User login
What to expect in the new concussion guidelines
This transcript has been edited for clarity.
Andrew N. Wilner, MD: I’m your host, Dr. Andrew Wilner, reporting virtually from the 2023 American Academy of Neurology meeting in Boston. It’s my pleasure today to speak with Dr. Shae Datta, codirector of the NYU Langone Concussion Center.
She’s also a clinical assistant professor of neurology at NYU School of Medicine. Dr. Datta is chair of the AAN Sports Neurology Section, and she’s leading a panel on concussion at this year’s meeting. She’s going to give us an update. Welcome, Dr. Datta.
Shae Datta, MD: Thank you so much, Andrew. I really love the fact that I’m here speaking to you about all of the new, exciting developments in the field.
Dr. Wilner: Before we get too deep, tell us how you got interested in this topic.
Dr. Datta: I initially thought, when I was in training as a resident, that I wanted to do something like neurocritical care or EEG. It also puzzled me why these seemingly smaller head injuries that didn’t end up in the hospital or ICU were bounced from neurology headache clinic to neuro-ophthalmology headache clinic to neurovestibular headache clinic, and nobody seemed to be able to put together the dots about why they’re having so many different issues — but at the same time, nobody could help them.
At that time, this field was very new. I was on a plane to Paris to a neurocritical care conference as a resident, and I saw the movie Concussion with Will Smith.
It featured one of my current mentors who taught at the fellowship that I graduated from, and it was a fascinating field. I just started looking deeply into it, and I saw that there was a new training fellowship for sports neurology and concussion management, and this is basically why we’re here today.
New concussion consensus guidelines coming
Dr. Wilner: I think this field has really exploded. It used to be that you banged your head, you did a CT scan – remember, I trained about 45 years ago – and if there was nothing on the CT scan, you were done. If you had headaches, you took Tylenol until they went away.
Now, we do MRI, and we realized that it’s really a syndrome. I understand that there are going to be some formal guidelines that have been put together. Is that correct?
Dr. Datta: That’s correct. The 6th International Consensus Conference on Concussion in Sport, in Amsterdam, where I attended and presented a poster, was really a meeting of all the best minds – clinicians and researchers in brain injury – to form a consensus on the newest guidelines that are going to direct our treatment going forward.
Dr. Wilner: I’m going to ask you a trick question because the last time I looked it up I did not get a satisfying answer. What is a concussion?
Dr. Datta: That’s a very good question, and everyone always asks. A concussion is an external force that is emitted upon the head or the neck, or the body, in general, that may cause temporary loss of function. It’s a functional problem.
We don’t see much on CT. We can do MRI. We can do SPECT or we can do these very fancy images, sometimes, of high-velocity head injuries and see small microhemorrhages.
Often, we don’t see anything, but still the patient is loopy. They can’t see straight. They are double-visioned. They have vertigo. Why is that happening? On the cellular level, we have an energy deficit in the sodium-potassium-ATPase pump of the neurons themselves.
Dr. Wilner: Suppose you do see diffuse axonal injury; does that take it out of concussion, or can you have a concussion with visible injury?
Dr. Datta: I think you can have overlap in the symptoms. The diffuse axonal injury would put it into a higher grade of head injury as opposed to a mild traumatic brain injury. Definitely, we would need to work together with our trauma doctors to ensure that patients are not on blood thinners or anything until they heal well enough. Obviously, I would pick them up as an outpatient and follow them until we resolve or rehab them as best as possible.
Concussion assessment tools
Dr. Wilner: There are many sports out there where concussions are fairly frequent, like American football and hockey, for example. Are there any statements in the new guidelines?
Dr. Datta: There are no statements for or against a particular sport because that would really make too much of a bold statement about cause and effect. There is a cause and effect in long-term, repetitive exposure, I would say, in terms of someone being able to play or sustain injury.
Right now, at least at the concussion conference I went to and in the upcoming consensus statement, they will not comment on a specific sport. Obviously, we know that the higher-impact sports are a little more dangerous.
Let’s be honest. At the high school, middle school, or even younger level, some kids are not necessarily the most athletic, right? They play because their friends are playing. If they’re repeatedly getting injured, it’s time for an astute clinician, or a coach, and a whole team to assess them to see if maybe this person is just going to continue to get hurt if they’re not taken out of the game and perhaps they should go to a lower-impact sport.
Dr. Wilner: In schools, often there’s a big size and weight difference. There are 14-year-olds who are 6 fett 2 inches and 200 pounds, and there are 14-year-olds who are 5 feet 2 inches and 110 pounds. Obviously, they’re mismatched on the football field.
You mentioned coaches. Is there anything in the guidelines about training coaches?
Dr. Datta: Specifically, there was nothing in the guidelines about that. There’s a tool for coaches at every level to use, which is called the Sports Concussion Assessment Tool, or SCAT, which is going to be updated to the SCAT6. At the NCAA level, they must receive annual training on concussion management and be given an NCAA concussion handout for coaches.
Obviously, there are more rigorous protocols for national-level coaching. As it stands now, it is not mandatory, but they are given tools to assess someone once they’ve gotten a hit to take them out of the game.
Dr. Wilner: I’ve been following the concussion research through the years. They did some neuropsychological testing on athletes who’ve had this many concussions or that many concussions, and they would find deficits here or subtle deficits there, but they had no baseline.
Then, there was a movement to start testing athletes before the season starts so that they could do a repeat test after concussion and see if there is any difference. Is that something we’re recommending?
Dr. Datta: Most of the time, NCAA-level – certainly where I trained – and national-level sports do testing, but it’s not everywhere. Prior guidelines have indicated that preseason testing is not required. That is largely because there has been no standardized neuropsychological testing established.
There are computerized testing options where the validity and reliability are questionable. Also, let’s say it’s a college student; they didn’t sleep all night and then they took this computer test. They would probably do worse than they would if they had received a head hit.
Just to be on the safe side, most places that have collegiate-level sports that are at a high level do preseason testing. If I were to speak personally, aside from the guidelines, I would say that it’s been helpful for me to look at the before and after, in general, overall, to make a decision about my treatment protocol.
Dr. Wilner: Let’s talk about the patient. You have a 20-year-old guy. He’s playing football. There’s a big play. Bonk, he gets hit on the head. He’s on the ground. He’s dazed, staggers a little bit, gets up, and you ask how he is feeling. He says he’s fine and then he wobbles off to the sideline. What do you do with that kid?
Dr. Datta: Obviously, the first thing is to remove him from the play environment to a quiet space. Second, either an athletic trainer or a coach would administer basic screening neurologic tests, such as “where are you, what’s today’s date, what is your name?” and other orientation questions.
They’ll also go through the SCAT – that’ll be SCAT6 starting in July – the SCAT5 symptom questionnaire to see what symptoms they have. Often, they’re using sideline testing software.
There are two things that can be used on a card to test eye movements, to see if they’re slower. They come out of NYU, coincidentally – the Memory Image Completion (MIC) and the Mobile Universal Lexicon Evaluation System (MULES) – and are used to determine whether eye movements are slower. That way, you can tell whether someone is, compared with before they got their head hit, slower than before.
Based on this composite information, usually the teammates and the head people on the team will know if a player looks different.
They need to be taken out, obviously, if there is nausea or vomiting, any neurologic signs and symptoms, or a neck injury that needs to be stabilized. ABCs first, right? If there’s any vomiting or seizures, they should be taken to the ER right away.
The first thing is to take them out, then do a sideline assessment. Third, see if they need to immediately go to the ED versus follow-up outpatient with me within a day or two.
Dr. Wilner: I think it’s the subtle injuries that are the tough ones. Back to our 20-year-old. He says: “Oh, I’m fine. I want to go back in the game.” Everybody can tell he’s not quite right, even though he passed all the tests. What do you do then?
Dr. Datta: You have to make a judgment call for the safety of the player. They always want to go back, right? This is also an issue when they’re competing for college scholarships and things of that nature. Sometimes they’re sandbagging, where they memorize the answers.
Everything’s on the Internet nowadays, right? We have to make a judgment call as members of the healthcare community and the sports community to keep that player safe.
Just keep them out. Don’t bring them back in the game. Keep them out for a reasonable amount of time. There’s a test called the Buffalo Concussion Treadmill Test; Dr. John Leddy from University of Buffalo has developed a way for us to put athletes through a screening protocol.
This can be part of their vestibular and ocular rehabilitation, where if they don’t have symptoms when we bring their heart rate to certain levels, then we can slowly clear them for return to play as long as they’re nonsymptomatic.
Dr. Wilner: I spoke with your colleague, Dr. Riggins, who is also on your panel, and we were talking about when they can go back. She said they can go back when they don’t have any symptoms. No more headache, no more dizziness, no more lightheadedness, no more trouble concentrating or with memory – all those things have gone away.
Sometimes these symptoms are stubborn. If you have, say, 100 patients like our 20-year-old who got bonked on the head, has some headaches, and doesn’t feel quite right, what usually happens? How many are back to play the next day, the next week, or the next month? How many are out for the season? How does that play out?
Dr. Datta: It depends on a couple of different factors. One, have they had previous head injuries? Two, do they have preexisting symptoms or signs, or diagnoses like migraines, which are likely to get worse after a head injury? Anything that’s preexisting, like a mood disorder, anxiety, depression, or trouble sleeping, is going to get worse.
If they were compensating for untreated ADD or borderline personality or bipolar, I’ve seen many people who’ve developed them. These are not the norm, but I’m saying that you have to be very careful.
Getting back to the question, you treat them. Reasonably, if they’re healthy and they don’t have preexisting signs and symptoms, I would say more than half are back in about 2 weeks.. I would say 60%-70%. It all depends. If they have preexisting issues, then it’s going to take much longer.
From SCAT to SCOAT
Dr. Wilner: This has been very informative. Before we wrap up, tell us what to expect from these guidelines in July. How are they really going to help?
Dr. Datta: We’ve been using the SCAT, which was meant for more sideline assessment because that’s all we had, and it’s worked perfectly well.
This will be better because we often see them within 24-48 hours, when the symptoms are sometimes a little bit better.
We also will see the sport and concussion group come up with added athlete perspectives, ethics discussion, power-sport athlete considerations, and development of this new SCOAT.
Dr. Wilner: Dr. Datta, this is very exciting. I look forward to reading these guidelines in July. I want to thank you for your hard work. I also look forward to talking to you at next year’s meeting. Thank you very much for giving us this update.
Dr. Datta: No problem. It’s my pleasure.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Andrew N. Wilner, MD: I’m your host, Dr. Andrew Wilner, reporting virtually from the 2023 American Academy of Neurology meeting in Boston. It’s my pleasure today to speak with Dr. Shae Datta, codirector of the NYU Langone Concussion Center.
She’s also a clinical assistant professor of neurology at NYU School of Medicine. Dr. Datta is chair of the AAN Sports Neurology Section, and she’s leading a panel on concussion at this year’s meeting. She’s going to give us an update. Welcome, Dr. Datta.
Shae Datta, MD: Thank you so much, Andrew. I really love the fact that I’m here speaking to you about all of the new, exciting developments in the field.
Dr. Wilner: Before we get too deep, tell us how you got interested in this topic.
Dr. Datta: I initially thought, when I was in training as a resident, that I wanted to do something like neurocritical care or EEG. It also puzzled me why these seemingly smaller head injuries that didn’t end up in the hospital or ICU were bounced from neurology headache clinic to neuro-ophthalmology headache clinic to neurovestibular headache clinic, and nobody seemed to be able to put together the dots about why they’re having so many different issues — but at the same time, nobody could help them.
At that time, this field was very new. I was on a plane to Paris to a neurocritical care conference as a resident, and I saw the movie Concussion with Will Smith.
It featured one of my current mentors who taught at the fellowship that I graduated from, and it was a fascinating field. I just started looking deeply into it, and I saw that there was a new training fellowship for sports neurology and concussion management, and this is basically why we’re here today.
New concussion consensus guidelines coming
Dr. Wilner: I think this field has really exploded. It used to be that you banged your head, you did a CT scan – remember, I trained about 45 years ago – and if there was nothing on the CT scan, you were done. If you had headaches, you took Tylenol until they went away.
Now, we do MRI, and we realized that it’s really a syndrome. I understand that there are going to be some formal guidelines that have been put together. Is that correct?
Dr. Datta: That’s correct. The 6th International Consensus Conference on Concussion in Sport, in Amsterdam, where I attended and presented a poster, was really a meeting of all the best minds – clinicians and researchers in brain injury – to form a consensus on the newest guidelines that are going to direct our treatment going forward.
Dr. Wilner: I’m going to ask you a trick question because the last time I looked it up I did not get a satisfying answer. What is a concussion?
Dr. Datta: That’s a very good question, and everyone always asks. A concussion is an external force that is emitted upon the head or the neck, or the body, in general, that may cause temporary loss of function. It’s a functional problem.
We don’t see much on CT. We can do MRI. We can do SPECT or we can do these very fancy images, sometimes, of high-velocity head injuries and see small microhemorrhages.
Often, we don’t see anything, but still the patient is loopy. They can’t see straight. They are double-visioned. They have vertigo. Why is that happening? On the cellular level, we have an energy deficit in the sodium-potassium-ATPase pump of the neurons themselves.
Dr. Wilner: Suppose you do see diffuse axonal injury; does that take it out of concussion, or can you have a concussion with visible injury?
Dr. Datta: I think you can have overlap in the symptoms. The diffuse axonal injury would put it into a higher grade of head injury as opposed to a mild traumatic brain injury. Definitely, we would need to work together with our trauma doctors to ensure that patients are not on blood thinners or anything until they heal well enough. Obviously, I would pick them up as an outpatient and follow them until we resolve or rehab them as best as possible.
Concussion assessment tools
Dr. Wilner: There are many sports out there where concussions are fairly frequent, like American football and hockey, for example. Are there any statements in the new guidelines?
Dr. Datta: There are no statements for or against a particular sport because that would really make too much of a bold statement about cause and effect. There is a cause and effect in long-term, repetitive exposure, I would say, in terms of someone being able to play or sustain injury.
Right now, at least at the concussion conference I went to and in the upcoming consensus statement, they will not comment on a specific sport. Obviously, we know that the higher-impact sports are a little more dangerous.
Let’s be honest. At the high school, middle school, or even younger level, some kids are not necessarily the most athletic, right? They play because their friends are playing. If they’re repeatedly getting injured, it’s time for an astute clinician, or a coach, and a whole team to assess them to see if maybe this person is just going to continue to get hurt if they’re not taken out of the game and perhaps they should go to a lower-impact sport.
Dr. Wilner: In schools, often there’s a big size and weight difference. There are 14-year-olds who are 6 fett 2 inches and 200 pounds, and there are 14-year-olds who are 5 feet 2 inches and 110 pounds. Obviously, they’re mismatched on the football field.
You mentioned coaches. Is there anything in the guidelines about training coaches?
Dr. Datta: Specifically, there was nothing in the guidelines about that. There’s a tool for coaches at every level to use, which is called the Sports Concussion Assessment Tool, or SCAT, which is going to be updated to the SCAT6. At the NCAA level, they must receive annual training on concussion management and be given an NCAA concussion handout for coaches.
Obviously, there are more rigorous protocols for national-level coaching. As it stands now, it is not mandatory, but they are given tools to assess someone once they’ve gotten a hit to take them out of the game.
Dr. Wilner: I’ve been following the concussion research through the years. They did some neuropsychological testing on athletes who’ve had this many concussions or that many concussions, and they would find deficits here or subtle deficits there, but they had no baseline.
Then, there was a movement to start testing athletes before the season starts so that they could do a repeat test after concussion and see if there is any difference. Is that something we’re recommending?
Dr. Datta: Most of the time, NCAA-level – certainly where I trained – and national-level sports do testing, but it’s not everywhere. Prior guidelines have indicated that preseason testing is not required. That is largely because there has been no standardized neuropsychological testing established.
There are computerized testing options where the validity and reliability are questionable. Also, let’s say it’s a college student; they didn’t sleep all night and then they took this computer test. They would probably do worse than they would if they had received a head hit.
Just to be on the safe side, most places that have collegiate-level sports that are at a high level do preseason testing. If I were to speak personally, aside from the guidelines, I would say that it’s been helpful for me to look at the before and after, in general, overall, to make a decision about my treatment protocol.
Dr. Wilner: Let’s talk about the patient. You have a 20-year-old guy. He’s playing football. There’s a big play. Bonk, he gets hit on the head. He’s on the ground. He’s dazed, staggers a little bit, gets up, and you ask how he is feeling. He says he’s fine and then he wobbles off to the sideline. What do you do with that kid?
Dr. Datta: Obviously, the first thing is to remove him from the play environment to a quiet space. Second, either an athletic trainer or a coach would administer basic screening neurologic tests, such as “where are you, what’s today’s date, what is your name?” and other orientation questions.
They’ll also go through the SCAT – that’ll be SCAT6 starting in July – the SCAT5 symptom questionnaire to see what symptoms they have. Often, they’re using sideline testing software.
There are two things that can be used on a card to test eye movements, to see if they’re slower. They come out of NYU, coincidentally – the Memory Image Completion (MIC) and the Mobile Universal Lexicon Evaluation System (MULES) – and are used to determine whether eye movements are slower. That way, you can tell whether someone is, compared with before they got their head hit, slower than before.
Based on this composite information, usually the teammates and the head people on the team will know if a player looks different.
They need to be taken out, obviously, if there is nausea or vomiting, any neurologic signs and symptoms, or a neck injury that needs to be stabilized. ABCs first, right? If there’s any vomiting or seizures, they should be taken to the ER right away.
The first thing is to take them out, then do a sideline assessment. Third, see if they need to immediately go to the ED versus follow-up outpatient with me within a day or two.
Dr. Wilner: I think it’s the subtle injuries that are the tough ones. Back to our 20-year-old. He says: “Oh, I’m fine. I want to go back in the game.” Everybody can tell he’s not quite right, even though he passed all the tests. What do you do then?
Dr. Datta: You have to make a judgment call for the safety of the player. They always want to go back, right? This is also an issue when they’re competing for college scholarships and things of that nature. Sometimes they’re sandbagging, where they memorize the answers.
Everything’s on the Internet nowadays, right? We have to make a judgment call as members of the healthcare community and the sports community to keep that player safe.
Just keep them out. Don’t bring them back in the game. Keep them out for a reasonable amount of time. There’s a test called the Buffalo Concussion Treadmill Test; Dr. John Leddy from University of Buffalo has developed a way for us to put athletes through a screening protocol.
This can be part of their vestibular and ocular rehabilitation, where if they don’t have symptoms when we bring their heart rate to certain levels, then we can slowly clear them for return to play as long as they’re nonsymptomatic.
Dr. Wilner: I spoke with your colleague, Dr. Riggins, who is also on your panel, and we were talking about when they can go back. She said they can go back when they don’t have any symptoms. No more headache, no more dizziness, no more lightheadedness, no more trouble concentrating or with memory – all those things have gone away.
Sometimes these symptoms are stubborn. If you have, say, 100 patients like our 20-year-old who got bonked on the head, has some headaches, and doesn’t feel quite right, what usually happens? How many are back to play the next day, the next week, or the next month? How many are out for the season? How does that play out?
Dr. Datta: It depends on a couple of different factors. One, have they had previous head injuries? Two, do they have preexisting symptoms or signs, or diagnoses like migraines, which are likely to get worse after a head injury? Anything that’s preexisting, like a mood disorder, anxiety, depression, or trouble sleeping, is going to get worse.
If they were compensating for untreated ADD or borderline personality or bipolar, I’ve seen many people who’ve developed them. These are not the norm, but I’m saying that you have to be very careful.
Getting back to the question, you treat them. Reasonably, if they’re healthy and they don’t have preexisting signs and symptoms, I would say more than half are back in about 2 weeks.. I would say 60%-70%. It all depends. If they have preexisting issues, then it’s going to take much longer.
From SCAT to SCOAT
Dr. Wilner: This has been very informative. Before we wrap up, tell us what to expect from these guidelines in July. How are they really going to help?
Dr. Datta: We’ve been using the SCAT, which was meant for more sideline assessment because that’s all we had, and it’s worked perfectly well.
This will be better because we often see them within 24-48 hours, when the symptoms are sometimes a little bit better.
We also will see the sport and concussion group come up with added athlete perspectives, ethics discussion, power-sport athlete considerations, and development of this new SCOAT.
Dr. Wilner: Dr. Datta, this is very exciting. I look forward to reading these guidelines in July. I want to thank you for your hard work. I also look forward to talking to you at next year’s meeting. Thank you very much for giving us this update.
Dr. Datta: No problem. It’s my pleasure.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Andrew N. Wilner, MD: I’m your host, Dr. Andrew Wilner, reporting virtually from the 2023 American Academy of Neurology meeting in Boston. It’s my pleasure today to speak with Dr. Shae Datta, codirector of the NYU Langone Concussion Center.
She’s also a clinical assistant professor of neurology at NYU School of Medicine. Dr. Datta is chair of the AAN Sports Neurology Section, and she’s leading a panel on concussion at this year’s meeting. She’s going to give us an update. Welcome, Dr. Datta.
Shae Datta, MD: Thank you so much, Andrew. I really love the fact that I’m here speaking to you about all of the new, exciting developments in the field.
Dr. Wilner: Before we get too deep, tell us how you got interested in this topic.
Dr. Datta: I initially thought, when I was in training as a resident, that I wanted to do something like neurocritical care or EEG. It also puzzled me why these seemingly smaller head injuries that didn’t end up in the hospital or ICU were bounced from neurology headache clinic to neuro-ophthalmology headache clinic to neurovestibular headache clinic, and nobody seemed to be able to put together the dots about why they’re having so many different issues — but at the same time, nobody could help them.
At that time, this field was very new. I was on a plane to Paris to a neurocritical care conference as a resident, and I saw the movie Concussion with Will Smith.
It featured one of my current mentors who taught at the fellowship that I graduated from, and it was a fascinating field. I just started looking deeply into it, and I saw that there was a new training fellowship for sports neurology and concussion management, and this is basically why we’re here today.
New concussion consensus guidelines coming
Dr. Wilner: I think this field has really exploded. It used to be that you banged your head, you did a CT scan – remember, I trained about 45 years ago – and if there was nothing on the CT scan, you were done. If you had headaches, you took Tylenol until they went away.
Now, we do MRI, and we realized that it’s really a syndrome. I understand that there are going to be some formal guidelines that have been put together. Is that correct?
Dr. Datta: That’s correct. The 6th International Consensus Conference on Concussion in Sport, in Amsterdam, where I attended and presented a poster, was really a meeting of all the best minds – clinicians and researchers in brain injury – to form a consensus on the newest guidelines that are going to direct our treatment going forward.
Dr. Wilner: I’m going to ask you a trick question because the last time I looked it up I did not get a satisfying answer. What is a concussion?
Dr. Datta: That’s a very good question, and everyone always asks. A concussion is an external force that is emitted upon the head or the neck, or the body, in general, that may cause temporary loss of function. It’s a functional problem.
We don’t see much on CT. We can do MRI. We can do SPECT or we can do these very fancy images, sometimes, of high-velocity head injuries and see small microhemorrhages.
Often, we don’t see anything, but still the patient is loopy. They can’t see straight. They are double-visioned. They have vertigo. Why is that happening? On the cellular level, we have an energy deficit in the sodium-potassium-ATPase pump of the neurons themselves.
Dr. Wilner: Suppose you do see diffuse axonal injury; does that take it out of concussion, or can you have a concussion with visible injury?
Dr. Datta: I think you can have overlap in the symptoms. The diffuse axonal injury would put it into a higher grade of head injury as opposed to a mild traumatic brain injury. Definitely, we would need to work together with our trauma doctors to ensure that patients are not on blood thinners or anything until they heal well enough. Obviously, I would pick them up as an outpatient and follow them until we resolve or rehab them as best as possible.
Concussion assessment tools
Dr. Wilner: There are many sports out there where concussions are fairly frequent, like American football and hockey, for example. Are there any statements in the new guidelines?
Dr. Datta: There are no statements for or against a particular sport because that would really make too much of a bold statement about cause and effect. There is a cause and effect in long-term, repetitive exposure, I would say, in terms of someone being able to play or sustain injury.
Right now, at least at the concussion conference I went to and in the upcoming consensus statement, they will not comment on a specific sport. Obviously, we know that the higher-impact sports are a little more dangerous.
Let’s be honest. At the high school, middle school, or even younger level, some kids are not necessarily the most athletic, right? They play because their friends are playing. If they’re repeatedly getting injured, it’s time for an astute clinician, or a coach, and a whole team to assess them to see if maybe this person is just going to continue to get hurt if they’re not taken out of the game and perhaps they should go to a lower-impact sport.
Dr. Wilner: In schools, often there’s a big size and weight difference. There are 14-year-olds who are 6 fett 2 inches and 200 pounds, and there are 14-year-olds who are 5 feet 2 inches and 110 pounds. Obviously, they’re mismatched on the football field.
You mentioned coaches. Is there anything in the guidelines about training coaches?
Dr. Datta: Specifically, there was nothing in the guidelines about that. There’s a tool for coaches at every level to use, which is called the Sports Concussion Assessment Tool, or SCAT, which is going to be updated to the SCAT6. At the NCAA level, they must receive annual training on concussion management and be given an NCAA concussion handout for coaches.
Obviously, there are more rigorous protocols for national-level coaching. As it stands now, it is not mandatory, but they are given tools to assess someone once they’ve gotten a hit to take them out of the game.
Dr. Wilner: I’ve been following the concussion research through the years. They did some neuropsychological testing on athletes who’ve had this many concussions or that many concussions, and they would find deficits here or subtle deficits there, but they had no baseline.
Then, there was a movement to start testing athletes before the season starts so that they could do a repeat test after concussion and see if there is any difference. Is that something we’re recommending?
Dr. Datta: Most of the time, NCAA-level – certainly where I trained – and national-level sports do testing, but it’s not everywhere. Prior guidelines have indicated that preseason testing is not required. That is largely because there has been no standardized neuropsychological testing established.
There are computerized testing options where the validity and reliability are questionable. Also, let’s say it’s a college student; they didn’t sleep all night and then they took this computer test. They would probably do worse than they would if they had received a head hit.
Just to be on the safe side, most places that have collegiate-level sports that are at a high level do preseason testing. If I were to speak personally, aside from the guidelines, I would say that it’s been helpful for me to look at the before and after, in general, overall, to make a decision about my treatment protocol.
Dr. Wilner: Let’s talk about the patient. You have a 20-year-old guy. He’s playing football. There’s a big play. Bonk, he gets hit on the head. He’s on the ground. He’s dazed, staggers a little bit, gets up, and you ask how he is feeling. He says he’s fine and then he wobbles off to the sideline. What do you do with that kid?
Dr. Datta: Obviously, the first thing is to remove him from the play environment to a quiet space. Second, either an athletic trainer or a coach would administer basic screening neurologic tests, such as “where are you, what’s today’s date, what is your name?” and other orientation questions.
They’ll also go through the SCAT – that’ll be SCAT6 starting in July – the SCAT5 symptom questionnaire to see what symptoms they have. Often, they’re using sideline testing software.
There are two things that can be used on a card to test eye movements, to see if they’re slower. They come out of NYU, coincidentally – the Memory Image Completion (MIC) and the Mobile Universal Lexicon Evaluation System (MULES) – and are used to determine whether eye movements are slower. That way, you can tell whether someone is, compared with before they got their head hit, slower than before.
Based on this composite information, usually the teammates and the head people on the team will know if a player looks different.
They need to be taken out, obviously, if there is nausea or vomiting, any neurologic signs and symptoms, or a neck injury that needs to be stabilized. ABCs first, right? If there’s any vomiting or seizures, they should be taken to the ER right away.
The first thing is to take them out, then do a sideline assessment. Third, see if they need to immediately go to the ED versus follow-up outpatient with me within a day or two.
Dr. Wilner: I think it’s the subtle injuries that are the tough ones. Back to our 20-year-old. He says: “Oh, I’m fine. I want to go back in the game.” Everybody can tell he’s not quite right, even though he passed all the tests. What do you do then?
Dr. Datta: You have to make a judgment call for the safety of the player. They always want to go back, right? This is also an issue when they’re competing for college scholarships and things of that nature. Sometimes they’re sandbagging, where they memorize the answers.
Everything’s on the Internet nowadays, right? We have to make a judgment call as members of the healthcare community and the sports community to keep that player safe.
Just keep them out. Don’t bring them back in the game. Keep them out for a reasonable amount of time. There’s a test called the Buffalo Concussion Treadmill Test; Dr. John Leddy from University of Buffalo has developed a way for us to put athletes through a screening protocol.
This can be part of their vestibular and ocular rehabilitation, where if they don’t have symptoms when we bring their heart rate to certain levels, then we can slowly clear them for return to play as long as they’re nonsymptomatic.
Dr. Wilner: I spoke with your colleague, Dr. Riggins, who is also on your panel, and we were talking about when they can go back. She said they can go back when they don’t have any symptoms. No more headache, no more dizziness, no more lightheadedness, no more trouble concentrating or with memory – all those things have gone away.
Sometimes these symptoms are stubborn. If you have, say, 100 patients like our 20-year-old who got bonked on the head, has some headaches, and doesn’t feel quite right, what usually happens? How many are back to play the next day, the next week, or the next month? How many are out for the season? How does that play out?
Dr. Datta: It depends on a couple of different factors. One, have they had previous head injuries? Two, do they have preexisting symptoms or signs, or diagnoses like migraines, which are likely to get worse after a head injury? Anything that’s preexisting, like a mood disorder, anxiety, depression, or trouble sleeping, is going to get worse.
If they were compensating for untreated ADD or borderline personality or bipolar, I’ve seen many people who’ve developed them. These are not the norm, but I’m saying that you have to be very careful.
Getting back to the question, you treat them. Reasonably, if they’re healthy and they don’t have preexisting signs and symptoms, I would say more than half are back in about 2 weeks.. I would say 60%-70%. It all depends. If they have preexisting issues, then it’s going to take much longer.
From SCAT to SCOAT
Dr. Wilner: This has been very informative. Before we wrap up, tell us what to expect from these guidelines in July. How are they really going to help?
Dr. Datta: We’ve been using the SCAT, which was meant for more sideline assessment because that’s all we had, and it’s worked perfectly well.
This will be better because we often see them within 24-48 hours, when the symptoms are sometimes a little bit better.
We also will see the sport and concussion group come up with added athlete perspectives, ethics discussion, power-sport athlete considerations, and development of this new SCOAT.
Dr. Wilner: Dr. Datta, this is very exciting. I look forward to reading these guidelines in July. I want to thank you for your hard work. I also look forward to talking to you at next year’s meeting. Thank you very much for giving us this update.
Dr. Datta: No problem. It’s my pleasure.
A version of this article originally appeared on Medscape.com.
Morning PT
Tuesdays and Fridays are tough. Not so much because of clinic, but rather because of the 32 minutes before clinic that I’m on the Peloton bike. They are the mornings I dedicate to training VO2max.
Training VO2max, or maximal oxygen consumption, is simple. Spin for a leisurely, easy-breathing, 4 minutes, then for 4 minutes push yourself until you see the light of heaven and wish for death to come. Then relax for 4 minutes again. Repeat this cycle four to six times. Done justly, you will dread Tuesdays and Fridays too. The punishing cycle of a 4-minute push, then 4-minute recovery is, however, an excellent way to improve cardiovascular fitness. And no, I’m not training for the Boston Marathon, so why am I working so hard? Because I’m training for marathon clinic days for the next 20 years.
Now more than ever, I feel we have to be physically fit to deal with a physicians’ day’s work. It’s exhausting. The root cause is too much work, yes, but I believe being physically fit could help.
I was talking to an 86-year-old patient about this very topic recently. He was short, with a well-manicured goatee and shiny head. He stuck his arm out to shake my hand. “Glad we’re back to handshakes again, doc.” His grip was that of a 30-year-old. “Buff” you’d likely describe him: He is noticeably muscular, not a skinny old man. He’s an old Navy Master Chief who started a business in wholesale flowers, which distributes all over the United States. And he’s still working full time. Impressed, I asked his secret for such vigor. PT, he replied.
PT, or physical training, is a foundational element of the Navy. Every sailor starts his or her day with morning PT before carrying out their duties. Some 30 years later, this guy is still getting after it. He does push-ups, sit-ups, and pull-ups nearly every morning. Morning PT is what he attributes to his success not only in health, but also business. As he sees it, he has the business savvy and experience of an old guy and the energy and stamina of a college kid. A good combination for a successful life.
I’ve always been pretty fit. Lately, I’ve been trying to take it to the next level, to not just be “physically active,” but rather “high-performance fit.” There are plenty of sources for instruction; how to stay young and healthy isn’t a new idea after all. I mean, Herodotus wrote of finding the Fountain of Youth in the 5th century BCE. A couple thousand years later, it’s still on trend. One of my favorite sages giving health span advice is Peter Attia, MD. I’ve been a fan since I met him at TEDMED in 2013 and I marvel at the astounding body of work he has created since. A Johns Hopkins–trained surgeon, he has spent his career reviewing the scientific literature about longevity and sharing it as actionable content. His book, “Outlive: The Science and Art of Longevity” (New York: Penguin Random House, 2023) is a nice summary of his work. I recommend it.
Right now I’m switching between type 2 muscle fiber work (lots of jumping like my 2-year-old) and cardiovascular training including the aforementioned VO2max work. I cannot say that my patient inbox is any cleaner, or that I’m faster in the office, but I’m not flagging by the end of the day anymore. Master Chief challenged me to match his 10 pull-ups before he returns for his follow up visit. I’ll gladly give up Peloton sprints to work on that.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Tuesdays and Fridays are tough. Not so much because of clinic, but rather because of the 32 minutes before clinic that I’m on the Peloton bike. They are the mornings I dedicate to training VO2max.
Training VO2max, or maximal oxygen consumption, is simple. Spin for a leisurely, easy-breathing, 4 minutes, then for 4 minutes push yourself until you see the light of heaven and wish for death to come. Then relax for 4 minutes again. Repeat this cycle four to six times. Done justly, you will dread Tuesdays and Fridays too. The punishing cycle of a 4-minute push, then 4-minute recovery is, however, an excellent way to improve cardiovascular fitness. And no, I’m not training for the Boston Marathon, so why am I working so hard? Because I’m training for marathon clinic days for the next 20 years.
Now more than ever, I feel we have to be physically fit to deal with a physicians’ day’s work. It’s exhausting. The root cause is too much work, yes, but I believe being physically fit could help.
I was talking to an 86-year-old patient about this very topic recently. He was short, with a well-manicured goatee and shiny head. He stuck his arm out to shake my hand. “Glad we’re back to handshakes again, doc.” His grip was that of a 30-year-old. “Buff” you’d likely describe him: He is noticeably muscular, not a skinny old man. He’s an old Navy Master Chief who started a business in wholesale flowers, which distributes all over the United States. And he’s still working full time. Impressed, I asked his secret for such vigor. PT, he replied.
PT, or physical training, is a foundational element of the Navy. Every sailor starts his or her day with morning PT before carrying out their duties. Some 30 years later, this guy is still getting after it. He does push-ups, sit-ups, and pull-ups nearly every morning. Morning PT is what he attributes to his success not only in health, but also business. As he sees it, he has the business savvy and experience of an old guy and the energy and stamina of a college kid. A good combination for a successful life.
I’ve always been pretty fit. Lately, I’ve been trying to take it to the next level, to not just be “physically active,” but rather “high-performance fit.” There are plenty of sources for instruction; how to stay young and healthy isn’t a new idea after all. I mean, Herodotus wrote of finding the Fountain of Youth in the 5th century BCE. A couple thousand years later, it’s still on trend. One of my favorite sages giving health span advice is Peter Attia, MD. I’ve been a fan since I met him at TEDMED in 2013 and I marvel at the astounding body of work he has created since. A Johns Hopkins–trained surgeon, he has spent his career reviewing the scientific literature about longevity and sharing it as actionable content. His book, “Outlive: The Science and Art of Longevity” (New York: Penguin Random House, 2023) is a nice summary of his work. I recommend it.
Right now I’m switching between type 2 muscle fiber work (lots of jumping like my 2-year-old) and cardiovascular training including the aforementioned VO2max work. I cannot say that my patient inbox is any cleaner, or that I’m faster in the office, but I’m not flagging by the end of the day anymore. Master Chief challenged me to match his 10 pull-ups before he returns for his follow up visit. I’ll gladly give up Peloton sprints to work on that.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Tuesdays and Fridays are tough. Not so much because of clinic, but rather because of the 32 minutes before clinic that I’m on the Peloton bike. They are the mornings I dedicate to training VO2max.
Training VO2max, or maximal oxygen consumption, is simple. Spin for a leisurely, easy-breathing, 4 minutes, then for 4 minutes push yourself until you see the light of heaven and wish for death to come. Then relax for 4 minutes again. Repeat this cycle four to six times. Done justly, you will dread Tuesdays and Fridays too. The punishing cycle of a 4-minute push, then 4-minute recovery is, however, an excellent way to improve cardiovascular fitness. And no, I’m not training for the Boston Marathon, so why am I working so hard? Because I’m training for marathon clinic days for the next 20 years.
Now more than ever, I feel we have to be physically fit to deal with a physicians’ day’s work. It’s exhausting. The root cause is too much work, yes, but I believe being physically fit could help.
I was talking to an 86-year-old patient about this very topic recently. He was short, with a well-manicured goatee and shiny head. He stuck his arm out to shake my hand. “Glad we’re back to handshakes again, doc.” His grip was that of a 30-year-old. “Buff” you’d likely describe him: He is noticeably muscular, not a skinny old man. He’s an old Navy Master Chief who started a business in wholesale flowers, which distributes all over the United States. And he’s still working full time. Impressed, I asked his secret for such vigor. PT, he replied.
PT, or physical training, is a foundational element of the Navy. Every sailor starts his or her day with morning PT before carrying out their duties. Some 30 years later, this guy is still getting after it. He does push-ups, sit-ups, and pull-ups nearly every morning. Morning PT is what he attributes to his success not only in health, but also business. As he sees it, he has the business savvy and experience of an old guy and the energy and stamina of a college kid. A good combination for a successful life.
I’ve always been pretty fit. Lately, I’ve been trying to take it to the next level, to not just be “physically active,” but rather “high-performance fit.” There are plenty of sources for instruction; how to stay young and healthy isn’t a new idea after all. I mean, Herodotus wrote of finding the Fountain of Youth in the 5th century BCE. A couple thousand years later, it’s still on trend. One of my favorite sages giving health span advice is Peter Attia, MD. I’ve been a fan since I met him at TEDMED in 2013 and I marvel at the astounding body of work he has created since. A Johns Hopkins–trained surgeon, he has spent his career reviewing the scientific literature about longevity and sharing it as actionable content. His book, “Outlive: The Science and Art of Longevity” (New York: Penguin Random House, 2023) is a nice summary of his work. I recommend it.
Right now I’m switching between type 2 muscle fiber work (lots of jumping like my 2-year-old) and cardiovascular training including the aforementioned VO2max work. I cannot say that my patient inbox is any cleaner, or that I’m faster in the office, but I’m not flagging by the end of the day anymore. Master Chief challenged me to match his 10 pull-ups before he returns for his follow up visit. I’ll gladly give up Peloton sprints to work on that.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
The antimicrobial peptide that even Pharma can love
Fastest peptide north, south, east, aaaaand west of the Pecos
Bacterial infections are supposed to be simple. You get infected, you get an antibiotic to treat it. Easy. Some bacteria, though, don’t play by the rules. Those antibiotics may kill 99.9% of germs, but what about the 0.1% that gets left behind? With their fallen comrades out of the way, the accidentally drug resistant species are free to inherit the Earth.
Antibiotic resistance is thus a major concern for the medical community. Naturally, anything that prevents doctors from successfully curing sick people is a priority. Unless you’re a major pharmaceutical company that has been loath to develop new drugs that can beat antibiotic-resistant bacteria. Blah blah, time and money, blah blah, long time between development and market application, blah blah, no profit. We all know the story with pharmaceutical companies.
Research from other sources has continued, however, and Brazilian scientists recently published research involving a peptide known as plantaricin 149. This peptide, derived from the bacterium Lactobacillus plantarum, has been known for nearly 30 years to have antibacterial properties. Pln149 in its natural state, though, is not particularly efficient at bacteria-killing. Fortunately, we have science and technology on our side.
The researchers synthesized 20 analogs of Pln149, of which Pln149-PEP20 had the best results. The elegantly named compound is less than half the size of the original peptide, less toxic, and far better at killing any and all drug-resistant bacteria the researchers threw at it. How much better? Pln149-PEP20 started killing bacteria less than an hour after being introduced in lab trials.
The research is just in its early days – just because something is less toxic doesn’t necessarily mean you want to go and help yourself to it – but we can only hope that those lovely pharmaceutical companies deign to look down upon us and actually develop a drug utilizing Pln149-PEP20 to, you know, actually help sick people, instead of trying to build monopolies or avoiding paying billions in taxes. Yeah, we couldn’t keep a straight face through that last sentence either.
Speed healing: The wavy wound gets the swirl
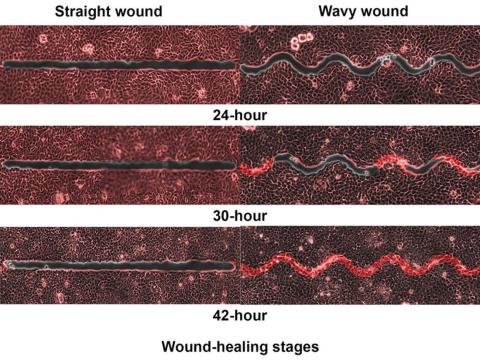
Did you know that wavy wounds heal faster than straight wounds? Well, we didn’t, but apparently quite a few people did, because somebody has been trying to figure out why wavy wounds heal faster than straight ones. Do the surgeons know about this? How about you dermatologists? Wavy over straight? We’re the media. We’re supposed to report this kind of stuff. Maybe hit us with a tweet next time you do something important, or push a TikTok our way, okay?
You could be more like the investigators at Nanyang Technological University in Singapore, who figured out the why and then released a statement about it.
They created synthetic wounds – some straight, some wavy – in micropatterned hydrogel substrates that mimicked human skin. Then they used an advanced optical technique known as particle image velocimetry to measure fluid flow and learn how cells moved to close the wound gaps.
The wavy wounds “induced more complex collective cell movements, such as a swirly, vortex-like motion,” according to the written statement from NTU Singapore. In the straight wounds, cell movements paralleled the wound front, “moving in straight lines like a marching band,” they pointed out, unlike some researchers who never call us unless they need money.
Complex epithelial cell movements are better, it turns out. Over an observation period of 64 hours the NTU team found that the healing efficiency of wavy gaps – measured by the area covered by the cells over time – is nearly five times faster than straight gaps.
The complex motion “enabled cells to quickly connect with similar cells on the opposite site of the wound edge, forming a bridge and closing the wavy wound gaps faster than straight gaps,” explained lead author Xu Hongmei, a doctoral student at NTU’s School of Mechanical and Aerospace Engineering, who seems to have time to toss out a tumblr or two to keep the press informed.
As for the rest of you, would it kill you to pick up a phone once in a while? Maybe let a journalist know that you’re still alive? We have feelings too, you know, and we worry.
A little Jekyll, a little Hyde, and a little shop of horrors
More “Little Shop of Horrors” references are coming, so be prepared.
We begin with Triphyophyllum peltatum. This woody vine is of great interest to medical and pharmaceutical researchers because its constituents have shown promise against pancreatic cancer and leukemia cells, among others, along with the pathogens that cause malaria and other diseases. There is another side, however. T. peltatum also has a tendency to turn into a realistic Audrey II when deprived.
No, of course they’re not craving human flesh, but it does become … carnivorous in its appetite.
T. peltatum, native to the West African tropics and not found in a New York florist shop, has the unique ability to change its diet and development based on the environmental circumstances. For some unknown reason, the leaves would develop adhesive traps in the form of sticky drops that capture insect prey. The plant is notoriously hard to grow, however, so no one could study the transformation under lab conditions. Until now.
A group of German scientists “exposed the plant to different stress factors, including deficiencies of various nutrients, and studied how it responded to each,” said Dr. Traud Winkelmann of Leibniz University Hannover. “Only in one case were we able to observe the formation of traps: in the case of a lack of phosphorus.”
Well, there you have it: phosphorus. We need it for healthy bones and teeth, which this plant doesn’t have to worry about, unlike its Tony Award–nominated counterpart. The investigators hope that their findings could lead to “future molecular analyses that will help understand the origins of carnivory,” but we’re guessing that a certain singing alien species will be left out of that research.
Fastest peptide north, south, east, aaaaand west of the Pecos
Bacterial infections are supposed to be simple. You get infected, you get an antibiotic to treat it. Easy. Some bacteria, though, don’t play by the rules. Those antibiotics may kill 99.9% of germs, but what about the 0.1% that gets left behind? With their fallen comrades out of the way, the accidentally drug resistant species are free to inherit the Earth.
Antibiotic resistance is thus a major concern for the medical community. Naturally, anything that prevents doctors from successfully curing sick people is a priority. Unless you’re a major pharmaceutical company that has been loath to develop new drugs that can beat antibiotic-resistant bacteria. Blah blah, time and money, blah blah, long time between development and market application, blah blah, no profit. We all know the story with pharmaceutical companies.
Research from other sources has continued, however, and Brazilian scientists recently published research involving a peptide known as plantaricin 149. This peptide, derived from the bacterium Lactobacillus plantarum, has been known for nearly 30 years to have antibacterial properties. Pln149 in its natural state, though, is not particularly efficient at bacteria-killing. Fortunately, we have science and technology on our side.
The researchers synthesized 20 analogs of Pln149, of which Pln149-PEP20 had the best results. The elegantly named compound is less than half the size of the original peptide, less toxic, and far better at killing any and all drug-resistant bacteria the researchers threw at it. How much better? Pln149-PEP20 started killing bacteria less than an hour after being introduced in lab trials.
The research is just in its early days – just because something is less toxic doesn’t necessarily mean you want to go and help yourself to it – but we can only hope that those lovely pharmaceutical companies deign to look down upon us and actually develop a drug utilizing Pln149-PEP20 to, you know, actually help sick people, instead of trying to build monopolies or avoiding paying billions in taxes. Yeah, we couldn’t keep a straight face through that last sentence either.
Speed healing: The wavy wound gets the swirl
Did you know that wavy wounds heal faster than straight wounds? Well, we didn’t, but apparently quite a few people did, because somebody has been trying to figure out why wavy wounds heal faster than straight ones. Do the surgeons know about this? How about you dermatologists? Wavy over straight? We’re the media. We’re supposed to report this kind of stuff. Maybe hit us with a tweet next time you do something important, or push a TikTok our way, okay?
You could be more like the investigators at Nanyang Technological University in Singapore, who figured out the why and then released a statement about it.
They created synthetic wounds – some straight, some wavy – in micropatterned hydrogel substrates that mimicked human skin. Then they used an advanced optical technique known as particle image velocimetry to measure fluid flow and learn how cells moved to close the wound gaps.
The wavy wounds “induced more complex collective cell movements, such as a swirly, vortex-like motion,” according to the written statement from NTU Singapore. In the straight wounds, cell movements paralleled the wound front, “moving in straight lines like a marching band,” they pointed out, unlike some researchers who never call us unless they need money.
Complex epithelial cell movements are better, it turns out. Over an observation period of 64 hours the NTU team found that the healing efficiency of wavy gaps – measured by the area covered by the cells over time – is nearly five times faster than straight gaps.
The complex motion “enabled cells to quickly connect with similar cells on the opposite site of the wound edge, forming a bridge and closing the wavy wound gaps faster than straight gaps,” explained lead author Xu Hongmei, a doctoral student at NTU’s School of Mechanical and Aerospace Engineering, who seems to have time to toss out a tumblr or two to keep the press informed.
As for the rest of you, would it kill you to pick up a phone once in a while? Maybe let a journalist know that you’re still alive? We have feelings too, you know, and we worry.
A little Jekyll, a little Hyde, and a little shop of horrors
More “Little Shop of Horrors” references are coming, so be prepared.
We begin with Triphyophyllum peltatum. This woody vine is of great interest to medical and pharmaceutical researchers because its constituents have shown promise against pancreatic cancer and leukemia cells, among others, along with the pathogens that cause malaria and other diseases. There is another side, however. T. peltatum also has a tendency to turn into a realistic Audrey II when deprived.
No, of course they’re not craving human flesh, but it does become … carnivorous in its appetite.
T. peltatum, native to the West African tropics and not found in a New York florist shop, has the unique ability to change its diet and development based on the environmental circumstances. For some unknown reason, the leaves would develop adhesive traps in the form of sticky drops that capture insect prey. The plant is notoriously hard to grow, however, so no one could study the transformation under lab conditions. Until now.
A group of German scientists “exposed the plant to different stress factors, including deficiencies of various nutrients, and studied how it responded to each,” said Dr. Traud Winkelmann of Leibniz University Hannover. “Only in one case were we able to observe the formation of traps: in the case of a lack of phosphorus.”
Well, there you have it: phosphorus. We need it for healthy bones and teeth, which this plant doesn’t have to worry about, unlike its Tony Award–nominated counterpart. The investigators hope that their findings could lead to “future molecular analyses that will help understand the origins of carnivory,” but we’re guessing that a certain singing alien species will be left out of that research.
Fastest peptide north, south, east, aaaaand west of the Pecos
Bacterial infections are supposed to be simple. You get infected, you get an antibiotic to treat it. Easy. Some bacteria, though, don’t play by the rules. Those antibiotics may kill 99.9% of germs, but what about the 0.1% that gets left behind? With their fallen comrades out of the way, the accidentally drug resistant species are free to inherit the Earth.
Antibiotic resistance is thus a major concern for the medical community. Naturally, anything that prevents doctors from successfully curing sick people is a priority. Unless you’re a major pharmaceutical company that has been loath to develop new drugs that can beat antibiotic-resistant bacteria. Blah blah, time and money, blah blah, long time between development and market application, blah blah, no profit. We all know the story with pharmaceutical companies.
Research from other sources has continued, however, and Brazilian scientists recently published research involving a peptide known as plantaricin 149. This peptide, derived from the bacterium Lactobacillus plantarum, has been known for nearly 30 years to have antibacterial properties. Pln149 in its natural state, though, is not particularly efficient at bacteria-killing. Fortunately, we have science and technology on our side.
The researchers synthesized 20 analogs of Pln149, of which Pln149-PEP20 had the best results. The elegantly named compound is less than half the size of the original peptide, less toxic, and far better at killing any and all drug-resistant bacteria the researchers threw at it. How much better? Pln149-PEP20 started killing bacteria less than an hour after being introduced in lab trials.
The research is just in its early days – just because something is less toxic doesn’t necessarily mean you want to go and help yourself to it – but we can only hope that those lovely pharmaceutical companies deign to look down upon us and actually develop a drug utilizing Pln149-PEP20 to, you know, actually help sick people, instead of trying to build monopolies or avoiding paying billions in taxes. Yeah, we couldn’t keep a straight face through that last sentence either.
Speed healing: The wavy wound gets the swirl
Did you know that wavy wounds heal faster than straight wounds? Well, we didn’t, but apparently quite a few people did, because somebody has been trying to figure out why wavy wounds heal faster than straight ones. Do the surgeons know about this? How about you dermatologists? Wavy over straight? We’re the media. We’re supposed to report this kind of stuff. Maybe hit us with a tweet next time you do something important, or push a TikTok our way, okay?
You could be more like the investigators at Nanyang Technological University in Singapore, who figured out the why and then released a statement about it.
They created synthetic wounds – some straight, some wavy – in micropatterned hydrogel substrates that mimicked human skin. Then they used an advanced optical technique known as particle image velocimetry to measure fluid flow and learn how cells moved to close the wound gaps.
The wavy wounds “induced more complex collective cell movements, such as a swirly, vortex-like motion,” according to the written statement from NTU Singapore. In the straight wounds, cell movements paralleled the wound front, “moving in straight lines like a marching band,” they pointed out, unlike some researchers who never call us unless they need money.
Complex epithelial cell movements are better, it turns out. Over an observation period of 64 hours the NTU team found that the healing efficiency of wavy gaps – measured by the area covered by the cells over time – is nearly five times faster than straight gaps.
The complex motion “enabled cells to quickly connect with similar cells on the opposite site of the wound edge, forming a bridge and closing the wavy wound gaps faster than straight gaps,” explained lead author Xu Hongmei, a doctoral student at NTU’s School of Mechanical and Aerospace Engineering, who seems to have time to toss out a tumblr or two to keep the press informed.
As for the rest of you, would it kill you to pick up a phone once in a while? Maybe let a journalist know that you’re still alive? We have feelings too, you know, and we worry.
A little Jekyll, a little Hyde, and a little shop of horrors
More “Little Shop of Horrors” references are coming, so be prepared.
We begin with Triphyophyllum peltatum. This woody vine is of great interest to medical and pharmaceutical researchers because its constituents have shown promise against pancreatic cancer and leukemia cells, among others, along with the pathogens that cause malaria and other diseases. There is another side, however. T. peltatum also has a tendency to turn into a realistic Audrey II when deprived.
No, of course they’re not craving human flesh, but it does become … carnivorous in its appetite.
T. peltatum, native to the West African tropics and not found in a New York florist shop, has the unique ability to change its diet and development based on the environmental circumstances. For some unknown reason, the leaves would develop adhesive traps in the form of sticky drops that capture insect prey. The plant is notoriously hard to grow, however, so no one could study the transformation under lab conditions. Until now.
A group of German scientists “exposed the plant to different stress factors, including deficiencies of various nutrients, and studied how it responded to each,” said Dr. Traud Winkelmann of Leibniz University Hannover. “Only in one case were we able to observe the formation of traps: in the case of a lack of phosphorus.”
Well, there you have it: phosphorus. We need it for healthy bones and teeth, which this plant doesn’t have to worry about, unlike its Tony Award–nominated counterpart. The investigators hope that their findings could lead to “future molecular analyses that will help understand the origins of carnivory,” but we’re guessing that a certain singing alien species will be left out of that research.
Expunging ‘penicillin allergy’: Your questions answered
Last month, I described a 28-year-old patient with a history of injection drug use who presented with pain in his left forearm. His history showed that, within the past 2 years, he’d been seen for cutaneous infections multiple times as an outpatient and in the emergency department. His records indicated that he was diagnosed with a penicillin allergy as a child when he developed a rash after receiving amoxicillin. I believed the next course of action should be to test for a penicillin allergy with an oral amoxicillin challenge.
Thank you for your excellent questions regarding this case. Great to hear the enthusiasm for testing for penicillin allergy!
One question focused on the course of action in the case of a mild or moderate IgE-mediated reaction after a single dose test with amoxicillin. Treatment for these reactions should include an antihistamine. I would reserve intravenous antihistamines for more severe cases, which also require treatment with a course of corticosteroids. However, the risk for a moderate to severe reaction to amoxicillin on retesting is quite low.
Clinicians need to exercise caution in the use of systemic corticosteroids. These drugs can be lifesaving, but even short courses of corticosteroids are associated with potentially serious adverse events. In a review of adverse events associated with short-course systemic corticosteroids among children, the rate of vomiting was 5.4%; behavioral change, 4.7%; and sleep disturbance, 4.3%. One child died after contracting herpes zoster, more than one-third of children developed elevated blood pressure, and 81.1% had evidence of suppression of the hypothalamic-pituitary-adrenal axis.
Among adults, short courses of systemic corticosteroids are associated with acute increases in the risks for gastrointestinal bleeding and hypertension. Cumulative exposure to short courses of corticosteroids over time results in higher risks for obesity, type 2 diabetes, and osteoporosis.
Another question prompted by this young man’s case focused on the durability of IgE reactions against penicillin. The IgE response to penicillin does indeed wane over time; 80% of patients with a previous true penicillin allergy can tolerate the antibiotic after 10 years. Thus, about 95% of patients with a remote history of penicillin allergy are tolerant of penicillin, and testing can be performed using the algorithm described.
Clinicians should avoid applying current guidelines for the evaluation of patients with penicillin allergy to other common drug allergies. The overall prevalence of sulfonamide allergy is 3%-8%, and the vast majority of these reactions follow treatment with trimethoprim-sulfamethoxazole. Sulfa allergy is even more common among persons living with HIV infection. The natural history of sulfa allergy is not as well established as penicillin allergy. Allergy testing is encouraged in these cases. Graded oral challenge testing is best reserved for patients who are unlikely to have a true sulfa allergy based on their history.
A version of this article first appeared on Medscape.com.
Last month, I described a 28-year-old patient with a history of injection drug use who presented with pain in his left forearm. His history showed that, within the past 2 years, he’d been seen for cutaneous infections multiple times as an outpatient and in the emergency department. His records indicated that he was diagnosed with a penicillin allergy as a child when he developed a rash after receiving amoxicillin. I believed the next course of action should be to test for a penicillin allergy with an oral amoxicillin challenge.
Thank you for your excellent questions regarding this case. Great to hear the enthusiasm for testing for penicillin allergy!
One question focused on the course of action in the case of a mild or moderate IgE-mediated reaction after a single dose test with amoxicillin. Treatment for these reactions should include an antihistamine. I would reserve intravenous antihistamines for more severe cases, which also require treatment with a course of corticosteroids. However, the risk for a moderate to severe reaction to amoxicillin on retesting is quite low.
Clinicians need to exercise caution in the use of systemic corticosteroids. These drugs can be lifesaving, but even short courses of corticosteroids are associated with potentially serious adverse events. In a review of adverse events associated with short-course systemic corticosteroids among children, the rate of vomiting was 5.4%; behavioral change, 4.7%; and sleep disturbance, 4.3%. One child died after contracting herpes zoster, more than one-third of children developed elevated blood pressure, and 81.1% had evidence of suppression of the hypothalamic-pituitary-adrenal axis.
Among adults, short courses of systemic corticosteroids are associated with acute increases in the risks for gastrointestinal bleeding and hypertension. Cumulative exposure to short courses of corticosteroids over time results in higher risks for obesity, type 2 diabetes, and osteoporosis.
Another question prompted by this young man’s case focused on the durability of IgE reactions against penicillin. The IgE response to penicillin does indeed wane over time; 80% of patients with a previous true penicillin allergy can tolerate the antibiotic after 10 years. Thus, about 95% of patients with a remote history of penicillin allergy are tolerant of penicillin, and testing can be performed using the algorithm described.
Clinicians should avoid applying current guidelines for the evaluation of patients with penicillin allergy to other common drug allergies. The overall prevalence of sulfonamide allergy is 3%-8%, and the vast majority of these reactions follow treatment with trimethoprim-sulfamethoxazole. Sulfa allergy is even more common among persons living with HIV infection. The natural history of sulfa allergy is not as well established as penicillin allergy. Allergy testing is encouraged in these cases. Graded oral challenge testing is best reserved for patients who are unlikely to have a true sulfa allergy based on their history.
A version of this article first appeared on Medscape.com.
Last month, I described a 28-year-old patient with a history of injection drug use who presented with pain in his left forearm. His history showed that, within the past 2 years, he’d been seen for cutaneous infections multiple times as an outpatient and in the emergency department. His records indicated that he was diagnosed with a penicillin allergy as a child when he developed a rash after receiving amoxicillin. I believed the next course of action should be to test for a penicillin allergy with an oral amoxicillin challenge.
Thank you for your excellent questions regarding this case. Great to hear the enthusiasm for testing for penicillin allergy!
One question focused on the course of action in the case of a mild or moderate IgE-mediated reaction after a single dose test with amoxicillin. Treatment for these reactions should include an antihistamine. I would reserve intravenous antihistamines for more severe cases, which also require treatment with a course of corticosteroids. However, the risk for a moderate to severe reaction to amoxicillin on retesting is quite low.
Clinicians need to exercise caution in the use of systemic corticosteroids. These drugs can be lifesaving, but even short courses of corticosteroids are associated with potentially serious adverse events. In a review of adverse events associated with short-course systemic corticosteroids among children, the rate of vomiting was 5.4%; behavioral change, 4.7%; and sleep disturbance, 4.3%. One child died after contracting herpes zoster, more than one-third of children developed elevated blood pressure, and 81.1% had evidence of suppression of the hypothalamic-pituitary-adrenal axis.
Among adults, short courses of systemic corticosteroids are associated with acute increases in the risks for gastrointestinal bleeding and hypertension. Cumulative exposure to short courses of corticosteroids over time results in higher risks for obesity, type 2 diabetes, and osteoporosis.
Another question prompted by this young man’s case focused on the durability of IgE reactions against penicillin. The IgE response to penicillin does indeed wane over time; 80% of patients with a previous true penicillin allergy can tolerate the antibiotic after 10 years. Thus, about 95% of patients with a remote history of penicillin allergy are tolerant of penicillin, and testing can be performed using the algorithm described.
Clinicians should avoid applying current guidelines for the evaluation of patients with penicillin allergy to other common drug allergies. The overall prevalence of sulfonamide allergy is 3%-8%, and the vast majority of these reactions follow treatment with trimethoprim-sulfamethoxazole. Sulfa allergy is even more common among persons living with HIV infection. The natural history of sulfa allergy is not as well established as penicillin allergy. Allergy testing is encouraged in these cases. Graded oral challenge testing is best reserved for patients who are unlikely to have a true sulfa allergy based on their history.
A version of this article first appeared on Medscape.com.
Emotional eating isn’t all emotional
“Food gives me ‘hugs,’ ” Ms. S* said as her eyes lit up. Finally, after weeks of working together, she could articulate her complex relationship with food. She had been struggling to explain why she continued to eat when she was full or consumed foods she knew wouldn’t help her health.
Like millions of people struggling with their weight or the disease of obesity, Ms. S had tried multiple diets and programs but continued to return to unhelpful eating patterns. Ms. S was an emotional eater, and the pandemic only worsened her emotional eating. As a single professional forced to work from home during the pandemic, she became lonely. She went from working in a busy downtown office, training for half-marathons, and teaching live workout sessions to being alone daily. Her only “real” human interaction was when she ordered daily delivery meals of her favorite comfort foods. As a person with type 2 diabetes, she knew that her delivery habit was wrecking her health, but willpower wasn’t enough to make her stop.
Her psychologist referred her to our virtual integrative obesity practice to help her lose weight and find long-term solutions. Ms. S admitted that she knew what she was doing as an emotional eater. But like many emotional eaters, she didn’t know why or how to switch from emotional eating to eating based on her biological hunger signals. As a trained obesity expert and recovering emotional eater of 8 years, personally and professionally I can appreciate the challenges of emotional eating and how it can sabotage even the best weight loss plan. In this article, I will share facts and feelings that drive emotional eating. I aim to empower clinicians seeking to help patients with emotional eating.
Fact: Emotional eating isn’t all emotional
It’s important not to dismiss emotional eating as all emotion driven. Recall that hunger is hormonally regulated. There are two main hunger pathways: the homeostatic pathway and the hedonic pathway. The homeostatic pathway is our biological hunger pathway and is driven by the need for energy in calories. Conversely, hedonic eating is pleasure-driven and uses emotional stimuli to “bypass” the physical hunger/satisfaction signals.
Emotional eating falls under the hedonic pathway. As clinicians, the first step in helping a patient struggling with emotional eating is empathetically listening, then assessing for any physiologic causes.
Several factors can disrupt physiologic appetite regulation, such as sleep disturbances; high stress levels; and many medical conditions, including but not limited to obesity, diabetes, and polycystic ovarian syndrome. Such factors as insulin resistance and inflammation are a common link in these conditions. Both contribute to the pathophysiology of the changes in appetite and can influence other hormones that lead to reduced satisfaction after eating. Furthermore, mental health conditions may disrupt levels of neurotransmitters such as serotonin and dopamine, which can also cause appetite changes.
These settings of physiologically disrupted appetite can trigger hedonic eating. But the relationship is complex. For example, one way to research hedonic eating is by using the Power of Food Scale. Functional MRI studies show that people with higher Power of Food Scale readings have more brain activity in the visual cortex when they see highly palatable foods. While more studies are needed to better understand the clinical implications of this finding, it’s yet another indicator that “emotional” eating isn’t all emotional. It’s also physiologic.
Feelings: Patterns, personality, places, psychological factors
Physiology only explains part of emotional eating. Like Ms. S, emotional eaters have strong emotional connections to food and behavior patterns. Often, physiologic cues have been coupled with psychological habits.
For example, menses is a common physiologic trigger for stress-eating for many of my patients. Studies have shown that in addition to iron levels changing during menses, calcium, magnesium, and phosphorous levels also change. Emotionally, the discomfort of “that time of the month” can lead to solace in comfort foods such as chocolate in different forms. But this isn’t surprising, as cacao and its derivative, chocolate, are rich in iron and other minerals. The chocolate is actually addressing a physical and emotional need. It can be helpful to point out this association to your patients. Suggest choosing a lower-sugar form of chocolate, such as dark chocolate, or even trying cacao nibs, while addressing any emotions.
But physiologic conditions and patterns aren’t the only emotional eating triggers. Places and psychological conditions can also trigger emotional eating.
Places and people
Celebrations, vacations, proximity to certain restaurants, exposure to food marketing, and major life shifts can lead to increased hedonic eating. Helping patients recognize this connection opens the door to advance preparation for these situations.
Psychological conditions can be connected to emotional eating. It’s important to screen for mental health conditions and past traumas. For example, emotional eating could be a symptom of binge eating disorder, major depression, or generalized anxiety disorder. Childhood trauma is associated with disordered eating. The adverse childhood events quiz can be used clinically.
Emotional eating can lead to feelings of guilt, shame, and negative self-talk. It’s helpful to offer patients reassurance and encourage self-compassion. After all, it’s natural to eat. The goal isn’t to stop eating but to eat on the basis of physiologic needs.
Putting it together: Addressing the facts and feelings of emotional eating
1. Treat biological causes that impact physiologic hunger and trigger emotional eating.
2. Triggers: Address patterns, places/people, psychological events.
3. Transition to non-food rewards; the key to emotional eating is eating. While healthier substitutes can be a short-term solution for improving eating behaviors, ultimately, helping patients find non-food ways to address emotions is invaluable.
4. Stress management: Offer your patients ways to decrease stress levels through mindfulness and other techniques.
5. Professional support: Creating a multidisciplinary team is helpful, given the complexity of emotional eating. In addition to the primary care physician/clinician, other team members may include:
- Psychologist
- Psychiatrist
- coach and/or certified wellness coaches
- Obesity specialist
Back to Ms. S
Ms. S is doing well. We started her on a GLP-1 agonist to address her underlying insulin resistance. Together we’ve found creative ways to satisfy her loneliness, such as volunteering and teaching virtual workout classes. Her emotional eating has decreased by over 60%, and we continue to discover new strategies to address her emotional eating triggers.
Conclusion
Despite being common, the impact of emotional eating is often minimized. With no DSM-5 criteria or ICD-11 code, it’s easy to dismiss emotional eating clinically. However, emotional eating is common and associated with weight gain.
In light of the obesity epidemic, this significance can’t be overlooked. Thankfully we have groundbreaking medications to address the homeostatic hunger pathway and physiologic drivers of emotional eating, but they’re not a substitute for addressing the psychosocial components of emotional eating.
As clinicians, we can have a meaningful impact on our patients’ lives beyond writing a prescription.
*Name/initial changed for privacy.
Sylvia Gonsahn-Bollie, MD, DipABOM, is an integrative obesity specialist focused on individualized solutions for emotional and biological overeating.
A version of this article first appeared on Medscape.com.
“Food gives me ‘hugs,’ ” Ms. S* said as her eyes lit up. Finally, after weeks of working together, she could articulate her complex relationship with food. She had been struggling to explain why she continued to eat when she was full or consumed foods she knew wouldn’t help her health.
Like millions of people struggling with their weight or the disease of obesity, Ms. S had tried multiple diets and programs but continued to return to unhelpful eating patterns. Ms. S was an emotional eater, and the pandemic only worsened her emotional eating. As a single professional forced to work from home during the pandemic, she became lonely. She went from working in a busy downtown office, training for half-marathons, and teaching live workout sessions to being alone daily. Her only “real” human interaction was when she ordered daily delivery meals of her favorite comfort foods. As a person with type 2 diabetes, she knew that her delivery habit was wrecking her health, but willpower wasn’t enough to make her stop.
Her psychologist referred her to our virtual integrative obesity practice to help her lose weight and find long-term solutions. Ms. S admitted that she knew what she was doing as an emotional eater. But like many emotional eaters, she didn’t know why or how to switch from emotional eating to eating based on her biological hunger signals. As a trained obesity expert and recovering emotional eater of 8 years, personally and professionally I can appreciate the challenges of emotional eating and how it can sabotage even the best weight loss plan. In this article, I will share facts and feelings that drive emotional eating. I aim to empower clinicians seeking to help patients with emotional eating.
Fact: Emotional eating isn’t all emotional
It’s important not to dismiss emotional eating as all emotion driven. Recall that hunger is hormonally regulated. There are two main hunger pathways: the homeostatic pathway and the hedonic pathway. The homeostatic pathway is our biological hunger pathway and is driven by the need for energy in calories. Conversely, hedonic eating is pleasure-driven and uses emotional stimuli to “bypass” the physical hunger/satisfaction signals.
Emotional eating falls under the hedonic pathway. As clinicians, the first step in helping a patient struggling with emotional eating is empathetically listening, then assessing for any physiologic causes.
Several factors can disrupt physiologic appetite regulation, such as sleep disturbances; high stress levels; and many medical conditions, including but not limited to obesity, diabetes, and polycystic ovarian syndrome. Such factors as insulin resistance and inflammation are a common link in these conditions. Both contribute to the pathophysiology of the changes in appetite and can influence other hormones that lead to reduced satisfaction after eating. Furthermore, mental health conditions may disrupt levels of neurotransmitters such as serotonin and dopamine, which can also cause appetite changes.
These settings of physiologically disrupted appetite can trigger hedonic eating. But the relationship is complex. For example, one way to research hedonic eating is by using the Power of Food Scale. Functional MRI studies show that people with higher Power of Food Scale readings have more brain activity in the visual cortex when they see highly palatable foods. While more studies are needed to better understand the clinical implications of this finding, it’s yet another indicator that “emotional” eating isn’t all emotional. It’s also physiologic.
Feelings: Patterns, personality, places, psychological factors
Physiology only explains part of emotional eating. Like Ms. S, emotional eaters have strong emotional connections to food and behavior patterns. Often, physiologic cues have been coupled with psychological habits.
For example, menses is a common physiologic trigger for stress-eating for many of my patients. Studies have shown that in addition to iron levels changing during menses, calcium, magnesium, and phosphorous levels also change. Emotionally, the discomfort of “that time of the month” can lead to solace in comfort foods such as chocolate in different forms. But this isn’t surprising, as cacao and its derivative, chocolate, are rich in iron and other minerals. The chocolate is actually addressing a physical and emotional need. It can be helpful to point out this association to your patients. Suggest choosing a lower-sugar form of chocolate, such as dark chocolate, or even trying cacao nibs, while addressing any emotions.
But physiologic conditions and patterns aren’t the only emotional eating triggers. Places and psychological conditions can also trigger emotional eating.
Places and people
Celebrations, vacations, proximity to certain restaurants, exposure to food marketing, and major life shifts can lead to increased hedonic eating. Helping patients recognize this connection opens the door to advance preparation for these situations.
Psychological conditions can be connected to emotional eating. It’s important to screen for mental health conditions and past traumas. For example, emotional eating could be a symptom of binge eating disorder, major depression, or generalized anxiety disorder. Childhood trauma is associated with disordered eating. The adverse childhood events quiz can be used clinically.
Emotional eating can lead to feelings of guilt, shame, and negative self-talk. It’s helpful to offer patients reassurance and encourage self-compassion. After all, it’s natural to eat. The goal isn’t to stop eating but to eat on the basis of physiologic needs.
Putting it together: Addressing the facts and feelings of emotional eating
1. Treat biological causes that impact physiologic hunger and trigger emotional eating.
2. Triggers: Address patterns, places/people, psychological events.
3. Transition to non-food rewards; the key to emotional eating is eating. While healthier substitutes can be a short-term solution for improving eating behaviors, ultimately, helping patients find non-food ways to address emotions is invaluable.
4. Stress management: Offer your patients ways to decrease stress levels through mindfulness and other techniques.
5. Professional support: Creating a multidisciplinary team is helpful, given the complexity of emotional eating. In addition to the primary care physician/clinician, other team members may include:
- Psychologist
- Psychiatrist
- coach and/or certified wellness coaches
- Obesity specialist
Back to Ms. S
Ms. S is doing well. We started her on a GLP-1 agonist to address her underlying insulin resistance. Together we’ve found creative ways to satisfy her loneliness, such as volunteering and teaching virtual workout classes. Her emotional eating has decreased by over 60%, and we continue to discover new strategies to address her emotional eating triggers.
Conclusion
Despite being common, the impact of emotional eating is often minimized. With no DSM-5 criteria or ICD-11 code, it’s easy to dismiss emotional eating clinically. However, emotional eating is common and associated with weight gain.
In light of the obesity epidemic, this significance can’t be overlooked. Thankfully we have groundbreaking medications to address the homeostatic hunger pathway and physiologic drivers of emotional eating, but they’re not a substitute for addressing the psychosocial components of emotional eating.
As clinicians, we can have a meaningful impact on our patients’ lives beyond writing a prescription.
*Name/initial changed for privacy.
Sylvia Gonsahn-Bollie, MD, DipABOM, is an integrative obesity specialist focused on individualized solutions for emotional and biological overeating.
A version of this article first appeared on Medscape.com.
“Food gives me ‘hugs,’ ” Ms. S* said as her eyes lit up. Finally, after weeks of working together, she could articulate her complex relationship with food. She had been struggling to explain why she continued to eat when she was full or consumed foods she knew wouldn’t help her health.
Like millions of people struggling with their weight or the disease of obesity, Ms. S had tried multiple diets and programs but continued to return to unhelpful eating patterns. Ms. S was an emotional eater, and the pandemic only worsened her emotional eating. As a single professional forced to work from home during the pandemic, she became lonely. She went from working in a busy downtown office, training for half-marathons, and teaching live workout sessions to being alone daily. Her only “real” human interaction was when she ordered daily delivery meals of her favorite comfort foods. As a person with type 2 diabetes, she knew that her delivery habit was wrecking her health, but willpower wasn’t enough to make her stop.
Her psychologist referred her to our virtual integrative obesity practice to help her lose weight and find long-term solutions. Ms. S admitted that she knew what she was doing as an emotional eater. But like many emotional eaters, she didn’t know why or how to switch from emotional eating to eating based on her biological hunger signals. As a trained obesity expert and recovering emotional eater of 8 years, personally and professionally I can appreciate the challenges of emotional eating and how it can sabotage even the best weight loss plan. In this article, I will share facts and feelings that drive emotional eating. I aim to empower clinicians seeking to help patients with emotional eating.
Fact: Emotional eating isn’t all emotional
It’s important not to dismiss emotional eating as all emotion driven. Recall that hunger is hormonally regulated. There are two main hunger pathways: the homeostatic pathway and the hedonic pathway. The homeostatic pathway is our biological hunger pathway and is driven by the need for energy in calories. Conversely, hedonic eating is pleasure-driven and uses emotional stimuli to “bypass” the physical hunger/satisfaction signals.
Emotional eating falls under the hedonic pathway. As clinicians, the first step in helping a patient struggling with emotional eating is empathetically listening, then assessing for any physiologic causes.
Several factors can disrupt physiologic appetite regulation, such as sleep disturbances; high stress levels; and many medical conditions, including but not limited to obesity, diabetes, and polycystic ovarian syndrome. Such factors as insulin resistance and inflammation are a common link in these conditions. Both contribute to the pathophysiology of the changes in appetite and can influence other hormones that lead to reduced satisfaction after eating. Furthermore, mental health conditions may disrupt levels of neurotransmitters such as serotonin and dopamine, which can also cause appetite changes.
These settings of physiologically disrupted appetite can trigger hedonic eating. But the relationship is complex. For example, one way to research hedonic eating is by using the Power of Food Scale. Functional MRI studies show that people with higher Power of Food Scale readings have more brain activity in the visual cortex when they see highly palatable foods. While more studies are needed to better understand the clinical implications of this finding, it’s yet another indicator that “emotional” eating isn’t all emotional. It’s also physiologic.
Feelings: Patterns, personality, places, psychological factors
Physiology only explains part of emotional eating. Like Ms. S, emotional eaters have strong emotional connections to food and behavior patterns. Often, physiologic cues have been coupled with psychological habits.
For example, menses is a common physiologic trigger for stress-eating for many of my patients. Studies have shown that in addition to iron levels changing during menses, calcium, magnesium, and phosphorous levels also change. Emotionally, the discomfort of “that time of the month” can lead to solace in comfort foods such as chocolate in different forms. But this isn’t surprising, as cacao and its derivative, chocolate, are rich in iron and other minerals. The chocolate is actually addressing a physical and emotional need. It can be helpful to point out this association to your patients. Suggest choosing a lower-sugar form of chocolate, such as dark chocolate, or even trying cacao nibs, while addressing any emotions.
But physiologic conditions and patterns aren’t the only emotional eating triggers. Places and psychological conditions can also trigger emotional eating.
Places and people
Celebrations, vacations, proximity to certain restaurants, exposure to food marketing, and major life shifts can lead to increased hedonic eating. Helping patients recognize this connection opens the door to advance preparation for these situations.
Psychological conditions can be connected to emotional eating. It’s important to screen for mental health conditions and past traumas. For example, emotional eating could be a symptom of binge eating disorder, major depression, or generalized anxiety disorder. Childhood trauma is associated with disordered eating. The adverse childhood events quiz can be used clinically.
Emotional eating can lead to feelings of guilt, shame, and negative self-talk. It’s helpful to offer patients reassurance and encourage self-compassion. After all, it’s natural to eat. The goal isn’t to stop eating but to eat on the basis of physiologic needs.
Putting it together: Addressing the facts and feelings of emotional eating
1. Treat biological causes that impact physiologic hunger and trigger emotional eating.
2. Triggers: Address patterns, places/people, psychological events.
3. Transition to non-food rewards; the key to emotional eating is eating. While healthier substitutes can be a short-term solution for improving eating behaviors, ultimately, helping patients find non-food ways to address emotions is invaluable.
4. Stress management: Offer your patients ways to decrease stress levels through mindfulness and other techniques.
5. Professional support: Creating a multidisciplinary team is helpful, given the complexity of emotional eating. In addition to the primary care physician/clinician, other team members may include:
- Psychologist
- Psychiatrist
- coach and/or certified wellness coaches
- Obesity specialist
Back to Ms. S
Ms. S is doing well. We started her on a GLP-1 agonist to address her underlying insulin resistance. Together we’ve found creative ways to satisfy her loneliness, such as volunteering and teaching virtual workout classes. Her emotional eating has decreased by over 60%, and we continue to discover new strategies to address her emotional eating triggers.
Conclusion
Despite being common, the impact of emotional eating is often minimized. With no DSM-5 criteria or ICD-11 code, it’s easy to dismiss emotional eating clinically. However, emotional eating is common and associated with weight gain.
In light of the obesity epidemic, this significance can’t be overlooked. Thankfully we have groundbreaking medications to address the homeostatic hunger pathway and physiologic drivers of emotional eating, but they’re not a substitute for addressing the psychosocial components of emotional eating.
As clinicians, we can have a meaningful impact on our patients’ lives beyond writing a prescription.
*Name/initial changed for privacy.
Sylvia Gonsahn-Bollie, MD, DipABOM, is an integrative obesity specialist focused on individualized solutions for emotional and biological overeating.
A version of this article first appeared on Medscape.com.
Nonpharmacologic therapies for T2D: Five things to know
According to the Centers for Disease Control and Prevention National Diabetes Statistic Report, there are more than 37 million adults aged 18 years or older with diabetes in the United States, representing 14.7% of the adult population. Approximately 90%-95% of people diagnosed with diabetes have type 2 diabetes (T2D). An increasing aging population with T2D and a disparate incidence and burden of disease in African American and Hispanic populations raise important care considerations in effective disease assessment and management, especially in primary care, where the majority of diabetes management occurs.
This extends to the need for quality patient education in an effort to give persons with diabetes a better understanding of what it’s like to live with the disease.
Here are five things to know about nonpharmacologic therapies for effective T2D management.
1. Understand and treat the person before the disease.
Diabetes is a complex and unrelenting disease of self-management, requiring an individualized care approach to achieve optimal health outcomes and quality of life for persons living with this condition. Over 90% of care is provided by the person with diabetes, therefore understanding the lived world of the person with diabetes and its connected impact on self-care is critical to establishing effective treatment recommendations, especially for people from racial and ethnic minority groups and lower socioeconomic status where diabetes disparities are highest. Disease prevalence, cost of care, and disease burden are driven by social determinants of health (SDOH) factors that need to be assessed, and strategies addressing causative factors need to be implemented. SDOH factors, including the built environment, safety, financial status, education, food access, health care access, and social support, directly affect the ability of a person with diabetes to effectively implement treatment recommendations, including access to new medications. The adoption of a shared decision-making approach is key to person-centered care. Shared decision-making promotes a positive communication feedback loop, therapeutic patient-care team relationship, and collaborative plan of care between the person with diabetes and the care team. It also supports the establishment of mutual respect between the person with diabetes and the care team members. This cultivates the strong, open, and authentic partnership needed for effective chronic disease management.
2. Quality diabetes education is the foundation for effective self-care.
Diabetes self-management education and support (DSMES) is a fundamental component of diabetes care and ensures patients have the knowledge, skills, motivation, and resources necessary for effectively managing this condition. Despite treatment advances and the evidence base for DSMES, less than 5% of Medicare beneficiaries and 6.8% of privately insured beneficiaries have utilized its services, and this is a likely contributor to the lack of improvement for achieving national diabetes clinical targets. The Association of Diabetes Care and Education Specialists (ADCES7) Self-Care Behaviors provides an evidence-based framework for an optimal DSMES curriculum, incorporating the self-care behaviors of healthy coping (e.g., having a positive attitude toward diabetes self-management), nutritious eating, being active, taking medication, monitoring, reducing risk, and problem-solving.
There are four core times to implement and adapt referral for DSMES: (1) at diagnosis, (2) annually or when not meeting targets, (3) when complications arise, and (4) with transitions in life and care. DSMES referrals should be made for programs accredited by the ADCES or American Diabetes Association (ADA) and led by expert Certified Diabetes Care and Education Specialists (CDCES). The multidisciplinary composition and clinical skill level of CDCES make them a highly valued member of the diabetes care team. CDCES have demonstrated not only diabetes education expertise but are involved in broader health care roles to include population health management, technology integration, mitigation of therapeutic inertia, quality improvement activity, and delivery of cost-effective care.
3. Establish a strong foundation in lifestyle medicine.
Lifestyle medicine encompasses healthy eating, physical activity, restorative sleep, stress management, avoidance of risky behaviors, and positive social connections. It has also been strongly connected as a primary modality to prevent and treat chronic conditions like T2D. Lifestyle modifications have been noted in reducing the incidence of developing diabetes, reversing disease, improving clinical markers such as A1c and lipids, weight reduction, reducing use of medications, and improving quality of life. The multidisciplinary care team and CDCES can support the empowerment of individuals with T2D to develop the life skills and knowledge needed to establish positive self-care behaviors and successfully achieve health goals. Lifestyle medicine is not a replacement for pharmacologic interventions but rather serves as an adjunct when medication management is required.
4. Harness technology in diabetes treatment and care delivery.
Diabetes technology is advancing swiftly and includes glucose monitors, medication delivery devices, data-sharing platforms, and disease self-management applications. Combined with education and support, diabetes technology has been shown to have a positive clinical and personal impact on disease outcomes and quality of life. Regardless of its benefits, at times technology can seem overwhelming for the person with diabetes and the care team. Diabetes Care and Education Specialists (DCES) can support the care team and people living with diabetes to effectively identify, implement, and evaluate patient-centered diabetes technologies, as well as implement processes to drive clinical efficiencies and sustainability. Patient-generated health data reports can provide the care team with effective and proficient evaluation of diabetes care and needed treatment changes.
The expansion of telehealth during the COVID-19 pandemic, including real-time and asynchronous approaches, coupled with in-person care team visits, has resulted in improved access to diabetes care and education. Moreover, there continues to be an expanding health system focus on improving access to care beyond traditional brick and mortar solutions. Telehealth poses one possible access solution for people living with diabetes for whom factors such as transportation, remote geographies, and physical limitations affect their ability to attend in-person care visits.
5. Assess and address diabetes-related distress.
The persistent nature of diabetes self-care expectations and the impact on lifestyle behaviors, medication adherence, and glycemic control demands the need for assessment and treatment of diabetes-related distress (DRD). DRD can be expressed as shame, guilt, anger, fear, and frustration in combination with the everyday context of life priorities and stressors. An assessment of diabetes distress, utilizing a simple scale, should be included as part of an annual therapeutic diabetes care plan. The ADA Standards of Care in Diabetes recommends assessing patients’ psychological and social situations as an ongoing part of medical management, including an annual screening for depression and other psychological problems. The prevalence of depression is nearly twice as high in people with T2D than in the general population and can significantly influence patients’ ability to self-manage their diabetes and achieve healthy outcomes. Assessment and treatment of psychosocial components of care can result in significant improvements in A1c and other positive outcomes, including quality of life.
Kellie M. Rodriguez, director of the global diabetes program at Parkland Health, Dallas, Tex., disclosed ties with the Association of Diabetes Care and Education Specialists.
A version of this article originally appeared on Medscape.com.
According to the Centers for Disease Control and Prevention National Diabetes Statistic Report, there are more than 37 million adults aged 18 years or older with diabetes in the United States, representing 14.7% of the adult population. Approximately 90%-95% of people diagnosed with diabetes have type 2 diabetes (T2D). An increasing aging population with T2D and a disparate incidence and burden of disease in African American and Hispanic populations raise important care considerations in effective disease assessment and management, especially in primary care, where the majority of diabetes management occurs.
This extends to the need for quality patient education in an effort to give persons with diabetes a better understanding of what it’s like to live with the disease.
Here are five things to know about nonpharmacologic therapies for effective T2D management.
1. Understand and treat the person before the disease.
Diabetes is a complex and unrelenting disease of self-management, requiring an individualized care approach to achieve optimal health outcomes and quality of life for persons living with this condition. Over 90% of care is provided by the person with diabetes, therefore understanding the lived world of the person with diabetes and its connected impact on self-care is critical to establishing effective treatment recommendations, especially for people from racial and ethnic minority groups and lower socioeconomic status where diabetes disparities are highest. Disease prevalence, cost of care, and disease burden are driven by social determinants of health (SDOH) factors that need to be assessed, and strategies addressing causative factors need to be implemented. SDOH factors, including the built environment, safety, financial status, education, food access, health care access, and social support, directly affect the ability of a person with diabetes to effectively implement treatment recommendations, including access to new medications. The adoption of a shared decision-making approach is key to person-centered care. Shared decision-making promotes a positive communication feedback loop, therapeutic patient-care team relationship, and collaborative plan of care between the person with diabetes and the care team. It also supports the establishment of mutual respect between the person with diabetes and the care team members. This cultivates the strong, open, and authentic partnership needed for effective chronic disease management.
2. Quality diabetes education is the foundation for effective self-care.
Diabetes self-management education and support (DSMES) is a fundamental component of diabetes care and ensures patients have the knowledge, skills, motivation, and resources necessary for effectively managing this condition. Despite treatment advances and the evidence base for DSMES, less than 5% of Medicare beneficiaries and 6.8% of privately insured beneficiaries have utilized its services, and this is a likely contributor to the lack of improvement for achieving national diabetes clinical targets. The Association of Diabetes Care and Education Specialists (ADCES7) Self-Care Behaviors provides an evidence-based framework for an optimal DSMES curriculum, incorporating the self-care behaviors of healthy coping (e.g., having a positive attitude toward diabetes self-management), nutritious eating, being active, taking medication, monitoring, reducing risk, and problem-solving.
There are four core times to implement and adapt referral for DSMES: (1) at diagnosis, (2) annually or when not meeting targets, (3) when complications arise, and (4) with transitions in life and care. DSMES referrals should be made for programs accredited by the ADCES or American Diabetes Association (ADA) and led by expert Certified Diabetes Care and Education Specialists (CDCES). The multidisciplinary composition and clinical skill level of CDCES make them a highly valued member of the diabetes care team. CDCES have demonstrated not only diabetes education expertise but are involved in broader health care roles to include population health management, technology integration, mitigation of therapeutic inertia, quality improvement activity, and delivery of cost-effective care.
3. Establish a strong foundation in lifestyle medicine.
Lifestyle medicine encompasses healthy eating, physical activity, restorative sleep, stress management, avoidance of risky behaviors, and positive social connections. It has also been strongly connected as a primary modality to prevent and treat chronic conditions like T2D. Lifestyle modifications have been noted in reducing the incidence of developing diabetes, reversing disease, improving clinical markers such as A1c and lipids, weight reduction, reducing use of medications, and improving quality of life. The multidisciplinary care team and CDCES can support the empowerment of individuals with T2D to develop the life skills and knowledge needed to establish positive self-care behaviors and successfully achieve health goals. Lifestyle medicine is not a replacement for pharmacologic interventions but rather serves as an adjunct when medication management is required.
4. Harness technology in diabetes treatment and care delivery.
Diabetes technology is advancing swiftly and includes glucose monitors, medication delivery devices, data-sharing platforms, and disease self-management applications. Combined with education and support, diabetes technology has been shown to have a positive clinical and personal impact on disease outcomes and quality of life. Regardless of its benefits, at times technology can seem overwhelming for the person with diabetes and the care team. Diabetes Care and Education Specialists (DCES) can support the care team and people living with diabetes to effectively identify, implement, and evaluate patient-centered diabetes technologies, as well as implement processes to drive clinical efficiencies and sustainability. Patient-generated health data reports can provide the care team with effective and proficient evaluation of diabetes care and needed treatment changes.
The expansion of telehealth during the COVID-19 pandemic, including real-time and asynchronous approaches, coupled with in-person care team visits, has resulted in improved access to diabetes care and education. Moreover, there continues to be an expanding health system focus on improving access to care beyond traditional brick and mortar solutions. Telehealth poses one possible access solution for people living with diabetes for whom factors such as transportation, remote geographies, and physical limitations affect their ability to attend in-person care visits.
5. Assess and address diabetes-related distress.
The persistent nature of diabetes self-care expectations and the impact on lifestyle behaviors, medication adherence, and glycemic control demands the need for assessment and treatment of diabetes-related distress (DRD). DRD can be expressed as shame, guilt, anger, fear, and frustration in combination with the everyday context of life priorities and stressors. An assessment of diabetes distress, utilizing a simple scale, should be included as part of an annual therapeutic diabetes care plan. The ADA Standards of Care in Diabetes recommends assessing patients’ psychological and social situations as an ongoing part of medical management, including an annual screening for depression and other psychological problems. The prevalence of depression is nearly twice as high in people with T2D than in the general population and can significantly influence patients’ ability to self-manage their diabetes and achieve healthy outcomes. Assessment and treatment of psychosocial components of care can result in significant improvements in A1c and other positive outcomes, including quality of life.
Kellie M. Rodriguez, director of the global diabetes program at Parkland Health, Dallas, Tex., disclosed ties with the Association of Diabetes Care and Education Specialists.
A version of this article originally appeared on Medscape.com.
According to the Centers for Disease Control and Prevention National Diabetes Statistic Report, there are more than 37 million adults aged 18 years or older with diabetes in the United States, representing 14.7% of the adult population. Approximately 90%-95% of people diagnosed with diabetes have type 2 diabetes (T2D). An increasing aging population with T2D and a disparate incidence and burden of disease in African American and Hispanic populations raise important care considerations in effective disease assessment and management, especially in primary care, where the majority of diabetes management occurs.
This extends to the need for quality patient education in an effort to give persons with diabetes a better understanding of what it’s like to live with the disease.
Here are five things to know about nonpharmacologic therapies for effective T2D management.
1. Understand and treat the person before the disease.
Diabetes is a complex and unrelenting disease of self-management, requiring an individualized care approach to achieve optimal health outcomes and quality of life for persons living with this condition. Over 90% of care is provided by the person with diabetes, therefore understanding the lived world of the person with diabetes and its connected impact on self-care is critical to establishing effective treatment recommendations, especially for people from racial and ethnic minority groups and lower socioeconomic status where diabetes disparities are highest. Disease prevalence, cost of care, and disease burden are driven by social determinants of health (SDOH) factors that need to be assessed, and strategies addressing causative factors need to be implemented. SDOH factors, including the built environment, safety, financial status, education, food access, health care access, and social support, directly affect the ability of a person with diabetes to effectively implement treatment recommendations, including access to new medications. The adoption of a shared decision-making approach is key to person-centered care. Shared decision-making promotes a positive communication feedback loop, therapeutic patient-care team relationship, and collaborative plan of care between the person with diabetes and the care team. It also supports the establishment of mutual respect between the person with diabetes and the care team members. This cultivates the strong, open, and authentic partnership needed for effective chronic disease management.
2. Quality diabetes education is the foundation for effective self-care.
Diabetes self-management education and support (DSMES) is a fundamental component of diabetes care and ensures patients have the knowledge, skills, motivation, and resources necessary for effectively managing this condition. Despite treatment advances and the evidence base for DSMES, less than 5% of Medicare beneficiaries and 6.8% of privately insured beneficiaries have utilized its services, and this is a likely contributor to the lack of improvement for achieving national diabetes clinical targets. The Association of Diabetes Care and Education Specialists (ADCES7) Self-Care Behaviors provides an evidence-based framework for an optimal DSMES curriculum, incorporating the self-care behaviors of healthy coping (e.g., having a positive attitude toward diabetes self-management), nutritious eating, being active, taking medication, monitoring, reducing risk, and problem-solving.
There are four core times to implement and adapt referral for DSMES: (1) at diagnosis, (2) annually or when not meeting targets, (3) when complications arise, and (4) with transitions in life and care. DSMES referrals should be made for programs accredited by the ADCES or American Diabetes Association (ADA) and led by expert Certified Diabetes Care and Education Specialists (CDCES). The multidisciplinary composition and clinical skill level of CDCES make them a highly valued member of the diabetes care team. CDCES have demonstrated not only diabetes education expertise but are involved in broader health care roles to include population health management, technology integration, mitigation of therapeutic inertia, quality improvement activity, and delivery of cost-effective care.
3. Establish a strong foundation in lifestyle medicine.
Lifestyle medicine encompasses healthy eating, physical activity, restorative sleep, stress management, avoidance of risky behaviors, and positive social connections. It has also been strongly connected as a primary modality to prevent and treat chronic conditions like T2D. Lifestyle modifications have been noted in reducing the incidence of developing diabetes, reversing disease, improving clinical markers such as A1c and lipids, weight reduction, reducing use of medications, and improving quality of life. The multidisciplinary care team and CDCES can support the empowerment of individuals with T2D to develop the life skills and knowledge needed to establish positive self-care behaviors and successfully achieve health goals. Lifestyle medicine is not a replacement for pharmacologic interventions but rather serves as an adjunct when medication management is required.
4. Harness technology in diabetes treatment and care delivery.
Diabetes technology is advancing swiftly and includes glucose monitors, medication delivery devices, data-sharing platforms, and disease self-management applications. Combined with education and support, diabetes technology has been shown to have a positive clinical and personal impact on disease outcomes and quality of life. Regardless of its benefits, at times technology can seem overwhelming for the person with diabetes and the care team. Diabetes Care and Education Specialists (DCES) can support the care team and people living with diabetes to effectively identify, implement, and evaluate patient-centered diabetes technologies, as well as implement processes to drive clinical efficiencies and sustainability. Patient-generated health data reports can provide the care team with effective and proficient evaluation of diabetes care and needed treatment changes.
The expansion of telehealth during the COVID-19 pandemic, including real-time and asynchronous approaches, coupled with in-person care team visits, has resulted in improved access to diabetes care and education. Moreover, there continues to be an expanding health system focus on improving access to care beyond traditional brick and mortar solutions. Telehealth poses one possible access solution for people living with diabetes for whom factors such as transportation, remote geographies, and physical limitations affect their ability to attend in-person care visits.
5. Assess and address diabetes-related distress.
The persistent nature of diabetes self-care expectations and the impact on lifestyle behaviors, medication adherence, and glycemic control demands the need for assessment and treatment of diabetes-related distress (DRD). DRD can be expressed as shame, guilt, anger, fear, and frustration in combination with the everyday context of life priorities and stressors. An assessment of diabetes distress, utilizing a simple scale, should be included as part of an annual therapeutic diabetes care plan. The ADA Standards of Care in Diabetes recommends assessing patients’ psychological and social situations as an ongoing part of medical management, including an annual screening for depression and other psychological problems. The prevalence of depression is nearly twice as high in people with T2D than in the general population and can significantly influence patients’ ability to self-manage their diabetes and achieve healthy outcomes. Assessment and treatment of psychosocial components of care can result in significant improvements in A1c and other positive outcomes, including quality of life.
Kellie M. Rodriguez, director of the global diabetes program at Parkland Health, Dallas, Tex., disclosed ties with the Association of Diabetes Care and Education Specialists.
A version of this article originally appeared on Medscape.com.
Overcoming death anxiety: Understanding our lives and legacies
Disappointment – “I failed this exam, my life is ruined” or regret – “I am getting a divorce, I wasted so much of my life.” Patients present with a wide variety of complaints that can be understood as a form of death anxiety.
Fundamentally, patients come to see us to understand and explain their lives. One can reinterpret this as a patient asking, “If I died today, would my life have been good enough?” or “When I die, how will I look back at this moment in time and judge the choices I made?”
Other patients come to us attempting to use the same maladaptive defenses that did not serve them well in the past in the hopes of achieving a new outcome that will validate their lives. While it may be understandable that a child dissociates when facing abuse, hoping that this defense mechanism – as an adult – will work, it is unlikely to be fruitful and will certainly not validate or repair the past. This hope to repair one’s past can be interpreted as a fear of death – “I cannot die without correcting this.” This psychic conflict can intensify if one does not adopt a more adaptive understanding of his or her life.
Death anxiety is the feeling associated with the finality of life. Not only is life final, but a constant reminder of that fact is the idea that any one moment is final. Other than in science fiction, one cannot return to a prior moment and repair the past in the hope of a better future. Time goes only in one direction and death is the natural outcome of all life.
Death may have some evolutionary purpose that encourages the promotion of newer and more fitter genes, but one doesn’t have to consider its origin and reason to admit death’s constancy throughout humanity. People die and that is an anxiety-provoking fact of life. Death anxiety can feel especially tangible in our connected world. In a world of constant news, it can feel – for many people – that if your house wasn’t displaced because of global warming or that you are not a war refugee, you don’t deserve to be seen and heard.
This can be a particularly strong feeling for and among physicians, who don’t think that the mental health challenges generated by their own tough circumstances deserve to be labeled a mental disorder, so they designate themselves as having “burnout”1 – as they don’t deserve the sympathy of having the clinically significant impairments of “depression.” Our traumas don’t seem important enough to deserve notice, and thus we may feel like we could die without ever having truly mattered.
This can also be applied in the reverse fashion. Certain individuals, like celebrities, live such extravagant lives that our simpler achievements can feel futile in comparison. While the neighbor’s grass has always felt greener, we are now constantly exposed to perfectly manicured lawns on social media. When compounded, the idea that our successes and our pains are both simultaneously irrelevant can lead one to have very palpable death anxiety – my life will never matter if none of the things I do matter, or my life will never matter because I will never achieve the requisite number of “likes” or “views” on social media required to believe that one’s life was worth living.
A way of alleviating death anxiety can be through the concept of legacy, or what we leave behind. How will people remember me? Will people remember me, or will I disappear like a shadow into the distant memory of my near and dear ones? The idea of being forgotten or lost to memory is intolerable to some and can be a strong driving force to “make a name” for oneself. For those who crave fame, whether a celebrity or a generous alumnus, part of this is likely related to remaining well known after death. After all, one can argue that you are not truly dead as long as you continue to live in the memory and/or genes of others.
Legacy thus serves as a form of posthumous transitional object; a way of calming our fears about how we will be remembered. For many, reconciling their feelings towards their legacy is an avenue to tame death anxiety.
A case study
The case of Mr. B illustrates this. As a 72-year-old male with a long history of generalized anxiety, he once had a nightmare as a child, similar to the plot of Sleeping Beauty. In his dream, he walks up a spiral staircase in a castle and touches the spindle on a spinning wheel, thus ending his life. The dream was vivid and marked him.
His fear of death has subsequently reared its head throughout his life. In more recent years, he has suffered from cardiovascular disease. Although he is now quite stable on his current cardiac medications, he is constantly fearful that he will experience a cardiac event while asleep and suddenly die. He is so anxious about not waking up in the morning that falling asleep is nearly impossible.
Mr. B is single, with no close family besides a sister who lives in another state. He has a dog and few friends. He worries about what will happen to his dog if he doesn’t wake up in the morning, but perhaps most distressing to him is “there’s so much left for me to do, I have so much to write!” As an accomplished author, he continues to write, and hopes to publish many more novels in his lifetime. It is unsurprising that someone without a strong social network may fear death and feel pressured to somehow make a mark on the world before the curtain falls. It is scary to think that even without us, life goes on.
By bringing to Mr. B’s attention that his ever-present anxiety is rooted in fear of death, he was able to gain more insight into his own defensive behaviors. By confronting his death anxiety and processing his definition of a life well lived together in therapy, he’s acknowledged his lack of social connection as demoralizing, and has made significant strides to remedy this. He’s been able to focus on a more fulfilling life day to day, with less emphasis on his to-do list and aspirations. Instead, he’s connected more with his faith and members of his church. He’s gotten close to several neighbors and enjoys long dinners with them on his back patio.
At a recent meeting, he confessed that he feels “lighter” and not as fearful about sudden cardiac death, and thus has noticed that his overall anxiety has diminished greatly. He concluded that experiencing meaningful relationships in the present moment would give him greater joy than spending his remaining time engaged in preserving a future identity for himself. It seems elementary, but if we look within, we may find that we all suffer similarly: How much of our daily actions, thoughts, and fears are tied to the looming threat of death?
Conclusion
While modern psychiatry continues to advance with better understandings of our neurobiology, improved knowledge of pathophysiological processes of mental illness, and expanding discovery of novel pharmacotherapeutics, the modern psychiatrist should not forget fundamental truths of behavior and humanity that were once the staple of psychiatry.
Death anxiety is one of those truths; it is the ultimate stressor that we will all face and should be regular study and practice for psychiatrists. In this article, we explored some of those facets most meaningful to us but recommend you expand your study to the many more available.
Patients often come to physicians seeking validation of their lives or trying to use the same maladaptive defense mechanisms that did not serve them well in the past to achieve a better outcome.
In today’s world, death anxiety can feel palpable due to the constant exposure to global news and social media that can make us feel irrelevant. However, legacy, or what we leave behind, can serve as a way to alleviate death anxiety. For many, reconciling their feelings toward their legacy is an avenue to tame death anxiety. Therapy can help individuals gain insight into their defensive behaviors and process their definition of a life well lived. By focusing on a life worth living, individuals can alleviate their death anxiety and gain a sense of fulfillment.
Dr. Akkoor is a psychiatry resident at the University of California, San Diego. She is interested in immigrant mental health, ethics, consultation-liaison psychiatry, and medical education. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Badre and Dr. Akkoor have no conflicts of interest.
Reference
1. Badre N. Burnout: A concept that rebrands mental illness for professionals. Clinical Psychiatry News. 2020 Mar 5.
Disappointment – “I failed this exam, my life is ruined” or regret – “I am getting a divorce, I wasted so much of my life.” Patients present with a wide variety of complaints that can be understood as a form of death anxiety.
Fundamentally, patients come to see us to understand and explain their lives. One can reinterpret this as a patient asking, “If I died today, would my life have been good enough?” or “When I die, how will I look back at this moment in time and judge the choices I made?”
Other patients come to us attempting to use the same maladaptive defenses that did not serve them well in the past in the hopes of achieving a new outcome that will validate their lives. While it may be understandable that a child dissociates when facing abuse, hoping that this defense mechanism – as an adult – will work, it is unlikely to be fruitful and will certainly not validate or repair the past. This hope to repair one’s past can be interpreted as a fear of death – “I cannot die without correcting this.” This psychic conflict can intensify if one does not adopt a more adaptive understanding of his or her life.
Death anxiety is the feeling associated with the finality of life. Not only is life final, but a constant reminder of that fact is the idea that any one moment is final. Other than in science fiction, one cannot return to a prior moment and repair the past in the hope of a better future. Time goes only in one direction and death is the natural outcome of all life.
Death may have some evolutionary purpose that encourages the promotion of newer and more fitter genes, but one doesn’t have to consider its origin and reason to admit death’s constancy throughout humanity. People die and that is an anxiety-provoking fact of life. Death anxiety can feel especially tangible in our connected world. In a world of constant news, it can feel – for many people – that if your house wasn’t displaced because of global warming or that you are not a war refugee, you don’t deserve to be seen and heard.
This can be a particularly strong feeling for and among physicians, who don’t think that the mental health challenges generated by their own tough circumstances deserve to be labeled a mental disorder, so they designate themselves as having “burnout”1 – as they don’t deserve the sympathy of having the clinically significant impairments of “depression.” Our traumas don’t seem important enough to deserve notice, and thus we may feel like we could die without ever having truly mattered.
This can also be applied in the reverse fashion. Certain individuals, like celebrities, live such extravagant lives that our simpler achievements can feel futile in comparison. While the neighbor’s grass has always felt greener, we are now constantly exposed to perfectly manicured lawns on social media. When compounded, the idea that our successes and our pains are both simultaneously irrelevant can lead one to have very palpable death anxiety – my life will never matter if none of the things I do matter, or my life will never matter because I will never achieve the requisite number of “likes” or “views” on social media required to believe that one’s life was worth living.
A way of alleviating death anxiety can be through the concept of legacy, or what we leave behind. How will people remember me? Will people remember me, or will I disappear like a shadow into the distant memory of my near and dear ones? The idea of being forgotten or lost to memory is intolerable to some and can be a strong driving force to “make a name” for oneself. For those who crave fame, whether a celebrity or a generous alumnus, part of this is likely related to remaining well known after death. After all, one can argue that you are not truly dead as long as you continue to live in the memory and/or genes of others.
Legacy thus serves as a form of posthumous transitional object; a way of calming our fears about how we will be remembered. For many, reconciling their feelings towards their legacy is an avenue to tame death anxiety.
A case study
The case of Mr. B illustrates this. As a 72-year-old male with a long history of generalized anxiety, he once had a nightmare as a child, similar to the plot of Sleeping Beauty. In his dream, he walks up a spiral staircase in a castle and touches the spindle on a spinning wheel, thus ending his life. The dream was vivid and marked him.
His fear of death has subsequently reared its head throughout his life. In more recent years, he has suffered from cardiovascular disease. Although he is now quite stable on his current cardiac medications, he is constantly fearful that he will experience a cardiac event while asleep and suddenly die. He is so anxious about not waking up in the morning that falling asleep is nearly impossible.
Mr. B is single, with no close family besides a sister who lives in another state. He has a dog and few friends. He worries about what will happen to his dog if he doesn’t wake up in the morning, but perhaps most distressing to him is “there’s so much left for me to do, I have so much to write!” As an accomplished author, he continues to write, and hopes to publish many more novels in his lifetime. It is unsurprising that someone without a strong social network may fear death and feel pressured to somehow make a mark on the world before the curtain falls. It is scary to think that even without us, life goes on.
By bringing to Mr. B’s attention that his ever-present anxiety is rooted in fear of death, he was able to gain more insight into his own defensive behaviors. By confronting his death anxiety and processing his definition of a life well lived together in therapy, he’s acknowledged his lack of social connection as demoralizing, and has made significant strides to remedy this. He’s been able to focus on a more fulfilling life day to day, with less emphasis on his to-do list and aspirations. Instead, he’s connected more with his faith and members of his church. He’s gotten close to several neighbors and enjoys long dinners with them on his back patio.
At a recent meeting, he confessed that he feels “lighter” and not as fearful about sudden cardiac death, and thus has noticed that his overall anxiety has diminished greatly. He concluded that experiencing meaningful relationships in the present moment would give him greater joy than spending his remaining time engaged in preserving a future identity for himself. It seems elementary, but if we look within, we may find that we all suffer similarly: How much of our daily actions, thoughts, and fears are tied to the looming threat of death?
Conclusion
While modern psychiatry continues to advance with better understandings of our neurobiology, improved knowledge of pathophysiological processes of mental illness, and expanding discovery of novel pharmacotherapeutics, the modern psychiatrist should not forget fundamental truths of behavior and humanity that were once the staple of psychiatry.
Death anxiety is one of those truths; it is the ultimate stressor that we will all face and should be regular study and practice for psychiatrists. In this article, we explored some of those facets most meaningful to us but recommend you expand your study to the many more available.
Patients often come to physicians seeking validation of their lives or trying to use the same maladaptive defense mechanisms that did not serve them well in the past to achieve a better outcome.
In today’s world, death anxiety can feel palpable due to the constant exposure to global news and social media that can make us feel irrelevant. However, legacy, or what we leave behind, can serve as a way to alleviate death anxiety. For many, reconciling their feelings toward their legacy is an avenue to tame death anxiety. Therapy can help individuals gain insight into their defensive behaviors and process their definition of a life well lived. By focusing on a life worth living, individuals can alleviate their death anxiety and gain a sense of fulfillment.
Dr. Akkoor is a psychiatry resident at the University of California, San Diego. She is interested in immigrant mental health, ethics, consultation-liaison psychiatry, and medical education. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Badre and Dr. Akkoor have no conflicts of interest.
Reference
1. Badre N. Burnout: A concept that rebrands mental illness for professionals. Clinical Psychiatry News. 2020 Mar 5.
Disappointment – “I failed this exam, my life is ruined” or regret – “I am getting a divorce, I wasted so much of my life.” Patients present with a wide variety of complaints that can be understood as a form of death anxiety.
Fundamentally, patients come to see us to understand and explain their lives. One can reinterpret this as a patient asking, “If I died today, would my life have been good enough?” or “When I die, how will I look back at this moment in time and judge the choices I made?”
Other patients come to us attempting to use the same maladaptive defenses that did not serve them well in the past in the hopes of achieving a new outcome that will validate their lives. While it may be understandable that a child dissociates when facing abuse, hoping that this defense mechanism – as an adult – will work, it is unlikely to be fruitful and will certainly not validate or repair the past. This hope to repair one’s past can be interpreted as a fear of death – “I cannot die without correcting this.” This psychic conflict can intensify if one does not adopt a more adaptive understanding of his or her life.
Death anxiety is the feeling associated with the finality of life. Not only is life final, but a constant reminder of that fact is the idea that any one moment is final. Other than in science fiction, one cannot return to a prior moment and repair the past in the hope of a better future. Time goes only in one direction and death is the natural outcome of all life.
Death may have some evolutionary purpose that encourages the promotion of newer and more fitter genes, but one doesn’t have to consider its origin and reason to admit death’s constancy throughout humanity. People die and that is an anxiety-provoking fact of life. Death anxiety can feel especially tangible in our connected world. In a world of constant news, it can feel – for many people – that if your house wasn’t displaced because of global warming or that you are not a war refugee, you don’t deserve to be seen and heard.
This can be a particularly strong feeling for and among physicians, who don’t think that the mental health challenges generated by their own tough circumstances deserve to be labeled a mental disorder, so they designate themselves as having “burnout”1 – as they don’t deserve the sympathy of having the clinically significant impairments of “depression.” Our traumas don’t seem important enough to deserve notice, and thus we may feel like we could die without ever having truly mattered.
This can also be applied in the reverse fashion. Certain individuals, like celebrities, live such extravagant lives that our simpler achievements can feel futile in comparison. While the neighbor’s grass has always felt greener, we are now constantly exposed to perfectly manicured lawns on social media. When compounded, the idea that our successes and our pains are both simultaneously irrelevant can lead one to have very palpable death anxiety – my life will never matter if none of the things I do matter, or my life will never matter because I will never achieve the requisite number of “likes” or “views” on social media required to believe that one’s life was worth living.
A way of alleviating death anxiety can be through the concept of legacy, or what we leave behind. How will people remember me? Will people remember me, or will I disappear like a shadow into the distant memory of my near and dear ones? The idea of being forgotten or lost to memory is intolerable to some and can be a strong driving force to “make a name” for oneself. For those who crave fame, whether a celebrity or a generous alumnus, part of this is likely related to remaining well known after death. After all, one can argue that you are not truly dead as long as you continue to live in the memory and/or genes of others.
Legacy thus serves as a form of posthumous transitional object; a way of calming our fears about how we will be remembered. For many, reconciling their feelings towards their legacy is an avenue to tame death anxiety.
A case study
The case of Mr. B illustrates this. As a 72-year-old male with a long history of generalized anxiety, he once had a nightmare as a child, similar to the plot of Sleeping Beauty. In his dream, he walks up a spiral staircase in a castle and touches the spindle on a spinning wheel, thus ending his life. The dream was vivid and marked him.
His fear of death has subsequently reared its head throughout his life. In more recent years, he has suffered from cardiovascular disease. Although he is now quite stable on his current cardiac medications, he is constantly fearful that he will experience a cardiac event while asleep and suddenly die. He is so anxious about not waking up in the morning that falling asleep is nearly impossible.
Mr. B is single, with no close family besides a sister who lives in another state. He has a dog and few friends. He worries about what will happen to his dog if he doesn’t wake up in the morning, but perhaps most distressing to him is “there’s so much left for me to do, I have so much to write!” As an accomplished author, he continues to write, and hopes to publish many more novels in his lifetime. It is unsurprising that someone without a strong social network may fear death and feel pressured to somehow make a mark on the world before the curtain falls. It is scary to think that even without us, life goes on.
By bringing to Mr. B’s attention that his ever-present anxiety is rooted in fear of death, he was able to gain more insight into his own defensive behaviors. By confronting his death anxiety and processing his definition of a life well lived together in therapy, he’s acknowledged his lack of social connection as demoralizing, and has made significant strides to remedy this. He’s been able to focus on a more fulfilling life day to day, with less emphasis on his to-do list and aspirations. Instead, he’s connected more with his faith and members of his church. He’s gotten close to several neighbors and enjoys long dinners with them on his back patio.
At a recent meeting, he confessed that he feels “lighter” and not as fearful about sudden cardiac death, and thus has noticed that his overall anxiety has diminished greatly. He concluded that experiencing meaningful relationships in the present moment would give him greater joy than spending his remaining time engaged in preserving a future identity for himself. It seems elementary, but if we look within, we may find that we all suffer similarly: How much of our daily actions, thoughts, and fears are tied to the looming threat of death?
Conclusion
While modern psychiatry continues to advance with better understandings of our neurobiology, improved knowledge of pathophysiological processes of mental illness, and expanding discovery of novel pharmacotherapeutics, the modern psychiatrist should not forget fundamental truths of behavior and humanity that were once the staple of psychiatry.
Death anxiety is one of those truths; it is the ultimate stressor that we will all face and should be regular study and practice for psychiatrists. In this article, we explored some of those facets most meaningful to us but recommend you expand your study to the many more available.
Patients often come to physicians seeking validation of their lives or trying to use the same maladaptive defense mechanisms that did not serve them well in the past to achieve a better outcome.
In today’s world, death anxiety can feel palpable due to the constant exposure to global news and social media that can make us feel irrelevant. However, legacy, or what we leave behind, can serve as a way to alleviate death anxiety. For many, reconciling their feelings toward their legacy is an avenue to tame death anxiety. Therapy can help individuals gain insight into their defensive behaviors and process their definition of a life well lived. By focusing on a life worth living, individuals can alleviate their death anxiety and gain a sense of fulfillment.
Dr. Akkoor is a psychiatry resident at the University of California, San Diego. She is interested in immigrant mental health, ethics, consultation-liaison psychiatry, and medical education. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Badre and Dr. Akkoor have no conflicts of interest.
Reference
1. Badre N. Burnout: A concept that rebrands mental illness for professionals. Clinical Psychiatry News. 2020 Mar 5.
Does the current age cutoff for screening miss too many cases of cervical cancer in older women?
Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
EXPERT COMMENTARY
Cervical cancer screening guidelines recommend screening cessation at age 65 once specific exit criteria are met. (According to the American Cancer Society, individuals aged >65 years who have no history of cervical intraepithelial neoplasia [CIN] grade 2 or more severe disease within the past 25 years, and who have documented adequate negative prior screening in the prior 10 years, discontinue all cervical cancer screening.)1 We know, however, that about one-fifth of all cervical cancer cases are diagnosed among individuals aged 65 or older, and for Black women that proportion is even higher when data are appropriately adjusted to account for the increased rate of hysterectomy among Black versus White women.2-4
Early-stage cervical cancer is largely a curable disease with very high 5-year overall survival rates. Unfortunately, more than half of all cervical cancer is diagnosed at a more advanced stage, and survival rates are much lower for this population.5
Cervical cancer incidence rates plummeted in the United States after the introduction of the Pap test for cervical cancer screening. However, the percentage of women who are not up to date with cervical cancer screening may now be increasing, from 14% in 2005 to 23% in 2019 according to one study from the US Preventive Services Task Force.6 When looking at cervical cancer screening rates by age, researchers from the Centers for Disease Control and Prevention estimate that the proportion of patients who have not been recently screened goes up as patients get older, with approximately 845,000 American women aged 61 to 65 not adequately screened in 2015 alone.7
Details of the study
Cooley and colleagues sought to better characterize the cohort of women diagnosed with cervical cancer at a later age, specifically the stage at diagnosis and survival.8 They used data from the California Cancer Registry (CCR), a large state-mandated, population-based data repository that is affiliated with the Surveillance, Epidemiology, and End Results (SEER) program.
The researchers identified 12,442 womenin the CCR who were newly diagnosed with cervical cancer from 2009 to 2018, 17.4% of whom were age 65 or older. They looked at cancer stage at diagnosis as it relates to relative survival rate (“the ratio of the observed survival rate among those who have cancer divided by the expected survival rate for people of the same sex, race/ethnicity, and age who do not have cancer”), Charlson comorbidity score, socioeconomic status, health insurance status, urbanicity, and race/ethnicity.
Results. In this study, 71% of women aged 65 or older presented with advanced-stage disease (FIGO [International Federation of Gynecology and Obstetrics] stage II–IV) as compared with only 48% in those aged 21 to 64. Five-year relative survival rates also were lower in the older cohort—23% to 37%, compared with 42% to 52% in the younger patients. In a sensitivity analysis, late-stage disease was associated with older age, increasing medical comorbidities, and nonadenocarcinoma histology.
Interestingly, older women of Hispanic ethnicity were less likely to be diagnosed with late-stage disease when compared with non-Hispanic White women.
Study strengths and limitations
Although this study’s conclusions—that patients with advanced-stage cancer are more likely to do poorly than those with early-stage cancer—may seem obvious to some even without the proven data, it is still important to highlight what a clinician may intuit with data to support that intuition. It is particularly important to emphasize this risk in older women in light of the aging population in the United States, with adults older than age 65 expected to account for more than 20% of the nation’s population by 2030.9
The study by Cooley and colleagues adds value to the existing literature due to its large study population, which included more than 12,000 patients diagnosed with cervical cancer.8 And although its results may not be completely generalizable as the data were gathered from only a California-specific population, the sample was diverse with significant portions of Hispanic and Black patients. This study supports previous data that showed high rates of advanced cervical cancer in women older than age 65, with resultant worse 5-year relative survival in this population of older women specifically.4 ●
Cervical cancer is both common and deadly in older women. Although current cervical cancer screening guidelines recommend screening cessation after age 65, remember that this is based on strict exit criteria. Consider screening older women (especially with human papillomavirus [HPV] testing) for cervical cancer if they have risk factors (such as smoking, multiple sexual partners, inconsistent or infrequent screening, history of abnormal Pap or HPV tests), and keep cervical cancer on your differential diagnosis in women who present with postmenopausal bleeding, vaginal discharge, pelvic pain, recurrent urinary tract infections, or other concerning symptoms.
SARAH DILLEY, MD, MPH, AND WARNER HUH, MD
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- Dilley S, Huh W, Blechter B, et al. It’s time to re-evaluate cervical cancer screening after age 65. Gynecol Oncol. 2021;162:200-202. doi:10.1016/j.ygyno.2021.04.027.
- Rositch AF, Nowak RG, Gravitt PE. Increased age and racespecific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120:2032-2038. doi:10.1002/cncr.28548.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050. doi:10.1002 /cncr.30507.
- Cancer Stat Facts. National Cancer Institute Surveillance, Epidemiology, and End Results Program. https://seer.cancer .gov/statfacts/html/cervix.html
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001 /jamanetworkopen.2021.43582.
- White MC, Shoemaker ML, Benard VB. Cervical cancer screening and incidence by age: unmet needs near and after the stopping age for screening. Am J Prev Med. 2017;53:392395. doi:10.1016/j.amepre.2017.02.024.
- Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. May 2014. United States Census Bureau. Accessed April 12, 2023. https://www.census .gov/library/publications/2014/demo/p25-1140.html
Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
EXPERT COMMENTARY
Cervical cancer screening guidelines recommend screening cessation at age 65 once specific exit criteria are met. (According to the American Cancer Society, individuals aged >65 years who have no history of cervical intraepithelial neoplasia [CIN] grade 2 or more severe disease within the past 25 years, and who have documented adequate negative prior screening in the prior 10 years, discontinue all cervical cancer screening.)1 We know, however, that about one-fifth of all cervical cancer cases are diagnosed among individuals aged 65 or older, and for Black women that proportion is even higher when data are appropriately adjusted to account for the increased rate of hysterectomy among Black versus White women.2-4
Early-stage cervical cancer is largely a curable disease with very high 5-year overall survival rates. Unfortunately, more than half of all cervical cancer is diagnosed at a more advanced stage, and survival rates are much lower for this population.5
Cervical cancer incidence rates plummeted in the United States after the introduction of the Pap test for cervical cancer screening. However, the percentage of women who are not up to date with cervical cancer screening may now be increasing, from 14% in 2005 to 23% in 2019 according to one study from the US Preventive Services Task Force.6 When looking at cervical cancer screening rates by age, researchers from the Centers for Disease Control and Prevention estimate that the proportion of patients who have not been recently screened goes up as patients get older, with approximately 845,000 American women aged 61 to 65 not adequately screened in 2015 alone.7
Details of the study
Cooley and colleagues sought to better characterize the cohort of women diagnosed with cervical cancer at a later age, specifically the stage at diagnosis and survival.8 They used data from the California Cancer Registry (CCR), a large state-mandated, population-based data repository that is affiliated with the Surveillance, Epidemiology, and End Results (SEER) program.
The researchers identified 12,442 womenin the CCR who were newly diagnosed with cervical cancer from 2009 to 2018, 17.4% of whom were age 65 or older. They looked at cancer stage at diagnosis as it relates to relative survival rate (“the ratio of the observed survival rate among those who have cancer divided by the expected survival rate for people of the same sex, race/ethnicity, and age who do not have cancer”), Charlson comorbidity score, socioeconomic status, health insurance status, urbanicity, and race/ethnicity.
Results. In this study, 71% of women aged 65 or older presented with advanced-stage disease (FIGO [International Federation of Gynecology and Obstetrics] stage II–IV) as compared with only 48% in those aged 21 to 64. Five-year relative survival rates also were lower in the older cohort—23% to 37%, compared with 42% to 52% in the younger patients. In a sensitivity analysis, late-stage disease was associated with older age, increasing medical comorbidities, and nonadenocarcinoma histology.
Interestingly, older women of Hispanic ethnicity were less likely to be diagnosed with late-stage disease when compared with non-Hispanic White women.
Study strengths and limitations
Although this study’s conclusions—that patients with advanced-stage cancer are more likely to do poorly than those with early-stage cancer—may seem obvious to some even without the proven data, it is still important to highlight what a clinician may intuit with data to support that intuition. It is particularly important to emphasize this risk in older women in light of the aging population in the United States, with adults older than age 65 expected to account for more than 20% of the nation’s population by 2030.9
The study by Cooley and colleagues adds value to the existing literature due to its large study population, which included more than 12,000 patients diagnosed with cervical cancer.8 And although its results may not be completely generalizable as the data were gathered from only a California-specific population, the sample was diverse with significant portions of Hispanic and Black patients. This study supports previous data that showed high rates of advanced cervical cancer in women older than age 65, with resultant worse 5-year relative survival in this population of older women specifically.4 ●
Cervical cancer is both common and deadly in older women. Although current cervical cancer screening guidelines recommend screening cessation after age 65, remember that this is based on strict exit criteria. Consider screening older women (especially with human papillomavirus [HPV] testing) for cervical cancer if they have risk factors (such as smoking, multiple sexual partners, inconsistent or infrequent screening, history of abnormal Pap or HPV tests), and keep cervical cancer on your differential diagnosis in women who present with postmenopausal bleeding, vaginal discharge, pelvic pain, recurrent urinary tract infections, or other concerning symptoms.
SARAH DILLEY, MD, MPH, AND WARNER HUH, MD
Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
EXPERT COMMENTARY
Cervical cancer screening guidelines recommend screening cessation at age 65 once specific exit criteria are met. (According to the American Cancer Society, individuals aged >65 years who have no history of cervical intraepithelial neoplasia [CIN] grade 2 or more severe disease within the past 25 years, and who have documented adequate negative prior screening in the prior 10 years, discontinue all cervical cancer screening.)1 We know, however, that about one-fifth of all cervical cancer cases are diagnosed among individuals aged 65 or older, and for Black women that proportion is even higher when data are appropriately adjusted to account for the increased rate of hysterectomy among Black versus White women.2-4
Early-stage cervical cancer is largely a curable disease with very high 5-year overall survival rates. Unfortunately, more than half of all cervical cancer is diagnosed at a more advanced stage, and survival rates are much lower for this population.5
Cervical cancer incidence rates plummeted in the United States after the introduction of the Pap test for cervical cancer screening. However, the percentage of women who are not up to date with cervical cancer screening may now be increasing, from 14% in 2005 to 23% in 2019 according to one study from the US Preventive Services Task Force.6 When looking at cervical cancer screening rates by age, researchers from the Centers for Disease Control and Prevention estimate that the proportion of patients who have not been recently screened goes up as patients get older, with approximately 845,000 American women aged 61 to 65 not adequately screened in 2015 alone.7
Details of the study
Cooley and colleagues sought to better characterize the cohort of women diagnosed with cervical cancer at a later age, specifically the stage at diagnosis and survival.8 They used data from the California Cancer Registry (CCR), a large state-mandated, population-based data repository that is affiliated with the Surveillance, Epidemiology, and End Results (SEER) program.
The researchers identified 12,442 womenin the CCR who were newly diagnosed with cervical cancer from 2009 to 2018, 17.4% of whom were age 65 or older. They looked at cancer stage at diagnosis as it relates to relative survival rate (“the ratio of the observed survival rate among those who have cancer divided by the expected survival rate for people of the same sex, race/ethnicity, and age who do not have cancer”), Charlson comorbidity score, socioeconomic status, health insurance status, urbanicity, and race/ethnicity.
Results. In this study, 71% of women aged 65 or older presented with advanced-stage disease (FIGO [International Federation of Gynecology and Obstetrics] stage II–IV) as compared with only 48% in those aged 21 to 64. Five-year relative survival rates also were lower in the older cohort—23% to 37%, compared with 42% to 52% in the younger patients. In a sensitivity analysis, late-stage disease was associated with older age, increasing medical comorbidities, and nonadenocarcinoma histology.
Interestingly, older women of Hispanic ethnicity were less likely to be diagnosed with late-stage disease when compared with non-Hispanic White women.
Study strengths and limitations
Although this study’s conclusions—that patients with advanced-stage cancer are more likely to do poorly than those with early-stage cancer—may seem obvious to some even without the proven data, it is still important to highlight what a clinician may intuit with data to support that intuition. It is particularly important to emphasize this risk in older women in light of the aging population in the United States, with adults older than age 65 expected to account for more than 20% of the nation’s population by 2030.9
The study by Cooley and colleagues adds value to the existing literature due to its large study population, which included more than 12,000 patients diagnosed with cervical cancer.8 And although its results may not be completely generalizable as the data were gathered from only a California-specific population, the sample was diverse with significant portions of Hispanic and Black patients. This study supports previous data that showed high rates of advanced cervical cancer in women older than age 65, with resultant worse 5-year relative survival in this population of older women specifically.4 ●
Cervical cancer is both common and deadly in older women. Although current cervical cancer screening guidelines recommend screening cessation after age 65, remember that this is based on strict exit criteria. Consider screening older women (especially with human papillomavirus [HPV] testing) for cervical cancer if they have risk factors (such as smoking, multiple sexual partners, inconsistent or infrequent screening, history of abnormal Pap or HPV tests), and keep cervical cancer on your differential diagnosis in women who present with postmenopausal bleeding, vaginal discharge, pelvic pain, recurrent urinary tract infections, or other concerning symptoms.
SARAH DILLEY, MD, MPH, AND WARNER HUH, MD
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- Dilley S, Huh W, Blechter B, et al. It’s time to re-evaluate cervical cancer screening after age 65. Gynecol Oncol. 2021;162:200-202. doi:10.1016/j.ygyno.2021.04.027.
- Rositch AF, Nowak RG, Gravitt PE. Increased age and racespecific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120:2032-2038. doi:10.1002/cncr.28548.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050. doi:10.1002 /cncr.30507.
- Cancer Stat Facts. National Cancer Institute Surveillance, Epidemiology, and End Results Program. https://seer.cancer .gov/statfacts/html/cervix.html
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001 /jamanetworkopen.2021.43582.
- White MC, Shoemaker ML, Benard VB. Cervical cancer screening and incidence by age: unmet needs near and after the stopping age for screening. Am J Prev Med. 2017;53:392395. doi:10.1016/j.amepre.2017.02.024.
- Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. May 2014. United States Census Bureau. Accessed April 12, 2023. https://www.census .gov/library/publications/2014/demo/p25-1140.html
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- Dilley S, Huh W, Blechter B, et al. It’s time to re-evaluate cervical cancer screening after age 65. Gynecol Oncol. 2021;162:200-202. doi:10.1016/j.ygyno.2021.04.027.
- Rositch AF, Nowak RG, Gravitt PE. Increased age and racespecific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120:2032-2038. doi:10.1002/cncr.28548.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050. doi:10.1002 /cncr.30507.
- Cancer Stat Facts. National Cancer Institute Surveillance, Epidemiology, and End Results Program. https://seer.cancer .gov/statfacts/html/cervix.html
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001 /jamanetworkopen.2021.43582.
- White MC, Shoemaker ML, Benard VB. Cervical cancer screening and incidence by age: unmet needs near and after the stopping age for screening. Am J Prev Med. 2017;53:392395. doi:10.1016/j.amepre.2017.02.024.
- Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. May 2014. United States Census Bureau. Accessed April 12, 2023. https://www.census .gov/library/publications/2014/demo/p25-1140.html
Doctor spots a gunshot victim staggering down his street
It was a quiet day. I got up around 3 o’clock in the afternoon for my shift at 6 p.m. I was shaking off the cobwebs and making coffee at our front window that overlooked Brown Street in North Philadelphia. There was nobody else around so I went outside to see what was going on.
He was in his 50s or 60s, bleeding and obviously in distress. I had him sit down. Then I ran back inside and grabbed a dish towel and some exam gloves that I had in the house.
I ran back out and assessed him. A bullet had gone through one of his hands, but he had other wounds. I had to expose him, so I trauma stripped him on the sidewalk. I got his pants and his shirt off and saw a gunshot going through his lower pelvis. He was bleeding out from there.
I got the towel and started applying deep pressure down into the iliac vein in case they hit something, which I found out later, they had. I held it there. The man was just lying there begging not to die.
I’m someone who is very calm, maybe abnormally calm, as people tell me. I try to use that during my resuscitations and traumas. Just keeping everybody calm makes the situation easier. Afterwards, people asked me, “Weren’t you worried that you were going to get shot?” That does happen in North Philadelphia. But it didn’t even cross my mind.
I didn’t have to think at all about what I was doing. We saw so many gunshots, especially at Einstein Medical Center. We saw them daily. I’d sometimes get more than half a dozen gunshots in one shift.
So, I was holding pressure and some people started to come over. I got somebody to call 911 and asked the man about his medical history. I found out he had diabetes. Five or 10 minutes later, EMS showed up. They looked pretty stunned when I was able to give the handoff presentation to them. I told them what happened and his back-story. I wanted to make sure they would check his sugar and take extra precautions.
They got him on the stretcher, and he eventually made it to the hospital where he had surgery. They had to have a vascular surgeon work on him. I called later, and they told me, “Yeah, he’s alive.” But that’s about the extent of the update I got.
After the ambulance left, it was kind of chaos. All the neighbors poured out of their houses. People were panicked, talking and getting excited about it. I didn’t know, but everyone else had actually been home the whole time. They didn’t come out until then.
I went back inside and tried to get ready for work. I wasn’t planning on talking to the media, but my next door neighbor just walked the news camera crew over to my house and knocked on my door. I wasn’t exactly dressed to be on TV, but they talked to me on camera, and it was on the news later that night.
I went to work and didn’t say anything about it. To be honest, I was trying to avoid telling anyone. Our team had a close-knit bond, and we would often tease each other when we received any type of recognition.
Naturally one of my attendings saw it on the local news and told everybody. So, I got a lot of happy harassment for quite some time. Someone baked me a cake that said, “Hero of Fairmount” (the Philly neighborhood in which I live). Someone else printed out a photo of me that said, “Stop the Bleed Hero of Fairmount,” and put it on every single computer screen.
The man came to see me about 2 weeks later (a neighbor told him where I lived). The man was very tearful and gave me a big hug. We just embraced for a while, and he said how thankful he was. He brought me a bottle of wine, which I thought was really nice.
He told me what happened to him: There was a lot of construction on our street and he was the contractor overseeing a couple of home remodels and demolitions. Sometimes he paid workers in cash and carried it with him. Somebody had tipped off somebody else that he was going to be there that day. The contractor walked into one of the houses and a guy in a ski mask waited there with a gun. The guy shot him and took the cash. The bullet went through his hand into his pelvis.
I had never had to deal with something that intense before outside of work. Most of it really comes down to the basics – the ABCs and bleeding control. You do whatever you can with what you have. In this case, it was just a dish towel, gloves, and my hands to put as much pressure as possible.
It really was strange that I happened to be looking out the window at that moment. I don’t know if it was just a coincidence. The man told me he believed God had put somebody there at the right place at the right time to save his life. I just felt very fortunate to have been able to help him. I never saw him again.
I think something like this gives you a little confidence that you can actually do something and make a meaningful impact anywhere when it’s needed. It lets you know that you’re capable of doing it. You always think about it, but you don’t know until it happens.
A version of this article first appeared on Medscape.com.
It was a quiet day. I got up around 3 o’clock in the afternoon for my shift at 6 p.m. I was shaking off the cobwebs and making coffee at our front window that overlooked Brown Street in North Philadelphia. There was nobody else around so I went outside to see what was going on.
He was in his 50s or 60s, bleeding and obviously in distress. I had him sit down. Then I ran back inside and grabbed a dish towel and some exam gloves that I had in the house.
I ran back out and assessed him. A bullet had gone through one of his hands, but he had other wounds. I had to expose him, so I trauma stripped him on the sidewalk. I got his pants and his shirt off and saw a gunshot going through his lower pelvis. He was bleeding out from there.
I got the towel and started applying deep pressure down into the iliac vein in case they hit something, which I found out later, they had. I held it there. The man was just lying there begging not to die.
I’m someone who is very calm, maybe abnormally calm, as people tell me. I try to use that during my resuscitations and traumas. Just keeping everybody calm makes the situation easier. Afterwards, people asked me, “Weren’t you worried that you were going to get shot?” That does happen in North Philadelphia. But it didn’t even cross my mind.
I didn’t have to think at all about what I was doing. We saw so many gunshots, especially at Einstein Medical Center. We saw them daily. I’d sometimes get more than half a dozen gunshots in one shift.
So, I was holding pressure and some people started to come over. I got somebody to call 911 and asked the man about his medical history. I found out he had diabetes. Five or 10 minutes later, EMS showed up. They looked pretty stunned when I was able to give the handoff presentation to them. I told them what happened and his back-story. I wanted to make sure they would check his sugar and take extra precautions.
They got him on the stretcher, and he eventually made it to the hospital where he had surgery. They had to have a vascular surgeon work on him. I called later, and they told me, “Yeah, he’s alive.” But that’s about the extent of the update I got.
After the ambulance left, it was kind of chaos. All the neighbors poured out of their houses. People were panicked, talking and getting excited about it. I didn’t know, but everyone else had actually been home the whole time. They didn’t come out until then.
I went back inside and tried to get ready for work. I wasn’t planning on talking to the media, but my next door neighbor just walked the news camera crew over to my house and knocked on my door. I wasn’t exactly dressed to be on TV, but they talked to me on camera, and it was on the news later that night.
I went to work and didn’t say anything about it. To be honest, I was trying to avoid telling anyone. Our team had a close-knit bond, and we would often tease each other when we received any type of recognition.
Naturally one of my attendings saw it on the local news and told everybody. So, I got a lot of happy harassment for quite some time. Someone baked me a cake that said, “Hero of Fairmount” (the Philly neighborhood in which I live). Someone else printed out a photo of me that said, “Stop the Bleed Hero of Fairmount,” and put it on every single computer screen.
The man came to see me about 2 weeks later (a neighbor told him where I lived). The man was very tearful and gave me a big hug. We just embraced for a while, and he said how thankful he was. He brought me a bottle of wine, which I thought was really nice.
He told me what happened to him: There was a lot of construction on our street and he was the contractor overseeing a couple of home remodels and demolitions. Sometimes he paid workers in cash and carried it with him. Somebody had tipped off somebody else that he was going to be there that day. The contractor walked into one of the houses and a guy in a ski mask waited there with a gun. The guy shot him and took the cash. The bullet went through his hand into his pelvis.
I had never had to deal with something that intense before outside of work. Most of it really comes down to the basics – the ABCs and bleeding control. You do whatever you can with what you have. In this case, it was just a dish towel, gloves, and my hands to put as much pressure as possible.
It really was strange that I happened to be looking out the window at that moment. I don’t know if it was just a coincidence. The man told me he believed God had put somebody there at the right place at the right time to save his life. I just felt very fortunate to have been able to help him. I never saw him again.
I think something like this gives you a little confidence that you can actually do something and make a meaningful impact anywhere when it’s needed. It lets you know that you’re capable of doing it. You always think about it, but you don’t know until it happens.
A version of this article first appeared on Medscape.com.
It was a quiet day. I got up around 3 o’clock in the afternoon for my shift at 6 p.m. I was shaking off the cobwebs and making coffee at our front window that overlooked Brown Street in North Philadelphia. There was nobody else around so I went outside to see what was going on.
He was in his 50s or 60s, bleeding and obviously in distress. I had him sit down. Then I ran back inside and grabbed a dish towel and some exam gloves that I had in the house.
I ran back out and assessed him. A bullet had gone through one of his hands, but he had other wounds. I had to expose him, so I trauma stripped him on the sidewalk. I got his pants and his shirt off and saw a gunshot going through his lower pelvis. He was bleeding out from there.
I got the towel and started applying deep pressure down into the iliac vein in case they hit something, which I found out later, they had. I held it there. The man was just lying there begging not to die.
I’m someone who is very calm, maybe abnormally calm, as people tell me. I try to use that during my resuscitations and traumas. Just keeping everybody calm makes the situation easier. Afterwards, people asked me, “Weren’t you worried that you were going to get shot?” That does happen in North Philadelphia. But it didn’t even cross my mind.
I didn’t have to think at all about what I was doing. We saw so many gunshots, especially at Einstein Medical Center. We saw them daily. I’d sometimes get more than half a dozen gunshots in one shift.
So, I was holding pressure and some people started to come over. I got somebody to call 911 and asked the man about his medical history. I found out he had diabetes. Five or 10 minutes later, EMS showed up. They looked pretty stunned when I was able to give the handoff presentation to them. I told them what happened and his back-story. I wanted to make sure they would check his sugar and take extra precautions.
They got him on the stretcher, and he eventually made it to the hospital where he had surgery. They had to have a vascular surgeon work on him. I called later, and they told me, “Yeah, he’s alive.” But that’s about the extent of the update I got.
After the ambulance left, it was kind of chaos. All the neighbors poured out of their houses. People were panicked, talking and getting excited about it. I didn’t know, but everyone else had actually been home the whole time. They didn’t come out until then.
I went back inside and tried to get ready for work. I wasn’t planning on talking to the media, but my next door neighbor just walked the news camera crew over to my house and knocked on my door. I wasn’t exactly dressed to be on TV, but they talked to me on camera, and it was on the news later that night.
I went to work and didn’t say anything about it. To be honest, I was trying to avoid telling anyone. Our team had a close-knit bond, and we would often tease each other when we received any type of recognition.
Naturally one of my attendings saw it on the local news and told everybody. So, I got a lot of happy harassment for quite some time. Someone baked me a cake that said, “Hero of Fairmount” (the Philly neighborhood in which I live). Someone else printed out a photo of me that said, “Stop the Bleed Hero of Fairmount,” and put it on every single computer screen.
The man came to see me about 2 weeks later (a neighbor told him where I lived). The man was very tearful and gave me a big hug. We just embraced for a while, and he said how thankful he was. He brought me a bottle of wine, which I thought was really nice.
He told me what happened to him: There was a lot of construction on our street and he was the contractor overseeing a couple of home remodels and demolitions. Sometimes he paid workers in cash and carried it with him. Somebody had tipped off somebody else that he was going to be there that day. The contractor walked into one of the houses and a guy in a ski mask waited there with a gun. The guy shot him and took the cash. The bullet went through his hand into his pelvis.
I had never had to deal with something that intense before outside of work. Most of it really comes down to the basics – the ABCs and bleeding control. You do whatever you can with what you have. In this case, it was just a dish towel, gloves, and my hands to put as much pressure as possible.
It really was strange that I happened to be looking out the window at that moment. I don’t know if it was just a coincidence. The man told me he believed God had put somebody there at the right place at the right time to save his life. I just felt very fortunate to have been able to help him. I never saw him again.
I think something like this gives you a little confidence that you can actually do something and make a meaningful impact anywhere when it’s needed. It lets you know that you’re capable of doing it. You always think about it, but you don’t know until it happens.
A version of this article first appeared on Medscape.com.
Balancing needs and risks as the opioid pendulum swings
Recently, my family had a conversation about the volume of news reports on overdose deaths from the illicit use of opioid drugs—a phenomenon that is complex and stems from many factors. We decided, as a family, that we could have a small impact on the problem. How? By carrying naloxone with us and administering it if we encounter a person with potential opioid overdose. Our decision was made possible by the recent US Food and Drug Administration (FDA) approval of naloxone nasal spray for over-the-counter use.1 At a cost of about $50 for 2 nasal sprays, we decided it would be a reasonable price to pay to potentially save a life.
Prescribing opioids in clinical practice is a different side of the problem. The Centers for Disease Control and Prevention (CDC) reports that prescription opioids account for about one-quarter of opioid overdose deaths.2 This is not trivial, and much effort has gone into addressing how clinicians can do better by their patients. There are training programs and risk-mitigation strategies for opioid prescribing. States have developed prescribing registries to identify patients who receive controlled substances from multiple prescribers, at higher-than-recommended doses, and too early in the pain management process. These efforts have reduced the number of opioid prescriptions and rates of high-dose prescribing (> 90 morphine milligram equivalents). However, that hasn’t translated into a reduction in the number of deaths.2
The article by Posen et al3 in this issue further reminded me how trends in health care, including opioid prescribing, are like a pendulum—swinging from one extreme to the other before eventually centering. I recall conversations with colleagues about how often we undertreated pain—and then later, how relieved we were when new approaches to pain management, using newer opiates, emerged and were reported to be much safer, even for long-term use. We now know the rest of that story: more prescriptions, higher doses, longer duration, addiction, death, and deception by manufacturers.
In our efforts to prevent addiction and decrease opioid deaths, we tried to get patients off opioids completely, thereby increasing demand for addiction therapy, including medication-assisted recovery. This also drove many of our patients to seek opioids from nefarious suppliers, resulting in even more deaths from fentanyl-laced drugs.
At least one positive has arisen from the “no more opioids” movement: We have re-evaluated their true effect on managing pain. Initially, we were told opioids were safe and highly effective—and, having few tools to help our patients, we were Pollyanna-ish in accepting this. But many recent studies have demonstrated that using opioids for pain is no more effective than using other analgesics.4-9 In addition to overdose deaths and addiction, these studies show significantly higher rates of opioid discontinuation due to adverse effects.
We certainly can manage most patients’ pain effectively with other approaches. For some, though—patients whose pain is not adequately controlled and/or interferes with their ability to function, and those who are terminally ill—opioid nihilism has had unintended consequences. Recognizing these issues, the CDC updated its guideline for prescribing opioids in 2022.10 Four areas were addressed: whether to initiate opioids; opioid selection and dosing; duration of therapy and need for follow-up; and assessing risk and addressing potential harms of opioid use. The CDC encourages clinicians to find a balance of the potential benefits and harms and to avoid inflexibility. Finally, the CDC encourages clinicians to identify and treat patients with opioid use disorders.
Clearly, opioid overuse and overdose result from complex medical, economic, and societal factors. Individual clinicians are well equipped to manage things “in their own backyards.” However, what we do can be perceived as a bandage for a much larger problem. Our public health system has the potential for greater impact, but the “cure” will require multimodal solutions addressing many facets of society and government.11 At the very least, we should keep some naloxone close by and vote for political candidates who see broader solutions for addressing this life-and-death crisis.
1. FDA. FDA approves first over-the-counter naloxone nasal spray. Updated March 29, 2023. Accessed April 16, 2023. www.fda.gov/news-events/press-announcements/fda-approves-first-over-counter-naloxone-nasal-spray
2. CDC. Prescription opioid overdose death maps. Updated June 6, 2022. Accessed April 16, 2023. www.cdc.gov/drugoverdose/deaths/prescription/maps.html
3. Posen A, Keller E, Elmes At, et al. Medication-assisted recovery for opioid use disorder: a guide. J Fam Pract. 2023;72:164-171.
4. Fiore JF Jr, El-Kefraoui C, Chay MA, et al. Opioid versus opioid-free analgesia after surgical discharge: a systematic review and meta-analysis of randomised trials. Lancet. 2022;399:2280-2293. doi: 10.1016/S0140-6736(22)00582-7
5. Moutzouros V, Jildeh TR, Tramer JS, et al. Can we eliminate opioids after anterior cruciate ligament reconstruction? A prospective, randomized controlled trial. Am J Sports Med. 2021;49:3794-3801. doi: 10.1177/03635465211045394
6. Falk J, Thomas B, Kirkwood J, et al. PEER systematic review of randomized controlled trials: management of chronic neuropathic pain in primary care. Can Fam Physician. 2021;67:e130-e140. doi: 10.46747/cfp.6705e130
7. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191. doi: 10.7326/m17-0598
8. Kolber MR, Ton J, Thomas B, et al. PEER systematic review of randomized controlled trials: management of chronic low back pain in primary care. Can Fam Physician. 2021;67:e20-e30. doi: 10.46747/cfp.6701e20
9. O’Brien MDC, Wand APF. A systematic review of the evidence for the efficacy of opioids for chronic non-cancer pain in community-dwelling older adults. Age Ageing. 2020;49:175-183. doi: 10.1093/ageing/afz175
10. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
11. American Academy of Family Physicians. Chronic pain management and opioid misuse: a public health concern (position paper). Accessed April 16, 2023. www.aafp.org/about/policies/all/chronic-pain-management-opiod-misuse.html
Recently, my family had a conversation about the volume of news reports on overdose deaths from the illicit use of opioid drugs—a phenomenon that is complex and stems from many factors. We decided, as a family, that we could have a small impact on the problem. How? By carrying naloxone with us and administering it if we encounter a person with potential opioid overdose. Our decision was made possible by the recent US Food and Drug Administration (FDA) approval of naloxone nasal spray for over-the-counter use.1 At a cost of about $50 for 2 nasal sprays, we decided it would be a reasonable price to pay to potentially save a life.
Prescribing opioids in clinical practice is a different side of the problem. The Centers for Disease Control and Prevention (CDC) reports that prescription opioids account for about one-quarter of opioid overdose deaths.2 This is not trivial, and much effort has gone into addressing how clinicians can do better by their patients. There are training programs and risk-mitigation strategies for opioid prescribing. States have developed prescribing registries to identify patients who receive controlled substances from multiple prescribers, at higher-than-recommended doses, and too early in the pain management process. These efforts have reduced the number of opioid prescriptions and rates of high-dose prescribing (> 90 morphine milligram equivalents). However, that hasn’t translated into a reduction in the number of deaths.2
The article by Posen et al3 in this issue further reminded me how trends in health care, including opioid prescribing, are like a pendulum—swinging from one extreme to the other before eventually centering. I recall conversations with colleagues about how often we undertreated pain—and then later, how relieved we were when new approaches to pain management, using newer opiates, emerged and were reported to be much safer, even for long-term use. We now know the rest of that story: more prescriptions, higher doses, longer duration, addiction, death, and deception by manufacturers.
In our efforts to prevent addiction and decrease opioid deaths, we tried to get patients off opioids completely, thereby increasing demand for addiction therapy, including medication-assisted recovery. This also drove many of our patients to seek opioids from nefarious suppliers, resulting in even more deaths from fentanyl-laced drugs.
At least one positive has arisen from the “no more opioids” movement: We have re-evaluated their true effect on managing pain. Initially, we were told opioids were safe and highly effective—and, having few tools to help our patients, we were Pollyanna-ish in accepting this. But many recent studies have demonstrated that using opioids for pain is no more effective than using other analgesics.4-9 In addition to overdose deaths and addiction, these studies show significantly higher rates of opioid discontinuation due to adverse effects.
We certainly can manage most patients’ pain effectively with other approaches. For some, though—patients whose pain is not adequately controlled and/or interferes with their ability to function, and those who are terminally ill—opioid nihilism has had unintended consequences. Recognizing these issues, the CDC updated its guideline for prescribing opioids in 2022.10 Four areas were addressed: whether to initiate opioids; opioid selection and dosing; duration of therapy and need for follow-up; and assessing risk and addressing potential harms of opioid use. The CDC encourages clinicians to find a balance of the potential benefits and harms and to avoid inflexibility. Finally, the CDC encourages clinicians to identify and treat patients with opioid use disorders.
Clearly, opioid overuse and overdose result from complex medical, economic, and societal factors. Individual clinicians are well equipped to manage things “in their own backyards.” However, what we do can be perceived as a bandage for a much larger problem. Our public health system has the potential for greater impact, but the “cure” will require multimodal solutions addressing many facets of society and government.11 At the very least, we should keep some naloxone close by and vote for political candidates who see broader solutions for addressing this life-and-death crisis.
Recently, my family had a conversation about the volume of news reports on overdose deaths from the illicit use of opioid drugs—a phenomenon that is complex and stems from many factors. We decided, as a family, that we could have a small impact on the problem. How? By carrying naloxone with us and administering it if we encounter a person with potential opioid overdose. Our decision was made possible by the recent US Food and Drug Administration (FDA) approval of naloxone nasal spray for over-the-counter use.1 At a cost of about $50 for 2 nasal sprays, we decided it would be a reasonable price to pay to potentially save a life.
Prescribing opioids in clinical practice is a different side of the problem. The Centers for Disease Control and Prevention (CDC) reports that prescription opioids account for about one-quarter of opioid overdose deaths.2 This is not trivial, and much effort has gone into addressing how clinicians can do better by their patients. There are training programs and risk-mitigation strategies for opioid prescribing. States have developed prescribing registries to identify patients who receive controlled substances from multiple prescribers, at higher-than-recommended doses, and too early in the pain management process. These efforts have reduced the number of opioid prescriptions and rates of high-dose prescribing (> 90 morphine milligram equivalents). However, that hasn’t translated into a reduction in the number of deaths.2
The article by Posen et al3 in this issue further reminded me how trends in health care, including opioid prescribing, are like a pendulum—swinging from one extreme to the other before eventually centering. I recall conversations with colleagues about how often we undertreated pain—and then later, how relieved we were when new approaches to pain management, using newer opiates, emerged and were reported to be much safer, even for long-term use. We now know the rest of that story: more prescriptions, higher doses, longer duration, addiction, death, and deception by manufacturers.
In our efforts to prevent addiction and decrease opioid deaths, we tried to get patients off opioids completely, thereby increasing demand for addiction therapy, including medication-assisted recovery. This also drove many of our patients to seek opioids from nefarious suppliers, resulting in even more deaths from fentanyl-laced drugs.
At least one positive has arisen from the “no more opioids” movement: We have re-evaluated their true effect on managing pain. Initially, we were told opioids were safe and highly effective—and, having few tools to help our patients, we were Pollyanna-ish in accepting this. But many recent studies have demonstrated that using opioids for pain is no more effective than using other analgesics.4-9 In addition to overdose deaths and addiction, these studies show significantly higher rates of opioid discontinuation due to adverse effects.
We certainly can manage most patients’ pain effectively with other approaches. For some, though—patients whose pain is not adequately controlled and/or interferes with their ability to function, and those who are terminally ill—opioid nihilism has had unintended consequences. Recognizing these issues, the CDC updated its guideline for prescribing opioids in 2022.10 Four areas were addressed: whether to initiate opioids; opioid selection and dosing; duration of therapy and need for follow-up; and assessing risk and addressing potential harms of opioid use. The CDC encourages clinicians to find a balance of the potential benefits and harms and to avoid inflexibility. Finally, the CDC encourages clinicians to identify and treat patients with opioid use disorders.
Clearly, opioid overuse and overdose result from complex medical, economic, and societal factors. Individual clinicians are well equipped to manage things “in their own backyards.” However, what we do can be perceived as a bandage for a much larger problem. Our public health system has the potential for greater impact, but the “cure” will require multimodal solutions addressing many facets of society and government.11 At the very least, we should keep some naloxone close by and vote for political candidates who see broader solutions for addressing this life-and-death crisis.
1. FDA. FDA approves first over-the-counter naloxone nasal spray. Updated March 29, 2023. Accessed April 16, 2023. www.fda.gov/news-events/press-announcements/fda-approves-first-over-counter-naloxone-nasal-spray
2. CDC. Prescription opioid overdose death maps. Updated June 6, 2022. Accessed April 16, 2023. www.cdc.gov/drugoverdose/deaths/prescription/maps.html
3. Posen A, Keller E, Elmes At, et al. Medication-assisted recovery for opioid use disorder: a guide. J Fam Pract. 2023;72:164-171.
4. Fiore JF Jr, El-Kefraoui C, Chay MA, et al. Opioid versus opioid-free analgesia after surgical discharge: a systematic review and meta-analysis of randomised trials. Lancet. 2022;399:2280-2293. doi: 10.1016/S0140-6736(22)00582-7
5. Moutzouros V, Jildeh TR, Tramer JS, et al. Can we eliminate opioids after anterior cruciate ligament reconstruction? A prospective, randomized controlled trial. Am J Sports Med. 2021;49:3794-3801. doi: 10.1177/03635465211045394
6. Falk J, Thomas B, Kirkwood J, et al. PEER systematic review of randomized controlled trials: management of chronic neuropathic pain in primary care. Can Fam Physician. 2021;67:e130-e140. doi: 10.46747/cfp.6705e130
7. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191. doi: 10.7326/m17-0598
8. Kolber MR, Ton J, Thomas B, et al. PEER systematic review of randomized controlled trials: management of chronic low back pain in primary care. Can Fam Physician. 2021;67:e20-e30. doi: 10.46747/cfp.6701e20
9. O’Brien MDC, Wand APF. A systematic review of the evidence for the efficacy of opioids for chronic non-cancer pain in community-dwelling older adults. Age Ageing. 2020;49:175-183. doi: 10.1093/ageing/afz175
10. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
11. American Academy of Family Physicians. Chronic pain management and opioid misuse: a public health concern (position paper). Accessed April 16, 2023. www.aafp.org/about/policies/all/chronic-pain-management-opiod-misuse.html
1. FDA. FDA approves first over-the-counter naloxone nasal spray. Updated March 29, 2023. Accessed April 16, 2023. www.fda.gov/news-events/press-announcements/fda-approves-first-over-counter-naloxone-nasal-spray
2. CDC. Prescription opioid overdose death maps. Updated June 6, 2022. Accessed April 16, 2023. www.cdc.gov/drugoverdose/deaths/prescription/maps.html
3. Posen A, Keller E, Elmes At, et al. Medication-assisted recovery for opioid use disorder: a guide. J Fam Pract. 2023;72:164-171.
4. Fiore JF Jr, El-Kefraoui C, Chay MA, et al. Opioid versus opioid-free analgesia after surgical discharge: a systematic review and meta-analysis of randomised trials. Lancet. 2022;399:2280-2293. doi: 10.1016/S0140-6736(22)00582-7
5. Moutzouros V, Jildeh TR, Tramer JS, et al. Can we eliminate opioids after anterior cruciate ligament reconstruction? A prospective, randomized controlled trial. Am J Sports Med. 2021;49:3794-3801. doi: 10.1177/03635465211045394
6. Falk J, Thomas B, Kirkwood J, et al. PEER systematic review of randomized controlled trials: management of chronic neuropathic pain in primary care. Can Fam Physician. 2021;67:e130-e140. doi: 10.46747/cfp.6705e130
7. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191. doi: 10.7326/m17-0598
8. Kolber MR, Ton J, Thomas B, et al. PEER systematic review of randomized controlled trials: management of chronic low back pain in primary care. Can Fam Physician. 2021;67:e20-e30. doi: 10.46747/cfp.6701e20
9. O’Brien MDC, Wand APF. A systematic review of the evidence for the efficacy of opioids for chronic non-cancer pain in community-dwelling older adults. Age Ageing. 2020;49:175-183. doi: 10.1093/ageing/afz175
10. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
11. American Academy of Family Physicians. Chronic pain management and opioid misuse: a public health concern (position paper). Accessed April 16, 2023. www.aafp.org/about/policies/all/chronic-pain-management-opiod-misuse.html