User login
For Radiation ‘Downwinders,’ Cancer Compensation Is On Hold
As of 2022, more than 40,000 patients with cancer successfully applied for $2.6 billion in compensation. Recipients included “downwinders” who were eligible for $50,000 each if they lived in certain areas of Nevada, Utah, and Arizona during specified nuclear testing periods and developed a covered form of cancer.
In June 2024, however, the Radiation Exposure Compensation Program expired amid infighting among Republicans in Congress over whether to expand it. For now, no one can make a claim, even though many downwinders are still alive and continue to be diagnosed with covered cancers decades after they were exposed in the 1940s, 1950s, and 1960s.
There’s a glimmer of good news. The federal government continues to support free medical screenings for eligible people, including certain downwinders and uranium workers. Meanwhile, there are still important roles for clinicians across the country to play as politicians figure out what — if anything — to do next regarding those exposed to radiation.
“We are still here. We can still screen people,” Zachary Davis, program director for the Radiation Exposure Screening and Education Program, The University of New Mexico, in Albuquerque, New Mexico, said in an interview.
Still-Unfolding Legacy of Radiation Exposure
No one knew just how far radiation would spread when the first nuclear bomb was tested in New Mexico in July 1945. Would it cover the state? The entire Southwest? The whole nation?
It also wasn’t clear how radiation would affect people’s health. “There was an awareness that some cancers were caused by radiation, but there wasn’t a cohesive understanding of what the problem was,” Joseph Shonka, PhD, a health physicist who studies radiation exposure and has worked for decades in nuclear engineering, said in an interview.
Now, nearly eight decades later, scientists are still figuring out the full extent of radioactive fallout from nuclear testing. Just last year, a study suggested that radiation from 94 nuclear weapon tests in the Southwest from 1945 to 1962 reached 46 states along with Canada and Mexico.
Activists believe the tests triggered untold number of cancer cases in residents who were exposed in downwind areas:
“My brother died of stomach cancer; my mom died of bone cancer. One of my sisters is surviving brain tumors, and the other one is surviving thyroid cancer,” one New Mexico man recently told ABC-TV’s “Nightline.”
In Idaho, a downwinder advocate told Idaho Capital Sun that everyone who attended a reception for her newly married parents in 1952 — just weeks after a nuclear test — developed cancer or “weird medical complications.” That included her parents, who both had cancer. Her two older brothers, born in 1953 and 1955, also developed cancer, and she’s tracked many other cases in the small town of Emmett.
In Utah, another downwinder advocate told Utah News Dispatch that cancer was common in Salt Lake City neighborhood, where she grew up, which was exposed to fallout. She developed thyroid cancer, her younger sister developed stomach cancer, and an older sister died of lupus, which is connected to radiation exposure. But Salt Lake City isn’t in one of the regions of Utah covered by the federal compensation program, so the advocate can’t get a $50,000 payment.
Downwinders who lived in New Mexico, Idaho, and the Salt Lake City area of Utah are not covered by the federal compensation program. That means none of these people or their descendants are eligible for payments — yet.
Decades After Nuclear Testing, the Government Responds
In 1990, Congress passed the Radiation Exposure Compensation Act, which allowed compensation to people with cancer at several levels. It was later expanded. Downwinders — including those who’ve moved elsewhere over the years — were eligible for $50,000. Onsite participants in nuclear testing could get $75,000. Uranium miners, millers, and ore transporters in 11 states west of the Mississippi River could get $100,000.
Among downwinders, eligible cancers included blood cancers (leukemias with the exception of chronic lymphocytic leukemia, multiple myeloma, and non-Hodgkin’s lymphomas) and a long list of solid organ cancers such as thyroid, breast, stomach, brain, lung, colon, and liver cancers.
“When it comes to blood-related cancers, we do see leukemias, lymphomas, and multiple myeloma, but these cancers were more likely to occur sooner after fallout exposure,” said Laura Shaw, MD, principal investigator who oversees the radiation exposure screening program at the University of Nevada, Las Vegas. “At this point, we see more pancreatic, thyroid, lung, stomach, bladder, and breast cancer.”
The compensation program had major limitations, critics said. “It left out a lot of communities that were exposed,” said Lilly Adams, senior outreach coordinator with the Union of Concerned Scientists (UCS), which supports expanding the program. A national nonprofit organization, UCS was founded more than 50 years ago by scientists and students at the Massachusetts Institute of Technology.
“You have this pretty small amount of one-time compensation, and that’s it,” Adams said in an interview. “You can’t get reimbursed for medical costs or lost wages.” Still, “as flawed as the program is, it’s really valuable for the people who are eligible,” she noted.
Now Congress Is Divided on Next Steps
Some lawmakers have recognized the need to do more for those who developed cancer that’s potentially linked to radiation exposure. As the June 2024 expiration of the Radiation Exposure Compensation Act loomed, Democrats and Republicans in Congress worked together to extend and expand the program.
They introduced a bill for higher compensation — $100,000 per person — and the widening of covered downwinder areas to all of Arizona, Nevada, and Utah (which had only been partially covered), along with all of Colorado, Idaho, New Mexico, Montana, and Guam. Under the legislation, the program also would expand to cover some uranium workers who were on the job after 1971 and residents exposed to nuclear waste in Kentucky, Missouri, and Tennessee.
In March, the new legislation easily passed the US Senate by a vote of 69-30, with support from both political parties — but the Republican-led House hasn’t taken it up. As a result, the Radiation Exposure Compensation Act expired in June, and no one can submit new applications for compensation.
A spokesman for House Speaker Mike Johnson told Missouri Independent “unfortunately, the current Senate bill is estimated to cost $50-$60 billion in new mandatory spending with no offsets and was supported by only 20 of 49 Republicans in the Senate.”
Adams rejected these arguments. “The government spends literally trillions of dollars on our nuclear weapons. Whether or not you support that spending, the human cost of building those weapons should be factored in,” she said. She added that she hopes the House will act by the end of the year to pass the bill, but that’s uncertain.
As Compensation Is On Hold, Medical Screening Continues
A major benefit is still available for downwinders and uranium workers: Free medical screening and referrals for medical treatment. The Radiation Exposure Screening and Education Program’s funding has not been affected by the congressional impasse, so screenings are continuing for eligible people exposed to radiation.
Radiation exposure clinics offer screening in Arizona, Colorado, Nevada, New Mexico, and Utah, and health providers can get funding to offer screening in other affected states.
In Nevada, “we hold screening clinics throughout the state: Caliente, Ely, and Winnemucca. Also, in Reno and Las Vegas, which are not in designated downwind areas, but many downwinders have migrated there,” said Shaw in an interview. Among downwinders, “our youngest patients are in their 60s and range up to a few in their 90s,” she said.
Patients fill out questionnaires that ask about their medical problems, family history, and medications. “Ely patients in particular seem to have extensive family histories of cancer, and this may be due to their location directly downwind of the Nevada Test Site,” Shaw said. (Ely is a remote town in central eastern Nevada near the Utah border.)
The screenings cover both cancer and noncancer conditions. Shaw said clinicians often diagnose problems other than the covered cancers — new cases of atrial fibrillation, diabetes, and hypertension. “We see a ton of prostate and skin cancer” but don’t make patients eligible for the compensation program because they’re not covered, she said.
Even as compensation is on hold, doctors can get the word out that screenings are still available, Shaw said. “We continue to get contacted by individuals who in these communities who have never heard of this program, even though we’ve been holding clinics since 2005,” Shaw said. “Despite outreach activities and advertising through newspapers and radio, we find the most successful method of reaching these patients is through word of mouth — either from other patients or their doctors. That is why we feel it is so important to reach other physicians as well.”
Affected Patients Don’t Just Live in the West
On the outreach front, clinicians in states outside of the western US region can be helpful, too. Shaw urged oncologists nationwide to ask older patients where they lived in the 1950s and 1960s. “Did they live in Nevada, Arizona, Utah, and other Western states that are downwind? They may qualify for needed services and future compensation.”
With regard to compensation, she noted that applicants need to prove that they lived in affected areas many decades ago. And, of course, they must prove that they’ve had cancer. Locating residency records “has often been an enormous challenge.” Old utility bills, pay stubs, and high school annuals can be helpful, “but these records tend to disappear. People and their families throw stuff away.”
Even proving a cancer diagnosis can be a challenge because records can be missing. In Nevada, the law says clinicians only need to keep medical records for 5 years, Shaw said. “Imaging and pathology reports are destroyed. Patients that have been diagnosed with cancer can’t prove it.”
Shaw said she hopes oncologists will offer these messages to patients: “Be an advocate for your own health and keep copies of your own records. Discuss your diagnosis with your family and contact a cancer registry if you are diagnosed with cancer.”
A version of this article appeared on Medscape.com.
As of 2022, more than 40,000 patients with cancer successfully applied for $2.6 billion in compensation. Recipients included “downwinders” who were eligible for $50,000 each if they lived in certain areas of Nevada, Utah, and Arizona during specified nuclear testing periods and developed a covered form of cancer.
In June 2024, however, the Radiation Exposure Compensation Program expired amid infighting among Republicans in Congress over whether to expand it. For now, no one can make a claim, even though many downwinders are still alive and continue to be diagnosed with covered cancers decades after they were exposed in the 1940s, 1950s, and 1960s.
There’s a glimmer of good news. The federal government continues to support free medical screenings for eligible people, including certain downwinders and uranium workers. Meanwhile, there are still important roles for clinicians across the country to play as politicians figure out what — if anything — to do next regarding those exposed to radiation.
“We are still here. We can still screen people,” Zachary Davis, program director for the Radiation Exposure Screening and Education Program, The University of New Mexico, in Albuquerque, New Mexico, said in an interview.
Still-Unfolding Legacy of Radiation Exposure
No one knew just how far radiation would spread when the first nuclear bomb was tested in New Mexico in July 1945. Would it cover the state? The entire Southwest? The whole nation?
It also wasn’t clear how radiation would affect people’s health. “There was an awareness that some cancers were caused by radiation, but there wasn’t a cohesive understanding of what the problem was,” Joseph Shonka, PhD, a health physicist who studies radiation exposure and has worked for decades in nuclear engineering, said in an interview.
Now, nearly eight decades later, scientists are still figuring out the full extent of radioactive fallout from nuclear testing. Just last year, a study suggested that radiation from 94 nuclear weapon tests in the Southwest from 1945 to 1962 reached 46 states along with Canada and Mexico.
Activists believe the tests triggered untold number of cancer cases in residents who were exposed in downwind areas:
“My brother died of stomach cancer; my mom died of bone cancer. One of my sisters is surviving brain tumors, and the other one is surviving thyroid cancer,” one New Mexico man recently told ABC-TV’s “Nightline.”
In Idaho, a downwinder advocate told Idaho Capital Sun that everyone who attended a reception for her newly married parents in 1952 — just weeks after a nuclear test — developed cancer or “weird medical complications.” That included her parents, who both had cancer. Her two older brothers, born in 1953 and 1955, also developed cancer, and she’s tracked many other cases in the small town of Emmett.
In Utah, another downwinder advocate told Utah News Dispatch that cancer was common in Salt Lake City neighborhood, where she grew up, which was exposed to fallout. She developed thyroid cancer, her younger sister developed stomach cancer, and an older sister died of lupus, which is connected to radiation exposure. But Salt Lake City isn’t in one of the regions of Utah covered by the federal compensation program, so the advocate can’t get a $50,000 payment.
Downwinders who lived in New Mexico, Idaho, and the Salt Lake City area of Utah are not covered by the federal compensation program. That means none of these people or their descendants are eligible for payments — yet.
Decades After Nuclear Testing, the Government Responds
In 1990, Congress passed the Radiation Exposure Compensation Act, which allowed compensation to people with cancer at several levels. It was later expanded. Downwinders — including those who’ve moved elsewhere over the years — were eligible for $50,000. Onsite participants in nuclear testing could get $75,000. Uranium miners, millers, and ore transporters in 11 states west of the Mississippi River could get $100,000.
Among downwinders, eligible cancers included blood cancers (leukemias with the exception of chronic lymphocytic leukemia, multiple myeloma, and non-Hodgkin’s lymphomas) and a long list of solid organ cancers such as thyroid, breast, stomach, brain, lung, colon, and liver cancers.
“When it comes to blood-related cancers, we do see leukemias, lymphomas, and multiple myeloma, but these cancers were more likely to occur sooner after fallout exposure,” said Laura Shaw, MD, principal investigator who oversees the radiation exposure screening program at the University of Nevada, Las Vegas. “At this point, we see more pancreatic, thyroid, lung, stomach, bladder, and breast cancer.”
The compensation program had major limitations, critics said. “It left out a lot of communities that were exposed,” said Lilly Adams, senior outreach coordinator with the Union of Concerned Scientists (UCS), which supports expanding the program. A national nonprofit organization, UCS was founded more than 50 years ago by scientists and students at the Massachusetts Institute of Technology.
“You have this pretty small amount of one-time compensation, and that’s it,” Adams said in an interview. “You can’t get reimbursed for medical costs or lost wages.” Still, “as flawed as the program is, it’s really valuable for the people who are eligible,” she noted.
Now Congress Is Divided on Next Steps
Some lawmakers have recognized the need to do more for those who developed cancer that’s potentially linked to radiation exposure. As the June 2024 expiration of the Radiation Exposure Compensation Act loomed, Democrats and Republicans in Congress worked together to extend and expand the program.
They introduced a bill for higher compensation — $100,000 per person — and the widening of covered downwinder areas to all of Arizona, Nevada, and Utah (which had only been partially covered), along with all of Colorado, Idaho, New Mexico, Montana, and Guam. Under the legislation, the program also would expand to cover some uranium workers who were on the job after 1971 and residents exposed to nuclear waste in Kentucky, Missouri, and Tennessee.
In March, the new legislation easily passed the US Senate by a vote of 69-30, with support from both political parties — but the Republican-led House hasn’t taken it up. As a result, the Radiation Exposure Compensation Act expired in June, and no one can submit new applications for compensation.
A spokesman for House Speaker Mike Johnson told Missouri Independent “unfortunately, the current Senate bill is estimated to cost $50-$60 billion in new mandatory spending with no offsets and was supported by only 20 of 49 Republicans in the Senate.”
Adams rejected these arguments. “The government spends literally trillions of dollars on our nuclear weapons. Whether or not you support that spending, the human cost of building those weapons should be factored in,” she said. She added that she hopes the House will act by the end of the year to pass the bill, but that’s uncertain.
As Compensation Is On Hold, Medical Screening Continues
A major benefit is still available for downwinders and uranium workers: Free medical screening and referrals for medical treatment. The Radiation Exposure Screening and Education Program’s funding has not been affected by the congressional impasse, so screenings are continuing for eligible people exposed to radiation.
Radiation exposure clinics offer screening in Arizona, Colorado, Nevada, New Mexico, and Utah, and health providers can get funding to offer screening in other affected states.
In Nevada, “we hold screening clinics throughout the state: Caliente, Ely, and Winnemucca. Also, in Reno and Las Vegas, which are not in designated downwind areas, but many downwinders have migrated there,” said Shaw in an interview. Among downwinders, “our youngest patients are in their 60s and range up to a few in their 90s,” she said.
Patients fill out questionnaires that ask about their medical problems, family history, and medications. “Ely patients in particular seem to have extensive family histories of cancer, and this may be due to their location directly downwind of the Nevada Test Site,” Shaw said. (Ely is a remote town in central eastern Nevada near the Utah border.)
The screenings cover both cancer and noncancer conditions. Shaw said clinicians often diagnose problems other than the covered cancers — new cases of atrial fibrillation, diabetes, and hypertension. “We see a ton of prostate and skin cancer” but don’t make patients eligible for the compensation program because they’re not covered, she said.
Even as compensation is on hold, doctors can get the word out that screenings are still available, Shaw said. “We continue to get contacted by individuals who in these communities who have never heard of this program, even though we’ve been holding clinics since 2005,” Shaw said. “Despite outreach activities and advertising through newspapers and radio, we find the most successful method of reaching these patients is through word of mouth — either from other patients or their doctors. That is why we feel it is so important to reach other physicians as well.”
Affected Patients Don’t Just Live in the West
On the outreach front, clinicians in states outside of the western US region can be helpful, too. Shaw urged oncologists nationwide to ask older patients where they lived in the 1950s and 1960s. “Did they live in Nevada, Arizona, Utah, and other Western states that are downwind? They may qualify for needed services and future compensation.”
With regard to compensation, she noted that applicants need to prove that they lived in affected areas many decades ago. And, of course, they must prove that they’ve had cancer. Locating residency records “has often been an enormous challenge.” Old utility bills, pay stubs, and high school annuals can be helpful, “but these records tend to disappear. People and their families throw stuff away.”
Even proving a cancer diagnosis can be a challenge because records can be missing. In Nevada, the law says clinicians only need to keep medical records for 5 years, Shaw said. “Imaging and pathology reports are destroyed. Patients that have been diagnosed with cancer can’t prove it.”
Shaw said she hopes oncologists will offer these messages to patients: “Be an advocate for your own health and keep copies of your own records. Discuss your diagnosis with your family and contact a cancer registry if you are diagnosed with cancer.”
A version of this article appeared on Medscape.com.
As of 2022, more than 40,000 patients with cancer successfully applied for $2.6 billion in compensation. Recipients included “downwinders” who were eligible for $50,000 each if they lived in certain areas of Nevada, Utah, and Arizona during specified nuclear testing periods and developed a covered form of cancer.
In June 2024, however, the Radiation Exposure Compensation Program expired amid infighting among Republicans in Congress over whether to expand it. For now, no one can make a claim, even though many downwinders are still alive and continue to be diagnosed with covered cancers decades after they were exposed in the 1940s, 1950s, and 1960s.
There’s a glimmer of good news. The federal government continues to support free medical screenings for eligible people, including certain downwinders and uranium workers. Meanwhile, there are still important roles for clinicians across the country to play as politicians figure out what — if anything — to do next regarding those exposed to radiation.
“We are still here. We can still screen people,” Zachary Davis, program director for the Radiation Exposure Screening and Education Program, The University of New Mexico, in Albuquerque, New Mexico, said in an interview.
Still-Unfolding Legacy of Radiation Exposure
No one knew just how far radiation would spread when the first nuclear bomb was tested in New Mexico in July 1945. Would it cover the state? The entire Southwest? The whole nation?
It also wasn’t clear how radiation would affect people’s health. “There was an awareness that some cancers were caused by radiation, but there wasn’t a cohesive understanding of what the problem was,” Joseph Shonka, PhD, a health physicist who studies radiation exposure and has worked for decades in nuclear engineering, said in an interview.
Now, nearly eight decades later, scientists are still figuring out the full extent of radioactive fallout from nuclear testing. Just last year, a study suggested that radiation from 94 nuclear weapon tests in the Southwest from 1945 to 1962 reached 46 states along with Canada and Mexico.
Activists believe the tests triggered untold number of cancer cases in residents who were exposed in downwind areas:
“My brother died of stomach cancer; my mom died of bone cancer. One of my sisters is surviving brain tumors, and the other one is surviving thyroid cancer,” one New Mexico man recently told ABC-TV’s “Nightline.”
In Idaho, a downwinder advocate told Idaho Capital Sun that everyone who attended a reception for her newly married parents in 1952 — just weeks after a nuclear test — developed cancer or “weird medical complications.” That included her parents, who both had cancer. Her two older brothers, born in 1953 and 1955, also developed cancer, and she’s tracked many other cases in the small town of Emmett.
In Utah, another downwinder advocate told Utah News Dispatch that cancer was common in Salt Lake City neighborhood, where she grew up, which was exposed to fallout. She developed thyroid cancer, her younger sister developed stomach cancer, and an older sister died of lupus, which is connected to radiation exposure. But Salt Lake City isn’t in one of the regions of Utah covered by the federal compensation program, so the advocate can’t get a $50,000 payment.
Downwinders who lived in New Mexico, Idaho, and the Salt Lake City area of Utah are not covered by the federal compensation program. That means none of these people or their descendants are eligible for payments — yet.
Decades After Nuclear Testing, the Government Responds
In 1990, Congress passed the Radiation Exposure Compensation Act, which allowed compensation to people with cancer at several levels. It was later expanded. Downwinders — including those who’ve moved elsewhere over the years — were eligible for $50,000. Onsite participants in nuclear testing could get $75,000. Uranium miners, millers, and ore transporters in 11 states west of the Mississippi River could get $100,000.
Among downwinders, eligible cancers included blood cancers (leukemias with the exception of chronic lymphocytic leukemia, multiple myeloma, and non-Hodgkin’s lymphomas) and a long list of solid organ cancers such as thyroid, breast, stomach, brain, lung, colon, and liver cancers.
“When it comes to blood-related cancers, we do see leukemias, lymphomas, and multiple myeloma, but these cancers were more likely to occur sooner after fallout exposure,” said Laura Shaw, MD, principal investigator who oversees the radiation exposure screening program at the University of Nevada, Las Vegas. “At this point, we see more pancreatic, thyroid, lung, stomach, bladder, and breast cancer.”
The compensation program had major limitations, critics said. “It left out a lot of communities that were exposed,” said Lilly Adams, senior outreach coordinator with the Union of Concerned Scientists (UCS), which supports expanding the program. A national nonprofit organization, UCS was founded more than 50 years ago by scientists and students at the Massachusetts Institute of Technology.
“You have this pretty small amount of one-time compensation, and that’s it,” Adams said in an interview. “You can’t get reimbursed for medical costs or lost wages.” Still, “as flawed as the program is, it’s really valuable for the people who are eligible,” she noted.
Now Congress Is Divided on Next Steps
Some lawmakers have recognized the need to do more for those who developed cancer that’s potentially linked to radiation exposure. As the June 2024 expiration of the Radiation Exposure Compensation Act loomed, Democrats and Republicans in Congress worked together to extend and expand the program.
They introduced a bill for higher compensation — $100,000 per person — and the widening of covered downwinder areas to all of Arizona, Nevada, and Utah (which had only been partially covered), along with all of Colorado, Idaho, New Mexico, Montana, and Guam. Under the legislation, the program also would expand to cover some uranium workers who were on the job after 1971 and residents exposed to nuclear waste in Kentucky, Missouri, and Tennessee.
In March, the new legislation easily passed the US Senate by a vote of 69-30, with support from both political parties — but the Republican-led House hasn’t taken it up. As a result, the Radiation Exposure Compensation Act expired in June, and no one can submit new applications for compensation.
A spokesman for House Speaker Mike Johnson told Missouri Independent “unfortunately, the current Senate bill is estimated to cost $50-$60 billion in new mandatory spending with no offsets and was supported by only 20 of 49 Republicans in the Senate.”
Adams rejected these arguments. “The government spends literally trillions of dollars on our nuclear weapons. Whether or not you support that spending, the human cost of building those weapons should be factored in,” she said. She added that she hopes the House will act by the end of the year to pass the bill, but that’s uncertain.
As Compensation Is On Hold, Medical Screening Continues
A major benefit is still available for downwinders and uranium workers: Free medical screening and referrals for medical treatment. The Radiation Exposure Screening and Education Program’s funding has not been affected by the congressional impasse, so screenings are continuing for eligible people exposed to radiation.
Radiation exposure clinics offer screening in Arizona, Colorado, Nevada, New Mexico, and Utah, and health providers can get funding to offer screening in other affected states.
In Nevada, “we hold screening clinics throughout the state: Caliente, Ely, and Winnemucca. Also, in Reno and Las Vegas, which are not in designated downwind areas, but many downwinders have migrated there,” said Shaw in an interview. Among downwinders, “our youngest patients are in their 60s and range up to a few in their 90s,” she said.
Patients fill out questionnaires that ask about their medical problems, family history, and medications. “Ely patients in particular seem to have extensive family histories of cancer, and this may be due to their location directly downwind of the Nevada Test Site,” Shaw said. (Ely is a remote town in central eastern Nevada near the Utah border.)
The screenings cover both cancer and noncancer conditions. Shaw said clinicians often diagnose problems other than the covered cancers — new cases of atrial fibrillation, diabetes, and hypertension. “We see a ton of prostate and skin cancer” but don’t make patients eligible for the compensation program because they’re not covered, she said.
Even as compensation is on hold, doctors can get the word out that screenings are still available, Shaw said. “We continue to get contacted by individuals who in these communities who have never heard of this program, even though we’ve been holding clinics since 2005,” Shaw said. “Despite outreach activities and advertising through newspapers and radio, we find the most successful method of reaching these patients is through word of mouth — either from other patients or their doctors. That is why we feel it is so important to reach other physicians as well.”
Affected Patients Don’t Just Live in the West
On the outreach front, clinicians in states outside of the western US region can be helpful, too. Shaw urged oncologists nationwide to ask older patients where they lived in the 1950s and 1960s. “Did they live in Nevada, Arizona, Utah, and other Western states that are downwind? They may qualify for needed services and future compensation.”
With regard to compensation, she noted that applicants need to prove that they lived in affected areas many decades ago. And, of course, they must prove that they’ve had cancer. Locating residency records “has often been an enormous challenge.” Old utility bills, pay stubs, and high school annuals can be helpful, “but these records tend to disappear. People and their families throw stuff away.”
Even proving a cancer diagnosis can be a challenge because records can be missing. In Nevada, the law says clinicians only need to keep medical records for 5 years, Shaw said. “Imaging and pathology reports are destroyed. Patients that have been diagnosed with cancer can’t prove it.”
Shaw said she hopes oncologists will offer these messages to patients: “Be an advocate for your own health and keep copies of your own records. Discuss your diagnosis with your family and contact a cancer registry if you are diagnosed with cancer.”
A version of this article appeared on Medscape.com.
Increase in Troublesome Fungal Infections Requires All-Out Approach
As dermatologists, public health officials, and infectious disease specialists scramble to raise awareness about prevention and treatment, challenges ranging from a dearth of testing facilities and data to payer pushback over longer therapeutic courses remain.
Dermatophyte Discourse Changing
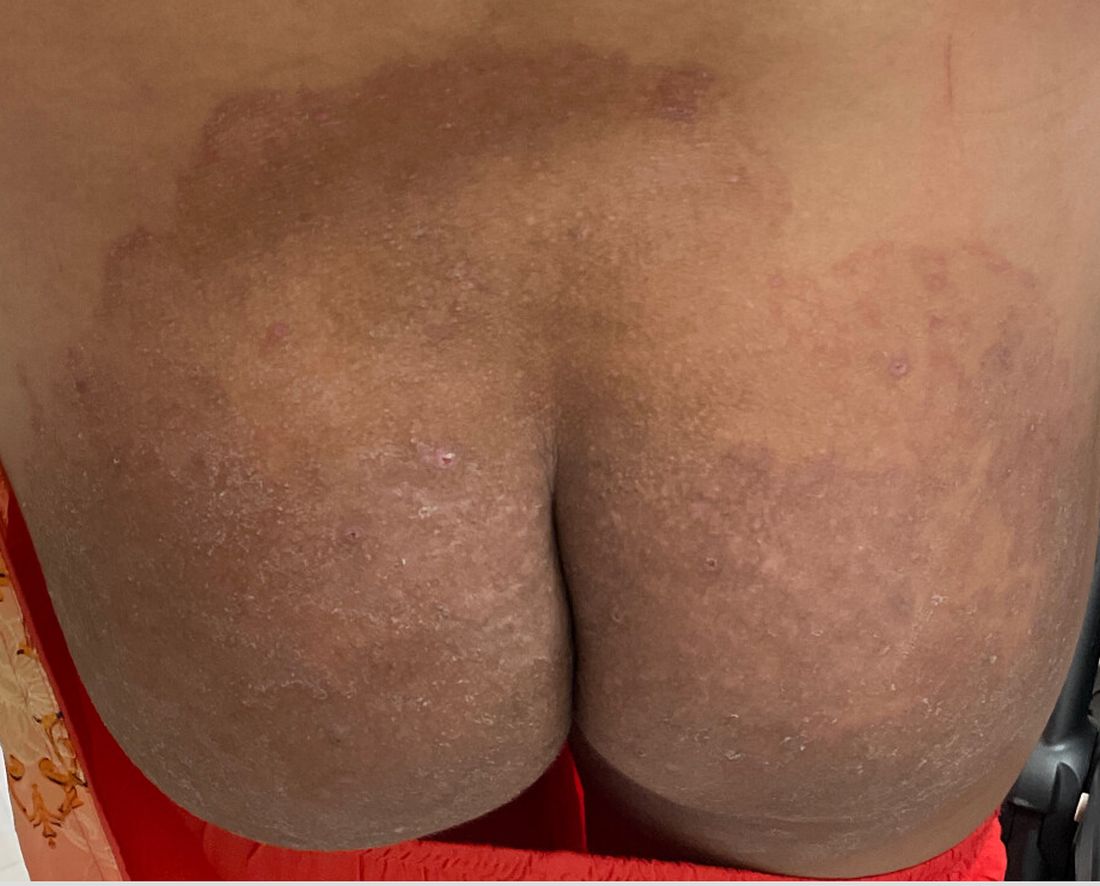
“Trichophyton indotineae is changing the way we talk about dermatophyte infections,” Avrom S. Caplan, MD, assistant professor in the Department of Dermatology at New York University, New York City, said in an interview. Called T mentagrophytes VIII (TMVIII) before a 2020 report in the journal Mycopathologia proposed the name T indotineae, this species requires clinicians to expand their conception of how tinea looks, acts, and responds to treatment.
Boni E. Elewski, MD, professor and chair of dermatology, at The University of Alabama at Birmingham, saw her first case of probable T indotineae in a patient in early 2020. “He was covered with fine scale, and he itched all over. I thought he had atopic dermatitis. This didn’t look like any tinea. His face, arms, back, and legs were scaly.”
Nevertheless, KOH and biopsy confirmed dermatophytosis. Culture (performed at the Center for Medical Mycology [CMM] in Cleveland) identified T mentagrophytes. Back then, Elewski told this news organization, labs did not routinely go beyond genus and species. But based on the patient’s symptoms, history of unresponsiveness to terbinafine, borderline sensitivity to fluconazole, and travel to India and Spain, Elewski strongly suspected T indotineae.
The patient refused itraconazole, to which the fungus was sensitive, and did not respond to fluconazole 400 mg daily. Ultimately, he was lost to follow-up. “Last I saw him,” said Elewski, “he was not cured.”
Tracking Cases
Because T indotineae does not require reporting to public health agencies, said Jeremy Gold, MD, MS, a medical officer with the US Centers for Disease Control and Prevention (CDC) Mycotic Diseases Branch in Atlanta, “there is no official public health surveillance keeping track of exactly how many cases have occurred.”
The same is true for TMVII and terbinafine-resistant T rubrum, which are also on the rise. Regarding T indotineae, authors from the University of Texas Health Science Center at San Antonio retrospectively reported 21 terbinafine-resistant isolates from North America in the July 2023 Journal of Clinical Microbiology .
Caplan has seen approximately 12 T indotineae cases to date, including the first two confirmed US cases, which he and co-authors, including Gold, reported in the CDC’s Morbidity and Mortality Weekly Report in May 2023. T indotineae is likely underreported, he said, because it eludes standard culture-based techniques, and identifying it requires molecular testing, which is available at only a handful of labs nationally.
To help educate providers, in July, the American Academy of Dermatology (AAD) and the International League of Dermatological Societies unveiled an Emerging Diseases Resource Center, which includes resources for providers and a registry for reporting confirmed and suspected resistant dermatophytes.
“Our goal is to provide easy-to-access and easy-to-understand resources to healthcare providers,” Esther Freeman, MD, PhD, told this news organization. She is director of Global Health Dermatology at Massachusetts General Hospital, associate professor of dermatology at Harvard Medical School, both in Boston, and chair of the AAD’s Emerging Diseases Task Force.
“Our resources include an algorithm for when to suspect a drug-resistant case and how to think through treatment options. We cover issues related to diagnosis and treatment, as well as linking to our case registry reporting system,” said Freeman.
The new registry resides within the AAD’s existing COVID-19, Mpox, and Emerging Infections Registry. “Our registry efforts have already captured 2500 COVID-19 and mpox cases from 72 different countries,” Freeman said. For all these infections, she added, “we hope that real-time data analysis of cases worldwide will provide information that helps physicians recognize and treat cases.”
Consistent with the registry’s approach, said Caplan and Gold, there is no silver bullet for battling dermatophyte resistance. What is needed, said Gold, is a coordinated approach involving public health officials, dermatologists, primary care providers, infectious disease specialists, pharmacists, and patients. “It’s going to be a team effort to address the challenge of emerging complex dermatophytosis,” he said.
Resistant T rubrum
“The biggest difference with T rubrum resistance is you may not see that widespread infection that we see with T indotineae,” said Caplan. T rubrum is probably the most common dermatophyte that dermatologists see, added Elewski, who encounters a resistant case at least monthly. One such patient, featured in a January 2021 British Journal of Dermatology research letter, cleared on itraconazole and ciclopirox cream but subsequently returned with itraconazole-resistant T rubrum because he had been doctor-shopping for the drug intermittently for years, she said. He cleared on posaconazole 300 mg daily, then was lost to follow-up.
TMVII
A 2023 Emerging Infectious Diseases report highlighted the potential for this dermatophyte to spread among men who have sex with men (MSM), presenting as an itchy, scaly rash affecting the pubic, genital, and buttocks skin. “People don’t generally think of a fungal infection as something that could behave like a sexually transmitted infection (STI),” said Gold.
Caplan and coauthors recently reported the first confirmed US TMVII case in JAMA Dermatology. Many experts suspect that unreported US cases existed previously, he said. “When it circulates in Europe and there’s so much travel, it’s probably here too.”
The fact that T indotineae was formerly called TMVIII has created confusion, added Caplan. “I’ve had patients say, ‘I’m worried I have that resistant ringworm that’s spreading among MSM.’ Whenever we talk about STIs and introduce the word ‘resistant,’ that comes with the potential for stigma, anxiety, and concern.” Fortunately, he said, TMVII has shown no resistance to first-line antifungals.
Why the Rise
Gold said, “We don’t know for sure why we’re seeing these different drug-resistant species popping up.” One possibility, he said, is the common misuse and overuse of topical antifungals — especially those available overseas in combination with high-potency steroids, such as clobetasol. Consumers use these products for a few weeks until symptoms resolve, then reapply them off and on over years, fueling resistance, said Gold.
“We are worried that with warming temperatures, there’s potential to see expansion of the geographic range of epidemic fungi,” he added. “That could be part of what has fueled recent increases in resistant dermatophytes. But it’s hard to prove.”
Climate change may be behind the emergence of Candida auris, according to a 2022 article in The Lancet Regional Health – Americas. This potentially fatal multidrug-resistant infection spreads easily among sick patients in healthcare facilities, according to a CDC information page on C auris.
Confirming Dermatophyte Infection
“A biopsy will only confirm the presence of fungus,” said Elewski. “Here you will need a lab that knows how to do a fungal culture.” Most state laboratories can do this, she said, as can some hospitals and special labs such as CMM in Cleveland.
It takes a Clinical Laboratory Improvement Amendments–certified lab to perform KOH prep in-house, added Caplan, plus up-to-date gear and knowledge of where and how to scrape and what to look for microscopically. Moreover, identifying T indotineae requires molecular testing available at only a handful of laboratories — listed on the AAD Emerging Dermatophytes webpage — nationwide.
Nevertheless, said Caplan, nailing down a diagnosis can guide treatment, often supplanting empirically prescribed antifungal steroid creams. “Those are probably not going to help. And people may be using those on areas of the body they shouldn’t. Both the clinical clues and the steps to make the diagnosis need to come together. But that’s often easier said than done, especially in a busy practice.”
Identifying resistance requires antifungal sensitivity testing, he added, which few labs perform. “Practically speaking,” said Elewski, “if the patient failed terbinafine, I would try itraconazole. You don’t necessarily need proof” of resistance. But if a patient does not respond to itraconazole and terbinafine clinically, she said that she might consider fungal susceptibility testing.
Treatment Tips
To address any resistant dermatophyte, Elewski recommended getting comfortable with itraconazole. For decades, she said, dermatologists have avoided itraconazole because terbinafine typically costs patients $10 for 3 months. “Itraconazole could be $200 per month,” said Elewski. Because of potential drug-drug interactions and absorption issues — and a boxed warning regarding congestive heart failure — physicians historically reserved itraconazole for severe fungal infections.
Itraconazole labeled dosing for onychomycosis is 200 mg daily for 12 weeks. Elewski favors a two-pronged attack, often combining an -azole antifungal with topical ciclopirox.
Another element that emerging tinea pathogens share is slower response to treatment. For T indotineae, reports appearing in the Journal of the American Academy of Dermatology in 2022 and 2024 suggest duration from 6-8 weeks up to 20 weeks.
To avoid recurrences of resistant T rubrum, Elewski treats for a year. However, she has problems getting itraconazole approved, when often it is the only agent that works. “I’ve written more letters than I like to insurance companies” to document terbinafine failure, she said.
Rarely, said Gold, dermatophyte infections resist both terbinafine and itraconazole. Next-line agents such as voriconazole, which some dermatologists have used for resistant T indotineae, can be much harder to tolerate, with more drug interactions, he said.
And because itraconazole, voriconazole, and posaconazole are all triazoles, added Elewski, the latter two might not work better than the former. But because these drugs might outperform itraconazole in selected cases, she said, “that’s when you want to do fungal susceptibility testing.”
TMVII is so new, said Caplan, that optimal therapy duration remains unclear. “One of the challenges with TMVII is when it gets into the genital skin, it’s a hair-bearing area. And based on various grooming practices, there’s an opportunity for the tinea to get deeper into the hair follicle and dermis. That may also be true of T indotineae.”
Anemic Arsenal
Unfortunately, said Gold, the arsenal of antifungals available in the United States remains limited. “Depending on how you count, there are only three to four classes of antifungal drugs designed to treat severe or invasive infections. So whenever we hear about a new fungal pathogen that’s causing resistant infections, it causes public health concern.”
Promising drugs in development include olorofim (F2G) and fosmanogepix (Basilea), according to Gold. However, he said, the development of these drugs to date has targeted invasive fungal infections such as aspergillosis. In June 2023, the Food and Drug Administration rejected the new drug application for olorofim, requesting additional data and analyses. Regarding fosmanogepix, a double-blinded noninferiority phase 3 trial in invasive yeast infections was recently launched, according to a September 24 press release.
Gold, Caplan, and Elewski reported no relevant financial disclosures. Freeman is a COVID-19 co-author for UpToDate and chair of the AAD Emerging Diseases Task Force.
A version of this article appeared on Medscape.com.
As dermatologists, public health officials, and infectious disease specialists scramble to raise awareness about prevention and treatment, challenges ranging from a dearth of testing facilities and data to payer pushback over longer therapeutic courses remain.
Dermatophyte Discourse Changing
“Trichophyton indotineae is changing the way we talk about dermatophyte infections,” Avrom S. Caplan, MD, assistant professor in the Department of Dermatology at New York University, New York City, said in an interview. Called T mentagrophytes VIII (TMVIII) before a 2020 report in the journal Mycopathologia proposed the name T indotineae, this species requires clinicians to expand their conception of how tinea looks, acts, and responds to treatment.
Boni E. Elewski, MD, professor and chair of dermatology, at The University of Alabama at Birmingham, saw her first case of probable T indotineae in a patient in early 2020. “He was covered with fine scale, and he itched all over. I thought he had atopic dermatitis. This didn’t look like any tinea. His face, arms, back, and legs were scaly.”
Nevertheless, KOH and biopsy confirmed dermatophytosis. Culture (performed at the Center for Medical Mycology [CMM] in Cleveland) identified T mentagrophytes. Back then, Elewski told this news organization, labs did not routinely go beyond genus and species. But based on the patient’s symptoms, history of unresponsiveness to terbinafine, borderline sensitivity to fluconazole, and travel to India and Spain, Elewski strongly suspected T indotineae.
The patient refused itraconazole, to which the fungus was sensitive, and did not respond to fluconazole 400 mg daily. Ultimately, he was lost to follow-up. “Last I saw him,” said Elewski, “he was not cured.”
Tracking Cases
Because T indotineae does not require reporting to public health agencies, said Jeremy Gold, MD, MS, a medical officer with the US Centers for Disease Control and Prevention (CDC) Mycotic Diseases Branch in Atlanta, “there is no official public health surveillance keeping track of exactly how many cases have occurred.”
The same is true for TMVII and terbinafine-resistant T rubrum, which are also on the rise. Regarding T indotineae, authors from the University of Texas Health Science Center at San Antonio retrospectively reported 21 terbinafine-resistant isolates from North America in the July 2023 Journal of Clinical Microbiology .
Caplan has seen approximately 12 T indotineae cases to date, including the first two confirmed US cases, which he and co-authors, including Gold, reported in the CDC’s Morbidity and Mortality Weekly Report in May 2023. T indotineae is likely underreported, he said, because it eludes standard culture-based techniques, and identifying it requires molecular testing, which is available at only a handful of labs nationally.
To help educate providers, in July, the American Academy of Dermatology (AAD) and the International League of Dermatological Societies unveiled an Emerging Diseases Resource Center, which includes resources for providers and a registry for reporting confirmed and suspected resistant dermatophytes.
“Our goal is to provide easy-to-access and easy-to-understand resources to healthcare providers,” Esther Freeman, MD, PhD, told this news organization. She is director of Global Health Dermatology at Massachusetts General Hospital, associate professor of dermatology at Harvard Medical School, both in Boston, and chair of the AAD’s Emerging Diseases Task Force.
“Our resources include an algorithm for when to suspect a drug-resistant case and how to think through treatment options. We cover issues related to diagnosis and treatment, as well as linking to our case registry reporting system,” said Freeman.
The new registry resides within the AAD’s existing COVID-19, Mpox, and Emerging Infections Registry. “Our registry efforts have already captured 2500 COVID-19 and mpox cases from 72 different countries,” Freeman said. For all these infections, she added, “we hope that real-time data analysis of cases worldwide will provide information that helps physicians recognize and treat cases.”
Consistent with the registry’s approach, said Caplan and Gold, there is no silver bullet for battling dermatophyte resistance. What is needed, said Gold, is a coordinated approach involving public health officials, dermatologists, primary care providers, infectious disease specialists, pharmacists, and patients. “It’s going to be a team effort to address the challenge of emerging complex dermatophytosis,” he said.
Resistant T rubrum
“The biggest difference with T rubrum resistance is you may not see that widespread infection that we see with T indotineae,” said Caplan. T rubrum is probably the most common dermatophyte that dermatologists see, added Elewski, who encounters a resistant case at least monthly. One such patient, featured in a January 2021 British Journal of Dermatology research letter, cleared on itraconazole and ciclopirox cream but subsequently returned with itraconazole-resistant T rubrum because he had been doctor-shopping for the drug intermittently for years, she said. He cleared on posaconazole 300 mg daily, then was lost to follow-up.
TMVII
A 2023 Emerging Infectious Diseases report highlighted the potential for this dermatophyte to spread among men who have sex with men (MSM), presenting as an itchy, scaly rash affecting the pubic, genital, and buttocks skin. “People don’t generally think of a fungal infection as something that could behave like a sexually transmitted infection (STI),” said Gold.
Caplan and coauthors recently reported the first confirmed US TMVII case in JAMA Dermatology. Many experts suspect that unreported US cases existed previously, he said. “When it circulates in Europe and there’s so much travel, it’s probably here too.”
The fact that T indotineae was formerly called TMVIII has created confusion, added Caplan. “I’ve had patients say, ‘I’m worried I have that resistant ringworm that’s spreading among MSM.’ Whenever we talk about STIs and introduce the word ‘resistant,’ that comes with the potential for stigma, anxiety, and concern.” Fortunately, he said, TMVII has shown no resistance to first-line antifungals.
Why the Rise
Gold said, “We don’t know for sure why we’re seeing these different drug-resistant species popping up.” One possibility, he said, is the common misuse and overuse of topical antifungals — especially those available overseas in combination with high-potency steroids, such as clobetasol. Consumers use these products for a few weeks until symptoms resolve, then reapply them off and on over years, fueling resistance, said Gold.
“We are worried that with warming temperatures, there’s potential to see expansion of the geographic range of epidemic fungi,” he added. “That could be part of what has fueled recent increases in resistant dermatophytes. But it’s hard to prove.”
Climate change may be behind the emergence of Candida auris, according to a 2022 article in The Lancet Regional Health – Americas. This potentially fatal multidrug-resistant infection spreads easily among sick patients in healthcare facilities, according to a CDC information page on C auris.
Confirming Dermatophyte Infection
“A biopsy will only confirm the presence of fungus,” said Elewski. “Here you will need a lab that knows how to do a fungal culture.” Most state laboratories can do this, she said, as can some hospitals and special labs such as CMM in Cleveland.
It takes a Clinical Laboratory Improvement Amendments–certified lab to perform KOH prep in-house, added Caplan, plus up-to-date gear and knowledge of where and how to scrape and what to look for microscopically. Moreover, identifying T indotineae requires molecular testing available at only a handful of laboratories — listed on the AAD Emerging Dermatophytes webpage — nationwide.
Nevertheless, said Caplan, nailing down a diagnosis can guide treatment, often supplanting empirically prescribed antifungal steroid creams. “Those are probably not going to help. And people may be using those on areas of the body they shouldn’t. Both the clinical clues and the steps to make the diagnosis need to come together. But that’s often easier said than done, especially in a busy practice.”
Identifying resistance requires antifungal sensitivity testing, he added, which few labs perform. “Practically speaking,” said Elewski, “if the patient failed terbinafine, I would try itraconazole. You don’t necessarily need proof” of resistance. But if a patient does not respond to itraconazole and terbinafine clinically, she said that she might consider fungal susceptibility testing.
Treatment Tips
To address any resistant dermatophyte, Elewski recommended getting comfortable with itraconazole. For decades, she said, dermatologists have avoided itraconazole because terbinafine typically costs patients $10 for 3 months. “Itraconazole could be $200 per month,” said Elewski. Because of potential drug-drug interactions and absorption issues — and a boxed warning regarding congestive heart failure — physicians historically reserved itraconazole for severe fungal infections.
Itraconazole labeled dosing for onychomycosis is 200 mg daily for 12 weeks. Elewski favors a two-pronged attack, often combining an -azole antifungal with topical ciclopirox.
Another element that emerging tinea pathogens share is slower response to treatment. For T indotineae, reports appearing in the Journal of the American Academy of Dermatology in 2022 and 2024 suggest duration from 6-8 weeks up to 20 weeks.
To avoid recurrences of resistant T rubrum, Elewski treats for a year. However, she has problems getting itraconazole approved, when often it is the only agent that works. “I’ve written more letters than I like to insurance companies” to document terbinafine failure, she said.
Rarely, said Gold, dermatophyte infections resist both terbinafine and itraconazole. Next-line agents such as voriconazole, which some dermatologists have used for resistant T indotineae, can be much harder to tolerate, with more drug interactions, he said.
And because itraconazole, voriconazole, and posaconazole are all triazoles, added Elewski, the latter two might not work better than the former. But because these drugs might outperform itraconazole in selected cases, she said, “that’s when you want to do fungal susceptibility testing.”
TMVII is so new, said Caplan, that optimal therapy duration remains unclear. “One of the challenges with TMVII is when it gets into the genital skin, it’s a hair-bearing area. And based on various grooming practices, there’s an opportunity for the tinea to get deeper into the hair follicle and dermis. That may also be true of T indotineae.”
Anemic Arsenal
Unfortunately, said Gold, the arsenal of antifungals available in the United States remains limited. “Depending on how you count, there are only three to four classes of antifungal drugs designed to treat severe or invasive infections. So whenever we hear about a new fungal pathogen that’s causing resistant infections, it causes public health concern.”
Promising drugs in development include olorofim (F2G) and fosmanogepix (Basilea), according to Gold. However, he said, the development of these drugs to date has targeted invasive fungal infections such as aspergillosis. In June 2023, the Food and Drug Administration rejected the new drug application for olorofim, requesting additional data and analyses. Regarding fosmanogepix, a double-blinded noninferiority phase 3 trial in invasive yeast infections was recently launched, according to a September 24 press release.
Gold, Caplan, and Elewski reported no relevant financial disclosures. Freeman is a COVID-19 co-author for UpToDate and chair of the AAD Emerging Diseases Task Force.
A version of this article appeared on Medscape.com.
As dermatologists, public health officials, and infectious disease specialists scramble to raise awareness about prevention and treatment, challenges ranging from a dearth of testing facilities and data to payer pushback over longer therapeutic courses remain.
Dermatophyte Discourse Changing
“Trichophyton indotineae is changing the way we talk about dermatophyte infections,” Avrom S. Caplan, MD, assistant professor in the Department of Dermatology at New York University, New York City, said in an interview. Called T mentagrophytes VIII (TMVIII) before a 2020 report in the journal Mycopathologia proposed the name T indotineae, this species requires clinicians to expand their conception of how tinea looks, acts, and responds to treatment.
Boni E. Elewski, MD, professor and chair of dermatology, at The University of Alabama at Birmingham, saw her first case of probable T indotineae in a patient in early 2020. “He was covered with fine scale, and he itched all over. I thought he had atopic dermatitis. This didn’t look like any tinea. His face, arms, back, and legs were scaly.”
Nevertheless, KOH and biopsy confirmed dermatophytosis. Culture (performed at the Center for Medical Mycology [CMM] in Cleveland) identified T mentagrophytes. Back then, Elewski told this news organization, labs did not routinely go beyond genus and species. But based on the patient’s symptoms, history of unresponsiveness to terbinafine, borderline sensitivity to fluconazole, and travel to India and Spain, Elewski strongly suspected T indotineae.
The patient refused itraconazole, to which the fungus was sensitive, and did not respond to fluconazole 400 mg daily. Ultimately, he was lost to follow-up. “Last I saw him,” said Elewski, “he was not cured.”
Tracking Cases
Because T indotineae does not require reporting to public health agencies, said Jeremy Gold, MD, MS, a medical officer with the US Centers for Disease Control and Prevention (CDC) Mycotic Diseases Branch in Atlanta, “there is no official public health surveillance keeping track of exactly how many cases have occurred.”
The same is true for TMVII and terbinafine-resistant T rubrum, which are also on the rise. Regarding T indotineae, authors from the University of Texas Health Science Center at San Antonio retrospectively reported 21 terbinafine-resistant isolates from North America in the July 2023 Journal of Clinical Microbiology .
Caplan has seen approximately 12 T indotineae cases to date, including the first two confirmed US cases, which he and co-authors, including Gold, reported in the CDC’s Morbidity and Mortality Weekly Report in May 2023. T indotineae is likely underreported, he said, because it eludes standard culture-based techniques, and identifying it requires molecular testing, which is available at only a handful of labs nationally.
To help educate providers, in July, the American Academy of Dermatology (AAD) and the International League of Dermatological Societies unveiled an Emerging Diseases Resource Center, which includes resources for providers and a registry for reporting confirmed and suspected resistant dermatophytes.
“Our goal is to provide easy-to-access and easy-to-understand resources to healthcare providers,” Esther Freeman, MD, PhD, told this news organization. She is director of Global Health Dermatology at Massachusetts General Hospital, associate professor of dermatology at Harvard Medical School, both in Boston, and chair of the AAD’s Emerging Diseases Task Force.
“Our resources include an algorithm for when to suspect a drug-resistant case and how to think through treatment options. We cover issues related to diagnosis and treatment, as well as linking to our case registry reporting system,” said Freeman.
The new registry resides within the AAD’s existing COVID-19, Mpox, and Emerging Infections Registry. “Our registry efforts have already captured 2500 COVID-19 and mpox cases from 72 different countries,” Freeman said. For all these infections, she added, “we hope that real-time data analysis of cases worldwide will provide information that helps physicians recognize and treat cases.”
Consistent with the registry’s approach, said Caplan and Gold, there is no silver bullet for battling dermatophyte resistance. What is needed, said Gold, is a coordinated approach involving public health officials, dermatologists, primary care providers, infectious disease specialists, pharmacists, and patients. “It’s going to be a team effort to address the challenge of emerging complex dermatophytosis,” he said.
Resistant T rubrum
“The biggest difference with T rubrum resistance is you may not see that widespread infection that we see with T indotineae,” said Caplan. T rubrum is probably the most common dermatophyte that dermatologists see, added Elewski, who encounters a resistant case at least monthly. One such patient, featured in a January 2021 British Journal of Dermatology research letter, cleared on itraconazole and ciclopirox cream but subsequently returned with itraconazole-resistant T rubrum because he had been doctor-shopping for the drug intermittently for years, she said. He cleared on posaconazole 300 mg daily, then was lost to follow-up.
TMVII
A 2023 Emerging Infectious Diseases report highlighted the potential for this dermatophyte to spread among men who have sex with men (MSM), presenting as an itchy, scaly rash affecting the pubic, genital, and buttocks skin. “People don’t generally think of a fungal infection as something that could behave like a sexually transmitted infection (STI),” said Gold.
Caplan and coauthors recently reported the first confirmed US TMVII case in JAMA Dermatology. Many experts suspect that unreported US cases existed previously, he said. “When it circulates in Europe and there’s so much travel, it’s probably here too.”
The fact that T indotineae was formerly called TMVIII has created confusion, added Caplan. “I’ve had patients say, ‘I’m worried I have that resistant ringworm that’s spreading among MSM.’ Whenever we talk about STIs and introduce the word ‘resistant,’ that comes with the potential for stigma, anxiety, and concern.” Fortunately, he said, TMVII has shown no resistance to first-line antifungals.
Why the Rise
Gold said, “We don’t know for sure why we’re seeing these different drug-resistant species popping up.” One possibility, he said, is the common misuse and overuse of topical antifungals — especially those available overseas in combination with high-potency steroids, such as clobetasol. Consumers use these products for a few weeks until symptoms resolve, then reapply them off and on over years, fueling resistance, said Gold.
“We are worried that with warming temperatures, there’s potential to see expansion of the geographic range of epidemic fungi,” he added. “That could be part of what has fueled recent increases in resistant dermatophytes. But it’s hard to prove.”
Climate change may be behind the emergence of Candida auris, according to a 2022 article in The Lancet Regional Health – Americas. This potentially fatal multidrug-resistant infection spreads easily among sick patients in healthcare facilities, according to a CDC information page on C auris.
Confirming Dermatophyte Infection
“A biopsy will only confirm the presence of fungus,” said Elewski. “Here you will need a lab that knows how to do a fungal culture.” Most state laboratories can do this, she said, as can some hospitals and special labs such as CMM in Cleveland.
It takes a Clinical Laboratory Improvement Amendments–certified lab to perform KOH prep in-house, added Caplan, plus up-to-date gear and knowledge of where and how to scrape and what to look for microscopically. Moreover, identifying T indotineae requires molecular testing available at only a handful of laboratories — listed on the AAD Emerging Dermatophytes webpage — nationwide.
Nevertheless, said Caplan, nailing down a diagnosis can guide treatment, often supplanting empirically prescribed antifungal steroid creams. “Those are probably not going to help. And people may be using those on areas of the body they shouldn’t. Both the clinical clues and the steps to make the diagnosis need to come together. But that’s often easier said than done, especially in a busy practice.”
Identifying resistance requires antifungal sensitivity testing, he added, which few labs perform. “Practically speaking,” said Elewski, “if the patient failed terbinafine, I would try itraconazole. You don’t necessarily need proof” of resistance. But if a patient does not respond to itraconazole and terbinafine clinically, she said that she might consider fungal susceptibility testing.
Treatment Tips
To address any resistant dermatophyte, Elewski recommended getting comfortable with itraconazole. For decades, she said, dermatologists have avoided itraconazole because terbinafine typically costs patients $10 for 3 months. “Itraconazole could be $200 per month,” said Elewski. Because of potential drug-drug interactions and absorption issues — and a boxed warning regarding congestive heart failure — physicians historically reserved itraconazole for severe fungal infections.
Itraconazole labeled dosing for onychomycosis is 200 mg daily for 12 weeks. Elewski favors a two-pronged attack, often combining an -azole antifungal with topical ciclopirox.
Another element that emerging tinea pathogens share is slower response to treatment. For T indotineae, reports appearing in the Journal of the American Academy of Dermatology in 2022 and 2024 suggest duration from 6-8 weeks up to 20 weeks.
To avoid recurrences of resistant T rubrum, Elewski treats for a year. However, she has problems getting itraconazole approved, when often it is the only agent that works. “I’ve written more letters than I like to insurance companies” to document terbinafine failure, she said.
Rarely, said Gold, dermatophyte infections resist both terbinafine and itraconazole. Next-line agents such as voriconazole, which some dermatologists have used for resistant T indotineae, can be much harder to tolerate, with more drug interactions, he said.
And because itraconazole, voriconazole, and posaconazole are all triazoles, added Elewski, the latter two might not work better than the former. But because these drugs might outperform itraconazole in selected cases, she said, “that’s when you want to do fungal susceptibility testing.”
TMVII is so new, said Caplan, that optimal therapy duration remains unclear. “One of the challenges with TMVII is when it gets into the genital skin, it’s a hair-bearing area. And based on various grooming practices, there’s an opportunity for the tinea to get deeper into the hair follicle and dermis. That may also be true of T indotineae.”
Anemic Arsenal
Unfortunately, said Gold, the arsenal of antifungals available in the United States remains limited. “Depending on how you count, there are only three to four classes of antifungal drugs designed to treat severe or invasive infections. So whenever we hear about a new fungal pathogen that’s causing resistant infections, it causes public health concern.”
Promising drugs in development include olorofim (F2G) and fosmanogepix (Basilea), according to Gold. However, he said, the development of these drugs to date has targeted invasive fungal infections such as aspergillosis. In June 2023, the Food and Drug Administration rejected the new drug application for olorofim, requesting additional data and analyses. Regarding fosmanogepix, a double-blinded noninferiority phase 3 trial in invasive yeast infections was recently launched, according to a September 24 press release.
Gold, Caplan, and Elewski reported no relevant financial disclosures. Freeman is a COVID-19 co-author for UpToDate and chair of the AAD Emerging Diseases Task Force.
A version of this article appeared on Medscape.com.
Should First-Line Dual Checkpoint Blockade Be Used for NSCLC With Specific Mutations?
according to the authors of a new paper.
These findings, drawn from a post hoc analysis of phase 3 data, are backed up by cell line and mouse data revealing clear mechanisms of efficacy, making the collective evidence compelling enough to reshape clinical practice, reported lead author Ferdinandos Skoulidis, MD, PhD, of The University of Texas MD Anderson Cancer Center, Houston.
“Although STK11 and KEAP1 mutations are associated with limited benefit from PD-1 or PD-L1 [PD-(L)1] inhibition, the association between these mutations and benefit from combinations of PD-(L)1 inhibitors with chemotherapy is not yet as well established,” the investigators wrote in Nature.
Skoulidis and colleagues conducted the subgroup analysis of POSEIDON trial data and characterized underlying biologic mechanisms using mouse models to address this knowledge gap.
What Were the Original Findings of POSEIDON?
The POSEIDON trial involved 1013 patients with metastatic NSCLC. Treatment arms included standard chemotherapy alone, chemotherapy plus programmed death ligand 1 (PD-L1) inhibitor durvalumab, and chemotherapy plus durvalumab and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor tremelimumab.
Adding durvalumab to chemotherapy significantly improved median progression-free survival (PFS) but not median overall survival (OS), while dual checkpoint blockade boosted both PFS and OS.
These findings provided support for the dual approach in the first-line setting, but not preferentially so. Experts called for more long-term data, questioned the survival benefit in terms of the increased toxicity, and noted the lack of biomarkers for patient selection.
What Did Post Hoc Analysis Highlight About POSEIDON?
The present analysis aimed to validate two actionable biomarkers.
“We and others have previously observed that alterations in STK11 and KEAP1 can promote an immunosuppressive tumor microenvironment and together might be responsible for half or more of the primary resistance to PD-(L)1 inhibition among patients with nsNSCLC when given as monotherapy,” Skoulidis and colleagues wrote.
From the original 1013 patients, 612 had non-squamous NSCLC and were evaluable for mutations. Among them, 87 had STK11 mutations and 37 had KEAP1 mutations.
As anticipated, patients in the STK11/KEAP1 subgroup saw little to no benefit from adding durvalumab to chemotherapy, but adding tremelimumab on top yielded notable improvement.
This was first observed in the objective response rate, which was 42.9% with dual checkpoint blockade plus chemotherapy vs 30.2% with single checkpoint blockade plus chemotherapy and 28% for chemotherapy alone. Durations of response improved in kind.
Survival outcomes also trended toward improvement in the dual checkpoint arm, which had a median OS of 15.8 months vs 7.3 months for durvalumab plus chemotherapy (hazard ratio [HR], 0.64; 95% CI, 0.40-1.04) and 10.5 months for chemotherapy alone (HR, 0.50; 95% CI, 0.29-0.87). PFS showed similar trends.
How Do Findings Relate to Previous NSCLC Subgroup Research?
Skoulidis and colleagues noted that their findings align with those of the CheckMate 9LA trial, which showed that patients with STK11 and/or KEAP1 mutations had better outcomes with dual checkpoint blockade plus chemotherapy than with chemotherapy alone.
“These data support the hypothesis that CTLA-4 inhibition can mitigate the resistance to chemotherapy plus PD-(L)1 inhibition observed in patients who have STK11 and/or KEAP1 mutations and suggest that this group of patients derives greater benefit from CTLA-4 inhibition than do patients who lack either alteration,” Skoulidis and colleagues wrote.
Grace Dy, MD, professor of oncology in the Department of Medicine at Roswell Park Comprehensive Cancer Center, Buffalo, New York, noted that in the present analysis, PD-L1 expression status did not predict outcomes; however, patients with STK11 and/or KEAP1 mutations typically have low or negative PD-L1 expression, which has been linked with better responses to CTLA-4 inhibition in multiple trials.
“In the CheckMate 227 and CheckMate 9LA studies, we have seen that patients with PD-L1–negative tumors appear to derive greater and more durable long-term overall survival benefit from dual immune checkpoint blockade compared to patients receiving anti-PD1-based therapy alone,” Dy said in a written comment. “While we take the necessary caveats on cross-trial comparisons, the same survival trend favoring CTLA-4-based immune checkpoint blockade is seen compared to the tail of the survival curves observed in PD-L1–negative patients enrolled in the KEYNOTE studies (KEYNOTE-189, KEYNOTE-407).”
Detecting improvements in survival within PD-L1 patients “may not be readily apparent until later when looking at the tail of the survival curves,” she added.
What Mechanisms of Action Explain Relative Benefits of Dual Checkpoint Blockade?
To elucidate underlying mechanisms of action, Skoulidis and colleagues conducted a series of experiments involving cell lines and mouse models of Stk11- and Keap1-deficient NSCLC.
“For us, it was critical to provide mechanistic support for the observed clinical benefit in POSEIDON, especially since this is based on a retrospective subgroup analysis,” Skoulidis said in an interview.
Their efforts revealed a strong link between the mutations and resistance to PD-(L)1 inhibition.
Inactivation of Stk11 and Keap1 promoted an immunosuppressive tumor microenvironment, marked by increased infiltration of suppressive myeloid cells and a reduction in CD8+ effector T cells. This immune imbalance contributed to evasion of immune destruction and limited the efficacy of programmed cell death protein 1 (PD-1) blockade.
Dual checkpoint blockade reprogrammed the immune microenvironment, leading to increased activation of CD4+ T helper (Th) cells, specifically the Th1 subtype, while inducing tumoricidal changes in myeloid cells. Consequently, antitumor responses improved, resulting in tumor regression and prolonged survival, compared with PD-1 monotherapy.
“Addition of CTLA-4 [inhibition] turns the two cardinal components of the suppressive microenvironment of these tumors on its head, and that’s why we believe we are observing this clinical benefit,” Skoulidis said. “This is not a mere association…but also based on very solid mechanistic data across a multitude of different models.”
Are Data Sufficient to Shift to First-Line Dual Checkpoint Blockade?
“Our work strengthens the available evidence that this regimen — and chemoimmunotherapy more broadly, with dual immune checkpoint blockade — constitutes a preferred approach for these patients,” Skoulidis said. “I personally, and I think physicians within MD Anderson, as well as a lot of physicians that I talk to, are already using — based on these data — the POSEIDON regimen, as well as, more broadly, chemoimmunotherapy with dual immune checkpoint for patients with these alterations.”
This view, however, remains contested by some oncologists.
Lei Deng, MD, assistant professor in the Division of Hematology and Oncology at the University of Washington, Fred Hutchinson Cancer Center, Seattle, called the new data “intriguing” and “hypothesis-generating,” but stopped short of supporting preferential first-line use.
“This study is a post hoc analysis, so you don’t have a lot of patients,” Deng said. “It is still not strong enough or definitive enough to make it standard of care to use dual checkpoint blockade for [patients with STK11 and/or KEAP1 mutations].”
The cell line and mouse data help explain biologic mechanisms of efficacy, he said, but these findings do not obviate toxicity concerns.
“You are adding one more agent, and this agent is more toxic than single checkpoint blockade,” Deng said. “So, if you weigh the risk, it is known, [but] the benefit is suggestive. I am not sure if the risk-benefit ratio would argue for routine implementation of this regimen yet.”
On the other hand, he noted, the combination is the US Food and Drug Administration–approved in this setting, so “it is not wrong to use it.”
Jyoti Malhotra, MD, director of thoracic medical oncology at City of Hope Orange County in Irvine, California, had a similar take.
“The clinical data presented so far is exploratory and limited by the small sample size,” Malhotra said in a written comment. “Data from an ongoing phase 3 trial (TRITON) is awaited before dual checkpoint blockade becomes the standard of care in this setting.”
Hossein Borghaei, DO, chief of the Division of Thoracic Medical Oncology at Fox Chase Cancer Center, Philadelphia, was also unequivocal when asked if dual checkpoint blockade with chemotherapy should be considered the preferred first-line treatment option in patients with STK11 and/or KEAP1 mutations.
“No,” he said in a written comment. “The data and the hypothesis are very strong, but it is all based on retrospective clinical data, cell line data, and mouse models. We need a randomized study to test the hypothesis.”
Incidentally, Borghaei is on the steering committee for the TRITON trial. He shared this potential conflict of interest before praising Skoulidis and colleagues for their efforts, noting that the present findings underscore the broader importance of widespread tumor profiling and access to resultant data.
“This is a beautiful story that has developed over the last few years based on the research by the group from MD Anderson who has reported the current Nature article,” he said. “This highlights the possible utility of collecting sequencing data on [all] patients’ tumors. These sorts of understandings and new ideas could arise only if there is access to this information.”
The study was supported by AstraZeneca, the National Cancer Institute, the Gunnigar Fund, and others. The investigators disclosed additional relationships with Novartis, Merck, Amgen, and others. Deng disclosed relationships with Merck, BridgeBio, MJH Life Sciences, and others. Dy disclosed relationships with Eli Lilly and Company, Janssen Pharmaceuticals, Meru, and others. Malhotra has previously served as a consultant for AstraZeneca. Borghaei has served as a consultant for AstraZeneca and is on the steering committee for the TRITON trial.
A version of this article appeared on Medscape.com.
according to the authors of a new paper.
These findings, drawn from a post hoc analysis of phase 3 data, are backed up by cell line and mouse data revealing clear mechanisms of efficacy, making the collective evidence compelling enough to reshape clinical practice, reported lead author Ferdinandos Skoulidis, MD, PhD, of The University of Texas MD Anderson Cancer Center, Houston.
“Although STK11 and KEAP1 mutations are associated with limited benefit from PD-1 or PD-L1 [PD-(L)1] inhibition, the association between these mutations and benefit from combinations of PD-(L)1 inhibitors with chemotherapy is not yet as well established,” the investigators wrote in Nature.
Skoulidis and colleagues conducted the subgroup analysis of POSEIDON trial data and characterized underlying biologic mechanisms using mouse models to address this knowledge gap.
What Were the Original Findings of POSEIDON?
The POSEIDON trial involved 1013 patients with metastatic NSCLC. Treatment arms included standard chemotherapy alone, chemotherapy plus programmed death ligand 1 (PD-L1) inhibitor durvalumab, and chemotherapy plus durvalumab and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor tremelimumab.
Adding durvalumab to chemotherapy significantly improved median progression-free survival (PFS) but not median overall survival (OS), while dual checkpoint blockade boosted both PFS and OS.
These findings provided support for the dual approach in the first-line setting, but not preferentially so. Experts called for more long-term data, questioned the survival benefit in terms of the increased toxicity, and noted the lack of biomarkers for patient selection.
What Did Post Hoc Analysis Highlight About POSEIDON?
The present analysis aimed to validate two actionable biomarkers.
“We and others have previously observed that alterations in STK11 and KEAP1 can promote an immunosuppressive tumor microenvironment and together might be responsible for half or more of the primary resistance to PD-(L)1 inhibition among patients with nsNSCLC when given as monotherapy,” Skoulidis and colleagues wrote.
From the original 1013 patients, 612 had non-squamous NSCLC and were evaluable for mutations. Among them, 87 had STK11 mutations and 37 had KEAP1 mutations.
As anticipated, patients in the STK11/KEAP1 subgroup saw little to no benefit from adding durvalumab to chemotherapy, but adding tremelimumab on top yielded notable improvement.
This was first observed in the objective response rate, which was 42.9% with dual checkpoint blockade plus chemotherapy vs 30.2% with single checkpoint blockade plus chemotherapy and 28% for chemotherapy alone. Durations of response improved in kind.
Survival outcomes also trended toward improvement in the dual checkpoint arm, which had a median OS of 15.8 months vs 7.3 months for durvalumab plus chemotherapy (hazard ratio [HR], 0.64; 95% CI, 0.40-1.04) and 10.5 months for chemotherapy alone (HR, 0.50; 95% CI, 0.29-0.87). PFS showed similar trends.
How Do Findings Relate to Previous NSCLC Subgroup Research?
Skoulidis and colleagues noted that their findings align with those of the CheckMate 9LA trial, which showed that patients with STK11 and/or KEAP1 mutations had better outcomes with dual checkpoint blockade plus chemotherapy than with chemotherapy alone.
“These data support the hypothesis that CTLA-4 inhibition can mitigate the resistance to chemotherapy plus PD-(L)1 inhibition observed in patients who have STK11 and/or KEAP1 mutations and suggest that this group of patients derives greater benefit from CTLA-4 inhibition than do patients who lack either alteration,” Skoulidis and colleagues wrote.
Grace Dy, MD, professor of oncology in the Department of Medicine at Roswell Park Comprehensive Cancer Center, Buffalo, New York, noted that in the present analysis, PD-L1 expression status did not predict outcomes; however, patients with STK11 and/or KEAP1 mutations typically have low or negative PD-L1 expression, which has been linked with better responses to CTLA-4 inhibition in multiple trials.
“In the CheckMate 227 and CheckMate 9LA studies, we have seen that patients with PD-L1–negative tumors appear to derive greater and more durable long-term overall survival benefit from dual immune checkpoint blockade compared to patients receiving anti-PD1-based therapy alone,” Dy said in a written comment. “While we take the necessary caveats on cross-trial comparisons, the same survival trend favoring CTLA-4-based immune checkpoint blockade is seen compared to the tail of the survival curves observed in PD-L1–negative patients enrolled in the KEYNOTE studies (KEYNOTE-189, KEYNOTE-407).”
Detecting improvements in survival within PD-L1 patients “may not be readily apparent until later when looking at the tail of the survival curves,” she added.
What Mechanisms of Action Explain Relative Benefits of Dual Checkpoint Blockade?
To elucidate underlying mechanisms of action, Skoulidis and colleagues conducted a series of experiments involving cell lines and mouse models of Stk11- and Keap1-deficient NSCLC.
“For us, it was critical to provide mechanistic support for the observed clinical benefit in POSEIDON, especially since this is based on a retrospective subgroup analysis,” Skoulidis said in an interview.
Their efforts revealed a strong link between the mutations and resistance to PD-(L)1 inhibition.
Inactivation of Stk11 and Keap1 promoted an immunosuppressive tumor microenvironment, marked by increased infiltration of suppressive myeloid cells and a reduction in CD8+ effector T cells. This immune imbalance contributed to evasion of immune destruction and limited the efficacy of programmed cell death protein 1 (PD-1) blockade.
Dual checkpoint blockade reprogrammed the immune microenvironment, leading to increased activation of CD4+ T helper (Th) cells, specifically the Th1 subtype, while inducing tumoricidal changes in myeloid cells. Consequently, antitumor responses improved, resulting in tumor regression and prolonged survival, compared with PD-1 monotherapy.
“Addition of CTLA-4 [inhibition] turns the two cardinal components of the suppressive microenvironment of these tumors on its head, and that’s why we believe we are observing this clinical benefit,” Skoulidis said. “This is not a mere association…but also based on very solid mechanistic data across a multitude of different models.”
Are Data Sufficient to Shift to First-Line Dual Checkpoint Blockade?
“Our work strengthens the available evidence that this regimen — and chemoimmunotherapy more broadly, with dual immune checkpoint blockade — constitutes a preferred approach for these patients,” Skoulidis said. “I personally, and I think physicians within MD Anderson, as well as a lot of physicians that I talk to, are already using — based on these data — the POSEIDON regimen, as well as, more broadly, chemoimmunotherapy with dual immune checkpoint for patients with these alterations.”
This view, however, remains contested by some oncologists.
Lei Deng, MD, assistant professor in the Division of Hematology and Oncology at the University of Washington, Fred Hutchinson Cancer Center, Seattle, called the new data “intriguing” and “hypothesis-generating,” but stopped short of supporting preferential first-line use.
“This study is a post hoc analysis, so you don’t have a lot of patients,” Deng said. “It is still not strong enough or definitive enough to make it standard of care to use dual checkpoint blockade for [patients with STK11 and/or KEAP1 mutations].”
The cell line and mouse data help explain biologic mechanisms of efficacy, he said, but these findings do not obviate toxicity concerns.
“You are adding one more agent, and this agent is more toxic than single checkpoint blockade,” Deng said. “So, if you weigh the risk, it is known, [but] the benefit is suggestive. I am not sure if the risk-benefit ratio would argue for routine implementation of this regimen yet.”
On the other hand, he noted, the combination is the US Food and Drug Administration–approved in this setting, so “it is not wrong to use it.”
Jyoti Malhotra, MD, director of thoracic medical oncology at City of Hope Orange County in Irvine, California, had a similar take.
“The clinical data presented so far is exploratory and limited by the small sample size,” Malhotra said in a written comment. “Data from an ongoing phase 3 trial (TRITON) is awaited before dual checkpoint blockade becomes the standard of care in this setting.”
Hossein Borghaei, DO, chief of the Division of Thoracic Medical Oncology at Fox Chase Cancer Center, Philadelphia, was also unequivocal when asked if dual checkpoint blockade with chemotherapy should be considered the preferred first-line treatment option in patients with STK11 and/or KEAP1 mutations.
“No,” he said in a written comment. “The data and the hypothesis are very strong, but it is all based on retrospective clinical data, cell line data, and mouse models. We need a randomized study to test the hypothesis.”
Incidentally, Borghaei is on the steering committee for the TRITON trial. He shared this potential conflict of interest before praising Skoulidis and colleagues for their efforts, noting that the present findings underscore the broader importance of widespread tumor profiling and access to resultant data.
“This is a beautiful story that has developed over the last few years based on the research by the group from MD Anderson who has reported the current Nature article,” he said. “This highlights the possible utility of collecting sequencing data on [all] patients’ tumors. These sorts of understandings and new ideas could arise only if there is access to this information.”
The study was supported by AstraZeneca, the National Cancer Institute, the Gunnigar Fund, and others. The investigators disclosed additional relationships with Novartis, Merck, Amgen, and others. Deng disclosed relationships with Merck, BridgeBio, MJH Life Sciences, and others. Dy disclosed relationships with Eli Lilly and Company, Janssen Pharmaceuticals, Meru, and others. Malhotra has previously served as a consultant for AstraZeneca. Borghaei has served as a consultant for AstraZeneca and is on the steering committee for the TRITON trial.
A version of this article appeared on Medscape.com.
according to the authors of a new paper.
These findings, drawn from a post hoc analysis of phase 3 data, are backed up by cell line and mouse data revealing clear mechanisms of efficacy, making the collective evidence compelling enough to reshape clinical practice, reported lead author Ferdinandos Skoulidis, MD, PhD, of The University of Texas MD Anderson Cancer Center, Houston.
“Although STK11 and KEAP1 mutations are associated with limited benefit from PD-1 or PD-L1 [PD-(L)1] inhibition, the association between these mutations and benefit from combinations of PD-(L)1 inhibitors with chemotherapy is not yet as well established,” the investigators wrote in Nature.
Skoulidis and colleagues conducted the subgroup analysis of POSEIDON trial data and characterized underlying biologic mechanisms using mouse models to address this knowledge gap.
What Were the Original Findings of POSEIDON?
The POSEIDON trial involved 1013 patients with metastatic NSCLC. Treatment arms included standard chemotherapy alone, chemotherapy plus programmed death ligand 1 (PD-L1) inhibitor durvalumab, and chemotherapy plus durvalumab and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor tremelimumab.
Adding durvalumab to chemotherapy significantly improved median progression-free survival (PFS) but not median overall survival (OS), while dual checkpoint blockade boosted both PFS and OS.
These findings provided support for the dual approach in the first-line setting, but not preferentially so. Experts called for more long-term data, questioned the survival benefit in terms of the increased toxicity, and noted the lack of biomarkers for patient selection.
What Did Post Hoc Analysis Highlight About POSEIDON?
The present analysis aimed to validate two actionable biomarkers.
“We and others have previously observed that alterations in STK11 and KEAP1 can promote an immunosuppressive tumor microenvironment and together might be responsible for half or more of the primary resistance to PD-(L)1 inhibition among patients with nsNSCLC when given as monotherapy,” Skoulidis and colleagues wrote.
From the original 1013 patients, 612 had non-squamous NSCLC and were evaluable for mutations. Among them, 87 had STK11 mutations and 37 had KEAP1 mutations.
As anticipated, patients in the STK11/KEAP1 subgroup saw little to no benefit from adding durvalumab to chemotherapy, but adding tremelimumab on top yielded notable improvement.
This was first observed in the objective response rate, which was 42.9% with dual checkpoint blockade plus chemotherapy vs 30.2% with single checkpoint blockade plus chemotherapy and 28% for chemotherapy alone. Durations of response improved in kind.
Survival outcomes also trended toward improvement in the dual checkpoint arm, which had a median OS of 15.8 months vs 7.3 months for durvalumab plus chemotherapy (hazard ratio [HR], 0.64; 95% CI, 0.40-1.04) and 10.5 months for chemotherapy alone (HR, 0.50; 95% CI, 0.29-0.87). PFS showed similar trends.
How Do Findings Relate to Previous NSCLC Subgroup Research?
Skoulidis and colleagues noted that their findings align with those of the CheckMate 9LA trial, which showed that patients with STK11 and/or KEAP1 mutations had better outcomes with dual checkpoint blockade plus chemotherapy than with chemotherapy alone.
“These data support the hypothesis that CTLA-4 inhibition can mitigate the resistance to chemotherapy plus PD-(L)1 inhibition observed in patients who have STK11 and/or KEAP1 mutations and suggest that this group of patients derives greater benefit from CTLA-4 inhibition than do patients who lack either alteration,” Skoulidis and colleagues wrote.
Grace Dy, MD, professor of oncology in the Department of Medicine at Roswell Park Comprehensive Cancer Center, Buffalo, New York, noted that in the present analysis, PD-L1 expression status did not predict outcomes; however, patients with STK11 and/or KEAP1 mutations typically have low or negative PD-L1 expression, which has been linked with better responses to CTLA-4 inhibition in multiple trials.
“In the CheckMate 227 and CheckMate 9LA studies, we have seen that patients with PD-L1–negative tumors appear to derive greater and more durable long-term overall survival benefit from dual immune checkpoint blockade compared to patients receiving anti-PD1-based therapy alone,” Dy said in a written comment. “While we take the necessary caveats on cross-trial comparisons, the same survival trend favoring CTLA-4-based immune checkpoint blockade is seen compared to the tail of the survival curves observed in PD-L1–negative patients enrolled in the KEYNOTE studies (KEYNOTE-189, KEYNOTE-407).”
Detecting improvements in survival within PD-L1 patients “may not be readily apparent until later when looking at the tail of the survival curves,” she added.
What Mechanisms of Action Explain Relative Benefits of Dual Checkpoint Blockade?
To elucidate underlying mechanisms of action, Skoulidis and colleagues conducted a series of experiments involving cell lines and mouse models of Stk11- and Keap1-deficient NSCLC.
“For us, it was critical to provide mechanistic support for the observed clinical benefit in POSEIDON, especially since this is based on a retrospective subgroup analysis,” Skoulidis said in an interview.
Their efforts revealed a strong link between the mutations and resistance to PD-(L)1 inhibition.
Inactivation of Stk11 and Keap1 promoted an immunosuppressive tumor microenvironment, marked by increased infiltration of suppressive myeloid cells and a reduction in CD8+ effector T cells. This immune imbalance contributed to evasion of immune destruction and limited the efficacy of programmed cell death protein 1 (PD-1) blockade.
Dual checkpoint blockade reprogrammed the immune microenvironment, leading to increased activation of CD4+ T helper (Th) cells, specifically the Th1 subtype, while inducing tumoricidal changes in myeloid cells. Consequently, antitumor responses improved, resulting in tumor regression and prolonged survival, compared with PD-1 monotherapy.
“Addition of CTLA-4 [inhibition] turns the two cardinal components of the suppressive microenvironment of these tumors on its head, and that’s why we believe we are observing this clinical benefit,” Skoulidis said. “This is not a mere association…but also based on very solid mechanistic data across a multitude of different models.”
Are Data Sufficient to Shift to First-Line Dual Checkpoint Blockade?
“Our work strengthens the available evidence that this regimen — and chemoimmunotherapy more broadly, with dual immune checkpoint blockade — constitutes a preferred approach for these patients,” Skoulidis said. “I personally, and I think physicians within MD Anderson, as well as a lot of physicians that I talk to, are already using — based on these data — the POSEIDON regimen, as well as, more broadly, chemoimmunotherapy with dual immune checkpoint for patients with these alterations.”
This view, however, remains contested by some oncologists.
Lei Deng, MD, assistant professor in the Division of Hematology and Oncology at the University of Washington, Fred Hutchinson Cancer Center, Seattle, called the new data “intriguing” and “hypothesis-generating,” but stopped short of supporting preferential first-line use.
“This study is a post hoc analysis, so you don’t have a lot of patients,” Deng said. “It is still not strong enough or definitive enough to make it standard of care to use dual checkpoint blockade for [patients with STK11 and/or KEAP1 mutations].”
The cell line and mouse data help explain biologic mechanisms of efficacy, he said, but these findings do not obviate toxicity concerns.
“You are adding one more agent, and this agent is more toxic than single checkpoint blockade,” Deng said. “So, if you weigh the risk, it is known, [but] the benefit is suggestive. I am not sure if the risk-benefit ratio would argue for routine implementation of this regimen yet.”
On the other hand, he noted, the combination is the US Food and Drug Administration–approved in this setting, so “it is not wrong to use it.”
Jyoti Malhotra, MD, director of thoracic medical oncology at City of Hope Orange County in Irvine, California, had a similar take.
“The clinical data presented so far is exploratory and limited by the small sample size,” Malhotra said in a written comment. “Data from an ongoing phase 3 trial (TRITON) is awaited before dual checkpoint blockade becomes the standard of care in this setting.”
Hossein Borghaei, DO, chief of the Division of Thoracic Medical Oncology at Fox Chase Cancer Center, Philadelphia, was also unequivocal when asked if dual checkpoint blockade with chemotherapy should be considered the preferred first-line treatment option in patients with STK11 and/or KEAP1 mutations.
“No,” he said in a written comment. “The data and the hypothesis are very strong, but it is all based on retrospective clinical data, cell line data, and mouse models. We need a randomized study to test the hypothesis.”
Incidentally, Borghaei is on the steering committee for the TRITON trial. He shared this potential conflict of interest before praising Skoulidis and colleagues for their efforts, noting that the present findings underscore the broader importance of widespread tumor profiling and access to resultant data.
“This is a beautiful story that has developed over the last few years based on the research by the group from MD Anderson who has reported the current Nature article,” he said. “This highlights the possible utility of collecting sequencing data on [all] patients’ tumors. These sorts of understandings and new ideas could arise only if there is access to this information.”
The study was supported by AstraZeneca, the National Cancer Institute, the Gunnigar Fund, and others. The investigators disclosed additional relationships with Novartis, Merck, Amgen, and others. Deng disclosed relationships with Merck, BridgeBio, MJH Life Sciences, and others. Dy disclosed relationships with Eli Lilly and Company, Janssen Pharmaceuticals, Meru, and others. Malhotra has previously served as a consultant for AstraZeneca. Borghaei has served as a consultant for AstraZeneca and is on the steering committee for the TRITON trial.
A version of this article appeared on Medscape.com.
Therapeutic Drug Monitoring in Rheumatology: A Promising Outlook But Many Barriers to Overcome
Therapeutic drug monitoring (TDM) — the practice of using laboratory testing to measure blood levels of drugs — has garnered growing interest among rheumatologists in managing patients on disease-modifying antirheumatic drugs (DMARDs), but that hasn’t exactly translated to widespread practice.
While TDM has made some inroads with patients taking monoclonal antibodies, specifically infliximab, its uptake has encountered a number of headwinds, not the least of which is a lack of evidence and clinical guidelines, uneven access and standards of assays, and even an uncertainty about how to interpret laboratory results.
“In some fields, such as neurology, TDM is accepted for antiepileptics,” Michelle Petri, MD, MPH, director of the Johns Hopkins Lupus Center, Baltimore, told Medscape Medical News. “In rheumatology, though, TDM is underutilized and not adequately championed by the American College of Rheumatology.”
She noted that TDM is most acutely needed for management of systemic lupus erythematosus, where nonadherence is a major problem. “Whole blood hydroxychloroquine monitoring has proven beneficial for identifying nonadherence, but also to pinpoint patients who are on too much, a risk factor for retinopathy,” Petri said.
“The state of therapeutic drug monitoring in general has been interesting when you think about its use in autoimmune disease because it’s very much used in gastroenterology and it’s been much less used in rheumatology,” Zachary Wallace, MD, codirector of the Rheumatology & Allergy Clinical Epidemiology Research Center at Massachusetts General Hospital in Boston, told Medscape Medical News. “Some of that may have to do with the interpretation of the availability of evidence, but I think it’s something clinicians will come across more and more often in their practice and wondering what its role might be,” he added.
The movement to precision medicine also portends to grow interest in TDM in rheumatology, said Stephen Balevic, MD, PhD, a rheumatologist and pharmacologist at Duke University and director of pharmacometrics at the Duke Clinical Research Institute, Durham, North Carolina.
“It’s a very exciting time for rheumatologists to begin thinking outside box on what it means to study precision medicine, and I think pharmacology is one of the most overlooked aspects of precision medicine in our community,” he told Medscape Medical News.
That may be because older DMARDs, namely hydroxychloroquine and methotrexate, came to market when regulatory requirements were different than they are today, Balevic said. “Many of the older conventional DMARDs were discovered incidentally and never really had the traditional pharmacokinetic-pharmacodynamic trials to determine optimal dosing, or perhaps that was extrapolated from other populations,” he said.
So, the “one-size-fits-all” approach does not work for prescribing older or even some of the newer DMARDs for rheumatologic disorders, Balevic said.
Reactive vs Proactive TDM
Among the few trials that examined TDM in rheumatology patients are the NOR-DRUM A and B trials in Norway. Marthe Brun, MD, PhD, a rheumatologist at the Center for Treatment of Rheumatic and Musculoskeletal Diseases at Diakonhjemmet Hospital in Oslo, Norway, and a coauthor of the NOR-DRUM trials, told Medscape Medical News that the trials found an overall benefit to TDM during infliximab maintenance therapy. The trials included not only patients with inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis) but also patients with inflammatory bowel disease and psoriasis, Brun said.
Brun explained that two types of TDM exist: Reactive and proactive. “Reactive TDM is when you use it to find the reason for a patient having a flare or disease worsening,” she told Medscape Medical News. “Proactive TDM would be regular testing to keep a patient within a therapeutic range to avoid flare because of low drug concentrations.”
Gastroenterologists are more inclined than rheumatologists and dermatologists to use reactive TDM, she said. “There have been no recommendations regarding proactive TDM because of the lack of data.”
In Europe, Wallace noted that European Alliance of Associations for Rheumatology (EULAR) recommendations consider the use of TDM in specific clinical scenarios, such as when treatment fails or to evaluate immunogenicity of a reaction, but they are limited. The American College of Rheumatology (ACR) does not have any recommendations for the use of TDM.
Based on the NOR-DRUM trials, rheumatologists in Norway have published their own guidelines for TDM for infliximab in rheumatologic disease, but they are in Norwegian and have not yet been taken up by EULAR, Brun noted. Publication of those recommendations in English is pending, she said.
“But for other subcutaneously administered TNF inhibitors, there’s a lack of data,” Brun added.
The State of the Evidence
NOR-DRUM A did not support the use of proactive TDM in the 30-week induction period as a way to improve disease remission in patients with chronic immune-mediated inflammatory disease. NOR-DRUM B, which evaluated TDM over a year, found the approach was more likely to lead to sustained disease control for that period.
Brun’s group recently published an analysis of the trials. “We did not find an overall effect during the initial phase of the treatment, the first 30 weeks,” she told Medscape Medical News.
“Then we looked at subgroups, and we found that the patients that developed antidrug antibodies [ADAs] had an effect, and ADA are associated with poorer outcomes as well as infusion reactions for patients treated with infliximab.
“So, it’s probably a benefit to be able to detect these ADA early before the patient experiences a disease flare or infusion reaction,” Brun added. “It facilitates for the clinician to take action to, for example, increase the dosing or switch therapy.”
However, the quality of the data supporting TDM in rheumatology is limited, Balevic said. “There’s very good observational data, but we have very few clinical trials that actually leverage TDM,” he said.
NOR-DRUM is the exception, he said. “Ideally, we need more of these dose-optimization trials to help guide clinical practice,” he said. But it stands alone.
Wallace noted several take-home messages from the NOR-DRUM trials, namely that using TDM to prevent ADA may be more effective during the maintenance phase of treatment than the induction phase. However, he said, the evidence is still emerging.
“It’s reasonable to say that we’re at an early stage of the evidence,” he said. “If you look at the large trials that have been done in rheumatology, they’ve combined patients with many different types of conditions, and a lot of our recommendations in rheumatology are disease-specific — in rheumatoid arthritis, in vasculitis. There’s a lack of data in specific diseases to guide or examine what the role of TDM might be.”
In the meantime, no fewer than four clinical trials evaluating TDM with tumor necrosis factor (TNF) inhibitors in rheumatologic diseases are ongoing or have completed but not yet released results, according to Wallace. Three Adalimumab Drug Optimization in Rheumatoid Arthritis trials are underway: The first is evaluating drug tapering vs disease activity score; the second is testing low or usual drug concentration; and the third is studying switches to etanercept or a non-TNF inhibitor drug (abatacept, rituximab, tocilizumab, or sarilumab) in patients failing treatment. Another trial called Tocilizumab Drug Levels to Optimized Treatment in RA is randomizing patients with high drug levels to dose maintenance or dose reduction. All four trials are sponsored by the Reade Rheumatology Research Institute, Amsterdam, the Netherlands.
Until clearer answers emerge from clinical trials, a number of barriers to and questions about the potential for TDM in rheumatology persist.
Barriers to Wider Use of TDM
“The biggest barrier with TDM is simply just a lack of what to do with the data,” Balevic said. “The clinician needs clear-cut guidance on what to do with the drug level. So, in other words, what is the target concentration for the drug? And if that target is not the goal, how should that dose be adjusted?”
The optimal drug levels, particularly for the older conventional synthetic DMARDs, simply have not been validated by clinical trials, he said.
“Different studies may report different target drug levels, and this could be due to different underlying population, or a different matrix — a measure of whole blood vs plasma — or even the timing of the sample,” he said. Balevic led a pharmacokinetic study earlier this year that proposed an algorithm for determining the number of missed hydroxychloroquine doses.
“This really goes back to the clinician needing to draw on a lot of pharmacology training to interpret the literature,” Balevic added.
That gets to the need for more education among rheumatologists, as Brun pointed out. “The physician needs to be educated about therapeutic ranges, when to assess concentrations of drug antibodies, and how to react to the results,” Brun said.
Which ADAs to identify is also problematic. “For antidrug antibodies, it’s especially challenging because there are so many assay formats in use, and it’s a bit complicated to analyze these antidrug antibodies,” Brun said. “There’s no consensus on what calibrators to use, and there’s no standardization of how to report the results, so you can’t really compare results from different assays. You need to know what your laboratory is using and how to interpret results from that particular assay, so that’s a challenge.”
Variability in drug tolerance also exists across assays, Wallace noted. “One of the challenges that have come up in the discussion of therapeutic drug monitoring is understanding what the target level is,” he said. “Defining what the target level might be for a specific condition is not something that’s well understood.”
Breaking down the science, he noted that an ADA can bind to a monoclonal antibody, forming an immune complex that avoids detection. Drug-sensitive assays may detect high concentrations of ADAs but miss low or moderate concentrations. Drug-tolerant assays may be more likely to detect low concentrations at ADAs, but the clinical significance is unclear.
Cost and Patient Trust as Barriers
“The costs vary a lot from assay to assay,” Brun said. “Some commercial assays can be really expensive.” In Norway, a dedicated lab with its own in-house assays helps to keep costs down, she said.
But that’s not the case in the United States, where insurance coverage can be a question mark, Shivani Garg, MD, a rheumatologist at the University of Wisconsin (UW)-Madison and director of the UW-Madison Health Lupus and Lupus Nephritis Clinics, told Medscape Medical News. “A lot of insurances are covering therapeutic drug monitoring, but for the high-deductible plans, there should be a way to offer these important tests to patients at a lower cost or figure out a way for coverage for those patients so that they can show that there are benefits of therapeutic drug monitoring without being sent a really big bill,” she said.
Patient trust could be another potential barrier, Garg said. “A lot of times there is not shared decision-making involved in why this test is being done, how those tests will help us as clinicians, and [patients’ understanding of] the use of the medicine,” Garg said.
“If the shared decision-making to build trust is not there, a lot of times patients worry that they’re being under surveillance or they’re being watched, so that might add to the lack of trust in the core issues that are critical threats to patients with chronic diseases because this is a lifelong partnership,” she said.
Convenience is another issue. “Particularly with mycophenolate levels, a lot of studies have used area under the curve, so getting an area under the curve level over a period of 12 hours would require several samples,” Garg said.
Testing protocols are also uncertain, Garg added. “A few data points ... are missing, like how we use the data over time,” she said. “If you do it for a given patient over several years, how often should you do it? How often do the levels fluctuate? How are the data used to inform dosing changes or monitoring changes?
“When those pieces are put together, then we are more likely to build up an intervention that clinicians can use in clinical practice, so they know how to order it and how frequently do it — every 6 months, 3 months, or every month. And then, over a period of time, how to adjust the dosing. That’s the big question.”
Who May Benefit Most From TDM?
In the NOR-DRUM trials, patients at risk of developing ADA early on, before a disease flare or infusion reaction, seemed to benefit most from TDM. But who are those patients?
“We looked at risk factors for developing antidrug antibodies, and we found that patients with high disease activity when starting treatment, smokers, and patients with rheumatoid arthritis had a higher risk than other patients, as did patients who are not using concomitant immunosuppressive therapy,” Brun said.
“During treatment, we also found that low serum drug levels and drug holidays above 11 weeks were also risk factors,” she added.
The NOR-DRUM researchers also evaluated genetic risk factors and found that patients with the HLA-DQ2 gene variant were also at increased risk of developing ADA.
While NOR-DRUM evaluated only infliximab, some of its lessons may be applied to other DMARDs, Brun said. “We think that for other subcutaneously administered TNF inhibitors, you would probably see the same effect of proactive TDM, but we currently do not have data on that,” she said. A study similar to the NOR-DRUM design will evaluate this in Norway, Brun added.
She explained why the findings with infliximab may extend to adalimumab, which may be the second most immunogenic TNF inhibitor after infliximab. “The administration is different; it’s administered more often than infliximab; that would also make the results more uncertain to generalize to the other treatments, but I would guess there are also benefits of using TDM in other treatments.”
Potential Risks for TDM
Wallace has noted that TDM, with the current state of evidence, carries a number of potential risks. “The potential risks might be that you unnecessarily discontinue a medication because you detected an antibody, or the level seems low and you’re not able to get it higher, but the patient is otherwise doing fine,” he said. “You might end up increasing doses of the medicine that would put the patient at potentially increased risk of infection, as well as obviously more costs.”
That would also lead to more utilization of resources and costs, he said. “Some of those reasons are why there has been hesitation with therapeutic drug monitoring,” Wallace added.
A number of questions also surround the use of biosimilars and ADA levels, Wallace said. While a review of clinical trials found no meaningful differences in terms of immunogenicity between biosimilars and reference products, it did note discrepancies in how the agents were evaluated.
What DMARDs Are Most Suitable for TDM?
Petri said TDM would be useful for monitoring patients on mycophenolate mofetil. “A trough level can at least tell us if a patient is taking it,” she said. “Tacrolimus, used for lupus nephritis, has well-accepted peak and trough trends due to widespread use in transplant.”
Drugs with a wide variability in pharmacokinetics may also be suitable for TDM, Balevic said. That would include hydroxychloroquine, azathioprine, mycophenolate, or even cyclophosphamide. Drugs that have a narrow therapeutic index, such as tacrolimus, cyclosporine, or again, cyclophosphamide, might also be amenable to TDM, he said.
Why Do TDM?
“The two main reasons why somebody would go on to detect drug levels: The first may be to assess medication adherence, and this applies virtually to any drug that rheumatologists use; the second reason is to optimize dozing, either for efficacy purposes or to prevent toxicity,” Balevic said.
“When it comes to optimizing dosing, you should really think about TDM as one tool in our toolbelt,” he said.
Dose is “just a surrogate,” he said. “When we prescribe a drug, what truly matters is the amount of active unbound drug at the site of action. That’s what’s responsible for a drug’s pharmacologic effect.”
However, the same dose, or even the same weight-based dose, does not necessarily mean similar patients will achieve the same amount of exposure to the drug, but TDM can help determine that, he said.
What’s Next
Studies into the use of TDM in rheumatology are ongoing. Brun said her group is currently conducting a cost-effective analysis from the NOR-DRUM trials.
“There’s going to be more studies coming out in the next few years, looking at what impact the use of therapeutic drug monitoring might have on outcomes,” Wallace said.
“As we accumulate more and more evidence, we might see organizations like ACR and EULAR start to weigh in more on whether or not therapeutic drug monitoring can or should be used.”
Petri, Brun, and Garg had no relevant disclosures. Wallace disclosed financial relationships with Amgen, Alexion, BioCryst, Boehringer Ingelheim, Bristol Myers Squibb, Medpace, Novartis, Sanofi, Viela Bio, Visterra, Xencor, and Zenas. Balevic disclosed relationships with the National Institutes of Health, the Childhood Arthritis and Rheumatology Research Alliance, and UCB.
A version of this article appeared on Medscape.com.
Therapeutic drug monitoring (TDM) — the practice of using laboratory testing to measure blood levels of drugs — has garnered growing interest among rheumatologists in managing patients on disease-modifying antirheumatic drugs (DMARDs), but that hasn’t exactly translated to widespread practice.
While TDM has made some inroads with patients taking monoclonal antibodies, specifically infliximab, its uptake has encountered a number of headwinds, not the least of which is a lack of evidence and clinical guidelines, uneven access and standards of assays, and even an uncertainty about how to interpret laboratory results.
“In some fields, such as neurology, TDM is accepted for antiepileptics,” Michelle Petri, MD, MPH, director of the Johns Hopkins Lupus Center, Baltimore, told Medscape Medical News. “In rheumatology, though, TDM is underutilized and not adequately championed by the American College of Rheumatology.”
She noted that TDM is most acutely needed for management of systemic lupus erythematosus, where nonadherence is a major problem. “Whole blood hydroxychloroquine monitoring has proven beneficial for identifying nonadherence, but also to pinpoint patients who are on too much, a risk factor for retinopathy,” Petri said.
“The state of therapeutic drug monitoring in general has been interesting when you think about its use in autoimmune disease because it’s very much used in gastroenterology and it’s been much less used in rheumatology,” Zachary Wallace, MD, codirector of the Rheumatology & Allergy Clinical Epidemiology Research Center at Massachusetts General Hospital in Boston, told Medscape Medical News. “Some of that may have to do with the interpretation of the availability of evidence, but I think it’s something clinicians will come across more and more often in their practice and wondering what its role might be,” he added.
The movement to precision medicine also portends to grow interest in TDM in rheumatology, said Stephen Balevic, MD, PhD, a rheumatologist and pharmacologist at Duke University and director of pharmacometrics at the Duke Clinical Research Institute, Durham, North Carolina.
“It’s a very exciting time for rheumatologists to begin thinking outside box on what it means to study precision medicine, and I think pharmacology is one of the most overlooked aspects of precision medicine in our community,” he told Medscape Medical News.
That may be because older DMARDs, namely hydroxychloroquine and methotrexate, came to market when regulatory requirements were different than they are today, Balevic said. “Many of the older conventional DMARDs were discovered incidentally and never really had the traditional pharmacokinetic-pharmacodynamic trials to determine optimal dosing, or perhaps that was extrapolated from other populations,” he said.
So, the “one-size-fits-all” approach does not work for prescribing older or even some of the newer DMARDs for rheumatologic disorders, Balevic said.
Reactive vs Proactive TDM
Among the few trials that examined TDM in rheumatology patients are the NOR-DRUM A and B trials in Norway. Marthe Brun, MD, PhD, a rheumatologist at the Center for Treatment of Rheumatic and Musculoskeletal Diseases at Diakonhjemmet Hospital in Oslo, Norway, and a coauthor of the NOR-DRUM trials, told Medscape Medical News that the trials found an overall benefit to TDM during infliximab maintenance therapy. The trials included not only patients with inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis) but also patients with inflammatory bowel disease and psoriasis, Brun said.
Brun explained that two types of TDM exist: Reactive and proactive. “Reactive TDM is when you use it to find the reason for a patient having a flare or disease worsening,” she told Medscape Medical News. “Proactive TDM would be regular testing to keep a patient within a therapeutic range to avoid flare because of low drug concentrations.”
Gastroenterologists are more inclined than rheumatologists and dermatologists to use reactive TDM, she said. “There have been no recommendations regarding proactive TDM because of the lack of data.”
In Europe, Wallace noted that European Alliance of Associations for Rheumatology (EULAR) recommendations consider the use of TDM in specific clinical scenarios, such as when treatment fails or to evaluate immunogenicity of a reaction, but they are limited. The American College of Rheumatology (ACR) does not have any recommendations for the use of TDM.
Based on the NOR-DRUM trials, rheumatologists in Norway have published their own guidelines for TDM for infliximab in rheumatologic disease, but they are in Norwegian and have not yet been taken up by EULAR, Brun noted. Publication of those recommendations in English is pending, she said.
“But for other subcutaneously administered TNF inhibitors, there’s a lack of data,” Brun added.
The State of the Evidence
NOR-DRUM A did not support the use of proactive TDM in the 30-week induction period as a way to improve disease remission in patients with chronic immune-mediated inflammatory disease. NOR-DRUM B, which evaluated TDM over a year, found the approach was more likely to lead to sustained disease control for that period.
Brun’s group recently published an analysis of the trials. “We did not find an overall effect during the initial phase of the treatment, the first 30 weeks,” she told Medscape Medical News.
“Then we looked at subgroups, and we found that the patients that developed antidrug antibodies [ADAs] had an effect, and ADA are associated with poorer outcomes as well as infusion reactions for patients treated with infliximab.
“So, it’s probably a benefit to be able to detect these ADA early before the patient experiences a disease flare or infusion reaction,” Brun added. “It facilitates for the clinician to take action to, for example, increase the dosing or switch therapy.”
However, the quality of the data supporting TDM in rheumatology is limited, Balevic said. “There’s very good observational data, but we have very few clinical trials that actually leverage TDM,” he said.
NOR-DRUM is the exception, he said. “Ideally, we need more of these dose-optimization trials to help guide clinical practice,” he said. But it stands alone.
Wallace noted several take-home messages from the NOR-DRUM trials, namely that using TDM to prevent ADA may be more effective during the maintenance phase of treatment than the induction phase. However, he said, the evidence is still emerging.
“It’s reasonable to say that we’re at an early stage of the evidence,” he said. “If you look at the large trials that have been done in rheumatology, they’ve combined patients with many different types of conditions, and a lot of our recommendations in rheumatology are disease-specific — in rheumatoid arthritis, in vasculitis. There’s a lack of data in specific diseases to guide or examine what the role of TDM might be.”
In the meantime, no fewer than four clinical trials evaluating TDM with tumor necrosis factor (TNF) inhibitors in rheumatologic diseases are ongoing or have completed but not yet released results, according to Wallace. Three Adalimumab Drug Optimization in Rheumatoid Arthritis trials are underway: The first is evaluating drug tapering vs disease activity score; the second is testing low or usual drug concentration; and the third is studying switches to etanercept or a non-TNF inhibitor drug (abatacept, rituximab, tocilizumab, or sarilumab) in patients failing treatment. Another trial called Tocilizumab Drug Levels to Optimized Treatment in RA is randomizing patients with high drug levels to dose maintenance or dose reduction. All four trials are sponsored by the Reade Rheumatology Research Institute, Amsterdam, the Netherlands.
Until clearer answers emerge from clinical trials, a number of barriers to and questions about the potential for TDM in rheumatology persist.
Barriers to Wider Use of TDM
“The biggest barrier with TDM is simply just a lack of what to do with the data,” Balevic said. “The clinician needs clear-cut guidance on what to do with the drug level. So, in other words, what is the target concentration for the drug? And if that target is not the goal, how should that dose be adjusted?”
The optimal drug levels, particularly for the older conventional synthetic DMARDs, simply have not been validated by clinical trials, he said.
“Different studies may report different target drug levels, and this could be due to different underlying population, or a different matrix — a measure of whole blood vs plasma — or even the timing of the sample,” he said. Balevic led a pharmacokinetic study earlier this year that proposed an algorithm for determining the number of missed hydroxychloroquine doses.
“This really goes back to the clinician needing to draw on a lot of pharmacology training to interpret the literature,” Balevic added.
That gets to the need for more education among rheumatologists, as Brun pointed out. “The physician needs to be educated about therapeutic ranges, when to assess concentrations of drug antibodies, and how to react to the results,” Brun said.
Which ADAs to identify is also problematic. “For antidrug antibodies, it’s especially challenging because there are so many assay formats in use, and it’s a bit complicated to analyze these antidrug antibodies,” Brun said. “There’s no consensus on what calibrators to use, and there’s no standardization of how to report the results, so you can’t really compare results from different assays. You need to know what your laboratory is using and how to interpret results from that particular assay, so that’s a challenge.”
Variability in drug tolerance also exists across assays, Wallace noted. “One of the challenges that have come up in the discussion of therapeutic drug monitoring is understanding what the target level is,” he said. “Defining what the target level might be for a specific condition is not something that’s well understood.”
Breaking down the science, he noted that an ADA can bind to a monoclonal antibody, forming an immune complex that avoids detection. Drug-sensitive assays may detect high concentrations of ADAs but miss low or moderate concentrations. Drug-tolerant assays may be more likely to detect low concentrations at ADAs, but the clinical significance is unclear.
Cost and Patient Trust as Barriers
“The costs vary a lot from assay to assay,” Brun said. “Some commercial assays can be really expensive.” In Norway, a dedicated lab with its own in-house assays helps to keep costs down, she said.
But that’s not the case in the United States, where insurance coverage can be a question mark, Shivani Garg, MD, a rheumatologist at the University of Wisconsin (UW)-Madison and director of the UW-Madison Health Lupus and Lupus Nephritis Clinics, told Medscape Medical News. “A lot of insurances are covering therapeutic drug monitoring, but for the high-deductible plans, there should be a way to offer these important tests to patients at a lower cost or figure out a way for coverage for those patients so that they can show that there are benefits of therapeutic drug monitoring without being sent a really big bill,” she said.
Patient trust could be another potential barrier, Garg said. “A lot of times there is not shared decision-making involved in why this test is being done, how those tests will help us as clinicians, and [patients’ understanding of] the use of the medicine,” Garg said.
“If the shared decision-making to build trust is not there, a lot of times patients worry that they’re being under surveillance or they’re being watched, so that might add to the lack of trust in the core issues that are critical threats to patients with chronic diseases because this is a lifelong partnership,” she said.
Convenience is another issue. “Particularly with mycophenolate levels, a lot of studies have used area under the curve, so getting an area under the curve level over a period of 12 hours would require several samples,” Garg said.
Testing protocols are also uncertain, Garg added. “A few data points ... are missing, like how we use the data over time,” she said. “If you do it for a given patient over several years, how often should you do it? How often do the levels fluctuate? How are the data used to inform dosing changes or monitoring changes?
“When those pieces are put together, then we are more likely to build up an intervention that clinicians can use in clinical practice, so they know how to order it and how frequently do it — every 6 months, 3 months, or every month. And then, over a period of time, how to adjust the dosing. That’s the big question.”
Who May Benefit Most From TDM?
In the NOR-DRUM trials, patients at risk of developing ADA early on, before a disease flare or infusion reaction, seemed to benefit most from TDM. But who are those patients?
“We looked at risk factors for developing antidrug antibodies, and we found that patients with high disease activity when starting treatment, smokers, and patients with rheumatoid arthritis had a higher risk than other patients, as did patients who are not using concomitant immunosuppressive therapy,” Brun said.
“During treatment, we also found that low serum drug levels and drug holidays above 11 weeks were also risk factors,” she added.
The NOR-DRUM researchers also evaluated genetic risk factors and found that patients with the HLA-DQ2 gene variant were also at increased risk of developing ADA.
While NOR-DRUM evaluated only infliximab, some of its lessons may be applied to other DMARDs, Brun said. “We think that for other subcutaneously administered TNF inhibitors, you would probably see the same effect of proactive TDM, but we currently do not have data on that,” she said. A study similar to the NOR-DRUM design will evaluate this in Norway, Brun added.
She explained why the findings with infliximab may extend to adalimumab, which may be the second most immunogenic TNF inhibitor after infliximab. “The administration is different; it’s administered more often than infliximab; that would also make the results more uncertain to generalize to the other treatments, but I would guess there are also benefits of using TDM in other treatments.”
Potential Risks for TDM
Wallace has noted that TDM, with the current state of evidence, carries a number of potential risks. “The potential risks might be that you unnecessarily discontinue a medication because you detected an antibody, or the level seems low and you’re not able to get it higher, but the patient is otherwise doing fine,” he said. “You might end up increasing doses of the medicine that would put the patient at potentially increased risk of infection, as well as obviously more costs.”
That would also lead to more utilization of resources and costs, he said. “Some of those reasons are why there has been hesitation with therapeutic drug monitoring,” Wallace added.
A number of questions also surround the use of biosimilars and ADA levels, Wallace said. While a review of clinical trials found no meaningful differences in terms of immunogenicity between biosimilars and reference products, it did note discrepancies in how the agents were evaluated.
What DMARDs Are Most Suitable for TDM?
Petri said TDM would be useful for monitoring patients on mycophenolate mofetil. “A trough level can at least tell us if a patient is taking it,” she said. “Tacrolimus, used for lupus nephritis, has well-accepted peak and trough trends due to widespread use in transplant.”
Drugs with a wide variability in pharmacokinetics may also be suitable for TDM, Balevic said. That would include hydroxychloroquine, azathioprine, mycophenolate, or even cyclophosphamide. Drugs that have a narrow therapeutic index, such as tacrolimus, cyclosporine, or again, cyclophosphamide, might also be amenable to TDM, he said.
Why Do TDM?
“The two main reasons why somebody would go on to detect drug levels: The first may be to assess medication adherence, and this applies virtually to any drug that rheumatologists use; the second reason is to optimize dozing, either for efficacy purposes or to prevent toxicity,” Balevic said.
“When it comes to optimizing dosing, you should really think about TDM as one tool in our toolbelt,” he said.
Dose is “just a surrogate,” he said. “When we prescribe a drug, what truly matters is the amount of active unbound drug at the site of action. That’s what’s responsible for a drug’s pharmacologic effect.”
However, the same dose, or even the same weight-based dose, does not necessarily mean similar patients will achieve the same amount of exposure to the drug, but TDM can help determine that, he said.
What’s Next
Studies into the use of TDM in rheumatology are ongoing. Brun said her group is currently conducting a cost-effective analysis from the NOR-DRUM trials.
“There’s going to be more studies coming out in the next few years, looking at what impact the use of therapeutic drug monitoring might have on outcomes,” Wallace said.
“As we accumulate more and more evidence, we might see organizations like ACR and EULAR start to weigh in more on whether or not therapeutic drug monitoring can or should be used.”
Petri, Brun, and Garg had no relevant disclosures. Wallace disclosed financial relationships with Amgen, Alexion, BioCryst, Boehringer Ingelheim, Bristol Myers Squibb, Medpace, Novartis, Sanofi, Viela Bio, Visterra, Xencor, and Zenas. Balevic disclosed relationships with the National Institutes of Health, the Childhood Arthritis and Rheumatology Research Alliance, and UCB.
A version of this article appeared on Medscape.com.
Therapeutic drug monitoring (TDM) — the practice of using laboratory testing to measure blood levels of drugs — has garnered growing interest among rheumatologists in managing patients on disease-modifying antirheumatic drugs (DMARDs), but that hasn’t exactly translated to widespread practice.
While TDM has made some inroads with patients taking monoclonal antibodies, specifically infliximab, its uptake has encountered a number of headwinds, not the least of which is a lack of evidence and clinical guidelines, uneven access and standards of assays, and even an uncertainty about how to interpret laboratory results.
“In some fields, such as neurology, TDM is accepted for antiepileptics,” Michelle Petri, MD, MPH, director of the Johns Hopkins Lupus Center, Baltimore, told Medscape Medical News. “In rheumatology, though, TDM is underutilized and not adequately championed by the American College of Rheumatology.”
She noted that TDM is most acutely needed for management of systemic lupus erythematosus, where nonadherence is a major problem. “Whole blood hydroxychloroquine monitoring has proven beneficial for identifying nonadherence, but also to pinpoint patients who are on too much, a risk factor for retinopathy,” Petri said.
“The state of therapeutic drug monitoring in general has been interesting when you think about its use in autoimmune disease because it’s very much used in gastroenterology and it’s been much less used in rheumatology,” Zachary Wallace, MD, codirector of the Rheumatology & Allergy Clinical Epidemiology Research Center at Massachusetts General Hospital in Boston, told Medscape Medical News. “Some of that may have to do with the interpretation of the availability of evidence, but I think it’s something clinicians will come across more and more often in their practice and wondering what its role might be,” he added.
The movement to precision medicine also portends to grow interest in TDM in rheumatology, said Stephen Balevic, MD, PhD, a rheumatologist and pharmacologist at Duke University and director of pharmacometrics at the Duke Clinical Research Institute, Durham, North Carolina.
“It’s a very exciting time for rheumatologists to begin thinking outside box on what it means to study precision medicine, and I think pharmacology is one of the most overlooked aspects of precision medicine in our community,” he told Medscape Medical News.
That may be because older DMARDs, namely hydroxychloroquine and methotrexate, came to market when regulatory requirements were different than they are today, Balevic said. “Many of the older conventional DMARDs were discovered incidentally and never really had the traditional pharmacokinetic-pharmacodynamic trials to determine optimal dosing, or perhaps that was extrapolated from other populations,” he said.
So, the “one-size-fits-all” approach does not work for prescribing older or even some of the newer DMARDs for rheumatologic disorders, Balevic said.
Reactive vs Proactive TDM
Among the few trials that examined TDM in rheumatology patients are the NOR-DRUM A and B trials in Norway. Marthe Brun, MD, PhD, a rheumatologist at the Center for Treatment of Rheumatic and Musculoskeletal Diseases at Diakonhjemmet Hospital in Oslo, Norway, and a coauthor of the NOR-DRUM trials, told Medscape Medical News that the trials found an overall benefit to TDM during infliximab maintenance therapy. The trials included not only patients with inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis) but also patients with inflammatory bowel disease and psoriasis, Brun said.
Brun explained that two types of TDM exist: Reactive and proactive. “Reactive TDM is when you use it to find the reason for a patient having a flare or disease worsening,” she told Medscape Medical News. “Proactive TDM would be regular testing to keep a patient within a therapeutic range to avoid flare because of low drug concentrations.”
Gastroenterologists are more inclined than rheumatologists and dermatologists to use reactive TDM, she said. “There have been no recommendations regarding proactive TDM because of the lack of data.”
In Europe, Wallace noted that European Alliance of Associations for Rheumatology (EULAR) recommendations consider the use of TDM in specific clinical scenarios, such as when treatment fails or to evaluate immunogenicity of a reaction, but they are limited. The American College of Rheumatology (ACR) does not have any recommendations for the use of TDM.
Based on the NOR-DRUM trials, rheumatologists in Norway have published their own guidelines for TDM for infliximab in rheumatologic disease, but they are in Norwegian and have not yet been taken up by EULAR, Brun noted. Publication of those recommendations in English is pending, she said.
“But for other subcutaneously administered TNF inhibitors, there’s a lack of data,” Brun added.
The State of the Evidence
NOR-DRUM A did not support the use of proactive TDM in the 30-week induction period as a way to improve disease remission in patients with chronic immune-mediated inflammatory disease. NOR-DRUM B, which evaluated TDM over a year, found the approach was more likely to lead to sustained disease control for that period.
Brun’s group recently published an analysis of the trials. “We did not find an overall effect during the initial phase of the treatment, the first 30 weeks,” she told Medscape Medical News.
“Then we looked at subgroups, and we found that the patients that developed antidrug antibodies [ADAs] had an effect, and ADA are associated with poorer outcomes as well as infusion reactions for patients treated with infliximab.
“So, it’s probably a benefit to be able to detect these ADA early before the patient experiences a disease flare or infusion reaction,” Brun added. “It facilitates for the clinician to take action to, for example, increase the dosing or switch therapy.”
However, the quality of the data supporting TDM in rheumatology is limited, Balevic said. “There’s very good observational data, but we have very few clinical trials that actually leverage TDM,” he said.
NOR-DRUM is the exception, he said. “Ideally, we need more of these dose-optimization trials to help guide clinical practice,” he said. But it stands alone.
Wallace noted several take-home messages from the NOR-DRUM trials, namely that using TDM to prevent ADA may be more effective during the maintenance phase of treatment than the induction phase. However, he said, the evidence is still emerging.
“It’s reasonable to say that we’re at an early stage of the evidence,” he said. “If you look at the large trials that have been done in rheumatology, they’ve combined patients with many different types of conditions, and a lot of our recommendations in rheumatology are disease-specific — in rheumatoid arthritis, in vasculitis. There’s a lack of data in specific diseases to guide or examine what the role of TDM might be.”
In the meantime, no fewer than four clinical trials evaluating TDM with tumor necrosis factor (TNF) inhibitors in rheumatologic diseases are ongoing or have completed but not yet released results, according to Wallace. Three Adalimumab Drug Optimization in Rheumatoid Arthritis trials are underway: The first is evaluating drug tapering vs disease activity score; the second is testing low or usual drug concentration; and the third is studying switches to etanercept or a non-TNF inhibitor drug (abatacept, rituximab, tocilizumab, or sarilumab) in patients failing treatment. Another trial called Tocilizumab Drug Levels to Optimized Treatment in RA is randomizing patients with high drug levels to dose maintenance or dose reduction. All four trials are sponsored by the Reade Rheumatology Research Institute, Amsterdam, the Netherlands.
Until clearer answers emerge from clinical trials, a number of barriers to and questions about the potential for TDM in rheumatology persist.
Barriers to Wider Use of TDM
“The biggest barrier with TDM is simply just a lack of what to do with the data,” Balevic said. “The clinician needs clear-cut guidance on what to do with the drug level. So, in other words, what is the target concentration for the drug? And if that target is not the goal, how should that dose be adjusted?”
The optimal drug levels, particularly for the older conventional synthetic DMARDs, simply have not been validated by clinical trials, he said.
“Different studies may report different target drug levels, and this could be due to different underlying population, or a different matrix — a measure of whole blood vs plasma — or even the timing of the sample,” he said. Balevic led a pharmacokinetic study earlier this year that proposed an algorithm for determining the number of missed hydroxychloroquine doses.
“This really goes back to the clinician needing to draw on a lot of pharmacology training to interpret the literature,” Balevic added.
That gets to the need for more education among rheumatologists, as Brun pointed out. “The physician needs to be educated about therapeutic ranges, when to assess concentrations of drug antibodies, and how to react to the results,” Brun said.
Which ADAs to identify is also problematic. “For antidrug antibodies, it’s especially challenging because there are so many assay formats in use, and it’s a bit complicated to analyze these antidrug antibodies,” Brun said. “There’s no consensus on what calibrators to use, and there’s no standardization of how to report the results, so you can’t really compare results from different assays. You need to know what your laboratory is using and how to interpret results from that particular assay, so that’s a challenge.”
Variability in drug tolerance also exists across assays, Wallace noted. “One of the challenges that have come up in the discussion of therapeutic drug monitoring is understanding what the target level is,” he said. “Defining what the target level might be for a specific condition is not something that’s well understood.”
Breaking down the science, he noted that an ADA can bind to a monoclonal antibody, forming an immune complex that avoids detection. Drug-sensitive assays may detect high concentrations of ADAs but miss low or moderate concentrations. Drug-tolerant assays may be more likely to detect low concentrations at ADAs, but the clinical significance is unclear.
Cost and Patient Trust as Barriers
“The costs vary a lot from assay to assay,” Brun said. “Some commercial assays can be really expensive.” In Norway, a dedicated lab with its own in-house assays helps to keep costs down, she said.
But that’s not the case in the United States, where insurance coverage can be a question mark, Shivani Garg, MD, a rheumatologist at the University of Wisconsin (UW)-Madison and director of the UW-Madison Health Lupus and Lupus Nephritis Clinics, told Medscape Medical News. “A lot of insurances are covering therapeutic drug monitoring, but for the high-deductible plans, there should be a way to offer these important tests to patients at a lower cost or figure out a way for coverage for those patients so that they can show that there are benefits of therapeutic drug monitoring without being sent a really big bill,” she said.
Patient trust could be another potential barrier, Garg said. “A lot of times there is not shared decision-making involved in why this test is being done, how those tests will help us as clinicians, and [patients’ understanding of] the use of the medicine,” Garg said.
“If the shared decision-making to build trust is not there, a lot of times patients worry that they’re being under surveillance or they’re being watched, so that might add to the lack of trust in the core issues that are critical threats to patients with chronic diseases because this is a lifelong partnership,” she said.
Convenience is another issue. “Particularly with mycophenolate levels, a lot of studies have used area under the curve, so getting an area under the curve level over a period of 12 hours would require several samples,” Garg said.
Testing protocols are also uncertain, Garg added. “A few data points ... are missing, like how we use the data over time,” she said. “If you do it for a given patient over several years, how often should you do it? How often do the levels fluctuate? How are the data used to inform dosing changes or monitoring changes?
“When those pieces are put together, then we are more likely to build up an intervention that clinicians can use in clinical practice, so they know how to order it and how frequently do it — every 6 months, 3 months, or every month. And then, over a period of time, how to adjust the dosing. That’s the big question.”
Who May Benefit Most From TDM?
In the NOR-DRUM trials, patients at risk of developing ADA early on, before a disease flare or infusion reaction, seemed to benefit most from TDM. But who are those patients?
“We looked at risk factors for developing antidrug antibodies, and we found that patients with high disease activity when starting treatment, smokers, and patients with rheumatoid arthritis had a higher risk than other patients, as did patients who are not using concomitant immunosuppressive therapy,” Brun said.
“During treatment, we also found that low serum drug levels and drug holidays above 11 weeks were also risk factors,” she added.
The NOR-DRUM researchers also evaluated genetic risk factors and found that patients with the HLA-DQ2 gene variant were also at increased risk of developing ADA.
While NOR-DRUM evaluated only infliximab, some of its lessons may be applied to other DMARDs, Brun said. “We think that for other subcutaneously administered TNF inhibitors, you would probably see the same effect of proactive TDM, but we currently do not have data on that,” she said. A study similar to the NOR-DRUM design will evaluate this in Norway, Brun added.
She explained why the findings with infliximab may extend to adalimumab, which may be the second most immunogenic TNF inhibitor after infliximab. “The administration is different; it’s administered more often than infliximab; that would also make the results more uncertain to generalize to the other treatments, but I would guess there are also benefits of using TDM in other treatments.”
Potential Risks for TDM
Wallace has noted that TDM, with the current state of evidence, carries a number of potential risks. “The potential risks might be that you unnecessarily discontinue a medication because you detected an antibody, or the level seems low and you’re not able to get it higher, but the patient is otherwise doing fine,” he said. “You might end up increasing doses of the medicine that would put the patient at potentially increased risk of infection, as well as obviously more costs.”
That would also lead to more utilization of resources and costs, he said. “Some of those reasons are why there has been hesitation with therapeutic drug monitoring,” Wallace added.
A number of questions also surround the use of biosimilars and ADA levels, Wallace said. While a review of clinical trials found no meaningful differences in terms of immunogenicity between biosimilars and reference products, it did note discrepancies in how the agents were evaluated.
What DMARDs Are Most Suitable for TDM?
Petri said TDM would be useful for monitoring patients on mycophenolate mofetil. “A trough level can at least tell us if a patient is taking it,” she said. “Tacrolimus, used for lupus nephritis, has well-accepted peak and trough trends due to widespread use in transplant.”
Drugs with a wide variability in pharmacokinetics may also be suitable for TDM, Balevic said. That would include hydroxychloroquine, azathioprine, mycophenolate, or even cyclophosphamide. Drugs that have a narrow therapeutic index, such as tacrolimus, cyclosporine, or again, cyclophosphamide, might also be amenable to TDM, he said.
Why Do TDM?
“The two main reasons why somebody would go on to detect drug levels: The first may be to assess medication adherence, and this applies virtually to any drug that rheumatologists use; the second reason is to optimize dozing, either for efficacy purposes or to prevent toxicity,” Balevic said.
“When it comes to optimizing dosing, you should really think about TDM as one tool in our toolbelt,” he said.
Dose is “just a surrogate,” he said. “When we prescribe a drug, what truly matters is the amount of active unbound drug at the site of action. That’s what’s responsible for a drug’s pharmacologic effect.”
However, the same dose, or even the same weight-based dose, does not necessarily mean similar patients will achieve the same amount of exposure to the drug, but TDM can help determine that, he said.
What’s Next
Studies into the use of TDM in rheumatology are ongoing. Brun said her group is currently conducting a cost-effective analysis from the NOR-DRUM trials.
“There’s going to be more studies coming out in the next few years, looking at what impact the use of therapeutic drug monitoring might have on outcomes,” Wallace said.
“As we accumulate more and more evidence, we might see organizations like ACR and EULAR start to weigh in more on whether or not therapeutic drug monitoring can or should be used.”
Petri, Brun, and Garg had no relevant disclosures. Wallace disclosed financial relationships with Amgen, Alexion, BioCryst, Boehringer Ingelheim, Bristol Myers Squibb, Medpace, Novartis, Sanofi, Viela Bio, Visterra, Xencor, and Zenas. Balevic disclosed relationships with the National Institutes of Health, the Childhood Arthritis and Rheumatology Research Alliance, and UCB.
A version of this article appeared on Medscape.com.
Cannabis in Cancer: What Oncologists and Patients Should Know
first, and oncologists may be hesitant to broach the topic with their patients.
Updated guidelines from the American Society of Clinical Oncology (ASCO) on the use of cannabis and cannabinoids in adults with cancer stress that it’s an important conversation to have.
According to the ASCO expert panel, access to and use of cannabis alongside cancer care have outpaced the science on evidence-based indications, and overall high-quality data on the effects of cannabis during cancer care are lacking. While several observational studies support cannabis use to help ease chemotherapy-related nausea and vomiting, the literature remains more divided on other potential benefits, such as alleviating cancer pain and sleep problems, and some evidence points to potential downsides of cannabis use.
Oncologists should “absolutely talk to patients” about cannabis, Brooke Worster, MD, medical director for the Master of Science in Medical Cannabis Science & Business program at Thomas Jefferson University, Philadelphia, told Medscape Medical News.
“Patients are interested, and they are going to find access to information. As a medical professional, it’s our job to help guide them through these spaces in a safe, nonjudgmental way.”
But, Worster noted, oncologists don’t have to be experts on cannabis to begin the conversation with patients.
So, “let yourself off the hook,” Worster urged.
Plus, avoiding the conversation won’t stop patients from using cannabis. In a recent study, Worster and her colleagues found that nearly one third of patients at 12 National Cancer Institute-designated cancer centers had used cannabis since their diagnosis — most often for sleep disturbance, pain, stress, and anxiety. Most (60%) felt somewhat or extremely comfortable talking to their healthcare provider about it, but only 21.5% said they had done so. Even fewer — about 10% — had talked to their treating oncologist.
Because patients may not discuss cannabis use, it’s especially important for oncologists to open up a line of communication, said Worster, also the enterprise director of supportive oncology at the Thomas Jefferson University.
Evidence on Cannabis During Cancer Care
A substantial proportion of people with cancer believe cannabis can help manage cancer-related symptoms.
In Worster’s recent survey study, regardless of whether patients had used cannabis, almost 90% of those surveyed reported a perceived benefit. Although 65% also reported perceived risks for cannabis use, including difficulty concentrating, lung damage, and impaired memory, the perceived benefits outweighed the risks.
Despite generally positive perceptions, the overall literature on the benefits of cannabis in patients with cancer paints a less clear picture.
The ASCO guidelines, which were based on 13 systematic reviews and five additional primary studies, reported that cannabis can improve refractory, chemotherapy-induced nausea or vomiting when added to guideline-concordant antiemetic regimens, but that there is no clear evidence of benefit or harm for other supportive care outcomes.
The “certainty of evidence for most outcomes was low or very low,” the ASCO authors wrote.
The ASCO experts explained that, outside the context of a clinical trial, the evidence is not sufficient to recommend cannabis or cannabinoids for managing cancer pain, sleep issues, appetite loss, or anxiety and depression. For these outcomes, some studies indicate a benefit, while others don’t.
Real-world data from a large registry study, for instance, have indicated that medical cannabis is “a safe and effective complementary treatment for pain relief in patients with cancer.” However, a 2020 meta-analysis found that, in studies with a low risk for bias, adding cannabinoids to opioids did not reduce cancer pain in adults with advanced cancer.
There can be downsides to cannabis use, too. In one recent study, some patients reported feeling worse physically and psychologically compared with those who didn’t use cannabis. Another study found that oral cannabis was associated with “bothersome” side effects, including sedation, dizziness, and transient anxiety.
The ASCO guidelines also made it clear that cannabis or cannabinoids should not be used as cancer-directed treatment, outside of a clinical trial.
Talking to Patients About Cannabis
Given the level of evidence and patient interest in cannabis, it is important for oncologists to raise the topic of cannabis use with their patients.
To help inform decision-making and approaches to care, the ASCO guidelines suggest that oncologists can guide care themselves or direct patients to appropriate “unbiased, evidence-based” resources. For those who use cannabis or cannabinoids outside of evidence-based indications or clinician recommendations, it’s important to explore patients’ goals, educate them, and try to minimize harm.
One strategy for broaching the topic, Worster suggested, is to simply ask patients if they have tried or considered trying cannabis to control symptoms like nausea and vomiting, loss of appetite, or cancer pain.
The conversation with patients should then include an overview of the potential benefits and potential risks for cannabis use as well as risk reduction strategies, Worster noted.
But “approach it in an open and nonjudgmental frame of mind,” she said. “Just have a conversation.”
Discussing the formulation and concentration of tetrahydrocannabinol (THC) and cannabidiol (CBD) in products matters as well.
Will the product be inhaled, ingested, or topical? Inhaled cannabis is not ideal but is sometimes what patients have access to, Worster explained. Inhaled formulations tend to have faster onset, which might be preferable for treating chemotherapy-related nausea and vomiting, whereas edible formulations may take a while to start working.
It’s also important to warn patients about taking too much, she said, explaining that inhaling THC at higher doses can increase the risk for cardiovascular effects, anxiety, paranoia, panic, and psychosis.
CBD, on the other hand, is anti-inflammatory, but early data suggest it may blunt immune responses in high doses and should be used cautiously by patients receiving immunotherapy.
Worster noted that as laws change and the science advances, new cannabis products and formulations will emerge, as will artificial intelligence tools for helping to guide patients and clinicians in optimal use of cannabis for cancer care. State websites are a particularly helpful tool for providing state-specific medical education related to cannabis laws and use, as well, she said.
The bottom line, she said, is that talking to patients about the ins and outs of cannabis use “really matters.”
Worster disclosed that she is a medical consultant for EO Care.
A version of this article appeared on Medscape.com.
first, and oncologists may be hesitant to broach the topic with their patients.
Updated guidelines from the American Society of Clinical Oncology (ASCO) on the use of cannabis and cannabinoids in adults with cancer stress that it’s an important conversation to have.
According to the ASCO expert panel, access to and use of cannabis alongside cancer care have outpaced the science on evidence-based indications, and overall high-quality data on the effects of cannabis during cancer care are lacking. While several observational studies support cannabis use to help ease chemotherapy-related nausea and vomiting, the literature remains more divided on other potential benefits, such as alleviating cancer pain and sleep problems, and some evidence points to potential downsides of cannabis use.
Oncologists should “absolutely talk to patients” about cannabis, Brooke Worster, MD, medical director for the Master of Science in Medical Cannabis Science & Business program at Thomas Jefferson University, Philadelphia, told Medscape Medical News.
“Patients are interested, and they are going to find access to information. As a medical professional, it’s our job to help guide them through these spaces in a safe, nonjudgmental way.”
But, Worster noted, oncologists don’t have to be experts on cannabis to begin the conversation with patients.
So, “let yourself off the hook,” Worster urged.
Plus, avoiding the conversation won’t stop patients from using cannabis. In a recent study, Worster and her colleagues found that nearly one third of patients at 12 National Cancer Institute-designated cancer centers had used cannabis since their diagnosis — most often for sleep disturbance, pain, stress, and anxiety. Most (60%) felt somewhat or extremely comfortable talking to their healthcare provider about it, but only 21.5% said they had done so. Even fewer — about 10% — had talked to their treating oncologist.
Because patients may not discuss cannabis use, it’s especially important for oncologists to open up a line of communication, said Worster, also the enterprise director of supportive oncology at the Thomas Jefferson University.
Evidence on Cannabis During Cancer Care
A substantial proportion of people with cancer believe cannabis can help manage cancer-related symptoms.
In Worster’s recent survey study, regardless of whether patients had used cannabis, almost 90% of those surveyed reported a perceived benefit. Although 65% also reported perceived risks for cannabis use, including difficulty concentrating, lung damage, and impaired memory, the perceived benefits outweighed the risks.
Despite generally positive perceptions, the overall literature on the benefits of cannabis in patients with cancer paints a less clear picture.
The ASCO guidelines, which were based on 13 systematic reviews and five additional primary studies, reported that cannabis can improve refractory, chemotherapy-induced nausea or vomiting when added to guideline-concordant antiemetic regimens, but that there is no clear evidence of benefit or harm for other supportive care outcomes.
The “certainty of evidence for most outcomes was low or very low,” the ASCO authors wrote.
The ASCO experts explained that, outside the context of a clinical trial, the evidence is not sufficient to recommend cannabis or cannabinoids for managing cancer pain, sleep issues, appetite loss, or anxiety and depression. For these outcomes, some studies indicate a benefit, while others don’t.
Real-world data from a large registry study, for instance, have indicated that medical cannabis is “a safe and effective complementary treatment for pain relief in patients with cancer.” However, a 2020 meta-analysis found that, in studies with a low risk for bias, adding cannabinoids to opioids did not reduce cancer pain in adults with advanced cancer.
There can be downsides to cannabis use, too. In one recent study, some patients reported feeling worse physically and psychologically compared with those who didn’t use cannabis. Another study found that oral cannabis was associated with “bothersome” side effects, including sedation, dizziness, and transient anxiety.
The ASCO guidelines also made it clear that cannabis or cannabinoids should not be used as cancer-directed treatment, outside of a clinical trial.
Talking to Patients About Cannabis
Given the level of evidence and patient interest in cannabis, it is important for oncologists to raise the topic of cannabis use with their patients.
To help inform decision-making and approaches to care, the ASCO guidelines suggest that oncologists can guide care themselves or direct patients to appropriate “unbiased, evidence-based” resources. For those who use cannabis or cannabinoids outside of evidence-based indications or clinician recommendations, it’s important to explore patients’ goals, educate them, and try to minimize harm.
One strategy for broaching the topic, Worster suggested, is to simply ask patients if they have tried or considered trying cannabis to control symptoms like nausea and vomiting, loss of appetite, or cancer pain.
The conversation with patients should then include an overview of the potential benefits and potential risks for cannabis use as well as risk reduction strategies, Worster noted.
But “approach it in an open and nonjudgmental frame of mind,” she said. “Just have a conversation.”
Discussing the formulation and concentration of tetrahydrocannabinol (THC) and cannabidiol (CBD) in products matters as well.
Will the product be inhaled, ingested, or topical? Inhaled cannabis is not ideal but is sometimes what patients have access to, Worster explained. Inhaled formulations tend to have faster onset, which might be preferable for treating chemotherapy-related nausea and vomiting, whereas edible formulations may take a while to start working.
It’s also important to warn patients about taking too much, she said, explaining that inhaling THC at higher doses can increase the risk for cardiovascular effects, anxiety, paranoia, panic, and psychosis.
CBD, on the other hand, is anti-inflammatory, but early data suggest it may blunt immune responses in high doses and should be used cautiously by patients receiving immunotherapy.
Worster noted that as laws change and the science advances, new cannabis products and formulations will emerge, as will artificial intelligence tools for helping to guide patients and clinicians in optimal use of cannabis for cancer care. State websites are a particularly helpful tool for providing state-specific medical education related to cannabis laws and use, as well, she said.
The bottom line, she said, is that talking to patients about the ins and outs of cannabis use “really matters.”
Worster disclosed that she is a medical consultant for EO Care.
A version of this article appeared on Medscape.com.
first, and oncologists may be hesitant to broach the topic with their patients.
Updated guidelines from the American Society of Clinical Oncology (ASCO) on the use of cannabis and cannabinoids in adults with cancer stress that it’s an important conversation to have.
According to the ASCO expert panel, access to and use of cannabis alongside cancer care have outpaced the science on evidence-based indications, and overall high-quality data on the effects of cannabis during cancer care are lacking. While several observational studies support cannabis use to help ease chemotherapy-related nausea and vomiting, the literature remains more divided on other potential benefits, such as alleviating cancer pain and sleep problems, and some evidence points to potential downsides of cannabis use.
Oncologists should “absolutely talk to patients” about cannabis, Brooke Worster, MD, medical director for the Master of Science in Medical Cannabis Science & Business program at Thomas Jefferson University, Philadelphia, told Medscape Medical News.
“Patients are interested, and they are going to find access to information. As a medical professional, it’s our job to help guide them through these spaces in a safe, nonjudgmental way.”
But, Worster noted, oncologists don’t have to be experts on cannabis to begin the conversation with patients.
So, “let yourself off the hook,” Worster urged.
Plus, avoiding the conversation won’t stop patients from using cannabis. In a recent study, Worster and her colleagues found that nearly one third of patients at 12 National Cancer Institute-designated cancer centers had used cannabis since their diagnosis — most often for sleep disturbance, pain, stress, and anxiety. Most (60%) felt somewhat or extremely comfortable talking to their healthcare provider about it, but only 21.5% said they had done so. Even fewer — about 10% — had talked to their treating oncologist.
Because patients may not discuss cannabis use, it’s especially important for oncologists to open up a line of communication, said Worster, also the enterprise director of supportive oncology at the Thomas Jefferson University.
Evidence on Cannabis During Cancer Care
A substantial proportion of people with cancer believe cannabis can help manage cancer-related symptoms.
In Worster’s recent survey study, regardless of whether patients had used cannabis, almost 90% of those surveyed reported a perceived benefit. Although 65% also reported perceived risks for cannabis use, including difficulty concentrating, lung damage, and impaired memory, the perceived benefits outweighed the risks.
Despite generally positive perceptions, the overall literature on the benefits of cannabis in patients with cancer paints a less clear picture.
The ASCO guidelines, which were based on 13 systematic reviews and five additional primary studies, reported that cannabis can improve refractory, chemotherapy-induced nausea or vomiting when added to guideline-concordant antiemetic regimens, but that there is no clear evidence of benefit or harm for other supportive care outcomes.
The “certainty of evidence for most outcomes was low or very low,” the ASCO authors wrote.
The ASCO experts explained that, outside the context of a clinical trial, the evidence is not sufficient to recommend cannabis or cannabinoids for managing cancer pain, sleep issues, appetite loss, or anxiety and depression. For these outcomes, some studies indicate a benefit, while others don’t.
Real-world data from a large registry study, for instance, have indicated that medical cannabis is “a safe and effective complementary treatment for pain relief in patients with cancer.” However, a 2020 meta-analysis found that, in studies with a low risk for bias, adding cannabinoids to opioids did not reduce cancer pain in adults with advanced cancer.
There can be downsides to cannabis use, too. In one recent study, some patients reported feeling worse physically and psychologically compared with those who didn’t use cannabis. Another study found that oral cannabis was associated with “bothersome” side effects, including sedation, dizziness, and transient anxiety.
The ASCO guidelines also made it clear that cannabis or cannabinoids should not be used as cancer-directed treatment, outside of a clinical trial.
Talking to Patients About Cannabis
Given the level of evidence and patient interest in cannabis, it is important for oncologists to raise the topic of cannabis use with their patients.
To help inform decision-making and approaches to care, the ASCO guidelines suggest that oncologists can guide care themselves or direct patients to appropriate “unbiased, evidence-based” resources. For those who use cannabis or cannabinoids outside of evidence-based indications or clinician recommendations, it’s important to explore patients’ goals, educate them, and try to minimize harm.
One strategy for broaching the topic, Worster suggested, is to simply ask patients if they have tried or considered trying cannabis to control symptoms like nausea and vomiting, loss of appetite, or cancer pain.
The conversation with patients should then include an overview of the potential benefits and potential risks for cannabis use as well as risk reduction strategies, Worster noted.
But “approach it in an open and nonjudgmental frame of mind,” she said. “Just have a conversation.”
Discussing the formulation and concentration of tetrahydrocannabinol (THC) and cannabidiol (CBD) in products matters as well.
Will the product be inhaled, ingested, or topical? Inhaled cannabis is not ideal but is sometimes what patients have access to, Worster explained. Inhaled formulations tend to have faster onset, which might be preferable for treating chemotherapy-related nausea and vomiting, whereas edible formulations may take a while to start working.
It’s also important to warn patients about taking too much, she said, explaining that inhaling THC at higher doses can increase the risk for cardiovascular effects, anxiety, paranoia, panic, and psychosis.
CBD, on the other hand, is anti-inflammatory, but early data suggest it may blunt immune responses in high doses and should be used cautiously by patients receiving immunotherapy.
Worster noted that as laws change and the science advances, new cannabis products and formulations will emerge, as will artificial intelligence tools for helping to guide patients and clinicians in optimal use of cannabis for cancer care. State websites are a particularly helpful tool for providing state-specific medical education related to cannabis laws and use, as well, she said.
The bottom line, she said, is that talking to patients about the ins and outs of cannabis use “really matters.”
Worster disclosed that she is a medical consultant for EO Care.
A version of this article appeared on Medscape.com.
How Are Doctors Using Tirzepatide vs Semaglutide? A Q&A
When prescribing glucagon-like peptide 1 (GLP-1) medications, many physicians prefer tirzepatide over the more well-known semaglutide due to its superior efficacy in weight loss and A1c reduction. Studies indicated that tirzepatide can lead to greater weight loss than semaglutide.
Factors like insurance coverage, drug availability, and side effects also influence physicians’ choices, with some patients benefiting from the broader dosing options that tirzepatide offers.
In this Q&A, Medscape Medical News explored how physicians can make the best decisions with their patients when choosing between GLP-1 medications tirzepatide and semaglutide for the treatment for type 2 diabetes and obesity.
We spoke to physicians who specialize in medical weight loss on things to consider when choosing between these two medications, such as patient profiles, drug access and availability, and financial considerations. We also discussed the side effect profiles of the medications based on current data in the literature.
Medscape Medical News: How are you deciding which of the two drugs to prescribe?
Caroline Messer, MD, endocrinologist at Lenox Hill Hospital, Northwell, New York City: To some degree, it’s based on insurance. But in general, I’m pushing most patients toward tirzepatide just because the data show that there’s more weight loss and more A1c reduction on tirzepatide. But the research shows that there are more side effects. But I think every practicing clinician who uses these medications knows that there are actually fewer side effects despite what the trial showed.
Sue Decotiis, MD, weight loss doctor, New York City: I think that many doctors that are prescribing these drugs are not really weight loss specialists. It’s just like one of many drugs that they prescribe. And semaglutide (Ozempic) is more well known. I think it’s because they don’t really know that it’s not as good as the other drugs. There are still massive shortages of these drugs. So that’s another reason why a doctor may choose one drug over another. Also, if a patient’s reliant on insurance to cover it, they may go with whatever the insurance company is willing to cover.
Kathleen Dungan, MD, professor of internal medicine, Division of Endocrinology, Diabetes and Metabolism, The Ohio State University Wexner Medical Center and College of Medicine: Some patients may have preferences with the delivery device. In the past year, in particular, availability of these drugs was limited and varied from time to time and geographically, and therefore, patients needed to substitute one drug for another in order to maintain treatment.
Maria Teresa Anton, MD, endocrinologist and educator, Pritikin Longevity Center, Miami: While I do not prescribe these medications, I do focus on integrating them into a comprehensive lifestyle program that empowers patients to make sustainable changes. By fostering an environment of education and support, we enhance their well-being and promote long-term health outcomes. In my practice, I’ve found that the most successful outcomes occur when these medications are combined with a comprehensive approach, including dietary changes, physical activity, and behavioral support.
Medscape Medical News: How do you make the decision of tirzepatide vs semaglutide?
Messer: There’s no guideline per se. Sometimes when I don’t want a patient to lose too much weight, I might consider Ozempic or Wegovy if you know they only have 5 lb to lose. If diabetes, then I might go for the Ozempic instead, just because the weight loss is so drastic with tirzepatide with any kind of appetite.
Decotiis: If somebody has a lot of weight to lose and they’re highly insulin resistant, as most people are when they start these drugs, I really prefer tirzepatide ... because I think patients are going to lose more weight, they’re going to lose more fat. I also see that patients have less side effects because before tirzepatide came out, I was prescribing mostly semaglutide, and there were a lot of side effects. But semaglutide is fine. I mean, it’s a good drug. Maybe it’s better for people that don’t have as much weight to lose. So I don’t have to worry about them hitting that wall after a certain period of time. But it’s a good drug. I mean, I certainly still use it.
Medscape Medical News: What of the data and the literature on the differences in the outcomes and the side effect profile?
Messer: In terms of outcomes, the weight loss is almost double [with tirzepatide]. It depends what trial you’re looking at, but we tend to see like about 15% of your body weight you lose with the semaglutide and 25%-30% with the tirzepatide. The big difference, I suppose…is semaglutide now has a cardiovascular indication and the tirzepatide doesn’t, but I’m very confident that tirzepatide is going to get the same indication.
Decotiis: When that first Lilly study came out in June of 2022, it really blew everybody away. I mean, some patients lost up to 25% of their weight on tirzepatide, whereas on Ozempic, it was really like 15%. Now, in my practice, I really monitor everyone with a body composition scale. I’m not just looking at somebody’s weight or body mass index, I am looking at how much body fat they have, how much muscle mass they have, how much water they have, and how much bone they have.
The golden rule here is make sure the patient loses fat, and you want to make sure they’re not losing muscle or too much water. The patient really needs to be adequately hydrated. So what I’m saying is a lot of people who have lost weight have not reached the promised land because they haven’t lost enough body fat to get them into that healthy zone. But once they reduce the body fat to a certain percentage, let’s say for a woman about 20%, or a man in the low teens, they’re less likely to regain that weight because they haven’t really lost fat. And that’s how we gain health.
A version of this article first appeared on Medscape.com.
When prescribing glucagon-like peptide 1 (GLP-1) medications, many physicians prefer tirzepatide over the more well-known semaglutide due to its superior efficacy in weight loss and A1c reduction. Studies indicated that tirzepatide can lead to greater weight loss than semaglutide.
Factors like insurance coverage, drug availability, and side effects also influence physicians’ choices, with some patients benefiting from the broader dosing options that tirzepatide offers.
In this Q&A, Medscape Medical News explored how physicians can make the best decisions with their patients when choosing between GLP-1 medications tirzepatide and semaglutide for the treatment for type 2 diabetes and obesity.
We spoke to physicians who specialize in medical weight loss on things to consider when choosing between these two medications, such as patient profiles, drug access and availability, and financial considerations. We also discussed the side effect profiles of the medications based on current data in the literature.
Medscape Medical News: How are you deciding which of the two drugs to prescribe?
Caroline Messer, MD, endocrinologist at Lenox Hill Hospital, Northwell, New York City: To some degree, it’s based on insurance. But in general, I’m pushing most patients toward tirzepatide just because the data show that there’s more weight loss and more A1c reduction on tirzepatide. But the research shows that there are more side effects. But I think every practicing clinician who uses these medications knows that there are actually fewer side effects despite what the trial showed.
Sue Decotiis, MD, weight loss doctor, New York City: I think that many doctors that are prescribing these drugs are not really weight loss specialists. It’s just like one of many drugs that they prescribe. And semaglutide (Ozempic) is more well known. I think it’s because they don’t really know that it’s not as good as the other drugs. There are still massive shortages of these drugs. So that’s another reason why a doctor may choose one drug over another. Also, if a patient’s reliant on insurance to cover it, they may go with whatever the insurance company is willing to cover.
Kathleen Dungan, MD, professor of internal medicine, Division of Endocrinology, Diabetes and Metabolism, The Ohio State University Wexner Medical Center and College of Medicine: Some patients may have preferences with the delivery device. In the past year, in particular, availability of these drugs was limited and varied from time to time and geographically, and therefore, patients needed to substitute one drug for another in order to maintain treatment.
Maria Teresa Anton, MD, endocrinologist and educator, Pritikin Longevity Center, Miami: While I do not prescribe these medications, I do focus on integrating them into a comprehensive lifestyle program that empowers patients to make sustainable changes. By fostering an environment of education and support, we enhance their well-being and promote long-term health outcomes. In my practice, I’ve found that the most successful outcomes occur when these medications are combined with a comprehensive approach, including dietary changes, physical activity, and behavioral support.
Medscape Medical News: How do you make the decision of tirzepatide vs semaglutide?
Messer: There’s no guideline per se. Sometimes when I don’t want a patient to lose too much weight, I might consider Ozempic or Wegovy if you know they only have 5 lb to lose. If diabetes, then I might go for the Ozempic instead, just because the weight loss is so drastic with tirzepatide with any kind of appetite.
Decotiis: If somebody has a lot of weight to lose and they’re highly insulin resistant, as most people are when they start these drugs, I really prefer tirzepatide ... because I think patients are going to lose more weight, they’re going to lose more fat. I also see that patients have less side effects because before tirzepatide came out, I was prescribing mostly semaglutide, and there were a lot of side effects. But semaglutide is fine. I mean, it’s a good drug. Maybe it’s better for people that don’t have as much weight to lose. So I don’t have to worry about them hitting that wall after a certain period of time. But it’s a good drug. I mean, I certainly still use it.
Medscape Medical News: What of the data and the literature on the differences in the outcomes and the side effect profile?
Messer: In terms of outcomes, the weight loss is almost double [with tirzepatide]. It depends what trial you’re looking at, but we tend to see like about 15% of your body weight you lose with the semaglutide and 25%-30% with the tirzepatide. The big difference, I suppose…is semaglutide now has a cardiovascular indication and the tirzepatide doesn’t, but I’m very confident that tirzepatide is going to get the same indication.
Decotiis: When that first Lilly study came out in June of 2022, it really blew everybody away. I mean, some patients lost up to 25% of their weight on tirzepatide, whereas on Ozempic, it was really like 15%. Now, in my practice, I really monitor everyone with a body composition scale. I’m not just looking at somebody’s weight or body mass index, I am looking at how much body fat they have, how much muscle mass they have, how much water they have, and how much bone they have.
The golden rule here is make sure the patient loses fat, and you want to make sure they’re not losing muscle or too much water. The patient really needs to be adequately hydrated. So what I’m saying is a lot of people who have lost weight have not reached the promised land because they haven’t lost enough body fat to get them into that healthy zone. But once they reduce the body fat to a certain percentage, let’s say for a woman about 20%, or a man in the low teens, they’re less likely to regain that weight because they haven’t really lost fat. And that’s how we gain health.
A version of this article first appeared on Medscape.com.
When prescribing glucagon-like peptide 1 (GLP-1) medications, many physicians prefer tirzepatide over the more well-known semaglutide due to its superior efficacy in weight loss and A1c reduction. Studies indicated that tirzepatide can lead to greater weight loss than semaglutide.
Factors like insurance coverage, drug availability, and side effects also influence physicians’ choices, with some patients benefiting from the broader dosing options that tirzepatide offers.
In this Q&A, Medscape Medical News explored how physicians can make the best decisions with their patients when choosing between GLP-1 medications tirzepatide and semaglutide for the treatment for type 2 diabetes and obesity.
We spoke to physicians who specialize in medical weight loss on things to consider when choosing between these two medications, such as patient profiles, drug access and availability, and financial considerations. We also discussed the side effect profiles of the medications based on current data in the literature.
Medscape Medical News: How are you deciding which of the two drugs to prescribe?
Caroline Messer, MD, endocrinologist at Lenox Hill Hospital, Northwell, New York City: To some degree, it’s based on insurance. But in general, I’m pushing most patients toward tirzepatide just because the data show that there’s more weight loss and more A1c reduction on tirzepatide. But the research shows that there are more side effects. But I think every practicing clinician who uses these medications knows that there are actually fewer side effects despite what the trial showed.
Sue Decotiis, MD, weight loss doctor, New York City: I think that many doctors that are prescribing these drugs are not really weight loss specialists. It’s just like one of many drugs that they prescribe. And semaglutide (Ozempic) is more well known. I think it’s because they don’t really know that it’s not as good as the other drugs. There are still massive shortages of these drugs. So that’s another reason why a doctor may choose one drug over another. Also, if a patient’s reliant on insurance to cover it, they may go with whatever the insurance company is willing to cover.
Kathleen Dungan, MD, professor of internal medicine, Division of Endocrinology, Diabetes and Metabolism, The Ohio State University Wexner Medical Center and College of Medicine: Some patients may have preferences with the delivery device. In the past year, in particular, availability of these drugs was limited and varied from time to time and geographically, and therefore, patients needed to substitute one drug for another in order to maintain treatment.
Maria Teresa Anton, MD, endocrinologist and educator, Pritikin Longevity Center, Miami: While I do not prescribe these medications, I do focus on integrating them into a comprehensive lifestyle program that empowers patients to make sustainable changes. By fostering an environment of education and support, we enhance their well-being and promote long-term health outcomes. In my practice, I’ve found that the most successful outcomes occur when these medications are combined with a comprehensive approach, including dietary changes, physical activity, and behavioral support.
Medscape Medical News: How do you make the decision of tirzepatide vs semaglutide?
Messer: There’s no guideline per se. Sometimes when I don’t want a patient to lose too much weight, I might consider Ozempic or Wegovy if you know they only have 5 lb to lose. If diabetes, then I might go for the Ozempic instead, just because the weight loss is so drastic with tirzepatide with any kind of appetite.
Decotiis: If somebody has a lot of weight to lose and they’re highly insulin resistant, as most people are when they start these drugs, I really prefer tirzepatide ... because I think patients are going to lose more weight, they’re going to lose more fat. I also see that patients have less side effects because before tirzepatide came out, I was prescribing mostly semaglutide, and there were a lot of side effects. But semaglutide is fine. I mean, it’s a good drug. Maybe it’s better for people that don’t have as much weight to lose. So I don’t have to worry about them hitting that wall after a certain period of time. But it’s a good drug. I mean, I certainly still use it.
Medscape Medical News: What of the data and the literature on the differences in the outcomes and the side effect profile?
Messer: In terms of outcomes, the weight loss is almost double [with tirzepatide]. It depends what trial you’re looking at, but we tend to see like about 15% of your body weight you lose with the semaglutide and 25%-30% with the tirzepatide. The big difference, I suppose…is semaglutide now has a cardiovascular indication and the tirzepatide doesn’t, but I’m very confident that tirzepatide is going to get the same indication.
Decotiis: When that first Lilly study came out in June of 2022, it really blew everybody away. I mean, some patients lost up to 25% of their weight on tirzepatide, whereas on Ozempic, it was really like 15%. Now, in my practice, I really monitor everyone with a body composition scale. I’m not just looking at somebody’s weight or body mass index, I am looking at how much body fat they have, how much muscle mass they have, how much water they have, and how much bone they have.
The golden rule here is make sure the patient loses fat, and you want to make sure they’re not losing muscle or too much water. The patient really needs to be adequately hydrated. So what I’m saying is a lot of people who have lost weight have not reached the promised land because they haven’t lost enough body fat to get them into that healthy zone. But once they reduce the body fat to a certain percentage, let’s say for a woman about 20%, or a man in the low teens, they’re less likely to regain that weight because they haven’t really lost fat. And that’s how we gain health.
A version of this article first appeared on Medscape.com.
More Mobile Clinics Are Bringing Long-Acting Birth Control to Rural Areas
Twice a month, a 40-foot-long truck transformed into a mobile clinic travels the Rio Grande Valley to provide rural Texans with women’s health care, including birth control.
The clinic, called the UniMóvil, is part of the Healthy Mujeres program at the University of Texas Rio Grande Valley School of Medicine in Edinburg.
The United States has about 3000 mobile health programs. But Saul Rivas, an ob.gyn., said he wasn’t aware of any that shared the specific mission of Healthy Mujeres when he helped launch the initiative in 2017. “Mujeres” means “women” in Spanish.
It’s now part of a small but growing number of mobile programs aimed at increasing rural access to women’s health services, including long-acting reversible contraception.
There are two kinds of these highly effective methods: intrauterine devices, known as IUDs, and hormonal implants inserted into the upper arm. These birth control options can be especially difficult to obtain — or have removed — in rural areas.
“Women who want to prevent an unintended pregnancy should have whatever works best for them,” said Kelly Conroy, senior director of mobile and maternal health programs at the University of Arkansas for Medical Sciences, Little Rock.
The school is launching a mobile women’s health and contraception program in rural parts of the state in October.
Rural areas have disproportionately fewer doctors, including ob.gyns., than urban areas. And rural providers may not be able to afford to stock long-acting birth control devices or may not be trained in administering them, program leaders say.
Mobile clinics help shrink that gap in rural care, but they can be challenging to operate, said Elizabeth Jones, a senior director at the National Family Planning & Reproductive Health Association.
Money is the greatest obstacle, Jones said. The Texas program costs up to $400,000 a year. A 2020 study of 173 mobile clinics found they cost an average of more than $630,000 a year. Mobile dental programs were the most expensive, averaging more than $1 million.
While many programs launch with the help of grants, they can be difficult to sustain, especially with over a decade of decreased or stagnant funding to Title X, a federal money stream that helps low-income people receive family planning services.
For example, a mobile contraception program serving rural Pennsylvania lasted less than 3 years before closing in 2023. It shut down after losing federal funding, said a spokesperson for the clinic that ran it.
Rural mobile programs aren’t as efficient or profitable as brick-and-mortar clinics. That’s because staff members may have to make hours-long trips to reach towns where they’ll probably see fewer patients than they would at a traditional site, Jones said.
She said organizations that can’t afford mobile programs can consider setting up “pop-up clinics” at existing health and community sites in rural areas.
Maria Briones is a patient who has benefited from the Healthy Mujeres program in southern Texas. The 41-year-old day care worker was concerned because she wasn’t getting her menstrual period with her IUD.
She considered going to Mexico to have the device removed because few doctors take her insurance on the US side of the Rio Grande Valley.
But Briones learned that the UniMóvil was visiting a small Texas city about 20 minutes from her home. She told the staff there that she doesn’t want more kids but was worried about the IUD.
Briones decided to keep the device after learning it’s safe and normal not to have periods while using an IUD. She won’t get billed for her appointment with the mobile clinic, even though the university health system doesn’t take her insurance.
“They have a lot of patience, and they answered all the questions that I had,” Briones said.
IUDs and hormonal implants are highly effective and can last up to 10 years. But they’re also expensive — devices can cost more than $1,000 without insurance — and inserting an IUD can be painful.
Patient-rights advocates are also concerned that some providers pressure people to use these devices.
They say ethical birth control programs aim to empower patients to choose the contraceptive method — if any — that is best for them, instead of promoting long-acting methods in an attempt to lower birth and poverty rates. They point to the history of eugenics-inspired sterilization and even more recent incidents.
For example, an investigation by Time magazine found doctors are more likely to push Black, Latina, young, and low-income women than other patients to use long-acting birth control — and to refuse to remove the devices.
Rivas said Healthy Mujeres staffers are trained on this issue.
“Our goal isn’t necessarily to place IUDs and implants,” he said. It’s to “provide education and help patients make the best decisions for themselves.”
David Wise, a spokesperson for the University of Arkansas for Medical Sciences, said staff members with the university’s mobile program will ask patients if they want to get pregnant in the next year, and will support their choice. The Arkansas and Texas programs also remove IUDs and hormonal arm implants if patients aren’t happy with them.
The Arkansas initiative will visit 14 rural counties with four vehicles the size of food trucks that were used in previous mobile health efforts. Staffing and equipment will be covered by a 2-year, $431,000 grant from an anonymous donor, Wise said.
In addition to contraception, faculty and medical residents staffing the vehicles will offer women’s health screenings, vaccinations, prenatal care, and testing and treatment for sexually transmitted infections.
Rivas said the Texas program was inspired by a study that found that, 6 months after giving birth, 34% of surveyed Texas mothers said long-acting contraception is their preferred birth control option — but only 13% were using that method.
“We started thinking about ways to address that gap,” Rivas said.
Healthy Mujeres, which is funded through multiple grants, started with a focus on contraception. It later expanded to services such as pregnancy ultrasounds, cervical cancer screenings, and testing for sexually transmitted infections.
While the Texas and Arkansas programs can bill insurance, they also have funding to help uninsured and underinsured patients afford their services. Both use community health workers — called promotoras in largely Spanish-speaking communities like the Rio Grande Valley — to connect patients with food, transportation, additional medical services, and other needs.
They partner with organizations that locals trust, such as food pantries and community colleges, which let the mobile units set up in their parking lots. And to further increase the availability of long-acting contraception in rural areas, the universities are training their students and local providers on how to insert, remove, and get reimbursed for the devices.
One difference between the programs is dictated by state laws. The Arkansas program can provide birth control to minors without a parent or guardian’s consent. But in Texas, most minors need consent before receiving health care, including contraception.
Advocates say these initiatives might help lower the rates of unintended and teen pregnancies in both states, which are higher than the national average.
Rivas and Conroy said their programs haven’t received much pushback. But Rivas said some churches that had asked the UniMóvil to visit their congregations changed their minds after learning the services included birth control.
Catherine Phillips, director of the Respect Life Office at Arkansas’ Catholic diocese, said the diocese supports efforts to achieve health care equity and she’s personally interested in mobile programs that visit rural areas such as where she lives.
But Phillips said the Arkansas program’s focus on birth control, especially long-acting methods, violates the teachings of the Catholic Church. Offering these services to minors without parental consent “makes it more egregious,” she said.
Jones said that, while these programs have hefty costs and other challenges, they also have benefits that can’t be measured in numbers.
“Building community trust and making an impact in the communities most impacted by health inequities — that’s invaluable,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Twice a month, a 40-foot-long truck transformed into a mobile clinic travels the Rio Grande Valley to provide rural Texans with women’s health care, including birth control.
The clinic, called the UniMóvil, is part of the Healthy Mujeres program at the University of Texas Rio Grande Valley School of Medicine in Edinburg.
The United States has about 3000 mobile health programs. But Saul Rivas, an ob.gyn., said he wasn’t aware of any that shared the specific mission of Healthy Mujeres when he helped launch the initiative in 2017. “Mujeres” means “women” in Spanish.
It’s now part of a small but growing number of mobile programs aimed at increasing rural access to women’s health services, including long-acting reversible contraception.
There are two kinds of these highly effective methods: intrauterine devices, known as IUDs, and hormonal implants inserted into the upper arm. These birth control options can be especially difficult to obtain — or have removed — in rural areas.
“Women who want to prevent an unintended pregnancy should have whatever works best for them,” said Kelly Conroy, senior director of mobile and maternal health programs at the University of Arkansas for Medical Sciences, Little Rock.
The school is launching a mobile women’s health and contraception program in rural parts of the state in October.
Rural areas have disproportionately fewer doctors, including ob.gyns., than urban areas. And rural providers may not be able to afford to stock long-acting birth control devices or may not be trained in administering them, program leaders say.
Mobile clinics help shrink that gap in rural care, but they can be challenging to operate, said Elizabeth Jones, a senior director at the National Family Planning & Reproductive Health Association.
Money is the greatest obstacle, Jones said. The Texas program costs up to $400,000 a year. A 2020 study of 173 mobile clinics found they cost an average of more than $630,000 a year. Mobile dental programs were the most expensive, averaging more than $1 million.
While many programs launch with the help of grants, they can be difficult to sustain, especially with over a decade of decreased or stagnant funding to Title X, a federal money stream that helps low-income people receive family planning services.
For example, a mobile contraception program serving rural Pennsylvania lasted less than 3 years before closing in 2023. It shut down after losing federal funding, said a spokesperson for the clinic that ran it.
Rural mobile programs aren’t as efficient or profitable as brick-and-mortar clinics. That’s because staff members may have to make hours-long trips to reach towns where they’ll probably see fewer patients than they would at a traditional site, Jones said.
She said organizations that can’t afford mobile programs can consider setting up “pop-up clinics” at existing health and community sites in rural areas.
Maria Briones is a patient who has benefited from the Healthy Mujeres program in southern Texas. The 41-year-old day care worker was concerned because she wasn’t getting her menstrual period with her IUD.
She considered going to Mexico to have the device removed because few doctors take her insurance on the US side of the Rio Grande Valley.
But Briones learned that the UniMóvil was visiting a small Texas city about 20 minutes from her home. She told the staff there that she doesn’t want more kids but was worried about the IUD.
Briones decided to keep the device after learning it’s safe and normal not to have periods while using an IUD. She won’t get billed for her appointment with the mobile clinic, even though the university health system doesn’t take her insurance.
“They have a lot of patience, and they answered all the questions that I had,” Briones said.
IUDs and hormonal implants are highly effective and can last up to 10 years. But they’re also expensive — devices can cost more than $1,000 without insurance — and inserting an IUD can be painful.
Patient-rights advocates are also concerned that some providers pressure people to use these devices.
They say ethical birth control programs aim to empower patients to choose the contraceptive method — if any — that is best for them, instead of promoting long-acting methods in an attempt to lower birth and poverty rates. They point to the history of eugenics-inspired sterilization and even more recent incidents.
For example, an investigation by Time magazine found doctors are more likely to push Black, Latina, young, and low-income women than other patients to use long-acting birth control — and to refuse to remove the devices.
Rivas said Healthy Mujeres staffers are trained on this issue.
“Our goal isn’t necessarily to place IUDs and implants,” he said. It’s to “provide education and help patients make the best decisions for themselves.”
David Wise, a spokesperson for the University of Arkansas for Medical Sciences, said staff members with the university’s mobile program will ask patients if they want to get pregnant in the next year, and will support their choice. The Arkansas and Texas programs also remove IUDs and hormonal arm implants if patients aren’t happy with them.
The Arkansas initiative will visit 14 rural counties with four vehicles the size of food trucks that were used in previous mobile health efforts. Staffing and equipment will be covered by a 2-year, $431,000 grant from an anonymous donor, Wise said.
In addition to contraception, faculty and medical residents staffing the vehicles will offer women’s health screenings, vaccinations, prenatal care, and testing and treatment for sexually transmitted infections.
Rivas said the Texas program was inspired by a study that found that, 6 months after giving birth, 34% of surveyed Texas mothers said long-acting contraception is their preferred birth control option — but only 13% were using that method.
“We started thinking about ways to address that gap,” Rivas said.
Healthy Mujeres, which is funded through multiple grants, started with a focus on contraception. It later expanded to services such as pregnancy ultrasounds, cervical cancer screenings, and testing for sexually transmitted infections.
While the Texas and Arkansas programs can bill insurance, they also have funding to help uninsured and underinsured patients afford their services. Both use community health workers — called promotoras in largely Spanish-speaking communities like the Rio Grande Valley — to connect patients with food, transportation, additional medical services, and other needs.
They partner with organizations that locals trust, such as food pantries and community colleges, which let the mobile units set up in their parking lots. And to further increase the availability of long-acting contraception in rural areas, the universities are training their students and local providers on how to insert, remove, and get reimbursed for the devices.
One difference between the programs is dictated by state laws. The Arkansas program can provide birth control to minors without a parent or guardian’s consent. But in Texas, most minors need consent before receiving health care, including contraception.
Advocates say these initiatives might help lower the rates of unintended and teen pregnancies in both states, which are higher than the national average.
Rivas and Conroy said their programs haven’t received much pushback. But Rivas said some churches that had asked the UniMóvil to visit their congregations changed their minds after learning the services included birth control.
Catherine Phillips, director of the Respect Life Office at Arkansas’ Catholic diocese, said the diocese supports efforts to achieve health care equity and she’s personally interested in mobile programs that visit rural areas such as where she lives.
But Phillips said the Arkansas program’s focus on birth control, especially long-acting methods, violates the teachings of the Catholic Church. Offering these services to minors without parental consent “makes it more egregious,” she said.
Jones said that, while these programs have hefty costs and other challenges, they also have benefits that can’t be measured in numbers.
“Building community trust and making an impact in the communities most impacted by health inequities — that’s invaluable,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Twice a month, a 40-foot-long truck transformed into a mobile clinic travels the Rio Grande Valley to provide rural Texans with women’s health care, including birth control.
The clinic, called the UniMóvil, is part of the Healthy Mujeres program at the University of Texas Rio Grande Valley School of Medicine in Edinburg.
The United States has about 3000 mobile health programs. But Saul Rivas, an ob.gyn., said he wasn’t aware of any that shared the specific mission of Healthy Mujeres when he helped launch the initiative in 2017. “Mujeres” means “women” in Spanish.
It’s now part of a small but growing number of mobile programs aimed at increasing rural access to women’s health services, including long-acting reversible contraception.
There are two kinds of these highly effective methods: intrauterine devices, known as IUDs, and hormonal implants inserted into the upper arm. These birth control options can be especially difficult to obtain — or have removed — in rural areas.
“Women who want to prevent an unintended pregnancy should have whatever works best for them,” said Kelly Conroy, senior director of mobile and maternal health programs at the University of Arkansas for Medical Sciences, Little Rock.
The school is launching a mobile women’s health and contraception program in rural parts of the state in October.
Rural areas have disproportionately fewer doctors, including ob.gyns., than urban areas. And rural providers may not be able to afford to stock long-acting birth control devices or may not be trained in administering them, program leaders say.
Mobile clinics help shrink that gap in rural care, but they can be challenging to operate, said Elizabeth Jones, a senior director at the National Family Planning & Reproductive Health Association.
Money is the greatest obstacle, Jones said. The Texas program costs up to $400,000 a year. A 2020 study of 173 mobile clinics found they cost an average of more than $630,000 a year. Mobile dental programs were the most expensive, averaging more than $1 million.
While many programs launch with the help of grants, they can be difficult to sustain, especially with over a decade of decreased or stagnant funding to Title X, a federal money stream that helps low-income people receive family planning services.
For example, a mobile contraception program serving rural Pennsylvania lasted less than 3 years before closing in 2023. It shut down after losing federal funding, said a spokesperson for the clinic that ran it.
Rural mobile programs aren’t as efficient or profitable as brick-and-mortar clinics. That’s because staff members may have to make hours-long trips to reach towns where they’ll probably see fewer patients than they would at a traditional site, Jones said.
She said organizations that can’t afford mobile programs can consider setting up “pop-up clinics” at existing health and community sites in rural areas.
Maria Briones is a patient who has benefited from the Healthy Mujeres program in southern Texas. The 41-year-old day care worker was concerned because she wasn’t getting her menstrual period with her IUD.
She considered going to Mexico to have the device removed because few doctors take her insurance on the US side of the Rio Grande Valley.
But Briones learned that the UniMóvil was visiting a small Texas city about 20 minutes from her home. She told the staff there that she doesn’t want more kids but was worried about the IUD.
Briones decided to keep the device after learning it’s safe and normal not to have periods while using an IUD. She won’t get billed for her appointment with the mobile clinic, even though the university health system doesn’t take her insurance.
“They have a lot of patience, and they answered all the questions that I had,” Briones said.
IUDs and hormonal implants are highly effective and can last up to 10 years. But they’re also expensive — devices can cost more than $1,000 without insurance — and inserting an IUD can be painful.
Patient-rights advocates are also concerned that some providers pressure people to use these devices.
They say ethical birth control programs aim to empower patients to choose the contraceptive method — if any — that is best for them, instead of promoting long-acting methods in an attempt to lower birth and poverty rates. They point to the history of eugenics-inspired sterilization and even more recent incidents.
For example, an investigation by Time magazine found doctors are more likely to push Black, Latina, young, and low-income women than other patients to use long-acting birth control — and to refuse to remove the devices.
Rivas said Healthy Mujeres staffers are trained on this issue.
“Our goal isn’t necessarily to place IUDs and implants,” he said. It’s to “provide education and help patients make the best decisions for themselves.”
David Wise, a spokesperson for the University of Arkansas for Medical Sciences, said staff members with the university’s mobile program will ask patients if they want to get pregnant in the next year, and will support their choice. The Arkansas and Texas programs also remove IUDs and hormonal arm implants if patients aren’t happy with them.
The Arkansas initiative will visit 14 rural counties with four vehicles the size of food trucks that were used in previous mobile health efforts. Staffing and equipment will be covered by a 2-year, $431,000 grant from an anonymous donor, Wise said.
In addition to contraception, faculty and medical residents staffing the vehicles will offer women’s health screenings, vaccinations, prenatal care, and testing and treatment for sexually transmitted infections.
Rivas said the Texas program was inspired by a study that found that, 6 months after giving birth, 34% of surveyed Texas mothers said long-acting contraception is their preferred birth control option — but only 13% were using that method.
“We started thinking about ways to address that gap,” Rivas said.
Healthy Mujeres, which is funded through multiple grants, started with a focus on contraception. It later expanded to services such as pregnancy ultrasounds, cervical cancer screenings, and testing for sexually transmitted infections.
While the Texas and Arkansas programs can bill insurance, they also have funding to help uninsured and underinsured patients afford their services. Both use community health workers — called promotoras in largely Spanish-speaking communities like the Rio Grande Valley — to connect patients with food, transportation, additional medical services, and other needs.
They partner with organizations that locals trust, such as food pantries and community colleges, which let the mobile units set up in their parking lots. And to further increase the availability of long-acting contraception in rural areas, the universities are training their students and local providers on how to insert, remove, and get reimbursed for the devices.
One difference between the programs is dictated by state laws. The Arkansas program can provide birth control to minors without a parent or guardian’s consent. But in Texas, most minors need consent before receiving health care, including contraception.
Advocates say these initiatives might help lower the rates of unintended and teen pregnancies in both states, which are higher than the national average.
Rivas and Conroy said their programs haven’t received much pushback. But Rivas said some churches that had asked the UniMóvil to visit their congregations changed their minds after learning the services included birth control.
Catherine Phillips, director of the Respect Life Office at Arkansas’ Catholic diocese, said the diocese supports efforts to achieve health care equity and she’s personally interested in mobile programs that visit rural areas such as where she lives.
But Phillips said the Arkansas program’s focus on birth control, especially long-acting methods, violates the teachings of the Catholic Church. Offering these services to minors without parental consent “makes it more egregious,” she said.
Jones said that, while these programs have hefty costs and other challenges, they also have benefits that can’t be measured in numbers.
“Building community trust and making an impact in the communities most impacted by health inequities — that’s invaluable,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
The Heavy Physical and Psychological Burden of Premenstrual Dysphoric Disorder
Premenstrual disorders (PMDs), including premenstrual dysphoric disorder (PMDD), adversely affect the lives of millions of women worldwide. Most girls and women — as many as 80%-90%— will experience some premenstrual discomfort such as irritability, depressed mood, food or alcohol cravings, bloating, body aches, breast pain, constipation, or fatigue.
Diagnosable menstrual disorders include, collectively, premenstrual syndrome (PMS); PMDD, formerly called late luteal phase dysphoric disorder; and premenstrual worsening of another medical condition.
The most debilitating of these is PMDD, which has an estimated prevalence of about 4%-8% in women of reproductive age, according to obstetrician/gynecologist Hoosna Haque, MD, assistant professor of medicine at Columbia University Irving Medical Center in New York City.
“It’s difficult to be sure because this condition is underreported,” said Luu D. Ireland, MD, MPH, assistant professor of obstetrics and gynecology at UMass Memorial Medical Center in Worcester, Massachusetts. “But more women are coming forward, and there’s more discussion and media coverage of this condition.”
Occurring in the same post-follicular timeframe as PMS, PMDD takes cyclical discomfort to a more intense level, with a trifecta of affective comorbidities, somatic manifestations, and behavioral changes, all of which can seriously impair daily functioning, including work, physical activities, and personal relationships. Romantic and marital relationships can be particularly impaired.
Although recent cost figures are lacking, PMDs exact a considerable economic toll with increased direct healthcare costs from doctor visits and pharmaceuticals. A 2010 study found that US women with PMS were more likely to accrue in excess of $500 in healthcare visit costs over 2 years, and the figure would likely be higher today. PMDs also increase work/school absenteeism and reduce productivity.
Etiology
Brain areas that regulate emotion and behavior contain receptors for estrogen, progesterone, and other sex hormones, which affect the functioning of neurotransmitter systems influencing mood and thinking. Although the precise pathophysiology remains unclear, PMDD is likely multifactorial and results in a heightened sensitivity to normal fluctuations in estrogen and progesterone during the luteal phase of the menstrual cycle and dysfunction of the serotonin and gamma-aminobutyric acid neurotransmitter systems.
Patients with PMDD have lower levels of cortisol and beta-endorphins during both the follicular and luteal phases, suggesting abnormalities in the hypothalamic-pituitary-gonadal axis (HPGA), which is consistent with dysregulation in mood disorders.
Risk Factors
These include family history, past traumatic events, smoking, chronic pain syndrome, and obesity. There may be a genetic component as recent studies have suggested the involvement of the gene that codes for the serotonergic 5HT1A receptor and allelic variants of ESR1 in the development of PMS/PMDD.
A particularly concerning aspect of PMDs of any sort is their possible association with a higher risk for death from non-natural causes. In a recent Swedish study, which did not distinguish between PMDs in general and PMDD in particular, patients had an almost 60% greater risk for death from non-natural causes and nearly twice the risk for death by suicide compared with women without PMDs.
Those diagnosed with a PMD at an early age showed excess mortality, and the risk for suicide was elevated regardless of age. “These findings support the need for careful follow-up for young women with PMDs and the need for suicide prevention strategies,” wrote lead author Marion Opatowski, PhD, a medical epidemiologist at Karolinska Institutet in Stockholm, Sweden. “Women with severe PMDD should definitely be monitored for suicidal thoughts or behavior and they should have an emergency outreach plan in place,” Haque added.
Diagnosis
Although the somatic manifestations of PMDD resemble those of PMS, they are more severe and associated psychological symptoms are greater. “In my experience, PMDD symptoms can last the whole 2 weeks of the luteal phase, whereas PMS might occur a couple of days before menstruation,” said Ireland.
Symptoms include labile mood, nervousness, hopelessness, anger and aggressiveness, as well as tension and irritability. Those affected may have suicidal thoughts or even behaviors. In addition to a lethargic loss of interest in normal activities, patients with PMDD may feel paranoid, confused, exhausted, or out of control and experience insomnia or hypersomnia. They may have trouble concentrating or remembering. Some patients with PMDD may already be prone to attention-deficit/hyperactivity disorder and non–cycle-related depression, anxiety, and panic attacks.
Diagnosis is based on the presence of any five of the typical affective, somatic, or behavioral symptoms outlined above in the week before onset of menses.
“It’s important to do a careful diagnosis for PMDD and rule out other underlying conditions such as existing depressive or anxiety disorders,” said Haque. “Symptoms tend to be more intense in periods of high hormonal fluctuation such as in the postpartum and perimenopause periods. Women with PMDD should be monitored for postpartum depression.”
PMDD is considered both a gynecologic-genitourinary disorder and an affective condition.
In 2013, it was controversially included as a depressive disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Strongly advocated by some patients, psychiatrists, and pharmaceutical companies, its inclusion was criticized by psychologists and generalists, who feared it would lead to overdiagnosis and pathologization of normal female hormonal changes. Women’s advocates protested that this inclusion would stigmatize female biology and harm their advance in society and the workplace, while some doctors continued to dismiss PMDD as not a serious concern.
Treatments
In its latest clinical practice guideline on PMDs, the American College of Obstetricians and Gynecologists (ACOG), for which Ireland served as the lead author, recommends that most patients with PMDD get medical treatment and outlines the following therapies, based on varying degrees of evidence strength.
Antidepressants. These may benefit patients with strong affective symptoms. Selective serotonin reuptake inhibitors such as sertraline (Zoloft), citalopram (Celexa), escitalopram (Lexapro), or fluoxetine (Prozac) are first choices.
Antidepressants may interrupt aberrant signaling in the HPGA, the circuit linking brain and ovaries and regulating the reproductive cycle. Serotonin norepinephrine reuptake inhibitor venlafaxine (Effexor) may also improve symptoms, but other types of antidepressants have not proven effective.
“The response to these well-tolerated drugs is rapid and can happen in the first 2 days,” said Ireland. The drugs may be taken either just in the luteal period or over the month, especially by patients with chronic depression or anxiety.
Hormonal therapy. ACOG recommends the use of combined oral contraceptives (COCs), gonadotropin-releasing hormone (GnRH) agonists to induce anovulation (with combined add-back hormones), progestin-only methods, and noncontraceptive continuous estrogen formulations. It notes, however, that COCs have not been more effective than placebo in reducing depressive symptom scores.
If symptoms do not improve over two to three cycles, an alternate therapy should be considered. Haque recommends an assessment after three cycles and then yearly.
Some women in her practice take both antidepressant and hormone therapy. “Unfortunately, there are no new pharmaceutical treatments on the horizon, but we have good ones already and we would love for patients to utilize them more often,” Ireland said.
Nonsteroidal anti-inflammatory drugs. Limited evidence shows these may reduce physical symptoms such as abdominal cramps, headaches, and general body aches, as well as some mood-related symptoms, which may be an indirect effect of pain alleviation.
Surgery. For women with the most severe intractable symptoms, bilateral oophorectomy with or without hysterectomy may be a last-resort option when medical management has failed. A trial period of GnRH agonist therapy (with or without adjunctive estrogen add-back treatment) is advised before surgery to predict a patient’s response to surgical management.
Acupuncture. ACOG suggests that acupuncture may help manage physical and affective premenstrual symptoms.
Diet. The usual dietary advice for premenstrual symptoms — such as consuming less caffeine, sugar, or alcohol and eating smaller, more frequent meals — is unlikely to help women with PMDD.
Exercise. Although it has not been well studied for PMDD, aerobic exercises such as walking, swimming, and biking tend to improve mood and energy levels in general. Exercise may reduce symptoms through several pathways, including effects on beta-endorphin, cortisol, and ovarian hormone levels.
Supplements. Vitamin B6, calcium and magnesium supplements, and herbal remedies are not supported by consistent or compelling evidence of efficacy. ACOG conditionally recommends calcium supplementation of 100-200 mg/d in adults to help manage physical and affective symptoms.
A small study suggested that supplemental zinc may improve both physical and psychological symptoms.
Cognitive-behavioral therapy. This treatment aims to interrupt negative and irrational thought patterns and may include awareness and education, as well as relaxation techniques, problem-solving and coping skills, and stress management. It has been associated with small to moderate improvement in anxiety and depression, said Ireland.
Peer support. Patients should consider joining a support group. The International Association for Premenstrual Disorders can help patients connect and develop coping skills.
The bottom line is that people with strong symptomatic evidence of PMDD should have medical intervention — to the benefit of their health and quality of life. Screening for PMDD should be part of women’s wellness examinations, said Ireland. “The impact of PMDD should not be minimized or dismissed,” said Haque. “And patients need to know there are very effective treatments.”
Ireland and Haque had no competing interests with regard to their comments.
A version of this article first appeared on Medscape.com.
Premenstrual disorders (PMDs), including premenstrual dysphoric disorder (PMDD), adversely affect the lives of millions of women worldwide. Most girls and women — as many as 80%-90%— will experience some premenstrual discomfort such as irritability, depressed mood, food or alcohol cravings, bloating, body aches, breast pain, constipation, or fatigue.
Diagnosable menstrual disorders include, collectively, premenstrual syndrome (PMS); PMDD, formerly called late luteal phase dysphoric disorder; and premenstrual worsening of another medical condition.
The most debilitating of these is PMDD, which has an estimated prevalence of about 4%-8% in women of reproductive age, according to obstetrician/gynecologist Hoosna Haque, MD, assistant professor of medicine at Columbia University Irving Medical Center in New York City.
“It’s difficult to be sure because this condition is underreported,” said Luu D. Ireland, MD, MPH, assistant professor of obstetrics and gynecology at UMass Memorial Medical Center in Worcester, Massachusetts. “But more women are coming forward, and there’s more discussion and media coverage of this condition.”
Occurring in the same post-follicular timeframe as PMS, PMDD takes cyclical discomfort to a more intense level, with a trifecta of affective comorbidities, somatic manifestations, and behavioral changes, all of which can seriously impair daily functioning, including work, physical activities, and personal relationships. Romantic and marital relationships can be particularly impaired.
Although recent cost figures are lacking, PMDs exact a considerable economic toll with increased direct healthcare costs from doctor visits and pharmaceuticals. A 2010 study found that US women with PMS were more likely to accrue in excess of $500 in healthcare visit costs over 2 years, and the figure would likely be higher today. PMDs also increase work/school absenteeism and reduce productivity.
Etiology
Brain areas that regulate emotion and behavior contain receptors for estrogen, progesterone, and other sex hormones, which affect the functioning of neurotransmitter systems influencing mood and thinking. Although the precise pathophysiology remains unclear, PMDD is likely multifactorial and results in a heightened sensitivity to normal fluctuations in estrogen and progesterone during the luteal phase of the menstrual cycle and dysfunction of the serotonin and gamma-aminobutyric acid neurotransmitter systems.
Patients with PMDD have lower levels of cortisol and beta-endorphins during both the follicular and luteal phases, suggesting abnormalities in the hypothalamic-pituitary-gonadal axis (HPGA), which is consistent with dysregulation in mood disorders.
Risk Factors
These include family history, past traumatic events, smoking, chronic pain syndrome, and obesity. There may be a genetic component as recent studies have suggested the involvement of the gene that codes for the serotonergic 5HT1A receptor and allelic variants of ESR1 in the development of PMS/PMDD.
A particularly concerning aspect of PMDs of any sort is their possible association with a higher risk for death from non-natural causes. In a recent Swedish study, which did not distinguish between PMDs in general and PMDD in particular, patients had an almost 60% greater risk for death from non-natural causes and nearly twice the risk for death by suicide compared with women without PMDs.
Those diagnosed with a PMD at an early age showed excess mortality, and the risk for suicide was elevated regardless of age. “These findings support the need for careful follow-up for young women with PMDs and the need for suicide prevention strategies,” wrote lead author Marion Opatowski, PhD, a medical epidemiologist at Karolinska Institutet in Stockholm, Sweden. “Women with severe PMDD should definitely be monitored for suicidal thoughts or behavior and they should have an emergency outreach plan in place,” Haque added.
Diagnosis
Although the somatic manifestations of PMDD resemble those of PMS, they are more severe and associated psychological symptoms are greater. “In my experience, PMDD symptoms can last the whole 2 weeks of the luteal phase, whereas PMS might occur a couple of days before menstruation,” said Ireland.
Symptoms include labile mood, nervousness, hopelessness, anger and aggressiveness, as well as tension and irritability. Those affected may have suicidal thoughts or even behaviors. In addition to a lethargic loss of interest in normal activities, patients with PMDD may feel paranoid, confused, exhausted, or out of control and experience insomnia or hypersomnia. They may have trouble concentrating or remembering. Some patients with PMDD may already be prone to attention-deficit/hyperactivity disorder and non–cycle-related depression, anxiety, and panic attacks.
Diagnosis is based on the presence of any five of the typical affective, somatic, or behavioral symptoms outlined above in the week before onset of menses.
“It’s important to do a careful diagnosis for PMDD and rule out other underlying conditions such as existing depressive or anxiety disorders,” said Haque. “Symptoms tend to be more intense in periods of high hormonal fluctuation such as in the postpartum and perimenopause periods. Women with PMDD should be monitored for postpartum depression.”
PMDD is considered both a gynecologic-genitourinary disorder and an affective condition.
In 2013, it was controversially included as a depressive disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Strongly advocated by some patients, psychiatrists, and pharmaceutical companies, its inclusion was criticized by psychologists and generalists, who feared it would lead to overdiagnosis and pathologization of normal female hormonal changes. Women’s advocates protested that this inclusion would stigmatize female biology and harm their advance in society and the workplace, while some doctors continued to dismiss PMDD as not a serious concern.
Treatments
In its latest clinical practice guideline on PMDs, the American College of Obstetricians and Gynecologists (ACOG), for which Ireland served as the lead author, recommends that most patients with PMDD get medical treatment and outlines the following therapies, based on varying degrees of evidence strength.
Antidepressants. These may benefit patients with strong affective symptoms. Selective serotonin reuptake inhibitors such as sertraline (Zoloft), citalopram (Celexa), escitalopram (Lexapro), or fluoxetine (Prozac) are first choices.
Antidepressants may interrupt aberrant signaling in the HPGA, the circuit linking brain and ovaries and regulating the reproductive cycle. Serotonin norepinephrine reuptake inhibitor venlafaxine (Effexor) may also improve symptoms, but other types of antidepressants have not proven effective.
“The response to these well-tolerated drugs is rapid and can happen in the first 2 days,” said Ireland. The drugs may be taken either just in the luteal period or over the month, especially by patients with chronic depression or anxiety.
Hormonal therapy. ACOG recommends the use of combined oral contraceptives (COCs), gonadotropin-releasing hormone (GnRH) agonists to induce anovulation (with combined add-back hormones), progestin-only methods, and noncontraceptive continuous estrogen formulations. It notes, however, that COCs have not been more effective than placebo in reducing depressive symptom scores.
If symptoms do not improve over two to three cycles, an alternate therapy should be considered. Haque recommends an assessment after three cycles and then yearly.
Some women in her practice take both antidepressant and hormone therapy. “Unfortunately, there are no new pharmaceutical treatments on the horizon, but we have good ones already and we would love for patients to utilize them more often,” Ireland said.
Nonsteroidal anti-inflammatory drugs. Limited evidence shows these may reduce physical symptoms such as abdominal cramps, headaches, and general body aches, as well as some mood-related symptoms, which may be an indirect effect of pain alleviation.
Surgery. For women with the most severe intractable symptoms, bilateral oophorectomy with or without hysterectomy may be a last-resort option when medical management has failed. A trial period of GnRH agonist therapy (with or without adjunctive estrogen add-back treatment) is advised before surgery to predict a patient’s response to surgical management.
Acupuncture. ACOG suggests that acupuncture may help manage physical and affective premenstrual symptoms.
Diet. The usual dietary advice for premenstrual symptoms — such as consuming less caffeine, sugar, or alcohol and eating smaller, more frequent meals — is unlikely to help women with PMDD.
Exercise. Although it has not been well studied for PMDD, aerobic exercises such as walking, swimming, and biking tend to improve mood and energy levels in general. Exercise may reduce symptoms through several pathways, including effects on beta-endorphin, cortisol, and ovarian hormone levels.
Supplements. Vitamin B6, calcium and magnesium supplements, and herbal remedies are not supported by consistent or compelling evidence of efficacy. ACOG conditionally recommends calcium supplementation of 100-200 mg/d in adults to help manage physical and affective symptoms.
A small study suggested that supplemental zinc may improve both physical and psychological symptoms.
Cognitive-behavioral therapy. This treatment aims to interrupt negative and irrational thought patterns and may include awareness and education, as well as relaxation techniques, problem-solving and coping skills, and stress management. It has been associated with small to moderate improvement in anxiety and depression, said Ireland.
Peer support. Patients should consider joining a support group. The International Association for Premenstrual Disorders can help patients connect and develop coping skills.
The bottom line is that people with strong symptomatic evidence of PMDD should have medical intervention — to the benefit of their health and quality of life. Screening for PMDD should be part of women’s wellness examinations, said Ireland. “The impact of PMDD should not be minimized or dismissed,” said Haque. “And patients need to know there are very effective treatments.”
Ireland and Haque had no competing interests with regard to their comments.
A version of this article first appeared on Medscape.com.
Premenstrual disorders (PMDs), including premenstrual dysphoric disorder (PMDD), adversely affect the lives of millions of women worldwide. Most girls and women — as many as 80%-90%— will experience some premenstrual discomfort such as irritability, depressed mood, food or alcohol cravings, bloating, body aches, breast pain, constipation, or fatigue.
Diagnosable menstrual disorders include, collectively, premenstrual syndrome (PMS); PMDD, formerly called late luteal phase dysphoric disorder; and premenstrual worsening of another medical condition.
The most debilitating of these is PMDD, which has an estimated prevalence of about 4%-8% in women of reproductive age, according to obstetrician/gynecologist Hoosna Haque, MD, assistant professor of medicine at Columbia University Irving Medical Center in New York City.
“It’s difficult to be sure because this condition is underreported,” said Luu D. Ireland, MD, MPH, assistant professor of obstetrics and gynecology at UMass Memorial Medical Center in Worcester, Massachusetts. “But more women are coming forward, and there’s more discussion and media coverage of this condition.”
Occurring in the same post-follicular timeframe as PMS, PMDD takes cyclical discomfort to a more intense level, with a trifecta of affective comorbidities, somatic manifestations, and behavioral changes, all of which can seriously impair daily functioning, including work, physical activities, and personal relationships. Romantic and marital relationships can be particularly impaired.
Although recent cost figures are lacking, PMDs exact a considerable economic toll with increased direct healthcare costs from doctor visits and pharmaceuticals. A 2010 study found that US women with PMS were more likely to accrue in excess of $500 in healthcare visit costs over 2 years, and the figure would likely be higher today. PMDs also increase work/school absenteeism and reduce productivity.
Etiology
Brain areas that regulate emotion and behavior contain receptors for estrogen, progesterone, and other sex hormones, which affect the functioning of neurotransmitter systems influencing mood and thinking. Although the precise pathophysiology remains unclear, PMDD is likely multifactorial and results in a heightened sensitivity to normal fluctuations in estrogen and progesterone during the luteal phase of the menstrual cycle and dysfunction of the serotonin and gamma-aminobutyric acid neurotransmitter systems.
Patients with PMDD have lower levels of cortisol and beta-endorphins during both the follicular and luteal phases, suggesting abnormalities in the hypothalamic-pituitary-gonadal axis (HPGA), which is consistent with dysregulation in mood disorders.
Risk Factors
These include family history, past traumatic events, smoking, chronic pain syndrome, and obesity. There may be a genetic component as recent studies have suggested the involvement of the gene that codes for the serotonergic 5HT1A receptor and allelic variants of ESR1 in the development of PMS/PMDD.
A particularly concerning aspect of PMDs of any sort is their possible association with a higher risk for death from non-natural causes. In a recent Swedish study, which did not distinguish between PMDs in general and PMDD in particular, patients had an almost 60% greater risk for death from non-natural causes and nearly twice the risk for death by suicide compared with women without PMDs.
Those diagnosed with a PMD at an early age showed excess mortality, and the risk for suicide was elevated regardless of age. “These findings support the need for careful follow-up for young women with PMDs and the need for suicide prevention strategies,” wrote lead author Marion Opatowski, PhD, a medical epidemiologist at Karolinska Institutet in Stockholm, Sweden. “Women with severe PMDD should definitely be monitored for suicidal thoughts or behavior and they should have an emergency outreach plan in place,” Haque added.
Diagnosis
Although the somatic manifestations of PMDD resemble those of PMS, they are more severe and associated psychological symptoms are greater. “In my experience, PMDD symptoms can last the whole 2 weeks of the luteal phase, whereas PMS might occur a couple of days before menstruation,” said Ireland.
Symptoms include labile mood, nervousness, hopelessness, anger and aggressiveness, as well as tension and irritability. Those affected may have suicidal thoughts or even behaviors. In addition to a lethargic loss of interest in normal activities, patients with PMDD may feel paranoid, confused, exhausted, or out of control and experience insomnia or hypersomnia. They may have trouble concentrating or remembering. Some patients with PMDD may already be prone to attention-deficit/hyperactivity disorder and non–cycle-related depression, anxiety, and panic attacks.
Diagnosis is based on the presence of any five of the typical affective, somatic, or behavioral symptoms outlined above in the week before onset of menses.
“It’s important to do a careful diagnosis for PMDD and rule out other underlying conditions such as existing depressive or anxiety disorders,” said Haque. “Symptoms tend to be more intense in periods of high hormonal fluctuation such as in the postpartum and perimenopause periods. Women with PMDD should be monitored for postpartum depression.”
PMDD is considered both a gynecologic-genitourinary disorder and an affective condition.
In 2013, it was controversially included as a depressive disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Strongly advocated by some patients, psychiatrists, and pharmaceutical companies, its inclusion was criticized by psychologists and generalists, who feared it would lead to overdiagnosis and pathologization of normal female hormonal changes. Women’s advocates protested that this inclusion would stigmatize female biology and harm their advance in society and the workplace, while some doctors continued to dismiss PMDD as not a serious concern.
Treatments
In its latest clinical practice guideline on PMDs, the American College of Obstetricians and Gynecologists (ACOG), for which Ireland served as the lead author, recommends that most patients with PMDD get medical treatment and outlines the following therapies, based on varying degrees of evidence strength.
Antidepressants. These may benefit patients with strong affective symptoms. Selective serotonin reuptake inhibitors such as sertraline (Zoloft), citalopram (Celexa), escitalopram (Lexapro), or fluoxetine (Prozac) are first choices.
Antidepressants may interrupt aberrant signaling in the HPGA, the circuit linking brain and ovaries and regulating the reproductive cycle. Serotonin norepinephrine reuptake inhibitor venlafaxine (Effexor) may also improve symptoms, but other types of antidepressants have not proven effective.
“The response to these well-tolerated drugs is rapid and can happen in the first 2 days,” said Ireland. The drugs may be taken either just in the luteal period or over the month, especially by patients with chronic depression or anxiety.
Hormonal therapy. ACOG recommends the use of combined oral contraceptives (COCs), gonadotropin-releasing hormone (GnRH) agonists to induce anovulation (with combined add-back hormones), progestin-only methods, and noncontraceptive continuous estrogen formulations. It notes, however, that COCs have not been more effective than placebo in reducing depressive symptom scores.
If symptoms do not improve over two to three cycles, an alternate therapy should be considered. Haque recommends an assessment after three cycles and then yearly.
Some women in her practice take both antidepressant and hormone therapy. “Unfortunately, there are no new pharmaceutical treatments on the horizon, but we have good ones already and we would love for patients to utilize them more often,” Ireland said.
Nonsteroidal anti-inflammatory drugs. Limited evidence shows these may reduce physical symptoms such as abdominal cramps, headaches, and general body aches, as well as some mood-related symptoms, which may be an indirect effect of pain alleviation.
Surgery. For women with the most severe intractable symptoms, bilateral oophorectomy with or without hysterectomy may be a last-resort option when medical management has failed. A trial period of GnRH agonist therapy (with or without adjunctive estrogen add-back treatment) is advised before surgery to predict a patient’s response to surgical management.
Acupuncture. ACOG suggests that acupuncture may help manage physical and affective premenstrual symptoms.
Diet. The usual dietary advice for premenstrual symptoms — such as consuming less caffeine, sugar, or alcohol and eating smaller, more frequent meals — is unlikely to help women with PMDD.
Exercise. Although it has not been well studied for PMDD, aerobic exercises such as walking, swimming, and biking tend to improve mood and energy levels in general. Exercise may reduce symptoms through several pathways, including effects on beta-endorphin, cortisol, and ovarian hormone levels.
Supplements. Vitamin B6, calcium and magnesium supplements, and herbal remedies are not supported by consistent or compelling evidence of efficacy. ACOG conditionally recommends calcium supplementation of 100-200 mg/d in adults to help manage physical and affective symptoms.
A small study suggested that supplemental zinc may improve both physical and psychological symptoms.
Cognitive-behavioral therapy. This treatment aims to interrupt negative and irrational thought patterns and may include awareness and education, as well as relaxation techniques, problem-solving and coping skills, and stress management. It has been associated with small to moderate improvement in anxiety and depression, said Ireland.
Peer support. Patients should consider joining a support group. The International Association for Premenstrual Disorders can help patients connect and develop coping skills.
The bottom line is that people with strong symptomatic evidence of PMDD should have medical intervention — to the benefit of their health and quality of life. Screening for PMDD should be part of women’s wellness examinations, said Ireland. “The impact of PMDD should not be minimized or dismissed,” said Haque. “And patients need to know there are very effective treatments.”
Ireland and Haque had no competing interests with regard to their comments.
A version of this article first appeared on Medscape.com.
MDMA Is Off the Table, So What’s Next for PTSD?
It has been 24 years since a pharmaceutical was last approved for posttraumatic stress disorder (PTSD). The condition is notoriously difficult to treat, with up to 40% patients finding no relief from symptoms through psychotherapy or current medications.
Many clinicians, advocates, and patients had pinned their hopes on the psychedelic drug midomafetamine with assisted therapy (MDMA-AT). However, in August, the US Food and Drug Administration (FDA) rejected it. At this point, it’s unclear when the therapy will be available, if ever.
“Not getting the FDA approval of any drug at this point is a setback for the field,” Lori Davis, MD, a senior research psychiatrist at the Birmingham Veterans Affairs (VA) Health Care System in Birmingham, Alabama, told Medscape Medical News.
Having an FDA-approved product would have helped increase public awareness of PTSD and driven interest in developing new therapies, said Davis, who is also adjunct professor of psychiatry at the Heersink School of Medicine, University of Alabama at Birmingham.
A Treatable Condition
So with MDMA-AT off the table, where does the field go next?
A public meeting in September hosted by the Reagan-Udall Foundation for the FDA in sought to answer that question. Agency officials joined representatives from the Department of Defense (DoD) and VA, patients, advocates, and industry representatives to discuss the current treatment landscape and what can be done to accelerate development of PTSD treatment.
Despite the common belief that PTSD is intractable, it “is a treatable condition,” Paula P. Schnurr, PhD, executive director of the VA National Center for PTSD, said at the meeting.
“There are effective treatments that work well for a lot of people, although not everyone has a satisfactory response,” she added.
The most effective psychotherapies are “trauma-focused,” and include cognitive processing therapy, eye movement desensitization and reprocessing, and prolonged exposure, according to the VA National Center for PTSD.
Three drugs have been approved by the FDA for PTSD: Venlafaxine (Effexor) in 1993, sertraline (Zoloft) in 1999, and paroxetine (Paxil) in 2000.
However, as the September meeting demonstrated, more therapies are needed.
“It’s clear to FDA and the federal government at large that there is an unmet need for safe and effective therapies to treat PTSD,” Bernard Fischer, MD, deputy director of the Division of Psychiatry in the Office of New Drugs at FDA’s Center for Drug Evaluation and Research, said at the meeting.
There is no shortage of research, Fischer added. Nearly 500 trials focused on PTSD are listed on clinicaltrials.gov are recruiting participants now or plan to soon.
Unsurprisingly, one of the primary drivers of PTSD therapeutics research is the VA. About 14% of the 5.7 million veterans who received care through the VA in 2023 had a diagnosis of PTSD.
“The US military is currently losing thousands of service members each year to PTSD- related disability discharges,” US Army Maj. Aaron Wolfgang, MD, a psychiatrist at the Walter Reed National Military Medical Center, said at the meeting. Only about 12%-20% of patients achieve remission with conventional therapies, added Wolfgang, who also is an assistant professor at the Uniformed Services University.
“For these reasons, establishing better treatments for PTSD is not only a matter of humanitarianism but also a pressing matter of national security,” he said.
The VA has committed at least $230 million to more than 140 active research projects in PTSD, Miriam J. Smyth, PhD, acting director of the clinical science, research and development service at the VA, said at the Reagan-Udall meeting.
One of the VA projects is the PTSD psychopharmacology initiative, which began in 2017 and now has 14 active clinical trials, said Smyth, who is also acting director for brain behavior and mental health at the VA. The first study should be finished by 2025.
The Million Veteran Program, with more than 1 million enrollees, has led to the discovery of genes related to re-experiencing traumatic memories and has confirmed that both PTSD and traumatic brain injury are risk factors for dementia, Smyth said.
The DoD has created a novel platform that establishes a common infrastructure for testing multiple drugs, called M-PACT. The platform allows sharing of placebo data across treatment arms. Drugs cycle off the platform if evidence indicates probability of success or failure.
Four trials are actively recruiting veterans and current service members. One is looking at vilazodone, approved in 2011 for major depressive disorder. It is being compared with placebo and fluoxetine in a trial that is currently recruiting.
Another trial will study daridorexant (sold as Quviviq), an orexin receptor antagonist, against placebo. The FDA approved daridorexant in 2022 as an insomnia treatment. A core issue in PTSD is sleep disruption, noted Davis.
New Therapies on the Way
Separately, Davis and colleagues are also studying methylphenidate, the stimulant used for attention-deficit/hyperactivity disorder. It may help with neurocognitive complaints and reduce PTSD symptoms, said Davis.
Because it is generic, few pharmaceutical manufacturers are likely to test it for PTSD, she said. But eventually, their work may lead a company to test newer stimulants for PTSD, she said.
Another potential therapeutic, BNC210, received Fast Track designation for PTSD from the FDA in 2019. Bionomics Limited in Australia will soon launch phase 3 trials of the investigational oral drug, which is a negative allosteric modulator of the alpha-7 nicotinic acetylcholine receptor. In late July, the company announced “ favorable feedback” from the agency on its phase 2 study, which led to the decision to move forward with larger trials.
Researchers at Brigham and Women’s Hospital have just reported that they may have found a target within the brain that will allow for transcranial magnetic stimulation (TMS) to ameliorate PTSD symptoms. They published results of a mapping effort in Nature Neuroscience and reported on one patient who had improved symptoms after receiving TMS for severe PTSD.
But perhaps one of the most promising treatments is a combination of sertraline and the new psychiatric medication brexpiprazole.
Brexpiprazole was developed by Otsuka Pharmaceutical and approved in the United States in 2015 as an adjunctive therapy to antidepressants for major depressive disorder and as a treatment for schizophrenia. In 2023, the FDA approved it for Alzheimer’s-related agitation. However, according to Otsuka, its mechanism of action is unknown.
Its efficacy may be mediated through a combination of partial agonist activity at serotonin 5-HT1A and dopamine D2 receptors, antagonist activity at serotonin 5-HT2A receptors, as well as antagonism of alpha-1B/2C receptors, said the company.
“It is the combination, rather than either alone, that’s going to have that broad synergistic pharmacology that is obviously potent for ameliorating the symptoms of PTSD,” said Davis, who has received consulting fees from Otsuka. “That’s an exciting development.”
Otsuka and partner Lundbeck Pharmaceuticals reported results in May from the companies’ phase 2 and 3 randomized clinical trials. The therapy achieved a statistically significant reduction (P <.05) in PTSD symptoms compared with sertraline plus placebo. This was without any supplemental psychotherapy.
The FDA accepted the companies’ new drug application in June and is expected to make a decision on approval in February 2025.
The Potential of Psychedelics
Though Lykos Therapeutics may have to go back to the drawing board on its MDMA-AT, psychedelics still have potential as PTSD therapies, Smyth said, who added that the VA is continuing to encourage study of MDMA and other psychedelic agents.
The VA issued a call for proposals for research on psychedelics in January, focused on MDMA or psilocybin in combination with psychotherapy. The administration received the first wave of applications early in the summer.
Scientific peer review panels made up of research experts from within and outside the VA have reviewed the applications and funding announcements are expected this fall, Smyth said.
Wolfgang, the Army psychiatrist, said, “Under the psychedelic treatment research clinical trial award, we welcome investigators to apply to what we anticipate will usher in a new era of innovation and hope for service members and their families who need it the most.”
Psychedelic studies are also proceeding without VA funding, as they have for years, when most of the trials were backed by universities or foundations or other private money. Johns Hopkins University is recruiting for a study in which patients would receive psilocybin along with trauma-focused psychotherapy, as is Ohio State University.
London-based Compass Pathways said in May that it successfully completed a phase 2 trial of Comp360, its synthetic psilocybin, in PTSD. The company has started a phase 3 study in treatment-resistant depression but has not given any further updates on PTSD.
Davis said that she believes that the FDA’s rejection of Lykos won’t lead to a shutdown of exploration of psychedelics.
“I think it informs these designs going forward, but it doesn’t eliminate that whole field of research,” she said.
Davis reported receiving consulting fees from Boehringer Ingelheim and Otsuka and research funding from Alkermes, the Patient-Centered Outcomes Research Institute, and the VA. Schnurr, Fischer, Smyth, and Wolfgang reported no relevant disclosures.
A version of this article appeared on Medscape.com.
It has been 24 years since a pharmaceutical was last approved for posttraumatic stress disorder (PTSD). The condition is notoriously difficult to treat, with up to 40% patients finding no relief from symptoms through psychotherapy or current medications.
Many clinicians, advocates, and patients had pinned their hopes on the psychedelic drug midomafetamine with assisted therapy (MDMA-AT). However, in August, the US Food and Drug Administration (FDA) rejected it. At this point, it’s unclear when the therapy will be available, if ever.
“Not getting the FDA approval of any drug at this point is a setback for the field,” Lori Davis, MD, a senior research psychiatrist at the Birmingham Veterans Affairs (VA) Health Care System in Birmingham, Alabama, told Medscape Medical News.
Having an FDA-approved product would have helped increase public awareness of PTSD and driven interest in developing new therapies, said Davis, who is also adjunct professor of psychiatry at the Heersink School of Medicine, University of Alabama at Birmingham.
A Treatable Condition
So with MDMA-AT off the table, where does the field go next?
A public meeting in September hosted by the Reagan-Udall Foundation for the FDA in sought to answer that question. Agency officials joined representatives from the Department of Defense (DoD) and VA, patients, advocates, and industry representatives to discuss the current treatment landscape and what can be done to accelerate development of PTSD treatment.
Despite the common belief that PTSD is intractable, it “is a treatable condition,” Paula P. Schnurr, PhD, executive director of the VA National Center for PTSD, said at the meeting.
“There are effective treatments that work well for a lot of people, although not everyone has a satisfactory response,” she added.
The most effective psychotherapies are “trauma-focused,” and include cognitive processing therapy, eye movement desensitization and reprocessing, and prolonged exposure, according to the VA National Center for PTSD.
Three drugs have been approved by the FDA for PTSD: Venlafaxine (Effexor) in 1993, sertraline (Zoloft) in 1999, and paroxetine (Paxil) in 2000.
However, as the September meeting demonstrated, more therapies are needed.
“It’s clear to FDA and the federal government at large that there is an unmet need for safe and effective therapies to treat PTSD,” Bernard Fischer, MD, deputy director of the Division of Psychiatry in the Office of New Drugs at FDA’s Center for Drug Evaluation and Research, said at the meeting.
There is no shortage of research, Fischer added. Nearly 500 trials focused on PTSD are listed on clinicaltrials.gov are recruiting participants now or plan to soon.
Unsurprisingly, one of the primary drivers of PTSD therapeutics research is the VA. About 14% of the 5.7 million veterans who received care through the VA in 2023 had a diagnosis of PTSD.
“The US military is currently losing thousands of service members each year to PTSD- related disability discharges,” US Army Maj. Aaron Wolfgang, MD, a psychiatrist at the Walter Reed National Military Medical Center, said at the meeting. Only about 12%-20% of patients achieve remission with conventional therapies, added Wolfgang, who also is an assistant professor at the Uniformed Services University.
“For these reasons, establishing better treatments for PTSD is not only a matter of humanitarianism but also a pressing matter of national security,” he said.
The VA has committed at least $230 million to more than 140 active research projects in PTSD, Miriam J. Smyth, PhD, acting director of the clinical science, research and development service at the VA, said at the Reagan-Udall meeting.
One of the VA projects is the PTSD psychopharmacology initiative, which began in 2017 and now has 14 active clinical trials, said Smyth, who is also acting director for brain behavior and mental health at the VA. The first study should be finished by 2025.
The Million Veteran Program, with more than 1 million enrollees, has led to the discovery of genes related to re-experiencing traumatic memories and has confirmed that both PTSD and traumatic brain injury are risk factors for dementia, Smyth said.
The DoD has created a novel platform that establishes a common infrastructure for testing multiple drugs, called M-PACT. The platform allows sharing of placebo data across treatment arms. Drugs cycle off the platform if evidence indicates probability of success or failure.
Four trials are actively recruiting veterans and current service members. One is looking at vilazodone, approved in 2011 for major depressive disorder. It is being compared with placebo and fluoxetine in a trial that is currently recruiting.
Another trial will study daridorexant (sold as Quviviq), an orexin receptor antagonist, against placebo. The FDA approved daridorexant in 2022 as an insomnia treatment. A core issue in PTSD is sleep disruption, noted Davis.
New Therapies on the Way
Separately, Davis and colleagues are also studying methylphenidate, the stimulant used for attention-deficit/hyperactivity disorder. It may help with neurocognitive complaints and reduce PTSD symptoms, said Davis.
Because it is generic, few pharmaceutical manufacturers are likely to test it for PTSD, she said. But eventually, their work may lead a company to test newer stimulants for PTSD, she said.
Another potential therapeutic, BNC210, received Fast Track designation for PTSD from the FDA in 2019. Bionomics Limited in Australia will soon launch phase 3 trials of the investigational oral drug, which is a negative allosteric modulator of the alpha-7 nicotinic acetylcholine receptor. In late July, the company announced “ favorable feedback” from the agency on its phase 2 study, which led to the decision to move forward with larger trials.
Researchers at Brigham and Women’s Hospital have just reported that they may have found a target within the brain that will allow for transcranial magnetic stimulation (TMS) to ameliorate PTSD symptoms. They published results of a mapping effort in Nature Neuroscience and reported on one patient who had improved symptoms after receiving TMS for severe PTSD.
But perhaps one of the most promising treatments is a combination of sertraline and the new psychiatric medication brexpiprazole.
Brexpiprazole was developed by Otsuka Pharmaceutical and approved in the United States in 2015 as an adjunctive therapy to antidepressants for major depressive disorder and as a treatment for schizophrenia. In 2023, the FDA approved it for Alzheimer’s-related agitation. However, according to Otsuka, its mechanism of action is unknown.
Its efficacy may be mediated through a combination of partial agonist activity at serotonin 5-HT1A and dopamine D2 receptors, antagonist activity at serotonin 5-HT2A receptors, as well as antagonism of alpha-1B/2C receptors, said the company.
“It is the combination, rather than either alone, that’s going to have that broad synergistic pharmacology that is obviously potent for ameliorating the symptoms of PTSD,” said Davis, who has received consulting fees from Otsuka. “That’s an exciting development.”
Otsuka and partner Lundbeck Pharmaceuticals reported results in May from the companies’ phase 2 and 3 randomized clinical trials. The therapy achieved a statistically significant reduction (P <.05) in PTSD symptoms compared with sertraline plus placebo. This was without any supplemental psychotherapy.
The FDA accepted the companies’ new drug application in June and is expected to make a decision on approval in February 2025.
The Potential of Psychedelics
Though Lykos Therapeutics may have to go back to the drawing board on its MDMA-AT, psychedelics still have potential as PTSD therapies, Smyth said, who added that the VA is continuing to encourage study of MDMA and other psychedelic agents.
The VA issued a call for proposals for research on psychedelics in January, focused on MDMA or psilocybin in combination with psychotherapy. The administration received the first wave of applications early in the summer.
Scientific peer review panels made up of research experts from within and outside the VA have reviewed the applications and funding announcements are expected this fall, Smyth said.
Wolfgang, the Army psychiatrist, said, “Under the psychedelic treatment research clinical trial award, we welcome investigators to apply to what we anticipate will usher in a new era of innovation and hope for service members and their families who need it the most.”
Psychedelic studies are also proceeding without VA funding, as they have for years, when most of the trials were backed by universities or foundations or other private money. Johns Hopkins University is recruiting for a study in which patients would receive psilocybin along with trauma-focused psychotherapy, as is Ohio State University.
London-based Compass Pathways said in May that it successfully completed a phase 2 trial of Comp360, its synthetic psilocybin, in PTSD. The company has started a phase 3 study in treatment-resistant depression but has not given any further updates on PTSD.
Davis said that she believes that the FDA’s rejection of Lykos won’t lead to a shutdown of exploration of psychedelics.
“I think it informs these designs going forward, but it doesn’t eliminate that whole field of research,” she said.
Davis reported receiving consulting fees from Boehringer Ingelheim and Otsuka and research funding from Alkermes, the Patient-Centered Outcomes Research Institute, and the VA. Schnurr, Fischer, Smyth, and Wolfgang reported no relevant disclosures.
A version of this article appeared on Medscape.com.
It has been 24 years since a pharmaceutical was last approved for posttraumatic stress disorder (PTSD). The condition is notoriously difficult to treat, with up to 40% patients finding no relief from symptoms through psychotherapy or current medications.
Many clinicians, advocates, and patients had pinned their hopes on the psychedelic drug midomafetamine with assisted therapy (MDMA-AT). However, in August, the US Food and Drug Administration (FDA) rejected it. At this point, it’s unclear when the therapy will be available, if ever.
“Not getting the FDA approval of any drug at this point is a setback for the field,” Lori Davis, MD, a senior research psychiatrist at the Birmingham Veterans Affairs (VA) Health Care System in Birmingham, Alabama, told Medscape Medical News.
Having an FDA-approved product would have helped increase public awareness of PTSD and driven interest in developing new therapies, said Davis, who is also adjunct professor of psychiatry at the Heersink School of Medicine, University of Alabama at Birmingham.
A Treatable Condition
So with MDMA-AT off the table, where does the field go next?
A public meeting in September hosted by the Reagan-Udall Foundation for the FDA in sought to answer that question. Agency officials joined representatives from the Department of Defense (DoD) and VA, patients, advocates, and industry representatives to discuss the current treatment landscape and what can be done to accelerate development of PTSD treatment.
Despite the common belief that PTSD is intractable, it “is a treatable condition,” Paula P. Schnurr, PhD, executive director of the VA National Center for PTSD, said at the meeting.
“There are effective treatments that work well for a lot of people, although not everyone has a satisfactory response,” she added.
The most effective psychotherapies are “trauma-focused,” and include cognitive processing therapy, eye movement desensitization and reprocessing, and prolonged exposure, according to the VA National Center for PTSD.
Three drugs have been approved by the FDA for PTSD: Venlafaxine (Effexor) in 1993, sertraline (Zoloft) in 1999, and paroxetine (Paxil) in 2000.
However, as the September meeting demonstrated, more therapies are needed.
“It’s clear to FDA and the federal government at large that there is an unmet need for safe and effective therapies to treat PTSD,” Bernard Fischer, MD, deputy director of the Division of Psychiatry in the Office of New Drugs at FDA’s Center for Drug Evaluation and Research, said at the meeting.
There is no shortage of research, Fischer added. Nearly 500 trials focused on PTSD are listed on clinicaltrials.gov are recruiting participants now or plan to soon.
Unsurprisingly, one of the primary drivers of PTSD therapeutics research is the VA. About 14% of the 5.7 million veterans who received care through the VA in 2023 had a diagnosis of PTSD.
“The US military is currently losing thousands of service members each year to PTSD- related disability discharges,” US Army Maj. Aaron Wolfgang, MD, a psychiatrist at the Walter Reed National Military Medical Center, said at the meeting. Only about 12%-20% of patients achieve remission with conventional therapies, added Wolfgang, who also is an assistant professor at the Uniformed Services University.
“For these reasons, establishing better treatments for PTSD is not only a matter of humanitarianism but also a pressing matter of national security,” he said.
The VA has committed at least $230 million to more than 140 active research projects in PTSD, Miriam J. Smyth, PhD, acting director of the clinical science, research and development service at the VA, said at the Reagan-Udall meeting.
One of the VA projects is the PTSD psychopharmacology initiative, which began in 2017 and now has 14 active clinical trials, said Smyth, who is also acting director for brain behavior and mental health at the VA. The first study should be finished by 2025.
The Million Veteran Program, with more than 1 million enrollees, has led to the discovery of genes related to re-experiencing traumatic memories and has confirmed that both PTSD and traumatic brain injury are risk factors for dementia, Smyth said.
The DoD has created a novel platform that establishes a common infrastructure for testing multiple drugs, called M-PACT. The platform allows sharing of placebo data across treatment arms. Drugs cycle off the platform if evidence indicates probability of success or failure.
Four trials are actively recruiting veterans and current service members. One is looking at vilazodone, approved in 2011 for major depressive disorder. It is being compared with placebo and fluoxetine in a trial that is currently recruiting.
Another trial will study daridorexant (sold as Quviviq), an orexin receptor antagonist, against placebo. The FDA approved daridorexant in 2022 as an insomnia treatment. A core issue in PTSD is sleep disruption, noted Davis.
New Therapies on the Way
Separately, Davis and colleagues are also studying methylphenidate, the stimulant used for attention-deficit/hyperactivity disorder. It may help with neurocognitive complaints and reduce PTSD symptoms, said Davis.
Because it is generic, few pharmaceutical manufacturers are likely to test it for PTSD, she said. But eventually, their work may lead a company to test newer stimulants for PTSD, she said.
Another potential therapeutic, BNC210, received Fast Track designation for PTSD from the FDA in 2019. Bionomics Limited in Australia will soon launch phase 3 trials of the investigational oral drug, which is a negative allosteric modulator of the alpha-7 nicotinic acetylcholine receptor. In late July, the company announced “ favorable feedback” from the agency on its phase 2 study, which led to the decision to move forward with larger trials.
Researchers at Brigham and Women’s Hospital have just reported that they may have found a target within the brain that will allow for transcranial magnetic stimulation (TMS) to ameliorate PTSD symptoms. They published results of a mapping effort in Nature Neuroscience and reported on one patient who had improved symptoms after receiving TMS for severe PTSD.
But perhaps one of the most promising treatments is a combination of sertraline and the new psychiatric medication brexpiprazole.
Brexpiprazole was developed by Otsuka Pharmaceutical and approved in the United States in 2015 as an adjunctive therapy to antidepressants for major depressive disorder and as a treatment for schizophrenia. In 2023, the FDA approved it for Alzheimer’s-related agitation. However, according to Otsuka, its mechanism of action is unknown.
Its efficacy may be mediated through a combination of partial agonist activity at serotonin 5-HT1A and dopamine D2 receptors, antagonist activity at serotonin 5-HT2A receptors, as well as antagonism of alpha-1B/2C receptors, said the company.
“It is the combination, rather than either alone, that’s going to have that broad synergistic pharmacology that is obviously potent for ameliorating the symptoms of PTSD,” said Davis, who has received consulting fees from Otsuka. “That’s an exciting development.”
Otsuka and partner Lundbeck Pharmaceuticals reported results in May from the companies’ phase 2 and 3 randomized clinical trials. The therapy achieved a statistically significant reduction (P <.05) in PTSD symptoms compared with sertraline plus placebo. This was without any supplemental psychotherapy.
The FDA accepted the companies’ new drug application in June and is expected to make a decision on approval in February 2025.
The Potential of Psychedelics
Though Lykos Therapeutics may have to go back to the drawing board on its MDMA-AT, psychedelics still have potential as PTSD therapies, Smyth said, who added that the VA is continuing to encourage study of MDMA and other psychedelic agents.
The VA issued a call for proposals for research on psychedelics in January, focused on MDMA or psilocybin in combination with psychotherapy. The administration received the first wave of applications early in the summer.
Scientific peer review panels made up of research experts from within and outside the VA have reviewed the applications and funding announcements are expected this fall, Smyth said.
Wolfgang, the Army psychiatrist, said, “Under the psychedelic treatment research clinical trial award, we welcome investigators to apply to what we anticipate will usher in a new era of innovation and hope for service members and their families who need it the most.”
Psychedelic studies are also proceeding without VA funding, as they have for years, when most of the trials were backed by universities or foundations or other private money. Johns Hopkins University is recruiting for a study in which patients would receive psilocybin along with trauma-focused psychotherapy, as is Ohio State University.
London-based Compass Pathways said in May that it successfully completed a phase 2 trial of Comp360, its synthetic psilocybin, in PTSD. The company has started a phase 3 study in treatment-resistant depression but has not given any further updates on PTSD.
Davis said that she believes that the FDA’s rejection of Lykos won’t lead to a shutdown of exploration of psychedelics.
“I think it informs these designs going forward, but it doesn’t eliminate that whole field of research,” she said.
Davis reported receiving consulting fees from Boehringer Ingelheim and Otsuka and research funding from Alkermes, the Patient-Centered Outcomes Research Institute, and the VA. Schnurr, Fischer, Smyth, and Wolfgang reported no relevant disclosures.
A version of this article appeared on Medscape.com.
State of Confusion: Should All Children Get Lipid Labs for High Cholesterol?
Clinicians receive conflicting advice on whether to order blood tests to screen for lipids in children. A new study could add to the confusion. Researchers found that a combination of physical proxy measures such as hypertension and body mass index (BMI) predicted the risk for future cardiovascular events as well as the physical model plus lipid labs, questioning the value of those blood tests.
Some medical organizations advise screening only for high-risk children because more research is needed to define the harms and benefits of universal screening. Diet and behavioral changes are sufficient for most children, and universal screening could lead to false positives and unnecessary further testing, they said.
Groups that favor lipid tests for all children say these measurements detect familial hypercholesterolemia (FH) that would not otherwise be diagnosed, leading to treatment with drugs like statins and a greater chance of preventing cardiovascular disease (CVD) in adulthood.
Researchers from the new study said their findings do not address screenings for FH, which affects 1 in 250 US children and puts them at a risk for atherosclerotic CVD.
Recommending Blood Tests in Age Groups
One of the seminal guidelines on screening lipids in children came from the National Heart, Lung, and Blood Institute (NHLBI), which in 2011 recommended children undergo dyslipidemia screening between the ages of 9 and 11 years and again between 17 and 21 years. Children should receive a screening starting at age 2 years if they have a family history of CVD or dyslipidemia or have diabetes, an elevated BMI, or hypertension. The American Academy of Pediatrics shortly followed suit, issuing similar recommendations.
Screening for the two subsets of ages was an expansion from the original 1992 guidelines from the National Cholesterol Education Program, which recommended screening only for children with either a family history of early CVD or elevated total cholesterol levels.
A 2011 panel for the NHLBI said the older approach identified significantly fewer children with abnormal levels of low-density lipoprotein cholesterol (LDL-C) than the addition of two age groups for screening, adding that many children do not have a complete family history. The American College of Cardiology and American Heart Association later supported NHLBI’s stance in their joint guidelines on the management of cholesterol.
Mark Corkins, MD, chair of the AAP’s Committee on Nutrition, told Medscape Medical News that if children are screened only because they have obesity or a family history of FH, some with elevated lipid levels will be missed. For instance, studies indicate caregiver recall of FH often is inaccurate, and the genetic disorder that causes the condition is not related to obesity.
“The screening is to find familial hypercholesterolemia, to try to find the ones that need therapy,” that would not be caught by the risk-based screening earlier on in childhood, Corkins said.
Only Screen Children With Risk Factors
But other groups do not agree. The US Preventive Services Task Force (USPSTF) found insufficient evidence to recommend for or against screening for lipid disorders in asymptomatic children and teens.
The group also said it found inadequate evidence that lipid-lowering interventions in the general pediatric population lead to reductions in cardiovascular events or all-cause mortality once they reached adulthood. USPSTF also raised questions about the safety of lipid-lowering drugs in children.
“The current evidence is insufficient to assess the balance of benefits and harms of screening for lipid disorders in children and adolescents 20 years or younger,” the panel wrote.
The American Academy of Family Physicians supports USPSTF’s recommendations.
Low Rate of Screening
While the uncertainty over screening in children continues, the practice has been adopted by a minority of clinicians.
A study published in JAMA Network Open in July found 9% of 700,000 9- to 11-year-olds had a documented result from a lipid screening. Among more than 1.3 million 17- to 21-year-olds, 13% had received a screening.
As BMI went up, so did screening rates. A little over 9% children and teens with a healthy weight were screened compared with 14.7% of those with moderate obesity and 21.9% of those with severe obesity.
Among those screened, 32.3% of 9- to 11-year-olds and 30.2% of 17- to 21-year-olds had abnormal lipid levels, defined as having one elevated measure out of five, including total cholesterol of 200 mg/dL or higher or LDL-C levels of 130 mg/dL or higher.
Justin Zachariah, MD, MPH, an associate professor of pediatrics-cardiology at Baylor College of Medicine in Houston, spoke about physicians screening children based only on factors like obesity during a presentation at the recent annual meeting of the American Academy of Pediatrics. He cited research showing roughly one in four children with abnormal lipids had a normal weight.
If a clinician is reserving a lipid screening for a child who is overweight or has obesity, “you’re missing nearly half the problem,” Zachariah said during his presentation.
One reason for the low rate of universal screening may be inattention to FH by clinicians, according to Samuel S. Gidding, MD, a professor in the Department of Genomic Health at Geisinger College of Health Sciences in Bridgewater Corners, Vermont.
For instance, a clinician has only a set amount of time during a well-child visit and other issues may take precedence, “so it doesn’t make sense to broach preventive screening for something that could happen 30 or 40 years from now, vs this [other] very immediate problem,” he said.
Clinicians “are triggered to act on the LDL level, but don’t think about FH as a possible diagnosis,” Gidding told Medscape Medical News.
Another barrier is that in some settings, caregivers must take children and teens to another facility on a different day to fulfill an order for a lipid test.
“It’s reluctance of doctors to order it, knowing patients won’t go through with it,” Gidding said.
Gidding is a consultant for Esperion Therapeutics. Other sources in this story reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Clinicians receive conflicting advice on whether to order blood tests to screen for lipids in children. A new study could add to the confusion. Researchers found that a combination of physical proxy measures such as hypertension and body mass index (BMI) predicted the risk for future cardiovascular events as well as the physical model plus lipid labs, questioning the value of those blood tests.
Some medical organizations advise screening only for high-risk children because more research is needed to define the harms and benefits of universal screening. Diet and behavioral changes are sufficient for most children, and universal screening could lead to false positives and unnecessary further testing, they said.
Groups that favor lipid tests for all children say these measurements detect familial hypercholesterolemia (FH) that would not otherwise be diagnosed, leading to treatment with drugs like statins and a greater chance of preventing cardiovascular disease (CVD) in adulthood.
Researchers from the new study said their findings do not address screenings for FH, which affects 1 in 250 US children and puts them at a risk for atherosclerotic CVD.
Recommending Blood Tests in Age Groups
One of the seminal guidelines on screening lipids in children came from the National Heart, Lung, and Blood Institute (NHLBI), which in 2011 recommended children undergo dyslipidemia screening between the ages of 9 and 11 years and again between 17 and 21 years. Children should receive a screening starting at age 2 years if they have a family history of CVD or dyslipidemia or have diabetes, an elevated BMI, or hypertension. The American Academy of Pediatrics shortly followed suit, issuing similar recommendations.
Screening for the two subsets of ages was an expansion from the original 1992 guidelines from the National Cholesterol Education Program, which recommended screening only for children with either a family history of early CVD or elevated total cholesterol levels.
A 2011 panel for the NHLBI said the older approach identified significantly fewer children with abnormal levels of low-density lipoprotein cholesterol (LDL-C) than the addition of two age groups for screening, adding that many children do not have a complete family history. The American College of Cardiology and American Heart Association later supported NHLBI’s stance in their joint guidelines on the management of cholesterol.
Mark Corkins, MD, chair of the AAP’s Committee on Nutrition, told Medscape Medical News that if children are screened only because they have obesity or a family history of FH, some with elevated lipid levels will be missed. For instance, studies indicate caregiver recall of FH often is inaccurate, and the genetic disorder that causes the condition is not related to obesity.
“The screening is to find familial hypercholesterolemia, to try to find the ones that need therapy,” that would not be caught by the risk-based screening earlier on in childhood, Corkins said.
Only Screen Children With Risk Factors
But other groups do not agree. The US Preventive Services Task Force (USPSTF) found insufficient evidence to recommend for or against screening for lipid disorders in asymptomatic children and teens.
The group also said it found inadequate evidence that lipid-lowering interventions in the general pediatric population lead to reductions in cardiovascular events or all-cause mortality once they reached adulthood. USPSTF also raised questions about the safety of lipid-lowering drugs in children.
“The current evidence is insufficient to assess the balance of benefits and harms of screening for lipid disorders in children and adolescents 20 years or younger,” the panel wrote.
The American Academy of Family Physicians supports USPSTF’s recommendations.
Low Rate of Screening
While the uncertainty over screening in children continues, the practice has been adopted by a minority of clinicians.
A study published in JAMA Network Open in July found 9% of 700,000 9- to 11-year-olds had a documented result from a lipid screening. Among more than 1.3 million 17- to 21-year-olds, 13% had received a screening.
As BMI went up, so did screening rates. A little over 9% children and teens with a healthy weight were screened compared with 14.7% of those with moderate obesity and 21.9% of those with severe obesity.
Among those screened, 32.3% of 9- to 11-year-olds and 30.2% of 17- to 21-year-olds had abnormal lipid levels, defined as having one elevated measure out of five, including total cholesterol of 200 mg/dL or higher or LDL-C levels of 130 mg/dL or higher.
Justin Zachariah, MD, MPH, an associate professor of pediatrics-cardiology at Baylor College of Medicine in Houston, spoke about physicians screening children based only on factors like obesity during a presentation at the recent annual meeting of the American Academy of Pediatrics. He cited research showing roughly one in four children with abnormal lipids had a normal weight.
If a clinician is reserving a lipid screening for a child who is overweight or has obesity, “you’re missing nearly half the problem,” Zachariah said during his presentation.
One reason for the low rate of universal screening may be inattention to FH by clinicians, according to Samuel S. Gidding, MD, a professor in the Department of Genomic Health at Geisinger College of Health Sciences in Bridgewater Corners, Vermont.
For instance, a clinician has only a set amount of time during a well-child visit and other issues may take precedence, “so it doesn’t make sense to broach preventive screening for something that could happen 30 or 40 years from now, vs this [other] very immediate problem,” he said.
Clinicians “are triggered to act on the LDL level, but don’t think about FH as a possible diagnosis,” Gidding told Medscape Medical News.
Another barrier is that in some settings, caregivers must take children and teens to another facility on a different day to fulfill an order for a lipid test.
“It’s reluctance of doctors to order it, knowing patients won’t go through with it,” Gidding said.
Gidding is a consultant for Esperion Therapeutics. Other sources in this story reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Clinicians receive conflicting advice on whether to order blood tests to screen for lipids in children. A new study could add to the confusion. Researchers found that a combination of physical proxy measures such as hypertension and body mass index (BMI) predicted the risk for future cardiovascular events as well as the physical model plus lipid labs, questioning the value of those blood tests.
Some medical organizations advise screening only for high-risk children because more research is needed to define the harms and benefits of universal screening. Diet and behavioral changes are sufficient for most children, and universal screening could lead to false positives and unnecessary further testing, they said.
Groups that favor lipid tests for all children say these measurements detect familial hypercholesterolemia (FH) that would not otherwise be diagnosed, leading to treatment with drugs like statins and a greater chance of preventing cardiovascular disease (CVD) in adulthood.
Researchers from the new study said their findings do not address screenings for FH, which affects 1 in 250 US children and puts them at a risk for atherosclerotic CVD.
Recommending Blood Tests in Age Groups
One of the seminal guidelines on screening lipids in children came from the National Heart, Lung, and Blood Institute (NHLBI), which in 2011 recommended children undergo dyslipidemia screening between the ages of 9 and 11 years and again between 17 and 21 years. Children should receive a screening starting at age 2 years if they have a family history of CVD or dyslipidemia or have diabetes, an elevated BMI, or hypertension. The American Academy of Pediatrics shortly followed suit, issuing similar recommendations.
Screening for the two subsets of ages was an expansion from the original 1992 guidelines from the National Cholesterol Education Program, which recommended screening only for children with either a family history of early CVD or elevated total cholesterol levels.
A 2011 panel for the NHLBI said the older approach identified significantly fewer children with abnormal levels of low-density lipoprotein cholesterol (LDL-C) than the addition of two age groups for screening, adding that many children do not have a complete family history. The American College of Cardiology and American Heart Association later supported NHLBI’s stance in their joint guidelines on the management of cholesterol.
Mark Corkins, MD, chair of the AAP’s Committee on Nutrition, told Medscape Medical News that if children are screened only because they have obesity or a family history of FH, some with elevated lipid levels will be missed. For instance, studies indicate caregiver recall of FH often is inaccurate, and the genetic disorder that causes the condition is not related to obesity.
“The screening is to find familial hypercholesterolemia, to try to find the ones that need therapy,” that would not be caught by the risk-based screening earlier on in childhood, Corkins said.
Only Screen Children With Risk Factors
But other groups do not agree. The US Preventive Services Task Force (USPSTF) found insufficient evidence to recommend for or against screening for lipid disorders in asymptomatic children and teens.
The group also said it found inadequate evidence that lipid-lowering interventions in the general pediatric population lead to reductions in cardiovascular events or all-cause mortality once they reached adulthood. USPSTF also raised questions about the safety of lipid-lowering drugs in children.
“The current evidence is insufficient to assess the balance of benefits and harms of screening for lipid disorders in children and adolescents 20 years or younger,” the panel wrote.
The American Academy of Family Physicians supports USPSTF’s recommendations.
Low Rate of Screening
While the uncertainty over screening in children continues, the practice has been adopted by a minority of clinicians.
A study published in JAMA Network Open in July found 9% of 700,000 9- to 11-year-olds had a documented result from a lipid screening. Among more than 1.3 million 17- to 21-year-olds, 13% had received a screening.
As BMI went up, so did screening rates. A little over 9% children and teens with a healthy weight were screened compared with 14.7% of those with moderate obesity and 21.9% of those with severe obesity.
Among those screened, 32.3% of 9- to 11-year-olds and 30.2% of 17- to 21-year-olds had abnormal lipid levels, defined as having one elevated measure out of five, including total cholesterol of 200 mg/dL or higher or LDL-C levels of 130 mg/dL or higher.
Justin Zachariah, MD, MPH, an associate professor of pediatrics-cardiology at Baylor College of Medicine in Houston, spoke about physicians screening children based only on factors like obesity during a presentation at the recent annual meeting of the American Academy of Pediatrics. He cited research showing roughly one in four children with abnormal lipids had a normal weight.
If a clinician is reserving a lipid screening for a child who is overweight or has obesity, “you’re missing nearly half the problem,” Zachariah said during his presentation.
One reason for the low rate of universal screening may be inattention to FH by clinicians, according to Samuel S. Gidding, MD, a professor in the Department of Genomic Health at Geisinger College of Health Sciences in Bridgewater Corners, Vermont.
For instance, a clinician has only a set amount of time during a well-child visit and other issues may take precedence, “so it doesn’t make sense to broach preventive screening for something that could happen 30 or 40 years from now, vs this [other] very immediate problem,” he said.
Clinicians “are triggered to act on the LDL level, but don’t think about FH as a possible diagnosis,” Gidding told Medscape Medical News.
Another barrier is that in some settings, caregivers must take children and teens to another facility on a different day to fulfill an order for a lipid test.
“It’s reluctance of doctors to order it, knowing patients won’t go through with it,” Gidding said.
Gidding is a consultant for Esperion Therapeutics. Other sources in this story reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.