User login
Tide turns in favor of multivessel PCI in STEMI
SNOWMASS, COLO. – Recent data seem to refute the 2013 American Heart Association/American College of Cardiology class III recommendation to avoid multivessel percutaneous coronary intervention at the time of primary PCI for ST-elevation MI, Dr. David R. Holmes Jr. observed at the Annual Cardiovascular Conference at Snowmass.
“The current AHA/ACC guidelines for STEMI should be and are being reevaluated regarding clarifications for the indications and timing of non–infarct artery revascularization,” according to Dr. Holmes, a cardiologist at the Mayo Clinic in Rochester, Minn., and an ACC past president.
Indeed, the ACC has already withdrawn from its ‘Choosing Wisely’ campaign its former recommendation discouraging multivessel revascularization at the time of primary PCI for STEMI. The college cited “new science showing that complete revascularization of all significant blocked arteries leads to better outcomes in some heart attack patients.”
Dr. Holmes was coauthor of a meta-analysis of 4 prospective and 14 retrospective studies involving more than 40,000 patients that concluded multivessel PCI for STEMI should be discouraged, and that significant nonculprit lesions should only be treated during staged procedures (J. Am. Coll. Cardiol. 2011;58:692-703). This meta-analysis was influential in the creation of the class III ‘don’t do it’ recommendation in the AHA/ACC guidelines. But Dr. Holmes said that in hindsight, the data included in the meta-analysis were something of a mishmash and “wound up being very hard to interpret.”
Greater clarity has been brought by two more recent randomized trials: PRAMI and CvLPRIT. Both were relatively small by cardiology standards, but they ended up showing similarly striking advantages in favor of using the STEMI hospitalization to perform preventive PCI of both the infarct-related artery and non–infarct arteries with major stenoses.
PRAMI included 465 acute STEMI patients who underwent infarct artery PCI and were then randomized to preventive PCI or infarct artery–only PCI. At a mean follow-up of 23 months, the preventive multivessel PCI group had a 65% reduction in the relative risk of the primary outcome, a composite of cardiac death, nonfatal MI, or refractory angina (N. Engl. J. Med. 2013;369:1115-23).
The yet-to-be-published CvLPRIT study was presented at the 2014 European Society of Cardiology meeting in Barcelona. The multicenter study included 296 STEMI patients with angiographically established significant multivessel disease who were randomized to primary PCI of the culprit vessel only or to complete revascularization. The primary outcome, the 12-month composite of all-cause mortality, recurrent MI, heart failure, or ischemia-driven revascularization, occurred in 10% of the complete revascularization group, compared with 21.2% of patients assigned to culprit artery–only PCI.
Also at the ESC conference, CvLPRIT investigator Dr. Anthony Gershlick of the University of Leicester (England) presented a meta-analysis combining the weighted results of PRAMI, CvLPRIT, and two earlier randomized trials: HELP AMI (Int. J. Cardiovasc. Intervent. 2004;6:128-33) and an Italian trial (Heart 2010;96:662-7). The results strongly favored multivessel PCI, with a 45% reduction in mortality and a 61% decrease in recurrent MI, compared with culprit vessel–only PCI at the time of admission for STEMI.
“Maybe there aren’t any innocent bystanders,” commented Dr. Holmes. “Maybe if you have somebody who has multivessel disease and you see something you think might be an innocent bystander but is a significant lesion, maybe it’s not so innocent. Maybe by treating them all at the time of the initial intervention the patient is going to do better.”
He reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – Recent data seem to refute the 2013 American Heart Association/American College of Cardiology class III recommendation to avoid multivessel percutaneous coronary intervention at the time of primary PCI for ST-elevation MI, Dr. David R. Holmes Jr. observed at the Annual Cardiovascular Conference at Snowmass.
“The current AHA/ACC guidelines for STEMI should be and are being reevaluated regarding clarifications for the indications and timing of non–infarct artery revascularization,” according to Dr. Holmes, a cardiologist at the Mayo Clinic in Rochester, Minn., and an ACC past president.
Indeed, the ACC has already withdrawn from its ‘Choosing Wisely’ campaign its former recommendation discouraging multivessel revascularization at the time of primary PCI for STEMI. The college cited “new science showing that complete revascularization of all significant blocked arteries leads to better outcomes in some heart attack patients.”
Dr. Holmes was coauthor of a meta-analysis of 4 prospective and 14 retrospective studies involving more than 40,000 patients that concluded multivessel PCI for STEMI should be discouraged, and that significant nonculprit lesions should only be treated during staged procedures (J. Am. Coll. Cardiol. 2011;58:692-703). This meta-analysis was influential in the creation of the class III ‘don’t do it’ recommendation in the AHA/ACC guidelines. But Dr. Holmes said that in hindsight, the data included in the meta-analysis were something of a mishmash and “wound up being very hard to interpret.”
Greater clarity has been brought by two more recent randomized trials: PRAMI and CvLPRIT. Both were relatively small by cardiology standards, but they ended up showing similarly striking advantages in favor of using the STEMI hospitalization to perform preventive PCI of both the infarct-related artery and non–infarct arteries with major stenoses.
PRAMI included 465 acute STEMI patients who underwent infarct artery PCI and were then randomized to preventive PCI or infarct artery–only PCI. At a mean follow-up of 23 months, the preventive multivessel PCI group had a 65% reduction in the relative risk of the primary outcome, a composite of cardiac death, nonfatal MI, or refractory angina (N. Engl. J. Med. 2013;369:1115-23).
The yet-to-be-published CvLPRIT study was presented at the 2014 European Society of Cardiology meeting in Barcelona. The multicenter study included 296 STEMI patients with angiographically established significant multivessel disease who were randomized to primary PCI of the culprit vessel only or to complete revascularization. The primary outcome, the 12-month composite of all-cause mortality, recurrent MI, heart failure, or ischemia-driven revascularization, occurred in 10% of the complete revascularization group, compared with 21.2% of patients assigned to culprit artery–only PCI.
Also at the ESC conference, CvLPRIT investigator Dr. Anthony Gershlick of the University of Leicester (England) presented a meta-analysis combining the weighted results of PRAMI, CvLPRIT, and two earlier randomized trials: HELP AMI (Int. J. Cardiovasc. Intervent. 2004;6:128-33) and an Italian trial (Heart 2010;96:662-7). The results strongly favored multivessel PCI, with a 45% reduction in mortality and a 61% decrease in recurrent MI, compared with culprit vessel–only PCI at the time of admission for STEMI.
“Maybe there aren’t any innocent bystanders,” commented Dr. Holmes. “Maybe if you have somebody who has multivessel disease and you see something you think might be an innocent bystander but is a significant lesion, maybe it’s not so innocent. Maybe by treating them all at the time of the initial intervention the patient is going to do better.”
He reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – Recent data seem to refute the 2013 American Heart Association/American College of Cardiology class III recommendation to avoid multivessel percutaneous coronary intervention at the time of primary PCI for ST-elevation MI, Dr. David R. Holmes Jr. observed at the Annual Cardiovascular Conference at Snowmass.
“The current AHA/ACC guidelines for STEMI should be and are being reevaluated regarding clarifications for the indications and timing of non–infarct artery revascularization,” according to Dr. Holmes, a cardiologist at the Mayo Clinic in Rochester, Minn., and an ACC past president.
Indeed, the ACC has already withdrawn from its ‘Choosing Wisely’ campaign its former recommendation discouraging multivessel revascularization at the time of primary PCI for STEMI. The college cited “new science showing that complete revascularization of all significant blocked arteries leads to better outcomes in some heart attack patients.”
Dr. Holmes was coauthor of a meta-analysis of 4 prospective and 14 retrospective studies involving more than 40,000 patients that concluded multivessel PCI for STEMI should be discouraged, and that significant nonculprit lesions should only be treated during staged procedures (J. Am. Coll. Cardiol. 2011;58:692-703). This meta-analysis was influential in the creation of the class III ‘don’t do it’ recommendation in the AHA/ACC guidelines. But Dr. Holmes said that in hindsight, the data included in the meta-analysis were something of a mishmash and “wound up being very hard to interpret.”
Greater clarity has been brought by two more recent randomized trials: PRAMI and CvLPRIT. Both were relatively small by cardiology standards, but they ended up showing similarly striking advantages in favor of using the STEMI hospitalization to perform preventive PCI of both the infarct-related artery and non–infarct arteries with major stenoses.
PRAMI included 465 acute STEMI patients who underwent infarct artery PCI and were then randomized to preventive PCI or infarct artery–only PCI. At a mean follow-up of 23 months, the preventive multivessel PCI group had a 65% reduction in the relative risk of the primary outcome, a composite of cardiac death, nonfatal MI, or refractory angina (N. Engl. J. Med. 2013;369:1115-23).
The yet-to-be-published CvLPRIT study was presented at the 2014 European Society of Cardiology meeting in Barcelona. The multicenter study included 296 STEMI patients with angiographically established significant multivessel disease who were randomized to primary PCI of the culprit vessel only or to complete revascularization. The primary outcome, the 12-month composite of all-cause mortality, recurrent MI, heart failure, or ischemia-driven revascularization, occurred in 10% of the complete revascularization group, compared with 21.2% of patients assigned to culprit artery–only PCI.
Also at the ESC conference, CvLPRIT investigator Dr. Anthony Gershlick of the University of Leicester (England) presented a meta-analysis combining the weighted results of PRAMI, CvLPRIT, and two earlier randomized trials: HELP AMI (Int. J. Cardiovasc. Intervent. 2004;6:128-33) and an Italian trial (Heart 2010;96:662-7). The results strongly favored multivessel PCI, with a 45% reduction in mortality and a 61% decrease in recurrent MI, compared with culprit vessel–only PCI at the time of admission for STEMI.
“Maybe there aren’t any innocent bystanders,” commented Dr. Holmes. “Maybe if you have somebody who has multivessel disease and you see something you think might be an innocent bystander but is a significant lesion, maybe it’s not so innocent. Maybe by treating them all at the time of the initial intervention the patient is going to do better.”
He reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Emergency department holds key to early readmissions for heart failure
SNOWMASS, COLO. – Under intense fiscal pressure to curb early hospital readmissions for heart failure, cardiologists and hospital administrators are taking a hard look at the traditional role of the emergency department as the point of triage for patients with decompensated heart failure.
“Alternatives to the emergency department for ambulatory triage and intervention are essential,” Dr. Akshay Desai said at the Annual Cardiovascular Conference at Snowmass.
“Traditionally, when our patients become decompensated, we send them from our office or clinic to the ED. And 80%-90% of those who present to the ED with the diagnosis of heart failure are admitted to the hospital. So this means that the ED is a pretty ineffective triage point for heart failure patients. Most ED staff are concerned about ambulatory follow-up and feel it’s safer to follow patients in the hospital. The message here is we need a more robust ambulatory framework to manage patients with milder decompensation so they don’t all need to come into the hospital,” said Dr. Desai of Brigham and Women’s Hospital and Harvard Medical School, Boston.
Hospital admission is of questionable value for a large fraction of decompensating heart failure patients. After all, not that much happens to them in the hospital that couldn’t take place in a less costly setting.
“For the most part, our patients in the hospital for decompensated heart failure get IV diuretics, with an average weight loss of about 4 kg. Surveillance is typically once- or twice-daily laboratory tests and a bedside clinical visit by a physician at 7:30 in the morning. Few patients get much else. There’s little diagnostic testing, few other therapies that require intensive monitoring, and most patients feel a little better by their first day in the hospital,” he said.
This suggests the need for what he called “an evolved model of heart failure care” in which the ED is replaced as the point of service by some form of ambulatory center that can serve as a buffer limiting the number of patients who need to come into the hospital.
“It could be a home-based strategy of IV diuretic administration, a clinic-based strategy of outpatient diuretic administration, or an observation unit based in the ED. All of these are now being tested in various models across the country as alternatives to help manage the readmission problem, and also to make life better for our patients, who’d prefer not to be in the hospital if they could be managed in other ways,” Dr. Desai continued.
Reducing 30-day readmission rates after a hospitalization for heart failure is seen by health policy makers as an opportunity to simultaneously improve care and reduce costs. But studies show only about half of readmissions in patients with heart failure are cardiovascular related, just half of those cardiovascular readmissions are heart failure related, and only about 30% of heart failure readmissions are truly preventable. However, wide variation exists across the country in risk-adjusted 30-day readmission rates, suggesting there is an opportunity for improvement in outlier hospitals.
Numerous factors have been linked to high heart failure readmission rates, including patient sociodemographic characteristics, comorbid conditions, and serum markers of heart failure severity. One underappreciated factor, in Dr. Desai’s view, is that readmission rates are significantly higher in hospitals that are financially and clinically resource poor, as shown in a Harvard School of Public Health analysis of Medicare claims data for more than 900,000 heart failure discharges. These resource-poor hospitals provide care for underserved populations, and they are experiencing a disproportionate burden of the financial penalties imposed for early readmission.
“As we seek to improve care for patients with heart failure, we should ensure that penalties for poor performance do not worsen disparities in quality of care,” according to the Harvard investigators (Circ. Cardiovasc. Qual. Outcomes 2011;4:53-9).
Dr. Desai reported serving as a consultant to 5AM Ventures, AtCor Medical, Novartis, and St. Jude Medical.
SNOWMASS, COLO. – Under intense fiscal pressure to curb early hospital readmissions for heart failure, cardiologists and hospital administrators are taking a hard look at the traditional role of the emergency department as the point of triage for patients with decompensated heart failure.
“Alternatives to the emergency department for ambulatory triage and intervention are essential,” Dr. Akshay Desai said at the Annual Cardiovascular Conference at Snowmass.
“Traditionally, when our patients become decompensated, we send them from our office or clinic to the ED. And 80%-90% of those who present to the ED with the diagnosis of heart failure are admitted to the hospital. So this means that the ED is a pretty ineffective triage point for heart failure patients. Most ED staff are concerned about ambulatory follow-up and feel it’s safer to follow patients in the hospital. The message here is we need a more robust ambulatory framework to manage patients with milder decompensation so they don’t all need to come into the hospital,” said Dr. Desai of Brigham and Women’s Hospital and Harvard Medical School, Boston.
Hospital admission is of questionable value for a large fraction of decompensating heart failure patients. After all, not that much happens to them in the hospital that couldn’t take place in a less costly setting.
“For the most part, our patients in the hospital for decompensated heart failure get IV diuretics, with an average weight loss of about 4 kg. Surveillance is typically once- or twice-daily laboratory tests and a bedside clinical visit by a physician at 7:30 in the morning. Few patients get much else. There’s little diagnostic testing, few other therapies that require intensive monitoring, and most patients feel a little better by their first day in the hospital,” he said.
This suggests the need for what he called “an evolved model of heart failure care” in which the ED is replaced as the point of service by some form of ambulatory center that can serve as a buffer limiting the number of patients who need to come into the hospital.
“It could be a home-based strategy of IV diuretic administration, a clinic-based strategy of outpatient diuretic administration, or an observation unit based in the ED. All of these are now being tested in various models across the country as alternatives to help manage the readmission problem, and also to make life better for our patients, who’d prefer not to be in the hospital if they could be managed in other ways,” Dr. Desai continued.
Reducing 30-day readmission rates after a hospitalization for heart failure is seen by health policy makers as an opportunity to simultaneously improve care and reduce costs. But studies show only about half of readmissions in patients with heart failure are cardiovascular related, just half of those cardiovascular readmissions are heart failure related, and only about 30% of heart failure readmissions are truly preventable. However, wide variation exists across the country in risk-adjusted 30-day readmission rates, suggesting there is an opportunity for improvement in outlier hospitals.
Numerous factors have been linked to high heart failure readmission rates, including patient sociodemographic characteristics, comorbid conditions, and serum markers of heart failure severity. One underappreciated factor, in Dr. Desai’s view, is that readmission rates are significantly higher in hospitals that are financially and clinically resource poor, as shown in a Harvard School of Public Health analysis of Medicare claims data for more than 900,000 heart failure discharges. These resource-poor hospitals provide care for underserved populations, and they are experiencing a disproportionate burden of the financial penalties imposed for early readmission.
“As we seek to improve care for patients with heart failure, we should ensure that penalties for poor performance do not worsen disparities in quality of care,” according to the Harvard investigators (Circ. Cardiovasc. Qual. Outcomes 2011;4:53-9).
Dr. Desai reported serving as a consultant to 5AM Ventures, AtCor Medical, Novartis, and St. Jude Medical.
SNOWMASS, COLO. – Under intense fiscal pressure to curb early hospital readmissions for heart failure, cardiologists and hospital administrators are taking a hard look at the traditional role of the emergency department as the point of triage for patients with decompensated heart failure.
“Alternatives to the emergency department for ambulatory triage and intervention are essential,” Dr. Akshay Desai said at the Annual Cardiovascular Conference at Snowmass.
“Traditionally, when our patients become decompensated, we send them from our office or clinic to the ED. And 80%-90% of those who present to the ED with the diagnosis of heart failure are admitted to the hospital. So this means that the ED is a pretty ineffective triage point for heart failure patients. Most ED staff are concerned about ambulatory follow-up and feel it’s safer to follow patients in the hospital. The message here is we need a more robust ambulatory framework to manage patients with milder decompensation so they don’t all need to come into the hospital,” said Dr. Desai of Brigham and Women’s Hospital and Harvard Medical School, Boston.
Hospital admission is of questionable value for a large fraction of decompensating heart failure patients. After all, not that much happens to them in the hospital that couldn’t take place in a less costly setting.
“For the most part, our patients in the hospital for decompensated heart failure get IV diuretics, with an average weight loss of about 4 kg. Surveillance is typically once- or twice-daily laboratory tests and a bedside clinical visit by a physician at 7:30 in the morning. Few patients get much else. There’s little diagnostic testing, few other therapies that require intensive monitoring, and most patients feel a little better by their first day in the hospital,” he said.
This suggests the need for what he called “an evolved model of heart failure care” in which the ED is replaced as the point of service by some form of ambulatory center that can serve as a buffer limiting the number of patients who need to come into the hospital.
“It could be a home-based strategy of IV diuretic administration, a clinic-based strategy of outpatient diuretic administration, or an observation unit based in the ED. All of these are now being tested in various models across the country as alternatives to help manage the readmission problem, and also to make life better for our patients, who’d prefer not to be in the hospital if they could be managed in other ways,” Dr. Desai continued.
Reducing 30-day readmission rates after a hospitalization for heart failure is seen by health policy makers as an opportunity to simultaneously improve care and reduce costs. But studies show only about half of readmissions in patients with heart failure are cardiovascular related, just half of those cardiovascular readmissions are heart failure related, and only about 30% of heart failure readmissions are truly preventable. However, wide variation exists across the country in risk-adjusted 30-day readmission rates, suggesting there is an opportunity for improvement in outlier hospitals.
Numerous factors have been linked to high heart failure readmission rates, including patient sociodemographic characteristics, comorbid conditions, and serum markers of heart failure severity. One underappreciated factor, in Dr. Desai’s view, is that readmission rates are significantly higher in hospitals that are financially and clinically resource poor, as shown in a Harvard School of Public Health analysis of Medicare claims data for more than 900,000 heart failure discharges. These resource-poor hospitals provide care for underserved populations, and they are experiencing a disproportionate burden of the financial penalties imposed for early readmission.
“As we seek to improve care for patients with heart failure, we should ensure that penalties for poor performance do not worsen disparities in quality of care,” according to the Harvard investigators (Circ. Cardiovasc. Qual. Outcomes 2011;4:53-9).
Dr. Desai reported serving as a consultant to 5AM Ventures, AtCor Medical, Novartis, and St. Jude Medical.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Environmental factors could increase U.S. anthrax cases
WASHINGTON– Recent isolated cases of anthrax in Minnesota and elsewhere, along with the disease’s relative ease of transmission from animals or plants to humans, should heighten U.S. physicians’ awareness of anthrax’s symptoms and treatments, warned Dr. Jason K. Blackburn.
“[Anthrax] is something that our international partners deal with on an annual basis [as] we can see the disease reemerging, or at least increasing, in annual reports on humans in a number of countries,” explained Dr. Blackburn of the University of Florida in Gainesville, at a meeting on biodefense and emerging diseases sponsored by the American Society for Microbiology. “Here in the United States, we’re seeing it shift from a livestock disease [to] a wildlife disease, where we have these populations that we can’t reach with vaccines, and where surveillance is very logistically challenging.”
Environmental factors can drive higher incidences of anthrax cases. Temperature, precipitation, and vegetation indices are key variables that facilitate anthrax transmission and spread of the disease. Geographically, lowland areas also have higher prevalences of the disease.
For example, Dr. Blackburn and his colleagues used predictive models to quantify the theory that anthrax case rates increase during years that have wet springs followed by hot, dry summers in the region of western Texas.
Using these data would allow scientists and health care providers to predict which years would have an increased risk for anthrax cases in humans, Dr. Blackburn said, and could help hospitals and clinics effectively prepare to treat a higher influx of these cases and prevent possible outbreaks.
Although relatively large numbers of human anthrax cases persist in parts of world – particularly in Africa and central Asia – cases in the United States have been primarily relegated to livestock.
However, during the last decade, there has been a noticeable shift in cases from livestock to wildlife, particularly in western Texas and Montana, where local populations of elk, bison, and white-tailed deer have been affected. The newfound prevalence in wildlife species, along with continued presence in domestic animals such as cattle and sheep, mean that transmission to humans could become even easier.
“Human cases are usually driven by direct human interaction with mammalian hosts,” said Dr. Blackburn, citing farms and meat factories as prime examples of where the Bacillus anthracis organism would easily spread. In addition, Dr. Blackburn specifically noted a scenario in which flies can transmit the disease from sheep to humans, and have also been found to carry anthrax from carcasses to wildlife and vegetation.
From 2010 to 2012, anthrax cases in Europe, particularly Georgia and Turkey, increased, compared with numbers over a similar time frame between 2000 and 2009. While case reporting can be partly attributed to this increase, Dr. Blackburn indicated that it was most likely evidence of an associative trend between livestock and human anthrax cases.
Dr. Blackburn did not report any disclosures.
WASHINGTON– Recent isolated cases of anthrax in Minnesota and elsewhere, along with the disease’s relative ease of transmission from animals or plants to humans, should heighten U.S. physicians’ awareness of anthrax’s symptoms and treatments, warned Dr. Jason K. Blackburn.
“[Anthrax] is something that our international partners deal with on an annual basis [as] we can see the disease reemerging, or at least increasing, in annual reports on humans in a number of countries,” explained Dr. Blackburn of the University of Florida in Gainesville, at a meeting on biodefense and emerging diseases sponsored by the American Society for Microbiology. “Here in the United States, we’re seeing it shift from a livestock disease [to] a wildlife disease, where we have these populations that we can’t reach with vaccines, and where surveillance is very logistically challenging.”
Environmental factors can drive higher incidences of anthrax cases. Temperature, precipitation, and vegetation indices are key variables that facilitate anthrax transmission and spread of the disease. Geographically, lowland areas also have higher prevalences of the disease.
For example, Dr. Blackburn and his colleagues used predictive models to quantify the theory that anthrax case rates increase during years that have wet springs followed by hot, dry summers in the region of western Texas.
Using these data would allow scientists and health care providers to predict which years would have an increased risk for anthrax cases in humans, Dr. Blackburn said, and could help hospitals and clinics effectively prepare to treat a higher influx of these cases and prevent possible outbreaks.
Although relatively large numbers of human anthrax cases persist in parts of world – particularly in Africa and central Asia – cases in the United States have been primarily relegated to livestock.
However, during the last decade, there has been a noticeable shift in cases from livestock to wildlife, particularly in western Texas and Montana, where local populations of elk, bison, and white-tailed deer have been affected. The newfound prevalence in wildlife species, along with continued presence in domestic animals such as cattle and sheep, mean that transmission to humans could become even easier.
“Human cases are usually driven by direct human interaction with mammalian hosts,” said Dr. Blackburn, citing farms and meat factories as prime examples of where the Bacillus anthracis organism would easily spread. In addition, Dr. Blackburn specifically noted a scenario in which flies can transmit the disease from sheep to humans, and have also been found to carry anthrax from carcasses to wildlife and vegetation.
From 2010 to 2012, anthrax cases in Europe, particularly Georgia and Turkey, increased, compared with numbers over a similar time frame between 2000 and 2009. While case reporting can be partly attributed to this increase, Dr. Blackburn indicated that it was most likely evidence of an associative trend between livestock and human anthrax cases.
Dr. Blackburn did not report any disclosures.
WASHINGTON– Recent isolated cases of anthrax in Minnesota and elsewhere, along with the disease’s relative ease of transmission from animals or plants to humans, should heighten U.S. physicians’ awareness of anthrax’s symptoms and treatments, warned Dr. Jason K. Blackburn.
“[Anthrax] is something that our international partners deal with on an annual basis [as] we can see the disease reemerging, or at least increasing, in annual reports on humans in a number of countries,” explained Dr. Blackburn of the University of Florida in Gainesville, at a meeting on biodefense and emerging diseases sponsored by the American Society for Microbiology. “Here in the United States, we’re seeing it shift from a livestock disease [to] a wildlife disease, where we have these populations that we can’t reach with vaccines, and where surveillance is very logistically challenging.”
Environmental factors can drive higher incidences of anthrax cases. Temperature, precipitation, and vegetation indices are key variables that facilitate anthrax transmission and spread of the disease. Geographically, lowland areas also have higher prevalences of the disease.
For example, Dr. Blackburn and his colleagues used predictive models to quantify the theory that anthrax case rates increase during years that have wet springs followed by hot, dry summers in the region of western Texas.
Using these data would allow scientists and health care providers to predict which years would have an increased risk for anthrax cases in humans, Dr. Blackburn said, and could help hospitals and clinics effectively prepare to treat a higher influx of these cases and prevent possible outbreaks.
Although relatively large numbers of human anthrax cases persist in parts of world – particularly in Africa and central Asia – cases in the United States have been primarily relegated to livestock.
However, during the last decade, there has been a noticeable shift in cases from livestock to wildlife, particularly in western Texas and Montana, where local populations of elk, bison, and white-tailed deer have been affected. The newfound prevalence in wildlife species, along with continued presence in domestic animals such as cattle and sheep, mean that transmission to humans could become even easier.
“Human cases are usually driven by direct human interaction with mammalian hosts,” said Dr. Blackburn, citing farms and meat factories as prime examples of where the Bacillus anthracis organism would easily spread. In addition, Dr. Blackburn specifically noted a scenario in which flies can transmit the disease from sheep to humans, and have also been found to carry anthrax from carcasses to wildlife and vegetation.
From 2010 to 2012, anthrax cases in Europe, particularly Georgia and Turkey, increased, compared with numbers over a similar time frame between 2000 and 2009. While case reporting can be partly attributed to this increase, Dr. Blackburn indicated that it was most likely evidence of an associative trend between livestock and human anthrax cases.
Dr. Blackburn did not report any disclosures.
AT THE ASM BIODEFENSE MEETING
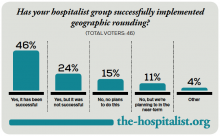
Geographic Rounding of Hospital Nurses Challenges Unit-Based Theory
Nurses, of course, have always been assigned by unit—that is, geographically. So it should come as no surprise that searching “unit-based” at the-hospitalist.org returns many articles about assigning hospitalists geographically, but not nurses, partly because few would consider it a new idea. But this article is about a new wrinkle in assigning nurses.
Although there likely are a number of hospitals doing something similar, I’ll describe a place I was lucky enough to see up close.
Bassett Medical Center
On a cold day last December, I was part of a team that spent a few days in Cooperstown, N.Y. This is a place that is so pretty that I didn’t immediately recognize we had arrived at the Bassett Medical Center Campus, since the entrance we used looked more like a library topped by a pretty cupola and warmly decorated for the holidays. We met so many nice people, including Kai Mebust, MD, FHM, who I’m convinced works full-time for the local Chamber of Commerce and tourism industry. If he doesn’t, then they should put him on their payroll.
Not long after our arrival, Dr. Mebust led us outside in the winter air without our coats to see the very beautiful view from the patio adjacent to the hospital cafeteria. And before we left for home he climbed in our car to direct us on a tour of the town. I’m sold. What a beautiful place. So much more than the Baseball Hall of Fame for which Cooperstown is known.
When not promoting his town’s tourism or enthusiastically describing his eighth-grade son playing with the Preservation Hall Jazz Band in New Orleans, he seems to find time to serve as the chief of this academic hospital’s hospital medicine practice. He was the principal engineer of the geographic assignment of nurses and describes it with an enthusiasm that matches his service as tour guide.
Geographic Care: Single RN Caring for Five Adjacent Patients
The idea is simple and best described using an illustration. A single nurse cares for five patients in adjacent rooms referred to as a “pod.” A second nurse is responsible for the next pod of five consecutive patients, and a single hospitalist cares for all 10 in both pods. There are currently four pods on a single floor of 36 beds; however, they hope to expand this system to most of the medical-surgical beds in the hospital.
The nurses eligible to care for patients in these pods are all trained to be able to provide “step down” level of care, meaning patients don’t need to transfer to a different location for more frequent monitoring and such therapies as vasopressors, mask ventilation, and the like.
Each hospitalist caring for two pods of 10 adjacent patients will typically have additional patients in other locations. This is the hospital’s way of finding the sweet spot between the competing interests of “load leveling” patient volume across hospitalists and rigidly assigning each doctor to a single location, though if they succeed in expanding the model through most of the hospital, the hospitalists will likely need to figure out how to assign themselves more rigidly to three or four pods.
Additional Components
Each morning, the hospitalist meets with the two pod nurses. They briefly discuss overnight events and plans for the day.
Much later in the morning, they also conduct daily multidisciplinary rounds involving nurse, case manager, pharmacist, dietician, social worker, respiratory therapist, and hospitalist. These follow a standard format, which is posted on the wall, and are done in a workroom that allows most participants to be in front of a computer, so they can enter notes and orders into the electronic health record (EHR) as they discuss patients.
What Is the Big Deal Here?
A lot of smart people have developed and written about systems that assign hospitalists geographically, but in most cases this has not been accompanied by adjustments in the way nurses are assigned. On nursing units at most hospitals, this means that even if a hospitalist has all of her patients on the same floor, she is still interacting with five to seven nurses caring for her patients. That usually means the hospitalist and nurse have less awareness of each other’s thinking and doing than if the ratio is reduced to no more than three or four nurses for a single hospitalist.
Dr. Mebust provided a document enumerating the goals for the program:
- Improve communication;
- Reduce patient bed moves;
- Improve patient and staff satisfaction; and
- Provide more efficient care as measured in time-of-discharge, decreased physician time-per-patient, and possibly length of stay.
Because of a number of problems teasing out the effects of this program and its limited duration to this point, Dr. Mebust and staff can’t provide robust statistics to document success in these goals. But anecdotal information is very encouraging, and clearly the nurses love it.
A major barrier to assigning nurses based rigidly on patients in adjacent rooms is the inability to ensure that each nurse has a workload of roughly equivalent complexity, but they’ve found this is a much less significant problem than feared. The nurse I spoke with said any risk of ending up with unusually complex and time-consuming patients is essentially offset by the efficiency gained by having the same attending hospitalist for all of her patients.
In fact, the nurses love it so much that they much prefer being assigned to a pod rather than a traditional assortment of patients with different attending physicians, even if the latter offers a chance to address uneven acuity.
The Big Picture
I’ve often wished that I could incorporate into hospitalist work some of the efficient ways a doctor and nurse can work together seeing scheduled patients in an outpatient setting. Surely assigning hospitalists geographically does this to some degree and has a number of advantages that others have written about. But it comes at the cost of difficult tradeoffs for hospitalists, and I know of many groups that have abandoned it after concluding that the challenges of the system exceeded its benefits.
But when it is coupled with assigning nurses geographically, I think the benefits are even greater, not only for the hospitalists, but also for patients, nurses, and other hospital staff.
Next time you’re in Cooperstown, be sure you don’t just visit the Baseball Hall of Fame. Look up Dr. Mebust, Komron Ostovar, MD, and their colleagues at Bassett Medical Center. I betcha you’ll be persuaded to see the value of their geographic model.
And maybe you’ll even fall so far under the spell of how they all talk about where they work and live that you’ll be ready to move there and join them.

Nurses, of course, have always been assigned by unit—that is, geographically. So it should come as no surprise that searching “unit-based” at the-hospitalist.org returns many articles about assigning hospitalists geographically, but not nurses, partly because few would consider it a new idea. But this article is about a new wrinkle in assigning nurses.
Although there likely are a number of hospitals doing something similar, I’ll describe a place I was lucky enough to see up close.
Bassett Medical Center
On a cold day last December, I was part of a team that spent a few days in Cooperstown, N.Y. This is a place that is so pretty that I didn’t immediately recognize we had arrived at the Bassett Medical Center Campus, since the entrance we used looked more like a library topped by a pretty cupola and warmly decorated for the holidays. We met so many nice people, including Kai Mebust, MD, FHM, who I’m convinced works full-time for the local Chamber of Commerce and tourism industry. If he doesn’t, then they should put him on their payroll.
Not long after our arrival, Dr. Mebust led us outside in the winter air without our coats to see the very beautiful view from the patio adjacent to the hospital cafeteria. And before we left for home he climbed in our car to direct us on a tour of the town. I’m sold. What a beautiful place. So much more than the Baseball Hall of Fame for which Cooperstown is known.
When not promoting his town’s tourism or enthusiastically describing his eighth-grade son playing with the Preservation Hall Jazz Band in New Orleans, he seems to find time to serve as the chief of this academic hospital’s hospital medicine practice. He was the principal engineer of the geographic assignment of nurses and describes it with an enthusiasm that matches his service as tour guide.
Geographic Care: Single RN Caring for Five Adjacent Patients
The idea is simple and best described using an illustration. A single nurse cares for five patients in adjacent rooms referred to as a “pod.” A second nurse is responsible for the next pod of five consecutive patients, and a single hospitalist cares for all 10 in both pods. There are currently four pods on a single floor of 36 beds; however, they hope to expand this system to most of the medical-surgical beds in the hospital.
The nurses eligible to care for patients in these pods are all trained to be able to provide “step down” level of care, meaning patients don’t need to transfer to a different location for more frequent monitoring and such therapies as vasopressors, mask ventilation, and the like.
Each hospitalist caring for two pods of 10 adjacent patients will typically have additional patients in other locations. This is the hospital’s way of finding the sweet spot between the competing interests of “load leveling” patient volume across hospitalists and rigidly assigning each doctor to a single location, though if they succeed in expanding the model through most of the hospital, the hospitalists will likely need to figure out how to assign themselves more rigidly to three or four pods.
Additional Components
Each morning, the hospitalist meets with the two pod nurses. They briefly discuss overnight events and plans for the day.
Much later in the morning, they also conduct daily multidisciplinary rounds involving nurse, case manager, pharmacist, dietician, social worker, respiratory therapist, and hospitalist. These follow a standard format, which is posted on the wall, and are done in a workroom that allows most participants to be in front of a computer, so they can enter notes and orders into the electronic health record (EHR) as they discuss patients.
What Is the Big Deal Here?
A lot of smart people have developed and written about systems that assign hospitalists geographically, but in most cases this has not been accompanied by adjustments in the way nurses are assigned. On nursing units at most hospitals, this means that even if a hospitalist has all of her patients on the same floor, she is still interacting with five to seven nurses caring for her patients. That usually means the hospitalist and nurse have less awareness of each other’s thinking and doing than if the ratio is reduced to no more than three or four nurses for a single hospitalist.
Dr. Mebust provided a document enumerating the goals for the program:
- Improve communication;
- Reduce patient bed moves;
- Improve patient and staff satisfaction; and
- Provide more efficient care as measured in time-of-discharge, decreased physician time-per-patient, and possibly length of stay.
Because of a number of problems teasing out the effects of this program and its limited duration to this point, Dr. Mebust and staff can’t provide robust statistics to document success in these goals. But anecdotal information is very encouraging, and clearly the nurses love it.
A major barrier to assigning nurses based rigidly on patients in adjacent rooms is the inability to ensure that each nurse has a workload of roughly equivalent complexity, but they’ve found this is a much less significant problem than feared. The nurse I spoke with said any risk of ending up with unusually complex and time-consuming patients is essentially offset by the efficiency gained by having the same attending hospitalist for all of her patients.
In fact, the nurses love it so much that they much prefer being assigned to a pod rather than a traditional assortment of patients with different attending physicians, even if the latter offers a chance to address uneven acuity.
The Big Picture
I’ve often wished that I could incorporate into hospitalist work some of the efficient ways a doctor and nurse can work together seeing scheduled patients in an outpatient setting. Surely assigning hospitalists geographically does this to some degree and has a number of advantages that others have written about. But it comes at the cost of difficult tradeoffs for hospitalists, and I know of many groups that have abandoned it after concluding that the challenges of the system exceeded its benefits.
But when it is coupled with assigning nurses geographically, I think the benefits are even greater, not only for the hospitalists, but also for patients, nurses, and other hospital staff.
Next time you’re in Cooperstown, be sure you don’t just visit the Baseball Hall of Fame. Look up Dr. Mebust, Komron Ostovar, MD, and their colleagues at Bassett Medical Center. I betcha you’ll be persuaded to see the value of their geographic model.
And maybe you’ll even fall so far under the spell of how they all talk about where they work and live that you’ll be ready to move there and join them.

Nurses, of course, have always been assigned by unit—that is, geographically. So it should come as no surprise that searching “unit-based” at the-hospitalist.org returns many articles about assigning hospitalists geographically, but not nurses, partly because few would consider it a new idea. But this article is about a new wrinkle in assigning nurses.
Although there likely are a number of hospitals doing something similar, I’ll describe a place I was lucky enough to see up close.
Bassett Medical Center
On a cold day last December, I was part of a team that spent a few days in Cooperstown, N.Y. This is a place that is so pretty that I didn’t immediately recognize we had arrived at the Bassett Medical Center Campus, since the entrance we used looked more like a library topped by a pretty cupola and warmly decorated for the holidays. We met so many nice people, including Kai Mebust, MD, FHM, who I’m convinced works full-time for the local Chamber of Commerce and tourism industry. If he doesn’t, then they should put him on their payroll.
Not long after our arrival, Dr. Mebust led us outside in the winter air without our coats to see the very beautiful view from the patio adjacent to the hospital cafeteria. And before we left for home he climbed in our car to direct us on a tour of the town. I’m sold. What a beautiful place. So much more than the Baseball Hall of Fame for which Cooperstown is known.
When not promoting his town’s tourism or enthusiastically describing his eighth-grade son playing with the Preservation Hall Jazz Band in New Orleans, he seems to find time to serve as the chief of this academic hospital’s hospital medicine practice. He was the principal engineer of the geographic assignment of nurses and describes it with an enthusiasm that matches his service as tour guide.
Geographic Care: Single RN Caring for Five Adjacent Patients
The idea is simple and best described using an illustration. A single nurse cares for five patients in adjacent rooms referred to as a “pod.” A second nurse is responsible for the next pod of five consecutive patients, and a single hospitalist cares for all 10 in both pods. There are currently four pods on a single floor of 36 beds; however, they hope to expand this system to most of the medical-surgical beds in the hospital.
The nurses eligible to care for patients in these pods are all trained to be able to provide “step down” level of care, meaning patients don’t need to transfer to a different location for more frequent monitoring and such therapies as vasopressors, mask ventilation, and the like.
Each hospitalist caring for two pods of 10 adjacent patients will typically have additional patients in other locations. This is the hospital’s way of finding the sweet spot between the competing interests of “load leveling” patient volume across hospitalists and rigidly assigning each doctor to a single location, though if they succeed in expanding the model through most of the hospital, the hospitalists will likely need to figure out how to assign themselves more rigidly to three or four pods.
Additional Components
Each morning, the hospitalist meets with the two pod nurses. They briefly discuss overnight events and plans for the day.
Much later in the morning, they also conduct daily multidisciplinary rounds involving nurse, case manager, pharmacist, dietician, social worker, respiratory therapist, and hospitalist. These follow a standard format, which is posted on the wall, and are done in a workroom that allows most participants to be in front of a computer, so they can enter notes and orders into the electronic health record (EHR) as they discuss patients.
What Is the Big Deal Here?
A lot of smart people have developed and written about systems that assign hospitalists geographically, but in most cases this has not been accompanied by adjustments in the way nurses are assigned. On nursing units at most hospitals, this means that even if a hospitalist has all of her patients on the same floor, she is still interacting with five to seven nurses caring for her patients. That usually means the hospitalist and nurse have less awareness of each other’s thinking and doing than if the ratio is reduced to no more than three or four nurses for a single hospitalist.
Dr. Mebust provided a document enumerating the goals for the program:
- Improve communication;
- Reduce patient bed moves;
- Improve patient and staff satisfaction; and
- Provide more efficient care as measured in time-of-discharge, decreased physician time-per-patient, and possibly length of stay.
Because of a number of problems teasing out the effects of this program and its limited duration to this point, Dr. Mebust and staff can’t provide robust statistics to document success in these goals. But anecdotal information is very encouraging, and clearly the nurses love it.
A major barrier to assigning nurses based rigidly on patients in adjacent rooms is the inability to ensure that each nurse has a workload of roughly equivalent complexity, but they’ve found this is a much less significant problem than feared. The nurse I spoke with said any risk of ending up with unusually complex and time-consuming patients is essentially offset by the efficiency gained by having the same attending hospitalist for all of her patients.
In fact, the nurses love it so much that they much prefer being assigned to a pod rather than a traditional assortment of patients with different attending physicians, even if the latter offers a chance to address uneven acuity.
The Big Picture
I’ve often wished that I could incorporate into hospitalist work some of the efficient ways a doctor and nurse can work together seeing scheduled patients in an outpatient setting. Surely assigning hospitalists geographically does this to some degree and has a number of advantages that others have written about. But it comes at the cost of difficult tradeoffs for hospitalists, and I know of many groups that have abandoned it after concluding that the challenges of the system exceeded its benefits.
But when it is coupled with assigning nurses geographically, I think the benefits are even greater, not only for the hospitalists, but also for patients, nurses, and other hospital staff.
Next time you’re in Cooperstown, be sure you don’t just visit the Baseball Hall of Fame. Look up Dr. Mebust, Komron Ostovar, MD, and their colleagues at Bassett Medical Center. I betcha you’ll be persuaded to see the value of their geographic model.
And maybe you’ll even fall so far under the spell of how they all talk about where they work and live that you’ll be ready to move there and join them.

Does tight control of hypertension in pregnancy produce better perinatal outcomes?
The question of degree of control of hypertension during pregnancy has been debated for many years. The primary concern, which is mainly theoretical, is that tight control of hypertension may lead to underperfusion of the uterus, ultimately resulting in fetal growth restriction. This study adds to the available body of literature on this subject.
Details of the trial
In this pragmatic randomized clinical trial, 987 women with office diastolic BP of 90 to 105 mm Hg (or 85 to 105 mm Hg if they were taking a hypertensive medication) between 14 weeks, zero days of gestation and 33 weeks, 6 days of gestation were randomized to tight (n = 488) versus less-tight control of hypertension (n = 493).
Practitioners were encouraged to use labetalol for treatment. The primary outcome was pregnancy loss (miscarriage, ectopic pregnancy, pregnancy termination, stillbirth, or neonatal death) or the need for high-level neonatal care (defined as greater than normal newborn care for more than 48 hours until 28 days of life or discharge home). Secondary outcomes included serious maternal morbidity as late as 6 weeks postpartum. Statistical analysis was based on the intent-to-treat principle.
Adherence to assigned treatment was good, at approximately 75% in each arm. As stated above, the study found no differences in the combined primary endpoint between the two groups. It also found no differences in other perinatal outcomes, including small size for gestational age or other adverse neonatal outcomes. Maternal complications generally were similar as well, with the exception of severe hypertension, which was more common in the less-tight control group.
Strengths and weaknesses of the study
This trial has several important strengths, including its pragmatic design, making it more applicable to everyday practice. Other strengths include rigorous methods and a large sample size.
Two main weaknesses hamper the study, however:
- the inclusion of both chronic hypertension and gestational hypertension. In my opinion, the much more clinically relevant question concerns women with chronic hypertension, who have a long duration of treatment.
- the choice of high-level neonatal care as part of the composite endpoint. This aspect of the composite outcome drove the endpoint in terms of numbers, but it is unclear to me what its clinical relevance is. In my opinion, it is a poor surrogate for the neonatal outcomes we really care about.
What this evidence means for practice
This study does not establish a foundation for a change in clinical practice. At best, it supports the maternal safety of less-tight control of hypertension in pregnancy. That aspect of the trial may find its way into counseling of the patient.
–George Macones, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The question of degree of control of hypertension during pregnancy has been debated for many years. The primary concern, which is mainly theoretical, is that tight control of hypertension may lead to underperfusion of the uterus, ultimately resulting in fetal growth restriction. This study adds to the available body of literature on this subject.
Details of the trial
In this pragmatic randomized clinical trial, 987 women with office diastolic BP of 90 to 105 mm Hg (or 85 to 105 mm Hg if they were taking a hypertensive medication) between 14 weeks, zero days of gestation and 33 weeks, 6 days of gestation were randomized to tight (n = 488) versus less-tight control of hypertension (n = 493).
Practitioners were encouraged to use labetalol for treatment. The primary outcome was pregnancy loss (miscarriage, ectopic pregnancy, pregnancy termination, stillbirth, or neonatal death) or the need for high-level neonatal care (defined as greater than normal newborn care for more than 48 hours until 28 days of life or discharge home). Secondary outcomes included serious maternal morbidity as late as 6 weeks postpartum. Statistical analysis was based on the intent-to-treat principle.
Adherence to assigned treatment was good, at approximately 75% in each arm. As stated above, the study found no differences in the combined primary endpoint between the two groups. It also found no differences in other perinatal outcomes, including small size for gestational age or other adverse neonatal outcomes. Maternal complications generally were similar as well, with the exception of severe hypertension, which was more common in the less-tight control group.
Strengths and weaknesses of the study
This trial has several important strengths, including its pragmatic design, making it more applicable to everyday practice. Other strengths include rigorous methods and a large sample size.
Two main weaknesses hamper the study, however:
- the inclusion of both chronic hypertension and gestational hypertension. In my opinion, the much more clinically relevant question concerns women with chronic hypertension, who have a long duration of treatment.
- the choice of high-level neonatal care as part of the composite endpoint. This aspect of the composite outcome drove the endpoint in terms of numbers, but it is unclear to me what its clinical relevance is. In my opinion, it is a poor surrogate for the neonatal outcomes we really care about.
What this evidence means for practice
This study does not establish a foundation for a change in clinical practice. At best, it supports the maternal safety of less-tight control of hypertension in pregnancy. That aspect of the trial may find its way into counseling of the patient.
–George Macones, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The question of degree of control of hypertension during pregnancy has been debated for many years. The primary concern, which is mainly theoretical, is that tight control of hypertension may lead to underperfusion of the uterus, ultimately resulting in fetal growth restriction. This study adds to the available body of literature on this subject.
Details of the trial
In this pragmatic randomized clinical trial, 987 women with office diastolic BP of 90 to 105 mm Hg (or 85 to 105 mm Hg if they were taking a hypertensive medication) between 14 weeks, zero days of gestation and 33 weeks, 6 days of gestation were randomized to tight (n = 488) versus less-tight control of hypertension (n = 493).
Practitioners were encouraged to use labetalol for treatment. The primary outcome was pregnancy loss (miscarriage, ectopic pregnancy, pregnancy termination, stillbirth, or neonatal death) or the need for high-level neonatal care (defined as greater than normal newborn care for more than 48 hours until 28 days of life or discharge home). Secondary outcomes included serious maternal morbidity as late as 6 weeks postpartum. Statistical analysis was based on the intent-to-treat principle.
Adherence to assigned treatment was good, at approximately 75% in each arm. As stated above, the study found no differences in the combined primary endpoint between the two groups. It also found no differences in other perinatal outcomes, including small size for gestational age or other adverse neonatal outcomes. Maternal complications generally were similar as well, with the exception of severe hypertension, which was more common in the less-tight control group.
Strengths and weaknesses of the study
This trial has several important strengths, including its pragmatic design, making it more applicable to everyday practice. Other strengths include rigorous methods and a large sample size.
Two main weaknesses hamper the study, however:
- the inclusion of both chronic hypertension and gestational hypertension. In my opinion, the much more clinically relevant question concerns women with chronic hypertension, who have a long duration of treatment.
- the choice of high-level neonatal care as part of the composite endpoint. This aspect of the composite outcome drove the endpoint in terms of numbers, but it is unclear to me what its clinical relevance is. In my opinion, it is a poor surrogate for the neonatal outcomes we really care about.
What this evidence means for practice
This study does not establish a foundation for a change in clinical practice. At best, it supports the maternal safety of less-tight control of hypertension in pregnancy. That aspect of the trial may find its way into counseling of the patient.
–George Macones, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Is supplemental ultrasonography a valuable addition to breast cancer screening for women with dense breasts?
Screening mammography in women with dense breasts (ie, containing more than 50% fibroglandular tissue) is challenging for two reasons:
- Compared with women with less breast density, there is decreased cancer detection (sensitivity) with screening mammography.
- Women with dense breasts have an increased lifetime risk of breast cancer.1
Because nearly half of women in the United States undergoing screening mammography have dense breasts, it is vital that we provide them with accurate and useful counseling.
The challenge of managing women with dense breasts has become complicated by the fact that 21 states have passed laws requiring that women with dense breasts be informed through scripted messages of the decreased sensitivity of screening and increased risk of cancer and advised to
discuss with their provider whether additional testing (eg, with supplemental ultrasound) should be ordered. These laws may be well-intentioned, but they are problematic.
Although there are data documenting increased cancer detection with screening ultrasonography, there are no data currently available demonstrating that this increased detection adds value by improving important outcomes like disease-specific mortality. Further, the value proposition (improved outcomes/cost) of screening ultrasonography is unknown.
In this article, Sprague and colleagues attempt to fill this void by assessing the potential benefits, harms, and cost-effectivenessof supplemental ultrasonography following a negative screening mammogram for women with dense breasts.
Through the use of validated micro-simulation modeling, they calculate that the routine use of supplemental ultrasonography in women with dense breasts might result in 0.36 fewer deaths per 1,000 women screened. Compare this to 6 fewer deaths per 1,000 women undergoing screening mammography.
Moreover, the specificity of supplemental ultrasonography in this setting is poor, with 94% of recommended biopsies yielding benign findings (ie, positive predictive value of 6%).2
What this evidence means for practice
At present, there is little evidence that routine supplemental ultrasonography improves important outcomes such as disease-specific mortality at a rational cost. However, there may be hope on the horizon: Emerging data suggest that digital tomosynthesis as a primary screening modality may improve both specificity and sensitivity, compared with mammography, in women with dense breasts.
Initial experience with tomosynthesis demonstrates both fewer callbacks and improved cancer detection in women, compared with screening mammography.3,4 However, the value proposition of this new technology will ultimately depend on a careful analysis of its effect on mortality and cost.
–Mark D. Pearlman, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. American College of Obstetricians and Gynecologists. Management of women with dense breasts diagnosed by mammography. Committee Opinion No. 625. Obstet Gynecol. 2015;125(3):750–751.
2. Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology. 2012;265(1):59–69.
3. Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
4. Skaane PA, Bandos EB, Eben IN, et al. Two-view digital breast tomosynthesis screening with synthetically reconstructed projection images: comparison with digital breast tomosynthesis with full-field digital mammographic images. Radiology. 2014;271(3):655–663.
Screening mammography in women with dense breasts (ie, containing more than 50% fibroglandular tissue) is challenging for two reasons:
- Compared with women with less breast density, there is decreased cancer detection (sensitivity) with screening mammography.
- Women with dense breasts have an increased lifetime risk of breast cancer.1
Because nearly half of women in the United States undergoing screening mammography have dense breasts, it is vital that we provide them with accurate and useful counseling.
The challenge of managing women with dense breasts has become complicated by the fact that 21 states have passed laws requiring that women with dense breasts be informed through scripted messages of the decreased sensitivity of screening and increased risk of cancer and advised to
discuss with their provider whether additional testing (eg, with supplemental ultrasound) should be ordered. These laws may be well-intentioned, but they are problematic.
Although there are data documenting increased cancer detection with screening ultrasonography, there are no data currently available demonstrating that this increased detection adds value by improving important outcomes like disease-specific mortality. Further, the value proposition (improved outcomes/cost) of screening ultrasonography is unknown.
In this article, Sprague and colleagues attempt to fill this void by assessing the potential benefits, harms, and cost-effectivenessof supplemental ultrasonography following a negative screening mammogram for women with dense breasts.
Through the use of validated micro-simulation modeling, they calculate that the routine use of supplemental ultrasonography in women with dense breasts might result in 0.36 fewer deaths per 1,000 women screened. Compare this to 6 fewer deaths per 1,000 women undergoing screening mammography.
Moreover, the specificity of supplemental ultrasonography in this setting is poor, with 94% of recommended biopsies yielding benign findings (ie, positive predictive value of 6%).2
What this evidence means for practice
At present, there is little evidence that routine supplemental ultrasonography improves important outcomes such as disease-specific mortality at a rational cost. However, there may be hope on the horizon: Emerging data suggest that digital tomosynthesis as a primary screening modality may improve both specificity and sensitivity, compared with mammography, in women with dense breasts.
Initial experience with tomosynthesis demonstrates both fewer callbacks and improved cancer detection in women, compared with screening mammography.3,4 However, the value proposition of this new technology will ultimately depend on a careful analysis of its effect on mortality and cost.
–Mark D. Pearlman, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Screening mammography in women with dense breasts (ie, containing more than 50% fibroglandular tissue) is challenging for two reasons:
- Compared with women with less breast density, there is decreased cancer detection (sensitivity) with screening mammography.
- Women with dense breasts have an increased lifetime risk of breast cancer.1
Because nearly half of women in the United States undergoing screening mammography have dense breasts, it is vital that we provide them with accurate and useful counseling.
The challenge of managing women with dense breasts has become complicated by the fact that 21 states have passed laws requiring that women with dense breasts be informed through scripted messages of the decreased sensitivity of screening and increased risk of cancer and advised to
discuss with their provider whether additional testing (eg, with supplemental ultrasound) should be ordered. These laws may be well-intentioned, but they are problematic.
Although there are data documenting increased cancer detection with screening ultrasonography, there are no data currently available demonstrating that this increased detection adds value by improving important outcomes like disease-specific mortality. Further, the value proposition (improved outcomes/cost) of screening ultrasonography is unknown.
In this article, Sprague and colleagues attempt to fill this void by assessing the potential benefits, harms, and cost-effectivenessof supplemental ultrasonography following a negative screening mammogram for women with dense breasts.
Through the use of validated micro-simulation modeling, they calculate that the routine use of supplemental ultrasonography in women with dense breasts might result in 0.36 fewer deaths per 1,000 women screened. Compare this to 6 fewer deaths per 1,000 women undergoing screening mammography.
Moreover, the specificity of supplemental ultrasonography in this setting is poor, with 94% of recommended biopsies yielding benign findings (ie, positive predictive value of 6%).2
What this evidence means for practice
At present, there is little evidence that routine supplemental ultrasonography improves important outcomes such as disease-specific mortality at a rational cost. However, there may be hope on the horizon: Emerging data suggest that digital tomosynthesis as a primary screening modality may improve both specificity and sensitivity, compared with mammography, in women with dense breasts.
Initial experience with tomosynthesis demonstrates both fewer callbacks and improved cancer detection in women, compared with screening mammography.3,4 However, the value proposition of this new technology will ultimately depend on a careful analysis of its effect on mortality and cost.
–Mark D. Pearlman, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. American College of Obstetricians and Gynecologists. Management of women with dense breasts diagnosed by mammography. Committee Opinion No. 625. Obstet Gynecol. 2015;125(3):750–751.
2. Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology. 2012;265(1):59–69.
3. Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
4. Skaane PA, Bandos EB, Eben IN, et al. Two-view digital breast tomosynthesis screening with synthetically reconstructed projection images: comparison with digital breast tomosynthesis with full-field digital mammographic images. Radiology. 2014;271(3):655–663.
1. American College of Obstetricians and Gynecologists. Management of women with dense breasts diagnosed by mammography. Committee Opinion No. 625. Obstet Gynecol. 2015;125(3):750–751.
2. Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology. 2012;265(1):59–69.
3. Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
4. Skaane PA, Bandos EB, Eben IN, et al. Two-view digital breast tomosynthesis screening with synthetically reconstructed projection images: comparison with digital breast tomosynthesis with full-field digital mammographic images. Radiology. 2014;271(3):655–663.
Dr. Andrew M. Kaunitz on prescribing systemic HT to older women
Recorded at the 2014 meeting of the North American Menopause Society
Recorded at the 2014 meeting of the North American Menopause Society
Recorded at the 2014 meeting of the North American Menopause Society
Broadly implementing stroke embolectomy faces hurdles
NASHVILLE, TENN. – Results from three randomized controlled trials presented at the International Stroke Conference, plus the outcomes from a fourth trial first reported last fall, immediately established embolectomy as standard-of-care treatment for selected patients with acute ischemic stroke.
Stroke experts interviewed during the conference, however, said that making embolectomy routinely available to most U.S. stroke patients who would be candidates for the intervention will take months, if not years.
They envision challenges involving the availability of trained interventionalists, triage of patients to the right centers, and reimbursement issues as some of the obstacles to be dealt with before endovascular embolectomy aimed at removing intracerebral-artery occlusions in acute ischemic stroke patients becomes uniformly available.
Yet another challenge will arise when stroke-treatment groups that did not participate in the trials strive to replicate the success their colleagues reported by implementing the highly streamlined systems that were used in the trials for identifying appropriate stroke patients and for delivering treatment. Those systems were cited as an important reason why those studies succeeded in producing positive outcomes when similar embolectomy trials without the same efficiencies reported just a year or two ago failed to show benefit.
“The evidence makes it standard of care, but the challenge is that our systems are not set up. This is the big thing we will all go home to work on,” said Dr. Pooja Khatri, professor of neurology and director of acute stroke at the University of Cincinnati.
“You talk to everyone at this meeting, and what they want to go home and figure out is how can we deliver this care. It’s really challenging, at a myriad of levels,” said Dr. Colin P. Derdeyn, professor of neurology and director of the Center for Stroke and Cerebrovascular Disease at Washington University in St. Louis.
Growing endovascular availability
Arguably the most critical issue in rolling out endovascular stroke interventions more broadly is scaling up the number of centers that have the staff and systems in place to perform them. Clearly, the scope of providers able to deliver this treatment currently falls substantially short of what will be needed. “It’s kind of daunting to think about the [workforce] needs,” Dr. Khatri said in a talk at the conference, which was sponsored by the American Heart Association.
“In the United States, we’ve been building out a two-tier system, with comprehensive stroke centers capable of delivering this [endovascular embolectomy] treatment” and primary stroke centers capable of administering intravenous treatment with tissue plasminogen activator (TPA), the first treatment that patients eligible for embolectomy should receive, said Dr. Jeffrey L. Saver, professor of neurology and director of the stroke center at the University of California, Los Angeles, and lead investigator for one of the new embolectomy studies.
“Work groups have suggested about 60,000 U.S. stroke patients could potentially be treated with endovascular therapy, and we’d need about 300 comprehensive stroke centers to do this.” Dr. Saver estimated the current total of U.S. comprehensive stroke centers to be 75, a number that several others at the meeting pegged as more like 80, and they also noted that some centers are endovascular ready but have not received official comprehensive stroke center certification from the Joint Commission.
The extent to which availability of U.S. embolectomy remained limited through most of 2013 was apparent in data reported at the conference by Dr. Opeolu M. Adeoye, an emergency medicine physician and medical director of the telestroke program of the University of Cincinnati. During fiscal year 2013 (Oct. 2012 to Sept. 2013), 386,144 Medicare patients either older than 65 years or totally disabled had a primary hospital discharge diagnosis of stroke; of those, 5.1% had received thrombolytic therapy with intravenous TPA and 0.8% had undergone embolectomy. In a second analysis, he looked at stroke discharges and reperfusion treatments used in the 214 U.S. acute-care hospitals currently enrolled in StrokeNet, a program begun in 2013 by the National Institute of Neurological Disorders and Stroke to organize U.S. centers interested in participating in stroke trials.
During the same period, the 214 StrokeNet hospitals discharged 44,282 Medicare eligible patients who met the same age or disability criteria, with a TPA-treatment rate of 7.9% and an endovascular treatment rate of 2.2%. Although the StrokeNet hospitals treated roughly 11% of U.S. stroke patients in the specified demographic, they administered about 20% of the reperfusion treatment, Dr. Adeoye reported. He also highlighted that the 7.9% rate of TPA treatment among the StrokeNet hospitals correlated well with prior estimates that 6%-11% of stroke patients fulfill existing criteria for TPA treatment
A wide disparity existed in the rate of reperfusion use among StrokeNet hospitals. Sixty-seven hospitals, 31% of the StrokeNet group, treated at least 20 stroke patients with TPA during the study period, while 100 (47%) of the StrokeNet hospitals treated fewer than 10 acute stroke patients. The rate of those doing embolectomies was substantially lower, with 10 hospitals (5%) doing at least 20 endovascular procedures while 90% did fewer than 10.
Although Dr. Adeoye expressed confidence that the number of U.S. centers doing embolectomy cases will “change rapidly” following the new reports documenting the efficacy of the approach, he also acknowledged the challenges of growing the number of high-volume endovascular centers.
Centers that have been equivocal about embolectomy will now start doing it in a more concerted way, he predicted, but if cases get spread out and some sites do only a few patients a year, the quality of the procedures may suffer. “The more cases a site does, the better,” he noted, adding that regions that funnel all their stroke patients to a single endovascular site “do really well.”
“Right now, many hospitals want to do everything to get [fully] reimbursed and not send their patients down the line. There is a financial incentive to build up the stroke service at every hospital,” Dr. Derdeyn noted.
Another aspect to sorting out which centers start offering endovascular treatment will be the need to locate them in a rational way, as happened with trauma centers. Until now, placement of comprehensive stroke centers often depended on hospitals developing the capability as a marketing tool, noted Dr. Larry B. Goldstein, professor of neurology and director of the stroke center at Duke University in Durham, N.C. A hospital might achieve comprehensive stroke center certification, so a second center a few blocks away then follows suit seemingly to keep pace in a public-relations battle for cachet. The result has been an irrational clustering of centers with endovascular capability. He cited the situation in Cleveland, where comprehensive centers run by the Cleveland Clinic and Case Western stand a few dozen feet apart.
Challenges for triage
An analysis published last year by Dr. Adeoye and his colleagues showed that 56% of the U.S. population lived within a 60-minute drive of a hospital able to administer endovascular stroke treatment; by air, 85% had that access (Stroke 2014;45:3019-24). For TPA, 81% of people lived within a 60-minute drive of a center able to administer intravenous lytic treatment and 97% could reach these hospitals within an hour by air. While those numbers sound promising, though, fulfilling that potential depends on getting the right patients to the right hospitals.
Patient triage is perhaps the most vexing issue created by embolectomy’s success. For at least the short term, a limited number of centers will have the staffing and capacity to deliver endovascular embolectomy on a 24/7 basis to acute ischemic stroke patients who have a proximal blockage in a large cerebral artery. The relatively small number of sites able to offer embolectomy, and the much larger number of sites able to administer thrombolytic therapy with TPA, set the stage for some possible tension, or at least confusion, within communities over where an ambulance should bring an acute ischemic stroke patient.
“In some places they have trained the EMS [emergency medical services] to recognize severe strokes that are likely to benefit [from embolectomy], and they take those patients to places with endovascular capability. But there are some states with laws against doing this. There are major issues when EMS bypasses hospitals,” Dr. Derdeyn noted. “That’s the million-dollar question: How do you identify the stroke patients [with severe strokes who would benefit from embolectomy] and get them to where they need to go.” Like Dr. Adeoye, Dr. Derdeyn believes that endovascular treatment for stroke needs to be centralized at a relatively small number of high-volume centers.
“You can imagine that the fastest way to get stroke patients treated is to have them all go to one place, but that is much easier said than done,” Dr. Khatri said.
“Stent retrievers get cerebral arteries open, but that is not the biggest challenge. For the short term, the key issue is to get the correct patients to the correct hospitals where they can be treated by the correct team,” said Dr. Mayank Goyal, professor of diagnostic imaging at the University of Calgary (Alta.) and lead investigator for two of the three trials presented at the conference.
“You need a neurologist capable of deciding whether it really is a stroke, and pretty high-level imaging to identify the large-vessel occlusions that will benefit. Acquiring a CT angiography (CTA) image of the brain is a push-button process, but figuring out what the CTA says is not push button, especially the more sophisticated perfusion CT imaging to assess collateral circulation. I don’t see this capability happening in every catheterization laboratory,” Dr. Derdeyn said in an interview.
Another issue is volume. “Telemedicine may allow broader use of [more sophisticated] imaging, but if a place is only doing 20 endovascular procedures a year, they won’t have the best outcomes. Most small hospitals that today give patients TPA see 20 cases or fewer a year, and perhaps 5 patients will have a large-vessel occlusion,” Dr. Derdeyn said.
Before the new reports documenting the safety and efficacy of endovascular treatment, “we did not have the justification to bypass primary stroke centers and take patients directly to comprehensive stroke centers,” Dr. Khatri said. Now, “there is clear evidence that patients with severe strokes should not go to the nearby primary stroke center” but instead head directly for the centers capable of performing embolectomy. But Dr. Khatri also acknowledged that a complex calculation is needed to balance the trade off: Is it better to take a stroke patient more quickly to a nearby hospital that can only start TPA and perhaps later forward the patient to an embolectomy-ready hospital, or to transport the patient somewhat further to a facility able to deliver both TPA and embolectomy?
Dr. Khatri said that, in the Cincinnati area, “we have scheduled a retreat for March to start to plan how this will happen.” Her region includes just one comprehensive stroke center that already performs endovascular treatments for stroke, at the University of Cincinnati, which sits amid 16 other hospitals that perform acute stroke care and can administer TPA. “Ambulance-based triage will be a big issue,” she predicted.
Other aspects of improved triage will be training ambulance personal to better identify the more severe stroke patients who will most likely need endovascular treatment and improving communication between ambulance crews and receiving hospitals to further speed up a process that depends on quick treatment to get the best outcomes.
The ideal is “having paramedics call and tell us what is happening [in their ambulance] and give us as much information as possible so we can start planning for the patient’s arrival. Most hospitals don’t do this now; relatively few have their system well organized,” Dr. Goyal said in an interview. A finely orchestrated emergency transport system and hospital-based stroke team was part of the program developed at the University of Calgary by Dr. Goyal and his associates and which they credited with contributing to the successful embolectomy trial they led, called ESCAPE (Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times)(N. Engl. J. Med. 2015 Feb. 11 [doi:10.1056/NEJMoa1414905]). Dr. Goyal said that he is now visiting hospitals around the world to assist them in setting up stroke-response systems that mimic what was successful in Calgary and the other centers that participated in ESCAPE.
Improving triage with better screening
A key to improved ambulance triage will be identifying a simple, evidence-based method for assessing stroke severity that ambulance personnel can use to determine what sort of care a patient needs and where the patient needs to go to. Although a couple of U.S. sites have begun pilot studies of field-based CT units that allow stroke patients to undergo imaging-based assessment in the field, clinical evaluation remains the main tool used in the ambulance.
One possible tool is the Los Angeles Motor Scale (LAMS), a stroke-assessment scoring system developed by Dr. Saver and his associates for ambulance use about a decade ago (Prehosp. Emerg. Care 2004;8:46-50). “A LAMS score of 4 or 5 [on a scale of 0-5] is a good starting point, and with time it might improve,” Dr. Goyal said.
The National Institutes of Health Stroke Scale (NIHSS) is a clinical assessment tool not designed for prehospital use, but a new analysis reported at the meeting showed value in using the NIHSS to identify stroke patients who are good candidates for endovascular treatment, further suggesting that a simple screening tool could potentially work in the ambulance to identify patients who probably need embolectomy.
The new analysis combined data from two randomized trials: The IMS (Interventional Management of Stroke) III trial, the results of which, published in early 2013, showed no incremental benefit of endovascular therapy plus TPA over TPA alone for patients with acute ischemic stroke (N. Engl. J. Med. 2013;368:893-903); and the MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) trial, the results of which, published in January, showed a significant incremental benefit from endovascular treatment – it was the first of the four studies recently reported to show this benefit (N. Engl. J. Med. 2015;372:11-20).
The combined data included all patients from both studies with a NIHSS score of at least 20, indicating a very severe stroke. This produced a total of 342 patients, of whom 191 received intravenous TPA plus endovascular treatment and 152 received only TPA. After 90 days, 24% of the patients treated with endovascular treatment and 14% of those treated only with TPA had a modified Rankin Scale score of 0-2, indicating no or limited disability, Dr. Joseph P. Broderick reported at the conference. After adjustments for age and other potential confounders, treatment with endovascular therapy produced a statistically significant 85% improvement in patients achieving an acceptable modified Rankin Scale score at 90 days, said Dr. Broderick, professor of neurology and director of the neuroscience institute at the University of Cincinnati.
“The NIHSS score is a surrogate for clot size. It is an imperfect measure, especially at lower levels, but when the score is 20 or higher it means the patient has a big clot” that will likely not fully respond to TPA but potentially will respond to embolectomy, Dr. Broderick said in an interview. “A patient with a NIHSS score of 20 or higher has about a 95% risk of having an ongoing major artery occlusion despite TPA treatment.”
“The challenge is that we don’t have a fully validated [prehospital] scoring system. Several groups are trying to create one; in the meantime we may come up with certain clinical thresholds” that can reliably guide ambulance crews on the best place to take each stroke patient, Dr. Khatri said.
Dr. Khatri has received research support from Penumbra and Genentech. Dr. Derdeyn has received honoraria from Penumbra and holds equity in Pulse Therapeutics. Dr. Saver has been a consultant to and received research support from Covidien. Dr. Adeoye has been a speaker for Genentech. Dr. Goldstein had no disclosures. Dr. Goyal has been a consultant to and received research support from Covidien and holds a patent on using CT angiography to diagnose stroke. Dr. Broderick has received research support from Genentech.
[email protected]
On Twitter @mitchelzoler
NASHVILLE, TENN. – Results from three randomized controlled trials presented at the International Stroke Conference, plus the outcomes from a fourth trial first reported last fall, immediately established embolectomy as standard-of-care treatment for selected patients with acute ischemic stroke.
Stroke experts interviewed during the conference, however, said that making embolectomy routinely available to most U.S. stroke patients who would be candidates for the intervention will take months, if not years.
They envision challenges involving the availability of trained interventionalists, triage of patients to the right centers, and reimbursement issues as some of the obstacles to be dealt with before endovascular embolectomy aimed at removing intracerebral-artery occlusions in acute ischemic stroke patients becomes uniformly available.
Yet another challenge will arise when stroke-treatment groups that did not participate in the trials strive to replicate the success their colleagues reported by implementing the highly streamlined systems that were used in the trials for identifying appropriate stroke patients and for delivering treatment. Those systems were cited as an important reason why those studies succeeded in producing positive outcomes when similar embolectomy trials without the same efficiencies reported just a year or two ago failed to show benefit.
“The evidence makes it standard of care, but the challenge is that our systems are not set up. This is the big thing we will all go home to work on,” said Dr. Pooja Khatri, professor of neurology and director of acute stroke at the University of Cincinnati.
“You talk to everyone at this meeting, and what they want to go home and figure out is how can we deliver this care. It’s really challenging, at a myriad of levels,” said Dr. Colin P. Derdeyn, professor of neurology and director of the Center for Stroke and Cerebrovascular Disease at Washington University in St. Louis.
Growing endovascular availability
Arguably the most critical issue in rolling out endovascular stroke interventions more broadly is scaling up the number of centers that have the staff and systems in place to perform them. Clearly, the scope of providers able to deliver this treatment currently falls substantially short of what will be needed. “It’s kind of daunting to think about the [workforce] needs,” Dr. Khatri said in a talk at the conference, which was sponsored by the American Heart Association.
“In the United States, we’ve been building out a two-tier system, with comprehensive stroke centers capable of delivering this [endovascular embolectomy] treatment” and primary stroke centers capable of administering intravenous treatment with tissue plasminogen activator (TPA), the first treatment that patients eligible for embolectomy should receive, said Dr. Jeffrey L. Saver, professor of neurology and director of the stroke center at the University of California, Los Angeles, and lead investigator for one of the new embolectomy studies.
“Work groups have suggested about 60,000 U.S. stroke patients could potentially be treated with endovascular therapy, and we’d need about 300 comprehensive stroke centers to do this.” Dr. Saver estimated the current total of U.S. comprehensive stroke centers to be 75, a number that several others at the meeting pegged as more like 80, and they also noted that some centers are endovascular ready but have not received official comprehensive stroke center certification from the Joint Commission.
The extent to which availability of U.S. embolectomy remained limited through most of 2013 was apparent in data reported at the conference by Dr. Opeolu M. Adeoye, an emergency medicine physician and medical director of the telestroke program of the University of Cincinnati. During fiscal year 2013 (Oct. 2012 to Sept. 2013), 386,144 Medicare patients either older than 65 years or totally disabled had a primary hospital discharge diagnosis of stroke; of those, 5.1% had received thrombolytic therapy with intravenous TPA and 0.8% had undergone embolectomy. In a second analysis, he looked at stroke discharges and reperfusion treatments used in the 214 U.S. acute-care hospitals currently enrolled in StrokeNet, a program begun in 2013 by the National Institute of Neurological Disorders and Stroke to organize U.S. centers interested in participating in stroke trials.
During the same period, the 214 StrokeNet hospitals discharged 44,282 Medicare eligible patients who met the same age or disability criteria, with a TPA-treatment rate of 7.9% and an endovascular treatment rate of 2.2%. Although the StrokeNet hospitals treated roughly 11% of U.S. stroke patients in the specified demographic, they administered about 20% of the reperfusion treatment, Dr. Adeoye reported. He also highlighted that the 7.9% rate of TPA treatment among the StrokeNet hospitals correlated well with prior estimates that 6%-11% of stroke patients fulfill existing criteria for TPA treatment
A wide disparity existed in the rate of reperfusion use among StrokeNet hospitals. Sixty-seven hospitals, 31% of the StrokeNet group, treated at least 20 stroke patients with TPA during the study period, while 100 (47%) of the StrokeNet hospitals treated fewer than 10 acute stroke patients. The rate of those doing embolectomies was substantially lower, with 10 hospitals (5%) doing at least 20 endovascular procedures while 90% did fewer than 10.
Although Dr. Adeoye expressed confidence that the number of U.S. centers doing embolectomy cases will “change rapidly” following the new reports documenting the efficacy of the approach, he also acknowledged the challenges of growing the number of high-volume endovascular centers.
Centers that have been equivocal about embolectomy will now start doing it in a more concerted way, he predicted, but if cases get spread out and some sites do only a few patients a year, the quality of the procedures may suffer. “The more cases a site does, the better,” he noted, adding that regions that funnel all their stroke patients to a single endovascular site “do really well.”
“Right now, many hospitals want to do everything to get [fully] reimbursed and not send their patients down the line. There is a financial incentive to build up the stroke service at every hospital,” Dr. Derdeyn noted.
Another aspect to sorting out which centers start offering endovascular treatment will be the need to locate them in a rational way, as happened with trauma centers. Until now, placement of comprehensive stroke centers often depended on hospitals developing the capability as a marketing tool, noted Dr. Larry B. Goldstein, professor of neurology and director of the stroke center at Duke University in Durham, N.C. A hospital might achieve comprehensive stroke center certification, so a second center a few blocks away then follows suit seemingly to keep pace in a public-relations battle for cachet. The result has been an irrational clustering of centers with endovascular capability. He cited the situation in Cleveland, where comprehensive centers run by the Cleveland Clinic and Case Western stand a few dozen feet apart.
Challenges for triage
An analysis published last year by Dr. Adeoye and his colleagues showed that 56% of the U.S. population lived within a 60-minute drive of a hospital able to administer endovascular stroke treatment; by air, 85% had that access (Stroke 2014;45:3019-24). For TPA, 81% of people lived within a 60-minute drive of a center able to administer intravenous lytic treatment and 97% could reach these hospitals within an hour by air. While those numbers sound promising, though, fulfilling that potential depends on getting the right patients to the right hospitals.
Patient triage is perhaps the most vexing issue created by embolectomy’s success. For at least the short term, a limited number of centers will have the staffing and capacity to deliver endovascular embolectomy on a 24/7 basis to acute ischemic stroke patients who have a proximal blockage in a large cerebral artery. The relatively small number of sites able to offer embolectomy, and the much larger number of sites able to administer thrombolytic therapy with TPA, set the stage for some possible tension, or at least confusion, within communities over where an ambulance should bring an acute ischemic stroke patient.
“In some places they have trained the EMS [emergency medical services] to recognize severe strokes that are likely to benefit [from embolectomy], and they take those patients to places with endovascular capability. But there are some states with laws against doing this. There are major issues when EMS bypasses hospitals,” Dr. Derdeyn noted. “That’s the million-dollar question: How do you identify the stroke patients [with severe strokes who would benefit from embolectomy] and get them to where they need to go.” Like Dr. Adeoye, Dr. Derdeyn believes that endovascular treatment for stroke needs to be centralized at a relatively small number of high-volume centers.
“You can imagine that the fastest way to get stroke patients treated is to have them all go to one place, but that is much easier said than done,” Dr. Khatri said.
“Stent retrievers get cerebral arteries open, but that is not the biggest challenge. For the short term, the key issue is to get the correct patients to the correct hospitals where they can be treated by the correct team,” said Dr. Mayank Goyal, professor of diagnostic imaging at the University of Calgary (Alta.) and lead investigator for two of the three trials presented at the conference.
“You need a neurologist capable of deciding whether it really is a stroke, and pretty high-level imaging to identify the large-vessel occlusions that will benefit. Acquiring a CT angiography (CTA) image of the brain is a push-button process, but figuring out what the CTA says is not push button, especially the more sophisticated perfusion CT imaging to assess collateral circulation. I don’t see this capability happening in every catheterization laboratory,” Dr. Derdeyn said in an interview.
Another issue is volume. “Telemedicine may allow broader use of [more sophisticated] imaging, but if a place is only doing 20 endovascular procedures a year, they won’t have the best outcomes. Most small hospitals that today give patients TPA see 20 cases or fewer a year, and perhaps 5 patients will have a large-vessel occlusion,” Dr. Derdeyn said.
Before the new reports documenting the safety and efficacy of endovascular treatment, “we did not have the justification to bypass primary stroke centers and take patients directly to comprehensive stroke centers,” Dr. Khatri said. Now, “there is clear evidence that patients with severe strokes should not go to the nearby primary stroke center” but instead head directly for the centers capable of performing embolectomy. But Dr. Khatri also acknowledged that a complex calculation is needed to balance the trade off: Is it better to take a stroke patient more quickly to a nearby hospital that can only start TPA and perhaps later forward the patient to an embolectomy-ready hospital, or to transport the patient somewhat further to a facility able to deliver both TPA and embolectomy?
Dr. Khatri said that, in the Cincinnati area, “we have scheduled a retreat for March to start to plan how this will happen.” Her region includes just one comprehensive stroke center that already performs endovascular treatments for stroke, at the University of Cincinnati, which sits amid 16 other hospitals that perform acute stroke care and can administer TPA. “Ambulance-based triage will be a big issue,” she predicted.
Other aspects of improved triage will be training ambulance personal to better identify the more severe stroke patients who will most likely need endovascular treatment and improving communication between ambulance crews and receiving hospitals to further speed up a process that depends on quick treatment to get the best outcomes.
The ideal is “having paramedics call and tell us what is happening [in their ambulance] and give us as much information as possible so we can start planning for the patient’s arrival. Most hospitals don’t do this now; relatively few have their system well organized,” Dr. Goyal said in an interview. A finely orchestrated emergency transport system and hospital-based stroke team was part of the program developed at the University of Calgary by Dr. Goyal and his associates and which they credited with contributing to the successful embolectomy trial they led, called ESCAPE (Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times)(N. Engl. J. Med. 2015 Feb. 11 [doi:10.1056/NEJMoa1414905]). Dr. Goyal said that he is now visiting hospitals around the world to assist them in setting up stroke-response systems that mimic what was successful in Calgary and the other centers that participated in ESCAPE.
Improving triage with better screening
A key to improved ambulance triage will be identifying a simple, evidence-based method for assessing stroke severity that ambulance personnel can use to determine what sort of care a patient needs and where the patient needs to go to. Although a couple of U.S. sites have begun pilot studies of field-based CT units that allow stroke patients to undergo imaging-based assessment in the field, clinical evaluation remains the main tool used in the ambulance.
One possible tool is the Los Angeles Motor Scale (LAMS), a stroke-assessment scoring system developed by Dr. Saver and his associates for ambulance use about a decade ago (Prehosp. Emerg. Care 2004;8:46-50). “A LAMS score of 4 or 5 [on a scale of 0-5] is a good starting point, and with time it might improve,” Dr. Goyal said.
The National Institutes of Health Stroke Scale (NIHSS) is a clinical assessment tool not designed for prehospital use, but a new analysis reported at the meeting showed value in using the NIHSS to identify stroke patients who are good candidates for endovascular treatment, further suggesting that a simple screening tool could potentially work in the ambulance to identify patients who probably need embolectomy.
The new analysis combined data from two randomized trials: The IMS (Interventional Management of Stroke) III trial, the results of which, published in early 2013, showed no incremental benefit of endovascular therapy plus TPA over TPA alone for patients with acute ischemic stroke (N. Engl. J. Med. 2013;368:893-903); and the MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) trial, the results of which, published in January, showed a significant incremental benefit from endovascular treatment – it was the first of the four studies recently reported to show this benefit (N. Engl. J. Med. 2015;372:11-20).
The combined data included all patients from both studies with a NIHSS score of at least 20, indicating a very severe stroke. This produced a total of 342 patients, of whom 191 received intravenous TPA plus endovascular treatment and 152 received only TPA. After 90 days, 24% of the patients treated with endovascular treatment and 14% of those treated only with TPA had a modified Rankin Scale score of 0-2, indicating no or limited disability, Dr. Joseph P. Broderick reported at the conference. After adjustments for age and other potential confounders, treatment with endovascular therapy produced a statistically significant 85% improvement in patients achieving an acceptable modified Rankin Scale score at 90 days, said Dr. Broderick, professor of neurology and director of the neuroscience institute at the University of Cincinnati.
“The NIHSS score is a surrogate for clot size. It is an imperfect measure, especially at lower levels, but when the score is 20 or higher it means the patient has a big clot” that will likely not fully respond to TPA but potentially will respond to embolectomy, Dr. Broderick said in an interview. “A patient with a NIHSS score of 20 or higher has about a 95% risk of having an ongoing major artery occlusion despite TPA treatment.”
“The challenge is that we don’t have a fully validated [prehospital] scoring system. Several groups are trying to create one; in the meantime we may come up with certain clinical thresholds” that can reliably guide ambulance crews on the best place to take each stroke patient, Dr. Khatri said.
Dr. Khatri has received research support from Penumbra and Genentech. Dr. Derdeyn has received honoraria from Penumbra and holds equity in Pulse Therapeutics. Dr. Saver has been a consultant to and received research support from Covidien. Dr. Adeoye has been a speaker for Genentech. Dr. Goldstein had no disclosures. Dr. Goyal has been a consultant to and received research support from Covidien and holds a patent on using CT angiography to diagnose stroke. Dr. Broderick has received research support from Genentech.
[email protected]
On Twitter @mitchelzoler
NASHVILLE, TENN. – Results from three randomized controlled trials presented at the International Stroke Conference, plus the outcomes from a fourth trial first reported last fall, immediately established embolectomy as standard-of-care treatment for selected patients with acute ischemic stroke.
Stroke experts interviewed during the conference, however, said that making embolectomy routinely available to most U.S. stroke patients who would be candidates for the intervention will take months, if not years.
They envision challenges involving the availability of trained interventionalists, triage of patients to the right centers, and reimbursement issues as some of the obstacles to be dealt with before endovascular embolectomy aimed at removing intracerebral-artery occlusions in acute ischemic stroke patients becomes uniformly available.
Yet another challenge will arise when stroke-treatment groups that did not participate in the trials strive to replicate the success their colleagues reported by implementing the highly streamlined systems that were used in the trials for identifying appropriate stroke patients and for delivering treatment. Those systems were cited as an important reason why those studies succeeded in producing positive outcomes when similar embolectomy trials without the same efficiencies reported just a year or two ago failed to show benefit.
“The evidence makes it standard of care, but the challenge is that our systems are not set up. This is the big thing we will all go home to work on,” said Dr. Pooja Khatri, professor of neurology and director of acute stroke at the University of Cincinnati.
“You talk to everyone at this meeting, and what they want to go home and figure out is how can we deliver this care. It’s really challenging, at a myriad of levels,” said Dr. Colin P. Derdeyn, professor of neurology and director of the Center for Stroke and Cerebrovascular Disease at Washington University in St. Louis.
Growing endovascular availability
Arguably the most critical issue in rolling out endovascular stroke interventions more broadly is scaling up the number of centers that have the staff and systems in place to perform them. Clearly, the scope of providers able to deliver this treatment currently falls substantially short of what will be needed. “It’s kind of daunting to think about the [workforce] needs,” Dr. Khatri said in a talk at the conference, which was sponsored by the American Heart Association.
“In the United States, we’ve been building out a two-tier system, with comprehensive stroke centers capable of delivering this [endovascular embolectomy] treatment” and primary stroke centers capable of administering intravenous treatment with tissue plasminogen activator (TPA), the first treatment that patients eligible for embolectomy should receive, said Dr. Jeffrey L. Saver, professor of neurology and director of the stroke center at the University of California, Los Angeles, and lead investigator for one of the new embolectomy studies.
“Work groups have suggested about 60,000 U.S. stroke patients could potentially be treated with endovascular therapy, and we’d need about 300 comprehensive stroke centers to do this.” Dr. Saver estimated the current total of U.S. comprehensive stroke centers to be 75, a number that several others at the meeting pegged as more like 80, and they also noted that some centers are endovascular ready but have not received official comprehensive stroke center certification from the Joint Commission.
The extent to which availability of U.S. embolectomy remained limited through most of 2013 was apparent in data reported at the conference by Dr. Opeolu M. Adeoye, an emergency medicine physician and medical director of the telestroke program of the University of Cincinnati. During fiscal year 2013 (Oct. 2012 to Sept. 2013), 386,144 Medicare patients either older than 65 years or totally disabled had a primary hospital discharge diagnosis of stroke; of those, 5.1% had received thrombolytic therapy with intravenous TPA and 0.8% had undergone embolectomy. In a second analysis, he looked at stroke discharges and reperfusion treatments used in the 214 U.S. acute-care hospitals currently enrolled in StrokeNet, a program begun in 2013 by the National Institute of Neurological Disorders and Stroke to organize U.S. centers interested in participating in stroke trials.
During the same period, the 214 StrokeNet hospitals discharged 44,282 Medicare eligible patients who met the same age or disability criteria, with a TPA-treatment rate of 7.9% and an endovascular treatment rate of 2.2%. Although the StrokeNet hospitals treated roughly 11% of U.S. stroke patients in the specified demographic, they administered about 20% of the reperfusion treatment, Dr. Adeoye reported. He also highlighted that the 7.9% rate of TPA treatment among the StrokeNet hospitals correlated well with prior estimates that 6%-11% of stroke patients fulfill existing criteria for TPA treatment
A wide disparity existed in the rate of reperfusion use among StrokeNet hospitals. Sixty-seven hospitals, 31% of the StrokeNet group, treated at least 20 stroke patients with TPA during the study period, while 100 (47%) of the StrokeNet hospitals treated fewer than 10 acute stroke patients. The rate of those doing embolectomies was substantially lower, with 10 hospitals (5%) doing at least 20 endovascular procedures while 90% did fewer than 10.
Although Dr. Adeoye expressed confidence that the number of U.S. centers doing embolectomy cases will “change rapidly” following the new reports documenting the efficacy of the approach, he also acknowledged the challenges of growing the number of high-volume endovascular centers.
Centers that have been equivocal about embolectomy will now start doing it in a more concerted way, he predicted, but if cases get spread out and some sites do only a few patients a year, the quality of the procedures may suffer. “The more cases a site does, the better,” he noted, adding that regions that funnel all their stroke patients to a single endovascular site “do really well.”
“Right now, many hospitals want to do everything to get [fully] reimbursed and not send their patients down the line. There is a financial incentive to build up the stroke service at every hospital,” Dr. Derdeyn noted.
Another aspect to sorting out which centers start offering endovascular treatment will be the need to locate them in a rational way, as happened with trauma centers. Until now, placement of comprehensive stroke centers often depended on hospitals developing the capability as a marketing tool, noted Dr. Larry B. Goldstein, professor of neurology and director of the stroke center at Duke University in Durham, N.C. A hospital might achieve comprehensive stroke center certification, so a second center a few blocks away then follows suit seemingly to keep pace in a public-relations battle for cachet. The result has been an irrational clustering of centers with endovascular capability. He cited the situation in Cleveland, where comprehensive centers run by the Cleveland Clinic and Case Western stand a few dozen feet apart.
Challenges for triage
An analysis published last year by Dr. Adeoye and his colleagues showed that 56% of the U.S. population lived within a 60-minute drive of a hospital able to administer endovascular stroke treatment; by air, 85% had that access (Stroke 2014;45:3019-24). For TPA, 81% of people lived within a 60-minute drive of a center able to administer intravenous lytic treatment and 97% could reach these hospitals within an hour by air. While those numbers sound promising, though, fulfilling that potential depends on getting the right patients to the right hospitals.
Patient triage is perhaps the most vexing issue created by embolectomy’s success. For at least the short term, a limited number of centers will have the staffing and capacity to deliver endovascular embolectomy on a 24/7 basis to acute ischemic stroke patients who have a proximal blockage in a large cerebral artery. The relatively small number of sites able to offer embolectomy, and the much larger number of sites able to administer thrombolytic therapy with TPA, set the stage for some possible tension, or at least confusion, within communities over where an ambulance should bring an acute ischemic stroke patient.
“In some places they have trained the EMS [emergency medical services] to recognize severe strokes that are likely to benefit [from embolectomy], and they take those patients to places with endovascular capability. But there are some states with laws against doing this. There are major issues when EMS bypasses hospitals,” Dr. Derdeyn noted. “That’s the million-dollar question: How do you identify the stroke patients [with severe strokes who would benefit from embolectomy] and get them to where they need to go.” Like Dr. Adeoye, Dr. Derdeyn believes that endovascular treatment for stroke needs to be centralized at a relatively small number of high-volume centers.
“You can imagine that the fastest way to get stroke patients treated is to have them all go to one place, but that is much easier said than done,” Dr. Khatri said.
“Stent retrievers get cerebral arteries open, but that is not the biggest challenge. For the short term, the key issue is to get the correct patients to the correct hospitals where they can be treated by the correct team,” said Dr. Mayank Goyal, professor of diagnostic imaging at the University of Calgary (Alta.) and lead investigator for two of the three trials presented at the conference.
“You need a neurologist capable of deciding whether it really is a stroke, and pretty high-level imaging to identify the large-vessel occlusions that will benefit. Acquiring a CT angiography (CTA) image of the brain is a push-button process, but figuring out what the CTA says is not push button, especially the more sophisticated perfusion CT imaging to assess collateral circulation. I don’t see this capability happening in every catheterization laboratory,” Dr. Derdeyn said in an interview.
Another issue is volume. “Telemedicine may allow broader use of [more sophisticated] imaging, but if a place is only doing 20 endovascular procedures a year, they won’t have the best outcomes. Most small hospitals that today give patients TPA see 20 cases or fewer a year, and perhaps 5 patients will have a large-vessel occlusion,” Dr. Derdeyn said.
Before the new reports documenting the safety and efficacy of endovascular treatment, “we did not have the justification to bypass primary stroke centers and take patients directly to comprehensive stroke centers,” Dr. Khatri said. Now, “there is clear evidence that patients with severe strokes should not go to the nearby primary stroke center” but instead head directly for the centers capable of performing embolectomy. But Dr. Khatri also acknowledged that a complex calculation is needed to balance the trade off: Is it better to take a stroke patient more quickly to a nearby hospital that can only start TPA and perhaps later forward the patient to an embolectomy-ready hospital, or to transport the patient somewhat further to a facility able to deliver both TPA and embolectomy?
Dr. Khatri said that, in the Cincinnati area, “we have scheduled a retreat for March to start to plan how this will happen.” Her region includes just one comprehensive stroke center that already performs endovascular treatments for stroke, at the University of Cincinnati, which sits amid 16 other hospitals that perform acute stroke care and can administer TPA. “Ambulance-based triage will be a big issue,” she predicted.
Other aspects of improved triage will be training ambulance personal to better identify the more severe stroke patients who will most likely need endovascular treatment and improving communication between ambulance crews and receiving hospitals to further speed up a process that depends on quick treatment to get the best outcomes.
The ideal is “having paramedics call and tell us what is happening [in their ambulance] and give us as much information as possible so we can start planning for the patient’s arrival. Most hospitals don’t do this now; relatively few have their system well organized,” Dr. Goyal said in an interview. A finely orchestrated emergency transport system and hospital-based stroke team was part of the program developed at the University of Calgary by Dr. Goyal and his associates and which they credited with contributing to the successful embolectomy trial they led, called ESCAPE (Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times)(N. Engl. J. Med. 2015 Feb. 11 [doi:10.1056/NEJMoa1414905]). Dr. Goyal said that he is now visiting hospitals around the world to assist them in setting up stroke-response systems that mimic what was successful in Calgary and the other centers that participated in ESCAPE.
Improving triage with better screening
A key to improved ambulance triage will be identifying a simple, evidence-based method for assessing stroke severity that ambulance personnel can use to determine what sort of care a patient needs and where the patient needs to go to. Although a couple of U.S. sites have begun pilot studies of field-based CT units that allow stroke patients to undergo imaging-based assessment in the field, clinical evaluation remains the main tool used in the ambulance.
One possible tool is the Los Angeles Motor Scale (LAMS), a stroke-assessment scoring system developed by Dr. Saver and his associates for ambulance use about a decade ago (Prehosp. Emerg. Care 2004;8:46-50). “A LAMS score of 4 or 5 [on a scale of 0-5] is a good starting point, and with time it might improve,” Dr. Goyal said.
The National Institutes of Health Stroke Scale (NIHSS) is a clinical assessment tool not designed for prehospital use, but a new analysis reported at the meeting showed value in using the NIHSS to identify stroke patients who are good candidates for endovascular treatment, further suggesting that a simple screening tool could potentially work in the ambulance to identify patients who probably need embolectomy.
The new analysis combined data from two randomized trials: The IMS (Interventional Management of Stroke) III trial, the results of which, published in early 2013, showed no incremental benefit of endovascular therapy plus TPA over TPA alone for patients with acute ischemic stroke (N. Engl. J. Med. 2013;368:893-903); and the MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) trial, the results of which, published in January, showed a significant incremental benefit from endovascular treatment – it was the first of the four studies recently reported to show this benefit (N. Engl. J. Med. 2015;372:11-20).
The combined data included all patients from both studies with a NIHSS score of at least 20, indicating a very severe stroke. This produced a total of 342 patients, of whom 191 received intravenous TPA plus endovascular treatment and 152 received only TPA. After 90 days, 24% of the patients treated with endovascular treatment and 14% of those treated only with TPA had a modified Rankin Scale score of 0-2, indicating no or limited disability, Dr. Joseph P. Broderick reported at the conference. After adjustments for age and other potential confounders, treatment with endovascular therapy produced a statistically significant 85% improvement in patients achieving an acceptable modified Rankin Scale score at 90 days, said Dr. Broderick, professor of neurology and director of the neuroscience institute at the University of Cincinnati.
“The NIHSS score is a surrogate for clot size. It is an imperfect measure, especially at lower levels, but when the score is 20 or higher it means the patient has a big clot” that will likely not fully respond to TPA but potentially will respond to embolectomy, Dr. Broderick said in an interview. “A patient with a NIHSS score of 20 or higher has about a 95% risk of having an ongoing major artery occlusion despite TPA treatment.”
“The challenge is that we don’t have a fully validated [prehospital] scoring system. Several groups are trying to create one; in the meantime we may come up with certain clinical thresholds” that can reliably guide ambulance crews on the best place to take each stroke patient, Dr. Khatri said.
Dr. Khatri has received research support from Penumbra and Genentech. Dr. Derdeyn has received honoraria from Penumbra and holds equity in Pulse Therapeutics. Dr. Saver has been a consultant to and received research support from Covidien. Dr. Adeoye has been a speaker for Genentech. Dr. Goldstein had no disclosures. Dr. Goyal has been a consultant to and received research support from Covidien and holds a patent on using CT angiography to diagnose stroke. Dr. Broderick has received research support from Genentech.
[email protected]
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE INTERNATIONAL STROKE CONFERENCE
How to halt ‘a heart attack of the finger’
SNOWMASS, COLO. – Persistent, widespread, intense ischemic pain in the vicinity of a digital ulcer in a patient with secondary Raynaud’s phenomenon signals impending tissue infarction and gangrene, Dr. Fredrick M. Wigley warned at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“When you see this you’ve got a heart attack of the finger occurring. It’s a medical emergency,” emphasized Dr. Wigley, professor of medicine, associate head of rheumatology, and director of the scleroderma center at Johns Hopkins University, Baltimore.
The pain associated with this condition is severe enough that opiates are often required. Therefore, one of Dr. Wigley’s first moves in the office is often to administer a digital block for immediate pain relief. He sticks the needle into the web of the finger and infiltrates 2% lidocaine. It’s an easy procedure to do, and it often breaks the acute event.
If the digital block doesn’t succeed in terminating the ischemic event, however, then his go-to therapy is prostacyclin infusion.
“We’re very keen at our center, and at other centers around the country as well, to go right to a prostacyclin analogue. You can do that for prevention or during an acute event. Set up a peripheral line, run in prostacyclin for 3-5 days, and it has a magical effect that lasts for 10-12 weeks. It can really break an ischemic event quickly and may also have some preventive benefit. What we have available in the U.S. is epoprostenol. In can be administered on an inpatient basis, but we set it up as outpatient therapy. Patients come in for an 8-hour period for 3-5 days in a row,” the rheumatologist explained.
This is off-label therapy. Epoprostenol’s approved indication is in treating pulmonary arterial hypertension. But there is persuasive evidence of the effectiveness of epoprostenol (Flolan) and the prostacyclins available in other countries in aborting acute digital ischemic events in patients with Raynaud’s, he said.
One prostacyclin, treprostinil, is now available as Orenitram in an oral formulation approved for treatment of pulmonary arterial hypertension. Other oral prostacyclins are in the developmental pipeline. Despite their promise of much greater patient convenience, however, at this point their use in patients with acute digital critical ischemia isn’t supported by evidence.
During those multiple, daylong outpatient treatment sessions, Dr. Wigley emphasizes the importance of staying warm, resting, and avoiding stress. He also makes sure the patient is on an optimal vasodilatory medication program, the linchpin of which is a long-acting calcium channel blocker titrated to the maximum tolerated dose.
He also does special testing in search of a potential obstruction in a larger upstream vessel that might be amenable to a corrective procedure. These tests include Doppler ultrasound, the Allen’s test, magnetic resonance angiography, and/or digital subtraction x-ray.
This multiday period of prostacyclin therapy is an excellent opportunity to initiate the use of vasculoprotective medications. The ones he’s found most helpful include statins, low-dose aspirin or another antiplatelet agent, antioxidants, and in the case of a suspected early thrombotic or embolic event, 24-72 hours of low-molecular-weight or unfractionated heparin.
There is clinical trial evidence that bosentan (Tracleer) reduces the frequency of recurrent digital ulcers. Dr. Wigley rarely uses it, however, because it’s expensive and has lots of toxicity.
Surgical sympathectomy can be finger saving.
“Don’t dillydally,” urged Dr. Wigley. “If your patient is not responding to medical therapy, don’t say, ‘Come back in a few weeks, and we’ll see how you’re doing.’ You’ll lose the finger.”
Sympathectomy has several salutary effects: the procedure rips sympathetic nerve fibers from the over-sensitive blood vessels and also gets rid of fibrous material entrapping the vessels.
In a meta-analysis of studies that included 511 proximal sympathectomies in 449 patients with secondary Raynaud’s, 89% reported sustained improvement (J. Vasc. Surg. 2011;54:273-7). The available data on the less morbid alternative digital sympathectomy procedure are less extensive, but the rates of complete healing and/or decrease in digital ulcers are impressive.
Remember: Avoid tunnel vision, Dr. Wigley cautioned. Always consider the likely possibility of macrovascular disease in the setting of lower-extremity digital ischemia. Measurement of the ankle-brachial pressure index is considered a mandatory part of the clinical work-up in these patients. An ankle-brachial index of less than 0.9 has 95% sensitivity for the presence of angiographically evident cardiovascular disease.
Dr. Wigley reported serving as a consultant to Novartis and United Therapeutics and receiving research grants from Kinemed, MedImmune, and CSL Behring.
SNOWMASS, COLO. – Persistent, widespread, intense ischemic pain in the vicinity of a digital ulcer in a patient with secondary Raynaud’s phenomenon signals impending tissue infarction and gangrene, Dr. Fredrick M. Wigley warned at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“When you see this you’ve got a heart attack of the finger occurring. It’s a medical emergency,” emphasized Dr. Wigley, professor of medicine, associate head of rheumatology, and director of the scleroderma center at Johns Hopkins University, Baltimore.
The pain associated with this condition is severe enough that opiates are often required. Therefore, one of Dr. Wigley’s first moves in the office is often to administer a digital block for immediate pain relief. He sticks the needle into the web of the finger and infiltrates 2% lidocaine. It’s an easy procedure to do, and it often breaks the acute event.
If the digital block doesn’t succeed in terminating the ischemic event, however, then his go-to therapy is prostacyclin infusion.
“We’re very keen at our center, and at other centers around the country as well, to go right to a prostacyclin analogue. You can do that for prevention or during an acute event. Set up a peripheral line, run in prostacyclin for 3-5 days, and it has a magical effect that lasts for 10-12 weeks. It can really break an ischemic event quickly and may also have some preventive benefit. What we have available in the U.S. is epoprostenol. In can be administered on an inpatient basis, but we set it up as outpatient therapy. Patients come in for an 8-hour period for 3-5 days in a row,” the rheumatologist explained.
This is off-label therapy. Epoprostenol’s approved indication is in treating pulmonary arterial hypertension. But there is persuasive evidence of the effectiveness of epoprostenol (Flolan) and the prostacyclins available in other countries in aborting acute digital ischemic events in patients with Raynaud’s, he said.
One prostacyclin, treprostinil, is now available as Orenitram in an oral formulation approved for treatment of pulmonary arterial hypertension. Other oral prostacyclins are in the developmental pipeline. Despite their promise of much greater patient convenience, however, at this point their use in patients with acute digital critical ischemia isn’t supported by evidence.
During those multiple, daylong outpatient treatment sessions, Dr. Wigley emphasizes the importance of staying warm, resting, and avoiding stress. He also makes sure the patient is on an optimal vasodilatory medication program, the linchpin of which is a long-acting calcium channel blocker titrated to the maximum tolerated dose.
He also does special testing in search of a potential obstruction in a larger upstream vessel that might be amenable to a corrective procedure. These tests include Doppler ultrasound, the Allen’s test, magnetic resonance angiography, and/or digital subtraction x-ray.
This multiday period of prostacyclin therapy is an excellent opportunity to initiate the use of vasculoprotective medications. The ones he’s found most helpful include statins, low-dose aspirin or another antiplatelet agent, antioxidants, and in the case of a suspected early thrombotic or embolic event, 24-72 hours of low-molecular-weight or unfractionated heparin.
There is clinical trial evidence that bosentan (Tracleer) reduces the frequency of recurrent digital ulcers. Dr. Wigley rarely uses it, however, because it’s expensive and has lots of toxicity.
Surgical sympathectomy can be finger saving.
“Don’t dillydally,” urged Dr. Wigley. “If your patient is not responding to medical therapy, don’t say, ‘Come back in a few weeks, and we’ll see how you’re doing.’ You’ll lose the finger.”
Sympathectomy has several salutary effects: the procedure rips sympathetic nerve fibers from the over-sensitive blood vessels and also gets rid of fibrous material entrapping the vessels.
In a meta-analysis of studies that included 511 proximal sympathectomies in 449 patients with secondary Raynaud’s, 89% reported sustained improvement (J. Vasc. Surg. 2011;54:273-7). The available data on the less morbid alternative digital sympathectomy procedure are less extensive, but the rates of complete healing and/or decrease in digital ulcers are impressive.
Remember: Avoid tunnel vision, Dr. Wigley cautioned. Always consider the likely possibility of macrovascular disease in the setting of lower-extremity digital ischemia. Measurement of the ankle-brachial pressure index is considered a mandatory part of the clinical work-up in these patients. An ankle-brachial index of less than 0.9 has 95% sensitivity for the presence of angiographically evident cardiovascular disease.
Dr. Wigley reported serving as a consultant to Novartis and United Therapeutics and receiving research grants from Kinemed, MedImmune, and CSL Behring.
SNOWMASS, COLO. – Persistent, widespread, intense ischemic pain in the vicinity of a digital ulcer in a patient with secondary Raynaud’s phenomenon signals impending tissue infarction and gangrene, Dr. Fredrick M. Wigley warned at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“When you see this you’ve got a heart attack of the finger occurring. It’s a medical emergency,” emphasized Dr. Wigley, professor of medicine, associate head of rheumatology, and director of the scleroderma center at Johns Hopkins University, Baltimore.
The pain associated with this condition is severe enough that opiates are often required. Therefore, one of Dr. Wigley’s first moves in the office is often to administer a digital block for immediate pain relief. He sticks the needle into the web of the finger and infiltrates 2% lidocaine. It’s an easy procedure to do, and it often breaks the acute event.
If the digital block doesn’t succeed in terminating the ischemic event, however, then his go-to therapy is prostacyclin infusion.
“We’re very keen at our center, and at other centers around the country as well, to go right to a prostacyclin analogue. You can do that for prevention or during an acute event. Set up a peripheral line, run in prostacyclin for 3-5 days, and it has a magical effect that lasts for 10-12 weeks. It can really break an ischemic event quickly and may also have some preventive benefit. What we have available in the U.S. is epoprostenol. In can be administered on an inpatient basis, but we set it up as outpatient therapy. Patients come in for an 8-hour period for 3-5 days in a row,” the rheumatologist explained.
This is off-label therapy. Epoprostenol’s approved indication is in treating pulmonary arterial hypertension. But there is persuasive evidence of the effectiveness of epoprostenol (Flolan) and the prostacyclins available in other countries in aborting acute digital ischemic events in patients with Raynaud’s, he said.
One prostacyclin, treprostinil, is now available as Orenitram in an oral formulation approved for treatment of pulmonary arterial hypertension. Other oral prostacyclins are in the developmental pipeline. Despite their promise of much greater patient convenience, however, at this point their use in patients with acute digital critical ischemia isn’t supported by evidence.
During those multiple, daylong outpatient treatment sessions, Dr. Wigley emphasizes the importance of staying warm, resting, and avoiding stress. He also makes sure the patient is on an optimal vasodilatory medication program, the linchpin of which is a long-acting calcium channel blocker titrated to the maximum tolerated dose.
He also does special testing in search of a potential obstruction in a larger upstream vessel that might be amenable to a corrective procedure. These tests include Doppler ultrasound, the Allen’s test, magnetic resonance angiography, and/or digital subtraction x-ray.
This multiday period of prostacyclin therapy is an excellent opportunity to initiate the use of vasculoprotective medications. The ones he’s found most helpful include statins, low-dose aspirin or another antiplatelet agent, antioxidants, and in the case of a suspected early thrombotic or embolic event, 24-72 hours of low-molecular-weight or unfractionated heparin.
There is clinical trial evidence that bosentan (Tracleer) reduces the frequency of recurrent digital ulcers. Dr. Wigley rarely uses it, however, because it’s expensive and has lots of toxicity.
Surgical sympathectomy can be finger saving.
“Don’t dillydally,” urged Dr. Wigley. “If your patient is not responding to medical therapy, don’t say, ‘Come back in a few weeks, and we’ll see how you’re doing.’ You’ll lose the finger.”
Sympathectomy has several salutary effects: the procedure rips sympathetic nerve fibers from the over-sensitive blood vessels and also gets rid of fibrous material entrapping the vessels.
In a meta-analysis of studies that included 511 proximal sympathectomies in 449 patients with secondary Raynaud’s, 89% reported sustained improvement (J. Vasc. Surg. 2011;54:273-7). The available data on the less morbid alternative digital sympathectomy procedure are less extensive, but the rates of complete healing and/or decrease in digital ulcers are impressive.
Remember: Avoid tunnel vision, Dr. Wigley cautioned. Always consider the likely possibility of macrovascular disease in the setting of lower-extremity digital ischemia. Measurement of the ankle-brachial pressure index is considered a mandatory part of the clinical work-up in these patients. An ankle-brachial index of less than 0.9 has 95% sensitivity for the presence of angiographically evident cardiovascular disease.
Dr. Wigley reported serving as a consultant to Novartis and United Therapeutics and receiving research grants from Kinemed, MedImmune, and CSL Behring.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Cervical cancer screening: National snapshot reveals confusion over optimal intervals
Results of a recent survey of provider and patient attitudes toward and behaviors surrounding cervical cancer screening indicate there is much confusion, among both practitioners and patients, about the optimal screening interval for cervical cancer. Only 48% of women understood that HPV can cause cervical cancer.
The survey, of 2000 women and 750 providers, was conducted by the National Association of Nurse Practitioners in Women’s Health and Healthy Women.
This special audiocast is an interview with James S. Simon, MD, panel member for the survey release event in Washington, DC.
Listen to hear the survey results and Dr. Simon discuss:
• The roots of the highlighted confusion around cervical cancer screening among women and practitioners
• How often he screens patients for cervical cancer and what tests he employs
• His patient counseling strategy regarding HPV and other sexually transmitted infections
Dr. James A. Simon reports having served (within the last year) or currently serving as a consultant to or on the advisory boards of: AbbVie, Inc. (North Chicago, IL), Actavis, PLC. (Dublin, Ireland), Amgen Inc. (Thousand Oaks, CA), Amneal Pharmaceuticals (Bridgewater, NJ), Apotex, Inc. (Toronto, Canada), Ascend Therapeutics (Herndon, VA), Dr. Reddy Laboratories, Ltd. (Hyderabad, India), Everett Laboratories, Inc. (West Orange, NJ), Lupin Pharmaceuticals, (Baltimore, MD), Merck & Co., Inc. (Whitehouse Station, NJ), Novartis Pharmaceuticals Corporation (East Hanover, NJ), Noven Pharmaceuticals, Inc. (New York, NY), Novo Nordisk (Bagsvrerd, Denmark), Pfizer Inc. (New York, NY), Shionogi Inc. (Florham Park, NJ), Shippan Point Advisors LLC (Upper Saddle River, NJ), Sprout Pharmaceuticals (Raleigh, NC), and TherapeuticsMD (Boca Raton, FL).
He reports having received grant/research support in the last year or currently from: AbbVie, Inc. (North Chicago, IL), Actavis, PLC. (Dublin, Ireland), Agile Therapeutics (Princeton, NJ), Bayer Healthcare LLC., (Tarrytown, NY), New England Research Institute, Inc. (Watertown, MA), Novo Nordisk (Bagsvrerd, Denmark), Palatin Technologies (Cranbury, NJ), and Teva Pharmaceutical Industries Ltd (Jerusalem, Israel), and TherapeuticsMD (Boca Raton, FL).
He also reports having served or currently serving on the speakers' bureaus of: Amgen Inc. (Thousand Oaks, CA), Eisai, Inc. (Woodcliff Lake, NJ), Merck (Whitehouse Station, NJ), Noven Pharmaceuticals, Inc. (New York, NY), Novo Nordisk (Bagsvrerd, Denmark), and Shionogi Inc. (Florham Park, NJ).
Results of a recent survey of provider and patient attitudes toward and behaviors surrounding cervical cancer screening indicate there is much confusion, among both practitioners and patients, about the optimal screening interval for cervical cancer. Only 48% of women understood that HPV can cause cervical cancer.
The survey, of 2000 women and 750 providers, was conducted by the National Association of Nurse Practitioners in Women’s Health and Healthy Women.
This special audiocast is an interview with James S. Simon, MD, panel member for the survey release event in Washington, DC.
Listen to hear the survey results and Dr. Simon discuss:
• The roots of the highlighted confusion around cervical cancer screening among women and practitioners
• How often he screens patients for cervical cancer and what tests he employs
• His patient counseling strategy regarding HPV and other sexually transmitted infections
Dr. James A. Simon reports having served (within the last year) or currently serving as a consultant to or on the advisory boards of: AbbVie, Inc. (North Chicago, IL), Actavis, PLC. (Dublin, Ireland), Amgen Inc. (Thousand Oaks, CA), Amneal Pharmaceuticals (Bridgewater, NJ), Apotex, Inc. (Toronto, Canada), Ascend Therapeutics (Herndon, VA), Dr. Reddy Laboratories, Ltd. (Hyderabad, India), Everett Laboratories, Inc. (West Orange, NJ), Lupin Pharmaceuticals, (Baltimore, MD), Merck & Co., Inc. (Whitehouse Station, NJ), Novartis Pharmaceuticals Corporation (East Hanover, NJ), Noven Pharmaceuticals, Inc. (New York, NY), Novo Nordisk (Bagsvrerd, Denmark), Pfizer Inc. (New York, NY), Shionogi Inc. (Florham Park, NJ), Shippan Point Advisors LLC (Upper Saddle River, NJ), Sprout Pharmaceuticals (Raleigh, NC), and TherapeuticsMD (Boca Raton, FL).
He reports having received grant/research support in the last year or currently from: AbbVie, Inc. (North Chicago, IL), Actavis, PLC. (Dublin, Ireland), Agile Therapeutics (Princeton, NJ), Bayer Healthcare LLC., (Tarrytown, NY), New England Research Institute, Inc. (Watertown, MA), Novo Nordisk (Bagsvrerd, Denmark), Palatin Technologies (Cranbury, NJ), and Teva Pharmaceutical Industries Ltd (Jerusalem, Israel), and TherapeuticsMD (Boca Raton, FL).
He also reports having served or currently serving on the speakers' bureaus of: Amgen Inc. (Thousand Oaks, CA), Eisai, Inc. (Woodcliff Lake, NJ), Merck (Whitehouse Station, NJ), Noven Pharmaceuticals, Inc. (New York, NY), Novo Nordisk (Bagsvrerd, Denmark), and Shionogi Inc. (Florham Park, NJ).
Results of a recent survey of provider and patient attitudes toward and behaviors surrounding cervical cancer screening indicate there is much confusion, among both practitioners and patients, about the optimal screening interval for cervical cancer. Only 48% of women understood that HPV can cause cervical cancer.
The survey, of 2000 women and 750 providers, was conducted by the National Association of Nurse Practitioners in Women’s Health and Healthy Women.
This special audiocast is an interview with James S. Simon, MD, panel member for the survey release event in Washington, DC.
Listen to hear the survey results and Dr. Simon discuss:
• The roots of the highlighted confusion around cervical cancer screening among women and practitioners
• How often he screens patients for cervical cancer and what tests he employs
• His patient counseling strategy regarding HPV and other sexually transmitted infections
Dr. James A. Simon reports having served (within the last year) or currently serving as a consultant to or on the advisory boards of: AbbVie, Inc. (North Chicago, IL), Actavis, PLC. (Dublin, Ireland), Amgen Inc. (Thousand Oaks, CA), Amneal Pharmaceuticals (Bridgewater, NJ), Apotex, Inc. (Toronto, Canada), Ascend Therapeutics (Herndon, VA), Dr. Reddy Laboratories, Ltd. (Hyderabad, India), Everett Laboratories, Inc. (West Orange, NJ), Lupin Pharmaceuticals, (Baltimore, MD), Merck & Co., Inc. (Whitehouse Station, NJ), Novartis Pharmaceuticals Corporation (East Hanover, NJ), Noven Pharmaceuticals, Inc. (New York, NY), Novo Nordisk (Bagsvrerd, Denmark), Pfizer Inc. (New York, NY), Shionogi Inc. (Florham Park, NJ), Shippan Point Advisors LLC (Upper Saddle River, NJ), Sprout Pharmaceuticals (Raleigh, NC), and TherapeuticsMD (Boca Raton, FL).
He reports having received grant/research support in the last year or currently from: AbbVie, Inc. (North Chicago, IL), Actavis, PLC. (Dublin, Ireland), Agile Therapeutics (Princeton, NJ), Bayer Healthcare LLC., (Tarrytown, NY), New England Research Institute, Inc. (Watertown, MA), Novo Nordisk (Bagsvrerd, Denmark), Palatin Technologies (Cranbury, NJ), and Teva Pharmaceutical Industries Ltd (Jerusalem, Israel), and TherapeuticsMD (Boca Raton, FL).
He also reports having served or currently serving on the speakers' bureaus of: Amgen Inc. (Thousand Oaks, CA), Eisai, Inc. (Woodcliff Lake, NJ), Merck (Whitehouse Station, NJ), Noven Pharmaceuticals, Inc. (New York, NY), Novo Nordisk (Bagsvrerd, Denmark), and Shionogi Inc. (Florham Park, NJ).