User login
More on AI-generated content
In his recent editorial (“A ‘guest editorial’ … generated by ChatGPT?”
Sara Hartley, MD
Berkeley, California
I just read the “guest editorial” generated by ChatGPT. Thank you for this article. Although this is truly an amazing advancement in artificial intelligence (AI), I feel this guest editorial was very basic. It did not read like scientific writing. It read more like it was written at an 11th- or 12th-grade level, though I am fully aware that the question was simple, and thus the answer was not very deep. I can’t deny that if I had been tested, chances are good I would have fallen among the 32% of my peers who would not have recognized it as AI. I appreciate that you (and your team) are working on a protocol regarding how to include content generated by or with the help of AI. God knows if (most likely, when) people with evil minds will use AI to spread false information that may dispute the accredited scientific data and research that guide the medical world and many other fields. I wonder if AI can serve as a search engine that is better or easier to use than PubMed (for example) and the other services we use for research and learning.
Alex Mustachi, PMHNP-BC
Suffern, New York
I wanted to let you know how much I enjoyed reading your recent editorial on AI and scientific writing. Sharing the 4 AI-generated “articles” with readers (“For artificial intelligence, the future is finally here,”
Martha Sajatovic, MD
Cleveland, Ohio
Continue to: The AI-generated samples...
The Al-generated samples were fascinating. As far as I superficially noted, the spelling, grammar, and punctuation were correct. That is better than one gets from most student compositions. However, the articles were completely lacking in depth or apparent insight. The article on anosognosia mentioned it can be present in up to 50% of cases of schizophrenia. In my experience, it is present in approximately 99.9% of cases. It clearly did not consider if anosognosia is also present in alcoholics, codependents, abusers, or people with bizarre political beliefs. But I guess the “intelligence” wasn’t asked that. The other samples also show shallow thinking and repetitive wording—pretty much like my high school junior compositions.
Maybe an appropriate use for AI is a task such as evaluating suicide notes. AI’s success causes one to feel nonplussed. Much more disconcerting was a recent news article that reported AI made up nonexistent references to a professor’s alleged sexual harassment, and then generated citations to its own made-up reference.1 That is indeed frightening new territory. How does one fight against a machine to clear their own name?
Linda Miller, NP
Harrisonburg, Virginia
References
1. Verma P, Oremus W. ChatGPT invented a sexual harassment scandal and named a real law prof as the accused. The Washington Post. April 5, 2023. Accessed May 8, 2023. https://www.washingtonpost.com/technology/2023/04/05/chatgpt-lies/
Thank you, Dr. Nasrallah, for your latest thought-provoking articles on AI. Time and again you provide the profession with cutting-edge, relevant food for thought. Caveat emptor, indeed.
Lawrence E. Cormier, MD
Denver, Colorado
Continue to: We read with interest...
We read with interest Dr. Nasrallah’s editorial that invited readers to share their take on the quality of an AI-generated writing sample. I (MZP) was a computational neuroscience major at Columbia University and was accepted to medical school in 2022 at age 19. I identify with the character traits common among many young tech entrepreneurs driving the AI revolution—social awkwardness; discomfort with subjective emotions; restricted areas of interest; algorithmic thinking; strict, naive idealism; and an obsession with data. To gain a deeper understanding of Sam Altman, the CEO of OpenAI (the company that created ChatGPT), we analyzed a 2.5-hour interview that MIT research scientist Lex Fridman conducted with Altman.1 As a result, we began to discern why AI-generated text feels so stiff and bland compared to the superior fluidity and expressiveness of human communication. As of now, the creation is a reflection of its creator.
Generally speaking, computer scientists are not warm and fuzzy types. Hence, ChatGPT strives to be neutral, accurate, and objective compared to more biased and fallible humans, and, consequently, its language lacks the emotive flair we have come to relish in normal human interactions. In the interview, Altman discusses several solutions that will soon raise the quality of ChatGPT’s currently deficient emotional quotient to approximate its superior IQ. Altruistically, Altman has opened ChatGPT to all, so we can freely interact and utilize its potential to increase our productivity exponentially. As a result, ChatGPT interfaces with millions of humans through RLHF (reinforcement learning from human feedback), which makes each iteration more in tune with our sensibilities.2 Another initiative Altman is undertaking is to depart his Silicon Valley bubble for a road trip to interact with “regular people” and gain a better sense of how to make ChatGPT more user-friendly.1
What’s so saddening about Dr. Nasrallah’s homework assignment is that he is asking us to evaluate with our mature adult standards an article that was written at the emotional stage of a child in early high school. But our hubris and complacency are entirely unfounded because ChatGPT is learning much faster than we ever could, and it will quickly surpass us all as it continues to evolve.
It is also quite disconcerting to hear how Altman is naively relying upon governmental regulation and corporate responsibility to manage the potential misuse of future artificial general intelligence for social, economic, and political control and upheaval. We know well the harmful effects of the internet and social media, particularly on our youth, yet our laws still lag far behind the fact that these technological innovations are simultaneously enhancing our knowledge while destroying our souls. As custodians of our world, dedicated to promoting and preserving mental well-being, we cannot wait much longer to intervene in properly parenting AI along its wisest developmental trajectory before it is too late.
Maxwell Zachary Price, BA
Nutley, New Jersey
Richard Louis Price, MD
New York, New York
References
1. Sam Altman: OpenAI CEO on GPT-4, ChatGPT, and the Future of AI. Lex Fridman Podcast #367. March 25, 2023. Accessed April 5, 2023. https://www.youtube.com/watch?v=L_Guz73e6fw
2. Heikkilä M. How OpenAI is trying to make ChatGPT safer and less biased. MIT Technology Review. Published February 21, 2023. Accessed April 5, 2023. https://www.technologyreview.com/2023/02/21/1068893/how-openai-is-trying-to-make-chatgpt-safer-and-less-biased/
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
In his recent editorial (“A ‘guest editorial’ … generated by ChatGPT?”
Sara Hartley, MD
Berkeley, California
I just read the “guest editorial” generated by ChatGPT. Thank you for this article. Although this is truly an amazing advancement in artificial intelligence (AI), I feel this guest editorial was very basic. It did not read like scientific writing. It read more like it was written at an 11th- or 12th-grade level, though I am fully aware that the question was simple, and thus the answer was not very deep. I can’t deny that if I had been tested, chances are good I would have fallen among the 32% of my peers who would not have recognized it as AI. I appreciate that you (and your team) are working on a protocol regarding how to include content generated by or with the help of AI. God knows if (most likely, when) people with evil minds will use AI to spread false information that may dispute the accredited scientific data and research that guide the medical world and many other fields. I wonder if AI can serve as a search engine that is better or easier to use than PubMed (for example) and the other services we use for research and learning.
Alex Mustachi, PMHNP-BC
Suffern, New York
I wanted to let you know how much I enjoyed reading your recent editorial on AI and scientific writing. Sharing the 4 AI-generated “articles” with readers (“For artificial intelligence, the future is finally here,”
Martha Sajatovic, MD
Cleveland, Ohio
Continue to: The AI-generated samples...
The Al-generated samples were fascinating. As far as I superficially noted, the spelling, grammar, and punctuation were correct. That is better than one gets from most student compositions. However, the articles were completely lacking in depth or apparent insight. The article on anosognosia mentioned it can be present in up to 50% of cases of schizophrenia. In my experience, it is present in approximately 99.9% of cases. It clearly did not consider if anosognosia is also present in alcoholics, codependents, abusers, or people with bizarre political beliefs. But I guess the “intelligence” wasn’t asked that. The other samples also show shallow thinking and repetitive wording—pretty much like my high school junior compositions.
Maybe an appropriate use for AI is a task such as evaluating suicide notes. AI’s success causes one to feel nonplussed. Much more disconcerting was a recent news article that reported AI made up nonexistent references to a professor’s alleged sexual harassment, and then generated citations to its own made-up reference.1 That is indeed frightening new territory. How does one fight against a machine to clear their own name?
Linda Miller, NP
Harrisonburg, Virginia
References
1. Verma P, Oremus W. ChatGPT invented a sexual harassment scandal and named a real law prof as the accused. The Washington Post. April 5, 2023. Accessed May 8, 2023. https://www.washingtonpost.com/technology/2023/04/05/chatgpt-lies/
Thank you, Dr. Nasrallah, for your latest thought-provoking articles on AI. Time and again you provide the profession with cutting-edge, relevant food for thought. Caveat emptor, indeed.
Lawrence E. Cormier, MD
Denver, Colorado
Continue to: We read with interest...
We read with interest Dr. Nasrallah’s editorial that invited readers to share their take on the quality of an AI-generated writing sample. I (MZP) was a computational neuroscience major at Columbia University and was accepted to medical school in 2022 at age 19. I identify with the character traits common among many young tech entrepreneurs driving the AI revolution—social awkwardness; discomfort with subjective emotions; restricted areas of interest; algorithmic thinking; strict, naive idealism; and an obsession with data. To gain a deeper understanding of Sam Altman, the CEO of OpenAI (the company that created ChatGPT), we analyzed a 2.5-hour interview that MIT research scientist Lex Fridman conducted with Altman.1 As a result, we began to discern why AI-generated text feels so stiff and bland compared to the superior fluidity and expressiveness of human communication. As of now, the creation is a reflection of its creator.
Generally speaking, computer scientists are not warm and fuzzy types. Hence, ChatGPT strives to be neutral, accurate, and objective compared to more biased and fallible humans, and, consequently, its language lacks the emotive flair we have come to relish in normal human interactions. In the interview, Altman discusses several solutions that will soon raise the quality of ChatGPT’s currently deficient emotional quotient to approximate its superior IQ. Altruistically, Altman has opened ChatGPT to all, so we can freely interact and utilize its potential to increase our productivity exponentially. As a result, ChatGPT interfaces with millions of humans through RLHF (reinforcement learning from human feedback), which makes each iteration more in tune with our sensibilities.2 Another initiative Altman is undertaking is to depart his Silicon Valley bubble for a road trip to interact with “regular people” and gain a better sense of how to make ChatGPT more user-friendly.1
What’s so saddening about Dr. Nasrallah’s homework assignment is that he is asking us to evaluate with our mature adult standards an article that was written at the emotional stage of a child in early high school. But our hubris and complacency are entirely unfounded because ChatGPT is learning much faster than we ever could, and it will quickly surpass us all as it continues to evolve.
It is also quite disconcerting to hear how Altman is naively relying upon governmental regulation and corporate responsibility to manage the potential misuse of future artificial general intelligence for social, economic, and political control and upheaval. We know well the harmful effects of the internet and social media, particularly on our youth, yet our laws still lag far behind the fact that these technological innovations are simultaneously enhancing our knowledge while destroying our souls. As custodians of our world, dedicated to promoting and preserving mental well-being, we cannot wait much longer to intervene in properly parenting AI along its wisest developmental trajectory before it is too late.
Maxwell Zachary Price, BA
Nutley, New Jersey
Richard Louis Price, MD
New York, New York
References
1. Sam Altman: OpenAI CEO on GPT-4, ChatGPT, and the Future of AI. Lex Fridman Podcast #367. March 25, 2023. Accessed April 5, 2023. https://www.youtube.com/watch?v=L_Guz73e6fw
2. Heikkilä M. How OpenAI is trying to make ChatGPT safer and less biased. MIT Technology Review. Published February 21, 2023. Accessed April 5, 2023. https://www.technologyreview.com/2023/02/21/1068893/how-openai-is-trying-to-make-chatgpt-safer-and-less-biased/
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
In his recent editorial (“A ‘guest editorial’ … generated by ChatGPT?”
Sara Hartley, MD
Berkeley, California
I just read the “guest editorial” generated by ChatGPT. Thank you for this article. Although this is truly an amazing advancement in artificial intelligence (AI), I feel this guest editorial was very basic. It did not read like scientific writing. It read more like it was written at an 11th- or 12th-grade level, though I am fully aware that the question was simple, and thus the answer was not very deep. I can’t deny that if I had been tested, chances are good I would have fallen among the 32% of my peers who would not have recognized it as AI. I appreciate that you (and your team) are working on a protocol regarding how to include content generated by or with the help of AI. God knows if (most likely, when) people with evil minds will use AI to spread false information that may dispute the accredited scientific data and research that guide the medical world and many other fields. I wonder if AI can serve as a search engine that is better or easier to use than PubMed (for example) and the other services we use for research and learning.
Alex Mustachi, PMHNP-BC
Suffern, New York
I wanted to let you know how much I enjoyed reading your recent editorial on AI and scientific writing. Sharing the 4 AI-generated “articles” with readers (“For artificial intelligence, the future is finally here,”
Martha Sajatovic, MD
Cleveland, Ohio
Continue to: The AI-generated samples...
The Al-generated samples were fascinating. As far as I superficially noted, the spelling, grammar, and punctuation were correct. That is better than one gets from most student compositions. However, the articles were completely lacking in depth or apparent insight. The article on anosognosia mentioned it can be present in up to 50% of cases of schizophrenia. In my experience, it is present in approximately 99.9% of cases. It clearly did not consider if anosognosia is also present in alcoholics, codependents, abusers, or people with bizarre political beliefs. But I guess the “intelligence” wasn’t asked that. The other samples also show shallow thinking and repetitive wording—pretty much like my high school junior compositions.
Maybe an appropriate use for AI is a task such as evaluating suicide notes. AI’s success causes one to feel nonplussed. Much more disconcerting was a recent news article that reported AI made up nonexistent references to a professor’s alleged sexual harassment, and then generated citations to its own made-up reference.1 That is indeed frightening new territory. How does one fight against a machine to clear their own name?
Linda Miller, NP
Harrisonburg, Virginia
References
1. Verma P, Oremus W. ChatGPT invented a sexual harassment scandal and named a real law prof as the accused. The Washington Post. April 5, 2023. Accessed May 8, 2023. https://www.washingtonpost.com/technology/2023/04/05/chatgpt-lies/
Thank you, Dr. Nasrallah, for your latest thought-provoking articles on AI. Time and again you provide the profession with cutting-edge, relevant food for thought. Caveat emptor, indeed.
Lawrence E. Cormier, MD
Denver, Colorado
Continue to: We read with interest...
We read with interest Dr. Nasrallah’s editorial that invited readers to share their take on the quality of an AI-generated writing sample. I (MZP) was a computational neuroscience major at Columbia University and was accepted to medical school in 2022 at age 19. I identify with the character traits common among many young tech entrepreneurs driving the AI revolution—social awkwardness; discomfort with subjective emotions; restricted areas of interest; algorithmic thinking; strict, naive idealism; and an obsession with data. To gain a deeper understanding of Sam Altman, the CEO of OpenAI (the company that created ChatGPT), we analyzed a 2.5-hour interview that MIT research scientist Lex Fridman conducted with Altman.1 As a result, we began to discern why AI-generated text feels so stiff and bland compared to the superior fluidity and expressiveness of human communication. As of now, the creation is a reflection of its creator.
Generally speaking, computer scientists are not warm and fuzzy types. Hence, ChatGPT strives to be neutral, accurate, and objective compared to more biased and fallible humans, and, consequently, its language lacks the emotive flair we have come to relish in normal human interactions. In the interview, Altman discusses several solutions that will soon raise the quality of ChatGPT’s currently deficient emotional quotient to approximate its superior IQ. Altruistically, Altman has opened ChatGPT to all, so we can freely interact and utilize its potential to increase our productivity exponentially. As a result, ChatGPT interfaces with millions of humans through RLHF (reinforcement learning from human feedback), which makes each iteration more in tune with our sensibilities.2 Another initiative Altman is undertaking is to depart his Silicon Valley bubble for a road trip to interact with “regular people” and gain a better sense of how to make ChatGPT more user-friendly.1
What’s so saddening about Dr. Nasrallah’s homework assignment is that he is asking us to evaluate with our mature adult standards an article that was written at the emotional stage of a child in early high school. But our hubris and complacency are entirely unfounded because ChatGPT is learning much faster than we ever could, and it will quickly surpass us all as it continues to evolve.
It is also quite disconcerting to hear how Altman is naively relying upon governmental regulation and corporate responsibility to manage the potential misuse of future artificial general intelligence for social, economic, and political control and upheaval. We know well the harmful effects of the internet and social media, particularly on our youth, yet our laws still lag far behind the fact that these technological innovations are simultaneously enhancing our knowledge while destroying our souls. As custodians of our world, dedicated to promoting and preserving mental well-being, we cannot wait much longer to intervene in properly parenting AI along its wisest developmental trajectory before it is too late.
Maxwell Zachary Price, BA
Nutley, New Jersey
Richard Louis Price, MD
New York, New York
References
1. Sam Altman: OpenAI CEO on GPT-4, ChatGPT, and the Future of AI. Lex Fridman Podcast #367. March 25, 2023. Accessed April 5, 2023. https://www.youtube.com/watch?v=L_Guz73e6fw
2. Heikkilä M. How OpenAI is trying to make ChatGPT safer and less biased. MIT Technology Review. Published February 21, 2023. Accessed April 5, 2023. https://www.technologyreview.com/2023/02/21/1068893/how-openai-is-trying-to-make-chatgpt-safer-and-less-biased/
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
Membership priorities shape the AGA advocacy agenda
Here, we present key highlights from the survey findings and share opportunities for members to engage in GI advocacy.
AGA advocacy has contributed to significant recent successes that include lowering the average-risk of colorectal cancer screening age from 50 to 45 years, phasing out cost-sharing burdens associated with polypectomy at screening colonoscopy, encouraging federal support to focus on GI cancer disparities, ensuring coverage for telehealth services, expanding colonoscopy coverage after positive noninvasive colorectal cancer screening tests, and mitigating scheduled cuts in Medicare reimbursement for GI services.
Despite these important successes, the GI community faces significant challenges that include persisting GI health disparities; declines in reimbursement and increased prior authorization burdens for GI procedures and clinic visits, limited research funding to address the burden of GI disease, climate change, provider burnout, and increasing administrative burdens (such as insurance prior authorizations and step therapy policies.
The AGA sought to better understand policy priorities of the GI community by disseminating a 34-question policy priority survey to AGA members in December 2022. A total of 251 members responded to the survey with career stage and primary practice setting varying among respondents (Figure 1). The AGA vetted and selected 10 health policy issues of highest interest with 95% of survey respondents agreeing these 10 selected topics covered the top priority issues impacting gastroenterology (Figure 2).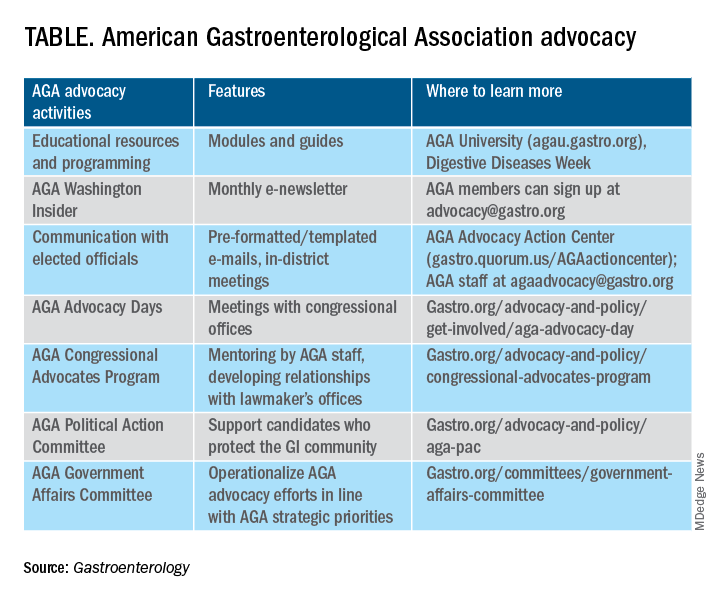
From these 10 policy issues, members were asked to identify the top 5 issues that AGA advocacy efforts should address.
The issues most frequently identified included reducing administrative burdens and patient delays in care because of increased prior authorizations (78%), ensuring fair reimbursement for GI providers (68%), reducing insurance-initiated switching of patient treatments for nonmedical reasons (58%), maintaining coverage of video and telephone evaluation and management visits (55%), and reducing delays in clinical care resulting from step therapy protocols (53%).
Other important issues included ensuring patients with pre-existing conditions have access to essential benefits and quality specialty care (43%); protecting providers from medical licensing restrictions and liability to deliver care across state lines (35%); addressing Medicare Quality Payment Program reporting requirements and lack of specialty advanced payment models (27%); increasing funding for GI health disparities (24%); and, increasing federal research funding to ensure greater opportunities for diverse early career investigators (20%).
Most problematic burdens
Survey respondents identified insurer prior authorization and step therapy burdens as especially problematic. 93% of respondents described the impact of prior authorization on their practices as “significantly burdensome” (61%) or “somewhat burdensome” (32%).
About 95% noted that prior authorization restrictions have impacted patient access to clinically appropriate treatments and patient clinical outcomes “significantly” (56%) or “somewhat” (39%) negatively. 84% described the burdens associated with prior authorization policies as having increased “significantly” (60%) or “somewhat” (24%) over the last 5 years.
Likewise, step therapy protocols were perceived by 84% of respondents as burdensome; by 88% as negatively impactful on patient access to clinically appropriate treatments; and, by 88% as negatively impactful on patient clinical outcomes.
About 84% of respondents noted increases in the frequency of nonmedical switching and dosing restrictions over the last 5 years, with 90% perceiving negative impacts on patient clinical outcomes. 73% of respondents reported increased burdens associated with compliance in the Medicare QPP over the last 5 years.
AGA’s advocacy work
About 76% of respondents were interested in learning more about the AGA’s advocacy work. We presented some of the various opportunities and resources for members to engage with and contribute to AGA advocacy efforts (see pie chart). Based on the tremendous efforts and dedication of AGA staff, some of these opportunities include educational modules on AGA University, DDW programming, the AGA Washington Insider monthly policy newsletter, preformatted communications available through the AGA Advocacy Action Center, participation in AGA Advocacy Days or the AGA Congressional Advocates Program, service on the AGA Government Affairs Committee, and/or contributing to the AGA Political Action Committee.
Overall, the survey respondents illustrate the diversity and enthusiasm of AGA membership. Importantly, 95% of AGA members responding to the survey agreed these 10 selected policy issues are inclusive of the current top priority issues of the GI community. Amidst an ever-shifting health care landscape, we – the AGA community – must remain vigilant and adaptable to best address expected and unexpected changes and challenges to our patients and colleagues. In this respect, we should encourage constructive communication and dialogue between AGA membership, leadership, other issue stakeholders, government representatives and entities, and payers.
Amit Patel, MD, is a gastroenterologist and associate professor of medicine at Duke University and the Durham Veterans Affairs Medical Center, both in Durham, N.C. He serves on the editorial review board of Gastroenterology. Rotonya McCants Carr, MD, is the Cyrus E. Rubin Chair and division head of gastroenterology at the University of Washington, Seattle. Both Dr. Patel and Dr. Carr serve on the AGA Government Affairs Committee. The contents of this article do not represent the views of the Department of Veterans Affairs.
Reference
Patel A et al. Gastroenterology. 2023 May;164[6]:847-50.
Here, we present key highlights from the survey findings and share opportunities for members to engage in GI advocacy.
AGA advocacy has contributed to significant recent successes that include lowering the average-risk of colorectal cancer screening age from 50 to 45 years, phasing out cost-sharing burdens associated with polypectomy at screening colonoscopy, encouraging federal support to focus on GI cancer disparities, ensuring coverage for telehealth services, expanding colonoscopy coverage after positive noninvasive colorectal cancer screening tests, and mitigating scheduled cuts in Medicare reimbursement for GI services.
Despite these important successes, the GI community faces significant challenges that include persisting GI health disparities; declines in reimbursement and increased prior authorization burdens for GI procedures and clinic visits, limited research funding to address the burden of GI disease, climate change, provider burnout, and increasing administrative burdens (such as insurance prior authorizations and step therapy policies.
The AGA sought to better understand policy priorities of the GI community by disseminating a 34-question policy priority survey to AGA members in December 2022. A total of 251 members responded to the survey with career stage and primary practice setting varying among respondents (Figure 1). The AGA vetted and selected 10 health policy issues of highest interest with 95% of survey respondents agreeing these 10 selected topics covered the top priority issues impacting gastroenterology (Figure 2).
From these 10 policy issues, members were asked to identify the top 5 issues that AGA advocacy efforts should address.
The issues most frequently identified included reducing administrative burdens and patient delays in care because of increased prior authorizations (78%), ensuring fair reimbursement for GI providers (68%), reducing insurance-initiated switching of patient treatments for nonmedical reasons (58%), maintaining coverage of video and telephone evaluation and management visits (55%), and reducing delays in clinical care resulting from step therapy protocols (53%).
Other important issues included ensuring patients with pre-existing conditions have access to essential benefits and quality specialty care (43%); protecting providers from medical licensing restrictions and liability to deliver care across state lines (35%); addressing Medicare Quality Payment Program reporting requirements and lack of specialty advanced payment models (27%); increasing funding for GI health disparities (24%); and, increasing federal research funding to ensure greater opportunities for diverse early career investigators (20%).
Most problematic burdens
Survey respondents identified insurer prior authorization and step therapy burdens as especially problematic. 93% of respondents described the impact of prior authorization on their practices as “significantly burdensome” (61%) or “somewhat burdensome” (32%).
About 95% noted that prior authorization restrictions have impacted patient access to clinically appropriate treatments and patient clinical outcomes “significantly” (56%) or “somewhat” (39%) negatively. 84% described the burdens associated with prior authorization policies as having increased “significantly” (60%) or “somewhat” (24%) over the last 5 years.
Likewise, step therapy protocols were perceived by 84% of respondents as burdensome; by 88% as negatively impactful on patient access to clinically appropriate treatments; and, by 88% as negatively impactful on patient clinical outcomes.
About 84% of respondents noted increases in the frequency of nonmedical switching and dosing restrictions over the last 5 years, with 90% perceiving negative impacts on patient clinical outcomes. 73% of respondents reported increased burdens associated with compliance in the Medicare QPP over the last 5 years.
AGA’s advocacy work
About 76% of respondents were interested in learning more about the AGA’s advocacy work. We presented some of the various opportunities and resources for members to engage with and contribute to AGA advocacy efforts (see pie chart). Based on the tremendous efforts and dedication of AGA staff, some of these opportunities include educational modules on AGA University, DDW programming, the AGA Washington Insider monthly policy newsletter, preformatted communications available through the AGA Advocacy Action Center, participation in AGA Advocacy Days or the AGA Congressional Advocates Program, service on the AGA Government Affairs Committee, and/or contributing to the AGA Political Action Committee.
Overall, the survey respondents illustrate the diversity and enthusiasm of AGA membership. Importantly, 95% of AGA members responding to the survey agreed these 10 selected policy issues are inclusive of the current top priority issues of the GI community. Amidst an ever-shifting health care landscape, we – the AGA community – must remain vigilant and adaptable to best address expected and unexpected changes and challenges to our patients and colleagues. In this respect, we should encourage constructive communication and dialogue between AGA membership, leadership, other issue stakeholders, government representatives and entities, and payers.
Amit Patel, MD, is a gastroenterologist and associate professor of medicine at Duke University and the Durham Veterans Affairs Medical Center, both in Durham, N.C. He serves on the editorial review board of Gastroenterology. Rotonya McCants Carr, MD, is the Cyrus E. Rubin Chair and division head of gastroenterology at the University of Washington, Seattle. Both Dr. Patel and Dr. Carr serve on the AGA Government Affairs Committee. The contents of this article do not represent the views of the Department of Veterans Affairs.
Reference
Patel A et al. Gastroenterology. 2023 May;164[6]:847-50.
Here, we present key highlights from the survey findings and share opportunities for members to engage in GI advocacy.
AGA advocacy has contributed to significant recent successes that include lowering the average-risk of colorectal cancer screening age from 50 to 45 years, phasing out cost-sharing burdens associated with polypectomy at screening colonoscopy, encouraging federal support to focus on GI cancer disparities, ensuring coverage for telehealth services, expanding colonoscopy coverage after positive noninvasive colorectal cancer screening tests, and mitigating scheduled cuts in Medicare reimbursement for GI services.
Despite these important successes, the GI community faces significant challenges that include persisting GI health disparities; declines in reimbursement and increased prior authorization burdens for GI procedures and clinic visits, limited research funding to address the burden of GI disease, climate change, provider burnout, and increasing administrative burdens (such as insurance prior authorizations and step therapy policies.
The AGA sought to better understand policy priorities of the GI community by disseminating a 34-question policy priority survey to AGA members in December 2022. A total of 251 members responded to the survey with career stage and primary practice setting varying among respondents (Figure 1). The AGA vetted and selected 10 health policy issues of highest interest with 95% of survey respondents agreeing these 10 selected topics covered the top priority issues impacting gastroenterology (Figure 2).
From these 10 policy issues, members were asked to identify the top 5 issues that AGA advocacy efforts should address.
The issues most frequently identified included reducing administrative burdens and patient delays in care because of increased prior authorizations (78%), ensuring fair reimbursement for GI providers (68%), reducing insurance-initiated switching of patient treatments for nonmedical reasons (58%), maintaining coverage of video and telephone evaluation and management visits (55%), and reducing delays in clinical care resulting from step therapy protocols (53%).
Other important issues included ensuring patients with pre-existing conditions have access to essential benefits and quality specialty care (43%); protecting providers from medical licensing restrictions and liability to deliver care across state lines (35%); addressing Medicare Quality Payment Program reporting requirements and lack of specialty advanced payment models (27%); increasing funding for GI health disparities (24%); and, increasing federal research funding to ensure greater opportunities for diverse early career investigators (20%).
Most problematic burdens
Survey respondents identified insurer prior authorization and step therapy burdens as especially problematic. 93% of respondents described the impact of prior authorization on their practices as “significantly burdensome” (61%) or “somewhat burdensome” (32%).
About 95% noted that prior authorization restrictions have impacted patient access to clinically appropriate treatments and patient clinical outcomes “significantly” (56%) or “somewhat” (39%) negatively. 84% described the burdens associated with prior authorization policies as having increased “significantly” (60%) or “somewhat” (24%) over the last 5 years.
Likewise, step therapy protocols were perceived by 84% of respondents as burdensome; by 88% as negatively impactful on patient access to clinically appropriate treatments; and, by 88% as negatively impactful on patient clinical outcomes.
About 84% of respondents noted increases in the frequency of nonmedical switching and dosing restrictions over the last 5 years, with 90% perceiving negative impacts on patient clinical outcomes. 73% of respondents reported increased burdens associated with compliance in the Medicare QPP over the last 5 years.
AGA’s advocacy work
About 76% of respondents were interested in learning more about the AGA’s advocacy work. We presented some of the various opportunities and resources for members to engage with and contribute to AGA advocacy efforts (see pie chart). Based on the tremendous efforts and dedication of AGA staff, some of these opportunities include educational modules on AGA University, DDW programming, the AGA Washington Insider monthly policy newsletter, preformatted communications available through the AGA Advocacy Action Center, participation in AGA Advocacy Days or the AGA Congressional Advocates Program, service on the AGA Government Affairs Committee, and/or contributing to the AGA Political Action Committee.
Overall, the survey respondents illustrate the diversity and enthusiasm of AGA membership. Importantly, 95% of AGA members responding to the survey agreed these 10 selected policy issues are inclusive of the current top priority issues of the GI community. Amidst an ever-shifting health care landscape, we – the AGA community – must remain vigilant and adaptable to best address expected and unexpected changes and challenges to our patients and colleagues. In this respect, we should encourage constructive communication and dialogue between AGA membership, leadership, other issue stakeholders, government representatives and entities, and payers.
Amit Patel, MD, is a gastroenterologist and associate professor of medicine at Duke University and the Durham Veterans Affairs Medical Center, both in Durham, N.C. He serves on the editorial review board of Gastroenterology. Rotonya McCants Carr, MD, is the Cyrus E. Rubin Chair and division head of gastroenterology at the University of Washington, Seattle. Both Dr. Patel and Dr. Carr serve on the AGA Government Affairs Committee. The contents of this article do not represent the views of the Department of Veterans Affairs.
Reference
Patel A et al. Gastroenterology. 2023 May;164[6]:847-50.
The power of mentorship
In a 2018 JAMA Viewpoint, Dr. Vineet Chopra, a former colleague of mine at the University of Michigan (now chair of medicine at the University of Colorado) and colleagues wrote about four archetypes of mentorship: mentor, coach, sponsor, and connector. along the way.
For me, DDW serves as an annual reminder of the power of mentorship in building and sustaining careers. Each May, trainees and early career faculty present their projects in oral or poster sessions, cheered on by their research mentors. Senior thought leaders offer career advice and guidance to more junior colleagues through structured sessions or informal conversations and facilitate introductions to new collaborators. Department chairs, division chiefs, and senior practice leaders take time to reconnect with their early mentors who believed in their potential and provided them with opportunities to take their careers to new heights. And, we see the incredible payoff of programs like AGA’s FORWARD and Future Leaders Programs in serving as springboards for career advancement and creating powerful role models and mentors for the future.
This year’s AGA presidential leadership transition served as a particularly poignant example of the power of mentorship as incoming AGA President Dr. Barbara Jung succeeded one of her early mentors, outgoing AGA President Dr. John Carethers, in this prestigious role. I hope you’ll join me in reflecting on the tremendous impact that mentors, coaches, sponsors, and connectors have had on your career, and continue to pay it forward to the next generation.
In this month’s issue, we feature several stories from DDW 2023, including summaries of the AGA presidential address and a study evaluating the impact of state Medicaid expansion on uptake of CRC screening in safety-net practices. From AGA’s flagship journals, we highlight a propensity-matched cohort study assessing the impact of pancreatic cancer surveillance of high-risk patients on important clinical outcomes and a new AGA CPU on management of extraesophageal GERD. In this month’s AGA Policy and Advocacy column, Dr. Amit Patel and Dr. Rotonya Carr review the results of a recent membership survey on policy priorities and outline the many ways you can get involved in advocacy efforts. Finally, our Member Spotlight column celebrates gastroenterologist and humanitarian Kadirawel Iswara, MD, recipient of this year’s AGA Distinguished Clinician Award in Private Practice, who is a cherished mentor to many prominent members of our field.
Megan A. Adams, M.D., J.D., MSc
Editor-in-Chief
In a 2018 JAMA Viewpoint, Dr. Vineet Chopra, a former colleague of mine at the University of Michigan (now chair of medicine at the University of Colorado) and colleagues wrote about four archetypes of mentorship: mentor, coach, sponsor, and connector. along the way.
For me, DDW serves as an annual reminder of the power of mentorship in building and sustaining careers. Each May, trainees and early career faculty present their projects in oral or poster sessions, cheered on by their research mentors. Senior thought leaders offer career advice and guidance to more junior colleagues through structured sessions or informal conversations and facilitate introductions to new collaborators. Department chairs, division chiefs, and senior practice leaders take time to reconnect with their early mentors who believed in their potential and provided them with opportunities to take their careers to new heights. And, we see the incredible payoff of programs like AGA’s FORWARD and Future Leaders Programs in serving as springboards for career advancement and creating powerful role models and mentors for the future.
This year’s AGA presidential leadership transition served as a particularly poignant example of the power of mentorship as incoming AGA President Dr. Barbara Jung succeeded one of her early mentors, outgoing AGA President Dr. John Carethers, in this prestigious role. I hope you’ll join me in reflecting on the tremendous impact that mentors, coaches, sponsors, and connectors have had on your career, and continue to pay it forward to the next generation.
In this month’s issue, we feature several stories from DDW 2023, including summaries of the AGA presidential address and a study evaluating the impact of state Medicaid expansion on uptake of CRC screening in safety-net practices. From AGA’s flagship journals, we highlight a propensity-matched cohort study assessing the impact of pancreatic cancer surveillance of high-risk patients on important clinical outcomes and a new AGA CPU on management of extraesophageal GERD. In this month’s AGA Policy and Advocacy column, Dr. Amit Patel and Dr. Rotonya Carr review the results of a recent membership survey on policy priorities and outline the many ways you can get involved in advocacy efforts. Finally, our Member Spotlight column celebrates gastroenterologist and humanitarian Kadirawel Iswara, MD, recipient of this year’s AGA Distinguished Clinician Award in Private Practice, who is a cherished mentor to many prominent members of our field.
Megan A. Adams, M.D., J.D., MSc
Editor-in-Chief
In a 2018 JAMA Viewpoint, Dr. Vineet Chopra, a former colleague of mine at the University of Michigan (now chair of medicine at the University of Colorado) and colleagues wrote about four archetypes of mentorship: mentor, coach, sponsor, and connector. along the way.
For me, DDW serves as an annual reminder of the power of mentorship in building and sustaining careers. Each May, trainees and early career faculty present their projects in oral or poster sessions, cheered on by their research mentors. Senior thought leaders offer career advice and guidance to more junior colleagues through structured sessions or informal conversations and facilitate introductions to new collaborators. Department chairs, division chiefs, and senior practice leaders take time to reconnect with their early mentors who believed in their potential and provided them with opportunities to take their careers to new heights. And, we see the incredible payoff of programs like AGA’s FORWARD and Future Leaders Programs in serving as springboards for career advancement and creating powerful role models and mentors for the future.
This year’s AGA presidential leadership transition served as a particularly poignant example of the power of mentorship as incoming AGA President Dr. Barbara Jung succeeded one of her early mentors, outgoing AGA President Dr. John Carethers, in this prestigious role. I hope you’ll join me in reflecting on the tremendous impact that mentors, coaches, sponsors, and connectors have had on your career, and continue to pay it forward to the next generation.
In this month’s issue, we feature several stories from DDW 2023, including summaries of the AGA presidential address and a study evaluating the impact of state Medicaid expansion on uptake of CRC screening in safety-net practices. From AGA’s flagship journals, we highlight a propensity-matched cohort study assessing the impact of pancreatic cancer surveillance of high-risk patients on important clinical outcomes and a new AGA CPU on management of extraesophageal GERD. In this month’s AGA Policy and Advocacy column, Dr. Amit Patel and Dr. Rotonya Carr review the results of a recent membership survey on policy priorities and outline the many ways you can get involved in advocacy efforts. Finally, our Member Spotlight column celebrates gastroenterologist and humanitarian Kadirawel Iswara, MD, recipient of this year’s AGA Distinguished Clinician Award in Private Practice, who is a cherished mentor to many prominent members of our field.
Megan A. Adams, M.D., J.D., MSc
Editor-in-Chief
COVID boosters effective, but not for long
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
I am here today to talk about the effectiveness of COVID vaccine boosters in the midst of 2023. The reason I want to talk about this isn’t necessarily to dig into exactly how effective vaccines are. This is an area that’s been trod upon multiple times. But it does give me an opportunity to talk about a neat study design called the “test-negative case-control” design, which has some unique properties when you’re trying to evaluate the effect of something outside of the context of a randomized trial.
So, just a little bit of background to remind everyone where we are. These are the number of doses of COVID vaccines administered over time throughout the pandemic.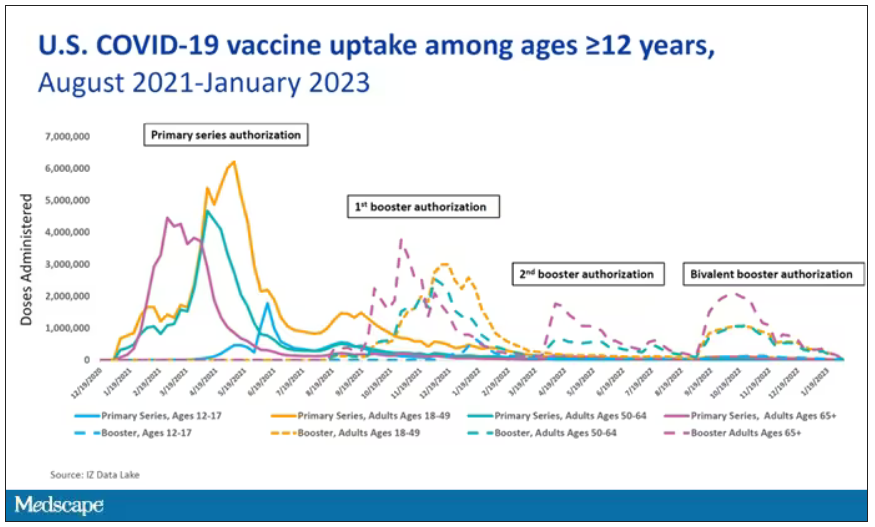
You can see that it’s stratified by age. The orange lines are adults ages 18-49, for example. You can see a big wave of vaccination when the vaccine first came out at the start of 2021. Then subsequently, you can see smaller waves after the first and second booster authorizations, and maybe a bit of a pickup, particularly among older adults, when the bivalent boosters were authorized. But still very little overall pickup of the bivalent booster, compared with the monovalent vaccines, which might suggest vaccine fatigue going on this far into the pandemic. But it’s important to try to understand exactly how effective those new boosters are, at least at this point in time.
I’m talking about Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022–January 2023, which came out in the Morbidity and Mortality Weekly Report very recently, which uses this test-negative case-control design to evaluate the ability of bivalent mRNA vaccines to prevent hospitalization.
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death? That may not be the question that is of interest to everyone. I know people are interested in symptoms, missed work, and transmission, but this paper was looking at hospitalization, ICU stay, and death.
What’s kind of tricky here is that the data they’re using are in people who are hospitalized with various diseases. You might look at that on the surface and say: “Well, you can’t – that’s impossible.” But you can, actually, with this cool test-negative case-control design.
Here’s basically how it works. You take a population of people who are hospitalized and confirmed to have COVID. Some of them will be vaccinated and some of them will be unvaccinated. And the proportion of vaccinated and unvaccinated people doesn’t tell you very much because it depends on how that compares with the rates in the general population, for instance. Let me clarify this. If 100% of the population were vaccinated, then 100% of the people hospitalized with COVID would be vaccinated. That doesn’t mean vaccines are bad. Put another way, if 90% of the population were vaccinated and 60% of people hospitalized with COVID were vaccinated, that would actually show that the vaccines were working to some extent, all else being equal. So it’s not just the raw percentages that tell you anything. Some people are vaccinated, some people aren’t. You need to understand what the baseline rate is.
The test-negative case-control design looks at people who are hospitalized without COVID. Now who those people are (who the controls are, in this case) is something you really need to think about. In the case of this CDC study, they used people who were hospitalized with COVID-like illnesses – flu-like illnesses, respiratory illnesses, pneumonia, influenza, etc. This is a pretty good idea because it standardizes a little bit for people who have access to healthcare. They can get to a hospital and they’re the type of person who would go to a hospital when they’re feeling sick. That’s a better control than the general population overall, which is something I like about this design.
Some of those people who don’t have COVID (they’re in the hospital for flu or whatever) will have been vaccinated for COVID, and some will not have been vaccinated for COVID. And of course, we don’t expect COVID vaccines necessarily to protect against the flu or pneumonia, but that gives us a way to standardize.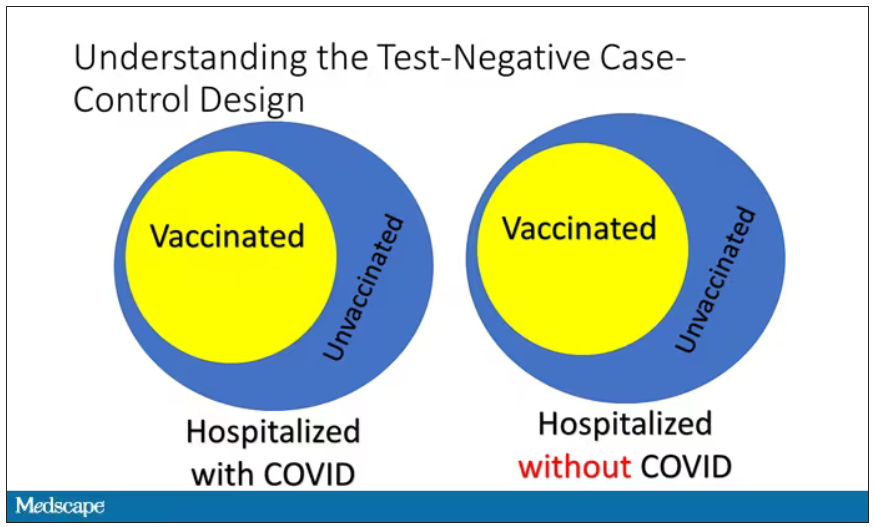
If you look at these Venn diagrams, I’ve got vaccinated/unvaccinated being exactly the same proportion, which would suggest that you’re just as likely to be hospitalized with COVID if you’re vaccinated as you are to be hospitalized with some other respiratory illness, which suggests that the vaccine isn’t particularly effective.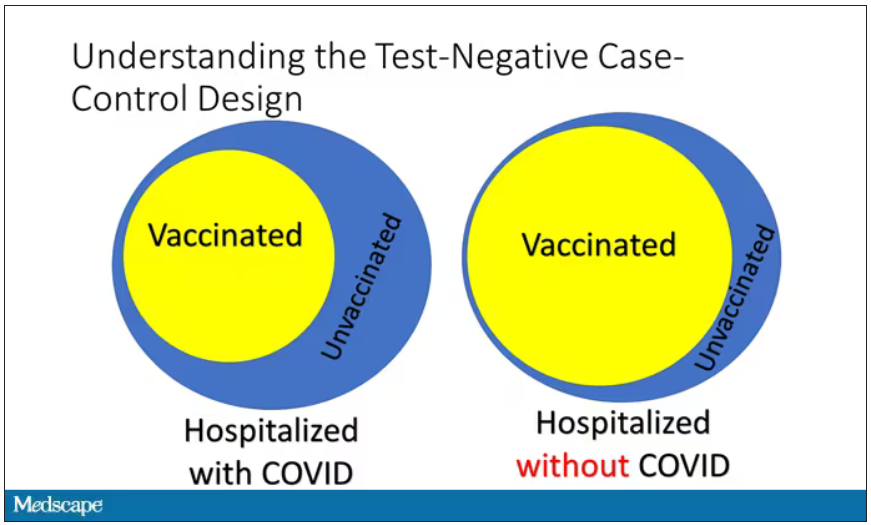
However, if you saw something like this, looking at all those patients with flu and other non-COVID illnesses, a lot more of them had been vaccinated for COVID. What that tells you is that we’re seeing fewer vaccinated people hospitalized with COVID than we would expect because we have this standardization from other respiratory infections. We expect this many vaccinated people because that’s how many vaccinated people there are who show up with flu. But in the COVID population, there are fewer, and that would suggest that the vaccines are effective. So that is the test-negative case-control design. You can do the same thing with ICU stays and death.
There are some assumptions here which you might already be thinking about. The most important one is that vaccination status is not associated with the risk for the disease. I always think of older people in this context. During the pandemic, at least in the United States, older people were much more likely to be vaccinated but were also much more likely to contract COVID and be hospitalized with COVID. The test-negative design actually accounts for this in some sense, because older people are also more likely to be hospitalized for things like flu and pneumonia. So there’s some control there.
But to the extent that older people are uniquely susceptible to COVID compared with other respiratory illnesses, that would bias your results to make the vaccines look worse. So the standard approach here is to adjust for these things. I think the CDC adjusted for age, sex, race, ethnicity, and a few other things to settle down and see how effective the vaccines were.
Let’s get to a worked example.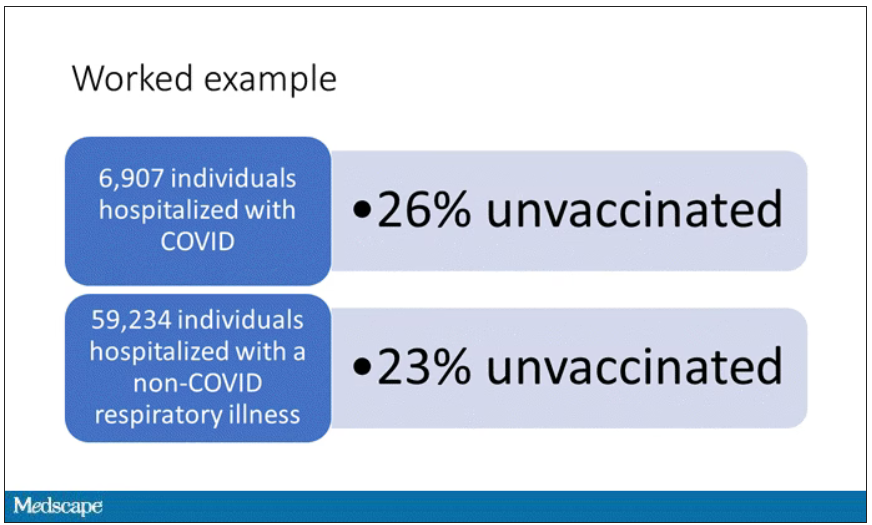
This is the actual data from the CDC paper. They had 6,907 individuals who were hospitalized with COVID, and 26% of them were unvaccinated. What’s the baseline rate that we would expect to be unvaccinated? A total of 59,234 individuals were hospitalized with a non-COVID respiratory illness, and 23% of them were unvaccinated. So you can see that there were more unvaccinated people than you would think in the COVID group. In other words, fewer vaccinated people, which suggests that the vaccine works to some degree because it’s keeping some people out of the hospital.
Now, 26% versus 23% is not a very impressive difference. But it gets more interesting when you break it down by the type of vaccine and how long ago the individual was vaccinated.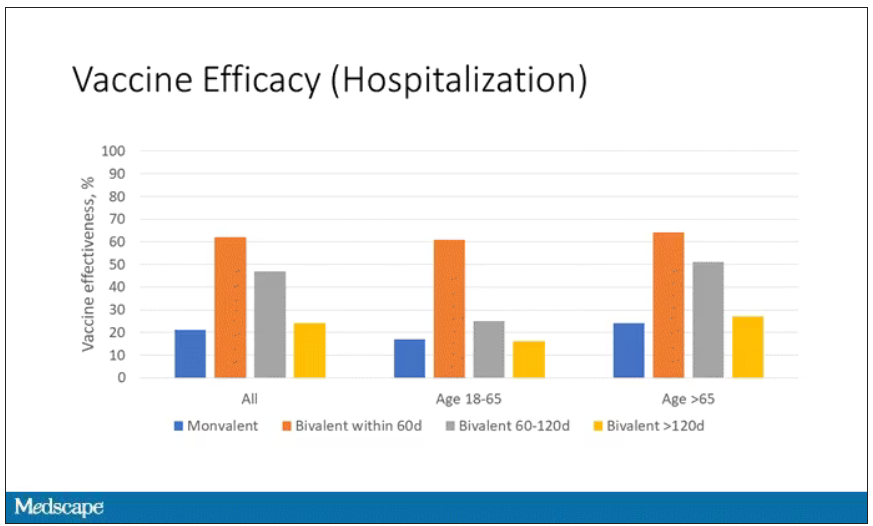
Let’s walk through the “all” group on this figure. What you can see is the calculated vaccine effectiveness. If you look at just the monovalent vaccine here, we see a 20% vaccine effectiveness. This means that you’re preventing 20% of hospitalizations basically due to COVID by people getting vaccinated. That’s okay but it’s certainly not anything to write home about. But we see much better vaccine effectiveness with the bivalent vaccine if it had been received within 60 days.
This compares people who received the bivalent vaccine within 60 days in the COVID group and the non-COVID group. The concern that the vaccine was given very recently affects both groups equally so it shouldn’t result in bias there. You see a step-off in vaccine effectiveness from 60 days, 60-120 days, and greater than 120 days. This is 4 months, and you’ve gone from 60% to 20%. When you break that down by age, you can see a similar pattern in the 18-to-65 group and potentially some more protection the greater than 65 age group.
Why is vaccine efficacy going down? The study doesn’t tell us, but we can hypothesize that this might be an immunologic effect – the antibodies or the protective T cells are waning over time. This could also reflect changes in the virus in the environment as the virus seeks to evade certain immune responses. But overall, this suggests that waiting a year between booster doses may leave you exposed for quite some time, although the take-home here is that bivalent vaccines in general are probably a good idea for the proportion of people who haven’t gotten them.
When we look at critical illness and death, the numbers look a little bit better.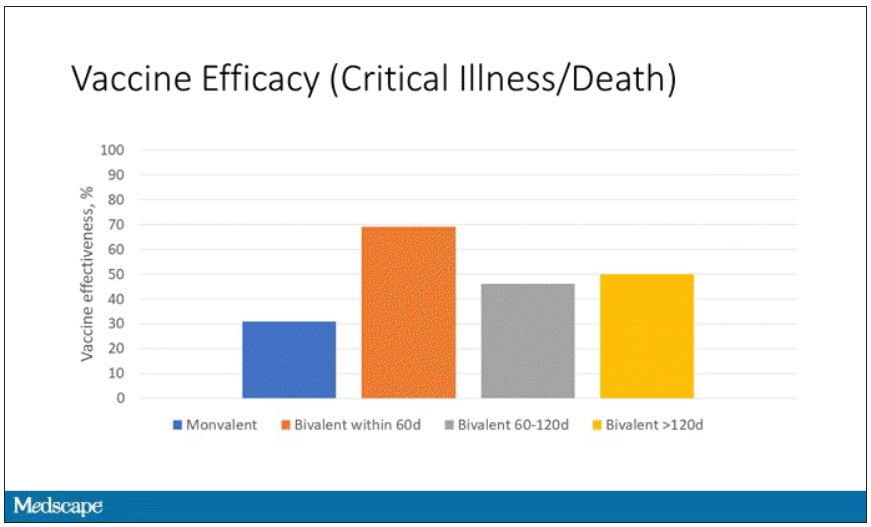
You can see that bivalent is better than monovalent – certainly pretty good if you’ve received it within 60 days. It does tend to wane a little bit, but not nearly as much. You’ve still got about 50% vaccine efficacy beyond 120 days when we’re looking at critical illness, which is stays in the ICU and death.
The overriding thing to think about when we think about vaccine policy is that the way you get immunized against COVID is either by vaccine or by getting infected with COVID, or both.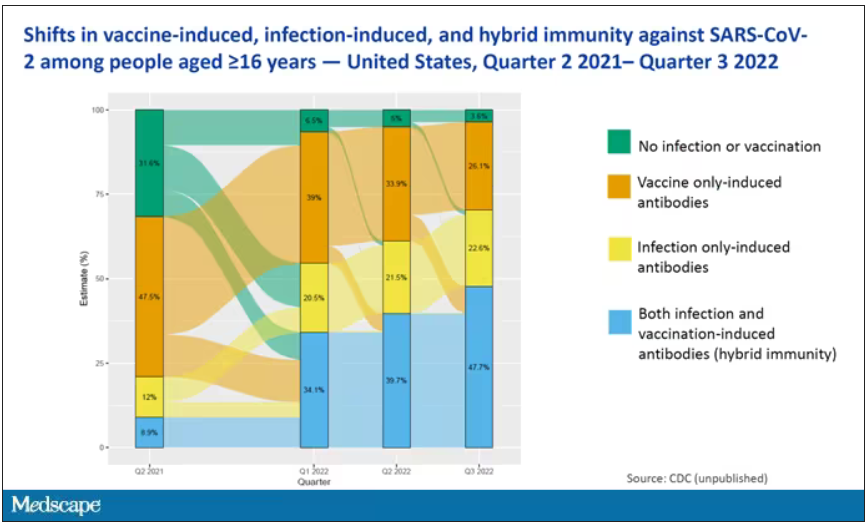
This really interesting graph from the CDC (although it’s updated only through quarter three of 2022) shows the proportion of Americans, based on routine lab tests, who have varying degrees of protection against COVID. What you can see is that, by quarter three of 2022, just 3.6% of people who had blood drawn at a commercial laboratory had no evidence of infection or vaccination. In other words, almost no one was totally naive. Then 26% of people had never been infected – they only have vaccine antibodies – plus 22% of people had only been infected but had never been vaccinated. And then 50% of people had both. So there’s a tremendous amount of existing immunity out there.
The really interesting question about future vaccination and future booster doses is, how does it work on the background of this pattern? The CDC study doesn’t tell us, and I don’t think they have the data to tell us the vaccine efficacy in these different groups. Is it more effective in people who have only had an infection, for example? Is it more effective in people who have only had vaccination versus people who had both, or people who have no protection whatsoever? Those are the really interesting questions that need to be answered going forward as vaccine policy gets developed in the future.
I hope this was a helpful primer on how the test-negative case-control design can answer questions that seem a little bit unanswerable.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
I am here today to talk about the effectiveness of COVID vaccine boosters in the midst of 2023. The reason I want to talk about this isn’t necessarily to dig into exactly how effective vaccines are. This is an area that’s been trod upon multiple times. But it does give me an opportunity to talk about a neat study design called the “test-negative case-control” design, which has some unique properties when you’re trying to evaluate the effect of something outside of the context of a randomized trial.
So, just a little bit of background to remind everyone where we are. These are the number of doses of COVID vaccines administered over time throughout the pandemic.
You can see that it’s stratified by age. The orange lines are adults ages 18-49, for example. You can see a big wave of vaccination when the vaccine first came out at the start of 2021. Then subsequently, you can see smaller waves after the first and second booster authorizations, and maybe a bit of a pickup, particularly among older adults, when the bivalent boosters were authorized. But still very little overall pickup of the bivalent booster, compared with the monovalent vaccines, which might suggest vaccine fatigue going on this far into the pandemic. But it’s important to try to understand exactly how effective those new boosters are, at least at this point in time.
I’m talking about Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022–January 2023, which came out in the Morbidity and Mortality Weekly Report very recently, which uses this test-negative case-control design to evaluate the ability of bivalent mRNA vaccines to prevent hospitalization.
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death? That may not be the question that is of interest to everyone. I know people are interested in symptoms, missed work, and transmission, but this paper was looking at hospitalization, ICU stay, and death.
What’s kind of tricky here is that the data they’re using are in people who are hospitalized with various diseases. You might look at that on the surface and say: “Well, you can’t – that’s impossible.” But you can, actually, with this cool test-negative case-control design.
Here’s basically how it works. You take a population of people who are hospitalized and confirmed to have COVID. Some of them will be vaccinated and some of them will be unvaccinated. And the proportion of vaccinated and unvaccinated people doesn’t tell you very much because it depends on how that compares with the rates in the general population, for instance. Let me clarify this. If 100% of the population were vaccinated, then 100% of the people hospitalized with COVID would be vaccinated. That doesn’t mean vaccines are bad. Put another way, if 90% of the population were vaccinated and 60% of people hospitalized with COVID were vaccinated, that would actually show that the vaccines were working to some extent, all else being equal. So it’s not just the raw percentages that tell you anything. Some people are vaccinated, some people aren’t. You need to understand what the baseline rate is.
The test-negative case-control design looks at people who are hospitalized without COVID. Now who those people are (who the controls are, in this case) is something you really need to think about. In the case of this CDC study, they used people who were hospitalized with COVID-like illnesses – flu-like illnesses, respiratory illnesses, pneumonia, influenza, etc. This is a pretty good idea because it standardizes a little bit for people who have access to healthcare. They can get to a hospital and they’re the type of person who would go to a hospital when they’re feeling sick. That’s a better control than the general population overall, which is something I like about this design.
Some of those people who don’t have COVID (they’re in the hospital for flu or whatever) will have been vaccinated for COVID, and some will not have been vaccinated for COVID. And of course, we don’t expect COVID vaccines necessarily to protect against the flu or pneumonia, but that gives us a way to standardize.
If you look at these Venn diagrams, I’ve got vaccinated/unvaccinated being exactly the same proportion, which would suggest that you’re just as likely to be hospitalized with COVID if you’re vaccinated as you are to be hospitalized with some other respiratory illness, which suggests that the vaccine isn’t particularly effective.
However, if you saw something like this, looking at all those patients with flu and other non-COVID illnesses, a lot more of them had been vaccinated for COVID. What that tells you is that we’re seeing fewer vaccinated people hospitalized with COVID than we would expect because we have this standardization from other respiratory infections. We expect this many vaccinated people because that’s how many vaccinated people there are who show up with flu. But in the COVID population, there are fewer, and that would suggest that the vaccines are effective. So that is the test-negative case-control design. You can do the same thing with ICU stays and death.
There are some assumptions here which you might already be thinking about. The most important one is that vaccination status is not associated with the risk for the disease. I always think of older people in this context. During the pandemic, at least in the United States, older people were much more likely to be vaccinated but were also much more likely to contract COVID and be hospitalized with COVID. The test-negative design actually accounts for this in some sense, because older people are also more likely to be hospitalized for things like flu and pneumonia. So there’s some control there.
But to the extent that older people are uniquely susceptible to COVID compared with other respiratory illnesses, that would bias your results to make the vaccines look worse. So the standard approach here is to adjust for these things. I think the CDC adjusted for age, sex, race, ethnicity, and a few other things to settle down and see how effective the vaccines were.
Let’s get to a worked example.
This is the actual data from the CDC paper. They had 6,907 individuals who were hospitalized with COVID, and 26% of them were unvaccinated. What’s the baseline rate that we would expect to be unvaccinated? A total of 59,234 individuals were hospitalized with a non-COVID respiratory illness, and 23% of them were unvaccinated. So you can see that there were more unvaccinated people than you would think in the COVID group. In other words, fewer vaccinated people, which suggests that the vaccine works to some degree because it’s keeping some people out of the hospital.
Now, 26% versus 23% is not a very impressive difference. But it gets more interesting when you break it down by the type of vaccine and how long ago the individual was vaccinated.
Let’s walk through the “all” group on this figure. What you can see is the calculated vaccine effectiveness. If you look at just the monovalent vaccine here, we see a 20% vaccine effectiveness. This means that you’re preventing 20% of hospitalizations basically due to COVID by people getting vaccinated. That’s okay but it’s certainly not anything to write home about. But we see much better vaccine effectiveness with the bivalent vaccine if it had been received within 60 days.
This compares people who received the bivalent vaccine within 60 days in the COVID group and the non-COVID group. The concern that the vaccine was given very recently affects both groups equally so it shouldn’t result in bias there. You see a step-off in vaccine effectiveness from 60 days, 60-120 days, and greater than 120 days. This is 4 months, and you’ve gone from 60% to 20%. When you break that down by age, you can see a similar pattern in the 18-to-65 group and potentially some more protection the greater than 65 age group.
Why is vaccine efficacy going down? The study doesn’t tell us, but we can hypothesize that this might be an immunologic effect – the antibodies or the protective T cells are waning over time. This could also reflect changes in the virus in the environment as the virus seeks to evade certain immune responses. But overall, this suggests that waiting a year between booster doses may leave you exposed for quite some time, although the take-home here is that bivalent vaccines in general are probably a good idea for the proportion of people who haven’t gotten them.
When we look at critical illness and death, the numbers look a little bit better.
You can see that bivalent is better than monovalent – certainly pretty good if you’ve received it within 60 days. It does tend to wane a little bit, but not nearly as much. You’ve still got about 50% vaccine efficacy beyond 120 days when we’re looking at critical illness, which is stays in the ICU and death.
The overriding thing to think about when we think about vaccine policy is that the way you get immunized against COVID is either by vaccine or by getting infected with COVID, or both.
This really interesting graph from the CDC (although it’s updated only through quarter three of 2022) shows the proportion of Americans, based on routine lab tests, who have varying degrees of protection against COVID. What you can see is that, by quarter three of 2022, just 3.6% of people who had blood drawn at a commercial laboratory had no evidence of infection or vaccination. In other words, almost no one was totally naive. Then 26% of people had never been infected – they only have vaccine antibodies – plus 22% of people had only been infected but had never been vaccinated. And then 50% of people had both. So there’s a tremendous amount of existing immunity out there.
The really interesting question about future vaccination and future booster doses is, how does it work on the background of this pattern? The CDC study doesn’t tell us, and I don’t think they have the data to tell us the vaccine efficacy in these different groups. Is it more effective in people who have only had an infection, for example? Is it more effective in people who have only had vaccination versus people who had both, or people who have no protection whatsoever? Those are the really interesting questions that need to be answered going forward as vaccine policy gets developed in the future.
I hope this was a helpful primer on how the test-negative case-control design can answer questions that seem a little bit unanswerable.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
I am here today to talk about the effectiveness of COVID vaccine boosters in the midst of 2023. The reason I want to talk about this isn’t necessarily to dig into exactly how effective vaccines are. This is an area that’s been trod upon multiple times. But it does give me an opportunity to talk about a neat study design called the “test-negative case-control” design, which has some unique properties when you’re trying to evaluate the effect of something outside of the context of a randomized trial.
So, just a little bit of background to remind everyone where we are. These are the number of doses of COVID vaccines administered over time throughout the pandemic.
You can see that it’s stratified by age. The orange lines are adults ages 18-49, for example. You can see a big wave of vaccination when the vaccine first came out at the start of 2021. Then subsequently, you can see smaller waves after the first and second booster authorizations, and maybe a bit of a pickup, particularly among older adults, when the bivalent boosters were authorized. But still very little overall pickup of the bivalent booster, compared with the monovalent vaccines, which might suggest vaccine fatigue going on this far into the pandemic. But it’s important to try to understand exactly how effective those new boosters are, at least at this point in time.
I’m talking about Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022–January 2023, which came out in the Morbidity and Mortality Weekly Report very recently, which uses this test-negative case-control design to evaluate the ability of bivalent mRNA vaccines to prevent hospitalization.
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death? That may not be the question that is of interest to everyone. I know people are interested in symptoms, missed work, and transmission, but this paper was looking at hospitalization, ICU stay, and death.
What’s kind of tricky here is that the data they’re using are in people who are hospitalized with various diseases. You might look at that on the surface and say: “Well, you can’t – that’s impossible.” But you can, actually, with this cool test-negative case-control design.
Here’s basically how it works. You take a population of people who are hospitalized and confirmed to have COVID. Some of them will be vaccinated and some of them will be unvaccinated. And the proportion of vaccinated and unvaccinated people doesn’t tell you very much because it depends on how that compares with the rates in the general population, for instance. Let me clarify this. If 100% of the population were vaccinated, then 100% of the people hospitalized with COVID would be vaccinated. That doesn’t mean vaccines are bad. Put another way, if 90% of the population were vaccinated and 60% of people hospitalized with COVID were vaccinated, that would actually show that the vaccines were working to some extent, all else being equal. So it’s not just the raw percentages that tell you anything. Some people are vaccinated, some people aren’t. You need to understand what the baseline rate is.
The test-negative case-control design looks at people who are hospitalized without COVID. Now who those people are (who the controls are, in this case) is something you really need to think about. In the case of this CDC study, they used people who were hospitalized with COVID-like illnesses – flu-like illnesses, respiratory illnesses, pneumonia, influenza, etc. This is a pretty good idea because it standardizes a little bit for people who have access to healthcare. They can get to a hospital and they’re the type of person who would go to a hospital when they’re feeling sick. That’s a better control than the general population overall, which is something I like about this design.
Some of those people who don’t have COVID (they’re in the hospital for flu or whatever) will have been vaccinated for COVID, and some will not have been vaccinated for COVID. And of course, we don’t expect COVID vaccines necessarily to protect against the flu or pneumonia, but that gives us a way to standardize.
If you look at these Venn diagrams, I’ve got vaccinated/unvaccinated being exactly the same proportion, which would suggest that you’re just as likely to be hospitalized with COVID if you’re vaccinated as you are to be hospitalized with some other respiratory illness, which suggests that the vaccine isn’t particularly effective.
However, if you saw something like this, looking at all those patients with flu and other non-COVID illnesses, a lot more of them had been vaccinated for COVID. What that tells you is that we’re seeing fewer vaccinated people hospitalized with COVID than we would expect because we have this standardization from other respiratory infections. We expect this many vaccinated people because that’s how many vaccinated people there are who show up with flu. But in the COVID population, there are fewer, and that would suggest that the vaccines are effective. So that is the test-negative case-control design. You can do the same thing with ICU stays and death.
There are some assumptions here which you might already be thinking about. The most important one is that vaccination status is not associated with the risk for the disease. I always think of older people in this context. During the pandemic, at least in the United States, older people were much more likely to be vaccinated but were also much more likely to contract COVID and be hospitalized with COVID. The test-negative design actually accounts for this in some sense, because older people are also more likely to be hospitalized for things like flu and pneumonia. So there’s some control there.
But to the extent that older people are uniquely susceptible to COVID compared with other respiratory illnesses, that would bias your results to make the vaccines look worse. So the standard approach here is to adjust for these things. I think the CDC adjusted for age, sex, race, ethnicity, and a few other things to settle down and see how effective the vaccines were.
Let’s get to a worked example.
This is the actual data from the CDC paper. They had 6,907 individuals who were hospitalized with COVID, and 26% of them were unvaccinated. What’s the baseline rate that we would expect to be unvaccinated? A total of 59,234 individuals were hospitalized with a non-COVID respiratory illness, and 23% of them were unvaccinated. So you can see that there were more unvaccinated people than you would think in the COVID group. In other words, fewer vaccinated people, which suggests that the vaccine works to some degree because it’s keeping some people out of the hospital.
Now, 26% versus 23% is not a very impressive difference. But it gets more interesting when you break it down by the type of vaccine and how long ago the individual was vaccinated.
Let’s walk through the “all” group on this figure. What you can see is the calculated vaccine effectiveness. If you look at just the monovalent vaccine here, we see a 20% vaccine effectiveness. This means that you’re preventing 20% of hospitalizations basically due to COVID by people getting vaccinated. That’s okay but it’s certainly not anything to write home about. But we see much better vaccine effectiveness with the bivalent vaccine if it had been received within 60 days.
This compares people who received the bivalent vaccine within 60 days in the COVID group and the non-COVID group. The concern that the vaccine was given very recently affects both groups equally so it shouldn’t result in bias there. You see a step-off in vaccine effectiveness from 60 days, 60-120 days, and greater than 120 days. This is 4 months, and you’ve gone from 60% to 20%. When you break that down by age, you can see a similar pattern in the 18-to-65 group and potentially some more protection the greater than 65 age group.
Why is vaccine efficacy going down? The study doesn’t tell us, but we can hypothesize that this might be an immunologic effect – the antibodies or the protective T cells are waning over time. This could also reflect changes in the virus in the environment as the virus seeks to evade certain immune responses. But overall, this suggests that waiting a year between booster doses may leave you exposed for quite some time, although the take-home here is that bivalent vaccines in general are probably a good idea for the proportion of people who haven’t gotten them.
When we look at critical illness and death, the numbers look a little bit better.
You can see that bivalent is better than monovalent – certainly pretty good if you’ve received it within 60 days. It does tend to wane a little bit, but not nearly as much. You’ve still got about 50% vaccine efficacy beyond 120 days when we’re looking at critical illness, which is stays in the ICU and death.
The overriding thing to think about when we think about vaccine policy is that the way you get immunized against COVID is either by vaccine or by getting infected with COVID, or both.
This really interesting graph from the CDC (although it’s updated only through quarter three of 2022) shows the proportion of Americans, based on routine lab tests, who have varying degrees of protection against COVID. What you can see is that, by quarter three of 2022, just 3.6% of people who had blood drawn at a commercial laboratory had no evidence of infection or vaccination. In other words, almost no one was totally naive. Then 26% of people had never been infected – they only have vaccine antibodies – plus 22% of people had only been infected but had never been vaccinated. And then 50% of people had both. So there’s a tremendous amount of existing immunity out there.
The really interesting question about future vaccination and future booster doses is, how does it work on the background of this pattern? The CDC study doesn’t tell us, and I don’t think they have the data to tell us the vaccine efficacy in these different groups. Is it more effective in people who have only had an infection, for example? Is it more effective in people who have only had vaccination versus people who had both, or people who have no protection whatsoever? Those are the really interesting questions that need to be answered going forward as vaccine policy gets developed in the future.
I hope this was a helpful primer on how the test-negative case-control design can answer questions that seem a little bit unanswerable.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Talking tobacco with youth? Ask the right questions
There is good news and bad news regarding the use of tobacco products by young people in the United States, according to the recently released findings from the 2021 Youth Risk Behavior Survey (YRBS).1 The use of cigarettes among high school students declined from 36.4% in 1997 to 6.0% in 2019.2 However, young people have replaced cigarettes with other tobacco products, including electronic vapor products (EVPs). So we need to ask specifically about these products.
Known by many names. EVPs are referred to as e-cigarettes, vapes, hookah pens, and mods. They usually contain nicotine, which is highly addictive, can affect brain development, and may lead to smoking of cigarettes.3 The most common reasons young people say they use EVPs are feelings of anxiety, stress, and depression, as well as the “high” associated with nicotine use.4
Use of EVPs among youth. The YRBS, which includes a representative sample of public and private school students in grades 9 to 12 in the 50 states, categorizes the use of EVPs as
- ever use
- current use (≥ 1 use during the 30 days before the survey), and
- daily use (during the 30 days before the survey).
In 2021, 36.2% of young people reported ever use of EVPs (40.9% of females; 32.1% of males), 18% reported current use (21.4% of females; 14.9% of males), and 5% reported daily use (5.6% of females; 4.5% of males). Differences between racial and ethnic groups were minor, except for markedly lower rates in Asian youth (19.5% ever use, 5.5% current use, and 1.2% daily use).5
Current recommendations. The US Preventive Services Task Force (USPSTF) recommends education and brief counseling for school-age children and adolescents to prevent them from starting to use tobacco (including use of EVPs).6 The USPSTF also recommends tobacco cessation using behavioral interventions and/or pharmacotherapy for those ages 18 years and older.7
The USPSTF makes no recommendation on cessation for those younger than 18 years, citing weak evidence. However, it would be reasonable to offer behavioral interventions to younger current users. (Pharmacotherapy is not approved for use in children and adolescents.)
The take-home message. When we ask children and adolescents about use of tobacco products, we need to specifically mention EVPs and advise against their use.
1. CDC. Youth Risk Behavior Surveillance—United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(suppl 1):1-93. Accessed May 24, 2023. www.cdc.gov/mmwr/volumes/72/su/pdfs/su7201-h.pdf
2. Creamer MR, Everett Jones S, Gentzke AS, et al. Tobacco product use among high school students—Youth Risk Behavior Survey, United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(suppl 1):56-63. doi: 10.15585/mmwr.su6901a7
3. National Academies of Sciences, Engineering, and Medicine. Public Health Consequences of E-Cigarettes. Washington, DC: National Academies Press; 2018. Accessed May 24, 2023. https://nap.nationalacademies.org/catalog/24952/public-health-consequences-of-e-cigarettes
4. Gentzke AS, Wang TW, Cornelius M, et al. Tobacco product use and associated factors among middle and high school students—National Youth Tobacco Survey, United States, 2021. MMWR Surveill Summ. 2022;71(no. SS-5):1-29. doi: 10.15585/mmwr.ss7105a1
5. Oliver BE, Jones SE, Hops ED, et al. Electronic vapor product use among high school students—Youth Risk Behavior Survey, United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(suppl 1):93-99. doi: 10.15585/mmwr.su7201a11
6. USPSTF. Tobacco use in children and adolescents: primary care interventions. Final recommendation statement. Published April 28, 2020. Accessed May 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
7. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Final recommendation statement. Published January 19, 2021. Accessed May 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
There is good news and bad news regarding the use of tobacco products by young people in the United States, according to the recently released findings from the 2021 Youth Risk Behavior Survey (YRBS).1 The use of cigarettes among high school students declined from 36.4% in 1997 to 6.0% in 2019.2 However, young people have replaced cigarettes with other tobacco products, including electronic vapor products (EVPs). So we need to ask specifically about these products.
Known by many names. EVPs are referred to as e-cigarettes, vapes, hookah pens, and mods. They usually contain nicotine, which is highly addictive, can affect brain development, and may lead to smoking of cigarettes.3 The most common reasons young people say they use EVPs are feelings of anxiety, stress, and depression, as well as the “high” associated with nicotine use.4
Use of EVPs among youth. The YRBS, which includes a representative sample of public and private school students in grades 9 to 12 in the 50 states, categorizes the use of EVPs as
- ever use
- current use (≥ 1 use during the 30 days before the survey), and
- daily use (during the 30 days before the survey).
In 2021, 36.2% of young people reported ever use of EVPs (40.9% of females; 32.1% of males), 18% reported current use (21.4% of females; 14.9% of males), and 5% reported daily use (5.6% of females; 4.5% of males). Differences between racial and ethnic groups were minor, except for markedly lower rates in Asian youth (19.5% ever use, 5.5% current use, and 1.2% daily use).5
Current recommendations. The US Preventive Services Task Force (USPSTF) recommends education and brief counseling for school-age children and adolescents to prevent them from starting to use tobacco (including use of EVPs).6 The USPSTF also recommends tobacco cessation using behavioral interventions and/or pharmacotherapy for those ages 18 years and older.7
The USPSTF makes no recommendation on cessation for those younger than 18 years, citing weak evidence. However, it would be reasonable to offer behavioral interventions to younger current users. (Pharmacotherapy is not approved for use in children and adolescents.)
The take-home message. When we ask children and adolescents about use of tobacco products, we need to specifically mention EVPs and advise against their use.
There is good news and bad news regarding the use of tobacco products by young people in the United States, according to the recently released findings from the 2021 Youth Risk Behavior Survey (YRBS).1 The use of cigarettes among high school students declined from 36.4% in 1997 to 6.0% in 2019.2 However, young people have replaced cigarettes with other tobacco products, including electronic vapor products (EVPs). So we need to ask specifically about these products.
Known by many names. EVPs are referred to as e-cigarettes, vapes, hookah pens, and mods. They usually contain nicotine, which is highly addictive, can affect brain development, and may lead to smoking of cigarettes.3 The most common reasons young people say they use EVPs are feelings of anxiety, stress, and depression, as well as the “high” associated with nicotine use.4
Use of EVPs among youth. The YRBS, which includes a representative sample of public and private school students in grades 9 to 12 in the 50 states, categorizes the use of EVPs as
- ever use
- current use (≥ 1 use during the 30 days before the survey), and
- daily use (during the 30 days before the survey).
In 2021, 36.2% of young people reported ever use of EVPs (40.9% of females; 32.1% of males), 18% reported current use (21.4% of females; 14.9% of males), and 5% reported daily use (5.6% of females; 4.5% of males). Differences between racial and ethnic groups were minor, except for markedly lower rates in Asian youth (19.5% ever use, 5.5% current use, and 1.2% daily use).5
Current recommendations. The US Preventive Services Task Force (USPSTF) recommends education and brief counseling for school-age children and adolescents to prevent them from starting to use tobacco (including use of EVPs).6 The USPSTF also recommends tobacco cessation using behavioral interventions and/or pharmacotherapy for those ages 18 years and older.7
The USPSTF makes no recommendation on cessation for those younger than 18 years, citing weak evidence. However, it would be reasonable to offer behavioral interventions to younger current users. (Pharmacotherapy is not approved for use in children and adolescents.)
The take-home message. When we ask children and adolescents about use of tobacco products, we need to specifically mention EVPs and advise against their use.
1. CDC. Youth Risk Behavior Surveillance—United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(suppl 1):1-93. Accessed May 24, 2023. www.cdc.gov/mmwr/volumes/72/su/pdfs/su7201-h.pdf
2. Creamer MR, Everett Jones S, Gentzke AS, et al. Tobacco product use among high school students—Youth Risk Behavior Survey, United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(suppl 1):56-63. doi: 10.15585/mmwr.su6901a7
3. National Academies of Sciences, Engineering, and Medicine. Public Health Consequences of E-Cigarettes. Washington, DC: National Academies Press; 2018. Accessed May 24, 2023. https://nap.nationalacademies.org/catalog/24952/public-health-consequences-of-e-cigarettes
4. Gentzke AS, Wang TW, Cornelius M, et al. Tobacco product use and associated factors among middle and high school students—National Youth Tobacco Survey, United States, 2021. MMWR Surveill Summ. 2022;71(no. SS-5):1-29. doi: 10.15585/mmwr.ss7105a1
5. Oliver BE, Jones SE, Hops ED, et al. Electronic vapor product use among high school students—Youth Risk Behavior Survey, United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(suppl 1):93-99. doi: 10.15585/mmwr.su7201a11
6. USPSTF. Tobacco use in children and adolescents: primary care interventions. Final recommendation statement. Published April 28, 2020. Accessed May 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
7. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Final recommendation statement. Published January 19, 2021. Accessed May 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
1. CDC. Youth Risk Behavior Surveillance—United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(suppl 1):1-93. Accessed May 24, 2023. www.cdc.gov/mmwr/volumes/72/su/pdfs/su7201-h.pdf
2. Creamer MR, Everett Jones S, Gentzke AS, et al. Tobacco product use among high school students—Youth Risk Behavior Survey, United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(suppl 1):56-63. doi: 10.15585/mmwr.su6901a7
3. National Academies of Sciences, Engineering, and Medicine. Public Health Consequences of E-Cigarettes. Washington, DC: National Academies Press; 2018. Accessed May 24, 2023. https://nap.nationalacademies.org/catalog/24952/public-health-consequences-of-e-cigarettes
4. Gentzke AS, Wang TW, Cornelius M, et al. Tobacco product use and associated factors among middle and high school students—National Youth Tobacco Survey, United States, 2021. MMWR Surveill Summ. 2022;71(no. SS-5):1-29. doi: 10.15585/mmwr.ss7105a1
5. Oliver BE, Jones SE, Hops ED, et al. Electronic vapor product use among high school students—Youth Risk Behavior Survey, United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(suppl 1):93-99. doi: 10.15585/mmwr.su7201a11
6. USPSTF. Tobacco use in children and adolescents: primary care interventions. Final recommendation statement. Published April 28, 2020. Accessed May 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
7. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Final recommendation statement. Published January 19, 2021. Accessed May 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
Investigating the etiology of recurrent pregnancy loss
With attention to the timing of loss
Introduction: Reassurance through pregnancy loss and workups
Pregnancy loss is not an uncommon complication but it can be associated with significant stress among parents and loved ones when it occurs. Especially when recurrent, it also becomes a medical dilemma for physicians and nurses because the cause is not always obvious immediately, and even with exploration, the cause may not always be found.
First and foremost, it is important that physicians provide counseling and reassurance to families experiencing loss, and that they encourage a level of patience while the investigation for loss is done. Investigations tend not to be linear. One must look at a number of diagnostic areas including genetics, anatomy, immunology, and infections.
Even with an extensive workup, what often is found are potential associations rather than precise causes. For instance, one may find that certain antibodies or certain conditions are present, or that certain anatomic structures are abnormal. While such findings are not necessarily causative, there are therapeutic interventions that we can offer to address many of the conditions (e.g., surgical correction of the septate uterus, and low-dose aspirin and heparin for antiphospholipid syndrome).
Less than 1% of couples experience recurrent pregnancy loss (traditionally defined as three or more losses), so parents who experience one loss should be given reassurance that their loss was likely a sporadic miscarriage and that chances of recurrence will be low. Even as workups proceed, reassurance is important.
For this month’s Master Class in Obstetrics we’ve invited Wendy L. Kinzler, MD, and Anthony Vintzileos, MD, both of whom have expertise in the area of recurrent pregnancy loss, to review the potential causes and the management approach. They focus on the first trimester, when genetic causes predominate, and the early second trimester, when undetected uterine factors can be at play. They explain that the gestational age at which recurrent losses occur is an important factor in decoding etiology and management.
Dr. Kinzler is associate dean, graduate medical education, and professor of obstetrics and gynecology at NYU Long Island School of Medicine, Mineola, N.Y., and Dr. Vintzileos is chief patient safety officer for obstetrics, Northwell Health–Western Region, and professor in the department of obstetrics and gynecology in the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y. Dr. Kinzler and Dr. Vintzileos reported no relevant disclosures.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Investigating the etiology of recurrent pregnancy loss
Pregnancy loss is defined as a loss occurring at < 20 weeks’ gestation.
Consideration of the timing of the pregnancy loss can provide a useful guide to this evaluation, as etiologies vary depending on when in gestation losses occur. In this Master Class, we will address the evaluation of pregnancy loss at < 20 weeks’ gestation, with particular attention to first trimester and early second trimester causes. Literature on the role of the cervix and intra-amniotic infection in midtrimester loss is extensive and will not be covered here.
Although early first trimester miscarriage is common, occurring in approximately 15% of clinically recognized pregnancies, only 2%-3% of couples experience two or more miscarriages and 0.5%-1% experience three or more.
When to begin a diagnostic workup should be part of a shared decision-making process, taking into consideration future family planning, parity, number of previous losses, and notably, the gestational age at which loss(es) occurred. Recurrence rates for first trimester miscarriage are similar after two versus three consecutive losses and either situation usually prompts an evaluation, whereas any second-trimester loss should be evaluated.
Increasingly, we are appreciating the value of a more targeted, gestational age–driven approach to the evaluation of pregnancy loss in an attempt to provide grieving parents with useful information without subjecting them to a wide array of expensive and unnecessary tests.
Genetic causes
The earlier the pregnancy loss, the more likely it is the result of abnormal fetal genetics. Genetic factors should be considered as the most likely cause for first trimester pregnancy losses (especially those occurring at < 10 weeks’ gestation), the most frequent being autosomal trisomies or monosomy X. The vast majority of trisomy conceptuses are sporadic in nature and are related to the natural aging process of the ovary (increasing the rate of meiotic nondisjunction).
If fetal aneuploidy is identified in a pregnancy loss, couples can be counseled about the definitive cause of the loss and can be counseled about recurrence based on age-related risks and/or tests of ovarian reserve. Recurrent pregnancy loss (RPL) is only rarely associated with a parental translocation (< 5%). Testing parental karyotypes should be reserved for cases in which the fetal karyotypes are unknown or in which an unbalanced translocation was identified in the fetus.
When a first trimester pregnancy loss is diagnosed, chorionic villus sampling (CVS) with microarray testing is the most reliable and comprehensive method for evaluating potential genetic causes. It provides valuable information even when cells are not viable and reduces the risk of maternal cell contamination – two significant limitations to standard karyotype analysis performed on tissue passed spontaneously or at the time of D&C. Studies of products of conception (POC) testing with microarray have documented the detection of abnormalities in an additional 10%-15% of cases compared with traditional karyotype analysis.
When CVS is not feasible, testing maternal serum for cell-free DNA is a reasonable alternative. In a prospective cohort study of 50 maternal blood samples taken after fetal demise, 76% of samples yielded cell-free DNA results, meaning fetal fractions were within the detectable range. The higher the gestational age at the time of loss, the higher the chance of obtaining a result: Findings in the study were possible in 88% of samples when the gestational age was 8 weeks or greater, and in 53% of cases involving a lower gestational age. The time from demise to blood draw did not affect the likelihood of obtaining a result (Obstet Gynecol. 2015 Jun;125[6]:1321-29).
When neither CVS nor cell-free DNA analysis is feasible, analysis of either spontaneously passed tissue or tissue obtained at the time of a D&C may still be worthwhile. Maternal cell contamination, of course, is the main downside.
A paper published in 2020 documented the value of refocusing the initial workup. Researchers reported that 57% of 1,400 cases of recurrent pregnancy loss went unexplained using the 2012 ASRM guidelines, which included parental karyotyping but not POC cytogenetic analysis. When parental karyotypes were omitted from the initial workup and POC analysis with 24-chromosome microarray was added, at least one potential explanation for loss could be identified in 92% of cases. Only 8% were left “unexplained” (Curr Opin Obstet Gynecol. 2020 Oct;32[5]:371-9).
When genetics are ruled out
Issues that are top of mind when we lack genetic information or when genetic causes are ruled out include maternal metabolic disorders (uncontrolled diabetes, obesity, uncontrolled thyroid disease), environmental exposures (smoking), uterine abnormalities, and antiphospholipid syndrome.
Thorough evaluation of the uterine cavity after recurrent first trimester miscarriage – or after any second trimester loss – is too often a missing element of investigation. It is a vital component of the evaluation, and information about uterine structure is easily obtained.
A saline infusion sonogram (SIS) allows us to look at the external contour of the uterus, assess the myometrium for muscular abnormalities, visualize the uterine lining, and assess uterine orientation. Performed in the nonpregnant state, and ideally coupled with 3D technology, this relatively simple test can identify congenital uterine anomalies, intracavitary abnormalities (such as fibroids, polyps, or synechiae) which can surgically be removed prior to another pregnancy, a retroverted uterus that may be predisposed to incarceration during pregnancy, and other potentially impactful conditions, such as adenomyosis.
Structural anomalies
Congenital uterine anomalies are associated with first trimester miscarriage, second trimester pregnancy loss, and preterm birth. A uterine septum is of particular concern for early miscarriage, as the early embryo can implant on the relatively avascular septum.
Other congenital uterine anomalies (bicornuate, didelphys, unicornuate) can be associated with concomitant cervical structural abnormalities leading to cervical insufficiency and/or result in pathologic uterine stretch of a space-limited cavity, leading to midtrimester loss or preterm birth. The diagnosis of these anomalies is an important part of the evaluation of pregnancy loss, as it can guide monitoring in future pregnancies, or can be surgically corrected, as in the case of a uterine septum, significantly improving pregnancy outcomes.
A short cervix can result either congenitally or from injury or trauma and may also be associated with cervical insufficiency and miscarriage. It can be evaluated and monitored by ultrasound and, in some cases, treated by surgical cerclage. Pregnancy losses due to cervical insufficiency usually occur after 16 weeks of gestation and frequently are associated with intra-amniotic infections.
Incarcerated uterus and adenomyosis
Other uterine factors that can contribute to pregnancy loss and that are largely underdiagnosed or undiagnosed are an incarcerated retroverted uterus and adenomyosis.
Most of the time, a retroverted uterus naturally assumes an anteverted position by the late first trimester or early second trimester, allowing for continued growth of the uterus and developing fetus. In approximately 10% of cases, however, the retroverted uterus becomes “stuck” or incarcerated in the posterior pelvis. This is more likely if there are large uterine fibroids or in the presence of pelvic adhesions due to endometriosis or previous pelvic surgery.
When this occurs, the fundus is wedged on the sacral promontory (may cause pelvic discomfort and constipation) and the cervix is markedly displaced anteriorly under the pubic symphysis (causing bladder outlet obstruction and urinary retention).
It is critical that ob.gyns. and emergency medicine providers are aware of this condition, which typically presents between 12 and 16 weeks’ gestation. The most frequent complaint is lower abdominal discomfort due to distended bladder and inability to void, which usually leads to bladder catheterization with drainage of large amounts of urine. An incarcerated uterus can predispose to pregnancy loss during this time (few other conditions cause loss during this time window), presumably due to impaired uterine artery blood flow.
Once the diagnosis is made, uterine incarceration may be corrected by elevating the gravid uterus out of the pelvis either manually, or by using a transvaginal ultrasound probe. (The latter minimally invasive approach was described in March at the American Institute of Ultrasound in Medicine’s annual conference by Martin Chavez, MD, and coinvestigators. More invasive approaches are rarely required but include CO2 intraperitoneal insufflation, as used prior to laparoscopy, or laparotomy.
The later in gestation the condition is allowed to persist, the less likely correction will be possible due to the enlarging fundus. Correction between 14 and 16 weeks, or earlier if symptoms develop, is recommended.
Adenomyosis, another poorly understood condition impacting pregnancy outcomes, has been associated with increased rates of miscarriage after in vitro fertilization (in addition to lower implantation rates); a meta-analysis published almost a decade ago found a risk ratio of miscarriage of 2.12 (95% confidence interval, 1.2-3.75) in women with adenomyosis versus those without (Hum Reprod. 2014 May;29[5]:964-77). However, outside of reproductive endocrinology, its impact on pregnancy outcomes in the obstetrical literature is not well recognized.
Although more research is necessary, we believe that adenomyosis should be considered a risk factor for pregnancy loss in the second trimester. The presence of endometrial glands within the myometrium, predisposing for an inflammatory environment, can lead to abnormal implantation, poor uterine distensibility, sterile inflammation, and early cervical insufficiency. As the prevalence of adenomyosis increases with age and maternal ages are increasing, this is an important condition to consider.
Diagnosis is typically made with MRI (although pathology of a hysterectomy specimen is the gold standard). Ultrasound findings consistent with adenomyosis are not routinely assessed and have not been studied in a gravid uterus. Nonetheless, a heightened sense of awareness about this possible contributor to pregnancy loss is very important.
A word about antiphospholipid syndrome
Antiphospholipid syndrome can cause a variety of adverse pregnancy outcomes, including first and second trimester pregnancy loss, fetal demise, fetal growth restriction, preeclampsia, preterm birth, and maternal thromboembolism. The classical presentation of miscarriage due to untreated antiphospholipid antibody syndrome is early severe fetal growth restriction, oligohydramnios, and IUFD in the second trimester.
The diagnosis requires at least one clinical criterion and one laboratory criterion. The mere presence of low level antibodies does not make the diagnosis of antiphospholipid antibody syndrome, and care should be taken to consider both the clinical and laboratory diagnostic criteria to make an accurate diagnosis.
When present, close maternal and fetal surveillance and a combination of low-dose aspirin and heparin are mainstays of treatment. The majority of studies suggest that low-molecular weight heparin (LMWH) and unfractionated heparin have comparable clinical efficacy. However, if a recurrent loss is experienced despite treatment with LMWH, the use of unfractionated heparin in a subsequent pregnancy should be considered.
With attention to the timing of loss
With attention to the timing of loss
Introduction: Reassurance through pregnancy loss and workups
Pregnancy loss is not an uncommon complication but it can be associated with significant stress among parents and loved ones when it occurs. Especially when recurrent, it also becomes a medical dilemma for physicians and nurses because the cause is not always obvious immediately, and even with exploration, the cause may not always be found.
First and foremost, it is important that physicians provide counseling and reassurance to families experiencing loss, and that they encourage a level of patience while the investigation for loss is done. Investigations tend not to be linear. One must look at a number of diagnostic areas including genetics, anatomy, immunology, and infections.
Even with an extensive workup, what often is found are potential associations rather than precise causes. For instance, one may find that certain antibodies or certain conditions are present, or that certain anatomic structures are abnormal. While such findings are not necessarily causative, there are therapeutic interventions that we can offer to address many of the conditions (e.g., surgical correction of the septate uterus, and low-dose aspirin and heparin for antiphospholipid syndrome).
Less than 1% of couples experience recurrent pregnancy loss (traditionally defined as three or more losses), so parents who experience one loss should be given reassurance that their loss was likely a sporadic miscarriage and that chances of recurrence will be low. Even as workups proceed, reassurance is important.
For this month’s Master Class in Obstetrics we’ve invited Wendy L. Kinzler, MD, and Anthony Vintzileos, MD, both of whom have expertise in the area of recurrent pregnancy loss, to review the potential causes and the management approach. They focus on the first trimester, when genetic causes predominate, and the early second trimester, when undetected uterine factors can be at play. They explain that the gestational age at which recurrent losses occur is an important factor in decoding etiology and management.
Dr. Kinzler is associate dean, graduate medical education, and professor of obstetrics and gynecology at NYU Long Island School of Medicine, Mineola, N.Y., and Dr. Vintzileos is chief patient safety officer for obstetrics, Northwell Health–Western Region, and professor in the department of obstetrics and gynecology in the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y. Dr. Kinzler and Dr. Vintzileos reported no relevant disclosures.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Investigating the etiology of recurrent pregnancy loss
Pregnancy loss is defined as a loss occurring at < 20 weeks’ gestation.
Consideration of the timing of the pregnancy loss can provide a useful guide to this evaluation, as etiologies vary depending on when in gestation losses occur. In this Master Class, we will address the evaluation of pregnancy loss at < 20 weeks’ gestation, with particular attention to first trimester and early second trimester causes. Literature on the role of the cervix and intra-amniotic infection in midtrimester loss is extensive and will not be covered here.
Although early first trimester miscarriage is common, occurring in approximately 15% of clinically recognized pregnancies, only 2%-3% of couples experience two or more miscarriages and 0.5%-1% experience three or more.
When to begin a diagnostic workup should be part of a shared decision-making process, taking into consideration future family planning, parity, number of previous losses, and notably, the gestational age at which loss(es) occurred. Recurrence rates for first trimester miscarriage are similar after two versus three consecutive losses and either situation usually prompts an evaluation, whereas any second-trimester loss should be evaluated.
Increasingly, we are appreciating the value of a more targeted, gestational age–driven approach to the evaluation of pregnancy loss in an attempt to provide grieving parents with useful information without subjecting them to a wide array of expensive and unnecessary tests.
Genetic causes
The earlier the pregnancy loss, the more likely it is the result of abnormal fetal genetics. Genetic factors should be considered as the most likely cause for first trimester pregnancy losses (especially those occurring at < 10 weeks’ gestation), the most frequent being autosomal trisomies or monosomy X. The vast majority of trisomy conceptuses are sporadic in nature and are related to the natural aging process of the ovary (increasing the rate of meiotic nondisjunction).
If fetal aneuploidy is identified in a pregnancy loss, couples can be counseled about the definitive cause of the loss and can be counseled about recurrence based on age-related risks and/or tests of ovarian reserve. Recurrent pregnancy loss (RPL) is only rarely associated with a parental translocation (< 5%). Testing parental karyotypes should be reserved for cases in which the fetal karyotypes are unknown or in which an unbalanced translocation was identified in the fetus.
When a first trimester pregnancy loss is diagnosed, chorionic villus sampling (CVS) with microarray testing is the most reliable and comprehensive method for evaluating potential genetic causes. It provides valuable information even when cells are not viable and reduces the risk of maternal cell contamination – two significant limitations to standard karyotype analysis performed on tissue passed spontaneously or at the time of D&C. Studies of products of conception (POC) testing with microarray have documented the detection of abnormalities in an additional 10%-15% of cases compared with traditional karyotype analysis.
When CVS is not feasible, testing maternal serum for cell-free DNA is a reasonable alternative. In a prospective cohort study of 50 maternal blood samples taken after fetal demise, 76% of samples yielded cell-free DNA results, meaning fetal fractions were within the detectable range. The higher the gestational age at the time of loss, the higher the chance of obtaining a result: Findings in the study were possible in 88% of samples when the gestational age was 8 weeks or greater, and in 53% of cases involving a lower gestational age. The time from demise to blood draw did not affect the likelihood of obtaining a result (Obstet Gynecol. 2015 Jun;125[6]:1321-29).
When neither CVS nor cell-free DNA analysis is feasible, analysis of either spontaneously passed tissue or tissue obtained at the time of a D&C may still be worthwhile. Maternal cell contamination, of course, is the main downside.
A paper published in 2020 documented the value of refocusing the initial workup. Researchers reported that 57% of 1,400 cases of recurrent pregnancy loss went unexplained using the 2012 ASRM guidelines, which included parental karyotyping but not POC cytogenetic analysis. When parental karyotypes were omitted from the initial workup and POC analysis with 24-chromosome microarray was added, at least one potential explanation for loss could be identified in 92% of cases. Only 8% were left “unexplained” (Curr Opin Obstet Gynecol. 2020 Oct;32[5]:371-9).
When genetics are ruled out
Issues that are top of mind when we lack genetic information or when genetic causes are ruled out include maternal metabolic disorders (uncontrolled diabetes, obesity, uncontrolled thyroid disease), environmental exposures (smoking), uterine abnormalities, and antiphospholipid syndrome.
Thorough evaluation of the uterine cavity after recurrent first trimester miscarriage – or after any second trimester loss – is too often a missing element of investigation. It is a vital component of the evaluation, and information about uterine structure is easily obtained.
A saline infusion sonogram (SIS) allows us to look at the external contour of the uterus, assess the myometrium for muscular abnormalities, visualize the uterine lining, and assess uterine orientation. Performed in the nonpregnant state, and ideally coupled with 3D technology, this relatively simple test can identify congenital uterine anomalies, intracavitary abnormalities (such as fibroids, polyps, or synechiae) which can surgically be removed prior to another pregnancy, a retroverted uterus that may be predisposed to incarceration during pregnancy, and other potentially impactful conditions, such as adenomyosis.
Structural anomalies
Congenital uterine anomalies are associated with first trimester miscarriage, second trimester pregnancy loss, and preterm birth. A uterine septum is of particular concern for early miscarriage, as the early embryo can implant on the relatively avascular septum.
Other congenital uterine anomalies (bicornuate, didelphys, unicornuate) can be associated with concomitant cervical structural abnormalities leading to cervical insufficiency and/or result in pathologic uterine stretch of a space-limited cavity, leading to midtrimester loss or preterm birth. The diagnosis of these anomalies is an important part of the evaluation of pregnancy loss, as it can guide monitoring in future pregnancies, or can be surgically corrected, as in the case of a uterine septum, significantly improving pregnancy outcomes.
A short cervix can result either congenitally or from injury or trauma and may also be associated with cervical insufficiency and miscarriage. It can be evaluated and monitored by ultrasound and, in some cases, treated by surgical cerclage. Pregnancy losses due to cervical insufficiency usually occur after 16 weeks of gestation and frequently are associated with intra-amniotic infections.
Incarcerated uterus and adenomyosis
Other uterine factors that can contribute to pregnancy loss and that are largely underdiagnosed or undiagnosed are an incarcerated retroverted uterus and adenomyosis.

Most of the time, a retroverted uterus naturally assumes an anteverted position by the late first trimester or early second trimester, allowing for continued growth of the uterus and developing fetus. In approximately 10% of cases, however, the retroverted uterus becomes “stuck” or incarcerated in the posterior pelvis. This is more likely if there are large uterine fibroids or in the presence of pelvic adhesions due to endometriosis or previous pelvic surgery.
When this occurs, the fundus is wedged on the sacral promontory (may cause pelvic discomfort and constipation) and the cervix is markedly displaced anteriorly under the pubic symphysis (causing bladder outlet obstruction and urinary retention).
It is critical that ob.gyns. and emergency medicine providers are aware of this condition, which typically presents between 12 and 16 weeks’ gestation. The most frequent complaint is lower abdominal discomfort due to distended bladder and inability to void, which usually leads to bladder catheterization with drainage of large amounts of urine. An incarcerated uterus can predispose to pregnancy loss during this time (few other conditions cause loss during this time window), presumably due to impaired uterine artery blood flow.
Once the diagnosis is made, uterine incarceration may be corrected by elevating the gravid uterus out of the pelvis either manually, or by using a transvaginal ultrasound probe. (The latter minimally invasive approach was described in March at the American Institute of Ultrasound in Medicine’s annual conference by Martin Chavez, MD, and coinvestigators. More invasive approaches are rarely required but include CO2 intraperitoneal insufflation, as used prior to laparoscopy, or laparotomy.
The later in gestation the condition is allowed to persist, the less likely correction will be possible due to the enlarging fundus. Correction between 14 and 16 weeks, or earlier if symptoms develop, is recommended.
Adenomyosis, another poorly understood condition impacting pregnancy outcomes, has been associated with increased rates of miscarriage after in vitro fertilization (in addition to lower implantation rates); a meta-analysis published almost a decade ago found a risk ratio of miscarriage of 2.12 (95% confidence interval, 1.2-3.75) in women with adenomyosis versus those without (Hum Reprod. 2014 May;29[5]:964-77). However, outside of reproductive endocrinology, its impact on pregnancy outcomes in the obstetrical literature is not well recognized.
Although more research is necessary, we believe that adenomyosis should be considered a risk factor for pregnancy loss in the second trimester. The presence of endometrial glands within the myometrium, predisposing for an inflammatory environment, can lead to abnormal implantation, poor uterine distensibility, sterile inflammation, and early cervical insufficiency. As the prevalence of adenomyosis increases with age and maternal ages are increasing, this is an important condition to consider.
Diagnosis is typically made with MRI (although pathology of a hysterectomy specimen is the gold standard). Ultrasound findings consistent with adenomyosis are not routinely assessed and have not been studied in a gravid uterus. Nonetheless, a heightened sense of awareness about this possible contributor to pregnancy loss is very important.
A word about antiphospholipid syndrome
Antiphospholipid syndrome can cause a variety of adverse pregnancy outcomes, including first and second trimester pregnancy loss, fetal demise, fetal growth restriction, preeclampsia, preterm birth, and maternal thromboembolism. The classical presentation of miscarriage due to untreated antiphospholipid antibody syndrome is early severe fetal growth restriction, oligohydramnios, and IUFD in the second trimester.
The diagnosis requires at least one clinical criterion and one laboratory criterion. The mere presence of low level antibodies does not make the diagnosis of antiphospholipid antibody syndrome, and care should be taken to consider both the clinical and laboratory diagnostic criteria to make an accurate diagnosis.
When present, close maternal and fetal surveillance and a combination of low-dose aspirin and heparin are mainstays of treatment. The majority of studies suggest that low-molecular weight heparin (LMWH) and unfractionated heparin have comparable clinical efficacy. However, if a recurrent loss is experienced despite treatment with LMWH, the use of unfractionated heparin in a subsequent pregnancy should be considered.
Introduction: Reassurance through pregnancy loss and workups
Pregnancy loss is not an uncommon complication but it can be associated with significant stress among parents and loved ones when it occurs. Especially when recurrent, it also becomes a medical dilemma for physicians and nurses because the cause is not always obvious immediately, and even with exploration, the cause may not always be found.
First and foremost, it is important that physicians provide counseling and reassurance to families experiencing loss, and that they encourage a level of patience while the investigation for loss is done. Investigations tend not to be linear. One must look at a number of diagnostic areas including genetics, anatomy, immunology, and infections.
Even with an extensive workup, what often is found are potential associations rather than precise causes. For instance, one may find that certain antibodies or certain conditions are present, or that certain anatomic structures are abnormal. While such findings are not necessarily causative, there are therapeutic interventions that we can offer to address many of the conditions (e.g., surgical correction of the septate uterus, and low-dose aspirin and heparin for antiphospholipid syndrome).
Less than 1% of couples experience recurrent pregnancy loss (traditionally defined as three or more losses), so parents who experience one loss should be given reassurance that their loss was likely a sporadic miscarriage and that chances of recurrence will be low. Even as workups proceed, reassurance is important.
For this month’s Master Class in Obstetrics we’ve invited Wendy L. Kinzler, MD, and Anthony Vintzileos, MD, both of whom have expertise in the area of recurrent pregnancy loss, to review the potential causes and the management approach. They focus on the first trimester, when genetic causes predominate, and the early second trimester, when undetected uterine factors can be at play. They explain that the gestational age at which recurrent losses occur is an important factor in decoding etiology and management.
Dr. Kinzler is associate dean, graduate medical education, and professor of obstetrics and gynecology at NYU Long Island School of Medicine, Mineola, N.Y., and Dr. Vintzileos is chief patient safety officer for obstetrics, Northwell Health–Western Region, and professor in the department of obstetrics and gynecology in the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y. Dr. Kinzler and Dr. Vintzileos reported no relevant disclosures.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Investigating the etiology of recurrent pregnancy loss
Pregnancy loss is defined as a loss occurring at < 20 weeks’ gestation.
Consideration of the timing of the pregnancy loss can provide a useful guide to this evaluation, as etiologies vary depending on when in gestation losses occur. In this Master Class, we will address the evaluation of pregnancy loss at < 20 weeks’ gestation, with particular attention to first trimester and early second trimester causes. Literature on the role of the cervix and intra-amniotic infection in midtrimester loss is extensive and will not be covered here.
Although early first trimester miscarriage is common, occurring in approximately 15% of clinically recognized pregnancies, only 2%-3% of couples experience two or more miscarriages and 0.5%-1% experience three or more.
When to begin a diagnostic workup should be part of a shared decision-making process, taking into consideration future family planning, parity, number of previous losses, and notably, the gestational age at which loss(es) occurred. Recurrence rates for first trimester miscarriage are similar after two versus three consecutive losses and either situation usually prompts an evaluation, whereas any second-trimester loss should be evaluated.
Increasingly, we are appreciating the value of a more targeted, gestational age–driven approach to the evaluation of pregnancy loss in an attempt to provide grieving parents with useful information without subjecting them to a wide array of expensive and unnecessary tests.
Genetic causes
The earlier the pregnancy loss, the more likely it is the result of abnormal fetal genetics. Genetic factors should be considered as the most likely cause for first trimester pregnancy losses (especially those occurring at < 10 weeks’ gestation), the most frequent being autosomal trisomies or monosomy X. The vast majority of trisomy conceptuses are sporadic in nature and are related to the natural aging process of the ovary (increasing the rate of meiotic nondisjunction).
If fetal aneuploidy is identified in a pregnancy loss, couples can be counseled about the definitive cause of the loss and can be counseled about recurrence based on age-related risks and/or tests of ovarian reserve. Recurrent pregnancy loss (RPL) is only rarely associated with a parental translocation (< 5%). Testing parental karyotypes should be reserved for cases in which the fetal karyotypes are unknown or in which an unbalanced translocation was identified in the fetus.
When a first trimester pregnancy loss is diagnosed, chorionic villus sampling (CVS) with microarray testing is the most reliable and comprehensive method for evaluating potential genetic causes. It provides valuable information even when cells are not viable and reduces the risk of maternal cell contamination – two significant limitations to standard karyotype analysis performed on tissue passed spontaneously or at the time of D&C. Studies of products of conception (POC) testing with microarray have documented the detection of abnormalities in an additional 10%-15% of cases compared with traditional karyotype analysis.
When CVS is not feasible, testing maternal serum for cell-free DNA is a reasonable alternative. In a prospective cohort study of 50 maternal blood samples taken after fetal demise, 76% of samples yielded cell-free DNA results, meaning fetal fractions were within the detectable range. The higher the gestational age at the time of loss, the higher the chance of obtaining a result: Findings in the study were possible in 88% of samples when the gestational age was 8 weeks or greater, and in 53% of cases involving a lower gestational age. The time from demise to blood draw did not affect the likelihood of obtaining a result (Obstet Gynecol. 2015 Jun;125[6]:1321-29).
When neither CVS nor cell-free DNA analysis is feasible, analysis of either spontaneously passed tissue or tissue obtained at the time of a D&C may still be worthwhile. Maternal cell contamination, of course, is the main downside.
A paper published in 2020 documented the value of refocusing the initial workup. Researchers reported that 57% of 1,400 cases of recurrent pregnancy loss went unexplained using the 2012 ASRM guidelines, which included parental karyotyping but not POC cytogenetic analysis. When parental karyotypes were omitted from the initial workup and POC analysis with 24-chromosome microarray was added, at least one potential explanation for loss could be identified in 92% of cases. Only 8% were left “unexplained” (Curr Opin Obstet Gynecol. 2020 Oct;32[5]:371-9).
When genetics are ruled out
Issues that are top of mind when we lack genetic information or when genetic causes are ruled out include maternal metabolic disorders (uncontrolled diabetes, obesity, uncontrolled thyroid disease), environmental exposures (smoking), uterine abnormalities, and antiphospholipid syndrome.
Thorough evaluation of the uterine cavity after recurrent first trimester miscarriage – or after any second trimester loss – is too often a missing element of investigation. It is a vital component of the evaluation, and information about uterine structure is easily obtained.
A saline infusion sonogram (SIS) allows us to look at the external contour of the uterus, assess the myometrium for muscular abnormalities, visualize the uterine lining, and assess uterine orientation. Performed in the nonpregnant state, and ideally coupled with 3D technology, this relatively simple test can identify congenital uterine anomalies, intracavitary abnormalities (such as fibroids, polyps, or synechiae) which can surgically be removed prior to another pregnancy, a retroverted uterus that may be predisposed to incarceration during pregnancy, and other potentially impactful conditions, such as adenomyosis.
Structural anomalies
Congenital uterine anomalies are associated with first trimester miscarriage, second trimester pregnancy loss, and preterm birth. A uterine septum is of particular concern for early miscarriage, as the early embryo can implant on the relatively avascular septum.
Other congenital uterine anomalies (bicornuate, didelphys, unicornuate) can be associated with concomitant cervical structural abnormalities leading to cervical insufficiency and/or result in pathologic uterine stretch of a space-limited cavity, leading to midtrimester loss or preterm birth. The diagnosis of these anomalies is an important part of the evaluation of pregnancy loss, as it can guide monitoring in future pregnancies, or can be surgically corrected, as in the case of a uterine septum, significantly improving pregnancy outcomes.
A short cervix can result either congenitally or from injury or trauma and may also be associated with cervical insufficiency and miscarriage. It can be evaluated and monitored by ultrasound and, in some cases, treated by surgical cerclage. Pregnancy losses due to cervical insufficiency usually occur after 16 weeks of gestation and frequently are associated with intra-amniotic infections.
Incarcerated uterus and adenomyosis
Other uterine factors that can contribute to pregnancy loss and that are largely underdiagnosed or undiagnosed are an incarcerated retroverted uterus and adenomyosis.

Most of the time, a retroverted uterus naturally assumes an anteverted position by the late first trimester or early second trimester, allowing for continued growth of the uterus and developing fetus. In approximately 10% of cases, however, the retroverted uterus becomes “stuck” or incarcerated in the posterior pelvis. This is more likely if there are large uterine fibroids or in the presence of pelvic adhesions due to endometriosis or previous pelvic surgery.
When this occurs, the fundus is wedged on the sacral promontory (may cause pelvic discomfort and constipation) and the cervix is markedly displaced anteriorly under the pubic symphysis (causing bladder outlet obstruction and urinary retention).
It is critical that ob.gyns. and emergency medicine providers are aware of this condition, which typically presents between 12 and 16 weeks’ gestation. The most frequent complaint is lower abdominal discomfort due to distended bladder and inability to void, which usually leads to bladder catheterization with drainage of large amounts of urine. An incarcerated uterus can predispose to pregnancy loss during this time (few other conditions cause loss during this time window), presumably due to impaired uterine artery blood flow.
Once the diagnosis is made, uterine incarceration may be corrected by elevating the gravid uterus out of the pelvis either manually, or by using a transvaginal ultrasound probe. (The latter minimally invasive approach was described in March at the American Institute of Ultrasound in Medicine’s annual conference by Martin Chavez, MD, and coinvestigators. More invasive approaches are rarely required but include CO2 intraperitoneal insufflation, as used prior to laparoscopy, or laparotomy.
The later in gestation the condition is allowed to persist, the less likely correction will be possible due to the enlarging fundus. Correction between 14 and 16 weeks, or earlier if symptoms develop, is recommended.
Adenomyosis, another poorly understood condition impacting pregnancy outcomes, has been associated with increased rates of miscarriage after in vitro fertilization (in addition to lower implantation rates); a meta-analysis published almost a decade ago found a risk ratio of miscarriage of 2.12 (95% confidence interval, 1.2-3.75) in women with adenomyosis versus those without (Hum Reprod. 2014 May;29[5]:964-77). However, outside of reproductive endocrinology, its impact on pregnancy outcomes in the obstetrical literature is not well recognized.
Although more research is necessary, we believe that adenomyosis should be considered a risk factor for pregnancy loss in the second trimester. The presence of endometrial glands within the myometrium, predisposing for an inflammatory environment, can lead to abnormal implantation, poor uterine distensibility, sterile inflammation, and early cervical insufficiency. As the prevalence of adenomyosis increases with age and maternal ages are increasing, this is an important condition to consider.
Diagnosis is typically made with MRI (although pathology of a hysterectomy specimen is the gold standard). Ultrasound findings consistent with adenomyosis are not routinely assessed and have not been studied in a gravid uterus. Nonetheless, a heightened sense of awareness about this possible contributor to pregnancy loss is very important.
A word about antiphospholipid syndrome
Antiphospholipid syndrome can cause a variety of adverse pregnancy outcomes, including first and second trimester pregnancy loss, fetal demise, fetal growth restriction, preeclampsia, preterm birth, and maternal thromboembolism. The classical presentation of miscarriage due to untreated antiphospholipid antibody syndrome is early severe fetal growth restriction, oligohydramnios, and IUFD in the second trimester.
The diagnosis requires at least one clinical criterion and one laboratory criterion. The mere presence of low level antibodies does not make the diagnosis of antiphospholipid antibody syndrome, and care should be taken to consider both the clinical and laboratory diagnostic criteria to make an accurate diagnosis.
When present, close maternal and fetal surveillance and a combination of low-dose aspirin and heparin are mainstays of treatment. The majority of studies suggest that low-molecular weight heparin (LMWH) and unfractionated heparin have comparable clinical efficacy. However, if a recurrent loss is experienced despite treatment with LMWH, the use of unfractionated heparin in a subsequent pregnancy should be considered.
We can reduce suicide with enforced treatment and eyesight supervision
The old man was restrained at the last moment from jumping off the hospital’s fifth floor atrium parapet. He was suffering from terminal cancer and had been racked with chronic, severe pain for months.
The consult recognized symptoms of depression arising from his continuous physical suffering, advising that a male aide be dispatched to sit with the man and that he be put on a regimen of 10 mg of methadone twice a day to alleviate the pain. The following day the man was calm; he no longer wanted to kill himself. He expressed a strong desire to go home, return to gardening, and to play with his grandchildren.
Most of the 47,000 suicides that occur in the United States every year are preventable.1 Our national policy on this front has been nothing short of an abject failure. The government implemented a system with limited effect on completed suicides – a telephone hotline. This hotline is not called by the most common suicide victims: male, old, and quiet.2
The consequences of this national policy disaster have been profound, resulting in the biggest loss of productive years of life for any fatal condition.3 The grief experienced by the families of those who commit suicide is far greater than normal bereavement: The cause of death of their loved ones was not an unfortunate accident or a disease, it was an intentional act, and families take it personally.
One of the greatest achievements of psychiatry during the 20th century was the lowering of suicide rates in prisons by 70% with no treatment, no additional staffing, and no additional expenditures of funds. Today if inmates threaten suicide, they are immediately placed under eyesight supervision by guards. The federal pamphlet that describes this protocol was published in 1995 and is freely available online.4
Half of all suicide attempts are made by individuals who are legally drunk.5 Watch them for 6 hours and then ask them if they want to kill themselves and the response will almost invariably be “Of course not,” with the risk of further attempts dissipating in step with their blood alcohol level. The best resources to provide this kind of intervention are responsible adult family members, at no cost to the government. Indeed, in many cases family supervision is superior to that provided by a locked psychiatric ward with three staff members chasing after 20 agitated people all night long. The one-on-one attention that a family member can provide is free as well as far more personal and insightful, and more sincerely caring.
Guarantees in the field of medicine are rare. But, one such guarantee is that after their mood has improved, 100% of people will be thankful that they did not hurt themselves.6 This means that successful treatment will prevent 100% of all suicides.7 Not all treatments are successful, but 95% can be.8 At autopsy, few successful suicide victims have psychiatric medications in their system.9 The urge to kill oneself might best be characterized as a temporary chemical alteration of the brain causing delusional thoughts, including the ultimate delusion that life is not worth living.10 This alteration suppresses the strongest, most fundamental urge of all, namely, the survival instinct.
We must overturn the catastrophic decision of the U.S. Supreme Court that requires the showing of a dangerous act and the holding of a trial employing at least three lawyers for involuntary commitment to be authorized. Rather, involuntary treatment is justified by medical necessity as determined by two licensed professionals with no conflicts of interest. It can be outpatient.
The Supreme Court’s decision in O’Connor v. Donaldson in 1975 remedied an illusory wrong, addressing an act of blatant malpractice, not policy inequity.11 The superintendent of the state facility in that case was not even a doctor. He kept O’Connor prisoner for more than a decade, perhaps to keep a bed filled. Over the past half-century, this one decision has resulted in 1 million preventable suicides12 and half a million senseless murders by paranoid individuals, including many rampage shootings.13
More than two-thirds of homeless individuals suffer from an untreated mental condition.14 The vast majority of them will refuse all offers of treatment because they also have anosognosia, a brain-based disorder causing denial of illness.15 By referring to these individuals as “homeless,” we are also lowering real estate values for a square block around where they happen to be camped out. That cost has never been calculated, but it is another real consequence of this devastating Supreme Court decision.16
Detractors and mental health rights activists may argue that individual rights cannot be infringed. In that case, those same detractors and mental health rights activists must take responsibility for the thousands of lives and billions of dollars in economic damage caused by their refusal to allow an effective solution to the suicide epidemic to be implemented. Enforced outpatient treatment by the U.S. Air Force dropped its suicide rate by 60%. As an unintended benefit, the murder rate dropped by 50%.17
It is high time we did so.
Dr. Behar is a psychiatrist in Lower Merion, Pa. He graduated from Hahnemann Medical College, Philadelphia, in 1975, and has had postgraduate training at SUNY Stony Brook, University of Iowa, the National Institute of Mental Health, and Columbia University. His practice focuses on difficult, treatment-resistant cases.
References
1. U.S. Centers for Disease Control and Prevention. Suicide Prevention. 2020.
2. Luoma JB et al. Contact with mental health and primary care providers before suicide: A review of the evidence. Am J Psychiatry. 2002 Jun 1. doi: 10.1176/appi.ajp.159.6.909.
3. World Health Organization. Suicide. 2021 Jun 17.
4. National Institute of Corrections. Correctional suicide prevention: Policies and procedures. 1995.
5. Hufford MR. Alcohol and suicidal behavior. Clin Psychol Rev. 2001 Jul;21(5):797-811.
6. Stanley B and Brown GK. Safety planning intervention: A brief intervention to mitigate suicide risk. Cogn Behav Pract. 2012 May;19(2):256-64.
7. Ibid.
8. Brown GK and Jager-Hyman S. Evidence-based psychotherapies for suicide prevention: Future directions. Am J Prev Med. 2014 Sep;47(3 Suppl 2):S186-94.
9. Isometsä ET. Psychological autopsy studies – A review. Eur Psychiatry. 2001 Nov;16(7):379-85.
10. Van Orden KA et al. The interpersonal theory of suicide. Psychol Rev. 2010 Apr; 117(2):575-600.
11. O’Connor v. Donaldson, 422 U.S. 563 (1975).
12. Calculated based on annual suicide statistics from the CDC and the time elapsed since the Supreme Court decision in O’Connor v. Donaldson.
13. Metzl JM and MacLeish KT. Mental illness, mass shootings, and the politics of American firearms. Am J Public Health. 2015 Feb;105(2):240-9.
14. Fazel S et al. The prevalence of mental disorders among the homeless in western countries: Systematic review and meta-regression analysis. PLoS Med. 2008 Dec 2;5(12):e225.
15. Amador XF and David AS. (eds.) Insight and psychosis: Awareness of illness in schizophrenia and related disorders (2nd ed.). Oxford Univ Press. 2004.
16. Calculated based on the potential impact of homelessness on property values and the relationship between untreated mental illness and homelessness.
17. Armed Forces Health Surveillance Branch. Surveillance snapshot: Manner and cause of death, active component, U.S. Armed Forces, 1998-2015. Medical Surveillance Monthly Report. 2016 Apr;23(4):19.
The old man was restrained at the last moment from jumping off the hospital’s fifth floor atrium parapet. He was suffering from terminal cancer and had been racked with chronic, severe pain for months.
The consult recognized symptoms of depression arising from his continuous physical suffering, advising that a male aide be dispatched to sit with the man and that he be put on a regimen of 10 mg of methadone twice a day to alleviate the pain. The following day the man was calm; he no longer wanted to kill himself. He expressed a strong desire to go home, return to gardening, and to play with his grandchildren.
Most of the 47,000 suicides that occur in the United States every year are preventable.1 Our national policy on this front has been nothing short of an abject failure. The government implemented a system with limited effect on completed suicides – a telephone hotline. This hotline is not called by the most common suicide victims: male, old, and quiet.2
The consequences of this national policy disaster have been profound, resulting in the biggest loss of productive years of life for any fatal condition.3 The grief experienced by the families of those who commit suicide is far greater than normal bereavement: The cause of death of their loved ones was not an unfortunate accident or a disease, it was an intentional act, and families take it personally.
One of the greatest achievements of psychiatry during the 20th century was the lowering of suicide rates in prisons by 70% with no treatment, no additional staffing, and no additional expenditures of funds. Today if inmates threaten suicide, they are immediately placed under eyesight supervision by guards. The federal pamphlet that describes this protocol was published in 1995 and is freely available online.4
Half of all suicide attempts are made by individuals who are legally drunk.5 Watch them for 6 hours and then ask them if they want to kill themselves and the response will almost invariably be “Of course not,” with the risk of further attempts dissipating in step with their blood alcohol level. The best resources to provide this kind of intervention are responsible adult family members, at no cost to the government. Indeed, in many cases family supervision is superior to that provided by a locked psychiatric ward with three staff members chasing after 20 agitated people all night long. The one-on-one attention that a family member can provide is free as well as far more personal and insightful, and more sincerely caring.
Guarantees in the field of medicine are rare. But, one such guarantee is that after their mood has improved, 100% of people will be thankful that they did not hurt themselves.6 This means that successful treatment will prevent 100% of all suicides.7 Not all treatments are successful, but 95% can be.8 At autopsy, few successful suicide victims have psychiatric medications in their system.9 The urge to kill oneself might best be characterized as a temporary chemical alteration of the brain causing delusional thoughts, including the ultimate delusion that life is not worth living.10 This alteration suppresses the strongest, most fundamental urge of all, namely, the survival instinct.
We must overturn the catastrophic decision of the U.S. Supreme Court that requires the showing of a dangerous act and the holding of a trial employing at least three lawyers for involuntary commitment to be authorized. Rather, involuntary treatment is justified by medical necessity as determined by two licensed professionals with no conflicts of interest. It can be outpatient.
The Supreme Court’s decision in O’Connor v. Donaldson in 1975 remedied an illusory wrong, addressing an act of blatant malpractice, not policy inequity.11 The superintendent of the state facility in that case was not even a doctor. He kept O’Connor prisoner for more than a decade, perhaps to keep a bed filled. Over the past half-century, this one decision has resulted in 1 million preventable suicides12 and half a million senseless murders by paranoid individuals, including many rampage shootings.13
More than two-thirds of homeless individuals suffer from an untreated mental condition.14 The vast majority of them will refuse all offers of treatment because they also have anosognosia, a brain-based disorder causing denial of illness.15 By referring to these individuals as “homeless,” we are also lowering real estate values for a square block around where they happen to be camped out. That cost has never been calculated, but it is another real consequence of this devastating Supreme Court decision.16
Detractors and mental health rights activists may argue that individual rights cannot be infringed. In that case, those same detractors and mental health rights activists must take responsibility for the thousands of lives and billions of dollars in economic damage caused by their refusal to allow an effective solution to the suicide epidemic to be implemented. Enforced outpatient treatment by the U.S. Air Force dropped its suicide rate by 60%. As an unintended benefit, the murder rate dropped by 50%.17
It is high time we did so.
Dr. Behar is a psychiatrist in Lower Merion, Pa. He graduated from Hahnemann Medical College, Philadelphia, in 1975, and has had postgraduate training at SUNY Stony Brook, University of Iowa, the National Institute of Mental Health, and Columbia University. His practice focuses on difficult, treatment-resistant cases.
References
1. U.S. Centers for Disease Control and Prevention. Suicide Prevention. 2020.
2. Luoma JB et al. Contact with mental health and primary care providers before suicide: A review of the evidence. Am J Psychiatry. 2002 Jun 1. doi: 10.1176/appi.ajp.159.6.909.
3. World Health Organization. Suicide. 2021 Jun 17.
4. National Institute of Corrections. Correctional suicide prevention: Policies and procedures. 1995.
5. Hufford MR. Alcohol and suicidal behavior. Clin Psychol Rev. 2001 Jul;21(5):797-811.
6. Stanley B and Brown GK. Safety planning intervention: A brief intervention to mitigate suicide risk. Cogn Behav Pract. 2012 May;19(2):256-64.
7. Ibid.
8. Brown GK and Jager-Hyman S. Evidence-based psychotherapies for suicide prevention: Future directions. Am J Prev Med. 2014 Sep;47(3 Suppl 2):S186-94.
9. Isometsä ET. Psychological autopsy studies – A review. Eur Psychiatry. 2001 Nov;16(7):379-85.
10. Van Orden KA et al. The interpersonal theory of suicide. Psychol Rev. 2010 Apr; 117(2):575-600.
11. O’Connor v. Donaldson, 422 U.S. 563 (1975).
12. Calculated based on annual suicide statistics from the CDC and the time elapsed since the Supreme Court decision in O’Connor v. Donaldson.
13. Metzl JM and MacLeish KT. Mental illness, mass shootings, and the politics of American firearms. Am J Public Health. 2015 Feb;105(2):240-9.
14. Fazel S et al. The prevalence of mental disorders among the homeless in western countries: Systematic review and meta-regression analysis. PLoS Med. 2008 Dec 2;5(12):e225.
15. Amador XF and David AS. (eds.) Insight and psychosis: Awareness of illness in schizophrenia and related disorders (2nd ed.). Oxford Univ Press. 2004.
16. Calculated based on the potential impact of homelessness on property values and the relationship between untreated mental illness and homelessness.
17. Armed Forces Health Surveillance Branch. Surveillance snapshot: Manner and cause of death, active component, U.S. Armed Forces, 1998-2015. Medical Surveillance Monthly Report. 2016 Apr;23(4):19.
The old man was restrained at the last moment from jumping off the hospital’s fifth floor atrium parapet. He was suffering from terminal cancer and had been racked with chronic, severe pain for months.
The consult recognized symptoms of depression arising from his continuous physical suffering, advising that a male aide be dispatched to sit with the man and that he be put on a regimen of 10 mg of methadone twice a day to alleviate the pain. The following day the man was calm; he no longer wanted to kill himself. He expressed a strong desire to go home, return to gardening, and to play with his grandchildren.
Most of the 47,000 suicides that occur in the United States every year are preventable.1 Our national policy on this front has been nothing short of an abject failure. The government implemented a system with limited effect on completed suicides – a telephone hotline. This hotline is not called by the most common suicide victims: male, old, and quiet.2
The consequences of this national policy disaster have been profound, resulting in the biggest loss of productive years of life for any fatal condition.3 The grief experienced by the families of those who commit suicide is far greater than normal bereavement: The cause of death of their loved ones was not an unfortunate accident or a disease, it was an intentional act, and families take it personally.
One of the greatest achievements of psychiatry during the 20th century was the lowering of suicide rates in prisons by 70% with no treatment, no additional staffing, and no additional expenditures of funds. Today if inmates threaten suicide, they are immediately placed under eyesight supervision by guards. The federal pamphlet that describes this protocol was published in 1995 and is freely available online.4
Half of all suicide attempts are made by individuals who are legally drunk.5 Watch them for 6 hours and then ask them if they want to kill themselves and the response will almost invariably be “Of course not,” with the risk of further attempts dissipating in step with their blood alcohol level. The best resources to provide this kind of intervention are responsible adult family members, at no cost to the government. Indeed, in many cases family supervision is superior to that provided by a locked psychiatric ward with three staff members chasing after 20 agitated people all night long. The one-on-one attention that a family member can provide is free as well as far more personal and insightful, and more sincerely caring.
Guarantees in the field of medicine are rare. But, one such guarantee is that after their mood has improved, 100% of people will be thankful that they did not hurt themselves.6 This means that successful treatment will prevent 100% of all suicides.7 Not all treatments are successful, but 95% can be.8 At autopsy, few successful suicide victims have psychiatric medications in their system.9 The urge to kill oneself might best be characterized as a temporary chemical alteration of the brain causing delusional thoughts, including the ultimate delusion that life is not worth living.10 This alteration suppresses the strongest, most fundamental urge of all, namely, the survival instinct.
We must overturn the catastrophic decision of the U.S. Supreme Court that requires the showing of a dangerous act and the holding of a trial employing at least three lawyers for involuntary commitment to be authorized. Rather, involuntary treatment is justified by medical necessity as determined by two licensed professionals with no conflicts of interest. It can be outpatient.
The Supreme Court’s decision in O’Connor v. Donaldson in 1975 remedied an illusory wrong, addressing an act of blatant malpractice, not policy inequity.11 The superintendent of the state facility in that case was not even a doctor. He kept O’Connor prisoner for more than a decade, perhaps to keep a bed filled. Over the past half-century, this one decision has resulted in 1 million preventable suicides12 and half a million senseless murders by paranoid individuals, including many rampage shootings.13
More than two-thirds of homeless individuals suffer from an untreated mental condition.14 The vast majority of them will refuse all offers of treatment because they also have anosognosia, a brain-based disorder causing denial of illness.15 By referring to these individuals as “homeless,” we are also lowering real estate values for a square block around where they happen to be camped out. That cost has never been calculated, but it is another real consequence of this devastating Supreme Court decision.16
Detractors and mental health rights activists may argue that individual rights cannot be infringed. In that case, those same detractors and mental health rights activists must take responsibility for the thousands of lives and billions of dollars in economic damage caused by their refusal to allow an effective solution to the suicide epidemic to be implemented. Enforced outpatient treatment by the U.S. Air Force dropped its suicide rate by 60%. As an unintended benefit, the murder rate dropped by 50%.17
It is high time we did so.
Dr. Behar is a psychiatrist in Lower Merion, Pa. He graduated from Hahnemann Medical College, Philadelphia, in 1975, and has had postgraduate training at SUNY Stony Brook, University of Iowa, the National Institute of Mental Health, and Columbia University. His practice focuses on difficult, treatment-resistant cases.
References
1. U.S. Centers for Disease Control and Prevention. Suicide Prevention. 2020.
2. Luoma JB et al. Contact with mental health and primary care providers before suicide: A review of the evidence. Am J Psychiatry. 2002 Jun 1. doi: 10.1176/appi.ajp.159.6.909.
3. World Health Organization. Suicide. 2021 Jun 17.
4. National Institute of Corrections. Correctional suicide prevention: Policies and procedures. 1995.
5. Hufford MR. Alcohol and suicidal behavior. Clin Psychol Rev. 2001 Jul;21(5):797-811.
6. Stanley B and Brown GK. Safety planning intervention: A brief intervention to mitigate suicide risk. Cogn Behav Pract. 2012 May;19(2):256-64.
7. Ibid.
8. Brown GK and Jager-Hyman S. Evidence-based psychotherapies for suicide prevention: Future directions. Am J Prev Med. 2014 Sep;47(3 Suppl 2):S186-94.
9. Isometsä ET. Psychological autopsy studies – A review. Eur Psychiatry. 2001 Nov;16(7):379-85.
10. Van Orden KA et al. The interpersonal theory of suicide. Psychol Rev. 2010 Apr; 117(2):575-600.
11. O’Connor v. Donaldson, 422 U.S. 563 (1975).
12. Calculated based on annual suicide statistics from the CDC and the time elapsed since the Supreme Court decision in O’Connor v. Donaldson.
13. Metzl JM and MacLeish KT. Mental illness, mass shootings, and the politics of American firearms. Am J Public Health. 2015 Feb;105(2):240-9.
14. Fazel S et al. The prevalence of mental disorders among the homeless in western countries: Systematic review and meta-regression analysis. PLoS Med. 2008 Dec 2;5(12):e225.
15. Amador XF and David AS. (eds.) Insight and psychosis: Awareness of illness in schizophrenia and related disorders (2nd ed.). Oxford Univ Press. 2004.
16. Calculated based on the potential impact of homelessness on property values and the relationship between untreated mental illness and homelessness.
17. Armed Forces Health Surveillance Branch. Surveillance snapshot: Manner and cause of death, active component, U.S. Armed Forces, 1998-2015. Medical Surveillance Monthly Report. 2016 Apr;23(4):19.
HFpEF: New guidelines are pertinent for primary care
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. The incidence of HFpEF is increasing, yet it’s underrecognized. Now that there are evidence-based treatment approaches that improve outcomes, we’ve started to look for this condition and are diagnosing it more often. HFpEF is commonly encountered in primary care.
We should be thinking about HFpEF when we see adults with shortness of breath and/or fatigue and reduced exercise capacity, particularly in the settings of obesity, hypertension, or diabetes. It may not be simple deconditioning; it could be HFpEF.
I’ll organize this discussion into three topics: when to think about HFpEF, how to diagnosis it. and how to treat it.
When to think about HFpEF. When we see a person with risk factors (e.g., older age, obesity, diabetes, hypertension) experiencing dyspnea or fatigue with physical activity, their symptoms are not always from simple deconditioning. HFpEF should be on our differential as well as chronic obstructive pulmonary disease (COPD).
Making the diagnosis. HFpEF is defined as a clinical diagnosis of HR with left ventricular EF (LVEF) greater than 50%. Remember, in HF with reduced EF (HFrEF), the EF is less than 40%, and the EF in midrange HF is 40%-50%. See this recent HF review for more details on reduced and midrange ejection fractions.
For practical purposes, to diagnose HFpEF, check for an elevated N-terminal pro B-type natriuretic peptide (NT-proBNP) (> 125 pg/mL) and evidence of diastolic dysfunction on echocardiogram. Be aware that patients with obesity and HFpEF have lower BNP concentrations than those without obesity, and one professional society has suggested that a 50% reduction in BNP cutoff values should be used when making the diagnosis in patients with obesity.
Of course, we evaluate for other causes of dyspnea and/or edema including lung (most commonly COPD), liver, or kidney disease. When the diagnosis of HFpEF is made, consider whether further evaluation is warranted for specific underlying causes of HFpEF, such as amyloidosis, sarcoid, hemochromatosis, or hypertrophic cardiomyopathy.
Treatment. The evolution of the management of HFpEF has been intriguing. I recommend that people take a look at the guidelines and read the supporting trials. Finding effective therapies has taken longer than it did for HFrEF, but finally, an effective therapy for HFpEF is available.
To quote the guidelines, diuretics should be used “judiciously as needed” to reduce pulmonary congestion and improve symptoms. But here’s the big deal. The mainstays of treatment for HFpEF are the sodium-glucose cotransporter 2 (SGLT2) inhibitors on the basis of the findings of two trials: DELIVER (dapagliflozin) and EMPEROR-Preserved (empagliflozin), both of which have shown very impressive levels of benefit.
Both trials lasted a little over 2 years and found a statistically significant approximately 30% decline in HF hospitalizations and a numerical reduction of about 10% in cardiovascular death, which was statistically significant in meta-analysis. That’s over 2 years! That’s a large level of effect. They also showed improvements in symptoms and health status. Therefore, SGLT2 inhibitors are first-line treatment for all individuals with HFpEF, currently graded as a Class 2a (moderate) recommendation, but likely soon to be upgraded to Class 1 (strong) recommendation.
After the SGLT2 inhibitors, treatment is based on evidence which is not as strong and the recommendations are graded as Class 2b (weak) recommendations. In men with an LVEF less than 55%-60% and for women with any EF, use of a mineralocorticoid antagonist (MRA), an angiotensin receptor-neprilysin inhibitor, or if an ARN inhibitor is not feasible, an angiotensin receptor blocker (ARB) may be considered.
Nonpharmacologic management is also important. Exercise and weight loss (if the patient is overweight) can improve symptoms and quality of life. A new intervention, an implantable ambulatory pulmonary artery sensor, called CardioMEMS, has been evaluated in two trials, showing a decrease in HF hospitalizations. This may be considered for those who experience hospitalizations for HF and continue to experience New York Heart Association functional Class 3 symptoms despite optimal guideline-directed medical therapy or those who have lability in volume status or other medical problems (such as obesity or COPD) that make it difficult to tell whether their symptoms are from HFpEF or a comorbid condition.
In summary:
- Have a low threshold to evaluate for HFpEF in any patients who have shortness of breath, fatigue with exertion, or fluid overload.
- Initially evaluate with an NT-proBNP level and an echocardiogram.
- First-line treatment is an evidence-based SGLT2 inhibitor along with exercise and perhaps weight loss if needed. A loop diuretic can be used as needed to control volume status. Then you can consider, based on symptoms and details discussed above, an MRA, ARN inhibitor, or ARB.
This is important information for a diagnosis that is common in primary care, HFpEF, and for which we now have impressive, effective treatment.
Dr. Skolnik is a professor in the department of family medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the department of family medicine at Abington (Pa.) Jefferson Health. He disclosed conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim; Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. The incidence of HFpEF is increasing, yet it’s underrecognized. Now that there are evidence-based treatment approaches that improve outcomes, we’ve started to look for this condition and are diagnosing it more often. HFpEF is commonly encountered in primary care.
We should be thinking about HFpEF when we see adults with shortness of breath and/or fatigue and reduced exercise capacity, particularly in the settings of obesity, hypertension, or diabetes. It may not be simple deconditioning; it could be HFpEF.
I’ll organize this discussion into three topics: when to think about HFpEF, how to diagnosis it. and how to treat it.
When to think about HFpEF. When we see a person with risk factors (e.g., older age, obesity, diabetes, hypertension) experiencing dyspnea or fatigue with physical activity, their symptoms are not always from simple deconditioning. HFpEF should be on our differential as well as chronic obstructive pulmonary disease (COPD).
Making the diagnosis. HFpEF is defined as a clinical diagnosis of HR with left ventricular EF (LVEF) greater than 50%. Remember, in HF with reduced EF (HFrEF), the EF is less than 40%, and the EF in midrange HF is 40%-50%. See this recent HF review for more details on reduced and midrange ejection fractions.
For practical purposes, to diagnose HFpEF, check for an elevated N-terminal pro B-type natriuretic peptide (NT-proBNP) (> 125 pg/mL) and evidence of diastolic dysfunction on echocardiogram. Be aware that patients with obesity and HFpEF have lower BNP concentrations than those without obesity, and one professional society has suggested that a 50% reduction in BNP cutoff values should be used when making the diagnosis in patients with obesity.
Of course, we evaluate for other causes of dyspnea and/or edema including lung (most commonly COPD), liver, or kidney disease. When the diagnosis of HFpEF is made, consider whether further evaluation is warranted for specific underlying causes of HFpEF, such as amyloidosis, sarcoid, hemochromatosis, or hypertrophic cardiomyopathy.
Treatment. The evolution of the management of HFpEF has been intriguing. I recommend that people take a look at the guidelines and read the supporting trials. Finding effective therapies has taken longer than it did for HFrEF, but finally, an effective therapy for HFpEF is available.
To quote the guidelines, diuretics should be used “judiciously as needed” to reduce pulmonary congestion and improve symptoms. But here’s the big deal. The mainstays of treatment for HFpEF are the sodium-glucose cotransporter 2 (SGLT2) inhibitors on the basis of the findings of two trials: DELIVER (dapagliflozin) and EMPEROR-Preserved (empagliflozin), both of which have shown very impressive levels of benefit.
Both trials lasted a little over 2 years and found a statistically significant approximately 30% decline in HF hospitalizations and a numerical reduction of about 10% in cardiovascular death, which was statistically significant in meta-analysis. That’s over 2 years! That’s a large level of effect. They also showed improvements in symptoms and health status. Therefore, SGLT2 inhibitors are first-line treatment for all individuals with HFpEF, currently graded as a Class 2a (moderate) recommendation, but likely soon to be upgraded to Class 1 (strong) recommendation.
After the SGLT2 inhibitors, treatment is based on evidence which is not as strong and the recommendations are graded as Class 2b (weak) recommendations. In men with an LVEF less than 55%-60% and for women with any EF, use of a mineralocorticoid antagonist (MRA), an angiotensin receptor-neprilysin inhibitor, or if an ARN inhibitor is not feasible, an angiotensin receptor blocker (ARB) may be considered.
Nonpharmacologic management is also important. Exercise and weight loss (if the patient is overweight) can improve symptoms and quality of life. A new intervention, an implantable ambulatory pulmonary artery sensor, called CardioMEMS, has been evaluated in two trials, showing a decrease in HF hospitalizations. This may be considered for those who experience hospitalizations for HF and continue to experience New York Heart Association functional Class 3 symptoms despite optimal guideline-directed medical therapy or those who have lability in volume status or other medical problems (such as obesity or COPD) that make it difficult to tell whether their symptoms are from HFpEF or a comorbid condition.
In summary:
- Have a low threshold to evaluate for HFpEF in any patients who have shortness of breath, fatigue with exertion, or fluid overload.
- Initially evaluate with an NT-proBNP level and an echocardiogram.
- First-line treatment is an evidence-based SGLT2 inhibitor along with exercise and perhaps weight loss if needed. A loop diuretic can be used as needed to control volume status. Then you can consider, based on symptoms and details discussed above, an MRA, ARN inhibitor, or ARB.
This is important information for a diagnosis that is common in primary care, HFpEF, and for which we now have impressive, effective treatment.
Dr. Skolnik is a professor in the department of family medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the department of family medicine at Abington (Pa.) Jefferson Health. He disclosed conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim; Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. The incidence of HFpEF is increasing, yet it’s underrecognized. Now that there are evidence-based treatment approaches that improve outcomes, we’ve started to look for this condition and are diagnosing it more often. HFpEF is commonly encountered in primary care.
We should be thinking about HFpEF when we see adults with shortness of breath and/or fatigue and reduced exercise capacity, particularly in the settings of obesity, hypertension, or diabetes. It may not be simple deconditioning; it could be HFpEF.
I’ll organize this discussion into three topics: when to think about HFpEF, how to diagnosis it. and how to treat it.
When to think about HFpEF. When we see a person with risk factors (e.g., older age, obesity, diabetes, hypertension) experiencing dyspnea or fatigue with physical activity, their symptoms are not always from simple deconditioning. HFpEF should be on our differential as well as chronic obstructive pulmonary disease (COPD).
Making the diagnosis. HFpEF is defined as a clinical diagnosis of HR with left ventricular EF (LVEF) greater than 50%. Remember, in HF with reduced EF (HFrEF), the EF is less than 40%, and the EF in midrange HF is 40%-50%. See this recent HF review for more details on reduced and midrange ejection fractions.
For practical purposes, to diagnose HFpEF, check for an elevated N-terminal pro B-type natriuretic peptide (NT-proBNP) (> 125 pg/mL) and evidence of diastolic dysfunction on echocardiogram. Be aware that patients with obesity and HFpEF have lower BNP concentrations than those without obesity, and one professional society has suggested that a 50% reduction in BNP cutoff values should be used when making the diagnosis in patients with obesity.
Of course, we evaluate for other causes of dyspnea and/or edema including lung (most commonly COPD), liver, or kidney disease. When the diagnosis of HFpEF is made, consider whether further evaluation is warranted for specific underlying causes of HFpEF, such as amyloidosis, sarcoid, hemochromatosis, or hypertrophic cardiomyopathy.
Treatment. The evolution of the management of HFpEF has been intriguing. I recommend that people take a look at the guidelines and read the supporting trials. Finding effective therapies has taken longer than it did for HFrEF, but finally, an effective therapy for HFpEF is available.
To quote the guidelines, diuretics should be used “judiciously as needed” to reduce pulmonary congestion and improve symptoms. But here’s the big deal. The mainstays of treatment for HFpEF are the sodium-glucose cotransporter 2 (SGLT2) inhibitors on the basis of the findings of two trials: DELIVER (dapagliflozin) and EMPEROR-Preserved (empagliflozin), both of which have shown very impressive levels of benefit.
Both trials lasted a little over 2 years and found a statistically significant approximately 30% decline in HF hospitalizations and a numerical reduction of about 10% in cardiovascular death, which was statistically significant in meta-analysis. That’s over 2 years! That’s a large level of effect. They also showed improvements in symptoms and health status. Therefore, SGLT2 inhibitors are first-line treatment for all individuals with HFpEF, currently graded as a Class 2a (moderate) recommendation, but likely soon to be upgraded to Class 1 (strong) recommendation.
After the SGLT2 inhibitors, treatment is based on evidence which is not as strong and the recommendations are graded as Class 2b (weak) recommendations. In men with an LVEF less than 55%-60% and for women with any EF, use of a mineralocorticoid antagonist (MRA), an angiotensin receptor-neprilysin inhibitor, or if an ARN inhibitor is not feasible, an angiotensin receptor blocker (ARB) may be considered.
Nonpharmacologic management is also important. Exercise and weight loss (if the patient is overweight) can improve symptoms and quality of life. A new intervention, an implantable ambulatory pulmonary artery sensor, called CardioMEMS, has been evaluated in two trials, showing a decrease in HF hospitalizations. This may be considered for those who experience hospitalizations for HF and continue to experience New York Heart Association functional Class 3 symptoms despite optimal guideline-directed medical therapy or those who have lability in volume status or other medical problems (such as obesity or COPD) that make it difficult to tell whether their symptoms are from HFpEF or a comorbid condition.
In summary:
- Have a low threshold to evaluate for HFpEF in any patients who have shortness of breath, fatigue with exertion, or fluid overload.
- Initially evaluate with an NT-proBNP level and an echocardiogram.
- First-line treatment is an evidence-based SGLT2 inhibitor along with exercise and perhaps weight loss if needed. A loop diuretic can be used as needed to control volume status. Then you can consider, based on symptoms and details discussed above, an MRA, ARN inhibitor, or ARB.
This is important information for a diagnosis that is common in primary care, HFpEF, and for which we now have impressive, effective treatment.
Dr. Skolnik is a professor in the department of family medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the department of family medicine at Abington (Pa.) Jefferson Health. He disclosed conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim; Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article first appeared on Medscape.com.
No expiration date for sex
For health professionals, the thought that our parents and grandparents don’t have sex – or didn’t – might be comforting.
The reality is that, for a significant proportion of our older patients, sex has no use-by date. Humans are sexual beings throughout their lives, yet the culture has concealed that fact.
According to Rome, the purpose of sex is to make children. According to Hollywood, sex is only for the young, the healthy, and the beautiful. For the medical profession, sex consists mainly of risks or dysfunctions.
The results of these biases? Sexuality and intimacy are essential elements for quality of life, with clear physical, emotional, and relational benefits.
Let’s look at the data when researchers dared to ask seniors about their sexuality.
We start with the 2015 U.K. national research on sexuality. The study found a link between age and a decline in various aspects of sexual activity – but not a zeroing-out. For example, among men aged 70-79, 59% reported having had sex in the past year, with 19% having intercourse at least twice a month and 18% masturbating at least that often. Above age 80, those numbers dropped to 39%, 6%, and 5%, respectively. The reason behind the declines? A combination of taboo, fear of disease, use of medications or other interventions that disrupt sexual function or cause disfigurement, and a little bit of age itself.
What about women? Among women ages 70-79, 39% said they’d had sex in the past year, with 6% having intercourse at least twice per month and 5% masturbating two times or more monthly. Above age 80, those numbers were 10%, 4.5%, and 1%, respectively. Driving the falloff in women were the same factors as for men, plus the sad reality that many heterosexual women become widowed because their older male partners die earlier.
The male-female difference also reflects lower levels of testosterone in women. And, because women say they value intimacy more than performance, we have two explanations for their lower frequency of masturbation. After all, a lot of intimacy occurs without either intercourse or masturbation.
Surprising and relevant is the amount of distress – or rather, their relative lack thereof – older patients report because of sexual problems. At age 18-44, 11% of U.S. women indicated sexual distress; at age 45-64, the figure was 15%; and at age 65 and up, 9%.
For clinicians, those figures should prompt us to look more closely at alternative forms of sexual expression – those not involving intercourse or masturbation – in the aged, a field physicians typically do not consider.
Although dyspareunia or erectile problems affect many in long-standing relationships, neither is a reason to abstain from sexual pleasure. Indeed, in many couples, oral sex will replace vaginal intercourse, and if urinary, fecal, or flatal incontinence intrude, couples often waive oral sex in favor of more cuddling, kissing, digital stimulation, and other forms of sexual pleasure.
What about the expiry date for sex?
Fascinating research from Nils Beckman, PhD, and colleagues found that the sex drive persists even as people (and men in particular) reach their 100th year. Dr. Beckman’s group interviewed 269 Swedish seniors, all without dementia, at age 97. Sexual desire was affirmed by 27% of men and 5% of women in the survey. Among the men, 32% said they still had sexual thoughts, compared with 18% of women. Meanwhile, 26% of the men and 15% of the women said they missed sexual activity.
What should clinicians do with this information? First, we could start talking about sex with our older patients. According to the 97-year-old Swedes, most want us to! More than 8 in 10 of both women and men in the survey expressed positive views about questions on sexuality. And please don’t be scared to address the subject in the single senior. They, too, can have a sexual or relationship issue and are happy when we raise the subject. They’re not scared to talk about masturbation, either.
When caring for those with chronic diseases, cancer, in the course of physical rehabilitation, and even in the last phase of life, the clinical experience indicates that our patients are happy when we address sexuality and intimacy. Doing so can open the door to the admission of a problem and a corresponding solution, a lubricant or a PDE5 inhibitor.
But sometimes the solution is the conversation itself: Roughly 25% of patients are sufficiently helped simply by talking about sex. Addressing the importance of sexual pleasure is nearly always valuable.
Here are a few ice-breakers I find helpful:
- Did taking this medication change aspects of sexuality? If so, does that bother you?
- Knowing that continuing intimacy is healthy, do you mind if I address that subject?
- We know that aspects of sexuality and intimacy are healthy. Without a partner, some people become sexually isolated. Would you like to talk about that?’
If addressing sexuality has benefits, what about sex itself?
We are gradually learning more about the many short-, intermediate-, and long-term health benefits of solo and joint sexual activity. Short-term benefits include muscle relaxation, pain relief (even, perhaps ironically, for headaches), and better sleep – all pretty valuable for older adults. Examples of intermediate-term benefits include stress relief and less depression. Research from the United States has found that hugging can reduce the concentrations of proinflammatory cytokines, and kissing positively influences cholesterol levels.
Finally, while the long-term benefits of sex might be less relevant for seniors, they do exist.
Among them are delayed onset of dementia and a substantial reduction in cardiovascular and cerebrovascular problems in men. More sex has been linked to longevity, with men benefiting a bit more than women from going through the entire process, including an orgasm, whereas women appear to gain from having a “satisfying” sex life, which does not always require an orgasm.
Let us not forget that these benefits apply to both patients and clinicians alike. Addressing intimacy and sexuality can ease eventual sexual concerns and potentially create a stronger clinician-patient relationship.
Dr. Gianotten, MD is emeritus senior lecturer in medical sexology, Erasmus University Medical Centre, Rotterdam, the Netherlands. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
For health professionals, the thought that our parents and grandparents don’t have sex – or didn’t – might be comforting.
The reality is that, for a significant proportion of our older patients, sex has no use-by date. Humans are sexual beings throughout their lives, yet the culture has concealed that fact.
According to Rome, the purpose of sex is to make children. According to Hollywood, sex is only for the young, the healthy, and the beautiful. For the medical profession, sex consists mainly of risks or dysfunctions.
The results of these biases? Sexuality and intimacy are essential elements for quality of life, with clear physical, emotional, and relational benefits.
Let’s look at the data when researchers dared to ask seniors about their sexuality.
We start with the 2015 U.K. national research on sexuality. The study found a link between age and a decline in various aspects of sexual activity – but not a zeroing-out. For example, among men aged 70-79, 59% reported having had sex in the past year, with 19% having intercourse at least twice a month and 18% masturbating at least that often. Above age 80, those numbers dropped to 39%, 6%, and 5%, respectively. The reason behind the declines? A combination of taboo, fear of disease, use of medications or other interventions that disrupt sexual function or cause disfigurement, and a little bit of age itself.
What about women? Among women ages 70-79, 39% said they’d had sex in the past year, with 6% having intercourse at least twice per month and 5% masturbating two times or more monthly. Above age 80, those numbers were 10%, 4.5%, and 1%, respectively. Driving the falloff in women were the same factors as for men, plus the sad reality that many heterosexual women become widowed because their older male partners die earlier.
The male-female difference also reflects lower levels of testosterone in women. And, because women say they value intimacy more than performance, we have two explanations for their lower frequency of masturbation. After all, a lot of intimacy occurs without either intercourse or masturbation.
Surprising and relevant is the amount of distress – or rather, their relative lack thereof – older patients report because of sexual problems. At age 18-44, 11% of U.S. women indicated sexual distress; at age 45-64, the figure was 15%; and at age 65 and up, 9%.
For clinicians, those figures should prompt us to look more closely at alternative forms of sexual expression – those not involving intercourse or masturbation – in the aged, a field physicians typically do not consider.
Although dyspareunia or erectile problems affect many in long-standing relationships, neither is a reason to abstain from sexual pleasure. Indeed, in many couples, oral sex will replace vaginal intercourse, and if urinary, fecal, or flatal incontinence intrude, couples often waive oral sex in favor of more cuddling, kissing, digital stimulation, and other forms of sexual pleasure.
What about the expiry date for sex?
Fascinating research from Nils Beckman, PhD, and colleagues found that the sex drive persists even as people (and men in particular) reach their 100th year. Dr. Beckman’s group interviewed 269 Swedish seniors, all without dementia, at age 97. Sexual desire was affirmed by 27% of men and 5% of women in the survey. Among the men, 32% said they still had sexual thoughts, compared with 18% of women. Meanwhile, 26% of the men and 15% of the women said they missed sexual activity.
What should clinicians do with this information? First, we could start talking about sex with our older patients. According to the 97-year-old Swedes, most want us to! More than 8 in 10 of both women and men in the survey expressed positive views about questions on sexuality. And please don’t be scared to address the subject in the single senior. They, too, can have a sexual or relationship issue and are happy when we raise the subject. They’re not scared to talk about masturbation, either.
When caring for those with chronic diseases, cancer, in the course of physical rehabilitation, and even in the last phase of life, the clinical experience indicates that our patients are happy when we address sexuality and intimacy. Doing so can open the door to the admission of a problem and a corresponding solution, a lubricant or a PDE5 inhibitor.
But sometimes the solution is the conversation itself: Roughly 25% of patients are sufficiently helped simply by talking about sex. Addressing the importance of sexual pleasure is nearly always valuable.
Here are a few ice-breakers I find helpful:
- Did taking this medication change aspects of sexuality? If so, does that bother you?
- Knowing that continuing intimacy is healthy, do you mind if I address that subject?
- We know that aspects of sexuality and intimacy are healthy. Without a partner, some people become sexually isolated. Would you like to talk about that?’
If addressing sexuality has benefits, what about sex itself?
We are gradually learning more about the many short-, intermediate-, and long-term health benefits of solo and joint sexual activity. Short-term benefits include muscle relaxation, pain relief (even, perhaps ironically, for headaches), and better sleep – all pretty valuable for older adults. Examples of intermediate-term benefits include stress relief and less depression. Research from the United States has found that hugging can reduce the concentrations of proinflammatory cytokines, and kissing positively influences cholesterol levels.
Finally, while the long-term benefits of sex might be less relevant for seniors, they do exist.
Among them are delayed onset of dementia and a substantial reduction in cardiovascular and cerebrovascular problems in men. More sex has been linked to longevity, with men benefiting a bit more than women from going through the entire process, including an orgasm, whereas women appear to gain from having a “satisfying” sex life, which does not always require an orgasm.
Let us not forget that these benefits apply to both patients and clinicians alike. Addressing intimacy and sexuality can ease eventual sexual concerns and potentially create a stronger clinician-patient relationship.
Dr. Gianotten, MD is emeritus senior lecturer in medical sexology, Erasmus University Medical Centre, Rotterdam, the Netherlands. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
For health professionals, the thought that our parents and grandparents don’t have sex – or didn’t – might be comforting.
The reality is that, for a significant proportion of our older patients, sex has no use-by date. Humans are sexual beings throughout their lives, yet the culture has concealed that fact.
According to Rome, the purpose of sex is to make children. According to Hollywood, sex is only for the young, the healthy, and the beautiful. For the medical profession, sex consists mainly of risks or dysfunctions.
The results of these biases? Sexuality and intimacy are essential elements for quality of life, with clear physical, emotional, and relational benefits.
Let’s look at the data when researchers dared to ask seniors about their sexuality.
We start with the 2015 U.K. national research on sexuality. The study found a link between age and a decline in various aspects of sexual activity – but not a zeroing-out. For example, among men aged 70-79, 59% reported having had sex in the past year, with 19% having intercourse at least twice a month and 18% masturbating at least that often. Above age 80, those numbers dropped to 39%, 6%, and 5%, respectively. The reason behind the declines? A combination of taboo, fear of disease, use of medications or other interventions that disrupt sexual function or cause disfigurement, and a little bit of age itself.
What about women? Among women ages 70-79, 39% said they’d had sex in the past year, with 6% having intercourse at least twice per month and 5% masturbating two times or more monthly. Above age 80, those numbers were 10%, 4.5%, and 1%, respectively. Driving the falloff in women were the same factors as for men, plus the sad reality that many heterosexual women become widowed because their older male partners die earlier.
The male-female difference also reflects lower levels of testosterone in women. And, because women say they value intimacy more than performance, we have two explanations for their lower frequency of masturbation. After all, a lot of intimacy occurs without either intercourse or masturbation.
Surprising and relevant is the amount of distress – or rather, their relative lack thereof – older patients report because of sexual problems. At age 18-44, 11% of U.S. women indicated sexual distress; at age 45-64, the figure was 15%; and at age 65 and up, 9%.
For clinicians, those figures should prompt us to look more closely at alternative forms of sexual expression – those not involving intercourse or masturbation – in the aged, a field physicians typically do not consider.
Although dyspareunia or erectile problems affect many in long-standing relationships, neither is a reason to abstain from sexual pleasure. Indeed, in many couples, oral sex will replace vaginal intercourse, and if urinary, fecal, or flatal incontinence intrude, couples often waive oral sex in favor of more cuddling, kissing, digital stimulation, and other forms of sexual pleasure.
What about the expiry date for sex?
Fascinating research from Nils Beckman, PhD, and colleagues found that the sex drive persists even as people (and men in particular) reach their 100th year. Dr. Beckman’s group interviewed 269 Swedish seniors, all without dementia, at age 97. Sexual desire was affirmed by 27% of men and 5% of women in the survey. Among the men, 32% said they still had sexual thoughts, compared with 18% of women. Meanwhile, 26% of the men and 15% of the women said they missed sexual activity.
What should clinicians do with this information? First, we could start talking about sex with our older patients. According to the 97-year-old Swedes, most want us to! More than 8 in 10 of both women and men in the survey expressed positive views about questions on sexuality. And please don’t be scared to address the subject in the single senior. They, too, can have a sexual or relationship issue and are happy when we raise the subject. They’re not scared to talk about masturbation, either.
When caring for those with chronic diseases, cancer, in the course of physical rehabilitation, and even in the last phase of life, the clinical experience indicates that our patients are happy when we address sexuality and intimacy. Doing so can open the door to the admission of a problem and a corresponding solution, a lubricant or a PDE5 inhibitor.
But sometimes the solution is the conversation itself: Roughly 25% of patients are sufficiently helped simply by talking about sex. Addressing the importance of sexual pleasure is nearly always valuable.
Here are a few ice-breakers I find helpful:
- Did taking this medication change aspects of sexuality? If so, does that bother you?
- Knowing that continuing intimacy is healthy, do you mind if I address that subject?
- We know that aspects of sexuality and intimacy are healthy. Without a partner, some people become sexually isolated. Would you like to talk about that?’
If addressing sexuality has benefits, what about sex itself?
We are gradually learning more about the many short-, intermediate-, and long-term health benefits of solo and joint sexual activity. Short-term benefits include muscle relaxation, pain relief (even, perhaps ironically, for headaches), and better sleep – all pretty valuable for older adults. Examples of intermediate-term benefits include stress relief and less depression. Research from the United States has found that hugging can reduce the concentrations of proinflammatory cytokines, and kissing positively influences cholesterol levels.
Finally, while the long-term benefits of sex might be less relevant for seniors, they do exist.
Among them are delayed onset of dementia and a substantial reduction in cardiovascular and cerebrovascular problems in men. More sex has been linked to longevity, with men benefiting a bit more than women from going through the entire process, including an orgasm, whereas women appear to gain from having a “satisfying” sex life, which does not always require an orgasm.
Let us not forget that these benefits apply to both patients and clinicians alike. Addressing intimacy and sexuality can ease eventual sexual concerns and potentially create a stronger clinician-patient relationship.
Dr. Gianotten, MD is emeritus senior lecturer in medical sexology, Erasmus University Medical Centre, Rotterdam, the Netherlands. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Choosing our terms: The diagnostic words we use can be harmful
We are living in an era of increasing sensitivity to our diversity and the ways we interact, but also an era of growing resistance to change and accommodation. As clinicians, we hope to be among the sensitive and the progressive, open to improving our views and interactions. And as part of our respect for those we treat, we seek to speak clearly with them about our assessment of what is disrupting their lives and about their options.
Using the right words is crucial in that work. Well-chosen words can be heard and understood. Poorly chosen words can be confusing or off-putting; they may miscommunicate or be offensive. Careful choice of words is also important among colleagues, who may not always mean the same things when using the same words.
In psychiatry, consumer knowledge and access are growing. There are effective standard treatments and promising new ones. But our terminology is often antique and obscure. This is so despite a recognition that some terms we use may communicate poorly and some are deprecating.
A notable example is “schizophrenia.” Originally referring to cognitive phenomena that were not adequately coherent with reality or one another, it has gone through periods of describing most psychosis to particular subsets of psychoses. Debates persist on specific criteria for key symptoms and typical course. Even two clinicians trained in the same site may not agree on the defining criteria, and the public, mostly informed by books, movies, and newspapers, is even more confused, often believing schizophrenia is multiple-personality disorder. In addition, the press and public often associate schizophrenia with violent behavior and uniformly bad outcomes, and for those reasons, a diagnosis is not only frightening but also stigmatizing.1
Many papers have presented the case for retiring “schizophrenia.”2 And practical efforts to rename schizophrenia have been made. These efforts have occurred in countries in which English is not the primary language.3 In Japan, schizophrenia was replaced by “integration disorder.” In Hong Kong, “disorder of thought and perception” was implemented. Korea chose “attunement disorder.” A recent large survey of stakeholders, including clinicians, researchers, and consumers in the United States, explored alternatives in English.4 Terms receiving approval included: “psychosis spectrum syndrome,” “altered perception syndrome,” and “neuro-emotional integration disorder.”
Despite these recommendations, the standard manuals of diagnosis, the ICD and DSM, have maintained the century-old term “schizophrenia” in their most recent editions, released in 2022. Aside from the inertia commonly associated with long-standing practices, it has been noted that many of the alternatives suggested or, in some places, implemented, are complex, somewhat vague, or too inclusive to distinguish different clinical presentations requiring different treatment approaches. They might not be compelling for use or optimal to guide caregiving.
Perhaps more concerning than “schizophrenia” are terms used to describe personality disorders.5 “Personality disorder” itself is problematic, implying a core and possibly unalterable fault in an individual. And among the personality disorders, words for the related group of disorders called “Cluster B” in the DSM raise issues. This includes the terms narcissistic, antisocial, histrionic, and borderline in DSM-5-TR. The first three terms are clearly pejorative. The last is unclear: What is the border between? Originally, it was bordering on psychosis, but as explained in DSM and ICD, borderline disorder is much more closely related to other personality disorders.
Notably, the “Cluster B” disorders run together in families, but men are more likely to be called antisocial and women borderline, even though the overlap in signs and symptoms is profound, suggesting marginally different manifestations of the same condition. The ICD has made changes to address the problems associated with some of these terms. ICD proposes personality “difficulty” to replace personality “disorder”; a modest change but less offensive. And it proposes seeing all, or at least most, personality disorders as being related to one another. Most share features of disturbances in sense-of-self and relationships with others. As descriptors, ICD kept “borderline pattern,” but replaced “antisocial” with “dissocial,” in an effort to be accurate but less demeaning. Other descriptors it proposes are negative affectivity, detachment, disinhibition, and anankastia, the last referring to compulsions.
These are notable advances. Can the field find even better terms to communicate hard to hear information, with words that are less problematic? In search of options, we surveyed clinicians at academic centers about the terms they preferred to avoid and the ones they prefer to use in talking with patients.6 Their practices may be informative.
Briefly summarized, these clinicians preferred not to use “schizophrenia” and very few used “antisocial,” “histrionic,” or “narcissistic.” Most avoided using “borderline” as well. Instead, they recommended discussing specific symptoms and manifestations of illness or dysfunctional behavior and relationships with their patients. They employed terms including “psychosis,” “hallucination,” “delusion,” “thinking disorder,” and “mood disorder.” They explained these terms, as needed, and found that patients understood them.
For Cluster B personality disorders, they spoke of personality traits and styles and specifically about “conduct,” “rule breaking,” “coping,” “self-focus,” “emotionality,” and “reactivity.” Those choices are not perfect, of course. Medical terms are often not standard words used in a conversational way. But the words chosen by these clinicians are generally straightforward and may communicate in a clear and acceptable fashion. It is also notable that the terms match how the clinicians assess and treat their patients, as observed in a separate study of their practices.7 That is, the clinicians advised that they look for and suggest treatments for the specific symptoms they see that most disrupt an individual’s life, such as delusions or mood instability. They are not much guided by diagnoses, like schizophrenia or borderline disorder. That makes the chosen terms not only less confusing or off-putting but also more practical.
Changing terminology in any field is difficult. We are trained to use standard terms. Clearly, however, many clinicians avoid some terms and use alternatives in their work. Asked why, they responded that they did so precisely to communicate more effectively and more respectfully. That is key to their treatment goals. Perhaps others will consider these choices useful in their work. And perhaps both the DSM and the ICD will not only continue to consider but will decide to implement alternatives for problematic terms in the years ahead, as they discuss their next revisions.
Dr. Cohen is director of the Program for Neuropsychiatric Research at McLean Hospital, Belmont, Mass., and Robertson-Steele Professor of Psychiatry at Harvard Medical School, Boston.
References
1. Lasalvia A et al. Renaming schizophrenia? A survey among psychiatrists, mental health service users and family members in Italy. Schizophr Res. 2021;228:502-9.
2. Gülöksüz S et al. Renaming schizophrenia: 5 x 5. Epidemiol Psychiatr Sci. 2019;28(3):254-7.
3. Sartorius N et al. Name change for schizophrenia. Schizophr Bull. 2014;40(2):255-8.
4. Mesholam-Gately RI et al. Are we ready for a name change for schizophrenia? A survey of multiple stakeholders. Schizophr Res. 2021;238:152-60.
5. Mulder R. The evolving nosology of personality disorder and its clinical utility. World Psychiatry. 2021 Oct;20(3):361-2.
6. Cohen BM et al. Diagnostic terms psychiatrists prefer to use for common psychotic and personality disorders. J Psychiatr Res. 2022 Sep 5;155:226-31.
7. Cohen BM, et al. Use of DSM-5 diagnoses vs. other clinical information by US academic-affiliated psychiatrists in assessing and treating psychotic disorders. World Psychiatry. 2021 Oct;20(3):447-8.
We are living in an era of increasing sensitivity to our diversity and the ways we interact, but also an era of growing resistance to change and accommodation. As clinicians, we hope to be among the sensitive and the progressive, open to improving our views and interactions. And as part of our respect for those we treat, we seek to speak clearly with them about our assessment of what is disrupting their lives and about their options.
Using the right words is crucial in that work. Well-chosen words can be heard and understood. Poorly chosen words can be confusing or off-putting; they may miscommunicate or be offensive. Careful choice of words is also important among colleagues, who may not always mean the same things when using the same words.
In psychiatry, consumer knowledge and access are growing. There are effective standard treatments and promising new ones. But our terminology is often antique and obscure. This is so despite a recognition that some terms we use may communicate poorly and some are deprecating.
A notable example is “schizophrenia.” Originally referring to cognitive phenomena that were not adequately coherent with reality or one another, it has gone through periods of describing most psychosis to particular subsets of psychoses. Debates persist on specific criteria for key symptoms and typical course. Even two clinicians trained in the same site may not agree on the defining criteria, and the public, mostly informed by books, movies, and newspapers, is even more confused, often believing schizophrenia is multiple-personality disorder. In addition, the press and public often associate schizophrenia with violent behavior and uniformly bad outcomes, and for those reasons, a diagnosis is not only frightening but also stigmatizing.1
Many papers have presented the case for retiring “schizophrenia.”2 And practical efforts to rename schizophrenia have been made. These efforts have occurred in countries in which English is not the primary language.3 In Japan, schizophrenia was replaced by “integration disorder.” In Hong Kong, “disorder of thought and perception” was implemented. Korea chose “attunement disorder.” A recent large survey of stakeholders, including clinicians, researchers, and consumers in the United States, explored alternatives in English.4 Terms receiving approval included: “psychosis spectrum syndrome,” “altered perception syndrome,” and “neuro-emotional integration disorder.”
Despite these recommendations, the standard manuals of diagnosis, the ICD and DSM, have maintained the century-old term “schizophrenia” in their most recent editions, released in 2022. Aside from the inertia commonly associated with long-standing practices, it has been noted that many of the alternatives suggested or, in some places, implemented, are complex, somewhat vague, or too inclusive to distinguish different clinical presentations requiring different treatment approaches. They might not be compelling for use or optimal to guide caregiving.
Perhaps more concerning than “schizophrenia” are terms used to describe personality disorders.5 “Personality disorder” itself is problematic, implying a core and possibly unalterable fault in an individual. And among the personality disorders, words for the related group of disorders called “Cluster B” in the DSM raise issues. This includes the terms narcissistic, antisocial, histrionic, and borderline in DSM-5-TR. The first three terms are clearly pejorative. The last is unclear: What is the border between? Originally, it was bordering on psychosis, but as explained in DSM and ICD, borderline disorder is much more closely related to other personality disorders.
Notably, the “Cluster B” disorders run together in families, but men are more likely to be called antisocial and women borderline, even though the overlap in signs and symptoms is profound, suggesting marginally different manifestations of the same condition. The ICD has made changes to address the problems associated with some of these terms. ICD proposes personality “difficulty” to replace personality “disorder”; a modest change but less offensive. And it proposes seeing all, or at least most, personality disorders as being related to one another. Most share features of disturbances in sense-of-self and relationships with others. As descriptors, ICD kept “borderline pattern,” but replaced “antisocial” with “dissocial,” in an effort to be accurate but less demeaning. Other descriptors it proposes are negative affectivity, detachment, disinhibition, and anankastia, the last referring to compulsions.
These are notable advances. Can the field find even better terms to communicate hard to hear information, with words that are less problematic? In search of options, we surveyed clinicians at academic centers about the terms they preferred to avoid and the ones they prefer to use in talking with patients.6 Their practices may be informative.
Briefly summarized, these clinicians preferred not to use “schizophrenia” and very few used “antisocial,” “histrionic,” or “narcissistic.” Most avoided using “borderline” as well. Instead, they recommended discussing specific symptoms and manifestations of illness or dysfunctional behavior and relationships with their patients. They employed terms including “psychosis,” “hallucination,” “delusion,” “thinking disorder,” and “mood disorder.” They explained these terms, as needed, and found that patients understood them.
For Cluster B personality disorders, they spoke of personality traits and styles and specifically about “conduct,” “rule breaking,” “coping,” “self-focus,” “emotionality,” and “reactivity.” Those choices are not perfect, of course. Medical terms are often not standard words used in a conversational way. But the words chosen by these clinicians are generally straightforward and may communicate in a clear and acceptable fashion. It is also notable that the terms match how the clinicians assess and treat their patients, as observed in a separate study of their practices.7 That is, the clinicians advised that they look for and suggest treatments for the specific symptoms they see that most disrupt an individual’s life, such as delusions or mood instability. They are not much guided by diagnoses, like schizophrenia or borderline disorder. That makes the chosen terms not only less confusing or off-putting but also more practical.
Changing terminology in any field is difficult. We are trained to use standard terms. Clearly, however, many clinicians avoid some terms and use alternatives in their work. Asked why, they responded that they did so precisely to communicate more effectively and more respectfully. That is key to their treatment goals. Perhaps others will consider these choices useful in their work. And perhaps both the DSM and the ICD will not only continue to consider but will decide to implement alternatives for problematic terms in the years ahead, as they discuss their next revisions.
Dr. Cohen is director of the Program for Neuropsychiatric Research at McLean Hospital, Belmont, Mass., and Robertson-Steele Professor of Psychiatry at Harvard Medical School, Boston.
References
1. Lasalvia A et al. Renaming schizophrenia? A survey among psychiatrists, mental health service users and family members in Italy. Schizophr Res. 2021;228:502-9.
2. Gülöksüz S et al. Renaming schizophrenia: 5 x 5. Epidemiol Psychiatr Sci. 2019;28(3):254-7.
3. Sartorius N et al. Name change for schizophrenia. Schizophr Bull. 2014;40(2):255-8.
4. Mesholam-Gately RI et al. Are we ready for a name change for schizophrenia? A survey of multiple stakeholders. Schizophr Res. 2021;238:152-60.
5. Mulder R. The evolving nosology of personality disorder and its clinical utility. World Psychiatry. 2021 Oct;20(3):361-2.
6. Cohen BM et al. Diagnostic terms psychiatrists prefer to use for common psychotic and personality disorders. J Psychiatr Res. 2022 Sep 5;155:226-31.
7. Cohen BM, et al. Use of DSM-5 diagnoses vs. other clinical information by US academic-affiliated psychiatrists in assessing and treating psychotic disorders. World Psychiatry. 2021 Oct;20(3):447-8.
We are living in an era of increasing sensitivity to our diversity and the ways we interact, but also an era of growing resistance to change and accommodation. As clinicians, we hope to be among the sensitive and the progressive, open to improving our views and interactions. And as part of our respect for those we treat, we seek to speak clearly with them about our assessment of what is disrupting their lives and about their options.
Using the right words is crucial in that work. Well-chosen words can be heard and understood. Poorly chosen words can be confusing or off-putting; they may miscommunicate or be offensive. Careful choice of words is also important among colleagues, who may not always mean the same things when using the same words.
In psychiatry, consumer knowledge and access are growing. There are effective standard treatments and promising new ones. But our terminology is often antique and obscure. This is so despite a recognition that some terms we use may communicate poorly and some are deprecating.
A notable example is “schizophrenia.” Originally referring to cognitive phenomena that were not adequately coherent with reality or one another, it has gone through periods of describing most psychosis to particular subsets of psychoses. Debates persist on specific criteria for key symptoms and typical course. Even two clinicians trained in the same site may not agree on the defining criteria, and the public, mostly informed by books, movies, and newspapers, is even more confused, often believing schizophrenia is multiple-personality disorder. In addition, the press and public often associate schizophrenia with violent behavior and uniformly bad outcomes, and for those reasons, a diagnosis is not only frightening but also stigmatizing.1
Many papers have presented the case for retiring “schizophrenia.”2 And practical efforts to rename schizophrenia have been made. These efforts have occurred in countries in which English is not the primary language.3 In Japan, schizophrenia was replaced by “integration disorder.” In Hong Kong, “disorder of thought and perception” was implemented. Korea chose “attunement disorder.” A recent large survey of stakeholders, including clinicians, researchers, and consumers in the United States, explored alternatives in English.4 Terms receiving approval included: “psychosis spectrum syndrome,” “altered perception syndrome,” and “neuro-emotional integration disorder.”
Despite these recommendations, the standard manuals of diagnosis, the ICD and DSM, have maintained the century-old term “schizophrenia” in their most recent editions, released in 2022. Aside from the inertia commonly associated with long-standing practices, it has been noted that many of the alternatives suggested or, in some places, implemented, are complex, somewhat vague, or too inclusive to distinguish different clinical presentations requiring different treatment approaches. They might not be compelling for use or optimal to guide caregiving.
Perhaps more concerning than “schizophrenia” are terms used to describe personality disorders.5 “Personality disorder” itself is problematic, implying a core and possibly unalterable fault in an individual. And among the personality disorders, words for the related group of disorders called “Cluster B” in the DSM raise issues. This includes the terms narcissistic, antisocial, histrionic, and borderline in DSM-5-TR. The first three terms are clearly pejorative. The last is unclear: What is the border between? Originally, it was bordering on psychosis, but as explained in DSM and ICD, borderline disorder is much more closely related to other personality disorders.
Notably, the “Cluster B” disorders run together in families, but men are more likely to be called antisocial and women borderline, even though the overlap in signs and symptoms is profound, suggesting marginally different manifestations of the same condition. The ICD has made changes to address the problems associated with some of these terms. ICD proposes personality “difficulty” to replace personality “disorder”; a modest change but less offensive. And it proposes seeing all, or at least most, personality disorders as being related to one another. Most share features of disturbances in sense-of-self and relationships with others. As descriptors, ICD kept “borderline pattern,” but replaced “antisocial” with “dissocial,” in an effort to be accurate but less demeaning. Other descriptors it proposes are negative affectivity, detachment, disinhibition, and anankastia, the last referring to compulsions.
These are notable advances. Can the field find even better terms to communicate hard to hear information, with words that are less problematic? In search of options, we surveyed clinicians at academic centers about the terms they preferred to avoid and the ones they prefer to use in talking with patients.6 Their practices may be informative.
Briefly summarized, these clinicians preferred not to use “schizophrenia” and very few used “antisocial,” “histrionic,” or “narcissistic.” Most avoided using “borderline” as well. Instead, they recommended discussing specific symptoms and manifestations of illness or dysfunctional behavior and relationships with their patients. They employed terms including “psychosis,” “hallucination,” “delusion,” “thinking disorder,” and “mood disorder.” They explained these terms, as needed, and found that patients understood them.
For Cluster B personality disorders, they spoke of personality traits and styles and specifically about “conduct,” “rule breaking,” “coping,” “self-focus,” “emotionality,” and “reactivity.” Those choices are not perfect, of course. Medical terms are often not standard words used in a conversational way. But the words chosen by these clinicians are generally straightforward and may communicate in a clear and acceptable fashion. It is also notable that the terms match how the clinicians assess and treat their patients, as observed in a separate study of their practices.7 That is, the clinicians advised that they look for and suggest treatments for the specific symptoms they see that most disrupt an individual’s life, such as delusions or mood instability. They are not much guided by diagnoses, like schizophrenia or borderline disorder. That makes the chosen terms not only less confusing or off-putting but also more practical.
Changing terminology in any field is difficult. We are trained to use standard terms. Clearly, however, many clinicians avoid some terms and use alternatives in their work. Asked why, they responded that they did so precisely to communicate more effectively and more respectfully. That is key to their treatment goals. Perhaps others will consider these choices useful in their work. And perhaps both the DSM and the ICD will not only continue to consider but will decide to implement alternatives for problematic terms in the years ahead, as they discuss their next revisions.
Dr. Cohen is director of the Program for Neuropsychiatric Research at McLean Hospital, Belmont, Mass., and Robertson-Steele Professor of Psychiatry at Harvard Medical School, Boston.
References
1. Lasalvia A et al. Renaming schizophrenia? A survey among psychiatrists, mental health service users and family members in Italy. Schizophr Res. 2021;228:502-9.
2. Gülöksüz S et al. Renaming schizophrenia: 5 x 5. Epidemiol Psychiatr Sci. 2019;28(3):254-7.
3. Sartorius N et al. Name change for schizophrenia. Schizophr Bull. 2014;40(2):255-8.
4. Mesholam-Gately RI et al. Are we ready for a name change for schizophrenia? A survey of multiple stakeholders. Schizophr Res. 2021;238:152-60.
5. Mulder R. The evolving nosology of personality disorder and its clinical utility. World Psychiatry. 2021 Oct;20(3):361-2.
6. Cohen BM et al. Diagnostic terms psychiatrists prefer to use for common psychotic and personality disorders. J Psychiatr Res. 2022 Sep 5;155:226-31.
7. Cohen BM, et al. Use of DSM-5 diagnoses vs. other clinical information by US academic-affiliated psychiatrists in assessing and treating psychotic disorders. World Psychiatry. 2021 Oct;20(3):447-8.