User login
Accident or incident?
In May 2016, a 4-year-old managed to get into the gorilla enclosure at the Cincinnati Zoo. In a successful attempt to remove the child before he could be seriously injured, the zoo officials shot and killed the gorilla. While there has been some debate as to whether the situation warranted the use of deadly force, there is general agreement that we should value a human life over that of an animal.
However, the debate that continues to simmer focuses on whether the child’s parents share in any culpability for the event. I suspect we will never know enough of the details to make a judgment on this question. And faced with that uncertainty, what I am going to say in the next few paragraphs does not apply to the situation at the Cincinnati Zoo. I am using that event only as a place to start a discussion.
You and I have seen scores of children who, because of their immaturity and to a larger extent to their temperament, are adventuresome and resistant to attempts by those who would like to keep them safe. You could label them as risk takers, but I don’t think those children are perceptive enough to understand that what they are doing is risky.
You could call them accident prone, but this raises the question of how many events that end in injury or death are really accidental. Almost all of those tragedies aren’t intentional. But how many were preventable? Of course, that depends on how broadly you define preventable. How far back in the chain of events that preceded an incident are you willing to realistically assign causality or culpability?
For example, a mother is standing on the sidewalk of busy intersection with a traffic light, holding her 3-year-old’s hand. Her cell phone alerts her of a text, she drops his hand to check it, and in an instant he darts out into the travel lane to grab what turns out to be an empty candy wrapper and is struck by a car. Was this an accident?
I suspect that if we interviewed family friends, neighbors, and maybe even the child’s pediatrician, we would learn of several dozen examples of the child’s unusually impulsive behavior. We also might learn that he had been resistant to his mother’s attempts to set limits and modify his behavior. In other words, his past history suggests that he was an “accident” waiting to happen.
Who is culpable here? Should the driver have been more aware of the pedestrians who were waiting to cross and, seeing that one was a young child, realized that some little children are more impulsive than others and driven more prudently? Should the child’s mother have accepted the fact that while some 3-year-olds can be under “voice control,” her son was certainly not one of those and ignored her phone?
Should the child’s pediatrician, who was aware of his temperament and impulsivity, have spent more time with the family on how to manage his behavior? Dr. William J. Turtle published a book in the 1970s titled, “Dr. Turtle’s Babies,” in which among other sage advice, he suggests most if not all toddlers should be fitted with a harness until they can be trusted. While you and I may have trouble selling this concept to parents – particularly parents of post toddlers – a harness or wrist-restraining tether might have saved this 3-year-old’s life. Whether a child’s impulsivity is age appropriate or pathologic, we must include advice on its management in our anticipatory guidance. There are very few true accidents, many are incidents that were predictable – and to some extent – preventable.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
In May 2016, a 4-year-old managed to get into the gorilla enclosure at the Cincinnati Zoo. In a successful attempt to remove the child before he could be seriously injured, the zoo officials shot and killed the gorilla. While there has been some debate as to whether the situation warranted the use of deadly force, there is general agreement that we should value a human life over that of an animal.
However, the debate that continues to simmer focuses on whether the child’s parents share in any culpability for the event. I suspect we will never know enough of the details to make a judgment on this question. And faced with that uncertainty, what I am going to say in the next few paragraphs does not apply to the situation at the Cincinnati Zoo. I am using that event only as a place to start a discussion.
You and I have seen scores of children who, because of their immaturity and to a larger extent to their temperament, are adventuresome and resistant to attempts by those who would like to keep them safe. You could label them as risk takers, but I don’t think those children are perceptive enough to understand that what they are doing is risky.
You could call them accident prone, but this raises the question of how many events that end in injury or death are really accidental. Almost all of those tragedies aren’t intentional. But how many were preventable? Of course, that depends on how broadly you define preventable. How far back in the chain of events that preceded an incident are you willing to realistically assign causality or culpability?
For example, a mother is standing on the sidewalk of busy intersection with a traffic light, holding her 3-year-old’s hand. Her cell phone alerts her of a text, she drops his hand to check it, and in an instant he darts out into the travel lane to grab what turns out to be an empty candy wrapper and is struck by a car. Was this an accident?
I suspect that if we interviewed family friends, neighbors, and maybe even the child’s pediatrician, we would learn of several dozen examples of the child’s unusually impulsive behavior. We also might learn that he had been resistant to his mother’s attempts to set limits and modify his behavior. In other words, his past history suggests that he was an “accident” waiting to happen.
Who is culpable here? Should the driver have been more aware of the pedestrians who were waiting to cross and, seeing that one was a young child, realized that some little children are more impulsive than others and driven more prudently? Should the child’s mother have accepted the fact that while some 3-year-olds can be under “voice control,” her son was certainly not one of those and ignored her phone?
Should the child’s pediatrician, who was aware of his temperament and impulsivity, have spent more time with the family on how to manage his behavior? Dr. William J. Turtle published a book in the 1970s titled, “Dr. Turtle’s Babies,” in which among other sage advice, he suggests most if not all toddlers should be fitted with a harness until they can be trusted. While you and I may have trouble selling this concept to parents – particularly parents of post toddlers – a harness or wrist-restraining tether might have saved this 3-year-old’s life. Whether a child’s impulsivity is age appropriate or pathologic, we must include advice on its management in our anticipatory guidance. There are very few true accidents, many are incidents that were predictable – and to some extent – preventable.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
In May 2016, a 4-year-old managed to get into the gorilla enclosure at the Cincinnati Zoo. In a successful attempt to remove the child before he could be seriously injured, the zoo officials shot and killed the gorilla. While there has been some debate as to whether the situation warranted the use of deadly force, there is general agreement that we should value a human life over that of an animal.
However, the debate that continues to simmer focuses on whether the child’s parents share in any culpability for the event. I suspect we will never know enough of the details to make a judgment on this question. And faced with that uncertainty, what I am going to say in the next few paragraphs does not apply to the situation at the Cincinnati Zoo. I am using that event only as a place to start a discussion.
You and I have seen scores of children who, because of their immaturity and to a larger extent to their temperament, are adventuresome and resistant to attempts by those who would like to keep them safe. You could label them as risk takers, but I don’t think those children are perceptive enough to understand that what they are doing is risky.
You could call them accident prone, but this raises the question of how many events that end in injury or death are really accidental. Almost all of those tragedies aren’t intentional. But how many were preventable? Of course, that depends on how broadly you define preventable. How far back in the chain of events that preceded an incident are you willing to realistically assign causality or culpability?
For example, a mother is standing on the sidewalk of busy intersection with a traffic light, holding her 3-year-old’s hand. Her cell phone alerts her of a text, she drops his hand to check it, and in an instant he darts out into the travel lane to grab what turns out to be an empty candy wrapper and is struck by a car. Was this an accident?
I suspect that if we interviewed family friends, neighbors, and maybe even the child’s pediatrician, we would learn of several dozen examples of the child’s unusually impulsive behavior. We also might learn that he had been resistant to his mother’s attempts to set limits and modify his behavior. In other words, his past history suggests that he was an “accident” waiting to happen.
Who is culpable here? Should the driver have been more aware of the pedestrians who were waiting to cross and, seeing that one was a young child, realized that some little children are more impulsive than others and driven more prudently? Should the child’s mother have accepted the fact that while some 3-year-olds can be under “voice control,” her son was certainly not one of those and ignored her phone?
Should the child’s pediatrician, who was aware of his temperament and impulsivity, have spent more time with the family on how to manage his behavior? Dr. William J. Turtle published a book in the 1970s titled, “Dr. Turtle’s Babies,” in which among other sage advice, he suggests most if not all toddlers should be fitted with a harness until they can be trusted. While you and I may have trouble selling this concept to parents – particularly parents of post toddlers – a harness or wrist-restraining tether might have saved this 3-year-old’s life. Whether a child’s impulsivity is age appropriate or pathologic, we must include advice on its management in our anticipatory guidance. There are very few true accidents, many are incidents that were predictable – and to some extent – preventable.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
50 years of gynecologic surgery: A large dose of ingenuity, a small dose of controversy
Over the past 50 years, there has been explosive change in gynecologic surgery. Ob.Gyn. News has been at the forefront of capturing and chronicling this paradigm shift in the treatment of the female patient.
Our beginnings
From antiquity, physicians and surgeons have struggled with pelvic prolapse, uterine fibroids, ovarian cysts, urinary incontinence, vesicovaginal fistulas, pelvic pain, and abnormal uterine bleeding. At the time of the first edition of Ob.Gyn. News, it had been less than a century since Thomas Edison invented the light bulb; just over 50 years since Hans Christian Jacobaeus first created air pneumoperitoneum using a trocar, followed by the Nitze cystoscope; about 40 years since Richard Zollikofer created a carbon dioxide pneumoperitoneum; 25 years since F.H. Powers and A.C. Barnes had first described laparoscopic tubal sterilization by cautery; and about 20 years since Raoul Palmer, considered the father of modern laparoscopy, had first described the technique – left upper quadrant entry, testing insufflation, Trendelenburg positioning, and simple laparoscopic instrumentation.
In the 1950s, Hans Frangenheim would bring monopolar electrosurgery to laparoscopy and Harold Hopkins would introduce fiber optics. It was not until 1967 that Patrick Steptoe would publish the first textbook on laparoscopy in the English language.
Although usage as a diagnostic tool and a method of sterilization increased popularity of laparoscopy in the 1960s and early 1970s, there were few advances. In fact, a review of early editions of Ob.Gyn. News during that time period shows that the majority of articles involving laparoscopy dealt with sterilization; including the introduction of clips for tubal sterilization by Jaroslav Hulka in 1972. This did not deter the efforts of Jordan Phillips, who along with Jacques Rioux, Louis Keith, Richard Soderstrom – four early laparoscopists – incorporated a new society, the American Association of Gynecologic Laparoscopists (the AAGL) in 1971.
Simultaneously, in 1979, James Daniell in the United States, Maurice Bruhart in France, and Yona Tadir in Israel were promoting efforts to couple the carbon dioxide laser to the laparoscope to treat pelvic adhesions and endometriosis. Later on, fiber lasers, KTP, Nd:YAG, and Argon lasers would be utilized in our field. Still, only a few extirpative procedures were being performed via a laparoscope route. This included linear salpingostomy for the treatment of ectopic pregnancy, championed by Professor Bruhart and H. Manhes in Europe, and Alan DeCherney in the United States.
During the 1980s, laparoscopic surgery was at its innovative best. Through the pioneering efforts of Professor Kurt Semm and his protégée, Liselotte Mettler, the gynecologic laparoscopist was introduced to endoloops, simple suturing techniques, and mechanical morcellation techniques.
Procedures such as salpingo-oophorectomy, appendectomy, and myomectomy could now be performed via the laparoscope. Dr. Camran Nezhat coupled the carbon dioxide laser, the laparoscope, and the television monitor, coining the term laparoscopy. Most importantly, the laparoscopic surgeon was liberated; he or she could remain upright and perform surgery with both hands. Through the 1980s and 1990s, Dr. Nezhat, Dr. Harry Reich, and other innovators pushed the envelope in increasing the ability to extirpate endometriosis, excise severe pelvic adhesions, and perform discoid and segmental bowel resection.
The day the earth stood still
Every gynecologic laparoscopic surgeon should remember Jan. 26, 1988, as that was the date that Dr. Harry Reich performed the first total laparoscopic hysterectomy. Now, little more than 25 years later, in many parts of the country, a laparoscopic approach to hysterectomy is indeed the most common route. Over the years, with the evolution of instrumentation, including new energy systems (ultrasonic, advanced bipolar) and the introduction of barbed sutures, hysterectomy can now be performed via minilaparoscopy, single-site laparoscopy, robot-assisted, and robotic single site, all of which have been featured in the Ob.Gyn. News’ Master Class in Gynecologic Surgery.
But hysteroscopy came first
Abulkasim utilized a mirror to reflect light into the vaginal vault in 1,000 A.D. In 1806, Philipp Bozzini originated the idea of illuminating body cavities by an external light source. Through a system of mirrors and tubes, candlelight could be reflected into the body. In 1869, D.C. Pantaleoni used a cystoscope developed by Antoine Desormeaux – who has been called the father of endoscopy – to treat endometrial polyps with silver nitrate.
Through the 50 years of Ob.Gyn. News and over the past 12 years of the Master Class in Gynecologic Surgery, our community has been consistently updated as to advances in hysteroscopy, not only to enhance treatment efficacy, but safety as well. This has included such advances as the continuous flow hysteroscope, the Hamou contact hysteroscope, and fluid management systems to enhance visualization.
In 1978, Robert Neuwirth introduced loops to perform hysteroscopic myomectomy. The loop resectoscope was quickly followed by the rollerball to perform endometrial ablation. In the late 1990s, hysteroscopic bipolar cutting loops were introduced. This enabled use of ionic distension media saline, instead of nonionic media, thus decreasing risks related to hyponatremia.
In 2003, Mark Emanuel introduced hysteroscopic morcellation systems, which enabled more gynecologists to perform operative hysteroscopy safely. Resected tissue is removed immediately to allow superior visualization. The flexible hysteroscope coupled with vaginoscopy has enabled hysteroscopy to be done with minimal to no anesthesia in an in-office setting.
With advances in hysteroscopy over the past 35 years, hysteroscopic procedures such as polypectomy, myomectomy, lysis of adhesions, transection of endometriosis, evacuation of retained products of conception, and endometrial ablation/resection have become routine.
And now, the controversy
Since its inception, laparoscopic surgery has not been without controversy. In 1933, Karl Fervers described explosion and flashes of light from a combination of high frequency electric current and oxygen distension gas while performing laparoscopic adhesiolysis with the coagulation probe of the ureterocystoscope.
In the early 1970s, Professor Kurt Semm’s pioneering effort was not rewarded by his department, in Kiel, Germany, which instead recommended he schedule a brain scan and psychological testing.
Nearly 20 years later, in a 1992 edition of Current Science, Professor Semm, along with Alan DeCherney, stated that “over 80% of gynecological operations can now be performed by laparoscopy.” Shortly thereafter, however, Dr. Roy Pitkin, who at the time was president of the American College of Obstetricians and Gynecologists, wrote an editorial in the Journal of Obstetrics and Gynecology – “Operative Laparoscopy: Surgical Advance or Technical Gimmick?” (Obstet Gynecol. 1992 Mar;79[3]:441-2).
Fortunately, 18 years later, with the continued advances in laparoscopic surgery making it less expensive, safer, and more accessible, Dr. Pitkin did retract his statement (Obstet Gynecol. 2010 May;115[5]:890-1).
Currently, the gynecologic community is embroiled in controversies involving the use of the robot to assist in the performance of laparoscopic surgery, the incorporation of synthetic mesh to enhance urogynecologic procedures, the placement of Essure micro-inserts to occlude fallopian tubes, and the use of electronic power morcellation at time of laparoscopic or robot-assisted hysterectomy, myomectomy, or sacrocolpopexy.
After reading the 2013 article by Dr. Jason Wright, published in JAMA, comparing laparoscopic hysterectomy to robotic hysterectomy, no one can deny that the rise in a minimally invasive route to hysterectomy has coincided with the advent of the robot (JAMA. 2013 Feb 20;309[7]:689-98). On the other hand, many detractors, including Dr. James Breeden (past ACOG president 2012-2013), find the higher cost of robotic surgery very problematic. In fact, many of these detractors cite the paucity of data showing a significant advantage to use of robotics.
While certainly cost, more than ever, must be a major consideration, remember that during the 1990s, there were multiple articles in Ob.Gyn. News raising concerns about the cost of laparoscopic hysterectomy. Interestingly, studies over the past decade by Warren and Jonsdottir show a cost savings when hysterectomy is done laparoscopically as opposed to its being done by laparotomy. Thus, it certainly can be anticipated that with more physician experience, improved instrumentation, and robotic industry competition, the overall cost will become more comparable to a laparoscopic route.
In 1995, Ulf Ulmsten first described the use of tension-free tape (TVT) to treat stress urinary incontinence. In 1998, the Food and Drug Administration approved the use of the TVT sling in the United States. Since then, transobturator tension-free vaginal tape (TVT-O) and single incision mini-slings have been introduced. All of these techniques have been shown to be successful and have been well adapted into the armamentarium of physicians treating stress urinary incontinence.
With the success of synthetic mesh for the treatment of stress urinary incontinence, its use was extended to pelvic prolapse. In 2002, the first mesh device with indications for the treatment of pelvic organ prolapse was approved by the FDA. While the erosion rate utilizing synthetic mesh for stress urinary incontinence has been noted to be 2%, rates up to 8.3% have been noted in patients treated for pelvic prolapse.
In 2008, the FDA issued a warning regarding the use of mesh for prolapse and incontinence repair secondary to the sequelae of mesh erosion. Subsequently, in 2011, the concern was limited to vaginal mesh to correct pelvic organ prolapse. Finally, on Jan. 4, 2016, the FDA issued an order to reclassify surgical mesh to repair pelvic organ prolapse from class II, which includes moderate-risk devices, to class III, which includes high-risk devices. Moreover, the FDA issued a second order to manufacturers to submit a premarket approval application to support the safety and effectiveness of synthetic mesh for transvaginal repair of pelvic organ prolapse.
Essure micro-inserts for permanent birth control received initial approval from the FDA in November 2002. Despite the fact that Essure can be easily placed, is highly effective, and has seemingly low complication rates, concerns have been raised by the Facebook group “Essure Problems” and Erin Brockovich, the focus of the 2000 biographical film starring Julia Roberts.
After more than 5,000 women filed grievances with the FDA between November 2002 and May 2015, based on unintended pregnancies, miscarriages, stillbirths, severe pain, and bleeding, the FDA announced in 2016 that it would require a boxed warning label for Essure. The FDA also called upon Bayer, which makes and markets Essure, to conduct surveillance to assess “risks of the device in a real-world environment.” The agency stated it will use the results to “determine what, if any, further actions related to Essure are needed to protect public health.”
While Jan. 26, 1988, is a very special date in minimally invasive gynecologic surgery, April 17, 2014, is a day of infamy for the gynecologic laparoscopist. For on this day, the FDA announced a warning regarding electronic power morcellation. Many hospitals and hospital systems throughout the country issued bans on electronic power morcellation, leading to needless open laparotomy procedures and thus, introducing prolonged recovery times and increased risk.
At a time when the recent introduction of barbed suture had made both closure of the vaginal cuff at time of hysterectomy and repair of the hysterotomy at myomectomy easier and faster, the gynecologic laparoscopist was taking a step backward. The FDA based this decision and a subsequent boxed warning – issued in November 2014 – on a small number of studies showing potential upstaging of leiomyosarcoma post electronic power morcellation. Interestingly, many of the morcellation procedures cited did not use power morcellation. Furthermore, a more comprehensive meta-analysis by Elizabeth A. Pritts and colleagues, showed a far lower risk than suggested by the FDA (Gynecol Surg. 2015;12[3]:165-77).
Recently, an article by William Parker and colleagues recommended that the FDA reverse its position (Obstet Gynecol. 2016 Jan;127[1]:18-22). Many believe that ultimately, the solution will be morcellation in a containment bag, which I and my colleagues have been performing in virtually every power morcellation procedure since May 2014. During this current power morcellation controversy, the Master Class in Gynecologic Surgery has continued to update its readers with three different articles related to the subject.
And in conclusion
Without a doubt, the past 50 years of gynecologic surgery has been a time of unparalleled innovation with occasional controversy thrown in. Ob.Gyn. News and more recently, the Master Class in Gynecologic Surgery, has had a major leadership role in bringing this profound ingenuity to the gynecology community by introducing this explosion of surgical creativity to its readers.
And what will the next 50 years bring? I believe we will continue to see tremendous advancements in minimally invasive gynecologic surgery. There will be a definite impact of costs on the marketplace. Thus, many of the minor minimally invasive procedures currently performed in the hospital or surgery center will be brought into office settings. In addition, secondary to reimbursement, the more complex cases will be carried out by fewer gynecologic surgeons who have undergone more intense training in pelvic surgery and who can perform these cases more efficiently and with fewer complications. Our ability to perform surgery and what type of procedures we do will not only be based on randomized, controlled trials, but big data collection as well.
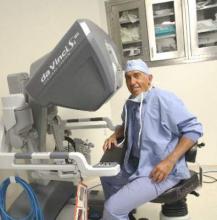
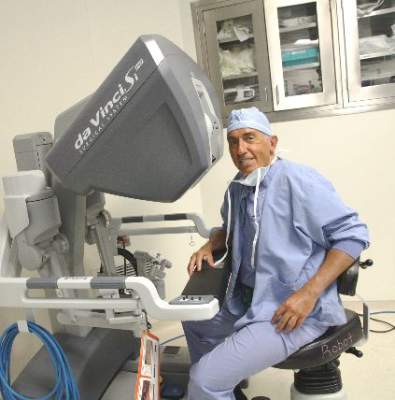
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and is on the speakers bureau for Ethicon.
Over the past 50 years, there has been explosive change in gynecologic surgery. Ob.Gyn. News has been at the forefront of capturing and chronicling this paradigm shift in the treatment of the female patient.
Our beginnings
From antiquity, physicians and surgeons have struggled with pelvic prolapse, uterine fibroids, ovarian cysts, urinary incontinence, vesicovaginal fistulas, pelvic pain, and abnormal uterine bleeding. At the time of the first edition of Ob.Gyn. News, it had been less than a century since Thomas Edison invented the light bulb; just over 50 years since Hans Christian Jacobaeus first created air pneumoperitoneum using a trocar, followed by the Nitze cystoscope; about 40 years since Richard Zollikofer created a carbon dioxide pneumoperitoneum; 25 years since F.H. Powers and A.C. Barnes had first described laparoscopic tubal sterilization by cautery; and about 20 years since Raoul Palmer, considered the father of modern laparoscopy, had first described the technique – left upper quadrant entry, testing insufflation, Trendelenburg positioning, and simple laparoscopic instrumentation.
In the 1950s, Hans Frangenheim would bring monopolar electrosurgery to laparoscopy and Harold Hopkins would introduce fiber optics. It was not until 1967 that Patrick Steptoe would publish the first textbook on laparoscopy in the English language.
Although usage as a diagnostic tool and a method of sterilization increased popularity of laparoscopy in the 1960s and early 1970s, there were few advances. In fact, a review of early editions of Ob.Gyn. News during that time period shows that the majority of articles involving laparoscopy dealt with sterilization; including the introduction of clips for tubal sterilization by Jaroslav Hulka in 1972. This did not deter the efforts of Jordan Phillips, who along with Jacques Rioux, Louis Keith, Richard Soderstrom – four early laparoscopists – incorporated a new society, the American Association of Gynecologic Laparoscopists (the AAGL) in 1971.
Simultaneously, in 1979, James Daniell in the United States, Maurice Bruhart in France, and Yona Tadir in Israel were promoting efforts to couple the carbon dioxide laser to the laparoscope to treat pelvic adhesions and endometriosis. Later on, fiber lasers, KTP, Nd:YAG, and Argon lasers would be utilized in our field. Still, only a few extirpative procedures were being performed via a laparoscope route. This included linear salpingostomy for the treatment of ectopic pregnancy, championed by Professor Bruhart and H. Manhes in Europe, and Alan DeCherney in the United States.
During the 1980s, laparoscopic surgery was at its innovative best. Through the pioneering efforts of Professor Kurt Semm and his protégée, Liselotte Mettler, the gynecologic laparoscopist was introduced to endoloops, simple suturing techniques, and mechanical morcellation techniques.
Procedures such as salpingo-oophorectomy, appendectomy, and myomectomy could now be performed via the laparoscope. Dr. Camran Nezhat coupled the carbon dioxide laser, the laparoscope, and the television monitor, coining the term laparoscopy. Most importantly, the laparoscopic surgeon was liberated; he or she could remain upright and perform surgery with both hands. Through the 1980s and 1990s, Dr. Nezhat, Dr. Harry Reich, and other innovators pushed the envelope in increasing the ability to extirpate endometriosis, excise severe pelvic adhesions, and perform discoid and segmental bowel resection.
The day the earth stood still
Every gynecologic laparoscopic surgeon should remember Jan. 26, 1988, as that was the date that Dr. Harry Reich performed the first total laparoscopic hysterectomy. Now, little more than 25 years later, in many parts of the country, a laparoscopic approach to hysterectomy is indeed the most common route. Over the years, with the evolution of instrumentation, including new energy systems (ultrasonic, advanced bipolar) and the introduction of barbed sutures, hysterectomy can now be performed via minilaparoscopy, single-site laparoscopy, robot-assisted, and robotic single site, all of which have been featured in the Ob.Gyn. News’ Master Class in Gynecologic Surgery.
But hysteroscopy came first
Abulkasim utilized a mirror to reflect light into the vaginal vault in 1,000 A.D. In 1806, Philipp Bozzini originated the idea of illuminating body cavities by an external light source. Through a system of mirrors and tubes, candlelight could be reflected into the body. In 1869, D.C. Pantaleoni used a cystoscope developed by Antoine Desormeaux – who has been called the father of endoscopy – to treat endometrial polyps with silver nitrate.
Through the 50 years of Ob.Gyn. News and over the past 12 years of the Master Class in Gynecologic Surgery, our community has been consistently updated as to advances in hysteroscopy, not only to enhance treatment efficacy, but safety as well. This has included such advances as the continuous flow hysteroscope, the Hamou contact hysteroscope, and fluid management systems to enhance visualization.
In 1978, Robert Neuwirth introduced loops to perform hysteroscopic myomectomy. The loop resectoscope was quickly followed by the rollerball to perform endometrial ablation. In the late 1990s, hysteroscopic bipolar cutting loops were introduced. This enabled use of ionic distension media saline, instead of nonionic media, thus decreasing risks related to hyponatremia.
In 2003, Mark Emanuel introduced hysteroscopic morcellation systems, which enabled more gynecologists to perform operative hysteroscopy safely. Resected tissue is removed immediately to allow superior visualization. The flexible hysteroscope coupled with vaginoscopy has enabled hysteroscopy to be done with minimal to no anesthesia in an in-office setting.
With advances in hysteroscopy over the past 35 years, hysteroscopic procedures such as polypectomy, myomectomy, lysis of adhesions, transection of endometriosis, evacuation of retained products of conception, and endometrial ablation/resection have become routine.
And now, the controversy
Since its inception, laparoscopic surgery has not been without controversy. In 1933, Karl Fervers described explosion and flashes of light from a combination of high frequency electric current and oxygen distension gas while performing laparoscopic adhesiolysis with the coagulation probe of the ureterocystoscope.
In the early 1970s, Professor Kurt Semm’s pioneering effort was not rewarded by his department, in Kiel, Germany, which instead recommended he schedule a brain scan and psychological testing.
Nearly 20 years later, in a 1992 edition of Current Science, Professor Semm, along with Alan DeCherney, stated that “over 80% of gynecological operations can now be performed by laparoscopy.” Shortly thereafter, however, Dr. Roy Pitkin, who at the time was president of the American College of Obstetricians and Gynecologists, wrote an editorial in the Journal of Obstetrics and Gynecology – “Operative Laparoscopy: Surgical Advance or Technical Gimmick?” (Obstet Gynecol. 1992 Mar;79[3]:441-2).
Fortunately, 18 years later, with the continued advances in laparoscopic surgery making it less expensive, safer, and more accessible, Dr. Pitkin did retract his statement (Obstet Gynecol. 2010 May;115[5]:890-1).
Currently, the gynecologic community is embroiled in controversies involving the use of the robot to assist in the performance of laparoscopic surgery, the incorporation of synthetic mesh to enhance urogynecologic procedures, the placement of Essure micro-inserts to occlude fallopian tubes, and the use of electronic power morcellation at time of laparoscopic or robot-assisted hysterectomy, myomectomy, or sacrocolpopexy.
After reading the 2013 article by Dr. Jason Wright, published in JAMA, comparing laparoscopic hysterectomy to robotic hysterectomy, no one can deny that the rise in a minimally invasive route to hysterectomy has coincided with the advent of the robot (JAMA. 2013 Feb 20;309[7]:689-98). On the other hand, many detractors, including Dr. James Breeden (past ACOG president 2012-2013), find the higher cost of robotic surgery very problematic. In fact, many of these detractors cite the paucity of data showing a significant advantage to use of robotics.
While certainly cost, more than ever, must be a major consideration, remember that during the 1990s, there were multiple articles in Ob.Gyn. News raising concerns about the cost of laparoscopic hysterectomy. Interestingly, studies over the past decade by Warren and Jonsdottir show a cost savings when hysterectomy is done laparoscopically as opposed to its being done by laparotomy. Thus, it certainly can be anticipated that with more physician experience, improved instrumentation, and robotic industry competition, the overall cost will become more comparable to a laparoscopic route.
In 1995, Ulf Ulmsten first described the use of tension-free tape (TVT) to treat stress urinary incontinence. In 1998, the Food and Drug Administration approved the use of the TVT sling in the United States. Since then, transobturator tension-free vaginal tape (TVT-O) and single incision mini-slings have been introduced. All of these techniques have been shown to be successful and have been well adapted into the armamentarium of physicians treating stress urinary incontinence.
With the success of synthetic mesh for the treatment of stress urinary incontinence, its use was extended to pelvic prolapse. In 2002, the first mesh device with indications for the treatment of pelvic organ prolapse was approved by the FDA. While the erosion rate utilizing synthetic mesh for stress urinary incontinence has been noted to be 2%, rates up to 8.3% have been noted in patients treated for pelvic prolapse.
In 2008, the FDA issued a warning regarding the use of mesh for prolapse and incontinence repair secondary to the sequelae of mesh erosion. Subsequently, in 2011, the concern was limited to vaginal mesh to correct pelvic organ prolapse. Finally, on Jan. 4, 2016, the FDA issued an order to reclassify surgical mesh to repair pelvic organ prolapse from class II, which includes moderate-risk devices, to class III, which includes high-risk devices. Moreover, the FDA issued a second order to manufacturers to submit a premarket approval application to support the safety and effectiveness of synthetic mesh for transvaginal repair of pelvic organ prolapse.
Essure micro-inserts for permanent birth control received initial approval from the FDA in November 2002. Despite the fact that Essure can be easily placed, is highly effective, and has seemingly low complication rates, concerns have been raised by the Facebook group “Essure Problems” and Erin Brockovich, the focus of the 2000 biographical film starring Julia Roberts.
After more than 5,000 women filed grievances with the FDA between November 2002 and May 2015, based on unintended pregnancies, miscarriages, stillbirths, severe pain, and bleeding, the FDA announced in 2016 that it would require a boxed warning label for Essure. The FDA also called upon Bayer, which makes and markets Essure, to conduct surveillance to assess “risks of the device in a real-world environment.” The agency stated it will use the results to “determine what, if any, further actions related to Essure are needed to protect public health.”
While Jan. 26, 1988, is a very special date in minimally invasive gynecologic surgery, April 17, 2014, is a day of infamy for the gynecologic laparoscopist. For on this day, the FDA announced a warning regarding electronic power morcellation. Many hospitals and hospital systems throughout the country issued bans on electronic power morcellation, leading to needless open laparotomy procedures and thus, introducing prolonged recovery times and increased risk.
At a time when the recent introduction of barbed suture had made both closure of the vaginal cuff at time of hysterectomy and repair of the hysterotomy at myomectomy easier and faster, the gynecologic laparoscopist was taking a step backward. The FDA based this decision and a subsequent boxed warning – issued in November 2014 – on a small number of studies showing potential upstaging of leiomyosarcoma post electronic power morcellation. Interestingly, many of the morcellation procedures cited did not use power morcellation. Furthermore, a more comprehensive meta-analysis by Elizabeth A. Pritts and colleagues, showed a far lower risk than suggested by the FDA (Gynecol Surg. 2015;12[3]:165-77).
Recently, an article by William Parker and colleagues recommended that the FDA reverse its position (Obstet Gynecol. 2016 Jan;127[1]:18-22). Many believe that ultimately, the solution will be morcellation in a containment bag, which I and my colleagues have been performing in virtually every power morcellation procedure since May 2014. During this current power morcellation controversy, the Master Class in Gynecologic Surgery has continued to update its readers with three different articles related to the subject.
And in conclusion
Without a doubt, the past 50 years of gynecologic surgery has been a time of unparalleled innovation with occasional controversy thrown in. Ob.Gyn. News and more recently, the Master Class in Gynecologic Surgery, has had a major leadership role in bringing this profound ingenuity to the gynecology community by introducing this explosion of surgical creativity to its readers.
And what will the next 50 years bring? I believe we will continue to see tremendous advancements in minimally invasive gynecologic surgery. There will be a definite impact of costs on the marketplace. Thus, many of the minor minimally invasive procedures currently performed in the hospital or surgery center will be brought into office settings. In addition, secondary to reimbursement, the more complex cases will be carried out by fewer gynecologic surgeons who have undergone more intense training in pelvic surgery and who can perform these cases more efficiently and with fewer complications. Our ability to perform surgery and what type of procedures we do will not only be based on randomized, controlled trials, but big data collection as well.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and is on the speakers bureau for Ethicon.
Over the past 50 years, there has been explosive change in gynecologic surgery. Ob.Gyn. News has been at the forefront of capturing and chronicling this paradigm shift in the treatment of the female patient.
Our beginnings
From antiquity, physicians and surgeons have struggled with pelvic prolapse, uterine fibroids, ovarian cysts, urinary incontinence, vesicovaginal fistulas, pelvic pain, and abnormal uterine bleeding. At the time of the first edition of Ob.Gyn. News, it had been less than a century since Thomas Edison invented the light bulb; just over 50 years since Hans Christian Jacobaeus first created air pneumoperitoneum using a trocar, followed by the Nitze cystoscope; about 40 years since Richard Zollikofer created a carbon dioxide pneumoperitoneum; 25 years since F.H. Powers and A.C. Barnes had first described laparoscopic tubal sterilization by cautery; and about 20 years since Raoul Palmer, considered the father of modern laparoscopy, had first described the technique – left upper quadrant entry, testing insufflation, Trendelenburg positioning, and simple laparoscopic instrumentation.
In the 1950s, Hans Frangenheim would bring monopolar electrosurgery to laparoscopy and Harold Hopkins would introduce fiber optics. It was not until 1967 that Patrick Steptoe would publish the first textbook on laparoscopy in the English language.
Although usage as a diagnostic tool and a method of sterilization increased popularity of laparoscopy in the 1960s and early 1970s, there were few advances. In fact, a review of early editions of Ob.Gyn. News during that time period shows that the majority of articles involving laparoscopy dealt with sterilization; including the introduction of clips for tubal sterilization by Jaroslav Hulka in 1972. This did not deter the efforts of Jordan Phillips, who along with Jacques Rioux, Louis Keith, Richard Soderstrom – four early laparoscopists – incorporated a new society, the American Association of Gynecologic Laparoscopists (the AAGL) in 1971.
Simultaneously, in 1979, James Daniell in the United States, Maurice Bruhart in France, and Yona Tadir in Israel were promoting efforts to couple the carbon dioxide laser to the laparoscope to treat pelvic adhesions and endometriosis. Later on, fiber lasers, KTP, Nd:YAG, and Argon lasers would be utilized in our field. Still, only a few extirpative procedures were being performed via a laparoscope route. This included linear salpingostomy for the treatment of ectopic pregnancy, championed by Professor Bruhart and H. Manhes in Europe, and Alan DeCherney in the United States.
During the 1980s, laparoscopic surgery was at its innovative best. Through the pioneering efforts of Professor Kurt Semm and his protégée, Liselotte Mettler, the gynecologic laparoscopist was introduced to endoloops, simple suturing techniques, and mechanical morcellation techniques.
Procedures such as salpingo-oophorectomy, appendectomy, and myomectomy could now be performed via the laparoscope. Dr. Camran Nezhat coupled the carbon dioxide laser, the laparoscope, and the television monitor, coining the term laparoscopy. Most importantly, the laparoscopic surgeon was liberated; he or she could remain upright and perform surgery with both hands. Through the 1980s and 1990s, Dr. Nezhat, Dr. Harry Reich, and other innovators pushed the envelope in increasing the ability to extirpate endometriosis, excise severe pelvic adhesions, and perform discoid and segmental bowel resection.
The day the earth stood still
Every gynecologic laparoscopic surgeon should remember Jan. 26, 1988, as that was the date that Dr. Harry Reich performed the first total laparoscopic hysterectomy. Now, little more than 25 years later, in many parts of the country, a laparoscopic approach to hysterectomy is indeed the most common route. Over the years, with the evolution of instrumentation, including new energy systems (ultrasonic, advanced bipolar) and the introduction of barbed sutures, hysterectomy can now be performed via minilaparoscopy, single-site laparoscopy, robot-assisted, and robotic single site, all of which have been featured in the Ob.Gyn. News’ Master Class in Gynecologic Surgery.
But hysteroscopy came first
Abulkasim utilized a mirror to reflect light into the vaginal vault in 1,000 A.D. In 1806, Philipp Bozzini originated the idea of illuminating body cavities by an external light source. Through a system of mirrors and tubes, candlelight could be reflected into the body. In 1869, D.C. Pantaleoni used a cystoscope developed by Antoine Desormeaux – who has been called the father of endoscopy – to treat endometrial polyps with silver nitrate.
Through the 50 years of Ob.Gyn. News and over the past 12 years of the Master Class in Gynecologic Surgery, our community has been consistently updated as to advances in hysteroscopy, not only to enhance treatment efficacy, but safety as well. This has included such advances as the continuous flow hysteroscope, the Hamou contact hysteroscope, and fluid management systems to enhance visualization.
In 1978, Robert Neuwirth introduced loops to perform hysteroscopic myomectomy. The loop resectoscope was quickly followed by the rollerball to perform endometrial ablation. In the late 1990s, hysteroscopic bipolar cutting loops were introduced. This enabled use of ionic distension media saline, instead of nonionic media, thus decreasing risks related to hyponatremia.
In 2003, Mark Emanuel introduced hysteroscopic morcellation systems, which enabled more gynecologists to perform operative hysteroscopy safely. Resected tissue is removed immediately to allow superior visualization. The flexible hysteroscope coupled with vaginoscopy has enabled hysteroscopy to be done with minimal to no anesthesia in an in-office setting.
With advances in hysteroscopy over the past 35 years, hysteroscopic procedures such as polypectomy, myomectomy, lysis of adhesions, transection of endometriosis, evacuation of retained products of conception, and endometrial ablation/resection have become routine.
And now, the controversy
Since its inception, laparoscopic surgery has not been without controversy. In 1933, Karl Fervers described explosion and flashes of light from a combination of high frequency electric current and oxygen distension gas while performing laparoscopic adhesiolysis with the coagulation probe of the ureterocystoscope.
In the early 1970s, Professor Kurt Semm’s pioneering effort was not rewarded by his department, in Kiel, Germany, which instead recommended he schedule a brain scan and psychological testing.
Nearly 20 years later, in a 1992 edition of Current Science, Professor Semm, along with Alan DeCherney, stated that “over 80% of gynecological operations can now be performed by laparoscopy.” Shortly thereafter, however, Dr. Roy Pitkin, who at the time was president of the American College of Obstetricians and Gynecologists, wrote an editorial in the Journal of Obstetrics and Gynecology – “Operative Laparoscopy: Surgical Advance or Technical Gimmick?” (Obstet Gynecol. 1992 Mar;79[3]:441-2).
Fortunately, 18 years later, with the continued advances in laparoscopic surgery making it less expensive, safer, and more accessible, Dr. Pitkin did retract his statement (Obstet Gynecol. 2010 May;115[5]:890-1).
Currently, the gynecologic community is embroiled in controversies involving the use of the robot to assist in the performance of laparoscopic surgery, the incorporation of synthetic mesh to enhance urogynecologic procedures, the placement of Essure micro-inserts to occlude fallopian tubes, and the use of electronic power morcellation at time of laparoscopic or robot-assisted hysterectomy, myomectomy, or sacrocolpopexy.
After reading the 2013 article by Dr. Jason Wright, published in JAMA, comparing laparoscopic hysterectomy to robotic hysterectomy, no one can deny that the rise in a minimally invasive route to hysterectomy has coincided with the advent of the robot (JAMA. 2013 Feb 20;309[7]:689-98). On the other hand, many detractors, including Dr. James Breeden (past ACOG president 2012-2013), find the higher cost of robotic surgery very problematic. In fact, many of these detractors cite the paucity of data showing a significant advantage to use of robotics.
While certainly cost, more than ever, must be a major consideration, remember that during the 1990s, there were multiple articles in Ob.Gyn. News raising concerns about the cost of laparoscopic hysterectomy. Interestingly, studies over the past decade by Warren and Jonsdottir show a cost savings when hysterectomy is done laparoscopically as opposed to its being done by laparotomy. Thus, it certainly can be anticipated that with more physician experience, improved instrumentation, and robotic industry competition, the overall cost will become more comparable to a laparoscopic route.
In 1995, Ulf Ulmsten first described the use of tension-free tape (TVT) to treat stress urinary incontinence. In 1998, the Food and Drug Administration approved the use of the TVT sling in the United States. Since then, transobturator tension-free vaginal tape (TVT-O) and single incision mini-slings have been introduced. All of these techniques have been shown to be successful and have been well adapted into the armamentarium of physicians treating stress urinary incontinence.
With the success of synthetic mesh for the treatment of stress urinary incontinence, its use was extended to pelvic prolapse. In 2002, the first mesh device with indications for the treatment of pelvic organ prolapse was approved by the FDA. While the erosion rate utilizing synthetic mesh for stress urinary incontinence has been noted to be 2%, rates up to 8.3% have been noted in patients treated for pelvic prolapse.
In 2008, the FDA issued a warning regarding the use of mesh for prolapse and incontinence repair secondary to the sequelae of mesh erosion. Subsequently, in 2011, the concern was limited to vaginal mesh to correct pelvic organ prolapse. Finally, on Jan. 4, 2016, the FDA issued an order to reclassify surgical mesh to repair pelvic organ prolapse from class II, which includes moderate-risk devices, to class III, which includes high-risk devices. Moreover, the FDA issued a second order to manufacturers to submit a premarket approval application to support the safety and effectiveness of synthetic mesh for transvaginal repair of pelvic organ prolapse.
Essure micro-inserts for permanent birth control received initial approval from the FDA in November 2002. Despite the fact that Essure can be easily placed, is highly effective, and has seemingly low complication rates, concerns have been raised by the Facebook group “Essure Problems” and Erin Brockovich, the focus of the 2000 biographical film starring Julia Roberts.
After more than 5,000 women filed grievances with the FDA between November 2002 and May 2015, based on unintended pregnancies, miscarriages, stillbirths, severe pain, and bleeding, the FDA announced in 2016 that it would require a boxed warning label for Essure. The FDA also called upon Bayer, which makes and markets Essure, to conduct surveillance to assess “risks of the device in a real-world environment.” The agency stated it will use the results to “determine what, if any, further actions related to Essure are needed to protect public health.”
While Jan. 26, 1988, is a very special date in minimally invasive gynecologic surgery, April 17, 2014, is a day of infamy for the gynecologic laparoscopist. For on this day, the FDA announced a warning regarding electronic power morcellation. Many hospitals and hospital systems throughout the country issued bans on electronic power morcellation, leading to needless open laparotomy procedures and thus, introducing prolonged recovery times and increased risk.
At a time when the recent introduction of barbed suture had made both closure of the vaginal cuff at time of hysterectomy and repair of the hysterotomy at myomectomy easier and faster, the gynecologic laparoscopist was taking a step backward. The FDA based this decision and a subsequent boxed warning – issued in November 2014 – on a small number of studies showing potential upstaging of leiomyosarcoma post electronic power morcellation. Interestingly, many of the morcellation procedures cited did not use power morcellation. Furthermore, a more comprehensive meta-analysis by Elizabeth A. Pritts and colleagues, showed a far lower risk than suggested by the FDA (Gynecol Surg. 2015;12[3]:165-77).
Recently, an article by William Parker and colleagues recommended that the FDA reverse its position (Obstet Gynecol. 2016 Jan;127[1]:18-22). Many believe that ultimately, the solution will be morcellation in a containment bag, which I and my colleagues have been performing in virtually every power morcellation procedure since May 2014. During this current power morcellation controversy, the Master Class in Gynecologic Surgery has continued to update its readers with three different articles related to the subject.
And in conclusion
Without a doubt, the past 50 years of gynecologic surgery has been a time of unparalleled innovation with occasional controversy thrown in. Ob.Gyn. News and more recently, the Master Class in Gynecologic Surgery, has had a major leadership role in bringing this profound ingenuity to the gynecology community by introducing this explosion of surgical creativity to its readers.
And what will the next 50 years bring? I believe we will continue to see tremendous advancements in minimally invasive gynecologic surgery. There will be a definite impact of costs on the marketplace. Thus, many of the minor minimally invasive procedures currently performed in the hospital or surgery center will be brought into office settings. In addition, secondary to reimbursement, the more complex cases will be carried out by fewer gynecologic surgeons who have undergone more intense training in pelvic surgery and who can perform these cases more efficiently and with fewer complications. Our ability to perform surgery and what type of procedures we do will not only be based on randomized, controlled trials, but big data collection as well.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and is on the speakers bureau for Ethicon.
New heart failure interventions face outcomes test
A history of trials where a well-reasoned heart failure intervention did not have the expected results is now coloring the way some clinicians view potential new treatments.
A couple of examples of this cautious, skeptical mindset cropped up during the annual meeting of the Heart Failure Association of the European Society of Cardiology in Florence, Italy, in May.
I previously reported on one of the major talks at the sessions, results from a pivotal trial of a phrenic nerve stimulation device in 151 patients with some type of cardiovascular disease (more than half had heart failure) and central sleep apnea. These patients generally had significant improvement in their apnea-hypopnea index while on active treatment with the phrenic nerve stimulator, designed to produce rhythmic contractions of the diaphragm to create negative chest pressure and enhance breathing in a way that mimics natural respiration and avoids the apparent danger from a positive-pressure intervention in patients with central sleep apnea and heart failure with reduced ejection fraction (HFrEF).
The positive-pressure danger in such patients occurred unexpectedly and dramatically in the form of significantly increased mortality among HFrEF patients with central sleep apnea enrolled in the SERVE-HF trial. Based on that chilling experience, “our understanding of central sleep apnea is imperfect,” said Dr. Martin R. Cowie, lead investigator of SERVE-HF, who rehashed his experience with that study during the recent meeting. “A really important message was that just because patients say they feel better [with the adaptive servo-ventilation tested in SERVE-HF], that doesn’t necessarily translate into benefit,” Dr. Cowie noted.
Dr. Mariell L. Jessup, a leading U.S. heart failure expert, had a similar reaction when I spoke with her during the meeting.
“A lot of things made a lot of sense, including treating central sleep apnea in a patient with HFrEF with positive air pressure that made them feel better,” she said, also invoking the specter of SERVE-HF. “We have to now demand that sleep trials show benefit in clinical outcomes,” such as reduced mortality or a cut in heart failure hospitalizations, and certainly no increase in mortality. Until that’s shown for phrenic nerve stimulation she’ll stay a skeptic, she told me.
Another intervention recently available for U.S. heart failure patients that seems on track to confront this “show-me-the-outcomes” attitude involves new drugs that cut serum potassium levels by binding to potassium in the gastrointestinal tract. This class includes patiromer (Veltassa), approved by the Food and Drug Administration in October 2015. Another potassium binder, sodium zirconium cyclosilicate (ZS-9), seems on track to soon receive FDA approval.
Several speakers at the meeting spoke of the potential to use these drugs to control the hyperkalemia that often complicates treatment of heart failure patients with an ACE inhibitor, angiotensin receptor blocker, or a mineralocorticoid receptor antagonist, especially heart failure patients with concomitant renal disease. Study results showed treatment with patiromer or ZS-9 reversed hyperkalemia, thereby allowing patients to remain on these drugs that are known to significantly cut rates of mortality and heart failure hospitalizations in heart failure patients.
“Right now, a lot of patients don’t get these lifesaving drugs” because of hyperkalemia, noted Dr. Bertram Pitt, a University of Michigan cardiologist who has been involved in several patiromer trials (and is a consultant to Relypsa, the company that markets patiromer).
The problem is that no studies of patiromer or ZS-9 treatment have so far shown that these drugs lead to reduced mortality or heart failure hospitalizations in heart failure patients. All that’s been shown is that patiromer and ZS-9 are effective at lowering potassium levels in patients with hyperkalemia out of the danger zone, levels above 5 mEq/L.
“I want to see outcome trials,” said Dr. Jessup.
Dr. Pitt agreed that outcomes data would be ideal, but also noted that currently no studies aimed at collecting these data are underway.
Without these data, clinicians need to decide whether they believe proven potassium lowering alone is a good enough reason to prescribe patiromer or ZS-9, or whether they need to see proof that these drugs give patients clinically meaningful benefits.
If they demand outcomes evidence they may need to wait quite a while.
On Twitter @mitchelzoler
A history of trials where a well-reasoned heart failure intervention did not have the expected results is now coloring the way some clinicians view potential new treatments.
A couple of examples of this cautious, skeptical mindset cropped up during the annual meeting of the Heart Failure Association of the European Society of Cardiology in Florence, Italy, in May.
I previously reported on one of the major talks at the sessions, results from a pivotal trial of a phrenic nerve stimulation device in 151 patients with some type of cardiovascular disease (more than half had heart failure) and central sleep apnea. These patients generally had significant improvement in their apnea-hypopnea index while on active treatment with the phrenic nerve stimulator, designed to produce rhythmic contractions of the diaphragm to create negative chest pressure and enhance breathing in a way that mimics natural respiration and avoids the apparent danger from a positive-pressure intervention in patients with central sleep apnea and heart failure with reduced ejection fraction (HFrEF).
The positive-pressure danger in such patients occurred unexpectedly and dramatically in the form of significantly increased mortality among HFrEF patients with central sleep apnea enrolled in the SERVE-HF trial. Based on that chilling experience, “our understanding of central sleep apnea is imperfect,” said Dr. Martin R. Cowie, lead investigator of SERVE-HF, who rehashed his experience with that study during the recent meeting. “A really important message was that just because patients say they feel better [with the adaptive servo-ventilation tested in SERVE-HF], that doesn’t necessarily translate into benefit,” Dr. Cowie noted.
Dr. Mariell L. Jessup, a leading U.S. heart failure expert, had a similar reaction when I spoke with her during the meeting.
“A lot of things made a lot of sense, including treating central sleep apnea in a patient with HFrEF with positive air pressure that made them feel better,” she said, also invoking the specter of SERVE-HF. “We have to now demand that sleep trials show benefit in clinical outcomes,” such as reduced mortality or a cut in heart failure hospitalizations, and certainly no increase in mortality. Until that’s shown for phrenic nerve stimulation she’ll stay a skeptic, she told me.
Another intervention recently available for U.S. heart failure patients that seems on track to confront this “show-me-the-outcomes” attitude involves new drugs that cut serum potassium levels by binding to potassium in the gastrointestinal tract. This class includes patiromer (Veltassa), approved by the Food and Drug Administration in October 2015. Another potassium binder, sodium zirconium cyclosilicate (ZS-9), seems on track to soon receive FDA approval.
Several speakers at the meeting spoke of the potential to use these drugs to control the hyperkalemia that often complicates treatment of heart failure patients with an ACE inhibitor, angiotensin receptor blocker, or a mineralocorticoid receptor antagonist, especially heart failure patients with concomitant renal disease. Study results showed treatment with patiromer or ZS-9 reversed hyperkalemia, thereby allowing patients to remain on these drugs that are known to significantly cut rates of mortality and heart failure hospitalizations in heart failure patients.
“Right now, a lot of patients don’t get these lifesaving drugs” because of hyperkalemia, noted Dr. Bertram Pitt, a University of Michigan cardiologist who has been involved in several patiromer trials (and is a consultant to Relypsa, the company that markets patiromer).
The problem is that no studies of patiromer or ZS-9 treatment have so far shown that these drugs lead to reduced mortality or heart failure hospitalizations in heart failure patients. All that’s been shown is that patiromer and ZS-9 are effective at lowering potassium levels in patients with hyperkalemia out of the danger zone, levels above 5 mEq/L.
“I want to see outcome trials,” said Dr. Jessup.
Dr. Pitt agreed that outcomes data would be ideal, but also noted that currently no studies aimed at collecting these data are underway.
Without these data, clinicians need to decide whether they believe proven potassium lowering alone is a good enough reason to prescribe patiromer or ZS-9, or whether they need to see proof that these drugs give patients clinically meaningful benefits.
If they demand outcomes evidence they may need to wait quite a while.
On Twitter @mitchelzoler
A history of trials where a well-reasoned heart failure intervention did not have the expected results is now coloring the way some clinicians view potential new treatments.
A couple of examples of this cautious, skeptical mindset cropped up during the annual meeting of the Heart Failure Association of the European Society of Cardiology in Florence, Italy, in May.
I previously reported on one of the major talks at the sessions, results from a pivotal trial of a phrenic nerve stimulation device in 151 patients with some type of cardiovascular disease (more than half had heart failure) and central sleep apnea. These patients generally had significant improvement in their apnea-hypopnea index while on active treatment with the phrenic nerve stimulator, designed to produce rhythmic contractions of the diaphragm to create negative chest pressure and enhance breathing in a way that mimics natural respiration and avoids the apparent danger from a positive-pressure intervention in patients with central sleep apnea and heart failure with reduced ejection fraction (HFrEF).
The positive-pressure danger in such patients occurred unexpectedly and dramatically in the form of significantly increased mortality among HFrEF patients with central sleep apnea enrolled in the SERVE-HF trial. Based on that chilling experience, “our understanding of central sleep apnea is imperfect,” said Dr. Martin R. Cowie, lead investigator of SERVE-HF, who rehashed his experience with that study during the recent meeting. “A really important message was that just because patients say they feel better [with the adaptive servo-ventilation tested in SERVE-HF], that doesn’t necessarily translate into benefit,” Dr. Cowie noted.
Dr. Mariell L. Jessup, a leading U.S. heart failure expert, had a similar reaction when I spoke with her during the meeting.
“A lot of things made a lot of sense, including treating central sleep apnea in a patient with HFrEF with positive air pressure that made them feel better,” she said, also invoking the specter of SERVE-HF. “We have to now demand that sleep trials show benefit in clinical outcomes,” such as reduced mortality or a cut in heart failure hospitalizations, and certainly no increase in mortality. Until that’s shown for phrenic nerve stimulation she’ll stay a skeptic, she told me.
Another intervention recently available for U.S. heart failure patients that seems on track to confront this “show-me-the-outcomes” attitude involves new drugs that cut serum potassium levels by binding to potassium in the gastrointestinal tract. This class includes patiromer (Veltassa), approved by the Food and Drug Administration in October 2015. Another potassium binder, sodium zirconium cyclosilicate (ZS-9), seems on track to soon receive FDA approval.
Several speakers at the meeting spoke of the potential to use these drugs to control the hyperkalemia that often complicates treatment of heart failure patients with an ACE inhibitor, angiotensin receptor blocker, or a mineralocorticoid receptor antagonist, especially heart failure patients with concomitant renal disease. Study results showed treatment with patiromer or ZS-9 reversed hyperkalemia, thereby allowing patients to remain on these drugs that are known to significantly cut rates of mortality and heart failure hospitalizations in heart failure patients.
“Right now, a lot of patients don’t get these lifesaving drugs” because of hyperkalemia, noted Dr. Bertram Pitt, a University of Michigan cardiologist who has been involved in several patiromer trials (and is a consultant to Relypsa, the company that markets patiromer).
The problem is that no studies of patiromer or ZS-9 treatment have so far shown that these drugs lead to reduced mortality or heart failure hospitalizations in heart failure patients. All that’s been shown is that patiromer and ZS-9 are effective at lowering potassium levels in patients with hyperkalemia out of the danger zone, levels above 5 mEq/L.
“I want to see outcome trials,” said Dr. Jessup.
Dr. Pitt agreed that outcomes data would be ideal, but also noted that currently no studies aimed at collecting these data are underway.
Without these data, clinicians need to decide whether they believe proven potassium lowering alone is a good enough reason to prescribe patiromer or ZS-9, or whether they need to see proof that these drugs give patients clinically meaningful benefits.
If they demand outcomes evidence they may need to wait quite a while.
On Twitter @mitchelzoler
Weather or not
There was an outdoor equipment designer for a well-known Maine-based outfitter who was fond of saying that there was no such thing as bad weather, just bad or inappropriate clothing. The origin of this pearl is claimed by the Norwegians, the Germans, and the English – all nations that have some experience with inclement weather.
The folks who continue to go about their business regardless of the weather usually place a high value on the benefits of being outdoors. They often claim that they simply feel healthier when they are breathing fresh air. It is likely they have grown up in a place, in a family, and in a culture in which waiting for good weather to get something done means it’s not going to get done.
For example, my daughter-in-law’s visual impairment prevents her from driving a car. As a result, she and my two grandchildren get to almost all of their in-town destinations here in Brunswick, Maine, by bicycle or on foot ... 12 months a year ... rain or shine. They can afford appropriate clothing, but they still get wet and cold from time to time. I have never heard them complain. They accept bad weather as a given just as much as they accept a sunny day as something to enjoy.
While my grandchildren’s attitude toward the weather may not be typical of most 9- and 11-year-olds, they may not be alone in a few decades. Preschools are popping up around the country that not only accept the vagaries in the weather, but embrace the outdoors as an educational tool (“Preschool Without Walls,” by Lillian Mongeau, the New York Times, Dec. 29, 2015). The Natural Start Alliance, founded in 2013, has a membership of 92 preschools whose students spend a significant portion of their school day playing and exploring outdoors rain or shine. While most of these schools emphasize the value of learning from the natural environment, one of my granddaughters attended an outdoors-rain-or-shine preschool in urban San Francisco.
At the other end of the spectrum are the schools that feel obligated to shield their students from the realities of meteorological variability. While excessive sun exposure and tissue-freezing wind chills are to be avoided, a crisp calm sunny day with temperatures in the middle teens can be fun, particularly if there is some dry snow to tromp around in. However, during the months from November 2014 to March 2015, the children who attended Public School 126 in New York City were not given the opportunity to play outside on more than 40 school days. The situation is complicated because their school, which lacks its own playground, must rely on nearby parks (“A Casualty of a Frigid New York Winter: Outdoor School Recess,” by Ginia Bellafante, the New York Times, March 6, 2015). But we aren’t talking Siberia. It was a colder-than-usual winter, but I suspect there must have been more than a few missed opportunities to go outside and enjoy the snow. I wonder how often the decision to stay inside was influenced by teachers and administrators who hadn’t come prepared to spend any more time outside than it took them to walk from the parking lot or bus stop.
The data are accumulating that adding physical activity to the school day improves student behavior and even promotes learning. The evidence that being outside is beneficial is a bit more difficult to find. Early in the last century, when indoor air was saturated with smoke from cooking and open combustion heating sources, many physicians recommended that even for infants, raising a healthy child meant having the child spend a large part of the day outdoors.
It’s time for pediatricians to spread the word to parents that their little darlings won’t catch pneumonia from playing outdoors on a cool damp day. Nor will they shrink if they get a little wet on a rainy day ... but they will run ... and the running will be good for them.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
There was an outdoor equipment designer for a well-known Maine-based outfitter who was fond of saying that there was no such thing as bad weather, just bad or inappropriate clothing. The origin of this pearl is claimed by the Norwegians, the Germans, and the English – all nations that have some experience with inclement weather.
The folks who continue to go about their business regardless of the weather usually place a high value on the benefits of being outdoors. They often claim that they simply feel healthier when they are breathing fresh air. It is likely they have grown up in a place, in a family, and in a culture in which waiting for good weather to get something done means it’s not going to get done.
For example, my daughter-in-law’s visual impairment prevents her from driving a car. As a result, she and my two grandchildren get to almost all of their in-town destinations here in Brunswick, Maine, by bicycle or on foot ... 12 months a year ... rain or shine. They can afford appropriate clothing, but they still get wet and cold from time to time. I have never heard them complain. They accept bad weather as a given just as much as they accept a sunny day as something to enjoy.
While my grandchildren’s attitude toward the weather may not be typical of most 9- and 11-year-olds, they may not be alone in a few decades. Preschools are popping up around the country that not only accept the vagaries in the weather, but embrace the outdoors as an educational tool (“Preschool Without Walls,” by Lillian Mongeau, the New York Times, Dec. 29, 2015). The Natural Start Alliance, founded in 2013, has a membership of 92 preschools whose students spend a significant portion of their school day playing and exploring outdoors rain or shine. While most of these schools emphasize the value of learning from the natural environment, one of my granddaughters attended an outdoors-rain-or-shine preschool in urban San Francisco.
At the other end of the spectrum are the schools that feel obligated to shield their students from the realities of meteorological variability. While excessive sun exposure and tissue-freezing wind chills are to be avoided, a crisp calm sunny day with temperatures in the middle teens can be fun, particularly if there is some dry snow to tromp around in. However, during the months from November 2014 to March 2015, the children who attended Public School 126 in New York City were not given the opportunity to play outside on more than 40 school days. The situation is complicated because their school, which lacks its own playground, must rely on nearby parks (“A Casualty of a Frigid New York Winter: Outdoor School Recess,” by Ginia Bellafante, the New York Times, March 6, 2015). But we aren’t talking Siberia. It was a colder-than-usual winter, but I suspect there must have been more than a few missed opportunities to go outside and enjoy the snow. I wonder how often the decision to stay inside was influenced by teachers and administrators who hadn’t come prepared to spend any more time outside than it took them to walk from the parking lot or bus stop.
The data are accumulating that adding physical activity to the school day improves student behavior and even promotes learning. The evidence that being outside is beneficial is a bit more difficult to find. Early in the last century, when indoor air was saturated with smoke from cooking and open combustion heating sources, many physicians recommended that even for infants, raising a healthy child meant having the child spend a large part of the day outdoors.
It’s time for pediatricians to spread the word to parents that their little darlings won’t catch pneumonia from playing outdoors on a cool damp day. Nor will they shrink if they get a little wet on a rainy day ... but they will run ... and the running will be good for them.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
There was an outdoor equipment designer for a well-known Maine-based outfitter who was fond of saying that there was no such thing as bad weather, just bad or inappropriate clothing. The origin of this pearl is claimed by the Norwegians, the Germans, and the English – all nations that have some experience with inclement weather.
The folks who continue to go about their business regardless of the weather usually place a high value on the benefits of being outdoors. They often claim that they simply feel healthier when they are breathing fresh air. It is likely they have grown up in a place, in a family, and in a culture in which waiting for good weather to get something done means it’s not going to get done.
For example, my daughter-in-law’s visual impairment prevents her from driving a car. As a result, she and my two grandchildren get to almost all of their in-town destinations here in Brunswick, Maine, by bicycle or on foot ... 12 months a year ... rain or shine. They can afford appropriate clothing, but they still get wet and cold from time to time. I have never heard them complain. They accept bad weather as a given just as much as they accept a sunny day as something to enjoy.
While my grandchildren’s attitude toward the weather may not be typical of most 9- and 11-year-olds, they may not be alone in a few decades. Preschools are popping up around the country that not only accept the vagaries in the weather, but embrace the outdoors as an educational tool (“Preschool Without Walls,” by Lillian Mongeau, the New York Times, Dec. 29, 2015). The Natural Start Alliance, founded in 2013, has a membership of 92 preschools whose students spend a significant portion of their school day playing and exploring outdoors rain or shine. While most of these schools emphasize the value of learning from the natural environment, one of my granddaughters attended an outdoors-rain-or-shine preschool in urban San Francisco.
At the other end of the spectrum are the schools that feel obligated to shield their students from the realities of meteorological variability. While excessive sun exposure and tissue-freezing wind chills are to be avoided, a crisp calm sunny day with temperatures in the middle teens can be fun, particularly if there is some dry snow to tromp around in. However, during the months from November 2014 to March 2015, the children who attended Public School 126 in New York City were not given the opportunity to play outside on more than 40 school days. The situation is complicated because their school, which lacks its own playground, must rely on nearby parks (“A Casualty of a Frigid New York Winter: Outdoor School Recess,” by Ginia Bellafante, the New York Times, March 6, 2015). But we aren’t talking Siberia. It was a colder-than-usual winter, but I suspect there must have been more than a few missed opportunities to go outside and enjoy the snow. I wonder how often the decision to stay inside was influenced by teachers and administrators who hadn’t come prepared to spend any more time outside than it took them to walk from the parking lot or bus stop.
The data are accumulating that adding physical activity to the school day improves student behavior and even promotes learning. The evidence that being outside is beneficial is a bit more difficult to find. Early in the last century, when indoor air was saturated with smoke from cooking and open combustion heating sources, many physicians recommended that even for infants, raising a healthy child meant having the child spend a large part of the day outdoors.
It’s time for pediatricians to spread the word to parents that their little darlings won’t catch pneumonia from playing outdoors on a cool damp day. Nor will they shrink if they get a little wet on a rainy day ... but they will run ... and the running will be good for them.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
Déjà vu: An FDA warning about oral ketoconazole ... again
The Food and Drug Administration issued a health warning on May 16th, 2016, regarding the use of oral ketoconazole for the treatment of skin and nail dermatophyte and candidal infections – wait what? Why is this even an active discussion? Let’s take a step back: In July 2013, the FDA strengthened its warnings and withdrew FDA indications for ketoconazole, specifically stating that its use for Candida and dermatophyte infections is no longer indicated and that it should only be considered in fungal infections, such as blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis, when other antifungals are not available.
The reality is that for, most clinicians, it has been well accepted for years that this drug has significant toxicities, probably the most among the azole class, especially in terms of liver issues and drug interactions being one of the most potent inhibitors of the hepatic CYP (cytochrome P450) system.
Endocrinologists have been preaching that the drug can impair adrenal function, causing insufficiency. So this warning begs the question … who is still actively prescribing this medication? Sadly, this advisory was in response to data showing that this drug was still being prescribed for skin and nail fungal infections in 2015, as well as one documented death associated with its use for this indication. Seriously? Terbinafine (250 mg daily for 6 weeks for fingernails, 12 weeks for toenails) and fluconazole (300 mg weekly for 6 months for fingernails and 9 months for toenails) are both safe and cheap means to treat onychomycosis. If you can get it covered, we even have effective topical treatments for onychomycosis, not to mention we have ALWAYS had topical options for superficial cutaneous mycoses. This is a no brainer. Just say NO to oral ketoconazole.
Dr. Adam Friedman is the residency program director and director of translational research in the department of dermatology, George Washington University, Washington. He is on the editorial advisory board of Dermatology News. He has no related disclosures.
The Food and Drug Administration issued a health warning on May 16th, 2016, regarding the use of oral ketoconazole for the treatment of skin and nail dermatophyte and candidal infections – wait what? Why is this even an active discussion? Let’s take a step back: In July 2013, the FDA strengthened its warnings and withdrew FDA indications for ketoconazole, specifically stating that its use for Candida and dermatophyte infections is no longer indicated and that it should only be considered in fungal infections, such as blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis, when other antifungals are not available.
The reality is that for, most clinicians, it has been well accepted for years that this drug has significant toxicities, probably the most among the azole class, especially in terms of liver issues and drug interactions being one of the most potent inhibitors of the hepatic CYP (cytochrome P450) system.
Endocrinologists have been preaching that the drug can impair adrenal function, causing insufficiency. So this warning begs the question … who is still actively prescribing this medication? Sadly, this advisory was in response to data showing that this drug was still being prescribed for skin and nail fungal infections in 2015, as well as one documented death associated with its use for this indication. Seriously? Terbinafine (250 mg daily for 6 weeks for fingernails, 12 weeks for toenails) and fluconazole (300 mg weekly for 6 months for fingernails and 9 months for toenails) are both safe and cheap means to treat onychomycosis. If you can get it covered, we even have effective topical treatments for onychomycosis, not to mention we have ALWAYS had topical options for superficial cutaneous mycoses. This is a no brainer. Just say NO to oral ketoconazole.
Dr. Adam Friedman is the residency program director and director of translational research in the department of dermatology, George Washington University, Washington. He is on the editorial advisory board of Dermatology News. He has no related disclosures.
The Food and Drug Administration issued a health warning on May 16th, 2016, regarding the use of oral ketoconazole for the treatment of skin and nail dermatophyte and candidal infections – wait what? Why is this even an active discussion? Let’s take a step back: In July 2013, the FDA strengthened its warnings and withdrew FDA indications for ketoconazole, specifically stating that its use for Candida and dermatophyte infections is no longer indicated and that it should only be considered in fungal infections, such as blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis, when other antifungals are not available.
The reality is that for, most clinicians, it has been well accepted for years that this drug has significant toxicities, probably the most among the azole class, especially in terms of liver issues and drug interactions being one of the most potent inhibitors of the hepatic CYP (cytochrome P450) system.
Endocrinologists have been preaching that the drug can impair adrenal function, causing insufficiency. So this warning begs the question … who is still actively prescribing this medication? Sadly, this advisory was in response to data showing that this drug was still being prescribed for skin and nail fungal infections in 2015, as well as one documented death associated with its use for this indication. Seriously? Terbinafine (250 mg daily for 6 weeks for fingernails, 12 weeks for toenails) and fluconazole (300 mg weekly for 6 months for fingernails and 9 months for toenails) are both safe and cheap means to treat onychomycosis. If you can get it covered, we even have effective topical treatments for onychomycosis, not to mention we have ALWAYS had topical options for superficial cutaneous mycoses. This is a no brainer. Just say NO to oral ketoconazole.
Dr. Adam Friedman is the residency program director and director of translational research in the department of dermatology, George Washington University, Washington. He is on the editorial advisory board of Dermatology News. He has no related disclosures.
Peter Kramer returns to his role in defending antidepressants in ‘Ordinarily Well’
With his 1993 landmark book, “Listening to Prozac,” psychiatrist Peter D. Kramer became one of the most famous psychiatrists in America – second perhaps only to the fictional Frasier Crane of primetime TV. Since then, Dr. Kramer has continued to write – including a novel, books on other psychiatric topics, a blog, and articles for mainstream media, including the New York Times. In his latest book, “Ordinarily Well: The Case for Antidepressants,” Dr. Kramer will return to the role he left behind 23 years ago: defending the use of antidepressants.
Dr. Kramer notes that he doesn’t do this easily. In the preface, with a bit of dismay, he talks about how reviewers have called him “Dr. Prozac.” He’s felt stuck on a wave that didn’t reflect his diverse interests, and he didn’t want to be covered by the spreading stain of Big Pharma.
“Against all indications, I remained hopeful that I might walk free by day, alter my obituary,” Dr. Kramer tells the reader.
With “Ordinarily Well,” Peter Kramer is back as Dr. Prozac. The book is written as a response to the “research”– now seen so often in headlines – that antidepressants are as effective as sugar pills for mild to moderate depression, and they should be prescribed only for severe major depression. He gets even more specific: The book is written partly in response to an article by Dr. Marcia Angell, former editor in chief of the New England Journal of Medicine, published in June 2011 in the New York Review of Books, and Dr. Angell’s assertion that psychiatric medications are no more effective than placebo. Since psychotropics come with more side effects than placebos, the next logical conclusion is that they are not only ineffective – they are harmful.
As a clinical psychiatrist, I’ve found this evidence-based stance to be perplexing. People get better on antidepressants, even if you aren’t measuring Hamilton rating scores and even if you aren’t limiting treatment to those with severe major depression. I would estimate that at least some of the people, some of the time, get better, and when you progress to strategies of switching and augmenting, most of the the people, most of the time, get significant relief from their major depression.
Obviously, this is my clinical impression and not research, and Dr. Kramer takes a more ardent stance: Most patients with depression, be it mild, moderate, severe, or long-standing dysthymia, have a good response to antidepressants. It’s the minority who don’t respond.
Dr. Kramer goes through the “science” that would suggest that antidepressants are not effective for milder forms of depression, dysthymia, and neuroticism. He does a systematic and comprehensive review of how pharmaceutical studies are conducted, and what factors might skew results, and there is plenty here to fill the pages. He explains complex issues – such as meta-analyses and numbers needed to treat – such that the lay reader can follow.
As just one example, Dr. Kramer talks about screening research subjects for participation in antidepressant studies: “If raters have a sense of the minimum Hamilton score for admission to a study, and if they are under pressure to fill an enrollment quota, they will be inclined to tack on questionable Hamilton points. The boost will not be uniform. There’s no need to raise rating in the very ill. Scores for least afflicted participants will be most inflated.
“When off-site raters, with no stake in the pace of enrollment, analyze tapes of admission interviews,” he continues, “they find patients to be much healthier than the on-site Hamilton scores suggest. According to off-site assessments, many patients admitted to drug studies simply are not depressed.”
Dr. Kramer methodically marches through problems with finding patients for the studies, shortcomings of the Hamilton rating scale, which gives suicidal ideation the same point as a somatic symptom; the bias some studies have of excluding people with severe depression; substance abuse, or comorbid disorders; and “the floor effect,” which underestimates efficacy in patients with fewer symptoms.
He follows subjects at an unnamed for-profit research center and praises the skills of everyone who comes in contact with the research subjects, including the friendly van driver who fetches patients from their homes.
In a chapter titled “How We’re Doing,” Dr. Kramer goes into detail about specific studies, including the STAR*D trials, where patients were recruited from primary care and psychiatric clinics with the guarantee that they would be provided active medication, and those with comorbid conditions were not excluded.
“Only the sickest came. For nearly 80% of the participants (more than 2,800 were tested), the disorder was chronic. The average length of the current depressive episode was more than 2 years, generally despite attempts at treatment. The average enrollee had lived with depression on and off for more than15 years and was now in a seventh episode. Most patients were alcoholics or had other forms of mental illness.
“In the first phase, patients were put on Celexa [citalopram], managed by their own doctors. About 30% of patients achieved remission within weeks – with virtually no symptoms. Responses (including remissions) ran at just under 50%.
“Commentators considered this outcome disappointing, but is it?”
So this is Dr. Kramer’s strength: He writes an engaging book about a complex topic, arguing throughout that antidepressants work well and have been given a bum rap by flawed research and careless journalism that enjoy the sensationalism of villainizing psychotropics. There are no headlines, he points out, singing the praises of antidepressants for milder forms of depression, even when the evidence is there. Despite the complexity of the topic and the breadth of his research reviews, Dr. Kramer tells the story of antidepressant research in a way that a lay reader can follow. There are no mice or moleculars, no genetic loci, and no explanations of cytochrome P450 metabolism, neurotransmission, or synaptic blockade. The complexities are explained without medical lingo and, in the end, he concludes what psychiatrists see every day: Antidepressants work. They work for the sickest of the sick, and they work for those who are suffering from less-severe forms of depression.
Dr. Kramer ends the book with a discussion of his own clinical experiences, which are not always in tune with what the “science” declares to be true. He cites studies that show that psychotherapy adds nothing to the treatment of depression, yet still, he treats his own patients with psychotherapy. He notes studies that show maximal efficacy when medications are used at high doses and continue for the long haul at these high doses, yet in his own practice, he sometimes uses lower doses and weans patients off medications. He does a wonderful job of pointing out the disconnect of the promise of evidence-based medicine and how its usefulness has limits in clinical practice.
In our communications, Dr. Kramer wrote to me: “There were many reasons not to write this book, and I was reluctant. I took up the topic only after declining a series of opportunities to weigh in. This book is the only one of mine that I wrote primarily out of a sense of duty. The debunking of antidepressants had gone too far and been too widely accepted, and I believed that the underlying research was shaky.”
He worried that I saw his view of antidepressants as more favorable than he intended it to be, and in fact, his perception is correct: While I prescribe antidepressants and see their benefits (as well as their side effects) with many of my patients, I believe Peter Kramer is more enthusiastic than I am about the efficacy of antidepressants for milder forms of depression, dysthymia, and neuroticism.
“My view is the one expressed in the title,” Dr. Kramer countered, “Our medications work ordinarily well, and they bring patients to a state of ordinary wellness.”
“Ordinarily Well” will be available on June 7; it is available for preorder on Amazon now.
Dr. Miller is a coauthor of “Shrink Rap: Three Psychiatrists Explain Their Work” (Baltimore: Johns Hopkins University Press, 2011).
With his 1993 landmark book, “Listening to Prozac,” psychiatrist Peter D. Kramer became one of the most famous psychiatrists in America – second perhaps only to the fictional Frasier Crane of primetime TV. Since then, Dr. Kramer has continued to write – including a novel, books on other psychiatric topics, a blog, and articles for mainstream media, including the New York Times. In his latest book, “Ordinarily Well: The Case for Antidepressants,” Dr. Kramer will return to the role he left behind 23 years ago: defending the use of antidepressants.
Dr. Kramer notes that he doesn’t do this easily. In the preface, with a bit of dismay, he talks about how reviewers have called him “Dr. Prozac.” He’s felt stuck on a wave that didn’t reflect his diverse interests, and he didn’t want to be covered by the spreading stain of Big Pharma.
“Against all indications, I remained hopeful that I might walk free by day, alter my obituary,” Dr. Kramer tells the reader.
With “Ordinarily Well,” Peter Kramer is back as Dr. Prozac. The book is written as a response to the “research”– now seen so often in headlines – that antidepressants are as effective as sugar pills for mild to moderate depression, and they should be prescribed only for severe major depression. He gets even more specific: The book is written partly in response to an article by Dr. Marcia Angell, former editor in chief of the New England Journal of Medicine, published in June 2011 in the New York Review of Books, and Dr. Angell’s assertion that psychiatric medications are no more effective than placebo. Since psychotropics come with more side effects than placebos, the next logical conclusion is that they are not only ineffective – they are harmful.
As a clinical psychiatrist, I’ve found this evidence-based stance to be perplexing. People get better on antidepressants, even if you aren’t measuring Hamilton rating scores and even if you aren’t limiting treatment to those with severe major depression. I would estimate that at least some of the people, some of the time, get better, and when you progress to strategies of switching and augmenting, most of the the people, most of the time, get significant relief from their major depression.
Obviously, this is my clinical impression and not research, and Dr. Kramer takes a more ardent stance: Most patients with depression, be it mild, moderate, severe, or long-standing dysthymia, have a good response to antidepressants. It’s the minority who don’t respond.
Dr. Kramer goes through the “science” that would suggest that antidepressants are not effective for milder forms of depression, dysthymia, and neuroticism. He does a systematic and comprehensive review of how pharmaceutical studies are conducted, and what factors might skew results, and there is plenty here to fill the pages. He explains complex issues – such as meta-analyses and numbers needed to treat – such that the lay reader can follow.
As just one example, Dr. Kramer talks about screening research subjects for participation in antidepressant studies: “If raters have a sense of the minimum Hamilton score for admission to a study, and if they are under pressure to fill an enrollment quota, they will be inclined to tack on questionable Hamilton points. The boost will not be uniform. There’s no need to raise rating in the very ill. Scores for least afflicted participants will be most inflated.
“When off-site raters, with no stake in the pace of enrollment, analyze tapes of admission interviews,” he continues, “they find patients to be much healthier than the on-site Hamilton scores suggest. According to off-site assessments, many patients admitted to drug studies simply are not depressed.”
Dr. Kramer methodically marches through problems with finding patients for the studies, shortcomings of the Hamilton rating scale, which gives suicidal ideation the same point as a somatic symptom; the bias some studies have of excluding people with severe depression; substance abuse, or comorbid disorders; and “the floor effect,” which underestimates efficacy in patients with fewer symptoms.
He follows subjects at an unnamed for-profit research center and praises the skills of everyone who comes in contact with the research subjects, including the friendly van driver who fetches patients from their homes.
In a chapter titled “How We’re Doing,” Dr. Kramer goes into detail about specific studies, including the STAR*D trials, where patients were recruited from primary care and psychiatric clinics with the guarantee that they would be provided active medication, and those with comorbid conditions were not excluded.
“Only the sickest came. For nearly 80% of the participants (more than 2,800 were tested), the disorder was chronic. The average length of the current depressive episode was more than 2 years, generally despite attempts at treatment. The average enrollee had lived with depression on and off for more than15 years and was now in a seventh episode. Most patients were alcoholics or had other forms of mental illness.
“In the first phase, patients were put on Celexa [citalopram], managed by their own doctors. About 30% of patients achieved remission within weeks – with virtually no symptoms. Responses (including remissions) ran at just under 50%.
“Commentators considered this outcome disappointing, but is it?”
So this is Dr. Kramer’s strength: He writes an engaging book about a complex topic, arguing throughout that antidepressants work well and have been given a bum rap by flawed research and careless journalism that enjoy the sensationalism of villainizing psychotropics. There are no headlines, he points out, singing the praises of antidepressants for milder forms of depression, even when the evidence is there. Despite the complexity of the topic and the breadth of his research reviews, Dr. Kramer tells the story of antidepressant research in a way that a lay reader can follow. There are no mice or moleculars, no genetic loci, and no explanations of cytochrome P450 metabolism, neurotransmission, or synaptic blockade. The complexities are explained without medical lingo and, in the end, he concludes what psychiatrists see every day: Antidepressants work. They work for the sickest of the sick, and they work for those who are suffering from less-severe forms of depression.
Dr. Kramer ends the book with a discussion of his own clinical experiences, which are not always in tune with what the “science” declares to be true. He cites studies that show that psychotherapy adds nothing to the treatment of depression, yet still, he treats his own patients with psychotherapy. He notes studies that show maximal efficacy when medications are used at high doses and continue for the long haul at these high doses, yet in his own practice, he sometimes uses lower doses and weans patients off medications. He does a wonderful job of pointing out the disconnect of the promise of evidence-based medicine and how its usefulness has limits in clinical practice.
In our communications, Dr. Kramer wrote to me: “There were many reasons not to write this book, and I was reluctant. I took up the topic only after declining a series of opportunities to weigh in. This book is the only one of mine that I wrote primarily out of a sense of duty. The debunking of antidepressants had gone too far and been too widely accepted, and I believed that the underlying research was shaky.”
He worried that I saw his view of antidepressants as more favorable than he intended it to be, and in fact, his perception is correct: While I prescribe antidepressants and see their benefits (as well as their side effects) with many of my patients, I believe Peter Kramer is more enthusiastic than I am about the efficacy of antidepressants for milder forms of depression, dysthymia, and neuroticism.
“My view is the one expressed in the title,” Dr. Kramer countered, “Our medications work ordinarily well, and they bring patients to a state of ordinary wellness.”
“Ordinarily Well” will be available on June 7; it is available for preorder on Amazon now.
Dr. Miller is a coauthor of “Shrink Rap: Three Psychiatrists Explain Their Work” (Baltimore: Johns Hopkins University Press, 2011).
With his 1993 landmark book, “Listening to Prozac,” psychiatrist Peter D. Kramer became one of the most famous psychiatrists in America – second perhaps only to the fictional Frasier Crane of primetime TV. Since then, Dr. Kramer has continued to write – including a novel, books on other psychiatric topics, a blog, and articles for mainstream media, including the New York Times. In his latest book, “Ordinarily Well: The Case for Antidepressants,” Dr. Kramer will return to the role he left behind 23 years ago: defending the use of antidepressants.
Dr. Kramer notes that he doesn’t do this easily. In the preface, with a bit of dismay, he talks about how reviewers have called him “Dr. Prozac.” He’s felt stuck on a wave that didn’t reflect his diverse interests, and he didn’t want to be covered by the spreading stain of Big Pharma.
“Against all indications, I remained hopeful that I might walk free by day, alter my obituary,” Dr. Kramer tells the reader.
With “Ordinarily Well,” Peter Kramer is back as Dr. Prozac. The book is written as a response to the “research”– now seen so often in headlines – that antidepressants are as effective as sugar pills for mild to moderate depression, and they should be prescribed only for severe major depression. He gets even more specific: The book is written partly in response to an article by Dr. Marcia Angell, former editor in chief of the New England Journal of Medicine, published in June 2011 in the New York Review of Books, and Dr. Angell’s assertion that psychiatric medications are no more effective than placebo. Since psychotropics come with more side effects than placebos, the next logical conclusion is that they are not only ineffective – they are harmful.
As a clinical psychiatrist, I’ve found this evidence-based stance to be perplexing. People get better on antidepressants, even if you aren’t measuring Hamilton rating scores and even if you aren’t limiting treatment to those with severe major depression. I would estimate that at least some of the people, some of the time, get better, and when you progress to strategies of switching and augmenting, most of the the people, most of the time, get significant relief from their major depression.
Obviously, this is my clinical impression and not research, and Dr. Kramer takes a more ardent stance: Most patients with depression, be it mild, moderate, severe, or long-standing dysthymia, have a good response to antidepressants. It’s the minority who don’t respond.
Dr. Kramer goes through the “science” that would suggest that antidepressants are not effective for milder forms of depression, dysthymia, and neuroticism. He does a systematic and comprehensive review of how pharmaceutical studies are conducted, and what factors might skew results, and there is plenty here to fill the pages. He explains complex issues – such as meta-analyses and numbers needed to treat – such that the lay reader can follow.
As just one example, Dr. Kramer talks about screening research subjects for participation in antidepressant studies: “If raters have a sense of the minimum Hamilton score for admission to a study, and if they are under pressure to fill an enrollment quota, they will be inclined to tack on questionable Hamilton points. The boost will not be uniform. There’s no need to raise rating in the very ill. Scores for least afflicted participants will be most inflated.
“When off-site raters, with no stake in the pace of enrollment, analyze tapes of admission interviews,” he continues, “they find patients to be much healthier than the on-site Hamilton scores suggest. According to off-site assessments, many patients admitted to drug studies simply are not depressed.”
Dr. Kramer methodically marches through problems with finding patients for the studies, shortcomings of the Hamilton rating scale, which gives suicidal ideation the same point as a somatic symptom; the bias some studies have of excluding people with severe depression; substance abuse, or comorbid disorders; and “the floor effect,” which underestimates efficacy in patients with fewer symptoms.
He follows subjects at an unnamed for-profit research center and praises the skills of everyone who comes in contact with the research subjects, including the friendly van driver who fetches patients from their homes.
In a chapter titled “How We’re Doing,” Dr. Kramer goes into detail about specific studies, including the STAR*D trials, where patients were recruited from primary care and psychiatric clinics with the guarantee that they would be provided active medication, and those with comorbid conditions were not excluded.
“Only the sickest came. For nearly 80% of the participants (more than 2,800 were tested), the disorder was chronic. The average length of the current depressive episode was more than 2 years, generally despite attempts at treatment. The average enrollee had lived with depression on and off for more than15 years and was now in a seventh episode. Most patients were alcoholics or had other forms of mental illness.
“In the first phase, patients were put on Celexa [citalopram], managed by their own doctors. About 30% of patients achieved remission within weeks – with virtually no symptoms. Responses (including remissions) ran at just under 50%.
“Commentators considered this outcome disappointing, but is it?”
So this is Dr. Kramer’s strength: He writes an engaging book about a complex topic, arguing throughout that antidepressants work well and have been given a bum rap by flawed research and careless journalism that enjoy the sensationalism of villainizing psychotropics. There are no headlines, he points out, singing the praises of antidepressants for milder forms of depression, even when the evidence is there. Despite the complexity of the topic and the breadth of his research reviews, Dr. Kramer tells the story of antidepressant research in a way that a lay reader can follow. There are no mice or moleculars, no genetic loci, and no explanations of cytochrome P450 metabolism, neurotransmission, or synaptic blockade. The complexities are explained without medical lingo and, in the end, he concludes what psychiatrists see every day: Antidepressants work. They work for the sickest of the sick, and they work for those who are suffering from less-severe forms of depression.
Dr. Kramer ends the book with a discussion of his own clinical experiences, which are not always in tune with what the “science” declares to be true. He cites studies that show that psychotherapy adds nothing to the treatment of depression, yet still, he treats his own patients with psychotherapy. He notes studies that show maximal efficacy when medications are used at high doses and continue for the long haul at these high doses, yet in his own practice, he sometimes uses lower doses and weans patients off medications. He does a wonderful job of pointing out the disconnect of the promise of evidence-based medicine and how its usefulness has limits in clinical practice.
In our communications, Dr. Kramer wrote to me: “There were many reasons not to write this book, and I was reluctant. I took up the topic only after declining a series of opportunities to weigh in. This book is the only one of mine that I wrote primarily out of a sense of duty. The debunking of antidepressants had gone too far and been too widely accepted, and I believed that the underlying research was shaky.”
He worried that I saw his view of antidepressants as more favorable than he intended it to be, and in fact, his perception is correct: While I prescribe antidepressants and see their benefits (as well as their side effects) with many of my patients, I believe Peter Kramer is more enthusiastic than I am about the efficacy of antidepressants for milder forms of depression, dysthymia, and neuroticism.
“My view is the one expressed in the title,” Dr. Kramer countered, “Our medications work ordinarily well, and they bring patients to a state of ordinary wellness.”
“Ordinarily Well” will be available on June 7; it is available for preorder on Amazon now.
Dr. Miller is a coauthor of “Shrink Rap: Three Psychiatrists Explain Their Work” (Baltimore: Johns Hopkins University Press, 2011).
More Hospitals to Be Replaced by FSEDs
If an ED is considered the “front door” to the hospital, how do we regard a free-standing emergency department (FSED) with no hospital attached to it? Fueled by continued hospital closures in the face of steadily increasing demands for emergency care, FSEDs are now replacing hospitals in previously well-served urban areas in addition to serving rural areas lacking alternative facilities.
According to The New York Times (http://nyti.ms/1TB8Z44), since 2000, 19 New York City hospitals “have either closed or overhauled how they operate.” As this issue of Emergency Medicine went to press, plans had been announced to replace Manhattan’s Beth Israel and Brooklyn’s Wyckoff Heights hospitals with FSEDs and expanded outpatient facilities. These hospitals and many others that have recently closed, including St Vincent’s (2010) and the Long Island College Hospital (2014), had been part of the health care landscape in New York for over 125 years.
What do FSEDs mean for emergency medicine (EM) and emergency physicians (EPs), and are they safe alternatives to traditional hospital-based EDs? Newer technologies and treatments, coupled with steadily increasing pressures to reduce inpatient stays, razor-thin hospital operating margins, and the refusal of state and local governments to bail out financially failing hospitals, have created a disconnect between the increasing need for emergency care and the decreasing number of inpatient beds.
On one end of the EM patient care spectrum, urgent care centers (UCCs) and retail pharmacy clinics—collectively referred to as “convenient care” centers—are rapidly proliferating to offer care to those with urgent, episodic, and relatively minor medical and surgical problems. (See “Urgent Care and the Urgent Need for Care” at http://bit.ly/1OSrHSA). With little or no regulatory oversight, convenient care centers staffed by EPs, family practitioners, internists, NPs, and PAs, offer extended hour care—but not 24/7 care—to anyone with adequate health insurance or the ability to pay for the care.
On the other end of the EM patient care spectrum are the FSEDs, now divided into two types: satellite EDs of nearby hospitals, and “FS”-FSEDs with no direct hospital connections. Almost all FSEDs receive 911 ambulances, are staffed at all times by trained and certified EPs and registered nurses (RNs) provide acute care and stabilization consistent with the standards for hospital-based EDs, and are open 24/7—a hallmark that distinguishes EDs from UCCs. FSEDs code and bill both for facility and provider services in the same way hospital-based EDs do. Although organized EM has enthusiastically embraced and endorsed FSEDs, its position on UCCs has been decidedly mixed.
Are FSEDs safe for patients requiring emergency care? The lack of uniform definitions and federal and state regulatory requirements make it difficult to gather and interpret meaningful clinical data on FSEDs and convenient care centers. But a well-equipped FSED, served by state-of-the-art pre- and inter-facility ambulances, and staffed by qualified EPs and RNs, should provide a safe alternative to hospital-based EDs for almost all patients in need of emergency care—especially when no hospital-based ED is available.
Specialty designations of qualifying area hospitals such as “Level I trauma center” will minimize but not completely eliminate bad outcomes of cases where even seconds may make the difference between life and death. In the end though, the real question may be is an FSED better than no ED at all?
Ideally, a hospital-based ED should be the epicenter of a network of both satellite convenient care centers and FSEDs, coordinating services, providing management and staffing for all parts of the network, and arranging safe, appropriate intranetwork ambulance transport.
Should you think that FSEDs are a new phenomenon, you might be surprised to discover that in 1875, after New York Hospital (now part of New York Presbyterian) closed its original lower Manhattan site to move further uptown, it opened a “House of Relief” in its old neighborhood that contained an emergency treatment center, an operating room, an isolation area, a dispensary, a reception area, examination rooms, an ambulance entrance, and wards to observe and treat patients until they could be safely transported to the new main hospital. FSEDs served 19th-century patients well, and in the 21st century may serve as a reminder that sometimes even in medicine, “everything old is new again!” (See http://bit.ly/1NSPlDG.)
Editor’s Note: Portions of this editorial were previously published in Emergency Medicine.
If an ED is considered the “front door” to the hospital, how do we regard a free-standing emergency department (FSED) with no hospital attached to it? Fueled by continued hospital closures in the face of steadily increasing demands for emergency care, FSEDs are now replacing hospitals in previously well-served urban areas in addition to serving rural areas lacking alternative facilities.
According to The New York Times (http://nyti.ms/1TB8Z44), since 2000, 19 New York City hospitals “have either closed or overhauled how they operate.” As this issue of Emergency Medicine went to press, plans had been announced to replace Manhattan’s Beth Israel and Brooklyn’s Wyckoff Heights hospitals with FSEDs and expanded outpatient facilities. These hospitals and many others that have recently closed, including St Vincent’s (2010) and the Long Island College Hospital (2014), had been part of the health care landscape in New York for over 125 years.
What do FSEDs mean for emergency medicine (EM) and emergency physicians (EPs), and are they safe alternatives to traditional hospital-based EDs? Newer technologies and treatments, coupled with steadily increasing pressures to reduce inpatient stays, razor-thin hospital operating margins, and the refusal of state and local governments to bail out financially failing hospitals, have created a disconnect between the increasing need for emergency care and the decreasing number of inpatient beds.
On one end of the EM patient care spectrum, urgent care centers (UCCs) and retail pharmacy clinics—collectively referred to as “convenient care” centers—are rapidly proliferating to offer care to those with urgent, episodic, and relatively minor medical and surgical problems. (See “Urgent Care and the Urgent Need for Care” at http://bit.ly/1OSrHSA). With little or no regulatory oversight, convenient care centers staffed by EPs, family practitioners, internists, NPs, and PAs, offer extended hour care—but not 24/7 care—to anyone with adequate health insurance or the ability to pay for the care.
On the other end of the EM patient care spectrum are the FSEDs, now divided into two types: satellite EDs of nearby hospitals, and “FS”-FSEDs with no direct hospital connections. Almost all FSEDs receive 911 ambulances, are staffed at all times by trained and certified EPs and registered nurses (RNs) provide acute care and stabilization consistent with the standards for hospital-based EDs, and are open 24/7—a hallmark that distinguishes EDs from UCCs. FSEDs code and bill both for facility and provider services in the same way hospital-based EDs do. Although organized EM has enthusiastically embraced and endorsed FSEDs, its position on UCCs has been decidedly mixed.
Are FSEDs safe for patients requiring emergency care? The lack of uniform definitions and federal and state regulatory requirements make it difficult to gather and interpret meaningful clinical data on FSEDs and convenient care centers. But a well-equipped FSED, served by state-of-the-art pre- and inter-facility ambulances, and staffed by qualified EPs and RNs, should provide a safe alternative to hospital-based EDs for almost all patients in need of emergency care—especially when no hospital-based ED is available.
Specialty designations of qualifying area hospitals such as “Level I trauma center” will minimize but not completely eliminate bad outcomes of cases where even seconds may make the difference between life and death. In the end though, the real question may be is an FSED better than no ED at all?
Ideally, a hospital-based ED should be the epicenter of a network of both satellite convenient care centers and FSEDs, coordinating services, providing management and staffing for all parts of the network, and arranging safe, appropriate intranetwork ambulance transport.
Should you think that FSEDs are a new phenomenon, you might be surprised to discover that in 1875, after New York Hospital (now part of New York Presbyterian) closed its original lower Manhattan site to move further uptown, it opened a “House of Relief” in its old neighborhood that contained an emergency treatment center, an operating room, an isolation area, a dispensary, a reception area, examination rooms, an ambulance entrance, and wards to observe and treat patients until they could be safely transported to the new main hospital. FSEDs served 19th-century patients well, and in the 21st century may serve as a reminder that sometimes even in medicine, “everything old is new again!” (See http://bit.ly/1NSPlDG.)
Editor’s Note: Portions of this editorial were previously published in Emergency Medicine.
If an ED is considered the “front door” to the hospital, how do we regard a free-standing emergency department (FSED) with no hospital attached to it? Fueled by continued hospital closures in the face of steadily increasing demands for emergency care, FSEDs are now replacing hospitals in previously well-served urban areas in addition to serving rural areas lacking alternative facilities.
According to The New York Times (http://nyti.ms/1TB8Z44), since 2000, 19 New York City hospitals “have either closed or overhauled how they operate.” As this issue of Emergency Medicine went to press, plans had been announced to replace Manhattan’s Beth Israel and Brooklyn’s Wyckoff Heights hospitals with FSEDs and expanded outpatient facilities. These hospitals and many others that have recently closed, including St Vincent’s (2010) and the Long Island College Hospital (2014), had been part of the health care landscape in New York for over 125 years.
What do FSEDs mean for emergency medicine (EM) and emergency physicians (EPs), and are they safe alternatives to traditional hospital-based EDs? Newer technologies and treatments, coupled with steadily increasing pressures to reduce inpatient stays, razor-thin hospital operating margins, and the refusal of state and local governments to bail out financially failing hospitals, have created a disconnect between the increasing need for emergency care and the decreasing number of inpatient beds.
On one end of the EM patient care spectrum, urgent care centers (UCCs) and retail pharmacy clinics—collectively referred to as “convenient care” centers—are rapidly proliferating to offer care to those with urgent, episodic, and relatively minor medical and surgical problems. (See “Urgent Care and the Urgent Need for Care” at http://bit.ly/1OSrHSA). With little or no regulatory oversight, convenient care centers staffed by EPs, family practitioners, internists, NPs, and PAs, offer extended hour care—but not 24/7 care—to anyone with adequate health insurance or the ability to pay for the care.
On the other end of the EM patient care spectrum are the FSEDs, now divided into two types: satellite EDs of nearby hospitals, and “FS”-FSEDs with no direct hospital connections. Almost all FSEDs receive 911 ambulances, are staffed at all times by trained and certified EPs and registered nurses (RNs) provide acute care and stabilization consistent with the standards for hospital-based EDs, and are open 24/7—a hallmark that distinguishes EDs from UCCs. FSEDs code and bill both for facility and provider services in the same way hospital-based EDs do. Although organized EM has enthusiastically embraced and endorsed FSEDs, its position on UCCs has been decidedly mixed.
Are FSEDs safe for patients requiring emergency care? The lack of uniform definitions and federal and state regulatory requirements make it difficult to gather and interpret meaningful clinical data on FSEDs and convenient care centers. But a well-equipped FSED, served by state-of-the-art pre- and inter-facility ambulances, and staffed by qualified EPs and RNs, should provide a safe alternative to hospital-based EDs for almost all patients in need of emergency care—especially when no hospital-based ED is available.
Specialty designations of qualifying area hospitals such as “Level I trauma center” will minimize but not completely eliminate bad outcomes of cases where even seconds may make the difference between life and death. In the end though, the real question may be is an FSED better than no ED at all?
Ideally, a hospital-based ED should be the epicenter of a network of both satellite convenient care centers and FSEDs, coordinating services, providing management and staffing for all parts of the network, and arranging safe, appropriate intranetwork ambulance transport.
Should you think that FSEDs are a new phenomenon, you might be surprised to discover that in 1875, after New York Hospital (now part of New York Presbyterian) closed its original lower Manhattan site to move further uptown, it opened a “House of Relief” in its old neighborhood that contained an emergency treatment center, an operating room, an isolation area, a dispensary, a reception area, examination rooms, an ambulance entrance, and wards to observe and treat patients until they could be safely transported to the new main hospital. FSEDs served 19th-century patients well, and in the 21st century may serve as a reminder that sometimes even in medicine, “everything old is new again!” (See http://bit.ly/1NSPlDG.)
Editor’s Note: Portions of this editorial were previously published in Emergency Medicine.
Coldiron Truth: Beware the state pharmacy board
What does the pharmacy board have to do with me? I’m a physician, regulated by the state medical board. Well heads up. If regulations coming your way are adopted, you will have the additional privilege of being licensed, inspected by, and financially supporting your state pharmacy board.
How did all this happen? In 2012, a compounding pharmacy inadequately sterilized multiple lots of methylprednisolone, which were sold around the country and used for intrathecal injections. As a result, 753 patients developed fungal infections, including 386 cases of meningitis, and 64 of them died. The owner of the pharmacy and the head pharmacist are up on second degree murder charges.
But what does this have to do with you?
After a media bonfire, a congressional hearing complete with the taking of the fifth amendment, and a major rewrite of pharmacy regulations with increased scrutiny and oversight, the State of Ohio Board of Pharmacy rushed to adopt rules before reasonable regulations could be worked out by the Food and Drug Administration, the American Medical Association, and the Federation of State Medical Boards. The Ohio board of pharmacy adopted the U.S. Pharmacopeial Convention (USP) regulations, written for compounding pharmacies, and applied them to physicians’ offices.
In an overreaching bureaucratic coup de grace, any practitioners who reconstitute any drug in their offices is considered to be a compounding pharmacy, ordered to pay compounding pharmacy registration fees ($112 yearly), and to undergo the same inspections as compounding pharmacies. You can’t be too safe, you know, and all those registration fees (totaling about $2 million per year in Ohio alone) will decrease what would have been an onerous registration and inspection expense for true compounding pharmacies.
This is the reality we are facing in Ohio, and this situation may soon be “coming to a theater near you.” I understand that several pharmacy boards in other states are preparing to roll out similar regulations.
As a kicker, if the product you reconstitute is preservative free (botulinum toxin anyone?), you must use it or dispose of it within one hour. Yes, one hour. If you dilute bleomycin or 5-fluorouracil (5-FU), you must install an outside vented laminar flow hood, and wear level 5 hazmat gear while drawing it up.
Is this situation insane or what? As the result of a pharmacy in Massachusetts that skirted existing regulations and sold contaminated drugs that killed patients, doctors now need more regulations, licensing, inspections, and fees?
The real problem here, of course, is not the $112 fee. It will be the loss of many drugs and therapies that can be used inexpensively in the office, but will now either be unavailable to patients or available at a greatly increased cost. I pointed this situation out at a pharmacy board meeting, and they helpfully responded that I can have my friendly local pharmacist compound any drug I need in a specific strength and unit dose. Who is going to pay for this? I can make diclofenac or 5-FU cream in my office for less than $20. Instead, it will cost over $700 at the pharmacy! Further, making something fiscally impossible, like installing a laminar flow hood, is not different that denying it outright. I consider this to be restraint of trade.
Don’t allow yourselves to be compromised as Ohio physicians have been. You must be vigilant. Attend the public hearings and testify. In Ohio, the hearings were held over the Christmas holidays. Guess what? No one came to the hearings! You must show up and complain. Loudly. You must point out how patients are going to be hurt, not helped, by these rules. You must point out the superb safety record of physicians when using in-office pharmaceuticals. You must alert your neurology, ophthalmology, gynecology, and urology colleagues to the problem since they all use neurotoxins, too. The primary care doctors all reconstitute drugs (think antibiotics) for office use, too.
These efforts are also part of a larger campaign to give pharmacists a larger clinical role in patient care. If pharmacists license you, if they inspect your office, how can you oppose them when they want clinical privileges?
The fix is to enact a moratorium on regulations until the FDA rules come out. These will be more reasonable than the rules issued by the USP. Another fix is a legislative change that instructs that physicians, not pharmacists, will define what is considered to be a dangerous drug.
It is time to be alert, vigilant, and outspoken. You must do this to preserve your ability to do what is best for patients, to be able to deliver care in an expeditious, efficient, and cost-effective manner. This is what being physician is all about! Keep your state board of pharmacy off your license and out of your office.
Dr. Coldiron is a past president of the American Academy of Dermatology. He is currently in private practice, but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. Reach him at [email protected].
What does the pharmacy board have to do with me? I’m a physician, regulated by the state medical board. Well heads up. If regulations coming your way are adopted, you will have the additional privilege of being licensed, inspected by, and financially supporting your state pharmacy board.
How did all this happen? In 2012, a compounding pharmacy inadequately sterilized multiple lots of methylprednisolone, which were sold around the country and used for intrathecal injections. As a result, 753 patients developed fungal infections, including 386 cases of meningitis, and 64 of them died. The owner of the pharmacy and the head pharmacist are up on second degree murder charges.
But what does this have to do with you?
After a media bonfire, a congressional hearing complete with the taking of the fifth amendment, and a major rewrite of pharmacy regulations with increased scrutiny and oversight, the State of Ohio Board of Pharmacy rushed to adopt rules before reasonable regulations could be worked out by the Food and Drug Administration, the American Medical Association, and the Federation of State Medical Boards. The Ohio board of pharmacy adopted the U.S. Pharmacopeial Convention (USP) regulations, written for compounding pharmacies, and applied them to physicians’ offices.
In an overreaching bureaucratic coup de grace, any practitioners who reconstitute any drug in their offices is considered to be a compounding pharmacy, ordered to pay compounding pharmacy registration fees ($112 yearly), and to undergo the same inspections as compounding pharmacies. You can’t be too safe, you know, and all those registration fees (totaling about $2 million per year in Ohio alone) will decrease what would have been an onerous registration and inspection expense for true compounding pharmacies.
This is the reality we are facing in Ohio, and this situation may soon be “coming to a theater near you.” I understand that several pharmacy boards in other states are preparing to roll out similar regulations.
As a kicker, if the product you reconstitute is preservative free (botulinum toxin anyone?), you must use it or dispose of it within one hour. Yes, one hour. If you dilute bleomycin or 5-fluorouracil (5-FU), you must install an outside vented laminar flow hood, and wear level 5 hazmat gear while drawing it up.
Is this situation insane or what? As the result of a pharmacy in Massachusetts that skirted existing regulations and sold contaminated drugs that killed patients, doctors now need more regulations, licensing, inspections, and fees?
The real problem here, of course, is not the $112 fee. It will be the loss of many drugs and therapies that can be used inexpensively in the office, but will now either be unavailable to patients or available at a greatly increased cost. I pointed this situation out at a pharmacy board meeting, and they helpfully responded that I can have my friendly local pharmacist compound any drug I need in a specific strength and unit dose. Who is going to pay for this? I can make diclofenac or 5-FU cream in my office for less than $20. Instead, it will cost over $700 at the pharmacy! Further, making something fiscally impossible, like installing a laminar flow hood, is not different that denying it outright. I consider this to be restraint of trade.
Don’t allow yourselves to be compromised as Ohio physicians have been. You must be vigilant. Attend the public hearings and testify. In Ohio, the hearings were held over the Christmas holidays. Guess what? No one came to the hearings! You must show up and complain. Loudly. You must point out how patients are going to be hurt, not helped, by these rules. You must point out the superb safety record of physicians when using in-office pharmaceuticals. You must alert your neurology, ophthalmology, gynecology, and urology colleagues to the problem since they all use neurotoxins, too. The primary care doctors all reconstitute drugs (think antibiotics) for office use, too.
These efforts are also part of a larger campaign to give pharmacists a larger clinical role in patient care. If pharmacists license you, if they inspect your office, how can you oppose them when they want clinical privileges?
The fix is to enact a moratorium on regulations until the FDA rules come out. These will be more reasonable than the rules issued by the USP. Another fix is a legislative change that instructs that physicians, not pharmacists, will define what is considered to be a dangerous drug.
It is time to be alert, vigilant, and outspoken. You must do this to preserve your ability to do what is best for patients, to be able to deliver care in an expeditious, efficient, and cost-effective manner. This is what being physician is all about! Keep your state board of pharmacy off your license and out of your office.
Dr. Coldiron is a past president of the American Academy of Dermatology. He is currently in private practice, but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. Reach him at [email protected].
What does the pharmacy board have to do with me? I’m a physician, regulated by the state medical board. Well heads up. If regulations coming your way are adopted, you will have the additional privilege of being licensed, inspected by, and financially supporting your state pharmacy board.
How did all this happen? In 2012, a compounding pharmacy inadequately sterilized multiple lots of methylprednisolone, which were sold around the country and used for intrathecal injections. As a result, 753 patients developed fungal infections, including 386 cases of meningitis, and 64 of them died. The owner of the pharmacy and the head pharmacist are up on second degree murder charges.
But what does this have to do with you?
After a media bonfire, a congressional hearing complete with the taking of the fifth amendment, and a major rewrite of pharmacy regulations with increased scrutiny and oversight, the State of Ohio Board of Pharmacy rushed to adopt rules before reasonable regulations could be worked out by the Food and Drug Administration, the American Medical Association, and the Federation of State Medical Boards. The Ohio board of pharmacy adopted the U.S. Pharmacopeial Convention (USP) regulations, written for compounding pharmacies, and applied them to physicians’ offices.
In an overreaching bureaucratic coup de grace, any practitioners who reconstitute any drug in their offices is considered to be a compounding pharmacy, ordered to pay compounding pharmacy registration fees ($112 yearly), and to undergo the same inspections as compounding pharmacies. You can’t be too safe, you know, and all those registration fees (totaling about $2 million per year in Ohio alone) will decrease what would have been an onerous registration and inspection expense for true compounding pharmacies.
This is the reality we are facing in Ohio, and this situation may soon be “coming to a theater near you.” I understand that several pharmacy boards in other states are preparing to roll out similar regulations.
As a kicker, if the product you reconstitute is preservative free (botulinum toxin anyone?), you must use it or dispose of it within one hour. Yes, one hour. If you dilute bleomycin or 5-fluorouracil (5-FU), you must install an outside vented laminar flow hood, and wear level 5 hazmat gear while drawing it up.
Is this situation insane or what? As the result of a pharmacy in Massachusetts that skirted existing regulations and sold contaminated drugs that killed patients, doctors now need more regulations, licensing, inspections, and fees?
The real problem here, of course, is not the $112 fee. It will be the loss of many drugs and therapies that can be used inexpensively in the office, but will now either be unavailable to patients or available at a greatly increased cost. I pointed this situation out at a pharmacy board meeting, and they helpfully responded that I can have my friendly local pharmacist compound any drug I need in a specific strength and unit dose. Who is going to pay for this? I can make diclofenac or 5-FU cream in my office for less than $20. Instead, it will cost over $700 at the pharmacy! Further, making something fiscally impossible, like installing a laminar flow hood, is not different that denying it outright. I consider this to be restraint of trade.
Don’t allow yourselves to be compromised as Ohio physicians have been. You must be vigilant. Attend the public hearings and testify. In Ohio, the hearings were held over the Christmas holidays. Guess what? No one came to the hearings! You must show up and complain. Loudly. You must point out how patients are going to be hurt, not helped, by these rules. You must point out the superb safety record of physicians when using in-office pharmaceuticals. You must alert your neurology, ophthalmology, gynecology, and urology colleagues to the problem since they all use neurotoxins, too. The primary care doctors all reconstitute drugs (think antibiotics) for office use, too.
These efforts are also part of a larger campaign to give pharmacists a larger clinical role in patient care. If pharmacists license you, if they inspect your office, how can you oppose them when they want clinical privileges?
The fix is to enact a moratorium on regulations until the FDA rules come out. These will be more reasonable than the rules issued by the USP. Another fix is a legislative change that instructs that physicians, not pharmacists, will define what is considered to be a dangerous drug.
It is time to be alert, vigilant, and outspoken. You must do this to preserve your ability to do what is best for patients, to be able to deliver care in an expeditious, efficient, and cost-effective manner. This is what being physician is all about! Keep your state board of pharmacy off your license and out of your office.
Dr. Coldiron is a past president of the American Academy of Dermatology. He is currently in private practice, but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. Reach him at [email protected].
Give serious thought before starting antipsychotics in elders
The page filled me with dread: “Your elderly patient is confused, getting out of bed, and threw her entire dinner tray at a nurse just now.” Because this morning my patient was polite and appropriate, the now-angry, dinner-splattered nurse means only one thing: delirium.
Delirium is one of the most difficult problems for hospitalists to manage, in part because our management of delirium is often learned on the fly during residency and early years of practice. This post-hoc approach toward delirium misses the most important aspect of treatment: Prevention.
Interventions like early mobilization, environmental interventions, careful oversight of drugs, hydration protocols, and reinforcing the day/night cycle are crucial. Unfortunately, few hospitals can provide these resources or the trained multidisciplinary team with geriatrics expertise to administer them. The result is that delirium occurs more frequently than it should, and hospitalists often face a patient who is a risk to themselves or others.
In this situation, antipsychotics (APs) are often prescribed. However, long term use of APs by elders is highly discouraged by many organizations, including the Society of Hospital Medicine, because of risks like cardiac events (e.g., QT prolongation), stroke, extrapyramidal symptoms, falls, somnolence, and increased mortality in older patients with dementia.
One unanswered question regarding the use of APs is whether starting the medications in the hospital results in long-term use. To answer this, we performed a retrospective study of 300 elderly hospitalized patients who were treated for the first time with APs during their hospitalization. Of these 300 patients, 10% died during that first hospitalization, and almost half (48%) remained on APs at discharge. We found that most of the prescriptions were to treat delirium (J Hosp Med. 2014 Dec;9[12]:802-4. doi:10.1002/jhm.2277).
In a more recent study, we looked at outcomes for patients discharged from Baystate Medical Center (Springfield, Mass.) on APs. Within a year of discharge, 40% of these patients were readmitted at least once and approximately two-thirds were still taking the same APs on which they had been discharged (J Hosp Med. 2016 Apr 6. doi: 10.1002/jhm.2585). Thus, if I start my patient described above (who threw the dinner tray) on an AP today, she is very likely to be readmitted the next year still taking that same medication. Starting an AP in the short term can lead to the very thing we have been warned against: long-term use of an AP in an elderly person.
Even more striking than the continuation rate was the incredibly high 1-year mortality rate. Of the 260 patients discharged from the original admission on an AP, one-third had died at the 1-year mark. This group of patients had a wide range of diagnoses, but nearly as many died as if they all had stage IV heart failure. Because most had an agitated delirium at the time of AP prescription, these findings suggest that onset of in-hospital delirium should trigger a closer examination of the patient’s current burden of illness, prognosis, functional and cognitive status, treatment options, and goals of care.
Prevention is key
Our study also supports the prevention of delirium as the most important strategy to improve patient outcomes. Since conducting this study, Baystate Medical Center has implemented an “ACE” (Acute Care for Elders) pilot project and will soon open a full ACE unit. This unit, which employs many of the behavioral interventions described above (early discharge planning, drug oversight, team-based care, early mobilization, optimizing vision and hearing, sleep-wake cycle preservation, and hydration) has resulted in declines in both delirium rates and use of APs. Use of restraints has been virtually eliminated.
Our ACE program was a combined effort between geriatrics, hospital medicine, nursing, pharmacy, and others, but hospitalists often lead acute care quality improvement (QI) initiatives, and are superbly positioned to champion programs like ACE, NICHE (Nurses Improving Care for Health System Elders), and HELP (Hospital Elder Life Program) to benefit this vulnerable population.
Some important questions about AP use remain unanswered. First, there is very limited clinical trial evidence that APs actually improve outcomes in patients with delirium. Second, our study fails to answer one all-important question: does long-term AP use increase mortality in elders? Prior studies are largely retrospective, and results have been mixed.
Our study highlights the difficulty of teasing apart the baseline risks of the patients, the risk of the medications themselves, and confounding variables. There may be an association between APs and death, but it is quite possible that patients who require APs are simply at higher risk of death independent of the drugs’ effect; this confounding by indication cannot be adjusted away.
This leaves hospitalists in a difficult position. At Baystate Medical Center, hospitalists have opted to focus on prevention, but when delirium occurs, some patients are still treated with APs. Clinicians reserve the medications for patients who are suffering and fail to respond to nonpharmacologic interventions or are a risk to themselves or others. Still, Baystate plans to reduce use even in this population by instituting behavioral response teams to devise nondrug care plans, and hospitalists are encouraged to avoid discharging patients on APs.
Finally, even though patients who require APs may lack a clear terminal diagnosis, we encourage clinicians to recognize that delirium should prompt a discussion of prognosis and clarification of values, goals, and realistic treatment options.
Dr. Loh is a fellow at the James Wilmot Cancer Center, University of Rochester (N.Y.) Medical Center. Dr. Brennan is chief of geriatrics and post-acute medicine at Baystate Medical Center, Springfield, Mass. Dr. Lagu is an academic hospitalist in the Center for Quality of Care Research at Baystate Medical Center.
The page filled me with dread: “Your elderly patient is confused, getting out of bed, and threw her entire dinner tray at a nurse just now.” Because this morning my patient was polite and appropriate, the now-angry, dinner-splattered nurse means only one thing: delirium.
Delirium is one of the most difficult problems for hospitalists to manage, in part because our management of delirium is often learned on the fly during residency and early years of practice. This post-hoc approach toward delirium misses the most important aspect of treatment: Prevention.
Interventions like early mobilization, environmental interventions, careful oversight of drugs, hydration protocols, and reinforcing the day/night cycle are crucial. Unfortunately, few hospitals can provide these resources or the trained multidisciplinary team with geriatrics expertise to administer them. The result is that delirium occurs more frequently than it should, and hospitalists often face a patient who is a risk to themselves or others.
In this situation, antipsychotics (APs) are often prescribed. However, long term use of APs by elders is highly discouraged by many organizations, including the Society of Hospital Medicine, because of risks like cardiac events (e.g., QT prolongation), stroke, extrapyramidal symptoms, falls, somnolence, and increased mortality in older patients with dementia.
One unanswered question regarding the use of APs is whether starting the medications in the hospital results in long-term use. To answer this, we performed a retrospective study of 300 elderly hospitalized patients who were treated for the first time with APs during their hospitalization. Of these 300 patients, 10% died during that first hospitalization, and almost half (48%) remained on APs at discharge. We found that most of the prescriptions were to treat delirium (J Hosp Med. 2014 Dec;9[12]:802-4. doi:10.1002/jhm.2277).
In a more recent study, we looked at outcomes for patients discharged from Baystate Medical Center (Springfield, Mass.) on APs. Within a year of discharge, 40% of these patients were readmitted at least once and approximately two-thirds were still taking the same APs on which they had been discharged (J Hosp Med. 2016 Apr 6. doi: 10.1002/jhm.2585). Thus, if I start my patient described above (who threw the dinner tray) on an AP today, she is very likely to be readmitted the next year still taking that same medication. Starting an AP in the short term can lead to the very thing we have been warned against: long-term use of an AP in an elderly person.
Even more striking than the continuation rate was the incredibly high 1-year mortality rate. Of the 260 patients discharged from the original admission on an AP, one-third had died at the 1-year mark. This group of patients had a wide range of diagnoses, but nearly as many died as if they all had stage IV heart failure. Because most had an agitated delirium at the time of AP prescription, these findings suggest that onset of in-hospital delirium should trigger a closer examination of the patient’s current burden of illness, prognosis, functional and cognitive status, treatment options, and goals of care.
Prevention is key
Our study also supports the prevention of delirium as the most important strategy to improve patient outcomes. Since conducting this study, Baystate Medical Center has implemented an “ACE” (Acute Care for Elders) pilot project and will soon open a full ACE unit. This unit, which employs many of the behavioral interventions described above (early discharge planning, drug oversight, team-based care, early mobilization, optimizing vision and hearing, sleep-wake cycle preservation, and hydration) has resulted in declines in both delirium rates and use of APs. Use of restraints has been virtually eliminated.
Our ACE program was a combined effort between geriatrics, hospital medicine, nursing, pharmacy, and others, but hospitalists often lead acute care quality improvement (QI) initiatives, and are superbly positioned to champion programs like ACE, NICHE (Nurses Improving Care for Health System Elders), and HELP (Hospital Elder Life Program) to benefit this vulnerable population.
Some important questions about AP use remain unanswered. First, there is very limited clinical trial evidence that APs actually improve outcomes in patients with delirium. Second, our study fails to answer one all-important question: does long-term AP use increase mortality in elders? Prior studies are largely retrospective, and results have been mixed.
Our study highlights the difficulty of teasing apart the baseline risks of the patients, the risk of the medications themselves, and confounding variables. There may be an association between APs and death, but it is quite possible that patients who require APs are simply at higher risk of death independent of the drugs’ effect; this confounding by indication cannot be adjusted away.
This leaves hospitalists in a difficult position. At Baystate Medical Center, hospitalists have opted to focus on prevention, but when delirium occurs, some patients are still treated with APs. Clinicians reserve the medications for patients who are suffering and fail to respond to nonpharmacologic interventions or are a risk to themselves or others. Still, Baystate plans to reduce use even in this population by instituting behavioral response teams to devise nondrug care plans, and hospitalists are encouraged to avoid discharging patients on APs.
Finally, even though patients who require APs may lack a clear terminal diagnosis, we encourage clinicians to recognize that delirium should prompt a discussion of prognosis and clarification of values, goals, and realistic treatment options.
Dr. Loh is a fellow at the James Wilmot Cancer Center, University of Rochester (N.Y.) Medical Center. Dr. Brennan is chief of geriatrics and post-acute medicine at Baystate Medical Center, Springfield, Mass. Dr. Lagu is an academic hospitalist in the Center for Quality of Care Research at Baystate Medical Center.
The page filled me with dread: “Your elderly patient is confused, getting out of bed, and threw her entire dinner tray at a nurse just now.” Because this morning my patient was polite and appropriate, the now-angry, dinner-splattered nurse means only one thing: delirium.
Delirium is one of the most difficult problems for hospitalists to manage, in part because our management of delirium is often learned on the fly during residency and early years of practice. This post-hoc approach toward delirium misses the most important aspect of treatment: Prevention.
Interventions like early mobilization, environmental interventions, careful oversight of drugs, hydration protocols, and reinforcing the day/night cycle are crucial. Unfortunately, few hospitals can provide these resources or the trained multidisciplinary team with geriatrics expertise to administer them. The result is that delirium occurs more frequently than it should, and hospitalists often face a patient who is a risk to themselves or others.
In this situation, antipsychotics (APs) are often prescribed. However, long term use of APs by elders is highly discouraged by many organizations, including the Society of Hospital Medicine, because of risks like cardiac events (e.g., QT prolongation), stroke, extrapyramidal symptoms, falls, somnolence, and increased mortality in older patients with dementia.
One unanswered question regarding the use of APs is whether starting the medications in the hospital results in long-term use. To answer this, we performed a retrospective study of 300 elderly hospitalized patients who were treated for the first time with APs during their hospitalization. Of these 300 patients, 10% died during that first hospitalization, and almost half (48%) remained on APs at discharge. We found that most of the prescriptions were to treat delirium (J Hosp Med. 2014 Dec;9[12]:802-4. doi:10.1002/jhm.2277).
In a more recent study, we looked at outcomes for patients discharged from Baystate Medical Center (Springfield, Mass.) on APs. Within a year of discharge, 40% of these patients were readmitted at least once and approximately two-thirds were still taking the same APs on which they had been discharged (J Hosp Med. 2016 Apr 6. doi: 10.1002/jhm.2585). Thus, if I start my patient described above (who threw the dinner tray) on an AP today, she is very likely to be readmitted the next year still taking that same medication. Starting an AP in the short term can lead to the very thing we have been warned against: long-term use of an AP in an elderly person.
Even more striking than the continuation rate was the incredibly high 1-year mortality rate. Of the 260 patients discharged from the original admission on an AP, one-third had died at the 1-year mark. This group of patients had a wide range of diagnoses, but nearly as many died as if they all had stage IV heart failure. Because most had an agitated delirium at the time of AP prescription, these findings suggest that onset of in-hospital delirium should trigger a closer examination of the patient’s current burden of illness, prognosis, functional and cognitive status, treatment options, and goals of care.
Prevention is key
Our study also supports the prevention of delirium as the most important strategy to improve patient outcomes. Since conducting this study, Baystate Medical Center has implemented an “ACE” (Acute Care for Elders) pilot project and will soon open a full ACE unit. This unit, which employs many of the behavioral interventions described above (early discharge planning, drug oversight, team-based care, early mobilization, optimizing vision and hearing, sleep-wake cycle preservation, and hydration) has resulted in declines in both delirium rates and use of APs. Use of restraints has been virtually eliminated.
Our ACE program was a combined effort between geriatrics, hospital medicine, nursing, pharmacy, and others, but hospitalists often lead acute care quality improvement (QI) initiatives, and are superbly positioned to champion programs like ACE, NICHE (Nurses Improving Care for Health System Elders), and HELP (Hospital Elder Life Program) to benefit this vulnerable population.
Some important questions about AP use remain unanswered. First, there is very limited clinical trial evidence that APs actually improve outcomes in patients with delirium. Second, our study fails to answer one all-important question: does long-term AP use increase mortality in elders? Prior studies are largely retrospective, and results have been mixed.
Our study highlights the difficulty of teasing apart the baseline risks of the patients, the risk of the medications themselves, and confounding variables. There may be an association between APs and death, but it is quite possible that patients who require APs are simply at higher risk of death independent of the drugs’ effect; this confounding by indication cannot be adjusted away.
This leaves hospitalists in a difficult position. At Baystate Medical Center, hospitalists have opted to focus on prevention, but when delirium occurs, some patients are still treated with APs. Clinicians reserve the medications for patients who are suffering and fail to respond to nonpharmacologic interventions or are a risk to themselves or others. Still, Baystate plans to reduce use even in this population by instituting behavioral response teams to devise nondrug care plans, and hospitalists are encouraged to avoid discharging patients on APs.
Finally, even though patients who require APs may lack a clear terminal diagnosis, we encourage clinicians to recognize that delirium should prompt a discussion of prognosis and clarification of values, goals, and realistic treatment options.
Dr. Loh is a fellow at the James Wilmot Cancer Center, University of Rochester (N.Y.) Medical Center. Dr. Brennan is chief of geriatrics and post-acute medicine at Baystate Medical Center, Springfield, Mass. Dr. Lagu is an academic hospitalist in the Center for Quality of Care Research at Baystate Medical Center.
Sell skin care products to protect your patients
The ethics behind selling skin care products to patients has been hotly debated within the field of cosmetic dermatology for several decades. In 15 years of practice, I have come to the conclusion that patients want you to and need you to because otherwise they are easily taken advantage of. Other physicians are doing it but we – the dermatologists – are the most qualified to offer skin care advice. This article will discuss the reasons that you need to get over the ethical dilemma and offer skin care to your patients.
Using the correct skin care regimen for the face and body will improve outcomes
Whether a patient suffers from acne, rosacea, melasma, psoriasis, eczema, contact dermatitis, or even tinea versicolor, using the proper skin care regimen will improve outcomes by affecting the skin barrier, pH, hydration level, and function of the keratinocytes and fibroblasts. In fact, every personal care product that touches the skin has an impact on skin health. For example, if a patient uses a detergent-laden bar soap, the skin barrier will be impaired, which can cause them to react to allergens and irritants. Personal care products can affect the skin pH; this is shown to play a role in Malassezia colonization in atopic dermatitis patients (J Clin Med. 2015;4[6]:1217-28). As dermatologists, we know better than anyone that daily use of SPF improves skin health and lowers the risk of postinflammatory pigmentation. We all agree that patients should cleanse the skin and apply a SPF every day. Giving them guidance about which to choose is very important.
Giving the patient exact instructions will lead to improved compliance
Why should recommending skin care products be perceived differently than prescribing a prescription medication? We should prescribe to our patients in writing the exact skin care regimen they should use for their face or body to ensure that they understand the directions. I have been surprised by patients who have said, “I did not know I was supposed to wash my cleanser off,” or “I wash my face with hand soap.” We can help them by educating them and giving them specific instructions. Improved education and communication results in increased compliance. When you do surgery on a wound, you probably tell them to apply a topical antibiotic ointment, but do you direct them to what cleanser to use or tell them which SPF to use on the stitched wound? Providing written instructions for all dermatologic disorders and postprocedure care is necessary to improve compliance and outcomes.
Combine cosmeceuticals, prescription medications, and medical procedures
You (unlike the cosmetic counter salesperson) have the ability to combine cosmeceuticals with prescription medications and medical procedures. In fact, selling your patients the right skin care products to use after a procedure saves them a trip to the store and ensures that they use the correct products. Of course it makes sense that patients getting toxins and fillers should use a retinoid to improve skin aging; however, many general dermatologic diseases would improve with the proper skin care. For example, do you use biologics for psoriasis? Using the proper skin care to regulate skin pH and improve the skin barrier may help prevent colonization of yeast, fungus, and bacteria. The same is true for atopic patients. Do you use liquid nitrogen? Studies show that using a retinoid before a procedure speeds healing. Skin care goes way beyond wrinkles and dark circles under the eyes, so if you are not prescribing the patient an exact regimen, you are not maximizing outcomes.
I don’t have time to talk to my patients about skin care
The missing piece is that most of us don’t have the time to spend discussing skin care. This is where using a standardized scientific methodology is crucial. I developed and use a skin typing methodology in my office and have seen improved physician/patient relationships and increased patient satisfaction resulting in a significant amount of referrals. We also have noted decreased call backs and fewer adverse events from products because the patients have a better understanding of how to properly apply the cosmeceuticals and prescription products. The best part is, it does not add any time onto the patient visit when standardized methodologies are properly adopted.
What if I still do not feel comfortable profiting from the sale of skin care products?
First you need to realize that time is money and you are saving the patient the cost in time it would have taken them to go to a store, park, and shop for the correct product. I have seen data presented from several companies that show that patients usually spend a large amount of money on skin care products after they see their dermatologist. Without guidance, they will likely buy the incorrect products. If they buy the wrong product, you save them the hassle of having to make another office visit and the aggravation of the side effects from the incorrect product. These are often of poor quality or not appropriate for their skin issues. Counterfeit products are rampant on the Internet and many new companies tout worthless products with stem cells and other nonsense. Only you can help your patients make sure that money is spent on the proper products.
Conclusion
Do you really want someone else giving your patients skin care advice? Your patients deserve to have someone with your insights, knowledge, compassion, and honesty help them achieve optimal skin health through use of the proper cosmeceuticals and prescription medications. It is up to you and your staff to save your patients from falling prey to persuasive salespeople with no scientific knowledge or concern for long-term skin health.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever. Dr. Baumann also developed and owns the Baumann Skin Type Solution skin typing systems and related products.
The ethics behind selling skin care products to patients has been hotly debated within the field of cosmetic dermatology for several decades. In 15 years of practice, I have come to the conclusion that patients want you to and need you to because otherwise they are easily taken advantage of. Other physicians are doing it but we – the dermatologists – are the most qualified to offer skin care advice. This article will discuss the reasons that you need to get over the ethical dilemma and offer skin care to your patients.
Using the correct skin care regimen for the face and body will improve outcomes
Whether a patient suffers from acne, rosacea, melasma, psoriasis, eczema, contact dermatitis, or even tinea versicolor, using the proper skin care regimen will improve outcomes by affecting the skin barrier, pH, hydration level, and function of the keratinocytes and fibroblasts. In fact, every personal care product that touches the skin has an impact on skin health. For example, if a patient uses a detergent-laden bar soap, the skin barrier will be impaired, which can cause them to react to allergens and irritants. Personal care products can affect the skin pH; this is shown to play a role in Malassezia colonization in atopic dermatitis patients (J Clin Med. 2015;4[6]:1217-28). As dermatologists, we know better than anyone that daily use of SPF improves skin health and lowers the risk of postinflammatory pigmentation. We all agree that patients should cleanse the skin and apply a SPF every day. Giving them guidance about which to choose is very important.
Giving the patient exact instructions will lead to improved compliance
Why should recommending skin care products be perceived differently than prescribing a prescription medication? We should prescribe to our patients in writing the exact skin care regimen they should use for their face or body to ensure that they understand the directions. I have been surprised by patients who have said, “I did not know I was supposed to wash my cleanser off,” or “I wash my face with hand soap.” We can help them by educating them and giving them specific instructions. Improved education and communication results in increased compliance. When you do surgery on a wound, you probably tell them to apply a topical antibiotic ointment, but do you direct them to what cleanser to use or tell them which SPF to use on the stitched wound? Providing written instructions for all dermatologic disorders and postprocedure care is necessary to improve compliance and outcomes.
Combine cosmeceuticals, prescription medications, and medical procedures
You (unlike the cosmetic counter salesperson) have the ability to combine cosmeceuticals with prescription medications and medical procedures. In fact, selling your patients the right skin care products to use after a procedure saves them a trip to the store and ensures that they use the correct products. Of course it makes sense that patients getting toxins and fillers should use a retinoid to improve skin aging; however, many general dermatologic diseases would improve with the proper skin care. For example, do you use biologics for psoriasis? Using the proper skin care to regulate skin pH and improve the skin barrier may help prevent colonization of yeast, fungus, and bacteria. The same is true for atopic patients. Do you use liquid nitrogen? Studies show that using a retinoid before a procedure speeds healing. Skin care goes way beyond wrinkles and dark circles under the eyes, so if you are not prescribing the patient an exact regimen, you are not maximizing outcomes.
I don’t have time to talk to my patients about skin care
The missing piece is that most of us don’t have the time to spend discussing skin care. This is where using a standardized scientific methodology is crucial. I developed and use a skin typing methodology in my office and have seen improved physician/patient relationships and increased patient satisfaction resulting in a significant amount of referrals. We also have noted decreased call backs and fewer adverse events from products because the patients have a better understanding of how to properly apply the cosmeceuticals and prescription products. The best part is, it does not add any time onto the patient visit when standardized methodologies are properly adopted.
What if I still do not feel comfortable profiting from the sale of skin care products?
First you need to realize that time is money and you are saving the patient the cost in time it would have taken them to go to a store, park, and shop for the correct product. I have seen data presented from several companies that show that patients usually spend a large amount of money on skin care products after they see their dermatologist. Without guidance, they will likely buy the incorrect products. If they buy the wrong product, you save them the hassle of having to make another office visit and the aggravation of the side effects from the incorrect product. These are often of poor quality or not appropriate for their skin issues. Counterfeit products are rampant on the Internet and many new companies tout worthless products with stem cells and other nonsense. Only you can help your patients make sure that money is spent on the proper products.
Conclusion
Do you really want someone else giving your patients skin care advice? Your patients deserve to have someone with your insights, knowledge, compassion, and honesty help them achieve optimal skin health through use of the proper cosmeceuticals and prescription medications. It is up to you and your staff to save your patients from falling prey to persuasive salespeople with no scientific knowledge or concern for long-term skin health.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever. Dr. Baumann also developed and owns the Baumann Skin Type Solution skin typing systems and related products.
The ethics behind selling skin care products to patients has been hotly debated within the field of cosmetic dermatology for several decades. In 15 years of practice, I have come to the conclusion that patients want you to and need you to because otherwise they are easily taken advantage of. Other physicians are doing it but we – the dermatologists – are the most qualified to offer skin care advice. This article will discuss the reasons that you need to get over the ethical dilemma and offer skin care to your patients.
Using the correct skin care regimen for the face and body will improve outcomes
Whether a patient suffers from acne, rosacea, melasma, psoriasis, eczema, contact dermatitis, or even tinea versicolor, using the proper skin care regimen will improve outcomes by affecting the skin barrier, pH, hydration level, and function of the keratinocytes and fibroblasts. In fact, every personal care product that touches the skin has an impact on skin health. For example, if a patient uses a detergent-laden bar soap, the skin barrier will be impaired, which can cause them to react to allergens and irritants. Personal care products can affect the skin pH; this is shown to play a role in Malassezia colonization in atopic dermatitis patients (J Clin Med. 2015;4[6]:1217-28). As dermatologists, we know better than anyone that daily use of SPF improves skin health and lowers the risk of postinflammatory pigmentation. We all agree that patients should cleanse the skin and apply a SPF every day. Giving them guidance about which to choose is very important.
Giving the patient exact instructions will lead to improved compliance
Why should recommending skin care products be perceived differently than prescribing a prescription medication? We should prescribe to our patients in writing the exact skin care regimen they should use for their face or body to ensure that they understand the directions. I have been surprised by patients who have said, “I did not know I was supposed to wash my cleanser off,” or “I wash my face with hand soap.” We can help them by educating them and giving them specific instructions. Improved education and communication results in increased compliance. When you do surgery on a wound, you probably tell them to apply a topical antibiotic ointment, but do you direct them to what cleanser to use or tell them which SPF to use on the stitched wound? Providing written instructions for all dermatologic disorders and postprocedure care is necessary to improve compliance and outcomes.
Combine cosmeceuticals, prescription medications, and medical procedures
You (unlike the cosmetic counter salesperson) have the ability to combine cosmeceuticals with prescription medications and medical procedures. In fact, selling your patients the right skin care products to use after a procedure saves them a trip to the store and ensures that they use the correct products. Of course it makes sense that patients getting toxins and fillers should use a retinoid to improve skin aging; however, many general dermatologic diseases would improve with the proper skin care. For example, do you use biologics for psoriasis? Using the proper skin care to regulate skin pH and improve the skin barrier may help prevent colonization of yeast, fungus, and bacteria. The same is true for atopic patients. Do you use liquid nitrogen? Studies show that using a retinoid before a procedure speeds healing. Skin care goes way beyond wrinkles and dark circles under the eyes, so if you are not prescribing the patient an exact regimen, you are not maximizing outcomes.
I don’t have time to talk to my patients about skin care
The missing piece is that most of us don’t have the time to spend discussing skin care. This is where using a standardized scientific methodology is crucial. I developed and use a skin typing methodology in my office and have seen improved physician/patient relationships and increased patient satisfaction resulting in a significant amount of referrals. We also have noted decreased call backs and fewer adverse events from products because the patients have a better understanding of how to properly apply the cosmeceuticals and prescription products. The best part is, it does not add any time onto the patient visit when standardized methodologies are properly adopted.
What if I still do not feel comfortable profiting from the sale of skin care products?
First you need to realize that time is money and you are saving the patient the cost in time it would have taken them to go to a store, park, and shop for the correct product. I have seen data presented from several companies that show that patients usually spend a large amount of money on skin care products after they see their dermatologist. Without guidance, they will likely buy the incorrect products. If they buy the wrong product, you save them the hassle of having to make another office visit and the aggravation of the side effects from the incorrect product. These are often of poor quality or not appropriate for their skin issues. Counterfeit products are rampant on the Internet and many new companies tout worthless products with stem cells and other nonsense. Only you can help your patients make sure that money is spent on the proper products.
Conclusion
Do you really want someone else giving your patients skin care advice? Your patients deserve to have someone with your insights, knowledge, compassion, and honesty help them achieve optimal skin health through use of the proper cosmeceuticals and prescription medications. It is up to you and your staff to save your patients from falling prey to persuasive salespeople with no scientific knowledge or concern for long-term skin health.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever. Dr. Baumann also developed and owns the Baumann Skin Type Solution skin typing systems and related products.
• Most skin care products that patients buy are not appropriate for their skin issues.
• Dermatologists have the most knowledge and insights to prescribe skin care.
• Giving specific skin care instructions helps improve communication.
• Increased communication improves outcomes.