User login
Will Testosterone Replacement Therapy Kill Your Patient?
At first it seems to be a fairly straightforward proposition. The older gentleman you are seeing in the clinic reports that he has been running rather low on energy in recent weeks, and he also mentions that there’s not much lead in his pencil these days. As a conscientious clinician, you immediately entertain the possibility that hypogonadism might explain some of his symptoms. You dutifully order up a total testosterone level, and then a free testosterone level when the total comes back low, recognizing that binding protein abnormalities might produce a low total even when the clinically relevant free level is still normal. Both levels do come back well below the age-adjusted lower limits of normal, which gives you some transient level of satisfaction that you have identified a very significant factor contributing to your patient’s difficulties.
You have confirmed a deficiency of a major hormone, and it seems logical that you would want to restore the hormone level to normal in this particular patient. But before you reach for your prescription pad (or your mouse), a fundamental question hangs uneasily in the air. Are you going to be doing more harm than good by prescribing testosterone replacement therapy (TRT) to this rather trusting older fellow? In light of recent studies, might you actually increase this patent’s chances of a heart attack or a stroke? That would not be a nice thing to do to this pleasant older gentleman. (As a newly minted senior citizen, I pray mightily that my own caregivers adhere rigorously to Hippocrates’ hoary admonition to, above all, do no harm.)
I’m not going to be able to resolve this clinical conundrum definitively in this editorial. (Please don’t stop reading just yet!) But maybe a review of the pros and cons for testosterone replacement therapy can help you faithful readers gain just a little bit better sense of the operative risk/benefit considerations at play here.
Let’s look first at the case for prescribing TRT when the laboratory test values show definitive evidence of low testosterone levels. I don’t want to delve into the distracting issue of which form of testosterone replacement to consider, which pits injections vs gels vs patches vs pills (don’t use the potentially hepatotoxic methyltestosterone pills passed out like candy by some urologists). Apart from the possibly relevant issue of peaks and troughs seen with injection therapy, the same risk/benefit considerations pretty much apply to all forms of TRT.
The benefits of TRT clearly include an increase in lean muscle mass, an increase in red blood cell concentration due to the hematopoetic effects of the male hormone, and a reduction in both the total amount and the percentage of body fat. A number of studies have shown that testosterone enhances insulin sensitivity—surely a good thing given the massive number of older patients with either prediabetes or full-blown type 2 diabetes. Some men also report a significant increase in their hard-to-define-but-still important sense of manliness, and sometimes a major improvement in their ability to perform in the sack. The latter effects, though, are often very modest and of considerably less potency (sorry, pun intended) than seen with sildenafil or one of the other PDE-5 inhibitors. In spite of all these seemingly positive effects, the clear majority of men report that they really don’t feel much different after starting on TRT, and many discontinue it on their own after relatively short periods, especially those enduring intramuscular injections every 2 weeks.
So the benefits derived from TRT are not really very impressive in many patients. What about the downside of giving testosterone? Surely there can’t be any problems associated with simply replacing an important hormone that has fallen to low levels? After all, we don’t hesitate to give thyroid hormone to hypothyroid patients, to give growth hormone to children with low levels of this critical hormone, or to give insulin to diabetic patients whose pancreases are not producing enough of that life-saving hormone.
For a very long time the risk/benefit arguments over whether or not to give TRT were almost entirely theoretical. Those in favor cited the several aforementioned benefits, and those in opposition decried replacement therapy as a perverse form of tinkering with nature by trying to alter the natural decline in the levels of certain hormones that were part and parcel of the natural aging process.
Then along came 3 rather worrisome studies in fairly rapid-fire succession, which seamed collectively to deliver a true body blow to TRT. However, a closer examination of these studies reveals that each is so severely flawed that no meaningful conclusions can be derived from any of them.
The Testosterone in Older Men with Sarcopenia (TOM) trial was a randomized trial of TRT vs placebo in older men (mean age 74 years) with mobility limitations (sarcopenia, after all, means decreased muscle bulk) and a high prevalence of chronic disease.1 The trial was stopped early because of a much higher occurrence of self-reported cardiovascular-related adverse events. However, these adverse events were extremely disparate and were all self-reported; none had been prespecified outcomes. Any objective observer would have to conclude that the study was poorly designed and that no meaningful conclusions can be drawn from its premature termination.
The second trial that seemed to cast doubt on the safety of TRT suffered from an even worse design. It was a retrospective cohort study of 8,709 veterans aged 60 to 64 years with low testosterone levels who were undergoing coronary artery angiography. The authors reported in the Journal of the American Medical Association that those receiving testosterone therapy had a higher risk of experiencing a composite outcome of all-cause mortality, myocardial infarction (MI), or cerebrovascular accident than did those who had not received testosterone therapy (hazard ratio [HR] = 1.29; 95% confidence interval [CI]: 1.04-1.58).2 Right off the bat, you should be very wary of any HR emanating from a retrospective study that shows a small increase in risk of 29%; it’s only when a HR is 2.0 or more that it’s likely you’re looking at a real phenomenon. But to add insult to injury, the percentage of actual adverse outcomes was actually SMALLER in those taking testosterone than in those who did not get any! The authors had used such an incredibly tortured series of risk adjustments for a variety of comorbidities that they actually managed to stand the raw numbers on their head.
The third study, which had seamed at first blush to demonstrate cardiovascular toxicity of TRT, was a much larger retrospective cohort study of 55,793 men who had received replacement testosterone.3 The authors reported an increase in the relative risk of MI in the first 3 months after starting testosterone compared with the risk of MI in the same men in the prior year (relative risk [RR] = 1.36). However, the much more important absolute risk increase was very, very low, with only an additional 1.25 cases of MI seen over 1,000 patient-years. Apart from the fact that a RR of 1.36 is most unimpressive in a retrospective study, the simple fact that the men were older by a few months after TRT is probably more than adequate to explain this tiny increase in apparent risk.
The FDA has monitored these studies closely and has chosen not to make a determination that there is an increased risk of cardiovascular events associated with TRT. That is not at all the same as saying that it has been proven to be completely free of cardiovascular risk; rather it is a common-sense acknowledgment that there is not any convincing evidence to date of such a risk.
Thus, the conscientious clinician is left to conclude that TRT is a reasonable option in symptomatic patients who have been shown to have low levels of free testosterone. It has not been conclusively demonstrated that TRT will have significant beneficial effects, but neither has it been proven to have any true cardiovascular toxicity. It is a therapy worth trying in those symptomatic patients who understand that they will be receiving therapy of uncertain benefit, if any, and with the possibility of uncertain risk, if any.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men; A randomized, double-blind, placebo controlled trial. J Clin Endocrinol Metab. 2010;95(2):639-650.
2. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
3. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PloS One. 2014;9(1):e85805 Epub.
At first it seems to be a fairly straightforward proposition. The older gentleman you are seeing in the clinic reports that he has been running rather low on energy in recent weeks, and he also mentions that there’s not much lead in his pencil these days. As a conscientious clinician, you immediately entertain the possibility that hypogonadism might explain some of his symptoms. You dutifully order up a total testosterone level, and then a free testosterone level when the total comes back low, recognizing that binding protein abnormalities might produce a low total even when the clinically relevant free level is still normal. Both levels do come back well below the age-adjusted lower limits of normal, which gives you some transient level of satisfaction that you have identified a very significant factor contributing to your patient’s difficulties.
You have confirmed a deficiency of a major hormone, and it seems logical that you would want to restore the hormone level to normal in this particular patient. But before you reach for your prescription pad (or your mouse), a fundamental question hangs uneasily in the air. Are you going to be doing more harm than good by prescribing testosterone replacement therapy (TRT) to this rather trusting older fellow? In light of recent studies, might you actually increase this patent’s chances of a heart attack or a stroke? That would not be a nice thing to do to this pleasant older gentleman. (As a newly minted senior citizen, I pray mightily that my own caregivers adhere rigorously to Hippocrates’ hoary admonition to, above all, do no harm.)
I’m not going to be able to resolve this clinical conundrum definitively in this editorial. (Please don’t stop reading just yet!) But maybe a review of the pros and cons for testosterone replacement therapy can help you faithful readers gain just a little bit better sense of the operative risk/benefit considerations at play here.
Let’s look first at the case for prescribing TRT when the laboratory test values show definitive evidence of low testosterone levels. I don’t want to delve into the distracting issue of which form of testosterone replacement to consider, which pits injections vs gels vs patches vs pills (don’t use the potentially hepatotoxic methyltestosterone pills passed out like candy by some urologists). Apart from the possibly relevant issue of peaks and troughs seen with injection therapy, the same risk/benefit considerations pretty much apply to all forms of TRT.
The benefits of TRT clearly include an increase in lean muscle mass, an increase in red blood cell concentration due to the hematopoetic effects of the male hormone, and a reduction in both the total amount and the percentage of body fat. A number of studies have shown that testosterone enhances insulin sensitivity—surely a good thing given the massive number of older patients with either prediabetes or full-blown type 2 diabetes. Some men also report a significant increase in their hard-to-define-but-still important sense of manliness, and sometimes a major improvement in their ability to perform in the sack. The latter effects, though, are often very modest and of considerably less potency (sorry, pun intended) than seen with sildenafil or one of the other PDE-5 inhibitors. In spite of all these seemingly positive effects, the clear majority of men report that they really don’t feel much different after starting on TRT, and many discontinue it on their own after relatively short periods, especially those enduring intramuscular injections every 2 weeks.
So the benefits derived from TRT are not really very impressive in many patients. What about the downside of giving testosterone? Surely there can’t be any problems associated with simply replacing an important hormone that has fallen to low levels? After all, we don’t hesitate to give thyroid hormone to hypothyroid patients, to give growth hormone to children with low levels of this critical hormone, or to give insulin to diabetic patients whose pancreases are not producing enough of that life-saving hormone.
For a very long time the risk/benefit arguments over whether or not to give TRT were almost entirely theoretical. Those in favor cited the several aforementioned benefits, and those in opposition decried replacement therapy as a perverse form of tinkering with nature by trying to alter the natural decline in the levels of certain hormones that were part and parcel of the natural aging process.
Then along came 3 rather worrisome studies in fairly rapid-fire succession, which seamed collectively to deliver a true body blow to TRT. However, a closer examination of these studies reveals that each is so severely flawed that no meaningful conclusions can be derived from any of them.
The Testosterone in Older Men with Sarcopenia (TOM) trial was a randomized trial of TRT vs placebo in older men (mean age 74 years) with mobility limitations (sarcopenia, after all, means decreased muscle bulk) and a high prevalence of chronic disease.1 The trial was stopped early because of a much higher occurrence of self-reported cardiovascular-related adverse events. However, these adverse events were extremely disparate and were all self-reported; none had been prespecified outcomes. Any objective observer would have to conclude that the study was poorly designed and that no meaningful conclusions can be drawn from its premature termination.
The second trial that seemed to cast doubt on the safety of TRT suffered from an even worse design. It was a retrospective cohort study of 8,709 veterans aged 60 to 64 years with low testosterone levels who were undergoing coronary artery angiography. The authors reported in the Journal of the American Medical Association that those receiving testosterone therapy had a higher risk of experiencing a composite outcome of all-cause mortality, myocardial infarction (MI), or cerebrovascular accident than did those who had not received testosterone therapy (hazard ratio [HR] = 1.29; 95% confidence interval [CI]: 1.04-1.58).2 Right off the bat, you should be very wary of any HR emanating from a retrospective study that shows a small increase in risk of 29%; it’s only when a HR is 2.0 or more that it’s likely you’re looking at a real phenomenon. But to add insult to injury, the percentage of actual adverse outcomes was actually SMALLER in those taking testosterone than in those who did not get any! The authors had used such an incredibly tortured series of risk adjustments for a variety of comorbidities that they actually managed to stand the raw numbers on their head.
The third study, which had seamed at first blush to demonstrate cardiovascular toxicity of TRT, was a much larger retrospective cohort study of 55,793 men who had received replacement testosterone.3 The authors reported an increase in the relative risk of MI in the first 3 months after starting testosterone compared with the risk of MI in the same men in the prior year (relative risk [RR] = 1.36). However, the much more important absolute risk increase was very, very low, with only an additional 1.25 cases of MI seen over 1,000 patient-years. Apart from the fact that a RR of 1.36 is most unimpressive in a retrospective study, the simple fact that the men were older by a few months after TRT is probably more than adequate to explain this tiny increase in apparent risk.
The FDA has monitored these studies closely and has chosen not to make a determination that there is an increased risk of cardiovascular events associated with TRT. That is not at all the same as saying that it has been proven to be completely free of cardiovascular risk; rather it is a common-sense acknowledgment that there is not any convincing evidence to date of such a risk.
Thus, the conscientious clinician is left to conclude that TRT is a reasonable option in symptomatic patients who have been shown to have low levels of free testosterone. It has not been conclusively demonstrated that TRT will have significant beneficial effects, but neither has it been proven to have any true cardiovascular toxicity. It is a therapy worth trying in those symptomatic patients who understand that they will be receiving therapy of uncertain benefit, if any, and with the possibility of uncertain risk, if any.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
At first it seems to be a fairly straightforward proposition. The older gentleman you are seeing in the clinic reports that he has been running rather low on energy in recent weeks, and he also mentions that there’s not much lead in his pencil these days. As a conscientious clinician, you immediately entertain the possibility that hypogonadism might explain some of his symptoms. You dutifully order up a total testosterone level, and then a free testosterone level when the total comes back low, recognizing that binding protein abnormalities might produce a low total even when the clinically relevant free level is still normal. Both levels do come back well below the age-adjusted lower limits of normal, which gives you some transient level of satisfaction that you have identified a very significant factor contributing to your patient’s difficulties.
You have confirmed a deficiency of a major hormone, and it seems logical that you would want to restore the hormone level to normal in this particular patient. But before you reach for your prescription pad (or your mouse), a fundamental question hangs uneasily in the air. Are you going to be doing more harm than good by prescribing testosterone replacement therapy (TRT) to this rather trusting older fellow? In light of recent studies, might you actually increase this patent’s chances of a heart attack or a stroke? That would not be a nice thing to do to this pleasant older gentleman. (As a newly minted senior citizen, I pray mightily that my own caregivers adhere rigorously to Hippocrates’ hoary admonition to, above all, do no harm.)
I’m not going to be able to resolve this clinical conundrum definitively in this editorial. (Please don’t stop reading just yet!) But maybe a review of the pros and cons for testosterone replacement therapy can help you faithful readers gain just a little bit better sense of the operative risk/benefit considerations at play here.
Let’s look first at the case for prescribing TRT when the laboratory test values show definitive evidence of low testosterone levels. I don’t want to delve into the distracting issue of which form of testosterone replacement to consider, which pits injections vs gels vs patches vs pills (don’t use the potentially hepatotoxic methyltestosterone pills passed out like candy by some urologists). Apart from the possibly relevant issue of peaks and troughs seen with injection therapy, the same risk/benefit considerations pretty much apply to all forms of TRT.
The benefits of TRT clearly include an increase in lean muscle mass, an increase in red blood cell concentration due to the hematopoetic effects of the male hormone, and a reduction in both the total amount and the percentage of body fat. A number of studies have shown that testosterone enhances insulin sensitivity—surely a good thing given the massive number of older patients with either prediabetes or full-blown type 2 diabetes. Some men also report a significant increase in their hard-to-define-but-still important sense of manliness, and sometimes a major improvement in their ability to perform in the sack. The latter effects, though, are often very modest and of considerably less potency (sorry, pun intended) than seen with sildenafil or one of the other PDE-5 inhibitors. In spite of all these seemingly positive effects, the clear majority of men report that they really don’t feel much different after starting on TRT, and many discontinue it on their own after relatively short periods, especially those enduring intramuscular injections every 2 weeks.
So the benefits derived from TRT are not really very impressive in many patients. What about the downside of giving testosterone? Surely there can’t be any problems associated with simply replacing an important hormone that has fallen to low levels? After all, we don’t hesitate to give thyroid hormone to hypothyroid patients, to give growth hormone to children with low levels of this critical hormone, or to give insulin to diabetic patients whose pancreases are not producing enough of that life-saving hormone.
For a very long time the risk/benefit arguments over whether or not to give TRT were almost entirely theoretical. Those in favor cited the several aforementioned benefits, and those in opposition decried replacement therapy as a perverse form of tinkering with nature by trying to alter the natural decline in the levels of certain hormones that were part and parcel of the natural aging process.
Then along came 3 rather worrisome studies in fairly rapid-fire succession, which seamed collectively to deliver a true body blow to TRT. However, a closer examination of these studies reveals that each is so severely flawed that no meaningful conclusions can be derived from any of them.
The Testosterone in Older Men with Sarcopenia (TOM) trial was a randomized trial of TRT vs placebo in older men (mean age 74 years) with mobility limitations (sarcopenia, after all, means decreased muscle bulk) and a high prevalence of chronic disease.1 The trial was stopped early because of a much higher occurrence of self-reported cardiovascular-related adverse events. However, these adverse events were extremely disparate and were all self-reported; none had been prespecified outcomes. Any objective observer would have to conclude that the study was poorly designed and that no meaningful conclusions can be drawn from its premature termination.
The second trial that seemed to cast doubt on the safety of TRT suffered from an even worse design. It was a retrospective cohort study of 8,709 veterans aged 60 to 64 years with low testosterone levels who were undergoing coronary artery angiography. The authors reported in the Journal of the American Medical Association that those receiving testosterone therapy had a higher risk of experiencing a composite outcome of all-cause mortality, myocardial infarction (MI), or cerebrovascular accident than did those who had not received testosterone therapy (hazard ratio [HR] = 1.29; 95% confidence interval [CI]: 1.04-1.58).2 Right off the bat, you should be very wary of any HR emanating from a retrospective study that shows a small increase in risk of 29%; it’s only when a HR is 2.0 or more that it’s likely you’re looking at a real phenomenon. But to add insult to injury, the percentage of actual adverse outcomes was actually SMALLER in those taking testosterone than in those who did not get any! The authors had used such an incredibly tortured series of risk adjustments for a variety of comorbidities that they actually managed to stand the raw numbers on their head.
The third study, which had seamed at first blush to demonstrate cardiovascular toxicity of TRT, was a much larger retrospective cohort study of 55,793 men who had received replacement testosterone.3 The authors reported an increase in the relative risk of MI in the first 3 months after starting testosterone compared with the risk of MI in the same men in the prior year (relative risk [RR] = 1.36). However, the much more important absolute risk increase was very, very low, with only an additional 1.25 cases of MI seen over 1,000 patient-years. Apart from the fact that a RR of 1.36 is most unimpressive in a retrospective study, the simple fact that the men were older by a few months after TRT is probably more than adequate to explain this tiny increase in apparent risk.
The FDA has monitored these studies closely and has chosen not to make a determination that there is an increased risk of cardiovascular events associated with TRT. That is not at all the same as saying that it has been proven to be completely free of cardiovascular risk; rather it is a common-sense acknowledgment that there is not any convincing evidence to date of such a risk.
Thus, the conscientious clinician is left to conclude that TRT is a reasonable option in symptomatic patients who have been shown to have low levels of free testosterone. It has not been conclusively demonstrated that TRT will have significant beneficial effects, but neither has it been proven to have any true cardiovascular toxicity. It is a therapy worth trying in those symptomatic patients who understand that they will be receiving therapy of uncertain benefit, if any, and with the possibility of uncertain risk, if any.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men; A randomized, double-blind, placebo controlled trial. J Clin Endocrinol Metab. 2010;95(2):639-650.
2. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
3. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PloS One. 2014;9(1):e85805 Epub.
1. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men; A randomized, double-blind, placebo controlled trial. J Clin Endocrinol Metab. 2010;95(2):639-650.
2. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
3. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PloS One. 2014;9(1):e85805 Epub.
Acting Surgeon General Confident in the Battle Against Tobacco, Ebola, and Preventable Diseases
Not many health care leaders can transition smoothly from discussing the importance of walking 30 minutes per day to the need to send PHS officers to help control the Ebola epidemic in West Africa. The Surgeon General has to. As the most prominent public health official, the Surgeon General must offer a reassuring voice on health care issues big and small. With over 26 years at the PHS, Rear Admiral (RADM) Boris D. Lushniak, MD, MPH, is well equipped to handle the challenging role.
A year after assuming the role and just before delivering a plenary address at the 2014 AMSUS meeting, RADM Lushniak agreed to a wide-ranging conversation with Federal Practitioner. The following is condensed and edited, but the complete interview can be found at http://www.fedprac.com.
The 50th anniversary of the Surgeon General’s report on smoking
RADM Boris D. Lushniak, Acting Surgeon General. Go back to January 1964 and realize what a different world we lived in back then. In fact, that report, which came out after a year and a half of scientific deliberations, of looking at facts, of searching through the literature, came up with a very important conclusion. That conclusion, simply put was: Smoking is bad for you.
Now it really was a landmark report from that perspective, but when we look back 50 years, what did it prove? It proved that cigarette smoking was directly associated with only 1 cancer at that point, specifically lung cancer in men. The report had a very simple but beautiful conclusion. It said that cigarette smoking is a health hazard of significant importance in the U.S. to warrant appropriate remedial action....
A half-century later, the social norms of our society have changed. We don’t have ashtrays all around. We don’t smoke on airplanes anymore. We oftentimes can’t smoke in bars and restaurants and establishments like that. We’ve moved from 43% of our population that smoked in 1964 to 18% currently.
We’ve had 32 Surgeons’ General reports since that first one....We brought up the issue of secondhand smoke 25 years ago. We talked about the successes and failures over these years, but 50 years later smoking remains a major public health problem in this country....
When we look to the future, what’s the goal? Well we really want to get to a zero point. We reannounced with the 50th anniversary report, which was released in January 2014, that this is an endgame strategy. At some point we have to realize that it’s not good enough to get down to 18% because of the health impact. Cigarettes and tobacco use in this country bring no good; no good to the individual, no good to the individual who has to deal with secondhand smoke, and no good for the future of our nation. So we’re really talking about an endgame strategy....
Our 50th anniversary report wasn’t just looking backward....It contains current data that now show us that we’re up to 13 different cancers caused by tobacco use. We know the impact on the whole human body. In essence, it affects almost every single system of the human body now. Brand-new diseases, formerly not associated with smoking, are still being discovered.
Most recently, we’ve seen diabetes and colon and rectal cancers as some of those diseases. We’re talking about blindness associated with smoking. We’re talking about diseases such as erectile dysfunction, which are associated with smoking. This product has brought nothing but grief and sorrow into our society and continues to do so.
Now it’s not only an impact on the United States for the Office of the Surgeon General’s to speak, but also in essence we know that internationally people look at the Surgeon General reports that come out of this office as that stellar scientific information that then can be translated worldwide.
Not only do we see leadership of public health here within the United States, but we also see leadership on an international level by profiling some of the major public health issues.
The PHS response to Ebola in the U.S. and Africa
RADM Lushniak. As many of the readers may know, the Surgeon General is the commander of the U.S. Public Health Service Commissioned Corps. We have 6,800 public health professionals. These are officers working in 11 different categories working across the government, to protect, promote, and advance the health and safety of the nation.
[In October] I was in Anniston, Alabama, seeing about 70 of my officers being trained for deployment to Liberia. And in fact, in the next weeks, we will have a full team in Liberia who will be serving in the Monrovia Medical Unit and providing health care to both Liberian as well as foreign health care workers. I want to get that message out, because this battle against Ebola is occurring here in the U.S. and being done very well by the CDC and the NIH and elements of the Commissioned Corps who are working with the CDC.
At the same time, we know that the real success of eliminating Ebola and stopping the epidemic lies in Western Africa. Dr. Frieden has said that. We’re confident that there will not be an Ebola outbreak on U.S. soil; however, we need to be able to stop this outbreak. Therefore, I’m very proud of my officers who are heading off to Western Africa.
The role of the PHS Commissioned Corps
RADM Lushniak. Most of my officers are dedicated to who? To serving the underserved and vulnerable populations. Many of my clinical officers are assigned, for example, to the Indian Health Service and are providing care to that important population of our nation. They’re assigned to the Federal Bureau of Prisons and, therefore, working with the Department of Justice in getting health care to, again, a vulnerable and underserved population. They’re working at the NIH in a clinical perspective. They’re treating the Coast Guard as the main medical and dental and environment health officers. So I have officers scattered all around, and in essence, they see everything that any other practitioner sees in this country.
The emphasis certainly from the Office of the Surgeon General has been on prevention. It’s prevention of preventable diseases; many of them are chronic diseases. And certainly, my officers not only are out there treating those individual patients, but at the same time are implementing and taking to task on the importance of prevention as a general theme. We make sure that the word of the Surgeon General’s office gets spread to local communities through our practitioners.
Raising the Commissioned Corps profile
RADM Lushniak. We need to get the word out. Part of our issue, I’ll be honest with you, is that oftentimes people don’t even know the U.S. Public Health Service Commissioned Corps exists. Therefore, even when my officers are part of a Centers for Disease Control and Prevention response, they’re embedded with other facets of CDC.
What I want to proudly say is that right now this Monrovia Medical Unit will be run by U.S. Public Health Service Commissioned Corps. This is the only entity of the U.S. government that will actually have direct patient care responsibilities in Western Africa. That being said, we’re also proud that this year is the 125th anniversary of the Commissioned Corps as a uniformed service in this country. So 125 years ago an act was passed by Congress to be able to establish this uniformed service.
Finally, I’d like to say that no other nation has a uniformed service like this. I keep saying that I love my sister services. I love the Army, the Navy, the Air Force, the Marines, the Coast Guard; but many other nations have similar type entities.
The reality of the situation is that no other nation on this planet has a uniformed service purely dedicated to public health. We are an unarmed service, and we are part of the Department of Health and Human Services, but we are just as proud to be officers. We are just as proud to be serving our nation in uniform on a slightly different mission but one that has, again, a noble cause associated with it.
Reaching the top of the PHS
RADM Lushniak. I’m honored and humbled to be in this position at this stage of my career. I came in 26, almost 27 years ago into the United States Public Health Service as a young lieutenant. My goal at that time was to be an Epidemic Intelligence Service Officer at the Centers for Disease Control and Prevention. That’s how I started my career, doing what’s deemed to be shoe leather epidemiology, going out there and getting my hands dirty and being able to try to make this nation a better place and to protect the public’s health.
It’s been a great ride from the CDC to the FDA, and then ultimately, to the Office of the Secretary here within the Office of the Surgeon General as the Deputy and now as the Acting Surgeon General. The message is everyone should, first of all, acknowledge the fact that we have an incredible mission to undertake. The mission of the Commissioned Corps of the PHS is to protect, promote, and advance the health and safety of our nation. And I dare say although we captured that as our mission, that mission is translatable to almost every federal practitioner that is out there.
The burden of that is apparent—to protect, promote, and advance the health and safety of our nation. And yet it’s a bold and noble mission, one that is achievable. We’ve had incredible successes. We still have a lot of work ahead of us.
So first and foremost, I tell my young officers and I tell everyone who may be exposed to this conversation is the sense that do your job and do it well. That’s really the prime thing I’m asking my officers to do: Be dedicated to the mission and realize that incredible things are still achievable.
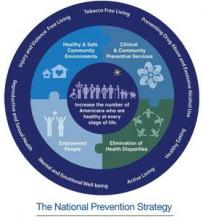
The National Prevention Strategy
The goal is for us to have a healthy population at every stage of life. And so 20 federal partners...came up with a National Prevention Strategy, which is a focus of priming our nation towards prevention and wellness. It’s based on 4 strategic aims, which includes the importance of healthy and safe communities. It also entails the idea of clinical community preventive services. It talks about the empowerment of people, which is a key component of change in this nation, and the elimination of health disparities throughout the nation. It focuses on the really important preventable diseases. And among them, include the elimination of tobacco use, the importance of our really looking at alcohol and substance use in general. It’s looking at the concept of active living, the importance that we move our bodies, and the importance of healthy eating.
Office of the Surgeon General initiatives
RADM Lushniak. First and foremost, the smoking issue still continues, and there will be more on tobacco use and smoking from us. We won’t give up that fight until we’re zeroed out.
In addition, recently we released a call to action on skin cancer prevention. That’s, I think, an important issue as well because we have over 5 million people in the United States each and every year who are treated for skin cancers. We have over 60,000 people who are diagnosed with the most deadly form of skin cancer, melanoma, and 9,000 people, that’s 1 person every hour, dying of melanoma. It has an incredible impact on our country, and it is, again, one of those preventable diseases. So we look at the idea of getting the message out that we, in the Office of Surgeon General, want people to live an active lifestyle. That’s an important part of the National Prevention Strategy.
I want people to be outdoors, I want them to be runners and walkers and enjoying nature; but at the same time, I need to get the message out that we need to be wary of ultraviolet radiation from sunlight, that we can protect ourselves, seek shade when possible, put on a big hat that produces shade on your face and neck and ears. Wear glasses, put on protective clothing, and then use sunscreens, broad-spectrum sunscreens of a UV protective factor of at least 15. That’s one of the initiatives that we recently released.
In the future, where we’re priming, we’re really getting back into the fitness mode. One of the things that we’re working on, and it really simplifies, I think, what has become too complex a message—the idea of how do we have a healthy and fit nation?...
I want people to start walking 30 minutes a day, 5 days a week. Do you realize just by that simple act of walking how good our nation could do in the future? How healthy we can be as a people. So we’re really looking at an emphasis on walking and walkable communities, because not every community is walkable at this stage.
Speaking at the AMSUS Continuing Education Meeting in Washington, D.C.
RADM Lushniak. AMSUS has always provided an excellent forum for the United States Public Health Service Commissioned Corps, of which I am the commander, to be able to share our information with other federal practitioners, with other parties within the federal family that are interested in health care, in public health, in contact with patients on the clinical side and the scientific side....
I’ve been a member over many years, and I’ve been a regular attendee at the meetings. It allows us to cross-fertilize, to have that ability to sit down with our sister services, to be able to sit down with nonuniformed professionals who serve in the federal system under the flag of health care or under the flag of medical care or under the big flag of science, medical science.
Not many health care leaders can transition smoothly from discussing the importance of walking 30 minutes per day to the need to send PHS officers to help control the Ebola epidemic in West Africa. The Surgeon General has to. As the most prominent public health official, the Surgeon General must offer a reassuring voice on health care issues big and small. With over 26 years at the PHS, Rear Admiral (RADM) Boris D. Lushniak, MD, MPH, is well equipped to handle the challenging role.
A year after assuming the role and just before delivering a plenary address at the 2014 AMSUS meeting, RADM Lushniak agreed to a wide-ranging conversation with Federal Practitioner. The following is condensed and edited, but the complete interview can be found at http://www.fedprac.com.
The 50th anniversary of the Surgeon General’s report on smoking
RADM Boris D. Lushniak, Acting Surgeon General. Go back to January 1964 and realize what a different world we lived in back then. In fact, that report, which came out after a year and a half of scientific deliberations, of looking at facts, of searching through the literature, came up with a very important conclusion. That conclusion, simply put was: Smoking is bad for you.
Now it really was a landmark report from that perspective, but when we look back 50 years, what did it prove? It proved that cigarette smoking was directly associated with only 1 cancer at that point, specifically lung cancer in men. The report had a very simple but beautiful conclusion. It said that cigarette smoking is a health hazard of significant importance in the U.S. to warrant appropriate remedial action....
A half-century later, the social norms of our society have changed. We don’t have ashtrays all around. We don’t smoke on airplanes anymore. We oftentimes can’t smoke in bars and restaurants and establishments like that. We’ve moved from 43% of our population that smoked in 1964 to 18% currently.
We’ve had 32 Surgeons’ General reports since that first one....We brought up the issue of secondhand smoke 25 years ago. We talked about the successes and failures over these years, but 50 years later smoking remains a major public health problem in this country....
When we look to the future, what’s the goal? Well we really want to get to a zero point. We reannounced with the 50th anniversary report, which was released in January 2014, that this is an endgame strategy. At some point we have to realize that it’s not good enough to get down to 18% because of the health impact. Cigarettes and tobacco use in this country bring no good; no good to the individual, no good to the individual who has to deal with secondhand smoke, and no good for the future of our nation. So we’re really talking about an endgame strategy....
Our 50th anniversary report wasn’t just looking backward....It contains current data that now show us that we’re up to 13 different cancers caused by tobacco use. We know the impact on the whole human body. In essence, it affects almost every single system of the human body now. Brand-new diseases, formerly not associated with smoking, are still being discovered.
Most recently, we’ve seen diabetes and colon and rectal cancers as some of those diseases. We’re talking about blindness associated with smoking. We’re talking about diseases such as erectile dysfunction, which are associated with smoking. This product has brought nothing but grief and sorrow into our society and continues to do so.
Now it’s not only an impact on the United States for the Office of the Surgeon General’s to speak, but also in essence we know that internationally people look at the Surgeon General reports that come out of this office as that stellar scientific information that then can be translated worldwide.
Not only do we see leadership of public health here within the United States, but we also see leadership on an international level by profiling some of the major public health issues.
The PHS response to Ebola in the U.S. and Africa
RADM Lushniak. As many of the readers may know, the Surgeon General is the commander of the U.S. Public Health Service Commissioned Corps. We have 6,800 public health professionals. These are officers working in 11 different categories working across the government, to protect, promote, and advance the health and safety of the nation.
[In October] I was in Anniston, Alabama, seeing about 70 of my officers being trained for deployment to Liberia. And in fact, in the next weeks, we will have a full team in Liberia who will be serving in the Monrovia Medical Unit and providing health care to both Liberian as well as foreign health care workers. I want to get that message out, because this battle against Ebola is occurring here in the U.S. and being done very well by the CDC and the NIH and elements of the Commissioned Corps who are working with the CDC.
At the same time, we know that the real success of eliminating Ebola and stopping the epidemic lies in Western Africa. Dr. Frieden has said that. We’re confident that there will not be an Ebola outbreak on U.S. soil; however, we need to be able to stop this outbreak. Therefore, I’m very proud of my officers who are heading off to Western Africa.
The role of the PHS Commissioned Corps
RADM Lushniak. Most of my officers are dedicated to who? To serving the underserved and vulnerable populations. Many of my clinical officers are assigned, for example, to the Indian Health Service and are providing care to that important population of our nation. They’re assigned to the Federal Bureau of Prisons and, therefore, working with the Department of Justice in getting health care to, again, a vulnerable and underserved population. They’re working at the NIH in a clinical perspective. They’re treating the Coast Guard as the main medical and dental and environment health officers. So I have officers scattered all around, and in essence, they see everything that any other practitioner sees in this country.
The emphasis certainly from the Office of the Surgeon General has been on prevention. It’s prevention of preventable diseases; many of them are chronic diseases. And certainly, my officers not only are out there treating those individual patients, but at the same time are implementing and taking to task on the importance of prevention as a general theme. We make sure that the word of the Surgeon General’s office gets spread to local communities through our practitioners.
Raising the Commissioned Corps profile
RADM Lushniak. We need to get the word out. Part of our issue, I’ll be honest with you, is that oftentimes people don’t even know the U.S. Public Health Service Commissioned Corps exists. Therefore, even when my officers are part of a Centers for Disease Control and Prevention response, they’re embedded with other facets of CDC.
What I want to proudly say is that right now this Monrovia Medical Unit will be run by U.S. Public Health Service Commissioned Corps. This is the only entity of the U.S. government that will actually have direct patient care responsibilities in Western Africa. That being said, we’re also proud that this year is the 125th anniversary of the Commissioned Corps as a uniformed service in this country. So 125 years ago an act was passed by Congress to be able to establish this uniformed service.
Finally, I’d like to say that no other nation has a uniformed service like this. I keep saying that I love my sister services. I love the Army, the Navy, the Air Force, the Marines, the Coast Guard; but many other nations have similar type entities.
The reality of the situation is that no other nation on this planet has a uniformed service purely dedicated to public health. We are an unarmed service, and we are part of the Department of Health and Human Services, but we are just as proud to be officers. We are just as proud to be serving our nation in uniform on a slightly different mission but one that has, again, a noble cause associated with it.
Reaching the top of the PHS
RADM Lushniak. I’m honored and humbled to be in this position at this stage of my career. I came in 26, almost 27 years ago into the United States Public Health Service as a young lieutenant. My goal at that time was to be an Epidemic Intelligence Service Officer at the Centers for Disease Control and Prevention. That’s how I started my career, doing what’s deemed to be shoe leather epidemiology, going out there and getting my hands dirty and being able to try to make this nation a better place and to protect the public’s health.
It’s been a great ride from the CDC to the FDA, and then ultimately, to the Office of the Secretary here within the Office of the Surgeon General as the Deputy and now as the Acting Surgeon General. The message is everyone should, first of all, acknowledge the fact that we have an incredible mission to undertake. The mission of the Commissioned Corps of the PHS is to protect, promote, and advance the health and safety of our nation. And I dare say although we captured that as our mission, that mission is translatable to almost every federal practitioner that is out there.
The burden of that is apparent—to protect, promote, and advance the health and safety of our nation. And yet it’s a bold and noble mission, one that is achievable. We’ve had incredible successes. We still have a lot of work ahead of us.
So first and foremost, I tell my young officers and I tell everyone who may be exposed to this conversation is the sense that do your job and do it well. That’s really the prime thing I’m asking my officers to do: Be dedicated to the mission and realize that incredible things are still achievable.
The National Prevention Strategy
The goal is for us to have a healthy population at every stage of life. And so 20 federal partners...came up with a National Prevention Strategy, which is a focus of priming our nation towards prevention and wellness. It’s based on 4 strategic aims, which includes the importance of healthy and safe communities. It also entails the idea of clinical community preventive services. It talks about the empowerment of people, which is a key component of change in this nation, and the elimination of health disparities throughout the nation. It focuses on the really important preventable diseases. And among them, include the elimination of tobacco use, the importance of our really looking at alcohol and substance use in general. It’s looking at the concept of active living, the importance that we move our bodies, and the importance of healthy eating.
Office of the Surgeon General initiatives
RADM Lushniak. First and foremost, the smoking issue still continues, and there will be more on tobacco use and smoking from us. We won’t give up that fight until we’re zeroed out.
In addition, recently we released a call to action on skin cancer prevention. That’s, I think, an important issue as well because we have over 5 million people in the United States each and every year who are treated for skin cancers. We have over 60,000 people who are diagnosed with the most deadly form of skin cancer, melanoma, and 9,000 people, that’s 1 person every hour, dying of melanoma. It has an incredible impact on our country, and it is, again, one of those preventable diseases. So we look at the idea of getting the message out that we, in the Office of Surgeon General, want people to live an active lifestyle. That’s an important part of the National Prevention Strategy.
I want people to be outdoors, I want them to be runners and walkers and enjoying nature; but at the same time, I need to get the message out that we need to be wary of ultraviolet radiation from sunlight, that we can protect ourselves, seek shade when possible, put on a big hat that produces shade on your face and neck and ears. Wear glasses, put on protective clothing, and then use sunscreens, broad-spectrum sunscreens of a UV protective factor of at least 15. That’s one of the initiatives that we recently released.
In the future, where we’re priming, we’re really getting back into the fitness mode. One of the things that we’re working on, and it really simplifies, I think, what has become too complex a message—the idea of how do we have a healthy and fit nation?...
I want people to start walking 30 minutes a day, 5 days a week. Do you realize just by that simple act of walking how good our nation could do in the future? How healthy we can be as a people. So we’re really looking at an emphasis on walking and walkable communities, because not every community is walkable at this stage.
Speaking at the AMSUS Continuing Education Meeting in Washington, D.C.
RADM Lushniak. AMSUS has always provided an excellent forum for the United States Public Health Service Commissioned Corps, of which I am the commander, to be able to share our information with other federal practitioners, with other parties within the federal family that are interested in health care, in public health, in contact with patients on the clinical side and the scientific side....
I’ve been a member over many years, and I’ve been a regular attendee at the meetings. It allows us to cross-fertilize, to have that ability to sit down with our sister services, to be able to sit down with nonuniformed professionals who serve in the federal system under the flag of health care or under the flag of medical care or under the big flag of science, medical science.
Not many health care leaders can transition smoothly from discussing the importance of walking 30 minutes per day to the need to send PHS officers to help control the Ebola epidemic in West Africa. The Surgeon General has to. As the most prominent public health official, the Surgeon General must offer a reassuring voice on health care issues big and small. With over 26 years at the PHS, Rear Admiral (RADM) Boris D. Lushniak, MD, MPH, is well equipped to handle the challenging role.
A year after assuming the role and just before delivering a plenary address at the 2014 AMSUS meeting, RADM Lushniak agreed to a wide-ranging conversation with Federal Practitioner. The following is condensed and edited, but the complete interview can be found at http://www.fedprac.com.
The 50th anniversary of the Surgeon General’s report on smoking
RADM Boris D. Lushniak, Acting Surgeon General. Go back to January 1964 and realize what a different world we lived in back then. In fact, that report, which came out after a year and a half of scientific deliberations, of looking at facts, of searching through the literature, came up with a very important conclusion. That conclusion, simply put was: Smoking is bad for you.
Now it really was a landmark report from that perspective, but when we look back 50 years, what did it prove? It proved that cigarette smoking was directly associated with only 1 cancer at that point, specifically lung cancer in men. The report had a very simple but beautiful conclusion. It said that cigarette smoking is a health hazard of significant importance in the U.S. to warrant appropriate remedial action....
A half-century later, the social norms of our society have changed. We don’t have ashtrays all around. We don’t smoke on airplanes anymore. We oftentimes can’t smoke in bars and restaurants and establishments like that. We’ve moved from 43% of our population that smoked in 1964 to 18% currently.
We’ve had 32 Surgeons’ General reports since that first one....We brought up the issue of secondhand smoke 25 years ago. We talked about the successes and failures over these years, but 50 years later smoking remains a major public health problem in this country....
When we look to the future, what’s the goal? Well we really want to get to a zero point. We reannounced with the 50th anniversary report, which was released in January 2014, that this is an endgame strategy. At some point we have to realize that it’s not good enough to get down to 18% because of the health impact. Cigarettes and tobacco use in this country bring no good; no good to the individual, no good to the individual who has to deal with secondhand smoke, and no good for the future of our nation. So we’re really talking about an endgame strategy....
Our 50th anniversary report wasn’t just looking backward....It contains current data that now show us that we’re up to 13 different cancers caused by tobacco use. We know the impact on the whole human body. In essence, it affects almost every single system of the human body now. Brand-new diseases, formerly not associated with smoking, are still being discovered.
Most recently, we’ve seen diabetes and colon and rectal cancers as some of those diseases. We’re talking about blindness associated with smoking. We’re talking about diseases such as erectile dysfunction, which are associated with smoking. This product has brought nothing but grief and sorrow into our society and continues to do so.
Now it’s not only an impact on the United States for the Office of the Surgeon General’s to speak, but also in essence we know that internationally people look at the Surgeon General reports that come out of this office as that stellar scientific information that then can be translated worldwide.
Not only do we see leadership of public health here within the United States, but we also see leadership on an international level by profiling some of the major public health issues.
The PHS response to Ebola in the U.S. and Africa
RADM Lushniak. As many of the readers may know, the Surgeon General is the commander of the U.S. Public Health Service Commissioned Corps. We have 6,800 public health professionals. These are officers working in 11 different categories working across the government, to protect, promote, and advance the health and safety of the nation.
[In October] I was in Anniston, Alabama, seeing about 70 of my officers being trained for deployment to Liberia. And in fact, in the next weeks, we will have a full team in Liberia who will be serving in the Monrovia Medical Unit and providing health care to both Liberian as well as foreign health care workers. I want to get that message out, because this battle against Ebola is occurring here in the U.S. and being done very well by the CDC and the NIH and elements of the Commissioned Corps who are working with the CDC.
At the same time, we know that the real success of eliminating Ebola and stopping the epidemic lies in Western Africa. Dr. Frieden has said that. We’re confident that there will not be an Ebola outbreak on U.S. soil; however, we need to be able to stop this outbreak. Therefore, I’m very proud of my officers who are heading off to Western Africa.
The role of the PHS Commissioned Corps
RADM Lushniak. Most of my officers are dedicated to who? To serving the underserved and vulnerable populations. Many of my clinical officers are assigned, for example, to the Indian Health Service and are providing care to that important population of our nation. They’re assigned to the Federal Bureau of Prisons and, therefore, working with the Department of Justice in getting health care to, again, a vulnerable and underserved population. They’re working at the NIH in a clinical perspective. They’re treating the Coast Guard as the main medical and dental and environment health officers. So I have officers scattered all around, and in essence, they see everything that any other practitioner sees in this country.
The emphasis certainly from the Office of the Surgeon General has been on prevention. It’s prevention of preventable diseases; many of them are chronic diseases. And certainly, my officers not only are out there treating those individual patients, but at the same time are implementing and taking to task on the importance of prevention as a general theme. We make sure that the word of the Surgeon General’s office gets spread to local communities through our practitioners.
Raising the Commissioned Corps profile
RADM Lushniak. We need to get the word out. Part of our issue, I’ll be honest with you, is that oftentimes people don’t even know the U.S. Public Health Service Commissioned Corps exists. Therefore, even when my officers are part of a Centers for Disease Control and Prevention response, they’re embedded with other facets of CDC.
What I want to proudly say is that right now this Monrovia Medical Unit will be run by U.S. Public Health Service Commissioned Corps. This is the only entity of the U.S. government that will actually have direct patient care responsibilities in Western Africa. That being said, we’re also proud that this year is the 125th anniversary of the Commissioned Corps as a uniformed service in this country. So 125 years ago an act was passed by Congress to be able to establish this uniformed service.
Finally, I’d like to say that no other nation has a uniformed service like this. I keep saying that I love my sister services. I love the Army, the Navy, the Air Force, the Marines, the Coast Guard; but many other nations have similar type entities.
The reality of the situation is that no other nation on this planet has a uniformed service purely dedicated to public health. We are an unarmed service, and we are part of the Department of Health and Human Services, but we are just as proud to be officers. We are just as proud to be serving our nation in uniform on a slightly different mission but one that has, again, a noble cause associated with it.
Reaching the top of the PHS
RADM Lushniak. I’m honored and humbled to be in this position at this stage of my career. I came in 26, almost 27 years ago into the United States Public Health Service as a young lieutenant. My goal at that time was to be an Epidemic Intelligence Service Officer at the Centers for Disease Control and Prevention. That’s how I started my career, doing what’s deemed to be shoe leather epidemiology, going out there and getting my hands dirty and being able to try to make this nation a better place and to protect the public’s health.
It’s been a great ride from the CDC to the FDA, and then ultimately, to the Office of the Secretary here within the Office of the Surgeon General as the Deputy and now as the Acting Surgeon General. The message is everyone should, first of all, acknowledge the fact that we have an incredible mission to undertake. The mission of the Commissioned Corps of the PHS is to protect, promote, and advance the health and safety of our nation. And I dare say although we captured that as our mission, that mission is translatable to almost every federal practitioner that is out there.
The burden of that is apparent—to protect, promote, and advance the health and safety of our nation. And yet it’s a bold and noble mission, one that is achievable. We’ve had incredible successes. We still have a lot of work ahead of us.
So first and foremost, I tell my young officers and I tell everyone who may be exposed to this conversation is the sense that do your job and do it well. That’s really the prime thing I’m asking my officers to do: Be dedicated to the mission and realize that incredible things are still achievable.
The National Prevention Strategy
The goal is for us to have a healthy population at every stage of life. And so 20 federal partners...came up with a National Prevention Strategy, which is a focus of priming our nation towards prevention and wellness. It’s based on 4 strategic aims, which includes the importance of healthy and safe communities. It also entails the idea of clinical community preventive services. It talks about the empowerment of people, which is a key component of change in this nation, and the elimination of health disparities throughout the nation. It focuses on the really important preventable diseases. And among them, include the elimination of tobacco use, the importance of our really looking at alcohol and substance use in general. It’s looking at the concept of active living, the importance that we move our bodies, and the importance of healthy eating.
Office of the Surgeon General initiatives
RADM Lushniak. First and foremost, the smoking issue still continues, and there will be more on tobacco use and smoking from us. We won’t give up that fight until we’re zeroed out.
In addition, recently we released a call to action on skin cancer prevention. That’s, I think, an important issue as well because we have over 5 million people in the United States each and every year who are treated for skin cancers. We have over 60,000 people who are diagnosed with the most deadly form of skin cancer, melanoma, and 9,000 people, that’s 1 person every hour, dying of melanoma. It has an incredible impact on our country, and it is, again, one of those preventable diseases. So we look at the idea of getting the message out that we, in the Office of Surgeon General, want people to live an active lifestyle. That’s an important part of the National Prevention Strategy.
I want people to be outdoors, I want them to be runners and walkers and enjoying nature; but at the same time, I need to get the message out that we need to be wary of ultraviolet radiation from sunlight, that we can protect ourselves, seek shade when possible, put on a big hat that produces shade on your face and neck and ears. Wear glasses, put on protective clothing, and then use sunscreens, broad-spectrum sunscreens of a UV protective factor of at least 15. That’s one of the initiatives that we recently released.
In the future, where we’re priming, we’re really getting back into the fitness mode. One of the things that we’re working on, and it really simplifies, I think, what has become too complex a message—the idea of how do we have a healthy and fit nation?...
I want people to start walking 30 minutes a day, 5 days a week. Do you realize just by that simple act of walking how good our nation could do in the future? How healthy we can be as a people. So we’re really looking at an emphasis on walking and walkable communities, because not every community is walkable at this stage.
Speaking at the AMSUS Continuing Education Meeting in Washington, D.C.
RADM Lushniak. AMSUS has always provided an excellent forum for the United States Public Health Service Commissioned Corps, of which I am the commander, to be able to share our information with other federal practitioners, with other parties within the federal family that are interested in health care, in public health, in contact with patients on the clinical side and the scientific side....
I’ve been a member over many years, and I’ve been a regular attendee at the meetings. It allows us to cross-fertilize, to have that ability to sit down with our sister services, to be able to sit down with nonuniformed professionals who serve in the federal system under the flag of health care or under the flag of medical care or under the big flag of science, medical science.
The 'Spidey Sense' of doctors
Of all the things you learn in training, one of the most nebulous, but useful, is “Spidey Sense.”
In comics, Spider-Man had a power called Spidey Sense, which caused a skull-base tingling when danger was present. It was a prescient, clairvoyant ability that allowed him to take action to protect himself.
Somewhere along the line, most doctors I know get a similar ability, but it warns us when something is seriously wrong with a patient. Often, it fires before you even have a rational reason to be worried, and it’s almost never wrong.
As a senior in medical school, I heard a conversation between a resident and an attending. The resident was talking about how she’d seen a patient in the emergency department who she sent to the ICU without a concrete reason. An hour after arriving, the patient suffered a cardiac arrest and was successfully resuscitated. The attending told her that this was one of the most critical skills to develop: knowing when patients are really sick, even before you have any obvious reason to think they are.
I have no idea when I learned it. At some point it was just there. I assume it’s a result of years of medical training making you subconsciously recognize a bad situation. It doesn’t fire very often, but when it does it can’t be ignored. Sometimes even a few words typed on my schedule will set it off.
My staff knows when it’s hit me because I’ll bring an MRI order up to the front desk before I’ve completed the appointment and tell them to start working on it.
Not every sick patient sets it off. In fact, obviously sick people never do. In those cases, it’s not needed. But when the tingling starts when you first start talking to someone … don’t ignore it.
There are a lot of intangibles in medicine, and this is one of them. I can’t explain it, but it’s one of the most important skills I’ve learned, although I have no idea when I did. I’m just glad it’s there.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Of all the things you learn in training, one of the most nebulous, but useful, is “Spidey Sense.”
In comics, Spider-Man had a power called Spidey Sense, which caused a skull-base tingling when danger was present. It was a prescient, clairvoyant ability that allowed him to take action to protect himself.
Somewhere along the line, most doctors I know get a similar ability, but it warns us when something is seriously wrong with a patient. Often, it fires before you even have a rational reason to be worried, and it’s almost never wrong.
As a senior in medical school, I heard a conversation between a resident and an attending. The resident was talking about how she’d seen a patient in the emergency department who she sent to the ICU without a concrete reason. An hour after arriving, the patient suffered a cardiac arrest and was successfully resuscitated. The attending told her that this was one of the most critical skills to develop: knowing when patients are really sick, even before you have any obvious reason to think they are.
I have no idea when I learned it. At some point it was just there. I assume it’s a result of years of medical training making you subconsciously recognize a bad situation. It doesn’t fire very often, but when it does it can’t be ignored. Sometimes even a few words typed on my schedule will set it off.
My staff knows when it’s hit me because I’ll bring an MRI order up to the front desk before I’ve completed the appointment and tell them to start working on it.
Not every sick patient sets it off. In fact, obviously sick people never do. In those cases, it’s not needed. But when the tingling starts when you first start talking to someone … don’t ignore it.
There are a lot of intangibles in medicine, and this is one of them. I can’t explain it, but it’s one of the most important skills I’ve learned, although I have no idea when I did. I’m just glad it’s there.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Of all the things you learn in training, one of the most nebulous, but useful, is “Spidey Sense.”
In comics, Spider-Man had a power called Spidey Sense, which caused a skull-base tingling when danger was present. It was a prescient, clairvoyant ability that allowed him to take action to protect himself.
Somewhere along the line, most doctors I know get a similar ability, but it warns us when something is seriously wrong with a patient. Often, it fires before you even have a rational reason to be worried, and it’s almost never wrong.
As a senior in medical school, I heard a conversation between a resident and an attending. The resident was talking about how she’d seen a patient in the emergency department who she sent to the ICU without a concrete reason. An hour after arriving, the patient suffered a cardiac arrest and was successfully resuscitated. The attending told her that this was one of the most critical skills to develop: knowing when patients are really sick, even before you have any obvious reason to think they are.
I have no idea when I learned it. At some point it was just there. I assume it’s a result of years of medical training making you subconsciously recognize a bad situation. It doesn’t fire very often, but when it does it can’t be ignored. Sometimes even a few words typed on my schedule will set it off.
My staff knows when it’s hit me because I’ll bring an MRI order up to the front desk before I’ve completed the appointment and tell them to start working on it.
Not every sick patient sets it off. In fact, obviously sick people never do. In those cases, it’s not needed. But when the tingling starts when you first start talking to someone … don’t ignore it.
There are a lot of intangibles in medicine, and this is one of them. I can’t explain it, but it’s one of the most important skills I’ve learned, although I have no idea when I did. I’m just glad it’s there.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Laser treatments for men
In our final segment on male dermatology, we will be focusing on laser treatments in men. There has been a steady increase in cosmetic procedures in men over the last decade, and laser procedures tend to be some of the most popular. In general, laser treatments provide faster results than topical or oral treatments and offer subtle aesthetic improvements with little to no downtime depending on the procedure. These factors appeal to male patients, who generally are generally less risk tolerant than women, and want masculinizing treatments with little downtime and natural results.
• Hair growth. Men tend to have highly pigmented, thicker hair in contrast to women, and often seek laser hair removal for excess body hair. Common sites include the back, upper arms, posterior hairline, lower beardline, and chest. Similar precautions apply to both men and women, such as proper cooling of the skin and avoidance of tanned skin. However, laser settings for male patients may need to be adjusted given the thicker, darkly pigmented hairs and often lower pain threshold. In addition, proper counseling of men is necessary with laser hair removal, because men often need more treatments than women and may need a topical anesthetic for highly sensitive areas.
• Body contouring. Men tend to deposit fat in hard-to-lose areas, such as the central abdomen and flanks. The expanding array of noninvasive devices using cold temperatures to freeze the fat, or ultrasound and radiofrequency devices to heat and thereby tighten the subcutaneous tissue have made body contouring one of the fastest growing cosmetic markets for men. Men are great candidates for these procedures given the fast results, minor discomfort, and noninvasive nature. Although many men have visceral abdominal fat that does not respond to these treatments, areas often treated with great long-term results include the upper and lower abdomen, flanks, arms, chest, and back.
• Rosacea. Men have a higher density of facial blood vessels than women, and they often seek treatment for telangiectasias and overall facial erythema. For noninflammatory erythematotelangiectatic rosacea, vascular laser treatments are the most effective treatments. Pulsed dye laser is often the best laser to target both large and small facial blood vessels and flushing erythema. Intense pulsed light (IPL) lasers are often a more popular choice for men because they involve less downtime and can treat brown spots as well. However, IPL must be used with caution in skin of color and tanned skin because of the risks of scarring and hyperpigmentation. Men may need more treatments and higher energy settings than women. Men also prefer minimal downtime and thus more frequent nonpurpuric settings are often preferred with any vascular laser. In addition, with IPL, men should be warned of the possibility of the laser temporarily stunting hair growth or causing hair to grow in patchy temporarily when using the device in the beard or mustache area.
• Laser resurfacing. Laser skin resurfacing can be performed for acne scars, rhytids, age sports, sun spots, melasma, and overall skin laxity. Options include ablative and nonablative skin resurfacing. The choice of procedure depends on the type of problem being treated, skin type, and downtime. Ablative CO2, erbium:YAG, and fractional ablative lasers provide the best results for deep rhytids, acne scars, surgical scars, and skin laxity. However, men often shy away from these procedures given the pain, postprocedure care necessary, and downtime. Nonablative lasers may be a better choice for men, particularly for fine rhytids, melasma, and sun spots. With multiple treatments, they also may be used for scars and skin laxity. Postprocedure skincare and downtime are the critical factors for men when choosing resurfacing procedures, and detailed review of the care, complexity, and side effects are essential in the care of male patients.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
In our final segment on male dermatology, we will be focusing on laser treatments in men. There has been a steady increase in cosmetic procedures in men over the last decade, and laser procedures tend to be some of the most popular. In general, laser treatments provide faster results than topical or oral treatments and offer subtle aesthetic improvements with little to no downtime depending on the procedure. These factors appeal to male patients, who generally are generally less risk tolerant than women, and want masculinizing treatments with little downtime and natural results.
• Hair growth. Men tend to have highly pigmented, thicker hair in contrast to women, and often seek laser hair removal for excess body hair. Common sites include the back, upper arms, posterior hairline, lower beardline, and chest. Similar precautions apply to both men and women, such as proper cooling of the skin and avoidance of tanned skin. However, laser settings for male patients may need to be adjusted given the thicker, darkly pigmented hairs and often lower pain threshold. In addition, proper counseling of men is necessary with laser hair removal, because men often need more treatments than women and may need a topical anesthetic for highly sensitive areas.
• Body contouring. Men tend to deposit fat in hard-to-lose areas, such as the central abdomen and flanks. The expanding array of noninvasive devices using cold temperatures to freeze the fat, or ultrasound and radiofrequency devices to heat and thereby tighten the subcutaneous tissue have made body contouring one of the fastest growing cosmetic markets for men. Men are great candidates for these procedures given the fast results, minor discomfort, and noninvasive nature. Although many men have visceral abdominal fat that does not respond to these treatments, areas often treated with great long-term results include the upper and lower abdomen, flanks, arms, chest, and back.
• Rosacea. Men have a higher density of facial blood vessels than women, and they often seek treatment for telangiectasias and overall facial erythema. For noninflammatory erythematotelangiectatic rosacea, vascular laser treatments are the most effective treatments. Pulsed dye laser is often the best laser to target both large and small facial blood vessels and flushing erythema. Intense pulsed light (IPL) lasers are often a more popular choice for men because they involve less downtime and can treat brown spots as well. However, IPL must be used with caution in skin of color and tanned skin because of the risks of scarring and hyperpigmentation. Men may need more treatments and higher energy settings than women. Men also prefer minimal downtime and thus more frequent nonpurpuric settings are often preferred with any vascular laser. In addition, with IPL, men should be warned of the possibility of the laser temporarily stunting hair growth or causing hair to grow in patchy temporarily when using the device in the beard or mustache area.
• Laser resurfacing. Laser skin resurfacing can be performed for acne scars, rhytids, age sports, sun spots, melasma, and overall skin laxity. Options include ablative and nonablative skin resurfacing. The choice of procedure depends on the type of problem being treated, skin type, and downtime. Ablative CO2, erbium:YAG, and fractional ablative lasers provide the best results for deep rhytids, acne scars, surgical scars, and skin laxity. However, men often shy away from these procedures given the pain, postprocedure care necessary, and downtime. Nonablative lasers may be a better choice for men, particularly for fine rhytids, melasma, and sun spots. With multiple treatments, they also may be used for scars and skin laxity. Postprocedure skincare and downtime are the critical factors for men when choosing resurfacing procedures, and detailed review of the care, complexity, and side effects are essential in the care of male patients.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
In our final segment on male dermatology, we will be focusing on laser treatments in men. There has been a steady increase in cosmetic procedures in men over the last decade, and laser procedures tend to be some of the most popular. In general, laser treatments provide faster results than topical or oral treatments and offer subtle aesthetic improvements with little to no downtime depending on the procedure. These factors appeal to male patients, who generally are generally less risk tolerant than women, and want masculinizing treatments with little downtime and natural results.
• Hair growth. Men tend to have highly pigmented, thicker hair in contrast to women, and often seek laser hair removal for excess body hair. Common sites include the back, upper arms, posterior hairline, lower beardline, and chest. Similar precautions apply to both men and women, such as proper cooling of the skin and avoidance of tanned skin. However, laser settings for male patients may need to be adjusted given the thicker, darkly pigmented hairs and often lower pain threshold. In addition, proper counseling of men is necessary with laser hair removal, because men often need more treatments than women and may need a topical anesthetic for highly sensitive areas.
• Body contouring. Men tend to deposit fat in hard-to-lose areas, such as the central abdomen and flanks. The expanding array of noninvasive devices using cold temperatures to freeze the fat, or ultrasound and radiofrequency devices to heat and thereby tighten the subcutaneous tissue have made body contouring one of the fastest growing cosmetic markets for men. Men are great candidates for these procedures given the fast results, minor discomfort, and noninvasive nature. Although many men have visceral abdominal fat that does not respond to these treatments, areas often treated with great long-term results include the upper and lower abdomen, flanks, arms, chest, and back.
• Rosacea. Men have a higher density of facial blood vessels than women, and they often seek treatment for telangiectasias and overall facial erythema. For noninflammatory erythematotelangiectatic rosacea, vascular laser treatments are the most effective treatments. Pulsed dye laser is often the best laser to target both large and small facial blood vessels and flushing erythema. Intense pulsed light (IPL) lasers are often a more popular choice for men because they involve less downtime and can treat brown spots as well. However, IPL must be used with caution in skin of color and tanned skin because of the risks of scarring and hyperpigmentation. Men may need more treatments and higher energy settings than women. Men also prefer minimal downtime and thus more frequent nonpurpuric settings are often preferred with any vascular laser. In addition, with IPL, men should be warned of the possibility of the laser temporarily stunting hair growth or causing hair to grow in patchy temporarily when using the device in the beard or mustache area.
• Laser resurfacing. Laser skin resurfacing can be performed for acne scars, rhytids, age sports, sun spots, melasma, and overall skin laxity. Options include ablative and nonablative skin resurfacing. The choice of procedure depends on the type of problem being treated, skin type, and downtime. Ablative CO2, erbium:YAG, and fractional ablative lasers provide the best results for deep rhytids, acne scars, surgical scars, and skin laxity. However, men often shy away from these procedures given the pain, postprocedure care necessary, and downtime. Nonablative lasers may be a better choice for men, particularly for fine rhytids, melasma, and sun spots. With multiple treatments, they also may be used for scars and skin laxity. Postprocedure skincare and downtime are the critical factors for men when choosing resurfacing procedures, and detailed review of the care, complexity, and side effects are essential in the care of male patients.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
Preop risk assessment, prophylaxis for VTE
The majority of women with gynecologic cancer will undergo surgery for their disease. Deep vein thrombosis and pulmonary embolism, or venous thromboembolic events are common, serious complications. The rate of pulmonary embolism in women with gynecologic malignancy may be as high as 6.8%, with the case fatality rate being 11%-12%. Hence, one key strategy to lower the rate of fatal pulmonary embolism depends on proper prophylaxis for deep vein thrombosis prevention.
Factors associated with the development of venous thromboembolic events (VTE) include prior VTE, malignancy, older age, African American race, prolonged operative time, and prior radiation therapy (Obstet. Gynecol. 1987;69:146-50). The risk of pulmonary embolism (PE) in women undergoing gynecologic surgery is quadrupled in the presence of malignancy (Obstet. Gynecol. 2006;107:666-71) and these patients are twice as likely to die from a VTE, compared with matched controls (Gynecol. Oncol. 2007;106:439-45). In addition, cancer patients are typically older and have longer and more complex surgeries. Furthermore, the presence of a pelvic mass further contributes to venous stasis (Obstet. Gynecol. 2012;119:155-67).
Other risk factors associated with the development of VTE include hormone replacement therapy, oral contraceptives, use of tamoxifen, and inherited thrombophilias. The most common is factor V Leiden deficiency, affecting up to 20% of patients with VTE. Affected heterozygotes have a 3- to 8-fold increased risk of VTE, whereas homozygotes have a 50- to 80-fold increased risk (Blood 1995;85:1504-8).
Depending on additional risk factors, both the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin and guidelines published by the American College of Chest Physicians (ACCP) place women with gynecologic cancers into "high" or "highest" risk categories (Obstet. Gynecol. 2007;110:429-40; Chest 2012;141:e227S-77S).
Currently, thromboprophylaxis regimens include mechanical and pharmacologic methods. Mechanical devices include graduated compression stockings and intermittent pneumatic compression, which reduce venous stasis and may promote endogenous fibrinolysis. Pharmacologic prophylaxis includes unfractionated heparin (UFH) and low-molecular weight heparin (LMWH). Prospective controlled trials have shown that UFH reduces VTE in patients with gynecologic cancer. Trials comparing LMWH with UFH have demonstrated equivalent efficacy and similar bleeding complications. The recommended prophylactic dose for LMWH is 40 mg subcutaneous injection daily. However, this dose may need to be adjusted in morbidly obese patients (body mass index greater than 40 kg/m2) as well as in women with abnormal renal clearance. UFH should be administered as a dose of 5,000 units subcutaneously three times daily. Intermittent pneumatic compression also has been shown to reduce the incidence of VTE in this patient population.
A combined regimen of pharmacologic and mechanical prophylaxis may improve efficacy, especially in the highest-risk patients, such as women with gynecologic cancer. Although limited data exist to support this approach in gynecology patients, studies from other surgical disciplines suggest benefit from a combined regimen. With regards to addressing the timing of initiation, a large retrospective trial of patients undergoing hysterectomy for benign indications concluded that postoperative rather than preoperative administration of UFH or LMWH may reduce the risk of bleeding complications without apparent risk of increased VTE (Acta. Obstet. Gynecol. Scand. 2008;87:1039-47).
In summary, the majority of gynecologic oncology patients are considered to be at the highest risk for developing VTE. For this group of women, double prophylaxis with either UFH or LMWH, and a mechanical method (intermittent pneumatic compression) are recommended in the perioperative setting. In addition, ACCP further recommends that these patients receive extended postoperative prophylaxis with LMWH for 4 weeks. Further evidence is needed to determine acceptable timing for initiation of therapy in order to find a balance between adequate thromboprophylaxis and bleeding complications.
Dr. Roque is a fellow in the gynecologic oncology program at the University of North Carolina at Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology and a professor in the division of gynecologic oncology at the university. Dr. Roque and Dr. Clarke-Pearson said they had no relevant disclosures. Scan this QR code or go to obgynnews.com to view similar columns.
The majority of women with gynecologic cancer will undergo surgery for their disease. Deep vein thrombosis and pulmonary embolism, or venous thromboembolic events are common, serious complications. The rate of pulmonary embolism in women with gynecologic malignancy may be as high as 6.8%, with the case fatality rate being 11%-12%. Hence, one key strategy to lower the rate of fatal pulmonary embolism depends on proper prophylaxis for deep vein thrombosis prevention.
Factors associated with the development of venous thromboembolic events (VTE) include prior VTE, malignancy, older age, African American race, prolonged operative time, and prior radiation therapy (Obstet. Gynecol. 1987;69:146-50). The risk of pulmonary embolism (PE) in women undergoing gynecologic surgery is quadrupled in the presence of malignancy (Obstet. Gynecol. 2006;107:666-71) and these patients are twice as likely to die from a VTE, compared with matched controls (Gynecol. Oncol. 2007;106:439-45). In addition, cancer patients are typically older and have longer and more complex surgeries. Furthermore, the presence of a pelvic mass further contributes to venous stasis (Obstet. Gynecol. 2012;119:155-67).
Other risk factors associated with the development of VTE include hormone replacement therapy, oral contraceptives, use of tamoxifen, and inherited thrombophilias. The most common is factor V Leiden deficiency, affecting up to 20% of patients with VTE. Affected heterozygotes have a 3- to 8-fold increased risk of VTE, whereas homozygotes have a 50- to 80-fold increased risk (Blood 1995;85:1504-8).
Depending on additional risk factors, both the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin and guidelines published by the American College of Chest Physicians (ACCP) place women with gynecologic cancers into "high" or "highest" risk categories (Obstet. Gynecol. 2007;110:429-40; Chest 2012;141:e227S-77S).
Currently, thromboprophylaxis regimens include mechanical and pharmacologic methods. Mechanical devices include graduated compression stockings and intermittent pneumatic compression, which reduce venous stasis and may promote endogenous fibrinolysis. Pharmacologic prophylaxis includes unfractionated heparin (UFH) and low-molecular weight heparin (LMWH). Prospective controlled trials have shown that UFH reduces VTE in patients with gynecologic cancer. Trials comparing LMWH with UFH have demonstrated equivalent efficacy and similar bleeding complications. The recommended prophylactic dose for LMWH is 40 mg subcutaneous injection daily. However, this dose may need to be adjusted in morbidly obese patients (body mass index greater than 40 kg/m2) as well as in women with abnormal renal clearance. UFH should be administered as a dose of 5,000 units subcutaneously three times daily. Intermittent pneumatic compression also has been shown to reduce the incidence of VTE in this patient population.
A combined regimen of pharmacologic and mechanical prophylaxis may improve efficacy, especially in the highest-risk patients, such as women with gynecologic cancer. Although limited data exist to support this approach in gynecology patients, studies from other surgical disciplines suggest benefit from a combined regimen. With regards to addressing the timing of initiation, a large retrospective trial of patients undergoing hysterectomy for benign indications concluded that postoperative rather than preoperative administration of UFH or LMWH may reduce the risk of bleeding complications without apparent risk of increased VTE (Acta. Obstet. Gynecol. Scand. 2008;87:1039-47).
In summary, the majority of gynecologic oncology patients are considered to be at the highest risk for developing VTE. For this group of women, double prophylaxis with either UFH or LMWH, and a mechanical method (intermittent pneumatic compression) are recommended in the perioperative setting. In addition, ACCP further recommends that these patients receive extended postoperative prophylaxis with LMWH for 4 weeks. Further evidence is needed to determine acceptable timing for initiation of therapy in order to find a balance between adequate thromboprophylaxis and bleeding complications.
Dr. Roque is a fellow in the gynecologic oncology program at the University of North Carolina at Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology and a professor in the division of gynecologic oncology at the university. Dr. Roque and Dr. Clarke-Pearson said they had no relevant disclosures. Scan this QR code or go to obgynnews.com to view similar columns.
The majority of women with gynecologic cancer will undergo surgery for their disease. Deep vein thrombosis and pulmonary embolism, or venous thromboembolic events are common, serious complications. The rate of pulmonary embolism in women with gynecologic malignancy may be as high as 6.8%, with the case fatality rate being 11%-12%. Hence, one key strategy to lower the rate of fatal pulmonary embolism depends on proper prophylaxis for deep vein thrombosis prevention.
Factors associated with the development of venous thromboembolic events (VTE) include prior VTE, malignancy, older age, African American race, prolonged operative time, and prior radiation therapy (Obstet. Gynecol. 1987;69:146-50). The risk of pulmonary embolism (PE) in women undergoing gynecologic surgery is quadrupled in the presence of malignancy (Obstet. Gynecol. 2006;107:666-71) and these patients are twice as likely to die from a VTE, compared with matched controls (Gynecol. Oncol. 2007;106:439-45). In addition, cancer patients are typically older and have longer and more complex surgeries. Furthermore, the presence of a pelvic mass further contributes to venous stasis (Obstet. Gynecol. 2012;119:155-67).
Other risk factors associated with the development of VTE include hormone replacement therapy, oral contraceptives, use of tamoxifen, and inherited thrombophilias. The most common is factor V Leiden deficiency, affecting up to 20% of patients with VTE. Affected heterozygotes have a 3- to 8-fold increased risk of VTE, whereas homozygotes have a 50- to 80-fold increased risk (Blood 1995;85:1504-8).
Depending on additional risk factors, both the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin and guidelines published by the American College of Chest Physicians (ACCP) place women with gynecologic cancers into "high" or "highest" risk categories (Obstet. Gynecol. 2007;110:429-40; Chest 2012;141:e227S-77S).
Currently, thromboprophylaxis regimens include mechanical and pharmacologic methods. Mechanical devices include graduated compression stockings and intermittent pneumatic compression, which reduce venous stasis and may promote endogenous fibrinolysis. Pharmacologic prophylaxis includes unfractionated heparin (UFH) and low-molecular weight heparin (LMWH). Prospective controlled trials have shown that UFH reduces VTE in patients with gynecologic cancer. Trials comparing LMWH with UFH have demonstrated equivalent efficacy and similar bleeding complications. The recommended prophylactic dose for LMWH is 40 mg subcutaneous injection daily. However, this dose may need to be adjusted in morbidly obese patients (body mass index greater than 40 kg/m2) as well as in women with abnormal renal clearance. UFH should be administered as a dose of 5,000 units subcutaneously three times daily. Intermittent pneumatic compression also has been shown to reduce the incidence of VTE in this patient population.
A combined regimen of pharmacologic and mechanical prophylaxis may improve efficacy, especially in the highest-risk patients, such as women with gynecologic cancer. Although limited data exist to support this approach in gynecology patients, studies from other surgical disciplines suggest benefit from a combined regimen. With regards to addressing the timing of initiation, a large retrospective trial of patients undergoing hysterectomy for benign indications concluded that postoperative rather than preoperative administration of UFH or LMWH may reduce the risk of bleeding complications without apparent risk of increased VTE (Acta. Obstet. Gynecol. Scand. 2008;87:1039-47).
In summary, the majority of gynecologic oncology patients are considered to be at the highest risk for developing VTE. For this group of women, double prophylaxis with either UFH or LMWH, and a mechanical method (intermittent pneumatic compression) are recommended in the perioperative setting. In addition, ACCP further recommends that these patients receive extended postoperative prophylaxis with LMWH for 4 weeks. Further evidence is needed to determine acceptable timing for initiation of therapy in order to find a balance between adequate thromboprophylaxis and bleeding complications.
Dr. Roque is a fellow in the gynecologic oncology program at the University of North Carolina at Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology and a professor in the division of gynecologic oncology at the university. Dr. Roque and Dr. Clarke-Pearson said they had no relevant disclosures. Scan this QR code or go to obgynnews.com to view similar columns.
Burnout prevention
If you manage to struggle your way to the back of the October 2014 Pediatrics, you will find a clinical report authored by members of the American Academy of Pediatrics Section on Integrative Medicine Executive Committee and the AAP Committee on Practice and Ambulatory Medicine (Pediatrics 2014;134:830-35 [doi: 10.1542/peds.2014-2278]). Under the title Physician Health and Wellness, the authors have carved out an approach to physician burnout, not by addressing its treatment but instead addressing it from the perspective of prevention.
Although the authors refer to two systems-based initiatives that have been launched in the last several years, unfortunately they shy away from making any specific recommendations themselves.
Not surprisingly, they observe that a mentally healthy physician is usually a physically healthy physician who eats well, sleeps well, and exercises regularly. He probably has supportive friends and/or family, and knows how to partition his life in a way that allows him time for one or two pursuits that he enjoys outside of his professional activities. Unless, of course, he is one of those lucky few who derives enough enjoyment from his patients. I would add that a mentally healthy, burnout-resistant physician is one whose expectations for his life are well within his abilities and the situation in which he finds himself.
But, why not give us some specifics on how we might guide young physicians onto paths that will maximize their resistance to burnout while it is still a preventable condition? For starters, why not consider a potential medical student’s ability to make healthy lifestyle choices? How many medical school admissions officers ask about the applicant’s exercise and sleep history ... and hobbies? An urban legend has it that the admissions director where I attended medical school occasionally performed his own little stress tests by among other things, asking the interviewee to open a window that had been painted shut. His efforts may have been crude and cruel, but why not give more attention to seeking out medical students who have already demonstrated some ability to find balance in their lives?
Once in medical school, students should have mentors or coaches who are good role models of wellness and who have the commitment to meet with the students on a regular basis. Encourage medical students to keep and share with their mentors diaries that include their exercise, sleep, and dietary schedules as well as observations on their own mental health. It’s gotta be easy on a smart phone. This may sound like smothering with mothering, but the magnitude of the problem of burnout deserves a more hands-on approach.
As students approach the last 2 years of school, they should be coached on what to expect from postgraduate training and the career paths they are considering. They should be encouraged to consider their own strengths and vulnerabilities, and how these will mesh with the realities of life as a practicing physician.
In the generation just ahead of me, some of the elite house officer training programs required that their house officers be single, because the program administrators felt that the life of a house officer was not compatible with married life. I’m not suggesting that we return to those monastic days, but medical students and young physicians need to be coached on how to be more realistic when making career choices and family planning decisions. One survey noted by the authors of this report has shown that not having minor children in the home was associated with less stress.
The authors offer “mindfulness” as an avenue worthy of consideration. It is a concept that I have trouble grasping, other than seeing it as a reminder to folks to stop just going through the motions and make a broad and honest assessment of their current situations. I guess if it helps a young physician to realize that if he moved a half-hour closer to work he would gain an hour a day with his family, “mindfulness” makes sense.
Finally, this AAP clinical report ignores one of the most serious contributors to physician burnout: electronic medical records. Everyone on the front lines knows it and talks about it. But, for the decision makers it continues to be the elephant in the room. It may take someone with a heavy hand and some common sense, but with good leadership a national electronic health record system that works is within reach.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” E-mail him at [email protected].
If you manage to struggle your way to the back of the October 2014 Pediatrics, you will find a clinical report authored by members of the American Academy of Pediatrics Section on Integrative Medicine Executive Committee and the AAP Committee on Practice and Ambulatory Medicine (Pediatrics 2014;134:830-35 [doi: 10.1542/peds.2014-2278]). Under the title Physician Health and Wellness, the authors have carved out an approach to physician burnout, not by addressing its treatment but instead addressing it from the perspective of prevention.
Although the authors refer to two systems-based initiatives that have been launched in the last several years, unfortunately they shy away from making any specific recommendations themselves.
Not surprisingly, they observe that a mentally healthy physician is usually a physically healthy physician who eats well, sleeps well, and exercises regularly. He probably has supportive friends and/or family, and knows how to partition his life in a way that allows him time for one or two pursuits that he enjoys outside of his professional activities. Unless, of course, he is one of those lucky few who derives enough enjoyment from his patients. I would add that a mentally healthy, burnout-resistant physician is one whose expectations for his life are well within his abilities and the situation in which he finds himself.
But, why not give us some specifics on how we might guide young physicians onto paths that will maximize their resistance to burnout while it is still a preventable condition? For starters, why not consider a potential medical student’s ability to make healthy lifestyle choices? How many medical school admissions officers ask about the applicant’s exercise and sleep history ... and hobbies? An urban legend has it that the admissions director where I attended medical school occasionally performed his own little stress tests by among other things, asking the interviewee to open a window that had been painted shut. His efforts may have been crude and cruel, but why not give more attention to seeking out medical students who have already demonstrated some ability to find balance in their lives?
Once in medical school, students should have mentors or coaches who are good role models of wellness and who have the commitment to meet with the students on a regular basis. Encourage medical students to keep and share with their mentors diaries that include their exercise, sleep, and dietary schedules as well as observations on their own mental health. It’s gotta be easy on a smart phone. This may sound like smothering with mothering, but the magnitude of the problem of burnout deserves a more hands-on approach.
As students approach the last 2 years of school, they should be coached on what to expect from postgraduate training and the career paths they are considering. They should be encouraged to consider their own strengths and vulnerabilities, and how these will mesh with the realities of life as a practicing physician.
In the generation just ahead of me, some of the elite house officer training programs required that their house officers be single, because the program administrators felt that the life of a house officer was not compatible with married life. I’m not suggesting that we return to those monastic days, but medical students and young physicians need to be coached on how to be more realistic when making career choices and family planning decisions. One survey noted by the authors of this report has shown that not having minor children in the home was associated with less stress.
The authors offer “mindfulness” as an avenue worthy of consideration. It is a concept that I have trouble grasping, other than seeing it as a reminder to folks to stop just going through the motions and make a broad and honest assessment of their current situations. I guess if it helps a young physician to realize that if he moved a half-hour closer to work he would gain an hour a day with his family, “mindfulness” makes sense.
Finally, this AAP clinical report ignores one of the most serious contributors to physician burnout: electronic medical records. Everyone on the front lines knows it and talks about it. But, for the decision makers it continues to be the elephant in the room. It may take someone with a heavy hand and some common sense, but with good leadership a national electronic health record system that works is within reach.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” E-mail him at [email protected].
If you manage to struggle your way to the back of the October 2014 Pediatrics, you will find a clinical report authored by members of the American Academy of Pediatrics Section on Integrative Medicine Executive Committee and the AAP Committee on Practice and Ambulatory Medicine (Pediatrics 2014;134:830-35 [doi: 10.1542/peds.2014-2278]). Under the title Physician Health and Wellness, the authors have carved out an approach to physician burnout, not by addressing its treatment but instead addressing it from the perspective of prevention.
Although the authors refer to two systems-based initiatives that have been launched in the last several years, unfortunately they shy away from making any specific recommendations themselves.
Not surprisingly, they observe that a mentally healthy physician is usually a physically healthy physician who eats well, sleeps well, and exercises regularly. He probably has supportive friends and/or family, and knows how to partition his life in a way that allows him time for one or two pursuits that he enjoys outside of his professional activities. Unless, of course, he is one of those lucky few who derives enough enjoyment from his patients. I would add that a mentally healthy, burnout-resistant physician is one whose expectations for his life are well within his abilities and the situation in which he finds himself.
But, why not give us some specifics on how we might guide young physicians onto paths that will maximize their resistance to burnout while it is still a preventable condition? For starters, why not consider a potential medical student’s ability to make healthy lifestyle choices? How many medical school admissions officers ask about the applicant’s exercise and sleep history ... and hobbies? An urban legend has it that the admissions director where I attended medical school occasionally performed his own little stress tests by among other things, asking the interviewee to open a window that had been painted shut. His efforts may have been crude and cruel, but why not give more attention to seeking out medical students who have already demonstrated some ability to find balance in their lives?
Once in medical school, students should have mentors or coaches who are good role models of wellness and who have the commitment to meet with the students on a regular basis. Encourage medical students to keep and share with their mentors diaries that include their exercise, sleep, and dietary schedules as well as observations on their own mental health. It’s gotta be easy on a smart phone. This may sound like smothering with mothering, but the magnitude of the problem of burnout deserves a more hands-on approach.
As students approach the last 2 years of school, they should be coached on what to expect from postgraduate training and the career paths they are considering. They should be encouraged to consider their own strengths and vulnerabilities, and how these will mesh with the realities of life as a practicing physician.
In the generation just ahead of me, some of the elite house officer training programs required that their house officers be single, because the program administrators felt that the life of a house officer was not compatible with married life. I’m not suggesting that we return to those monastic days, but medical students and young physicians need to be coached on how to be more realistic when making career choices and family planning decisions. One survey noted by the authors of this report has shown that not having minor children in the home was associated with less stress.
The authors offer “mindfulness” as an avenue worthy of consideration. It is a concept that I have trouble grasping, other than seeing it as a reminder to folks to stop just going through the motions and make a broad and honest assessment of their current situations. I guess if it helps a young physician to realize that if he moved a half-hour closer to work he would gain an hour a day with his family, “mindfulness” makes sense.
Finally, this AAP clinical report ignores one of the most serious contributors to physician burnout: electronic medical records. Everyone on the front lines knows it and talks about it. But, for the decision makers it continues to be the elephant in the room. It may take someone with a heavy hand and some common sense, but with good leadership a national electronic health record system that works is within reach.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” E-mail him at [email protected].
Inspiring TED talks
December is the time for reflection. For the last few years, I have found a great way to do that – by watching TED talks. The TED talk phenomenon is an example of how digital media enable the spread of ideas. In an effort to inspire you, both in life and in practice, I’m sharing nine of my favorite TED talks that range from digital medicine to meditation, from healthcare costs to healthcare transformation, and from happiness to introspection. Find the time in the next month or so to watch a few of these. You will be grateful for 2014 and inspired for 2015.
Stefan Larsson, “What Doctors Can Learn From Each Other,” October 2013
A physician and value-based health care advocate, Dr. Larsson argues for a paradigm shift in health care. He asks, “With our ever-increasing focus on costs, are we forgetting about the patient?” Through concrete examples, he argues that health care leaders should focus not only on quality over cost, but also that doing so will lead to overall lower costs and better care delivery.
Daniel Kraft, “Medicine’s future? There’s an app for that,” April 2011
In this fast-paced, energetic presentation, Kraft, a physician, scientist, and innovator, explores exponential technologies such as robotics and artificial intelligence, and how they are radically transforming health care. He argues that these technologies will usher in an “era of digital medicine,” (which has already begun), and will ultimately make care delivery faster, smaller, cheaper, and better.
Atul Gawande, “How do we heal medicine?” February 2012
Dr. Gawande, a Harvard surgeon, researcher, and writer, argues that health care needs fewer cowboys and more pit crews. The problem is that physicians have been trained, hired, and rewarded to be cowboys, or rugged individuals. Gawande says that medicine is obsessed with components – we want the best specialists, the best drugs, the best tests. But at what cost? He calls for medicine to be a system in which we can recognize both success and failure, and design solutions for the failures. His answer: a checklist. In a study of eight hospitals in eight different countries that implemented checklists for surgery, they found complication rates fell 35% and death rates fell 47%. The truth: As individualistic as we want to be, complexity requires group success.
Rebecca Onie, “What if our health care system kept us healthy?” June 2012
What if a physician could write a prescription for food, shelter, or heat for their patients to give them the basic resources they needed to be healthy? That’s exactly what’s happening in clinics in which Health Leads operate. Ms. Onie, a cofounder of Health Leads, has helped more than 9,000 families to receive the basic necessities for their health. She argues that this system not only allows physicians to manage patients’ diseases, but also to improve patients’ health.
Dan Pink, “The Puzzle of motivation,” July 2009
If you want your employees to work better, faster, and more creatively, then you should dangle a sweeter carrot in front of them. Right? Not so fast. In this intriguing talk, Pink blasts a hole in the belief that bigger rewards produce better results. In tasks that require heuristic thinking, larger rewards typically lead to poorer performance. Therefore, he advocates developing intrinsic motivation by focusing on autonomy, mastery, and purpose.
Matthieu Ricard, “The Habits of Happiness,” February 2004
With dozens of headlines screaming about doctor dissatisfaction, it might not be a bad idea to watch Matthieu Ricard’s video about achieving happiness. We all seek happiness and avoid suffering, yet for most us happiness comes in fleeting glimpses. What if happiness was something you could experience daily? Mr. Ricard, a French biochemist turned Buddhist monk, says you can. He believes that practicing meditation can put us in touch with our emotions (both good and bad), cultivate compassion toward others, and ultimately achieve happiness and fulfillment.
Graham Hill, “Less stuff, more happiness,” March 2011
In this video, Mr. Hill, the founder of LifeEdited, shares his story of buying a 420-square-foot apartment then designing it so it can include an office, a bed, a kitchen, and a table large enough for a dinner party of 10 people. (Spoiler alert: He gets it all.) His premise is simple: Having less stuff gives you more freedom and time, which will ultimately make room for more of the good stuff in life.
Louie Schwartzberg, “Nature. Beauty. Gratitude,” June 2011
In this beautiful and poignant video, Schwartzberg, a cinematographer who has been shooting time-lapsed flowers 24 hours a day, 7 days a week, for more than 30 years, shares his illuminating images and encourages us to more fully and mindfully connect with the people, places, and things around us. He shares two interviews about nature and gratitude, one from the perspective of a young child, the other from an elderly man. The underlying message in both is that nature’s beauty is a gift that cultivates appreciation and gratitude in us that we can then pass on to others.
OK, I’m going to include one more TED talk, which is mine. I presented “Reinventing physicians” in 2011 at TEDxPennQuarter. I hope you enjoy it.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is@dermdoc on Twitter.
December is the time for reflection. For the last few years, I have found a great way to do that – by watching TED talks. The TED talk phenomenon is an example of how digital media enable the spread of ideas. In an effort to inspire you, both in life and in practice, I’m sharing nine of my favorite TED talks that range from digital medicine to meditation, from healthcare costs to healthcare transformation, and from happiness to introspection. Find the time in the next month or so to watch a few of these. You will be grateful for 2014 and inspired for 2015.
Stefan Larsson, “What Doctors Can Learn From Each Other,” October 2013
A physician and value-based health care advocate, Dr. Larsson argues for a paradigm shift in health care. He asks, “With our ever-increasing focus on costs, are we forgetting about the patient?” Through concrete examples, he argues that health care leaders should focus not only on quality over cost, but also that doing so will lead to overall lower costs and better care delivery.
Daniel Kraft, “Medicine’s future? There’s an app for that,” April 2011
In this fast-paced, energetic presentation, Kraft, a physician, scientist, and innovator, explores exponential technologies such as robotics and artificial intelligence, and how they are radically transforming health care. He argues that these technologies will usher in an “era of digital medicine,” (which has already begun), and will ultimately make care delivery faster, smaller, cheaper, and better.
Atul Gawande, “How do we heal medicine?” February 2012
Dr. Gawande, a Harvard surgeon, researcher, and writer, argues that health care needs fewer cowboys and more pit crews. The problem is that physicians have been trained, hired, and rewarded to be cowboys, or rugged individuals. Gawande says that medicine is obsessed with components – we want the best specialists, the best drugs, the best tests. But at what cost? He calls for medicine to be a system in which we can recognize both success and failure, and design solutions for the failures. His answer: a checklist. In a study of eight hospitals in eight different countries that implemented checklists for surgery, they found complication rates fell 35% and death rates fell 47%. The truth: As individualistic as we want to be, complexity requires group success.
Rebecca Onie, “What if our health care system kept us healthy?” June 2012
What if a physician could write a prescription for food, shelter, or heat for their patients to give them the basic resources they needed to be healthy? That’s exactly what’s happening in clinics in which Health Leads operate. Ms. Onie, a cofounder of Health Leads, has helped more than 9,000 families to receive the basic necessities for their health. She argues that this system not only allows physicians to manage patients’ diseases, but also to improve patients’ health.
Dan Pink, “The Puzzle of motivation,” July 2009
If you want your employees to work better, faster, and more creatively, then you should dangle a sweeter carrot in front of them. Right? Not so fast. In this intriguing talk, Pink blasts a hole in the belief that bigger rewards produce better results. In tasks that require heuristic thinking, larger rewards typically lead to poorer performance. Therefore, he advocates developing intrinsic motivation by focusing on autonomy, mastery, and purpose.
Matthieu Ricard, “The Habits of Happiness,” February 2004
With dozens of headlines screaming about doctor dissatisfaction, it might not be a bad idea to watch Matthieu Ricard’s video about achieving happiness. We all seek happiness and avoid suffering, yet for most us happiness comes in fleeting glimpses. What if happiness was something you could experience daily? Mr. Ricard, a French biochemist turned Buddhist monk, says you can. He believes that practicing meditation can put us in touch with our emotions (both good and bad), cultivate compassion toward others, and ultimately achieve happiness and fulfillment.
Graham Hill, “Less stuff, more happiness,” March 2011
In this video, Mr. Hill, the founder of LifeEdited, shares his story of buying a 420-square-foot apartment then designing it so it can include an office, a bed, a kitchen, and a table large enough for a dinner party of 10 people. (Spoiler alert: He gets it all.) His premise is simple: Having less stuff gives you more freedom and time, which will ultimately make room for more of the good stuff in life.
Louie Schwartzberg, “Nature. Beauty. Gratitude,” June 2011
In this beautiful and poignant video, Schwartzberg, a cinematographer who has been shooting time-lapsed flowers 24 hours a day, 7 days a week, for more than 30 years, shares his illuminating images and encourages us to more fully and mindfully connect with the people, places, and things around us. He shares two interviews about nature and gratitude, one from the perspective of a young child, the other from an elderly man. The underlying message in both is that nature’s beauty is a gift that cultivates appreciation and gratitude in us that we can then pass on to others.
OK, I’m going to include one more TED talk, which is mine. I presented “Reinventing physicians” in 2011 at TEDxPennQuarter. I hope you enjoy it.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is@dermdoc on Twitter.
December is the time for reflection. For the last few years, I have found a great way to do that – by watching TED talks. The TED talk phenomenon is an example of how digital media enable the spread of ideas. In an effort to inspire you, both in life and in practice, I’m sharing nine of my favorite TED talks that range from digital medicine to meditation, from healthcare costs to healthcare transformation, and from happiness to introspection. Find the time in the next month or so to watch a few of these. You will be grateful for 2014 and inspired for 2015.
Stefan Larsson, “What Doctors Can Learn From Each Other,” October 2013
A physician and value-based health care advocate, Dr. Larsson argues for a paradigm shift in health care. He asks, “With our ever-increasing focus on costs, are we forgetting about the patient?” Through concrete examples, he argues that health care leaders should focus not only on quality over cost, but also that doing so will lead to overall lower costs and better care delivery.
Daniel Kraft, “Medicine’s future? There’s an app for that,” April 2011
In this fast-paced, energetic presentation, Kraft, a physician, scientist, and innovator, explores exponential technologies such as robotics and artificial intelligence, and how they are radically transforming health care. He argues that these technologies will usher in an “era of digital medicine,” (which has already begun), and will ultimately make care delivery faster, smaller, cheaper, and better.
Atul Gawande, “How do we heal medicine?” February 2012
Dr. Gawande, a Harvard surgeon, researcher, and writer, argues that health care needs fewer cowboys and more pit crews. The problem is that physicians have been trained, hired, and rewarded to be cowboys, or rugged individuals. Gawande says that medicine is obsessed with components – we want the best specialists, the best drugs, the best tests. But at what cost? He calls for medicine to be a system in which we can recognize both success and failure, and design solutions for the failures. His answer: a checklist. In a study of eight hospitals in eight different countries that implemented checklists for surgery, they found complication rates fell 35% and death rates fell 47%. The truth: As individualistic as we want to be, complexity requires group success.
Rebecca Onie, “What if our health care system kept us healthy?” June 2012
What if a physician could write a prescription for food, shelter, or heat for their patients to give them the basic resources they needed to be healthy? That’s exactly what’s happening in clinics in which Health Leads operate. Ms. Onie, a cofounder of Health Leads, has helped more than 9,000 families to receive the basic necessities for their health. She argues that this system not only allows physicians to manage patients’ diseases, but also to improve patients’ health.
Dan Pink, “The Puzzle of motivation,” July 2009
If you want your employees to work better, faster, and more creatively, then you should dangle a sweeter carrot in front of them. Right? Not so fast. In this intriguing talk, Pink blasts a hole in the belief that bigger rewards produce better results. In tasks that require heuristic thinking, larger rewards typically lead to poorer performance. Therefore, he advocates developing intrinsic motivation by focusing on autonomy, mastery, and purpose.
Matthieu Ricard, “The Habits of Happiness,” February 2004
With dozens of headlines screaming about doctor dissatisfaction, it might not be a bad idea to watch Matthieu Ricard’s video about achieving happiness. We all seek happiness and avoid suffering, yet for most us happiness comes in fleeting glimpses. What if happiness was something you could experience daily? Mr. Ricard, a French biochemist turned Buddhist monk, says you can. He believes that practicing meditation can put us in touch with our emotions (both good and bad), cultivate compassion toward others, and ultimately achieve happiness and fulfillment.
Graham Hill, “Less stuff, more happiness,” March 2011
In this video, Mr. Hill, the founder of LifeEdited, shares his story of buying a 420-square-foot apartment then designing it so it can include an office, a bed, a kitchen, and a table large enough for a dinner party of 10 people. (Spoiler alert: He gets it all.) His premise is simple: Having less stuff gives you more freedom and time, which will ultimately make room for more of the good stuff in life.
Louie Schwartzberg, “Nature. Beauty. Gratitude,” June 2011
In this beautiful and poignant video, Schwartzberg, a cinematographer who has been shooting time-lapsed flowers 24 hours a day, 7 days a week, for more than 30 years, shares his illuminating images and encourages us to more fully and mindfully connect with the people, places, and things around us. He shares two interviews about nature and gratitude, one from the perspective of a young child, the other from an elderly man. The underlying message in both is that nature’s beauty is a gift that cultivates appreciation and gratitude in us that we can then pass on to others.
OK, I’m going to include one more TED talk, which is mine. I presented “Reinventing physicians” in 2011 at TEDxPennQuarter. I hope you enjoy it.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is@dermdoc on Twitter.
Sunrise calls
When your pager vibrates at 7 o’clock in the morning, it is unlikely to be alerting you of good news. It may be a parent who assumes that because his child’s medical home has evening office hours that there will be a receptionist sitting there at sunup to help him make an appointment for a nonurgent complaint. Or, it may be a call from a parent who knows that she doesn’t have a medical emergency on her hands, but who would like your advice about whether she should take a day off from work or send her child to day care.
But, an uncomfortable number of daybreak calls come from parents with what eventually turns out to be a desperately ill child. I have witnessed those scenarios often enough that even though I am retired, I break into a cold sweat when the home phone rings anytime between 6 and 7 in the morning.
A study from Wake Forest University, Winston-Salem, N.C., published in the November 2014 issue of Pediatrics, supports my long-standing discomfort with patients who present in the early morning. (McCrory et al. “Off-Hours Admission to Pediatric Intensive Care and Mortality,” Pediatrics 2014;134:e1345-e1353 [doi: 10.1542/peds.2014-1071]). In a retrospective study of nearly a quarter of a million admissions to 99 perinatal ICUs over a 3-year period, the investigators discovered that admission in off-hours and weekends “does not independently increase the odds of mortality.”
However, they found that admission from 6 to 11 in the morning is “associated with an increased risk of death.” This may not have been the result the investigators were expecting, and their analyses don’t suggest a cause. In the discussion portion of the paper, they offer some explanations that are in sync with my observations. First, ICUs are generally fully staffed 24-7-365. While the lights maybe dimmed slightly, there is seldom a diurnal variation in the attentiveness and quality of the caregivers in an ICU. Contrast this to an ordinary medical/surgical floor on which the staffing levels drop precipitously when the sun goes down. The skeletal staff is usually working in the dark, resorting to flashlights and ankle-level lighting to make their observations. And ... things are missed. Things like skin-color changes and the quality of respirations that become obvious when the morning shift arrives and the lights go on. “Holy s**t! This patient needs to be in the PICU!” And, the PICU now receives a patient at 7:30 a.m. who has a greater odds of mortality because the illness has percolated in the dark overnight.
The same phenomenon occurs in the outpatient setting. At night, sleep deprivation may cloud a parent’s observational skills. The lights in the bedroom may have been left off in hopes of keeping the child more comfortable. The parent may have called the doctor and been shunted to a triage nurse who is unfamiliar with the family and whose algorithm fails at a critical branch point. Or, the call may have been fielded by an answering service that is more interested in protecting its client’s sleep than serving the needs of the caller.
Or the parent may have spoken to the doctor early in the evening, but was hesitant to call again and wake her when the child’s condition changed. House officers can fall into the same trap when their misplaced concern about the sleep needs of the physician to whom they report prevents them from making a critical call for help.
Again, the result is that when the sun comes up, a child whose illness might have been more easily managed in the PICU at 2:30 a.m. doesn’t arrive in the unit until those deadly hours between 6 a.m. and 11 a.m. While there may be diurnal variations in the inherent mortality of some pathological conditions, this study from North Carolina suggests that when the lights go out, critical observations go unmade and so do wise decisions.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” E-mail him at [email protected].
When your pager vibrates at 7 o’clock in the morning, it is unlikely to be alerting you of good news. It may be a parent who assumes that because his child’s medical home has evening office hours that there will be a receptionist sitting there at sunup to help him make an appointment for a nonurgent complaint. Or, it may be a call from a parent who knows that she doesn’t have a medical emergency on her hands, but who would like your advice about whether she should take a day off from work or send her child to day care.
But, an uncomfortable number of daybreak calls come from parents with what eventually turns out to be a desperately ill child. I have witnessed those scenarios often enough that even though I am retired, I break into a cold sweat when the home phone rings anytime between 6 and 7 in the morning.
A study from Wake Forest University, Winston-Salem, N.C., published in the November 2014 issue of Pediatrics, supports my long-standing discomfort with patients who present in the early morning. (McCrory et al. “Off-Hours Admission to Pediatric Intensive Care and Mortality,” Pediatrics 2014;134:e1345-e1353 [doi: 10.1542/peds.2014-1071]). In a retrospective study of nearly a quarter of a million admissions to 99 perinatal ICUs over a 3-year period, the investigators discovered that admission in off-hours and weekends “does not independently increase the odds of mortality.”
However, they found that admission from 6 to 11 in the morning is “associated with an increased risk of death.” This may not have been the result the investigators were expecting, and their analyses don’t suggest a cause. In the discussion portion of the paper, they offer some explanations that are in sync with my observations. First, ICUs are generally fully staffed 24-7-365. While the lights maybe dimmed slightly, there is seldom a diurnal variation in the attentiveness and quality of the caregivers in an ICU. Contrast this to an ordinary medical/surgical floor on which the staffing levels drop precipitously when the sun goes down. The skeletal staff is usually working in the dark, resorting to flashlights and ankle-level lighting to make their observations. And ... things are missed. Things like skin-color changes and the quality of respirations that become obvious when the morning shift arrives and the lights go on. “Holy s**t! This patient needs to be in the PICU!” And, the PICU now receives a patient at 7:30 a.m. who has a greater odds of mortality because the illness has percolated in the dark overnight.
The same phenomenon occurs in the outpatient setting. At night, sleep deprivation may cloud a parent’s observational skills. The lights in the bedroom may have been left off in hopes of keeping the child more comfortable. The parent may have called the doctor and been shunted to a triage nurse who is unfamiliar with the family and whose algorithm fails at a critical branch point. Or, the call may have been fielded by an answering service that is more interested in protecting its client’s sleep than serving the needs of the caller.
Or the parent may have spoken to the doctor early in the evening, but was hesitant to call again and wake her when the child’s condition changed. House officers can fall into the same trap when their misplaced concern about the sleep needs of the physician to whom they report prevents them from making a critical call for help.
Again, the result is that when the sun comes up, a child whose illness might have been more easily managed in the PICU at 2:30 a.m. doesn’t arrive in the unit until those deadly hours between 6 a.m. and 11 a.m. While there may be diurnal variations in the inherent mortality of some pathological conditions, this study from North Carolina suggests that when the lights go out, critical observations go unmade and so do wise decisions.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” E-mail him at [email protected].
When your pager vibrates at 7 o’clock in the morning, it is unlikely to be alerting you of good news. It may be a parent who assumes that because his child’s medical home has evening office hours that there will be a receptionist sitting there at sunup to help him make an appointment for a nonurgent complaint. Or, it may be a call from a parent who knows that she doesn’t have a medical emergency on her hands, but who would like your advice about whether she should take a day off from work or send her child to day care.
But, an uncomfortable number of daybreak calls come from parents with what eventually turns out to be a desperately ill child. I have witnessed those scenarios often enough that even though I am retired, I break into a cold sweat when the home phone rings anytime between 6 and 7 in the morning.
A study from Wake Forest University, Winston-Salem, N.C., published in the November 2014 issue of Pediatrics, supports my long-standing discomfort with patients who present in the early morning. (McCrory et al. “Off-Hours Admission to Pediatric Intensive Care and Mortality,” Pediatrics 2014;134:e1345-e1353 [doi: 10.1542/peds.2014-1071]). In a retrospective study of nearly a quarter of a million admissions to 99 perinatal ICUs over a 3-year period, the investigators discovered that admission in off-hours and weekends “does not independently increase the odds of mortality.”
However, they found that admission from 6 to 11 in the morning is “associated with an increased risk of death.” This may not have been the result the investigators were expecting, and their analyses don’t suggest a cause. In the discussion portion of the paper, they offer some explanations that are in sync with my observations. First, ICUs are generally fully staffed 24-7-365. While the lights maybe dimmed slightly, there is seldom a diurnal variation in the attentiveness and quality of the caregivers in an ICU. Contrast this to an ordinary medical/surgical floor on which the staffing levels drop precipitously when the sun goes down. The skeletal staff is usually working in the dark, resorting to flashlights and ankle-level lighting to make their observations. And ... things are missed. Things like skin-color changes and the quality of respirations that become obvious when the morning shift arrives and the lights go on. “Holy s**t! This patient needs to be in the PICU!” And, the PICU now receives a patient at 7:30 a.m. who has a greater odds of mortality because the illness has percolated in the dark overnight.
The same phenomenon occurs in the outpatient setting. At night, sleep deprivation may cloud a parent’s observational skills. The lights in the bedroom may have been left off in hopes of keeping the child more comfortable. The parent may have called the doctor and been shunted to a triage nurse who is unfamiliar with the family and whose algorithm fails at a critical branch point. Or, the call may have been fielded by an answering service that is more interested in protecting its client’s sleep than serving the needs of the caller.
Or the parent may have spoken to the doctor early in the evening, but was hesitant to call again and wake her when the child’s condition changed. House officers can fall into the same trap when their misplaced concern about the sleep needs of the physician to whom they report prevents them from making a critical call for help.
Again, the result is that when the sun comes up, a child whose illness might have been more easily managed in the PICU at 2:30 a.m. doesn’t arrive in the unit until those deadly hours between 6 a.m. and 11 a.m. While there may be diurnal variations in the inherent mortality of some pathological conditions, this study from North Carolina suggests that when the lights go out, critical observations go unmade and so do wise decisions.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” E-mail him at [email protected].
Paleo-Parenting
Two years ago, I saw a young man in my office who had decided not to return to college after his freshman year. He had been a very good high school hockey player, and I asked him what he was doing now to stay fit. He replied that he had joined a fitness facility, part of a national franchise system, and “I’m going on the Paleo Diet.” As I was among the clueless at that time, I quizzed him about his diet.
He told me that it was an attempt to duplicate the diet of our ancestors prior to the development of agriculture (thought to be about 10,000 years ago). This meant no processed food, no dairy products, no grains or legumes, no refined sugars. Lean meat, nuts, fruits, and low-starch vegetables were okay.
When I saw him for a follow-up visit 2 months later, I asked how he was doing with what I called his “caveman diet.” He said, “I lasted about 6 weeks, but I’m still working out four times a week.” It turns out that while my patient had drifted away from his paleolithic diet, enough other people have climbed on the bandwagon that there are now a couple of magazines devoted what has broadened beyond diet to what could be called a paleo lifestyle.
Devotees of the live-like-our-ancestors movement hope to avoid the “diseases of civilization by exercising frequently, particularly doing things that mimic our ancestors activities such as running, jumping, climbing, and throwing. A committed paleo person should wear a minimum of clothes and try to go barefoot as often as possible. He should have frequent contact with nature and get plenty of sun exposure for his source of vitamin D. His sleep patterns should be in sync with the sun cycle, and he should avoid stress by simplifying and downsizing his life.
This sounds like a lifestyle most toddlers strive for everyday. They prefer to run around nude and shoeless, climb just for fun, and throw anything within reach. It got me wondering what paleo parenting might be look like. Certainly, it would begin with breastfeeding. But, for how long? I don’t think we know the answer to that. It may not have been as long some breastfeeding advocates believe.
I suspect young children are smart enough to find shade in the middle of day to take a nap if we allow them. My obsession with the hazards of sleep deprivation makes the paleo’s attempt to link sleep to the sun cycle particularly appealing. It would benefit parents as well as the children for them to all go to bed when the sun went down. Paleo parenting would mean no TV. What a concept!
Of course, there are several flies in this ancestral ointment. First, I suspect that our prehistoric ancestors seldom lived into their fourth decade. How many of the “diseases of civilization” are simply the effect of aging on bodies that were not genetically engineered for longevity? How much do we really know about the diet and lifestyle of our paleo ancestors? Carbon isotope studies and microscopic analysis of ancient stool samples are pretty scanty evidence.
And, why choose to set our target to emulate before the development of agriculture? Some grain and a few root vegetables aren’t going to send our children on the road to obesity if they are active and getting adequate amounts of sleep.
There can be many advantages to adopting an “ancestral lifestyle,” but we don’t have to peel the onion all the way back to prehistory to reap the benefits. Heck, I bet if we rolled back to pretelevision, we would be a much healthier society.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” E-mail him at [email protected].
Two years ago, I saw a young man in my office who had decided not to return to college after his freshman year. He had been a very good high school hockey player, and I asked him what he was doing now to stay fit. He replied that he had joined a fitness facility, part of a national franchise system, and “I’m going on the Paleo Diet.” As I was among the clueless at that time, I quizzed him about his diet.
He told me that it was an attempt to duplicate the diet of our ancestors prior to the development of agriculture (thought to be about 10,000 years ago). This meant no processed food, no dairy products, no grains or legumes, no refined sugars. Lean meat, nuts, fruits, and low-starch vegetables were okay.
When I saw him for a follow-up visit 2 months later, I asked how he was doing with what I called his “caveman diet.” He said, “I lasted about 6 weeks, but I’m still working out four times a week.” It turns out that while my patient had drifted away from his paleolithic diet, enough other people have climbed on the bandwagon that there are now a couple of magazines devoted what has broadened beyond diet to what could be called a paleo lifestyle.
Devotees of the live-like-our-ancestors movement hope to avoid the “diseases of civilization by exercising frequently, particularly doing things that mimic our ancestors activities such as running, jumping, climbing, and throwing. A committed paleo person should wear a minimum of clothes and try to go barefoot as often as possible. He should have frequent contact with nature and get plenty of sun exposure for his source of vitamin D. His sleep patterns should be in sync with the sun cycle, and he should avoid stress by simplifying and downsizing his life.
This sounds like a lifestyle most toddlers strive for everyday. They prefer to run around nude and shoeless, climb just for fun, and throw anything within reach. It got me wondering what paleo parenting might be look like. Certainly, it would begin with breastfeeding. But, for how long? I don’t think we know the answer to that. It may not have been as long some breastfeeding advocates believe.
I suspect young children are smart enough to find shade in the middle of day to take a nap if we allow them. My obsession with the hazards of sleep deprivation makes the paleo’s attempt to link sleep to the sun cycle particularly appealing. It would benefit parents as well as the children for them to all go to bed when the sun went down. Paleo parenting would mean no TV. What a concept!
Of course, there are several flies in this ancestral ointment. First, I suspect that our prehistoric ancestors seldom lived into their fourth decade. How many of the “diseases of civilization” are simply the effect of aging on bodies that were not genetically engineered for longevity? How much do we really know about the diet and lifestyle of our paleo ancestors? Carbon isotope studies and microscopic analysis of ancient stool samples are pretty scanty evidence.
And, why choose to set our target to emulate before the development of agriculture? Some grain and a few root vegetables aren’t going to send our children on the road to obesity if they are active and getting adequate amounts of sleep.
There can be many advantages to adopting an “ancestral lifestyle,” but we don’t have to peel the onion all the way back to prehistory to reap the benefits. Heck, I bet if we rolled back to pretelevision, we would be a much healthier society.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” E-mail him at [email protected].
Two years ago, I saw a young man in my office who had decided not to return to college after his freshman year. He had been a very good high school hockey player, and I asked him what he was doing now to stay fit. He replied that he had joined a fitness facility, part of a national franchise system, and “I’m going on the Paleo Diet.” As I was among the clueless at that time, I quizzed him about his diet.
He told me that it was an attempt to duplicate the diet of our ancestors prior to the development of agriculture (thought to be about 10,000 years ago). This meant no processed food, no dairy products, no grains or legumes, no refined sugars. Lean meat, nuts, fruits, and low-starch vegetables were okay.
When I saw him for a follow-up visit 2 months later, I asked how he was doing with what I called his “caveman diet.” He said, “I lasted about 6 weeks, but I’m still working out four times a week.” It turns out that while my patient had drifted away from his paleolithic diet, enough other people have climbed on the bandwagon that there are now a couple of magazines devoted what has broadened beyond diet to what could be called a paleo lifestyle.
Devotees of the live-like-our-ancestors movement hope to avoid the “diseases of civilization by exercising frequently, particularly doing things that mimic our ancestors activities such as running, jumping, climbing, and throwing. A committed paleo person should wear a minimum of clothes and try to go barefoot as often as possible. He should have frequent contact with nature and get plenty of sun exposure for his source of vitamin D. His sleep patterns should be in sync with the sun cycle, and he should avoid stress by simplifying and downsizing his life.
This sounds like a lifestyle most toddlers strive for everyday. They prefer to run around nude and shoeless, climb just for fun, and throw anything within reach. It got me wondering what paleo parenting might be look like. Certainly, it would begin with breastfeeding. But, for how long? I don’t think we know the answer to that. It may not have been as long some breastfeeding advocates believe.
I suspect young children are smart enough to find shade in the middle of day to take a nap if we allow them. My obsession with the hazards of sleep deprivation makes the paleo’s attempt to link sleep to the sun cycle particularly appealing. It would benefit parents as well as the children for them to all go to bed when the sun went down. Paleo parenting would mean no TV. What a concept!
Of course, there are several flies in this ancestral ointment. First, I suspect that our prehistoric ancestors seldom lived into their fourth decade. How many of the “diseases of civilization” are simply the effect of aging on bodies that were not genetically engineered for longevity? How much do we really know about the diet and lifestyle of our paleo ancestors? Carbon isotope studies and microscopic analysis of ancient stool samples are pretty scanty evidence.
And, why choose to set our target to emulate before the development of agriculture? Some grain and a few root vegetables aren’t going to send our children on the road to obesity if they are active and getting adequate amounts of sleep.
There can be many advantages to adopting an “ancestral lifestyle,” but we don’t have to peel the onion all the way back to prehistory to reap the benefits. Heck, I bet if we rolled back to pretelevision, we would be a much healthier society.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” E-mail him at [email protected].
Pedunculagin
Pedunculagin is an ellagitannin, a group of polyphenolic hydrolyzable tannins, found in various plants, including Emblica officinalis, Pimenta dioica, and several others (Arch. Pharm. Res. 2014, Feb 7. [Epub ahead of print]; Curr. Drug Targets 2012;13:1900-06). The substance is reported to exhibit anti-inflammatory, anticancer, and antimicrobial activities, and it is considered a potent dietary antioxidant (Z. Naturforsch C. 2007;62:526-36; J. Org. Chem. 1996;61:2606-12; J. Nutr. 2014;144(4 Suppl):555S-60S). Purified from the Manchurian alder (Alnus hirsuta), pedunculagin is also a novel immunomodulating agent (Skin Res. Technol. 2010;16:371-7). Pedunculagin is also one of the hydrolyzable tannins found in Punica granatum (pomegranate), fruit extracts of which have been shown by Afaq et al. to exert photochemopreventive effects against the deleterious effects of ultraviolet B radiation (Photochem. Photobiol. 2005;81:38-45). Pedunculagin was first synthesized (in 2,3- and 4,6-coupled form) in 1996 (J. Org. Chem. 1996;61:2606-12).
Anticancer and antioxidant activity
In a study of 57 tannins and related compounds, Kashiwada et al. noted in a 1992 study that pedunculagin exhibited selective cytotoxicity against melanoma cells (J. Nat. Prod. 1992;55:1033-43).
According to a 2007 report by Marzouk et al., pedunculagin is among one of several tannins identified in the leaves of Pimenta dioica, and it is among the most potent free radical scavengers, as well as one of the most cytotoxic substances against solid tumor cancer cells. Pedunculagin also was found to significantly suppress nitric oxide production and spur the proliferation of T-lymphocytes and macrophages (Z. Naturforsch C. 2007;62:526-36).
In 2012, Kähkönen et al. observed that red raspberry and cloudberry ellagitannins, including pedunculagin, acted as effective radical scavengers, substantially contributing to the antioxidant activity of the berries in lipoprotein and lipid emulsion environments (J. Agric. Food Chem. 2012;60:1167-74).
A 2014 review by Hardman summarized several studies suggesting that potent anticancer properties, including antiproliferative and antiangiogenic activities, have been linked to walnuts. She noted that pedunculagin is one of the key constituents in walnuts to which such characteristics have been attributed (J. Nutr. 2014;144(4 Suppl):555S-60S).
Potential cutaneous applications
In 2010, Lee et al. assessed the effects of pedunculagin on 2,4,6-trinitrochlorobenzene (TNCB)-induced atopic dermatitis-like lesions in NC/Nga mice. Investigators applied a cream containing 0.1% or 0.5% pedunculagin to the positive treatment group; the negative treatment group received the base cream without pedunculagin, with no topical formulations administered to a control group. The investigators found, 4 weeks after treatment, that greater and more rapid improvement in the lesions was experienced by the group that received the higher concentration of pedunculagin (Skin Res. Technol. 2010;16:371-7).
Kim et al., in 2014, isolated pedunculagin and five other phenolic compounds from the leaves of Quercus mongolica (Mongolian oak). They found that pedunculagin exhibited strong in vitro inhibition against the expression of matrix metalloproteinase (MMP)-1 and increased type I procollagen in human fibroblasts exposed to UVB. The Q. mongolica constituent was also found to concentration-dependently exhibit potent scavenging activity against the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical. The investigators suggested that the ellagitannin shows promise for use in preventing and treating cutaneous aging (Arch. Pharm. Res. 2014 Feb. 7. [Epub ahead of print]).
Conclusion
Pedunculagin shows some promise as an agent that can yield dermatologic benefits. However, the body of research on this natural compound is relatively scant. More expansive follow-up work is needed to determine the extent to which pedunculagin can be reasonably incorporated into the dermatologic armamentarium.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy,Topix Pharmaceuticals, and Unilever.
Pedunculagin is an ellagitannin, a group of polyphenolic hydrolyzable tannins, found in various plants, including Emblica officinalis, Pimenta dioica, and several others (Arch. Pharm. Res. 2014, Feb 7. [Epub ahead of print]; Curr. Drug Targets 2012;13:1900-06). The substance is reported to exhibit anti-inflammatory, anticancer, and antimicrobial activities, and it is considered a potent dietary antioxidant (Z. Naturforsch C. 2007;62:526-36; J. Org. Chem. 1996;61:2606-12; J. Nutr. 2014;144(4 Suppl):555S-60S). Purified from the Manchurian alder (Alnus hirsuta), pedunculagin is also a novel immunomodulating agent (Skin Res. Technol. 2010;16:371-7). Pedunculagin is also one of the hydrolyzable tannins found in Punica granatum (pomegranate), fruit extracts of which have been shown by Afaq et al. to exert photochemopreventive effects against the deleterious effects of ultraviolet B radiation (Photochem. Photobiol. 2005;81:38-45). Pedunculagin was first synthesized (in 2,3- and 4,6-coupled form) in 1996 (J. Org. Chem. 1996;61:2606-12).
Anticancer and antioxidant activity
In a study of 57 tannins and related compounds, Kashiwada et al. noted in a 1992 study that pedunculagin exhibited selective cytotoxicity against melanoma cells (J. Nat. Prod. 1992;55:1033-43).
According to a 2007 report by Marzouk et al., pedunculagin is among one of several tannins identified in the leaves of Pimenta dioica, and it is among the most potent free radical scavengers, as well as one of the most cytotoxic substances against solid tumor cancer cells. Pedunculagin also was found to significantly suppress nitric oxide production and spur the proliferation of T-lymphocytes and macrophages (Z. Naturforsch C. 2007;62:526-36).
In 2012, Kähkönen et al. observed that red raspberry and cloudberry ellagitannins, including pedunculagin, acted as effective radical scavengers, substantially contributing to the antioxidant activity of the berries in lipoprotein and lipid emulsion environments (J. Agric. Food Chem. 2012;60:1167-74).
A 2014 review by Hardman summarized several studies suggesting that potent anticancer properties, including antiproliferative and antiangiogenic activities, have been linked to walnuts. She noted that pedunculagin is one of the key constituents in walnuts to which such characteristics have been attributed (J. Nutr. 2014;144(4 Suppl):555S-60S).
Potential cutaneous applications
In 2010, Lee et al. assessed the effects of pedunculagin on 2,4,6-trinitrochlorobenzene (TNCB)-induced atopic dermatitis-like lesions in NC/Nga mice. Investigators applied a cream containing 0.1% or 0.5% pedunculagin to the positive treatment group; the negative treatment group received the base cream without pedunculagin, with no topical formulations administered to a control group. The investigators found, 4 weeks after treatment, that greater and more rapid improvement in the lesions was experienced by the group that received the higher concentration of pedunculagin (Skin Res. Technol. 2010;16:371-7).
Kim et al., in 2014, isolated pedunculagin and five other phenolic compounds from the leaves of Quercus mongolica (Mongolian oak). They found that pedunculagin exhibited strong in vitro inhibition against the expression of matrix metalloproteinase (MMP)-1 and increased type I procollagen in human fibroblasts exposed to UVB. The Q. mongolica constituent was also found to concentration-dependently exhibit potent scavenging activity against the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical. The investigators suggested that the ellagitannin shows promise for use in preventing and treating cutaneous aging (Arch. Pharm. Res. 2014 Feb. 7. [Epub ahead of print]).
Conclusion
Pedunculagin shows some promise as an agent that can yield dermatologic benefits. However, the body of research on this natural compound is relatively scant. More expansive follow-up work is needed to determine the extent to which pedunculagin can be reasonably incorporated into the dermatologic armamentarium.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy,Topix Pharmaceuticals, and Unilever.
Pedunculagin is an ellagitannin, a group of polyphenolic hydrolyzable tannins, found in various plants, including Emblica officinalis, Pimenta dioica, and several others (Arch. Pharm. Res. 2014, Feb 7. [Epub ahead of print]; Curr. Drug Targets 2012;13:1900-06). The substance is reported to exhibit anti-inflammatory, anticancer, and antimicrobial activities, and it is considered a potent dietary antioxidant (Z. Naturforsch C. 2007;62:526-36; J. Org. Chem. 1996;61:2606-12; J. Nutr. 2014;144(4 Suppl):555S-60S). Purified from the Manchurian alder (Alnus hirsuta), pedunculagin is also a novel immunomodulating agent (Skin Res. Technol. 2010;16:371-7). Pedunculagin is also one of the hydrolyzable tannins found in Punica granatum (pomegranate), fruit extracts of which have been shown by Afaq et al. to exert photochemopreventive effects against the deleterious effects of ultraviolet B radiation (Photochem. Photobiol. 2005;81:38-45). Pedunculagin was first synthesized (in 2,3- and 4,6-coupled form) in 1996 (J. Org. Chem. 1996;61:2606-12).
Anticancer and antioxidant activity
In a study of 57 tannins and related compounds, Kashiwada et al. noted in a 1992 study that pedunculagin exhibited selective cytotoxicity against melanoma cells (J. Nat. Prod. 1992;55:1033-43).
According to a 2007 report by Marzouk et al., pedunculagin is among one of several tannins identified in the leaves of Pimenta dioica, and it is among the most potent free radical scavengers, as well as one of the most cytotoxic substances against solid tumor cancer cells. Pedunculagin also was found to significantly suppress nitric oxide production and spur the proliferation of T-lymphocytes and macrophages (Z. Naturforsch C. 2007;62:526-36).
In 2012, Kähkönen et al. observed that red raspberry and cloudberry ellagitannins, including pedunculagin, acted as effective radical scavengers, substantially contributing to the antioxidant activity of the berries in lipoprotein and lipid emulsion environments (J. Agric. Food Chem. 2012;60:1167-74).
A 2014 review by Hardman summarized several studies suggesting that potent anticancer properties, including antiproliferative and antiangiogenic activities, have been linked to walnuts. She noted that pedunculagin is one of the key constituents in walnuts to which such characteristics have been attributed (J. Nutr. 2014;144(4 Suppl):555S-60S).
Potential cutaneous applications
In 2010, Lee et al. assessed the effects of pedunculagin on 2,4,6-trinitrochlorobenzene (TNCB)-induced atopic dermatitis-like lesions in NC/Nga mice. Investigators applied a cream containing 0.1% or 0.5% pedunculagin to the positive treatment group; the negative treatment group received the base cream without pedunculagin, with no topical formulations administered to a control group. The investigators found, 4 weeks after treatment, that greater and more rapid improvement in the lesions was experienced by the group that received the higher concentration of pedunculagin (Skin Res. Technol. 2010;16:371-7).
Kim et al., in 2014, isolated pedunculagin and five other phenolic compounds from the leaves of Quercus mongolica (Mongolian oak). They found that pedunculagin exhibited strong in vitro inhibition against the expression of matrix metalloproteinase (MMP)-1 and increased type I procollagen in human fibroblasts exposed to UVB. The Q. mongolica constituent was also found to concentration-dependently exhibit potent scavenging activity against the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical. The investigators suggested that the ellagitannin shows promise for use in preventing and treating cutaneous aging (Arch. Pharm. Res. 2014 Feb. 7. [Epub ahead of print]).
Conclusion
Pedunculagin shows some promise as an agent that can yield dermatologic benefits. However, the body of research on this natural compound is relatively scant. More expansive follow-up work is needed to determine the extent to which pedunculagin can be reasonably incorporated into the dermatologic armamentarium.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy,Topix Pharmaceuticals, and Unilever.