User login
Up in the air
I find it scary to travel without my kids, even just to a meeting. I’m not sure what I fear more: that something is going to happen to me (like what, I’ll freeze to death in a conference room?) or that something will happen to one of them (with someone else driving them to school, they really will finally strangle each other). The person I should really be worried about is the sitter left to watch five kids all week! I’ll be relieved if when we get back she still has all her nose rings.
Learning curveball
Have you ever kept doing something you knew was stupid, even after the thing everyone told you was going to happen happened? If you’ve ever had a hangover -- twice -- then are you really surprised that a resurgence of vaccine-preventable disease hasn’t done a thing to improve vaccination rates? People don’t make unwise choices accidentally; they work really hard at it.
If you’re like me, then ever since 1998 you’ve been saying, “Just wait until vaccine-preventable diseases make a comeback. Then those vaccine deniers will come to their senses.” Remember how innocent we were back in 1998? Just in case you had some lingering glimmer of hope for humanity, researchers from Seattle Children’s presented new findings at the Pediatric Academic Societies’ meeting earlier this month regarding a whooping cough outbreak in Washington State in 2011.
A team led by Dr. Elizabeth Wolf compared local pertussis vaccination rates before and during the outbreak, presuming that when people realized that their own babies were threatened by a deadly disease, they would, you know, respond. As it turns out, they did respond, either by denying that there was a threat or by ignoring that there was anything to do about it. I suppose these data don’t bode well for vaccine acceptance or, for that matter, for other problems where science points to an obvious solution that some people don’t want to accept, like gun safety and global warming. It’s enough to make me court a hangover -- my 23rd.
Well rounded
Have you gotten caught up in this fad for “life-hacking,” trying to save time and effort with tricks like using paper clips to organize electrical cables, painting look-alike house keys with colored nail polish, or not having five children? Now a study suggests a new way for pediatricians and parents to simplify: Don’t treat moderate to severe positional plagiocephaly with skull-molding helmets. I know, it’s not as cool as using a sawed-off water bottle to seal your unused chocolate chips, but it’ll save a lot more money, and you’re less likely to need stitches.
A group of Dutch researchers randomized 84 otherwise normal 5- to 6-month-old infants with moderate to severe positional plagiocephaly to 6 months of helmet therapy vs. 6 months of, well, not wearing helmets. (There’s just no feasible way to double-blind a helmet study.) End points involved careful measurements of the skull at 24 months of age as well as secondary findings like ear deviation, facial asymmetry, and whether parents argued over where they were going to find $2,500 for the helmet.
In the end, helmets made no difference whatsoever in outcomes. Helmet makers were swift to point out that the study excluded premature infants younger than 36 weeks and those with dysmorphic features or torticollis. It’s too early to gauge whether this study will actually lead providers and parents to turn to skull molding helmets less often, but think about it: With each helmet not prescribed, we’ll be able to save enough money to paint 10,000 previously indistinguishable keys with nail polish!
Short changed
Have you ever noticed that in every monster movie there’s that one scene where a cop, faced with a scaly creature roughly the size of the Staples Center, unloads the magazine of a handgun at it and then stares in terror to see that the beast is unfazed or, even worse, annoyed? When the American Academy of Pediatrics announced that its latest child health priority is poverty, some of us thought of the new AAP President James Perrin in the role of that ambitious but outgunned officer. (Full disclosure: I’ve met Dr. Perrin and, at the time, he was wearing neither dark blue nor a sidearm.)
For those who wonder why the AAP would take on a leviathan like poverty, there is a new study in the Proceedings of the National Academy of Sciences comparing African American children from severely underprivileged and privileged backgrounds. The investigators found a difference much deeper than whether family members used SAT words around the dinner table or, for that matter, could afford dinner. They discovered that destitution actually shortens children’s telomeres, leading to poorer health and a reduced life expectancy at the genetic level. These findings might lend us some perspective when our kids don’t get accepted to the first choice colleges.
These results make me want to go home at the end of this trip and hug my children, assuming we all survive. I already got them presents, and I even picked up something for the sitter. I hope she loves her new nose ring!
David L. Hill, M.D., FAAP is the author of Dad to Dad: Parenting Like a Pro (AAP Publishing, 2012). He is also vice president of Cape Fear Pediatrics in Wilmington, N.C., and adjunct assistant professor of pediatrics at the University of North Carolina at Chapel Hill. He serves as Program Director for the AAP Council on Communications and Media and as an executive committee member of the North Carolina Pediatric Society. He has recorded commentaries for NPR's All Things Considered and provided content for various print, television, and Internet outlets.
I find it scary to travel without my kids, even just to a meeting. I’m not sure what I fear more: that something is going to happen to me (like what, I’ll freeze to death in a conference room?) or that something will happen to one of them (with someone else driving them to school, they really will finally strangle each other). The person I should really be worried about is the sitter left to watch five kids all week! I’ll be relieved if when we get back she still has all her nose rings.
Learning curveball
Have you ever kept doing something you knew was stupid, even after the thing everyone told you was going to happen happened? If you’ve ever had a hangover -- twice -- then are you really surprised that a resurgence of vaccine-preventable disease hasn’t done a thing to improve vaccination rates? People don’t make unwise choices accidentally; they work really hard at it.
If you’re like me, then ever since 1998 you’ve been saying, “Just wait until vaccine-preventable diseases make a comeback. Then those vaccine deniers will come to their senses.” Remember how innocent we were back in 1998? Just in case you had some lingering glimmer of hope for humanity, researchers from Seattle Children’s presented new findings at the Pediatric Academic Societies’ meeting earlier this month regarding a whooping cough outbreak in Washington State in 2011.
A team led by Dr. Elizabeth Wolf compared local pertussis vaccination rates before and during the outbreak, presuming that when people realized that their own babies were threatened by a deadly disease, they would, you know, respond. As it turns out, they did respond, either by denying that there was a threat or by ignoring that there was anything to do about it. I suppose these data don’t bode well for vaccine acceptance or, for that matter, for other problems where science points to an obvious solution that some people don’t want to accept, like gun safety and global warming. It’s enough to make me court a hangover -- my 23rd.
Well rounded
Have you gotten caught up in this fad for “life-hacking,” trying to save time and effort with tricks like using paper clips to organize electrical cables, painting look-alike house keys with colored nail polish, or not having five children? Now a study suggests a new way for pediatricians and parents to simplify: Don’t treat moderate to severe positional plagiocephaly with skull-molding helmets. I know, it’s not as cool as using a sawed-off water bottle to seal your unused chocolate chips, but it’ll save a lot more money, and you’re less likely to need stitches.
A group of Dutch researchers randomized 84 otherwise normal 5- to 6-month-old infants with moderate to severe positional plagiocephaly to 6 months of helmet therapy vs. 6 months of, well, not wearing helmets. (There’s just no feasible way to double-blind a helmet study.) End points involved careful measurements of the skull at 24 months of age as well as secondary findings like ear deviation, facial asymmetry, and whether parents argued over where they were going to find $2,500 for the helmet.
In the end, helmets made no difference whatsoever in outcomes. Helmet makers were swift to point out that the study excluded premature infants younger than 36 weeks and those with dysmorphic features or torticollis. It’s too early to gauge whether this study will actually lead providers and parents to turn to skull molding helmets less often, but think about it: With each helmet not prescribed, we’ll be able to save enough money to paint 10,000 previously indistinguishable keys with nail polish!
Short changed
Have you ever noticed that in every monster movie there’s that one scene where a cop, faced with a scaly creature roughly the size of the Staples Center, unloads the magazine of a handgun at it and then stares in terror to see that the beast is unfazed or, even worse, annoyed? When the American Academy of Pediatrics announced that its latest child health priority is poverty, some of us thought of the new AAP President James Perrin in the role of that ambitious but outgunned officer. (Full disclosure: I’ve met Dr. Perrin and, at the time, he was wearing neither dark blue nor a sidearm.)
For those who wonder why the AAP would take on a leviathan like poverty, there is a new study in the Proceedings of the National Academy of Sciences comparing African American children from severely underprivileged and privileged backgrounds. The investigators found a difference much deeper than whether family members used SAT words around the dinner table or, for that matter, could afford dinner. They discovered that destitution actually shortens children’s telomeres, leading to poorer health and a reduced life expectancy at the genetic level. These findings might lend us some perspective when our kids don’t get accepted to the first choice colleges.
These results make me want to go home at the end of this trip and hug my children, assuming we all survive. I already got them presents, and I even picked up something for the sitter. I hope she loves her new nose ring!
David L. Hill, M.D., FAAP is the author of Dad to Dad: Parenting Like a Pro (AAP Publishing, 2012). He is also vice president of Cape Fear Pediatrics in Wilmington, N.C., and adjunct assistant professor of pediatrics at the University of North Carolina at Chapel Hill. He serves as Program Director for the AAP Council on Communications and Media and as an executive committee member of the North Carolina Pediatric Society. He has recorded commentaries for NPR's All Things Considered and provided content for various print, television, and Internet outlets.
I find it scary to travel without my kids, even just to a meeting. I’m not sure what I fear more: that something is going to happen to me (like what, I’ll freeze to death in a conference room?) or that something will happen to one of them (with someone else driving them to school, they really will finally strangle each other). The person I should really be worried about is the sitter left to watch five kids all week! I’ll be relieved if when we get back she still has all her nose rings.
Learning curveball
Have you ever kept doing something you knew was stupid, even after the thing everyone told you was going to happen happened? If you’ve ever had a hangover -- twice -- then are you really surprised that a resurgence of vaccine-preventable disease hasn’t done a thing to improve vaccination rates? People don’t make unwise choices accidentally; they work really hard at it.
If you’re like me, then ever since 1998 you’ve been saying, “Just wait until vaccine-preventable diseases make a comeback. Then those vaccine deniers will come to their senses.” Remember how innocent we were back in 1998? Just in case you had some lingering glimmer of hope for humanity, researchers from Seattle Children’s presented new findings at the Pediatric Academic Societies’ meeting earlier this month regarding a whooping cough outbreak in Washington State in 2011.
A team led by Dr. Elizabeth Wolf compared local pertussis vaccination rates before and during the outbreak, presuming that when people realized that their own babies were threatened by a deadly disease, they would, you know, respond. As it turns out, they did respond, either by denying that there was a threat or by ignoring that there was anything to do about it. I suppose these data don’t bode well for vaccine acceptance or, for that matter, for other problems where science points to an obvious solution that some people don’t want to accept, like gun safety and global warming. It’s enough to make me court a hangover -- my 23rd.
Well rounded
Have you gotten caught up in this fad for “life-hacking,” trying to save time and effort with tricks like using paper clips to organize electrical cables, painting look-alike house keys with colored nail polish, or not having five children? Now a study suggests a new way for pediatricians and parents to simplify: Don’t treat moderate to severe positional plagiocephaly with skull-molding helmets. I know, it’s not as cool as using a sawed-off water bottle to seal your unused chocolate chips, but it’ll save a lot more money, and you’re less likely to need stitches.
A group of Dutch researchers randomized 84 otherwise normal 5- to 6-month-old infants with moderate to severe positional plagiocephaly to 6 months of helmet therapy vs. 6 months of, well, not wearing helmets. (There’s just no feasible way to double-blind a helmet study.) End points involved careful measurements of the skull at 24 months of age as well as secondary findings like ear deviation, facial asymmetry, and whether parents argued over where they were going to find $2,500 for the helmet.
In the end, helmets made no difference whatsoever in outcomes. Helmet makers were swift to point out that the study excluded premature infants younger than 36 weeks and those with dysmorphic features or torticollis. It’s too early to gauge whether this study will actually lead providers and parents to turn to skull molding helmets less often, but think about it: With each helmet not prescribed, we’ll be able to save enough money to paint 10,000 previously indistinguishable keys with nail polish!
Short changed
Have you ever noticed that in every monster movie there’s that one scene where a cop, faced with a scaly creature roughly the size of the Staples Center, unloads the magazine of a handgun at it and then stares in terror to see that the beast is unfazed or, even worse, annoyed? When the American Academy of Pediatrics announced that its latest child health priority is poverty, some of us thought of the new AAP President James Perrin in the role of that ambitious but outgunned officer. (Full disclosure: I’ve met Dr. Perrin and, at the time, he was wearing neither dark blue nor a sidearm.)
For those who wonder why the AAP would take on a leviathan like poverty, there is a new study in the Proceedings of the National Academy of Sciences comparing African American children from severely underprivileged and privileged backgrounds. The investigators found a difference much deeper than whether family members used SAT words around the dinner table or, for that matter, could afford dinner. They discovered that destitution actually shortens children’s telomeres, leading to poorer health and a reduced life expectancy at the genetic level. These findings might lend us some perspective when our kids don’t get accepted to the first choice colleges.
These results make me want to go home at the end of this trip and hug my children, assuming we all survive. I already got them presents, and I even picked up something for the sitter. I hope she loves her new nose ring!
David L. Hill, M.D., FAAP is the author of Dad to Dad: Parenting Like a Pro (AAP Publishing, 2012). He is also vice president of Cape Fear Pediatrics in Wilmington, N.C., and adjunct assistant professor of pediatrics at the University of North Carolina at Chapel Hill. He serves as Program Director for the AAP Council on Communications and Media and as an executive committee member of the North Carolina Pediatric Society. He has recorded commentaries for NPR's All Things Considered and provided content for various print, television, and Internet outlets.
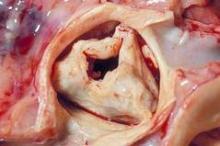
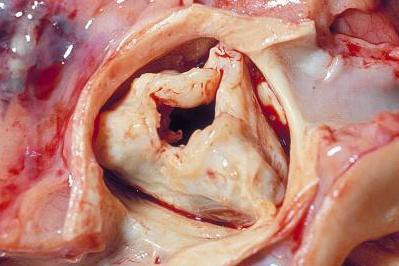
TAVR quickly penetrates to low-risk patients
Have too many low-risk U.S. patients undergone transcatheter aortic-valve replacement since the procedure became available in October 2012 to U.S. patients who are also judged eligible for surgical aortic-valve replacement?
Furthermore, regardless of the answer, are the tools currently available to cardiologists and cardiac surgeons to estimate a patient’s risk for undergoing aortic-valve surgery too limited and flawed to even allow clinicians to reasonably judge who is at high risk for surgical valve replacement and who isn’t?
And finally, have the benefits of transcatheter aortic valve replacement (TAVR) as an alternative to surgery become so compelling that patients, cardiologists, and surgeons are all now willing to ignore the possible downside that still remains to TAVR and the risk-level ground rules that the field set up just a few years ago?
Answering the third question is probably the easiest, and the answer seems to be yes, at least based on U.S. use of TAVR since the first valve system came onto the U.S. market for inoperable patients in November 2011, as well as on what happened in the latest big TAVR trial. Last November, researchers published a report on the first 7,710 U.S. TAVR patients, while results from the latest big trial, the CoreValve pivotal trial, came out in March.
A key finding in the JAMA report last November on nearly 8,000 TAVR recipients, most of whom were operable patients once this indication received U.S. approval in 2012, was that the median Predicted Risk of Operative Mortality score by the formula crafted by the Society of Thoracic Surgeons (the STS PROM score) was 7%, and a quarter of all U.S. patients had a score of 5% or less. Those risk levels are quite low relative to the levels in the first TAVR pivotal trial, the PARTNER I trial, and relative to how TAVR developers viewed the role for this technology when it first entered the U.S. market a couple of years ago.
In the first U.S. pivotal trial for TAVR in patients judged operable, a head-to-head comparison of TAVR and surgical aortic valve replacement (SAVR), all enrolled patients had to have an STS PROM score of at least 10%, and the average score of enrolled patients was 11.8%. Labeling for the first U.S. approved TAVR system was for patients with an STS PROM score of at least 8%. The follow-up trial designed to test a second-generation TAVR device, PARTNER II, launched about 3 years ago and not scheduled to finish until the end of 2015, specifically targeted "intermediate-risk" patients with aortic stenosis, those with an STS PROM of 4%-8%. Even this next-generation-device trial, PARTNER II, wasn’t designed to target patients with risk levels of less than 4%, yet patients of that very sort have already received treatment with the first-generation device based on the registry results.
It’s not just the registry that shows a shift toward lower-risk patients. The CoreValve pivotal trial that pitted a different TAVR system head to head against SAVR showed more of the same. The trial was designed to enroll operable patients with a predicted 30-day mortality risk after SAVR of at least 15%, though the study left it up to clinicians to decide how to measure risk and gave them free rein to use parameters in addition to the STS PROM score. The result was that the average STS PROM score of enrolled patients in the CoreValve trial was 7%, and roughly 10% of enrolled patients had a score of less than 4%. The temptation to use TAVR on lower-risk patients seems to have been inescapable, happening in both the CoreValve trial as well as in the registry’s Sapien experience.
Of course, the CoreValve results also showed significant survival benefit from TAVR using the CoreValve system, a game-changing result.
Part of what has been going on with risk assessment is that in the CoreValve study as well as in routine practice, clinicians have been fudging their use of the STS PROM score when sizing up patients for TAVR. I asked several interventional cardiologists about this at the ACC meeting in March, and their answers were all variants of what Dr. James Hermiller told me: "It’s frailty that often gets a patient to TAVR, and frailty is hard to quantify. Frailty can exist even when the STS score is not high." And even though labeling for the first-generation TAVR system that all 7,710 of the first U.S. patients received specified an STS PROM score of at least 8%, Dr. David Holmes told me that for Medicare reimbursement, all that’s needed is for two cardiac surgeons to sign off on saying that the patient’s status warrants TAVR. "That’s what carries the day," he said.
Data from the new CoreValve study underscore how limited the STS PROM score is right now. The average score of the patients enrolled in the surgical arm of this study was 7.5%, which means that 7.5 % of the patients who underwent SAVR were predicted by the scoring system to die during the first 30 days after surgery. But the actual rate was 4.5%, "substantially lower," said the CoreValve report. STS PROM scoring resulted in a substantial overcall on predicted risk.
When TAVR was first introduced, experts had two caveats about its potential to completely replace SAVR. The first was uncertainty about the long-term durability (think 10 or more years) of TAVR. The second was uncertainty about the short- and intermediate-term safety and efficacy of TAVR, especially for the patients for whom conventional SAVR was a reasonable option.
Doubts about short- and intermediate-term efficacy arose with the first-generation TAVR device, Sapien, because of the issue of paravalvular leak and the inability of TAVR to surpass SAVR outcomes in the PARTNER I results, but those doubts have now been mostly swept away from by the CoreValve results, which established CoreValve as superior to SAVR and made it the current standard for essentially all patients who need their aortic valve replaced. Even if paravalvular leak is still an issue for some patients, patients treated with CoreValve, TAVR overall did significantly better after 1 year than SAVR in the CoreValve trial, which means that TAVR was best regardless of whether paravalvular leak was an issue for some patients. And this was in patients who represented a wide range of STS PROM risk, with close to 10% of enrolled patients having a score of less than 4%. A subanalysis showed that the low-risk patients derived as much benefit from CoreValve TAVR, compared with SAVR, as did higher-risk patients.
The long-term durability question still remains for now, but the substantial mortality benefit in the CoreValve trial seen after 1 year probably trumps that.
Researchers designed the TAVR trials to methodically progress through a spectrum of patient risk levels. As recently as a year ago, several experts told me that no way in the near future could TAVR be an option for low-risk patients with STS PROM scores of less than 4%. But that is not how it has worked out. Patients, cardiac surgeons, and cardiologists embraced TAVR way faster and tighter than anyone expected just a few years ago.
On Twitter @mitchelzoler
Have too many low-risk U.S. patients undergone transcatheter aortic-valve replacement since the procedure became available in October 2012 to U.S. patients who are also judged eligible for surgical aortic-valve replacement?
Furthermore, regardless of the answer, are the tools currently available to cardiologists and cardiac surgeons to estimate a patient’s risk for undergoing aortic-valve surgery too limited and flawed to even allow clinicians to reasonably judge who is at high risk for surgical valve replacement and who isn’t?
And finally, have the benefits of transcatheter aortic valve replacement (TAVR) as an alternative to surgery become so compelling that patients, cardiologists, and surgeons are all now willing to ignore the possible downside that still remains to TAVR and the risk-level ground rules that the field set up just a few years ago?
Answering the third question is probably the easiest, and the answer seems to be yes, at least based on U.S. use of TAVR since the first valve system came onto the U.S. market for inoperable patients in November 2011, as well as on what happened in the latest big TAVR trial. Last November, researchers published a report on the first 7,710 U.S. TAVR patients, while results from the latest big trial, the CoreValve pivotal trial, came out in March.
A key finding in the JAMA report last November on nearly 8,000 TAVR recipients, most of whom were operable patients once this indication received U.S. approval in 2012, was that the median Predicted Risk of Operative Mortality score by the formula crafted by the Society of Thoracic Surgeons (the STS PROM score) was 7%, and a quarter of all U.S. patients had a score of 5% or less. Those risk levels are quite low relative to the levels in the first TAVR pivotal trial, the PARTNER I trial, and relative to how TAVR developers viewed the role for this technology when it first entered the U.S. market a couple of years ago.
In the first U.S. pivotal trial for TAVR in patients judged operable, a head-to-head comparison of TAVR and surgical aortic valve replacement (SAVR), all enrolled patients had to have an STS PROM score of at least 10%, and the average score of enrolled patients was 11.8%. Labeling for the first U.S. approved TAVR system was for patients with an STS PROM score of at least 8%. The follow-up trial designed to test a second-generation TAVR device, PARTNER II, launched about 3 years ago and not scheduled to finish until the end of 2015, specifically targeted "intermediate-risk" patients with aortic stenosis, those with an STS PROM of 4%-8%. Even this next-generation-device trial, PARTNER II, wasn’t designed to target patients with risk levels of less than 4%, yet patients of that very sort have already received treatment with the first-generation device based on the registry results.
It’s not just the registry that shows a shift toward lower-risk patients. The CoreValve pivotal trial that pitted a different TAVR system head to head against SAVR showed more of the same. The trial was designed to enroll operable patients with a predicted 30-day mortality risk after SAVR of at least 15%, though the study left it up to clinicians to decide how to measure risk and gave them free rein to use parameters in addition to the STS PROM score. The result was that the average STS PROM score of enrolled patients in the CoreValve trial was 7%, and roughly 10% of enrolled patients had a score of less than 4%. The temptation to use TAVR on lower-risk patients seems to have been inescapable, happening in both the CoreValve trial as well as in the registry’s Sapien experience.
Of course, the CoreValve results also showed significant survival benefit from TAVR using the CoreValve system, a game-changing result.
Part of what has been going on with risk assessment is that in the CoreValve study as well as in routine practice, clinicians have been fudging their use of the STS PROM score when sizing up patients for TAVR. I asked several interventional cardiologists about this at the ACC meeting in March, and their answers were all variants of what Dr. James Hermiller told me: "It’s frailty that often gets a patient to TAVR, and frailty is hard to quantify. Frailty can exist even when the STS score is not high." And even though labeling for the first-generation TAVR system that all 7,710 of the first U.S. patients received specified an STS PROM score of at least 8%, Dr. David Holmes told me that for Medicare reimbursement, all that’s needed is for two cardiac surgeons to sign off on saying that the patient’s status warrants TAVR. "That’s what carries the day," he said.
Data from the new CoreValve study underscore how limited the STS PROM score is right now. The average score of the patients enrolled in the surgical arm of this study was 7.5%, which means that 7.5 % of the patients who underwent SAVR were predicted by the scoring system to die during the first 30 days after surgery. But the actual rate was 4.5%, "substantially lower," said the CoreValve report. STS PROM scoring resulted in a substantial overcall on predicted risk.
When TAVR was first introduced, experts had two caveats about its potential to completely replace SAVR. The first was uncertainty about the long-term durability (think 10 or more years) of TAVR. The second was uncertainty about the short- and intermediate-term safety and efficacy of TAVR, especially for the patients for whom conventional SAVR was a reasonable option.
Doubts about short- and intermediate-term efficacy arose with the first-generation TAVR device, Sapien, because of the issue of paravalvular leak and the inability of TAVR to surpass SAVR outcomes in the PARTNER I results, but those doubts have now been mostly swept away from by the CoreValve results, which established CoreValve as superior to SAVR and made it the current standard for essentially all patients who need their aortic valve replaced. Even if paravalvular leak is still an issue for some patients, patients treated with CoreValve, TAVR overall did significantly better after 1 year than SAVR in the CoreValve trial, which means that TAVR was best regardless of whether paravalvular leak was an issue for some patients. And this was in patients who represented a wide range of STS PROM risk, with close to 10% of enrolled patients having a score of less than 4%. A subanalysis showed that the low-risk patients derived as much benefit from CoreValve TAVR, compared with SAVR, as did higher-risk patients.
The long-term durability question still remains for now, but the substantial mortality benefit in the CoreValve trial seen after 1 year probably trumps that.
Researchers designed the TAVR trials to methodically progress through a spectrum of patient risk levels. As recently as a year ago, several experts told me that no way in the near future could TAVR be an option for low-risk patients with STS PROM scores of less than 4%. But that is not how it has worked out. Patients, cardiac surgeons, and cardiologists embraced TAVR way faster and tighter than anyone expected just a few years ago.
On Twitter @mitchelzoler
Have too many low-risk U.S. patients undergone transcatheter aortic-valve replacement since the procedure became available in October 2012 to U.S. patients who are also judged eligible for surgical aortic-valve replacement?
Furthermore, regardless of the answer, are the tools currently available to cardiologists and cardiac surgeons to estimate a patient’s risk for undergoing aortic-valve surgery too limited and flawed to even allow clinicians to reasonably judge who is at high risk for surgical valve replacement and who isn’t?
And finally, have the benefits of transcatheter aortic valve replacement (TAVR) as an alternative to surgery become so compelling that patients, cardiologists, and surgeons are all now willing to ignore the possible downside that still remains to TAVR and the risk-level ground rules that the field set up just a few years ago?
Answering the third question is probably the easiest, and the answer seems to be yes, at least based on U.S. use of TAVR since the first valve system came onto the U.S. market for inoperable patients in November 2011, as well as on what happened in the latest big TAVR trial. Last November, researchers published a report on the first 7,710 U.S. TAVR patients, while results from the latest big trial, the CoreValve pivotal trial, came out in March.
A key finding in the JAMA report last November on nearly 8,000 TAVR recipients, most of whom were operable patients once this indication received U.S. approval in 2012, was that the median Predicted Risk of Operative Mortality score by the formula crafted by the Society of Thoracic Surgeons (the STS PROM score) was 7%, and a quarter of all U.S. patients had a score of 5% or less. Those risk levels are quite low relative to the levels in the first TAVR pivotal trial, the PARTNER I trial, and relative to how TAVR developers viewed the role for this technology when it first entered the U.S. market a couple of years ago.
In the first U.S. pivotal trial for TAVR in patients judged operable, a head-to-head comparison of TAVR and surgical aortic valve replacement (SAVR), all enrolled patients had to have an STS PROM score of at least 10%, and the average score of enrolled patients was 11.8%. Labeling for the first U.S. approved TAVR system was for patients with an STS PROM score of at least 8%. The follow-up trial designed to test a second-generation TAVR device, PARTNER II, launched about 3 years ago and not scheduled to finish until the end of 2015, specifically targeted "intermediate-risk" patients with aortic stenosis, those with an STS PROM of 4%-8%. Even this next-generation-device trial, PARTNER II, wasn’t designed to target patients with risk levels of less than 4%, yet patients of that very sort have already received treatment with the first-generation device based on the registry results.
It’s not just the registry that shows a shift toward lower-risk patients. The CoreValve pivotal trial that pitted a different TAVR system head to head against SAVR showed more of the same. The trial was designed to enroll operable patients with a predicted 30-day mortality risk after SAVR of at least 15%, though the study left it up to clinicians to decide how to measure risk and gave them free rein to use parameters in addition to the STS PROM score. The result was that the average STS PROM score of enrolled patients in the CoreValve trial was 7%, and roughly 10% of enrolled patients had a score of less than 4%. The temptation to use TAVR on lower-risk patients seems to have been inescapable, happening in both the CoreValve trial as well as in the registry’s Sapien experience.
Of course, the CoreValve results also showed significant survival benefit from TAVR using the CoreValve system, a game-changing result.
Part of what has been going on with risk assessment is that in the CoreValve study as well as in routine practice, clinicians have been fudging their use of the STS PROM score when sizing up patients for TAVR. I asked several interventional cardiologists about this at the ACC meeting in March, and their answers were all variants of what Dr. James Hermiller told me: "It’s frailty that often gets a patient to TAVR, and frailty is hard to quantify. Frailty can exist even when the STS score is not high." And even though labeling for the first-generation TAVR system that all 7,710 of the first U.S. patients received specified an STS PROM score of at least 8%, Dr. David Holmes told me that for Medicare reimbursement, all that’s needed is for two cardiac surgeons to sign off on saying that the patient’s status warrants TAVR. "That’s what carries the day," he said.
Data from the new CoreValve study underscore how limited the STS PROM score is right now. The average score of the patients enrolled in the surgical arm of this study was 7.5%, which means that 7.5 % of the patients who underwent SAVR were predicted by the scoring system to die during the first 30 days after surgery. But the actual rate was 4.5%, "substantially lower," said the CoreValve report. STS PROM scoring resulted in a substantial overcall on predicted risk.
When TAVR was first introduced, experts had two caveats about its potential to completely replace SAVR. The first was uncertainty about the long-term durability (think 10 or more years) of TAVR. The second was uncertainty about the short- and intermediate-term safety and efficacy of TAVR, especially for the patients for whom conventional SAVR was a reasonable option.
Doubts about short- and intermediate-term efficacy arose with the first-generation TAVR device, Sapien, because of the issue of paravalvular leak and the inability of TAVR to surpass SAVR outcomes in the PARTNER I results, but those doubts have now been mostly swept away from by the CoreValve results, which established CoreValve as superior to SAVR and made it the current standard for essentially all patients who need their aortic valve replaced. Even if paravalvular leak is still an issue for some patients, patients treated with CoreValve, TAVR overall did significantly better after 1 year than SAVR in the CoreValve trial, which means that TAVR was best regardless of whether paravalvular leak was an issue for some patients. And this was in patients who represented a wide range of STS PROM risk, with close to 10% of enrolled patients having a score of less than 4%. A subanalysis showed that the low-risk patients derived as much benefit from CoreValve TAVR, compared with SAVR, as did higher-risk patients.
The long-term durability question still remains for now, but the substantial mortality benefit in the CoreValve trial seen after 1 year probably trumps that.
Researchers designed the TAVR trials to methodically progress through a spectrum of patient risk levels. As recently as a year ago, several experts told me that no way in the near future could TAVR be an option for low-risk patients with STS PROM scores of less than 4%. But that is not how it has worked out. Patients, cardiac surgeons, and cardiologists embraced TAVR way faster and tighter than anyone expected just a few years ago.
On Twitter @mitchelzoler
Mobile health validation efforts in infancy
Hardly a day goes by anymore without an announcement of a new mobile health app, but there are precious few data to show which apps are useful in clinical or financial terms. Lots of people would like to change that, but how?
Three experts offered ideas in a recent opinion article in the Journal of the American Medical Association, and some partnerships between academia and industry may be laying the groundwork for greater validation of new mobile health (mHealth) tools.
Of the more than 40,000 health, fitness, and medical apps on the market, reviews "have largely focused on personal impressions, rather than evidence-based, unbiased assessments of clinical performance and data security," Adam C. Powell, Ph.D., Dr. Adam B. Landman, and Dr. David W. Bates wrote in their article, "In Search of a Few Good Apps" (JAMA 2014;311:1851-2).
The Office of the National Coordinator for Health Information Technology (ONC) could play a greater role supporting development of mHealth app guidelines and, eventually, commission non-profit or for-profit entities to certify apps, as it does now for electronic health records, they suggested.
Dr. Powell is a Boston-based consultant. Dr. Landman, an emergency medicine specialist, is chief medical information officer for health information innovation and integration at Brigham and Women’s Hospital, Boston. Dr. Bates is chief of general internal medicine and the chief quality officer at Brigham and Women’s. Dr. Landman and Dr. Bates are both at Harvard Medical School in Boston.
The National Institutes of Health mHealth Training Institute is educating an interdisciplinary group of researchers about the potential of mHealth and the need to evaluate these new tools. "If this effort is coupled with increased funding for mHealth research, it may help galvanize a larger body of evidence to inform mHealth app development and certification," the authors wrote.
Meanwhile, a new partnership between the University of California, San Francisco (UCSF), and Samsung Electronics aims to validate and commercialize promising digital tools for health care, including apps, in a Digital Health Innovation Lab. A few other universities recently entered into similar partnerships with industry.
Both UCSF and Samsung are making a "significant investment" to fund the lab but are not ready to release financial details, Dr. Michael Blum said in an interview.
The partnership initially will focus on preventive health, said Dr. Blum, who will direct the lab to be located at UCSF’s Mission Bay campus. "There is no better way to treat disease than by avoiding it in the first place. [Moe than] 70% of our health care dollars are spent on avoidable disease," said Dr. Blum, a cardiologist who has been leading UCSF’s relatively new Center for Digital Health Innovation.
UCSF already has begun testing medical apps in clinical trials, such as a smoking cessation app, and will pursue clinical testing of tools including health sensors, wearable computing, and cloud-based analytics.
"There are many sites designing medical apps but very few are rigorously validated," Dr. Blum said. "We believe that validation is critical to the success of these apps and products. It is important for health care providers to know that they can trust the data and information which will lead to more consistent use and, hopefully, better outcomes for the users."
In Michigan, the William Davidson Foundation in January 2014 awarded $3 million to the Henry Ford Health System to create the William Davidson Center for Entrepreneurs in Digital Health. The center hopes to attract corporate partners and others to create, clinically validate, and commercialize digital health tools and to create a curriculum integrating health care, digital technologies, and entrepreneurship, according to a statement released by the Henry Ford Innovation Institute.
At the University of Colorado, Denver, the Center for Information Technology Innovation recently launched a Digital Health Consortium to bring its business school faculty together with entrepreneurs, health care providers, researchers, educators, and others to develop and clinically validate the next generation of digital health tools. The Center is funded by its members, which include the university and more than 30 Colorado information technology business leaders.
As each of these initiatives and others like them report results from their validation efforts, we’ll bring you the latest news on medical apps. Watch this space.
On Twitter @sherryboschert
Hardly a day goes by anymore without an announcement of a new mobile health app, but there are precious few data to show which apps are useful in clinical or financial terms. Lots of people would like to change that, but how?
Three experts offered ideas in a recent opinion article in the Journal of the American Medical Association, and some partnerships between academia and industry may be laying the groundwork for greater validation of new mobile health (mHealth) tools.
Of the more than 40,000 health, fitness, and medical apps on the market, reviews "have largely focused on personal impressions, rather than evidence-based, unbiased assessments of clinical performance and data security," Adam C. Powell, Ph.D., Dr. Adam B. Landman, and Dr. David W. Bates wrote in their article, "In Search of a Few Good Apps" (JAMA 2014;311:1851-2).
The Office of the National Coordinator for Health Information Technology (ONC) could play a greater role supporting development of mHealth app guidelines and, eventually, commission non-profit or for-profit entities to certify apps, as it does now for electronic health records, they suggested.
Dr. Powell is a Boston-based consultant. Dr. Landman, an emergency medicine specialist, is chief medical information officer for health information innovation and integration at Brigham and Women’s Hospital, Boston. Dr. Bates is chief of general internal medicine and the chief quality officer at Brigham and Women’s. Dr. Landman and Dr. Bates are both at Harvard Medical School in Boston.
The National Institutes of Health mHealth Training Institute is educating an interdisciplinary group of researchers about the potential of mHealth and the need to evaluate these new tools. "If this effort is coupled with increased funding for mHealth research, it may help galvanize a larger body of evidence to inform mHealth app development and certification," the authors wrote.
Meanwhile, a new partnership between the University of California, San Francisco (UCSF), and Samsung Electronics aims to validate and commercialize promising digital tools for health care, including apps, in a Digital Health Innovation Lab. A few other universities recently entered into similar partnerships with industry.
Both UCSF and Samsung are making a "significant investment" to fund the lab but are not ready to release financial details, Dr. Michael Blum said in an interview.
The partnership initially will focus on preventive health, said Dr. Blum, who will direct the lab to be located at UCSF’s Mission Bay campus. "There is no better way to treat disease than by avoiding it in the first place. [Moe than] 70% of our health care dollars are spent on avoidable disease," said Dr. Blum, a cardiologist who has been leading UCSF’s relatively new Center for Digital Health Innovation.
UCSF already has begun testing medical apps in clinical trials, such as a smoking cessation app, and will pursue clinical testing of tools including health sensors, wearable computing, and cloud-based analytics.
"There are many sites designing medical apps but very few are rigorously validated," Dr. Blum said. "We believe that validation is critical to the success of these apps and products. It is important for health care providers to know that they can trust the data and information which will lead to more consistent use and, hopefully, better outcomes for the users."
In Michigan, the William Davidson Foundation in January 2014 awarded $3 million to the Henry Ford Health System to create the William Davidson Center for Entrepreneurs in Digital Health. The center hopes to attract corporate partners and others to create, clinically validate, and commercialize digital health tools and to create a curriculum integrating health care, digital technologies, and entrepreneurship, according to a statement released by the Henry Ford Innovation Institute.
At the University of Colorado, Denver, the Center for Information Technology Innovation recently launched a Digital Health Consortium to bring its business school faculty together with entrepreneurs, health care providers, researchers, educators, and others to develop and clinically validate the next generation of digital health tools. The Center is funded by its members, which include the university and more than 30 Colorado information technology business leaders.
As each of these initiatives and others like them report results from their validation efforts, we’ll bring you the latest news on medical apps. Watch this space.
On Twitter @sherryboschert
Hardly a day goes by anymore without an announcement of a new mobile health app, but there are precious few data to show which apps are useful in clinical or financial terms. Lots of people would like to change that, but how?
Three experts offered ideas in a recent opinion article in the Journal of the American Medical Association, and some partnerships between academia and industry may be laying the groundwork for greater validation of new mobile health (mHealth) tools.
Of the more than 40,000 health, fitness, and medical apps on the market, reviews "have largely focused on personal impressions, rather than evidence-based, unbiased assessments of clinical performance and data security," Adam C. Powell, Ph.D., Dr. Adam B. Landman, and Dr. David W. Bates wrote in their article, "In Search of a Few Good Apps" (JAMA 2014;311:1851-2).
The Office of the National Coordinator for Health Information Technology (ONC) could play a greater role supporting development of mHealth app guidelines and, eventually, commission non-profit or for-profit entities to certify apps, as it does now for electronic health records, they suggested.
Dr. Powell is a Boston-based consultant. Dr. Landman, an emergency medicine specialist, is chief medical information officer for health information innovation and integration at Brigham and Women’s Hospital, Boston. Dr. Bates is chief of general internal medicine and the chief quality officer at Brigham and Women’s. Dr. Landman and Dr. Bates are both at Harvard Medical School in Boston.
The National Institutes of Health mHealth Training Institute is educating an interdisciplinary group of researchers about the potential of mHealth and the need to evaluate these new tools. "If this effort is coupled with increased funding for mHealth research, it may help galvanize a larger body of evidence to inform mHealth app development and certification," the authors wrote.
Meanwhile, a new partnership between the University of California, San Francisco (UCSF), and Samsung Electronics aims to validate and commercialize promising digital tools for health care, including apps, in a Digital Health Innovation Lab. A few other universities recently entered into similar partnerships with industry.
Both UCSF and Samsung are making a "significant investment" to fund the lab but are not ready to release financial details, Dr. Michael Blum said in an interview.
The partnership initially will focus on preventive health, said Dr. Blum, who will direct the lab to be located at UCSF’s Mission Bay campus. "There is no better way to treat disease than by avoiding it in the first place. [Moe than] 70% of our health care dollars are spent on avoidable disease," said Dr. Blum, a cardiologist who has been leading UCSF’s relatively new Center for Digital Health Innovation.
UCSF already has begun testing medical apps in clinical trials, such as a smoking cessation app, and will pursue clinical testing of tools including health sensors, wearable computing, and cloud-based analytics.
"There are many sites designing medical apps but very few are rigorously validated," Dr. Blum said. "We believe that validation is critical to the success of these apps and products. It is important for health care providers to know that they can trust the data and information which will lead to more consistent use and, hopefully, better outcomes for the users."
In Michigan, the William Davidson Foundation in January 2014 awarded $3 million to the Henry Ford Health System to create the William Davidson Center for Entrepreneurs in Digital Health. The center hopes to attract corporate partners and others to create, clinically validate, and commercialize digital health tools and to create a curriculum integrating health care, digital technologies, and entrepreneurship, according to a statement released by the Henry Ford Innovation Institute.
At the University of Colorado, Denver, the Center for Information Technology Innovation recently launched a Digital Health Consortium to bring its business school faculty together with entrepreneurs, health care providers, researchers, educators, and others to develop and clinically validate the next generation of digital health tools. The Center is funded by its members, which include the university and more than 30 Colorado information technology business leaders.
As each of these initiatives and others like them report results from their validation efforts, we’ll bring you the latest news on medical apps. Watch this space.
On Twitter @sherryboschert
RealSelf
If you have patients who express interest in cosmetic procedures, and especially if you are a cosmetic dermatologist or a plastic surgeon, you might want to familiarize yourself with RealSelf.com. Founded in 2006, RealSelf is an online community for learning and sharing information about cosmetic surgery, dermatology, dentistry, and other elective treatments. In 2013, the site had 36 million unique visitors, and it is expected to grow.
Why might RealSelf be relevant for you? Simply put, it’s another channel to market you and your practice. It works by allowing physicians to answer users’ questions about cosmetic procedures ranging from rhinoplasty and liposuction to tattoo removal and Botox. Over time, your participation can lead to new consultations at your practice.
To ensure credibility, physicians must be board-certified in order to join RealSelf’s physician community. There is an element of game mechanics: The more active the physician, the more exposure his or her profile and practice receives. Similarly, paid subscriptions lead to more exposure than free subscriptions (more on this later.) Although this model does not appeal to some physicians, many of them do like the platform, and see it as a way to build a reputation as an expert and to market their practices.
Unlike doctor review sites that focus on the physician, RealSelf focuses on the procedure. For each procedure, users will find actual patient reviews and before and after photos, as well as Q&A’s with board-certified physicians. Users will also find licensed physicians in their area as well as the average cost for the procedure. RealSelf believes that patients value transparency, and including prices creates transparency.
Since most patients genuinely want to help other patients make informed medical decisions, the reviews tend to be thoughtful and thorough, and many of them contain multiple before-and-after photos. As a physician perusing the patient reviews, you’ll start to notice that most of them are reasonable. For example, customer satisfaction with laser treatment for melasma was 51%, whereas satisfaction for laser treatment for rosacea was 80%.
Patients and prospective patients are flocking to the site because it allows them to share their experiences, interact with other patients, and gain access to physician experts in the field. Many patients have difficulty making decisions about cosmetic procedures; RealSelf aims to alleviate their fears and help them "make confident health and beauty decisions." If a prospective patient wants to see a video of tattoo removal or Botox injections, he or she can. If a patient wants to ask physicians their opinions, he or she can. According to RealSelf, physicians have answered over 500,000 questions on the site.
Of course, all this isn’t free for physicians. RealSelf is a business. They have a tiered membership – free, pro, and spotlight. To obtain free membership, you simply visit the site and follow the prompts to "claim your profile." Once your profile is completed, you will have access to a "doctor advisor" who can help you "optimize your visibility on the site." Both "pro" and "spotlight" offer additional benefits, such as integrating patient reviews on your practice website, promotions on Facebook and Twitter, extended directory listings, and exposure in your local area. RealSelf does not discuss costs of membership until you have claimed your profile.
Only you can determine if RealSelf is beneficial to you and your practice. If, for example, you’re not looking for new patients, then you might find it unnecessary. But at the very least, you’ll know what RealSelf is the next time a fellow cosmetic physician brings it up at a conference. And it’s never a bad idea to be familiar with current social technologies that may affect your livelihood.
If you’ve used RealSelf, let us know what you think. For more information, visit RealSelf.com.
Disclaimer: I have no financial interest in RealSelf and am not an active member.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @Dermdoc on Twitter.
If you have patients who express interest in cosmetic procedures, and especially if you are a cosmetic dermatologist or a plastic surgeon, you might want to familiarize yourself with RealSelf.com. Founded in 2006, RealSelf is an online community for learning and sharing information about cosmetic surgery, dermatology, dentistry, and other elective treatments. In 2013, the site had 36 million unique visitors, and it is expected to grow.
Why might RealSelf be relevant for you? Simply put, it’s another channel to market you and your practice. It works by allowing physicians to answer users’ questions about cosmetic procedures ranging from rhinoplasty and liposuction to tattoo removal and Botox. Over time, your participation can lead to new consultations at your practice.
To ensure credibility, physicians must be board-certified in order to join RealSelf’s physician community. There is an element of game mechanics: The more active the physician, the more exposure his or her profile and practice receives. Similarly, paid subscriptions lead to more exposure than free subscriptions (more on this later.) Although this model does not appeal to some physicians, many of them do like the platform, and see it as a way to build a reputation as an expert and to market their practices.
Unlike doctor review sites that focus on the physician, RealSelf focuses on the procedure. For each procedure, users will find actual patient reviews and before and after photos, as well as Q&A’s with board-certified physicians. Users will also find licensed physicians in their area as well as the average cost for the procedure. RealSelf believes that patients value transparency, and including prices creates transparency.
Since most patients genuinely want to help other patients make informed medical decisions, the reviews tend to be thoughtful and thorough, and many of them contain multiple before-and-after photos. As a physician perusing the patient reviews, you’ll start to notice that most of them are reasonable. For example, customer satisfaction with laser treatment for melasma was 51%, whereas satisfaction for laser treatment for rosacea was 80%.
Patients and prospective patients are flocking to the site because it allows them to share their experiences, interact with other patients, and gain access to physician experts in the field. Many patients have difficulty making decisions about cosmetic procedures; RealSelf aims to alleviate their fears and help them "make confident health and beauty decisions." If a prospective patient wants to see a video of tattoo removal or Botox injections, he or she can. If a patient wants to ask physicians their opinions, he or she can. According to RealSelf, physicians have answered over 500,000 questions on the site.
Of course, all this isn’t free for physicians. RealSelf is a business. They have a tiered membership – free, pro, and spotlight. To obtain free membership, you simply visit the site and follow the prompts to "claim your profile." Once your profile is completed, you will have access to a "doctor advisor" who can help you "optimize your visibility on the site." Both "pro" and "spotlight" offer additional benefits, such as integrating patient reviews on your practice website, promotions on Facebook and Twitter, extended directory listings, and exposure in your local area. RealSelf does not discuss costs of membership until you have claimed your profile.
Only you can determine if RealSelf is beneficial to you and your practice. If, for example, you’re not looking for new patients, then you might find it unnecessary. But at the very least, you’ll know what RealSelf is the next time a fellow cosmetic physician brings it up at a conference. And it’s never a bad idea to be familiar with current social technologies that may affect your livelihood.
If you’ve used RealSelf, let us know what you think. For more information, visit RealSelf.com.
Disclaimer: I have no financial interest in RealSelf and am not an active member.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @Dermdoc on Twitter.
If you have patients who express interest in cosmetic procedures, and especially if you are a cosmetic dermatologist or a plastic surgeon, you might want to familiarize yourself with RealSelf.com. Founded in 2006, RealSelf is an online community for learning and sharing information about cosmetic surgery, dermatology, dentistry, and other elective treatments. In 2013, the site had 36 million unique visitors, and it is expected to grow.
Why might RealSelf be relevant for you? Simply put, it’s another channel to market you and your practice. It works by allowing physicians to answer users’ questions about cosmetic procedures ranging from rhinoplasty and liposuction to tattoo removal and Botox. Over time, your participation can lead to new consultations at your practice.
To ensure credibility, physicians must be board-certified in order to join RealSelf’s physician community. There is an element of game mechanics: The more active the physician, the more exposure his or her profile and practice receives. Similarly, paid subscriptions lead to more exposure than free subscriptions (more on this later.) Although this model does not appeal to some physicians, many of them do like the platform, and see it as a way to build a reputation as an expert and to market their practices.
Unlike doctor review sites that focus on the physician, RealSelf focuses on the procedure. For each procedure, users will find actual patient reviews and before and after photos, as well as Q&A’s with board-certified physicians. Users will also find licensed physicians in their area as well as the average cost for the procedure. RealSelf believes that patients value transparency, and including prices creates transparency.
Since most patients genuinely want to help other patients make informed medical decisions, the reviews tend to be thoughtful and thorough, and many of them contain multiple before-and-after photos. As a physician perusing the patient reviews, you’ll start to notice that most of them are reasonable. For example, customer satisfaction with laser treatment for melasma was 51%, whereas satisfaction for laser treatment for rosacea was 80%.
Patients and prospective patients are flocking to the site because it allows them to share their experiences, interact with other patients, and gain access to physician experts in the field. Many patients have difficulty making decisions about cosmetic procedures; RealSelf aims to alleviate their fears and help them "make confident health and beauty decisions." If a prospective patient wants to see a video of tattoo removal or Botox injections, he or she can. If a patient wants to ask physicians their opinions, he or she can. According to RealSelf, physicians have answered over 500,000 questions on the site.
Of course, all this isn’t free for physicians. RealSelf is a business. They have a tiered membership – free, pro, and spotlight. To obtain free membership, you simply visit the site and follow the prompts to "claim your profile." Once your profile is completed, you will have access to a "doctor advisor" who can help you "optimize your visibility on the site." Both "pro" and "spotlight" offer additional benefits, such as integrating patient reviews on your practice website, promotions on Facebook and Twitter, extended directory listings, and exposure in your local area. RealSelf does not discuss costs of membership until you have claimed your profile.
Only you can determine if RealSelf is beneficial to you and your practice. If, for example, you’re not looking for new patients, then you might find it unnecessary. But at the very least, you’ll know what RealSelf is the next time a fellow cosmetic physician brings it up at a conference. And it’s never a bad idea to be familiar with current social technologies that may affect your livelihood.
If you’ve used RealSelf, let us know what you think. For more information, visit RealSelf.com.
Disclaimer: I have no financial interest in RealSelf and am not an active member.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @Dermdoc on Twitter.
Law & Medicine: Antitrust issues in health care, part 2
Question: On antitrust, the U.S. courts have made the following statements, except:
A. To agree to prices is to fix them.
B. There is no learned profession exception to the antitrust laws.
C. To fix maximum price may amount to a fix of minimum price.
D. A group boycott of chiropractors violates the Sherman Act.
E. Tying arrangement in the health care industry is per se illegal.
Answer: E (see Jefferson Parish Hospital below). In the second part of the 20th century, the U.S. Supreme Court and other appellate courts began issuing a number of landmark opinions regarding health care economics and antitrust. Group boycotts were a major target, as was price fixing. This article briefly reviews a few of these decisions to impart a sense of how the judicial system views free market competition in health care.
In AMA v. United States (317 U.S. 519 [1943]), the issue was whether the medical profession’s leading organization, the American Medical Association, could be allowed to expel its salaried doctors or those who associated professionally with salaried doctors. Those who were denied AMA membership were naturally less able to compete (hospital privileges, consultations, etc.).
The U.S. Supreme Court held that such a group boycott of all salaried doctors was illegal because of its anticompetitive purpose, even if it allegedly promoted professional competence and public welfare.
Wilk v. AMA (895 F.2d 352 [1990]) was the culmination of a number of lawsuits surrounding the AMA and chiropractic. In 1963, the AMA had formed a Committee on Quackery aimed at eliminating chiropractic as a profession. The AMA Code of Ethics, Principle 3, opined that it was unethical for a physician to associate professionally with chiropractors. In 1976, Dr. Wilk and four other licensed chiropractors filed suit against the AMA, and a jury trial found that the purpose of the boycott was to eliminate substantial competition without corresponding procompetitive benefits.
The U.S. Court of Appeals for the Seventh Circuit subsequently affirmed the lower court’s finding that the AMA violated Section 1 of the Sherman Act in its illegal boycott of chiropractors, although the court did not answer the question as to whether chiropractic theory was in fact scientific. The court inquired into whether there was a genuine reasonable concern for the use of the scientific method in the doctor-patient relationship, and whether that concern was the dominating, motivating factor in the boycott, and if so, whether it could have been satisfied without restraining competition.
The court found that the AMA’s motive for the boycott was anticompetitive, believing that concern for patient care could be expressed, for example, through public-education campaigns. Although the AMA had formally removed Principle 3 in 1980, it nonetheless appealed this adverse decision to the U.S. Supreme Court on three separate occasions, but the latter declined to hear the case.
In Goldfarb v. Virginia State Bar (421 U.S. 773 [1975]), the Virginia State Bar enforced an "advisory" minimum fee schedule for legal services. The U.S. Supreme Court found that this was an agreement to fix prices, holding, "This is not merely a case of an agreement that may be inferred from an exchange of price information ... for here a naked agreement was clearly shown, and the effect on prices is plain."
The court rejected the defendant’s argument that the practice of law was not a trade or commerce intended to be under Sherman Act scrutiny, declaring there was to be no "learned profession" exemption.
However, it noted that special considerations might apply, holding that "It would be unrealistic to view the practice of professions as interchangeable with other business activities, and automatically to apply to the professions antitrust concepts, which originated in other areas. The public service aspect, and other features of the professions, may require that a particular practice, which could properly be viewed as a violation of the Sherman Act in another context, be treated differently."
Following Goldfarb, there remains no doubt that professional services – legal, medical, and other services – are all to be governed by the antitrust laws.
A flurry of health care–related antitrust cases, including Patrick v. Burget (to be discussed in part 3), reached the courts in the 1980s. In Arizona v. Maricopa County Medical Society (457 U.S. 332 [1982]), the county medical society set maximum allowable fees that member physicians could charge their patients, presumably to guard against price gouging. However, the U.S. Supreme Court, using the tough illegal per se standard, characterized the agreement as price fixing, despite it being for maximum rather than minimum fees.
The court ruled, "Maximum and minimum price fixing may have different consequences in many situations. But schemes to fix maximum prices, by substituting perhaps the erroneous judgment of a seller for the forces of the competitive market, may severely intrude upon the ability of buyers to compete and survive in that market. ... Maximum prices may be fixed too low ... may channel distribution through a few large or specifically advantaged dealers. ... Moreover, if the actual price charged under a maximum price scheme is nearly always the fixed maximum price, which is increasingly likely as the maximum price approaches the actual cost of the dealer, the scheme tends to acquire all the attributes of an arrangement fixing minimum prices."
At issue in Jefferson Parish Hospital District No. 2 v. Hyde (466 U.S. 2 [1984]) was an exclusive contract between a group of four anesthesiologists and Jefferson Parish Hospital in the New Orleans area. Dr. Hyde was an independent board-certified anesthesiologist who was denied medical staff privileges at the hospital because of this exclusive contract. The exclusive arrangement in effect required patients at the hospital to use the services of the four anesthesiologists and none others, raising the issue of unlawful "tying," where a seller requires a customer to purchase one product or service as a condition of being allowed to purchase another.
In a rare unanimous decision, the U.S. Supreme Court, while agreeing that the contract was a tying arrangement, nonetheless rejected the argument that it was per se illegal or that it unreasonably restrained competition among anesthesiologists. The court reasoned that the hospital’s 30% share of the market did not amount to sufficient market power in the provision of hospital services in the Jefferson Parish area. Pointing out that every patient undergoing surgery needed anesthesia, the court found no evidence that any patient received unnecessary services, and it noted that the tying arrangement that was generally employed in the health care industry improved patient care and promoted hospital efficiency.
Tying arrangements in health care are frequently analyzed under a rule of reason standard instead of the strict per se standard, and the favorable decision in this specific case depended heavily on the hospitals’ relatively small market power.
Finally, consider a case on insurance reimbursement and a group boycott against a third-party payer. In Federal Trade Commission v. Indiana Federation of Dentists (476 U.S. 447 [1986]), dental health insurers in Indiana attempted to contain the cost of dental treatment by limiting payments to the least expensive yet adequate treatment suitable to the needs of the patient. The insurers required the submission of x-rays by treating dentists for review of their insurance claims.
Viewing such review of diagnostic and treatment decisions as a threat to their professional independence and economic well-being, members of the Indiana Dental Association and later the Indiana Federation of Dentists agreed collectively to refuse to submit the requested x-rays. These concerted activities resulted in the denial of information that dental customers had requested and had a right to know, and forced them to choose between acquiring the information in a more costly manner or forgoing it altogether.
The lower court had ruled in favor of the dentists, but the U.S. Supreme Court reversed. It agreed that in the absence of concerted behavior, an individual dentist would have been subject to market forces of competition, creating incentives for him or her to comply with the requests of patients’ third-party insurers. But the conduct of the federation was tantamount to a group boycott, which unreasonably restrained trade. The court noted that while this was not price fixing as such, no elaborate industry analysis was required to demonstrate the anticompetitive character of such an agreement.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, "Medical Malpractice: Understanding the Law, Managing the Risk", and his 2012 Halsbury treatise, "Medical Negligence and Professional Misconduct." For additional information, readers may contact the author at [email protected].
Question: On antitrust, the U.S. courts have made the following statements, except:
A. To agree to prices is to fix them.
B. There is no learned profession exception to the antitrust laws.
C. To fix maximum price may amount to a fix of minimum price.
D. A group boycott of chiropractors violates the Sherman Act.
E. Tying arrangement in the health care industry is per se illegal.
Answer: E (see Jefferson Parish Hospital below). In the second part of the 20th century, the U.S. Supreme Court and other appellate courts began issuing a number of landmark opinions regarding health care economics and antitrust. Group boycotts were a major target, as was price fixing. This article briefly reviews a few of these decisions to impart a sense of how the judicial system views free market competition in health care.
In AMA v. United States (317 U.S. 519 [1943]), the issue was whether the medical profession’s leading organization, the American Medical Association, could be allowed to expel its salaried doctors or those who associated professionally with salaried doctors. Those who were denied AMA membership were naturally less able to compete (hospital privileges, consultations, etc.).
The U.S. Supreme Court held that such a group boycott of all salaried doctors was illegal because of its anticompetitive purpose, even if it allegedly promoted professional competence and public welfare.
Wilk v. AMA (895 F.2d 352 [1990]) was the culmination of a number of lawsuits surrounding the AMA and chiropractic. In 1963, the AMA had formed a Committee on Quackery aimed at eliminating chiropractic as a profession. The AMA Code of Ethics, Principle 3, opined that it was unethical for a physician to associate professionally with chiropractors. In 1976, Dr. Wilk and four other licensed chiropractors filed suit against the AMA, and a jury trial found that the purpose of the boycott was to eliminate substantial competition without corresponding procompetitive benefits.
The U.S. Court of Appeals for the Seventh Circuit subsequently affirmed the lower court’s finding that the AMA violated Section 1 of the Sherman Act in its illegal boycott of chiropractors, although the court did not answer the question as to whether chiropractic theory was in fact scientific. The court inquired into whether there was a genuine reasonable concern for the use of the scientific method in the doctor-patient relationship, and whether that concern was the dominating, motivating factor in the boycott, and if so, whether it could have been satisfied without restraining competition.
The court found that the AMA’s motive for the boycott was anticompetitive, believing that concern for patient care could be expressed, for example, through public-education campaigns. Although the AMA had formally removed Principle 3 in 1980, it nonetheless appealed this adverse decision to the U.S. Supreme Court on three separate occasions, but the latter declined to hear the case.
In Goldfarb v. Virginia State Bar (421 U.S. 773 [1975]), the Virginia State Bar enforced an "advisory" minimum fee schedule for legal services. The U.S. Supreme Court found that this was an agreement to fix prices, holding, "This is not merely a case of an agreement that may be inferred from an exchange of price information ... for here a naked agreement was clearly shown, and the effect on prices is plain."
The court rejected the defendant’s argument that the practice of law was not a trade or commerce intended to be under Sherman Act scrutiny, declaring there was to be no "learned profession" exemption.
However, it noted that special considerations might apply, holding that "It would be unrealistic to view the practice of professions as interchangeable with other business activities, and automatically to apply to the professions antitrust concepts, which originated in other areas. The public service aspect, and other features of the professions, may require that a particular practice, which could properly be viewed as a violation of the Sherman Act in another context, be treated differently."
Following Goldfarb, there remains no doubt that professional services – legal, medical, and other services – are all to be governed by the antitrust laws.
A flurry of health care–related antitrust cases, including Patrick v. Burget (to be discussed in part 3), reached the courts in the 1980s. In Arizona v. Maricopa County Medical Society (457 U.S. 332 [1982]), the county medical society set maximum allowable fees that member physicians could charge their patients, presumably to guard against price gouging. However, the U.S. Supreme Court, using the tough illegal per se standard, characterized the agreement as price fixing, despite it being for maximum rather than minimum fees.
The court ruled, "Maximum and minimum price fixing may have different consequences in many situations. But schemes to fix maximum prices, by substituting perhaps the erroneous judgment of a seller for the forces of the competitive market, may severely intrude upon the ability of buyers to compete and survive in that market. ... Maximum prices may be fixed too low ... may channel distribution through a few large or specifically advantaged dealers. ... Moreover, if the actual price charged under a maximum price scheme is nearly always the fixed maximum price, which is increasingly likely as the maximum price approaches the actual cost of the dealer, the scheme tends to acquire all the attributes of an arrangement fixing minimum prices."
At issue in Jefferson Parish Hospital District No. 2 v. Hyde (466 U.S. 2 [1984]) was an exclusive contract between a group of four anesthesiologists and Jefferson Parish Hospital in the New Orleans area. Dr. Hyde was an independent board-certified anesthesiologist who was denied medical staff privileges at the hospital because of this exclusive contract. The exclusive arrangement in effect required patients at the hospital to use the services of the four anesthesiologists and none others, raising the issue of unlawful "tying," where a seller requires a customer to purchase one product or service as a condition of being allowed to purchase another.
In a rare unanimous decision, the U.S. Supreme Court, while agreeing that the contract was a tying arrangement, nonetheless rejected the argument that it was per se illegal or that it unreasonably restrained competition among anesthesiologists. The court reasoned that the hospital’s 30% share of the market did not amount to sufficient market power in the provision of hospital services in the Jefferson Parish area. Pointing out that every patient undergoing surgery needed anesthesia, the court found no evidence that any patient received unnecessary services, and it noted that the tying arrangement that was generally employed in the health care industry improved patient care and promoted hospital efficiency.
Tying arrangements in health care are frequently analyzed under a rule of reason standard instead of the strict per se standard, and the favorable decision in this specific case depended heavily on the hospitals’ relatively small market power.
Finally, consider a case on insurance reimbursement and a group boycott against a third-party payer. In Federal Trade Commission v. Indiana Federation of Dentists (476 U.S. 447 [1986]), dental health insurers in Indiana attempted to contain the cost of dental treatment by limiting payments to the least expensive yet adequate treatment suitable to the needs of the patient. The insurers required the submission of x-rays by treating dentists for review of their insurance claims.
Viewing such review of diagnostic and treatment decisions as a threat to their professional independence and economic well-being, members of the Indiana Dental Association and later the Indiana Federation of Dentists agreed collectively to refuse to submit the requested x-rays. These concerted activities resulted in the denial of information that dental customers had requested and had a right to know, and forced them to choose between acquiring the information in a more costly manner or forgoing it altogether.
The lower court had ruled in favor of the dentists, but the U.S. Supreme Court reversed. It agreed that in the absence of concerted behavior, an individual dentist would have been subject to market forces of competition, creating incentives for him or her to comply with the requests of patients’ third-party insurers. But the conduct of the federation was tantamount to a group boycott, which unreasonably restrained trade. The court noted that while this was not price fixing as such, no elaborate industry analysis was required to demonstrate the anticompetitive character of such an agreement.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, "Medical Malpractice: Understanding the Law, Managing the Risk", and his 2012 Halsbury treatise, "Medical Negligence and Professional Misconduct." For additional information, readers may contact the author at [email protected].
Question: On antitrust, the U.S. courts have made the following statements, except:
A. To agree to prices is to fix them.
B. There is no learned profession exception to the antitrust laws.
C. To fix maximum price may amount to a fix of minimum price.
D. A group boycott of chiropractors violates the Sherman Act.
E. Tying arrangement in the health care industry is per se illegal.
Answer: E (see Jefferson Parish Hospital below). In the second part of the 20th century, the U.S. Supreme Court and other appellate courts began issuing a number of landmark opinions regarding health care economics and antitrust. Group boycotts were a major target, as was price fixing. This article briefly reviews a few of these decisions to impart a sense of how the judicial system views free market competition in health care.
In AMA v. United States (317 U.S. 519 [1943]), the issue was whether the medical profession’s leading organization, the American Medical Association, could be allowed to expel its salaried doctors or those who associated professionally with salaried doctors. Those who were denied AMA membership were naturally less able to compete (hospital privileges, consultations, etc.).
The U.S. Supreme Court held that such a group boycott of all salaried doctors was illegal because of its anticompetitive purpose, even if it allegedly promoted professional competence and public welfare.
Wilk v. AMA (895 F.2d 352 [1990]) was the culmination of a number of lawsuits surrounding the AMA and chiropractic. In 1963, the AMA had formed a Committee on Quackery aimed at eliminating chiropractic as a profession. The AMA Code of Ethics, Principle 3, opined that it was unethical for a physician to associate professionally with chiropractors. In 1976, Dr. Wilk and four other licensed chiropractors filed suit against the AMA, and a jury trial found that the purpose of the boycott was to eliminate substantial competition without corresponding procompetitive benefits.
The U.S. Court of Appeals for the Seventh Circuit subsequently affirmed the lower court’s finding that the AMA violated Section 1 of the Sherman Act in its illegal boycott of chiropractors, although the court did not answer the question as to whether chiropractic theory was in fact scientific. The court inquired into whether there was a genuine reasonable concern for the use of the scientific method in the doctor-patient relationship, and whether that concern was the dominating, motivating factor in the boycott, and if so, whether it could have been satisfied without restraining competition.
The court found that the AMA’s motive for the boycott was anticompetitive, believing that concern for patient care could be expressed, for example, through public-education campaigns. Although the AMA had formally removed Principle 3 in 1980, it nonetheless appealed this adverse decision to the U.S. Supreme Court on three separate occasions, but the latter declined to hear the case.
In Goldfarb v. Virginia State Bar (421 U.S. 773 [1975]), the Virginia State Bar enforced an "advisory" minimum fee schedule for legal services. The U.S. Supreme Court found that this was an agreement to fix prices, holding, "This is not merely a case of an agreement that may be inferred from an exchange of price information ... for here a naked agreement was clearly shown, and the effect on prices is plain."
The court rejected the defendant’s argument that the practice of law was not a trade or commerce intended to be under Sherman Act scrutiny, declaring there was to be no "learned profession" exemption.
However, it noted that special considerations might apply, holding that "It would be unrealistic to view the practice of professions as interchangeable with other business activities, and automatically to apply to the professions antitrust concepts, which originated in other areas. The public service aspect, and other features of the professions, may require that a particular practice, which could properly be viewed as a violation of the Sherman Act in another context, be treated differently."
Following Goldfarb, there remains no doubt that professional services – legal, medical, and other services – are all to be governed by the antitrust laws.
A flurry of health care–related antitrust cases, including Patrick v. Burget (to be discussed in part 3), reached the courts in the 1980s. In Arizona v. Maricopa County Medical Society (457 U.S. 332 [1982]), the county medical society set maximum allowable fees that member physicians could charge their patients, presumably to guard against price gouging. However, the U.S. Supreme Court, using the tough illegal per se standard, characterized the agreement as price fixing, despite it being for maximum rather than minimum fees.
The court ruled, "Maximum and minimum price fixing may have different consequences in many situations. But schemes to fix maximum prices, by substituting perhaps the erroneous judgment of a seller for the forces of the competitive market, may severely intrude upon the ability of buyers to compete and survive in that market. ... Maximum prices may be fixed too low ... may channel distribution through a few large or specifically advantaged dealers. ... Moreover, if the actual price charged under a maximum price scheme is nearly always the fixed maximum price, which is increasingly likely as the maximum price approaches the actual cost of the dealer, the scheme tends to acquire all the attributes of an arrangement fixing minimum prices."
At issue in Jefferson Parish Hospital District No. 2 v. Hyde (466 U.S. 2 [1984]) was an exclusive contract between a group of four anesthesiologists and Jefferson Parish Hospital in the New Orleans area. Dr. Hyde was an independent board-certified anesthesiologist who was denied medical staff privileges at the hospital because of this exclusive contract. The exclusive arrangement in effect required patients at the hospital to use the services of the four anesthesiologists and none others, raising the issue of unlawful "tying," where a seller requires a customer to purchase one product or service as a condition of being allowed to purchase another.
In a rare unanimous decision, the U.S. Supreme Court, while agreeing that the contract was a tying arrangement, nonetheless rejected the argument that it was per se illegal or that it unreasonably restrained competition among anesthesiologists. The court reasoned that the hospital’s 30% share of the market did not amount to sufficient market power in the provision of hospital services in the Jefferson Parish area. Pointing out that every patient undergoing surgery needed anesthesia, the court found no evidence that any patient received unnecessary services, and it noted that the tying arrangement that was generally employed in the health care industry improved patient care and promoted hospital efficiency.
Tying arrangements in health care are frequently analyzed under a rule of reason standard instead of the strict per se standard, and the favorable decision in this specific case depended heavily on the hospitals’ relatively small market power.
Finally, consider a case on insurance reimbursement and a group boycott against a third-party payer. In Federal Trade Commission v. Indiana Federation of Dentists (476 U.S. 447 [1986]), dental health insurers in Indiana attempted to contain the cost of dental treatment by limiting payments to the least expensive yet adequate treatment suitable to the needs of the patient. The insurers required the submission of x-rays by treating dentists for review of their insurance claims.
Viewing such review of diagnostic and treatment decisions as a threat to their professional independence and economic well-being, members of the Indiana Dental Association and later the Indiana Federation of Dentists agreed collectively to refuse to submit the requested x-rays. These concerted activities resulted in the denial of information that dental customers had requested and had a right to know, and forced them to choose between acquiring the information in a more costly manner or forgoing it altogether.
The lower court had ruled in favor of the dentists, but the U.S. Supreme Court reversed. It agreed that in the absence of concerted behavior, an individual dentist would have been subject to market forces of competition, creating incentives for him or her to comply with the requests of patients’ third-party insurers. But the conduct of the federation was tantamount to a group boycott, which unreasonably restrained trade. The court noted that while this was not price fixing as such, no elaborate industry analysis was required to demonstrate the anticompetitive character of such an agreement.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, "Medical Malpractice: Understanding the Law, Managing the Risk", and his 2012 Halsbury treatise, "Medical Negligence and Professional Misconduct." For additional information, readers may contact the author at [email protected].
Cosmeceutical Critique: Benzoyl peroxide
Benzoyl peroxide (BPO) has been used for more than 45 years for the treatment of acne, and has recently been enjoying renewed popularity, thanks to its performance in recent studies of both prescription and over-the-counter formulations (J. Drugs Dermatol. 2013;12:180-5). In fact, BPO is one of the two most common ingredients in OTC acne products (Semin. Cutan. Med. Surg. 2008;27:170-6). The prescription form is used alone or in combination with tretinoin, adapalene, or clindamycin. BPO, originally sourced from the coal tar component chlorhydroxyquinoline, is now typically prepared by treating hydrogen peroxide with benzoyl chloride (Dermatol. Clin. 2009;27:17-24). Because it can generate reactive oxygen species and commonly leads to skin irritation, its use is somewhat limited.
Antibacterial uses
BPO imparts bactericidal activity by releasing highly reactive oxygen (free radicals) that can oxidize proteins in bacterial cell membranes. It also exhibits antibacterial action against Propionibacterium acnes and Corynebacterium acnes, the bacteria implicated in the pathophysiology of acne (Dermatol. Ther. 2012;25:6-11), as well as Staphylococcus capitis, S. epidermis, S. hominis, P. avidum, P. granulosum, and the yeast Pityrosporum ovale (J. Appl. Bacteriol. 1983;54:379-82).
Acne
Many studies over the years have shown that topically applied BPO effectively treats acne (Expert Opin. Pharmacother. 2009;10:2555-62). These ameliorative results, which include enhancing the benefits of other topical antimicrobials, are thought to arise because BPO, a highly lipophilic molecule, penetrates through the sebum and into the pilosebaceous unit, and exerts bactericidal, keratolytic, and anti-inflammatory activity (Skin Pharmacol. Physiol. 2006;19:283-9). BPO may contribute to the antiacne efficacy of other antimicrobials by preventing bacterial resistance and promoting penetration into the sebum, keratin, and polysaccharides to reach the target bacteria. Specifically, the oxidative activity of BPO helps eliminate the biofilm polysaccharides secreted by P. acnes, thus expediting the delivery of other agents to the bacteria (Int. J. Dermatol. 2006;45:872; Int. J. Dermatol. 2003;42:925-7).
Not surprisingly, several studies have shown that the antiacne efficacy of a combination of BPO with other antimicrobials, such as clindamycin, is greater than that of either agent used alone. Simpson et al. demonstrated that the use of clindamycin and BPO together led to a 61% decline in inflammatory lesions after 3 months, as compared with 39% and 35%, respectively, when the agents were used alone (J. Am. Acad. Dermatol. 1997;37:590-5). BPO is frequently paired with salicylic acid to treat acne (Clin. Exp. Dermatol. 2011;36:840-3).
Acne often improves more rapidly with BPO treatment than with retinoids and other acne therapies, and data suggest that the faster clearing of acne lesions and comedones is most likely because of its keratolytic activity (Dermatol. Clin. 2009;27:17-24; J. Dermatolog. Treat. 2003;14:166-71). However, the dryness and irritation associated with BPO usage may undermine patient compliance. Several studies have suggested that BPO is effective in cleanser formulations, which seem to reduce irritation (Clin. Exp. Dermatol. 2011;36:840-3).
Photocarcinogenicity
Reports that BPO predisposed mice to skin cancer, particularly when they were exposed to ultraviolet radiation, prompted the Food and Drug Administration to form an advisory committee in 1992 to review the safety of BPO. The committee called for additional photocarcinogenicity studies while suggesting that BPO products include animal safety data on the labels. BPO-containing acne products were kept on the market. In the ensuing two decades, newer safety studies have led the FDA to change the classification of BPO to category I, deeming the OTC topical treatment of acne to be generally recognized as safe and effective (GRASE) (Fed. Regist. 2010;75:9767-77).
Photoaging
When BPO breaks down into benzoic acid in the skin, benzoyloxy, a free radical, forms as an intermediate (Prog. Clin. Biol. Res. 1995;391:245). Benzoyloxy can decarboxylate into a phenyl radical. These free radicals produce oxidative stress, which may cause DNA strand breaks in keratinocytes or may harm proteins or lipids. In addition to becoming a free radical, BPO depletes membrane and cytosolic antioxidants (Toxicology 2001;165:225-34). No retrospective trials looking at the effects of long-term use of BP on photoaging have been performed, so the role of BPO in photoaging is not clear. One study in mice found that topical BP has some of the same effects on skin as UVB (J. Invest. Dermatol. 1999;112:933-38).
Other safety issues
Acne is not uncommon among pregnant women. Although safety studies of BPO use by pregnant women have not been performed, various authors suggest that only about 5% of topically applied BPO is absorbed systemically, implying that topical BPO can be safely used during pregnancy (Int. J. Dermatol. 2002;41:197-203; Can. Fam. Physician 2011;57:665-7; Drugs 2013;73:779-87; Dermatol. Ther. 2013;26:302-11).
In approximately 1% of patients, topical BPO causes contact or irritant dermatitis (Contact Dermatitis 1999;41:233; Contact Dermatitis 1996;34:68-9). The use of barrier repair moisturizers may reduce the incidence of irritation, though this has not been proven.
Usage considerations
BPO use for acne is linked to a reduction in antibiotic resistance (J. Drugs Dermatol. 2013;12:s73-6). Because BPO, a potent oxidizer, eliminates bacteria by generating reactive oxygen species in the sebaceous follicle, it is important to consider the chemical compatibility of BPO with other agents (J. Am. Acad. Dermatol. 1981;4:31-7). Martin et al. showed that BPO tends to degrade tretinoin to about 80% of initial content, an effect that is markedly enhanced by indoor light. However, even in the presence of light, adapalene is not degraded by BPO (Br. J. Dermatol. 1998;139 Suppl 52:8-11). But the order in which products are applied is important, given that BPO can inactivate other ingredients.
Studies have demonstrated that the use of BPO in body washes leads to greater efficacy when the product is left on for 5 minutes before rinsing (J. Drugs Dermatol. 2010;9:622-5; J. Clin. Aesthet. Dermatol. 2010;3:26-9). Notably, the efficacy of BPO in cleansing products is comparable to that observed in leave-on products, but BPO provokes less irritation than leave-on formulations (J. Drugs Dermatol. 2009;8:657-61; Skinmed. 2005;4:370).
Conclusion
BPO remains quite effective in acne therapy, and it is one of the few acne medications available both over the counter and by prescription in the United States. BPO helps prevent antibiotic resistance to erythromycin and clindamycin, which makes it an important ingredient in many acne skin care regimens. However, it is pro-oxidant, and clinicians and patients should take into account the risk of BPO contributing to skin aging because of the free radicals it produces.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Benzoyl peroxide (BPO) has been used for more than 45 years for the treatment of acne, and has recently been enjoying renewed popularity, thanks to its performance in recent studies of both prescription and over-the-counter formulations (J. Drugs Dermatol. 2013;12:180-5). In fact, BPO is one of the two most common ingredients in OTC acne products (Semin. Cutan. Med. Surg. 2008;27:170-6). The prescription form is used alone or in combination with tretinoin, adapalene, or clindamycin. BPO, originally sourced from the coal tar component chlorhydroxyquinoline, is now typically prepared by treating hydrogen peroxide with benzoyl chloride (Dermatol. Clin. 2009;27:17-24). Because it can generate reactive oxygen species and commonly leads to skin irritation, its use is somewhat limited.
Antibacterial uses
BPO imparts bactericidal activity by releasing highly reactive oxygen (free radicals) that can oxidize proteins in bacterial cell membranes. It also exhibits antibacterial action against Propionibacterium acnes and Corynebacterium acnes, the bacteria implicated in the pathophysiology of acne (Dermatol. Ther. 2012;25:6-11), as well as Staphylococcus capitis, S. epidermis, S. hominis, P. avidum, P. granulosum, and the yeast Pityrosporum ovale (J. Appl. Bacteriol. 1983;54:379-82).
Acne
Many studies over the years have shown that topically applied BPO effectively treats acne (Expert Opin. Pharmacother. 2009;10:2555-62). These ameliorative results, which include enhancing the benefits of other topical antimicrobials, are thought to arise because BPO, a highly lipophilic molecule, penetrates through the sebum and into the pilosebaceous unit, and exerts bactericidal, keratolytic, and anti-inflammatory activity (Skin Pharmacol. Physiol. 2006;19:283-9). BPO may contribute to the antiacne efficacy of other antimicrobials by preventing bacterial resistance and promoting penetration into the sebum, keratin, and polysaccharides to reach the target bacteria. Specifically, the oxidative activity of BPO helps eliminate the biofilm polysaccharides secreted by P. acnes, thus expediting the delivery of other agents to the bacteria (Int. J. Dermatol. 2006;45:872; Int. J. Dermatol. 2003;42:925-7).
Not surprisingly, several studies have shown that the antiacne efficacy of a combination of BPO with other antimicrobials, such as clindamycin, is greater than that of either agent used alone. Simpson et al. demonstrated that the use of clindamycin and BPO together led to a 61% decline in inflammatory lesions after 3 months, as compared with 39% and 35%, respectively, when the agents were used alone (J. Am. Acad. Dermatol. 1997;37:590-5). BPO is frequently paired with salicylic acid to treat acne (Clin. Exp. Dermatol. 2011;36:840-3).
Acne often improves more rapidly with BPO treatment than with retinoids and other acne therapies, and data suggest that the faster clearing of acne lesions and comedones is most likely because of its keratolytic activity (Dermatol. Clin. 2009;27:17-24; J. Dermatolog. Treat. 2003;14:166-71). However, the dryness and irritation associated with BPO usage may undermine patient compliance. Several studies have suggested that BPO is effective in cleanser formulations, which seem to reduce irritation (Clin. Exp. Dermatol. 2011;36:840-3).
Photocarcinogenicity
Reports that BPO predisposed mice to skin cancer, particularly when they were exposed to ultraviolet radiation, prompted the Food and Drug Administration to form an advisory committee in 1992 to review the safety of BPO. The committee called for additional photocarcinogenicity studies while suggesting that BPO products include animal safety data on the labels. BPO-containing acne products were kept on the market. In the ensuing two decades, newer safety studies have led the FDA to change the classification of BPO to category I, deeming the OTC topical treatment of acne to be generally recognized as safe and effective (GRASE) (Fed. Regist. 2010;75:9767-77).
Photoaging
When BPO breaks down into benzoic acid in the skin, benzoyloxy, a free radical, forms as an intermediate (Prog. Clin. Biol. Res. 1995;391:245). Benzoyloxy can decarboxylate into a phenyl radical. These free radicals produce oxidative stress, which may cause DNA strand breaks in keratinocytes or may harm proteins or lipids. In addition to becoming a free radical, BPO depletes membrane and cytosolic antioxidants (Toxicology 2001;165:225-34). No retrospective trials looking at the effects of long-term use of BP on photoaging have been performed, so the role of BPO in photoaging is not clear. One study in mice found that topical BP has some of the same effects on skin as UVB (J. Invest. Dermatol. 1999;112:933-38).
Other safety issues
Acne is not uncommon among pregnant women. Although safety studies of BPO use by pregnant women have not been performed, various authors suggest that only about 5% of topically applied BPO is absorbed systemically, implying that topical BPO can be safely used during pregnancy (Int. J. Dermatol. 2002;41:197-203; Can. Fam. Physician 2011;57:665-7; Drugs 2013;73:779-87; Dermatol. Ther. 2013;26:302-11).
In approximately 1% of patients, topical BPO causes contact or irritant dermatitis (Contact Dermatitis 1999;41:233; Contact Dermatitis 1996;34:68-9). The use of barrier repair moisturizers may reduce the incidence of irritation, though this has not been proven.
Usage considerations
BPO use for acne is linked to a reduction in antibiotic resistance (J. Drugs Dermatol. 2013;12:s73-6). Because BPO, a potent oxidizer, eliminates bacteria by generating reactive oxygen species in the sebaceous follicle, it is important to consider the chemical compatibility of BPO with other agents (J. Am. Acad. Dermatol. 1981;4:31-7). Martin et al. showed that BPO tends to degrade tretinoin to about 80% of initial content, an effect that is markedly enhanced by indoor light. However, even in the presence of light, adapalene is not degraded by BPO (Br. J. Dermatol. 1998;139 Suppl 52:8-11). But the order in which products are applied is important, given that BPO can inactivate other ingredients.
Studies have demonstrated that the use of BPO in body washes leads to greater efficacy when the product is left on for 5 minutes before rinsing (J. Drugs Dermatol. 2010;9:622-5; J. Clin. Aesthet. Dermatol. 2010;3:26-9). Notably, the efficacy of BPO in cleansing products is comparable to that observed in leave-on products, but BPO provokes less irritation than leave-on formulations (J. Drugs Dermatol. 2009;8:657-61; Skinmed. 2005;4:370).
Conclusion
BPO remains quite effective in acne therapy, and it is one of the few acne medications available both over the counter and by prescription in the United States. BPO helps prevent antibiotic resistance to erythromycin and clindamycin, which makes it an important ingredient in many acne skin care regimens. However, it is pro-oxidant, and clinicians and patients should take into account the risk of BPO contributing to skin aging because of the free radicals it produces.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Benzoyl peroxide (BPO) has been used for more than 45 years for the treatment of acne, and has recently been enjoying renewed popularity, thanks to its performance in recent studies of both prescription and over-the-counter formulations (J. Drugs Dermatol. 2013;12:180-5). In fact, BPO is one of the two most common ingredients in OTC acne products (Semin. Cutan. Med. Surg. 2008;27:170-6). The prescription form is used alone or in combination with tretinoin, adapalene, or clindamycin. BPO, originally sourced from the coal tar component chlorhydroxyquinoline, is now typically prepared by treating hydrogen peroxide with benzoyl chloride (Dermatol. Clin. 2009;27:17-24). Because it can generate reactive oxygen species and commonly leads to skin irritation, its use is somewhat limited.
Antibacterial uses
BPO imparts bactericidal activity by releasing highly reactive oxygen (free radicals) that can oxidize proteins in bacterial cell membranes. It also exhibits antibacterial action against Propionibacterium acnes and Corynebacterium acnes, the bacteria implicated in the pathophysiology of acne (Dermatol. Ther. 2012;25:6-11), as well as Staphylococcus capitis, S. epidermis, S. hominis, P. avidum, P. granulosum, and the yeast Pityrosporum ovale (J. Appl. Bacteriol. 1983;54:379-82).
Acne
Many studies over the years have shown that topically applied BPO effectively treats acne (Expert Opin. Pharmacother. 2009;10:2555-62). These ameliorative results, which include enhancing the benefits of other topical antimicrobials, are thought to arise because BPO, a highly lipophilic molecule, penetrates through the sebum and into the pilosebaceous unit, and exerts bactericidal, keratolytic, and anti-inflammatory activity (Skin Pharmacol. Physiol. 2006;19:283-9). BPO may contribute to the antiacne efficacy of other antimicrobials by preventing bacterial resistance and promoting penetration into the sebum, keratin, and polysaccharides to reach the target bacteria. Specifically, the oxidative activity of BPO helps eliminate the biofilm polysaccharides secreted by P. acnes, thus expediting the delivery of other agents to the bacteria (Int. J. Dermatol. 2006;45:872; Int. J. Dermatol. 2003;42:925-7).
Not surprisingly, several studies have shown that the antiacne efficacy of a combination of BPO with other antimicrobials, such as clindamycin, is greater than that of either agent used alone. Simpson et al. demonstrated that the use of clindamycin and BPO together led to a 61% decline in inflammatory lesions after 3 months, as compared with 39% and 35%, respectively, when the agents were used alone (J. Am. Acad. Dermatol. 1997;37:590-5). BPO is frequently paired with salicylic acid to treat acne (Clin. Exp. Dermatol. 2011;36:840-3).
Acne often improves more rapidly with BPO treatment than with retinoids and other acne therapies, and data suggest that the faster clearing of acne lesions and comedones is most likely because of its keratolytic activity (Dermatol. Clin. 2009;27:17-24; J. Dermatolog. Treat. 2003;14:166-71). However, the dryness and irritation associated with BPO usage may undermine patient compliance. Several studies have suggested that BPO is effective in cleanser formulations, which seem to reduce irritation (Clin. Exp. Dermatol. 2011;36:840-3).
Photocarcinogenicity
Reports that BPO predisposed mice to skin cancer, particularly when they were exposed to ultraviolet radiation, prompted the Food and Drug Administration to form an advisory committee in 1992 to review the safety of BPO. The committee called for additional photocarcinogenicity studies while suggesting that BPO products include animal safety data on the labels. BPO-containing acne products were kept on the market. In the ensuing two decades, newer safety studies have led the FDA to change the classification of BPO to category I, deeming the OTC topical treatment of acne to be generally recognized as safe and effective (GRASE) (Fed. Regist. 2010;75:9767-77).
Photoaging
When BPO breaks down into benzoic acid in the skin, benzoyloxy, a free radical, forms as an intermediate (Prog. Clin. Biol. Res. 1995;391:245). Benzoyloxy can decarboxylate into a phenyl radical. These free radicals produce oxidative stress, which may cause DNA strand breaks in keratinocytes or may harm proteins or lipids. In addition to becoming a free radical, BPO depletes membrane and cytosolic antioxidants (Toxicology 2001;165:225-34). No retrospective trials looking at the effects of long-term use of BP on photoaging have been performed, so the role of BPO in photoaging is not clear. One study in mice found that topical BP has some of the same effects on skin as UVB (J. Invest. Dermatol. 1999;112:933-38).
Other safety issues
Acne is not uncommon among pregnant women. Although safety studies of BPO use by pregnant women have not been performed, various authors suggest that only about 5% of topically applied BPO is absorbed systemically, implying that topical BPO can be safely used during pregnancy (Int. J. Dermatol. 2002;41:197-203; Can. Fam. Physician 2011;57:665-7; Drugs 2013;73:779-87; Dermatol. Ther. 2013;26:302-11).
In approximately 1% of patients, topical BPO causes contact or irritant dermatitis (Contact Dermatitis 1999;41:233; Contact Dermatitis 1996;34:68-9). The use of barrier repair moisturizers may reduce the incidence of irritation, though this has not been proven.
Usage considerations
BPO use for acne is linked to a reduction in antibiotic resistance (J. Drugs Dermatol. 2013;12:s73-6). Because BPO, a potent oxidizer, eliminates bacteria by generating reactive oxygen species in the sebaceous follicle, it is important to consider the chemical compatibility of BPO with other agents (J. Am. Acad. Dermatol. 1981;4:31-7). Martin et al. showed that BPO tends to degrade tretinoin to about 80% of initial content, an effect that is markedly enhanced by indoor light. However, even in the presence of light, adapalene is not degraded by BPO (Br. J. Dermatol. 1998;139 Suppl 52:8-11). But the order in which products are applied is important, given that BPO can inactivate other ingredients.
Studies have demonstrated that the use of BPO in body washes leads to greater efficacy when the product is left on for 5 minutes before rinsing (J. Drugs Dermatol. 2010;9:622-5; J. Clin. Aesthet. Dermatol. 2010;3:26-9). Notably, the efficacy of BPO in cleansing products is comparable to that observed in leave-on products, but BPO provokes less irritation than leave-on formulations (J. Drugs Dermatol. 2009;8:657-61; Skinmed. 2005;4:370).
Conclusion
BPO remains quite effective in acne therapy, and it is one of the few acne medications available both over the counter and by prescription in the United States. BPO helps prevent antibiotic resistance to erythromycin and clindamycin, which makes it an important ingredient in many acne skin care regimens. However, it is pro-oxidant, and clinicians and patients should take into account the risk of BPO contributing to skin aging because of the free radicals it produces.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Unnecessary checking of epilepsy drug levels
I hate epilepsy drug levels.
Other neurologists know what I’m talking about: Dilantin, Tegretol, and Depakote are the main ones we deal with. Levels for newer medications aren’t checked much, and phenobarbital is fading into the background.
I rarely check levels because 90% of the time they’re immaterial. If the patient isn’t having seizures and isn’t having side effects, who cares what the level is? It’s obviously just right for that patient (sort of like Goldilocks) regardless of what a number tells me it is.
Certainly there are exceptions to this: the well-controlled patient who suddenly goes downhill, for example. But in most cases, routinely checking drug levels serves no purpose, and often causes more harm them good.
In medical school, I was taught that you should never order a test unless the results will affect your plan of care. In a stable seizure patient, are you going to base your medication adjustments on how they’re doing, or a number?
Worse still, these levels are often used by specialties that don’t understand them. How many times have you seen well-controlled patients who had an unneeded level ordered – along with their annual lipid profile or thyroid panel, or because they went to the emergency department with pneumonia – and then had a nonneurologist adjust their dose based solely on the result? You often don’t find out about it until the patient calls you to report they seized or are suddenly drug-toxic.
I’m not an internist. In fact, I stink at general medicine, and don’t even pretend to understand the many drugs they have to start, adjust, and stop on an everyday basis. I don’t expect them to know as much about seizure medications as I do, anymore than they expect me to understand treatments for hypertension or diabetes. So I tell my patients that unless it’s an emergency, don’t let anyone else adjust their seizure medications. Just have them fax me the lab report, and I’ll deal with it.
At first, I was afraid this approach would ruffle feathers, but it hasn’t. Local internists have told me they’re glad to be able to take something off their list of concerns and punt it to me.
Modern medicine is full of things we can do, even when they’re just minor drug levels from a lab. But it’s still important to focus on what we should do, and to me, routinely checking drug levels should always be questioned.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I hate epilepsy drug levels.
Other neurologists know what I’m talking about: Dilantin, Tegretol, and Depakote are the main ones we deal with. Levels for newer medications aren’t checked much, and phenobarbital is fading into the background.
I rarely check levels because 90% of the time they’re immaterial. If the patient isn’t having seizures and isn’t having side effects, who cares what the level is? It’s obviously just right for that patient (sort of like Goldilocks) regardless of what a number tells me it is.
Certainly there are exceptions to this: the well-controlled patient who suddenly goes downhill, for example. But in most cases, routinely checking drug levels serves no purpose, and often causes more harm them good.
In medical school, I was taught that you should never order a test unless the results will affect your plan of care. In a stable seizure patient, are you going to base your medication adjustments on how they’re doing, or a number?
Worse still, these levels are often used by specialties that don’t understand them. How many times have you seen well-controlled patients who had an unneeded level ordered – along with their annual lipid profile or thyroid panel, or because they went to the emergency department with pneumonia – and then had a nonneurologist adjust their dose based solely on the result? You often don’t find out about it until the patient calls you to report they seized or are suddenly drug-toxic.
I’m not an internist. In fact, I stink at general medicine, and don’t even pretend to understand the many drugs they have to start, adjust, and stop on an everyday basis. I don’t expect them to know as much about seizure medications as I do, anymore than they expect me to understand treatments for hypertension or diabetes. So I tell my patients that unless it’s an emergency, don’t let anyone else adjust their seizure medications. Just have them fax me the lab report, and I’ll deal with it.
At first, I was afraid this approach would ruffle feathers, but it hasn’t. Local internists have told me they’re glad to be able to take something off their list of concerns and punt it to me.
Modern medicine is full of things we can do, even when they’re just minor drug levels from a lab. But it’s still important to focus on what we should do, and to me, routinely checking drug levels should always be questioned.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I hate epilepsy drug levels.
Other neurologists know what I’m talking about: Dilantin, Tegretol, and Depakote are the main ones we deal with. Levels for newer medications aren’t checked much, and phenobarbital is fading into the background.
I rarely check levels because 90% of the time they’re immaterial. If the patient isn’t having seizures and isn’t having side effects, who cares what the level is? It’s obviously just right for that patient (sort of like Goldilocks) regardless of what a number tells me it is.
Certainly there are exceptions to this: the well-controlled patient who suddenly goes downhill, for example. But in most cases, routinely checking drug levels serves no purpose, and often causes more harm them good.
In medical school, I was taught that you should never order a test unless the results will affect your plan of care. In a stable seizure patient, are you going to base your medication adjustments on how they’re doing, or a number?
Worse still, these levels are often used by specialties that don’t understand them. How many times have you seen well-controlled patients who had an unneeded level ordered – along with their annual lipid profile or thyroid panel, or because they went to the emergency department with pneumonia – and then had a nonneurologist adjust their dose based solely on the result? You often don’t find out about it until the patient calls you to report they seized or are suddenly drug-toxic.
I’m not an internist. In fact, I stink at general medicine, and don’t even pretend to understand the many drugs they have to start, adjust, and stop on an everyday basis. I don’t expect them to know as much about seizure medications as I do, anymore than they expect me to understand treatments for hypertension or diabetes. So I tell my patients that unless it’s an emergency, don’t let anyone else adjust their seizure medications. Just have them fax me the lab report, and I’ll deal with it.
At first, I was afraid this approach would ruffle feathers, but it hasn’t. Local internists have told me they’re glad to be able to take something off their list of concerns and punt it to me.
Modern medicine is full of things we can do, even when they’re just minor drug levels from a lab. But it’s still important to focus on what we should do, and to me, routinely checking drug levels should always be questioned.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
You’ll Have a Dickens of a Time
Most of you are no doubt familiar with the opening of Charles Dickens’ classic novel A Tale of Two Cities: “It was the best of times, it was the worst of times.” In many ways Dickens has presaged my current thinking about the new lipid guidelines that were recently issued. On the one hand, they are truly the very best guidelines that could possibly be produced at the present time; on the other hand, they may also be the worst set of guidelines that could possibly be promulgated on the practice community at the present moment.
Let’s first take a good look at what the new guidelines actually recommend. First, it’s important to understand that the parentage of the new guidelines has changed in a very important way from that of earlier recommendations. The previous lipid guidelines—the National Cholesterol Education Panel (NCEP) recommendations—were issued in 2001 and were sponsored and endorsed by the National Institutes of Health (NIH) as the federal government’s best effort at lipid recommendations for the general practice community. An update in 2004 further advised that a low-density lipoprotein cholesterol (LDL-C) goal of < 70 mg/dL is appropriate for many patients with preexisting vascular disease.
New guidelines were clearly overdue. Indeed, an expert panel had already been convened and was hard at work. However, a year or so ago the NIH made a critical strategic decision that it no longer wanted to be in the guideline business. The NIH rather abruptly decided that there would be no further iterations of the NCEP guidelines.
Fortunately the NIH did not simply drop the ball. Rather, it decided to pass the baton (mixed metaphor—sorry!) to a joint task force of the American Heart Association (AHA) and the American College of Cardiology (ACC). After all, who better to ponder lipid goals than the vascular experts who populate these 2 august societies? These 2 groups took a good look at the work that had been done by the expert panel, and decided that they would bless the new recommendations.
The fruit of the years of labor were finally presented at the annual meeting of the AHA held in Dallas in 2013. I didn’t make it to all of the heart sessions during that meeting. (I was distracted to a considerable extent by the 50th anniversary commemoration of President John F. Kennedy’s assassination going on just a few blocks away from the convention center at the Texas School Book Depository site.) But I can tell you I was definitely at the AHA session where the guidelines were formally presented, and it was indeed a lively and controversial session.
By far the biggest change in the new guidelines, representing both its greatest strength and its greatest weakness, is the new emphasis on overall cardiovascular risk assessment rather than on the attainment of a certain defined LDL-C goal. Indeed, a feature of the new guidelines, which many find disconcerting, is that there is no longer any mention whatsoever of LDL-C goals or targets!
The guidelines are also heavily statin-centric; other classes of lipid-lowering agents, such as fibrates or niacin, receive short shrift indeed. The recommendations are that statins should be prescribed routinely for each of the following “statin benefit groups”:
1. Patients who have clinical atherosclerotic cardiovascular disease and thus fall into the “secondary prevention” category.
2. Those with LDL-C levels of ≥ 190 mg/dL and who have no secondary cause, such as certain medications or diseases such as hypothyroidism or nephrotic syndrome.
3. Patients with diabetes without established cardiovascular disease aged 40 to 75 years with LDL-C levels between 70 mg/dL and 189 mg/dL.
4. Patients without diabetes with established atherosclerotic cardiovascular disease aged 40 to 75 years with LDL-C levels from 70 mg/dL to 189 mg/dL and a calculated cardiovascular risk of at least 7.5% over the next 10 years.
The fourth category is potentially the most confusing for conscientious providers. The risk calculator that determines whether or not someone has a risk of > 7.5% over the next year is not the traditional Framingham risk calculator, with which many providers are familiar. Rather, it is a brand-new, improved risk calculator devised by the panel. The calculator can be found on both the AHA and ACC websites and in iOS and Android apps (See App Corner, p.38).
To make things even more confusing, once it has been determined that a statin is indicated, the dosing of the statin, either low, moderate, or high intensity, must be selected on the basis of determined risk level. Fortunately, the panel has given us a nice table defining which statins qualify for inclusion in each of these 3 intensity categories. As a general rule, the low-intensity statins should almost always be avoided. But the determination of whether moderate or high-intensity statins are indicated gets somewhat murky. The first two aforementioned classes both deserve high-intensity statins. However, patients with diabetes who haven’t had an event could go with either moderate- or high-intensity statins, and those patients without diabetes or LDL-C levels ≥ 190, but with a 10-year risk of at least 7.5%, can also receive either moderate- or high-intensity statins.
So there it is, and it all does make a certain amount of sense. You first determine the patient’s risk category, which determines whether or not statins are indicated. If they are, you then decide what level of potency your prescribed statin should possess. There is no need to go checking LDL-C levels later, because your therapy is not targeted at any particular LDL-C level. You might want to check occasionally, though, just as a way of assessing patient compliance.
So what should we make of all of this? From a purely scientific point of view, it seems abundantly clear that these are the most scientifically valid set of guidelines that have ever been produced, generated by genuine experts who bent over backward to examine every possible relevant study. The new risk calculator is clearly a broader-based tool than the Framingham calculator, which was based on now-dated data from a very narrow heavily-white population basis. Although the new risk calculator has been criticized by some as a very imperfect tool that overestimates risk in some subpopulations, I firmly believe it is considerably less imperfect than the Framingham tool. It is, quite frankly, the best risk calculator anyone could come up with at this time, and the cutoff for treatment at a risk of 7.5% or higher over 10 years seems eminently reasonable to me.
So what’s the problem with the new guidelines? I think you astute readers already know what the problem is: These guidelines simply represent way too radical a change for the huge bulk of busy, harried providers out there. The average primary care provider is currently struggling to complete a multifaceted patient encounter in 15 minutes or less and then document it in excruciating detail. He or she is going to be extremely hard-pressed to master and implement the new guidelines. The guidelines are indeed the most scientifically accurate and thorough guidelines that could be humanly produced, but they represent such a radical change from previous guidelines that a huge number of providers are going to be playing catch-up for a long time. I hope that their learning curve can be a very rapid one, but I worry that these scientifically pristine guidelines will be slow to find their way into general practice.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Most of you are no doubt familiar with the opening of Charles Dickens’ classic novel A Tale of Two Cities: “It was the best of times, it was the worst of times.” In many ways Dickens has presaged my current thinking about the new lipid guidelines that were recently issued. On the one hand, they are truly the very best guidelines that could possibly be produced at the present time; on the other hand, they may also be the worst set of guidelines that could possibly be promulgated on the practice community at the present moment.
Let’s first take a good look at what the new guidelines actually recommend. First, it’s important to understand that the parentage of the new guidelines has changed in a very important way from that of earlier recommendations. The previous lipid guidelines—the National Cholesterol Education Panel (NCEP) recommendations—were issued in 2001 and were sponsored and endorsed by the National Institutes of Health (NIH) as the federal government’s best effort at lipid recommendations for the general practice community. An update in 2004 further advised that a low-density lipoprotein cholesterol (LDL-C) goal of < 70 mg/dL is appropriate for many patients with preexisting vascular disease.
New guidelines were clearly overdue. Indeed, an expert panel had already been convened and was hard at work. However, a year or so ago the NIH made a critical strategic decision that it no longer wanted to be in the guideline business. The NIH rather abruptly decided that there would be no further iterations of the NCEP guidelines.
Fortunately the NIH did not simply drop the ball. Rather, it decided to pass the baton (mixed metaphor—sorry!) to a joint task force of the American Heart Association (AHA) and the American College of Cardiology (ACC). After all, who better to ponder lipid goals than the vascular experts who populate these 2 august societies? These 2 groups took a good look at the work that had been done by the expert panel, and decided that they would bless the new recommendations.
The fruit of the years of labor were finally presented at the annual meeting of the AHA held in Dallas in 2013. I didn’t make it to all of the heart sessions during that meeting. (I was distracted to a considerable extent by the 50th anniversary commemoration of President John F. Kennedy’s assassination going on just a few blocks away from the convention center at the Texas School Book Depository site.) But I can tell you I was definitely at the AHA session where the guidelines were formally presented, and it was indeed a lively and controversial session.
By far the biggest change in the new guidelines, representing both its greatest strength and its greatest weakness, is the new emphasis on overall cardiovascular risk assessment rather than on the attainment of a certain defined LDL-C goal. Indeed, a feature of the new guidelines, which many find disconcerting, is that there is no longer any mention whatsoever of LDL-C goals or targets!
The guidelines are also heavily statin-centric; other classes of lipid-lowering agents, such as fibrates or niacin, receive short shrift indeed. The recommendations are that statins should be prescribed routinely for each of the following “statin benefit groups”:
1. Patients who have clinical atherosclerotic cardiovascular disease and thus fall into the “secondary prevention” category.
2. Those with LDL-C levels of ≥ 190 mg/dL and who have no secondary cause, such as certain medications or diseases such as hypothyroidism or nephrotic syndrome.
3. Patients with diabetes without established cardiovascular disease aged 40 to 75 years with LDL-C levels between 70 mg/dL and 189 mg/dL.
4. Patients without diabetes with established atherosclerotic cardiovascular disease aged 40 to 75 years with LDL-C levels from 70 mg/dL to 189 mg/dL and a calculated cardiovascular risk of at least 7.5% over the next 10 years.
The fourth category is potentially the most confusing for conscientious providers. The risk calculator that determines whether or not someone has a risk of > 7.5% over the next year is not the traditional Framingham risk calculator, with which many providers are familiar. Rather, it is a brand-new, improved risk calculator devised by the panel. The calculator can be found on both the AHA and ACC websites and in iOS and Android apps (See App Corner, p.38).
To make things even more confusing, once it has been determined that a statin is indicated, the dosing of the statin, either low, moderate, or high intensity, must be selected on the basis of determined risk level. Fortunately, the panel has given us a nice table defining which statins qualify for inclusion in each of these 3 intensity categories. As a general rule, the low-intensity statins should almost always be avoided. But the determination of whether moderate or high-intensity statins are indicated gets somewhat murky. The first two aforementioned classes both deserve high-intensity statins. However, patients with diabetes who haven’t had an event could go with either moderate- or high-intensity statins, and those patients without diabetes or LDL-C levels ≥ 190, but with a 10-year risk of at least 7.5%, can also receive either moderate- or high-intensity statins.
So there it is, and it all does make a certain amount of sense. You first determine the patient’s risk category, which determines whether or not statins are indicated. If they are, you then decide what level of potency your prescribed statin should possess. There is no need to go checking LDL-C levels later, because your therapy is not targeted at any particular LDL-C level. You might want to check occasionally, though, just as a way of assessing patient compliance.
So what should we make of all of this? From a purely scientific point of view, it seems abundantly clear that these are the most scientifically valid set of guidelines that have ever been produced, generated by genuine experts who bent over backward to examine every possible relevant study. The new risk calculator is clearly a broader-based tool than the Framingham calculator, which was based on now-dated data from a very narrow heavily-white population basis. Although the new risk calculator has been criticized by some as a very imperfect tool that overestimates risk in some subpopulations, I firmly believe it is considerably less imperfect than the Framingham tool. It is, quite frankly, the best risk calculator anyone could come up with at this time, and the cutoff for treatment at a risk of 7.5% or higher over 10 years seems eminently reasonable to me.
So what’s the problem with the new guidelines? I think you astute readers already know what the problem is: These guidelines simply represent way too radical a change for the huge bulk of busy, harried providers out there. The average primary care provider is currently struggling to complete a multifaceted patient encounter in 15 minutes or less and then document it in excruciating detail. He or she is going to be extremely hard-pressed to master and implement the new guidelines. The guidelines are indeed the most scientifically accurate and thorough guidelines that could be humanly produced, but they represent such a radical change from previous guidelines that a huge number of providers are going to be playing catch-up for a long time. I hope that their learning curve can be a very rapid one, but I worry that these scientifically pristine guidelines will be slow to find their way into general practice.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Most of you are no doubt familiar with the opening of Charles Dickens’ classic novel A Tale of Two Cities: “It was the best of times, it was the worst of times.” In many ways Dickens has presaged my current thinking about the new lipid guidelines that were recently issued. On the one hand, they are truly the very best guidelines that could possibly be produced at the present time; on the other hand, they may also be the worst set of guidelines that could possibly be promulgated on the practice community at the present moment.
Let’s first take a good look at what the new guidelines actually recommend. First, it’s important to understand that the parentage of the new guidelines has changed in a very important way from that of earlier recommendations. The previous lipid guidelines—the National Cholesterol Education Panel (NCEP) recommendations—were issued in 2001 and were sponsored and endorsed by the National Institutes of Health (NIH) as the federal government’s best effort at lipid recommendations for the general practice community. An update in 2004 further advised that a low-density lipoprotein cholesterol (LDL-C) goal of < 70 mg/dL is appropriate for many patients with preexisting vascular disease.
New guidelines were clearly overdue. Indeed, an expert panel had already been convened and was hard at work. However, a year or so ago the NIH made a critical strategic decision that it no longer wanted to be in the guideline business. The NIH rather abruptly decided that there would be no further iterations of the NCEP guidelines.
Fortunately the NIH did not simply drop the ball. Rather, it decided to pass the baton (mixed metaphor—sorry!) to a joint task force of the American Heart Association (AHA) and the American College of Cardiology (ACC). After all, who better to ponder lipid goals than the vascular experts who populate these 2 august societies? These 2 groups took a good look at the work that had been done by the expert panel, and decided that they would bless the new recommendations.
The fruit of the years of labor were finally presented at the annual meeting of the AHA held in Dallas in 2013. I didn’t make it to all of the heart sessions during that meeting. (I was distracted to a considerable extent by the 50th anniversary commemoration of President John F. Kennedy’s assassination going on just a few blocks away from the convention center at the Texas School Book Depository site.) But I can tell you I was definitely at the AHA session where the guidelines were formally presented, and it was indeed a lively and controversial session.
By far the biggest change in the new guidelines, representing both its greatest strength and its greatest weakness, is the new emphasis on overall cardiovascular risk assessment rather than on the attainment of a certain defined LDL-C goal. Indeed, a feature of the new guidelines, which many find disconcerting, is that there is no longer any mention whatsoever of LDL-C goals or targets!
The guidelines are also heavily statin-centric; other classes of lipid-lowering agents, such as fibrates or niacin, receive short shrift indeed. The recommendations are that statins should be prescribed routinely for each of the following “statin benefit groups”:
1. Patients who have clinical atherosclerotic cardiovascular disease and thus fall into the “secondary prevention” category.
2. Those with LDL-C levels of ≥ 190 mg/dL and who have no secondary cause, such as certain medications or diseases such as hypothyroidism or nephrotic syndrome.
3. Patients with diabetes without established cardiovascular disease aged 40 to 75 years with LDL-C levels between 70 mg/dL and 189 mg/dL.
4. Patients without diabetes with established atherosclerotic cardiovascular disease aged 40 to 75 years with LDL-C levels from 70 mg/dL to 189 mg/dL and a calculated cardiovascular risk of at least 7.5% over the next 10 years.
The fourth category is potentially the most confusing for conscientious providers. The risk calculator that determines whether or not someone has a risk of > 7.5% over the next year is not the traditional Framingham risk calculator, with which many providers are familiar. Rather, it is a brand-new, improved risk calculator devised by the panel. The calculator can be found on both the AHA and ACC websites and in iOS and Android apps (See App Corner, p.38).
To make things even more confusing, once it has been determined that a statin is indicated, the dosing of the statin, either low, moderate, or high intensity, must be selected on the basis of determined risk level. Fortunately, the panel has given us a nice table defining which statins qualify for inclusion in each of these 3 intensity categories. As a general rule, the low-intensity statins should almost always be avoided. But the determination of whether moderate or high-intensity statins are indicated gets somewhat murky. The first two aforementioned classes both deserve high-intensity statins. However, patients with diabetes who haven’t had an event could go with either moderate- or high-intensity statins, and those patients without diabetes or LDL-C levels ≥ 190, but with a 10-year risk of at least 7.5%, can also receive either moderate- or high-intensity statins.
So there it is, and it all does make a certain amount of sense. You first determine the patient’s risk category, which determines whether or not statins are indicated. If they are, you then decide what level of potency your prescribed statin should possess. There is no need to go checking LDL-C levels later, because your therapy is not targeted at any particular LDL-C level. You might want to check occasionally, though, just as a way of assessing patient compliance.
So what should we make of all of this? From a purely scientific point of view, it seems abundantly clear that these are the most scientifically valid set of guidelines that have ever been produced, generated by genuine experts who bent over backward to examine every possible relevant study. The new risk calculator is clearly a broader-based tool than the Framingham calculator, which was based on now-dated data from a very narrow heavily-white population basis. Although the new risk calculator has been criticized by some as a very imperfect tool that overestimates risk in some subpopulations, I firmly believe it is considerably less imperfect than the Framingham tool. It is, quite frankly, the best risk calculator anyone could come up with at this time, and the cutoff for treatment at a risk of 7.5% or higher over 10 years seems eminently reasonable to me.
So what’s the problem with the new guidelines? I think you astute readers already know what the problem is: These guidelines simply represent way too radical a change for the huge bulk of busy, harried providers out there. The average primary care provider is currently struggling to complete a multifaceted patient encounter in 15 minutes or less and then document it in excruciating detail. He or she is going to be extremely hard-pressed to master and implement the new guidelines. The guidelines are indeed the most scientifically accurate and thorough guidelines that could be humanly produced, but they represent such a radical change from previous guidelines that a huge number of providers are going to be playing catch-up for a long time. I hope that their learning curve can be a very rapid one, but I worry that these scientifically pristine guidelines will be slow to find their way into general practice.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
MERS: New scourge poses old ID challenge
There are hardly any infectious diseases that a little soap and water can’t help.
That old chestnut resurfaced when Dr. Keiji Fukuda of the World Health Organization announced on May 14 that while a special WHO panel had determined that the worldwide spread of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) did not yet constitute an international public health emergency, its spread right now seems to be driven largely by poor infection-control measures.
"Hospital outbreaks have been a key feature of this virus," which has produced nearly 600 confirmed cases worldwide, mostly in Saudi Arabia and the United Arab Emirates, but also with at least two confirmed cases in the United States, Dr. Fukuda said.
The recent uptick in cases – half of all cases to date were diagnosed in April – as well as their spread to new countries led the WHO special panel on MERS-CoV to meet May 13-14.
The panel concluded that no emergency exists yet. And they urged hospitals to put a much greater emphasis on the principles of Infection Fighting 101: handwashing, using masks and gloves, and changing gloves between patients.
In late April and early May, WHO staffers inspected several Saudi Arabian hospitals where MERS-CoV clusters had appeared and found "infection control practices were not up to standards," and that issues such as overcrowded emergency departments "amplified" the spread of MERS-CoV, Dr. Fukuda, WHO assistant director general, said during a press conference in Geneva.
MERS-CoV cases in the community also increased in recent weeks, which may reflect a seasonal rise in infections, improved surveillance and case recognition, or increasing person-to-person transmission. But Dr. Fukuda stressed that for now there is "no convincing evidence" of any change in the transmissibility of MERS-CoV or of its genetic makeup, and no evidence that MERS-CoV is cutting infectious swaths through communities.
The WHO committee put all the MERS-CoV evidence "on the table to see how it adds up," to produce "a very sober and critical assessment," a process Dr. Fukuda said could not have happened even 10 years ago.
On Twitter @mitchelzoler
There are hardly any infectious diseases that a little soap and water can’t help.
That old chestnut resurfaced when Dr. Keiji Fukuda of the World Health Organization announced on May 14 that while a special WHO panel had determined that the worldwide spread of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) did not yet constitute an international public health emergency, its spread right now seems to be driven largely by poor infection-control measures.
"Hospital outbreaks have been a key feature of this virus," which has produced nearly 600 confirmed cases worldwide, mostly in Saudi Arabia and the United Arab Emirates, but also with at least two confirmed cases in the United States, Dr. Fukuda said.
The recent uptick in cases – half of all cases to date were diagnosed in April – as well as their spread to new countries led the WHO special panel on MERS-CoV to meet May 13-14.
The panel concluded that no emergency exists yet. And they urged hospitals to put a much greater emphasis on the principles of Infection Fighting 101: handwashing, using masks and gloves, and changing gloves between patients.
In late April and early May, WHO staffers inspected several Saudi Arabian hospitals where MERS-CoV clusters had appeared and found "infection control practices were not up to standards," and that issues such as overcrowded emergency departments "amplified" the spread of MERS-CoV, Dr. Fukuda, WHO assistant director general, said during a press conference in Geneva.
MERS-CoV cases in the community also increased in recent weeks, which may reflect a seasonal rise in infections, improved surveillance and case recognition, or increasing person-to-person transmission. But Dr. Fukuda stressed that for now there is "no convincing evidence" of any change in the transmissibility of MERS-CoV or of its genetic makeup, and no evidence that MERS-CoV is cutting infectious swaths through communities.
The WHO committee put all the MERS-CoV evidence "on the table to see how it adds up," to produce "a very sober and critical assessment," a process Dr. Fukuda said could not have happened even 10 years ago.
On Twitter @mitchelzoler
There are hardly any infectious diseases that a little soap and water can’t help.
That old chestnut resurfaced when Dr. Keiji Fukuda of the World Health Organization announced on May 14 that while a special WHO panel had determined that the worldwide spread of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) did not yet constitute an international public health emergency, its spread right now seems to be driven largely by poor infection-control measures.
"Hospital outbreaks have been a key feature of this virus," which has produced nearly 600 confirmed cases worldwide, mostly in Saudi Arabia and the United Arab Emirates, but also with at least two confirmed cases in the United States, Dr. Fukuda said.
The recent uptick in cases – half of all cases to date were diagnosed in April – as well as their spread to new countries led the WHO special panel on MERS-CoV to meet May 13-14.
The panel concluded that no emergency exists yet. And they urged hospitals to put a much greater emphasis on the principles of Infection Fighting 101: handwashing, using masks and gloves, and changing gloves between patients.
In late April and early May, WHO staffers inspected several Saudi Arabian hospitals where MERS-CoV clusters had appeared and found "infection control practices were not up to standards," and that issues such as overcrowded emergency departments "amplified" the spread of MERS-CoV, Dr. Fukuda, WHO assistant director general, said during a press conference in Geneva.
MERS-CoV cases in the community also increased in recent weeks, which may reflect a seasonal rise in infections, improved surveillance and case recognition, or increasing person-to-person transmission. But Dr. Fukuda stressed that for now there is "no convincing evidence" of any change in the transmissibility of MERS-CoV or of its genetic makeup, and no evidence that MERS-CoV is cutting infectious swaths through communities.
The WHO committee put all the MERS-CoV evidence "on the table to see how it adds up," to produce "a very sober and critical assessment," a process Dr. Fukuda said could not have happened even 10 years ago.
On Twitter @mitchelzoler
Letters to the Editor
Peripheral interventions editorial
I read Dr. Samson’s recent critique of nonvascular surgeons foray into the world of peripheral vascular interventions with interest. He points out in his article how the American College of Cardiology has changed its guidelines for percutaneous coronary interventions.
Suddenly, what once lacked evidence-based medicine now becomes justified. Is it a surprise to anyone that these same "vascular specialists" use the "rarely appropriate" indication to justify countless unnecessary peripheral vascular interventions?
Until the advent of percutaneous endovascular interventions, these "vascular specialists" had little to no interest in the treatment of peripheral vascular disease. It is only within the past decade that we have seen the emergence of "vascular specialists" who portray themselves as experts in the field of peripheral vascular disease.
Even more astonishing is that hospitals have embraced the concept of "heart and vascular centers" to bring these "vascular specialists" into the mainstream treatment of what was once the domain of vascular surgery.
I think three factors have led to Dr. Samson’s poignant set of observations. First, the number of cardiology trainees continues to outpace the need for coronary interventions. Since our cardiology colleagues already have access to patients with peripheral vascular disease, it is only logical that there would be interest in peripheral interventions.
Second, industry-sponsored trials have sought out high-volume physicians to enroll patients. Vascular surgeons are generally a conservative group, which is comfortable with the medical management of intermittent claudication and asymptomatic carotid stenosis, to name just a few examples.
The medical device industry quickly moved to physicians with a more aggressive approach to peripheral vascular disease. Lastly, hospitals needed to maintain the lucrative revenue stream afforded by coronary interventions and cardiac surgery.
With declining revenue it made sense for hospitals to economically credential "vascular specialists" to be the spokespersons for their "heart and vascular centers."
Proponents of "vascular specialists" argue that with an aging population, we will need a substantial influx of providers who can manage peripheral vascular disease. The flaw in this argument is that all of these patients are going to need procedures. Most vascular surgeons would argue that substantial numbers of patients with peripheral vascular disease would do quite well with medical management alone.
Certainly, we need to deal with symptomatic carotid disease, large aneurysms, and critical limb ischemia, but these patients account for a small proportion of those with vascular disease. It may well be that with shrinking Medicare, Medicaid, and third-party insurance coffers, government regulations will finally put an end to the countless number of unnecessary endovascular procedures.
Unfortunately for Dr. Samson and the rest of us, the future is not bright. Whereas inside the university there may be harmony among vascular surgeons and "vascular specialists," that is surely not the case in the community where vascular surgeons are faced with increasing competition.
With the tacit approval of hospitals and their hired consulting firms – who champion profits over delivery of evidence-based vascular care – the role of vascular surgery is becoming marginalized.
I would suggest that there has been deafening silence from our societies about the unique role vascular surgery has in treating peripheral vascular disease.
Who else has the training and skill to treat vascular disease with the most appropriate open or endovascular procedures – and more importantly, knows when to apply each method?
Perhaps the unbiased observer might ask the obvious, who needs a "vascular specialist"?
Dr. Samson’s observations point us to the answer in my view – the hospital.
– Richard David Edrington, M.D. Raleigh, N.C.
Carotid screening
I read your commentary article published in the online Vascular Specialist re: carotid screening and I have to say that you nailed it. I would think that any conclusion must be suspect if it is drawn on the basis of studies that are poorly performed by technologists of questionable skills who are usually not RVT’s, then are interpreted (if we are lucky) by internists or radiologists with no particular expertise in vascular disease. Worst-case scenario is that the techs actually interpret the study, as you noted. These are the same people that perform a duplex scan of the leg arterial system to evaluate the significance of arterial occlusive disease – no pressure studies at all!
I have had the same experience you have noted many times of having a patient come into the office holding a carotid scan report claiming a critical stenosis and our RVT finds no significant disease at all. The opposite is also true.
As is your practice, we never initiate treatment based on results from an outside lab. I believe that the only way we’ll see any change in this "open season" on performing noninvasive studies is to make reimbursement contingent upon lab accreditation.
This whole situation is reminiscent of that in the ’90s when carotid endarterectomy came under assault from studies designed and conducted by neurologists who had already drawn their conclusions before they enrolled the first patients.
Good job. Keep up the fight to inject a bit of reason into this, so far, one-sided debate.
– William M. Blackshear Jr., M.D.
Director, Vascular Institute of Florida, St. Petersburg
Peripheral interventions editorial
I read Dr. Samson’s recent critique of nonvascular surgeons foray into the world of peripheral vascular interventions with interest. He points out in his article how the American College of Cardiology has changed its guidelines for percutaneous coronary interventions.
Suddenly, what once lacked evidence-based medicine now becomes justified. Is it a surprise to anyone that these same "vascular specialists" use the "rarely appropriate" indication to justify countless unnecessary peripheral vascular interventions?
Until the advent of percutaneous endovascular interventions, these "vascular specialists" had little to no interest in the treatment of peripheral vascular disease. It is only within the past decade that we have seen the emergence of "vascular specialists" who portray themselves as experts in the field of peripheral vascular disease.
Even more astonishing is that hospitals have embraced the concept of "heart and vascular centers" to bring these "vascular specialists" into the mainstream treatment of what was once the domain of vascular surgery.
I think three factors have led to Dr. Samson’s poignant set of observations. First, the number of cardiology trainees continues to outpace the need for coronary interventions. Since our cardiology colleagues already have access to patients with peripheral vascular disease, it is only logical that there would be interest in peripheral interventions.
Second, industry-sponsored trials have sought out high-volume physicians to enroll patients. Vascular surgeons are generally a conservative group, which is comfortable with the medical management of intermittent claudication and asymptomatic carotid stenosis, to name just a few examples.
The medical device industry quickly moved to physicians with a more aggressive approach to peripheral vascular disease. Lastly, hospitals needed to maintain the lucrative revenue stream afforded by coronary interventions and cardiac surgery.
With declining revenue it made sense for hospitals to economically credential "vascular specialists" to be the spokespersons for their "heart and vascular centers."
Proponents of "vascular specialists" argue that with an aging population, we will need a substantial influx of providers who can manage peripheral vascular disease. The flaw in this argument is that all of these patients are going to need procedures. Most vascular surgeons would argue that substantial numbers of patients with peripheral vascular disease would do quite well with medical management alone.
Certainly, we need to deal with symptomatic carotid disease, large aneurysms, and critical limb ischemia, but these patients account for a small proportion of those with vascular disease. It may well be that with shrinking Medicare, Medicaid, and third-party insurance coffers, government regulations will finally put an end to the countless number of unnecessary endovascular procedures.
Unfortunately for Dr. Samson and the rest of us, the future is not bright. Whereas inside the university there may be harmony among vascular surgeons and "vascular specialists," that is surely not the case in the community where vascular surgeons are faced with increasing competition.
With the tacit approval of hospitals and their hired consulting firms – who champion profits over delivery of evidence-based vascular care – the role of vascular surgery is becoming marginalized.
I would suggest that there has been deafening silence from our societies about the unique role vascular surgery has in treating peripheral vascular disease.
Who else has the training and skill to treat vascular disease with the most appropriate open or endovascular procedures – and more importantly, knows when to apply each method?
Perhaps the unbiased observer might ask the obvious, who needs a "vascular specialist"?
Dr. Samson’s observations point us to the answer in my view – the hospital.
– Richard David Edrington, M.D. Raleigh, N.C.
Carotid screening
I read your commentary article published in the online Vascular Specialist re: carotid screening and I have to say that you nailed it. I would think that any conclusion must be suspect if it is drawn on the basis of studies that are poorly performed by technologists of questionable skills who are usually not RVT’s, then are interpreted (if we are lucky) by internists or radiologists with no particular expertise in vascular disease. Worst-case scenario is that the techs actually interpret the study, as you noted. These are the same people that perform a duplex scan of the leg arterial system to evaluate the significance of arterial occlusive disease – no pressure studies at all!
I have had the same experience you have noted many times of having a patient come into the office holding a carotid scan report claiming a critical stenosis and our RVT finds no significant disease at all. The opposite is also true.
As is your practice, we never initiate treatment based on results from an outside lab. I believe that the only way we’ll see any change in this "open season" on performing noninvasive studies is to make reimbursement contingent upon lab accreditation.
This whole situation is reminiscent of that in the ’90s when carotid endarterectomy came under assault from studies designed and conducted by neurologists who had already drawn their conclusions before they enrolled the first patients.
Good job. Keep up the fight to inject a bit of reason into this, so far, one-sided debate.
– William M. Blackshear Jr., M.D.
Director, Vascular Institute of Florida, St. Petersburg
Peripheral interventions editorial
I read Dr. Samson’s recent critique of nonvascular surgeons foray into the world of peripheral vascular interventions with interest. He points out in his article how the American College of Cardiology has changed its guidelines for percutaneous coronary interventions.
Suddenly, what once lacked evidence-based medicine now becomes justified. Is it a surprise to anyone that these same "vascular specialists" use the "rarely appropriate" indication to justify countless unnecessary peripheral vascular interventions?
Until the advent of percutaneous endovascular interventions, these "vascular specialists" had little to no interest in the treatment of peripheral vascular disease. It is only within the past decade that we have seen the emergence of "vascular specialists" who portray themselves as experts in the field of peripheral vascular disease.
Even more astonishing is that hospitals have embraced the concept of "heart and vascular centers" to bring these "vascular specialists" into the mainstream treatment of what was once the domain of vascular surgery.
I think three factors have led to Dr. Samson’s poignant set of observations. First, the number of cardiology trainees continues to outpace the need for coronary interventions. Since our cardiology colleagues already have access to patients with peripheral vascular disease, it is only logical that there would be interest in peripheral interventions.
Second, industry-sponsored trials have sought out high-volume physicians to enroll patients. Vascular surgeons are generally a conservative group, which is comfortable with the medical management of intermittent claudication and asymptomatic carotid stenosis, to name just a few examples.
The medical device industry quickly moved to physicians with a more aggressive approach to peripheral vascular disease. Lastly, hospitals needed to maintain the lucrative revenue stream afforded by coronary interventions and cardiac surgery.
With declining revenue it made sense for hospitals to economically credential "vascular specialists" to be the spokespersons for their "heart and vascular centers."
Proponents of "vascular specialists" argue that with an aging population, we will need a substantial influx of providers who can manage peripheral vascular disease. The flaw in this argument is that all of these patients are going to need procedures. Most vascular surgeons would argue that substantial numbers of patients with peripheral vascular disease would do quite well with medical management alone.
Certainly, we need to deal with symptomatic carotid disease, large aneurysms, and critical limb ischemia, but these patients account for a small proportion of those with vascular disease. It may well be that with shrinking Medicare, Medicaid, and third-party insurance coffers, government regulations will finally put an end to the countless number of unnecessary endovascular procedures.
Unfortunately for Dr. Samson and the rest of us, the future is not bright. Whereas inside the university there may be harmony among vascular surgeons and "vascular specialists," that is surely not the case in the community where vascular surgeons are faced with increasing competition.
With the tacit approval of hospitals and their hired consulting firms – who champion profits over delivery of evidence-based vascular care – the role of vascular surgery is becoming marginalized.
I would suggest that there has been deafening silence from our societies about the unique role vascular surgery has in treating peripheral vascular disease.
Who else has the training and skill to treat vascular disease with the most appropriate open or endovascular procedures – and more importantly, knows when to apply each method?
Perhaps the unbiased observer might ask the obvious, who needs a "vascular specialist"?
Dr. Samson’s observations point us to the answer in my view – the hospital.
– Richard David Edrington, M.D. Raleigh, N.C.
Carotid screening
I read your commentary article published in the online Vascular Specialist re: carotid screening and I have to say that you nailed it. I would think that any conclusion must be suspect if it is drawn on the basis of studies that are poorly performed by technologists of questionable skills who are usually not RVT’s, then are interpreted (if we are lucky) by internists or radiologists with no particular expertise in vascular disease. Worst-case scenario is that the techs actually interpret the study, as you noted. These are the same people that perform a duplex scan of the leg arterial system to evaluate the significance of arterial occlusive disease – no pressure studies at all!
I have had the same experience you have noted many times of having a patient come into the office holding a carotid scan report claiming a critical stenosis and our RVT finds no significant disease at all. The opposite is also true.
As is your practice, we never initiate treatment based on results from an outside lab. I believe that the only way we’ll see any change in this "open season" on performing noninvasive studies is to make reimbursement contingent upon lab accreditation.
This whole situation is reminiscent of that in the ’90s when carotid endarterectomy came under assault from studies designed and conducted by neurologists who had already drawn their conclusions before they enrolled the first patients.
Good job. Keep up the fight to inject a bit of reason into this, so far, one-sided debate.
– William M. Blackshear Jr., M.D.
Director, Vascular Institute of Florida, St. Petersburg