User login
Letter to the Editor: Therapeutic hypothermia for newborns
“PROTECTING THE NEWBORN BRAIN—THE FINAL FRONTIER IN OBSTETRIC AND NEONATAL CARE”
ROBERT L. BARBIERI, MD (AUGUST 2016)
Therapeutic hypothermia
I practice in a small community hospital without a neonatal intensive care unit (NICU). We have always paid attention to warming neonates. Although we cannot start neonatal therapeutic hypothermia, as Dr. Barbieri discusses in his August Editorial, would there be any benefit to avoiding purposefully warming infants who are depressed at birth? NICU care requires a pediatric transport team, which takes at least an hour to arrive.
Jane Dawson, MD
Maryville, Missouri
Dr. Barbieri responds
I thank Dr. Dawson for her observations and query. I agree that at a hospital without a NICU, pending the arrival of a pediatric transport team, clinicians should strive to prevent hyperthermia in a newborn with encephalopathy because hyperthermia might exacerbate the ischemic injury. It may make sense to avoid aggressive warming of the newborn to permit the core temperature to decrease in order to begin the hypothermia process.
“PROTECTING THE NEWBORN BRAIN—THE FINAL FRONTIER IN OBSTETRIC AND NEONATAL CARE”
ROBERT L. BARBIERI, MD (AUGUST 2016)
Therapeutic hypothermia
I practice in a small community hospital without a neonatal intensive care unit (NICU). We have always paid attention to warming neonates. Although we cannot start neonatal therapeutic hypothermia, as Dr. Barbieri discusses in his August Editorial, would there be any benefit to avoiding purposefully warming infants who are depressed at birth? NICU care requires a pediatric transport team, which takes at least an hour to arrive.
Jane Dawson, MD
Maryville, Missouri
Dr. Barbieri responds
I thank Dr. Dawson for her observations and query. I agree that at a hospital without a NICU, pending the arrival of a pediatric transport team, clinicians should strive to prevent hyperthermia in a newborn with encephalopathy because hyperthermia might exacerbate the ischemic injury. It may make sense to avoid aggressive warming of the newborn to permit the core temperature to decrease in order to begin the hypothermia process.
“PROTECTING THE NEWBORN BRAIN—THE FINAL FRONTIER IN OBSTETRIC AND NEONATAL CARE”
ROBERT L. BARBIERI, MD (AUGUST 2016)
Therapeutic hypothermia
I practice in a small community hospital without a neonatal intensive care unit (NICU). We have always paid attention to warming neonates. Although we cannot start neonatal therapeutic hypothermia, as Dr. Barbieri discusses in his August Editorial, would there be any benefit to avoiding purposefully warming infants who are depressed at birth? NICU care requires a pediatric transport team, which takes at least an hour to arrive.
Jane Dawson, MD
Maryville, Missouri
Dr. Barbieri responds
I thank Dr. Dawson for her observations and query. I agree that at a hospital without a NICU, pending the arrival of a pediatric transport team, clinicians should strive to prevent hyperthermia in a newborn with encephalopathy because hyperthermia might exacerbate the ischemic injury. It may make sense to avoid aggressive warming of the newborn to permit the core temperature to decrease in order to begin the hypothermia process.
Letters to the Editor: Managing impacted fetal head at cesarean
“STOP USING INSTRUMENTS TO ASSIST WITH DELIVERY OF THE HEAD AT CESAREAN; START DISENGAGING THE HEAD PRIOR TO SURGERY”
ERROL R. NORWITZ, MD, PHD, MBA (AUGUST 2016)
Patient positioning helps in managing impacted fetal head
As a general practice ObGyn, I have seen an increasing incidence of difficult cesareans as a result of prolonged second stage of labor. Dr. Norwitz cites this increase in his article. I have found that trying to elevate the fetal head prior to the start of surgery has been remarkably ineffective. In my practice, I place all my patients with second-stage arrest in low lithotomy stirrups (“blue fins”); this allows the nurses easier access to the vagina to elevate the head at surgery while I am reaching down from above. Usually, this facilitates delivery. It also allows better assessment of blood loss through the vagina as the cesarean progresses, and it makes placement of a Bakri balloon easier if necessary. If stirrups are not available, the patient can be placed in frog leg positioning so that my assistant can reach down and elevate the head if necessary. I find that in a patient with a very small pelvis, it is hard to get my hand down to the baby’s head. I have not yet done a breech extraction, but I know it is possible. I would probably try nitroglycerin first.
I think that difficult cesarean delivery is much more common than difficult shoulder dystocia, and we should develop standard procedures for addressing the issue and use simulation models to practice. In my time-out prior to surgery, I discuss my concerns so that everyone is ready for it, including the anesthesiologist/CRNA, and we have nitroglycerin available to relax the uterus if necessary. I hope that the American College of Obstetricians and Gynecologists (ACOG) will develop a committee opinion about this very important issue.
Marguerite P. Cohen, MD
Portland, Oregon
Assistant is key in disengaging fetal head
Disengaging the head by an assistant during a cesarean delivery is probably the most successful and useful method for managing an impacted fetal head at cesarean. The disengagement of the head prior to cesarean is practiced routinely in Europe, where forceps delivery is frequently performed. However, the disengagement should be done in the operating room (OR) just prior to or during the cesarean. To perform this in the delivery room, as suggested in Dr. Norwitz’s article, risks the associated fetal bradycardia due to head compression that might compromise an already compromised fetus. In addition, there is a risk of cord prolapse or release of excessive amniotic fluid resulting in cord compression. Also, in many hospitals in the United States, there is some delay to perform the cesarean because the OR is on a different floor from the labor and delivery room and the OR staff come from home.
Vacuum extraction can be safely used for the extraction of the head if it is not possible to deliver it manually. However, the head should be manually disimpacted and rotated to occiput anterior prior to application of the vacuum. But the presence of caput might pose some difficulty with proper application and traction.
It is important to remember that the risk factors for an impacted fetal head are also risk factors for postoperative infection. Therefore, vaginal preparation with antiseptic solution should be considered prior to cesarean delivery for all patients in labor.1
Raymond Michael, MD
Marshall, Minnesota
Reference
- Haas DM, Morgan Al Darei S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2010;3:CD007892.
“STOP USING INSTRUMENTS TO ASSIST WITH DELIVERY OF THE HEAD AT CESAREAN; START DISENGAGING THE HEAD PRIOR TO SURGERY”
ERROL R. NORWITZ, MD, PHD, MBA (AUGUST 2016)
Patient positioning helps in managing impacted fetal head
As a general practice ObGyn, I have seen an increasing incidence of difficult cesareans as a result of prolonged second stage of labor. Dr. Norwitz cites this increase in his article. I have found that trying to elevate the fetal head prior to the start of surgery has been remarkably ineffective. In my practice, I place all my patients with second-stage arrest in low lithotomy stirrups (“blue fins”); this allows the nurses easier access to the vagina to elevate the head at surgery while I am reaching down from above. Usually, this facilitates delivery. It also allows better assessment of blood loss through the vagina as the cesarean progresses, and it makes placement of a Bakri balloon easier if necessary. If stirrups are not available, the patient can be placed in frog leg positioning so that my assistant can reach down and elevate the head if necessary. I find that in a patient with a very small pelvis, it is hard to get my hand down to the baby’s head. I have not yet done a breech extraction, but I know it is possible. I would probably try nitroglycerin first.
I think that difficult cesarean delivery is much more common than difficult shoulder dystocia, and we should develop standard procedures for addressing the issue and use simulation models to practice. In my time-out prior to surgery, I discuss my concerns so that everyone is ready for it, including the anesthesiologist/CRNA, and we have nitroglycerin available to relax the uterus if necessary. I hope that the American College of Obstetricians and Gynecologists (ACOG) will develop a committee opinion about this very important issue.
Marguerite P. Cohen, MD
Portland, Oregon
Assistant is key in disengaging fetal head
Disengaging the head by an assistant during a cesarean delivery is probably the most successful and useful method for managing an impacted fetal head at cesarean. The disengagement of the head prior to cesarean is practiced routinely in Europe, where forceps delivery is frequently performed. However, the disengagement should be done in the operating room (OR) just prior to or during the cesarean. To perform this in the delivery room, as suggested in Dr. Norwitz’s article, risks the associated fetal bradycardia due to head compression that might compromise an already compromised fetus. In addition, there is a risk of cord prolapse or release of excessive amniotic fluid resulting in cord compression. Also, in many hospitals in the United States, there is some delay to perform the cesarean because the OR is on a different floor from the labor and delivery room and the OR staff come from home.
Vacuum extraction can be safely used for the extraction of the head if it is not possible to deliver it manually. However, the head should be manually disimpacted and rotated to occiput anterior prior to application of the vacuum. But the presence of caput might pose some difficulty with proper application and traction.
It is important to remember that the risk factors for an impacted fetal head are also risk factors for postoperative infection. Therefore, vaginal preparation with antiseptic solution should be considered prior to cesarean delivery for all patients in labor.1
Raymond Michael, MD
Marshall, Minnesota
Reference
- Haas DM, Morgan Al Darei S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2010;3:CD007892.
“STOP USING INSTRUMENTS TO ASSIST WITH DELIVERY OF THE HEAD AT CESAREAN; START DISENGAGING THE HEAD PRIOR TO SURGERY”
ERROL R. NORWITZ, MD, PHD, MBA (AUGUST 2016)
Patient positioning helps in managing impacted fetal head
As a general practice ObGyn, I have seen an increasing incidence of difficult cesareans as a result of prolonged second stage of labor. Dr. Norwitz cites this increase in his article. I have found that trying to elevate the fetal head prior to the start of surgery has been remarkably ineffective. In my practice, I place all my patients with second-stage arrest in low lithotomy stirrups (“blue fins”); this allows the nurses easier access to the vagina to elevate the head at surgery while I am reaching down from above. Usually, this facilitates delivery. It also allows better assessment of blood loss through the vagina as the cesarean progresses, and it makes placement of a Bakri balloon easier if necessary. If stirrups are not available, the patient can be placed in frog leg positioning so that my assistant can reach down and elevate the head if necessary. I find that in a patient with a very small pelvis, it is hard to get my hand down to the baby’s head. I have not yet done a breech extraction, but I know it is possible. I would probably try nitroglycerin first.
I think that difficult cesarean delivery is much more common than difficult shoulder dystocia, and we should develop standard procedures for addressing the issue and use simulation models to practice. In my time-out prior to surgery, I discuss my concerns so that everyone is ready for it, including the anesthesiologist/CRNA, and we have nitroglycerin available to relax the uterus if necessary. I hope that the American College of Obstetricians and Gynecologists (ACOG) will develop a committee opinion about this very important issue.
Marguerite P. Cohen, MD
Portland, Oregon
Assistant is key in disengaging fetal head
Disengaging the head by an assistant during a cesarean delivery is probably the most successful and useful method for managing an impacted fetal head at cesarean. The disengagement of the head prior to cesarean is practiced routinely in Europe, where forceps delivery is frequently performed. However, the disengagement should be done in the operating room (OR) just prior to or during the cesarean. To perform this in the delivery room, as suggested in Dr. Norwitz’s article, risks the associated fetal bradycardia due to head compression that might compromise an already compromised fetus. In addition, there is a risk of cord prolapse or release of excessive amniotic fluid resulting in cord compression. Also, in many hospitals in the United States, there is some delay to perform the cesarean because the OR is on a different floor from the labor and delivery room and the OR staff come from home.
Vacuum extraction can be safely used for the extraction of the head if it is not possible to deliver it manually. However, the head should be manually disimpacted and rotated to occiput anterior prior to application of the vacuum. But the presence of caput might pose some difficulty with proper application and traction.
It is important to remember that the risk factors for an impacted fetal head are also risk factors for postoperative infection. Therefore, vaginal preparation with antiseptic solution should be considered prior to cesarean delivery for all patients in labor.1
Raymond Michael, MD
Marshall, Minnesota
Reference
- Haas DM, Morgan Al Darei S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2010;3:CD007892.
Forget the myths and help your psychiatric patients quit smoking
The National Ambulatory Medical Care Survey1,2 (NAMCS) indicates that less than 1 out of 4 (23%) psychiatrists provide smoking cessation counseling to their patients, and even fewer prescribe medications.
What gives? How is it that so many psychiatrists endorse having recently helped a patient quit smoking when the data from large-scale surveys1,2 indicate they do not?
From the “glass is half-full” perspective, the discrepancy might indicate that psychiatrists finally have bought into the message put forth 20 years ago when the American Psychiatric Association first published its clinical practice guidelines for treating nicotine dependence.3 Because the figures I cited from NAMCS reflect data from 2006 to 2010, it is possible that in the last 5 years more psychiatrists have started to help their patients quit smoking. Such an hypothesis is further supported by the increasing number of research papers on smoking cessation in individuals with mental illness published over the past 8 years—a period that coincides with the release of the second edition of the Treating tobacco use and dependence clinical practice guideline from the U.S. Agency for Healthcare Research and Quality, which highlighted the need for more research in this population of smokers.4
Regardless of the reason, the fact that my informal surveys indicate a likely uptick in activity among psychiatrists to help their patients quit smoking is welcome news. With nearly 1 out of 2 cigarettes sold in the United States being smoked by individuals with psychiatric and substance use disorders,5 psychiatrists and other mental health professionals play a vital role in addressing this epidemic. That our patients smoke at rates 2- to 4-times that of the general population and die decades earlier than their non-smoking, non-mentally ill counterparts6 are compelling reasons urging us to end our complacency and help our patients quit smoking.
EAGLES trial results help debunk the latest myth about smoking cessation
In an article that I wrote for
In addition to applying the “black-box” warning, the FDA issued a post-marketing requirement to the manufacturers of bupropion and varenicline to conduct a large randomized controlled trial—Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES)—the top-line results of which were published in The Lancet this spring.12
Key results of the EAGLES trial
The researchers found no significant increase in serious neuropsychiatric AEs—a composite measure assessing depression, anxiety, suicidality, and 13 other symptom clusters—attributable to varenicline or bupropion compared with placebo or the nicotine patch in smokers with or without psychiatric disorders. The study did detect a significant difference—approximately 4% (2% in non-psychiatric cohort vs 6% in psychiatric cohort)—in the rate of serious neuropsychiatric AEs regardless of treatment condition. In both cohorts, varenicline was more effective than bupropion, which had similar efficacy to the nicotine patch; all interventions were superior to placebo. Importantly, all 3 medications significantly improved quit rates in smokers with and without psychiatric disorders. Although the efficacy of medications in smokers with or without psychiatric disorders was similar in terms of odds ratios, overall, those with psychiatric disorders had 20% to 30% lower quit rates compared with non-psychiatrically ill smokers.
The EAGLES study results, when viewed in the context of findings from other clinical trials and large-scale observational studies, provide further evidence that smokers with stable mental illness can use bupropion and varenicline safely. It also demonstrates that moderate to severe neuropsychiatric AEs occur during a smoking cessation attempt regardless of the medication used, therefore, monitoring smokers—especially those with psychiatric disorders—is important, a role that psychiatrists are uniquely poised to play.
That all 3 smoking cessation medications are effective in patients with mood, anxiety, and psychotic disorders is good news for our patients. Combined with the EAGLES safety findings, there is no better time to intervene in tobacco dependence
1. Rogers E, Sherman S. Tobacco use screening and treatment by outpatient psychiatrists before and after release of the American Psychiatric Association treatment guidelines for nicotine dependence. Am J Public Health. 2014;104(1):90-95.
2. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry. 2003;160(12):2228-2230.
3. Practice guideline for the treatment of patients with nicotine dependence. American Psychiatric Association. Am J Psychiatry. 1996;53;153(suppl 10):1-31.
4. U.S. Department of Health and Human Services. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. http://www.ahrq.gov/sites/default/files/wysiwyg/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf. Published May 2008. Accessed September 12, 2016.
5. Grant BF, Hasin DS, Chou SP, et al. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107-1115.
6. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
7. Anthenelli RM. How—and why—to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
8. Zyban [package insert]. Research Triangle Park, NC; GlaxoSmithKline; 2016.
9. Chantix [package insert]. New York, NY: Pfizer; 2016.
10. U.S. Department of Health and Human Services. The health consequences of smoking – 50 years of progress: a report of the surgeon general, 2014. Rockville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
11. World Health Organization. WHO report on the global tobacco epidemic, 2011: warning about the dangers of tobacco. http://www.who.int/tobacco/global_report/2011/en/index.html. Published 2011. Accessed December 1, 2015.
12. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomized, placebo-controlled clinical trial. Lancet. 2016;18;387(10037):2507-2520.
13. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
14. First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002.
15. First M, Gibbon M, Spitzer RL, et al. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II). Washington, DC: American Psychiatric Press, Inc.; 1997.
16. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370.
17. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2001;168(12):1266-1277.
The National Ambulatory Medical Care Survey1,2 (NAMCS) indicates that less than 1 out of 4 (23%) psychiatrists provide smoking cessation counseling to their patients, and even fewer prescribe medications.
What gives? How is it that so many psychiatrists endorse having recently helped a patient quit smoking when the data from large-scale surveys1,2 indicate they do not?
From the “glass is half-full” perspective, the discrepancy might indicate that psychiatrists finally have bought into the message put forth 20 years ago when the American Psychiatric Association first published its clinical practice guidelines for treating nicotine dependence.3 Because the figures I cited from NAMCS reflect data from 2006 to 2010, it is possible that in the last 5 years more psychiatrists have started to help their patients quit smoking. Such an hypothesis is further supported by the increasing number of research papers on smoking cessation in individuals with mental illness published over the past 8 years—a period that coincides with the release of the second edition of the Treating tobacco use and dependence clinical practice guideline from the U.S. Agency for Healthcare Research and Quality, which highlighted the need for more research in this population of smokers.4
Regardless of the reason, the fact that my informal surveys indicate a likely uptick in activity among psychiatrists to help their patients quit smoking is welcome news. With nearly 1 out of 2 cigarettes sold in the United States being smoked by individuals with psychiatric and substance use disorders,5 psychiatrists and other mental health professionals play a vital role in addressing this epidemic. That our patients smoke at rates 2- to 4-times that of the general population and die decades earlier than their non-smoking, non-mentally ill counterparts6 are compelling reasons urging us to end our complacency and help our patients quit smoking.
EAGLES trial results help debunk the latest myth about smoking cessation
In an article that I wrote for
In addition to applying the “black-box” warning, the FDA issued a post-marketing requirement to the manufacturers of bupropion and varenicline to conduct a large randomized controlled trial—Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES)—the top-line results of which were published in The Lancet this spring.12
Key results of the EAGLES trial
The researchers found no significant increase in serious neuropsychiatric AEs—a composite measure assessing depression, anxiety, suicidality, and 13 other symptom clusters—attributable to varenicline or bupropion compared with placebo or the nicotine patch in smokers with or without psychiatric disorders. The study did detect a significant difference—approximately 4% (2% in non-psychiatric cohort vs 6% in psychiatric cohort)—in the rate of serious neuropsychiatric AEs regardless of treatment condition. In both cohorts, varenicline was more effective than bupropion, which had similar efficacy to the nicotine patch; all interventions were superior to placebo. Importantly, all 3 medications significantly improved quit rates in smokers with and without psychiatric disorders. Although the efficacy of medications in smokers with or without psychiatric disorders was similar in terms of odds ratios, overall, those with psychiatric disorders had 20% to 30% lower quit rates compared with non-psychiatrically ill smokers.
The EAGLES study results, when viewed in the context of findings from other clinical trials and large-scale observational studies, provide further evidence that smokers with stable mental illness can use bupropion and varenicline safely. It also demonstrates that moderate to severe neuropsychiatric AEs occur during a smoking cessation attempt regardless of the medication used, therefore, monitoring smokers—especially those with psychiatric disorders—is important, a role that psychiatrists are uniquely poised to play.
That all 3 smoking cessation medications are effective in patients with mood, anxiety, and psychotic disorders is good news for our patients. Combined with the EAGLES safety findings, there is no better time to intervene in tobacco dependence
The National Ambulatory Medical Care Survey1,2 (NAMCS) indicates that less than 1 out of 4 (23%) psychiatrists provide smoking cessation counseling to their patients, and even fewer prescribe medications.
What gives? How is it that so many psychiatrists endorse having recently helped a patient quit smoking when the data from large-scale surveys1,2 indicate they do not?
From the “glass is half-full” perspective, the discrepancy might indicate that psychiatrists finally have bought into the message put forth 20 years ago when the American Psychiatric Association first published its clinical practice guidelines for treating nicotine dependence.3 Because the figures I cited from NAMCS reflect data from 2006 to 2010, it is possible that in the last 5 years more psychiatrists have started to help their patients quit smoking. Such an hypothesis is further supported by the increasing number of research papers on smoking cessation in individuals with mental illness published over the past 8 years—a period that coincides with the release of the second edition of the Treating tobacco use and dependence clinical practice guideline from the U.S. Agency for Healthcare Research and Quality, which highlighted the need for more research in this population of smokers.4
Regardless of the reason, the fact that my informal surveys indicate a likely uptick in activity among psychiatrists to help their patients quit smoking is welcome news. With nearly 1 out of 2 cigarettes sold in the United States being smoked by individuals with psychiatric and substance use disorders,5 psychiatrists and other mental health professionals play a vital role in addressing this epidemic. That our patients smoke at rates 2- to 4-times that of the general population and die decades earlier than their non-smoking, non-mentally ill counterparts6 are compelling reasons urging us to end our complacency and help our patients quit smoking.
EAGLES trial results help debunk the latest myth about smoking cessation
In an article that I wrote for
In addition to applying the “black-box” warning, the FDA issued a post-marketing requirement to the manufacturers of bupropion and varenicline to conduct a large randomized controlled trial—Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES)—the top-line results of which were published in The Lancet this spring.12
Key results of the EAGLES trial
The researchers found no significant increase in serious neuropsychiatric AEs—a composite measure assessing depression, anxiety, suicidality, and 13 other symptom clusters—attributable to varenicline or bupropion compared with placebo or the nicotine patch in smokers with or without psychiatric disorders. The study did detect a significant difference—approximately 4% (2% in non-psychiatric cohort vs 6% in psychiatric cohort)—in the rate of serious neuropsychiatric AEs regardless of treatment condition. In both cohorts, varenicline was more effective than bupropion, which had similar efficacy to the nicotine patch; all interventions were superior to placebo. Importantly, all 3 medications significantly improved quit rates in smokers with and without psychiatric disorders. Although the efficacy of medications in smokers with or without psychiatric disorders was similar in terms of odds ratios, overall, those with psychiatric disorders had 20% to 30% lower quit rates compared with non-psychiatrically ill smokers.
The EAGLES study results, when viewed in the context of findings from other clinical trials and large-scale observational studies, provide further evidence that smokers with stable mental illness can use bupropion and varenicline safely. It also demonstrates that moderate to severe neuropsychiatric AEs occur during a smoking cessation attempt regardless of the medication used, therefore, monitoring smokers—especially those with psychiatric disorders—is important, a role that psychiatrists are uniquely poised to play.
That all 3 smoking cessation medications are effective in patients with mood, anxiety, and psychotic disorders is good news for our patients. Combined with the EAGLES safety findings, there is no better time to intervene in tobacco dependence
1. Rogers E, Sherman S. Tobacco use screening and treatment by outpatient psychiatrists before and after release of the American Psychiatric Association treatment guidelines for nicotine dependence. Am J Public Health. 2014;104(1):90-95.
2. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry. 2003;160(12):2228-2230.
3. Practice guideline for the treatment of patients with nicotine dependence. American Psychiatric Association. Am J Psychiatry. 1996;53;153(suppl 10):1-31.
4. U.S. Department of Health and Human Services. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. http://www.ahrq.gov/sites/default/files/wysiwyg/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf. Published May 2008. Accessed September 12, 2016.
5. Grant BF, Hasin DS, Chou SP, et al. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107-1115.
6. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
7. Anthenelli RM. How—and why—to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
8. Zyban [package insert]. Research Triangle Park, NC; GlaxoSmithKline; 2016.
9. Chantix [package insert]. New York, NY: Pfizer; 2016.
10. U.S. Department of Health and Human Services. The health consequences of smoking – 50 years of progress: a report of the surgeon general, 2014. Rockville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
11. World Health Organization. WHO report on the global tobacco epidemic, 2011: warning about the dangers of tobacco. http://www.who.int/tobacco/global_report/2011/en/index.html. Published 2011. Accessed December 1, 2015.
12. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomized, placebo-controlled clinical trial. Lancet. 2016;18;387(10037):2507-2520.
13. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
14. First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002.
15. First M, Gibbon M, Spitzer RL, et al. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II). Washington, DC: American Psychiatric Press, Inc.; 1997.
16. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370.
17. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2001;168(12):1266-1277.
1. Rogers E, Sherman S. Tobacco use screening and treatment by outpatient psychiatrists before and after release of the American Psychiatric Association treatment guidelines for nicotine dependence. Am J Public Health. 2014;104(1):90-95.
2. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry. 2003;160(12):2228-2230.
3. Practice guideline for the treatment of patients with nicotine dependence. American Psychiatric Association. Am J Psychiatry. 1996;53;153(suppl 10):1-31.
4. U.S. Department of Health and Human Services. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. http://www.ahrq.gov/sites/default/files/wysiwyg/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf. Published May 2008. Accessed September 12, 2016.
5. Grant BF, Hasin DS, Chou SP, et al. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107-1115.
6. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
7. Anthenelli RM. How—and why—to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
8. Zyban [package insert]. Research Triangle Park, NC; GlaxoSmithKline; 2016.
9. Chantix [package insert]. New York, NY: Pfizer; 2016.
10. U.S. Department of Health and Human Services. The health consequences of smoking – 50 years of progress: a report of the surgeon general, 2014. Rockville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
11. World Health Organization. WHO report on the global tobacco epidemic, 2011: warning about the dangers of tobacco. http://www.who.int/tobacco/global_report/2011/en/index.html. Published 2011. Accessed December 1, 2015.
12. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomized, placebo-controlled clinical trial. Lancet. 2016;18;387(10037):2507-2520.
13. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
14. First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002.
15. First M, Gibbon M, Spitzer RL, et al. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II). Washington, DC: American Psychiatric Press, Inc.; 1997.
16. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370.
17. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2001;168(12):1266-1277.
HPV vaccine and adolescents: What we say really does matter
It has been almost 10 years since the Advisory Committee on Immunization Practices (ACIP) recommended administration of human papillomavirus (HPV) vaccine for 11- to 12-year-old girls and young women up to 26 years of age. Routine administration in preteen boys and young adult males up to 21 years of age was recommended in 2011. An HPV series should be completed by 13 years. So how well are we protecting our patients?
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually among adolescents 13-17 years. Data are obtained from individuals from the 50 states, the District of Columbia, the U.S. Virgin Islands, and six major urban areas (MMWR. 2016 Aug 26;65[33]:850-8).
HPV vaccination continues to lag behind Tdap and the meningococcal conjugate vaccine (MCV), although each one is recommended to be administered at the 11- to 12-year visit. In 2015, coverage for receiving at least one dose of HPV vaccine among females was almost 62.8 % and for at least three doses was 41.9%; among males, coverage with at least one dose was 49.8% and for at least three doses was 28.1%. Compared with 2014, coverage for at least one dose of HPV vaccine increased 2.8% in females and 8.1% in males. Males also had a 7.6% for receipt of at least two doses of HPV vaccine, compared with 2014. HPV vaccine coverage in females aged 13 and younger also was lower than for those aged 15 and older. Coverage did not differ for males based on age.
HPV vaccination coverage also differed by state. In 2015, 28 states reported increased coverage in males, but only 7 states had increased coverage in females. Among all adolescents, coverage with at least one dose of HPV vaccine was 56.1%, at least two doses was 45.4%, and at least three doses was 34.9%. In contrast, 86.4% of all adolescents received at least one dose of Tdap, and 81.3% received at least one dose of MCV.
HPV-associated cancers
HPV is the most common sexually transmitted infection in both men and women. It is estimated that 79 million Americans are infected and 14 million new infections occur annually, usually in teens and young adults. Although most infections are asymptomatic and clear spontaneously, persistent infection with oncogenic types can progress to cancer. Cervical and oropharyngeal cancer were the most common HPV-associated cancers in women and men, respectively, in 2008-2012 (MMWR 2016;65:661-6).
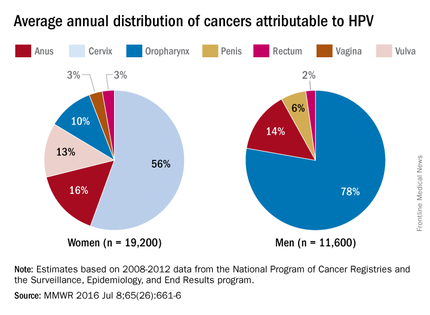
All three HPV vaccines protect against HPV types 16 and 18. These types are estimated to account for the majority of cervical and oropharyngeal cancers, 66% and 62%, respectively. The additional types in the 9-valent HPV will protect against HPV types that cause approximately 15% of cervical cancers.
The association between HPV and cancer is clear. So why isn’t this vaccine being embraced? HPV vaccine is all about cancer prevention. Isn’t it? What are the barriers to HPV vaccination? Are parental concerns the only barrier? Are we recommending this vaccine as strongly as others?
Vaccine safety and efficacy
Safety has been a concern voiced by some parents. Collectively, HPV vaccines were studied in more than 95,000 individuals prior to licensure. Almost 90 million doses of vaccine have been distributed in the United States and more than 183 million, worldwide. The federal government utilizes three systems to monitor vaccine safety once a vaccine is licensed: The Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD), and the Clinical Immunization Safety Assessment (CISA) Network. Ongoing safety studies also are conducted by vaccine manufacturers. Since licensure, no serious safety concerns have been identified. Postvaccination syncope, first identified in the VAERS database in 2006, has declined since observation post injection was recommended by ACIP. Multiple studies in the United States and abroad have not demonstrated a causal association with HPV vaccine and any autoimmune and/or neurologic condition or increased risk for thromboembolism.
Mélanie Drolet, PhD, and her colleagues reviewed 20 studies in nine countries with at least 50% coverage in female adolescents aged 13-19 years. There was a 68% reduction in the prevalence of HPV types 16 and 18 and a 61% reduction in anal warts in the postvaccine era (Lancet Infect Dis. 2015 May;15[5]:565-80). Studies also indicate there is no indication of waning immunity.
Parental perceptions
Some parents feel the vaccine is not necessary because their child is not sexually active and/or is not at risk for acquiring a sexually transmitted infection. Others opt to delay initiation. NHANES (National Health and Nutrition Examination Survey) data from 2011 to 2014 revealed that among females aged 14-26 years whose age was known at the time of their first dose of HPV vaccine, 43% had reported having sex before or in the same year that they received their first dose.
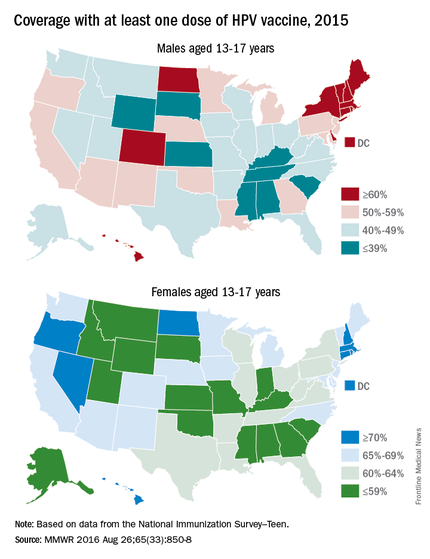
One consistent reason parents indicate for not vaccinating is the lack of recommendation from their child’s health provider. Differences in age and sex recommendations also are reported. NIS-Teen 2013 demonstrated that parents of girls were more likely than parents of boys to receive a provider recommendation (65% vs.42%.) Only 29% of female parents indicated they’d received a provider recommendation to have their child vaccinated with HPV by ages 11-12 years.
Mandy A. Allison, MD, and her colleagues reviewed primary care physician perspectives about HPV vaccine in a national survey among 364 pediatricians and 218 family physicians (FPs). Although 84% of pediatricians and 75% of FPs indicated they always discuss HPV vaccination, only 60% of pediatricians and 59% of FPs strongly recommend HPV vaccine for 11- to 12-year-old girls; for boys it was 52% and 41%. More than half reported parental deferral. For pediatricians who almost never discussed the topic, the reasons included that the patient was not sexually active (54%), the child was young (38%), and the patient was already receiving other vaccines (35%) (Pediatrics. 2016 Feb;137[2]:e20152488).
Providers can be influenced by their perceptions of what value parents place on vaccines. In one study, parents were asked to put a value on specific vaccines. Providers were then asked to estimate how parents ranked the vaccines on a scale of 0-10. Providers underestimated the value placed on HPV vaccine (9.3 vs 5.2) (Vaccine 2014;32:579-84).
Improving HPV coverage: Preventing future HPV-related cancers
HPV vaccine should be recommended with as much conviction as Tdap and MCV at the 11- to 12-year visit for both girls and boys. Administration of all three should occur on the same day. Clinician recommendation is the No. 1 reason parents decide to vaccinate. The mantra “same way, same day” should become synonymous with the 11- to 12-year visit. All who have contact with the patient, beginning with the front desk staff, should know the importance of HPV vaccine, and when and why it is recommended. Often, families spend more time with support staff and have discussions prior to interacting with you.
Anticipate questions about HPV. Why give the vaccine when the child is so young and not sexually active? Is my child really at risk? Is it safe? I read on the Internet. … Questions should be interpreted as a need for additional information and reassurance from you.
Remember to emphasize that HPV vaccine is important because it prevents cancer and it is most effective prior to exposure to HPV.
Additional resources to facilitate your discussions about HPV can be found at www.cdc.gov/hpv.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
It has been almost 10 years since the Advisory Committee on Immunization Practices (ACIP) recommended administration of human papillomavirus (HPV) vaccine for 11- to 12-year-old girls and young women up to 26 years of age. Routine administration in preteen boys and young adult males up to 21 years of age was recommended in 2011. An HPV series should be completed by 13 years. So how well are we protecting our patients?
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually among adolescents 13-17 years. Data are obtained from individuals from the 50 states, the District of Columbia, the U.S. Virgin Islands, and six major urban areas (MMWR. 2016 Aug 26;65[33]:850-8).
HPV vaccination continues to lag behind Tdap and the meningococcal conjugate vaccine (MCV), although each one is recommended to be administered at the 11- to 12-year visit. In 2015, coverage for receiving at least one dose of HPV vaccine among females was almost 62.8 % and for at least three doses was 41.9%; among males, coverage with at least one dose was 49.8% and for at least three doses was 28.1%. Compared with 2014, coverage for at least one dose of HPV vaccine increased 2.8% in females and 8.1% in males. Males also had a 7.6% for receipt of at least two doses of HPV vaccine, compared with 2014. HPV vaccine coverage in females aged 13 and younger also was lower than for those aged 15 and older. Coverage did not differ for males based on age.
HPV vaccination coverage also differed by state. In 2015, 28 states reported increased coverage in males, but only 7 states had increased coverage in females. Among all adolescents, coverage with at least one dose of HPV vaccine was 56.1%, at least two doses was 45.4%, and at least three doses was 34.9%. In contrast, 86.4% of all adolescents received at least one dose of Tdap, and 81.3% received at least one dose of MCV.
HPV-associated cancers
HPV is the most common sexually transmitted infection in both men and women. It is estimated that 79 million Americans are infected and 14 million new infections occur annually, usually in teens and young adults. Although most infections are asymptomatic and clear spontaneously, persistent infection with oncogenic types can progress to cancer. Cervical and oropharyngeal cancer were the most common HPV-associated cancers in women and men, respectively, in 2008-2012 (MMWR 2016;65:661-6).

All three HPV vaccines protect against HPV types 16 and 18. These types are estimated to account for the majority of cervical and oropharyngeal cancers, 66% and 62%, respectively. The additional types in the 9-valent HPV will protect against HPV types that cause approximately 15% of cervical cancers.
The association between HPV and cancer is clear. So why isn’t this vaccine being embraced? HPV vaccine is all about cancer prevention. Isn’t it? What are the barriers to HPV vaccination? Are parental concerns the only barrier? Are we recommending this vaccine as strongly as others?
Vaccine safety and efficacy
Safety has been a concern voiced by some parents. Collectively, HPV vaccines were studied in more than 95,000 individuals prior to licensure. Almost 90 million doses of vaccine have been distributed in the United States and more than 183 million, worldwide. The federal government utilizes three systems to monitor vaccine safety once a vaccine is licensed: The Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD), and the Clinical Immunization Safety Assessment (CISA) Network. Ongoing safety studies also are conducted by vaccine manufacturers. Since licensure, no serious safety concerns have been identified. Postvaccination syncope, first identified in the VAERS database in 2006, has declined since observation post injection was recommended by ACIP. Multiple studies in the United States and abroad have not demonstrated a causal association with HPV vaccine and any autoimmune and/or neurologic condition or increased risk for thromboembolism.
Mélanie Drolet, PhD, and her colleagues reviewed 20 studies in nine countries with at least 50% coverage in female adolescents aged 13-19 years. There was a 68% reduction in the prevalence of HPV types 16 and 18 and a 61% reduction in anal warts in the postvaccine era (Lancet Infect Dis. 2015 May;15[5]:565-80). Studies also indicate there is no indication of waning immunity.
Parental perceptions
Some parents feel the vaccine is not necessary because their child is not sexually active and/or is not at risk for acquiring a sexually transmitted infection. Others opt to delay initiation. NHANES (National Health and Nutrition Examination Survey) data from 2011 to 2014 revealed that among females aged 14-26 years whose age was known at the time of their first dose of HPV vaccine, 43% had reported having sex before or in the same year that they received their first dose.

One consistent reason parents indicate for not vaccinating is the lack of recommendation from their child’s health provider. Differences in age and sex recommendations also are reported. NIS-Teen 2013 demonstrated that parents of girls were more likely than parents of boys to receive a provider recommendation (65% vs.42%.) Only 29% of female parents indicated they’d received a provider recommendation to have their child vaccinated with HPV by ages 11-12 years.
Mandy A. Allison, MD, and her colleagues reviewed primary care physician perspectives about HPV vaccine in a national survey among 364 pediatricians and 218 family physicians (FPs). Although 84% of pediatricians and 75% of FPs indicated they always discuss HPV vaccination, only 60% of pediatricians and 59% of FPs strongly recommend HPV vaccine for 11- to 12-year-old girls; for boys it was 52% and 41%. More than half reported parental deferral. For pediatricians who almost never discussed the topic, the reasons included that the patient was not sexually active (54%), the child was young (38%), and the patient was already receiving other vaccines (35%) (Pediatrics. 2016 Feb;137[2]:e20152488).
Providers can be influenced by their perceptions of what value parents place on vaccines. In one study, parents were asked to put a value on specific vaccines. Providers were then asked to estimate how parents ranked the vaccines on a scale of 0-10. Providers underestimated the value placed on HPV vaccine (9.3 vs 5.2) (Vaccine 2014;32:579-84).
Improving HPV coverage: Preventing future HPV-related cancers
HPV vaccine should be recommended with as much conviction as Tdap and MCV at the 11- to 12-year visit for both girls and boys. Administration of all three should occur on the same day. Clinician recommendation is the No. 1 reason parents decide to vaccinate. The mantra “same way, same day” should become synonymous with the 11- to 12-year visit. All who have contact with the patient, beginning with the front desk staff, should know the importance of HPV vaccine, and when and why it is recommended. Often, families spend more time with support staff and have discussions prior to interacting with you.
Anticipate questions about HPV. Why give the vaccine when the child is so young and not sexually active? Is my child really at risk? Is it safe? I read on the Internet. … Questions should be interpreted as a need for additional information and reassurance from you.
Remember to emphasize that HPV vaccine is important because it prevents cancer and it is most effective prior to exposure to HPV.
Additional resources to facilitate your discussions about HPV can be found at www.cdc.gov/hpv.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
It has been almost 10 years since the Advisory Committee on Immunization Practices (ACIP) recommended administration of human papillomavirus (HPV) vaccine for 11- to 12-year-old girls and young women up to 26 years of age. Routine administration in preteen boys and young adult males up to 21 years of age was recommended in 2011. An HPV series should be completed by 13 years. So how well are we protecting our patients?
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually among adolescents 13-17 years. Data are obtained from individuals from the 50 states, the District of Columbia, the U.S. Virgin Islands, and six major urban areas (MMWR. 2016 Aug 26;65[33]:850-8).
HPV vaccination continues to lag behind Tdap and the meningococcal conjugate vaccine (MCV), although each one is recommended to be administered at the 11- to 12-year visit. In 2015, coverage for receiving at least one dose of HPV vaccine among females was almost 62.8 % and for at least three doses was 41.9%; among males, coverage with at least one dose was 49.8% and for at least three doses was 28.1%. Compared with 2014, coverage for at least one dose of HPV vaccine increased 2.8% in females and 8.1% in males. Males also had a 7.6% for receipt of at least two doses of HPV vaccine, compared with 2014. HPV vaccine coverage in females aged 13 and younger also was lower than for those aged 15 and older. Coverage did not differ for males based on age.
HPV vaccination coverage also differed by state. In 2015, 28 states reported increased coverage in males, but only 7 states had increased coverage in females. Among all adolescents, coverage with at least one dose of HPV vaccine was 56.1%, at least two doses was 45.4%, and at least three doses was 34.9%. In contrast, 86.4% of all adolescents received at least one dose of Tdap, and 81.3% received at least one dose of MCV.
HPV-associated cancers
HPV is the most common sexually transmitted infection in both men and women. It is estimated that 79 million Americans are infected and 14 million new infections occur annually, usually in teens and young adults. Although most infections are asymptomatic and clear spontaneously, persistent infection with oncogenic types can progress to cancer. Cervical and oropharyngeal cancer were the most common HPV-associated cancers in women and men, respectively, in 2008-2012 (MMWR 2016;65:661-6).

All three HPV vaccines protect against HPV types 16 and 18. These types are estimated to account for the majority of cervical and oropharyngeal cancers, 66% and 62%, respectively. The additional types in the 9-valent HPV will protect against HPV types that cause approximately 15% of cervical cancers.
The association between HPV and cancer is clear. So why isn’t this vaccine being embraced? HPV vaccine is all about cancer prevention. Isn’t it? What are the barriers to HPV vaccination? Are parental concerns the only barrier? Are we recommending this vaccine as strongly as others?
Vaccine safety and efficacy
Safety has been a concern voiced by some parents. Collectively, HPV vaccines were studied in more than 95,000 individuals prior to licensure. Almost 90 million doses of vaccine have been distributed in the United States and more than 183 million, worldwide. The federal government utilizes three systems to monitor vaccine safety once a vaccine is licensed: The Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD), and the Clinical Immunization Safety Assessment (CISA) Network. Ongoing safety studies also are conducted by vaccine manufacturers. Since licensure, no serious safety concerns have been identified. Postvaccination syncope, first identified in the VAERS database in 2006, has declined since observation post injection was recommended by ACIP. Multiple studies in the United States and abroad have not demonstrated a causal association with HPV vaccine and any autoimmune and/or neurologic condition or increased risk for thromboembolism.
Mélanie Drolet, PhD, and her colleagues reviewed 20 studies in nine countries with at least 50% coverage in female adolescents aged 13-19 years. There was a 68% reduction in the prevalence of HPV types 16 and 18 and a 61% reduction in anal warts in the postvaccine era (Lancet Infect Dis. 2015 May;15[5]:565-80). Studies also indicate there is no indication of waning immunity.
Parental perceptions
Some parents feel the vaccine is not necessary because their child is not sexually active and/or is not at risk for acquiring a sexually transmitted infection. Others opt to delay initiation. NHANES (National Health and Nutrition Examination Survey) data from 2011 to 2014 revealed that among females aged 14-26 years whose age was known at the time of their first dose of HPV vaccine, 43% had reported having sex before or in the same year that they received their first dose.

One consistent reason parents indicate for not vaccinating is the lack of recommendation from their child’s health provider. Differences in age and sex recommendations also are reported. NIS-Teen 2013 demonstrated that parents of girls were more likely than parents of boys to receive a provider recommendation (65% vs.42%.) Only 29% of female parents indicated they’d received a provider recommendation to have their child vaccinated with HPV by ages 11-12 years.
Mandy A. Allison, MD, and her colleagues reviewed primary care physician perspectives about HPV vaccine in a national survey among 364 pediatricians and 218 family physicians (FPs). Although 84% of pediatricians and 75% of FPs indicated they always discuss HPV vaccination, only 60% of pediatricians and 59% of FPs strongly recommend HPV vaccine for 11- to 12-year-old girls; for boys it was 52% and 41%. More than half reported parental deferral. For pediatricians who almost never discussed the topic, the reasons included that the patient was not sexually active (54%), the child was young (38%), and the patient was already receiving other vaccines (35%) (Pediatrics. 2016 Feb;137[2]:e20152488).
Providers can be influenced by their perceptions of what value parents place on vaccines. In one study, parents were asked to put a value on specific vaccines. Providers were then asked to estimate how parents ranked the vaccines on a scale of 0-10. Providers underestimated the value placed on HPV vaccine (9.3 vs 5.2) (Vaccine 2014;32:579-84).
Improving HPV coverage: Preventing future HPV-related cancers
HPV vaccine should be recommended with as much conviction as Tdap and MCV at the 11- to 12-year visit for both girls and boys. Administration of all three should occur on the same day. Clinician recommendation is the No. 1 reason parents decide to vaccinate. The mantra “same way, same day” should become synonymous with the 11- to 12-year visit. All who have contact with the patient, beginning with the front desk staff, should know the importance of HPV vaccine, and when and why it is recommended. Often, families spend more time with support staff and have discussions prior to interacting with you.
Anticipate questions about HPV. Why give the vaccine when the child is so young and not sexually active? Is my child really at risk? Is it safe? I read on the Internet. … Questions should be interpreted as a need for additional information and reassurance from you.
Remember to emphasize that HPV vaccine is important because it prevents cancer and it is most effective prior to exposure to HPV.
Additional resources to facilitate your discussions about HPV can be found at www.cdc.gov/hpv.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Miscellany
Many interesting things happen in a medical office, most of which don’t merit a full column. Here are some from my own past few months:
Endocrine Knee? I was hard put to explain the calluses on both my patient’s knees. As I tried to formulate a question, he rescued me by saying, “I’m an endocrinologist. I spend a lot of my time on my knees, trimming the toenails of elderly diabetics.”
Who knew? At least bending the knee to insurers and regulators doesn’t require keratolytics ...
You can get anything online. My patient was about to graduate with a degree in psychoanalysis. “I have to set up my office,” she said, “drapes, analyst couch, and so forth.”
“Where do you buy an analyst couch?” I asked.
“Analyticcouch.com,” she explained. “Available in a variety of colors.”
What a country!
No I’m not, Officer! Many patients consider removing facial red spots that make them self-conscious, but Harriet’s reason was unique. “I got pulled over by a cop for an illegal change of lanes,” she said. “When he saw the red spot under my eye, he assumed I was a drunk. ‘Get over there, punk,’ he said.”
The other bathroom is upstairs. Stan listed his occupation as “muralist.” Picturing him sneaking up to blank walls on street corners in the middle of the night with a can of Benjamin Moore to ply his trade, I asked where he draws his murals.
“Most of my work is residential,” he said. “For instance, last year I did a bathroom in Framingham. The motif they wanted was ancient Egypt. I had to do a lot of research on the 18th dynasty, to get the details exactly right.”
That made sense. You wouldn’t want a dangling hieroglyphic participle in your downstairs lavatory. I asked him how it worked out.
“The client was delighted,” he said, “only there was one problem. Whenever guests came over for a dinner party, there was always a long line, because whoever was in the bathroom wouldn’t come out.”
There are always alternatives. By now I am used to hearing patients extol the virtues of exotic treatments: Vicks VapoRub for toenail tinea, tea tree oil for most anything. Apple cider vinegar for everything else.
Then the other day Marcy surprised me with this:
“I stopped the minocycline,” she said, “Instead I started using celery, which I ground up and boiled and then froze and then applied to the face.”
A little bit of a production, perhaps – grinding, boiling, freezing. As long as it works ...
You need a different kind of doctor. “I see I won’t be able to shower for 3 days,” said the new patient.
My jaw dropped, but no words came out.
“It’s that sign you put up,” he said, “right on the exam room door.”
As I don’t usually read my own signs, I turned to look. The sign read:
“If you have no-showed without notice three times, we reserve the right to reschedule you at our convenience.”
“It says, ‘No-Showed,” I said. Not ‘No Showers.”
I resisted the urge to refer him to an optometrist.
This reminded me of another episode some time ago, when a patient listed his Chief Complaint as, “I want Lasik Surgery.”
“Forgive me,” I said, “but why would you ask a dermatologist for Lasik surgery?”
“Doesn’t the sign on your door say, “Boston Ophthalmology?” he asked.
“Upstairs,” I said. “Seventh floor.”
Negotiating with Father Time. We suspected porphyria, and ordered a 24-hour urine collection. “I’m a busy executive,” said the patient. “I haven’t got time to collect it for that long.”
“But it has to be a whole day ...”
“Fifteen hours,” he said. “I’ll give you 15 hours.”
“But we need ...”
“Eighteen hours. OK?”
“Well, not really. You see, the test has to be a whole day ...”
“All right, 21 hours. That’s my best offer.”
Maybe if I could get him to spend the day in that Egyptian bathroom ...
Dr. Rockoff practices dermatology in Brookline, Mass, and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His new book, “Act Like a Doctor, Think Like a Patient,” is now available on amazon.com and barnesandnoble.com. This is his second book. Write to him at [email protected].
Many interesting things happen in a medical office, most of which don’t merit a full column. Here are some from my own past few months:
Endocrine Knee? I was hard put to explain the calluses on both my patient’s knees. As I tried to formulate a question, he rescued me by saying, “I’m an endocrinologist. I spend a lot of my time on my knees, trimming the toenails of elderly diabetics.”
Who knew? At least bending the knee to insurers and regulators doesn’t require keratolytics ...
You can get anything online. My patient was about to graduate with a degree in psychoanalysis. “I have to set up my office,” she said, “drapes, analyst couch, and so forth.”
“Where do you buy an analyst couch?” I asked.
“Analyticcouch.com,” she explained. “Available in a variety of colors.”
What a country!
No I’m not, Officer! Many patients consider removing facial red spots that make them self-conscious, but Harriet’s reason was unique. “I got pulled over by a cop for an illegal change of lanes,” she said. “When he saw the red spot under my eye, he assumed I was a drunk. ‘Get over there, punk,’ he said.”
The other bathroom is upstairs. Stan listed his occupation as “muralist.” Picturing him sneaking up to blank walls on street corners in the middle of the night with a can of Benjamin Moore to ply his trade, I asked where he draws his murals.
“Most of my work is residential,” he said. “For instance, last year I did a bathroom in Framingham. The motif they wanted was ancient Egypt. I had to do a lot of research on the 18th dynasty, to get the details exactly right.”
That made sense. You wouldn’t want a dangling hieroglyphic participle in your downstairs lavatory. I asked him how it worked out.
“The client was delighted,” he said, “only there was one problem. Whenever guests came over for a dinner party, there was always a long line, because whoever was in the bathroom wouldn’t come out.”
There are always alternatives. By now I am used to hearing patients extol the virtues of exotic treatments: Vicks VapoRub for toenail tinea, tea tree oil for most anything. Apple cider vinegar for everything else.
Then the other day Marcy surprised me with this:
“I stopped the minocycline,” she said, “Instead I started using celery, which I ground up and boiled and then froze and then applied to the face.”
A little bit of a production, perhaps – grinding, boiling, freezing. As long as it works ...
You need a different kind of doctor. “I see I won’t be able to shower for 3 days,” said the new patient.
My jaw dropped, but no words came out.
“It’s that sign you put up,” he said, “right on the exam room door.”
As I don’t usually read my own signs, I turned to look. The sign read:
“If you have no-showed without notice three times, we reserve the right to reschedule you at our convenience.”
“It says, ‘No-Showed,” I said. Not ‘No Showers.”
I resisted the urge to refer him to an optometrist.
This reminded me of another episode some time ago, when a patient listed his Chief Complaint as, “I want Lasik Surgery.”
“Forgive me,” I said, “but why would you ask a dermatologist for Lasik surgery?”
“Doesn’t the sign on your door say, “Boston Ophthalmology?” he asked.
“Upstairs,” I said. “Seventh floor.”
Negotiating with Father Time. We suspected porphyria, and ordered a 24-hour urine collection. “I’m a busy executive,” said the patient. “I haven’t got time to collect it for that long.”
“But it has to be a whole day ...”
“Fifteen hours,” he said. “I’ll give you 15 hours.”
“But we need ...”
“Eighteen hours. OK?”
“Well, not really. You see, the test has to be a whole day ...”
“All right, 21 hours. That’s my best offer.”
Maybe if I could get him to spend the day in that Egyptian bathroom ...
Dr. Rockoff practices dermatology in Brookline, Mass, and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His new book, “Act Like a Doctor, Think Like a Patient,” is now available on amazon.com and barnesandnoble.com. This is his second book. Write to him at [email protected].
Many interesting things happen in a medical office, most of which don’t merit a full column. Here are some from my own past few months:
Endocrine Knee? I was hard put to explain the calluses on both my patient’s knees. As I tried to formulate a question, he rescued me by saying, “I’m an endocrinologist. I spend a lot of my time on my knees, trimming the toenails of elderly diabetics.”
Who knew? At least bending the knee to insurers and regulators doesn’t require keratolytics ...
You can get anything online. My patient was about to graduate with a degree in psychoanalysis. “I have to set up my office,” she said, “drapes, analyst couch, and so forth.”
“Where do you buy an analyst couch?” I asked.
“Analyticcouch.com,” she explained. “Available in a variety of colors.”
What a country!
No I’m not, Officer! Many patients consider removing facial red spots that make them self-conscious, but Harriet’s reason was unique. “I got pulled over by a cop for an illegal change of lanes,” she said. “When he saw the red spot under my eye, he assumed I was a drunk. ‘Get over there, punk,’ he said.”
The other bathroom is upstairs. Stan listed his occupation as “muralist.” Picturing him sneaking up to blank walls on street corners in the middle of the night with a can of Benjamin Moore to ply his trade, I asked where he draws his murals.
“Most of my work is residential,” he said. “For instance, last year I did a bathroom in Framingham. The motif they wanted was ancient Egypt. I had to do a lot of research on the 18th dynasty, to get the details exactly right.”
That made sense. You wouldn’t want a dangling hieroglyphic participle in your downstairs lavatory. I asked him how it worked out.
“The client was delighted,” he said, “only there was one problem. Whenever guests came over for a dinner party, there was always a long line, because whoever was in the bathroom wouldn’t come out.”
There are always alternatives. By now I am used to hearing patients extol the virtues of exotic treatments: Vicks VapoRub for toenail tinea, tea tree oil for most anything. Apple cider vinegar for everything else.
Then the other day Marcy surprised me with this:
“I stopped the minocycline,” she said, “Instead I started using celery, which I ground up and boiled and then froze and then applied to the face.”
A little bit of a production, perhaps – grinding, boiling, freezing. As long as it works ...
You need a different kind of doctor. “I see I won’t be able to shower for 3 days,” said the new patient.
My jaw dropped, but no words came out.
“It’s that sign you put up,” he said, “right on the exam room door.”
As I don’t usually read my own signs, I turned to look. The sign read:
“If you have no-showed without notice three times, we reserve the right to reschedule you at our convenience.”
“It says, ‘No-Showed,” I said. Not ‘No Showers.”
I resisted the urge to refer him to an optometrist.
This reminded me of another episode some time ago, when a patient listed his Chief Complaint as, “I want Lasik Surgery.”
“Forgive me,” I said, “but why would you ask a dermatologist for Lasik surgery?”
“Doesn’t the sign on your door say, “Boston Ophthalmology?” he asked.
“Upstairs,” I said. “Seventh floor.”
Negotiating with Father Time. We suspected porphyria, and ordered a 24-hour urine collection. “I’m a busy executive,” said the patient. “I haven’t got time to collect it for that long.”
“But it has to be a whole day ...”
“Fifteen hours,” he said. “I’ll give you 15 hours.”
“But we need ...”
“Eighteen hours. OK?”
“Well, not really. You see, the test has to be a whole day ...”
“All right, 21 hours. That’s my best offer.”
Maybe if I could get him to spend the day in that Egyptian bathroom ...
Dr. Rockoff practices dermatology in Brookline, Mass, and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His new book, “Act Like a Doctor, Think Like a Patient,” is now available on amazon.com and barnesandnoble.com. This is his second book. Write to him at [email protected].
Do as I say, not as I do! A futile plea
I am constantly amazed when parents come in complaining about their child’s nail biting or irritable attitude “no matter how many times I tell her” as they do these same things in front of me!
We have not evolved that far from our nonverbal ancestors to expect that words will speak louder than actions. Looking closely, you can see even very young infants gazing closely at their parents, then mirroring their facial expressions a few minutes later (because of slower processing). Mirroring is probably the correct word for this as the mirror neuron system of the brain has as its primary and crucial function allowing humans to copy what they see in others.
Children look to model, especially those who are slightly older and more adept than they are. Older siblings bask in this adoration at times and squeal in frustration at other times that their younger sister is “mocking” them by copying their speech and actions. When children are picking up serious negative behaviors from siblings or peers, particularly in adolescence, we need to coach parents to take action.
But watching parents is the most powerful or “salient” stimulus for learning. Some theorize that the long period of childhood evolved to allow children to learn the incredible amount of information necessary to live independently in our complex culture. This learning begins very early and requires close contact and careful observation of the minute details of how the parent survives every day.
Eating is a great primitive example of why children must model their parents. How do animals know which plants are poisonous? By watching others eat and spit, choke, or vomit. Entire families have nonpreferred foods passed on by modeling refusal as well as lack of exposure on the table. Conversely, picky eaters need to observe others, preferably admired peers and parents, eating those vegetables. (Tasting is also necessary, but that’s a topic for another day!) It is worth asking about family meals, without the distraction of a TV, as they are key moments to model nutritious eating for their lifetime.
In “underdeveloped” countries, infants are naturally carried everywhere and observing constantly. In our “developed” country, many infants spend hours each day at day care, modeling their caregivers or watching media examples of people interacting, which may not be the models parents would consciously choose. Parents often ask us about childcare, anxious about the extremely rare threat of abduction, when we should instead be advising them about what models they want for their children during this critical learning period.
Emotion cueing is a crucial component of modeling and an untaught constant of typical parent-child interaction. Crawling infants placed on a clear surface over a “visual cliff” that appeared to be a sudden precipice look to the parent’s affect to decide how to act. The mother was instructed to show fear or joy when her baby reached the apparent danger and looked up for a warning. When fear was shown, the infants backed off and cried. When joy was shown, the baby crawled gaily across the “cliff.” For parents who do not come by signaling confidence naturally but want to model this for their children, I advise they “fake it until you make it!”
Parents are instructing their progeny in how to feel and act in every situation, whether they know it or not. Confident parents model bravery; kind parents model compassion; flexible parents model resilience; patient parents model tolerance; anxious parents model caution; angry parents model aggression. Ignoring parents (think: on their cellphone, distracted, depressed, inebriated, or high) leave their children to feel confused and insecure. An adaptive child of an ignoring parent may demand information by crying, clinging, fighting with siblings, or hitting the parent. They are desperate for the parental attention to teach them and keep them safe. A more passive child may become increasingly inhibited in their exploration of the world. We need to consider possible modeling failures when such child reaction patterns are the complaint, and remember that the adverse model may not be in the room, requiring us to ask, “What other adult models does he see?”
Studies have shown that infants learn resilience when experiencing “mistakes” in parent-infant interaction; learning how to tolerate and repair an interaction that is not perfect. This is really good news for parents who feel that they must be perfect models for their children! For parents of anxious or obsessive children, I sometimes prescribe making mistakes and saying “Oh well,” as well as rewarding the child when they can say “Oh well” themselves. No child is too old to benefit from observing a parent apologize sincerely for a mistake.
Language is modeled, right down to accent. But when parents complain about their child cussing, raising their voice in anger, having an “attitude,” or “talking back,” it is worth asking (parent and child) “Where do you think they/you have heard talk like that?” It may be childcare providers, peers, TV, video games or online media (all of which may warrant a change), but it also may reflect interactions at home.
Children make stronger memories when emotions are high as these may signal danger, making recall more salient to survival. This salience helps explain the lasting detriments to learning and health of growing up with psychological abuse, marital discord, partner violence, mental illness, or criminal behavior (among the Adverse Childhood Experiences). Such experiences cause stress and a sense of the world as a dangerous place, but also become models for the child’s own later relationships as adults. While unavoidable, they can be buffered by parents’ explaining them and providing alternative positive modeling.
Watching the parent conduct their craft, a key component of apprenticeship and family businesses in the past, has been replaced by YouTube and avatars for learning physical skills. But “modeling the process” of pride in craftsmanship, persistence in a task, and recovering and starting over when things go awry are omitted from training videos. These are good reasons to assure that parents do chores, crafts, cooking, or camping with their children as some things will surely go wrong, giving parents the chance to model resilience and problem solving!
Although teens may protest conversations and activities, they are watching their parents for how to be a spouse, a neighbor, a friend, a leader, a citizen. Parents, who may be cutting their teens loose, need to continue to expect/require participation in family meals and outings. Those are opportunities to model adult-level interactions with each other and with the community as well as to talk about their activities at work, in volunteering, in charity, and in religious practice. The moral development of the adolescent is shaped by what they see to the degree that when parents state one moral code but violate it themselves (for example, cheating on taxes, running red lights), the teen is less likely to follow the principles long term that the parents have verbalized.
Parents often relate to us as though we were their own parents. While this “projection” can interfere with disclosure on touchy subjects, it is also an opportunity for us to model ways of relating and reacting from sympathizing with the 4-year-old screaming about vaccines to asking an 8-year-old why he thinks his parents are getting a divorce.
Parenting (and being a pediatrician) is an opportunity to enjoy reliving your youth or to get a “do over” of parts you would like to have different for your child. Playfulness and silliness model joy for the child that can last a lifetime.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. Email her at [email protected].
I am constantly amazed when parents come in complaining about their child’s nail biting or irritable attitude “no matter how many times I tell her” as they do these same things in front of me!
We have not evolved that far from our nonverbal ancestors to expect that words will speak louder than actions. Looking closely, you can see even very young infants gazing closely at their parents, then mirroring their facial expressions a few minutes later (because of slower processing). Mirroring is probably the correct word for this as the mirror neuron system of the brain has as its primary and crucial function allowing humans to copy what they see in others.
Children look to model, especially those who are slightly older and more adept than they are. Older siblings bask in this adoration at times and squeal in frustration at other times that their younger sister is “mocking” them by copying their speech and actions. When children are picking up serious negative behaviors from siblings or peers, particularly in adolescence, we need to coach parents to take action.
But watching parents is the most powerful or “salient” stimulus for learning. Some theorize that the long period of childhood evolved to allow children to learn the incredible amount of information necessary to live independently in our complex culture. This learning begins very early and requires close contact and careful observation of the minute details of how the parent survives every day.
Eating is a great primitive example of why children must model their parents. How do animals know which plants are poisonous? By watching others eat and spit, choke, or vomit. Entire families have nonpreferred foods passed on by modeling refusal as well as lack of exposure on the table. Conversely, picky eaters need to observe others, preferably admired peers and parents, eating those vegetables. (Tasting is also necessary, but that’s a topic for another day!) It is worth asking about family meals, without the distraction of a TV, as they are key moments to model nutritious eating for their lifetime.
In “underdeveloped” countries, infants are naturally carried everywhere and observing constantly. In our “developed” country, many infants spend hours each day at day care, modeling their caregivers or watching media examples of people interacting, which may not be the models parents would consciously choose. Parents often ask us about childcare, anxious about the extremely rare threat of abduction, when we should instead be advising them about what models they want for their children during this critical learning period.
Emotion cueing is a crucial component of modeling and an untaught constant of typical parent-child interaction. Crawling infants placed on a clear surface over a “visual cliff” that appeared to be a sudden precipice look to the parent’s affect to decide how to act. The mother was instructed to show fear or joy when her baby reached the apparent danger and looked up for a warning. When fear was shown, the infants backed off and cried. When joy was shown, the baby crawled gaily across the “cliff.” For parents who do not come by signaling confidence naturally but want to model this for their children, I advise they “fake it until you make it!”
Parents are instructing their progeny in how to feel and act in every situation, whether they know it or not. Confident parents model bravery; kind parents model compassion; flexible parents model resilience; patient parents model tolerance; anxious parents model caution; angry parents model aggression. Ignoring parents (think: on their cellphone, distracted, depressed, inebriated, or high) leave their children to feel confused and insecure. An adaptive child of an ignoring parent may demand information by crying, clinging, fighting with siblings, or hitting the parent. They are desperate for the parental attention to teach them and keep them safe. A more passive child may become increasingly inhibited in their exploration of the world. We need to consider possible modeling failures when such child reaction patterns are the complaint, and remember that the adverse model may not be in the room, requiring us to ask, “What other adult models does he see?”
Studies have shown that infants learn resilience when experiencing “mistakes” in parent-infant interaction; learning how to tolerate and repair an interaction that is not perfect. This is really good news for parents who feel that they must be perfect models for their children! For parents of anxious or obsessive children, I sometimes prescribe making mistakes and saying “Oh well,” as well as rewarding the child when they can say “Oh well” themselves. No child is too old to benefit from observing a parent apologize sincerely for a mistake.
Language is modeled, right down to accent. But when parents complain about their child cussing, raising their voice in anger, having an “attitude,” or “talking back,” it is worth asking (parent and child) “Where do you think they/you have heard talk like that?” It may be childcare providers, peers, TV, video games or online media (all of which may warrant a change), but it also may reflect interactions at home.
Children make stronger memories when emotions are high as these may signal danger, making recall more salient to survival. This salience helps explain the lasting detriments to learning and health of growing up with psychological abuse, marital discord, partner violence, mental illness, or criminal behavior (among the Adverse Childhood Experiences). Such experiences cause stress and a sense of the world as a dangerous place, but also become models for the child’s own later relationships as adults. While unavoidable, they can be buffered by parents’ explaining them and providing alternative positive modeling.
Watching the parent conduct their craft, a key component of apprenticeship and family businesses in the past, has been replaced by YouTube and avatars for learning physical skills. But “modeling the process” of pride in craftsmanship, persistence in a task, and recovering and starting over when things go awry are omitted from training videos. These are good reasons to assure that parents do chores, crafts, cooking, or camping with their children as some things will surely go wrong, giving parents the chance to model resilience and problem solving!
Although teens may protest conversations and activities, they are watching their parents for how to be a spouse, a neighbor, a friend, a leader, a citizen. Parents, who may be cutting their teens loose, need to continue to expect/require participation in family meals and outings. Those are opportunities to model adult-level interactions with each other and with the community as well as to talk about their activities at work, in volunteering, in charity, and in religious practice. The moral development of the adolescent is shaped by what they see to the degree that when parents state one moral code but violate it themselves (for example, cheating on taxes, running red lights), the teen is less likely to follow the principles long term that the parents have verbalized.
Parents often relate to us as though we were their own parents. While this “projection” can interfere with disclosure on touchy subjects, it is also an opportunity for us to model ways of relating and reacting from sympathizing with the 4-year-old screaming about vaccines to asking an 8-year-old why he thinks his parents are getting a divorce.
Parenting (and being a pediatrician) is an opportunity to enjoy reliving your youth or to get a “do over” of parts you would like to have different for your child. Playfulness and silliness model joy for the child that can last a lifetime.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. Email her at [email protected].
I am constantly amazed when parents come in complaining about their child’s nail biting or irritable attitude “no matter how many times I tell her” as they do these same things in front of me!
We have not evolved that far from our nonverbal ancestors to expect that words will speak louder than actions. Looking closely, you can see even very young infants gazing closely at their parents, then mirroring their facial expressions a few minutes later (because of slower processing). Mirroring is probably the correct word for this as the mirror neuron system of the brain has as its primary and crucial function allowing humans to copy what they see in others.
Children look to model, especially those who are slightly older and more adept than they are. Older siblings bask in this adoration at times and squeal in frustration at other times that their younger sister is “mocking” them by copying their speech and actions. When children are picking up serious negative behaviors from siblings or peers, particularly in adolescence, we need to coach parents to take action.
But watching parents is the most powerful or “salient” stimulus for learning. Some theorize that the long period of childhood evolved to allow children to learn the incredible amount of information necessary to live independently in our complex culture. This learning begins very early and requires close contact and careful observation of the minute details of how the parent survives every day.
Eating is a great primitive example of why children must model their parents. How do animals know which plants are poisonous? By watching others eat and spit, choke, or vomit. Entire families have nonpreferred foods passed on by modeling refusal as well as lack of exposure on the table. Conversely, picky eaters need to observe others, preferably admired peers and parents, eating those vegetables. (Tasting is also necessary, but that’s a topic for another day!) It is worth asking about family meals, without the distraction of a TV, as they are key moments to model nutritious eating for their lifetime.
In “underdeveloped” countries, infants are naturally carried everywhere and observing constantly. In our “developed” country, many infants spend hours each day at day care, modeling their caregivers or watching media examples of people interacting, which may not be the models parents would consciously choose. Parents often ask us about childcare, anxious about the extremely rare threat of abduction, when we should instead be advising them about what models they want for their children during this critical learning period.
Emotion cueing is a crucial component of modeling and an untaught constant of typical parent-child interaction. Crawling infants placed on a clear surface over a “visual cliff” that appeared to be a sudden precipice look to the parent’s affect to decide how to act. The mother was instructed to show fear or joy when her baby reached the apparent danger and looked up for a warning. When fear was shown, the infants backed off and cried. When joy was shown, the baby crawled gaily across the “cliff.” For parents who do not come by signaling confidence naturally but want to model this for their children, I advise they “fake it until you make it!”
Parents are instructing their progeny in how to feel and act in every situation, whether they know it or not. Confident parents model bravery; kind parents model compassion; flexible parents model resilience; patient parents model tolerance; anxious parents model caution; angry parents model aggression. Ignoring parents (think: on their cellphone, distracted, depressed, inebriated, or high) leave their children to feel confused and insecure. An adaptive child of an ignoring parent may demand information by crying, clinging, fighting with siblings, or hitting the parent. They are desperate for the parental attention to teach them and keep them safe. A more passive child may become increasingly inhibited in their exploration of the world. We need to consider possible modeling failures when such child reaction patterns are the complaint, and remember that the adverse model may not be in the room, requiring us to ask, “What other adult models does he see?”
Studies have shown that infants learn resilience when experiencing “mistakes” in parent-infant interaction; learning how to tolerate and repair an interaction that is not perfect. This is really good news for parents who feel that they must be perfect models for their children! For parents of anxious or obsessive children, I sometimes prescribe making mistakes and saying “Oh well,” as well as rewarding the child when they can say “Oh well” themselves. No child is too old to benefit from observing a parent apologize sincerely for a mistake.
Language is modeled, right down to accent. But when parents complain about their child cussing, raising their voice in anger, having an “attitude,” or “talking back,” it is worth asking (parent and child) “Where do you think they/you have heard talk like that?” It may be childcare providers, peers, TV, video games or online media (all of which may warrant a change), but it also may reflect interactions at home.
Children make stronger memories when emotions are high as these may signal danger, making recall more salient to survival. This salience helps explain the lasting detriments to learning and health of growing up with psychological abuse, marital discord, partner violence, mental illness, or criminal behavior (among the Adverse Childhood Experiences). Such experiences cause stress and a sense of the world as a dangerous place, but also become models for the child’s own later relationships as adults. While unavoidable, they can be buffered by parents’ explaining them and providing alternative positive modeling.
Watching the parent conduct their craft, a key component of apprenticeship and family businesses in the past, has been replaced by YouTube and avatars for learning physical skills. But “modeling the process” of pride in craftsmanship, persistence in a task, and recovering and starting over when things go awry are omitted from training videos. These are good reasons to assure that parents do chores, crafts, cooking, or camping with their children as some things will surely go wrong, giving parents the chance to model resilience and problem solving!
Although teens may protest conversations and activities, they are watching their parents for how to be a spouse, a neighbor, a friend, a leader, a citizen. Parents, who may be cutting their teens loose, need to continue to expect/require participation in family meals and outings. Those are opportunities to model adult-level interactions with each other and with the community as well as to talk about their activities at work, in volunteering, in charity, and in religious practice. The moral development of the adolescent is shaped by what they see to the degree that when parents state one moral code but violate it themselves (for example, cheating on taxes, running red lights), the teen is less likely to follow the principles long term that the parents have verbalized.
Parents often relate to us as though we were their own parents. While this “projection” can interfere with disclosure on touchy subjects, it is also an opportunity for us to model ways of relating and reacting from sympathizing with the 4-year-old screaming about vaccines to asking an 8-year-old why he thinks his parents are getting a divorce.
Parenting (and being a pediatrician) is an opportunity to enjoy reliving your youth or to get a “do over” of parts you would like to have different for your child. Playfulness and silliness model joy for the child that can last a lifetime.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. Email her at [email protected].
Hospital managers identify barriers to antimicrobial stewardship
Antimicrobial stewardship programs are being introduced in hospitals internationally amidst the problem of escalating antimicrobial resistance. But sustained behavioral change in the area of antibiotic prescribing has been difficult to achieve.
While we have an understanding of doctors’ roles in antibiotic optimization within hospital contexts, the role of hospital management in successes or failures of antimicrobial stewardship programs (and optimization of antibiotics more broadly) has not been explored. Our new study published in the Journal of Hospital Infection examines this very question – the role of the manager as an enabler, or indeed a barrier, to antibiotic optimization.
Researchers in the study performed semistructured interviews with 23 hospital managers at three hospitals in two different states in Australia to specifically examine their opinions on antibiotic resistance, antibiotic governance, and their roles as senior management. The results illustrate how hospital managers prioritize financial pressures and immediate clinical demands over longer-term issues such as antibiotic prescribing and resistance. Here is an example of those pressures, described by one manager:
“I think the problem is [antimicrobial stewardship] in a competitive market. Are the waiting lists more newsworthy than antibiotic prescribing? Absolutely. You get more adverse events happening because of the waiting lists. … So, of course it’s not going to be the [antibiotic] prescribing that comes up to the top of that.” –Departmental Director
The study results also showed how managers find it challenging to comprehend, or act on the basis of, antibiotic-prescribing audits and had little faith in the value of data on antibiotic use and appropriateness. Other clinical areas with more clearly defined targets (and consequences for failing to meet targets) were prioritized over antibiotic prescribing in medical management decision making. Managers also found it difficult to influence the behavior of doctors and thought that it was a clinical responsibility to improve practice. In the words of one:
“I am a believer in delegated accountability and people on the shop floor knowing what they’re doing and being held accountable for it.” – Divisional Director
Managers perceived that there was limited accountability among doctors for antibiotic use and limited education and feedback to doctors:
“Those figures [on suboptimal prescribing] you give me, I haven’t heard them before. So, that in itself is a problem, and I would suggest you’d probably find a large number of medical staff haven’t been exposed to that.”
– Divisional Director
This study was performed in three hospitals with active antimicrobial stewardship programs. In Australia, as is becoming the pattern in countries within the Organisation for Economic Co-operation and Development (OECD), there is a legislative requirement for hospitals to have an effective antimicrobial stewardship program. And yet, meaningful sustained change in antibiotic prescribing is elusive, as evidenced by national antibiotic-prescribing data. The study results raise the important question of who is perceived as responsible for antibiotic-prescribing improvement and the actual and potential role of hospital managers in enacting change. It seems likely that both “top-down” influence (by managers and executive) and “bottom-up” influence (clinician-driven processes) will be required for effective and sustained practice change.
It is also clear from the results of this study that hospital managers do not perceive clear or immediate consequences for failing to improve antibiotic prescribing, and the perceived “distant” threat of antimicrobial resistance is not prioritized among other competing pressures. In addition, the widespread nature of antibiotic use makes it difficult to audit and even more difficult to communicate the extent of the problem.
These data would suggest that to move forward we need to look at an incentive structure for antibiotic-prescribing improvements or consequences in the short term for failing to optimize antibiotic use, and clearly defined goals for antibiotic optimization in hospitals.
Jennifer Broom, MBChB, PhD, is an infectious diseases physician at the Sunshine Coast Hospital and Health Service and an associate professor of medicine at the University of Queensland, Brisbane, Australia. Alex Broom, PhD, is professor of sociology in the School of Social Sciences at the University of New South Wales, Sydney.
Antimicrobial stewardship programs are being introduced in hospitals internationally amidst the problem of escalating antimicrobial resistance. But sustained behavioral change in the area of antibiotic prescribing has been difficult to achieve.
While we have an understanding of doctors’ roles in antibiotic optimization within hospital contexts, the role of hospital management in successes or failures of antimicrobial stewardship programs (and optimization of antibiotics more broadly) has not been explored. Our new study published in the Journal of Hospital Infection examines this very question – the role of the manager as an enabler, or indeed a barrier, to antibiotic optimization.
Researchers in the study performed semistructured interviews with 23 hospital managers at three hospitals in two different states in Australia to specifically examine their opinions on antibiotic resistance, antibiotic governance, and their roles as senior management. The results illustrate how hospital managers prioritize financial pressures and immediate clinical demands over longer-term issues such as antibiotic prescribing and resistance. Here is an example of those pressures, described by one manager:
“I think the problem is [antimicrobial stewardship] in a competitive market. Are the waiting lists more newsworthy than antibiotic prescribing? Absolutely. You get more adverse events happening because of the waiting lists. … So, of course it’s not going to be the [antibiotic] prescribing that comes up to the top of that.” –Departmental Director
The study results also showed how managers find it challenging to comprehend, or act on the basis of, antibiotic-prescribing audits and had little faith in the value of data on antibiotic use and appropriateness. Other clinical areas with more clearly defined targets (and consequences for failing to meet targets) were prioritized over antibiotic prescribing in medical management decision making. Managers also found it difficult to influence the behavior of doctors and thought that it was a clinical responsibility to improve practice. In the words of one:
“I am a believer in delegated accountability and people on the shop floor knowing what they’re doing and being held accountable for it.” – Divisional Director
Managers perceived that there was limited accountability among doctors for antibiotic use and limited education and feedback to doctors:
“Those figures [on suboptimal prescribing] you give me, I haven’t heard them before. So, that in itself is a problem, and I would suggest you’d probably find a large number of medical staff haven’t been exposed to that.”
– Divisional Director
This study was performed in three hospitals with active antimicrobial stewardship programs. In Australia, as is becoming the pattern in countries within the Organisation for Economic Co-operation and Development (OECD), there is a legislative requirement for hospitals to have an effective antimicrobial stewardship program. And yet, meaningful sustained change in antibiotic prescribing is elusive, as evidenced by national antibiotic-prescribing data. The study results raise the important question of who is perceived as responsible for antibiotic-prescribing improvement and the actual and potential role of hospital managers in enacting change. It seems likely that both “top-down” influence (by managers and executive) and “bottom-up” influence (clinician-driven processes) will be required for effective and sustained practice change.
It is also clear from the results of this study that hospital managers do not perceive clear or immediate consequences for failing to improve antibiotic prescribing, and the perceived “distant” threat of antimicrobial resistance is not prioritized among other competing pressures. In addition, the widespread nature of antibiotic use makes it difficult to audit and even more difficult to communicate the extent of the problem.
These data would suggest that to move forward we need to look at an incentive structure for antibiotic-prescribing improvements or consequences in the short term for failing to optimize antibiotic use, and clearly defined goals for antibiotic optimization in hospitals.
Jennifer Broom, MBChB, PhD, is an infectious diseases physician at the Sunshine Coast Hospital and Health Service and an associate professor of medicine at the University of Queensland, Brisbane, Australia. Alex Broom, PhD, is professor of sociology in the School of Social Sciences at the University of New South Wales, Sydney.
Antimicrobial stewardship programs are being introduced in hospitals internationally amidst the problem of escalating antimicrobial resistance. But sustained behavioral change in the area of antibiotic prescribing has been difficult to achieve.
While we have an understanding of doctors’ roles in antibiotic optimization within hospital contexts, the role of hospital management in successes or failures of antimicrobial stewardship programs (and optimization of antibiotics more broadly) has not been explored. Our new study published in the Journal of Hospital Infection examines this very question – the role of the manager as an enabler, or indeed a barrier, to antibiotic optimization.
Researchers in the study performed semistructured interviews with 23 hospital managers at three hospitals in two different states in Australia to specifically examine their opinions on antibiotic resistance, antibiotic governance, and their roles as senior management. The results illustrate how hospital managers prioritize financial pressures and immediate clinical demands over longer-term issues such as antibiotic prescribing and resistance. Here is an example of those pressures, described by one manager:
“I think the problem is [antimicrobial stewardship] in a competitive market. Are the waiting lists more newsworthy than antibiotic prescribing? Absolutely. You get more adverse events happening because of the waiting lists. … So, of course it’s not going to be the [antibiotic] prescribing that comes up to the top of that.” –Departmental Director
The study results also showed how managers find it challenging to comprehend, or act on the basis of, antibiotic-prescribing audits and had little faith in the value of data on antibiotic use and appropriateness. Other clinical areas with more clearly defined targets (and consequences for failing to meet targets) were prioritized over antibiotic prescribing in medical management decision making. Managers also found it difficult to influence the behavior of doctors and thought that it was a clinical responsibility to improve practice. In the words of one:
“I am a believer in delegated accountability and people on the shop floor knowing what they’re doing and being held accountable for it.” – Divisional Director
Managers perceived that there was limited accountability among doctors for antibiotic use and limited education and feedback to doctors:
“Those figures [on suboptimal prescribing] you give me, I haven’t heard them before. So, that in itself is a problem, and I would suggest you’d probably find a large number of medical staff haven’t been exposed to that.”
– Divisional Director
This study was performed in three hospitals with active antimicrobial stewardship programs. In Australia, as is becoming the pattern in countries within the Organisation for Economic Co-operation and Development (OECD), there is a legislative requirement for hospitals to have an effective antimicrobial stewardship program. And yet, meaningful sustained change in antibiotic prescribing is elusive, as evidenced by national antibiotic-prescribing data. The study results raise the important question of who is perceived as responsible for antibiotic-prescribing improvement and the actual and potential role of hospital managers in enacting change. It seems likely that both “top-down” influence (by managers and executive) and “bottom-up” influence (clinician-driven processes) will be required for effective and sustained practice change.
It is also clear from the results of this study that hospital managers do not perceive clear or immediate consequences for failing to improve antibiotic prescribing, and the perceived “distant” threat of antimicrobial resistance is not prioritized among other competing pressures. In addition, the widespread nature of antibiotic use makes it difficult to audit and even more difficult to communicate the extent of the problem.
These data would suggest that to move forward we need to look at an incentive structure for antibiotic-prescribing improvements or consequences in the short term for failing to optimize antibiotic use, and clearly defined goals for antibiotic optimization in hospitals.
Jennifer Broom, MBChB, PhD, is an infectious diseases physician at the Sunshine Coast Hospital and Health Service and an associate professor of medicine at the University of Queensland, Brisbane, Australia. Alex Broom, PhD, is professor of sociology in the School of Social Sciences at the University of New South Wales, Sydney.
Parenting: Tips on discussing a tough but important topic
It seems like the field of psychiatry has been all over the map when it comes to viewing the importance of parenting with regard to child behavioral problems and disorders. For decades, we heard that parents, particularly mothers, were to blame for everything from childhood autism to excessive temper tantrums.1 Then, parenting somehow got somewhat pushed aside as the genetic and biological underpinnings of behavior became increasingly appreciated. For a while, parenting was nearly relegated to epiphenomenon status – that is, an almost irrelevant reaction to genetically driven child behavior.
More recently, it appears that some semblance of balance has been restored with parenting behavior being appreciated as critically important in the development of a child, but in the context of many other mutually interacting factors.2 There also is a far greater understanding that child behavior and parent behavior is very much a two-way street.
These more nuanced and neuroscience-backed perspectives, however, don’t make bringing up the subject of parenting any easier. In part because of how seriously most mothers, fathers, and other caretakers take their job as a parent, it can be easy to put parents on the defensive, especially when one of their children is struggling behaviorally. At the same time, taking the easy way out by giving boilerplate advice, or even avoiding the topic of parenting completely, is a huge missed opportunity to engage families who often are desperately seeking some guidance.
Case summary
Emily is a healthy 6-year-old girl who comes in with her single mother and her two younger siblings for an annual exam. Her mother proudly reports that she is doing great at school, but seems reluctant to say much about her home life. The mother seems somewhat frazzled, and the interview is difficult because the three siblings are arguing with each other. After Emily and her sister fight over reading the same book, the mother suddenly and quite loudly says, “Can you just let me talk for 1 second!”
Discussion
Pediatricians often have strong suspicions that parents are struggling with a child’s behavior but can have trouble knowing how exactly to bring up the subject of parenting. Some specific suggestions for having productive discussions on parenting include the following:
• Think about the statements embedded in your questions. A screening question about parenting such as, “Can you tell me about the areas of parenting that you are most proud of and the areas where you feel you need the most help?” helps a parent understand that you assume that no parent is perfect and that everyone has areas of strength and weakness.
• Compliment when you can. Related to the above, find those areas of positive parenting, even if it involves effort more than results, and communicate that you have noticed them. This can make talking about the weaknesses a little easier to hear for the parent.
• Frame the issue in terms of surpluses rather than deficits. Instead of coming from the perspective that a parent is deficient in their basic parenting skills, reframe the challenge as someone needing “superparent” skills to manage multiple or more challenging children. The often-heard statements that “kids don’t come with instruction books” or “you need to earn a license to drive a car but not raise a child” are almost cliché these days, but still convey to parents that you understand how difficult parenting can be. In some cases, it may be appropriate to disclose some parenting challenges you have experienced firsthand.
• Get details. Before launching into specific recommendations, ask yourself if you are able to really see the issue a parent is describing. Rather than reviewing a laundry list of sleep hygiene recommendations, for example, it can be very worthwhile to ask, “How exactly does bedtime work at your home?” Getting all the details can not only build empathy, but allow you to really see specific areas for improvement. If you can’t paint a picture of how a scene might really look at this patient’s home, there likely is more to learn.
Of course, one of the key challenges here is time. Really giving these parenting concerns the time they deserve usually means going beyond the precious few minutes pediatricians have for a well visit. In these instances, it may be worth scheduling a future appointment that is exclusively devoted to this issue. Alternatively, a referral can be made to a therapist, counselor, or parent “coach” to give a family greater opportunity to work 1:1 with a professional. When you do this, be clear that you are looking for a therapist to work with the whole family, ideally using many of the evidence-based techniques that have been shown to be effective. A list of manual-based treatments as well as some books that parents could read on their own to address oppositional-defiant behavior is available, including a guide for families from the American Academy from Child and Adolescent Psychiatry.3
Case follow-up
The pediatrician finds another book to satisfy the younger sibling and says to the mother, “I’m glad to see that at least they are fighting over a book. That’s great that you have taught them to like reading.” They commiserate about how difficult it is to raise three young children as a single parent, and the mother then begins to open up about Emily’s defiant and disrespectful behavior at home that the mother blames on herself. The pediatrician offers a referral to see a local family therapist, which the mother gratefully accepts.
References
1. Am J Orthopsychiatry. 1985 Jul;55(3):345-53.
2. Child Adolesc Psychiatr Clin N Am. 2016 Apr;25(2):167-78.
3. American Academy of Child and Adolescent Psychiatry. (2009). Oppositional Defiant Disorder: A Guide for Families.
Dr. Rettew is associate professor of psychiatry and pediatrics at the University of Vermont, Burlington. Email him at [email protected].
It seems like the field of psychiatry has been all over the map when it comes to viewing the importance of parenting with regard to child behavioral problems and disorders. For decades, we heard that parents, particularly mothers, were to blame for everything from childhood autism to excessive temper tantrums.1 Then, parenting somehow got somewhat pushed aside as the genetic and biological underpinnings of behavior became increasingly appreciated. For a while, parenting was nearly relegated to epiphenomenon status – that is, an almost irrelevant reaction to genetically driven child behavior.
More recently, it appears that some semblance of balance has been restored with parenting behavior being appreciated as critically important in the development of a child, but in the context of many other mutually interacting factors.2 There also is a far greater understanding that child behavior and parent behavior is very much a two-way street.
These more nuanced and neuroscience-backed perspectives, however, don’t make bringing up the subject of parenting any easier. In part because of how seriously most mothers, fathers, and other caretakers take their job as a parent, it can be easy to put parents on the defensive, especially when one of their children is struggling behaviorally. At the same time, taking the easy way out by giving boilerplate advice, or even avoiding the topic of parenting completely, is a huge missed opportunity to engage families who often are desperately seeking some guidance.
Case summary
Emily is a healthy 6-year-old girl who comes in with her single mother and her two younger siblings for an annual exam. Her mother proudly reports that she is doing great at school, but seems reluctant to say much about her home life. The mother seems somewhat frazzled, and the interview is difficult because the three siblings are arguing with each other. After Emily and her sister fight over reading the same book, the mother suddenly and quite loudly says, “Can you just let me talk for 1 second!”
Discussion
Pediatricians often have strong suspicions that parents are struggling with a child’s behavior but can have trouble knowing how exactly to bring up the subject of parenting. Some specific suggestions for having productive discussions on parenting include the following:
• Think about the statements embedded in your questions. A screening question about parenting such as, “Can you tell me about the areas of parenting that you are most proud of and the areas where you feel you need the most help?” helps a parent understand that you assume that no parent is perfect and that everyone has areas of strength and weakness.
• Compliment when you can. Related to the above, find those areas of positive parenting, even if it involves effort more than results, and communicate that you have noticed them. This can make talking about the weaknesses a little easier to hear for the parent.
• Frame the issue in terms of surpluses rather than deficits. Instead of coming from the perspective that a parent is deficient in their basic parenting skills, reframe the challenge as someone needing “superparent” skills to manage multiple or more challenging children. The often-heard statements that “kids don’t come with instruction books” or “you need to earn a license to drive a car but not raise a child” are almost cliché these days, but still convey to parents that you understand how difficult parenting can be. In some cases, it may be appropriate to disclose some parenting challenges you have experienced firsthand.
• Get details. Before launching into specific recommendations, ask yourself if you are able to really see the issue a parent is describing. Rather than reviewing a laundry list of sleep hygiene recommendations, for example, it can be very worthwhile to ask, “How exactly does bedtime work at your home?” Getting all the details can not only build empathy, but allow you to really see specific areas for improvement. If you can’t paint a picture of how a scene might really look at this patient’s home, there likely is more to learn.
Of course, one of the key challenges here is time. Really giving these parenting concerns the time they deserve usually means going beyond the precious few minutes pediatricians have for a well visit. In these instances, it may be worth scheduling a future appointment that is exclusively devoted to this issue. Alternatively, a referral can be made to a therapist, counselor, or parent “coach” to give a family greater opportunity to work 1:1 with a professional. When you do this, be clear that you are looking for a therapist to work with the whole family, ideally using many of the evidence-based techniques that have been shown to be effective. A list of manual-based treatments as well as some books that parents could read on their own to address oppositional-defiant behavior is available, including a guide for families from the American Academy from Child and Adolescent Psychiatry.3
Case follow-up
The pediatrician finds another book to satisfy the younger sibling and says to the mother, “I’m glad to see that at least they are fighting over a book. That’s great that you have taught them to like reading.” They commiserate about how difficult it is to raise three young children as a single parent, and the mother then begins to open up about Emily’s defiant and disrespectful behavior at home that the mother blames on herself. The pediatrician offers a referral to see a local family therapist, which the mother gratefully accepts.
References
1. Am J Orthopsychiatry. 1985 Jul;55(3):345-53.
2. Child Adolesc Psychiatr Clin N Am. 2016 Apr;25(2):167-78.
3. American Academy of Child and Adolescent Psychiatry. (2009). Oppositional Defiant Disorder: A Guide for Families.
Dr. Rettew is associate professor of psychiatry and pediatrics at the University of Vermont, Burlington. Email him at [email protected].
It seems like the field of psychiatry has been all over the map when it comes to viewing the importance of parenting with regard to child behavioral problems and disorders. For decades, we heard that parents, particularly mothers, were to blame for everything from childhood autism to excessive temper tantrums.1 Then, parenting somehow got somewhat pushed aside as the genetic and biological underpinnings of behavior became increasingly appreciated. For a while, parenting was nearly relegated to epiphenomenon status – that is, an almost irrelevant reaction to genetically driven child behavior.
More recently, it appears that some semblance of balance has been restored with parenting behavior being appreciated as critically important in the development of a child, but in the context of many other mutually interacting factors.2 There also is a far greater understanding that child behavior and parent behavior is very much a two-way street.
These more nuanced and neuroscience-backed perspectives, however, don’t make bringing up the subject of parenting any easier. In part because of how seriously most mothers, fathers, and other caretakers take their job as a parent, it can be easy to put parents on the defensive, especially when one of their children is struggling behaviorally. At the same time, taking the easy way out by giving boilerplate advice, or even avoiding the topic of parenting completely, is a huge missed opportunity to engage families who often are desperately seeking some guidance.
Case summary
Emily is a healthy 6-year-old girl who comes in with her single mother and her two younger siblings for an annual exam. Her mother proudly reports that she is doing great at school, but seems reluctant to say much about her home life. The mother seems somewhat frazzled, and the interview is difficult because the three siblings are arguing with each other. After Emily and her sister fight over reading the same book, the mother suddenly and quite loudly says, “Can you just let me talk for 1 second!”
Discussion
Pediatricians often have strong suspicions that parents are struggling with a child’s behavior but can have trouble knowing how exactly to bring up the subject of parenting. Some specific suggestions for having productive discussions on parenting include the following:
• Think about the statements embedded in your questions. A screening question about parenting such as, “Can you tell me about the areas of parenting that you are most proud of and the areas where you feel you need the most help?” helps a parent understand that you assume that no parent is perfect and that everyone has areas of strength and weakness.
• Compliment when you can. Related to the above, find those areas of positive parenting, even if it involves effort more than results, and communicate that you have noticed them. This can make talking about the weaknesses a little easier to hear for the parent.
• Frame the issue in terms of surpluses rather than deficits. Instead of coming from the perspective that a parent is deficient in their basic parenting skills, reframe the challenge as someone needing “superparent” skills to manage multiple or more challenging children. The often-heard statements that “kids don’t come with instruction books” or “you need to earn a license to drive a car but not raise a child” are almost cliché these days, but still convey to parents that you understand how difficult parenting can be. In some cases, it may be appropriate to disclose some parenting challenges you have experienced firsthand.
• Get details. Before launching into specific recommendations, ask yourself if you are able to really see the issue a parent is describing. Rather than reviewing a laundry list of sleep hygiene recommendations, for example, it can be very worthwhile to ask, “How exactly does bedtime work at your home?” Getting all the details can not only build empathy, but allow you to really see specific areas for improvement. If you can’t paint a picture of how a scene might really look at this patient’s home, there likely is more to learn.
Of course, one of the key challenges here is time. Really giving these parenting concerns the time they deserve usually means going beyond the precious few minutes pediatricians have for a well visit. In these instances, it may be worth scheduling a future appointment that is exclusively devoted to this issue. Alternatively, a referral can be made to a therapist, counselor, or parent “coach” to give a family greater opportunity to work 1:1 with a professional. When you do this, be clear that you are looking for a therapist to work with the whole family, ideally using many of the evidence-based techniques that have been shown to be effective. A list of manual-based treatments as well as some books that parents could read on their own to address oppositional-defiant behavior is available, including a guide for families from the American Academy from Child and Adolescent Psychiatry.3
Case follow-up
The pediatrician finds another book to satisfy the younger sibling and says to the mother, “I’m glad to see that at least they are fighting over a book. That’s great that you have taught them to like reading.” They commiserate about how difficult it is to raise three young children as a single parent, and the mother then begins to open up about Emily’s defiant and disrespectful behavior at home that the mother blames on herself. The pediatrician offers a referral to see a local family therapist, which the mother gratefully accepts.
References
1. Am J Orthopsychiatry. 1985 Jul;55(3):345-53.
2. Child Adolesc Psychiatr Clin N Am. 2016 Apr;25(2):167-78.
3. American Academy of Child and Adolescent Psychiatry. (2009). Oppositional Defiant Disorder: A Guide for Families.
Dr. Rettew is associate professor of psychiatry and pediatrics at the University of Vermont, Burlington. Email him at [email protected].
Back pain: Let’s get it straight!
Back pain is a common complaint among adolescents. But there are several misconceptions about back pain that lead to excessive referrals and unnecessary imaging.
One of the most common of these misconceptions is that back pain in adolescents is caused by their carrying a heavy backpack. When this was researched, carrying a heavy backpack had a weak association with back pain,1,2 but because so many relate the two, the American Academy of Pediatrics came out with recommendations limiting the weight to 20% of the child’s body weight.3
Another misconception regarding back pain in children is that the cause is likely serious and needs a prompt work-up. A study followed 560 children who were diagnosed with low back pain and idiopathic scoliosis. In only 9% of cases was an underlying pathologic condition found: 29 patients had spondylolysis or spondylolisthesis, 9 had Scheuermann’s kyphosis, 5 had a syrinx, 2 had a herniated disk, 1 had hydromyelia, 1 had a tethered cord, and 1 had an intraspinal tumor.4
As with all diagnoses, a detailed history is very important. Is the pain acute or chronic? Does the pain radiate? Is there nighttime pain? Where exactly is the pain? Commonly, with acute onset there is an association of new activity or trauma. A good social history is also important because complaints of back pain are high on the list with psychosomatic issues as well.
The physical exam should include inspection of the back with the patient standing, bent forward, and in a hyperextended position. Identifying curvatures of the spine and pinpointing what increases the pain are helpful in getting the correct diagnosis. Middle back pain associated with an excessively rounded back, or kyphosis of the thoracic spine, is known as Scheuermann’s kyphosis. This is due to the vertebrae becoming wedged.5
Body habitus also is important to note. Adolescents with large breasts may complain of upper back pain especially, if they are not wearing a supportive bra. Weak abdominal muscles can also give rise to back pain, most commonly in the lower back. Tight hamstrings can cause posterior rotation of the pelvis, which can result in lumbosacral pain.
More worrisome causes of back pain are spondylosis or stress fractures; these are usually caused by a sports trauma such as gymnastics or diving, but also can be caused by a rapid growth spurt.1 Pain is usually mild initially. Spondylolisthesis is the forward movement of one vertebra on another. This causes pain in the lumbosacral area, with radiation into the lower extremities and weakness.4
Infection and tumor are rare causes of low back pain. Both can cause nighttime pain. Infection in the intervertebral disk space, or diskitis, is more common in younger children. Pain can be described in the back or the abdominal area.1 Limping or refusal to walk are also noted.
Tumors, although rare, are what parents worry the most about. Osteoid osteoma is the most common form, and should be considered whenever a scoliosis is of new onset or advances quickly.6
A work-up for acute low back pain associated with minor trauma and no radiation or limitation to movement can be done conservatively. If the physical exam reveals muscle imbalances or tightness and abdominal weakness, referral to physical therapy is warranted.
If low back pain is associated with abnormal findings, then an x-ray should be done. Anteroposterior and lateral views are usually sufficient. Oblique views should be added if there is suspicion of a stress fracture.
Referral to an orthopedist is warranted if there are any bowel or bladder changes, significant weakness, and/or sensory changes. A bone scan, CT scan and MRI are more definitive tests. The bone scan is not very sensitive (61%) but has a high specificity (80%). CT scans show soft tissue, but no marrow elements are seen, so it is not as helpful with a herniated disk.7
In this ever-changing medical arena, taking a detailed history and doing a good exam will not only save a lot of time but also decrease the number of unnecessary referrals, which is the name of the game!
References
1. J Pediatr Orthop. 2006 May-Jun;26(3):358-63.
2. Pediatrics. 2003 Apr;111(4 Pt 1):822-8.
3. American Academy of Pediatrics. Backpack safety.
4. J Bone Joint Surg Am. 1997 Mar;79(3):364-8.
6. J Paediatr Child Health. 2000 Jun;36(3):208-12.
7. Skeletal Radiol. 2005 Feb;34(2):63-73.
Dr. Pearce is a pediatrician in Frankfort, Ill.
Back pain is a common complaint among adolescents. But there are several misconceptions about back pain that lead to excessive referrals and unnecessary imaging.
One of the most common of these misconceptions is that back pain in adolescents is caused by their carrying a heavy backpack. When this was researched, carrying a heavy backpack had a weak association with back pain,1,2 but because so many relate the two, the American Academy of Pediatrics came out with recommendations limiting the weight to 20% of the child’s body weight.3
Another misconception regarding back pain in children is that the cause is likely serious and needs a prompt work-up. A study followed 560 children who were diagnosed with low back pain and idiopathic scoliosis. In only 9% of cases was an underlying pathologic condition found: 29 patients had spondylolysis or spondylolisthesis, 9 had Scheuermann’s kyphosis, 5 had a syrinx, 2 had a herniated disk, 1 had hydromyelia, 1 had a tethered cord, and 1 had an intraspinal tumor.4
As with all diagnoses, a detailed history is very important. Is the pain acute or chronic? Does the pain radiate? Is there nighttime pain? Where exactly is the pain? Commonly, with acute onset there is an association of new activity or trauma. A good social history is also important because complaints of back pain are high on the list with psychosomatic issues as well.
The physical exam should include inspection of the back with the patient standing, bent forward, and in a hyperextended position. Identifying curvatures of the spine and pinpointing what increases the pain are helpful in getting the correct diagnosis. Middle back pain associated with an excessively rounded back, or kyphosis of the thoracic spine, is known as Scheuermann’s kyphosis. This is due to the vertebrae becoming wedged.5
Body habitus also is important to note. Adolescents with large breasts may complain of upper back pain especially, if they are not wearing a supportive bra. Weak abdominal muscles can also give rise to back pain, most commonly in the lower back. Tight hamstrings can cause posterior rotation of the pelvis, which can result in lumbosacral pain.
More worrisome causes of back pain are spondylosis or stress fractures; these are usually caused by a sports trauma such as gymnastics or diving, but also can be caused by a rapid growth spurt.1 Pain is usually mild initially. Spondylolisthesis is the forward movement of one vertebra on another. This causes pain in the lumbosacral area, with radiation into the lower extremities and weakness.4
Infection and tumor are rare causes of low back pain. Both can cause nighttime pain. Infection in the intervertebral disk space, or diskitis, is more common in younger children. Pain can be described in the back or the abdominal area.1 Limping or refusal to walk are also noted.
Tumors, although rare, are what parents worry the most about. Osteoid osteoma is the most common form, and should be considered whenever a scoliosis is of new onset or advances quickly.6
A work-up for acute low back pain associated with minor trauma and no radiation or limitation to movement can be done conservatively. If the physical exam reveals muscle imbalances or tightness and abdominal weakness, referral to physical therapy is warranted.
If low back pain is associated with abnormal findings, then an x-ray should be done. Anteroposterior and lateral views are usually sufficient. Oblique views should be added if there is suspicion of a stress fracture.
Referral to an orthopedist is warranted if there are any bowel or bladder changes, significant weakness, and/or sensory changes. A bone scan, CT scan and MRI are more definitive tests. The bone scan is not very sensitive (61%) but has a high specificity (80%). CT scans show soft tissue, but no marrow elements are seen, so it is not as helpful with a herniated disk.7
In this ever-changing medical arena, taking a detailed history and doing a good exam will not only save a lot of time but also decrease the number of unnecessary referrals, which is the name of the game!
References
1. J Pediatr Orthop. 2006 May-Jun;26(3):358-63.
2. Pediatrics. 2003 Apr;111(4 Pt 1):822-8.
3. American Academy of Pediatrics. Backpack safety.
4. J Bone Joint Surg Am. 1997 Mar;79(3):364-8.
6. J Paediatr Child Health. 2000 Jun;36(3):208-12.
7. Skeletal Radiol. 2005 Feb;34(2):63-73.
Dr. Pearce is a pediatrician in Frankfort, Ill.
Back pain is a common complaint among adolescents. But there are several misconceptions about back pain that lead to excessive referrals and unnecessary imaging.
One of the most common of these misconceptions is that back pain in adolescents is caused by their carrying a heavy backpack. When this was researched, carrying a heavy backpack had a weak association with back pain,1,2 but because so many relate the two, the American Academy of Pediatrics came out with recommendations limiting the weight to 20% of the child’s body weight.3
Another misconception regarding back pain in children is that the cause is likely serious and needs a prompt work-up. A study followed 560 children who were diagnosed with low back pain and idiopathic scoliosis. In only 9% of cases was an underlying pathologic condition found: 29 patients had spondylolysis or spondylolisthesis, 9 had Scheuermann’s kyphosis, 5 had a syrinx, 2 had a herniated disk, 1 had hydromyelia, 1 had a tethered cord, and 1 had an intraspinal tumor.4
As with all diagnoses, a detailed history is very important. Is the pain acute or chronic? Does the pain radiate? Is there nighttime pain? Where exactly is the pain? Commonly, with acute onset there is an association of new activity or trauma. A good social history is also important because complaints of back pain are high on the list with psychosomatic issues as well.
The physical exam should include inspection of the back with the patient standing, bent forward, and in a hyperextended position. Identifying curvatures of the spine and pinpointing what increases the pain are helpful in getting the correct diagnosis. Middle back pain associated with an excessively rounded back, or kyphosis of the thoracic spine, is known as Scheuermann’s kyphosis. This is due to the vertebrae becoming wedged.5
Body habitus also is important to note. Adolescents with large breasts may complain of upper back pain especially, if they are not wearing a supportive bra. Weak abdominal muscles can also give rise to back pain, most commonly in the lower back. Tight hamstrings can cause posterior rotation of the pelvis, which can result in lumbosacral pain.
More worrisome causes of back pain are spondylosis or stress fractures; these are usually caused by a sports trauma such as gymnastics or diving, but also can be caused by a rapid growth spurt.1 Pain is usually mild initially. Spondylolisthesis is the forward movement of one vertebra on another. This causes pain in the lumbosacral area, with radiation into the lower extremities and weakness.4
Infection and tumor are rare causes of low back pain. Both can cause nighttime pain. Infection in the intervertebral disk space, or diskitis, is more common in younger children. Pain can be described in the back or the abdominal area.1 Limping or refusal to walk are also noted.
Tumors, although rare, are what parents worry the most about. Osteoid osteoma is the most common form, and should be considered whenever a scoliosis is of new onset or advances quickly.6
A work-up for acute low back pain associated with minor trauma and no radiation or limitation to movement can be done conservatively. If the physical exam reveals muscle imbalances or tightness and abdominal weakness, referral to physical therapy is warranted.
If low back pain is associated with abnormal findings, then an x-ray should be done. Anteroposterior and lateral views are usually sufficient. Oblique views should be added if there is suspicion of a stress fracture.
Referral to an orthopedist is warranted if there are any bowel or bladder changes, significant weakness, and/or sensory changes. A bone scan, CT scan and MRI are more definitive tests. The bone scan is not very sensitive (61%) but has a high specificity (80%). CT scans show soft tissue, but no marrow elements are seen, so it is not as helpful with a herniated disk.7
In this ever-changing medical arena, taking a detailed history and doing a good exam will not only save a lot of time but also decrease the number of unnecessary referrals, which is the name of the game!
References
1. J Pediatr Orthop. 2006 May-Jun;26(3):358-63.
2. Pediatrics. 2003 Apr;111(4 Pt 1):822-8.
3. American Academy of Pediatrics. Backpack safety.
4. J Bone Joint Surg Am. 1997 Mar;79(3):364-8.
6. J Paediatr Child Health. 2000 Jun;36(3):208-12.
7. Skeletal Radiol. 2005 Feb;34(2):63-73.
Dr. Pearce is a pediatrician in Frankfort, Ill.
DEA licenses: Is it time for a graduated approach?
I sometimes think about giving up my DEA certificate. I can’t be the only one.
It’s not strictly about the money. $731 every 3 years is small change, compared with my rent, staff salaries, and malpractice insurance, although the savings would be nice.
Part of it is the desire to unfetter myself from prescribing controlled substances. I wouldn’t have to worry about drug seekers hitting me up, or dealing with the paperwork and pharmacy calls, or doing background checks on the state monitoring site.
But, realistically, I can’t do that. Even though I try to limit my narcotic scripts, the DEA number covers many other things, such as Lyrica, Vimpat, and Klonopin for epilepsy patients; Provigil for MS fatigue; and Valium to help someone get through an MRI scan.
There are, of course, the occasional narcotics. I don’t have many patients on chronic narcotics any more, but I’m sure all of us have a few migraine patients who keep an emergency supply of some narcotic (say, 5-10 pills) on hand to be used in lieu of an ER trip. They may only get it filled once a year, which doesn’t seem unreasonable.
I think it would be unfair to make patients, many of whom I’ve seen for many years, have to find a new neurologist over such things.
But what if there were a graduated DEA license? Say, one that limited me to schedules III-V or even just level IV and V substances? That would probably make life easier. The latter would still allow tramadol and Stadol-NS for breakthrough migraines, while adding level III would still allow Tylenol with codeine No. 3. Either way, it would take oxycodone, hydrocodone, morphine, amphetamines, and other drugs of greater abuse potential out of my hands. A few less things to worry about.
There are pros and cons of this. From one view, it would limit the number of docs prescribing higher level substances, making them easier to track and control. On the other hand, patients who need them, many of whom are legitimately in pain, would be forced to change to a dwindling number of narcotic prescribers. Many of these doctors would likely start to work on a cash-only basis knowing the demand they’d be in. Who knows where that would lead? Like so many things in medicine, there are always going to be unintended consequences.
For the time being I have no plans to drop my DEA license, but it would be nice to have options besides all or nothing.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I sometimes think about giving up my DEA certificate. I can’t be the only one.
It’s not strictly about the money. $731 every 3 years is small change, compared with my rent, staff salaries, and malpractice insurance, although the savings would be nice.
Part of it is the desire to unfetter myself from prescribing controlled substances. I wouldn’t have to worry about drug seekers hitting me up, or dealing with the paperwork and pharmacy calls, or doing background checks on the state monitoring site.
But, realistically, I can’t do that. Even though I try to limit my narcotic scripts, the DEA number covers many other things, such as Lyrica, Vimpat, and Klonopin for epilepsy patients; Provigil for MS fatigue; and Valium to help someone get through an MRI scan.
There are, of course, the occasional narcotics. I don’t have many patients on chronic narcotics any more, but I’m sure all of us have a few migraine patients who keep an emergency supply of some narcotic (say, 5-10 pills) on hand to be used in lieu of an ER trip. They may only get it filled once a year, which doesn’t seem unreasonable.
I think it would be unfair to make patients, many of whom I’ve seen for many years, have to find a new neurologist over such things.
But what if there were a graduated DEA license? Say, one that limited me to schedules III-V or even just level IV and V substances? That would probably make life easier. The latter would still allow tramadol and Stadol-NS for breakthrough migraines, while adding level III would still allow Tylenol with codeine No. 3. Either way, it would take oxycodone, hydrocodone, morphine, amphetamines, and other drugs of greater abuse potential out of my hands. A few less things to worry about.
There are pros and cons of this. From one view, it would limit the number of docs prescribing higher level substances, making them easier to track and control. On the other hand, patients who need them, many of whom are legitimately in pain, would be forced to change to a dwindling number of narcotic prescribers. Many of these doctors would likely start to work on a cash-only basis knowing the demand they’d be in. Who knows where that would lead? Like so many things in medicine, there are always going to be unintended consequences.
For the time being I have no plans to drop my DEA license, but it would be nice to have options besides all or nothing.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I sometimes think about giving up my DEA certificate. I can’t be the only one.
It’s not strictly about the money. $731 every 3 years is small change, compared with my rent, staff salaries, and malpractice insurance, although the savings would be nice.
Part of it is the desire to unfetter myself from prescribing controlled substances. I wouldn’t have to worry about drug seekers hitting me up, or dealing with the paperwork and pharmacy calls, or doing background checks on the state monitoring site.
But, realistically, I can’t do that. Even though I try to limit my narcotic scripts, the DEA number covers many other things, such as Lyrica, Vimpat, and Klonopin for epilepsy patients; Provigil for MS fatigue; and Valium to help someone get through an MRI scan.
There are, of course, the occasional narcotics. I don’t have many patients on chronic narcotics any more, but I’m sure all of us have a few migraine patients who keep an emergency supply of some narcotic (say, 5-10 pills) on hand to be used in lieu of an ER trip. They may only get it filled once a year, which doesn’t seem unreasonable.
I think it would be unfair to make patients, many of whom I’ve seen for many years, have to find a new neurologist over such things.
But what if there were a graduated DEA license? Say, one that limited me to schedules III-V or even just level IV and V substances? That would probably make life easier. The latter would still allow tramadol and Stadol-NS for breakthrough migraines, while adding level III would still allow Tylenol with codeine No. 3. Either way, it would take oxycodone, hydrocodone, morphine, amphetamines, and other drugs of greater abuse potential out of my hands. A few less things to worry about.
There are pros and cons of this. From one view, it would limit the number of docs prescribing higher level substances, making them easier to track and control. On the other hand, patients who need them, many of whom are legitimately in pain, would be forced to change to a dwindling number of narcotic prescribers. Many of these doctors would likely start to work on a cash-only basis knowing the demand they’d be in. Who knows where that would lead? Like so many things in medicine, there are always going to be unintended consequences.
For the time being I have no plans to drop my DEA license, but it would be nice to have options besides all or nothing.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
















