User login
Age-Friendly Health Systems and Meeting the Principles of High Reliability Organizations in the VHA
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing care to more than 9 million enrolled veterans at 1298 facilities.1 In February 2019, the VHA identified key action steps to become a high reliability organization (HRO), transforming how employees think about patient safety and care quality.2 The VHA is also working toward becoming the largest age-friendly health system in the US to be recognized by the Institute for Healthcare Improvement (IHI) for its commitment to providing care guided by the 4Ms (what matters, medication, mentation, and mobility), causing no harm, and aligning care with what matters to older veterans.3 In this article, we describe how the Age-Friendly Health Systems (AFHS) movement supports the culture shift observed in HROs.
Age-Friendly Veteran Care
By 2060, the US population of adults aged ≥ 65 years is projected to increase to about 95 million.3 In the VHA, nearly half of veteran enrollees are aged ≥ 65 years, necessitating evidence-based models of care, such as the 4Ms, to meet their complex care needs.3 Historically, the VHA has been a leader in caring for older adults, recognizing the value of age-friendly care for veterans.4 In 1975, the VHA established the Geriatric Research, Education, and Clinical Centers (GRECCs) to serve as catalysts for developing, implementing, and refining enduring models of geriatric care.4 For 5 decades, GRECCs have driven innovations related to the 4Ms.
The VHA is well positioned to be a leader in the AFHS movement, building on decades of GRECC innovations and geriatric programs that align with the 4Ms and providing specialized geriatric training for health care professionals to expand age-friendly care to new settings and health systems.4 The AFHS movement organizes the 4Ms into a simple framework for frontline staff, and the VHA has recently begun tracking 4Ms care in the electronic health record (EHR) to facilitate evaluation and continuous improvement.
AFHS use the 4Ms as a framework to be implemented in every care setting, from the emergency department to inpatient units, outpatient settings, and postacute and long-term care. By assessing and acting on each M and practicing the 4Ms collectively, all members of the care team work to improve health outcomes and prevent avoidable harm.5
The 4Ms
What matters, is the driver of this person-centered approach. Any member of the care team may initiate a what matters conversation with the older adult to understand their personal values, health goals, and care preferences. When compared with usual care, care aligned with the older adult’s health priorities has been shown to decrease the use of high-risk medications and reduce treatment burden.6 The VHA has adopted Whole Health principles of care and the Patient Priorities Care approach to identify and support what matters to veterans.7,8
Addressing polypharmacy and identifying and deprescribing potentially inappropriate medications are essential in preventing adverse drug events, drug-drug interactions, and medication nonadherence.9 In the VHA, VIONE (Vital, Important, Optional, Not indicated, Every medication has an indication) is a rapidly expanding medication deprescribing program that exemplifies HRO principles.9 VIONE provides medication management that supports shared decision making, reducing risk and improving patient safety and quality of life.9 As of June 2023, > 600,000 unique veterans have benefited from VIONE, with an average of 2.2 medications deprescribed per patient with an annual cost avoidance of > $100 million.10
Assessing and acting on mentation includes preventing, identifying, and managing depression and dementia in outpatient settings and delirium in hospital and long-term care settings.5 There are many tools and clinical reminders available in the EHR so that interdisciplinary teams can document changes to mentation and identify opportunities for continuous improvement.
Closely aligned with mentation is mobility, with evidence suggesting that regular physical activity reduces the risk of falls (preventing associated complications), maintains physical functioning, and lowers the risk of cognitive impairment and depression.5 Ensuring early, frequent, and safe mobility helps patients achieve better health outcomes and prevent injury.5 Mobility programs within the VHA include the STRIDE program for the inpatient setting and Gerofit for outpatient settings.11,12
HRO Principles
An HRO is a complex environment of care that experiences fewer than anticipated accidents or adverse events by (1) establishing trust among leaders and staff by balancing individual accountability with systems thinking; (2) empowering staff to lead continuous process improvements; and (3) creating an environment where employees feel safe to report harm or near misses, focusing on the reasons errors occur.13 The work of AFHS incorporates HRO principles with an emphasis on 3 elements. First, it involves interactive systems and processes needed to support 4Ms care across care settings. Second, AFHS acknowledge the complexity of age-friendly work and deference to the expertise of interdisciplinary team members. Finally, AFHS are committed to resilience by overcoming failures and challenges to implementation and long-term sustainment as a standard of practice.
Case study
The names and details in this case have been modified to protect patient privacy. It is representative of many Community Living Centers (CLCs) involved in AFHS that work to create a safe, person-centered environment for veterans.
In a CLC team workroom, 2 nurses were discussing a long-term care resident. The nurses approached the attending physician and explained that they were worried about Sgt Johnson, who seemed depressed and sometimes combative. They had noticed a change in his behavior when they helped him clean up after an episode of incontinence and were concerned that he would try to get out of bed on his own and fall. The attending physician thanked them for sharing their concerns. Sgt Johnson was a retired Army veteran who had a long, decorated military career. His chronic health conditions had led to muscle weakness, and he fell and broke a hip before this admission. He had an uneventful hip replacement but was showing signs of depression due to his limited mobility, loss of independence, and inability to live at home without additional support.
The attending physician knocked on the door of his room, sat down next to the bed, and asked, “How are you feeling today?” Sgt Johnson tersely replied, “About the same.” The physician asked, “Sgt Johnson, what matters most to you related to your recovery? What is important to you?” Sgt Johnson responded, “Feeling like a man!” The doctor replied, “So what makes you feel ‘not like a man’?” The Sgt replied, “Having to be cleaned up by the nurses and not being able to use the toilet on my own.” The physician surmised that his decline in physical functioning had a connection to his worsening depression and combativeness and said to the Sgt, “Let’s get the team together and work out a plan to get you strong enough to use a bedside commode by yourself. Let’s make that the first goal in our plan to get you back to using the toilet independently. Can you work with us on that?” He smiled and said, “Sir, yes Sir!”
At the weekly interdisciplinary team meeting, the team discussed Sgt Johnson’s wishes and the nurses’ safety concerns. The physician reported to the team what mattered to the veteran. The nurses arranged for a bedside commode and supplies to be placed in his room, encouraged and assisted him, and provided a privacy screen. The physical therapist continued to support his mobility needs, concentrating on transfers, small steps like standing and turning with a walker to get in position to use the bedside commode, and later the bathroom toilet. The psychologist addressed what matters to Sgt Johnson and his mentation, health goals, and coping strategies. The social worker provided support and counseling for the veteran and his family. The pharmacist checked his medications to be sure that none were affecting his gastrointestinal tract and his ability to move safely and do what matters to him. Knowing what mattered to Sgt Johnson was the driver of the interdisciplinary care plan to provide 4Ms care.
The team worked collaboratively with the veteran to develop and set attainable goals around toileting and regaining his dignity. This improved his overall recovery. As Sgt Johnson became more independent, his mood gradually improved and he began to participate in other activities and interact with other residents on the unit, and he did not experience any falls. By addressing the 4Ms, the interdisciplinary team coordinated efforts to provide high-quality, person-centered care. They built trust with the veteran, shared accountability, and followed HRO principles to keep the veteran safe.
Becoming an Age-Friendly HRO
Becoming an HRO is a dynamic, ever-changing process to maintain high standards, improve care quality, and cause no harm. There are 3 pillars and 5 principles that guide an HRO. The pillars are critical areas of focus and include leadership commitment, culture of safety, and continuous process improvement.14 The first of 5 HRO principles is sensitivity to operations. This is defined as an awareness of how processes and systems impact the entire organization, the downstream impact.15 Focusing on the 4Ms helps develop the capability of frontline staff to provide high-quality care for older adults while ensuring that processes are in place to support the work. The 4Ms provide an efficient way to organize interdisciplinary team meetings, provide warm handoffs using Situation-Background-Assessment-Recommendation, and standardize documentation. Involvement in the AFHS movement improves communication, care quality, and patient and staff satisfaction to meet this HRO principle.15
The second HRO principle, reluctance to simplify, ensures that direct care staff and leaders delve further into issues to find solutions.15 AFHS use the Plan-Do-Study-Act cycle to put the 4Ms into practice; this cycle helps teams test small increments of change, study their performance, and act to ensure that all 4Ms are being practiced as a set. AFHS teams are encouraged to review at least 3 months of data after implementation of the 4Ms, working to find solutions if there are gaps or issues identified.
The third principle, preoccupation with failure, refers to shared attentiveness—being prepared for the unexpected and learning from mistakes.15 The entire AFHS team shares responsibility for providing 4Ms care, where staff are empowered to report any safety concerns or close calls. The fourth principle of deference to expertise includes listening to staff who have the most knowledge for the task at hand, which aligns with the collaborative interdisciplinary teamwork of age-friendly teams.15
The final HRO principle, commitment to resilience, includes continuous learning, interdisciplinary team training, and sharing of lessons learned.15 Although IHI offers 2 levels of AFHS recognition, teams are continuously learning to improve and sustain care beyond level 2, Committed to Care Excellence recognition.16
The Table shows the VHA’s AFHS implementation strategies and the HRO principles adapted from the Joint Commission’s High Reliability Health Care Maturity Model and the IHI’s Framework for Safe, Reliable, and Effective Care. The VHA is developing a national dashboard to capture age-friendly processes and health outcome measures that address patient safety and care quality.
Conclusions
AFHS empowers VHA teams to honor veterans’ care preferences and values, supporting their independence, dignity, and quality of life across care settings. The adoption of AFHS brings evidence-based practices to the point of care by addressing common pitfalls in the care of older adults, drawing attention to, and calling for action on inappropriate medication use, physical inactivity, and assessment of the vulnerable brain. The 4Ms also serve as a framework to continuously improve care and cause zero harm, reinforcing HRO pillars and principles across the VHA, and ensuring that older adults reliably receive the evidence-based, high-quality care they deserve.
1. Veterans Health Administration. Providing healthcare for veterans. Updated June 20, 2023. Accessed June 26, 2023. https://www.va.gov/health
2. Veazie S, Peterson K, Bourne D. Evidence brief: implementation of high reliability organization principles. Washington, DC: Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP Project #09-199; 2019. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/esp/high-reliability-org.cfm
3. Church K, Munro S, Shaughnessy M, Clancy C. Age-Friendly Health Systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58(suppl 1):5-8. doi:10.1111/1475-6773.14110
4. Farrell TW, Volden TA, Butler JM, et al. Age-friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. 2023;71(1):18-25. doi:10.1111/jgs.18070
5. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
6. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: A nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688-1697. doi:10.1001/jamainternmed.2019.4235
7. US Department of Veterans Affairs. What is whole health? Updated: October 31, 2023. November 30, 2023. https://www.va.gov/wholehealth
8. Patient Priorities Care. Updated 2019. Accessed November 30, 2023. https://patientprioritiescare.org
9. Battar S, Watson Dickerson KR, Sedgwick C, Cmelik T. Understanding principles of high reliability organizations through the eyes of VIONE: a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
10. VA Diffusion Marketplace. VIONE- medication optimization and polypharmacy reduction initiative. Accessed November 30, 2023. https://marketplace.va.gov/innovations/vione
11. US Department of Veterans Affairs, Office of Research and Development. STRIDE program to keep hospitalized veterans mobile. Updated November 6, 2018. Accessed November 30, 2023. https://www.research.va.gov/research_in_action/STRIDE-program-to-keep-hospitalized-Veterans-mobile.cfm
12. US Department of Veterans Affairs, VA Geriatrics and Extended Care. Gerofit: a program promoting exercise and health for older veterans. Updated August 2, 2023. Accessed November 30, 2023. https://www.va.gov/GERIATRICS/pages/gerofit_Home.asp
13. US Department of Veterans Affairs, Health Services Research and Development. VHA’s vision for a high reliability organization. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. US Department of Veterans Affairs, Health Services Research and Development. Three HRO evaluation priorities. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-2
15. Oster CA, Deakins S. Practical application of high-reliability principles in healthcare to optimize quality and safety outcomes. J Nurs Adm. 2018;48(1):50-55. doi:10.1097/NNA.0000000000000570
16. Institute for Healthcare Improvement. Age-Friendly Health Systems recognitions. Accessed November 30, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/Recognition.aspx
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing care to more than 9 million enrolled veterans at 1298 facilities.1 In February 2019, the VHA identified key action steps to become a high reliability organization (HRO), transforming how employees think about patient safety and care quality.2 The VHA is also working toward becoming the largest age-friendly health system in the US to be recognized by the Institute for Healthcare Improvement (IHI) for its commitment to providing care guided by the 4Ms (what matters, medication, mentation, and mobility), causing no harm, and aligning care with what matters to older veterans.3 In this article, we describe how the Age-Friendly Health Systems (AFHS) movement supports the culture shift observed in HROs.
Age-Friendly Veteran Care
By 2060, the US population of adults aged ≥ 65 years is projected to increase to about 95 million.3 In the VHA, nearly half of veteran enrollees are aged ≥ 65 years, necessitating evidence-based models of care, such as the 4Ms, to meet their complex care needs.3 Historically, the VHA has been a leader in caring for older adults, recognizing the value of age-friendly care for veterans.4 In 1975, the VHA established the Geriatric Research, Education, and Clinical Centers (GRECCs) to serve as catalysts for developing, implementing, and refining enduring models of geriatric care.4 For 5 decades, GRECCs have driven innovations related to the 4Ms.
The VHA is well positioned to be a leader in the AFHS movement, building on decades of GRECC innovations and geriatric programs that align with the 4Ms and providing specialized geriatric training for health care professionals to expand age-friendly care to new settings and health systems.4 The AFHS movement organizes the 4Ms into a simple framework for frontline staff, and the VHA has recently begun tracking 4Ms care in the electronic health record (EHR) to facilitate evaluation and continuous improvement.
AFHS use the 4Ms as a framework to be implemented in every care setting, from the emergency department to inpatient units, outpatient settings, and postacute and long-term care. By assessing and acting on each M and practicing the 4Ms collectively, all members of the care team work to improve health outcomes and prevent avoidable harm.5
The 4Ms
What matters, is the driver of this person-centered approach. Any member of the care team may initiate a what matters conversation with the older adult to understand their personal values, health goals, and care preferences. When compared with usual care, care aligned with the older adult’s health priorities has been shown to decrease the use of high-risk medications and reduce treatment burden.6 The VHA has adopted Whole Health principles of care and the Patient Priorities Care approach to identify and support what matters to veterans.7,8
Addressing polypharmacy and identifying and deprescribing potentially inappropriate medications are essential in preventing adverse drug events, drug-drug interactions, and medication nonadherence.9 In the VHA, VIONE (Vital, Important, Optional, Not indicated, Every medication has an indication) is a rapidly expanding medication deprescribing program that exemplifies HRO principles.9 VIONE provides medication management that supports shared decision making, reducing risk and improving patient safety and quality of life.9 As of June 2023, > 600,000 unique veterans have benefited from VIONE, with an average of 2.2 medications deprescribed per patient with an annual cost avoidance of > $100 million.10
Assessing and acting on mentation includes preventing, identifying, and managing depression and dementia in outpatient settings and delirium in hospital and long-term care settings.5 There are many tools and clinical reminders available in the EHR so that interdisciplinary teams can document changes to mentation and identify opportunities for continuous improvement.
Closely aligned with mentation is mobility, with evidence suggesting that regular physical activity reduces the risk of falls (preventing associated complications), maintains physical functioning, and lowers the risk of cognitive impairment and depression.5 Ensuring early, frequent, and safe mobility helps patients achieve better health outcomes and prevent injury.5 Mobility programs within the VHA include the STRIDE program for the inpatient setting and Gerofit for outpatient settings.11,12
HRO Principles
An HRO is a complex environment of care that experiences fewer than anticipated accidents or adverse events by (1) establishing trust among leaders and staff by balancing individual accountability with systems thinking; (2) empowering staff to lead continuous process improvements; and (3) creating an environment where employees feel safe to report harm or near misses, focusing on the reasons errors occur.13 The work of AFHS incorporates HRO principles with an emphasis on 3 elements. First, it involves interactive systems and processes needed to support 4Ms care across care settings. Second, AFHS acknowledge the complexity of age-friendly work and deference to the expertise of interdisciplinary team members. Finally, AFHS are committed to resilience by overcoming failures and challenges to implementation and long-term sustainment as a standard of practice.
Case study
The names and details in this case have been modified to protect patient privacy. It is representative of many Community Living Centers (CLCs) involved in AFHS that work to create a safe, person-centered environment for veterans.
In a CLC team workroom, 2 nurses were discussing a long-term care resident. The nurses approached the attending physician and explained that they were worried about Sgt Johnson, who seemed depressed and sometimes combative. They had noticed a change in his behavior when they helped him clean up after an episode of incontinence and were concerned that he would try to get out of bed on his own and fall. The attending physician thanked them for sharing their concerns. Sgt Johnson was a retired Army veteran who had a long, decorated military career. His chronic health conditions had led to muscle weakness, and he fell and broke a hip before this admission. He had an uneventful hip replacement but was showing signs of depression due to his limited mobility, loss of independence, and inability to live at home without additional support.
The attending physician knocked on the door of his room, sat down next to the bed, and asked, “How are you feeling today?” Sgt Johnson tersely replied, “About the same.” The physician asked, “Sgt Johnson, what matters most to you related to your recovery? What is important to you?” Sgt Johnson responded, “Feeling like a man!” The doctor replied, “So what makes you feel ‘not like a man’?” The Sgt replied, “Having to be cleaned up by the nurses and not being able to use the toilet on my own.” The physician surmised that his decline in physical functioning had a connection to his worsening depression and combativeness and said to the Sgt, “Let’s get the team together and work out a plan to get you strong enough to use a bedside commode by yourself. Let’s make that the first goal in our plan to get you back to using the toilet independently. Can you work with us on that?” He smiled and said, “Sir, yes Sir!”
At the weekly interdisciplinary team meeting, the team discussed Sgt Johnson’s wishes and the nurses’ safety concerns. The physician reported to the team what mattered to the veteran. The nurses arranged for a bedside commode and supplies to be placed in his room, encouraged and assisted him, and provided a privacy screen. The physical therapist continued to support his mobility needs, concentrating on transfers, small steps like standing and turning with a walker to get in position to use the bedside commode, and later the bathroom toilet. The psychologist addressed what matters to Sgt Johnson and his mentation, health goals, and coping strategies. The social worker provided support and counseling for the veteran and his family. The pharmacist checked his medications to be sure that none were affecting his gastrointestinal tract and his ability to move safely and do what matters to him. Knowing what mattered to Sgt Johnson was the driver of the interdisciplinary care plan to provide 4Ms care.
The team worked collaboratively with the veteran to develop and set attainable goals around toileting and regaining his dignity. This improved his overall recovery. As Sgt Johnson became more independent, his mood gradually improved and he began to participate in other activities and interact with other residents on the unit, and he did not experience any falls. By addressing the 4Ms, the interdisciplinary team coordinated efforts to provide high-quality, person-centered care. They built trust with the veteran, shared accountability, and followed HRO principles to keep the veteran safe.
Becoming an Age-Friendly HRO
Becoming an HRO is a dynamic, ever-changing process to maintain high standards, improve care quality, and cause no harm. There are 3 pillars and 5 principles that guide an HRO. The pillars are critical areas of focus and include leadership commitment, culture of safety, and continuous process improvement.14 The first of 5 HRO principles is sensitivity to operations. This is defined as an awareness of how processes and systems impact the entire organization, the downstream impact.15 Focusing on the 4Ms helps develop the capability of frontline staff to provide high-quality care for older adults while ensuring that processes are in place to support the work. The 4Ms provide an efficient way to organize interdisciplinary team meetings, provide warm handoffs using Situation-Background-Assessment-Recommendation, and standardize documentation. Involvement in the AFHS movement improves communication, care quality, and patient and staff satisfaction to meet this HRO principle.15
The second HRO principle, reluctance to simplify, ensures that direct care staff and leaders delve further into issues to find solutions.15 AFHS use the Plan-Do-Study-Act cycle to put the 4Ms into practice; this cycle helps teams test small increments of change, study their performance, and act to ensure that all 4Ms are being practiced as a set. AFHS teams are encouraged to review at least 3 months of data after implementation of the 4Ms, working to find solutions if there are gaps or issues identified.
The third principle, preoccupation with failure, refers to shared attentiveness—being prepared for the unexpected and learning from mistakes.15 The entire AFHS team shares responsibility for providing 4Ms care, where staff are empowered to report any safety concerns or close calls. The fourth principle of deference to expertise includes listening to staff who have the most knowledge for the task at hand, which aligns with the collaborative interdisciplinary teamwork of age-friendly teams.15
The final HRO principle, commitment to resilience, includes continuous learning, interdisciplinary team training, and sharing of lessons learned.15 Although IHI offers 2 levels of AFHS recognition, teams are continuously learning to improve and sustain care beyond level 2, Committed to Care Excellence recognition.16
The Table shows the VHA’s AFHS implementation strategies and the HRO principles adapted from the Joint Commission’s High Reliability Health Care Maturity Model and the IHI’s Framework for Safe, Reliable, and Effective Care. The VHA is developing a national dashboard to capture age-friendly processes and health outcome measures that address patient safety and care quality.
Conclusions
AFHS empowers VHA teams to honor veterans’ care preferences and values, supporting their independence, dignity, and quality of life across care settings. The adoption of AFHS brings evidence-based practices to the point of care by addressing common pitfalls in the care of older adults, drawing attention to, and calling for action on inappropriate medication use, physical inactivity, and assessment of the vulnerable brain. The 4Ms also serve as a framework to continuously improve care and cause zero harm, reinforcing HRO pillars and principles across the VHA, and ensuring that older adults reliably receive the evidence-based, high-quality care they deserve.
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing care to more than 9 million enrolled veterans at 1298 facilities.1 In February 2019, the VHA identified key action steps to become a high reliability organization (HRO), transforming how employees think about patient safety and care quality.2 The VHA is also working toward becoming the largest age-friendly health system in the US to be recognized by the Institute for Healthcare Improvement (IHI) for its commitment to providing care guided by the 4Ms (what matters, medication, mentation, and mobility), causing no harm, and aligning care with what matters to older veterans.3 In this article, we describe how the Age-Friendly Health Systems (AFHS) movement supports the culture shift observed in HROs.
Age-Friendly Veteran Care
By 2060, the US population of adults aged ≥ 65 years is projected to increase to about 95 million.3 In the VHA, nearly half of veteran enrollees are aged ≥ 65 years, necessitating evidence-based models of care, such as the 4Ms, to meet their complex care needs.3 Historically, the VHA has been a leader in caring for older adults, recognizing the value of age-friendly care for veterans.4 In 1975, the VHA established the Geriatric Research, Education, and Clinical Centers (GRECCs) to serve as catalysts for developing, implementing, and refining enduring models of geriatric care.4 For 5 decades, GRECCs have driven innovations related to the 4Ms.
The VHA is well positioned to be a leader in the AFHS movement, building on decades of GRECC innovations and geriatric programs that align with the 4Ms and providing specialized geriatric training for health care professionals to expand age-friendly care to new settings and health systems.4 The AFHS movement organizes the 4Ms into a simple framework for frontline staff, and the VHA has recently begun tracking 4Ms care in the electronic health record (EHR) to facilitate evaluation and continuous improvement.
AFHS use the 4Ms as a framework to be implemented in every care setting, from the emergency department to inpatient units, outpatient settings, and postacute and long-term care. By assessing and acting on each M and practicing the 4Ms collectively, all members of the care team work to improve health outcomes and prevent avoidable harm.5
The 4Ms
What matters, is the driver of this person-centered approach. Any member of the care team may initiate a what matters conversation with the older adult to understand their personal values, health goals, and care preferences. When compared with usual care, care aligned with the older adult’s health priorities has been shown to decrease the use of high-risk medications and reduce treatment burden.6 The VHA has adopted Whole Health principles of care and the Patient Priorities Care approach to identify and support what matters to veterans.7,8
Addressing polypharmacy and identifying and deprescribing potentially inappropriate medications are essential in preventing adverse drug events, drug-drug interactions, and medication nonadherence.9 In the VHA, VIONE (Vital, Important, Optional, Not indicated, Every medication has an indication) is a rapidly expanding medication deprescribing program that exemplifies HRO principles.9 VIONE provides medication management that supports shared decision making, reducing risk and improving patient safety and quality of life.9 As of June 2023, > 600,000 unique veterans have benefited from VIONE, with an average of 2.2 medications deprescribed per patient with an annual cost avoidance of > $100 million.10
Assessing and acting on mentation includes preventing, identifying, and managing depression and dementia in outpatient settings and delirium in hospital and long-term care settings.5 There are many tools and clinical reminders available in the EHR so that interdisciplinary teams can document changes to mentation and identify opportunities for continuous improvement.
Closely aligned with mentation is mobility, with evidence suggesting that regular physical activity reduces the risk of falls (preventing associated complications), maintains physical functioning, and lowers the risk of cognitive impairment and depression.5 Ensuring early, frequent, and safe mobility helps patients achieve better health outcomes and prevent injury.5 Mobility programs within the VHA include the STRIDE program for the inpatient setting and Gerofit for outpatient settings.11,12
HRO Principles
An HRO is a complex environment of care that experiences fewer than anticipated accidents or adverse events by (1) establishing trust among leaders and staff by balancing individual accountability with systems thinking; (2) empowering staff to lead continuous process improvements; and (3) creating an environment where employees feel safe to report harm or near misses, focusing on the reasons errors occur.13 The work of AFHS incorporates HRO principles with an emphasis on 3 elements. First, it involves interactive systems and processes needed to support 4Ms care across care settings. Second, AFHS acknowledge the complexity of age-friendly work and deference to the expertise of interdisciplinary team members. Finally, AFHS are committed to resilience by overcoming failures and challenges to implementation and long-term sustainment as a standard of practice.
Case study
The names and details in this case have been modified to protect patient privacy. It is representative of many Community Living Centers (CLCs) involved in AFHS that work to create a safe, person-centered environment for veterans.
In a CLC team workroom, 2 nurses were discussing a long-term care resident. The nurses approached the attending physician and explained that they were worried about Sgt Johnson, who seemed depressed and sometimes combative. They had noticed a change in his behavior when they helped him clean up after an episode of incontinence and were concerned that he would try to get out of bed on his own and fall. The attending physician thanked them for sharing their concerns. Sgt Johnson was a retired Army veteran who had a long, decorated military career. His chronic health conditions had led to muscle weakness, and he fell and broke a hip before this admission. He had an uneventful hip replacement but was showing signs of depression due to his limited mobility, loss of independence, and inability to live at home without additional support.
The attending physician knocked on the door of his room, sat down next to the bed, and asked, “How are you feeling today?” Sgt Johnson tersely replied, “About the same.” The physician asked, “Sgt Johnson, what matters most to you related to your recovery? What is important to you?” Sgt Johnson responded, “Feeling like a man!” The doctor replied, “So what makes you feel ‘not like a man’?” The Sgt replied, “Having to be cleaned up by the nurses and not being able to use the toilet on my own.” The physician surmised that his decline in physical functioning had a connection to his worsening depression and combativeness and said to the Sgt, “Let’s get the team together and work out a plan to get you strong enough to use a bedside commode by yourself. Let’s make that the first goal in our plan to get you back to using the toilet independently. Can you work with us on that?” He smiled and said, “Sir, yes Sir!”
At the weekly interdisciplinary team meeting, the team discussed Sgt Johnson’s wishes and the nurses’ safety concerns. The physician reported to the team what mattered to the veteran. The nurses arranged for a bedside commode and supplies to be placed in his room, encouraged and assisted him, and provided a privacy screen. The physical therapist continued to support his mobility needs, concentrating on transfers, small steps like standing and turning with a walker to get in position to use the bedside commode, and later the bathroom toilet. The psychologist addressed what matters to Sgt Johnson and his mentation, health goals, and coping strategies. The social worker provided support and counseling for the veteran and his family. The pharmacist checked his medications to be sure that none were affecting his gastrointestinal tract and his ability to move safely and do what matters to him. Knowing what mattered to Sgt Johnson was the driver of the interdisciplinary care plan to provide 4Ms care.
The team worked collaboratively with the veteran to develop and set attainable goals around toileting and regaining his dignity. This improved his overall recovery. As Sgt Johnson became more independent, his mood gradually improved and he began to participate in other activities and interact with other residents on the unit, and he did not experience any falls. By addressing the 4Ms, the interdisciplinary team coordinated efforts to provide high-quality, person-centered care. They built trust with the veteran, shared accountability, and followed HRO principles to keep the veteran safe.
Becoming an Age-Friendly HRO
Becoming an HRO is a dynamic, ever-changing process to maintain high standards, improve care quality, and cause no harm. There are 3 pillars and 5 principles that guide an HRO. The pillars are critical areas of focus and include leadership commitment, culture of safety, and continuous process improvement.14 The first of 5 HRO principles is sensitivity to operations. This is defined as an awareness of how processes and systems impact the entire organization, the downstream impact.15 Focusing on the 4Ms helps develop the capability of frontline staff to provide high-quality care for older adults while ensuring that processes are in place to support the work. The 4Ms provide an efficient way to organize interdisciplinary team meetings, provide warm handoffs using Situation-Background-Assessment-Recommendation, and standardize documentation. Involvement in the AFHS movement improves communication, care quality, and patient and staff satisfaction to meet this HRO principle.15
The second HRO principle, reluctance to simplify, ensures that direct care staff and leaders delve further into issues to find solutions.15 AFHS use the Plan-Do-Study-Act cycle to put the 4Ms into practice; this cycle helps teams test small increments of change, study their performance, and act to ensure that all 4Ms are being practiced as a set. AFHS teams are encouraged to review at least 3 months of data after implementation of the 4Ms, working to find solutions if there are gaps or issues identified.
The third principle, preoccupation with failure, refers to shared attentiveness—being prepared for the unexpected and learning from mistakes.15 The entire AFHS team shares responsibility for providing 4Ms care, where staff are empowered to report any safety concerns or close calls. The fourth principle of deference to expertise includes listening to staff who have the most knowledge for the task at hand, which aligns with the collaborative interdisciplinary teamwork of age-friendly teams.15
The final HRO principle, commitment to resilience, includes continuous learning, interdisciplinary team training, and sharing of lessons learned.15 Although IHI offers 2 levels of AFHS recognition, teams are continuously learning to improve and sustain care beyond level 2, Committed to Care Excellence recognition.16
The Table shows the VHA’s AFHS implementation strategies and the HRO principles adapted from the Joint Commission’s High Reliability Health Care Maturity Model and the IHI’s Framework for Safe, Reliable, and Effective Care. The VHA is developing a national dashboard to capture age-friendly processes and health outcome measures that address patient safety and care quality.
Conclusions
AFHS empowers VHA teams to honor veterans’ care preferences and values, supporting their independence, dignity, and quality of life across care settings. The adoption of AFHS brings evidence-based practices to the point of care by addressing common pitfalls in the care of older adults, drawing attention to, and calling for action on inappropriate medication use, physical inactivity, and assessment of the vulnerable brain. The 4Ms also serve as a framework to continuously improve care and cause zero harm, reinforcing HRO pillars and principles across the VHA, and ensuring that older adults reliably receive the evidence-based, high-quality care they deserve.
1. Veterans Health Administration. Providing healthcare for veterans. Updated June 20, 2023. Accessed June 26, 2023. https://www.va.gov/health
2. Veazie S, Peterson K, Bourne D. Evidence brief: implementation of high reliability organization principles. Washington, DC: Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP Project #09-199; 2019. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/esp/high-reliability-org.cfm
3. Church K, Munro S, Shaughnessy M, Clancy C. Age-Friendly Health Systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58(suppl 1):5-8. doi:10.1111/1475-6773.14110
4. Farrell TW, Volden TA, Butler JM, et al. Age-friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. 2023;71(1):18-25. doi:10.1111/jgs.18070
5. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
6. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: A nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688-1697. doi:10.1001/jamainternmed.2019.4235
7. US Department of Veterans Affairs. What is whole health? Updated: October 31, 2023. November 30, 2023. https://www.va.gov/wholehealth
8. Patient Priorities Care. Updated 2019. Accessed November 30, 2023. https://patientprioritiescare.org
9. Battar S, Watson Dickerson KR, Sedgwick C, Cmelik T. Understanding principles of high reliability organizations through the eyes of VIONE: a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
10. VA Diffusion Marketplace. VIONE- medication optimization and polypharmacy reduction initiative. Accessed November 30, 2023. https://marketplace.va.gov/innovations/vione
11. US Department of Veterans Affairs, Office of Research and Development. STRIDE program to keep hospitalized veterans mobile. Updated November 6, 2018. Accessed November 30, 2023. https://www.research.va.gov/research_in_action/STRIDE-program-to-keep-hospitalized-Veterans-mobile.cfm
12. US Department of Veterans Affairs, VA Geriatrics and Extended Care. Gerofit: a program promoting exercise and health for older veterans. Updated August 2, 2023. Accessed November 30, 2023. https://www.va.gov/GERIATRICS/pages/gerofit_Home.asp
13. US Department of Veterans Affairs, Health Services Research and Development. VHA’s vision for a high reliability organization. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. US Department of Veterans Affairs, Health Services Research and Development. Three HRO evaluation priorities. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-2
15. Oster CA, Deakins S. Practical application of high-reliability principles in healthcare to optimize quality and safety outcomes. J Nurs Adm. 2018;48(1):50-55. doi:10.1097/NNA.0000000000000570
16. Institute for Healthcare Improvement. Age-Friendly Health Systems recognitions. Accessed November 30, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/Recognition.aspx
1. Veterans Health Administration. Providing healthcare for veterans. Updated June 20, 2023. Accessed June 26, 2023. https://www.va.gov/health
2. Veazie S, Peterson K, Bourne D. Evidence brief: implementation of high reliability organization principles. Washington, DC: Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP Project #09-199; 2019. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/esp/high-reliability-org.cfm
3. Church K, Munro S, Shaughnessy M, Clancy C. Age-Friendly Health Systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58(suppl 1):5-8. doi:10.1111/1475-6773.14110
4. Farrell TW, Volden TA, Butler JM, et al. Age-friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. 2023;71(1):18-25. doi:10.1111/jgs.18070
5. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
6. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: A nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688-1697. doi:10.1001/jamainternmed.2019.4235
7. US Department of Veterans Affairs. What is whole health? Updated: October 31, 2023. November 30, 2023. https://www.va.gov/wholehealth
8. Patient Priorities Care. Updated 2019. Accessed November 30, 2023. https://patientprioritiescare.org
9. Battar S, Watson Dickerson KR, Sedgwick C, Cmelik T. Understanding principles of high reliability organizations through the eyes of VIONE: a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
10. VA Diffusion Marketplace. VIONE- medication optimization and polypharmacy reduction initiative. Accessed November 30, 2023. https://marketplace.va.gov/innovations/vione
11. US Department of Veterans Affairs, Office of Research and Development. STRIDE program to keep hospitalized veterans mobile. Updated November 6, 2018. Accessed November 30, 2023. https://www.research.va.gov/research_in_action/STRIDE-program-to-keep-hospitalized-Veterans-mobile.cfm
12. US Department of Veterans Affairs, VA Geriatrics and Extended Care. Gerofit: a program promoting exercise and health for older veterans. Updated August 2, 2023. Accessed November 30, 2023. https://www.va.gov/GERIATRICS/pages/gerofit_Home.asp
13. US Department of Veterans Affairs, Health Services Research and Development. VHA’s vision for a high reliability organization. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. US Department of Veterans Affairs, Health Services Research and Development. Three HRO evaluation priorities. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-2
15. Oster CA, Deakins S. Practical application of high-reliability principles in healthcare to optimize quality and safety outcomes. J Nurs Adm. 2018;48(1):50-55. doi:10.1097/NNA.0000000000000570
16. Institute for Healthcare Improvement. Age-Friendly Health Systems recognitions. Accessed November 30, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/Recognition.aspx
Perinatal Psychiatry in 2024: Helping More Patients Access Care
The past year has been a challenging time for many, both at the local level and globally, with divisive undercurrents across many communities. Many times, the end of the year is an opportunity for reflection. As I reflect on the state of perinatal psychiatry in the new year, I see several evolving issues that I’d like to share in this first column of 2024.
In 2023, the American College of Obstetricians and Gynecologists published new recommendations meant to enhance the well-being of pregnant and postpartum women and families. A main message from discussion papers borne out of these recommendations was that as a field, we should be doing more than identifying perinatal illness. We should be screening women at risk for postpartum psychiatric illness and see that those suffering from posttraumatic stress disorder (PTSD) have access to care and “wrap-around services” from clinicians with varying expertise.
Screening is a primary way we identify patients at risk for psychiatric illness and also those who are suffering at the time of a screen. One problem I see in the near future is our disparate collection and management of data. When we look closely across health care systems, it’s not clear how screening data are captured, let alone managed. What is being done in one hospital system may be very different from what is being done elsewhere. Some clinicians are adopting digital platforms to identify those with postpartum depression, while others are practicing as they always have, either through a paper screening process or with queries as part of a clinical encounter.
Given this amalgam of methods for collecting and storing information, there does not appear to be a systematic way clinicians and researchers are recording whether women are meeting criteria for significant depressive symptoms or frank postpartum psychiatric illness. It is clear a more cohesive method for collection and management is needed to optimize the likelihood that next steps can be taken to get patients the care they need.
However, screening is only one part of the story. Certainly, in our own center, one of our greatest interests, both clinically and on the research side, is what happens after screening. Through our center’s initiation of the Screening and Treatment Enhancement for Postpartum Depression (STEPS for PPD) project funded by the Marriott Foundation, we are evaluating the outcomes of women who are screened at 6 weeks postpartum with significant depressive symptoms, and who are then given an opportunity to engage with a perinatal social worker who can assist with direct psychotherapy, arranging for referrals, and navigating care for a new mother.
What we are learning as we enroll women through the initial stages of STEPS for PPD is that screening and identifying women who likely suffer from PPD simply is not enough. In fact, once identified with a depression screening tool, women who are suffering from postpartum depression can be very challenging to engage clinically. What I am learning decades after starting to work with perinatal patients is that even with a screening system and effective tools for treatment of PPD, optimizing engagement with these depressed women seems a critical and understudied step on the road to optimizing positive clinical outcomes.
A recent study published in the Journal of Women’s Health explored gaps in care for perinatal depression and found that patients without a history of psychiatric illness prior to pregnancy were less likely to be screened for depression and 80% less likely to receive care if they developed depression compared with women with a previous history of psychiatric illness (J Womens Health (Larchmt). 2023 Oct;32[10]:1111-9).
That history may help women navigate to care, while women for whom psychiatric illness is a new experience may be less likely to engage, be referred for care, and receive appropriate treatment. The study indicates that, as a field, we must strive to ensure universal screening for depression in perinatal populations.
While we have always been particularly interested in populations of patients at highest risk for PPD, helping women at risk for PPD in the general population without a history of psychiatric illness is a large public health issue and will be an even larger undertaking. As women’s mental health is gaining more appropriate focus, both at the local level and even in the recent White House Initiative on Women’s Health Research, the focus has been on screening and developing new treatments.
We are not lacking in pharmacologic agents nor nonpharmacologic options as treatments for women experiencing PPD. Newer alternative treatments are being explored, such as transcranial magnetic stimulation (TMS) and even psychedelics as a potential therapy for PPD. But perhaps what we’ve learned in 2023 and as we move into a new year, is that the problem of tackling PPD is not only about having the right tools, but is about helping women navigate to the care that they need.
The COVID-19 pandemic brought with it an explosion of telehealth options that have enhanced the odds women can find support during such a challenging time; as society has returned to some semblance of normal, nearly all support groups for postpartum women have remained online.
When we set up Virtual Rounds at the Center for Women’s Mental Health at the beginning of the pandemic, I was struck by the community of colleagues at various stages of their careers dedicated to mitigating the suffering associated with perinatal psychiatric illness. As I’ve often said, it takes a village to care for these patients. We need help from colleagues with varying expertise — from lactation consultants, psychiatrists, psychologists, obstetricians, nurse practitioners, support group leaders, and a host of others — who can help reach these women.
At the end of the day, helping depressed women find resources is a challenge that we have not met in this country. We should be excited that we have so many treatment options to offer patients — whether it be a new first-in-class medication, TMS, or digital apps to ensure patients are receiving effective treatment. But there should also be a focus on reaching women who still need treatment, particularly in underserved communities where resources are sparse or nonexistent. Identifying the path to reaching these women where they are and getting them well should be a top priority in 2024.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. STEPS for PPD is funded by the Marriott Foundation. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
The past year has been a challenging time for many, both at the local level and globally, with divisive undercurrents across many communities. Many times, the end of the year is an opportunity for reflection. As I reflect on the state of perinatal psychiatry in the new year, I see several evolving issues that I’d like to share in this first column of 2024.
In 2023, the American College of Obstetricians and Gynecologists published new recommendations meant to enhance the well-being of pregnant and postpartum women and families. A main message from discussion papers borne out of these recommendations was that as a field, we should be doing more than identifying perinatal illness. We should be screening women at risk for postpartum psychiatric illness and see that those suffering from posttraumatic stress disorder (PTSD) have access to care and “wrap-around services” from clinicians with varying expertise.
Screening is a primary way we identify patients at risk for psychiatric illness and also those who are suffering at the time of a screen. One problem I see in the near future is our disparate collection and management of data. When we look closely across health care systems, it’s not clear how screening data are captured, let alone managed. What is being done in one hospital system may be very different from what is being done elsewhere. Some clinicians are adopting digital platforms to identify those with postpartum depression, while others are practicing as they always have, either through a paper screening process or with queries as part of a clinical encounter.
Given this amalgam of methods for collecting and storing information, there does not appear to be a systematic way clinicians and researchers are recording whether women are meeting criteria for significant depressive symptoms or frank postpartum psychiatric illness. It is clear a more cohesive method for collection and management is needed to optimize the likelihood that next steps can be taken to get patients the care they need.
However, screening is only one part of the story. Certainly, in our own center, one of our greatest interests, both clinically and on the research side, is what happens after screening. Through our center’s initiation of the Screening and Treatment Enhancement for Postpartum Depression (STEPS for PPD) project funded by the Marriott Foundation, we are evaluating the outcomes of women who are screened at 6 weeks postpartum with significant depressive symptoms, and who are then given an opportunity to engage with a perinatal social worker who can assist with direct psychotherapy, arranging for referrals, and navigating care for a new mother.
What we are learning as we enroll women through the initial stages of STEPS for PPD is that screening and identifying women who likely suffer from PPD simply is not enough. In fact, once identified with a depression screening tool, women who are suffering from postpartum depression can be very challenging to engage clinically. What I am learning decades after starting to work with perinatal patients is that even with a screening system and effective tools for treatment of PPD, optimizing engagement with these depressed women seems a critical and understudied step on the road to optimizing positive clinical outcomes.
A recent study published in the Journal of Women’s Health explored gaps in care for perinatal depression and found that patients without a history of psychiatric illness prior to pregnancy were less likely to be screened for depression and 80% less likely to receive care if they developed depression compared with women with a previous history of psychiatric illness (J Womens Health (Larchmt). 2023 Oct;32[10]:1111-9).
That history may help women navigate to care, while women for whom psychiatric illness is a new experience may be less likely to engage, be referred for care, and receive appropriate treatment. The study indicates that, as a field, we must strive to ensure universal screening for depression in perinatal populations.
While we have always been particularly interested in populations of patients at highest risk for PPD, helping women at risk for PPD in the general population without a history of psychiatric illness is a large public health issue and will be an even larger undertaking. As women’s mental health is gaining more appropriate focus, both at the local level and even in the recent White House Initiative on Women’s Health Research, the focus has been on screening and developing new treatments.
We are not lacking in pharmacologic agents nor nonpharmacologic options as treatments for women experiencing PPD. Newer alternative treatments are being explored, such as transcranial magnetic stimulation (TMS) and even psychedelics as a potential therapy for PPD. But perhaps what we’ve learned in 2023 and as we move into a new year, is that the problem of tackling PPD is not only about having the right tools, but is about helping women navigate to the care that they need.
The COVID-19 pandemic brought with it an explosion of telehealth options that have enhanced the odds women can find support during such a challenging time; as society has returned to some semblance of normal, nearly all support groups for postpartum women have remained online.
When we set up Virtual Rounds at the Center for Women’s Mental Health at the beginning of the pandemic, I was struck by the community of colleagues at various stages of their careers dedicated to mitigating the suffering associated with perinatal psychiatric illness. As I’ve often said, it takes a village to care for these patients. We need help from colleagues with varying expertise — from lactation consultants, psychiatrists, psychologists, obstetricians, nurse practitioners, support group leaders, and a host of others — who can help reach these women.
At the end of the day, helping depressed women find resources is a challenge that we have not met in this country. We should be excited that we have so many treatment options to offer patients — whether it be a new first-in-class medication, TMS, or digital apps to ensure patients are receiving effective treatment. But there should also be a focus on reaching women who still need treatment, particularly in underserved communities where resources are sparse or nonexistent. Identifying the path to reaching these women where they are and getting them well should be a top priority in 2024.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. STEPS for PPD is funded by the Marriott Foundation. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
The past year has been a challenging time for many, both at the local level and globally, with divisive undercurrents across many communities. Many times, the end of the year is an opportunity for reflection. As I reflect on the state of perinatal psychiatry in the new year, I see several evolving issues that I’d like to share in this first column of 2024.
In 2023, the American College of Obstetricians and Gynecologists published new recommendations meant to enhance the well-being of pregnant and postpartum women and families. A main message from discussion papers borne out of these recommendations was that as a field, we should be doing more than identifying perinatal illness. We should be screening women at risk for postpartum psychiatric illness and see that those suffering from posttraumatic stress disorder (PTSD) have access to care and “wrap-around services” from clinicians with varying expertise.
Screening is a primary way we identify patients at risk for psychiatric illness and also those who are suffering at the time of a screen. One problem I see in the near future is our disparate collection and management of data. When we look closely across health care systems, it’s not clear how screening data are captured, let alone managed. What is being done in one hospital system may be very different from what is being done elsewhere. Some clinicians are adopting digital platforms to identify those with postpartum depression, while others are practicing as they always have, either through a paper screening process or with queries as part of a clinical encounter.
Given this amalgam of methods for collecting and storing information, there does not appear to be a systematic way clinicians and researchers are recording whether women are meeting criteria for significant depressive symptoms or frank postpartum psychiatric illness. It is clear a more cohesive method for collection and management is needed to optimize the likelihood that next steps can be taken to get patients the care they need.
However, screening is only one part of the story. Certainly, in our own center, one of our greatest interests, both clinically and on the research side, is what happens after screening. Through our center’s initiation of the Screening and Treatment Enhancement for Postpartum Depression (STEPS for PPD) project funded by the Marriott Foundation, we are evaluating the outcomes of women who are screened at 6 weeks postpartum with significant depressive symptoms, and who are then given an opportunity to engage with a perinatal social worker who can assist with direct psychotherapy, arranging for referrals, and navigating care for a new mother.
What we are learning as we enroll women through the initial stages of STEPS for PPD is that screening and identifying women who likely suffer from PPD simply is not enough. In fact, once identified with a depression screening tool, women who are suffering from postpartum depression can be very challenging to engage clinically. What I am learning decades after starting to work with perinatal patients is that even with a screening system and effective tools for treatment of PPD, optimizing engagement with these depressed women seems a critical and understudied step on the road to optimizing positive clinical outcomes.
A recent study published in the Journal of Women’s Health explored gaps in care for perinatal depression and found that patients without a history of psychiatric illness prior to pregnancy were less likely to be screened for depression and 80% less likely to receive care if they developed depression compared with women with a previous history of psychiatric illness (J Womens Health (Larchmt). 2023 Oct;32[10]:1111-9).
That history may help women navigate to care, while women for whom psychiatric illness is a new experience may be less likely to engage, be referred for care, and receive appropriate treatment. The study indicates that, as a field, we must strive to ensure universal screening for depression in perinatal populations.
While we have always been particularly interested in populations of patients at highest risk for PPD, helping women at risk for PPD in the general population without a history of psychiatric illness is a large public health issue and will be an even larger undertaking. As women’s mental health is gaining more appropriate focus, both at the local level and even in the recent White House Initiative on Women’s Health Research, the focus has been on screening and developing new treatments.
We are not lacking in pharmacologic agents nor nonpharmacologic options as treatments for women experiencing PPD. Newer alternative treatments are being explored, such as transcranial magnetic stimulation (TMS) and even psychedelics as a potential therapy for PPD. But perhaps what we’ve learned in 2023 and as we move into a new year, is that the problem of tackling PPD is not only about having the right tools, but is about helping women navigate to the care that they need.
The COVID-19 pandemic brought with it an explosion of telehealth options that have enhanced the odds women can find support during such a challenging time; as society has returned to some semblance of normal, nearly all support groups for postpartum women have remained online.
When we set up Virtual Rounds at the Center for Women’s Mental Health at the beginning of the pandemic, I was struck by the community of colleagues at various stages of their careers dedicated to mitigating the suffering associated with perinatal psychiatric illness. As I’ve often said, it takes a village to care for these patients. We need help from colleagues with varying expertise — from lactation consultants, psychiatrists, psychologists, obstetricians, nurse practitioners, support group leaders, and a host of others — who can help reach these women.
At the end of the day, helping depressed women find resources is a challenge that we have not met in this country. We should be excited that we have so many treatment options to offer patients — whether it be a new first-in-class medication, TMS, or digital apps to ensure patients are receiving effective treatment. But there should also be a focus on reaching women who still need treatment, particularly in underserved communities where resources are sparse or nonexistent. Identifying the path to reaching these women where they are and getting them well should be a top priority in 2024.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. STEPS for PPD is funded by the Marriott Foundation. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
Nasal Tanning Sprays: Illuminating the Risks of a Popular TikTok Trend
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
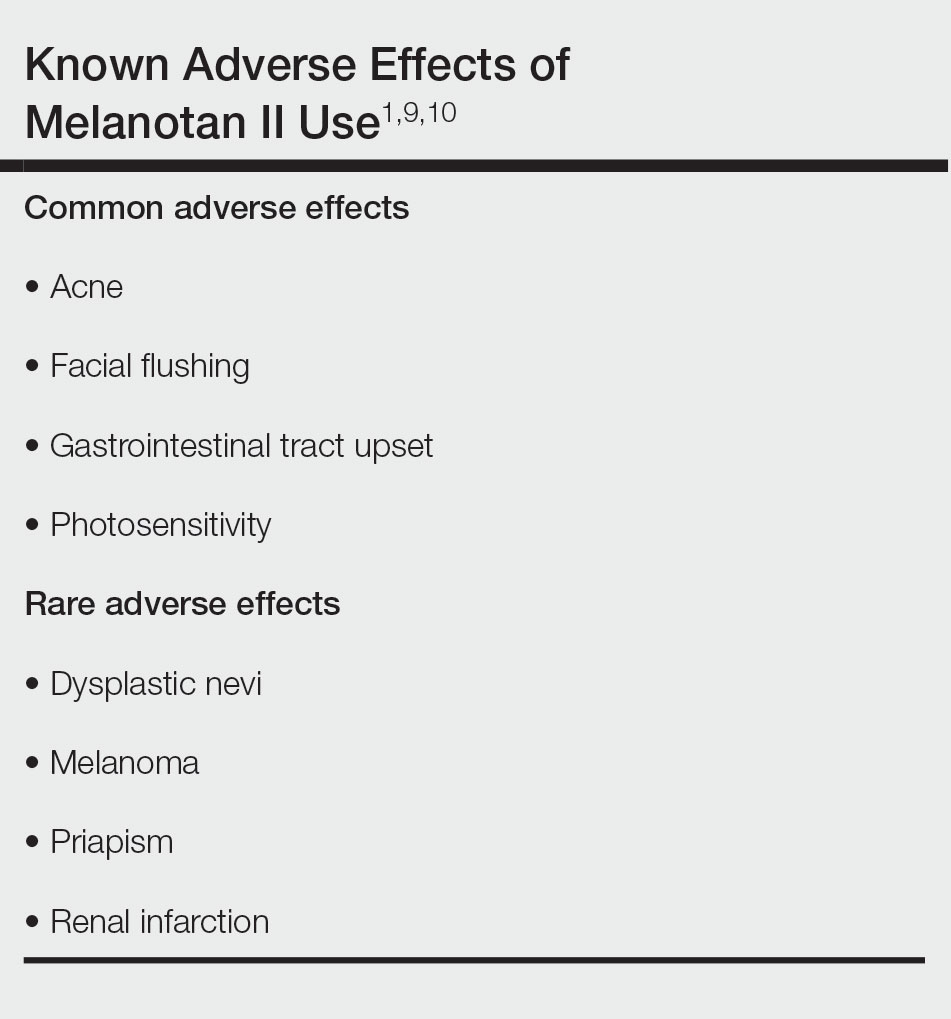
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10
Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
PRACTICE POINTS
- Although tanning beds are arguably the most common and dangerous method used by patients to tan their skin, dermatologists should be aware of the other means by which patients may artificially increase skin pigmentation and the risks imposed by undertaking such practices.
- We challenge dermatologists to note the influence of social media on tanning trends and consider creating a platform on these mediums to combat misinformation and promote sun safety and skin health.
- We encourage dermatologists to diligently stay informed about the popular societal trends related to the skin such as the use of nasal tanning products (eg, melanotan I and II) and be proactive in discussing their risks with patients as deemed appropriate.
Ascending Thoracic Aortic Aneurysms: A ‘Silver Lining’?
Often known as a “silent killer,” ascending thoracic aortic aneurysms (ATAAs) may grow asymptomatically until they rupture, at which point, mortality is over 90%.
But and by extension, for those who have one, a significantly reduced risk for coronary artery disease and myocardial infarction (MI).
“We noticed in the operating room that many patients we worked on who had an ATAA had pristine arteries, like a teenager’s,” said John Elefteriades, MD, William W.L. Glenn Professor of Cardiothoracic Surgery and former chief of cardiothoracic surgery at Yale University and Yale New Haven Hospital, New Haven, Connecticut. “The same was true of the femoral artery, which we use to hook up to the heart-lung machine.”
Elefteriades and colleagues have been investigating the implications of this association for more than two decades. Many of their studies are highlighted in a recent review of the evidence supporting the protective relationship between ATAAs and the development of atherosclerosis and the possible mechanisms driving the relationship.
“We see four different layers of protection,” said Sandip Mukherjee, MD, medical director of the Aortic Institute at Yale New Haven Hospital and a senior editor of the journal AORTA. Mukherjee collaborated with Elefteriades on many of the studies.
The first layer of protection is lower intima-media thickness, specifically, 0.131 mm lower than in individuals without an ATAA. “It may not seem like very much, but one point can actually translate into a 13%-15% decline in the rate of myocardial infarction or stroke,” Dr. Mukherjee said.
The second layer is lower levels of low-density lipoprotein (LDL) cholesterol. Lower LDL cholesterol levels (75 mg/dL) were associated with increased odds of ATAAs (odds ratio [OR], 1.21), whereas elevated levels (150 mg/dL and 200 mg/dL) were associated with decreased odds of ATAAs (OR, 0.62 and 0.29, respectively).
Lower calcification scores for the coronary arteries are the third layer of protection (6.73 vs 9.36 in one study).
The fourth protective layer is a significantly reduced prevalence of coronary artery disease. A study of individuals with ATAA compared to controls found 61 of those with ATAA had coronary artery disease vs 140 of controls, and 11 vs 83 had experienced an MI. Of note, patients with ATAAs were protected despite having higher body mass indices than controls.
Other MI risk factors such as age increased the risk even among those with an ATAA but, again, much less so than among controls; a multivariable binary logistic regression of data in the team’s review showed that patients with ATAAs were 298, 250, and 232 times less likely to have an MI than if they had a family history of MI, dyslipidemia, or hypertension, respectively.
Why the Protection?
The ligamentum arteriosum separates the ascending from the descending (thoracoabdominal) aorta. ATAAs, located above the ligamentum, tend to be pro-aneurysmal but anti-atherosclerotic. In the descending aorta, below the ligamentum, atherosclerotic aneurysms develop.
The differences between the two sections of the aorta originate in the germ layer in the embryo, Dr. Elefteriades said. “The fundamental difference in tissue of origin translates into marked differences in the character of aneurysms in the different aortic segments.”
What specifically underlies the reduced cardiovascular risk? “We don’t really know, but we think that there may be two possible etiologies,” Dr. Mukherjee said. One hypothesis involves transforming growth factor–beta (TGF-beta), which is overexpressed in patients with ATAA and seems to increase their vulnerability to aneurysms while also conferring protection from coronary disease risk.
Some studies have shown differences in cellular responses to TGF-beta between the thoracic and abdominal aorta, including collagen production and contractility. Others have shown that some patients who have had an MI have polymorphisms that decrease their levels of TGF-beta.
Furthermore, TGF-beta plays a key role in the development of the intimal layer, which could underpin the lack of intimal thickening in patients with ATAA.
But overall, studies have been mixed and challenging to interpret, Dr. Elefteriades and Dr. Mukherjee agreed. TGF-beta has multiple remodeling roles in the body, and it is difficult at this point to isolate its exact role in aortic disease.
Another hypothesis involves matrix metalloproteinases (MMPs), which are dysregulated in patients with ATAA and may confer some protection, Mukherjee said. Several studies have shown higher plasma levels of certain MMPs in patients with ATAAs. MMPs also were found to be elevated in the thoracic aortic walls of patients with ATAA who had an aortic dissection, as well as in the aortic smooth muscle cells in the intima and media.
In addition, some studies have shown increased levels of MMP-2 in the aortas of patients with ATAAs compared with patients with coronary artery disease.
Adding to the mix of possibilities, “We recently found a gene that’s dysregulated in our aneurysm patients that is very intimately related to atherosclerosis,” Dr. Elefteriades said. “But the work is too preliminary to say anything more at this point.”
“It would be fabulous to prove what it is causing this protection,” Dr. Mukherjee added. “But the truth is we don’t know. These are hypotheses.”
“The most important message from our work is that most clinicians need to dissociate an ATAA from the concept of atherosclerosis,” Dr. Elefteriades said. “The ascending aorta is not an atherosclerotic phenomenon.”
How to Manage Patients With ATAA
What does the distinct character of ATAAs mean for patient management? “Finding a drug to treat ATAAs — to prevent growth, rupture, or dissection — has been like a search for the Holy Grail,” Dr. Elefteriades said. “Statins are not necessary, as this is a non-atherosclerotic process. Although sporadic studies have reported beneficial effects from beta-blockers or angiotensin II receptor blockers (ARBs), this has often been based on ‘soft’ evidence, requiring a combination of outcome measures to achieve significance.”
That said, he noted, “The mainstay, common sense treatment is to keep blood pressure controlled. This is usually achieved by a beta-blocker and an ARB, even if the benefit is not via a direct biologic effect on the aneurysmal degenerative process, but via simple hemodynamics — discouraging rupture by keeping pressure in the aorta low.”
Dr. Mukherjee suggested that these patients should be referred to a specialty aneurysm center where their genes will be evaluated, and then the aneurysm will be followed very closely.
“If the aneurysm is larger than 4.5 cm, we screen the patient every single year, and if they have chest pain, we treat them the same way as we treat other aneurysms,” he said. “As a rule of thumb, if the aneurysm reaches 5 cm, it should come out, although the size at which this should happen may differ between 4.5 cm and 5.5 cm, depending on the patient’s body size.”
As for lifestyle management, Dr. Elefteriades said, “Protection from atherosclerosis and MI won’t go away after the aneurysm is removed. We think it’s in the body’s chemistry. But even though it’s very hard for those patients to have a heart attack, we don’t recommend they eat roast beef every night — although I do think they’d be protected from such lifestyle aberrations.”
For now, he added, “Our team is on a hunt to find a drug to treat ascending disease directly and effectively. We have ongoing laboratory experiments with two drugs undergoing investigation at some level. We hope to embark soon on clinical trials.”
‘A Milestone’
James Hamilton Black III, MD, vice chair of the writing committee for the 2022 American College of Cardiology/American Heart Association Aortic Disease Guideline and chief of Division of Vascular Surgery and Endovascular Therapy at Johns Hopkins Medicine, Baltimore, commented on the review and the concept of ATAA’s atherosclerotic protection.
“The association of ascending aortic aneurysms with a lower risk for MI is an interesting one, but it’s probably influenced, at least in part, by the patient population.” That population is at least partially curated since people are coming to an academic center. In addition, Dr. Black noted, “the patients with ATAAs are younger, and so age may be a confounding factor in the analyses. We wouldn’t expect them to have the same burden of atherosclerosis” as older patients.
Nevertheless, he said, “the findings speak to an emerging body of literature suggesting that although the aorta is a single organ, there are certainly different areas, and these would respond quite differently to environmental or genetic or heritable stressors. This isn’t surprising, and there probably are a lot of factors involved.”
Overall, he said, the findings underscore “the precision medicine approaches we need to take with patients with aortic diseases.”
In a commentary on the team’s review article, published in 2022, John G.T. Augoustides, MD, professor of anesthesiology and critical care at the Perelman School of Medicine in Philadelphia, Pennsylvania, suggested that ATAA’s “silver lining” could advance the understanding of thoracic aortic aneurysm (TAA) management, be integrated with the expanding horizons in hereditary thoracic aortic disease, and might be explored in the context of bicuspid aortic valve disease.
Highlighting the “relative absence” of atherosclerosis in ascending aortic aneurysms and its importance is a “milestone in our understanding,” he concluded. “It is likely that future advances in TAAs will be significantly influenced by this observation.”
Dr. Elefteriades, Dr. Mukherjee, and Dr. Black have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Often known as a “silent killer,” ascending thoracic aortic aneurysms (ATAAs) may grow asymptomatically until they rupture, at which point, mortality is over 90%.
But and by extension, for those who have one, a significantly reduced risk for coronary artery disease and myocardial infarction (MI).
“We noticed in the operating room that many patients we worked on who had an ATAA had pristine arteries, like a teenager’s,” said John Elefteriades, MD, William W.L. Glenn Professor of Cardiothoracic Surgery and former chief of cardiothoracic surgery at Yale University and Yale New Haven Hospital, New Haven, Connecticut. “The same was true of the femoral artery, which we use to hook up to the heart-lung machine.”
Elefteriades and colleagues have been investigating the implications of this association for more than two decades. Many of their studies are highlighted in a recent review of the evidence supporting the protective relationship between ATAAs and the development of atherosclerosis and the possible mechanisms driving the relationship.
“We see four different layers of protection,” said Sandip Mukherjee, MD, medical director of the Aortic Institute at Yale New Haven Hospital and a senior editor of the journal AORTA. Mukherjee collaborated with Elefteriades on many of the studies.
The first layer of protection is lower intima-media thickness, specifically, 0.131 mm lower than in individuals without an ATAA. “It may not seem like very much, but one point can actually translate into a 13%-15% decline in the rate of myocardial infarction or stroke,” Dr. Mukherjee said.
The second layer is lower levels of low-density lipoprotein (LDL) cholesterol. Lower LDL cholesterol levels (75 mg/dL) were associated with increased odds of ATAAs (odds ratio [OR], 1.21), whereas elevated levels (150 mg/dL and 200 mg/dL) were associated with decreased odds of ATAAs (OR, 0.62 and 0.29, respectively).
Lower calcification scores for the coronary arteries are the third layer of protection (6.73 vs 9.36 in one study).
The fourth protective layer is a significantly reduced prevalence of coronary artery disease. A study of individuals with ATAA compared to controls found 61 of those with ATAA had coronary artery disease vs 140 of controls, and 11 vs 83 had experienced an MI. Of note, patients with ATAAs were protected despite having higher body mass indices than controls.
Other MI risk factors such as age increased the risk even among those with an ATAA but, again, much less so than among controls; a multivariable binary logistic regression of data in the team’s review showed that patients with ATAAs were 298, 250, and 232 times less likely to have an MI than if they had a family history of MI, dyslipidemia, or hypertension, respectively.
Why the Protection?
The ligamentum arteriosum separates the ascending from the descending (thoracoabdominal) aorta. ATAAs, located above the ligamentum, tend to be pro-aneurysmal but anti-atherosclerotic. In the descending aorta, below the ligamentum, atherosclerotic aneurysms develop.
The differences between the two sections of the aorta originate in the germ layer in the embryo, Dr. Elefteriades said. “The fundamental difference in tissue of origin translates into marked differences in the character of aneurysms in the different aortic segments.”
What specifically underlies the reduced cardiovascular risk? “We don’t really know, but we think that there may be two possible etiologies,” Dr. Mukherjee said. One hypothesis involves transforming growth factor–beta (TGF-beta), which is overexpressed in patients with ATAA and seems to increase their vulnerability to aneurysms while also conferring protection from coronary disease risk.
Some studies have shown differences in cellular responses to TGF-beta between the thoracic and abdominal aorta, including collagen production and contractility. Others have shown that some patients who have had an MI have polymorphisms that decrease their levels of TGF-beta.
Furthermore, TGF-beta plays a key role in the development of the intimal layer, which could underpin the lack of intimal thickening in patients with ATAA.
But overall, studies have been mixed and challenging to interpret, Dr. Elefteriades and Dr. Mukherjee agreed. TGF-beta has multiple remodeling roles in the body, and it is difficult at this point to isolate its exact role in aortic disease.
Another hypothesis involves matrix metalloproteinases (MMPs), which are dysregulated in patients with ATAA and may confer some protection, Mukherjee said. Several studies have shown higher plasma levels of certain MMPs in patients with ATAAs. MMPs also were found to be elevated in the thoracic aortic walls of patients with ATAA who had an aortic dissection, as well as in the aortic smooth muscle cells in the intima and media.
In addition, some studies have shown increased levels of MMP-2 in the aortas of patients with ATAAs compared with patients with coronary artery disease.
Adding to the mix of possibilities, “We recently found a gene that’s dysregulated in our aneurysm patients that is very intimately related to atherosclerosis,” Dr. Elefteriades said. “But the work is too preliminary to say anything more at this point.”
“It would be fabulous to prove what it is causing this protection,” Dr. Mukherjee added. “But the truth is we don’t know. These are hypotheses.”
“The most important message from our work is that most clinicians need to dissociate an ATAA from the concept of atherosclerosis,” Dr. Elefteriades said. “The ascending aorta is not an atherosclerotic phenomenon.”
How to Manage Patients With ATAA
What does the distinct character of ATAAs mean for patient management? “Finding a drug to treat ATAAs — to prevent growth, rupture, or dissection — has been like a search for the Holy Grail,” Dr. Elefteriades said. “Statins are not necessary, as this is a non-atherosclerotic process. Although sporadic studies have reported beneficial effects from beta-blockers or angiotensin II receptor blockers (ARBs), this has often been based on ‘soft’ evidence, requiring a combination of outcome measures to achieve significance.”
That said, he noted, “The mainstay, common sense treatment is to keep blood pressure controlled. This is usually achieved by a beta-blocker and an ARB, even if the benefit is not via a direct biologic effect on the aneurysmal degenerative process, but via simple hemodynamics — discouraging rupture by keeping pressure in the aorta low.”
Dr. Mukherjee suggested that these patients should be referred to a specialty aneurysm center where their genes will be evaluated, and then the aneurysm will be followed very closely.
“If the aneurysm is larger than 4.5 cm, we screen the patient every single year, and if they have chest pain, we treat them the same way as we treat other aneurysms,” he said. “As a rule of thumb, if the aneurysm reaches 5 cm, it should come out, although the size at which this should happen may differ between 4.5 cm and 5.5 cm, depending on the patient’s body size.”
As for lifestyle management, Dr. Elefteriades said, “Protection from atherosclerosis and MI won’t go away after the aneurysm is removed. We think it’s in the body’s chemistry. But even though it’s very hard for those patients to have a heart attack, we don’t recommend they eat roast beef every night — although I do think they’d be protected from such lifestyle aberrations.”
For now, he added, “Our team is on a hunt to find a drug to treat ascending disease directly and effectively. We have ongoing laboratory experiments with two drugs undergoing investigation at some level. We hope to embark soon on clinical trials.”
‘A Milestone’
James Hamilton Black III, MD, vice chair of the writing committee for the 2022 American College of Cardiology/American Heart Association Aortic Disease Guideline and chief of Division of Vascular Surgery and Endovascular Therapy at Johns Hopkins Medicine, Baltimore, commented on the review and the concept of ATAA’s atherosclerotic protection.
“The association of ascending aortic aneurysms with a lower risk for MI is an interesting one, but it’s probably influenced, at least in part, by the patient population.” That population is at least partially curated since people are coming to an academic center. In addition, Dr. Black noted, “the patients with ATAAs are younger, and so age may be a confounding factor in the analyses. We wouldn’t expect them to have the same burden of atherosclerosis” as older patients.
Nevertheless, he said, “the findings speak to an emerging body of literature suggesting that although the aorta is a single organ, there are certainly different areas, and these would respond quite differently to environmental or genetic or heritable stressors. This isn’t surprising, and there probably are a lot of factors involved.”
Overall, he said, the findings underscore “the precision medicine approaches we need to take with patients with aortic diseases.”
In a commentary on the team’s review article, published in 2022, John G.T. Augoustides, MD, professor of anesthesiology and critical care at the Perelman School of Medicine in Philadelphia, Pennsylvania, suggested that ATAA’s “silver lining” could advance the understanding of thoracic aortic aneurysm (TAA) management, be integrated with the expanding horizons in hereditary thoracic aortic disease, and might be explored in the context of bicuspid aortic valve disease.
Highlighting the “relative absence” of atherosclerosis in ascending aortic aneurysms and its importance is a “milestone in our understanding,” he concluded. “It is likely that future advances in TAAs will be significantly influenced by this observation.”
Dr. Elefteriades, Dr. Mukherjee, and Dr. Black have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Often known as a “silent killer,” ascending thoracic aortic aneurysms (ATAAs) may grow asymptomatically until they rupture, at which point, mortality is over 90%.
But and by extension, for those who have one, a significantly reduced risk for coronary artery disease and myocardial infarction (MI).
“We noticed in the operating room that many patients we worked on who had an ATAA had pristine arteries, like a teenager’s,” said John Elefteriades, MD, William W.L. Glenn Professor of Cardiothoracic Surgery and former chief of cardiothoracic surgery at Yale University and Yale New Haven Hospital, New Haven, Connecticut. “The same was true of the femoral artery, which we use to hook up to the heart-lung machine.”
Elefteriades and colleagues have been investigating the implications of this association for more than two decades. Many of their studies are highlighted in a recent review of the evidence supporting the protective relationship between ATAAs and the development of atherosclerosis and the possible mechanisms driving the relationship.
“We see four different layers of protection,” said Sandip Mukherjee, MD, medical director of the Aortic Institute at Yale New Haven Hospital and a senior editor of the journal AORTA. Mukherjee collaborated with Elefteriades on many of the studies.
The first layer of protection is lower intima-media thickness, specifically, 0.131 mm lower than in individuals without an ATAA. “It may not seem like very much, but one point can actually translate into a 13%-15% decline in the rate of myocardial infarction or stroke,” Dr. Mukherjee said.
The second layer is lower levels of low-density lipoprotein (LDL) cholesterol. Lower LDL cholesterol levels (75 mg/dL) were associated with increased odds of ATAAs (odds ratio [OR], 1.21), whereas elevated levels (150 mg/dL and 200 mg/dL) were associated with decreased odds of ATAAs (OR, 0.62 and 0.29, respectively).
Lower calcification scores for the coronary arteries are the third layer of protection (6.73 vs 9.36 in one study).
The fourth protective layer is a significantly reduced prevalence of coronary artery disease. A study of individuals with ATAA compared to controls found 61 of those with ATAA had coronary artery disease vs 140 of controls, and 11 vs 83 had experienced an MI. Of note, patients with ATAAs were protected despite having higher body mass indices than controls.
Other MI risk factors such as age increased the risk even among those with an ATAA but, again, much less so than among controls; a multivariable binary logistic regression of data in the team’s review showed that patients with ATAAs were 298, 250, and 232 times less likely to have an MI than if they had a family history of MI, dyslipidemia, or hypertension, respectively.
Why the Protection?
The ligamentum arteriosum separates the ascending from the descending (thoracoabdominal) aorta. ATAAs, located above the ligamentum, tend to be pro-aneurysmal but anti-atherosclerotic. In the descending aorta, below the ligamentum, atherosclerotic aneurysms develop.
The differences between the two sections of the aorta originate in the germ layer in the embryo, Dr. Elefteriades said. “The fundamental difference in tissue of origin translates into marked differences in the character of aneurysms in the different aortic segments.”
What specifically underlies the reduced cardiovascular risk? “We don’t really know, but we think that there may be two possible etiologies,” Dr. Mukherjee said. One hypothesis involves transforming growth factor–beta (TGF-beta), which is overexpressed in patients with ATAA and seems to increase their vulnerability to aneurysms while also conferring protection from coronary disease risk.
Some studies have shown differences in cellular responses to TGF-beta between the thoracic and abdominal aorta, including collagen production and contractility. Others have shown that some patients who have had an MI have polymorphisms that decrease their levels of TGF-beta.
Furthermore, TGF-beta plays a key role in the development of the intimal layer, which could underpin the lack of intimal thickening in patients with ATAA.
But overall, studies have been mixed and challenging to interpret, Dr. Elefteriades and Dr. Mukherjee agreed. TGF-beta has multiple remodeling roles in the body, and it is difficult at this point to isolate its exact role in aortic disease.
Another hypothesis involves matrix metalloproteinases (MMPs), which are dysregulated in patients with ATAA and may confer some protection, Mukherjee said. Several studies have shown higher plasma levels of certain MMPs in patients with ATAAs. MMPs also were found to be elevated in the thoracic aortic walls of patients with ATAA who had an aortic dissection, as well as in the aortic smooth muscle cells in the intima and media.
In addition, some studies have shown increased levels of MMP-2 in the aortas of patients with ATAAs compared with patients with coronary artery disease.
Adding to the mix of possibilities, “We recently found a gene that’s dysregulated in our aneurysm patients that is very intimately related to atherosclerosis,” Dr. Elefteriades said. “But the work is too preliminary to say anything more at this point.”
“It would be fabulous to prove what it is causing this protection,” Dr. Mukherjee added. “But the truth is we don’t know. These are hypotheses.”
“The most important message from our work is that most clinicians need to dissociate an ATAA from the concept of atherosclerosis,” Dr. Elefteriades said. “The ascending aorta is not an atherosclerotic phenomenon.”
How to Manage Patients With ATAA
What does the distinct character of ATAAs mean for patient management? “Finding a drug to treat ATAAs — to prevent growth, rupture, or dissection — has been like a search for the Holy Grail,” Dr. Elefteriades said. “Statins are not necessary, as this is a non-atherosclerotic process. Although sporadic studies have reported beneficial effects from beta-blockers or angiotensin II receptor blockers (ARBs), this has often been based on ‘soft’ evidence, requiring a combination of outcome measures to achieve significance.”
That said, he noted, “The mainstay, common sense treatment is to keep blood pressure controlled. This is usually achieved by a beta-blocker and an ARB, even if the benefit is not via a direct biologic effect on the aneurysmal degenerative process, but via simple hemodynamics — discouraging rupture by keeping pressure in the aorta low.”
Dr. Mukherjee suggested that these patients should be referred to a specialty aneurysm center where their genes will be evaluated, and then the aneurysm will be followed very closely.
“If the aneurysm is larger than 4.5 cm, we screen the patient every single year, and if they have chest pain, we treat them the same way as we treat other aneurysms,” he said. “As a rule of thumb, if the aneurysm reaches 5 cm, it should come out, although the size at which this should happen may differ between 4.5 cm and 5.5 cm, depending on the patient’s body size.”
As for lifestyle management, Dr. Elefteriades said, “Protection from atherosclerosis and MI won’t go away after the aneurysm is removed. We think it’s in the body’s chemistry. But even though it’s very hard for those patients to have a heart attack, we don’t recommend they eat roast beef every night — although I do think they’d be protected from such lifestyle aberrations.”
For now, he added, “Our team is on a hunt to find a drug to treat ascending disease directly and effectively. We have ongoing laboratory experiments with two drugs undergoing investigation at some level. We hope to embark soon on clinical trials.”
‘A Milestone’
James Hamilton Black III, MD, vice chair of the writing committee for the 2022 American College of Cardiology/American Heart Association Aortic Disease Guideline and chief of Division of Vascular Surgery and Endovascular Therapy at Johns Hopkins Medicine, Baltimore, commented on the review and the concept of ATAA’s atherosclerotic protection.
“The association of ascending aortic aneurysms with a lower risk for MI is an interesting one, but it’s probably influenced, at least in part, by the patient population.” That population is at least partially curated since people are coming to an academic center. In addition, Dr. Black noted, “the patients with ATAAs are younger, and so age may be a confounding factor in the analyses. We wouldn’t expect them to have the same burden of atherosclerosis” as older patients.
Nevertheless, he said, “the findings speak to an emerging body of literature suggesting that although the aorta is a single organ, there are certainly different areas, and these would respond quite differently to environmental or genetic or heritable stressors. This isn’t surprising, and there probably are a lot of factors involved.”
Overall, he said, the findings underscore “the precision medicine approaches we need to take with patients with aortic diseases.”
In a commentary on the team’s review article, published in 2022, John G.T. Augoustides, MD, professor of anesthesiology and critical care at the Perelman School of Medicine in Philadelphia, Pennsylvania, suggested that ATAA’s “silver lining” could advance the understanding of thoracic aortic aneurysm (TAA) management, be integrated with the expanding horizons in hereditary thoracic aortic disease, and might be explored in the context of bicuspid aortic valve disease.
Highlighting the “relative absence” of atherosclerosis in ascending aortic aneurysms and its importance is a “milestone in our understanding,” he concluded. “It is likely that future advances in TAAs will be significantly influenced by this observation.”
Dr. Elefteriades, Dr. Mukherjee, and Dr. Black have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
JAMA Internal Medicine Editor Recaps 2023’s High-Impact Research
Harvard Medical School’s Sharon K. Inouye, MD, MPH, is editor in chief of JAMA Internal Medicine and a leading voice in American gerontology. We asked her to choose five of the influential journal’s most impactful studies from 2023 and highlight important take-home messages for internists and their colleagues.
Q: One of the studies you chose suggests that the antiviral nirmatrelvir (Paxlovid) can ward off long COVID. Could you recap the findings?
A: Researchers followed a group of more than 280,000 Department of Veterans Affairs patients who were seen in 2022, had a positive COVID test, and had at least one risk factor for severe COVID. They focused on those who survived to 30 days after their COVID infection and compared those who received the drug within the first 5 days of a positive test with an equivalent control group.
They found that 13 long COVID symptoms were all significantly less common (relative risk = 0.74) in those who received nirmatrelvir. This was true no matter whether they’d ever had a COVID vaccination.
Q: How should this research affect clinical practice?
A: You can’t generalize from this to everyone because, of course, not everyone was included in this study. But it is highly suggestive that this drug is very effective for preventing long COVID.
Nirmatrelvir was touted as being able to shorten duration of illness and prevent hospitalization. But if you were low risk or you were already well into your COVID course, it wasn’t like rush, rush, rush to the doctor to get it.
This changes that equation because we know long COVID is such a huge issue. The vast majority of doctors who work with COVID patients and know this are now being more aggressive about prescribing it.
Q: What about patients whom the CDC considers to be at less risk — people with up-to-date vaccinations who are under 50 with mild-to-moderate COVID and no higher-risk medical conditions? Should they take nirmatrelvir?
A: The evidence is not 100% in yet. A study like this one needs to be repeated and include younger people without any risk factors to see if we see the same thing. So it’s a personal choice, and a personal calculus needs to be done. A lot of people are making that choice [to take the drug], and it can be a rational decision.
Q: You also chose a study that links high thyroid hormone levels to higher rates of dementia. What did it reveal?
A: This study looks at patients who had thyrotoxicosis — a thyroid level that’s too high — from hormone produced endogenously, and exogenously. Researchers tracked almost 66,000 patients aged 65 and older and found that thyrotoxicosis from all causes, whether it was endogenous or exogenous, was linked to an increased risk of dementia in a dose-response relationship (adjusted hazard ratio = 1.39).
Q: Is there a clinical take-home message here?
A: When we start patients on thyroid medication, they don’t always get reassessed on a regular basis. Given this finding, a TSH [thyroid-stimulating hormone] level is indicated during the annual wellness check that patients on Medicare can get every year.
Q: Is TSH measured as part of routine blood tests?
A: No it’s not. It has to be ordered. I think that’s why we’re seeing this problem to begin with — because it’s not something we all have awareness about. I wasn’t aware myself that mildly high levels of thyroid could increase the risk of cognitive impairment. Certainly, I’m going to be much more aware in my practice.
Q: You also picked a study about silicosis in workers who are exposed to dust when they make engineered stone countertops, also known as quartz countertops. What were the findings?
A: Silicosis is a very serious lung condition that develops from exposure to crystalline silica. Essentially, sand gets inhaled into the lungs. Workers can be exposed when they’re making engineered stone countertops, the most popular countertops now in the United States.
This study is based on statewide surveys from 2019 to 2022 that the California Department of Public Health does routinely. They gathered cases of silicosis and found 52 — all men with an average age of 45. All but one were Latino immigrants, and most either had no insurance or very poor insurance.
Q: The study found that “diagnosis was delayed in 58%, with 38% presenting with advanced disease (progressive massive fibrosis), and 19% died.” What does that tell you?
A: It’s a very serious condition. Once it gets to the advanced stage, it will just continue to progress, and the person will die. That’s why it’s so important to know that it’s absolutely preventable.
Q: Is there a message here for internists?
A: If you treat a lot of immigrants or work in an area where there are a lot of industrial workers, you’re going to want to have a very high suspicion about it. If you see an atypical pattern on the chest x-ray or via diffusion scoring, have a low threshold for getting a pulmonary function test.
Doctors need to be aware and diagnose this very quickly. When patients present, you can pull them out of that work environment or put mitigation systems into place.
Q: California regulators were expected to put emergency rules into place in late December to protect workers. Did this study play a role in focusing attention on the problem?
A: This article, along with a commentary and podcast that we put out, really helped with advocacy to improve health and safety for workers at stone-cutting and fabrication shops.
Q: You were impressed by another study about airborne dangers, this one linking air pollution to dementia. What did researchers discover?
A: [This analysis] of more than 27,000 people in the Health and Retirement Study, a respected and rich database, found that exposure to air pollution was associated with greater rates of dementia — an increase of about 8% a year. Exposure to agricultural emissions and wildfire smoke were most robustly associated with a greater risk of dementia.
Q: How are these findings important, especially in light of the unhealthy air spawned by recent wildfires in the United States and Canada?
A: Studies like this will make it even more compelling that we are better prepared for air quality issues.
I grew up in Los Angeles, where smog and pollution were very big issues. I was constantly hearing about various mitigation strategies that were going into place. But after I moved to the East Coast, I almost never heard about prevention.
Now, I’m hoping we can keep this topic in the national conversation.
Q: You also highlighted a systematic review of the use of restraints in the emergency department. Why did you choose this research?
A: At JAMA Internal Medicine, we’re really focused on ways we can address health disparities and raise awareness of potential unconscious bias.
This review looked at 10 studies that included more than 2.5 million patient encounters, including 24,000 incidents of physical restraint use. They found that the overall rate of use of restraints was low at below 1%.
But when they are used, Black patients were 1.3 times more likely to be restrained than White patients.
Q: What’s the message here?
A: This is an important start to recognizing these differences and then changing our behavior. Perhaps restraints don’t need to be used as often in light of evidence, for example, of increased rates of misdiagnosis of psychosis in the Black population.
Q: How should physicians change their approach to restraints?
A: Restraints are not to be used to control disruption — wild behavior or verbal outbursts. They’re for when someone is a danger to themselves or others.
Dr. Inouye has no conflicts of interest.
Harvard Medical School’s Sharon K. Inouye, MD, MPH, is editor in chief of JAMA Internal Medicine and a leading voice in American gerontology. We asked her to choose five of the influential journal’s most impactful studies from 2023 and highlight important take-home messages for internists and their colleagues.
Q: One of the studies you chose suggests that the antiviral nirmatrelvir (Paxlovid) can ward off long COVID. Could you recap the findings?
A: Researchers followed a group of more than 280,000 Department of Veterans Affairs patients who were seen in 2022, had a positive COVID test, and had at least one risk factor for severe COVID. They focused on those who survived to 30 days after their COVID infection and compared those who received the drug within the first 5 days of a positive test with an equivalent control group.
They found that 13 long COVID symptoms were all significantly less common (relative risk = 0.74) in those who received nirmatrelvir. This was true no matter whether they’d ever had a COVID vaccination.
Q: How should this research affect clinical practice?
A: You can’t generalize from this to everyone because, of course, not everyone was included in this study. But it is highly suggestive that this drug is very effective for preventing long COVID.
Nirmatrelvir was touted as being able to shorten duration of illness and prevent hospitalization. But if you were low risk or you were already well into your COVID course, it wasn’t like rush, rush, rush to the doctor to get it.
This changes that equation because we know long COVID is such a huge issue. The vast majority of doctors who work with COVID patients and know this are now being more aggressive about prescribing it.
Q: What about patients whom the CDC considers to be at less risk — people with up-to-date vaccinations who are under 50 with mild-to-moderate COVID and no higher-risk medical conditions? Should they take nirmatrelvir?
A: The evidence is not 100% in yet. A study like this one needs to be repeated and include younger people without any risk factors to see if we see the same thing. So it’s a personal choice, and a personal calculus needs to be done. A lot of people are making that choice [to take the drug], and it can be a rational decision.
Q: You also chose a study that links high thyroid hormone levels to higher rates of dementia. What did it reveal?
A: This study looks at patients who had thyrotoxicosis — a thyroid level that’s too high — from hormone produced endogenously, and exogenously. Researchers tracked almost 66,000 patients aged 65 and older and found that thyrotoxicosis from all causes, whether it was endogenous or exogenous, was linked to an increased risk of dementia in a dose-response relationship (adjusted hazard ratio = 1.39).
Q: Is there a clinical take-home message here?
A: When we start patients on thyroid medication, they don’t always get reassessed on a regular basis. Given this finding, a TSH [thyroid-stimulating hormone] level is indicated during the annual wellness check that patients on Medicare can get every year.
Q: Is TSH measured as part of routine blood tests?
A: No it’s not. It has to be ordered. I think that’s why we’re seeing this problem to begin with — because it’s not something we all have awareness about. I wasn’t aware myself that mildly high levels of thyroid could increase the risk of cognitive impairment. Certainly, I’m going to be much more aware in my practice.
Q: You also picked a study about silicosis in workers who are exposed to dust when they make engineered stone countertops, also known as quartz countertops. What were the findings?
A: Silicosis is a very serious lung condition that develops from exposure to crystalline silica. Essentially, sand gets inhaled into the lungs. Workers can be exposed when they’re making engineered stone countertops, the most popular countertops now in the United States.
This study is based on statewide surveys from 2019 to 2022 that the California Department of Public Health does routinely. They gathered cases of silicosis and found 52 — all men with an average age of 45. All but one were Latino immigrants, and most either had no insurance or very poor insurance.
Q: The study found that “diagnosis was delayed in 58%, with 38% presenting with advanced disease (progressive massive fibrosis), and 19% died.” What does that tell you?
A: It’s a very serious condition. Once it gets to the advanced stage, it will just continue to progress, and the person will die. That’s why it’s so important to know that it’s absolutely preventable.
Q: Is there a message here for internists?
A: If you treat a lot of immigrants or work in an area where there are a lot of industrial workers, you’re going to want to have a very high suspicion about it. If you see an atypical pattern on the chest x-ray or via diffusion scoring, have a low threshold for getting a pulmonary function test.
Doctors need to be aware and diagnose this very quickly. When patients present, you can pull them out of that work environment or put mitigation systems into place.
Q: California regulators were expected to put emergency rules into place in late December to protect workers. Did this study play a role in focusing attention on the problem?
A: This article, along with a commentary and podcast that we put out, really helped with advocacy to improve health and safety for workers at stone-cutting and fabrication shops.
Q: You were impressed by another study about airborne dangers, this one linking air pollution to dementia. What did researchers discover?
A: [This analysis] of more than 27,000 people in the Health and Retirement Study, a respected and rich database, found that exposure to air pollution was associated with greater rates of dementia — an increase of about 8% a year. Exposure to agricultural emissions and wildfire smoke were most robustly associated with a greater risk of dementia.
Q: How are these findings important, especially in light of the unhealthy air spawned by recent wildfires in the United States and Canada?
A: Studies like this will make it even more compelling that we are better prepared for air quality issues.
I grew up in Los Angeles, where smog and pollution were very big issues. I was constantly hearing about various mitigation strategies that were going into place. But after I moved to the East Coast, I almost never heard about prevention.
Now, I’m hoping we can keep this topic in the national conversation.
Q: You also highlighted a systematic review of the use of restraints in the emergency department. Why did you choose this research?
A: At JAMA Internal Medicine, we’re really focused on ways we can address health disparities and raise awareness of potential unconscious bias.
This review looked at 10 studies that included more than 2.5 million patient encounters, including 24,000 incidents of physical restraint use. They found that the overall rate of use of restraints was low at below 1%.
But when they are used, Black patients were 1.3 times more likely to be restrained than White patients.
Q: What’s the message here?
A: This is an important start to recognizing these differences and then changing our behavior. Perhaps restraints don’t need to be used as often in light of evidence, for example, of increased rates of misdiagnosis of psychosis in the Black population.
Q: How should physicians change their approach to restraints?
A: Restraints are not to be used to control disruption — wild behavior or verbal outbursts. They’re for when someone is a danger to themselves or others.
Dr. Inouye has no conflicts of interest.
Harvard Medical School’s Sharon K. Inouye, MD, MPH, is editor in chief of JAMA Internal Medicine and a leading voice in American gerontology. We asked her to choose five of the influential journal’s most impactful studies from 2023 and highlight important take-home messages for internists and their colleagues.
Q: One of the studies you chose suggests that the antiviral nirmatrelvir (Paxlovid) can ward off long COVID. Could you recap the findings?
A: Researchers followed a group of more than 280,000 Department of Veterans Affairs patients who were seen in 2022, had a positive COVID test, and had at least one risk factor for severe COVID. They focused on those who survived to 30 days after their COVID infection and compared those who received the drug within the first 5 days of a positive test with an equivalent control group.
They found that 13 long COVID symptoms were all significantly less common (relative risk = 0.74) in those who received nirmatrelvir. This was true no matter whether they’d ever had a COVID vaccination.
Q: How should this research affect clinical practice?
A: You can’t generalize from this to everyone because, of course, not everyone was included in this study. But it is highly suggestive that this drug is very effective for preventing long COVID.
Nirmatrelvir was touted as being able to shorten duration of illness and prevent hospitalization. But if you were low risk or you were already well into your COVID course, it wasn’t like rush, rush, rush to the doctor to get it.
This changes that equation because we know long COVID is such a huge issue. The vast majority of doctors who work with COVID patients and know this are now being more aggressive about prescribing it.
Q: What about patients whom the CDC considers to be at less risk — people with up-to-date vaccinations who are under 50 with mild-to-moderate COVID and no higher-risk medical conditions? Should they take nirmatrelvir?
A: The evidence is not 100% in yet. A study like this one needs to be repeated and include younger people without any risk factors to see if we see the same thing. So it’s a personal choice, and a personal calculus needs to be done. A lot of people are making that choice [to take the drug], and it can be a rational decision.
Q: You also chose a study that links high thyroid hormone levels to higher rates of dementia. What did it reveal?
A: This study looks at patients who had thyrotoxicosis — a thyroid level that’s too high — from hormone produced endogenously, and exogenously. Researchers tracked almost 66,000 patients aged 65 and older and found that thyrotoxicosis from all causes, whether it was endogenous or exogenous, was linked to an increased risk of dementia in a dose-response relationship (adjusted hazard ratio = 1.39).
Q: Is there a clinical take-home message here?
A: When we start patients on thyroid medication, they don’t always get reassessed on a regular basis. Given this finding, a TSH [thyroid-stimulating hormone] level is indicated during the annual wellness check that patients on Medicare can get every year.
Q: Is TSH measured as part of routine blood tests?
A: No it’s not. It has to be ordered. I think that’s why we’re seeing this problem to begin with — because it’s not something we all have awareness about. I wasn’t aware myself that mildly high levels of thyroid could increase the risk of cognitive impairment. Certainly, I’m going to be much more aware in my practice.
Q: You also picked a study about silicosis in workers who are exposed to dust when they make engineered stone countertops, also known as quartz countertops. What were the findings?
A: Silicosis is a very serious lung condition that develops from exposure to crystalline silica. Essentially, sand gets inhaled into the lungs. Workers can be exposed when they’re making engineered stone countertops, the most popular countertops now in the United States.
This study is based on statewide surveys from 2019 to 2022 that the California Department of Public Health does routinely. They gathered cases of silicosis and found 52 — all men with an average age of 45. All but one were Latino immigrants, and most either had no insurance or very poor insurance.
Q: The study found that “diagnosis was delayed in 58%, with 38% presenting with advanced disease (progressive massive fibrosis), and 19% died.” What does that tell you?
A: It’s a very serious condition. Once it gets to the advanced stage, it will just continue to progress, and the person will die. That’s why it’s so important to know that it’s absolutely preventable.
Q: Is there a message here for internists?
A: If you treat a lot of immigrants or work in an area where there are a lot of industrial workers, you’re going to want to have a very high suspicion about it. If you see an atypical pattern on the chest x-ray or via diffusion scoring, have a low threshold for getting a pulmonary function test.
Doctors need to be aware and diagnose this very quickly. When patients present, you can pull them out of that work environment or put mitigation systems into place.
Q: California regulators were expected to put emergency rules into place in late December to protect workers. Did this study play a role in focusing attention on the problem?
A: This article, along with a commentary and podcast that we put out, really helped with advocacy to improve health and safety for workers at stone-cutting and fabrication shops.
Q: You were impressed by another study about airborne dangers, this one linking air pollution to dementia. What did researchers discover?
A: [This analysis] of more than 27,000 people in the Health and Retirement Study, a respected and rich database, found that exposure to air pollution was associated with greater rates of dementia — an increase of about 8% a year. Exposure to agricultural emissions and wildfire smoke were most robustly associated with a greater risk of dementia.
Q: How are these findings important, especially in light of the unhealthy air spawned by recent wildfires in the United States and Canada?
A: Studies like this will make it even more compelling that we are better prepared for air quality issues.
I grew up in Los Angeles, where smog and pollution were very big issues. I was constantly hearing about various mitigation strategies that were going into place. But after I moved to the East Coast, I almost never heard about prevention.
Now, I’m hoping we can keep this topic in the national conversation.
Q: You also highlighted a systematic review of the use of restraints in the emergency department. Why did you choose this research?
A: At JAMA Internal Medicine, we’re really focused on ways we can address health disparities and raise awareness of potential unconscious bias.
This review looked at 10 studies that included more than 2.5 million patient encounters, including 24,000 incidents of physical restraint use. They found that the overall rate of use of restraints was low at below 1%.
But when they are used, Black patients were 1.3 times more likely to be restrained than White patients.
Q: What’s the message here?
A: This is an important start to recognizing these differences and then changing our behavior. Perhaps restraints don’t need to be used as often in light of evidence, for example, of increased rates of misdiagnosis of psychosis in the Black population.
Q: How should physicians change their approach to restraints?
A: Restraints are not to be used to control disruption — wild behavior or verbal outbursts. They’re for when someone is a danger to themselves or others.
Dr. Inouye has no conflicts of interest.
The Art of Seeing
People are surprised when they learn I was an art history major in college. Most folks assume I had majored in biology or chemistry. Their assumption was based on strong odds. The U.S. Bureau of Labor Statistics reports that nearly half of all physicians practicing in this country were biology majors.
I headed off to college clueless about my future. I was hoping to succeed as a walk-on to the football team and beyond that I figured someone or something would guide me toward a career. Had you asked me, “physician” it would have been a definite “Never.”
I flirted with a psychology major, but after a semester I realized that the department was more interested in the behavior of rats rather than humans. I got an “easy A” in the intro to art history and that was the open door I was looking for.
By my senior year I was applying for fellowships to study in faraway places. However, the world situation in 1965 was unsettling for a young man in this country. I had had a strong high school science education and had continued to take a some science courses. Fortunately, I had banked just enough credits so that I could apply to medical school, again without really planning to become a physician.
Even during the sharpest turns in my circuitous path to becoming a small town pediatrician, including a year doing research in exercise physiology in Denmark, I never once regretted my years spent studying art history. I credit them with making me a more sensitive observer.
You can probably understand why I was intrigued by an article I recently read that described a program in which the radiology residents that the Brigham and Women’s Hospital in Boston take a year-long course in art history using the Art Museum at Harvard University as a resource. Titled “Seeing in Art and Medical Imaging,” the program is now 6 years old. Hyewon Hyun, MD, a radiologist and one of its cofounders, observes that “art is the starting point for in-depth conversations about medicine, humanity, and different ways of seeing the world.”
Radiology and dermatology are obviously the two specialties in which the physician relies most heavily on his or her powers of observation. However, every doctor can benefit from learning to really “see” what they are looking at. Looking and seeing are two very different activities. There is obviously the forest-from the-trees phenomenon. Can the physician in a hurried clinical situation muster up the discipline to shift focus back and forth from the lesion or painful body part to the entire patient and beyond? How is the parent responding to the child’s discomfort? How are they dressed? Does this wider view suggest some additional questions to ask that may help you understand how this patient or family will be able to cope with diagnosis or follow up with your treatment plan?
The art historian sees every object in its historical context. What has come before? How have the societal conditions influenced the artist choice of subject and use of materials? How has his or her emotions at the time of creation influenced his or her style? The astute physician must likewise see the patients and their complaints in the broader context of their emotional health and socioeconomic situation. This requires sensitive listening and careful observation.
One doesn’t have to major in art history or spend years roaming through the sometimes dark and dusty halls of the world’s museums to progress from being one who simply looks to a person who really sees the environment and its inhabitants. It is really a state of mind and a commitment to improvement.
As physicians, we often complain or sometimes brag about how many patients we “see” in a day. I fear that too often we mean “looked at.” How frequently did we make the effort to really see the patient?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
People are surprised when they learn I was an art history major in college. Most folks assume I had majored in biology or chemistry. Their assumption was based on strong odds. The U.S. Bureau of Labor Statistics reports that nearly half of all physicians practicing in this country were biology majors.
I headed off to college clueless about my future. I was hoping to succeed as a walk-on to the football team and beyond that I figured someone or something would guide me toward a career. Had you asked me, “physician” it would have been a definite “Never.”
I flirted with a psychology major, but after a semester I realized that the department was more interested in the behavior of rats rather than humans. I got an “easy A” in the intro to art history and that was the open door I was looking for.
By my senior year I was applying for fellowships to study in faraway places. However, the world situation in 1965 was unsettling for a young man in this country. I had had a strong high school science education and had continued to take a some science courses. Fortunately, I had banked just enough credits so that I could apply to medical school, again without really planning to become a physician.
Even during the sharpest turns in my circuitous path to becoming a small town pediatrician, including a year doing research in exercise physiology in Denmark, I never once regretted my years spent studying art history. I credit them with making me a more sensitive observer.
You can probably understand why I was intrigued by an article I recently read that described a program in which the radiology residents that the Brigham and Women’s Hospital in Boston take a year-long course in art history using the Art Museum at Harvard University as a resource. Titled “Seeing in Art and Medical Imaging,” the program is now 6 years old. Hyewon Hyun, MD, a radiologist and one of its cofounders, observes that “art is the starting point for in-depth conversations about medicine, humanity, and different ways of seeing the world.”
Radiology and dermatology are obviously the two specialties in which the physician relies most heavily on his or her powers of observation. However, every doctor can benefit from learning to really “see” what they are looking at. Looking and seeing are two very different activities. There is obviously the forest-from the-trees phenomenon. Can the physician in a hurried clinical situation muster up the discipline to shift focus back and forth from the lesion or painful body part to the entire patient and beyond? How is the parent responding to the child’s discomfort? How are they dressed? Does this wider view suggest some additional questions to ask that may help you understand how this patient or family will be able to cope with diagnosis or follow up with your treatment plan?
The art historian sees every object in its historical context. What has come before? How have the societal conditions influenced the artist choice of subject and use of materials? How has his or her emotions at the time of creation influenced his or her style? The astute physician must likewise see the patients and their complaints in the broader context of their emotional health and socioeconomic situation. This requires sensitive listening and careful observation.
One doesn’t have to major in art history or spend years roaming through the sometimes dark and dusty halls of the world’s museums to progress from being one who simply looks to a person who really sees the environment and its inhabitants. It is really a state of mind and a commitment to improvement.
As physicians, we often complain or sometimes brag about how many patients we “see” in a day. I fear that too often we mean “looked at.” How frequently did we make the effort to really see the patient?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
People are surprised when they learn I was an art history major in college. Most folks assume I had majored in biology or chemistry. Their assumption was based on strong odds. The U.S. Bureau of Labor Statistics reports that nearly half of all physicians practicing in this country were biology majors.
I headed off to college clueless about my future. I was hoping to succeed as a walk-on to the football team and beyond that I figured someone or something would guide me toward a career. Had you asked me, “physician” it would have been a definite “Never.”
I flirted with a psychology major, but after a semester I realized that the department was more interested in the behavior of rats rather than humans. I got an “easy A” in the intro to art history and that was the open door I was looking for.
By my senior year I was applying for fellowships to study in faraway places. However, the world situation in 1965 was unsettling for a young man in this country. I had had a strong high school science education and had continued to take a some science courses. Fortunately, I had banked just enough credits so that I could apply to medical school, again without really planning to become a physician.
Even during the sharpest turns in my circuitous path to becoming a small town pediatrician, including a year doing research in exercise physiology in Denmark, I never once regretted my years spent studying art history. I credit them with making me a more sensitive observer.
You can probably understand why I was intrigued by an article I recently read that described a program in which the radiology residents that the Brigham and Women’s Hospital in Boston take a year-long course in art history using the Art Museum at Harvard University as a resource. Titled “Seeing in Art and Medical Imaging,” the program is now 6 years old. Hyewon Hyun, MD, a radiologist and one of its cofounders, observes that “art is the starting point for in-depth conversations about medicine, humanity, and different ways of seeing the world.”
Radiology and dermatology are obviously the two specialties in which the physician relies most heavily on his or her powers of observation. However, every doctor can benefit from learning to really “see” what they are looking at. Looking and seeing are two very different activities. There is obviously the forest-from the-trees phenomenon. Can the physician in a hurried clinical situation muster up the discipline to shift focus back and forth from the lesion or painful body part to the entire patient and beyond? How is the parent responding to the child’s discomfort? How are they dressed? Does this wider view suggest some additional questions to ask that may help you understand how this patient or family will be able to cope with diagnosis or follow up with your treatment plan?
The art historian sees every object in its historical context. What has come before? How have the societal conditions influenced the artist choice of subject and use of materials? How has his or her emotions at the time of creation influenced his or her style? The astute physician must likewise see the patients and their complaints in the broader context of their emotional health and socioeconomic situation. This requires sensitive listening and careful observation.
One doesn’t have to major in art history or spend years roaming through the sometimes dark and dusty halls of the world’s museums to progress from being one who simply looks to a person who really sees the environment and its inhabitants. It is really a state of mind and a commitment to improvement.
As physicians, we often complain or sometimes brag about how many patients we “see” in a day. I fear that too often we mean “looked at.” How frequently did we make the effort to really see the patient?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
New Insights, New Standards: How 2023 Changed Care for Internists
The past year brought major changes in preventive standards for anxiety, HIV, and RSV along with new guidelines for the treatment of atrial fibrillation. For insight into the effect on internal medicine, we turned to Sarah Candler, MD, MPH, a Houston internist who specializes in the care of high-risk older adults.
Q: Which new prevention guidelines had the most impact on you over the past year?
A: I’m a primary care doctor, and most of the internal medicine updates that are interesting to me focus on how we can keep people from getting sick in the first place. That’s especially important in light of the fact that we had a decrease in life expectancy of 2 years [it finally rose slightly in 2022] and widening of the gender gap in life expectancy for men and women.
I’m excited to see new recommendations from the U.S. Preventive Services Task Force, including a new one about using PREP [pre-exposure prophylaxis] to preventively treat anyone who’s at risk for getting HIV. That’s a big one because it’s one of the first times that we’ve identified at-risk groups for screening based on social risk factors, not gender, age, or genetics.
The new recommendation is PREP for anyone who’s at risk for getting HIV because they have a partner with HIV, had an sexually transmitted infection in the last 6 months, or a history of inconsistent or no condom use with partners with unknown HIV status.
PREP therapy is something that most primary care physicians can either do or learn how to do pretty easily. But the treatment does require maintenance and monitoring.
Q: How firm is this recommendation?
A: The task force gives different grades for their recommendations based on how strong the evidence is. For the guidelines about PREP, they give a grade of A. That means this is top of the class: You should definitely do this.
Q: What are the best strategies to ask patients personal questions about their sex lives in order to evaluate their risk?
A: A lot of internal medicine physicians are getting pretty good at this. We see it as part of our job just the same way as we asked things like, “How often are you walking?” and “Have you been feeling down?”
There’s no one right way to have a conversation like that. But it’s key to say, as I do to my patients, that “I’m not here to judge anything. I am truly here to gather information and make recommendations to you as a partner in your care.”
Q: What other guidelines made an impact in 2023?
A: The U.S. Preventive Services Task Force made a recommendation to screen adults aged 18-64 for anxiety, and this guidance got a B grade. [The task force said there’s not enough evidence to support routine anxiety screening in adults 65 and older.]
The new recommendations is a sign that we’re doing a better job at making treatment of those diseases more acceptable. This is also another example of the medical community recognizing that internal medicine physicians are pretty good at identifying and treating mental health.
Q: How do you figure out whether to treat depression/anxiety yourself or refer patients to specialists?
A: As a primary care physician, I feel comfortable diagnosing and managing some mental health disease in my own practice. There are FDA-approved medications for both anxiety and depression that are easily managed by a primary care physician.
And there’s something to the therapeutic relationship, to naming and identifying these conditions with your patients. Some patients feel a bit of relief just knowing that they have a diagnosis.
Q: What should internists know about the new CDC guidelines that promote discussing RSV vaccines with patients who are over 60?
A: The vaccines are recommended for folks who have underlying conditions like lung disease or heart disease. Those are the ones who end up getting really, really sick. There are two adult vaccines that are available, and there’s not a preference for one over the other.
The vaccines are both protein-based, like the old-school versions of vaccines, not the mRNA vaccines that we’ve all been hearing more about through COVID. Anybody who’s reluctant to take an mRNA vaccine can rest assured that the RSV is not protein-based. And they are single-dose vaccines, which is helpful.
Q: What else should internists know about that was new in 2023?
A: I’m super excited about how cardiologists are thinking about atrial fibrillation. In 2023, the American College of Cardiology and the American Heart Association came up with a giant overhaul of how they look at atrial fibrillation. They classify it in stages and allows us to think about stopping it before it starts.
They’re talking about something they’re calling preclinical or subclinical atrial fibrillation, which you may detect on wearables like somebody’s watch or another tool used to monitor heart rate or exercise. It might be the first harbinger that there’s something wrong with the heart rate, and they may not even have symptoms of it. [A 2023 study in The New England Journal of Medicine linked the anticoagulant apixaban, or Eliquis, to a 37% lower risk of stroke and systemic embolism rates in older patients with subclinical atrial fibrillation but an 80% higher risk of major bleeding vs. aspirin therapy.]
And they’re now recommending early rhythm control.
Q: What does early rhythm control mean for patients and physicians?
A: For the longest time, we have thought about atrial fibrillation treatment in terms of rate control and not worrying too much about the rhythm. But now we recognize that it’s actually really important that we get the rhythm under control because physical changes to the heart can lead to permanent damage.
So now they’re recommending catheter ablation as first-line therapy in some patients as a class 1 recommendation because heart function is already decreased. Improving the ability of the heart to beat with a regular rhythm can lead to improvement of function. This was unheard of even 5 years ago.
Q: Should internists be more willing to refer patients with atrial fibrillation to cardiologists?
A: Yes, I think so. One of the biggest changes for me is that I am going to refer new diagnoses of atrial fibrillation to a cardiologist. And I’m going to ask patients if they have wearable devices because sometimes those things might tell me about something like subclinical atrial fibrillation.
Q: There’s also detailed data about atrial fibrillation risk factors, which include older age, smoking, sedentary lifestyle, alcohol use, diabetes, height, obesity, diabetes, and others. Is this information useful?
A: It’s a really great tool to have in the arsenal because it helps me have shared decision-making conversations with my patients in a way that’s much more convincing. A patient might say, “Why do you care if I drink so much? My liver levels are fine.” And I can say, “It’s going to be a risk factor for having problems with your heart.”
For better or worse, people really take the heart very seriously, I am an internal medicine physician, so I love all the organs equally. But man, people get pretty scared when you tell them something can affect their heart. So when I talk to patients about their risk factors, it’s going to really be helpful that I can remind them of the impact that some of these lifestyle behaviors can have on their heart health.
Dr. Candler has no disclosures.
The past year brought major changes in preventive standards for anxiety, HIV, and RSV along with new guidelines for the treatment of atrial fibrillation. For insight into the effect on internal medicine, we turned to Sarah Candler, MD, MPH, a Houston internist who specializes in the care of high-risk older adults.
Q: Which new prevention guidelines had the most impact on you over the past year?
A: I’m a primary care doctor, and most of the internal medicine updates that are interesting to me focus on how we can keep people from getting sick in the first place. That’s especially important in light of the fact that we had a decrease in life expectancy of 2 years [it finally rose slightly in 2022] and widening of the gender gap in life expectancy for men and women.
I’m excited to see new recommendations from the U.S. Preventive Services Task Force, including a new one about using PREP [pre-exposure prophylaxis] to preventively treat anyone who’s at risk for getting HIV. That’s a big one because it’s one of the first times that we’ve identified at-risk groups for screening based on social risk factors, not gender, age, or genetics.
The new recommendation is PREP for anyone who’s at risk for getting HIV because they have a partner with HIV, had an sexually transmitted infection in the last 6 months, or a history of inconsistent or no condom use with partners with unknown HIV status.
PREP therapy is something that most primary care physicians can either do or learn how to do pretty easily. But the treatment does require maintenance and monitoring.
Q: How firm is this recommendation?
A: The task force gives different grades for their recommendations based on how strong the evidence is. For the guidelines about PREP, they give a grade of A. That means this is top of the class: You should definitely do this.
Q: What are the best strategies to ask patients personal questions about their sex lives in order to evaluate their risk?
A: A lot of internal medicine physicians are getting pretty good at this. We see it as part of our job just the same way as we asked things like, “How often are you walking?” and “Have you been feeling down?”
There’s no one right way to have a conversation like that. But it’s key to say, as I do to my patients, that “I’m not here to judge anything. I am truly here to gather information and make recommendations to you as a partner in your care.”
Q: What other guidelines made an impact in 2023?
A: The U.S. Preventive Services Task Force made a recommendation to screen adults aged 18-64 for anxiety, and this guidance got a B grade. [The task force said there’s not enough evidence to support routine anxiety screening in adults 65 and older.]
The new recommendations is a sign that we’re doing a better job at making treatment of those diseases more acceptable. This is also another example of the medical community recognizing that internal medicine physicians are pretty good at identifying and treating mental health.
Q: How do you figure out whether to treat depression/anxiety yourself or refer patients to specialists?
A: As a primary care physician, I feel comfortable diagnosing and managing some mental health disease in my own practice. There are FDA-approved medications for both anxiety and depression that are easily managed by a primary care physician.
And there’s something to the therapeutic relationship, to naming and identifying these conditions with your patients. Some patients feel a bit of relief just knowing that they have a diagnosis.
Q: What should internists know about the new CDC guidelines that promote discussing RSV vaccines with patients who are over 60?
A: The vaccines are recommended for folks who have underlying conditions like lung disease or heart disease. Those are the ones who end up getting really, really sick. There are two adult vaccines that are available, and there’s not a preference for one over the other.
The vaccines are both protein-based, like the old-school versions of vaccines, not the mRNA vaccines that we’ve all been hearing more about through COVID. Anybody who’s reluctant to take an mRNA vaccine can rest assured that the RSV is not protein-based. And they are single-dose vaccines, which is helpful.
Q: What else should internists know about that was new in 2023?
A: I’m super excited about how cardiologists are thinking about atrial fibrillation. In 2023, the American College of Cardiology and the American Heart Association came up with a giant overhaul of how they look at atrial fibrillation. They classify it in stages and allows us to think about stopping it before it starts.
They’re talking about something they’re calling preclinical or subclinical atrial fibrillation, which you may detect on wearables like somebody’s watch or another tool used to monitor heart rate or exercise. It might be the first harbinger that there’s something wrong with the heart rate, and they may not even have symptoms of it. [A 2023 study in The New England Journal of Medicine linked the anticoagulant apixaban, or Eliquis, to a 37% lower risk of stroke and systemic embolism rates in older patients with subclinical atrial fibrillation but an 80% higher risk of major bleeding vs. aspirin therapy.]
And they’re now recommending early rhythm control.
Q: What does early rhythm control mean for patients and physicians?
A: For the longest time, we have thought about atrial fibrillation treatment in terms of rate control and not worrying too much about the rhythm. But now we recognize that it’s actually really important that we get the rhythm under control because physical changes to the heart can lead to permanent damage.
So now they’re recommending catheter ablation as first-line therapy in some patients as a class 1 recommendation because heart function is already decreased. Improving the ability of the heart to beat with a regular rhythm can lead to improvement of function. This was unheard of even 5 years ago.
Q: Should internists be more willing to refer patients with atrial fibrillation to cardiologists?
A: Yes, I think so. One of the biggest changes for me is that I am going to refer new diagnoses of atrial fibrillation to a cardiologist. And I’m going to ask patients if they have wearable devices because sometimes those things might tell me about something like subclinical atrial fibrillation.
Q: There’s also detailed data about atrial fibrillation risk factors, which include older age, smoking, sedentary lifestyle, alcohol use, diabetes, height, obesity, diabetes, and others. Is this information useful?
A: It’s a really great tool to have in the arsenal because it helps me have shared decision-making conversations with my patients in a way that’s much more convincing. A patient might say, “Why do you care if I drink so much? My liver levels are fine.” And I can say, “It’s going to be a risk factor for having problems with your heart.”
For better or worse, people really take the heart very seriously, I am an internal medicine physician, so I love all the organs equally. But man, people get pretty scared when you tell them something can affect their heart. So when I talk to patients about their risk factors, it’s going to really be helpful that I can remind them of the impact that some of these lifestyle behaviors can have on their heart health.
Dr. Candler has no disclosures.
The past year brought major changes in preventive standards for anxiety, HIV, and RSV along with new guidelines for the treatment of atrial fibrillation. For insight into the effect on internal medicine, we turned to Sarah Candler, MD, MPH, a Houston internist who specializes in the care of high-risk older adults.
Q: Which new prevention guidelines had the most impact on you over the past year?
A: I’m a primary care doctor, and most of the internal medicine updates that are interesting to me focus on how we can keep people from getting sick in the first place. That’s especially important in light of the fact that we had a decrease in life expectancy of 2 years [it finally rose slightly in 2022] and widening of the gender gap in life expectancy for men and women.
I’m excited to see new recommendations from the U.S. Preventive Services Task Force, including a new one about using PREP [pre-exposure prophylaxis] to preventively treat anyone who’s at risk for getting HIV. That’s a big one because it’s one of the first times that we’ve identified at-risk groups for screening based on social risk factors, not gender, age, or genetics.
The new recommendation is PREP for anyone who’s at risk for getting HIV because they have a partner with HIV, had an sexually transmitted infection in the last 6 months, or a history of inconsistent or no condom use with partners with unknown HIV status.
PREP therapy is something that most primary care physicians can either do or learn how to do pretty easily. But the treatment does require maintenance and monitoring.
Q: How firm is this recommendation?
A: The task force gives different grades for their recommendations based on how strong the evidence is. For the guidelines about PREP, they give a grade of A. That means this is top of the class: You should definitely do this.
Q: What are the best strategies to ask patients personal questions about their sex lives in order to evaluate their risk?
A: A lot of internal medicine physicians are getting pretty good at this. We see it as part of our job just the same way as we asked things like, “How often are you walking?” and “Have you been feeling down?”
There’s no one right way to have a conversation like that. But it’s key to say, as I do to my patients, that “I’m not here to judge anything. I am truly here to gather information and make recommendations to you as a partner in your care.”
Q: What other guidelines made an impact in 2023?
A: The U.S. Preventive Services Task Force made a recommendation to screen adults aged 18-64 for anxiety, and this guidance got a B grade. [The task force said there’s not enough evidence to support routine anxiety screening in adults 65 and older.]
The new recommendations is a sign that we’re doing a better job at making treatment of those diseases more acceptable. This is also another example of the medical community recognizing that internal medicine physicians are pretty good at identifying and treating mental health.
Q: How do you figure out whether to treat depression/anxiety yourself or refer patients to specialists?
A: As a primary care physician, I feel comfortable diagnosing and managing some mental health disease in my own practice. There are FDA-approved medications for both anxiety and depression that are easily managed by a primary care physician.
And there’s something to the therapeutic relationship, to naming and identifying these conditions with your patients. Some patients feel a bit of relief just knowing that they have a diagnosis.
Q: What should internists know about the new CDC guidelines that promote discussing RSV vaccines with patients who are over 60?
A: The vaccines are recommended for folks who have underlying conditions like lung disease or heart disease. Those are the ones who end up getting really, really sick. There are two adult vaccines that are available, and there’s not a preference for one over the other.
The vaccines are both protein-based, like the old-school versions of vaccines, not the mRNA vaccines that we’ve all been hearing more about through COVID. Anybody who’s reluctant to take an mRNA vaccine can rest assured that the RSV is not protein-based. And they are single-dose vaccines, which is helpful.
Q: What else should internists know about that was new in 2023?
A: I’m super excited about how cardiologists are thinking about atrial fibrillation. In 2023, the American College of Cardiology and the American Heart Association came up with a giant overhaul of how they look at atrial fibrillation. They classify it in stages and allows us to think about stopping it before it starts.
They’re talking about something they’re calling preclinical or subclinical atrial fibrillation, which you may detect on wearables like somebody’s watch or another tool used to monitor heart rate or exercise. It might be the first harbinger that there’s something wrong with the heart rate, and they may not even have symptoms of it. [A 2023 study in The New England Journal of Medicine linked the anticoagulant apixaban, or Eliquis, to a 37% lower risk of stroke and systemic embolism rates in older patients with subclinical atrial fibrillation but an 80% higher risk of major bleeding vs. aspirin therapy.]
And they’re now recommending early rhythm control.
Q: What does early rhythm control mean for patients and physicians?
A: For the longest time, we have thought about atrial fibrillation treatment in terms of rate control and not worrying too much about the rhythm. But now we recognize that it’s actually really important that we get the rhythm under control because physical changes to the heart can lead to permanent damage.
So now they’re recommending catheter ablation as first-line therapy in some patients as a class 1 recommendation because heart function is already decreased. Improving the ability of the heart to beat with a regular rhythm can lead to improvement of function. This was unheard of even 5 years ago.
Q: Should internists be more willing to refer patients with atrial fibrillation to cardiologists?
A: Yes, I think so. One of the biggest changes for me is that I am going to refer new diagnoses of atrial fibrillation to a cardiologist. And I’m going to ask patients if they have wearable devices because sometimes those things might tell me about something like subclinical atrial fibrillation.
Q: There’s also detailed data about atrial fibrillation risk factors, which include older age, smoking, sedentary lifestyle, alcohol use, diabetes, height, obesity, diabetes, and others. Is this information useful?
A: It’s a really great tool to have in the arsenal because it helps me have shared decision-making conversations with my patients in a way that’s much more convincing. A patient might say, “Why do you care if I drink so much? My liver levels are fine.” And I can say, “It’s going to be a risk factor for having problems with your heart.”
For better or worse, people really take the heart very seriously, I am an internal medicine physician, so I love all the organs equally. But man, people get pretty scared when you tell them something can affect their heart. So when I talk to patients about their risk factors, it’s going to really be helpful that I can remind them of the impact that some of these lifestyle behaviors can have on their heart health.
Dr. Candler has no disclosures.
We Want to Hear From You, Our Readers
Happy New Year, everyone. It’s hard to believe, but we are nearing the mid-point of our five-year term on the GI & Hepatology News (GIHN) board of editors. Our central goal over the past two-and-a-half years has been to curate thought-provoking content for GIHN that helps to inform clinical practice and keeps you up-to-date on emerging scientific innovations and policy changes impacting patients with digestive and liver diseases.
Your feedback is critical to ensuring the continued success of the newspaper as your go-to source for cutting edge news relevant to our field.
To start, we welcome your thoughts on the following questions:
- What do you want to see more of in the newspaper (e.g., a particular column, topic)?
- How can we continue to serve you best as a reader?
Please email your feedback to us at [email protected]. Your input is greatly appreciated by both the board and our larger editorial team and will help inform future coverage.
In this month’s issue of GIHN, we update you on the proceedings of AGA’s 2023 Innovation Conference, highlight a new Clinical Practice Guideline focused on the role of biomarkers in Crohn’s disease management, and summarize key AGA journal content.
The AGA Government Affairs Committee also details 2024 updates to Medicare payment rules, including a new add-on code for complex care, increased facility payment for POEM procedures, and continuation of expanded telehealth coverage through the end of 2024.
GIHN associate editor Dr. Avi Ketwaroo introduces this month’s Perspectives column focused on the impact of substance use (specifically alcohol and marijuana) on liver transplant candidacy.
In our January Member Spotlight, we feature Dr. Sonali Paul, a hepatologist and co-founder of Rainbows in Gastro. She shares her passion for promoting health equity in sexual and gender minority populations.
We hope you enjoy this, and all the exciting content included in our January issue.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Happy New Year, everyone. It’s hard to believe, but we are nearing the mid-point of our five-year term on the GI & Hepatology News (GIHN) board of editors. Our central goal over the past two-and-a-half years has been to curate thought-provoking content for GIHN that helps to inform clinical practice and keeps you up-to-date on emerging scientific innovations and policy changes impacting patients with digestive and liver diseases.
Your feedback is critical to ensuring the continued success of the newspaper as your go-to source for cutting edge news relevant to our field.
To start, we welcome your thoughts on the following questions:
- What do you want to see more of in the newspaper (e.g., a particular column, topic)?
- How can we continue to serve you best as a reader?
Please email your feedback to us at [email protected]. Your input is greatly appreciated by both the board and our larger editorial team and will help inform future coverage.
In this month’s issue of GIHN, we update you on the proceedings of AGA’s 2023 Innovation Conference, highlight a new Clinical Practice Guideline focused on the role of biomarkers in Crohn’s disease management, and summarize key AGA journal content.
The AGA Government Affairs Committee also details 2024 updates to Medicare payment rules, including a new add-on code for complex care, increased facility payment for POEM procedures, and continuation of expanded telehealth coverage through the end of 2024.
GIHN associate editor Dr. Avi Ketwaroo introduces this month’s Perspectives column focused on the impact of substance use (specifically alcohol and marijuana) on liver transplant candidacy.
In our January Member Spotlight, we feature Dr. Sonali Paul, a hepatologist and co-founder of Rainbows in Gastro. She shares her passion for promoting health equity in sexual and gender minority populations.
We hope you enjoy this, and all the exciting content included in our January issue.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Happy New Year, everyone. It’s hard to believe, but we are nearing the mid-point of our five-year term on the GI & Hepatology News (GIHN) board of editors. Our central goal over the past two-and-a-half years has been to curate thought-provoking content for GIHN that helps to inform clinical practice and keeps you up-to-date on emerging scientific innovations and policy changes impacting patients with digestive and liver diseases.
Your feedback is critical to ensuring the continued success of the newspaper as your go-to source for cutting edge news relevant to our field.
To start, we welcome your thoughts on the following questions:
- What do you want to see more of in the newspaper (e.g., a particular column, topic)?
- How can we continue to serve you best as a reader?
Please email your feedback to us at [email protected]. Your input is greatly appreciated by both the board and our larger editorial team and will help inform future coverage.
In this month’s issue of GIHN, we update you on the proceedings of AGA’s 2023 Innovation Conference, highlight a new Clinical Practice Guideline focused on the role of biomarkers in Crohn’s disease management, and summarize key AGA journal content.
The AGA Government Affairs Committee also details 2024 updates to Medicare payment rules, including a new add-on code for complex care, increased facility payment for POEM procedures, and continuation of expanded telehealth coverage through the end of 2024.
GIHN associate editor Dr. Avi Ketwaroo introduces this month’s Perspectives column focused on the impact of substance use (specifically alcohol and marijuana) on liver transplant candidacy.
In our January Member Spotlight, we feature Dr. Sonali Paul, a hepatologist and co-founder of Rainbows in Gastro. She shares her passion for promoting health equity in sexual and gender minority populations.
We hope you enjoy this, and all the exciting content included in our January issue.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Physician-Owned Hospitals: The Answer for Better Care?
This discussion was recorded on November 16, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today is Dr. Brian J. Miller, a hospitalist with Johns Hopkins University School of Medicine and a health policy expert, to discuss the current and renewed interest in physician-owned hospitals.
Welcome, Dr. Miller. It’s a pleasure to have you join me today.
Brian J. Miller, MD, MBA, MPH: Thank you for having me.
History and Controversies Surrounding Physician-Owned Hospitals
Dr. Glatter: I want to start off by having you describe the history associated with the moratorium on new physician-owned hospitals in 2010 that’s related ultimately to the Affordable Care Act, but also, the current and renewed media interest in physician-owned hospitals that’s linked to recent congressional hearings last month.
Dr. Miller: Thank you. I should note that my views are my own and don’t represent those of Hopkins or the American Enterprise Institute, where I’m a nonresident fellow nor the Medicare Payment Advisory Commission, of which I’m a Commissioner.
The story about physician-owned hospitals is an interesting one. Hospitals turned into health systems in the 1980s and 1990s, and physicians started to shift purely from an independent model into a more organized group practice or employed model. Physicians realized that they wanted an alternative operating arrangement. You want a choice of how you practice and what your employment is. And as community hospitals started to buy physicians and also establish their own physician groups de novo, physicians opened physician-owned hospitals.
Physician-owned hospitals fell into a couple of buckets. One is what we call community hospitals, or what the antitrust lawyers would call general acute care hospitals: those offering emergency room (ER) services, labor and delivery, primary care, general surgery — the whole regular gamut, except that some of the owners were physicians.
The other half of the marketplace ended up being specialty hospitals: those built around a specific medical specialty and series of procedures and chronic care. For example, cardiac hospitals often do CABG, TAVR, maybe abdominal aortic aneurysm (triple A) repairs, and they have cardiology clinics, cath labs, a cardiac intensive care unit (ICU), ER, etc. There were also orthopedic surgical specialty hospitals, which were sort of like an ambulatory surgery center (ASC) plus several beds. Then there were general surgical specialty hospitals. At one point, there were some women’s health–focused specialty hospitals.
The hospital industry, of course, as you can understand, didn’t exactly like this. They had a series of concerns about what we would historically call cherry-picking or lemon-dropping of patients. They were worried that physician-owned facilities didn’t want to serve public payer patients, and there was a whole series of reports and investigations.
Around the time the Affordable Care Act passed, the hospital industry had many concerns about physician-owned specialty hospitals, and there was a moratorium as part of the 2003 Medicare Modernization Act. As part of the bargaining over the hospital industry support for the Affordable Care Act, they traded their support for, among other things, their number one priority, which is a statutory prohibition on new or expanded physician-owned hospitals from participating in Medicare. That included both physician-owned community hospitals and physician-owned specialty hospitals.
Dr. Glatter: That was part of the impetus to prevent physicians from referring patients where they had an ownership stake. Certainly, hospitals can be owned by attorneys and nonprofit organizations, and certainly, ASCs can be owned by physicians. There is an ongoing issue in terms of physicians not being able to have an ownership stake. In terms of equity ownership, we know that certain other models allow this, but basically, it sounds like this is an issue with Medicare. That seems to be the crux of it, correct?
Dr. Miller: Yes. I would also add that it’s interesting when we look at other professions. When we look at lawyers, nonlawyers are actually not allowed to own an equity stake in a law practice. In many other professions, you either have corporate ownership or professional ownership, or the alternative is you have only professional ownership. I would say the hospital industry is one of the few areas where professional ownership not only is not allowed, but also is statutorily prohibited functionally through the Medicare program.
Unveiling the Dynamics of Hospital Ownership
Dr. Glatter: A recent study done by two PhDs looked at 2019 data on 20 of the most expensive diagnosis-related groups (DRGs). It examined the cost savings, and we’re talking over $1 billion in expenditures when you look at the data from general acute care hospitals vs physician-owned hospitals. This is what appears to me to be a key driver of the push to loosen restrictions on physician-owned hospitals. Isn’t that correct?
Dr. Miller: I would say that’s one of many components. There’s more history to this issue. I remember sitting at a think tank talking to someone several years ago about hospital consolidation as an issue. We went through the usual levers that us policy wonks go through. We talked about antitrust enforcement, certificate of need, rising hospital costs from consolidation, lower quality (or at least no quality gains, as shown by a New England Journal of Medicine study), and decrements in patient experience that result from the diseconomies of scale. They sort of pooh-poohed many of the policy ideas. They basically said that there was no hope for hospital consolidation as an issue.
Well, what about physician ownership? I started with my research team to comb through the literature and found a variety of studies — some of which were sort of entertaining, because they’d do things like study physician-owned specialty hospitals, nonprofit-owned specialty hospitals, and for-profit specialty hospitals and compare them with nonprofit or for-profit community hospitals, and then say physician-owned hospitals that were specialty were bad.
They mixed ownership and service markets right there in so many ways, I’m not sure where to start. My team did a systematic review of around 30 years of research, looking at the evidence base in this space. We found a couple of things.
We found that physician-owned community hospitals did not have a cost or quality difference, meaning that there was no definitive evidence that the physician-owned community hospitals were cheaper based on historical evidence, which was very old. That means there’s not specific harm from them. When you permit market entry for community hospitals, that promotes competition, which results in lower prices and higher quality.
Then we also looked at the specialty hospital markets — surgical specialty hospitals, orthopedic surgical specialty hospitals, and cardiac hospitals. We noted for cardiac hospitals, there wasn’t clear evidence about cost savings, but there was definitive evidence of higher quality, from things like 30-day mortality for significant procedures like treatment of acute MI, triple A repair, stuff like that.
For orthopedic surgical specialty hospitals, we noted lower costs and higher quality, which again fits with operationally what we would know. If you have a facility that’s doing 20 total hips a day, you’re creating a focused factory. Just like if you think about it for interventional cardiology, your boards have a minimum number of procedures that you have to do to stay certified because we know about the volume-quality relationship.
Then we looked at general surgical specialty hospitals. There wasn’t enough evidence to make a conclusive thought about costs, and there was a clear trend toward higher quality. I would say this recent study is important, but there is a whole bunch of other literature out there, too.
Exploring the Scope of Emergency Care in Physician-Owned Hospitals
Dr. Glatter: Certainly, your colleague Wang from Johns Hopkins has done important research in this sector. The paper, “Reconsidering the Ban on Physician-Owned Hospitals to Combat Consolidation,” by you and several colleagues, mentions and highlights the issues that you just described. I understand that it’s going to be published in the NYU Journal of Legislation and Public Policy.
One thing I want to bring up — and this is an important issue — is that the risk for patients has been talked about by the American Hospital Association and the Federation of American Hospitals, in terms of limited or no emergency services at such physician-owned hospitals and having to call 911 when patients need emergent care or stabilization. That’s been the rebuttal, along with an Office of Inspector General (OIG) report from 2008. Almost, I guess, three quarters of the patients that needed emergent care got this at publicly funded hospitals.
Dr. Miller: I’m familiar with the argument about emergency care. If you actually go and look at it, it differs by specialty market. Physician-owned community hospitals have ERs because that’s how they get their business. If you are running a hospital medicine floor, a general surgical specialty floor, you have a labor delivery unit, a primary care clinic, and a cardiology clinic. You have all the things that all the other hospitals have. The physician-owned community hospitals almost uniformly have an ER.
When you look at the physician-owned specialty hospitals, it’s a little more granular. If you look at the cardiac hospitals, they have ERs. They also have cardiac ICUs, operating rooms, etc. The area where the hospital industry had concerns — which I think is valid to point out — is that physician-owned orthopedic surgical specialty hospitals don’t have ERs. But this makes sense because of what that hospital functionally is: a factory for whatever the scope of procedures is, be it joint replacements or shoulder arthroscopy. The orthopedic surgical specialty hospital is like an ASC plus several hospital beds. Many of those did not have ERs because clinically it didn’t make sense.
What’s interesting, though, is that the hospital industry also operates specialty hospitals. If you go into many of the large systems, they have cardiac specialty hospitals and cancer specialty hospitals. I would say that some of them have ERs, as they appropriately should, and some of those specialty hospitals do not. They might have a community hospital down the street that’s part of that health system that has an ER, but some of the specialty hospitals don’t necessarily have a dedicated ER.
I agree, that’s a valid concern. I would say, though, the question is, what are the scope of services in that hospital? Is an ER required? Community hospitals should have ERs. It makes sense also for a cardiac hospital to have one. If you’re running a total joint replacement factory, it might not make clinical sense.
Dr. Glatter: The patients who are treated at that hospital, if they do have emergent conditions, need to have board-certified emergency physicians treating them, in my view because I’m an ER physician. Having surgeons that are not emergency physicians staff a department at a specialty orthopedic hospital or, say, a cancer hospital is not acceptable from my standpoint. That›s my opinion and recommendation, coming from emergency medicine.
Dr. Miller: I would say that anesthesiologists are actually highly qualified in critical care. The question is about clinical decompensation; if you’re doing a procedure, you have an anesthesiologist right there who is capable of critical care. The function of the ER is to either serve as a window into the hospital for patient volume or to serve as a referral for emergent complaints.
Dr. Glatter: An anesthesiologist — I’ll take issue with that — does not have the training of an emergency physician in terms of scope of practice.
Dr. Miller: My anesthesiology colleagues would probably disagree for managing an emergency during an operating room case.
Dr. Glatter: Fair enough, but I think in the general sense. The other issue is that, in terms of emergent responses to patients that decompensate, when you have to transfer a patient, that violates Medicare requirements. How is that even a valid issue or argument if you’re going to have to transfer a patient from your specialty hospital? That happens. Again, I know that you’re saying these hospitals are completely independent and can function, stabilize patients, and treat emergencies, but that’s not the reality across the country, in my opinion.
Dr. Miller: I don’t think that’s the case for the physician-owned specialty cardiac hospitals, for starters. Many of those have ICUs in addition to operating rooms as a matter of routine in addition to ERs. I don’t think that’s the case for physician-owned community hospitals, which have ERs, ICUs, medicine floors, and surgical floors. Physician-owned community hospitals are around half the market. Of that remaining market, a significant percentage are cardiac hospitals. If you’re taking an issue with orthopedic surgical specialty hospitals, that’s a clinical operational question that can and should be answered.
I’d also posit that the nonprofit and for-profit hospital industries also operate specialty hospitals. Any of these questions, we shouldn’t just be asking about physician-owned facilities; we should be asking about them across ownership types, because we’re talking about scope of service and quality and safety. The ownership in that case doesn’t matter. The broader question is, are orthopedic surgical specialty hospitals owned by physicians, tax-exempt hospitals, or tax-paying hospitals? Is that a valid clinical business model? Is it safe? Does it meet Medicare conditions of participation? I would say that’s what that question is, because other ownership models do operate those facilities.
Dr. Glatter: You make some valid points, and I do agree on some of them. I think that, ultimately, these models of care, and certainly cost and quality, are issues. Again, it goes back to being able, in my opinion, to provide emergent care, which seems to me a very important issue.
Dr. Miller: I agree that providing emergent care is an issue. It›s an issue in any site of care. The hospital industry posits that all hospital outpatient departments (HOPDs) have emergent care. I can tell you, having worked in HOPDs (I›ve trained in them during residency), the response if something emergent happens is to either call 911 or wheel the patient down to the ER in a wheelchair or stretcher. I think that these hospital claims about emergency care coverage — these are important questions, but we should be asking them across all clinical settings and say what is the appropriate scope of care provided? What is the appropriate level of acuity and ability to provide emergent or critical care? That›s an important question regardless of ownership model across the entire industry.
Deeper Dive Into Data on Physician-Owned Hospitals
Dr. Glatter: We need to really focus on that. I’ll agree with you on that.
There was a March 2023 report from Dobson | DaVanzo. It showed that physician-owned hospitals had lower Medicaid, dual-eligible, and uncompensated care and charity care discharges than full-service acute care hospitals. Physician-owned hospitals had less than half the proportion of Medicaid discharges compared with non–physician-owned hospitals. They were also less likely to care for dual-eligible patients overall compared with non–physician-owned hospitals.
In addition, when COVID hit, the physician-owned hospitals overall — and again, there may be exceptions — were not equipped to handle these patient surges in the acute setting of a public health emergency. There was a hospital in Texas that did pivot that I’m aware of — Renaissance Hospital, which ramped up a long-term care facility to become a COVID hospital — but I think that’s the exception. I think this report raises some valid concerns; I’ll let you rebut that.
Dr. Miller: A couple of things. One, I am not aware that there’s any clear market evidence or a systematic study that shows that physician-owned hospitals had trouble responding to COVID. I don’t think that assertion has been proven. The study was funded by the hospital industry. First of all, it was not a peer-reviewed study; it was funded by an industry that paid a consulting firm. It doesn’t mean that we still shouldn’t read it, but that brings bias into question. The joke in Washington is, pick your favorite statistician or economist, and they can say what you want and have a battle of economists and statisticians.
For example, in that study, they didn’t include the entire ownership universe of physician-owned hospitals. If we go to the peer-reviewed literature, there’s a great 2015 BMJ paper showing that the Medicaid payer mix is actually the same between physician-owned hospitals vs not. The mix of patients by ethnicity — for example, think about African American patients — was the same. I would be more inclined to believe the peer-reviewed literature in BMJ as opposed to an industry-funded study that was not peer-reviewed and not independent and has methodological questions.
Dr. Glatter: Those data are 8 years old, so I’d like to see more recent data. It would be interesting, just as a follow-up to that, to see where the needle has moved — if it has, for that matter — in terms of Medicaid patients that you’re referring to.
Dr. Miller: I tend to be skeptical of all industry research, regardless of who published it, because they have an economic incentive. If they’re selecting certain age groups or excluding certain hospitals, that makes you wonder about the validity of the study. Your job as an industry-funded researcher is that, essentially, you’re being paid to look for an answer. It’s not necessarily an honest evaluation of the data.
Dr. Glatter: I want to bring up another point about the Hospital Readmissions Reduction Program (HRRP) and the data on how physician-owned hospitals compared with acute care hospitals that are non–physician-owned and have you comment on that. The Dobson | DaVanzo study called into question that physician-owned hospitals treat fewer patients who are dual-eligible, which we know.
Dr. Miller: I don’t think we do know that.
Dr. Glatter: There are data that point to that, again, looking at the studies.
Dr. Miller: I’m saying that’s a single study funded by industry as opposed to an independent, academic, peer-reviewed literature paper. That would be like saying, during the debate of the Inflation Reduction Act (IRA), that you should read the pharmaceutical industries research but take any of it at pure face value as factual. Yes, we should read it. Yes, we should evaluate it on its own merits. I think, again, appropriately, you need to be concerned when people have an economic incentive.
The question about the HRRP I’m going to take a little broader, because I think that program is unfair to the industry overall. There are many factors that drive hospital readmission. Whether Mrs Smith went home and ate potato chips and then took her Lasix, that’s very much outside of the hospital industry’s control, and there’s some evidence that the HRRP increases mortality in some patient populations.
In terms of a quality metric, it’s unfair to the industry. I think we took an operating process, internal metric for the hospital industry, turned it into a quality metric, and attached it to a financial bonus, which is an inappropriate policy decision.
Rethinking Ownership Models and Empowering Clinicians
Dr. Glatter: I agree with you on that. One thing I do want to bring up is that whether the physician-owned hospitals are subject to many of the quality measures that full-service, acute care hospitals are. That really is, I think, a broader context.
Dr. Miller: Fifty-five percent of physician-owned hospitals are full-service community hospitals, so I would say at least half the market is 100% subject to that.
Dr. Glatter: If only 50% are, that’s already an issue.
Dr. Miller: Cardiac specialty hospitals — which, as I said, nonprofit and for-profit hospital chains also operate — are also subject to the appropriate quality measures, readmissions, etc. Just because we don’t necessarily have the best quality measurement in the system in the country, it doesn’t mean that we shouldn’t allow care specialization. As I’d point out, if we’re concerned about specialty hospitals, the concern shouldn’t just be about physician-owned specialty hospitals; it should be about specialty hospitals by and large. Many health systems run cardiac specialty hospitals, cancer specialty hospitals, and orthopedic specialty hospitals. If we’re going to have a discussion about concerns there, it should be about the entire industry of specialty hospitals.
I think specialty hospitals serve an important role in society, allowing for specialization and exploiting in a positive way the volume-quality relationship. Whether those are owned by a for-profit publicly traded company, a tax-exempt facility, or physicians, I think that is an important way to have innovation and care delivery because frankly, we haven’t had much innovation in care delivery. Much of what we do in terms of how we practice clinically hasn’t really changed in the 50 years since my late father graduated from medical school. We still have rounds, we’re still taking notes, we’re still operating in the same way. Many processes are manual. We don’t have the mass production and mass customization of care that we need.
When you have a focused factory, it allows you to design care in a way that drives up quality, not just for the average patient but also the patients at the tail ends, because you have time to focus on that specific service line and that specific patient population.
Physician-owned community hospitals offer an important opportunity for a different employment model. I remember going to the dermatologist and the dermatologist was depressed, shuffling around the room, sad, and I asked him why. He said he didn’t really like his employer, and I said, “Why don’t you pick another one?” He’s like, “There are only two large health systems I can work for. They all have the same clinical practice environment and functionally the same value.”
Physicians are increasingly burned out. They face monopsony power in who purchases their labor. They have little control. They don’t want to go through five committees, seven administrators, and attend 25 meetings just to change a single small process in clinical operations. If you’re an owner operator, you have a much better ability to do it.
Frankly, when many facilities do well now, when they do well clinically and do well financially, who benefits? The hospital administration and the hospital executives. The doctors aren’t benefiting. The nurses aren’t benefiting. The CNA is not benefiting. The secretary is not benefiting. The custodian is not benefiting. Shouldn’t the workers have a right to own and operate the business and do well when the business does well serving the community? That puts me in the weird space of agreeing with both conservatives and progressives.
Dr. Glatter: I agree with you. I think an ownership stake is always attractive. It helps with retention of employed persons. There›s no question that, when they have a stake, when they have skin in the game, they feel more empowered. I will not argue with you about that.
Dr. Miller: We don’t have business models where workers have that option in healthcare. Like the National Academy of Medicine said, one of the key drivers of burnout is the externalization of the locus of control over clinical practice, and the current business operating models guarantee an externalization of the locus of control over clinical practice.
If you actually look at the recent American Medical Association (AMA) meeting, there was a resolution to ban the corporate practice of medicine. They wanted to go more toward the legal professions model where only physicians can own and operate care delivery.
Dr. Glatter: Well, I think the shift is certainly something that the AMA would like and physicians collectively would agree with. Having a better lifestyle and being able to have control are factors in burnout.
Dr. Miller: It’s not just doctors. I think nurses want a better lifestyle. The nurses are treated as interchangeable lines on a spreadsheet. The nurses are an integral part of our clinical team. Why don’t we work together as a clinical unit to build a better delivery system? What better way to do that than to have clinicians in charge of it, right?
My favorite bakery that’s about 30 minutes away is owned by a baker. It is not owned by a large tax-exempt corporation. It’s owned by an owner operator who takes pride in their work. I think that is something that the profession would do well to return to. When I was a resident, one of my colleagues was already planning their retirement. That’s how depressed they were.
I went into medicine to actually care for patients. I think that we can make the world a better place for our patients. What that means is not only treating them with drugs and devices, but also creating a delivery system where they don’t have to wander from lobby to lobby in a 200,000 square-foot facility, wait in line for hours on end, get bills 6 months later, and fill out endless paper forms over and over again.
All of these basic processes in healthcare delivery that are broken could have and should have been fixed — and have been fixed in almost every other industry. I had to replace one of my car tires because I had a flat tire. The local tire shop has an app, and it sends me SMS text messages telling me when my appointment is and when my car is ready. We have solved all of these problems in many other businesses.
We have not solved them in healthcare delivery because, one, we have massive monopolies that are raising prices, have lower quality, and deliver a crappy patient experience, and we have also subjugated the clinical worker into a corporate automaton. We are functionally drones. We don’t have the agency and the authority to improve clinical operations anymore. It’s really depressing, and we should have that option again.
I trust my doctor. I trust the nurses that I work with, and I would like them to help make clinical decisions in a financially responsible and a sensible operational manner. We need to empower our workforce in order to do that so we can recapture the value of what it means to be a clinician again.
The current model of corporate employment: massive scale, more administrators, more processes, more emails, more meetings, more PowerPoint decks, more federal subsidies. The hospital industry has choices. It can improve clinical operations. It can show up in Washington and lobby for increased subsidies. It can invest in the market and not pay taxes for the tax-exempt facilities. Obviously, it makes the logical choices as an economic actor to show up, lobby for increased subsidies, and then also invest in the stock market.
Improving clinical operations is hard. It hasn’t happened. The Bureau of Labor Statistics shows that the private community hospital industry has had flat labor productivity growth, on average, for the past 25 years, and for some years it even declined. This is totally atypical across the economy.
We have failed our clinicians, and most importantly, we have failed our patients. I’ve been sick. My relatives have been sick, waiting hours, not able to get appointments, and redoing forms. It’s a total disaster. It’s time and reasonable to try an alternative ownership and operating model. There are obviously problems. The problems can and should be addressed, but it doesn’t mean that we should have a statutory prohibition on professionals owning and operating their own business.
Dr. Glatter: There was a report that $500 million was saved by limiting or banning or putting a moratorium on physician-owned hospitals by the Congressional Budget Office.
Dr. Miller: Yes, I’m very aware of those data. I’d say that the CBO also is off by 50% on the estimation of the implementation of the Part D program. They overestimated the Affordable Care Act market enrollment by over 10 million people — again, around 50%. They also estimated that the CMS Innovation Center initially would be a savings. Now they’ve re-estimated it as a 10-year expenditure and it has actually cost the taxpayers money.
The CBO is not transparent about what its assumptions are or its analysis and methods. As a researcher, we have to publish our information. It has to go through peer review. I want to know what goes into that $500 million figure — what the assumptions are and what the model is. It’s hard to comment without knowing how they came up with it.
Dr. Glatter: The points you make are very valid. Physicians and nurses want a better lifestyle.
Dr. Miller: It’s not even a better lifestyle. It’s about having a say in how clinical operations work and helping make them better. We want the delivery system to work better. This is an opportunity for us to do so.
Dr. Glatter: That translates into technology: obviously, generative artificial intelligence (AI) coming into the forefront, as we know, and changing care delivery models as you’re referring to, which is going to happen. It’s going to be a slow process. I think that the evolution is happening and will happen, as you accurately described.
Dr. Miller: The other thing that’s different now vs 20 years ago is that managed care is here, there, and everywhere, as Dr Seuss would say. You have utilization review and prior authorization, which I’ve experienced as a patient and a physician, and boy, is it not a fun process. There’s a large amount of friction that needs to be improved. If we’re worried about induced demand or inappropriate utilization, we have managed care right there to help police bad behavior.
Reforming Healthcare Systems and Restoring Patient-Centric Focus
Dr. Glatter: If you were to come up with, say, three bullet points of how we can work our way out of this current morass of where our healthcare systems exist, where do you see the solutions or how can we make and effect change?
Dr. Miller: I’d say there are a couple of things. One is, let business models compete fairly on an equal playing field. Let the physician-owned hospital compete with the tax-exempt hospital and the nonprofit hospital. Put them on an equal playing field. We have things like 340B, which favors tax-exempt hospitals. For-profit or tax-paying hospitals are not able to participate in that. That doesn’t make any sense just from a public policy perspective. Tax-paying hospitals and physician-owned hospitals pay taxes on investments, but tax-exempt hospitals don’t. I think, in public policy, we need to equalize the playing field between business models. Let the best business model win.
The other thing we need to do is to encourage the adoption of technology. The physician will eventually be an arbiter of tech-driven or AI-driven tools. In fact, at some point, the standard of care might be to use those tools. Not using those tools would be seen as negligence. If you think about placing a jugular or central venous catheter, to not use ultrasound would be considered insane. Thirty years ago, to use ultrasound would be considered novel. I think technology and AI will get us to that point of helping make care more efficient and more customized.
Those are the two biggest interventions, I would say. Third, every time we have a conversation in public policy, we need to remember what it is to be a patient. The decision should be driven not around any one industry’s profitability, but what it is to be a patient and how we can make that experience less burdensome, less expensive, or in plain English, suck less.
Dr. Glatter: Safety net hospitals and critical access hospitals are part of this discussion that, yes, we want everything to, in an ideal world, function more efficiently and effectively, with less cost and less red tape. The safety net of our nation is struggling.
Dr. Miller: I 100% agree. The Cook County hospitals of the world are deserving of our support and, frankly, our gratitude. Facilities like that have huge burdens of patients with Medicaid. We also still have millions of uninsured patients. The neighborhoods that they serve are also poorer. I think facilities like that are deserving of public support.
I also think we need to clearly define what those hospitals are. One of the challenges I’ve realized as I waded into this space is that market definitions of what a service market is for a hospital, its specialty type or what a safety net hospital is need to be more clearly defined because those facilities 100% are deserving of our support. We just need to be clear about what they are.
Regarding critical access hospitals, when you practice in a rural area, you have to think differently about care delivery. I’d say many of the rural systems are highly creative in how they structure clinical operations. Before the public health emergency, during the COVID pandemic, when we had a massive change in telehealth, rural hospitals were using — within the very narrow confines — as much telehealth as they could and should.
Rural hospitals also make greater use of nurse practitioners (NPs) and physician assistants (PAs). For many of the specialty services, I remember, your first call was an NP or a PA because the physician was downstairs doing procedures. They’d come up and assess the patient before the procedure, but most of your consult questions were answered by the NP or PA. I’m not saying that’s the model we should use nationwide, but that rural systems are highly innovative and creative; they’re deserving of our time, attention, and support, and frankly, we can learn from them.
Dr. Glatter: I want to thank you for your time and your expertise in this area. We’ll see how the congressional hearings affect the industry as a whole, how the needle moves, and whether the ban or moratorium on physician-owned hospitals continues to exist going forward.
Dr. Miller: I appreciate you having me. The hospital industry is one of the most important industries for health care. This is a time of inflection, right? We need to go back to the value of what it means to be a clinician and serve patients. Hospitals need to reorient themselves around that core concern. How do we help support clinicians — doctors, nurses, pharmacists, whomever it is — in serving patients? Hospitals have become too corporate, so I think that this is an expected pushback.
Dr. Glatter: Again, I want to thank you for your time. This was a very important discussion. Thank you for your expertise.
Robert D. Glatter, MD, is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is a medical advisor for Medscape and hosts the Hot Topics in EM series. He disclosed no relevant financial relationships.Brian J. Miller, MD, MBA, MPH, is a hospitalist and an assistant professor of medicine at the Johns Hopkins University School of Medicine. He is also a nonresident fellow at the American Enterprise Institute. From 2014 to 2017, Dr. Miller worked at four federal regulatory agencies: Federal Trade Commission (FTC), Federal Communications Commission (FCC), Centers for Medicare & Medicaid Services (CMS), and the Food & Drug Administration (FDA). Dr. Miller disclosed ties with the Medicare Payment Advisory Commission.
A version of this article appeared on Medscape.com.
This discussion was recorded on November 16, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today is Dr. Brian J. Miller, a hospitalist with Johns Hopkins University School of Medicine and a health policy expert, to discuss the current and renewed interest in physician-owned hospitals.
Welcome, Dr. Miller. It’s a pleasure to have you join me today.
Brian J. Miller, MD, MBA, MPH: Thank you for having me.
History and Controversies Surrounding Physician-Owned Hospitals
Dr. Glatter: I want to start off by having you describe the history associated with the moratorium on new physician-owned hospitals in 2010 that’s related ultimately to the Affordable Care Act, but also, the current and renewed media interest in physician-owned hospitals that’s linked to recent congressional hearings last month.
Dr. Miller: Thank you. I should note that my views are my own and don’t represent those of Hopkins or the American Enterprise Institute, where I’m a nonresident fellow nor the Medicare Payment Advisory Commission, of which I’m a Commissioner.
The story about physician-owned hospitals is an interesting one. Hospitals turned into health systems in the 1980s and 1990s, and physicians started to shift purely from an independent model into a more organized group practice or employed model. Physicians realized that they wanted an alternative operating arrangement. You want a choice of how you practice and what your employment is. And as community hospitals started to buy physicians and also establish their own physician groups de novo, physicians opened physician-owned hospitals.
Physician-owned hospitals fell into a couple of buckets. One is what we call community hospitals, or what the antitrust lawyers would call general acute care hospitals: those offering emergency room (ER) services, labor and delivery, primary care, general surgery — the whole regular gamut, except that some of the owners were physicians.
The other half of the marketplace ended up being specialty hospitals: those built around a specific medical specialty and series of procedures and chronic care. For example, cardiac hospitals often do CABG, TAVR, maybe abdominal aortic aneurysm (triple A) repairs, and they have cardiology clinics, cath labs, a cardiac intensive care unit (ICU), ER, etc. There were also orthopedic surgical specialty hospitals, which were sort of like an ambulatory surgery center (ASC) plus several beds. Then there were general surgical specialty hospitals. At one point, there were some women’s health–focused specialty hospitals.
The hospital industry, of course, as you can understand, didn’t exactly like this. They had a series of concerns about what we would historically call cherry-picking or lemon-dropping of patients. They were worried that physician-owned facilities didn’t want to serve public payer patients, and there was a whole series of reports and investigations.
Around the time the Affordable Care Act passed, the hospital industry had many concerns about physician-owned specialty hospitals, and there was a moratorium as part of the 2003 Medicare Modernization Act. As part of the bargaining over the hospital industry support for the Affordable Care Act, they traded their support for, among other things, their number one priority, which is a statutory prohibition on new or expanded physician-owned hospitals from participating in Medicare. That included both physician-owned community hospitals and physician-owned specialty hospitals.
Dr. Glatter: That was part of the impetus to prevent physicians from referring patients where they had an ownership stake. Certainly, hospitals can be owned by attorneys and nonprofit organizations, and certainly, ASCs can be owned by physicians. There is an ongoing issue in terms of physicians not being able to have an ownership stake. In terms of equity ownership, we know that certain other models allow this, but basically, it sounds like this is an issue with Medicare. That seems to be the crux of it, correct?
Dr. Miller: Yes. I would also add that it’s interesting when we look at other professions. When we look at lawyers, nonlawyers are actually not allowed to own an equity stake in a law practice. In many other professions, you either have corporate ownership or professional ownership, or the alternative is you have only professional ownership. I would say the hospital industry is one of the few areas where professional ownership not only is not allowed, but also is statutorily prohibited functionally through the Medicare program.
Unveiling the Dynamics of Hospital Ownership
Dr. Glatter: A recent study done by two PhDs looked at 2019 data on 20 of the most expensive diagnosis-related groups (DRGs). It examined the cost savings, and we’re talking over $1 billion in expenditures when you look at the data from general acute care hospitals vs physician-owned hospitals. This is what appears to me to be a key driver of the push to loosen restrictions on physician-owned hospitals. Isn’t that correct?
Dr. Miller: I would say that’s one of many components. There’s more history to this issue. I remember sitting at a think tank talking to someone several years ago about hospital consolidation as an issue. We went through the usual levers that us policy wonks go through. We talked about antitrust enforcement, certificate of need, rising hospital costs from consolidation, lower quality (or at least no quality gains, as shown by a New England Journal of Medicine study), and decrements in patient experience that result from the diseconomies of scale. They sort of pooh-poohed many of the policy ideas. They basically said that there was no hope for hospital consolidation as an issue.
Well, what about physician ownership? I started with my research team to comb through the literature and found a variety of studies — some of which were sort of entertaining, because they’d do things like study physician-owned specialty hospitals, nonprofit-owned specialty hospitals, and for-profit specialty hospitals and compare them with nonprofit or for-profit community hospitals, and then say physician-owned hospitals that were specialty were bad.
They mixed ownership and service markets right there in so many ways, I’m not sure where to start. My team did a systematic review of around 30 years of research, looking at the evidence base in this space. We found a couple of things.
We found that physician-owned community hospitals did not have a cost or quality difference, meaning that there was no definitive evidence that the physician-owned community hospitals were cheaper based on historical evidence, which was very old. That means there’s not specific harm from them. When you permit market entry for community hospitals, that promotes competition, which results in lower prices and higher quality.
Then we also looked at the specialty hospital markets — surgical specialty hospitals, orthopedic surgical specialty hospitals, and cardiac hospitals. We noted for cardiac hospitals, there wasn’t clear evidence about cost savings, but there was definitive evidence of higher quality, from things like 30-day mortality for significant procedures like treatment of acute MI, triple A repair, stuff like that.
For orthopedic surgical specialty hospitals, we noted lower costs and higher quality, which again fits with operationally what we would know. If you have a facility that’s doing 20 total hips a day, you’re creating a focused factory. Just like if you think about it for interventional cardiology, your boards have a minimum number of procedures that you have to do to stay certified because we know about the volume-quality relationship.
Then we looked at general surgical specialty hospitals. There wasn’t enough evidence to make a conclusive thought about costs, and there was a clear trend toward higher quality. I would say this recent study is important, but there is a whole bunch of other literature out there, too.
Exploring the Scope of Emergency Care in Physician-Owned Hospitals
Dr. Glatter: Certainly, your colleague Wang from Johns Hopkins has done important research in this sector. The paper, “Reconsidering the Ban on Physician-Owned Hospitals to Combat Consolidation,” by you and several colleagues, mentions and highlights the issues that you just described. I understand that it’s going to be published in the NYU Journal of Legislation and Public Policy.
One thing I want to bring up — and this is an important issue — is that the risk for patients has been talked about by the American Hospital Association and the Federation of American Hospitals, in terms of limited or no emergency services at such physician-owned hospitals and having to call 911 when patients need emergent care or stabilization. That’s been the rebuttal, along with an Office of Inspector General (OIG) report from 2008. Almost, I guess, three quarters of the patients that needed emergent care got this at publicly funded hospitals.
Dr. Miller: I’m familiar with the argument about emergency care. If you actually go and look at it, it differs by specialty market. Physician-owned community hospitals have ERs because that’s how they get their business. If you are running a hospital medicine floor, a general surgical specialty floor, you have a labor delivery unit, a primary care clinic, and a cardiology clinic. You have all the things that all the other hospitals have. The physician-owned community hospitals almost uniformly have an ER.
When you look at the physician-owned specialty hospitals, it’s a little more granular. If you look at the cardiac hospitals, they have ERs. They also have cardiac ICUs, operating rooms, etc. The area where the hospital industry had concerns — which I think is valid to point out — is that physician-owned orthopedic surgical specialty hospitals don’t have ERs. But this makes sense because of what that hospital functionally is: a factory for whatever the scope of procedures is, be it joint replacements or shoulder arthroscopy. The orthopedic surgical specialty hospital is like an ASC plus several hospital beds. Many of those did not have ERs because clinically it didn’t make sense.
What’s interesting, though, is that the hospital industry also operates specialty hospitals. If you go into many of the large systems, they have cardiac specialty hospitals and cancer specialty hospitals. I would say that some of them have ERs, as they appropriately should, and some of those specialty hospitals do not. They might have a community hospital down the street that’s part of that health system that has an ER, but some of the specialty hospitals don’t necessarily have a dedicated ER.
I agree, that’s a valid concern. I would say, though, the question is, what are the scope of services in that hospital? Is an ER required? Community hospitals should have ERs. It makes sense also for a cardiac hospital to have one. If you’re running a total joint replacement factory, it might not make clinical sense.
Dr. Glatter: The patients who are treated at that hospital, if they do have emergent conditions, need to have board-certified emergency physicians treating them, in my view because I’m an ER physician. Having surgeons that are not emergency physicians staff a department at a specialty orthopedic hospital or, say, a cancer hospital is not acceptable from my standpoint. That›s my opinion and recommendation, coming from emergency medicine.
Dr. Miller: I would say that anesthesiologists are actually highly qualified in critical care. The question is about clinical decompensation; if you’re doing a procedure, you have an anesthesiologist right there who is capable of critical care. The function of the ER is to either serve as a window into the hospital for patient volume or to serve as a referral for emergent complaints.
Dr. Glatter: An anesthesiologist — I’ll take issue with that — does not have the training of an emergency physician in terms of scope of practice.
Dr. Miller: My anesthesiology colleagues would probably disagree for managing an emergency during an operating room case.
Dr. Glatter: Fair enough, but I think in the general sense. The other issue is that, in terms of emergent responses to patients that decompensate, when you have to transfer a patient, that violates Medicare requirements. How is that even a valid issue or argument if you’re going to have to transfer a patient from your specialty hospital? That happens. Again, I know that you’re saying these hospitals are completely independent and can function, stabilize patients, and treat emergencies, but that’s not the reality across the country, in my opinion.
Dr. Miller: I don’t think that’s the case for the physician-owned specialty cardiac hospitals, for starters. Many of those have ICUs in addition to operating rooms as a matter of routine in addition to ERs. I don’t think that’s the case for physician-owned community hospitals, which have ERs, ICUs, medicine floors, and surgical floors. Physician-owned community hospitals are around half the market. Of that remaining market, a significant percentage are cardiac hospitals. If you’re taking an issue with orthopedic surgical specialty hospitals, that’s a clinical operational question that can and should be answered.
I’d also posit that the nonprofit and for-profit hospital industries also operate specialty hospitals. Any of these questions, we shouldn’t just be asking about physician-owned facilities; we should be asking about them across ownership types, because we’re talking about scope of service and quality and safety. The ownership in that case doesn’t matter. The broader question is, are orthopedic surgical specialty hospitals owned by physicians, tax-exempt hospitals, or tax-paying hospitals? Is that a valid clinical business model? Is it safe? Does it meet Medicare conditions of participation? I would say that’s what that question is, because other ownership models do operate those facilities.
Dr. Glatter: You make some valid points, and I do agree on some of them. I think that, ultimately, these models of care, and certainly cost and quality, are issues. Again, it goes back to being able, in my opinion, to provide emergent care, which seems to me a very important issue.
Dr. Miller: I agree that providing emergent care is an issue. It›s an issue in any site of care. The hospital industry posits that all hospital outpatient departments (HOPDs) have emergent care. I can tell you, having worked in HOPDs (I›ve trained in them during residency), the response if something emergent happens is to either call 911 or wheel the patient down to the ER in a wheelchair or stretcher. I think that these hospital claims about emergency care coverage — these are important questions, but we should be asking them across all clinical settings and say what is the appropriate scope of care provided? What is the appropriate level of acuity and ability to provide emergent or critical care? That›s an important question regardless of ownership model across the entire industry.
Deeper Dive Into Data on Physician-Owned Hospitals
Dr. Glatter: We need to really focus on that. I’ll agree with you on that.
There was a March 2023 report from Dobson | DaVanzo. It showed that physician-owned hospitals had lower Medicaid, dual-eligible, and uncompensated care and charity care discharges than full-service acute care hospitals. Physician-owned hospitals had less than half the proportion of Medicaid discharges compared with non–physician-owned hospitals. They were also less likely to care for dual-eligible patients overall compared with non–physician-owned hospitals.
In addition, when COVID hit, the physician-owned hospitals overall — and again, there may be exceptions — were not equipped to handle these patient surges in the acute setting of a public health emergency. There was a hospital in Texas that did pivot that I’m aware of — Renaissance Hospital, which ramped up a long-term care facility to become a COVID hospital — but I think that’s the exception. I think this report raises some valid concerns; I’ll let you rebut that.
Dr. Miller: A couple of things. One, I am not aware that there’s any clear market evidence or a systematic study that shows that physician-owned hospitals had trouble responding to COVID. I don’t think that assertion has been proven. The study was funded by the hospital industry. First of all, it was not a peer-reviewed study; it was funded by an industry that paid a consulting firm. It doesn’t mean that we still shouldn’t read it, but that brings bias into question. The joke in Washington is, pick your favorite statistician or economist, and they can say what you want and have a battle of economists and statisticians.
For example, in that study, they didn’t include the entire ownership universe of physician-owned hospitals. If we go to the peer-reviewed literature, there’s a great 2015 BMJ paper showing that the Medicaid payer mix is actually the same between physician-owned hospitals vs not. The mix of patients by ethnicity — for example, think about African American patients — was the same. I would be more inclined to believe the peer-reviewed literature in BMJ as opposed to an industry-funded study that was not peer-reviewed and not independent and has methodological questions.
Dr. Glatter: Those data are 8 years old, so I’d like to see more recent data. It would be interesting, just as a follow-up to that, to see where the needle has moved — if it has, for that matter — in terms of Medicaid patients that you’re referring to.
Dr. Miller: I tend to be skeptical of all industry research, regardless of who published it, because they have an economic incentive. If they’re selecting certain age groups or excluding certain hospitals, that makes you wonder about the validity of the study. Your job as an industry-funded researcher is that, essentially, you’re being paid to look for an answer. It’s not necessarily an honest evaluation of the data.
Dr. Glatter: I want to bring up another point about the Hospital Readmissions Reduction Program (HRRP) and the data on how physician-owned hospitals compared with acute care hospitals that are non–physician-owned and have you comment on that. The Dobson | DaVanzo study called into question that physician-owned hospitals treat fewer patients who are dual-eligible, which we know.
Dr. Miller: I don’t think we do know that.
Dr. Glatter: There are data that point to that, again, looking at the studies.
Dr. Miller: I’m saying that’s a single study funded by industry as opposed to an independent, academic, peer-reviewed literature paper. That would be like saying, during the debate of the Inflation Reduction Act (IRA), that you should read the pharmaceutical industries research but take any of it at pure face value as factual. Yes, we should read it. Yes, we should evaluate it on its own merits. I think, again, appropriately, you need to be concerned when people have an economic incentive.
The question about the HRRP I’m going to take a little broader, because I think that program is unfair to the industry overall. There are many factors that drive hospital readmission. Whether Mrs Smith went home and ate potato chips and then took her Lasix, that’s very much outside of the hospital industry’s control, and there’s some evidence that the HRRP increases mortality in some patient populations.
In terms of a quality metric, it’s unfair to the industry. I think we took an operating process, internal metric for the hospital industry, turned it into a quality metric, and attached it to a financial bonus, which is an inappropriate policy decision.
Rethinking Ownership Models and Empowering Clinicians
Dr. Glatter: I agree with you on that. One thing I do want to bring up is that whether the physician-owned hospitals are subject to many of the quality measures that full-service, acute care hospitals are. That really is, I think, a broader context.
Dr. Miller: Fifty-five percent of physician-owned hospitals are full-service community hospitals, so I would say at least half the market is 100% subject to that.
Dr. Glatter: If only 50% are, that’s already an issue.
Dr. Miller: Cardiac specialty hospitals — which, as I said, nonprofit and for-profit hospital chains also operate — are also subject to the appropriate quality measures, readmissions, etc. Just because we don’t necessarily have the best quality measurement in the system in the country, it doesn’t mean that we shouldn’t allow care specialization. As I’d point out, if we’re concerned about specialty hospitals, the concern shouldn’t just be about physician-owned specialty hospitals; it should be about specialty hospitals by and large. Many health systems run cardiac specialty hospitals, cancer specialty hospitals, and orthopedic specialty hospitals. If we’re going to have a discussion about concerns there, it should be about the entire industry of specialty hospitals.
I think specialty hospitals serve an important role in society, allowing for specialization and exploiting in a positive way the volume-quality relationship. Whether those are owned by a for-profit publicly traded company, a tax-exempt facility, or physicians, I think that is an important way to have innovation and care delivery because frankly, we haven’t had much innovation in care delivery. Much of what we do in terms of how we practice clinically hasn’t really changed in the 50 years since my late father graduated from medical school. We still have rounds, we’re still taking notes, we’re still operating in the same way. Many processes are manual. We don’t have the mass production and mass customization of care that we need.
When you have a focused factory, it allows you to design care in a way that drives up quality, not just for the average patient but also the patients at the tail ends, because you have time to focus on that specific service line and that specific patient population.
Physician-owned community hospitals offer an important opportunity for a different employment model. I remember going to the dermatologist and the dermatologist was depressed, shuffling around the room, sad, and I asked him why. He said he didn’t really like his employer, and I said, “Why don’t you pick another one?” He’s like, “There are only two large health systems I can work for. They all have the same clinical practice environment and functionally the same value.”
Physicians are increasingly burned out. They face monopsony power in who purchases their labor. They have little control. They don’t want to go through five committees, seven administrators, and attend 25 meetings just to change a single small process in clinical operations. If you’re an owner operator, you have a much better ability to do it.
Frankly, when many facilities do well now, when they do well clinically and do well financially, who benefits? The hospital administration and the hospital executives. The doctors aren’t benefiting. The nurses aren’t benefiting. The CNA is not benefiting. The secretary is not benefiting. The custodian is not benefiting. Shouldn’t the workers have a right to own and operate the business and do well when the business does well serving the community? That puts me in the weird space of agreeing with both conservatives and progressives.
Dr. Glatter: I agree with you. I think an ownership stake is always attractive. It helps with retention of employed persons. There›s no question that, when they have a stake, when they have skin in the game, they feel more empowered. I will not argue with you about that.
Dr. Miller: We don’t have business models where workers have that option in healthcare. Like the National Academy of Medicine said, one of the key drivers of burnout is the externalization of the locus of control over clinical practice, and the current business operating models guarantee an externalization of the locus of control over clinical practice.
If you actually look at the recent American Medical Association (AMA) meeting, there was a resolution to ban the corporate practice of medicine. They wanted to go more toward the legal professions model where only physicians can own and operate care delivery.
Dr. Glatter: Well, I think the shift is certainly something that the AMA would like and physicians collectively would agree with. Having a better lifestyle and being able to have control are factors in burnout.
Dr. Miller: It’s not just doctors. I think nurses want a better lifestyle. The nurses are treated as interchangeable lines on a spreadsheet. The nurses are an integral part of our clinical team. Why don’t we work together as a clinical unit to build a better delivery system? What better way to do that than to have clinicians in charge of it, right?
My favorite bakery that’s about 30 minutes away is owned by a baker. It is not owned by a large tax-exempt corporation. It’s owned by an owner operator who takes pride in their work. I think that is something that the profession would do well to return to. When I was a resident, one of my colleagues was already planning their retirement. That’s how depressed they were.
I went into medicine to actually care for patients. I think that we can make the world a better place for our patients. What that means is not only treating them with drugs and devices, but also creating a delivery system where they don’t have to wander from lobby to lobby in a 200,000 square-foot facility, wait in line for hours on end, get bills 6 months later, and fill out endless paper forms over and over again.
All of these basic processes in healthcare delivery that are broken could have and should have been fixed — and have been fixed in almost every other industry. I had to replace one of my car tires because I had a flat tire. The local tire shop has an app, and it sends me SMS text messages telling me when my appointment is and when my car is ready. We have solved all of these problems in many other businesses.
We have not solved them in healthcare delivery because, one, we have massive monopolies that are raising prices, have lower quality, and deliver a crappy patient experience, and we have also subjugated the clinical worker into a corporate automaton. We are functionally drones. We don’t have the agency and the authority to improve clinical operations anymore. It’s really depressing, and we should have that option again.
I trust my doctor. I trust the nurses that I work with, and I would like them to help make clinical decisions in a financially responsible and a sensible operational manner. We need to empower our workforce in order to do that so we can recapture the value of what it means to be a clinician again.
The current model of corporate employment: massive scale, more administrators, more processes, more emails, more meetings, more PowerPoint decks, more federal subsidies. The hospital industry has choices. It can improve clinical operations. It can show up in Washington and lobby for increased subsidies. It can invest in the market and not pay taxes for the tax-exempt facilities. Obviously, it makes the logical choices as an economic actor to show up, lobby for increased subsidies, and then also invest in the stock market.
Improving clinical operations is hard. It hasn’t happened. The Bureau of Labor Statistics shows that the private community hospital industry has had flat labor productivity growth, on average, for the past 25 years, and for some years it even declined. This is totally atypical across the economy.
We have failed our clinicians, and most importantly, we have failed our patients. I’ve been sick. My relatives have been sick, waiting hours, not able to get appointments, and redoing forms. It’s a total disaster. It’s time and reasonable to try an alternative ownership and operating model. There are obviously problems. The problems can and should be addressed, but it doesn’t mean that we should have a statutory prohibition on professionals owning and operating their own business.
Dr. Glatter: There was a report that $500 million was saved by limiting or banning or putting a moratorium on physician-owned hospitals by the Congressional Budget Office.
Dr. Miller: Yes, I’m very aware of those data. I’d say that the CBO also is off by 50% on the estimation of the implementation of the Part D program. They overestimated the Affordable Care Act market enrollment by over 10 million people — again, around 50%. They also estimated that the CMS Innovation Center initially would be a savings. Now they’ve re-estimated it as a 10-year expenditure and it has actually cost the taxpayers money.
The CBO is not transparent about what its assumptions are or its analysis and methods. As a researcher, we have to publish our information. It has to go through peer review. I want to know what goes into that $500 million figure — what the assumptions are and what the model is. It’s hard to comment without knowing how they came up with it.
Dr. Glatter: The points you make are very valid. Physicians and nurses want a better lifestyle.
Dr. Miller: It’s not even a better lifestyle. It’s about having a say in how clinical operations work and helping make them better. We want the delivery system to work better. This is an opportunity for us to do so.
Dr. Glatter: That translates into technology: obviously, generative artificial intelligence (AI) coming into the forefront, as we know, and changing care delivery models as you’re referring to, which is going to happen. It’s going to be a slow process. I think that the evolution is happening and will happen, as you accurately described.
Dr. Miller: The other thing that’s different now vs 20 years ago is that managed care is here, there, and everywhere, as Dr Seuss would say. You have utilization review and prior authorization, which I’ve experienced as a patient and a physician, and boy, is it not a fun process. There’s a large amount of friction that needs to be improved. If we’re worried about induced demand or inappropriate utilization, we have managed care right there to help police bad behavior.
Reforming Healthcare Systems and Restoring Patient-Centric Focus
Dr. Glatter: If you were to come up with, say, three bullet points of how we can work our way out of this current morass of where our healthcare systems exist, where do you see the solutions or how can we make and effect change?
Dr. Miller: I’d say there are a couple of things. One is, let business models compete fairly on an equal playing field. Let the physician-owned hospital compete with the tax-exempt hospital and the nonprofit hospital. Put them on an equal playing field. We have things like 340B, which favors tax-exempt hospitals. For-profit or tax-paying hospitals are not able to participate in that. That doesn’t make any sense just from a public policy perspective. Tax-paying hospitals and physician-owned hospitals pay taxes on investments, but tax-exempt hospitals don’t. I think, in public policy, we need to equalize the playing field between business models. Let the best business model win.
The other thing we need to do is to encourage the adoption of technology. The physician will eventually be an arbiter of tech-driven or AI-driven tools. In fact, at some point, the standard of care might be to use those tools. Not using those tools would be seen as negligence. If you think about placing a jugular or central venous catheter, to not use ultrasound would be considered insane. Thirty years ago, to use ultrasound would be considered novel. I think technology and AI will get us to that point of helping make care more efficient and more customized.
Those are the two biggest interventions, I would say. Third, every time we have a conversation in public policy, we need to remember what it is to be a patient. The decision should be driven not around any one industry’s profitability, but what it is to be a patient and how we can make that experience less burdensome, less expensive, or in plain English, suck less.
Dr. Glatter: Safety net hospitals and critical access hospitals are part of this discussion that, yes, we want everything to, in an ideal world, function more efficiently and effectively, with less cost and less red tape. The safety net of our nation is struggling.
Dr. Miller: I 100% agree. The Cook County hospitals of the world are deserving of our support and, frankly, our gratitude. Facilities like that have huge burdens of patients with Medicaid. We also still have millions of uninsured patients. The neighborhoods that they serve are also poorer. I think facilities like that are deserving of public support.
I also think we need to clearly define what those hospitals are. One of the challenges I’ve realized as I waded into this space is that market definitions of what a service market is for a hospital, its specialty type or what a safety net hospital is need to be more clearly defined because those facilities 100% are deserving of our support. We just need to be clear about what they are.
Regarding critical access hospitals, when you practice in a rural area, you have to think differently about care delivery. I’d say many of the rural systems are highly creative in how they structure clinical operations. Before the public health emergency, during the COVID pandemic, when we had a massive change in telehealth, rural hospitals were using — within the very narrow confines — as much telehealth as they could and should.
Rural hospitals also make greater use of nurse practitioners (NPs) and physician assistants (PAs). For many of the specialty services, I remember, your first call was an NP or a PA because the physician was downstairs doing procedures. They’d come up and assess the patient before the procedure, but most of your consult questions were answered by the NP or PA. I’m not saying that’s the model we should use nationwide, but that rural systems are highly innovative and creative; they’re deserving of our time, attention, and support, and frankly, we can learn from them.
Dr. Glatter: I want to thank you for your time and your expertise in this area. We’ll see how the congressional hearings affect the industry as a whole, how the needle moves, and whether the ban or moratorium on physician-owned hospitals continues to exist going forward.
Dr. Miller: I appreciate you having me. The hospital industry is one of the most important industries for health care. This is a time of inflection, right? We need to go back to the value of what it means to be a clinician and serve patients. Hospitals need to reorient themselves around that core concern. How do we help support clinicians — doctors, nurses, pharmacists, whomever it is — in serving patients? Hospitals have become too corporate, so I think that this is an expected pushback.
Dr. Glatter: Again, I want to thank you for your time. This was a very important discussion. Thank you for your expertise.
Robert D. Glatter, MD, is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is a medical advisor for Medscape and hosts the Hot Topics in EM series. He disclosed no relevant financial relationships.Brian J. Miller, MD, MBA, MPH, is a hospitalist and an assistant professor of medicine at the Johns Hopkins University School of Medicine. He is also a nonresident fellow at the American Enterprise Institute. From 2014 to 2017, Dr. Miller worked at four federal regulatory agencies: Federal Trade Commission (FTC), Federal Communications Commission (FCC), Centers for Medicare & Medicaid Services (CMS), and the Food & Drug Administration (FDA). Dr. Miller disclosed ties with the Medicare Payment Advisory Commission.
A version of this article appeared on Medscape.com.
This discussion was recorded on November 16, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today is Dr. Brian J. Miller, a hospitalist with Johns Hopkins University School of Medicine and a health policy expert, to discuss the current and renewed interest in physician-owned hospitals.
Welcome, Dr. Miller. It’s a pleasure to have you join me today.
Brian J. Miller, MD, MBA, MPH: Thank you for having me.
History and Controversies Surrounding Physician-Owned Hospitals
Dr. Glatter: I want to start off by having you describe the history associated with the moratorium on new physician-owned hospitals in 2010 that’s related ultimately to the Affordable Care Act, but also, the current and renewed media interest in physician-owned hospitals that’s linked to recent congressional hearings last month.
Dr. Miller: Thank you. I should note that my views are my own and don’t represent those of Hopkins or the American Enterprise Institute, where I’m a nonresident fellow nor the Medicare Payment Advisory Commission, of which I’m a Commissioner.
The story about physician-owned hospitals is an interesting one. Hospitals turned into health systems in the 1980s and 1990s, and physicians started to shift purely from an independent model into a more organized group practice or employed model. Physicians realized that they wanted an alternative operating arrangement. You want a choice of how you practice and what your employment is. And as community hospitals started to buy physicians and also establish their own physician groups de novo, physicians opened physician-owned hospitals.
Physician-owned hospitals fell into a couple of buckets. One is what we call community hospitals, or what the antitrust lawyers would call general acute care hospitals: those offering emergency room (ER) services, labor and delivery, primary care, general surgery — the whole regular gamut, except that some of the owners were physicians.
The other half of the marketplace ended up being specialty hospitals: those built around a specific medical specialty and series of procedures and chronic care. For example, cardiac hospitals often do CABG, TAVR, maybe abdominal aortic aneurysm (triple A) repairs, and they have cardiology clinics, cath labs, a cardiac intensive care unit (ICU), ER, etc. There were also orthopedic surgical specialty hospitals, which were sort of like an ambulatory surgery center (ASC) plus several beds. Then there were general surgical specialty hospitals. At one point, there were some women’s health–focused specialty hospitals.
The hospital industry, of course, as you can understand, didn’t exactly like this. They had a series of concerns about what we would historically call cherry-picking or lemon-dropping of patients. They were worried that physician-owned facilities didn’t want to serve public payer patients, and there was a whole series of reports and investigations.
Around the time the Affordable Care Act passed, the hospital industry had many concerns about physician-owned specialty hospitals, and there was a moratorium as part of the 2003 Medicare Modernization Act. As part of the bargaining over the hospital industry support for the Affordable Care Act, they traded their support for, among other things, their number one priority, which is a statutory prohibition on new or expanded physician-owned hospitals from participating in Medicare. That included both physician-owned community hospitals and physician-owned specialty hospitals.
Dr. Glatter: That was part of the impetus to prevent physicians from referring patients where they had an ownership stake. Certainly, hospitals can be owned by attorneys and nonprofit organizations, and certainly, ASCs can be owned by physicians. There is an ongoing issue in terms of physicians not being able to have an ownership stake. In terms of equity ownership, we know that certain other models allow this, but basically, it sounds like this is an issue with Medicare. That seems to be the crux of it, correct?
Dr. Miller: Yes. I would also add that it’s interesting when we look at other professions. When we look at lawyers, nonlawyers are actually not allowed to own an equity stake in a law practice. In many other professions, you either have corporate ownership or professional ownership, or the alternative is you have only professional ownership. I would say the hospital industry is one of the few areas where professional ownership not only is not allowed, but also is statutorily prohibited functionally through the Medicare program.
Unveiling the Dynamics of Hospital Ownership
Dr. Glatter: A recent study done by two PhDs looked at 2019 data on 20 of the most expensive diagnosis-related groups (DRGs). It examined the cost savings, and we’re talking over $1 billion in expenditures when you look at the data from general acute care hospitals vs physician-owned hospitals. This is what appears to me to be a key driver of the push to loosen restrictions on physician-owned hospitals. Isn’t that correct?
Dr. Miller: I would say that’s one of many components. There’s more history to this issue. I remember sitting at a think tank talking to someone several years ago about hospital consolidation as an issue. We went through the usual levers that us policy wonks go through. We talked about antitrust enforcement, certificate of need, rising hospital costs from consolidation, lower quality (or at least no quality gains, as shown by a New England Journal of Medicine study), and decrements in patient experience that result from the diseconomies of scale. They sort of pooh-poohed many of the policy ideas. They basically said that there was no hope for hospital consolidation as an issue.
Well, what about physician ownership? I started with my research team to comb through the literature and found a variety of studies — some of which were sort of entertaining, because they’d do things like study physician-owned specialty hospitals, nonprofit-owned specialty hospitals, and for-profit specialty hospitals and compare them with nonprofit or for-profit community hospitals, and then say physician-owned hospitals that were specialty were bad.
They mixed ownership and service markets right there in so many ways, I’m not sure where to start. My team did a systematic review of around 30 years of research, looking at the evidence base in this space. We found a couple of things.
We found that physician-owned community hospitals did not have a cost or quality difference, meaning that there was no definitive evidence that the physician-owned community hospitals were cheaper based on historical evidence, which was very old. That means there’s not specific harm from them. When you permit market entry for community hospitals, that promotes competition, which results in lower prices and higher quality.
Then we also looked at the specialty hospital markets — surgical specialty hospitals, orthopedic surgical specialty hospitals, and cardiac hospitals. We noted for cardiac hospitals, there wasn’t clear evidence about cost savings, but there was definitive evidence of higher quality, from things like 30-day mortality for significant procedures like treatment of acute MI, triple A repair, stuff like that.
For orthopedic surgical specialty hospitals, we noted lower costs and higher quality, which again fits with operationally what we would know. If you have a facility that’s doing 20 total hips a day, you’re creating a focused factory. Just like if you think about it for interventional cardiology, your boards have a minimum number of procedures that you have to do to stay certified because we know about the volume-quality relationship.
Then we looked at general surgical specialty hospitals. There wasn’t enough evidence to make a conclusive thought about costs, and there was a clear trend toward higher quality. I would say this recent study is important, but there is a whole bunch of other literature out there, too.
Exploring the Scope of Emergency Care in Physician-Owned Hospitals
Dr. Glatter: Certainly, your colleague Wang from Johns Hopkins has done important research in this sector. The paper, “Reconsidering the Ban on Physician-Owned Hospitals to Combat Consolidation,” by you and several colleagues, mentions and highlights the issues that you just described. I understand that it’s going to be published in the NYU Journal of Legislation and Public Policy.
One thing I want to bring up — and this is an important issue — is that the risk for patients has been talked about by the American Hospital Association and the Federation of American Hospitals, in terms of limited or no emergency services at such physician-owned hospitals and having to call 911 when patients need emergent care or stabilization. That’s been the rebuttal, along with an Office of Inspector General (OIG) report from 2008. Almost, I guess, three quarters of the patients that needed emergent care got this at publicly funded hospitals.
Dr. Miller: I’m familiar with the argument about emergency care. If you actually go and look at it, it differs by specialty market. Physician-owned community hospitals have ERs because that’s how they get their business. If you are running a hospital medicine floor, a general surgical specialty floor, you have a labor delivery unit, a primary care clinic, and a cardiology clinic. You have all the things that all the other hospitals have. The physician-owned community hospitals almost uniformly have an ER.
When you look at the physician-owned specialty hospitals, it’s a little more granular. If you look at the cardiac hospitals, they have ERs. They also have cardiac ICUs, operating rooms, etc. The area where the hospital industry had concerns — which I think is valid to point out — is that physician-owned orthopedic surgical specialty hospitals don’t have ERs. But this makes sense because of what that hospital functionally is: a factory for whatever the scope of procedures is, be it joint replacements or shoulder arthroscopy. The orthopedic surgical specialty hospital is like an ASC plus several hospital beds. Many of those did not have ERs because clinically it didn’t make sense.
What’s interesting, though, is that the hospital industry also operates specialty hospitals. If you go into many of the large systems, they have cardiac specialty hospitals and cancer specialty hospitals. I would say that some of them have ERs, as they appropriately should, and some of those specialty hospitals do not. They might have a community hospital down the street that’s part of that health system that has an ER, but some of the specialty hospitals don’t necessarily have a dedicated ER.
I agree, that’s a valid concern. I would say, though, the question is, what are the scope of services in that hospital? Is an ER required? Community hospitals should have ERs. It makes sense also for a cardiac hospital to have one. If you’re running a total joint replacement factory, it might not make clinical sense.
Dr. Glatter: The patients who are treated at that hospital, if they do have emergent conditions, need to have board-certified emergency physicians treating them, in my view because I’m an ER physician. Having surgeons that are not emergency physicians staff a department at a specialty orthopedic hospital or, say, a cancer hospital is not acceptable from my standpoint. That›s my opinion and recommendation, coming from emergency medicine.
Dr. Miller: I would say that anesthesiologists are actually highly qualified in critical care. The question is about clinical decompensation; if you’re doing a procedure, you have an anesthesiologist right there who is capable of critical care. The function of the ER is to either serve as a window into the hospital for patient volume or to serve as a referral for emergent complaints.
Dr. Glatter: An anesthesiologist — I’ll take issue with that — does not have the training of an emergency physician in terms of scope of practice.
Dr. Miller: My anesthesiology colleagues would probably disagree for managing an emergency during an operating room case.
Dr. Glatter: Fair enough, but I think in the general sense. The other issue is that, in terms of emergent responses to patients that decompensate, when you have to transfer a patient, that violates Medicare requirements. How is that even a valid issue or argument if you’re going to have to transfer a patient from your specialty hospital? That happens. Again, I know that you’re saying these hospitals are completely independent and can function, stabilize patients, and treat emergencies, but that’s not the reality across the country, in my opinion.
Dr. Miller: I don’t think that’s the case for the physician-owned specialty cardiac hospitals, for starters. Many of those have ICUs in addition to operating rooms as a matter of routine in addition to ERs. I don’t think that’s the case for physician-owned community hospitals, which have ERs, ICUs, medicine floors, and surgical floors. Physician-owned community hospitals are around half the market. Of that remaining market, a significant percentage are cardiac hospitals. If you’re taking an issue with orthopedic surgical specialty hospitals, that’s a clinical operational question that can and should be answered.
I’d also posit that the nonprofit and for-profit hospital industries also operate specialty hospitals. Any of these questions, we shouldn’t just be asking about physician-owned facilities; we should be asking about them across ownership types, because we’re talking about scope of service and quality and safety. The ownership in that case doesn’t matter. The broader question is, are orthopedic surgical specialty hospitals owned by physicians, tax-exempt hospitals, or tax-paying hospitals? Is that a valid clinical business model? Is it safe? Does it meet Medicare conditions of participation? I would say that’s what that question is, because other ownership models do operate those facilities.
Dr. Glatter: You make some valid points, and I do agree on some of them. I think that, ultimately, these models of care, and certainly cost and quality, are issues. Again, it goes back to being able, in my opinion, to provide emergent care, which seems to me a very important issue.
Dr. Miller: I agree that providing emergent care is an issue. It›s an issue in any site of care. The hospital industry posits that all hospital outpatient departments (HOPDs) have emergent care. I can tell you, having worked in HOPDs (I›ve trained in them during residency), the response if something emergent happens is to either call 911 or wheel the patient down to the ER in a wheelchair or stretcher. I think that these hospital claims about emergency care coverage — these are important questions, but we should be asking them across all clinical settings and say what is the appropriate scope of care provided? What is the appropriate level of acuity and ability to provide emergent or critical care? That›s an important question regardless of ownership model across the entire industry.
Deeper Dive Into Data on Physician-Owned Hospitals
Dr. Glatter: We need to really focus on that. I’ll agree with you on that.
There was a March 2023 report from Dobson | DaVanzo. It showed that physician-owned hospitals had lower Medicaid, dual-eligible, and uncompensated care and charity care discharges than full-service acute care hospitals. Physician-owned hospitals had less than half the proportion of Medicaid discharges compared with non–physician-owned hospitals. They were also less likely to care for dual-eligible patients overall compared with non–physician-owned hospitals.
In addition, when COVID hit, the physician-owned hospitals overall — and again, there may be exceptions — were not equipped to handle these patient surges in the acute setting of a public health emergency. There was a hospital in Texas that did pivot that I’m aware of — Renaissance Hospital, which ramped up a long-term care facility to become a COVID hospital — but I think that’s the exception. I think this report raises some valid concerns; I’ll let you rebut that.
Dr. Miller: A couple of things. One, I am not aware that there’s any clear market evidence or a systematic study that shows that physician-owned hospitals had trouble responding to COVID. I don’t think that assertion has been proven. The study was funded by the hospital industry. First of all, it was not a peer-reviewed study; it was funded by an industry that paid a consulting firm. It doesn’t mean that we still shouldn’t read it, but that brings bias into question. The joke in Washington is, pick your favorite statistician or economist, and they can say what you want and have a battle of economists and statisticians.
For example, in that study, they didn’t include the entire ownership universe of physician-owned hospitals. If we go to the peer-reviewed literature, there’s a great 2015 BMJ paper showing that the Medicaid payer mix is actually the same between physician-owned hospitals vs not. The mix of patients by ethnicity — for example, think about African American patients — was the same. I would be more inclined to believe the peer-reviewed literature in BMJ as opposed to an industry-funded study that was not peer-reviewed and not independent and has methodological questions.
Dr. Glatter: Those data are 8 years old, so I’d like to see more recent data. It would be interesting, just as a follow-up to that, to see where the needle has moved — if it has, for that matter — in terms of Medicaid patients that you’re referring to.
Dr. Miller: I tend to be skeptical of all industry research, regardless of who published it, because they have an economic incentive. If they’re selecting certain age groups or excluding certain hospitals, that makes you wonder about the validity of the study. Your job as an industry-funded researcher is that, essentially, you’re being paid to look for an answer. It’s not necessarily an honest evaluation of the data.
Dr. Glatter: I want to bring up another point about the Hospital Readmissions Reduction Program (HRRP) and the data on how physician-owned hospitals compared with acute care hospitals that are non–physician-owned and have you comment on that. The Dobson | DaVanzo study called into question that physician-owned hospitals treat fewer patients who are dual-eligible, which we know.
Dr. Miller: I don’t think we do know that.
Dr. Glatter: There are data that point to that, again, looking at the studies.
Dr. Miller: I’m saying that’s a single study funded by industry as opposed to an independent, academic, peer-reviewed literature paper. That would be like saying, during the debate of the Inflation Reduction Act (IRA), that you should read the pharmaceutical industries research but take any of it at pure face value as factual. Yes, we should read it. Yes, we should evaluate it on its own merits. I think, again, appropriately, you need to be concerned when people have an economic incentive.
The question about the HRRP I’m going to take a little broader, because I think that program is unfair to the industry overall. There are many factors that drive hospital readmission. Whether Mrs Smith went home and ate potato chips and then took her Lasix, that’s very much outside of the hospital industry’s control, and there’s some evidence that the HRRP increases mortality in some patient populations.
In terms of a quality metric, it’s unfair to the industry. I think we took an operating process, internal metric for the hospital industry, turned it into a quality metric, and attached it to a financial bonus, which is an inappropriate policy decision.
Rethinking Ownership Models and Empowering Clinicians
Dr. Glatter: I agree with you on that. One thing I do want to bring up is that whether the physician-owned hospitals are subject to many of the quality measures that full-service, acute care hospitals are. That really is, I think, a broader context.
Dr. Miller: Fifty-five percent of physician-owned hospitals are full-service community hospitals, so I would say at least half the market is 100% subject to that.
Dr. Glatter: If only 50% are, that’s already an issue.
Dr. Miller: Cardiac specialty hospitals — which, as I said, nonprofit and for-profit hospital chains also operate — are also subject to the appropriate quality measures, readmissions, etc. Just because we don’t necessarily have the best quality measurement in the system in the country, it doesn’t mean that we shouldn’t allow care specialization. As I’d point out, if we’re concerned about specialty hospitals, the concern shouldn’t just be about physician-owned specialty hospitals; it should be about specialty hospitals by and large. Many health systems run cardiac specialty hospitals, cancer specialty hospitals, and orthopedic specialty hospitals. If we’re going to have a discussion about concerns there, it should be about the entire industry of specialty hospitals.
I think specialty hospitals serve an important role in society, allowing for specialization and exploiting in a positive way the volume-quality relationship. Whether those are owned by a for-profit publicly traded company, a tax-exempt facility, or physicians, I think that is an important way to have innovation and care delivery because frankly, we haven’t had much innovation in care delivery. Much of what we do in terms of how we practice clinically hasn’t really changed in the 50 years since my late father graduated from medical school. We still have rounds, we’re still taking notes, we’re still operating in the same way. Many processes are manual. We don’t have the mass production and mass customization of care that we need.
When you have a focused factory, it allows you to design care in a way that drives up quality, not just for the average patient but also the patients at the tail ends, because you have time to focus on that specific service line and that specific patient population.
Physician-owned community hospitals offer an important opportunity for a different employment model. I remember going to the dermatologist and the dermatologist was depressed, shuffling around the room, sad, and I asked him why. He said he didn’t really like his employer, and I said, “Why don’t you pick another one?” He’s like, “There are only two large health systems I can work for. They all have the same clinical practice environment and functionally the same value.”
Physicians are increasingly burned out. They face monopsony power in who purchases their labor. They have little control. They don’t want to go through five committees, seven administrators, and attend 25 meetings just to change a single small process in clinical operations. If you’re an owner operator, you have a much better ability to do it.
Frankly, when many facilities do well now, when they do well clinically and do well financially, who benefits? The hospital administration and the hospital executives. The doctors aren’t benefiting. The nurses aren’t benefiting. The CNA is not benefiting. The secretary is not benefiting. The custodian is not benefiting. Shouldn’t the workers have a right to own and operate the business and do well when the business does well serving the community? That puts me in the weird space of agreeing with both conservatives and progressives.
Dr. Glatter: I agree with you. I think an ownership stake is always attractive. It helps with retention of employed persons. There›s no question that, when they have a stake, when they have skin in the game, they feel more empowered. I will not argue with you about that.
Dr. Miller: We don’t have business models where workers have that option in healthcare. Like the National Academy of Medicine said, one of the key drivers of burnout is the externalization of the locus of control over clinical practice, and the current business operating models guarantee an externalization of the locus of control over clinical practice.
If you actually look at the recent American Medical Association (AMA) meeting, there was a resolution to ban the corporate practice of medicine. They wanted to go more toward the legal professions model where only physicians can own and operate care delivery.
Dr. Glatter: Well, I think the shift is certainly something that the AMA would like and physicians collectively would agree with. Having a better lifestyle and being able to have control are factors in burnout.
Dr. Miller: It’s not just doctors. I think nurses want a better lifestyle. The nurses are treated as interchangeable lines on a spreadsheet. The nurses are an integral part of our clinical team. Why don’t we work together as a clinical unit to build a better delivery system? What better way to do that than to have clinicians in charge of it, right?
My favorite bakery that’s about 30 minutes away is owned by a baker. It is not owned by a large tax-exempt corporation. It’s owned by an owner operator who takes pride in their work. I think that is something that the profession would do well to return to. When I was a resident, one of my colleagues was already planning their retirement. That’s how depressed they were.
I went into medicine to actually care for patients. I think that we can make the world a better place for our patients. What that means is not only treating them with drugs and devices, but also creating a delivery system where they don’t have to wander from lobby to lobby in a 200,000 square-foot facility, wait in line for hours on end, get bills 6 months later, and fill out endless paper forms over and over again.
All of these basic processes in healthcare delivery that are broken could have and should have been fixed — and have been fixed in almost every other industry. I had to replace one of my car tires because I had a flat tire. The local tire shop has an app, and it sends me SMS text messages telling me when my appointment is and when my car is ready. We have solved all of these problems in many other businesses.
We have not solved them in healthcare delivery because, one, we have massive monopolies that are raising prices, have lower quality, and deliver a crappy patient experience, and we have also subjugated the clinical worker into a corporate automaton. We are functionally drones. We don’t have the agency and the authority to improve clinical operations anymore. It’s really depressing, and we should have that option again.
I trust my doctor. I trust the nurses that I work with, and I would like them to help make clinical decisions in a financially responsible and a sensible operational manner. We need to empower our workforce in order to do that so we can recapture the value of what it means to be a clinician again.
The current model of corporate employment: massive scale, more administrators, more processes, more emails, more meetings, more PowerPoint decks, more federal subsidies. The hospital industry has choices. It can improve clinical operations. It can show up in Washington and lobby for increased subsidies. It can invest in the market and not pay taxes for the tax-exempt facilities. Obviously, it makes the logical choices as an economic actor to show up, lobby for increased subsidies, and then also invest in the stock market.
Improving clinical operations is hard. It hasn’t happened. The Bureau of Labor Statistics shows that the private community hospital industry has had flat labor productivity growth, on average, for the past 25 years, and for some years it even declined. This is totally atypical across the economy.
We have failed our clinicians, and most importantly, we have failed our patients. I’ve been sick. My relatives have been sick, waiting hours, not able to get appointments, and redoing forms. It’s a total disaster. It’s time and reasonable to try an alternative ownership and operating model. There are obviously problems. The problems can and should be addressed, but it doesn’t mean that we should have a statutory prohibition on professionals owning and operating their own business.
Dr. Glatter: There was a report that $500 million was saved by limiting or banning or putting a moratorium on physician-owned hospitals by the Congressional Budget Office.
Dr. Miller: Yes, I’m very aware of those data. I’d say that the CBO also is off by 50% on the estimation of the implementation of the Part D program. They overestimated the Affordable Care Act market enrollment by over 10 million people — again, around 50%. They also estimated that the CMS Innovation Center initially would be a savings. Now they’ve re-estimated it as a 10-year expenditure and it has actually cost the taxpayers money.
The CBO is not transparent about what its assumptions are or its analysis and methods. As a researcher, we have to publish our information. It has to go through peer review. I want to know what goes into that $500 million figure — what the assumptions are and what the model is. It’s hard to comment without knowing how they came up with it.
Dr. Glatter: The points you make are very valid. Physicians and nurses want a better lifestyle.
Dr. Miller: It’s not even a better lifestyle. It’s about having a say in how clinical operations work and helping make them better. We want the delivery system to work better. This is an opportunity for us to do so.
Dr. Glatter: That translates into technology: obviously, generative artificial intelligence (AI) coming into the forefront, as we know, and changing care delivery models as you’re referring to, which is going to happen. It’s going to be a slow process. I think that the evolution is happening and will happen, as you accurately described.
Dr. Miller: The other thing that’s different now vs 20 years ago is that managed care is here, there, and everywhere, as Dr Seuss would say. You have utilization review and prior authorization, which I’ve experienced as a patient and a physician, and boy, is it not a fun process. There’s a large amount of friction that needs to be improved. If we’re worried about induced demand or inappropriate utilization, we have managed care right there to help police bad behavior.
Reforming Healthcare Systems and Restoring Patient-Centric Focus
Dr. Glatter: If you were to come up with, say, three bullet points of how we can work our way out of this current morass of where our healthcare systems exist, where do you see the solutions or how can we make and effect change?
Dr. Miller: I’d say there are a couple of things. One is, let business models compete fairly on an equal playing field. Let the physician-owned hospital compete with the tax-exempt hospital and the nonprofit hospital. Put them on an equal playing field. We have things like 340B, which favors tax-exempt hospitals. For-profit or tax-paying hospitals are not able to participate in that. That doesn’t make any sense just from a public policy perspective. Tax-paying hospitals and physician-owned hospitals pay taxes on investments, but tax-exempt hospitals don’t. I think, in public policy, we need to equalize the playing field between business models. Let the best business model win.
The other thing we need to do is to encourage the adoption of technology. The physician will eventually be an arbiter of tech-driven or AI-driven tools. In fact, at some point, the standard of care might be to use those tools. Not using those tools would be seen as negligence. If you think about placing a jugular or central venous catheter, to not use ultrasound would be considered insane. Thirty years ago, to use ultrasound would be considered novel. I think technology and AI will get us to that point of helping make care more efficient and more customized.
Those are the two biggest interventions, I would say. Third, every time we have a conversation in public policy, we need to remember what it is to be a patient. The decision should be driven not around any one industry’s profitability, but what it is to be a patient and how we can make that experience less burdensome, less expensive, or in plain English, suck less.
Dr. Glatter: Safety net hospitals and critical access hospitals are part of this discussion that, yes, we want everything to, in an ideal world, function more efficiently and effectively, with less cost and less red tape. The safety net of our nation is struggling.
Dr. Miller: I 100% agree. The Cook County hospitals of the world are deserving of our support and, frankly, our gratitude. Facilities like that have huge burdens of patients with Medicaid. We also still have millions of uninsured patients. The neighborhoods that they serve are also poorer. I think facilities like that are deserving of public support.
I also think we need to clearly define what those hospitals are. One of the challenges I’ve realized as I waded into this space is that market definitions of what a service market is for a hospital, its specialty type or what a safety net hospital is need to be more clearly defined because those facilities 100% are deserving of our support. We just need to be clear about what they are.
Regarding critical access hospitals, when you practice in a rural area, you have to think differently about care delivery. I’d say many of the rural systems are highly creative in how they structure clinical operations. Before the public health emergency, during the COVID pandemic, when we had a massive change in telehealth, rural hospitals were using — within the very narrow confines — as much telehealth as they could and should.
Rural hospitals also make greater use of nurse practitioners (NPs) and physician assistants (PAs). For many of the specialty services, I remember, your first call was an NP or a PA because the physician was downstairs doing procedures. They’d come up and assess the patient before the procedure, but most of your consult questions were answered by the NP or PA. I’m not saying that’s the model we should use nationwide, but that rural systems are highly innovative and creative; they’re deserving of our time, attention, and support, and frankly, we can learn from them.
Dr. Glatter: I want to thank you for your time and your expertise in this area. We’ll see how the congressional hearings affect the industry as a whole, how the needle moves, and whether the ban or moratorium on physician-owned hospitals continues to exist going forward.
Dr. Miller: I appreciate you having me. The hospital industry is one of the most important industries for health care. This is a time of inflection, right? We need to go back to the value of what it means to be a clinician and serve patients. Hospitals need to reorient themselves around that core concern. How do we help support clinicians — doctors, nurses, pharmacists, whomever it is — in serving patients? Hospitals have become too corporate, so I think that this is an expected pushback.
Dr. Glatter: Again, I want to thank you for your time. This was a very important discussion. Thank you for your expertise.
Robert D. Glatter, MD, is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is a medical advisor for Medscape and hosts the Hot Topics in EM series. He disclosed no relevant financial relationships.Brian J. Miller, MD, MBA, MPH, is a hospitalist and an assistant professor of medicine at the Johns Hopkins University School of Medicine. He is also a nonresident fellow at the American Enterprise Institute. From 2014 to 2017, Dr. Miller worked at four federal regulatory agencies: Federal Trade Commission (FTC), Federal Communications Commission (FCC), Centers for Medicare & Medicaid Services (CMS), and the Food & Drug Administration (FDA). Dr. Miller disclosed ties with the Medicare Payment Advisory Commission.
A version of this article appeared on Medscape.com.
Navigating Hair Loss in Medical School: Experiences of 2 Young Black Women
As medical students, we often assume we are exempt from the diagnoses we learn about. During the first 2 years of medical school, we learn about alopecia as a condition that may be associated with stress, hormonal imbalances, nutrient deficiencies, and aging. However, our curricula do not explore the subtypes, psychosocial impact, or even the overwhelming number of Black women who are disproportionately affected by alopecia. For Black women, hair is a colossal part of their cultural identity, learning from a young age how to nurture and style natural coils. It becomes devastating when women begin to lose them.
The diagnosis of alopecia subtypes in Black women has been explored in the literature; however, understanding the unique experiences of young Black women is an important part of patient care, as alopecia often is destructive to the patient’s self-image. Therefore, it is important to shed light on these experiences so others feel empowered and supported in their journeys. Herein, we share the experiences of 2 authors (J.D. and C.A.V.O.)—both young Black women—who navigated unexpected hair loss in medical school.
Jewell’s Story
During my first year of medical school, I noticed my hair was shedding more than usual, and my ponytail was not as thick as it once was. I also had an area in my crown that was abnormally thin. My parents suggested that it was a consequence of stress, but I knew something was not right. With only 1 Black dermatologist within 2 hours of Nashville, Tennessee, I remember worrying about seeing a dermatologist who did not understand Black hair. I still scheduled an appointment, but I remember debating if I should straighten my hair or wear my naturally curly Afro. The first dermatologist I saw diagnosed me with seborrheic dermatitis—without even examining my scalp. She told me that I had a “full head of hair” and that I had nothing to worry about. I was unconvinced. Weeks later, I met with another dermatologist who took the time to listen to my concerns. After a scalp biopsy and laboratory work, she diagnosed me with telogen effluvium and androgenetic alopecia. Months later, I had the opportunity to visit the Black dermatologist, and she diagnosed me with central centrifugal cicatricial alopecia. I am grateful for the earlier dermatologists I saw, but I finally feel at ease with my diagnosis and treatment plan after being seen by the latter.
Chidubem’s Story
From a young age, I was conditioned to think my hair was thick, unmanageable, and a nuisance. I grew accustomed to people yanking on my hair, and my gentle whispers of “this hurts” and “the braid is too tight” being ignored. That continued into adulthood. While studying for the US Medical Licensing Examination, I noticed a burning sensation on my scalp. I decided to ignore it. However, as the days progressed, the slight burning sensation turned into intense burning and itching. I still ignored it. Not only did I lack the funds for a dermatology appointment, but my licensing examination was approaching, and it was more important than anything related to my hair. After the examination, I eventually made an appointment with my primary care physician, who attributed my symptoms to the stressors of medical school. “I think you are having migraines,” she told me. So, I continued to ignore my symptoms. A year passed, and a hair braider pointed out that I had 2 well-defined bald patches on my scalp. I remember feeling angry and confused as to how I missed those findings. I could no longer ignore it—it bothered me less when no one else knew about it. I quickly made a dermatology appointment. Although I opted out of a biopsy, we decided to treat my hair loss empirically, and I have experienced drastic improvement.
Final Thoughts
We are 2 Black women living more than 500 miles away from each other at different medical institutions, yet we share the same experience, which many other women unfortunately face alone. It is not uncommon for us to feel unheard, dismissed, or misdiagnosed. We write this for the Black woman sorting through the feelings of confusion and shock as she traces the hairless spot on her scalp. We write this for the medical student ignoring their symptoms until after their examination. We even write this for any nondermatologists uncomfortable with diagnosing and treating textured hair. To improve patient satisfaction and overall health outcomes, physicians must approach patients with both knowledge and cultural competency. Most importantly, dermatologists (and other physicians) should be appropriately trained in not only the structural differences of textured hair but also the unique practices and beliefs among Black women in relation to their hair.
Acknowledgments—Jewell Dinkins is the inaugural recipient of the Janssen–Skin of Color Research Fellowship at Howard University (Washington, DC), and Chidubem A.V. Okeke is the inaugural recipient of the Women’s Dermatologic Society–La Roche-Posay dermatology fellowship at Howard University.
As medical students, we often assume we are exempt from the diagnoses we learn about. During the first 2 years of medical school, we learn about alopecia as a condition that may be associated with stress, hormonal imbalances, nutrient deficiencies, and aging. However, our curricula do not explore the subtypes, psychosocial impact, or even the overwhelming number of Black women who are disproportionately affected by alopecia. For Black women, hair is a colossal part of their cultural identity, learning from a young age how to nurture and style natural coils. It becomes devastating when women begin to lose them.
The diagnosis of alopecia subtypes in Black women has been explored in the literature; however, understanding the unique experiences of young Black women is an important part of patient care, as alopecia often is destructive to the patient’s self-image. Therefore, it is important to shed light on these experiences so others feel empowered and supported in their journeys. Herein, we share the experiences of 2 authors (J.D. and C.A.V.O.)—both young Black women—who navigated unexpected hair loss in medical school.
Jewell’s Story
During my first year of medical school, I noticed my hair was shedding more than usual, and my ponytail was not as thick as it once was. I also had an area in my crown that was abnormally thin. My parents suggested that it was a consequence of stress, but I knew something was not right. With only 1 Black dermatologist within 2 hours of Nashville, Tennessee, I remember worrying about seeing a dermatologist who did not understand Black hair. I still scheduled an appointment, but I remember debating if I should straighten my hair or wear my naturally curly Afro. The first dermatologist I saw diagnosed me with seborrheic dermatitis—without even examining my scalp. She told me that I had a “full head of hair” and that I had nothing to worry about. I was unconvinced. Weeks later, I met with another dermatologist who took the time to listen to my concerns. After a scalp biopsy and laboratory work, she diagnosed me with telogen effluvium and androgenetic alopecia. Months later, I had the opportunity to visit the Black dermatologist, and she diagnosed me with central centrifugal cicatricial alopecia. I am grateful for the earlier dermatologists I saw, but I finally feel at ease with my diagnosis and treatment plan after being seen by the latter.
Chidubem’s Story
From a young age, I was conditioned to think my hair was thick, unmanageable, and a nuisance. I grew accustomed to people yanking on my hair, and my gentle whispers of “this hurts” and “the braid is too tight” being ignored. That continued into adulthood. While studying for the US Medical Licensing Examination, I noticed a burning sensation on my scalp. I decided to ignore it. However, as the days progressed, the slight burning sensation turned into intense burning and itching. I still ignored it. Not only did I lack the funds for a dermatology appointment, but my licensing examination was approaching, and it was more important than anything related to my hair. After the examination, I eventually made an appointment with my primary care physician, who attributed my symptoms to the stressors of medical school. “I think you are having migraines,” she told me. So, I continued to ignore my symptoms. A year passed, and a hair braider pointed out that I had 2 well-defined bald patches on my scalp. I remember feeling angry and confused as to how I missed those findings. I could no longer ignore it—it bothered me less when no one else knew about it. I quickly made a dermatology appointment. Although I opted out of a biopsy, we decided to treat my hair loss empirically, and I have experienced drastic improvement.
Final Thoughts
We are 2 Black women living more than 500 miles away from each other at different medical institutions, yet we share the same experience, which many other women unfortunately face alone. It is not uncommon for us to feel unheard, dismissed, or misdiagnosed. We write this for the Black woman sorting through the feelings of confusion and shock as she traces the hairless spot on her scalp. We write this for the medical student ignoring their symptoms until after their examination. We even write this for any nondermatologists uncomfortable with diagnosing and treating textured hair. To improve patient satisfaction and overall health outcomes, physicians must approach patients with both knowledge and cultural competency. Most importantly, dermatologists (and other physicians) should be appropriately trained in not only the structural differences of textured hair but also the unique practices and beliefs among Black women in relation to their hair.
Acknowledgments—Jewell Dinkins is the inaugural recipient of the Janssen–Skin of Color Research Fellowship at Howard University (Washington, DC), and Chidubem A.V. Okeke is the inaugural recipient of the Women’s Dermatologic Society–La Roche-Posay dermatology fellowship at Howard University.
As medical students, we often assume we are exempt from the diagnoses we learn about. During the first 2 years of medical school, we learn about alopecia as a condition that may be associated with stress, hormonal imbalances, nutrient deficiencies, and aging. However, our curricula do not explore the subtypes, psychosocial impact, or even the overwhelming number of Black women who are disproportionately affected by alopecia. For Black women, hair is a colossal part of their cultural identity, learning from a young age how to nurture and style natural coils. It becomes devastating when women begin to lose them.
The diagnosis of alopecia subtypes in Black women has been explored in the literature; however, understanding the unique experiences of young Black women is an important part of patient care, as alopecia often is destructive to the patient’s self-image. Therefore, it is important to shed light on these experiences so others feel empowered and supported in their journeys. Herein, we share the experiences of 2 authors (J.D. and C.A.V.O.)—both young Black women—who navigated unexpected hair loss in medical school.
Jewell’s Story
During my first year of medical school, I noticed my hair was shedding more than usual, and my ponytail was not as thick as it once was. I also had an area in my crown that was abnormally thin. My parents suggested that it was a consequence of stress, but I knew something was not right. With only 1 Black dermatologist within 2 hours of Nashville, Tennessee, I remember worrying about seeing a dermatologist who did not understand Black hair. I still scheduled an appointment, but I remember debating if I should straighten my hair or wear my naturally curly Afro. The first dermatologist I saw diagnosed me with seborrheic dermatitis—without even examining my scalp. She told me that I had a “full head of hair” and that I had nothing to worry about. I was unconvinced. Weeks later, I met with another dermatologist who took the time to listen to my concerns. After a scalp biopsy and laboratory work, she diagnosed me with telogen effluvium and androgenetic alopecia. Months later, I had the opportunity to visit the Black dermatologist, and she diagnosed me with central centrifugal cicatricial alopecia. I am grateful for the earlier dermatologists I saw, but I finally feel at ease with my diagnosis and treatment plan after being seen by the latter.
Chidubem’s Story
From a young age, I was conditioned to think my hair was thick, unmanageable, and a nuisance. I grew accustomed to people yanking on my hair, and my gentle whispers of “this hurts” and “the braid is too tight” being ignored. That continued into adulthood. While studying for the US Medical Licensing Examination, I noticed a burning sensation on my scalp. I decided to ignore it. However, as the days progressed, the slight burning sensation turned into intense burning and itching. I still ignored it. Not only did I lack the funds for a dermatology appointment, but my licensing examination was approaching, and it was more important than anything related to my hair. After the examination, I eventually made an appointment with my primary care physician, who attributed my symptoms to the stressors of medical school. “I think you are having migraines,” she told me. So, I continued to ignore my symptoms. A year passed, and a hair braider pointed out that I had 2 well-defined bald patches on my scalp. I remember feeling angry and confused as to how I missed those findings. I could no longer ignore it—it bothered me less when no one else knew about it. I quickly made a dermatology appointment. Although I opted out of a biopsy, we decided to treat my hair loss empirically, and I have experienced drastic improvement.
Final Thoughts
We are 2 Black women living more than 500 miles away from each other at different medical institutions, yet we share the same experience, which many other women unfortunately face alone. It is not uncommon for us to feel unheard, dismissed, or misdiagnosed. We write this for the Black woman sorting through the feelings of confusion and shock as she traces the hairless spot on her scalp. We write this for the medical student ignoring their symptoms until after their examination. We even write this for any nondermatologists uncomfortable with diagnosing and treating textured hair. To improve patient satisfaction and overall health outcomes, physicians must approach patients with both knowledge and cultural competency. Most importantly, dermatologists (and other physicians) should be appropriately trained in not only the structural differences of textured hair but also the unique practices and beliefs among Black women in relation to their hair.
Acknowledgments—Jewell Dinkins is the inaugural recipient of the Janssen–Skin of Color Research Fellowship at Howard University (Washington, DC), and Chidubem A.V. Okeke is the inaugural recipient of the Women’s Dermatologic Society–La Roche-Posay dermatology fellowship at Howard University.
Practice Points
- Hair loss is a common dermatologic concern among Black women and can represent a diagnostic challenge to dermatologists who may not be familiar with textured hair.
- Dermatologists should practice cultural sensitivity and provide relevant recommendations to Black patients dealing with hair loss.






