obviously limits to any study, and it was a convenience sample. This was an unmasked trial, so people knew if they were getting this green whistle, which is popular in Australia. People would be familiar with this device, and they didn’t compare it with a sham or placebo group.
Pharmacology of Penthrox: Its Role and Mechanism of Action
Dr. Glatter: The primary outcome wasn’t met, but certainly secondary outcomes were. There was, again, a relatively small number of patients in this trial. That said, there was significant pain relief. I think there are issues with the trial, as with any trial limitations.
Getting to the pharmacology of Penthrox, can you describe this inhaled anesthetic and how we use it, specifically its role at the subanesthetic doses?
Sergey M. Motov, MD: Methoxyflurane is embedded in the green whistle package, and that whole contraption is called Penthrox. It’s an inhaled volatile fluorinated hydrocarbon anesthetic that was predominantly used, I’d say 40, 50 years ago, for general anesthesia and slowly but surely fell out of favor due to the fact that, when used for prolonged duration or in supratherapeutic doses, there were cases of severe or even fatal nephrotoxicity and hepatotoxicity.
In the late ‘70s and early ‘80s, all the fluranes came on board that are slightly different as general anesthetics, and methoxyflurane started slowly falling out of favor. Because of this paucity and then a subsequent slightly greater number of cases of nephrotoxicity and hepatotoxicity, [the US Food and Drug Administration] FDA made a decision to pull the drug off the market in 2005. FDA successfully accomplished its mission and since then has pretty much banned the use of inhaled methoxyflurane in any shape, form, or color in the United States.
Going back to the green whistle, it has been used in Australia probably for about 50-60 years, and has been used in Europe for probably 10-20 years. Ken can attest that it has been used in Canada for at least a decade and the track record is phenomenal.
We are using subanesthetic, even supratherapeutic doses that, based on available literature, has no incidence of this fatal hepatotoxicity or nephrotoxicity. We’re talking about 10 million doses administered worldwide, except in the United States. There are 40-plus randomized clinical trials with over 30,000 patients enrolled that prove efficacy and safety.
That’s where we are right now, in a conundrum. We have a great deal of data all over the world, except in the United States, that push for the use of this noninvasive, patient-controlled nonopioid inhaled anesthetic. We just don’t have the access in North America, with the exception of Canada.
Regulatory Hurdles: Challenges in FDA Approval
Dr. Glatter: Absolutely. The FDA wants to be cautious, but if you look at the evidence base of data on this, it really indicates otherwise. Do you think that these roadblocks can be somehow overcome?
Dr. Milne: In the 2000s and 2010s, everybody was focused on opioids and all the dangers and potential adverse events. Opioids are great drugs like many other drugs; it depends on dose and duration. If used properly, it’s an excellent drug. Well, here’s another excellent drug if it’s used properly, and the adverse events are dependent on their dose and duration. Penthrox, or methoxyflurane, is a subtherapeutic, small dose and there have been no reported cases of addiction or abuse related to these inhalers.
Dr. Glatter: That argues for the point — and I’ll turn this over to you, Sergey — of how can this not, in my mind, be an issue that the FDA can overcome.
Dr. Motov: I agree with you. It’s very hard for me to speak on behalf of the FDA, to allude to their thinking processes, but we need to be up to speed with the evidence. The first thing is, why don’t you study the drug in the United States? I’m not asking you to lift the ban, which you put in 2005, but why don’t you honor what has been done over two decades and at least open the door a little bit and let us do what we do best? Why don’t you allow us to do the research in a controlled setting with a carefully, properly selected group of patients without underlying renal or hepatic insufficiency and see where we’re at?
Let’s compare it against placebo. If that’s not ethical, let’s compare it against active comparators — God knows we have 15-20 drugs we can use — and let’s see where we’re at. Ken has been nothing short of superb when it comes to evidence. Let us put the evidence together.
Dr. Milne: If there were concerns decades ago, those need to be addressed. As science is iterative and as other information becomes available, the scientific method would say, Let’s reexamine this and let’s reexamine our position, and do that with evidence. To do that, it has to have validity within the US system. Someone like you doing the research, you are a pain research guru; you should be doing this research to say, “Does it work or not? Does this nonapproval still stand today in 2024?”
Dr. Motov: Thank you for the shout-out, and I agree with you. All of us, those who are interested, on the frontiers of emergency care — as present clinicians — we should be doing this. There is nothing that will convince the FDA more than properly and rightly conducted research, time to reassess the evidence, and time to be less rigid. I understand that you placed a ban 20 years ago, but let’s go with the science. We cannot be behind it.
Exploring the Ecological Footprint of Methoxyflurane
Dr. Milne: There was an Austrian study in 2022 and a very interesting study out of the UK looking at life-cycle impact assessment on the environment. If we’re not just concerned about patient care —obviously, we want to provide patients with a safe and effective product, compared with other products that are available that might not have as good a safety profile — this looks at the impact on the environment.
Dr. Glatter: Ken, can you tell me about some of your recent research regarding the environmental effects related to use of Penthrox, but also its utility pharmacologically and its mechanism of action?
Dr. Milne: There was a really interesting study published this year by Martindale in the Emergency Medicine Journal. It took a different approach to this question about could we be using this drug, and why should we be using this drug? Sergey and I have already talked about the potential benefits and the potential harms. I mentioned opioids and some of the concerns about that. For this drug, if we’re using it in the prehospital setting in this little green whistle, the potential benefits look really good, and we haven’t seen any of the potential harms come through in the literature.
This was another line of evidence of why this might be a good drug, because of the environmental impact of this low-dose methoxyflurane. They compared it with nitrous oxide and said, “Well, what about the life-cycle impact on the environment of using this and the overall cradle-to-grave environmental impacts?”
Obviously, Sergey and I are interested in patient care, and we treat patients one at a time. But we have a larger responsibility to social determinants of health, like our environment. If you look at the overall cradle-to-grave environmental impact of this drug, it was better than for nitrous oxide when looking specifically at climate-change impact. That might be another reason, another line of argument, that could be put forward in the United States to say, “We want to have a healthy environment and a healthy option for patients.”
I’ll let Sergey speak to mechanisms of action and those types of things.
Dr. Motov: As a general anesthetic and hydrocarbonated volatile ones, I’m just going to say that it causes this generalized diffuse cortical depression, and there are no particular channels, receptors, or enzymes we need to worry much about. In short, it’s an inhaled gas used to put patients or people to sleep.
Over the past 30 or 40 years — and I’ll go back to the past decade — there have been numerous studies in different countries (outside of the United States, of course), and with the recent study that Ken just cited, there were comparisons for managing predominantly acute traumatic injuries in pediatric and adult populations presenting to EDs in various regions of the world that compared Penthrox, or the green whistle, with either placebo or active comparators, which included parenteral opioids, oral opioids, and NSAIDs.
The recent systematic review by Fabbri, out of Italy, showed that for ultra–short-term pain — we’re talking about 5, 10, or 15 minutes — inhaled methoxyflurane was found to be equal or even superior to standard of care, primarily related to parenteral opioids, and safety was off the hook. Interestingly, with respect to analgesia, they found that geriatric patients seemed to be responding more, with respect to changing pain score, than younger adults — we’re talking about ages 18-64 vs 65 or older. Again, we need to make sure that we carefully select those elderly people without underlying renal or hepatic insufficiency.
To wrap this up, there is evidence clearly supporting its analgesic efficacy and safety, even in comparison to commonly used and traditionally accepted analgesic modalities that we use for managing acute pain.
US Military Use and Implications for Civilian Practice
Dr. Glatter: Do you think that methoxyflurane’s use in the military will help propel its use in clinical settings in the US, and possibly convince the FDA to look at this closer? The military is currently using it in deployed combat veterans in an ongoing fashion.
Dr. Motov: I’m excited that the Department of Defense in the United States has taken the lead, and they’re being very progressive. There are data that we’ve adapted to the civilian environment by use of intranasal opioids and intranasal ketamine with more doctors who came out of the military. In the military, it’s a kingdom within a kingdom. I don’t know their relationship with the FDA, but I support the military’s pharmacologic initiative by honoring and disseminating their research once it becomes available.
For us nonmilitary folks, we still need to work with the FDA. We need to convince the FDA to let us study the drug, and then we need to pile the evidence within the United States so that the FDA will start looking at this favorably. It wouldn’t hurt and it wouldn’t harm. Any piece of evidence will add to the existing body of literature that we need to allow this medication to be available to us.
Safety Considerations and Aerosolization Concerns
Dr. Glatter: Its safety in children is well established in Australia and throughout the world. I think it deserves a careful look, and the evidence that you’ve both presented argues for the use of this prehospital but also in hospital. I guess there was concern in the hospital with underventilation and healthcare workers being exposed to the fumes, and then getting headaches, dizziness, and so forth. I don’t know if that’s borne out, Ken, in any of your experience in Canada at all.
Dr. Milne: We currently don’t have it in our shop. It’s being used in British Columbia right now in the prehospital setting, and I’m not aware of anybody using it in their department. It’s used prehospital as far as I know.
Dr. Motov: I can attest to it, if I may, because I had familiarized myself with the device. I actually was able to hold it in my hands. I have not used it yet but I had the prototype. The way it’s set up, there is an activated charcoal chamber that sits right on top of the device, which serves as the scavenger for exhaled air that contains particles of methoxyflurane. In theory, but I’m telling how it is in practicality, it significantly reduces occupational exposure, based on data that lacks specifics.
Although most of the researchers did not measure the concentration of methoxyflurane in ambient air within the treatment room in the EDs, I believe the additional data sources clearly stating that it’s within or even below the detectable level that would cause any harm. Once again, we need to honor pathology. We need to make sure that pregnant women will not be exposed to it.
Dr. Milne: In 2024, we also need to be concerned about aerosolizing procedures and aerosolizing treatments, and just take that into account because we should be considering all the potential benefits and all the potential harms. Going through the COVID-19 pandemic, there was concern about transmission and whether or not it was droplet or aerosolized.
There was an observational study published in 2022 in Austria by Trimmel in BMC Emergency Medicine showing similar results. It seemed to work well and potential harms didn’t get picked up. They had to stop the study early because of COVID-19.
We need to always focus in on the potential benefits, the potential harms; where does the science land? Where do the data lie? Then we move forward from that and make informed decisions.
Final Thoughts
Dr. Glatter: Are there any key takeaways you’d like to share with our audience?
Dr. Milne: One of the takeaways from this whole conversation is that science is iterative and science changes. When new evidence becomes available, and we’ve seen it accumulate around the world, we as scientists, as a researcher, as somebody committed to great patient care should revisit our positions on this. Since there is a prohibition against this medication, I think it’s time to reassess that stance and move forward to see if it still is accurate today.
Dr. Motov: I wholeheartedly agree with this. Thank you, Ken, for bringing this up. Good point.
Dr. Glatter: This has been a really informative discussion. I think our audience will certainly embrace this. Thank you very much for your time; it’s much appreciated.
Dr. Glatter is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is a medical adviser for Medscape and hosts the Hot Topics in EM series. Dr. Milne is an emergency physician at Strathroy Middlesex General Hospital in Ontario, Canada, and the founder of the well-known podcast The Skeptics’ Guide to Emergency Medicine (SGEM). Dr. Motov is professor of emergency medicine and director of research in the Department of Emergency Medicine at Maimonides Medical Center in Brooklyn, New York. He is passionate about safe and effective pain management in the emergency department, and has numerous publications on the subject of opioid alternatives in pain management. Dr. Glatter, Dr. Milne, and Dr. Motov had no conflicts of interest to disclose.
A version of this article appeared on Medscape.com.
This discussion was recorded on March 29, 2024. The transcript has been edited for clarity.
Robert D. Glatter, MD: Joining me today to discuss the use of methoxyflurane (Penthrox), an inhaled nonopioid analgesic for the relief of acute pain, is Dr. William Kenneth (Ken) Milne, an emergency physician at Strathroy Middlesex General Hospital in Ontario, Canada, and the founder of the well-known podcast The Skeptics’ Guide to Emergency Medicine (SGEM).
Also joining me is Dr. Sergey Motov, an emergency physician and research director at Maimonides Medical Center in Brooklyn, New York, and an expert in pain management. I want to welcome both of you and thank you for joining me.
RAMPED Trial: Evaluating the Efficacy of Methoxyflurane
Dr. Glatter: Ken, your recent post on Twitter [now X] regarding the utility of Penthrox in the RAMPED trial really caught my attention. While the trial was from 2021, it really is relevant regarding the prehospital management of pain in the practice of emergency medicine, and certainly in-hospital practice. I was hoping you could review the study design but also get into the rationale behind the use of this novel agent.
William Kenneth (Ken) Milne, MD, MSc: Sure. I’d be happy to kick this episode off with talking about a study that was published in 2020 in Academic Emergency Medicine. It was an Australian study by Brichko et al., and they were doing a randomized controlled trial looking at methoxyflurane vs standard care.
They selected out a population of adults, which they defined as 18-75 years of age. They were in the prehospital setting and they had a pain score of greater than 8. They gave the participants methoxyflurane, which is also called the “green whistle.” They had the subjects take that for their prehospital pain, and they compared that with whatever your standard analgesic in the prehospital setting would be.
Their primary outcome was how many patients had at least 50% reduction in their pain score within 30 minutes. They recruited about 120 people, and they found that there was no statistical difference in the primary outcome between methoxyflurane and standard care. Again, that primary outcome was a reduction in pain score by greater than 50% at 30 minutes, and there wasn’t a statistical difference between the two.
There are obviously limits to any study, and it was a convenience sample. This was an unmasked trial, so people knew if they were getting this green whistle, which is popular in Australia. People would be familiar with this device, and they didn’t compare it with a sham or placebo group.
Pharmacology of Penthrox: Its Role and Mechanism of Action
Dr. Glatter: The primary outcome wasn’t met, but certainly secondary outcomes were. There was, again, a relatively small number of patients in this trial. That said, there was significant pain relief. I think there are issues with the trial, as with any trial limitations.
Getting to the pharmacology of Penthrox, can you describe this inhaled anesthetic and how we use it, specifically its role at the subanesthetic doses?
Sergey M. Motov, MD: Methoxyflurane is embedded in the green whistle package, and that whole contraption is called Penthrox. It’s an inhaled volatile fluorinated hydrocarbon anesthetic that was predominantly used, I’d say 40, 50 years ago, for general anesthesia and slowly but surely fell out of favor due to the fact that, when used for prolonged duration or in supratherapeutic doses, there were cases of severe or even fatal nephrotoxicity and hepatotoxicity.
In the late ‘70s and early ‘80s, all the fluranes came on board that are slightly different as general anesthetics, and methoxyflurane started slowly falling out of favor. Because of this paucity and then a subsequent slightly greater number of cases of nephrotoxicity and hepatotoxicity, [the US Food and Drug Administration] FDA made a decision to pull the drug off the market in 2005. FDA successfully accomplished its mission and since then has pretty much banned the use of inhaled methoxyflurane in any shape, form, or color in the United States.
Going back to the green whistle, it has been used in Australia probably for about 50-60 years, and has been used in Europe for probably 10-20 years. Ken can attest that it has been used in Canada for at least a decade and the track record is phenomenal.
We are using subanesthetic, even supratherapeutic doses that, based on available literature, has no incidence of this fatal hepatotoxicity or nephrotoxicity. We’re talking about 10 million doses administered worldwide, except in the United States. There are 40-plus randomized clinical trials with over 30,000 patients enrolled that prove efficacy and safety.
That’s where we are right now, in a conundrum. We have a great deal of data all over the world, except in the United States, that push for the use of this noninvasive, patient-controlled nonopioid inhaled anesthetic. We just don’t have the access in North America, with the exception of Canada.
Regulatory Hurdles: Challenges in FDA Approval
Dr. Glatter: Absolutely. The FDA wants to be cautious, but if you look at the evidence base of data on this, it really indicates otherwise. Do you think that these roadblocks can be somehow overcome?
Dr. Milne: In the 2000s and 2010s, everybody was focused on opioids and all the dangers and potential adverse events. Opioids are great drugs like many other drugs; it depends on dose and duration. If used properly, it’s an excellent drug. Well, here’s another excellent drug if it’s used properly, and the adverse events are dependent on their dose and duration. Penthrox, or methoxyflurane, is a subtherapeutic, small dose and there have been no reported cases of addiction or abuse related to these inhalers.
Dr. Glatter: That argues for the point — and I’ll turn this over to you, Sergey — of how can this not, in my mind, be an issue that the FDA can overcome.
Dr. Motov: I agree with you. It’s very hard for me to speak on behalf of the FDA, to allude to their thinking processes, but we need to be up to speed with the evidence. The first thing is, why don’t you study the drug in the United States? I’m not asking you to lift the ban, which you put in 2005, but why don’t you honor what has been done over two decades and at least open the door a little bit and let us do what we do best? Why don’t you allow us to do the research in a controlled setting with a carefully, properly selected group of patients without underlying renal or hepatic insufficiency and see where we’re at?
Let’s compare it against placebo. If that’s not ethical, let’s compare it against active comparators — God knows we have 15-20 drugs we can use — and let’s see where we’re at. Ken has been nothing short of superb when it comes to evidence. Let us put the evidence together.
Dr. Milne: If there were concerns decades ago, those need to be addressed. As science is iterative and as other information becomes available, the scientific method would say, Let’s reexamine this and let’s reexamine our position, and do that with evidence. To do that, it has to have validity within the US system. Someone like you doing the research, you are a pain research guru; you should be doing this research to say, “Does it work or not? Does this nonapproval still stand today in 2024?”
Dr. Motov: Thank you for the shout-out, and I agree with you. All of us, those who are interested, on the frontiers of emergency care — as present clinicians — we should be doing this. There is nothing that will convince the FDA more than properly and rightly conducted research, time to reassess the evidence, and time to be less rigid. I understand that you placed a ban 20 years ago, but let’s go with the science. We cannot be behind it.
Exploring the Ecological Footprint of Methoxyflurane
Dr. Milne: There was an Austrian study in 2022 and a very interesting study out of the UK looking at life-cycle impact assessment on the environment. If we’re not just concerned about patient care —obviously, we want to provide patients with a safe and effective product, compared with other products that are available that might not have as good a safety profile — this looks at the impact on the environment.
Dr. Glatter: Ken, can you tell me about some of your recent research regarding the environmental effects related to use of Penthrox, but also its utility pharmacologically and its mechanism of action?
Dr. Milne: There was a really interesting study published this year by Martindale in the Emergency Medicine Journal. It took a different approach to this question about could we be using this drug, and why should we be using this drug? Sergey and I have already talked about the potential benefits and the potential harms. I mentioned opioids and some of the concerns about that. For this drug, if we’re using it in the prehospital setting in this little green whistle, the potential benefits look really good, and we haven’t seen any of the potential harms come through in the literature.
This was another line of evidence of why this might be a good drug, because of the environmental impact of this low-dose methoxyflurane. They compared it with nitrous oxide and said, “Well, what about the life-cycle impact on the environment of using this and the overall cradle-to-grave environmental impacts?”
Obviously, Sergey and I are interested in patient care, and we treat patients one at a time. But we have a larger responsibility to social determinants of health, like our environment. If you look at the overall cradle-to-grave environmental impact of this drug, it was better than for nitrous oxide when looking specifically at climate-change impact. That might be another reason, another line of argument, that could be put forward in the United States to say, “We want to have a healthy environment and a healthy option for patients.”
I’ll let Sergey speak to mechanisms of action and those types of things.
Dr. Motov: As a general anesthetic and hydrocarbonated volatile ones, I’m just going to say that it causes this generalized diffuse cortical depression, and there are no particular channels, receptors, or enzymes we need to worry much about. In short, it’s an inhaled gas used to put patients or people to sleep.
Over the past 30 or 40 years — and I’ll go back to the past decade — there have been numerous studies in different countries (outside of the United States, of course), and with the recent study that Ken just cited, there were comparisons for managing predominantly acute traumatic injuries in pediatric and adult populations presenting to EDs in various regions of the world that compared Penthrox, or the green whistle, with either placebo or active comparators, which included parenteral opioids, oral opioids, and NSAIDs.
The recent systematic review by Fabbri, out of Italy, showed that for ultra–short-term pain — we’re talking about 5, 10, or 15 minutes — inhaled methoxyflurane was found to be equal or even superior to standard of care, primarily related to parenteral opioids, and safety was off the hook. Interestingly, with respect to analgesia, they found that geriatric patients seemed to be responding more, with respect to changing pain score, than younger adults — we’re talking about ages 18-64 vs 65 or older. Again, we need to make sure that we carefully select those elderly people without underlying renal or hepatic insufficiency.
To wrap this up, there is evidence clearly supporting its analgesic efficacy and safety, even in comparison to commonly used and traditionally accepted analgesic modalities that we use for managing acute pain.
US Military Use and Implications for Civilian Practice
Dr. Glatter: Do you think that methoxyflurane’s use in the military will help propel its use in clinical settings in the US, and possibly convince the FDA to look at this closer? The military is currently using it in deployed combat veterans in an ongoing fashion.
Dr. Motov: I’m excited that the Department of Defense in the United States has taken the lead, and they’re being very progressive. There are data that we’ve adapted to the civilian environment by use of intranasal opioids and intranasal ketamine with more doctors who came out of the military. In the military, it’s a kingdom within a kingdom. I don’t know their relationship with the FDA, but I support the military’s pharmacologic initiative by honoring and disseminating their research once it becomes available.
For us nonmilitary folks, we still need to work with the FDA. We need to convince the FDA to let us study the drug, and then we need to pile the evidence within the United States so that the FDA will start looking at this favorably. It wouldn’t hurt and it wouldn’t harm. Any piece of evidence will add to the existing body of literature that we need to allow this medication to be available to us.
Safety Considerations and Aerosolization Concerns
Dr. Glatter: Its safety in children is well established in Australia and throughout the world. I think it deserves a careful look, and the evidence that you’ve both presented argues for the use of this prehospital but also in hospital. I guess there was concern in the hospital with underventilation and healthcare workers being exposed to the fumes, and then getting headaches, dizziness, and so forth. I don’t know if that’s borne out, Ken, in any of your experience in Canada at all.
Dr. Milne: We currently don’t have it in our shop. It’s being used in British Columbia right now in the prehospital setting, and I’m not aware of anybody using it in their department. It’s used prehospital as far as I know.
Dr. Motov: I can attest to it, if I may, because I had familiarized myself with the device. I actually was able to hold it in my hands. I have not used it yet but I had the prototype. The way it’s set up, there is an activated charcoal chamber that sits right on top of the device, which serves as the scavenger for exhaled air that contains particles of methoxyflurane. In theory, but I’m telling how it is in practicality, it significantly reduces occupational exposure, based on data that lacks specifics.
Although most of the researchers did not measure the concentration of methoxyflurane in ambient air within the treatment room in the EDs, I believe the additional data sources clearly stating that it’s within or even below the detectable level that would cause any harm. Once again, we need to honor pathology. We need to make sure that pregnant women will not be exposed to it.
Dr. Milne: In 2024, we also need to be concerned about aerosolizing procedures and aerosolizing treatments, and just take that into account because we should be considering all the potential benefits and all the potential harms. Going through the COVID-19 pandemic, there was concern about transmission and whether or not it was droplet or aerosolized.
There was an observational study published in 2022 in Austria by Trimmel in BMC Emergency Medicine showing similar results. It seemed to work well and potential harms didn’t get picked up. They had to stop the study early because of COVID-19.
We need to always focus in on the potential benefits, the potential harms; where does the science land? Where do the data lie? Then we move forward from that and make informed decisions.
Final Thoughts
Dr. Glatter: Are there any key takeaways you’d like to share with our audience?
Dr. Milne: One of the takeaways from this whole conversation is that science is iterative and science changes. When new evidence becomes available, and we’ve seen it accumulate around the world, we as scientists, as a researcher, as somebody committed to great patient care should revisit our positions on this. Since there is a prohibition against this medication, I think it’s time to reassess that stance and move forward to see if it still is accurate today.
Dr. Motov: I wholeheartedly agree with this. Thank you, Ken, for bringing this up. Good point.
Dr. Glatter: This has been a really informative discussion. I think our audience will certainly embrace this. Thank you very much for your time; it’s much appreciated.
Dr. Glatter is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is a medical adviser for Medscape and hosts the Hot Topics in EM series. Dr. Milne is an emergency physician at Strathroy Middlesex General Hospital in Ontario, Canada, and the founder of the well-known podcast The Skeptics’ Guide to Emergency Medicine (SGEM). Dr. Motov is professor of emergency medicine and director of research in the Department of Emergency Medicine at Maimonides Medical Center in Brooklyn, New York. He is passionate about safe and effective pain management in the emergency department, and has numerous publications on the subject of opioid alternatives in pain management. Dr. Glatter, Dr. Milne, and Dr. Motov had no conflicts of interest to disclose.
A version of this article appeared on Medscape.com.
This discussion was recorded on March 29, 2024. The transcript has been edited for clarity.
Robert D. Glatter, MD: Joining me today to discuss the use of methoxyflurane (Penthrox), an inhaled nonopioid analgesic for the relief of acute pain, is Dr. William Kenneth (Ken) Milne, an emergency physician at Strathroy Middlesex General Hospital in Ontario, Canada, and the founder of the well-known podcast The Skeptics’ Guide to Emergency Medicine (SGEM).
Also joining me is Dr. Sergey Motov, an emergency physician and research director at Maimonides Medical Center in Brooklyn, New York, and an expert in pain management. I want to welcome both of you and thank you for joining me.
RAMPED Trial: Evaluating the Efficacy of Methoxyflurane
Dr. Glatter: Ken, your recent post on Twitter [now X] regarding the utility of Penthrox in the RAMPED trial really caught my attention. While the trial was from 2021, it really is relevant regarding the prehospital management of pain in the practice of emergency medicine, and certainly in-hospital practice. I was hoping you could review the study design but also get into the rationale behind the use of this novel agent.
William Kenneth (Ken) Milne, MD, MSc: Sure. I’d be happy to kick this episode off with talking about a study that was published in 2020 in Academic Emergency Medicine. It was an Australian study by Brichko et al., and they were doing a randomized controlled trial looking at methoxyflurane vs standard care.
They selected out a population of adults, which they defined as 18-75 years of age. They were in the prehospital setting and they had a pain score of greater than 8. They gave the participants methoxyflurane, which is also called the “green whistle.” They had the subjects take that for their prehospital pain, and they compared that with whatever your standard analgesic in the prehospital setting would be.
Their primary outcome was how many patients had at least 50% reduction in their pain score within 30 minutes. They recruited about 120 people, and they found that there was no statistical difference in the primary outcome between methoxyflurane and standard care. Again, that primary outcome was a reduction in pain score by greater than 50% at 30 minutes, and there wasn’t a statistical difference between the two.
There are obviously limits to any study, and it was a convenience sample. This was an unmasked trial, so people knew if they were getting this green whistle, which is popular in Australia. People would be familiar with this device, and they didn’t compare it with a sham or placebo group.
Pharmacology of Penthrox: Its Role and Mechanism of Action
Dr. Glatter: The primary outcome wasn’t met, but certainly secondary outcomes were. There was, again, a relatively small number of patients in this trial. That said, there was significant pain relief. I think there are issues with the trial, as with any trial limitations.
Getting to the pharmacology of Penthrox, can you describe this inhaled anesthetic and how we use it, specifically its role at the subanesthetic doses?
Sergey M. Motov, MD: Methoxyflurane is embedded in the green whistle package, and that whole contraption is called Penthrox. It’s an inhaled volatile fluorinated hydrocarbon anesthetic that was predominantly used, I’d say 40, 50 years ago, for general anesthesia and slowly but surely fell out of favor due to the fact that, when used for prolonged duration or in supratherapeutic doses, there were cases of severe or even fatal nephrotoxicity and hepatotoxicity.
In the late ‘70s and early ‘80s, all the fluranes came on board that are slightly different as general anesthetics, and methoxyflurane started slowly falling out of favor. Because of this paucity and then a subsequent slightly greater number of cases of nephrotoxicity and hepatotoxicity, [the US Food and Drug Administration] FDA made a decision to pull the drug off the market in 2005. FDA successfully accomplished its mission and since then has pretty much banned the use of inhaled methoxyflurane in any shape, form, or color in the United States.
Going back to the green whistle, it has been used in Australia probably for about 50-60 years, and has been used in Europe for probably 10-20 years. Ken can attest that it has been used in Canada for at least a decade and the track record is phenomenal.
We are using subanesthetic, even supratherapeutic doses that, based on available literature, has no incidence of this fatal hepatotoxicity or nephrotoxicity. We’re talking about 10 million doses administered worldwide, except in the United States. There are 40-plus randomized clinical trials with over 30,000 patients enrolled that prove efficacy and safety.
That’s where we are right now, in a conundrum. We have a great deal of data all over the world, except in the United States, that push for the use of this noninvasive, patient-controlled nonopioid inhaled anesthetic. We just don’t have the access in North America, with the exception of Canada.
Regulatory Hurdles: Challenges in FDA Approval
Dr. Glatter: Absolutely. The FDA wants to be cautious, but if you look at the evidence base of data on this, it really indicates otherwise. Do you think that these roadblocks can be somehow overcome?
Dr. Milne: In the 2000s and 2010s, everybody was focused on opioids and all the dangers and potential adverse events. Opioids are great drugs like many other drugs; it depends on dose and duration. If used properly, it’s an excellent drug. Well, here’s another excellent drug if it’s used properly, and the adverse events are dependent on their dose and duration. Penthrox, or methoxyflurane, is a subtherapeutic, small dose and there have been no reported cases of addiction or abuse related to these inhalers.
Dr. Glatter: That argues for the point — and I’ll turn this over to you, Sergey — of how can this not, in my mind, be an issue that the FDA can overcome.
Dr. Motov: I agree with you. It’s very hard for me to speak on behalf of the FDA, to allude to their thinking processes, but we need to be up to speed with the evidence. The first thing is, why don’t you study the drug in the United States? I’m not asking you to lift the ban, which you put in 2005, but why don’t you honor what has been done over two decades and at least open the door a little bit and let us do what we do best? Why don’t you allow us to do the research in a controlled setting with a carefully, properly selected group of patients without underlying renal or hepatic insufficiency and see where we’re at?
Let’s compare it against placebo. If that’s not ethical, let’s compare it against active comparators — God knows we have 15-20 drugs we can use — and let’s see where we’re at. Ken has been nothing short of superb when it comes to evidence. Let us put the evidence together.
Dr. Milne: If there were concerns decades ago, those need to be addressed. As science is iterative and as other information becomes available, the scientific method would say, Let’s reexamine this and let’s reexamine our position, and do that with evidence. To do that, it has to have validity within the US system. Someone like you doing the research, you are a pain research guru; you should be doing this research to say, “Does it work or not? Does this nonapproval still stand today in 2024?”
Dr. Motov: Thank you for the shout-out, and I agree with you. All of us, those who are interested, on the frontiers of emergency care — as present clinicians — we should be doing this. There is nothing that will convince the FDA more than properly and rightly conducted research, time to reassess the evidence, and time to be less rigid. I understand that you placed a ban 20 years ago, but let’s go with the science. We cannot be behind it.
Exploring the Ecological Footprint of Methoxyflurane
Dr. Milne: There was an Austrian study in 2022 and a very interesting study out of the UK looking at life-cycle impact assessment on the environment. If we’re not just concerned about patient care —obviously, we want to provide patients with a safe and effective product, compared with other products that are available that might not have as good a safety profile — this looks at the impact on the environment.
Dr. Glatter: Ken, can you tell me about some of your recent research regarding the environmental effects related to use of Penthrox, but also its utility pharmacologically and its mechanism of action?
Dr. Milne: There was a really interesting study published this year by Martindale in the Emergency Medicine Journal. It took a different approach to this question about could we be using this drug, and why should we be using this drug? Sergey and I have already talked about the potential benefits and the potential harms. I mentioned opioids and some of the concerns about that. For this drug, if we’re using it in the prehospital setting in this little green whistle, the potential benefits look really good, and we haven’t seen any of the potential harms come through in the literature.
This was another line of evidence of why this might be a good drug, because of the environmental impact of this low-dose methoxyflurane. They compared it with nitrous oxide and said, “Well, what about the life-cycle impact on the environment of using this and the overall cradle-to-grave environmental impacts?”
Obviously, Sergey and I are interested in patient care, and we treat patients one at a time. But we have a larger responsibility to social determinants of health, like our environment. If you look at the overall cradle-to-grave environmental impact of this drug, it was better than for nitrous oxide when looking specifically at climate-change impact. That might be another reason, another line of argument, that could be put forward in the United States to say, “We want to have a healthy environment and a healthy option for patients.”
I’ll let Sergey speak to mechanisms of action and those types of things.
Dr. Motov: As a general anesthetic and hydrocarbonated volatile ones, I’m just going to say that it causes this generalized diffuse cortical depression, and there are no particular channels, receptors, or enzymes we need to worry much about. In short, it’s an inhaled gas used to put patients or people to sleep.
Over the past 30 or 40 years — and I’ll go back to the past decade — there have been numerous studies in different countries (outside of the United States, of course), and with the recent study that Ken just cited, there were comparisons for managing predominantly acute traumatic injuries in pediatric and adult populations presenting to EDs in various regions of the world that compared Penthrox, or the green whistle, with either placebo or active comparators, which included parenteral opioids, oral opioids, and NSAIDs.
The recent systematic review by Fabbri, out of Italy, showed that for ultra–short-term pain — we’re talking about 5, 10, or 15 minutes — inhaled methoxyflurane was found to be equal or even superior to standard of care, primarily related to parenteral opioids, and safety was off the hook. Interestingly, with respect to analgesia, they found that geriatric patients seemed to be responding more, with respect to changing pain score, than younger adults — we’re talking about ages 18-64 vs 65 or older. Again, we need to make sure that we carefully select those elderly people without underlying renal or hepatic insufficiency.
To wrap this up, there is evidence clearly supporting its analgesic efficacy and safety, even in comparison to commonly used and traditionally accepted analgesic modalities that we use for managing acute pain.
US Military Use and Implications for Civilian Practice
Dr. Glatter: Do you think that methoxyflurane’s use in the military will help propel its use in clinical settings in the US, and possibly convince the FDA to look at this closer? The military is currently using it in deployed combat veterans in an ongoing fashion.
Dr. Motov: I’m excited that the Department of Defense in the United States has taken the lead, and they’re being very progressive. There are data that we’ve adapted to the civilian environment by use of intranasal opioids and intranasal ketamine with more doctors who came out of the military. In the military, it’s a kingdom within a kingdom. I don’t know their relationship with the FDA, but I support the military’s pharmacologic initiative by honoring and disseminating their research once it becomes available.
For us nonmilitary folks, we still need to work with the FDA. We need to convince the FDA to let us study the drug, and then we need to pile the evidence within the United States so that the FDA will start looking at this favorably. It wouldn’t hurt and it wouldn’t harm. Any piece of evidence will add to the existing body of literature that we need to allow this medication to be available to us.
Safety Considerations and Aerosolization Concerns
Dr. Glatter: Its safety in children is well established in Australia and throughout the world. I think it deserves a careful look, and the evidence that you’ve both presented argues for the use of this prehospital but also in hospital. I guess there was concern in the hospital with underventilation and healthcare workers being exposed to the fumes, and then getting headaches, dizziness, and so forth. I don’t know if that’s borne out, Ken, in any of your experience in Canada at all.
Dr. Milne: We currently don’t have it in our shop. It’s being used in British Columbia right now in the prehospital setting, and I’m not aware of anybody using it in their department. It’s used prehospital as far as I know.
Dr. Motov: I can attest to it, if I may, because I had familiarized myself with the device. I actually was able to hold it in my hands. I have not used it yet but I had the prototype. The way it’s set up, there is an activated charcoal chamber that sits right on top of the device, which serves as the scavenger for exhaled air that contains particles of methoxyflurane. In theory, but I’m telling how it is in practicality, it significantly reduces occupational exposure, based on data that lacks specifics.
Although most of the researchers did not measure the concentration of methoxyflurane in ambient air within the treatment room in the EDs, I believe the additional data sources clearly stating that it’s within or even below the detectable level that would cause any harm. Once again, we need to honor pathology. We need to make sure that pregnant women will not be exposed to it.
Dr. Milne: In 2024, we also need to be concerned about aerosolizing procedures and aerosolizing treatments, and just take that into account because we should be considering all the potential benefits and all the potential harms. Going through the COVID-19 pandemic, there was concern about transmission and whether or not it was droplet or aerosolized.
There was an observational study published in 2022 in Austria by Trimmel in BMC Emergency Medicine showing similar results. It seemed to work well and potential harms didn’t get picked up. They had to stop the study early because of COVID-19.
We need to always focus in on the potential benefits, the potential harms; where does the science land? Where do the data lie? Then we move forward from that and make informed decisions.
Final Thoughts
Dr. Glatter: Are there any key takeaways you’d like to share with our audience?
Dr. Milne: One of the takeaways from this whole conversation is that science is iterative and science changes. When new evidence becomes available, and we’ve seen it accumulate around the world, we as scientists, as a researcher, as somebody committed to great patient care should revisit our positions on this. Since there is a prohibition against this medication, I think it’s time to reassess that stance and move forward to see if it still is accurate today.
Dr. Motov: I wholeheartedly agree with this. Thank you, Ken, for bringing this up. Good point.
Dr. Glatter: This has been a really informative discussion. I think our audience will certainly embrace this. Thank you very much for your time; it’s much appreciated.
Dr. Glatter is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is a medical adviser for Medscape and hosts the Hot Topics in EM series. Dr. Milne is an emergency physician at Strathroy Middlesex General Hospital in Ontario, Canada, and the founder of the well-known podcast The Skeptics’ Guide to Emergency Medicine (SGEM). Dr. Motov is professor of emergency medicine and director of research in the Department of Emergency Medicine at Maimonides Medical Center in Brooklyn, New York. He is passionate about safe and effective pain management in the emergency department, and has numerous publications on the subject of opioid alternatives in pain management. Dr. Glatter, Dr. Milne, and Dr. Motov had no conflicts of interest to disclose.
A version of this article appeared on Medscape.com.






