User login
MDedge conference coverage features onsite reporting of the latest study results and expert perspectives from leading researchers.
Circulating Tumor DNA Hints at BC Recurrence Risk
CHICAGO — Circulating tumor DNA (ctDNA) can predict relapse risk in some cases of early, high-risk breast cancer, but it’s too soon to use it to guide adjuvant therapy decisions, according to a study presented at the American Society of Clinical Oncology annual meeting.
Detectable ctDNA is “highly prognostic of worse outcomes, particularly in patients who [remain] persistently positive,” but the correlation isn’t perfect, said lead investigator Sherene Loi, MMBS, PhD, a breast cancer specialist at the Peter MacCallum Cancer Centre in Melbourne, Australia.
Although less likely, relapses also occurred in the study among women without ctDNA elevation. Conversely, there were women with elevated ctDNA who did not relapse, she said. The study was a subanalysis of the monarchE trial of adjuvant abemaciclib, a CDK 4/6 inhibitor.
Eventually, “we would like to use” ctDNA to guide adjuvant treatment decisions, but the research isn’t there yet, Dr. Loi said. It’s possible, for instance, that persistently detectable ctDNA indicates early treatment failure and the need for treatment intensification. Future research should tackle the issue.
Study discussant Francois-Clement Bidard, MD, PhD, a breast cancer specialist at Institut Curie, Paris, agreed that ctDNA isn’t ready for primetime in adjuvant early, high-risk breast cancer.
“There is no clinical evidence to suggest that there is clinical utility in this setting. There are several trials that are ongoing,” he said, but for now “you shouldn’t,” for example, “use ctDNA to de-escalate adjuvant CDK4/6 [inhibitors]. It could be in the future that we could have data on this, but at the moment, [the] clear clinical message [is] no way.”
At 5-year follow-up, the monarchE trial found a 7.6% invasive disease-free survival (IDFS) improvement when abemaciclib was added to the first 2 years of endocrine therapy in women with HR+, HER2-, node positive, high-risk early breast cancer. The combination is now a standard adjuvant option for the disease.
The ctDNA study focused on a subset of 910 subjects with adequate ctDNA testing to run the analysis. The study population was also selected to be enriched for overall IDFS events (27% versus 18% across the trial’s 5,637 subjects). An IDFS event was defined as a local, regional, contralateral or distant invasive recurrence; a new primary tumor; or death from any cause.
Testing was performed using the Signatera ctDNA assay. Baseline samples were taken after completion of adjuvant chemotherapy, then again at 3, 6, or 24 months.
Overall, ctDNA detection was infrequent. Just 8% of patients were positive at baseline and 17% were positive at any point during the trial. Even so, ctDNA detection at any point was adversely prognostic.
Patients who were ctDNA positive at baseline were more likely to experience an IDFS event, compared with those who were ctDNA negative at baseline (80% at 4 years follow-up versus 23%).
Similarly, those who remained positive or became positive during testing were more likely to experience an IDFS event compared with those who became negative or remained negative throughout testing.
For instance, all 34 patients who were positive at baseline and remained positive had an IDFS event by year 4, versus just 40% who started positive but then cleared their ctDNA.
Among women who were negative at baseline and remained negative, 13% had an IDFS event versus 89% who started negative but then turned positive. Subjects who turned positive also had the shortest time to an IDFS event, a median of 7 months.
Among women who recurred, those who were ctDNA negative tended to have local, regional, or contralateral recurrences, while ctDNA positive patients tended to have distant recurrences.
The finding “really highlights that ctDNA antedates the metastatic clinical relapse. What the ctDNA is telling you is that the metastatic process has been completed, and metastases are about to grow,” Dr. Bidard said.
The work was funded by Eli Lilly, maker of abemaciclib, with collaboration from Natera, maker of the Signatera assay. Dr. Loi is an adviser and researcher for Lilly, among other industry ties. Dr. Bidard is a speaker and consultant for Lilly, among other ties.
CHICAGO — Circulating tumor DNA (ctDNA) can predict relapse risk in some cases of early, high-risk breast cancer, but it’s too soon to use it to guide adjuvant therapy decisions, according to a study presented at the American Society of Clinical Oncology annual meeting.
Detectable ctDNA is “highly prognostic of worse outcomes, particularly in patients who [remain] persistently positive,” but the correlation isn’t perfect, said lead investigator Sherene Loi, MMBS, PhD, a breast cancer specialist at the Peter MacCallum Cancer Centre in Melbourne, Australia.
Although less likely, relapses also occurred in the study among women without ctDNA elevation. Conversely, there were women with elevated ctDNA who did not relapse, she said. The study was a subanalysis of the monarchE trial of adjuvant abemaciclib, a CDK 4/6 inhibitor.
Eventually, “we would like to use” ctDNA to guide adjuvant treatment decisions, but the research isn’t there yet, Dr. Loi said. It’s possible, for instance, that persistently detectable ctDNA indicates early treatment failure and the need for treatment intensification. Future research should tackle the issue.
Study discussant Francois-Clement Bidard, MD, PhD, a breast cancer specialist at Institut Curie, Paris, agreed that ctDNA isn’t ready for primetime in adjuvant early, high-risk breast cancer.
“There is no clinical evidence to suggest that there is clinical utility in this setting. There are several trials that are ongoing,” he said, but for now “you shouldn’t,” for example, “use ctDNA to de-escalate adjuvant CDK4/6 [inhibitors]. It could be in the future that we could have data on this, but at the moment, [the] clear clinical message [is] no way.”
At 5-year follow-up, the monarchE trial found a 7.6% invasive disease-free survival (IDFS) improvement when abemaciclib was added to the first 2 years of endocrine therapy in women with HR+, HER2-, node positive, high-risk early breast cancer. The combination is now a standard adjuvant option for the disease.
The ctDNA study focused on a subset of 910 subjects with adequate ctDNA testing to run the analysis. The study population was also selected to be enriched for overall IDFS events (27% versus 18% across the trial’s 5,637 subjects). An IDFS event was defined as a local, regional, contralateral or distant invasive recurrence; a new primary tumor; or death from any cause.
Testing was performed using the Signatera ctDNA assay. Baseline samples were taken after completion of adjuvant chemotherapy, then again at 3, 6, or 24 months.
Overall, ctDNA detection was infrequent. Just 8% of patients were positive at baseline and 17% were positive at any point during the trial. Even so, ctDNA detection at any point was adversely prognostic.
Patients who were ctDNA positive at baseline were more likely to experience an IDFS event, compared with those who were ctDNA negative at baseline (80% at 4 years follow-up versus 23%).
Similarly, those who remained positive or became positive during testing were more likely to experience an IDFS event compared with those who became negative or remained negative throughout testing.
For instance, all 34 patients who were positive at baseline and remained positive had an IDFS event by year 4, versus just 40% who started positive but then cleared their ctDNA.
Among women who were negative at baseline and remained negative, 13% had an IDFS event versus 89% who started negative but then turned positive. Subjects who turned positive also had the shortest time to an IDFS event, a median of 7 months.
Among women who recurred, those who were ctDNA negative tended to have local, regional, or contralateral recurrences, while ctDNA positive patients tended to have distant recurrences.
The finding “really highlights that ctDNA antedates the metastatic clinical relapse. What the ctDNA is telling you is that the metastatic process has been completed, and metastases are about to grow,” Dr. Bidard said.
The work was funded by Eli Lilly, maker of abemaciclib, with collaboration from Natera, maker of the Signatera assay. Dr. Loi is an adviser and researcher for Lilly, among other industry ties. Dr. Bidard is a speaker and consultant for Lilly, among other ties.
CHICAGO — Circulating tumor DNA (ctDNA) can predict relapse risk in some cases of early, high-risk breast cancer, but it’s too soon to use it to guide adjuvant therapy decisions, according to a study presented at the American Society of Clinical Oncology annual meeting.
Detectable ctDNA is “highly prognostic of worse outcomes, particularly in patients who [remain] persistently positive,” but the correlation isn’t perfect, said lead investigator Sherene Loi, MMBS, PhD, a breast cancer specialist at the Peter MacCallum Cancer Centre in Melbourne, Australia.
Although less likely, relapses also occurred in the study among women without ctDNA elevation. Conversely, there were women with elevated ctDNA who did not relapse, she said. The study was a subanalysis of the monarchE trial of adjuvant abemaciclib, a CDK 4/6 inhibitor.
Eventually, “we would like to use” ctDNA to guide adjuvant treatment decisions, but the research isn’t there yet, Dr. Loi said. It’s possible, for instance, that persistently detectable ctDNA indicates early treatment failure and the need for treatment intensification. Future research should tackle the issue.
Study discussant Francois-Clement Bidard, MD, PhD, a breast cancer specialist at Institut Curie, Paris, agreed that ctDNA isn’t ready for primetime in adjuvant early, high-risk breast cancer.
“There is no clinical evidence to suggest that there is clinical utility in this setting. There are several trials that are ongoing,” he said, but for now “you shouldn’t,” for example, “use ctDNA to de-escalate adjuvant CDK4/6 [inhibitors]. It could be in the future that we could have data on this, but at the moment, [the] clear clinical message [is] no way.”
At 5-year follow-up, the monarchE trial found a 7.6% invasive disease-free survival (IDFS) improvement when abemaciclib was added to the first 2 years of endocrine therapy in women with HR+, HER2-, node positive, high-risk early breast cancer. The combination is now a standard adjuvant option for the disease.
The ctDNA study focused on a subset of 910 subjects with adequate ctDNA testing to run the analysis. The study population was also selected to be enriched for overall IDFS events (27% versus 18% across the trial’s 5,637 subjects). An IDFS event was defined as a local, regional, contralateral or distant invasive recurrence; a new primary tumor; or death from any cause.
Testing was performed using the Signatera ctDNA assay. Baseline samples were taken after completion of adjuvant chemotherapy, then again at 3, 6, or 24 months.
Overall, ctDNA detection was infrequent. Just 8% of patients were positive at baseline and 17% were positive at any point during the trial. Even so, ctDNA detection at any point was adversely prognostic.
Patients who were ctDNA positive at baseline were more likely to experience an IDFS event, compared with those who were ctDNA negative at baseline (80% at 4 years follow-up versus 23%).
Similarly, those who remained positive or became positive during testing were more likely to experience an IDFS event compared with those who became negative or remained negative throughout testing.
For instance, all 34 patients who were positive at baseline and remained positive had an IDFS event by year 4, versus just 40% who started positive but then cleared their ctDNA.
Among women who were negative at baseline and remained negative, 13% had an IDFS event versus 89% who started negative but then turned positive. Subjects who turned positive also had the shortest time to an IDFS event, a median of 7 months.
Among women who recurred, those who were ctDNA negative tended to have local, regional, or contralateral recurrences, while ctDNA positive patients tended to have distant recurrences.
The finding “really highlights that ctDNA antedates the metastatic clinical relapse. What the ctDNA is telling you is that the metastatic process has been completed, and metastases are about to grow,” Dr. Bidard said.
The work was funded by Eli Lilly, maker of abemaciclib, with collaboration from Natera, maker of the Signatera assay. Dr. Loi is an adviser and researcher for Lilly, among other industry ties. Dr. Bidard is a speaker and consultant for Lilly, among other ties.
FROM ASCO 2024
Dermatoporosis in Older Adults: A Condition That Requires Holistic, Creative Management
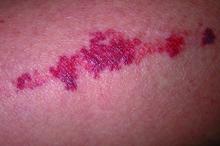
WASHINGTON — and conveys the skin’s vulnerability to serious medical complications, said Adam Friedman, MD, at the ElderDerm conference on dermatology in the older patient.
Key features of dermatoporosis include atrophic skin, solar purpura, white pseudoscars, easily acquired skin lacerations and tears, bruises, and delayed healing. “We’re going to see more of this, and it will more and more be a chief complaint of patients,” said Dr. Friedman, professor and chair of dermatology at George Washington University (GWU) in Washington, and co-chair of the meeting. GWU hosted the conference, describing it as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Dermatoporosis was described in the literature in 2007 by dermatologists at the University of Geneva in Switzerland. “It is not only a cosmetic problem,” Dr. Friedman said. “This is a medical problem ... which can absolutely lead to comorbidities [such as deep dissecting hematomas] that are a huge strain on the healthcare system.”
Dermatologists can meet the moment with holistic, creative combination treatment and counseling approaches aimed at improving the mechanical strength of skin and preventing potential complications in older patients, Dr. Friedman said at the meeting.
He described the case of a 76-year-old woman who presented with dermatoporosis on her arms involving pronounced skin atrophy, solar purpura, and a small covered laceration. “This was a patient who was both devastated by the appearance” and impacted by the pain and burden of dressing frequent wounds, said Dr. Friedman, who is also the director of the Residency Program, of Translational Research, and of Supportive Oncodermatology, all within the Department of Dermatology at GWU.
With 11 months of topical treatment that included daily application of calcipotriene 0.05% ointment and nightly application of tazarotene 0.045% lotion and oral supplementation with 1000-mg vitamin C twice daily and 1000-mg citrus bioflavonoid complex daily, as well as no changes to the medications she took for various comorbidities, the solar purpura improved significantly and “we made a huge difference in the integrity of her skin,” he said.
Dr. Friedman also described this case in a recently published article in the Journal of Drugs in Dermatology titled “What’s Old Is New: An Emerging Focus on Dermatoporosis”.
Likely Pathophysiology
Advancing age and chronic ultraviolet (UV) radiation exposure are the chief drivers of dermatoporosis. In addition to UVA and UVB light, other secondary drivers include genetic susceptibility, topical and systematic corticosteroid use, and anticoagulant treatment.
Its pathogenesis is not well described in the literature but is easy to envision, Dr. Friedman said. For one, both advancing age and exposure to UV light lead to a reduction in hygroscopic glycosaminoglycans, including hyaluronate (HA), and the impact of this diminishment is believed to go “beyond [the loss of] buoyancy,” he noted. Researchers have “been showing these are not just water-loving molecules, they also have some biologic properties” relating to keratinocyte production and epidermal turnover that appear to be intricately linked to the pathogenesis of dermatoporosis.
HAs have been shown to interact with the cell surface receptor CD44 to stimulate keratinocyte proliferation, and low levels of CD44 have been reported in skin with dermatoporosis compared with a younger control population. (A newly characterized organelle, the hyaluronosome, serves as an HA factory and contains CD44 and heparin-binding epidermal growth factor, Dr. Friedman noted. Inadequate functioning may be involved in skin atrophy.)
Advancing age also brings an increase in matrix metalloproteinases (MMPs)–1, –2, and –3, which are “the demolition workers of the skin,” and downregulation of a tissue inhibitor of MMPs, he said.
Adding insult to injury, dermis-penetrating UVA also activates MMPs, “obliterating collagen and elastin.” UVB generates DNA photoproducts, including oxidative stress and damaging skin cell DNA. “That UV light induces breakdown [of the skin] through different mechanisms and inhibits buildup is a simple concept I think our patients can understand,” Dr. Friedman said.
Multifaceted Treatment
For an older adult, “there is never a wrong time to start sun-protective measures” to prevent or try to halt the progression of dermatoporosis, Dr. Friedman said, noting that “UV radiation is an immunosuppressant, so there are many good reasons to start” if the adult is not already taking measures on a regular basis.
Potential treatments for the syndrome of dermatoporosis are backed by few clinical studies, but dermatologists are skilled at translating the use of products from one disease state to another based on understandings of pathophysiology and mechanistic pathways, Dr. Friedman commented in an interview after the meeting.
For instance, “from decades of research, we know what retinoids will do to the skin,” he said in the interview. “We know they will turn on collagen-1 and -3 genes in the skin, and that they will increase the production of glycosaminoglycans ... By understanding the biology, we can translate this to dermatoporosis.” These changes were demonstrated, for instance, in a small study of topical retinol in older adults.
Studies of topical alpha hydroxy acid (AHA), moreover, have demonstrated epidermal thickening and firmness, and “some studies show they can limit steroid-induced atrophy,” Dr. Friedman said at the meeting. “And things like lactic acid and urea are super accessible.”
Topical dehydroepiandrosterone is backed by even less data than retinoids or AHAs are, “but it’s still something to consider” as part of a multimechanistic approach to dermatoporosis, Dr. Friedman shared, noting that a small study demonstrated beneficial effects on epidermal atrophy in aging skin.
The use of vitamin D analogues such as calcipotriene, which is approved for the treatment of psoriasis, may also be promising. “One concept is that [vitamin D analogues] increase calcium concentrations in the epidermis, and calcium is so central to keratinocyte differentiation” and epidermal function that calcipotriene in combination with topical steroid therapy has been shown to limit skin atrophy, he noted.
Nutritionally, low protein intake is a known problem in the older population and is associated with increased skin fragility and poorer healing. From a prevention and treatment standpoint, therefore, patients can be counseled to be attentive to their diets, Dr. Friedman said. Experts have recommended a higher protein intake for older adults than for younger adults; in 2013, an international group recommended a protein intake of 1-1.5 g/kg/d for healthy older adults and more for those with acute or chronic illness.
“Patients love talking about diet and skin disease ... and they love over-the-counter nutraceuticals as well because they want something natural,” Dr. Friedman said. “I like using bioflavonoids in combination with vitamin C, which can be effective especially for solar purpura.”
A 6-week randomized, placebo-controlled, double-blind trial involving 67 patients with purpura associated with aging found a 50% reduction in purpura lesions among those took a particular citrus bioflavonoid blend twice daily. “I thought this was a pretty well-done study,” he said, noting that patient self-assessment and investigator global assessment were utilized.
Skin Injury and Wound Prevention
In addition to recommending gentle skin cleansers and daily moisturizing, dermatologists should talk to their older patients with dermatoporosis about their home environments. “What is it like? Is there furniture with sharp edges?” Dr. Friedman advised. If so, could they use sleeves or protectors on their arms or legs “to protect against injury?”
In a later meeting session about lower-extremity wounds on geriatric patients, Michael Stempel, DPM, assistant professor of medicine and surgery and chief of podiatry at GWU, said that he was happy to hear the term dermatoporosis being used because like diabetes, it’s a risk factor for developing lower-extremity wounds and poor wound healing.
He shared the case of an older woman with dermatoporosis who “tripped and skinned her knee against a step and then self-treated it for over a month by pouring hydrogen peroxide over it and letting air get to it.” The wound developed into “full-thickness tissue loss,” said Dr. Stempel, also medical director of the Wound Healing and Limb Preservation Center at GWU Hospital.
Misperceptions are common among older patients about how a simple wound should be managed; for instance, the adage “just let it get air” is not uncommon. This makes anticipatory guidance about basic wound care — such as the importance of a moist and occlusive environment and the safe use of hydrogen peroxide — especially important for patients with dermatoporosis, Dr. Friedman commented after the meeting.
Dermatoporosis is quantifiable, Dr. Friedman said during the meeting, with a scoring system having been developed by the researchers in Switzerland who originally coined the term. Its use in practice is unnecessary, but its existence is “nice to share with patients who feel bothered because oftentimes, patients feel it’s been dismissed by other providers,” he said. “Telling your patients there’s an actual name for their problem, and that there are ways to quantify and measure changes over time, is validating.”
Its recognition as a medical condition, Dr. Friedman added, also enables the dermatologist to bring it up and counsel appropriately — without a patient feeling shame — when it is identified in the context of a skin excision, treatment of a primary inflammatory skin disease, or management of another dermatologic problem.
Dr. Friedman disclosed that he is a consultant/advisory board member for L’Oréal, La Roche-Posay, Galderma, and other companies; a speaker for Regeneron/Sanofi, Incyte, BMD, and Janssen; and has grants from Pfizer, Lilly, Incyte, and other companies. Dr. Stempel reported no disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON — and conveys the skin’s vulnerability to serious medical complications, said Adam Friedman, MD, at the ElderDerm conference on dermatology in the older patient.
Key features of dermatoporosis include atrophic skin, solar purpura, white pseudoscars, easily acquired skin lacerations and tears, bruises, and delayed healing. “We’re going to see more of this, and it will more and more be a chief complaint of patients,” said Dr. Friedman, professor and chair of dermatology at George Washington University (GWU) in Washington, and co-chair of the meeting. GWU hosted the conference, describing it as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Dermatoporosis was described in the literature in 2007 by dermatologists at the University of Geneva in Switzerland. “It is not only a cosmetic problem,” Dr. Friedman said. “This is a medical problem ... which can absolutely lead to comorbidities [such as deep dissecting hematomas] that are a huge strain on the healthcare system.”
Dermatologists can meet the moment with holistic, creative combination treatment and counseling approaches aimed at improving the mechanical strength of skin and preventing potential complications in older patients, Dr. Friedman said at the meeting.
He described the case of a 76-year-old woman who presented with dermatoporosis on her arms involving pronounced skin atrophy, solar purpura, and a small covered laceration. “This was a patient who was both devastated by the appearance” and impacted by the pain and burden of dressing frequent wounds, said Dr. Friedman, who is also the director of the Residency Program, of Translational Research, and of Supportive Oncodermatology, all within the Department of Dermatology at GWU.
With 11 months of topical treatment that included daily application of calcipotriene 0.05% ointment and nightly application of tazarotene 0.045% lotion and oral supplementation with 1000-mg vitamin C twice daily and 1000-mg citrus bioflavonoid complex daily, as well as no changes to the medications she took for various comorbidities, the solar purpura improved significantly and “we made a huge difference in the integrity of her skin,” he said.
Dr. Friedman also described this case in a recently published article in the Journal of Drugs in Dermatology titled “What’s Old Is New: An Emerging Focus on Dermatoporosis”.
Likely Pathophysiology
Advancing age and chronic ultraviolet (UV) radiation exposure are the chief drivers of dermatoporosis. In addition to UVA and UVB light, other secondary drivers include genetic susceptibility, topical and systematic corticosteroid use, and anticoagulant treatment.
Its pathogenesis is not well described in the literature but is easy to envision, Dr. Friedman said. For one, both advancing age and exposure to UV light lead to a reduction in hygroscopic glycosaminoglycans, including hyaluronate (HA), and the impact of this diminishment is believed to go “beyond [the loss of] buoyancy,” he noted. Researchers have “been showing these are not just water-loving molecules, they also have some biologic properties” relating to keratinocyte production and epidermal turnover that appear to be intricately linked to the pathogenesis of dermatoporosis.
HAs have been shown to interact with the cell surface receptor CD44 to stimulate keratinocyte proliferation, and low levels of CD44 have been reported in skin with dermatoporosis compared with a younger control population. (A newly characterized organelle, the hyaluronosome, serves as an HA factory and contains CD44 and heparin-binding epidermal growth factor, Dr. Friedman noted. Inadequate functioning may be involved in skin atrophy.)
Advancing age also brings an increase in matrix metalloproteinases (MMPs)–1, –2, and –3, which are “the demolition workers of the skin,” and downregulation of a tissue inhibitor of MMPs, he said.
Adding insult to injury, dermis-penetrating UVA also activates MMPs, “obliterating collagen and elastin.” UVB generates DNA photoproducts, including oxidative stress and damaging skin cell DNA. “That UV light induces breakdown [of the skin] through different mechanisms and inhibits buildup is a simple concept I think our patients can understand,” Dr. Friedman said.
Multifaceted Treatment
For an older adult, “there is never a wrong time to start sun-protective measures” to prevent or try to halt the progression of dermatoporosis, Dr. Friedman said, noting that “UV radiation is an immunosuppressant, so there are many good reasons to start” if the adult is not already taking measures on a regular basis.
Potential treatments for the syndrome of dermatoporosis are backed by few clinical studies, but dermatologists are skilled at translating the use of products from one disease state to another based on understandings of pathophysiology and mechanistic pathways, Dr. Friedman commented in an interview after the meeting.
For instance, “from decades of research, we know what retinoids will do to the skin,” he said in the interview. “We know they will turn on collagen-1 and -3 genes in the skin, and that they will increase the production of glycosaminoglycans ... By understanding the biology, we can translate this to dermatoporosis.” These changes were demonstrated, for instance, in a small study of topical retinol in older adults.
Studies of topical alpha hydroxy acid (AHA), moreover, have demonstrated epidermal thickening and firmness, and “some studies show they can limit steroid-induced atrophy,” Dr. Friedman said at the meeting. “And things like lactic acid and urea are super accessible.”
Topical dehydroepiandrosterone is backed by even less data than retinoids or AHAs are, “but it’s still something to consider” as part of a multimechanistic approach to dermatoporosis, Dr. Friedman shared, noting that a small study demonstrated beneficial effects on epidermal atrophy in aging skin.
The use of vitamin D analogues such as calcipotriene, which is approved for the treatment of psoriasis, may also be promising. “One concept is that [vitamin D analogues] increase calcium concentrations in the epidermis, and calcium is so central to keratinocyte differentiation” and epidermal function that calcipotriene in combination with topical steroid therapy has been shown to limit skin atrophy, he noted.
Nutritionally, low protein intake is a known problem in the older population and is associated with increased skin fragility and poorer healing. From a prevention and treatment standpoint, therefore, patients can be counseled to be attentive to their diets, Dr. Friedman said. Experts have recommended a higher protein intake for older adults than for younger adults; in 2013, an international group recommended a protein intake of 1-1.5 g/kg/d for healthy older adults and more for those with acute or chronic illness.
“Patients love talking about diet and skin disease ... and they love over-the-counter nutraceuticals as well because they want something natural,” Dr. Friedman said. “I like using bioflavonoids in combination with vitamin C, which can be effective especially for solar purpura.”
A 6-week randomized, placebo-controlled, double-blind trial involving 67 patients with purpura associated with aging found a 50% reduction in purpura lesions among those took a particular citrus bioflavonoid blend twice daily. “I thought this was a pretty well-done study,” he said, noting that patient self-assessment and investigator global assessment were utilized.
Skin Injury and Wound Prevention
In addition to recommending gentle skin cleansers and daily moisturizing, dermatologists should talk to their older patients with dermatoporosis about their home environments. “What is it like? Is there furniture with sharp edges?” Dr. Friedman advised. If so, could they use sleeves or protectors on their arms or legs “to protect against injury?”
In a later meeting session about lower-extremity wounds on geriatric patients, Michael Stempel, DPM, assistant professor of medicine and surgery and chief of podiatry at GWU, said that he was happy to hear the term dermatoporosis being used because like diabetes, it’s a risk factor for developing lower-extremity wounds and poor wound healing.
He shared the case of an older woman with dermatoporosis who “tripped and skinned her knee against a step and then self-treated it for over a month by pouring hydrogen peroxide over it and letting air get to it.” The wound developed into “full-thickness tissue loss,” said Dr. Stempel, also medical director of the Wound Healing and Limb Preservation Center at GWU Hospital.
Misperceptions are common among older patients about how a simple wound should be managed; for instance, the adage “just let it get air” is not uncommon. This makes anticipatory guidance about basic wound care — such as the importance of a moist and occlusive environment and the safe use of hydrogen peroxide — especially important for patients with dermatoporosis, Dr. Friedman commented after the meeting.
Dermatoporosis is quantifiable, Dr. Friedman said during the meeting, with a scoring system having been developed by the researchers in Switzerland who originally coined the term. Its use in practice is unnecessary, but its existence is “nice to share with patients who feel bothered because oftentimes, patients feel it’s been dismissed by other providers,” he said. “Telling your patients there’s an actual name for their problem, and that there are ways to quantify and measure changes over time, is validating.”
Its recognition as a medical condition, Dr. Friedman added, also enables the dermatologist to bring it up and counsel appropriately — without a patient feeling shame — when it is identified in the context of a skin excision, treatment of a primary inflammatory skin disease, or management of another dermatologic problem.
Dr. Friedman disclosed that he is a consultant/advisory board member for L’Oréal, La Roche-Posay, Galderma, and other companies; a speaker for Regeneron/Sanofi, Incyte, BMD, and Janssen; and has grants from Pfizer, Lilly, Incyte, and other companies. Dr. Stempel reported no disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON — and conveys the skin’s vulnerability to serious medical complications, said Adam Friedman, MD, at the ElderDerm conference on dermatology in the older patient.
Key features of dermatoporosis include atrophic skin, solar purpura, white pseudoscars, easily acquired skin lacerations and tears, bruises, and delayed healing. “We’re going to see more of this, and it will more and more be a chief complaint of patients,” said Dr. Friedman, professor and chair of dermatology at George Washington University (GWU) in Washington, and co-chair of the meeting. GWU hosted the conference, describing it as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Dermatoporosis was described in the literature in 2007 by dermatologists at the University of Geneva in Switzerland. “It is not only a cosmetic problem,” Dr. Friedman said. “This is a medical problem ... which can absolutely lead to comorbidities [such as deep dissecting hematomas] that are a huge strain on the healthcare system.”
Dermatologists can meet the moment with holistic, creative combination treatment and counseling approaches aimed at improving the mechanical strength of skin and preventing potential complications in older patients, Dr. Friedman said at the meeting.
He described the case of a 76-year-old woman who presented with dermatoporosis on her arms involving pronounced skin atrophy, solar purpura, and a small covered laceration. “This was a patient who was both devastated by the appearance” and impacted by the pain and burden of dressing frequent wounds, said Dr. Friedman, who is also the director of the Residency Program, of Translational Research, and of Supportive Oncodermatology, all within the Department of Dermatology at GWU.
With 11 months of topical treatment that included daily application of calcipotriene 0.05% ointment and nightly application of tazarotene 0.045% lotion and oral supplementation with 1000-mg vitamin C twice daily and 1000-mg citrus bioflavonoid complex daily, as well as no changes to the medications she took for various comorbidities, the solar purpura improved significantly and “we made a huge difference in the integrity of her skin,” he said.
Dr. Friedman also described this case in a recently published article in the Journal of Drugs in Dermatology titled “What’s Old Is New: An Emerging Focus on Dermatoporosis”.
Likely Pathophysiology
Advancing age and chronic ultraviolet (UV) radiation exposure are the chief drivers of dermatoporosis. In addition to UVA and UVB light, other secondary drivers include genetic susceptibility, topical and systematic corticosteroid use, and anticoagulant treatment.
Its pathogenesis is not well described in the literature but is easy to envision, Dr. Friedman said. For one, both advancing age and exposure to UV light lead to a reduction in hygroscopic glycosaminoglycans, including hyaluronate (HA), and the impact of this diminishment is believed to go “beyond [the loss of] buoyancy,” he noted. Researchers have “been showing these are not just water-loving molecules, they also have some biologic properties” relating to keratinocyte production and epidermal turnover that appear to be intricately linked to the pathogenesis of dermatoporosis.
HAs have been shown to interact with the cell surface receptor CD44 to stimulate keratinocyte proliferation, and low levels of CD44 have been reported in skin with dermatoporosis compared with a younger control population. (A newly characterized organelle, the hyaluronosome, serves as an HA factory and contains CD44 and heparin-binding epidermal growth factor, Dr. Friedman noted. Inadequate functioning may be involved in skin atrophy.)
Advancing age also brings an increase in matrix metalloproteinases (MMPs)–1, –2, and –3, which are “the demolition workers of the skin,” and downregulation of a tissue inhibitor of MMPs, he said.
Adding insult to injury, dermis-penetrating UVA also activates MMPs, “obliterating collagen and elastin.” UVB generates DNA photoproducts, including oxidative stress and damaging skin cell DNA. “That UV light induces breakdown [of the skin] through different mechanisms and inhibits buildup is a simple concept I think our patients can understand,” Dr. Friedman said.
Multifaceted Treatment
For an older adult, “there is never a wrong time to start sun-protective measures” to prevent or try to halt the progression of dermatoporosis, Dr. Friedman said, noting that “UV radiation is an immunosuppressant, so there are many good reasons to start” if the adult is not already taking measures on a regular basis.
Potential treatments for the syndrome of dermatoporosis are backed by few clinical studies, but dermatologists are skilled at translating the use of products from one disease state to another based on understandings of pathophysiology and mechanistic pathways, Dr. Friedman commented in an interview after the meeting.
For instance, “from decades of research, we know what retinoids will do to the skin,” he said in the interview. “We know they will turn on collagen-1 and -3 genes in the skin, and that they will increase the production of glycosaminoglycans ... By understanding the biology, we can translate this to dermatoporosis.” These changes were demonstrated, for instance, in a small study of topical retinol in older adults.
Studies of topical alpha hydroxy acid (AHA), moreover, have demonstrated epidermal thickening and firmness, and “some studies show they can limit steroid-induced atrophy,” Dr. Friedman said at the meeting. “And things like lactic acid and urea are super accessible.”
Topical dehydroepiandrosterone is backed by even less data than retinoids or AHAs are, “but it’s still something to consider” as part of a multimechanistic approach to dermatoporosis, Dr. Friedman shared, noting that a small study demonstrated beneficial effects on epidermal atrophy in aging skin.
The use of vitamin D analogues such as calcipotriene, which is approved for the treatment of psoriasis, may also be promising. “One concept is that [vitamin D analogues] increase calcium concentrations in the epidermis, and calcium is so central to keratinocyte differentiation” and epidermal function that calcipotriene in combination with topical steroid therapy has been shown to limit skin atrophy, he noted.
Nutritionally, low protein intake is a known problem in the older population and is associated with increased skin fragility and poorer healing. From a prevention and treatment standpoint, therefore, patients can be counseled to be attentive to their diets, Dr. Friedman said. Experts have recommended a higher protein intake for older adults than for younger adults; in 2013, an international group recommended a protein intake of 1-1.5 g/kg/d for healthy older adults and more for those with acute or chronic illness.
“Patients love talking about diet and skin disease ... and they love over-the-counter nutraceuticals as well because they want something natural,” Dr. Friedman said. “I like using bioflavonoids in combination with vitamin C, which can be effective especially for solar purpura.”
A 6-week randomized, placebo-controlled, double-blind trial involving 67 patients with purpura associated with aging found a 50% reduction in purpura lesions among those took a particular citrus bioflavonoid blend twice daily. “I thought this was a pretty well-done study,” he said, noting that patient self-assessment and investigator global assessment were utilized.
Skin Injury and Wound Prevention
In addition to recommending gentle skin cleansers and daily moisturizing, dermatologists should talk to their older patients with dermatoporosis about their home environments. “What is it like? Is there furniture with sharp edges?” Dr. Friedman advised. If so, could they use sleeves or protectors on their arms or legs “to protect against injury?”
In a later meeting session about lower-extremity wounds on geriatric patients, Michael Stempel, DPM, assistant professor of medicine and surgery and chief of podiatry at GWU, said that he was happy to hear the term dermatoporosis being used because like diabetes, it’s a risk factor for developing lower-extremity wounds and poor wound healing.
He shared the case of an older woman with dermatoporosis who “tripped and skinned her knee against a step and then self-treated it for over a month by pouring hydrogen peroxide over it and letting air get to it.” The wound developed into “full-thickness tissue loss,” said Dr. Stempel, also medical director of the Wound Healing and Limb Preservation Center at GWU Hospital.
Misperceptions are common among older patients about how a simple wound should be managed; for instance, the adage “just let it get air” is not uncommon. This makes anticipatory guidance about basic wound care — such as the importance of a moist and occlusive environment and the safe use of hydrogen peroxide — especially important for patients with dermatoporosis, Dr. Friedman commented after the meeting.
Dermatoporosis is quantifiable, Dr. Friedman said during the meeting, with a scoring system having been developed by the researchers in Switzerland who originally coined the term. Its use in practice is unnecessary, but its existence is “nice to share with patients who feel bothered because oftentimes, patients feel it’s been dismissed by other providers,” he said. “Telling your patients there’s an actual name for their problem, and that there are ways to quantify and measure changes over time, is validating.”
Its recognition as a medical condition, Dr. Friedman added, also enables the dermatologist to bring it up and counsel appropriately — without a patient feeling shame — when it is identified in the context of a skin excision, treatment of a primary inflammatory skin disease, or management of another dermatologic problem.
Dr. Friedman disclosed that he is a consultant/advisory board member for L’Oréal, La Roche-Posay, Galderma, and other companies; a speaker for Regeneron/Sanofi, Incyte, BMD, and Janssen; and has grants from Pfizer, Lilly, Incyte, and other companies. Dr. Stempel reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM ELDERDERM 2024
Managing Atopic Dermatitis in Older Adults: A Common, Unique Challenge
WASHINGTON, DC — Jonathan I. Silverberg, MD, PhD, MPH, said at the ElderDerm Conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“I walked out of residency under the impression that if it didn’t start in the first year or two of life, it’s not AD,” said Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University. “The numbers tell us a very different story.”
The prevalence of AD in the United States fluctuates between 6% and 8% through adulthood, including age categories up to 81-85 years, according to 2012 National Health Interview Survey data. And while persistence of childhood-onset AD is common, a systematic review and meta-analysis published in 2018 concluded that one in four adults with AD report adult-onset disease.
The investigators, including Dr. Silverberg, identified 25 observational studies — studies conducted across 16 countries and published during 1956-2017 — that included an analysis of age of onset beyond 10 years of age, and other inclusion criteria. Of the 25 studies, 17 reported age of onset after 16 years of age and had sufficient data for the meta-analysis. Using random-effects weighting, the investigators found a pooled proportion of adult-onset AD of 26.1% (95% CI, 16.5%-37.2%).
The research demonstrates that “the age of onset is distributed well throughout the lifespan,” Dr. Silverberg said, with the data “indicating there are many elderly-onset cases of true AD as well.” (Thirteen of the studies analyzed an age of onset from age ≥ 65, and several looked beyond age 80).
A 2021 study of a primary care database in the United Kingdom of 3.85 million children and adults found a “fascinating” bimodal distribution of incidence across the lifespan, with peaks in both infancy and older adulthood, he said. Incidence in adulthood was relatively stable from ages 18-49 years, after which, “into the 50s, 60s and beyond, you started to see a steady climb again.”
Also intriguing, Dr. Silverberg continued, are findings from a study of outpatient healthcare utilization for AD in which he and his coinvestigator analyzed data from the National Ambulatory Medical Care Survey (NAMCS). In the article, published in 2023 covering data from the 1993-2015 NAMCS, they reported that AD visits were more common among children aged 0-4 years (32.0%) and 5-9 years of age (10.6%), then decreased in adolescents aged 10-19 years (11.6%), remained fairly steady in patients aged 20-89 years (1.0%-4.7%), and increased in patients aged > 90 years (20.7%).
“The peak usage for dermatologists, primary care physicians, etc., is happening in the first few years of life, partially because that’s when the disease is more common and more severe but also partially because that’s when parents and caregivers are first learning [about] the disease and trying to understand how to gain control,” Dr. Silverberg said at the meeting, presenting data from an expanded, unpublished analysis of NAMCS data showing these same outpatient utilization patterns.
“It’s fascinating — there’s a much greater utilization in the elderly population. Why? The short answer is, we don’t know,” he said.
Risk Factors, Immune Differences
People with adult-onset AD were more likely to be women, smokers in adulthood, and have a lower childhood socioeconomic status than those whose AD started in childhood in a longitudinal study of two large birth cohorts from the United Kingdom , Dr. Silverberg pointed out.
Patients with childhood-onset AD, meanwhile, were more likely to have asthma, allergen-specific immunoglobulin E (IgE), and known genetic polymorphisms previously associated with AD. (Each cohort — the 1958 British Cohort Study and the 1970 British Cohort Study — had more than 17,000 participants who were followed from birth through middle age.)
Data is limited, but “mechanistically,” AD in much older adults appears to have a unique serum cytokine pattern, Dr. Silverberg said. He pointed to a cross-sectional study in China of 1312 children and adults with AD in which researchers analyzed clinical features, serum samples, and skin biopsy samples.
Adults aged > 60 years showed more lesions on the trunk and extensor sites of the extremities and lower levels of serum IgE and peripheral eosinophil counts than those in younger age groups. And “interestingly,” compared with healthy controls, older patients with AD had “higher levels of serum expression of a variety of cytokines, including IL [interleukin]-4 but also high TARC levels ... and a variety of cytokines related to the Th17, TH1 axes, etc.,” he said.
“So, we’re seeing a fascinating new profile that may be a little different than younger-onset cases,” he said, noting that TARC (thymus and activation-regulated chemokine) is regarded as a “decent biomarker” for AD.
In addition to higher levels of IL-4 and TARC, the study investigators reported significantly higher levels of IL-17A, IL-6, IL-22, IL-33, and thymic stromal lymphopoietin in older patients, compared with healthy controls.
Research also suggests that air pollution may play a role in the onset of AD in older age, Dr. Silverberg said, referencing a 2023 study that explored the association of air pollution and genetic risk with the onset of AD after age 50. The study analyzed 337,910 participants from the UK Biobank, with a median 12-year follow-up. Genetic risks were assessed as low, intermediate, and high, based on tertiles of polygenic risk scores. Exposure to various air pollutants was assessed using weighted quantile sum and also categorized into tertiles.
The incidence of older adult-onset AD was associated with medium and high air pollution compared with low air pollution, with hazard ratios (HRs) of 1.182 (P = .003) and 1.359 (P < .001), respectively. And “to a lesser extent,” Dr. Silverberg said, incidence was associated with medium and high genetic susceptibility, with HRs of 1.065 (P = .249) and 1.153 (P = .008).
The researchers calculated a greater population-attributable fraction of air pollution (15.5%) than genetic risk (6.4%). “This means that yes, genetics can contribute even to later-onset disease ... but environment may play an even more important role,” Dr. Silverberg said.
In the Clinic
In all patients, and especially in older adults, sleep disturbance associated with AD is a consideration for care. Data collected at the eczema clinic of Northwestern University, Chicago, Illinois, between 2014 and 2019 through previsit, self-administered questionnaires show that patients ≥ 65 years of age have more profound sleep disturbance (especially trouble staying asleep) than patients aged 18-64 years, despite having similar AD severity, said Dr. Silverberg, a coinvestigator of the study.
Older age was associated with having an increased number of nights of sleep disturbance (3-7 nights in the previous week) because of eczema (adjusted odds ratio [aOR], 2.14; 95% CI, 1.16-3.92). It was also associated with itching-attributed delays in falling asleep and nighttime awakenings in the prior 2 weeks (aOR, 1.88; 95% CI, 1.05-3.39).
“The aging population has dysregulated sleep patterns and altered circadian rhythms, so some of this is just natural predisposition,” Dr. Silverberg said. “But it’s amplified [with AD and itching], and it becomes a big clinical problem when we get into treatment because it’s our natural inclination to prescribe antihistamines for their sedative properties.”
Antihistamines can cause more profound sedation, more forgetfulness, and more anticholinergic side effects, he said, noting that “there’s some evidence that high-dose antihistamines may exacerbate dementia.”
Medication side effects and medication interactions, comorbidities, and decreased renal and hepatic clearance all can complicate treatment of AD in older adults. So can mobility, the extent of social/caregiving support, and other aspects of aging. For example, “I’m a big fan of ‘soak and smears’ ... but you have to ask, can you get out of a bathtub safely?” Dr. Silverberg said. “And you have to ask, can you reach the areas you need to [in order] to apply topicals?”
With oral Janus kinase inhibitors and other systemic medications, as with other drugs, “our older population is the most vulnerable from a safety perspective,” he said. A recently published post hoc analysis of four randomized trials of dupilumab in adults ≥ 60 years of age with moderate to severe AD demonstrated efficacy comparable with that in younger patients and “a really clean safety profile,” said Dr. Silverberg, the lead author. “We really need more of these types of post hocs to have some relative contextualization” for older adults.
Dr. Silverberg reported being a speaker for AbbVie, Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi-Genzyme; a consultant and/or advisory board member for Regeneron, Sanofi-Genzyme, and other companies; and an investigator for several companies.
A version of this article first appeared on Medscape.com.
WASHINGTON, DC — Jonathan I. Silverberg, MD, PhD, MPH, said at the ElderDerm Conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“I walked out of residency under the impression that if it didn’t start in the first year or two of life, it’s not AD,” said Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University. “The numbers tell us a very different story.”
The prevalence of AD in the United States fluctuates between 6% and 8% through adulthood, including age categories up to 81-85 years, according to 2012 National Health Interview Survey data. And while persistence of childhood-onset AD is common, a systematic review and meta-analysis published in 2018 concluded that one in four adults with AD report adult-onset disease.
The investigators, including Dr. Silverberg, identified 25 observational studies — studies conducted across 16 countries and published during 1956-2017 — that included an analysis of age of onset beyond 10 years of age, and other inclusion criteria. Of the 25 studies, 17 reported age of onset after 16 years of age and had sufficient data for the meta-analysis. Using random-effects weighting, the investigators found a pooled proportion of adult-onset AD of 26.1% (95% CI, 16.5%-37.2%).
The research demonstrates that “the age of onset is distributed well throughout the lifespan,” Dr. Silverberg said, with the data “indicating there are many elderly-onset cases of true AD as well.” (Thirteen of the studies analyzed an age of onset from age ≥ 65, and several looked beyond age 80).
A 2021 study of a primary care database in the United Kingdom of 3.85 million children and adults found a “fascinating” bimodal distribution of incidence across the lifespan, with peaks in both infancy and older adulthood, he said. Incidence in adulthood was relatively stable from ages 18-49 years, after which, “into the 50s, 60s and beyond, you started to see a steady climb again.”
Also intriguing, Dr. Silverberg continued, are findings from a study of outpatient healthcare utilization for AD in which he and his coinvestigator analyzed data from the National Ambulatory Medical Care Survey (NAMCS). In the article, published in 2023 covering data from the 1993-2015 NAMCS, they reported that AD visits were more common among children aged 0-4 years (32.0%) and 5-9 years of age (10.6%), then decreased in adolescents aged 10-19 years (11.6%), remained fairly steady in patients aged 20-89 years (1.0%-4.7%), and increased in patients aged > 90 years (20.7%).
“The peak usage for dermatologists, primary care physicians, etc., is happening in the first few years of life, partially because that’s when the disease is more common and more severe but also partially because that’s when parents and caregivers are first learning [about] the disease and trying to understand how to gain control,” Dr. Silverberg said at the meeting, presenting data from an expanded, unpublished analysis of NAMCS data showing these same outpatient utilization patterns.
“It’s fascinating — there’s a much greater utilization in the elderly population. Why? The short answer is, we don’t know,” he said.
Risk Factors, Immune Differences
People with adult-onset AD were more likely to be women, smokers in adulthood, and have a lower childhood socioeconomic status than those whose AD started in childhood in a longitudinal study of two large birth cohorts from the United Kingdom , Dr. Silverberg pointed out.
Patients with childhood-onset AD, meanwhile, were more likely to have asthma, allergen-specific immunoglobulin E (IgE), and known genetic polymorphisms previously associated with AD. (Each cohort — the 1958 British Cohort Study and the 1970 British Cohort Study — had more than 17,000 participants who were followed from birth through middle age.)
Data is limited, but “mechanistically,” AD in much older adults appears to have a unique serum cytokine pattern, Dr. Silverberg said. He pointed to a cross-sectional study in China of 1312 children and adults with AD in which researchers analyzed clinical features, serum samples, and skin biopsy samples.
Adults aged > 60 years showed more lesions on the trunk and extensor sites of the extremities and lower levels of serum IgE and peripheral eosinophil counts than those in younger age groups. And “interestingly,” compared with healthy controls, older patients with AD had “higher levels of serum expression of a variety of cytokines, including IL [interleukin]-4 but also high TARC levels ... and a variety of cytokines related to the Th17, TH1 axes, etc.,” he said.
“So, we’re seeing a fascinating new profile that may be a little different than younger-onset cases,” he said, noting that TARC (thymus and activation-regulated chemokine) is regarded as a “decent biomarker” for AD.
In addition to higher levels of IL-4 and TARC, the study investigators reported significantly higher levels of IL-17A, IL-6, IL-22, IL-33, and thymic stromal lymphopoietin in older patients, compared with healthy controls.
Research also suggests that air pollution may play a role in the onset of AD in older age, Dr. Silverberg said, referencing a 2023 study that explored the association of air pollution and genetic risk with the onset of AD after age 50. The study analyzed 337,910 participants from the UK Biobank, with a median 12-year follow-up. Genetic risks were assessed as low, intermediate, and high, based on tertiles of polygenic risk scores. Exposure to various air pollutants was assessed using weighted quantile sum and also categorized into tertiles.
The incidence of older adult-onset AD was associated with medium and high air pollution compared with low air pollution, with hazard ratios (HRs) of 1.182 (P = .003) and 1.359 (P < .001), respectively. And “to a lesser extent,” Dr. Silverberg said, incidence was associated with medium and high genetic susceptibility, with HRs of 1.065 (P = .249) and 1.153 (P = .008).
The researchers calculated a greater population-attributable fraction of air pollution (15.5%) than genetic risk (6.4%). “This means that yes, genetics can contribute even to later-onset disease ... but environment may play an even more important role,” Dr. Silverberg said.
In the Clinic
In all patients, and especially in older adults, sleep disturbance associated with AD is a consideration for care. Data collected at the eczema clinic of Northwestern University, Chicago, Illinois, between 2014 and 2019 through previsit, self-administered questionnaires show that patients ≥ 65 years of age have more profound sleep disturbance (especially trouble staying asleep) than patients aged 18-64 years, despite having similar AD severity, said Dr. Silverberg, a coinvestigator of the study.
Older age was associated with having an increased number of nights of sleep disturbance (3-7 nights in the previous week) because of eczema (adjusted odds ratio [aOR], 2.14; 95% CI, 1.16-3.92). It was also associated with itching-attributed delays in falling asleep and nighttime awakenings in the prior 2 weeks (aOR, 1.88; 95% CI, 1.05-3.39).
“The aging population has dysregulated sleep patterns and altered circadian rhythms, so some of this is just natural predisposition,” Dr. Silverberg said. “But it’s amplified [with AD and itching], and it becomes a big clinical problem when we get into treatment because it’s our natural inclination to prescribe antihistamines for their sedative properties.”
Antihistamines can cause more profound sedation, more forgetfulness, and more anticholinergic side effects, he said, noting that “there’s some evidence that high-dose antihistamines may exacerbate dementia.”
Medication side effects and medication interactions, comorbidities, and decreased renal and hepatic clearance all can complicate treatment of AD in older adults. So can mobility, the extent of social/caregiving support, and other aspects of aging. For example, “I’m a big fan of ‘soak and smears’ ... but you have to ask, can you get out of a bathtub safely?” Dr. Silverberg said. “And you have to ask, can you reach the areas you need to [in order] to apply topicals?”
With oral Janus kinase inhibitors and other systemic medications, as with other drugs, “our older population is the most vulnerable from a safety perspective,” he said. A recently published post hoc analysis of four randomized trials of dupilumab in adults ≥ 60 years of age with moderate to severe AD demonstrated efficacy comparable with that in younger patients and “a really clean safety profile,” said Dr. Silverberg, the lead author. “We really need more of these types of post hocs to have some relative contextualization” for older adults.
Dr. Silverberg reported being a speaker for AbbVie, Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi-Genzyme; a consultant and/or advisory board member for Regeneron, Sanofi-Genzyme, and other companies; and an investigator for several companies.
A version of this article first appeared on Medscape.com.
WASHINGTON, DC — Jonathan I. Silverberg, MD, PhD, MPH, said at the ElderDerm Conference on dermatology in the older patient hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC.
“I walked out of residency under the impression that if it didn’t start in the first year or two of life, it’s not AD,” said Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University. “The numbers tell us a very different story.”
The prevalence of AD in the United States fluctuates between 6% and 8% through adulthood, including age categories up to 81-85 years, according to 2012 National Health Interview Survey data. And while persistence of childhood-onset AD is common, a systematic review and meta-analysis published in 2018 concluded that one in four adults with AD report adult-onset disease.
The investigators, including Dr. Silverberg, identified 25 observational studies — studies conducted across 16 countries and published during 1956-2017 — that included an analysis of age of onset beyond 10 years of age, and other inclusion criteria. Of the 25 studies, 17 reported age of onset after 16 years of age and had sufficient data for the meta-analysis. Using random-effects weighting, the investigators found a pooled proportion of adult-onset AD of 26.1% (95% CI, 16.5%-37.2%).
The research demonstrates that “the age of onset is distributed well throughout the lifespan,” Dr. Silverberg said, with the data “indicating there are many elderly-onset cases of true AD as well.” (Thirteen of the studies analyzed an age of onset from age ≥ 65, and several looked beyond age 80).
A 2021 study of a primary care database in the United Kingdom of 3.85 million children and adults found a “fascinating” bimodal distribution of incidence across the lifespan, with peaks in both infancy and older adulthood, he said. Incidence in adulthood was relatively stable from ages 18-49 years, after which, “into the 50s, 60s and beyond, you started to see a steady climb again.”
Also intriguing, Dr. Silverberg continued, are findings from a study of outpatient healthcare utilization for AD in which he and his coinvestigator analyzed data from the National Ambulatory Medical Care Survey (NAMCS). In the article, published in 2023 covering data from the 1993-2015 NAMCS, they reported that AD visits were more common among children aged 0-4 years (32.0%) and 5-9 years of age (10.6%), then decreased in adolescents aged 10-19 years (11.6%), remained fairly steady in patients aged 20-89 years (1.0%-4.7%), and increased in patients aged > 90 years (20.7%).
“The peak usage for dermatologists, primary care physicians, etc., is happening in the first few years of life, partially because that’s when the disease is more common and more severe but also partially because that’s when parents and caregivers are first learning [about] the disease and trying to understand how to gain control,” Dr. Silverberg said at the meeting, presenting data from an expanded, unpublished analysis of NAMCS data showing these same outpatient utilization patterns.
“It’s fascinating — there’s a much greater utilization in the elderly population. Why? The short answer is, we don’t know,” he said.
Risk Factors, Immune Differences
People with adult-onset AD were more likely to be women, smokers in adulthood, and have a lower childhood socioeconomic status than those whose AD started in childhood in a longitudinal study of two large birth cohorts from the United Kingdom , Dr. Silverberg pointed out.
Patients with childhood-onset AD, meanwhile, were more likely to have asthma, allergen-specific immunoglobulin E (IgE), and known genetic polymorphisms previously associated with AD. (Each cohort — the 1958 British Cohort Study and the 1970 British Cohort Study — had more than 17,000 participants who were followed from birth through middle age.)
Data is limited, but “mechanistically,” AD in much older adults appears to have a unique serum cytokine pattern, Dr. Silverberg said. He pointed to a cross-sectional study in China of 1312 children and adults with AD in which researchers analyzed clinical features, serum samples, and skin biopsy samples.
Adults aged > 60 years showed more lesions on the trunk and extensor sites of the extremities and lower levels of serum IgE and peripheral eosinophil counts than those in younger age groups. And “interestingly,” compared with healthy controls, older patients with AD had “higher levels of serum expression of a variety of cytokines, including IL [interleukin]-4 but also high TARC levels ... and a variety of cytokines related to the Th17, TH1 axes, etc.,” he said.
“So, we’re seeing a fascinating new profile that may be a little different than younger-onset cases,” he said, noting that TARC (thymus and activation-regulated chemokine) is regarded as a “decent biomarker” for AD.
In addition to higher levels of IL-4 and TARC, the study investigators reported significantly higher levels of IL-17A, IL-6, IL-22, IL-33, and thymic stromal lymphopoietin in older patients, compared with healthy controls.
Research also suggests that air pollution may play a role in the onset of AD in older age, Dr. Silverberg said, referencing a 2023 study that explored the association of air pollution and genetic risk with the onset of AD after age 50. The study analyzed 337,910 participants from the UK Biobank, with a median 12-year follow-up. Genetic risks were assessed as low, intermediate, and high, based on tertiles of polygenic risk scores. Exposure to various air pollutants was assessed using weighted quantile sum and also categorized into tertiles.
The incidence of older adult-onset AD was associated with medium and high air pollution compared with low air pollution, with hazard ratios (HRs) of 1.182 (P = .003) and 1.359 (P < .001), respectively. And “to a lesser extent,” Dr. Silverberg said, incidence was associated with medium and high genetic susceptibility, with HRs of 1.065 (P = .249) and 1.153 (P = .008).
The researchers calculated a greater population-attributable fraction of air pollution (15.5%) than genetic risk (6.4%). “This means that yes, genetics can contribute even to later-onset disease ... but environment may play an even more important role,” Dr. Silverberg said.
In the Clinic
In all patients, and especially in older adults, sleep disturbance associated with AD is a consideration for care. Data collected at the eczema clinic of Northwestern University, Chicago, Illinois, between 2014 and 2019 through previsit, self-administered questionnaires show that patients ≥ 65 years of age have more profound sleep disturbance (especially trouble staying asleep) than patients aged 18-64 years, despite having similar AD severity, said Dr. Silverberg, a coinvestigator of the study.
Older age was associated with having an increased number of nights of sleep disturbance (3-7 nights in the previous week) because of eczema (adjusted odds ratio [aOR], 2.14; 95% CI, 1.16-3.92). It was also associated with itching-attributed delays in falling asleep and nighttime awakenings in the prior 2 weeks (aOR, 1.88; 95% CI, 1.05-3.39).
“The aging population has dysregulated sleep patterns and altered circadian rhythms, so some of this is just natural predisposition,” Dr. Silverberg said. “But it’s amplified [with AD and itching], and it becomes a big clinical problem when we get into treatment because it’s our natural inclination to prescribe antihistamines for their sedative properties.”
Antihistamines can cause more profound sedation, more forgetfulness, and more anticholinergic side effects, he said, noting that “there’s some evidence that high-dose antihistamines may exacerbate dementia.”
Medication side effects and medication interactions, comorbidities, and decreased renal and hepatic clearance all can complicate treatment of AD in older adults. So can mobility, the extent of social/caregiving support, and other aspects of aging. For example, “I’m a big fan of ‘soak and smears’ ... but you have to ask, can you get out of a bathtub safely?” Dr. Silverberg said. “And you have to ask, can you reach the areas you need to [in order] to apply topicals?”
With oral Janus kinase inhibitors and other systemic medications, as with other drugs, “our older population is the most vulnerable from a safety perspective,” he said. A recently published post hoc analysis of four randomized trials of dupilumab in adults ≥ 60 years of age with moderate to severe AD demonstrated efficacy comparable with that in younger patients and “a really clean safety profile,” said Dr. Silverberg, the lead author. “We really need more of these types of post hocs to have some relative contextualization” for older adults.
Dr. Silverberg reported being a speaker for AbbVie, Eli Lilly, Leo Pharma, Pfizer, Regeneron, and Sanofi-Genzyme; a consultant and/or advisory board member for Regeneron, Sanofi-Genzyme, and other companies; and an investigator for several companies.
A version of this article first appeared on Medscape.com.
FROM ELDERDERM 2024
Debate: Should Dermatologists or Rheumatologists Manage Musculoskeletal Symptoms in Patients With Psoriasis?
SEATTLE — That was the subject of a debate between a dermatologist and a rheumatologist at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Fabian Proft, MD, the rheumatologist, spoke first and emphasized the potential that MSK symptoms are a sign of psoriatic arthritis (PsA) and therefore should be managed by a rheumatologist.
“Obviously, the rheumatologist perspective [is that] I should be in the driver’s seat when taking care of patient with psoriasis and MSK symptoms, but I will still need to have a copilot there: [The dermatologist] will have a slot,” said Dr. Proft, who is a rheumatologist at Charité — Universitätsmedizin Berlin.
“It’s so important that we make the correct and early diagnosis of [psoriatic arthritis and psoriasis] symptoms,” said Dr. Proft. He specifically called out cases where patients have symptoms that are difficult to determine, whether the cause is inflammatory, and when experience with imaging can be a key factor in the diagnosis.
It’s important not to overdiagnose or overtreat patients, he said, providing an example of a patient with psoriasis who had been training for a marathon. The MRI image suggested that his Achilles tendonitis pain was related to his athletic training, not PsA-associated inflammation. “So I think this is very important that you have the knowledge to read MRIs, and especially also carefully assessing them so as not to overdiagnose patients,” said Dr. Proft.
Dermatologist Rebuttal
In her rebuttal, Laura Savage, MD, PhD, emphasized the need for more of a coequal partnership between the two specialties because of the ability of dermatologists to intervene early in the treatment and prevention of PsA.
“Traditionally, I agree rheumatologists would solely be responsible for the assessment and the management of psoriatic arthritis, but I think that paradigm has shifted in part due to the increased recognition of the need for earlier intervention to limit disease progression and to reduce or even prevent functional limitation,” said Dr. Savage, who is a consultant dermatologist at Leeds Teaching Hospitals NHS Trust and a senior lecturer at the University of Leeds, Leeds, England.
Ideally, molecular biomarkers would be available to predict the development of PsA, but there aren’t any. Still, “we have a huge biomarker in the form of the skin, and it’s recognized that the majority of patients who will develop psoriatic arthritis will have antecedent psoriasis in about 70% of cases,” Dr. Savage said. “There’s a typical time delay of around 7-12 years between the onset of the skin [disease] and the patients developing psoriatic arthritis, and so many of them are going to be into the care of other healthcare practitioners, and particularly the care of dermatologists.”
Dermatologists may also be able to play a role in the prevention of PsA, according to Dr. Savage. In one retrospective study, treatment of skin lesions with biologics was associated with a reduced frequency of progression to PsA (11.1% vs 16.4%) over 10 years (P = .0006). Studies with tumor necrosis factor inhibitors and other interventions have shown similar results.
Such findings have led to the treat intercept strategy, which targets patients with psoriasis who have risk factors for transition to PsA — such as nail pitting, gluteal cleft disease, scalp disease, type 2 diabetes, obesity, and a first-degree relative with PsA — as well as symptoms of prodromal PSA, such as arthralgia and fatigue.
“I think dermatologists are aware of the need to not leave our patients languishing on these therapies and actually escalating them onto effective treatments that may also be able to treat early psoriatic arthritis. We could be more mindful about our choice of treatments for these patients, going on to thinking about their increased risk of PSA and trying to intercept,” Dr. Savage said. “What we don’t want is our patients to be developing these musculoskeletal symptoms of pain and stiffness and functional limitation and disability. We want to be treating the patients with musculoskeletal symptoms of that earlier prodromal phase when they’re developing arthralgia and fatigue.”
She conceded that more complicated patients are good candidates for care by the rheumatologist. “You can do your fancy imaging, and we’ll leave that to you, and the difficult-to-treat patients to [the rheumatologist], but actually we need to just get on and treat them,” she said. “One could argue as well that as a dermatologist, I’m likely to broaden my horizons in terms of choice of therapy and treat all of the domains of the patient. So I would argue that actually it should be the dermatologist who is in that driving seat, particularly when it comes to the management of early psoriatic arthritis, and actually what we should be doing is driving our patients and steering them to earlier intervention and better control for all domains of disease.”
Collaborative Care
During the follow-up discussion, both Dr. Proft and Dr. Savage agreed that dermatologists and rheumatologists should be working together in managing patients. “What we need to do is steer our patients toward collaborative care with our rheumatologists by trying to minimize delays to treatment, by working together in parallel clinics, combined clinics, and on virtual [multidisciplinary teams],” said Dr. Savage.
Dr. Proft agreed. “We should join forces and make decisions together.”
Dr. Savage and Dr. Proft did not provide any financial disclosures.
A version of this article appeared on Medscape.com.
SEATTLE — That was the subject of a debate between a dermatologist and a rheumatologist at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Fabian Proft, MD, the rheumatologist, spoke first and emphasized the potential that MSK symptoms are a sign of psoriatic arthritis (PsA) and therefore should be managed by a rheumatologist.
“Obviously, the rheumatologist perspective [is that] I should be in the driver’s seat when taking care of patient with psoriasis and MSK symptoms, but I will still need to have a copilot there: [The dermatologist] will have a slot,” said Dr. Proft, who is a rheumatologist at Charité — Universitätsmedizin Berlin.
“It’s so important that we make the correct and early diagnosis of [psoriatic arthritis and psoriasis] symptoms,” said Dr. Proft. He specifically called out cases where patients have symptoms that are difficult to determine, whether the cause is inflammatory, and when experience with imaging can be a key factor in the diagnosis.
It’s important not to overdiagnose or overtreat patients, he said, providing an example of a patient with psoriasis who had been training for a marathon. The MRI image suggested that his Achilles tendonitis pain was related to his athletic training, not PsA-associated inflammation. “So I think this is very important that you have the knowledge to read MRIs, and especially also carefully assessing them so as not to overdiagnose patients,” said Dr. Proft.
Dermatologist Rebuttal
In her rebuttal, Laura Savage, MD, PhD, emphasized the need for more of a coequal partnership between the two specialties because of the ability of dermatologists to intervene early in the treatment and prevention of PsA.
“Traditionally, I agree rheumatologists would solely be responsible for the assessment and the management of psoriatic arthritis, but I think that paradigm has shifted in part due to the increased recognition of the need for earlier intervention to limit disease progression and to reduce or even prevent functional limitation,” said Dr. Savage, who is a consultant dermatologist at Leeds Teaching Hospitals NHS Trust and a senior lecturer at the University of Leeds, Leeds, England.
Ideally, molecular biomarkers would be available to predict the development of PsA, but there aren’t any. Still, “we have a huge biomarker in the form of the skin, and it’s recognized that the majority of patients who will develop psoriatic arthritis will have antecedent psoriasis in about 70% of cases,” Dr. Savage said. “There’s a typical time delay of around 7-12 years between the onset of the skin [disease] and the patients developing psoriatic arthritis, and so many of them are going to be into the care of other healthcare practitioners, and particularly the care of dermatologists.”
Dermatologists may also be able to play a role in the prevention of PsA, according to Dr. Savage. In one retrospective study, treatment of skin lesions with biologics was associated with a reduced frequency of progression to PsA (11.1% vs 16.4%) over 10 years (P = .0006). Studies with tumor necrosis factor inhibitors and other interventions have shown similar results.
Such findings have led to the treat intercept strategy, which targets patients with psoriasis who have risk factors for transition to PsA — such as nail pitting, gluteal cleft disease, scalp disease, type 2 diabetes, obesity, and a first-degree relative with PsA — as well as symptoms of prodromal PSA, such as arthralgia and fatigue.
“I think dermatologists are aware of the need to not leave our patients languishing on these therapies and actually escalating them onto effective treatments that may also be able to treat early psoriatic arthritis. We could be more mindful about our choice of treatments for these patients, going on to thinking about their increased risk of PSA and trying to intercept,” Dr. Savage said. “What we don’t want is our patients to be developing these musculoskeletal symptoms of pain and stiffness and functional limitation and disability. We want to be treating the patients with musculoskeletal symptoms of that earlier prodromal phase when they’re developing arthralgia and fatigue.”
She conceded that more complicated patients are good candidates for care by the rheumatologist. “You can do your fancy imaging, and we’ll leave that to you, and the difficult-to-treat patients to [the rheumatologist], but actually we need to just get on and treat them,” she said. “One could argue as well that as a dermatologist, I’m likely to broaden my horizons in terms of choice of therapy and treat all of the domains of the patient. So I would argue that actually it should be the dermatologist who is in that driving seat, particularly when it comes to the management of early psoriatic arthritis, and actually what we should be doing is driving our patients and steering them to earlier intervention and better control for all domains of disease.”
Collaborative Care
During the follow-up discussion, both Dr. Proft and Dr. Savage agreed that dermatologists and rheumatologists should be working together in managing patients. “What we need to do is steer our patients toward collaborative care with our rheumatologists by trying to minimize delays to treatment, by working together in parallel clinics, combined clinics, and on virtual [multidisciplinary teams],” said Dr. Savage.
Dr. Proft agreed. “We should join forces and make decisions together.”
Dr. Savage and Dr. Proft did not provide any financial disclosures.
A version of this article appeared on Medscape.com.
SEATTLE — That was the subject of a debate between a dermatologist and a rheumatologist at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Fabian Proft, MD, the rheumatologist, spoke first and emphasized the potential that MSK symptoms are a sign of psoriatic arthritis (PsA) and therefore should be managed by a rheumatologist.
“Obviously, the rheumatologist perspective [is that] I should be in the driver’s seat when taking care of patient with psoriasis and MSK symptoms, but I will still need to have a copilot there: [The dermatologist] will have a slot,” said Dr. Proft, who is a rheumatologist at Charité — Universitätsmedizin Berlin.
“It’s so important that we make the correct and early diagnosis of [psoriatic arthritis and psoriasis] symptoms,” said Dr. Proft. He specifically called out cases where patients have symptoms that are difficult to determine, whether the cause is inflammatory, and when experience with imaging can be a key factor in the diagnosis.
It’s important not to overdiagnose or overtreat patients, he said, providing an example of a patient with psoriasis who had been training for a marathon. The MRI image suggested that his Achilles tendonitis pain was related to his athletic training, not PsA-associated inflammation. “So I think this is very important that you have the knowledge to read MRIs, and especially also carefully assessing them so as not to overdiagnose patients,” said Dr. Proft.
Dermatologist Rebuttal
In her rebuttal, Laura Savage, MD, PhD, emphasized the need for more of a coequal partnership between the two specialties because of the ability of dermatologists to intervene early in the treatment and prevention of PsA.
“Traditionally, I agree rheumatologists would solely be responsible for the assessment and the management of psoriatic arthritis, but I think that paradigm has shifted in part due to the increased recognition of the need for earlier intervention to limit disease progression and to reduce or even prevent functional limitation,” said Dr. Savage, who is a consultant dermatologist at Leeds Teaching Hospitals NHS Trust and a senior lecturer at the University of Leeds, Leeds, England.
Ideally, molecular biomarkers would be available to predict the development of PsA, but there aren’t any. Still, “we have a huge biomarker in the form of the skin, and it’s recognized that the majority of patients who will develop psoriatic arthritis will have antecedent psoriasis in about 70% of cases,” Dr. Savage said. “There’s a typical time delay of around 7-12 years between the onset of the skin [disease] and the patients developing psoriatic arthritis, and so many of them are going to be into the care of other healthcare practitioners, and particularly the care of dermatologists.”
Dermatologists may also be able to play a role in the prevention of PsA, according to Dr. Savage. In one retrospective study, treatment of skin lesions with biologics was associated with a reduced frequency of progression to PsA (11.1% vs 16.4%) over 10 years (P = .0006). Studies with tumor necrosis factor inhibitors and other interventions have shown similar results.
Such findings have led to the treat intercept strategy, which targets patients with psoriasis who have risk factors for transition to PsA — such as nail pitting, gluteal cleft disease, scalp disease, type 2 diabetes, obesity, and a first-degree relative with PsA — as well as symptoms of prodromal PSA, such as arthralgia and fatigue.
“I think dermatologists are aware of the need to not leave our patients languishing on these therapies and actually escalating them onto effective treatments that may also be able to treat early psoriatic arthritis. We could be more mindful about our choice of treatments for these patients, going on to thinking about their increased risk of PSA and trying to intercept,” Dr. Savage said. “What we don’t want is our patients to be developing these musculoskeletal symptoms of pain and stiffness and functional limitation and disability. We want to be treating the patients with musculoskeletal symptoms of that earlier prodromal phase when they’re developing arthralgia and fatigue.”
She conceded that more complicated patients are good candidates for care by the rheumatologist. “You can do your fancy imaging, and we’ll leave that to you, and the difficult-to-treat patients to [the rheumatologist], but actually we need to just get on and treat them,” she said. “One could argue as well that as a dermatologist, I’m likely to broaden my horizons in terms of choice of therapy and treat all of the domains of the patient. So I would argue that actually it should be the dermatologist who is in that driving seat, particularly when it comes to the management of early psoriatic arthritis, and actually what we should be doing is driving our patients and steering them to earlier intervention and better control for all domains of disease.”
Collaborative Care
During the follow-up discussion, both Dr. Proft and Dr. Savage agreed that dermatologists and rheumatologists should be working together in managing patients. “What we need to do is steer our patients toward collaborative care with our rheumatologists by trying to minimize delays to treatment, by working together in parallel clinics, combined clinics, and on virtual [multidisciplinary teams],” said Dr. Savage.
Dr. Proft agreed. “We should join forces and make decisions together.”
Dr. Savage and Dr. Proft did not provide any financial disclosures.
A version of this article appeared on Medscape.com.
FROM GRAPPA 2024
Greater Transparency of Oncologists’ Pharma Relationships Needed
The findings reflect limited awareness in low-income countries about what scenarios constitute a conflict of interest, first author, Khalid El Bairi, MD, said during an interview. “There is a lack of training in ethics and integrity in medical schools [in countries in Africa], so people are not informed about conflicts of interest,” continued Dr. El Bairi, who presented the new research at the annual meeting of the American Society of Clinical Oncology. “There is also a lack of policies in universities and hospitals to guide clinicians about conflict of interest reporting.”
Overall, 58.5% of survey participants categorized honoraria as a conflict of interest that required disclosure, while 50% said the same of gifts from pharmaceutical representatives, and 44.5% identified travel grants for attending conferences as conflicts of interests. The report was published in JCO Global Oncology. Less often considered conflicts of interest were personal and institutional research funding, trips to conferences, consulting or advisory roles, food and beverages, expert testimony, and sample drugs provided by the pharmaceutical industry.
Just 24% of participants indicated that all of the listed items were deemed conflicts of interest. The survey — called Oncology Transparency Under Scrutiny and Tracking, or ONCOTRUST-1 — considered the perceptions of 200 oncologists, about 70% of whom practice in low- and middle-income countries.
What’s more, 37.5% of respondents identified fear of losing financial support as a reason not to report a conflict of interest. Still, 75% indicated that industry-sponsored speaking does not affect treatment decisions, and 60% said conflicts of interest do not impair objective appraisal of clinical trials.
Dr. El Bairi, a research associate in the department of medical oncology at Mohammed VI University Hospital, Oujda, Morocco, and his colleagues undertook the study in part because of an editorial published in The Lancet Oncology last year. First author Fidel Rubagumya, MD, a consultant oncologist and director of research at Rwanda Military Hospital, Kigali, and colleagues called for more research on the ties between oncologists and industry in Africa. The ONCOTRUST-1 findings set the stage for a planned follow-up study, which aims to compare views surrounding conflicts of interests between oncologists in different economic settings.
Open Payments Houses US Physicians’ Conflicts of Interest
To be sure, many authors of research published in major US journals are based outside of the United States. According to JAMA Network Open, 69% of submissions to the journal are from international authors. However, Dr. El Bairi also raised other potential signs of industry influence that he said need global discussion, such as the role of pharmaceutical companies in presentations of clinical trial findings at large cancer societies’ conferences, a shift toward progression-free survival as the endpoint in clinical cancer trials, and the rise of third-party writing assistance.
“There are two sides of the story,” Dr. El Bairi said. “The good side is that unfortunately, sometimes [industry money is] the only way for African oncologists to go abroad for training, to conferences for their continuous medical education. The bad is now we may harm patients, we might harm science by having conflicts of interest not reported.”
Unlike other countries, the United States has plentiful data on the scale of physicians’ financial conflicts of interest in the form of the Open Payments platform. Championed by Sen. Chuck Grassley (R-Iowa), the federal repository of payments to doctors and teaching hospitals by drug and medical device companies was established as part of the Affordable Care Act (ACA).
The health care reform law, which passed in 2010, requires pharmaceutical companies and medical device makers to report this information.
From 2013 to 2021, the pharmaceutical and medical device industry paid physicians $12.1 billion, according to a research letter published in JAMA in March of 2024 that reviewed Open Payments data.
Ranked by specialty, hematologists and oncologists received the fourth-largest amount of money in aggregate, the study shows. Their total of $825.8 million trailed only physicians in orthopedics ($1.36 billion), neurology and psychiatry ($1.32 billion) and cardiology ($1.29 billion). What’s more, this specialty had the biggest share of physicians taking industry money, with 74.2% of hematologists and oncologists receiving payments.
The payments from industry include fees for consulting services and speaking, as well as food and beverages, travel and lodging, education, gifts, grants, and honoraria.
Joseph S. Ross, MD, MHS, one of the JAMA study’s coauthors, said in an interview that the continued prevalence of such funding runs counter to the expectation behind the measure, which was that transparency would lead to physicians’ becoming less likely to accept a payment.
“We as a profession need to take a cold hard look in the mirror,” he said, referring to physicians in general.
Dr. Ross, professor of medicine at Yale University School of Medicine, New Haven, Connecticut, said he hopes that the profession will self-police, and that patients will make a bigger deal of the issue. Still, he acknowledged that “the vast majority” of patient advocacy groups, too, are funded by the pharmaceutical industry.
Exposing Industry Payments May Have Perverse Effect
A growing body of research explores the effect that physicians’ financial relationships with pharmaceutical companies can have on their prescribing practices. Indeed, oncologists taking industry payments seem to be more likely to prescribe nonrecommended and low-value drugs in some clinical settings, according to a study published in The BMJ last year.
That study’s first author, Aaron P. Mitchell, MD, a medical oncologist and assistant attending physician at Memorial Sloan Kettering Cancer Center, New York City, suggested in an interview that exposing industry payments to the sunlight may have had a perverse effect on physicians.
“There’s this idea of having license to do something,” Dr. Mitchell said, speaking broadly about human psychology rather than drawing on empirical data. “You might feel a little less bad about then prescribing more of that company’s drug, because the disclosure has already been done.”
The influence of pharmaceutical industry money on oncologists goes beyond what’s prescribed to which treatments get studied, approved, and recommended by guidelines, Dr. Mitchell said. He was also first author of a 2016 paper published in JAMA Oncology that found 86% of authors of the National Comprehensive Cancer Network guidelines had at least one conflict of interest reported on Open Systems in 2014.
Meanwhile, the fact that physicians’ payments from industry are a matter of public record on Open Systems has not guaranteed that doctors will disclose their conflicts of interest in other forums. A study published in JAMA earlier this year, for which Dr. Mitchell served as first author, found that almost one in three physicians endorsing drugs and devices on the social media platform X failed to disclose that the manufacturer paid them.
The lack of disclosure seems to extend beyond social media. A 2018 study published in JAMA Oncology found that 32% of oncologist authors of clinical drug trials for drugs approved over a 20-month period from 2016 to 2017 did not fully disclose payments from the trial sponsor when checked against the Open Payments database.
A lion’s share of industry payments within oncology appears to be going to a small group of high-profile physicians, suggested a 2022 study published in JCO Oncology Practice. It found that just 1% of all US oncologists accounted for 37% of industry payments, with each receiving more than $100,000 a year.
Experts: Professional Societies Should Further Limit Industry Payments
While partnerships between drug companies and physicians are necessary and have often been positive, more than disclosure is needed to minimize the risk of patient harm, according to an editorial published in March in JCO Oncology Practice. In it, Nina Niu Sanford, MD, a radiation oncologist UT Southwestern Medical Center, Dallas, and Bishal Gyawali, MD, PhD, a medical oncologist at Queen’s University, Kingston, Ontario, Canada, argue that following a specific blueprint could help mitigate financial conflicts of interest.
For starters, Dr. Sanford and Dr. Gyawali contend in the editorial that the maximum general payment NCCN members are allowed to receive from industry should be $0, compared with a current bar of $20,000 from a single entity or $50,000 from all external entities combined. They also urge professional societies to follow the current policy of the American Society of Clinical Oncology and ban members serving in their leadership from receiving any general payments from the industry.
The authors further suggest that investigators of clinical trials should be barred from holding stock for the drug or product while it is under study and that editorialists should not have conflicts of interest with the company whose drug or product they are discussing.
Pharmaceutical money can harm patients in ways that are not always obvious, Dr. Gyawali said in an interview.
“It can dominate the conversation by removing critical viewpoints from these top people about certain drugs,” he said. “It’s not always about saying good things about the drug.”
For instance, he suggested, a doctor receiving payments from Pfizer might openly criticize perceived flaws in drugs from other companies but refrain from weighing in negatively on a Pfizer drug.
From 2016 to 2018, industry made general payments to more than 52,000 physicians for 137 unique cancer drugs, according to a separate 2021 study published in the Journal of Cancer Policy, for which Dr. Gyawali served as one of the coauthors.
The results suggest that pharmaceutical money affects the entire cancer system, not relatively few oncology leaders. The amounts and dollar values grew each year covered by the study, to nearly 466,000 payments totaling $98.5 million in 2018.
Adriane Fugh-Berman, MD, professor of pharmacology and physiology at Georgetown University, Washington, DC, and director of PharmedOut, a Georgetown-based project that advances evidence-based prescribing and educates healthcare professionals about pharmaceutical marketing practices, has called for a ban on industry gifts to physicians.
When a publication asks physicians to disclose relevant conflicts of interest, physicians may choose not to disclose, because they don’t feel that their conflicts are relevant, Dr. Fugh-Berman said. Drug and device makers have also grown sophisticated about how they work with physicians, she suggested. “It’s illegal to market a drug before it comes on the market, but it’s not illegal to market the disease,” said Dr. Fugh-Berman, noting that drugmakers often work on long timelines.
“The doctor is going around saying we don’t have good therapies. They’re not pushing a drug. And so they feel totally fine about it.”
Anecdotally, Dr. Fugh-Berman noted that, if anything, speaking fees and similar payments only improve doctors’ reputations. She said that’s especially true if the physicians are paid by multiple companies, on the supposed theory that their conflicts of interest cancel each other out.
“I’m not defending this,” added Dr. Fugh-Berman, observing that, at the end of the day, such conflicts may go against the interests of patients.
“Sometimes the best drugs are older, generic, cheap drugs, and if oncologists or other specialists are only choosing among the most promoted drugs, they’re not necessarily choosing the best drugs.”
Beyond any prestige, doctors have other possible nonfinancial incentives for receiving industry payments. “It’s the relationships,” Dr. Fugh-Berman said. “Companies are very good at offering friendship.”
Dr. El Bairi reported NCODA leadership and honoraria along with expert testimony through techspert.io. Dr. Ross reported that he is a deputy editor of JAMA but was not involved in decisions regarding acceptance of or the review of the manuscript he authored and discussed in this article. Dr. Ross also reported receiving grants from the Food and Drug Administration, Johnson & Johnson, the Medical Device Innovation Consortium, the Agency for Healthcare Research and Quality, and the National Heart, Lung, and Blood Institute. He was an expert witness in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen that was settled in 2022. Dr. Mitchell reported no relevant financial relationships. Dr. Gyawali reported a consulting or advisory role with Vivio Health. Dr. Fugh-Berman reported being an expert witness for plaintiffs in complaints about drug and device marketing practices.
The findings reflect limited awareness in low-income countries about what scenarios constitute a conflict of interest, first author, Khalid El Bairi, MD, said during an interview. “There is a lack of training in ethics and integrity in medical schools [in countries in Africa], so people are not informed about conflicts of interest,” continued Dr. El Bairi, who presented the new research at the annual meeting of the American Society of Clinical Oncology. “There is also a lack of policies in universities and hospitals to guide clinicians about conflict of interest reporting.”
Overall, 58.5% of survey participants categorized honoraria as a conflict of interest that required disclosure, while 50% said the same of gifts from pharmaceutical representatives, and 44.5% identified travel grants for attending conferences as conflicts of interests. The report was published in JCO Global Oncology. Less often considered conflicts of interest were personal and institutional research funding, trips to conferences, consulting or advisory roles, food and beverages, expert testimony, and sample drugs provided by the pharmaceutical industry.
Just 24% of participants indicated that all of the listed items were deemed conflicts of interest. The survey — called Oncology Transparency Under Scrutiny and Tracking, or ONCOTRUST-1 — considered the perceptions of 200 oncologists, about 70% of whom practice in low- and middle-income countries.
What’s more, 37.5% of respondents identified fear of losing financial support as a reason not to report a conflict of interest. Still, 75% indicated that industry-sponsored speaking does not affect treatment decisions, and 60% said conflicts of interest do not impair objective appraisal of clinical trials.
Dr. El Bairi, a research associate in the department of medical oncology at Mohammed VI University Hospital, Oujda, Morocco, and his colleagues undertook the study in part because of an editorial published in The Lancet Oncology last year. First author Fidel Rubagumya, MD, a consultant oncologist and director of research at Rwanda Military Hospital, Kigali, and colleagues called for more research on the ties between oncologists and industry in Africa. The ONCOTRUST-1 findings set the stage for a planned follow-up study, which aims to compare views surrounding conflicts of interests between oncologists in different economic settings.
Open Payments Houses US Physicians’ Conflicts of Interest
To be sure, many authors of research published in major US journals are based outside of the United States. According to JAMA Network Open, 69% of submissions to the journal are from international authors. However, Dr. El Bairi also raised other potential signs of industry influence that he said need global discussion, such as the role of pharmaceutical companies in presentations of clinical trial findings at large cancer societies’ conferences, a shift toward progression-free survival as the endpoint in clinical cancer trials, and the rise of third-party writing assistance.
“There are two sides of the story,” Dr. El Bairi said. “The good side is that unfortunately, sometimes [industry money is] the only way for African oncologists to go abroad for training, to conferences for their continuous medical education. The bad is now we may harm patients, we might harm science by having conflicts of interest not reported.”
Unlike other countries, the United States has plentiful data on the scale of physicians’ financial conflicts of interest in the form of the Open Payments platform. Championed by Sen. Chuck Grassley (R-Iowa), the federal repository of payments to doctors and teaching hospitals by drug and medical device companies was established as part of the Affordable Care Act (ACA).
The health care reform law, which passed in 2010, requires pharmaceutical companies and medical device makers to report this information.
From 2013 to 2021, the pharmaceutical and medical device industry paid physicians $12.1 billion, according to a research letter published in JAMA in March of 2024 that reviewed Open Payments data.
Ranked by specialty, hematologists and oncologists received the fourth-largest amount of money in aggregate, the study shows. Their total of $825.8 million trailed only physicians in orthopedics ($1.36 billion), neurology and psychiatry ($1.32 billion) and cardiology ($1.29 billion). What’s more, this specialty had the biggest share of physicians taking industry money, with 74.2% of hematologists and oncologists receiving payments.
The payments from industry include fees for consulting services and speaking, as well as food and beverages, travel and lodging, education, gifts, grants, and honoraria.
Joseph S. Ross, MD, MHS, one of the JAMA study’s coauthors, said in an interview that the continued prevalence of such funding runs counter to the expectation behind the measure, which was that transparency would lead to physicians’ becoming less likely to accept a payment.
“We as a profession need to take a cold hard look in the mirror,” he said, referring to physicians in general.
Dr. Ross, professor of medicine at Yale University School of Medicine, New Haven, Connecticut, said he hopes that the profession will self-police, and that patients will make a bigger deal of the issue. Still, he acknowledged that “the vast majority” of patient advocacy groups, too, are funded by the pharmaceutical industry.
Exposing Industry Payments May Have Perverse Effect
A growing body of research explores the effect that physicians’ financial relationships with pharmaceutical companies can have on their prescribing practices. Indeed, oncologists taking industry payments seem to be more likely to prescribe nonrecommended and low-value drugs in some clinical settings, according to a study published in The BMJ last year.
That study’s first author, Aaron P. Mitchell, MD, a medical oncologist and assistant attending physician at Memorial Sloan Kettering Cancer Center, New York City, suggested in an interview that exposing industry payments to the sunlight may have had a perverse effect on physicians.
“There’s this idea of having license to do something,” Dr. Mitchell said, speaking broadly about human psychology rather than drawing on empirical data. “You might feel a little less bad about then prescribing more of that company’s drug, because the disclosure has already been done.”
The influence of pharmaceutical industry money on oncologists goes beyond what’s prescribed to which treatments get studied, approved, and recommended by guidelines, Dr. Mitchell said. He was also first author of a 2016 paper published in JAMA Oncology that found 86% of authors of the National Comprehensive Cancer Network guidelines had at least one conflict of interest reported on Open Systems in 2014.
Meanwhile, the fact that physicians’ payments from industry are a matter of public record on Open Systems has not guaranteed that doctors will disclose their conflicts of interest in other forums. A study published in JAMA earlier this year, for which Dr. Mitchell served as first author, found that almost one in three physicians endorsing drugs and devices on the social media platform X failed to disclose that the manufacturer paid them.
The lack of disclosure seems to extend beyond social media. A 2018 study published in JAMA Oncology found that 32% of oncologist authors of clinical drug trials for drugs approved over a 20-month period from 2016 to 2017 did not fully disclose payments from the trial sponsor when checked against the Open Payments database.
A lion’s share of industry payments within oncology appears to be going to a small group of high-profile physicians, suggested a 2022 study published in JCO Oncology Practice. It found that just 1% of all US oncologists accounted for 37% of industry payments, with each receiving more than $100,000 a year.
Experts: Professional Societies Should Further Limit Industry Payments
While partnerships between drug companies and physicians are necessary and have often been positive, more than disclosure is needed to minimize the risk of patient harm, according to an editorial published in March in JCO Oncology Practice. In it, Nina Niu Sanford, MD, a radiation oncologist UT Southwestern Medical Center, Dallas, and Bishal Gyawali, MD, PhD, a medical oncologist at Queen’s University, Kingston, Ontario, Canada, argue that following a specific blueprint could help mitigate financial conflicts of interest.
For starters, Dr. Sanford and Dr. Gyawali contend in the editorial that the maximum general payment NCCN members are allowed to receive from industry should be $0, compared with a current bar of $20,000 from a single entity or $50,000 from all external entities combined. They also urge professional societies to follow the current policy of the American Society of Clinical Oncology and ban members serving in their leadership from receiving any general payments from the industry.
The authors further suggest that investigators of clinical trials should be barred from holding stock for the drug or product while it is under study and that editorialists should not have conflicts of interest with the company whose drug or product they are discussing.
Pharmaceutical money can harm patients in ways that are not always obvious, Dr. Gyawali said in an interview.
“It can dominate the conversation by removing critical viewpoints from these top people about certain drugs,” he said. “It’s not always about saying good things about the drug.”
For instance, he suggested, a doctor receiving payments from Pfizer might openly criticize perceived flaws in drugs from other companies but refrain from weighing in negatively on a Pfizer drug.
From 2016 to 2018, industry made general payments to more than 52,000 physicians for 137 unique cancer drugs, according to a separate 2021 study published in the Journal of Cancer Policy, for which Dr. Gyawali served as one of the coauthors.
The results suggest that pharmaceutical money affects the entire cancer system, not relatively few oncology leaders. The amounts and dollar values grew each year covered by the study, to nearly 466,000 payments totaling $98.5 million in 2018.
Adriane Fugh-Berman, MD, professor of pharmacology and physiology at Georgetown University, Washington, DC, and director of PharmedOut, a Georgetown-based project that advances evidence-based prescribing and educates healthcare professionals about pharmaceutical marketing practices, has called for a ban on industry gifts to physicians.
When a publication asks physicians to disclose relevant conflicts of interest, physicians may choose not to disclose, because they don’t feel that their conflicts are relevant, Dr. Fugh-Berman said. Drug and device makers have also grown sophisticated about how they work with physicians, she suggested. “It’s illegal to market a drug before it comes on the market, but it’s not illegal to market the disease,” said Dr. Fugh-Berman, noting that drugmakers often work on long timelines.
“The doctor is going around saying we don’t have good therapies. They’re not pushing a drug. And so they feel totally fine about it.”
Anecdotally, Dr. Fugh-Berman noted that, if anything, speaking fees and similar payments only improve doctors’ reputations. She said that’s especially true if the physicians are paid by multiple companies, on the supposed theory that their conflicts of interest cancel each other out.
“I’m not defending this,” added Dr. Fugh-Berman, observing that, at the end of the day, such conflicts may go against the interests of patients.
“Sometimes the best drugs are older, generic, cheap drugs, and if oncologists or other specialists are only choosing among the most promoted drugs, they’re not necessarily choosing the best drugs.”
Beyond any prestige, doctors have other possible nonfinancial incentives for receiving industry payments. “It’s the relationships,” Dr. Fugh-Berman said. “Companies are very good at offering friendship.”
Dr. El Bairi reported NCODA leadership and honoraria along with expert testimony through techspert.io. Dr. Ross reported that he is a deputy editor of JAMA but was not involved in decisions regarding acceptance of or the review of the manuscript he authored and discussed in this article. Dr. Ross also reported receiving grants from the Food and Drug Administration, Johnson & Johnson, the Medical Device Innovation Consortium, the Agency for Healthcare Research and Quality, and the National Heart, Lung, and Blood Institute. He was an expert witness in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen that was settled in 2022. Dr. Mitchell reported no relevant financial relationships. Dr. Gyawali reported a consulting or advisory role with Vivio Health. Dr. Fugh-Berman reported being an expert witness for plaintiffs in complaints about drug and device marketing practices.
The findings reflect limited awareness in low-income countries about what scenarios constitute a conflict of interest, first author, Khalid El Bairi, MD, said during an interview. “There is a lack of training in ethics and integrity in medical schools [in countries in Africa], so people are not informed about conflicts of interest,” continued Dr. El Bairi, who presented the new research at the annual meeting of the American Society of Clinical Oncology. “There is also a lack of policies in universities and hospitals to guide clinicians about conflict of interest reporting.”
Overall, 58.5% of survey participants categorized honoraria as a conflict of interest that required disclosure, while 50% said the same of gifts from pharmaceutical representatives, and 44.5% identified travel grants for attending conferences as conflicts of interests. The report was published in JCO Global Oncology. Less often considered conflicts of interest were personal and institutional research funding, trips to conferences, consulting or advisory roles, food and beverages, expert testimony, and sample drugs provided by the pharmaceutical industry.
Just 24% of participants indicated that all of the listed items were deemed conflicts of interest. The survey — called Oncology Transparency Under Scrutiny and Tracking, or ONCOTRUST-1 — considered the perceptions of 200 oncologists, about 70% of whom practice in low- and middle-income countries.
What’s more, 37.5% of respondents identified fear of losing financial support as a reason not to report a conflict of interest. Still, 75% indicated that industry-sponsored speaking does not affect treatment decisions, and 60% said conflicts of interest do not impair objective appraisal of clinical trials.
Dr. El Bairi, a research associate in the department of medical oncology at Mohammed VI University Hospital, Oujda, Morocco, and his colleagues undertook the study in part because of an editorial published in The Lancet Oncology last year. First author Fidel Rubagumya, MD, a consultant oncologist and director of research at Rwanda Military Hospital, Kigali, and colleagues called for more research on the ties between oncologists and industry in Africa. The ONCOTRUST-1 findings set the stage for a planned follow-up study, which aims to compare views surrounding conflicts of interests between oncologists in different economic settings.
Open Payments Houses US Physicians’ Conflicts of Interest
To be sure, many authors of research published in major US journals are based outside of the United States. According to JAMA Network Open, 69% of submissions to the journal are from international authors. However, Dr. El Bairi also raised other potential signs of industry influence that he said need global discussion, such as the role of pharmaceutical companies in presentations of clinical trial findings at large cancer societies’ conferences, a shift toward progression-free survival as the endpoint in clinical cancer trials, and the rise of third-party writing assistance.
“There are two sides of the story,” Dr. El Bairi said. “The good side is that unfortunately, sometimes [industry money is] the only way for African oncologists to go abroad for training, to conferences for their continuous medical education. The bad is now we may harm patients, we might harm science by having conflicts of interest not reported.”
Unlike other countries, the United States has plentiful data on the scale of physicians’ financial conflicts of interest in the form of the Open Payments platform. Championed by Sen. Chuck Grassley (R-Iowa), the federal repository of payments to doctors and teaching hospitals by drug and medical device companies was established as part of the Affordable Care Act (ACA).
The health care reform law, which passed in 2010, requires pharmaceutical companies and medical device makers to report this information.
From 2013 to 2021, the pharmaceutical and medical device industry paid physicians $12.1 billion, according to a research letter published in JAMA in March of 2024 that reviewed Open Payments data.
Ranked by specialty, hematologists and oncologists received the fourth-largest amount of money in aggregate, the study shows. Their total of $825.8 million trailed only physicians in orthopedics ($1.36 billion), neurology and psychiatry ($1.32 billion) and cardiology ($1.29 billion). What’s more, this specialty had the biggest share of physicians taking industry money, with 74.2% of hematologists and oncologists receiving payments.
The payments from industry include fees for consulting services and speaking, as well as food and beverages, travel and lodging, education, gifts, grants, and honoraria.
Joseph S. Ross, MD, MHS, one of the JAMA study’s coauthors, said in an interview that the continued prevalence of such funding runs counter to the expectation behind the measure, which was that transparency would lead to physicians’ becoming less likely to accept a payment.
“We as a profession need to take a cold hard look in the mirror,” he said, referring to physicians in general.
Dr. Ross, professor of medicine at Yale University School of Medicine, New Haven, Connecticut, said he hopes that the profession will self-police, and that patients will make a bigger deal of the issue. Still, he acknowledged that “the vast majority” of patient advocacy groups, too, are funded by the pharmaceutical industry.
Exposing Industry Payments May Have Perverse Effect
A growing body of research explores the effect that physicians’ financial relationships with pharmaceutical companies can have on their prescribing practices. Indeed, oncologists taking industry payments seem to be more likely to prescribe nonrecommended and low-value drugs in some clinical settings, according to a study published in The BMJ last year.
That study’s first author, Aaron P. Mitchell, MD, a medical oncologist and assistant attending physician at Memorial Sloan Kettering Cancer Center, New York City, suggested in an interview that exposing industry payments to the sunlight may have had a perverse effect on physicians.
“There’s this idea of having license to do something,” Dr. Mitchell said, speaking broadly about human psychology rather than drawing on empirical data. “You might feel a little less bad about then prescribing more of that company’s drug, because the disclosure has already been done.”
The influence of pharmaceutical industry money on oncologists goes beyond what’s prescribed to which treatments get studied, approved, and recommended by guidelines, Dr. Mitchell said. He was also first author of a 2016 paper published in JAMA Oncology that found 86% of authors of the National Comprehensive Cancer Network guidelines had at least one conflict of interest reported on Open Systems in 2014.
Meanwhile, the fact that physicians’ payments from industry are a matter of public record on Open Systems has not guaranteed that doctors will disclose their conflicts of interest in other forums. A study published in JAMA earlier this year, for which Dr. Mitchell served as first author, found that almost one in three physicians endorsing drugs and devices on the social media platform X failed to disclose that the manufacturer paid them.
The lack of disclosure seems to extend beyond social media. A 2018 study published in JAMA Oncology found that 32% of oncologist authors of clinical drug trials for drugs approved over a 20-month period from 2016 to 2017 did not fully disclose payments from the trial sponsor when checked against the Open Payments database.
A lion’s share of industry payments within oncology appears to be going to a small group of high-profile physicians, suggested a 2022 study published in JCO Oncology Practice. It found that just 1% of all US oncologists accounted for 37% of industry payments, with each receiving more than $100,000 a year.
Experts: Professional Societies Should Further Limit Industry Payments
While partnerships between drug companies and physicians are necessary and have often been positive, more than disclosure is needed to minimize the risk of patient harm, according to an editorial published in March in JCO Oncology Practice. In it, Nina Niu Sanford, MD, a radiation oncologist UT Southwestern Medical Center, Dallas, and Bishal Gyawali, MD, PhD, a medical oncologist at Queen’s University, Kingston, Ontario, Canada, argue that following a specific blueprint could help mitigate financial conflicts of interest.
For starters, Dr. Sanford and Dr. Gyawali contend in the editorial that the maximum general payment NCCN members are allowed to receive from industry should be $0, compared with a current bar of $20,000 from a single entity or $50,000 from all external entities combined. They also urge professional societies to follow the current policy of the American Society of Clinical Oncology and ban members serving in their leadership from receiving any general payments from the industry.
The authors further suggest that investigators of clinical trials should be barred from holding stock for the drug or product while it is under study and that editorialists should not have conflicts of interest with the company whose drug or product they are discussing.
Pharmaceutical money can harm patients in ways that are not always obvious, Dr. Gyawali said in an interview.
“It can dominate the conversation by removing critical viewpoints from these top people about certain drugs,” he said. “It’s not always about saying good things about the drug.”
For instance, he suggested, a doctor receiving payments from Pfizer might openly criticize perceived flaws in drugs from other companies but refrain from weighing in negatively on a Pfizer drug.
From 2016 to 2018, industry made general payments to more than 52,000 physicians for 137 unique cancer drugs, according to a separate 2021 study published in the Journal of Cancer Policy, for which Dr. Gyawali served as one of the coauthors.
The results suggest that pharmaceutical money affects the entire cancer system, not relatively few oncology leaders. The amounts and dollar values grew each year covered by the study, to nearly 466,000 payments totaling $98.5 million in 2018.
Adriane Fugh-Berman, MD, professor of pharmacology and physiology at Georgetown University, Washington, DC, and director of PharmedOut, a Georgetown-based project that advances evidence-based prescribing and educates healthcare professionals about pharmaceutical marketing practices, has called for a ban on industry gifts to physicians.
When a publication asks physicians to disclose relevant conflicts of interest, physicians may choose not to disclose, because they don’t feel that their conflicts are relevant, Dr. Fugh-Berman said. Drug and device makers have also grown sophisticated about how they work with physicians, she suggested. “It’s illegal to market a drug before it comes on the market, but it’s not illegal to market the disease,” said Dr. Fugh-Berman, noting that drugmakers often work on long timelines.
“The doctor is going around saying we don’t have good therapies. They’re not pushing a drug. And so they feel totally fine about it.”
Anecdotally, Dr. Fugh-Berman noted that, if anything, speaking fees and similar payments only improve doctors’ reputations. She said that’s especially true if the physicians are paid by multiple companies, on the supposed theory that their conflicts of interest cancel each other out.
“I’m not defending this,” added Dr. Fugh-Berman, observing that, at the end of the day, such conflicts may go against the interests of patients.
“Sometimes the best drugs are older, generic, cheap drugs, and if oncologists or other specialists are only choosing among the most promoted drugs, they’re not necessarily choosing the best drugs.”
Beyond any prestige, doctors have other possible nonfinancial incentives for receiving industry payments. “It’s the relationships,” Dr. Fugh-Berman said. “Companies are very good at offering friendship.”
Dr. El Bairi reported NCODA leadership and honoraria along with expert testimony through techspert.io. Dr. Ross reported that he is a deputy editor of JAMA but was not involved in decisions regarding acceptance of or the review of the manuscript he authored and discussed in this article. Dr. Ross also reported receiving grants from the Food and Drug Administration, Johnson & Johnson, the Medical Device Innovation Consortium, the Agency for Healthcare Research and Quality, and the National Heart, Lung, and Blood Institute. He was an expert witness in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen that was settled in 2022. Dr. Mitchell reported no relevant financial relationships. Dr. Gyawali reported a consulting or advisory role with Vivio Health. Dr. Fugh-Berman reported being an expert witness for plaintiffs in complaints about drug and device marketing practices.
FROM ASCO 2024
Munchausen Syndrome by Proxy: Be Aware of Cutaneous Signs
TORONTO — Be suspicious if a child with a severe dermatologic condition is unresponsive to treatment, especially if their parent or caregiver exhibits deceptive behavior.
These could be red flags for Munchausen syndrome by proxy (MSBP), also known as factitious disorder.
“The No. 1 thing dermatologists can do in situations like this is be open to thinking outside the box and ask themselves the difficult question: Could this be something the parent is inflicting on the child,” Kelly Frasier, DO, a dermatology clinical trials and epidemiology research fellow at Northwell Health, Poughkeepsie, New York, said in an interview.
She provided a review on advancing the understanding of the dermatologic manifestations of MSBP during a poster session at the annual meeting of the Society for Pediatric Dermatology (SPD). Dr. Frasier has a particular interest in psychodermatology — she was a mental health therapist before going to medical school.
MSBP is a type of abuse intentionally inflicted by a caregiver typically on their child “for some ulterior motive,” usually to seek attention or sympathy and not for material or financial gain, explained Dr. Frasier. People with MSBP seek medical help for exaggerated or fabricated symptoms in their child. They may alter medical tests, falsify medical records, or induce symptoms in their child.
To do this, these abusers may apply any number of caustic household products, including glue, directly to the child’s skin or even in formula. Dr. Frasier shared a picture of a baby whose formula had been doctored with a caustic substance that had dripped onto his neck and face, causing a rash with blisters.
In addition to blistering, cutaneous manifestations of MSBP can include severe bruising. Or the child may present with signs similar to those of granuloma annulare (a benign condition characterized by small, raised bumps) or cicatricial pemphigoid (a rare, chronic autoimmune blistering disorder) or may have recurrent nail avulsion, purpura, or coagulopathy, said Dr. Frasier.
In almost all cases of MSBP (an estimated 96%), the abuse is inflicted by the mother, who may have a preexisting mental illness. “Usually, a psychological disorder is at play, such as depression or anxiety,” said Dr. Frasier.
Some evidence suggests that, in cases of MSBP, the caregiver may have a personality disorder such as borderline or histrionic personality disorder — or may have suffered abuse or neglect as a child or is experiencing major stress, which some evidence suggests can trigger MSPB, she added.
This type of abuse is rarely seen in children older than 6 years, likely because they get wise to what’s going on and are better able to fight back or resist as they get older, Dr. Fraser noted.
High Mortality Rate
It’s critical that cases of MSBP are identified early. While a small proportion of child abuse cases involve MSBP, the mortality rate is extremely high, about 10%, research suggests, said Dr. Frasier.
Dermatologists should be skeptical if the child’s condition hasn’t improved despite trying numerous treatments that normally would have some effect. “If you’re doing everything you can to treat something that’s usually pretty simple in terms of what you normally see clinically and how you treat it, and you’re not seeing any improvement or things continue to get worse, that’s definitely a sign something else may be going on,” Dr. Frasier said.
Another suspicious sign is inflammation that continues “for weeks or months” and “doesn’t match up with actual lab markers and lab values,” said Dr. Frasier.
Other signs of possible MSBP include evidence of chemicals in the child’s blood, stool, or urine, or the child’s condition improves while in the hospital, but symptoms return after returning home.
Also be aware of the interaction between the parent and child, said Dr. Frasier. “See if you can pick up that something else might be going on, especially if the symptoms aren’t lining up very well with what you’re physically seeing and what your clinical impression is.”
And be suspicious of a parent’s inappropriate behavior; for example, they seem to be deliberately making symptoms worse or appear overly distraught. The seemingly caring parent could be overcompensating for what she’s doing at home, “and she wants to make sure it doesn’t appear that way,” said Dr. Frasier.
To help determine if some sort of trauma is occurring at home, the child would ideally be separated from the caregiver, perhaps with a nurse or other member of the interdisciplinary medical team, Dr. Frasier said.
It appears that pediatric dermatologists are already aware of the importance of protecting children from abuse. During a presentation at the meeting on child abuse and maltreatment in dermatology, not specifically on MSBP, Romy Cho, MD, assistant professor, Department of Pediatrics, University of Toronto, who is involved with the SCAN Program at The Hospital for Sick Children, Toronto, Canada, polled the audience on whether they had ever contacted child protective services (CPS). Almost 80% said they had.
That’s good news for Dr. Frasier. “We have to be willing to contact CPS if we think there’s something going on, and be more open to that because it’s better to be safe than sorry, especially in cases involving children.”
Dr. Frasier and Dr. Cho had no relevant disclosures.
A version of this article first appeared on Medscape.com.
TORONTO — Be suspicious if a child with a severe dermatologic condition is unresponsive to treatment, especially if their parent or caregiver exhibits deceptive behavior.
These could be red flags for Munchausen syndrome by proxy (MSBP), also known as factitious disorder.
“The No. 1 thing dermatologists can do in situations like this is be open to thinking outside the box and ask themselves the difficult question: Could this be something the parent is inflicting on the child,” Kelly Frasier, DO, a dermatology clinical trials and epidemiology research fellow at Northwell Health, Poughkeepsie, New York, said in an interview.
She provided a review on advancing the understanding of the dermatologic manifestations of MSBP during a poster session at the annual meeting of the Society for Pediatric Dermatology (SPD). Dr. Frasier has a particular interest in psychodermatology — she was a mental health therapist before going to medical school.
MSBP is a type of abuse intentionally inflicted by a caregiver typically on their child “for some ulterior motive,” usually to seek attention or sympathy and not for material or financial gain, explained Dr. Frasier. People with MSBP seek medical help for exaggerated or fabricated symptoms in their child. They may alter medical tests, falsify medical records, or induce symptoms in their child.
To do this, these abusers may apply any number of caustic household products, including glue, directly to the child’s skin or even in formula. Dr. Frasier shared a picture of a baby whose formula had been doctored with a caustic substance that had dripped onto his neck and face, causing a rash with blisters.
In addition to blistering, cutaneous manifestations of MSBP can include severe bruising. Or the child may present with signs similar to those of granuloma annulare (a benign condition characterized by small, raised bumps) or cicatricial pemphigoid (a rare, chronic autoimmune blistering disorder) or may have recurrent nail avulsion, purpura, or coagulopathy, said Dr. Frasier.
In almost all cases of MSBP (an estimated 96%), the abuse is inflicted by the mother, who may have a preexisting mental illness. “Usually, a psychological disorder is at play, such as depression or anxiety,” said Dr. Frasier.
Some evidence suggests that, in cases of MSBP, the caregiver may have a personality disorder such as borderline or histrionic personality disorder — or may have suffered abuse or neglect as a child or is experiencing major stress, which some evidence suggests can trigger MSPB, she added.
This type of abuse is rarely seen in children older than 6 years, likely because they get wise to what’s going on and are better able to fight back or resist as they get older, Dr. Fraser noted.
High Mortality Rate
It’s critical that cases of MSBP are identified early. While a small proportion of child abuse cases involve MSBP, the mortality rate is extremely high, about 10%, research suggests, said Dr. Frasier.
Dermatologists should be skeptical if the child’s condition hasn’t improved despite trying numerous treatments that normally would have some effect. “If you’re doing everything you can to treat something that’s usually pretty simple in terms of what you normally see clinically and how you treat it, and you’re not seeing any improvement or things continue to get worse, that’s definitely a sign something else may be going on,” Dr. Frasier said.
Another suspicious sign is inflammation that continues “for weeks or months” and “doesn’t match up with actual lab markers and lab values,” said Dr. Frasier.
Other signs of possible MSBP include evidence of chemicals in the child’s blood, stool, or urine, or the child’s condition improves while in the hospital, but symptoms return after returning home.
Also be aware of the interaction between the parent and child, said Dr. Frasier. “See if you can pick up that something else might be going on, especially if the symptoms aren’t lining up very well with what you’re physically seeing and what your clinical impression is.”
And be suspicious of a parent’s inappropriate behavior; for example, they seem to be deliberately making symptoms worse or appear overly distraught. The seemingly caring parent could be overcompensating for what she’s doing at home, “and she wants to make sure it doesn’t appear that way,” said Dr. Frasier.
To help determine if some sort of trauma is occurring at home, the child would ideally be separated from the caregiver, perhaps with a nurse or other member of the interdisciplinary medical team, Dr. Frasier said.
It appears that pediatric dermatologists are already aware of the importance of protecting children from abuse. During a presentation at the meeting on child abuse and maltreatment in dermatology, not specifically on MSBP, Romy Cho, MD, assistant professor, Department of Pediatrics, University of Toronto, who is involved with the SCAN Program at The Hospital for Sick Children, Toronto, Canada, polled the audience on whether they had ever contacted child protective services (CPS). Almost 80% said they had.
That’s good news for Dr. Frasier. “We have to be willing to contact CPS if we think there’s something going on, and be more open to that because it’s better to be safe than sorry, especially in cases involving children.”
Dr. Frasier and Dr. Cho had no relevant disclosures.
A version of this article first appeared on Medscape.com.
TORONTO — Be suspicious if a child with a severe dermatologic condition is unresponsive to treatment, especially if their parent or caregiver exhibits deceptive behavior.
These could be red flags for Munchausen syndrome by proxy (MSBP), also known as factitious disorder.
“The No. 1 thing dermatologists can do in situations like this is be open to thinking outside the box and ask themselves the difficult question: Could this be something the parent is inflicting on the child,” Kelly Frasier, DO, a dermatology clinical trials and epidemiology research fellow at Northwell Health, Poughkeepsie, New York, said in an interview.
She provided a review on advancing the understanding of the dermatologic manifestations of MSBP during a poster session at the annual meeting of the Society for Pediatric Dermatology (SPD). Dr. Frasier has a particular interest in psychodermatology — she was a mental health therapist before going to medical school.
MSBP is a type of abuse intentionally inflicted by a caregiver typically on their child “for some ulterior motive,” usually to seek attention or sympathy and not for material or financial gain, explained Dr. Frasier. People with MSBP seek medical help for exaggerated or fabricated symptoms in their child. They may alter medical tests, falsify medical records, or induce symptoms in their child.
To do this, these abusers may apply any number of caustic household products, including glue, directly to the child’s skin or even in formula. Dr. Frasier shared a picture of a baby whose formula had been doctored with a caustic substance that had dripped onto his neck and face, causing a rash with blisters.
In addition to blistering, cutaneous manifestations of MSBP can include severe bruising. Or the child may present with signs similar to those of granuloma annulare (a benign condition characterized by small, raised bumps) or cicatricial pemphigoid (a rare, chronic autoimmune blistering disorder) or may have recurrent nail avulsion, purpura, or coagulopathy, said Dr. Frasier.
In almost all cases of MSBP (an estimated 96%), the abuse is inflicted by the mother, who may have a preexisting mental illness. “Usually, a psychological disorder is at play, such as depression or anxiety,” said Dr. Frasier.
Some evidence suggests that, in cases of MSBP, the caregiver may have a personality disorder such as borderline or histrionic personality disorder — or may have suffered abuse or neglect as a child or is experiencing major stress, which some evidence suggests can trigger MSPB, she added.
This type of abuse is rarely seen in children older than 6 years, likely because they get wise to what’s going on and are better able to fight back or resist as they get older, Dr. Fraser noted.
High Mortality Rate
It’s critical that cases of MSBP are identified early. While a small proportion of child abuse cases involve MSBP, the mortality rate is extremely high, about 10%, research suggests, said Dr. Frasier.
Dermatologists should be skeptical if the child’s condition hasn’t improved despite trying numerous treatments that normally would have some effect. “If you’re doing everything you can to treat something that’s usually pretty simple in terms of what you normally see clinically and how you treat it, and you’re not seeing any improvement or things continue to get worse, that’s definitely a sign something else may be going on,” Dr. Frasier said.
Another suspicious sign is inflammation that continues “for weeks or months” and “doesn’t match up with actual lab markers and lab values,” said Dr. Frasier.
Other signs of possible MSBP include evidence of chemicals in the child’s blood, stool, or urine, or the child’s condition improves while in the hospital, but symptoms return after returning home.
Also be aware of the interaction between the parent and child, said Dr. Frasier. “See if you can pick up that something else might be going on, especially if the symptoms aren’t lining up very well with what you’re physically seeing and what your clinical impression is.”
And be suspicious of a parent’s inappropriate behavior; for example, they seem to be deliberately making symptoms worse or appear overly distraught. The seemingly caring parent could be overcompensating for what she’s doing at home, “and she wants to make sure it doesn’t appear that way,” said Dr. Frasier.
To help determine if some sort of trauma is occurring at home, the child would ideally be separated from the caregiver, perhaps with a nurse or other member of the interdisciplinary medical team, Dr. Frasier said.
It appears that pediatric dermatologists are already aware of the importance of protecting children from abuse. During a presentation at the meeting on child abuse and maltreatment in dermatology, not specifically on MSBP, Romy Cho, MD, assistant professor, Department of Pediatrics, University of Toronto, who is involved with the SCAN Program at The Hospital for Sick Children, Toronto, Canada, polled the audience on whether they had ever contacted child protective services (CPS). Almost 80% said they had.
That’s good news for Dr. Frasier. “We have to be willing to contact CPS if we think there’s something going on, and be more open to that because it’s better to be safe than sorry, especially in cases involving children.”
Dr. Frasier and Dr. Cho had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SPD 2024
Cognitive Decline Minimal After Endocrine + CDK4/6 Inhibition in BC
“Patients who are diagnosed with advanced breast cancer and start their first-line treatment already show cognitive impairments due to their previous treatments. And luckily, our results show that during first-line treatment for advanced breast cancer with endocrine therapy, with or without a CDK4/6 inhibitor, further cognitive decline is minimal,” lead investigator Maryse Luijendijk, said during her presentation at the annual meeting of the American Society of Clinical Oncology (ASCO).
“It is well known that cancer patients can experience cognitive problems, such as memory loss, problems with concentration or with planning, during or following their treatment,” explained Ms. Luijendijk, a PhD candidate in the department of Psychosocial Research and Epidemiology at the Netherlands Cancer Institute, in Amsterdam. “Much is known about the effects of chemotherapy or irradiation to the brain, but evidence into endocrine therapy is scarce, which is surprising because cognitive effects are biologically plausible.
“We know that estrogen plays an important role in neuronal functioning and that certain types of endocrine therapies are able to cross the blood-brain barrier, where they may interact with estrogen receptors distributed widely throughout the brain … We know that CDK4/6 inhibitors may either negatively affect cognitive function by increased fatigue due to cytokine release or by interrupting the cell cycle of healthy cells, or positively, as they have been associated with reduced inflammation and remyelination.”
Initial results of the SONIA trial, reported at ASCO last year, examined overall and progression-free survival in patients with HR-positive, HER2-negative metastatic breast cancer and no prior treatment for advanced disease. Findings for those who were randomized to treatment with nonsteroidal aromatase inhibition either with or without the addition of CDK4/6 inhibitors showed no between-group differences, explained Ms. Luijendijk.
The new results, described as being from the SONIA-EfFECT (Evaluation of cognitive functioning in patients with metastatic breast cancer treated with endocrine or combined therapy) trial, were based on the authors investigating cognitive functioning in the same cohort used in the SONIA trial plus a control group.
In SONIA-EfFECT, patients who participated in SONIA were asked to identify a female relative or friend without cancer to serve as a cancer-free control. Members of the 130-patient control group were matched for age, education, and computer use.
Participants in the SONIA trial and control group were asked to complete the Amsterdam Cognition Scan, an online neuropsychological test battery at baseline and again after 9 months of treatment. Of those patients from SONIA, 130 had received first-line treatment with aromatase inhibitors with CDK4/6 inhibition (Arm A) and 130 had received aromatase inhibitors without CDK4/6 inhibition (Arm B).
Baseline assessments for SONIA-EfFECT were completed for 260 patients from SONIA and the full 130-person control group. Follow-up assessments were completed for 119 members of the control group and 199 patients from the original SONIA trial (108 from Arm A, and 91 from Arm B). Patients from SONIA who switched to second-line treatment within 9 months were not retested.
Patients in both SONIA arms performed significantly worse than the controls on the domains of verbal memory, working memory, processing speed, executive function, and motor function. In both patient arms and the controls, standardized regression-based change scores showed limited decline in cognitive function over the 9-month interval. Minimal differences in cognitive change were observed between the patients treated with and without CDK4/6 inhibitors, and between patients and the controls, according to the abstract for SONIA-EfFECT, published in the program for the annual meeting of ASCO.
“At baseline, patients show worse cognitive function across all domains compared to the controls. And as expected, there were no differences between the two treatment arms,” Ms. Luijendijk explained. After 9 months of treatment, the testing showed limited further decline among patients, “and even some improvement on some tests,” with minimal differences between treatment arms “implying that cognitive function does not need to be an aspect when deciding on treatment.”
Ms. Luijendijk reported no relevant disclosures.
“Patients who are diagnosed with advanced breast cancer and start their first-line treatment already show cognitive impairments due to their previous treatments. And luckily, our results show that during first-line treatment for advanced breast cancer with endocrine therapy, with or without a CDK4/6 inhibitor, further cognitive decline is minimal,” lead investigator Maryse Luijendijk, said during her presentation at the annual meeting of the American Society of Clinical Oncology (ASCO).
“It is well known that cancer patients can experience cognitive problems, such as memory loss, problems with concentration or with planning, during or following their treatment,” explained Ms. Luijendijk, a PhD candidate in the department of Psychosocial Research and Epidemiology at the Netherlands Cancer Institute, in Amsterdam. “Much is known about the effects of chemotherapy or irradiation to the brain, but evidence into endocrine therapy is scarce, which is surprising because cognitive effects are biologically plausible.
“We know that estrogen plays an important role in neuronal functioning and that certain types of endocrine therapies are able to cross the blood-brain barrier, where they may interact with estrogen receptors distributed widely throughout the brain … We know that CDK4/6 inhibitors may either negatively affect cognitive function by increased fatigue due to cytokine release or by interrupting the cell cycle of healthy cells, or positively, as they have been associated with reduced inflammation and remyelination.”
Initial results of the SONIA trial, reported at ASCO last year, examined overall and progression-free survival in patients with HR-positive, HER2-negative metastatic breast cancer and no prior treatment for advanced disease. Findings for those who were randomized to treatment with nonsteroidal aromatase inhibition either with or without the addition of CDK4/6 inhibitors showed no between-group differences, explained Ms. Luijendijk.
The new results, described as being from the SONIA-EfFECT (Evaluation of cognitive functioning in patients with metastatic breast cancer treated with endocrine or combined therapy) trial, were based on the authors investigating cognitive functioning in the same cohort used in the SONIA trial plus a control group.
In SONIA-EfFECT, patients who participated in SONIA were asked to identify a female relative or friend without cancer to serve as a cancer-free control. Members of the 130-patient control group were matched for age, education, and computer use.
Participants in the SONIA trial and control group were asked to complete the Amsterdam Cognition Scan, an online neuropsychological test battery at baseline and again after 9 months of treatment. Of those patients from SONIA, 130 had received first-line treatment with aromatase inhibitors with CDK4/6 inhibition (Arm A) and 130 had received aromatase inhibitors without CDK4/6 inhibition (Arm B).
Baseline assessments for SONIA-EfFECT were completed for 260 patients from SONIA and the full 130-person control group. Follow-up assessments were completed for 119 members of the control group and 199 patients from the original SONIA trial (108 from Arm A, and 91 from Arm B). Patients from SONIA who switched to second-line treatment within 9 months were not retested.
Patients in both SONIA arms performed significantly worse than the controls on the domains of verbal memory, working memory, processing speed, executive function, and motor function. In both patient arms and the controls, standardized regression-based change scores showed limited decline in cognitive function over the 9-month interval. Minimal differences in cognitive change were observed between the patients treated with and without CDK4/6 inhibitors, and between patients and the controls, according to the abstract for SONIA-EfFECT, published in the program for the annual meeting of ASCO.
“At baseline, patients show worse cognitive function across all domains compared to the controls. And as expected, there were no differences between the two treatment arms,” Ms. Luijendijk explained. After 9 months of treatment, the testing showed limited further decline among patients, “and even some improvement on some tests,” with minimal differences between treatment arms “implying that cognitive function does not need to be an aspect when deciding on treatment.”
Ms. Luijendijk reported no relevant disclosures.
“Patients who are diagnosed with advanced breast cancer and start their first-line treatment already show cognitive impairments due to their previous treatments. And luckily, our results show that during first-line treatment for advanced breast cancer with endocrine therapy, with or without a CDK4/6 inhibitor, further cognitive decline is minimal,” lead investigator Maryse Luijendijk, said during her presentation at the annual meeting of the American Society of Clinical Oncology (ASCO).
“It is well known that cancer patients can experience cognitive problems, such as memory loss, problems with concentration or with planning, during or following their treatment,” explained Ms. Luijendijk, a PhD candidate in the department of Psychosocial Research and Epidemiology at the Netherlands Cancer Institute, in Amsterdam. “Much is known about the effects of chemotherapy or irradiation to the brain, but evidence into endocrine therapy is scarce, which is surprising because cognitive effects are biologically plausible.
“We know that estrogen plays an important role in neuronal functioning and that certain types of endocrine therapies are able to cross the blood-brain barrier, where they may interact with estrogen receptors distributed widely throughout the brain … We know that CDK4/6 inhibitors may either negatively affect cognitive function by increased fatigue due to cytokine release or by interrupting the cell cycle of healthy cells, or positively, as they have been associated with reduced inflammation and remyelination.”
Initial results of the SONIA trial, reported at ASCO last year, examined overall and progression-free survival in patients with HR-positive, HER2-negative metastatic breast cancer and no prior treatment for advanced disease. Findings for those who were randomized to treatment with nonsteroidal aromatase inhibition either with or without the addition of CDK4/6 inhibitors showed no between-group differences, explained Ms. Luijendijk.
The new results, described as being from the SONIA-EfFECT (Evaluation of cognitive functioning in patients with metastatic breast cancer treated with endocrine or combined therapy) trial, were based on the authors investigating cognitive functioning in the same cohort used in the SONIA trial plus a control group.
In SONIA-EfFECT, patients who participated in SONIA were asked to identify a female relative or friend without cancer to serve as a cancer-free control. Members of the 130-patient control group were matched for age, education, and computer use.
Participants in the SONIA trial and control group were asked to complete the Amsterdam Cognition Scan, an online neuropsychological test battery at baseline and again after 9 months of treatment. Of those patients from SONIA, 130 had received first-line treatment with aromatase inhibitors with CDK4/6 inhibition (Arm A) and 130 had received aromatase inhibitors without CDK4/6 inhibition (Arm B).
Baseline assessments for SONIA-EfFECT were completed for 260 patients from SONIA and the full 130-person control group. Follow-up assessments were completed for 119 members of the control group and 199 patients from the original SONIA trial (108 from Arm A, and 91 from Arm B). Patients from SONIA who switched to second-line treatment within 9 months were not retested.
Patients in both SONIA arms performed significantly worse than the controls on the domains of verbal memory, working memory, processing speed, executive function, and motor function. In both patient arms and the controls, standardized regression-based change scores showed limited decline in cognitive function over the 9-month interval. Minimal differences in cognitive change were observed between the patients treated with and without CDK4/6 inhibitors, and between patients and the controls, according to the abstract for SONIA-EfFECT, published in the program for the annual meeting of ASCO.
“At baseline, patients show worse cognitive function across all domains compared to the controls. And as expected, there were no differences between the two treatment arms,” Ms. Luijendijk explained. After 9 months of treatment, the testing showed limited further decline among patients, “and even some improvement on some tests,” with minimal differences between treatment arms “implying that cognitive function does not need to be an aspect when deciding on treatment.”
Ms. Luijendijk reported no relevant disclosures.
FROM ASCO 2024
New Parkinson’s Disease Gene Discovered
HELSINKI, FINLAND — , a discovery that experts believe will have important clinical implications in the not-too-distant future.
A variant in PMSF1, a proteasome regulator, was identified in 15 families from 13 countries around the world, with 22 affected individuals.
“These families were ethnically diverse, and in all of them, the variant in PMSF1 correlated with the neurologic phenotype. We know this is very clear cut — the genotype/phenotype correlation — with the patients carrying the missense mutation having ‘mild’ symptoms, while those with the progressive loss-of-function variant had the most severe phenotype,” she noted.
“Our findings unequivocally link defective PSMF1 to early-onset PD and neurodegeneration and suggest mitochondrial dysfunction as a mechanistic contributor,” study investigator Francesca Magrinelli, MD, PhD, of University College London (UCL) Queen Square Institute of Neurology, UCL, London, told delegates at the 2024 Congress of the European Academy of Neurology.
Managing Patient Expectations
Those “mildly” affected had an early-onset Parkinson’s disease starting between the second and fifth decade of life with pyramidal tract signs, dysphasia, psychiatric comorbidity, and early levodopa-induced dyskinesia.
In those with the intermediate type, Parkinson’s disease symptoms start in childhood and include, among other things, global hypokinesia, developmental delay, cerebellar signs, and in some, associated epilepsy.
In most cases, there was evidence on brain MRI of a hypoplasia of the corpus callosum, Dr. Magrinelli said. In the most severely affected individuals, there was perinatal lethality with neurologic manifestations.
While it may seem that the genetics of Parkinson’s disease is an academic exercise for the most part, it won’t be too much longer before it yields practical information that will inform how patients are treated, said Parkinson’s disease expert Christine Klein, MD, of the Institute of Neurogenetics and Department of Neurology, University of Lübeck, Helsinki, Finland.
The genetics of Parkinson’s disease are complicated, even within a single family. So, it’s very important to assess the pathogenicity of different variants, Dr. Klein noted.
“I am sure that you have all had a Parkinson’s disease [gene] panel back, and it says, ‘variant of uncertain significance.’ This is the worst thing that can happen. The lab does not know what it means. You don’t know what it means, and you don’t know what to tell the patient. So how do you get around this?”
Dr. Klein said that before conducting any genetic testing, clinicians should inform the patient that they may have a genetic variant of uncertain significance. It doesn’t solve the problem, but it does help physicians manage patient expectations.
Clinical Relevance on the Way?
While it may seem that all of the identified variants that predict Parkinson’s disease which, in addition to PSMF1, include the well-established LRRK2 and GBA1, may look the same, this is not true when patient history is taken into account, said Dr. Klein.
For example, age-of-onset of Parkinson’s disease can differ between identified variants, and this has led to “a paradigm change” whereby a purely genetic finding is called a disease.
This first occurred in Huntington’s disease, when researchers gave individuals at high genetic risk of developing the illness, but who currently had no clinical symptoms, the label of “Stage Zero disease.”
This is important to note “because if we get to the stage of having drugs that can slow down, or even prevent, progression to Parkinson’s disease, then it will be key to have patients we know are going to develop it to participate in clinical trials for such agents,” said Dr. Klein.
She cited the example of a family that she recently encountered that had genetic test results that showed variants of unknown significance, so Dr. Klein had the family’s samples sent to a specialized lab in Dundee, Scotland, for further analysis.
“The biochemists found that this variant was indeed pathogenic, and kinase-activating, so this is very helpful and very important because there are now clinical trials in Parkinson’s disease with kinase inhibitors,” she noted.
“If you think there is something else [over and above the finding of uncertain significance] in your Parkinson’s disease panel, and you are not happy with the genetic report, send it somewhere else,” Dr. Klein advised.
“We will see a lot more patients with genetic Parkinson’s disease in the future,” she predicted, while citing two recent preliminary clinical trials that have shown some promise in terms of neuroprotection in patients with early Parkinson’s disease.
“It remains to be seen whether there will be light at the end of the tunnel,” she said, but it may soon be possible to find treatments that delay, or even prevent, Parkinson’s disease onset.
Dr. Magrinelli reported receiving speaker’s honoraria from MJFF Edmond J. Safra Clinical Research Fellowship in Movement Disorders (Class of 2023), MJFF Edmond J. Safra Movement Disorders Research Career Development Award 2023 (Grant ID MJFF-023893), American Parkinson Disease Association (Research Grant 2024), and the David Blank Charitable Foundation. Dr. Klein reported being a medical advisor to Retromer Therapeutics, Takeda, and Centogene and speakers’ honoraria from Desitin and Bial.
A version of this article first appeared on Medscape.com.
HELSINKI, FINLAND — , a discovery that experts believe will have important clinical implications in the not-too-distant future.
A variant in PMSF1, a proteasome regulator, was identified in 15 families from 13 countries around the world, with 22 affected individuals.
“These families were ethnically diverse, and in all of them, the variant in PMSF1 correlated with the neurologic phenotype. We know this is very clear cut — the genotype/phenotype correlation — with the patients carrying the missense mutation having ‘mild’ symptoms, while those with the progressive loss-of-function variant had the most severe phenotype,” she noted.
“Our findings unequivocally link defective PSMF1 to early-onset PD and neurodegeneration and suggest mitochondrial dysfunction as a mechanistic contributor,” study investigator Francesca Magrinelli, MD, PhD, of University College London (UCL) Queen Square Institute of Neurology, UCL, London, told delegates at the 2024 Congress of the European Academy of Neurology.
Managing Patient Expectations
Those “mildly” affected had an early-onset Parkinson’s disease starting between the second and fifth decade of life with pyramidal tract signs, dysphasia, psychiatric comorbidity, and early levodopa-induced dyskinesia.
In those with the intermediate type, Parkinson’s disease symptoms start in childhood and include, among other things, global hypokinesia, developmental delay, cerebellar signs, and in some, associated epilepsy.
In most cases, there was evidence on brain MRI of a hypoplasia of the corpus callosum, Dr. Magrinelli said. In the most severely affected individuals, there was perinatal lethality with neurologic manifestations.
While it may seem that the genetics of Parkinson’s disease is an academic exercise for the most part, it won’t be too much longer before it yields practical information that will inform how patients are treated, said Parkinson’s disease expert Christine Klein, MD, of the Institute of Neurogenetics and Department of Neurology, University of Lübeck, Helsinki, Finland.
The genetics of Parkinson’s disease are complicated, even within a single family. So, it’s very important to assess the pathogenicity of different variants, Dr. Klein noted.
“I am sure that you have all had a Parkinson’s disease [gene] panel back, and it says, ‘variant of uncertain significance.’ This is the worst thing that can happen. The lab does not know what it means. You don’t know what it means, and you don’t know what to tell the patient. So how do you get around this?”
Dr. Klein said that before conducting any genetic testing, clinicians should inform the patient that they may have a genetic variant of uncertain significance. It doesn’t solve the problem, but it does help physicians manage patient expectations.
Clinical Relevance on the Way?
While it may seem that all of the identified variants that predict Parkinson’s disease which, in addition to PSMF1, include the well-established LRRK2 and GBA1, may look the same, this is not true when patient history is taken into account, said Dr. Klein.
For example, age-of-onset of Parkinson’s disease can differ between identified variants, and this has led to “a paradigm change” whereby a purely genetic finding is called a disease.
This first occurred in Huntington’s disease, when researchers gave individuals at high genetic risk of developing the illness, but who currently had no clinical symptoms, the label of “Stage Zero disease.”
This is important to note “because if we get to the stage of having drugs that can slow down, or even prevent, progression to Parkinson’s disease, then it will be key to have patients we know are going to develop it to participate in clinical trials for such agents,” said Dr. Klein.
She cited the example of a family that she recently encountered that had genetic test results that showed variants of unknown significance, so Dr. Klein had the family’s samples sent to a specialized lab in Dundee, Scotland, for further analysis.
“The biochemists found that this variant was indeed pathogenic, and kinase-activating, so this is very helpful and very important because there are now clinical trials in Parkinson’s disease with kinase inhibitors,” she noted.
“If you think there is something else [over and above the finding of uncertain significance] in your Parkinson’s disease panel, and you are not happy with the genetic report, send it somewhere else,” Dr. Klein advised.
“We will see a lot more patients with genetic Parkinson’s disease in the future,” she predicted, while citing two recent preliminary clinical trials that have shown some promise in terms of neuroprotection in patients with early Parkinson’s disease.
“It remains to be seen whether there will be light at the end of the tunnel,” she said, but it may soon be possible to find treatments that delay, or even prevent, Parkinson’s disease onset.
Dr. Magrinelli reported receiving speaker’s honoraria from MJFF Edmond J. Safra Clinical Research Fellowship in Movement Disorders (Class of 2023), MJFF Edmond J. Safra Movement Disorders Research Career Development Award 2023 (Grant ID MJFF-023893), American Parkinson Disease Association (Research Grant 2024), and the David Blank Charitable Foundation. Dr. Klein reported being a medical advisor to Retromer Therapeutics, Takeda, and Centogene and speakers’ honoraria from Desitin and Bial.
A version of this article first appeared on Medscape.com.
HELSINKI, FINLAND — , a discovery that experts believe will have important clinical implications in the not-too-distant future.
A variant in PMSF1, a proteasome regulator, was identified in 15 families from 13 countries around the world, with 22 affected individuals.
“These families were ethnically diverse, and in all of them, the variant in PMSF1 correlated with the neurologic phenotype. We know this is very clear cut — the genotype/phenotype correlation — with the patients carrying the missense mutation having ‘mild’ symptoms, while those with the progressive loss-of-function variant had the most severe phenotype,” she noted.
“Our findings unequivocally link defective PSMF1 to early-onset PD and neurodegeneration and suggest mitochondrial dysfunction as a mechanistic contributor,” study investigator Francesca Magrinelli, MD, PhD, of University College London (UCL) Queen Square Institute of Neurology, UCL, London, told delegates at the 2024 Congress of the European Academy of Neurology.
Managing Patient Expectations
Those “mildly” affected had an early-onset Parkinson’s disease starting between the second and fifth decade of life with pyramidal tract signs, dysphasia, psychiatric comorbidity, and early levodopa-induced dyskinesia.
In those with the intermediate type, Parkinson’s disease symptoms start in childhood and include, among other things, global hypokinesia, developmental delay, cerebellar signs, and in some, associated epilepsy.
In most cases, there was evidence on brain MRI of a hypoplasia of the corpus callosum, Dr. Magrinelli said. In the most severely affected individuals, there was perinatal lethality with neurologic manifestations.
While it may seem that the genetics of Parkinson’s disease is an academic exercise for the most part, it won’t be too much longer before it yields practical information that will inform how patients are treated, said Parkinson’s disease expert Christine Klein, MD, of the Institute of Neurogenetics and Department of Neurology, University of Lübeck, Helsinki, Finland.
The genetics of Parkinson’s disease are complicated, even within a single family. So, it’s very important to assess the pathogenicity of different variants, Dr. Klein noted.
“I am sure that you have all had a Parkinson’s disease [gene] panel back, and it says, ‘variant of uncertain significance.’ This is the worst thing that can happen. The lab does not know what it means. You don’t know what it means, and you don’t know what to tell the patient. So how do you get around this?”
Dr. Klein said that before conducting any genetic testing, clinicians should inform the patient that they may have a genetic variant of uncertain significance. It doesn’t solve the problem, but it does help physicians manage patient expectations.
Clinical Relevance on the Way?
While it may seem that all of the identified variants that predict Parkinson’s disease which, in addition to PSMF1, include the well-established LRRK2 and GBA1, may look the same, this is not true when patient history is taken into account, said Dr. Klein.
For example, age-of-onset of Parkinson’s disease can differ between identified variants, and this has led to “a paradigm change” whereby a purely genetic finding is called a disease.
This first occurred in Huntington’s disease, when researchers gave individuals at high genetic risk of developing the illness, but who currently had no clinical symptoms, the label of “Stage Zero disease.”
This is important to note “because if we get to the stage of having drugs that can slow down, or even prevent, progression to Parkinson’s disease, then it will be key to have patients we know are going to develop it to participate in clinical trials for such agents,” said Dr. Klein.
She cited the example of a family that she recently encountered that had genetic test results that showed variants of unknown significance, so Dr. Klein had the family’s samples sent to a specialized lab in Dundee, Scotland, for further analysis.
“The biochemists found that this variant was indeed pathogenic, and kinase-activating, so this is very helpful and very important because there are now clinical trials in Parkinson’s disease with kinase inhibitors,” she noted.
“If you think there is something else [over and above the finding of uncertain significance] in your Parkinson’s disease panel, and you are not happy with the genetic report, send it somewhere else,” Dr. Klein advised.
“We will see a lot more patients with genetic Parkinson’s disease in the future,” she predicted, while citing two recent preliminary clinical trials that have shown some promise in terms of neuroprotection in patients with early Parkinson’s disease.
“It remains to be seen whether there will be light at the end of the tunnel,” she said, but it may soon be possible to find treatments that delay, or even prevent, Parkinson’s disease onset.
Dr. Magrinelli reported receiving speaker’s honoraria from MJFF Edmond J. Safra Clinical Research Fellowship in Movement Disorders (Class of 2023), MJFF Edmond J. Safra Movement Disorders Research Career Development Award 2023 (Grant ID MJFF-023893), American Parkinson Disease Association (Research Grant 2024), and the David Blank Charitable Foundation. Dr. Klein reported being a medical advisor to Retromer Therapeutics, Takeda, and Centogene and speakers’ honoraria from Desitin and Bial.
A version of this article first appeared on Medscape.com.
FROM EAN 2024
The SOPHIA Project Conceives of Obesity Beyond BMI
During a lecture at the 2024 International Congress on Obesity in São Paulo, Brazil, Dr. Carel Le Roux, a South African researcher, reflected on the Stratification of Obesity Phenotypes to Optimize Future Therapy (SOPHIA) project. The effort, of which Dr. Le Roux is a leader, involves using federated data and reframing obesity as a set of diseases, each with its own peculiarities and treatment needs.
A collaborative research initiative led by the European Union, the SOPHIA project is a public-private partnership that brings together healthcare professionals, universities, industry leaders, and patient organizations to rethink how we understand and treat obesity, considering factors beyond body mass index (BMI).
“We need to ask ourselves, ‘Is obesity a disease? Or, in fact, does ‘obesity’ refer to multiple diseases that lead to excess adipose tissue?’ ” Dr. Le Roux asked at the beginning of his presentation.
The researcher, who is also the director of the Obesity and Metabolic Medicine Group, stated that obesity can no longer be seen as a single homogeneous pathology but rather should be viewed as clinical conditions affecting various subpopulations that respond differently to treatments.
Patients are currently diagnosed with obesity based on BMI value or waist measurement, as recommended by current clinical guidelines, but this method contributes to treating obesity subtypes as if they were identical.
“By taking into account the patient’s specificities, we can identify individuals who are likely to progress rapidly with the disease and those who will respond well to targeted interventions,” said Dr. Le Roux, emphasizing that this approach also contributes to reducing public health system costs.
Researchers proposed creating a map that allows the visualization of the distinct characteristics of patients with obesity, such as the presence of associated diseases like hypertension and diabetes. One of the main challenges of the project was finding a way to share sensitive data among SOPHIA partners without compromising individual privacy. The solution was the creation of a federated database.
In practice, this system allows academic and industry partners to send data to a central server, which keeps them protected. “We wanted to reach the optimal point, where we can have maximum utility and maximum privacy protection using technology. Researchers can then obtain statistics, enabling the analysis of large data sets without compromising security,” Dr. Le Roux explained.
Most patients analyzed in the project fall into the main group, where “the higher the weight, the greater the risk” for associated diseases, he added. However, the project allows for specifically visualizing patients with alterations related to high blood pressure, liver function, lipid profile, blood glucose, and inflammation.
“Subclassifying diseases helps us better understand the various mechanisms by which these pathologies arise and why some individuals exhibit unexpected phenotypic patterns of increased susceptibility or resilience. For example, patients with inflammation changes have a much higher risk for developing type 2 diabetes, rheumatoid arthritis, and liver failure,” said Dr. Le Roux.
In addition to visualizing the associated diseases of each participant, SOPHIA, in which 30 partners in Europe, the Middle East, and the United States participate, also features treatment overlap, which allows researchers to track individual responses to the treatment.
“With this overlap, we confirm something that many know: When treating people with type 2 diabetes, whether through lifestyle changes, medication, or surgery, weight loss is lower. But, to our surprise, we found that patients with inflammation-related changes had greater weight loss. This finding tells us that some groups benefit more, and others less,” said Dr. Le Roux.
This analysis is particularly interesting when it comes to bariatric surgery, he continued. “Often, the surgeon performs an incredibly well-done gastric bypass, and the response is not as expected. In this case, we can say that it is purely biology,” said Dr. Le Roux, who concluded the presentation by discussing the benefits of this approach for good patient counseling.
“When we talk about ‘obesities’ and not ‘obesity,’ we can also conduct our consultations more carefully by explaining to our patients that if they do not respond to treatment, it is not their fault, not because they did something wrong, but because of something that is not usually taken into account, such as the presence of comorbidities, or even personal characteristics and lifestyle, such as age and smoking,” said Dr. Le Roux.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
During a lecture at the 2024 International Congress on Obesity in São Paulo, Brazil, Dr. Carel Le Roux, a South African researcher, reflected on the Stratification of Obesity Phenotypes to Optimize Future Therapy (SOPHIA) project. The effort, of which Dr. Le Roux is a leader, involves using federated data and reframing obesity as a set of diseases, each with its own peculiarities and treatment needs.
A collaborative research initiative led by the European Union, the SOPHIA project is a public-private partnership that brings together healthcare professionals, universities, industry leaders, and patient organizations to rethink how we understand and treat obesity, considering factors beyond body mass index (BMI).
“We need to ask ourselves, ‘Is obesity a disease? Or, in fact, does ‘obesity’ refer to multiple diseases that lead to excess adipose tissue?’ ” Dr. Le Roux asked at the beginning of his presentation.
The researcher, who is also the director of the Obesity and Metabolic Medicine Group, stated that obesity can no longer be seen as a single homogeneous pathology but rather should be viewed as clinical conditions affecting various subpopulations that respond differently to treatments.
Patients are currently diagnosed with obesity based on BMI value or waist measurement, as recommended by current clinical guidelines, but this method contributes to treating obesity subtypes as if they were identical.
“By taking into account the patient’s specificities, we can identify individuals who are likely to progress rapidly with the disease and those who will respond well to targeted interventions,” said Dr. Le Roux, emphasizing that this approach also contributes to reducing public health system costs.
Researchers proposed creating a map that allows the visualization of the distinct characteristics of patients with obesity, such as the presence of associated diseases like hypertension and diabetes. One of the main challenges of the project was finding a way to share sensitive data among SOPHIA partners without compromising individual privacy. The solution was the creation of a federated database.
In practice, this system allows academic and industry partners to send data to a central server, which keeps them protected. “We wanted to reach the optimal point, where we can have maximum utility and maximum privacy protection using technology. Researchers can then obtain statistics, enabling the analysis of large data sets without compromising security,” Dr. Le Roux explained.
Most patients analyzed in the project fall into the main group, where “the higher the weight, the greater the risk” for associated diseases, he added. However, the project allows for specifically visualizing patients with alterations related to high blood pressure, liver function, lipid profile, blood glucose, and inflammation.
“Subclassifying diseases helps us better understand the various mechanisms by which these pathologies arise and why some individuals exhibit unexpected phenotypic patterns of increased susceptibility or resilience. For example, patients with inflammation changes have a much higher risk for developing type 2 diabetes, rheumatoid arthritis, and liver failure,” said Dr. Le Roux.
In addition to visualizing the associated diseases of each participant, SOPHIA, in which 30 partners in Europe, the Middle East, and the United States participate, also features treatment overlap, which allows researchers to track individual responses to the treatment.
“With this overlap, we confirm something that many know: When treating people with type 2 diabetes, whether through lifestyle changes, medication, or surgery, weight loss is lower. But, to our surprise, we found that patients with inflammation-related changes had greater weight loss. This finding tells us that some groups benefit more, and others less,” said Dr. Le Roux.
This analysis is particularly interesting when it comes to bariatric surgery, he continued. “Often, the surgeon performs an incredibly well-done gastric bypass, and the response is not as expected. In this case, we can say that it is purely biology,” said Dr. Le Roux, who concluded the presentation by discussing the benefits of this approach for good patient counseling.
“When we talk about ‘obesities’ and not ‘obesity,’ we can also conduct our consultations more carefully by explaining to our patients that if they do not respond to treatment, it is not their fault, not because they did something wrong, but because of something that is not usually taken into account, such as the presence of comorbidities, or even personal characteristics and lifestyle, such as age and smoking,” said Dr. Le Roux.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
During a lecture at the 2024 International Congress on Obesity in São Paulo, Brazil, Dr. Carel Le Roux, a South African researcher, reflected on the Stratification of Obesity Phenotypes to Optimize Future Therapy (SOPHIA) project. The effort, of which Dr. Le Roux is a leader, involves using federated data and reframing obesity as a set of diseases, each with its own peculiarities and treatment needs.
A collaborative research initiative led by the European Union, the SOPHIA project is a public-private partnership that brings together healthcare professionals, universities, industry leaders, and patient organizations to rethink how we understand and treat obesity, considering factors beyond body mass index (BMI).
“We need to ask ourselves, ‘Is obesity a disease? Or, in fact, does ‘obesity’ refer to multiple diseases that lead to excess adipose tissue?’ ” Dr. Le Roux asked at the beginning of his presentation.
The researcher, who is also the director of the Obesity and Metabolic Medicine Group, stated that obesity can no longer be seen as a single homogeneous pathology but rather should be viewed as clinical conditions affecting various subpopulations that respond differently to treatments.
Patients are currently diagnosed with obesity based on BMI value or waist measurement, as recommended by current clinical guidelines, but this method contributes to treating obesity subtypes as if they were identical.
“By taking into account the patient’s specificities, we can identify individuals who are likely to progress rapidly with the disease and those who will respond well to targeted interventions,” said Dr. Le Roux, emphasizing that this approach also contributes to reducing public health system costs.
Researchers proposed creating a map that allows the visualization of the distinct characteristics of patients with obesity, such as the presence of associated diseases like hypertension and diabetes. One of the main challenges of the project was finding a way to share sensitive data among SOPHIA partners without compromising individual privacy. The solution was the creation of a federated database.
In practice, this system allows academic and industry partners to send data to a central server, which keeps them protected. “We wanted to reach the optimal point, where we can have maximum utility and maximum privacy protection using technology. Researchers can then obtain statistics, enabling the analysis of large data sets without compromising security,” Dr. Le Roux explained.
Most patients analyzed in the project fall into the main group, where “the higher the weight, the greater the risk” for associated diseases, he added. However, the project allows for specifically visualizing patients with alterations related to high blood pressure, liver function, lipid profile, blood glucose, and inflammation.
“Subclassifying diseases helps us better understand the various mechanisms by which these pathologies arise and why some individuals exhibit unexpected phenotypic patterns of increased susceptibility or resilience. For example, patients with inflammation changes have a much higher risk for developing type 2 diabetes, rheumatoid arthritis, and liver failure,” said Dr. Le Roux.
In addition to visualizing the associated diseases of each participant, SOPHIA, in which 30 partners in Europe, the Middle East, and the United States participate, also features treatment overlap, which allows researchers to track individual responses to the treatment.
“With this overlap, we confirm something that many know: When treating people with type 2 diabetes, whether through lifestyle changes, medication, or surgery, weight loss is lower. But, to our surprise, we found that patients with inflammation-related changes had greater weight loss. This finding tells us that some groups benefit more, and others less,” said Dr. Le Roux.
This analysis is particularly interesting when it comes to bariatric surgery, he continued. “Often, the surgeon performs an incredibly well-done gastric bypass, and the response is not as expected. In this case, we can say that it is purely biology,” said Dr. Le Roux, who concluded the presentation by discussing the benefits of this approach for good patient counseling.
“When we talk about ‘obesities’ and not ‘obesity,’ we can also conduct our consultations more carefully by explaining to our patients that if they do not respond to treatment, it is not their fault, not because they did something wrong, but because of something that is not usually taken into account, such as the presence of comorbidities, or even personal characteristics and lifestyle, such as age and smoking,” said Dr. Le Roux.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Prescribing Epilepsy Meds in Pregnancy: ‘We Can Do Better,’ Experts Say
HELSINKI, FINLAND — When it comes to caring for women with epilepsy who become pregnant, there is a great deal of room for improvement, experts say.
“Too many women with epilepsy receive information about epilepsy and pregnancy only after pregnancy. We can do better,” Torbjörn Tomson, MD, PhD, senior professor of neurology and epileptology, Karolinska Institutet, Stockholm, Sweden, told delegates attending the Congress of the European Academy of Neurology 2024.
The goal in epilepsy is to maintain seizure control while minimizing exposure to potentially teratogenic medications, Dr. Tomson said. He added that pregnancy planning in women with epilepsy is important but also conceded that most pregnancies in this patient population are unplanned.
Overall, it’s important to tell patients that “there is a high likelihood of an uneventful pregnancy and a healthy offspring,” he said.
In recent years, new data have emerged on the risks to the fetus with exposure to different antiseizure medications (ASMs), said Dr. Tomson. This has led regulators, such as the US Food and Drug Administration and the European Medicines Agency, to issue restrictions on the use of some ASMs, particularly valproate and topiramate, in females of childbearing age.
Session chair Marte Bjørk, MD, PhD, of the Department of Neurology of Haukeland University Hospital, Bergen, Norway, questioned whether the latest recommendations from regulatory authorities have “sacrificed seizure control at the expense of teratogenic safety.”
To an extent, this is true, said Dr. Tomson, “as the regulations prioritize fetal health over women’s health.” However, “we have not seen poorer seizure control with newer medications” in recent datasets.
It’s about good planning, said Dr. Bjork, who is responsible for the clinical guidelines for treatment of epilepsy in pregnancy in Norway.
Start With Folic Acid
One simple measure is to ensure that all women with epilepsy of childbearing age are prescribed low-dose folic acid, Dr. Tomson said — even those who report that they are not considering pregnancy.
When it comes to folic acid, recently published guidelines on ASM use during pregnancy are relatively straightforward, he said.
The data do not show that folic acid reduces the risk for major congenital malformations, but they do show that it improves neurocognitive outcomes in children of mothers who received folic acid supplements prior to and throughout pregnancy.
Dr. Tomson said the new American Academy of Neurology (AAN) guidelines recommend a dosage of 0.4 mg/d, which balances the demonstrated benefits of supplementation and potential negative consequences of high doses of folic acid.
“Consider 0.4 mg of folic acid for all women on ASMs that are of childbearing potential, whether they become pregnant or not,” he said. However, well-designed, preferably randomized, studies are needed to better define the optimal folic acid dosing for pregnancy in women with epilepsy.
Choosing the Right ASM
The choice of the most appropriate ASM in pregnancy is based on the potential for an individual drug to cause major congenital malformations and, in more recent years, the likelihood that a woman with epilepsy is using any other medications associated with neurodevelopmental disorders in offspring.
Balanced against this must be the effect of pregnancy on seizure control, and the maternal and fetal risks associated with seizures during pregnancy.
“There are ways to optimize seizure control and to reduce teratogenic risks,” said Dr. Tomson, adding that the new AAN guidelines provide updated evidence-based conclusions on this topic.
The good news is that “there has been almost a 40% decline in the rate of major congenital malformations associated with ASM use in pregnancy, in parallel with a shift from use of ASMs such as carbamazepine and valproate to lamotrigine and levetiracetam.” The latter two medications are associated with a much lower risk for such birth defects, he added.
This is based on the average rate of major congenital malformations in the EURAP registry that tracks the comparative risk for major fetal malformations after ASM use during pregnancy in over 40 countries. The latest reporting from the registry shows that this risk has decreased from 6.1% in 1998-2004 to 3.7% in 2015-2022.
Taking valproate during pregnancy is associated with a significantly increased risk for neurodevelopmental outcomes, including autism spectrum disorder. However, the jury is still out on whether topiramate escalates the risk for neurodevelopmental disorders, because findings across studies have been inconsistent.
Overall, the AAN guidance, and similar advice from European regulatory authorities, is that valproate is associated with high risk for major congenital malformations and neurodevelopmental disorders. Topiramate has also been shown to increase the risk for major congenital malformations. Consequently, these two anticonvulsants are generally contraindicated in pregnancy, Dr. Tomson noted.
On the other hand, levetiracetam, lamotrigine, and oxcarbazepine seem to be the safest ASMs with respect to congenital malformation risk, and lamotrigine has the best documented safety profile when it comes to the risk for neurodevelopmental disorders.
Although there are newer ASMs on the market, including brivaracetam, cannabidiol, cenobamate, eslicarbazepine acetate, fenfluramine, lacosamide, perampanel, and zonisamide, at this juncture data on the risk potential of these agents are insufficient.
“For some of these newer meds, we don’t even have a single exposure in our large databases, even if you combine them all. We need to collect more data, and that will take time,” Dr. Tomson said.
Dose Optimization
Dose optimization of ASMs is also important — and for this to be accurate, it’s important to document an individual’s optimal ASM serum levels before pregnancy that can be used as a baseline target during pregnancy. However, Dr. Tomson noted, this information is not always available.
He pointed out that, with many ASMs, there can be a significant decline in serum concentration levels during pregnancy, which can increase seizure risk.
To address the uncertainty surrounding this issue, Dr. Tomson recommended that physicians consider future pregnancy when prescribing ASMs to women of childbearing age. He also advised discussing contraception with these patients, even if they indicate they are not currently planning to conceive.
The data clearly show the importance of planning a pregnancy so that the most appropriate and safest medications are prescribed, he said.
Dr. Tomson reported receiving research support, on behalf of EURAP, from Accord, Angelini, Bial, EcuPharma, Eisai, GlaxoSmithKline, Glenmark, GW Pharma, Hazz, Sanofi, Teva, USB, Zentiva, and SF Group. He has received speakers’ honoraria from Angelini, Eisai, and UCB. Dr. Bjørk reports receiving speakers’ honoraria from Pfizer, Eisai, AbbVie, Best Practice, Lilly, Novartis, and Teva. She has received unrestricted educational grants from The Research Council of Norway, the Research Council of the Nordic Countries (NordForsk), and the Norwegian Epilepsy Association. She has received consulting honoraria from Novartis and is on the advisory board of Eisai, Lundbeck, Angelini Pharma, and Jazz Pharmaceuticals. Dr. Bjørk also received institutional grants from marked authorization holders of valproate.
A version of this article first appeared on Medscape.com.
HELSINKI, FINLAND — When it comes to caring for women with epilepsy who become pregnant, there is a great deal of room for improvement, experts say.
“Too many women with epilepsy receive information about epilepsy and pregnancy only after pregnancy. We can do better,” Torbjörn Tomson, MD, PhD, senior professor of neurology and epileptology, Karolinska Institutet, Stockholm, Sweden, told delegates attending the Congress of the European Academy of Neurology 2024.
The goal in epilepsy is to maintain seizure control while minimizing exposure to potentially teratogenic medications, Dr. Tomson said. He added that pregnancy planning in women with epilepsy is important but also conceded that most pregnancies in this patient population are unplanned.
Overall, it’s important to tell patients that “there is a high likelihood of an uneventful pregnancy and a healthy offspring,” he said.
In recent years, new data have emerged on the risks to the fetus with exposure to different antiseizure medications (ASMs), said Dr. Tomson. This has led regulators, such as the US Food and Drug Administration and the European Medicines Agency, to issue restrictions on the use of some ASMs, particularly valproate and topiramate, in females of childbearing age.
Session chair Marte Bjørk, MD, PhD, of the Department of Neurology of Haukeland University Hospital, Bergen, Norway, questioned whether the latest recommendations from regulatory authorities have “sacrificed seizure control at the expense of teratogenic safety.”
To an extent, this is true, said Dr. Tomson, “as the regulations prioritize fetal health over women’s health.” However, “we have not seen poorer seizure control with newer medications” in recent datasets.
It’s about good planning, said Dr. Bjork, who is responsible for the clinical guidelines for treatment of epilepsy in pregnancy in Norway.
Start With Folic Acid
One simple measure is to ensure that all women with epilepsy of childbearing age are prescribed low-dose folic acid, Dr. Tomson said — even those who report that they are not considering pregnancy.
When it comes to folic acid, recently published guidelines on ASM use during pregnancy are relatively straightforward, he said.
The data do not show that folic acid reduces the risk for major congenital malformations, but they do show that it improves neurocognitive outcomes in children of mothers who received folic acid supplements prior to and throughout pregnancy.
Dr. Tomson said the new American Academy of Neurology (AAN) guidelines recommend a dosage of 0.4 mg/d, which balances the demonstrated benefits of supplementation and potential negative consequences of high doses of folic acid.
“Consider 0.4 mg of folic acid for all women on ASMs that are of childbearing potential, whether they become pregnant or not,” he said. However, well-designed, preferably randomized, studies are needed to better define the optimal folic acid dosing for pregnancy in women with epilepsy.
Choosing the Right ASM
The choice of the most appropriate ASM in pregnancy is based on the potential for an individual drug to cause major congenital malformations and, in more recent years, the likelihood that a woman with epilepsy is using any other medications associated with neurodevelopmental disorders in offspring.
Balanced against this must be the effect of pregnancy on seizure control, and the maternal and fetal risks associated with seizures during pregnancy.
“There are ways to optimize seizure control and to reduce teratogenic risks,” said Dr. Tomson, adding that the new AAN guidelines provide updated evidence-based conclusions on this topic.
The good news is that “there has been almost a 40% decline in the rate of major congenital malformations associated with ASM use in pregnancy, in parallel with a shift from use of ASMs such as carbamazepine and valproate to lamotrigine and levetiracetam.” The latter two medications are associated with a much lower risk for such birth defects, he added.
This is based on the average rate of major congenital malformations in the EURAP registry that tracks the comparative risk for major fetal malformations after ASM use during pregnancy in over 40 countries. The latest reporting from the registry shows that this risk has decreased from 6.1% in 1998-2004 to 3.7% in 2015-2022.
Taking valproate during pregnancy is associated with a significantly increased risk for neurodevelopmental outcomes, including autism spectrum disorder. However, the jury is still out on whether topiramate escalates the risk for neurodevelopmental disorders, because findings across studies have been inconsistent.
Overall, the AAN guidance, and similar advice from European regulatory authorities, is that valproate is associated with high risk for major congenital malformations and neurodevelopmental disorders. Topiramate has also been shown to increase the risk for major congenital malformations. Consequently, these two anticonvulsants are generally contraindicated in pregnancy, Dr. Tomson noted.
On the other hand, levetiracetam, lamotrigine, and oxcarbazepine seem to be the safest ASMs with respect to congenital malformation risk, and lamotrigine has the best documented safety profile when it comes to the risk for neurodevelopmental disorders.
Although there are newer ASMs on the market, including brivaracetam, cannabidiol, cenobamate, eslicarbazepine acetate, fenfluramine, lacosamide, perampanel, and zonisamide, at this juncture data on the risk potential of these agents are insufficient.
“For some of these newer meds, we don’t even have a single exposure in our large databases, even if you combine them all. We need to collect more data, and that will take time,” Dr. Tomson said.
Dose Optimization
Dose optimization of ASMs is also important — and for this to be accurate, it’s important to document an individual’s optimal ASM serum levels before pregnancy that can be used as a baseline target during pregnancy. However, Dr. Tomson noted, this information is not always available.
He pointed out that, with many ASMs, there can be a significant decline in serum concentration levels during pregnancy, which can increase seizure risk.
To address the uncertainty surrounding this issue, Dr. Tomson recommended that physicians consider future pregnancy when prescribing ASMs to women of childbearing age. He also advised discussing contraception with these patients, even if they indicate they are not currently planning to conceive.
The data clearly show the importance of planning a pregnancy so that the most appropriate and safest medications are prescribed, he said.
Dr. Tomson reported receiving research support, on behalf of EURAP, from Accord, Angelini, Bial, EcuPharma, Eisai, GlaxoSmithKline, Glenmark, GW Pharma, Hazz, Sanofi, Teva, USB, Zentiva, and SF Group. He has received speakers’ honoraria from Angelini, Eisai, and UCB. Dr. Bjørk reports receiving speakers’ honoraria from Pfizer, Eisai, AbbVie, Best Practice, Lilly, Novartis, and Teva. She has received unrestricted educational grants from The Research Council of Norway, the Research Council of the Nordic Countries (NordForsk), and the Norwegian Epilepsy Association. She has received consulting honoraria from Novartis and is on the advisory board of Eisai, Lundbeck, Angelini Pharma, and Jazz Pharmaceuticals. Dr. Bjørk also received institutional grants from marked authorization holders of valproate.
A version of this article first appeared on Medscape.com.
HELSINKI, FINLAND — When it comes to caring for women with epilepsy who become pregnant, there is a great deal of room for improvement, experts say.
“Too many women with epilepsy receive information about epilepsy and pregnancy only after pregnancy. We can do better,” Torbjörn Tomson, MD, PhD, senior professor of neurology and epileptology, Karolinska Institutet, Stockholm, Sweden, told delegates attending the Congress of the European Academy of Neurology 2024.
The goal in epilepsy is to maintain seizure control while minimizing exposure to potentially teratogenic medications, Dr. Tomson said. He added that pregnancy planning in women with epilepsy is important but also conceded that most pregnancies in this patient population are unplanned.
Overall, it’s important to tell patients that “there is a high likelihood of an uneventful pregnancy and a healthy offspring,” he said.
In recent years, new data have emerged on the risks to the fetus with exposure to different antiseizure medications (ASMs), said Dr. Tomson. This has led regulators, such as the US Food and Drug Administration and the European Medicines Agency, to issue restrictions on the use of some ASMs, particularly valproate and topiramate, in females of childbearing age.
Session chair Marte Bjørk, MD, PhD, of the Department of Neurology of Haukeland University Hospital, Bergen, Norway, questioned whether the latest recommendations from regulatory authorities have “sacrificed seizure control at the expense of teratogenic safety.”
To an extent, this is true, said Dr. Tomson, “as the regulations prioritize fetal health over women’s health.” However, “we have not seen poorer seizure control with newer medications” in recent datasets.
It’s about good planning, said Dr. Bjork, who is responsible for the clinical guidelines for treatment of epilepsy in pregnancy in Norway.
Start With Folic Acid
One simple measure is to ensure that all women with epilepsy of childbearing age are prescribed low-dose folic acid, Dr. Tomson said — even those who report that they are not considering pregnancy.
When it comes to folic acid, recently published guidelines on ASM use during pregnancy are relatively straightforward, he said.
The data do not show that folic acid reduces the risk for major congenital malformations, but they do show that it improves neurocognitive outcomes in children of mothers who received folic acid supplements prior to and throughout pregnancy.
Dr. Tomson said the new American Academy of Neurology (AAN) guidelines recommend a dosage of 0.4 mg/d, which balances the demonstrated benefits of supplementation and potential negative consequences of high doses of folic acid.
“Consider 0.4 mg of folic acid for all women on ASMs that are of childbearing potential, whether they become pregnant or not,” he said. However, well-designed, preferably randomized, studies are needed to better define the optimal folic acid dosing for pregnancy in women with epilepsy.
Choosing the Right ASM
The choice of the most appropriate ASM in pregnancy is based on the potential for an individual drug to cause major congenital malformations and, in more recent years, the likelihood that a woman with epilepsy is using any other medications associated with neurodevelopmental disorders in offspring.
Balanced against this must be the effect of pregnancy on seizure control, and the maternal and fetal risks associated with seizures during pregnancy.
“There are ways to optimize seizure control and to reduce teratogenic risks,” said Dr. Tomson, adding that the new AAN guidelines provide updated evidence-based conclusions on this topic.
The good news is that “there has been almost a 40% decline in the rate of major congenital malformations associated with ASM use in pregnancy, in parallel with a shift from use of ASMs such as carbamazepine and valproate to lamotrigine and levetiracetam.” The latter two medications are associated with a much lower risk for such birth defects, he added.
This is based on the average rate of major congenital malformations in the EURAP registry that tracks the comparative risk for major fetal malformations after ASM use during pregnancy in over 40 countries. The latest reporting from the registry shows that this risk has decreased from 6.1% in 1998-2004 to 3.7% in 2015-2022.
Taking valproate during pregnancy is associated with a significantly increased risk for neurodevelopmental outcomes, including autism spectrum disorder. However, the jury is still out on whether topiramate escalates the risk for neurodevelopmental disorders, because findings across studies have been inconsistent.
Overall, the AAN guidance, and similar advice from European regulatory authorities, is that valproate is associated with high risk for major congenital malformations and neurodevelopmental disorders. Topiramate has also been shown to increase the risk for major congenital malformations. Consequently, these two anticonvulsants are generally contraindicated in pregnancy, Dr. Tomson noted.
On the other hand, levetiracetam, lamotrigine, and oxcarbazepine seem to be the safest ASMs with respect to congenital malformation risk, and lamotrigine has the best documented safety profile when it comes to the risk for neurodevelopmental disorders.
Although there are newer ASMs on the market, including brivaracetam, cannabidiol, cenobamate, eslicarbazepine acetate, fenfluramine, lacosamide, perampanel, and zonisamide, at this juncture data on the risk potential of these agents are insufficient.
“For some of these newer meds, we don’t even have a single exposure in our large databases, even if you combine them all. We need to collect more data, and that will take time,” Dr. Tomson said.
Dose Optimization
Dose optimization of ASMs is also important — and for this to be accurate, it’s important to document an individual’s optimal ASM serum levels before pregnancy that can be used as a baseline target during pregnancy. However, Dr. Tomson noted, this information is not always available.
He pointed out that, with many ASMs, there can be a significant decline in serum concentration levels during pregnancy, which can increase seizure risk.
To address the uncertainty surrounding this issue, Dr. Tomson recommended that physicians consider future pregnancy when prescribing ASMs to women of childbearing age. He also advised discussing contraception with these patients, even if they indicate they are not currently planning to conceive.
The data clearly show the importance of planning a pregnancy so that the most appropriate and safest medications are prescribed, he said.
Dr. Tomson reported receiving research support, on behalf of EURAP, from Accord, Angelini, Bial, EcuPharma, Eisai, GlaxoSmithKline, Glenmark, GW Pharma, Hazz, Sanofi, Teva, USB, Zentiva, and SF Group. He has received speakers’ honoraria from Angelini, Eisai, and UCB. Dr. Bjørk reports receiving speakers’ honoraria from Pfizer, Eisai, AbbVie, Best Practice, Lilly, Novartis, and Teva. She has received unrestricted educational grants from The Research Council of Norway, the Research Council of the Nordic Countries (NordForsk), and the Norwegian Epilepsy Association. She has received consulting honoraria from Novartis and is on the advisory board of Eisai, Lundbeck, Angelini Pharma, and Jazz Pharmaceuticals. Dr. Bjørk also received institutional grants from marked authorization holders of valproate.
A version of this article first appeared on Medscape.com.
FROM EAN 2024